User login
Do NSAIDs impede fracture healing?
NO, ALTHOUGH THE EVIDENCE VARIES. Nonsteroidal anti-inflammatory drugs (NSAIDs) don’t appear to impair clinical fracture healing (strength of recommendation [SOR]: B, inconsistent evidence from a randomized controlled trial [RCT] and retrospective studies). Even though animal studies show delayed healing and nonunion with NSAID use, evidence in humans doesn’t merit avoiding NSAIDs in patients with fractures who need the drugs’ analgesic and anti-inflammatory benefits.
Evidence summary
NSAIDs are commonly prescribed to control pain in patients with fractures. Laboratory studies have found that their antiprostaglandin properties delay callus formation and subsequent healing.1 However, human studies evaluating the effects of NSAIDs on fracture healing have found variable results (TABLE).
TABLE
Fracture healing with NSAIDs: What the studies show
| Type of study | Population | Intervention | Outcome and results |
|---|---|---|---|
| Randomized controlled trial2 | Postmenopausal women with Colles’ fractures (N=42) | Piroxicam | No delay in fracture healing |
| Retrospective3 | Patients with long-bone fractures (N=112) | Indomethacin | Rate of nonunion 29% vs 7% (P=.004) |
| Retrospective4 | Patients with femoral shaft fractures (N=99) | Diclofenac or ibuprofen | OR for nonunion=10.7 (95% CI, 3.5-33.2) |
| Retrospective5 | Postoperative spinal fusion patients (N=288) | Ketoralac | OR for nonunion=4.9 (95% CI, 1.8-16.6) |
| Retrospective6 | Patients with tibial fractures (N=94) | Multiple NSAIDs | Increased mean time to union of 7.6 wk (P=.0003) |
| Retrospective7 | Patients with humeral shaft fractures (N=9995) | Multiple NSAIDs | Increased risk of nonunion with exposure to NSAIDs 60-90 days postfracture (RR=3.9; 95% CI, 2.0-6.2) |
| CI, confidence interval; OR, odds ratio; RR, relative risk. | |||
An RCT finds no delay in healing
An RCT of 42 postmenopausal women with displaced Colles’ fractures who were given piroxicam or placebo found no difference in the rate of healing between the intervention and control groups.2 After 8 weeks, the bone mineral content of the radius and ulna, measured by bone density, was similar in both groups. Patients in the piroxicam group had significantly less pain at 10 days and 4 weeks, and used significantly less rescue medication.
Other studies beg to differ
Three observational studies of patients with different types of fractures found an increase in nonunion associated with NSAIDs.3-5 Two retrospective studies of patients with long-bone fractures reported a higher rate of nonunion among patients taking indomethacin, diclofenac, or ibuprofen.3,4 The third study, a retrospective analysis of postoperative spinal fusion patients who took ketorolac, also found an association between increased risk of nonunion and NSAIDs5 (TABLE).
A retrospective study of 94 patients with tibial fractures reported delayed healing in patients who had taken any NSAID. This association persisted after elimination for age, sex, fracture severity, and high-energy injuries.6
A relationship, but is it causal?
A larger retrospective cohort study of 9995 patients with humeral shaft fractures found an increased risk of nonunion in patients exposed to NSAIDs during the 90 days after the fracture. On further analysis, however, only NSAID exposure 60 to 90 days after the fracture was significantly associated with nonunion. Because patients with painful nonunion fractures are likely to use more NSAIDs, the relationship may not be causal.7
Benefits of NSAIDs outweigh concerns
Three reviews of the effect of NSAIDs on fracture healing all come to the same conclusion: Although animal studies raise theoretical concerns that NSAIDs affect fracture healing, no conclusive evidence supports denying patients the analgesic benefits of these drugs for managing fractures.8-10
Recommendations
The American Academy of Family Physicians recommends using NSAIDs temporarily along with other measures—such as stretching, ice, and a steady return to the aggravating exercise—to relieve the pain of stress fractures until the patient is pain-free.11
The American College of Sports Medicine, The American Academy of Orthopedic Surgeons, and the American Academy of Physical Medicine and Rehabilitation haven’t issued definitive guidelines concerning whether to use NSAIDs in managing fractures.
1. Gerstenfeld LC, Al-Ghawas M, Alkhiary YM, et al. Selective and nonselective cyclooxygenase-2 inhibitors and experimental fracture-healing. Reversibility of effects after short-term treatment. J Bone Joint Surg Am. 2007;89:114-125.
2. Adolphson P, Abbaszadegan H, Jonnson U, et al. No effects of piroxicam on osteopenia and recovery after Colles’ fracture. A randomized, double-blind, placebo-controlled prospective trial. Arch Orthop Trauma Surg. 1993;112:127-130.
3. Burd TA, Hughes MS, Anglen JO. Heterotopic ossification prophylaxis with indomethacin increases the risk of long-bone nonunion. J Bone Joint Surg Br. 2003;85:700-705.
4. Giannoudis PV, MacDonald DA, Matthews SJ. Nonunion of the femoral diaphysis: the influence of reaming and nonsteroidal anti-inflammatory drugs. J Bone Joint Surg Br. 2000;82:655-658.
5. Glassman SD, Rose SM, Dimar JR, et al. The effect of postoperative nonsteroidal anti-inflammatory drug administration on spinal fusion. Spine. 1998;23:834-838.
6. Butcher CK, Marsh DR. Nonsteroidal anti-inflammatory drugs delay tibial fracture union. Injury. 1996;27:375.-
7. Bhattacharyya T, Levin R, Vrahas MS, et al. Nonsteroidal anti-inflammatory drugs and nonunion of humeral shaft fractures. Arthritis Rheum. 2005;53:364-367.
8. Wheeler P, Batt ME. Do nonsteroidal anti-inflammatory drugs adversely affect stress fracture healing? A short review. Br J Sports Med. 2005;39:65-69.
9. Clarke S, Lecky F. Best evidence topic report. Do nonsteroidal anti-inflammatory drugs cause a delay in fracture healing? Emerg Med J. 2005;22:652-653.
10. Koester MC, Spindler KP. NSAIDs and fracture healing: what’s the evidence? Curr Sports Med Rep. 2005;4:289-290.
11. Sanderlin BW, Raspa RF. Common stress fractures. Am Fam Physician. 2003;68:1527-1532.
NO, ALTHOUGH THE EVIDENCE VARIES. Nonsteroidal anti-inflammatory drugs (NSAIDs) don’t appear to impair clinical fracture healing (strength of recommendation [SOR]: B, inconsistent evidence from a randomized controlled trial [RCT] and retrospective studies). Even though animal studies show delayed healing and nonunion with NSAID use, evidence in humans doesn’t merit avoiding NSAIDs in patients with fractures who need the drugs’ analgesic and anti-inflammatory benefits.
Evidence summary
NSAIDs are commonly prescribed to control pain in patients with fractures. Laboratory studies have found that their antiprostaglandin properties delay callus formation and subsequent healing.1 However, human studies evaluating the effects of NSAIDs on fracture healing have found variable results (TABLE).
TABLE
Fracture healing with NSAIDs: What the studies show
| Type of study | Population | Intervention | Outcome and results |
|---|---|---|---|
| Randomized controlled trial2 | Postmenopausal women with Colles’ fractures (N=42) | Piroxicam | No delay in fracture healing |
| Retrospective3 | Patients with long-bone fractures (N=112) | Indomethacin | Rate of nonunion 29% vs 7% (P=.004) |
| Retrospective4 | Patients with femoral shaft fractures (N=99) | Diclofenac or ibuprofen | OR for nonunion=10.7 (95% CI, 3.5-33.2) |
| Retrospective5 | Postoperative spinal fusion patients (N=288) | Ketoralac | OR for nonunion=4.9 (95% CI, 1.8-16.6) |
| Retrospective6 | Patients with tibial fractures (N=94) | Multiple NSAIDs | Increased mean time to union of 7.6 wk (P=.0003) |
| Retrospective7 | Patients with humeral shaft fractures (N=9995) | Multiple NSAIDs | Increased risk of nonunion with exposure to NSAIDs 60-90 days postfracture (RR=3.9; 95% CI, 2.0-6.2) |
| CI, confidence interval; OR, odds ratio; RR, relative risk. | |||
An RCT finds no delay in healing
An RCT of 42 postmenopausal women with displaced Colles’ fractures who were given piroxicam or placebo found no difference in the rate of healing between the intervention and control groups.2 After 8 weeks, the bone mineral content of the radius and ulna, measured by bone density, was similar in both groups. Patients in the piroxicam group had significantly less pain at 10 days and 4 weeks, and used significantly less rescue medication.
Other studies beg to differ
Three observational studies of patients with different types of fractures found an increase in nonunion associated with NSAIDs.3-5 Two retrospective studies of patients with long-bone fractures reported a higher rate of nonunion among patients taking indomethacin, diclofenac, or ibuprofen.3,4 The third study, a retrospective analysis of postoperative spinal fusion patients who took ketorolac, also found an association between increased risk of nonunion and NSAIDs5 (TABLE).
A retrospective study of 94 patients with tibial fractures reported delayed healing in patients who had taken any NSAID. This association persisted after elimination for age, sex, fracture severity, and high-energy injuries.6
A relationship, but is it causal?
A larger retrospective cohort study of 9995 patients with humeral shaft fractures found an increased risk of nonunion in patients exposed to NSAIDs during the 90 days after the fracture. On further analysis, however, only NSAID exposure 60 to 90 days after the fracture was significantly associated with nonunion. Because patients with painful nonunion fractures are likely to use more NSAIDs, the relationship may not be causal.7
Benefits of NSAIDs outweigh concerns
Three reviews of the effect of NSAIDs on fracture healing all come to the same conclusion: Although animal studies raise theoretical concerns that NSAIDs affect fracture healing, no conclusive evidence supports denying patients the analgesic benefits of these drugs for managing fractures.8-10
Recommendations
The American Academy of Family Physicians recommends using NSAIDs temporarily along with other measures—such as stretching, ice, and a steady return to the aggravating exercise—to relieve the pain of stress fractures until the patient is pain-free.11
The American College of Sports Medicine, The American Academy of Orthopedic Surgeons, and the American Academy of Physical Medicine and Rehabilitation haven’t issued definitive guidelines concerning whether to use NSAIDs in managing fractures.
NO, ALTHOUGH THE EVIDENCE VARIES. Nonsteroidal anti-inflammatory drugs (NSAIDs) don’t appear to impair clinical fracture healing (strength of recommendation [SOR]: B, inconsistent evidence from a randomized controlled trial [RCT] and retrospective studies). Even though animal studies show delayed healing and nonunion with NSAID use, evidence in humans doesn’t merit avoiding NSAIDs in patients with fractures who need the drugs’ analgesic and anti-inflammatory benefits.
Evidence summary
NSAIDs are commonly prescribed to control pain in patients with fractures. Laboratory studies have found that their antiprostaglandin properties delay callus formation and subsequent healing.1 However, human studies evaluating the effects of NSAIDs on fracture healing have found variable results (TABLE).
TABLE
Fracture healing with NSAIDs: What the studies show
| Type of study | Population | Intervention | Outcome and results |
|---|---|---|---|
| Randomized controlled trial2 | Postmenopausal women with Colles’ fractures (N=42) | Piroxicam | No delay in fracture healing |
| Retrospective3 | Patients with long-bone fractures (N=112) | Indomethacin | Rate of nonunion 29% vs 7% (P=.004) |
| Retrospective4 | Patients with femoral shaft fractures (N=99) | Diclofenac or ibuprofen | OR for nonunion=10.7 (95% CI, 3.5-33.2) |
| Retrospective5 | Postoperative spinal fusion patients (N=288) | Ketoralac | OR for nonunion=4.9 (95% CI, 1.8-16.6) |
| Retrospective6 | Patients with tibial fractures (N=94) | Multiple NSAIDs | Increased mean time to union of 7.6 wk (P=.0003) |
| Retrospective7 | Patients with humeral shaft fractures (N=9995) | Multiple NSAIDs | Increased risk of nonunion with exposure to NSAIDs 60-90 days postfracture (RR=3.9; 95% CI, 2.0-6.2) |
| CI, confidence interval; OR, odds ratio; RR, relative risk. | |||
An RCT finds no delay in healing
An RCT of 42 postmenopausal women with displaced Colles’ fractures who were given piroxicam or placebo found no difference in the rate of healing between the intervention and control groups.2 After 8 weeks, the bone mineral content of the radius and ulna, measured by bone density, was similar in both groups. Patients in the piroxicam group had significantly less pain at 10 days and 4 weeks, and used significantly less rescue medication.
Other studies beg to differ
Three observational studies of patients with different types of fractures found an increase in nonunion associated with NSAIDs.3-5 Two retrospective studies of patients with long-bone fractures reported a higher rate of nonunion among patients taking indomethacin, diclofenac, or ibuprofen.3,4 The third study, a retrospective analysis of postoperative spinal fusion patients who took ketorolac, also found an association between increased risk of nonunion and NSAIDs5 (TABLE).
A retrospective study of 94 patients with tibial fractures reported delayed healing in patients who had taken any NSAID. This association persisted after elimination for age, sex, fracture severity, and high-energy injuries.6
A relationship, but is it causal?
A larger retrospective cohort study of 9995 patients with humeral shaft fractures found an increased risk of nonunion in patients exposed to NSAIDs during the 90 days after the fracture. On further analysis, however, only NSAID exposure 60 to 90 days after the fracture was significantly associated with nonunion. Because patients with painful nonunion fractures are likely to use more NSAIDs, the relationship may not be causal.7
Benefits of NSAIDs outweigh concerns
Three reviews of the effect of NSAIDs on fracture healing all come to the same conclusion: Although animal studies raise theoretical concerns that NSAIDs affect fracture healing, no conclusive evidence supports denying patients the analgesic benefits of these drugs for managing fractures.8-10
Recommendations
The American Academy of Family Physicians recommends using NSAIDs temporarily along with other measures—such as stretching, ice, and a steady return to the aggravating exercise—to relieve the pain of stress fractures until the patient is pain-free.11
The American College of Sports Medicine, The American Academy of Orthopedic Surgeons, and the American Academy of Physical Medicine and Rehabilitation haven’t issued definitive guidelines concerning whether to use NSAIDs in managing fractures.
1. Gerstenfeld LC, Al-Ghawas M, Alkhiary YM, et al. Selective and nonselective cyclooxygenase-2 inhibitors and experimental fracture-healing. Reversibility of effects after short-term treatment. J Bone Joint Surg Am. 2007;89:114-125.
2. Adolphson P, Abbaszadegan H, Jonnson U, et al. No effects of piroxicam on osteopenia and recovery after Colles’ fracture. A randomized, double-blind, placebo-controlled prospective trial. Arch Orthop Trauma Surg. 1993;112:127-130.
3. Burd TA, Hughes MS, Anglen JO. Heterotopic ossification prophylaxis with indomethacin increases the risk of long-bone nonunion. J Bone Joint Surg Br. 2003;85:700-705.
4. Giannoudis PV, MacDonald DA, Matthews SJ. Nonunion of the femoral diaphysis: the influence of reaming and nonsteroidal anti-inflammatory drugs. J Bone Joint Surg Br. 2000;82:655-658.
5. Glassman SD, Rose SM, Dimar JR, et al. The effect of postoperative nonsteroidal anti-inflammatory drug administration on spinal fusion. Spine. 1998;23:834-838.
6. Butcher CK, Marsh DR. Nonsteroidal anti-inflammatory drugs delay tibial fracture union. Injury. 1996;27:375.-
7. Bhattacharyya T, Levin R, Vrahas MS, et al. Nonsteroidal anti-inflammatory drugs and nonunion of humeral shaft fractures. Arthritis Rheum. 2005;53:364-367.
8. Wheeler P, Batt ME. Do nonsteroidal anti-inflammatory drugs adversely affect stress fracture healing? A short review. Br J Sports Med. 2005;39:65-69.
9. Clarke S, Lecky F. Best evidence topic report. Do nonsteroidal anti-inflammatory drugs cause a delay in fracture healing? Emerg Med J. 2005;22:652-653.
10. Koester MC, Spindler KP. NSAIDs and fracture healing: what’s the evidence? Curr Sports Med Rep. 2005;4:289-290.
11. Sanderlin BW, Raspa RF. Common stress fractures. Am Fam Physician. 2003;68:1527-1532.
1. Gerstenfeld LC, Al-Ghawas M, Alkhiary YM, et al. Selective and nonselective cyclooxygenase-2 inhibitors and experimental fracture-healing. Reversibility of effects after short-term treatment. J Bone Joint Surg Am. 2007;89:114-125.
2. Adolphson P, Abbaszadegan H, Jonnson U, et al. No effects of piroxicam on osteopenia and recovery after Colles’ fracture. A randomized, double-blind, placebo-controlled prospective trial. Arch Orthop Trauma Surg. 1993;112:127-130.
3. Burd TA, Hughes MS, Anglen JO. Heterotopic ossification prophylaxis with indomethacin increases the risk of long-bone nonunion. J Bone Joint Surg Br. 2003;85:700-705.
4. Giannoudis PV, MacDonald DA, Matthews SJ. Nonunion of the femoral diaphysis: the influence of reaming and nonsteroidal anti-inflammatory drugs. J Bone Joint Surg Br. 2000;82:655-658.
5. Glassman SD, Rose SM, Dimar JR, et al. The effect of postoperative nonsteroidal anti-inflammatory drug administration on spinal fusion. Spine. 1998;23:834-838.
6. Butcher CK, Marsh DR. Nonsteroidal anti-inflammatory drugs delay tibial fracture union. Injury. 1996;27:375.-
7. Bhattacharyya T, Levin R, Vrahas MS, et al. Nonsteroidal anti-inflammatory drugs and nonunion of humeral shaft fractures. Arthritis Rheum. 2005;53:364-367.
8. Wheeler P, Batt ME. Do nonsteroidal anti-inflammatory drugs adversely affect stress fracture healing? A short review. Br J Sports Med. 2005;39:65-69.
9. Clarke S, Lecky F. Best evidence topic report. Do nonsteroidal anti-inflammatory drugs cause a delay in fracture healing? Emerg Med J. 2005;22:652-653.
10. Koester MC, Spindler KP. NSAIDs and fracture healing: what’s the evidence? Curr Sports Med Rep. 2005;4:289-290.
11. Sanderlin BW, Raspa RF. Common stress fractures. Am Fam Physician. 2003;68:1527-1532.
Evidence-based answers from the Family Physicians Inquiries Network
Do nonmedicated topicals relieve childhood eczema?
Yes. Emollients are effective first-line treatment to decrease symptoms of eczema and reduce the need to use steroids in children (strength of recommendation [SOR]: A, consistent randomized, controlled trials [RCTs]).
Tar preparations work, but compliance may be limited (SOR: B, single small RCT). Gamma-linoleic acid preparations, borage oil, and evening primrose oil show efficacy in small studies (SOR: B, small RCTs). MAS063DP cream (Atopiclair) is effective (SOR: B, single RCT).
Chamomile (SOR: B, inconsistent RCTs) and bathing in acidic hot spring water (SOR: C, case-control study) may be effective, but these treatments have not been adequately evaluated. Wet wrap dressings may be effective but increase the risk of skin infections (SOR: B, single RCT).
Hamamelis distillate creams (SOR: B, limited RCT) and massage with essential oils/aromatherapy are ineffective (SOR: C, case-control study).
Evidence summary
Eczema is a chronic, inflammatory, pruritic skin disorder that affects infants, children, and adults. Therapeutic efficacy is defined as symptom relief and decreased inflammation. Topical corticosteroids and calcineurin inhibitors (such as tacrolimus and pimecrolimus) are the standard of care for prescription therapy in children, but their potentially harmful side effects argue for safer, nonmedicated treatments.
Topical treatments that work
Emollients have demonstrated efficacy in several RCTs compared with placebo and corticosteroids alone. No 1 preparation has proved superior to another; all reduce steroid use and improve skin hydration.1-3
Tar. Only 1 study has evaluated the use of tar: a comparison of 30 patients (mean age 11.8 years) who were treated with tar on one side of the body and 1% hydrocortisone on the other. Both treatments produced comparable results and were well tolerated. But compliance can be a problem with tar products because they smell unpleasant and stain clothing.4
Gamma-linoleic acid. Small studies have evaluated the efficacy of gamma-linoleic acid (GLA)—including borage oil (24% GLA) and evening primrose oil (7%-10% GLA). An RCT of 12 patients (ages 4-46 years, mean 18 years) that compared evening primrose oil with placebo found that patients treated with primrose oil showed a subjective improvement in skin scaling, dryness, redness, and itching.5
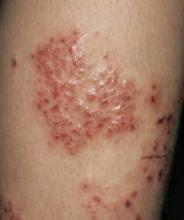
Eczema on the leg of a 9-year-old boy.A double-blind, placebo-controlled trial of 32 children that assessed the effects of undershirts coated with borage oil compared with noncoated undershirts found statistically significant improvements in both itching and erythema.6
MAS063DP is a nonsteroidal, hydrolipidic cream containing glycyrrhetinic acid (GrA), vitis vinifera (grapevine extract), and telmestine. A recent multicenter RCT of 142 children compared MAS063DP to vehicle cream alone. The primary outcome was treatment success defined as an Investigator’s Global Assessment score of ≤1 (range 0-5), measured on day 22. Therapy was successful in 77% of the treatment group vs 0% of the vehicle-only group (number needed to treat=1).7
Hot spring baths, chamomile may help
In a case control study of 70 patients (ages 12-80 years, mean 23 years,) bathing in acidic hot spring water (42° C) helped control edema, erythema, exudation, and excoriation in refractory cases of eczema.8
Several adult and mixed adult-child studies have found mild efficacy for chamomile extracts. One RCT demonstrated topical chamomile to be equivalent to 0.25% hydrocortisone cream for treating mild eczema.9
Wet wraps may help, but may raise skin infection risk
A critical review suggests that short-term use of wet wraps in combination with topical steroids and emollients is effective for severe eczema. However, a small RCT of 50 children found no additional benefit over standard care and an increased risk of skin infection (95% CI, 5%-42%; P=.05) with a number needed to harm of 5.10,11
Essential oils, hamamelis distillate don’t work
In 1 case control study, massage with essential oils didn’t improve eczema compared with massage without essential oils.12 Hamamelis (witch hazel) distillate cream was inferior to steroid creams.13
Recommendations
The American Academy of Dermatology guidelines state that emollients are the standard of care for childhood eczema and have a steroid-sparing effect (level of evidence [LOE]: A). Tar preparations have therapeutic benefits, but compliance is a major limitation (LOE: B). Not enough evidence exists to recommend acidic baths. The guidelines make no recommendations about other topical therapies.
A task force to formulate practice parameters has been created by the American College of Allergy, Asthma, and Immunology; the American Academy of Allergy, Asthma, and Immunology; and the Joint Council of Allergy, Asthma, and Immunology. The task force’s latest recommendations suggest that emollients, tar preparations, and wet dressings are beneficial for treating eczema.2
1. Grimalt R, Mengeaud V, Cambazard F. Study Investigators’ Group. The steroid-sparing effect of an emollient therapy in infants with atopic dermatitis: a randomized controlled study. Dermatology. 2007;214:61-67.
2. Leung DY, Nicklas RA, Li JT, et al. Disease management of atopic dermatitis: an updated practice parameter. Joint Task Force on Practice Parameters. Ann Allergy Asthma Immunol. 2004;93(3 suppl 2):S1-S21.
3. Hanifin JM, Cooper KD, Ho VC, et al. Guidelines of care for atopic dermatitis, developed in accordance with the American Academy of Dermatology (ADA)/American Academy of Dermatology Association “Administrative Regulations for Evidence-Based Clinical Practice Guidelines.” J Am Acad Dermatol. 2004;50:391-404.
4. Munkvad M. A comparative trial of Clinitar versus hydrocortisone cream in the treatment of atopic eczema. Br J Dermatol. 1989;121:763-766.
5. Anstey A, Quigley M, Wilkinson JD. Topical evening primrose oil as treatment for atopic eczema. J Dermatol Treat. 1990;1:199-201.
6. Kanehara S, Ohtani T, Uede K, et al. Clinical effects of undershirts coated with borage oil on children with atopic dermatitis: a double-blind, placebo-controlled trial. J Dermatol. 2007;34:811-815.
7. Boguniewicz M, Ziechner JA, Eichenfield LF, et al. MAS063DP is effective monotherapy for mild to moderate atopic dermatitis in infants and children: a multicenter, randomized, vehicle-controlled study. J Pediatr. 2008;152:854-859.
8. Kubota K, Machida I, Tamura K, et al. Treatment of refractory cases of atopic dermatitis with acidic hot-spring bathing. Acta Derm Venereol. 1997;77:452-454.
9. Ross SM. An integrative approach to eczema atopic dermatitis. Holist Nurs Pract. 2003;17:56-62.
10. Devillers AC, Oranje AP. Efficacy and safety of “wet-wrap” dressings as an intervention treatment in children with severe and/or refractory atopic dermatitis: a critical review of the literature. Br J Dermatol. 2006;154:579-585.
11. Hindley D, Galloway G, Murray J, et al. A randomised study of “wet wraps” versus conventional treatment for atopic eczema. Arch Dis Child. 2006;91:164-168.
12. Anderson C, Lis-Balchin M, Kirk-Smith M. Evaluation of massage with essential oils on childhood eczema. Phytother Res. 2000;14:452-456.
13. Korting HC, Schäfer-Korting M, Klövekorn W, et al. Comparative efficacy of hamamelis distillate and hydrocortisone cream in atopic eczema. Eur J Clin Pharmacol. 1995;48:461-465.
Yes. Emollients are effective first-line treatment to decrease symptoms of eczema and reduce the need to use steroids in children (strength of recommendation [SOR]: A, consistent randomized, controlled trials [RCTs]).
Tar preparations work, but compliance may be limited (SOR: B, single small RCT). Gamma-linoleic acid preparations, borage oil, and evening primrose oil show efficacy in small studies (SOR: B, small RCTs). MAS063DP cream (Atopiclair) is effective (SOR: B, single RCT).
Chamomile (SOR: B, inconsistent RCTs) and bathing in acidic hot spring water (SOR: C, case-control study) may be effective, but these treatments have not been adequately evaluated. Wet wrap dressings may be effective but increase the risk of skin infections (SOR: B, single RCT).
Hamamelis distillate creams (SOR: B, limited RCT) and massage with essential oils/aromatherapy are ineffective (SOR: C, case-control study).
Evidence summary
Eczema is a chronic, inflammatory, pruritic skin disorder that affects infants, children, and adults. Therapeutic efficacy is defined as symptom relief and decreased inflammation. Topical corticosteroids and calcineurin inhibitors (such as tacrolimus and pimecrolimus) are the standard of care for prescription therapy in children, but their potentially harmful side effects argue for safer, nonmedicated treatments.
Topical treatments that work
Emollients have demonstrated efficacy in several RCTs compared with placebo and corticosteroids alone. No 1 preparation has proved superior to another; all reduce steroid use and improve skin hydration.1-3
Tar. Only 1 study has evaluated the use of tar: a comparison of 30 patients (mean age 11.8 years) who were treated with tar on one side of the body and 1% hydrocortisone on the other. Both treatments produced comparable results and were well tolerated. But compliance can be a problem with tar products because they smell unpleasant and stain clothing.4
Gamma-linoleic acid. Small studies have evaluated the efficacy of gamma-linoleic acid (GLA)—including borage oil (24% GLA) and evening primrose oil (7%-10% GLA). An RCT of 12 patients (ages 4-46 years, mean 18 years) that compared evening primrose oil with placebo found that patients treated with primrose oil showed a subjective improvement in skin scaling, dryness, redness, and itching.5
Eczema on the leg of a 9-year-old boy.A double-blind, placebo-controlled trial of 32 children that assessed the effects of undershirts coated with borage oil compared with noncoated undershirts found statistically significant improvements in both itching and erythema.6
MAS063DP is a nonsteroidal, hydrolipidic cream containing glycyrrhetinic acid (GrA), vitis vinifera (grapevine extract), and telmestine. A recent multicenter RCT of 142 children compared MAS063DP to vehicle cream alone. The primary outcome was treatment success defined as an Investigator’s Global Assessment score of ≤1 (range 0-5), measured on day 22. Therapy was successful in 77% of the treatment group vs 0% of the vehicle-only group (number needed to treat=1).7
Hot spring baths, chamomile may help
In a case control study of 70 patients (ages 12-80 years, mean 23 years,) bathing in acidic hot spring water (42° C) helped control edema, erythema, exudation, and excoriation in refractory cases of eczema.8
Several adult and mixed adult-child studies have found mild efficacy for chamomile extracts. One RCT demonstrated topical chamomile to be equivalent to 0.25% hydrocortisone cream for treating mild eczema.9
Wet wraps may help, but may raise skin infection risk
A critical review suggests that short-term use of wet wraps in combination with topical steroids and emollients is effective for severe eczema. However, a small RCT of 50 children found no additional benefit over standard care and an increased risk of skin infection (95% CI, 5%-42%; P=.05) with a number needed to harm of 5.10,11
Essential oils, hamamelis distillate don’t work
In 1 case control study, massage with essential oils didn’t improve eczema compared with massage without essential oils.12 Hamamelis (witch hazel) distillate cream was inferior to steroid creams.13
Recommendations
The American Academy of Dermatology guidelines state that emollients are the standard of care for childhood eczema and have a steroid-sparing effect (level of evidence [LOE]: A). Tar preparations have therapeutic benefits, but compliance is a major limitation (LOE: B). Not enough evidence exists to recommend acidic baths. The guidelines make no recommendations about other topical therapies.
A task force to formulate practice parameters has been created by the American College of Allergy, Asthma, and Immunology; the American Academy of Allergy, Asthma, and Immunology; and the Joint Council of Allergy, Asthma, and Immunology. The task force’s latest recommendations suggest that emollients, tar preparations, and wet dressings are beneficial for treating eczema.2
Yes. Emollients are effective first-line treatment to decrease symptoms of eczema and reduce the need to use steroids in children (strength of recommendation [SOR]: A, consistent randomized, controlled trials [RCTs]).
Tar preparations work, but compliance may be limited (SOR: B, single small RCT). Gamma-linoleic acid preparations, borage oil, and evening primrose oil show efficacy in small studies (SOR: B, small RCTs). MAS063DP cream (Atopiclair) is effective (SOR: B, single RCT).
Chamomile (SOR: B, inconsistent RCTs) and bathing in acidic hot spring water (SOR: C, case-control study) may be effective, but these treatments have not been adequately evaluated. Wet wrap dressings may be effective but increase the risk of skin infections (SOR: B, single RCT).
Hamamelis distillate creams (SOR: B, limited RCT) and massage with essential oils/aromatherapy are ineffective (SOR: C, case-control study).
Evidence summary
Eczema is a chronic, inflammatory, pruritic skin disorder that affects infants, children, and adults. Therapeutic efficacy is defined as symptom relief and decreased inflammation. Topical corticosteroids and calcineurin inhibitors (such as tacrolimus and pimecrolimus) are the standard of care for prescription therapy in children, but their potentially harmful side effects argue for safer, nonmedicated treatments.
Topical treatments that work
Emollients have demonstrated efficacy in several RCTs compared with placebo and corticosteroids alone. No 1 preparation has proved superior to another; all reduce steroid use and improve skin hydration.1-3
Tar. Only 1 study has evaluated the use of tar: a comparison of 30 patients (mean age 11.8 years) who were treated with tar on one side of the body and 1% hydrocortisone on the other. Both treatments produced comparable results and were well tolerated. But compliance can be a problem with tar products because they smell unpleasant and stain clothing.4
Gamma-linoleic acid. Small studies have evaluated the efficacy of gamma-linoleic acid (GLA)—including borage oil (24% GLA) and evening primrose oil (7%-10% GLA). An RCT of 12 patients (ages 4-46 years, mean 18 years) that compared evening primrose oil with placebo found that patients treated with primrose oil showed a subjective improvement in skin scaling, dryness, redness, and itching.5
Eczema on the leg of a 9-year-old boy.A double-blind, placebo-controlled trial of 32 children that assessed the effects of undershirts coated with borage oil compared with noncoated undershirts found statistically significant improvements in both itching and erythema.6
MAS063DP is a nonsteroidal, hydrolipidic cream containing glycyrrhetinic acid (GrA), vitis vinifera (grapevine extract), and telmestine. A recent multicenter RCT of 142 children compared MAS063DP to vehicle cream alone. The primary outcome was treatment success defined as an Investigator’s Global Assessment score of ≤1 (range 0-5), measured on day 22. Therapy was successful in 77% of the treatment group vs 0% of the vehicle-only group (number needed to treat=1).7
Hot spring baths, chamomile may help
In a case control study of 70 patients (ages 12-80 years, mean 23 years,) bathing in acidic hot spring water (42° C) helped control edema, erythema, exudation, and excoriation in refractory cases of eczema.8
Several adult and mixed adult-child studies have found mild efficacy for chamomile extracts. One RCT demonstrated topical chamomile to be equivalent to 0.25% hydrocortisone cream for treating mild eczema.9
Wet wraps may help, but may raise skin infection risk
A critical review suggests that short-term use of wet wraps in combination with topical steroids and emollients is effective for severe eczema. However, a small RCT of 50 children found no additional benefit over standard care and an increased risk of skin infection (95% CI, 5%-42%; P=.05) with a number needed to harm of 5.10,11
Essential oils, hamamelis distillate don’t work
In 1 case control study, massage with essential oils didn’t improve eczema compared with massage without essential oils.12 Hamamelis (witch hazel) distillate cream was inferior to steroid creams.13
Recommendations
The American Academy of Dermatology guidelines state that emollients are the standard of care for childhood eczema and have a steroid-sparing effect (level of evidence [LOE]: A). Tar preparations have therapeutic benefits, but compliance is a major limitation (LOE: B). Not enough evidence exists to recommend acidic baths. The guidelines make no recommendations about other topical therapies.
A task force to formulate practice parameters has been created by the American College of Allergy, Asthma, and Immunology; the American Academy of Allergy, Asthma, and Immunology; and the Joint Council of Allergy, Asthma, and Immunology. The task force’s latest recommendations suggest that emollients, tar preparations, and wet dressings are beneficial for treating eczema.2
1. Grimalt R, Mengeaud V, Cambazard F. Study Investigators’ Group. The steroid-sparing effect of an emollient therapy in infants with atopic dermatitis: a randomized controlled study. Dermatology. 2007;214:61-67.
2. Leung DY, Nicklas RA, Li JT, et al. Disease management of atopic dermatitis: an updated practice parameter. Joint Task Force on Practice Parameters. Ann Allergy Asthma Immunol. 2004;93(3 suppl 2):S1-S21.
3. Hanifin JM, Cooper KD, Ho VC, et al. Guidelines of care for atopic dermatitis, developed in accordance with the American Academy of Dermatology (ADA)/American Academy of Dermatology Association “Administrative Regulations for Evidence-Based Clinical Practice Guidelines.” J Am Acad Dermatol. 2004;50:391-404.
4. Munkvad M. A comparative trial of Clinitar versus hydrocortisone cream in the treatment of atopic eczema. Br J Dermatol. 1989;121:763-766.
5. Anstey A, Quigley M, Wilkinson JD. Topical evening primrose oil as treatment for atopic eczema. J Dermatol Treat. 1990;1:199-201.
6. Kanehara S, Ohtani T, Uede K, et al. Clinical effects of undershirts coated with borage oil on children with atopic dermatitis: a double-blind, placebo-controlled trial. J Dermatol. 2007;34:811-815.
7. Boguniewicz M, Ziechner JA, Eichenfield LF, et al. MAS063DP is effective monotherapy for mild to moderate atopic dermatitis in infants and children: a multicenter, randomized, vehicle-controlled study. J Pediatr. 2008;152:854-859.
8. Kubota K, Machida I, Tamura K, et al. Treatment of refractory cases of atopic dermatitis with acidic hot-spring bathing. Acta Derm Venereol. 1997;77:452-454.
9. Ross SM. An integrative approach to eczema atopic dermatitis. Holist Nurs Pract. 2003;17:56-62.
10. Devillers AC, Oranje AP. Efficacy and safety of “wet-wrap” dressings as an intervention treatment in children with severe and/or refractory atopic dermatitis: a critical review of the literature. Br J Dermatol. 2006;154:579-585.
11. Hindley D, Galloway G, Murray J, et al. A randomised study of “wet wraps” versus conventional treatment for atopic eczema. Arch Dis Child. 2006;91:164-168.
12. Anderson C, Lis-Balchin M, Kirk-Smith M. Evaluation of massage with essential oils on childhood eczema. Phytother Res. 2000;14:452-456.
13. Korting HC, Schäfer-Korting M, Klövekorn W, et al. Comparative efficacy of hamamelis distillate and hydrocortisone cream in atopic eczema. Eur J Clin Pharmacol. 1995;48:461-465.
1. Grimalt R, Mengeaud V, Cambazard F. Study Investigators’ Group. The steroid-sparing effect of an emollient therapy in infants with atopic dermatitis: a randomized controlled study. Dermatology. 2007;214:61-67.
2. Leung DY, Nicklas RA, Li JT, et al. Disease management of atopic dermatitis: an updated practice parameter. Joint Task Force on Practice Parameters. Ann Allergy Asthma Immunol. 2004;93(3 suppl 2):S1-S21.
3. Hanifin JM, Cooper KD, Ho VC, et al. Guidelines of care for atopic dermatitis, developed in accordance with the American Academy of Dermatology (ADA)/American Academy of Dermatology Association “Administrative Regulations for Evidence-Based Clinical Practice Guidelines.” J Am Acad Dermatol. 2004;50:391-404.
4. Munkvad M. A comparative trial of Clinitar versus hydrocortisone cream in the treatment of atopic eczema. Br J Dermatol. 1989;121:763-766.
5. Anstey A, Quigley M, Wilkinson JD. Topical evening primrose oil as treatment for atopic eczema. J Dermatol Treat. 1990;1:199-201.
6. Kanehara S, Ohtani T, Uede K, et al. Clinical effects of undershirts coated with borage oil on children with atopic dermatitis: a double-blind, placebo-controlled trial. J Dermatol. 2007;34:811-815.
7. Boguniewicz M, Ziechner JA, Eichenfield LF, et al. MAS063DP is effective monotherapy for mild to moderate atopic dermatitis in infants and children: a multicenter, randomized, vehicle-controlled study. J Pediatr. 2008;152:854-859.
8. Kubota K, Machida I, Tamura K, et al. Treatment of refractory cases of atopic dermatitis with acidic hot-spring bathing. Acta Derm Venereol. 1997;77:452-454.
9. Ross SM. An integrative approach to eczema atopic dermatitis. Holist Nurs Pract. 2003;17:56-62.
10. Devillers AC, Oranje AP. Efficacy and safety of “wet-wrap” dressings as an intervention treatment in children with severe and/or refractory atopic dermatitis: a critical review of the literature. Br J Dermatol. 2006;154:579-585.
11. Hindley D, Galloway G, Murray J, et al. A randomised study of “wet wraps” versus conventional treatment for atopic eczema. Arch Dis Child. 2006;91:164-168.
12. Anderson C, Lis-Balchin M, Kirk-Smith M. Evaluation of massage with essential oils on childhood eczema. Phytother Res. 2000;14:452-456.
13. Korting HC, Schäfer-Korting M, Klövekorn W, et al. Comparative efficacy of hamamelis distillate and hydrocortisone cream in atopic eczema. Eur J Clin Pharmacol. 1995;48:461-465.
Evidence-based answers from the Family Physicians Inquiries Network
Alcoholic liver disease: Is acetaminophen safe?
Yes—acetaminophen is a safe and effective analgesic that can be appropriately used for adult patients with stable chronic alcoholic liver disease for at least a short period of time (studies have been limited to a maximum of 48–72 hours), up to the maximum recommended dosage of 4 g daily (strength of recommendation: A, based on 2 RCTs and other studies). There are little data to guide longer-term use of acetaminophen in this situation.
“Lesser of all evils”
Joseph J. Saseen, PharmD, FCCP, BCPS
University of Colorado at Denver and Health Sciences Center, Denver
Selecting an appropriate analgesic for patients with chronic alcoholic liver disease is complicated. Narcotics are potentially addictive, and nonsteroidal anti-inflammatory drugs (NSAIDs) can cause gastrointestinal bleeding and other adverse events. Alcoholic liver disease predisposes patients to these potential drug-related complications, so these options are not ideal.
Acetaminophen is the “lesser of all evils” in this population, based on some data suggesting it is safe when used within approved dosing parameters. However, these parameters vary significantly.
Although a maximum daily dose of 4 g is widely accepted as normal, the American Geriatric Society recommends no more than 2 to 3 g daily for older patients with hepatic insufficiency or a history of alcohol abuse.
Moreover, the American Liver Foundation issued a warning to not exceed 3 g daily for any prolonged period of time in response to a 2006 clinical trial that demonstrated aminotransferase increases in healthy volunteers treated with 4 g of acetaminophen daily for 14 days.1
Regardless of the exact maximum dose, none are greater than 4 g daily.
Always judiciously monitor dosing of acetaminophen because patients continue to experience unintentional overdose and hepatic failure caused by inadvertent use of multiple acetaminophen-containing products.2
Evidence summary
Acetaminophen, while widely used, is hepatotoxic in supra-therapeutic doses.3 Many studies purporting to show evidence of hepatic damage from therapeutic doses of acetaminophen have also been reported. Particularly in the 1970s and 1980s, there were a number of case reports and small literature reviews indicating that hepatic injury among regular users of alcohol (particularly chronic alcoholics) who take acetaminophen with therapeutic intent could be a “therapeutic misadventure.”4
Recent studies suggest short-term safety
- A systematic review (published in 2000) identified reports of acetaminophen toxicity, poisoning, or adverse events for alcohol patients.5
- In a randomized, double-blinded, placebo-controlled study, 102 alcoholic patients were given 4 g of acetaminophen daily for 2 days.6
Recommendations from others
American College of Gastroenterology. The American College of Gastroenterology states that it’s generally safe to take acetaminophen in the amount specified in the package labeling. Furthermore, they recommend that patients diagnosed with liver conditions consult their physician for advice on dosing for acetaminophen or any other pain reliever.7
In Liver and Biliary Disease, the author concludes that chronic alcoholics are at increased risk for hepatotoxicity secondary to acetaminophen even at therapeutic doses; therefore, advise them to take no more than 2 g daily.8
1. Watkins PB, Kaplowitz N, Slattery JT, et al. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily. A randomized controlled trial. JAMA 2006;296:87-93.
2. Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: Results of a United States multicenter, prospective study. Hepatology 2005;42:1364-1372.
3. Barker JD, Jr, de Carle DJ, Anuras S. Chronic excessive acetaminophen use and liver damage. Ann Intern Med 1977;87:299-301.
4. Zimmerman HJ, Maddery WC. Acetaminophen (paracetamol) hepatotoxicity with regular intake of alcohol: analysis of instances of therapeutic misadventure. Hepatology 1995;22:767-773.
5. Dart RC, Kuffner EK, Rumack BH. Treatment of pain or fever with paracetamol (acetaminophen) in the alcoholic patient: a systematic review. Am J Therapeutics 2000;7:123-134.
6. Kuffner EK, Dart RC, Bogdan GM, Hill RE, Casper E, Darton L. Effect of maximal daily doses of acetaminophen on the liver of alcoholic patients: a randomized, double-blind, placebo-controlled trial. Arch Inter Med 2001;161:2247-2252.
7. Herrera JL, O’Brien BL. Important information for patients with chronic liver disease and/or cirrhosis. In McNally PR, DeVault KR, Surawicz CM, eds. Common GI Problems. vol 3. Available at: www.acg.gi.org/patients/cgp/cgpvol3.asp#liver. Accessed on June 26, 2007.
8. Kaplowitz N. Liver and Biliary Diseases. Baltimore, Md: Williams and Wilkins; 1996.
Yes—acetaminophen is a safe and effective analgesic that can be appropriately used for adult patients with stable chronic alcoholic liver disease for at least a short period of time (studies have been limited to a maximum of 48–72 hours), up to the maximum recommended dosage of 4 g daily (strength of recommendation: A, based on 2 RCTs and other studies). There are little data to guide longer-term use of acetaminophen in this situation.
“Lesser of all evils”
Joseph J. Saseen, PharmD, FCCP, BCPS
University of Colorado at Denver and Health Sciences Center, Denver
Selecting an appropriate analgesic for patients with chronic alcoholic liver disease is complicated. Narcotics are potentially addictive, and nonsteroidal anti-inflammatory drugs (NSAIDs) can cause gastrointestinal bleeding and other adverse events. Alcoholic liver disease predisposes patients to these potential drug-related complications, so these options are not ideal.
Acetaminophen is the “lesser of all evils” in this population, based on some data suggesting it is safe when used within approved dosing parameters. However, these parameters vary significantly.
Although a maximum daily dose of 4 g is widely accepted as normal, the American Geriatric Society recommends no more than 2 to 3 g daily for older patients with hepatic insufficiency or a history of alcohol abuse.
Moreover, the American Liver Foundation issued a warning to not exceed 3 g daily for any prolonged period of time in response to a 2006 clinical trial that demonstrated aminotransferase increases in healthy volunteers treated with 4 g of acetaminophen daily for 14 days.1
Regardless of the exact maximum dose, none are greater than 4 g daily.
Always judiciously monitor dosing of acetaminophen because patients continue to experience unintentional overdose and hepatic failure caused by inadvertent use of multiple acetaminophen-containing products.2
Evidence summary
Acetaminophen, while widely used, is hepatotoxic in supra-therapeutic doses.3 Many studies purporting to show evidence of hepatic damage from therapeutic doses of acetaminophen have also been reported. Particularly in the 1970s and 1980s, there were a number of case reports and small literature reviews indicating that hepatic injury among regular users of alcohol (particularly chronic alcoholics) who take acetaminophen with therapeutic intent could be a “therapeutic misadventure.”4
Recent studies suggest short-term safety
- A systematic review (published in 2000) identified reports of acetaminophen toxicity, poisoning, or adverse events for alcohol patients.5
- In a randomized, double-blinded, placebo-controlled study, 102 alcoholic patients were given 4 g of acetaminophen daily for 2 days.6
Recommendations from others
American College of Gastroenterology. The American College of Gastroenterology states that it’s generally safe to take acetaminophen in the amount specified in the package labeling. Furthermore, they recommend that patients diagnosed with liver conditions consult their physician for advice on dosing for acetaminophen or any other pain reliever.7
In Liver and Biliary Disease, the author concludes that chronic alcoholics are at increased risk for hepatotoxicity secondary to acetaminophen even at therapeutic doses; therefore, advise them to take no more than 2 g daily.8
Yes—acetaminophen is a safe and effective analgesic that can be appropriately used for adult patients with stable chronic alcoholic liver disease for at least a short period of time (studies have been limited to a maximum of 48–72 hours), up to the maximum recommended dosage of 4 g daily (strength of recommendation: A, based on 2 RCTs and other studies). There are little data to guide longer-term use of acetaminophen in this situation.
“Lesser of all evils”
Joseph J. Saseen, PharmD, FCCP, BCPS
University of Colorado at Denver and Health Sciences Center, Denver
Selecting an appropriate analgesic for patients with chronic alcoholic liver disease is complicated. Narcotics are potentially addictive, and nonsteroidal anti-inflammatory drugs (NSAIDs) can cause gastrointestinal bleeding and other adverse events. Alcoholic liver disease predisposes patients to these potential drug-related complications, so these options are not ideal.
Acetaminophen is the “lesser of all evils” in this population, based on some data suggesting it is safe when used within approved dosing parameters. However, these parameters vary significantly.
Although a maximum daily dose of 4 g is widely accepted as normal, the American Geriatric Society recommends no more than 2 to 3 g daily for older patients with hepatic insufficiency or a history of alcohol abuse.
Moreover, the American Liver Foundation issued a warning to not exceed 3 g daily for any prolonged period of time in response to a 2006 clinical trial that demonstrated aminotransferase increases in healthy volunteers treated with 4 g of acetaminophen daily for 14 days.1
Regardless of the exact maximum dose, none are greater than 4 g daily.
Always judiciously monitor dosing of acetaminophen because patients continue to experience unintentional overdose and hepatic failure caused by inadvertent use of multiple acetaminophen-containing products.2
Evidence summary
Acetaminophen, while widely used, is hepatotoxic in supra-therapeutic doses.3 Many studies purporting to show evidence of hepatic damage from therapeutic doses of acetaminophen have also been reported. Particularly in the 1970s and 1980s, there were a number of case reports and small literature reviews indicating that hepatic injury among regular users of alcohol (particularly chronic alcoholics) who take acetaminophen with therapeutic intent could be a “therapeutic misadventure.”4
Recent studies suggest short-term safety
- A systematic review (published in 2000) identified reports of acetaminophen toxicity, poisoning, or adverse events for alcohol patients.5
- In a randomized, double-blinded, placebo-controlled study, 102 alcoholic patients were given 4 g of acetaminophen daily for 2 days.6
Recommendations from others
American College of Gastroenterology. The American College of Gastroenterology states that it’s generally safe to take acetaminophen in the amount specified in the package labeling. Furthermore, they recommend that patients diagnosed with liver conditions consult their physician for advice on dosing for acetaminophen or any other pain reliever.7
In Liver and Biliary Disease, the author concludes that chronic alcoholics are at increased risk for hepatotoxicity secondary to acetaminophen even at therapeutic doses; therefore, advise them to take no more than 2 g daily.8
1. Watkins PB, Kaplowitz N, Slattery JT, et al. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily. A randomized controlled trial. JAMA 2006;296:87-93.
2. Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: Results of a United States multicenter, prospective study. Hepatology 2005;42:1364-1372.
3. Barker JD, Jr, de Carle DJ, Anuras S. Chronic excessive acetaminophen use and liver damage. Ann Intern Med 1977;87:299-301.
4. Zimmerman HJ, Maddery WC. Acetaminophen (paracetamol) hepatotoxicity with regular intake of alcohol: analysis of instances of therapeutic misadventure. Hepatology 1995;22:767-773.
5. Dart RC, Kuffner EK, Rumack BH. Treatment of pain or fever with paracetamol (acetaminophen) in the alcoholic patient: a systematic review. Am J Therapeutics 2000;7:123-134.
6. Kuffner EK, Dart RC, Bogdan GM, Hill RE, Casper E, Darton L. Effect of maximal daily doses of acetaminophen on the liver of alcoholic patients: a randomized, double-blind, placebo-controlled trial. Arch Inter Med 2001;161:2247-2252.
7. Herrera JL, O’Brien BL. Important information for patients with chronic liver disease and/or cirrhosis. In McNally PR, DeVault KR, Surawicz CM, eds. Common GI Problems. vol 3. Available at: www.acg.gi.org/patients/cgp/cgpvol3.asp#liver. Accessed on June 26, 2007.
8. Kaplowitz N. Liver and Biliary Diseases. Baltimore, Md: Williams and Wilkins; 1996.
1. Watkins PB, Kaplowitz N, Slattery JT, et al. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily. A randomized controlled trial. JAMA 2006;296:87-93.
2. Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: Results of a United States multicenter, prospective study. Hepatology 2005;42:1364-1372.
3. Barker JD, Jr, de Carle DJ, Anuras S. Chronic excessive acetaminophen use and liver damage. Ann Intern Med 1977;87:299-301.
4. Zimmerman HJ, Maddery WC. Acetaminophen (paracetamol) hepatotoxicity with regular intake of alcohol: analysis of instances of therapeutic misadventure. Hepatology 1995;22:767-773.
5. Dart RC, Kuffner EK, Rumack BH. Treatment of pain or fever with paracetamol (acetaminophen) in the alcoholic patient: a systematic review. Am J Therapeutics 2000;7:123-134.
6. Kuffner EK, Dart RC, Bogdan GM, Hill RE, Casper E, Darton L. Effect of maximal daily doses of acetaminophen on the liver of alcoholic patients: a randomized, double-blind, placebo-controlled trial. Arch Inter Med 2001;161:2247-2252.
7. Herrera JL, O’Brien BL. Important information for patients with chronic liver disease and/or cirrhosis. In McNally PR, DeVault KR, Surawicz CM, eds. Common GI Problems. vol 3. Available at: www.acg.gi.org/patients/cgp/cgpvol3.asp#liver. Accessed on June 26, 2007.
8. Kaplowitz N. Liver and Biliary Diseases. Baltimore, Md: Williams and Wilkins; 1996.
Evidence-based answers from the Family Physicians Inquiries Network