User login
Hormones after cancer: Are they safe?
The impact of a gynecologic cancer diagnosis reaches beyond the obvious side effects of surgery, chemotherapy, and radiation. Many of our patients experience the quality-of-life–limiting side effects of abrupt hormone withdrawal as a consequence of our treatments. Assumptions are common, by both patients and providers, that hormonal therapy is unsafe after a gynecologic cancer diagnosis and that it is associated with an increased risk for recurrence. This sentiment likely originates from the fallout of the Womens’ Health Initiative (WHI) studies which showed an increased risk of breast cancer among users of combined estrogen and progesterone therapy.1 While this may be true for breast cancer risk, when initiated early, hormonal therapy is safe, even beneficial, for many patients with a history of gynecologic cancer, and can significantly improve their quality of life in addition to reducing all-cause mortality and incidence of osteoporosis, dementia, and cardiovascular disease.2
Premenopausal women undergoing surgery for endometrial cancer or preinvasive hyperplasia should be considered for ovarian preservation at the time of surgery. This strategy has been shown to be safe and not associated with an increased risk of recurrence. If oophorectomy is performed, hormonal therapy has been shown to be a safe remedy to the side effects of surgical menopause and the deleterious acceleration of bone loss and cardiovascular aging. The safety of hormone therapy for early-stage endometrial cancer has been thoroughly studied, including in a randomized controlled trial of more than 1,200 patients.3 This study showed no difference in the recurrence rate in users when compared with nonusers.
While hormone therapy is safe, from an oncologic standpoint, for women with a history of early-stage endometrial cancer other risks must also be considered. Given the association between endometrial cancer and obesity, these patients are at higher risk for venous thromboembolic (VTE) events, more so with the addition of exogenous hormone therapy. While not an overt contraindication to hormone prescription, obese patients who are prescribed these agents should be counseled regarding their risks for VTE.
The subgroup of patients with endometrial cancer in whom hormones should not be prescribed are those with advanced or recurrent disease. It is common for these tumors to express estrogen receptors, as evidenced by the responsiveness of these tumors to progesterone and antiestrogen treatments. Therefore, there is a theoretical risk for progression while using estrogen. In addition, as stated above, the risk of VTE is particularly elevated for women with metastatic malignancy receiving systemic therapies.
Cervical cancer commonly affects women of premenopausal age; therefore, early ovarian failure is particularly deleterious for this group of patients. Early-stage cervical cancer is most commonly treated with radical or extrafascial hysterectomy. Oophorectomy is not obligatory for the majority of these cases, and can be omitted in pre-, or perimenopausal patients to prevent surgical menopause. Ovarian metastases have been reported in cases of cervical adenocarcinoma, which led to the concern that ovarian preservation was not safe for this histology. However, recent data dispute this concern. A contemporary retrospective series of 105 patients with cervical adenocarcinoma identified no significant difference in overall survival when comparing those who had undergone ovarian preservation versus bilateral salpingo-oophorectomy.4
Ovarian preservation during cervical cancer surgery may not be enough to prevent early menopause. Approximately 20% of cervical cancer patients may require postoperative radiation for high- or intermediate-risk disease (such as positive lymph nodes, or adverse features in the tumor). For these women, ovarian ablation results, even if the ovaries were preserved at the time of surgery. Transposition of the ovaries to a location outside of the potential radiation fields is a strategy to mitigate this risk. To achieve this, the preserved ovaries and their vascular pedicles are skeletonized. The ovaries are then sutured to the paracolic gutter peritoneum or similar location above the pelvic brim, taking care to ensure that the vascular pedicle is not compromised or twisted. Placement of radio-opaque surgical clips on the caudad aspect of the transposed ovary aids in their identification by radiation oncologists when planning their treatment fields.
Ovarian transposition is most commonly used for women who are undergoing definitive surgery for cervical cancer. However, this strategy can also be used as a lead-in procedure for young women with advanced cervical cancer in whom definitive chemoradiation is planned. If the ovaries cannot be spared or moved out of “harm’s way” for premenopausal women undergoing treatment with definitive radiation, hormone therapy may be necessary and is safe for patients with cervical cancer, including those with adenocarcinoma. If the patient has not undergone hysterectomy, a regimen that includes a combination of estrogen and progesterone is necessary to avoid carcinogenic effects of unopposed estrogen on an intact endometrium, even after radiation has ablated those tissues.
When ovarian and fallopian cancers arise in premenopausal patients and appear confined to a single adnexa, contralateral ovarian preservation can be considered. However, for advanced disease, this is usually not possible or appropriate. Given that most ovarian cancers arise in a postmenopausal population, these patients may be preexisting users of hormone therapy. The data, including a randomized controlled trial, would suggest that it is safe to continue to use hormone therapy during or following a diagnosis of ovarian, fallopian tube, or primary peritoneal cancer and that it is not associated with worse outcomes from their cancer.5
Once again, patients should be carefully counseled about the additive risks for VTE that come from metastatic ovarian cancer, surgery via laparotomy, and exogenous hormonal therapy. However, these patients need not be subjected to an abrupt transition to menopause, because level I evidence suggests that these therapies are not associated with worse oncologic outcomes. All patients with ovarian, fallopian tube, and primary peritoneal cancer should receive genetic testing, and if deleterious mutations are found in BRCA 1 or 2 genes indicating an elevated risk for breast cancer, decision making regarding continued exogenous hormonal therapy is complicated. The most contemporary data, including long-term follow-up from the Women’s Health Initiative clinical trials, do not suggest an increased risk for breast cancer with estrogen-only preparations of hormone therapy.6 Given that most women with gynecologic cancers have undergone hysterectomy as part of their treatment, these estrogen-only preparations are appropriate for most.
For patients with rare tumors, such as endometrial stromal tumors or uterine leiomyosarcoma, the safety of exogenous hormone therapy should be dictated by the receptor profile of their particular cancer. Many of these cancers express estrogen receptors; therefore, current guidelines recommend against the use of hormones after these diagnoses when estrogen receptors are expressed.
Gynecologic cancer treatments induce many toxicities with long-term deleterious effects on quality of life. Use of hormones to mitigate the symptoms of menopause is an important tool in the toolkit for gynecologists. Assumptions should not be made that hormonal therapies are always unsafe for all of these patients. It is important to closely evaluate the patient’s tumor and other risk factors before withholding potentially valuable therapies.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant financial disclosures. Email her at [email protected].
References
1. Chlebowski R et al. JAMA. 2010 Oct 20;304(15):1684-92.
2. Sinno AK et al. Gynecol Oncol. 2020;157(2):303-6.
3. Barakat et al. J Clin Oncol. 2006;24(4):587-92.
4. Hu Jun et al. J Obstet Gynaecol. 2017 Nov;37(8):1065-9.
5. Eeles R et al. J Clin Oncol. 2015 Dec 10;33(35):4138-44.
6. Chlebowski R et al. JAMA Jul 28 2020;324(4):369-80.
The impact of a gynecologic cancer diagnosis reaches beyond the obvious side effects of surgery, chemotherapy, and radiation. Many of our patients experience the quality-of-life–limiting side effects of abrupt hormone withdrawal as a consequence of our treatments. Assumptions are common, by both patients and providers, that hormonal therapy is unsafe after a gynecologic cancer diagnosis and that it is associated with an increased risk for recurrence. This sentiment likely originates from the fallout of the Womens’ Health Initiative (WHI) studies which showed an increased risk of breast cancer among users of combined estrogen and progesterone therapy.1 While this may be true for breast cancer risk, when initiated early, hormonal therapy is safe, even beneficial, for many patients with a history of gynecologic cancer, and can significantly improve their quality of life in addition to reducing all-cause mortality and incidence of osteoporosis, dementia, and cardiovascular disease.2
Premenopausal women undergoing surgery for endometrial cancer or preinvasive hyperplasia should be considered for ovarian preservation at the time of surgery. This strategy has been shown to be safe and not associated with an increased risk of recurrence. If oophorectomy is performed, hormonal therapy has been shown to be a safe remedy to the side effects of surgical menopause and the deleterious acceleration of bone loss and cardiovascular aging. The safety of hormone therapy for early-stage endometrial cancer has been thoroughly studied, including in a randomized controlled trial of more than 1,200 patients.3 This study showed no difference in the recurrence rate in users when compared with nonusers.
While hormone therapy is safe, from an oncologic standpoint, for women with a history of early-stage endometrial cancer other risks must also be considered. Given the association between endometrial cancer and obesity, these patients are at higher risk for venous thromboembolic (VTE) events, more so with the addition of exogenous hormone therapy. While not an overt contraindication to hormone prescription, obese patients who are prescribed these agents should be counseled regarding their risks for VTE.
The subgroup of patients with endometrial cancer in whom hormones should not be prescribed are those with advanced or recurrent disease. It is common for these tumors to express estrogen receptors, as evidenced by the responsiveness of these tumors to progesterone and antiestrogen treatments. Therefore, there is a theoretical risk for progression while using estrogen. In addition, as stated above, the risk of VTE is particularly elevated for women with metastatic malignancy receiving systemic therapies.
Cervical cancer commonly affects women of premenopausal age; therefore, early ovarian failure is particularly deleterious for this group of patients. Early-stage cervical cancer is most commonly treated with radical or extrafascial hysterectomy. Oophorectomy is not obligatory for the majority of these cases, and can be omitted in pre-, or perimenopausal patients to prevent surgical menopause. Ovarian metastases have been reported in cases of cervical adenocarcinoma, which led to the concern that ovarian preservation was not safe for this histology. However, recent data dispute this concern. A contemporary retrospective series of 105 patients with cervical adenocarcinoma identified no significant difference in overall survival when comparing those who had undergone ovarian preservation versus bilateral salpingo-oophorectomy.4
Ovarian preservation during cervical cancer surgery may not be enough to prevent early menopause. Approximately 20% of cervical cancer patients may require postoperative radiation for high- or intermediate-risk disease (such as positive lymph nodes, or adverse features in the tumor). For these women, ovarian ablation results, even if the ovaries were preserved at the time of surgery. Transposition of the ovaries to a location outside of the potential radiation fields is a strategy to mitigate this risk. To achieve this, the preserved ovaries and their vascular pedicles are skeletonized. The ovaries are then sutured to the paracolic gutter peritoneum or similar location above the pelvic brim, taking care to ensure that the vascular pedicle is not compromised or twisted. Placement of radio-opaque surgical clips on the caudad aspect of the transposed ovary aids in their identification by radiation oncologists when planning their treatment fields.
Ovarian transposition is most commonly used for women who are undergoing definitive surgery for cervical cancer. However, this strategy can also be used as a lead-in procedure for young women with advanced cervical cancer in whom definitive chemoradiation is planned. If the ovaries cannot be spared or moved out of “harm’s way” for premenopausal women undergoing treatment with definitive radiation, hormone therapy may be necessary and is safe for patients with cervical cancer, including those with adenocarcinoma. If the patient has not undergone hysterectomy, a regimen that includes a combination of estrogen and progesterone is necessary to avoid carcinogenic effects of unopposed estrogen on an intact endometrium, even after radiation has ablated those tissues.
When ovarian and fallopian cancers arise in premenopausal patients and appear confined to a single adnexa, contralateral ovarian preservation can be considered. However, for advanced disease, this is usually not possible or appropriate. Given that most ovarian cancers arise in a postmenopausal population, these patients may be preexisting users of hormone therapy. The data, including a randomized controlled trial, would suggest that it is safe to continue to use hormone therapy during or following a diagnosis of ovarian, fallopian tube, or primary peritoneal cancer and that it is not associated with worse outcomes from their cancer.5
Once again, patients should be carefully counseled about the additive risks for VTE that come from metastatic ovarian cancer, surgery via laparotomy, and exogenous hormonal therapy. However, these patients need not be subjected to an abrupt transition to menopause, because level I evidence suggests that these therapies are not associated with worse oncologic outcomes. All patients with ovarian, fallopian tube, and primary peritoneal cancer should receive genetic testing, and if deleterious mutations are found in BRCA 1 or 2 genes indicating an elevated risk for breast cancer, decision making regarding continued exogenous hormonal therapy is complicated. The most contemporary data, including long-term follow-up from the Women’s Health Initiative clinical trials, do not suggest an increased risk for breast cancer with estrogen-only preparations of hormone therapy.6 Given that most women with gynecologic cancers have undergone hysterectomy as part of their treatment, these estrogen-only preparations are appropriate for most.
For patients with rare tumors, such as endometrial stromal tumors or uterine leiomyosarcoma, the safety of exogenous hormone therapy should be dictated by the receptor profile of their particular cancer. Many of these cancers express estrogen receptors; therefore, current guidelines recommend against the use of hormones after these diagnoses when estrogen receptors are expressed.
Gynecologic cancer treatments induce many toxicities with long-term deleterious effects on quality of life. Use of hormones to mitigate the symptoms of menopause is an important tool in the toolkit for gynecologists. Assumptions should not be made that hormonal therapies are always unsafe for all of these patients. It is important to closely evaluate the patient’s tumor and other risk factors before withholding potentially valuable therapies.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant financial disclosures. Email her at [email protected].
References
1. Chlebowski R et al. JAMA. 2010 Oct 20;304(15):1684-92.
2. Sinno AK et al. Gynecol Oncol. 2020;157(2):303-6.
3. Barakat et al. J Clin Oncol. 2006;24(4):587-92.
4. Hu Jun et al. J Obstet Gynaecol. 2017 Nov;37(8):1065-9.
5. Eeles R et al. J Clin Oncol. 2015 Dec 10;33(35):4138-44.
6. Chlebowski R et al. JAMA Jul 28 2020;324(4):369-80.
The impact of a gynecologic cancer diagnosis reaches beyond the obvious side effects of surgery, chemotherapy, and radiation. Many of our patients experience the quality-of-life–limiting side effects of abrupt hormone withdrawal as a consequence of our treatments. Assumptions are common, by both patients and providers, that hormonal therapy is unsafe after a gynecologic cancer diagnosis and that it is associated with an increased risk for recurrence. This sentiment likely originates from the fallout of the Womens’ Health Initiative (WHI) studies which showed an increased risk of breast cancer among users of combined estrogen and progesterone therapy.1 While this may be true for breast cancer risk, when initiated early, hormonal therapy is safe, even beneficial, for many patients with a history of gynecologic cancer, and can significantly improve their quality of life in addition to reducing all-cause mortality and incidence of osteoporosis, dementia, and cardiovascular disease.2
Premenopausal women undergoing surgery for endometrial cancer or preinvasive hyperplasia should be considered for ovarian preservation at the time of surgery. This strategy has been shown to be safe and not associated with an increased risk of recurrence. If oophorectomy is performed, hormonal therapy has been shown to be a safe remedy to the side effects of surgical menopause and the deleterious acceleration of bone loss and cardiovascular aging. The safety of hormone therapy for early-stage endometrial cancer has been thoroughly studied, including in a randomized controlled trial of more than 1,200 patients.3 This study showed no difference in the recurrence rate in users when compared with nonusers.
While hormone therapy is safe, from an oncologic standpoint, for women with a history of early-stage endometrial cancer other risks must also be considered. Given the association between endometrial cancer and obesity, these patients are at higher risk for venous thromboembolic (VTE) events, more so with the addition of exogenous hormone therapy. While not an overt contraindication to hormone prescription, obese patients who are prescribed these agents should be counseled regarding their risks for VTE.
The subgroup of patients with endometrial cancer in whom hormones should not be prescribed are those with advanced or recurrent disease. It is common for these tumors to express estrogen receptors, as evidenced by the responsiveness of these tumors to progesterone and antiestrogen treatments. Therefore, there is a theoretical risk for progression while using estrogen. In addition, as stated above, the risk of VTE is particularly elevated for women with metastatic malignancy receiving systemic therapies.
Cervical cancer commonly affects women of premenopausal age; therefore, early ovarian failure is particularly deleterious for this group of patients. Early-stage cervical cancer is most commonly treated with radical or extrafascial hysterectomy. Oophorectomy is not obligatory for the majority of these cases, and can be omitted in pre-, or perimenopausal patients to prevent surgical menopause. Ovarian metastases have been reported in cases of cervical adenocarcinoma, which led to the concern that ovarian preservation was not safe for this histology. However, recent data dispute this concern. A contemporary retrospective series of 105 patients with cervical adenocarcinoma identified no significant difference in overall survival when comparing those who had undergone ovarian preservation versus bilateral salpingo-oophorectomy.4
Ovarian preservation during cervical cancer surgery may not be enough to prevent early menopause. Approximately 20% of cervical cancer patients may require postoperative radiation for high- or intermediate-risk disease (such as positive lymph nodes, or adverse features in the tumor). For these women, ovarian ablation results, even if the ovaries were preserved at the time of surgery. Transposition of the ovaries to a location outside of the potential radiation fields is a strategy to mitigate this risk. To achieve this, the preserved ovaries and their vascular pedicles are skeletonized. The ovaries are then sutured to the paracolic gutter peritoneum or similar location above the pelvic brim, taking care to ensure that the vascular pedicle is not compromised or twisted. Placement of radio-opaque surgical clips on the caudad aspect of the transposed ovary aids in their identification by radiation oncologists when planning their treatment fields.
Ovarian transposition is most commonly used for women who are undergoing definitive surgery for cervical cancer. However, this strategy can also be used as a lead-in procedure for young women with advanced cervical cancer in whom definitive chemoradiation is planned. If the ovaries cannot be spared or moved out of “harm’s way” for premenopausal women undergoing treatment with definitive radiation, hormone therapy may be necessary and is safe for patients with cervical cancer, including those with adenocarcinoma. If the patient has not undergone hysterectomy, a regimen that includes a combination of estrogen and progesterone is necessary to avoid carcinogenic effects of unopposed estrogen on an intact endometrium, even after radiation has ablated those tissues.
When ovarian and fallopian cancers arise in premenopausal patients and appear confined to a single adnexa, contralateral ovarian preservation can be considered. However, for advanced disease, this is usually not possible or appropriate. Given that most ovarian cancers arise in a postmenopausal population, these patients may be preexisting users of hormone therapy. The data, including a randomized controlled trial, would suggest that it is safe to continue to use hormone therapy during or following a diagnosis of ovarian, fallopian tube, or primary peritoneal cancer and that it is not associated with worse outcomes from their cancer.5
Once again, patients should be carefully counseled about the additive risks for VTE that come from metastatic ovarian cancer, surgery via laparotomy, and exogenous hormonal therapy. However, these patients need not be subjected to an abrupt transition to menopause, because level I evidence suggests that these therapies are not associated with worse oncologic outcomes. All patients with ovarian, fallopian tube, and primary peritoneal cancer should receive genetic testing, and if deleterious mutations are found in BRCA 1 or 2 genes indicating an elevated risk for breast cancer, decision making regarding continued exogenous hormonal therapy is complicated. The most contemporary data, including long-term follow-up from the Women’s Health Initiative clinical trials, do not suggest an increased risk for breast cancer with estrogen-only preparations of hormone therapy.6 Given that most women with gynecologic cancers have undergone hysterectomy as part of their treatment, these estrogen-only preparations are appropriate for most.
For patients with rare tumors, such as endometrial stromal tumors or uterine leiomyosarcoma, the safety of exogenous hormone therapy should be dictated by the receptor profile of their particular cancer. Many of these cancers express estrogen receptors; therefore, current guidelines recommend against the use of hormones after these diagnoses when estrogen receptors are expressed.
Gynecologic cancer treatments induce many toxicities with long-term deleterious effects on quality of life. Use of hormones to mitigate the symptoms of menopause is an important tool in the toolkit for gynecologists. Assumptions should not be made that hormonal therapies are always unsafe for all of these patients. It is important to closely evaluate the patient’s tumor and other risk factors before withholding potentially valuable therapies.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant financial disclosures. Email her at [email protected].
References
1. Chlebowski R et al. JAMA. 2010 Oct 20;304(15):1684-92.
2. Sinno AK et al. Gynecol Oncol. 2020;157(2):303-6.
3. Barakat et al. J Clin Oncol. 2006;24(4):587-92.
4. Hu Jun et al. J Obstet Gynaecol. 2017 Nov;37(8):1065-9.
5. Eeles R et al. J Clin Oncol. 2015 Dec 10;33(35):4138-44.
6. Chlebowski R et al. JAMA Jul 28 2020;324(4):369-80.
The medical management of early-stage endometrial cancer: When surgery isn’t possible, or desired
The standard management for early-stage endometrial cancer involves surgery with hysterectomy, salpingectomy with or without oophorectomy, and staging lymph node sampling. Surgery serves as both a therapeutic and diagnostic intervention because surgical pathology results are in turn used to predict the likelihood of relapse and guide adjuvant therapy decisions. However, in some cases, surgical intervention is not feasible or desired, particularly if fertility preservation is a goal. Fortunately, there are nonsurgical options that are associated with favorable outcomes to offer these patients.
Endometrial cancer is associated with obesity attributable to causative mechanisms that promote endometrial hyperplasia, cellular proliferation, and heightened hormonal and growth factor signaling. Not only does obesity drive the development of endometrial cancer, but it also complicates the treatment of the disease. For example, endometrial cancer staging surgery is less successfully completed through a minimally invasive route as body mass index increases, primarily because of limitations in surgical exposure.1 In fact, obesity can prevent surgery from being offered through any route. In addition to body habitus, determination of inoperability is also significantly influenced by the presence of coronary artery disease, hypertension, and diabetes.2 Given that these comorbidities are more commonly experienced by women who are overweight, obesity creates a perfect storm of causative and complicating factors for optimal treatment.
While surgeons may determine the candidacy of patients for hysterectomy, patients themselves also drive this decision-making, particularly in the case of young patients who desire fertility preservation. Approximately 10% of patients with endometrial cancer are premenopausal, a number that is increasing over time. These women may have experienced infertility prior to their diagnosis, yet still strongly desire the attempt to conceive, particularly if they have suffered from anovulatory menstrual cycles or polycystic ovarian disease. Women with Lynch syndrome are at a higher risk for developing their cancer in premenopausal years. Therefore, it is critical that gynecologic oncologists consider nonsurgical remedies for these women and understand their potential for success.
Certain criteria should be met for women undergoing nonsurgical management of endometrial cancer, particularly if chosen electively for fertility preservation. Diagnosis should be obtained with a curettage specimen (rather than a pipelle) to optimize the accuracy of establishing tumor grade and to “debulk” the endometrial tissue. Pretreatment imaging is necessary to rule out distant metastatic disease. MRI is particularly helpful in approximating the depth of myometrial invasion of the malignancy and is recommended for patients desiring fertility preservation. Patients who have an endometrial cancer that is deeply invasive into the myometrium are poor candidates for fertility preservation and have a higher risk for metastatic disease, particularly to lymph nodes, and treatment decisions (such as surgery, or, if inoperable, radiation which treats nodal basins) should be considered for these women.
Hormonal therapy has long been identified as a highly effective systemic therapy for endometrial cancers, particularly those that are low grade and express estrogen and progesterone receptors. Progesterone can be administered orally in preparations such as megestrol or medroxyprogesterone or “locally” with levonorgestrel-releasing intrauterine devices. Oral preparations are straightforward, typically low-cost agents. Likelihood of success is 50%-75%. However, the systemic side effects of these agents, which include increased venous thromboembolism risk and appetite stimulation, are particularly problematic in this population. Therefore, many providers prefer to place progestin-releasing intrauterine devices to “bypass” these side effects, avoid issues with adherence to dosing, and provide some preventative endometrial coverage after resolution of the cancer. Recent trials have observed elimination of endometrial cancer on repeat sampling in 67%-76% of cases.3-5 This strategy may be more successful when it is paired with the addition of GnRH agonists.4
When hormonal therapy is chosen for primary endometrial cancer treatment, it is typically monitored for efficacy with repeat endometrial samplings, most commonly with pipelle biopsies to avoid displacement of an intrauterine device, though repeat D&C may be more effective in achieving a complete pathologic response to treatment. Most providers recommend resampling the endometrium at 3-month intervals until resolution of the malignancy has been documented, and thereafter if any new bleeding events develop. For women who have demonstrated resolution of carcinoma on repeat sampling, data are lacking to guide decision-making regarding resumption of conception efforts, ongoing surveillance, and completion hysterectomy after they finish childbearing. If malignancy continues to be identified after 6 months of hormonal therapy, consideration should be made of a more definitive treatment (such as surgery, if feasible, or radiation if not). Continued hormonal therapy can also be considered, as delayed responses remain common even 1 year after starting therapy.6 If hormonal therapy is prolonged for persistent disease, repeat MRI is recommended at 6 months to document lack of progression.
Radiation, preferably with both intracavitary and external beam treatment, is the most definitive intervention for inoperable early-stage endometrial cancer. Unfortunately, fertility is not preserved with this approach. However, for patients with high-grade tumors that are less likely to express hormone receptors or respond to hormonal therapies, this may be the only treatment option available. Typical treatment courses include 5 weeks of external beam radiation treatments, focused on treating the pelvis as a whole, including occult metastases not identified on imaging. Optimal therapy also includes placement of intracavitary radiation implants, such as Heymans capsules, to concentrate the dose at the uterine fundus, while minimizing toxicity to the adjacent bladder and bowel structures. While definitive radiation is considered inferior to a primary surgical effort, disease-specific survival can be observed in more than 80% of patients treated this way.7
While surgery remains the standard intervention for women with early-stage endometrial cancer, hormonal therapy or radiation remain viable options with high rates of success for women who are not surgical candidates or who desire fertility preservation.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Walker JL et al. J Clin Oncol. 2009;27(32):5331-6.
2. Ertel M et al. Ann Surg Oncol. 2021;28(13):8987-95.
3. Janda M et al. Gynecol Oncol. 2021;161(1):143-51.
4. Novikova OV et al. Gynecol Oncol. 2021;161(1):152-9.
5. Westin SN et al. Am J Obstet Gynecol. 2021;224(2):191.e1-15.
6. Cho A et al. Gynecol Oncol. 2021;160(2):413-17.
7. Dutta SW et al. Brachytherapy. 2017;16(3):526-33.
The standard management for early-stage endometrial cancer involves surgery with hysterectomy, salpingectomy with or without oophorectomy, and staging lymph node sampling. Surgery serves as both a therapeutic and diagnostic intervention because surgical pathology results are in turn used to predict the likelihood of relapse and guide adjuvant therapy decisions. However, in some cases, surgical intervention is not feasible or desired, particularly if fertility preservation is a goal. Fortunately, there are nonsurgical options that are associated with favorable outcomes to offer these patients.
Endometrial cancer is associated with obesity attributable to causative mechanisms that promote endometrial hyperplasia, cellular proliferation, and heightened hormonal and growth factor signaling. Not only does obesity drive the development of endometrial cancer, but it also complicates the treatment of the disease. For example, endometrial cancer staging surgery is less successfully completed through a minimally invasive route as body mass index increases, primarily because of limitations in surgical exposure.1 In fact, obesity can prevent surgery from being offered through any route. In addition to body habitus, determination of inoperability is also significantly influenced by the presence of coronary artery disease, hypertension, and diabetes.2 Given that these comorbidities are more commonly experienced by women who are overweight, obesity creates a perfect storm of causative and complicating factors for optimal treatment.
While surgeons may determine the candidacy of patients for hysterectomy, patients themselves also drive this decision-making, particularly in the case of young patients who desire fertility preservation. Approximately 10% of patients with endometrial cancer are premenopausal, a number that is increasing over time. These women may have experienced infertility prior to their diagnosis, yet still strongly desire the attempt to conceive, particularly if they have suffered from anovulatory menstrual cycles or polycystic ovarian disease. Women with Lynch syndrome are at a higher risk for developing their cancer in premenopausal years. Therefore, it is critical that gynecologic oncologists consider nonsurgical remedies for these women and understand their potential for success.
Certain criteria should be met for women undergoing nonsurgical management of endometrial cancer, particularly if chosen electively for fertility preservation. Diagnosis should be obtained with a curettage specimen (rather than a pipelle) to optimize the accuracy of establishing tumor grade and to “debulk” the endometrial tissue. Pretreatment imaging is necessary to rule out distant metastatic disease. MRI is particularly helpful in approximating the depth of myometrial invasion of the malignancy and is recommended for patients desiring fertility preservation. Patients who have an endometrial cancer that is deeply invasive into the myometrium are poor candidates for fertility preservation and have a higher risk for metastatic disease, particularly to lymph nodes, and treatment decisions (such as surgery, or, if inoperable, radiation which treats nodal basins) should be considered for these women.
Hormonal therapy has long been identified as a highly effective systemic therapy for endometrial cancers, particularly those that are low grade and express estrogen and progesterone receptors. Progesterone can be administered orally in preparations such as megestrol or medroxyprogesterone or “locally” with levonorgestrel-releasing intrauterine devices. Oral preparations are straightforward, typically low-cost agents. Likelihood of success is 50%-75%. However, the systemic side effects of these agents, which include increased venous thromboembolism risk and appetite stimulation, are particularly problematic in this population. Therefore, many providers prefer to place progestin-releasing intrauterine devices to “bypass” these side effects, avoid issues with adherence to dosing, and provide some preventative endometrial coverage after resolution of the cancer. Recent trials have observed elimination of endometrial cancer on repeat sampling in 67%-76% of cases.3-5 This strategy may be more successful when it is paired with the addition of GnRH agonists.4
When hormonal therapy is chosen for primary endometrial cancer treatment, it is typically monitored for efficacy with repeat endometrial samplings, most commonly with pipelle biopsies to avoid displacement of an intrauterine device, though repeat D&C may be more effective in achieving a complete pathologic response to treatment. Most providers recommend resampling the endometrium at 3-month intervals until resolution of the malignancy has been documented, and thereafter if any new bleeding events develop. For women who have demonstrated resolution of carcinoma on repeat sampling, data are lacking to guide decision-making regarding resumption of conception efforts, ongoing surveillance, and completion hysterectomy after they finish childbearing. If malignancy continues to be identified after 6 months of hormonal therapy, consideration should be made of a more definitive treatment (such as surgery, if feasible, or radiation if not). Continued hormonal therapy can also be considered, as delayed responses remain common even 1 year after starting therapy.6 If hormonal therapy is prolonged for persistent disease, repeat MRI is recommended at 6 months to document lack of progression.
Radiation, preferably with both intracavitary and external beam treatment, is the most definitive intervention for inoperable early-stage endometrial cancer. Unfortunately, fertility is not preserved with this approach. However, for patients with high-grade tumors that are less likely to express hormone receptors or respond to hormonal therapies, this may be the only treatment option available. Typical treatment courses include 5 weeks of external beam radiation treatments, focused on treating the pelvis as a whole, including occult metastases not identified on imaging. Optimal therapy also includes placement of intracavitary radiation implants, such as Heymans capsules, to concentrate the dose at the uterine fundus, while minimizing toxicity to the adjacent bladder and bowel structures. While definitive radiation is considered inferior to a primary surgical effort, disease-specific survival can be observed in more than 80% of patients treated this way.7
While surgery remains the standard intervention for women with early-stage endometrial cancer, hormonal therapy or radiation remain viable options with high rates of success for women who are not surgical candidates or who desire fertility preservation.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Walker JL et al. J Clin Oncol. 2009;27(32):5331-6.
2. Ertel M et al. Ann Surg Oncol. 2021;28(13):8987-95.
3. Janda M et al. Gynecol Oncol. 2021;161(1):143-51.
4. Novikova OV et al. Gynecol Oncol. 2021;161(1):152-9.
5. Westin SN et al. Am J Obstet Gynecol. 2021;224(2):191.e1-15.
6. Cho A et al. Gynecol Oncol. 2021;160(2):413-17.
7. Dutta SW et al. Brachytherapy. 2017;16(3):526-33.
The standard management for early-stage endometrial cancer involves surgery with hysterectomy, salpingectomy with or without oophorectomy, and staging lymph node sampling. Surgery serves as both a therapeutic and diagnostic intervention because surgical pathology results are in turn used to predict the likelihood of relapse and guide adjuvant therapy decisions. However, in some cases, surgical intervention is not feasible or desired, particularly if fertility preservation is a goal. Fortunately, there are nonsurgical options that are associated with favorable outcomes to offer these patients.
Endometrial cancer is associated with obesity attributable to causative mechanisms that promote endometrial hyperplasia, cellular proliferation, and heightened hormonal and growth factor signaling. Not only does obesity drive the development of endometrial cancer, but it also complicates the treatment of the disease. For example, endometrial cancer staging surgery is less successfully completed through a minimally invasive route as body mass index increases, primarily because of limitations in surgical exposure.1 In fact, obesity can prevent surgery from being offered through any route. In addition to body habitus, determination of inoperability is also significantly influenced by the presence of coronary artery disease, hypertension, and diabetes.2 Given that these comorbidities are more commonly experienced by women who are overweight, obesity creates a perfect storm of causative and complicating factors for optimal treatment.
While surgeons may determine the candidacy of patients for hysterectomy, patients themselves also drive this decision-making, particularly in the case of young patients who desire fertility preservation. Approximately 10% of patients with endometrial cancer are premenopausal, a number that is increasing over time. These women may have experienced infertility prior to their diagnosis, yet still strongly desire the attempt to conceive, particularly if they have suffered from anovulatory menstrual cycles or polycystic ovarian disease. Women with Lynch syndrome are at a higher risk for developing their cancer in premenopausal years. Therefore, it is critical that gynecologic oncologists consider nonsurgical remedies for these women and understand their potential for success.
Certain criteria should be met for women undergoing nonsurgical management of endometrial cancer, particularly if chosen electively for fertility preservation. Diagnosis should be obtained with a curettage specimen (rather than a pipelle) to optimize the accuracy of establishing tumor grade and to “debulk” the endometrial tissue. Pretreatment imaging is necessary to rule out distant metastatic disease. MRI is particularly helpful in approximating the depth of myometrial invasion of the malignancy and is recommended for patients desiring fertility preservation. Patients who have an endometrial cancer that is deeply invasive into the myometrium are poor candidates for fertility preservation and have a higher risk for metastatic disease, particularly to lymph nodes, and treatment decisions (such as surgery, or, if inoperable, radiation which treats nodal basins) should be considered for these women.
Hormonal therapy has long been identified as a highly effective systemic therapy for endometrial cancers, particularly those that are low grade and express estrogen and progesterone receptors. Progesterone can be administered orally in preparations such as megestrol or medroxyprogesterone or “locally” with levonorgestrel-releasing intrauterine devices. Oral preparations are straightforward, typically low-cost agents. Likelihood of success is 50%-75%. However, the systemic side effects of these agents, which include increased venous thromboembolism risk and appetite stimulation, are particularly problematic in this population. Therefore, many providers prefer to place progestin-releasing intrauterine devices to “bypass” these side effects, avoid issues with adherence to dosing, and provide some preventative endometrial coverage after resolution of the cancer. Recent trials have observed elimination of endometrial cancer on repeat sampling in 67%-76% of cases.3-5 This strategy may be more successful when it is paired with the addition of GnRH agonists.4
When hormonal therapy is chosen for primary endometrial cancer treatment, it is typically monitored for efficacy with repeat endometrial samplings, most commonly with pipelle biopsies to avoid displacement of an intrauterine device, though repeat D&C may be more effective in achieving a complete pathologic response to treatment. Most providers recommend resampling the endometrium at 3-month intervals until resolution of the malignancy has been documented, and thereafter if any new bleeding events develop. For women who have demonstrated resolution of carcinoma on repeat sampling, data are lacking to guide decision-making regarding resumption of conception efforts, ongoing surveillance, and completion hysterectomy after they finish childbearing. If malignancy continues to be identified after 6 months of hormonal therapy, consideration should be made of a more definitive treatment (such as surgery, if feasible, or radiation if not). Continued hormonal therapy can also be considered, as delayed responses remain common even 1 year after starting therapy.6 If hormonal therapy is prolonged for persistent disease, repeat MRI is recommended at 6 months to document lack of progression.
Radiation, preferably with both intracavitary and external beam treatment, is the most definitive intervention for inoperable early-stage endometrial cancer. Unfortunately, fertility is not preserved with this approach. However, for patients with high-grade tumors that are less likely to express hormone receptors or respond to hormonal therapies, this may be the only treatment option available. Typical treatment courses include 5 weeks of external beam radiation treatments, focused on treating the pelvis as a whole, including occult metastases not identified on imaging. Optimal therapy also includes placement of intracavitary radiation implants, such as Heymans capsules, to concentrate the dose at the uterine fundus, while minimizing toxicity to the adjacent bladder and bowel structures. While definitive radiation is considered inferior to a primary surgical effort, disease-specific survival can be observed in more than 80% of patients treated this way.7
While surgery remains the standard intervention for women with early-stage endometrial cancer, hormonal therapy or radiation remain viable options with high rates of success for women who are not surgical candidates or who desire fertility preservation.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Walker JL et al. J Clin Oncol. 2009;27(32):5331-6.
2. Ertel M et al. Ann Surg Oncol. 2021;28(13):8987-95.
3. Janda M et al. Gynecol Oncol. 2021;161(1):143-51.
4. Novikova OV et al. Gynecol Oncol. 2021;161(1):152-9.
5. Westin SN et al. Am J Obstet Gynecol. 2021;224(2):191.e1-15.
6. Cho A et al. Gynecol Oncol. 2021;160(2):413-17.
7. Dutta SW et al. Brachytherapy. 2017;16(3):526-33.
Optimizing ‘optimal’ in ovarian cancer cytoreduction
The goal of advanced ovarian cancer surgery is to remove all gross disease, or all visible and palpable disease implants. This became the established standard when improved survival was consistently observed among patients who had undergone complete surgical resection. Traditionally, definitions of no gross residual disease have been left in the hands, and eyes, of the surgeon. However, new technology has emerged which affords surgeons the ability to visualize ovarian cancer deposits that are imperceptible to the naked eye. But will this improve upon the poor cure rates for advanced ovarian cancer?
Many are familiar with the traditional definitions of “optimal” (less than 1 cm–sized deposits at any one location) and “suboptimal” (greater than 1 cm–sized deposits remaining) when referring to surgical cytoreduction of ovarian cancer. This nomenclature was introduced to define, categorize, and prognosticate patient groups after surgery. In recent years we have moved away from these descriptive definitions of ovarian cancer resection, borrowing from surgical oncology measures of surgical outcomes where “R0” defines surgical resection with negative margins, “R1” includes resection with positive microscopic margins (negative for tumor intraoperatively, but positive on microscopic pathology), and “R2” refers to macroscopic residual disease remaining.1
In ovarian cancer, surgeons have adopted the expression R0 to include patients in whom there is no gross visible or palpable residual disease, a special, favorable subgrouping of the previous “optimal” group. R1 is applied to patients with macroscopic, residual disease that fits within the traditional “optimal” cytoreduction classification (<1 cm in any one location). Obviously, these are significant variations to the traditional surgical oncology definitions, but not without supporting data. For example, patients with no gross residual disease (now defined as “R0”) have been observed to have improved survival, compared with patients who are “optimally” debulked but with R1 (<1 cm) residual disease.2 Therefore, this new goal of complete surgical resection has replaced the previous standard of “optimal” cytoreduction in which small macroscopic residual disease was acceptable.
Whether or not a surgery is completed with no gross residual disease is a subjective assessment made by the surgeon, and in practice, highly inaccurate. When a posttrial ad hoc analysis of 1,873 patients with advanced ovarian cancer who had been enrolled in a Gynecologic Oncology Group cooperative trial correlated surgeons’ assessments of “optimal” cytoreduction with objective postoperative radiographic findings (performed, on average, less than 1 month postoperatively) they found that postoperative CT scans identified lesions >1 cm in 40% of cases that had been characterized by surgeons as an “optimal” cytoreduction.3 Most commonly, discrepant lesions were identified in the upper abdominal quadrants and retroperitoneal aortic nodal regions. Therefore, surgeons’ subjective assessment of cytoreduction is prone to error, and given how important the completeness of cytoreduction is for clinical outcomes, there is interest in discovering methods to improve upon surgeons’ ability to discriminate volume of disease.
Pafolacianine (Cytalux, On Target Laboratories) is a novel drug that binds a fluorescent molecule to folic acid targeting the folate alpha receptors which are overexpressed on nonmucinous epithelial ovarian cancer cells compared with adjacent nonmalignant tissues.4 The drug is intravenously infused preoperatively and then visualized with companion near-infrared imaging devices during surgery to visualize its fluorescent signal where it is bound to ovarian cancer implants. In a phase 2 study of 178 patients with confirmed or suspected ovarian cancer, pafolacianine was able to detect implants of ovarian cancer in 26.9% of cases where the surgeon’s visual inspection was negative.5 Of note, the false-positive rate of this drug was not trivial, at 20%. Based on this efficacy data, the drug has been granted FDA approved for use in ovarian cancer surgery to augment the surgeon’s visualization of cancer. However, important questions remain unanswered by these preliminary data.
Will removal of additional microscopic ovarian cancer implants, only seen by pafolacianine, improve the survival of patients with ovarian cancer, and what effect will the addition of this extra surgery have on their surgical morbidity and risk? The use of pafolacianine to augment ovarian cancer debulking surgeries pivots on the premise that ovarian cancer outcomes are determined by surgical “effort” more than the biology of the disease. Otherwise said: The more we surgically remove, the more we cure. But this seems an old-fashioned notion, increasingly challenged by data. It has been shown that, when ovarian cancer debulking surgeries are necessarily more radical because of extensive disease distribution, prognosis is worse, compared with those patients with less extensive disease distribution.6 The effect of surgical effort contributes less than that of predetermined patterns of disease presentation. Additionally, genomic traits are different in tumors that are objectively determined to be not amenable to optimal cytoreduction, compared with resectable tumors.7 These data suggest that it is the disease, more than the surgeon, that most influences outcomes.
Additionally, the question of whether surgical removal of microscopic disease improves ovarian cancer survival has already been addressed with negative findings. The LION trial randomized 647 women with advanced ovarian cancer to primary cytoreductive surgery either with or without routine lymphadenectomy of clinically negative nodes.8 This study found no survival benefit to resecting clinically negative, microscopically positive nodes. In light of these data, it is difficult to imagine that there would be different results with the resection of microscopic peritoneal disease implants identified by pafolacianine.
While pafolacianine promises to move us closer to a true “R0” (negative margins) resection of ovarian cancer, is this even a feasible goal in a disease that is widely metastatic, particularly in the peritoneal cavity? What do “negative margins” mean in the peritoneal cavity? The sensitivity of pafolacianine in detecting microscopic disease is obviously not so high that it can guarantee patients a complete resection of a disseminated disease, and we still do not know what absolute benefit is derived from moving a little bit further on the continuum of surgical resection.
Perhaps augmentation of debulking is not the only, or best, use of pafolacianine for ovarian cancer surgery. Perhaps it might serve a role in diagnostics or staging of the disease rather than for a therapeutic purpose. In the meantime, we await ongoing clinical trials in this space to better inform clinicians what benefits, or harms, they might expect from the addition of this new drug as we continue to define the “optimal” surgical procedure for advanced ovarian cancer.
Dr. Emma Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest.
References
1. Hermanek P, Wittekind C. Semin Surg Oncol 1994;10:12-20.
2. Elattar A et al. Cochrane Database Syst Rev 2011 Aug 10;2011(8):CD007565.
3. Eskander RN et al. Gynecol Oncol 2018;149:525-30.
4. Randall LM et al. Gynecol Oncol 2019;155:63-8.
5. Food and Drug Administration. FDA approves pafolacianine for identifying malignant ovarian cancer lesions. 2021 Dec 1.
6. Horowitz NS et al. J Clin Oncol 2015;33:937-43.
7. Lee S et al. Cell Rep. 2020;31:107502.
8. Harter P et al. N Engl J Med 2019;380:822-32.
The goal of advanced ovarian cancer surgery is to remove all gross disease, or all visible and palpable disease implants. This became the established standard when improved survival was consistently observed among patients who had undergone complete surgical resection. Traditionally, definitions of no gross residual disease have been left in the hands, and eyes, of the surgeon. However, new technology has emerged which affords surgeons the ability to visualize ovarian cancer deposits that are imperceptible to the naked eye. But will this improve upon the poor cure rates for advanced ovarian cancer?
Many are familiar with the traditional definitions of “optimal” (less than 1 cm–sized deposits at any one location) and “suboptimal” (greater than 1 cm–sized deposits remaining) when referring to surgical cytoreduction of ovarian cancer. This nomenclature was introduced to define, categorize, and prognosticate patient groups after surgery. In recent years we have moved away from these descriptive definitions of ovarian cancer resection, borrowing from surgical oncology measures of surgical outcomes where “R0” defines surgical resection with negative margins, “R1” includes resection with positive microscopic margins (negative for tumor intraoperatively, but positive on microscopic pathology), and “R2” refers to macroscopic residual disease remaining.1
In ovarian cancer, surgeons have adopted the expression R0 to include patients in whom there is no gross visible or palpable residual disease, a special, favorable subgrouping of the previous “optimal” group. R1 is applied to patients with macroscopic, residual disease that fits within the traditional “optimal” cytoreduction classification (<1 cm in any one location). Obviously, these are significant variations to the traditional surgical oncology definitions, but not without supporting data. For example, patients with no gross residual disease (now defined as “R0”) have been observed to have improved survival, compared with patients who are “optimally” debulked but with R1 (<1 cm) residual disease.2 Therefore, this new goal of complete surgical resection has replaced the previous standard of “optimal” cytoreduction in which small macroscopic residual disease was acceptable.
Whether or not a surgery is completed with no gross residual disease is a subjective assessment made by the surgeon, and in practice, highly inaccurate. When a posttrial ad hoc analysis of 1,873 patients with advanced ovarian cancer who had been enrolled in a Gynecologic Oncology Group cooperative trial correlated surgeons’ assessments of “optimal” cytoreduction with objective postoperative radiographic findings (performed, on average, less than 1 month postoperatively) they found that postoperative CT scans identified lesions >1 cm in 40% of cases that had been characterized by surgeons as an “optimal” cytoreduction.3 Most commonly, discrepant lesions were identified in the upper abdominal quadrants and retroperitoneal aortic nodal regions. Therefore, surgeons’ subjective assessment of cytoreduction is prone to error, and given how important the completeness of cytoreduction is for clinical outcomes, there is interest in discovering methods to improve upon surgeons’ ability to discriminate volume of disease.
Pafolacianine (Cytalux, On Target Laboratories) is a novel drug that binds a fluorescent molecule to folic acid targeting the folate alpha receptors which are overexpressed on nonmucinous epithelial ovarian cancer cells compared with adjacent nonmalignant tissues.4 The drug is intravenously infused preoperatively and then visualized with companion near-infrared imaging devices during surgery to visualize its fluorescent signal where it is bound to ovarian cancer implants. In a phase 2 study of 178 patients with confirmed or suspected ovarian cancer, pafolacianine was able to detect implants of ovarian cancer in 26.9% of cases where the surgeon’s visual inspection was negative.5 Of note, the false-positive rate of this drug was not trivial, at 20%. Based on this efficacy data, the drug has been granted FDA approved for use in ovarian cancer surgery to augment the surgeon’s visualization of cancer. However, important questions remain unanswered by these preliminary data.
Will removal of additional microscopic ovarian cancer implants, only seen by pafolacianine, improve the survival of patients with ovarian cancer, and what effect will the addition of this extra surgery have on their surgical morbidity and risk? The use of pafolacianine to augment ovarian cancer debulking surgeries pivots on the premise that ovarian cancer outcomes are determined by surgical “effort” more than the biology of the disease. Otherwise said: The more we surgically remove, the more we cure. But this seems an old-fashioned notion, increasingly challenged by data. It has been shown that, when ovarian cancer debulking surgeries are necessarily more radical because of extensive disease distribution, prognosis is worse, compared with those patients with less extensive disease distribution.6 The effect of surgical effort contributes less than that of predetermined patterns of disease presentation. Additionally, genomic traits are different in tumors that are objectively determined to be not amenable to optimal cytoreduction, compared with resectable tumors.7 These data suggest that it is the disease, more than the surgeon, that most influences outcomes.
Additionally, the question of whether surgical removal of microscopic disease improves ovarian cancer survival has already been addressed with negative findings. The LION trial randomized 647 women with advanced ovarian cancer to primary cytoreductive surgery either with or without routine lymphadenectomy of clinically negative nodes.8 This study found no survival benefit to resecting clinically negative, microscopically positive nodes. In light of these data, it is difficult to imagine that there would be different results with the resection of microscopic peritoneal disease implants identified by pafolacianine.
While pafolacianine promises to move us closer to a true “R0” (negative margins) resection of ovarian cancer, is this even a feasible goal in a disease that is widely metastatic, particularly in the peritoneal cavity? What do “negative margins” mean in the peritoneal cavity? The sensitivity of pafolacianine in detecting microscopic disease is obviously not so high that it can guarantee patients a complete resection of a disseminated disease, and we still do not know what absolute benefit is derived from moving a little bit further on the continuum of surgical resection.
Perhaps augmentation of debulking is not the only, or best, use of pafolacianine for ovarian cancer surgery. Perhaps it might serve a role in diagnostics or staging of the disease rather than for a therapeutic purpose. In the meantime, we await ongoing clinical trials in this space to better inform clinicians what benefits, or harms, they might expect from the addition of this new drug as we continue to define the “optimal” surgical procedure for advanced ovarian cancer.
Dr. Emma Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest.
References
1. Hermanek P, Wittekind C. Semin Surg Oncol 1994;10:12-20.
2. Elattar A et al. Cochrane Database Syst Rev 2011 Aug 10;2011(8):CD007565.
3. Eskander RN et al. Gynecol Oncol 2018;149:525-30.
4. Randall LM et al. Gynecol Oncol 2019;155:63-8.
5. Food and Drug Administration. FDA approves pafolacianine for identifying malignant ovarian cancer lesions. 2021 Dec 1.
6. Horowitz NS et al. J Clin Oncol 2015;33:937-43.
7. Lee S et al. Cell Rep. 2020;31:107502.
8. Harter P et al. N Engl J Med 2019;380:822-32.
The goal of advanced ovarian cancer surgery is to remove all gross disease, or all visible and palpable disease implants. This became the established standard when improved survival was consistently observed among patients who had undergone complete surgical resection. Traditionally, definitions of no gross residual disease have been left in the hands, and eyes, of the surgeon. However, new technology has emerged which affords surgeons the ability to visualize ovarian cancer deposits that are imperceptible to the naked eye. But will this improve upon the poor cure rates for advanced ovarian cancer?
Many are familiar with the traditional definitions of “optimal” (less than 1 cm–sized deposits at any one location) and “suboptimal” (greater than 1 cm–sized deposits remaining) when referring to surgical cytoreduction of ovarian cancer. This nomenclature was introduced to define, categorize, and prognosticate patient groups after surgery. In recent years we have moved away from these descriptive definitions of ovarian cancer resection, borrowing from surgical oncology measures of surgical outcomes where “R0” defines surgical resection with negative margins, “R1” includes resection with positive microscopic margins (negative for tumor intraoperatively, but positive on microscopic pathology), and “R2” refers to macroscopic residual disease remaining.1
In ovarian cancer, surgeons have adopted the expression R0 to include patients in whom there is no gross visible or palpable residual disease, a special, favorable subgrouping of the previous “optimal” group. R1 is applied to patients with macroscopic, residual disease that fits within the traditional “optimal” cytoreduction classification (<1 cm in any one location). Obviously, these are significant variations to the traditional surgical oncology definitions, but not without supporting data. For example, patients with no gross residual disease (now defined as “R0”) have been observed to have improved survival, compared with patients who are “optimally” debulked but with R1 (<1 cm) residual disease.2 Therefore, this new goal of complete surgical resection has replaced the previous standard of “optimal” cytoreduction in which small macroscopic residual disease was acceptable.
Whether or not a surgery is completed with no gross residual disease is a subjective assessment made by the surgeon, and in practice, highly inaccurate. When a posttrial ad hoc analysis of 1,873 patients with advanced ovarian cancer who had been enrolled in a Gynecologic Oncology Group cooperative trial correlated surgeons’ assessments of “optimal” cytoreduction with objective postoperative radiographic findings (performed, on average, less than 1 month postoperatively) they found that postoperative CT scans identified lesions >1 cm in 40% of cases that had been characterized by surgeons as an “optimal” cytoreduction.3 Most commonly, discrepant lesions were identified in the upper abdominal quadrants and retroperitoneal aortic nodal regions. Therefore, surgeons’ subjective assessment of cytoreduction is prone to error, and given how important the completeness of cytoreduction is for clinical outcomes, there is interest in discovering methods to improve upon surgeons’ ability to discriminate volume of disease.
Pafolacianine (Cytalux, On Target Laboratories) is a novel drug that binds a fluorescent molecule to folic acid targeting the folate alpha receptors which are overexpressed on nonmucinous epithelial ovarian cancer cells compared with adjacent nonmalignant tissues.4 The drug is intravenously infused preoperatively and then visualized with companion near-infrared imaging devices during surgery to visualize its fluorescent signal where it is bound to ovarian cancer implants. In a phase 2 study of 178 patients with confirmed or suspected ovarian cancer, pafolacianine was able to detect implants of ovarian cancer in 26.9% of cases where the surgeon’s visual inspection was negative.5 Of note, the false-positive rate of this drug was not trivial, at 20%. Based on this efficacy data, the drug has been granted FDA approved for use in ovarian cancer surgery to augment the surgeon’s visualization of cancer. However, important questions remain unanswered by these preliminary data.
Will removal of additional microscopic ovarian cancer implants, only seen by pafolacianine, improve the survival of patients with ovarian cancer, and what effect will the addition of this extra surgery have on their surgical morbidity and risk? The use of pafolacianine to augment ovarian cancer debulking surgeries pivots on the premise that ovarian cancer outcomes are determined by surgical “effort” more than the biology of the disease. Otherwise said: The more we surgically remove, the more we cure. But this seems an old-fashioned notion, increasingly challenged by data. It has been shown that, when ovarian cancer debulking surgeries are necessarily more radical because of extensive disease distribution, prognosis is worse, compared with those patients with less extensive disease distribution.6 The effect of surgical effort contributes less than that of predetermined patterns of disease presentation. Additionally, genomic traits are different in tumors that are objectively determined to be not amenable to optimal cytoreduction, compared with resectable tumors.7 These data suggest that it is the disease, more than the surgeon, that most influences outcomes.
Additionally, the question of whether surgical removal of microscopic disease improves ovarian cancer survival has already been addressed with negative findings. The LION trial randomized 647 women with advanced ovarian cancer to primary cytoreductive surgery either with or without routine lymphadenectomy of clinically negative nodes.8 This study found no survival benefit to resecting clinically negative, microscopically positive nodes. In light of these data, it is difficult to imagine that there would be different results with the resection of microscopic peritoneal disease implants identified by pafolacianine.
While pafolacianine promises to move us closer to a true “R0” (negative margins) resection of ovarian cancer, is this even a feasible goal in a disease that is widely metastatic, particularly in the peritoneal cavity? What do “negative margins” mean in the peritoneal cavity? The sensitivity of pafolacianine in detecting microscopic disease is obviously not so high that it can guarantee patients a complete resection of a disseminated disease, and we still do not know what absolute benefit is derived from moving a little bit further on the continuum of surgical resection.
Perhaps augmentation of debulking is not the only, or best, use of pafolacianine for ovarian cancer surgery. Perhaps it might serve a role in diagnostics or staging of the disease rather than for a therapeutic purpose. In the meantime, we await ongoing clinical trials in this space to better inform clinicians what benefits, or harms, they might expect from the addition of this new drug as we continue to define the “optimal” surgical procedure for advanced ovarian cancer.
Dr. Emma Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest.
References
1. Hermanek P, Wittekind C. Semin Surg Oncol 1994;10:12-20.
2. Elattar A et al. Cochrane Database Syst Rev 2011 Aug 10;2011(8):CD007565.
3. Eskander RN et al. Gynecol Oncol 2018;149:525-30.
4. Randall LM et al. Gynecol Oncol 2019;155:63-8.
5. Food and Drug Administration. FDA approves pafolacianine for identifying malignant ovarian cancer lesions. 2021 Dec 1.
6. Horowitz NS et al. J Clin Oncol 2015;33:937-43.
7. Lee S et al. Cell Rep. 2020;31:107502.
8. Harter P et al. N Engl J Med 2019;380:822-32.
Evaluating phantom hCG and low-level hCG elevations in the nonpregnant patient
A human chorionic gonadotropin (hCG) test is commonly ordered by gynecologists prior to surgical procedures, in the workup of bleeding abnormalities, and in the follow-up of ectopic and molar pregnancies, to name a few indications. In doing so, occasionally clinicians will find themselves in the diagnostic dilemma of discovering an inexplicable low-level elevation in hCG, such as in a postmenopausal patient. This clinical picture can be confusing and can be concerning for conditions such as postmolar gestational trophoblastic neoplasia (GTN). However, there can be benign causes of this phenomenon.1 To prevent unnecessary worry, investigation of treatments is important. In fact, misdiagnosis and inappropriate treatment of benign, low-level hCG levels with unnecessary chemotherapy is problematic mismanagement of gestational trophoblastic disease (GTD), and a major cause of litigation.
Human chorionic gonadotropin is a glycoprotein hormone with two subunits (alpha and beta). It can come from multiple sources, including trophoblastic cells, malignant trophoblastic cells, the pituitary gland, and exogenous sources.1 Its alpha-subunit is identical to that of follicle stimulating hormone (FSH), luteinizing hormone (LH), and thyroid-stimulating hormone (TSH). Its beta-subunit is unique, though very similar to that of LH. The free hCG beta subunit can be produced by nontrophoblastic neoplasms. The gene for the beta subunit of hCG is in close proximity to the beta subunit of LH and increases in gonadotropin-releasing hormone (GnRH) in menopause can result in the stimulation of both genes. Understanding the sources of hCG-like glycoproteins and mechanisms for testing is important when considering possible causes for falsely elevated hCG.
Most commercially available serum hCG assays detect normal intact hCG and free beta subunits. They are typically sandwich assays utilizing antibody binding sites in which a solid-phase anti-hCG antibody to a specific hCG target is then mixed with the patient’s serum, trapping or binding the hCG, which is then treated with an indicator antibody. After being washed with the indicator or “capture” antibody, its relative (quantitative) levels can be measured.1
Urine hCG testing (such as urine pregnancy tests) work through capillary action, drawing the patient’s urine across absorbent pads before reaching a pad which contains anti-hCG antibodies (the detection zone) in the test line. These tests are less sensitive than serum tests, but many can detect hCG levels <15-20 mIU/mL.1
When ob.gyns. are asked to consult on or evaluate persistently low-level elevations of hCG in nonpregnant patients they should consider both malignant and nonmalignant etiologies. Malignant causes include GTN or quiescent GTD (e.g., after treatment of a molar pregnancy or GTN), choriocarcinoma (e.g., ovarian germ cell tumors), and nonchoriocarcinoma malignancies (such as cervical, pancreatic, breast, renal). Nonmalignant causes of hCG elevations in nonpregnant patients include pituitary hCG (in postmenopausal patients), exogenous hCG, and phantom hCG.
The first step in diagnostic workup is to perform a urine pregnancy test. Provided that the serum hCG level is > 20 mIU/mL, the urine HCG should be positive unless the cause of elevated levels is “phantom hCG” from heterophilic antibodies. When patients are exposed to animal antigens (such as in vaccines) they can develop antibodies such as human anti-mouse antibody. These antibodies have affinity to the binding antibodies used in many hCG sandwich assays and form a linkage between the solid phase antibody and the detection antibody creating a false-positive result. This false-positive test is only present in serum testing but not urine tests because the patient’s heterophilic antibodies are not excreted by the kidney and thus not available to create a false-positive result. An alternative method to make the diagnosis of phantom hCG is to request that the hCG testing be run at a different lab with a different assay (which may not react with the same affinity to the patient’s anti-animal heterophile antibodies), or to request that the lab perform serial dilutions. If phantom hCG from heterophile antibodies is at play, serial dilutions will result in a nonlinear dilution response.
If the patient’s urine hCG test is positive, then pregnancy should be ruled out with a transvaginal ultrasound. If negative, an ectopic pregnancy should still be considered (unless not medically plausible, such as in postmenopausal women or women who have undergone hysterectomy). In the absence of an intrauterine or ectopic pregnancy, a positive serum and urine pregnancy test could be from exogenous hCG, from malignancy or pituitary hCG. Use of exogenous hCG can be ruled out by taking a thorough history, with particular focus on asking about weight loss medications and muscle building therapies.
If pregnancy and exogenous hCG are ruled out, clinicians should assess for an occult hCG-secreting malignancy. The lab should be asked to measure the proportion of the free beta subunit of hCG, as this is typically what is secreted by malignancies. CT imaging of the chest, abdomen, and pelvis to search for an occult primary tumor should take place. If the patient has been recently treated for molar pregnancy or GTN, and serum hCG levels reside between 100 and 300 mIU/mL, quiescent GTD should be considered the diagnosis. Determination of the proportion of hyperglycosylated hCG to total hCG can help differentiate active choriocarcinoma from quiescent GTD. After restaging imaging has been done to confirm no measurable metastatic foci, observation can follow with monthly hCG measurements. The majority of these cases will eventually resolve without intervention within a year. Quiescent GTD and persistent low-level HCG in the absence of measurable GTN on imaging or symptoms does not require treatment with chemotherapy or hysterectomy, particularly in women who desire future fertility.2
Once occult malignancy has been ruled out, the remaining potential source of hCG is the pituitary gland. As mentioned earlier, hCG shares its morphology with TSH, LH, and FSH. This can result in cross reactivity and false positives. In the menopausal state, GnRH levels increase and thus so do pituitary LH and hCG levels. To confirm that the pituitary is the source of the low-level hCG levels, the provider should prescribe a course of hormonal treatment such as an oral contraceptive pill for a 2- to 3-month period. This should result in suppression of pituitary hCG, and serum hCG levels, as part of a negative feedback loop. Pituitary source of hCG is a benign condition, and, like quiescent GTD, phantom hCG or exogenous hCG does not require intervention.
Getting to the bottom of persistent low-level hCG elevations can be challenging. By following the step-wise algorithm listed here, clinicians can sequentially test for urine hCG, heterophilic antibodies, elevated free beta-subunit, occult malignancy, and pituitary hCG.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest. Email her at [email protected].
References
1. Oyatogun O et al. Ther Adv Reprod Health 2021 Jun 13. doi: 10.1177/2F26334941211016412.
2. Soper JT. Obstet Gynecol. 2021 Feb 1;137(2):355-70.
A human chorionic gonadotropin (hCG) test is commonly ordered by gynecologists prior to surgical procedures, in the workup of bleeding abnormalities, and in the follow-up of ectopic and molar pregnancies, to name a few indications. In doing so, occasionally clinicians will find themselves in the diagnostic dilemma of discovering an inexplicable low-level elevation in hCG, such as in a postmenopausal patient. This clinical picture can be confusing and can be concerning for conditions such as postmolar gestational trophoblastic neoplasia (GTN). However, there can be benign causes of this phenomenon.1 To prevent unnecessary worry, investigation of treatments is important. In fact, misdiagnosis and inappropriate treatment of benign, low-level hCG levels with unnecessary chemotherapy is problematic mismanagement of gestational trophoblastic disease (GTD), and a major cause of litigation.
Human chorionic gonadotropin is a glycoprotein hormone with two subunits (alpha and beta). It can come from multiple sources, including trophoblastic cells, malignant trophoblastic cells, the pituitary gland, and exogenous sources.1 Its alpha-subunit is identical to that of follicle stimulating hormone (FSH), luteinizing hormone (LH), and thyroid-stimulating hormone (TSH). Its beta-subunit is unique, though very similar to that of LH. The free hCG beta subunit can be produced by nontrophoblastic neoplasms. The gene for the beta subunit of hCG is in close proximity to the beta subunit of LH and increases in gonadotropin-releasing hormone (GnRH) in menopause can result in the stimulation of both genes. Understanding the sources of hCG-like glycoproteins and mechanisms for testing is important when considering possible causes for falsely elevated hCG.
Most commercially available serum hCG assays detect normal intact hCG and free beta subunits. They are typically sandwich assays utilizing antibody binding sites in which a solid-phase anti-hCG antibody to a specific hCG target is then mixed with the patient’s serum, trapping or binding the hCG, which is then treated with an indicator antibody. After being washed with the indicator or “capture” antibody, its relative (quantitative) levels can be measured.1
Urine hCG testing (such as urine pregnancy tests) work through capillary action, drawing the patient’s urine across absorbent pads before reaching a pad which contains anti-hCG antibodies (the detection zone) in the test line. These tests are less sensitive than serum tests, but many can detect hCG levels <15-20 mIU/mL.1
When ob.gyns. are asked to consult on or evaluate persistently low-level elevations of hCG in nonpregnant patients they should consider both malignant and nonmalignant etiologies. Malignant causes include GTN or quiescent GTD (e.g., after treatment of a molar pregnancy or GTN), choriocarcinoma (e.g., ovarian germ cell tumors), and nonchoriocarcinoma malignancies (such as cervical, pancreatic, breast, renal). Nonmalignant causes of hCG elevations in nonpregnant patients include pituitary hCG (in postmenopausal patients), exogenous hCG, and phantom hCG.
The first step in diagnostic workup is to perform a urine pregnancy test. Provided that the serum hCG level is > 20 mIU/mL, the urine HCG should be positive unless the cause of elevated levels is “phantom hCG” from heterophilic antibodies. When patients are exposed to animal antigens (such as in vaccines) they can develop antibodies such as human anti-mouse antibody. These antibodies have affinity to the binding antibodies used in many hCG sandwich assays and form a linkage between the solid phase antibody and the detection antibody creating a false-positive result. This false-positive test is only present in serum testing but not urine tests because the patient’s heterophilic antibodies are not excreted by the kidney and thus not available to create a false-positive result. An alternative method to make the diagnosis of phantom hCG is to request that the hCG testing be run at a different lab with a different assay (which may not react with the same affinity to the patient’s anti-animal heterophile antibodies), or to request that the lab perform serial dilutions. If phantom hCG from heterophile antibodies is at play, serial dilutions will result in a nonlinear dilution response.
If the patient’s urine hCG test is positive, then pregnancy should be ruled out with a transvaginal ultrasound. If negative, an ectopic pregnancy should still be considered (unless not medically plausible, such as in postmenopausal women or women who have undergone hysterectomy). In the absence of an intrauterine or ectopic pregnancy, a positive serum and urine pregnancy test could be from exogenous hCG, from malignancy or pituitary hCG. Use of exogenous hCG can be ruled out by taking a thorough history, with particular focus on asking about weight loss medications and muscle building therapies.
If pregnancy and exogenous hCG are ruled out, clinicians should assess for an occult hCG-secreting malignancy. The lab should be asked to measure the proportion of the free beta subunit of hCG, as this is typically what is secreted by malignancies. CT imaging of the chest, abdomen, and pelvis to search for an occult primary tumor should take place. If the patient has been recently treated for molar pregnancy or GTN, and serum hCG levels reside between 100 and 300 mIU/mL, quiescent GTD should be considered the diagnosis. Determination of the proportion of hyperglycosylated hCG to total hCG can help differentiate active choriocarcinoma from quiescent GTD. After restaging imaging has been done to confirm no measurable metastatic foci, observation can follow with monthly hCG measurements. The majority of these cases will eventually resolve without intervention within a year. Quiescent GTD and persistent low-level HCG in the absence of measurable GTN on imaging or symptoms does not require treatment with chemotherapy or hysterectomy, particularly in women who desire future fertility.2
Once occult malignancy has been ruled out, the remaining potential source of hCG is the pituitary gland. As mentioned earlier, hCG shares its morphology with TSH, LH, and FSH. This can result in cross reactivity and false positives. In the menopausal state, GnRH levels increase and thus so do pituitary LH and hCG levels. To confirm that the pituitary is the source of the low-level hCG levels, the provider should prescribe a course of hormonal treatment such as an oral contraceptive pill for a 2- to 3-month period. This should result in suppression of pituitary hCG, and serum hCG levels, as part of a negative feedback loop. Pituitary source of hCG is a benign condition, and, like quiescent GTD, phantom hCG or exogenous hCG does not require intervention.
Getting to the bottom of persistent low-level hCG elevations can be challenging. By following the step-wise algorithm listed here, clinicians can sequentially test for urine hCG, heterophilic antibodies, elevated free beta-subunit, occult malignancy, and pituitary hCG.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest. Email her at [email protected].
References
1. Oyatogun O et al. Ther Adv Reprod Health 2021 Jun 13. doi: 10.1177/2F26334941211016412.
2. Soper JT. Obstet Gynecol. 2021 Feb 1;137(2):355-70.
A human chorionic gonadotropin (hCG) test is commonly ordered by gynecologists prior to surgical procedures, in the workup of bleeding abnormalities, and in the follow-up of ectopic and molar pregnancies, to name a few indications. In doing so, occasionally clinicians will find themselves in the diagnostic dilemma of discovering an inexplicable low-level elevation in hCG, such as in a postmenopausal patient. This clinical picture can be confusing and can be concerning for conditions such as postmolar gestational trophoblastic neoplasia (GTN). However, there can be benign causes of this phenomenon.1 To prevent unnecessary worry, investigation of treatments is important. In fact, misdiagnosis and inappropriate treatment of benign, low-level hCG levels with unnecessary chemotherapy is problematic mismanagement of gestational trophoblastic disease (GTD), and a major cause of litigation.
Human chorionic gonadotropin is a glycoprotein hormone with two subunits (alpha and beta). It can come from multiple sources, including trophoblastic cells, malignant trophoblastic cells, the pituitary gland, and exogenous sources.1 Its alpha-subunit is identical to that of follicle stimulating hormone (FSH), luteinizing hormone (LH), and thyroid-stimulating hormone (TSH). Its beta-subunit is unique, though very similar to that of LH. The free hCG beta subunit can be produced by nontrophoblastic neoplasms. The gene for the beta subunit of hCG is in close proximity to the beta subunit of LH and increases in gonadotropin-releasing hormone (GnRH) in menopause can result in the stimulation of both genes. Understanding the sources of hCG-like glycoproteins and mechanisms for testing is important when considering possible causes for falsely elevated hCG.
Most commercially available serum hCG assays detect normal intact hCG and free beta subunits. They are typically sandwich assays utilizing antibody binding sites in which a solid-phase anti-hCG antibody to a specific hCG target is then mixed with the patient’s serum, trapping or binding the hCG, which is then treated with an indicator antibody. After being washed with the indicator or “capture” antibody, its relative (quantitative) levels can be measured.1
Urine hCG testing (such as urine pregnancy tests) work through capillary action, drawing the patient’s urine across absorbent pads before reaching a pad which contains anti-hCG antibodies (the detection zone) in the test line. These tests are less sensitive than serum tests, but many can detect hCG levels <15-20 mIU/mL.1
When ob.gyns. are asked to consult on or evaluate persistently low-level elevations of hCG in nonpregnant patients they should consider both malignant and nonmalignant etiologies. Malignant causes include GTN or quiescent GTD (e.g., after treatment of a molar pregnancy or GTN), choriocarcinoma (e.g., ovarian germ cell tumors), and nonchoriocarcinoma malignancies (such as cervical, pancreatic, breast, renal). Nonmalignant causes of hCG elevations in nonpregnant patients include pituitary hCG (in postmenopausal patients), exogenous hCG, and phantom hCG.
The first step in diagnostic workup is to perform a urine pregnancy test. Provided that the serum hCG level is > 20 mIU/mL, the urine HCG should be positive unless the cause of elevated levels is “phantom hCG” from heterophilic antibodies. When patients are exposed to animal antigens (such as in vaccines) they can develop antibodies such as human anti-mouse antibody. These antibodies have affinity to the binding antibodies used in many hCG sandwich assays and form a linkage between the solid phase antibody and the detection antibody creating a false-positive result. This false-positive test is only present in serum testing but not urine tests because the patient’s heterophilic antibodies are not excreted by the kidney and thus not available to create a false-positive result. An alternative method to make the diagnosis of phantom hCG is to request that the hCG testing be run at a different lab with a different assay (which may not react with the same affinity to the patient’s anti-animal heterophile antibodies), or to request that the lab perform serial dilutions. If phantom hCG from heterophile antibodies is at play, serial dilutions will result in a nonlinear dilution response.
If the patient’s urine hCG test is positive, then pregnancy should be ruled out with a transvaginal ultrasound. If negative, an ectopic pregnancy should still be considered (unless not medically plausible, such as in postmenopausal women or women who have undergone hysterectomy). In the absence of an intrauterine or ectopic pregnancy, a positive serum and urine pregnancy test could be from exogenous hCG, from malignancy or pituitary hCG. Use of exogenous hCG can be ruled out by taking a thorough history, with particular focus on asking about weight loss medications and muscle building therapies.
If pregnancy and exogenous hCG are ruled out, clinicians should assess for an occult hCG-secreting malignancy. The lab should be asked to measure the proportion of the free beta subunit of hCG, as this is typically what is secreted by malignancies. CT imaging of the chest, abdomen, and pelvis to search for an occult primary tumor should take place. If the patient has been recently treated for molar pregnancy or GTN, and serum hCG levels reside between 100 and 300 mIU/mL, quiescent GTD should be considered the diagnosis. Determination of the proportion of hyperglycosylated hCG to total hCG can help differentiate active choriocarcinoma from quiescent GTD. After restaging imaging has been done to confirm no measurable metastatic foci, observation can follow with monthly hCG measurements. The majority of these cases will eventually resolve without intervention within a year. Quiescent GTD and persistent low-level HCG in the absence of measurable GTN on imaging or symptoms does not require treatment with chemotherapy or hysterectomy, particularly in women who desire future fertility.2
Once occult malignancy has been ruled out, the remaining potential source of hCG is the pituitary gland. As mentioned earlier, hCG shares its morphology with TSH, LH, and FSH. This can result in cross reactivity and false positives. In the menopausal state, GnRH levels increase and thus so do pituitary LH and hCG levels. To confirm that the pituitary is the source of the low-level hCG levels, the provider should prescribe a course of hormonal treatment such as an oral contraceptive pill for a 2- to 3-month period. This should result in suppression of pituitary hCG, and serum hCG levels, as part of a negative feedback loop. Pituitary source of hCG is a benign condition, and, like quiescent GTD, phantom hCG or exogenous hCG does not require intervention.
Getting to the bottom of persistent low-level hCG elevations can be challenging. By following the step-wise algorithm listed here, clinicians can sequentially test for urine hCG, heterophilic antibodies, elevated free beta-subunit, occult malignancy, and pituitary hCG.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest. Email her at [email protected].
References
1. Oyatogun O et al. Ther Adv Reprod Health 2021 Jun 13. doi: 10.1177/2F26334941211016412.
2. Soper JT. Obstet Gynecol. 2021 Feb 1;137(2):355-70.
Management of advanced endometrial cancer
Endometrial cancer is most commonly diagnosed at an early stage. Unfortunately, there is a trend toward the diagnosis of more advanced disease, for which cure is rare, and this is an important contributing factor toward the overall increasing mortality trend for endometrial cancer.
Histology is a major risk factor for advanced disease. For example, serous carcinoma, which accounts for approximately only 10% of all endometrial cancer diagnoses, comprises 25% of cases of advanced cases. Similarly, carcinosarcoma, a cell type known to be particularly aggressive, is relatively overrepresented among cases of advanced disease.
Advanced endometrial cancer includes cases of stage III (involvement of lymph nodes, ovaries, and vagina) and stage IV disease (with direct extension into pelvic viscera and distant metastases). In most cases of stage III disease, extrauterine metastases are microscopic and are detected only at the time of surgical staging. Bulky nodal disease within the pelvic and para-aortic nodal basins is less common but associated with worse prognosis than for patients with microscopic nodal metastases. Stage IV disease usually presents with peritoneal spread of disease including carcinomatosis, omental disease, and involvement of the small and large intestine.
Once advanced, endometrial cancer requires more than surgery alone, relying heavily on adjuvant therapies to achieve responses, particularly systemic therapy with platinum and taxane chemotherapy. In some cases, molecularly targeted therapy (such as trastuzumab for serous carcinomas that demonstrate overexpression of HER2) has been shown to be superior in efficacy.1 Surgery may involve either radical nodal dissections to the infrarenal aortic basin, and/or peritoneal debulking procedures similar to that required for ovarian cancer. Perhaps because of patterns of disease distribution so similar to ovarian cancer, historically, sequencing of therapy focused on radical primary debulking surgery (PDS) followed by chemotherapy.
In 2000, a retrospective series from Johns Hopkins University documented the outcomes of 65 patients with advanced endometrial cancer who had undergone primary debulking surgery followed by chemotherapy.2 They noted that survival was directly associated with degree of cytoreduction, with the best outcomes seen for those patients whose surgery resulted in no gross residual disease. Following these data, PDS with complete resection of all disease became the goal of primary therapy.
However, unlike ovarian cancer (which shares a similar disease distribution with advanced endometrial cancer) patients with endometrial cancer are more obese, older, and typically have more comorbidities. Therefore, radical primary debulking surgeries may be associated with poor patient perioperative outcomes, and feasibility of complete cytoreduction, particularly in very obese patients, can be limited. For this reason, neoadjuvant chemotherapy (NACT) has been explored as an option. The potential virtue of NACT is that it allows for tumor deposits to decrease in size, or be eliminated, prior to surgery, resulting in a less morbid procedure for the patient.
Observed outcomes for NACT relative to PDS are mixed. When small series have compared the two for the treatment of advanced serous endometrial cancer, NACT was associated with decreased perioperative morbidity, with similar overall survival observed.3,4
However, in larger series exploring patients within the National Cancer Database (a collection of over 1,500 hospitals accredited by the Commission on Cancer) outcomes appear different for the two approaches.5,6 While PDS was initially associated with worse survival, at approximately 5-6 months from diagnosis, this changed and survival was observed to be consistently superior for this group. These data suggest that patients undergoing primary surgical cytoreduction may experience an early mortality risk, possibly secondary to the impact of surgery, but that if they are to survive beyond this point, they experience better outcomes. While the researchers attempted to control for risk factors of poor outcomes that might have systematically differed between the two groups, this specific database is limited in its ability to account for all fundamental differences between them. Only approximately 15% of women with advanced endometrial cancer were offered NACT during those time periods. This observation alone suggests that this likely represents a group specially selected for their poor candidacy for upfront debulking surgery, and inherently increased risk for death from all causes.
The question remains, is NACT appropriate for all patients or just those who are considered poor surgical candidates? Could all patients benefit from the decreased morbidity associated with surgery after NACT without compromising survival? Randomized controlled trials are necessary to answer this question as they are the only way to ensure that risk factors for poor outcomes (such as histology, disease distribution, medical comorbidities) are equally distributed among both groups.
In the meantime, gynecologic oncologists should take a cautious approach to decision making regarding sequencing of surgery and chemotherapy in the setting of a new diagnosis of advanced endometrial cancer. Arguably more important than surgical interventions, access to molecularly targeted systemic therapy is likely to bring the best outcomes for advanced endometrial cancer. Carboplatin and paclitaxel are the current gold standard of care for frontline systemic therapy; however, response rates with this regimen are less favorable for endometrial cancer than for ovarian cancer. Work is being done to test novel therapies against actionable targets to use as alternatives or as adjuncts to traditional chemotherapy regimens. In doing so, clinicians are learning to distinguish endometrial cancers by more than simply their histologic features, but also by their molecular profiles.
Advanced endometrial cancer is a serious disease with high lethality. Future research should focus on ways to ensure toxicities of therapy, including surgery, are minimized while improving upon existing poor clinical outcomes.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no financial disclosures.
References
1. Fader AN et al. J Clin Oncol 2018;36(20):2044-51.
2. Bristow RE et al. Gynecol Oncol 2000;78(2):85-91.
3. Bogani G et al. Tumori 2019;105(1):92-97.
4. Wilkinson-Ryan I et al. Int J Gynecol Cancer. 2015;25(1):63-8.
5. Tobias CJ et al. JAMA Netw Open 2020;3(12):e2028612.
6. Chambers LM et al. Gynecol Oncol 2021;160(2):405-12.
Endometrial cancer is most commonly diagnosed at an early stage. Unfortunately, there is a trend toward the diagnosis of more advanced disease, for which cure is rare, and this is an important contributing factor toward the overall increasing mortality trend for endometrial cancer.
Histology is a major risk factor for advanced disease. For example, serous carcinoma, which accounts for approximately only 10% of all endometrial cancer diagnoses, comprises 25% of cases of advanced cases. Similarly, carcinosarcoma, a cell type known to be particularly aggressive, is relatively overrepresented among cases of advanced disease.
Advanced endometrial cancer includes cases of stage III (involvement of lymph nodes, ovaries, and vagina) and stage IV disease (with direct extension into pelvic viscera and distant metastases). In most cases of stage III disease, extrauterine metastases are microscopic and are detected only at the time of surgical staging. Bulky nodal disease within the pelvic and para-aortic nodal basins is less common but associated with worse prognosis than for patients with microscopic nodal metastases. Stage IV disease usually presents with peritoneal spread of disease including carcinomatosis, omental disease, and involvement of the small and large intestine.
Once advanced, endometrial cancer requires more than surgery alone, relying heavily on adjuvant therapies to achieve responses, particularly systemic therapy with platinum and taxane chemotherapy. In some cases, molecularly targeted therapy (such as trastuzumab for serous carcinomas that demonstrate overexpression of HER2) has been shown to be superior in efficacy.1 Surgery may involve either radical nodal dissections to the infrarenal aortic basin, and/or peritoneal debulking procedures similar to that required for ovarian cancer. Perhaps because of patterns of disease distribution so similar to ovarian cancer, historically, sequencing of therapy focused on radical primary debulking surgery (PDS) followed by chemotherapy.
In 2000, a retrospective series from Johns Hopkins University documented the outcomes of 65 patients with advanced endometrial cancer who had undergone primary debulking surgery followed by chemotherapy.2 They noted that survival was directly associated with degree of cytoreduction, with the best outcomes seen for those patients whose surgery resulted in no gross residual disease. Following these data, PDS with complete resection of all disease became the goal of primary therapy.
However, unlike ovarian cancer (which shares a similar disease distribution with advanced endometrial cancer) patients with endometrial cancer are more obese, older, and typically have more comorbidities. Therefore, radical primary debulking surgeries may be associated with poor patient perioperative outcomes, and feasibility of complete cytoreduction, particularly in very obese patients, can be limited. For this reason, neoadjuvant chemotherapy (NACT) has been explored as an option. The potential virtue of NACT is that it allows for tumor deposits to decrease in size, or be eliminated, prior to surgery, resulting in a less morbid procedure for the patient.
Observed outcomes for NACT relative to PDS are mixed. When small series have compared the two for the treatment of advanced serous endometrial cancer, NACT was associated with decreased perioperative morbidity, with similar overall survival observed.3,4
However, in larger series exploring patients within the National Cancer Database (a collection of over 1,500 hospitals accredited by the Commission on Cancer) outcomes appear different for the two approaches.5,6 While PDS was initially associated with worse survival, at approximately 5-6 months from diagnosis, this changed and survival was observed to be consistently superior for this group. These data suggest that patients undergoing primary surgical cytoreduction may experience an early mortality risk, possibly secondary to the impact of surgery, but that if they are to survive beyond this point, they experience better outcomes. While the researchers attempted to control for risk factors of poor outcomes that might have systematically differed between the two groups, this specific database is limited in its ability to account for all fundamental differences between them. Only approximately 15% of women with advanced endometrial cancer were offered NACT during those time periods. This observation alone suggests that this likely represents a group specially selected for their poor candidacy for upfront debulking surgery, and inherently increased risk for death from all causes.
The question remains, is NACT appropriate for all patients or just those who are considered poor surgical candidates? Could all patients benefit from the decreased morbidity associated with surgery after NACT without compromising survival? Randomized controlled trials are necessary to answer this question as they are the only way to ensure that risk factors for poor outcomes (such as histology, disease distribution, medical comorbidities) are equally distributed among both groups.
In the meantime, gynecologic oncologists should take a cautious approach to decision making regarding sequencing of surgery and chemotherapy in the setting of a new diagnosis of advanced endometrial cancer. Arguably more important than surgical interventions, access to molecularly targeted systemic therapy is likely to bring the best outcomes for advanced endometrial cancer. Carboplatin and paclitaxel are the current gold standard of care for frontline systemic therapy; however, response rates with this regimen are less favorable for endometrial cancer than for ovarian cancer. Work is being done to test novel therapies against actionable targets to use as alternatives or as adjuncts to traditional chemotherapy regimens. In doing so, clinicians are learning to distinguish endometrial cancers by more than simply their histologic features, but also by their molecular profiles.
Advanced endometrial cancer is a serious disease with high lethality. Future research should focus on ways to ensure toxicities of therapy, including surgery, are minimized while improving upon existing poor clinical outcomes.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no financial disclosures.
References
1. Fader AN et al. J Clin Oncol 2018;36(20):2044-51.
2. Bristow RE et al. Gynecol Oncol 2000;78(2):85-91.
3. Bogani G et al. Tumori 2019;105(1):92-97.
4. Wilkinson-Ryan I et al. Int J Gynecol Cancer. 2015;25(1):63-8.
5. Tobias CJ et al. JAMA Netw Open 2020;3(12):e2028612.
6. Chambers LM et al. Gynecol Oncol 2021;160(2):405-12.
Endometrial cancer is most commonly diagnosed at an early stage. Unfortunately, there is a trend toward the diagnosis of more advanced disease, for which cure is rare, and this is an important contributing factor toward the overall increasing mortality trend for endometrial cancer.
Histology is a major risk factor for advanced disease. For example, serous carcinoma, which accounts for approximately only 10% of all endometrial cancer diagnoses, comprises 25% of cases of advanced cases. Similarly, carcinosarcoma, a cell type known to be particularly aggressive, is relatively overrepresented among cases of advanced disease.
Advanced endometrial cancer includes cases of stage III (involvement of lymph nodes, ovaries, and vagina) and stage IV disease (with direct extension into pelvic viscera and distant metastases). In most cases of stage III disease, extrauterine metastases are microscopic and are detected only at the time of surgical staging. Bulky nodal disease within the pelvic and para-aortic nodal basins is less common but associated with worse prognosis than for patients with microscopic nodal metastases. Stage IV disease usually presents with peritoneal spread of disease including carcinomatosis, omental disease, and involvement of the small and large intestine.
Once advanced, endometrial cancer requires more than surgery alone, relying heavily on adjuvant therapies to achieve responses, particularly systemic therapy with platinum and taxane chemotherapy. In some cases, molecularly targeted therapy (such as trastuzumab for serous carcinomas that demonstrate overexpression of HER2) has been shown to be superior in efficacy.1 Surgery may involve either radical nodal dissections to the infrarenal aortic basin, and/or peritoneal debulking procedures similar to that required for ovarian cancer. Perhaps because of patterns of disease distribution so similar to ovarian cancer, historically, sequencing of therapy focused on radical primary debulking surgery (PDS) followed by chemotherapy.
In 2000, a retrospective series from Johns Hopkins University documented the outcomes of 65 patients with advanced endometrial cancer who had undergone primary debulking surgery followed by chemotherapy.2 They noted that survival was directly associated with degree of cytoreduction, with the best outcomes seen for those patients whose surgery resulted in no gross residual disease. Following these data, PDS with complete resection of all disease became the goal of primary therapy.
However, unlike ovarian cancer (which shares a similar disease distribution with advanced endometrial cancer) patients with endometrial cancer are more obese, older, and typically have more comorbidities. Therefore, radical primary debulking surgeries may be associated with poor patient perioperative outcomes, and feasibility of complete cytoreduction, particularly in very obese patients, can be limited. For this reason, neoadjuvant chemotherapy (NACT) has been explored as an option. The potential virtue of NACT is that it allows for tumor deposits to decrease in size, or be eliminated, prior to surgery, resulting in a less morbid procedure for the patient.
Observed outcomes for NACT relative to PDS are mixed. When small series have compared the two for the treatment of advanced serous endometrial cancer, NACT was associated with decreased perioperative morbidity, with similar overall survival observed.3,4
However, in larger series exploring patients within the National Cancer Database (a collection of over 1,500 hospitals accredited by the Commission on Cancer) outcomes appear different for the two approaches.5,6 While PDS was initially associated with worse survival, at approximately 5-6 months from diagnosis, this changed and survival was observed to be consistently superior for this group. These data suggest that patients undergoing primary surgical cytoreduction may experience an early mortality risk, possibly secondary to the impact of surgery, but that if they are to survive beyond this point, they experience better outcomes. While the researchers attempted to control for risk factors of poor outcomes that might have systematically differed between the two groups, this specific database is limited in its ability to account for all fundamental differences between them. Only approximately 15% of women with advanced endometrial cancer were offered NACT during those time periods. This observation alone suggests that this likely represents a group specially selected for their poor candidacy for upfront debulking surgery, and inherently increased risk for death from all causes.
The question remains, is NACT appropriate for all patients or just those who are considered poor surgical candidates? Could all patients benefit from the decreased morbidity associated with surgery after NACT without compromising survival? Randomized controlled trials are necessary to answer this question as they are the only way to ensure that risk factors for poor outcomes (such as histology, disease distribution, medical comorbidities) are equally distributed among both groups.
In the meantime, gynecologic oncologists should take a cautious approach to decision making regarding sequencing of surgery and chemotherapy in the setting of a new diagnosis of advanced endometrial cancer. Arguably more important than surgical interventions, access to molecularly targeted systemic therapy is likely to bring the best outcomes for advanced endometrial cancer. Carboplatin and paclitaxel are the current gold standard of care for frontline systemic therapy; however, response rates with this regimen are less favorable for endometrial cancer than for ovarian cancer. Work is being done to test novel therapies against actionable targets to use as alternatives or as adjuncts to traditional chemotherapy regimens. In doing so, clinicians are learning to distinguish endometrial cancers by more than simply their histologic features, but also by their molecular profiles.
Advanced endometrial cancer is a serious disease with high lethality. Future research should focus on ways to ensure toxicities of therapy, including surgery, are minimized while improving upon existing poor clinical outcomes.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no financial disclosures.
References
1. Fader AN et al. J Clin Oncol 2018;36(20):2044-51.
2. Bristow RE et al. Gynecol Oncol 2000;78(2):85-91.
3. Bogani G et al. Tumori 2019;105(1):92-97.
4. Wilkinson-Ryan I et al. Int J Gynecol Cancer. 2015;25(1):63-8.
5. Tobias CJ et al. JAMA Netw Open 2020;3(12):e2028612.
6. Chambers LM et al. Gynecol Oncol 2021;160(2):405-12.
Recommendations from a gynecologic oncologist to a general ob.gyn., part 2
In this month’s column we continue to discuss recommendations from the gynecologic oncologist to the general gynecologist.
Don’t screen average-risk women for ovarian cancer.
Ovarian cancer is most often diagnosed at an advanced stage, which limits the curability of the disease. Consequently, there is a strong focus on attempting to diagnose the disease at earlier, more curable stages. This leads to the impulse by some well-intentioned providers to implement screening tests, such as ultrasounds and tumor markers, for all women. Unfortunately, the screening of “average risk” women for ovarian cancer is not recommended. Randomized controlled trials of tens of thousands of women have not observed a clinically significant decrease in ovarian cancer mortality with the addition of screening with tumor markers and ultrasound.1 These studies did observe a false-positive rate of 5%. While that may seem like a low rate of false-positive testing, the definitive diagnostic test which follows is a major abdominal surgery (oophorectomy) and serious complications are encountered in 15% of patients undergoing surgery for false-positive ovarian cancer screening.1 Therefore, quite simply, the harms are not balanced by benefits.
The key to offering patients appropriate and effective screening is case selection. It is important to identify which patients are at higher risk for ovarian cancer and offer those women testing for germline mutations and screening strategies. An important component of a well-woman visit is to take a thorough family history of cancer. Women are considered at high risk for having hereditary predisposition to ovarian cancer if they have a first- or second-degree relative with breast cancer younger than 45-50 years, or any age if Ashkenazi Jewish, triple-negative breast cancer younger than 60 years of age, two or more primary breast cancers with the first diagnosed at less than 50 years of age, male breast cancer, ovarian cancer, pancreatic cancer, a known BRCA 1/2 mutation, or a personal history of those same conditions. These women should be recommended to undergo genetic testing for BRCA 1, 2, and Lynch syndrome. They should not automatically be offered ovarian cancer screening. If a patient has a more remote family history for ovarian cancer, their personal risk may be somewhat elevated above the baseline population risk, however, not substantially enough to justify implementing screening in the absence of a confirmed genetic mutation.
While screening tests may not be appropriate for all patients, all patients should be asked about the early symptoms of ovarian cancer because these are consistently present, and frequently overlooked, prior to the eventual diagnosis of advanced disease. Those symptoms include abdominal discomfort, abdominal swelling and bloating, and urinary urgency.2 Consider offering all patients a dedicated ovarian cancer specific review of systems that includes inquiries about these symptoms at their annual wellness visits.
Opt for vertical midline incisions when surgery is anticipated to be complex
What is the first thing gynecologic oncologists do when called in to assist in a difficult gynecologic procedure? Get better exposure. Exposure is the cornerstone of safe, effective surgery. Sometimes this simply means placing a more effective retractor. In other cases, it might mean extending the incision. However, if the incision is a low transverse incision (the go-to for many gynecologists because of its favorable cosmetic and pain-producing profile) this proves to be difficult. Attempting to assist in a complicated case, such as a frozen pelvis, severed ureter or rectal injury, through a pfannensteil incision can be extraordinarily difficult, and while these incisions can be extended by incising the rectus muscle bellies, upper abdominal visualization remains elusive in most patients. This is particularly problematic if the ureter or splenic flexure need to be mobilized, or if extensive lysis of adhesions is necessary to ensure there is no occult enterotomy. As my mentor Dr. John Soper once described to me: “It’s like trying to scratch your armpit by reaching through your fly.”
While pfannensteil incisions come naturally, and comfortably, to most gynecologists, likely because of their frequent application during cesarean section, all gynecologists should be confident in the steps and anatomy for vertical midline, or paramedian incisions. This is not only beneficial for complex gynecologic cases, but also in the event of vascular emergency. In the hands of an experienced abdominal/pelvic surgeon, the vertical midline incision is the quickest way to safely enter the abdomen, and provides the kind of exposure that may be critical in safely repairing or controlling hemorrhage from a major vessel.
While low transverse incisions may be more cosmetic, less painful, and associated with fewer wound complications, our first concern as surgeons should be mitigating complications. In situations where risks of complications are high, it is best to not handicap ourselves with the incision location. And always remember, wound complications are highest when a transverse incision needs to be converted to a vertical one with a “T.”
It’s not just about diagnosis of cancer, it’s also prevention
Detection of cancer is an important role of the obstetrician gynecologist. However, equally important is being able to seize opportunities for cancer prevention. Cervical, vulvar, endometrial and ovarian cancer are all known to have preventative strategies.
All patients up to the age of 45 should be offered vaccination against HPV. Initial indications for HPV vaccination were for women up to age 26; however, recent data support the safety and efficacy of the vaccine in older women.3 HPV vaccination is most effective at preventing cancer when administered prior to exposure (ideally age 9-11), leaving this in the hands of our pediatrician colleagues. However, we must be vigilant to inquire about vaccination status for all our patients and encourage vaccines for those who were missed earlier in their life.
Patients should be counseled regarding the significant risk reduction for cancer that is gained from use of oral hormonal contraceptives and progestin-releasing IUDs (especially for endometrial and ovarian cancers). Providing them with knowledge of this information when considering options for contraception or menstrual cycle management is important in their decision-making process.
Endometrial cancer incidence is sadly on the rise in the United States, likely secondary to increasing rates of obesity. Pregnancy is a time when many women begin to gain, and accumulate, weight and therefore obstetric providers have a unique opportunity to assist patients in strategies to normalize their weight after pregnancy. Many of my patients with endometrial cancer state that they have never heard that it is associated with obesity. This suggests that more can be done to educate patients on the carcinogenic effect of obesity (for both endometrial and breast cancer), which may aid in motivating change of modifiable behaviors.
The fallopian tubes are the source of many ovarian cancers and knowledge of this has led to the recommendation to perform opportunistic salpingectomy as a cancer risk-reducing strategy. Hysterectomy and sterilization procedures are most apropos for this modification. While prospective data to confirm a reduced risk of ovarian cancer with opportunistic salpingectomy are lacking, a reduced incidence of cancer has been observed when the tubes have been removed for indicated surgeries; there appear to be no significant deleterious sequelae.4,5 A focus should be made on removal of the entire distal third of the tube, particularly the fimbriated ends, as this is the portion most implicated in malignancy.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant disclosures. Contact her at [email protected].
References
1. Buys SS et al. JAMA. 2011;305(22):2295.
2. Goff BA et al. JAMA. 2004;291(22):2705.
3. Castellsagué X et al. Br J Cancer. 2011;105(1):28.
4. Yoon SH et al. Eur J Cancer. 2016 Mar;55:38-46.
5. Hanley GE et al. Am J Obstet Gynecol. 2018;219(2):172.
In this month’s column we continue to discuss recommendations from the gynecologic oncologist to the general gynecologist.
Don’t screen average-risk women for ovarian cancer.
Ovarian cancer is most often diagnosed at an advanced stage, which limits the curability of the disease. Consequently, there is a strong focus on attempting to diagnose the disease at earlier, more curable stages. This leads to the impulse by some well-intentioned providers to implement screening tests, such as ultrasounds and tumor markers, for all women. Unfortunately, the screening of “average risk” women for ovarian cancer is not recommended. Randomized controlled trials of tens of thousands of women have not observed a clinically significant decrease in ovarian cancer mortality with the addition of screening with tumor markers and ultrasound.1 These studies did observe a false-positive rate of 5%. While that may seem like a low rate of false-positive testing, the definitive diagnostic test which follows is a major abdominal surgery (oophorectomy) and serious complications are encountered in 15% of patients undergoing surgery for false-positive ovarian cancer screening.1 Therefore, quite simply, the harms are not balanced by benefits.
The key to offering patients appropriate and effective screening is case selection. It is important to identify which patients are at higher risk for ovarian cancer and offer those women testing for germline mutations and screening strategies. An important component of a well-woman visit is to take a thorough family history of cancer. Women are considered at high risk for having hereditary predisposition to ovarian cancer if they have a first- or second-degree relative with breast cancer younger than 45-50 years, or any age if Ashkenazi Jewish, triple-negative breast cancer younger than 60 years of age, two or more primary breast cancers with the first diagnosed at less than 50 years of age, male breast cancer, ovarian cancer, pancreatic cancer, a known BRCA 1/2 mutation, or a personal history of those same conditions. These women should be recommended to undergo genetic testing for BRCA 1, 2, and Lynch syndrome. They should not automatically be offered ovarian cancer screening. If a patient has a more remote family history for ovarian cancer, their personal risk may be somewhat elevated above the baseline population risk, however, not substantially enough to justify implementing screening in the absence of a confirmed genetic mutation.
While screening tests may not be appropriate for all patients, all patients should be asked about the early symptoms of ovarian cancer because these are consistently present, and frequently overlooked, prior to the eventual diagnosis of advanced disease. Those symptoms include abdominal discomfort, abdominal swelling and bloating, and urinary urgency.2 Consider offering all patients a dedicated ovarian cancer specific review of systems that includes inquiries about these symptoms at their annual wellness visits.
Opt for vertical midline incisions when surgery is anticipated to be complex
What is the first thing gynecologic oncologists do when called in to assist in a difficult gynecologic procedure? Get better exposure. Exposure is the cornerstone of safe, effective surgery. Sometimes this simply means placing a more effective retractor. In other cases, it might mean extending the incision. However, if the incision is a low transverse incision (the go-to for many gynecologists because of its favorable cosmetic and pain-producing profile) this proves to be difficult. Attempting to assist in a complicated case, such as a frozen pelvis, severed ureter or rectal injury, through a pfannensteil incision can be extraordinarily difficult, and while these incisions can be extended by incising the rectus muscle bellies, upper abdominal visualization remains elusive in most patients. This is particularly problematic if the ureter or splenic flexure need to be mobilized, or if extensive lysis of adhesions is necessary to ensure there is no occult enterotomy. As my mentor Dr. John Soper once described to me: “It’s like trying to scratch your armpit by reaching through your fly.”
While pfannensteil incisions come naturally, and comfortably, to most gynecologists, likely because of their frequent application during cesarean section, all gynecologists should be confident in the steps and anatomy for vertical midline, or paramedian incisions. This is not only beneficial for complex gynecologic cases, but also in the event of vascular emergency. In the hands of an experienced abdominal/pelvic surgeon, the vertical midline incision is the quickest way to safely enter the abdomen, and provides the kind of exposure that may be critical in safely repairing or controlling hemorrhage from a major vessel.
While low transverse incisions may be more cosmetic, less painful, and associated with fewer wound complications, our first concern as surgeons should be mitigating complications. In situations where risks of complications are high, it is best to not handicap ourselves with the incision location. And always remember, wound complications are highest when a transverse incision needs to be converted to a vertical one with a “T.”
It’s not just about diagnosis of cancer, it’s also prevention
Detection of cancer is an important role of the obstetrician gynecologist. However, equally important is being able to seize opportunities for cancer prevention. Cervical, vulvar, endometrial and ovarian cancer are all known to have preventative strategies.
All patients up to the age of 45 should be offered vaccination against HPV. Initial indications for HPV vaccination were for women up to age 26; however, recent data support the safety and efficacy of the vaccine in older women.3 HPV vaccination is most effective at preventing cancer when administered prior to exposure (ideally age 9-11), leaving this in the hands of our pediatrician colleagues. However, we must be vigilant to inquire about vaccination status for all our patients and encourage vaccines for those who were missed earlier in their life.
Patients should be counseled regarding the significant risk reduction for cancer that is gained from use of oral hormonal contraceptives and progestin-releasing IUDs (especially for endometrial and ovarian cancers). Providing them with knowledge of this information when considering options for contraception or menstrual cycle management is important in their decision-making process.
Endometrial cancer incidence is sadly on the rise in the United States, likely secondary to increasing rates of obesity. Pregnancy is a time when many women begin to gain, and accumulate, weight and therefore obstetric providers have a unique opportunity to assist patients in strategies to normalize their weight after pregnancy. Many of my patients with endometrial cancer state that they have never heard that it is associated with obesity. This suggests that more can be done to educate patients on the carcinogenic effect of obesity (for both endometrial and breast cancer), which may aid in motivating change of modifiable behaviors.
The fallopian tubes are the source of many ovarian cancers and knowledge of this has led to the recommendation to perform opportunistic salpingectomy as a cancer risk-reducing strategy. Hysterectomy and sterilization procedures are most apropos for this modification. While prospective data to confirm a reduced risk of ovarian cancer with opportunistic salpingectomy are lacking, a reduced incidence of cancer has been observed when the tubes have been removed for indicated surgeries; there appear to be no significant deleterious sequelae.4,5 A focus should be made on removal of the entire distal third of the tube, particularly the fimbriated ends, as this is the portion most implicated in malignancy.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant disclosures. Contact her at [email protected].
References
1. Buys SS et al. JAMA. 2011;305(22):2295.
2. Goff BA et al. JAMA. 2004;291(22):2705.
3. Castellsagué X et al. Br J Cancer. 2011;105(1):28.
4. Yoon SH et al. Eur J Cancer. 2016 Mar;55:38-46.
5. Hanley GE et al. Am J Obstet Gynecol. 2018;219(2):172.
In this month’s column we continue to discuss recommendations from the gynecologic oncologist to the general gynecologist.
Don’t screen average-risk women for ovarian cancer.
Ovarian cancer is most often diagnosed at an advanced stage, which limits the curability of the disease. Consequently, there is a strong focus on attempting to diagnose the disease at earlier, more curable stages. This leads to the impulse by some well-intentioned providers to implement screening tests, such as ultrasounds and tumor markers, for all women. Unfortunately, the screening of “average risk” women for ovarian cancer is not recommended. Randomized controlled trials of tens of thousands of women have not observed a clinically significant decrease in ovarian cancer mortality with the addition of screening with tumor markers and ultrasound.1 These studies did observe a false-positive rate of 5%. While that may seem like a low rate of false-positive testing, the definitive diagnostic test which follows is a major abdominal surgery (oophorectomy) and serious complications are encountered in 15% of patients undergoing surgery for false-positive ovarian cancer screening.1 Therefore, quite simply, the harms are not balanced by benefits.
The key to offering patients appropriate and effective screening is case selection. It is important to identify which patients are at higher risk for ovarian cancer and offer those women testing for germline mutations and screening strategies. An important component of a well-woman visit is to take a thorough family history of cancer. Women are considered at high risk for having hereditary predisposition to ovarian cancer if they have a first- or second-degree relative with breast cancer younger than 45-50 years, or any age if Ashkenazi Jewish, triple-negative breast cancer younger than 60 years of age, two or more primary breast cancers with the first diagnosed at less than 50 years of age, male breast cancer, ovarian cancer, pancreatic cancer, a known BRCA 1/2 mutation, or a personal history of those same conditions. These women should be recommended to undergo genetic testing for BRCA 1, 2, and Lynch syndrome. They should not automatically be offered ovarian cancer screening. If a patient has a more remote family history for ovarian cancer, their personal risk may be somewhat elevated above the baseline population risk, however, not substantially enough to justify implementing screening in the absence of a confirmed genetic mutation.
While screening tests may not be appropriate for all patients, all patients should be asked about the early symptoms of ovarian cancer because these are consistently present, and frequently overlooked, prior to the eventual diagnosis of advanced disease. Those symptoms include abdominal discomfort, abdominal swelling and bloating, and urinary urgency.2 Consider offering all patients a dedicated ovarian cancer specific review of systems that includes inquiries about these symptoms at their annual wellness visits.
Opt for vertical midline incisions when surgery is anticipated to be complex
What is the first thing gynecologic oncologists do when called in to assist in a difficult gynecologic procedure? Get better exposure. Exposure is the cornerstone of safe, effective surgery. Sometimes this simply means placing a more effective retractor. In other cases, it might mean extending the incision. However, if the incision is a low transverse incision (the go-to for many gynecologists because of its favorable cosmetic and pain-producing profile) this proves to be difficult. Attempting to assist in a complicated case, such as a frozen pelvis, severed ureter or rectal injury, through a pfannensteil incision can be extraordinarily difficult, and while these incisions can be extended by incising the rectus muscle bellies, upper abdominal visualization remains elusive in most patients. This is particularly problematic if the ureter or splenic flexure need to be mobilized, or if extensive lysis of adhesions is necessary to ensure there is no occult enterotomy. As my mentor Dr. John Soper once described to me: “It’s like trying to scratch your armpit by reaching through your fly.”
While pfannensteil incisions come naturally, and comfortably, to most gynecologists, likely because of their frequent application during cesarean section, all gynecologists should be confident in the steps and anatomy for vertical midline, or paramedian incisions. This is not only beneficial for complex gynecologic cases, but also in the event of vascular emergency. In the hands of an experienced abdominal/pelvic surgeon, the vertical midline incision is the quickest way to safely enter the abdomen, and provides the kind of exposure that may be critical in safely repairing or controlling hemorrhage from a major vessel.
While low transverse incisions may be more cosmetic, less painful, and associated with fewer wound complications, our first concern as surgeons should be mitigating complications. In situations where risks of complications are high, it is best to not handicap ourselves with the incision location. And always remember, wound complications are highest when a transverse incision needs to be converted to a vertical one with a “T.”
It’s not just about diagnosis of cancer, it’s also prevention
Detection of cancer is an important role of the obstetrician gynecologist. However, equally important is being able to seize opportunities for cancer prevention. Cervical, vulvar, endometrial and ovarian cancer are all known to have preventative strategies.
All patients up to the age of 45 should be offered vaccination against HPV. Initial indications for HPV vaccination were for women up to age 26; however, recent data support the safety and efficacy of the vaccine in older women.3 HPV vaccination is most effective at preventing cancer when administered prior to exposure (ideally age 9-11), leaving this in the hands of our pediatrician colleagues. However, we must be vigilant to inquire about vaccination status for all our patients and encourage vaccines for those who were missed earlier in their life.
Patients should be counseled regarding the significant risk reduction for cancer that is gained from use of oral hormonal contraceptives and progestin-releasing IUDs (especially for endometrial and ovarian cancers). Providing them with knowledge of this information when considering options for contraception or menstrual cycle management is important in their decision-making process.
Endometrial cancer incidence is sadly on the rise in the United States, likely secondary to increasing rates of obesity. Pregnancy is a time when many women begin to gain, and accumulate, weight and therefore obstetric providers have a unique opportunity to assist patients in strategies to normalize their weight after pregnancy. Many of my patients with endometrial cancer state that they have never heard that it is associated with obesity. This suggests that more can be done to educate patients on the carcinogenic effect of obesity (for both endometrial and breast cancer), which may aid in motivating change of modifiable behaviors.
The fallopian tubes are the source of many ovarian cancers and knowledge of this has led to the recommendation to perform opportunistic salpingectomy as a cancer risk-reducing strategy. Hysterectomy and sterilization procedures are most apropos for this modification. While prospective data to confirm a reduced risk of ovarian cancer with opportunistic salpingectomy are lacking, a reduced incidence of cancer has been observed when the tubes have been removed for indicated surgeries; there appear to be no significant deleterious sequelae.4,5 A focus should be made on removal of the entire distal third of the tube, particularly the fimbriated ends, as this is the portion most implicated in malignancy.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant disclosures. Contact her at [email protected].
References
1. Buys SS et al. JAMA. 2011;305(22):2295.
2. Goff BA et al. JAMA. 2004;291(22):2705.
3. Castellsagué X et al. Br J Cancer. 2011;105(1):28.
4. Yoon SH et al. Eur J Cancer. 2016 Mar;55:38-46.
5. Hanley GE et al. Am J Obstet Gynecol. 2018;219(2):172.
Decision making regarding LEEP versus cone biopsy for excision of cervical dysplasia
Loop electrosurgical excision procedure (LEEP) or cold knife conization of the cervix (CKC) is the standard of care approach for women with cervical intra-epithelial neoplasia (CIN 3) because it achieves both disease control and diagnostic evaluation to rule out invasive carcinoma. While both techniques are associated with equivalent efficacy in disease control, each has its virtues and advantages, and clinical judgment is necessary when choosing a technique.1
LEEP, or large loop electrosurgical excision of the transformation zone (LLETZ) involves use of electrosurgical current directed through wire loops to excise pieces of cervical tissue. The equipment for this technique is widely available and this procedure can most often be performed safely and comfortably in an outpatient office setting, making it a cost-effective strategy. Its ease of access means that it can be employed in “see-and-treat” programs where there is concern regarding follow-up. The loop from the device has a tendency to take more shallow pieces of tissue, preserving more cervical stroma. This may be why LEEP has been associated with decreased risk for obstetric complications associated with cervical insufficiency when compared with CKC.2,3
The shallowness and standardized, preset shapes of the loops present challenges with this technique. It can be more difficult to tailor the shape of the excision for particular lesions, and surgeons may need to add a second “top hat” endocervical LEEP after the first ectocervical excision to adequately excise the endocervical canal. If the “coagulation” setting is used instead of “blend” or “cut,” excessive drag and resistance can develop during the procedure, which can result in the specimen’s being amputated, fragmented, or interrupted mid-sweep. This can severely limit pathologic interpretation of the specimen. Orienting these multiple fragments for pathology to specify margin status can be limited or impossible. Electrosurgical effect (“thermal effect”) at the margins of the specimen can limit accurate interpretation of adequacy of the excision.
CKC of the cervix is a procedure in which a narrow scalpel (typically an 11-blade) is used to excise the ecto- and endocervical tissues in a cone-shaped specimen that ensures maximal inclusion of ectocervical and endocervical mucosa but minimization of stromal excision. Absence of electrosurgery in the primary excision means that pathologists have clean edges to evaluate for margin status. Because the shape of the incision is unique for each patient, the surgeon can tailor the shape and extent of the cone to focus on known or suspected areas of disease. It is particularly useful when there is an endocervical lesion, such as in cases of adenocarcinoma in situ and in postmenopausal women whose transformation zone is frequently within the canal. In cases of a distorted, atrophic cervix, or one that is flush with the vagina, a conization procedure in the operating room affords surgeons greater control and precision. Major limitations of this procedure are that it is typically performed in an operating room setting because of the potential for intraoperative bleeding, and its increased risk for early and late complications. The conization procedure is associated with increased obstetric risk in later pregnancies, possibly because of more significant disturbance of cervical stroma.2,3
As mentioned earlier, both procedures are associated with equivalent outcomes with respect to control of disease.1 CKC procedures are associated with more complications, including bleeding (intraoperatively and postoperatively) than are LEEPs. Traditionally, adenocarcinoma in situ (AIS) has been preferentially treated with CKC because of the propensity of this lesion to reside within the endocervical canal, a region more readily and extensively sampled with the CKC. However, provided that the LEEP specimen achieves negative margin status, there is no specific benefit of CKC over LEEP. Guidelines recommend that AIS is excised as a single specimen (without a “top hat”) to achieve accurate pathology regarding margins in the endocervical canal.4 Considering that a specimen depth between 10 and 20 mm is ideal in the setting of AIS, it may be difficult to achieve this depth with a single-pass LEEP depending upon the dimensions of the cervix. It is due to these technical challenges associated with LEEP that CKC is typically preferred in the treatment of AIS.
Ultimately, the decision regarding when to choose LEEP versus CKC is nuanced and should be tailored for each patient. Factors to consider include the patient’s ease of follow-up, financial limitations, preexisting distortion of anatomy, and the need to minimize obstetrics risks or achieve wider margins. For example, a young, nulliparous patient with an ectocervical lesion of squamous dysplasia would likely best be served by a LEEP, which preserves her cervical stroma and affords her easy access and affordability of the procedure. A patient with a bleeding diathesis including iatrogenic anticoagulant therapy may also benefit from a LEEP to achieve better hemostasis and lower risk of bleeding complications.
A postmenopausal woman with a narrow upper vagina and cervix flush with the vagina from prior excisional procedures may benefit from a conization in the operating room where adequate retraction and exposure can minimize the risk of damage to adjacent structures, and the shape and size of the excision can be tailored to the long, narrow segment that is indicated. The table highlights some of the factors to consider when choosing these options. 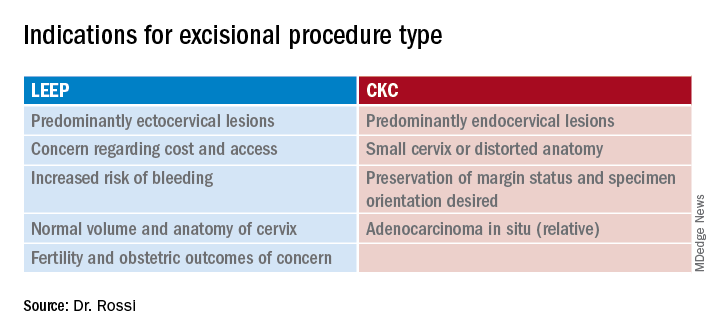
In summary, LEEP and CKC are both highly effective excisional procedures that can be considered for all patients with cervical dysplasia. Decisions regarding which is preferred for patients are nuanced and should consider individualized anatomic, pathologic, functional and financial implications.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest. Contact her at [email protected].
References
1. Martin-Hirsch PL et al. Cochrane Database Syst Rev 2000;(2):CD001318.
2. Arbyn M et al. BMJ. 2008;337:a1284.
3. Jin G et al. Arch Gynecol Obstet. 2014 Jan;289(1):85-99.
4. Perkins RB et al. J Low Genit Tract Dis. 2020;24(2):102.
Loop electrosurgical excision procedure (LEEP) or cold knife conization of the cervix (CKC) is the standard of care approach for women with cervical intra-epithelial neoplasia (CIN 3) because it achieves both disease control and diagnostic evaluation to rule out invasive carcinoma. While both techniques are associated with equivalent efficacy in disease control, each has its virtues and advantages, and clinical judgment is necessary when choosing a technique.1
LEEP, or large loop electrosurgical excision of the transformation zone (LLETZ) involves use of electrosurgical current directed through wire loops to excise pieces of cervical tissue. The equipment for this technique is widely available and this procedure can most often be performed safely and comfortably in an outpatient office setting, making it a cost-effective strategy. Its ease of access means that it can be employed in “see-and-treat” programs where there is concern regarding follow-up. The loop from the device has a tendency to take more shallow pieces of tissue, preserving more cervical stroma. This may be why LEEP has been associated with decreased risk for obstetric complications associated with cervical insufficiency when compared with CKC.2,3
The shallowness and standardized, preset shapes of the loops present challenges with this technique. It can be more difficult to tailor the shape of the excision for particular lesions, and surgeons may need to add a second “top hat” endocervical LEEP after the first ectocervical excision to adequately excise the endocervical canal. If the “coagulation” setting is used instead of “blend” or “cut,” excessive drag and resistance can develop during the procedure, which can result in the specimen’s being amputated, fragmented, or interrupted mid-sweep. This can severely limit pathologic interpretation of the specimen. Orienting these multiple fragments for pathology to specify margin status can be limited or impossible. Electrosurgical effect (“thermal effect”) at the margins of the specimen can limit accurate interpretation of adequacy of the excision.
CKC of the cervix is a procedure in which a narrow scalpel (typically an 11-blade) is used to excise the ecto- and endocervical tissues in a cone-shaped specimen that ensures maximal inclusion of ectocervical and endocervical mucosa but minimization of stromal excision. Absence of electrosurgery in the primary excision means that pathologists have clean edges to evaluate for margin status. Because the shape of the incision is unique for each patient, the surgeon can tailor the shape and extent of the cone to focus on known or suspected areas of disease. It is particularly useful when there is an endocervical lesion, such as in cases of adenocarcinoma in situ and in postmenopausal women whose transformation zone is frequently within the canal. In cases of a distorted, atrophic cervix, or one that is flush with the vagina, a conization procedure in the operating room affords surgeons greater control and precision. Major limitations of this procedure are that it is typically performed in an operating room setting because of the potential for intraoperative bleeding, and its increased risk for early and late complications. The conization procedure is associated with increased obstetric risk in later pregnancies, possibly because of more significant disturbance of cervical stroma.2,3
As mentioned earlier, both procedures are associated with equivalent outcomes with respect to control of disease.1 CKC procedures are associated with more complications, including bleeding (intraoperatively and postoperatively) than are LEEPs. Traditionally, adenocarcinoma in situ (AIS) has been preferentially treated with CKC because of the propensity of this lesion to reside within the endocervical canal, a region more readily and extensively sampled with the CKC. However, provided that the LEEP specimen achieves negative margin status, there is no specific benefit of CKC over LEEP. Guidelines recommend that AIS is excised as a single specimen (without a “top hat”) to achieve accurate pathology regarding margins in the endocervical canal.4 Considering that a specimen depth between 10 and 20 mm is ideal in the setting of AIS, it may be difficult to achieve this depth with a single-pass LEEP depending upon the dimensions of the cervix. It is due to these technical challenges associated with LEEP that CKC is typically preferred in the treatment of AIS.
Ultimately, the decision regarding when to choose LEEP versus CKC is nuanced and should be tailored for each patient. Factors to consider include the patient’s ease of follow-up, financial limitations, preexisting distortion of anatomy, and the need to minimize obstetrics risks or achieve wider margins. For example, a young, nulliparous patient with an ectocervical lesion of squamous dysplasia would likely best be served by a LEEP, which preserves her cervical stroma and affords her easy access and affordability of the procedure. A patient with a bleeding diathesis including iatrogenic anticoagulant therapy may also benefit from a LEEP to achieve better hemostasis and lower risk of bleeding complications.
A postmenopausal woman with a narrow upper vagina and cervix flush with the vagina from prior excisional procedures may benefit from a conization in the operating room where adequate retraction and exposure can minimize the risk of damage to adjacent structures, and the shape and size of the excision can be tailored to the long, narrow segment that is indicated. The table highlights some of the factors to consider when choosing these options. 
In summary, LEEP and CKC are both highly effective excisional procedures that can be considered for all patients with cervical dysplasia. Decisions regarding which is preferred for patients are nuanced and should consider individualized anatomic, pathologic, functional and financial implications.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest. Contact her at [email protected].
References
1. Martin-Hirsch PL et al. Cochrane Database Syst Rev 2000;(2):CD001318.
2. Arbyn M et al. BMJ. 2008;337:a1284.
3. Jin G et al. Arch Gynecol Obstet. 2014 Jan;289(1):85-99.
4. Perkins RB et al. J Low Genit Tract Dis. 2020;24(2):102.
Loop electrosurgical excision procedure (LEEP) or cold knife conization of the cervix (CKC) is the standard of care approach for women with cervical intra-epithelial neoplasia (CIN 3) because it achieves both disease control and diagnostic evaluation to rule out invasive carcinoma. While both techniques are associated with equivalent efficacy in disease control, each has its virtues and advantages, and clinical judgment is necessary when choosing a technique.1
LEEP, or large loop electrosurgical excision of the transformation zone (LLETZ) involves use of electrosurgical current directed through wire loops to excise pieces of cervical tissue. The equipment for this technique is widely available and this procedure can most often be performed safely and comfortably in an outpatient office setting, making it a cost-effective strategy. Its ease of access means that it can be employed in “see-and-treat” programs where there is concern regarding follow-up. The loop from the device has a tendency to take more shallow pieces of tissue, preserving more cervical stroma. This may be why LEEP has been associated with decreased risk for obstetric complications associated with cervical insufficiency when compared with CKC.2,3
The shallowness and standardized, preset shapes of the loops present challenges with this technique. It can be more difficult to tailor the shape of the excision for particular lesions, and surgeons may need to add a second “top hat” endocervical LEEP after the first ectocervical excision to adequately excise the endocervical canal. If the “coagulation” setting is used instead of “blend” or “cut,” excessive drag and resistance can develop during the procedure, which can result in the specimen’s being amputated, fragmented, or interrupted mid-sweep. This can severely limit pathologic interpretation of the specimen. Orienting these multiple fragments for pathology to specify margin status can be limited or impossible. Electrosurgical effect (“thermal effect”) at the margins of the specimen can limit accurate interpretation of adequacy of the excision.
CKC of the cervix is a procedure in which a narrow scalpel (typically an 11-blade) is used to excise the ecto- and endocervical tissues in a cone-shaped specimen that ensures maximal inclusion of ectocervical and endocervical mucosa but minimization of stromal excision. Absence of electrosurgery in the primary excision means that pathologists have clean edges to evaluate for margin status. Because the shape of the incision is unique for each patient, the surgeon can tailor the shape and extent of the cone to focus on known or suspected areas of disease. It is particularly useful when there is an endocervical lesion, such as in cases of adenocarcinoma in situ and in postmenopausal women whose transformation zone is frequently within the canal. In cases of a distorted, atrophic cervix, or one that is flush with the vagina, a conization procedure in the operating room affords surgeons greater control and precision. Major limitations of this procedure are that it is typically performed in an operating room setting because of the potential for intraoperative bleeding, and its increased risk for early and late complications. The conization procedure is associated with increased obstetric risk in later pregnancies, possibly because of more significant disturbance of cervical stroma.2,3
As mentioned earlier, both procedures are associated with equivalent outcomes with respect to control of disease.1 CKC procedures are associated with more complications, including bleeding (intraoperatively and postoperatively) than are LEEPs. Traditionally, adenocarcinoma in situ (AIS) has been preferentially treated with CKC because of the propensity of this lesion to reside within the endocervical canal, a region more readily and extensively sampled with the CKC. However, provided that the LEEP specimen achieves negative margin status, there is no specific benefit of CKC over LEEP. Guidelines recommend that AIS is excised as a single specimen (without a “top hat”) to achieve accurate pathology regarding margins in the endocervical canal.4 Considering that a specimen depth between 10 and 20 mm is ideal in the setting of AIS, it may be difficult to achieve this depth with a single-pass LEEP depending upon the dimensions of the cervix. It is due to these technical challenges associated with LEEP that CKC is typically preferred in the treatment of AIS.
Ultimately, the decision regarding when to choose LEEP versus CKC is nuanced and should be tailored for each patient. Factors to consider include the patient’s ease of follow-up, financial limitations, preexisting distortion of anatomy, and the need to minimize obstetrics risks or achieve wider margins. For example, a young, nulliparous patient with an ectocervical lesion of squamous dysplasia would likely best be served by a LEEP, which preserves her cervical stroma and affords her easy access and affordability of the procedure. A patient with a bleeding diathesis including iatrogenic anticoagulant therapy may also benefit from a LEEP to achieve better hemostasis and lower risk of bleeding complications.
A postmenopausal woman with a narrow upper vagina and cervix flush with the vagina from prior excisional procedures may benefit from a conization in the operating room where adequate retraction and exposure can minimize the risk of damage to adjacent structures, and the shape and size of the excision can be tailored to the long, narrow segment that is indicated. The table highlights some of the factors to consider when choosing these options. 
In summary, LEEP and CKC are both highly effective excisional procedures that can be considered for all patients with cervical dysplasia. Decisions regarding which is preferred for patients are nuanced and should consider individualized anatomic, pathologic, functional and financial implications.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest. Contact her at [email protected].
References
1. Martin-Hirsch PL et al. Cochrane Database Syst Rev 2000;(2):CD001318.
2. Arbyn M et al. BMJ. 2008;337:a1284.
3. Jin G et al. Arch Gynecol Obstet. 2014 Jan;289(1):85-99.
4. Perkins RB et al. J Low Genit Tract Dis. 2020;24(2):102.
Endometriosis-associated ovarian cancer
Endometriosis, which affects 1 in 10 women, is one of the most common conditions that gynecologists treat. It is known to cause pain, pelvic adhesive disease, endometriotic cyst formation, and infertility. However, even more sinister, it also increases a woman’s risk for the development of epithelial ovarian cancer (known as endometriosis-associated ovarian cancer or EAOC). A woman with endometriosis has a two- to threefold increased risk of developing epithelial ovarian cancer, compared with nonaffected women.1 This risk appears to be concentrated in the premenopausal age group, particularly the fifth decade of life. After menopause their risk of developing cancer returns to a baseline level.
EAOC classically presents as clear cell or endometrioid adenocarcinomas, rather than high-grade serous carcinomas. However, low-grade serous carcinomas are also frequently observed in this cohort.2,3 Unlike high-grade serous carcinoma, EAOC is more likely to be diagnosed at an early stage, with the majority at stage I or II, and prognosis is better. After matching for age and stage with cases of high-grade serous carcinoma, there is improved disease-free and overall survival observed among cases of EAOC of clear cell and endometrioid histologic cell types.4 The phenomenon of dual primaries (synchronous endometrial and ovarian cancer) occurs more frequently in EAOC than it does in patients with nonendometriosis-related high-grade serous cancer (25% vs. 4%).
The genomics of these endometriosis-associated cancers are quite distinct. Similar to benign endometriosis implants, EAOC is associated with genomic mutations in ARID1A, PIK3CA, and PTEN, as well as progesterone resistance.1,2 Multiple studies have shown that the adjacent eutopic endometrium carries similar gene mutations as those found in both benign endometriotic implants and EAOC.2 This may explain the higher incidence (twofold) of endometrial cancer in patients with endometriosis as well as the increased incidence of dual ovarian and endometrial cancer primaries.
Just as there are multiple theories regarding the mechanism of benign endometriosis, we have theories rather than conclusions regarding the origins of EAOC. One such theory is that it develops from malignant transformation in an existing endometriotic cyst.5 Endometriotic cysts provide an iron-rich environment which promotes reactive oxygen species that promote carcinogenesis by inducing gene mutations and epigenetic alterations. However, if prolonged exposure to oxidative stress within endometriotic cysts were to be the cause for EAOC, we would expect to see a progressively increasing incidence of ovarian cancer over time in patients with expectantly managed cysts. However, in cases of expectant management, an initial, early, increased risk for cancer within the first 5 years is followed by a subsequent decreasing incidence over time.6 This early incidence spike suggests that some endometriotic cysts may have been misclassified as benign, then rapidly declare themselves as malignant during the observation period rather than a transformation into malignancy from a benign endometrioma over time.
An alternative, and favored, theory for the origins of EAOC are that endometrial cells with carcinogenic genomic alterations reflux through the fallopian tubes during menstruation and settle onto the ovarian epithelium which itself is damaged from recent ovulation thus providing an environment that is highly suitable for oncogenesis.2 Genomic analyses of both the eutopic endometrium and malignant cells in patients with EAOC have shown that both tissues contain the same genomic alterations.1 Given that menstruation, including retrograde menstruation, ends after menopause, this mechanism supports the observation that EAOC is predominantly a malignancy of premenopausal women. Additionally, salpingectomy and hysterectomy confers a protective effect on the development of EAOC, theoretically by preventing the retrograde transfer of these mutant progenitor endometrial cells. Furthermore, the factors that increase the number of menstrual cycles (such as an early age of menarche and delayed or nonchildbearing states) increases the risk for EAOC and factors that inhibit menstruation, such as oral contraceptive pill use, appear to decrease its risk.
EAOC most commonly arises in the ovary, and not in the deep endometriosis implants of adjacent pelvic structures (such as the anterior and posterior cul de sac and pelvic peritoneum). It is suggested that the ovary itself provides a uniquely favorable environment for carcinogenesis. As stated above, it is hypothesized that refluxed endometrial cells, carrying important progenitor mutations, may become trapped in the tissues of traumatized ovarian epithelium, ripe with inflammatory changes, post ovulation.2 This microenvironment may promote the development of malignancy.
Given these theories and their supporting evidence, how can we attempt to reduce the incidence of this cancer for our patients with endometriosis? Despite their increased risk for ovarian and endometrial cancers, current recommendations do not support routine cancer screening in women with endometriosis.7 However, risk-mitigation strategies can still be pursued. Hormonal contraceptives to decrease ovulation and menstrual cycling are protective against ovarian cancer and are also helpful in mitigating the symptoms of endometriosis. While removal of endometriotic cysts may not, in and of itself, be a strategy to prevent EAOC, it is still generally recommended because these cysts are commonly a source of pain and infertility. While they do not appear to undergo malignant transformation, it can be difficult to definitively rule out an early ovarian cancer in these complex ovarian cysts, particularly as they are often associated with tumor marker abnormalities such as elevations in CA 125. Therefore, if surgical excision of an endometriotic cyst is not performed, it should be closely followed for at least 5 years to ensure it is a benign structure. If surgery is pursued and ovarian preservation is desired, removal of the fallopian tubes and uterus can help mitigate the risk for EAOC.8
Endometriosis is a morbid condition for many young women. In addition to causing pain and infertility it increases a woman’s risk for ovarian and endometrial cancer, particularly ovarian clear cell, endometrioid, and low-grade serous cancers and synchronous endometrial and ovarian cancers. Endometriotic cysts should be removed or closely monitored, and clinicians should discuss treatment options that minimize frequency of ovulation and menstruation events as a preventative strategy.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Endocrinology. 2019;160(3):626-38.
2. Cancers. 2020;12(6):1676.
3. Lancet Oncol. 2012;13:385-94.
4. Gynecol Oncol. 2014;132(3):760-6.
5. Redox Rep. 2016;21:119-26.
6. Int. J Clin Oncol. 2020;25:51-8.
7. Hum Reprod. 2013;28:1552-68.
8. J Natl Cancer Inst. 2019;111:1097-103.
Endometriosis, which affects 1 in 10 women, is one of the most common conditions that gynecologists treat. It is known to cause pain, pelvic adhesive disease, endometriotic cyst formation, and infertility. However, even more sinister, it also increases a woman’s risk for the development of epithelial ovarian cancer (known as endometriosis-associated ovarian cancer or EAOC). A woman with endometriosis has a two- to threefold increased risk of developing epithelial ovarian cancer, compared with nonaffected women.1 This risk appears to be concentrated in the premenopausal age group, particularly the fifth decade of life. After menopause their risk of developing cancer returns to a baseline level.
EAOC classically presents as clear cell or endometrioid adenocarcinomas, rather than high-grade serous carcinomas. However, low-grade serous carcinomas are also frequently observed in this cohort.2,3 Unlike high-grade serous carcinoma, EAOC is more likely to be diagnosed at an early stage, with the majority at stage I or II, and prognosis is better. After matching for age and stage with cases of high-grade serous carcinoma, there is improved disease-free and overall survival observed among cases of EAOC of clear cell and endometrioid histologic cell types.4 The phenomenon of dual primaries (synchronous endometrial and ovarian cancer) occurs more frequently in EAOC than it does in patients with nonendometriosis-related high-grade serous cancer (25% vs. 4%).
The genomics of these endometriosis-associated cancers are quite distinct. Similar to benign endometriosis implants, EAOC is associated with genomic mutations in ARID1A, PIK3CA, and PTEN, as well as progesterone resistance.1,2 Multiple studies have shown that the adjacent eutopic endometrium carries similar gene mutations as those found in both benign endometriotic implants and EAOC.2 This may explain the higher incidence (twofold) of endometrial cancer in patients with endometriosis as well as the increased incidence of dual ovarian and endometrial cancer primaries.
Just as there are multiple theories regarding the mechanism of benign endometriosis, we have theories rather than conclusions regarding the origins of EAOC. One such theory is that it develops from malignant transformation in an existing endometriotic cyst.5 Endometriotic cysts provide an iron-rich environment which promotes reactive oxygen species that promote carcinogenesis by inducing gene mutations and epigenetic alterations. However, if prolonged exposure to oxidative stress within endometriotic cysts were to be the cause for EAOC, we would expect to see a progressively increasing incidence of ovarian cancer over time in patients with expectantly managed cysts. However, in cases of expectant management, an initial, early, increased risk for cancer within the first 5 years is followed by a subsequent decreasing incidence over time.6 This early incidence spike suggests that some endometriotic cysts may have been misclassified as benign, then rapidly declare themselves as malignant during the observation period rather than a transformation into malignancy from a benign endometrioma over time.
An alternative, and favored, theory for the origins of EAOC are that endometrial cells with carcinogenic genomic alterations reflux through the fallopian tubes during menstruation and settle onto the ovarian epithelium which itself is damaged from recent ovulation thus providing an environment that is highly suitable for oncogenesis.2 Genomic analyses of both the eutopic endometrium and malignant cells in patients with EAOC have shown that both tissues contain the same genomic alterations.1 Given that menstruation, including retrograde menstruation, ends after menopause, this mechanism supports the observation that EAOC is predominantly a malignancy of premenopausal women. Additionally, salpingectomy and hysterectomy confers a protective effect on the development of EAOC, theoretically by preventing the retrograde transfer of these mutant progenitor endometrial cells. Furthermore, the factors that increase the number of menstrual cycles (such as an early age of menarche and delayed or nonchildbearing states) increases the risk for EAOC and factors that inhibit menstruation, such as oral contraceptive pill use, appear to decrease its risk.
EAOC most commonly arises in the ovary, and not in the deep endometriosis implants of adjacent pelvic structures (such as the anterior and posterior cul de sac and pelvic peritoneum). It is suggested that the ovary itself provides a uniquely favorable environment for carcinogenesis. As stated above, it is hypothesized that refluxed endometrial cells, carrying important progenitor mutations, may become trapped in the tissues of traumatized ovarian epithelium, ripe with inflammatory changes, post ovulation.2 This microenvironment may promote the development of malignancy.
Given these theories and their supporting evidence, how can we attempt to reduce the incidence of this cancer for our patients with endometriosis? Despite their increased risk for ovarian and endometrial cancers, current recommendations do not support routine cancer screening in women with endometriosis.7 However, risk-mitigation strategies can still be pursued. Hormonal contraceptives to decrease ovulation and menstrual cycling are protective against ovarian cancer and are also helpful in mitigating the symptoms of endometriosis. While removal of endometriotic cysts may not, in and of itself, be a strategy to prevent EAOC, it is still generally recommended because these cysts are commonly a source of pain and infertility. While they do not appear to undergo malignant transformation, it can be difficult to definitively rule out an early ovarian cancer in these complex ovarian cysts, particularly as they are often associated with tumor marker abnormalities such as elevations in CA 125. Therefore, if surgical excision of an endometriotic cyst is not performed, it should be closely followed for at least 5 years to ensure it is a benign structure. If surgery is pursued and ovarian preservation is desired, removal of the fallopian tubes and uterus can help mitigate the risk for EAOC.8
Endometriosis is a morbid condition for many young women. In addition to causing pain and infertility it increases a woman’s risk for ovarian and endometrial cancer, particularly ovarian clear cell, endometrioid, and low-grade serous cancers and synchronous endometrial and ovarian cancers. Endometriotic cysts should be removed or closely monitored, and clinicians should discuss treatment options that minimize frequency of ovulation and menstruation events as a preventative strategy.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Endocrinology. 2019;160(3):626-38.
2. Cancers. 2020;12(6):1676.
3. Lancet Oncol. 2012;13:385-94.
4. Gynecol Oncol. 2014;132(3):760-6.
5. Redox Rep. 2016;21:119-26.
6. Int. J Clin Oncol. 2020;25:51-8.
7. Hum Reprod. 2013;28:1552-68.
8. J Natl Cancer Inst. 2019;111:1097-103.
Endometriosis, which affects 1 in 10 women, is one of the most common conditions that gynecologists treat. It is known to cause pain, pelvic adhesive disease, endometriotic cyst formation, and infertility. However, even more sinister, it also increases a woman’s risk for the development of epithelial ovarian cancer (known as endometriosis-associated ovarian cancer or EAOC). A woman with endometriosis has a two- to threefold increased risk of developing epithelial ovarian cancer, compared with nonaffected women.1 This risk appears to be concentrated in the premenopausal age group, particularly the fifth decade of life. After menopause their risk of developing cancer returns to a baseline level.
EAOC classically presents as clear cell or endometrioid adenocarcinomas, rather than high-grade serous carcinomas. However, low-grade serous carcinomas are also frequently observed in this cohort.2,3 Unlike high-grade serous carcinoma, EAOC is more likely to be diagnosed at an early stage, with the majority at stage I or II, and prognosis is better. After matching for age and stage with cases of high-grade serous carcinoma, there is improved disease-free and overall survival observed among cases of EAOC of clear cell and endometrioid histologic cell types.4 The phenomenon of dual primaries (synchronous endometrial and ovarian cancer) occurs more frequently in EAOC than it does in patients with nonendometriosis-related high-grade serous cancer (25% vs. 4%).
The genomics of these endometriosis-associated cancers are quite distinct. Similar to benign endometriosis implants, EAOC is associated with genomic mutations in ARID1A, PIK3CA, and PTEN, as well as progesterone resistance.1,2 Multiple studies have shown that the adjacent eutopic endometrium carries similar gene mutations as those found in both benign endometriotic implants and EAOC.2 This may explain the higher incidence (twofold) of endometrial cancer in patients with endometriosis as well as the increased incidence of dual ovarian and endometrial cancer primaries.
Just as there are multiple theories regarding the mechanism of benign endometriosis, we have theories rather than conclusions regarding the origins of EAOC. One such theory is that it develops from malignant transformation in an existing endometriotic cyst.5 Endometriotic cysts provide an iron-rich environment which promotes reactive oxygen species that promote carcinogenesis by inducing gene mutations and epigenetic alterations. However, if prolonged exposure to oxidative stress within endometriotic cysts were to be the cause for EAOC, we would expect to see a progressively increasing incidence of ovarian cancer over time in patients with expectantly managed cysts. However, in cases of expectant management, an initial, early, increased risk for cancer within the first 5 years is followed by a subsequent decreasing incidence over time.6 This early incidence spike suggests that some endometriotic cysts may have been misclassified as benign, then rapidly declare themselves as malignant during the observation period rather than a transformation into malignancy from a benign endometrioma over time.
An alternative, and favored, theory for the origins of EAOC are that endometrial cells with carcinogenic genomic alterations reflux through the fallopian tubes during menstruation and settle onto the ovarian epithelium which itself is damaged from recent ovulation thus providing an environment that is highly suitable for oncogenesis.2 Genomic analyses of both the eutopic endometrium and malignant cells in patients with EAOC have shown that both tissues contain the same genomic alterations.1 Given that menstruation, including retrograde menstruation, ends after menopause, this mechanism supports the observation that EAOC is predominantly a malignancy of premenopausal women. Additionally, salpingectomy and hysterectomy confers a protective effect on the development of EAOC, theoretically by preventing the retrograde transfer of these mutant progenitor endometrial cells. Furthermore, the factors that increase the number of menstrual cycles (such as an early age of menarche and delayed or nonchildbearing states) increases the risk for EAOC and factors that inhibit menstruation, such as oral contraceptive pill use, appear to decrease its risk.
EAOC most commonly arises in the ovary, and not in the deep endometriosis implants of adjacent pelvic structures (such as the anterior and posterior cul de sac and pelvic peritoneum). It is suggested that the ovary itself provides a uniquely favorable environment for carcinogenesis. As stated above, it is hypothesized that refluxed endometrial cells, carrying important progenitor mutations, may become trapped in the tissues of traumatized ovarian epithelium, ripe with inflammatory changes, post ovulation.2 This microenvironment may promote the development of malignancy.
Given these theories and their supporting evidence, how can we attempt to reduce the incidence of this cancer for our patients with endometriosis? Despite their increased risk for ovarian and endometrial cancers, current recommendations do not support routine cancer screening in women with endometriosis.7 However, risk-mitigation strategies can still be pursued. Hormonal contraceptives to decrease ovulation and menstrual cycling are protective against ovarian cancer and are also helpful in mitigating the symptoms of endometriosis. While removal of endometriotic cysts may not, in and of itself, be a strategy to prevent EAOC, it is still generally recommended because these cysts are commonly a source of pain and infertility. While they do not appear to undergo malignant transformation, it can be difficult to definitively rule out an early ovarian cancer in these complex ovarian cysts, particularly as they are often associated with tumor marker abnormalities such as elevations in CA 125. Therefore, if surgical excision of an endometriotic cyst is not performed, it should be closely followed for at least 5 years to ensure it is a benign structure. If surgery is pursued and ovarian preservation is desired, removal of the fallopian tubes and uterus can help mitigate the risk for EAOC.8
Endometriosis is a morbid condition for many young women. In addition to causing pain and infertility it increases a woman’s risk for ovarian and endometrial cancer, particularly ovarian clear cell, endometrioid, and low-grade serous cancers and synchronous endometrial and ovarian cancers. Endometriotic cysts should be removed or closely monitored, and clinicians should discuss treatment options that minimize frequency of ovulation and menstruation events as a preventative strategy.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Endocrinology. 2019;160(3):626-38.
2. Cancers. 2020;12(6):1676.
3. Lancet Oncol. 2012;13:385-94.
4. Gynecol Oncol. 2014;132(3):760-6.
5. Redox Rep. 2016;21:119-26.
6. Int. J Clin Oncol. 2020;25:51-8.
7. Hum Reprod. 2013;28:1552-68.
8. J Natl Cancer Inst. 2019;111:1097-103.
Intraoperative rupture of ovarian cancer: Does it worsen outcomes?
Intact removal of an ovarian cyst is a well-established gynecologic surgical principle because ovarian cancer is definitively diagnosed only in retrospect (after ovarian extraction) and intraoperative cyst rupture upstages an otherwise nonmetastatic cancer to stage IC. This lumps cancers that are ruptured during surgical extraction together with those that have spontaneously ruptured or have surface excrescences. The theoretical rationale for this “lumping” is that contact between malignant cells from the ruptured cyst may take hold on peritoneal surfaces resulting in development of metastases. To offset this theoretical risk, it has been recommended that all stage IC ovarian cancer is treated with chemotherapy, whereas low-grade stage IA and IB cancers generally are not. No conscientious surgeon wants their surgical intervention to be the cause of a patient needing toxic chemotherapy. But is the contact between malignant cyst fluid and the peritoneum truly as bad as a spontaneous breach of the surface of the tumor? Or is cyst rupture a confounder for other adverse prognostic features, such as histologic cell type and dense pelvic attachments? If ovarian cyst rupture is an independent risk factor for patients with stage I ovarian cancer, strategies should be employed to avoid this occurrence, and we should understand how to counsel and treat patients in whom this has occurred.
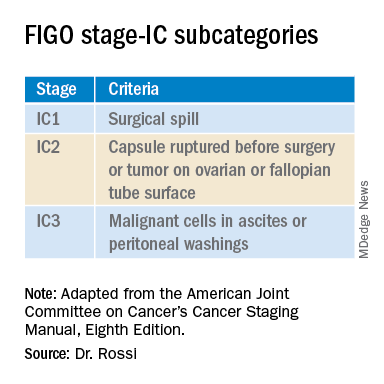
In 2017 the International Federation of Gynecology and Obstetrics (FIGO) staging of epithelial ovarian cancer subcategorized stage IC. This group encompasses women with contact between malignant cells and the peritoneum in the absence of other extraovarian disease. The table includes these distinct groupings. Stage IC1 includes patients in whom intraoperative spill occurred. Stage IC2 includes women with preoperative cyst rupture, and or microscopic or macroscopic surface involvement because the data support that these cases carry a poorer prognosis, compared with those with intraoperative rupture (IC1).1 The final subcategory, IC3, includes women who have washings (obtained at the onset of surgery, prior to manipulation of the tumor) that were positive for malignant cells, denoting preexisting contact between the tumor and peritoneum and a phenotypically more aggressive tumor.
The clinical significance of ovarian cancer capsule rupture has been evaluated in multiple studies with some mixed results.1 Consistently, it is reported that preoperative rupture, surface or capsular involvement, and preexisting peritoneal circulation of metastatic cells all portend a poorer prognosis; however, it is less clear that iatrogenic surgical rupture has the same deleterious association. In a large retrospective series from Japan, the authors evaluated 15,163 cases of stage I ovarian cancer and identified 7,227 cases of iatrogenic (intraoperative) cyst rupture.2 These cases were significantly more likely to occur among clear cell cancers, and were more likely to occur in younger patients. Worse prognosis was associated with cell type (clear cell cancers), but non–clear cell cancers (such as serous, mucinous, and endometrioid) did not have a higher hazard ratio for death when intraoperative rupture occurred. But why would intraoperative cyst rupture result in worse prognosis for only one histologic cell type? The authors hypothesized that perhaps rupture was more likely to occur during extraction of these clear cell tumors because they were associated with dense adhesions from associated endometriosis, and perhaps an adverse biologic phenomenon associated with infiltrative endometriosis is driving the behavior of this cancer.
The Japanese study also looked at the effect of chemotherapy on these same patients’ outcomes. Interestingly, the addition of chemotherapy did not improve survival for the patients with stage IC1 cancers, which was in contrast to the improved survival seen when chemotherapy was given to those with spontaneous rupture or ovarian surface involvement (IC2, IC3). These data support differentiating the subgroups of stage IC cancer in treatment decision-making, and suggest that adjuvant chemotherapy might be avoided for patients with nonclear cell stage IC1 ovarian cancer. While the outcomes are worse for patients with ruptured clear cell cancers, current therapeutic options for clear cell cancers are limited because of their known resistance to traditional agents, and outcomes for women with clear cell cancer can be worse across all stages.
While cyst rupture may not always negatively affect prognosis, the goal of surgery remains an intact removal, which influences decisions regarding surgical approach. Most adnexal masses are removed via minimally invasive surgery (MIS). MIS is associated with benefits of morbidity and cost, and therefore should be considered wherever feasible. However, MIS is associated with an increased risk of ovarian cyst rupture, likely because of the rigid instrumentation used when approaching a curved structure, in addition to the disparity in size of the pathology, compared with the extraction site incision.3 When weighing the benefits and risks of different surgical approaches, it is important to gauge the probability of malignancy. Not all complex ovarian masses associated with elevations in tumor markers are malignant, and certainly most that are associated with normal tumor markers are not. If the preoperative clinical data suggest that the mass is more likely to be malignant (e.g., mostly solid, vascular tumors with very elevated tumor markers), consideration might be made to abandoning a purely minimally invasive approach to a hand-assisted MIS or laparotomy approach. However, it would seem that abandoning an MIS approach to remove every ovarian cyst is unwise given that there is clear patient benefit with MIS and, as discussed above, most cases of iatrogenic malignant cyst rupture are unavoidable even with laparotomy, and do not necessarily independently portend poorer survival or mandate chemotherapy.
Surgeons should be both nuanced and flexible and apply some basic rules of thumb when approaching the diagnostically uncertain adnexal mass. Peritoneal washings should be obtained at the commencement of the case to discriminate those cases of true stage IC3. The peritoneum parallel to the ovarian vessel should be extensively opened to a level above the pelvic brim. In order to do this, the physiological attachments between the sigmoid colon or cecum and the suspensory ligament of the ovary may need to be carefully mobilized. This allows for retroperitoneal identification of the ureter and skeletonization of the ovarian vessels at least 2 cm proximal to their insertion into the ovary and avoidance of contact with the ovary itself (which may have a fragile capsule) or incomplete ovarian resection. If the ovary remains invested close to the sidewall or colonic structures and the appropriate peritoneal and retroperitoneal mobilization has not occurred, the surgeon may unavoidably rupture the ovarian cyst as they try to “hug” the ovary with their bites of tissue in an attempt to avoid visceral injury. There is little role for an ovarian cystectomy in a postmenopausal woman undergoing surgery for a complex adnexal mass, particularly if she has elevated tumor markers, because the process of performing ovarian cystectomy commonly invokes cyst rupture or fragmentation. Ovarian cystectomy should be reserved for premenopausal women with adnexal masses at low suspicion for malignancy. If the adnexa appears densely adherent to adjacent structures – for example, associated with infiltrative endometriosis – consideration for laparotomy or a hand-assisted approach may be necessary; in such cases, even open surgery can result in cyst rupture, and the morbidity of conversion to laparotomy should be weighed for individual cases.
Finally, retrieval of the ovarian specimen should occur intact without morcellation. There should be no uncontained morcellation of adnexal structures during retrieval of even normal-appearing ovaries. The preferred retrieval method is to place the adnexa in an appropriately sized retrieval bag, after which contained morcellation or drainage can occur to facilitate removal through a laparoscopic incision. Contained morcellation is very difficult for large solid masses through a laparoscopic port site; in these cases, extension of the incision may be necessary.
While operative spill of an ovarian cancer does upstage nonmetastatic ovarian cancer, it is unclear that, in most cases, this is independently associated with worse prognosis, and chemotherapy may not always be of added value. However, best surgical practice should always include strategies to minimize the chance of rupture when approaching adnexal masses, particularly those at highest likelihood of malignancy.
References
1. Kim HS et al. Eur J Surg Oncol. 2013 Mar 39(3):279-89.
2. Matsuo K et al. Obstet Gynecol. 2019 Nov;134(5):1017-26.
3. Matsuo K et al. JAMA Oncol. 2020 Jul 1;6(7):1110-3.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
Intact removal of an ovarian cyst is a well-established gynecologic surgical principle because ovarian cancer is definitively diagnosed only in retrospect (after ovarian extraction) and intraoperative cyst rupture upstages an otherwise nonmetastatic cancer to stage IC. This lumps cancers that are ruptured during surgical extraction together with those that have spontaneously ruptured or have surface excrescences. The theoretical rationale for this “lumping” is that contact between malignant cells from the ruptured cyst may take hold on peritoneal surfaces resulting in development of metastases. To offset this theoretical risk, it has been recommended that all stage IC ovarian cancer is treated with chemotherapy, whereas low-grade stage IA and IB cancers generally are not. No conscientious surgeon wants their surgical intervention to be the cause of a patient needing toxic chemotherapy. But is the contact between malignant cyst fluid and the peritoneum truly as bad as a spontaneous breach of the surface of the tumor? Or is cyst rupture a confounder for other adverse prognostic features, such as histologic cell type and dense pelvic attachments? If ovarian cyst rupture is an independent risk factor for patients with stage I ovarian cancer, strategies should be employed to avoid this occurrence, and we should understand how to counsel and treat patients in whom this has occurred.

In 2017 the International Federation of Gynecology and Obstetrics (FIGO) staging of epithelial ovarian cancer subcategorized stage IC. This group encompasses women with contact between malignant cells and the peritoneum in the absence of other extraovarian disease. The table includes these distinct groupings. Stage IC1 includes patients in whom intraoperative spill occurred. Stage IC2 includes women with preoperative cyst rupture, and or microscopic or macroscopic surface involvement because the data support that these cases carry a poorer prognosis, compared with those with intraoperative rupture (IC1).1 The final subcategory, IC3, includes women who have washings (obtained at the onset of surgery, prior to manipulation of the tumor) that were positive for malignant cells, denoting preexisting contact between the tumor and peritoneum and a phenotypically more aggressive tumor.
The clinical significance of ovarian cancer capsule rupture has been evaluated in multiple studies with some mixed results.1 Consistently, it is reported that preoperative rupture, surface or capsular involvement, and preexisting peritoneal circulation of metastatic cells all portend a poorer prognosis; however, it is less clear that iatrogenic surgical rupture has the same deleterious association. In a large retrospective series from Japan, the authors evaluated 15,163 cases of stage I ovarian cancer and identified 7,227 cases of iatrogenic (intraoperative) cyst rupture.2 These cases were significantly more likely to occur among clear cell cancers, and were more likely to occur in younger patients. Worse prognosis was associated with cell type (clear cell cancers), but non–clear cell cancers (such as serous, mucinous, and endometrioid) did not have a higher hazard ratio for death when intraoperative rupture occurred. But why would intraoperative cyst rupture result in worse prognosis for only one histologic cell type? The authors hypothesized that perhaps rupture was more likely to occur during extraction of these clear cell tumors because they were associated with dense adhesions from associated endometriosis, and perhaps an adverse biologic phenomenon associated with infiltrative endometriosis is driving the behavior of this cancer.
The Japanese study also looked at the effect of chemotherapy on these same patients’ outcomes. Interestingly, the addition of chemotherapy did not improve survival for the patients with stage IC1 cancers, which was in contrast to the improved survival seen when chemotherapy was given to those with spontaneous rupture or ovarian surface involvement (IC2, IC3). These data support differentiating the subgroups of stage IC cancer in treatment decision-making, and suggest that adjuvant chemotherapy might be avoided for patients with nonclear cell stage IC1 ovarian cancer. While the outcomes are worse for patients with ruptured clear cell cancers, current therapeutic options for clear cell cancers are limited because of their known resistance to traditional agents, and outcomes for women with clear cell cancer can be worse across all stages.
While cyst rupture may not always negatively affect prognosis, the goal of surgery remains an intact removal, which influences decisions regarding surgical approach. Most adnexal masses are removed via minimally invasive surgery (MIS). MIS is associated with benefits of morbidity and cost, and therefore should be considered wherever feasible. However, MIS is associated with an increased risk of ovarian cyst rupture, likely because of the rigid instrumentation used when approaching a curved structure, in addition to the disparity in size of the pathology, compared with the extraction site incision.3 When weighing the benefits and risks of different surgical approaches, it is important to gauge the probability of malignancy. Not all complex ovarian masses associated with elevations in tumor markers are malignant, and certainly most that are associated with normal tumor markers are not. If the preoperative clinical data suggest that the mass is more likely to be malignant (e.g., mostly solid, vascular tumors with very elevated tumor markers), consideration might be made to abandoning a purely minimally invasive approach to a hand-assisted MIS or laparotomy approach. However, it would seem that abandoning an MIS approach to remove every ovarian cyst is unwise given that there is clear patient benefit with MIS and, as discussed above, most cases of iatrogenic malignant cyst rupture are unavoidable even with laparotomy, and do not necessarily independently portend poorer survival or mandate chemotherapy.
Surgeons should be both nuanced and flexible and apply some basic rules of thumb when approaching the diagnostically uncertain adnexal mass. Peritoneal washings should be obtained at the commencement of the case to discriminate those cases of true stage IC3. The peritoneum parallel to the ovarian vessel should be extensively opened to a level above the pelvic brim. In order to do this, the physiological attachments between the sigmoid colon or cecum and the suspensory ligament of the ovary may need to be carefully mobilized. This allows for retroperitoneal identification of the ureter and skeletonization of the ovarian vessels at least 2 cm proximal to their insertion into the ovary and avoidance of contact with the ovary itself (which may have a fragile capsule) or incomplete ovarian resection. If the ovary remains invested close to the sidewall or colonic structures and the appropriate peritoneal and retroperitoneal mobilization has not occurred, the surgeon may unavoidably rupture the ovarian cyst as they try to “hug” the ovary with their bites of tissue in an attempt to avoid visceral injury. There is little role for an ovarian cystectomy in a postmenopausal woman undergoing surgery for a complex adnexal mass, particularly if she has elevated tumor markers, because the process of performing ovarian cystectomy commonly invokes cyst rupture or fragmentation. Ovarian cystectomy should be reserved for premenopausal women with adnexal masses at low suspicion for malignancy. If the adnexa appears densely adherent to adjacent structures – for example, associated with infiltrative endometriosis – consideration for laparotomy or a hand-assisted approach may be necessary; in such cases, even open surgery can result in cyst rupture, and the morbidity of conversion to laparotomy should be weighed for individual cases.
Finally, retrieval of the ovarian specimen should occur intact without morcellation. There should be no uncontained morcellation of adnexal structures during retrieval of even normal-appearing ovaries. The preferred retrieval method is to place the adnexa in an appropriately sized retrieval bag, after which contained morcellation or drainage can occur to facilitate removal through a laparoscopic incision. Contained morcellation is very difficult for large solid masses through a laparoscopic port site; in these cases, extension of the incision may be necessary.
While operative spill of an ovarian cancer does upstage nonmetastatic ovarian cancer, it is unclear that, in most cases, this is independently associated with worse prognosis, and chemotherapy may not always be of added value. However, best surgical practice should always include strategies to minimize the chance of rupture when approaching adnexal masses, particularly those at highest likelihood of malignancy.
References
1. Kim HS et al. Eur J Surg Oncol. 2013 Mar 39(3):279-89.
2. Matsuo K et al. Obstet Gynecol. 2019 Nov;134(5):1017-26.
3. Matsuo K et al. JAMA Oncol. 2020 Jul 1;6(7):1110-3.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
Intact removal of an ovarian cyst is a well-established gynecologic surgical principle because ovarian cancer is definitively diagnosed only in retrospect (after ovarian extraction) and intraoperative cyst rupture upstages an otherwise nonmetastatic cancer to stage IC. This lumps cancers that are ruptured during surgical extraction together with those that have spontaneously ruptured or have surface excrescences. The theoretical rationale for this “lumping” is that contact between malignant cells from the ruptured cyst may take hold on peritoneal surfaces resulting in development of metastases. To offset this theoretical risk, it has been recommended that all stage IC ovarian cancer is treated with chemotherapy, whereas low-grade stage IA and IB cancers generally are not. No conscientious surgeon wants their surgical intervention to be the cause of a patient needing toxic chemotherapy. But is the contact between malignant cyst fluid and the peritoneum truly as bad as a spontaneous breach of the surface of the tumor? Or is cyst rupture a confounder for other adverse prognostic features, such as histologic cell type and dense pelvic attachments? If ovarian cyst rupture is an independent risk factor for patients with stage I ovarian cancer, strategies should be employed to avoid this occurrence, and we should understand how to counsel and treat patients in whom this has occurred.

In 2017 the International Federation of Gynecology and Obstetrics (FIGO) staging of epithelial ovarian cancer subcategorized stage IC. This group encompasses women with contact between malignant cells and the peritoneum in the absence of other extraovarian disease. The table includes these distinct groupings. Stage IC1 includes patients in whom intraoperative spill occurred. Stage IC2 includes women with preoperative cyst rupture, and or microscopic or macroscopic surface involvement because the data support that these cases carry a poorer prognosis, compared with those with intraoperative rupture (IC1).1 The final subcategory, IC3, includes women who have washings (obtained at the onset of surgery, prior to manipulation of the tumor) that were positive for malignant cells, denoting preexisting contact between the tumor and peritoneum and a phenotypically more aggressive tumor.
The clinical significance of ovarian cancer capsule rupture has been evaluated in multiple studies with some mixed results.1 Consistently, it is reported that preoperative rupture, surface or capsular involvement, and preexisting peritoneal circulation of metastatic cells all portend a poorer prognosis; however, it is less clear that iatrogenic surgical rupture has the same deleterious association. In a large retrospective series from Japan, the authors evaluated 15,163 cases of stage I ovarian cancer and identified 7,227 cases of iatrogenic (intraoperative) cyst rupture.2 These cases were significantly more likely to occur among clear cell cancers, and were more likely to occur in younger patients. Worse prognosis was associated with cell type (clear cell cancers), but non–clear cell cancers (such as serous, mucinous, and endometrioid) did not have a higher hazard ratio for death when intraoperative rupture occurred. But why would intraoperative cyst rupture result in worse prognosis for only one histologic cell type? The authors hypothesized that perhaps rupture was more likely to occur during extraction of these clear cell tumors because they were associated with dense adhesions from associated endometriosis, and perhaps an adverse biologic phenomenon associated with infiltrative endometriosis is driving the behavior of this cancer.
The Japanese study also looked at the effect of chemotherapy on these same patients’ outcomes. Interestingly, the addition of chemotherapy did not improve survival for the patients with stage IC1 cancers, which was in contrast to the improved survival seen when chemotherapy was given to those with spontaneous rupture or ovarian surface involvement (IC2, IC3). These data support differentiating the subgroups of stage IC cancer in treatment decision-making, and suggest that adjuvant chemotherapy might be avoided for patients with nonclear cell stage IC1 ovarian cancer. While the outcomes are worse for patients with ruptured clear cell cancers, current therapeutic options for clear cell cancers are limited because of their known resistance to traditional agents, and outcomes for women with clear cell cancer can be worse across all stages.
While cyst rupture may not always negatively affect prognosis, the goal of surgery remains an intact removal, which influences decisions regarding surgical approach. Most adnexal masses are removed via minimally invasive surgery (MIS). MIS is associated with benefits of morbidity and cost, and therefore should be considered wherever feasible. However, MIS is associated with an increased risk of ovarian cyst rupture, likely because of the rigid instrumentation used when approaching a curved structure, in addition to the disparity in size of the pathology, compared with the extraction site incision.3 When weighing the benefits and risks of different surgical approaches, it is important to gauge the probability of malignancy. Not all complex ovarian masses associated with elevations in tumor markers are malignant, and certainly most that are associated with normal tumor markers are not. If the preoperative clinical data suggest that the mass is more likely to be malignant (e.g., mostly solid, vascular tumors with very elevated tumor markers), consideration might be made to abandoning a purely minimally invasive approach to a hand-assisted MIS or laparotomy approach. However, it would seem that abandoning an MIS approach to remove every ovarian cyst is unwise given that there is clear patient benefit with MIS and, as discussed above, most cases of iatrogenic malignant cyst rupture are unavoidable even with laparotomy, and do not necessarily independently portend poorer survival or mandate chemotherapy.
Surgeons should be both nuanced and flexible and apply some basic rules of thumb when approaching the diagnostically uncertain adnexal mass. Peritoneal washings should be obtained at the commencement of the case to discriminate those cases of true stage IC3. The peritoneum parallel to the ovarian vessel should be extensively opened to a level above the pelvic brim. In order to do this, the physiological attachments between the sigmoid colon or cecum and the suspensory ligament of the ovary may need to be carefully mobilized. This allows for retroperitoneal identification of the ureter and skeletonization of the ovarian vessels at least 2 cm proximal to their insertion into the ovary and avoidance of contact with the ovary itself (which may have a fragile capsule) or incomplete ovarian resection. If the ovary remains invested close to the sidewall or colonic structures and the appropriate peritoneal and retroperitoneal mobilization has not occurred, the surgeon may unavoidably rupture the ovarian cyst as they try to “hug” the ovary with their bites of tissue in an attempt to avoid visceral injury. There is little role for an ovarian cystectomy in a postmenopausal woman undergoing surgery for a complex adnexal mass, particularly if she has elevated tumor markers, because the process of performing ovarian cystectomy commonly invokes cyst rupture or fragmentation. Ovarian cystectomy should be reserved for premenopausal women with adnexal masses at low suspicion for malignancy. If the adnexa appears densely adherent to adjacent structures – for example, associated with infiltrative endometriosis – consideration for laparotomy or a hand-assisted approach may be necessary; in such cases, even open surgery can result in cyst rupture, and the morbidity of conversion to laparotomy should be weighed for individual cases.
Finally, retrieval of the ovarian specimen should occur intact without morcellation. There should be no uncontained morcellation of adnexal structures during retrieval of even normal-appearing ovaries. The preferred retrieval method is to place the adnexa in an appropriately sized retrieval bag, after which contained morcellation or drainage can occur to facilitate removal through a laparoscopic incision. Contained morcellation is very difficult for large solid masses through a laparoscopic port site; in these cases, extension of the incision may be necessary.
While operative spill of an ovarian cancer does upstage nonmetastatic ovarian cancer, it is unclear that, in most cases, this is independently associated with worse prognosis, and chemotherapy may not always be of added value. However, best surgical practice should always include strategies to minimize the chance of rupture when approaching adnexal masses, particularly those at highest likelihood of malignancy.
References
1. Kim HS et al. Eur J Surg Oncol. 2013 Mar 39(3):279-89.
2. Matsuo K et al. Obstet Gynecol. 2019 Nov;134(5):1017-26.
3. Matsuo K et al. JAMA Oncol. 2020 Jul 1;6(7):1110-3.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
Are uterine manipulators safe for gynecologic cancer surgery?
Over the past 4 decades there has been increasing use of minimally invasive surgery (MIS) for gynecologic cancer, particularly endometrial and cervical cancers. Uterine manipulators are a device inserted into the uterine cavity during MIS approaches to aid in directing the uterus within the pelvis, facilitating access to the uterine blood supply, defining the cardinal ligaments, lateralizing the ureters, and delineating the cervicovaginal junction. However, concerns have been raised regarding whether these devices are safe to use when the uterine corpus or cervix contains cancer.
In 2018, the LACC trial was published and demonstrated decreased survival for patients with cervical cancer who had undergone radical hysterectomy via a minimally invasive route.1 Several hypotheses were proposed to explain this finding including possible tumor disruption from use of a uterine manipulator. Regrettably, this study did not document manipulator use, and therefore its influence on outcomes could not be measured. However, since that time there has been honed interest into the potential negative influence of uterine manipulators on endometrial and cervical cancer surgery.
Uterine manipulators typically are inserted through the uterine cervix and reside in the endometrial cavity. It is often an inflated balloon which stabilizes the device within the cavity. Hypotheses for how they may contribute to the spread of malignancy include the massage of endometrial tumor from the pressure of the inflated balloon, facilitation of tumor dissemination through cervical lymphatics or vasculature as the manipulator traverses or punctures a cervical cancer, and possibly perforation of the uterine cavity during placement of the manipulator, and in doing so, contaminating the peritoneal cavity with endometrial or cervical cancer cells that have been dragged through with the device.
Interestingly, uterine manipulator placement is not the only time during which endometrial or cervical cancers may be disturbed prior to resection. Many diagnostic procedures such as cervical excisional procedures (loop electrosurgical excision procedure and conizations) or hysteroscopic resections cause significant intentional disruption of tumor. In the case of hysteroscopy for endometrial cancer, endometrial cancer cells have been detected in the peritoneal washings of endometrial cancer patients who have undergone this procedure, however, no worse outcomes have been associated when hysteroscopy was included as part of the diagnostic work-up, suggesting that more than simply efflux into the peritoneal cavity is necessary for those tumor cells to have metastatic potential.2
Indeed the data is mixed regarding oncologic outcomes with uterine manipulator use, especially for endometrial cancer. In one recent study the outcomes of 951 patients with endometrial cancer from seven Italian centers were evaluated.3 There was no difference in recurrence rates or disease-specific survival between the 579 patients in whom manipulators were used and the 372 patients in which surgery was performed without manipulators. More recently a Spanish study reported retrospectively on 2,661 patients at 15 centers and determined that use of a uterine manipulator (two-thirds of the cohort) was associated with a hazard ratio of 1.74 (95% confidence interval, 1.07-2.83) for risk of death.4 Unfortunately, in this study there were substantial differences between sites that used manipulators and those that did not. Additionally, while one would expect different patterns of recurrence if the manipulator was introducing a unique mechanism for metastasis, this was not observed between the manipulator and nonmanipulator arms. Finally, the groups were intrinsically different with respect to important risk factors such as lymphovascular space invasion, which might have contributed to the observed outcomes. It is important to recognize that, in both the LAP-2 and LACE trials, minimally invasive hysterectomy for endometrial cancer had been shown to have noninferior survival outcomes, compared with open hysterectomy.5,6 While these large randomized, controlled trials did not capture uterine manipulator usage, presumably it was utilized in at least some or most cases, and without apparent significant negative effect.
In cervical cancer, there is more competing data raising concern regarding manipulator use. The SUCCOR study was completed in 2020 and included a retrospective evaluation of 1,272 patients who had undergone open or MIS radical hysterectomy for early stage cervical cancer across 126 European centers during 2013-2014.7 They were able to evaluate for variables, such as uterine manipulator use. While they found that recurrence was higher for patients who had MIS hysterectomy, the HR (2.07) was similar to the HR for recurrence (2.76) among those who had uterine manipulator use. Conversely, the hazard ratio for recurrence following MIS radical hysterectomy without a manipulator was comparable with the superior rates seen with open surgery. This study was retrospective and therefore is largely hypothesis generating, however it does raise the question of whether the technique of MIS radical hysterectomy can be performed safely if particular steps, such as avoidance of a uterine manipulator, are followed. We await definitive results from prospective trials to determine this.
As mentioned earlier, the uterine manipulator is an important safety and feasibility tool for MIS hysterectomy. When not utilized, surgeons may need to add additional ports and instrumentation to maneuver the uterus and may have difficulty completing hysterectomy via a MIS approach for obese patients. There are additional urologic safety concerns when uterine elevation and cervicovaginal delineation is missing. Therefore, While the wealth of prospective data suggests that manipulators are most likely safe in hysterectomy for endometrial cancer, they should be avoided if a minimally invasive approach to cervical cancer is employed.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest to report. Email her at [email protected].
References
1. N Engl J Med. 2018 Nov 15. doi: 10.1056/NEJMoa1806395.
2. Fertil Steril. 2011 Oct. doi: 10.1016/j.fertnstert.2011.07.1146.
3. Am J Obstet Gynecol. 2017 Jun. doi: 10.1016/j.ajog.2017.01.027.
4. Am J Obstet Gynecol. 2020 Jul 18. doi: 10.1016/j.ajog.2020.07.025.
5. J Clin Oncol. 2009 Nov 10. doi: 10.1200/JCO.2009.22.3248.
6. JAMA. 2017 Mar 28. doi: 10.1001/jama.2017.2068.
7. Int J Gynecol Cancer. 2020. doi: 10.1136/ijgc-2020-001506.
Over the past 4 decades there has been increasing use of minimally invasive surgery (MIS) for gynecologic cancer, particularly endometrial and cervical cancers. Uterine manipulators are a device inserted into the uterine cavity during MIS approaches to aid in directing the uterus within the pelvis, facilitating access to the uterine blood supply, defining the cardinal ligaments, lateralizing the ureters, and delineating the cervicovaginal junction. However, concerns have been raised regarding whether these devices are safe to use when the uterine corpus or cervix contains cancer.
In 2018, the LACC trial was published and demonstrated decreased survival for patients with cervical cancer who had undergone radical hysterectomy via a minimally invasive route.1 Several hypotheses were proposed to explain this finding including possible tumor disruption from use of a uterine manipulator. Regrettably, this study did not document manipulator use, and therefore its influence on outcomes could not be measured. However, since that time there has been honed interest into the potential negative influence of uterine manipulators on endometrial and cervical cancer surgery.
Uterine manipulators typically are inserted through the uterine cervix and reside in the endometrial cavity. It is often an inflated balloon which stabilizes the device within the cavity. Hypotheses for how they may contribute to the spread of malignancy include the massage of endometrial tumor from the pressure of the inflated balloon, facilitation of tumor dissemination through cervical lymphatics or vasculature as the manipulator traverses or punctures a cervical cancer, and possibly perforation of the uterine cavity during placement of the manipulator, and in doing so, contaminating the peritoneal cavity with endometrial or cervical cancer cells that have been dragged through with the device.
Interestingly, uterine manipulator placement is not the only time during which endometrial or cervical cancers may be disturbed prior to resection. Many diagnostic procedures such as cervical excisional procedures (loop electrosurgical excision procedure and conizations) or hysteroscopic resections cause significant intentional disruption of tumor. In the case of hysteroscopy for endometrial cancer, endometrial cancer cells have been detected in the peritoneal washings of endometrial cancer patients who have undergone this procedure, however, no worse outcomes have been associated when hysteroscopy was included as part of the diagnostic work-up, suggesting that more than simply efflux into the peritoneal cavity is necessary for those tumor cells to have metastatic potential.2
Indeed the data is mixed regarding oncologic outcomes with uterine manipulator use, especially for endometrial cancer. In one recent study the outcomes of 951 patients with endometrial cancer from seven Italian centers were evaluated.3 There was no difference in recurrence rates or disease-specific survival between the 579 patients in whom manipulators were used and the 372 patients in which surgery was performed without manipulators. More recently a Spanish study reported retrospectively on 2,661 patients at 15 centers and determined that use of a uterine manipulator (two-thirds of the cohort) was associated with a hazard ratio of 1.74 (95% confidence interval, 1.07-2.83) for risk of death.4 Unfortunately, in this study there were substantial differences between sites that used manipulators and those that did not. Additionally, while one would expect different patterns of recurrence if the manipulator was introducing a unique mechanism for metastasis, this was not observed between the manipulator and nonmanipulator arms. Finally, the groups were intrinsically different with respect to important risk factors such as lymphovascular space invasion, which might have contributed to the observed outcomes. It is important to recognize that, in both the LAP-2 and LACE trials, minimally invasive hysterectomy for endometrial cancer had been shown to have noninferior survival outcomes, compared with open hysterectomy.5,6 While these large randomized, controlled trials did not capture uterine manipulator usage, presumably it was utilized in at least some or most cases, and without apparent significant negative effect.
In cervical cancer, there is more competing data raising concern regarding manipulator use. The SUCCOR study was completed in 2020 and included a retrospective evaluation of 1,272 patients who had undergone open or MIS radical hysterectomy for early stage cervical cancer across 126 European centers during 2013-2014.7 They were able to evaluate for variables, such as uterine manipulator use. While they found that recurrence was higher for patients who had MIS hysterectomy, the HR (2.07) was similar to the HR for recurrence (2.76) among those who had uterine manipulator use. Conversely, the hazard ratio for recurrence following MIS radical hysterectomy without a manipulator was comparable with the superior rates seen with open surgery. This study was retrospective and therefore is largely hypothesis generating, however it does raise the question of whether the technique of MIS radical hysterectomy can be performed safely if particular steps, such as avoidance of a uterine manipulator, are followed. We await definitive results from prospective trials to determine this.
As mentioned earlier, the uterine manipulator is an important safety and feasibility tool for MIS hysterectomy. When not utilized, surgeons may need to add additional ports and instrumentation to maneuver the uterus and may have difficulty completing hysterectomy via a MIS approach for obese patients. There are additional urologic safety concerns when uterine elevation and cervicovaginal delineation is missing. Therefore, While the wealth of prospective data suggests that manipulators are most likely safe in hysterectomy for endometrial cancer, they should be avoided if a minimally invasive approach to cervical cancer is employed.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest to report. Email her at [email protected].
References
1. N Engl J Med. 2018 Nov 15. doi: 10.1056/NEJMoa1806395.
2. Fertil Steril. 2011 Oct. doi: 10.1016/j.fertnstert.2011.07.1146.
3. Am J Obstet Gynecol. 2017 Jun. doi: 10.1016/j.ajog.2017.01.027.
4. Am J Obstet Gynecol. 2020 Jul 18. doi: 10.1016/j.ajog.2020.07.025.
5. J Clin Oncol. 2009 Nov 10. doi: 10.1200/JCO.2009.22.3248.
6. JAMA. 2017 Mar 28. doi: 10.1001/jama.2017.2068.
7. Int J Gynecol Cancer. 2020. doi: 10.1136/ijgc-2020-001506.
Over the past 4 decades there has been increasing use of minimally invasive surgery (MIS) for gynecologic cancer, particularly endometrial and cervical cancers. Uterine manipulators are a device inserted into the uterine cavity during MIS approaches to aid in directing the uterus within the pelvis, facilitating access to the uterine blood supply, defining the cardinal ligaments, lateralizing the ureters, and delineating the cervicovaginal junction. However, concerns have been raised regarding whether these devices are safe to use when the uterine corpus or cervix contains cancer.
In 2018, the LACC trial was published and demonstrated decreased survival for patients with cervical cancer who had undergone radical hysterectomy via a minimally invasive route.1 Several hypotheses were proposed to explain this finding including possible tumor disruption from use of a uterine manipulator. Regrettably, this study did not document manipulator use, and therefore its influence on outcomes could not be measured. However, since that time there has been honed interest into the potential negative influence of uterine manipulators on endometrial and cervical cancer surgery.
Uterine manipulators typically are inserted through the uterine cervix and reside in the endometrial cavity. It is often an inflated balloon which stabilizes the device within the cavity. Hypotheses for how they may contribute to the spread of malignancy include the massage of endometrial tumor from the pressure of the inflated balloon, facilitation of tumor dissemination through cervical lymphatics or vasculature as the manipulator traverses or punctures a cervical cancer, and possibly perforation of the uterine cavity during placement of the manipulator, and in doing so, contaminating the peritoneal cavity with endometrial or cervical cancer cells that have been dragged through with the device.
Interestingly, uterine manipulator placement is not the only time during which endometrial or cervical cancers may be disturbed prior to resection. Many diagnostic procedures such as cervical excisional procedures (loop electrosurgical excision procedure and conizations) or hysteroscopic resections cause significant intentional disruption of tumor. In the case of hysteroscopy for endometrial cancer, endometrial cancer cells have been detected in the peritoneal washings of endometrial cancer patients who have undergone this procedure, however, no worse outcomes have been associated when hysteroscopy was included as part of the diagnostic work-up, suggesting that more than simply efflux into the peritoneal cavity is necessary for those tumor cells to have metastatic potential.2
Indeed the data is mixed regarding oncologic outcomes with uterine manipulator use, especially for endometrial cancer. In one recent study the outcomes of 951 patients with endometrial cancer from seven Italian centers were evaluated.3 There was no difference in recurrence rates or disease-specific survival between the 579 patients in whom manipulators were used and the 372 patients in which surgery was performed without manipulators. More recently a Spanish study reported retrospectively on 2,661 patients at 15 centers and determined that use of a uterine manipulator (two-thirds of the cohort) was associated with a hazard ratio of 1.74 (95% confidence interval, 1.07-2.83) for risk of death.4 Unfortunately, in this study there were substantial differences between sites that used manipulators and those that did not. Additionally, while one would expect different patterns of recurrence if the manipulator was introducing a unique mechanism for metastasis, this was not observed between the manipulator and nonmanipulator arms. Finally, the groups were intrinsically different with respect to important risk factors such as lymphovascular space invasion, which might have contributed to the observed outcomes. It is important to recognize that, in both the LAP-2 and LACE trials, minimally invasive hysterectomy for endometrial cancer had been shown to have noninferior survival outcomes, compared with open hysterectomy.5,6 While these large randomized, controlled trials did not capture uterine manipulator usage, presumably it was utilized in at least some or most cases, and without apparent significant negative effect.
In cervical cancer, there is more competing data raising concern regarding manipulator use. The SUCCOR study was completed in 2020 and included a retrospective evaluation of 1,272 patients who had undergone open or MIS radical hysterectomy for early stage cervical cancer across 126 European centers during 2013-2014.7 They were able to evaluate for variables, such as uterine manipulator use. While they found that recurrence was higher for patients who had MIS hysterectomy, the HR (2.07) was similar to the HR for recurrence (2.76) among those who had uterine manipulator use. Conversely, the hazard ratio for recurrence following MIS radical hysterectomy without a manipulator was comparable with the superior rates seen with open surgery. This study was retrospective and therefore is largely hypothesis generating, however it does raise the question of whether the technique of MIS radical hysterectomy can be performed safely if particular steps, such as avoidance of a uterine manipulator, are followed. We await definitive results from prospective trials to determine this.
As mentioned earlier, the uterine manipulator is an important safety and feasibility tool for MIS hysterectomy. When not utilized, surgeons may need to add additional ports and instrumentation to maneuver the uterus and may have difficulty completing hysterectomy via a MIS approach for obese patients. There are additional urologic safety concerns when uterine elevation and cervicovaginal delineation is missing. Therefore, While the wealth of prospective data suggests that manipulators are most likely safe in hysterectomy for endometrial cancer, they should be avoided if a minimally invasive approach to cervical cancer is employed.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest to report. Email her at [email protected].
References
1. N Engl J Med. 2018 Nov 15. doi: 10.1056/NEJMoa1806395.
2. Fertil Steril. 2011 Oct. doi: 10.1016/j.fertnstert.2011.07.1146.
3. Am J Obstet Gynecol. 2017 Jun. doi: 10.1016/j.ajog.2017.01.027.
4. Am J Obstet Gynecol. 2020 Jul 18. doi: 10.1016/j.ajog.2020.07.025.
5. J Clin Oncol. 2009 Nov 10. doi: 10.1200/JCO.2009.22.3248.
6. JAMA. 2017 Mar 28. doi: 10.1001/jama.2017.2068.
7. Int J Gynecol Cancer. 2020. doi: 10.1136/ijgc-2020-001506.

