User login
M. Alexander Otto began his reporting career early in 1999 covering the pharmaceutical industry for a national pharmacists' magazine and freelancing for the Washington Post and other newspapers. He then joined BNA, now part of Bloomberg News, covering health law and the protection of people and animals in medical research. Alex next worked for the McClatchy Company. Based on his work, Alex won a year-long Knight Science Journalism Fellowship to MIT in 2008-2009. He joined the company shortly thereafter. Alex has a newspaper journalism degree from Syracuse (N.Y.) University and a master's degree in medical science -- a physician assistant degree -- from George Washington University. Alex is based in Seattle.
Anti-Müllerian hormone identified PCOS in small study
SAN FRANCISCO – An anti-Müllerian hormone level of 3.4 ng/mL or greater identified polycystic ovary syndrome in a study of 31 nonobese adolescents.
That cutoff was determined to best discriminate between PCOS and controls, with a positive predictive value of 75% and a negative predictive value of 61% in a study of 15 nonobese adolescents with PCOS aged 13-21 years, and 16 controls, reported Dr. Aviva Sopher at the Endocrine Society’s Annual Meeting.
The goal of the study wasn’t to define a definitive anti-Müllerian hormone (AMH) cutoff; that may come later as Dr. Sopher’s group and others continue to investigate the matter. Instead, the project was a preliminary proof-of-concept effort to gauge the utility of AMH in adolescent PCOS diagnosis.
For now, because there was "overlap in AMH values between PCOS and controls" and "a normal adolescent girl with polyfollicular ovaries and no other symptoms can have an AMH in the range that we think of [as signifying] PCOS, I wouldn’t use AMH on its own. I am suggesting the use of AMH in conjunction with clinical presentation and lab work," said Dr. Sopher, a pediatric endocrinologist at Columbia University in New York.
The hope, however, is that AMH will eventually replace the need for ultrasound; the transabdominal approach is "suboptimal" in adolescents, and transvaginal ultrasound is "overly invasive in this age group," she said.
The hormone is produced by growing follicles and is a marker of their number. Blood levels were assessed in the study by enzyme-linked immunosorbent assay (ELISA). PCOS was diagnosed by National Institutes of Health criteria (Fertil. Steril. 2010; 93:1938-41).
AMH was significantly higher in subjects with PCOS (4.4 ng/mL) than in controls (2.4 ng/mL), and correlated significantly with average ovarian size, the appearance of polycystic ovaries, free testosterone, and androstenedione.
The PCOS participants were 1.5-fold more likely to have an AMH level of more that 3.4 ng/mL than were the healthy controls, and that cutoff had a positive predictive value for PCOS of 75% and a negative predictive value of non-PCOS of 61%.
Mean ovarian size was similar in both groups (7.1 cc in subjects with PCOS versus 6.7 cc in controls), as were body mass index z-scores (0.45 vs. 0.19) and percent body fat (36.6% vs. 34.2%). The differences were not significant.
The subjects were at least 2 years post menarche. Exclusion criteria included premature birth; other potential causes of hirsutism and irregular menses; and birth control pill use within 3 months of enrollment. Normal-weight girls were selected "to exclude the confounding factor of obesity," Dr. Sopher said.
AMH is a useful adjunct to diagnose adolescent PCOS, and it has the potential to replace ultrasound as a marker of follicle count, she concluded.
The authors said they had nothing to disclose. The study was funded by the National Institutes of Health.
"I think AMH will diagnose women with an increased number of follicles, irrespective of their androgens. If in addition they have elevated androgen levels, they will be most probably have PCOS," said Dr. Rodolfo Rey.
But the value proposed in this study "is quite low. Many apparently normal girls have values over 3.4 ng/mL. The range of AMH is quite large" in teenagers and young women – perhaps up to 6-7 ng/mL. "This study needs more controls [to capture] the whole range of normal," he said.
Several investigators have proposed replacing ultrasound with AMH to diagnose PCOS in adult women, but "the problem is always the cutoff level. I haven’t seen studies that give cutoff values that could replace ultrasound. We don’t have enough data," he said.
Also, not all PCOS patients have polycystic ovaries. "AMH is reflecting the number of follicles rather than the levels of androgens." By relying on it too heavily, it’s possible to miss hyperandrogenism without polycystic ovaries.
Dr. Rey is a pediatric endocrinologist at Children’s Hospital in Buenos Aires, Argentina. He has received honoraria from Beckman Coulter for his work on AMH assays.
"I think AMH will diagnose women with an increased number of follicles, irrespective of their androgens. If in addition they have elevated androgen levels, they will be most probably have PCOS," said Dr. Rodolfo Rey.
But the value proposed in this study "is quite low. Many apparently normal girls have values over 3.4 ng/mL. The range of AMH is quite large" in teenagers and young women – perhaps up to 6-7 ng/mL. "This study needs more controls [to capture] the whole range of normal," he said.
Several investigators have proposed replacing ultrasound with AMH to diagnose PCOS in adult women, but "the problem is always the cutoff level. I haven’t seen studies that give cutoff values that could replace ultrasound. We don’t have enough data," he said.
Also, not all PCOS patients have polycystic ovaries. "AMH is reflecting the number of follicles rather than the levels of androgens." By relying on it too heavily, it’s possible to miss hyperandrogenism without polycystic ovaries.
Dr. Rey is a pediatric endocrinologist at Children’s Hospital in Buenos Aires, Argentina. He has received honoraria from Beckman Coulter for his work on AMH assays.
"I think AMH will diagnose women with an increased number of follicles, irrespective of their androgens. If in addition they have elevated androgen levels, they will be most probably have PCOS," said Dr. Rodolfo Rey.
But the value proposed in this study "is quite low. Many apparently normal girls have values over 3.4 ng/mL. The range of AMH is quite large" in teenagers and young women – perhaps up to 6-7 ng/mL. "This study needs more controls [to capture] the whole range of normal," he said.
Several investigators have proposed replacing ultrasound with AMH to diagnose PCOS in adult women, but "the problem is always the cutoff level. I haven’t seen studies that give cutoff values that could replace ultrasound. We don’t have enough data," he said.
Also, not all PCOS patients have polycystic ovaries. "AMH is reflecting the number of follicles rather than the levels of androgens." By relying on it too heavily, it’s possible to miss hyperandrogenism without polycystic ovaries.
Dr. Rey is a pediatric endocrinologist at Children’s Hospital in Buenos Aires, Argentina. He has received honoraria from Beckman Coulter for his work on AMH assays.
SAN FRANCISCO – An anti-Müllerian hormone level of 3.4 ng/mL or greater identified polycystic ovary syndrome in a study of 31 nonobese adolescents.
That cutoff was determined to best discriminate between PCOS and controls, with a positive predictive value of 75% and a negative predictive value of 61% in a study of 15 nonobese adolescents with PCOS aged 13-21 years, and 16 controls, reported Dr. Aviva Sopher at the Endocrine Society’s Annual Meeting.
The goal of the study wasn’t to define a definitive anti-Müllerian hormone (AMH) cutoff; that may come later as Dr. Sopher’s group and others continue to investigate the matter. Instead, the project was a preliminary proof-of-concept effort to gauge the utility of AMH in adolescent PCOS diagnosis.
For now, because there was "overlap in AMH values between PCOS and controls" and "a normal adolescent girl with polyfollicular ovaries and no other symptoms can have an AMH in the range that we think of [as signifying] PCOS, I wouldn’t use AMH on its own. I am suggesting the use of AMH in conjunction with clinical presentation and lab work," said Dr. Sopher, a pediatric endocrinologist at Columbia University in New York.
The hope, however, is that AMH will eventually replace the need for ultrasound; the transabdominal approach is "suboptimal" in adolescents, and transvaginal ultrasound is "overly invasive in this age group," she said.
The hormone is produced by growing follicles and is a marker of their number. Blood levels were assessed in the study by enzyme-linked immunosorbent assay (ELISA). PCOS was diagnosed by National Institutes of Health criteria (Fertil. Steril. 2010; 93:1938-41).
AMH was significantly higher in subjects with PCOS (4.4 ng/mL) than in controls (2.4 ng/mL), and correlated significantly with average ovarian size, the appearance of polycystic ovaries, free testosterone, and androstenedione.
The PCOS participants were 1.5-fold more likely to have an AMH level of more that 3.4 ng/mL than were the healthy controls, and that cutoff had a positive predictive value for PCOS of 75% and a negative predictive value of non-PCOS of 61%.
Mean ovarian size was similar in both groups (7.1 cc in subjects with PCOS versus 6.7 cc in controls), as were body mass index z-scores (0.45 vs. 0.19) and percent body fat (36.6% vs. 34.2%). The differences were not significant.
The subjects were at least 2 years post menarche. Exclusion criteria included premature birth; other potential causes of hirsutism and irregular menses; and birth control pill use within 3 months of enrollment. Normal-weight girls were selected "to exclude the confounding factor of obesity," Dr. Sopher said.
AMH is a useful adjunct to diagnose adolescent PCOS, and it has the potential to replace ultrasound as a marker of follicle count, she concluded.
The authors said they had nothing to disclose. The study was funded by the National Institutes of Health.
SAN FRANCISCO – An anti-Müllerian hormone level of 3.4 ng/mL or greater identified polycystic ovary syndrome in a study of 31 nonobese adolescents.
That cutoff was determined to best discriminate between PCOS and controls, with a positive predictive value of 75% and a negative predictive value of 61% in a study of 15 nonobese adolescents with PCOS aged 13-21 years, and 16 controls, reported Dr. Aviva Sopher at the Endocrine Society’s Annual Meeting.
The goal of the study wasn’t to define a definitive anti-Müllerian hormone (AMH) cutoff; that may come later as Dr. Sopher’s group and others continue to investigate the matter. Instead, the project was a preliminary proof-of-concept effort to gauge the utility of AMH in adolescent PCOS diagnosis.
For now, because there was "overlap in AMH values between PCOS and controls" and "a normal adolescent girl with polyfollicular ovaries and no other symptoms can have an AMH in the range that we think of [as signifying] PCOS, I wouldn’t use AMH on its own. I am suggesting the use of AMH in conjunction with clinical presentation and lab work," said Dr. Sopher, a pediatric endocrinologist at Columbia University in New York.
The hope, however, is that AMH will eventually replace the need for ultrasound; the transabdominal approach is "suboptimal" in adolescents, and transvaginal ultrasound is "overly invasive in this age group," she said.
The hormone is produced by growing follicles and is a marker of their number. Blood levels were assessed in the study by enzyme-linked immunosorbent assay (ELISA). PCOS was diagnosed by National Institutes of Health criteria (Fertil. Steril. 2010; 93:1938-41).
AMH was significantly higher in subjects with PCOS (4.4 ng/mL) than in controls (2.4 ng/mL), and correlated significantly with average ovarian size, the appearance of polycystic ovaries, free testosterone, and androstenedione.
The PCOS participants were 1.5-fold more likely to have an AMH level of more that 3.4 ng/mL than were the healthy controls, and that cutoff had a positive predictive value for PCOS of 75% and a negative predictive value of non-PCOS of 61%.
Mean ovarian size was similar in both groups (7.1 cc in subjects with PCOS versus 6.7 cc in controls), as were body mass index z-scores (0.45 vs. 0.19) and percent body fat (36.6% vs. 34.2%). The differences were not significant.
The subjects were at least 2 years post menarche. Exclusion criteria included premature birth; other potential causes of hirsutism and irregular menses; and birth control pill use within 3 months of enrollment. Normal-weight girls were selected "to exclude the confounding factor of obesity," Dr. Sopher said.
AMH is a useful adjunct to diagnose adolescent PCOS, and it has the potential to replace ultrasound as a marker of follicle count, she concluded.
The authors said they had nothing to disclose. The study was funded by the National Institutes of Health.
AT ENDO 2013
Major finding: An anti-Müllerian hormone cutoff level of 3.4 ng/mL discriminated between PCOS patients and controls.
Data source: Prospective, case-control trial with 31 subjects.
Disclosures: The investigators said they had nothing to disclose. The study was funded by the National Institutes of Health.
Androgens helped even young boys with Klinefelter's
SAN FRANCISCO – Boys with Klinefelter’s syndrome felt better about themselves and had a better quality of life after 2 years of low-dose androgen therapy, in a randomized placebo-controlled trial.
At the end of 2 years, treated boys scored about 10 points better on questionnaires measuring aspects of anxiety, depression, and behavioral problems. "A standard deviation is about 15 points, so a difference of 10 points represents a moderate effect size. Families noticed quite dramatic improvements in social function, depression symptoms, and anxiety symptoms," said lead investigator Dr. Judith Ross, a pediatric endocrinologist at the duPont Hospital for Children in Wilmington, Del.
Although "it’s important that the findings are replicated with longer-term studies, gradual, age-appropriate androgen replacement should be considered to optimize self-image and quality of life in childhood," she said at the Endocrine Society annual meeting.
Androgen replacement is already standard practice in adolescents and adults with the condition, but not in boys. To see if it would make a difference, investigators from the duPont Hospital for Children randomized 39 boys aged 4-12 years old to 0.06 mg/kg per day of oxandrolone, and 41 to placebo capsules.
Her team monitored oxandrolone patients carefully for undue bone age advancement, lipid-level changes, liver function elevations, blood pressure increases, pubertal changes before age 8, and other possible complications. The only significant problem they found was a drop in six boys of HDL cholesterol below 20 mg/dL, which led to dose reductions.
Doses were dropped a bit in other subjects, too; by the end of the study, placebo patients were receiving an average of about 0.045 mg/kg per day and oxandrolone patients were taking an average of about 0.042 mg/kg per day. "Dose individualization is key to avoid side effects and to optimize positive outcomes. I can’t overemphasize how important this is. Physician consultation and dose adjustment are very important in managing the use of oxandrolone in boys with Klinefelter’s syndrome," Dr. Ross said.
Scores on the self-reported Revised Children's Manifest Anxiety Scale improved among oxandrolone boys about 10 points from the upper 90s at baseline, with 100 as the population mean. Boys on placebo stayed in the mid-90s throughout the trial. The results were similar for worry scores.
On the Children's Depression Inventory, also a self-reported questionnaire with 100 as the population mean, the scores of treated boys improved more than 10 points from the mid-90s on measures of success in interpersonal relationships, with smaller gains in measurements of self-like and confidence in the future. Boys on placebo started with similar scores, but made no significant gains.
Treated boys remained in the mid-80s on the parent-reported Child Behavior Checklist's measures of social problems such as getting teased, acting too young, and being disliked. Boys on placebo dropped about 5 points; 100 again represented the population mean.
The differences were statistically significant, and the groups well matched for karyotype, socioeconomic status, and race, Dr. Ross. The oxandrolone group was about 1.3 years younger on average, which was taken into account in the statistical analysis. Neurocognitive outcomes are currently under analysis.
The authors said they had no relevant financial disclosures. The National Institutes of Health funded the work.
SAN FRANCISCO – Boys with Klinefelter’s syndrome felt better about themselves and had a better quality of life after 2 years of low-dose androgen therapy, in a randomized placebo-controlled trial.
At the end of 2 years, treated boys scored about 10 points better on questionnaires measuring aspects of anxiety, depression, and behavioral problems. "A standard deviation is about 15 points, so a difference of 10 points represents a moderate effect size. Families noticed quite dramatic improvements in social function, depression symptoms, and anxiety symptoms," said lead investigator Dr. Judith Ross, a pediatric endocrinologist at the duPont Hospital for Children in Wilmington, Del.
Although "it’s important that the findings are replicated with longer-term studies, gradual, age-appropriate androgen replacement should be considered to optimize self-image and quality of life in childhood," she said at the Endocrine Society annual meeting.
Androgen replacement is already standard practice in adolescents and adults with the condition, but not in boys. To see if it would make a difference, investigators from the duPont Hospital for Children randomized 39 boys aged 4-12 years old to 0.06 mg/kg per day of oxandrolone, and 41 to placebo capsules.
Her team monitored oxandrolone patients carefully for undue bone age advancement, lipid-level changes, liver function elevations, blood pressure increases, pubertal changes before age 8, and other possible complications. The only significant problem they found was a drop in six boys of HDL cholesterol below 20 mg/dL, which led to dose reductions.
Doses were dropped a bit in other subjects, too; by the end of the study, placebo patients were receiving an average of about 0.045 mg/kg per day and oxandrolone patients were taking an average of about 0.042 mg/kg per day. "Dose individualization is key to avoid side effects and to optimize positive outcomes. I can’t overemphasize how important this is. Physician consultation and dose adjustment are very important in managing the use of oxandrolone in boys with Klinefelter’s syndrome," Dr. Ross said.
Scores on the self-reported Revised Children's Manifest Anxiety Scale improved among oxandrolone boys about 10 points from the upper 90s at baseline, with 100 as the population mean. Boys on placebo stayed in the mid-90s throughout the trial. The results were similar for worry scores.
On the Children's Depression Inventory, also a self-reported questionnaire with 100 as the population mean, the scores of treated boys improved more than 10 points from the mid-90s on measures of success in interpersonal relationships, with smaller gains in measurements of self-like and confidence in the future. Boys on placebo started with similar scores, but made no significant gains.
Treated boys remained in the mid-80s on the parent-reported Child Behavior Checklist's measures of social problems such as getting teased, acting too young, and being disliked. Boys on placebo dropped about 5 points; 100 again represented the population mean.
The differences were statistically significant, and the groups well matched for karyotype, socioeconomic status, and race, Dr. Ross. The oxandrolone group was about 1.3 years younger on average, which was taken into account in the statistical analysis. Neurocognitive outcomes are currently under analysis.
The authors said they had no relevant financial disclosures. The National Institutes of Health funded the work.
SAN FRANCISCO – Boys with Klinefelter’s syndrome felt better about themselves and had a better quality of life after 2 years of low-dose androgen therapy, in a randomized placebo-controlled trial.
At the end of 2 years, treated boys scored about 10 points better on questionnaires measuring aspects of anxiety, depression, and behavioral problems. "A standard deviation is about 15 points, so a difference of 10 points represents a moderate effect size. Families noticed quite dramatic improvements in social function, depression symptoms, and anxiety symptoms," said lead investigator Dr. Judith Ross, a pediatric endocrinologist at the duPont Hospital for Children in Wilmington, Del.
Although "it’s important that the findings are replicated with longer-term studies, gradual, age-appropriate androgen replacement should be considered to optimize self-image and quality of life in childhood," she said at the Endocrine Society annual meeting.
Androgen replacement is already standard practice in adolescents and adults with the condition, but not in boys. To see if it would make a difference, investigators from the duPont Hospital for Children randomized 39 boys aged 4-12 years old to 0.06 mg/kg per day of oxandrolone, and 41 to placebo capsules.
Her team monitored oxandrolone patients carefully for undue bone age advancement, lipid-level changes, liver function elevations, blood pressure increases, pubertal changes before age 8, and other possible complications. The only significant problem they found was a drop in six boys of HDL cholesterol below 20 mg/dL, which led to dose reductions.
Doses were dropped a bit in other subjects, too; by the end of the study, placebo patients were receiving an average of about 0.045 mg/kg per day and oxandrolone patients were taking an average of about 0.042 mg/kg per day. "Dose individualization is key to avoid side effects and to optimize positive outcomes. I can’t overemphasize how important this is. Physician consultation and dose adjustment are very important in managing the use of oxandrolone in boys with Klinefelter’s syndrome," Dr. Ross said.
Scores on the self-reported Revised Children's Manifest Anxiety Scale improved among oxandrolone boys about 10 points from the upper 90s at baseline, with 100 as the population mean. Boys on placebo stayed in the mid-90s throughout the trial. The results were similar for worry scores.
On the Children's Depression Inventory, also a self-reported questionnaire with 100 as the population mean, the scores of treated boys improved more than 10 points from the mid-90s on measures of success in interpersonal relationships, with smaller gains in measurements of self-like and confidence in the future. Boys on placebo started with similar scores, but made no significant gains.
Treated boys remained in the mid-80s on the parent-reported Child Behavior Checklist's measures of social problems such as getting teased, acting too young, and being disliked. Boys on placebo dropped about 5 points; 100 again represented the population mean.
The differences were statistically significant, and the groups well matched for karyotype, socioeconomic status, and race, Dr. Ross. The oxandrolone group was about 1.3 years younger on average, which was taken into account in the statistical analysis. Neurocognitive outcomes are currently under analysis.
The authors said they had no relevant financial disclosures. The National Institutes of Health funded the work.
AT ENDO 2013
Major finding: Klinefelter’s patients had a moderate improvement in various social adjustment scores after 2 years of low-dose androgen therapy.
Data Source: A randomized controlled trial in 80 boys with Klinefelter’s syndrome aged 4-12 years.
Disclosures: The authors said they had no relevant financial disclosures. The National Institutes of Health funded the work.
Allergy risk in obese children may be related to vitamin D deficiency
SAN FRANCISCO – Vitamin D deficiency may help explain why obese children are more at risk than others for allergies and asthma, according to an observational cross-sectional study.
"In our population, we found that the allergic profile was increased in obese adolescents," said lead investigator Dr. Candace Percival, a pediatric endocrinology fellow at the Uniformed Services University of the Health Sciences in Bethesda, Md.
Fifty-four boys and girls 10-18 years old who were at or above the 85th percentile for age- and sex-adjusted body mass index and 32 normal-weight controls participated in the project. The investigators tested for blood levels of vitamin D, immunoglobulin E (IgE), leptin, adiponectin, and cytokines that contribute to allergy and asthma.
As expected, based on previous obesity studies, higher BMIs correlated significantly with higher leptin and lower adiponectin levels, both of which have been linked to enhanced allergic response. Obesity correlated significantly with increased levels of IgE, IL (interleukin)-6 and IL-13 as well; the obese subjects were all also vitamin D deficient.
However, "when we ran a multivariate analysis controlling for vitamin D deficiency, we found that the statistical significance of correlations between cytokines and IgE with BMI disappeared. The relationship between BMI and ... markers of allergic disease seemed to depend on vitamin D deficiency; it was a dependent cofactor for adolescents having this allergy profile. It’s hard to say if there’s any sort of causal relationship, [but] this makes us wonder if vitamin D may be a mediator of the increased risk for allergy in the setting of obesity," Dr. Percival said at the Endocrine Society’s annual meeting.
It’s not an unreasonable thought. "Past research has shown that vitamin D is important for normal immune system function. It has both anti-inflammatory and antiallergic effects. Vitamin D deficiency is very common in obese individuals [and] is associated with an increased risk for allergy and asthma; it may skew the immune system towards an allergic response," she said.
The next step is to see if vitamin D supplements make a difference in biochemical markers and symptoms of allergic response in obese adolescents. Dr. Percival said she hopes to initiate that trial.
The authors said they had no relevant financial disclosures. The study was funded by Walter Reed National Military Medical Center and the Diabetes Action Research and Education Foundation in Washington, D.C.
SAN FRANCISCO – Vitamin D deficiency may help explain why obese children are more at risk than others for allergies and asthma, according to an observational cross-sectional study.
"In our population, we found that the allergic profile was increased in obese adolescents," said lead investigator Dr. Candace Percival, a pediatric endocrinology fellow at the Uniformed Services University of the Health Sciences in Bethesda, Md.
Fifty-four boys and girls 10-18 years old who were at or above the 85th percentile for age- and sex-adjusted body mass index and 32 normal-weight controls participated in the project. The investigators tested for blood levels of vitamin D, immunoglobulin E (IgE), leptin, adiponectin, and cytokines that contribute to allergy and asthma.
As expected, based on previous obesity studies, higher BMIs correlated significantly with higher leptin and lower adiponectin levels, both of which have been linked to enhanced allergic response. Obesity correlated significantly with increased levels of IgE, IL (interleukin)-6 and IL-13 as well; the obese subjects were all also vitamin D deficient.
However, "when we ran a multivariate analysis controlling for vitamin D deficiency, we found that the statistical significance of correlations between cytokines and IgE with BMI disappeared. The relationship between BMI and ... markers of allergic disease seemed to depend on vitamin D deficiency; it was a dependent cofactor for adolescents having this allergy profile. It’s hard to say if there’s any sort of causal relationship, [but] this makes us wonder if vitamin D may be a mediator of the increased risk for allergy in the setting of obesity," Dr. Percival said at the Endocrine Society’s annual meeting.
It’s not an unreasonable thought. "Past research has shown that vitamin D is important for normal immune system function. It has both anti-inflammatory and antiallergic effects. Vitamin D deficiency is very common in obese individuals [and] is associated with an increased risk for allergy and asthma; it may skew the immune system towards an allergic response," she said.
The next step is to see if vitamin D supplements make a difference in biochemical markers and symptoms of allergic response in obese adolescents. Dr. Percival said she hopes to initiate that trial.
The authors said they had no relevant financial disclosures. The study was funded by Walter Reed National Military Medical Center and the Diabetes Action Research and Education Foundation in Washington, D.C.
SAN FRANCISCO – Vitamin D deficiency may help explain why obese children are more at risk than others for allergies and asthma, according to an observational cross-sectional study.
"In our population, we found that the allergic profile was increased in obese adolescents," said lead investigator Dr. Candace Percival, a pediatric endocrinology fellow at the Uniformed Services University of the Health Sciences in Bethesda, Md.
Fifty-four boys and girls 10-18 years old who were at or above the 85th percentile for age- and sex-adjusted body mass index and 32 normal-weight controls participated in the project. The investigators tested for blood levels of vitamin D, immunoglobulin E (IgE), leptin, adiponectin, and cytokines that contribute to allergy and asthma.
As expected, based on previous obesity studies, higher BMIs correlated significantly with higher leptin and lower adiponectin levels, both of which have been linked to enhanced allergic response. Obesity correlated significantly with increased levels of IgE, IL (interleukin)-6 and IL-13 as well; the obese subjects were all also vitamin D deficient.
However, "when we ran a multivariate analysis controlling for vitamin D deficiency, we found that the statistical significance of correlations between cytokines and IgE with BMI disappeared. The relationship between BMI and ... markers of allergic disease seemed to depend on vitamin D deficiency; it was a dependent cofactor for adolescents having this allergy profile. It’s hard to say if there’s any sort of causal relationship, [but] this makes us wonder if vitamin D may be a mediator of the increased risk for allergy in the setting of obesity," Dr. Percival said at the Endocrine Society’s annual meeting.
It’s not an unreasonable thought. "Past research has shown that vitamin D is important for normal immune system function. It has both anti-inflammatory and antiallergic effects. Vitamin D deficiency is very common in obese individuals [and] is associated with an increased risk for allergy and asthma; it may skew the immune system towards an allergic response," she said.
The next step is to see if vitamin D supplements make a difference in biochemical markers and symptoms of allergic response in obese adolescents. Dr. Percival said she hopes to initiate that trial.
The authors said they had no relevant financial disclosures. The study was funded by Walter Reed National Military Medical Center and the Diabetes Action Research and Education Foundation in Washington, D.C.
AT ENDO 2013
Major finding: Vitamin D deficiency is a dependent cofactor for allergic biochemical profiles in obese adolescents.
Data Source: An observational cross-sectional study involving 54 overweight and 32 normal-weight adolescents.
Disclosures: The authors said they had no relevant financial disclosures. The study was funded by the Walter Reed National Military Medical Center and the Diabetes Action Research and Education Foundation in Washington, D.C.
Low-dose paroxetine approved for hot flashes
The Food and Drug Administration June 28 approved paroxetine (Brisdelle) for moderate to severe hot flashes, but in a dose lower than paroxetine formulations indicated for depression, obsessive-compulsive disorder, and other psychiatric problems.
"There are a significant number of women who suffer from hot flashes associated with menopause and who cannot or do not want to use hormonal treatments. Today’s approval provides women with the first FDA-approved, nonhormonal therapeutic option to help ease the hot flashes that are so common in menopause," Dr. Hylton Joffe, director of the Division of Bone, Reproductive and Urologic Products in the agency’s Center for Drug Evaluation and Research, said in a statement. All other approved treatments contain estrogen or estrogen plus a progestin.
Brisdelle is to be taken in a 7.5-mg dose once daily at bedtime. Approval came after two randomized, double-blind, placebo-controlled studies in 1,175 postmenopausal women with at least seven to eight hot flashes per day, or 50-60 per week. Treatment lasted 12 weeks in one study and 24 weeks in the other. At week 4 in the 24 week study, treated women had 28.9 fewer hot flashes per week, vs. 19 fewer on placebo; at week 12, they had 37.2 fewer, vs. 27.6 fewer on placebo. Differences persisted at 24 weeks and were statistically significant. Results were similar in the 12 week trial.
Headache, fatigue, and nausea/vomiting were the most common side effects.
"All medications that are approved for treating depression, including Paxil and Pexeva, have a boxed warning about an increased risk of suicide in children and young adults. Because Brisdelle contains the same active ingredient as Paxil and Pexeva, a boxed warning about suicidality is included in the Brisdelle label," the agency noted.
Brisdelle’s label also warns of a possible reduction in the effectiveness of tamoxifen if both medications are used together, an increased risk of bleeding; and a risk of developing serotonin syndrome. The drug will come with a Medication Guide for patients.
Brisdelle and Pexeva are marketed by Noven Therapeutics. GlaxoSmithKline markets Paxil.
The Food and Drug Administration June 28 approved paroxetine (Brisdelle) for moderate to severe hot flashes, but in a dose lower than paroxetine formulations indicated for depression, obsessive-compulsive disorder, and other psychiatric problems.
"There are a significant number of women who suffer from hot flashes associated with menopause and who cannot or do not want to use hormonal treatments. Today’s approval provides women with the first FDA-approved, nonhormonal therapeutic option to help ease the hot flashes that are so common in menopause," Dr. Hylton Joffe, director of the Division of Bone, Reproductive and Urologic Products in the agency’s Center for Drug Evaluation and Research, said in a statement. All other approved treatments contain estrogen or estrogen plus a progestin.
Brisdelle is to be taken in a 7.5-mg dose once daily at bedtime. Approval came after two randomized, double-blind, placebo-controlled studies in 1,175 postmenopausal women with at least seven to eight hot flashes per day, or 50-60 per week. Treatment lasted 12 weeks in one study and 24 weeks in the other. At week 4 in the 24 week study, treated women had 28.9 fewer hot flashes per week, vs. 19 fewer on placebo; at week 12, they had 37.2 fewer, vs. 27.6 fewer on placebo. Differences persisted at 24 weeks and were statistically significant. Results were similar in the 12 week trial.
Headache, fatigue, and nausea/vomiting were the most common side effects.
"All medications that are approved for treating depression, including Paxil and Pexeva, have a boxed warning about an increased risk of suicide in children and young adults. Because Brisdelle contains the same active ingredient as Paxil and Pexeva, a boxed warning about suicidality is included in the Brisdelle label," the agency noted.
Brisdelle’s label also warns of a possible reduction in the effectiveness of tamoxifen if both medications are used together, an increased risk of bleeding; and a risk of developing serotonin syndrome. The drug will come with a Medication Guide for patients.
Brisdelle and Pexeva are marketed by Noven Therapeutics. GlaxoSmithKline markets Paxil.
The Food and Drug Administration June 28 approved paroxetine (Brisdelle) for moderate to severe hot flashes, but in a dose lower than paroxetine formulations indicated for depression, obsessive-compulsive disorder, and other psychiatric problems.
"There are a significant number of women who suffer from hot flashes associated with menopause and who cannot or do not want to use hormonal treatments. Today’s approval provides women with the first FDA-approved, nonhormonal therapeutic option to help ease the hot flashes that are so common in menopause," Dr. Hylton Joffe, director of the Division of Bone, Reproductive and Urologic Products in the agency’s Center for Drug Evaluation and Research, said in a statement. All other approved treatments contain estrogen or estrogen plus a progestin.
Brisdelle is to be taken in a 7.5-mg dose once daily at bedtime. Approval came after two randomized, double-blind, placebo-controlled studies in 1,175 postmenopausal women with at least seven to eight hot flashes per day, or 50-60 per week. Treatment lasted 12 weeks in one study and 24 weeks in the other. At week 4 in the 24 week study, treated women had 28.9 fewer hot flashes per week, vs. 19 fewer on placebo; at week 12, they had 37.2 fewer, vs. 27.6 fewer on placebo. Differences persisted at 24 weeks and were statistically significant. Results were similar in the 12 week trial.
Headache, fatigue, and nausea/vomiting were the most common side effects.
"All medications that are approved for treating depression, including Paxil and Pexeva, have a boxed warning about an increased risk of suicide in children and young adults. Because Brisdelle contains the same active ingredient as Paxil and Pexeva, a boxed warning about suicidality is included in the Brisdelle label," the agency noted.
Brisdelle’s label also warns of a possible reduction in the effectiveness of tamoxifen if both medications are used together, an increased risk of bleeding; and a risk of developing serotonin syndrome. The drug will come with a Medication Guide for patients.
Brisdelle and Pexeva are marketed by Noven Therapeutics. GlaxoSmithKline markets Paxil.
If babies don't sleep at night, check feeding schedule
PORTLAND, ORE. – Sometimes, parents who get great advice in the hospital about how often to feed their newborns are in the pediatrician’s office a few months later because their baby isn’t sleeping more than a couple hours at a time, and they don’t know what to do about it.
They might have a "trained night-feeder" on their hands, according to Dr. Barbara Howard of the department of pediatrics at Johns Hopkins University in Baltimore.
In the nursery, parents are often told to feed on demand, but "we don’t remember to tell them to change that" after the first several weeks of life, so the child keeps to the schedule. At 4 months, he might still be waking up every 90 minutes to be fed, she said.
It’s not necessary. Half of kids at a month and a half and 95% by 4 months of gestational term age, can go 8 hours without feeding, Dr. Howard said at a conference sponsored by the North Pacific Pediatric Society.
The trick to breaking the pattern is to lengthen the daytime feeding interval. "Make [the baby] wait just a little bit longer when he starts fussing, until you’re aiming for about 4 hours between feedings. Make sure he is getting a feeding before the parents go to bed," as well. Don’t go cold turkey, either, if the child is used to an 8-ounce feeding at 2 a.m.; it’s kinder to slowly decrease the amount over a week or so, Dr. Howard said.
The fix is a bit more complex if the child is used to falling asleep with the bottle or breast in her mouth; she might need those familiar comforts to fall asleep after awakening for her 2 a.m. feeding.
To prevent the association – or hopefully break it if it’s already established – "advise parents to put the baby in bed at least a little bit awake, starting at about 2 months of age. Let her fuss just a little bit, make sure she opens her eyes," she said.
"Does that mean [mothers] can’t breastfeed the baby until she is asleep? Well, it’s okay to do that, but they need to wake her back up to put her in bed," she said.
A lot of mothers and fathers might also want to share their bed with their child for the first few months, which also isn’t necessarily a bad thing.
In many places, "it’s become our policy [that] we say ‘No, you shouldn’t sleep with your baby,’ [but] really, it’s often about parents" wanting to give their child a "cozy first few months of life, and I don’t think that’s a terrible thing," Dr. Howard said.
Plus, parents are going to do it anyway, "so I think it’s better to be on the up and up with [them] and say ‘here are some things you need to be careful about,’ " she said.
Entrapment in soft bedding is a real danger, as is "a parent who sleeps too deeply. Usually they are obese, or using substances or alcohol, and they overlie their child," she said.
Eight months is about the limit for cosleeping. Developmental night waking starts about then and will make breaking the habit "more of a struggle," she said.
Dr. Howard is the creator of CHADIS and president of Total Child Health, the management company that licenses its use.
PORTLAND, ORE. – Sometimes, parents who get great advice in the hospital about how often to feed their newborns are in the pediatrician’s office a few months later because their baby isn’t sleeping more than a couple hours at a time, and they don’t know what to do about it.
They might have a "trained night-feeder" on their hands, according to Dr. Barbara Howard of the department of pediatrics at Johns Hopkins University in Baltimore.
In the nursery, parents are often told to feed on demand, but "we don’t remember to tell them to change that" after the first several weeks of life, so the child keeps to the schedule. At 4 months, he might still be waking up every 90 minutes to be fed, she said.
It’s not necessary. Half of kids at a month and a half and 95% by 4 months of gestational term age, can go 8 hours without feeding, Dr. Howard said at a conference sponsored by the North Pacific Pediatric Society.
The trick to breaking the pattern is to lengthen the daytime feeding interval. "Make [the baby] wait just a little bit longer when he starts fussing, until you’re aiming for about 4 hours between feedings. Make sure he is getting a feeding before the parents go to bed," as well. Don’t go cold turkey, either, if the child is used to an 8-ounce feeding at 2 a.m.; it’s kinder to slowly decrease the amount over a week or so, Dr. Howard said.
The fix is a bit more complex if the child is used to falling asleep with the bottle or breast in her mouth; she might need those familiar comforts to fall asleep after awakening for her 2 a.m. feeding.
To prevent the association – or hopefully break it if it’s already established – "advise parents to put the baby in bed at least a little bit awake, starting at about 2 months of age. Let her fuss just a little bit, make sure she opens her eyes," she said.
"Does that mean [mothers] can’t breastfeed the baby until she is asleep? Well, it’s okay to do that, but they need to wake her back up to put her in bed," she said.
A lot of mothers and fathers might also want to share their bed with their child for the first few months, which also isn’t necessarily a bad thing.
In many places, "it’s become our policy [that] we say ‘No, you shouldn’t sleep with your baby,’ [but] really, it’s often about parents" wanting to give their child a "cozy first few months of life, and I don’t think that’s a terrible thing," Dr. Howard said.
Plus, parents are going to do it anyway, "so I think it’s better to be on the up and up with [them] and say ‘here are some things you need to be careful about,’ " she said.
Entrapment in soft bedding is a real danger, as is "a parent who sleeps too deeply. Usually they are obese, or using substances or alcohol, and they overlie their child," she said.
Eight months is about the limit for cosleeping. Developmental night waking starts about then and will make breaking the habit "more of a struggle," she said.
Dr. Howard is the creator of CHADIS and president of Total Child Health, the management company that licenses its use.
PORTLAND, ORE. – Sometimes, parents who get great advice in the hospital about how often to feed their newborns are in the pediatrician’s office a few months later because their baby isn’t sleeping more than a couple hours at a time, and they don’t know what to do about it.
They might have a "trained night-feeder" on their hands, according to Dr. Barbara Howard of the department of pediatrics at Johns Hopkins University in Baltimore.
In the nursery, parents are often told to feed on demand, but "we don’t remember to tell them to change that" after the first several weeks of life, so the child keeps to the schedule. At 4 months, he might still be waking up every 90 minutes to be fed, she said.
It’s not necessary. Half of kids at a month and a half and 95% by 4 months of gestational term age, can go 8 hours without feeding, Dr. Howard said at a conference sponsored by the North Pacific Pediatric Society.
The trick to breaking the pattern is to lengthen the daytime feeding interval. "Make [the baby] wait just a little bit longer when he starts fussing, until you’re aiming for about 4 hours between feedings. Make sure he is getting a feeding before the parents go to bed," as well. Don’t go cold turkey, either, if the child is used to an 8-ounce feeding at 2 a.m.; it’s kinder to slowly decrease the amount over a week or so, Dr. Howard said.
The fix is a bit more complex if the child is used to falling asleep with the bottle or breast in her mouth; she might need those familiar comforts to fall asleep after awakening for her 2 a.m. feeding.
To prevent the association – or hopefully break it if it’s already established – "advise parents to put the baby in bed at least a little bit awake, starting at about 2 months of age. Let her fuss just a little bit, make sure she opens her eyes," she said.
"Does that mean [mothers] can’t breastfeed the baby until she is asleep? Well, it’s okay to do that, but they need to wake her back up to put her in bed," she said.
A lot of mothers and fathers might also want to share their bed with their child for the first few months, which also isn’t necessarily a bad thing.
In many places, "it’s become our policy [that] we say ‘No, you shouldn’t sleep with your baby,’ [but] really, it’s often about parents" wanting to give their child a "cozy first few months of life, and I don’t think that’s a terrible thing," Dr. Howard said.
Plus, parents are going to do it anyway, "so I think it’s better to be on the up and up with [them] and say ‘here are some things you need to be careful about,’ " she said.
Entrapment in soft bedding is a real danger, as is "a parent who sleeps too deeply. Usually they are obese, or using substances or alcohol, and they overlie their child," she said.
Eight months is about the limit for cosleeping. Developmental night waking starts about then and will make breaking the habit "more of a struggle," she said.
Dr. Howard is the creator of CHADIS and president of Total Child Health, the management company that licenses its use.
EXPERT ANALYSIS FROM NPPS SCIENTIFIC CONFERENCE
Infection protocols contain hospital-acquired MERS outbreak in Saudi Arabia
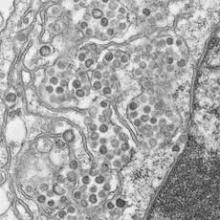
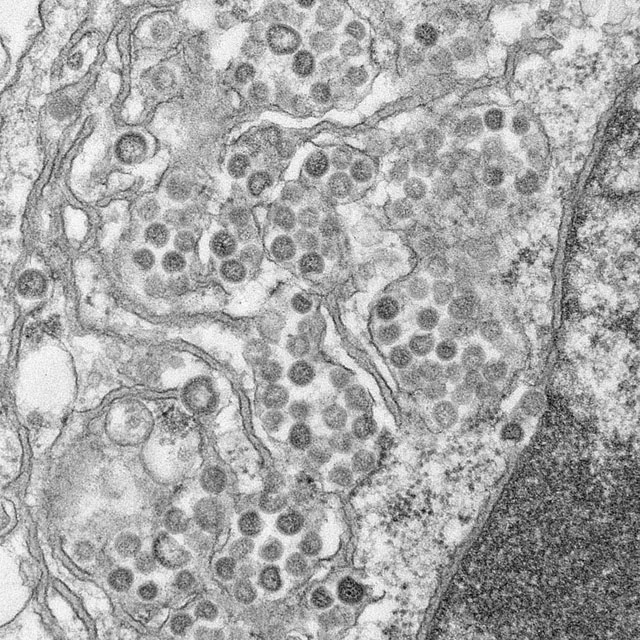
A coronavirus documented in four Saudi hospitals has caught the attention of investigators, who say its potential for easy transmission poses a challenge, particularly in health care settings. The team of experts investigating the recent cases of Middle East respiratory syndrome also investigated a deadly 2003 outbreak of severe acute respiratory syndrome (SARS) in Toronto hospitals and noted similarities between the two events, according to a report published online in the New England Journal of Medicine June 19.
Fifteen of 23 people with laboratory-confirmed Middle East respiratory syndrome coronavirus infections (MERS-CoV) died in the outbreak at four centers in eastern Saudi Arabia this spring
Fourteen of the 23 cases were in patients who caught the virus in the hemodialysis or, less frequently, intensive care or other units of a single 150-bed general hospital in Al-Hofuf. Some of those patients were transferred or sought hemodialysis at other facilities, spreading the infection. The outbreak likely originated from a probable case admitted originally for dizziness and diaphoresis, whose room was adjacent to a hemodialysis patient’s room (N. Engl. J. Med. 19 June 2013 [doi:10.1056/NEJMoa1306742]).
Proximity was the common denominator. In most cases, patients picked up the infection when they shared space or had a room near someone who was already symptomatic. "We are unable to determine whether person-to-person transmission occurred through respiratory droplets or through direct or indirect contact, and whether the virus was transmitted when contact was more than 1 meter away from the case patient," noted investigators from the Ministry of Health in Riyadh, Saudi Arabia; Johns Hopkins University in Baltimore; University College London; and elsewhere.
"Because some patients presented with gastrointestinal symptoms, and transmission appeared to occur between rooms on the ward, the current WHO [World Health Organization] recommendations for surveillance and control should be regarded as minimum standards. Hospitals should use contact and droplet precautions and should consider the follow-up of persons who were in the same ward as a patient with MERS-CoV infection,"they said.
WHO reported two cases of severe community-acquired pneumonia caused by MERS-CoV in September 2012. Since then, it’s been reported as a cause of pneumonia in Saudi Arabia, Qatar, Jordan, the United Kingdom, Germany, France, Tunisia, and Italy. The natural host and reservoir "remain unknown," they noted.
Three confirmed cases and two probable cases were in family members who visited symptomatic patients; an additional confirmed case was in a doctor who cared for them and a fifth in a nurse administrator who had face-to-face contact with the physician once he was symptomatic. No other cases were detected among 217 family members and more than 200 health care workers who came in contact with sick patients.
Once it was recognized, infection control measures contained the outbreak. The efforts included monitored hand hygiene, droplet and contact precautions for febrile patients, testing febrile patients for MERS-CoV, masking all patients undergoing hemodialysis, enhanced environmental cleaning, excluding patients with suspected infection from hemodialysis units, and banning contact with visitors and nonessential staff.
Symptoms included fever in 20 (87%) patients, cough in 20 (87%), shortness of breath in 11 (48%), and gastrointestinal symptoms in 8 (35%). "It is not yet known what proportion of ill patients shed virus in their stool," investigators said.
Twelve patients (52%) had end-stage renal disease, 17 (74%) had diabetes, 9 (39%) heart disease, and 10 (43%) lung disease. The majority had unilateral or bilateral infiltrates on chest x-ray; 17 (74%) cases were in men and 17 (74%) patients were 50 years or older. As of June 12, six patients had recovered from the infection and two remained hospitalized.
Among those who progressed, the median time for symptom onset to ICU admission was 5 days and median time to death was 11 days. The median incubation period was 5.2 days.
Infection was confirmed by reverse-transcriptase polymerase chain reaction. "Results of throat swabs were occasionally negative and repeat testing" was required. "It is not clear whether sputum of nasopharyngeal samples might be superior," the authors noted. There were 11 probable but not confirmed cases in the outbreak.
The investigators said that several similarities between MERS-CoV and SARS, also caused by a coronavirus, include a range of severity from "mild to fulminate"; a nonspecific early phase; heterogeneity in transmission, "with many infected patients not transmitting disease at all and one patient transmitting disease to seven others"; and gastrointestinal symptoms.
"The 65% case-fatality rate in this outbreak is of concern," but it may come down if more cases are identified, including those with mild symptoms, they said.
A coronavirus documented in four Saudi hospitals has caught the attention of investigators, who say its potential for easy transmission poses a challenge, particularly in health care settings. The team of experts investigating the recent cases of Middle East respiratory syndrome also investigated a deadly 2003 outbreak of severe acute respiratory syndrome (SARS) in Toronto hospitals and noted similarities between the two events, according to a report published online in the New England Journal of Medicine June 19.
Fifteen of 23 people with laboratory-confirmed Middle East respiratory syndrome coronavirus infections (MERS-CoV) died in the outbreak at four centers in eastern Saudi Arabia this spring
Fourteen of the 23 cases were in patients who caught the virus in the hemodialysis or, less frequently, intensive care or other units of a single 150-bed general hospital in Al-Hofuf. Some of those patients were transferred or sought hemodialysis at other facilities, spreading the infection. The outbreak likely originated from a probable case admitted originally for dizziness and diaphoresis, whose room was adjacent to a hemodialysis patient’s room (N. Engl. J. Med. 19 June 2013 [doi:10.1056/NEJMoa1306742]).
Proximity was the common denominator. In most cases, patients picked up the infection when they shared space or had a room near someone who was already symptomatic. "We are unable to determine whether person-to-person transmission occurred through respiratory droplets or through direct or indirect contact, and whether the virus was transmitted when contact was more than 1 meter away from the case patient," noted investigators from the Ministry of Health in Riyadh, Saudi Arabia; Johns Hopkins University in Baltimore; University College London; and elsewhere.
"Because some patients presented with gastrointestinal symptoms, and transmission appeared to occur between rooms on the ward, the current WHO [World Health Organization] recommendations for surveillance and control should be regarded as minimum standards. Hospitals should use contact and droplet precautions and should consider the follow-up of persons who were in the same ward as a patient with MERS-CoV infection,"they said.
WHO reported two cases of severe community-acquired pneumonia caused by MERS-CoV in September 2012. Since then, it’s been reported as a cause of pneumonia in Saudi Arabia, Qatar, Jordan, the United Kingdom, Germany, France, Tunisia, and Italy. The natural host and reservoir "remain unknown," they noted.
Three confirmed cases and two probable cases were in family members who visited symptomatic patients; an additional confirmed case was in a doctor who cared for them and a fifth in a nurse administrator who had face-to-face contact with the physician once he was symptomatic. No other cases were detected among 217 family members and more than 200 health care workers who came in contact with sick patients.
Once it was recognized, infection control measures contained the outbreak. The efforts included monitored hand hygiene, droplet and contact precautions for febrile patients, testing febrile patients for MERS-CoV, masking all patients undergoing hemodialysis, enhanced environmental cleaning, excluding patients with suspected infection from hemodialysis units, and banning contact with visitors and nonessential staff.
Symptoms included fever in 20 (87%) patients, cough in 20 (87%), shortness of breath in 11 (48%), and gastrointestinal symptoms in 8 (35%). "It is not yet known what proportion of ill patients shed virus in their stool," investigators said.
Twelve patients (52%) had end-stage renal disease, 17 (74%) had diabetes, 9 (39%) heart disease, and 10 (43%) lung disease. The majority had unilateral or bilateral infiltrates on chest x-ray; 17 (74%) cases were in men and 17 (74%) patients were 50 years or older. As of June 12, six patients had recovered from the infection and two remained hospitalized.
Among those who progressed, the median time for symptom onset to ICU admission was 5 days and median time to death was 11 days. The median incubation period was 5.2 days.
Infection was confirmed by reverse-transcriptase polymerase chain reaction. "Results of throat swabs were occasionally negative and repeat testing" was required. "It is not clear whether sputum of nasopharyngeal samples might be superior," the authors noted. There were 11 probable but not confirmed cases in the outbreak.
The investigators said that several similarities between MERS-CoV and SARS, also caused by a coronavirus, include a range of severity from "mild to fulminate"; a nonspecific early phase; heterogeneity in transmission, "with many infected patients not transmitting disease at all and one patient transmitting disease to seven others"; and gastrointestinal symptoms.
"The 65% case-fatality rate in this outbreak is of concern," but it may come down if more cases are identified, including those with mild symptoms, they said.
A coronavirus documented in four Saudi hospitals has caught the attention of investigators, who say its potential for easy transmission poses a challenge, particularly in health care settings. The team of experts investigating the recent cases of Middle East respiratory syndrome also investigated a deadly 2003 outbreak of severe acute respiratory syndrome (SARS) in Toronto hospitals and noted similarities between the two events, according to a report published online in the New England Journal of Medicine June 19.
Fifteen of 23 people with laboratory-confirmed Middle East respiratory syndrome coronavirus infections (MERS-CoV) died in the outbreak at four centers in eastern Saudi Arabia this spring
Fourteen of the 23 cases were in patients who caught the virus in the hemodialysis or, less frequently, intensive care or other units of a single 150-bed general hospital in Al-Hofuf. Some of those patients were transferred or sought hemodialysis at other facilities, spreading the infection. The outbreak likely originated from a probable case admitted originally for dizziness and diaphoresis, whose room was adjacent to a hemodialysis patient’s room (N. Engl. J. Med. 19 June 2013 [doi:10.1056/NEJMoa1306742]).
Proximity was the common denominator. In most cases, patients picked up the infection when they shared space or had a room near someone who was already symptomatic. "We are unable to determine whether person-to-person transmission occurred through respiratory droplets or through direct or indirect contact, and whether the virus was transmitted when contact was more than 1 meter away from the case patient," noted investigators from the Ministry of Health in Riyadh, Saudi Arabia; Johns Hopkins University in Baltimore; University College London; and elsewhere.
"Because some patients presented with gastrointestinal symptoms, and transmission appeared to occur between rooms on the ward, the current WHO [World Health Organization] recommendations for surveillance and control should be regarded as minimum standards. Hospitals should use contact and droplet precautions and should consider the follow-up of persons who were in the same ward as a patient with MERS-CoV infection,"they said.
WHO reported two cases of severe community-acquired pneumonia caused by MERS-CoV in September 2012. Since then, it’s been reported as a cause of pneumonia in Saudi Arabia, Qatar, Jordan, the United Kingdom, Germany, France, Tunisia, and Italy. The natural host and reservoir "remain unknown," they noted.
Three confirmed cases and two probable cases were in family members who visited symptomatic patients; an additional confirmed case was in a doctor who cared for them and a fifth in a nurse administrator who had face-to-face contact with the physician once he was symptomatic. No other cases were detected among 217 family members and more than 200 health care workers who came in contact with sick patients.
Once it was recognized, infection control measures contained the outbreak. The efforts included monitored hand hygiene, droplet and contact precautions for febrile patients, testing febrile patients for MERS-CoV, masking all patients undergoing hemodialysis, enhanced environmental cleaning, excluding patients with suspected infection from hemodialysis units, and banning contact with visitors and nonessential staff.
Symptoms included fever in 20 (87%) patients, cough in 20 (87%), shortness of breath in 11 (48%), and gastrointestinal symptoms in 8 (35%). "It is not yet known what proportion of ill patients shed virus in their stool," investigators said.
Twelve patients (52%) had end-stage renal disease, 17 (74%) had diabetes, 9 (39%) heart disease, and 10 (43%) lung disease. The majority had unilateral or bilateral infiltrates on chest x-ray; 17 (74%) cases were in men and 17 (74%) patients were 50 years or older. As of June 12, six patients had recovered from the infection and two remained hospitalized.
Among those who progressed, the median time for symptom onset to ICU admission was 5 days and median time to death was 11 days. The median incubation period was 5.2 days.
Infection was confirmed by reverse-transcriptase polymerase chain reaction. "Results of throat swabs were occasionally negative and repeat testing" was required. "It is not clear whether sputum of nasopharyngeal samples might be superior," the authors noted. There were 11 probable but not confirmed cases in the outbreak.
The investigators said that several similarities between MERS-CoV and SARS, also caused by a coronavirus, include a range of severity from "mild to fulminate"; a nonspecific early phase; heterogeneity in transmission, "with many infected patients not transmitting disease at all and one patient transmitting disease to seven others"; and gastrointestinal symptoms.
"The 65% case-fatality rate in this outbreak is of concern," but it may come down if more cases are identified, including those with mild symptoms, they said.
FROM NEJM
Major finding: Fifteen of 23 people with laboratory-confirmed Middle East respiratory syndrome coronavirus infections died in an outbreak at four hospitals in eastern Saudi Arabia this spring.
Data source: Epidemiologic investigation.
Disclosures: The authors reported having no relevant financial conflicts of interest.
Estrogen replacement eases weight-gain anxiety in girls with anorexia
SAN FRANCISCO – Teenage girls with anorexia have less anxiety about gaining weight if their estrogen levels have been returned to normal, according to a small, randomized study, researchers from Massachusetts General Hospital and Harvard Medical School in Boston reported.
If confirmed in additional studies, it is an important finding, because anxiety about weight gain can sabotage anorexia nervosa treatment. "Physiological estrogen replacement is a potential strategy to reduce ... anxiety and improve chances of successful treatment," said lead investigator Dr. Madhusmita Misra, a pediatric endocrinologist and associate professor of pediatrics at Harvard Medical School in Boston.
Her team randomized female patients aged 13-18 years to 100-mcg transdermal estradiol twice weekly with cyclic progesterone 10 days/month, or to placebo patches and pills.
After 18 months, 20 girls on active treatment, when compared with 17 on placebo, had a significant decrease in trait anxiety, the tendency to experience anxiety as assessed by the State-Trait Anxiety Inventory for Children (–3.05 vs. 2.07, P = .01). Patients with the greatest estrogen level increases had the greatest reductions in trait anxiety scores (P = .001).
The girls were amenorrheic, with baseline estrogen levels far below normal; the intervention restored them to physiologic levels. They all met DSM-IV anorexia criteria, had bone ages of at least 15 years old, and were under treatment.
There were no significant between-group baseline differences in body mass index, psychotropic medication use, age, estrogen levels, questionnaire scores, and other factors, including weight gain over the 18 months. "We did various multiple adjustments to be sure there were not any confounders driving this association," Dr. Misra said.
Estrogen replacement did not directly affect eating attitudes assessed by the Eating Disorders Inventory II questionnaire, or body-shape perceptions assessed by the Body Shape Questionnaire (BSQ-34). However, it did seem to prevent an increase in body dissatisfaction and state anxiety – current anxiety – with weight gain, something noted in the placebo group and common in anorexia nervosa treatment.
"We have shown" before "that physiologic estrogen replacement is very important for normalizing the rate of bone accrual" in teenage girls with the condition (J. Bone. Miner. Res. 2011;26:2430-8). "This seems to be an additional benefit. These findings have the potential to [affect] therapy in anorexia nervosa with early implementation of estrogen replacement in girls who are estrogen deficient. [But,] more studies are necessary to confirm these findings; it would be premature to advise estrogen replacement just for anxiety issues," Dr. Misra said.
Estradiol levels during the study increased a mean of 117.0 pg/mL in treated girls, and 22.4 pg/mL in the placebo group.
The investigators said they have nothing to disclose. The National Institutes of Health funded the work.
SAN FRANCISCO – Teenage girls with anorexia have less anxiety about gaining weight if their estrogen levels have been returned to normal, according to a small, randomized study, researchers from Massachusetts General Hospital and Harvard Medical School in Boston reported.
If confirmed in additional studies, it is an important finding, because anxiety about weight gain can sabotage anorexia nervosa treatment. "Physiological estrogen replacement is a potential strategy to reduce ... anxiety and improve chances of successful treatment," said lead investigator Dr. Madhusmita Misra, a pediatric endocrinologist and associate professor of pediatrics at Harvard Medical School in Boston.
Her team randomized female patients aged 13-18 years to 100-mcg transdermal estradiol twice weekly with cyclic progesterone 10 days/month, or to placebo patches and pills.
After 18 months, 20 girls on active treatment, when compared with 17 on placebo, had a significant decrease in trait anxiety, the tendency to experience anxiety as assessed by the State-Trait Anxiety Inventory for Children (–3.05 vs. 2.07, P = .01). Patients with the greatest estrogen level increases had the greatest reductions in trait anxiety scores (P = .001).
The girls were amenorrheic, with baseline estrogen levels far below normal; the intervention restored them to physiologic levels. They all met DSM-IV anorexia criteria, had bone ages of at least 15 years old, and were under treatment.
There were no significant between-group baseline differences in body mass index, psychotropic medication use, age, estrogen levels, questionnaire scores, and other factors, including weight gain over the 18 months. "We did various multiple adjustments to be sure there were not any confounders driving this association," Dr. Misra said.
Estrogen replacement did not directly affect eating attitudes assessed by the Eating Disorders Inventory II questionnaire, or body-shape perceptions assessed by the Body Shape Questionnaire (BSQ-34). However, it did seem to prevent an increase in body dissatisfaction and state anxiety – current anxiety – with weight gain, something noted in the placebo group and common in anorexia nervosa treatment.
"We have shown" before "that physiologic estrogen replacement is very important for normalizing the rate of bone accrual" in teenage girls with the condition (J. Bone. Miner. Res. 2011;26:2430-8). "This seems to be an additional benefit. These findings have the potential to [affect] therapy in anorexia nervosa with early implementation of estrogen replacement in girls who are estrogen deficient. [But,] more studies are necessary to confirm these findings; it would be premature to advise estrogen replacement just for anxiety issues," Dr. Misra said.
Estradiol levels during the study increased a mean of 117.0 pg/mL in treated girls, and 22.4 pg/mL in the placebo group.
The investigators said they have nothing to disclose. The National Institutes of Health funded the work.
SAN FRANCISCO – Teenage girls with anorexia have less anxiety about gaining weight if their estrogen levels have been returned to normal, according to a small, randomized study, researchers from Massachusetts General Hospital and Harvard Medical School in Boston reported.
If confirmed in additional studies, it is an important finding, because anxiety about weight gain can sabotage anorexia nervosa treatment. "Physiological estrogen replacement is a potential strategy to reduce ... anxiety and improve chances of successful treatment," said lead investigator Dr. Madhusmita Misra, a pediatric endocrinologist and associate professor of pediatrics at Harvard Medical School in Boston.
Her team randomized female patients aged 13-18 years to 100-mcg transdermal estradiol twice weekly with cyclic progesterone 10 days/month, or to placebo patches and pills.
After 18 months, 20 girls on active treatment, when compared with 17 on placebo, had a significant decrease in trait anxiety, the tendency to experience anxiety as assessed by the State-Trait Anxiety Inventory for Children (–3.05 vs. 2.07, P = .01). Patients with the greatest estrogen level increases had the greatest reductions in trait anxiety scores (P = .001).
The girls were amenorrheic, with baseline estrogen levels far below normal; the intervention restored them to physiologic levels. They all met DSM-IV anorexia criteria, had bone ages of at least 15 years old, and were under treatment.
There were no significant between-group baseline differences in body mass index, psychotropic medication use, age, estrogen levels, questionnaire scores, and other factors, including weight gain over the 18 months. "We did various multiple adjustments to be sure there were not any confounders driving this association," Dr. Misra said.
Estrogen replacement did not directly affect eating attitudes assessed by the Eating Disorders Inventory II questionnaire, or body-shape perceptions assessed by the Body Shape Questionnaire (BSQ-34). However, it did seem to prevent an increase in body dissatisfaction and state anxiety – current anxiety – with weight gain, something noted in the placebo group and common in anorexia nervosa treatment.
"We have shown" before "that physiologic estrogen replacement is very important for normalizing the rate of bone accrual" in teenage girls with the condition (J. Bone. Miner. Res. 2011;26:2430-8). "This seems to be an additional benefit. These findings have the potential to [affect] therapy in anorexia nervosa with early implementation of estrogen replacement in girls who are estrogen deficient. [But,] more studies are necessary to confirm these findings; it would be premature to advise estrogen replacement just for anxiety issues," Dr. Misra said.
Estradiol levels during the study increased a mean of 117.0 pg/mL in treated girls, and 22.4 pg/mL in the placebo group.
The investigators said they have nothing to disclose. The National Institutes of Health funded the work.
AT ENDO 2013
Major finding: After 18 months of estrogen replacement therapy, 20 anorexic teenage girls, compared with 17 on placebo, had a significant decrease in trait anxiety as assessed by the State-Trait Anxiety Inventory for Children (–3.05 vs. 2.07).
Data source: Randomized, controlled trial with 37 female anorexic patients 13-18 years old.
Disclosures: The investigators said they have nothing to disclose. The National Institutes of Health funded the work.
Dual biomarkers not always predictive of Alzheimer's
The jury is still out on the ability of brain biomarkers to predict Alzheimer’s disease in people with mild cognitive impairment, according to a study published May 20 online in Annals of Neurology.
The study was a field test of the National Institute on Aging-Alzheimer’s Association (NIA-AA) diagnostic guidelines for mild cognitive impairment (MCI) from Alzheimer’s disease, which propose that the presence of two neuroimaging biomarkers – amyloid and neurodegeneration – makes it highly likely that MCI will progress to Alzheimer’s, and that the presence of one, if the other is untested, conveys an intermediate risk (Alzheimers Dement. 2011;7:270-9).
The investigators found both of the markers in 46% of 212 MCI patients, one of the findings that led them to conclude that the "NIA-AA criteria apply to most MCI subjects in both the community and clinical trials settings" and that the "the proposed addition of biomarkers to the clinical diagnosis of MCI is largely valid."
However, they also found that "a sizeable proportion of subjects had conflicting biomarkers" that didn’t fit neatly into the model and require further exploration (Ann. Neurol. 2013 May 20 [doi: 10.1002/ana.23931]).
"Do these biomarkers work? Maybe, yes. Time will tell," lead investigator Dr. Ronald C. Petersen told an audience May 9 at Northwestern University in Chicago, explaining the findings."Conflicting markers are a problem. We clearly need more data," said Dr. Peterson, who is director of the Alzheimer’s Disease Research Center at the Mayo Clinic in Rochester, Minn.
His team enrolled 154 MCI patients from the Mayo Clinic Study of Aging (MCSA), a longitudinal study of patients without dementia, aged 70-89 years at enrollment, who are randomly selected from Olmsted County, Minn. In addition, 58 amnestic MCI patients (median age, 75 years) were enrolled from the Alzheimer’s Disease Neuroimaging Initiative-1 (ADNI-1), a longitudinal, multisite observational study into the early detection of Alzheimer’s. After their initial evaluation, the MCSA patients were reassessed at 15 months and the ADNI-1 patients at 12 months.
Among the findings inconsistent with the proposed NIA-AA mode, "29% of the MCSA subjects and 17% of the ADNI-1 subjects had evidence for neurodegeneration without amyloid deposition," the authors wrote. The model would suggest that "by the time of symptomatic impairment with MCI, both amyloid and neurodegeneration should be present," they noted.
Adding to the complexity, they also found that the neurodegeneration-positive but amyloid-negative group "had the highest rate of progression to dementia" on follow-up in the MCSA (7/36) cohort and the second highest in the ADNI-1 (2/10) cohort, bringing into question the salience of amyloid.
"These are very preliminary numbers; we don’t put a lot of faith in them," said Dr. Petersen. The final validation of the use of biomarkers will come from longitudinal studies.
Even so, biomarker research "is starting to tell us a pattern," he said. The cumulative acquisition of markers with clinical symptoms "does portend the likelihood that somebody is going to progress. But we have to sort out" the details, he said.
The presence of amyloid was assessed by Pittsburgh Compound B-PET scans; neurodegenerative findings were assessed by fluorodeoxyglucose PET scans and MRI measures of hippocampal volume. "We considered a subject positive for evidence of neurodegeneration if one or both measures" were positive, the investigators noted.
Dr. Petersen is the chair of Pfizer’s data monitoring committee, the chair of Janssen Alzheimer Immunotherapy’s data monitoring committee, and a consultant to Elan and GE Healthcare. The study was funded by the National Institute on Aging, the Robert H. and Clarice Smith and Abigail van Buren Alzheimer’s Disease Research Program, and other sources.
The jury is still out on the ability of brain biomarkers to predict Alzheimer’s disease in people with mild cognitive impairment, according to a study published May 20 online in Annals of Neurology.
The study was a field test of the National Institute on Aging-Alzheimer’s Association (NIA-AA) diagnostic guidelines for mild cognitive impairment (MCI) from Alzheimer’s disease, which propose that the presence of two neuroimaging biomarkers – amyloid and neurodegeneration – makes it highly likely that MCI will progress to Alzheimer’s, and that the presence of one, if the other is untested, conveys an intermediate risk (Alzheimers Dement. 2011;7:270-9).
The investigators found both of the markers in 46% of 212 MCI patients, one of the findings that led them to conclude that the "NIA-AA criteria apply to most MCI subjects in both the community and clinical trials settings" and that the "the proposed addition of biomarkers to the clinical diagnosis of MCI is largely valid."
However, they also found that "a sizeable proportion of subjects had conflicting biomarkers" that didn’t fit neatly into the model and require further exploration (Ann. Neurol. 2013 May 20 [doi: 10.1002/ana.23931]).
"Do these biomarkers work? Maybe, yes. Time will tell," lead investigator Dr. Ronald C. Petersen told an audience May 9 at Northwestern University in Chicago, explaining the findings."Conflicting markers are a problem. We clearly need more data," said Dr. Peterson, who is director of the Alzheimer’s Disease Research Center at the Mayo Clinic in Rochester, Minn.
His team enrolled 154 MCI patients from the Mayo Clinic Study of Aging (MCSA), a longitudinal study of patients without dementia, aged 70-89 years at enrollment, who are randomly selected from Olmsted County, Minn. In addition, 58 amnestic MCI patients (median age, 75 years) were enrolled from the Alzheimer’s Disease Neuroimaging Initiative-1 (ADNI-1), a longitudinal, multisite observational study into the early detection of Alzheimer’s. After their initial evaluation, the MCSA patients were reassessed at 15 months and the ADNI-1 patients at 12 months.
Among the findings inconsistent with the proposed NIA-AA mode, "29% of the MCSA subjects and 17% of the ADNI-1 subjects had evidence for neurodegeneration without amyloid deposition," the authors wrote. The model would suggest that "by the time of symptomatic impairment with MCI, both amyloid and neurodegeneration should be present," they noted.
Adding to the complexity, they also found that the neurodegeneration-positive but amyloid-negative group "had the highest rate of progression to dementia" on follow-up in the MCSA (7/36) cohort and the second highest in the ADNI-1 (2/10) cohort, bringing into question the salience of amyloid.
"These are very preliminary numbers; we don’t put a lot of faith in them," said Dr. Petersen. The final validation of the use of biomarkers will come from longitudinal studies.
Even so, biomarker research "is starting to tell us a pattern," he said. The cumulative acquisition of markers with clinical symptoms "does portend the likelihood that somebody is going to progress. But we have to sort out" the details, he said.
The presence of amyloid was assessed by Pittsburgh Compound B-PET scans; neurodegenerative findings were assessed by fluorodeoxyglucose PET scans and MRI measures of hippocampal volume. "We considered a subject positive for evidence of neurodegeneration if one or both measures" were positive, the investigators noted.
Dr. Petersen is the chair of Pfizer’s data monitoring committee, the chair of Janssen Alzheimer Immunotherapy’s data monitoring committee, and a consultant to Elan and GE Healthcare. The study was funded by the National Institute on Aging, the Robert H. and Clarice Smith and Abigail van Buren Alzheimer’s Disease Research Program, and other sources.
The jury is still out on the ability of brain biomarkers to predict Alzheimer’s disease in people with mild cognitive impairment, according to a study published May 20 online in Annals of Neurology.
The study was a field test of the National Institute on Aging-Alzheimer’s Association (NIA-AA) diagnostic guidelines for mild cognitive impairment (MCI) from Alzheimer’s disease, which propose that the presence of two neuroimaging biomarkers – amyloid and neurodegeneration – makes it highly likely that MCI will progress to Alzheimer’s, and that the presence of one, if the other is untested, conveys an intermediate risk (Alzheimers Dement. 2011;7:270-9).
The investigators found both of the markers in 46% of 212 MCI patients, one of the findings that led them to conclude that the "NIA-AA criteria apply to most MCI subjects in both the community and clinical trials settings" and that the "the proposed addition of biomarkers to the clinical diagnosis of MCI is largely valid."
However, they also found that "a sizeable proportion of subjects had conflicting biomarkers" that didn’t fit neatly into the model and require further exploration (Ann. Neurol. 2013 May 20 [doi: 10.1002/ana.23931]).
"Do these biomarkers work? Maybe, yes. Time will tell," lead investigator Dr. Ronald C. Petersen told an audience May 9 at Northwestern University in Chicago, explaining the findings."Conflicting markers are a problem. We clearly need more data," said Dr. Peterson, who is director of the Alzheimer’s Disease Research Center at the Mayo Clinic in Rochester, Minn.
His team enrolled 154 MCI patients from the Mayo Clinic Study of Aging (MCSA), a longitudinal study of patients without dementia, aged 70-89 years at enrollment, who are randomly selected from Olmsted County, Minn. In addition, 58 amnestic MCI patients (median age, 75 years) were enrolled from the Alzheimer’s Disease Neuroimaging Initiative-1 (ADNI-1), a longitudinal, multisite observational study into the early detection of Alzheimer’s. After their initial evaluation, the MCSA patients were reassessed at 15 months and the ADNI-1 patients at 12 months.
Among the findings inconsistent with the proposed NIA-AA mode, "29% of the MCSA subjects and 17% of the ADNI-1 subjects had evidence for neurodegeneration without amyloid deposition," the authors wrote. The model would suggest that "by the time of symptomatic impairment with MCI, both amyloid and neurodegeneration should be present," they noted.
Adding to the complexity, they also found that the neurodegeneration-positive but amyloid-negative group "had the highest rate of progression to dementia" on follow-up in the MCSA (7/36) cohort and the second highest in the ADNI-1 (2/10) cohort, bringing into question the salience of amyloid.
"These are very preliminary numbers; we don’t put a lot of faith in them," said Dr. Petersen. The final validation of the use of biomarkers will come from longitudinal studies.
Even so, biomarker research "is starting to tell us a pattern," he said. The cumulative acquisition of markers with clinical symptoms "does portend the likelihood that somebody is going to progress. But we have to sort out" the details, he said.
The presence of amyloid was assessed by Pittsburgh Compound B-PET scans; neurodegenerative findings were assessed by fluorodeoxyglucose PET scans and MRI measures of hippocampal volume. "We considered a subject positive for evidence of neurodegeneration if one or both measures" were positive, the investigators noted.
Dr. Petersen is the chair of Pfizer’s data monitoring committee, the chair of Janssen Alzheimer Immunotherapy’s data monitoring committee, and a consultant to Elan and GE Healthcare. The study was funded by the National Institute on Aging, the Robert H. and Clarice Smith and Abigail van Buren Alzheimer’s Disease Research Program, and other sources.
FROM ANNALS OF NEUROLOGY
Major finding: Of patients with mild cognitive impairment, 46% had both amyloid and neurodegeneration biomarkers.
Data source: Neuroimaging of 212 patients with mild cognitive impairment.
Disclosures: Dr. Petersen is the chair of Pfizer’s data monitoring committee, the chair of Janssen Alzheimer Immunotherapy’s data monitoring committee, and a consultant to Elan and GE Healthcare. The study was funded by the National Institute on Aging, the Robert H. and Clarice Smith and Abigail van Buren Alzheimer’s Disease Research Program, and other sources.
For empiric candidemia treatment, echinocandin tops fluconazole
LAS VEGAS – An echinocandin should be used for empiric therapy in critically ill candidemia patients awaiting culture results, according to investigators from Wayne State University in Detroit.
The reason is that Candida glabrata is on the rise in the critically ill, and it’s often resistant to fluconazole, the usual empiric choice, said Dr. Lisa Flynn, a vascular surgeon in the department of surgery at the university.
Dr. Flynn and her colleagues came to their conclusion after reviewing outcomes in 91 critically ill candidemia patients.
Just 40% (36) had the historic cause of candidemia, Candida albicans, which remains generally susceptible to fluconazole; 25% (23) had C. glabrata, and the rest had C. parapsilosis or other species.
Before those results were known, 53% (48) of patients were treated empirically with fluconazole and 36% (33), with the echinocandin micafungin. Most of the others received no treatment.
Seventy percent (16) of C. glabrata patients got fluconazole, the highest rate in the study of inappropriate initial antifungal therapy; probably not coincidently, 56% (13) of the C. glabrata patients died; the mortality rate in patients with other candida species was 32% (22). On univariate analysis, mortality increased from 18% to 37% if C. glabrata was cultured (P = .04).
"When we looked at glabrata versus all other candida species, we found significant increases in in-hospital mortality" that corresponded to a greater likelihood of inappropriate initial treatment, she said at the annual meeting of the Surgical Infection Society.
For that reason, "we are proposing that initial empiric antifungal therapy start with an echinocandin in the critically ill patient and then deescalate to fluconazole if [indicated by] culture data," she said.
It’s sound advice, so long as "your incidence of Candida glabrata is high," session moderator Dr. Addison May said after the presentation.
"It really depends on your hospital’s rate, and how frequently it’s [isolated]. It’s important to understand what you need to empirically treat with," but also important to use newer agents like micafungin judiciously, to prevent resistance, said Dr. May, professor of surgery and anesthesiology at Vanderbilt University in Nashville, Tenn.
C. glabrata patients were more likely than others to be over 60 years old; they had longer hospital and ICU stays, as well.
The mean APACHE II (Acute Physiology and Chronic Health Evaluation II) score in the study was 25, and the mean age was 57 years; 54% (49) of patients were men, and 68% (62) were black. In the previous month, almost half had surgery and a quarter had been on total parenteral nutrition.
Central lines were the source of infection in 84% (76).
On multivariate analysis, inappropriate initial antifungal treatment, vasopressor therapy, mechanical ventilation, and end-stage renal disease were all significant risk factors for death.
Dr. May and Dr. Flynn said they had no relevant financial disclosures.
LAS VEGAS – An echinocandin should be used for empiric therapy in critically ill candidemia patients awaiting culture results, according to investigators from Wayne State University in Detroit.
The reason is that Candida glabrata is on the rise in the critically ill, and it’s often resistant to fluconazole, the usual empiric choice, said Dr. Lisa Flynn, a vascular surgeon in the department of surgery at the university.
Dr. Flynn and her colleagues came to their conclusion after reviewing outcomes in 91 critically ill candidemia patients.
Just 40% (36) had the historic cause of candidemia, Candida albicans, which remains generally susceptible to fluconazole; 25% (23) had C. glabrata, and the rest had C. parapsilosis or other species.
Before those results were known, 53% (48) of patients were treated empirically with fluconazole and 36% (33), with the echinocandin micafungin. Most of the others received no treatment.
Seventy percent (16) of C. glabrata patients got fluconazole, the highest rate in the study of inappropriate initial antifungal therapy; probably not coincidently, 56% (13) of the C. glabrata patients died; the mortality rate in patients with other candida species was 32% (22). On univariate analysis, mortality increased from 18% to 37% if C. glabrata was cultured (P = .04).
"When we looked at glabrata versus all other candida species, we found significant increases in in-hospital mortality" that corresponded to a greater likelihood of inappropriate initial treatment, she said at the annual meeting of the Surgical Infection Society.
For that reason, "we are proposing that initial empiric antifungal therapy start with an echinocandin in the critically ill patient and then deescalate to fluconazole if [indicated by] culture data," she said.
It’s sound advice, so long as "your incidence of Candida glabrata is high," session moderator Dr. Addison May said after the presentation.
"It really depends on your hospital’s rate, and how frequently it’s [isolated]. It’s important to understand what you need to empirically treat with," but also important to use newer agents like micafungin judiciously, to prevent resistance, said Dr. May, professor of surgery and anesthesiology at Vanderbilt University in Nashville, Tenn.
C. glabrata patients were more likely than others to be over 60 years old; they had longer hospital and ICU stays, as well.
The mean APACHE II (Acute Physiology and Chronic Health Evaluation II) score in the study was 25, and the mean age was 57 years; 54% (49) of patients were men, and 68% (62) were black. In the previous month, almost half had surgery and a quarter had been on total parenteral nutrition.
Central lines were the source of infection in 84% (76).
On multivariate analysis, inappropriate initial antifungal treatment, vasopressor therapy, mechanical ventilation, and end-stage renal disease were all significant risk factors for death.
Dr. May and Dr. Flynn said they had no relevant financial disclosures.
LAS VEGAS – An echinocandin should be used for empiric therapy in critically ill candidemia patients awaiting culture results, according to investigators from Wayne State University in Detroit.
The reason is that Candida glabrata is on the rise in the critically ill, and it’s often resistant to fluconazole, the usual empiric choice, said Dr. Lisa Flynn, a vascular surgeon in the department of surgery at the university.
Dr. Flynn and her colleagues came to their conclusion after reviewing outcomes in 91 critically ill candidemia patients.
Just 40% (36) had the historic cause of candidemia, Candida albicans, which remains generally susceptible to fluconazole; 25% (23) had C. glabrata, and the rest had C. parapsilosis or other species.
Before those results were known, 53% (48) of patients were treated empirically with fluconazole and 36% (33), with the echinocandin micafungin. Most of the others received no treatment.
Seventy percent (16) of C. glabrata patients got fluconazole, the highest rate in the study of inappropriate initial antifungal therapy; probably not coincidently, 56% (13) of the C. glabrata patients died; the mortality rate in patients with other candida species was 32% (22). On univariate analysis, mortality increased from 18% to 37% if C. glabrata was cultured (P = .04).
"When we looked at glabrata versus all other candida species, we found significant increases in in-hospital mortality" that corresponded to a greater likelihood of inappropriate initial treatment, she said at the annual meeting of the Surgical Infection Society.
For that reason, "we are proposing that initial empiric antifungal therapy start with an echinocandin in the critically ill patient and then deescalate to fluconazole if [indicated by] culture data," she said.
It’s sound advice, so long as "your incidence of Candida glabrata is high," session moderator Dr. Addison May said after the presentation.
"It really depends on your hospital’s rate, and how frequently it’s [isolated]. It’s important to understand what you need to empirically treat with," but also important to use newer agents like micafungin judiciously, to prevent resistance, said Dr. May, professor of surgery and anesthesiology at Vanderbilt University in Nashville, Tenn.
C. glabrata patients were more likely than others to be over 60 years old; they had longer hospital and ICU stays, as well.
The mean APACHE II (Acute Physiology and Chronic Health Evaluation II) score in the study was 25, and the mean age was 57 years; 54% (49) of patients were men, and 68% (62) were black. In the previous month, almost half had surgery and a quarter had been on total parenteral nutrition.
Central lines were the source of infection in 84% (76).
On multivariate analysis, inappropriate initial antifungal treatment, vasopressor therapy, mechanical ventilation, and end-stage renal disease were all significant risk factors for death.
Dr. May and Dr. Flynn said they had no relevant financial disclosures.
AT THE SIS ANNUAL MEETING
Major finding: Of candidemia patients, 25% had C. glabrata, which is resistant to fluconazole and is associated with in-hospital mortality.
Data Source: A retrospective study of 91 candidemia patients
Disclosures: Dr. May and Dr. Flynn said they had no relevant financial disclosures.
Cholinesterase inhibitors may protect the heart
Patients who used cholinesterase inhibitors had a 38% lower risk of heart attacks, based on a study of Swedish Alzheimer’s disease patients published online June 4 in the European Heart Journal.
"This is an observational study, [so] we cannot say that cholinesterase inhibitor use is causing the reduction in risk, only that it is associated with a reduction. It would be of great value if a meta-analysis of previous, randomized controlled trials could be performed, as this might produce answers on which clinical recommendations could be based," lead investigator Dr. Peter Nordstrom, of Umea University in Sweden, said in a statement.
The findings were not adjusted for statin use, which was unknown.
The investigators cross-linked the records of 7,073 patients in the Swedish Dementia Registry to myocardial infarction and death records in national registries. The results were then adjusted for age, gender, weight, height, dementia type, living conditions, home care, cognitive function, cardiovascular disease history, baseline health, and the use of antidepressants, neuroleptics, antihypertensives, and antidiabetics.
Compared with 1,914 Alzheimer’s patients who never took cholinesterase inhibitors, the 5,159 patients who used the drugs during a mean period of 495 days had a 38% lower risk of myocardial infarction (hazard ratio, 0.62; 95% confidence interval, 0.40-0.95), a 34% lower risk of MI or death (HR, 0.66; 95% CI, 0.56-0.78), a 26% lower risk of death from all cardiovascular causes (HR, 0.74; 95% CI, 0.57-0.97), and a 36% lower risk of death from any cause (HR, 0.64; 95% CI, 0.54-0.76). A case-control analysis produced similar results (Eur. Heart J. 2013 June 4 [doi: 10.1093/eurheartj/eht182]).
Patients who, in their last recorded prescription, were taking higher doses of cholinesterase inhibitors – 10 mg of donepezil, 24 mg of galantamine, or more than 6 mg of rivastigmine daily – had the lowest risk of MI (HR, 0.35; 95% CI, 0.19-0.64) and death (HR, 0.54; 95% CI 0.43-0.67). Among those with baseline cardiovascular disease, high doses of cholinesterase inhibitors reduced MI risk by 71% (HR, 0.29; 95% CI, 0.09-0.94).
Memantine, an Alzheimer’s drug with a different mechanism, was not associated with lower rates of death.
"If you translate these reductions in risk into absolute figures, it means that for every 100,000 people with Alzheimer’s disease, there would be 180 fewer heart attacks – 295 as opposed to 475 – and 1,125 fewer deaths from all causes – 2,000 versus 3,125 – every year among those taking cholinesterase inhibitors compared to those not using them," Dr. Nordstrom said in a statement.
If cholinesterase inhibitors really do protect the heart, it might have something to do with their known anti-inflammatory properties or their effects on the vagus nerve, the investigators noted.
The subjects were enrolled in the Swedish Dementia Registry between May 2007 and December 2010 with newly diagnosed Alzheimer’s disease. Their mean age was 79 years at baseline, about 60% were women, and 831 had an MI or died.
The investigators reported no conflicts of interest. The Swedish Research Council, the Swedish Association of Local Authorities and Regions, and other groups paid for the work.
Patients who used cholinesterase inhibitors had a 38% lower risk of heart attacks, based on a study of Swedish Alzheimer’s disease patients published online June 4 in the European Heart Journal.
"This is an observational study, [so] we cannot say that cholinesterase inhibitor use is causing the reduction in risk, only that it is associated with a reduction. It would be of great value if a meta-analysis of previous, randomized controlled trials could be performed, as this might produce answers on which clinical recommendations could be based," lead investigator Dr. Peter Nordstrom, of Umea University in Sweden, said in a statement.
The findings were not adjusted for statin use, which was unknown.
The investigators cross-linked the records of 7,073 patients in the Swedish Dementia Registry to myocardial infarction and death records in national registries. The results were then adjusted for age, gender, weight, height, dementia type, living conditions, home care, cognitive function, cardiovascular disease history, baseline health, and the use of antidepressants, neuroleptics, antihypertensives, and antidiabetics.
Compared with 1,914 Alzheimer’s patients who never took cholinesterase inhibitors, the 5,159 patients who used the drugs during a mean period of 495 days had a 38% lower risk of myocardial infarction (hazard ratio, 0.62; 95% confidence interval, 0.40-0.95), a 34% lower risk of MI or death (HR, 0.66; 95% CI, 0.56-0.78), a 26% lower risk of death from all cardiovascular causes (HR, 0.74; 95% CI, 0.57-0.97), and a 36% lower risk of death from any cause (HR, 0.64; 95% CI, 0.54-0.76). A case-control analysis produced similar results (Eur. Heart J. 2013 June 4 [doi: 10.1093/eurheartj/eht182]).
Patients who, in their last recorded prescription, were taking higher doses of cholinesterase inhibitors – 10 mg of donepezil, 24 mg of galantamine, or more than 6 mg of rivastigmine daily – had the lowest risk of MI (HR, 0.35; 95% CI, 0.19-0.64) and death (HR, 0.54; 95% CI 0.43-0.67). Among those with baseline cardiovascular disease, high doses of cholinesterase inhibitors reduced MI risk by 71% (HR, 0.29; 95% CI, 0.09-0.94).
Memantine, an Alzheimer’s drug with a different mechanism, was not associated with lower rates of death.
"If you translate these reductions in risk into absolute figures, it means that for every 100,000 people with Alzheimer’s disease, there would be 180 fewer heart attacks – 295 as opposed to 475 – and 1,125 fewer deaths from all causes – 2,000 versus 3,125 – every year among those taking cholinesterase inhibitors compared to those not using them," Dr. Nordstrom said in a statement.
If cholinesterase inhibitors really do protect the heart, it might have something to do with their known anti-inflammatory properties or their effects on the vagus nerve, the investigators noted.
The subjects were enrolled in the Swedish Dementia Registry between May 2007 and December 2010 with newly diagnosed Alzheimer’s disease. Their mean age was 79 years at baseline, about 60% were women, and 831 had an MI or died.
The investigators reported no conflicts of interest. The Swedish Research Council, the Swedish Association of Local Authorities and Regions, and other groups paid for the work.
Patients who used cholinesterase inhibitors had a 38% lower risk of heart attacks, based on a study of Swedish Alzheimer’s disease patients published online June 4 in the European Heart Journal.
"This is an observational study, [so] we cannot say that cholinesterase inhibitor use is causing the reduction in risk, only that it is associated with a reduction. It would be of great value if a meta-analysis of previous, randomized controlled trials could be performed, as this might produce answers on which clinical recommendations could be based," lead investigator Dr. Peter Nordstrom, of Umea University in Sweden, said in a statement.
The findings were not adjusted for statin use, which was unknown.
The investigators cross-linked the records of 7,073 patients in the Swedish Dementia Registry to myocardial infarction and death records in national registries. The results were then adjusted for age, gender, weight, height, dementia type, living conditions, home care, cognitive function, cardiovascular disease history, baseline health, and the use of antidepressants, neuroleptics, antihypertensives, and antidiabetics.
Compared with 1,914 Alzheimer’s patients who never took cholinesterase inhibitors, the 5,159 patients who used the drugs during a mean period of 495 days had a 38% lower risk of myocardial infarction (hazard ratio, 0.62; 95% confidence interval, 0.40-0.95), a 34% lower risk of MI or death (HR, 0.66; 95% CI, 0.56-0.78), a 26% lower risk of death from all cardiovascular causes (HR, 0.74; 95% CI, 0.57-0.97), and a 36% lower risk of death from any cause (HR, 0.64; 95% CI, 0.54-0.76). A case-control analysis produced similar results (Eur. Heart J. 2013 June 4 [doi: 10.1093/eurheartj/eht182]).
Patients who, in their last recorded prescription, were taking higher doses of cholinesterase inhibitors – 10 mg of donepezil, 24 mg of galantamine, or more than 6 mg of rivastigmine daily – had the lowest risk of MI (HR, 0.35; 95% CI, 0.19-0.64) and death (HR, 0.54; 95% CI 0.43-0.67). Among those with baseline cardiovascular disease, high doses of cholinesterase inhibitors reduced MI risk by 71% (HR, 0.29; 95% CI, 0.09-0.94).
Memantine, an Alzheimer’s drug with a different mechanism, was not associated with lower rates of death.
"If you translate these reductions in risk into absolute figures, it means that for every 100,000 people with Alzheimer’s disease, there would be 180 fewer heart attacks – 295 as opposed to 475 – and 1,125 fewer deaths from all causes – 2,000 versus 3,125 – every year among those taking cholinesterase inhibitors compared to those not using them," Dr. Nordstrom said in a statement.
If cholinesterase inhibitors really do protect the heart, it might have something to do with their known anti-inflammatory properties or their effects on the vagus nerve, the investigators noted.
The subjects were enrolled in the Swedish Dementia Registry between May 2007 and December 2010 with newly diagnosed Alzheimer’s disease. Their mean age was 79 years at baseline, about 60% were women, and 831 had an MI or died.
The investigators reported no conflicts of interest. The Swedish Research Council, the Swedish Association of Local Authorities and Regions, and other groups paid for the work.
FROM THE EUROPEAN HEART JOURNAL
Major finding: Alzheimer’s patients who use cholinesterase inhibitors, compared with those who do not, have a 38% reduction in heart attack risk and a 26% reduction in the risk of dying from cardiovascular causes.
Data source: Swedish retrospective, observational study of 7,073 Alzheimer’s patients.
Disclosures: The investigators reported no conflicts of interest. The Swedish Research Council, the Swedish Association of Local Authorities and Regions, and other groups paid for the work.