User login
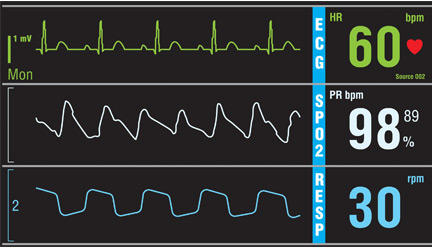
Do all hospital inpatients need cardiac telemetry?
No. Continuous monitoring for changes in heart rhythm with cardiac telemetry is recommended for all patients admitted to an intensive care unit (ICU). But routine telemetry monitoring for patients in non-ICU beds is not recommended, as it leads to unnecessary testing and treatment, increasing the cost of care and hospital length of stay.
RISK STRATIFICATION AND INDICATIONS
Telemetry is generally recommended for patients admitted with any type of heart disease, including:
- Acute myocardial infarction with ST-segment elevation or Q waves on 12-lead electrocardiography (ECG)
- Acute ischemia suggested by ST-segment depression or T-wave inversion on ECG
- Systolic blood pressure less than 100 mm Hg
- Acute decompensated heart failure with bilateral rales above the lung bases
- Chest pain that is worse than or the same as that in prior angina or myocardial infarction.1,2
Indications for telemetry are less clear in patients with no history of heart disease. The American Heart Association (AHA)3 has classified admitted patients based on the presence or absence of heart disease3:
- Class I (high risk of arrhythmia): acute coronary syndrome, new arrhythmia (eg, atrial fibrillation or flutter), severe electrolyte imbalance; telemetry is warranted
- Class II (moderate risk): acute decompensated heart failure with stable hemodynamic status, a surgical or medical diagnosis with underlying paced rhythms (ie, with a pacemaker), and chronic arrhythmia (atrial fibrillation or flutter); in these cases, telemetry monitoring may be considered
- Class III (low risk): no history of cardiac disease or arrhythmias, admitted for medical or surgical reasons; in these cases, telemetry is generally not indicated3
Telemetry should also be considered in patients admitted with syncope or stroke, critical illness, or palpitations.
Syncope and stroke
Despite the wide use of telemetry for patients admitted with syncope, current evidence does not support this practice. However, the AHA recommends routine telemetry for patients admitted with idiopathic syncope when there is a high level of suspicion for underlying cardiac arrhythmias as a cause of syncope (risk class II-b).3 In 30% of patients admitted with stroke or transient ischemic attack, the cause is cardioembolic. Therefore, telemetry is indicated to rule out an underlying cardiac cause.4
Critical illness
Patients hospitalized with major trauma, acute respiratory failure, sepsis, shock, or acute pulmonary embolism or for major noncardiac surgery (especially elderly patients with coronary artery disease or at high risk of coronary events) require cardiac telemetry (risk class I-b). Patients admitted with kidney failure, significant electrolyte abnormalities, drug or substance toxicity (especially with known arrhythmogenic drugs) also require cardiac telemetry at the time of admission (risk class I-b).
Recurrent palpitations, arrhythmia
Most patients with palpitations can be evaluated in an outpatient setting.5 However, patients hospitalized for recurrent palpitations or for suspected underlying cardiac disease require telemetric monitoring (risk class II-b).3 Patients with high-degree atrioventricular block admitted after percutaneous temporary pacemaker implantation should be monitored, as should patients with a permanent pacemaker for 12 to 24 hours after implantation (risk class I-c). Also, patients hospitalized after implantable cardioverter-defibrillator (ICD) shock need to be monitored.3,6
Patients with a paced rhythm who do not meet the above criteria do not require routine telemetric monitoring (risk class III-c).7
TELEMETRY IS OVERUSED
Off-site telemetry monitoring can identify significant arrhythmias during hospitalization. It also saves time on nursing staff to focus on bedside patient care. However, its convenience can lower the threshold for ordering it. This can lead to overuse with a major impact on healthcare costs.
Routine use of cardiac telemetry is associated with increased hospitalization costs with little benefit.8 The use of off-site services for continuous monitoring can activate many alarms throughout the day, triggering unnecessary workups and leading to densensitization to alarms (“alarm fatigue”).9
Despite the precise indications outlined in the AHA updated practice standards for inpatient electrocardiographic monitoring,10 telemetry use is expanding to non-ICU units without evidence of benefit,8 and this overuse can result in harmful clinical outcomes and a financial burden. Telemetry monitoring of low-risk patients can cause delays in emergency department and ICU admissions and transfers8,11 of patients who may be sicker and need intensive care.
In a prospective observational study,12 only 11 (6%) of 182 patients admitted to a general medical floor met AHA class I criteria for telemetry; very few patients developed a significant telemetry event such as atrial fibrillation or flutter that necessitated a change in management. Most overprescribers of telemetry monitoring reason that it will catch arrhythmias early.12 In fact, in a study of patients in a cardiac unit, telemetry detected just 50% of in-house cardiac arrest cases, with a potential survival benefit of only 0.02%.13
Another study showed that only 0.01% of all telemetry alarms represented a real emergency. Only 37.2% of emergency alarms were classified as clinically important, and only 48.3% of these led to a change in management within 1 hour.14
Moreover, in a report of trauma patients with abnormal results on ECG at the time of admission, telemetry had negligible clinical benefit.15 And in a study of 414 patients, only 4% of those admitted with chest pain and normal initial ECG had cardiac interventions.16
Another study8 showed that hospital intervention to restrict the use of telemetry guided by AHA recommendations resulted in a 43% reduction in telemetry orders, a 47% reduction in telemetry duration, and a 70% reduction in the mean daily number of patients monitored, with no changes in hospital census or rates of code blue, death, or rapid response team activation.8
The financial cost can be seen in the backup of patients in the emergency department. A study showed that 91% of patients being admitted for chest pain were delayed by more than 3 hours while waiting for monitored beds. This translated into an annual cost of $168,300 to the hospital.17 Adherence to guidelines for appropriate use of telemetry can significantly decrease costs. Applying a simple algorithm for telemetry use was shown8 to decrease daily non-ICU cardiac telemetry costs from $18,971 to $5,772.
CURRENT GUIDELINES ARE LIMITED
The current American College of Cardiology and AHA guidelines are based mostly on expert opinion rather than randomized clinical trials, while most telemetry trials have been performed on patients with a cardiac or possible cardiac diagnosis.3 Current guidelines need to be updated, and more studies are needed to specify the optimal duration of cardiac monitoring in indicated cases. Many noncardiac conditions raise a legitimate concern of dysrhythmia, an indication for cardiac monitoring, but precise recommendations for telemetry for such conditions are lacking.
RECOMMENDATIONS
Raising awareness of the clinical and financial burdens associated with unwise telemetry utilization is critical. We suggest use of a pop-up notification in the electronic medical record to remind the provider of the existing telemetry order and to specify the duration of telemetry monitoring when placing the initial order. The goal is to identify patients in true need of a telemetry bed, to decrease unnecessary testing, and to reduce hospitalization costs.
- Recommended guidelines for in-hospital cardiac monitoring of adults for detection of arrhythmia. Emergency Cardiac Care Committee members. J Am Coll Cardiol 1991; 18(6):1431–1433. pmid:1939942
- Goldman L, Cook EF, Johnson PA, Brand DA, Rouan GW, Lee TH. Prediction of the need for intensive care in patients who come to emergency departments with acute chest pain. N Engl J Med 1996; 334(23):1498–1504. doi:10.1056/NEJM199606063342303
- Drew BJ, Califf RM, Funk M, et al; American Heart Association; Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation 2004;110(17):2721–2746. doi:10.1161/01.CIR.0000145144.56673.59
- Ustrell X, Pellise A. Cardiac workup of ischemic stroke. Curr Cardiol Rev 2010; 6(3):175-183. doi:10.2174/157340310791658721
- Olson JA, Fouts AM, Padanilam BJ, Prystowsky EN. Utility of mobile cardiac outpatient telemetry for the diagnosis of palpitations, presyncope, syncope, and the assessment of therapy efficacy. J Cardiovasc Electrophysiol 2007; 18(5):473–477. doi:10.1111/j.1540-8167.2007.00779.x
- Chen EH, Hollander JE. When do patients need admission to a telemetry bed? J Emerg Med 2007; 33(1):53–60. doi:10.1016/j.jemermed.2007.01.017
- Sandau KE, Funk M, Auerbach A, et al; American Heart Association Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; and Council on Cardiovascular Disease in the Young. Update to practice standards for electrocardiographic monitoring in hospital settings: a scientific statement from the American Heart Association. Circulation 2017; 136(19):e273–e344. doi:10.1161/CIR.0000000000000527
- Dressler R, Dryer MM, Coletti C, Mahoney D, Doorey AJ. Altering overuse of cardiac telemetry in non-intensive care unit settings by hardwiring the use of American Heart Association guidelines. JAMA Intern Med 2014; 174(11):1852–1854. doi:10.1001/jamainternmed.2014.4491
- Cantillon DJ, Loy M, Burkle A, et al. Association between off-site central monitoring using standardized cardiac telemetry and clinical outcomes among non–critically ill patients. JAMA 2016; 316(5):519–524. doi:10.1001/jama.2016.10258
- Sandau KE, Funk M, Auerbach A, et al. Update to practice standards for electrocardiographic monitoring in hospital settings: a scientific statement from the American Heart Association. Circulation 2017; 136(19):e273–e344. doi:10.1161/CIR.0000000000000527
- Atzema C, Schull MJ, Borgundvaag B, Slaughter GR, Lee CK. ALARMED: adverse events in low-risk patients with chest pain receiving continuous electrocardiographic monitoring in the emergency department. A pilot study. Am J Emerg Med 2006; 24(1):62–67. doi:10.1016/j.ajem.2005.05.015
- Najafi N, Auerbach A. Use and outcomes of telemetry monitoring on a medicine service. Arch Intern Med 2012; 172(17):1349–1350. doi:10.1001/archinternmed.2012.3163
- Schull MJ, Redelmeier DA. Continuous electrocardiographic monitoring and cardiac arrest outcomes in 8,932 telemetry ward patients. Acad Emerg Med 2000; 7(6):647–652. pmid:10905643
- Kansara P, Jackson K, Dressler R, et al. Potential of missing life-threatening arrhythmias after limiting the use of cardiac telemetry. JAMA Intern Med 2015; 175(8):1416–1418. doi:10.1001/jamainternmed.2015.2387
- Nagy KK, Krosner SM, Roberts RR, Joseph KT, Smith RF, Barrett J. Determining which patients require evaluation for blunt cardiac injury following blunt chest trauma. World J Surg 2001; 25(1):108–111. pmid:11213149
- Snider A, Papaleo M, Beldner S, et al. Is telemetry monitoring necessary in low-risk suspected acute chest pain syndromes? Chest 2002; 122(2):517–523. pmid:12171825
- Bayley MD, Schwartz JS, Shofer FS, et al. The financial burden of emergency department congestion and hospital crowding for chest pain patients awaiting admission. Ann Emerg Med 2005; 45(2):110–117. doi:10.1016/j.annemergmed.2004.09.010
No. Continuous monitoring for changes in heart rhythm with cardiac telemetry is recommended for all patients admitted to an intensive care unit (ICU). But routine telemetry monitoring for patients in non-ICU beds is not recommended, as it leads to unnecessary testing and treatment, increasing the cost of care and hospital length of stay.
RISK STRATIFICATION AND INDICATIONS
Telemetry is generally recommended for patients admitted with any type of heart disease, including:
- Acute myocardial infarction with ST-segment elevation or Q waves on 12-lead electrocardiography (ECG)
- Acute ischemia suggested by ST-segment depression or T-wave inversion on ECG
- Systolic blood pressure less than 100 mm Hg
- Acute decompensated heart failure with bilateral rales above the lung bases
- Chest pain that is worse than or the same as that in prior angina or myocardial infarction.1,2
Indications for telemetry are less clear in patients with no history of heart disease. The American Heart Association (AHA)3 has classified admitted patients based on the presence or absence of heart disease3:
- Class I (high risk of arrhythmia): acute coronary syndrome, new arrhythmia (eg, atrial fibrillation or flutter), severe electrolyte imbalance; telemetry is warranted
- Class II (moderate risk): acute decompensated heart failure with stable hemodynamic status, a surgical or medical diagnosis with underlying paced rhythms (ie, with a pacemaker), and chronic arrhythmia (atrial fibrillation or flutter); in these cases, telemetry monitoring may be considered
- Class III (low risk): no history of cardiac disease or arrhythmias, admitted for medical or surgical reasons; in these cases, telemetry is generally not indicated3
Telemetry should also be considered in patients admitted with syncope or stroke, critical illness, or palpitations.
Syncope and stroke
Despite the wide use of telemetry for patients admitted with syncope, current evidence does not support this practice. However, the AHA recommends routine telemetry for patients admitted with idiopathic syncope when there is a high level of suspicion for underlying cardiac arrhythmias as a cause of syncope (risk class II-b).3 In 30% of patients admitted with stroke or transient ischemic attack, the cause is cardioembolic. Therefore, telemetry is indicated to rule out an underlying cardiac cause.4
Critical illness
Patients hospitalized with major trauma, acute respiratory failure, sepsis, shock, or acute pulmonary embolism or for major noncardiac surgery (especially elderly patients with coronary artery disease or at high risk of coronary events) require cardiac telemetry (risk class I-b). Patients admitted with kidney failure, significant electrolyte abnormalities, drug or substance toxicity (especially with known arrhythmogenic drugs) also require cardiac telemetry at the time of admission (risk class I-b).
Recurrent palpitations, arrhythmia
Most patients with palpitations can be evaluated in an outpatient setting.5 However, patients hospitalized for recurrent palpitations or for suspected underlying cardiac disease require telemetric monitoring (risk class II-b).3 Patients with high-degree atrioventricular block admitted after percutaneous temporary pacemaker implantation should be monitored, as should patients with a permanent pacemaker for 12 to 24 hours after implantation (risk class I-c). Also, patients hospitalized after implantable cardioverter-defibrillator (ICD) shock need to be monitored.3,6
Patients with a paced rhythm who do not meet the above criteria do not require routine telemetric monitoring (risk class III-c).7
TELEMETRY IS OVERUSED
Off-site telemetry monitoring can identify significant arrhythmias during hospitalization. It also saves time on nursing staff to focus on bedside patient care. However, its convenience can lower the threshold for ordering it. This can lead to overuse with a major impact on healthcare costs.
Routine use of cardiac telemetry is associated with increased hospitalization costs with little benefit.8 The use of off-site services for continuous monitoring can activate many alarms throughout the day, triggering unnecessary workups and leading to densensitization to alarms (“alarm fatigue”).9
Despite the precise indications outlined in the AHA updated practice standards for inpatient electrocardiographic monitoring,10 telemetry use is expanding to non-ICU units without evidence of benefit,8 and this overuse can result in harmful clinical outcomes and a financial burden. Telemetry monitoring of low-risk patients can cause delays in emergency department and ICU admissions and transfers8,11 of patients who may be sicker and need intensive care.
In a prospective observational study,12 only 11 (6%) of 182 patients admitted to a general medical floor met AHA class I criteria for telemetry; very few patients developed a significant telemetry event such as atrial fibrillation or flutter that necessitated a change in management. Most overprescribers of telemetry monitoring reason that it will catch arrhythmias early.12 In fact, in a study of patients in a cardiac unit, telemetry detected just 50% of in-house cardiac arrest cases, with a potential survival benefit of only 0.02%.13
Another study showed that only 0.01% of all telemetry alarms represented a real emergency. Only 37.2% of emergency alarms were classified as clinically important, and only 48.3% of these led to a change in management within 1 hour.14
Moreover, in a report of trauma patients with abnormal results on ECG at the time of admission, telemetry had negligible clinical benefit.15 And in a study of 414 patients, only 4% of those admitted with chest pain and normal initial ECG had cardiac interventions.16
Another study8 showed that hospital intervention to restrict the use of telemetry guided by AHA recommendations resulted in a 43% reduction in telemetry orders, a 47% reduction in telemetry duration, and a 70% reduction in the mean daily number of patients monitored, with no changes in hospital census or rates of code blue, death, or rapid response team activation.8
The financial cost can be seen in the backup of patients in the emergency department. A study showed that 91% of patients being admitted for chest pain were delayed by more than 3 hours while waiting for monitored beds. This translated into an annual cost of $168,300 to the hospital.17 Adherence to guidelines for appropriate use of telemetry can significantly decrease costs. Applying a simple algorithm for telemetry use was shown8 to decrease daily non-ICU cardiac telemetry costs from $18,971 to $5,772.
CURRENT GUIDELINES ARE LIMITED
The current American College of Cardiology and AHA guidelines are based mostly on expert opinion rather than randomized clinical trials, while most telemetry trials have been performed on patients with a cardiac or possible cardiac diagnosis.3 Current guidelines need to be updated, and more studies are needed to specify the optimal duration of cardiac monitoring in indicated cases. Many noncardiac conditions raise a legitimate concern of dysrhythmia, an indication for cardiac monitoring, but precise recommendations for telemetry for such conditions are lacking.
RECOMMENDATIONS
Raising awareness of the clinical and financial burdens associated with unwise telemetry utilization is critical. We suggest use of a pop-up notification in the electronic medical record to remind the provider of the existing telemetry order and to specify the duration of telemetry monitoring when placing the initial order. The goal is to identify patients in true need of a telemetry bed, to decrease unnecessary testing, and to reduce hospitalization costs.
No. Continuous monitoring for changes in heart rhythm with cardiac telemetry is recommended for all patients admitted to an intensive care unit (ICU). But routine telemetry monitoring for patients in non-ICU beds is not recommended, as it leads to unnecessary testing and treatment, increasing the cost of care and hospital length of stay.
RISK STRATIFICATION AND INDICATIONS
Telemetry is generally recommended for patients admitted with any type of heart disease, including:
- Acute myocardial infarction with ST-segment elevation or Q waves on 12-lead electrocardiography (ECG)
- Acute ischemia suggested by ST-segment depression or T-wave inversion on ECG
- Systolic blood pressure less than 100 mm Hg
- Acute decompensated heart failure with bilateral rales above the lung bases
- Chest pain that is worse than or the same as that in prior angina or myocardial infarction.1,2
Indications for telemetry are less clear in patients with no history of heart disease. The American Heart Association (AHA)3 has classified admitted patients based on the presence or absence of heart disease3:
- Class I (high risk of arrhythmia): acute coronary syndrome, new arrhythmia (eg, atrial fibrillation or flutter), severe electrolyte imbalance; telemetry is warranted
- Class II (moderate risk): acute decompensated heart failure with stable hemodynamic status, a surgical or medical diagnosis with underlying paced rhythms (ie, with a pacemaker), and chronic arrhythmia (atrial fibrillation or flutter); in these cases, telemetry monitoring may be considered
- Class III (low risk): no history of cardiac disease or arrhythmias, admitted for medical or surgical reasons; in these cases, telemetry is generally not indicated3
Telemetry should also be considered in patients admitted with syncope or stroke, critical illness, or palpitations.
Syncope and stroke
Despite the wide use of telemetry for patients admitted with syncope, current evidence does not support this practice. However, the AHA recommends routine telemetry for patients admitted with idiopathic syncope when there is a high level of suspicion for underlying cardiac arrhythmias as a cause of syncope (risk class II-b).3 In 30% of patients admitted with stroke or transient ischemic attack, the cause is cardioembolic. Therefore, telemetry is indicated to rule out an underlying cardiac cause.4
Critical illness
Patients hospitalized with major trauma, acute respiratory failure, sepsis, shock, or acute pulmonary embolism or for major noncardiac surgery (especially elderly patients with coronary artery disease or at high risk of coronary events) require cardiac telemetry (risk class I-b). Patients admitted with kidney failure, significant electrolyte abnormalities, drug or substance toxicity (especially with known arrhythmogenic drugs) also require cardiac telemetry at the time of admission (risk class I-b).
Recurrent palpitations, arrhythmia
Most patients with palpitations can be evaluated in an outpatient setting.5 However, patients hospitalized for recurrent palpitations or for suspected underlying cardiac disease require telemetric monitoring (risk class II-b).3 Patients with high-degree atrioventricular block admitted after percutaneous temporary pacemaker implantation should be monitored, as should patients with a permanent pacemaker for 12 to 24 hours after implantation (risk class I-c). Also, patients hospitalized after implantable cardioverter-defibrillator (ICD) shock need to be monitored.3,6
Patients with a paced rhythm who do not meet the above criteria do not require routine telemetric monitoring (risk class III-c).7
TELEMETRY IS OVERUSED
Off-site telemetry monitoring can identify significant arrhythmias during hospitalization. It also saves time on nursing staff to focus on bedside patient care. However, its convenience can lower the threshold for ordering it. This can lead to overuse with a major impact on healthcare costs.
Routine use of cardiac telemetry is associated with increased hospitalization costs with little benefit.8 The use of off-site services for continuous monitoring can activate many alarms throughout the day, triggering unnecessary workups and leading to densensitization to alarms (“alarm fatigue”).9
Despite the precise indications outlined in the AHA updated practice standards for inpatient electrocardiographic monitoring,10 telemetry use is expanding to non-ICU units without evidence of benefit,8 and this overuse can result in harmful clinical outcomes and a financial burden. Telemetry monitoring of low-risk patients can cause delays in emergency department and ICU admissions and transfers8,11 of patients who may be sicker and need intensive care.
In a prospective observational study,12 only 11 (6%) of 182 patients admitted to a general medical floor met AHA class I criteria for telemetry; very few patients developed a significant telemetry event such as atrial fibrillation or flutter that necessitated a change in management. Most overprescribers of telemetry monitoring reason that it will catch arrhythmias early.12 In fact, in a study of patients in a cardiac unit, telemetry detected just 50% of in-house cardiac arrest cases, with a potential survival benefit of only 0.02%.13
Another study showed that only 0.01% of all telemetry alarms represented a real emergency. Only 37.2% of emergency alarms were classified as clinically important, and only 48.3% of these led to a change in management within 1 hour.14
Moreover, in a report of trauma patients with abnormal results on ECG at the time of admission, telemetry had negligible clinical benefit.15 And in a study of 414 patients, only 4% of those admitted with chest pain and normal initial ECG had cardiac interventions.16
Another study8 showed that hospital intervention to restrict the use of telemetry guided by AHA recommendations resulted in a 43% reduction in telemetry orders, a 47% reduction in telemetry duration, and a 70% reduction in the mean daily number of patients monitored, with no changes in hospital census or rates of code blue, death, or rapid response team activation.8
The financial cost can be seen in the backup of patients in the emergency department. A study showed that 91% of patients being admitted for chest pain were delayed by more than 3 hours while waiting for monitored beds. This translated into an annual cost of $168,300 to the hospital.17 Adherence to guidelines for appropriate use of telemetry can significantly decrease costs. Applying a simple algorithm for telemetry use was shown8 to decrease daily non-ICU cardiac telemetry costs from $18,971 to $5,772.
CURRENT GUIDELINES ARE LIMITED
The current American College of Cardiology and AHA guidelines are based mostly on expert opinion rather than randomized clinical trials, while most telemetry trials have been performed on patients with a cardiac or possible cardiac diagnosis.3 Current guidelines need to be updated, and more studies are needed to specify the optimal duration of cardiac monitoring in indicated cases. Many noncardiac conditions raise a legitimate concern of dysrhythmia, an indication for cardiac monitoring, but precise recommendations for telemetry for such conditions are lacking.
RECOMMENDATIONS
Raising awareness of the clinical and financial burdens associated with unwise telemetry utilization is critical. We suggest use of a pop-up notification in the electronic medical record to remind the provider of the existing telemetry order and to specify the duration of telemetry monitoring when placing the initial order. The goal is to identify patients in true need of a telemetry bed, to decrease unnecessary testing, and to reduce hospitalization costs.
- Recommended guidelines for in-hospital cardiac monitoring of adults for detection of arrhythmia. Emergency Cardiac Care Committee members. J Am Coll Cardiol 1991; 18(6):1431–1433. pmid:1939942
- Goldman L, Cook EF, Johnson PA, Brand DA, Rouan GW, Lee TH. Prediction of the need for intensive care in patients who come to emergency departments with acute chest pain. N Engl J Med 1996; 334(23):1498–1504. doi:10.1056/NEJM199606063342303
- Drew BJ, Califf RM, Funk M, et al; American Heart Association; Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation 2004;110(17):2721–2746. doi:10.1161/01.CIR.0000145144.56673.59
- Ustrell X, Pellise A. Cardiac workup of ischemic stroke. Curr Cardiol Rev 2010; 6(3):175-183. doi:10.2174/157340310791658721
- Olson JA, Fouts AM, Padanilam BJ, Prystowsky EN. Utility of mobile cardiac outpatient telemetry for the diagnosis of palpitations, presyncope, syncope, and the assessment of therapy efficacy. J Cardiovasc Electrophysiol 2007; 18(5):473–477. doi:10.1111/j.1540-8167.2007.00779.x
- Chen EH, Hollander JE. When do patients need admission to a telemetry bed? J Emerg Med 2007; 33(1):53–60. doi:10.1016/j.jemermed.2007.01.017
- Sandau KE, Funk M, Auerbach A, et al; American Heart Association Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; and Council on Cardiovascular Disease in the Young. Update to practice standards for electrocardiographic monitoring in hospital settings: a scientific statement from the American Heart Association. Circulation 2017; 136(19):e273–e344. doi:10.1161/CIR.0000000000000527
- Dressler R, Dryer MM, Coletti C, Mahoney D, Doorey AJ. Altering overuse of cardiac telemetry in non-intensive care unit settings by hardwiring the use of American Heart Association guidelines. JAMA Intern Med 2014; 174(11):1852–1854. doi:10.1001/jamainternmed.2014.4491
- Cantillon DJ, Loy M, Burkle A, et al. Association between off-site central monitoring using standardized cardiac telemetry and clinical outcomes among non–critically ill patients. JAMA 2016; 316(5):519–524. doi:10.1001/jama.2016.10258
- Sandau KE, Funk M, Auerbach A, et al. Update to practice standards for electrocardiographic monitoring in hospital settings: a scientific statement from the American Heart Association. Circulation 2017; 136(19):e273–e344. doi:10.1161/CIR.0000000000000527
- Atzema C, Schull MJ, Borgundvaag B, Slaughter GR, Lee CK. ALARMED: adverse events in low-risk patients with chest pain receiving continuous electrocardiographic monitoring in the emergency department. A pilot study. Am J Emerg Med 2006; 24(1):62–67. doi:10.1016/j.ajem.2005.05.015
- Najafi N, Auerbach A. Use and outcomes of telemetry monitoring on a medicine service. Arch Intern Med 2012; 172(17):1349–1350. doi:10.1001/archinternmed.2012.3163
- Schull MJ, Redelmeier DA. Continuous electrocardiographic monitoring and cardiac arrest outcomes in 8,932 telemetry ward patients. Acad Emerg Med 2000; 7(6):647–652. pmid:10905643
- Kansara P, Jackson K, Dressler R, et al. Potential of missing life-threatening arrhythmias after limiting the use of cardiac telemetry. JAMA Intern Med 2015; 175(8):1416–1418. doi:10.1001/jamainternmed.2015.2387
- Nagy KK, Krosner SM, Roberts RR, Joseph KT, Smith RF, Barrett J. Determining which patients require evaluation for blunt cardiac injury following blunt chest trauma. World J Surg 2001; 25(1):108–111. pmid:11213149
- Snider A, Papaleo M, Beldner S, et al. Is telemetry monitoring necessary in low-risk suspected acute chest pain syndromes? Chest 2002; 122(2):517–523. pmid:12171825
- Bayley MD, Schwartz JS, Shofer FS, et al. The financial burden of emergency department congestion and hospital crowding for chest pain patients awaiting admission. Ann Emerg Med 2005; 45(2):110–117. doi:10.1016/j.annemergmed.2004.09.010
- Recommended guidelines for in-hospital cardiac monitoring of adults for detection of arrhythmia. Emergency Cardiac Care Committee members. J Am Coll Cardiol 1991; 18(6):1431–1433. pmid:1939942
- Goldman L, Cook EF, Johnson PA, Brand DA, Rouan GW, Lee TH. Prediction of the need for intensive care in patients who come to emergency departments with acute chest pain. N Engl J Med 1996; 334(23):1498–1504. doi:10.1056/NEJM199606063342303
- Drew BJ, Califf RM, Funk M, et al; American Heart Association; Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation 2004;110(17):2721–2746. doi:10.1161/01.CIR.0000145144.56673.59
- Ustrell X, Pellise A. Cardiac workup of ischemic stroke. Curr Cardiol Rev 2010; 6(3):175-183. doi:10.2174/157340310791658721
- Olson JA, Fouts AM, Padanilam BJ, Prystowsky EN. Utility of mobile cardiac outpatient telemetry for the diagnosis of palpitations, presyncope, syncope, and the assessment of therapy efficacy. J Cardiovasc Electrophysiol 2007; 18(5):473–477. doi:10.1111/j.1540-8167.2007.00779.x
- Chen EH, Hollander JE. When do patients need admission to a telemetry bed? J Emerg Med 2007; 33(1):53–60. doi:10.1016/j.jemermed.2007.01.017
- Sandau KE, Funk M, Auerbach A, et al; American Heart Association Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; and Council on Cardiovascular Disease in the Young. Update to practice standards for electrocardiographic monitoring in hospital settings: a scientific statement from the American Heart Association. Circulation 2017; 136(19):e273–e344. doi:10.1161/CIR.0000000000000527
- Dressler R, Dryer MM, Coletti C, Mahoney D, Doorey AJ. Altering overuse of cardiac telemetry in non-intensive care unit settings by hardwiring the use of American Heart Association guidelines. JAMA Intern Med 2014; 174(11):1852–1854. doi:10.1001/jamainternmed.2014.4491
- Cantillon DJ, Loy M, Burkle A, et al. Association between off-site central monitoring using standardized cardiac telemetry and clinical outcomes among non–critically ill patients. JAMA 2016; 316(5):519–524. doi:10.1001/jama.2016.10258
- Sandau KE, Funk M, Auerbach A, et al. Update to practice standards for electrocardiographic monitoring in hospital settings: a scientific statement from the American Heart Association. Circulation 2017; 136(19):e273–e344. doi:10.1161/CIR.0000000000000527
- Atzema C, Schull MJ, Borgundvaag B, Slaughter GR, Lee CK. ALARMED: adverse events in low-risk patients with chest pain receiving continuous electrocardiographic monitoring in the emergency department. A pilot study. Am J Emerg Med 2006; 24(1):62–67. doi:10.1016/j.ajem.2005.05.015
- Najafi N, Auerbach A. Use and outcomes of telemetry monitoring on a medicine service. Arch Intern Med 2012; 172(17):1349–1350. doi:10.1001/archinternmed.2012.3163
- Schull MJ, Redelmeier DA. Continuous electrocardiographic monitoring and cardiac arrest outcomes in 8,932 telemetry ward patients. Acad Emerg Med 2000; 7(6):647–652. pmid:10905643
- Kansara P, Jackson K, Dressler R, et al. Potential of missing life-threatening arrhythmias after limiting the use of cardiac telemetry. JAMA Intern Med 2015; 175(8):1416–1418. doi:10.1001/jamainternmed.2015.2387
- Nagy KK, Krosner SM, Roberts RR, Joseph KT, Smith RF, Barrett J. Determining which patients require evaluation for blunt cardiac injury following blunt chest trauma. World J Surg 2001; 25(1):108–111. pmid:11213149
- Snider A, Papaleo M, Beldner S, et al. Is telemetry monitoring necessary in low-risk suspected acute chest pain syndromes? Chest 2002; 122(2):517–523. pmid:12171825
- Bayley MD, Schwartz JS, Shofer FS, et al. The financial burden of emergency department congestion and hospital crowding for chest pain patients awaiting admission. Ann Emerg Med 2005; 45(2):110–117. doi:10.1016/j.annemergmed.2004.09.010
Which patients with pulmonary embolism need echocardiography?
Most patients admitted with pulmonary embolism (PE) do not need transthoracic echocardiography (TTE); it should be performed in hemodynamically unstable patients, as well as in hemodynamically stable patients with specific elevated cardiac biomarkers and imaging features.
The decision to perform TTE should be based on clinical presentation, PE burden, and imaging findings (eg, computed tomographic angiography). TTE helps to stratify risk, guide management, monitor response to therapy, and give prognostic information for a subset of patients at increased risk for PE-related adverse events.
RISK STRATIFICATION IN PULMONARY EMBOLISM
PE has a spectrum of presentations ranging from no symptoms to shock. Based on the clinical presentation, PE can be categorized as high, intermediate, or low risk.
High-risk PE, often referred to as “massive” PE, is defined in current American Heart Association guidelines as acute PE with sustained hypotension (systolic blood pressure < 90 mm Hg for at least 15 minutes or requiring inotropic support), persistent profound bradycardia (heart rate < 40 beats per minute with signs or symptoms of shock), syncope, or cardiac arrest.1
Intermediate-risk or “submassive” PE is more challenging to identify because patients are more hemodynamically stable, yet have evidence on electrocardiography, TTE, computed tomography, or cardiac biomarker testing—ie, N-terminal pro-B-type natriuretic peptide (NT-proBNP) or troponin—that indicates myocardial injury or volume overload.1
Low-risk PE is acute PE in the absence of clinical markers of adverse prognosis that define massive or submassive PE.1
ECHOCARDIOGRAPHIC FEATURES OF HIGH-RISK PULMONARY EMBOLISM
Certain TTE findings suggest increased risk of a poor outcome and may warrant therapy that is more invasive and aggressive. High-risk features include the following:
- Impaired right ventricular function
- Interventricular septum bulging into the left ventricle (“D-shaped” septum)
- Dilated proximal pulmonary arteries
- Increased severity of tricuspid regurgitation
- Elevated right atrial pressure
- Elevated pulmonary artery pressure
- Free-floating right ventricular thrombi, which are associated with a mortality rate of up to 45% and can be detected in 7% to 18% of patients6
- Tricuspid annular plane systolic excursion, an echocardiographic measure of right ventricular function1; a value less than 17 mm suggests impaired right ventricular systolic function7
- The McConnell sign, a feature of acute massive PE: akinesia of the mid-free wall of the right ventricle and hypercontractility of the apex.
These TTE findings often lead to treatment with thrombolysis, transfer to the intensive care unit, and activation of the interventional team for catheter-based therapies.1,8 Free-floating right heart thrombi or thrombus straddling the interatrial septum (“thrombus in transit”) through a patent foramen ovale may require surgical embolectomy.8
PATIENT SELECTION AND INDICATIONS FOR ECHOCARDIOGRAPHY
- Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension. Circulation 2011; 123:1788–1830. doi:10.1161/CIR.0b013e318214914f
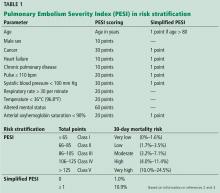
- Jiménez D, Aujesky D, Moores L, et al; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–1389. doi:10.1001/archinternmed.2010.199
- Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005; 172:1041–1046. doi:10.1164/rccm.200506-862OC
- Bova C, Pesavento R, Marchiori A, et al; TELESIO Study Group. Risk stratification and outcomes in hemodynamically stable patients with acute pulmonary embolism. J Thromb Haemost 2009; 7:938–944. doi:10.1111/j.1538-7836.2009.03345.x
- Fernandez C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest 2015; 148:211–218. doi:10.1378/chest.14-2551
- Chartier L, Bera J, Delomez M, et al. Free-floating thrombi in the right heart: diagnosis, management, and prognostic indexes in 38 consecutive patients. Circulation 1999; 99:2779–2783. pmid:10351972
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults. J Am Soc Echocardiogr 2010; 23:685–713. doi:10.1016/j.echo.2010.05.010
- Konstantinides S, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35:3033–3069a–k. doi:10.1093/eurheartj/ehu283
- Saric M, Armour AC, Arnaout MS, et al. Guidelines for the use of echocardiography in the evaluation of a cardiac source of embolism. J Am Soc Echocardiogr 2016; 29:1–42. doi:10.1016/j.echo.2015.09.011
Most patients admitted with pulmonary embolism (PE) do not need transthoracic echocardiography (TTE); it should be performed in hemodynamically unstable patients, as well as in hemodynamically stable patients with specific elevated cardiac biomarkers and imaging features.
The decision to perform TTE should be based on clinical presentation, PE burden, and imaging findings (eg, computed tomographic angiography). TTE helps to stratify risk, guide management, monitor response to therapy, and give prognostic information for a subset of patients at increased risk for PE-related adverse events.
RISK STRATIFICATION IN PULMONARY EMBOLISM
PE has a spectrum of presentations ranging from no symptoms to shock. Based on the clinical presentation, PE can be categorized as high, intermediate, or low risk.
High-risk PE, often referred to as “massive” PE, is defined in current American Heart Association guidelines as acute PE with sustained hypotension (systolic blood pressure < 90 mm Hg for at least 15 minutes or requiring inotropic support), persistent profound bradycardia (heart rate < 40 beats per minute with signs or symptoms of shock), syncope, or cardiac arrest.1
Intermediate-risk or “submassive” PE is more challenging to identify because patients are more hemodynamically stable, yet have evidence on electrocardiography, TTE, computed tomography, or cardiac biomarker testing—ie, N-terminal pro-B-type natriuretic peptide (NT-proBNP) or troponin—that indicates myocardial injury or volume overload.1
Low-risk PE is acute PE in the absence of clinical markers of adverse prognosis that define massive or submassive PE.1
ECHOCARDIOGRAPHIC FEATURES OF HIGH-RISK PULMONARY EMBOLISM
Certain TTE findings suggest increased risk of a poor outcome and may warrant therapy that is more invasive and aggressive. High-risk features include the following:
- Impaired right ventricular function
- Interventricular septum bulging into the left ventricle (“D-shaped” septum)
- Dilated proximal pulmonary arteries
- Increased severity of tricuspid regurgitation
- Elevated right atrial pressure
- Elevated pulmonary artery pressure
- Free-floating right ventricular thrombi, which are associated with a mortality rate of up to 45% and can be detected in 7% to 18% of patients6
- Tricuspid annular plane systolic excursion, an echocardiographic measure of right ventricular function1; a value less than 17 mm suggests impaired right ventricular systolic function7
- The McConnell sign, a feature of acute massive PE: akinesia of the mid-free wall of the right ventricle and hypercontractility of the apex.
These TTE findings often lead to treatment with thrombolysis, transfer to the intensive care unit, and activation of the interventional team for catheter-based therapies.1,8 Free-floating right heart thrombi or thrombus straddling the interatrial septum (“thrombus in transit”) through a patent foramen ovale may require surgical embolectomy.8
PATIENT SELECTION AND INDICATIONS FOR ECHOCARDIOGRAPHY
Most patients admitted with pulmonary embolism (PE) do not need transthoracic echocardiography (TTE); it should be performed in hemodynamically unstable patients, as well as in hemodynamically stable patients with specific elevated cardiac biomarkers and imaging features.
The decision to perform TTE should be based on clinical presentation, PE burden, and imaging findings (eg, computed tomographic angiography). TTE helps to stratify risk, guide management, monitor response to therapy, and give prognostic information for a subset of patients at increased risk for PE-related adverse events.
RISK STRATIFICATION IN PULMONARY EMBOLISM
PE has a spectrum of presentations ranging from no symptoms to shock. Based on the clinical presentation, PE can be categorized as high, intermediate, or low risk.
High-risk PE, often referred to as “massive” PE, is defined in current American Heart Association guidelines as acute PE with sustained hypotension (systolic blood pressure < 90 mm Hg for at least 15 minutes or requiring inotropic support), persistent profound bradycardia (heart rate < 40 beats per minute with signs or symptoms of shock), syncope, or cardiac arrest.1
Intermediate-risk or “submassive” PE is more challenging to identify because patients are more hemodynamically stable, yet have evidence on electrocardiography, TTE, computed tomography, or cardiac biomarker testing—ie, N-terminal pro-B-type natriuretic peptide (NT-proBNP) or troponin—that indicates myocardial injury or volume overload.1
Low-risk PE is acute PE in the absence of clinical markers of adverse prognosis that define massive or submassive PE.1
ECHOCARDIOGRAPHIC FEATURES OF HIGH-RISK PULMONARY EMBOLISM
Certain TTE findings suggest increased risk of a poor outcome and may warrant therapy that is more invasive and aggressive. High-risk features include the following:
- Impaired right ventricular function
- Interventricular septum bulging into the left ventricle (“D-shaped” septum)
- Dilated proximal pulmonary arteries
- Increased severity of tricuspid regurgitation
- Elevated right atrial pressure
- Elevated pulmonary artery pressure
- Free-floating right ventricular thrombi, which are associated with a mortality rate of up to 45% and can be detected in 7% to 18% of patients6
- Tricuspid annular plane systolic excursion, an echocardiographic measure of right ventricular function1; a value less than 17 mm suggests impaired right ventricular systolic function7
- The McConnell sign, a feature of acute massive PE: akinesia of the mid-free wall of the right ventricle and hypercontractility of the apex.
These TTE findings often lead to treatment with thrombolysis, transfer to the intensive care unit, and activation of the interventional team for catheter-based therapies.1,8 Free-floating right heart thrombi or thrombus straddling the interatrial septum (“thrombus in transit”) through a patent foramen ovale may require surgical embolectomy.8
PATIENT SELECTION AND INDICATIONS FOR ECHOCARDIOGRAPHY
- Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension. Circulation 2011; 123:1788–1830. doi:10.1161/CIR.0b013e318214914f
- Jiménez D, Aujesky D, Moores L, et al; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–1389. doi:10.1001/archinternmed.2010.199
- Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005; 172:1041–1046. doi:10.1164/rccm.200506-862OC
- Bova C, Pesavento R, Marchiori A, et al; TELESIO Study Group. Risk stratification and outcomes in hemodynamically stable patients with acute pulmonary embolism. J Thromb Haemost 2009; 7:938–944. doi:10.1111/j.1538-7836.2009.03345.x
- Fernandez C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest 2015; 148:211–218. doi:10.1378/chest.14-2551
- Chartier L, Bera J, Delomez M, et al. Free-floating thrombi in the right heart: diagnosis, management, and prognostic indexes in 38 consecutive patients. Circulation 1999; 99:2779–2783. pmid:10351972
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults. J Am Soc Echocardiogr 2010; 23:685–713. doi:10.1016/j.echo.2010.05.010
- Konstantinides S, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35:3033–3069a–k. doi:10.1093/eurheartj/ehu283
- Saric M, Armour AC, Arnaout MS, et al. Guidelines for the use of echocardiography in the evaluation of a cardiac source of embolism. J Am Soc Echocardiogr 2016; 29:1–42. doi:10.1016/j.echo.2015.09.011
- Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension. Circulation 2011; 123:1788–1830. doi:10.1161/CIR.0b013e318214914f
- Jiménez D, Aujesky D, Moores L, et al; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–1389. doi:10.1001/archinternmed.2010.199
- Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005; 172:1041–1046. doi:10.1164/rccm.200506-862OC
- Bova C, Pesavento R, Marchiori A, et al; TELESIO Study Group. Risk stratification and outcomes in hemodynamically stable patients with acute pulmonary embolism. J Thromb Haemost 2009; 7:938–944. doi:10.1111/j.1538-7836.2009.03345.x
- Fernandez C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest 2015; 148:211–218. doi:10.1378/chest.14-2551
- Chartier L, Bera J, Delomez M, et al. Free-floating thrombi in the right heart: diagnosis, management, and prognostic indexes in 38 consecutive patients. Circulation 1999; 99:2779–2783. pmid:10351972
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults. J Am Soc Echocardiogr 2010; 23:685–713. doi:10.1016/j.echo.2010.05.010
- Konstantinides S, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35:3033–3069a–k. doi:10.1093/eurheartj/ehu283
- Saric M, Armour AC, Arnaout MS, et al. Guidelines for the use of echocardiography in the evaluation of a cardiac source of embolism. J Am Soc Echocardiogr 2016; 29:1–42. doi:10.1016/j.echo.2015.09.011
How soon should patients with infective endocarditis be referred for valve surgery?
WHAT IS ‘EARLY’ SURGERY?
More than 50% of patients with infective endocarditis undergo cardiac surgery during their initial presentation.1
The 2017 guidelines of the American Association for Thoracic Surgery (AATS) recommend surgery once a surgical indication has been established and effective antimicrobial therapy has been started.2
The American Heart Association/American College of Cardiology (ACC/AHA) guidelines recommend surgery during the initial hospitalization before completion of a full course of antibiotics.3
The European Society of Cardiology guidelines define surgery according to the time since the patient received intravenous antibiotic therapy: emergency surgery is performed within 24 hours of therapy, urgent surgery is performed within a few days, and elective surgery is performed after at least 1 to 2 weeks.4
These slight differences are due to the dearth of large randomized trials addressing this question.
INDICATIONS FOR EARLY SURGERY
Left ventricular dysfunction and heart failure
Of all the complications of infectious endocarditis, concomitant heart failure has the greatest impact on prognosis5 and is one of the most frequent indications for surgery.6
The guidelines recommend emergency surgery during the initial hospitalization for all patients with infective endocarditis who present with refractory pulmonary edema, worsening left ventricular dysfunction, or cardiogenic shock, regardless of whether they have completed a full course of antibiotics. This applies to both native valve endocarditis and prosthetic valve endocarditis.
Uncontrolled persistent infection
Persistent infection is defined as fever and positive cultures persisting after 1 week of appropriate antibiotic treatment.4 However, 1 week is a long time. Persistence of positive blood cultures more than 48 to 72 hours after starting antibiotic therapy is associated with poor outcome and is an independent predictor of in-hospital mortality.7
The ACC/AHA guidelines recommend early surgery in patients with left-sided infective endocarditis caused by fungi or highly resistant organisms such as vancomycin-resistant enterococci or multidrug-resistant gram-negative bacilli.3 Nonetheless, antibiotic resistance is an unusual reason for expediting surgery unless there are additional indications for it.
Extension of the infection beyond the valve annulus, which occurs in about 30% of cases of native valve endocarditis and 50% of cases of prosthetic valve endocarditis,8 is considered a more valid reason to expedite surgery. Similarly, urgent surgery should be considered if there is any evidence of locally uncontrolled infection causing perivalvular abscess, fistula, pseudoaneurysm, or conduction system abnormalities causing atrioventricular nodal block.2–4
Some authors suggest reviewing the surgical pathology and microbial sequencing of excised cardiac valves after surgery to confirm the diagnosis and identify the culprit pathogen.9,10
Right-sided infective endocarditis
Right-sided infective endocarditis has a more favorable prognosis than left-sided infective endocarditis and usually responds well to medical therapy.11
Nevertheless, surgery for right-sided infective endocarditis should be expedited in patients with right heart failure secondary to severe tricuspid regurgitation with poor response to medical therapy or in the case of large tricuspid valve vegetations.12 Likewise, recurrent septic pulmonary emboli can be encountered in the setting of right-sided infective endocarditis and are an indication for early surgery.4,12
Since many patients with right-sided infective endocarditis acquire the infection by intravenous drug use, there is often a reluctance to recommend surgery, given the risk of prosthetic valve infection if they continue to use intravenous drugs.4,12 One study showed that the risk of death or reoperation between 3 and 6 months after surgery for infective endocarditis was 10 times higher in intravenous drug users. Yet their survival after surgery beyond this period was similar to that of patients with endocarditis who did not inject drugs.13 Therefore, the AATS guidelines recommend applying normal indications for surgery to those patients, with emphasis on the need for strict follow-up aimed at addiction treatment.2
Prevention of embolic events
Neurologic embolic events are a frequent complication of infective endocarditis, with the highest risk during the first few days after antibiotics are started. However, this risk decreases significantly after 2 weeks.14
The timing of surgery largely depends on whether the patient has had previous neurologic embolic events and on the size and mobility of the vegetation. The current guidelines recommend early surgery for recurrent emboli and persistent or enlarging vegetations despite appropriate antibiotic therapy, or in case of large vegetations (> 10 mm) on a native valve even in the absence of embolic events.4
A randomized trial by Kang et al15 demonstrated that, compared with conventional care, early surgery (within 48 hours of diagnosis) in patients with native valve endocarditis with large vegetations (> 10 mm) and severe valve dysfunction was associated with a significant reduction in the risk of death and embolic events.
Timing of surgery after a neurologic complication
Determining the right time for surgery is challenging in patients with infective endocarditis who have had neurologic complications, given the risk of hemorrhagic conversion of existing stroke with anticoagulation or exacerbation of cerebral ischemia in case of intraoperative hypotension. The decision should take into account the severity of cardiac decompensation, weighed against the severity of neurologic symptoms.
In general, surgery should be postponed for at least 4 weeks after intracerebral hemorrhage. However, it should be expedited in the event of silent cerebral embolism or transient ischemic attack, or in patients with infective endocarditis with stroke who have other indications for early surgery, as long as cerebral hemorrhage has been excluded by appropriate imaging.4
Early surgery for prosthetic valve endocarditis
The timing of surgery for prosthetic valve endocarditis follows the same general principles as for native valve endocarditis.2–4,12
One study showed that early surgery for prosthetic valve endocarditis was not associated with lower in-hospital and 1-year mortality rates compared with medical therapy.16 On the other hand, a subgroup analysis demonstrated surgery to be significantly beneficial in those with the strongest indications for surgery, including severe valve regurgitation, heart failure, paravalvular abscess, fistula, or prosthetic valve dehiscence.
The decision to proceed with surgery in prosthetic valve endocarditis should be weighed carefully, taking into consideration the patient’s overall clinical condition and estimated surgical risk.16
COLLABORATION IS HELPFUL
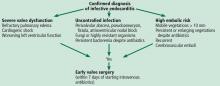
Early surgery is indicated for infective endocarditis patients presenting with:
- Refractory heart failure symptoms
- Persistent infection
- Large vegetations with a high risk of embolism.
Expeditious and successful treatment entails multidisciplinary collaboration among experts in cardiology and infectious diseases with access to cardiac surgery input early in the evaluation.
- Lalani T, Cabell CH, Benjamin DK, et al; International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators. Analysis of the impact of early surgery on in-hospital mortality of native valve endocarditis: use of propensity score and instrumental variable methods to adjust for treatment-selection bias. Circulation 2010; 121(8):1005–1013. doi:10.1161/CIRCULATIONAHA.109.864488
- AATS Surgical Treatment of Infective Endocarditis Consensus Guidelines Writing Committee Chairs; Pettersson GB, Coselli JS; Writing Committee, et al. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: surgical treatment of infective endocarditis: executive summary. J Thorac Cardiovasc Surg 2017; 153(6):1241–1258.e29. doi:10.1016/j.jtcvs.2016.09.093
- Nishimura RA, Otto CM, Bonow RO, et al; ACC/AHA Task Force Members. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(23):2440–2492. doi:10.1161/CIR.0000000000000029
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis. Eur Heart J 2015; 36(44):3075–3128. doi:10.1093/eurheartj/ehv319
- Prendergast BD, Tornos P. Surgery for infective endocarditis. Who and when? Circulation 2010; 121(9):1141–1152. doi:10.1161/CIRCULATIONAHA.108.773598
- Tornos P, Iung B, Permanyer-Miralda G, et al. Infective endocarditis in Europe: lessons from the Euro heart survey. Heart 2005; 91(5):571–575. doi:10.1136/hrt.2003.032128
- López J, Sevilla T, Vilacosta I, et al. Prognostic role of persistent positive blood cultures after initiation of antibiotic therapy in left-sided infective endocarditis. Eur Heart J 2013; 34(23):1749–1754. doi:10.1093/eurheartj/ehs379
- Graupner C, Vilacosta I, SanRoman J, et al. Periannular extension of infective endocarditis. J Am Coll Cardiol 2002; 39(7):1204–1211. doi:10.1016/S0735-1097(02)01747-3
- Shrestha NK, Ledtke CS, Wang H, et al. Heart valve culture and sequencing to identify the infective endocarditis pathogen in surgically treated patients. Ann Thorac Surg 2015; 99(1):33–37. doi:10.1016/j.athoracsur.2014.07.028
- Shapira N, Merin O, Rosenmann E, et al. Latent infective endocarditis: epidemiology and clinical characteristics of patients with unsuspected endocarditis detected after elective valve replacement. Ann Thorac Surg 2004; 78(5):1623–1629. doi:10.1016/j.athoracsur.2004.05.052
- Hecht SR, Berger M. Right-sided endocarditis in intravenous drug users. Prognostic features in 102 episodes. Ann Intern Med 1992; 117(7):560–566. doi:10.7326/0003-4819-117-7-560
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Shrestha NK, Jue J, Hussain ST, et al. Injection drug use and outcomes after surgical intervention for infective endocarditis. Ann Thorac Surg 2015; 100(3):875–882. doi:10.1016/j.athoracsur.2015.03.019
- Garcia-Cabrera E, Fernandez-Hidalgo N, Almirante B, et al. Neurological complications of infective endocarditis: risk factors, outcome, and impact of cardiac surgery: a multicenter observational study. Circulation 2013; 127(23):2272–2284. doi:10.1161/CIRCULATIONAHA.112.000813
- Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med 2012; 366(26):2466–2473. doi:10.1056/NEJMoa1112843
- Lalani T, Chu VH, Park LP, et al; International Collaboration on Endocarditis–Prospective Cohort Study Investigators. In-hospital and 1-year mortality in patients undergoing early surgery for prosthetic valve endocarditis. JAMA Intern Med 2013; 173(16):1495–1504. doi:10.1001/jamainternmed.2013.8203
WHAT IS ‘EARLY’ SURGERY?
More than 50% of patients with infective endocarditis undergo cardiac surgery during their initial presentation.1
The 2017 guidelines of the American Association for Thoracic Surgery (AATS) recommend surgery once a surgical indication has been established and effective antimicrobial therapy has been started.2
The American Heart Association/American College of Cardiology (ACC/AHA) guidelines recommend surgery during the initial hospitalization before completion of a full course of antibiotics.3
The European Society of Cardiology guidelines define surgery according to the time since the patient received intravenous antibiotic therapy: emergency surgery is performed within 24 hours of therapy, urgent surgery is performed within a few days, and elective surgery is performed after at least 1 to 2 weeks.4
These slight differences are due to the dearth of large randomized trials addressing this question.
INDICATIONS FOR EARLY SURGERY
Left ventricular dysfunction and heart failure
Of all the complications of infectious endocarditis, concomitant heart failure has the greatest impact on prognosis5 and is one of the most frequent indications for surgery.6
The guidelines recommend emergency surgery during the initial hospitalization for all patients with infective endocarditis who present with refractory pulmonary edema, worsening left ventricular dysfunction, or cardiogenic shock, regardless of whether they have completed a full course of antibiotics. This applies to both native valve endocarditis and prosthetic valve endocarditis.
Uncontrolled persistent infection
Persistent infection is defined as fever and positive cultures persisting after 1 week of appropriate antibiotic treatment.4 However, 1 week is a long time. Persistence of positive blood cultures more than 48 to 72 hours after starting antibiotic therapy is associated with poor outcome and is an independent predictor of in-hospital mortality.7
The ACC/AHA guidelines recommend early surgery in patients with left-sided infective endocarditis caused by fungi or highly resistant organisms such as vancomycin-resistant enterococci or multidrug-resistant gram-negative bacilli.3 Nonetheless, antibiotic resistance is an unusual reason for expediting surgery unless there are additional indications for it.
Extension of the infection beyond the valve annulus, which occurs in about 30% of cases of native valve endocarditis and 50% of cases of prosthetic valve endocarditis,8 is considered a more valid reason to expedite surgery. Similarly, urgent surgery should be considered if there is any evidence of locally uncontrolled infection causing perivalvular abscess, fistula, pseudoaneurysm, or conduction system abnormalities causing atrioventricular nodal block.2–4
Some authors suggest reviewing the surgical pathology and microbial sequencing of excised cardiac valves after surgery to confirm the diagnosis and identify the culprit pathogen.9,10
Right-sided infective endocarditis
Right-sided infective endocarditis has a more favorable prognosis than left-sided infective endocarditis and usually responds well to medical therapy.11
Nevertheless, surgery for right-sided infective endocarditis should be expedited in patients with right heart failure secondary to severe tricuspid regurgitation with poor response to medical therapy or in the case of large tricuspid valve vegetations.12 Likewise, recurrent septic pulmonary emboli can be encountered in the setting of right-sided infective endocarditis and are an indication for early surgery.4,12
Since many patients with right-sided infective endocarditis acquire the infection by intravenous drug use, there is often a reluctance to recommend surgery, given the risk of prosthetic valve infection if they continue to use intravenous drugs.4,12 One study showed that the risk of death or reoperation between 3 and 6 months after surgery for infective endocarditis was 10 times higher in intravenous drug users. Yet their survival after surgery beyond this period was similar to that of patients with endocarditis who did not inject drugs.13 Therefore, the AATS guidelines recommend applying normal indications for surgery to those patients, with emphasis on the need for strict follow-up aimed at addiction treatment.2
Prevention of embolic events
Neurologic embolic events are a frequent complication of infective endocarditis, with the highest risk during the first few days after antibiotics are started. However, this risk decreases significantly after 2 weeks.14
The timing of surgery largely depends on whether the patient has had previous neurologic embolic events and on the size and mobility of the vegetation. The current guidelines recommend early surgery for recurrent emboli and persistent or enlarging vegetations despite appropriate antibiotic therapy, or in case of large vegetations (> 10 mm) on a native valve even in the absence of embolic events.4
A randomized trial by Kang et al15 demonstrated that, compared with conventional care, early surgery (within 48 hours of diagnosis) in patients with native valve endocarditis with large vegetations (> 10 mm) and severe valve dysfunction was associated with a significant reduction in the risk of death and embolic events.
Timing of surgery after a neurologic complication
Determining the right time for surgery is challenging in patients with infective endocarditis who have had neurologic complications, given the risk of hemorrhagic conversion of existing stroke with anticoagulation or exacerbation of cerebral ischemia in case of intraoperative hypotension. The decision should take into account the severity of cardiac decompensation, weighed against the severity of neurologic symptoms.
In general, surgery should be postponed for at least 4 weeks after intracerebral hemorrhage. However, it should be expedited in the event of silent cerebral embolism or transient ischemic attack, or in patients with infective endocarditis with stroke who have other indications for early surgery, as long as cerebral hemorrhage has been excluded by appropriate imaging.4
Early surgery for prosthetic valve endocarditis
The timing of surgery for prosthetic valve endocarditis follows the same general principles as for native valve endocarditis.2–4,12
One study showed that early surgery for prosthetic valve endocarditis was not associated with lower in-hospital and 1-year mortality rates compared with medical therapy.16 On the other hand, a subgroup analysis demonstrated surgery to be significantly beneficial in those with the strongest indications for surgery, including severe valve regurgitation, heart failure, paravalvular abscess, fistula, or prosthetic valve dehiscence.
The decision to proceed with surgery in prosthetic valve endocarditis should be weighed carefully, taking into consideration the patient’s overall clinical condition and estimated surgical risk.16
COLLABORATION IS HELPFUL
Early surgery is indicated for infective endocarditis patients presenting with:
- Refractory heart failure symptoms
- Persistent infection
- Large vegetations with a high risk of embolism.
Expeditious and successful treatment entails multidisciplinary collaboration among experts in cardiology and infectious diseases with access to cardiac surgery input early in the evaluation.
WHAT IS ‘EARLY’ SURGERY?
More than 50% of patients with infective endocarditis undergo cardiac surgery during their initial presentation.1
The 2017 guidelines of the American Association for Thoracic Surgery (AATS) recommend surgery once a surgical indication has been established and effective antimicrobial therapy has been started.2
The American Heart Association/American College of Cardiology (ACC/AHA) guidelines recommend surgery during the initial hospitalization before completion of a full course of antibiotics.3
The European Society of Cardiology guidelines define surgery according to the time since the patient received intravenous antibiotic therapy: emergency surgery is performed within 24 hours of therapy, urgent surgery is performed within a few days, and elective surgery is performed after at least 1 to 2 weeks.4
These slight differences are due to the dearth of large randomized trials addressing this question.
INDICATIONS FOR EARLY SURGERY
Left ventricular dysfunction and heart failure
Of all the complications of infectious endocarditis, concomitant heart failure has the greatest impact on prognosis5 and is one of the most frequent indications for surgery.6
The guidelines recommend emergency surgery during the initial hospitalization for all patients with infective endocarditis who present with refractory pulmonary edema, worsening left ventricular dysfunction, or cardiogenic shock, regardless of whether they have completed a full course of antibiotics. This applies to both native valve endocarditis and prosthetic valve endocarditis.
Uncontrolled persistent infection
Persistent infection is defined as fever and positive cultures persisting after 1 week of appropriate antibiotic treatment.4 However, 1 week is a long time. Persistence of positive blood cultures more than 48 to 72 hours after starting antibiotic therapy is associated with poor outcome and is an independent predictor of in-hospital mortality.7
The ACC/AHA guidelines recommend early surgery in patients with left-sided infective endocarditis caused by fungi or highly resistant organisms such as vancomycin-resistant enterococci or multidrug-resistant gram-negative bacilli.3 Nonetheless, antibiotic resistance is an unusual reason for expediting surgery unless there are additional indications for it.
Extension of the infection beyond the valve annulus, which occurs in about 30% of cases of native valve endocarditis and 50% of cases of prosthetic valve endocarditis,8 is considered a more valid reason to expedite surgery. Similarly, urgent surgery should be considered if there is any evidence of locally uncontrolled infection causing perivalvular abscess, fistula, pseudoaneurysm, or conduction system abnormalities causing atrioventricular nodal block.2–4
Some authors suggest reviewing the surgical pathology and microbial sequencing of excised cardiac valves after surgery to confirm the diagnosis and identify the culprit pathogen.9,10
Right-sided infective endocarditis
Right-sided infective endocarditis has a more favorable prognosis than left-sided infective endocarditis and usually responds well to medical therapy.11
Nevertheless, surgery for right-sided infective endocarditis should be expedited in patients with right heart failure secondary to severe tricuspid regurgitation with poor response to medical therapy or in the case of large tricuspid valve vegetations.12 Likewise, recurrent septic pulmonary emboli can be encountered in the setting of right-sided infective endocarditis and are an indication for early surgery.4,12
Since many patients with right-sided infective endocarditis acquire the infection by intravenous drug use, there is often a reluctance to recommend surgery, given the risk of prosthetic valve infection if they continue to use intravenous drugs.4,12 One study showed that the risk of death or reoperation between 3 and 6 months after surgery for infective endocarditis was 10 times higher in intravenous drug users. Yet their survival after surgery beyond this period was similar to that of patients with endocarditis who did not inject drugs.13 Therefore, the AATS guidelines recommend applying normal indications for surgery to those patients, with emphasis on the need for strict follow-up aimed at addiction treatment.2
Prevention of embolic events
Neurologic embolic events are a frequent complication of infective endocarditis, with the highest risk during the first few days after antibiotics are started. However, this risk decreases significantly after 2 weeks.14
The timing of surgery largely depends on whether the patient has had previous neurologic embolic events and on the size and mobility of the vegetation. The current guidelines recommend early surgery for recurrent emboli and persistent or enlarging vegetations despite appropriate antibiotic therapy, or in case of large vegetations (> 10 mm) on a native valve even in the absence of embolic events.4
A randomized trial by Kang et al15 demonstrated that, compared with conventional care, early surgery (within 48 hours of diagnosis) in patients with native valve endocarditis with large vegetations (> 10 mm) and severe valve dysfunction was associated with a significant reduction in the risk of death and embolic events.
Timing of surgery after a neurologic complication
Determining the right time for surgery is challenging in patients with infective endocarditis who have had neurologic complications, given the risk of hemorrhagic conversion of existing stroke with anticoagulation or exacerbation of cerebral ischemia in case of intraoperative hypotension. The decision should take into account the severity of cardiac decompensation, weighed against the severity of neurologic symptoms.
In general, surgery should be postponed for at least 4 weeks after intracerebral hemorrhage. However, it should be expedited in the event of silent cerebral embolism or transient ischemic attack, or in patients with infective endocarditis with stroke who have other indications for early surgery, as long as cerebral hemorrhage has been excluded by appropriate imaging.4
Early surgery for prosthetic valve endocarditis
The timing of surgery for prosthetic valve endocarditis follows the same general principles as for native valve endocarditis.2–4,12
One study showed that early surgery for prosthetic valve endocarditis was not associated with lower in-hospital and 1-year mortality rates compared with medical therapy.16 On the other hand, a subgroup analysis demonstrated surgery to be significantly beneficial in those with the strongest indications for surgery, including severe valve regurgitation, heart failure, paravalvular abscess, fistula, or prosthetic valve dehiscence.
The decision to proceed with surgery in prosthetic valve endocarditis should be weighed carefully, taking into consideration the patient’s overall clinical condition and estimated surgical risk.16
COLLABORATION IS HELPFUL
Early surgery is indicated for infective endocarditis patients presenting with:
- Refractory heart failure symptoms
- Persistent infection
- Large vegetations with a high risk of embolism.
Expeditious and successful treatment entails multidisciplinary collaboration among experts in cardiology and infectious diseases with access to cardiac surgery input early in the evaluation.
- Lalani T, Cabell CH, Benjamin DK, et al; International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators. Analysis of the impact of early surgery on in-hospital mortality of native valve endocarditis: use of propensity score and instrumental variable methods to adjust for treatment-selection bias. Circulation 2010; 121(8):1005–1013. doi:10.1161/CIRCULATIONAHA.109.864488
- AATS Surgical Treatment of Infective Endocarditis Consensus Guidelines Writing Committee Chairs; Pettersson GB, Coselli JS; Writing Committee, et al. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: surgical treatment of infective endocarditis: executive summary. J Thorac Cardiovasc Surg 2017; 153(6):1241–1258.e29. doi:10.1016/j.jtcvs.2016.09.093
- Nishimura RA, Otto CM, Bonow RO, et al; ACC/AHA Task Force Members. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(23):2440–2492. doi:10.1161/CIR.0000000000000029
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis. Eur Heart J 2015; 36(44):3075–3128. doi:10.1093/eurheartj/ehv319
- Prendergast BD, Tornos P. Surgery for infective endocarditis. Who and when? Circulation 2010; 121(9):1141–1152. doi:10.1161/CIRCULATIONAHA.108.773598
- Tornos P, Iung B, Permanyer-Miralda G, et al. Infective endocarditis in Europe: lessons from the Euro heart survey. Heart 2005; 91(5):571–575. doi:10.1136/hrt.2003.032128
- López J, Sevilla T, Vilacosta I, et al. Prognostic role of persistent positive blood cultures after initiation of antibiotic therapy in left-sided infective endocarditis. Eur Heart J 2013; 34(23):1749–1754. doi:10.1093/eurheartj/ehs379
- Graupner C, Vilacosta I, SanRoman J, et al. Periannular extension of infective endocarditis. J Am Coll Cardiol 2002; 39(7):1204–1211. doi:10.1016/S0735-1097(02)01747-3
- Shrestha NK, Ledtke CS, Wang H, et al. Heart valve culture and sequencing to identify the infective endocarditis pathogen in surgically treated patients. Ann Thorac Surg 2015; 99(1):33–37. doi:10.1016/j.athoracsur.2014.07.028
- Shapira N, Merin O, Rosenmann E, et al. Latent infective endocarditis: epidemiology and clinical characteristics of patients with unsuspected endocarditis detected after elective valve replacement. Ann Thorac Surg 2004; 78(5):1623–1629. doi:10.1016/j.athoracsur.2004.05.052
- Hecht SR, Berger M. Right-sided endocarditis in intravenous drug users. Prognostic features in 102 episodes. Ann Intern Med 1992; 117(7):560–566. doi:10.7326/0003-4819-117-7-560
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Shrestha NK, Jue J, Hussain ST, et al. Injection drug use and outcomes after surgical intervention for infective endocarditis. Ann Thorac Surg 2015; 100(3):875–882. doi:10.1016/j.athoracsur.2015.03.019
- Garcia-Cabrera E, Fernandez-Hidalgo N, Almirante B, et al. Neurological complications of infective endocarditis: risk factors, outcome, and impact of cardiac surgery: a multicenter observational study. Circulation 2013; 127(23):2272–2284. doi:10.1161/CIRCULATIONAHA.112.000813
- Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med 2012; 366(26):2466–2473. doi:10.1056/NEJMoa1112843
- Lalani T, Chu VH, Park LP, et al; International Collaboration on Endocarditis–Prospective Cohort Study Investigators. In-hospital and 1-year mortality in patients undergoing early surgery for prosthetic valve endocarditis. JAMA Intern Med 2013; 173(16):1495–1504. doi:10.1001/jamainternmed.2013.8203
- Lalani T, Cabell CH, Benjamin DK, et al; International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators. Analysis of the impact of early surgery on in-hospital mortality of native valve endocarditis: use of propensity score and instrumental variable methods to adjust for treatment-selection bias. Circulation 2010; 121(8):1005–1013. doi:10.1161/CIRCULATIONAHA.109.864488
- AATS Surgical Treatment of Infective Endocarditis Consensus Guidelines Writing Committee Chairs; Pettersson GB, Coselli JS; Writing Committee, et al. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: surgical treatment of infective endocarditis: executive summary. J Thorac Cardiovasc Surg 2017; 153(6):1241–1258.e29. doi:10.1016/j.jtcvs.2016.09.093
- Nishimura RA, Otto CM, Bonow RO, et al; ACC/AHA Task Force Members. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(23):2440–2492. doi:10.1161/CIR.0000000000000029
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis. Eur Heart J 2015; 36(44):3075–3128. doi:10.1093/eurheartj/ehv319
- Prendergast BD, Tornos P. Surgery for infective endocarditis. Who and when? Circulation 2010; 121(9):1141–1152. doi:10.1161/CIRCULATIONAHA.108.773598
- Tornos P, Iung B, Permanyer-Miralda G, et al. Infective endocarditis in Europe: lessons from the Euro heart survey. Heart 2005; 91(5):571–575. doi:10.1136/hrt.2003.032128
- López J, Sevilla T, Vilacosta I, et al. Prognostic role of persistent positive blood cultures after initiation of antibiotic therapy in left-sided infective endocarditis. Eur Heart J 2013; 34(23):1749–1754. doi:10.1093/eurheartj/ehs379
- Graupner C, Vilacosta I, SanRoman J, et al. Periannular extension of infective endocarditis. J Am Coll Cardiol 2002; 39(7):1204–1211. doi:10.1016/S0735-1097(02)01747-3
- Shrestha NK, Ledtke CS, Wang H, et al. Heart valve culture and sequencing to identify the infective endocarditis pathogen in surgically treated patients. Ann Thorac Surg 2015; 99(1):33–37. doi:10.1016/j.athoracsur.2014.07.028
- Shapira N, Merin O, Rosenmann E, et al. Latent infective endocarditis: epidemiology and clinical characteristics of patients with unsuspected endocarditis detected after elective valve replacement. Ann Thorac Surg 2004; 78(5):1623–1629. doi:10.1016/j.athoracsur.2004.05.052
- Hecht SR, Berger M. Right-sided endocarditis in intravenous drug users. Prognostic features in 102 episodes. Ann Intern Med 1992; 117(7):560–566. doi:10.7326/0003-4819-117-7-560
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Shrestha NK, Jue J, Hussain ST, et al. Injection drug use and outcomes after surgical intervention for infective endocarditis. Ann Thorac Surg 2015; 100(3):875–882. doi:10.1016/j.athoracsur.2015.03.019
- Garcia-Cabrera E, Fernandez-Hidalgo N, Almirante B, et al. Neurological complications of infective endocarditis: risk factors, outcome, and impact of cardiac surgery: a multicenter observational study. Circulation 2013; 127(23):2272–2284. doi:10.1161/CIRCULATIONAHA.112.000813
- Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med 2012; 366(26):2466–2473. doi:10.1056/NEJMoa1112843
- Lalani T, Chu VH, Park LP, et al; International Collaboration on Endocarditis–Prospective Cohort Study Investigators. In-hospital and 1-year mortality in patients undergoing early surgery for prosthetic valve endocarditis. JAMA Intern Med 2013; 173(16):1495–1504. doi:10.1001/jamainternmed.2013.8203