User login
Pediatric Hospital Medicine Core Competencies
Introduction
The Society of Hospital Medicine (SHM) defines hospitalists as physicians whose primary professional focus is the comprehensive general medical care of hospitalized patients. Their activities include patient care, teaching, research, and leadership related to Hospital Medicine.1 It is estimated that there are up to 2500 pediatric hospitalists in the United States, with continued growth due to the converging needs for a dedicated focus on patient safety, quality improvement, hospital throughput, and inpatient teaching.2‐9 (Pediatric Hospital Medicine (PHM), as defined today, has been practiced in the United States for at least 30 years10 and continues to evolve as an area of specialization, with the refinement of a distinct knowledgebase and skill set focused on the provision of high quality general pediatric care in the inpatient setting. PHM is the latest site‐specific specialty to emerge from the field of general pediatrics it's development analogous to the evolution of critical care or emergency medicine during previous decades.11 Adult hospital medicine has defined itself within the field of general internal medicine12 and has recently received approval to provide a recognized focus of practice exam in 2010 for those re‐certifying with the American Board of Internal Medicine,13 PHM is creating an identity as a subspecialty practice with distinct focus on inpatient care for children within the larger context of general pediatric care.8, 14
The Pediatric Hospital Medicine Core Competencies were created to help define the roles and expectations for pediatric hospitalists, regardless of practice setting. The intent is to provide a unified approach toward identifying the specific body of knowledge and measurable skills needed to assure delivery of the highest quality of care for all hospitalized pediatric patients. Most children requiring hospitalization in the United States are hospitalized in community settings where subspecialty support is more limited and many pediatric services may be unavailable. Children with complex, chronic medical problems, however, are more likely to be hospitalized at a tertiary care or academic institutions. In order to unify pediatric hospitalists who work in different practice environments, the PHM Core Competencies were constructed to represent the knowledge, skills, attitudes, and systems improvements that all pediatric hospitalists can be expected to acquire and maintain.
Furthermore, the content of the PHM Core Competencies reflect the fact that children are a vulnerable population. Their care requires attention to many elements which distinguishes it from that given to the majority of the adult population: dependency, differences in developmental physiology and behavior, occurrence of congenital genetic disorders and age‐based clinical conditions, impact of chronic disease states on whole child development, and weight‐based medication dosing often with limited guidance from pediatric studies, to name a few. Awareness of these needs must be heightened when a child enters the hospital where diagnoses, procedures, and treatments often include use of high‐risk modalities and require coordination of care across multiple providers.
Pediatric hospitalists commonly work to improve the systems of care in which they operate and therefore both clinical and non‐clinical topics are included. The 54 chapters address the fundamental and most common components of inpatient care but are not an extensive review of all aspects of inpatient medicine encountered by those caring for hospitalized children. Finally, the PHM Core Competencies are not intended for use in assessing proficiency immediately post‐residency, but do provide a framework for the education and evaluation of both physicians‐in‐training and practicing hospitalists. Meeting these competencies is anticipated to take from one to three years of active practice in pediatric hospital medicine, and may be reached through a combination of practice experience, course work, self‐directed work, and/or formalized training.
Methods
Timeline
In 2002, SHM convened an educational summit from which there was a resolution to create core competencies. Following the summit, the SHM Pediatric Core Curriculum Task Force (CCTF) was created, which included 12 pediatric hospitalists practicing in academic and community facilities, as well as teaching and non‐teaching settings, and occupying leadership positions within institutions of varied size and geographic location. Shortly thereafter, in November 2003, approximately 130 pediatric hospitalists attended the first PHM meeting in San Antonio, Texas.11 At this meeting, with support from leaders in pediatric emergency medicine, first discussions regarding PHM scope of practice were held.
Formal development of the competencies began in 2005 in parallel to but distinct from SHM's adult work, which culminated in The Core Competencies in Hospital Medicine: A Framework for Curriculum Development published in 2006. The CCTF divided into three groups, focused on clinical, procedural, and systems‐based topics. Face‐to‐face meetings were held at the SHM annual meetings, with most work being completed by phone and electronically in the interim periods. In 2007, due to the overlapping interests of the three core pediatric societies, the work was transferred to leaders within the APA. In 2008 the work was transferred back to the leadership within SHM. Since that time, external reviewers were solicited, new chapters created, sections re‐aligned, internal and external reviewer comments incorporated, and final edits for taxonomy, content, and formatting were completed (Table 1).
| Date | Event |
|---|---|
| Feb 2002 | SHM Educational Summit held and CCTF created |
| Oct 2003 | 1st PHM meeting held in San Antonio |
| 2003‐2007 | Chapter focus determined; contributors engaged |
| 2007‐2008 | APA PHM Special Interest Group (SIG) review; creation of separate PHM Fellowship Competencies (not in this document) |
| Aug 2008‐Oct 2008 | SHM Pediatric Committee and CCTF members resume work; editorial review |
| Oct 2008‐Mar 2009 | Internal review: PHM Fellowship Director, AAP, APA, and SHM section/committee leader, and key national PHM leader reviews solicited and returned |
| Mar 2009 | PHM Fellowship Director comments addressed; editorial review |
| Mar‐Apr 2009 | External reviewers solicited from national agencies and societies relevant to PHM |
| Apr‐July 2009 | External reviewer comments returned |
| July‐Oct 2009 | Contributor review of all comments; editorial review, sections revised |
| Oct 2009 | Final review: Chapters to SHM subcommittees and Board |
Areas of Focused Practice
The PHM Core Competencies were conceptualized similarly to the SHM adult core competencies. Initial sections were divided into clinical conditions, procedures, and systems. However as content developed and reviewer comments were addressed, the four final sections were modified to those noted in Table 2. For the Common Clinical Diagnoses and Conditions, the goal was to select conditions most commonly encountered by pediatric hospitalists. Non‐surgical diagnosis‐related group (DRG) conditions were selected from the following sources: The Joint Commission's (TJC) Oryx Performance Measures Report15‐16 (asthma, abdominal pain, acute gastroenteritis, simple pneumonia); Child Health Corporation of America's Pediatric Health Information System Dataset (CHCA PHIS, Shawnee Mission, KS), and relevant publications on common pediatric hospitalizations.17 These data were compared to billing data from randomly‐selected practicing hospitalists representing free‐standing children's and community hospitals, teaching and non‐teaching settings, and urban and rural locations. The 22 clinical conditions chosen by the CCTF were those most relevant to the practice of pediatric hospital medicine.
| Common Clinical Diagnoses and Conditions | Specialized Clinical Services | Core Skills | Healthcare Systems: Supporting and Advancing Child Health | |
|---|---|---|---|---|
| Acute abdominal pain and the acute abdomen | Neonatal fever | Child abuse and neglect | Bladder catheterization/suprapubic bladder tap | Advocacy |
| Apparent life‐threatening event | Neonatal jaundice | Hospice and palliative care | Electrocardiogram interpretation | Business practices |
| Asthma | Pneumonia | Leading a healthcare team | Feeding tubes | Communication |
| Bone and joint infections | Respiratory failure | Newborn care and delivery room management | Fluids and electrolyte management | Continuous quality improvement |
| Bronchiolitis | Seizures | Technology‐dependent children | Intravenous access and phlebotomy | Cost‐effective care |
| Central nervous system infections | Shock | Transport of the critically ill child | Lumbar puncture | Education |
| Diabetes mellitus | Sickle cell disease | Non‐invasive monitoring | Ethics | |
| Failure to thrive | Skin and soft tissue infection | Nutrition | Evidence‐based medicine | |
| Fever of unknown origin | Toxic ingestion | Oxygen delivery and airway management | Health information systems | |
| Gastroenteritis | Upper airway infections | Pain management | Legal issues/risk management | |
| Kawasaki disease | Urinary tract infections | Pediatric advanced life support | Patient safety |
The Specialized Clinical Servicessection addresses important components of care that are not DRG‐based and reflect the unique needs of hospitalized children, as assessed by the CCTF, editors, and contributors. Core Skillswere chosen based on the HCUP Factbook 2 Procedures,18 billing data from randomly‐selected practicing hospitalists representing the same settings listed above, and critical input from reviewers. Depending on the individual setting, pediatric hospitalists may require skills in areas not found in these 11 chapters, such as chest tube placement or ventilator management. The list is therefore not exhaustive, but rather representative of skills most pediatric hospitalists should maintain.
The Healthcare Systems: Supporting and Advancing Child Healthchapters are likely the most dissimilar to any core content taught in traditional residency programs. While residency graduates are versed in some components listed in these chapters, comprehensive education in most of these competencies is currently lacking. Improvement of healthcare systems is an essential element of pediatric hospital medicine, and unifies all pediatric hospitalists regardless of practice environment or patient population. Therefore, this section includes chapters that not only focus on systems of care, but also on advancing child health through advocacy, research, education, evidence‐based medicine, and ethical practice. These chapters were drawn from a combination of several sources: expectations of external agencies (TJC, Center for Medicaid and Medicare) related to the specific nonclinical work in which pediatric hospitalists are integrally involved; expectations for advocacy as best defined by the AAP and the National Association of Children's Hospitals and Related Institutions (NACHRI); the six core competency domains mandated by the Accrediting Council on Graduate Medical Education (ACGME), the American Board of Pediatrics (ABP), and hospital medical staff offices as part of Focused Professional Practice Evaluation (FPPE) and Ongoing Professional Practice Evaluation (OPPE)16; and assessment of responsibilities and leadership roles fulfilled by pediatric hospitalists in all venues. In keeping with the intent of the competencies to be timeless, the competency elements call out the need to attend to the changing goals of these groups as well as those of the Institute of Healthcare Improvement (IHI), the Alliance for Pediatric Quality (which consists of ABP, AAP, TJC, CHCA, NACHRI), and local hospital systems leaders.
Contributors and Review
The CCTF selected section (associate) editors from SHM based on established expertise in each area, with input from the SHM Pediatric and Education Committees and the SHM Board. As a collaborative effort, authors for various chapters were solicited in consultation with experts from the AAP, APA, and SHM, and included non‐hospitalists with reputations as experts in various fields. Numerous SHM Pediatric Committee and CCTF conference calls were held to review hospital and academic appointments, presentations given, and affiliations relevant to the practice of pediatric hospital medicine. This vetting process resulted in a robust author list representing diverse geographic and practice settings. Contributors were provided with structure (Knowledge, Skills, Attitudes, and Systems subsections) and content (timeless, competency based) guidelines.
The review process was rigorous, and included both internal and external reviewers. The APA review in 2007 included the PHM Special Interest Group as well as the PHM Fellowship Directors (Table 1). After return to SHM and further editing, the internal review commenced which focused on content and scope. The editors addressed the resulting suggestions and worked to standardize formatting and use of Bloom's taxonomy.19 A list of common terms and phrases were created to add consistency between chapters. External reviewers were first mailed a letter requesting interest, which was followed up by emails, letters, and phone calls to encourage feedback. External review included 29 solicited agencies and societies (Table 3), with overall response rate of 66% (41% for Groups I and II). Individual contributors then reviewed comments specific to their chapters, with associate editor overview of their respective sections. The editors reviewed each chapter individually multiple times throughout the 2007‐2009 years, contacting individual contributors and reviewers by email and phone. Editors concluded a final comprehensive review of all chapters in late 2009.
| I. Academic and certifying societies |
| Academic Pediatric Association |
| Accreditation Council for Graduate Medical Education, Pediatric Residency Review Committee |
| American Academy of Family Physicians |
| American Academy of Pediatrics Board |
| American Academy of Pediatrics National Committee on Hospital Care |
| American Association of Critical Care Nursing |
| American Board of Family Medicine |
| American Board of Pediatrics |
| American College of Emergency Physicians |
| American Pediatric Society |
| Association of American Medical Colleges |
| Association of Medical School Pediatric Department Chairs (AMSPDC) |
| Association of Pediatric Program Directors |
| Council on Teaching Hospitals |
| Society of Pediatric Research |
| II. Stakeholder agencies |
| Agency for Healthcare Research and Quality |
| American Association of Critical Care Nursing |
| American College of Emergency Physicians |
| American Hospital Association (AHA) |
| American Nurses Association |
| American Society of Health‐System Pharmacists |
| Child Health Corporation of America (CHCA) |
| Institute for Healthcare Improvement |
| National Association for Children's Hospitals and Related Institutions (NACHRI) |
| National Association of Pediatric Nurse Practitioners (NAPNAP) |
| National Initiative for Children's Healthcare Quality (NICHQ) |
| National Quality Forum |
| Quality Resources International |
| Robert Wood Johnson Foundation |
| The Joint Commission for Accreditation of Hospitals and Organizations (TJC) |
| III. Pediatric hospital medicine fellowship directors |
| Boston Children's |
| Children's Hospital Los Angeles |
| Children's National D.C. |
| Emory |
| Hospital for Sick Kids Toronto |
| Rady Children's San Diego University of California San Diego |
| Riley Children's Hospital Indiana |
| University of South Florida, All Children's Hospital |
| Texas Children's Hospital, Baylor College of Medicine |
| IV. SHM, APA, AAP Leadership and committee chairs |
| American Academy of Pediatrics Section on Hospital Medicine |
| Academic Pediatric Association PHM Special Interest Group |
| SHM Board |
| SHM Education Committee |
| SHM Family Practice Committee |
| SHM Hospital Quality and Patient Safety Committee |
| SHM IT Task Force |
| SHM Journal Editorial Board |
| SHM Palliative Care Task Force |
| SHM Practice Analysis Committee |
| SHM Public Policy Committee |
| SHM Research Committee |
Chapter Content
Each of the 54 chapters within the four sections of these competencies is presented in the educational theory of learning domains: Knowledge, Skills, Attitudes, with a final Systems domain added to reflect the emphasis of hospitalist practice on improving healthcare systems. Each chapter is designed to stand alone, which may assist with development of curriculum at individual practice locations. Certain key phrases are apparent throughout, such as lead, coordinate, or participate in and work with hospital and community leaders to which were designed to note the varied roles in different practice settings. Some chapters specifically comment on the application of competency bullets given the unique and differing roles and expectations of pediatric hospitalists, such as research and education. Chapters state specific proficiencies expected wherever possible, with phrases and wording selected to help guide learning activities to achieve the competency.
Application and Future Directions
Although pediatric hospitalists care for children in many settings, these core competencies address the common expectations for any venue. Pediatric hospital medicine requires skills in acute care clinical medicine that attend to the changing needs of hospitalized children. The core of pediatric hospital medicine is dedicated to the care of children in the geographic hospital environment between emergency medicine and tertiary pediatric and neonatal intensive care units. Pediatric hospitalists provide care in related clinical service programs that are linked to hospital systems. In performing these activities, pediatric hospitalists consistently partner with ambulatory providers and subspecialists to render coordinated care across the continuum for a given child. Pediatric hospital medicine is an interdisciplinary practice, with focus on processes of care and clinical quality outcomes based in evidence. Engagement in local, state, and national initiatives to improve child health outcomes is a cornerstone of pediatric hospitalists' practice. These competencies provide the framework for creation of curricula that can reflect local issues and react to changing evidence.
As providers of systems‐based care, pediatric hospitalists are called upon more and more to render care and provide leadership in clinical arenas that are integral to healthcare organizations, such as home health care, sub‐acute care facilities, and hospice and palliative care programs. The practice of pediatric hospital medicine has evolved to its current state through efforts of many represented in the competencies as contributors, associate editors, editors, and reviewers. Pediatric hospitalists are committed to leading change in healthcare for hospitalized children, and are positioned well to address the interests and needs of community and urban, teaching and non‐teaching facilities, and the children and families they serve. These competencies reflect the areas of focused practice which, similar to pediatric emergency medicine, will no doubt be refined but not fundamentally changed in future years. The intent, we hope, is clear: to provide excellence in clinical care, accountability for practice, and lead improvements in healthcare for hospitalized children.
- Society of Hospital Medicine (SHM). Definition of a Hospitalist. http://www.hospitalmedicine.org/AM/Template.cfm?Section=General_Information 2009.
- .Pediatric Hospitalists Membership Numbers. In.Philadelphia:Society of Hospital Medicine, PA 19130;2009.
- , .The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- .The future of hospital medicine: evolution or revolution?.Am J Med.2004;117:446–450.
- , .The hospitalist movement 5 years later.JAMA.2002;287:487–494.
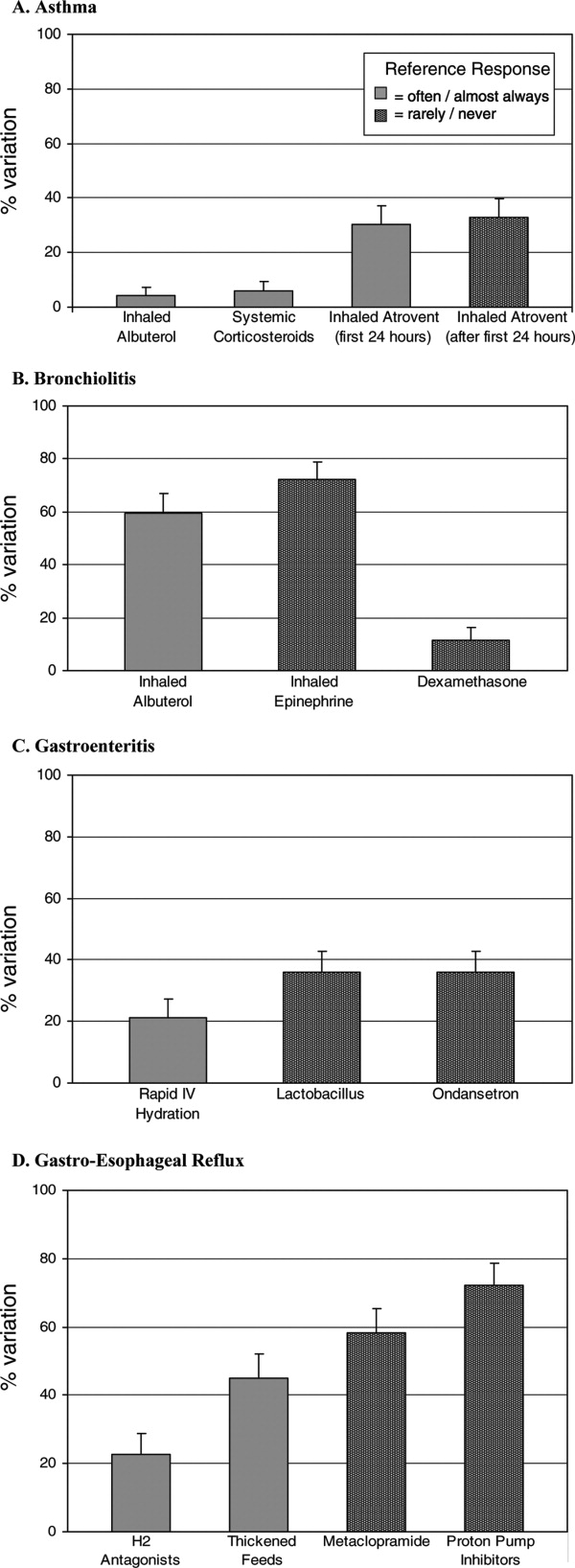
- , , , , .Variation in pediatric hospitalists' use of proven and unproven therapies: A study from the Pediatric Research in Inpatient Settings (PRIS) network.J Hosp Med.2008;3(4):292–298.
- , , .Pediatric hospitalists: Training, current practice, and career goals.J Hosp Med.2009;4(3):179–186.
- , .Standardize to excellence: improving the quality and safety of care with clinical pathways.Pediatr Clin North Am.2009;56(4):893–904.
- .Evolution of a new specialty ‐ a twenty year pediatric hospitalist experience [Abstract]. In:National Association of Inpatient Physicians (now Society of Hospital Medicine).New Orleans, Louisiana;1999.
- , , , , , , et al.Pediatric hospitalists: report of a leadership conference.Pediatrics.2006;117(4):1122–1130.
- , , , , e.The core competencies in hospital medicine: a framework for curriculum development.J Hosp Med.2006;1(Suppl 1).
- American Board of Internal Medicine. Questions and answers regarding ABIM recognition of focused practice in hospital medicine through maintenance of certification. http://www.abim.org/news/news/focused‐practice‐hospital‐medicine‐qa.aspx. Published 2010. Accessed January 6,2010.
- .Comprehensive pediatric hospital medicine.N Engl J Med.2008;358(21):2301–2302.
- The Joint Commission. Performance measurement initiatives. http://www. jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/. Published 2010. Accessed December 5,2010.
- The Joint Commission. Standards frequently asked questions: comprehensive accreditation manual for critical access hospitals (CAMCAH). http://www.jointcommission.org/AccreditationPrograms/CriticalAccess Hospitals/Standards/09_FAQs/default.htm. Accessed December 5,2008; December 14, 2009.
- , , , , .Infectious disease hospitalizations among infants in the United States.Pediatrics.2008;121(2):244–252.
- , , , .Procedures in U.S. hospitals, 1997.HCUP fact book no. 2. In:agency for healthcare research and quality,Rockville, MD;2001.
- Anderson L, Krathwohl DR, Airasian PW, Cruikshank KA, Mayer RE, Pintrich PR, et al., editors.A taxonomy for learning, teaching, and assessing. In: A Revision of Bloom's Taxonomy of Educational Objectives.Upper Saddle River, NJ: Addison Wesley Longman, Inc. Pearson Education USA;2001.
Introduction
The Society of Hospital Medicine (SHM) defines hospitalists as physicians whose primary professional focus is the comprehensive general medical care of hospitalized patients. Their activities include patient care, teaching, research, and leadership related to Hospital Medicine.1 It is estimated that there are up to 2500 pediatric hospitalists in the United States, with continued growth due to the converging needs for a dedicated focus on patient safety, quality improvement, hospital throughput, and inpatient teaching.2‐9 (Pediatric Hospital Medicine (PHM), as defined today, has been practiced in the United States for at least 30 years10 and continues to evolve as an area of specialization, with the refinement of a distinct knowledgebase and skill set focused on the provision of high quality general pediatric care in the inpatient setting. PHM is the latest site‐specific specialty to emerge from the field of general pediatrics it's development analogous to the evolution of critical care or emergency medicine during previous decades.11 Adult hospital medicine has defined itself within the field of general internal medicine12 and has recently received approval to provide a recognized focus of practice exam in 2010 for those re‐certifying with the American Board of Internal Medicine,13 PHM is creating an identity as a subspecialty practice with distinct focus on inpatient care for children within the larger context of general pediatric care.8, 14
The Pediatric Hospital Medicine Core Competencies were created to help define the roles and expectations for pediatric hospitalists, regardless of practice setting. The intent is to provide a unified approach toward identifying the specific body of knowledge and measurable skills needed to assure delivery of the highest quality of care for all hospitalized pediatric patients. Most children requiring hospitalization in the United States are hospitalized in community settings where subspecialty support is more limited and many pediatric services may be unavailable. Children with complex, chronic medical problems, however, are more likely to be hospitalized at a tertiary care or academic institutions. In order to unify pediatric hospitalists who work in different practice environments, the PHM Core Competencies were constructed to represent the knowledge, skills, attitudes, and systems improvements that all pediatric hospitalists can be expected to acquire and maintain.
Furthermore, the content of the PHM Core Competencies reflect the fact that children are a vulnerable population. Their care requires attention to many elements which distinguishes it from that given to the majority of the adult population: dependency, differences in developmental physiology and behavior, occurrence of congenital genetic disorders and age‐based clinical conditions, impact of chronic disease states on whole child development, and weight‐based medication dosing often with limited guidance from pediatric studies, to name a few. Awareness of these needs must be heightened when a child enters the hospital where diagnoses, procedures, and treatments often include use of high‐risk modalities and require coordination of care across multiple providers.
Pediatric hospitalists commonly work to improve the systems of care in which they operate and therefore both clinical and non‐clinical topics are included. The 54 chapters address the fundamental and most common components of inpatient care but are not an extensive review of all aspects of inpatient medicine encountered by those caring for hospitalized children. Finally, the PHM Core Competencies are not intended for use in assessing proficiency immediately post‐residency, but do provide a framework for the education and evaluation of both physicians‐in‐training and practicing hospitalists. Meeting these competencies is anticipated to take from one to three years of active practice in pediatric hospital medicine, and may be reached through a combination of practice experience, course work, self‐directed work, and/or formalized training.
Methods
Timeline
In 2002, SHM convened an educational summit from which there was a resolution to create core competencies. Following the summit, the SHM Pediatric Core Curriculum Task Force (CCTF) was created, which included 12 pediatric hospitalists practicing in academic and community facilities, as well as teaching and non‐teaching settings, and occupying leadership positions within institutions of varied size and geographic location. Shortly thereafter, in November 2003, approximately 130 pediatric hospitalists attended the first PHM meeting in San Antonio, Texas.11 At this meeting, with support from leaders in pediatric emergency medicine, first discussions regarding PHM scope of practice were held.
Formal development of the competencies began in 2005 in parallel to but distinct from SHM's adult work, which culminated in The Core Competencies in Hospital Medicine: A Framework for Curriculum Development published in 2006. The CCTF divided into three groups, focused on clinical, procedural, and systems‐based topics. Face‐to‐face meetings were held at the SHM annual meetings, with most work being completed by phone and electronically in the interim periods. In 2007, due to the overlapping interests of the three core pediatric societies, the work was transferred to leaders within the APA. In 2008 the work was transferred back to the leadership within SHM. Since that time, external reviewers were solicited, new chapters created, sections re‐aligned, internal and external reviewer comments incorporated, and final edits for taxonomy, content, and formatting were completed (Table 1).
| Date | Event |
|---|---|
| Feb 2002 | SHM Educational Summit held and CCTF created |
| Oct 2003 | 1st PHM meeting held in San Antonio |
| 2003‐2007 | Chapter focus determined; contributors engaged |
| 2007‐2008 | APA PHM Special Interest Group (SIG) review; creation of separate PHM Fellowship Competencies (not in this document) |
| Aug 2008‐Oct 2008 | SHM Pediatric Committee and CCTF members resume work; editorial review |
| Oct 2008‐Mar 2009 | Internal review: PHM Fellowship Director, AAP, APA, and SHM section/committee leader, and key national PHM leader reviews solicited and returned |
| Mar 2009 | PHM Fellowship Director comments addressed; editorial review |
| Mar‐Apr 2009 | External reviewers solicited from national agencies and societies relevant to PHM |
| Apr‐July 2009 | External reviewer comments returned |
| July‐Oct 2009 | Contributor review of all comments; editorial review, sections revised |
| Oct 2009 | Final review: Chapters to SHM subcommittees and Board |
Areas of Focused Practice
The PHM Core Competencies were conceptualized similarly to the SHM adult core competencies. Initial sections were divided into clinical conditions, procedures, and systems. However as content developed and reviewer comments were addressed, the four final sections were modified to those noted in Table 2. For the Common Clinical Diagnoses and Conditions, the goal was to select conditions most commonly encountered by pediatric hospitalists. Non‐surgical diagnosis‐related group (DRG) conditions were selected from the following sources: The Joint Commission's (TJC) Oryx Performance Measures Report15‐16 (asthma, abdominal pain, acute gastroenteritis, simple pneumonia); Child Health Corporation of America's Pediatric Health Information System Dataset (CHCA PHIS, Shawnee Mission, KS), and relevant publications on common pediatric hospitalizations.17 These data were compared to billing data from randomly‐selected practicing hospitalists representing free‐standing children's and community hospitals, teaching and non‐teaching settings, and urban and rural locations. The 22 clinical conditions chosen by the CCTF were those most relevant to the practice of pediatric hospital medicine.
| Common Clinical Diagnoses and Conditions | Specialized Clinical Services | Core Skills | Healthcare Systems: Supporting and Advancing Child Health | |
|---|---|---|---|---|
| Acute abdominal pain and the acute abdomen | Neonatal fever | Child abuse and neglect | Bladder catheterization/suprapubic bladder tap | Advocacy |
| Apparent life‐threatening event | Neonatal jaundice | Hospice and palliative care | Electrocardiogram interpretation | Business practices |
| Asthma | Pneumonia | Leading a healthcare team | Feeding tubes | Communication |
| Bone and joint infections | Respiratory failure | Newborn care and delivery room management | Fluids and electrolyte management | Continuous quality improvement |
| Bronchiolitis | Seizures | Technology‐dependent children | Intravenous access and phlebotomy | Cost‐effective care |
| Central nervous system infections | Shock | Transport of the critically ill child | Lumbar puncture | Education |
| Diabetes mellitus | Sickle cell disease | Non‐invasive monitoring | Ethics | |
| Failure to thrive | Skin and soft tissue infection | Nutrition | Evidence‐based medicine | |
| Fever of unknown origin | Toxic ingestion | Oxygen delivery and airway management | Health information systems | |
| Gastroenteritis | Upper airway infections | Pain management | Legal issues/risk management | |
| Kawasaki disease | Urinary tract infections | Pediatric advanced life support | Patient safety |
The Specialized Clinical Servicessection addresses important components of care that are not DRG‐based and reflect the unique needs of hospitalized children, as assessed by the CCTF, editors, and contributors. Core Skillswere chosen based on the HCUP Factbook 2 Procedures,18 billing data from randomly‐selected practicing hospitalists representing the same settings listed above, and critical input from reviewers. Depending on the individual setting, pediatric hospitalists may require skills in areas not found in these 11 chapters, such as chest tube placement or ventilator management. The list is therefore not exhaustive, but rather representative of skills most pediatric hospitalists should maintain.
The Healthcare Systems: Supporting and Advancing Child Healthchapters are likely the most dissimilar to any core content taught in traditional residency programs. While residency graduates are versed in some components listed in these chapters, comprehensive education in most of these competencies is currently lacking. Improvement of healthcare systems is an essential element of pediatric hospital medicine, and unifies all pediatric hospitalists regardless of practice environment or patient population. Therefore, this section includes chapters that not only focus on systems of care, but also on advancing child health through advocacy, research, education, evidence‐based medicine, and ethical practice. These chapters were drawn from a combination of several sources: expectations of external agencies (TJC, Center for Medicaid and Medicare) related to the specific nonclinical work in which pediatric hospitalists are integrally involved; expectations for advocacy as best defined by the AAP and the National Association of Children's Hospitals and Related Institutions (NACHRI); the six core competency domains mandated by the Accrediting Council on Graduate Medical Education (ACGME), the American Board of Pediatrics (ABP), and hospital medical staff offices as part of Focused Professional Practice Evaluation (FPPE) and Ongoing Professional Practice Evaluation (OPPE)16; and assessment of responsibilities and leadership roles fulfilled by pediatric hospitalists in all venues. In keeping with the intent of the competencies to be timeless, the competency elements call out the need to attend to the changing goals of these groups as well as those of the Institute of Healthcare Improvement (IHI), the Alliance for Pediatric Quality (which consists of ABP, AAP, TJC, CHCA, NACHRI), and local hospital systems leaders.
Contributors and Review
The CCTF selected section (associate) editors from SHM based on established expertise in each area, with input from the SHM Pediatric and Education Committees and the SHM Board. As a collaborative effort, authors for various chapters were solicited in consultation with experts from the AAP, APA, and SHM, and included non‐hospitalists with reputations as experts in various fields. Numerous SHM Pediatric Committee and CCTF conference calls were held to review hospital and academic appointments, presentations given, and affiliations relevant to the practice of pediatric hospital medicine. This vetting process resulted in a robust author list representing diverse geographic and practice settings. Contributors were provided with structure (Knowledge, Skills, Attitudes, and Systems subsections) and content (timeless, competency based) guidelines.
The review process was rigorous, and included both internal and external reviewers. The APA review in 2007 included the PHM Special Interest Group as well as the PHM Fellowship Directors (Table 1). After return to SHM and further editing, the internal review commenced which focused on content and scope. The editors addressed the resulting suggestions and worked to standardize formatting and use of Bloom's taxonomy.19 A list of common terms and phrases were created to add consistency between chapters. External reviewers were first mailed a letter requesting interest, which was followed up by emails, letters, and phone calls to encourage feedback. External review included 29 solicited agencies and societies (Table 3), with overall response rate of 66% (41% for Groups I and II). Individual contributors then reviewed comments specific to their chapters, with associate editor overview of their respective sections. The editors reviewed each chapter individually multiple times throughout the 2007‐2009 years, contacting individual contributors and reviewers by email and phone. Editors concluded a final comprehensive review of all chapters in late 2009.
| I. Academic and certifying societies |
| Academic Pediatric Association |
| Accreditation Council for Graduate Medical Education, Pediatric Residency Review Committee |
| American Academy of Family Physicians |
| American Academy of Pediatrics Board |
| American Academy of Pediatrics National Committee on Hospital Care |
| American Association of Critical Care Nursing |
| American Board of Family Medicine |
| American Board of Pediatrics |
| American College of Emergency Physicians |
| American Pediatric Society |
| Association of American Medical Colleges |
| Association of Medical School Pediatric Department Chairs (AMSPDC) |
| Association of Pediatric Program Directors |
| Council on Teaching Hospitals |
| Society of Pediatric Research |
| II. Stakeholder agencies |
| Agency for Healthcare Research and Quality |
| American Association of Critical Care Nursing |
| American College of Emergency Physicians |
| American Hospital Association (AHA) |
| American Nurses Association |
| American Society of Health‐System Pharmacists |
| Child Health Corporation of America (CHCA) |
| Institute for Healthcare Improvement |
| National Association for Children's Hospitals and Related Institutions (NACHRI) |
| National Association of Pediatric Nurse Practitioners (NAPNAP) |
| National Initiative for Children's Healthcare Quality (NICHQ) |
| National Quality Forum |
| Quality Resources International |
| Robert Wood Johnson Foundation |
| The Joint Commission for Accreditation of Hospitals and Organizations (TJC) |
| III. Pediatric hospital medicine fellowship directors |
| Boston Children's |
| Children's Hospital Los Angeles |
| Children's National D.C. |
| Emory |
| Hospital for Sick Kids Toronto |
| Rady Children's San Diego University of California San Diego |
| Riley Children's Hospital Indiana |
| University of South Florida, All Children's Hospital |
| Texas Children's Hospital, Baylor College of Medicine |
| IV. SHM, APA, AAP Leadership and committee chairs |
| American Academy of Pediatrics Section on Hospital Medicine |
| Academic Pediatric Association PHM Special Interest Group |
| SHM Board |
| SHM Education Committee |
| SHM Family Practice Committee |
| SHM Hospital Quality and Patient Safety Committee |
| SHM IT Task Force |
| SHM Journal Editorial Board |
| SHM Palliative Care Task Force |
| SHM Practice Analysis Committee |
| SHM Public Policy Committee |
| SHM Research Committee |
Chapter Content
Each of the 54 chapters within the four sections of these competencies is presented in the educational theory of learning domains: Knowledge, Skills, Attitudes, with a final Systems domain added to reflect the emphasis of hospitalist practice on improving healthcare systems. Each chapter is designed to stand alone, which may assist with development of curriculum at individual practice locations. Certain key phrases are apparent throughout, such as lead, coordinate, or participate in and work with hospital and community leaders to which were designed to note the varied roles in different practice settings. Some chapters specifically comment on the application of competency bullets given the unique and differing roles and expectations of pediatric hospitalists, such as research and education. Chapters state specific proficiencies expected wherever possible, with phrases and wording selected to help guide learning activities to achieve the competency.
Application and Future Directions
Although pediatric hospitalists care for children in many settings, these core competencies address the common expectations for any venue. Pediatric hospital medicine requires skills in acute care clinical medicine that attend to the changing needs of hospitalized children. The core of pediatric hospital medicine is dedicated to the care of children in the geographic hospital environment between emergency medicine and tertiary pediatric and neonatal intensive care units. Pediatric hospitalists provide care in related clinical service programs that are linked to hospital systems. In performing these activities, pediatric hospitalists consistently partner with ambulatory providers and subspecialists to render coordinated care across the continuum for a given child. Pediatric hospital medicine is an interdisciplinary practice, with focus on processes of care and clinical quality outcomes based in evidence. Engagement in local, state, and national initiatives to improve child health outcomes is a cornerstone of pediatric hospitalists' practice. These competencies provide the framework for creation of curricula that can reflect local issues and react to changing evidence.
As providers of systems‐based care, pediatric hospitalists are called upon more and more to render care and provide leadership in clinical arenas that are integral to healthcare organizations, such as home health care, sub‐acute care facilities, and hospice and palliative care programs. The practice of pediatric hospital medicine has evolved to its current state through efforts of many represented in the competencies as contributors, associate editors, editors, and reviewers. Pediatric hospitalists are committed to leading change in healthcare for hospitalized children, and are positioned well to address the interests and needs of community and urban, teaching and non‐teaching facilities, and the children and families they serve. These competencies reflect the areas of focused practice which, similar to pediatric emergency medicine, will no doubt be refined but not fundamentally changed in future years. The intent, we hope, is clear: to provide excellence in clinical care, accountability for practice, and lead improvements in healthcare for hospitalized children.
Introduction
The Society of Hospital Medicine (SHM) defines hospitalists as physicians whose primary professional focus is the comprehensive general medical care of hospitalized patients. Their activities include patient care, teaching, research, and leadership related to Hospital Medicine.1 It is estimated that there are up to 2500 pediatric hospitalists in the United States, with continued growth due to the converging needs for a dedicated focus on patient safety, quality improvement, hospital throughput, and inpatient teaching.2‐9 (Pediatric Hospital Medicine (PHM), as defined today, has been practiced in the United States for at least 30 years10 and continues to evolve as an area of specialization, with the refinement of a distinct knowledgebase and skill set focused on the provision of high quality general pediatric care in the inpatient setting. PHM is the latest site‐specific specialty to emerge from the field of general pediatrics it's development analogous to the evolution of critical care or emergency medicine during previous decades.11 Adult hospital medicine has defined itself within the field of general internal medicine12 and has recently received approval to provide a recognized focus of practice exam in 2010 for those re‐certifying with the American Board of Internal Medicine,13 PHM is creating an identity as a subspecialty practice with distinct focus on inpatient care for children within the larger context of general pediatric care.8, 14
The Pediatric Hospital Medicine Core Competencies were created to help define the roles and expectations for pediatric hospitalists, regardless of practice setting. The intent is to provide a unified approach toward identifying the specific body of knowledge and measurable skills needed to assure delivery of the highest quality of care for all hospitalized pediatric patients. Most children requiring hospitalization in the United States are hospitalized in community settings where subspecialty support is more limited and many pediatric services may be unavailable. Children with complex, chronic medical problems, however, are more likely to be hospitalized at a tertiary care or academic institutions. In order to unify pediatric hospitalists who work in different practice environments, the PHM Core Competencies were constructed to represent the knowledge, skills, attitudes, and systems improvements that all pediatric hospitalists can be expected to acquire and maintain.
Furthermore, the content of the PHM Core Competencies reflect the fact that children are a vulnerable population. Their care requires attention to many elements which distinguishes it from that given to the majority of the adult population: dependency, differences in developmental physiology and behavior, occurrence of congenital genetic disorders and age‐based clinical conditions, impact of chronic disease states on whole child development, and weight‐based medication dosing often with limited guidance from pediatric studies, to name a few. Awareness of these needs must be heightened when a child enters the hospital where diagnoses, procedures, and treatments often include use of high‐risk modalities and require coordination of care across multiple providers.
Pediatric hospitalists commonly work to improve the systems of care in which they operate and therefore both clinical and non‐clinical topics are included. The 54 chapters address the fundamental and most common components of inpatient care but are not an extensive review of all aspects of inpatient medicine encountered by those caring for hospitalized children. Finally, the PHM Core Competencies are not intended for use in assessing proficiency immediately post‐residency, but do provide a framework for the education and evaluation of both physicians‐in‐training and practicing hospitalists. Meeting these competencies is anticipated to take from one to three years of active practice in pediatric hospital medicine, and may be reached through a combination of practice experience, course work, self‐directed work, and/or formalized training.
Methods
Timeline
In 2002, SHM convened an educational summit from which there was a resolution to create core competencies. Following the summit, the SHM Pediatric Core Curriculum Task Force (CCTF) was created, which included 12 pediatric hospitalists practicing in academic and community facilities, as well as teaching and non‐teaching settings, and occupying leadership positions within institutions of varied size and geographic location. Shortly thereafter, in November 2003, approximately 130 pediatric hospitalists attended the first PHM meeting in San Antonio, Texas.11 At this meeting, with support from leaders in pediatric emergency medicine, first discussions regarding PHM scope of practice were held.
Formal development of the competencies began in 2005 in parallel to but distinct from SHM's adult work, which culminated in The Core Competencies in Hospital Medicine: A Framework for Curriculum Development published in 2006. The CCTF divided into three groups, focused on clinical, procedural, and systems‐based topics. Face‐to‐face meetings were held at the SHM annual meetings, with most work being completed by phone and electronically in the interim periods. In 2007, due to the overlapping interests of the three core pediatric societies, the work was transferred to leaders within the APA. In 2008 the work was transferred back to the leadership within SHM. Since that time, external reviewers were solicited, new chapters created, sections re‐aligned, internal and external reviewer comments incorporated, and final edits for taxonomy, content, and formatting were completed (Table 1).
| Date | Event |
|---|---|
| Feb 2002 | SHM Educational Summit held and CCTF created |
| Oct 2003 | 1st PHM meeting held in San Antonio |
| 2003‐2007 | Chapter focus determined; contributors engaged |
| 2007‐2008 | APA PHM Special Interest Group (SIG) review; creation of separate PHM Fellowship Competencies (not in this document) |
| Aug 2008‐Oct 2008 | SHM Pediatric Committee and CCTF members resume work; editorial review |
| Oct 2008‐Mar 2009 | Internal review: PHM Fellowship Director, AAP, APA, and SHM section/committee leader, and key national PHM leader reviews solicited and returned |
| Mar 2009 | PHM Fellowship Director comments addressed; editorial review |
| Mar‐Apr 2009 | External reviewers solicited from national agencies and societies relevant to PHM |
| Apr‐July 2009 | External reviewer comments returned |
| July‐Oct 2009 | Contributor review of all comments; editorial review, sections revised |
| Oct 2009 | Final review: Chapters to SHM subcommittees and Board |
Areas of Focused Practice
The PHM Core Competencies were conceptualized similarly to the SHM adult core competencies. Initial sections were divided into clinical conditions, procedures, and systems. However as content developed and reviewer comments were addressed, the four final sections were modified to those noted in Table 2. For the Common Clinical Diagnoses and Conditions, the goal was to select conditions most commonly encountered by pediatric hospitalists. Non‐surgical diagnosis‐related group (DRG) conditions were selected from the following sources: The Joint Commission's (TJC) Oryx Performance Measures Report15‐16 (asthma, abdominal pain, acute gastroenteritis, simple pneumonia); Child Health Corporation of America's Pediatric Health Information System Dataset (CHCA PHIS, Shawnee Mission, KS), and relevant publications on common pediatric hospitalizations.17 These data were compared to billing data from randomly‐selected practicing hospitalists representing free‐standing children's and community hospitals, teaching and non‐teaching settings, and urban and rural locations. The 22 clinical conditions chosen by the CCTF were those most relevant to the practice of pediatric hospital medicine.
| Common Clinical Diagnoses and Conditions | Specialized Clinical Services | Core Skills | Healthcare Systems: Supporting and Advancing Child Health | |
|---|---|---|---|---|
| Acute abdominal pain and the acute abdomen | Neonatal fever | Child abuse and neglect | Bladder catheterization/suprapubic bladder tap | Advocacy |
| Apparent life‐threatening event | Neonatal jaundice | Hospice and palliative care | Electrocardiogram interpretation | Business practices |
| Asthma | Pneumonia | Leading a healthcare team | Feeding tubes | Communication |
| Bone and joint infections | Respiratory failure | Newborn care and delivery room management | Fluids and electrolyte management | Continuous quality improvement |
| Bronchiolitis | Seizures | Technology‐dependent children | Intravenous access and phlebotomy | Cost‐effective care |
| Central nervous system infections | Shock | Transport of the critically ill child | Lumbar puncture | Education |
| Diabetes mellitus | Sickle cell disease | Non‐invasive monitoring | Ethics | |
| Failure to thrive | Skin and soft tissue infection | Nutrition | Evidence‐based medicine | |
| Fever of unknown origin | Toxic ingestion | Oxygen delivery and airway management | Health information systems | |
| Gastroenteritis | Upper airway infections | Pain management | Legal issues/risk management | |
| Kawasaki disease | Urinary tract infections | Pediatric advanced life support | Patient safety |
The Specialized Clinical Servicessection addresses important components of care that are not DRG‐based and reflect the unique needs of hospitalized children, as assessed by the CCTF, editors, and contributors. Core Skillswere chosen based on the HCUP Factbook 2 Procedures,18 billing data from randomly‐selected practicing hospitalists representing the same settings listed above, and critical input from reviewers. Depending on the individual setting, pediatric hospitalists may require skills in areas not found in these 11 chapters, such as chest tube placement or ventilator management. The list is therefore not exhaustive, but rather representative of skills most pediatric hospitalists should maintain.
The Healthcare Systems: Supporting and Advancing Child Healthchapters are likely the most dissimilar to any core content taught in traditional residency programs. While residency graduates are versed in some components listed in these chapters, comprehensive education in most of these competencies is currently lacking. Improvement of healthcare systems is an essential element of pediatric hospital medicine, and unifies all pediatric hospitalists regardless of practice environment or patient population. Therefore, this section includes chapters that not only focus on systems of care, but also on advancing child health through advocacy, research, education, evidence‐based medicine, and ethical practice. These chapters were drawn from a combination of several sources: expectations of external agencies (TJC, Center for Medicaid and Medicare) related to the specific nonclinical work in which pediatric hospitalists are integrally involved; expectations for advocacy as best defined by the AAP and the National Association of Children's Hospitals and Related Institutions (NACHRI); the six core competency domains mandated by the Accrediting Council on Graduate Medical Education (ACGME), the American Board of Pediatrics (ABP), and hospital medical staff offices as part of Focused Professional Practice Evaluation (FPPE) and Ongoing Professional Practice Evaluation (OPPE)16; and assessment of responsibilities and leadership roles fulfilled by pediatric hospitalists in all venues. In keeping with the intent of the competencies to be timeless, the competency elements call out the need to attend to the changing goals of these groups as well as those of the Institute of Healthcare Improvement (IHI), the Alliance for Pediatric Quality (which consists of ABP, AAP, TJC, CHCA, NACHRI), and local hospital systems leaders.
Contributors and Review
The CCTF selected section (associate) editors from SHM based on established expertise in each area, with input from the SHM Pediatric and Education Committees and the SHM Board. As a collaborative effort, authors for various chapters were solicited in consultation with experts from the AAP, APA, and SHM, and included non‐hospitalists with reputations as experts in various fields. Numerous SHM Pediatric Committee and CCTF conference calls were held to review hospital and academic appointments, presentations given, and affiliations relevant to the practice of pediatric hospital medicine. This vetting process resulted in a robust author list representing diverse geographic and practice settings. Contributors were provided with structure (Knowledge, Skills, Attitudes, and Systems subsections) and content (timeless, competency based) guidelines.
The review process was rigorous, and included both internal and external reviewers. The APA review in 2007 included the PHM Special Interest Group as well as the PHM Fellowship Directors (Table 1). After return to SHM and further editing, the internal review commenced which focused on content and scope. The editors addressed the resulting suggestions and worked to standardize formatting and use of Bloom's taxonomy.19 A list of common terms and phrases were created to add consistency between chapters. External reviewers were first mailed a letter requesting interest, which was followed up by emails, letters, and phone calls to encourage feedback. External review included 29 solicited agencies and societies (Table 3), with overall response rate of 66% (41% for Groups I and II). Individual contributors then reviewed comments specific to their chapters, with associate editor overview of their respective sections. The editors reviewed each chapter individually multiple times throughout the 2007‐2009 years, contacting individual contributors and reviewers by email and phone. Editors concluded a final comprehensive review of all chapters in late 2009.
| I. Academic and certifying societies |
| Academic Pediatric Association |
| Accreditation Council for Graduate Medical Education, Pediatric Residency Review Committee |
| American Academy of Family Physicians |
| American Academy of Pediatrics Board |
| American Academy of Pediatrics National Committee on Hospital Care |
| American Association of Critical Care Nursing |
| American Board of Family Medicine |
| American Board of Pediatrics |
| American College of Emergency Physicians |
| American Pediatric Society |
| Association of American Medical Colleges |
| Association of Medical School Pediatric Department Chairs (AMSPDC) |
| Association of Pediatric Program Directors |
| Council on Teaching Hospitals |
| Society of Pediatric Research |
| II. Stakeholder agencies |
| Agency for Healthcare Research and Quality |
| American Association of Critical Care Nursing |
| American College of Emergency Physicians |
| American Hospital Association (AHA) |
| American Nurses Association |
| American Society of Health‐System Pharmacists |
| Child Health Corporation of America (CHCA) |
| Institute for Healthcare Improvement |
| National Association for Children's Hospitals and Related Institutions (NACHRI) |
| National Association of Pediatric Nurse Practitioners (NAPNAP) |
| National Initiative for Children's Healthcare Quality (NICHQ) |
| National Quality Forum |
| Quality Resources International |
| Robert Wood Johnson Foundation |
| The Joint Commission for Accreditation of Hospitals and Organizations (TJC) |
| III. Pediatric hospital medicine fellowship directors |
| Boston Children's |
| Children's Hospital Los Angeles |
| Children's National D.C. |
| Emory |
| Hospital for Sick Kids Toronto |
| Rady Children's San Diego University of California San Diego |
| Riley Children's Hospital Indiana |
| University of South Florida, All Children's Hospital |
| Texas Children's Hospital, Baylor College of Medicine |
| IV. SHM, APA, AAP Leadership and committee chairs |
| American Academy of Pediatrics Section on Hospital Medicine |
| Academic Pediatric Association PHM Special Interest Group |
| SHM Board |
| SHM Education Committee |
| SHM Family Practice Committee |
| SHM Hospital Quality and Patient Safety Committee |
| SHM IT Task Force |
| SHM Journal Editorial Board |
| SHM Palliative Care Task Force |
| SHM Practice Analysis Committee |
| SHM Public Policy Committee |
| SHM Research Committee |
Chapter Content
Each of the 54 chapters within the four sections of these competencies is presented in the educational theory of learning domains: Knowledge, Skills, Attitudes, with a final Systems domain added to reflect the emphasis of hospitalist practice on improving healthcare systems. Each chapter is designed to stand alone, which may assist with development of curriculum at individual practice locations. Certain key phrases are apparent throughout, such as lead, coordinate, or participate in and work with hospital and community leaders to which were designed to note the varied roles in different practice settings. Some chapters specifically comment on the application of competency bullets given the unique and differing roles and expectations of pediatric hospitalists, such as research and education. Chapters state specific proficiencies expected wherever possible, with phrases and wording selected to help guide learning activities to achieve the competency.
Application and Future Directions
Although pediatric hospitalists care for children in many settings, these core competencies address the common expectations for any venue. Pediatric hospital medicine requires skills in acute care clinical medicine that attend to the changing needs of hospitalized children. The core of pediatric hospital medicine is dedicated to the care of children in the geographic hospital environment between emergency medicine and tertiary pediatric and neonatal intensive care units. Pediatric hospitalists provide care in related clinical service programs that are linked to hospital systems. In performing these activities, pediatric hospitalists consistently partner with ambulatory providers and subspecialists to render coordinated care across the continuum for a given child. Pediatric hospital medicine is an interdisciplinary practice, with focus on processes of care and clinical quality outcomes based in evidence. Engagement in local, state, and national initiatives to improve child health outcomes is a cornerstone of pediatric hospitalists' practice. These competencies provide the framework for creation of curricula that can reflect local issues and react to changing evidence.
As providers of systems‐based care, pediatric hospitalists are called upon more and more to render care and provide leadership in clinical arenas that are integral to healthcare organizations, such as home health care, sub‐acute care facilities, and hospice and palliative care programs. The practice of pediatric hospital medicine has evolved to its current state through efforts of many represented in the competencies as contributors, associate editors, editors, and reviewers. Pediatric hospitalists are committed to leading change in healthcare for hospitalized children, and are positioned well to address the interests and needs of community and urban, teaching and non‐teaching facilities, and the children and families they serve. These competencies reflect the areas of focused practice which, similar to pediatric emergency medicine, will no doubt be refined but not fundamentally changed in future years. The intent, we hope, is clear: to provide excellence in clinical care, accountability for practice, and lead improvements in healthcare for hospitalized children.
- Society of Hospital Medicine (SHM). Definition of a Hospitalist. http://www.hospitalmedicine.org/AM/Template.cfm?Section=General_Information 2009.
- .Pediatric Hospitalists Membership Numbers. In.Philadelphia:Society of Hospital Medicine, PA 19130;2009.
- , .The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- .The future of hospital medicine: evolution or revolution?.Am J Med.2004;117:446–450.
- , .The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- , , , , .Variation in pediatric hospitalists' use of proven and unproven therapies: A study from the Pediatric Research in Inpatient Settings (PRIS) network.J Hosp Med.2008;3(4):292–298.
- , , .Pediatric hospitalists: Training, current practice, and career goals.J Hosp Med.2009;4(3):179–186.
- , .Standardize to excellence: improving the quality and safety of care with clinical pathways.Pediatr Clin North Am.2009;56(4):893–904.
- .Evolution of a new specialty ‐ a twenty year pediatric hospitalist experience [Abstract]. In:National Association of Inpatient Physicians (now Society of Hospital Medicine).New Orleans, Louisiana;1999.
- , , , , , , et al.Pediatric hospitalists: report of a leadership conference.Pediatrics.2006;117(4):1122–1130.
- , , , , e.The core competencies in hospital medicine: a framework for curriculum development.J Hosp Med.2006;1(Suppl 1).
- American Board of Internal Medicine. Questions and answers regarding ABIM recognition of focused practice in hospital medicine through maintenance of certification. http://www.abim.org/news/news/focused‐practice‐hospital‐medicine‐qa.aspx. Published 2010. Accessed January 6,2010.
- .Comprehensive pediatric hospital medicine.N Engl J Med.2008;358(21):2301–2302.
- The Joint Commission. Performance measurement initiatives. http://www. jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/. Published 2010. Accessed December 5,2010.
- The Joint Commission. Standards frequently asked questions: comprehensive accreditation manual for critical access hospitals (CAMCAH). http://www.jointcommission.org/AccreditationPrograms/CriticalAccess Hospitals/Standards/09_FAQs/default.htm. Accessed December 5,2008; December 14, 2009.
- , , , , .Infectious disease hospitalizations among infants in the United States.Pediatrics.2008;121(2):244–252.
- , , , .Procedures in U.S. hospitals, 1997.HCUP fact book no. 2. In:agency for healthcare research and quality,Rockville, MD;2001.
- Anderson L, Krathwohl DR, Airasian PW, Cruikshank KA, Mayer RE, Pintrich PR, et al., editors.A taxonomy for learning, teaching, and assessing. In: A Revision of Bloom's Taxonomy of Educational Objectives.Upper Saddle River, NJ: Addison Wesley Longman, Inc. Pearson Education USA;2001.
- Society of Hospital Medicine (SHM). Definition of a Hospitalist. http://www.hospitalmedicine.org/AM/Template.cfm?Section=General_Information 2009.
- .Pediatric Hospitalists Membership Numbers. In.Philadelphia:Society of Hospital Medicine, PA 19130;2009.
- , .The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- .The future of hospital medicine: evolution or revolution?.Am J Med.2004;117:446–450.
- , .The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- , , , , .Variation in pediatric hospitalists' use of proven and unproven therapies: A study from the Pediatric Research in Inpatient Settings (PRIS) network.J Hosp Med.2008;3(4):292–298.
- , , .Pediatric hospitalists: Training, current practice, and career goals.J Hosp Med.2009;4(3):179–186.
- , .Standardize to excellence: improving the quality and safety of care with clinical pathways.Pediatr Clin North Am.2009;56(4):893–904.
- .Evolution of a new specialty ‐ a twenty year pediatric hospitalist experience [Abstract]. In:National Association of Inpatient Physicians (now Society of Hospital Medicine).New Orleans, Louisiana;1999.
- , , , , , , et al.Pediatric hospitalists: report of a leadership conference.Pediatrics.2006;117(4):1122–1130.
- , , , , e.The core competencies in hospital medicine: a framework for curriculum development.J Hosp Med.2006;1(Suppl 1).
- American Board of Internal Medicine. Questions and answers regarding ABIM recognition of focused practice in hospital medicine through maintenance of certification. http://www.abim.org/news/news/focused‐practice‐hospital‐medicine‐qa.aspx. Published 2010. Accessed January 6,2010.
- .Comprehensive pediatric hospital medicine.N Engl J Med.2008;358(21):2301–2302.
- The Joint Commission. Performance measurement initiatives. http://www. jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/. Published 2010. Accessed December 5,2010.
- The Joint Commission. Standards frequently asked questions: comprehensive accreditation manual for critical access hospitals (CAMCAH). http://www.jointcommission.org/AccreditationPrograms/CriticalAccess Hospitals/Standards/09_FAQs/default.htm. Accessed December 5,2008; December 14, 2009.
- , , , , .Infectious disease hospitalizations among infants in the United States.Pediatrics.2008;121(2):244–252.
- , , , .Procedures in U.S. hospitals, 1997.HCUP fact book no. 2. In:agency for healthcare research and quality,Rockville, MD;2001.
- Anderson L, Krathwohl DR, Airasian PW, Cruikshank KA, Mayer RE, Pintrich PR, et al., editors.A taxonomy for learning, teaching, and assessing. In: A Revision of Bloom's Taxonomy of Educational Objectives.Upper Saddle River, NJ: Addison Wesley Longman, Inc. Pearson Education USA;2001.
Copyright © 2010 Society of Hospital Medicine
Pediatric Hospital Medicine Core Competencies
Introduction
The Society of Hospital Medicine (SHM) defines hospitalists as physicians whose primary professional focus is the comprehensive general medical care of hospitalized patients. Their activities include patient care, teaching, research, and leadership related to Hospital Medicine.1 It is estimated that there are up to 2500 pediatric hospitalists in the United States, with continued growth due to the converging needs for a dedicated focus on patient safety, quality improvement, hospital throughput, and inpatient teaching.2‐9 (Pediatric Hospital Medicine (PHM), as defined today, has been practiced in the United States for at least 30 years10 and continues to evolve as an area of specialization, with the refinement of a distinct knowledgebase and skill set focused on the provision of high quality general pediatric care in the inpatient setting. PHM is the latest site‐specific specialty to emerge from the field of general pediatrics it's development analogous to the evolution of critical care or emergency medicine during previous decades.11 Adult hospital medicine has defined itself within the field of general internal medicine12 and has recently received approval to provide a recognized focus of practice exam in 2010 for those re‐certifying with the American Board of Internal Medicine,13 PHM is creating an identity as a subspecialty practice with distinct focus on inpatient care for children within the larger context of general pediatric care.8, 14
The Pediatric Hospital Medicine Core Competencies were created to help define the roles and expectations for pediatric hospitalists, regardless of practice setting. The intent is to provide a unified approach toward identifying the specific body of knowledge and measurable skills needed to assure delivery of the highest quality of care for all hospitalized pediatric patients. Most children requiring hospitalization in the United States are hospitalized in community settings where subspecialty support is more limited and many pediatric services may be unavailable. Children with complex, chronic medical problems, however, are more likely to be hospitalized at a tertiary care or academic institutions. In order to unify pediatric hospitalists who work in different practice environments, the PHM Core Competencies were constructed to represent the knowledge, skills, attitudes, and systems improvements that all pediatric hospitalists can be expected to acquire and maintain.
Furthermore, the content of the PHM Core Competencies reflect the fact that children are a vulnerable population. Their care requires attention to many elements which distinguishes it from that given to the majority of the adult population: dependency, differences in developmental physiology and behavior, occurrence of congenital genetic disorders and age‐based clinical conditions, impact of chronic disease states on whole child development, and weight‐based medication dosing often with limited guidance from pediatric studies, to name a few. Awareness of these needs must be heightened when a child enters the hospital where diagnoses, procedures, and treatments often include use of high‐risk modalities and require coordination of care across multiple providers.
Pediatric hospitalists commonly work to improve the systems of care in which they operate and therefore both clinical and non‐clinical topics are included. The 54 chapters address the fundamental and most common components of inpatient care but are not an extensive review of all aspects of inpatient medicine encountered by those caring for hospitalized children. Finally, the PHM Core Competencies are not intended for use in assessing proficiency immediately post‐residency, but do provide a framework for the education and evaluation of both physicians‐in‐training and practicing hospitalists. Meeting these competencies is anticipated to take from one to three years of active practice in pediatric hospital medicine, and may be reached through a combination of practice experience, course work, self‐directed work, and/or formalized training.
Methods
Timeline
In 2002, SHM convened an educational summit from which there was a resolution to create core competencies. Following the summit, the SHM Pediatric Core Curriculum Task Force (CCTF) was created, which included 12 pediatric hospitalists practicing in academic and community facilities, as well as teaching and non‐teaching settings, and occupying leadership positions within institutions of varied size and geographic location. Shortly thereafter, in November 2003, approximately 130 pediatric hospitalists attended the first PHM meeting in San Antonio, Texas.11 At this meeting, with support from leaders in pediatric emergency medicine, first discussions regarding PHM scope of practice were held.
Formal development of the competencies began in 2005 in parallel to but distinct from SHM's adult work, which culminated in The Core Competencies in Hospital Medicine: A Framework for Curriculum Development published in 2006. The CCTF divided into three groups, focused on clinical, procedural, and systems‐based topics. Face‐to‐face meetings were held at the SHM annual meetings, with most work being completed by phone and electronically in the interim periods. In 2007, due to the overlapping interests of the three core pediatric societies, the work was transferred to leaders within the APA. In 2008 the work was transferred back to the leadership within SHM. Since that time, external reviewers were solicited, new chapters created, sections re‐aligned, internal and external reviewer comments incorporated, and final edits for taxonomy, content, and formatting were completed (Table 1).
| Date | Event |
|---|---|
| Feb 2002 | SHM Educational Summit held and CCTF created |
| Oct 2003 | 1st PHM meeting held in San Antonio |
| 2003‐2007 | Chapter focus determined; contributors engaged |
| 2007‐2008 | APA PHM Special Interest Group (SIG) review; creation of separate PHM Fellowship Competencies (not in this document) |
| Aug 2008‐Oct 2008 | SHM Pediatric Committee and CCTF members resume work; editorial review |
| Oct 2008‐Mar 2009 | Internal review: PHM Fellowship Director, AAP, APA, and SHM section/committee leader, and key national PHM leader reviews solicited and returned |
| Mar 2009 | PHM Fellowship Director comments addressed; editorial review |
| Mar‐Apr 2009 | External reviewers solicited from national agencies and societies relevant to PHM |
| Apr‐July 2009 | External reviewer comments returned |
| July‐Oct 2009 | Contributor review of all comments; editorial review, sections revised |
| Oct 2009 | Final review: Chapters to SHM subcommittees and Board |
Areas of Focused Practice
The PHM Core Competencies were conceptualized similarly to the SHM adult core competencies. Initial sections were divided into clinical conditions, procedures, and systems. However as content developed and reviewer comments were addressed, the four final sections were modified to those noted in Table 2. For the Common Clinical Diagnoses and Conditions, the goal was to select conditions most commonly encountered by pediatric hospitalists. Non‐surgical diagnosis‐related group (DRG) conditions were selected from the following sources: The Joint Commission's (TJC) Oryx Performance Measures Report15‐16 (asthma, abdominal pain, acute gastroenteritis, simple pneumonia); Child Health Corporation of America's Pediatric Health Information System Dataset (CHCA PHIS, Shawnee Mission, KS), and relevant publications on common pediatric hospitalizations.17 These data were compared to billing data from randomly‐selected practicing hospitalists representing free‐standing children's and community hospitals, teaching and non‐teaching settings, and urban and rural locations. The 22 clinical conditions chosen by the CCTF were those most relevant to the practice of pediatric hospital medicine.
| Common Clinical Diagnoses and Conditions | Specialized Clinical Services | Core Skills | Healthcare Systems: Supporting and Advancing Child Health | |
|---|---|---|---|---|
| Acute abdominal pain and the acute abdomen | Neonatal fever | Child abuse and neglect | Bladder catheterization/suprapubic bladder tap | Advocacy |
| Apparent life‐threatening event | Neonatal Jaundice | Hospice and palliative care | Electrocardiogram interpretation | Business practices |
| Asthma | Pneumonia | Leading a healthcare team | Feeding Tubes | Communication |
| Bone and joint infections | Respiratory Failure | Newborn care and delivery room management | Fluids and Electrolyte Management | Continuous quality improvement |
| Bronchiolitis | Seizures | Technology dependent children | Intravenous access and phlebotomy | Cost‐effective care |
| Central nervous system infections | Shock | Transport of the critically ill child | Lumbar puncture | Education |
| Diabetes mellitus | Sickle cell disease | Non‐invasive monitoring | Ethics | |
| Failure to thrive | Skin and soft tissue infection | Nutrition | Evidence based medicine | |
| Fever of unknown origin | Toxic ingestion | Oxygen delivery and airway management | Health Information Systems | |
| Gastroenteritis | Upper airway infections | Pain management | Legal issues/risk management | |
| Kawasaki disease | Urinary Tract infections | Pediatric Advanced Life Support | Patient safety |
The Specialized Clinical Servicessection addresses important components of care that are not DRG‐based and reflect the unique needs of hospitalized children, as assessed by the CCTF, editors, and contributors. Core Skillswere chosen based on the HCUP Factbook 2 Procedures,18 billing data from randomly‐selected practicing hospitalists representing the same settings listed above, and critical input from reviewers. Depending on the individual setting, pediatric hospitalists may require skills in areas not found in these 11 chapters, such as chest tube placement or ventilator management. The list is therefore not exhaustive, but rather representative of skills most pediatric hospitalists should maintain.
The Healthcare Systems: Supporting and Advancing Child Healthchapters are likely the most dissimilar to any core content taught in traditional residency programs. While residency graduates are versed in some components listed in these chapters, comprehensive education in most of these competencies is currently lacking. Improvement of healthcare systems is an essential element of pediatric hospital medicine, and unifies all pediatric hospitalists regardless of practice environment or patient population. Therefore, this section includes chapters that not only focus on systems of care, but also on advancing child health through advocacy, research, education, evidence‐based medicine, and ethical practice. These chapters were drawn from a combination of several sources: expectations of external agencies (TJC, Center for Medicaid and Medicare) related to the specific nonclinical work in which pediatric hospitalists are integrally involved; expectations for advocacy as best defined by the AAP and the National Association of Children's Hospitals and Related Institutions (NACHRI); the six core competency domains mandated by the Accrediting Council on Graduate Medical Education (ACGME), the American Board of Pediatrics (ABP), and hospital medical staff offices as part of Focused Professional Practice Evaluation (FPPE) and Ongoing Professional Practice Evaluation (OPPE)16; and assessment of responsibilities and leadership roles fulfilled by pediatric hospitalists in all venues. In keeping with the intent of the competencies to be timeless, the competency elements call out the need to attend to the changing goals of these groups as well as those of the Institute of Healthcare Improvement (IHI), the Alliance for Pediatric Quality (which consists of ABP, AAP, TJC, CHCA, NACHRI), and local hospital systems leaders.
Contributors and Review
The CCTF selected section (associate) editors from SHM based on established expertise in each area, with input from the SHM Pediatric and Education Committees and the SHM Board. As a collaborative effort, authors for various chapters were solicited in consultation with experts from the AAP, APA, and SHM, and included non‐hospitalists with reputations as experts in various fields. Numerous SHM Pediatric Committee and CCTF conference calls were held to review hospital and academic appointments, presentations given, and affiliations relevant to the practice of pediatric hospital medicine. This vetting process resulted in a robust author list representing diverse geographic and practice settings. Contributors were provided with structure (Knowledge, Skills, Attitudes, and Systems subsections) and content (timeless, competency based) guidelines.
The review process was rigorous, and included both internal and external reviewers. The APA review in 2007 included the PHM Special Interest Group as well as the PHM Fellowship Directors (Table 1). After return to SHM and further editing, the internal review commenced which focused on content and scope. The editors addressed the resulting suggestions and worked to standardize formatting and use of Bloom's taxonomy.19 A list of common terms and phrases were created to add consistency between chapters. External reviewers were first mailed a letter requesting interest, which was followed up by emails, letters, and phone calls to encourage feedback. External review included 29 solicited agencies and societies (Table 3), with overall response rate of 66% (41% for Groups I and II). Individual contributors then reviewed comments specific to their chapters, with associate editor overview of their respective sections. The editors reviewed each chapter individually multiple times throughout the 2007‐2009 years, contacting individual contributors and reviewers by email and phone. Editors concluded a final comprehensive review of all chapters in late 2009.
| I. Academic and Certifying Societies |
| Academic Pediatric Association |
| Accreditation Council for Graduate Medical Education, Pediatric Residency Review Committee |
| American Academy of Family Physicians |
| American Academy of Pediatrics Board |
| American Academy of Pediatrics National Committee on Hospital Care |
| American Association of Critical Care Nursing |
| American Board of Family Medicine |
| American Board of Pediatrics |
| American College of Emergency Physicians |
| American Pediatric Society |
| Association of American Medical Colleges |
| Association of Medical School Pediatric Department Chairs (AMSPDC) |
| Association of Pediatric Program Directors |
| Council on Teaching Hospitals |
| Society of Pediatric Research |
| II. Stakeholder agencies |
| Agency for Healthcare Research and Quality |
| American Association of Critical Care Nursing |
| American College of Emergency Physicians |
| American Hospital Association (AHA) |
| American Nurses Association |
| American Society of Health‐System Pharmacists |
| Child Health Corporation of America (CHCA) |
| Institute for Healthcare Improvement |
| National Association for Children's Hospitals and Related Institutions (NACHRI) |
| National Association of Pediatric Nurse Practitioners (NAPNAP) |
| National Initiative for Children's Healthcare Quality (NICHQ) |
| National Quality Forum |
| Quality Resources International |
| Robert Wood Johnson Foundation |
| The Joint Commission for Accreditation of Hospitals and Organizations (TJC) |
| III. Pediatric Hospital Medicine Fellowship Directors |
| Boston Children's |
| Children's Hospital Los Angeles |
| Children's National D.C. |
| Emory |
| Hospital for Sick Kids Toronto |
| Rady Children's San Diego University of California San Diego |
| Riley Children's Hospital Indiana |
| University of South Florida, All Children's Hospital |
| Texas Children's Hospital, Baylor College of Medicine |
| IV. SHM, APA, AAP Leadership and committee chairs |
| American Academy of Pediatrics Section on Hospital Medicine |
| Academic Pediatric Association PHM Special Interest Group |
| SHM Board |
| SHM Education Committee |
| SHM Family Practice Committee |
| SHM Hospital Quality and Patient Safety Committee |
| SHM IT Task Force |
| SHM Journal Editorial Board |
| SHM Palliative Care Task Force |
| SHM Practice Analysis Committee |
| SHM Public Policy Committee |
| SHM Research Committee |
Chapter Content
Each of the 54 chapters within the four sections of these competencies is presented in the educational theory of learning domains: Knowledge, Skills, Attitudes, with a final Systems domain added to reflect the emphasis of hospitalist practice on improving healthcare systems. Each chapter is designed to stand alone, which may assist with development of curriculum at individual practice locations. Certain key phrases are apparent throughout, such as lead, coordinate, or participate in and work with hospital and community leaders to which were designed to note the varied roles in different practice settings. Some chapters specifically comment on the application of competency bullets given the unique and differing roles and expectations of pediatric hospitalists, such as research and education. Chapters state specific proficiencies expected wherever possible, with phrases and wording selected to help guide learning activities to achieve the competency.
Application and Future Directions
Although pediatric hospitalists care for children in many settings, these core competencies address the common expectations for any venue. Pediatric hospital medicine requires skills in acute care clinical medicine that attend to the changing needs of hospitalized children. The core of pediatric hospital medicine is dedicated to the care of children in the geographic hospital environment between emergency medicine and tertiary pediatric and neonatal intensive care units. Pediatric hospitalists provide care in related clinical service programs that are linked to hospital systems. In performing these activities, pediatric hospitalists consistently partner with ambulatory providers and subspecialists to render coordinated care across the continuum for a given child. Pediatric hospital medicine is an interdisciplinary practice, with focus on processes of care and clinical quality outcomes based in evidence. Engagement in local, state, and national initiatives to improve child health outcomes is a cornerstone of pediatric hospitalists' practice. These competencies provide the framework for creation of curricula that can reflect local issues and react to changing evidence.
As providers of systems‐based care, pediatric hospitalists are called upon more and more to render care and provide leadership in clinical arenas that are integral to healthcare organizations, such as home health care, sub‐acute care facilities, and hospice and palliative care programs. The practice of pediatric hospital medicine has evolved to its current state through efforts of many represented in the competencies as contributors, associate editors, editors, and reviewers. Pediatric hospitalists are committed to leading change in healthcare for hospitalized children, and are positioned well to address the interests and needs of community and urban, teaching and non‐teaching facilities, and the children and families they serve. These competencies reflect the areas of focused practice which, similar to pediatric emergency medicine, will no doubt be refined but not fundamentally changed in future years. The intent, we hope, is clear: to provide excellence in clinical care, accountability for practice, and lead improvements in healthcare for hospitalized children.
- Society of Hospital Medicine (SHM). Definition of a Hospitalist. http://www.hospitalmedicine.org/AM/Template.cfm?Section=General_Information 2009.
- Todd von Deak MBA CAE Vice President Membership and Marketing.Pediatric Hospitalists Membership Numbers. In.Philadelphia:Society of Hospital Medicine National Office 1500 Spring Garden, Suite 501, Philadelphia, PA 19130;2009.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- .The future of hospital medicine: evolution or revolution?Am J Med.2004;117:446–450.
- ,.The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- ,,,,.Variation in pediatric hospitalists' use of proven and unproven therapies: A study from the Pediatric Research in Inpatient Settings (PRIS) network.Journal of Hospital Medicine.2008;3(4):292–298.
- ,,.Pediatric hospitalists: Training, current practice, and career goals.Journal of Hospital Medicine.2009;4(3):179–186.
- ,.Standardize to Excellence: Improving the Quality and Safety of Care with Clinical Pathways.Pediatric Clinics of North America.2009;56(4):893–904.
- .Evolution of a new specialty ‐ a twenty year pediatric hospitalist experience [Abstract]. In:National Association of Inpatient Physicians (now Society of Hospital Medicine).New Orleans, Louisiana;1999.
- ,,,,,, et al.Pediatric Hospitalists: Report of a Leadership Conference.Pediatrics.2006;117(4):1122–1130.
- ,,,, e.The Core Competencies in Hospital Medicine: A Framework for Curriculum Development.J Hosp Med.2006;1(Suppl 1).
- American Board of Internal Medicine. Questions and Answers regarding ABIM Recognition of Focused Practice in Hospital Medicine through Maintenance of Certification. http://www.abim.org/news/news/focused‐practice‐hospital‐medicine‐qa.aspx. Published 2010. Accessed January 6,2010.
- .Comprehensive Pediatric Hospital Medicine.N Engl J Med.2008;358(21):2301–2302.
- The Joint Commission. Performance Measurement Initiatives. http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/. Published 2010. Accessed December 5,2010.
- The Joint Commission. Standards Frequently Asked Questions: Comprehensive Accreditation Manual for Critical Access Hospitals (CAMCAH). http://www.jointcommission.org/AccreditationPrograms/CriticalAccessHospitals/Standards/09_FAQs/default.htm. Accessed December 5,2008; December 14, 2009.
- ,,,,.Infectious Disease Hospitalizations Among Infants in the United States.Pediatrics.2008;121(2):244–252.
- ,,,.Procedures in U.S. Hospitals, 1997.HCUP Fact Book No. 2. In:Agency for Healthcare Research and Quality,Rockville, MD;2001.
- Anderson L,Krathwohl DR,Airasian PW,Cruikshank KA,Mayer RE,Pintrich PR, et al., editors.A Taxonomy for Learning, Teaching, and Assessing — A Revision of Bloom's Taxonomy of Educational Objectives.Addison Wesley Longman, Inc.Pearson Education USA, One Lake Street Upper Saddle River, NJ; (2001).
Introduction
The Society of Hospital Medicine (SHM) defines hospitalists as physicians whose primary professional focus is the comprehensive general medical care of hospitalized patients. Their activities include patient care, teaching, research, and leadership related to Hospital Medicine.1 It is estimated that there are up to 2500 pediatric hospitalists in the United States, with continued growth due to the converging needs for a dedicated focus on patient safety, quality improvement, hospital throughput, and inpatient teaching.2‐9 (Pediatric Hospital Medicine (PHM), as defined today, has been practiced in the United States for at least 30 years10 and continues to evolve as an area of specialization, with the refinement of a distinct knowledgebase and skill set focused on the provision of high quality general pediatric care in the inpatient setting. PHM is the latest site‐specific specialty to emerge from the field of general pediatrics it's development analogous to the evolution of critical care or emergency medicine during previous decades.11 Adult hospital medicine has defined itself within the field of general internal medicine12 and has recently received approval to provide a recognized focus of practice exam in 2010 for those re‐certifying with the American Board of Internal Medicine,13 PHM is creating an identity as a subspecialty practice with distinct focus on inpatient care for children within the larger context of general pediatric care.8, 14
The Pediatric Hospital Medicine Core Competencies were created to help define the roles and expectations for pediatric hospitalists, regardless of practice setting. The intent is to provide a unified approach toward identifying the specific body of knowledge and measurable skills needed to assure delivery of the highest quality of care for all hospitalized pediatric patients. Most children requiring hospitalization in the United States are hospitalized in community settings where subspecialty support is more limited and many pediatric services may be unavailable. Children with complex, chronic medical problems, however, are more likely to be hospitalized at a tertiary care or academic institutions. In order to unify pediatric hospitalists who work in different practice environments, the PHM Core Competencies were constructed to represent the knowledge, skills, attitudes, and systems improvements that all pediatric hospitalists can be expected to acquire and maintain.
Furthermore, the content of the PHM Core Competencies reflect the fact that children are a vulnerable population. Their care requires attention to many elements which distinguishes it from that given to the majority of the adult population: dependency, differences in developmental physiology and behavior, occurrence of congenital genetic disorders and age‐based clinical conditions, impact of chronic disease states on whole child development, and weight‐based medication dosing often with limited guidance from pediatric studies, to name a few. Awareness of these needs must be heightened when a child enters the hospital where diagnoses, procedures, and treatments often include use of high‐risk modalities and require coordination of care across multiple providers.
Pediatric hospitalists commonly work to improve the systems of care in which they operate and therefore both clinical and non‐clinical topics are included. The 54 chapters address the fundamental and most common components of inpatient care but are not an extensive review of all aspects of inpatient medicine encountered by those caring for hospitalized children. Finally, the PHM Core Competencies are not intended for use in assessing proficiency immediately post‐residency, but do provide a framework for the education and evaluation of both physicians‐in‐training and practicing hospitalists. Meeting these competencies is anticipated to take from one to three years of active practice in pediatric hospital medicine, and may be reached through a combination of practice experience, course work, self‐directed work, and/or formalized training.
Methods
Timeline
In 2002, SHM convened an educational summit from which there was a resolution to create core competencies. Following the summit, the SHM Pediatric Core Curriculum Task Force (CCTF) was created, which included 12 pediatric hospitalists practicing in academic and community facilities, as well as teaching and non‐teaching settings, and occupying leadership positions within institutions of varied size and geographic location. Shortly thereafter, in November 2003, approximately 130 pediatric hospitalists attended the first PHM meeting in San Antonio, Texas.11 At this meeting, with support from leaders in pediatric emergency medicine, first discussions regarding PHM scope of practice were held.
Formal development of the competencies began in 2005 in parallel to but distinct from SHM's adult work, which culminated in The Core Competencies in Hospital Medicine: A Framework for Curriculum Development published in 2006. The CCTF divided into three groups, focused on clinical, procedural, and systems‐based topics. Face‐to‐face meetings were held at the SHM annual meetings, with most work being completed by phone and electronically in the interim periods. In 2007, due to the overlapping interests of the three core pediatric societies, the work was transferred to leaders within the APA. In 2008 the work was transferred back to the leadership within SHM. Since that time, external reviewers were solicited, new chapters created, sections re‐aligned, internal and external reviewer comments incorporated, and final edits for taxonomy, content, and formatting were completed (Table 1).
| Date | Event |
|---|---|
| Feb 2002 | SHM Educational Summit held and CCTF created |
| Oct 2003 | 1st PHM meeting held in San Antonio |
| 2003‐2007 | Chapter focus determined; contributors engaged |
| 2007‐2008 | APA PHM Special Interest Group (SIG) review; creation of separate PHM Fellowship Competencies (not in this document) |
| Aug 2008‐Oct 2008 | SHM Pediatric Committee and CCTF members resume work; editorial review |
| Oct 2008‐Mar 2009 | Internal review: PHM Fellowship Director, AAP, APA, and SHM section/committee leader, and key national PHM leader reviews solicited and returned |
| Mar 2009 | PHM Fellowship Director comments addressed; editorial review |
| Mar‐Apr 2009 | External reviewers solicited from national agencies and societies relevant to PHM |
| Apr‐July 2009 | External reviewer comments returned |
| July‐Oct 2009 | Contributor review of all comments; editorial review, sections revised |
| Oct 2009 | Final review: Chapters to SHM subcommittees and Board |
Areas of Focused Practice
The PHM Core Competencies were conceptualized similarly to the SHM adult core competencies. Initial sections were divided into clinical conditions, procedures, and systems. However as content developed and reviewer comments were addressed, the four final sections were modified to those noted in Table 2. For the Common Clinical Diagnoses and Conditions, the goal was to select conditions most commonly encountered by pediatric hospitalists. Non‐surgical diagnosis‐related group (DRG) conditions were selected from the following sources: The Joint Commission's (TJC) Oryx Performance Measures Report15‐16 (asthma, abdominal pain, acute gastroenteritis, simple pneumonia); Child Health Corporation of America's Pediatric Health Information System Dataset (CHCA PHIS, Shawnee Mission, KS), and relevant publications on common pediatric hospitalizations.17 These data were compared to billing data from randomly‐selected practicing hospitalists representing free‐standing children's and community hospitals, teaching and non‐teaching settings, and urban and rural locations. The 22 clinical conditions chosen by the CCTF were those most relevant to the practice of pediatric hospital medicine.
| Common Clinical Diagnoses and Conditions | Specialized Clinical Services | Core Skills | Healthcare Systems: Supporting and Advancing Child Health | |
|---|---|---|---|---|
| Acute abdominal pain and the acute abdomen | Neonatal fever | Child abuse and neglect | Bladder catheterization/suprapubic bladder tap | Advocacy |
| Apparent life‐threatening event | Neonatal Jaundice | Hospice and palliative care | Electrocardiogram interpretation | Business practices |
| Asthma | Pneumonia | Leading a healthcare team | Feeding Tubes | Communication |
| Bone and joint infections | Respiratory Failure | Newborn care and delivery room management | Fluids and Electrolyte Management | Continuous quality improvement |
| Bronchiolitis | Seizures | Technology dependent children | Intravenous access and phlebotomy | Cost‐effective care |
| Central nervous system infections | Shock | Transport of the critically ill child | Lumbar puncture | Education |
| Diabetes mellitus | Sickle cell disease | Non‐invasive monitoring | Ethics | |
| Failure to thrive | Skin and soft tissue infection | Nutrition | Evidence based medicine | |
| Fever of unknown origin | Toxic ingestion | Oxygen delivery and airway management | Health Information Systems | |
| Gastroenteritis | Upper airway infections | Pain management | Legal issues/risk management | |
| Kawasaki disease | Urinary Tract infections | Pediatric Advanced Life Support | Patient safety |
The Specialized Clinical Servicessection addresses important components of care that are not DRG‐based and reflect the unique needs of hospitalized children, as assessed by the CCTF, editors, and contributors. Core Skillswere chosen based on the HCUP Factbook 2 Procedures,18 billing data from randomly‐selected practicing hospitalists representing the same settings listed above, and critical input from reviewers. Depending on the individual setting, pediatric hospitalists may require skills in areas not found in these 11 chapters, such as chest tube placement or ventilator management. The list is therefore not exhaustive, but rather representative of skills most pediatric hospitalists should maintain.
The Healthcare Systems: Supporting and Advancing Child Healthchapters are likely the most dissimilar to any core content taught in traditional residency programs. While residency graduates are versed in some components listed in these chapters, comprehensive education in most of these competencies is currently lacking. Improvement of healthcare systems is an essential element of pediatric hospital medicine, and unifies all pediatric hospitalists regardless of practice environment or patient population. Therefore, this section includes chapters that not only focus on systems of care, but also on advancing child health through advocacy, research, education, evidence‐based medicine, and ethical practice. These chapters were drawn from a combination of several sources: expectations of external agencies (TJC, Center for Medicaid and Medicare) related to the specific nonclinical work in which pediatric hospitalists are integrally involved; expectations for advocacy as best defined by the AAP and the National Association of Children's Hospitals and Related Institutions (NACHRI); the six core competency domains mandated by the Accrediting Council on Graduate Medical Education (ACGME), the American Board of Pediatrics (ABP), and hospital medical staff offices as part of Focused Professional Practice Evaluation (FPPE) and Ongoing Professional Practice Evaluation (OPPE)16; and assessment of responsibilities and leadership roles fulfilled by pediatric hospitalists in all venues. In keeping with the intent of the competencies to be timeless, the competency elements call out the need to attend to the changing goals of these groups as well as those of the Institute of Healthcare Improvement (IHI), the Alliance for Pediatric Quality (which consists of ABP, AAP, TJC, CHCA, NACHRI), and local hospital systems leaders.
Contributors and Review
The CCTF selected section (associate) editors from SHM based on established expertise in each area, with input from the SHM Pediatric and Education Committees and the SHM Board. As a collaborative effort, authors for various chapters were solicited in consultation with experts from the AAP, APA, and SHM, and included non‐hospitalists with reputations as experts in various fields. Numerous SHM Pediatric Committee and CCTF conference calls were held to review hospital and academic appointments, presentations given, and affiliations relevant to the practice of pediatric hospital medicine. This vetting process resulted in a robust author list representing diverse geographic and practice settings. Contributors were provided with structure (Knowledge, Skills, Attitudes, and Systems subsections) and content (timeless, competency based) guidelines.
The review process was rigorous, and included both internal and external reviewers. The APA review in 2007 included the PHM Special Interest Group as well as the PHM Fellowship Directors (Table 1). After return to SHM and further editing, the internal review commenced which focused on content and scope. The editors addressed the resulting suggestions and worked to standardize formatting and use of Bloom's taxonomy.19 A list of common terms and phrases were created to add consistency between chapters. External reviewers were first mailed a letter requesting interest, which was followed up by emails, letters, and phone calls to encourage feedback. External review included 29 solicited agencies and societies (Table 3), with overall response rate of 66% (41% for Groups I and II). Individual contributors then reviewed comments specific to their chapters, with associate editor overview of their respective sections. The editors reviewed each chapter individually multiple times throughout the 2007‐2009 years, contacting individual contributors and reviewers by email and phone. Editors concluded a final comprehensive review of all chapters in late 2009.
| I. Academic and Certifying Societies |
| Academic Pediatric Association |
| Accreditation Council for Graduate Medical Education, Pediatric Residency Review Committee |
| American Academy of Family Physicians |
| American Academy of Pediatrics Board |
| American Academy of Pediatrics National Committee on Hospital Care |
| American Association of Critical Care Nursing |
| American Board of Family Medicine |
| American Board of Pediatrics |
| American College of Emergency Physicians |
| American Pediatric Society |
| Association of American Medical Colleges |
| Association of Medical School Pediatric Department Chairs (AMSPDC) |
| Association of Pediatric Program Directors |
| Council on Teaching Hospitals |
| Society of Pediatric Research |
| II. Stakeholder agencies |
| Agency for Healthcare Research and Quality |
| American Association of Critical Care Nursing |
| American College of Emergency Physicians |
| American Hospital Association (AHA) |
| American Nurses Association |
| American Society of Health‐System Pharmacists |
| Child Health Corporation of America (CHCA) |
| Institute for Healthcare Improvement |
| National Association for Children's Hospitals and Related Institutions (NACHRI) |
| National Association of Pediatric Nurse Practitioners (NAPNAP) |
| National Initiative for Children's Healthcare Quality (NICHQ) |
| National Quality Forum |
| Quality Resources International |
| Robert Wood Johnson Foundation |
| The Joint Commission for Accreditation of Hospitals and Organizations (TJC) |
| III. Pediatric Hospital Medicine Fellowship Directors |
| Boston Children's |
| Children's Hospital Los Angeles |
| Children's National D.C. |
| Emory |
| Hospital for Sick Kids Toronto |
| Rady Children's San Diego University of California San Diego |
| Riley Children's Hospital Indiana |
| University of South Florida, All Children's Hospital |
| Texas Children's Hospital, Baylor College of Medicine |
| IV. SHM, APA, AAP Leadership and committee chairs |
| American Academy of Pediatrics Section on Hospital Medicine |
| Academic Pediatric Association PHM Special Interest Group |
| SHM Board |
| SHM Education Committee |
| SHM Family Practice Committee |
| SHM Hospital Quality and Patient Safety Committee |
| SHM IT Task Force |
| SHM Journal Editorial Board |
| SHM Palliative Care Task Force |
| SHM Practice Analysis Committee |
| SHM Public Policy Committee |
| SHM Research Committee |
Chapter Content
Each of the 54 chapters within the four sections of these competencies is presented in the educational theory of learning domains: Knowledge, Skills, Attitudes, with a final Systems domain added to reflect the emphasis of hospitalist practice on improving healthcare systems. Each chapter is designed to stand alone, which may assist with development of curriculum at individual practice locations. Certain key phrases are apparent throughout, such as lead, coordinate, or participate in and work with hospital and community leaders to which were designed to note the varied roles in different practice settings. Some chapters specifically comment on the application of competency bullets given the unique and differing roles and expectations of pediatric hospitalists, such as research and education. Chapters state specific proficiencies expected wherever possible, with phrases and wording selected to help guide learning activities to achieve the competency.
Application and Future Directions
Although pediatric hospitalists care for children in many settings, these core competencies address the common expectations for any venue. Pediatric hospital medicine requires skills in acute care clinical medicine that attend to the changing needs of hospitalized children. The core of pediatric hospital medicine is dedicated to the care of children in the geographic hospital environment between emergency medicine and tertiary pediatric and neonatal intensive care units. Pediatric hospitalists provide care in related clinical service programs that are linked to hospital systems. In performing these activities, pediatric hospitalists consistently partner with ambulatory providers and subspecialists to render coordinated care across the continuum for a given child. Pediatric hospital medicine is an interdisciplinary practice, with focus on processes of care and clinical quality outcomes based in evidence. Engagement in local, state, and national initiatives to improve child health outcomes is a cornerstone of pediatric hospitalists' practice. These competencies provide the framework for creation of curricula that can reflect local issues and react to changing evidence.
As providers of systems‐based care, pediatric hospitalists are called upon more and more to render care and provide leadership in clinical arenas that are integral to healthcare organizations, such as home health care, sub‐acute care facilities, and hospice and palliative care programs. The practice of pediatric hospital medicine has evolved to its current state through efforts of many represented in the competencies as contributors, associate editors, editors, and reviewers. Pediatric hospitalists are committed to leading change in healthcare for hospitalized children, and are positioned well to address the interests and needs of community and urban, teaching and non‐teaching facilities, and the children and families they serve. These competencies reflect the areas of focused practice which, similar to pediatric emergency medicine, will no doubt be refined but not fundamentally changed in future years. The intent, we hope, is clear: to provide excellence in clinical care, accountability for practice, and lead improvements in healthcare for hospitalized children.
Introduction
The Society of Hospital Medicine (SHM) defines hospitalists as physicians whose primary professional focus is the comprehensive general medical care of hospitalized patients. Their activities include patient care, teaching, research, and leadership related to Hospital Medicine.1 It is estimated that there are up to 2500 pediatric hospitalists in the United States, with continued growth due to the converging needs for a dedicated focus on patient safety, quality improvement, hospital throughput, and inpatient teaching.2‐9 (Pediatric Hospital Medicine (PHM), as defined today, has been practiced in the United States for at least 30 years10 and continues to evolve as an area of specialization, with the refinement of a distinct knowledgebase and skill set focused on the provision of high quality general pediatric care in the inpatient setting. PHM is the latest site‐specific specialty to emerge from the field of general pediatrics it's development analogous to the evolution of critical care or emergency medicine during previous decades.11 Adult hospital medicine has defined itself within the field of general internal medicine12 and has recently received approval to provide a recognized focus of practice exam in 2010 for those re‐certifying with the American Board of Internal Medicine,13 PHM is creating an identity as a subspecialty practice with distinct focus on inpatient care for children within the larger context of general pediatric care.8, 14
The Pediatric Hospital Medicine Core Competencies were created to help define the roles and expectations for pediatric hospitalists, regardless of practice setting. The intent is to provide a unified approach toward identifying the specific body of knowledge and measurable skills needed to assure delivery of the highest quality of care for all hospitalized pediatric patients. Most children requiring hospitalization in the United States are hospitalized in community settings where subspecialty support is more limited and many pediatric services may be unavailable. Children with complex, chronic medical problems, however, are more likely to be hospitalized at a tertiary care or academic institutions. In order to unify pediatric hospitalists who work in different practice environments, the PHM Core Competencies were constructed to represent the knowledge, skills, attitudes, and systems improvements that all pediatric hospitalists can be expected to acquire and maintain.
Furthermore, the content of the PHM Core Competencies reflect the fact that children are a vulnerable population. Their care requires attention to many elements which distinguishes it from that given to the majority of the adult population: dependency, differences in developmental physiology and behavior, occurrence of congenital genetic disorders and age‐based clinical conditions, impact of chronic disease states on whole child development, and weight‐based medication dosing often with limited guidance from pediatric studies, to name a few. Awareness of these needs must be heightened when a child enters the hospital where diagnoses, procedures, and treatments often include use of high‐risk modalities and require coordination of care across multiple providers.
Pediatric hospitalists commonly work to improve the systems of care in which they operate and therefore both clinical and non‐clinical topics are included. The 54 chapters address the fundamental and most common components of inpatient care but are not an extensive review of all aspects of inpatient medicine encountered by those caring for hospitalized children. Finally, the PHM Core Competencies are not intended for use in assessing proficiency immediately post‐residency, but do provide a framework for the education and evaluation of both physicians‐in‐training and practicing hospitalists. Meeting these competencies is anticipated to take from one to three years of active practice in pediatric hospital medicine, and may be reached through a combination of practice experience, course work, self‐directed work, and/or formalized training.
Methods
Timeline
In 2002, SHM convened an educational summit from which there was a resolution to create core competencies. Following the summit, the SHM Pediatric Core Curriculum Task Force (CCTF) was created, which included 12 pediatric hospitalists practicing in academic and community facilities, as well as teaching and non‐teaching settings, and occupying leadership positions within institutions of varied size and geographic location. Shortly thereafter, in November 2003, approximately 130 pediatric hospitalists attended the first PHM meeting in San Antonio, Texas.11 At this meeting, with support from leaders in pediatric emergency medicine, first discussions regarding PHM scope of practice were held.
Formal development of the competencies began in 2005 in parallel to but distinct from SHM's adult work, which culminated in The Core Competencies in Hospital Medicine: A Framework for Curriculum Development published in 2006. The CCTF divided into three groups, focused on clinical, procedural, and systems‐based topics. Face‐to‐face meetings were held at the SHM annual meetings, with most work being completed by phone and electronically in the interim periods. In 2007, due to the overlapping interests of the three core pediatric societies, the work was transferred to leaders within the APA. In 2008 the work was transferred back to the leadership within SHM. Since that time, external reviewers were solicited, new chapters created, sections re‐aligned, internal and external reviewer comments incorporated, and final edits for taxonomy, content, and formatting were completed (Table 1).
| Date | Event |
|---|---|
| Feb 2002 | SHM Educational Summit held and CCTF created |
| Oct 2003 | 1st PHM meeting held in San Antonio |
| 2003‐2007 | Chapter focus determined; contributors engaged |
| 2007‐2008 | APA PHM Special Interest Group (SIG) review; creation of separate PHM Fellowship Competencies (not in this document) |
| Aug 2008‐Oct 2008 | SHM Pediatric Committee and CCTF members resume work; editorial review |
| Oct 2008‐Mar 2009 | Internal review: PHM Fellowship Director, AAP, APA, and SHM section/committee leader, and key national PHM leader reviews solicited and returned |
| Mar 2009 | PHM Fellowship Director comments addressed; editorial review |
| Mar‐Apr 2009 | External reviewers solicited from national agencies and societies relevant to PHM |
| Apr‐July 2009 | External reviewer comments returned |
| July‐Oct 2009 | Contributor review of all comments; editorial review, sections revised |
| Oct 2009 | Final review: Chapters to SHM subcommittees and Board |
Areas of Focused Practice
The PHM Core Competencies were conceptualized similarly to the SHM adult core competencies. Initial sections were divided into clinical conditions, procedures, and systems. However as content developed and reviewer comments were addressed, the four final sections were modified to those noted in Table 2. For the Common Clinical Diagnoses and Conditions, the goal was to select conditions most commonly encountered by pediatric hospitalists. Non‐surgical diagnosis‐related group (DRG) conditions were selected from the following sources: The Joint Commission's (TJC) Oryx Performance Measures Report15‐16 (asthma, abdominal pain, acute gastroenteritis, simple pneumonia); Child Health Corporation of America's Pediatric Health Information System Dataset (CHCA PHIS, Shawnee Mission, KS), and relevant publications on common pediatric hospitalizations.17 These data were compared to billing data from randomly‐selected practicing hospitalists representing free‐standing children's and community hospitals, teaching and non‐teaching settings, and urban and rural locations. The 22 clinical conditions chosen by the CCTF were those most relevant to the practice of pediatric hospital medicine.
| Common Clinical Diagnoses and Conditions | Specialized Clinical Services | Core Skills | Healthcare Systems: Supporting and Advancing Child Health | |
|---|---|---|---|---|
| Acute abdominal pain and the acute abdomen | Neonatal fever | Child abuse and neglect | Bladder catheterization/suprapubic bladder tap | Advocacy |
| Apparent life‐threatening event | Neonatal Jaundice | Hospice and palliative care | Electrocardiogram interpretation | Business practices |
| Asthma | Pneumonia | Leading a healthcare team | Feeding Tubes | Communication |
| Bone and joint infections | Respiratory Failure | Newborn care and delivery room management | Fluids and Electrolyte Management | Continuous quality improvement |
| Bronchiolitis | Seizures | Technology dependent children | Intravenous access and phlebotomy | Cost‐effective care |
| Central nervous system infections | Shock | Transport of the critically ill child | Lumbar puncture | Education |
| Diabetes mellitus | Sickle cell disease | Non‐invasive monitoring | Ethics | |
| Failure to thrive | Skin and soft tissue infection | Nutrition | Evidence based medicine | |
| Fever of unknown origin | Toxic ingestion | Oxygen delivery and airway management | Health Information Systems | |
| Gastroenteritis | Upper airway infections | Pain management | Legal issues/risk management | |
| Kawasaki disease | Urinary Tract infections | Pediatric Advanced Life Support | Patient safety |
The Specialized Clinical Servicessection addresses important components of care that are not DRG‐based and reflect the unique needs of hospitalized children, as assessed by the CCTF, editors, and contributors. Core Skillswere chosen based on the HCUP Factbook 2 Procedures,18 billing data from randomly‐selected practicing hospitalists representing the same settings listed above, and critical input from reviewers. Depending on the individual setting, pediatric hospitalists may require skills in areas not found in these 11 chapters, such as chest tube placement or ventilator management. The list is therefore not exhaustive, but rather representative of skills most pediatric hospitalists should maintain.
The Healthcare Systems: Supporting and Advancing Child Healthchapters are likely the most dissimilar to any core content taught in traditional residency programs. While residency graduates are versed in some components listed in these chapters, comprehensive education in most of these competencies is currently lacking. Improvement of healthcare systems is an essential element of pediatric hospital medicine, and unifies all pediatric hospitalists regardless of practice environment or patient population. Therefore, this section includes chapters that not only focus on systems of care, but also on advancing child health through advocacy, research, education, evidence‐based medicine, and ethical practice. These chapters were drawn from a combination of several sources: expectations of external agencies (TJC, Center for Medicaid and Medicare) related to the specific nonclinical work in which pediatric hospitalists are integrally involved; expectations for advocacy as best defined by the AAP and the National Association of Children's Hospitals and Related Institutions (NACHRI); the six core competency domains mandated by the Accrediting Council on Graduate Medical Education (ACGME), the American Board of Pediatrics (ABP), and hospital medical staff offices as part of Focused Professional Practice Evaluation (FPPE) and Ongoing Professional Practice Evaluation (OPPE)16; and assessment of responsibilities and leadership roles fulfilled by pediatric hospitalists in all venues. In keeping with the intent of the competencies to be timeless, the competency elements call out the need to attend to the changing goals of these groups as well as those of the Institute of Healthcare Improvement (IHI), the Alliance for Pediatric Quality (which consists of ABP, AAP, TJC, CHCA, NACHRI), and local hospital systems leaders.
Contributors and Review
The CCTF selected section (associate) editors from SHM based on established expertise in each area, with input from the SHM Pediatric and Education Committees and the SHM Board. As a collaborative effort, authors for various chapters were solicited in consultation with experts from the AAP, APA, and SHM, and included non‐hospitalists with reputations as experts in various fields. Numerous SHM Pediatric Committee and CCTF conference calls were held to review hospital and academic appointments, presentations given, and affiliations relevant to the practice of pediatric hospital medicine. This vetting process resulted in a robust author list representing diverse geographic and practice settings. Contributors were provided with structure (Knowledge, Skills, Attitudes, and Systems subsections) and content (timeless, competency based) guidelines.
The review process was rigorous, and included both internal and external reviewers. The APA review in 2007 included the PHM Special Interest Group as well as the PHM Fellowship Directors (Table 1). After return to SHM and further editing, the internal review commenced which focused on content and scope. The editors addressed the resulting suggestions and worked to standardize formatting and use of Bloom's taxonomy.19 A list of common terms and phrases were created to add consistency between chapters. External reviewers were first mailed a letter requesting interest, which was followed up by emails, letters, and phone calls to encourage feedback. External review included 29 solicited agencies and societies (Table 3), with overall response rate of 66% (41% for Groups I and II). Individual contributors then reviewed comments specific to their chapters, with associate editor overview of their respective sections. The editors reviewed each chapter individually multiple times throughout the 2007‐2009 years, contacting individual contributors and reviewers by email and phone. Editors concluded a final comprehensive review of all chapters in late 2009.
| I. Academic and Certifying Societies |
| Academic Pediatric Association |
| Accreditation Council for Graduate Medical Education, Pediatric Residency Review Committee |
| American Academy of Family Physicians |
| American Academy of Pediatrics Board |
| American Academy of Pediatrics National Committee on Hospital Care |
| American Association of Critical Care Nursing |
| American Board of Family Medicine |
| American Board of Pediatrics |
| American College of Emergency Physicians |
| American Pediatric Society |
| Association of American Medical Colleges |
| Association of Medical School Pediatric Department Chairs (AMSPDC) |
| Association of Pediatric Program Directors |
| Council on Teaching Hospitals |
| Society of Pediatric Research |
| II. Stakeholder agencies |
| Agency for Healthcare Research and Quality |
| American Association of Critical Care Nursing |
| American College of Emergency Physicians |
| American Hospital Association (AHA) |
| American Nurses Association |
| American Society of Health‐System Pharmacists |
| Child Health Corporation of America (CHCA) |
| Institute for Healthcare Improvement |
| National Association for Children's Hospitals and Related Institutions (NACHRI) |
| National Association of Pediatric Nurse Practitioners (NAPNAP) |
| National Initiative for Children's Healthcare Quality (NICHQ) |
| National Quality Forum |
| Quality Resources International |
| Robert Wood Johnson Foundation |
| The Joint Commission for Accreditation of Hospitals and Organizations (TJC) |
| III. Pediatric Hospital Medicine Fellowship Directors |
| Boston Children's |
| Children's Hospital Los Angeles |
| Children's National D.C. |
| Emory |
| Hospital for Sick Kids Toronto |
| Rady Children's San Diego University of California San Diego |
| Riley Children's Hospital Indiana |
| University of South Florida, All Children's Hospital |
| Texas Children's Hospital, Baylor College of Medicine |
| IV. SHM, APA, AAP Leadership and committee chairs |
| American Academy of Pediatrics Section on Hospital Medicine |
| Academic Pediatric Association PHM Special Interest Group |
| SHM Board |
| SHM Education Committee |
| SHM Family Practice Committee |
| SHM Hospital Quality and Patient Safety Committee |
| SHM IT Task Force |
| SHM Journal Editorial Board |
| SHM Palliative Care Task Force |
| SHM Practice Analysis Committee |
| SHM Public Policy Committee |
| SHM Research Committee |
Chapter Content
Each of the 54 chapters within the four sections of these competencies is presented in the educational theory of learning domains: Knowledge, Skills, Attitudes, with a final Systems domain added to reflect the emphasis of hospitalist practice on improving healthcare systems. Each chapter is designed to stand alone, which may assist with development of curriculum at individual practice locations. Certain key phrases are apparent throughout, such as lead, coordinate, or participate in and work with hospital and community leaders to which were designed to note the varied roles in different practice settings. Some chapters specifically comment on the application of competency bullets given the unique and differing roles and expectations of pediatric hospitalists, such as research and education. Chapters state specific proficiencies expected wherever possible, with phrases and wording selected to help guide learning activities to achieve the competency.
Application and Future Directions
Although pediatric hospitalists care for children in many settings, these core competencies address the common expectations for any venue. Pediatric hospital medicine requires skills in acute care clinical medicine that attend to the changing needs of hospitalized children. The core of pediatric hospital medicine is dedicated to the care of children in the geographic hospital environment between emergency medicine and tertiary pediatric and neonatal intensive care units. Pediatric hospitalists provide care in related clinical service programs that are linked to hospital systems. In performing these activities, pediatric hospitalists consistently partner with ambulatory providers and subspecialists to render coordinated care across the continuum for a given child. Pediatric hospital medicine is an interdisciplinary practice, with focus on processes of care and clinical quality outcomes based in evidence. Engagement in local, state, and national initiatives to improve child health outcomes is a cornerstone of pediatric hospitalists' practice. These competencies provide the framework for creation of curricula that can reflect local issues and react to changing evidence.
As providers of systems‐based care, pediatric hospitalists are called upon more and more to render care and provide leadership in clinical arenas that are integral to healthcare organizations, such as home health care, sub‐acute care facilities, and hospice and palliative care programs. The practice of pediatric hospital medicine has evolved to its current state through efforts of many represented in the competencies as contributors, associate editors, editors, and reviewers. Pediatric hospitalists are committed to leading change in healthcare for hospitalized children, and are positioned well to address the interests and needs of community and urban, teaching and non‐teaching facilities, and the children and families they serve. These competencies reflect the areas of focused practice which, similar to pediatric emergency medicine, will no doubt be refined but not fundamentally changed in future years. The intent, we hope, is clear: to provide excellence in clinical care, accountability for practice, and lead improvements in healthcare for hospitalized children.
- Society of Hospital Medicine (SHM). Definition of a Hospitalist. http://www.hospitalmedicine.org/AM/Template.cfm?Section=General_Information 2009.
- Todd von Deak MBA CAE Vice President Membership and Marketing.Pediatric Hospitalists Membership Numbers. In.Philadelphia:Society of Hospital Medicine National Office 1500 Spring Garden, Suite 501, Philadelphia, PA 19130;2009.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- .The future of hospital medicine: evolution or revolution?Am J Med.2004;117:446–450.
- ,.The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- ,,,,.Variation in pediatric hospitalists' use of proven and unproven therapies: A study from the Pediatric Research in Inpatient Settings (PRIS) network.Journal of Hospital Medicine.2008;3(4):292–298.
- ,,.Pediatric hospitalists: Training, current practice, and career goals.Journal of Hospital Medicine.2009;4(3):179–186.
- ,.Standardize to Excellence: Improving the Quality and Safety of Care with Clinical Pathways.Pediatric Clinics of North America.2009;56(4):893–904.
- .Evolution of a new specialty ‐ a twenty year pediatric hospitalist experience [Abstract]. In:National Association of Inpatient Physicians (now Society of Hospital Medicine).New Orleans, Louisiana;1999.
- ,,,,,, et al.Pediatric Hospitalists: Report of a Leadership Conference.Pediatrics.2006;117(4):1122–1130.
- ,,,, e.The Core Competencies in Hospital Medicine: A Framework for Curriculum Development.J Hosp Med.2006;1(Suppl 1).
- American Board of Internal Medicine. Questions and Answers regarding ABIM Recognition of Focused Practice in Hospital Medicine through Maintenance of Certification. http://www.abim.org/news/news/focused‐practice‐hospital‐medicine‐qa.aspx. Published 2010. Accessed January 6,2010.
- .Comprehensive Pediatric Hospital Medicine.N Engl J Med.2008;358(21):2301–2302.
- The Joint Commission. Performance Measurement Initiatives. http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/. Published 2010. Accessed December 5,2010.
- The Joint Commission. Standards Frequently Asked Questions: Comprehensive Accreditation Manual for Critical Access Hospitals (CAMCAH). http://www.jointcommission.org/AccreditationPrograms/CriticalAccessHospitals/Standards/09_FAQs/default.htm. Accessed December 5,2008; December 14, 2009.
- ,,,,.Infectious Disease Hospitalizations Among Infants in the United States.Pediatrics.2008;121(2):244–252.
- ,,,.Procedures in U.S. Hospitals, 1997.HCUP Fact Book No. 2. In:Agency for Healthcare Research and Quality,Rockville, MD;2001.
- Anderson L,Krathwohl DR,Airasian PW,Cruikshank KA,Mayer RE,Pintrich PR, et al., editors.A Taxonomy for Learning, Teaching, and Assessing — A Revision of Bloom's Taxonomy of Educational Objectives.Addison Wesley Longman, Inc.Pearson Education USA, One Lake Street Upper Saddle River, NJ; (2001).
- Society of Hospital Medicine (SHM). Definition of a Hospitalist. http://www.hospitalmedicine.org/AM/Template.cfm?Section=General_Information 2009.
- Todd von Deak MBA CAE Vice President Membership and Marketing.Pediatric Hospitalists Membership Numbers. In.Philadelphia:Society of Hospital Medicine National Office 1500 Spring Garden, Suite 501, Philadelphia, PA 19130;2009.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- .The future of hospital medicine: evolution or revolution?Am J Med.2004;117:446–450.
- ,.The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- ,,,,.Variation in pediatric hospitalists' use of proven and unproven therapies: A study from the Pediatric Research in Inpatient Settings (PRIS) network.Journal of Hospital Medicine.2008;3(4):292–298.
- ,,.Pediatric hospitalists: Training, current practice, and career goals.Journal of Hospital Medicine.2009;4(3):179–186.
- ,.Standardize to Excellence: Improving the Quality and Safety of Care with Clinical Pathways.Pediatric Clinics of North America.2009;56(4):893–904.
- .Evolution of a new specialty ‐ a twenty year pediatric hospitalist experience [Abstract]. In:National Association of Inpatient Physicians (now Society of Hospital Medicine).New Orleans, Louisiana;1999.
- ,,,,,, et al.Pediatric Hospitalists: Report of a Leadership Conference.Pediatrics.2006;117(4):1122–1130.
- ,,,, e.The Core Competencies in Hospital Medicine: A Framework for Curriculum Development.J Hosp Med.2006;1(Suppl 1).
- American Board of Internal Medicine. Questions and Answers regarding ABIM Recognition of Focused Practice in Hospital Medicine through Maintenance of Certification. http://www.abim.org/news/news/focused‐practice‐hospital‐medicine‐qa.aspx. Published 2010. Accessed January 6,2010.
- .Comprehensive Pediatric Hospital Medicine.N Engl J Med.2008;358(21):2301–2302.
- The Joint Commission. Performance Measurement Initiatives. http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/. Published 2010. Accessed December 5,2010.
- The Joint Commission. Standards Frequently Asked Questions: Comprehensive Accreditation Manual for Critical Access Hospitals (CAMCAH). http://www.jointcommission.org/AccreditationPrograms/CriticalAccessHospitals/Standards/09_FAQs/default.htm. Accessed December 5,2008; December 14, 2009.
- ,,,,.Infectious Disease Hospitalizations Among Infants in the United States.Pediatrics.2008;121(2):244–252.
- ,,,.Procedures in U.S. Hospitals, 1997.HCUP Fact Book No. 2. In:Agency for Healthcare Research and Quality,Rockville, MD;2001.
- Anderson L,Krathwohl DR,Airasian PW,Cruikshank KA,Mayer RE,Pintrich PR, et al., editors.A Taxonomy for Learning, Teaching, and Assessing — A Revision of Bloom's Taxonomy of Educational Objectives.Addison Wesley Longman, Inc.Pearson Education USA, One Lake Street Upper Saddle River, NJ; (2001).
SHM Medication Reconciliation Survey Results
The Joint Commission's (TJC) National Patient Safety Goal (NPSG) #8Accurately and completely reconcile medications across the continuum of carechallenges hospitals to design and implement new medication management processes. With medication errors contributing to patient morbidity and mortality,1 establishing a comprehensive process for reconciling a patient's medications during the hospitalization episode is an important quality improvement and patient safety goal.
However, the current state of inpatient medication management is highly fragmented. Standard documentation is lacking, as is integration of information between care settings.2 There are now reports describing implementation of various medication reconciliation processes for admissions,3 transfers,4 and discharges.5
Hospitalists are well‐positioned to contribute to the implementation of medication reconciliation. Indeed, because TJC does not explicitly specify what type of health care provider (eg, physician, nurse, etc.) should assume responsibility for this process, institutions have designed workflows to suit their own needs, while striving to comply with national standards.
Given the complexity and lack of standardization around this NPSG, a survey was distributed to attendees of a Society of Hospital Medicine (SHM) national meeting to determine the various processes implemented thus far, and to ascertain existing challenges to implementation. We report here on the results.
METHODS
A survey tool (Appendix) was designed to query demographic and institutional factors, involvement in the process, and barriers to implementation of medication reconciliation. Surveys were included in all attendees' registration materials, resulting in the distributions of approximately 800 surveys.
Responses were entered into an Excel spreadsheet. Simple descriptive statistics were used to determine proportions for providers, processes, and barriers to implementation. Where appropriate, variables were dichotomized, allowing for paired t‐test analysis. Statistical significance was defined as a P value less than .05. Subgroup analyses by hospital type, provider type, and process method were performed.
RESULTS
A total of 295 completed surveys were collected. The responses are tabulated in Table 1.
| |
| Primary practice setting | |
| Academic tertiary center | 23% |
| Community teaching hospital | 29% |
| Non‐academic hospital | 43% |
| Patient population | |
| Adults only | 90% |
| Pediatrics only | 5% |
| Adults and pediatrics | 5% |
| State of implementation | |
| Fully implemented | 48% |
| Partially implemented | 35% |
| Planning stages | 11% |
| Unaware of plans to implement | 2% |
| Unaware of med reconciliation | 4% |
| Hospitalist involvement | |
| Active role | 36% |
| Peripheral role | 24% |
| No role | 31% |
| Process format | |
| Paper | 47% |
| Computer | 11% |
| Both paper and computer | 31% |
| Don't know | 2% |
| Measuring compliance | |
| Yes | 42% |
| No | 14% |
| Don't know | 34% |
| Measuring outcomes | |
| Yes | 22% |
| No | 25% |
| Don't know | 41% |
| Impact of medication reconciliation | |
| No impact | 9% |
| Positive impact | 58% |
| Negative impact | 7% |
| Don't know | 14% |
Process
A paper process was used most often (47%), followed by a combined process (31%), and computers alone in just 11% of cases. Measurement of process compliance was reported in less than half (42%), with 34% unaware if their institutions were monitoring compliance. Outcome measurement was recorded as not performed (25%) or unknown (41%) in a majority of cases. Respondents reported a favorable view of the future impact of medication reconciliation, with 58% citing likely positive impacts on patient safety and patient care; fewer were unsure (14%) or anticipated no impact (9%) or negative impact (7%). Survey results regarding responsibility for individual process steps are detailed in Table 2. Notably, respondents often indicated that both physicians and nurses would share responsibility for a given step. Physicians were more often responsible for reconciling home medications, updating discharge medication lists, and communicating to outpatient providers. Nursing performed reconciliation in only 10% of cases. Results across all steps demonstrated very low participation rates by pharmacists, with pharmacist responsibility for reconciliation only 6% of the time.
| Process Step | Physician | Nurse | Physician and Nurse | Pharmacist | Other |
|---|---|---|---|---|---|
| |||||
| Obtaining home med list | 15% | 39% | 41% | 3% | 2% |
| Documenting home med list | 17% | 41% | 37% | 2% | 3% |
| Reconciling medications | 56% | 10% | 21% | 6% | 7% |
| Updating discharge med list | 64% | 6% | 17% | 3% | 10% |
| Providing instructions at discharge | 15% | 46% | 32% | 2% | 5% |
| Communicating changes at follow‐up | 84% | 6% | 4% | 6% | 1% |
Hospital Type
Results of subgroup analyses by hospital type are detailed in Table 3. Community teaching hospitals (CTHs) were significantly more likely (57%) than nonteaching hospitals (NTHs) (49%) or tertiary academic centers (TACs) (35%) to have achieved full implementation. NTHs were significantly less likely to have involved hospitalists in implementation. Use of computer‐based processes at TACs was more common (27%) than in CTHs (9%) or NTHs (7%). TACs were significantly more likely to have a physician obtain the medication list (33%, compared with 15% and 7% for CTHs and NTHs, respectively), whereas NTHs were more likely to use nurses (50%) than were CTHs (31%) or TACs (26%). Similar significant differences were found among hospital types with regard to obtaining the preadmission medication list. Physicians in TACs (25%) were more likely to be responsible for giving discharge medication instructions than in CTHs (10%) or NTHs (14%, not significant compared with TACs).
| Academic Centers [AC] | Community Teaching Hospitals [CT] | Non‐Teaching Hospitals [NT] | P values (2‐tailed) | |||
|---|---|---|---|---|---|---|
| AC vs. CT | AC vs. NT | CT vs. NT | ||||
| ||||||
| State of implementation | ||||||
| Fully implemented | 25/71 (35) | 48/84 (57) | 68/139 (49) | 0.007 | 0.06 | 0.25 |
| Partially implemented | 31/71 (44) | 25/84 (30) | 48/139 (35) | 0.07 | 0.21 | 0.44 |
| Planning stages | 9/71 (13) | 9/84 (11) | 14/139 (10) | 0.70 | 0.51 | 0.81 |
| Unaware of plans to implement | 2/71 (3) | 1/84 (1) | 3/139 (2) | 0.37 | 0.65 | 0.57 |
| Unaware of med reconciliation | 4/71 (5) | 1/84 (1) | 6/139 (4) | 0.14 | 0.74 | 0.19 |
| Hospitalist involvement | ||||||
| Active role | 28/59 (47) | 34/80 (43) | 43/127 (34) | 0.64 | 0.09 | 0.19 |
| Peripheral role | 12/59 (20) | 25/80 (31) | 34/127 (27) | 0.15 | 0.30 | 0.54 |
| No role | 19/59 (32) | 19/80 (24) | 50/127 (39) | 0.30 | 0.36 | 0.03 |
| Process format | ||||||
| Paper | 26/59 (44) | 47/81 (58) | 63/127 (50) | 0.10 | 0.45 | 0.26 |
| Computer | 16/59 (27) | 7/81 (9) | 9/127 (7) | 0.005 | 0.001 | 0.60 |
| Both paper and computer | 17/59 (29) | 25/81 (31) | 51/127 (40) | 0.80 | 0.15 | 0.19 |
| Don't know | 0/59 (0) | 2/81 (2) | 4/127 (3) | 0.28 | 0.18 | 0.66 |
| Process steps (selected questions) | ||||||
| Obtaining home med list | ||||||
| Physician | 19/58 (33) | 12/80 (15) | 9/125 (7) | 0.013 | 0.001 | 0.07 |
| Physician and Nurse | 19/58 (33) | 39/80 (49) | 49/125 (39) | 0.47 | 0.44 | 0.16 |
| Nurse | 15/58 (26) | 25/80 (31) | 62/125 (50) | 0.005 | 0.003 | 0.008 |
| Pharmacist | 5/58 (9) | 1/80 (1) | 2/125 (2) | 0.06 | 0.03 | 0.58 |
| Documenting home med list | ||||||
| Physician | 22/58 (38) | 11/80 (14) | 11/125 (9) | 0.001 | 0.001 | 0.26 |
| Physician and Nurse | 15/58 (26) | 37/80 (46) | 45/125 (36) | 0.02 | 0.18 | 0.16 |
| Nurse | 18/58 (31) | 26/80 (32) | 64/125 (51) | 0.90 | 0.012 | 0.008 |
| Pharmacist | 3/58 (5) | 2/80 (3) | 1/125 (1) | 0.55 | 0.09 | 0.29 |
| Reconciling medications | ||||||
| Physician | 33/58 (57) | 51/80 (64) | 63/125 (50) | 0.41 | 0.42 | 0.051 |
| Physician and Nurse | 8/58 (14) | 14/80 (18) | 32/125 (26) | 0.53 | 0.09 | 0.18 |
| Nurse | 6/58 (10) | 6/80 (8) | 15/125 (12) | 0.68 | 0.71 | 0.36 |
| Pharmacist | 8/58 (14) | 5/80 (6) | 3/125 (2) | 0.11 | 0.007 | 0.13 |
| Updating discharge med list | ||||||
| Physician | 42/58 (72) | 50/80 (63) | 76/125 (61) | 0.27 | 0.15 | 0.77 |
| Physician and Nurse | 7/58 (12) | 16/80 (20) | 23/125 (18) | 0.22 | 0.31 | 0.72 |
| Nurse | 2/58 (3) | 5/80 (6) | 10/125 (8) | 0.41 | 0.20 | 0.59 |
| Pharmacist | 3/58 (5) | 3/80 (4) | 3/125 (2) | 0.78 | 0.27 | 0.40 |
| Providing instructions at discharge | ||||||
| Physician | 14/57 (25) | 8/80 (10) | 17/125 (14) | 0.02 | 0.07 | 0.40 |
| Physician and Nurse | 14/57 (25) | 30/80 (38) | 39/125 (31) | 0.11 | 0.41 | 0.30 |
| Nurse | 25/57 (44) | 37/80 (46) | 60/125 (48) | 0.82 | 0.62 | 0.80 |
| Pharmacist | 4/57 (7) | 1/80 (1) | 0/125 (0) | 0.06 | 0.003 | 0.26 |
Barriers
Results regarding barriers to successful implementation are shown in Table 4. Patient lack of knowledge of medications (87%) and absence of a preadmission medication list from other sources (80%) were common. Both paper and computer medication reconciliation processes were associated with respondents citing cumbersome hospital systems as a barrier; this barrier was cited more often when the implemented process was paper‐only (Table 5). Respondents who stated the medication reconciliation process takes too long did so regardless of whether the implemented process was paper‐based or computer‐based. Despite these barriers, only 16% of respondents stated that medication reconciliation was not worth the effort of implementation. Barriers reported were similar across hospital type (Table 6) with 2 exceptions. Formulary differences were noted to be a barrier more often in CTHs (78%) compared with NTHs (60%) and TACs (64%, not significant compared with CTHs). Language barriers were problematic more often in TACs (48%) than in NTHs (28%) or CTHs (36%, not significant compared with TACs).
| Barrier to Implementation | Yes | No | Unsure |
|---|---|---|---|
| |||
| Patient not knowing meds | 87% | 2% | 0% |
| Process takes too long | 53% | 28% | 8% |
| Med list not available | 80% | 9% | 0% |
| Process not worth effort | 16% | 60% | 12% |
| Cumbersome hospital systems | 52% | 33% | 4% |
| Formulary differences | 59% | 24% | 5% |
| Language barriers | 31% | 53% | 4% |
| No access to outside records | 63% | 23% | 2% |
| Lack of job clarity in process | 38% | 48% | 3% |
| Availability of med list at discharge | 27% | 57% | 3% |
| Barriers (Selected Questions) | Paper Only [P] | Computer Only [C] | Paper and Computer [PC] | P values (2‐tailed) | ||
|---|---|---|---|---|---|---|
| P vs. C | P vs. PC | C vs. PC | ||||
| ||||||
| Process takes too long | ||||||
| Yes | 77/134 (57) | 19/31 (61) | 55/91 (60) | 0.69 | 0.65 | 0.92 |
| No | 43/134 (32) | 11/31 (35) | 28/91 (31) | 0.75 | 0.87 | 0.68 |
| Unsure | 14/134 (10) | 1/31 (3) | 8/91 (9) | 0.21 | 0.80 | 0.27 |
| Process not worth effort | ||||||
| Yes | 24/133 (18) | 3/31 (10) | 17/91 (19) | 0.28 | 0.85 | 0.25 |
| No | 93/133 (70) | 22/31 (71) | 62/91 (68) | 0.91 | 0.75 | 0.76 |
| Unsure | 16/133 (12) | 6/31 (19) | 12/91 (13) | 0.30 | 0.82 | 0.41 |
| Cumbersome hospital systems | ||||||
| Yes | 86/133 (65) | 16/31 (52) | 46/92 (50) | 0.18 | 0.03 | 0.85 |
| No | 42/133 (32) | 13/31 (42) | 42/92 (46) | 0.29 | 0.03 | 0.70 |
| Unsure | 5/133 (4) | 2/31 (6) | 4/92 (4) | 0.62 | 0.82 | 0.64 |
| Barrier to Implementation (Selected Questions) | Academic Centers [AC] | Community Teaching Hospitals [CT] | Non‐Teaching Hospitals [NT] | P values | ||
|---|---|---|---|---|---|---|
| AC vs. CT | AC vs. NT | CT vs. NT | ||||
| ||||||
| Process takes too long | ||||||
| Yes | 37/58 (64) | 49/78 (63) | 70/124 (56) | 0.90 | 0.31 | 0.37 |
| No | 15/58 (26) | 24/78 (31) | 42/124 (34) | 0.53 | 0.28 | 0.66 |
| Unsure | 6/58 (10) | 5/78 (6) | 12/124 (10) | 0.39 | 0.88 | 0.32 |
| Process not worth effort | ||||||
| Yes | 7/58 (12) | 16/78 (21) | 23/123 (19) | 0.17 | 0.24 | 0.73 |
| No | 42/58 (72) | 52/78 (67) | 84/123 (68) | 0.53 | 0.59 | 0.88 |
| Unsure | 9/58 (16) | 10/78 (12) | 16/123 (13) | 0.50 | 0.59 | 0.84 |
| Cumbersome hospital systems | ||||||
| Yes | 36/58 (62) | 46/79 (58) | 69/123 (56) | 0.64 | 0.45 | 0.78 |
| No | 19/58 (33) | 32/79 (41) | 46/123 (37) | 0.34 | 0.60 | 0.57 |
| Unsure | 3/58 (5) | 1/79 (1) | 8/123 (7) | 0.16 | 0.61 | 0.049 |
| Formulary differences | ||||||
| Yes | 37/58 (64) | 61/78 (78) | 74/123 (60) | 0.07 | 0.61 | 0.009 |
| No | 16/58 (28) | 14/78 (18) | 41/123 (33) | 0.17 | 0.50 | 0.02 |
| Unsure | 5/58 (8) | 2/78 (3) | 8/123 (7) | 0.19 | 0.81 | 0.22 |
| Language barriers | ||||||
| Yes | 28/58 (48) | 28/77 (36) | 34/123 (28) | 0.16 | 0.009 | 0.24 |
| No | 28/58 (48) | 46/77 (60) | 82/123 (67) | 0.17 | 0.016 | 0.32 |
| Unsure | 2/58 (3) | 3/77 (4) | 7/123 (5) | 0.76 | 0.54 | 0.74 |
| No access to outside records | ||||||
| Yes | 38/58 (66) | 60/79 (76) | 87/123 (71) | 0.20 | 0.50 | 0.44 |
| No | 18/58 (31) | 18/79 (23) | 33/123 (27) | 0.30 | 0.58 | 0.52 |
| Unsure | 2/58 (3) | 1/79 (1) | 3/123 (2) | 0.39 | 0.68 | 0.58 |
| Lack of job clarity in process | ||||||
| Yes | 26/58 (45) | 31/79 (39) | 49/121 (40) | 0.48 | 0.53 | 0.89 |
| No | 28/58 (48) | 46/79 (58) | 68/121 (56) | 0.25 | 0.32 | 0.78 |
| Unsure | 4/58 (7) | 2/79 (3) | 4/121 (3) | 0.28 | 0.22 | 0.75 |
| Availability of med list at discharge | ||||||
| Yes | 20/58 (34) | 24/79 (30) | 35/120 (29) | 0.62 | 0.50 | 0.88 |
| No | 36/58 (62) | 54/79 (68) | 78/120 (65) | 0.47 | 0.70 | 0.66 |
| Unsure | 0/58 (0) | 1/79 (1) | 7/120 (6) | 0.45 | 0.06 | 0.08 |
DISCUSSION
Managing medication information for inpatients is an extremely complex task. On admission, home medication lists are often inaccurate or absent,6 requiring extra time and effort to discover this information. By discharge, medication regimens have frequently been altered,7 making communication of changes to the next provider essential. One study described myriad provider, patient, and health system issues in maintaining accurate outpatient medication lists.8 These issues are further compounded by the multiple prescribers, necessary hand‐offs, and formulary differences in the inpatient setting.
Over half of the hospitalists in this survey reported hospitalist involvement in design and implementation of medication reconciliation. Given the familiarity with hospital systems and inpatient workflow, hospitalists are well‐positioned to contribute to successful implementation. Nonetheless, many were unaware of efforts to implement this NPSG.
Measurement of both process and outcome measures is important when determining value in quality improvement. Beyond process measures, outcome measures such as adverse drug events, readmission rates, mortality, patient satisfaction, and outpatient provider satisfaction may be appropriate in evaluating medication reconciliation strategies. Even measuring the accuracy of the process with respect to the admission orders written would be a valuable source of information for further improvement. Unfortunately, respondents indicated that evaluation was occurring infrequently. Potentially more problematic is the apparent lack of clarity regarding identification of healthcare provider responsibility for specific process steps. By far the least uniformity is in the acquisition and documentation of the preadmission medication list. There is variability in who is assigned to perform this task, but a substantial number of respondents indicated that their process involved a shared responsibility between physicians and nurses. It is unclear whether this phenomenon reflects the complexity of inpatient medication information management, or is simply an attempt to distribute the work among providers. Sharing the work between physicians and nurses may increase the overall likelihood for compliance and possibly improve the safety and accuracy of the process, especially if the physicians and nurses take the medication history in a redundant fashion and share their findings. Conversely, compliance may decrease if each provider merely expects the other to complete the process. Optimally, an interdisciplinary workflow for medication history taking would be in place, involving both physicians and nurses, with the availability of pharmacist consultation in complex cases. However, our survey data suggest this is infrequent; resident physicians appear to be the ones shouldering substantial responsibility for medication reconciliation in tertiary academic centers. Further research into the accuracy of medication reconciliation processes involving different strategies for medication information collection would be useful.
We documented several barriers to successful implementation of medication reconciliation. Physicians cited a lack of medication knowledge on the part of the patient and unavailable prior medication lists as substantial barriers to success. Many medication reconciliation processes are limited by issues of poor health literacy or inadequate patient knowledge about medications. This lack of medication knowledge is especially problematic for patients new to a healthcare system. It will be important to implement processes that not only reconcile medications accurately, but also make medication information available for future care episodes.
Time required to complete the process was also important. Certain elements of the medication reconciliation process are new work, and integrating the process into existing workflows is crucial. Given the significant time commitment required, the rare involvement of pharmacists at most institutions is striking. It appears that hospital pharmacists do not currently own any of the medication reconciliation process steps at most facilities, despite having formal training in medication history‐taking. In the 2006 ASHP national hospital pharmacy survey, one‐third of pharmacists stated that there were not enough pharmacy resources to meet medication reconciliation demands; only 19% of those surveyed stated pharmacists provided medication education at discharge to more than 25% of their patients.9
This report has several limitations. The survey used was not comprehensive, and only represents a convenience sample of hospitalists attending anational meeting. Nearly 300 physicians responded, representing both teaching and private hospital settings. We consider the response rate of 37% reasonable for a survey of this nature, and the variety of processes described is likely indicative of the overall status of medication reconciliation implementation. The over‐representation of certain institutions in our survey is possible, especially those with large or influential hospital medicine programs. Our survey did not ask respondents to name their home institutions. In addition, this design is open to a convenience sample bias, in that surveying only national meeting attendees (rather than the entire SHM membership) risks overinclusion of those hospitalists involved in leadership roles and quality improvement projects. Despite this, the variety of processes described is likely indicative of the overall status of medication reconciliation implementation in mid‐2006. It is possible that processes have become more uniform nationwide in the interim.
Our survey results reflect the complexity surrounding medication reconciliation. It appears that full implementation has not yet occurred everywhere, significant barriers remain, and outcome measurement is limited. Importantly, physicians, nurses, and pharmacists do not have standardized roles. Responsibility for medication reconciliation has predominantly been added to the existing duties of inpatient physicians and nurses, with limited involvement of pharmacists. Hospitalists are well‐positioned to lead the ongoing implementation of medication reconciliation processes and should take advantage of their systems knowledge to effectively partner with other physicians, nurses, and pharmacists to achieve success in medication reconciliation.
Acknowledgements
The authors thank Ken Epstein, MD, and Renee Meadows, MD, along with the entire SHM Medication Reconciliation Task Force for their helpful review and comments on the article.
Appendix
|
|
- Kohn LT,Corrigan JM,Donaldson MS, eds.To Err Is Human: Building a Safer Health System.Washington, DC:National Academy Press;1999.
- .Medication reconciliation: transfer of medication information across settings – keeping it free from error.Am J Nurs.2005;105(3 Suppl):31–36.
- ,,, et al.Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients.Am J Health‐Syst Pharm.2004;61(16):1689–1695.
- ,,, et al.Medication reconciliation: a practical tool to reduce the risk of medication errors.J Crit Care.2003;18(4):201–205.
- ,,,.Evaluation of a new integrated discharge prescription form.Ann Pharmacother.2001;35(7‐8):953–958.
- ,,, et al.Patient safety standardization as a mechanism to improve safety in health care.Jt Comm J Qual Saf.2004;30(1):5–14.
- ,,.What happens to long‐term medication when general practice patients are referred to hospital?Eur J Clin Pharmacol.1996;50(4):253–257.
- ,,, et al.An experiential interdisciplinary quality improvement education initiative.Am J Med Qual.2006;21(5):317–322.
- ,,.ASHP national survey of pharmacy practice in hospital settings: monitoring and patient education‐2006.Am J Health‐Syst Pharm.2007;64(5):507–520.
The Joint Commission's (TJC) National Patient Safety Goal (NPSG) #8Accurately and completely reconcile medications across the continuum of carechallenges hospitals to design and implement new medication management processes. With medication errors contributing to patient morbidity and mortality,1 establishing a comprehensive process for reconciling a patient's medications during the hospitalization episode is an important quality improvement and patient safety goal.
However, the current state of inpatient medication management is highly fragmented. Standard documentation is lacking, as is integration of information between care settings.2 There are now reports describing implementation of various medication reconciliation processes for admissions,3 transfers,4 and discharges.5
Hospitalists are well‐positioned to contribute to the implementation of medication reconciliation. Indeed, because TJC does not explicitly specify what type of health care provider (eg, physician, nurse, etc.) should assume responsibility for this process, institutions have designed workflows to suit their own needs, while striving to comply with national standards.
Given the complexity and lack of standardization around this NPSG, a survey was distributed to attendees of a Society of Hospital Medicine (SHM) national meeting to determine the various processes implemented thus far, and to ascertain existing challenges to implementation. We report here on the results.
METHODS
A survey tool (Appendix) was designed to query demographic and institutional factors, involvement in the process, and barriers to implementation of medication reconciliation. Surveys were included in all attendees' registration materials, resulting in the distributions of approximately 800 surveys.
Responses were entered into an Excel spreadsheet. Simple descriptive statistics were used to determine proportions for providers, processes, and barriers to implementation. Where appropriate, variables were dichotomized, allowing for paired t‐test analysis. Statistical significance was defined as a P value less than .05. Subgroup analyses by hospital type, provider type, and process method were performed.
RESULTS
A total of 295 completed surveys were collected. The responses are tabulated in Table 1.
| |
| Primary practice setting | |
| Academic tertiary center | 23% |
| Community teaching hospital | 29% |
| Non‐academic hospital | 43% |
| Patient population | |
| Adults only | 90% |
| Pediatrics only | 5% |
| Adults and pediatrics | 5% |
| State of implementation | |
| Fully implemented | 48% |
| Partially implemented | 35% |
| Planning stages | 11% |
| Unaware of plans to implement | 2% |
| Unaware of med reconciliation | 4% |
| Hospitalist involvement | |
| Active role | 36% |
| Peripheral role | 24% |
| No role | 31% |
| Process format | |
| Paper | 47% |
| Computer | 11% |
| Both paper and computer | 31% |
| Don't know | 2% |
| Measuring compliance | |
| Yes | 42% |
| No | 14% |
| Don't know | 34% |
| Measuring outcomes | |
| Yes | 22% |
| No | 25% |
| Don't know | 41% |
| Impact of medication reconciliation | |
| No impact | 9% |
| Positive impact | 58% |
| Negative impact | 7% |
| Don't know | 14% |
Process
A paper process was used most often (47%), followed by a combined process (31%), and computers alone in just 11% of cases. Measurement of process compliance was reported in less than half (42%), with 34% unaware if their institutions were monitoring compliance. Outcome measurement was recorded as not performed (25%) or unknown (41%) in a majority of cases. Respondents reported a favorable view of the future impact of medication reconciliation, with 58% citing likely positive impacts on patient safety and patient care; fewer were unsure (14%) or anticipated no impact (9%) or negative impact (7%). Survey results regarding responsibility for individual process steps are detailed in Table 2. Notably, respondents often indicated that both physicians and nurses would share responsibility for a given step. Physicians were more often responsible for reconciling home medications, updating discharge medication lists, and communicating to outpatient providers. Nursing performed reconciliation in only 10% of cases. Results across all steps demonstrated very low participation rates by pharmacists, with pharmacist responsibility for reconciliation only 6% of the time.
| Process Step | Physician | Nurse | Physician and Nurse | Pharmacist | Other |
|---|---|---|---|---|---|
| |||||
| Obtaining home med list | 15% | 39% | 41% | 3% | 2% |
| Documenting home med list | 17% | 41% | 37% | 2% | 3% |
| Reconciling medications | 56% | 10% | 21% | 6% | 7% |
| Updating discharge med list | 64% | 6% | 17% | 3% | 10% |
| Providing instructions at discharge | 15% | 46% | 32% | 2% | 5% |
| Communicating changes at follow‐up | 84% | 6% | 4% | 6% | 1% |
Hospital Type
Results of subgroup analyses by hospital type are detailed in Table 3. Community teaching hospitals (CTHs) were significantly more likely (57%) than nonteaching hospitals (NTHs) (49%) or tertiary academic centers (TACs) (35%) to have achieved full implementation. NTHs were significantly less likely to have involved hospitalists in implementation. Use of computer‐based processes at TACs was more common (27%) than in CTHs (9%) or NTHs (7%). TACs were significantly more likely to have a physician obtain the medication list (33%, compared with 15% and 7% for CTHs and NTHs, respectively), whereas NTHs were more likely to use nurses (50%) than were CTHs (31%) or TACs (26%). Similar significant differences were found among hospital types with regard to obtaining the preadmission medication list. Physicians in TACs (25%) were more likely to be responsible for giving discharge medication instructions than in CTHs (10%) or NTHs (14%, not significant compared with TACs).
| Academic Centers [AC] | Community Teaching Hospitals [CT] | Non‐Teaching Hospitals [NT] | P values (2‐tailed) | |||
|---|---|---|---|---|---|---|
| AC vs. CT | AC vs. NT | CT vs. NT | ||||
| ||||||
| State of implementation | ||||||
| Fully implemented | 25/71 (35) | 48/84 (57) | 68/139 (49) | 0.007 | 0.06 | 0.25 |
| Partially implemented | 31/71 (44) | 25/84 (30) | 48/139 (35) | 0.07 | 0.21 | 0.44 |
| Planning stages | 9/71 (13) | 9/84 (11) | 14/139 (10) | 0.70 | 0.51 | 0.81 |
| Unaware of plans to implement | 2/71 (3) | 1/84 (1) | 3/139 (2) | 0.37 | 0.65 | 0.57 |
| Unaware of med reconciliation | 4/71 (5) | 1/84 (1) | 6/139 (4) | 0.14 | 0.74 | 0.19 |
| Hospitalist involvement | ||||||
| Active role | 28/59 (47) | 34/80 (43) | 43/127 (34) | 0.64 | 0.09 | 0.19 |
| Peripheral role | 12/59 (20) | 25/80 (31) | 34/127 (27) | 0.15 | 0.30 | 0.54 |
| No role | 19/59 (32) | 19/80 (24) | 50/127 (39) | 0.30 | 0.36 | 0.03 |
| Process format | ||||||
| Paper | 26/59 (44) | 47/81 (58) | 63/127 (50) | 0.10 | 0.45 | 0.26 |
| Computer | 16/59 (27) | 7/81 (9) | 9/127 (7) | 0.005 | 0.001 | 0.60 |
| Both paper and computer | 17/59 (29) | 25/81 (31) | 51/127 (40) | 0.80 | 0.15 | 0.19 |
| Don't know | 0/59 (0) | 2/81 (2) | 4/127 (3) | 0.28 | 0.18 | 0.66 |
| Process steps (selected questions) | ||||||
| Obtaining home med list | ||||||
| Physician | 19/58 (33) | 12/80 (15) | 9/125 (7) | 0.013 | 0.001 | 0.07 |
| Physician and Nurse | 19/58 (33) | 39/80 (49) | 49/125 (39) | 0.47 | 0.44 | 0.16 |
| Nurse | 15/58 (26) | 25/80 (31) | 62/125 (50) | 0.005 | 0.003 | 0.008 |
| Pharmacist | 5/58 (9) | 1/80 (1) | 2/125 (2) | 0.06 | 0.03 | 0.58 |
| Documenting home med list | ||||||
| Physician | 22/58 (38) | 11/80 (14) | 11/125 (9) | 0.001 | 0.001 | 0.26 |
| Physician and Nurse | 15/58 (26) | 37/80 (46) | 45/125 (36) | 0.02 | 0.18 | 0.16 |
| Nurse | 18/58 (31) | 26/80 (32) | 64/125 (51) | 0.90 | 0.012 | 0.008 |
| Pharmacist | 3/58 (5) | 2/80 (3) | 1/125 (1) | 0.55 | 0.09 | 0.29 |
| Reconciling medications | ||||||
| Physician | 33/58 (57) | 51/80 (64) | 63/125 (50) | 0.41 | 0.42 | 0.051 |
| Physician and Nurse | 8/58 (14) | 14/80 (18) | 32/125 (26) | 0.53 | 0.09 | 0.18 |
| Nurse | 6/58 (10) | 6/80 (8) | 15/125 (12) | 0.68 | 0.71 | 0.36 |
| Pharmacist | 8/58 (14) | 5/80 (6) | 3/125 (2) | 0.11 | 0.007 | 0.13 |
| Updating discharge med list | ||||||
| Physician | 42/58 (72) | 50/80 (63) | 76/125 (61) | 0.27 | 0.15 | 0.77 |
| Physician and Nurse | 7/58 (12) | 16/80 (20) | 23/125 (18) | 0.22 | 0.31 | 0.72 |
| Nurse | 2/58 (3) | 5/80 (6) | 10/125 (8) | 0.41 | 0.20 | 0.59 |
| Pharmacist | 3/58 (5) | 3/80 (4) | 3/125 (2) | 0.78 | 0.27 | 0.40 |
| Providing instructions at discharge | ||||||
| Physician | 14/57 (25) | 8/80 (10) | 17/125 (14) | 0.02 | 0.07 | 0.40 |
| Physician and Nurse | 14/57 (25) | 30/80 (38) | 39/125 (31) | 0.11 | 0.41 | 0.30 |
| Nurse | 25/57 (44) | 37/80 (46) | 60/125 (48) | 0.82 | 0.62 | 0.80 |
| Pharmacist | 4/57 (7) | 1/80 (1) | 0/125 (0) | 0.06 | 0.003 | 0.26 |
Barriers
Results regarding barriers to successful implementation are shown in Table 4. Patient lack of knowledge of medications (87%) and absence of a preadmission medication list from other sources (80%) were common. Both paper and computer medication reconciliation processes were associated with respondents citing cumbersome hospital systems as a barrier; this barrier was cited more often when the implemented process was paper‐only (Table 5). Respondents who stated the medication reconciliation process takes too long did so regardless of whether the implemented process was paper‐based or computer‐based. Despite these barriers, only 16% of respondents stated that medication reconciliation was not worth the effort of implementation. Barriers reported were similar across hospital type (Table 6) with 2 exceptions. Formulary differences were noted to be a barrier more often in CTHs (78%) compared with NTHs (60%) and TACs (64%, not significant compared with CTHs). Language barriers were problematic more often in TACs (48%) than in NTHs (28%) or CTHs (36%, not significant compared with TACs).
| Barrier to Implementation | Yes | No | Unsure |
|---|---|---|---|
| |||
| Patient not knowing meds | 87% | 2% | 0% |
| Process takes too long | 53% | 28% | 8% |
| Med list not available | 80% | 9% | 0% |
| Process not worth effort | 16% | 60% | 12% |
| Cumbersome hospital systems | 52% | 33% | 4% |
| Formulary differences | 59% | 24% | 5% |
| Language barriers | 31% | 53% | 4% |
| No access to outside records | 63% | 23% | 2% |
| Lack of job clarity in process | 38% | 48% | 3% |
| Availability of med list at discharge | 27% | 57% | 3% |
| Barriers (Selected Questions) | Paper Only [P] | Computer Only [C] | Paper and Computer [PC] | P values (2‐tailed) | ||
|---|---|---|---|---|---|---|
| P vs. C | P vs. PC | C vs. PC | ||||
| ||||||
| Process takes too long | ||||||
| Yes | 77/134 (57) | 19/31 (61) | 55/91 (60) | 0.69 | 0.65 | 0.92 |
| No | 43/134 (32) | 11/31 (35) | 28/91 (31) | 0.75 | 0.87 | 0.68 |
| Unsure | 14/134 (10) | 1/31 (3) | 8/91 (9) | 0.21 | 0.80 | 0.27 |
| Process not worth effort | ||||||
| Yes | 24/133 (18) | 3/31 (10) | 17/91 (19) | 0.28 | 0.85 | 0.25 |
| No | 93/133 (70) | 22/31 (71) | 62/91 (68) | 0.91 | 0.75 | 0.76 |
| Unsure | 16/133 (12) | 6/31 (19) | 12/91 (13) | 0.30 | 0.82 | 0.41 |
| Cumbersome hospital systems | ||||||
| Yes | 86/133 (65) | 16/31 (52) | 46/92 (50) | 0.18 | 0.03 | 0.85 |
| No | 42/133 (32) | 13/31 (42) | 42/92 (46) | 0.29 | 0.03 | 0.70 |
| Unsure | 5/133 (4) | 2/31 (6) | 4/92 (4) | 0.62 | 0.82 | 0.64 |
| Barrier to Implementation (Selected Questions) | Academic Centers [AC] | Community Teaching Hospitals [CT] | Non‐Teaching Hospitals [NT] | P values | ||
|---|---|---|---|---|---|---|
| AC vs. CT | AC vs. NT | CT vs. NT | ||||
| ||||||
| Process takes too long | ||||||
| Yes | 37/58 (64) | 49/78 (63) | 70/124 (56) | 0.90 | 0.31 | 0.37 |
| No | 15/58 (26) | 24/78 (31) | 42/124 (34) | 0.53 | 0.28 | 0.66 |
| Unsure | 6/58 (10) | 5/78 (6) | 12/124 (10) | 0.39 | 0.88 | 0.32 |
| Process not worth effort | ||||||
| Yes | 7/58 (12) | 16/78 (21) | 23/123 (19) | 0.17 | 0.24 | 0.73 |
| No | 42/58 (72) | 52/78 (67) | 84/123 (68) | 0.53 | 0.59 | 0.88 |
| Unsure | 9/58 (16) | 10/78 (12) | 16/123 (13) | 0.50 | 0.59 | 0.84 |
| Cumbersome hospital systems | ||||||
| Yes | 36/58 (62) | 46/79 (58) | 69/123 (56) | 0.64 | 0.45 | 0.78 |
| No | 19/58 (33) | 32/79 (41) | 46/123 (37) | 0.34 | 0.60 | 0.57 |
| Unsure | 3/58 (5) | 1/79 (1) | 8/123 (7) | 0.16 | 0.61 | 0.049 |
| Formulary differences | ||||||
| Yes | 37/58 (64) | 61/78 (78) | 74/123 (60) | 0.07 | 0.61 | 0.009 |
| No | 16/58 (28) | 14/78 (18) | 41/123 (33) | 0.17 | 0.50 | 0.02 |
| Unsure | 5/58 (8) | 2/78 (3) | 8/123 (7) | 0.19 | 0.81 | 0.22 |
| Language barriers | ||||||
| Yes | 28/58 (48) | 28/77 (36) | 34/123 (28) | 0.16 | 0.009 | 0.24 |
| No | 28/58 (48) | 46/77 (60) | 82/123 (67) | 0.17 | 0.016 | 0.32 |
| Unsure | 2/58 (3) | 3/77 (4) | 7/123 (5) | 0.76 | 0.54 | 0.74 |
| No access to outside records | ||||||
| Yes | 38/58 (66) | 60/79 (76) | 87/123 (71) | 0.20 | 0.50 | 0.44 |
| No | 18/58 (31) | 18/79 (23) | 33/123 (27) | 0.30 | 0.58 | 0.52 |
| Unsure | 2/58 (3) | 1/79 (1) | 3/123 (2) | 0.39 | 0.68 | 0.58 |
| Lack of job clarity in process | ||||||
| Yes | 26/58 (45) | 31/79 (39) | 49/121 (40) | 0.48 | 0.53 | 0.89 |
| No | 28/58 (48) | 46/79 (58) | 68/121 (56) | 0.25 | 0.32 | 0.78 |
| Unsure | 4/58 (7) | 2/79 (3) | 4/121 (3) | 0.28 | 0.22 | 0.75 |
| Availability of med list at discharge | ||||||
| Yes | 20/58 (34) | 24/79 (30) | 35/120 (29) | 0.62 | 0.50 | 0.88 |
| No | 36/58 (62) | 54/79 (68) | 78/120 (65) | 0.47 | 0.70 | 0.66 |
| Unsure | 0/58 (0) | 1/79 (1) | 7/120 (6) | 0.45 | 0.06 | 0.08 |
DISCUSSION
Managing medication information for inpatients is an extremely complex task. On admission, home medication lists are often inaccurate or absent,6 requiring extra time and effort to discover this information. By discharge, medication regimens have frequently been altered,7 making communication of changes to the next provider essential. One study described myriad provider, patient, and health system issues in maintaining accurate outpatient medication lists.8 These issues are further compounded by the multiple prescribers, necessary hand‐offs, and formulary differences in the inpatient setting.
Over half of the hospitalists in this survey reported hospitalist involvement in design and implementation of medication reconciliation. Given the familiarity with hospital systems and inpatient workflow, hospitalists are well‐positioned to contribute to successful implementation. Nonetheless, many were unaware of efforts to implement this NPSG.
Measurement of both process and outcome measures is important when determining value in quality improvement. Beyond process measures, outcome measures such as adverse drug events, readmission rates, mortality, patient satisfaction, and outpatient provider satisfaction may be appropriate in evaluating medication reconciliation strategies. Even measuring the accuracy of the process with respect to the admission orders written would be a valuable source of information for further improvement. Unfortunately, respondents indicated that evaluation was occurring infrequently. Potentially more problematic is the apparent lack of clarity regarding identification of healthcare provider responsibility for specific process steps. By far the least uniformity is in the acquisition and documentation of the preadmission medication list. There is variability in who is assigned to perform this task, but a substantial number of respondents indicated that their process involved a shared responsibility between physicians and nurses. It is unclear whether this phenomenon reflects the complexity of inpatient medication information management, or is simply an attempt to distribute the work among providers. Sharing the work between physicians and nurses may increase the overall likelihood for compliance and possibly improve the safety and accuracy of the process, especially if the physicians and nurses take the medication history in a redundant fashion and share their findings. Conversely, compliance may decrease if each provider merely expects the other to complete the process. Optimally, an interdisciplinary workflow for medication history taking would be in place, involving both physicians and nurses, with the availability of pharmacist consultation in complex cases. However, our survey data suggest this is infrequent; resident physicians appear to be the ones shouldering substantial responsibility for medication reconciliation in tertiary academic centers. Further research into the accuracy of medication reconciliation processes involving different strategies for medication information collection would be useful.
We documented several barriers to successful implementation of medication reconciliation. Physicians cited a lack of medication knowledge on the part of the patient and unavailable prior medication lists as substantial barriers to success. Many medication reconciliation processes are limited by issues of poor health literacy or inadequate patient knowledge about medications. This lack of medication knowledge is especially problematic for patients new to a healthcare system. It will be important to implement processes that not only reconcile medications accurately, but also make medication information available for future care episodes.
Time required to complete the process was also important. Certain elements of the medication reconciliation process are new work, and integrating the process into existing workflows is crucial. Given the significant time commitment required, the rare involvement of pharmacists at most institutions is striking. It appears that hospital pharmacists do not currently own any of the medication reconciliation process steps at most facilities, despite having formal training in medication history‐taking. In the 2006 ASHP national hospital pharmacy survey, one‐third of pharmacists stated that there were not enough pharmacy resources to meet medication reconciliation demands; only 19% of those surveyed stated pharmacists provided medication education at discharge to more than 25% of their patients.9
This report has several limitations. The survey used was not comprehensive, and only represents a convenience sample of hospitalists attending anational meeting. Nearly 300 physicians responded, representing both teaching and private hospital settings. We consider the response rate of 37% reasonable for a survey of this nature, and the variety of processes described is likely indicative of the overall status of medication reconciliation implementation. The over‐representation of certain institutions in our survey is possible, especially those with large or influential hospital medicine programs. Our survey did not ask respondents to name their home institutions. In addition, this design is open to a convenience sample bias, in that surveying only national meeting attendees (rather than the entire SHM membership) risks overinclusion of those hospitalists involved in leadership roles and quality improvement projects. Despite this, the variety of processes described is likely indicative of the overall status of medication reconciliation implementation in mid‐2006. It is possible that processes have become more uniform nationwide in the interim.
Our survey results reflect the complexity surrounding medication reconciliation. It appears that full implementation has not yet occurred everywhere, significant barriers remain, and outcome measurement is limited. Importantly, physicians, nurses, and pharmacists do not have standardized roles. Responsibility for medication reconciliation has predominantly been added to the existing duties of inpatient physicians and nurses, with limited involvement of pharmacists. Hospitalists are well‐positioned to lead the ongoing implementation of medication reconciliation processes and should take advantage of their systems knowledge to effectively partner with other physicians, nurses, and pharmacists to achieve success in medication reconciliation.
Acknowledgements
The authors thank Ken Epstein, MD, and Renee Meadows, MD, along with the entire SHM Medication Reconciliation Task Force for their helpful review and comments on the article.
Appendix
|
|
The Joint Commission's (TJC) National Patient Safety Goal (NPSG) #8Accurately and completely reconcile medications across the continuum of carechallenges hospitals to design and implement new medication management processes. With medication errors contributing to patient morbidity and mortality,1 establishing a comprehensive process for reconciling a patient's medications during the hospitalization episode is an important quality improvement and patient safety goal.
However, the current state of inpatient medication management is highly fragmented. Standard documentation is lacking, as is integration of information between care settings.2 There are now reports describing implementation of various medication reconciliation processes for admissions,3 transfers,4 and discharges.5
Hospitalists are well‐positioned to contribute to the implementation of medication reconciliation. Indeed, because TJC does not explicitly specify what type of health care provider (eg, physician, nurse, etc.) should assume responsibility for this process, institutions have designed workflows to suit their own needs, while striving to comply with national standards.
Given the complexity and lack of standardization around this NPSG, a survey was distributed to attendees of a Society of Hospital Medicine (SHM) national meeting to determine the various processes implemented thus far, and to ascertain existing challenges to implementation. We report here on the results.
METHODS
A survey tool (Appendix) was designed to query demographic and institutional factors, involvement in the process, and barriers to implementation of medication reconciliation. Surveys were included in all attendees' registration materials, resulting in the distributions of approximately 800 surveys.
Responses were entered into an Excel spreadsheet. Simple descriptive statistics were used to determine proportions for providers, processes, and barriers to implementation. Where appropriate, variables were dichotomized, allowing for paired t‐test analysis. Statistical significance was defined as a P value less than .05. Subgroup analyses by hospital type, provider type, and process method were performed.
RESULTS
A total of 295 completed surveys were collected. The responses are tabulated in Table 1.
| |
| Primary practice setting | |
| Academic tertiary center | 23% |
| Community teaching hospital | 29% |
| Non‐academic hospital | 43% |
| Patient population | |
| Adults only | 90% |
| Pediatrics only | 5% |
| Adults and pediatrics | 5% |
| State of implementation | |
| Fully implemented | 48% |
| Partially implemented | 35% |
| Planning stages | 11% |
| Unaware of plans to implement | 2% |
| Unaware of med reconciliation | 4% |
| Hospitalist involvement | |
| Active role | 36% |
| Peripheral role | 24% |
| No role | 31% |
| Process format | |
| Paper | 47% |
| Computer | 11% |
| Both paper and computer | 31% |
| Don't know | 2% |
| Measuring compliance | |
| Yes | 42% |
| No | 14% |
| Don't know | 34% |
| Measuring outcomes | |
| Yes | 22% |
| No | 25% |
| Don't know | 41% |
| Impact of medication reconciliation | |
| No impact | 9% |
| Positive impact | 58% |
| Negative impact | 7% |
| Don't know | 14% |
Process
A paper process was used most often (47%), followed by a combined process (31%), and computers alone in just 11% of cases. Measurement of process compliance was reported in less than half (42%), with 34% unaware if their institutions were monitoring compliance. Outcome measurement was recorded as not performed (25%) or unknown (41%) in a majority of cases. Respondents reported a favorable view of the future impact of medication reconciliation, with 58% citing likely positive impacts on patient safety and patient care; fewer were unsure (14%) or anticipated no impact (9%) or negative impact (7%). Survey results regarding responsibility for individual process steps are detailed in Table 2. Notably, respondents often indicated that both physicians and nurses would share responsibility for a given step. Physicians were more often responsible for reconciling home medications, updating discharge medication lists, and communicating to outpatient providers. Nursing performed reconciliation in only 10% of cases. Results across all steps demonstrated very low participation rates by pharmacists, with pharmacist responsibility for reconciliation only 6% of the time.
| Process Step | Physician | Nurse | Physician and Nurse | Pharmacist | Other |
|---|---|---|---|---|---|
| |||||
| Obtaining home med list | 15% | 39% | 41% | 3% | 2% |
| Documenting home med list | 17% | 41% | 37% | 2% | 3% |
| Reconciling medications | 56% | 10% | 21% | 6% | 7% |
| Updating discharge med list | 64% | 6% | 17% | 3% | 10% |
| Providing instructions at discharge | 15% | 46% | 32% | 2% | 5% |
| Communicating changes at follow‐up | 84% | 6% | 4% | 6% | 1% |
Hospital Type
Results of subgroup analyses by hospital type are detailed in Table 3. Community teaching hospitals (CTHs) were significantly more likely (57%) than nonteaching hospitals (NTHs) (49%) or tertiary academic centers (TACs) (35%) to have achieved full implementation. NTHs were significantly less likely to have involved hospitalists in implementation. Use of computer‐based processes at TACs was more common (27%) than in CTHs (9%) or NTHs (7%). TACs were significantly more likely to have a physician obtain the medication list (33%, compared with 15% and 7% for CTHs and NTHs, respectively), whereas NTHs were more likely to use nurses (50%) than were CTHs (31%) or TACs (26%). Similar significant differences were found among hospital types with regard to obtaining the preadmission medication list. Physicians in TACs (25%) were more likely to be responsible for giving discharge medication instructions than in CTHs (10%) or NTHs (14%, not significant compared with TACs).
| Academic Centers [AC] | Community Teaching Hospitals [CT] | Non‐Teaching Hospitals [NT] | P values (2‐tailed) | |||
|---|---|---|---|---|---|---|
| AC vs. CT | AC vs. NT | CT vs. NT | ||||
| ||||||
| State of implementation | ||||||
| Fully implemented | 25/71 (35) | 48/84 (57) | 68/139 (49) | 0.007 | 0.06 | 0.25 |
| Partially implemented | 31/71 (44) | 25/84 (30) | 48/139 (35) | 0.07 | 0.21 | 0.44 |
| Planning stages | 9/71 (13) | 9/84 (11) | 14/139 (10) | 0.70 | 0.51 | 0.81 |
| Unaware of plans to implement | 2/71 (3) | 1/84 (1) | 3/139 (2) | 0.37 | 0.65 | 0.57 |
| Unaware of med reconciliation | 4/71 (5) | 1/84 (1) | 6/139 (4) | 0.14 | 0.74 | 0.19 |
| Hospitalist involvement | ||||||
| Active role | 28/59 (47) | 34/80 (43) | 43/127 (34) | 0.64 | 0.09 | 0.19 |
| Peripheral role | 12/59 (20) | 25/80 (31) | 34/127 (27) | 0.15 | 0.30 | 0.54 |
| No role | 19/59 (32) | 19/80 (24) | 50/127 (39) | 0.30 | 0.36 | 0.03 |
| Process format | ||||||
| Paper | 26/59 (44) | 47/81 (58) | 63/127 (50) | 0.10 | 0.45 | 0.26 |
| Computer | 16/59 (27) | 7/81 (9) | 9/127 (7) | 0.005 | 0.001 | 0.60 |
| Both paper and computer | 17/59 (29) | 25/81 (31) | 51/127 (40) | 0.80 | 0.15 | 0.19 |
| Don't know | 0/59 (0) | 2/81 (2) | 4/127 (3) | 0.28 | 0.18 | 0.66 |
| Process steps (selected questions) | ||||||
| Obtaining home med list | ||||||
| Physician | 19/58 (33) | 12/80 (15) | 9/125 (7) | 0.013 | 0.001 | 0.07 |
| Physician and Nurse | 19/58 (33) | 39/80 (49) | 49/125 (39) | 0.47 | 0.44 | 0.16 |
| Nurse | 15/58 (26) | 25/80 (31) | 62/125 (50) | 0.005 | 0.003 | 0.008 |
| Pharmacist | 5/58 (9) | 1/80 (1) | 2/125 (2) | 0.06 | 0.03 | 0.58 |
| Documenting home med list | ||||||
| Physician | 22/58 (38) | 11/80 (14) | 11/125 (9) | 0.001 | 0.001 | 0.26 |
| Physician and Nurse | 15/58 (26) | 37/80 (46) | 45/125 (36) | 0.02 | 0.18 | 0.16 |
| Nurse | 18/58 (31) | 26/80 (32) | 64/125 (51) | 0.90 | 0.012 | 0.008 |
| Pharmacist | 3/58 (5) | 2/80 (3) | 1/125 (1) | 0.55 | 0.09 | 0.29 |
| Reconciling medications | ||||||
| Physician | 33/58 (57) | 51/80 (64) | 63/125 (50) | 0.41 | 0.42 | 0.051 |
| Physician and Nurse | 8/58 (14) | 14/80 (18) | 32/125 (26) | 0.53 | 0.09 | 0.18 |
| Nurse | 6/58 (10) | 6/80 (8) | 15/125 (12) | 0.68 | 0.71 | 0.36 |
| Pharmacist | 8/58 (14) | 5/80 (6) | 3/125 (2) | 0.11 | 0.007 | 0.13 |
| Updating discharge med list | ||||||
| Physician | 42/58 (72) | 50/80 (63) | 76/125 (61) | 0.27 | 0.15 | 0.77 |
| Physician and Nurse | 7/58 (12) | 16/80 (20) | 23/125 (18) | 0.22 | 0.31 | 0.72 |
| Nurse | 2/58 (3) | 5/80 (6) | 10/125 (8) | 0.41 | 0.20 | 0.59 |
| Pharmacist | 3/58 (5) | 3/80 (4) | 3/125 (2) | 0.78 | 0.27 | 0.40 |
| Providing instructions at discharge | ||||||
| Physician | 14/57 (25) | 8/80 (10) | 17/125 (14) | 0.02 | 0.07 | 0.40 |
| Physician and Nurse | 14/57 (25) | 30/80 (38) | 39/125 (31) | 0.11 | 0.41 | 0.30 |
| Nurse | 25/57 (44) | 37/80 (46) | 60/125 (48) | 0.82 | 0.62 | 0.80 |
| Pharmacist | 4/57 (7) | 1/80 (1) | 0/125 (0) | 0.06 | 0.003 | 0.26 |
Barriers
Results regarding barriers to successful implementation are shown in Table 4. Patient lack of knowledge of medications (87%) and absence of a preadmission medication list from other sources (80%) were common. Both paper and computer medication reconciliation processes were associated with respondents citing cumbersome hospital systems as a barrier; this barrier was cited more often when the implemented process was paper‐only (Table 5). Respondents who stated the medication reconciliation process takes too long did so regardless of whether the implemented process was paper‐based or computer‐based. Despite these barriers, only 16% of respondents stated that medication reconciliation was not worth the effort of implementation. Barriers reported were similar across hospital type (Table 6) with 2 exceptions. Formulary differences were noted to be a barrier more often in CTHs (78%) compared with NTHs (60%) and TACs (64%, not significant compared with CTHs). Language barriers were problematic more often in TACs (48%) than in NTHs (28%) or CTHs (36%, not significant compared with TACs).
| Barrier to Implementation | Yes | No | Unsure |
|---|---|---|---|
| |||
| Patient not knowing meds | 87% | 2% | 0% |
| Process takes too long | 53% | 28% | 8% |
| Med list not available | 80% | 9% | 0% |
| Process not worth effort | 16% | 60% | 12% |
| Cumbersome hospital systems | 52% | 33% | 4% |
| Formulary differences | 59% | 24% | 5% |
| Language barriers | 31% | 53% | 4% |
| No access to outside records | 63% | 23% | 2% |
| Lack of job clarity in process | 38% | 48% | 3% |
| Availability of med list at discharge | 27% | 57% | 3% |
| Barriers (Selected Questions) | Paper Only [P] | Computer Only [C] | Paper and Computer [PC] | P values (2‐tailed) | ||
|---|---|---|---|---|---|---|
| P vs. C | P vs. PC | C vs. PC | ||||
| ||||||
| Process takes too long | ||||||
| Yes | 77/134 (57) | 19/31 (61) | 55/91 (60) | 0.69 | 0.65 | 0.92 |
| No | 43/134 (32) | 11/31 (35) | 28/91 (31) | 0.75 | 0.87 | 0.68 |
| Unsure | 14/134 (10) | 1/31 (3) | 8/91 (9) | 0.21 | 0.80 | 0.27 |
| Process not worth effort | ||||||
| Yes | 24/133 (18) | 3/31 (10) | 17/91 (19) | 0.28 | 0.85 | 0.25 |
| No | 93/133 (70) | 22/31 (71) | 62/91 (68) | 0.91 | 0.75 | 0.76 |
| Unsure | 16/133 (12) | 6/31 (19) | 12/91 (13) | 0.30 | 0.82 | 0.41 |
| Cumbersome hospital systems | ||||||
| Yes | 86/133 (65) | 16/31 (52) | 46/92 (50) | 0.18 | 0.03 | 0.85 |
| No | 42/133 (32) | 13/31 (42) | 42/92 (46) | 0.29 | 0.03 | 0.70 |
| Unsure | 5/133 (4) | 2/31 (6) | 4/92 (4) | 0.62 | 0.82 | 0.64 |
| Barrier to Implementation (Selected Questions) | Academic Centers [AC] | Community Teaching Hospitals [CT] | Non‐Teaching Hospitals [NT] | P values | ||
|---|---|---|---|---|---|---|
| AC vs. CT | AC vs. NT | CT vs. NT | ||||
| ||||||
| Process takes too long | ||||||
| Yes | 37/58 (64) | 49/78 (63) | 70/124 (56) | 0.90 | 0.31 | 0.37 |
| No | 15/58 (26) | 24/78 (31) | 42/124 (34) | 0.53 | 0.28 | 0.66 |
| Unsure | 6/58 (10) | 5/78 (6) | 12/124 (10) | 0.39 | 0.88 | 0.32 |
| Process not worth effort | ||||||
| Yes | 7/58 (12) | 16/78 (21) | 23/123 (19) | 0.17 | 0.24 | 0.73 |
| No | 42/58 (72) | 52/78 (67) | 84/123 (68) | 0.53 | 0.59 | 0.88 |
| Unsure | 9/58 (16) | 10/78 (12) | 16/123 (13) | 0.50 | 0.59 | 0.84 |
| Cumbersome hospital systems | ||||||
| Yes | 36/58 (62) | 46/79 (58) | 69/123 (56) | 0.64 | 0.45 | 0.78 |
| No | 19/58 (33) | 32/79 (41) | 46/123 (37) | 0.34 | 0.60 | 0.57 |
| Unsure | 3/58 (5) | 1/79 (1) | 8/123 (7) | 0.16 | 0.61 | 0.049 |
| Formulary differences | ||||||
| Yes | 37/58 (64) | 61/78 (78) | 74/123 (60) | 0.07 | 0.61 | 0.009 |
| No | 16/58 (28) | 14/78 (18) | 41/123 (33) | 0.17 | 0.50 | 0.02 |
| Unsure | 5/58 (8) | 2/78 (3) | 8/123 (7) | 0.19 | 0.81 | 0.22 |
| Language barriers | ||||||
| Yes | 28/58 (48) | 28/77 (36) | 34/123 (28) | 0.16 | 0.009 | 0.24 |
| No | 28/58 (48) | 46/77 (60) | 82/123 (67) | 0.17 | 0.016 | 0.32 |
| Unsure | 2/58 (3) | 3/77 (4) | 7/123 (5) | 0.76 | 0.54 | 0.74 |
| No access to outside records | ||||||
| Yes | 38/58 (66) | 60/79 (76) | 87/123 (71) | 0.20 | 0.50 | 0.44 |
| No | 18/58 (31) | 18/79 (23) | 33/123 (27) | 0.30 | 0.58 | 0.52 |
| Unsure | 2/58 (3) | 1/79 (1) | 3/123 (2) | 0.39 | 0.68 | 0.58 |
| Lack of job clarity in process | ||||||
| Yes | 26/58 (45) | 31/79 (39) | 49/121 (40) | 0.48 | 0.53 | 0.89 |
| No | 28/58 (48) | 46/79 (58) | 68/121 (56) | 0.25 | 0.32 | 0.78 |
| Unsure | 4/58 (7) | 2/79 (3) | 4/121 (3) | 0.28 | 0.22 | 0.75 |
| Availability of med list at discharge | ||||||
| Yes | 20/58 (34) | 24/79 (30) | 35/120 (29) | 0.62 | 0.50 | 0.88 |
| No | 36/58 (62) | 54/79 (68) | 78/120 (65) | 0.47 | 0.70 | 0.66 |
| Unsure | 0/58 (0) | 1/79 (1) | 7/120 (6) | 0.45 | 0.06 | 0.08 |
DISCUSSION
Managing medication information for inpatients is an extremely complex task. On admission, home medication lists are often inaccurate or absent,6 requiring extra time and effort to discover this information. By discharge, medication regimens have frequently been altered,7 making communication of changes to the next provider essential. One study described myriad provider, patient, and health system issues in maintaining accurate outpatient medication lists.8 These issues are further compounded by the multiple prescribers, necessary hand‐offs, and formulary differences in the inpatient setting.
Over half of the hospitalists in this survey reported hospitalist involvement in design and implementation of medication reconciliation. Given the familiarity with hospital systems and inpatient workflow, hospitalists are well‐positioned to contribute to successful implementation. Nonetheless, many were unaware of efforts to implement this NPSG.
Measurement of both process and outcome measures is important when determining value in quality improvement. Beyond process measures, outcome measures such as adverse drug events, readmission rates, mortality, patient satisfaction, and outpatient provider satisfaction may be appropriate in evaluating medication reconciliation strategies. Even measuring the accuracy of the process with respect to the admission orders written would be a valuable source of information for further improvement. Unfortunately, respondents indicated that evaluation was occurring infrequently. Potentially more problematic is the apparent lack of clarity regarding identification of healthcare provider responsibility for specific process steps. By far the least uniformity is in the acquisition and documentation of the preadmission medication list. There is variability in who is assigned to perform this task, but a substantial number of respondents indicated that their process involved a shared responsibility between physicians and nurses. It is unclear whether this phenomenon reflects the complexity of inpatient medication information management, or is simply an attempt to distribute the work among providers. Sharing the work between physicians and nurses may increase the overall likelihood for compliance and possibly improve the safety and accuracy of the process, especially if the physicians and nurses take the medication history in a redundant fashion and share their findings. Conversely, compliance may decrease if each provider merely expects the other to complete the process. Optimally, an interdisciplinary workflow for medication history taking would be in place, involving both physicians and nurses, with the availability of pharmacist consultation in complex cases. However, our survey data suggest this is infrequent; resident physicians appear to be the ones shouldering substantial responsibility for medication reconciliation in tertiary academic centers. Further research into the accuracy of medication reconciliation processes involving different strategies for medication information collection would be useful.
We documented several barriers to successful implementation of medication reconciliation. Physicians cited a lack of medication knowledge on the part of the patient and unavailable prior medication lists as substantial barriers to success. Many medication reconciliation processes are limited by issues of poor health literacy or inadequate patient knowledge about medications. This lack of medication knowledge is especially problematic for patients new to a healthcare system. It will be important to implement processes that not only reconcile medications accurately, but also make medication information available for future care episodes.
Time required to complete the process was also important. Certain elements of the medication reconciliation process are new work, and integrating the process into existing workflows is crucial. Given the significant time commitment required, the rare involvement of pharmacists at most institutions is striking. It appears that hospital pharmacists do not currently own any of the medication reconciliation process steps at most facilities, despite having formal training in medication history‐taking. In the 2006 ASHP national hospital pharmacy survey, one‐third of pharmacists stated that there were not enough pharmacy resources to meet medication reconciliation demands; only 19% of those surveyed stated pharmacists provided medication education at discharge to more than 25% of their patients.9
This report has several limitations. The survey used was not comprehensive, and only represents a convenience sample of hospitalists attending anational meeting. Nearly 300 physicians responded, representing both teaching and private hospital settings. We consider the response rate of 37% reasonable for a survey of this nature, and the variety of processes described is likely indicative of the overall status of medication reconciliation implementation. The over‐representation of certain institutions in our survey is possible, especially those with large or influential hospital medicine programs. Our survey did not ask respondents to name their home institutions. In addition, this design is open to a convenience sample bias, in that surveying only national meeting attendees (rather than the entire SHM membership) risks overinclusion of those hospitalists involved in leadership roles and quality improvement projects. Despite this, the variety of processes described is likely indicative of the overall status of medication reconciliation implementation in mid‐2006. It is possible that processes have become more uniform nationwide in the interim.
Our survey results reflect the complexity surrounding medication reconciliation. It appears that full implementation has not yet occurred everywhere, significant barriers remain, and outcome measurement is limited. Importantly, physicians, nurses, and pharmacists do not have standardized roles. Responsibility for medication reconciliation has predominantly been added to the existing duties of inpatient physicians and nurses, with limited involvement of pharmacists. Hospitalists are well‐positioned to lead the ongoing implementation of medication reconciliation processes and should take advantage of their systems knowledge to effectively partner with other physicians, nurses, and pharmacists to achieve success in medication reconciliation.
Acknowledgements
The authors thank Ken Epstein, MD, and Renee Meadows, MD, along with the entire SHM Medication Reconciliation Task Force for their helpful review and comments on the article.
Appendix
|
|
- Kohn LT,Corrigan JM,Donaldson MS, eds.To Err Is Human: Building a Safer Health System.Washington, DC:National Academy Press;1999.
- .Medication reconciliation: transfer of medication information across settings – keeping it free from error.Am J Nurs.2005;105(3 Suppl):31–36.
- ,,, et al.Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients.Am J Health‐Syst Pharm.2004;61(16):1689–1695.
- ,,, et al.Medication reconciliation: a practical tool to reduce the risk of medication errors.J Crit Care.2003;18(4):201–205.
- ,,,.Evaluation of a new integrated discharge prescription form.Ann Pharmacother.2001;35(7‐8):953–958.
- ,,, et al.Patient safety standardization as a mechanism to improve safety in health care.Jt Comm J Qual Saf.2004;30(1):5–14.
- ,,.What happens to long‐term medication when general practice patients are referred to hospital?Eur J Clin Pharmacol.1996;50(4):253–257.
- ,,, et al.An experiential interdisciplinary quality improvement education initiative.Am J Med Qual.2006;21(5):317–322.
- ,,.ASHP national survey of pharmacy practice in hospital settings: monitoring and patient education‐2006.Am J Health‐Syst Pharm.2007;64(5):507–520.
- Kohn LT,Corrigan JM,Donaldson MS, eds.To Err Is Human: Building a Safer Health System.Washington, DC:National Academy Press;1999.
- .Medication reconciliation: transfer of medication information across settings – keeping it free from error.Am J Nurs.2005;105(3 Suppl):31–36.
- ,,, et al.Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients.Am J Health‐Syst Pharm.2004;61(16):1689–1695.
- ,,, et al.Medication reconciliation: a practical tool to reduce the risk of medication errors.J Crit Care.2003;18(4):201–205.
- ,,,.Evaluation of a new integrated discharge prescription form.Ann Pharmacother.2001;35(7‐8):953–958.
- ,,, et al.Patient safety standardization as a mechanism to improve safety in health care.Jt Comm J Qual Saf.2004;30(1):5–14.
- ,,.What happens to long‐term medication when general practice patients are referred to hospital?Eur J Clin Pharmacol.1996;50(4):253–257.
- ,,, et al.An experiential interdisciplinary quality improvement education initiative.Am J Med Qual.2006;21(5):317–322.
- ,,.ASHP national survey of pharmacy practice in hospital settings: monitoring and patient education‐2006.Am J Health‐Syst Pharm.2007;64(5):507–520.
Pediatric Hospitalist Variation in Care
Reduction of undesirable variation in care has been a major focus of systematic efforts to improve the quality of the healthcare system.13 The emergence of hospitalists, physicians specializing in the care of hospitalized patients, was spurred by a desire to streamline care and reduce variability in hospital management of common diseases.4, 5 Over the past decade, hospitalist systems have become a leading vehicle for care delivery.4, 6, 7 It remains unclear, however, whether implementation of hospitalist systems has lessened undesirable variation in the inpatient management of common diseases.
While systematic reviews have found costs and hospital length of stay to be 10‐15% lower in both pediatric and internal medicine hospitalist systems, few studies have adequately assessed the processes or quality of care in hospitalist systems.8, 9 Two internal medicine studies have found decreased mortality in hospitalist systems, but the mechanism by which hospitalists apparently achieved these gains is unclear.10, 11 Even less is known about care processes or quality in pediatric hospitalist systems. Death is a rare occurrence in pediatric ward settings, and the seven studies conducted to date comparing pediatric hospitalist and traditional systems have been universally underpowered to detect differences in mortality.9, 1218 There is a need to better understand care processes as a first step in understanding and improving quality of care in hospitalist systems.19
The Pediatric Research in Inpatient Settings (PRIS) Network was formed to improve the quality of care for hospitalized children through collaborative clinical research. In this study, we sought to study variation in the care of common pediatric conditions among a cohort of pediatric hospitalists. We have previously reported that less variability exists in hospitalists' reported management of inpatient conditions than in the reported management of these same conditions by community‐based pediatricians,20 but we were concerned that substantial undesirable variation (ie, variation in practice due to uncertainty or unsubstantiated local practice traditions, rather than justified variation in care based on different risks of harms or benefits in different patients) may still exist among hospitalists. We therefore conducted a study: 1) to investigate variation in hospitalists' reported use of common inpatient therapies, and 2) to test the hypothesis that greater variation exists in hospitalists' reported use of inpatient therapies of unproven benefit than in those therapies proven to be beneficial.
METHODS
Survey Design and Administration
In 2003, we designed the PRIS Survey to collect data on hospitalists' backgrounds, practices, and training needs, as well as their management of common pediatric conditions. For the current study, we chose a priori to evaluate hospitalists' use of 14 therapies in the management of 4 common conditions: asthma, bronchiolitis, gastroenteritis, and gastro‐esophageal reflux disease (GERD) (Table 1). These four conditions were chosen for study because they were among the top discharge diagnoses (primary and secondary) from the inpatient services at 2 of the authors' institutions (Children's Hospital Boston and Children's Hospital San Diego) during the year before administration of the survey, and because a discrete set of therapeutic agents are commonly used in their management. Respondents were asked to report the frequency with which they used each of the 14 therapies of interest on 5‐point Likert scales (from 1=never to 5=almost always). The survey initially developed was piloted with a small group of hospitalists and pediatricians, and a final version incorporating revisions was subsequently administered to all pediatric hospitalists in the US and Canada identified through any of 3 sources: 1) the Pediatric Research in Inpatient Settings (PRIS) list of participants; 2) the Society for Hospital Medicine (SHM) pediatric hospital medicine e‐mail listserv; and 3) the list of all attendees of the first national pediatric hospitalist conference sponsored by the Ambulatory Pediatrics Association (APA), SHM, and American Academy of Pediatrics (AAP); this meeting was held in San Antonio, Texas, USA in November 2003. Individuals identified through more than 1 of these groups were counted only once. Potential participants were assured that individual responses would be kept confidential, and were e‐mailed an access code to participate in the online survey, using a secure web‐based interface; a paper‐based version was also made available to those who preferred to respond in this manner. Regular reminder notices were sent to all non‐responders. Further details regarding PRIS Survey recruitment and study methods have been published previously.20
| Condition | Therapy | BMJ clinical evidence Treatment effect categorization* | Study classification |
|---|---|---|---|
| |||
| Asthma | Inhaled albuterol | Beneficial | Proven |
| Systemic corticosteroids | Beneficial | Proven | |
| Inhaled ipratropium in the first 24 hours of hospitalization | Beneficial | Proven | |
| Inhaled ipratropium after the first 24 hours of hospitalization | Unknown effectiveness | Unproven | |
| Bronchiolitis | Inhaled albuterol | Unknown effectiveness | Unproven |
| Inhaled epinephrine | Unknown effectiveness | Unproven | |
| Systemic corticosteroids | Unknown effectiveness | Unproven | |
| Gastroenteritis | Intravenous hydration | Beneficial | Proven |
| Lactobacillus | Not assessed | Unproven | |
| Ondansetron | Not assessed | Unproven | |
| Gastro‐Esophageal Reflux Disease (GERD) | H2 histamine‐receptor antagonists | Unknown effectiveness | Unproven |
| Thickened feeds | Unknown effectiveness Likely to be beneficial | Unproven Proven | |
| Metoclopramide | Unknown effectiveness | Unproven | |
| Proton‐pump inhibitors | Unknown effectiveness | Unproven | |
DefinitionsReference Responses and Percent Variation
To measure variation in reported management, we first sought to determine a reference response for each therapy of interest. Since the evidence base for most of the therapies we studied is weak, it was not possible to determine a gold standard response for each therapy. Instead, we sought to measure the degree of divergence from a reference response for each therapy in the following manner. First, to simplify analyses, we collapsed our five‐category Likert scale into three categories (never/rarely, sometimes, and often/almost always). We then defined the reference response for each therapy to be never/rarely or often/almost always, whichever of the 2 was more frequently selected by respondents; sometimes was not used as a reference category, as reporting use of a particular therapy sometimes indicated substantial variability even within an individual's own practice.
Classification of therapies as proven or unproven.
To classify each of the 14 studied therapies as being of proven or unproven, we used the British Medical Journal's publication Clinical Evidence.19 We chose to use Clinical Evidence as an evidence‐based reference because it provides rigorously developed, systematic analyses of therapeutic management options for multiple common pediatric conditions, and organizes recommendations in a straightforward manner. Four of the 14 therapies had been determined on systematic review to be proven beneficial at the time of study design: systemic corticosteroids, inhaled albuterol, and ipratropium (in the first 24 h) in the care of children with asthma; and IV hydration in the care of children with acute gastroenteritis. The remaining 10 therapies were either considered to be of unknown effectiveness or had not been formally evaluated by Clinical Evidence, and were hence considered unproven for this study (Table 1). Of note, the use of thickened feeds in the treatment of children with GERD had been determined to be of unknown effectiveness at the time of study design, but was reclassified as likely to be beneficial during the course of the study.
Analyses
Descriptive statistics were used to report respondents' demographic characteristics and work environments, as well as variation in their reported use of each of the 14 therapies. Variation in hospitalists' use of proven versus unproven therapies was compared using the Wilcoxon rank sum test, as it was distributed non‐normally. For our primary analysis, the use of thickened feeds in GERD was considered unproven, but a sensitivity analysis was conducted reclassifying it as proven in light of the evolving literature on its use and its consequent reclassification in Clinical Evidence.(SAS Version 9.1, Cary, NC) was used for statistical analyses.
RESULTS
213 of the 320 individuals identified through the 3 lists of pediatric hospitalists (67%) responded to the survey. Of these, 198 (93%) identified themselves as hospitalists and were therefore included. As previously reported,20 53% of respondents were male, 55% worked in academic training environments, and 47% had completed advanced training (fellowship) beyond their core pediatric training (residency training); respondents reported completing residency training 11 9 (mean, standard deviation) years prior to the survey, and spending 176 72 days per year in the care of hospitalized patients.
Variation in reported management: asthma
(Figure 1, Panel A). Relatively little variation existed in reported use of the 4 asthma therapies studied. Only 4.4% (95% CI, 1.4‐7.4%) of respondents did not provide the reference response of using inhaled albuterol often or almost always in the care of inpatients with asthma, and only 6.0% (2.5‐9.5%) of respondents did not report using systemic corticosteroids often or almost always. Variation in reported use of ipratropium was somewhat higher.
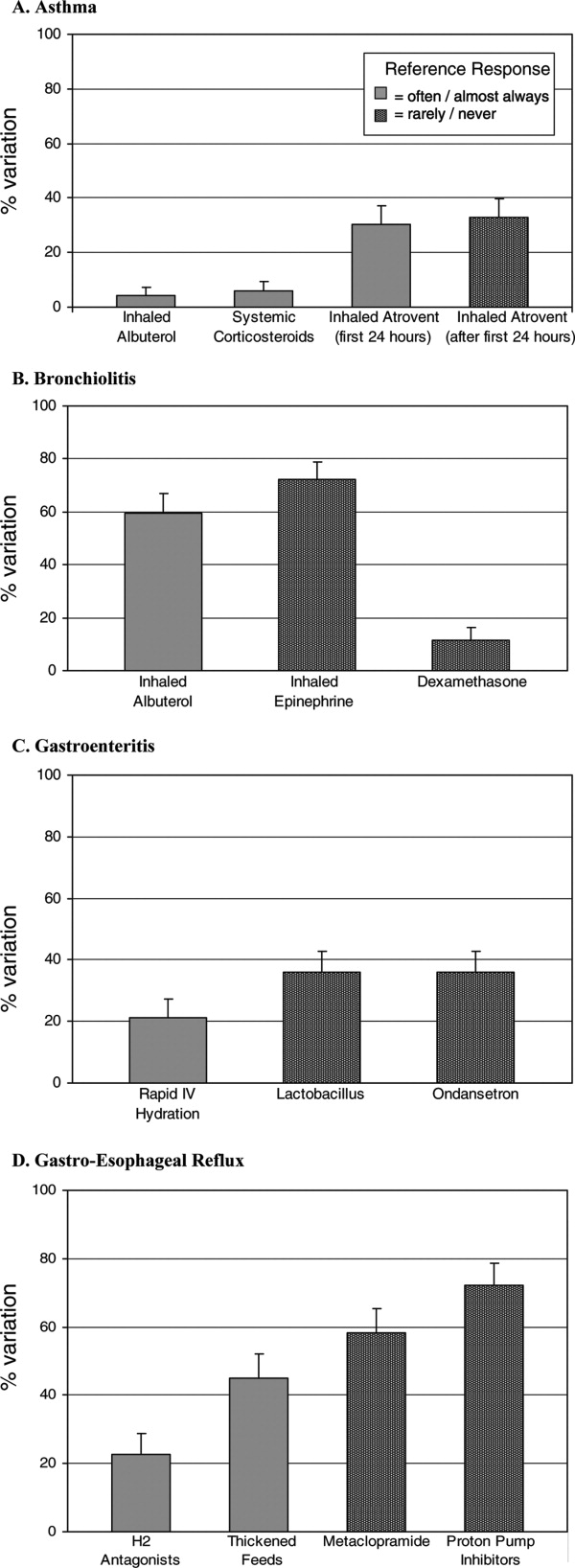
Bronchiolitis
(Figure 1B). By contrast, variation in reported use of inhaled therapies for bronchiolitis was high, with many respondents reporting that they often or always used inhaled albuterol or epinephrine, while many others reported rarely or never using them. There was 59.6% (52.4‐66.8%) variation from the reference response of often/almost always using inhaled albuterol, and 72.2% (65.6‐78.8%) variation from the reference response of never/rarely using inhaled epinephrine. Only 11.6% (6.9‐16.3%) of respondents, however, varied from the reference response of using dexamethasone more than rarely in the care of children with bronchiolitis.
Gastroenteritis
(Figure 1C). Moderate variability existed in the reported use of the 3 studied therapies for children hospitalized with gastroenteritis. 21.1% (15.1‐27.1%) of respondents did not provide the reference response of often/almost always using IV hydration; 35.9% (28.9‐42.9%) did not provide the reference response of never or rarely using lactobacillus; likewise, 35.9% (28.9‐42.9%) did not provide the reference response of never or rarely using ondansetron.
Gastro‐Esophageal Reflux Disease
(Figure 1, Panel D). There was moderate to high variability in the reported management of GERD. 22.8% (16.7‐28.9%) of respondents did not provide the reference response of often/almost always using H2 antagonists, and 44.9% (37.6‐52.2%) did not report often/almost always using thickened feeds in the care of these children. 58.3% (51.1‐65.5%) and 72.1% (65.5‐78.7%) of respondents did not provide the reference response of never/rarely using metoclopramide and proton pump inhibitors, respectively.
Proven vs. Unproven Therapies
(Figure 2). Variation in reported use of therapies of unproven benefit was significantly higher than variation in reported use of the 4 proven therapies (albuterol, corticosteroids, and ipratropium in the first 24 h for asthma; IV re‐hydration for gastroenteritis). The mean variation in reported use of unproven therapies was 44.6 20.5%, compared with 15.5 12.5% variation in reported use of therapies of proven benefit (p = 0.02).
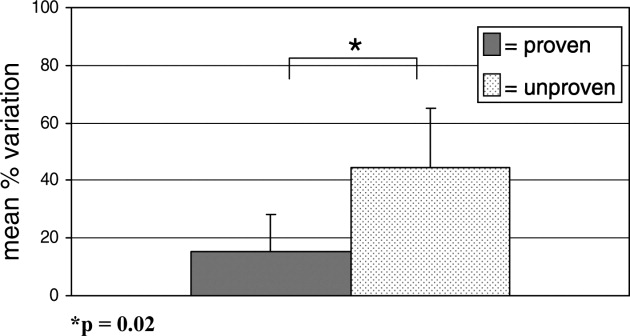
As a sensitivity analysis, the use of thickened feeds as a therapy for GERD was re‐categorized as proven and the above analysis repeated, for the reasons outlined in the methods section. This did not alter the identified relationship between variability and the evidence base fundamentally; hospitalists' reported variation in use of therapies of unproven benefit in this sensitivity analysis was 44.6 21.7%, compared with 21.4 17.0% variation in reported use of proven therapies (p = 0.05).
DISCUSSION
Substantial variation exists in the inpatient management of common pediatric diseases. Although we have previously found less reported variability in pediatric hospitalists' practices than in those of community‐based pediatricians,20 the current study demonstrates a high degree of reported variation even among a cohort of inpatient specialists. Importantly, however, reported variation was found to be significantly less for those inpatient therapies supported by a robust evidence base.
Bronchiolitis, gastroenteritis, asthma, and GERD are extremely common causes of pediatric hospitalization throughout the developed world.2125 Our finding of high reported variability in the routine care of all of these conditions except asthma is concerning, as it suggests that experts do not agree on how to manage children hospitalized with even the most common childhood diseases. While we hypothesized that there would be some variation in the use of therapies whose benefit has not been well established, the high degree of variation observed is of concern because it indicates that an insufficient evidentiary base exists to support much of our day‐to‐day practice. Some variation in practice in response to differing clinical presentations is both expected and desirable, but it is remarkable that variance in practice was significantly less for the most evidence‐based therapies than for those grounded less firmly in science, suggesting that the variation identified here is not justifiable variation based on appropriate responses to atypical clinical presentations, but uncertainty in the absence of clear data. Such undesired variability may decrease system reliability (introducing avoidable opportunity for error),26 and lead to under‐use of needed therapies as well as overuse of unnecessary therapies.1
Our work extends prior research that has identified wide variation in patterns of hospital admission, use of hospital resources, and processes of inpatient care,2732 by documenting reported variation in the use of common inpatient therapies. Rates of hospital admission may vary by as much as 7‐fold across regions.33 Our study demonstrates that wide variation exists not only in admission rates, but in reported inpatient care processes for some of the most common diseases seen in pediatric hospitals. Our study also supports the hypothesis that variation in care may be driven by gaps in knowledge.32 Among hospitalists, we found the strength of the evidence base to be a major determinant of reported variability.
Our study has several limitations. First, the data presented here are derived from provider self‐reports, which may not fully reflect actual practice. In the case of the few proven therapies studied, reporting bias could lead to an over‐reporting of adherence to evidence‐based standards of care. Like our study, however, prior studies have found that hospital‐based providers fairly consistently comply with evidence‐based practice recommendations for acute asthma care,34, 35 supporting our finding that variation in acute asthma care (which represented 3 of our 4 proven therapies) is low in this setting.
Another limitation is that classifications of therapies as proven or unproven change as the evidence base evolves. Of particular relevance to this study, the use of thickened feeds as a therapy for GERD, originally classified as being of unknown effectiveness, was reclassified by Clinical Evidence during the course of the study as likely to be beneficial. The relationship we identified between proven therapies and degree of variability in care did not change when we conducted a sensitivity analysis re‐categorizing this therapy as proven, but precisely quantifying variation is complicated by continuous changes in the state of the evidence.
Pediatric hospitalist systems have been found consistently to improve the efficiency of care,9 yet this study suggests that considerable variation in hospitalists' management of key conditions remains. The Pediatric Research in Inpatient Settings (PRIS) Network was formed in 2002 to improve the care of hospitalized children and the quality of inpatient practice by developing an evidence base for inpatient pediatric care. Ongoing multi‐center research efforts through PRIS and other research networks are beginning to critically evaluate therapies used in the management of common pediatric conditions. Rigorous studies of the processes and outcomes of pediatric hospital care will inform inpatient pediatric practice, and ultimately improve the care of hospitalized children. The current study strongly affirms the urgent need to establish such an evidence base. Without data to inform optimal care, efforts to reduce undesirable variation in care and improve care quality cannot be fully realized.
Acknowledgements
The authors would like to extend their thanks to the hospitalists and members of the Pediatric Research in Inpatient Settings Network who participated in this research, as well as the Children's National Medical Center and Children's Hospital Boston Inpatient Pediatrics Services, who provided funding to support this study. Special thanks to the Ambulatory Pediatrics Association (APA), for its core support of the PRIS Network. Dr. Landrigan is the recipient of a career development award from the Agency for Healthcare Research and Quality (AHRQ K08 HS13333). Dr. Conway is the recipient of a Robert Wood Johnson Clinical Scholars Grant. All researchers were independent from the funding agencies; the academic medical centers named above, APA, and AHRQ had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
- Institute of Medicine: Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, D.C.:National Academic Press,2001.
- ..Reducing variation in surgical care.BMJ2005;330:1401–1402.
- ,,,.Variation in use of video assisted thoracic surgery in the United Kingdom.BMJ2004;329:1011–1012.
- ,..The emerging role of “hospitalists” in the American health care system.N. Engl J Med1996;335:514–517.
- ,..Hospitalism in the USA.Lancet1999;353:1902.
- Society of Hospital Medicine. Growth of Hospital Medicine Nationwide. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/Media/GrowthofHospitalMedicineNationwide/Growth_of_Hospital_M.htm. Accessed April 11,2007.
- .The changing face of hospital practice.Med Econ2002;79:72–79.
- ,..The hospitalist movement 5 years later.JAMA2002;287:487–494.
- ,,,.Pediatric hospitalists: a systematic review of the literature.Pediatrics2006;117:1736–1744.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med2002;137:859–865.
- ,,,,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med2002;137:866–874.
- ,.Evaluation of a pediatric hospitalist service: impact on length of stay and hospital charges.Pediatrics2000;105:478–484.
- ,,,,,,, and .Impact of an HMO hospitalist system in academic pediatrics.Pediatrics2002;110:720–728.
- ,, and .Evaluation of a pediatric hospitalist service by APR‐DRG's: impact on length of stay and hospital charges.Pediatr Research2001;49(suppl),691.
- ,,.Pediatric hospitalists: quality care for the underserved?Am J Med Qual2001;16:174–180.
- ,,,,,.Restructuring an academic pediatric inpatient service using concepts developed by hospitalists.Clin Pediatr (Phila)2001;40:653–660.
- ,,, and .Hospitalist care of medically complex children.Pediatr Research2004;55(suppl),1789.
- ,,.Hospital‐based and community pediatricians: comparing outcomes for asthma and bronchiolitis.J Clin Outcomes Manage1997;4:21–24.
- Godlee F,Tovey D,Bedford M, et al., eds.Clinical Evidence: The International Source of the Best Available Evidence for Effective Health Care.London, United Kingdom:BMJ Publishing Group;2004.
- ,,,,,.Variations in management of common inpatient pediatric illnesses: hospitalists and community pediatricians.Pediatrics2006;118:441–447.
- ,,,,.Contribution of RSV to bronchiolitis and pneumonia‐associated hospitalizations in English children, April 1995‐March 1998.Epidemiol Infect2002;129:99–106.
- ,,.Direct medical costs of bronchiolitis hospitalizations in the United States.Pediatrics2006;118:2418–2423.
- ,,,,,.Multicenter Prospective Study of the Burden of Rotavirus Acute Gastroenteritis in Europe, 2004‐2005: The REVEAL Study.J Infect Dis2007;195Suppl 1:S4–S16.
- .The state of childhood asthma, United States, 1980‐2005.Adv.Data.2006;1–24.
- ,.Gastroesophageal reflux in children: pathogenesis, prevalence, diagnosis, and role of proton pump inhibitors in treatment.Paediatr Drugs2002;4:673–685.
- ,,,.Reliability science and patient safety.Pediatr Clin North Am2006;53:1121–1133.
- Wennberg JE and McAndrew Cooper M, eds.The Dartmouth Atlas of Health Care in the United States.Hanover, NH, USA:Health Forum, Inc.,1999.
- ,,,,,.Variations in rates of hospitalization of children in three urban communities.N Engl J Med1989;320:1183–1187.
- ,,,,,.Use of hospitals, physician visits, and hospice care during last six months of life among cohorts loyal to highly respected hospitals in the United States.BMJ2004;328:607.
- ,,,,,, et al.Variations in practice and outcomes in the Canadian NICU network: 1996‐1997.Pediatrics2000;106:1070–1079.
- ,,,.Evaluation of febrile children with petechial rashes: is there consensus among pediatricians?Pediatr Infect Dis J1998;17:1135–1140.
- ,,,,,, et al.Practice variation among pediatric emergency departments in the treatment of bronchiolitis.Acad Emerg Med2004;11:353–360.
- ,,,.Paediatric inpatient utilisation in a district general hospital.Arch Dis Child1994;70:488–492.
- ,,,.Emergency department asthma: compliance with an evidence‐based management algorithm.Ann Acad Med Singapore2002;31:419–424.
- ,,.Is the practice of paediatric inpatient medicine evidence‐based?J Paediatr Child Health2002;38:347–351.
Reduction of undesirable variation in care has been a major focus of systematic efforts to improve the quality of the healthcare system.13 The emergence of hospitalists, physicians specializing in the care of hospitalized patients, was spurred by a desire to streamline care and reduce variability in hospital management of common diseases.4, 5 Over the past decade, hospitalist systems have become a leading vehicle for care delivery.4, 6, 7 It remains unclear, however, whether implementation of hospitalist systems has lessened undesirable variation in the inpatient management of common diseases.
While systematic reviews have found costs and hospital length of stay to be 10‐15% lower in both pediatric and internal medicine hospitalist systems, few studies have adequately assessed the processes or quality of care in hospitalist systems.8, 9 Two internal medicine studies have found decreased mortality in hospitalist systems, but the mechanism by which hospitalists apparently achieved these gains is unclear.10, 11 Even less is known about care processes or quality in pediatric hospitalist systems. Death is a rare occurrence in pediatric ward settings, and the seven studies conducted to date comparing pediatric hospitalist and traditional systems have been universally underpowered to detect differences in mortality.9, 1218 There is a need to better understand care processes as a first step in understanding and improving quality of care in hospitalist systems.19
The Pediatric Research in Inpatient Settings (PRIS) Network was formed to improve the quality of care for hospitalized children through collaborative clinical research. In this study, we sought to study variation in the care of common pediatric conditions among a cohort of pediatric hospitalists. We have previously reported that less variability exists in hospitalists' reported management of inpatient conditions than in the reported management of these same conditions by community‐based pediatricians,20 but we were concerned that substantial undesirable variation (ie, variation in practice due to uncertainty or unsubstantiated local practice traditions, rather than justified variation in care based on different risks of harms or benefits in different patients) may still exist among hospitalists. We therefore conducted a study: 1) to investigate variation in hospitalists' reported use of common inpatient therapies, and 2) to test the hypothesis that greater variation exists in hospitalists' reported use of inpatient therapies of unproven benefit than in those therapies proven to be beneficial.
METHODS
Survey Design and Administration
In 2003, we designed the PRIS Survey to collect data on hospitalists' backgrounds, practices, and training needs, as well as their management of common pediatric conditions. For the current study, we chose a priori to evaluate hospitalists' use of 14 therapies in the management of 4 common conditions: asthma, bronchiolitis, gastroenteritis, and gastro‐esophageal reflux disease (GERD) (Table 1). These four conditions were chosen for study because they were among the top discharge diagnoses (primary and secondary) from the inpatient services at 2 of the authors' institutions (Children's Hospital Boston and Children's Hospital San Diego) during the year before administration of the survey, and because a discrete set of therapeutic agents are commonly used in their management. Respondents were asked to report the frequency with which they used each of the 14 therapies of interest on 5‐point Likert scales (from 1=never to 5=almost always). The survey initially developed was piloted with a small group of hospitalists and pediatricians, and a final version incorporating revisions was subsequently administered to all pediatric hospitalists in the US and Canada identified through any of 3 sources: 1) the Pediatric Research in Inpatient Settings (PRIS) list of participants; 2) the Society for Hospital Medicine (SHM) pediatric hospital medicine e‐mail listserv; and 3) the list of all attendees of the first national pediatric hospitalist conference sponsored by the Ambulatory Pediatrics Association (APA), SHM, and American Academy of Pediatrics (AAP); this meeting was held in San Antonio, Texas, USA in November 2003. Individuals identified through more than 1 of these groups were counted only once. Potential participants were assured that individual responses would be kept confidential, and were e‐mailed an access code to participate in the online survey, using a secure web‐based interface; a paper‐based version was also made available to those who preferred to respond in this manner. Regular reminder notices were sent to all non‐responders. Further details regarding PRIS Survey recruitment and study methods have been published previously.20
| Condition | Therapy | BMJ clinical evidence Treatment effect categorization* | Study classification |
|---|---|---|---|
| |||
| Asthma | Inhaled albuterol | Beneficial | Proven |
| Systemic corticosteroids | Beneficial | Proven | |
| Inhaled ipratropium in the first 24 hours of hospitalization | Beneficial | Proven | |
| Inhaled ipratropium after the first 24 hours of hospitalization | Unknown effectiveness | Unproven | |
| Bronchiolitis | Inhaled albuterol | Unknown effectiveness | Unproven |
| Inhaled epinephrine | Unknown effectiveness | Unproven | |
| Systemic corticosteroids | Unknown effectiveness | Unproven | |
| Gastroenteritis | Intravenous hydration | Beneficial | Proven |
| Lactobacillus | Not assessed | Unproven | |
| Ondansetron | Not assessed | Unproven | |
| Gastro‐Esophageal Reflux Disease (GERD) | H2 histamine‐receptor antagonists | Unknown effectiveness | Unproven |
| Thickened feeds | Unknown effectiveness Likely to be beneficial | Unproven Proven | |
| Metoclopramide | Unknown effectiveness | Unproven | |
| Proton‐pump inhibitors | Unknown effectiveness | Unproven | |
DefinitionsReference Responses and Percent Variation
To measure variation in reported management, we first sought to determine a reference response for each therapy of interest. Since the evidence base for most of the therapies we studied is weak, it was not possible to determine a gold standard response for each therapy. Instead, we sought to measure the degree of divergence from a reference response for each therapy in the following manner. First, to simplify analyses, we collapsed our five‐category Likert scale into three categories (never/rarely, sometimes, and often/almost always). We then defined the reference response for each therapy to be never/rarely or often/almost always, whichever of the 2 was more frequently selected by respondents; sometimes was not used as a reference category, as reporting use of a particular therapy sometimes indicated substantial variability even within an individual's own practice.
Classification of therapies as proven or unproven.
To classify each of the 14 studied therapies as being of proven or unproven, we used the British Medical Journal's publication Clinical Evidence.19 We chose to use Clinical Evidence as an evidence‐based reference because it provides rigorously developed, systematic analyses of therapeutic management options for multiple common pediatric conditions, and organizes recommendations in a straightforward manner. Four of the 14 therapies had been determined on systematic review to be proven beneficial at the time of study design: systemic corticosteroids, inhaled albuterol, and ipratropium (in the first 24 h) in the care of children with asthma; and IV hydration in the care of children with acute gastroenteritis. The remaining 10 therapies were either considered to be of unknown effectiveness or had not been formally evaluated by Clinical Evidence, and were hence considered unproven for this study (Table 1). Of note, the use of thickened feeds in the treatment of children with GERD had been determined to be of unknown effectiveness at the time of study design, but was reclassified as likely to be beneficial during the course of the study.
Analyses
Descriptive statistics were used to report respondents' demographic characteristics and work environments, as well as variation in their reported use of each of the 14 therapies. Variation in hospitalists' use of proven versus unproven therapies was compared using the Wilcoxon rank sum test, as it was distributed non‐normally. For our primary analysis, the use of thickened feeds in GERD was considered unproven, but a sensitivity analysis was conducted reclassifying it as proven in light of the evolving literature on its use and its consequent reclassification in Clinical Evidence.(SAS Version 9.1, Cary, NC) was used for statistical analyses.
RESULTS
213 of the 320 individuals identified through the 3 lists of pediatric hospitalists (67%) responded to the survey. Of these, 198 (93%) identified themselves as hospitalists and were therefore included. As previously reported,20 53% of respondents were male, 55% worked in academic training environments, and 47% had completed advanced training (fellowship) beyond their core pediatric training (residency training); respondents reported completing residency training 11 9 (mean, standard deviation) years prior to the survey, and spending 176 72 days per year in the care of hospitalized patients.
Variation in reported management: asthma
(Figure 1, Panel A). Relatively little variation existed in reported use of the 4 asthma therapies studied. Only 4.4% (95% CI, 1.4‐7.4%) of respondents did not provide the reference response of using inhaled albuterol often or almost always in the care of inpatients with asthma, and only 6.0% (2.5‐9.5%) of respondents did not report using systemic corticosteroids often or almost always. Variation in reported use of ipratropium was somewhat higher.

Bronchiolitis
(Figure 1B). By contrast, variation in reported use of inhaled therapies for bronchiolitis was high, with many respondents reporting that they often or always used inhaled albuterol or epinephrine, while many others reported rarely or never using them. There was 59.6% (52.4‐66.8%) variation from the reference response of often/almost always using inhaled albuterol, and 72.2% (65.6‐78.8%) variation from the reference response of never/rarely using inhaled epinephrine. Only 11.6% (6.9‐16.3%) of respondents, however, varied from the reference response of using dexamethasone more than rarely in the care of children with bronchiolitis.
Gastroenteritis
(Figure 1C). Moderate variability existed in the reported use of the 3 studied therapies for children hospitalized with gastroenteritis. 21.1% (15.1‐27.1%) of respondents did not provide the reference response of often/almost always using IV hydration; 35.9% (28.9‐42.9%) did not provide the reference response of never or rarely using lactobacillus; likewise, 35.9% (28.9‐42.9%) did not provide the reference response of never or rarely using ondansetron.
Gastro‐Esophageal Reflux Disease
(Figure 1, Panel D). There was moderate to high variability in the reported management of GERD. 22.8% (16.7‐28.9%) of respondents did not provide the reference response of often/almost always using H2 antagonists, and 44.9% (37.6‐52.2%) did not report often/almost always using thickened feeds in the care of these children. 58.3% (51.1‐65.5%) and 72.1% (65.5‐78.7%) of respondents did not provide the reference response of never/rarely using metoclopramide and proton pump inhibitors, respectively.
Proven vs. Unproven Therapies
(Figure 2). Variation in reported use of therapies of unproven benefit was significantly higher than variation in reported use of the 4 proven therapies (albuterol, corticosteroids, and ipratropium in the first 24 h for asthma; IV re‐hydration for gastroenteritis). The mean variation in reported use of unproven therapies was 44.6 20.5%, compared with 15.5 12.5% variation in reported use of therapies of proven benefit (p = 0.02).

As a sensitivity analysis, the use of thickened feeds as a therapy for GERD was re‐categorized as proven and the above analysis repeated, for the reasons outlined in the methods section. This did not alter the identified relationship between variability and the evidence base fundamentally; hospitalists' reported variation in use of therapies of unproven benefit in this sensitivity analysis was 44.6 21.7%, compared with 21.4 17.0% variation in reported use of proven therapies (p = 0.05).
DISCUSSION
Substantial variation exists in the inpatient management of common pediatric diseases. Although we have previously found less reported variability in pediatric hospitalists' practices than in those of community‐based pediatricians,20 the current study demonstrates a high degree of reported variation even among a cohort of inpatient specialists. Importantly, however, reported variation was found to be significantly less for those inpatient therapies supported by a robust evidence base.
Bronchiolitis, gastroenteritis, asthma, and GERD are extremely common causes of pediatric hospitalization throughout the developed world.2125 Our finding of high reported variability in the routine care of all of these conditions except asthma is concerning, as it suggests that experts do not agree on how to manage children hospitalized with even the most common childhood diseases. While we hypothesized that there would be some variation in the use of therapies whose benefit has not been well established, the high degree of variation observed is of concern because it indicates that an insufficient evidentiary base exists to support much of our day‐to‐day practice. Some variation in practice in response to differing clinical presentations is both expected and desirable, but it is remarkable that variance in practice was significantly less for the most evidence‐based therapies than for those grounded less firmly in science, suggesting that the variation identified here is not justifiable variation based on appropriate responses to atypical clinical presentations, but uncertainty in the absence of clear data. Such undesired variability may decrease system reliability (introducing avoidable opportunity for error),26 and lead to under‐use of needed therapies as well as overuse of unnecessary therapies.1
Our work extends prior research that has identified wide variation in patterns of hospital admission, use of hospital resources, and processes of inpatient care,2732 by documenting reported variation in the use of common inpatient therapies. Rates of hospital admission may vary by as much as 7‐fold across regions.33 Our study demonstrates that wide variation exists not only in admission rates, but in reported inpatient care processes for some of the most common diseases seen in pediatric hospitals. Our study also supports the hypothesis that variation in care may be driven by gaps in knowledge.32 Among hospitalists, we found the strength of the evidence base to be a major determinant of reported variability.
Our study has several limitations. First, the data presented here are derived from provider self‐reports, which may not fully reflect actual practice. In the case of the few proven therapies studied, reporting bias could lead to an over‐reporting of adherence to evidence‐based standards of care. Like our study, however, prior studies have found that hospital‐based providers fairly consistently comply with evidence‐based practice recommendations for acute asthma care,34, 35 supporting our finding that variation in acute asthma care (which represented 3 of our 4 proven therapies) is low in this setting.
Another limitation is that classifications of therapies as proven or unproven change as the evidence base evolves. Of particular relevance to this study, the use of thickened feeds as a therapy for GERD, originally classified as being of unknown effectiveness, was reclassified by Clinical Evidence during the course of the study as likely to be beneficial. The relationship we identified between proven therapies and degree of variability in care did not change when we conducted a sensitivity analysis re‐categorizing this therapy as proven, but precisely quantifying variation is complicated by continuous changes in the state of the evidence.
Pediatric hospitalist systems have been found consistently to improve the efficiency of care,9 yet this study suggests that considerable variation in hospitalists' management of key conditions remains. The Pediatric Research in Inpatient Settings (PRIS) Network was formed in 2002 to improve the care of hospitalized children and the quality of inpatient practice by developing an evidence base for inpatient pediatric care. Ongoing multi‐center research efforts through PRIS and other research networks are beginning to critically evaluate therapies used in the management of common pediatric conditions. Rigorous studies of the processes and outcomes of pediatric hospital care will inform inpatient pediatric practice, and ultimately improve the care of hospitalized children. The current study strongly affirms the urgent need to establish such an evidence base. Without data to inform optimal care, efforts to reduce undesirable variation in care and improve care quality cannot be fully realized.
Acknowledgements
The authors would like to extend their thanks to the hospitalists and members of the Pediatric Research in Inpatient Settings Network who participated in this research, as well as the Children's National Medical Center and Children's Hospital Boston Inpatient Pediatrics Services, who provided funding to support this study. Special thanks to the Ambulatory Pediatrics Association (APA), for its core support of the PRIS Network. Dr. Landrigan is the recipient of a career development award from the Agency for Healthcare Research and Quality (AHRQ K08 HS13333). Dr. Conway is the recipient of a Robert Wood Johnson Clinical Scholars Grant. All researchers were independent from the funding agencies; the academic medical centers named above, APA, and AHRQ had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Reduction of undesirable variation in care has been a major focus of systematic efforts to improve the quality of the healthcare system.13 The emergence of hospitalists, physicians specializing in the care of hospitalized patients, was spurred by a desire to streamline care and reduce variability in hospital management of common diseases.4, 5 Over the past decade, hospitalist systems have become a leading vehicle for care delivery.4, 6, 7 It remains unclear, however, whether implementation of hospitalist systems has lessened undesirable variation in the inpatient management of common diseases.
While systematic reviews have found costs and hospital length of stay to be 10‐15% lower in both pediatric and internal medicine hospitalist systems, few studies have adequately assessed the processes or quality of care in hospitalist systems.8, 9 Two internal medicine studies have found decreased mortality in hospitalist systems, but the mechanism by which hospitalists apparently achieved these gains is unclear.10, 11 Even less is known about care processes or quality in pediatric hospitalist systems. Death is a rare occurrence in pediatric ward settings, and the seven studies conducted to date comparing pediatric hospitalist and traditional systems have been universally underpowered to detect differences in mortality.9, 1218 There is a need to better understand care processes as a first step in understanding and improving quality of care in hospitalist systems.19
The Pediatric Research in Inpatient Settings (PRIS) Network was formed to improve the quality of care for hospitalized children through collaborative clinical research. In this study, we sought to study variation in the care of common pediatric conditions among a cohort of pediatric hospitalists. We have previously reported that less variability exists in hospitalists' reported management of inpatient conditions than in the reported management of these same conditions by community‐based pediatricians,20 but we were concerned that substantial undesirable variation (ie, variation in practice due to uncertainty or unsubstantiated local practice traditions, rather than justified variation in care based on different risks of harms or benefits in different patients) may still exist among hospitalists. We therefore conducted a study: 1) to investigate variation in hospitalists' reported use of common inpatient therapies, and 2) to test the hypothesis that greater variation exists in hospitalists' reported use of inpatient therapies of unproven benefit than in those therapies proven to be beneficial.
METHODS
Survey Design and Administration
In 2003, we designed the PRIS Survey to collect data on hospitalists' backgrounds, practices, and training needs, as well as their management of common pediatric conditions. For the current study, we chose a priori to evaluate hospitalists' use of 14 therapies in the management of 4 common conditions: asthma, bronchiolitis, gastroenteritis, and gastro‐esophageal reflux disease (GERD) (Table 1). These four conditions were chosen for study because they were among the top discharge diagnoses (primary and secondary) from the inpatient services at 2 of the authors' institutions (Children's Hospital Boston and Children's Hospital San Diego) during the year before administration of the survey, and because a discrete set of therapeutic agents are commonly used in their management. Respondents were asked to report the frequency with which they used each of the 14 therapies of interest on 5‐point Likert scales (from 1=never to 5=almost always). The survey initially developed was piloted with a small group of hospitalists and pediatricians, and a final version incorporating revisions was subsequently administered to all pediatric hospitalists in the US and Canada identified through any of 3 sources: 1) the Pediatric Research in Inpatient Settings (PRIS) list of participants; 2) the Society for Hospital Medicine (SHM) pediatric hospital medicine e‐mail listserv; and 3) the list of all attendees of the first national pediatric hospitalist conference sponsored by the Ambulatory Pediatrics Association (APA), SHM, and American Academy of Pediatrics (AAP); this meeting was held in San Antonio, Texas, USA in November 2003. Individuals identified through more than 1 of these groups were counted only once. Potential participants were assured that individual responses would be kept confidential, and were e‐mailed an access code to participate in the online survey, using a secure web‐based interface; a paper‐based version was also made available to those who preferred to respond in this manner. Regular reminder notices were sent to all non‐responders. Further details regarding PRIS Survey recruitment and study methods have been published previously.20
| Condition | Therapy | BMJ clinical evidence Treatment effect categorization* | Study classification |
|---|---|---|---|
| |||
| Asthma | Inhaled albuterol | Beneficial | Proven |
| Systemic corticosteroids | Beneficial | Proven | |
| Inhaled ipratropium in the first 24 hours of hospitalization | Beneficial | Proven | |
| Inhaled ipratropium after the first 24 hours of hospitalization | Unknown effectiveness | Unproven | |
| Bronchiolitis | Inhaled albuterol | Unknown effectiveness | Unproven |
| Inhaled epinephrine | Unknown effectiveness | Unproven | |
| Systemic corticosteroids | Unknown effectiveness | Unproven | |
| Gastroenteritis | Intravenous hydration | Beneficial | Proven |
| Lactobacillus | Not assessed | Unproven | |
| Ondansetron | Not assessed | Unproven | |
| Gastro‐Esophageal Reflux Disease (GERD) | H2 histamine‐receptor antagonists | Unknown effectiveness | Unproven |
| Thickened feeds | Unknown effectiveness Likely to be beneficial | Unproven Proven | |
| Metoclopramide | Unknown effectiveness | Unproven | |
| Proton‐pump inhibitors | Unknown effectiveness | Unproven | |
DefinitionsReference Responses and Percent Variation
To measure variation in reported management, we first sought to determine a reference response for each therapy of interest. Since the evidence base for most of the therapies we studied is weak, it was not possible to determine a gold standard response for each therapy. Instead, we sought to measure the degree of divergence from a reference response for each therapy in the following manner. First, to simplify analyses, we collapsed our five‐category Likert scale into three categories (never/rarely, sometimes, and often/almost always). We then defined the reference response for each therapy to be never/rarely or often/almost always, whichever of the 2 was more frequently selected by respondents; sometimes was not used as a reference category, as reporting use of a particular therapy sometimes indicated substantial variability even within an individual's own practice.
Classification of therapies as proven or unproven.
To classify each of the 14 studied therapies as being of proven or unproven, we used the British Medical Journal's publication Clinical Evidence.19 We chose to use Clinical Evidence as an evidence‐based reference because it provides rigorously developed, systematic analyses of therapeutic management options for multiple common pediatric conditions, and organizes recommendations in a straightforward manner. Four of the 14 therapies had been determined on systematic review to be proven beneficial at the time of study design: systemic corticosteroids, inhaled albuterol, and ipratropium (in the first 24 h) in the care of children with asthma; and IV hydration in the care of children with acute gastroenteritis. The remaining 10 therapies were either considered to be of unknown effectiveness or had not been formally evaluated by Clinical Evidence, and were hence considered unproven for this study (Table 1). Of note, the use of thickened feeds in the treatment of children with GERD had been determined to be of unknown effectiveness at the time of study design, but was reclassified as likely to be beneficial during the course of the study.
Analyses
Descriptive statistics were used to report respondents' demographic characteristics and work environments, as well as variation in their reported use of each of the 14 therapies. Variation in hospitalists' use of proven versus unproven therapies was compared using the Wilcoxon rank sum test, as it was distributed non‐normally. For our primary analysis, the use of thickened feeds in GERD was considered unproven, but a sensitivity analysis was conducted reclassifying it as proven in light of the evolving literature on its use and its consequent reclassification in Clinical Evidence.(SAS Version 9.1, Cary, NC) was used for statistical analyses.
RESULTS
213 of the 320 individuals identified through the 3 lists of pediatric hospitalists (67%) responded to the survey. Of these, 198 (93%) identified themselves as hospitalists and were therefore included. As previously reported,20 53% of respondents were male, 55% worked in academic training environments, and 47% had completed advanced training (fellowship) beyond their core pediatric training (residency training); respondents reported completing residency training 11 9 (mean, standard deviation) years prior to the survey, and spending 176 72 days per year in the care of hospitalized patients.
Variation in reported management: asthma
(Figure 1, Panel A). Relatively little variation existed in reported use of the 4 asthma therapies studied. Only 4.4% (95% CI, 1.4‐7.4%) of respondents did not provide the reference response of using inhaled albuterol often or almost always in the care of inpatients with asthma, and only 6.0% (2.5‐9.5%) of respondents did not report using systemic corticosteroids often or almost always. Variation in reported use of ipratropium was somewhat higher.

Bronchiolitis
(Figure 1B). By contrast, variation in reported use of inhaled therapies for bronchiolitis was high, with many respondents reporting that they often or always used inhaled albuterol or epinephrine, while many others reported rarely or never using them. There was 59.6% (52.4‐66.8%) variation from the reference response of often/almost always using inhaled albuterol, and 72.2% (65.6‐78.8%) variation from the reference response of never/rarely using inhaled epinephrine. Only 11.6% (6.9‐16.3%) of respondents, however, varied from the reference response of using dexamethasone more than rarely in the care of children with bronchiolitis.
Gastroenteritis
(Figure 1C). Moderate variability existed in the reported use of the 3 studied therapies for children hospitalized with gastroenteritis. 21.1% (15.1‐27.1%) of respondents did not provide the reference response of often/almost always using IV hydration; 35.9% (28.9‐42.9%) did not provide the reference response of never or rarely using lactobacillus; likewise, 35.9% (28.9‐42.9%) did not provide the reference response of never or rarely using ondansetron.
Gastro‐Esophageal Reflux Disease
(Figure 1, Panel D). There was moderate to high variability in the reported management of GERD. 22.8% (16.7‐28.9%) of respondents did not provide the reference response of often/almost always using H2 antagonists, and 44.9% (37.6‐52.2%) did not report often/almost always using thickened feeds in the care of these children. 58.3% (51.1‐65.5%) and 72.1% (65.5‐78.7%) of respondents did not provide the reference response of never/rarely using metoclopramide and proton pump inhibitors, respectively.
Proven vs. Unproven Therapies
(Figure 2). Variation in reported use of therapies of unproven benefit was significantly higher than variation in reported use of the 4 proven therapies (albuterol, corticosteroids, and ipratropium in the first 24 h for asthma; IV re‐hydration for gastroenteritis). The mean variation in reported use of unproven therapies was 44.6 20.5%, compared with 15.5 12.5% variation in reported use of therapies of proven benefit (p = 0.02).

As a sensitivity analysis, the use of thickened feeds as a therapy for GERD was re‐categorized as proven and the above analysis repeated, for the reasons outlined in the methods section. This did not alter the identified relationship between variability and the evidence base fundamentally; hospitalists' reported variation in use of therapies of unproven benefit in this sensitivity analysis was 44.6 21.7%, compared with 21.4 17.0% variation in reported use of proven therapies (p = 0.05).
DISCUSSION
Substantial variation exists in the inpatient management of common pediatric diseases. Although we have previously found less reported variability in pediatric hospitalists' practices than in those of community‐based pediatricians,20 the current study demonstrates a high degree of reported variation even among a cohort of inpatient specialists. Importantly, however, reported variation was found to be significantly less for those inpatient therapies supported by a robust evidence base.
Bronchiolitis, gastroenteritis, asthma, and GERD are extremely common causes of pediatric hospitalization throughout the developed world.2125 Our finding of high reported variability in the routine care of all of these conditions except asthma is concerning, as it suggests that experts do not agree on how to manage children hospitalized with even the most common childhood diseases. While we hypothesized that there would be some variation in the use of therapies whose benefit has not been well established, the high degree of variation observed is of concern because it indicates that an insufficient evidentiary base exists to support much of our day‐to‐day practice. Some variation in practice in response to differing clinical presentations is both expected and desirable, but it is remarkable that variance in practice was significantly less for the most evidence‐based therapies than for those grounded less firmly in science, suggesting that the variation identified here is not justifiable variation based on appropriate responses to atypical clinical presentations, but uncertainty in the absence of clear data. Such undesired variability may decrease system reliability (introducing avoidable opportunity for error),26 and lead to under‐use of needed therapies as well as overuse of unnecessary therapies.1
Our work extends prior research that has identified wide variation in patterns of hospital admission, use of hospital resources, and processes of inpatient care,2732 by documenting reported variation in the use of common inpatient therapies. Rates of hospital admission may vary by as much as 7‐fold across regions.33 Our study demonstrates that wide variation exists not only in admission rates, but in reported inpatient care processes for some of the most common diseases seen in pediatric hospitals. Our study also supports the hypothesis that variation in care may be driven by gaps in knowledge.32 Among hospitalists, we found the strength of the evidence base to be a major determinant of reported variability.
Our study has several limitations. First, the data presented here are derived from provider self‐reports, which may not fully reflect actual practice. In the case of the few proven therapies studied, reporting bias could lead to an over‐reporting of adherence to evidence‐based standards of care. Like our study, however, prior studies have found that hospital‐based providers fairly consistently comply with evidence‐based practice recommendations for acute asthma care,34, 35 supporting our finding that variation in acute asthma care (which represented 3 of our 4 proven therapies) is low in this setting.
Another limitation is that classifications of therapies as proven or unproven change as the evidence base evolves. Of particular relevance to this study, the use of thickened feeds as a therapy for GERD, originally classified as being of unknown effectiveness, was reclassified by Clinical Evidence during the course of the study as likely to be beneficial. The relationship we identified between proven therapies and degree of variability in care did not change when we conducted a sensitivity analysis re‐categorizing this therapy as proven, but precisely quantifying variation is complicated by continuous changes in the state of the evidence.
Pediatric hospitalist systems have been found consistently to improve the efficiency of care,9 yet this study suggests that considerable variation in hospitalists' management of key conditions remains. The Pediatric Research in Inpatient Settings (PRIS) Network was formed in 2002 to improve the care of hospitalized children and the quality of inpatient practice by developing an evidence base for inpatient pediatric care. Ongoing multi‐center research efforts through PRIS and other research networks are beginning to critically evaluate therapies used in the management of common pediatric conditions. Rigorous studies of the processes and outcomes of pediatric hospital care will inform inpatient pediatric practice, and ultimately improve the care of hospitalized children. The current study strongly affirms the urgent need to establish such an evidence base. Without data to inform optimal care, efforts to reduce undesirable variation in care and improve care quality cannot be fully realized.
Acknowledgements
The authors would like to extend their thanks to the hospitalists and members of the Pediatric Research in Inpatient Settings Network who participated in this research, as well as the Children's National Medical Center and Children's Hospital Boston Inpatient Pediatrics Services, who provided funding to support this study. Special thanks to the Ambulatory Pediatrics Association (APA), for its core support of the PRIS Network. Dr. Landrigan is the recipient of a career development award from the Agency for Healthcare Research and Quality (AHRQ K08 HS13333). Dr. Conway is the recipient of a Robert Wood Johnson Clinical Scholars Grant. All researchers were independent from the funding agencies; the academic medical centers named above, APA, and AHRQ had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
- Institute of Medicine: Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, D.C.:National Academic Press,2001.
- ..Reducing variation in surgical care.BMJ2005;330:1401–1402.
- ,,,.Variation in use of video assisted thoracic surgery in the United Kingdom.BMJ2004;329:1011–1012.
- ,..The emerging role of “hospitalists” in the American health care system.N. Engl J Med1996;335:514–517.
- ,..Hospitalism in the USA.Lancet1999;353:1902.
- Society of Hospital Medicine. Growth of Hospital Medicine Nationwide. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/Media/GrowthofHospitalMedicineNationwide/Growth_of_Hospital_M.htm. Accessed April 11,2007.
- .The changing face of hospital practice.Med Econ2002;79:72–79.
- ,..The hospitalist movement 5 years later.JAMA2002;287:487–494.
- ,,,.Pediatric hospitalists: a systematic review of the literature.Pediatrics2006;117:1736–1744.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med2002;137:859–865.
- ,,,,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med2002;137:866–874.
- ,.Evaluation of a pediatric hospitalist service: impact on length of stay and hospital charges.Pediatrics2000;105:478–484.
- ,,,,,,, and .Impact of an HMO hospitalist system in academic pediatrics.Pediatrics2002;110:720–728.
- ,, and .Evaluation of a pediatric hospitalist service by APR‐DRG's: impact on length of stay and hospital charges.Pediatr Research2001;49(suppl),691.
- ,,.Pediatric hospitalists: quality care for the underserved?Am J Med Qual2001;16:174–180.
- ,,,,,.Restructuring an academic pediatric inpatient service using concepts developed by hospitalists.Clin Pediatr (Phila)2001;40:653–660.
- ,,, and .Hospitalist care of medically complex children.Pediatr Research2004;55(suppl),1789.
- ,,.Hospital‐based and community pediatricians: comparing outcomes for asthma and bronchiolitis.J Clin Outcomes Manage1997;4:21–24.
- Godlee F,Tovey D,Bedford M, et al., eds.Clinical Evidence: The International Source of the Best Available Evidence for Effective Health Care.London, United Kingdom:BMJ Publishing Group;2004.
- ,,,,,.Variations in management of common inpatient pediatric illnesses: hospitalists and community pediatricians.Pediatrics2006;118:441–447.
- ,,,,.Contribution of RSV to bronchiolitis and pneumonia‐associated hospitalizations in English children, April 1995‐March 1998.Epidemiol Infect2002;129:99–106.
- ,,.Direct medical costs of bronchiolitis hospitalizations in the United States.Pediatrics2006;118:2418–2423.
- ,,,,,.Multicenter Prospective Study of the Burden of Rotavirus Acute Gastroenteritis in Europe, 2004‐2005: The REVEAL Study.J Infect Dis2007;195Suppl 1:S4–S16.
- .The state of childhood asthma, United States, 1980‐2005.Adv.Data.2006;1–24.
- ,.Gastroesophageal reflux in children: pathogenesis, prevalence, diagnosis, and role of proton pump inhibitors in treatment.Paediatr Drugs2002;4:673–685.
- ,,,.Reliability science and patient safety.Pediatr Clin North Am2006;53:1121–1133.
- Wennberg JE and McAndrew Cooper M, eds.The Dartmouth Atlas of Health Care in the United States.Hanover, NH, USA:Health Forum, Inc.,1999.
- ,,,,,.Variations in rates of hospitalization of children in three urban communities.N Engl J Med1989;320:1183–1187.
- ,,,,,.Use of hospitals, physician visits, and hospice care during last six months of life among cohorts loyal to highly respected hospitals in the United States.BMJ2004;328:607.
- ,,,,,, et al.Variations in practice and outcomes in the Canadian NICU network: 1996‐1997.Pediatrics2000;106:1070–1079.
- ,,,.Evaluation of febrile children with petechial rashes: is there consensus among pediatricians?Pediatr Infect Dis J1998;17:1135–1140.
- ,,,,,, et al.Practice variation among pediatric emergency departments in the treatment of bronchiolitis.Acad Emerg Med2004;11:353–360.
- ,,,.Paediatric inpatient utilisation in a district general hospital.Arch Dis Child1994;70:488–492.
- ,,,.Emergency department asthma: compliance with an evidence‐based management algorithm.Ann Acad Med Singapore2002;31:419–424.
- ,,.Is the practice of paediatric inpatient medicine evidence‐based?J Paediatr Child Health2002;38:347–351.
- Institute of Medicine: Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, D.C.:National Academic Press,2001.
- ..Reducing variation in surgical care.BMJ2005;330:1401–1402.
- ,,,.Variation in use of video assisted thoracic surgery in the United Kingdom.BMJ2004;329:1011–1012.
- ,..The emerging role of “hospitalists” in the American health care system.N. Engl J Med1996;335:514–517.
- ,..Hospitalism in the USA.Lancet1999;353:1902.
- Society of Hospital Medicine. Growth of Hospital Medicine Nationwide. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/Media/GrowthofHospitalMedicineNationwide/Growth_of_Hospital_M.htm. Accessed April 11,2007.
- .The changing face of hospital practice.Med Econ2002;79:72–79.
- ,..The hospitalist movement 5 years later.JAMA2002;287:487–494.
- ,,,.Pediatric hospitalists: a systematic review of the literature.Pediatrics2006;117:1736–1744.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med2002;137:859–865.
- ,,,,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med2002;137:866–874.
- ,.Evaluation of a pediatric hospitalist service: impact on length of stay and hospital charges.Pediatrics2000;105:478–484.
- ,,,,,,, and .Impact of an HMO hospitalist system in academic pediatrics.Pediatrics2002;110:720–728.
- ,, and .Evaluation of a pediatric hospitalist service by APR‐DRG's: impact on length of stay and hospital charges.Pediatr Research2001;49(suppl),691.
- ,,.Pediatric hospitalists: quality care for the underserved?Am J Med Qual2001;16:174–180.
- ,,,,,.Restructuring an academic pediatric inpatient service using concepts developed by hospitalists.Clin Pediatr (Phila)2001;40:653–660.
- ,,, and .Hospitalist care of medically complex children.Pediatr Research2004;55(suppl),1789.
- ,,.Hospital‐based and community pediatricians: comparing outcomes for asthma and bronchiolitis.J Clin Outcomes Manage1997;4:21–24.
- Godlee F,Tovey D,Bedford M, et al., eds.Clinical Evidence: The International Source of the Best Available Evidence for Effective Health Care.London, United Kingdom:BMJ Publishing Group;2004.
- ,,,,,.Variations in management of common inpatient pediatric illnesses: hospitalists and community pediatricians.Pediatrics2006;118:441–447.
- ,,,,.Contribution of RSV to bronchiolitis and pneumonia‐associated hospitalizations in English children, April 1995‐March 1998.Epidemiol Infect2002;129:99–106.
- ,,.Direct medical costs of bronchiolitis hospitalizations in the United States.Pediatrics2006;118:2418–2423.
- ,,,,,.Multicenter Prospective Study of the Burden of Rotavirus Acute Gastroenteritis in Europe, 2004‐2005: The REVEAL Study.J Infect Dis2007;195Suppl 1:S4–S16.
- .The state of childhood asthma, United States, 1980‐2005.Adv.Data.2006;1–24.
- ,.Gastroesophageal reflux in children: pathogenesis, prevalence, diagnosis, and role of proton pump inhibitors in treatment.Paediatr Drugs2002;4:673–685.
- ,,,.Reliability science and patient safety.Pediatr Clin North Am2006;53:1121–1133.
- Wennberg JE and McAndrew Cooper M, eds.The Dartmouth Atlas of Health Care in the United States.Hanover, NH, USA:Health Forum, Inc.,1999.
- ,,,,,.Variations in rates of hospitalization of children in three urban communities.N Engl J Med1989;320:1183–1187.
- ,,,,,.Use of hospitals, physician visits, and hospice care during last six months of life among cohorts loyal to highly respected hospitals in the United States.BMJ2004;328:607.
- ,,,,,, et al.Variations in practice and outcomes in the Canadian NICU network: 1996‐1997.Pediatrics2000;106:1070–1079.
- ,,,.Evaluation of febrile children with petechial rashes: is there consensus among pediatricians?Pediatr Infect Dis J1998;17:1135–1140.
- ,,,,,, et al.Practice variation among pediatric emergency departments in the treatment of bronchiolitis.Acad Emerg Med2004;11:353–360.
- ,,,.Paediatric inpatient utilisation in a district general hospital.Arch Dis Child1994;70:488–492.
- ,,,.Emergency department asthma: compliance with an evidence‐based management algorithm.Ann Acad Med Singapore2002;31:419–424.
- ,,.Is the practice of paediatric inpatient medicine evidence‐based?J Paediatr Child Health2002;38:347–351.
Copyright © 2008 Society of Hospital Medicine
Case Report
A 5 year‐old girl presented to an emergency department (ED) with a 2‐day history of more than 12 episodes of nonbilious emesis. She received intravenous (IV) promethazine and 400 cc of normal saline and was discharged home. When emesis recurred the next morning, her pediatrician referred her for admission to our hospital for further hydration. The patient had neither fever nor rash but did report periumbilical pain and a few loose stools. She did not receive any medications other than the single dose of promethazine. Parents denied other toxic ingestion. The patient's family members had had a diarrheal illness over the past days.
The patient was born at term, small for gestational age, with uncomplicated pregnancy and delivery. She had normal growth and development and previously had been healthy, although her mother reported several prior ED visits for vomiting, including emesis with upper respiratory illnesses (URIs). There was no family history of gastrointestinal, metabolic, or renal disease. The patient lived with her parents and brother and attended kindergarten.
On examination, her temperature was 98.6F, blood pressure was 103/66, heart rate was 88, and respiratory rate was 24. She weighed 17.8 kg and was 115 cm tall (35th and 75th percentiles, respectively). She was alert and cooperative. Her breath had a ketotic odor. She had somewhat sunken eyes and dry mucous membranes. Her neck was supple, without lymphadenopathy. Capillary refill time was 3 seconds. Her lungs were clear to auscultation. Her abdomen was soft, nontender to palpation, and nondistended, and bowel sounds were hyperactive. Liver and spleen were of normal size. There was no edema, clubbing, nor cyanosis in the extremities. The patient was very fair‐skinned and did not have rashes, bruises, or other skin lesions. The results of her neurologic exam were entirely normal. Initial serum chemistry results were: sodium, 140 mEq/L; potassium, 5.9 mEq/L (hemolyzed); chloride, 107 mEq/L; bicarbonate, 13 mEq/L; glucose, 52 mg/dL; BUN, 19 mg/dL; creatinine, 0.5 mg/dL; and calcium, 10.5 mg/dL. Other laboratory analyses were ordered including urinalysis (UA), serum lactate, stool culture, rotaviral test, and fecal white blood cell count.
RESULTS
Further laboratory analysis revealed a urine pH of 5.5, specific gravity of 1.023, and 3+ ketones, with an otherwise normal UA. Blood lactic acid and pyruvic acid levels were normal at 0.8 and 0.11 mmol/L, respectively. All stool studies were negative. Tests to determine plasma quantitative amino acid and urine quantitative organic acid levels were ordered. The clinical course was benign, with recovery and normalization of blood chemistry values within 24 hours of IV hydration. The patient was discharged home the next day. Results of biochemical genetics laboratory testing were available 5 days later (Table 1). Plasma amino acids showed a decreased level of alanine, with elevated levels of leucine, isoleucine, and valine. L‐alloisoleucine, which is not normally in plasma but is pathognomic for maple syrup urine disease (MSUD), was detected. Urine organic acid test results were notable for ketonuria, with elevated branched‐chain 2‐hydroxy and 2‐oxo acids consistent with a diagnosis of MSUD (Table 1).
| Measured (mol/L) | Normal Range | |
|---|---|---|
| Plasma amino acids | ||
| Alanine | 103 | 246‐486 |
| Leucine | 434 | 61‐168 |
| Valine | 576 | 110‐279 |
| Isoleucine | 280 | 39‐88 |
| L‐Alloisoleucine | 28 | 0 |
| Measured (mmol/mol creatinine) | Normal Range | |
| Urine organic acids | ||
| 3‐Hydroxybutyric | 13,000 | 0‐10 |
| 2‐Hydroxyisovaleric | 16 | 0‐6 |
| 2‐Hydroxyisocaproic | 0 | 0‐2 |
| 2‐Hydroxy‐3‐methylvaleric | 0 | 0‐2 |
| 2‐Oxoisovaleric | 0 | 0‐2 |
| 2‐Oxoisocaproic | 0 | 0‐2 |
| 2‐Oxo‐3‐methylvaleric | 41 | 0‐2 |
The patient has done very well on follow‐up. Modest restriction of protein intake was instituted (2 g/kg daily), and approximately 1 month after hospitalization a trial of thiamine was started. Plasma amino acid and urine organic acids have been normal on subsequent testing while the patient has been clinically well. Whole‐body leucine oxidation was estimated by quantitation of 13CO2 after administration of an oral bolus of 1‐13C‐leucine, following the protocol of Elsas et al.1 The enrichment of 13CO2 indicated oxidation of approximately 11% of the administered leucine over 3 hours, which was at the lower end of the normal range, with no increase after thiamine supplementation for 1 month. The patient did have 2 episodes of vomiting associated with mild intercurrent illness 1 month and 1 year after hospitalization. Care was sought promptly at urgent care centers. Ketonuria was documented; however, no blood tests were ordered. There were no changes in mental status, and oral rehydration was successful. Molecular analysis of the branched chain ketoacid dehydrogenase complex E1, E1, and E2 subunit genes did not reveal any mutations in the coding regions, although 3 sequence variations were observed in E1 (2 silent changes, c.972C>T [Phe324Phe] and c.1221A>G [Leu407Leu], and c.376G>T [Gly126Cys], at this time of unclear significance).
DISCUSSION
We report the case of a young girl who presented with what was initially labeled simple gastroenteritis. It is important to note, however, that she had several days of repeated emesis, no fever, and minimal diarrhea, along with multiple previous episodes of vomiting illnesses requiring ED visits. Combined, these prompted further evaluation of her acidosis. Maple syrup urine disease, a congenital condition that can be lethal, went undiagnosed in this patient until this admission when she was 5 years old.
Metabolic Acidosis
Metabolic acidosis is a very common laboratory abnormality caused by 1 of 3 basic mechanisms: loss of bicarbonate, impaired renal acid excretion, or the addition of either endogenous or exogenous acids to the body. Common causes of nonanion gap metabolic acidosis in children include diarrhea and renal tubular acidosis (RTA). Increased anion gap is associated with lactic acidosis, ketoacidosis, and ingestion of such substances as methanol, ethylene glycol, acetylsalicylic acid, and bismuth subsalicylate. Inborn errors of metabolism cause production of ketoacids, lactic acid, and other organic anions. This can occur chronically or during acute decompensation with illness, stress, or therapy noncompliance.2 Serum anion gap, glucose, ketones, lactate, and ammonia can help to elucidate the specific etiology of metabolic acidosis (Fig. 1).
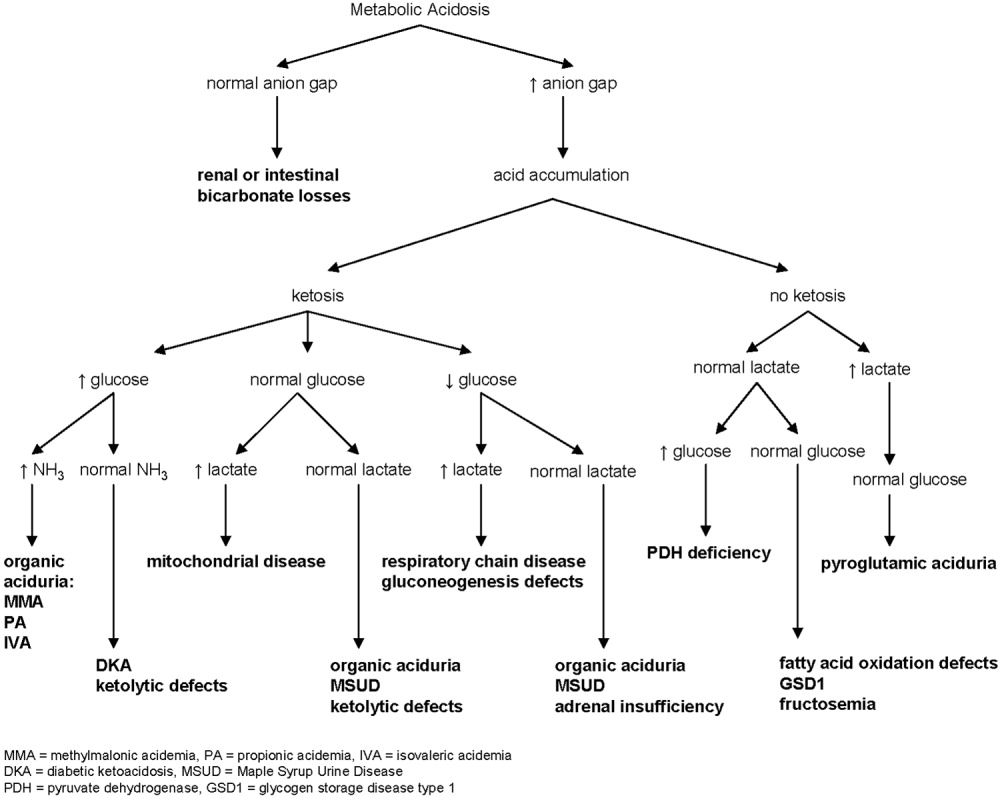
In our patient whose anion gap was at the upper limit of normal, both increased gap and non‐anion gap were considered as causes of the metabolic acidosis. Diarrhea was minimal, and there was no history of toxin or medication ingestion other than promethazine. RTA was unlikely given the borderline high serum anion gap and normal UA. Lactate level was normal, and examination did not find evidence of profound dehydration, both refuting that she had lactic acidosis severe enough to account for a serum bicarbonate of 13 mEq/L. Inborn errors of metabolism were therefore strongly considered.
Maple Syrup Urine Disease
Background
MSUD, or branched‐chain ketoaciduria, is a disease resulting from defects in the catabolic pathway of the branched‐chain amino acids (BCAAs) isoleucine, leucine, and valine. The deficient enzyme is the branched‐chain alpha‐ketoacid dehydrogenase complex (BCKDC), an enzyme system responsible for oxidative decarboxylation of the 2‐oxoacid transamination products of isoleucine, leucine, and valine. BCKDC is made up of 4 subunits (E1, E1, E2, and E3); its coenzymes include thiamine (vitamin B1) and lipoic acid. Deficiency of BCKDC leads to accumulation of BCAAs and the related branched chain oxoacids and organic acid intermediates, including one (sotalone) that lends a sweet odor reminiscent of maple syrup to sweat, cerumen, and urine. MSUD is autosomal recessive, with more than 100 specific mutations identified in the 4 genes encoding BCKDC.3 MSUD is a rare disease, occurring in 1 of every 180,000 newborns in the United States.2
Clinical Phenotypes
MSUD has been divided into at least 5 clinical phenotypes,4 although in several cases distinctions are not clear. Differences result from variation in the severity of enzyme deficiency. Classic MSUD is the most severe form, with less than 2% of normal BCKDC function; it presents in the first week of life with poor feeding and neurologic signs such as hypo/hypertonia, seizures, lethargy, and coma.5 General laboratory findings are nonspecific except for ketoacidosis. This form is rapidly fatal in the first months of life if not treated. Intermediate MSUD is milder, having 3%‐30% of BCKDC activity. Patients manifest variable degrees of retardation, developmental delay, and failure to thrive, often without signs of ketoacidosis. Thiamine‐responsive MSUD is distinguished by the favorable response to high‐dose thiamine supplementation with significant reduction in BCAA level.6 Although it is reasonable to try treatment with thiamine in most cases of MSUD, responsive patients are very rare. MSUD due to a deficiency of the E3 subunit is the rarest form, described in fewer than 10 patients.7 Intermittent MSUD is the least severe form, with 5%‐20% BCKDC activity. Children develop with normal growth and intelligence but are at risk of acute metabolic decompensation during catabolic states such as stress, infection, or surgery. Recurrent episodes of ketoacidosis, ataxia, and lethargy can lead to coma and death if untreated.8 Initial symptoms usually occur by 2 years of age but have appeared as late as the fifth decade of life. Between episodes, a normal diet is tolerated without elevation of BCAA level.
Long‐term morbidity and mortality in MSUD is neurologic. Death is often a result of brain edema that is generally attributed to the osmotic effects of leucine and amino acid imbalance; however, pathophysiologic mechanisms remain unclear.9 Progressive white matter changes are thought to result from chronic exposure to leucine. Levels of some amino acids and neurotransmitters are reduced in MSUD, which may play a role in causing encephalopathies and coma.10
Diagnosis
Elevation of plasma BCAA level can be directly assessed by standard plasma amino acid analysis; reduction of alanine is also characteristic. L‐alloisoleucine is the most sensitive and specific marker of MSUD and is pathognomic for MSUD.1112 In most cases of intermittent MSUD it is detectable at all times, including when BCAA level is normal, but in some cases it may be absent between episodes. Urine organic acids show elevation of the 2‐oxoacids corresponding to leucine, valine, and isoleucine and the corresponding 2‐hydroxy‐acids. Modern newborn screening programs generally ascertain MSUD by liquid chromatographytandem mass spectrometry, which detects elevated levels of leucine, isoleucine, and L‐alloisoleucine or the ratio of these to alanine. BCAA concentration is elevated in plasma within hours of birth; however, it is unclear how often intermittent MSUD might be missed in newborn screening.
Treatment
Therapy consists of dietary control (limited protein intake) and, in some cases, thiamine supplementation. The goal is to limit BCAA so as not to overwhelm the capacity of the BCKDC. However, BCAAs are essential, and the challenge is therefore to provide appropriate amounts of protein to sustain growth without exceeding the individual's metabolic capacity. Generally, the amount of dietary protein tolerated is insufficient to provide enough of the other essential amino acids, so supplemental amino acids are needed. During acute episodes a BCAA‐free diet, sometimes with insulin and glucose, is used to encourage BCAA removal. If this anabolic approach is unsuccessful, dialysis may be used. Orthotopic liver transplantation has also been performed.1315 In all cases so far, BCAA level normalized after transplantation, and metabolic control was sustainable on a regular diet without protein restriction.13, 1618
Lessons for the Physician
Vomiting without true diarrhea deserves careful evaluation. Inborn errors of metabolism are individually rare but as a group are fairly common. One study noted an incidence of 15.7 per 100,000 births detected on tandem mass spectrometry screening, whereas rates of clinical detection are lower.19 This case illustrates how symptoms may be nonspecific and can easily mimic simple gastroenteritis. The following may be helpful when evaluating a patient with isolated emesis:
-
History: Does the patient have a history of emesis with illnesses that usually do not cause vomiting, such as an asthma flare or URI? Has the patient required emergency treatment or IV fluids for bouts of emesis in the past? Could toxins or medications that cause metabolic acidosis have been ingested?
-
Physical exam: Skin lesions, odors, and hepatomegaly are sometimes found in metabolic disorders. Commonly recognized odors include the musty smell of phenylketonuria and the sweet maple syrup smell of MSUD.
-
Laboratory studies: UA and calculation of anion gap are first steps to take to eliminate more common causes of metabolic acidosis in children.
-
Reliance on newborn screening: Patients with intermittent MSUD may have normal BCAA levels between acute episodes. Newborn screening tests may not identify these patients. The physician must maintain a degree of suspicion in approaching an acute illness that might indicate a metabolic disease, even in a child who has had negative expanded newborn screening.
Unusual disorders may masquerade as a simple problem. Common laboratory tests and a thorough history and exam can help to differentiate between simple gastroenteritis and inborn metabolic error and guide further diagnostic evaluation.
- ,,.Practical methods to estimate whole body leucine oxidation in maple syrup urine disease.Pediatr Res.1993;33:445–451.
- Behrman RE,Kliegman RM,Jenson HB, editors.Nelson Textbook of Pediatrics.Philadelphia:Saunders;2004:224–235,409–418.
- ,,.Lessons from genetic disorders of branched‐chain amino acid metabolism.J Nutr.2006;136(suppl 1):243S–249S.
- ,.Maple syrup urine disease (branched‐chain ketoaciduria). In:Scriver CR,Beaudet AL,Sly WS,Valle D,Vogelstein B,Childs B, editors.The Metabolic and Molecular Basis of Inherited Disease.New York:McGraw‐Hill;2001:1971–2006.
- ,,.A new syndrome: progressive familial infantile cerebral dysfunction associated with an unusual urinary substance.Pediatrics.1954;14:462–467.
- ,,,.Thiamine‐responsive maple‐syrup‐urine disease.Lancet.1971;1:310–312.
- ,,.Deficiency of dihydrolipoyl dehydrogenase (a component of the pyruvate and ‐ketoglutarate dehydrogenase complexes): a cause of congenital chronic lactic acidosis in infancy.Pediatr Res.1977;11:1198–202.
- ,,.Late‐onset branched‐chain ketoaciduria: (maple syrup urine disease).J Lancet.1966;86(3):149–152.
- ,,,.Cerebral edema causing death in children with maple syrup urine disease.J Pediatr.1991;119:42–45.
- ,,,,,,.Glutamate and γ‐aminobutyric acid neurotransmitter systems in the acute phase of maple syrup urine disease and citrullinemia encephalopathies in newborn calves.J Neurochem.1992;59:582–590.
- ,,,Maple syrup urine disease, with particular reference to dietotherapy.Pediatrics.1964;34:454–472.
- ,,,.Significance of L‐alloisoleucine in plasma for diagnosis of maple syrup urine disease.Clin Chem.1999;45:1734–40.
- ,,, et al.Mid‐term outcome of 2 cases with maple syrup urine disease: role of liver transplantation in the treatment.Arch Pediatr.1994;1:730–734.
- , et al.Transplantation for maple syrup urine disease (MSUD) and methylmalonic acidopathy (MMA).J Inherit Metab Dis.1997;20(suppl 1):37.
- ,,,.Liver transplantation in maple syrup urine disease.Eur J Pediatrics.1999;158(suppl 2):S60–S64.
- ,,, et al.Elective liver transplantation for the treatment of classical maple syrup urine disease.Am J Transplant.2006;6:557–564.
- ,,,,,.Domino liver transplantation in maple syrup urine disease.Liver Transpl.2006;12:876–882.
- ,,,.Branched‐chain L‐amino acid metabolism in classical maple syrup urine disease after orthotopic liver transplantation.J Inherit Metab Dis.2000;23:805–818.
- ,,,.Screening newborns for inborn errors of metabolism by tandem mass spectrometry.N Engl J Med.2003;348:2304–2312.
A 5 year‐old girl presented to an emergency department (ED) with a 2‐day history of more than 12 episodes of nonbilious emesis. She received intravenous (IV) promethazine and 400 cc of normal saline and was discharged home. When emesis recurred the next morning, her pediatrician referred her for admission to our hospital for further hydration. The patient had neither fever nor rash but did report periumbilical pain and a few loose stools. She did not receive any medications other than the single dose of promethazine. Parents denied other toxic ingestion. The patient's family members had had a diarrheal illness over the past days.
The patient was born at term, small for gestational age, with uncomplicated pregnancy and delivery. She had normal growth and development and previously had been healthy, although her mother reported several prior ED visits for vomiting, including emesis with upper respiratory illnesses (URIs). There was no family history of gastrointestinal, metabolic, or renal disease. The patient lived with her parents and brother and attended kindergarten.
On examination, her temperature was 98.6F, blood pressure was 103/66, heart rate was 88, and respiratory rate was 24. She weighed 17.8 kg and was 115 cm tall (35th and 75th percentiles, respectively). She was alert and cooperative. Her breath had a ketotic odor. She had somewhat sunken eyes and dry mucous membranes. Her neck was supple, without lymphadenopathy. Capillary refill time was 3 seconds. Her lungs were clear to auscultation. Her abdomen was soft, nontender to palpation, and nondistended, and bowel sounds were hyperactive. Liver and spleen were of normal size. There was no edema, clubbing, nor cyanosis in the extremities. The patient was very fair‐skinned and did not have rashes, bruises, or other skin lesions. The results of her neurologic exam were entirely normal. Initial serum chemistry results were: sodium, 140 mEq/L; potassium, 5.9 mEq/L (hemolyzed); chloride, 107 mEq/L; bicarbonate, 13 mEq/L; glucose, 52 mg/dL; BUN, 19 mg/dL; creatinine, 0.5 mg/dL; and calcium, 10.5 mg/dL. Other laboratory analyses were ordered including urinalysis (UA), serum lactate, stool culture, rotaviral test, and fecal white blood cell count.
RESULTS
Further laboratory analysis revealed a urine pH of 5.5, specific gravity of 1.023, and 3+ ketones, with an otherwise normal UA. Blood lactic acid and pyruvic acid levels were normal at 0.8 and 0.11 mmol/L, respectively. All stool studies were negative. Tests to determine plasma quantitative amino acid and urine quantitative organic acid levels were ordered. The clinical course was benign, with recovery and normalization of blood chemistry values within 24 hours of IV hydration. The patient was discharged home the next day. Results of biochemical genetics laboratory testing were available 5 days later (Table 1). Plasma amino acids showed a decreased level of alanine, with elevated levels of leucine, isoleucine, and valine. L‐alloisoleucine, which is not normally in plasma but is pathognomic for maple syrup urine disease (MSUD), was detected. Urine organic acid test results were notable for ketonuria, with elevated branched‐chain 2‐hydroxy and 2‐oxo acids consistent with a diagnosis of MSUD (Table 1).
| Measured (mol/L) | Normal Range | |
|---|---|---|
| Plasma amino acids | ||
| Alanine | 103 | 246‐486 |
| Leucine | 434 | 61‐168 |
| Valine | 576 | 110‐279 |
| Isoleucine | 280 | 39‐88 |
| L‐Alloisoleucine | 28 | 0 |
| Measured (mmol/mol creatinine) | Normal Range | |
| Urine organic acids | ||
| 3‐Hydroxybutyric | 13,000 | 0‐10 |
| 2‐Hydroxyisovaleric | 16 | 0‐6 |
| 2‐Hydroxyisocaproic | 0 | 0‐2 |
| 2‐Hydroxy‐3‐methylvaleric | 0 | 0‐2 |
| 2‐Oxoisovaleric | 0 | 0‐2 |
| 2‐Oxoisocaproic | 0 | 0‐2 |
| 2‐Oxo‐3‐methylvaleric | 41 | 0‐2 |
The patient has done very well on follow‐up. Modest restriction of protein intake was instituted (2 g/kg daily), and approximately 1 month after hospitalization a trial of thiamine was started. Plasma amino acid and urine organic acids have been normal on subsequent testing while the patient has been clinically well. Whole‐body leucine oxidation was estimated by quantitation of 13CO2 after administration of an oral bolus of 1‐13C‐leucine, following the protocol of Elsas et al.1 The enrichment of 13CO2 indicated oxidation of approximately 11% of the administered leucine over 3 hours, which was at the lower end of the normal range, with no increase after thiamine supplementation for 1 month. The patient did have 2 episodes of vomiting associated with mild intercurrent illness 1 month and 1 year after hospitalization. Care was sought promptly at urgent care centers. Ketonuria was documented; however, no blood tests were ordered. There were no changes in mental status, and oral rehydration was successful. Molecular analysis of the branched chain ketoacid dehydrogenase complex E1, E1, and E2 subunit genes did not reveal any mutations in the coding regions, although 3 sequence variations were observed in E1 (2 silent changes, c.972C>T [Phe324Phe] and c.1221A>G [Leu407Leu], and c.376G>T [Gly126Cys], at this time of unclear significance).
DISCUSSION
We report the case of a young girl who presented with what was initially labeled simple gastroenteritis. It is important to note, however, that she had several days of repeated emesis, no fever, and minimal diarrhea, along with multiple previous episodes of vomiting illnesses requiring ED visits. Combined, these prompted further evaluation of her acidosis. Maple syrup urine disease, a congenital condition that can be lethal, went undiagnosed in this patient until this admission when she was 5 years old.
Metabolic Acidosis
Metabolic acidosis is a very common laboratory abnormality caused by 1 of 3 basic mechanisms: loss of bicarbonate, impaired renal acid excretion, or the addition of either endogenous or exogenous acids to the body. Common causes of nonanion gap metabolic acidosis in children include diarrhea and renal tubular acidosis (RTA). Increased anion gap is associated with lactic acidosis, ketoacidosis, and ingestion of such substances as methanol, ethylene glycol, acetylsalicylic acid, and bismuth subsalicylate. Inborn errors of metabolism cause production of ketoacids, lactic acid, and other organic anions. This can occur chronically or during acute decompensation with illness, stress, or therapy noncompliance.2 Serum anion gap, glucose, ketones, lactate, and ammonia can help to elucidate the specific etiology of metabolic acidosis (Fig. 1).

In our patient whose anion gap was at the upper limit of normal, both increased gap and non‐anion gap were considered as causes of the metabolic acidosis. Diarrhea was minimal, and there was no history of toxin or medication ingestion other than promethazine. RTA was unlikely given the borderline high serum anion gap and normal UA. Lactate level was normal, and examination did not find evidence of profound dehydration, both refuting that she had lactic acidosis severe enough to account for a serum bicarbonate of 13 mEq/L. Inborn errors of metabolism were therefore strongly considered.
Maple Syrup Urine Disease
Background
MSUD, or branched‐chain ketoaciduria, is a disease resulting from defects in the catabolic pathway of the branched‐chain amino acids (BCAAs) isoleucine, leucine, and valine. The deficient enzyme is the branched‐chain alpha‐ketoacid dehydrogenase complex (BCKDC), an enzyme system responsible for oxidative decarboxylation of the 2‐oxoacid transamination products of isoleucine, leucine, and valine. BCKDC is made up of 4 subunits (E1, E1, E2, and E3); its coenzymes include thiamine (vitamin B1) and lipoic acid. Deficiency of BCKDC leads to accumulation of BCAAs and the related branched chain oxoacids and organic acid intermediates, including one (sotalone) that lends a sweet odor reminiscent of maple syrup to sweat, cerumen, and urine. MSUD is autosomal recessive, with more than 100 specific mutations identified in the 4 genes encoding BCKDC.3 MSUD is a rare disease, occurring in 1 of every 180,000 newborns in the United States.2
Clinical Phenotypes
MSUD has been divided into at least 5 clinical phenotypes,4 although in several cases distinctions are not clear. Differences result from variation in the severity of enzyme deficiency. Classic MSUD is the most severe form, with less than 2% of normal BCKDC function; it presents in the first week of life with poor feeding and neurologic signs such as hypo/hypertonia, seizures, lethargy, and coma.5 General laboratory findings are nonspecific except for ketoacidosis. This form is rapidly fatal in the first months of life if not treated. Intermediate MSUD is milder, having 3%‐30% of BCKDC activity. Patients manifest variable degrees of retardation, developmental delay, and failure to thrive, often without signs of ketoacidosis. Thiamine‐responsive MSUD is distinguished by the favorable response to high‐dose thiamine supplementation with significant reduction in BCAA level.6 Although it is reasonable to try treatment with thiamine in most cases of MSUD, responsive patients are very rare. MSUD due to a deficiency of the E3 subunit is the rarest form, described in fewer than 10 patients.7 Intermittent MSUD is the least severe form, with 5%‐20% BCKDC activity. Children develop with normal growth and intelligence but are at risk of acute metabolic decompensation during catabolic states such as stress, infection, or surgery. Recurrent episodes of ketoacidosis, ataxia, and lethargy can lead to coma and death if untreated.8 Initial symptoms usually occur by 2 years of age but have appeared as late as the fifth decade of life. Between episodes, a normal diet is tolerated without elevation of BCAA level.
Long‐term morbidity and mortality in MSUD is neurologic. Death is often a result of brain edema that is generally attributed to the osmotic effects of leucine and amino acid imbalance; however, pathophysiologic mechanisms remain unclear.9 Progressive white matter changes are thought to result from chronic exposure to leucine. Levels of some amino acids and neurotransmitters are reduced in MSUD, which may play a role in causing encephalopathies and coma.10
Diagnosis
Elevation of plasma BCAA level can be directly assessed by standard plasma amino acid analysis; reduction of alanine is also characteristic. L‐alloisoleucine is the most sensitive and specific marker of MSUD and is pathognomic for MSUD.1112 In most cases of intermittent MSUD it is detectable at all times, including when BCAA level is normal, but in some cases it may be absent between episodes. Urine organic acids show elevation of the 2‐oxoacids corresponding to leucine, valine, and isoleucine and the corresponding 2‐hydroxy‐acids. Modern newborn screening programs generally ascertain MSUD by liquid chromatographytandem mass spectrometry, which detects elevated levels of leucine, isoleucine, and L‐alloisoleucine or the ratio of these to alanine. BCAA concentration is elevated in plasma within hours of birth; however, it is unclear how often intermittent MSUD might be missed in newborn screening.
Treatment
Therapy consists of dietary control (limited protein intake) and, in some cases, thiamine supplementation. The goal is to limit BCAA so as not to overwhelm the capacity of the BCKDC. However, BCAAs are essential, and the challenge is therefore to provide appropriate amounts of protein to sustain growth without exceeding the individual's metabolic capacity. Generally, the amount of dietary protein tolerated is insufficient to provide enough of the other essential amino acids, so supplemental amino acids are needed. During acute episodes a BCAA‐free diet, sometimes with insulin and glucose, is used to encourage BCAA removal. If this anabolic approach is unsuccessful, dialysis may be used. Orthotopic liver transplantation has also been performed.1315 In all cases so far, BCAA level normalized after transplantation, and metabolic control was sustainable on a regular diet without protein restriction.13, 1618
Lessons for the Physician
Vomiting without true diarrhea deserves careful evaluation. Inborn errors of metabolism are individually rare but as a group are fairly common. One study noted an incidence of 15.7 per 100,000 births detected on tandem mass spectrometry screening, whereas rates of clinical detection are lower.19 This case illustrates how symptoms may be nonspecific and can easily mimic simple gastroenteritis. The following may be helpful when evaluating a patient with isolated emesis:
-
History: Does the patient have a history of emesis with illnesses that usually do not cause vomiting, such as an asthma flare or URI? Has the patient required emergency treatment or IV fluids for bouts of emesis in the past? Could toxins or medications that cause metabolic acidosis have been ingested?
-
Physical exam: Skin lesions, odors, and hepatomegaly are sometimes found in metabolic disorders. Commonly recognized odors include the musty smell of phenylketonuria and the sweet maple syrup smell of MSUD.
-
Laboratory studies: UA and calculation of anion gap are first steps to take to eliminate more common causes of metabolic acidosis in children.
-
Reliance on newborn screening: Patients with intermittent MSUD may have normal BCAA levels between acute episodes. Newborn screening tests may not identify these patients. The physician must maintain a degree of suspicion in approaching an acute illness that might indicate a metabolic disease, even in a child who has had negative expanded newborn screening.
Unusual disorders may masquerade as a simple problem. Common laboratory tests and a thorough history and exam can help to differentiate between simple gastroenteritis and inborn metabolic error and guide further diagnostic evaluation.
A 5 year‐old girl presented to an emergency department (ED) with a 2‐day history of more than 12 episodes of nonbilious emesis. She received intravenous (IV) promethazine and 400 cc of normal saline and was discharged home. When emesis recurred the next morning, her pediatrician referred her for admission to our hospital for further hydration. The patient had neither fever nor rash but did report periumbilical pain and a few loose stools. She did not receive any medications other than the single dose of promethazine. Parents denied other toxic ingestion. The patient's family members had had a diarrheal illness over the past days.
The patient was born at term, small for gestational age, with uncomplicated pregnancy and delivery. She had normal growth and development and previously had been healthy, although her mother reported several prior ED visits for vomiting, including emesis with upper respiratory illnesses (URIs). There was no family history of gastrointestinal, metabolic, or renal disease. The patient lived with her parents and brother and attended kindergarten.
On examination, her temperature was 98.6F, blood pressure was 103/66, heart rate was 88, and respiratory rate was 24. She weighed 17.8 kg and was 115 cm tall (35th and 75th percentiles, respectively). She was alert and cooperative. Her breath had a ketotic odor. She had somewhat sunken eyes and dry mucous membranes. Her neck was supple, without lymphadenopathy. Capillary refill time was 3 seconds. Her lungs were clear to auscultation. Her abdomen was soft, nontender to palpation, and nondistended, and bowel sounds were hyperactive. Liver and spleen were of normal size. There was no edema, clubbing, nor cyanosis in the extremities. The patient was very fair‐skinned and did not have rashes, bruises, or other skin lesions. The results of her neurologic exam were entirely normal. Initial serum chemistry results were: sodium, 140 mEq/L; potassium, 5.9 mEq/L (hemolyzed); chloride, 107 mEq/L; bicarbonate, 13 mEq/L; glucose, 52 mg/dL; BUN, 19 mg/dL; creatinine, 0.5 mg/dL; and calcium, 10.5 mg/dL. Other laboratory analyses were ordered including urinalysis (UA), serum lactate, stool culture, rotaviral test, and fecal white blood cell count.
RESULTS
Further laboratory analysis revealed a urine pH of 5.5, specific gravity of 1.023, and 3+ ketones, with an otherwise normal UA. Blood lactic acid and pyruvic acid levels were normal at 0.8 and 0.11 mmol/L, respectively. All stool studies were negative. Tests to determine plasma quantitative amino acid and urine quantitative organic acid levels were ordered. The clinical course was benign, with recovery and normalization of blood chemistry values within 24 hours of IV hydration. The patient was discharged home the next day. Results of biochemical genetics laboratory testing were available 5 days later (Table 1). Plasma amino acids showed a decreased level of alanine, with elevated levels of leucine, isoleucine, and valine. L‐alloisoleucine, which is not normally in plasma but is pathognomic for maple syrup urine disease (MSUD), was detected. Urine organic acid test results were notable for ketonuria, with elevated branched‐chain 2‐hydroxy and 2‐oxo acids consistent with a diagnosis of MSUD (Table 1).
| Measured (mol/L) | Normal Range | |
|---|---|---|
| Plasma amino acids | ||
| Alanine | 103 | 246‐486 |
| Leucine | 434 | 61‐168 |
| Valine | 576 | 110‐279 |
| Isoleucine | 280 | 39‐88 |
| L‐Alloisoleucine | 28 | 0 |
| Measured (mmol/mol creatinine) | Normal Range | |
| Urine organic acids | ||
| 3‐Hydroxybutyric | 13,000 | 0‐10 |
| 2‐Hydroxyisovaleric | 16 | 0‐6 |
| 2‐Hydroxyisocaproic | 0 | 0‐2 |
| 2‐Hydroxy‐3‐methylvaleric | 0 | 0‐2 |
| 2‐Oxoisovaleric | 0 | 0‐2 |
| 2‐Oxoisocaproic | 0 | 0‐2 |
| 2‐Oxo‐3‐methylvaleric | 41 | 0‐2 |
The patient has done very well on follow‐up. Modest restriction of protein intake was instituted (2 g/kg daily), and approximately 1 month after hospitalization a trial of thiamine was started. Plasma amino acid and urine organic acids have been normal on subsequent testing while the patient has been clinically well. Whole‐body leucine oxidation was estimated by quantitation of 13CO2 after administration of an oral bolus of 1‐13C‐leucine, following the protocol of Elsas et al.1 The enrichment of 13CO2 indicated oxidation of approximately 11% of the administered leucine over 3 hours, which was at the lower end of the normal range, with no increase after thiamine supplementation for 1 month. The patient did have 2 episodes of vomiting associated with mild intercurrent illness 1 month and 1 year after hospitalization. Care was sought promptly at urgent care centers. Ketonuria was documented; however, no blood tests were ordered. There were no changes in mental status, and oral rehydration was successful. Molecular analysis of the branched chain ketoacid dehydrogenase complex E1, E1, and E2 subunit genes did not reveal any mutations in the coding regions, although 3 sequence variations were observed in E1 (2 silent changes, c.972C>T [Phe324Phe] and c.1221A>G [Leu407Leu], and c.376G>T [Gly126Cys], at this time of unclear significance).
DISCUSSION
We report the case of a young girl who presented with what was initially labeled simple gastroenteritis. It is important to note, however, that she had several days of repeated emesis, no fever, and minimal diarrhea, along with multiple previous episodes of vomiting illnesses requiring ED visits. Combined, these prompted further evaluation of her acidosis. Maple syrup urine disease, a congenital condition that can be lethal, went undiagnosed in this patient until this admission when she was 5 years old.
Metabolic Acidosis
Metabolic acidosis is a very common laboratory abnormality caused by 1 of 3 basic mechanisms: loss of bicarbonate, impaired renal acid excretion, or the addition of either endogenous or exogenous acids to the body. Common causes of nonanion gap metabolic acidosis in children include diarrhea and renal tubular acidosis (RTA). Increased anion gap is associated with lactic acidosis, ketoacidosis, and ingestion of such substances as methanol, ethylene glycol, acetylsalicylic acid, and bismuth subsalicylate. Inborn errors of metabolism cause production of ketoacids, lactic acid, and other organic anions. This can occur chronically or during acute decompensation with illness, stress, or therapy noncompliance.2 Serum anion gap, glucose, ketones, lactate, and ammonia can help to elucidate the specific etiology of metabolic acidosis (Fig. 1).

In our patient whose anion gap was at the upper limit of normal, both increased gap and non‐anion gap were considered as causes of the metabolic acidosis. Diarrhea was minimal, and there was no history of toxin or medication ingestion other than promethazine. RTA was unlikely given the borderline high serum anion gap and normal UA. Lactate level was normal, and examination did not find evidence of profound dehydration, both refuting that she had lactic acidosis severe enough to account for a serum bicarbonate of 13 mEq/L. Inborn errors of metabolism were therefore strongly considered.
Maple Syrup Urine Disease
Background
MSUD, or branched‐chain ketoaciduria, is a disease resulting from defects in the catabolic pathway of the branched‐chain amino acids (BCAAs) isoleucine, leucine, and valine. The deficient enzyme is the branched‐chain alpha‐ketoacid dehydrogenase complex (BCKDC), an enzyme system responsible for oxidative decarboxylation of the 2‐oxoacid transamination products of isoleucine, leucine, and valine. BCKDC is made up of 4 subunits (E1, E1, E2, and E3); its coenzymes include thiamine (vitamin B1) and lipoic acid. Deficiency of BCKDC leads to accumulation of BCAAs and the related branched chain oxoacids and organic acid intermediates, including one (sotalone) that lends a sweet odor reminiscent of maple syrup to sweat, cerumen, and urine. MSUD is autosomal recessive, with more than 100 specific mutations identified in the 4 genes encoding BCKDC.3 MSUD is a rare disease, occurring in 1 of every 180,000 newborns in the United States.2
Clinical Phenotypes
MSUD has been divided into at least 5 clinical phenotypes,4 although in several cases distinctions are not clear. Differences result from variation in the severity of enzyme deficiency. Classic MSUD is the most severe form, with less than 2% of normal BCKDC function; it presents in the first week of life with poor feeding and neurologic signs such as hypo/hypertonia, seizures, lethargy, and coma.5 General laboratory findings are nonspecific except for ketoacidosis. This form is rapidly fatal in the first months of life if not treated. Intermediate MSUD is milder, having 3%‐30% of BCKDC activity. Patients manifest variable degrees of retardation, developmental delay, and failure to thrive, often without signs of ketoacidosis. Thiamine‐responsive MSUD is distinguished by the favorable response to high‐dose thiamine supplementation with significant reduction in BCAA level.6 Although it is reasonable to try treatment with thiamine in most cases of MSUD, responsive patients are very rare. MSUD due to a deficiency of the E3 subunit is the rarest form, described in fewer than 10 patients.7 Intermittent MSUD is the least severe form, with 5%‐20% BCKDC activity. Children develop with normal growth and intelligence but are at risk of acute metabolic decompensation during catabolic states such as stress, infection, or surgery. Recurrent episodes of ketoacidosis, ataxia, and lethargy can lead to coma and death if untreated.8 Initial symptoms usually occur by 2 years of age but have appeared as late as the fifth decade of life. Between episodes, a normal diet is tolerated without elevation of BCAA level.
Long‐term morbidity and mortality in MSUD is neurologic. Death is often a result of brain edema that is generally attributed to the osmotic effects of leucine and amino acid imbalance; however, pathophysiologic mechanisms remain unclear.9 Progressive white matter changes are thought to result from chronic exposure to leucine. Levels of some amino acids and neurotransmitters are reduced in MSUD, which may play a role in causing encephalopathies and coma.10
Diagnosis
Elevation of plasma BCAA level can be directly assessed by standard plasma amino acid analysis; reduction of alanine is also characteristic. L‐alloisoleucine is the most sensitive and specific marker of MSUD and is pathognomic for MSUD.1112 In most cases of intermittent MSUD it is detectable at all times, including when BCAA level is normal, but in some cases it may be absent between episodes. Urine organic acids show elevation of the 2‐oxoacids corresponding to leucine, valine, and isoleucine and the corresponding 2‐hydroxy‐acids. Modern newborn screening programs generally ascertain MSUD by liquid chromatographytandem mass spectrometry, which detects elevated levels of leucine, isoleucine, and L‐alloisoleucine or the ratio of these to alanine. BCAA concentration is elevated in plasma within hours of birth; however, it is unclear how often intermittent MSUD might be missed in newborn screening.
Treatment
Therapy consists of dietary control (limited protein intake) and, in some cases, thiamine supplementation. The goal is to limit BCAA so as not to overwhelm the capacity of the BCKDC. However, BCAAs are essential, and the challenge is therefore to provide appropriate amounts of protein to sustain growth without exceeding the individual's metabolic capacity. Generally, the amount of dietary protein tolerated is insufficient to provide enough of the other essential amino acids, so supplemental amino acids are needed. During acute episodes a BCAA‐free diet, sometimes with insulin and glucose, is used to encourage BCAA removal. If this anabolic approach is unsuccessful, dialysis may be used. Orthotopic liver transplantation has also been performed.1315 In all cases so far, BCAA level normalized after transplantation, and metabolic control was sustainable on a regular diet without protein restriction.13, 1618
Lessons for the Physician
Vomiting without true diarrhea deserves careful evaluation. Inborn errors of metabolism are individually rare but as a group are fairly common. One study noted an incidence of 15.7 per 100,000 births detected on tandem mass spectrometry screening, whereas rates of clinical detection are lower.19 This case illustrates how symptoms may be nonspecific and can easily mimic simple gastroenteritis. The following may be helpful when evaluating a patient with isolated emesis:
-
History: Does the patient have a history of emesis with illnesses that usually do not cause vomiting, such as an asthma flare or URI? Has the patient required emergency treatment or IV fluids for bouts of emesis in the past? Could toxins or medications that cause metabolic acidosis have been ingested?
-
Physical exam: Skin lesions, odors, and hepatomegaly are sometimes found in metabolic disorders. Commonly recognized odors include the musty smell of phenylketonuria and the sweet maple syrup smell of MSUD.
-
Laboratory studies: UA and calculation of anion gap are first steps to take to eliminate more common causes of metabolic acidosis in children.
-
Reliance on newborn screening: Patients with intermittent MSUD may have normal BCAA levels between acute episodes. Newborn screening tests may not identify these patients. The physician must maintain a degree of suspicion in approaching an acute illness that might indicate a metabolic disease, even in a child who has had negative expanded newborn screening.
Unusual disorders may masquerade as a simple problem. Common laboratory tests and a thorough history and exam can help to differentiate between simple gastroenteritis and inborn metabolic error and guide further diagnostic evaluation.
- ,,.Practical methods to estimate whole body leucine oxidation in maple syrup urine disease.Pediatr Res.1993;33:445–451.
- Behrman RE,Kliegman RM,Jenson HB, editors.Nelson Textbook of Pediatrics.Philadelphia:Saunders;2004:224–235,409–418.
- ,,.Lessons from genetic disorders of branched‐chain amino acid metabolism.J Nutr.2006;136(suppl 1):243S–249S.
- ,.Maple syrup urine disease (branched‐chain ketoaciduria). In:Scriver CR,Beaudet AL,Sly WS,Valle D,Vogelstein B,Childs B, editors.The Metabolic and Molecular Basis of Inherited Disease.New York:McGraw‐Hill;2001:1971–2006.
- ,,.A new syndrome: progressive familial infantile cerebral dysfunction associated with an unusual urinary substance.Pediatrics.1954;14:462–467.
- ,,,.Thiamine‐responsive maple‐syrup‐urine disease.Lancet.1971;1:310–312.
- ,,.Deficiency of dihydrolipoyl dehydrogenase (a component of the pyruvate and ‐ketoglutarate dehydrogenase complexes): a cause of congenital chronic lactic acidosis in infancy.Pediatr Res.1977;11:1198–202.
- ,,.Late‐onset branched‐chain ketoaciduria: (maple syrup urine disease).J Lancet.1966;86(3):149–152.
- ,,,.Cerebral edema causing death in children with maple syrup urine disease.J Pediatr.1991;119:42–45.
- ,,,,,,.Glutamate and γ‐aminobutyric acid neurotransmitter systems in the acute phase of maple syrup urine disease and citrullinemia encephalopathies in newborn calves.J Neurochem.1992;59:582–590.
- ,,,Maple syrup urine disease, with particular reference to dietotherapy.Pediatrics.1964;34:454–472.
- ,,,.Significance of L‐alloisoleucine in plasma for diagnosis of maple syrup urine disease.Clin Chem.1999;45:1734–40.
- ,,, et al.Mid‐term outcome of 2 cases with maple syrup urine disease: role of liver transplantation in the treatment.Arch Pediatr.1994;1:730–734.
- , et al.Transplantation for maple syrup urine disease (MSUD) and methylmalonic acidopathy (MMA).J Inherit Metab Dis.1997;20(suppl 1):37.
- ,,,.Liver transplantation in maple syrup urine disease.Eur J Pediatrics.1999;158(suppl 2):S60–S64.
- ,,, et al.Elective liver transplantation for the treatment of classical maple syrup urine disease.Am J Transplant.2006;6:557–564.
- ,,,,,.Domino liver transplantation in maple syrup urine disease.Liver Transpl.2006;12:876–882.
- ,,,.Branched‐chain L‐amino acid metabolism in classical maple syrup urine disease after orthotopic liver transplantation.J Inherit Metab Dis.2000;23:805–818.
- ,,,.Screening newborns for inborn errors of metabolism by tandem mass spectrometry.N Engl J Med.2003;348:2304–2312.
- ,,.Practical methods to estimate whole body leucine oxidation in maple syrup urine disease.Pediatr Res.1993;33:445–451.
- Behrman RE,Kliegman RM,Jenson HB, editors.Nelson Textbook of Pediatrics.Philadelphia:Saunders;2004:224–235,409–418.
- ,,.Lessons from genetic disorders of branched‐chain amino acid metabolism.J Nutr.2006;136(suppl 1):243S–249S.
- ,.Maple syrup urine disease (branched‐chain ketoaciduria). In:Scriver CR,Beaudet AL,Sly WS,Valle D,Vogelstein B,Childs B, editors.The Metabolic and Molecular Basis of Inherited Disease.New York:McGraw‐Hill;2001:1971–2006.
- ,,.A new syndrome: progressive familial infantile cerebral dysfunction associated with an unusual urinary substance.Pediatrics.1954;14:462–467.
- ,,,.Thiamine‐responsive maple‐syrup‐urine disease.Lancet.1971;1:310–312.
- ,,.Deficiency of dihydrolipoyl dehydrogenase (a component of the pyruvate and ‐ketoglutarate dehydrogenase complexes): a cause of congenital chronic lactic acidosis in infancy.Pediatr Res.1977;11:1198–202.
- ,,.Late‐onset branched‐chain ketoaciduria: (maple syrup urine disease).J Lancet.1966;86(3):149–152.
- ,,,.Cerebral edema causing death in children with maple syrup urine disease.J Pediatr.1991;119:42–45.
- ,,,,,,.Glutamate and γ‐aminobutyric acid neurotransmitter systems in the acute phase of maple syrup urine disease and citrullinemia encephalopathies in newborn calves.J Neurochem.1992;59:582–590.
- ,,,Maple syrup urine disease, with particular reference to dietotherapy.Pediatrics.1964;34:454–472.
- ,,,.Significance of L‐alloisoleucine in plasma for diagnosis of maple syrup urine disease.Clin Chem.1999;45:1734–40.
- ,,, et al.Mid‐term outcome of 2 cases with maple syrup urine disease: role of liver transplantation in the treatment.Arch Pediatr.1994;1:730–734.
- , et al.Transplantation for maple syrup urine disease (MSUD) and methylmalonic acidopathy (MMA).J Inherit Metab Dis.1997;20(suppl 1):37.
- ,,,.Liver transplantation in maple syrup urine disease.Eur J Pediatrics.1999;158(suppl 2):S60–S64.
- ,,, et al.Elective liver transplantation for the treatment of classical maple syrup urine disease.Am J Transplant.2006;6:557–564.
- ,,,,,.Domino liver transplantation in maple syrup urine disease.Liver Transpl.2006;12:876–882.
- ,,,.Branched‐chain L‐amino acid metabolism in classical maple syrup urine disease after orthotopic liver transplantation.J Inherit Metab Dis.2000;23:805–818.
- ,,,.Screening newborns for inborn errors of metabolism by tandem mass spectrometry.N Engl J Med.2003;348:2304–2312.
Inpatient Management of Urinary Tract Infections in Infants and Young Children
Introduction
Urinary tract infections (UTIs) are serious bacterial infections and a common cause for hospital admission of infants and young children. The prevalence of UTI in infants younger than 1 year of age ranges from 3.3% to 6.5%, and between 1 and 2 years of age from 1.9% to 8.1%. Females outpace males across all age groups, with the exception of the first 3 months of life (1). Without appropriate treatment and management, UTI can result in dehydration, urosepsis, and long-term medical problems including hypertension, renal scarring, and decreased renal function. This review will focus on the inpatient management of first-episode UTI in infants and young children.
Diagnosis
Presenting symptoms in older children include urgency, frequency, dysuria, and complaints of back pain. In contrast, symptoms in infants and young children are often nonspecific and include irritability, diarrhea, vomiting, poor feeding, poor weight gain, crying on urination, and foul-smelling urine. The presence of a fever in infants and young children with UTI has been accepted as a marker of pyelonephritis, which occurs when infection has ascended to the upper collecting system of the kidney. Urinalysis (UA) and culture should be collected by suprapubic aspiration or transurethral catheterization, or by appropriately performed clean catch method for children of appropriate age and developmental ability. The use of a bag-collected urine specimen is insufficient and unreliable and should not be used in making the diagnosis of UTI. While suprapubic aspiration is considered the gold standard with a specificity and sensitivity of 100%, there is often resistance from parents and from physicians who are not properly trained to do this procedure.
The most accepted method of obtaining urine is sterile transurethral catheterization, results of which have 95% sensitivity and 99% specificity (2). When interpreting the UA, the most useful components for the diagnosis of a UTI include a positive leukocyte esterase, nitrite test, or gram stain on unspun urine, and microscopy revealing >10 white blood cells per high-powered field of spun urine. However, neonates under 30 days old may have no abnormality noted on initial UA (3,4). The presence of any bacteria on gram-stained urine offers the best sensitivity and specificity (5). Final diagnosis depends upon isolation of >105 of a single organism from a clean-catch specimen, or >104 of a single organism from a catheterized specimen.
Admission Criteria
Guidelines for evaluation of serious bacterial infection and parenteral antibiotic use for febrile infants under 60 days of age should be followed. All febrile neonates less than 30 days of age should be admitted for parenteral antibiotics (6–11). Controversy exists on the need to use corrected or postconceptual age when evaluating and determining need for admission for febrile preterm infants, particularly for those under 35 weeks of gestation. Factors that can be considered by the practitioner include severity of Neonatal ICU course, severity of prematurity, and combined disease burden of UTI with common preterm comorbidities (anemia, apnea of prematurity, chronic lung disease).
Consider admitting and initiating parenteral antibiotic treatment using Table 1 as a guideline. Exercise a lower threshold for admitting infants and toxic-appearing young children due to concern for urosepsis, complications, and the need for appropriate and aggressive initial therapy.
Initial Inpatient Management
The 3 goals of inhospital treatment of UTIs are to effectively treat and eliminate the acute infection, prevent urosepsis in infants and immunocompromised children, and prevent and reduce long-term complications such as renal scarring, hypertension, and decreased renal function. Initial antibiotic treatment should be administered parenterally to ensure optimal antimicrobial levels and aimed at the most common organisms, including Escherichia coli, Klebsiella, Proteus, and Enterobacter spp. Less common organisms to consider include Pseudomonas, Enterococcus, Staphylococcus aureus, and group B Streptococcus. Organisms will differ on many factors, such as age, underlying disease, prior colonization, and antibiotic exposure.
Table 2 outlines the initial choices of antibiotics until culture and sensitivities are known.
The choices and dosage of antibiotics are dependent on the age of the patient and are selected based on the other most likely organisms and their expected sensitivities (12). Ampicillin is added to the less than 2-month age group not only to cover Enterococcus, but also as part of broader neonatal sepsis coverage for Listeria. The third-generation cephalosporins are felt to be adequate initial coverage for most of the common organisms causing UTI. Children with congenital anomalies known to be associated with genitourinary abnormalities may be infected with less common organisms. In these situations, consider tailoring initial antibiotic coverage.
Complications
The major complication of UTIs in infants is bacteremia. The rate of bacteremia in infants 0–3 months has variably been reported as 10% (13), 21–31% (14), and 36% (15). Infants with and without bacteremia are often clinically indistinguishable, making early determination of bacteremia difficult. A recent comprehensive review of 17 studies by Malik noted both C-reactive protein (CRP) and procalcitonin (PCT) results were highly variable in infants under 90 days old with known positive bacterial cultures (16). These inflammatory markers are therefore currently not useful to predict bacteremia. In addition to blood stream infection, other acute complications include meningitis, renal and perinephric abscess, and infected calculi. Longer-term complications include reflux nephropathy, renal scarring, hypertension, decreased renal function, and renal failure.
Duration of Antibiotic Therapy
While most uncomplicated UTIs are successfully treated with a 10-day treatment course, many experts prefer a 14-day course for neonates, infants, and ill-appearing young children. Despite effectiveness in adults, very-short-course therapy (≤3 days) for pediatric patients is associated with more treatment failures and reinfections (17). Although it may be considered in older children with cystitis, at this time it is not appropriate for treating infants and younger children in whom pyelonephritis cannot be distinguished from isolated lower tract infection (17,18).
Total treatment time and total days of parenteral therapy needed continue to be debated. Hoberman randomized children as young as 1 month of age to either entirely oral treatment or parenteral therapy for 3 days followed by oral treatment (19). In both arms of this study, children received 14 days of total therapy as was the standard at the time. He suggested, however, that a 10-day course of antibiotics should be adequate therapy for noncomplicated acute pyelonephritis. Of the 306 children, only 13 were under 2 months of age. Although only 13 positive blood cultures were reported, 10 of these occurred in children under 6 months of age. Given the limited number of children less than 2 months of age and the prevalence of positive blood cultures noted, conclusions cannot be drawn on the safety of entirely oral treatment for young infants. Parenteral antibiotic therapy should be continued for all hospitalized children until the patient is afebrile and free from signs of toxicity. Most hospitalized pediatric patients defervesce quickly on parenteral therapy—89% within 48 hours and 97% within 72 hours (20). Longer parenteral therapy of at least 10 days should be considered for neonates and infants with urosepsis, because they are immunologically immature, at greater risk of complications, have higher incidence of urinary tract anomalies, and have less reliable absorption of oral antibiotics.
Delayed or lack of response to antibiotic therapy may indicate the presence of urinary tract obstruction, resistant organisms, or renal or perinephric abscess. A repeat urine culture and immediate renal ultrasound or CT should be performed if the patient is not improving within 48 hours.
Radiological Studies
Renal Ultrasound (RUS)
Recent studies have questioned the value of performing routine RUS after a first-time UTI because of the low sensitivity in detecting vesicoureteral reflux (VUR) and a lack of significant influence in altering management (21,22). Patients who have had a normal late (30-32 weeks’ gestation) prenatal ultrasound with a good view of the kidneys may not require a repeat postnatal renal ultrasound (21,22). Further studies are needed to evaluate the costs and value of routine RUS. Until these studies are completed, renal and bladder ultrasound early during hospitalization continues to be recommended for all patients admitted with a first-time UTI to identify hydronephrosis, duplicating collecting systems, ureteral dilatation, calculi, and other structural anomalies.
Voiding Cystourethrogram (VCUG) or Radionuclide Cystography (RNC)
Either a VCUG or RNC should be performed to detect vesico-ureteral reflux in infants and young children. The AAP practice parameter and more recent literature clearly state the need for this evaluation in children under the age of 2 years (2,21). Additional data on incidence of anomalies by age suggest studying children under the age of 6 years (23,24). Recommendations for evaluation of children over age 6 may vary depending on age, patient, and family history, and comorbidities. Alternate methods such as voiding sonogram may also be options for this age group, and is not part of this discussion (25).
RNC exposes the patient to less radiation but does not show urethral or bladder anomalies. RNC is more often used for females with normal RUS and no voiding dysfunction, or to follow the progress of known VUR. The VCUG is often preferred because it provides more anatomic detail and is better for grading VUR and demonstrating posterior urethral vales in males (26). It is suggested that infants with antenatal renal pelvis dilation who have 2 normal renal sonograms in the first month of life are at low risk for abnormalities and may not require a VCUG (27). The rate of detection of VUR with a first episode of UTI does not increase when the VCUG is done early, within the first 7 days of diagnosis (28,29). Performing the VCUG as an inpatient should be considered if outpatient follow-up is of significant concern, or if the RUS suggests bilateral ureteral obstruction. If done as an inpatient procedure, it should be performed preferably during day 3–5 of antibiotic therapy and when the patient is clinically responding to the appropriate antibiotic. The overall value of the VCUG is being reviewed, as its usefulness is most significant only if VUR antimicrobial prophylaxis is effective in reducing reinfections and renal scarring (21,30). Until further studies are performed, the VCUG should continue as part of the initial UTI evaluation for infants and young children.
Renal Cortical Scintigraphy (RCS)
This is the imaging study of choice for the detection of acute pyelonephritis and renal scarring. As children are treated for presumptive upper-tract infection empirically, DMSA scan for diagnosis of pyelonephritis has limited utility (21). Scans have more often been performed at 6 months’ postinfection to document scar formation. Hoberman demonstrated that only 15% of children with abnormal scintigraphy at diagnosis have renal scarring on repeat RCS at 6 months. The importance of these scars is unclear. Association of scars with ultimate development of hypertension, renal insufficiency, and end-stage renal disease is based on studies performed in the 1980s using intravenous pyelogram. RCS is much more sensitive, finding more minor scars of uncertain significance.
Table 3 may be of value when considering imaging options.
Other Considerations
CRP and PCT use in UTI have been evaluated by Pratt. Values at diagnosis are potentially helpful in ruling out scar formation at 6 months’ postinfection. Values under 1.0 ng/mL for PCT and 20 mg/L for CRP had a negative predictive value of 97.5% and 95%, respectively (31). Further studies are warranted to confirm the usefulness of these inflammatory markers to rule out future scar formation.
Consultations
Consider urology consultation if the RUS, VCUG, voiding history, or examination demonstrated concern for significant genitourinary abnormalities, abnormal voiding function or neurogenic bladder (23,32). Consider infectious disease consultation if the patient is not responding to conventional therapy without obstruction, unusual organisms are identified, or the patient is having recurrent urinary tract infections in the presence of normal urological structure and function.
Discharge Criteria and Processes
Consider discharge under the following conditions:
- The patient is comfortable and tolerating oral fluids well.
- The patient has been afebrile or has significantly decreasing fever for 24 hours.
- Appropriate radiological studies and consultations have been completed or arranged for as an outpatient.
- For patients requiring parenterally administered medications at home, long-term IV access must be obtained to assessment of home care service availability, benefits, family home resources, and caregiver education completed.
- Appropriate prophylactic antibiotic prescription has been given to the caregiver with education on use after completion of acute antibiotic therapy. Prophylactic antibiotics should be administered until imaging studies have been completed and assessed.
Conclusion
UTI is a common bacterial infection requiring hospital admission for infants and young children. Admission decisions should take into consideration goals for inpatient care and special age or clinical circumstances. Treatment mode and duration must address avoidance of both acute and chronic complications. Radiologic studies offer both anatomic view and functional information. Clinical relevance of scars, utility of radiologic studies, and value of inflammatory markers are some of the many areas requiring further study.
References
- Long SS, Klein J. Bacterial infections of the urinary tract. In: Remington JSand Klein JO(eds.). Infectious Diseases of the Fetus and Newborn Infant. 5th ed. Philadelphia, Pa: WB Saunders; 2001:1035-46.
- Committee on Quality Improvement, Subcommittee on Urinary Tract Infection. Practice parameter: the diagnosis, treatment, and evaluation of the initial urinary tract infection in febrile infants and young children. Pediatrics. 1999;103:843-52.
- Dayan PS, Bennett J, Best R, et al. Test characteristics of the urine gram stain in infants ≤60 days of age with fever. Pediatr Emerg Care. 2002;18:12-4.
- Huicho L, Campos-Sanchez M, Alamo C. Metaanalysis of urine screening tests for determining the risk of urinary tract infection in children. Pediatr Infect Dis J. 2002;21:1-11.
- Gorelick M, Shaw KN. Screening tests for urinary tract infections in Children: a meta-analysis. Pediatrics. 1999;104:e54.
- Byington CL, Enriquez F, Hoff C, et al. Serious bacterial infections in febrile infants 1 to 90 days old with and without viral infections. Pediatrics. 2004:113:1662-6.
- Baraff L. Management of fever without source in infants and children. Ann Emerg Med. 2000;36:602-14.
- Baraff LJ, Oslund SA, Schriger D, Stephen ML. Probability of bacterial infections in febrile infants less than three months of age: a meta-analysis. Pediatr Infect Dis J. 1992;11:257-64.
- Klein JO. Management of the febrile child without a focus of infection in the era of universal pneumococcal immunization. Pediatr Infect Dis J. 2002;21:584-8.
- Syrogiannopoulos G, Grieva I, Anastassiou E, Triga M, Dimitracopoulos G, Beratis N. Sterile cerebrospinal fluid pleocytosis in young infants with urinary tract infections. Pediatr Infect Dis J. 2001;20:927-30.
- Jaskiewicz JA, Mc Carthy CA, Richardson AC, ET AL. Febrile infants at low risk for serious bacterial infection--an appraisal of the Rochester criteria and implications for management. Febrile Collaborative Study Group. Pediatrics. 1994;94:390-6.
- AAP Redbook. Report of the committee on infectious diseases, 2003:700.
- Newman TB, Bernzweig JA, Takayama JI, Finch SA, Wasserman RC, Pantell RH. Urine testing and urinary tract infections in febrile infants seen in the office setting: the Pediatric Research in Office Settings’ Febrile Infant Study Arch Pediatr Adolesc Med. 2002;156:44-54.
- Ginsberg CM, McCracken GH Jr. Urinary tract infection in young infants. Pediatrics. 1982;69:409-12.
- Wiswell T, Geschke D. Risks from circumcision during the first month of life compared to uncircumcised boys. Pediatrics. 1989;83:1011-15.
- Malik A, Hui C, Pennie RA, Kirpalani H. Beyond the complete blood cell count and C-reactive protein: a systematic review of modern diagnostic tests for neonatal sepsis. Arch Pediatr Adolesc Med. 2003;157:511-6.
- Keren R, Chan E. A meta-analysis of randomized, controlled trials comparing short- and long-course antibiotic therapy for urinary tract infections in children. Pediatrics. 2002;109:e70.
- Michael M, Hodson EM, Craig JC, Martin S, Moyer VA. Short versus standard duration oral antibiotic therapy for acute urinary tract infection in children. Cochrane Database of Syst Rev.2003.
- Hoberman A, Wald ER, Hickey RW, et al. Oral versus intravenous therapy for urinary tract infections in young children. Pediatrics. 1999;104:79-86.
- Bachur R. Nonresponders: prolonged fever among infants with urinary tract infections. Pediatrics. 2000;105:E59.
- Hoberman A, Charron M, Hickey RW, Baskin M, Kearney DH, Wald ER. Imaging studies after a first febrile urinary tract infection in young children. N Engl J Med. 2003;348:195-202.
- Zamir G, Sakran W, Horowitz Y, Koren A, Miron D. Urinary tract infection: is there a need for routine renal ultrasonography? Arch Dis Child. 2004;89:466-8.
- Johnson CE. New advances in childhood urinary tract infections. Pediatr Rev. 1999:20:335-43.
- Thompson M, Simon S, Sharma V, Alon US. Timing of follow-up voiding cystourethrogram in children with primary vesicoureteral reflux: development and application of a clinical algorithm. Pediatrics. 2005:115:426-34.
- Darge K, Moeller RT, Trusen A, BuĴer F, Gordjani N, Riedmiller H. Diagnosis of vesicoureteral reflux with low-dose contrast-enhanced harmonic ultrasound imaging. Pediatr Radiol. 2005:35:73-8.
- Kraus S. Genitourinary imaging in children. Pediatr Clin North Am. 2001;48:1381-1424.
- Ismaili K, Avni F, Hall M; Brussels Free University Perinatal Nephrology (BFUPN) Study Group. Results of systematic voiding cystourethrography in infants with antenatally diagnosed renal pelvis dilation. J Pediatr. 2002;141: 21-4.
- Mahant S, To T, Friedman J. Timing of voiding cystourethrogram in the investigation of urinary tract infections in children. J Pediatr. 2001;39:568-71.
- McDonald A, Scranton M, Gillespie R, Mahajan V, Edwards GA. Voiding cystourethrograms and urinary tract infections: how long to wait? Pediatrics. 2000:105:E50.
- Williams G, Lee A, Craig J. Antibiotics for the prevention of urinary tract infection in children: a systematic review of randomized controlled trials. J Pediatr. 2001;138:868-74.
- Prat C, Dominguez J, Rodrigo C, et al. Elevated serum procalcitonin values correlate with renal scarring in children with urinary tract infection. Pediatr Infect Dis J. 2003;22:438-42.
- Roberts KB. Urinary tract infection treatment and evaluation update. Pediatr Infect Dis J. 2004: 23:1163-4.
Introduction
Urinary tract infections (UTIs) are serious bacterial infections and a common cause for hospital admission of infants and young children. The prevalence of UTI in infants younger than 1 year of age ranges from 3.3% to 6.5%, and between 1 and 2 years of age from 1.9% to 8.1%. Females outpace males across all age groups, with the exception of the first 3 months of life (1). Without appropriate treatment and management, UTI can result in dehydration, urosepsis, and long-term medical problems including hypertension, renal scarring, and decreased renal function. This review will focus on the inpatient management of first-episode UTI in infants and young children.
Diagnosis
Presenting symptoms in older children include urgency, frequency, dysuria, and complaints of back pain. In contrast, symptoms in infants and young children are often nonspecific and include irritability, diarrhea, vomiting, poor feeding, poor weight gain, crying on urination, and foul-smelling urine. The presence of a fever in infants and young children with UTI has been accepted as a marker of pyelonephritis, which occurs when infection has ascended to the upper collecting system of the kidney. Urinalysis (UA) and culture should be collected by suprapubic aspiration or transurethral catheterization, or by appropriately performed clean catch method for children of appropriate age and developmental ability. The use of a bag-collected urine specimen is insufficient and unreliable and should not be used in making the diagnosis of UTI. While suprapubic aspiration is considered the gold standard with a specificity and sensitivity of 100%, there is often resistance from parents and from physicians who are not properly trained to do this procedure.
The most accepted method of obtaining urine is sterile transurethral catheterization, results of which have 95% sensitivity and 99% specificity (2). When interpreting the UA, the most useful components for the diagnosis of a UTI include a positive leukocyte esterase, nitrite test, or gram stain on unspun urine, and microscopy revealing >10 white blood cells per high-powered field of spun urine. However, neonates under 30 days old may have no abnormality noted on initial UA (3,4). The presence of any bacteria on gram-stained urine offers the best sensitivity and specificity (5). Final diagnosis depends upon isolation of >105 of a single organism from a clean-catch specimen, or >104 of a single organism from a catheterized specimen.
Admission Criteria
Guidelines for evaluation of serious bacterial infection and parenteral antibiotic use for febrile infants under 60 days of age should be followed. All febrile neonates less than 30 days of age should be admitted for parenteral antibiotics (6–11). Controversy exists on the need to use corrected or postconceptual age when evaluating and determining need for admission for febrile preterm infants, particularly for those under 35 weeks of gestation. Factors that can be considered by the practitioner include severity of Neonatal ICU course, severity of prematurity, and combined disease burden of UTI with common preterm comorbidities (anemia, apnea of prematurity, chronic lung disease).
Consider admitting and initiating parenteral antibiotic treatment using Table 1 as a guideline. Exercise a lower threshold for admitting infants and toxic-appearing young children due to concern for urosepsis, complications, and the need for appropriate and aggressive initial therapy.
Initial Inpatient Management
The 3 goals of inhospital treatment of UTIs are to effectively treat and eliminate the acute infection, prevent urosepsis in infants and immunocompromised children, and prevent and reduce long-term complications such as renal scarring, hypertension, and decreased renal function. Initial antibiotic treatment should be administered parenterally to ensure optimal antimicrobial levels and aimed at the most common organisms, including Escherichia coli, Klebsiella, Proteus, and Enterobacter spp. Less common organisms to consider include Pseudomonas, Enterococcus, Staphylococcus aureus, and group B Streptococcus. Organisms will differ on many factors, such as age, underlying disease, prior colonization, and antibiotic exposure.
Table 2 outlines the initial choices of antibiotics until culture and sensitivities are known.
The choices and dosage of antibiotics are dependent on the age of the patient and are selected based on the other most likely organisms and their expected sensitivities (12). Ampicillin is added to the less than 2-month age group not only to cover Enterococcus, but also as part of broader neonatal sepsis coverage for Listeria. The third-generation cephalosporins are felt to be adequate initial coverage for most of the common organisms causing UTI. Children with congenital anomalies known to be associated with genitourinary abnormalities may be infected with less common organisms. In these situations, consider tailoring initial antibiotic coverage.
Complications
The major complication of UTIs in infants is bacteremia. The rate of bacteremia in infants 0–3 months has variably been reported as 10% (13), 21–31% (14), and 36% (15). Infants with and without bacteremia are often clinically indistinguishable, making early determination of bacteremia difficult. A recent comprehensive review of 17 studies by Malik noted both C-reactive protein (CRP) and procalcitonin (PCT) results were highly variable in infants under 90 days old with known positive bacterial cultures (16). These inflammatory markers are therefore currently not useful to predict bacteremia. In addition to blood stream infection, other acute complications include meningitis, renal and perinephric abscess, and infected calculi. Longer-term complications include reflux nephropathy, renal scarring, hypertension, decreased renal function, and renal failure.
Duration of Antibiotic Therapy
While most uncomplicated UTIs are successfully treated with a 10-day treatment course, many experts prefer a 14-day course for neonates, infants, and ill-appearing young children. Despite effectiveness in adults, very-short-course therapy (≤3 days) for pediatric patients is associated with more treatment failures and reinfections (17). Although it may be considered in older children with cystitis, at this time it is not appropriate for treating infants and younger children in whom pyelonephritis cannot be distinguished from isolated lower tract infection (17,18).
Total treatment time and total days of parenteral therapy needed continue to be debated. Hoberman randomized children as young as 1 month of age to either entirely oral treatment or parenteral therapy for 3 days followed by oral treatment (19). In both arms of this study, children received 14 days of total therapy as was the standard at the time. He suggested, however, that a 10-day course of antibiotics should be adequate therapy for noncomplicated acute pyelonephritis. Of the 306 children, only 13 were under 2 months of age. Although only 13 positive blood cultures were reported, 10 of these occurred in children under 6 months of age. Given the limited number of children less than 2 months of age and the prevalence of positive blood cultures noted, conclusions cannot be drawn on the safety of entirely oral treatment for young infants. Parenteral antibiotic therapy should be continued for all hospitalized children until the patient is afebrile and free from signs of toxicity. Most hospitalized pediatric patients defervesce quickly on parenteral therapy—89% within 48 hours and 97% within 72 hours (20). Longer parenteral therapy of at least 10 days should be considered for neonates and infants with urosepsis, because they are immunologically immature, at greater risk of complications, have higher incidence of urinary tract anomalies, and have less reliable absorption of oral antibiotics.
Delayed or lack of response to antibiotic therapy may indicate the presence of urinary tract obstruction, resistant organisms, or renal or perinephric abscess. A repeat urine culture and immediate renal ultrasound or CT should be performed if the patient is not improving within 48 hours.
Radiological Studies
Renal Ultrasound (RUS)
Recent studies have questioned the value of performing routine RUS after a first-time UTI because of the low sensitivity in detecting vesicoureteral reflux (VUR) and a lack of significant influence in altering management (21,22). Patients who have had a normal late (30-32 weeks’ gestation) prenatal ultrasound with a good view of the kidneys may not require a repeat postnatal renal ultrasound (21,22). Further studies are needed to evaluate the costs and value of routine RUS. Until these studies are completed, renal and bladder ultrasound early during hospitalization continues to be recommended for all patients admitted with a first-time UTI to identify hydronephrosis, duplicating collecting systems, ureteral dilatation, calculi, and other structural anomalies.
Voiding Cystourethrogram (VCUG) or Radionuclide Cystography (RNC)
Either a VCUG or RNC should be performed to detect vesico-ureteral reflux in infants and young children. The AAP practice parameter and more recent literature clearly state the need for this evaluation in children under the age of 2 years (2,21). Additional data on incidence of anomalies by age suggest studying children under the age of 6 years (23,24). Recommendations for evaluation of children over age 6 may vary depending on age, patient, and family history, and comorbidities. Alternate methods such as voiding sonogram may also be options for this age group, and is not part of this discussion (25).
RNC exposes the patient to less radiation but does not show urethral or bladder anomalies. RNC is more often used for females with normal RUS and no voiding dysfunction, or to follow the progress of known VUR. The VCUG is often preferred because it provides more anatomic detail and is better for grading VUR and demonstrating posterior urethral vales in males (26). It is suggested that infants with antenatal renal pelvis dilation who have 2 normal renal sonograms in the first month of life are at low risk for abnormalities and may not require a VCUG (27). The rate of detection of VUR with a first episode of UTI does not increase when the VCUG is done early, within the first 7 days of diagnosis (28,29). Performing the VCUG as an inpatient should be considered if outpatient follow-up is of significant concern, or if the RUS suggests bilateral ureteral obstruction. If done as an inpatient procedure, it should be performed preferably during day 3–5 of antibiotic therapy and when the patient is clinically responding to the appropriate antibiotic. The overall value of the VCUG is being reviewed, as its usefulness is most significant only if VUR antimicrobial prophylaxis is effective in reducing reinfections and renal scarring (21,30). Until further studies are performed, the VCUG should continue as part of the initial UTI evaluation for infants and young children.
Renal Cortical Scintigraphy (RCS)
This is the imaging study of choice for the detection of acute pyelonephritis and renal scarring. As children are treated for presumptive upper-tract infection empirically, DMSA scan for diagnosis of pyelonephritis has limited utility (21). Scans have more often been performed at 6 months’ postinfection to document scar formation. Hoberman demonstrated that only 15% of children with abnormal scintigraphy at diagnosis have renal scarring on repeat RCS at 6 months. The importance of these scars is unclear. Association of scars with ultimate development of hypertension, renal insufficiency, and end-stage renal disease is based on studies performed in the 1980s using intravenous pyelogram. RCS is much more sensitive, finding more minor scars of uncertain significance.
Table 3 may be of value when considering imaging options.
Other Considerations
CRP and PCT use in UTI have been evaluated by Pratt. Values at diagnosis are potentially helpful in ruling out scar formation at 6 months’ postinfection. Values under 1.0 ng/mL for PCT and 20 mg/L for CRP had a negative predictive value of 97.5% and 95%, respectively (31). Further studies are warranted to confirm the usefulness of these inflammatory markers to rule out future scar formation.
Consultations
Consider urology consultation if the RUS, VCUG, voiding history, or examination demonstrated concern for significant genitourinary abnormalities, abnormal voiding function or neurogenic bladder (23,32). Consider infectious disease consultation if the patient is not responding to conventional therapy without obstruction, unusual organisms are identified, or the patient is having recurrent urinary tract infections in the presence of normal urological structure and function.
Discharge Criteria and Processes
Consider discharge under the following conditions:
- The patient is comfortable and tolerating oral fluids well.
- The patient has been afebrile or has significantly decreasing fever for 24 hours.
- Appropriate radiological studies and consultations have been completed or arranged for as an outpatient.
- For patients requiring parenterally administered medications at home, long-term IV access must be obtained to assessment of home care service availability, benefits, family home resources, and caregiver education completed.
- Appropriate prophylactic antibiotic prescription has been given to the caregiver with education on use after completion of acute antibiotic therapy. Prophylactic antibiotics should be administered until imaging studies have been completed and assessed.
Conclusion
UTI is a common bacterial infection requiring hospital admission for infants and young children. Admission decisions should take into consideration goals for inpatient care and special age or clinical circumstances. Treatment mode and duration must address avoidance of both acute and chronic complications. Radiologic studies offer both anatomic view and functional information. Clinical relevance of scars, utility of radiologic studies, and value of inflammatory markers are some of the many areas requiring further study.
References
- Long SS, Klein J. Bacterial infections of the urinary tract. In: Remington JSand Klein JO(eds.). Infectious Diseases of the Fetus and Newborn Infant. 5th ed. Philadelphia, Pa: WB Saunders; 2001:1035-46.
- Committee on Quality Improvement, Subcommittee on Urinary Tract Infection. Practice parameter: the diagnosis, treatment, and evaluation of the initial urinary tract infection in febrile infants and young children. Pediatrics. 1999;103:843-52.
- Dayan PS, Bennett J, Best R, et al. Test characteristics of the urine gram stain in infants ≤60 days of age with fever. Pediatr Emerg Care. 2002;18:12-4.
- Huicho L, Campos-Sanchez M, Alamo C. Metaanalysis of urine screening tests for determining the risk of urinary tract infection in children. Pediatr Infect Dis J. 2002;21:1-11.
- Gorelick M, Shaw KN. Screening tests for urinary tract infections in Children: a meta-analysis. Pediatrics. 1999;104:e54.
- Byington CL, Enriquez F, Hoff C, et al. Serious bacterial infections in febrile infants 1 to 90 days old with and without viral infections. Pediatrics. 2004:113:1662-6.
- Baraff L. Management of fever without source in infants and children. Ann Emerg Med. 2000;36:602-14.
- Baraff LJ, Oslund SA, Schriger D, Stephen ML. Probability of bacterial infections in febrile infants less than three months of age: a meta-analysis. Pediatr Infect Dis J. 1992;11:257-64.
- Klein JO. Management of the febrile child without a focus of infection in the era of universal pneumococcal immunization. Pediatr Infect Dis J. 2002;21:584-8.
- Syrogiannopoulos G, Grieva I, Anastassiou E, Triga M, Dimitracopoulos G, Beratis N. Sterile cerebrospinal fluid pleocytosis in young infants with urinary tract infections. Pediatr Infect Dis J. 2001;20:927-30.
- Jaskiewicz JA, Mc Carthy CA, Richardson AC, ET AL. Febrile infants at low risk for serious bacterial infection--an appraisal of the Rochester criteria and implications for management. Febrile Collaborative Study Group. Pediatrics. 1994;94:390-6.
- AAP Redbook. Report of the committee on infectious diseases, 2003:700.
- Newman TB, Bernzweig JA, Takayama JI, Finch SA, Wasserman RC, Pantell RH. Urine testing and urinary tract infections in febrile infants seen in the office setting: the Pediatric Research in Office Settings’ Febrile Infant Study Arch Pediatr Adolesc Med. 2002;156:44-54.
- Ginsberg CM, McCracken GH Jr. Urinary tract infection in young infants. Pediatrics. 1982;69:409-12.
- Wiswell T, Geschke D. Risks from circumcision during the first month of life compared to uncircumcised boys. Pediatrics. 1989;83:1011-15.
- Malik A, Hui C, Pennie RA, Kirpalani H. Beyond the complete blood cell count and C-reactive protein: a systematic review of modern diagnostic tests for neonatal sepsis. Arch Pediatr Adolesc Med. 2003;157:511-6.
- Keren R, Chan E. A meta-analysis of randomized, controlled trials comparing short- and long-course antibiotic therapy for urinary tract infections in children. Pediatrics. 2002;109:e70.
- Michael M, Hodson EM, Craig JC, Martin S, Moyer VA. Short versus standard duration oral antibiotic therapy for acute urinary tract infection in children. Cochrane Database of Syst Rev.2003.
- Hoberman A, Wald ER, Hickey RW, et al. Oral versus intravenous therapy for urinary tract infections in young children. Pediatrics. 1999;104:79-86.
- Bachur R. Nonresponders: prolonged fever among infants with urinary tract infections. Pediatrics. 2000;105:E59.
- Hoberman A, Charron M, Hickey RW, Baskin M, Kearney DH, Wald ER. Imaging studies after a first febrile urinary tract infection in young children. N Engl J Med. 2003;348:195-202.
- Zamir G, Sakran W, Horowitz Y, Koren A, Miron D. Urinary tract infection: is there a need for routine renal ultrasonography? Arch Dis Child. 2004;89:466-8.
- Johnson CE. New advances in childhood urinary tract infections. Pediatr Rev. 1999:20:335-43.
- Thompson M, Simon S, Sharma V, Alon US. Timing of follow-up voiding cystourethrogram in children with primary vesicoureteral reflux: development and application of a clinical algorithm. Pediatrics. 2005:115:426-34.
- Darge K, Moeller RT, Trusen A, BuĴer F, Gordjani N, Riedmiller H. Diagnosis of vesicoureteral reflux with low-dose contrast-enhanced harmonic ultrasound imaging. Pediatr Radiol. 2005:35:73-8.
- Kraus S. Genitourinary imaging in children. Pediatr Clin North Am. 2001;48:1381-1424.
- Ismaili K, Avni F, Hall M; Brussels Free University Perinatal Nephrology (BFUPN) Study Group. Results of systematic voiding cystourethrography in infants with antenatally diagnosed renal pelvis dilation. J Pediatr. 2002;141: 21-4.
- Mahant S, To T, Friedman J. Timing of voiding cystourethrogram in the investigation of urinary tract infections in children. J Pediatr. 2001;39:568-71.
- McDonald A, Scranton M, Gillespie R, Mahajan V, Edwards GA. Voiding cystourethrograms and urinary tract infections: how long to wait? Pediatrics. 2000:105:E50.
- Williams G, Lee A, Craig J. Antibiotics for the prevention of urinary tract infection in children: a systematic review of randomized controlled trials. J Pediatr. 2001;138:868-74.
- Prat C, Dominguez J, Rodrigo C, et al. Elevated serum procalcitonin values correlate with renal scarring in children with urinary tract infection. Pediatr Infect Dis J. 2003;22:438-42.
- Roberts KB. Urinary tract infection treatment and evaluation update. Pediatr Infect Dis J. 2004: 23:1163-4.
Introduction
Urinary tract infections (UTIs) are serious bacterial infections and a common cause for hospital admission of infants and young children. The prevalence of UTI in infants younger than 1 year of age ranges from 3.3% to 6.5%, and between 1 and 2 years of age from 1.9% to 8.1%. Females outpace males across all age groups, with the exception of the first 3 months of life (1). Without appropriate treatment and management, UTI can result in dehydration, urosepsis, and long-term medical problems including hypertension, renal scarring, and decreased renal function. This review will focus on the inpatient management of first-episode UTI in infants and young children.
Diagnosis
Presenting symptoms in older children include urgency, frequency, dysuria, and complaints of back pain. In contrast, symptoms in infants and young children are often nonspecific and include irritability, diarrhea, vomiting, poor feeding, poor weight gain, crying on urination, and foul-smelling urine. The presence of a fever in infants and young children with UTI has been accepted as a marker of pyelonephritis, which occurs when infection has ascended to the upper collecting system of the kidney. Urinalysis (UA) and culture should be collected by suprapubic aspiration or transurethral catheterization, or by appropriately performed clean catch method for children of appropriate age and developmental ability. The use of a bag-collected urine specimen is insufficient and unreliable and should not be used in making the diagnosis of UTI. While suprapubic aspiration is considered the gold standard with a specificity and sensitivity of 100%, there is often resistance from parents and from physicians who are not properly trained to do this procedure.
The most accepted method of obtaining urine is sterile transurethral catheterization, results of which have 95% sensitivity and 99% specificity (2). When interpreting the UA, the most useful components for the diagnosis of a UTI include a positive leukocyte esterase, nitrite test, or gram stain on unspun urine, and microscopy revealing >10 white blood cells per high-powered field of spun urine. However, neonates under 30 days old may have no abnormality noted on initial UA (3,4). The presence of any bacteria on gram-stained urine offers the best sensitivity and specificity (5). Final diagnosis depends upon isolation of >105 of a single organism from a clean-catch specimen, or >104 of a single organism from a catheterized specimen.
Admission Criteria
Guidelines for evaluation of serious bacterial infection and parenteral antibiotic use for febrile infants under 60 days of age should be followed. All febrile neonates less than 30 days of age should be admitted for parenteral antibiotics (6–11). Controversy exists on the need to use corrected or postconceptual age when evaluating and determining need for admission for febrile preterm infants, particularly for those under 35 weeks of gestation. Factors that can be considered by the practitioner include severity of Neonatal ICU course, severity of prematurity, and combined disease burden of UTI with common preterm comorbidities (anemia, apnea of prematurity, chronic lung disease).
Consider admitting and initiating parenteral antibiotic treatment using Table 1 as a guideline. Exercise a lower threshold for admitting infants and toxic-appearing young children due to concern for urosepsis, complications, and the need for appropriate and aggressive initial therapy.
Initial Inpatient Management
The 3 goals of inhospital treatment of UTIs are to effectively treat and eliminate the acute infection, prevent urosepsis in infants and immunocompromised children, and prevent and reduce long-term complications such as renal scarring, hypertension, and decreased renal function. Initial antibiotic treatment should be administered parenterally to ensure optimal antimicrobial levels and aimed at the most common organisms, including Escherichia coli, Klebsiella, Proteus, and Enterobacter spp. Less common organisms to consider include Pseudomonas, Enterococcus, Staphylococcus aureus, and group B Streptococcus. Organisms will differ on many factors, such as age, underlying disease, prior colonization, and antibiotic exposure.
Table 2 outlines the initial choices of antibiotics until culture and sensitivities are known.
The choices and dosage of antibiotics are dependent on the age of the patient and are selected based on the other most likely organisms and their expected sensitivities (12). Ampicillin is added to the less than 2-month age group not only to cover Enterococcus, but also as part of broader neonatal sepsis coverage for Listeria. The third-generation cephalosporins are felt to be adequate initial coverage for most of the common organisms causing UTI. Children with congenital anomalies known to be associated with genitourinary abnormalities may be infected with less common organisms. In these situations, consider tailoring initial antibiotic coverage.
Complications
The major complication of UTIs in infants is bacteremia. The rate of bacteremia in infants 0–3 months has variably been reported as 10% (13), 21–31% (14), and 36% (15). Infants with and without bacteremia are often clinically indistinguishable, making early determination of bacteremia difficult. A recent comprehensive review of 17 studies by Malik noted both C-reactive protein (CRP) and procalcitonin (PCT) results were highly variable in infants under 90 days old with known positive bacterial cultures (16). These inflammatory markers are therefore currently not useful to predict bacteremia. In addition to blood stream infection, other acute complications include meningitis, renal and perinephric abscess, and infected calculi. Longer-term complications include reflux nephropathy, renal scarring, hypertension, decreased renal function, and renal failure.
Duration of Antibiotic Therapy
While most uncomplicated UTIs are successfully treated with a 10-day treatment course, many experts prefer a 14-day course for neonates, infants, and ill-appearing young children. Despite effectiveness in adults, very-short-course therapy (≤3 days) for pediatric patients is associated with more treatment failures and reinfections (17). Although it may be considered in older children with cystitis, at this time it is not appropriate for treating infants and younger children in whom pyelonephritis cannot be distinguished from isolated lower tract infection (17,18).
Total treatment time and total days of parenteral therapy needed continue to be debated. Hoberman randomized children as young as 1 month of age to either entirely oral treatment or parenteral therapy for 3 days followed by oral treatment (19). In both arms of this study, children received 14 days of total therapy as was the standard at the time. He suggested, however, that a 10-day course of antibiotics should be adequate therapy for noncomplicated acute pyelonephritis. Of the 306 children, only 13 were under 2 months of age. Although only 13 positive blood cultures were reported, 10 of these occurred in children under 6 months of age. Given the limited number of children less than 2 months of age and the prevalence of positive blood cultures noted, conclusions cannot be drawn on the safety of entirely oral treatment for young infants. Parenteral antibiotic therapy should be continued for all hospitalized children until the patient is afebrile and free from signs of toxicity. Most hospitalized pediatric patients defervesce quickly on parenteral therapy—89% within 48 hours and 97% within 72 hours (20). Longer parenteral therapy of at least 10 days should be considered for neonates and infants with urosepsis, because they are immunologically immature, at greater risk of complications, have higher incidence of urinary tract anomalies, and have less reliable absorption of oral antibiotics.
Delayed or lack of response to antibiotic therapy may indicate the presence of urinary tract obstruction, resistant organisms, or renal or perinephric abscess. A repeat urine culture and immediate renal ultrasound or CT should be performed if the patient is not improving within 48 hours.
Radiological Studies
Renal Ultrasound (RUS)
Recent studies have questioned the value of performing routine RUS after a first-time UTI because of the low sensitivity in detecting vesicoureteral reflux (VUR) and a lack of significant influence in altering management (21,22). Patients who have had a normal late (30-32 weeks’ gestation) prenatal ultrasound with a good view of the kidneys may not require a repeat postnatal renal ultrasound (21,22). Further studies are needed to evaluate the costs and value of routine RUS. Until these studies are completed, renal and bladder ultrasound early during hospitalization continues to be recommended for all patients admitted with a first-time UTI to identify hydronephrosis, duplicating collecting systems, ureteral dilatation, calculi, and other structural anomalies.
Voiding Cystourethrogram (VCUG) or Radionuclide Cystography (RNC)
Either a VCUG or RNC should be performed to detect vesico-ureteral reflux in infants and young children. The AAP practice parameter and more recent literature clearly state the need for this evaluation in children under the age of 2 years (2,21). Additional data on incidence of anomalies by age suggest studying children under the age of 6 years (23,24). Recommendations for evaluation of children over age 6 may vary depending on age, patient, and family history, and comorbidities. Alternate methods such as voiding sonogram may also be options for this age group, and is not part of this discussion (25).
RNC exposes the patient to less radiation but does not show urethral or bladder anomalies. RNC is more often used for females with normal RUS and no voiding dysfunction, or to follow the progress of known VUR. The VCUG is often preferred because it provides more anatomic detail and is better for grading VUR and demonstrating posterior urethral vales in males (26). It is suggested that infants with antenatal renal pelvis dilation who have 2 normal renal sonograms in the first month of life are at low risk for abnormalities and may not require a VCUG (27). The rate of detection of VUR with a first episode of UTI does not increase when the VCUG is done early, within the first 7 days of diagnosis (28,29). Performing the VCUG as an inpatient should be considered if outpatient follow-up is of significant concern, or if the RUS suggests bilateral ureteral obstruction. If done as an inpatient procedure, it should be performed preferably during day 3–5 of antibiotic therapy and when the patient is clinically responding to the appropriate antibiotic. The overall value of the VCUG is being reviewed, as its usefulness is most significant only if VUR antimicrobial prophylaxis is effective in reducing reinfections and renal scarring (21,30). Until further studies are performed, the VCUG should continue as part of the initial UTI evaluation for infants and young children.
Renal Cortical Scintigraphy (RCS)
This is the imaging study of choice for the detection of acute pyelonephritis and renal scarring. As children are treated for presumptive upper-tract infection empirically, DMSA scan for diagnosis of pyelonephritis has limited utility (21). Scans have more often been performed at 6 months’ postinfection to document scar formation. Hoberman demonstrated that only 15% of children with abnormal scintigraphy at diagnosis have renal scarring on repeat RCS at 6 months. The importance of these scars is unclear. Association of scars with ultimate development of hypertension, renal insufficiency, and end-stage renal disease is based on studies performed in the 1980s using intravenous pyelogram. RCS is much more sensitive, finding more minor scars of uncertain significance.
Table 3 may be of value when considering imaging options.
Other Considerations
CRP and PCT use in UTI have been evaluated by Pratt. Values at diagnosis are potentially helpful in ruling out scar formation at 6 months’ postinfection. Values under 1.0 ng/mL for PCT and 20 mg/L for CRP had a negative predictive value of 97.5% and 95%, respectively (31). Further studies are warranted to confirm the usefulness of these inflammatory markers to rule out future scar formation.
Consultations
Consider urology consultation if the RUS, VCUG, voiding history, or examination demonstrated concern for significant genitourinary abnormalities, abnormal voiding function or neurogenic bladder (23,32). Consider infectious disease consultation if the patient is not responding to conventional therapy without obstruction, unusual organisms are identified, or the patient is having recurrent urinary tract infections in the presence of normal urological structure and function.
Discharge Criteria and Processes
Consider discharge under the following conditions:
- The patient is comfortable and tolerating oral fluids well.
- The patient has been afebrile or has significantly decreasing fever for 24 hours.
- Appropriate radiological studies and consultations have been completed or arranged for as an outpatient.
- For patients requiring parenterally administered medications at home, long-term IV access must be obtained to assessment of home care service availability, benefits, family home resources, and caregiver education completed.
- Appropriate prophylactic antibiotic prescription has been given to the caregiver with education on use after completion of acute antibiotic therapy. Prophylactic antibiotics should be administered until imaging studies have been completed and assessed.
Conclusion
UTI is a common bacterial infection requiring hospital admission for infants and young children. Admission decisions should take into consideration goals for inpatient care and special age or clinical circumstances. Treatment mode and duration must address avoidance of both acute and chronic complications. Radiologic studies offer both anatomic view and functional information. Clinical relevance of scars, utility of radiologic studies, and value of inflammatory markers are some of the many areas requiring further study.
References
- Long SS, Klein J. Bacterial infections of the urinary tract. In: Remington JSand Klein JO(eds.). Infectious Diseases of the Fetus and Newborn Infant. 5th ed. Philadelphia, Pa: WB Saunders; 2001:1035-46.
- Committee on Quality Improvement, Subcommittee on Urinary Tract Infection. Practice parameter: the diagnosis, treatment, and evaluation of the initial urinary tract infection in febrile infants and young children. Pediatrics. 1999;103:843-52.
- Dayan PS, Bennett J, Best R, et al. Test characteristics of the urine gram stain in infants ≤60 days of age with fever. Pediatr Emerg Care. 2002;18:12-4.
- Huicho L, Campos-Sanchez M, Alamo C. Metaanalysis of urine screening tests for determining the risk of urinary tract infection in children. Pediatr Infect Dis J. 2002;21:1-11.
- Gorelick M, Shaw KN. Screening tests for urinary tract infections in Children: a meta-analysis. Pediatrics. 1999;104:e54.
- Byington CL, Enriquez F, Hoff C, et al. Serious bacterial infections in febrile infants 1 to 90 days old with and without viral infections. Pediatrics. 2004:113:1662-6.
- Baraff L. Management of fever without source in infants and children. Ann Emerg Med. 2000;36:602-14.
- Baraff LJ, Oslund SA, Schriger D, Stephen ML. Probability of bacterial infections in febrile infants less than three months of age: a meta-analysis. Pediatr Infect Dis J. 1992;11:257-64.
- Klein JO. Management of the febrile child without a focus of infection in the era of universal pneumococcal immunization. Pediatr Infect Dis J. 2002;21:584-8.
- Syrogiannopoulos G, Grieva I, Anastassiou E, Triga M, Dimitracopoulos G, Beratis N. Sterile cerebrospinal fluid pleocytosis in young infants with urinary tract infections. Pediatr Infect Dis J. 2001;20:927-30.
- Jaskiewicz JA, Mc Carthy CA, Richardson AC, ET AL. Febrile infants at low risk for serious bacterial infection--an appraisal of the Rochester criteria and implications for management. Febrile Collaborative Study Group. Pediatrics. 1994;94:390-6.
- AAP Redbook. Report of the committee on infectious diseases, 2003:700.
- Newman TB, Bernzweig JA, Takayama JI, Finch SA, Wasserman RC, Pantell RH. Urine testing and urinary tract infections in febrile infants seen in the office setting: the Pediatric Research in Office Settings’ Febrile Infant Study Arch Pediatr Adolesc Med. 2002;156:44-54.
- Ginsberg CM, McCracken GH Jr. Urinary tract infection in young infants. Pediatrics. 1982;69:409-12.
- Wiswell T, Geschke D. Risks from circumcision during the first month of life compared to uncircumcised boys. Pediatrics. 1989;83:1011-15.
- Malik A, Hui C, Pennie RA, Kirpalani H. Beyond the complete blood cell count and C-reactive protein: a systematic review of modern diagnostic tests for neonatal sepsis. Arch Pediatr Adolesc Med. 2003;157:511-6.
- Keren R, Chan E. A meta-analysis of randomized, controlled trials comparing short- and long-course antibiotic therapy for urinary tract infections in children. Pediatrics. 2002;109:e70.
- Michael M, Hodson EM, Craig JC, Martin S, Moyer VA. Short versus standard duration oral antibiotic therapy for acute urinary tract infection in children. Cochrane Database of Syst Rev.2003.
- Hoberman A, Wald ER, Hickey RW, et al. Oral versus intravenous therapy for urinary tract infections in young children. Pediatrics. 1999;104:79-86.
- Bachur R. Nonresponders: prolonged fever among infants with urinary tract infections. Pediatrics. 2000;105:E59.
- Hoberman A, Charron M, Hickey RW, Baskin M, Kearney DH, Wald ER. Imaging studies after a first febrile urinary tract infection in young children. N Engl J Med. 2003;348:195-202.
- Zamir G, Sakran W, Horowitz Y, Koren A, Miron D. Urinary tract infection: is there a need for routine renal ultrasonography? Arch Dis Child. 2004;89:466-8.
- Johnson CE. New advances in childhood urinary tract infections. Pediatr Rev. 1999:20:335-43.
- Thompson M, Simon S, Sharma V, Alon US. Timing of follow-up voiding cystourethrogram in children with primary vesicoureteral reflux: development and application of a clinical algorithm. Pediatrics. 2005:115:426-34.
- Darge K, Moeller RT, Trusen A, BuĴer F, Gordjani N, Riedmiller H. Diagnosis of vesicoureteral reflux with low-dose contrast-enhanced harmonic ultrasound imaging. Pediatr Radiol. 2005:35:73-8.
- Kraus S. Genitourinary imaging in children. Pediatr Clin North Am. 2001;48:1381-1424.
- Ismaili K, Avni F, Hall M; Brussels Free University Perinatal Nephrology (BFUPN) Study Group. Results of systematic voiding cystourethrography in infants with antenatally diagnosed renal pelvis dilation. J Pediatr. 2002;141: 21-4.
- Mahant S, To T, Friedman J. Timing of voiding cystourethrogram in the investigation of urinary tract infections in children. J Pediatr. 2001;39:568-71.
- McDonald A, Scranton M, Gillespie R, Mahajan V, Edwards GA. Voiding cystourethrograms and urinary tract infections: how long to wait? Pediatrics. 2000:105:E50.
- Williams G, Lee A, Craig J. Antibiotics for the prevention of urinary tract infection in children: a systematic review of randomized controlled trials. J Pediatr. 2001;138:868-74.
- Prat C, Dominguez J, Rodrigo C, et al. Elevated serum procalcitonin values correlate with renal scarring in children with urinary tract infection. Pediatr Infect Dis J. 2003;22:438-42.
- Roberts KB. Urinary tract infection treatment and evaluation update. Pediatr Infect Dis J. 2004: 23:1163-4.