User login
Colorism and dermatology
With the world currently really listening and engaged (hopefully) on making positive changes with regards to racism and systemic racial injustices, skin color has come to the forefront. Racism because of skin color has been an unfortunate part of our history and foundation of the United States with a capitalist society built and thriving on the profits of slavery, and a democracy founded on equality – unless you had black skin. These issues are at the forefront in the United States, but have also significantly impacted other parts of the world, including the Caribbean and South America having a significant African slave trade history and impacts, with Brazil currently facing the same systemic racial injustices and police brutality among black men, and King Leopold II of Belgium slaughtering an estimated 10-15 million Congolese people in the name of colonialism, slavery, and robbing resources (natural resources as well as servitude) in the Congo as late as the early 1900s.
These are just a few of the many historical examples of racial injustice, which remains ingrained in many parts of our society today. With this worldwide history, it has been advantageous for people to have lighter skin with regards to money, politics, jobs, education, the justice system, modeling/acting opportunities and contracts, home ownership, and opportunities for generational wealth for years to come. It has ingrained some unfortunate beliefs among some that having lighter skin is better, advantageous, and will make them more desirable or more beautiful.
and across the African continent. It is estimated that 77% of women in Nigeria and 55% of women in China use bleaching creams to achieve overall skin lightening. Unilever’s Fair & Lovely skin-whitening cream has long been a popular over-the-counter product in India, with an estimated market worth of 270 billion rupees ($4 billion USD). On June 25, 2020, Unilever vowed to rename and rebrand Fair & Lovely. With such an offensive name for a product that further promotes colorism, this is an effort in the right direction and has been a long time coming since its debut in 1975. Unilever’s Fair and Lovely Foundation for women’s causes still exists, and has not been renamed at the time of this writing.
Controversy remains on whether this product and other products such as these should exist for the purposes they are used for. Johnson & Johnson has decided that it will no longer produce and sell the Neutrogena Fine Fairness line, sold only in Asia and the Middle East, and the Clean & Clear Fairness line, sold in India. There are arguments to the contrary that halting production of skin-lightening products altogether may result in an influx of unsafe alternatives.
As dermatologists, we use skin-lightening products appropriately for the purposes of treating skin conditions such as postinflammatory hyperpigmentation, melasma, and photoaging. This is where the use of such products should largely end. While it is up to individuals about what they do with their skin and their bodies, we, as health care skin professionals, should be furthering the notion that all skin colors and types are beautiful. Moreover, we should not be encouraging the use of these products for overall skin whitening. Part of the issue is that these products are available often at high concentrations over the counter or in the illegal market, especially in parts of Asia and Africa where colorism is more common and skin whitening is more commonly practiced. The dangers are not only the risk of ochronosis with high concentrations or long term use of hydroquinone, but also what the Centre for Science and Environment found in a 2014 study, that 44% of the skin “fairness” creams in India contained mercury, which is illegal and a health concern.
In my practice, I have also had patients (several originally from Nigeria) who have admitted to long term use of skin-bleaching products for the purposes of all over face- and body-skin lightening who now suffer from very sensitive skin and experience bouts of eczematous dermatitis from time to time, despite having stopped using lightening cream. While there are adverse physical effects resulting from the use of these topicals for this purpose, the effects on the psyche are what concern me the most.
The beauty industry has also been an unfortunate part of furthering thoughts and attitudes concerning colorism over the years with lighter skin and Caucasian ideals being set as standards of beauty. One of many examples is a deodorant ad in the Middle East with the tagline “White is Purity” on a woman, which was pulled by Nivea in 2017 after it was slammed as racist. Another is the 2017 Dove ad for body wash that showed a smiling black woman peel off her brown shirt to reveal a white woman in a lighter-color shirt.
A shift has occurred in recent years with more ethnic images of beauty appearing in magazines and film. However, such opportunities are still less plentiful, pay discrepancies still occur, and sexual objectification of women of color as opposed to beautification is still rampant. As such, it is also up to us to do our part in studying and utilizing ethnic and racial differences in skin and beauty to maximize our efforts in promoting what is inherently beautiful as opposed to one standard of beauty. The education begins with the images we see, what we teach our children, loving ourselves, and as doctors, being knowledgeable about the right aesthetic choices for patients with different skin colors and types.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
With the world currently really listening and engaged (hopefully) on making positive changes with regards to racism and systemic racial injustices, skin color has come to the forefront. Racism because of skin color has been an unfortunate part of our history and foundation of the United States with a capitalist society built and thriving on the profits of slavery, and a democracy founded on equality – unless you had black skin. These issues are at the forefront in the United States, but have also significantly impacted other parts of the world, including the Caribbean and South America having a significant African slave trade history and impacts, with Brazil currently facing the same systemic racial injustices and police brutality among black men, and King Leopold II of Belgium slaughtering an estimated 10-15 million Congolese people in the name of colonialism, slavery, and robbing resources (natural resources as well as servitude) in the Congo as late as the early 1900s.
These are just a few of the many historical examples of racial injustice, which remains ingrained in many parts of our society today. With this worldwide history, it has been advantageous for people to have lighter skin with regards to money, politics, jobs, education, the justice system, modeling/acting opportunities and contracts, home ownership, and opportunities for generational wealth for years to come. It has ingrained some unfortunate beliefs among some that having lighter skin is better, advantageous, and will make them more desirable or more beautiful.
and across the African continent. It is estimated that 77% of women in Nigeria and 55% of women in China use bleaching creams to achieve overall skin lightening. Unilever’s Fair & Lovely skin-whitening cream has long been a popular over-the-counter product in India, with an estimated market worth of 270 billion rupees ($4 billion USD). On June 25, 2020, Unilever vowed to rename and rebrand Fair & Lovely. With such an offensive name for a product that further promotes colorism, this is an effort in the right direction and has been a long time coming since its debut in 1975. Unilever’s Fair and Lovely Foundation for women’s causes still exists, and has not been renamed at the time of this writing.
Controversy remains on whether this product and other products such as these should exist for the purposes they are used for. Johnson & Johnson has decided that it will no longer produce and sell the Neutrogena Fine Fairness line, sold only in Asia and the Middle East, and the Clean & Clear Fairness line, sold in India. There are arguments to the contrary that halting production of skin-lightening products altogether may result in an influx of unsafe alternatives.
As dermatologists, we use skin-lightening products appropriately for the purposes of treating skin conditions such as postinflammatory hyperpigmentation, melasma, and photoaging. This is where the use of such products should largely end. While it is up to individuals about what they do with their skin and their bodies, we, as health care skin professionals, should be furthering the notion that all skin colors and types are beautiful. Moreover, we should not be encouraging the use of these products for overall skin whitening. Part of the issue is that these products are available often at high concentrations over the counter or in the illegal market, especially in parts of Asia and Africa where colorism is more common and skin whitening is more commonly practiced. The dangers are not only the risk of ochronosis with high concentrations or long term use of hydroquinone, but also what the Centre for Science and Environment found in a 2014 study, that 44% of the skin “fairness” creams in India contained mercury, which is illegal and a health concern.
In my practice, I have also had patients (several originally from Nigeria) who have admitted to long term use of skin-bleaching products for the purposes of all over face- and body-skin lightening who now suffer from very sensitive skin and experience bouts of eczematous dermatitis from time to time, despite having stopped using lightening cream. While there are adverse physical effects resulting from the use of these topicals for this purpose, the effects on the psyche are what concern me the most.
The beauty industry has also been an unfortunate part of furthering thoughts and attitudes concerning colorism over the years with lighter skin and Caucasian ideals being set as standards of beauty. One of many examples is a deodorant ad in the Middle East with the tagline “White is Purity” on a woman, which was pulled by Nivea in 2017 after it was slammed as racist. Another is the 2017 Dove ad for body wash that showed a smiling black woman peel off her brown shirt to reveal a white woman in a lighter-color shirt.
A shift has occurred in recent years with more ethnic images of beauty appearing in magazines and film. However, such opportunities are still less plentiful, pay discrepancies still occur, and sexual objectification of women of color as opposed to beautification is still rampant. As such, it is also up to us to do our part in studying and utilizing ethnic and racial differences in skin and beauty to maximize our efforts in promoting what is inherently beautiful as opposed to one standard of beauty. The education begins with the images we see, what we teach our children, loving ourselves, and as doctors, being knowledgeable about the right aesthetic choices for patients with different skin colors and types.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
With the world currently really listening and engaged (hopefully) on making positive changes with regards to racism and systemic racial injustices, skin color has come to the forefront. Racism because of skin color has been an unfortunate part of our history and foundation of the United States with a capitalist society built and thriving on the profits of slavery, and a democracy founded on equality – unless you had black skin. These issues are at the forefront in the United States, but have also significantly impacted other parts of the world, including the Caribbean and South America having a significant African slave trade history and impacts, with Brazil currently facing the same systemic racial injustices and police brutality among black men, and King Leopold II of Belgium slaughtering an estimated 10-15 million Congolese people in the name of colonialism, slavery, and robbing resources (natural resources as well as servitude) in the Congo as late as the early 1900s.
These are just a few of the many historical examples of racial injustice, which remains ingrained in many parts of our society today. With this worldwide history, it has been advantageous for people to have lighter skin with regards to money, politics, jobs, education, the justice system, modeling/acting opportunities and contracts, home ownership, and opportunities for generational wealth for years to come. It has ingrained some unfortunate beliefs among some that having lighter skin is better, advantageous, and will make them more desirable or more beautiful.
and across the African continent. It is estimated that 77% of women in Nigeria and 55% of women in China use bleaching creams to achieve overall skin lightening. Unilever’s Fair & Lovely skin-whitening cream has long been a popular over-the-counter product in India, with an estimated market worth of 270 billion rupees ($4 billion USD). On June 25, 2020, Unilever vowed to rename and rebrand Fair & Lovely. With such an offensive name for a product that further promotes colorism, this is an effort in the right direction and has been a long time coming since its debut in 1975. Unilever’s Fair and Lovely Foundation for women’s causes still exists, and has not been renamed at the time of this writing.
Controversy remains on whether this product and other products such as these should exist for the purposes they are used for. Johnson & Johnson has decided that it will no longer produce and sell the Neutrogena Fine Fairness line, sold only in Asia and the Middle East, and the Clean & Clear Fairness line, sold in India. There are arguments to the contrary that halting production of skin-lightening products altogether may result in an influx of unsafe alternatives.
As dermatologists, we use skin-lightening products appropriately for the purposes of treating skin conditions such as postinflammatory hyperpigmentation, melasma, and photoaging. This is where the use of such products should largely end. While it is up to individuals about what they do with their skin and their bodies, we, as health care skin professionals, should be furthering the notion that all skin colors and types are beautiful. Moreover, we should not be encouraging the use of these products for overall skin whitening. Part of the issue is that these products are available often at high concentrations over the counter or in the illegal market, especially in parts of Asia and Africa where colorism is more common and skin whitening is more commonly practiced. The dangers are not only the risk of ochronosis with high concentrations or long term use of hydroquinone, but also what the Centre for Science and Environment found in a 2014 study, that 44% of the skin “fairness” creams in India contained mercury, which is illegal and a health concern.
In my practice, I have also had patients (several originally from Nigeria) who have admitted to long term use of skin-bleaching products for the purposes of all over face- and body-skin lightening who now suffer from very sensitive skin and experience bouts of eczematous dermatitis from time to time, despite having stopped using lightening cream. While there are adverse physical effects resulting from the use of these topicals for this purpose, the effects on the psyche are what concern me the most.
The beauty industry has also been an unfortunate part of furthering thoughts and attitudes concerning colorism over the years with lighter skin and Caucasian ideals being set as standards of beauty. One of many examples is a deodorant ad in the Middle East with the tagline “White is Purity” on a woman, which was pulled by Nivea in 2017 after it was slammed as racist. Another is the 2017 Dove ad for body wash that showed a smiling black woman peel off her brown shirt to reveal a white woman in a lighter-color shirt.
A shift has occurred in recent years with more ethnic images of beauty appearing in magazines and film. However, such opportunities are still less plentiful, pay discrepancies still occur, and sexual objectification of women of color as opposed to beautification is still rampant. As such, it is also up to us to do our part in studying and utilizing ethnic and racial differences in skin and beauty to maximize our efforts in promoting what is inherently beautiful as opposed to one standard of beauty. The education begins with the images we see, what we teach our children, loving ourselves, and as doctors, being knowledgeable about the right aesthetic choices for patients with different skin colors and types.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Your diet may be aging you
Recent studies have shown a correlation between many dietary elements and skin diseases including acne, rosacea, and perioral dermatitis. and there is now evidence that the aging process can also be slowed with a healthy diet. Previous studies have shown that intake of vegetables, fish, and foods high in vitamin C, carotenoids, olive oil, and linoleic acid are associated with decreased wrinkles.
In a Dutch population-based cohort study published in the Journal of the American Academy of Dermatology in 2019, Mekić et al. investigated the association between diet and facial wrinkles in an elderly population. Facial photographs were used to evaluate wrinkle severity and diet of the participants was assessed with the Food Frequency Questionnaire and adherence to the Dutch Healthy Diet Index (DHDI).
The DHDI is a measure of the ability to adhere to the Dutch Guidelines for a Healthy Diet. The guidelines recommend a daily intake in the diet of at least 200 g of vegetables daily; at least 200 g of fruit; 90 g of brown bread, wholemeal bread, or other whole-grain products; and at least 15 g of unsalted nuts. One serving of fish (preferably oily fish) per week and little to no dairy, alcohol, red meat, cooking fats, and sugar is also recommended.
The study revealed that better adherence to the DHDI was significantly associated with fewer wrinkles among women but not men. Women who ate more animal meat and fats and carbohydrates had more wrinkles than did those with a fruit-dominant diet.
Although other healthy behaviors such as exercise and alcohol are likely to play a role in confounding these data, UV exposure as a cause of wrinkling was accounted for, and in the study, increased outdoor exercise was associated with more wrinkles. Unhealthy food can induce oxidative stress, increased skin and gut inflammation, and glycation, which are some of the physiologic mechanisms suggested to increase wrinkle formation. In contrast, nutrients in fruits and vegetables stimulate collagen production and DNA repair and reduce oxidative stress on the skin.
Nutritional advice is largely rare in internal medicine, cardiology, and even endocrinology. We are developing better ways to assess and understand the way foods interact and cause inflammation of the gut and the body and skin. I highly recommend nutritional education be a part of our residency training programs and to make better guidelines on the prevention of skin disease and aging available for both practitioners and patients.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
References
Mekić S et al. J Am Acad Dermatol. 2019 May;80(5):1358-1363.e2.
Purba MB et al. J Am Coll Nutr. 2001;20(1):71‐80.
van Lee L et al. Nutr J. 2012 Jul 20;11:49.
Kromhout D et al. Eur J Clin Nutr. 2016 Aug;70(8):869‐78.
Recent studies have shown a correlation between many dietary elements and skin diseases including acne, rosacea, and perioral dermatitis. and there is now evidence that the aging process can also be slowed with a healthy diet. Previous studies have shown that intake of vegetables, fish, and foods high in vitamin C, carotenoids, olive oil, and linoleic acid are associated with decreased wrinkles.
In a Dutch population-based cohort study published in the Journal of the American Academy of Dermatology in 2019, Mekić et al. investigated the association between diet and facial wrinkles in an elderly population. Facial photographs were used to evaluate wrinkle severity and diet of the participants was assessed with the Food Frequency Questionnaire and adherence to the Dutch Healthy Diet Index (DHDI).
The DHDI is a measure of the ability to adhere to the Dutch Guidelines for a Healthy Diet. The guidelines recommend a daily intake in the diet of at least 200 g of vegetables daily; at least 200 g of fruit; 90 g of brown bread, wholemeal bread, or other whole-grain products; and at least 15 g of unsalted nuts. One serving of fish (preferably oily fish) per week and little to no dairy, alcohol, red meat, cooking fats, and sugar is also recommended.
The study revealed that better adherence to the DHDI was significantly associated with fewer wrinkles among women but not men. Women who ate more animal meat and fats and carbohydrates had more wrinkles than did those with a fruit-dominant diet.
Although other healthy behaviors such as exercise and alcohol are likely to play a role in confounding these data, UV exposure as a cause of wrinkling was accounted for, and in the study, increased outdoor exercise was associated with more wrinkles. Unhealthy food can induce oxidative stress, increased skin and gut inflammation, and glycation, which are some of the physiologic mechanisms suggested to increase wrinkle formation. In contrast, nutrients in fruits and vegetables stimulate collagen production and DNA repair and reduce oxidative stress on the skin.
Nutritional advice is largely rare in internal medicine, cardiology, and even endocrinology. We are developing better ways to assess and understand the way foods interact and cause inflammation of the gut and the body and skin. I highly recommend nutritional education be a part of our residency training programs and to make better guidelines on the prevention of skin disease and aging available for both practitioners and patients.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
References
Mekić S et al. J Am Acad Dermatol. 2019 May;80(5):1358-1363.e2.
Purba MB et al. J Am Coll Nutr. 2001;20(1):71‐80.
van Lee L et al. Nutr J. 2012 Jul 20;11:49.
Kromhout D et al. Eur J Clin Nutr. 2016 Aug;70(8):869‐78.
Recent studies have shown a correlation between many dietary elements and skin diseases including acne, rosacea, and perioral dermatitis. and there is now evidence that the aging process can also be slowed with a healthy diet. Previous studies have shown that intake of vegetables, fish, and foods high in vitamin C, carotenoids, olive oil, and linoleic acid are associated with decreased wrinkles.
In a Dutch population-based cohort study published in the Journal of the American Academy of Dermatology in 2019, Mekić et al. investigated the association between diet and facial wrinkles in an elderly population. Facial photographs were used to evaluate wrinkle severity and diet of the participants was assessed with the Food Frequency Questionnaire and adherence to the Dutch Healthy Diet Index (DHDI).
The DHDI is a measure of the ability to adhere to the Dutch Guidelines for a Healthy Diet. The guidelines recommend a daily intake in the diet of at least 200 g of vegetables daily; at least 200 g of fruit; 90 g of brown bread, wholemeal bread, or other whole-grain products; and at least 15 g of unsalted nuts. One serving of fish (preferably oily fish) per week and little to no dairy, alcohol, red meat, cooking fats, and sugar is also recommended.
The study revealed that better adherence to the DHDI was significantly associated with fewer wrinkles among women but not men. Women who ate more animal meat and fats and carbohydrates had more wrinkles than did those with a fruit-dominant diet.
Although other healthy behaviors such as exercise and alcohol are likely to play a role in confounding these data, UV exposure as a cause of wrinkling was accounted for, and in the study, increased outdoor exercise was associated with more wrinkles. Unhealthy food can induce oxidative stress, increased skin and gut inflammation, and glycation, which are some of the physiologic mechanisms suggested to increase wrinkle formation. In contrast, nutrients in fruits and vegetables stimulate collagen production and DNA repair and reduce oxidative stress on the skin.
Nutritional advice is largely rare in internal medicine, cardiology, and even endocrinology. We are developing better ways to assess and understand the way foods interact and cause inflammation of the gut and the body and skin. I highly recommend nutritional education be a part of our residency training programs and to make better guidelines on the prevention of skin disease and aging available for both practitioners and patients.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
References
Mekić S et al. J Am Acad Dermatol. 2019 May;80(5):1358-1363.e2.
Purba MB et al. J Am Coll Nutr. 2001;20(1):71‐80.
van Lee L et al. Nutr J. 2012 Jul 20;11:49.
Kromhout D et al. Eur J Clin Nutr. 2016 Aug;70(8):869‐78.
Laser surgery precautions as clinics begin to reopen amid COVID-19
Protective measures recommended for cosmetic procedures have recently been published by Dover et al. in Facial Plastic Surgery & Aesthetic Medicine. The manuscript, titled “A path to resume aesthetic care Project AesCert Guidance Supplement – practical considerations for aesthetic medicine professionals supporting clinic preparedness in response to the SARS-CoV-2 outbreak,” provides thorough, detailed recommendations on all aspects of protection and preparedness for aesthetic clinical practices.
in this uncharted territory. During the last pandemic, the 1918 Spanish flu, caused by an H1N1 virus, laser procedures didn’t exist. Discussion among dermatologists and laser surgeons, including the aforementioned publication, have led to the following initial office recommendations (subject to change).
Office preparation and safety including:
- Prescreening patients for symptoms.
- Social distancing in the office, including waiting room areas (or eliminating waiting areas and bringing patients into exam rooms upon arrival).
- Decreasing patient load and increasing length of appointment times.
- Having no additional visitors during patient appointments, unless necessary (minor, caregiver).
- Patients wearing masks to appointments and hand washing/sanitizing upon arrival/departure.
- Providers wearing appropriate personal protective equipment (PPE) during visits.
- Instituting office disinfectant checklists.
For nonablative laser surgery specifically, especially for therapy of the face and neck, recommendations include the following:
- Lasers and office areas are thoroughly sanitized between each procedure.
- Providers wear appropriate PPE, including N95 masks if possible, wraparound safety glasses, gloves, as well as strong consideration of face shields).
- The duration and number of procedures should be limited, as should intraprocedure conversations and close face-to-face proximity with patient’s airways.
- Lasers with increased plume, including laser tattoo removal and laser hair removal, are the procedures with the most concern with regards to viral particle or infection transmission.
PPE is recommended (including masks – N95 if available – gloves, and face shield), as well as evacuator suction systems of the two-stage filtration type, and/or negative room pressure if available. For air-filtration evacuator suction systems, the device vacuum must be held within 2 inches of the treatment area for the best efficacy. Some have suggested performing laser tattoo removal through a hydrogel patch to help eliminate plume, which may also increase the cost of the procedure and may depend on the availability of the patches themselves. Nothing has been published on the use of the hydrogel patch in laser hair removal. Shaving or trimming of hairs prior to the procedure is critical.
While pulse dye and intense pulsed light (IPL) lasers have generally been deemed safer to use during the COVID-19 pandemic – with appropriate protective gear and general office precautions – I would recommend being mindful of potential plume created when using these lasers in hair-bearing areas. IPL is generally avoided in these regions, unless specific filters are used for hair removal treatment. But if use an IPL in a hair-bearing region, shaving or trimming of the hairs with the above precautions should be done first to reduce plume. As with all face-to-face procedures, the above PPE, contact, and intraprocedure conversation precautions should be taken.
Nonablative fractional resurfacing lasers are areas in which more questions lie. Some providers are comfortable performing nonablative fractional lasers with protective gear and air filtration systems, while others are recommending delaying these procedures until more information is available. The question essentially involves whether infection risk is higher with these procedures because of plume and if depth of penetration of the laser can release viral particles.
In addition to the other precautions above, with the high transmissibility of COVID-19, I would recommend considering precleansing the treatment area with soap and water or a sterile prep that won’t irritate the skin, which has activity against coronaviruses. A study by Kampf et al. demonstrated that coronaviruses can persist on surfaces such as metal, glass, or plastic for up to 9 days (human skin surface unknown) but can be effectively inactivated by surface disinfection procedures with 62%-71% ethanol, 0.5% hydrogen peroxide, or 0.1% sodium hypochlorite within 1 minute. Other biocidal agents that may be more tolerable on the skin surface, such as 0.05%-0.2% benzalkonium chloride or 0.02% chlorhexidine digluconate were less effective. Washing the face with soap and water may be the most tolerated and easiest cleansing method. Face-to-face respiratory transmission should be mitigated by the aforementioned methods.
Ablative laser surgery
Most laser surgeons agree that ablative laser surgery procedures should likely be delayed until the virus has waned more, because of the increased invasiveness of and recovery of wound healing from the procedure. There is increased evidence of SARS-CoV-2 infecting endothelial cells, raising concern about transmission via blood. A study of the cardiovascular manifestations seen in COVID-19 infection, published in The Lancet, showed the virus directly targets the endothelial cells that line blood vessels. Ablative laser surgery (fractional and fully ablative) is associated with blood or serous fluid on the skin surface immediately after the procedure and for up to 5-7 days post procedure, particularly with Er:Yag than with the CO2 laser. Antibacterial and antiviral prophylaxis often is used with these procedures. While the aforementioned protocols for other nonablative lasers may help with ablative laser treatment, there is currently no known effective and available antiviral prophylactic medication against SARS-CoV-2, if needed.
PPE
Personal protective equipment shortages are still a concern. Many hospitals are sterilizing and reusing traditionally disposable N95 masks in the inpatient setting, which is unprecedented. Resterilization will likely be necessary in outpatient medical offices as well, if the supply of masks does not increase. The supply chain will be a factor in considering PPE use in outpatient offices affecting the availability of PPE for emergency medicine, inpatient hospital, and ICU providers in direct contact with known COVID-19 patients.
With asymptomatic spread and the lack of adequate testing for COVID-19, as practices reopen, all practitioners will be on the front lines and should treat their practice and protect their patients, staff and themselves as such.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They have no relevant disclosures.
References:
Dover JS et al. Facial Plast Surg Aesthet Med. 2020 May 5. doi: 10.1089/fpsam.2020.0239.
Kampf G et al. J Hosp Infect. 2020 Mar;104(3):246-51.
Varga Z et al. Lancet. 2020 May 2;395(10234):1417-8.
Protective measures recommended for cosmetic procedures have recently been published by Dover et al. in Facial Plastic Surgery & Aesthetic Medicine. The manuscript, titled “A path to resume aesthetic care Project AesCert Guidance Supplement – practical considerations for aesthetic medicine professionals supporting clinic preparedness in response to the SARS-CoV-2 outbreak,” provides thorough, detailed recommendations on all aspects of protection and preparedness for aesthetic clinical practices.
in this uncharted territory. During the last pandemic, the 1918 Spanish flu, caused by an H1N1 virus, laser procedures didn’t exist. Discussion among dermatologists and laser surgeons, including the aforementioned publication, have led to the following initial office recommendations (subject to change).
Office preparation and safety including:
- Prescreening patients for symptoms.
- Social distancing in the office, including waiting room areas (or eliminating waiting areas and bringing patients into exam rooms upon arrival).
- Decreasing patient load and increasing length of appointment times.
- Having no additional visitors during patient appointments, unless necessary (minor, caregiver).
- Patients wearing masks to appointments and hand washing/sanitizing upon arrival/departure.
- Providers wearing appropriate personal protective equipment (PPE) during visits.
- Instituting office disinfectant checklists.
For nonablative laser surgery specifically, especially for therapy of the face and neck, recommendations include the following:
- Lasers and office areas are thoroughly sanitized between each procedure.
- Providers wear appropriate PPE, including N95 masks if possible, wraparound safety glasses, gloves, as well as strong consideration of face shields).
- The duration and number of procedures should be limited, as should intraprocedure conversations and close face-to-face proximity with patient’s airways.
- Lasers with increased plume, including laser tattoo removal and laser hair removal, are the procedures with the most concern with regards to viral particle or infection transmission.
PPE is recommended (including masks – N95 if available – gloves, and face shield), as well as evacuator suction systems of the two-stage filtration type, and/or negative room pressure if available. For air-filtration evacuator suction systems, the device vacuum must be held within 2 inches of the treatment area for the best efficacy. Some have suggested performing laser tattoo removal through a hydrogel patch to help eliminate plume, which may also increase the cost of the procedure and may depend on the availability of the patches themselves. Nothing has been published on the use of the hydrogel patch in laser hair removal. Shaving or trimming of hairs prior to the procedure is critical.
While pulse dye and intense pulsed light (IPL) lasers have generally been deemed safer to use during the COVID-19 pandemic – with appropriate protective gear and general office precautions – I would recommend being mindful of potential plume created when using these lasers in hair-bearing areas. IPL is generally avoided in these regions, unless specific filters are used for hair removal treatment. But if use an IPL in a hair-bearing region, shaving or trimming of the hairs with the above precautions should be done first to reduce plume. As with all face-to-face procedures, the above PPE, contact, and intraprocedure conversation precautions should be taken.
Nonablative fractional resurfacing lasers are areas in which more questions lie. Some providers are comfortable performing nonablative fractional lasers with protective gear and air filtration systems, while others are recommending delaying these procedures until more information is available. The question essentially involves whether infection risk is higher with these procedures because of plume and if depth of penetration of the laser can release viral particles.
In addition to the other precautions above, with the high transmissibility of COVID-19, I would recommend considering precleansing the treatment area with soap and water or a sterile prep that won’t irritate the skin, which has activity against coronaviruses. A study by Kampf et al. demonstrated that coronaviruses can persist on surfaces such as metal, glass, or plastic for up to 9 days (human skin surface unknown) but can be effectively inactivated by surface disinfection procedures with 62%-71% ethanol, 0.5% hydrogen peroxide, or 0.1% sodium hypochlorite within 1 minute. Other biocidal agents that may be more tolerable on the skin surface, such as 0.05%-0.2% benzalkonium chloride or 0.02% chlorhexidine digluconate were less effective. Washing the face with soap and water may be the most tolerated and easiest cleansing method. Face-to-face respiratory transmission should be mitigated by the aforementioned methods.
Ablative laser surgery
Most laser surgeons agree that ablative laser surgery procedures should likely be delayed until the virus has waned more, because of the increased invasiveness of and recovery of wound healing from the procedure. There is increased evidence of SARS-CoV-2 infecting endothelial cells, raising concern about transmission via blood. A study of the cardiovascular manifestations seen in COVID-19 infection, published in The Lancet, showed the virus directly targets the endothelial cells that line blood vessels. Ablative laser surgery (fractional and fully ablative) is associated with blood or serous fluid on the skin surface immediately after the procedure and for up to 5-7 days post procedure, particularly with Er:Yag than with the CO2 laser. Antibacterial and antiviral prophylaxis often is used with these procedures. While the aforementioned protocols for other nonablative lasers may help with ablative laser treatment, there is currently no known effective and available antiviral prophylactic medication against SARS-CoV-2, if needed.
PPE
Personal protective equipment shortages are still a concern. Many hospitals are sterilizing and reusing traditionally disposable N95 masks in the inpatient setting, which is unprecedented. Resterilization will likely be necessary in outpatient medical offices as well, if the supply of masks does not increase. The supply chain will be a factor in considering PPE use in outpatient offices affecting the availability of PPE for emergency medicine, inpatient hospital, and ICU providers in direct contact with known COVID-19 patients.
With asymptomatic spread and the lack of adequate testing for COVID-19, as practices reopen, all practitioners will be on the front lines and should treat their practice and protect their patients, staff and themselves as such.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They have no relevant disclosures.
References:
Dover JS et al. Facial Plast Surg Aesthet Med. 2020 May 5. doi: 10.1089/fpsam.2020.0239.
Kampf G et al. J Hosp Infect. 2020 Mar;104(3):246-51.
Varga Z et al. Lancet. 2020 May 2;395(10234):1417-8.
Protective measures recommended for cosmetic procedures have recently been published by Dover et al. in Facial Plastic Surgery & Aesthetic Medicine. The manuscript, titled “A path to resume aesthetic care Project AesCert Guidance Supplement – practical considerations for aesthetic medicine professionals supporting clinic preparedness in response to the SARS-CoV-2 outbreak,” provides thorough, detailed recommendations on all aspects of protection and preparedness for aesthetic clinical practices.
in this uncharted territory. During the last pandemic, the 1918 Spanish flu, caused by an H1N1 virus, laser procedures didn’t exist. Discussion among dermatologists and laser surgeons, including the aforementioned publication, have led to the following initial office recommendations (subject to change).
Office preparation and safety including:
- Prescreening patients for symptoms.
- Social distancing in the office, including waiting room areas (or eliminating waiting areas and bringing patients into exam rooms upon arrival).
- Decreasing patient load and increasing length of appointment times.
- Having no additional visitors during patient appointments, unless necessary (minor, caregiver).
- Patients wearing masks to appointments and hand washing/sanitizing upon arrival/departure.
- Providers wearing appropriate personal protective equipment (PPE) during visits.
- Instituting office disinfectant checklists.
For nonablative laser surgery specifically, especially for therapy of the face and neck, recommendations include the following:
- Lasers and office areas are thoroughly sanitized between each procedure.
- Providers wear appropriate PPE, including N95 masks if possible, wraparound safety glasses, gloves, as well as strong consideration of face shields).
- The duration and number of procedures should be limited, as should intraprocedure conversations and close face-to-face proximity with patient’s airways.
- Lasers with increased plume, including laser tattoo removal and laser hair removal, are the procedures with the most concern with regards to viral particle or infection transmission.
PPE is recommended (including masks – N95 if available – gloves, and face shield), as well as evacuator suction systems of the two-stage filtration type, and/or negative room pressure if available. For air-filtration evacuator suction systems, the device vacuum must be held within 2 inches of the treatment area for the best efficacy. Some have suggested performing laser tattoo removal through a hydrogel patch to help eliminate plume, which may also increase the cost of the procedure and may depend on the availability of the patches themselves. Nothing has been published on the use of the hydrogel patch in laser hair removal. Shaving or trimming of hairs prior to the procedure is critical.
While pulse dye and intense pulsed light (IPL) lasers have generally been deemed safer to use during the COVID-19 pandemic – with appropriate protective gear and general office precautions – I would recommend being mindful of potential plume created when using these lasers in hair-bearing areas. IPL is generally avoided in these regions, unless specific filters are used for hair removal treatment. But if use an IPL in a hair-bearing region, shaving or trimming of the hairs with the above precautions should be done first to reduce plume. As with all face-to-face procedures, the above PPE, contact, and intraprocedure conversation precautions should be taken.
Nonablative fractional resurfacing lasers are areas in which more questions lie. Some providers are comfortable performing nonablative fractional lasers with protective gear and air filtration systems, while others are recommending delaying these procedures until more information is available. The question essentially involves whether infection risk is higher with these procedures because of plume and if depth of penetration of the laser can release viral particles.
In addition to the other precautions above, with the high transmissibility of COVID-19, I would recommend considering precleansing the treatment area with soap and water or a sterile prep that won’t irritate the skin, which has activity against coronaviruses. A study by Kampf et al. demonstrated that coronaviruses can persist on surfaces such as metal, glass, or plastic for up to 9 days (human skin surface unknown) but can be effectively inactivated by surface disinfection procedures with 62%-71% ethanol, 0.5% hydrogen peroxide, or 0.1% sodium hypochlorite within 1 minute. Other biocidal agents that may be more tolerable on the skin surface, such as 0.05%-0.2% benzalkonium chloride or 0.02% chlorhexidine digluconate were less effective. Washing the face with soap and water may be the most tolerated and easiest cleansing method. Face-to-face respiratory transmission should be mitigated by the aforementioned methods.
Ablative laser surgery
Most laser surgeons agree that ablative laser surgery procedures should likely be delayed until the virus has waned more, because of the increased invasiveness of and recovery of wound healing from the procedure. There is increased evidence of SARS-CoV-2 infecting endothelial cells, raising concern about transmission via blood. A study of the cardiovascular manifestations seen in COVID-19 infection, published in The Lancet, showed the virus directly targets the endothelial cells that line blood vessels. Ablative laser surgery (fractional and fully ablative) is associated with blood or serous fluid on the skin surface immediately after the procedure and for up to 5-7 days post procedure, particularly with Er:Yag than with the CO2 laser. Antibacterial and antiviral prophylaxis often is used with these procedures. While the aforementioned protocols for other nonablative lasers may help with ablative laser treatment, there is currently no known effective and available antiviral prophylactic medication against SARS-CoV-2, if needed.
PPE
Personal protective equipment shortages are still a concern. Many hospitals are sterilizing and reusing traditionally disposable N95 masks in the inpatient setting, which is unprecedented. Resterilization will likely be necessary in outpatient medical offices as well, if the supply of masks does not increase. The supply chain will be a factor in considering PPE use in outpatient offices affecting the availability of PPE for emergency medicine, inpatient hospital, and ICU providers in direct contact with known COVID-19 patients.
With asymptomatic spread and the lack of adequate testing for COVID-19, as practices reopen, all practitioners will be on the front lines and should treat their practice and protect their patients, staff and themselves as such.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They have no relevant disclosures.
References:
Dover JS et al. Facial Plast Surg Aesthet Med. 2020 May 5. doi: 10.1089/fpsam.2020.0239.
Kampf G et al. J Hosp Infect. 2020 Mar;104(3):246-51.
Varga Z et al. Lancet. 2020 May 2;395(10234):1417-8.
The resurgence of Plaquenil (hydroxychloroquine)
Two of the most unusual dermatologic drugs have resurged as possible first-line therapy for rescue treatment of hospitalized patients with SARS-CoV-2, despite extremely limited clinical data supporting their efficacy, optimal dose, treatment duration, and potential adverse effects.
Chloroquine and hydroxychloroquine were introduced as treatment and prophylaxis of malaria and approved by the Food and Drug Administration in 1949 and 1955, respectively. They belong to a class of drugs called 4-aminoquinolones and have a flat aromatic core and a basic side chain. The basic property of these drugs contribute to their ability to accumulate in lysosomes. They have a large volume of distribution in the blood and a half-life of 40-60 days. Important interactions include use with tamoxifen, proton pump inhibitors, and with smoking. Although both drugs cross the placenta, they don’t have any notable effects on the fetus.
Chloroquine and hydroxychloroquine enter the cell and accumulate in the lysosomes along a pH gradient. Within the lysosome, they increase the pH, thereby stabilizing lysosomes and inhibiting eosinophil and neutrophil chemotaxis and phagocytic activity. They also inhibit complement-mediated hemolysis, reduce acute phase reactants, and prevent MHC class II–mediated auto antigen presentation. Additionally, they decrease cell-mediated immunity by decreasing the production of interleukin-1 and plasma cell synthesis. Hydroxychloroquine can also accumulate in endosomes and inhibit toll-like receptor signaling, thereby reducing the production of proinflammatory cytokines.
One of the ways SARS-CoV-2 enters cells is by up-regulating and binding to ACE2. Chloroquine/hydroxychloroquine reduce glycosylation of ACE2 and thus inhibit viral entry. Additionally, by increasing the endosomal pH, they potentially inactivate enzymes that viruses require for replication. Their lifesaving benefits, however, are thought to involve blocking the proinflammatory cytokine IL-6 and suppressing the cytokine storm thought to induce acute respiratory distress syndrome. Interestingly, chloroquine has also been shown to allow zinc ions into the cell, and zinc is a potent inhibitor of coronavirus RNA polymerase.
Side effects of chloroquine and hydroxychloroquine include GI upset, retinal toxicity with long-term use, hypoglycemia, cardiomyopathy, QT prolongation, ventricular arrhythmias, and renal and liver toxicity. Adverse effects have been observed with long-term daily doses of more than 3.5 mg/kg of chloroquine or more than 6.5 mg/kg of hydroxychloroquine. Cutaneous effects include pruritus, morbilliform rashes (in an estimated 10% of those treated) and psoriasis flares, and blue-black hyperpigmentation (in about 25%) of the shins, face, oral palate, and nails.
Initial In February 2020, the first clinical results of 100 patients treated with chloroquine were reported in a news briefing by the Chinese government. On March 20, the first clinical trial was published offering guidelines for the treatment of COVID-19 using hydroxychloroquine and azithromycin combination therapy – albeit with many limitations and reported biases in the study. Despite the poorly designed studies and inconclusive evidence, on March 28, the FDA issued an Emergency Use Authorization that allows providers to request a supply of hydroxychloroquine or chloroquine for hospitalized patients with COVID-19 who are unable to join a clinical trial.
On April 2, the first clinical trial to evaluate the safety and efficacy of hydroxychloroquine in adults hospitalized with COVID-19 began at Vanderbilt University Medical Center, Nashville, Tenn. The ORCHID trial (Outcomes Related to COVID-19 Treated With Hydroxychloroquine Among In-patients With Symptomatic Disease), funded by the National Heart, Lung, and Blood Institute. This blinded, placebo-controlled study is evaluating hydroxychloroquine treatment of hospitalized patients with COVID-19 in hopes of treating the severe complications of acute respiratory distress syndrome. Participants are randomly assigned to receive 400 mg hydroxychloroquine twice daily as a loading dose and then 200 mg twice daily thereafter on days 2-5. As of this writing, this study is currently underway and outcomes are expected in the upcoming weeks.
There is now a shortage of chloroquine and hydroxychloroquine in patients who have severe dermatologic and rheumatologic diseases, which include some who been in remission for years because of these medications and are in grave danger of recurrence. During this crisis, we desperately need well-controlled, randomized studies to test the efficacy and prolonged safety profile of these drugs in COVID-19 patients, as well as appropriate funding to source these medications for hospitalized and nonhospitalized patients in need.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. They had no relevant disclosures. Write to them at [email protected].
Sources
Liu J et al. Cell Discov. 2020 Mar 18. doi: 10.1038/s41421-020-0156-0.
Vincent MJ et al. Virol J. 2005 Aug 22;2:69.
Gautret P et al. Int J Antimicrob Agents. 2020 Mar 20. doi: 10.1016/j.ijantimicag.2020.105949.
Devaux CA et al. Int J Antimicrob Agents. 2020 Mar 12:105938. doi: 10.1016/j.ijantimicag.2020.105938.
Aronson J et al. COVID-19 trials registered up to 8 March 2020 – an analysis of 382 studies. 2020. Centre for Evidence-Based Medicine. https://www.cebm.net/oxford-covid-19/covid-19-registered-trials-and-analysis/
Savarino A et al. Lancet Infect Dis. 2003 Nov;3(11):722-7.
Yazdany J, Kim AHJ. Ann Intern Med. 2020 Mar 31. doi: 10.7326/M20-1334.
Xue J et al. PLoS One. 2014 Oct 1;9(10):e109180.
te Velthuis AJ et al. PLoS Pathog. 2010 Nov 4;6(11):e1001176.
Two of the most unusual dermatologic drugs have resurged as possible first-line therapy for rescue treatment of hospitalized patients with SARS-CoV-2, despite extremely limited clinical data supporting their efficacy, optimal dose, treatment duration, and potential adverse effects.
Chloroquine and hydroxychloroquine were introduced as treatment and prophylaxis of malaria and approved by the Food and Drug Administration in 1949 and 1955, respectively. They belong to a class of drugs called 4-aminoquinolones and have a flat aromatic core and a basic side chain. The basic property of these drugs contribute to their ability to accumulate in lysosomes. They have a large volume of distribution in the blood and a half-life of 40-60 days. Important interactions include use with tamoxifen, proton pump inhibitors, and with smoking. Although both drugs cross the placenta, they don’t have any notable effects on the fetus.
Chloroquine and hydroxychloroquine enter the cell and accumulate in the lysosomes along a pH gradient. Within the lysosome, they increase the pH, thereby stabilizing lysosomes and inhibiting eosinophil and neutrophil chemotaxis and phagocytic activity. They also inhibit complement-mediated hemolysis, reduce acute phase reactants, and prevent MHC class II–mediated auto antigen presentation. Additionally, they decrease cell-mediated immunity by decreasing the production of interleukin-1 and plasma cell synthesis. Hydroxychloroquine can also accumulate in endosomes and inhibit toll-like receptor signaling, thereby reducing the production of proinflammatory cytokines.
One of the ways SARS-CoV-2 enters cells is by up-regulating and binding to ACE2. Chloroquine/hydroxychloroquine reduce glycosylation of ACE2 and thus inhibit viral entry. Additionally, by increasing the endosomal pH, they potentially inactivate enzymes that viruses require for replication. Their lifesaving benefits, however, are thought to involve blocking the proinflammatory cytokine IL-6 and suppressing the cytokine storm thought to induce acute respiratory distress syndrome. Interestingly, chloroquine has also been shown to allow zinc ions into the cell, and zinc is a potent inhibitor of coronavirus RNA polymerase.
Side effects of chloroquine and hydroxychloroquine include GI upset, retinal toxicity with long-term use, hypoglycemia, cardiomyopathy, QT prolongation, ventricular arrhythmias, and renal and liver toxicity. Adverse effects have been observed with long-term daily doses of more than 3.5 mg/kg of chloroquine or more than 6.5 mg/kg of hydroxychloroquine. Cutaneous effects include pruritus, morbilliform rashes (in an estimated 10% of those treated) and psoriasis flares, and blue-black hyperpigmentation (in about 25%) of the shins, face, oral palate, and nails.
Initial In February 2020, the first clinical results of 100 patients treated with chloroquine were reported in a news briefing by the Chinese government. On March 20, the first clinical trial was published offering guidelines for the treatment of COVID-19 using hydroxychloroquine and azithromycin combination therapy – albeit with many limitations and reported biases in the study. Despite the poorly designed studies and inconclusive evidence, on March 28, the FDA issued an Emergency Use Authorization that allows providers to request a supply of hydroxychloroquine or chloroquine for hospitalized patients with COVID-19 who are unable to join a clinical trial.
On April 2, the first clinical trial to evaluate the safety and efficacy of hydroxychloroquine in adults hospitalized with COVID-19 began at Vanderbilt University Medical Center, Nashville, Tenn. The ORCHID trial (Outcomes Related to COVID-19 Treated With Hydroxychloroquine Among In-patients With Symptomatic Disease), funded by the National Heart, Lung, and Blood Institute. This blinded, placebo-controlled study is evaluating hydroxychloroquine treatment of hospitalized patients with COVID-19 in hopes of treating the severe complications of acute respiratory distress syndrome. Participants are randomly assigned to receive 400 mg hydroxychloroquine twice daily as a loading dose and then 200 mg twice daily thereafter on days 2-5. As of this writing, this study is currently underway and outcomes are expected in the upcoming weeks.
There is now a shortage of chloroquine and hydroxychloroquine in patients who have severe dermatologic and rheumatologic diseases, which include some who been in remission for years because of these medications and are in grave danger of recurrence. During this crisis, we desperately need well-controlled, randomized studies to test the efficacy and prolonged safety profile of these drugs in COVID-19 patients, as well as appropriate funding to source these medications for hospitalized and nonhospitalized patients in need.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. They had no relevant disclosures. Write to them at [email protected].
Sources
Liu J et al. Cell Discov. 2020 Mar 18. doi: 10.1038/s41421-020-0156-0.
Vincent MJ et al. Virol J. 2005 Aug 22;2:69.
Gautret P et al. Int J Antimicrob Agents. 2020 Mar 20. doi: 10.1016/j.ijantimicag.2020.105949.
Devaux CA et al. Int J Antimicrob Agents. 2020 Mar 12:105938. doi: 10.1016/j.ijantimicag.2020.105938.
Aronson J et al. COVID-19 trials registered up to 8 March 2020 – an analysis of 382 studies. 2020. Centre for Evidence-Based Medicine. https://www.cebm.net/oxford-covid-19/covid-19-registered-trials-and-analysis/
Savarino A et al. Lancet Infect Dis. 2003 Nov;3(11):722-7.
Yazdany J, Kim AHJ. Ann Intern Med. 2020 Mar 31. doi: 10.7326/M20-1334.
Xue J et al. PLoS One. 2014 Oct 1;9(10):e109180.
te Velthuis AJ et al. PLoS Pathog. 2010 Nov 4;6(11):e1001176.
Two of the most unusual dermatologic drugs have resurged as possible first-line therapy for rescue treatment of hospitalized patients with SARS-CoV-2, despite extremely limited clinical data supporting their efficacy, optimal dose, treatment duration, and potential adverse effects.
Chloroquine and hydroxychloroquine were introduced as treatment and prophylaxis of malaria and approved by the Food and Drug Administration in 1949 and 1955, respectively. They belong to a class of drugs called 4-aminoquinolones and have a flat aromatic core and a basic side chain. The basic property of these drugs contribute to their ability to accumulate in lysosomes. They have a large volume of distribution in the blood and a half-life of 40-60 days. Important interactions include use with tamoxifen, proton pump inhibitors, and with smoking. Although both drugs cross the placenta, they don’t have any notable effects on the fetus.
Chloroquine and hydroxychloroquine enter the cell and accumulate in the lysosomes along a pH gradient. Within the lysosome, they increase the pH, thereby stabilizing lysosomes and inhibiting eosinophil and neutrophil chemotaxis and phagocytic activity. They also inhibit complement-mediated hemolysis, reduce acute phase reactants, and prevent MHC class II–mediated auto antigen presentation. Additionally, they decrease cell-mediated immunity by decreasing the production of interleukin-1 and plasma cell synthesis. Hydroxychloroquine can also accumulate in endosomes and inhibit toll-like receptor signaling, thereby reducing the production of proinflammatory cytokines.
One of the ways SARS-CoV-2 enters cells is by up-regulating and binding to ACE2. Chloroquine/hydroxychloroquine reduce glycosylation of ACE2 and thus inhibit viral entry. Additionally, by increasing the endosomal pH, they potentially inactivate enzymes that viruses require for replication. Their lifesaving benefits, however, are thought to involve blocking the proinflammatory cytokine IL-6 and suppressing the cytokine storm thought to induce acute respiratory distress syndrome. Interestingly, chloroquine has also been shown to allow zinc ions into the cell, and zinc is a potent inhibitor of coronavirus RNA polymerase.
Side effects of chloroquine and hydroxychloroquine include GI upset, retinal toxicity with long-term use, hypoglycemia, cardiomyopathy, QT prolongation, ventricular arrhythmias, and renal and liver toxicity. Adverse effects have been observed with long-term daily doses of more than 3.5 mg/kg of chloroquine or more than 6.5 mg/kg of hydroxychloroquine. Cutaneous effects include pruritus, morbilliform rashes (in an estimated 10% of those treated) and psoriasis flares, and blue-black hyperpigmentation (in about 25%) of the shins, face, oral palate, and nails.
Initial In February 2020, the first clinical results of 100 patients treated with chloroquine were reported in a news briefing by the Chinese government. On March 20, the first clinical trial was published offering guidelines for the treatment of COVID-19 using hydroxychloroquine and azithromycin combination therapy – albeit with many limitations and reported biases in the study. Despite the poorly designed studies and inconclusive evidence, on March 28, the FDA issued an Emergency Use Authorization that allows providers to request a supply of hydroxychloroquine or chloroquine for hospitalized patients with COVID-19 who are unable to join a clinical trial.
On April 2, the first clinical trial to evaluate the safety and efficacy of hydroxychloroquine in adults hospitalized with COVID-19 began at Vanderbilt University Medical Center, Nashville, Tenn. The ORCHID trial (Outcomes Related to COVID-19 Treated With Hydroxychloroquine Among In-patients With Symptomatic Disease), funded by the National Heart, Lung, and Blood Institute. This blinded, placebo-controlled study is evaluating hydroxychloroquine treatment of hospitalized patients with COVID-19 in hopes of treating the severe complications of acute respiratory distress syndrome. Participants are randomly assigned to receive 400 mg hydroxychloroquine twice daily as a loading dose and then 200 mg twice daily thereafter on days 2-5. As of this writing, this study is currently underway and outcomes are expected in the upcoming weeks.
There is now a shortage of chloroquine and hydroxychloroquine in patients who have severe dermatologic and rheumatologic diseases, which include some who been in remission for years because of these medications and are in grave danger of recurrence. During this crisis, we desperately need well-controlled, randomized studies to test the efficacy and prolonged safety profile of these drugs in COVID-19 patients, as well as appropriate funding to source these medications for hospitalized and nonhospitalized patients in need.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. They had no relevant disclosures. Write to them at [email protected].
Sources
Liu J et al. Cell Discov. 2020 Mar 18. doi: 10.1038/s41421-020-0156-0.
Vincent MJ et al. Virol J. 2005 Aug 22;2:69.
Gautret P et al. Int J Antimicrob Agents. 2020 Mar 20. doi: 10.1016/j.ijantimicag.2020.105949.
Devaux CA et al. Int J Antimicrob Agents. 2020 Mar 12:105938. doi: 10.1016/j.ijantimicag.2020.105938.
Aronson J et al. COVID-19 trials registered up to 8 March 2020 – an analysis of 382 studies. 2020. Centre for Evidence-Based Medicine. https://www.cebm.net/oxford-covid-19/covid-19-registered-trials-and-analysis/
Savarino A et al. Lancet Infect Dis. 2003 Nov;3(11):722-7.
Yazdany J, Kim AHJ. Ann Intern Med. 2020 Mar 31. doi: 10.7326/M20-1334.
Xue J et al. PLoS One. 2014 Oct 1;9(10):e109180.
te Velthuis AJ et al. PLoS Pathog. 2010 Nov 4;6(11):e1001176.
Hand washing and hand sanitizer on the skin and COVID-19 infection risk
As we deal with the effects of the COVID-19 pandemic, hand washing and the use of hand sanitizers have been key for infection prevention. With drier, colder weather in many of the communities initially affected by COVID-19, skin was already prone to dryness and a skin barrier compromised, and hand eczema was more prevalent because of these factors alone. This article explores the while maintaining the maximum possible degree of infection prevention.
With many viruses, including coronavirus, the virus is a self-assembled nanoparticle in which the most vulnerable structure is the outer lipid bilayer. Soaps dissolve the lipid membrane and the virus breaks apart, inactivating it; they are also alkaline surfactants that pick up particles – including dirt, bacteria, and viruses – which are removed from the surface of the skin when the soaps are rinsed off. In the process of washing, the alkalinity of the soap (pH approximately 9-10), compared with the normal outer skin pH of approximately 5.5 or lower, also can affect the skin barrier as well as the resident skin microflora. In a study by Lambers et al., it was found that an acid skin pH (4-4.5) keeps the resident bacterial flora attached to the skin, whereas an alkaline pH (8-9) promotes the dispersal from the skin in assessments of the volar forearm.
With regard to the effectiveness of hand washing against viruses, the length of time spent hand washing has been shown to have an impact on influenza-like illness. In a recent study of 2,082 participants by Bin Abdulrahman et al., those who spent only 5-10 seconds hand washing with soap and hand rubbing were at a higher risk of more frequent influenza-like illness (odds ratio, 1.37; 95% confidence interval, 1.08-1.75), compared with those who washed their hands for 15 seconds or longer. Moreover, hand washing with soap and rubbing after shaking hands was found to be an independent protective factor against frequent influenza-like illness (adjusted OR, 0.59; 95% confidence interval, 0.37-0.94). Previous studies on the impact of hand washing on bacterial and parasitic illnesses also found similar results: Hand washing for 15-20 seconds or longer reduces infection.
Alcohol, long known as a disinfectant, has been recommended for disinfecting the hands since the late 1800s. Most alcohol-based hand antiseptics contain isopropanol, ethanol, N-propanol, or a combination of two of these products. The antimicrobial activity of alcohols can be attributed to their ability to denature and coagulate proteins, thereby lysing microorganisms’ cells, and disrupting their cellular metabolism. Alcohol solutions containing 60%-95% alcohol are the most effective. Notably, very high concentrations of alcohol are less potent because less water is found in higher concentrations of alcohol and proteins are not denatured easily in the absence of water. Alcohol-based hand sanitizers also often contain humectants, such as glycerin and/or aloe vera, to help prevent skin dryness and replace water content that is stripped by the use of alcohol on the skin surface.
Other topical disinfectants can also be used to inactivate coronaviruses from surfaces, including the skin. A recently published analysis of 22 studies found that human coronaviruses – such as severe acute respiratory syndrome (SARS) coronavirus, Middle East respiratory syndrome (MERS) coronavirus, or endemic human coronaviruses (HCoV) – can persist on inanimate surfaces such as metal, glass, or plastic for up to 9 days (COVID-19 was found in a study to persist on metal for up to 2-3 days), but can be efficiently inactivated by surface disinfection procedures with 62%-71% ethanol, 0.5% hydrogen peroxide, or 0.1% sodium hypochlorite within 1 minute. Other biocidal agents, such as 0.05%-0.2% benzalkonium chloride or 0.02% chlorhexidine digluconate, are less effective.
In the case of SARS, treatment of SARS-CoV with povidone-iodine products for 2 minutes reduced virus infectivity to below the detectable level, equivalent to the effect of ethanol, in one study. Formalin fixation of the infected cells and heating the virus to 56° C, as used in routine tissue processing, were found to inactivate several coronaviruses as well. Based on this information, ethanol-based hand sanitizers, typically containing ethanol content of 60% or higher, can be used to inactivate coronaviruses on the skin, including COVID-19.
In patients with influenza-virus infections, whether pathogens were in wet or dried mucus played a role in whether hand washing or rubbing with hand sanitizer was more effective. In a study that examined the effects of hand washing versus antiseptic hand rubbing with an ethanol-based hand disinfectant on inactivation of influenza A virus adhered to the hands, the investigators showed that the effectiveness of the ethanol-based disinfectant against influenza A virus in mucus was reduced, compared with influenza A virus in saline. Influenza A in mucus remained active, despite 120 seconds of hand rubbing with hand sanitizer; however, influenza A in saline was completely inactivated within 30 seconds. Interestingly, rubbing hands with an ethanol-based disinfectant inactivated influenza A virus in mucus within 30 seconds with mucus that had dried completely because the hydrogel characteristics had been eliminated. Hand washing rapidly inactivated influenza A virus whether in mucus form, saline, or dried mucous.
It is important to note that in COVID-19 infections, a productive cough or rhinorrhea are not as common compared with dry cough. Regardless, the findings of the study described above should be considered if mucous symptoms develop during a COVID-19 infection when determining infection control. Luckily, with COVID-19, both hand washing and use of an ethanol-based hand sanitizer are seemingly effective in inactivating the virus or removing it from the skin surface.
After frequent hand washing, we all can experience dryness and potentially cracked skin as well. With hand sanitizer, the alcohol content can also cause burning of skin, especially compromised skin.
Vanilloid receptor-1 (VR1), a heat-gated ion channel, is responsible for the burning sensation caused by capsaicin. Ethanol lowers the amount of heat needed to turn on VR1 nocioceptive pain receptors by almost ten degrees, resulting in a potential burning sensation when applied.
Nails are affected as well with frequent hand washing and/or application of hand sanitizer and can become cracked or brittle. Contact dermatitis, both irritant and allergic, can occur with increased use of disinfectants, particularly household cleaners without proper barrier protection.
We’ve previously mentioned the effect of hand washing disrupting the resident skin microflora. Maintaining the skin microflora and barrier is an important component of skin health for preventing both dermatitis and infection. Hand washing or use of hand sanitizer is of paramount importance and effective in infection control for COVID-19. To maintain skin health and the skin barrier, applying lotion or cream after hand washing is recommended. It is recommended to avoid scrubbing hands while washing, since this causes breaks in the skin. Using water that is too hot is not recommended as it can inflame the skin further and disrupt the skin barrier.
Wearing gloves, if possible, is recommended when using household disinfectant products to further decrease skin irritation, barrier disruption, and risk of contact dermatitis. I have found hand emollients that contain ceramides or ingredients higher in omega 6 fatty acids, such as borage seed oil or other oils high in linoleic acid content, to be helpful. In addition to improving the skin barrier, emollients and perhaps those with topical pre- or probiotics, may help restore the skin microflora, potentially improving infection control further. Application of hand moisturizer each time after hand washing to maintain better infection control and barrier protection was also recommended by the recent consensus statement of Chinese experts on protection of skin and mucous membrane barrier for health care workers fighting against COVID-19.
We and our patients have remarked how it seems like our hands have aged 20-50 years in the previous 2 weeks. No one is complaining, everyone understands that protecting themselves and others against a potentially lethal virus is paramount. Maintaining skin health is of secondary concern, but maintaining healthy skin may also protect the skin barrier, another important component of potential infection control.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. They had no relevant disclosures. Write to them at [email protected].
Resources
Lambers H et al. Int J Cosmet Sci. 2006 Oct;28(5):359-70.
Bin Abdulrahman AK et al. BMC Public Health. 2019 Oct 22;19(1):1324. doi: 10.1186/s12889-019-77.
Kariwa H et al. Dermatology. 2006;212 Suppl 1:119-23.
HIrose R et al. mSphere. 2019 Sep 18;4(5). pii: e00474-19. doi: 10.1128/mSphere.00474-19.
Trevisani M et al. Nat Neurosci. 2002 Jun;5(6):546-51.
Yan Y et al. Dermatol Ther. 2020 Mar 13:e13310. doi: 10.1111/dth.13310.
As we deal with the effects of the COVID-19 pandemic, hand washing and the use of hand sanitizers have been key for infection prevention. With drier, colder weather in many of the communities initially affected by COVID-19, skin was already prone to dryness and a skin barrier compromised, and hand eczema was more prevalent because of these factors alone. This article explores the while maintaining the maximum possible degree of infection prevention.
With many viruses, including coronavirus, the virus is a self-assembled nanoparticle in which the most vulnerable structure is the outer lipid bilayer. Soaps dissolve the lipid membrane and the virus breaks apart, inactivating it; they are also alkaline surfactants that pick up particles – including dirt, bacteria, and viruses – which are removed from the surface of the skin when the soaps are rinsed off. In the process of washing, the alkalinity of the soap (pH approximately 9-10), compared with the normal outer skin pH of approximately 5.5 or lower, also can affect the skin barrier as well as the resident skin microflora. In a study by Lambers et al., it was found that an acid skin pH (4-4.5) keeps the resident bacterial flora attached to the skin, whereas an alkaline pH (8-9) promotes the dispersal from the skin in assessments of the volar forearm.
With regard to the effectiveness of hand washing against viruses, the length of time spent hand washing has been shown to have an impact on influenza-like illness. In a recent study of 2,082 participants by Bin Abdulrahman et al., those who spent only 5-10 seconds hand washing with soap and hand rubbing were at a higher risk of more frequent influenza-like illness (odds ratio, 1.37; 95% confidence interval, 1.08-1.75), compared with those who washed their hands for 15 seconds or longer. Moreover, hand washing with soap and rubbing after shaking hands was found to be an independent protective factor against frequent influenza-like illness (adjusted OR, 0.59; 95% confidence interval, 0.37-0.94). Previous studies on the impact of hand washing on bacterial and parasitic illnesses also found similar results: Hand washing for 15-20 seconds or longer reduces infection.
Alcohol, long known as a disinfectant, has been recommended for disinfecting the hands since the late 1800s. Most alcohol-based hand antiseptics contain isopropanol, ethanol, N-propanol, or a combination of two of these products. The antimicrobial activity of alcohols can be attributed to their ability to denature and coagulate proteins, thereby lysing microorganisms’ cells, and disrupting their cellular metabolism. Alcohol solutions containing 60%-95% alcohol are the most effective. Notably, very high concentrations of alcohol are less potent because less water is found in higher concentrations of alcohol and proteins are not denatured easily in the absence of water. Alcohol-based hand sanitizers also often contain humectants, such as glycerin and/or aloe vera, to help prevent skin dryness and replace water content that is stripped by the use of alcohol on the skin surface.
Other topical disinfectants can also be used to inactivate coronaviruses from surfaces, including the skin. A recently published analysis of 22 studies found that human coronaviruses – such as severe acute respiratory syndrome (SARS) coronavirus, Middle East respiratory syndrome (MERS) coronavirus, or endemic human coronaviruses (HCoV) – can persist on inanimate surfaces such as metal, glass, or plastic for up to 9 days (COVID-19 was found in a study to persist on metal for up to 2-3 days), but can be efficiently inactivated by surface disinfection procedures with 62%-71% ethanol, 0.5% hydrogen peroxide, or 0.1% sodium hypochlorite within 1 minute. Other biocidal agents, such as 0.05%-0.2% benzalkonium chloride or 0.02% chlorhexidine digluconate, are less effective.
In the case of SARS, treatment of SARS-CoV with povidone-iodine products for 2 minutes reduced virus infectivity to below the detectable level, equivalent to the effect of ethanol, in one study. Formalin fixation of the infected cells and heating the virus to 56° C, as used in routine tissue processing, were found to inactivate several coronaviruses as well. Based on this information, ethanol-based hand sanitizers, typically containing ethanol content of 60% or higher, can be used to inactivate coronaviruses on the skin, including COVID-19.
In patients with influenza-virus infections, whether pathogens were in wet or dried mucus played a role in whether hand washing or rubbing with hand sanitizer was more effective. In a study that examined the effects of hand washing versus antiseptic hand rubbing with an ethanol-based hand disinfectant on inactivation of influenza A virus adhered to the hands, the investigators showed that the effectiveness of the ethanol-based disinfectant against influenza A virus in mucus was reduced, compared with influenza A virus in saline. Influenza A in mucus remained active, despite 120 seconds of hand rubbing with hand sanitizer; however, influenza A in saline was completely inactivated within 30 seconds. Interestingly, rubbing hands with an ethanol-based disinfectant inactivated influenza A virus in mucus within 30 seconds with mucus that had dried completely because the hydrogel characteristics had been eliminated. Hand washing rapidly inactivated influenza A virus whether in mucus form, saline, or dried mucous.
It is important to note that in COVID-19 infections, a productive cough or rhinorrhea are not as common compared with dry cough. Regardless, the findings of the study described above should be considered if mucous symptoms develop during a COVID-19 infection when determining infection control. Luckily, with COVID-19, both hand washing and use of an ethanol-based hand sanitizer are seemingly effective in inactivating the virus or removing it from the skin surface.
After frequent hand washing, we all can experience dryness and potentially cracked skin as well. With hand sanitizer, the alcohol content can also cause burning of skin, especially compromised skin.
Vanilloid receptor-1 (VR1), a heat-gated ion channel, is responsible for the burning sensation caused by capsaicin. Ethanol lowers the amount of heat needed to turn on VR1 nocioceptive pain receptors by almost ten degrees, resulting in a potential burning sensation when applied.
Nails are affected as well with frequent hand washing and/or application of hand sanitizer and can become cracked or brittle. Contact dermatitis, both irritant and allergic, can occur with increased use of disinfectants, particularly household cleaners without proper barrier protection.
We’ve previously mentioned the effect of hand washing disrupting the resident skin microflora. Maintaining the skin microflora and barrier is an important component of skin health for preventing both dermatitis and infection. Hand washing or use of hand sanitizer is of paramount importance and effective in infection control for COVID-19. To maintain skin health and the skin barrier, applying lotion or cream after hand washing is recommended. It is recommended to avoid scrubbing hands while washing, since this causes breaks in the skin. Using water that is too hot is not recommended as it can inflame the skin further and disrupt the skin barrier.
Wearing gloves, if possible, is recommended when using household disinfectant products to further decrease skin irritation, barrier disruption, and risk of contact dermatitis. I have found hand emollients that contain ceramides or ingredients higher in omega 6 fatty acids, such as borage seed oil or other oils high in linoleic acid content, to be helpful. In addition to improving the skin barrier, emollients and perhaps those with topical pre- or probiotics, may help restore the skin microflora, potentially improving infection control further. Application of hand moisturizer each time after hand washing to maintain better infection control and barrier protection was also recommended by the recent consensus statement of Chinese experts on protection of skin and mucous membrane barrier for health care workers fighting against COVID-19.
We and our patients have remarked how it seems like our hands have aged 20-50 years in the previous 2 weeks. No one is complaining, everyone understands that protecting themselves and others against a potentially lethal virus is paramount. Maintaining skin health is of secondary concern, but maintaining healthy skin may also protect the skin barrier, another important component of potential infection control.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. They had no relevant disclosures. Write to them at [email protected].
Resources
Lambers H et al. Int J Cosmet Sci. 2006 Oct;28(5):359-70.
Bin Abdulrahman AK et al. BMC Public Health. 2019 Oct 22;19(1):1324. doi: 10.1186/s12889-019-77.
Kariwa H et al. Dermatology. 2006;212 Suppl 1:119-23.
HIrose R et al. mSphere. 2019 Sep 18;4(5). pii: e00474-19. doi: 10.1128/mSphere.00474-19.
Trevisani M et al. Nat Neurosci. 2002 Jun;5(6):546-51.
Yan Y et al. Dermatol Ther. 2020 Mar 13:e13310. doi: 10.1111/dth.13310.
As we deal with the effects of the COVID-19 pandemic, hand washing and the use of hand sanitizers have been key for infection prevention. With drier, colder weather in many of the communities initially affected by COVID-19, skin was already prone to dryness and a skin barrier compromised, and hand eczema was more prevalent because of these factors alone. This article explores the while maintaining the maximum possible degree of infection prevention.
With many viruses, including coronavirus, the virus is a self-assembled nanoparticle in which the most vulnerable structure is the outer lipid bilayer. Soaps dissolve the lipid membrane and the virus breaks apart, inactivating it; they are also alkaline surfactants that pick up particles – including dirt, bacteria, and viruses – which are removed from the surface of the skin when the soaps are rinsed off. In the process of washing, the alkalinity of the soap (pH approximately 9-10), compared with the normal outer skin pH of approximately 5.5 or lower, also can affect the skin barrier as well as the resident skin microflora. In a study by Lambers et al., it was found that an acid skin pH (4-4.5) keeps the resident bacterial flora attached to the skin, whereas an alkaline pH (8-9) promotes the dispersal from the skin in assessments of the volar forearm.
With regard to the effectiveness of hand washing against viruses, the length of time spent hand washing has been shown to have an impact on influenza-like illness. In a recent study of 2,082 participants by Bin Abdulrahman et al., those who spent only 5-10 seconds hand washing with soap and hand rubbing were at a higher risk of more frequent influenza-like illness (odds ratio, 1.37; 95% confidence interval, 1.08-1.75), compared with those who washed their hands for 15 seconds or longer. Moreover, hand washing with soap and rubbing after shaking hands was found to be an independent protective factor against frequent influenza-like illness (adjusted OR, 0.59; 95% confidence interval, 0.37-0.94). Previous studies on the impact of hand washing on bacterial and parasitic illnesses also found similar results: Hand washing for 15-20 seconds or longer reduces infection.
Alcohol, long known as a disinfectant, has been recommended for disinfecting the hands since the late 1800s. Most alcohol-based hand antiseptics contain isopropanol, ethanol, N-propanol, or a combination of two of these products. The antimicrobial activity of alcohols can be attributed to their ability to denature and coagulate proteins, thereby lysing microorganisms’ cells, and disrupting their cellular metabolism. Alcohol solutions containing 60%-95% alcohol are the most effective. Notably, very high concentrations of alcohol are less potent because less water is found in higher concentrations of alcohol and proteins are not denatured easily in the absence of water. Alcohol-based hand sanitizers also often contain humectants, such as glycerin and/or aloe vera, to help prevent skin dryness and replace water content that is stripped by the use of alcohol on the skin surface.
Other topical disinfectants can also be used to inactivate coronaviruses from surfaces, including the skin. A recently published analysis of 22 studies found that human coronaviruses – such as severe acute respiratory syndrome (SARS) coronavirus, Middle East respiratory syndrome (MERS) coronavirus, or endemic human coronaviruses (HCoV) – can persist on inanimate surfaces such as metal, glass, or plastic for up to 9 days (COVID-19 was found in a study to persist on metal for up to 2-3 days), but can be efficiently inactivated by surface disinfection procedures with 62%-71% ethanol, 0.5% hydrogen peroxide, or 0.1% sodium hypochlorite within 1 minute. Other biocidal agents, such as 0.05%-0.2% benzalkonium chloride or 0.02% chlorhexidine digluconate, are less effective.
In the case of SARS, treatment of SARS-CoV with povidone-iodine products for 2 minutes reduced virus infectivity to below the detectable level, equivalent to the effect of ethanol, in one study. Formalin fixation of the infected cells and heating the virus to 56° C, as used in routine tissue processing, were found to inactivate several coronaviruses as well. Based on this information, ethanol-based hand sanitizers, typically containing ethanol content of 60% or higher, can be used to inactivate coronaviruses on the skin, including COVID-19.
In patients with influenza-virus infections, whether pathogens were in wet or dried mucus played a role in whether hand washing or rubbing with hand sanitizer was more effective. In a study that examined the effects of hand washing versus antiseptic hand rubbing with an ethanol-based hand disinfectant on inactivation of influenza A virus adhered to the hands, the investigators showed that the effectiveness of the ethanol-based disinfectant against influenza A virus in mucus was reduced, compared with influenza A virus in saline. Influenza A in mucus remained active, despite 120 seconds of hand rubbing with hand sanitizer; however, influenza A in saline was completely inactivated within 30 seconds. Interestingly, rubbing hands with an ethanol-based disinfectant inactivated influenza A virus in mucus within 30 seconds with mucus that had dried completely because the hydrogel characteristics had been eliminated. Hand washing rapidly inactivated influenza A virus whether in mucus form, saline, or dried mucous.
It is important to note that in COVID-19 infections, a productive cough or rhinorrhea are not as common compared with dry cough. Regardless, the findings of the study described above should be considered if mucous symptoms develop during a COVID-19 infection when determining infection control. Luckily, with COVID-19, both hand washing and use of an ethanol-based hand sanitizer are seemingly effective in inactivating the virus or removing it from the skin surface.
After frequent hand washing, we all can experience dryness and potentially cracked skin as well. With hand sanitizer, the alcohol content can also cause burning of skin, especially compromised skin.
Vanilloid receptor-1 (VR1), a heat-gated ion channel, is responsible for the burning sensation caused by capsaicin. Ethanol lowers the amount of heat needed to turn on VR1 nocioceptive pain receptors by almost ten degrees, resulting in a potential burning sensation when applied.
Nails are affected as well with frequent hand washing and/or application of hand sanitizer and can become cracked or brittle. Contact dermatitis, both irritant and allergic, can occur with increased use of disinfectants, particularly household cleaners without proper barrier protection.
We’ve previously mentioned the effect of hand washing disrupting the resident skin microflora. Maintaining the skin microflora and barrier is an important component of skin health for preventing both dermatitis and infection. Hand washing or use of hand sanitizer is of paramount importance and effective in infection control for COVID-19. To maintain skin health and the skin barrier, applying lotion or cream after hand washing is recommended. It is recommended to avoid scrubbing hands while washing, since this causes breaks in the skin. Using water that is too hot is not recommended as it can inflame the skin further and disrupt the skin barrier.
Wearing gloves, if possible, is recommended when using household disinfectant products to further decrease skin irritation, barrier disruption, and risk of contact dermatitis. I have found hand emollients that contain ceramides or ingredients higher in omega 6 fatty acids, such as borage seed oil or other oils high in linoleic acid content, to be helpful. In addition to improving the skin barrier, emollients and perhaps those with topical pre- or probiotics, may help restore the skin microflora, potentially improving infection control further. Application of hand moisturizer each time after hand washing to maintain better infection control and barrier protection was also recommended by the recent consensus statement of Chinese experts on protection of skin and mucous membrane barrier for health care workers fighting against COVID-19.
We and our patients have remarked how it seems like our hands have aged 20-50 years in the previous 2 weeks. No one is complaining, everyone understands that protecting themselves and others against a potentially lethal virus is paramount. Maintaining skin health is of secondary concern, but maintaining healthy skin may also protect the skin barrier, another important component of potential infection control.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. They had no relevant disclosures. Write to them at [email protected].
Resources
Lambers H et al. Int J Cosmet Sci. 2006 Oct;28(5):359-70.
Bin Abdulrahman AK et al. BMC Public Health. 2019 Oct 22;19(1):1324. doi: 10.1186/s12889-019-77.
Kariwa H et al. Dermatology. 2006;212 Suppl 1:119-23.
HIrose R et al. mSphere. 2019 Sep 18;4(5). pii: e00474-19. doi: 10.1128/mSphere.00474-19.
Trevisani M et al. Nat Neurosci. 2002 Jun;5(6):546-51.
Yan Y et al. Dermatol Ther. 2020 Mar 13:e13310. doi: 10.1111/dth.13310.
Vascular occlusion management
The time course and proper management of vascular occlusion attributable to interarterial hyaluronic acid fillers is critical. Albeit a rare complication, off-label uses of HA fillers, lack of proper training of injectors, and lack of clear appropriate guidelines in the management of these complications are some of the causes of delayed treatment and necrotic complications.
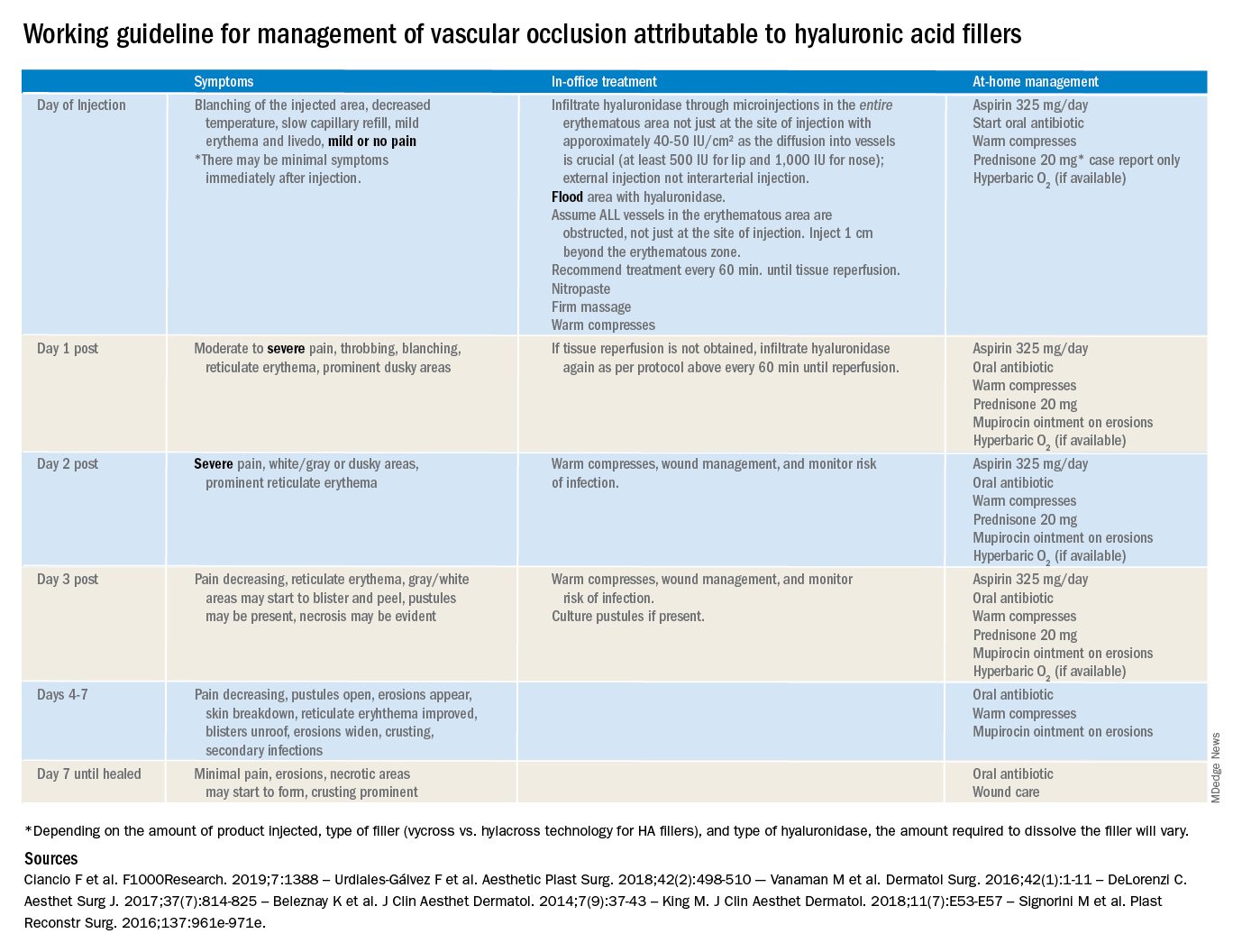
There are currently no definitive guidelines for the management of filler-associated cutaneous necrosis as experience with its treatment continues to evolve and be reported. In an attempt to consolidate the published data, as well as to give somewhat of a clear guideline of expectations, a time course and treatment guide has been outlined. The following is a working guideline for management of vascular occlusion attributable to HA fillers based on reports in the literature. This is not a consensus statement, rather it is a consolidation of the anecdotal reports and case studies outlined to help practitioners. It is also not inclusive of all the presentations of vascular occlusion. There are delayed cases of vascular occlusion beginning several days after injection, as well as alternative treatment options that may be considered.
These guidelines also are not for the devastating complication of blindness because of vascular occlusion secondary to fillers. Blindness is beyond the scope of the current article; however, we believe all experienced injectors should have emergency preparations in place and a relationship with an ophthalmologist or other trained surgeons experienced in performing retrobulbar hyaluronidase injections who can be reached in the event of a suspected occlusion. Any symptoms of eye pain, headache, or visual changes need to be immediately treated. Vascular occlusion is an emergency and timing is critical to prevent permanent blindness and facial deformities.
As with all filler injections, risks and complications can happen, and we cannot stress enough the appropriate level of training, as well as expert understanding of anatomy and injection technique, in minimizing potential risks. We encourage regulations and a required level of training to perform these procedures.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
The time course and proper management of vascular occlusion attributable to interarterial hyaluronic acid fillers is critical. Albeit a rare complication, off-label uses of HA fillers, lack of proper training of injectors, and lack of clear appropriate guidelines in the management of these complications are some of the causes of delayed treatment and necrotic complications.

There are currently no definitive guidelines for the management of filler-associated cutaneous necrosis as experience with its treatment continues to evolve and be reported. In an attempt to consolidate the published data, as well as to give somewhat of a clear guideline of expectations, a time course and treatment guide has been outlined. The following is a working guideline for management of vascular occlusion attributable to HA fillers based on reports in the literature. This is not a consensus statement, rather it is a consolidation of the anecdotal reports and case studies outlined to help practitioners. It is also not inclusive of all the presentations of vascular occlusion. There are delayed cases of vascular occlusion beginning several days after injection, as well as alternative treatment options that may be considered.
These guidelines also are not for the devastating complication of blindness because of vascular occlusion secondary to fillers. Blindness is beyond the scope of the current article; however, we believe all experienced injectors should have emergency preparations in place and a relationship with an ophthalmologist or other trained surgeons experienced in performing retrobulbar hyaluronidase injections who can be reached in the event of a suspected occlusion. Any symptoms of eye pain, headache, or visual changes need to be immediately treated. Vascular occlusion is an emergency and timing is critical to prevent permanent blindness and facial deformities.
As with all filler injections, risks and complications can happen, and we cannot stress enough the appropriate level of training, as well as expert understanding of anatomy and injection technique, in minimizing potential risks. We encourage regulations and a required level of training to perform these procedures.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
The time course and proper management of vascular occlusion attributable to interarterial hyaluronic acid fillers is critical. Albeit a rare complication, off-label uses of HA fillers, lack of proper training of injectors, and lack of clear appropriate guidelines in the management of these complications are some of the causes of delayed treatment and necrotic complications.

There are currently no definitive guidelines for the management of filler-associated cutaneous necrosis as experience with its treatment continues to evolve and be reported. In an attempt to consolidate the published data, as well as to give somewhat of a clear guideline of expectations, a time course and treatment guide has been outlined. The following is a working guideline for management of vascular occlusion attributable to HA fillers based on reports in the literature. This is not a consensus statement, rather it is a consolidation of the anecdotal reports and case studies outlined to help practitioners. It is also not inclusive of all the presentations of vascular occlusion. There are delayed cases of vascular occlusion beginning several days after injection, as well as alternative treatment options that may be considered.
These guidelines also are not for the devastating complication of blindness because of vascular occlusion secondary to fillers. Blindness is beyond the scope of the current article; however, we believe all experienced injectors should have emergency preparations in place and a relationship with an ophthalmologist or other trained surgeons experienced in performing retrobulbar hyaluronidase injections who can be reached in the event of a suspected occlusion. Any symptoms of eye pain, headache, or visual changes need to be immediately treated. Vascular occlusion is an emergency and timing is critical to prevent permanent blindness and facial deformities.
As with all filler injections, risks and complications can happen, and we cannot stress enough the appropriate level of training, as well as expert understanding of anatomy and injection technique, in minimizing potential risks. We encourage regulations and a required level of training to perform these procedures.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
CD1a and cosmetic-related contact dermatitis
As industries develop more chemical extraction techniques for synthetic or purified botanical ingredients to include in cosmetic and personal care products, the incidence of contact dermatitis is rising. Contact dermatitis (irritant or allergic) is the most common occupational skin disease, with current lifetime incidence exceeding 50%. For allergic contact dermatitis, type IV hypersensitivity (or delayed-type hypersensitivity) is thought to be the immunologic mediated pathway in which a T cell–mediated response occurs approximately 72 hours after exposure to the contact allergen. Diagnosis currently is predominately made clinically, after identifying the potential allergen or via patch testing. Treatment typically involves topical steroids or anti-inflammatories should a rash occur, and avoidance of the identified allergen.
In delayed-type hypersensitivity, most T-cell receptors recognize a peptide antigen bound to major histocompatibility complex (MHC) I or MHC II proteins, which stimulates a subsequent inflammatory immune response. However, in a recently published study, the authors wrote that “most known contact allergens are nonpeptidic small molecules, cations, or metals that are typically delivered to skin as drugs, oils, cosmetics, skin creams, or fragrances.” The chemical nature and structure of contact allergens “does not match the chemical structures of most antigens commonly recognized within the TCR-peptide-MHC axis,” they added. Thus, the mechanism by which nonpeptide molecules found in cosmetics cause a T cell–mediated hypersensitivity is poorly understood.
In that study, investigators from Brigham and Women’s Hospital, Boston; Columbia University, New York; and Monash University, Melbourne, looked at whether a protein found in immune cells – CD1a – could be involved in these allergic reactions. In a press release describing the results, cosenior author D. Branch Moody, MD, a principal investigator and physician in Brigham and Women’s division of rheumatology, inflammation, and immunity, noted that they “questioned the prevailing paradigm that T cell–mediated allergic reaction is only triggered when T cells respond to proteins or peptide antigens,” and found “a mechanism through which fragrance can initiate a T-cell response through a protein called CD1a.”
In their study, CD1a was identified as the and personal care products. Specifically, balsam of Peru (a tree oil commonly found in cosmetics and toothpaste), benzyl benzoate, benzyl cinnamate, and farnesol (often present in “fragrance”) after positive patch tests were found to elicit a CD1a-mediated immune response. Their findings suggest that, for these hydrophobic contact allergens, in forming CD1a-farnesol (or other) complexes, displacement of self-lipids normally bound to CD1a occurs, exposing T cell–stimulatory surface regions of CD1a that are normally hidden, thereby eliciting T cell–mediated hypersensitivity reactions.
The authors note that having a better understanding of how these ingredients elicit an immune response on a molecular level can help us potentially identify other molecules that can potentially block this response in humans, thereby treating or potentially mitigating allergic skin disease from these ingredients.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Resource
Nicolai S et al. Sci Immunol. 2020 Jan 3. doi: 10.1126/sciimmunol.aax5430.
As industries develop more chemical extraction techniques for synthetic or purified botanical ingredients to include in cosmetic and personal care products, the incidence of contact dermatitis is rising. Contact dermatitis (irritant or allergic) is the most common occupational skin disease, with current lifetime incidence exceeding 50%. For allergic contact dermatitis, type IV hypersensitivity (or delayed-type hypersensitivity) is thought to be the immunologic mediated pathway in which a T cell–mediated response occurs approximately 72 hours after exposure to the contact allergen. Diagnosis currently is predominately made clinically, after identifying the potential allergen or via patch testing. Treatment typically involves topical steroids or anti-inflammatories should a rash occur, and avoidance of the identified allergen.
In delayed-type hypersensitivity, most T-cell receptors recognize a peptide antigen bound to major histocompatibility complex (MHC) I or MHC II proteins, which stimulates a subsequent inflammatory immune response. However, in a recently published study, the authors wrote that “most known contact allergens are nonpeptidic small molecules, cations, or metals that are typically delivered to skin as drugs, oils, cosmetics, skin creams, or fragrances.” The chemical nature and structure of contact allergens “does not match the chemical structures of most antigens commonly recognized within the TCR-peptide-MHC axis,” they added. Thus, the mechanism by which nonpeptide molecules found in cosmetics cause a T cell–mediated hypersensitivity is poorly understood.
In that study, investigators from Brigham and Women’s Hospital, Boston; Columbia University, New York; and Monash University, Melbourne, looked at whether a protein found in immune cells – CD1a – could be involved in these allergic reactions. In a press release describing the results, cosenior author D. Branch Moody, MD, a principal investigator and physician in Brigham and Women’s division of rheumatology, inflammation, and immunity, noted that they “questioned the prevailing paradigm that T cell–mediated allergic reaction is only triggered when T cells respond to proteins or peptide antigens,” and found “a mechanism through which fragrance can initiate a T-cell response through a protein called CD1a.”
In their study, CD1a was identified as the and personal care products. Specifically, balsam of Peru (a tree oil commonly found in cosmetics and toothpaste), benzyl benzoate, benzyl cinnamate, and farnesol (often present in “fragrance”) after positive patch tests were found to elicit a CD1a-mediated immune response. Their findings suggest that, for these hydrophobic contact allergens, in forming CD1a-farnesol (or other) complexes, displacement of self-lipids normally bound to CD1a occurs, exposing T cell–stimulatory surface regions of CD1a that are normally hidden, thereby eliciting T cell–mediated hypersensitivity reactions.
The authors note that having a better understanding of how these ingredients elicit an immune response on a molecular level can help us potentially identify other molecules that can potentially block this response in humans, thereby treating or potentially mitigating allergic skin disease from these ingredients.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Resource
Nicolai S et al. Sci Immunol. 2020 Jan 3. doi: 10.1126/sciimmunol.aax5430.
As industries develop more chemical extraction techniques for synthetic or purified botanical ingredients to include in cosmetic and personal care products, the incidence of contact dermatitis is rising. Contact dermatitis (irritant or allergic) is the most common occupational skin disease, with current lifetime incidence exceeding 50%. For allergic contact dermatitis, type IV hypersensitivity (or delayed-type hypersensitivity) is thought to be the immunologic mediated pathway in which a T cell–mediated response occurs approximately 72 hours after exposure to the contact allergen. Diagnosis currently is predominately made clinically, after identifying the potential allergen or via patch testing. Treatment typically involves topical steroids or anti-inflammatories should a rash occur, and avoidance of the identified allergen.
In delayed-type hypersensitivity, most T-cell receptors recognize a peptide antigen bound to major histocompatibility complex (MHC) I or MHC II proteins, which stimulates a subsequent inflammatory immune response. However, in a recently published study, the authors wrote that “most known contact allergens are nonpeptidic small molecules, cations, or metals that are typically delivered to skin as drugs, oils, cosmetics, skin creams, or fragrances.” The chemical nature and structure of contact allergens “does not match the chemical structures of most antigens commonly recognized within the TCR-peptide-MHC axis,” they added. Thus, the mechanism by which nonpeptide molecules found in cosmetics cause a T cell–mediated hypersensitivity is poorly understood.
In that study, investigators from Brigham and Women’s Hospital, Boston; Columbia University, New York; and Monash University, Melbourne, looked at whether a protein found in immune cells – CD1a – could be involved in these allergic reactions. In a press release describing the results, cosenior author D. Branch Moody, MD, a principal investigator and physician in Brigham and Women’s division of rheumatology, inflammation, and immunity, noted that they “questioned the prevailing paradigm that T cell–mediated allergic reaction is only triggered when T cells respond to proteins or peptide antigens,” and found “a mechanism through which fragrance can initiate a T-cell response through a protein called CD1a.”
In their study, CD1a was identified as the and personal care products. Specifically, balsam of Peru (a tree oil commonly found in cosmetics and toothpaste), benzyl benzoate, benzyl cinnamate, and farnesol (often present in “fragrance”) after positive patch tests were found to elicit a CD1a-mediated immune response. Their findings suggest that, for these hydrophobic contact allergens, in forming CD1a-farnesol (or other) complexes, displacement of self-lipids normally bound to CD1a occurs, exposing T cell–stimulatory surface regions of CD1a that are normally hidden, thereby eliciting T cell–mediated hypersensitivity reactions.
The authors note that having a better understanding of how these ingredients elicit an immune response on a molecular level can help us potentially identify other molecules that can potentially block this response in humans, thereby treating or potentially mitigating allergic skin disease from these ingredients.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Resource
Nicolai S et al. Sci Immunol. 2020 Jan 3. doi: 10.1126/sciimmunol.aax5430.
Makeup is contaminated with pathogenic bacteria
Recalcitrant acne is a common, unwavering problem in dermatology practices nationwide. However, both gram positive and gram negative infections of the skin can go undiagnosed in patients with acne resistant to the armamentarium of oral and topical therapeutics. Although I often use isotretinoin in patients with cystic or recalcitrant acne, I almost always do a culture prior to initiating therapy, and more often than not, have discovered patients have gram negative and gram positive skin infections resistant to antibiotics commonly used to treat acne.
In a study by Bashir and Lambert published in the Journal of Applied Microbiology, 70%-90% of makeup products tested – including lipstick, lip gloss, beauty blenders, eyeliners, and mascara – were found to be contaminated with bacteria. Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli were the most common culprits, and the product with the highest contamination rates were beauty blenders (the small sponges used to apply makeup), which also had high rates of fungal contamination.
Expiration dates on cosmetic products are used to indicate the length of time a preservative in a product can control bacterial contamination. They are printed on packaging as an open jar symbol with the 3M, 6M, 9M, and 12M label for the number of months the product can be opened and used. Unfortunately and unknowingly, most consumers use products beyond the expiration date, and the most common offender is mascara.
Gram positive and gram negative skin infections should be ruled out in all cases of recalcitrant acne. A reminder to note on all culture requisitions to grow gram negatives because not all labs will grow gram negatives on a skin swab. Counseling should also be given to those patients who wear makeup, which should include techniques to clean and sanitize makeup applicators including brushes, tools, and towels. Blenders are known to be used “wet” and are not dried when washed.
It is my recommendation that blenders be a one-time-use-only tool and disposed of after EVERY application. Instructions provided in my clinic are to wash all devices and brushes once a week with hot soapy water, and blow dry with a hair dryer immediately afterward. Lipsticks, mascara wands, and lip glosses should be sanitized with alcohol once a month. Finally, all products need to be disposed of after their expiry.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
Resource
Basher A, Lambert P. J Appl Microbiol. 2019. doi: 10.1111/jam.14479.
Recalcitrant acne is a common, unwavering problem in dermatology practices nationwide. However, both gram positive and gram negative infections of the skin can go undiagnosed in patients with acne resistant to the armamentarium of oral and topical therapeutics. Although I often use isotretinoin in patients with cystic or recalcitrant acne, I almost always do a culture prior to initiating therapy, and more often than not, have discovered patients have gram negative and gram positive skin infections resistant to antibiotics commonly used to treat acne.
In a study by Bashir and Lambert published in the Journal of Applied Microbiology, 70%-90% of makeup products tested – including lipstick, lip gloss, beauty blenders, eyeliners, and mascara – were found to be contaminated with bacteria. Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli were the most common culprits, and the product with the highest contamination rates were beauty blenders (the small sponges used to apply makeup), which also had high rates of fungal contamination.
Expiration dates on cosmetic products are used to indicate the length of time a preservative in a product can control bacterial contamination. They are printed on packaging as an open jar symbol with the 3M, 6M, 9M, and 12M label for the number of months the product can be opened and used. Unfortunately and unknowingly, most consumers use products beyond the expiration date, and the most common offender is mascara.
Gram positive and gram negative skin infections should be ruled out in all cases of recalcitrant acne. A reminder to note on all culture requisitions to grow gram negatives because not all labs will grow gram negatives on a skin swab. Counseling should also be given to those patients who wear makeup, which should include techniques to clean and sanitize makeup applicators including brushes, tools, and towels. Blenders are known to be used “wet” and are not dried when washed.
It is my recommendation that blenders be a one-time-use-only tool and disposed of after EVERY application. Instructions provided in my clinic are to wash all devices and brushes once a week with hot soapy water, and blow dry with a hair dryer immediately afterward. Lipsticks, mascara wands, and lip glosses should be sanitized with alcohol once a month. Finally, all products need to be disposed of after their expiry.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
Resource
Basher A, Lambert P. J Appl Microbiol. 2019. doi: 10.1111/jam.14479.
Recalcitrant acne is a common, unwavering problem in dermatology practices nationwide. However, both gram positive and gram negative infections of the skin can go undiagnosed in patients with acne resistant to the armamentarium of oral and topical therapeutics. Although I often use isotretinoin in patients with cystic or recalcitrant acne, I almost always do a culture prior to initiating therapy, and more often than not, have discovered patients have gram negative and gram positive skin infections resistant to antibiotics commonly used to treat acne.
In a study by Bashir and Lambert published in the Journal of Applied Microbiology, 70%-90% of makeup products tested – including lipstick, lip gloss, beauty blenders, eyeliners, and mascara – were found to be contaminated with bacteria. Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli were the most common culprits, and the product with the highest contamination rates were beauty blenders (the small sponges used to apply makeup), which also had high rates of fungal contamination.
Expiration dates on cosmetic products are used to indicate the length of time a preservative in a product can control bacterial contamination. They are printed on packaging as an open jar symbol with the 3M, 6M, 9M, and 12M label for the number of months the product can be opened and used. Unfortunately and unknowingly, most consumers use products beyond the expiration date, and the most common offender is mascara.
Gram positive and gram negative skin infections should be ruled out in all cases of recalcitrant acne. A reminder to note on all culture requisitions to grow gram negatives because not all labs will grow gram negatives on a skin swab. Counseling should also be given to those patients who wear makeup, which should include techniques to clean and sanitize makeup applicators including brushes, tools, and towels. Blenders are known to be used “wet” and are not dried when washed.
It is my recommendation that blenders be a one-time-use-only tool and disposed of after EVERY application. Instructions provided in my clinic are to wash all devices and brushes once a week with hot soapy water, and blow dry with a hair dryer immediately afterward. Lipsticks, mascara wands, and lip glosses should be sanitized with alcohol once a month. Finally, all products need to be disposed of after their expiry.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
Resource
Basher A, Lambert P. J Appl Microbiol. 2019. doi: 10.1111/jam.14479.
Halal nail polish
Ever heard of halal nail polish? As an expert on all things hair, skin, and nails, I was dismayed when I walked into my local nail salon and saw this new category of nail polish I’d never heard of before. About 10 halal nail polishes were available in an array of beautiful colors. This nail salon was already branded as “nontoxic,” carrying only “8-free” nail polishes, vegan, and cruelty-free body and cleaning products – as well as no acrylics or UV light devices used for drying manicured nails or processing gel nails. With the salon already providing 8-free nail polishes, what was the difference between those and halal nail polishes?
As I did my Google search while sitting in the salon chair, I got the answer both from salon employees and the Internet, and also found several other brands of halal nail polishes sold on Amazon.
The main ingredient in traditional nail lacquer is nitrocellulose, a mixture of an indigestible plant fiber. Once used for gunpowder and blast mining in the 19th century, today, nitrocellulose is used for many purposes for holding materials together, such as photography film, and diagnostic tests that involve antigen-antibody binding, such as pregnancy tests. In a bottle of traditional nail polish, nitrocellulose is dissolved in a chemical solvent (typically ethyl acetate), along with pigment colors and plasticizers. The solvent quickly evaporates and is what gives nail polish its chemical smell. Once painted on the nail, the solvent gradually evaporates away entirely and the nitrocellulose is left behind, drying into a solid film on the nail. The same solvent molecule is in nonacetone nail polish remover, which simply redissolves the nitrocellulose back into a liquid so it can be wiped off.
Some nail polish may also include “pearl essence” to give a shiny look, like the silvery iridescence of fish scales. In fact, these polishes have contained ground up iridescent fish scales, but because of overfishing and cost, cheaper mineral alternatives are now more commonly used to give this shiny appearance.
Traditional nail polish contains tight molecular bonds that are impermeable to air and water. The tight bonds create fewer interstitial spaces for water to pass through. Nail polishes with polymer blends that help them withstand or make them more impermeable to water often chip less quickly and stay shinier longer.
While nail polishes are generally deemed safe, newer categories of 3-, 5-, or 8-free nail polishes containing fewer or different ingredients to preserve the product or give it it’s finish have been developed because of health concerns over some ingredients, for both users and cosmetologists. The 8-free nail polish does not contain dibutyl phthalate (DBP), toluene, formaldehyde, formaldehyde resin, camphor, ethyl tosylamide, parabens, or xylene. Three-, 5-, or 8- free doesn’t always mean that the lacquer has fewer chemicals; it may have alternative ingredients that also warrant study comparison to traditional ingredients.
Halal nail polish is in another category of “breathable nail polish,” which is not purely a function of the ingredient or lack of ingredients, but has to do with the way it is formulated. Compared with the tight molecular bonds of traditional nail polish, “breathable” polishes have a more staggered structure, which allows air and water molecules to pass through the polish. Halal nail polish is often free of the same ingredients as 8-free polishes, and some brands are even 13-free, animal product free, and do not require a base or top coat, but may also contain ingredients like bis (glycidoxyphenyl) propane/bisaminomethylnorbornae. Those ingredients are not typically used in traditional nail polish and may play a role in the unique staggered structure allowing air and water to pass through the polish. Halal nail polish may not last as long on nails as does traditional nail polish, usually a few days to a week.
In the past, some Islamic women would not wear nail polish because it is not porous, and so would interfere with wudu or ablution, the Islamic tradition of washing parts of the body before prayer. Halal translates to what is permissible and is most often associated with diet and procuring of meat. While the custom is not the same, the purpose is analogous to kosher preparation of foods in Judaism and dietary traditions in Hinduism. Having the opportunity to learn about this nail polish has been an interesting way to learn more about how different traditions, cultures, and faith affect skin and nail care.
Our nails are circulating breathing structures, with our nail plates being appendages over our nail beds with a rich pulse and blood supply. The main oxygen supply to the ends of our digits comes from our blood supply, not via oxygen through the nail plate, but wearing nail polish continuously can affect our nails. Oxygen saturation is detected through the end of our digits and nails when vital signs are being checked (less so when nail polish is present). As a continual wearer of nail polish for over 30 years, I can personally attest to certain types of onychodystrophy (white spots and discoloration on toe nails) from overuse of dark nail polish colors. Taking a break from polish and using these more “breathable” polishes could also potentially be a solution to this common complaint of nonfungal onychodystrophy.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Ever heard of halal nail polish? As an expert on all things hair, skin, and nails, I was dismayed when I walked into my local nail salon and saw this new category of nail polish I’d never heard of before. About 10 halal nail polishes were available in an array of beautiful colors. This nail salon was already branded as “nontoxic,” carrying only “8-free” nail polishes, vegan, and cruelty-free body and cleaning products – as well as no acrylics or UV light devices used for drying manicured nails or processing gel nails. With the salon already providing 8-free nail polishes, what was the difference between those and halal nail polishes?
As I did my Google search while sitting in the salon chair, I got the answer both from salon employees and the Internet, and also found several other brands of halal nail polishes sold on Amazon.
The main ingredient in traditional nail lacquer is nitrocellulose, a mixture of an indigestible plant fiber. Once used for gunpowder and blast mining in the 19th century, today, nitrocellulose is used for many purposes for holding materials together, such as photography film, and diagnostic tests that involve antigen-antibody binding, such as pregnancy tests. In a bottle of traditional nail polish, nitrocellulose is dissolved in a chemical solvent (typically ethyl acetate), along with pigment colors and plasticizers. The solvent quickly evaporates and is what gives nail polish its chemical smell. Once painted on the nail, the solvent gradually evaporates away entirely and the nitrocellulose is left behind, drying into a solid film on the nail. The same solvent molecule is in nonacetone nail polish remover, which simply redissolves the nitrocellulose back into a liquid so it can be wiped off.
Some nail polish may also include “pearl essence” to give a shiny look, like the silvery iridescence of fish scales. In fact, these polishes have contained ground up iridescent fish scales, but because of overfishing and cost, cheaper mineral alternatives are now more commonly used to give this shiny appearance.
Traditional nail polish contains tight molecular bonds that are impermeable to air and water. The tight bonds create fewer interstitial spaces for water to pass through. Nail polishes with polymer blends that help them withstand or make them more impermeable to water often chip less quickly and stay shinier longer.
While nail polishes are generally deemed safe, newer categories of 3-, 5-, or 8-free nail polishes containing fewer or different ingredients to preserve the product or give it it’s finish have been developed because of health concerns over some ingredients, for both users and cosmetologists. The 8-free nail polish does not contain dibutyl phthalate (DBP), toluene, formaldehyde, formaldehyde resin, camphor, ethyl tosylamide, parabens, or xylene. Three-, 5-, or 8- free doesn’t always mean that the lacquer has fewer chemicals; it may have alternative ingredients that also warrant study comparison to traditional ingredients.
Halal nail polish is in another category of “breathable nail polish,” which is not purely a function of the ingredient or lack of ingredients, but has to do with the way it is formulated. Compared with the tight molecular bonds of traditional nail polish, “breathable” polishes have a more staggered structure, which allows air and water molecules to pass through the polish. Halal nail polish is often free of the same ingredients as 8-free polishes, and some brands are even 13-free, animal product free, and do not require a base or top coat, but may also contain ingredients like bis (glycidoxyphenyl) propane/bisaminomethylnorbornae. Those ingredients are not typically used in traditional nail polish and may play a role in the unique staggered structure allowing air and water to pass through the polish. Halal nail polish may not last as long on nails as does traditional nail polish, usually a few days to a week.
In the past, some Islamic women would not wear nail polish because it is not porous, and so would interfere with wudu or ablution, the Islamic tradition of washing parts of the body before prayer. Halal translates to what is permissible and is most often associated with diet and procuring of meat. While the custom is not the same, the purpose is analogous to kosher preparation of foods in Judaism and dietary traditions in Hinduism. Having the opportunity to learn about this nail polish has been an interesting way to learn more about how different traditions, cultures, and faith affect skin and nail care.
Our nails are circulating breathing structures, with our nail plates being appendages over our nail beds with a rich pulse and blood supply. The main oxygen supply to the ends of our digits comes from our blood supply, not via oxygen through the nail plate, but wearing nail polish continuously can affect our nails. Oxygen saturation is detected through the end of our digits and nails when vital signs are being checked (less so when nail polish is present). As a continual wearer of nail polish for over 30 years, I can personally attest to certain types of onychodystrophy (white spots and discoloration on toe nails) from overuse of dark nail polish colors. Taking a break from polish and using these more “breathable” polishes could also potentially be a solution to this common complaint of nonfungal onychodystrophy.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Ever heard of halal nail polish? As an expert on all things hair, skin, and nails, I was dismayed when I walked into my local nail salon and saw this new category of nail polish I’d never heard of before. About 10 halal nail polishes were available in an array of beautiful colors. This nail salon was already branded as “nontoxic,” carrying only “8-free” nail polishes, vegan, and cruelty-free body and cleaning products – as well as no acrylics or UV light devices used for drying manicured nails or processing gel nails. With the salon already providing 8-free nail polishes, what was the difference between those and halal nail polishes?
As I did my Google search while sitting in the salon chair, I got the answer both from salon employees and the Internet, and also found several other brands of halal nail polishes sold on Amazon.
The main ingredient in traditional nail lacquer is nitrocellulose, a mixture of an indigestible plant fiber. Once used for gunpowder and blast mining in the 19th century, today, nitrocellulose is used for many purposes for holding materials together, such as photography film, and diagnostic tests that involve antigen-antibody binding, such as pregnancy tests. In a bottle of traditional nail polish, nitrocellulose is dissolved in a chemical solvent (typically ethyl acetate), along with pigment colors and plasticizers. The solvent quickly evaporates and is what gives nail polish its chemical smell. Once painted on the nail, the solvent gradually evaporates away entirely and the nitrocellulose is left behind, drying into a solid film on the nail. The same solvent molecule is in nonacetone nail polish remover, which simply redissolves the nitrocellulose back into a liquid so it can be wiped off.
Some nail polish may also include “pearl essence” to give a shiny look, like the silvery iridescence of fish scales. In fact, these polishes have contained ground up iridescent fish scales, but because of overfishing and cost, cheaper mineral alternatives are now more commonly used to give this shiny appearance.
Traditional nail polish contains tight molecular bonds that are impermeable to air and water. The tight bonds create fewer interstitial spaces for water to pass through. Nail polishes with polymer blends that help them withstand or make them more impermeable to water often chip less quickly and stay shinier longer.
While nail polishes are generally deemed safe, newer categories of 3-, 5-, or 8-free nail polishes containing fewer or different ingredients to preserve the product or give it it’s finish have been developed because of health concerns over some ingredients, for both users and cosmetologists. The 8-free nail polish does not contain dibutyl phthalate (DBP), toluene, formaldehyde, formaldehyde resin, camphor, ethyl tosylamide, parabens, or xylene. Three-, 5-, or 8- free doesn’t always mean that the lacquer has fewer chemicals; it may have alternative ingredients that also warrant study comparison to traditional ingredients.
Halal nail polish is in another category of “breathable nail polish,” which is not purely a function of the ingredient or lack of ingredients, but has to do with the way it is formulated. Compared with the tight molecular bonds of traditional nail polish, “breathable” polishes have a more staggered structure, which allows air and water molecules to pass through the polish. Halal nail polish is often free of the same ingredients as 8-free polishes, and some brands are even 13-free, animal product free, and do not require a base or top coat, but may also contain ingredients like bis (glycidoxyphenyl) propane/bisaminomethylnorbornae. Those ingredients are not typically used in traditional nail polish and may play a role in the unique staggered structure allowing air and water to pass through the polish. Halal nail polish may not last as long on nails as does traditional nail polish, usually a few days to a week.
In the past, some Islamic women would not wear nail polish because it is not porous, and so would interfere with wudu or ablution, the Islamic tradition of washing parts of the body before prayer. Halal translates to what is permissible and is most often associated with diet and procuring of meat. While the custom is not the same, the purpose is analogous to kosher preparation of foods in Judaism and dietary traditions in Hinduism. Having the opportunity to learn about this nail polish has been an interesting way to learn more about how different traditions, cultures, and faith affect skin and nail care.
Our nails are circulating breathing structures, with our nail plates being appendages over our nail beds with a rich pulse and blood supply. The main oxygen supply to the ends of our digits comes from our blood supply, not via oxygen through the nail plate, but wearing nail polish continuously can affect our nails. Oxygen saturation is detected through the end of our digits and nails when vital signs are being checked (less so when nail polish is present). As a continual wearer of nail polish for over 30 years, I can personally attest to certain types of onychodystrophy (white spots and discoloration on toe nails) from overuse of dark nail polish colors. Taking a break from polish and using these more “breathable” polishes could also potentially be a solution to this common complaint of nonfungal onychodystrophy.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
‘Clean’ and ‘natural’ beauty products
Clean beauty products have taken over the skin care market. A wave of new indie brands has entered the skin care market, some of which have garnered fame from bloggers and celebrities and via social media. There has also been a shift towards larger, more-established brands developing and marketing cleaner alternatives to their established skin care lines.
As consumers, physicians, and parents, we all want nontoxic products. However, as highlighted in a recent editorial by Bruce Brod, MD, and Courtney Blair Rubin, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, the Food and Drug Administration has “failed to define clean and natural, leaving these labels open to interpretation by nondermatologist retailers, bloggers, and celebrities who have set out to define clean beauty for themselves” (JAMA Dermatol. 2019 Sep 25. doi: 10.1001/jamadermatol.2019.2724). This vague interpretation has given rise to a billion-dollar industry of products that is unregulated and may, in fact, not be safer than other products.
For the last decade, to skin care products have also been on the rise. Some of the ingredients deemed toxic include petrolatum and parabens, which have good safety profiles and clinically, are among the least allergenic ingredients in skin products, particularly among patients with the most sensitive skin. In contrast, botanical oils, essential oils, and plant-based natural fragrances are chronic culprits of contact sensitivities and severe skin allergies.
I encourage all dermatologists to read this viewpoint as this topic will inevitably be a point of discussion with many patients. Large studies and expert consensus of safety profiles of chemicals – particularly those deemed carcinogenic, endocrine disruptors, and environmental hazards – are often lacking, leading to confusion for consumers. Our professional organizations and industry should be leading the efforts to establish standardized definitions and FDA regulations of skin care products deemed clean and natural so that the differentiation between marketing taglines and true, substantiated FDA-supported claims are clearer for consumers.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
Clean beauty products have taken over the skin care market. A wave of new indie brands has entered the skin care market, some of which have garnered fame from bloggers and celebrities and via social media. There has also been a shift towards larger, more-established brands developing and marketing cleaner alternatives to their established skin care lines.
As consumers, physicians, and parents, we all want nontoxic products. However, as highlighted in a recent editorial by Bruce Brod, MD, and Courtney Blair Rubin, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, the Food and Drug Administration has “failed to define clean and natural, leaving these labels open to interpretation by nondermatologist retailers, bloggers, and celebrities who have set out to define clean beauty for themselves” (JAMA Dermatol. 2019 Sep 25. doi: 10.1001/jamadermatol.2019.2724). This vague interpretation has given rise to a billion-dollar industry of products that is unregulated and may, in fact, not be safer than other products.
For the last decade, to skin care products have also been on the rise. Some of the ingredients deemed toxic include petrolatum and parabens, which have good safety profiles and clinically, are among the least allergenic ingredients in skin products, particularly among patients with the most sensitive skin. In contrast, botanical oils, essential oils, and plant-based natural fragrances are chronic culprits of contact sensitivities and severe skin allergies.
I encourage all dermatologists to read this viewpoint as this topic will inevitably be a point of discussion with many patients. Large studies and expert consensus of safety profiles of chemicals – particularly those deemed carcinogenic, endocrine disruptors, and environmental hazards – are often lacking, leading to confusion for consumers. Our professional organizations and industry should be leading the efforts to establish standardized definitions and FDA regulations of skin care products deemed clean and natural so that the differentiation between marketing taglines and true, substantiated FDA-supported claims are clearer for consumers.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
Clean beauty products have taken over the skin care market. A wave of new indie brands has entered the skin care market, some of which have garnered fame from bloggers and celebrities and via social media. There has also been a shift towards larger, more-established brands developing and marketing cleaner alternatives to their established skin care lines.
As consumers, physicians, and parents, we all want nontoxic products. However, as highlighted in a recent editorial by Bruce Brod, MD, and Courtney Blair Rubin, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, the Food and Drug Administration has “failed to define clean and natural, leaving these labels open to interpretation by nondermatologist retailers, bloggers, and celebrities who have set out to define clean beauty for themselves” (JAMA Dermatol. 2019 Sep 25. doi: 10.1001/jamadermatol.2019.2724). This vague interpretation has given rise to a billion-dollar industry of products that is unregulated and may, in fact, not be safer than other products.
For the last decade, to skin care products have also been on the rise. Some of the ingredients deemed toxic include petrolatum and parabens, which have good safety profiles and clinically, are among the least allergenic ingredients in skin products, particularly among patients with the most sensitive skin. In contrast, botanical oils, essential oils, and plant-based natural fragrances are chronic culprits of contact sensitivities and severe skin allergies.
I encourage all dermatologists to read this viewpoint as this topic will inevitably be a point of discussion with many patients. Large studies and expert consensus of safety profiles of chemicals – particularly those deemed carcinogenic, endocrine disruptors, and environmental hazards – are often lacking, leading to confusion for consumers. Our professional organizations and industry should be leading the efforts to establish standardized definitions and FDA regulations of skin care products deemed clean and natural so that the differentiation between marketing taglines and true, substantiated FDA-supported claims are clearer for consumers.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.











