User login
Influenza update
2018-2019 season retrospective
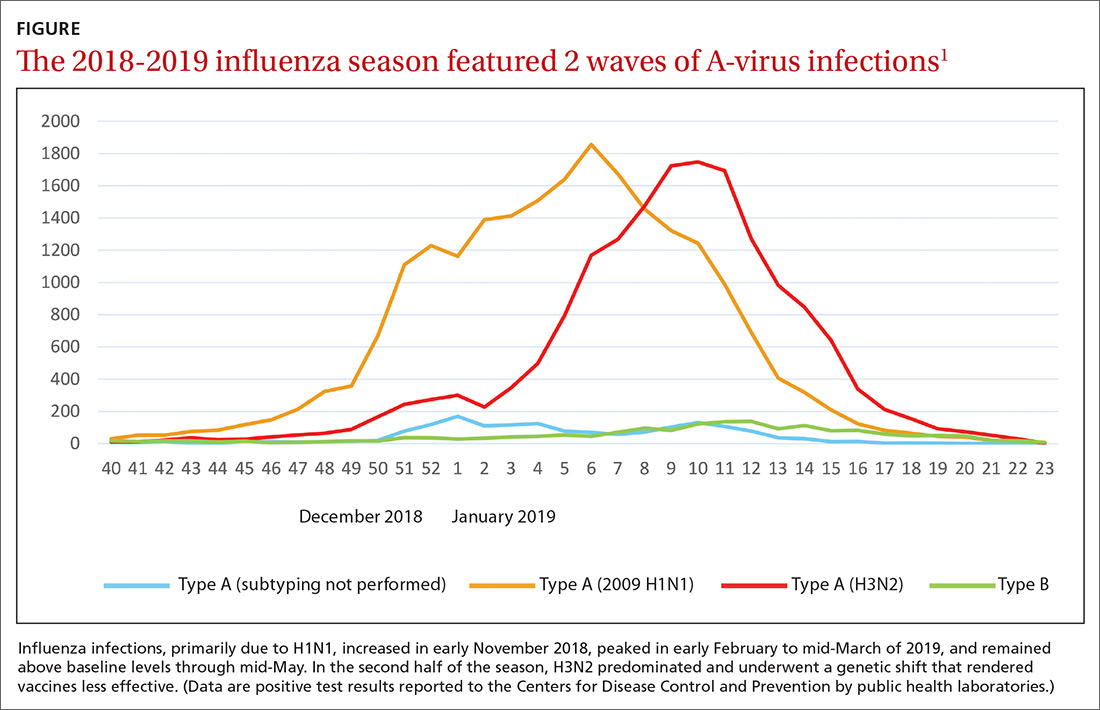
Last year’s influenza season was longer than usual. Infections, as measured by the percentage of outpatient visits due to influenza-like illness, increased in early November 2018, peaked in early February to mid-March of 2019, and remained above baseline levels through mid-May.1,2 Ninety six percent of influenza-positive samples were influenza A,1 and 57% of those were H1N1.2 In the second half of the season, H3N2 became the predominant circulating virus and there was a genetic shift in this strain that caused a decrease in the effectiveness of influenza vaccines (FIGURE).1 The influenza-confirmed hospitalization rate was 65.3/100,000, with the highest rate (221.7/100,000) occurring among those 65 years of age and older.2 Of those hospitalized with influenza, 93% of adults and 55% of children had an underlying medical condition and 29% of women of childbearing age were pregnant.2
Morbidity and mortality from influenza during the 2018-2019 influenza season were moderate compared with previous years. Pneumonia and influenza mortality reached close to 8% of all deaths during the peak of the season (considered a modest peak), but stayed above the epidemic threshold for 10 weeks.2 There were 119 pediatric deaths.1 Overall, in the United States, there were an estimated 37 to 43 million influenza-related illnesses, 17 to 20 million flu-related medical visits, 531,000 to 647,000 flu-related hospitalizations, and 36,400 to 61,200 deaths.1
Influenza viral resistance to oseltamivir remained very low throughout the season for both A and B viruses.2
Vaccine effectiveness was subpar
The effectiveness of influenza vaccine last season was disappointing. When assessed using laboratory-confirmed medically attended influenza, the vaccine was 29% effective; when assessed by age group, the confidence intervals included 0 in ages 9 to 17 years and 50 years and older.3 In the age group 6 months to 8 years, the vaccine was 49% effective.3 The vaccine was not effective against the predominant H3N2 strain circulating. It was 25% effective in preventing hospitalization, with a lack of benefit seen in individuals ages 18 to 49 years and those 65 and older.3
Vaccination was associated with increased rates of hospitalizations from infections cause by H3N2. It is not known if this finding was due to chance, unstable results from small numbers, an unknown bias, or some biological cause not yet understood. This is a topic of ongoing research.
Effectiveness in preventing pediatric hospitalizations was estimated at 31%, again with no effectiveness against H3N2.3 The estimate of vaccine effectiveness in the United States was similar to that in Canada.2
While these results are much lower than desired, influenza vaccine did prevent an estimated 40,000 to 90,000 hospitalizations and decreased influenza-like illnesses by 44%.3
Continue to: A look at vaccine safety
A look at vaccine safety
Numerous studies of influenza vaccine safety were presented at the June 2019 meeting of the Advisory Committee on Immunization Practices (ACIP).4 These studies included assessments using the Vaccine Adverse Events Reporting System; the Vaccine Safety Datalink (VSD), which conducts ongoing rapid analysis of adverse events throughout the influenza season; and Food and Drug Administration (FDA)-sponsored studies of Medicare patients. These vaccine safety monitoring systems have been described in a prior Practice Alert.5
Possible vaccine reactions studied included Guillain-Barre Syndrome (GBS), anaphylaxis, encephalitis, Bell’s palsy, febrile seizures, and pregnancy-related adverse events such as miscarriage and congenital anomalies. While preliminary safety signals were detected for anaphylaxis, Bell’s palsy, febrile seizures, and GBS, a more in-depth investigation found no association of any adverse events with vaccination except for febrile seizures, with an attributable risk of 4.24/100,000 doses in children ages 6 to 23 months and 1.8/100,000 in those ages 24 to 59 months.4 The incidence of febrile seizures was similar to that of previous seasons and primarily occurred when the vaccine was administered in conjunction with another vaccine. A preliminary FDA analysis found a small elevated risk of GBS with high-dose trivalent inactivated vaccine, with an attributable risk of 0.98 per million doses, but this was not confirmed by the VSD analysis.4
What you need to know about the upcoming season
ACIP recommendations on influenza vaccines for 2019 to 2020 are essentially unchanged from last year.6 All individuals ages 6 months and older, who do not have a contraindication, should receive a flu vaccine in the fall of 2019. The composition of this season’s vaccine contains new H1N1 and H3N2 variants to more closely match the circulating strains. ACIP has updated or clarified 4 logistical issues in this year’s recommendations:
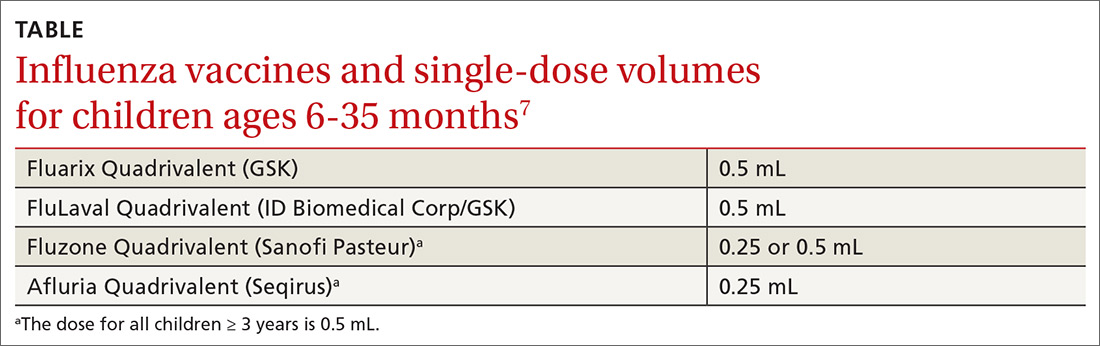
- Four inactivated-influenza vaccines are now available for children ages 6 to 35 months. Dose volumes are not the same for all 4 (TABLE).7
- Vaccination is now encouraged for September or later for those requiring only 1 dose of vaccine. Earlier administration can result in waning immunity by the end of the flu season, especially in older adults.7
- Children ages 6 months to 8 years may require 2 doses if they haven’t received any previous influenza vaccine, and the second dose should be given even if the child turns 9 between doses 1 and 2.7
- One adjuvanted influenza vaccine containing MF59—the trivalent inactivated influenza vaccine, Fluad—is approved for those ages 65 years and older. One note of caution is that licensed vaccines for other conditions also contain new nonaluminum adjuvants and there are few data on the safety and effectiveness of simultaneous or sequential administration of Fluad with the 2 novel nonaluminum adjuvant-containing vaccines. These vaccines are the recombinant zoster subunit vaccine (Shingrix), which contains the liposome-based adjuvant ASO1, and the recombinant hepatitis B surface antigen vaccine (Heplisav-B), which contains cytosine phosphoguanine oligodeoxynucleotide. Given the lack of data and the availability of other influenza vaccine options, ACIP advises that selecting a nonadjuvanted influenza vaccine may be the best option when an older adult needs both an influenza vaccine and either Shingrix or Heplisav-B. However, do not delay giving any vaccine if a specific alternate product is unavailable.7
All recommendations concerning the use of influenza vaccine for the 2019-2020 influenza season and a listing of all available influenza vaccine products can be found on the ACIP Web site (cdc.gov/vaccines/acip/index.html) or in the Morbidity and Mortality Weekly Report.8
1. Brammer L. Influenza Surveillance Update. Presented to the ACIP June 27, 2019. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/flu-2-Brammer-508.pdf. Accessed August 21, 2019.
2. Hammond A, Hundal K, Laurenson-Shafer H, et al. Review of the 2018–2019 influenza season in the northern hemisphere. WHO Wkly Epidemiol Record. 2019;94:345-364.
3. Flannery B, Chung J, Ferdinands J, et al. Preliminary estimates of the 2018-2019 seasonal influenza vaccine effectiveness against medically attended influenza from three U.S. networks. Presented to ACIP June 27, 2019. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/flu-3-flannery-508.pdf. Accessed August 21, 2019.
4. Shimabukuro T. End-of-season update: 2018-2019 influenza vaccine safety monitoring. Presented to the ACIP meeting June 27, 2019. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/flu-4-Shimabukuro-508.pdf. Accessed August 21, 2019.
5. Campos-Outcalt D. Facts to help you keep pace with the vaccine conversation. J Fam Pract. 2019;68:341-346.
6. Campos-Outcalt D. CDC recommendations for the 2018-2019 influenza season. J Fam Pract. 2018;67:550-553.
7. Grohskopf L. Influenza work group considerations and proposed 2019-2020 season recommendations. Presented to the ACIP June 27, 2019. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/flu-5-grohskopf-508.pdf. Accessed August 21, 2019.
8. Grohskopf LA, Alyanak E, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices —United States, 2019-20 influenza season. MMWR Recomm Rep. 2019;68:1-21.
2018-2019 season retrospective
Last year’s influenza season was longer than usual. Infections, as measured by the percentage of outpatient visits due to influenza-like illness, increased in early November 2018, peaked in early February to mid-March of 2019, and remained above baseline levels through mid-May.1,2 Ninety six percent of influenza-positive samples were influenza A,1 and 57% of those were H1N1.2 In the second half of the season, H3N2 became the predominant circulating virus and there was a genetic shift in this strain that caused a decrease in the effectiveness of influenza vaccines (FIGURE).1 The influenza-confirmed hospitalization rate was 65.3/100,000, with the highest rate (221.7/100,000) occurring among those 65 years of age and older.2 Of those hospitalized with influenza, 93% of adults and 55% of children had an underlying medical condition and 29% of women of childbearing age were pregnant.2

Morbidity and mortality from influenza during the 2018-2019 influenza season were moderate compared with previous years. Pneumonia and influenza mortality reached close to 8% of all deaths during the peak of the season (considered a modest peak), but stayed above the epidemic threshold for 10 weeks.2 There were 119 pediatric deaths.1 Overall, in the United States, there were an estimated 37 to 43 million influenza-related illnesses, 17 to 20 million flu-related medical visits, 531,000 to 647,000 flu-related hospitalizations, and 36,400 to 61,200 deaths.1
Influenza viral resistance to oseltamivir remained very low throughout the season for both A and B viruses.2
Vaccine effectiveness was subpar
The effectiveness of influenza vaccine last season was disappointing. When assessed using laboratory-confirmed medically attended influenza, the vaccine was 29% effective; when assessed by age group, the confidence intervals included 0 in ages 9 to 17 years and 50 years and older.3 In the age group 6 months to 8 years, the vaccine was 49% effective.3 The vaccine was not effective against the predominant H3N2 strain circulating. It was 25% effective in preventing hospitalization, with a lack of benefit seen in individuals ages 18 to 49 years and those 65 and older.3
Vaccination was associated with increased rates of hospitalizations from infections cause by H3N2. It is not known if this finding was due to chance, unstable results from small numbers, an unknown bias, or some biological cause not yet understood. This is a topic of ongoing research.
Effectiveness in preventing pediatric hospitalizations was estimated at 31%, again with no effectiveness against H3N2.3 The estimate of vaccine effectiveness in the United States was similar to that in Canada.2
While these results are much lower than desired, influenza vaccine did prevent an estimated 40,000 to 90,000 hospitalizations and decreased influenza-like illnesses by 44%.3
Continue to: A look at vaccine safety
A look at vaccine safety
Numerous studies of influenza vaccine safety were presented at the June 2019 meeting of the Advisory Committee on Immunization Practices (ACIP).4 These studies included assessments using the Vaccine Adverse Events Reporting System; the Vaccine Safety Datalink (VSD), which conducts ongoing rapid analysis of adverse events throughout the influenza season; and Food and Drug Administration (FDA)-sponsored studies of Medicare patients. These vaccine safety monitoring systems have been described in a prior Practice Alert.5
Possible vaccine reactions studied included Guillain-Barre Syndrome (GBS), anaphylaxis, encephalitis, Bell’s palsy, febrile seizures, and pregnancy-related adverse events such as miscarriage and congenital anomalies. While preliminary safety signals were detected for anaphylaxis, Bell’s palsy, febrile seizures, and GBS, a more in-depth investigation found no association of any adverse events with vaccination except for febrile seizures, with an attributable risk of 4.24/100,000 doses in children ages 6 to 23 months and 1.8/100,000 in those ages 24 to 59 months.4 The incidence of febrile seizures was similar to that of previous seasons and primarily occurred when the vaccine was administered in conjunction with another vaccine. A preliminary FDA analysis found a small elevated risk of GBS with high-dose trivalent inactivated vaccine, with an attributable risk of 0.98 per million doses, but this was not confirmed by the VSD analysis.4
What you need to know about the upcoming season
ACIP recommendations on influenza vaccines for 2019 to 2020 are essentially unchanged from last year.6 All individuals ages 6 months and older, who do not have a contraindication, should receive a flu vaccine in the fall of 2019. The composition of this season’s vaccine contains new H1N1 and H3N2 variants to more closely match the circulating strains. ACIP has updated or clarified 4 logistical issues in this year’s recommendations:
- Four inactivated-influenza vaccines are now available for children ages 6 to 35 months. Dose volumes are not the same for all 4 (TABLE).7
- Vaccination is now encouraged for September or later for those requiring only 1 dose of vaccine. Earlier administration can result in waning immunity by the end of the flu season, especially in older adults.7
- Children ages 6 months to 8 years may require 2 doses if they haven’t received any previous influenza vaccine, and the second dose should be given even if the child turns 9 between doses 1 and 2.7
- One adjuvanted influenza vaccine containing MF59—the trivalent inactivated influenza vaccine, Fluad—is approved for those ages 65 years and older. One note of caution is that licensed vaccines for other conditions also contain new nonaluminum adjuvants and there are few data on the safety and effectiveness of simultaneous or sequential administration of Fluad with the 2 novel nonaluminum adjuvant-containing vaccines. These vaccines are the recombinant zoster subunit vaccine (Shingrix), which contains the liposome-based adjuvant ASO1, and the recombinant hepatitis B surface antigen vaccine (Heplisav-B), which contains cytosine phosphoguanine oligodeoxynucleotide. Given the lack of data and the availability of other influenza vaccine options, ACIP advises that selecting a nonadjuvanted influenza vaccine may be the best option when an older adult needs both an influenza vaccine and either Shingrix or Heplisav-B. However, do not delay giving any vaccine if a specific alternate product is unavailable.7

All recommendations concerning the use of influenza vaccine for the 2019-2020 influenza season and a listing of all available influenza vaccine products can be found on the ACIP Web site (cdc.gov/vaccines/acip/index.html) or in the Morbidity and Mortality Weekly Report.8
2018-2019 season retrospective
Last year’s influenza season was longer than usual. Infections, as measured by the percentage of outpatient visits due to influenza-like illness, increased in early November 2018, peaked in early February to mid-March of 2019, and remained above baseline levels through mid-May.1,2 Ninety six percent of influenza-positive samples were influenza A,1 and 57% of those were H1N1.2 In the second half of the season, H3N2 became the predominant circulating virus and there was a genetic shift in this strain that caused a decrease in the effectiveness of influenza vaccines (FIGURE).1 The influenza-confirmed hospitalization rate was 65.3/100,000, with the highest rate (221.7/100,000) occurring among those 65 years of age and older.2 Of those hospitalized with influenza, 93% of adults and 55% of children had an underlying medical condition and 29% of women of childbearing age were pregnant.2

Morbidity and mortality from influenza during the 2018-2019 influenza season were moderate compared with previous years. Pneumonia and influenza mortality reached close to 8% of all deaths during the peak of the season (considered a modest peak), but stayed above the epidemic threshold for 10 weeks.2 There were 119 pediatric deaths.1 Overall, in the United States, there were an estimated 37 to 43 million influenza-related illnesses, 17 to 20 million flu-related medical visits, 531,000 to 647,000 flu-related hospitalizations, and 36,400 to 61,200 deaths.1
Influenza viral resistance to oseltamivir remained very low throughout the season for both A and B viruses.2
Vaccine effectiveness was subpar
The effectiveness of influenza vaccine last season was disappointing. When assessed using laboratory-confirmed medically attended influenza, the vaccine was 29% effective; when assessed by age group, the confidence intervals included 0 in ages 9 to 17 years and 50 years and older.3 In the age group 6 months to 8 years, the vaccine was 49% effective.3 The vaccine was not effective against the predominant H3N2 strain circulating. It was 25% effective in preventing hospitalization, with a lack of benefit seen in individuals ages 18 to 49 years and those 65 and older.3
Vaccination was associated with increased rates of hospitalizations from infections cause by H3N2. It is not known if this finding was due to chance, unstable results from small numbers, an unknown bias, or some biological cause not yet understood. This is a topic of ongoing research.
Effectiveness in preventing pediatric hospitalizations was estimated at 31%, again with no effectiveness against H3N2.3 The estimate of vaccine effectiveness in the United States was similar to that in Canada.2
While these results are much lower than desired, influenza vaccine did prevent an estimated 40,000 to 90,000 hospitalizations and decreased influenza-like illnesses by 44%.3
Continue to: A look at vaccine safety
A look at vaccine safety
Numerous studies of influenza vaccine safety were presented at the June 2019 meeting of the Advisory Committee on Immunization Practices (ACIP).4 These studies included assessments using the Vaccine Adverse Events Reporting System; the Vaccine Safety Datalink (VSD), which conducts ongoing rapid analysis of adverse events throughout the influenza season; and Food and Drug Administration (FDA)-sponsored studies of Medicare patients. These vaccine safety monitoring systems have been described in a prior Practice Alert.5
Possible vaccine reactions studied included Guillain-Barre Syndrome (GBS), anaphylaxis, encephalitis, Bell’s palsy, febrile seizures, and pregnancy-related adverse events such as miscarriage and congenital anomalies. While preliminary safety signals were detected for anaphylaxis, Bell’s palsy, febrile seizures, and GBS, a more in-depth investigation found no association of any adverse events with vaccination except for febrile seizures, with an attributable risk of 4.24/100,000 doses in children ages 6 to 23 months and 1.8/100,000 in those ages 24 to 59 months.4 The incidence of febrile seizures was similar to that of previous seasons and primarily occurred when the vaccine was administered in conjunction with another vaccine. A preliminary FDA analysis found a small elevated risk of GBS with high-dose trivalent inactivated vaccine, with an attributable risk of 0.98 per million doses, but this was not confirmed by the VSD analysis.4
What you need to know about the upcoming season
ACIP recommendations on influenza vaccines for 2019 to 2020 are essentially unchanged from last year.6 All individuals ages 6 months and older, who do not have a contraindication, should receive a flu vaccine in the fall of 2019. The composition of this season’s vaccine contains new H1N1 and H3N2 variants to more closely match the circulating strains. ACIP has updated or clarified 4 logistical issues in this year’s recommendations:
- Four inactivated-influenza vaccines are now available for children ages 6 to 35 months. Dose volumes are not the same for all 4 (TABLE).7
- Vaccination is now encouraged for September or later for those requiring only 1 dose of vaccine. Earlier administration can result in waning immunity by the end of the flu season, especially in older adults.7
- Children ages 6 months to 8 years may require 2 doses if they haven’t received any previous influenza vaccine, and the second dose should be given even if the child turns 9 between doses 1 and 2.7
- One adjuvanted influenza vaccine containing MF59—the trivalent inactivated influenza vaccine, Fluad—is approved for those ages 65 years and older. One note of caution is that licensed vaccines for other conditions also contain new nonaluminum adjuvants and there are few data on the safety and effectiveness of simultaneous or sequential administration of Fluad with the 2 novel nonaluminum adjuvant-containing vaccines. These vaccines are the recombinant zoster subunit vaccine (Shingrix), which contains the liposome-based adjuvant ASO1, and the recombinant hepatitis B surface antigen vaccine (Heplisav-B), which contains cytosine phosphoguanine oligodeoxynucleotide. Given the lack of data and the availability of other influenza vaccine options, ACIP advises that selecting a nonadjuvanted influenza vaccine may be the best option when an older adult needs both an influenza vaccine and either Shingrix or Heplisav-B. However, do not delay giving any vaccine if a specific alternate product is unavailable.7

All recommendations concerning the use of influenza vaccine for the 2019-2020 influenza season and a listing of all available influenza vaccine products can be found on the ACIP Web site (cdc.gov/vaccines/acip/index.html) or in the Morbidity and Mortality Weekly Report.8
1. Brammer L. Influenza Surveillance Update. Presented to the ACIP June 27, 2019. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/flu-2-Brammer-508.pdf. Accessed August 21, 2019.
2. Hammond A, Hundal K, Laurenson-Shafer H, et al. Review of the 2018–2019 influenza season in the northern hemisphere. WHO Wkly Epidemiol Record. 2019;94:345-364.
3. Flannery B, Chung J, Ferdinands J, et al. Preliminary estimates of the 2018-2019 seasonal influenza vaccine effectiveness against medically attended influenza from three U.S. networks. Presented to ACIP June 27, 2019. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/flu-3-flannery-508.pdf. Accessed August 21, 2019.
4. Shimabukuro T. End-of-season update: 2018-2019 influenza vaccine safety monitoring. Presented to the ACIP meeting June 27, 2019. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/flu-4-Shimabukuro-508.pdf. Accessed August 21, 2019.
5. Campos-Outcalt D. Facts to help you keep pace with the vaccine conversation. J Fam Pract. 2019;68:341-346.
6. Campos-Outcalt D. CDC recommendations for the 2018-2019 influenza season. J Fam Pract. 2018;67:550-553.
7. Grohskopf L. Influenza work group considerations and proposed 2019-2020 season recommendations. Presented to the ACIP June 27, 2019. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/flu-5-grohskopf-508.pdf. Accessed August 21, 2019.
8. Grohskopf LA, Alyanak E, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices —United States, 2019-20 influenza season. MMWR Recomm Rep. 2019;68:1-21.
1. Brammer L. Influenza Surveillance Update. Presented to the ACIP June 27, 2019. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/flu-2-Brammer-508.pdf. Accessed August 21, 2019.
2. Hammond A, Hundal K, Laurenson-Shafer H, et al. Review of the 2018–2019 influenza season in the northern hemisphere. WHO Wkly Epidemiol Record. 2019;94:345-364.
3. Flannery B, Chung J, Ferdinands J, et al. Preliminary estimates of the 2018-2019 seasonal influenza vaccine effectiveness against medically attended influenza from three U.S. networks. Presented to ACIP June 27, 2019. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/flu-3-flannery-508.pdf. Accessed August 21, 2019.
4. Shimabukuro T. End-of-season update: 2018-2019 influenza vaccine safety monitoring. Presented to the ACIP meeting June 27, 2019. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/flu-4-Shimabukuro-508.pdf. Accessed August 21, 2019.
5. Campos-Outcalt D. Facts to help you keep pace with the vaccine conversation. J Fam Pract. 2019;68:341-346.
6. Campos-Outcalt D. CDC recommendations for the 2018-2019 influenza season. J Fam Pract. 2018;67:550-553.
7. Grohskopf L. Influenza work group considerations and proposed 2019-2020 season recommendations. Presented to the ACIP June 27, 2019. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/flu-5-grohskopf-508.pdf. Accessed August 21, 2019.
8. Grohskopf LA, Alyanak E, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices —United States, 2019-20 influenza season. MMWR Recomm Rep. 2019;68:1-21.
USPSTF BRCA testing recs: 2 more groups require attention
Reference
1. US Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer. JAMA. 2019;322:652-665.
Reference
1. US Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer. JAMA. 2019;322:652-665.
Reference
1. US Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer. JAMA. 2019;322:652-665.
ACIP issues 2 new recs on HPV vaccination
References
1. Markowitz L. Overview and background (HPV). CDC Web site. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/HPV-2-Markowitz-508.pdf. Presented February 27, 2019. Accessed August 1, 2019.
2. Brisson M, Laprise J-F. Cost-effectiveness of extending HPV vaccination above age 26 years in the U.S. CDC Web site. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/HPV-3-Brisson-508.pdf. Presented February 2019. Accessed August 1, 2019.
3. Markowitz L. Recommendations for mid-adult HPV vaccination work group considerations. CDC Web site. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/HPV-7-Markowitz-508.pdf, Presented February 27, 2019. Accessed August 1, 2019.
4. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
References
1. Markowitz L. Overview and background (HPV). CDC Web site. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/HPV-2-Markowitz-508.pdf. Presented February 27, 2019. Accessed August 1, 2019.
2. Brisson M, Laprise J-F. Cost-effectiveness of extending HPV vaccination above age 26 years in the U.S. CDC Web site. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/HPV-3-Brisson-508.pdf. Presented February 2019. Accessed August 1, 2019.
3. Markowitz L. Recommendations for mid-adult HPV vaccination work group considerations. CDC Web site. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/HPV-7-Markowitz-508.pdf, Presented February 27, 2019. Accessed August 1, 2019.
4. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
References
1. Markowitz L. Overview and background (HPV). CDC Web site. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/HPV-2-Markowitz-508.pdf. Presented February 27, 2019. Accessed August 1, 2019.
2. Brisson M, Laprise J-F. Cost-effectiveness of extending HPV vaccination above age 26 years in the U.S. CDC Web site. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/HPV-3-Brisson-508.pdf. Presented February 2019. Accessed August 1, 2019.
3. Markowitz L. Recommendations for mid-adult HPV vaccination work group considerations. CDC Web site. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/HPV-7-Markowitz-508.pdf, Presented February 27, 2019. Accessed August 1, 2019.
4. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
Facts to help you keep pace with the vaccine conversation
The current increase in measles cases in the United States has sharpened the focus on antivaccine activities. While the percentage of US children who are fully vaccinated remains high (≥ 94%), the number of un- or undervaccinated children has been growing1 because of nonmedical exemptions from school vaccine requirements due to concerns about vaccine safety and an underappreciation of the benefits of vaccines. Family physicians need to be conversant with several important aspects of this matter, including the magnitude of benefits provided by childhood vaccines, as well as the systems already in place for
- assessing vaccine effectiveness and safety,
- making recommendations on the use of vaccines,
- monitoring safety after vaccine approval, and
- compensating those affected by rare but serious vaccine-related adverse events (AEs).
Familiarity with these issues will allow for informed discussions with parents who are vaccine hesitant and with those who have read or heard inaccurate information.
The benefits of vaccines are indisputable
In 1999, the Centers for Disease Control and Prevention (CDC) published a list of 9 selected childhood infectious diseases and compared their incidences before and after immunization was available.2 Each of these infections causes morbidity, sequelae, and mortality at predictable rates depending on the infectious agent. The comparisons were dramatic: Measles, with a baseline annual morbidity of 503,282 cases, fell to just 89 cases; poliomyelitis decreased from 16,316 to 0; and Haemophilus influenzae type b declined from 20,000 to 54. In a 2014 analysis, the CDC stated that “among 78.6 million children born during 1994–2013, routine childhood immunization was estimated to prevent 322 million illnesses (averaging 4.1 illnesses per child) and 21 million hospitalizations (0.27 per child) over the course of their lifetimes and avert 732,000 premature deaths from vaccine-preventable illnesses” (TABLE).3
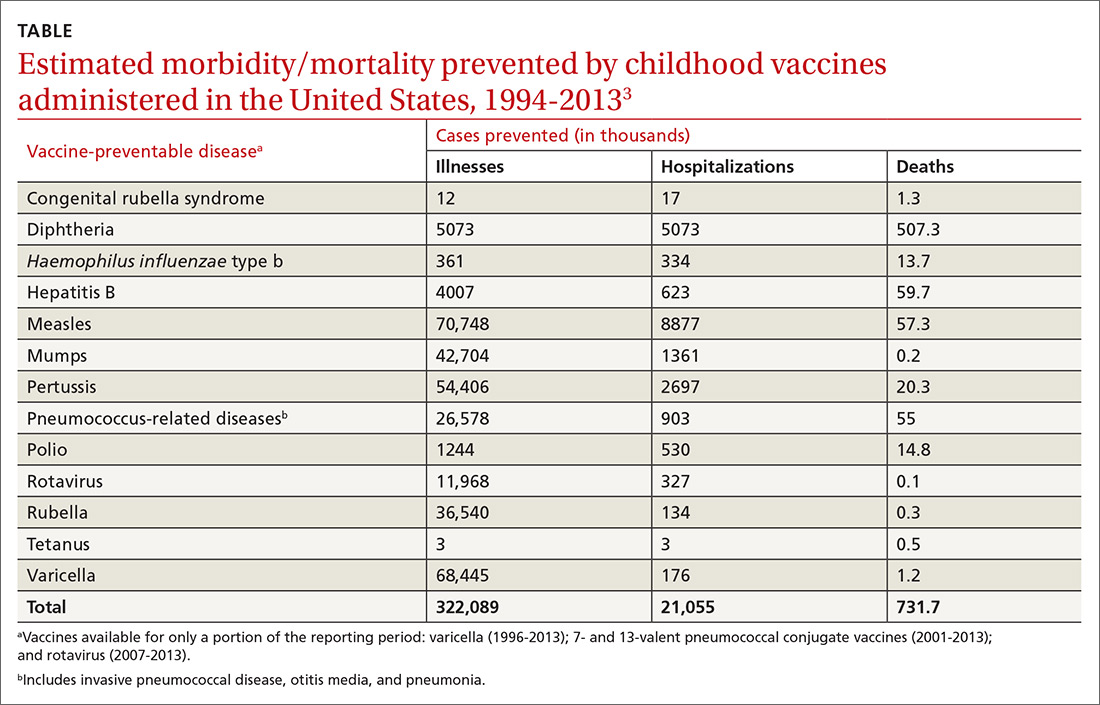
It is not unusual to hear a vaccine opponent say that childhood infectious diseases are not serious and that it is better for a child to contract the infection and let the immune system fight it naturally. Measles is often used as an example. This argument ignores some important aspects of vaccine benefits.
It is true in the United States that the average child who contracts measles will recover from it and not suffer immediate or long-term effects. However, it is also true that measles has a hospitalization rate of about 20% and a death rate of between 1/500 and 1/1000 cases.4 Mortality is much higher in developing countries. Prior to widespread use of measles vaccine, hundreds of thousands of cases of measles occurred each year. That translated into hundreds of preventable child deaths per year. An individual case does not tell the full story about the public health impact of infectious illnesses.
In addition, there are often unappreciated sequelae from child infections, such as shingles occurring years after resolution of a chickenpox infection. There are also societal consequences of child infections, such as deafness from congenital rubella and intergenerational transfer of infectious agents to family members at risk for serious consequences (influenza from a child to a grandparent). Finally, infected children pose a risk to those who cannot be vaccinated because of immune deficiencies and other medical conditions.
A multilayered US system monitors vaccine safety
Responsibility for assuring the safety of vaccines lies with the US Food and Drug Administration (FDA) Center for Biologics Evaluation and Research and with the CDC’s Immunization Safety Office (ISO). The FDA is responsible for the initial assessment of the effectiveness and safety of new vaccines and for ongoing monitoring of the manufacturing facilities where vaccines are produced. After FDA approval, safety is monitored using a multilayered system that includes the Vaccine Adverse Event Reporting System (VAERS), the Vaccine Safety Datalink (VSD) system, the Clinical Immunization Safety Assessment (CISA) Project, and periodic reviews by the National Academy of Medicine (NAM), previously the Institute of Medicine. In addition, there is a large number of studies published each year by the nation’s—and world’s—medical research community on vaccine effectiveness and safety.
Continue to: VAERS
VAERS (https://vaers.hhs.gov/) is a passive reporting system that allows patients, physicians, and other health care providers to record suspected vaccine-related adverse events.5 It was created in 1990 and is run by the FDA and the CDC. It is not intended to be a comprehensive or definitive list of proven vaccine-related harms. As a passive reporting system, it is subject to both over- and underreporting, and the data from it are often misinterpreted and used incorrectly by vaccine opponents—eg, wrongly declaring that VAERS reports of possible AEs are proven cases. It provides a sentinel system that is monitored for indications of possible serious AEs linked to a particular vaccine. When a suspected interaction is detected, it is investigated by the VSD system.
VSD is a collaboration of the CDC’s ISO and 8 geographically distributed health care organizations with complete electronic patient medical information on their members. VSD conducts studies when a question about vaccine safety arises, when new vaccines are licensed, or when there are new vaccine recommendations. A description of VSD sites, the research methods used, and a list of publications describing study results can be found at https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vsd/index.html#organizations. If the VSD system finds a link between serious AEs and a particular vaccine, this association is reported to the Advisory Committee on Immunization Practices (ACIP) for consideration in changing recommendations regarding that vaccine. This happens only rarely.
CISA was established in 2001 as a network of vaccine safety experts at 7 academic medical centers who collaborate with the CDC’s ISO. CISA conducts studies on specific questions related to vaccine safety and provides a consultation service to clinicians and researchers who have questions about vaccine safety. A description of the CISA sites, past publications on vaccine safety, and ongoing research priorities can be found at https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/cisa/index.html.
NAM (https://nam.edu/) conducts periodic reviews of vaccine safety and vaccine-caused AEs. The most recent was published in 2012 and looked at possible AEs of 8 vaccines containing 12 different antigens.6 The literature search for this review found more than 12,000 articles, which speaks to the volume of scientific work on vaccine safety. These NAM reports document the rarity of severe AEs to vaccines and are used with other information to construct the table for the Vaccine Injury Compensation Program (VICP), which is described below.
Are vaccines killing children?
Vaccine opponents frequently claim that vaccines cause much more harm than is documented, including the deaths of children. A vaccine opponent made this claim in my state (Arizona) at a legislative committee hearing even though our state child mortality review committee has been investigating all child deaths for decades and has never attributed a death to a vaccine.
Continue to: One study conducted...
One study conducted using the VSD system from January 1, 2005, to December 31, 2011, identified 1100 deaths occurring within 12 months of any vaccination among 2,189,504 VSD enrollees ages 9 to 26 years.7 They found that the risk of death in this age group was not increased during the 30 days after vaccination, and no deaths were found to be causally associated with vaccination. Deaths among children do occur and, due to the number of vaccines administered, some deaths will occur within a short time period after a vaccine. This temporal association does not prove the death was vaccine-caused, but vaccine opponents have claimed that it does.
The vaccine injury compensation system
In 1986, the federal government established a no-fault system—the National Vaccine Injury Compensation Program (VICP)—to compensate those who suffer a serious AE from a vaccine covered by the program. This system is administered by the Health Resources and Services Administration (HRSA) in the Department of Health and Human Services (DHHS). HRSA maintains a table of proven AEs of specific vaccines, based in part on the NAM report mentioned earlier. Petitions for compensation—with proof of an AE following the administration of a vaccine that is included on the HRSA table—are accepted and remunerated if the AE lasted > 6 months or resulted in hospitalization. Petitions that allege AEs following administration of a vaccine not included on the table are nevertheless reviewed by the staff of HRSA, who can still recommend compensation based on the medical evidence. If HRSA declines the petition, the petitioner can appeal the case in the US Court of Federal Claims, which makes the final decision on a petition’s validity and, if warranted, the type and amount of compensation.
From 2006 to 2017, > 3.4 billion doses of vaccines covered by VICP were distributed in the United States.8 During this period, 6293 petitions were adjudicated by the court; 4311 were compensated.8 For every 1 million doses of vaccine distributed, 1 individual was compensated. Seventy percent of these compensations were awarded to petitioners despite a lack of clear evidence that the patient’s condition was caused by a vaccine.8 The rate of compensation for conditions proven to be caused by a vaccine was 1/3.33 million.8
The VICP pays for attorney fees, in some cases even if the petition is denied, but does not allow contingency fees. Since the beginning of the program, more than $4 billion has been awarded.8 The program is funded by a 75-cent tax on each vaccine antigen. Because serious AEs are so rare, the trust fund established to administer the VICP finances has a surplus of about $6 billion.
The Advisory Committee on Immunization Practices
After a vaccine is approved for use by the FDA, ACIP makes recommendations for its use in the US civilian population.9,10 ACIP, created in 1964, was chartered as a federal advisory committee to provide expert external advice to the Director of the CDC and the Secretary of DHHS on the use of vaccines
Continue to: As an official...
As an official federal advisory committee governed by the Federal Advisory Committee Act, ACIP operates under strict requirements for public notification of meetings, allowing for written and oral public comment at its meetings, and timely publication of minutes. ACIP meeting minutes are posted soon after each meeting, along with draft recommendations. ACIP meeting agendas and slide presentations are available on the ACIP Web site (https://www.cdc.gov/vaccines/acip/index.html).
ACIP consists of 15 members serving overlapping 4-year terms, appointed by the Secretary of DHHS from a list of candidates proposed by the CDC. One member is a consumer representative; the other members have expertise in vaccinology, immunology, pediatrics, internal medicine, infectious diseases, preventive medicine, and public health. In the CDC, staff support for ACIP is provided by the National Center for Immunization and Respiratory Diseases, Office of Infectious Diseases.
ACIP holds 2-day meetings 3 times a year. Much of the work occurs between meetings, by work groups via phone conferences. Work groups are chaired by an ACIP member and staffed by one or more CDC programmatic, content-expert professionals. Membership of the work groups consists of at least 2 ACIP members, representatives from relevant professional clinical and public health organizations, and other individuals with specific expertise. Work groups propose recommendations to ACIP, which can adopt, revise, or reject them.
When formulating recommendations for a particular vaccine, ACIP considers the burden of disease prevented, the effectiveness and safety of the vaccine, cost effectiveness, and practical and logistical issues of implementing recommendations. ACIP also receives frequent reports from ISO regarding the safety of vaccines previously approved. Since 2011, ACIP has used a standardized, modified GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) system to assess the evidence regarding effectiveness and safety of new vaccines and an evidence-to-recommendation framework to transparently explain how it arrives at recommendations.11,12
We can recommend vaccines with confidence
In the United States, we have a secure supply of safe vaccines, a transparent method of making vaccine recommendations, a robust system to monitor vaccine safety, and an efficient system to compensate those who experience a rare, serious adverse reaction to a vaccine. The US public health system has achieved a marked reduction in morbidity and mortality from childhood infectious diseases, mostly because of vaccines. Many people today have not experienced or seen children with these once-common childhood infections and may not appreciate the seriousness of childhood infectious diseases or the full value of vaccines. As family physicians, we can help address this problem and recommend vaccines to our patients with confidence.
1. Mellerson JL, Maxwell CB, Knighton CL, et al. Vaccine coverage for selected vaccines and exemption rates among children in kindergarten—United States, 2017-18 school year. MMWR Morb Mortal Wkly Rep. 2018;67:1115-1122.
2. CDC. Ten great public health achievements—United States, 1900-1999. MMWR Morb Mortal Wkly Rep. 1999;48:241-243.
3. Whitney CG, Zhou F, Singleton J, et al. Benefits from immunization during the Vaccines for Children Program era—United States, 1994-2013. MMWR Morb Mortal Wkly Rep. 2014;63:352-355.
4. CDC. Complications of measles. https://www.cdc.gov/measles/symptoms/complications.html. Accessed July 16, 2019.
5. Shimabukuro TT, Nguyen M, Martin D, et al. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2015;33:4398-4405.
6. IOM (Institute of Medicine). Adverse Effects of Vaccines: Evidence and Causality. Washington, DC: The National Academies Press; 2012.
7. McCarthy NL, Gee J, Sukumaran L, et al. Vaccination and 30-day mortality risk in children, adolescents, and young adults. Pediatrics. 2016;137:1-8.
8. HRSA. Data and Statistics. https://www.hrsa.gov/sites/default/files/hrsa/vaccine-compensation/data/monthly-stats-may-2019.pdf. Accessed July 16, 2019.
9. Pickering LK, Orenstein WA, Sun W, et al. FDA licensure of and ACIP recommendations for vaccines. Vaccine. 2017;37:5027-5036.
10. Smith JC, Snider DE, Pickering LK. Immunization policy development in the United States: the role of the Advisory Committee on Immunization Practices. Ann Intern Med. 2009;150:45-49.
11. Ahmed F, Temte JL, Campos-Outcalt D, et al; for the ACIP Evidence Based Recommendations Work Group (EBRWG). Methods for developing evidence-based recommendations by the Advisory Committee on Immunization Practices (ACIP) of the U.S. Centers for Disease Control and Prevention (CDC). Vaccine. 2011;29:9171-9176.
12. Lee G, Carr W. Updated framework for development of evidence-based recommendations by the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2018:76:1271-1272.
The current increase in measles cases in the United States has sharpened the focus on antivaccine activities. While the percentage of US children who are fully vaccinated remains high (≥ 94%), the number of un- or undervaccinated children has been growing1 because of nonmedical exemptions from school vaccine requirements due to concerns about vaccine safety and an underappreciation of the benefits of vaccines. Family physicians need to be conversant with several important aspects of this matter, including the magnitude of benefits provided by childhood vaccines, as well as the systems already in place for
- assessing vaccine effectiveness and safety,
- making recommendations on the use of vaccines,
- monitoring safety after vaccine approval, and
- compensating those affected by rare but serious vaccine-related adverse events (AEs).
Familiarity with these issues will allow for informed discussions with parents who are vaccine hesitant and with those who have read or heard inaccurate information.
The benefits of vaccines are indisputable
In 1999, the Centers for Disease Control and Prevention (CDC) published a list of 9 selected childhood infectious diseases and compared their incidences before and after immunization was available.2 Each of these infections causes morbidity, sequelae, and mortality at predictable rates depending on the infectious agent. The comparisons were dramatic: Measles, with a baseline annual morbidity of 503,282 cases, fell to just 89 cases; poliomyelitis decreased from 16,316 to 0; and Haemophilus influenzae type b declined from 20,000 to 54. In a 2014 analysis, the CDC stated that “among 78.6 million children born during 1994–2013, routine childhood immunization was estimated to prevent 322 million illnesses (averaging 4.1 illnesses per child) and 21 million hospitalizations (0.27 per child) over the course of their lifetimes and avert 732,000 premature deaths from vaccine-preventable illnesses” (TABLE).3

It is not unusual to hear a vaccine opponent say that childhood infectious diseases are not serious and that it is better for a child to contract the infection and let the immune system fight it naturally. Measles is often used as an example. This argument ignores some important aspects of vaccine benefits.
It is true in the United States that the average child who contracts measles will recover from it and not suffer immediate or long-term effects. However, it is also true that measles has a hospitalization rate of about 20% and a death rate of between 1/500 and 1/1000 cases.4 Mortality is much higher in developing countries. Prior to widespread use of measles vaccine, hundreds of thousands of cases of measles occurred each year. That translated into hundreds of preventable child deaths per year. An individual case does not tell the full story about the public health impact of infectious illnesses.
In addition, there are often unappreciated sequelae from child infections, such as shingles occurring years after resolution of a chickenpox infection. There are also societal consequences of child infections, such as deafness from congenital rubella and intergenerational transfer of infectious agents to family members at risk for serious consequences (influenza from a child to a grandparent). Finally, infected children pose a risk to those who cannot be vaccinated because of immune deficiencies and other medical conditions.
A multilayered US system monitors vaccine safety
Responsibility for assuring the safety of vaccines lies with the US Food and Drug Administration (FDA) Center for Biologics Evaluation and Research and with the CDC’s Immunization Safety Office (ISO). The FDA is responsible for the initial assessment of the effectiveness and safety of new vaccines and for ongoing monitoring of the manufacturing facilities where vaccines are produced. After FDA approval, safety is monitored using a multilayered system that includes the Vaccine Adverse Event Reporting System (VAERS), the Vaccine Safety Datalink (VSD) system, the Clinical Immunization Safety Assessment (CISA) Project, and periodic reviews by the National Academy of Medicine (NAM), previously the Institute of Medicine. In addition, there is a large number of studies published each year by the nation’s—and world’s—medical research community on vaccine effectiveness and safety.
Continue to: VAERS
VAERS (https://vaers.hhs.gov/) is a passive reporting system that allows patients, physicians, and other health care providers to record suspected vaccine-related adverse events.5 It was created in 1990 and is run by the FDA and the CDC. It is not intended to be a comprehensive or definitive list of proven vaccine-related harms. As a passive reporting system, it is subject to both over- and underreporting, and the data from it are often misinterpreted and used incorrectly by vaccine opponents—eg, wrongly declaring that VAERS reports of possible AEs are proven cases. It provides a sentinel system that is monitored for indications of possible serious AEs linked to a particular vaccine. When a suspected interaction is detected, it is investigated by the VSD system.
VSD is a collaboration of the CDC’s ISO and 8 geographically distributed health care organizations with complete electronic patient medical information on their members. VSD conducts studies when a question about vaccine safety arises, when new vaccines are licensed, or when there are new vaccine recommendations. A description of VSD sites, the research methods used, and a list of publications describing study results can be found at https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vsd/index.html#organizations. If the VSD system finds a link between serious AEs and a particular vaccine, this association is reported to the Advisory Committee on Immunization Practices (ACIP) for consideration in changing recommendations regarding that vaccine. This happens only rarely.
CISA was established in 2001 as a network of vaccine safety experts at 7 academic medical centers who collaborate with the CDC’s ISO. CISA conducts studies on specific questions related to vaccine safety and provides a consultation service to clinicians and researchers who have questions about vaccine safety. A description of the CISA sites, past publications on vaccine safety, and ongoing research priorities can be found at https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/cisa/index.html.
NAM (https://nam.edu/) conducts periodic reviews of vaccine safety and vaccine-caused AEs. The most recent was published in 2012 and looked at possible AEs of 8 vaccines containing 12 different antigens.6 The literature search for this review found more than 12,000 articles, which speaks to the volume of scientific work on vaccine safety. These NAM reports document the rarity of severe AEs to vaccines and are used with other information to construct the table for the Vaccine Injury Compensation Program (VICP), which is described below.
Are vaccines killing children?
Vaccine opponents frequently claim that vaccines cause much more harm than is documented, including the deaths of children. A vaccine opponent made this claim in my state (Arizona) at a legislative committee hearing even though our state child mortality review committee has been investigating all child deaths for decades and has never attributed a death to a vaccine.
Continue to: One study conducted...
One study conducted using the VSD system from January 1, 2005, to December 31, 2011, identified 1100 deaths occurring within 12 months of any vaccination among 2,189,504 VSD enrollees ages 9 to 26 years.7 They found that the risk of death in this age group was not increased during the 30 days after vaccination, and no deaths were found to be causally associated with vaccination. Deaths among children do occur and, due to the number of vaccines administered, some deaths will occur within a short time period after a vaccine. This temporal association does not prove the death was vaccine-caused, but vaccine opponents have claimed that it does.
The vaccine injury compensation system
In 1986, the federal government established a no-fault system—the National Vaccine Injury Compensation Program (VICP)—to compensate those who suffer a serious AE from a vaccine covered by the program. This system is administered by the Health Resources and Services Administration (HRSA) in the Department of Health and Human Services (DHHS). HRSA maintains a table of proven AEs of specific vaccines, based in part on the NAM report mentioned earlier. Petitions for compensation—with proof of an AE following the administration of a vaccine that is included on the HRSA table—are accepted and remunerated if the AE lasted > 6 months or resulted in hospitalization. Petitions that allege AEs following administration of a vaccine not included on the table are nevertheless reviewed by the staff of HRSA, who can still recommend compensation based on the medical evidence. If HRSA declines the petition, the petitioner can appeal the case in the US Court of Federal Claims, which makes the final decision on a petition’s validity and, if warranted, the type and amount of compensation.
From 2006 to 2017, > 3.4 billion doses of vaccines covered by VICP were distributed in the United States.8 During this period, 6293 petitions were adjudicated by the court; 4311 were compensated.8 For every 1 million doses of vaccine distributed, 1 individual was compensated. Seventy percent of these compensations were awarded to petitioners despite a lack of clear evidence that the patient’s condition was caused by a vaccine.8 The rate of compensation for conditions proven to be caused by a vaccine was 1/3.33 million.8
The VICP pays for attorney fees, in some cases even if the petition is denied, but does not allow contingency fees. Since the beginning of the program, more than $4 billion has been awarded.8 The program is funded by a 75-cent tax on each vaccine antigen. Because serious AEs are so rare, the trust fund established to administer the VICP finances has a surplus of about $6 billion.
The Advisory Committee on Immunization Practices
After a vaccine is approved for use by the FDA, ACIP makes recommendations for its use in the US civilian population.9,10 ACIP, created in 1964, was chartered as a federal advisory committee to provide expert external advice to the Director of the CDC and the Secretary of DHHS on the use of vaccines
Continue to: As an official...
As an official federal advisory committee governed by the Federal Advisory Committee Act, ACIP operates under strict requirements for public notification of meetings, allowing for written and oral public comment at its meetings, and timely publication of minutes. ACIP meeting minutes are posted soon after each meeting, along with draft recommendations. ACIP meeting agendas and slide presentations are available on the ACIP Web site (https://www.cdc.gov/vaccines/acip/index.html).
ACIP consists of 15 members serving overlapping 4-year terms, appointed by the Secretary of DHHS from a list of candidates proposed by the CDC. One member is a consumer representative; the other members have expertise in vaccinology, immunology, pediatrics, internal medicine, infectious diseases, preventive medicine, and public health. In the CDC, staff support for ACIP is provided by the National Center for Immunization and Respiratory Diseases, Office of Infectious Diseases.
ACIP holds 2-day meetings 3 times a year. Much of the work occurs between meetings, by work groups via phone conferences. Work groups are chaired by an ACIP member and staffed by one or more CDC programmatic, content-expert professionals. Membership of the work groups consists of at least 2 ACIP members, representatives from relevant professional clinical and public health organizations, and other individuals with specific expertise. Work groups propose recommendations to ACIP, which can adopt, revise, or reject them.
When formulating recommendations for a particular vaccine, ACIP considers the burden of disease prevented, the effectiveness and safety of the vaccine, cost effectiveness, and practical and logistical issues of implementing recommendations. ACIP also receives frequent reports from ISO regarding the safety of vaccines previously approved. Since 2011, ACIP has used a standardized, modified GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) system to assess the evidence regarding effectiveness and safety of new vaccines and an evidence-to-recommendation framework to transparently explain how it arrives at recommendations.11,12
We can recommend vaccines with confidence
In the United States, we have a secure supply of safe vaccines, a transparent method of making vaccine recommendations, a robust system to monitor vaccine safety, and an efficient system to compensate those who experience a rare, serious adverse reaction to a vaccine. The US public health system has achieved a marked reduction in morbidity and mortality from childhood infectious diseases, mostly because of vaccines. Many people today have not experienced or seen children with these once-common childhood infections and may not appreciate the seriousness of childhood infectious diseases or the full value of vaccines. As family physicians, we can help address this problem and recommend vaccines to our patients with confidence.
The current increase in measles cases in the United States has sharpened the focus on antivaccine activities. While the percentage of US children who are fully vaccinated remains high (≥ 94%), the number of un- or undervaccinated children has been growing1 because of nonmedical exemptions from school vaccine requirements due to concerns about vaccine safety and an underappreciation of the benefits of vaccines. Family physicians need to be conversant with several important aspects of this matter, including the magnitude of benefits provided by childhood vaccines, as well as the systems already in place for
- assessing vaccine effectiveness and safety,
- making recommendations on the use of vaccines,
- monitoring safety after vaccine approval, and
- compensating those affected by rare but serious vaccine-related adverse events (AEs).
Familiarity with these issues will allow for informed discussions with parents who are vaccine hesitant and with those who have read or heard inaccurate information.
The benefits of vaccines are indisputable
In 1999, the Centers for Disease Control and Prevention (CDC) published a list of 9 selected childhood infectious diseases and compared their incidences before and after immunization was available.2 Each of these infections causes morbidity, sequelae, and mortality at predictable rates depending on the infectious agent. The comparisons were dramatic: Measles, with a baseline annual morbidity of 503,282 cases, fell to just 89 cases; poliomyelitis decreased from 16,316 to 0; and Haemophilus influenzae type b declined from 20,000 to 54. In a 2014 analysis, the CDC stated that “among 78.6 million children born during 1994–2013, routine childhood immunization was estimated to prevent 322 million illnesses (averaging 4.1 illnesses per child) and 21 million hospitalizations (0.27 per child) over the course of their lifetimes and avert 732,000 premature deaths from vaccine-preventable illnesses” (TABLE).3

It is not unusual to hear a vaccine opponent say that childhood infectious diseases are not serious and that it is better for a child to contract the infection and let the immune system fight it naturally. Measles is often used as an example. This argument ignores some important aspects of vaccine benefits.
It is true in the United States that the average child who contracts measles will recover from it and not suffer immediate or long-term effects. However, it is also true that measles has a hospitalization rate of about 20% and a death rate of between 1/500 and 1/1000 cases.4 Mortality is much higher in developing countries. Prior to widespread use of measles vaccine, hundreds of thousands of cases of measles occurred each year. That translated into hundreds of preventable child deaths per year. An individual case does not tell the full story about the public health impact of infectious illnesses.
In addition, there are often unappreciated sequelae from child infections, such as shingles occurring years after resolution of a chickenpox infection. There are also societal consequences of child infections, such as deafness from congenital rubella and intergenerational transfer of infectious agents to family members at risk for serious consequences (influenza from a child to a grandparent). Finally, infected children pose a risk to those who cannot be vaccinated because of immune deficiencies and other medical conditions.
A multilayered US system monitors vaccine safety
Responsibility for assuring the safety of vaccines lies with the US Food and Drug Administration (FDA) Center for Biologics Evaluation and Research and with the CDC’s Immunization Safety Office (ISO). The FDA is responsible for the initial assessment of the effectiveness and safety of new vaccines and for ongoing monitoring of the manufacturing facilities where vaccines are produced. After FDA approval, safety is monitored using a multilayered system that includes the Vaccine Adverse Event Reporting System (VAERS), the Vaccine Safety Datalink (VSD) system, the Clinical Immunization Safety Assessment (CISA) Project, and periodic reviews by the National Academy of Medicine (NAM), previously the Institute of Medicine. In addition, there is a large number of studies published each year by the nation’s—and world’s—medical research community on vaccine effectiveness and safety.
Continue to: VAERS
VAERS (https://vaers.hhs.gov/) is a passive reporting system that allows patients, physicians, and other health care providers to record suspected vaccine-related adverse events.5 It was created in 1990 and is run by the FDA and the CDC. It is not intended to be a comprehensive or definitive list of proven vaccine-related harms. As a passive reporting system, it is subject to both over- and underreporting, and the data from it are often misinterpreted and used incorrectly by vaccine opponents—eg, wrongly declaring that VAERS reports of possible AEs are proven cases. It provides a sentinel system that is monitored for indications of possible serious AEs linked to a particular vaccine. When a suspected interaction is detected, it is investigated by the VSD system.
VSD is a collaboration of the CDC’s ISO and 8 geographically distributed health care organizations with complete electronic patient medical information on their members. VSD conducts studies when a question about vaccine safety arises, when new vaccines are licensed, or when there are new vaccine recommendations. A description of VSD sites, the research methods used, and a list of publications describing study results can be found at https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vsd/index.html#organizations. If the VSD system finds a link between serious AEs and a particular vaccine, this association is reported to the Advisory Committee on Immunization Practices (ACIP) for consideration in changing recommendations regarding that vaccine. This happens only rarely.
CISA was established in 2001 as a network of vaccine safety experts at 7 academic medical centers who collaborate with the CDC’s ISO. CISA conducts studies on specific questions related to vaccine safety and provides a consultation service to clinicians and researchers who have questions about vaccine safety. A description of the CISA sites, past publications on vaccine safety, and ongoing research priorities can be found at https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/cisa/index.html.
NAM (https://nam.edu/) conducts periodic reviews of vaccine safety and vaccine-caused AEs. The most recent was published in 2012 and looked at possible AEs of 8 vaccines containing 12 different antigens.6 The literature search for this review found more than 12,000 articles, which speaks to the volume of scientific work on vaccine safety. These NAM reports document the rarity of severe AEs to vaccines and are used with other information to construct the table for the Vaccine Injury Compensation Program (VICP), which is described below.
Are vaccines killing children?
Vaccine opponents frequently claim that vaccines cause much more harm than is documented, including the deaths of children. A vaccine opponent made this claim in my state (Arizona) at a legislative committee hearing even though our state child mortality review committee has been investigating all child deaths for decades and has never attributed a death to a vaccine.
Continue to: One study conducted...
One study conducted using the VSD system from January 1, 2005, to December 31, 2011, identified 1100 deaths occurring within 12 months of any vaccination among 2,189,504 VSD enrollees ages 9 to 26 years.7 They found that the risk of death in this age group was not increased during the 30 days after vaccination, and no deaths were found to be causally associated with vaccination. Deaths among children do occur and, due to the number of vaccines administered, some deaths will occur within a short time period after a vaccine. This temporal association does not prove the death was vaccine-caused, but vaccine opponents have claimed that it does.
The vaccine injury compensation system
In 1986, the federal government established a no-fault system—the National Vaccine Injury Compensation Program (VICP)—to compensate those who suffer a serious AE from a vaccine covered by the program. This system is administered by the Health Resources and Services Administration (HRSA) in the Department of Health and Human Services (DHHS). HRSA maintains a table of proven AEs of specific vaccines, based in part on the NAM report mentioned earlier. Petitions for compensation—with proof of an AE following the administration of a vaccine that is included on the HRSA table—are accepted and remunerated if the AE lasted > 6 months or resulted in hospitalization. Petitions that allege AEs following administration of a vaccine not included on the table are nevertheless reviewed by the staff of HRSA, who can still recommend compensation based on the medical evidence. If HRSA declines the petition, the petitioner can appeal the case in the US Court of Federal Claims, which makes the final decision on a petition’s validity and, if warranted, the type and amount of compensation.
From 2006 to 2017, > 3.4 billion doses of vaccines covered by VICP were distributed in the United States.8 During this period, 6293 petitions were adjudicated by the court; 4311 were compensated.8 For every 1 million doses of vaccine distributed, 1 individual was compensated. Seventy percent of these compensations were awarded to petitioners despite a lack of clear evidence that the patient’s condition was caused by a vaccine.8 The rate of compensation for conditions proven to be caused by a vaccine was 1/3.33 million.8
The VICP pays for attorney fees, in some cases even if the petition is denied, but does not allow contingency fees. Since the beginning of the program, more than $4 billion has been awarded.8 The program is funded by a 75-cent tax on each vaccine antigen. Because serious AEs are so rare, the trust fund established to administer the VICP finances has a surplus of about $6 billion.
The Advisory Committee on Immunization Practices
After a vaccine is approved for use by the FDA, ACIP makes recommendations for its use in the US civilian population.9,10 ACIP, created in 1964, was chartered as a federal advisory committee to provide expert external advice to the Director of the CDC and the Secretary of DHHS on the use of vaccines
Continue to: As an official...
As an official federal advisory committee governed by the Federal Advisory Committee Act, ACIP operates under strict requirements for public notification of meetings, allowing for written and oral public comment at its meetings, and timely publication of minutes. ACIP meeting minutes are posted soon after each meeting, along with draft recommendations. ACIP meeting agendas and slide presentations are available on the ACIP Web site (https://www.cdc.gov/vaccines/acip/index.html).
ACIP consists of 15 members serving overlapping 4-year terms, appointed by the Secretary of DHHS from a list of candidates proposed by the CDC. One member is a consumer representative; the other members have expertise in vaccinology, immunology, pediatrics, internal medicine, infectious diseases, preventive medicine, and public health. In the CDC, staff support for ACIP is provided by the National Center for Immunization and Respiratory Diseases, Office of Infectious Diseases.
ACIP holds 2-day meetings 3 times a year. Much of the work occurs between meetings, by work groups via phone conferences. Work groups are chaired by an ACIP member and staffed by one or more CDC programmatic, content-expert professionals. Membership of the work groups consists of at least 2 ACIP members, representatives from relevant professional clinical and public health organizations, and other individuals with specific expertise. Work groups propose recommendations to ACIP, which can adopt, revise, or reject them.
When formulating recommendations for a particular vaccine, ACIP considers the burden of disease prevented, the effectiveness and safety of the vaccine, cost effectiveness, and practical and logistical issues of implementing recommendations. ACIP also receives frequent reports from ISO regarding the safety of vaccines previously approved. Since 2011, ACIP has used a standardized, modified GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) system to assess the evidence regarding effectiveness and safety of new vaccines and an evidence-to-recommendation framework to transparently explain how it arrives at recommendations.11,12
We can recommend vaccines with confidence
In the United States, we have a secure supply of safe vaccines, a transparent method of making vaccine recommendations, a robust system to monitor vaccine safety, and an efficient system to compensate those who experience a rare, serious adverse reaction to a vaccine. The US public health system has achieved a marked reduction in morbidity and mortality from childhood infectious diseases, mostly because of vaccines. Many people today have not experienced or seen children with these once-common childhood infections and may not appreciate the seriousness of childhood infectious diseases or the full value of vaccines. As family physicians, we can help address this problem and recommend vaccines to our patients with confidence.
1. Mellerson JL, Maxwell CB, Knighton CL, et al. Vaccine coverage for selected vaccines and exemption rates among children in kindergarten—United States, 2017-18 school year. MMWR Morb Mortal Wkly Rep. 2018;67:1115-1122.
2. CDC. Ten great public health achievements—United States, 1900-1999. MMWR Morb Mortal Wkly Rep. 1999;48:241-243.
3. Whitney CG, Zhou F, Singleton J, et al. Benefits from immunization during the Vaccines for Children Program era—United States, 1994-2013. MMWR Morb Mortal Wkly Rep. 2014;63:352-355.
4. CDC. Complications of measles. https://www.cdc.gov/measles/symptoms/complications.html. Accessed July 16, 2019.
5. Shimabukuro TT, Nguyen M, Martin D, et al. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2015;33:4398-4405.
6. IOM (Institute of Medicine). Adverse Effects of Vaccines: Evidence and Causality. Washington, DC: The National Academies Press; 2012.
7. McCarthy NL, Gee J, Sukumaran L, et al. Vaccination and 30-day mortality risk in children, adolescents, and young adults. Pediatrics. 2016;137:1-8.
8. HRSA. Data and Statistics. https://www.hrsa.gov/sites/default/files/hrsa/vaccine-compensation/data/monthly-stats-may-2019.pdf. Accessed July 16, 2019.
9. Pickering LK, Orenstein WA, Sun W, et al. FDA licensure of and ACIP recommendations for vaccines. Vaccine. 2017;37:5027-5036.
10. Smith JC, Snider DE, Pickering LK. Immunization policy development in the United States: the role of the Advisory Committee on Immunization Practices. Ann Intern Med. 2009;150:45-49.
11. Ahmed F, Temte JL, Campos-Outcalt D, et al; for the ACIP Evidence Based Recommendations Work Group (EBRWG). Methods for developing evidence-based recommendations by the Advisory Committee on Immunization Practices (ACIP) of the U.S. Centers for Disease Control and Prevention (CDC). Vaccine. 2011;29:9171-9176.
12. Lee G, Carr W. Updated framework for development of evidence-based recommendations by the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2018:76:1271-1272.
1. Mellerson JL, Maxwell CB, Knighton CL, et al. Vaccine coverage for selected vaccines and exemption rates among children in kindergarten—United States, 2017-18 school year. MMWR Morb Mortal Wkly Rep. 2018;67:1115-1122.
2. CDC. Ten great public health achievements—United States, 1900-1999. MMWR Morb Mortal Wkly Rep. 1999;48:241-243.
3. Whitney CG, Zhou F, Singleton J, et al. Benefits from immunization during the Vaccines for Children Program era—United States, 1994-2013. MMWR Morb Mortal Wkly Rep. 2014;63:352-355.
4. CDC. Complications of measles. https://www.cdc.gov/measles/symptoms/complications.html. Accessed July 16, 2019.
5. Shimabukuro TT, Nguyen M, Martin D, et al. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2015;33:4398-4405.
6. IOM (Institute of Medicine). Adverse Effects of Vaccines: Evidence and Causality. Washington, DC: The National Academies Press; 2012.
7. McCarthy NL, Gee J, Sukumaran L, et al. Vaccination and 30-day mortality risk in children, adolescents, and young adults. Pediatrics. 2016;137:1-8.
8. HRSA. Data and Statistics. https://www.hrsa.gov/sites/default/files/hrsa/vaccine-compensation/data/monthly-stats-may-2019.pdf. Accessed July 16, 2019.
9. Pickering LK, Orenstein WA, Sun W, et al. FDA licensure of and ACIP recommendations for vaccines. Vaccine. 2017;37:5027-5036.
10. Smith JC, Snider DE, Pickering LK. Immunization policy development in the United States: the role of the Advisory Committee on Immunization Practices. Ann Intern Med. 2009;150:45-49.
11. Ahmed F, Temte JL, Campos-Outcalt D, et al; for the ACIP Evidence Based Recommendations Work Group (EBRWG). Methods for developing evidence-based recommendations by the Advisory Committee on Immunization Practices (ACIP) of the U.S. Centers for Disease Control and Prevention (CDC). Vaccine. 2011;29:9171-9176.
12. Lee G, Carr W. Updated framework for development of evidence-based recommendations by the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2018:76:1271-1272.
Teens & tobacco use: USPSTF issues draft recs on prevention, cessation
Reference
1. US Preventive Services Task Force. Draft Evidence Review for Prevention and Cessation of Tobacco Use in Children and Adolescents: Primary Care Interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/draft-evidence-review/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions. Accessed July 8, 2019.
Reference
1. US Preventive Services Task Force. Draft Evidence Review for Prevention and Cessation of Tobacco Use in Children and Adolescents: Primary Care Interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/draft-evidence-review/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions. Accessed July 8, 2019.
Reference
1. US Preventive Services Task Force. Draft Evidence Review for Prevention and Cessation of Tobacco Use in Children and Adolescents: Primary Care Interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/draft-evidence-review/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions. Accessed July 8, 2019.
Water safety: Drowning isn’t the only concern
References
1. CDC Childhood Injury Report: Patterns of Unintentional Injuries among 0-19 Year Olds in the United States, 2000-2006. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/safechild/pdf/cdc-childhoodinjury.pdf. Accessed June 26, 2019.
2. World Health Organization. Global Report on Drowning: Preventing a Leading Killer. https://apps.who.int/iris/bitstream/handle/10665/143893/9789241564786_eng.pdf;jsessionid=0B51AAEB51E29A603A0CABB41FCD96B5?sequence=1. Accessed June 26, 2019.
3. Vanden Esschert K, Haileyesus T, Tarrier AL, et al. Pool chemical injuries in public and residential settings—United States, 2008–2017, and New York, 2018. MMWR Morb Mortal Wkly Rep. 2019;68:433–438.
4. Healthy Swimming. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/healthywater/swimming/index.html. Accessed June 26, 2019.
References
1. CDC Childhood Injury Report: Patterns of Unintentional Injuries among 0-19 Year Olds in the United States, 2000-2006. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/safechild/pdf/cdc-childhoodinjury.pdf. Accessed June 26, 2019.
2. World Health Organization. Global Report on Drowning: Preventing a Leading Killer. https://apps.who.int/iris/bitstream/handle/10665/143893/9789241564786_eng.pdf;jsessionid=0B51AAEB51E29A603A0CABB41FCD96B5?sequence=1. Accessed June 26, 2019.
3. Vanden Esschert K, Haileyesus T, Tarrier AL, et al. Pool chemical injuries in public and residential settings—United States, 2008–2017, and New York, 2018. MMWR Morb Mortal Wkly Rep. 2019;68:433–438.
4. Healthy Swimming. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/healthywater/swimming/index.html. Accessed June 26, 2019.
References
1. CDC Childhood Injury Report: Patterns of Unintentional Injuries among 0-19 Year Olds in the United States, 2000-2006. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/safechild/pdf/cdc-childhoodinjury.pdf. Accessed June 26, 2019.
2. World Health Organization. Global Report on Drowning: Preventing a Leading Killer. https://apps.who.int/iris/bitstream/handle/10665/143893/9789241564786_eng.pdf;jsessionid=0B51AAEB51E29A603A0CABB41FCD96B5?sequence=1. Accessed June 26, 2019.
3. Vanden Esschert K, Haileyesus T, Tarrier AL, et al. Pool chemical injuries in public and residential settings—United States, 2008–2017, and New York, 2018. MMWR Morb Mortal Wkly Rep. 2019;68:433–438.
4. Healthy Swimming. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/healthywater/swimming/index.html. Accessed June 26, 2019.
STIs may be overlooked if you fail to ask this question
Reference
Johnson Jones ML, Chapin-Bardales J, Bizune D. Extragenital chlamydia and gonorrhea among community venue – attending men who have sex with men – Five cities, United States, 2017. MMWR Morb Mortal Wkly Rep. 2019;68:321-325. https://www.cdc.gov/mmwr/volumes/68/wr/mm6814a1.htm?s_cid=mm6814a1_w. Accessed May 23, 2019.
Reference
Johnson Jones ML, Chapin-Bardales J, Bizune D. Extragenital chlamydia and gonorrhea among community venue – attending men who have sex with men – Five cities, United States, 2017. MMWR Morb Mortal Wkly Rep. 2019;68:321-325. https://www.cdc.gov/mmwr/volumes/68/wr/mm6814a1.htm?s_cid=mm6814a1_w. Accessed May 23, 2019.
Reference
Johnson Jones ML, Chapin-Bardales J, Bizune D. Extragenital chlamydia and gonorrhea among community venue – attending men who have sex with men – Five cities, United States, 2017. MMWR Morb Mortal Wkly Rep. 2019;68:321-325. https://www.cdc.gov/mmwr/volumes/68/wr/mm6814a1.htm?s_cid=mm6814a1_w. Accessed May 23, 2019.
2019 USPSTF update
Over the past year through early 2019, the US Preventive Services Task Force made 34 recommendations on 19 different topics. Twenty-six were reaffirmations of recommendations made in previous years (TABLE 11); the Task Force attempts to reassess topics every 7 years. Two new topics were addressed with 2 new recommendations, and 6 previous recommendations were revised or reversed (TABLE 22-9).
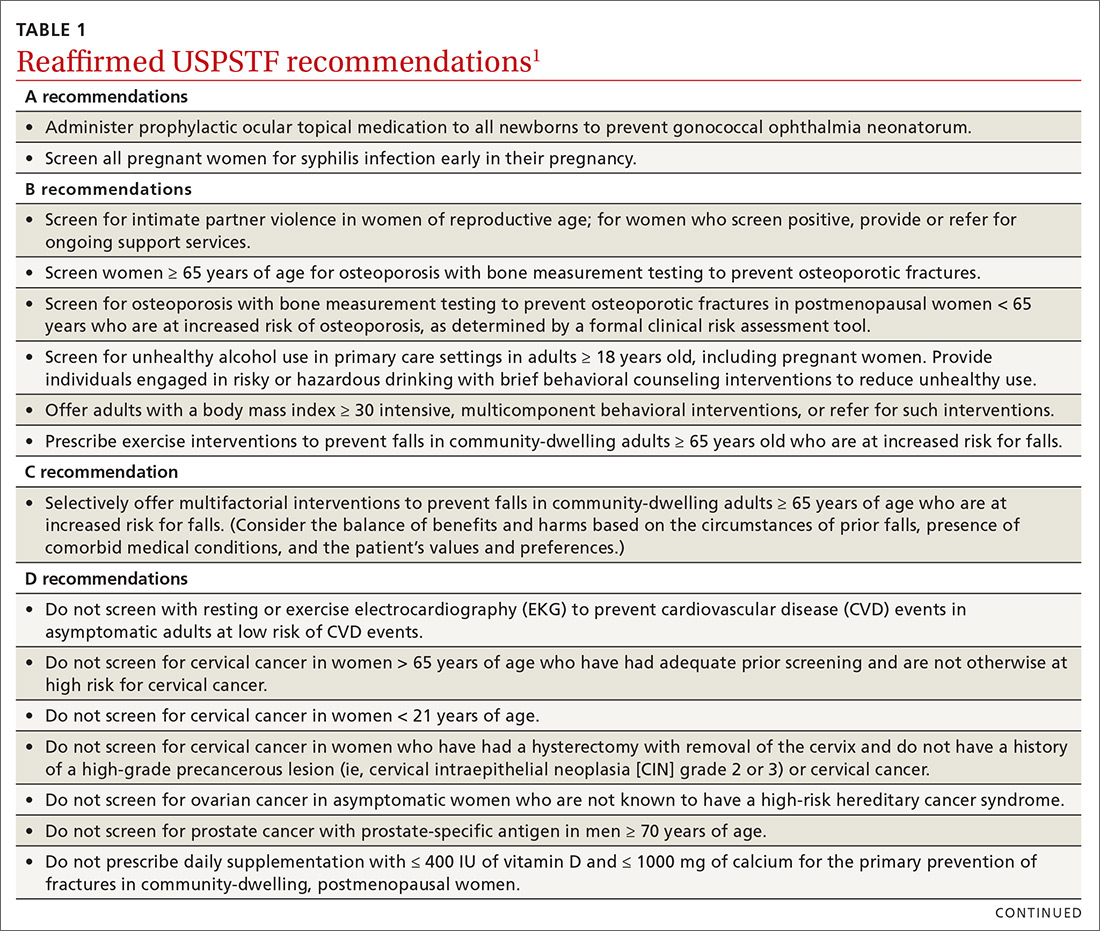
This Practice Alert discusses the new and the changed recommendations. (In 2018, the Practice Alert podcast series covered screening for ovarian cancer [April], prostate cancer [June], and cervical cancer [October], and EKG screening for cardiovascular disease [November].) All current Task Force recommendations are available on the USPSTF Web site.1
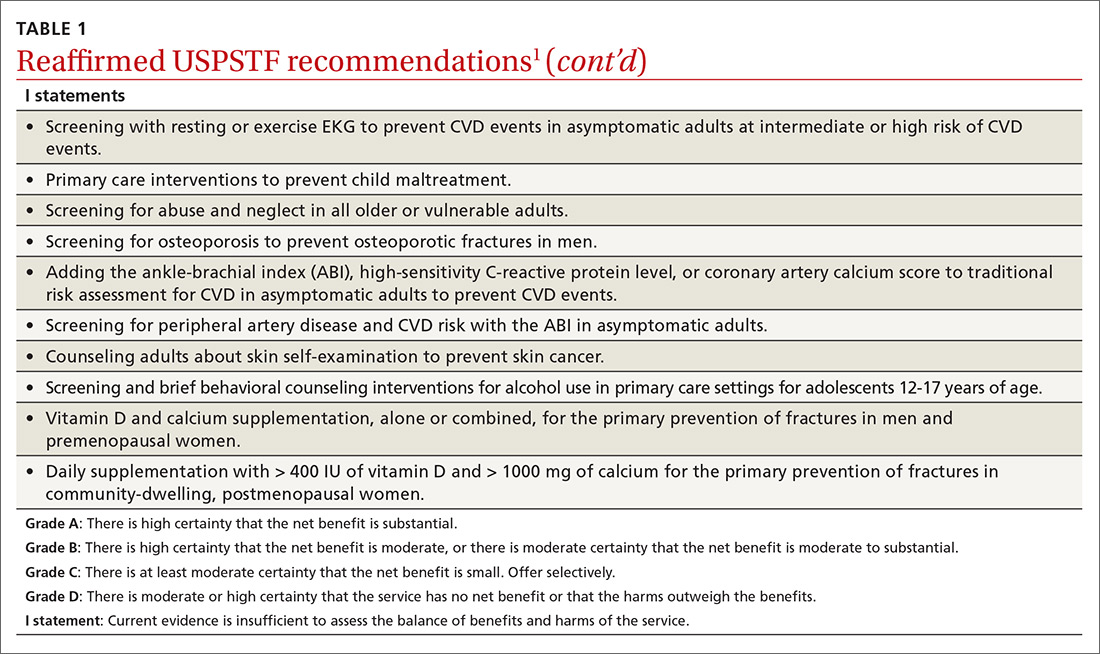
New topics
Perinatal depression prevention
The Task Force recommends that clinicians counsel pregnant women and women in the first year postpartum who are at increased risk for perinatal depression, or refer for such services. The recommendation applies to those who are not diagnosed with depression but are at increased risk.
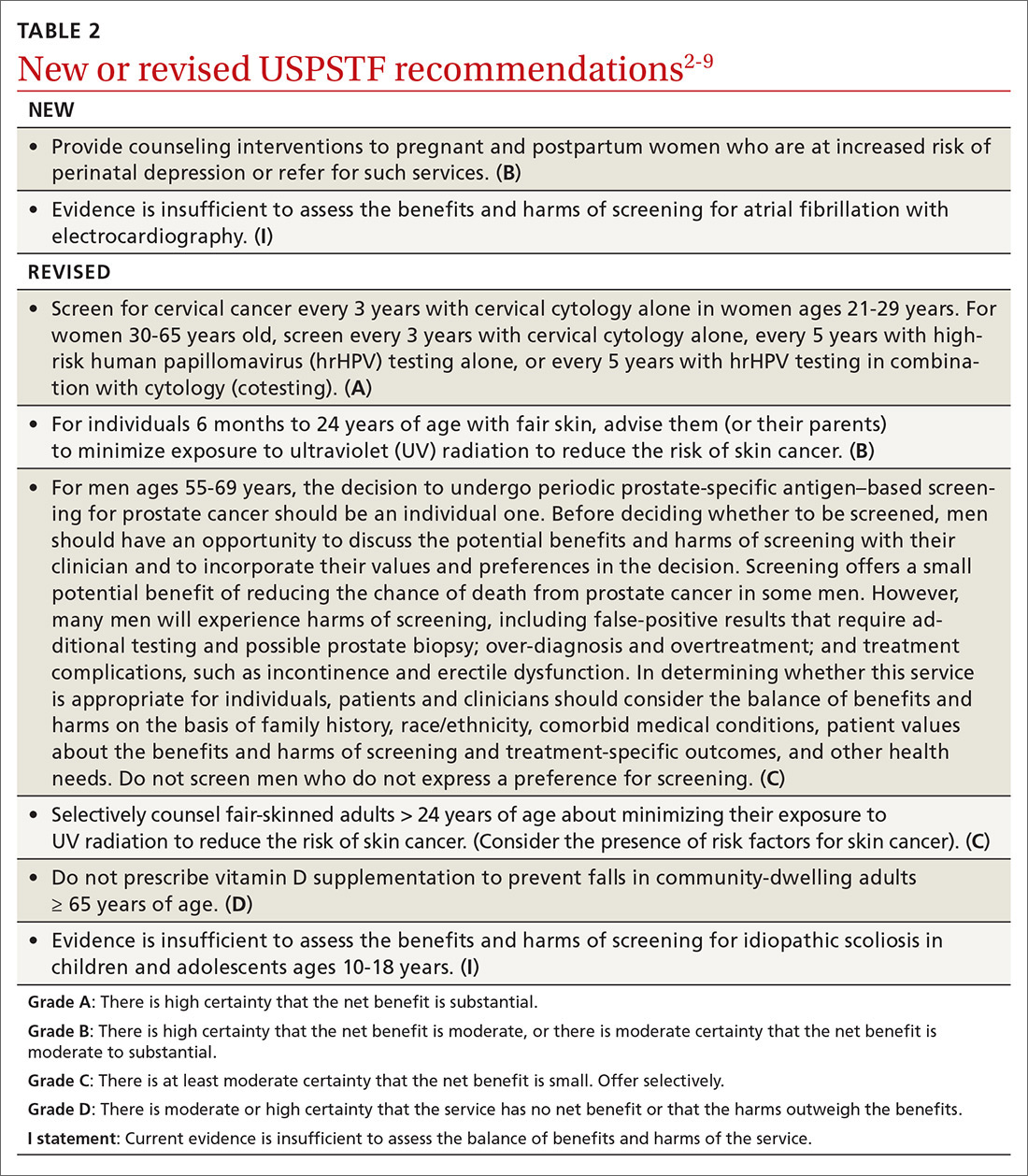
Perinatal depression can negatively affect both mother and child in several ways and occurs at a rate close to 9% during pregnancy and 37% during the first year postpartum.2 The interventions studied by the Task Force included cognitive behavioral therapy and interpersonal therapy; most sessions were initiated in the second trimester of pregnancy and varied in number of sessions and intensity. The Task Force includes the following in the list of risks that should prompt a referral: a history of depression, current depressive symptoms that fall short of that needed for a depression diagnosis, low income, adolescent or single parenthood, recent intimate partner violence, elevated anxiety symptoms, physical or sexual abuse, or a history of significant negative life events. (See “Postpartum anxiety: More common than you think,” in the April issue.)
Atrial fibrillation
The Task Force found insufficient evidence to recommend for or against the use of electrocardiography (EKG) to screen for atrial fibrillation (AF).3
Revisions of previous recommendations
Cervical cancer screening
Skin cancer prevention
The Task Force made 2 revisions to the 2012 recommendation on preventing skin cancer through behavioral counseling to avoid ultraviolet (UV) radiation.6 These recommendations continue to focus on those with fair skin. The first revision: The earliest age at which children (through their guardians) can benefit from counseling on UV avoidance has been lowered from age 10 years to 6 months. The second revision: Some adults older than age 24 can also benefit from such counseling if they have fair skin and other skin cancer risks such as using tanning beds, having a history of sunburns or previous skin cancer, having an increased number of nevi (moles) and atypical nevi, having human immunodeficiency virus (HIV) infection, having received an organ transplant, or having a family history of skin cancer.
Continue to: Those at risk...
Those at risk can reduce their chances of skin cancer by using broad-spectrum sunscreens and sun-protective clothing, and by avoiding sun exposure and indoor tanning beds.
Fall prevention
In a reversal of its 2012 recommendation, the Task Force now recommends against the use of vitamin D supplementation to prevent falls in community-dwelling adults 65 years or older.7 In a reanalysis of previous studies on this topic, along with new evidence, the Task Force concluded that vitamin D supplementation offers no benefit for preventing falls in adults who are not vitamin D deficient.
Screening for scoliosis in adolescents
In 2004 the USPSTF recommended against screening for idiopathic scoliosis in children and adolescents 10 to 18 years of age. In its most recent review, the Task Force continued to find no direct evidence of the benefit of screening and inadequate evidence on the long-term benefits of reduction in spinal curvature through exercise, surgery, and bracing. However, following a reanalysis of the potential harms of these treatments and the use of a new analytic framework, the Task Force concluded it is not possible at this time to assess the balance of benefits and harms of screening.8
Prostate cancer screening
In its most controversial action, the Task Force reversed its 2012 recommendation against routine prostate-specific antigen–based screening for prostate cancer in men ages 55 to 69 years and now lists this as a “C” recommendation.9 The potential benefits of screening include preventing 1.3 deaths from prostate cancer per 1000 men screened over 13 years and approximately 3 cases of metastatic prostate cancer. However, no trials have found a reduction in all-cause mortality from screening. Contrast that with the known harms of screening: 15% false positive results over 10 years; 1% hospitalization rate among those undergoing a prostate biopsy; over-diagnosis and resultant treatment of 20% to 50% of men diagnosed with prostate cancer through screening; and incontinence and erectile dysfunction in 20% and 67%, respectively, of men following prostatectomy.9
Based on these outcomes, the Task Force “does not recommend screening for prostate cancer unless men express a preference for screening after being informed of and understanding the benefits and risks.”9 The Task Force continues to recommend against screening men ages 70 years and older.
Continue to: The change in this recommendation...
The change in this recommendation and its wording present dilemmas for family physicians: whether to discuss potential screening with all men ages 55 to 69; to selectively discuss it with those at high risk (principally African Americans and those with a strong family history of prostate cancer); or to address the issue only if a patient asks about it. In addition, if a man requests screening, how often should it be performed? Most clinical trials have found equal benefit from testing less frequently than every year, with fewer harms. The Task Force provided little or no guidance on these issues.
Final advice: D recommendations
The Task Force reaffirmed that 7 services have either no benefit or cause more harm than benefit (TABLE 11). Family physicians should be familiar with these services, as well as all Task Force D recommendations, and avoid recommending them or providing them. High quality preventive care involves both providing services of proven benefit and avoiding those that do not.
1. USPSTF. Published recommendations. https://www.uspreventiveservicestaskforce.org/BrowseRec/Index/browse-recommendations. Accessed March 25, 2019.
2. USPSTF. Final recommendation statement. Perinatal depression: preventive interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/perinatal-depression-preventive-interventions. Accessed March 25, 2019.
3. USPSTF. Atrial fibrillation: screening with electrocardiography. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/atrial-fibrillation-screening-with-electrocardiography. Accessed March 25, 2019.
4. USPSTF. Screening for atrial fibrillation with electrocardiography. JAMA. 2018;320:478-484.
5. USPSTF. Cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cervical-cancer-screening2. Accessed March 25, 2019.
6. USPSTF. Skin cancer prevention: behavioral counseling. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/skin-cancer-counseling2. Accessed March 25, 2019.
7. USPSTF. Falls prevention in community-dwelling older adults: interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/falls-prevention-in-older-adults-interventions1. Accessed March 25, 2019.
8. USPSTF. Adolescent idiopathic scoliosis: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/adolescent-idiopathic-scoliosis-screening1. Accessed March 25, 2019.
9. USPSTF. Prostate cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1#consider. Accessed March 25, 2019.
Over the past year through early 2019, the US Preventive Services Task Force made 34 recommendations on 19 different topics. Twenty-six were reaffirmations of recommendations made in previous years (TABLE 11); the Task Force attempts to reassess topics every 7 years. Two new topics were addressed with 2 new recommendations, and 6 previous recommendations were revised or reversed (TABLE 22-9).

This Practice Alert discusses the new and the changed recommendations. (In 2018, the Practice Alert podcast series covered screening for ovarian cancer [April], prostate cancer [June], and cervical cancer [October], and EKG screening for cardiovascular disease [November].) All current Task Force recommendations are available on the USPSTF Web site.1

New topics
Perinatal depression prevention
The Task Force recommends that clinicians counsel pregnant women and women in the first year postpartum who are at increased risk for perinatal depression, or refer for such services. The recommendation applies to those who are not diagnosed with depression but are at increased risk.

Perinatal depression can negatively affect both mother and child in several ways and occurs at a rate close to 9% during pregnancy and 37% during the first year postpartum.2 The interventions studied by the Task Force included cognitive behavioral therapy and interpersonal therapy; most sessions were initiated in the second trimester of pregnancy and varied in number of sessions and intensity. The Task Force includes the following in the list of risks that should prompt a referral: a history of depression, current depressive symptoms that fall short of that needed for a depression diagnosis, low income, adolescent or single parenthood, recent intimate partner violence, elevated anxiety symptoms, physical or sexual abuse, or a history of significant negative life events. (See “Postpartum anxiety: More common than you think,” in the April issue.)
Atrial fibrillation
The Task Force found insufficient evidence to recommend for or against the use of electrocardiography (EKG) to screen for atrial fibrillation (AF).3
Revisions of previous recommendations
Cervical cancer screening
Skin cancer prevention
The Task Force made 2 revisions to the 2012 recommendation on preventing skin cancer through behavioral counseling to avoid ultraviolet (UV) radiation.6 These recommendations continue to focus on those with fair skin. The first revision: The earliest age at which children (through their guardians) can benefit from counseling on UV avoidance has been lowered from age 10 years to 6 months. The second revision: Some adults older than age 24 can also benefit from such counseling if they have fair skin and other skin cancer risks such as using tanning beds, having a history of sunburns or previous skin cancer, having an increased number of nevi (moles) and atypical nevi, having human immunodeficiency virus (HIV) infection, having received an organ transplant, or having a family history of skin cancer.
Continue to: Those at risk...
Those at risk can reduce their chances of skin cancer by using broad-spectrum sunscreens and sun-protective clothing, and by avoiding sun exposure and indoor tanning beds.
Fall prevention
In a reversal of its 2012 recommendation, the Task Force now recommends against the use of vitamin D supplementation to prevent falls in community-dwelling adults 65 years or older.7 In a reanalysis of previous studies on this topic, along with new evidence, the Task Force concluded that vitamin D supplementation offers no benefit for preventing falls in adults who are not vitamin D deficient.
Screening for scoliosis in adolescents
In 2004 the USPSTF recommended against screening for idiopathic scoliosis in children and adolescents 10 to 18 years of age. In its most recent review, the Task Force continued to find no direct evidence of the benefit of screening and inadequate evidence on the long-term benefits of reduction in spinal curvature through exercise, surgery, and bracing. However, following a reanalysis of the potential harms of these treatments and the use of a new analytic framework, the Task Force concluded it is not possible at this time to assess the balance of benefits and harms of screening.8
Prostate cancer screening
In its most controversial action, the Task Force reversed its 2012 recommendation against routine prostate-specific antigen–based screening for prostate cancer in men ages 55 to 69 years and now lists this as a “C” recommendation.9 The potential benefits of screening include preventing 1.3 deaths from prostate cancer per 1000 men screened over 13 years and approximately 3 cases of metastatic prostate cancer. However, no trials have found a reduction in all-cause mortality from screening. Contrast that with the known harms of screening: 15% false positive results over 10 years; 1% hospitalization rate among those undergoing a prostate biopsy; over-diagnosis and resultant treatment of 20% to 50% of men diagnosed with prostate cancer through screening; and incontinence and erectile dysfunction in 20% and 67%, respectively, of men following prostatectomy.9
Based on these outcomes, the Task Force “does not recommend screening for prostate cancer unless men express a preference for screening after being informed of and understanding the benefits and risks.”9 The Task Force continues to recommend against screening men ages 70 years and older.
Continue to: The change in this recommendation...
The change in this recommendation and its wording present dilemmas for family physicians: whether to discuss potential screening with all men ages 55 to 69; to selectively discuss it with those at high risk (principally African Americans and those with a strong family history of prostate cancer); or to address the issue only if a patient asks about it. In addition, if a man requests screening, how often should it be performed? Most clinical trials have found equal benefit from testing less frequently than every year, with fewer harms. The Task Force provided little or no guidance on these issues.
Final advice: D recommendations
The Task Force reaffirmed that 7 services have either no benefit or cause more harm than benefit (TABLE 11). Family physicians should be familiar with these services, as well as all Task Force D recommendations, and avoid recommending them or providing them. High quality preventive care involves both providing services of proven benefit and avoiding those that do not.
Over the past year through early 2019, the US Preventive Services Task Force made 34 recommendations on 19 different topics. Twenty-six were reaffirmations of recommendations made in previous years (TABLE 11); the Task Force attempts to reassess topics every 7 years. Two new topics were addressed with 2 new recommendations, and 6 previous recommendations were revised or reversed (TABLE 22-9).

This Practice Alert discusses the new and the changed recommendations. (In 2018, the Practice Alert podcast series covered screening for ovarian cancer [April], prostate cancer [June], and cervical cancer [October], and EKG screening for cardiovascular disease [November].) All current Task Force recommendations are available on the USPSTF Web site.1

New topics
Perinatal depression prevention
The Task Force recommends that clinicians counsel pregnant women and women in the first year postpartum who are at increased risk for perinatal depression, or refer for such services. The recommendation applies to those who are not diagnosed with depression but are at increased risk.

Perinatal depression can negatively affect both mother and child in several ways and occurs at a rate close to 9% during pregnancy and 37% during the first year postpartum.2 The interventions studied by the Task Force included cognitive behavioral therapy and interpersonal therapy; most sessions were initiated in the second trimester of pregnancy and varied in number of sessions and intensity. The Task Force includes the following in the list of risks that should prompt a referral: a history of depression, current depressive symptoms that fall short of that needed for a depression diagnosis, low income, adolescent or single parenthood, recent intimate partner violence, elevated anxiety symptoms, physical or sexual abuse, or a history of significant negative life events. (See “Postpartum anxiety: More common than you think,” in the April issue.)
Atrial fibrillation
The Task Force found insufficient evidence to recommend for or against the use of electrocardiography (EKG) to screen for atrial fibrillation (AF).3
Revisions of previous recommendations
Cervical cancer screening
Skin cancer prevention
The Task Force made 2 revisions to the 2012 recommendation on preventing skin cancer through behavioral counseling to avoid ultraviolet (UV) radiation.6 These recommendations continue to focus on those with fair skin. The first revision: The earliest age at which children (through their guardians) can benefit from counseling on UV avoidance has been lowered from age 10 years to 6 months. The second revision: Some adults older than age 24 can also benefit from such counseling if they have fair skin and other skin cancer risks such as using tanning beds, having a history of sunburns or previous skin cancer, having an increased number of nevi (moles) and atypical nevi, having human immunodeficiency virus (HIV) infection, having received an organ transplant, or having a family history of skin cancer.
Continue to: Those at risk...
Those at risk can reduce their chances of skin cancer by using broad-spectrum sunscreens and sun-protective clothing, and by avoiding sun exposure and indoor tanning beds.
Fall prevention
In a reversal of its 2012 recommendation, the Task Force now recommends against the use of vitamin D supplementation to prevent falls in community-dwelling adults 65 years or older.7 In a reanalysis of previous studies on this topic, along with new evidence, the Task Force concluded that vitamin D supplementation offers no benefit for preventing falls in adults who are not vitamin D deficient.
Screening for scoliosis in adolescents
In 2004 the USPSTF recommended against screening for idiopathic scoliosis in children and adolescents 10 to 18 years of age. In its most recent review, the Task Force continued to find no direct evidence of the benefit of screening and inadequate evidence on the long-term benefits of reduction in spinal curvature through exercise, surgery, and bracing. However, following a reanalysis of the potential harms of these treatments and the use of a new analytic framework, the Task Force concluded it is not possible at this time to assess the balance of benefits and harms of screening.8
Prostate cancer screening
In its most controversial action, the Task Force reversed its 2012 recommendation against routine prostate-specific antigen–based screening for prostate cancer in men ages 55 to 69 years and now lists this as a “C” recommendation.9 The potential benefits of screening include preventing 1.3 deaths from prostate cancer per 1000 men screened over 13 years and approximately 3 cases of metastatic prostate cancer. However, no trials have found a reduction in all-cause mortality from screening. Contrast that with the known harms of screening: 15% false positive results over 10 years; 1% hospitalization rate among those undergoing a prostate biopsy; over-diagnosis and resultant treatment of 20% to 50% of men diagnosed with prostate cancer through screening; and incontinence and erectile dysfunction in 20% and 67%, respectively, of men following prostatectomy.9
Based on these outcomes, the Task Force “does not recommend screening for prostate cancer unless men express a preference for screening after being informed of and understanding the benefits and risks.”9 The Task Force continues to recommend against screening men ages 70 years and older.
Continue to: The change in this recommendation...
The change in this recommendation and its wording present dilemmas for family physicians: whether to discuss potential screening with all men ages 55 to 69; to selectively discuss it with those at high risk (principally African Americans and those with a strong family history of prostate cancer); or to address the issue only if a patient asks about it. In addition, if a man requests screening, how often should it be performed? Most clinical trials have found equal benefit from testing less frequently than every year, with fewer harms. The Task Force provided little or no guidance on these issues.
Final advice: D recommendations
The Task Force reaffirmed that 7 services have either no benefit or cause more harm than benefit (TABLE 11). Family physicians should be familiar with these services, as well as all Task Force D recommendations, and avoid recommending them or providing them. High quality preventive care involves both providing services of proven benefit and avoiding those that do not.
1. USPSTF. Published recommendations. https://www.uspreventiveservicestaskforce.org/BrowseRec/Index/browse-recommendations. Accessed March 25, 2019.
2. USPSTF. Final recommendation statement. Perinatal depression: preventive interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/perinatal-depression-preventive-interventions. Accessed March 25, 2019.
3. USPSTF. Atrial fibrillation: screening with electrocardiography. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/atrial-fibrillation-screening-with-electrocardiography. Accessed March 25, 2019.
4. USPSTF. Screening for atrial fibrillation with electrocardiography. JAMA. 2018;320:478-484.
5. USPSTF. Cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cervical-cancer-screening2. Accessed March 25, 2019.
6. USPSTF. Skin cancer prevention: behavioral counseling. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/skin-cancer-counseling2. Accessed March 25, 2019.
7. USPSTF. Falls prevention in community-dwelling older adults: interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/falls-prevention-in-older-adults-interventions1. Accessed March 25, 2019.
8. USPSTF. Adolescent idiopathic scoliosis: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/adolescent-idiopathic-scoliosis-screening1. Accessed March 25, 2019.
9. USPSTF. Prostate cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1#consider. Accessed March 25, 2019.
1. USPSTF. Published recommendations. https://www.uspreventiveservicestaskforce.org/BrowseRec/Index/browse-recommendations. Accessed March 25, 2019.
2. USPSTF. Final recommendation statement. Perinatal depression: preventive interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/perinatal-depression-preventive-interventions. Accessed March 25, 2019.
3. USPSTF. Atrial fibrillation: screening with electrocardiography. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/atrial-fibrillation-screening-with-electrocardiography. Accessed March 25, 2019.
4. USPSTF. Screening for atrial fibrillation with electrocardiography. JAMA. 2018;320:478-484.
5. USPSTF. Cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cervical-cancer-screening2. Accessed March 25, 2019.
6. USPSTF. Skin cancer prevention: behavioral counseling. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/skin-cancer-counseling2. Accessed March 25, 2019.
7. USPSTF. Falls prevention in community-dwelling older adults: interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/falls-prevention-in-older-adults-interventions1. Accessed March 25, 2019.
8. USPSTF. Adolescent idiopathic scoliosis: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/adolescent-idiopathic-scoliosis-screening1. Accessed March 25, 2019.
9. USPSTF. Prostate cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1#consider. Accessed March 25, 2019.
Are you offering vaccines to adults for these 5 conditions?
References
1. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2018-19 influenza season. MMWR Recomm Rep. 2018;67:1-20.
2. Liang JL, Tiwari T, Moro P, et al. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2018;67:1-44.
3. Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108.
4. Kobayashi M, Bennett NM, Gierke R, et al. Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2015;64;944-947.
5. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405-1408.
6. Adult immunization schedule. Centers for Disease Control and Prevention website. https://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html. Reviewed February 5, 2019. Accessed April 23, 2019.
References
1. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2018-19 influenza season. MMWR Recomm Rep. 2018;67:1-20.
2. Liang JL, Tiwari T, Moro P, et al. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2018;67:1-44.
3. Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108.
4. Kobayashi M, Bennett NM, Gierke R, et al. Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2015;64;944-947.
5. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405-1408.
6. Adult immunization schedule. Centers for Disease Control and Prevention website. https://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html. Reviewed February 5, 2019. Accessed April 23, 2019.
References
1. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2018-19 influenza season. MMWR Recomm Rep. 2018;67:1-20.
2. Liang JL, Tiwari T, Moro P, et al. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2018;67:1-44.
3. Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108.
4. Kobayashi M, Bennett NM, Gierke R, et al. Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2015;64;944-947.
5. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405-1408.
6. Adult immunization schedule. Centers for Disease Control and Prevention website. https://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html. Reviewed February 5, 2019. Accessed April 23, 2019.
The 2018 AHA/ACC cholesterol guidelines: What’s changed?
References
1. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A report of The American College of Cardiology Foundation/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018 Nov 8. doi: 10.1016/j.jacc.2018.11.003. [Epub ahead of print].
2. Alenghat FJ, Davis AM. Management of blood cholesterol. JAMA. 2019;321:800-801.
3. Fanaroff AC, Califf RM, Windecker S, et al. Levels of evidence supporting American College of Cardiology/American Heart Association and European Society of Cardiology Guidelines, 2008-2018. JAMA. 2019;321:1069-1080. [ ]
4. US Preventive Services Task Force. Cardiovascular disease: risk assessment with nontraditional risk factors. July 2018. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cardiovascular-disease-screening-using-nontraditional-risk-assessment. Accessed March 26, 2019.
5. American Academy of Family Practitioners. Clinical Practice Guideline: Cholesterol. February 2019. https://www.aafp.org/patient-care/clinical-recommendations/all/cholesterol.html. Accessed March 26, 2019.
References
1. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A report of The American College of Cardiology Foundation/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018 Nov 8. doi: 10.1016/j.jacc.2018.11.003. [Epub ahead of print].
2. Alenghat FJ, Davis AM. Management of blood cholesterol. JAMA. 2019;321:800-801.
3. Fanaroff AC, Califf RM, Windecker S, et al. Levels of evidence supporting American College of Cardiology/American Heart Association and European Society of Cardiology Guidelines, 2008-2018. JAMA. 2019;321:1069-1080. [ ]
4. US Preventive Services Task Force. Cardiovascular disease: risk assessment with nontraditional risk factors. July 2018. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cardiovascular-disease-screening-using-nontraditional-risk-assessment. Accessed March 26, 2019.
5. American Academy of Family Practitioners. Clinical Practice Guideline: Cholesterol. February 2019. https://www.aafp.org/patient-care/clinical-recommendations/all/cholesterol.html. Accessed March 26, 2019.
References
1. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A report of The American College of Cardiology Foundation/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018 Nov 8. doi: 10.1016/j.jacc.2018.11.003. [Epub ahead of print].
2. Alenghat FJ, Davis AM. Management of blood cholesterol. JAMA. 2019;321:800-801.
3. Fanaroff AC, Califf RM, Windecker S, et al. Levels of evidence supporting American College of Cardiology/American Heart Association and European Society of Cardiology Guidelines, 2008-2018. JAMA. 2019;321:1069-1080. [ ]
4. US Preventive Services Task Force. Cardiovascular disease: risk assessment with nontraditional risk factors. July 2018. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cardiovascular-disease-screening-using-nontraditional-risk-assessment. Accessed March 26, 2019.
5. American Academy of Family Practitioners. Clinical Practice Guideline: Cholesterol. February 2019. https://www.aafp.org/patient-care/clinical-recommendations/all/cholesterol.html. Accessed March 26, 2019.