User login
Sarcoidosis and Squamous Cell Carcinoma: A Connection Documented in a Case Series of 3 Patients
Sarcoidosis is a multisystem granulomatous disease of unknown etiology that most commonly affects the lungs, eyes, and skin. Cutaneous involvement is reported in 25% to 35% of patients with sarcoidosis and may occur in a variety of forms including macules, papules, plaques, and lupus pernio.1,2 Dermatologists commonly are confronted with the diagnosis and management of sarcoidosis because of its high incidence of cutaneous involvement. Due to the protean nature of the disease, skin biopsy plays a key role in confirming the diagnosis. Histological evidence of noncaseating granulomas in combination with an appropriate clinical and radiographic picture is necessary for the diagnosis of sarcoidosis.1,2 Brincker and Wilbek
We describe 3 patients with sarcoidosis who developed squamous cell carcinoma (SCC) of the skin, including 2 black patients, which highlights the potential for SCC development.
Case Reports
Patient 1
A black woman in her 60s with a history of sarcoidosis affecting the lungs and skin that was well controlled with biweekly adalimumab 40 mg subcutaneous injections presented with a new dark painful lesion on the right third finger. She reported the lesion had been present for 1 to 2 years prior to the current presentation and was increasing in size. She had no history of prior skin cancers.
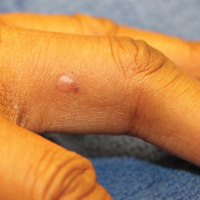
Physical examination revealed a waxy, brown-pigmented papule with overlying scale on the ulnar aspect of the right third digit near the web space (Figure 1A). A shave biopsy revealed atypical keratinocytes involving all layers of the epidermis along with associated parakeratotic scale consistent with a diagnosis of SCC in situ (Figure 1B). Human papillomavirus staining was negative. Due to the location of the lesion, the patient underwent Mohs micrographic surgery and the lesion was completely excised.

Patient 2
A black woman in her 60s with a history of cutaneous sarcoidosis that was maintained on minocycline 100 mg twice daily, chloroquine 250 mg daily, tacrolimus ointment 0.1%, tretinoin cream 0.025%, and intermittent intralesional triamcinolone acetonide injections to the nose, as well as quiescent pulmonary sarcoidosis, developed a new, growing, asymptomatic, hyperpigmented lesion on the left side of the submandibular neck over a period of a few months. A biopsy was performed and the lesion was found to be an SCC, which subsequently was completely excised.
Patient 3
A white man in his 60s with a history of prior quiescent pulmonary sarcoidosis, remote melanoma, and multiple nonmelanoma skin cancers developed scaly papules on the scalp for months, one that was interpreted by an outside pathologist as an invasive SCC (Figure 2A). He was referred to our institution for Mohs micrographic surgery. On presentation when his scalp was shaved for surgery, he was noted to have several violaceous, annular, thin plaques on the scalp (Figure 2B). A biopsy of an annular plaque demonstrated several areas of granulomatous dermatitis consistent with a diagnosis of cutaneous sarcoidosis (Figure 2C). The patient had clinical lymphadenopathy of the neck and supraclavicular region. Given the patient’s history, the differential diagnosis for these lesions included metastatic SCC, lymphoma, and sarcoidosis. The patient underwent a positron emission tomography scan, which demonstrated fluorodeoxyglucose-positive regions in both lungs and the right side of the neck. After evaluation by the pulmonary and otorhinolaryngology departments, including a lymph node biopsy, the positron emission tomography–enhancing lesions were ultimately determined to be consistent with sarcoidosis.
The patient underwent Mohs micrographic surgery for treatment of the scalp SCC and was started on triamcinolone cream 0.1% for the body, clobetasol propionate foam 0.05% for the scalp, and hydroxychloroquine sulfate 400 mg daily for the cutaneous sarcoidosis. His annular scalp lesions resolved, but over the following 12 months the patient had numerous clinically suspicious skin lesions that were biopsied and were consistent with multiple basal cell carcinomas, actinic keratoses, and SCC in situ. They were treated with surgery, cryosurgical destruction with liquid nitrogen, and 5-fluorouracil cream.

Over the 3 years subsequent to initial presentation, the patient developed ocular inflammation attributed to his sarcoidosis and atrial fibrillation, which was determined to be unrelated. He also developed 5 scaly hyperkeratotic plaques on the vertex aspect of the scalp. Biopsy of 2 lesions revealed mild keratinocyte atypia and epidermal hyperplasia, favored to represent SCC over pseudoepitheliomatous hyperplasia overlying associated granulomatous inflammation. These lesions ultimately were believed to represent new SCCs, while biopsies of 2 other lesions revealed isolated granulomatous inflammation that was believed to represent hyperkeratotic cutaneous sarcoidosis clinically resembling his SCCs. The patient was again referred for Mohs micrographic surgery and the malignancies were completely removed, while the cutaneous sarcoidosis was again treated with topical corticosteroids with complete resolution.
Comment
The potential increased risk for malignancy in patients with sarcoidosis has been well documented.3-6 Brincker and Wilbek3 first reported this association after studying 2544 patients with pulmonary sarcoidosis from 1962 to 1971. In particular, they noted a difference between the expected and observed number of cases of malignancy, particularly lung cancer and lymphoma, in the sarcoidosis population.3 In a study of 10,037 hospitalized sarcoidosis patients from 1964 to 2004, Ji et al5 noted a 40% overall increase in the incidence of cancer and found that the risk for malignancy was highest in the year following hospitalization. Interestingly, they found that the risk for developing cutaneous SCC was elevated in sarcoidosis patients even after the first year following hospitalization.5 In a retrospective cohort study examining more than 9000 patients, Askling et al4 also confirmed the increased incidence of malignancy in sarcoidosis patients. Specifically, the authors found a higher than expected occurrence of skin cancer, both melanoma (standardized incidence ratio, 1.6; 95% confidence interval, 1.1-2.3) and nonmelanoma skin cancer (standardized incidence ratio, 2.8; 95% confidence interval, 2.0-3.8) in patients with sarcoidosis.4 Reich et al7 cross-matched 30,000 cases from the Kaiser Permanente Northwest Region Tumor Registry against a sarcoidosis registry of 243 cases to evaluate for evidence of linkage between sarcoidosis and malignancy. They concluded that there may be an etiologic relationship between sarcoidosis and malignancy in at least one-quarter of cases in which both are present and hypothesized that granulomas may be the result of a cell-mediated reaction to tumor antigens.7
Few published studies specifically address the incidence of malignancy in patients with primarily cutaneous sarcoidosis. Cutaneous sarcoidosis includes nonspecific lesions, such as erythema nodosum, as well as specific lesions, such as papules, plaques, nodules, and lupus pernio.8 Alexandrescu et al6 evaluated 110 patients with a diagnosis of both sarcoidosis (cutaneous and noncutaneous) and malignancy. Through their analysis, they found that cutaneous sarcoidosis is seen more commonly in patients presenting with sarcoidosis and malignancy (56.4%) than in the total sarcoidosis population (20%–25%). From these findings, the authors concluded that cutaneous sarcoidosis appears to be a subtype of sarcoidosis associated with cancer.6
We report 3 cases that specifically illustrate a link between cutaneous sarcoidosis and an increased risk for cutaneous SCC. Because sarcoidosis commonly affects the skin, patients often present to dermatologists for care. Once the initial diagnosis of cutaneous sarcoidosis is made via biopsy, it is natural to be tempted to attribute any new skin lesions to worsening or active disease; however, as cutaneous sarcoidosis may take on a variety of nonspecific forms, it is important to biopsy any unusual lesions. In our case series, patient 3 presented at several different points with scaly scalp lesions. Upon biopsy, several of these lesions were found to be SCCs, while others demonstrated regions of granulomatous inflammation consistent with a diagnosis of cutaneous sarcoidosis. On further review of pathology during the preparation of this manuscript after the initial diagnoses were made, it was further noted that it is challenging to distinguish granulomatous inflammation with reactive pseudoepitheliomatous hyperplasia from SCC. The fact that these lesions were clinically indistinguishable illustrates the critical importance of appropriate-depth biopsy in this situation, and the histopathologic challenges highlighted herein are important for pathologists to remember.
Patients 1 and 2 were both black women, and the fact that these patients both presented with cutaneous SCCs—one of whom was immunosuppressed due to treatment with adalimumab, the other without systemic immunosuppression—exemplifies the need for comprehensive skin examinations in sarcoidosis patients as well as for biopsies of new or unusual lesions.
The mechanism for the development of malignancy in patients with sarcoidosis is unknown and likely is multifactorial. Multiple theories have been proposed.1,2,5,6,8 Sarcoidosis is marked by the development of granulomas secondary to the interaction between CD4+ T cells and antigen-presenting cells, which is mediated by various cytokines and chemokines, including IL-2 and IFN-γ. Patients with sarcoidosis have been found to have oligoclonal T-cell lineages with a limited receptor repertoire, suggestive of selective immune system activation, as well as a deficiency of certain types of regulatory cells, namely natural killer cells.1,2 This immune dysregulation has been postulated to play an etiologic role in the development of malignancy in sarcoidosis patients.1,2,5 Furthermore, the chronic inflammation found in the organs commonly affected by both sarcoidosis and malignancy is another possible mechanism.6,8 Finally, immunosuppression and mutagenesis secondary to the treatment modalities used in sarcoidosis may be another contributing factor.6
Conclusion
An association between sarcoidosis and malignancy has been suggested for several decades. We specifically report 3 cases of patients with cutaneous sarcoidosis who presented with concurrent cutaneous SCCs. Given the varied and often nonspecific nature of cutaneous sarcoidosis, these cases highlight the importance of biopsy when sarcoidosis patients present with new and unusual skin lesions. Additionally, they illustrate the importance of thorough skin examinations in sarcoidosis patients as well as some of the challenges these patients pose for dermatologists.
- Iannuzzi MC, Rybicki BA, Teirsten AS. Sarcoidosis. N Engl J Med. 2007;357:2153-2165.
- Iannuzzi MC, Fontana JR. Sarcoidosis: clinical presentation, immunopathogenesis and therapeutics. JAMA. 2011;305:391-399.
- Brincker H, Wilbek E. The incidence of malignant tumours in patients with respiratory sarcoidosis. Br J Cancer. 1974;29:247-251.
- Askling J, Grunewald J, Eklund A, et al. Increased risk for cancer following sarcoidosis. Am J Respir Crit Care Med. 1999;160(5, pt 1):1668-1672.
- Ji J, Shu X, Li X, et al. Cancer risk in hospitalized sarcoidosis patients: a follow-up study in Sweden. Ann Oncol. 2009;20:1121-1126.
- Alexandrescu DT, Kauffman CL, Ichim TE, et al. Cutaneous sarcoidosis and malignancy: an association between sarcoidosis with skin manifestations and systemic neoplasia. Dermatol Online J. 2011;17:2.
- Reich JM, Mullooly JP, Johnson RE. Linkage analysis of malignancy-associated sarcoidosis. Chest. 1995;107:605-613.
- Cohen PR, Kurzrock R. Sarcoidosis and malignancy. Clin Dermatol. 2007;25:326-333.
Sarcoidosis is a multisystem granulomatous disease of unknown etiology that most commonly affects the lungs, eyes, and skin. Cutaneous involvement is reported in 25% to 35% of patients with sarcoidosis and may occur in a variety of forms including macules, papules, plaques, and lupus pernio.1,2 Dermatologists commonly are confronted with the diagnosis and management of sarcoidosis because of its high incidence of cutaneous involvement. Due to the protean nature of the disease, skin biopsy plays a key role in confirming the diagnosis. Histological evidence of noncaseating granulomas in combination with an appropriate clinical and radiographic picture is necessary for the diagnosis of sarcoidosis.1,2 Brincker and Wilbek
We describe 3 patients with sarcoidosis who developed squamous cell carcinoma (SCC) of the skin, including 2 black patients, which highlights the potential for SCC development.
Case Reports
Patient 1
A black woman in her 60s with a history of sarcoidosis affecting the lungs and skin that was well controlled with biweekly adalimumab 40 mg subcutaneous injections presented with a new dark painful lesion on the right third finger. She reported the lesion had been present for 1 to 2 years prior to the current presentation and was increasing in size. She had no history of prior skin cancers.
Physical examination revealed a waxy, brown-pigmented papule with overlying scale on the ulnar aspect of the right third digit near the web space (Figure 1A). A shave biopsy revealed atypical keratinocytes involving all layers of the epidermis along with associated parakeratotic scale consistent with a diagnosis of SCC in situ (Figure 1B). Human papillomavirus staining was negative. Due to the location of the lesion, the patient underwent Mohs micrographic surgery and the lesion was completely excised.

Patient 2
A black woman in her 60s with a history of cutaneous sarcoidosis that was maintained on minocycline 100 mg twice daily, chloroquine 250 mg daily, tacrolimus ointment 0.1%, tretinoin cream 0.025%, and intermittent intralesional triamcinolone acetonide injections to the nose, as well as quiescent pulmonary sarcoidosis, developed a new, growing, asymptomatic, hyperpigmented lesion on the left side of the submandibular neck over a period of a few months. A biopsy was performed and the lesion was found to be an SCC, which subsequently was completely excised.
Patient 3
A white man in his 60s with a history of prior quiescent pulmonary sarcoidosis, remote melanoma, and multiple nonmelanoma skin cancers developed scaly papules on the scalp for months, one that was interpreted by an outside pathologist as an invasive SCC (Figure 2A). He was referred to our institution for Mohs micrographic surgery. On presentation when his scalp was shaved for surgery, he was noted to have several violaceous, annular, thin plaques on the scalp (Figure 2B). A biopsy of an annular plaque demonstrated several areas of granulomatous dermatitis consistent with a diagnosis of cutaneous sarcoidosis (Figure 2C). The patient had clinical lymphadenopathy of the neck and supraclavicular region. Given the patient’s history, the differential diagnosis for these lesions included metastatic SCC, lymphoma, and sarcoidosis. The patient underwent a positron emission tomography scan, which demonstrated fluorodeoxyglucose-positive regions in both lungs and the right side of the neck. After evaluation by the pulmonary and otorhinolaryngology departments, including a lymph node biopsy, the positron emission tomography–enhancing lesions were ultimately determined to be consistent with sarcoidosis.
The patient underwent Mohs micrographic surgery for treatment of the scalp SCC and was started on triamcinolone cream 0.1% for the body, clobetasol propionate foam 0.05% for the scalp, and hydroxychloroquine sulfate 400 mg daily for the cutaneous sarcoidosis. His annular scalp lesions resolved, but over the following 12 months the patient had numerous clinically suspicious skin lesions that were biopsied and were consistent with multiple basal cell carcinomas, actinic keratoses, and SCC in situ. They were treated with surgery, cryosurgical destruction with liquid nitrogen, and 5-fluorouracil cream.

Over the 3 years subsequent to initial presentation, the patient developed ocular inflammation attributed to his sarcoidosis and atrial fibrillation, which was determined to be unrelated. He also developed 5 scaly hyperkeratotic plaques on the vertex aspect of the scalp. Biopsy of 2 lesions revealed mild keratinocyte atypia and epidermal hyperplasia, favored to represent SCC over pseudoepitheliomatous hyperplasia overlying associated granulomatous inflammation. These lesions ultimately were believed to represent new SCCs, while biopsies of 2 other lesions revealed isolated granulomatous inflammation that was believed to represent hyperkeratotic cutaneous sarcoidosis clinically resembling his SCCs. The patient was again referred for Mohs micrographic surgery and the malignancies were completely removed, while the cutaneous sarcoidosis was again treated with topical corticosteroids with complete resolution.
Comment
The potential increased risk for malignancy in patients with sarcoidosis has been well documented.3-6 Brincker and Wilbek3 first reported this association after studying 2544 patients with pulmonary sarcoidosis from 1962 to 1971. In particular, they noted a difference between the expected and observed number of cases of malignancy, particularly lung cancer and lymphoma, in the sarcoidosis population.3 In a study of 10,037 hospitalized sarcoidosis patients from 1964 to 2004, Ji et al5 noted a 40% overall increase in the incidence of cancer and found that the risk for malignancy was highest in the year following hospitalization. Interestingly, they found that the risk for developing cutaneous SCC was elevated in sarcoidosis patients even after the first year following hospitalization.5 In a retrospective cohort study examining more than 9000 patients, Askling et al4 also confirmed the increased incidence of malignancy in sarcoidosis patients. Specifically, the authors found a higher than expected occurrence of skin cancer, both melanoma (standardized incidence ratio, 1.6; 95% confidence interval, 1.1-2.3) and nonmelanoma skin cancer (standardized incidence ratio, 2.8; 95% confidence interval, 2.0-3.8) in patients with sarcoidosis.4 Reich et al7 cross-matched 30,000 cases from the Kaiser Permanente Northwest Region Tumor Registry against a sarcoidosis registry of 243 cases to evaluate for evidence of linkage between sarcoidosis and malignancy. They concluded that there may be an etiologic relationship between sarcoidosis and malignancy in at least one-quarter of cases in which both are present and hypothesized that granulomas may be the result of a cell-mediated reaction to tumor antigens.7
Few published studies specifically address the incidence of malignancy in patients with primarily cutaneous sarcoidosis. Cutaneous sarcoidosis includes nonspecific lesions, such as erythema nodosum, as well as specific lesions, such as papules, plaques, nodules, and lupus pernio.8 Alexandrescu et al6 evaluated 110 patients with a diagnosis of both sarcoidosis (cutaneous and noncutaneous) and malignancy. Through their analysis, they found that cutaneous sarcoidosis is seen more commonly in patients presenting with sarcoidosis and malignancy (56.4%) than in the total sarcoidosis population (20%–25%). From these findings, the authors concluded that cutaneous sarcoidosis appears to be a subtype of sarcoidosis associated with cancer.6
We report 3 cases that specifically illustrate a link between cutaneous sarcoidosis and an increased risk for cutaneous SCC. Because sarcoidosis commonly affects the skin, patients often present to dermatologists for care. Once the initial diagnosis of cutaneous sarcoidosis is made via biopsy, it is natural to be tempted to attribute any new skin lesions to worsening or active disease; however, as cutaneous sarcoidosis may take on a variety of nonspecific forms, it is important to biopsy any unusual lesions. In our case series, patient 3 presented at several different points with scaly scalp lesions. Upon biopsy, several of these lesions were found to be SCCs, while others demonstrated regions of granulomatous inflammation consistent with a diagnosis of cutaneous sarcoidosis. On further review of pathology during the preparation of this manuscript after the initial diagnoses were made, it was further noted that it is challenging to distinguish granulomatous inflammation with reactive pseudoepitheliomatous hyperplasia from SCC. The fact that these lesions were clinically indistinguishable illustrates the critical importance of appropriate-depth biopsy in this situation, and the histopathologic challenges highlighted herein are important for pathologists to remember.
Patients 1 and 2 were both black women, and the fact that these patients both presented with cutaneous SCCs—one of whom was immunosuppressed due to treatment with adalimumab, the other without systemic immunosuppression—exemplifies the need for comprehensive skin examinations in sarcoidosis patients as well as for biopsies of new or unusual lesions.
The mechanism for the development of malignancy in patients with sarcoidosis is unknown and likely is multifactorial. Multiple theories have been proposed.1,2,5,6,8 Sarcoidosis is marked by the development of granulomas secondary to the interaction between CD4+ T cells and antigen-presenting cells, which is mediated by various cytokines and chemokines, including IL-2 and IFN-γ. Patients with sarcoidosis have been found to have oligoclonal T-cell lineages with a limited receptor repertoire, suggestive of selective immune system activation, as well as a deficiency of certain types of regulatory cells, namely natural killer cells.1,2 This immune dysregulation has been postulated to play an etiologic role in the development of malignancy in sarcoidosis patients.1,2,5 Furthermore, the chronic inflammation found in the organs commonly affected by both sarcoidosis and malignancy is another possible mechanism.6,8 Finally, immunosuppression and mutagenesis secondary to the treatment modalities used in sarcoidosis may be another contributing factor.6
Conclusion
An association between sarcoidosis and malignancy has been suggested for several decades. We specifically report 3 cases of patients with cutaneous sarcoidosis who presented with concurrent cutaneous SCCs. Given the varied and often nonspecific nature of cutaneous sarcoidosis, these cases highlight the importance of biopsy when sarcoidosis patients present with new and unusual skin lesions. Additionally, they illustrate the importance of thorough skin examinations in sarcoidosis patients as well as some of the challenges these patients pose for dermatologists.
Sarcoidosis is a multisystem granulomatous disease of unknown etiology that most commonly affects the lungs, eyes, and skin. Cutaneous involvement is reported in 25% to 35% of patients with sarcoidosis and may occur in a variety of forms including macules, papules, plaques, and lupus pernio.1,2 Dermatologists commonly are confronted with the diagnosis and management of sarcoidosis because of its high incidence of cutaneous involvement. Due to the protean nature of the disease, skin biopsy plays a key role in confirming the diagnosis. Histological evidence of noncaseating granulomas in combination with an appropriate clinical and radiographic picture is necessary for the diagnosis of sarcoidosis.1,2 Brincker and Wilbek
We describe 3 patients with sarcoidosis who developed squamous cell carcinoma (SCC) of the skin, including 2 black patients, which highlights the potential for SCC development.
Case Reports
Patient 1
A black woman in her 60s with a history of sarcoidosis affecting the lungs and skin that was well controlled with biweekly adalimumab 40 mg subcutaneous injections presented with a new dark painful lesion on the right third finger. She reported the lesion had been present for 1 to 2 years prior to the current presentation and was increasing in size. She had no history of prior skin cancers.
Physical examination revealed a waxy, brown-pigmented papule with overlying scale on the ulnar aspect of the right third digit near the web space (Figure 1A). A shave biopsy revealed atypical keratinocytes involving all layers of the epidermis along with associated parakeratotic scale consistent with a diagnosis of SCC in situ (Figure 1B). Human papillomavirus staining was negative. Due to the location of the lesion, the patient underwent Mohs micrographic surgery and the lesion was completely excised.

Patient 2
A black woman in her 60s with a history of cutaneous sarcoidosis that was maintained on minocycline 100 mg twice daily, chloroquine 250 mg daily, tacrolimus ointment 0.1%, tretinoin cream 0.025%, and intermittent intralesional triamcinolone acetonide injections to the nose, as well as quiescent pulmonary sarcoidosis, developed a new, growing, asymptomatic, hyperpigmented lesion on the left side of the submandibular neck over a period of a few months. A biopsy was performed and the lesion was found to be an SCC, which subsequently was completely excised.
Patient 3
A white man in his 60s with a history of prior quiescent pulmonary sarcoidosis, remote melanoma, and multiple nonmelanoma skin cancers developed scaly papules on the scalp for months, one that was interpreted by an outside pathologist as an invasive SCC (Figure 2A). He was referred to our institution for Mohs micrographic surgery. On presentation when his scalp was shaved for surgery, he was noted to have several violaceous, annular, thin plaques on the scalp (Figure 2B). A biopsy of an annular plaque demonstrated several areas of granulomatous dermatitis consistent with a diagnosis of cutaneous sarcoidosis (Figure 2C). The patient had clinical lymphadenopathy of the neck and supraclavicular region. Given the patient’s history, the differential diagnosis for these lesions included metastatic SCC, lymphoma, and sarcoidosis. The patient underwent a positron emission tomography scan, which demonstrated fluorodeoxyglucose-positive regions in both lungs and the right side of the neck. After evaluation by the pulmonary and otorhinolaryngology departments, including a lymph node biopsy, the positron emission tomography–enhancing lesions were ultimately determined to be consistent with sarcoidosis.
The patient underwent Mohs micrographic surgery for treatment of the scalp SCC and was started on triamcinolone cream 0.1% for the body, clobetasol propionate foam 0.05% for the scalp, and hydroxychloroquine sulfate 400 mg daily for the cutaneous sarcoidosis. His annular scalp lesions resolved, but over the following 12 months the patient had numerous clinically suspicious skin lesions that were biopsied and were consistent with multiple basal cell carcinomas, actinic keratoses, and SCC in situ. They were treated with surgery, cryosurgical destruction with liquid nitrogen, and 5-fluorouracil cream.

Over the 3 years subsequent to initial presentation, the patient developed ocular inflammation attributed to his sarcoidosis and atrial fibrillation, which was determined to be unrelated. He also developed 5 scaly hyperkeratotic plaques on the vertex aspect of the scalp. Biopsy of 2 lesions revealed mild keratinocyte atypia and epidermal hyperplasia, favored to represent SCC over pseudoepitheliomatous hyperplasia overlying associated granulomatous inflammation. These lesions ultimately were believed to represent new SCCs, while biopsies of 2 other lesions revealed isolated granulomatous inflammation that was believed to represent hyperkeratotic cutaneous sarcoidosis clinically resembling his SCCs. The patient was again referred for Mohs micrographic surgery and the malignancies were completely removed, while the cutaneous sarcoidosis was again treated with topical corticosteroids with complete resolution.
Comment
The potential increased risk for malignancy in patients with sarcoidosis has been well documented.3-6 Brincker and Wilbek3 first reported this association after studying 2544 patients with pulmonary sarcoidosis from 1962 to 1971. In particular, they noted a difference between the expected and observed number of cases of malignancy, particularly lung cancer and lymphoma, in the sarcoidosis population.3 In a study of 10,037 hospitalized sarcoidosis patients from 1964 to 2004, Ji et al5 noted a 40% overall increase in the incidence of cancer and found that the risk for malignancy was highest in the year following hospitalization. Interestingly, they found that the risk for developing cutaneous SCC was elevated in sarcoidosis patients even after the first year following hospitalization.5 In a retrospective cohort study examining more than 9000 patients, Askling et al4 also confirmed the increased incidence of malignancy in sarcoidosis patients. Specifically, the authors found a higher than expected occurrence of skin cancer, both melanoma (standardized incidence ratio, 1.6; 95% confidence interval, 1.1-2.3) and nonmelanoma skin cancer (standardized incidence ratio, 2.8; 95% confidence interval, 2.0-3.8) in patients with sarcoidosis.4 Reich et al7 cross-matched 30,000 cases from the Kaiser Permanente Northwest Region Tumor Registry against a sarcoidosis registry of 243 cases to evaluate for evidence of linkage between sarcoidosis and malignancy. They concluded that there may be an etiologic relationship between sarcoidosis and malignancy in at least one-quarter of cases in which both are present and hypothesized that granulomas may be the result of a cell-mediated reaction to tumor antigens.7
Few published studies specifically address the incidence of malignancy in patients with primarily cutaneous sarcoidosis. Cutaneous sarcoidosis includes nonspecific lesions, such as erythema nodosum, as well as specific lesions, such as papules, plaques, nodules, and lupus pernio.8 Alexandrescu et al6 evaluated 110 patients with a diagnosis of both sarcoidosis (cutaneous and noncutaneous) and malignancy. Through their analysis, they found that cutaneous sarcoidosis is seen more commonly in patients presenting with sarcoidosis and malignancy (56.4%) than in the total sarcoidosis population (20%–25%). From these findings, the authors concluded that cutaneous sarcoidosis appears to be a subtype of sarcoidosis associated with cancer.6
We report 3 cases that specifically illustrate a link between cutaneous sarcoidosis and an increased risk for cutaneous SCC. Because sarcoidosis commonly affects the skin, patients often present to dermatologists for care. Once the initial diagnosis of cutaneous sarcoidosis is made via biopsy, it is natural to be tempted to attribute any new skin lesions to worsening or active disease; however, as cutaneous sarcoidosis may take on a variety of nonspecific forms, it is important to biopsy any unusual lesions. In our case series, patient 3 presented at several different points with scaly scalp lesions. Upon biopsy, several of these lesions were found to be SCCs, while others demonstrated regions of granulomatous inflammation consistent with a diagnosis of cutaneous sarcoidosis. On further review of pathology during the preparation of this manuscript after the initial diagnoses were made, it was further noted that it is challenging to distinguish granulomatous inflammation with reactive pseudoepitheliomatous hyperplasia from SCC. The fact that these lesions were clinically indistinguishable illustrates the critical importance of appropriate-depth biopsy in this situation, and the histopathologic challenges highlighted herein are important for pathologists to remember.
Patients 1 and 2 were both black women, and the fact that these patients both presented with cutaneous SCCs—one of whom was immunosuppressed due to treatment with adalimumab, the other without systemic immunosuppression—exemplifies the need for comprehensive skin examinations in sarcoidosis patients as well as for biopsies of new or unusual lesions.
The mechanism for the development of malignancy in patients with sarcoidosis is unknown and likely is multifactorial. Multiple theories have been proposed.1,2,5,6,8 Sarcoidosis is marked by the development of granulomas secondary to the interaction between CD4+ T cells and antigen-presenting cells, which is mediated by various cytokines and chemokines, including IL-2 and IFN-γ. Patients with sarcoidosis have been found to have oligoclonal T-cell lineages with a limited receptor repertoire, suggestive of selective immune system activation, as well as a deficiency of certain types of regulatory cells, namely natural killer cells.1,2 This immune dysregulation has been postulated to play an etiologic role in the development of malignancy in sarcoidosis patients.1,2,5 Furthermore, the chronic inflammation found in the organs commonly affected by both sarcoidosis and malignancy is another possible mechanism.6,8 Finally, immunosuppression and mutagenesis secondary to the treatment modalities used in sarcoidosis may be another contributing factor.6
Conclusion
An association between sarcoidosis and malignancy has been suggested for several decades. We specifically report 3 cases of patients with cutaneous sarcoidosis who presented with concurrent cutaneous SCCs. Given the varied and often nonspecific nature of cutaneous sarcoidosis, these cases highlight the importance of biopsy when sarcoidosis patients present with new and unusual skin lesions. Additionally, they illustrate the importance of thorough skin examinations in sarcoidosis patients as well as some of the challenges these patients pose for dermatologists.
- Iannuzzi MC, Rybicki BA, Teirsten AS. Sarcoidosis. N Engl J Med. 2007;357:2153-2165.
- Iannuzzi MC, Fontana JR. Sarcoidosis: clinical presentation, immunopathogenesis and therapeutics. JAMA. 2011;305:391-399.
- Brincker H, Wilbek E. The incidence of malignant tumours in patients with respiratory sarcoidosis. Br J Cancer. 1974;29:247-251.
- Askling J, Grunewald J, Eklund A, et al. Increased risk for cancer following sarcoidosis. Am J Respir Crit Care Med. 1999;160(5, pt 1):1668-1672.
- Ji J, Shu X, Li X, et al. Cancer risk in hospitalized sarcoidosis patients: a follow-up study in Sweden. Ann Oncol. 2009;20:1121-1126.
- Alexandrescu DT, Kauffman CL, Ichim TE, et al. Cutaneous sarcoidosis and malignancy: an association between sarcoidosis with skin manifestations and systemic neoplasia. Dermatol Online J. 2011;17:2.
- Reich JM, Mullooly JP, Johnson RE. Linkage analysis of malignancy-associated sarcoidosis. Chest. 1995;107:605-613.
- Cohen PR, Kurzrock R. Sarcoidosis and malignancy. Clin Dermatol. 2007;25:326-333.
- Iannuzzi MC, Rybicki BA, Teirsten AS. Sarcoidosis. N Engl J Med. 2007;357:2153-2165.
- Iannuzzi MC, Fontana JR. Sarcoidosis: clinical presentation, immunopathogenesis and therapeutics. JAMA. 2011;305:391-399.
- Brincker H, Wilbek E. The incidence of malignant tumours in patients with respiratory sarcoidosis. Br J Cancer. 1974;29:247-251.
- Askling J, Grunewald J, Eklund A, et al. Increased risk for cancer following sarcoidosis. Am J Respir Crit Care Med. 1999;160(5, pt 1):1668-1672.
- Ji J, Shu X, Li X, et al. Cancer risk in hospitalized sarcoidosis patients: a follow-up study in Sweden. Ann Oncol. 2009;20:1121-1126.
- Alexandrescu DT, Kauffman CL, Ichim TE, et al. Cutaneous sarcoidosis and malignancy: an association between sarcoidosis with skin manifestations and systemic neoplasia. Dermatol Online J. 2011;17:2.
- Reich JM, Mullooly JP, Johnson RE. Linkage analysis of malignancy-associated sarcoidosis. Chest. 1995;107:605-613.
- Cohen PR, Kurzrock R. Sarcoidosis and malignancy. Clin Dermatol. 2007;25:326-333.
Practice Points
- There may be an increased risk of skin cancer in patients with sarcoidosis.
- Sarcoidosis may present with multiple morphologies, including verrucous or hyperkeratotic lesions; superficial biopsy of this type of lesion may be mistaken for a squamous cell carcinoma.
- A biopsy diagnosis of squamous cell carcinoma in a black patient with sarcoidosis should be carefully reviewed for evidence of deeper granulomatous inflammation.
What Is Your Diagnosis? Spiradenocarcinoma
Test your knowledge on diagnosing cancer with MD-IQ: the medical intelligence quiz. Click here to answer 5 questions.