User login
A Structured Approach for the Management of Orodynia (Burning Mouth Syndrome)
Practice Gap
Orodynia (OD)—together with glossodynia colloquially termed “burning mouth syndrome”—is a chronic disorder characterized by a burning sensation within the oral cavity without objective clinical signs. It is most common in perimenopausal and postmenopausal women.1,2
Orodynia is a diagnosis of exclusion and is considered after 4 to 6 months of normal imaging and laboratory test results.1,2 Its pathophysiology is poorly understood, as it can be intermittent or continuous, manifest with a variety of symptoms, and affect various entities of the oral cavity.3,4 The most common structure affected is the tongue, and symptoms may include xerostomia, dysgeusia, and discomfort.1,2 Orodynia is a frustrating condition, as many patients do not respond to treatment and experience symptoms for years.1-4
The current approach to management of OD typically involves a combination of psychosocial strategies and pharmacologic agents. The psychosocial component consists of coping mechanisms (eg, stress management techniques and behavioral therapies) aimed at alleviating the psychological impact of the condition. Pharmacologic agents such as antidepressants, anticonvulsants, and topical medications often are prescribed to address neuropathic pain and dry mouth symptoms.1,2 Additionally, oral rinses, saliva substitutes, and dietary supplements may be recommended to counteract the discomfort associated with xerostomia.1,2 However, there is no stepwise protocol, leaving these treatments to be trialed in a disorganized manner.2
The Tools
In our unique approach to managing OD, physicians may employ a variety of tools, including autoantibody profiles, noninvasive salivary gland analysis, saliva analysis, patch testing for allergens, and—if deemed necessary—a minor salivary gland biopsy. The use of specific prescription medications is included in the later stages of our approach.
The Technique
First, exclude inflammatory conditions such as geographic tongue, oral lichen planus, autoimmune bullous disorders, and other treatable conditions such as dyspepsia and Sjögren syndrome using the tools described above. Noninvasive modalities should be exhausted first, and dermatologists/clinicians should exercise clinical judgement to determine whether all options should be trialed, including more invasive/costly ones.
If symptoms persist, clinicians may want to obtain a culture for oral candida. If results are positive, candida may be treated quickly with oral fluconazole. If that treatment fails and fissuring is present, advise the patient on treating the tongue; we recommend lightly brushing the tongue once daily with a hydrogen peroxide 3% solution, followed by rinsing. Next, the patient can allow an active probiotic yogurt to sit on the tongue for at least 1 minute to repopulate it with healthy oral bacteria.
If symptoms persist, prescribe gabapentin 100 to 300 mg to be taken at bedtime. Cevimeline 30 mg 3 times daily can be added to treat symptoms of xerostomia. As a last resort, a low daily dose of trifluoperazine 1 to 2 mg may alleviate the dysesthesia of OD. Because this medication is an antipsychotic, there is an increased risk for adverse effects such as tardive dyskinesia; however, given that we recommend using at most one-twentieth of the dose recommended for psychiatric illnesses such as schizophrenia, the risk appears to be minimal.5
We have found this protocol to be more structured, and in our practice, it has led to better outcomes than previously described therapeutic interventions.
Practice Implications
As a chronic condition, OD can be frustrating for patients, as many of them have attempted multiple treatments without success. It also may be challenging for dermatologists who are unfamiliar with its management. This approach to OD provides simple step-by-step diagnostic and therapeutic plans for a condition with an often-uncertain etiology and stubborn response to initial treatments. By following this protocol, dermatologists can be confident in their ability to help patients find relief from OD.
- Klein B, Thoppay JR, De Rossi SS, et al. Burning mouth syndrome. Dermatol Clin. 2020;38:477-483. doi:10.1016/j.det.2020.05.008
- Bender SD. Burning mouth syndrome. Dent Clin North Am. 2018;62:585-596. doi:10.1016/j.cden.2018.05.006
- Javali MA. Burning mouth syndrome: an enigmatic disorder. Kathmandu Univ Med J. 2013;11:175-178. doi:10.3126/kumj.v11i2.12498
- Sardella A, Lodi G, Demarosi F, et al. Burning mouth syndrome: a retrospective study investigating spontaneous remission and response to treatments. Oral Dis. 2006;12:152-155. doi:10.1111/j.1601-0825.2005.01174
- Macdonald R, Watts TP. Trifluoperazine dihydrochloride (stelazine) in paranoid schizophrenia. Br Med J. 1959;1:549-550. doi:10.1136/bmj.1.5121.549
Practice Gap
Orodynia (OD)—together with glossodynia colloquially termed “burning mouth syndrome”—is a chronic disorder characterized by a burning sensation within the oral cavity without objective clinical signs. It is most common in perimenopausal and postmenopausal women.1,2
Orodynia is a diagnosis of exclusion and is considered after 4 to 6 months of normal imaging and laboratory test results.1,2 Its pathophysiology is poorly understood, as it can be intermittent or continuous, manifest with a variety of symptoms, and affect various entities of the oral cavity.3,4 The most common structure affected is the tongue, and symptoms may include xerostomia, dysgeusia, and discomfort.1,2 Orodynia is a frustrating condition, as many patients do not respond to treatment and experience symptoms for years.1-4
The current approach to management of OD typically involves a combination of psychosocial strategies and pharmacologic agents. The psychosocial component consists of coping mechanisms (eg, stress management techniques and behavioral therapies) aimed at alleviating the psychological impact of the condition. Pharmacologic agents such as antidepressants, anticonvulsants, and topical medications often are prescribed to address neuropathic pain and dry mouth symptoms.1,2 Additionally, oral rinses, saliva substitutes, and dietary supplements may be recommended to counteract the discomfort associated with xerostomia.1,2 However, there is no stepwise protocol, leaving these treatments to be trialed in a disorganized manner.2
The Tools
In our unique approach to managing OD, physicians may employ a variety of tools, including autoantibody profiles, noninvasive salivary gland analysis, saliva analysis, patch testing for allergens, and—if deemed necessary—a minor salivary gland biopsy. The use of specific prescription medications is included in the later stages of our approach.
The Technique
First, exclude inflammatory conditions such as geographic tongue, oral lichen planus, autoimmune bullous disorders, and other treatable conditions such as dyspepsia and Sjögren syndrome using the tools described above. Noninvasive modalities should be exhausted first, and dermatologists/clinicians should exercise clinical judgement to determine whether all options should be trialed, including more invasive/costly ones.
If symptoms persist, clinicians may want to obtain a culture for oral candida. If results are positive, candida may be treated quickly with oral fluconazole. If that treatment fails and fissuring is present, advise the patient on treating the tongue; we recommend lightly brushing the tongue once daily with a hydrogen peroxide 3% solution, followed by rinsing. Next, the patient can allow an active probiotic yogurt to sit on the tongue for at least 1 minute to repopulate it with healthy oral bacteria.
If symptoms persist, prescribe gabapentin 100 to 300 mg to be taken at bedtime. Cevimeline 30 mg 3 times daily can be added to treat symptoms of xerostomia. As a last resort, a low daily dose of trifluoperazine 1 to 2 mg may alleviate the dysesthesia of OD. Because this medication is an antipsychotic, there is an increased risk for adverse effects such as tardive dyskinesia; however, given that we recommend using at most one-twentieth of the dose recommended for psychiatric illnesses such as schizophrenia, the risk appears to be minimal.5
We have found this protocol to be more structured, and in our practice, it has led to better outcomes than previously described therapeutic interventions.
Practice Implications
As a chronic condition, OD can be frustrating for patients, as many of them have attempted multiple treatments without success. It also may be challenging for dermatologists who are unfamiliar with its management. This approach to OD provides simple step-by-step diagnostic and therapeutic plans for a condition with an often-uncertain etiology and stubborn response to initial treatments. By following this protocol, dermatologists can be confident in their ability to help patients find relief from OD.
Practice Gap
Orodynia (OD)—together with glossodynia colloquially termed “burning mouth syndrome”—is a chronic disorder characterized by a burning sensation within the oral cavity without objective clinical signs. It is most common in perimenopausal and postmenopausal women.1,2
Orodynia is a diagnosis of exclusion and is considered after 4 to 6 months of normal imaging and laboratory test results.1,2 Its pathophysiology is poorly understood, as it can be intermittent or continuous, manifest with a variety of symptoms, and affect various entities of the oral cavity.3,4 The most common structure affected is the tongue, and symptoms may include xerostomia, dysgeusia, and discomfort.1,2 Orodynia is a frustrating condition, as many patients do not respond to treatment and experience symptoms for years.1-4
The current approach to management of OD typically involves a combination of psychosocial strategies and pharmacologic agents. The psychosocial component consists of coping mechanisms (eg, stress management techniques and behavioral therapies) aimed at alleviating the psychological impact of the condition. Pharmacologic agents such as antidepressants, anticonvulsants, and topical medications often are prescribed to address neuropathic pain and dry mouth symptoms.1,2 Additionally, oral rinses, saliva substitutes, and dietary supplements may be recommended to counteract the discomfort associated with xerostomia.1,2 However, there is no stepwise protocol, leaving these treatments to be trialed in a disorganized manner.2
The Tools
In our unique approach to managing OD, physicians may employ a variety of tools, including autoantibody profiles, noninvasive salivary gland analysis, saliva analysis, patch testing for allergens, and—if deemed necessary—a minor salivary gland biopsy. The use of specific prescription medications is included in the later stages of our approach.
The Technique
First, exclude inflammatory conditions such as geographic tongue, oral lichen planus, autoimmune bullous disorders, and other treatable conditions such as dyspepsia and Sjögren syndrome using the tools described above. Noninvasive modalities should be exhausted first, and dermatologists/clinicians should exercise clinical judgement to determine whether all options should be trialed, including more invasive/costly ones.
If symptoms persist, clinicians may want to obtain a culture for oral candida. If results are positive, candida may be treated quickly with oral fluconazole. If that treatment fails and fissuring is present, advise the patient on treating the tongue; we recommend lightly brushing the tongue once daily with a hydrogen peroxide 3% solution, followed by rinsing. Next, the patient can allow an active probiotic yogurt to sit on the tongue for at least 1 minute to repopulate it with healthy oral bacteria.
If symptoms persist, prescribe gabapentin 100 to 300 mg to be taken at bedtime. Cevimeline 30 mg 3 times daily can be added to treat symptoms of xerostomia. As a last resort, a low daily dose of trifluoperazine 1 to 2 mg may alleviate the dysesthesia of OD. Because this medication is an antipsychotic, there is an increased risk for adverse effects such as tardive dyskinesia; however, given that we recommend using at most one-twentieth of the dose recommended for psychiatric illnesses such as schizophrenia, the risk appears to be minimal.5
We have found this protocol to be more structured, and in our practice, it has led to better outcomes than previously described therapeutic interventions.
Practice Implications
As a chronic condition, OD can be frustrating for patients, as many of them have attempted multiple treatments without success. It also may be challenging for dermatologists who are unfamiliar with its management. This approach to OD provides simple step-by-step diagnostic and therapeutic plans for a condition with an often-uncertain etiology and stubborn response to initial treatments. By following this protocol, dermatologists can be confident in their ability to help patients find relief from OD.
- Klein B, Thoppay JR, De Rossi SS, et al. Burning mouth syndrome. Dermatol Clin. 2020;38:477-483. doi:10.1016/j.det.2020.05.008
- Bender SD. Burning mouth syndrome. Dent Clin North Am. 2018;62:585-596. doi:10.1016/j.cden.2018.05.006
- Javali MA. Burning mouth syndrome: an enigmatic disorder. Kathmandu Univ Med J. 2013;11:175-178. doi:10.3126/kumj.v11i2.12498
- Sardella A, Lodi G, Demarosi F, et al. Burning mouth syndrome: a retrospective study investigating spontaneous remission and response to treatments. Oral Dis. 2006;12:152-155. doi:10.1111/j.1601-0825.2005.01174
- Macdonald R, Watts TP. Trifluoperazine dihydrochloride (stelazine) in paranoid schizophrenia. Br Med J. 1959;1:549-550. doi:10.1136/bmj.1.5121.549
- Klein B, Thoppay JR, De Rossi SS, et al. Burning mouth syndrome. Dermatol Clin. 2020;38:477-483. doi:10.1016/j.det.2020.05.008
- Bender SD. Burning mouth syndrome. Dent Clin North Am. 2018;62:585-596. doi:10.1016/j.cden.2018.05.006
- Javali MA. Burning mouth syndrome: an enigmatic disorder. Kathmandu Univ Med J. 2013;11:175-178. doi:10.3126/kumj.v11i2.12498
- Sardella A, Lodi G, Demarosi F, et al. Burning mouth syndrome: a retrospective study investigating spontaneous remission and response to treatments. Oral Dis. 2006;12:152-155. doi:10.1111/j.1601-0825.2005.01174
- Macdonald R, Watts TP. Trifluoperazine dihydrochloride (stelazine) in paranoid schizophrenia. Br Med J. 1959;1:549-550. doi:10.1136/bmj.1.5121.549
Efficacy of Cryosurgery and 5-Fluorouracil Cream 0.5% Combination Therapy for the Treatment of Actinic Keratosis
Actinic keratosis (AK) is regarded as a lesion on a continuum of progression to squamous cell carcinoma (SCC).1 Studies have estimated that 44% to 97% of SCCs were associated with AK lesions either in contiguous skin or within the same histologic section and that AK lesions progress to SCCs at a rate of 0.6% at 1 year.2 In 1993-1994 there were 3.7 million reported office visits for AK lesions, while in 2002 alone there were 8.2 million office visits.3,4 As the burden of disease from AKs has increased, so has the associated costs from office-based visits, treatments, and subsequent surveillance.
There are a number of highly effective approaches to AK treatment that are based on several factors such as the number of and extent of the lesions, history of skin cancer, provider practice characteristics (eg, location, appointment availability), patient preferences, cost, and tolerability. Cryosurgery is the most commonly used lesion-directed modality in the treatment of individual AKs based on its effectiveness and relative ease of use. Cryosurgery alone has been shown to have a success rate of 67% on AK lesions.5 Patients often experience erythema, edema, pain, and crusting at treated sites; there also is potential for ulceration, scarring, hypopigmentation, hyperpigmentation, and secondary infection, but these effects are less common. Recurrence may be an indicator of treatment-resistant lesions or new lesions appearing in the field.
A field-directed approach with topical 5-fluorouracil (5-FU) may be preferred in patients with a history of substantial photodamage, AKs that are resistant to cryosurgery, or multiple AKs. Field-directed treatments address multiple AKs simultaneously and treat subclinical lesions. Fluorouracil is a common therapy for AKs that often is implemented by dermatologists due to its efficacy and well-understood mechanism of action. Fluorouracil inhibits thymidylate synthase during DNA synthesis, thereby halting cellular proliferation. 5-Fluorouracil cream 0.5% has been approved for 1-, 2-, and 4-week treatment periods. In one study, resolution of AK lesions was greatest in the 4-week treatment group; however, side effects also were greatest in this group.6 Patients commonly may experience a range of local reactions including erythema, pruritus, erosions, ulcerations, scabbing, crusting, and facial irritation. For patients with substantial photodamage and AKs, a robust response can lead to perceived adverse events (AEs) and considerable downtime, possibly affecting patient satisfaction and treatment compliance.7
Many alternative and combination approaches have been studied to decrease AEs and improve compliance and efficacy in the treatment of AKs. In this study, we examined the efficacy and perceived side effects of cryosurgery and 5-FU cream 0.5% combination therapy in the treatment of AKs.
Methods
Study Design and Participants
This single-blind, single-center, comparator cream–controlled pilot study was parallel designed with a balanced randomization (1:1 frequency). The study protocol and consent form were approved by the Wake Forest University Health Sciences institutional review board (Winston-Salem, North Carolina). Participants were 18 years or older with 8 clinically typical, visible, and discrete AK lesions on the face (forehead and temples) or balding scalp. Typical inclusion and exclusion criteria were observed. No other topical agents or therapies were permitted to be applied to the affected areas at least 4 weeks prior to treatment, depending on the treatment modality.
Assessment
During the screening (baseline) visit, eligible participants provided informed consent, baseline lesion counts and investigator global assessments (IGAs) were performed, and cryosurgery was administered to all visible AK lesions in the study areas. Participants returned at weeks 3, 4, 8, and 26. Three weeks following cryosurgery, participants were randomized according to standard randomization tables into 1 of 2 treatment groups to receive once-daily treatment with either 5-FU cream 0.5% or a moisturizing comparator cream. The cream was applied at bedtime to the affected sites for 1 week. Randomization was investigator blinded, but participants and the study administrators were not blinded. Participants were instructed to record their treatment compliance in daily diary entries, which were reviewed at week 4 using the medication tolerability assessment rating for burning, stinging, and ulceration. Investigator global assessment, IGA of improvement, lesion counts, and quality of life (QOL) survey responses were gathered at weeks 3, 8, and 26. The IGA measured the overall severity of AK disease involvement on a 6-point scale (clear; very severe). The IGA of improvement measured the overall improvement from baseline on a 6-point scale (clear; worse). Adverse events were measured at each visit.
Efficacy End Points
The primary end point was 100% clearance of all AK lesions at the end of the study (week 26) relative to the baseline AK lesion count. Secondary end points included comparisons between the groups for the number of participants with greater than 75% reduction of baseline lesion counts at the end of the study as well as differences at each visit in medication tolerability assessments, QOL measures, IGA improvement scores, and medication adherence based on diary entries at week 4.
Statistical Analysis
An intention-to-treat analysis was performed. The number of participants with 100% or greater than 75% clearance of AK lesions by specified time points were compared using relative risks and risk differences with Poisson regression analysis log and identity link functions, respectively, to obtain robust error variance 95% confidence intervals. Medication tolerability assessment, QOL, and IGA improvement scores were compared between the 2 groups using the Mann-Whitney U test. The significance level was set at α=.05. All analyses were performed using SAS data analysis software.
Results
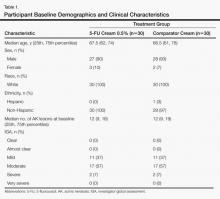
Sixty age-eligible participants were enrolled in the study with 30 participants in each treatment group. All of the participants completed the 26-week study period and were included in the intention-to-treat analysis. All of the participants were white with a median age of 67 years; the median number of baseline AK lesions was 12. Participant baseline demographics and clinical characteristics are provided in Table 1. Treatment compliance in both groups was good with only a few participants reporting missed doses.
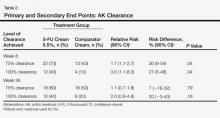
In our evaluation of the rate of change in the number of AK lesions at week 8 compared to baseline, the 5-FU cream 0.5% group showed an 84% reduction in the number of AK lesions versus a 69% reduction in the control group. At week 26, the 5-FU cream 0.5% group showed a 72% reduction in the number of lesions versus 73% in the control group. There was no significant difference between 5-FU cream 0.5% and the comparator cream for either 100% or 75% clearance of AK lesions by the end of the study; however, comparing the AK lesion count from baseline to 8 weeks following the initiation of the study, participants in the 5-FU cream 0.5% group were more likely than the control group to achieve 75% or 100% clearance on the relative risk and risk difference scales (Table 2).
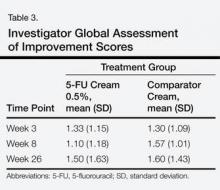
There were no significant differences between the 2 groups for the IGA of improvement at any time point (Table 3). On average, participants in the 5-FU cream 0.5% group experienced more dryness, erosion, fissuring, and redness than the control group but not more ulcerations by the end of week 4 (Table 4). All other QOL measures were statistically comparable between the 2 treatment groups for all time points.
A total of 25 AEs were reported throughout the study but none were considered to be serious. One AE (redness, burning, and itching over the eyebrow) was considered to be related to the study drug. No participants withdrew from the study due to AEs. A total of 12 participants in the 5-FU cream 0.5% group and 10 in the control group reported AEs.
Comment
After a 1-week course of 5-FU cream 0.5% following cryosurgery, a greater reduction in the number of AK lesions for a period of 2 months was noted in the treatment group compared to the control group. These findings are consistent with a similar study from 2006 that used 5-FU cream 0.5% or a vehicle 1 week prior to cryosurgery and then counted the number of AK lesions that remained.8 In the 2006 study, remarkable improvement out to week 26 was noted,8 unlike our study; however, there was insufficient power in our study to demonstrate a continued effect out to week 26.
Both the 2006 study and our current study support the benefit of using a combination treatment to clear AK lesions versus either treatment alone. Of note, these studies also show that combination treatments are equally effective, regardless of the order of treatments, in lowering AK lesion counts compared to cryosurgery alone.
Although participants in the 5-FU cream 0.5% group reported slightly more AEs on average at week 4, the rate of side effects was lower than those reported in a study documenting the side effects of a 4-week course of 5-FU.6 This rate of side effects must be considered in light of the added benefits this combination treatment has demonstrated.
Results of this pilot study suggest that a larger sample size would yield a difference in the study arms for all time periods (weeks 8 and 26). In an effort to maintain exchangeability of the study arms, patients were randomized at baseline treatment, but behaviors of patients in the 6 months following treatment, such as variation in sun exposure or other habits that promote AK lesion development, may have attenuated the results.
Key strengths of this study include no loss to follow-up and high medication adherence rates. The key limitation was the small sample size, which did not demonstrate a statistical advantage of the 5-FU cream 0.5% at 26 weeks; however, our study does show promise for larger future studies in illustrating this difference. A study by Krawtchenko et al9 noted that long-term efficacy of field therapy with 5-FU may ultimately be less than imiquimod cream 5%, suggesting that a possible alteration of the study protocol to compare the efficacy of different forms of field therapy may ultimately achieve better outcomes.
Conclusion
Overall, individuals with AK may benefit from a combination of treatment with cryosurgery and topical 5-FU to resolve lesions for longer periods than with cryosurgery alone. Although prior studies have found statistically significant differences in short-term and long-term treatment efficacy when cryosurgery is combined with an active field therapy versus a placebo vehicle,8,9 the current study aimed to find the best combination of efficacy with the fewest side effects. Therefore, the results of prior literature studies only further the feelings of the authors that with a protocol that looks at a slightly different treatment regimen within the treatment arm, the results can be extremely beneficial to patients. Further studies should be implemented to confirm the longer-term benefits of this combination therapy.
1. Lebwohl M. Actinic keratosis: epidemiology and progression to squamous cell carcinoma. Br J Dermatol. 2003;149(suppl 66):31-33.
2. Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
3. Smith ES, Feldman SR, Fleischer AB Jr, et al. Characteristics of office-based visits for skin cancer. dermatologists have more experience than other physicians in managing malignant and premalignant skin conditions. Dermatol Surg. 1998;24:981-985.
4. Shoimer I, Rosen N, Muhn C. Current management of actinic keratoses. Skin Therapy Lett. 2010;15:5-7.
5. Thai KE, Fergin P, Freeman M, et al. A prospective study of the use of cryosurgery for the treatment of actinic keratoses. Int J Dermatol. 2004;43:687-692.
6. Weiss J, Menter A, Hevia O, et al. Effective treatment of actinic keratosis with 0.5% fluorouracil cream for 1, 2, or 4 weeks. Cutis. 2002;70(suppl 2):22-29.
7. Jorizzo JL, Carney PS, Ko WT, et al. Treatment options in the management of actinic keratosis. Cutis. 2004;74 (suppl 6):9-17.
8. Jorizzo J, Weiss J, Vamvakias G. One-week treatment with 0.5% fluorouracil cream prior to cryosurgery in patients with actinic keratoses: a double-blind, vehicle-controlled, long-term study. J Drugs Dermatol. 2006;5:133-139.
9. Krawtchenko N, Roewert-Huber J, Ulrich M, et al. A randomised study of topical 5% imiquimod vs. topical 5-fluorouracil vs. cryosurgery in immunocompetent patients with actinic keratoses: a comparison of clinical and histological outcomes including 1-year follow-up. Br J Dermatol. 2007;157(suppl 2):34-40.
Actinic keratosis (AK) is regarded as a lesion on a continuum of progression to squamous cell carcinoma (SCC).1 Studies have estimated that 44% to 97% of SCCs were associated with AK lesions either in contiguous skin or within the same histologic section and that AK lesions progress to SCCs at a rate of 0.6% at 1 year.2 In 1993-1994 there were 3.7 million reported office visits for AK lesions, while in 2002 alone there were 8.2 million office visits.3,4 As the burden of disease from AKs has increased, so has the associated costs from office-based visits, treatments, and subsequent surveillance.
There are a number of highly effective approaches to AK treatment that are based on several factors such as the number of and extent of the lesions, history of skin cancer, provider practice characteristics (eg, location, appointment availability), patient preferences, cost, and tolerability. Cryosurgery is the most commonly used lesion-directed modality in the treatment of individual AKs based on its effectiveness and relative ease of use. Cryosurgery alone has been shown to have a success rate of 67% on AK lesions.5 Patients often experience erythema, edema, pain, and crusting at treated sites; there also is potential for ulceration, scarring, hypopigmentation, hyperpigmentation, and secondary infection, but these effects are less common. Recurrence may be an indicator of treatment-resistant lesions or new lesions appearing in the field.
A field-directed approach with topical 5-fluorouracil (5-FU) may be preferred in patients with a history of substantial photodamage, AKs that are resistant to cryosurgery, or multiple AKs. Field-directed treatments address multiple AKs simultaneously and treat subclinical lesions. Fluorouracil is a common therapy for AKs that often is implemented by dermatologists due to its efficacy and well-understood mechanism of action. Fluorouracil inhibits thymidylate synthase during DNA synthesis, thereby halting cellular proliferation. 5-Fluorouracil cream 0.5% has been approved for 1-, 2-, and 4-week treatment periods. In one study, resolution of AK lesions was greatest in the 4-week treatment group; however, side effects also were greatest in this group.6 Patients commonly may experience a range of local reactions including erythema, pruritus, erosions, ulcerations, scabbing, crusting, and facial irritation. For patients with substantial photodamage and AKs, a robust response can lead to perceived adverse events (AEs) and considerable downtime, possibly affecting patient satisfaction and treatment compliance.7
Many alternative and combination approaches have been studied to decrease AEs and improve compliance and efficacy in the treatment of AKs. In this study, we examined the efficacy and perceived side effects of cryosurgery and 5-FU cream 0.5% combination therapy in the treatment of AKs.
Methods
Study Design and Participants
This single-blind, single-center, comparator cream–controlled pilot study was parallel designed with a balanced randomization (1:1 frequency). The study protocol and consent form were approved by the Wake Forest University Health Sciences institutional review board (Winston-Salem, North Carolina). Participants were 18 years or older with 8 clinically typical, visible, and discrete AK lesions on the face (forehead and temples) or balding scalp. Typical inclusion and exclusion criteria were observed. No other topical agents or therapies were permitted to be applied to the affected areas at least 4 weeks prior to treatment, depending on the treatment modality.
Assessment
During the screening (baseline) visit, eligible participants provided informed consent, baseline lesion counts and investigator global assessments (IGAs) were performed, and cryosurgery was administered to all visible AK lesions in the study areas. Participants returned at weeks 3, 4, 8, and 26. Three weeks following cryosurgery, participants were randomized according to standard randomization tables into 1 of 2 treatment groups to receive once-daily treatment with either 5-FU cream 0.5% or a moisturizing comparator cream. The cream was applied at bedtime to the affected sites for 1 week. Randomization was investigator blinded, but participants and the study administrators were not blinded. Participants were instructed to record their treatment compliance in daily diary entries, which were reviewed at week 4 using the medication tolerability assessment rating for burning, stinging, and ulceration. Investigator global assessment, IGA of improvement, lesion counts, and quality of life (QOL) survey responses were gathered at weeks 3, 8, and 26. The IGA measured the overall severity of AK disease involvement on a 6-point scale (clear; very severe). The IGA of improvement measured the overall improvement from baseline on a 6-point scale (clear; worse). Adverse events were measured at each visit.
Efficacy End Points
The primary end point was 100% clearance of all AK lesions at the end of the study (week 26) relative to the baseline AK lesion count. Secondary end points included comparisons between the groups for the number of participants with greater than 75% reduction of baseline lesion counts at the end of the study as well as differences at each visit in medication tolerability assessments, QOL measures, IGA improvement scores, and medication adherence based on diary entries at week 4.
Statistical Analysis
An intention-to-treat analysis was performed. The number of participants with 100% or greater than 75% clearance of AK lesions by specified time points were compared using relative risks and risk differences with Poisson regression analysis log and identity link functions, respectively, to obtain robust error variance 95% confidence intervals. Medication tolerability assessment, QOL, and IGA improvement scores were compared between the 2 groups using the Mann-Whitney U test. The significance level was set at α=.05. All analyses were performed using SAS data analysis software.
Results
Sixty age-eligible participants were enrolled in the study with 30 participants in each treatment group. All of the participants completed the 26-week study period and were included in the intention-to-treat analysis. All of the participants were white with a median age of 67 years; the median number of baseline AK lesions was 12. Participant baseline demographics and clinical characteristics are provided in Table 1. Treatment compliance in both groups was good with only a few participants reporting missed doses.
In our evaluation of the rate of change in the number of AK lesions at week 8 compared to baseline, the 5-FU cream 0.5% group showed an 84% reduction in the number of AK lesions versus a 69% reduction in the control group. At week 26, the 5-FU cream 0.5% group showed a 72% reduction in the number of lesions versus 73% in the control group. There was no significant difference between 5-FU cream 0.5% and the comparator cream for either 100% or 75% clearance of AK lesions by the end of the study; however, comparing the AK lesion count from baseline to 8 weeks following the initiation of the study, participants in the 5-FU cream 0.5% group were more likely than the control group to achieve 75% or 100% clearance on the relative risk and risk difference scales (Table 2).
There were no significant differences between the 2 groups for the IGA of improvement at any time point (Table 3). On average, participants in the 5-FU cream 0.5% group experienced more dryness, erosion, fissuring, and redness than the control group but not more ulcerations by the end of week 4 (Table 4). All other QOL measures were statistically comparable between the 2 treatment groups for all time points.
A total of 25 AEs were reported throughout the study but none were considered to be serious. One AE (redness, burning, and itching over the eyebrow) was considered to be related to the study drug. No participants withdrew from the study due to AEs. A total of 12 participants in the 5-FU cream 0.5% group and 10 in the control group reported AEs.
Comment
After a 1-week course of 5-FU cream 0.5% following cryosurgery, a greater reduction in the number of AK lesions for a period of 2 months was noted in the treatment group compared to the control group. These findings are consistent with a similar study from 2006 that used 5-FU cream 0.5% or a vehicle 1 week prior to cryosurgery and then counted the number of AK lesions that remained.8 In the 2006 study, remarkable improvement out to week 26 was noted,8 unlike our study; however, there was insufficient power in our study to demonstrate a continued effect out to week 26.
Both the 2006 study and our current study support the benefit of using a combination treatment to clear AK lesions versus either treatment alone. Of note, these studies also show that combination treatments are equally effective, regardless of the order of treatments, in lowering AK lesion counts compared to cryosurgery alone.
Although participants in the 5-FU cream 0.5% group reported slightly more AEs on average at week 4, the rate of side effects was lower than those reported in a study documenting the side effects of a 4-week course of 5-FU.6 This rate of side effects must be considered in light of the added benefits this combination treatment has demonstrated.
Results of this pilot study suggest that a larger sample size would yield a difference in the study arms for all time periods (weeks 8 and 26). In an effort to maintain exchangeability of the study arms, patients were randomized at baseline treatment, but behaviors of patients in the 6 months following treatment, such as variation in sun exposure or other habits that promote AK lesion development, may have attenuated the results.
Key strengths of this study include no loss to follow-up and high medication adherence rates. The key limitation was the small sample size, which did not demonstrate a statistical advantage of the 5-FU cream 0.5% at 26 weeks; however, our study does show promise for larger future studies in illustrating this difference. A study by Krawtchenko et al9 noted that long-term efficacy of field therapy with 5-FU may ultimately be less than imiquimod cream 5%, suggesting that a possible alteration of the study protocol to compare the efficacy of different forms of field therapy may ultimately achieve better outcomes.
Conclusion
Overall, individuals with AK may benefit from a combination of treatment with cryosurgery and topical 5-FU to resolve lesions for longer periods than with cryosurgery alone. Although prior studies have found statistically significant differences in short-term and long-term treatment efficacy when cryosurgery is combined with an active field therapy versus a placebo vehicle,8,9 the current study aimed to find the best combination of efficacy with the fewest side effects. Therefore, the results of prior literature studies only further the feelings of the authors that with a protocol that looks at a slightly different treatment regimen within the treatment arm, the results can be extremely beneficial to patients. Further studies should be implemented to confirm the longer-term benefits of this combination therapy.
Actinic keratosis (AK) is regarded as a lesion on a continuum of progression to squamous cell carcinoma (SCC).1 Studies have estimated that 44% to 97% of SCCs were associated with AK lesions either in contiguous skin or within the same histologic section and that AK lesions progress to SCCs at a rate of 0.6% at 1 year.2 In 1993-1994 there were 3.7 million reported office visits for AK lesions, while in 2002 alone there were 8.2 million office visits.3,4 As the burden of disease from AKs has increased, so has the associated costs from office-based visits, treatments, and subsequent surveillance.
There are a number of highly effective approaches to AK treatment that are based on several factors such as the number of and extent of the lesions, history of skin cancer, provider practice characteristics (eg, location, appointment availability), patient preferences, cost, and tolerability. Cryosurgery is the most commonly used lesion-directed modality in the treatment of individual AKs based on its effectiveness and relative ease of use. Cryosurgery alone has been shown to have a success rate of 67% on AK lesions.5 Patients often experience erythema, edema, pain, and crusting at treated sites; there also is potential for ulceration, scarring, hypopigmentation, hyperpigmentation, and secondary infection, but these effects are less common. Recurrence may be an indicator of treatment-resistant lesions or new lesions appearing in the field.
A field-directed approach with topical 5-fluorouracil (5-FU) may be preferred in patients with a history of substantial photodamage, AKs that are resistant to cryosurgery, or multiple AKs. Field-directed treatments address multiple AKs simultaneously and treat subclinical lesions. Fluorouracil is a common therapy for AKs that often is implemented by dermatologists due to its efficacy and well-understood mechanism of action. Fluorouracil inhibits thymidylate synthase during DNA synthesis, thereby halting cellular proliferation. 5-Fluorouracil cream 0.5% has been approved for 1-, 2-, and 4-week treatment periods. In one study, resolution of AK lesions was greatest in the 4-week treatment group; however, side effects also were greatest in this group.6 Patients commonly may experience a range of local reactions including erythema, pruritus, erosions, ulcerations, scabbing, crusting, and facial irritation. For patients with substantial photodamage and AKs, a robust response can lead to perceived adverse events (AEs) and considerable downtime, possibly affecting patient satisfaction and treatment compliance.7
Many alternative and combination approaches have been studied to decrease AEs and improve compliance and efficacy in the treatment of AKs. In this study, we examined the efficacy and perceived side effects of cryosurgery and 5-FU cream 0.5% combination therapy in the treatment of AKs.
Methods
Study Design and Participants
This single-blind, single-center, comparator cream–controlled pilot study was parallel designed with a balanced randomization (1:1 frequency). The study protocol and consent form were approved by the Wake Forest University Health Sciences institutional review board (Winston-Salem, North Carolina). Participants were 18 years or older with 8 clinically typical, visible, and discrete AK lesions on the face (forehead and temples) or balding scalp. Typical inclusion and exclusion criteria were observed. No other topical agents or therapies were permitted to be applied to the affected areas at least 4 weeks prior to treatment, depending on the treatment modality.
Assessment
During the screening (baseline) visit, eligible participants provided informed consent, baseline lesion counts and investigator global assessments (IGAs) were performed, and cryosurgery was administered to all visible AK lesions in the study areas. Participants returned at weeks 3, 4, 8, and 26. Three weeks following cryosurgery, participants were randomized according to standard randomization tables into 1 of 2 treatment groups to receive once-daily treatment with either 5-FU cream 0.5% or a moisturizing comparator cream. The cream was applied at bedtime to the affected sites for 1 week. Randomization was investigator blinded, but participants and the study administrators were not blinded. Participants were instructed to record their treatment compliance in daily diary entries, which were reviewed at week 4 using the medication tolerability assessment rating for burning, stinging, and ulceration. Investigator global assessment, IGA of improvement, lesion counts, and quality of life (QOL) survey responses were gathered at weeks 3, 8, and 26. The IGA measured the overall severity of AK disease involvement on a 6-point scale (clear; very severe). The IGA of improvement measured the overall improvement from baseline on a 6-point scale (clear; worse). Adverse events were measured at each visit.
Efficacy End Points
The primary end point was 100% clearance of all AK lesions at the end of the study (week 26) relative to the baseline AK lesion count. Secondary end points included comparisons between the groups for the number of participants with greater than 75% reduction of baseline lesion counts at the end of the study as well as differences at each visit in medication tolerability assessments, QOL measures, IGA improvement scores, and medication adherence based on diary entries at week 4.
Statistical Analysis
An intention-to-treat analysis was performed. The number of participants with 100% or greater than 75% clearance of AK lesions by specified time points were compared using relative risks and risk differences with Poisson regression analysis log and identity link functions, respectively, to obtain robust error variance 95% confidence intervals. Medication tolerability assessment, QOL, and IGA improvement scores were compared between the 2 groups using the Mann-Whitney U test. The significance level was set at α=.05. All analyses were performed using SAS data analysis software.
Results
Sixty age-eligible participants were enrolled in the study with 30 participants in each treatment group. All of the participants completed the 26-week study period and were included in the intention-to-treat analysis. All of the participants were white with a median age of 67 years; the median number of baseline AK lesions was 12. Participant baseline demographics and clinical characteristics are provided in Table 1. Treatment compliance in both groups was good with only a few participants reporting missed doses.
In our evaluation of the rate of change in the number of AK lesions at week 8 compared to baseline, the 5-FU cream 0.5% group showed an 84% reduction in the number of AK lesions versus a 69% reduction in the control group. At week 26, the 5-FU cream 0.5% group showed a 72% reduction in the number of lesions versus 73% in the control group. There was no significant difference between 5-FU cream 0.5% and the comparator cream for either 100% or 75% clearance of AK lesions by the end of the study; however, comparing the AK lesion count from baseline to 8 weeks following the initiation of the study, participants in the 5-FU cream 0.5% group were more likely than the control group to achieve 75% or 100% clearance on the relative risk and risk difference scales (Table 2).
There were no significant differences between the 2 groups for the IGA of improvement at any time point (Table 3). On average, participants in the 5-FU cream 0.5% group experienced more dryness, erosion, fissuring, and redness than the control group but not more ulcerations by the end of week 4 (Table 4). All other QOL measures were statistically comparable between the 2 treatment groups for all time points.
A total of 25 AEs were reported throughout the study but none were considered to be serious. One AE (redness, burning, and itching over the eyebrow) was considered to be related to the study drug. No participants withdrew from the study due to AEs. A total of 12 participants in the 5-FU cream 0.5% group and 10 in the control group reported AEs.
Comment
After a 1-week course of 5-FU cream 0.5% following cryosurgery, a greater reduction in the number of AK lesions for a period of 2 months was noted in the treatment group compared to the control group. These findings are consistent with a similar study from 2006 that used 5-FU cream 0.5% or a vehicle 1 week prior to cryosurgery and then counted the number of AK lesions that remained.8 In the 2006 study, remarkable improvement out to week 26 was noted,8 unlike our study; however, there was insufficient power in our study to demonstrate a continued effect out to week 26.
Both the 2006 study and our current study support the benefit of using a combination treatment to clear AK lesions versus either treatment alone. Of note, these studies also show that combination treatments are equally effective, regardless of the order of treatments, in lowering AK lesion counts compared to cryosurgery alone.
Although participants in the 5-FU cream 0.5% group reported slightly more AEs on average at week 4, the rate of side effects was lower than those reported in a study documenting the side effects of a 4-week course of 5-FU.6 This rate of side effects must be considered in light of the added benefits this combination treatment has demonstrated.
Results of this pilot study suggest that a larger sample size would yield a difference in the study arms for all time periods (weeks 8 and 26). In an effort to maintain exchangeability of the study arms, patients were randomized at baseline treatment, but behaviors of patients in the 6 months following treatment, such as variation in sun exposure or other habits that promote AK lesion development, may have attenuated the results.
Key strengths of this study include no loss to follow-up and high medication adherence rates. The key limitation was the small sample size, which did not demonstrate a statistical advantage of the 5-FU cream 0.5% at 26 weeks; however, our study does show promise for larger future studies in illustrating this difference. A study by Krawtchenko et al9 noted that long-term efficacy of field therapy with 5-FU may ultimately be less than imiquimod cream 5%, suggesting that a possible alteration of the study protocol to compare the efficacy of different forms of field therapy may ultimately achieve better outcomes.
Conclusion
Overall, individuals with AK may benefit from a combination of treatment with cryosurgery and topical 5-FU to resolve lesions for longer periods than with cryosurgery alone. Although prior studies have found statistically significant differences in short-term and long-term treatment efficacy when cryosurgery is combined with an active field therapy versus a placebo vehicle,8,9 the current study aimed to find the best combination of efficacy with the fewest side effects. Therefore, the results of prior literature studies only further the feelings of the authors that with a protocol that looks at a slightly different treatment regimen within the treatment arm, the results can be extremely beneficial to patients. Further studies should be implemented to confirm the longer-term benefits of this combination therapy.
1. Lebwohl M. Actinic keratosis: epidemiology and progression to squamous cell carcinoma. Br J Dermatol. 2003;149(suppl 66):31-33.
2. Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
3. Smith ES, Feldman SR, Fleischer AB Jr, et al. Characteristics of office-based visits for skin cancer. dermatologists have more experience than other physicians in managing malignant and premalignant skin conditions. Dermatol Surg. 1998;24:981-985.
4. Shoimer I, Rosen N, Muhn C. Current management of actinic keratoses. Skin Therapy Lett. 2010;15:5-7.
5. Thai KE, Fergin P, Freeman M, et al. A prospective study of the use of cryosurgery for the treatment of actinic keratoses. Int J Dermatol. 2004;43:687-692.
6. Weiss J, Menter A, Hevia O, et al. Effective treatment of actinic keratosis with 0.5% fluorouracil cream for 1, 2, or 4 weeks. Cutis. 2002;70(suppl 2):22-29.
7. Jorizzo JL, Carney PS, Ko WT, et al. Treatment options in the management of actinic keratosis. Cutis. 2004;74 (suppl 6):9-17.
8. Jorizzo J, Weiss J, Vamvakias G. One-week treatment with 0.5% fluorouracil cream prior to cryosurgery in patients with actinic keratoses: a double-blind, vehicle-controlled, long-term study. J Drugs Dermatol. 2006;5:133-139.
9. Krawtchenko N, Roewert-Huber J, Ulrich M, et al. A randomised study of topical 5% imiquimod vs. topical 5-fluorouracil vs. cryosurgery in immunocompetent patients with actinic keratoses: a comparison of clinical and histological outcomes including 1-year follow-up. Br J Dermatol. 2007;157(suppl 2):34-40.
1. Lebwohl M. Actinic keratosis: epidemiology and progression to squamous cell carcinoma. Br J Dermatol. 2003;149(suppl 66):31-33.
2. Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
3. Smith ES, Feldman SR, Fleischer AB Jr, et al. Characteristics of office-based visits for skin cancer. dermatologists have more experience than other physicians in managing malignant and premalignant skin conditions. Dermatol Surg. 1998;24:981-985.
4. Shoimer I, Rosen N, Muhn C. Current management of actinic keratoses. Skin Therapy Lett. 2010;15:5-7.
5. Thai KE, Fergin P, Freeman M, et al. A prospective study of the use of cryosurgery for the treatment of actinic keratoses. Int J Dermatol. 2004;43:687-692.
6. Weiss J, Menter A, Hevia O, et al. Effective treatment of actinic keratosis with 0.5% fluorouracil cream for 1, 2, or 4 weeks. Cutis. 2002;70(suppl 2):22-29.
7. Jorizzo JL, Carney PS, Ko WT, et al. Treatment options in the management of actinic keratosis. Cutis. 2004;74 (suppl 6):9-17.
8. Jorizzo J, Weiss J, Vamvakias G. One-week treatment with 0.5% fluorouracil cream prior to cryosurgery in patients with actinic keratoses: a double-blind, vehicle-controlled, long-term study. J Drugs Dermatol. 2006;5:133-139.
9. Krawtchenko N, Roewert-Huber J, Ulrich M, et al. A randomised study of topical 5% imiquimod vs. topical 5-fluorouracil vs. cryosurgery in immunocompetent patients with actinic keratoses: a comparison of clinical and histological outcomes including 1-year follow-up. Br J Dermatol. 2007;157(suppl 2):34-40.