User login
FDA expands Abraxane approval to include advanced pancreatic cancer
The albumin-bound formulation of paclitaxel has been approved by the Food and Drug Administration as a first-line treatment for metastatic adenocarcinoma of the pancreas, in combination with gemcitabine, the agency announced on Sept. 6.
The expanded indication for Abraxane (paclitaxel protein-bound particles for injectable suspension, albumin-bound), a microtubule inhibitor, is based on a study that found survival and progression-free survival were a median of almost 2 months longer among those treated with Abraxane and gemcitabine, compared with those treated with gemcitabine alone, according to the FDA statement announcing the approval.
When pancreatic cancer is diagnosed in an advanced stage and is inoperable, which is often when pancreatic cancer is diagnosed, "and in situations when the cancer has progressed following surgery, options like Abraxane can help prolong a patient’s life," Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, said in the statement.
The international study compared Abraxane plus gemcitabine with gemcitabine alone as a first-line treatment in 861 people with metastatic adenocarcinoma of the pancreas. Their median age was 63 years, and almost half had three or more sites of metastasis (84% had liver metastases).
Median overall survival was 8.5 months in those on the combination vs. 6.7 months among those on gemcitabine alone, a highly statistically significant difference. Median progression-free survival was 5.5 months among those on the combination vs. 3.7 months for those on gemcitabine alone, also a highly statistically significant difference. In addition, 23% of those in the combination arm had a confirmed complete or partial overall response, compared with 7% of those on gemcitabine, according to the prescribing information.
The results of the study, IMPACT (Metastatic Pancreatic Adenocarcinoma Clinical Trial), have been submitted for publication, according to the Celgene statement announcing the approval, and were presented at the American Society of Clinical Oncology annual meeting this year.
Common adverse events associated with treatment with Abraxane and gemcitabine included neutropenia, thrombocytopenia, peripheral neuropathy, nausea, alopecia, peripheral edema, diarrhea, fever, vomiting, rash, and dehydration. Fever, dehydration, pneumonia, and vomiting were the most common serious adverse events. Other "clinically important" adverse events included sepsis and pneumonitis, according to the FDA.
According to the new prescribing information, for pancreatic cancer, Abraxane (125 mg/m2) is administered intravenously on days 1, 8, and 15 of each 28-day cycle, followed by gemcitabine immediately afterwards. In the United States, pancreatic cancer is the fourth-leading cause of cancer deaths, according to the FDA statement, which cites National Cancer Institute estimates that 45,220 patients will be diagnosed with pancreatic cancer and 38,460 will die from the disease in 2013.
Abraxane, marketed by Celgene, was approved for treating breast cancer in 2005 and non–small cell lung cancer in 2012. Gemcitabine, a nucleoside metabolic inhibitor marketed as Gemzar by Eli Lilly, was approved in 1996 and is also available in generic formulations. Gemcitabine is approved as a single agent to treat pancreatic cancer.
The revised label is available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021660s037lbl.pdf.
The albumin-bound formulation of paclitaxel has been approved by the Food and Drug Administration as a first-line treatment for metastatic adenocarcinoma of the pancreas, in combination with gemcitabine, the agency announced on Sept. 6.
The expanded indication for Abraxane (paclitaxel protein-bound particles for injectable suspension, albumin-bound), a microtubule inhibitor, is based on a study that found survival and progression-free survival were a median of almost 2 months longer among those treated with Abraxane and gemcitabine, compared with those treated with gemcitabine alone, according to the FDA statement announcing the approval.
When pancreatic cancer is diagnosed in an advanced stage and is inoperable, which is often when pancreatic cancer is diagnosed, "and in situations when the cancer has progressed following surgery, options like Abraxane can help prolong a patient’s life," Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, said in the statement.
The international study compared Abraxane plus gemcitabine with gemcitabine alone as a first-line treatment in 861 people with metastatic adenocarcinoma of the pancreas. Their median age was 63 years, and almost half had three or more sites of metastasis (84% had liver metastases).
Median overall survival was 8.5 months in those on the combination vs. 6.7 months among those on gemcitabine alone, a highly statistically significant difference. Median progression-free survival was 5.5 months among those on the combination vs. 3.7 months for those on gemcitabine alone, also a highly statistically significant difference. In addition, 23% of those in the combination arm had a confirmed complete or partial overall response, compared with 7% of those on gemcitabine, according to the prescribing information.
The results of the study, IMPACT (Metastatic Pancreatic Adenocarcinoma Clinical Trial), have been submitted for publication, according to the Celgene statement announcing the approval, and were presented at the American Society of Clinical Oncology annual meeting this year.
Common adverse events associated with treatment with Abraxane and gemcitabine included neutropenia, thrombocytopenia, peripheral neuropathy, nausea, alopecia, peripheral edema, diarrhea, fever, vomiting, rash, and dehydration. Fever, dehydration, pneumonia, and vomiting were the most common serious adverse events. Other "clinically important" adverse events included sepsis and pneumonitis, according to the FDA.
According to the new prescribing information, for pancreatic cancer, Abraxane (125 mg/m2) is administered intravenously on days 1, 8, and 15 of each 28-day cycle, followed by gemcitabine immediately afterwards. In the United States, pancreatic cancer is the fourth-leading cause of cancer deaths, according to the FDA statement, which cites National Cancer Institute estimates that 45,220 patients will be diagnosed with pancreatic cancer and 38,460 will die from the disease in 2013.
Abraxane, marketed by Celgene, was approved for treating breast cancer in 2005 and non–small cell lung cancer in 2012. Gemcitabine, a nucleoside metabolic inhibitor marketed as Gemzar by Eli Lilly, was approved in 1996 and is also available in generic formulations. Gemcitabine is approved as a single agent to treat pancreatic cancer.
The revised label is available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021660s037lbl.pdf.
The albumin-bound formulation of paclitaxel has been approved by the Food and Drug Administration as a first-line treatment for metastatic adenocarcinoma of the pancreas, in combination with gemcitabine, the agency announced on Sept. 6.
The expanded indication for Abraxane (paclitaxel protein-bound particles for injectable suspension, albumin-bound), a microtubule inhibitor, is based on a study that found survival and progression-free survival were a median of almost 2 months longer among those treated with Abraxane and gemcitabine, compared with those treated with gemcitabine alone, according to the FDA statement announcing the approval.
When pancreatic cancer is diagnosed in an advanced stage and is inoperable, which is often when pancreatic cancer is diagnosed, "and in situations when the cancer has progressed following surgery, options like Abraxane can help prolong a patient’s life," Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, said in the statement.
The international study compared Abraxane plus gemcitabine with gemcitabine alone as a first-line treatment in 861 people with metastatic adenocarcinoma of the pancreas. Their median age was 63 years, and almost half had three or more sites of metastasis (84% had liver metastases).
Median overall survival was 8.5 months in those on the combination vs. 6.7 months among those on gemcitabine alone, a highly statistically significant difference. Median progression-free survival was 5.5 months among those on the combination vs. 3.7 months for those on gemcitabine alone, also a highly statistically significant difference. In addition, 23% of those in the combination arm had a confirmed complete or partial overall response, compared with 7% of those on gemcitabine, according to the prescribing information.
The results of the study, IMPACT (Metastatic Pancreatic Adenocarcinoma Clinical Trial), have been submitted for publication, according to the Celgene statement announcing the approval, and were presented at the American Society of Clinical Oncology annual meeting this year.
Common adverse events associated with treatment with Abraxane and gemcitabine included neutropenia, thrombocytopenia, peripheral neuropathy, nausea, alopecia, peripheral edema, diarrhea, fever, vomiting, rash, and dehydration. Fever, dehydration, pneumonia, and vomiting were the most common serious adverse events. Other "clinically important" adverse events included sepsis and pneumonitis, according to the FDA.
According to the new prescribing information, for pancreatic cancer, Abraxane (125 mg/m2) is administered intravenously on days 1, 8, and 15 of each 28-day cycle, followed by gemcitabine immediately afterwards. In the United States, pancreatic cancer is the fourth-leading cause of cancer deaths, according to the FDA statement, which cites National Cancer Institute estimates that 45,220 patients will be diagnosed with pancreatic cancer and 38,460 will die from the disease in 2013.
Abraxane, marketed by Celgene, was approved for treating breast cancer in 2005 and non–small cell lung cancer in 2012. Gemcitabine, a nucleoside metabolic inhibitor marketed as Gemzar by Eli Lilly, was approved in 1996 and is also available in generic formulations. Gemcitabine is approved as a single agent to treat pancreatic cancer.
The revised label is available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021660s037lbl.pdf.
PML reported in multiple sclerosis patient on fingolimod
The first case of progressive multifocal leukoencephalopathy has occurred in a patient with multiple sclerosis treated with fingolimod, according to the Food and Drug Administration.
The patient was diagnosed with progressive multifocal leukoencephalopathy (PML), the rare, often fatal opportunistic demyelinating infection, after almost 8 months of treatment with fingolimod, the agency reported on Aug. 29.
Fingolimod is a sphingosine 1-phosphate receptor modulator that was approved in 2010 for treating relapsing forms of MS.
The patient had not been treated with natalizumab, the MS drug that is known to increase the risk of the brain infection, but did receive 1 month of treatment with interferon beta-1a and azathioprine prior to beginning treatment with fingolimod. Those drugs were stopped when treatment with fingolimod began. However, the patient was also treated with "multiple courses" of intravenous corticosteroids for several months before and during treatment with fingolimod, which is marketed by Novartis under the name Gilenya.
Treatment with fingolimod was stopped after the diagnosis of PML, made on the basis of clinical symptoms and detection of JC viral DNA in cerebrospinal fluid, according to the FDA statement, which does not say whether the patient survived. The case was reported in Europe.
PML is caused by the JC virus, a common virus that usually does not cause illness, but can cause PML in people who are immunocompromised or are taking medications with immunosuppressive effects.
After several PML cases were reported in patients with MS treated with natalizumab (Tysabri) months after it was approved for treating MS in 2004, it was taken off the market in 2005 and reintroduced in June 2006 with measures to address the risk of PML, including a restricted distribution program.
The FDA is working with Novartis to investigate this case and will make recommendations when the evaluation has been completed, the statement said. The FDA is advising patients not to stop treatment with fingolimod before discussing this with their health care professionals.
The precautions and warnings section of the fingolimod label includes a statement that the treatment causes a dose-dependent reduction in peripheral lymphocyte count, due to reversible sequestration of lymphocytes in lymphoid tissues, and the drug "may therefore increase the risk of infections, some serious in nature," but PML is not mentioned. The label also says that it has not been administered with antineoplastic, immunosuppressive, or immune modulating therapies used to treat MS and that use of fingolimod with any of these treatments "would be expected to increase the risk of immunosuppression."
Fingolimod was approved with a risk evaluation and mitigation strategy, addressing the serious risks associated with treatment, including bradyarrhythmia and atrioventricular block at the start of treatment, infections, macular edema, respiratory effects, hepatic effects, and fetal risk.
According to Novartis, about 71,000 patients worldwide have been treated with fingolimod.
The safety communication is available here. Serious adverse events associated with fingolimod should be reported to the FDA’s MedWatch program at 800-332-1088.
The first case of progressive multifocal leukoencephalopathy has occurred in a patient with multiple sclerosis treated with fingolimod, according to the Food and Drug Administration.
The patient was diagnosed with progressive multifocal leukoencephalopathy (PML), the rare, often fatal opportunistic demyelinating infection, after almost 8 months of treatment with fingolimod, the agency reported on Aug. 29.
Fingolimod is a sphingosine 1-phosphate receptor modulator that was approved in 2010 for treating relapsing forms of MS.
The patient had not been treated with natalizumab, the MS drug that is known to increase the risk of the brain infection, but did receive 1 month of treatment with interferon beta-1a and azathioprine prior to beginning treatment with fingolimod. Those drugs were stopped when treatment with fingolimod began. However, the patient was also treated with "multiple courses" of intravenous corticosteroids for several months before and during treatment with fingolimod, which is marketed by Novartis under the name Gilenya.
Treatment with fingolimod was stopped after the diagnosis of PML, made on the basis of clinical symptoms and detection of JC viral DNA in cerebrospinal fluid, according to the FDA statement, which does not say whether the patient survived. The case was reported in Europe.
PML is caused by the JC virus, a common virus that usually does not cause illness, but can cause PML in people who are immunocompromised or are taking medications with immunosuppressive effects.
After several PML cases were reported in patients with MS treated with natalizumab (Tysabri) months after it was approved for treating MS in 2004, it was taken off the market in 2005 and reintroduced in June 2006 with measures to address the risk of PML, including a restricted distribution program.
The FDA is working with Novartis to investigate this case and will make recommendations when the evaluation has been completed, the statement said. The FDA is advising patients not to stop treatment with fingolimod before discussing this with their health care professionals.
The precautions and warnings section of the fingolimod label includes a statement that the treatment causes a dose-dependent reduction in peripheral lymphocyte count, due to reversible sequestration of lymphocytes in lymphoid tissues, and the drug "may therefore increase the risk of infections, some serious in nature," but PML is not mentioned. The label also says that it has not been administered with antineoplastic, immunosuppressive, or immune modulating therapies used to treat MS and that use of fingolimod with any of these treatments "would be expected to increase the risk of immunosuppression."
Fingolimod was approved with a risk evaluation and mitigation strategy, addressing the serious risks associated with treatment, including bradyarrhythmia and atrioventricular block at the start of treatment, infections, macular edema, respiratory effects, hepatic effects, and fetal risk.
According to Novartis, about 71,000 patients worldwide have been treated with fingolimod.
The safety communication is available here. Serious adverse events associated with fingolimod should be reported to the FDA’s MedWatch program at 800-332-1088.
The first case of progressive multifocal leukoencephalopathy has occurred in a patient with multiple sclerosis treated with fingolimod, according to the Food and Drug Administration.
The patient was diagnosed with progressive multifocal leukoencephalopathy (PML), the rare, often fatal opportunistic demyelinating infection, after almost 8 months of treatment with fingolimod, the agency reported on Aug. 29.
Fingolimod is a sphingosine 1-phosphate receptor modulator that was approved in 2010 for treating relapsing forms of MS.
The patient had not been treated with natalizumab, the MS drug that is known to increase the risk of the brain infection, but did receive 1 month of treatment with interferon beta-1a and azathioprine prior to beginning treatment with fingolimod. Those drugs were stopped when treatment with fingolimod began. However, the patient was also treated with "multiple courses" of intravenous corticosteroids for several months before and during treatment with fingolimod, which is marketed by Novartis under the name Gilenya.
Treatment with fingolimod was stopped after the diagnosis of PML, made on the basis of clinical symptoms and detection of JC viral DNA in cerebrospinal fluid, according to the FDA statement, which does not say whether the patient survived. The case was reported in Europe.
PML is caused by the JC virus, a common virus that usually does not cause illness, but can cause PML in people who are immunocompromised or are taking medications with immunosuppressive effects.
After several PML cases were reported in patients with MS treated with natalizumab (Tysabri) months after it was approved for treating MS in 2004, it was taken off the market in 2005 and reintroduced in June 2006 with measures to address the risk of PML, including a restricted distribution program.
The FDA is working with Novartis to investigate this case and will make recommendations when the evaluation has been completed, the statement said. The FDA is advising patients not to stop treatment with fingolimod before discussing this with their health care professionals.
The precautions and warnings section of the fingolimod label includes a statement that the treatment causes a dose-dependent reduction in peripheral lymphocyte count, due to reversible sequestration of lymphocytes in lymphoid tissues, and the drug "may therefore increase the risk of infections, some serious in nature," but PML is not mentioned. The label also says that it has not been administered with antineoplastic, immunosuppressive, or immune modulating therapies used to treat MS and that use of fingolimod with any of these treatments "would be expected to increase the risk of immunosuppression."
Fingolimod was approved with a risk evaluation and mitigation strategy, addressing the serious risks associated with treatment, including bradyarrhythmia and atrioventricular block at the start of treatment, infections, macular edema, respiratory effects, hepatic effects, and fetal risk.
According to Novartis, about 71,000 patients worldwide have been treated with fingolimod.
The safety communication is available here. Serious adverse events associated with fingolimod should be reported to the FDA’s MedWatch program at 800-332-1088.
Policy statement outlines components of successful stroke care
Government agencies, health care authorities, and medical leaders should support the formation and certification of stroke centers and the use of telemedicine systems to improve care, according to a policy statement issued by the American Heart Association and the American Stroke Association.
Other recommendations in the statement – which describes the components needed for a modern system of stroke care in the United States – involve establishing a system that provides universal access to poststroke care and developing hospital protocols that reflect current stroke care guidelines.
These are among the 10 policy recommendations that describe concepts and elements to be included in "stroke systems of care that are intended to optimize patient care and management processes and improve patient outcomes, are practical to implement, and are supported by existing clinical data or expert consensus opinion," the statement said.
The paper, "Interactions Within Stroke Systems of Care," was published Aug. 29 in Stroke (doi:10.1161/STR.0b013e3182a6d2b2).
The lead authors are Dr. Randall Higashida, chair of the American Heart Association Advocacy Coordinating Committee, and Dr. Mark Alberts, cochair.
A "fully functional" system of stroke care would reduce the number of deaths by 20,000 in the United States and by about 400,000 worldwide. In addition, such a system would reduce disability after strokes, which would improve quality of life and would lower costs for patients, their families, third-party payers, and governments, according to the statement.
Recommendations for the main elements of a stroke system of care range from calling 911 to the interactions of different types of health care professionals involved in the care of patients in a stroke center to discharge and rehabilitation.
The first recommendation advises medical professionals and public health leaders to "design and implement" public education programs about stroke symptoms and the need to seek emergency care quickly.
Since designated stroke centers have been shown to improve patient care and outcomes, including lower death rates, another recommendation is for health care professionals, medical leaders, and government agencies to support the formation, operation, and certification of such centers. The statement includes descriptions of different acute inpatient stroke care facilities, including a comprehensive stroke center, primary stroke center and acute stroke–ready hospital.
In addition, hospitals caring for stroke patients "within a stroke system of care should develop, adopt and adhere to care protocols that reflect current care guidelines" that have been established by national and international professional organizations, and state and federal agencies.
Governments, payers, vendors, and health care institutions should support the use of telemedicine resources and "telestroke" systems to ensure that stroke patients in a variety of settings have adequate around-the-clock care, according to another recommendation that noted the "limited distribution and availability of neurological, neurosurgical, and radiological expertise."
Other recommendations pertain to transfer protocol and criteria, reimbursement issues, legal issues in stroke care, and rehabilitation.
The authors state that local and regional health care providers and government officials and related agencies should adopt the procedures and policies described in the statement. The statement concludes that "any system of care will only be as strong and efficient as its weakest link," and that by following the principles in the statement, "we hope to minimize or eliminate any weak links."
Dr. Higashida, chief of the division of interventional neurovascular radiology at the University of California San Francisco Medical Center, had no disclosures. Dr. Alberts, professor of neurology and neurotherapeutics at the University of Texas Southwestern Medical Center, Dallas, disclosed having received honoraria or serving on the speakers bureau for Genentech. Of the 15 remaining members of the committee, 6 had no disclosures. The remaining committee members’ disclosures included serving as a consultant or adviser to Genentech, W.L. Gore, Covidien, and/or the National Stroke Association, and receiving grants from the National Institutes of Health and/or the National Institute of Neurological Disorders and Stroke, the National Stroke Association, and Genentech.
Government agencies, health care authorities, and medical leaders should support the formation and certification of stroke centers and the use of telemedicine systems to improve care, according to a policy statement issued by the American Heart Association and the American Stroke Association.
Other recommendations in the statement – which describes the components needed for a modern system of stroke care in the United States – involve establishing a system that provides universal access to poststroke care and developing hospital protocols that reflect current stroke care guidelines.
These are among the 10 policy recommendations that describe concepts and elements to be included in "stroke systems of care that are intended to optimize patient care and management processes and improve patient outcomes, are practical to implement, and are supported by existing clinical data or expert consensus opinion," the statement said.
The paper, "Interactions Within Stroke Systems of Care," was published Aug. 29 in Stroke (doi:10.1161/STR.0b013e3182a6d2b2).
The lead authors are Dr. Randall Higashida, chair of the American Heart Association Advocacy Coordinating Committee, and Dr. Mark Alberts, cochair.
A "fully functional" system of stroke care would reduce the number of deaths by 20,000 in the United States and by about 400,000 worldwide. In addition, such a system would reduce disability after strokes, which would improve quality of life and would lower costs for patients, their families, third-party payers, and governments, according to the statement.
Recommendations for the main elements of a stroke system of care range from calling 911 to the interactions of different types of health care professionals involved in the care of patients in a stroke center to discharge and rehabilitation.
The first recommendation advises medical professionals and public health leaders to "design and implement" public education programs about stroke symptoms and the need to seek emergency care quickly.
Since designated stroke centers have been shown to improve patient care and outcomes, including lower death rates, another recommendation is for health care professionals, medical leaders, and government agencies to support the formation, operation, and certification of such centers. The statement includes descriptions of different acute inpatient stroke care facilities, including a comprehensive stroke center, primary stroke center and acute stroke–ready hospital.
In addition, hospitals caring for stroke patients "within a stroke system of care should develop, adopt and adhere to care protocols that reflect current care guidelines" that have been established by national and international professional organizations, and state and federal agencies.
Governments, payers, vendors, and health care institutions should support the use of telemedicine resources and "telestroke" systems to ensure that stroke patients in a variety of settings have adequate around-the-clock care, according to another recommendation that noted the "limited distribution and availability of neurological, neurosurgical, and radiological expertise."
Other recommendations pertain to transfer protocol and criteria, reimbursement issues, legal issues in stroke care, and rehabilitation.
The authors state that local and regional health care providers and government officials and related agencies should adopt the procedures and policies described in the statement. The statement concludes that "any system of care will only be as strong and efficient as its weakest link," and that by following the principles in the statement, "we hope to minimize or eliminate any weak links."
Dr. Higashida, chief of the division of interventional neurovascular radiology at the University of California San Francisco Medical Center, had no disclosures. Dr. Alberts, professor of neurology and neurotherapeutics at the University of Texas Southwestern Medical Center, Dallas, disclosed having received honoraria or serving on the speakers bureau for Genentech. Of the 15 remaining members of the committee, 6 had no disclosures. The remaining committee members’ disclosures included serving as a consultant or adviser to Genentech, W.L. Gore, Covidien, and/or the National Stroke Association, and receiving grants from the National Institutes of Health and/or the National Institute of Neurological Disorders and Stroke, the National Stroke Association, and Genentech.
Government agencies, health care authorities, and medical leaders should support the formation and certification of stroke centers and the use of telemedicine systems to improve care, according to a policy statement issued by the American Heart Association and the American Stroke Association.
Other recommendations in the statement – which describes the components needed for a modern system of stroke care in the United States – involve establishing a system that provides universal access to poststroke care and developing hospital protocols that reflect current stroke care guidelines.
These are among the 10 policy recommendations that describe concepts and elements to be included in "stroke systems of care that are intended to optimize patient care and management processes and improve patient outcomes, are practical to implement, and are supported by existing clinical data or expert consensus opinion," the statement said.
The paper, "Interactions Within Stroke Systems of Care," was published Aug. 29 in Stroke (doi:10.1161/STR.0b013e3182a6d2b2).
The lead authors are Dr. Randall Higashida, chair of the American Heart Association Advocacy Coordinating Committee, and Dr. Mark Alberts, cochair.
A "fully functional" system of stroke care would reduce the number of deaths by 20,000 in the United States and by about 400,000 worldwide. In addition, such a system would reduce disability after strokes, which would improve quality of life and would lower costs for patients, their families, third-party payers, and governments, according to the statement.
Recommendations for the main elements of a stroke system of care range from calling 911 to the interactions of different types of health care professionals involved in the care of patients in a stroke center to discharge and rehabilitation.
The first recommendation advises medical professionals and public health leaders to "design and implement" public education programs about stroke symptoms and the need to seek emergency care quickly.
Since designated stroke centers have been shown to improve patient care and outcomes, including lower death rates, another recommendation is for health care professionals, medical leaders, and government agencies to support the formation, operation, and certification of such centers. The statement includes descriptions of different acute inpatient stroke care facilities, including a comprehensive stroke center, primary stroke center and acute stroke–ready hospital.
In addition, hospitals caring for stroke patients "within a stroke system of care should develop, adopt and adhere to care protocols that reflect current care guidelines" that have been established by national and international professional organizations, and state and federal agencies.
Governments, payers, vendors, and health care institutions should support the use of telemedicine resources and "telestroke" systems to ensure that stroke patients in a variety of settings have adequate around-the-clock care, according to another recommendation that noted the "limited distribution and availability of neurological, neurosurgical, and radiological expertise."
Other recommendations pertain to transfer protocol and criteria, reimbursement issues, legal issues in stroke care, and rehabilitation.
The authors state that local and regional health care providers and government officials and related agencies should adopt the procedures and policies described in the statement. The statement concludes that "any system of care will only be as strong and efficient as its weakest link," and that by following the principles in the statement, "we hope to minimize or eliminate any weak links."
Dr. Higashida, chief of the division of interventional neurovascular radiology at the University of California San Francisco Medical Center, had no disclosures. Dr. Alberts, professor of neurology and neurotherapeutics at the University of Texas Southwestern Medical Center, Dallas, disclosed having received honoraria or serving on the speakers bureau for Genentech. Of the 15 remaining members of the committee, 6 had no disclosures. The remaining committee members’ disclosures included serving as a consultant or adviser to Genentech, W.L. Gore, Covidien, and/or the National Stroke Association, and receiving grants from the National Institutes of Health and/or the National Institute of Neurological Disorders and Stroke, the National Stroke Association, and Genentech.
FROM STROKE
No increased birth defect risk found with oral fluconazole, with one exception
The overall risk of birth defects was not increased among infants whose mothers took oral fluconazole during the first trimester of pregnancy, in a large registry study that provides "largely reassuring" results regarding the teratogenicity of this drug, according to the authors.
In the study that included data on more than 7,000 pregnancies exposed to fluconazole in Denmark, fluconazole (largely at the most commonly prescribed 150-mg or 300-mg single doses) was also not associated with an increased risk of 14 of the 15 specific birth defects that have been associated with azole antifungal drugs in human or animal studies. But the risk of tetralogy of Fallot was increased by about threefold, an association that needs to be studied further, concluded Ditte Mølgaard-Nielsen and her associates in the department of epidemiology research, Statens Serum Institut, Copenhagen.
The risk of birth defects also was not increased among the smaller number of pregnancies with first-trimester exposure to two other oral azole antifungals, ketoconazole and itraconazole. The study is being published in the Aug. 29 issue of the New England Journal of Medicine (2013;369:830-9).
Most previous studies of the teratogenicity of fluconazole have involved case reports and have suggested that long-term use of fluconazole at high doses may be associated with an increased risk of certain birth defects – the basis of a Food and Drug Administration safety communication in 2011. But while a few epidemiologic studies of oral fluconazole, mostly at the 150-mg dose, have not found an association with birth defects, the studies were not large enough to investigate the risk with different doses or the risk of certain defects, the authors said.
In August 2011, the FDA described the possible association of oral fluconazole, at doses of 400-800 mg/day during the first trimester, and an increased risk of a rare but "distinct set of birth defects," based on case reports. The defects included brachycephaly, abnormal facies, abnormal calvarial development, cleft palate, femoral bowing, thin ribs and long bones, arthrogryposis, and congenital heart disease, which were also seen in animals. As a result, the FDA changed the pregnancy risk category of fluconazole from category C (adverse effects seen in animals but no adequate human data) to D (evidence of human fetal risk based on human data but the potential benefits may justify use in pregnant women with serious or life-threatening conditions) for all indications, except for the 150-mg single dose used for vaginal candidiasis. Because the available human data on that dose do not indicate there is an association with an increased risk of birth defects, the 150-mg dose used for vaginal candidiasis remained in category C.
The Danish study used data on all live-born infants in Denmark between January 1996 and March 2011 from the national birth registry, prescription data from the national prescription registry, and information on birth defects from a national patient registry. The most common cumulative doses of fluconazole were 150 mg (56%) and 300 mg (31%); the remaining percentage of patients were exposed to cumulative doses from 350 mg to 6,000 mg.
Among the 7,352 pregnancies exposed to fluconazole in the first trimester, there were 210 birth defects (2.86%), compared with 25,159 birth defects (2.6%) among 968,236 unexposed pregnancies.
When the investigators looked at 15 different birth defects that have been linked to in utero exposure to fluconazole and other azole antifungal agents, the risk was not significantly increased for defects that included craniosynostosis, cleft palate, cleft lip with or without cleft palate, limb defects, polydactyly, syndactyly, diaphragmatic hernia, heart defects overall, or ventricular septal defects.
However, there were 7 cases of tetralogy of Fallot (0.1%) among pregnancies exposed to fluconazole, compared with 287 cases (0.03%) among those not exposed – a threefold increased risk that was statistically significant. The cases were found in infants exposed to all three cumulative dose categories: four (0.10%) exposed to 150 mg, two (0.09%) exposed to 300 mg, and one (0.10%) exposed to the 350- to 6,000-mg dose range.
Although the increased risk of tetralogy of Fallot could be a chance finding, this was among the birth defects previously associated with exposure to fluconazole, which made the finding more reliable than if this had been the first time the association with this particular birth defect had been reported, the authors noted.
Among 687 pregnancies exposed to itraconazole and 72 pregnancies exposed to ketoconazole in the registry, there was no significantly increased risk of birth defects overall, or any clustering of malformations.
"Our finding that a 150-mg dose of fluconazole was not associated with an increased risk of birth defects overall confirms the results of previous studies; our study also adds to these safety data by reporting risk estimates for doses higher than 150 mg," the authors wrote. "Although fluconazole may confer an increased risk of tetralogy of Fallot, the absolute risk was small and the association needs to be confirmed," they added.
The study’s strengths include the nationwide cohort, the 1-year follow-up of the infants, the high degree of accuracy of the registry data on birth detects, and the use of prescription data, which eliminates the chance of recall bias, the authors said. The limitations included not being able to account for all confounding factors, such as ascertainment of maternal illnesses.
The study was funded by the Danish Medical Research Council. The authors reported having no relevant financial disclosures.
Fluconazole is marketed as Diflucan, ketoconazole is marketed as Nizoral, and itraconazole is marketed as Sporanox; all three are available in generic formulations.
The overall risk of birth defects was not increased among infants whose mothers took oral fluconazole during the first trimester of pregnancy, in a large registry study that provides "largely reassuring" results regarding the teratogenicity of this drug, according to the authors.
In the study that included data on more than 7,000 pregnancies exposed to fluconazole in Denmark, fluconazole (largely at the most commonly prescribed 150-mg or 300-mg single doses) was also not associated with an increased risk of 14 of the 15 specific birth defects that have been associated with azole antifungal drugs in human or animal studies. But the risk of tetralogy of Fallot was increased by about threefold, an association that needs to be studied further, concluded Ditte Mølgaard-Nielsen and her associates in the department of epidemiology research, Statens Serum Institut, Copenhagen.
The risk of birth defects also was not increased among the smaller number of pregnancies with first-trimester exposure to two other oral azole antifungals, ketoconazole and itraconazole. The study is being published in the Aug. 29 issue of the New England Journal of Medicine (2013;369:830-9).
Most previous studies of the teratogenicity of fluconazole have involved case reports and have suggested that long-term use of fluconazole at high doses may be associated with an increased risk of certain birth defects – the basis of a Food and Drug Administration safety communication in 2011. But while a few epidemiologic studies of oral fluconazole, mostly at the 150-mg dose, have not found an association with birth defects, the studies were not large enough to investigate the risk with different doses or the risk of certain defects, the authors said.
In August 2011, the FDA described the possible association of oral fluconazole, at doses of 400-800 mg/day during the first trimester, and an increased risk of a rare but "distinct set of birth defects," based on case reports. The defects included brachycephaly, abnormal facies, abnormal calvarial development, cleft palate, femoral bowing, thin ribs and long bones, arthrogryposis, and congenital heart disease, which were also seen in animals. As a result, the FDA changed the pregnancy risk category of fluconazole from category C (adverse effects seen in animals but no adequate human data) to D (evidence of human fetal risk based on human data but the potential benefits may justify use in pregnant women with serious or life-threatening conditions) for all indications, except for the 150-mg single dose used for vaginal candidiasis. Because the available human data on that dose do not indicate there is an association with an increased risk of birth defects, the 150-mg dose used for vaginal candidiasis remained in category C.
The Danish study used data on all live-born infants in Denmark between January 1996 and March 2011 from the national birth registry, prescription data from the national prescription registry, and information on birth defects from a national patient registry. The most common cumulative doses of fluconazole were 150 mg (56%) and 300 mg (31%); the remaining percentage of patients were exposed to cumulative doses from 350 mg to 6,000 mg.
Among the 7,352 pregnancies exposed to fluconazole in the first trimester, there were 210 birth defects (2.86%), compared with 25,159 birth defects (2.6%) among 968,236 unexposed pregnancies.
When the investigators looked at 15 different birth defects that have been linked to in utero exposure to fluconazole and other azole antifungal agents, the risk was not significantly increased for defects that included craniosynostosis, cleft palate, cleft lip with or without cleft palate, limb defects, polydactyly, syndactyly, diaphragmatic hernia, heart defects overall, or ventricular septal defects.
However, there were 7 cases of tetralogy of Fallot (0.1%) among pregnancies exposed to fluconazole, compared with 287 cases (0.03%) among those not exposed – a threefold increased risk that was statistically significant. The cases were found in infants exposed to all three cumulative dose categories: four (0.10%) exposed to 150 mg, two (0.09%) exposed to 300 mg, and one (0.10%) exposed to the 350- to 6,000-mg dose range.
Although the increased risk of tetralogy of Fallot could be a chance finding, this was among the birth defects previously associated with exposure to fluconazole, which made the finding more reliable than if this had been the first time the association with this particular birth defect had been reported, the authors noted.
Among 687 pregnancies exposed to itraconazole and 72 pregnancies exposed to ketoconazole in the registry, there was no significantly increased risk of birth defects overall, or any clustering of malformations.
"Our finding that a 150-mg dose of fluconazole was not associated with an increased risk of birth defects overall confirms the results of previous studies; our study also adds to these safety data by reporting risk estimates for doses higher than 150 mg," the authors wrote. "Although fluconazole may confer an increased risk of tetralogy of Fallot, the absolute risk was small and the association needs to be confirmed," they added.
The study’s strengths include the nationwide cohort, the 1-year follow-up of the infants, the high degree of accuracy of the registry data on birth detects, and the use of prescription data, which eliminates the chance of recall bias, the authors said. The limitations included not being able to account for all confounding factors, such as ascertainment of maternal illnesses.
The study was funded by the Danish Medical Research Council. The authors reported having no relevant financial disclosures.
Fluconazole is marketed as Diflucan, ketoconazole is marketed as Nizoral, and itraconazole is marketed as Sporanox; all three are available in generic formulations.
The overall risk of birth defects was not increased among infants whose mothers took oral fluconazole during the first trimester of pregnancy, in a large registry study that provides "largely reassuring" results regarding the teratogenicity of this drug, according to the authors.
In the study that included data on more than 7,000 pregnancies exposed to fluconazole in Denmark, fluconazole (largely at the most commonly prescribed 150-mg or 300-mg single doses) was also not associated with an increased risk of 14 of the 15 specific birth defects that have been associated with azole antifungal drugs in human or animal studies. But the risk of tetralogy of Fallot was increased by about threefold, an association that needs to be studied further, concluded Ditte Mølgaard-Nielsen and her associates in the department of epidemiology research, Statens Serum Institut, Copenhagen.
The risk of birth defects also was not increased among the smaller number of pregnancies with first-trimester exposure to two other oral azole antifungals, ketoconazole and itraconazole. The study is being published in the Aug. 29 issue of the New England Journal of Medicine (2013;369:830-9).
Most previous studies of the teratogenicity of fluconazole have involved case reports and have suggested that long-term use of fluconazole at high doses may be associated with an increased risk of certain birth defects – the basis of a Food and Drug Administration safety communication in 2011. But while a few epidemiologic studies of oral fluconazole, mostly at the 150-mg dose, have not found an association with birth defects, the studies were not large enough to investigate the risk with different doses or the risk of certain defects, the authors said.
In August 2011, the FDA described the possible association of oral fluconazole, at doses of 400-800 mg/day during the first trimester, and an increased risk of a rare but "distinct set of birth defects," based on case reports. The defects included brachycephaly, abnormal facies, abnormal calvarial development, cleft palate, femoral bowing, thin ribs and long bones, arthrogryposis, and congenital heart disease, which were also seen in animals. As a result, the FDA changed the pregnancy risk category of fluconazole from category C (adverse effects seen in animals but no adequate human data) to D (evidence of human fetal risk based on human data but the potential benefits may justify use in pregnant women with serious or life-threatening conditions) for all indications, except for the 150-mg single dose used for vaginal candidiasis. Because the available human data on that dose do not indicate there is an association with an increased risk of birth defects, the 150-mg dose used for vaginal candidiasis remained in category C.
The Danish study used data on all live-born infants in Denmark between January 1996 and March 2011 from the national birth registry, prescription data from the national prescription registry, and information on birth defects from a national patient registry. The most common cumulative doses of fluconazole were 150 mg (56%) and 300 mg (31%); the remaining percentage of patients were exposed to cumulative doses from 350 mg to 6,000 mg.
Among the 7,352 pregnancies exposed to fluconazole in the first trimester, there were 210 birth defects (2.86%), compared with 25,159 birth defects (2.6%) among 968,236 unexposed pregnancies.
When the investigators looked at 15 different birth defects that have been linked to in utero exposure to fluconazole and other azole antifungal agents, the risk was not significantly increased for defects that included craniosynostosis, cleft palate, cleft lip with or without cleft palate, limb defects, polydactyly, syndactyly, diaphragmatic hernia, heart defects overall, or ventricular septal defects.
However, there were 7 cases of tetralogy of Fallot (0.1%) among pregnancies exposed to fluconazole, compared with 287 cases (0.03%) among those not exposed – a threefold increased risk that was statistically significant. The cases were found in infants exposed to all three cumulative dose categories: four (0.10%) exposed to 150 mg, two (0.09%) exposed to 300 mg, and one (0.10%) exposed to the 350- to 6,000-mg dose range.
Although the increased risk of tetralogy of Fallot could be a chance finding, this was among the birth defects previously associated with exposure to fluconazole, which made the finding more reliable than if this had been the first time the association with this particular birth defect had been reported, the authors noted.
Among 687 pregnancies exposed to itraconazole and 72 pregnancies exposed to ketoconazole in the registry, there was no significantly increased risk of birth defects overall, or any clustering of malformations.
"Our finding that a 150-mg dose of fluconazole was not associated with an increased risk of birth defects overall confirms the results of previous studies; our study also adds to these safety data by reporting risk estimates for doses higher than 150 mg," the authors wrote. "Although fluconazole may confer an increased risk of tetralogy of Fallot, the absolute risk was small and the association needs to be confirmed," they added.
The study’s strengths include the nationwide cohort, the 1-year follow-up of the infants, the high degree of accuracy of the registry data on birth detects, and the use of prescription data, which eliminates the chance of recall bias, the authors said. The limitations included not being able to account for all confounding factors, such as ascertainment of maternal illnesses.
The study was funded by the Danish Medical Research Council. The authors reported having no relevant financial disclosures.
Fluconazole is marketed as Diflucan, ketoconazole is marketed as Nizoral, and itraconazole is marketed as Sporanox; all three are available in generic formulations.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Major finding: Exposure to oral fluconazole in the first trimester was not associated with an increased risk of birth defects overall, but was associated with a threefold increased risk in tetralogy of Fallot, although the absolute risk was small.
Data source: A registry-based cohort study comparing the risk of birth defects overall, and the risk of specific birth defects associated with first-trimester exposure to different doses of oral fluconazole, in 7,352 fluconazole-exposed pregnancies and 968,236 unexposed pregnancies.
Disclosures: The study was funded by the Danish Medical Research Council. The authors reported having no relevant financial disclosures.
Drop in infertility rate among findings of national survey
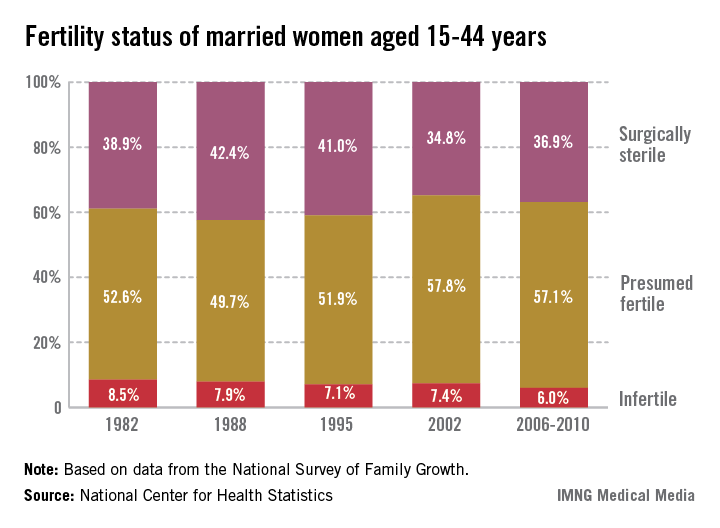
Recent trends in fertility problems in the United States include a drop in the proportion of married women aged 15-44 who were infertile from 8.5% in 1982 to 6% between 2006 and 2010, according to a report from the National Center for Health Statistics.
The corresponding number of women affected dropped from 2.4 million in 1982 to 1.5 million in the later period.
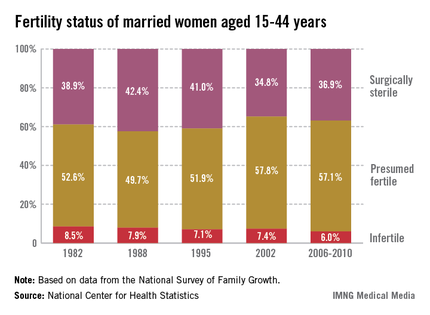
Among all the women surveyed, the proportion of women with impaired fecundity stabilized at 11% in 2006-2010. Prior to this period, the proportion had increased significantly from 8.4% in 1982 to 10% in 1995 and 12% in 2002. There was a similar pattern observed among married women, although the proportion affected was higher: In 1982 and 1988, 11% of married women had impaired fecundity, increasing to 15% in 2002 and dropping to 12% in 2006-2010, according to Anjanai Chandra, Ph.D., of the NCHS, and her coauthors.
The data are from the National Survey of Family Growth (NSFG), which provides nationally representative measures of fertility, based on 22,682 interviews with men and women aged 15-44, from June 2006 through June 2010. The report was published on Aug. 14 (Natl. Health Stat. Report. 2013;67:1-19). Data are also collected for men: Between 2006 and 2010, 9.4% of men aged 15-44 and 12% of men aged 25-44 reported having some form of infertility, which was similar to the proportion reported in 2002, when men were first included in the survey,
In women, the two infertility measures used since 1982 are infertility (defined as "a lack of pregnancy in the 12 months prior to the survey, despite having unprotected sexual intercourse in each of those months with the same husband or partner") and impaired fecundity ("physical difficulty in either getting pregnant or carrying a pregnancy to live birth"). The infertility measure has been limited to couples who are married or living together, because it does not distinguish between infertility in the male or female partner. Because of issues with the size of the sample, the authors reported infertility estimates in subgroups in married women only, but noted that in 2006-2010, the proportions of married and cohabiting women who were infertile were similar, at 6% and 4.9%, respectively.
The authors pointed out that among nulliparous women, infertility and impaired fecundity were closely associated with age: Among married nulliparous women aged 35-44, the proportion who were infertile dropped from 44% in 1982 to 27% in 2006-2010.
They also compared some data to data from the National Fertility Study in 1965: Among married women who were infertile, the rate of primary infertility – the proportion who were having problems having their first child – increased from 17% in 1965 to 41%-46% in 1982-2010, "which is consistent with patterns and trends in delayed childbearing over these years," the authors pointed out.
While there was no "clear association" between poverty level and education, women who were less educated and at poverty income levels were more likely to be surgically sterilized. For example, between 2006 and 2010, among women aged 25-44 years, 44% of those who had not completed high school had undergone surgical sterilization for contraceptive reasons, compared with 21% of those with a bachelor’s degree and 16% of those with a master’s degree or higher degree. The women with more education also were more likely to be fecund (65%-71%), compared with those with a high school education or less (42%-43%), "presumably because these latter women started and completed their fertility at younger ages and opted for surgical sterilization," the authors wrote.
The NSFG has been collecting data on impaired fecundity in all women aged *15-44 years since 1982 and 12-month infertility data in married women since 1973. The data provide "demographic ‘snapshots’ of the impact of societal trends such as delayed childbearing, and tracks the potential demand for infertility-related medical services," said Dr. Chandra and her associates.
There are no disclosures. The National Center for Health Statistics is part of the Centers for Disease Control and Prevention.
*Correction (8/29/13): A previous version of this story misstated the age range of the women involved in the study. This version has been updated.
Recent trends in fertility problems in the United States include a drop in the proportion of married women aged 15-44 who were infertile from 8.5% in 1982 to 6% between 2006 and 2010, according to a report from the National Center for Health Statistics.
The corresponding number of women affected dropped from 2.4 million in 1982 to 1.5 million in the later period.

Among all the women surveyed, the proportion of women with impaired fecundity stabilized at 11% in 2006-2010. Prior to this period, the proportion had increased significantly from 8.4% in 1982 to 10% in 1995 and 12% in 2002. There was a similar pattern observed among married women, although the proportion affected was higher: In 1982 and 1988, 11% of married women had impaired fecundity, increasing to 15% in 2002 and dropping to 12% in 2006-2010, according to Anjanai Chandra, Ph.D., of the NCHS, and her coauthors.
The data are from the National Survey of Family Growth (NSFG), which provides nationally representative measures of fertility, based on 22,682 interviews with men and women aged 15-44, from June 2006 through June 2010. The report was published on Aug. 14 (Natl. Health Stat. Report. 2013;67:1-19). Data are also collected for men: Between 2006 and 2010, 9.4% of men aged 15-44 and 12% of men aged 25-44 reported having some form of infertility, which was similar to the proportion reported in 2002, when men were first included in the survey,
In women, the two infertility measures used since 1982 are infertility (defined as "a lack of pregnancy in the 12 months prior to the survey, despite having unprotected sexual intercourse in each of those months with the same husband or partner") and impaired fecundity ("physical difficulty in either getting pregnant or carrying a pregnancy to live birth"). The infertility measure has been limited to couples who are married or living together, because it does not distinguish between infertility in the male or female partner. Because of issues with the size of the sample, the authors reported infertility estimates in subgroups in married women only, but noted that in 2006-2010, the proportions of married and cohabiting women who were infertile were similar, at 6% and 4.9%, respectively.
The authors pointed out that among nulliparous women, infertility and impaired fecundity were closely associated with age: Among married nulliparous women aged 35-44, the proportion who were infertile dropped from 44% in 1982 to 27% in 2006-2010.
They also compared some data to data from the National Fertility Study in 1965: Among married women who were infertile, the rate of primary infertility – the proportion who were having problems having their first child – increased from 17% in 1965 to 41%-46% in 1982-2010, "which is consistent with patterns and trends in delayed childbearing over these years," the authors pointed out.
While there was no "clear association" between poverty level and education, women who were less educated and at poverty income levels were more likely to be surgically sterilized. For example, between 2006 and 2010, among women aged 25-44 years, 44% of those who had not completed high school had undergone surgical sterilization for contraceptive reasons, compared with 21% of those with a bachelor’s degree and 16% of those with a master’s degree or higher degree. The women with more education also were more likely to be fecund (65%-71%), compared with those with a high school education or less (42%-43%), "presumably because these latter women started and completed their fertility at younger ages and opted for surgical sterilization," the authors wrote.
The NSFG has been collecting data on impaired fecundity in all women aged *15-44 years since 1982 and 12-month infertility data in married women since 1973. The data provide "demographic ‘snapshots’ of the impact of societal trends such as delayed childbearing, and tracks the potential demand for infertility-related medical services," said Dr. Chandra and her associates.
There are no disclosures. The National Center for Health Statistics is part of the Centers for Disease Control and Prevention.
*Correction (8/29/13): A previous version of this story misstated the age range of the women involved in the study. This version has been updated.
Recent trends in fertility problems in the United States include a drop in the proportion of married women aged 15-44 who were infertile from 8.5% in 1982 to 6% between 2006 and 2010, according to a report from the National Center for Health Statistics.
The corresponding number of women affected dropped from 2.4 million in 1982 to 1.5 million in the later period.

Among all the women surveyed, the proportion of women with impaired fecundity stabilized at 11% in 2006-2010. Prior to this period, the proportion had increased significantly from 8.4% in 1982 to 10% in 1995 and 12% in 2002. There was a similar pattern observed among married women, although the proportion affected was higher: In 1982 and 1988, 11% of married women had impaired fecundity, increasing to 15% in 2002 and dropping to 12% in 2006-2010, according to Anjanai Chandra, Ph.D., of the NCHS, and her coauthors.
The data are from the National Survey of Family Growth (NSFG), which provides nationally representative measures of fertility, based on 22,682 interviews with men and women aged 15-44, from June 2006 through June 2010. The report was published on Aug. 14 (Natl. Health Stat. Report. 2013;67:1-19). Data are also collected for men: Between 2006 and 2010, 9.4% of men aged 15-44 and 12% of men aged 25-44 reported having some form of infertility, which was similar to the proportion reported in 2002, when men were first included in the survey,
In women, the two infertility measures used since 1982 are infertility (defined as "a lack of pregnancy in the 12 months prior to the survey, despite having unprotected sexual intercourse in each of those months with the same husband or partner") and impaired fecundity ("physical difficulty in either getting pregnant or carrying a pregnancy to live birth"). The infertility measure has been limited to couples who are married or living together, because it does not distinguish between infertility in the male or female partner. Because of issues with the size of the sample, the authors reported infertility estimates in subgroups in married women only, but noted that in 2006-2010, the proportions of married and cohabiting women who were infertile were similar, at 6% and 4.9%, respectively.
The authors pointed out that among nulliparous women, infertility and impaired fecundity were closely associated with age: Among married nulliparous women aged 35-44, the proportion who were infertile dropped from 44% in 1982 to 27% in 2006-2010.
They also compared some data to data from the National Fertility Study in 1965: Among married women who were infertile, the rate of primary infertility – the proportion who were having problems having their first child – increased from 17% in 1965 to 41%-46% in 1982-2010, "which is consistent with patterns and trends in delayed childbearing over these years," the authors pointed out.
While there was no "clear association" between poverty level and education, women who were less educated and at poverty income levels were more likely to be surgically sterilized. For example, between 2006 and 2010, among women aged 25-44 years, 44% of those who had not completed high school had undergone surgical sterilization for contraceptive reasons, compared with 21% of those with a bachelor’s degree and 16% of those with a master’s degree or higher degree. The women with more education also were more likely to be fecund (65%-71%), compared with those with a high school education or less (42%-43%), "presumably because these latter women started and completed their fertility at younger ages and opted for surgical sterilization," the authors wrote.
The NSFG has been collecting data on impaired fecundity in all women aged *15-44 years since 1982 and 12-month infertility data in married women since 1973. The data provide "demographic ‘snapshots’ of the impact of societal trends such as delayed childbearing, and tracks the potential demand for infertility-related medical services," said Dr. Chandra and her associates.
There are no disclosures. The National Center for Health Statistics is part of the Centers for Disease Control and Prevention.
*Correction (8/29/13): A previous version of this story misstated the age range of the women involved in the study. This version has been updated.
FROM THE NATIONAL CENTER FOR HEALTH STATISTICS
Major finding: From 2006 to 2010, an estimated 6% of married women in the United States aged 15-44 were infertile, down from 8.5% in 1982.
Data source: The National Survey of Family Growth, which provides nationally representative measures of fertility, based on 22,682 interviews with men and women aged 15-44, from June 2006 through June 2010.
Disclosures: There are no disclosures. The National Center for Health Statistics is part of the Centers for Disease Control and Prevention.
Task force backs primary care measures to prevent smoking in children, teens
Primary care physicians should provide school-aged children and adolescents with education, brief counseling, or other interventions to prevent them from starting to use tobacco products, according to guidelines issued by the 2013 U.S. Preventive Services Task Force.
This recommendation is a change from the last USPSTF guidelines on tobacco use in children and adolescents, which in 2003 did not make a recommendation for or against such counseling for these age groups because of insufficient evidence to support either recommendation.
But the 2013 recommendation says that the task force "found adequate evidence that behavioral counseling interventions, such as face-to-face or phone interaction with a health care provider, print materials, and computer applications, can reduce the risk for smoking initiation in school-aged children and adolescents." There is "moderate certainty" that behavioral interventions aimed at preventing tobacco use in these two pediatric populations that are relevant to primary care settings will have a "moderate net benefit," the task force concluded.
The task force statement, which includes resources for primary care clinicians, is being published simultaneously in Annals of Internal Medicine and Pediatrics (2013;132:560-5) on Aug. 26.
Interventions relevant to primary care were defined as those targeted at children, parents, or both, and either were practiced in health care settings or were considered feasible for such settings.
Interventions aimed at preventing tobacco use ranged from those that did not involve any direct interaction with the health care professional to those that entailed seven group sessions of more than 15 hours in total. "Even very minimal interventions, such as mailing materials to a youth's home, had substantial effects on reducing smoking initiation," the report said.
The evidence in the report includes a meta-analysis of nine studies of more than 26,000 children and adolescents, nonsmokers at baseline, which found that the risk of starting smoking at 6- to 36-month follow-up was reduced by 19% among those who received behavioral interventions, compared with controls - a statistically significant reduction.
Among the task force's other recommendations were mobile phone-based interventions for tobacco cessation, telephone follow-up combined with patient education materials, increasing the cost of tobacco products, mass media campaigns, and school-based education programs.
In 2009, about 8% of middle school students and almost 24% of high school students reported currently using a tobacco product, according to the report. Almost 30% of male high school students smoke, vs. approximately 22% of female high school students. In addition, every day in the United States, more than 3,800 children and adolescents aged 12-17 years smoke a cigarette for the first time, and about 1,000 children and adolescents under aged 18 start smoking daily, according to 2012 data.
Resources for primary care physicians in the report include:
• http://betobaccofree.hhs.gov/index.html
• http://www2.aap.org/richmondcenter/TobaccoPreventionPolicyTool/TPPT_PracticeCessation.html
In an editorial that accompanied the USPSTF recommendations on preventing tobacco use in children and adolescents, Dr. Michael Steinberg and Christine Delnevo, Ph.D., advocated increasing the legal age for purchase of tobacco products from 18 to 21 years, as has been proposed in New York City.
It is "critical to prevent young people from ever taking a puff on that first cigarette," they wrote, noting that the proposed NYC policy "is grounded in strong epidemiologic evidence, given that nearly 90% of adults who smoke on a daily basis had their first cigarette by age 18 years, and the transition from tobacco experimentation to regular use typically occurs during young adulthood."
In addition, people aged 18-20 years buy 90% of the cigarettes purchased for minors. "Preventing persons aged 18-20 years from slipping down the undesirable path of lifelong tobacco addiction will certainly not be accomplished by this one piece of legislation alone, but it is a start and is the right thing to do."
Dr. Steinberg is in the division of general internal medicine at the Robert Wood Johnson Medical School, and Dr. Delnevo is with the department of health education and behavioral science at Rutgers School of Public Health; both are in New Brunswick, N.J. They said they had no relevant financial disclosures
In an editorial that accompanied the USPSTF recommendations on preventing tobacco use in children and adolescents, Dr. Michael Steinberg and Christine Delnevo, Ph.D., advocated increasing the legal age for purchase of tobacco products from 18 to 21 years, as has been proposed in New York City.
It is "critical to prevent young people from ever taking a puff on that first cigarette," they wrote, noting that the proposed NYC policy "is grounded in strong epidemiologic evidence, given that nearly 90% of adults who smoke on a daily basis had their first cigarette by age 18 years, and the transition from tobacco experimentation to regular use typically occurs during young adulthood."
In addition, people aged 18-20 years buy 90% of the cigarettes purchased for minors. "Preventing persons aged 18-20 years from slipping down the undesirable path of lifelong tobacco addiction will certainly not be accomplished by this one piece of legislation alone, but it is a start and is the right thing to do."
Dr. Steinberg is in the division of general internal medicine at the Robert Wood Johnson Medical School, and Dr. Delnevo is with the department of health education and behavioral science at Rutgers School of Public Health; both are in New Brunswick, N.J. They said they had no relevant financial disclosures
In an editorial that accompanied the USPSTF recommendations on preventing tobacco use in children and adolescents, Dr. Michael Steinberg and Christine Delnevo, Ph.D., advocated increasing the legal age for purchase of tobacco products from 18 to 21 years, as has been proposed in New York City.
It is "critical to prevent young people from ever taking a puff on that first cigarette," they wrote, noting that the proposed NYC policy "is grounded in strong epidemiologic evidence, given that nearly 90% of adults who smoke on a daily basis had their first cigarette by age 18 years, and the transition from tobacco experimentation to regular use typically occurs during young adulthood."
In addition, people aged 18-20 years buy 90% of the cigarettes purchased for minors. "Preventing persons aged 18-20 years from slipping down the undesirable path of lifelong tobacco addiction will certainly not be accomplished by this one piece of legislation alone, but it is a start and is the right thing to do."
Dr. Steinberg is in the division of general internal medicine at the Robert Wood Johnson Medical School, and Dr. Delnevo is with the department of health education and behavioral science at Rutgers School of Public Health; both are in New Brunswick, N.J. They said they had no relevant financial disclosures
Primary care physicians should provide school-aged children and adolescents with education, brief counseling, or other interventions to prevent them from starting to use tobacco products, according to guidelines issued by the 2013 U.S. Preventive Services Task Force.
This recommendation is a change from the last USPSTF guidelines on tobacco use in children and adolescents, which in 2003 did not make a recommendation for or against such counseling for these age groups because of insufficient evidence to support either recommendation.
But the 2013 recommendation says that the task force "found adequate evidence that behavioral counseling interventions, such as face-to-face or phone interaction with a health care provider, print materials, and computer applications, can reduce the risk for smoking initiation in school-aged children and adolescents." There is "moderate certainty" that behavioral interventions aimed at preventing tobacco use in these two pediatric populations that are relevant to primary care settings will have a "moderate net benefit," the task force concluded.
The task force statement, which includes resources for primary care clinicians, is being published simultaneously in Annals of Internal Medicine and Pediatrics (2013;132:560-5) on Aug. 26.
Interventions relevant to primary care were defined as those targeted at children, parents, or both, and either were practiced in health care settings or were considered feasible for such settings.
Interventions aimed at preventing tobacco use ranged from those that did not involve any direct interaction with the health care professional to those that entailed seven group sessions of more than 15 hours in total. "Even very minimal interventions, such as mailing materials to a youth's home, had substantial effects on reducing smoking initiation," the report said.
The evidence in the report includes a meta-analysis of nine studies of more than 26,000 children and adolescents, nonsmokers at baseline, which found that the risk of starting smoking at 6- to 36-month follow-up was reduced by 19% among those who received behavioral interventions, compared with controls - a statistically significant reduction.
Among the task force's other recommendations were mobile phone-based interventions for tobacco cessation, telephone follow-up combined with patient education materials, increasing the cost of tobacco products, mass media campaigns, and school-based education programs.
In 2009, about 8% of middle school students and almost 24% of high school students reported currently using a tobacco product, according to the report. Almost 30% of male high school students smoke, vs. approximately 22% of female high school students. In addition, every day in the United States, more than 3,800 children and adolescents aged 12-17 years smoke a cigarette for the first time, and about 1,000 children and adolescents under aged 18 start smoking daily, according to 2012 data.
Resources for primary care physicians in the report include:
• http://betobaccofree.hhs.gov/index.html
• http://www2.aap.org/richmondcenter/TobaccoPreventionPolicyTool/TPPT_PracticeCessation.html
Primary care physicians should provide school-aged children and adolescents with education, brief counseling, or other interventions to prevent them from starting to use tobacco products, according to guidelines issued by the 2013 U.S. Preventive Services Task Force.
This recommendation is a change from the last USPSTF guidelines on tobacco use in children and adolescents, which in 2003 did not make a recommendation for or against such counseling for these age groups because of insufficient evidence to support either recommendation.
But the 2013 recommendation says that the task force "found adequate evidence that behavioral counseling interventions, such as face-to-face or phone interaction with a health care provider, print materials, and computer applications, can reduce the risk for smoking initiation in school-aged children and adolescents." There is "moderate certainty" that behavioral interventions aimed at preventing tobacco use in these two pediatric populations that are relevant to primary care settings will have a "moderate net benefit," the task force concluded.
The task force statement, which includes resources for primary care clinicians, is being published simultaneously in Annals of Internal Medicine and Pediatrics (2013;132:560-5) on Aug. 26.
Interventions relevant to primary care were defined as those targeted at children, parents, or both, and either were practiced in health care settings or were considered feasible for such settings.
Interventions aimed at preventing tobacco use ranged from those that did not involve any direct interaction with the health care professional to those that entailed seven group sessions of more than 15 hours in total. "Even very minimal interventions, such as mailing materials to a youth's home, had substantial effects on reducing smoking initiation," the report said.
The evidence in the report includes a meta-analysis of nine studies of more than 26,000 children and adolescents, nonsmokers at baseline, which found that the risk of starting smoking at 6- to 36-month follow-up was reduced by 19% among those who received behavioral interventions, compared with controls - a statistically significant reduction.
Among the task force's other recommendations were mobile phone-based interventions for tobacco cessation, telephone follow-up combined with patient education materials, increasing the cost of tobacco products, mass media campaigns, and school-based education programs.
In 2009, about 8% of middle school students and almost 24% of high school students reported currently using a tobacco product, according to the report. Almost 30% of male high school students smoke, vs. approximately 22% of female high school students. In addition, every day in the United States, more than 3,800 children and adolescents aged 12-17 years smoke a cigarette for the first time, and about 1,000 children and adolescents under aged 18 start smoking daily, according to 2012 data.
Resources for primary care physicians in the report include:
• http://betobaccofree.hhs.gov/index.html
• http://www2.aap.org/richmondcenter/TobaccoPreventionPolicyTool/TPPT_PracticeCessation.html
FROM ANNALS OF INTERNAL MEDICINE AND PEDIATRICS
Consensus statement: Data on cancer, pancreatitis do not warrant change in prescribing of antihyperglycemics
Available data on a possible link between antihyperglycemic drugs and an increased risk of pancreatitis and certain cancers do not justify making changes in clinical practice, according to a consensus statement on diabetes and cancer released by the American Association of Clinical Endocrinologists and American College of Endocrinology.
The available data do not include data from large, randomized controlled studies and are "limited and conflicting," the statement said. Health care professionals "should have greater confidence in prescribing all FDA-approved antihyperglycemic medications according to current clinical practice recommendations."
The consensus cautioned that "clinicians should exercise caution when choosing medications implicated in the etiology of cancer for patients with the specific organ-related risk ... Clinicians should be alert to the potential risk and should monitor patients more closely."
The statement was published online and in print in the July/August issue of Endocrine Practice (2013;19:675-93).
In July, the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) issued statements on the associations. The consensus statement is "in line" with those statements, which said that the data on medications used to manage hyperglycemia are "not substantial enough to make the connection and there is no need to change the official labeling about potential safety risks," according to the Aug. 20 press release from the American Association of Clinical Endocrinologists (AACE) announcing publication of the consensus statement.
"The implication of various medications’ role in the development of cancer has concerned physicians and patients alike, but the sum of evidence presents a very compelling case and suggests the risk of cancer is unproven," Dr. Yehuda Handelsman, cochair of the consensus statement task force, said in that statement.
"The research conducted to date is incomplete and, thus, insufficient to warrant withholding treatment that will result in adverse outcomes for those who have diabetes or are obese," added Dr. Handelsman, the medical director of the Metabolic Institute of America, Tarzana, Calif. "Until more definitive evidence becomes available, the benefits of treatment should take precedence over any concerns for potential low-grade cancer risk."
The statement includes information on the FDA safety communication in March 2013, which said the agency was evaluating a new study that treatment with incretin mimetics was associated with an increased risk for precancerous cellular changes in people with type 2 diabetes, although the statement adds, "the quality, relevance, and importance of the study are not clear."
Metformin has been associated with a "neutral to decreased" effect on the incidence of cancer and cancer mortality, and there also are data associating its use with a decreased risk of colorectal, lung, and breast cancers. Pioglitazone, a thiazolidinedione (TZD), has been associated with an increased risk of bladder cancer, particularly with longer use and larger cumulative doses, but recent data indicate the risk of bladder cancer is small. Based on these data, clinicians "should be confident and continue to use TZDs," but should monitor patients on pioglitazone and, as recommended by the FDA, avoid prescribing it to patients with a high risk or history of bladder cancer.
A sodium-glucose cotransporter 2 (SGLT2) inhibitor not approved in the United States (dapagliflozin) has been associated with an increased incidence of breast and bladder cancer, which was not statistically significant. But other drugs in this class, including canagliflozin, the SGLT2 inhibitor approved in the United States, "have not shown any cancer signal and are not presently implicated in cancer development," the statement said.
Epidemiologic data support an association between an increased body mass index and an increased risk of various cancers. Based on epidemiologic data, the risk of cancer is significantly increased with obesity, insulin-resistant states, and diabetes, and basic research has "suggested plausible mechanisms linking these conditions to the development of cancer."
"Further collaborative research between clinicians, including endocrinologists and oncologists, as well as basic, clinical, and epidemiologic researchers, is necessary to complete the evidence on these complex issues," the statement said.
The associations with body mass index were strongest for endometrial, gall bladder, esophageal (adenocarcinoma), renal, thyroid, ovarian, breast, and colorectal cancer. This relationship "supports the need to advocate for improved diet, greater physical activity, and early cancer screening in obese patients," the statement said.
Furthermore, in March 2013, the FDA announced that it was evaluating unpublished reports suggesting that the risk of pancreatitis (included in the warning section of incretin mimetics) and pancreatic duct metaplasia was increased in patients with type 2 diabetes treated with those drugs. In July, the EMA announced that its investigation into these reports of pancreatitis and changes in pancreatic duct metaplasia associated with glucagonlike peptide–1 (GLP-1) agonists was completed, and concluded that the available data "do not confirm recent concerns over an increased risk of pancreatic adverse events with these medicines." Until the results of two large studies, funded by the European Commission, on the risk profile of diabetes treatments are available in 2014, and other data are available, the EMA is continuing to closely monitor and evaluate information on these medicines "to ensure that their benefit-risk balance remains positive."
The FDA is aware of the EMA’s analyses and believes that the EMA’s conclusions "are consistent with our current understanding of the data," an FDA spokesperson said in an interview. "FDA believes that the current labeling for approved GLP-1–based therapies reflects the extent of our understanding of the safety signals at this point in time," she added, noting that the FDA’s review is ongoing as pancreatitis and pancreatic cancer data are being collected in the cardiovascular outcome trials being conducted with this class of drugs, and there is an ongoing epidemiological study.
GLP-1 receptor agonists have been associated with an increase in thyroid C-cell carcinomas in rats, but there is no evidence these drugs are associated with medullary thyroid cancer in humans, data that include an analysis of 10 studies conducted by the EMA, according to the consensus statement.
The incretin-based treatments, GLP-1 receptor agonists, and dipeptidyl peptidase–4 (DPP-4) inhibitors have been associated with some reports of acute pancreatitis, but "causal mechanisms have not been established," and "the link to pancreatic cancer is unclear," the statement said.
The incretin mimetics available in the United States include exenatide (Byetta, Bydureon), liraglutide (Victoza), sitagliptin (Januvia, Janumet, Janumet XR, Juvisync), saxagliptin (Onglyza, Kombiglyze XR), alogliptin (Nesina, Kazano, Oseni), and linagliptin (Tradjenta, Jentadueto).
Dr. Handelsman disclosed having received research grant support, consulting fees, and speaker’s honoraria from various pharmaceutical companies. The disclosures of the remaining 11 authors and task force members included having received speaker’s fees, consulting fees, and research contracts from, and/or holding shares in, various pharmaceutical companies. The consensus statement and the AACE conference that was the basis of the statement were supported by AACE. The conference was partly funded with grants from Amylin Pharmaceuticals, Bristol-Myers Squibb, AstraZeneca, Eli Lilly, Merck, Novo Nordisk, and Sanofi-Aventis US.
Available data on a possible link between antihyperglycemic drugs and an increased risk of pancreatitis and certain cancers do not justify making changes in clinical practice, according to a consensus statement on diabetes and cancer released by the American Association of Clinical Endocrinologists and American College of Endocrinology.
The available data do not include data from large, randomized controlled studies and are "limited and conflicting," the statement said. Health care professionals "should have greater confidence in prescribing all FDA-approved antihyperglycemic medications according to current clinical practice recommendations."
The consensus cautioned that "clinicians should exercise caution when choosing medications implicated in the etiology of cancer for patients with the specific organ-related risk ... Clinicians should be alert to the potential risk and should monitor patients more closely."
The statement was published online and in print in the July/August issue of Endocrine Practice (2013;19:675-93).
In July, the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) issued statements on the associations. The consensus statement is "in line" with those statements, which said that the data on medications used to manage hyperglycemia are "not substantial enough to make the connection and there is no need to change the official labeling about potential safety risks," according to the Aug. 20 press release from the American Association of Clinical Endocrinologists (AACE) announcing publication of the consensus statement.
"The implication of various medications’ role in the development of cancer has concerned physicians and patients alike, but the sum of evidence presents a very compelling case and suggests the risk of cancer is unproven," Dr. Yehuda Handelsman, cochair of the consensus statement task force, said in that statement.
"The research conducted to date is incomplete and, thus, insufficient to warrant withholding treatment that will result in adverse outcomes for those who have diabetes or are obese," added Dr. Handelsman, the medical director of the Metabolic Institute of America, Tarzana, Calif. "Until more definitive evidence becomes available, the benefits of treatment should take precedence over any concerns for potential low-grade cancer risk."
The statement includes information on the FDA safety communication in March 2013, which said the agency was evaluating a new study that treatment with incretin mimetics was associated with an increased risk for precancerous cellular changes in people with type 2 diabetes, although the statement adds, "the quality, relevance, and importance of the study are not clear."
Metformin has been associated with a "neutral to decreased" effect on the incidence of cancer and cancer mortality, and there also are data associating its use with a decreased risk of colorectal, lung, and breast cancers. Pioglitazone, a thiazolidinedione (TZD), has been associated with an increased risk of bladder cancer, particularly with longer use and larger cumulative doses, but recent data indicate the risk of bladder cancer is small. Based on these data, clinicians "should be confident and continue to use TZDs," but should monitor patients on pioglitazone and, as recommended by the FDA, avoid prescribing it to patients with a high risk or history of bladder cancer.
A sodium-glucose cotransporter 2 (SGLT2) inhibitor not approved in the United States (dapagliflozin) has been associated with an increased incidence of breast and bladder cancer, which was not statistically significant. But other drugs in this class, including canagliflozin, the SGLT2 inhibitor approved in the United States, "have not shown any cancer signal and are not presently implicated in cancer development," the statement said.
Epidemiologic data support an association between an increased body mass index and an increased risk of various cancers. Based on epidemiologic data, the risk of cancer is significantly increased with obesity, insulin-resistant states, and diabetes, and basic research has "suggested plausible mechanisms linking these conditions to the development of cancer."
"Further collaborative research between clinicians, including endocrinologists and oncologists, as well as basic, clinical, and epidemiologic researchers, is necessary to complete the evidence on these complex issues," the statement said.
The associations with body mass index were strongest for endometrial, gall bladder, esophageal (adenocarcinoma), renal, thyroid, ovarian, breast, and colorectal cancer. This relationship "supports the need to advocate for improved diet, greater physical activity, and early cancer screening in obese patients," the statement said.
Furthermore, in March 2013, the FDA announced that it was evaluating unpublished reports suggesting that the risk of pancreatitis (included in the warning section of incretin mimetics) and pancreatic duct metaplasia was increased in patients with type 2 diabetes treated with those drugs. In July, the EMA announced that its investigation into these reports of pancreatitis and changes in pancreatic duct metaplasia associated with glucagonlike peptide–1 (GLP-1) agonists was completed, and concluded that the available data "do not confirm recent concerns over an increased risk of pancreatic adverse events with these medicines." Until the results of two large studies, funded by the European Commission, on the risk profile of diabetes treatments are available in 2014, and other data are available, the EMA is continuing to closely monitor and evaluate information on these medicines "to ensure that their benefit-risk balance remains positive."
The FDA is aware of the EMA’s analyses and believes that the EMA’s conclusions "are consistent with our current understanding of the data," an FDA spokesperson said in an interview. "FDA believes that the current labeling for approved GLP-1–based therapies reflects the extent of our understanding of the safety signals at this point in time," she added, noting that the FDA’s review is ongoing as pancreatitis and pancreatic cancer data are being collected in the cardiovascular outcome trials being conducted with this class of drugs, and there is an ongoing epidemiological study.
GLP-1 receptor agonists have been associated with an increase in thyroid C-cell carcinomas in rats, but there is no evidence these drugs are associated with medullary thyroid cancer in humans, data that include an analysis of 10 studies conducted by the EMA, according to the consensus statement.
The incretin-based treatments, GLP-1 receptor agonists, and dipeptidyl peptidase–4 (DPP-4) inhibitors have been associated with some reports of acute pancreatitis, but "causal mechanisms have not been established," and "the link to pancreatic cancer is unclear," the statement said.
The incretin mimetics available in the United States include exenatide (Byetta, Bydureon), liraglutide (Victoza), sitagliptin (Januvia, Janumet, Janumet XR, Juvisync), saxagliptin (Onglyza, Kombiglyze XR), alogliptin (Nesina, Kazano, Oseni), and linagliptin (Tradjenta, Jentadueto).
Dr. Handelsman disclosed having received research grant support, consulting fees, and speaker’s honoraria from various pharmaceutical companies. The disclosures of the remaining 11 authors and task force members included having received speaker’s fees, consulting fees, and research contracts from, and/or holding shares in, various pharmaceutical companies. The consensus statement and the AACE conference that was the basis of the statement were supported by AACE. The conference was partly funded with grants from Amylin Pharmaceuticals, Bristol-Myers Squibb, AstraZeneca, Eli Lilly, Merck, Novo Nordisk, and Sanofi-Aventis US.
Available data on a possible link between antihyperglycemic drugs and an increased risk of pancreatitis and certain cancers do not justify making changes in clinical practice, according to a consensus statement on diabetes and cancer released by the American Association of Clinical Endocrinologists and American College of Endocrinology.
The available data do not include data from large, randomized controlled studies and are "limited and conflicting," the statement said. Health care professionals "should have greater confidence in prescribing all FDA-approved antihyperglycemic medications according to current clinical practice recommendations."
The consensus cautioned that "clinicians should exercise caution when choosing medications implicated in the etiology of cancer for patients with the specific organ-related risk ... Clinicians should be alert to the potential risk and should monitor patients more closely."
The statement was published online and in print in the July/August issue of Endocrine Practice (2013;19:675-93).
In July, the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) issued statements on the associations. The consensus statement is "in line" with those statements, which said that the data on medications used to manage hyperglycemia are "not substantial enough to make the connection and there is no need to change the official labeling about potential safety risks," according to the Aug. 20 press release from the American Association of Clinical Endocrinologists (AACE) announcing publication of the consensus statement.
"The implication of various medications’ role in the development of cancer has concerned physicians and patients alike, but the sum of evidence presents a very compelling case and suggests the risk of cancer is unproven," Dr. Yehuda Handelsman, cochair of the consensus statement task force, said in that statement.
"The research conducted to date is incomplete and, thus, insufficient to warrant withholding treatment that will result in adverse outcomes for those who have diabetes or are obese," added Dr. Handelsman, the medical director of the Metabolic Institute of America, Tarzana, Calif. "Until more definitive evidence becomes available, the benefits of treatment should take precedence over any concerns for potential low-grade cancer risk."
The statement includes information on the FDA safety communication in March 2013, which said the agency was evaluating a new study that treatment with incretin mimetics was associated with an increased risk for precancerous cellular changes in people with type 2 diabetes, although the statement adds, "the quality, relevance, and importance of the study are not clear."
Metformin has been associated with a "neutral to decreased" effect on the incidence of cancer and cancer mortality, and there also are data associating its use with a decreased risk of colorectal, lung, and breast cancers. Pioglitazone, a thiazolidinedione (TZD), has been associated with an increased risk of bladder cancer, particularly with longer use and larger cumulative doses, but recent data indicate the risk of bladder cancer is small. Based on these data, clinicians "should be confident and continue to use TZDs," but should monitor patients on pioglitazone and, as recommended by the FDA, avoid prescribing it to patients with a high risk or history of bladder cancer.
A sodium-glucose cotransporter 2 (SGLT2) inhibitor not approved in the United States (dapagliflozin) has been associated with an increased incidence of breast and bladder cancer, which was not statistically significant. But other drugs in this class, including canagliflozin, the SGLT2 inhibitor approved in the United States, "have not shown any cancer signal and are not presently implicated in cancer development," the statement said.
Epidemiologic data support an association between an increased body mass index and an increased risk of various cancers. Based on epidemiologic data, the risk of cancer is significantly increased with obesity, insulin-resistant states, and diabetes, and basic research has "suggested plausible mechanisms linking these conditions to the development of cancer."
"Further collaborative research between clinicians, including endocrinologists and oncologists, as well as basic, clinical, and epidemiologic researchers, is necessary to complete the evidence on these complex issues," the statement said.
The associations with body mass index were strongest for endometrial, gall bladder, esophageal (adenocarcinoma), renal, thyroid, ovarian, breast, and colorectal cancer. This relationship "supports the need to advocate for improved diet, greater physical activity, and early cancer screening in obese patients," the statement said.
Furthermore, in March 2013, the FDA announced that it was evaluating unpublished reports suggesting that the risk of pancreatitis (included in the warning section of incretin mimetics) and pancreatic duct metaplasia was increased in patients with type 2 diabetes treated with those drugs. In July, the EMA announced that its investigation into these reports of pancreatitis and changes in pancreatic duct metaplasia associated with glucagonlike peptide–1 (GLP-1) agonists was completed, and concluded that the available data "do not confirm recent concerns over an increased risk of pancreatic adverse events with these medicines." Until the results of two large studies, funded by the European Commission, on the risk profile of diabetes treatments are available in 2014, and other data are available, the EMA is continuing to closely monitor and evaluate information on these medicines "to ensure that their benefit-risk balance remains positive."
The FDA is aware of the EMA’s analyses and believes that the EMA’s conclusions "are consistent with our current understanding of the data," an FDA spokesperson said in an interview. "FDA believes that the current labeling for approved GLP-1–based therapies reflects the extent of our understanding of the safety signals at this point in time," she added, noting that the FDA’s review is ongoing as pancreatitis and pancreatic cancer data are being collected in the cardiovascular outcome trials being conducted with this class of drugs, and there is an ongoing epidemiological study.
GLP-1 receptor agonists have been associated with an increase in thyroid C-cell carcinomas in rats, but there is no evidence these drugs are associated with medullary thyroid cancer in humans, data that include an analysis of 10 studies conducted by the EMA, according to the consensus statement.
The incretin-based treatments, GLP-1 receptor agonists, and dipeptidyl peptidase–4 (DPP-4) inhibitors have been associated with some reports of acute pancreatitis, but "causal mechanisms have not been established," and "the link to pancreatic cancer is unclear," the statement said.
The incretin mimetics available in the United States include exenatide (Byetta, Bydureon), liraglutide (Victoza), sitagliptin (Januvia, Janumet, Janumet XR, Juvisync), saxagliptin (Onglyza, Kombiglyze XR), alogliptin (Nesina, Kazano, Oseni), and linagliptin (Tradjenta, Jentadueto).
Dr. Handelsman disclosed having received research grant support, consulting fees, and speaker’s honoraria from various pharmaceutical companies. The disclosures of the remaining 11 authors and task force members included having received speaker’s fees, consulting fees, and research contracts from, and/or holding shares in, various pharmaceutical companies. The consensus statement and the AACE conference that was the basis of the statement were supported by AACE. The conference was partly funded with grants from Amylin Pharmaceuticals, Bristol-Myers Squibb, AstraZeneca, Eli Lilly, Merck, Novo Nordisk, and Sanofi-Aventis US.
Adhesiolysis linked to high morbidity, higher hospitalization costs
Adhesiolysis was associated with an increased risk for a variety of morbidities during repeat abdominal surgery including inadvertent bowel defects, seromuscular injuries, and postoperative sepsis, according to the results of a prospective study.
"All physicians treating patients with disorders of the abdominal cavity that might require surgery should be aware of the adverse effects of adhesiolysis," reported Dr. Richard P.G. ten Broek and his associates in the department of surgery at Radboud University Nijmegen (the Netherlands) Medical Center. The study was published in Annals of Surgery (2013;258:98-106).
The investigators conducted the prospective cohort study to evaluate the direct effects of adhesiolysis on unintentional organ damage, morbidity, and costs during repeat operations, to address the lack of information in this area. They collected data from a total of 755 elective laparotomy or laparoscopy procedures in 715 patients performed at the medical center between June 2008 and June 2010.
The removal of adhesions was undertaken in almost 63% (475) of these procedures. Median adhesiolysis time was 20 minutes, ranging from 1 minute to almost 13 hours. Previous intra-abdominal surgery and peritonitis were among the most common causes of adhesions.
"All physicians treating patients with disorders of the abdominal cavity that might require surgery should be aware of the adverse effects of adhesiolysis,"
In the adhesiolysis group, the rates of perioperative complications were statistically significantly higher, compared with those in patients who did not require adhesiolysis, including full-thickness bowel defect (10.5% in the adhesiolysis group, versus no cases in the nonadhesiolysis group), injury to the seromuscular layer (27.6% v. 3.9%), and injuries to other organs (8.6% vs 2.5%).
Overall, the rate of surgical complications was 23.4% among those who had adhesiolysis, compared with 17.5% of those in the nonadhesiolysis group, also a statistically significant difference.
Adhesiolysis was associated with more than a fivefold increased rate of sepsis (odds ratio, 5.12), almost a fourfold increased risk of intra-abdominal complications (OR, 3.46), and more than a twofold increased risk of incisional wound infection (OR, 2.45), all statistically significant differences. The differences in mortality, urinary tract infections, pneumonia, or hemorrhage were not significantly different.
Adhesiolysis also resulted in longer surgery time (a mean of 22.5 minutes longer), recovery time (a mean of about 2 hours longer), and total hospital stay (a mean of about 3 days longer), as well as 29% greater operative blood loss. Among the cases reviewed, the mean hospital cost when adhesiolysis was performed was $18,579, vs. $14,063 when it was not performed.
This is the first study "showing adhesiolysis as a risk factor for postoperative surgical complications, longer hospital stays, more readmissions, and increased costs," the authors pointed out. These data can be helpful "when counseling patients before surgery, when physicians and health care providers make decisions on implementing antiadhesive strategies, and for the reimbursement policy of insurance companies," they added, noting that fewer than 10% of surgeons counsel patients about the risk of adhesions.
"With the projected increase in more repeat abdominal surgeries because of a longer life expectancy and newer technologies, prevention of adhesiolysis-related morbidity might be even more cost effective," they added.
The study was sponsored by Radboud University Nijmegen Medical Center, with no external funding, and the authors said they had no relevant financial conflicts.
Adhesiolysis was associated with an increased risk for a variety of morbidities during repeat abdominal surgery including inadvertent bowel defects, seromuscular injuries, and postoperative sepsis, according to the results of a prospective study.
"All physicians treating patients with disorders of the abdominal cavity that might require surgery should be aware of the adverse effects of adhesiolysis," reported Dr. Richard P.G. ten Broek and his associates in the department of surgery at Radboud University Nijmegen (the Netherlands) Medical Center. The study was published in Annals of Surgery (2013;258:98-106).
The investigators conducted the prospective cohort study to evaluate the direct effects of adhesiolysis on unintentional organ damage, morbidity, and costs during repeat operations, to address the lack of information in this area. They collected data from a total of 755 elective laparotomy or laparoscopy procedures in 715 patients performed at the medical center between June 2008 and June 2010.
The removal of adhesions was undertaken in almost 63% (475) of these procedures. Median adhesiolysis time was 20 minutes, ranging from 1 minute to almost 13 hours. Previous intra-abdominal surgery and peritonitis were among the most common causes of adhesions.
"All physicians treating patients with disorders of the abdominal cavity that might require surgery should be aware of the adverse effects of adhesiolysis,"
In the adhesiolysis group, the rates of perioperative complications were statistically significantly higher, compared with those in patients who did not require adhesiolysis, including full-thickness bowel defect (10.5% in the adhesiolysis group, versus no cases in the nonadhesiolysis group), injury to the seromuscular layer (27.6% v. 3.9%), and injuries to other organs (8.6% vs 2.5%).
Overall, the rate of surgical complications was 23.4% among those who had adhesiolysis, compared with 17.5% of those in the nonadhesiolysis group, also a statistically significant difference.
Adhesiolysis was associated with more than a fivefold increased rate of sepsis (odds ratio, 5.12), almost a fourfold increased risk of intra-abdominal complications (OR, 3.46), and more than a twofold increased risk of incisional wound infection (OR, 2.45), all statistically significant differences. The differences in mortality, urinary tract infections, pneumonia, or hemorrhage were not significantly different.
Adhesiolysis also resulted in longer surgery time (a mean of 22.5 minutes longer), recovery time (a mean of about 2 hours longer), and total hospital stay (a mean of about 3 days longer), as well as 29% greater operative blood loss. Among the cases reviewed, the mean hospital cost when adhesiolysis was performed was $18,579, vs. $14,063 when it was not performed.
This is the first study "showing adhesiolysis as a risk factor for postoperative surgical complications, longer hospital stays, more readmissions, and increased costs," the authors pointed out. These data can be helpful "when counseling patients before surgery, when physicians and health care providers make decisions on implementing antiadhesive strategies, and for the reimbursement policy of insurance companies," they added, noting that fewer than 10% of surgeons counsel patients about the risk of adhesions.
"With the projected increase in more repeat abdominal surgeries because of a longer life expectancy and newer technologies, prevention of adhesiolysis-related morbidity might be even more cost effective," they added.
The study was sponsored by Radboud University Nijmegen Medical Center, with no external funding, and the authors said they had no relevant financial conflicts.
Adhesiolysis was associated with an increased risk for a variety of morbidities during repeat abdominal surgery including inadvertent bowel defects, seromuscular injuries, and postoperative sepsis, according to the results of a prospective study.
"All physicians treating patients with disorders of the abdominal cavity that might require surgery should be aware of the adverse effects of adhesiolysis," reported Dr. Richard P.G. ten Broek and his associates in the department of surgery at Radboud University Nijmegen (the Netherlands) Medical Center. The study was published in Annals of Surgery (2013;258:98-106).
The investigators conducted the prospective cohort study to evaluate the direct effects of adhesiolysis on unintentional organ damage, morbidity, and costs during repeat operations, to address the lack of information in this area. They collected data from a total of 755 elective laparotomy or laparoscopy procedures in 715 patients performed at the medical center between June 2008 and June 2010.
The removal of adhesions was undertaken in almost 63% (475) of these procedures. Median adhesiolysis time was 20 minutes, ranging from 1 minute to almost 13 hours. Previous intra-abdominal surgery and peritonitis were among the most common causes of adhesions.
"All physicians treating patients with disorders of the abdominal cavity that might require surgery should be aware of the adverse effects of adhesiolysis,"
In the adhesiolysis group, the rates of perioperative complications were statistically significantly higher, compared with those in patients who did not require adhesiolysis, including full-thickness bowel defect (10.5% in the adhesiolysis group, versus no cases in the nonadhesiolysis group), injury to the seromuscular layer (27.6% v. 3.9%), and injuries to other organs (8.6% vs 2.5%).
Overall, the rate of surgical complications was 23.4% among those who had adhesiolysis, compared with 17.5% of those in the nonadhesiolysis group, also a statistically significant difference.
Adhesiolysis was associated with more than a fivefold increased rate of sepsis (odds ratio, 5.12), almost a fourfold increased risk of intra-abdominal complications (OR, 3.46), and more than a twofold increased risk of incisional wound infection (OR, 2.45), all statistically significant differences. The differences in mortality, urinary tract infections, pneumonia, or hemorrhage were not significantly different.
Adhesiolysis also resulted in longer surgery time (a mean of 22.5 minutes longer), recovery time (a mean of about 2 hours longer), and total hospital stay (a mean of about 3 days longer), as well as 29% greater operative blood loss. Among the cases reviewed, the mean hospital cost when adhesiolysis was performed was $18,579, vs. $14,063 when it was not performed.
This is the first study "showing adhesiolysis as a risk factor for postoperative surgical complications, longer hospital stays, more readmissions, and increased costs," the authors pointed out. These data can be helpful "when counseling patients before surgery, when physicians and health care providers make decisions on implementing antiadhesive strategies, and for the reimbursement policy of insurance companies," they added, noting that fewer than 10% of surgeons counsel patients about the risk of adhesions.
"With the projected increase in more repeat abdominal surgeries because of a longer life expectancy and newer technologies, prevention of adhesiolysis-related morbidity might be even more cost effective," they added.
The study was sponsored by Radboud University Nijmegen Medical Center, with no external funding, and the authors said they had no relevant financial conflicts.
FROM ANNALS OF SURGERY
Major finding: The adverse effects of adhesiolysis during abdominal surgery included inadvertently incurred bowel defects, as well as a fivefold greater risk of sepsis, and significantly longer hospital stays and higher hospital costs.
Data source: A prospective cohort study of 755 elective abdominal surgeries in 715 patients, evaluating perioperative and postoperative outcomes associated with those that required adhesiolysis and those that did not.
Disclosures: The study was sponsored by Radboud University Nijmegen Medical Center, with no external funding. The authors said they had no relevant financial conflicts.
Colorectal cancer risk increased with bariatric surgery
The risk of colorectal cancer was significantly increased among people who had undergone obesity surgery in a retrospective cohort study of more than 77,000 obese patients enrolled in a Swedish registry.
The increased risk for colorectal cancer was associated with all three bariatric procedures – vertical banded gastroplasty, adjustable gastric banding, and Roux-en-Y gastric bypass – and increased further over time, reported Dr. Maryam Derogar, of the Karolinska Institutet, Stockholm, and her associates. No such pattern over time was seen among the obese patients who did not have surgery.
"Our data suggest that increased colorectal cancer risk may be a long-term consequence of such surgery," they concluded. If the association is confirmed, they added, "it should stimulate research addressing colonoscopic evaluation of the incidence of colorectal adenomatous polyps after obesity surgery with a view to defining an optimum colonoscopy surveillance strategy for the increasing number of patients who undergo obesity surgery. The study was published online in the Annals of Surgery (2013 [doi:10.1097/SLA.0b013e318288463a]).
To address their "unexpected" finding in an earlier study of an apparent increase in the risk of colorectal cancer after obesity surgery, but no increase in the risk of other cancers related to obesity, they conducted a retrospective cohort study using national registry data between 1980 and 2009, of 15,095 obese patients who had undergone obesity surgery and 62,016 patients who had been diagnosed with obesity but did not undergo surgery. They calculated the colorectal cancer risk using the standardized incidence ratio (SIR), the observed number of cases divided by the number of expected cases in that group.
Over a median of 10 years, there were 70 colorectal cancers in the obesity surgery group; and over a median of 7 years, 373 among those who had no surgery. The SIR for colorectal cancer among those who had surgery was 1.60, which was statistically significant. Among those who had no surgery, there was a small, insignificant increase in risk group (a SIR of 1.26). In the surgery group, the risk increased over time in men and women, up to a twofold increased risk among those patients followed for at least 10 years, a pattern than was not observed in the obese patients who had no surgery.
The "substantial increase in colorectal cancer risk, above that associated with excess body weight alone, more than 10 years after surgery is compatible with the long natural history of colorectal carcinogenesis from normal mucosa to a malignant colorectal cancer," the authors wrote. Why the risk was increased is not clear, but one possible explanation could be that the malabsorption effects of the gastric bypass procedure results in local mucosal changes, the authors speculated. Previously, they had identified rectal mucosal hyperproliferation in patients who had undergone obesity surgery, present at least 3 years after the procedure, a finding that was "associated with increased mucosal expression of the protumorigenic cytokine macrophage migration inhibitory factor," they wrote.
The study’s strengths included the size of the sample, long follow-up, and the validity of Swedish national registry data, while the limitations included the retrospective design and the lack of data on body weight over time.
As in the United States and other countries, obesity has been increasing in Sweden, with a corresponding increase in bariatric surgery. Over the last 20 years, the prevalence of obesity in Sweden has doubled, and the annual number of obesity operations performed has increased from 1,500 in 2006 to almost 4,000 in 2009, according to the authors.
The study was supported by the Swedish Research Council. The authors had no conflicts of interest to declare.
The risk of colorectal cancer was significantly increased among people who had undergone obesity surgery in a retrospective cohort study of more than 77,000 obese patients enrolled in a Swedish registry.
The increased risk for colorectal cancer was associated with all three bariatric procedures – vertical banded gastroplasty, adjustable gastric banding, and Roux-en-Y gastric bypass – and increased further over time, reported Dr. Maryam Derogar, of the Karolinska Institutet, Stockholm, and her associates. No such pattern over time was seen among the obese patients who did not have surgery.
"Our data suggest that increased colorectal cancer risk may be a long-term consequence of such surgery," they concluded. If the association is confirmed, they added, "it should stimulate research addressing colonoscopic evaluation of the incidence of colorectal adenomatous polyps after obesity surgery with a view to defining an optimum colonoscopy surveillance strategy for the increasing number of patients who undergo obesity surgery. The study was published online in the Annals of Surgery (2013 [doi:10.1097/SLA.0b013e318288463a]).
To address their "unexpected" finding in an earlier study of an apparent increase in the risk of colorectal cancer after obesity surgery, but no increase in the risk of other cancers related to obesity, they conducted a retrospective cohort study using national registry data between 1980 and 2009, of 15,095 obese patients who had undergone obesity surgery and 62,016 patients who had been diagnosed with obesity but did not undergo surgery. They calculated the colorectal cancer risk using the standardized incidence ratio (SIR), the observed number of cases divided by the number of expected cases in that group.
Over a median of 10 years, there were 70 colorectal cancers in the obesity surgery group; and over a median of 7 years, 373 among those who had no surgery. The SIR for colorectal cancer among those who had surgery was 1.60, which was statistically significant. Among those who had no surgery, there was a small, insignificant increase in risk group (a SIR of 1.26). In the surgery group, the risk increased over time in men and women, up to a twofold increased risk among those patients followed for at least 10 years, a pattern than was not observed in the obese patients who had no surgery.
The "substantial increase in colorectal cancer risk, above that associated with excess body weight alone, more than 10 years after surgery is compatible with the long natural history of colorectal carcinogenesis from normal mucosa to a malignant colorectal cancer," the authors wrote. Why the risk was increased is not clear, but one possible explanation could be that the malabsorption effects of the gastric bypass procedure results in local mucosal changes, the authors speculated. Previously, they had identified rectal mucosal hyperproliferation in patients who had undergone obesity surgery, present at least 3 years after the procedure, a finding that was "associated with increased mucosal expression of the protumorigenic cytokine macrophage migration inhibitory factor," they wrote.
The study’s strengths included the size of the sample, long follow-up, and the validity of Swedish national registry data, while the limitations included the retrospective design and the lack of data on body weight over time.
As in the United States and other countries, obesity has been increasing in Sweden, with a corresponding increase in bariatric surgery. Over the last 20 years, the prevalence of obesity in Sweden has doubled, and the annual number of obesity operations performed has increased from 1,500 in 2006 to almost 4,000 in 2009, according to the authors.
The study was supported by the Swedish Research Council. The authors had no conflicts of interest to declare.
The risk of colorectal cancer was significantly increased among people who had undergone obesity surgery in a retrospective cohort study of more than 77,000 obese patients enrolled in a Swedish registry.
The increased risk for colorectal cancer was associated with all three bariatric procedures – vertical banded gastroplasty, adjustable gastric banding, and Roux-en-Y gastric bypass – and increased further over time, reported Dr. Maryam Derogar, of the Karolinska Institutet, Stockholm, and her associates. No such pattern over time was seen among the obese patients who did not have surgery.
"Our data suggest that increased colorectal cancer risk may be a long-term consequence of such surgery," they concluded. If the association is confirmed, they added, "it should stimulate research addressing colonoscopic evaluation of the incidence of colorectal adenomatous polyps after obesity surgery with a view to defining an optimum colonoscopy surveillance strategy for the increasing number of patients who undergo obesity surgery. The study was published online in the Annals of Surgery (2013 [doi:10.1097/SLA.0b013e318288463a]).
To address their "unexpected" finding in an earlier study of an apparent increase in the risk of colorectal cancer after obesity surgery, but no increase in the risk of other cancers related to obesity, they conducted a retrospective cohort study using national registry data between 1980 and 2009, of 15,095 obese patients who had undergone obesity surgery and 62,016 patients who had been diagnosed with obesity but did not undergo surgery. They calculated the colorectal cancer risk using the standardized incidence ratio (SIR), the observed number of cases divided by the number of expected cases in that group.
Over a median of 10 years, there were 70 colorectal cancers in the obesity surgery group; and over a median of 7 years, 373 among those who had no surgery. The SIR for colorectal cancer among those who had surgery was 1.60, which was statistically significant. Among those who had no surgery, there was a small, insignificant increase in risk group (a SIR of 1.26). In the surgery group, the risk increased over time in men and women, up to a twofold increased risk among those patients followed for at least 10 years, a pattern than was not observed in the obese patients who had no surgery.
The "substantial increase in colorectal cancer risk, above that associated with excess body weight alone, more than 10 years after surgery is compatible with the long natural history of colorectal carcinogenesis from normal mucosa to a malignant colorectal cancer," the authors wrote. Why the risk was increased is not clear, but one possible explanation could be that the malabsorption effects of the gastric bypass procedure results in local mucosal changes, the authors speculated. Previously, they had identified rectal mucosal hyperproliferation in patients who had undergone obesity surgery, present at least 3 years after the procedure, a finding that was "associated with increased mucosal expression of the protumorigenic cytokine macrophage migration inhibitory factor," they wrote.
The study’s strengths included the size of the sample, long follow-up, and the validity of Swedish national registry data, while the limitations included the retrospective design and the lack of data on body weight over time.
As in the United States and other countries, obesity has been increasing in Sweden, with a corresponding increase in bariatric surgery. Over the last 20 years, the prevalence of obesity in Sweden has doubled, and the annual number of obesity operations performed has increased from 1,500 in 2006 to almost 4,000 in 2009, according to the authors.
The study was supported by the Swedish Research Council. The authors had no conflicts of interest to declare.
FROM THE ANNALS OF SURGERY
Colorectal cancer risk increased with bariatric surgery
The risk of colorectal cancer was significantly increased among people who had undergone obesity surgery in a retrospective cohort study of more than 77,000 obese patients enrolled in a Swedish registry.
The increased risk for colorectal cancer was associated with all three bariatric procedures – vertical banded gastroplasty, adjustable gastric banding, and Roux-en-Y gastric bypass – and increased further over time, reported Dr. Maryam Derogar, of the Karolinska Institutet, Stockholm, and her associates. No such pattern over time was seen among the obese patients who did not have surgery.
"Our data suggest that increased colorectal cancer risk may be a long-term consequence of such surgery," they concluded. If the association is confirmed, they added, "it should stimulate research addressing colonoscopic evaluation of the incidence of colorectal adenomatous polyps after obesity surgery with a view to defining an optimum colonoscopy surveillance strategy for the increasing number of patients who undergo obesity surgery. The study was published online in the Annals of Surgery (2013 [doi:10.1097/SLA.0b013e318288463a]).
To address their "unexpected" finding in an earlier study of an apparent increase in the risk of colorectal cancer after obesity surgery, but no increase in the risk of other cancers related to obesity, they conducted a retrospective cohort study using national registry data between 1980 and 2009, of 15,095 obese patients who had undergone obesity surgery and 62,016 patients who had been diagnosed with obesity but did not undergo surgery. They calculated the colorectal cancer risk using the standardized incidence ratio (SIR), the observed number of cases divided by the number of expected cases in that group.
Over a median of 10 years, there were 70 colorectal cancers in the obesity surgery group; and over a median of 7 years, 373 among those who had no surgery. The SIR for colorectal cancer among those who had surgery was 1.60, which was statistically significant. Among those who had no surgery, there was a small, insignificant increase in risk group (a SIR of 1.26). In the surgery group, the risk increased over time in men and women, up to a twofold increased risk among those patients followed for at least 10 years, a pattern than was not observed in the obese patients who had no surgery.
The "substantial increase in colorectal cancer risk, above that associated with excess body weight alone, more than 10 years after surgery is compatible with the long natural history of colorectal carcinogenesis from normal mucosa to a malignant colorectal cancer," the authors wrote. Why the risk was increased is not clear, but one possible explanation could be that the malabsorption effects of the gastric bypass procedure results in local mucosal changes, the authors speculated. Previously, they had identified rectal mucosal hyperproliferation in patients who had undergone obesity surgery, present at least 3 years after the procedure, a finding that was "associated with increased mucosal expression of the protumorigenic cytokine macrophage migration inhibitory factor," they wrote.
The study’s strengths included the size of the sample, long follow-up, and the validity of Swedish national registry data, while the limitations included the retrospective design and the lack of data on body weight over time.
As in the United States and other countries, obesity has been increasing in Sweden, with a corresponding increase in bariatric surgery. Over the last 20 years, the prevalence of obesity in Sweden has doubled, and the annual number of obesity operations performed has increased from 1,500 in 2006 to almost 4,000 in 2009, according to the authors.
The study was supported by the Swedish Research Council. The authors had no conflicts of interest to declare.
It is well established that overweight and obese individuals have a higher incidence of certain types of cancer; such as breast, colorectal, and endometrial to name a few. The exact mechanism is not known but it is generally linked with chronic inflammation associated with adipocyte release of inflammatory cytokines, an increase in sex steroid hormones, and an increase in insulin resistance. Therefore, it would seem logical to suggest that with significant and sustained weight loss (with or without bariatric surgery) the risk of cancer development may be reduced. Unfortunately, the data on weight loss and subsequent reduction in cancer risk are not solid. This is in part attributed to the fact that significant and sustained weight loss is extremely difficult to achieve without bariatric surgery.
There are several reports that show a protective effect of bariatric surgery on future cancer risk. Christou and colleagues (Surg. Obese Relat. Dis. 2008;4:691-5) reported that obese adults who undergo bariatric surgery may reduce their risk of developing some cancers by as much as 80%. Adams and colleagues (Obesity 2009;17:796-802) compared 6,596 patients who had gastric bypass with 9,442 severely obese individuals who had not, and found a significant decrease in the incidence of cancer and cancer-related deaths after bariatric surgery.
This report by Derogar and colleagues, published in Annals of Surgery, is the first to suggest that bariatric surgery is associated with an increased risk of colorectal cancer over time. It is difficult to interpret the results, however, as they directly contradict other reports and our general understanding of obesity and cancer. There are clearly limitations with the retrospective design of the study and the omission of any body weight or weight loss data. In addition, certain colorectal cancer risk factors, such as family history of cancer and prior adenomatous polyps, are not controlled for between the two groups.
Furthermore, there is a notion that individuals who undergo bariatric surgery tend to be more proactive about their health and take actions to prevent cancer. The authors suggest a possible mechanism that may be related to an increase in putative mucosal biomarkers of colorectal cancer risk and mucosal proinflammatory gene expression following Roux-en-Y gastric bypass. Also, a high-protein diet can promote detrimental metabolic profiles promoting carcinogenesis in the colon and rectum.
The exact mechanism remains elusive and as in most biologic systems, the answer in the end will be complex. Further research is needed to help answer some of these questions. This paper is important because it again highlights the increased risk of cancer associated with obesity and it is important for bariatric surgery programs to implement the proper screening protocols for cancer detection and prevention. Patients who undergo bariatric surgery should be screened both pre- and postoperatively as currently recommended depending on whether they are at average or high risk for certain cancers, such as breast or colon cancer, where screening has been shown to be effective.
Dr. Alex Nagle, FACS, is director of bariatric surgery, Northwestern Memorial Hospital, and associate professor of surgery, Northwestern University Feinberg School of Medicine, Chicago. He disclosed no conflicts of interest.
It is well established that overweight and obese individuals have a higher incidence of certain types of cancer; such as breast, colorectal, and endometrial to name a few. The exact mechanism is not known but it is generally linked with chronic inflammation associated with adipocyte release of inflammatory cytokines, an increase in sex steroid hormones, and an increase in insulin resistance. Therefore, it would seem logical to suggest that with significant and sustained weight loss (with or without bariatric surgery) the risk of cancer development may be reduced. Unfortunately, the data on weight loss and subsequent reduction in cancer risk are not solid. This is in part attributed to the fact that significant and sustained weight loss is extremely difficult to achieve without bariatric surgery.
There are several reports that show a protective effect of bariatric surgery on future cancer risk. Christou and colleagues (Surg. Obese Relat. Dis. 2008;4:691-5) reported that obese adults who undergo bariatric surgery may reduce their risk of developing some cancers by as much as 80%. Adams and colleagues (Obesity 2009;17:796-802) compared 6,596 patients who had gastric bypass with 9,442 severely obese individuals who had not, and found a significant decrease in the incidence of cancer and cancer-related deaths after bariatric surgery.
This report by Derogar and colleagues, published in Annals of Surgery, is the first to suggest that bariatric surgery is associated with an increased risk of colorectal cancer over time. It is difficult to interpret the results, however, as they directly contradict other reports and our general understanding of obesity and cancer. There are clearly limitations with the retrospective design of the study and the omission of any body weight or weight loss data. In addition, certain colorectal cancer risk factors, such as family history of cancer and prior adenomatous polyps, are not controlled for between the two groups.
Furthermore, there is a notion that individuals who undergo bariatric surgery tend to be more proactive about their health and take actions to prevent cancer. The authors suggest a possible mechanism that may be related to an increase in putative mucosal biomarkers of colorectal cancer risk and mucosal proinflammatory gene expression following Roux-en-Y gastric bypass. Also, a high-protein diet can promote detrimental metabolic profiles promoting carcinogenesis in the colon and rectum.
The exact mechanism remains elusive and as in most biologic systems, the answer in the end will be complex. Further research is needed to help answer some of these questions. This paper is important because it again highlights the increased risk of cancer associated with obesity and it is important for bariatric surgery programs to implement the proper screening protocols for cancer detection and prevention. Patients who undergo bariatric surgery should be screened both pre- and postoperatively as currently recommended depending on whether they are at average or high risk for certain cancers, such as breast or colon cancer, where screening has been shown to be effective.
Dr. Alex Nagle, FACS, is director of bariatric surgery, Northwestern Memorial Hospital, and associate professor of surgery, Northwestern University Feinberg School of Medicine, Chicago. He disclosed no conflicts of interest.
It is well established that overweight and obese individuals have a higher incidence of certain types of cancer; such as breast, colorectal, and endometrial to name a few. The exact mechanism is not known but it is generally linked with chronic inflammation associated with adipocyte release of inflammatory cytokines, an increase in sex steroid hormones, and an increase in insulin resistance. Therefore, it would seem logical to suggest that with significant and sustained weight loss (with or without bariatric surgery) the risk of cancer development may be reduced. Unfortunately, the data on weight loss and subsequent reduction in cancer risk are not solid. This is in part attributed to the fact that significant and sustained weight loss is extremely difficult to achieve without bariatric surgery.
There are several reports that show a protective effect of bariatric surgery on future cancer risk. Christou and colleagues (Surg. Obese Relat. Dis. 2008;4:691-5) reported that obese adults who undergo bariatric surgery may reduce their risk of developing some cancers by as much as 80%. Adams and colleagues (Obesity 2009;17:796-802) compared 6,596 patients who had gastric bypass with 9,442 severely obese individuals who had not, and found a significant decrease in the incidence of cancer and cancer-related deaths after bariatric surgery.
This report by Derogar and colleagues, published in Annals of Surgery, is the first to suggest that bariatric surgery is associated with an increased risk of colorectal cancer over time. It is difficult to interpret the results, however, as they directly contradict other reports and our general understanding of obesity and cancer. There are clearly limitations with the retrospective design of the study and the omission of any body weight or weight loss data. In addition, certain colorectal cancer risk factors, such as family history of cancer and prior adenomatous polyps, are not controlled for between the two groups.
Furthermore, there is a notion that individuals who undergo bariatric surgery tend to be more proactive about their health and take actions to prevent cancer. The authors suggest a possible mechanism that may be related to an increase in putative mucosal biomarkers of colorectal cancer risk and mucosal proinflammatory gene expression following Roux-en-Y gastric bypass. Also, a high-protein diet can promote detrimental metabolic profiles promoting carcinogenesis in the colon and rectum.
The exact mechanism remains elusive and as in most biologic systems, the answer in the end will be complex. Further research is needed to help answer some of these questions. This paper is important because it again highlights the increased risk of cancer associated with obesity and it is important for bariatric surgery programs to implement the proper screening protocols for cancer detection and prevention. Patients who undergo bariatric surgery should be screened both pre- and postoperatively as currently recommended depending on whether they are at average or high risk for certain cancers, such as breast or colon cancer, where screening has been shown to be effective.
Dr. Alex Nagle, FACS, is director of bariatric surgery, Northwestern Memorial Hospital, and associate professor of surgery, Northwestern University Feinberg School of Medicine, Chicago. He disclosed no conflicts of interest.
The risk of colorectal cancer was significantly increased among people who had undergone obesity surgery in a retrospective cohort study of more than 77,000 obese patients enrolled in a Swedish registry.
The increased risk for colorectal cancer was associated with all three bariatric procedures – vertical banded gastroplasty, adjustable gastric banding, and Roux-en-Y gastric bypass – and increased further over time, reported Dr. Maryam Derogar, of the Karolinska Institutet, Stockholm, and her associates. No such pattern over time was seen among the obese patients who did not have surgery.
"Our data suggest that increased colorectal cancer risk may be a long-term consequence of such surgery," they concluded. If the association is confirmed, they added, "it should stimulate research addressing colonoscopic evaluation of the incidence of colorectal adenomatous polyps after obesity surgery with a view to defining an optimum colonoscopy surveillance strategy for the increasing number of patients who undergo obesity surgery. The study was published online in the Annals of Surgery (2013 [doi:10.1097/SLA.0b013e318288463a]).
To address their "unexpected" finding in an earlier study of an apparent increase in the risk of colorectal cancer after obesity surgery, but no increase in the risk of other cancers related to obesity, they conducted a retrospective cohort study using national registry data between 1980 and 2009, of 15,095 obese patients who had undergone obesity surgery and 62,016 patients who had been diagnosed with obesity but did not undergo surgery. They calculated the colorectal cancer risk using the standardized incidence ratio (SIR), the observed number of cases divided by the number of expected cases in that group.
Over a median of 10 years, there were 70 colorectal cancers in the obesity surgery group; and over a median of 7 years, 373 among those who had no surgery. The SIR for colorectal cancer among those who had surgery was 1.60, which was statistically significant. Among those who had no surgery, there was a small, insignificant increase in risk group (a SIR of 1.26). In the surgery group, the risk increased over time in men and women, up to a twofold increased risk among those patients followed for at least 10 years, a pattern than was not observed in the obese patients who had no surgery.
The "substantial increase in colorectal cancer risk, above that associated with excess body weight alone, more than 10 years after surgery is compatible with the long natural history of colorectal carcinogenesis from normal mucosa to a malignant colorectal cancer," the authors wrote. Why the risk was increased is not clear, but one possible explanation could be that the malabsorption effects of the gastric bypass procedure results in local mucosal changes, the authors speculated. Previously, they had identified rectal mucosal hyperproliferation in patients who had undergone obesity surgery, present at least 3 years after the procedure, a finding that was "associated with increased mucosal expression of the protumorigenic cytokine macrophage migration inhibitory factor," they wrote.
The study’s strengths included the size of the sample, long follow-up, and the validity of Swedish national registry data, while the limitations included the retrospective design and the lack of data on body weight over time.
As in the United States and other countries, obesity has been increasing in Sweden, with a corresponding increase in bariatric surgery. Over the last 20 years, the prevalence of obesity in Sweden has doubled, and the annual number of obesity operations performed has increased from 1,500 in 2006 to almost 4,000 in 2009, according to the authors.
The study was supported by the Swedish Research Council. The authors had no conflicts of interest to declare.
The risk of colorectal cancer was significantly increased among people who had undergone obesity surgery in a retrospective cohort study of more than 77,000 obese patients enrolled in a Swedish registry.
The increased risk for colorectal cancer was associated with all three bariatric procedures – vertical banded gastroplasty, adjustable gastric banding, and Roux-en-Y gastric bypass – and increased further over time, reported Dr. Maryam Derogar, of the Karolinska Institutet, Stockholm, and her associates. No such pattern over time was seen among the obese patients who did not have surgery.
"Our data suggest that increased colorectal cancer risk may be a long-term consequence of such surgery," they concluded. If the association is confirmed, they added, "it should stimulate research addressing colonoscopic evaluation of the incidence of colorectal adenomatous polyps after obesity surgery with a view to defining an optimum colonoscopy surveillance strategy for the increasing number of patients who undergo obesity surgery. The study was published online in the Annals of Surgery (2013 [doi:10.1097/SLA.0b013e318288463a]).
To address their "unexpected" finding in an earlier study of an apparent increase in the risk of colorectal cancer after obesity surgery, but no increase in the risk of other cancers related to obesity, they conducted a retrospective cohort study using national registry data between 1980 and 2009, of 15,095 obese patients who had undergone obesity surgery and 62,016 patients who had been diagnosed with obesity but did not undergo surgery. They calculated the colorectal cancer risk using the standardized incidence ratio (SIR), the observed number of cases divided by the number of expected cases in that group.
Over a median of 10 years, there were 70 colorectal cancers in the obesity surgery group; and over a median of 7 years, 373 among those who had no surgery. The SIR for colorectal cancer among those who had surgery was 1.60, which was statistically significant. Among those who had no surgery, there was a small, insignificant increase in risk group (a SIR of 1.26). In the surgery group, the risk increased over time in men and women, up to a twofold increased risk among those patients followed for at least 10 years, a pattern than was not observed in the obese patients who had no surgery.
The "substantial increase in colorectal cancer risk, above that associated with excess body weight alone, more than 10 years after surgery is compatible with the long natural history of colorectal carcinogenesis from normal mucosa to a malignant colorectal cancer," the authors wrote. Why the risk was increased is not clear, but one possible explanation could be that the malabsorption effects of the gastric bypass procedure results in local mucosal changes, the authors speculated. Previously, they had identified rectal mucosal hyperproliferation in patients who had undergone obesity surgery, present at least 3 years after the procedure, a finding that was "associated with increased mucosal expression of the protumorigenic cytokine macrophage migration inhibitory factor," they wrote.
The study’s strengths included the size of the sample, long follow-up, and the validity of Swedish national registry data, while the limitations included the retrospective design and the lack of data on body weight over time.
As in the United States and other countries, obesity has been increasing in Sweden, with a corresponding increase in bariatric surgery. Over the last 20 years, the prevalence of obesity in Sweden has doubled, and the annual number of obesity operations performed has increased from 1,500 in 2006 to almost 4,000 in 2009, according to the authors.
The study was supported by the Swedish Research Council. The authors had no conflicts of interest to declare.
FROM THE ANNALS OF SURGERY
Major finding: The risk of colorectal cancer increased by 60% among obese patients, a mean of 7 years after bariatric surgery but was only slightly increased among obese patients who did not undergo surgery, over the expected rate.
Data source: A retrospective cohort study using national patient registry data in Sweden compared the risk of colorectal cancer in about 15,000 obese patients who had undergone bariatric surgery and among about 62,000 obese patients who did not have surgery, compared to matched controls.
Disclosures: The study was supported by the Swedish Research Council. The authors had no conflicts of interest to declare.