User login
Atopic Dermatitis Triggered by Omalizumab and Treated With Dupilumab
To the Editor:
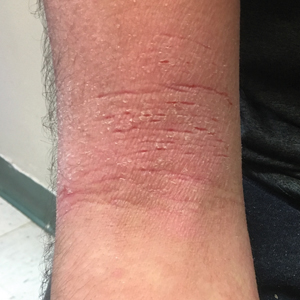
A 16-year-old adolescent boy presented to our pediatric dermatology clinic for evaluation of long-standing mild atopic dermatitis (AD) that had become severe over the last year after omalizumab was initiated for severe asthma. The patient had a history of multiple hospitalizations for severe asthma. Despite excellent control of asthma with omalizumab given every 2 weeks, he developed widespread eczematous plaques on the neck, trunk, and extremities over the course of a year. The AD often was complicated by superimposed folliculitis due to scratching from severe pruritus. Treatment with topical corticosteroids including triamcinolone ointment 0.1% to AD on the body, plus clobetasol ointment 0.05% for prurigolike lesions on the legs resulted in modest improvement; however, the AD consistently recurred within a few days after the biweekly omalizumab injection (Figure 1). When the omalizumab injections were delayed, the flares temporarily improved, and when injections were decreased to once monthly, the exacerbations subsided partially but not fully.

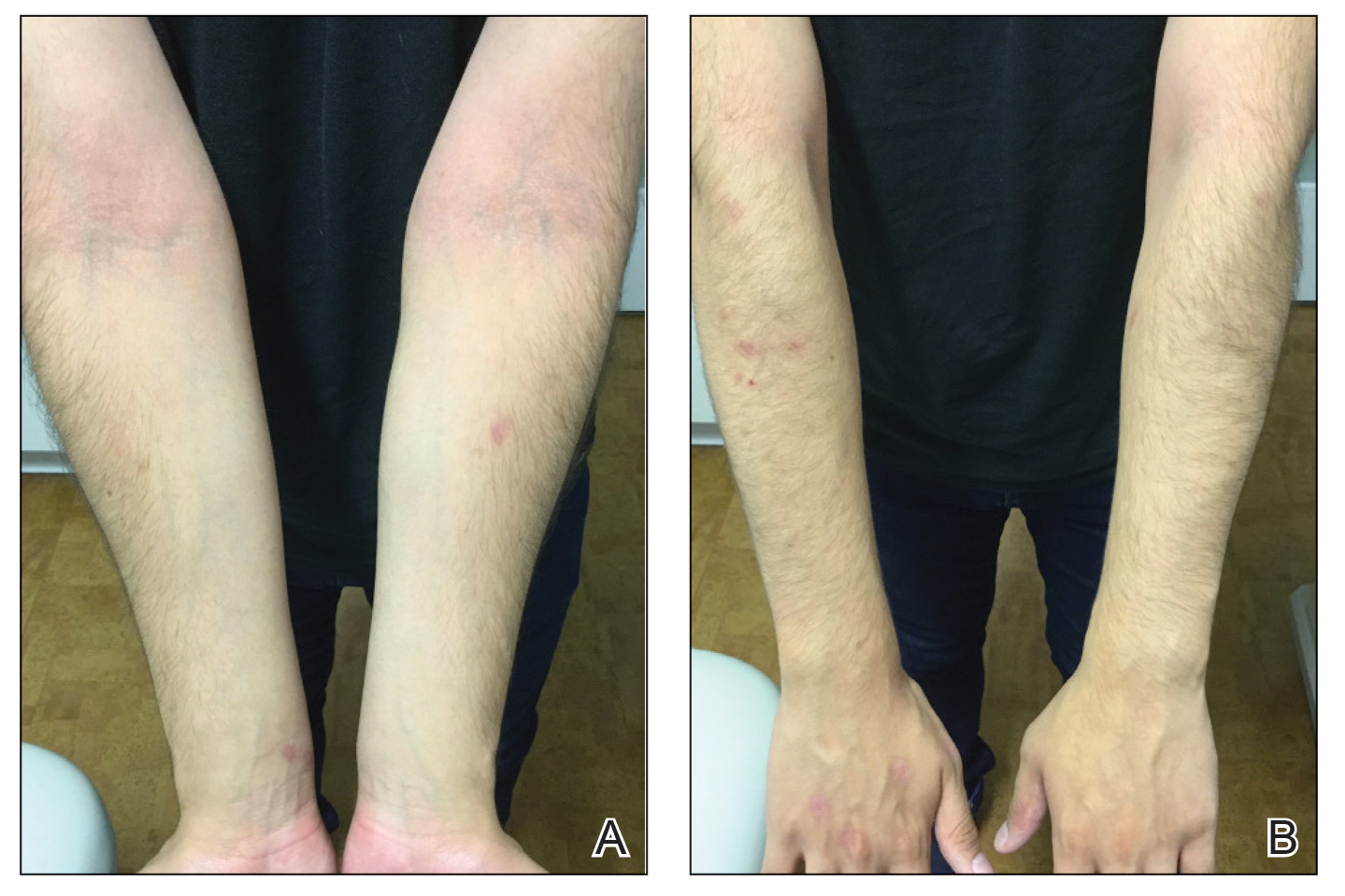
Because omalizumab resulted in dramatic improvement in the patient’s asthma, there was hesitation to discontinue it initially; however, the patient and his parents in conjunction with the dermatology and pulmonary teams decided to transition to dupilumab. The patient reported vast improvement of AD 1 month after initiation of dupilumab (Figure 2), which remained well controlled more than 1 year later. Mid-potency topical corticosteroids for the treatment of occasional mild eczematous flares on the extremities were used. The patient’s asthma has remained well controlled on dupilumab without any exacerbations.
Omalizumab is a recombinant DNA-derived humanized monoclonal antibody that binds both circulating and membrane-bound IgE. It has been proposed as a possible treatment for severe and/or recalcitrant AD, with mixed treatment results.1 A case series and review of 174 patients demonstrated a moderate to complete AD response to treatment with omalizumab in 74.1% of patients.2 The Atopic Dermatitis Anti-IgE Pediatric Trial (ADAPT) showed a statistically significant reduction in the Scoring Atopic Dermatitis (SCORAD) index (P=.01), along with improved quality of life in children treated with omalizumab vs those treated with placebo.3 However, a prior randomized, placebo-controlled, double-blind study did not show a significant difference in clinical disease parameters in patients treated with omalizumab.4
The humanized monoclonal antibody dupilumab, an anti–IL-4/IL-13 agent, has demonstrated more consistent efficacy for the treatment of AD in children and adults.1 Dupilumab is effective for both intrinsic and extrinsic AD1 because its clinical efficacy is unrelated to circulating levels of IgE in the bloodstream. Although IgE may have a role in childhood AD, our case demonstrated a different pathophysiologic mechanism independent of IgE. Our patient’s AD flares occurred within a few days of omalizumab injection, which may have resulted in a paradoxical increase in basophil sensitivity to other cytokines such as IL-335 and led to an increase in IL-4/IL-13 production within the skin. In our patient, this increase was successfully blocked by dupilumab. Furthermore, omalizumab has been shown to modulate helper T cell (TH2) cytokine response such as thymic stromal lymphopoietin.6 A cytokine imbalance could have exacerbated AD in our case.
Although additional work to clarify the pathogenesis of AD is needed, it is important to recognize the potential for the occurrence of paradoxical AD flares in patients treated with omalizumab, which is analogous to the well-documented entity of tumor necrosis factor α inhibitor–induced psoriasis. It is equally important to recognize the potential benefit for patients treated with dupilumab.
- Nygaard U, Vestergaard C, Deleuran M. Emerging treatment options in atopic dermatitis: systemic therapies. Dermatology. 2017;233:344-357.
- Holm JG, Agner T, Sand C, et al. Omalizumab for atopic dermatitis: case series and a systematic review of the literature. Int J Dermatol. 2017;56:18-26.
- Chan S, Cornelius V, Cro S, et al. Treatment effect of omalizumab on severe pediatric atopic dermatitis: the ADAPT randomized clinical trial. JAMA Pediatr. 2020;174:29-37.
- Heil PM, Maurer D, Klein B, et al. Omalizumab therapy in atopic dermatitis: depletion of IgE does not improve the clinical course – a randomized placebo-controlled and double blind pilot study. J Dtsch Dermatol Ges. 2010;8:990-998.
- Imai Y. Interleukin-33 in atopic dermatitis. J Dermatol Sci. 2019;96:2-7.
- Iyengar SR, Hoyte EG, Loza A, et al. Immunologic effects of omalizumab in children with severe refractory atopic dermatitis: a randomized, placebo-controlled clinical trial. Int Arch Allergy Immunol. 2013;162:89-93.
To the Editor:
A 16-year-old adolescent boy presented to our pediatric dermatology clinic for evaluation of long-standing mild atopic dermatitis (AD) that had become severe over the last year after omalizumab was initiated for severe asthma. The patient had a history of multiple hospitalizations for severe asthma. Despite excellent control of asthma with omalizumab given every 2 weeks, he developed widespread eczematous plaques on the neck, trunk, and extremities over the course of a year. The AD often was complicated by superimposed folliculitis due to scratching from severe pruritus. Treatment with topical corticosteroids including triamcinolone ointment 0.1% to AD on the body, plus clobetasol ointment 0.05% for prurigolike lesions on the legs resulted in modest improvement; however, the AD consistently recurred within a few days after the biweekly omalizumab injection (Figure 1). When the omalizumab injections were delayed, the flares temporarily improved, and when injections were decreased to once monthly, the exacerbations subsided partially but not fully.

Because omalizumab resulted in dramatic improvement in the patient’s asthma, there was hesitation to discontinue it initially; however, the patient and his parents in conjunction with the dermatology and pulmonary teams decided to transition to dupilumab. The patient reported vast improvement of AD 1 month after initiation of dupilumab (Figure 2), which remained well controlled more than 1 year later. Mid-potency topical corticosteroids for the treatment of occasional mild eczematous flares on the extremities were used. The patient’s asthma has remained well controlled on dupilumab without any exacerbations.

Omalizumab is a recombinant DNA-derived humanized monoclonal antibody that binds both circulating and membrane-bound IgE. It has been proposed as a possible treatment for severe and/or recalcitrant AD, with mixed treatment results.1 A case series and review of 174 patients demonstrated a moderate to complete AD response to treatment with omalizumab in 74.1% of patients.2 The Atopic Dermatitis Anti-IgE Pediatric Trial (ADAPT) showed a statistically significant reduction in the Scoring Atopic Dermatitis (SCORAD) index (P=.01), along with improved quality of life in children treated with omalizumab vs those treated with placebo.3 However, a prior randomized, placebo-controlled, double-blind study did not show a significant difference in clinical disease parameters in patients treated with omalizumab.4
The humanized monoclonal antibody dupilumab, an anti–IL-4/IL-13 agent, has demonstrated more consistent efficacy for the treatment of AD in children and adults.1 Dupilumab is effective for both intrinsic and extrinsic AD1 because its clinical efficacy is unrelated to circulating levels of IgE in the bloodstream. Although IgE may have a role in childhood AD, our case demonstrated a different pathophysiologic mechanism independent of IgE. Our patient’s AD flares occurred within a few days of omalizumab injection, which may have resulted in a paradoxical increase in basophil sensitivity to other cytokines such as IL-335 and led to an increase in IL-4/IL-13 production within the skin. In our patient, this increase was successfully blocked by dupilumab. Furthermore, omalizumab has been shown to modulate helper T cell (TH2) cytokine response such as thymic stromal lymphopoietin.6 A cytokine imbalance could have exacerbated AD in our case.
Although additional work to clarify the pathogenesis of AD is needed, it is important to recognize the potential for the occurrence of paradoxical AD flares in patients treated with omalizumab, which is analogous to the well-documented entity of tumor necrosis factor α inhibitor–induced psoriasis. It is equally important to recognize the potential benefit for patients treated with dupilumab.
To the Editor:
A 16-year-old adolescent boy presented to our pediatric dermatology clinic for evaluation of long-standing mild atopic dermatitis (AD) that had become severe over the last year after omalizumab was initiated for severe asthma. The patient had a history of multiple hospitalizations for severe asthma. Despite excellent control of asthma with omalizumab given every 2 weeks, he developed widespread eczematous plaques on the neck, trunk, and extremities over the course of a year. The AD often was complicated by superimposed folliculitis due to scratching from severe pruritus. Treatment with topical corticosteroids including triamcinolone ointment 0.1% to AD on the body, plus clobetasol ointment 0.05% for prurigolike lesions on the legs resulted in modest improvement; however, the AD consistently recurred within a few days after the biweekly omalizumab injection (Figure 1). When the omalizumab injections were delayed, the flares temporarily improved, and when injections were decreased to once monthly, the exacerbations subsided partially but not fully.

Because omalizumab resulted in dramatic improvement in the patient’s asthma, there was hesitation to discontinue it initially; however, the patient and his parents in conjunction with the dermatology and pulmonary teams decided to transition to dupilumab. The patient reported vast improvement of AD 1 month after initiation of dupilumab (Figure 2), which remained well controlled more than 1 year later. Mid-potency topical corticosteroids for the treatment of occasional mild eczematous flares on the extremities were used. The patient’s asthma has remained well controlled on dupilumab without any exacerbations.

Omalizumab is a recombinant DNA-derived humanized monoclonal antibody that binds both circulating and membrane-bound IgE. It has been proposed as a possible treatment for severe and/or recalcitrant AD, with mixed treatment results.1 A case series and review of 174 patients demonstrated a moderate to complete AD response to treatment with omalizumab in 74.1% of patients.2 The Atopic Dermatitis Anti-IgE Pediatric Trial (ADAPT) showed a statistically significant reduction in the Scoring Atopic Dermatitis (SCORAD) index (P=.01), along with improved quality of life in children treated with omalizumab vs those treated with placebo.3 However, a prior randomized, placebo-controlled, double-blind study did not show a significant difference in clinical disease parameters in patients treated with omalizumab.4
The humanized monoclonal antibody dupilumab, an anti–IL-4/IL-13 agent, has demonstrated more consistent efficacy for the treatment of AD in children and adults.1 Dupilumab is effective for both intrinsic and extrinsic AD1 because its clinical efficacy is unrelated to circulating levels of IgE in the bloodstream. Although IgE may have a role in childhood AD, our case demonstrated a different pathophysiologic mechanism independent of IgE. Our patient’s AD flares occurred within a few days of omalizumab injection, which may have resulted in a paradoxical increase in basophil sensitivity to other cytokines such as IL-335 and led to an increase in IL-4/IL-13 production within the skin. In our patient, this increase was successfully blocked by dupilumab. Furthermore, omalizumab has been shown to modulate helper T cell (TH2) cytokine response such as thymic stromal lymphopoietin.6 A cytokine imbalance could have exacerbated AD in our case.
Although additional work to clarify the pathogenesis of AD is needed, it is important to recognize the potential for the occurrence of paradoxical AD flares in patients treated with omalizumab, which is analogous to the well-documented entity of tumor necrosis factor α inhibitor–induced psoriasis. It is equally important to recognize the potential benefit for patients treated with dupilumab.
- Nygaard U, Vestergaard C, Deleuran M. Emerging treatment options in atopic dermatitis: systemic therapies. Dermatology. 2017;233:344-357.
- Holm JG, Agner T, Sand C, et al. Omalizumab for atopic dermatitis: case series and a systematic review of the literature. Int J Dermatol. 2017;56:18-26.
- Chan S, Cornelius V, Cro S, et al. Treatment effect of omalizumab on severe pediatric atopic dermatitis: the ADAPT randomized clinical trial. JAMA Pediatr. 2020;174:29-37.
- Heil PM, Maurer D, Klein B, et al. Omalizumab therapy in atopic dermatitis: depletion of IgE does not improve the clinical course – a randomized placebo-controlled and double blind pilot study. J Dtsch Dermatol Ges. 2010;8:990-998.
- Imai Y. Interleukin-33 in atopic dermatitis. J Dermatol Sci. 2019;96:2-7.
- Iyengar SR, Hoyte EG, Loza A, et al. Immunologic effects of omalizumab in children with severe refractory atopic dermatitis: a randomized, placebo-controlled clinical trial. Int Arch Allergy Immunol. 2013;162:89-93.
- Nygaard U, Vestergaard C, Deleuran M. Emerging treatment options in atopic dermatitis: systemic therapies. Dermatology. 2017;233:344-357.
- Holm JG, Agner T, Sand C, et al. Omalizumab for atopic dermatitis: case series and a systematic review of the literature. Int J Dermatol. 2017;56:18-26.
- Chan S, Cornelius V, Cro S, et al. Treatment effect of omalizumab on severe pediatric atopic dermatitis: the ADAPT randomized clinical trial. JAMA Pediatr. 2020;174:29-37.
- Heil PM, Maurer D, Klein B, et al. Omalizumab therapy in atopic dermatitis: depletion of IgE does not improve the clinical course – a randomized placebo-controlled and double blind pilot study. J Dtsch Dermatol Ges. 2010;8:990-998.
- Imai Y. Interleukin-33 in atopic dermatitis. J Dermatol Sci. 2019;96:2-7.
- Iyengar SR, Hoyte EG, Loza A, et al. Immunologic effects of omalizumab in children with severe refractory atopic dermatitis: a randomized, placebo-controlled clinical trial. Int Arch Allergy Immunol. 2013;162:89-93.
Practice Points
- Monoclonal antibodies are promising therapies for atopic conditions, although its efficacy for atopic dermatitis (AD) is debated and the side-effect profile is not entirely known.
- Omalizumab may cause a paradoxical exacerbation of AD in select patients analogous to tumor necrosis factor α inhibitor–induced psoriasis.
New Insights Into the Dermatology Residency Application Process Amid the COVID-19 Pandemic
Residency application is an arduous experience for many medical students. The National Resident Matching Program reported that US medical school seniors who matched into dermatology applied to a median of 90 programs and attended 9 interviews in 2019.1 High application and interview travel costs are a disadvantage for applicants from lower socioeconomic backgrounds. We propose that the coronavirus disease 2019 (COVID-19) pandemic should serve as a call to action for dermatology to update and promote a more equitable, time-effective, and cost-efficient residency interview process.
In light of COVID-19, dermatology residency program directors have recommended a holistic application review process, taking into consideration “disparities in strength of applications due to lack of opportunity for students with smaller home programs or in areas more affected by this crisis.”2 However, in a 2018 survey of 180 dermatology faculty members, 80% stated that time spent reviewing residency applications was already excessive.3 The Association of American Medical Colleges reported that for medical student applicants with US Medical Licensing Examination Step 1 scores lower than 237 or higher than 251, the value added by submitting one additional application beyond means of 43 (95% confidence interval [CI], 34-53) and 34 (95% CI, 28-41), respectively, is reduced relative to the value added by each application before reaching the point of diminishing returns.4 Therefore, we suggest limiting the number of applications per applicant to the upper bounds of the CI for the lower US Medical Licensing Examination Step 1 score (53), facilitating a more detailed review of fewer applications by each program and limiting student expenses.
On May 7, 2020, the Association of American Medical Colleges made a statement strongly encouraging medical school and teaching hospital faculty to conduct interviews through videoconferencing.5 Videoconferencing interviews (VCIs) minimize travel-associated health risks, providing a more equitable structure for applicants and programs in areas disproportionately impacted by the pandemic. In the 2018 survey of dermatology faculty members, only 11% believed that applicants interviewing virtually received equal consideration to those interviewing in person; a solution to this problem would be to mandate that all applicants use VCIs during the COVID-19 pandemic.3 This coming year, residency programs may elect to replace in-person interviews with VCIs, or they may utilize VCIs as screening tools to narrow down the applicant pool and for students to rank their preferred programs prior to an in-person interview. By inviting fewer applicants for in-person interviews, travel-associated health risks, financial costs, and missed educational activities would be minimized. Given that many medical students have had academic activities cancelled or postponed due to COVID-19, student opportunities for live clinical experiences should be maximized.
As programs plan for future application cycles beyond COVID-19, they must work to balance competing interests. Videoconferencing interviews allow for improved access to interviewing for applicants of lower socioeconomic classes, improved geographic mobility of applicants, and increased flexibility in accommodating faculty schedules with reduced time away from patient care and research; however, with VCIs one may lose the personal element that comes from the in-person interview, including interactions among applicants, faculty, current residents, and staff on the day of interview, as well as the departmental tour. Additionally, the quality of VCIs may be diminished by technical difficulties and the possibility of distractions, making standardization of the interview experience for applicants challenging.
The COVID-19 pandemic will surely leave its mark on the residency application cycle. We must take time now to collaborate and brainstorm creative solutions to maximize the equity and efficiency of the application process for both residency programs and students. We welcome reader feedback on these ideas and other possible solutions in the form of Letters to the Editor.
- National Resident Matching Program. Results of the 2019 NRMP Applicant Survey by Preferred Specialty and Applicant Type. Washington, DC: National Resident Matching Program; 2019. https://mk0nrmp3oyqui6wqfm.kinstacdn.com/wp-content/uploads/2019/06/Applicant-Survey-Report-2019.pdf. Accessed June 22, 2020.
- Association of American Medical Colleges. Specialty response to COVID-19: dermatology residency program director consensus statement on 2020-21 application cycle. https://aamc-orange.global.ssl.fastly.net/production/media/filer_
public/0f/7b/0f7b547e-65b5-4d93-8247-951206e7f726/updated_dermatology_program_director_
statement_on_2020-21_application_cycle_.pdf. Updated June 1, 2020. Accessed June 24, 2020. - Rojek NW, Shinkai K, Fett N. Dermatology faculty and residents’ perspectives on the dermatology residency application process: a nationwide survey. J Am Acad Dermatol. 2018;79:157-159.
- Association of American Medical Colleges. Apply smart: data to consider when applying to residency. https://www.students-residents.aamc.org/applying-residency/filteredresult/apply-smart-data-consider-when-applying-residency/. Accessed June 22, 2020.
- Association of American Medical Colleges. Conducting interviews during the coronavirus pandemic. https://www.aamc.org/what-we-do/mission-areas/medical-education/conducting-interviews-during-coronavirus-pandemic/. Published May 7, 2020. Accessed June 22, 2020.
Residency application is an arduous experience for many medical students. The National Resident Matching Program reported that US medical school seniors who matched into dermatology applied to a median of 90 programs and attended 9 interviews in 2019.1 High application and interview travel costs are a disadvantage for applicants from lower socioeconomic backgrounds. We propose that the coronavirus disease 2019 (COVID-19) pandemic should serve as a call to action for dermatology to update and promote a more equitable, time-effective, and cost-efficient residency interview process.
In light of COVID-19, dermatology residency program directors have recommended a holistic application review process, taking into consideration “disparities in strength of applications due to lack of opportunity for students with smaller home programs or in areas more affected by this crisis.”2 However, in a 2018 survey of 180 dermatology faculty members, 80% stated that time spent reviewing residency applications was already excessive.3 The Association of American Medical Colleges reported that for medical student applicants with US Medical Licensing Examination Step 1 scores lower than 237 or higher than 251, the value added by submitting one additional application beyond means of 43 (95% confidence interval [CI], 34-53) and 34 (95% CI, 28-41), respectively, is reduced relative to the value added by each application before reaching the point of diminishing returns.4 Therefore, we suggest limiting the number of applications per applicant to the upper bounds of the CI for the lower US Medical Licensing Examination Step 1 score (53), facilitating a more detailed review of fewer applications by each program and limiting student expenses.
On May 7, 2020, the Association of American Medical Colleges made a statement strongly encouraging medical school and teaching hospital faculty to conduct interviews through videoconferencing.5 Videoconferencing interviews (VCIs) minimize travel-associated health risks, providing a more equitable structure for applicants and programs in areas disproportionately impacted by the pandemic. In the 2018 survey of dermatology faculty members, only 11% believed that applicants interviewing virtually received equal consideration to those interviewing in person; a solution to this problem would be to mandate that all applicants use VCIs during the COVID-19 pandemic.3 This coming year, residency programs may elect to replace in-person interviews with VCIs, or they may utilize VCIs as screening tools to narrow down the applicant pool and for students to rank their preferred programs prior to an in-person interview. By inviting fewer applicants for in-person interviews, travel-associated health risks, financial costs, and missed educational activities would be minimized. Given that many medical students have had academic activities cancelled or postponed due to COVID-19, student opportunities for live clinical experiences should be maximized.
As programs plan for future application cycles beyond COVID-19, they must work to balance competing interests. Videoconferencing interviews allow for improved access to interviewing for applicants of lower socioeconomic classes, improved geographic mobility of applicants, and increased flexibility in accommodating faculty schedules with reduced time away from patient care and research; however, with VCIs one may lose the personal element that comes from the in-person interview, including interactions among applicants, faculty, current residents, and staff on the day of interview, as well as the departmental tour. Additionally, the quality of VCIs may be diminished by technical difficulties and the possibility of distractions, making standardization of the interview experience for applicants challenging.
The COVID-19 pandemic will surely leave its mark on the residency application cycle. We must take time now to collaborate and brainstorm creative solutions to maximize the equity and efficiency of the application process for both residency programs and students. We welcome reader feedback on these ideas and other possible solutions in the form of Letters to the Editor.
Residency application is an arduous experience for many medical students. The National Resident Matching Program reported that US medical school seniors who matched into dermatology applied to a median of 90 programs and attended 9 interviews in 2019.1 High application and interview travel costs are a disadvantage for applicants from lower socioeconomic backgrounds. We propose that the coronavirus disease 2019 (COVID-19) pandemic should serve as a call to action for dermatology to update and promote a more equitable, time-effective, and cost-efficient residency interview process.
In light of COVID-19, dermatology residency program directors have recommended a holistic application review process, taking into consideration “disparities in strength of applications due to lack of opportunity for students with smaller home programs or in areas more affected by this crisis.”2 However, in a 2018 survey of 180 dermatology faculty members, 80% stated that time spent reviewing residency applications was already excessive.3 The Association of American Medical Colleges reported that for medical student applicants with US Medical Licensing Examination Step 1 scores lower than 237 or higher than 251, the value added by submitting one additional application beyond means of 43 (95% confidence interval [CI], 34-53) and 34 (95% CI, 28-41), respectively, is reduced relative to the value added by each application before reaching the point of diminishing returns.4 Therefore, we suggest limiting the number of applications per applicant to the upper bounds of the CI for the lower US Medical Licensing Examination Step 1 score (53), facilitating a more detailed review of fewer applications by each program and limiting student expenses.
On May 7, 2020, the Association of American Medical Colleges made a statement strongly encouraging medical school and teaching hospital faculty to conduct interviews through videoconferencing.5 Videoconferencing interviews (VCIs) minimize travel-associated health risks, providing a more equitable structure for applicants and programs in areas disproportionately impacted by the pandemic. In the 2018 survey of dermatology faculty members, only 11% believed that applicants interviewing virtually received equal consideration to those interviewing in person; a solution to this problem would be to mandate that all applicants use VCIs during the COVID-19 pandemic.3 This coming year, residency programs may elect to replace in-person interviews with VCIs, or they may utilize VCIs as screening tools to narrow down the applicant pool and for students to rank their preferred programs prior to an in-person interview. By inviting fewer applicants for in-person interviews, travel-associated health risks, financial costs, and missed educational activities would be minimized. Given that many medical students have had academic activities cancelled or postponed due to COVID-19, student opportunities for live clinical experiences should be maximized.
As programs plan for future application cycles beyond COVID-19, they must work to balance competing interests. Videoconferencing interviews allow for improved access to interviewing for applicants of lower socioeconomic classes, improved geographic mobility of applicants, and increased flexibility in accommodating faculty schedules with reduced time away from patient care and research; however, with VCIs one may lose the personal element that comes from the in-person interview, including interactions among applicants, faculty, current residents, and staff on the day of interview, as well as the departmental tour. Additionally, the quality of VCIs may be diminished by technical difficulties and the possibility of distractions, making standardization of the interview experience for applicants challenging.
The COVID-19 pandemic will surely leave its mark on the residency application cycle. We must take time now to collaborate and brainstorm creative solutions to maximize the equity and efficiency of the application process for both residency programs and students. We welcome reader feedback on these ideas and other possible solutions in the form of Letters to the Editor.
- National Resident Matching Program. Results of the 2019 NRMP Applicant Survey by Preferred Specialty and Applicant Type. Washington, DC: National Resident Matching Program; 2019. https://mk0nrmp3oyqui6wqfm.kinstacdn.com/wp-content/uploads/2019/06/Applicant-Survey-Report-2019.pdf. Accessed June 22, 2020.
- Association of American Medical Colleges. Specialty response to COVID-19: dermatology residency program director consensus statement on 2020-21 application cycle. https://aamc-orange.global.ssl.fastly.net/production/media/filer_
public/0f/7b/0f7b547e-65b5-4d93-8247-951206e7f726/updated_dermatology_program_director_
statement_on_2020-21_application_cycle_.pdf. Updated June 1, 2020. Accessed June 24, 2020. - Rojek NW, Shinkai K, Fett N. Dermatology faculty and residents’ perspectives on the dermatology residency application process: a nationwide survey. J Am Acad Dermatol. 2018;79:157-159.
- Association of American Medical Colleges. Apply smart: data to consider when applying to residency. https://www.students-residents.aamc.org/applying-residency/filteredresult/apply-smart-data-consider-when-applying-residency/. Accessed June 22, 2020.
- Association of American Medical Colleges. Conducting interviews during the coronavirus pandemic. https://www.aamc.org/what-we-do/mission-areas/medical-education/conducting-interviews-during-coronavirus-pandemic/. Published May 7, 2020. Accessed June 22, 2020.
- National Resident Matching Program. Results of the 2019 NRMP Applicant Survey by Preferred Specialty and Applicant Type. Washington, DC: National Resident Matching Program; 2019. https://mk0nrmp3oyqui6wqfm.kinstacdn.com/wp-content/uploads/2019/06/Applicant-Survey-Report-2019.pdf. Accessed June 22, 2020.
- Association of American Medical Colleges. Specialty response to COVID-19: dermatology residency program director consensus statement on 2020-21 application cycle. https://aamc-orange.global.ssl.fastly.net/production/media/filer_
public/0f/7b/0f7b547e-65b5-4d93-8247-951206e7f726/updated_dermatology_program_director_
statement_on_2020-21_application_cycle_.pdf. Updated June 1, 2020. Accessed June 24, 2020. - Rojek NW, Shinkai K, Fett N. Dermatology faculty and residents’ perspectives on the dermatology residency application process: a nationwide survey. J Am Acad Dermatol. 2018;79:157-159.
- Association of American Medical Colleges. Apply smart: data to consider when applying to residency. https://www.students-residents.aamc.org/applying-residency/filteredresult/apply-smart-data-consider-when-applying-residency/. Accessed June 22, 2020.
- Association of American Medical Colleges. Conducting interviews during the coronavirus pandemic. https://www.aamc.org/what-we-do/mission-areas/medical-education/conducting-interviews-during-coronavirus-pandemic/. Published May 7, 2020. Accessed June 22, 2020.
Practice Points
- We propose that the coronavirus disease 2019 pandemic should serve as a call to action for dermatology to update and promote a more equitable, time-effective, and cost-efficient residency interview process.
- A limitation on the number of applications per candidate may lower expenses and allow for a more holistic review process by residency programs.
- The benefits and challenges of videoconferencing interviews must be weighed as residency programs decide on their continued use beyond this application cycle.