User login
A Fifth of American Adults Are at Risk for Gout
ATLANTA – An estimated 32 million adults in the United States have hyperuricemia, which often precedes gout, based on data from the National Health and Nutrition Examination Survey.
The results were presented during a press conference at the meeting.
Previous studies have suggested that the prevalence of gout and hyperuricemia are on the rise in the United States, possibly because of factors including obesity, the metabolic syndrome, and hypertension, said Yanyan Zhu, Ph.D., of Boston University.
Dr. Zhu and colleagues reviewed National Health and Nutrition Examination Survey (NHANES) data from 1999 through 2008 on 24,693 individuals who were aged 20 years and older. The group included 11,816 men and 12,877 women. The data were compared with U.S. population estimates from the U.S. Census Bureau.
Hyperuricemia was defined using the standard NHANES definition of serum urate levels greater than 7.0 mg/dL for men, and greater than 5.7 mg/dL for women.
The results suggest a substantial potential burden from gout, especially in older adults, said Dr. Zhu.
The prevalence of hyperuricemia was 31% in adults who were aged 65 years and older, vs. 18% in those aged 20-64 years.
Overall, the prevalence of hyperuricemia increased with age, ranging from 16% in individuals who were aged 20-29 years to 37% among those aged 80 years and older.
The estimates for hyperuricemia were similar for men and women (16.1 million vs. 15.8 million, respectively).
Gout rates in U.S. adults are rising, based on data from a related study that was also presented at the meeting.
Dr. Zhu and her colleagues used NHANES data to estimate 8.3 million cases of gout in U.S. adults who were aged 20 years and older during 2007-2008.
In this study, the researchers compared NHANES data from 1988 through 1994 with NHANES data from 2007 through 2008.
They found a 1.2% increase in gout among U.S. adults, from 2.7% during 1988-1994 to 3.9% during 2007-2008.
The increase was largely the result of the significant rise in gout among men and older adults, the researchers noted.
The prevalence of gout in men increased from 3.8% to 5.9% between the two time periods, and the prevalence in adults who were aged 80 years and older increased from 5.9% to 12.6%.
The NHANES data in the second study included 18,825 individuals from 1988 through 1994 and 5,707 from 2007 through 2008. These numbers also were compared with U.S. Census Bureau data.
Most physicians in the United States do not regularly check patients' uric acid levels, and fewer than 5% of adults with gout receive treatment, noted Dr. John Sundy of Duke University Medical Center in Durham, N.C.
Dr. Sundy served as moderator when the study findings were presented at the press conference.
More education is needed for doctors and patients so the available therapies can be used more effectively, he said.
Dr. Zhu said she had no financial conflicts to disclose.
Her coauthors on the study are employed by or have received consulting fees from Takeda Pharmaceuticals International.
Dr. Sundy has served as a consultant for multiple companies including Array Biopharma, Savient Pharmaceuticals, and Takeda Pharmaceuticals.
ATLANTA – An estimated 32 million adults in the United States have hyperuricemia, which often precedes gout, based on data from the National Health and Nutrition Examination Survey.
The results were presented during a press conference at the meeting.
Previous studies have suggested that the prevalence of gout and hyperuricemia are on the rise in the United States, possibly because of factors including obesity, the metabolic syndrome, and hypertension, said Yanyan Zhu, Ph.D., of Boston University.
Dr. Zhu and colleagues reviewed National Health and Nutrition Examination Survey (NHANES) data from 1999 through 2008 on 24,693 individuals who were aged 20 years and older. The group included 11,816 men and 12,877 women. The data were compared with U.S. population estimates from the U.S. Census Bureau.
Hyperuricemia was defined using the standard NHANES definition of serum urate levels greater than 7.0 mg/dL for men, and greater than 5.7 mg/dL for women.
The results suggest a substantial potential burden from gout, especially in older adults, said Dr. Zhu.
The prevalence of hyperuricemia was 31% in adults who were aged 65 years and older, vs. 18% in those aged 20-64 years.
Overall, the prevalence of hyperuricemia increased with age, ranging from 16% in individuals who were aged 20-29 years to 37% among those aged 80 years and older.
The estimates for hyperuricemia were similar for men and women (16.1 million vs. 15.8 million, respectively).
Gout rates in U.S. adults are rising, based on data from a related study that was also presented at the meeting.
Dr. Zhu and her colleagues used NHANES data to estimate 8.3 million cases of gout in U.S. adults who were aged 20 years and older during 2007-2008.
In this study, the researchers compared NHANES data from 1988 through 1994 with NHANES data from 2007 through 2008.
They found a 1.2% increase in gout among U.S. adults, from 2.7% during 1988-1994 to 3.9% during 2007-2008.
The increase was largely the result of the significant rise in gout among men and older adults, the researchers noted.
The prevalence of gout in men increased from 3.8% to 5.9% between the two time periods, and the prevalence in adults who were aged 80 years and older increased from 5.9% to 12.6%.
The NHANES data in the second study included 18,825 individuals from 1988 through 1994 and 5,707 from 2007 through 2008. These numbers also were compared with U.S. Census Bureau data.
Most physicians in the United States do not regularly check patients' uric acid levels, and fewer than 5% of adults with gout receive treatment, noted Dr. John Sundy of Duke University Medical Center in Durham, N.C.
Dr. Sundy served as moderator when the study findings were presented at the press conference.
More education is needed for doctors and patients so the available therapies can be used more effectively, he said.
Dr. Zhu said she had no financial conflicts to disclose.
Her coauthors on the study are employed by or have received consulting fees from Takeda Pharmaceuticals International.
Dr. Sundy has served as a consultant for multiple companies including Array Biopharma, Savient Pharmaceuticals, and Takeda Pharmaceuticals.
ATLANTA – An estimated 32 million adults in the United States have hyperuricemia, which often precedes gout, based on data from the National Health and Nutrition Examination Survey.
The results were presented during a press conference at the meeting.
Previous studies have suggested that the prevalence of gout and hyperuricemia are on the rise in the United States, possibly because of factors including obesity, the metabolic syndrome, and hypertension, said Yanyan Zhu, Ph.D., of Boston University.
Dr. Zhu and colleagues reviewed National Health and Nutrition Examination Survey (NHANES) data from 1999 through 2008 on 24,693 individuals who were aged 20 years and older. The group included 11,816 men and 12,877 women. The data were compared with U.S. population estimates from the U.S. Census Bureau.
Hyperuricemia was defined using the standard NHANES definition of serum urate levels greater than 7.0 mg/dL for men, and greater than 5.7 mg/dL for women.
The results suggest a substantial potential burden from gout, especially in older adults, said Dr. Zhu.
The prevalence of hyperuricemia was 31% in adults who were aged 65 years and older, vs. 18% in those aged 20-64 years.
Overall, the prevalence of hyperuricemia increased with age, ranging from 16% in individuals who were aged 20-29 years to 37% among those aged 80 years and older.
The estimates for hyperuricemia were similar for men and women (16.1 million vs. 15.8 million, respectively).
Gout rates in U.S. adults are rising, based on data from a related study that was also presented at the meeting.
Dr. Zhu and her colleagues used NHANES data to estimate 8.3 million cases of gout in U.S. adults who were aged 20 years and older during 2007-2008.
In this study, the researchers compared NHANES data from 1988 through 1994 with NHANES data from 2007 through 2008.
They found a 1.2% increase in gout among U.S. adults, from 2.7% during 1988-1994 to 3.9% during 2007-2008.
The increase was largely the result of the significant rise in gout among men and older adults, the researchers noted.
The prevalence of gout in men increased from 3.8% to 5.9% between the two time periods, and the prevalence in adults who were aged 80 years and older increased from 5.9% to 12.6%.
The NHANES data in the second study included 18,825 individuals from 1988 through 1994 and 5,707 from 2007 through 2008. These numbers also were compared with U.S. Census Bureau data.
Most physicians in the United States do not regularly check patients' uric acid levels, and fewer than 5% of adults with gout receive treatment, noted Dr. John Sundy of Duke University Medical Center in Durham, N.C.
Dr. Sundy served as moderator when the study findings were presented at the press conference.
More education is needed for doctors and patients so the available therapies can be used more effectively, he said.
Dr. Zhu said she had no financial conflicts to disclose.
Her coauthors on the study are employed by or have received consulting fees from Takeda Pharmaceuticals International.
Dr. Sundy has served as a consultant for multiple companies including Array Biopharma, Savient Pharmaceuticals, and Takeda Pharmaceuticals.
Spondyloarthritis Seen in 20% With Chronic Back Pain
ATLANTA – Approximately 20% of cases of chronic low back pain in younger adults seen in primary care settings might be caused by spondyloarthritis, based on data from 364 patients aged 19-45 years. The findings were presented at the annual meeting of the American College of Rheumatology.
In the cross-sectional study, 77 of 364 patients (22%) met the diagnosis of axial spondyloarthritis on examination by a rheumatologist. The average age of the patients was 36 years, 43% were male, and the average duration of chronic low back pain was 9 years.
The diagnostic techniques included a detailed patient questionnaire about inflammatory back pain; physical examination and patient history; blood tests to assess C-reactive protein levels and the presence of HLA-B27 (a gene that has been linked to spondyloarthritis); and conventional and MRI images of sacroiliac joints. Two radiologists reviewed the images for the signs of inflammation and bone lesions that might indicate axial spondyloarthritis.
In all, 52 patients were diagnosed according to MRI criteria plus one additional spondyloarthritis feature. The other 12 patients were diagnosed according to a positive HLA-B27 test plus two additional spondyloarthritis features, Dr. Angelique Weel of Maasstad Ziekenhuis in Rotterdam, the Netherlands, said at press conference.
In addition, 24 patients (6.6%) met the criteria for ankylosing spondylitis.
The results suggest that spondyloarthritis is underdiagnosed as a cause of chronic back pain in the general population. Dr. Weel recommended that primary care physicians suspect spondyloarthritis when they see younger adults with chronic back pain, and refer these patients to a rheumatologist if they suspect an inflammatory basis for the pain.
“We also tried to make a simple questionnaire for general practitioners, so they can determine which patient with chronic low back pain should be sent to a rheumatologist to investigate possible spondyloarthritis,” she noted. Possible red flags from the standardized questionnaire include the response to NSAIDs and a family history of spondyloarthritis, Dr. Weel said.
“Of course, we need to validate these data in another population, and we also have to validate our referral tool [for general physicians],” she noted.
Dr. Weel said that she had no financial conflicts to disclose.
Dr. Angelique Weel suggested that spondyloarthritis might be underdiagnosed in younger adults who have chronic back pain.
Source Heidi Splete/Elsevier Global Medical News
ATLANTA – Approximately 20% of cases of chronic low back pain in younger adults seen in primary care settings might be caused by spondyloarthritis, based on data from 364 patients aged 19-45 years. The findings were presented at the annual meeting of the American College of Rheumatology.
In the cross-sectional study, 77 of 364 patients (22%) met the diagnosis of axial spondyloarthritis on examination by a rheumatologist. The average age of the patients was 36 years, 43% were male, and the average duration of chronic low back pain was 9 years.
The diagnostic techniques included a detailed patient questionnaire about inflammatory back pain; physical examination and patient history; blood tests to assess C-reactive protein levels and the presence of HLA-B27 (a gene that has been linked to spondyloarthritis); and conventional and MRI images of sacroiliac joints. Two radiologists reviewed the images for the signs of inflammation and bone lesions that might indicate axial spondyloarthritis.
In all, 52 patients were diagnosed according to MRI criteria plus one additional spondyloarthritis feature. The other 12 patients were diagnosed according to a positive HLA-B27 test plus two additional spondyloarthritis features, Dr. Angelique Weel of Maasstad Ziekenhuis in Rotterdam, the Netherlands, said at press conference.
In addition, 24 patients (6.6%) met the criteria for ankylosing spondylitis.
The results suggest that spondyloarthritis is underdiagnosed as a cause of chronic back pain in the general population. Dr. Weel recommended that primary care physicians suspect spondyloarthritis when they see younger adults with chronic back pain, and refer these patients to a rheumatologist if they suspect an inflammatory basis for the pain.
“We also tried to make a simple questionnaire for general practitioners, so they can determine which patient with chronic low back pain should be sent to a rheumatologist to investigate possible spondyloarthritis,” she noted. Possible red flags from the standardized questionnaire include the response to NSAIDs and a family history of spondyloarthritis, Dr. Weel said.
“Of course, we need to validate these data in another population, and we also have to validate our referral tool [for general physicians],” she noted.
Dr. Weel said that she had no financial conflicts to disclose.
Dr. Angelique Weel suggested that spondyloarthritis might be underdiagnosed in younger adults who have chronic back pain.
Source Heidi Splete/Elsevier Global Medical News
ATLANTA – Approximately 20% of cases of chronic low back pain in younger adults seen in primary care settings might be caused by spondyloarthritis, based on data from 364 patients aged 19-45 years. The findings were presented at the annual meeting of the American College of Rheumatology.
In the cross-sectional study, 77 of 364 patients (22%) met the diagnosis of axial spondyloarthritis on examination by a rheumatologist. The average age of the patients was 36 years, 43% were male, and the average duration of chronic low back pain was 9 years.
The diagnostic techniques included a detailed patient questionnaire about inflammatory back pain; physical examination and patient history; blood tests to assess C-reactive protein levels and the presence of HLA-B27 (a gene that has been linked to spondyloarthritis); and conventional and MRI images of sacroiliac joints. Two radiologists reviewed the images for the signs of inflammation and bone lesions that might indicate axial spondyloarthritis.
In all, 52 patients were diagnosed according to MRI criteria plus one additional spondyloarthritis feature. The other 12 patients were diagnosed according to a positive HLA-B27 test plus two additional spondyloarthritis features, Dr. Angelique Weel of Maasstad Ziekenhuis in Rotterdam, the Netherlands, said at press conference.
In addition, 24 patients (6.6%) met the criteria for ankylosing spondylitis.
The results suggest that spondyloarthritis is underdiagnosed as a cause of chronic back pain in the general population. Dr. Weel recommended that primary care physicians suspect spondyloarthritis when they see younger adults with chronic back pain, and refer these patients to a rheumatologist if they suspect an inflammatory basis for the pain.
“We also tried to make a simple questionnaire for general practitioners, so they can determine which patient with chronic low back pain should be sent to a rheumatologist to investigate possible spondyloarthritis,” she noted. Possible red flags from the standardized questionnaire include the response to NSAIDs and a family history of spondyloarthritis, Dr. Weel said.
“Of course, we need to validate these data in another population, and we also have to validate our referral tool [for general physicians],” she noted.
Dr. Weel said that she had no financial conflicts to disclose.
Dr. Angelique Weel suggested that spondyloarthritis might be underdiagnosed in younger adults who have chronic back pain.
Source Heidi Splete/Elsevier Global Medical News
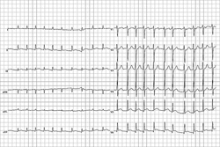
Atrial Fibrillation Research Spurs Updates to Treatment Guidelines
Strict heart rate control has no benefit over more lenient control in patients with atrial fibrillation, experts stated in an update of treatment guidelines.
The previous guidelines advised keeping the heart rate of an atrial fibrillation patient at less than 80 beats/min at rest and less than 110 beats/min during a 6-minute walk. The updated guidelines advise keeping a resting heart rate of less than 110 beats/min in patients with persistent atrial fibrillation who also have stable ventricular function and have no symptoms, or symptoms deemed acceptable, related to their arrhythmia.
The 2011 Focused Update on the Management of Patients With Atrial Fibrillation (Updating the 2006 Guideline) is a joint effort of the American College of Cardiology Foundation, the American Heart Association, and the Heart Rhythm Society. The guideline writing committee reviewed data from late-breaking clinical trials presented at scientific sessions of the AHA, ACC, and European Society of Cardiology in 2009, and other data published through April 2010 (Circulation 2010 Dec. 20 [doi:10.1161/CIR.0b013e3181fa3cf4]; Circulation 2011;123:104-23).
Another key update is the recommendation that a combination of clopidogrel and aspirin might be an option for atrial fibrillation patients who are poor candidates for warfarin. The recommendation is based on recent studies including the ACTIVE-A trial (Effect of Clopidogrel Added to Aspirin in Patients with Atrial Fibrillation). In this study, significantly fewer major vascular events occurred in patients randomized to receive clopidogrel plus aspirin, compared with those who received aspirin plus placebo.
“The clopidogrel/aspirin option is going to be of limited utility in most cases,” commented Dr. Anne B. Curtis, who was a member of the writing group, in an interview. The new thrombin inhibitors such as dabigatran will be used instead, she said. Because dabigatran was not approved by the Food and Drug Administration when the committee was making its decisions, it was not included in the guidelines, according to the report.
The updated guidelines also suggest the use of dronedarone for some atrial fibrillation patients to reduce hospitalizations for cardiovascular events.
Recent studies such as the DIONYSOS study (Efficacy and Safety of Dronedarone Versus Amiodarone for the Maintenance of Sinus Rhythm in Patients With Persistent Atrial Fibrillation) showed that dronedarone was less effective than amiodarone for reducing the recurrence of atrial fibrillation. However, in a placebo-controlled, double-blind, parallel-arm trial to assess the efficacy of dronedarone 400 mg b.i.d. for the prevention of cardiovascular hospitalization or death from any cause in patients with atrial fibrillation/atrial flutter (the ATHENA trial, dronedarone reduced the “the combined end point of death and cardiovascular hospitalizations, largely by reducing hospitalizations related to atrial fibrillation,” the reviewers wrote. However, dronedarone should not be given to patients with NYHA class IV heart failure or patients with decompensated heart failure within the past 4 weeks, they noted.
To maintain normal sinus rhythm, the updated guidelines support the use of catheter ablation based on data from more than 6,900 patients. In one multicenter study (the ThermoCool trial), symptomatic patients with paroxysmal atrial fibrillation who were treated with catheter ablation showed significant improvement after 3 months, compared with control patients. Currently, catheter ablation treatment is recommended for atrial fibrillation patients without severe lung disease who have not had success with drug therapy.
Dr. L. Samuel Wann, a cardiologist at Wheaton Franciscan Healthcare in Wauwatosa, Wis., served as chair of the 2011 Writing Group Committee. Dr. Wann and his colleagues wrote that the 2006 full-text guidelines for the management of atrial fibrillation that are not mentioned in the 2011 update remain unchanged at this time. “The guidelines attempt to define practices that meet the needs of most patients in most circumstances,” the reviewers said.
“The last time the guidelines were published was in 2006, and there have been several new drugs and treatments that have been developed since that time. Physicians want to know where they stand in terms of the hierarchy of treatment of patients with atrial fibrillation,” explained Dr. Curtis, chair of the department of medicine at the University of Buffalo, N.Y. “In order to be timely, we did a limited, focused update,” she said.
The clinical implications of more lenient heart rate control are unclear at this time, Dr. Curtis said. In the Rate Control Efficacy in Permanent Atrial Fibrillation (RACE II) study, on which the recommendations were based, the difference in heart rate control between lenient control and strict control groups was limited, she noted.
But the study emphasized the need for clinicians to be careful about going too far in trying to control patients' heart rates, Dr. Curtis said, because too much control could lead to bradycardia and unnecessary pacemaker use.
As for additional research, “We need to know how to prevent [atrial fibrillation] in the first place, and we need to understand thoroughly the long-term outcomes of ablation therapy,” Dr. Curtis said.
Dr. Wann had no financial conflicts to disclose. Several of the writing group members, including Dr. Curtis, disclosed serving as a speaker or consultant, or receiving research funding from multiple pharmaceutical companies including Medtronic, Boston Scientific, AstraZeneca, Sanofi-Aventis, and GlaxoSmithKline.
Strict heart rate control has no benefit over more lenient control in patients with atrial fibrillation, experts stated in an update of treatment guidelines.
The previous guidelines advised keeping the heart rate of an atrial fibrillation patient at less than 80 beats/min at rest and less than 110 beats/min during a 6-minute walk. The updated guidelines advise keeping a resting heart rate of less than 110 beats/min in patients with persistent atrial fibrillation who also have stable ventricular function and have no symptoms, or symptoms deemed acceptable, related to their arrhythmia.
The 2011 Focused Update on the Management of Patients With Atrial Fibrillation (Updating the 2006 Guideline) is a joint effort of the American College of Cardiology Foundation, the American Heart Association, and the Heart Rhythm Society. The guideline writing committee reviewed data from late-breaking clinical trials presented at scientific sessions of the AHA, ACC, and European Society of Cardiology in 2009, and other data published through April 2010 (Circulation 2010 Dec. 20 [doi:10.1161/CIR.0b013e3181fa3cf4]; Circulation 2011;123:104-23).
Another key update is the recommendation that a combination of clopidogrel and aspirin might be an option for atrial fibrillation patients who are poor candidates for warfarin. The recommendation is based on recent studies including the ACTIVE-A trial (Effect of Clopidogrel Added to Aspirin in Patients with Atrial Fibrillation). In this study, significantly fewer major vascular events occurred in patients randomized to receive clopidogrel plus aspirin, compared with those who received aspirin plus placebo.
“The clopidogrel/aspirin option is going to be of limited utility in most cases,” commented Dr. Anne B. Curtis, who was a member of the writing group, in an interview. The new thrombin inhibitors such as dabigatran will be used instead, she said. Because dabigatran was not approved by the Food and Drug Administration when the committee was making its decisions, it was not included in the guidelines, according to the report.
The updated guidelines also suggest the use of dronedarone for some atrial fibrillation patients to reduce hospitalizations for cardiovascular events.
Recent studies such as the DIONYSOS study (Efficacy and Safety of Dronedarone Versus Amiodarone for the Maintenance of Sinus Rhythm in Patients With Persistent Atrial Fibrillation) showed that dronedarone was less effective than amiodarone for reducing the recurrence of atrial fibrillation. However, in a placebo-controlled, double-blind, parallel-arm trial to assess the efficacy of dronedarone 400 mg b.i.d. for the prevention of cardiovascular hospitalization or death from any cause in patients with atrial fibrillation/atrial flutter (the ATHENA trial, dronedarone reduced the “the combined end point of death and cardiovascular hospitalizations, largely by reducing hospitalizations related to atrial fibrillation,” the reviewers wrote. However, dronedarone should not be given to patients with NYHA class IV heart failure or patients with decompensated heart failure within the past 4 weeks, they noted.
To maintain normal sinus rhythm, the updated guidelines support the use of catheter ablation based on data from more than 6,900 patients. In one multicenter study (the ThermoCool trial), symptomatic patients with paroxysmal atrial fibrillation who were treated with catheter ablation showed significant improvement after 3 months, compared with control patients. Currently, catheter ablation treatment is recommended for atrial fibrillation patients without severe lung disease who have not had success with drug therapy.
Dr. L. Samuel Wann, a cardiologist at Wheaton Franciscan Healthcare in Wauwatosa, Wis., served as chair of the 2011 Writing Group Committee. Dr. Wann and his colleagues wrote that the 2006 full-text guidelines for the management of atrial fibrillation that are not mentioned in the 2011 update remain unchanged at this time. “The guidelines attempt to define practices that meet the needs of most patients in most circumstances,” the reviewers said.
“The last time the guidelines were published was in 2006, and there have been several new drugs and treatments that have been developed since that time. Physicians want to know where they stand in terms of the hierarchy of treatment of patients with atrial fibrillation,” explained Dr. Curtis, chair of the department of medicine at the University of Buffalo, N.Y. “In order to be timely, we did a limited, focused update,” she said.
The clinical implications of more lenient heart rate control are unclear at this time, Dr. Curtis said. In the Rate Control Efficacy in Permanent Atrial Fibrillation (RACE II) study, on which the recommendations were based, the difference in heart rate control between lenient control and strict control groups was limited, she noted.
But the study emphasized the need for clinicians to be careful about going too far in trying to control patients' heart rates, Dr. Curtis said, because too much control could lead to bradycardia and unnecessary pacemaker use.
As for additional research, “We need to know how to prevent [atrial fibrillation] in the first place, and we need to understand thoroughly the long-term outcomes of ablation therapy,” Dr. Curtis said.
Dr. Wann had no financial conflicts to disclose. Several of the writing group members, including Dr. Curtis, disclosed serving as a speaker or consultant, or receiving research funding from multiple pharmaceutical companies including Medtronic, Boston Scientific, AstraZeneca, Sanofi-Aventis, and GlaxoSmithKline.
Strict heart rate control has no benefit over more lenient control in patients with atrial fibrillation, experts stated in an update of treatment guidelines.
The previous guidelines advised keeping the heart rate of an atrial fibrillation patient at less than 80 beats/min at rest and less than 110 beats/min during a 6-minute walk. The updated guidelines advise keeping a resting heart rate of less than 110 beats/min in patients with persistent atrial fibrillation who also have stable ventricular function and have no symptoms, or symptoms deemed acceptable, related to their arrhythmia.
The 2011 Focused Update on the Management of Patients With Atrial Fibrillation (Updating the 2006 Guideline) is a joint effort of the American College of Cardiology Foundation, the American Heart Association, and the Heart Rhythm Society. The guideline writing committee reviewed data from late-breaking clinical trials presented at scientific sessions of the AHA, ACC, and European Society of Cardiology in 2009, and other data published through April 2010 (Circulation 2010 Dec. 20 [doi:10.1161/CIR.0b013e3181fa3cf4]; Circulation 2011;123:104-23).
Another key update is the recommendation that a combination of clopidogrel and aspirin might be an option for atrial fibrillation patients who are poor candidates for warfarin. The recommendation is based on recent studies including the ACTIVE-A trial (Effect of Clopidogrel Added to Aspirin in Patients with Atrial Fibrillation). In this study, significantly fewer major vascular events occurred in patients randomized to receive clopidogrel plus aspirin, compared with those who received aspirin plus placebo.
“The clopidogrel/aspirin option is going to be of limited utility in most cases,” commented Dr. Anne B. Curtis, who was a member of the writing group, in an interview. The new thrombin inhibitors such as dabigatran will be used instead, she said. Because dabigatran was not approved by the Food and Drug Administration when the committee was making its decisions, it was not included in the guidelines, according to the report.
The updated guidelines also suggest the use of dronedarone for some atrial fibrillation patients to reduce hospitalizations for cardiovascular events.
Recent studies such as the DIONYSOS study (Efficacy and Safety of Dronedarone Versus Amiodarone for the Maintenance of Sinus Rhythm in Patients With Persistent Atrial Fibrillation) showed that dronedarone was less effective than amiodarone for reducing the recurrence of atrial fibrillation. However, in a placebo-controlled, double-blind, parallel-arm trial to assess the efficacy of dronedarone 400 mg b.i.d. for the prevention of cardiovascular hospitalization or death from any cause in patients with atrial fibrillation/atrial flutter (the ATHENA trial, dronedarone reduced the “the combined end point of death and cardiovascular hospitalizations, largely by reducing hospitalizations related to atrial fibrillation,” the reviewers wrote. However, dronedarone should not be given to patients with NYHA class IV heart failure or patients with decompensated heart failure within the past 4 weeks, they noted.
To maintain normal sinus rhythm, the updated guidelines support the use of catheter ablation based on data from more than 6,900 patients. In one multicenter study (the ThermoCool trial), symptomatic patients with paroxysmal atrial fibrillation who were treated with catheter ablation showed significant improvement after 3 months, compared with control patients. Currently, catheter ablation treatment is recommended for atrial fibrillation patients without severe lung disease who have not had success with drug therapy.
Dr. L. Samuel Wann, a cardiologist at Wheaton Franciscan Healthcare in Wauwatosa, Wis., served as chair of the 2011 Writing Group Committee. Dr. Wann and his colleagues wrote that the 2006 full-text guidelines for the management of atrial fibrillation that are not mentioned in the 2011 update remain unchanged at this time. “The guidelines attempt to define practices that meet the needs of most patients in most circumstances,” the reviewers said.
“The last time the guidelines were published was in 2006, and there have been several new drugs and treatments that have been developed since that time. Physicians want to know where they stand in terms of the hierarchy of treatment of patients with atrial fibrillation,” explained Dr. Curtis, chair of the department of medicine at the University of Buffalo, N.Y. “In order to be timely, we did a limited, focused update,” she said.
The clinical implications of more lenient heart rate control are unclear at this time, Dr. Curtis said. In the Rate Control Efficacy in Permanent Atrial Fibrillation (RACE II) study, on which the recommendations were based, the difference in heart rate control between lenient control and strict control groups was limited, she noted.
But the study emphasized the need for clinicians to be careful about going too far in trying to control patients' heart rates, Dr. Curtis said, because too much control could lead to bradycardia and unnecessary pacemaker use.
As for additional research, “We need to know how to prevent [atrial fibrillation] in the first place, and we need to understand thoroughly the long-term outcomes of ablation therapy,” Dr. Curtis said.
Dr. Wann had no financial conflicts to disclose. Several of the writing group members, including Dr. Curtis, disclosed serving as a speaker or consultant, or receiving research funding from multiple pharmaceutical companies including Medtronic, Boston Scientific, AstraZeneca, Sanofi-Aventis, and GlaxoSmithKline.
Survey: Daily Marijuana Use on Rise in Teens
WASHINGTON – The percentage of American teenagers reporting daily marijuana use increased significantly from 2009 to 2010, according to findings from the 2010 Monitoring the Future survey. The results were presented at a press conference Dec. 14.
"We are seeing a decrease in the number of teenagers perceiving marijuana use as harmful," said Dr. Nora D. Volkow, director of the National Institute on Drug Abuse. The survey is conducted for NIDA by the University of Michigan, Ann Arbor.
The percentage of 8th, 10th, and 12th graders reporting daily marijuana use increased from 1.0% to 1.2%, 2.8% to 3.3%, and 5.2% to 6.1%, respectively. In keeping with a gradual increase in use over the past 3 years, an increase was seen in all three grade levels measured in the survey. However, past-year and past-month reported use increased among 8th graders only.
In addition, Ecstasy appears to be gaining popularity after almost a decade of decline, said Lloyd D. Johnston, Ph.D., a research scientist at the University of Michigan and the principal investigator on the Monitoring the Future survey. In 2010, 2.4% of 8th graders and 4.7% of 10th graders reported past-year Ecstasy use, up from 1.3% and 3.7%, respectively, in the 2009 survey.
The decline in cigarette smoking appears to have stalled across all three grade levels, and nonmedical use of many prescription drugs, including OxyContin and Adderall, remained similar to last year’s levels among 12th graders.
On the positive side, past-year reports of alcohol consumption among 12th graders declined, from 43.5% to 41.2%, and binge drinking dropped from 25.2% to 23.2%, Dr. Johnston said. In addition, non-medical use of the prescription painkiller Vicodin decreased among 12th graders, he noted.
The changes in drug, cigarette, and alcohol use reflect changes in teens’ perceptions of the risks associated with these products, Dr. Volkow said. The 2010 results show a decline in the numbers of 8th graders who disapproved of marijuana use, and declines in the numbers of 10th and 12th graders who said they perceived a "great risk" of harm associated with smoking marijuana regularly. The mixed messages about the legalization of marijuana and its use as medicine might be contributing to the trend, Dr. Volkow noted.
It is important for doctors who treat teenagers to identify psychiatric problems, such as depression or anxiety, which could lead to the abuse of drugs if left untreated, Dr. Volkow said. In addition, physicians are in a unique position to help parents and teenagers understand the dangers of drugs, alcohol, and cigarette use, because they are neutral third parties, Dr. Johnston said. "I suspect that their advice would carry weight [with teens]," he said.
Monitoring the Future is an ongoing study of the opinions and beliefs of American adolescents. The 2010 survey included approximately 46,000 students in grades 8, 10, and 12. Monitoring the Future is funded by the National Institutes of Health. More detailed study results are available online.
WASHINGTON – The percentage of American teenagers reporting daily marijuana use increased significantly from 2009 to 2010, according to findings from the 2010 Monitoring the Future survey. The results were presented at a press conference Dec. 14.
"We are seeing a decrease in the number of teenagers perceiving marijuana use as harmful," said Dr. Nora D. Volkow, director of the National Institute on Drug Abuse. The survey is conducted for NIDA by the University of Michigan, Ann Arbor.
The percentage of 8th, 10th, and 12th graders reporting daily marijuana use increased from 1.0% to 1.2%, 2.8% to 3.3%, and 5.2% to 6.1%, respectively. In keeping with a gradual increase in use over the past 3 years, an increase was seen in all three grade levels measured in the survey. However, past-year and past-month reported use increased among 8th graders only.
In addition, Ecstasy appears to be gaining popularity after almost a decade of decline, said Lloyd D. Johnston, Ph.D., a research scientist at the University of Michigan and the principal investigator on the Monitoring the Future survey. In 2010, 2.4% of 8th graders and 4.7% of 10th graders reported past-year Ecstasy use, up from 1.3% and 3.7%, respectively, in the 2009 survey.
The decline in cigarette smoking appears to have stalled across all three grade levels, and nonmedical use of many prescription drugs, including OxyContin and Adderall, remained similar to last year’s levels among 12th graders.
On the positive side, past-year reports of alcohol consumption among 12th graders declined, from 43.5% to 41.2%, and binge drinking dropped from 25.2% to 23.2%, Dr. Johnston said. In addition, non-medical use of the prescription painkiller Vicodin decreased among 12th graders, he noted.
The changes in drug, cigarette, and alcohol use reflect changes in teens’ perceptions of the risks associated with these products, Dr. Volkow said. The 2010 results show a decline in the numbers of 8th graders who disapproved of marijuana use, and declines in the numbers of 10th and 12th graders who said they perceived a "great risk" of harm associated with smoking marijuana regularly. The mixed messages about the legalization of marijuana and its use as medicine might be contributing to the trend, Dr. Volkow noted.
It is important for doctors who treat teenagers to identify psychiatric problems, such as depression or anxiety, which could lead to the abuse of drugs if left untreated, Dr. Volkow said. In addition, physicians are in a unique position to help parents and teenagers understand the dangers of drugs, alcohol, and cigarette use, because they are neutral third parties, Dr. Johnston said. "I suspect that their advice would carry weight [with teens]," he said.
Monitoring the Future is an ongoing study of the opinions and beliefs of American adolescents. The 2010 survey included approximately 46,000 students in grades 8, 10, and 12. Monitoring the Future is funded by the National Institutes of Health. More detailed study results are available online.
WASHINGTON – The percentage of American teenagers reporting daily marijuana use increased significantly from 2009 to 2010, according to findings from the 2010 Monitoring the Future survey. The results were presented at a press conference Dec. 14.
"We are seeing a decrease in the number of teenagers perceiving marijuana use as harmful," said Dr. Nora D. Volkow, director of the National Institute on Drug Abuse. The survey is conducted for NIDA by the University of Michigan, Ann Arbor.
The percentage of 8th, 10th, and 12th graders reporting daily marijuana use increased from 1.0% to 1.2%, 2.8% to 3.3%, and 5.2% to 6.1%, respectively. In keeping with a gradual increase in use over the past 3 years, an increase was seen in all three grade levels measured in the survey. However, past-year and past-month reported use increased among 8th graders only.
In addition, Ecstasy appears to be gaining popularity after almost a decade of decline, said Lloyd D. Johnston, Ph.D., a research scientist at the University of Michigan and the principal investigator on the Monitoring the Future survey. In 2010, 2.4% of 8th graders and 4.7% of 10th graders reported past-year Ecstasy use, up from 1.3% and 3.7%, respectively, in the 2009 survey.
The decline in cigarette smoking appears to have stalled across all three grade levels, and nonmedical use of many prescription drugs, including OxyContin and Adderall, remained similar to last year’s levels among 12th graders.
On the positive side, past-year reports of alcohol consumption among 12th graders declined, from 43.5% to 41.2%, and binge drinking dropped from 25.2% to 23.2%, Dr. Johnston said. In addition, non-medical use of the prescription painkiller Vicodin decreased among 12th graders, he noted.
The changes in drug, cigarette, and alcohol use reflect changes in teens’ perceptions of the risks associated with these products, Dr. Volkow said. The 2010 results show a decline in the numbers of 8th graders who disapproved of marijuana use, and declines in the numbers of 10th and 12th graders who said they perceived a "great risk" of harm associated with smoking marijuana regularly. The mixed messages about the legalization of marijuana and its use as medicine might be contributing to the trend, Dr. Volkow noted.
It is important for doctors who treat teenagers to identify psychiatric problems, such as depression or anxiety, which could lead to the abuse of drugs if left untreated, Dr. Volkow said. In addition, physicians are in a unique position to help parents and teenagers understand the dangers of drugs, alcohol, and cigarette use, because they are neutral third parties, Dr. Johnston said. "I suspect that their advice would carry weight [with teens]," he said.
Monitoring the Future is an ongoing study of the opinions and beliefs of American adolescents. The 2010 survey included approximately 46,000 students in grades 8, 10, and 12. Monitoring the Future is funded by the National Institutes of Health. More detailed study results are available online.
FROM 2010 MONITORING THE FUTURE SURVEY
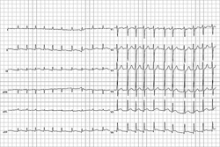
Heart Associations Update Atrial Fibrillation Guidelines
Strict heart rate control has no benefit over more lenient control in patients with atrial fibrillation, experts stated in an update of treatment guidelines. The update, which contained several new recommendations for the management of atrial fibrillation, was published online Dec. 20 in Circulation.
The previous guidelines advised keeping the heart rate of an atrial fibrillation patient at less than 80 beats/min at rest and less than 110 beats/min during a 6-minute walk. The updated guidelines advise keeping a resting heart rate of less than 110 beats/min in patients with persistent atrial fibrillation who also have stable ventricular function and have no symptoms, or symptoms deemed acceptable, related to their arrhythmia.
The 2011 Focused Update on the Management of Patients With Atrial Fibrillation (Updating the 2006 Guideline) is a joint effort of the American College of Cardiology Foundation, the American Heart Association, and the Heart Rhythm Society. The guideline writing committee reviewed data from late-breaking clinical trials presented at scientific sessions of the AHA, ACC, and European Society of Cardiology in 2009, and other data published through April 2010 (Circulation 2010 [doi:10.1161/CIR.0b013e3181fa3cf4]).
Another key update is the recommendation that a combination of clopidogrel and aspirin might be an option for atrial fibrillation patients who are poor candidates for warfarin. The recommendation is based on recent studies including the ACTIVE-A trial (Effect of Clopidogrel Added to Aspirin in Patients with Atrial Fibrillation). In this study, significantly fewer major vascular events occurred in patients randomized to receive clopidogrel plus aspirin, compared with those who received aspirin plus placebo.
"The clopidogrel/aspirin option is going to be of limited utility in most cases," commented Dr. Anne B. Curtis, who was a member of the writing group, in an interview. The new thrombin inhibitors such as dabigatran will be used instead, she said. Because dabigatran was not approved by the Food and Drug Administration when the committee was making its decisions, it was not included in the guidelines, according to the report.
The updated guidelines also suggest the use of dronedarone for some atrial fibrillation patients to reduce hospitalizations for cardiovascular events. Recent studies such as the DIONYSOS study (Efficacy and Safety of Dronedarone Versus Amiodarone for the Maintenance of Sinus Rhythm in Patients With Persistent Atrial Fibrillation) showed that dronedarone was less effective than amiodarone for reducing the recurrence of atrial fibrillation. However, in a placebo-controlled, double-blind, parallel-arm trial to assess the efficacy of dronedarone 400 mg b.i.d. for the prevention of cardiovascular hospitalization or death from any cause in patients with atrial fibrillation/atrial flutter (the ATHENA trial), dronedarone reduced the "the combined end point of death and cardiovascular hospitalizations, largely by reducing hospitalizations related to atrial fibrillation," the reviewers wrote. However, dronedarone should not be given to patients with NYHA class IV heart failure or patients with decompensated heart failure within the past 4 weeks, they noted.
To maintain normal sinus rhythm, the updated guidelines support the use of catheter ablation based on data from more than 6,900 patients. In one multicenter study (the ThermoCool trial), symptomatic patients with paroxysmal atrial fibrillation who were treated with catheter ablation showed significant improvement after 3 months, compared with control patients. Currently, catheter ablation treatment is recommended for atrial fibrillation patients without severe lung disease who have not had success with drug therapy.
Dr. L. Samuel Wann, a cardiologist at Wheaton Franciscan Healthcare in Wauwatosa, Wis., served as chair of the 2011 Writing Group Committee. Dr. Wann and his colleagues wrote that the 2006 full-text guidelines for the management of atrial fibrillation that are not mentioned in the 2011 update remain unchanged at this time. "The guidelines attempt to define practices that meet the needs of most patients in most circumstances," the reviewers said.
"The last time the guidelines were published was in 2006, and there have been several new drugs and treatments that have been developed since that time. Physicians want to know where they stand in terms of the hierarchy of treatment of patients with atrial fibrillation," explained Dr. Curtis, chair of the department of medicine at the University of Buffalo, N.Y. "In order to be timely, we did a limited, focused update," she said.
The clinical implications of more lenient heart rate control are unclear at this time, Dr. Curtis said. In the Rate Control Efficacy in Permanent Atrial Fibrillation (RACE II) study, on which the recommendations were based, the difference in heart rate control between lenient control and strict control groups was limited, she noted.
But the study emphasized the need for clinicians to be careful about going too far in trying to control patients’ heart rates, Dr. Curtis said, because too much control could lead to bradycardia and unnecessary pacemaker use.
As for additional research, "We need to know how to prevent [atrial fibrillation] in the first place, and we need to understand thoroughly the long-term outcomes of ablation therapy," Dr. Curtis said.
Dr. Wann had no financial conflicts to disclose. Several of the writing group members, including Dr. Curtis, disclosed serving as a speaker or consultant, or receiving research funding from multiple pharmaceutical companies including Medtronic, Boston Scientific, AstraZeneca, Sanofi-Aventis, and GlaxoSmithKline.
Strict heart rate control has no benefit over more lenient control in patients with atrial fibrillation, experts stated in an update of treatment guidelines. The update, which contained several new recommendations for the management of atrial fibrillation, was published online Dec. 20 in Circulation.
The previous guidelines advised keeping the heart rate of an atrial fibrillation patient at less than 80 beats/min at rest and less than 110 beats/min during a 6-minute walk. The updated guidelines advise keeping a resting heart rate of less than 110 beats/min in patients with persistent atrial fibrillation who also have stable ventricular function and have no symptoms, or symptoms deemed acceptable, related to their arrhythmia.
The 2011 Focused Update on the Management of Patients With Atrial Fibrillation (Updating the 2006 Guideline) is a joint effort of the American College of Cardiology Foundation, the American Heart Association, and the Heart Rhythm Society. The guideline writing committee reviewed data from late-breaking clinical trials presented at scientific sessions of the AHA, ACC, and European Society of Cardiology in 2009, and other data published through April 2010 (Circulation 2010 [doi:10.1161/CIR.0b013e3181fa3cf4]).
Another key update is the recommendation that a combination of clopidogrel and aspirin might be an option for atrial fibrillation patients who are poor candidates for warfarin. The recommendation is based on recent studies including the ACTIVE-A trial (Effect of Clopidogrel Added to Aspirin in Patients with Atrial Fibrillation). In this study, significantly fewer major vascular events occurred in patients randomized to receive clopidogrel plus aspirin, compared with those who received aspirin plus placebo.
"The clopidogrel/aspirin option is going to be of limited utility in most cases," commented Dr. Anne B. Curtis, who was a member of the writing group, in an interview. The new thrombin inhibitors such as dabigatran will be used instead, she said. Because dabigatran was not approved by the Food and Drug Administration when the committee was making its decisions, it was not included in the guidelines, according to the report.
The updated guidelines also suggest the use of dronedarone for some atrial fibrillation patients to reduce hospitalizations for cardiovascular events. Recent studies such as the DIONYSOS study (Efficacy and Safety of Dronedarone Versus Amiodarone for the Maintenance of Sinus Rhythm in Patients With Persistent Atrial Fibrillation) showed that dronedarone was less effective than amiodarone for reducing the recurrence of atrial fibrillation. However, in a placebo-controlled, double-blind, parallel-arm trial to assess the efficacy of dronedarone 400 mg b.i.d. for the prevention of cardiovascular hospitalization or death from any cause in patients with atrial fibrillation/atrial flutter (the ATHENA trial), dronedarone reduced the "the combined end point of death and cardiovascular hospitalizations, largely by reducing hospitalizations related to atrial fibrillation," the reviewers wrote. However, dronedarone should not be given to patients with NYHA class IV heart failure or patients with decompensated heart failure within the past 4 weeks, they noted.
To maintain normal sinus rhythm, the updated guidelines support the use of catheter ablation based on data from more than 6,900 patients. In one multicenter study (the ThermoCool trial), symptomatic patients with paroxysmal atrial fibrillation who were treated with catheter ablation showed significant improvement after 3 months, compared with control patients. Currently, catheter ablation treatment is recommended for atrial fibrillation patients without severe lung disease who have not had success with drug therapy.
Dr. L. Samuel Wann, a cardiologist at Wheaton Franciscan Healthcare in Wauwatosa, Wis., served as chair of the 2011 Writing Group Committee. Dr. Wann and his colleagues wrote that the 2006 full-text guidelines for the management of atrial fibrillation that are not mentioned in the 2011 update remain unchanged at this time. "The guidelines attempt to define practices that meet the needs of most patients in most circumstances," the reviewers said.
"The last time the guidelines were published was in 2006, and there have been several new drugs and treatments that have been developed since that time. Physicians want to know where they stand in terms of the hierarchy of treatment of patients with atrial fibrillation," explained Dr. Curtis, chair of the department of medicine at the University of Buffalo, N.Y. "In order to be timely, we did a limited, focused update," she said.
The clinical implications of more lenient heart rate control are unclear at this time, Dr. Curtis said. In the Rate Control Efficacy in Permanent Atrial Fibrillation (RACE II) study, on which the recommendations were based, the difference in heart rate control between lenient control and strict control groups was limited, she noted.
But the study emphasized the need for clinicians to be careful about going too far in trying to control patients’ heart rates, Dr. Curtis said, because too much control could lead to bradycardia and unnecessary pacemaker use.
As for additional research, "We need to know how to prevent [atrial fibrillation] in the first place, and we need to understand thoroughly the long-term outcomes of ablation therapy," Dr. Curtis said.
Dr. Wann had no financial conflicts to disclose. Several of the writing group members, including Dr. Curtis, disclosed serving as a speaker or consultant, or receiving research funding from multiple pharmaceutical companies including Medtronic, Boston Scientific, AstraZeneca, Sanofi-Aventis, and GlaxoSmithKline.
Strict heart rate control has no benefit over more lenient control in patients with atrial fibrillation, experts stated in an update of treatment guidelines. The update, which contained several new recommendations for the management of atrial fibrillation, was published online Dec. 20 in Circulation.
The previous guidelines advised keeping the heart rate of an atrial fibrillation patient at less than 80 beats/min at rest and less than 110 beats/min during a 6-minute walk. The updated guidelines advise keeping a resting heart rate of less than 110 beats/min in patients with persistent atrial fibrillation who also have stable ventricular function and have no symptoms, or symptoms deemed acceptable, related to their arrhythmia.
The 2011 Focused Update on the Management of Patients With Atrial Fibrillation (Updating the 2006 Guideline) is a joint effort of the American College of Cardiology Foundation, the American Heart Association, and the Heart Rhythm Society. The guideline writing committee reviewed data from late-breaking clinical trials presented at scientific sessions of the AHA, ACC, and European Society of Cardiology in 2009, and other data published through April 2010 (Circulation 2010 [doi:10.1161/CIR.0b013e3181fa3cf4]).
Another key update is the recommendation that a combination of clopidogrel and aspirin might be an option for atrial fibrillation patients who are poor candidates for warfarin. The recommendation is based on recent studies including the ACTIVE-A trial (Effect of Clopidogrel Added to Aspirin in Patients with Atrial Fibrillation). In this study, significantly fewer major vascular events occurred in patients randomized to receive clopidogrel plus aspirin, compared with those who received aspirin plus placebo.
"The clopidogrel/aspirin option is going to be of limited utility in most cases," commented Dr. Anne B. Curtis, who was a member of the writing group, in an interview. The new thrombin inhibitors such as dabigatran will be used instead, she said. Because dabigatran was not approved by the Food and Drug Administration when the committee was making its decisions, it was not included in the guidelines, according to the report.
The updated guidelines also suggest the use of dronedarone for some atrial fibrillation patients to reduce hospitalizations for cardiovascular events. Recent studies such as the DIONYSOS study (Efficacy and Safety of Dronedarone Versus Amiodarone for the Maintenance of Sinus Rhythm in Patients With Persistent Atrial Fibrillation) showed that dronedarone was less effective than amiodarone for reducing the recurrence of atrial fibrillation. However, in a placebo-controlled, double-blind, parallel-arm trial to assess the efficacy of dronedarone 400 mg b.i.d. for the prevention of cardiovascular hospitalization or death from any cause in patients with atrial fibrillation/atrial flutter (the ATHENA trial), dronedarone reduced the "the combined end point of death and cardiovascular hospitalizations, largely by reducing hospitalizations related to atrial fibrillation," the reviewers wrote. However, dronedarone should not be given to patients with NYHA class IV heart failure or patients with decompensated heart failure within the past 4 weeks, they noted.
To maintain normal sinus rhythm, the updated guidelines support the use of catheter ablation based on data from more than 6,900 patients. In one multicenter study (the ThermoCool trial), symptomatic patients with paroxysmal atrial fibrillation who were treated with catheter ablation showed significant improvement after 3 months, compared with control patients. Currently, catheter ablation treatment is recommended for atrial fibrillation patients without severe lung disease who have not had success with drug therapy.
Dr. L. Samuel Wann, a cardiologist at Wheaton Franciscan Healthcare in Wauwatosa, Wis., served as chair of the 2011 Writing Group Committee. Dr. Wann and his colleagues wrote that the 2006 full-text guidelines for the management of atrial fibrillation that are not mentioned in the 2011 update remain unchanged at this time. "The guidelines attempt to define practices that meet the needs of most patients in most circumstances," the reviewers said.
"The last time the guidelines were published was in 2006, and there have been several new drugs and treatments that have been developed since that time. Physicians want to know where they stand in terms of the hierarchy of treatment of patients with atrial fibrillation," explained Dr. Curtis, chair of the department of medicine at the University of Buffalo, N.Y. "In order to be timely, we did a limited, focused update," she said.
The clinical implications of more lenient heart rate control are unclear at this time, Dr. Curtis said. In the Rate Control Efficacy in Permanent Atrial Fibrillation (RACE II) study, on which the recommendations were based, the difference in heart rate control between lenient control and strict control groups was limited, she noted.
But the study emphasized the need for clinicians to be careful about going too far in trying to control patients’ heart rates, Dr. Curtis said, because too much control could lead to bradycardia and unnecessary pacemaker use.
As for additional research, "We need to know how to prevent [atrial fibrillation] in the first place, and we need to understand thoroughly the long-term outcomes of ablation therapy," Dr. Curtis said.
Dr. Wann had no financial conflicts to disclose. Several of the writing group members, including Dr. Curtis, disclosed serving as a speaker or consultant, or receiving research funding from multiple pharmaceutical companies including Medtronic, Boston Scientific, AstraZeneca, Sanofi-Aventis, and GlaxoSmithKline.
FROM CIRCULATION
Heart Associations Update Atrial Fibrillation Guidelines
Strict heart rate control has no benefit over more lenient control in patients with atrial fibrillation, experts stated in an update of treatment guidelines. The update, which contained several new recommendations for the management of atrial fibrillation, was published online Dec. 20 in Circulation.
The previous guidelines advised keeping the heart rate of an atrial fibrillation patient at less than 80 beats/min at rest and less than 110 beats/min during a 6-minute walk. The updated guidelines advise keeping a resting heart rate of less than 110 beats/min in patients with persistent atrial fibrillation who also have stable ventricular function and have no symptoms, or symptoms deemed acceptable, related to their arrhythmia.
The 2011 Focused Update on the Management of Patients With Atrial Fibrillation (Updating the 2006 Guideline) is a joint effort of the American College of Cardiology Foundation, the American Heart Association, and the Heart Rhythm Society. The guideline writing committee reviewed data from late-breaking clinical trials presented at scientific sessions of the AHA, ACC, and European Society of Cardiology in 2009, and other data published through April 2010 (Circulation 2010 [doi:10.1161/CIR.0b013e3181fa3cf4]).
Another key update is the recommendation that a combination of clopidogrel and aspirin might be an option for atrial fibrillation patients who are poor candidates for warfarin. The recommendation is based on recent studies including the ACTIVE-A trial (Effect of Clopidogrel Added to Aspirin in Patients with Atrial Fibrillation). In this study, significantly fewer major vascular events occurred in patients randomized to receive clopidogrel plus aspirin, compared with those who received aspirin plus placebo.
"The clopidogrel/aspirin option is going to be of limited utility in most cases," commented Dr. Anne B. Curtis, who was a member of the writing group, in an interview. The new thrombin inhibitors such as dabigatran will be used instead, she said. Because dabigatran was not approved by the Food and Drug Administration when the committee was making its decisions, it was not included in the guidelines, according to the report.
The updated guidelines also suggest the use of dronedarone for some atrial fibrillation patients to reduce hospitalizations for cardiovascular events. Recent studies such as the DIONYSOS study (Efficacy and Safety of Dronedarone Versus Amiodarone for the Maintenance of Sinus Rhythm in Patients With Persistent Atrial Fibrillation) showed that dronedarone was less effective than amiodarone for reducing the recurrence of atrial fibrillation. However, in a placebo-controlled, double-blind, parallel-arm trial to assess the efficacy of dronedarone 400 mg b.i.d. for the prevention of cardiovascular hospitalization or death from any cause in patients with atrial fibrillation/atrial flutter (the ATHENA trial), dronedarone reduced the "the combined end point of death and cardiovascular hospitalizations, largely by reducing hospitalizations related to atrial fibrillation," the reviewers wrote. However, dronedarone should not be given to patients with NYHA class IV heart failure or patients with decompensated heart failure within the past 4 weeks, they noted.
To maintain normal sinus rhythm, the updated guidelines support the use of catheter ablation based on data from more than 6,900 patients. In one multicenter study (the ThermoCool trial), symptomatic patients with paroxysmal atrial fibrillation who were treated with catheter ablation showed significant improvement after 3 months, compared with control patients. Currently, catheter ablation treatment is recommended for atrial fibrillation patients without severe lung disease who have not had success with drug therapy.
Dr. L. Samuel Wann, a cardiologist at Wheaton Franciscan Healthcare in Wauwatosa, Wis., served as chair of the 2011 Writing Group Committee. Dr. Wann and his colleagues wrote that the 2006 full-text guidelines for the management of atrial fibrillation that are not mentioned in the 2011 update remain unchanged at this time. "The guidelines attempt to define practices that meet the needs of most patients in most circumstances," the reviewers said.
"The last time the guidelines were published was in 2006, and there have been several new drugs and treatments that have been developed since that time. Physicians want to know where they stand in terms of the hierarchy of treatment of patients with atrial fibrillation," explained Dr. Curtis, chair of the department of medicine at the University of Buffalo, N.Y. "In order to be timely, we did a limited, focused update," she said.
The clinical implications of more lenient heart rate control are unclear at this time, Dr. Curtis said. In the Rate Control Efficacy in Permanent Atrial Fibrillation (RACE II) study, on which the recommendations were based, the difference in heart rate control between lenient control and strict control groups was limited, she noted.
But the study emphasized the need for clinicians to be careful about going too far in trying to control patients’ heart rates, Dr. Curtis said, because too much control could lead to bradycardia and unnecessary pacemaker use.
As for additional research, "We need to know how to prevent [atrial fibrillation] in the first place, and we need to understand thoroughly the long-term outcomes of ablation therapy," Dr. Curtis said.
Dr. Wann had no financial conflicts to disclose. Several of the writing group members, including Dr. Curtis, disclosed serving as a speaker or consultant, or receiving research funding from multiple pharmaceutical companies including Medtronic, Boston Scientific, AstraZeneca, Sanofi-Aventis, and GlaxoSmithKline.
Strict heart rate control has no benefit over more lenient control in patients with atrial fibrillation, experts stated in an update of treatment guidelines. The update, which contained several new recommendations for the management of atrial fibrillation, was published online Dec. 20 in Circulation.
The previous guidelines advised keeping the heart rate of an atrial fibrillation patient at less than 80 beats/min at rest and less than 110 beats/min during a 6-minute walk. The updated guidelines advise keeping a resting heart rate of less than 110 beats/min in patients with persistent atrial fibrillation who also have stable ventricular function and have no symptoms, or symptoms deemed acceptable, related to their arrhythmia.
The 2011 Focused Update on the Management of Patients With Atrial Fibrillation (Updating the 2006 Guideline) is a joint effort of the American College of Cardiology Foundation, the American Heart Association, and the Heart Rhythm Society. The guideline writing committee reviewed data from late-breaking clinical trials presented at scientific sessions of the AHA, ACC, and European Society of Cardiology in 2009, and other data published through April 2010 (Circulation 2010 [doi:10.1161/CIR.0b013e3181fa3cf4]).
Another key update is the recommendation that a combination of clopidogrel and aspirin might be an option for atrial fibrillation patients who are poor candidates for warfarin. The recommendation is based on recent studies including the ACTIVE-A trial (Effect of Clopidogrel Added to Aspirin in Patients with Atrial Fibrillation). In this study, significantly fewer major vascular events occurred in patients randomized to receive clopidogrel plus aspirin, compared with those who received aspirin plus placebo.
"The clopidogrel/aspirin option is going to be of limited utility in most cases," commented Dr. Anne B. Curtis, who was a member of the writing group, in an interview. The new thrombin inhibitors such as dabigatran will be used instead, she said. Because dabigatran was not approved by the Food and Drug Administration when the committee was making its decisions, it was not included in the guidelines, according to the report.
The updated guidelines also suggest the use of dronedarone for some atrial fibrillation patients to reduce hospitalizations for cardiovascular events. Recent studies such as the DIONYSOS study (Efficacy and Safety of Dronedarone Versus Amiodarone for the Maintenance of Sinus Rhythm in Patients With Persistent Atrial Fibrillation) showed that dronedarone was less effective than amiodarone for reducing the recurrence of atrial fibrillation. However, in a placebo-controlled, double-blind, parallel-arm trial to assess the efficacy of dronedarone 400 mg b.i.d. for the prevention of cardiovascular hospitalization or death from any cause in patients with atrial fibrillation/atrial flutter (the ATHENA trial), dronedarone reduced the "the combined end point of death and cardiovascular hospitalizations, largely by reducing hospitalizations related to atrial fibrillation," the reviewers wrote. However, dronedarone should not be given to patients with NYHA class IV heart failure or patients with decompensated heart failure within the past 4 weeks, they noted.
To maintain normal sinus rhythm, the updated guidelines support the use of catheter ablation based on data from more than 6,900 patients. In one multicenter study (the ThermoCool trial), symptomatic patients with paroxysmal atrial fibrillation who were treated with catheter ablation showed significant improvement after 3 months, compared with control patients. Currently, catheter ablation treatment is recommended for atrial fibrillation patients without severe lung disease who have not had success with drug therapy.
Dr. L. Samuel Wann, a cardiologist at Wheaton Franciscan Healthcare in Wauwatosa, Wis., served as chair of the 2011 Writing Group Committee. Dr. Wann and his colleagues wrote that the 2006 full-text guidelines for the management of atrial fibrillation that are not mentioned in the 2011 update remain unchanged at this time. "The guidelines attempt to define practices that meet the needs of most patients in most circumstances," the reviewers said.
"The last time the guidelines were published was in 2006, and there have been several new drugs and treatments that have been developed since that time. Physicians want to know where they stand in terms of the hierarchy of treatment of patients with atrial fibrillation," explained Dr. Curtis, chair of the department of medicine at the University of Buffalo, N.Y. "In order to be timely, we did a limited, focused update," she said.
The clinical implications of more lenient heart rate control are unclear at this time, Dr. Curtis said. In the Rate Control Efficacy in Permanent Atrial Fibrillation (RACE II) study, on which the recommendations were based, the difference in heart rate control between lenient control and strict control groups was limited, she noted.
But the study emphasized the need for clinicians to be careful about going too far in trying to control patients’ heart rates, Dr. Curtis said, because too much control could lead to bradycardia and unnecessary pacemaker use.
As for additional research, "We need to know how to prevent [atrial fibrillation] in the first place, and we need to understand thoroughly the long-term outcomes of ablation therapy," Dr. Curtis said.
Dr. Wann had no financial conflicts to disclose. Several of the writing group members, including Dr. Curtis, disclosed serving as a speaker or consultant, or receiving research funding from multiple pharmaceutical companies including Medtronic, Boston Scientific, AstraZeneca, Sanofi-Aventis, and GlaxoSmithKline.
Strict heart rate control has no benefit over more lenient control in patients with atrial fibrillation, experts stated in an update of treatment guidelines. The update, which contained several new recommendations for the management of atrial fibrillation, was published online Dec. 20 in Circulation.
The previous guidelines advised keeping the heart rate of an atrial fibrillation patient at less than 80 beats/min at rest and less than 110 beats/min during a 6-minute walk. The updated guidelines advise keeping a resting heart rate of less than 110 beats/min in patients with persistent atrial fibrillation who also have stable ventricular function and have no symptoms, or symptoms deemed acceptable, related to their arrhythmia.
The 2011 Focused Update on the Management of Patients With Atrial Fibrillation (Updating the 2006 Guideline) is a joint effort of the American College of Cardiology Foundation, the American Heart Association, and the Heart Rhythm Society. The guideline writing committee reviewed data from late-breaking clinical trials presented at scientific sessions of the AHA, ACC, and European Society of Cardiology in 2009, and other data published through April 2010 (Circulation 2010 [doi:10.1161/CIR.0b013e3181fa3cf4]).
Another key update is the recommendation that a combination of clopidogrel and aspirin might be an option for atrial fibrillation patients who are poor candidates for warfarin. The recommendation is based on recent studies including the ACTIVE-A trial (Effect of Clopidogrel Added to Aspirin in Patients with Atrial Fibrillation). In this study, significantly fewer major vascular events occurred in patients randomized to receive clopidogrel plus aspirin, compared with those who received aspirin plus placebo.
"The clopidogrel/aspirin option is going to be of limited utility in most cases," commented Dr. Anne B. Curtis, who was a member of the writing group, in an interview. The new thrombin inhibitors such as dabigatran will be used instead, she said. Because dabigatran was not approved by the Food and Drug Administration when the committee was making its decisions, it was not included in the guidelines, according to the report.
The updated guidelines also suggest the use of dronedarone for some atrial fibrillation patients to reduce hospitalizations for cardiovascular events. Recent studies such as the DIONYSOS study (Efficacy and Safety of Dronedarone Versus Amiodarone for the Maintenance of Sinus Rhythm in Patients With Persistent Atrial Fibrillation) showed that dronedarone was less effective than amiodarone for reducing the recurrence of atrial fibrillation. However, in a placebo-controlled, double-blind, parallel-arm trial to assess the efficacy of dronedarone 400 mg b.i.d. for the prevention of cardiovascular hospitalization or death from any cause in patients with atrial fibrillation/atrial flutter (the ATHENA trial), dronedarone reduced the "the combined end point of death and cardiovascular hospitalizations, largely by reducing hospitalizations related to atrial fibrillation," the reviewers wrote. However, dronedarone should not be given to patients with NYHA class IV heart failure or patients with decompensated heart failure within the past 4 weeks, they noted.
To maintain normal sinus rhythm, the updated guidelines support the use of catheter ablation based on data from more than 6,900 patients. In one multicenter study (the ThermoCool trial), symptomatic patients with paroxysmal atrial fibrillation who were treated with catheter ablation showed significant improvement after 3 months, compared with control patients. Currently, catheter ablation treatment is recommended for atrial fibrillation patients without severe lung disease who have not had success with drug therapy.
Dr. L. Samuel Wann, a cardiologist at Wheaton Franciscan Healthcare in Wauwatosa, Wis., served as chair of the 2011 Writing Group Committee. Dr. Wann and his colleagues wrote that the 2006 full-text guidelines for the management of atrial fibrillation that are not mentioned in the 2011 update remain unchanged at this time. "The guidelines attempt to define practices that meet the needs of most patients in most circumstances," the reviewers said.
"The last time the guidelines were published was in 2006, and there have been several new drugs and treatments that have been developed since that time. Physicians want to know where they stand in terms of the hierarchy of treatment of patients with atrial fibrillation," explained Dr. Curtis, chair of the department of medicine at the University of Buffalo, N.Y. "In order to be timely, we did a limited, focused update," she said.
The clinical implications of more lenient heart rate control are unclear at this time, Dr. Curtis said. In the Rate Control Efficacy in Permanent Atrial Fibrillation (RACE II) study, on which the recommendations were based, the difference in heart rate control between lenient control and strict control groups was limited, she noted.
But the study emphasized the need for clinicians to be careful about going too far in trying to control patients’ heart rates, Dr. Curtis said, because too much control could lead to bradycardia and unnecessary pacemaker use.
As for additional research, "We need to know how to prevent [atrial fibrillation] in the first place, and we need to understand thoroughly the long-term outcomes of ablation therapy," Dr. Curtis said.
Dr. Wann had no financial conflicts to disclose. Several of the writing group members, including Dr. Curtis, disclosed serving as a speaker or consultant, or receiving research funding from multiple pharmaceutical companies including Medtronic, Boston Scientific, AstraZeneca, Sanofi-Aventis, and GlaxoSmithKline.
FROM CIRCULATION
Thrombosis Risk Rises in Women With Myeloproliferative Disorders
ORLANDO – Thrombosis was significantly more prevalent in women, compared with men, in a review of data from 270 adults with JAK2 V617-F–positive myeloproliferative disorder, according to a poster at the annual meeting of the American Society of Hematology.
[Check out our comprehensive coverage of the American Society of Hematology's annual meeting.]
Vascular complications were identified in 29% of women vs. 18% of men in a cohort of patients seen at the center for myeloproliferative disorders of Johns Hopkins University in Baltimore, said Dr. Brady L. Stein of Northwestern University in Chicago and colleagues.
On average, the women were significantly younger (53 years vs. 57 years) and significantly less likely to smoke, compared with men (22% vs. 39%), which made the findings surprising, Dr. Stein noted. "This analysis supports the importance of individual variation on the thrombotic manifestations of the myeloproliferative disorder," he wrote.
Overall, 16 of 20 abdominal venous thrombotic events occurred in women, as did 11 of 12 abdominal venous thrombotic events in polycythemia vera, 6 of 7 cerebrovascular accidents, and 8 of 11 transient ischemic attacks.
However, significantly more men than women had vascular complications at or within 5 years of diagnosis (70% vs. 43%).
Women had a significantly lower incidence of dyslipidemia than did men (9% vs. 25%; P = .001) and a lower white blood cell count (9.5 x 103 mcL vs. 13.2 x 103 mcL; P = .017). Of nine patients who experienced multiple events, six were women.
No sex differences were noted in thrombosis prevalence based on disease class. Aspirin use, cytoreduction, and disease duration were not significantly different between men and women.
The results suggest that women are paradoxically at greater risk for thrombosis in myeloproliferative disorders, the investigators wrote, but more research is needed to determine the mechanism of action.
The researchers had no financial conflicts to disclose.
ORLANDO – Thrombosis was significantly more prevalent in women, compared with men, in a review of data from 270 adults with JAK2 V617-F–positive myeloproliferative disorder, according to a poster at the annual meeting of the American Society of Hematology.
[Check out our comprehensive coverage of the American Society of Hematology's annual meeting.]
Vascular complications were identified in 29% of women vs. 18% of men in a cohort of patients seen at the center for myeloproliferative disorders of Johns Hopkins University in Baltimore, said Dr. Brady L. Stein of Northwestern University in Chicago and colleagues.
On average, the women were significantly younger (53 years vs. 57 years) and significantly less likely to smoke, compared with men (22% vs. 39%), which made the findings surprising, Dr. Stein noted. "This analysis supports the importance of individual variation on the thrombotic manifestations of the myeloproliferative disorder," he wrote.
Overall, 16 of 20 abdominal venous thrombotic events occurred in women, as did 11 of 12 abdominal venous thrombotic events in polycythemia vera, 6 of 7 cerebrovascular accidents, and 8 of 11 transient ischemic attacks.
However, significantly more men than women had vascular complications at or within 5 years of diagnosis (70% vs. 43%).
Women had a significantly lower incidence of dyslipidemia than did men (9% vs. 25%; P = .001) and a lower white blood cell count (9.5 x 103 mcL vs. 13.2 x 103 mcL; P = .017). Of nine patients who experienced multiple events, six were women.
No sex differences were noted in thrombosis prevalence based on disease class. Aspirin use, cytoreduction, and disease duration were not significantly different between men and women.
The results suggest that women are paradoxically at greater risk for thrombosis in myeloproliferative disorders, the investigators wrote, but more research is needed to determine the mechanism of action.
The researchers had no financial conflicts to disclose.
ORLANDO – Thrombosis was significantly more prevalent in women, compared with men, in a review of data from 270 adults with JAK2 V617-F–positive myeloproliferative disorder, according to a poster at the annual meeting of the American Society of Hematology.
[Check out our comprehensive coverage of the American Society of Hematology's annual meeting.]
Vascular complications were identified in 29% of women vs. 18% of men in a cohort of patients seen at the center for myeloproliferative disorders of Johns Hopkins University in Baltimore, said Dr. Brady L. Stein of Northwestern University in Chicago and colleagues.
On average, the women were significantly younger (53 years vs. 57 years) and significantly less likely to smoke, compared with men (22% vs. 39%), which made the findings surprising, Dr. Stein noted. "This analysis supports the importance of individual variation on the thrombotic manifestations of the myeloproliferative disorder," he wrote.
Overall, 16 of 20 abdominal venous thrombotic events occurred in women, as did 11 of 12 abdominal venous thrombotic events in polycythemia vera, 6 of 7 cerebrovascular accidents, and 8 of 11 transient ischemic attacks.
However, significantly more men than women had vascular complications at or within 5 years of diagnosis (70% vs. 43%).
Women had a significantly lower incidence of dyslipidemia than did men (9% vs. 25%; P = .001) and a lower white blood cell count (9.5 x 103 mcL vs. 13.2 x 103 mcL; P = .017). Of nine patients who experienced multiple events, six were women.
No sex differences were noted in thrombosis prevalence based on disease class. Aspirin use, cytoreduction, and disease duration were not significantly different between men and women.
The results suggest that women are paradoxically at greater risk for thrombosis in myeloproliferative disorders, the investigators wrote, but more research is needed to determine the mechanism of action.
The researchers had no financial conflicts to disclose.
Major Finding: Vascular complications were seen in 29% of women vs. 18% of men with JAK2 V617F–positive myeloproliferative disorders.
Data Source: A review of data from 270 adults with myeloproliferative disorders
Disclosures: Dr. Stein had no financial conflicts to disclose.
Horse Beats Rabbit in Antithymocyte Globulin Race
ORLANDO – What might Aesop have written about a race between a horse and a rabbit? Researchers from the National Institutes of Health found that the horse outran the rabbit when it came to the successful use of antithymocyte globulin as a first-line treatment for patients with severe aplastic anemia.
In a randomized trial, rabbit antithymocyte globulin (ATG) was inferior to horse ATG for the initial treatment of these patients.
"Our findings were surprising to us," Dr. Phillip Scheinberg of the National Institutes of Health’s National Heart, Lung, and Blood Institute, Bethesda, Md., said in an interview at the annual meeting of the American Society of Hematology, where he presented the findings in a late-breaking abstract.
The hematologic response rate after 3 months of treatment was 62% in patients treated with horse ATG compared with 33% in those given rabbit ATG (P = .0017). Six-month hematologic response rates (the primary end point of the study) were still being evaluated at the time of the presentation, so no P value was available, but the difference in favor of horse vs. rabbit appeared to persist (69% vs. 35%).
Horse ATG is a standard treatment for patients with severe aplastic anemia (SAA) who are not candidates for stem cell transplantation, said Dr. Scheinberg. In recent studies, rabbit ATG has shown success in rescuing patients after horse ATG failures or in cases of relapse, but "there were no data showing the effectiveness of rabbit ATG as first-line therapy," he said.
Dr. Scheinberg and colleagues randomized 120 patients aged 2-78 years with treatment-naive SAA to receive either horse or rabbit ATG plus cyclosporine. Demographic and clinical characteristics were similar between the two groups.
At the time the findings were presented, 3 deaths had occurred in the horse group, compared with 14 deaths in the rabbit group. The rates of relapse and clonal evolution were similar between the groups.
The results "have immediate implications for patients and their treating physicians," Dr. Scheinberg said.
"We designed the study to show that rabbit would be superior to horse, and at the end of the study we found that it was the other way around."
Based on the study findings, horse ATG should be used as first-line therapy for all SAA patients, he said. Rabbit ATG is used in many parts of the world where horse ATG is not available, because of its presumed superiority, said Dr. Scheinberg. But the study results suggest that horse ATG should be reintroduced and made available worldwide, he said.
The results raised many questions for future research about why the rabbit ATG was not as effective as the horse ATG, Dr. Scheinberg emphasized. "Maybe the horse is doing something beneficial that we don’t appreciate. Or maybe the rabbit is doing something that is not good that we don’t understand," he said. Future studies will examine the mechanism of action of the different types of ATG, he added.
Dr. Scheinberg had no financial conflicts to disclose.
ORLANDO – What might Aesop have written about a race between a horse and a rabbit? Researchers from the National Institutes of Health found that the horse outran the rabbit when it came to the successful use of antithymocyte globulin as a first-line treatment for patients with severe aplastic anemia.
In a randomized trial, rabbit antithymocyte globulin (ATG) was inferior to horse ATG for the initial treatment of these patients.
"Our findings were surprising to us," Dr. Phillip Scheinberg of the National Institutes of Health’s National Heart, Lung, and Blood Institute, Bethesda, Md., said in an interview at the annual meeting of the American Society of Hematology, where he presented the findings in a late-breaking abstract.
The hematologic response rate after 3 months of treatment was 62% in patients treated with horse ATG compared with 33% in those given rabbit ATG (P = .0017). Six-month hematologic response rates (the primary end point of the study) were still being evaluated at the time of the presentation, so no P value was available, but the difference in favor of horse vs. rabbit appeared to persist (69% vs. 35%).
Horse ATG is a standard treatment for patients with severe aplastic anemia (SAA) who are not candidates for stem cell transplantation, said Dr. Scheinberg. In recent studies, rabbit ATG has shown success in rescuing patients after horse ATG failures or in cases of relapse, but "there were no data showing the effectiveness of rabbit ATG as first-line therapy," he said.
Dr. Scheinberg and colleagues randomized 120 patients aged 2-78 years with treatment-naive SAA to receive either horse or rabbit ATG plus cyclosporine. Demographic and clinical characteristics were similar between the two groups.
At the time the findings were presented, 3 deaths had occurred in the horse group, compared with 14 deaths in the rabbit group. The rates of relapse and clonal evolution were similar between the groups.
The results "have immediate implications for patients and their treating physicians," Dr. Scheinberg said.
"We designed the study to show that rabbit would be superior to horse, and at the end of the study we found that it was the other way around."
Based on the study findings, horse ATG should be used as first-line therapy for all SAA patients, he said. Rabbit ATG is used in many parts of the world where horse ATG is not available, because of its presumed superiority, said Dr. Scheinberg. But the study results suggest that horse ATG should be reintroduced and made available worldwide, he said.
The results raised many questions for future research about why the rabbit ATG was not as effective as the horse ATG, Dr. Scheinberg emphasized. "Maybe the horse is doing something beneficial that we don’t appreciate. Or maybe the rabbit is doing something that is not good that we don’t understand," he said. Future studies will examine the mechanism of action of the different types of ATG, he added.
Dr. Scheinberg had no financial conflicts to disclose.
ORLANDO – What might Aesop have written about a race between a horse and a rabbit? Researchers from the National Institutes of Health found that the horse outran the rabbit when it came to the successful use of antithymocyte globulin as a first-line treatment for patients with severe aplastic anemia.
In a randomized trial, rabbit antithymocyte globulin (ATG) was inferior to horse ATG for the initial treatment of these patients.
"Our findings were surprising to us," Dr. Phillip Scheinberg of the National Institutes of Health’s National Heart, Lung, and Blood Institute, Bethesda, Md., said in an interview at the annual meeting of the American Society of Hematology, where he presented the findings in a late-breaking abstract.
The hematologic response rate after 3 months of treatment was 62% in patients treated with horse ATG compared with 33% in those given rabbit ATG (P = .0017). Six-month hematologic response rates (the primary end point of the study) were still being evaluated at the time of the presentation, so no P value was available, but the difference in favor of horse vs. rabbit appeared to persist (69% vs. 35%).
Horse ATG is a standard treatment for patients with severe aplastic anemia (SAA) who are not candidates for stem cell transplantation, said Dr. Scheinberg. In recent studies, rabbit ATG has shown success in rescuing patients after horse ATG failures or in cases of relapse, but "there were no data showing the effectiveness of rabbit ATG as first-line therapy," he said.
Dr. Scheinberg and colleagues randomized 120 patients aged 2-78 years with treatment-naive SAA to receive either horse or rabbit ATG plus cyclosporine. Demographic and clinical characteristics were similar between the two groups.
At the time the findings were presented, 3 deaths had occurred in the horse group, compared with 14 deaths in the rabbit group. The rates of relapse and clonal evolution were similar between the groups.
The results "have immediate implications for patients and their treating physicians," Dr. Scheinberg said.
"We designed the study to show that rabbit would be superior to horse, and at the end of the study we found that it was the other way around."
Based on the study findings, horse ATG should be used as first-line therapy for all SAA patients, he said. Rabbit ATG is used in many parts of the world where horse ATG is not available, because of its presumed superiority, said Dr. Scheinberg. But the study results suggest that horse ATG should be reintroduced and made available worldwide, he said.
The results raised many questions for future research about why the rabbit ATG was not as effective as the horse ATG, Dr. Scheinberg emphasized. "Maybe the horse is doing something beneficial that we don’t appreciate. Or maybe the rabbit is doing something that is not good that we don’t understand," he said. Future studies will examine the mechanism of action of the different types of ATG, he added.
Dr. Scheinberg had no financial conflicts to disclose.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF HEMATOLOGY
Major Finding: The hematologic response rate after 3 months of treatment was 62% in patients treated with horse ATG compared with 33% in those given rabbit ATG (P = .0017).
Data Source: A randomized trial of 120 patients with severe aplastic anemia.
Disclosures: Dr. Scheinberg had no financial conflicts to disclose.
TNF Inhibitor Users Report Less Sick Leave
A significant 30% reduction in the number of sick leave days used per month was seen in adults with rheumatoid arthritis after using tumor necrosis factor antagonists for 6 months.
The finding was observed in a population-based study of 365 RA patients aged 18-85 years.
The study is among the first to address the quantitative impact of TNF inhibitors on sick leave and disability pension, said Dr. Tor Olofsson of Lund (Sweden) University, and colleagues.
They reviewed insurance database information on RA patients enrolled in the South Swedish Arthritis Treatment Group registry. Each patient was matched with four controls from the general population.
The study population averaged 9 sick days per month in the first month of anti-TNF treatment. The monthly rate dropped to an average of 6.5 days after 6 months and remained steady at an average of 6.6 days per month for months 6-12 (Ann. Rheum. Dis. 2010;69:2131-6).
Compared with the controls in the general population, the relative risk of being on sick leave in the RA group was 6.6 at the start of treatment, but dropped to 5.1 after 6 months, and remained at an average of 5.2 for the rest of the year. The relative risk of being on disability pension was 3.4 at the start of treatment and 3.2 after 1 year of treatment.
Approximately 98% of the patients had tried at least one disease-modifying antirheumatic drug (DMARD) before starting anti-TNF therapy. The average age of the patients was 46 years, and 82% were women. A total of 92 patients (25%) discontinued treatment, including 34 for adverse events, 32 for treatment failure, and 26 for other reasons.
"The reduction in sick leave during the first anti-TNF treatment year must be considered as a conservative measure in this cohort of patients who were severely ill, since there was a steady increase in sick leave prior to initiation of therapy," the researchers wrote. "Even a stabilization of the sick leave level could have been deemed as a successful outcome," they noted.
The results support findings from previous studies of the effects of TNF inhibitors on work outcomes, although the study was limited by the short follow-up period, the researchers noted. The findings conflict with data from a recent observational study that showed no difference in employment loss between RA patients using anti-TNF therapy and controls.
However, the average duration of RA in the observational study was 12 years, compared to an average duration of 4.5 years in the current study. This difference suggests a possible protective effect from anti-TNF therapy in a subset of patients with less than 11 years disease duration at the start of therapy, the researchers said.
The researchers said that they had no relevant financial conflicts to disclose. The study was funded by grants from multiple nonpharmaceutical organizations including the Swedish Research Council, Lund University, Region Skåne, the Swedish Social Insurance Agency, the Swedish Rheumatism Association, and the King Gustav V 80-Year Fund.
A significant 30% reduction in the number of sick leave days used per month was seen in adults with rheumatoid arthritis after using tumor necrosis factor antagonists for 6 months.
The finding was observed in a population-based study of 365 RA patients aged 18-85 years.
The study is among the first to address the quantitative impact of TNF inhibitors on sick leave and disability pension, said Dr. Tor Olofsson of Lund (Sweden) University, and colleagues.
They reviewed insurance database information on RA patients enrolled in the South Swedish Arthritis Treatment Group registry. Each patient was matched with four controls from the general population.
The study population averaged 9 sick days per month in the first month of anti-TNF treatment. The monthly rate dropped to an average of 6.5 days after 6 months and remained steady at an average of 6.6 days per month for months 6-12 (Ann. Rheum. Dis. 2010;69:2131-6).
Compared with the controls in the general population, the relative risk of being on sick leave in the RA group was 6.6 at the start of treatment, but dropped to 5.1 after 6 months, and remained at an average of 5.2 for the rest of the year. The relative risk of being on disability pension was 3.4 at the start of treatment and 3.2 after 1 year of treatment.
Approximately 98% of the patients had tried at least one disease-modifying antirheumatic drug (DMARD) before starting anti-TNF therapy. The average age of the patients was 46 years, and 82% were women. A total of 92 patients (25%) discontinued treatment, including 34 for adverse events, 32 for treatment failure, and 26 for other reasons.
"The reduction in sick leave during the first anti-TNF treatment year must be considered as a conservative measure in this cohort of patients who were severely ill, since there was a steady increase in sick leave prior to initiation of therapy," the researchers wrote. "Even a stabilization of the sick leave level could have been deemed as a successful outcome," they noted.
The results support findings from previous studies of the effects of TNF inhibitors on work outcomes, although the study was limited by the short follow-up period, the researchers noted. The findings conflict with data from a recent observational study that showed no difference in employment loss between RA patients using anti-TNF therapy and controls.
However, the average duration of RA in the observational study was 12 years, compared to an average duration of 4.5 years in the current study. This difference suggests a possible protective effect from anti-TNF therapy in a subset of patients with less than 11 years disease duration at the start of therapy, the researchers said.
The researchers said that they had no relevant financial conflicts to disclose. The study was funded by grants from multiple nonpharmaceutical organizations including the Swedish Research Council, Lund University, Region Skåne, the Swedish Social Insurance Agency, the Swedish Rheumatism Association, and the King Gustav V 80-Year Fund.
A significant 30% reduction in the number of sick leave days used per month was seen in adults with rheumatoid arthritis after using tumor necrosis factor antagonists for 6 months.
The finding was observed in a population-based study of 365 RA patients aged 18-85 years.
The study is among the first to address the quantitative impact of TNF inhibitors on sick leave and disability pension, said Dr. Tor Olofsson of Lund (Sweden) University, and colleagues.
They reviewed insurance database information on RA patients enrolled in the South Swedish Arthritis Treatment Group registry. Each patient was matched with four controls from the general population.
The study population averaged 9 sick days per month in the first month of anti-TNF treatment. The monthly rate dropped to an average of 6.5 days after 6 months and remained steady at an average of 6.6 days per month for months 6-12 (Ann. Rheum. Dis. 2010;69:2131-6).
Compared with the controls in the general population, the relative risk of being on sick leave in the RA group was 6.6 at the start of treatment, but dropped to 5.1 after 6 months, and remained at an average of 5.2 for the rest of the year. The relative risk of being on disability pension was 3.4 at the start of treatment and 3.2 after 1 year of treatment.
Approximately 98% of the patients had tried at least one disease-modifying antirheumatic drug (DMARD) before starting anti-TNF therapy. The average age of the patients was 46 years, and 82% were women. A total of 92 patients (25%) discontinued treatment, including 34 for adverse events, 32 for treatment failure, and 26 for other reasons.
"The reduction in sick leave during the first anti-TNF treatment year must be considered as a conservative measure in this cohort of patients who were severely ill, since there was a steady increase in sick leave prior to initiation of therapy," the researchers wrote. "Even a stabilization of the sick leave level could have been deemed as a successful outcome," they noted.
The results support findings from previous studies of the effects of TNF inhibitors on work outcomes, although the study was limited by the short follow-up period, the researchers noted. The findings conflict with data from a recent observational study that showed no difference in employment loss between RA patients using anti-TNF therapy and controls.
However, the average duration of RA in the observational study was 12 years, compared to an average duration of 4.5 years in the current study. This difference suggests a possible protective effect from anti-TNF therapy in a subset of patients with less than 11 years disease duration at the start of therapy, the researchers said.
The researchers said that they had no relevant financial conflicts to disclose. The study was funded by grants from multiple nonpharmaceutical organizations including the Swedish Research Council, Lund University, Region Skåne, the Swedish Social Insurance Agency, the Swedish Rheumatism Association, and the King Gustav V 80-Year Fund.
FROM ANNALS OF THE RHEUMATIC DISEASES
Major Finding: TNF-inhibitor users reduced the average sick leave time from 9.8 days per month at the start of treatment to 6.5 days after 6 months of treatment.
Data Source: A study of 365 Swedish adults aged 18-58 years with RA.
Disclosures: The researchers said that they had no relevant financial disclosures.
RA: TNF Inhibitor Users Report Less Sick Leave
A significant 30% reduction in the number of sick leave days used per month was seen in adults with rheumatoid arthritis after using tumor necrosis factor antagonists for 6 months.
The finding was observed in a population-based study of 365 RA patients aged 18-85 years.
[TNF Inhibitors Appear to Reduce Diabetes Risk in RA]
The study is among the first to address the quantitative impact of TNF inhibitors on sick leave and disability pension, said Dr. Tor Olofsson of Lund (Sweden) University, and colleagues, whose study was published in the December issue of Annals of the Rheumatic Diseases.
They reviewed insurance database information on RA patients enrolled in the South Swedish Arthritis Treatment Group registry. Each patient was matched with four controls from the general population.
The study population averaged 9 sick days per month in the first month of anti-TNF treatment. The monthly rate dropped to an average of 6.5 days after 6 months and remained steady at an average of 6.6 days per month for months 6-12 (Ann. Rheum. Dis. 2010;69:2131-6).
Compared with the controls in the general population, the relative risk of being on sick leave in the RA group was 6.6 at the start of treatment, but dropped to 5.1 after 6 months, and remained at an average of 5.2 for the rest of the year. The relative risk of being on disability pension was 3.4 at the start of treatment and 3.2 after 1 year of treatment.
Approximately 98% of the patients had tried at least one disease-modifying antirheumatic drug (DMARD) before starting anti-TNF therapy. The average age of the patients was 46 years, and 82% were women. A total of 92 patients (25%) discontinued treatment, including 34 for adverse events, 32 for treatment failure, and 26 for other reasons.
[TNF Inhibitors Protect Against Plaque Buildup in RA Patients]
"The reduction in sick leave during the first anti-TNF treatment year must be considered as a conservative measure in this cohort of patients who were severely ill, since there was a steady increase in sick leave prior to initiation of therapy," the researchers wrote. "Even a stabilization of the sick leave level could have been deemed as a successful outcome," they noted.
The results support findings from previous studies of the effects of TNF inhibitors on work outcomes, although the study was limited by the short follow-up period, the researchers noted. The findings conflict with data from a recent observational study that showed no difference in employment loss between RA patients using anti-TNF therapy and controls. However, the average duration of RA in the observational study was 12 years, compared to an average duration of 4.5 years in the current study. This difference suggests a possible protective effect from anti-TNF therapy in a subset of patients with less than 11 years disease duration at the start of therapy, the researchers said.
[Switching TNF Inhibitors Does Not Increase Serious Infection Rate in RA]
The researchers said that they had no relevant financial conflicts to disclose. The study was funded by grants from multiple nonpharmaceutical organizations including the Swedish Research Council, Lund University, Region Skåne, the Swedish Social Insurance Agency, the Swedish Rheumatism Association, and the King Gustav V 80-Year Fund.
A significant 30% reduction in the number of sick leave days used per month was seen in adults with rheumatoid arthritis after using tumor necrosis factor antagonists for 6 months.
The finding was observed in a population-based study of 365 RA patients aged 18-85 years.
[TNF Inhibitors Appear to Reduce Diabetes Risk in RA]
The study is among the first to address the quantitative impact of TNF inhibitors on sick leave and disability pension, said Dr. Tor Olofsson of Lund (Sweden) University, and colleagues, whose study was published in the December issue of Annals of the Rheumatic Diseases.
They reviewed insurance database information on RA patients enrolled in the South Swedish Arthritis Treatment Group registry. Each patient was matched with four controls from the general population.
The study population averaged 9 sick days per month in the first month of anti-TNF treatment. The monthly rate dropped to an average of 6.5 days after 6 months and remained steady at an average of 6.6 days per month for months 6-12 (Ann. Rheum. Dis. 2010;69:2131-6).
Compared with the controls in the general population, the relative risk of being on sick leave in the RA group was 6.6 at the start of treatment, but dropped to 5.1 after 6 months, and remained at an average of 5.2 for the rest of the year. The relative risk of being on disability pension was 3.4 at the start of treatment and 3.2 after 1 year of treatment.
Approximately 98% of the patients had tried at least one disease-modifying antirheumatic drug (DMARD) before starting anti-TNF therapy. The average age of the patients was 46 years, and 82% were women. A total of 92 patients (25%) discontinued treatment, including 34 for adverse events, 32 for treatment failure, and 26 for other reasons.
[TNF Inhibitors Protect Against Plaque Buildup in RA Patients]
"The reduction in sick leave during the first anti-TNF treatment year must be considered as a conservative measure in this cohort of patients who were severely ill, since there was a steady increase in sick leave prior to initiation of therapy," the researchers wrote. "Even a stabilization of the sick leave level could have been deemed as a successful outcome," they noted.
The results support findings from previous studies of the effects of TNF inhibitors on work outcomes, although the study was limited by the short follow-up period, the researchers noted. The findings conflict with data from a recent observational study that showed no difference in employment loss between RA patients using anti-TNF therapy and controls. However, the average duration of RA in the observational study was 12 years, compared to an average duration of 4.5 years in the current study. This difference suggests a possible protective effect from anti-TNF therapy in a subset of patients with less than 11 years disease duration at the start of therapy, the researchers said.
[Switching TNF Inhibitors Does Not Increase Serious Infection Rate in RA]
The researchers said that they had no relevant financial conflicts to disclose. The study was funded by grants from multiple nonpharmaceutical organizations including the Swedish Research Council, Lund University, Region Skåne, the Swedish Social Insurance Agency, the Swedish Rheumatism Association, and the King Gustav V 80-Year Fund.
A significant 30% reduction in the number of sick leave days used per month was seen in adults with rheumatoid arthritis after using tumor necrosis factor antagonists for 6 months.
The finding was observed in a population-based study of 365 RA patients aged 18-85 years.
[TNF Inhibitors Appear to Reduce Diabetes Risk in RA]
The study is among the first to address the quantitative impact of TNF inhibitors on sick leave and disability pension, said Dr. Tor Olofsson of Lund (Sweden) University, and colleagues, whose study was published in the December issue of Annals of the Rheumatic Diseases.
They reviewed insurance database information on RA patients enrolled in the South Swedish Arthritis Treatment Group registry. Each patient was matched with four controls from the general population.
The study population averaged 9 sick days per month in the first month of anti-TNF treatment. The monthly rate dropped to an average of 6.5 days after 6 months and remained steady at an average of 6.6 days per month for months 6-12 (Ann. Rheum. Dis. 2010;69:2131-6).
Compared with the controls in the general population, the relative risk of being on sick leave in the RA group was 6.6 at the start of treatment, but dropped to 5.1 after 6 months, and remained at an average of 5.2 for the rest of the year. The relative risk of being on disability pension was 3.4 at the start of treatment and 3.2 after 1 year of treatment.
Approximately 98% of the patients had tried at least one disease-modifying antirheumatic drug (DMARD) before starting anti-TNF therapy. The average age of the patients was 46 years, and 82% were women. A total of 92 patients (25%) discontinued treatment, including 34 for adverse events, 32 for treatment failure, and 26 for other reasons.
[TNF Inhibitors Protect Against Plaque Buildup in RA Patients]
"The reduction in sick leave during the first anti-TNF treatment year must be considered as a conservative measure in this cohort of patients who were severely ill, since there was a steady increase in sick leave prior to initiation of therapy," the researchers wrote. "Even a stabilization of the sick leave level could have been deemed as a successful outcome," they noted.
The results support findings from previous studies of the effects of TNF inhibitors on work outcomes, although the study was limited by the short follow-up period, the researchers noted. The findings conflict with data from a recent observational study that showed no difference in employment loss between RA patients using anti-TNF therapy and controls. However, the average duration of RA in the observational study was 12 years, compared to an average duration of 4.5 years in the current study. This difference suggests a possible protective effect from anti-TNF therapy in a subset of patients with less than 11 years disease duration at the start of therapy, the researchers said.
[Switching TNF Inhibitors Does Not Increase Serious Infection Rate in RA]
The researchers said that they had no relevant financial conflicts to disclose. The study was funded by grants from multiple nonpharmaceutical organizations including the Swedish Research Council, Lund University, Region Skåne, the Swedish Social Insurance Agency, the Swedish Rheumatism Association, and the King Gustav V 80-Year Fund.
FROM ANNALS OF THE RHEUMATIC DISEASES
Major Finding: TNF-inhibitor users reduced the average sick leave time from 9.8 days per month at the start of treatment to 6.5 days after 6 months of treatment.
Data Source: A study of 365 Swedish adults aged 18-58 years with rheumatoid arthritis
Disclosures: The researchers said that they had no relevant financial disclosures.