User login
Vaccine reduced risk for flu visits by 42%
Last year’s influenza vaccination reduced the overall risk for flu-related medical visits by 42%, according to the Centers for Disease Control and Prevention.
In an article summarizing influenza activity in the United States during October 2016–May 2017, investigators said that most of the viral strains antigenically characterized at the CDC “were similar to the reference viruses representing the recommended components for the 2016-2017 vaccine.”
The 2017-2018 influenza vaccine has been updated to include an additional influenza A (H1N1) component. This change was recommended by the Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee, based on data from global influenza virologic and epidemiologic surveillance, genetic and antigenic characterization, human serology studies, antiviral susceptibility, and the availability of candidate influenza viruses (MMWR. 2017;66[25]:668-76).
Preliminary data show that, during the 2016-2017 flu season, there were 18,184 laboratory-confirmed, flu-related hospitalizations, for an overall incidence of 65 per 100,000 population, more than double that for the 2015-2017 season (31/100,000). Broken down by age groups, the rates per 100,000 population in this past season were 44 at ages 0-4 years, 17 at ages 5-17 years, 20 at ages 18-49 years, and 65 at ages 50-64 years, compared with 291 at ages 65 years and older. Finalized estimates of the number of influenza illnesses, medical visits, and hospitalizations averted by vaccination during the 2016-2017 season will be published in December, the investigators said.
Last year’s influenza vaccination reduced the overall risk for flu-related medical visits by 42%, according to the Centers for Disease Control and Prevention.
In an article summarizing influenza activity in the United States during October 2016–May 2017, investigators said that most of the viral strains antigenically characterized at the CDC “were similar to the reference viruses representing the recommended components for the 2016-2017 vaccine.”
The 2017-2018 influenza vaccine has been updated to include an additional influenza A (H1N1) component. This change was recommended by the Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee, based on data from global influenza virologic and epidemiologic surveillance, genetic and antigenic characterization, human serology studies, antiviral susceptibility, and the availability of candidate influenza viruses (MMWR. 2017;66[25]:668-76).
Preliminary data show that, during the 2016-2017 flu season, there were 18,184 laboratory-confirmed, flu-related hospitalizations, for an overall incidence of 65 per 100,000 population, more than double that for the 2015-2017 season (31/100,000). Broken down by age groups, the rates per 100,000 population in this past season were 44 at ages 0-4 years, 17 at ages 5-17 years, 20 at ages 18-49 years, and 65 at ages 50-64 years, compared with 291 at ages 65 years and older. Finalized estimates of the number of influenza illnesses, medical visits, and hospitalizations averted by vaccination during the 2016-2017 season will be published in December, the investigators said.
Last year’s influenza vaccination reduced the overall risk for flu-related medical visits by 42%, according to the Centers for Disease Control and Prevention.
In an article summarizing influenza activity in the United States during October 2016–May 2017, investigators said that most of the viral strains antigenically characterized at the CDC “were similar to the reference viruses representing the recommended components for the 2016-2017 vaccine.”
The 2017-2018 influenza vaccine has been updated to include an additional influenza A (H1N1) component. This change was recommended by the Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee, based on data from global influenza virologic and epidemiologic surveillance, genetic and antigenic characterization, human serology studies, antiviral susceptibility, and the availability of candidate influenza viruses (MMWR. 2017;66[25]:668-76).
Preliminary data show that, during the 2016-2017 flu season, there were 18,184 laboratory-confirmed, flu-related hospitalizations, for an overall incidence of 65 per 100,000 population, more than double that for the 2015-2017 season (31/100,000). Broken down by age groups, the rates per 100,000 population in this past season were 44 at ages 0-4 years, 17 at ages 5-17 years, 20 at ages 18-49 years, and 65 at ages 50-64 years, compared with 291 at ages 65 years and older. Finalized estimates of the number of influenza illnesses, medical visits, and hospitalizations averted by vaccination during the 2016-2017 season will be published in December, the investigators said.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
Key clinical point: Last year’s influenza vaccination reduced the overall risk for flu-related medical visits by 42%.
Major finding: During the 2016-2017 flu season, there were 18,184 laboratory-confirmed, flu-related hospitalizations, for an overall incidence of 65 per 100,000 population.
Data source: A review of data submitted to the Centers for Disease Control and Prevention regarding the 2016-2017 influenza season.
Disclosures: This study was supported by the CDC. Dr. Blanton and her associates reported having no relevant financial disclosures.
Improving diet over time reduces mortality
Improving diet over time consistently reduces all-cause and cardiovascular mortality, according to an analysis of two large U.S. databases.
Researchers examined the association between dietary change over a 12-year period (1986-1998) and subsequent mortality during a further 12 years of follow-up (1998-2010), using data for 47,994 women participating in the Nurses’ Health Study and 25,745 men participating in the Health Professionals Follow-Up Study. They calculated dietary quality using three different methods: the Alternate Healthy Eating Index-2010 score, the Alternate Mediterranean Diet score, and the Dietary Approaches to Stop Hypertension (DASH) diet score.
Through numerous analyses of the data, they found a consistent inverse relationship between dietary quality and mortality. Overall, a 20-percentile increase in dietary quality over time was associated with an 8%-17% reduction in all-cause mortality, regardless of which scoring method was used. In contrast, a similar decline in dietary quality was associated with a 6%-12% increase in mortality.
“The pooled hazard ratios among participants with the greatest improvement in diet quality (13%-33% improvement), as compared with those whose diet quality remained relatively stable (0%-3% improvement) in the 12-year period, were the following: 0.91 according to changes in the Alternate Healthy Eating Index score, 0.84 according to changes in the Alternate Mediterranean Diet score, and 0.89 according to changes in the DASH score,” the investigators said (N Engl J Med. 2017 Jul 13. doi: 10.1056/NEJMoa1613502).
In addition, participants who maintained a high-quality diet throughout the study period also reduced their risk of death from any cause by 9%-14%, compared with those who maintained a poor-quality diet over time.
“Our findings provide support for the recommendation of the 2015 Dietary Guidelines Advisory Committee that it is not necessary to conform to a single diet plan to achieve healthy eating patterns. These three dietary patterns, although different in description and composition, capture the essential elements of a healthy diet. Common food groups in each score that contributed the most to improvements were whole grains, vegetables, fruits, and fish or n-3 fatty acids,” Dr. Sotos-Prieto and her associates noted.
Improving diet over time consistently reduces all-cause and cardiovascular mortality, according to an analysis of two large U.S. databases.
Researchers examined the association between dietary change over a 12-year period (1986-1998) and subsequent mortality during a further 12 years of follow-up (1998-2010), using data for 47,994 women participating in the Nurses’ Health Study and 25,745 men participating in the Health Professionals Follow-Up Study. They calculated dietary quality using three different methods: the Alternate Healthy Eating Index-2010 score, the Alternate Mediterranean Diet score, and the Dietary Approaches to Stop Hypertension (DASH) diet score.
Through numerous analyses of the data, they found a consistent inverse relationship between dietary quality and mortality. Overall, a 20-percentile increase in dietary quality over time was associated with an 8%-17% reduction in all-cause mortality, regardless of which scoring method was used. In contrast, a similar decline in dietary quality was associated with a 6%-12% increase in mortality.
“The pooled hazard ratios among participants with the greatest improvement in diet quality (13%-33% improvement), as compared with those whose diet quality remained relatively stable (0%-3% improvement) in the 12-year period, were the following: 0.91 according to changes in the Alternate Healthy Eating Index score, 0.84 according to changes in the Alternate Mediterranean Diet score, and 0.89 according to changes in the DASH score,” the investigators said (N Engl J Med. 2017 Jul 13. doi: 10.1056/NEJMoa1613502).
In addition, participants who maintained a high-quality diet throughout the study period also reduced their risk of death from any cause by 9%-14%, compared with those who maintained a poor-quality diet over time.
“Our findings provide support for the recommendation of the 2015 Dietary Guidelines Advisory Committee that it is not necessary to conform to a single diet plan to achieve healthy eating patterns. These three dietary patterns, although different in description and composition, capture the essential elements of a healthy diet. Common food groups in each score that contributed the most to improvements were whole grains, vegetables, fruits, and fish or n-3 fatty acids,” Dr. Sotos-Prieto and her associates noted.
Improving diet over time consistently reduces all-cause and cardiovascular mortality, according to an analysis of two large U.S. databases.
Researchers examined the association between dietary change over a 12-year period (1986-1998) and subsequent mortality during a further 12 years of follow-up (1998-2010), using data for 47,994 women participating in the Nurses’ Health Study and 25,745 men participating in the Health Professionals Follow-Up Study. They calculated dietary quality using three different methods: the Alternate Healthy Eating Index-2010 score, the Alternate Mediterranean Diet score, and the Dietary Approaches to Stop Hypertension (DASH) diet score.
Through numerous analyses of the data, they found a consistent inverse relationship between dietary quality and mortality. Overall, a 20-percentile increase in dietary quality over time was associated with an 8%-17% reduction in all-cause mortality, regardless of which scoring method was used. In contrast, a similar decline in dietary quality was associated with a 6%-12% increase in mortality.
“The pooled hazard ratios among participants with the greatest improvement in diet quality (13%-33% improvement), as compared with those whose diet quality remained relatively stable (0%-3% improvement) in the 12-year period, were the following: 0.91 according to changes in the Alternate Healthy Eating Index score, 0.84 according to changes in the Alternate Mediterranean Diet score, and 0.89 according to changes in the DASH score,” the investigators said (N Engl J Med. 2017 Jul 13. doi: 10.1056/NEJMoa1613502).
In addition, participants who maintained a high-quality diet throughout the study period also reduced their risk of death from any cause by 9%-14%, compared with those who maintained a poor-quality diet over time.
“Our findings provide support for the recommendation of the 2015 Dietary Guidelines Advisory Committee that it is not necessary to conform to a single diet plan to achieve healthy eating patterns. These three dietary patterns, although different in description and composition, capture the essential elements of a healthy diet. Common food groups in each score that contributed the most to improvements were whole grains, vegetables, fruits, and fish or n-3 fatty acids,” Dr. Sotos-Prieto and her associates noted.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Improving one’s diet over time consistently reduces all-cause and cardiovascular mortality.
Major finding: A 20-percentile increase in dietary quality over time was associated with an 8%-17% reduction in all-cause mortality, while a similar decline in dietary quality was associated with a 6%-12% increase in mortality.
Data source: A secondary analysis of data for 47,994 women participating in the Nurses’ Health Study and 25,745 men in the Health Professionals Follow-up Study.
Disclosures: This study was funded by the National Institutes of Health. Dr. Sotos-Prieto reported having no relevant financial disclosures. One of her associates reported ties to Metagenics and the California Walnut Commission.
CPAP doesn’t cut rates of CV events, death
Positive airway pressure, whether delivered continuously (CPAP) or as adaptive servoventilation, doesn’t reduce the rate of cardiovascular (CV) events or death in patients who have sleep apnea, according to a report published online July 11 in JAMA.
Positive airway pressure (PAP) relieves the symptoms of sleep apnea and has been reported to improve cardiovascular risk factors such as hypertension, insulin resistance, and endothelial dysfunction. However, whether the treatment improves “hard” vascular outcomes such as stroke and MI has never been established, said Jie Yu, MD, of the department of cardiology, Peking University and the Ministries of Health and Education, Beijing, and his associates.
They performed a systematic review of the literature and a meta-analysis of 10 randomized clinical trials that compared PAP against standard care or a sham treatment and had at least 6 months of follow-up for CV events. The meta-analysis involved 7,266 participants who had either obstructive (5,683 patients) or central (1,583 patients) sleep apnea. There were 356 major adverse CV events and 613 deaths during a median follow-up of 6-68 months.
The use of PAP showed no significant association with a range of outcomes: major adverse CV events (relative risk, 0.77; P = .19), major adverse CV events plus hospitalization for unstable angina (RR, 0.92; P = .54), cardiovascular death (RR, 1.15; P = .30), all-cause mortality (RR, 1.13; P = .08), noncardiovascular death (RR, 0.85; P = .33), acute coronary syndromes (RR, 1.00; P = .99), stroke (RR, 0.90; P = .47), and heart failure (RR, 1.03; P = .60). This lack of treatment benefit persisted regardless of length of follow-up, adherence to treatment, or baseline score on the apnea-hypopnea index, the investigators said (JAMA. 2017 July 11. doi: 10.1001/jama.2017.7967).
PAP also failed to improve blood pressure, body mass index, any lipid parameter, glycemia, or quality-of-life scores on the EQ-5D. It did improve sleepiness and some measures of physical and mental well-being.
“The evidence from these [randomized clinical trials] suggests that the association [between] sleep apnea and vascular outcomes and death ... may represent disease processes that cannot be ameliorated by PAP delivered at the average intensity achieved in these clinical trials or by currently feasible methods in clinical practice,” Dr. Yu and his associates said.
Their findings also “emphasize the importance of proven therapies, such as blood-pressure lowering, lipid lowering, and antiplatelet therapy, in patients with sleep apnea, who should be treated according to established guidelines for patients at elevated cardiovascular risk,” they added.
This study was supported by the National Health and Medical Research Council of Australia. Dr. Yu reported having no relevant financial disclosures. His associates reported ties to numerous industry sources.
The estimated relative risk for the association between PAP and the composite outcome of acute coronary events, stroke, or vascular death was 0.77 in the study by Yu et al. It did not reach statistical significance but is similar to the estimated risk reduction associated with antiplatelet therapy, statins, and beta-blockers in preventing recurrent vascular events.
This magnitude of benefit could be of substantial clinical importance. Far from discouraging further research, this meta-analysis should be an impetus for more studies examining whether treatment of sleep apnea reduces vascular disease risk.
Daniel J. Gottlieb, M.D., is in the Medical Service at the V.A. Boston Healthcare System and in the division of sleep medicine at Harvard. He reported receiving personal fees from VIVUS. Dr. Gottlieb made these remarks in an editorial accompanying Dr. Yu’s report (JAMA. 2017;318:128-30).
The estimated relative risk for the association between PAP and the composite outcome of acute coronary events, stroke, or vascular death was 0.77 in the study by Yu et al. It did not reach statistical significance but is similar to the estimated risk reduction associated with antiplatelet therapy, statins, and beta-blockers in preventing recurrent vascular events.
This magnitude of benefit could be of substantial clinical importance. Far from discouraging further research, this meta-analysis should be an impetus for more studies examining whether treatment of sleep apnea reduces vascular disease risk.
Daniel J. Gottlieb, M.D., is in the Medical Service at the V.A. Boston Healthcare System and in the division of sleep medicine at Harvard. He reported receiving personal fees from VIVUS. Dr. Gottlieb made these remarks in an editorial accompanying Dr. Yu’s report (JAMA. 2017;318:128-30).
The estimated relative risk for the association between PAP and the composite outcome of acute coronary events, stroke, or vascular death was 0.77 in the study by Yu et al. It did not reach statistical significance but is similar to the estimated risk reduction associated with antiplatelet therapy, statins, and beta-blockers in preventing recurrent vascular events.
This magnitude of benefit could be of substantial clinical importance. Far from discouraging further research, this meta-analysis should be an impetus for more studies examining whether treatment of sleep apnea reduces vascular disease risk.
Daniel J. Gottlieb, M.D., is in the Medical Service at the V.A. Boston Healthcare System and in the division of sleep medicine at Harvard. He reported receiving personal fees from VIVUS. Dr. Gottlieb made these remarks in an editorial accompanying Dr. Yu’s report (JAMA. 2017;318:128-30).
Positive airway pressure, whether delivered continuously (CPAP) or as adaptive servoventilation, doesn’t reduce the rate of cardiovascular (CV) events or death in patients who have sleep apnea, according to a report published online July 11 in JAMA.
Positive airway pressure (PAP) relieves the symptoms of sleep apnea and has been reported to improve cardiovascular risk factors such as hypertension, insulin resistance, and endothelial dysfunction. However, whether the treatment improves “hard” vascular outcomes such as stroke and MI has never been established, said Jie Yu, MD, of the department of cardiology, Peking University and the Ministries of Health and Education, Beijing, and his associates.
They performed a systematic review of the literature and a meta-analysis of 10 randomized clinical trials that compared PAP against standard care or a sham treatment and had at least 6 months of follow-up for CV events. The meta-analysis involved 7,266 participants who had either obstructive (5,683 patients) or central (1,583 patients) sleep apnea. There were 356 major adverse CV events and 613 deaths during a median follow-up of 6-68 months.
The use of PAP showed no significant association with a range of outcomes: major adverse CV events (relative risk, 0.77; P = .19), major adverse CV events plus hospitalization for unstable angina (RR, 0.92; P = .54), cardiovascular death (RR, 1.15; P = .30), all-cause mortality (RR, 1.13; P = .08), noncardiovascular death (RR, 0.85; P = .33), acute coronary syndromes (RR, 1.00; P = .99), stroke (RR, 0.90; P = .47), and heart failure (RR, 1.03; P = .60). This lack of treatment benefit persisted regardless of length of follow-up, adherence to treatment, or baseline score on the apnea-hypopnea index, the investigators said (JAMA. 2017 July 11. doi: 10.1001/jama.2017.7967).
PAP also failed to improve blood pressure, body mass index, any lipid parameter, glycemia, or quality-of-life scores on the EQ-5D. It did improve sleepiness and some measures of physical and mental well-being.
“The evidence from these [randomized clinical trials] suggests that the association [between] sleep apnea and vascular outcomes and death ... may represent disease processes that cannot be ameliorated by PAP delivered at the average intensity achieved in these clinical trials or by currently feasible methods in clinical practice,” Dr. Yu and his associates said.
Their findings also “emphasize the importance of proven therapies, such as blood-pressure lowering, lipid lowering, and antiplatelet therapy, in patients with sleep apnea, who should be treated according to established guidelines for patients at elevated cardiovascular risk,” they added.
This study was supported by the National Health and Medical Research Council of Australia. Dr. Yu reported having no relevant financial disclosures. His associates reported ties to numerous industry sources.
Positive airway pressure, whether delivered continuously (CPAP) or as adaptive servoventilation, doesn’t reduce the rate of cardiovascular (CV) events or death in patients who have sleep apnea, according to a report published online July 11 in JAMA.
Positive airway pressure (PAP) relieves the symptoms of sleep apnea and has been reported to improve cardiovascular risk factors such as hypertension, insulin resistance, and endothelial dysfunction. However, whether the treatment improves “hard” vascular outcomes such as stroke and MI has never been established, said Jie Yu, MD, of the department of cardiology, Peking University and the Ministries of Health and Education, Beijing, and his associates.
They performed a systematic review of the literature and a meta-analysis of 10 randomized clinical trials that compared PAP against standard care or a sham treatment and had at least 6 months of follow-up for CV events. The meta-analysis involved 7,266 participants who had either obstructive (5,683 patients) or central (1,583 patients) sleep apnea. There were 356 major adverse CV events and 613 deaths during a median follow-up of 6-68 months.
The use of PAP showed no significant association with a range of outcomes: major adverse CV events (relative risk, 0.77; P = .19), major adverse CV events plus hospitalization for unstable angina (RR, 0.92; P = .54), cardiovascular death (RR, 1.15; P = .30), all-cause mortality (RR, 1.13; P = .08), noncardiovascular death (RR, 0.85; P = .33), acute coronary syndromes (RR, 1.00; P = .99), stroke (RR, 0.90; P = .47), and heart failure (RR, 1.03; P = .60). This lack of treatment benefit persisted regardless of length of follow-up, adherence to treatment, or baseline score on the apnea-hypopnea index, the investigators said (JAMA. 2017 July 11. doi: 10.1001/jama.2017.7967).
PAP also failed to improve blood pressure, body mass index, any lipid parameter, glycemia, or quality-of-life scores on the EQ-5D. It did improve sleepiness and some measures of physical and mental well-being.
“The evidence from these [randomized clinical trials] suggests that the association [between] sleep apnea and vascular outcomes and death ... may represent disease processes that cannot be ameliorated by PAP delivered at the average intensity achieved in these clinical trials or by currently feasible methods in clinical practice,” Dr. Yu and his associates said.
Their findings also “emphasize the importance of proven therapies, such as blood-pressure lowering, lipid lowering, and antiplatelet therapy, in patients with sleep apnea, who should be treated according to established guidelines for patients at elevated cardiovascular risk,” they added.
This study was supported by the National Health and Medical Research Council of Australia. Dr. Yu reported having no relevant financial disclosures. His associates reported ties to numerous industry sources.
FROM JAMA
Key clinical point: Positive airway pressure, whether delivered continuously (CPAP) or as adaptive servoventilation, doesn’t reduce the rate of cardiovascular events or death in patients with sleep apnea.
Major finding: The use of PAP showed no association with a range of outcomes: major adverse CV events (RR, 0.77), major adverse CV events plus hospitalization for unstable angina (RR, 0.92), cardiovascular death (RR, 1.15), all-cause mortality (RR, 1.13), noncardiovascular death (RR, 0.85), acute coronary syndromes (RR, 1.00), stroke (RR, 0.90), and heart failure (RR, 1.03).
Data source: A meta-analysis of 10 randomized clinical trials involving 7,266 adults who had either central or obstructive sleep apnea.
Disclosures: This study was supported by the National Health and Medical Research Council of Australia. Dr. Yu reported having no relevant financial disclosures. His associates reported ties to numerous industry sources.
One-year cost of preeclampsia tops $2 billion
The cost of medical care for preeclampsia is estimated to be $2.18 billion for the first year surrounding delivery, split almost evenly between care for mothers and infants, according to a new report.
Preeclampsia is increasing at a more rapid rate than diabetes, ischemic heart failure, Alzheimer’s disease, obesity, and chronic kidney disease – disorders for which substantially more funding has been allocated for research and treatment, said Warren Stevens, PhD, of Precision Health Economics, Los Angeles, and his associates. Precision Health Economics provides consulting and other research services to pharmaceutical, device, government, and nongovernmental groups.
Compared with a healthy pregnancy, preeclampsia increased the probability that a mother would have at least one adverse outcome from 4.6% to 10.1%, and it increased the probability that an infant would have at least one adverse outcome from 7.9% to 14.2%. For mothers, preeclampsia was estimated to raise costs by $6,583 per birth, which translates to an increase of $1.03 billion for U.S. mothers during a single year (2012). For infants, the disorder raised costs by $1.15 billion during that year.
Infant costs varied according to the gestational age at delivery, ranging from $1,311 per birth at 36 weeks’ gestation to $150,000 per birth at 26 weeks’ gestation, Dr. Stevens and his associates reported (Am J Obstet Gynecol. 2017 Jul 11. doi: 10.1016/j.ajog.2017.04.032).
This study could not take into account longer-term adverse outcomes associated with preeclampsia. Women with a history of the disorder are at double the risk of developing ischemic heart disease or cerebrovascular disease compared with women without such a history, and at three times the risk of developing hypertension. Infants are at increased risk of developing stroke, metabolic syndrome, and chronic heart disease, the investigators noted.
The study was supported by rEVO Biologics. Five of the study authors are employees of rEVO Biologics and one author is a steering committee member for a clinical trial supported by the company; another study author is a consultant at Precision Health Economics.
Quantifying the total cost of a health problem helps to show the public, payers, and health care administrators the magnitude of the problem on a population level. By underscoring the economic burden of preeclampsia, Stevens et al. provided important information about the high costs of this condition.
However, we need to go beyond health burden and cost-of-illness studies when considering the value of interventions to prevent and better manage preeclampsia and its adverse outcomes. Cost of the intervention and the cost savings associated with preventing one case of the condition are important parameters that can be used to calculate the potential savings of interventions. In fact, if the lifetimes costs of caring for children with adverse outcomes of preeclampsia are included, the potential cost savings from effective interventions may be even greater.
Rui Li, PhD, and William M. Callaghan, MD, are in the division of reproductive health at the Centers for Disease Control and Prevention, Atlanta. Eleni Tsigas is with the Preeclampsia Foundation in Melbourne, Fla. They reported having no relevant financial disclosures. These remarks are adapted from an editorial (Am J Obstet Gynecol. 2017 Jul 11. doi: 10.1016/j.ajog.2017.06.011).
Quantifying the total cost of a health problem helps to show the public, payers, and health care administrators the magnitude of the problem on a population level. By underscoring the economic burden of preeclampsia, Stevens et al. provided important information about the high costs of this condition.
However, we need to go beyond health burden and cost-of-illness studies when considering the value of interventions to prevent and better manage preeclampsia and its adverse outcomes. Cost of the intervention and the cost savings associated with preventing one case of the condition are important parameters that can be used to calculate the potential savings of interventions. In fact, if the lifetimes costs of caring for children with adverse outcomes of preeclampsia are included, the potential cost savings from effective interventions may be even greater.
Rui Li, PhD, and William M. Callaghan, MD, are in the division of reproductive health at the Centers for Disease Control and Prevention, Atlanta. Eleni Tsigas is with the Preeclampsia Foundation in Melbourne, Fla. They reported having no relevant financial disclosures. These remarks are adapted from an editorial (Am J Obstet Gynecol. 2017 Jul 11. doi: 10.1016/j.ajog.2017.06.011).
Quantifying the total cost of a health problem helps to show the public, payers, and health care administrators the magnitude of the problem on a population level. By underscoring the economic burden of preeclampsia, Stevens et al. provided important information about the high costs of this condition.
However, we need to go beyond health burden and cost-of-illness studies when considering the value of interventions to prevent and better manage preeclampsia and its adverse outcomes. Cost of the intervention and the cost savings associated with preventing one case of the condition are important parameters that can be used to calculate the potential savings of interventions. In fact, if the lifetimes costs of caring for children with adverse outcomes of preeclampsia are included, the potential cost savings from effective interventions may be even greater.
Rui Li, PhD, and William M. Callaghan, MD, are in the division of reproductive health at the Centers for Disease Control and Prevention, Atlanta. Eleni Tsigas is with the Preeclampsia Foundation in Melbourne, Fla. They reported having no relevant financial disclosures. These remarks are adapted from an editorial (Am J Obstet Gynecol. 2017 Jul 11. doi: 10.1016/j.ajog.2017.06.011).
The cost of medical care for preeclampsia is estimated to be $2.18 billion for the first year surrounding delivery, split almost evenly between care for mothers and infants, according to a new report.
Preeclampsia is increasing at a more rapid rate than diabetes, ischemic heart failure, Alzheimer’s disease, obesity, and chronic kidney disease – disorders for which substantially more funding has been allocated for research and treatment, said Warren Stevens, PhD, of Precision Health Economics, Los Angeles, and his associates. Precision Health Economics provides consulting and other research services to pharmaceutical, device, government, and nongovernmental groups.
Compared with a healthy pregnancy, preeclampsia increased the probability that a mother would have at least one adverse outcome from 4.6% to 10.1%, and it increased the probability that an infant would have at least one adverse outcome from 7.9% to 14.2%. For mothers, preeclampsia was estimated to raise costs by $6,583 per birth, which translates to an increase of $1.03 billion for U.S. mothers during a single year (2012). For infants, the disorder raised costs by $1.15 billion during that year.
Infant costs varied according to the gestational age at delivery, ranging from $1,311 per birth at 36 weeks’ gestation to $150,000 per birth at 26 weeks’ gestation, Dr. Stevens and his associates reported (Am J Obstet Gynecol. 2017 Jul 11. doi: 10.1016/j.ajog.2017.04.032).
This study could not take into account longer-term adverse outcomes associated with preeclampsia. Women with a history of the disorder are at double the risk of developing ischemic heart disease or cerebrovascular disease compared with women without such a history, and at three times the risk of developing hypertension. Infants are at increased risk of developing stroke, metabolic syndrome, and chronic heart disease, the investigators noted.
The study was supported by rEVO Biologics. Five of the study authors are employees of rEVO Biologics and one author is a steering committee member for a clinical trial supported by the company; another study author is a consultant at Precision Health Economics.
The cost of medical care for preeclampsia is estimated to be $2.18 billion for the first year surrounding delivery, split almost evenly between care for mothers and infants, according to a new report.
Preeclampsia is increasing at a more rapid rate than diabetes, ischemic heart failure, Alzheimer’s disease, obesity, and chronic kidney disease – disorders for which substantially more funding has been allocated for research and treatment, said Warren Stevens, PhD, of Precision Health Economics, Los Angeles, and his associates. Precision Health Economics provides consulting and other research services to pharmaceutical, device, government, and nongovernmental groups.
Compared with a healthy pregnancy, preeclampsia increased the probability that a mother would have at least one adverse outcome from 4.6% to 10.1%, and it increased the probability that an infant would have at least one adverse outcome from 7.9% to 14.2%. For mothers, preeclampsia was estimated to raise costs by $6,583 per birth, which translates to an increase of $1.03 billion for U.S. mothers during a single year (2012). For infants, the disorder raised costs by $1.15 billion during that year.
Infant costs varied according to the gestational age at delivery, ranging from $1,311 per birth at 36 weeks’ gestation to $150,000 per birth at 26 weeks’ gestation, Dr. Stevens and his associates reported (Am J Obstet Gynecol. 2017 Jul 11. doi: 10.1016/j.ajog.2017.04.032).
This study could not take into account longer-term adverse outcomes associated with preeclampsia. Women with a history of the disorder are at double the risk of developing ischemic heart disease or cerebrovascular disease compared with women without such a history, and at three times the risk of developing hypertension. Infants are at increased risk of developing stroke, metabolic syndrome, and chronic heart disease, the investigators noted.
The study was supported by rEVO Biologics. Five of the study authors are employees of rEVO Biologics and one author is a steering committee member for a clinical trial supported by the company; another study author is a consultant at Precision Health Economics.
FROM THE AMERICAN JOURNAL OF OBSTETRICS & GYNECOLOGY
Key clinical point:
Major finding: Compared with healthy pregnancy, preeclampsia increased the probability that a mother would have at least one adverse outcome from 4.6% to 10.1%, and it increased the probability that an infant would have at least one adverse outcome from 7.9% to 14.2%.
Data source: A retrospective cohort study using secondary analysis of numerous data sets and extrapolating from approximately 2 million births in 2012 (1,918,498 births without preeclampsia and 69,193 with preeclampsia).
Disclosures: The study was supported by rEVO Biologics. Five of the study authors are employees of Precision Health Economics and one author is a consultant there; another author is on the steering committee for a clinical trial supported by rEVO Biologics.
OMV meningococcal vaccine also protected against gonorrhea
A group B meningococcal outer-membrane-vesicle (OMV) vaccine used during a meningitis outbreak in New Zealand also protected against gonorrhea, according to a report published online July 10 in the Lancet.
Even though Neisseria meningitidis and Neisseria gonorrhoeae cause distinctly different diseases, the bacteria are closely related and are genetically and antigenically very similar. Most of the virulence factors present in one pathogen have an equivalent in the other, “providing at least one biologically plausible mechanism for cross-protection,” said Helen Petousis-Harris, PhD, of the department of general practice and primary health care, University of Auckland (New Zealand), and her associates.
Approximately 1 million people – 81% of the New Zealand population younger than 20 years – received almost 3 million doses of the OMV meningococcal B vaccine (MeNZB) in a 2-year mass immunization program during the outbreak, allowing the investigators to compare the rate of gonorrhea between vaccinated and unvaccinated people. They performed a retrospective case-control study involving 14,730 participants, using information from a national health care database, a national immunization registry, and 11 sexual health clinics covering diverse geographic regions. This included 1,241 cases of gonorrhea (cases), 12,487 cases of chlamydia (controls), and 1,002 cases of gonorrhea plus chlamydia coinfection (categorized as controls or cases in separate analyses).
“The adjusted estimate for vaccine effectiveness of the MeNZB against confirmed cases of gonorrhea” was 31% (95% confidence interval, 21-39; P less than .0001), a finding that remained robust across several sensitivity analyses, Dr. Petousis-Harris and her associates said (Lancet. 2017 July 10. doi: 10.1016/S0140-6736(17)31449-6).
“To our knowledge, ours is the first study to show an association between a vaccine and a reduction in the risk of gonorrhea,” they noted. “The potential ability of an OMV group B meningococcal vaccine to provide even modest protection against gonorrhea would have substantial public health benefits in view of the prevalence of gonorrhea. Modeling suggests that a vaccine with 30% efficacy could decrease the prevalence of gonorrhea by more than 30% within 15 years, if immunity is maintained.”
These findings also are important in view of the organism’s increasing resistance to existing antibiotics. Moreover, if further study confirms that the MeNZB vaccine offers some degree of cross-protection against gonorrhea, these data can inform the development of a gonorrhea vaccine, the investigators added.
This study was funded by GlaxoSmithKline Vaccines and Auckland UniServices. Dr. Petousis-Harris reported serving as a consultant for GSK, Merck, and Pfizer, and one of her associates reported ties to Novartis Vaccines, GSK, Protein Sciences, and Merck.
Over decades of research, all of the attempts to create a vaccine against gonorrhea have failed, largely because of the variable nature of Neisseria gonorrhoeae antigens and the failure of the bacteria to induce a protective immune response, so the findings of Dr. Petousis-Harris and her associates are “a step in the right direction” and should reinvigorate interest and investment in this endeavor.
Although MeNZB is no longer available, another meningococcal vaccine (4CMenB, Bexsero) contains the same outer-membrane-vesicle antigen and three of the same recombinant proteins. As the authors pointed out, immunizing adolescents with this vaccine could reduce the rate of gonorrhea substantially, even if it has only moderate efficacy and duration of effect. In particular, reducing the pool of asymptomatic carriers would decrease both transmission and the severe sequelae that develop when the infection goes undetected.
Kate L. Seib, PhD, is a microbiologist at the Institute for Glycomics at Griffith University in Southport, Australia. She reported support by a career development fellowship from the Australian National Health and Medical Research Council. Dr. Seib made these remarks in an accompanying editorial comment (Lancet. 2017 July 10. doi: 10.1016/S0140-6736(17)31605-7).
Over decades of research, all of the attempts to create a vaccine against gonorrhea have failed, largely because of the variable nature of Neisseria gonorrhoeae antigens and the failure of the bacteria to induce a protective immune response, so the findings of Dr. Petousis-Harris and her associates are “a step in the right direction” and should reinvigorate interest and investment in this endeavor.
Although MeNZB is no longer available, another meningococcal vaccine (4CMenB, Bexsero) contains the same outer-membrane-vesicle antigen and three of the same recombinant proteins. As the authors pointed out, immunizing adolescents with this vaccine could reduce the rate of gonorrhea substantially, even if it has only moderate efficacy and duration of effect. In particular, reducing the pool of asymptomatic carriers would decrease both transmission and the severe sequelae that develop when the infection goes undetected.
Kate L. Seib, PhD, is a microbiologist at the Institute for Glycomics at Griffith University in Southport, Australia. She reported support by a career development fellowship from the Australian National Health and Medical Research Council. Dr. Seib made these remarks in an accompanying editorial comment (Lancet. 2017 July 10. doi: 10.1016/S0140-6736(17)31605-7).
Over decades of research, all of the attempts to create a vaccine against gonorrhea have failed, largely because of the variable nature of Neisseria gonorrhoeae antigens and the failure of the bacteria to induce a protective immune response, so the findings of Dr. Petousis-Harris and her associates are “a step in the right direction” and should reinvigorate interest and investment in this endeavor.
Although MeNZB is no longer available, another meningococcal vaccine (4CMenB, Bexsero) contains the same outer-membrane-vesicle antigen and three of the same recombinant proteins. As the authors pointed out, immunizing adolescents with this vaccine could reduce the rate of gonorrhea substantially, even if it has only moderate efficacy and duration of effect. In particular, reducing the pool of asymptomatic carriers would decrease both transmission and the severe sequelae that develop when the infection goes undetected.
Kate L. Seib, PhD, is a microbiologist at the Institute for Glycomics at Griffith University in Southport, Australia. She reported support by a career development fellowship from the Australian National Health and Medical Research Council. Dr. Seib made these remarks in an accompanying editorial comment (Lancet. 2017 July 10. doi: 10.1016/S0140-6736(17)31605-7).
A group B meningococcal outer-membrane-vesicle (OMV) vaccine used during a meningitis outbreak in New Zealand also protected against gonorrhea, according to a report published online July 10 in the Lancet.
Even though Neisseria meningitidis and Neisseria gonorrhoeae cause distinctly different diseases, the bacteria are closely related and are genetically and antigenically very similar. Most of the virulence factors present in one pathogen have an equivalent in the other, “providing at least one biologically plausible mechanism for cross-protection,” said Helen Petousis-Harris, PhD, of the department of general practice and primary health care, University of Auckland (New Zealand), and her associates.
Approximately 1 million people – 81% of the New Zealand population younger than 20 years – received almost 3 million doses of the OMV meningococcal B vaccine (MeNZB) in a 2-year mass immunization program during the outbreak, allowing the investigators to compare the rate of gonorrhea between vaccinated and unvaccinated people. They performed a retrospective case-control study involving 14,730 participants, using information from a national health care database, a national immunization registry, and 11 sexual health clinics covering diverse geographic regions. This included 1,241 cases of gonorrhea (cases), 12,487 cases of chlamydia (controls), and 1,002 cases of gonorrhea plus chlamydia coinfection (categorized as controls or cases in separate analyses).
“The adjusted estimate for vaccine effectiveness of the MeNZB against confirmed cases of gonorrhea” was 31% (95% confidence interval, 21-39; P less than .0001), a finding that remained robust across several sensitivity analyses, Dr. Petousis-Harris and her associates said (Lancet. 2017 July 10. doi: 10.1016/S0140-6736(17)31449-6).
“To our knowledge, ours is the first study to show an association between a vaccine and a reduction in the risk of gonorrhea,” they noted. “The potential ability of an OMV group B meningococcal vaccine to provide even modest protection against gonorrhea would have substantial public health benefits in view of the prevalence of gonorrhea. Modeling suggests that a vaccine with 30% efficacy could decrease the prevalence of gonorrhea by more than 30% within 15 years, if immunity is maintained.”
These findings also are important in view of the organism’s increasing resistance to existing antibiotics. Moreover, if further study confirms that the MeNZB vaccine offers some degree of cross-protection against gonorrhea, these data can inform the development of a gonorrhea vaccine, the investigators added.
This study was funded by GlaxoSmithKline Vaccines and Auckland UniServices. Dr. Petousis-Harris reported serving as a consultant for GSK, Merck, and Pfizer, and one of her associates reported ties to Novartis Vaccines, GSK, Protein Sciences, and Merck.
A group B meningococcal outer-membrane-vesicle (OMV) vaccine used during a meningitis outbreak in New Zealand also protected against gonorrhea, according to a report published online July 10 in the Lancet.
Even though Neisseria meningitidis and Neisseria gonorrhoeae cause distinctly different diseases, the bacteria are closely related and are genetically and antigenically very similar. Most of the virulence factors present in one pathogen have an equivalent in the other, “providing at least one biologically plausible mechanism for cross-protection,” said Helen Petousis-Harris, PhD, of the department of general practice and primary health care, University of Auckland (New Zealand), and her associates.
Approximately 1 million people – 81% of the New Zealand population younger than 20 years – received almost 3 million doses of the OMV meningococcal B vaccine (MeNZB) in a 2-year mass immunization program during the outbreak, allowing the investigators to compare the rate of gonorrhea between vaccinated and unvaccinated people. They performed a retrospective case-control study involving 14,730 participants, using information from a national health care database, a national immunization registry, and 11 sexual health clinics covering diverse geographic regions. This included 1,241 cases of gonorrhea (cases), 12,487 cases of chlamydia (controls), and 1,002 cases of gonorrhea plus chlamydia coinfection (categorized as controls or cases in separate analyses).
“The adjusted estimate for vaccine effectiveness of the MeNZB against confirmed cases of gonorrhea” was 31% (95% confidence interval, 21-39; P less than .0001), a finding that remained robust across several sensitivity analyses, Dr. Petousis-Harris and her associates said (Lancet. 2017 July 10. doi: 10.1016/S0140-6736(17)31449-6).
“To our knowledge, ours is the first study to show an association between a vaccine and a reduction in the risk of gonorrhea,” they noted. “The potential ability of an OMV group B meningococcal vaccine to provide even modest protection against gonorrhea would have substantial public health benefits in view of the prevalence of gonorrhea. Modeling suggests that a vaccine with 30% efficacy could decrease the prevalence of gonorrhea by more than 30% within 15 years, if immunity is maintained.”
These findings also are important in view of the organism’s increasing resistance to existing antibiotics. Moreover, if further study confirms that the MeNZB vaccine offers some degree of cross-protection against gonorrhea, these data can inform the development of a gonorrhea vaccine, the investigators added.
This study was funded by GlaxoSmithKline Vaccines and Auckland UniServices. Dr. Petousis-Harris reported serving as a consultant for GSK, Merck, and Pfizer, and one of her associates reported ties to Novartis Vaccines, GSK, Protein Sciences, and Merck.
FROM THE LANCET
Key clinical point:
Major finding: The adjusted estimate for the effectiveness of the MeNZB vaccine against cases of gonorrhea was 31%.
Data source: A retrospective case-control study involving 14,730 patients at 11 sexual health clinics across New Zealand.
Disclosures: This study was funded by GlaxoSmithKline Vaccines and Auckland UniServices. Dr. Petousis-Harris reported serving as a consultant for GSK, Merck, and Pfizer, and one of her associates reported ties to Novartis Vaccines, GSK, Protein Sciences, and Merck.
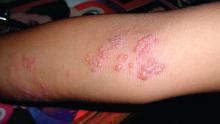
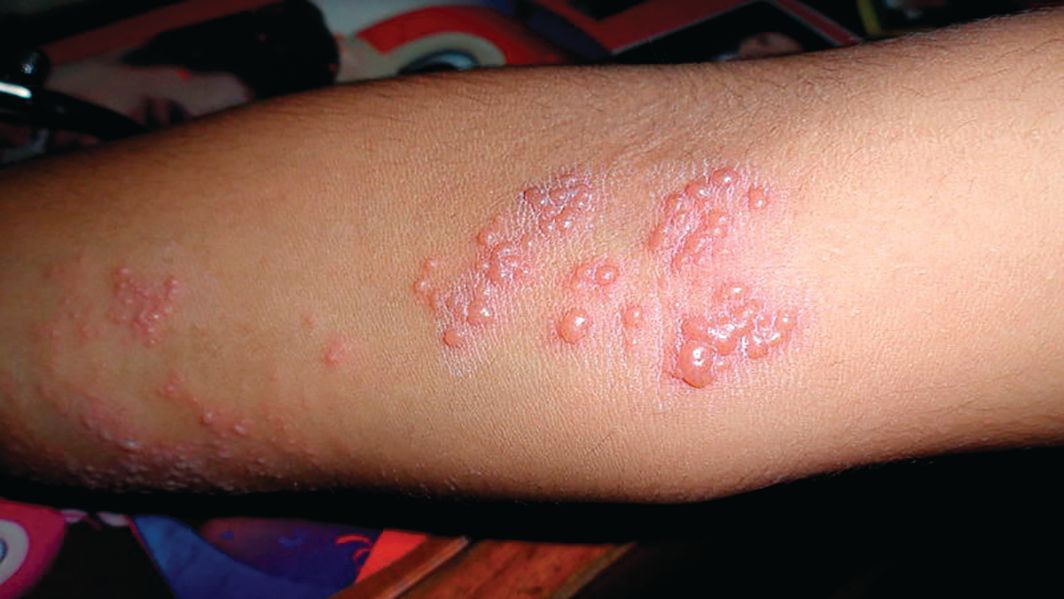
Subset of pediatric herpes simplex virus entails frequent episodes
There’s a newly identified, atypical, but substantial subset of patients: Young children who have frequent episodes of herpes simplex virus (HSV) that may not affect mucosal tissue, often are multifocal, and may require suppressive therapy, according to a report published in Pediatric Dermatology.
“Most of our knowledge of the clinical manifestations of HSV infections in children derives from cases of common mucosal HSV that present to and are treated by primary care providers,” said Julia K. Gittler, MD, and her associates at New York University.
These cases comprise primary herpetic gingivostomatitis or the well-known orolabial HSV. To characterize the more atypical presentations in the pediatric population, the investigators reviewed the charts of all 48 patients referred to their pediatric dermatology clinic in a 10-year period after receiving a diagnosis of HSV. Only 19% of these patients were older than 11 years of age, and more than 40% were younger than 2 years of age at presentation.
Dr. Gittler and her associates found a “substantial” subset of 19 patients (approximately 40% of the study sample) who had six or more outbreaks per year. Only four patients (8.3%) had one or fewer outbreaks per year. The mean frequency of outbreaks in their study population was seven per year. “Reported triggers for outbreaks included fever, viral infections, sun, cold, stress, vaccinations, and courses of oral corticosteroids for asthma flares,” the investigators said.
Given this high frequency of outbreaks, antiviral therapy was initiated in 16 patients (33%). The average age of this treatment initiation was only 6 years. “In the general population, only approximately 15%-40% of patients with serologic evidence of HSV-1 have recurrent HSV; most of these will have an average of 2 outbreaks per year, and only 5%-10% have more than 6 outbreaks per year,” the investigators wrote (Pediatr Dermatol. 2017. doi: 10.1111/pde.13190).
The majority of the study participants (29 patients) had no labial or mucosal involvement. Approximately 23% had cutaneous involvement of the cheek, “which may have been because the primary infection was acquired through the kiss of a caregiver,” they said. Other sites of cutaneous lesions that were frequent in this population but are considered atypical in general were the ear, forehead, chest, and knees.
Most commonly, the physical examination findings were vesicles or papulovesicles (52%), but crusting (33%), pustules (6%), and erosions (23%) also occurred.
Fully half of the study population had multifocal rather than unifocal presentations.
Atypical presentation of HSV is frequently misdiagnosed as impetigo or herpes zoster, according to reports in the literature. Fifteen (31%) patients in this study had a previous misdiagnosis of impetigo. “These less typical cutaneous presentations of HSV may evade accurate diagnosis and delay initiation of appropriate treatment,” Dr. Gittler and her associates noted.
No financial disclosures were provided for Dr. Gittler and her associates.
There’s a newly identified, atypical, but substantial subset of patients: Young children who have frequent episodes of herpes simplex virus (HSV) that may not affect mucosal tissue, often are multifocal, and may require suppressive therapy, according to a report published in Pediatric Dermatology.
“Most of our knowledge of the clinical manifestations of HSV infections in children derives from cases of common mucosal HSV that present to and are treated by primary care providers,” said Julia K. Gittler, MD, and her associates at New York University.
These cases comprise primary herpetic gingivostomatitis or the well-known orolabial HSV. To characterize the more atypical presentations in the pediatric population, the investigators reviewed the charts of all 48 patients referred to their pediatric dermatology clinic in a 10-year period after receiving a diagnosis of HSV. Only 19% of these patients were older than 11 years of age, and more than 40% were younger than 2 years of age at presentation.
Dr. Gittler and her associates found a “substantial” subset of 19 patients (approximately 40% of the study sample) who had six or more outbreaks per year. Only four patients (8.3%) had one or fewer outbreaks per year. The mean frequency of outbreaks in their study population was seven per year. “Reported triggers for outbreaks included fever, viral infections, sun, cold, stress, vaccinations, and courses of oral corticosteroids for asthma flares,” the investigators said.
Given this high frequency of outbreaks, antiviral therapy was initiated in 16 patients (33%). The average age of this treatment initiation was only 6 years. “In the general population, only approximately 15%-40% of patients with serologic evidence of HSV-1 have recurrent HSV; most of these will have an average of 2 outbreaks per year, and only 5%-10% have more than 6 outbreaks per year,” the investigators wrote (Pediatr Dermatol. 2017. doi: 10.1111/pde.13190).
The majority of the study participants (29 patients) had no labial or mucosal involvement. Approximately 23% had cutaneous involvement of the cheek, “which may have been because the primary infection was acquired through the kiss of a caregiver,” they said. Other sites of cutaneous lesions that were frequent in this population but are considered atypical in general were the ear, forehead, chest, and knees.
Most commonly, the physical examination findings were vesicles or papulovesicles (52%), but crusting (33%), pustules (6%), and erosions (23%) also occurred.
Fully half of the study population had multifocal rather than unifocal presentations.
Atypical presentation of HSV is frequently misdiagnosed as impetigo or herpes zoster, according to reports in the literature. Fifteen (31%) patients in this study had a previous misdiagnosis of impetigo. “These less typical cutaneous presentations of HSV may evade accurate diagnosis and delay initiation of appropriate treatment,” Dr. Gittler and her associates noted.
No financial disclosures were provided for Dr. Gittler and her associates.
There’s a newly identified, atypical, but substantial subset of patients: Young children who have frequent episodes of herpes simplex virus (HSV) that may not affect mucosal tissue, often are multifocal, and may require suppressive therapy, according to a report published in Pediatric Dermatology.
“Most of our knowledge of the clinical manifestations of HSV infections in children derives from cases of common mucosal HSV that present to and are treated by primary care providers,” said Julia K. Gittler, MD, and her associates at New York University.
These cases comprise primary herpetic gingivostomatitis or the well-known orolabial HSV. To characterize the more atypical presentations in the pediatric population, the investigators reviewed the charts of all 48 patients referred to their pediatric dermatology clinic in a 10-year period after receiving a diagnosis of HSV. Only 19% of these patients were older than 11 years of age, and more than 40% were younger than 2 years of age at presentation.
Dr. Gittler and her associates found a “substantial” subset of 19 patients (approximately 40% of the study sample) who had six or more outbreaks per year. Only four patients (8.3%) had one or fewer outbreaks per year. The mean frequency of outbreaks in their study population was seven per year. “Reported triggers for outbreaks included fever, viral infections, sun, cold, stress, vaccinations, and courses of oral corticosteroids for asthma flares,” the investigators said.
Given this high frequency of outbreaks, antiviral therapy was initiated in 16 patients (33%). The average age of this treatment initiation was only 6 years. “In the general population, only approximately 15%-40% of patients with serologic evidence of HSV-1 have recurrent HSV; most of these will have an average of 2 outbreaks per year, and only 5%-10% have more than 6 outbreaks per year,” the investigators wrote (Pediatr Dermatol. 2017. doi: 10.1111/pde.13190).
The majority of the study participants (29 patients) had no labial or mucosal involvement. Approximately 23% had cutaneous involvement of the cheek, “which may have been because the primary infection was acquired through the kiss of a caregiver,” they said. Other sites of cutaneous lesions that were frequent in this population but are considered atypical in general were the ear, forehead, chest, and knees.
Most commonly, the physical examination findings were vesicles or papulovesicles (52%), but crusting (33%), pustules (6%), and erosions (23%) also occurred.
Fully half of the study population had multifocal rather than unifocal presentations.
Atypical presentation of HSV is frequently misdiagnosed as impetigo or herpes zoster, according to reports in the literature. Fifteen (31%) patients in this study had a previous misdiagnosis of impetigo. “These less typical cutaneous presentations of HSV may evade accurate diagnosis and delay initiation of appropriate treatment,” Dr. Gittler and her associates noted.
No financial disclosures were provided for Dr. Gittler and her associates.
FROM PEDIATRIC DERMATOLOGY
Key clinical point:
Major finding: 19 of 48 (40%) patients had six or more HSV outbreaks per year, and the mean frequency of outbreaks in this study population was seven per year.
Data source: Chart review of 48 cases referred to a single pediatric dermatology clinic in a 10-year period.
Disclosures: No financial disclosures were provided for Dr. Gittler and her associates.
Herpes zoster raises risk of stroke, MI
Herpes zoster infection raised the risk of stroke by 35% and of myocardial infarction by 59%, in a South Korean nationwide study.
In addition, the risks of stroke and MI were highest in the first year following herpes zoster infection and decreased over time, said Min-Chul Kim, MD, the University of Ulsan, Seoul, South Korea, and associates.
The incidence of stroke was 1.34 per 1,000 person-years higher among patients with the infection than among those without it. Similarly, the incidence of MI was 0.80 per 1,000 person-years higher among patients with the infection than among those without it. The risks of both stroke and MI were highest during the first year after herpes onset and decreased over time. In contrast, the risks of stroke and MI were evenly distributed over time in the control group, the investigators said in a letter to the editor (J Am Coll Cardiol. 2017;70[2]:293-300). Several possible reasons have been proposed to explain a causal association between this infection and cardiovascular disease. Herpes zoster is “the only virus for which there is clear evidence of viral DNA and antigen in areas of ischemia or infarction in cerebral arteries,” Dr. Kim and associates noted.
Viral replication adjacent to a cerebral or cardiac artery could cause inflammation of the vessel, followed by subsequent thrombosis and rupture. Or, repeated subclinical reactivation of the virus could weaken the arteries. It is also possible that herpes zoster reactivation alters patients’ immunologic status, making them vulnerable to cerebrovascular or cardiovascular events, the investigators said.
This study was supported by the Korea Health Technology Research and Development Project, the Korea Health Industry Development Institute, and the Republic of Korea Ministry of Health & Welfare. Dr. Kim and associates reported having no relevant financial disclosures.
Herpes zoster infection raised the risk of stroke by 35% and of myocardial infarction by 59%, in a South Korean nationwide study.
In addition, the risks of stroke and MI were highest in the first year following herpes zoster infection and decreased over time, said Min-Chul Kim, MD, the University of Ulsan, Seoul, South Korea, and associates.
The incidence of stroke was 1.34 per 1,000 person-years higher among patients with the infection than among those without it. Similarly, the incidence of MI was 0.80 per 1,000 person-years higher among patients with the infection than among those without it. The risks of both stroke and MI were highest during the first year after herpes onset and decreased over time. In contrast, the risks of stroke and MI were evenly distributed over time in the control group, the investigators said in a letter to the editor (J Am Coll Cardiol. 2017;70[2]:293-300). Several possible reasons have been proposed to explain a causal association between this infection and cardiovascular disease. Herpes zoster is “the only virus for which there is clear evidence of viral DNA and antigen in areas of ischemia or infarction in cerebral arteries,” Dr. Kim and associates noted.
Viral replication adjacent to a cerebral or cardiac artery could cause inflammation of the vessel, followed by subsequent thrombosis and rupture. Or, repeated subclinical reactivation of the virus could weaken the arteries. It is also possible that herpes zoster reactivation alters patients’ immunologic status, making them vulnerable to cerebrovascular or cardiovascular events, the investigators said.
This study was supported by the Korea Health Technology Research and Development Project, the Korea Health Industry Development Institute, and the Republic of Korea Ministry of Health & Welfare. Dr. Kim and associates reported having no relevant financial disclosures.
Herpes zoster infection raised the risk of stroke by 35% and of myocardial infarction by 59%, in a South Korean nationwide study.
In addition, the risks of stroke and MI were highest in the first year following herpes zoster infection and decreased over time, said Min-Chul Kim, MD, the University of Ulsan, Seoul, South Korea, and associates.
The incidence of stroke was 1.34 per 1,000 person-years higher among patients with the infection than among those without it. Similarly, the incidence of MI was 0.80 per 1,000 person-years higher among patients with the infection than among those without it. The risks of both stroke and MI were highest during the first year after herpes onset and decreased over time. In contrast, the risks of stroke and MI were evenly distributed over time in the control group, the investigators said in a letter to the editor (J Am Coll Cardiol. 2017;70[2]:293-300). Several possible reasons have been proposed to explain a causal association between this infection and cardiovascular disease. Herpes zoster is “the only virus for which there is clear evidence of viral DNA and antigen in areas of ischemia or infarction in cerebral arteries,” Dr. Kim and associates noted.
Viral replication adjacent to a cerebral or cardiac artery could cause inflammation of the vessel, followed by subsequent thrombosis and rupture. Or, repeated subclinical reactivation of the virus could weaken the arteries. It is also possible that herpes zoster reactivation alters patients’ immunologic status, making them vulnerable to cerebrovascular or cardiovascular events, the investigators said.
This study was supported by the Korea Health Technology Research and Development Project, the Korea Health Industry Development Institute, and the Republic of Korea Ministry of Health & Welfare. Dr. Kim and associates reported having no relevant financial disclosures.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Key clinical point: Herpes zoster infection raised the risk of stroke by 35% and of MI by 59% in a nationwide study in South Korea.
Major finding: The incidence of stroke was 1.34 per 1,000 person-years higher among patients with the infection than among those without it, and the incidence of MI was 0.80 per 1,000 person-years higher.
Data source: A cohort study of CV risk in 519,880 adults in South Korea.
Disclosures: This study was supported by the Korea Health Technology Research and Development Project, the Korea Health Industry Development Institute, and the Republic of Korea Ministry of Health & Welfare. Dr. Kim and associates reported having no relevant financial disclosures.
Insulin degludec decreases rate, severity of hypoglycemic episodes
Insulin degludec decreased both the rate and the severity of hypoglycemic episodes in adults with type 1 and type 2 diabetes, compared with insulin glargine, in two head-to-head trials sponsored by the maker of insulin degludec and reported online July 3 in JAMA.
The two trials had identical randomized double-blind crossover designs. The first involved 501 adults with type 1 diabetes treated at 84 sites in the United States and 6 sites in Poland, and the second involved 721 adults with type 2 diabetes treated at 152 sites in the United States. All the study participants were at risk for hypoglycemia by virtue of experiencing one or more severe hypoglycemic episodes within the preceding year, having moderate chronic renal failure, having a 15-year or longer duration of diabetes, being unaware of their hypoglycemic symptoms, or experiencing severe hypoglycemia within the 3 months preceding baseline.
In both trials, patients were randomized to one type of once-daily insulin for 32 weeks (a 16-week titration period followed by a 16-week maintenance period) and then crossed over to the other type of insulin for 32 weeks. The primary end point in both studies was the rate of overall severe hypoglycemia during the maintenance period. This was defined as either an episode requiring the assistance of another person to administer aid or an episode in which blood glucose measured less than 56 mg/dL.
In the first trial, rates of hypoglycemia were significantly lower with insulin degludec (2,201 episodes per 100 person-years of exposure) than with insulin glargine (2,463 episodes per 100 PYE), demonstrating not just the noninferiority but also the superiority of insulin degludec. In addition, a significantly lower proportion of patients had hypoglycemia with insulin degludec (32.8%) than with insulin glargine (43.1%), said Wendy Lane, MD, of Mountain Diabetes and Endocrine Center, Asheville NC, and her associates.
Regarding the severity of hypoglycemia, the rate of severe episodes was significantly lower with insulin degludec (69 episodes per 100 PYE) than with insulin glargine (92 episodes per 100 PYE). In addition, the proportion of patients who experienced a severe hypoglycemic episode was significantly lower with insulin degludec (10.3%) than with insulin glargine (17.1%).
Both types of insulin reduced hemoglobin A1c levels to 7% or below. Rates of adverse events and serious adverse events were similar between the two study groups, and there were no differences between them in weight change, blood pressure, pulse rate, or laboratory findings, the investigators said (JAMA.2017;318[1]:33-44).
In the second trial, rates of hypoglycemia again were significantly lower with insulin degludec than with insulin glargine (186 vs. 265 episodes per 100 PYE). In addition, the proportion of patients who experienced a severe hypoglycemic episode was 1.6% vs. 2.4%, respectively, said Carol Wysham, MD, of Rockwood Clinic University of Washington, Spokane, and her associates.
As in the first trial, both types of insulin reduced HbA1c levels to the same degree, and rates of adverse events and of serious adverse events were comparable, Dr. Wysham and her associates said (JAMA. 2017;318[1]:45-56).
The dropout rates were similar and higher than expected in both trials, at approximately 20%. Dr. Lane and her associates noted that this may have resulted from “the demanding nature” of the studies, including their 64-week duration; demand for close monitoring of blood glucose; and the requirement of using vials and syringes to maintain treatment blinding, instead of more convenient injector devices.
Both trials were funded by Novo Nordisk, maker of insulin degludec. Dr. Lane reported ties to Novo Nordisk, Insulet Corporation, and Eli Lilly, and her associates reported ties to numerous industry sources. Dr. Wysham reported ties to Novo Nordisk, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, and Sanofi, and her associates reported ties to numerous industry sources.
Given the risks associated with hypoglycemia and the concerns about this adverse effect among patients and their families, any basal insulin that reduces the rate of hypoglycemia represents an advance in the treatment of diabetes.
Both studies were limited in that they had relatively high dropout rates of approximately 20% each. However, it appeared that the patients who completed the study were not substantially different from those who dropped out.
These remarks are from an editorial by Elizabeth R. Seaquist, MD, and Lisa S. Chow, MD, that was published along with the research reports (JAMA 2017;318[1]:31-2).
Dr Seaquist reported a variety of sources of funding from Eli Lilly, Locemia, Medtronic, Sanofi, and Zucera; serving as a member of the International Hypoglycemia Study Group; and serving on the examination committee for the American Board of Internal Medicine. Dr. Chow reported research funding from Eli Lilly.
Given the risks associated with hypoglycemia and the concerns about this adverse effect among patients and their families, any basal insulin that reduces the rate of hypoglycemia represents an advance in the treatment of diabetes.
Both studies were limited in that they had relatively high dropout rates of approximately 20% each. However, it appeared that the patients who completed the study were not substantially different from those who dropped out.
These remarks are from an editorial by Elizabeth R. Seaquist, MD, and Lisa S. Chow, MD, that was published along with the research reports (JAMA 2017;318[1]:31-2).
Dr Seaquist reported a variety of sources of funding from Eli Lilly, Locemia, Medtronic, Sanofi, and Zucera; serving as a member of the International Hypoglycemia Study Group; and serving on the examination committee for the American Board of Internal Medicine. Dr. Chow reported research funding from Eli Lilly.
Given the risks associated with hypoglycemia and the concerns about this adverse effect among patients and their families, any basal insulin that reduces the rate of hypoglycemia represents an advance in the treatment of diabetes.
Both studies were limited in that they had relatively high dropout rates of approximately 20% each. However, it appeared that the patients who completed the study were not substantially different from those who dropped out.
These remarks are from an editorial by Elizabeth R. Seaquist, MD, and Lisa S. Chow, MD, that was published along with the research reports (JAMA 2017;318[1]:31-2).
Dr Seaquist reported a variety of sources of funding from Eli Lilly, Locemia, Medtronic, Sanofi, and Zucera; serving as a member of the International Hypoglycemia Study Group; and serving on the examination committee for the American Board of Internal Medicine. Dr. Chow reported research funding from Eli Lilly.
Insulin degludec decreased both the rate and the severity of hypoglycemic episodes in adults with type 1 and type 2 diabetes, compared with insulin glargine, in two head-to-head trials sponsored by the maker of insulin degludec and reported online July 3 in JAMA.
The two trials had identical randomized double-blind crossover designs. The first involved 501 adults with type 1 diabetes treated at 84 sites in the United States and 6 sites in Poland, and the second involved 721 adults with type 2 diabetes treated at 152 sites in the United States. All the study participants were at risk for hypoglycemia by virtue of experiencing one or more severe hypoglycemic episodes within the preceding year, having moderate chronic renal failure, having a 15-year or longer duration of diabetes, being unaware of their hypoglycemic symptoms, or experiencing severe hypoglycemia within the 3 months preceding baseline.
In both trials, patients were randomized to one type of once-daily insulin for 32 weeks (a 16-week titration period followed by a 16-week maintenance period) and then crossed over to the other type of insulin for 32 weeks. The primary end point in both studies was the rate of overall severe hypoglycemia during the maintenance period. This was defined as either an episode requiring the assistance of another person to administer aid or an episode in which blood glucose measured less than 56 mg/dL.
In the first trial, rates of hypoglycemia were significantly lower with insulin degludec (2,201 episodes per 100 person-years of exposure) than with insulin glargine (2,463 episodes per 100 PYE), demonstrating not just the noninferiority but also the superiority of insulin degludec. In addition, a significantly lower proportion of patients had hypoglycemia with insulin degludec (32.8%) than with insulin glargine (43.1%), said Wendy Lane, MD, of Mountain Diabetes and Endocrine Center, Asheville NC, and her associates.
Regarding the severity of hypoglycemia, the rate of severe episodes was significantly lower with insulin degludec (69 episodes per 100 PYE) than with insulin glargine (92 episodes per 100 PYE). In addition, the proportion of patients who experienced a severe hypoglycemic episode was significantly lower with insulin degludec (10.3%) than with insulin glargine (17.1%).
Both types of insulin reduced hemoglobin A1c levels to 7% or below. Rates of adverse events and serious adverse events were similar between the two study groups, and there were no differences between them in weight change, blood pressure, pulse rate, or laboratory findings, the investigators said (JAMA.2017;318[1]:33-44).
In the second trial, rates of hypoglycemia again were significantly lower with insulin degludec than with insulin glargine (186 vs. 265 episodes per 100 PYE). In addition, the proportion of patients who experienced a severe hypoglycemic episode was 1.6% vs. 2.4%, respectively, said Carol Wysham, MD, of Rockwood Clinic University of Washington, Spokane, and her associates.
As in the first trial, both types of insulin reduced HbA1c levels to the same degree, and rates of adverse events and of serious adverse events were comparable, Dr. Wysham and her associates said (JAMA. 2017;318[1]:45-56).
The dropout rates were similar and higher than expected in both trials, at approximately 20%. Dr. Lane and her associates noted that this may have resulted from “the demanding nature” of the studies, including their 64-week duration; demand for close monitoring of blood glucose; and the requirement of using vials and syringes to maintain treatment blinding, instead of more convenient injector devices.
Both trials were funded by Novo Nordisk, maker of insulin degludec. Dr. Lane reported ties to Novo Nordisk, Insulet Corporation, and Eli Lilly, and her associates reported ties to numerous industry sources. Dr. Wysham reported ties to Novo Nordisk, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, and Sanofi, and her associates reported ties to numerous industry sources.
Insulin degludec decreased both the rate and the severity of hypoglycemic episodes in adults with type 1 and type 2 diabetes, compared with insulin glargine, in two head-to-head trials sponsored by the maker of insulin degludec and reported online July 3 in JAMA.
The two trials had identical randomized double-blind crossover designs. The first involved 501 adults with type 1 diabetes treated at 84 sites in the United States and 6 sites in Poland, and the second involved 721 adults with type 2 diabetes treated at 152 sites in the United States. All the study participants were at risk for hypoglycemia by virtue of experiencing one or more severe hypoglycemic episodes within the preceding year, having moderate chronic renal failure, having a 15-year or longer duration of diabetes, being unaware of their hypoglycemic symptoms, or experiencing severe hypoglycemia within the 3 months preceding baseline.
In both trials, patients were randomized to one type of once-daily insulin for 32 weeks (a 16-week titration period followed by a 16-week maintenance period) and then crossed over to the other type of insulin for 32 weeks. The primary end point in both studies was the rate of overall severe hypoglycemia during the maintenance period. This was defined as either an episode requiring the assistance of another person to administer aid or an episode in which blood glucose measured less than 56 mg/dL.
In the first trial, rates of hypoglycemia were significantly lower with insulin degludec (2,201 episodes per 100 person-years of exposure) than with insulin glargine (2,463 episodes per 100 PYE), demonstrating not just the noninferiority but also the superiority of insulin degludec. In addition, a significantly lower proportion of patients had hypoglycemia with insulin degludec (32.8%) than with insulin glargine (43.1%), said Wendy Lane, MD, of Mountain Diabetes and Endocrine Center, Asheville NC, and her associates.
Regarding the severity of hypoglycemia, the rate of severe episodes was significantly lower with insulin degludec (69 episodes per 100 PYE) than with insulin glargine (92 episodes per 100 PYE). In addition, the proportion of patients who experienced a severe hypoglycemic episode was significantly lower with insulin degludec (10.3%) than with insulin glargine (17.1%).
Both types of insulin reduced hemoglobin A1c levels to 7% or below. Rates of adverse events and serious adverse events were similar between the two study groups, and there were no differences between them in weight change, blood pressure, pulse rate, or laboratory findings, the investigators said (JAMA.2017;318[1]:33-44).
In the second trial, rates of hypoglycemia again were significantly lower with insulin degludec than with insulin glargine (186 vs. 265 episodes per 100 PYE). In addition, the proportion of patients who experienced a severe hypoglycemic episode was 1.6% vs. 2.4%, respectively, said Carol Wysham, MD, of Rockwood Clinic University of Washington, Spokane, and her associates.
As in the first trial, both types of insulin reduced HbA1c levels to the same degree, and rates of adverse events and of serious adverse events were comparable, Dr. Wysham and her associates said (JAMA. 2017;318[1]:45-56).
The dropout rates were similar and higher than expected in both trials, at approximately 20%. Dr. Lane and her associates noted that this may have resulted from “the demanding nature” of the studies, including their 64-week duration; demand for close monitoring of blood glucose; and the requirement of using vials and syringes to maintain treatment blinding, instead of more convenient injector devices.
Both trials were funded by Novo Nordisk, maker of insulin degludec. Dr. Lane reported ties to Novo Nordisk, Insulet Corporation, and Eli Lilly, and her associates reported ties to numerous industry sources. Dr. Wysham reported ties to Novo Nordisk, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, and Sanofi, and her associates reported ties to numerous industry sources.
FROM JAMA
Key clinical point: Insulin degludec decreases the rate and severity of hypoglycemic episodes in adults with type 1 and type 2 diabetes, compared with insulin glargine.
Major finding: Rates of hypoglycemia were significantly lower with insulin degludec (2,201 episodes per 100 person-years of exposure) than with insulin glargine (2,463 episodes per 100 PYE) in type 1 diabetes and in type 2 diabetes (185.6 vs. 265.4 episodes per 100 PYE).
Data source: Two separate multicenter, randomized, double-blind crossover trials involving 501 adults with type 1 and 721 with type 2 diabetes.
Disclosures: Both trials were funded by Novo Nordisk, maker of insulin degludec. Dr. Lane reported ties to Novo Nordisk, Insulet Corporation, and Eli Lilly, and her associates reported ties to numerous industry sources. Dr. Wysham reported ties to Novo Nordisk, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, and Sanofi, and her associates reported ties to numerous industry sources.
‘Chronic Lyme’: Serious bacterial infections reported with unproven treatments
Unproven treatments for so-called chronic Lyme disease can cause serious, even fatal, complications, according to five case reports published in the Morbidity and Mortality Weekly Report.
“Patients, clinicians, and public health practitioners should be aware that treatments for chronic Lyme disease can carry serious risks,” the authors wrote.
Chronic Lyme disease is a nonspecific diagnosis that has no consistent definition. Some clinicians use this label for patients who have a variety of debilitating conditions such as fatigue, generalized pain, and neurologic symptoms, even in the absence of laboratory evidence of Borrelia burgdorferi infection, objective signs of infection, or a history of tick exposure. People who support this diagnosis mistakenly believe that B. burgdorferi can cause longstanding disabling symptoms even when standard testing for the organism is negative, when the truth is that tests for the organism become more sensitive the longer the infection persists, according to Natalie S. Marzec, MD, a resident in preventive medicine at the University of Colorado, Aurora, and her associates.
Patients who cannot obtain symptom relief with conventional clinicians may consult “practitioners who might identify themselves as Lyme disease specialists (‘Lyme literate’ doctors) or from complementary and alternative medicine clinics,” where they are diagnosed with chronic Lyme disease. Such patients have been offered unproven treatments, including extended courses of intravenous antibiotics, infusions of hydrogen peroxide, immunoglobulin therapy, hyperbaric oxygen treatment, electromagnetic frequency therapy, garlic supplements, colloidal silver, and stem-cell transplantation.
Dr. Marzec and her associates presented case reports of five such patients diagnosed with chronic Lyme disease who sustained serious harm from such treatments (MMWR 2017;66[23]:607-9).
A woman in her late 30s with fatigue and joint pain was given a peripherally inserted central catheter (PICC) for IV delivery of ceftriaxone and cefotaxime. After 3 weeks, she developed fever, rash, hypotension, and tachycardia. In the intensive care unit (ICU), she was given broad-spectrum IV antibiotics and vasopressors, and was mechanically ventilated, but died of septic shock related to catheter-associated bacteremia.
An adolescent was told at an alternative medicine clinic that her years of muscle and joint pain, backaches, headaches, and lethargy were due to chronic Lyme disease. A PICC was placed to deliver IV antibiotics for 5 months. She developed pallor, chills, fever, hypotension, and tachycardia consistent with septic shock. Cultures demonstrated Acinetobacter species in her blood and on the PICC, and she required several weeks of ICU care.
A woman in her late 40s was diagnosed as having chronic Lyme disease based on unvalidated tests and was treated for months with intramuscular penicillin, IV ceftriazone, and IV azithromycin administered through a tunneled IV catheter; as well as doxycycline, and the antiparasitic drug tinidazole. She was hospitalized for back pain, shortness of breath, and malaise, and cultures of the catheter and her blood yielded Pseudomonas aeruginosa. She was found to have osteodiscitis caused by the same organism, with destruction of the 9th and 10th vertebrae, and was treated, and her back pain eventually improved.
A woman in her 50s was diagnosed as having amyotrophic lateral sclerosis, but sought a second opinion and was told that she had chronic Lyme disease (along with babesiosis, and Rocky Mountain spotted fever). She was treated with herbs and homeopathic remedies, followed by intensive antimicrobial and antiviral therapies. She developed intractable Clostridium difficile colitis that lasted for 2 years until she died from complications related to amyotrophic lateral sclerosis.
A woman in her 60s who had autoimmune neutropenia, mixed connective tissue disease, and degenerative arthritis was told her neuropathy was due to chronic Lyme disease. She was treated with immunoglobulin administered through an implanted subcutaneous port. After years of treatments, she was hospitalized for fever and back pain, had blood cultures positive for methicillin-sensitive Staphylococcus aureus, was found to have inflammation of the lumbar facet joints, epidural space, and paraspinal muscles – and eventually required surgical drainage of a paraspinal abscess.
“These cases highlight the severity and scope of adverse effects that can be caused by the use of unproven treatments for chronic Lyme disease,” Dr. Marzec and her associates said.
This work was supported by the CDC. Dr. Marzec and her associates reported having no relevant financial disclosures.
Unproven treatments for so-called chronic Lyme disease can cause serious, even fatal, complications, according to five case reports published in the Morbidity and Mortality Weekly Report.
“Patients, clinicians, and public health practitioners should be aware that treatments for chronic Lyme disease can carry serious risks,” the authors wrote.
Chronic Lyme disease is a nonspecific diagnosis that has no consistent definition. Some clinicians use this label for patients who have a variety of debilitating conditions such as fatigue, generalized pain, and neurologic symptoms, even in the absence of laboratory evidence of Borrelia burgdorferi infection, objective signs of infection, or a history of tick exposure. People who support this diagnosis mistakenly believe that B. burgdorferi can cause longstanding disabling symptoms even when standard testing for the organism is negative, when the truth is that tests for the organism become more sensitive the longer the infection persists, according to Natalie S. Marzec, MD, a resident in preventive medicine at the University of Colorado, Aurora, and her associates.
Patients who cannot obtain symptom relief with conventional clinicians may consult “practitioners who might identify themselves as Lyme disease specialists (‘Lyme literate’ doctors) or from complementary and alternative medicine clinics,” where they are diagnosed with chronic Lyme disease. Such patients have been offered unproven treatments, including extended courses of intravenous antibiotics, infusions of hydrogen peroxide, immunoglobulin therapy, hyperbaric oxygen treatment, electromagnetic frequency therapy, garlic supplements, colloidal silver, and stem-cell transplantation.
Dr. Marzec and her associates presented case reports of five such patients diagnosed with chronic Lyme disease who sustained serious harm from such treatments (MMWR 2017;66[23]:607-9).
A woman in her late 30s with fatigue and joint pain was given a peripherally inserted central catheter (PICC) for IV delivery of ceftriaxone and cefotaxime. After 3 weeks, she developed fever, rash, hypotension, and tachycardia. In the intensive care unit (ICU), she was given broad-spectrum IV antibiotics and vasopressors, and was mechanically ventilated, but died of septic shock related to catheter-associated bacteremia.
An adolescent was told at an alternative medicine clinic that her years of muscle and joint pain, backaches, headaches, and lethargy were due to chronic Lyme disease. A PICC was placed to deliver IV antibiotics for 5 months. She developed pallor, chills, fever, hypotension, and tachycardia consistent with septic shock. Cultures demonstrated Acinetobacter species in her blood and on the PICC, and she required several weeks of ICU care.
A woman in her late 40s was diagnosed as having chronic Lyme disease based on unvalidated tests and was treated for months with intramuscular penicillin, IV ceftriazone, and IV azithromycin administered through a tunneled IV catheter; as well as doxycycline, and the antiparasitic drug tinidazole. She was hospitalized for back pain, shortness of breath, and malaise, and cultures of the catheter and her blood yielded Pseudomonas aeruginosa. She was found to have osteodiscitis caused by the same organism, with destruction of the 9th and 10th vertebrae, and was treated, and her back pain eventually improved.
A woman in her 50s was diagnosed as having amyotrophic lateral sclerosis, but sought a second opinion and was told that she had chronic Lyme disease (along with babesiosis, and Rocky Mountain spotted fever). She was treated with herbs and homeopathic remedies, followed by intensive antimicrobial and antiviral therapies. She developed intractable Clostridium difficile colitis that lasted for 2 years until she died from complications related to amyotrophic lateral sclerosis.
A woman in her 60s who had autoimmune neutropenia, mixed connective tissue disease, and degenerative arthritis was told her neuropathy was due to chronic Lyme disease. She was treated with immunoglobulin administered through an implanted subcutaneous port. After years of treatments, she was hospitalized for fever and back pain, had blood cultures positive for methicillin-sensitive Staphylococcus aureus, was found to have inflammation of the lumbar facet joints, epidural space, and paraspinal muscles – and eventually required surgical drainage of a paraspinal abscess.
“These cases highlight the severity and scope of adverse effects that can be caused by the use of unproven treatments for chronic Lyme disease,” Dr. Marzec and her associates said.
This work was supported by the CDC. Dr. Marzec and her associates reported having no relevant financial disclosures.
Unproven treatments for so-called chronic Lyme disease can cause serious, even fatal, complications, according to five case reports published in the Morbidity and Mortality Weekly Report.
“Patients, clinicians, and public health practitioners should be aware that treatments for chronic Lyme disease can carry serious risks,” the authors wrote.
Chronic Lyme disease is a nonspecific diagnosis that has no consistent definition. Some clinicians use this label for patients who have a variety of debilitating conditions such as fatigue, generalized pain, and neurologic symptoms, even in the absence of laboratory evidence of Borrelia burgdorferi infection, objective signs of infection, or a history of tick exposure. People who support this diagnosis mistakenly believe that B. burgdorferi can cause longstanding disabling symptoms even when standard testing for the organism is negative, when the truth is that tests for the organism become more sensitive the longer the infection persists, according to Natalie S. Marzec, MD, a resident in preventive medicine at the University of Colorado, Aurora, and her associates.
Patients who cannot obtain symptom relief with conventional clinicians may consult “practitioners who might identify themselves as Lyme disease specialists (‘Lyme literate’ doctors) or from complementary and alternative medicine clinics,” where they are diagnosed with chronic Lyme disease. Such patients have been offered unproven treatments, including extended courses of intravenous antibiotics, infusions of hydrogen peroxide, immunoglobulin therapy, hyperbaric oxygen treatment, electromagnetic frequency therapy, garlic supplements, colloidal silver, and stem-cell transplantation.
Dr. Marzec and her associates presented case reports of five such patients diagnosed with chronic Lyme disease who sustained serious harm from such treatments (MMWR 2017;66[23]:607-9).
A woman in her late 30s with fatigue and joint pain was given a peripherally inserted central catheter (PICC) for IV delivery of ceftriaxone and cefotaxime. After 3 weeks, she developed fever, rash, hypotension, and tachycardia. In the intensive care unit (ICU), she was given broad-spectrum IV antibiotics and vasopressors, and was mechanically ventilated, but died of septic shock related to catheter-associated bacteremia.
An adolescent was told at an alternative medicine clinic that her years of muscle and joint pain, backaches, headaches, and lethargy were due to chronic Lyme disease. A PICC was placed to deliver IV antibiotics for 5 months. She developed pallor, chills, fever, hypotension, and tachycardia consistent with septic shock. Cultures demonstrated Acinetobacter species in her blood and on the PICC, and she required several weeks of ICU care.
A woman in her late 40s was diagnosed as having chronic Lyme disease based on unvalidated tests and was treated for months with intramuscular penicillin, IV ceftriazone, and IV azithromycin administered through a tunneled IV catheter; as well as doxycycline, and the antiparasitic drug tinidazole. She was hospitalized for back pain, shortness of breath, and malaise, and cultures of the catheter and her blood yielded Pseudomonas aeruginosa. She was found to have osteodiscitis caused by the same organism, with destruction of the 9th and 10th vertebrae, and was treated, and her back pain eventually improved.
A woman in her 50s was diagnosed as having amyotrophic lateral sclerosis, but sought a second opinion and was told that she had chronic Lyme disease (along with babesiosis, and Rocky Mountain spotted fever). She was treated with herbs and homeopathic remedies, followed by intensive antimicrobial and antiviral therapies. She developed intractable Clostridium difficile colitis that lasted for 2 years until she died from complications related to amyotrophic lateral sclerosis.
A woman in her 60s who had autoimmune neutropenia, mixed connective tissue disease, and degenerative arthritis was told her neuropathy was due to chronic Lyme disease. She was treated with immunoglobulin administered through an implanted subcutaneous port. After years of treatments, she was hospitalized for fever and back pain, had blood cultures positive for methicillin-sensitive Staphylococcus aureus, was found to have inflammation of the lumbar facet joints, epidural space, and paraspinal muscles – and eventually required surgical drainage of a paraspinal abscess.
“These cases highlight the severity and scope of adverse effects that can be caused by the use of unproven treatments for chronic Lyme disease,” Dr. Marzec and her associates said.
This work was supported by the CDC. Dr. Marzec and her associates reported having no relevant financial disclosures.
FROM MMWR
Key clinical point: Unproven treatments for so-called chronic Lyme disease cause serious, even fatal, complications.
Major finding:
Data source: Five case reports submitted to the CDC by clinicians, health departments, and individual patients.
Disclosures: This work was supported by the CDC. Dr. Marzec and her associates reported having no relevant financial disclosures.
Transcranial direct-current stimulation does not show noninferiority to escitalopram
Transcranial direct-current stimulation did not show noninferiority to escitalopram for major depressive disorder in a single-center trial. The results were published online June 29.
The randomized double-blind placebo-controlled noninferiority study involved 245 adults with moderate to severe depression, many of whom had coexisting anxiety disorder. This “reflects a typical clinical population in which treatment for depression is indicated,” said André R. Brunoni, MD, PhD, of the Service of Interdisciplinary Neuromodulation, Institute of Psychiatry and Laboratory of Neurosciences, University of São Paulo, Brazil, and his associates.
A total of 94 patients were assigned to receive active tDCS plus oral placebo (tDCS group), 91 to receive sham tDCS plus escitalopram (escitalopram group), and 60 to receive sham tDCS plus oral placebo (placebo group) for 10 weeks. The primary outcome – decrease in mean depression score on the 17-item Hamilton Depression Rating Scale – was 9.0 points with tDCS, 11.3 points with escitalopram, and 5.8 points with placebo. Thus, tDCS did not achieve noninferiority to escitalopram, the investigators said (N Engl J Med. 2017 June 29. doi: 10.1056/NEJMoa1612999).
Although tDCS was superior to placebo in some secondary outcomes, including rate of clinical response (defined as a reduction of 50% or more in the Hamilton or the Montgomery-Åsberg Depression Rating Scale), it was associated with significantly more adverse events. The tDCS group reported more tinnitus, more nervousness, and more itching, tingling, burning, and skin redness at the electrode sites than did the other study groups. The escitalopram group reported higher rates of sleepiness and obstipation than did the other study groups.
One concerning finding was that two patients in the tDCS group developed new-onset mania during treatment, Dr. Brunoni and his associates noted.
“Future studies of tDCS could investigate different total doses of electrical stimulation in patients with major depressive disorder,” they wrote.
This trial was supported by the Fundacão de Amparo à Pesquisa do Estado de São Paulo, the Brain and Behavior Research Foundation, the Sao Paulo State Foundation, the National Council for Scientific and Technological Development, the Associacao Beneficente Alzira Denise Hertzog de Silva, and the Brazilian Coordination for the Improvement of Higher Education Personnel. Soterix Medical supplied the tDCS devices, and Libbs Laboratory supplied the escitalopram used in this study free of charge. Dr. Brunoni reported ties to Soterix Medical, Libbs Laboratory, and Delta Medical. His associates reported ties to numerous industry sources.
Even though the antidepressant efficacy of tDCS remains uncertain, this study shows key knowledge gaps, particularly regarding dosing, that must be addressed in this and other forms of noninvasive brain stimulation before an effective therapy can be developed, Sarah H. Lisanby, MD, wrote in an accompanying editorial (N Engl J Med. 2017 June 29. doi: 10.1056/NEJMe1702492).
“Ultimately, the more we know about the ways in which noninvasive brain stimulation influences brain activity at a mechanistic level,” she wrote, “ the closer we come to determining the clinical usefulness of these new therapies.”
However, she said one important limitation in this trial was that most of the patients in the medication group became aware of their assigned therapy, presumably because they experienced side effects. This might have inflated the efficacy of escitalopram, which in turn may have invalidated the noninferiority comparison.
Dr. Lisanby is affiliated with the National Institute of Mental Health, Bethesda, Md. She reported ties to Oxford University Press, the Stanley Medical Research Foundation, Neosync, Brainsway, and the Brain Behavior Research Foundation. She also holds a patent for magnetic stimulation methods, apparatus, and systems.
Even though the antidepressant efficacy of tDCS remains uncertain, this study shows key knowledge gaps, particularly regarding dosing, that must be addressed in this and other forms of noninvasive brain stimulation before an effective therapy can be developed, Sarah H. Lisanby, MD, wrote in an accompanying editorial (N Engl J Med. 2017 June 29. doi: 10.1056/NEJMe1702492).
“Ultimately, the more we know about the ways in which noninvasive brain stimulation influences brain activity at a mechanistic level,” she wrote, “ the closer we come to determining the clinical usefulness of these new therapies.”
However, she said one important limitation in this trial was that most of the patients in the medication group became aware of their assigned therapy, presumably because they experienced side effects. This might have inflated the efficacy of escitalopram, which in turn may have invalidated the noninferiority comparison.
Dr. Lisanby is affiliated with the National Institute of Mental Health, Bethesda, Md. She reported ties to Oxford University Press, the Stanley Medical Research Foundation, Neosync, Brainsway, and the Brain Behavior Research Foundation. She also holds a patent for magnetic stimulation methods, apparatus, and systems.
Even though the antidepressant efficacy of tDCS remains uncertain, this study shows key knowledge gaps, particularly regarding dosing, that must be addressed in this and other forms of noninvasive brain stimulation before an effective therapy can be developed, Sarah H. Lisanby, MD, wrote in an accompanying editorial (N Engl J Med. 2017 June 29. doi: 10.1056/NEJMe1702492).
“Ultimately, the more we know about the ways in which noninvasive brain stimulation influences brain activity at a mechanistic level,” she wrote, “ the closer we come to determining the clinical usefulness of these new therapies.”
However, she said one important limitation in this trial was that most of the patients in the medication group became aware of their assigned therapy, presumably because they experienced side effects. This might have inflated the efficacy of escitalopram, which in turn may have invalidated the noninferiority comparison.
Dr. Lisanby is affiliated with the National Institute of Mental Health, Bethesda, Md. She reported ties to Oxford University Press, the Stanley Medical Research Foundation, Neosync, Brainsway, and the Brain Behavior Research Foundation. She also holds a patent for magnetic stimulation methods, apparatus, and systems.
Transcranial direct-current stimulation did not show noninferiority to escitalopram for major depressive disorder in a single-center trial. The results were published online June 29.
The randomized double-blind placebo-controlled noninferiority study involved 245 adults with moderate to severe depression, many of whom had coexisting anxiety disorder. This “reflects a typical clinical population in which treatment for depression is indicated,” said André R. Brunoni, MD, PhD, of the Service of Interdisciplinary Neuromodulation, Institute of Psychiatry and Laboratory of Neurosciences, University of São Paulo, Brazil, and his associates.
A total of 94 patients were assigned to receive active tDCS plus oral placebo (tDCS group), 91 to receive sham tDCS plus escitalopram (escitalopram group), and 60 to receive sham tDCS plus oral placebo (placebo group) for 10 weeks. The primary outcome – decrease in mean depression score on the 17-item Hamilton Depression Rating Scale – was 9.0 points with tDCS, 11.3 points with escitalopram, and 5.8 points with placebo. Thus, tDCS did not achieve noninferiority to escitalopram, the investigators said (N Engl J Med. 2017 June 29. doi: 10.1056/NEJMoa1612999).
Although tDCS was superior to placebo in some secondary outcomes, including rate of clinical response (defined as a reduction of 50% or more in the Hamilton or the Montgomery-Åsberg Depression Rating Scale), it was associated with significantly more adverse events. The tDCS group reported more tinnitus, more nervousness, and more itching, tingling, burning, and skin redness at the electrode sites than did the other study groups. The escitalopram group reported higher rates of sleepiness and obstipation than did the other study groups.
One concerning finding was that two patients in the tDCS group developed new-onset mania during treatment, Dr. Brunoni and his associates noted.
“Future studies of tDCS could investigate different total doses of electrical stimulation in patients with major depressive disorder,” they wrote.
This trial was supported by the Fundacão de Amparo à Pesquisa do Estado de São Paulo, the Brain and Behavior Research Foundation, the Sao Paulo State Foundation, the National Council for Scientific and Technological Development, the Associacao Beneficente Alzira Denise Hertzog de Silva, and the Brazilian Coordination for the Improvement of Higher Education Personnel. Soterix Medical supplied the tDCS devices, and Libbs Laboratory supplied the escitalopram used in this study free of charge. Dr. Brunoni reported ties to Soterix Medical, Libbs Laboratory, and Delta Medical. His associates reported ties to numerous industry sources.
Transcranial direct-current stimulation did not show noninferiority to escitalopram for major depressive disorder in a single-center trial. The results were published online June 29.
The randomized double-blind placebo-controlled noninferiority study involved 245 adults with moderate to severe depression, many of whom had coexisting anxiety disorder. This “reflects a typical clinical population in which treatment for depression is indicated,” said André R. Brunoni, MD, PhD, of the Service of Interdisciplinary Neuromodulation, Institute of Psychiatry and Laboratory of Neurosciences, University of São Paulo, Brazil, and his associates.
A total of 94 patients were assigned to receive active tDCS plus oral placebo (tDCS group), 91 to receive sham tDCS plus escitalopram (escitalopram group), and 60 to receive sham tDCS plus oral placebo (placebo group) for 10 weeks. The primary outcome – decrease in mean depression score on the 17-item Hamilton Depression Rating Scale – was 9.0 points with tDCS, 11.3 points with escitalopram, and 5.8 points with placebo. Thus, tDCS did not achieve noninferiority to escitalopram, the investigators said (N Engl J Med. 2017 June 29. doi: 10.1056/NEJMoa1612999).
Although tDCS was superior to placebo in some secondary outcomes, including rate of clinical response (defined as a reduction of 50% or more in the Hamilton or the Montgomery-Åsberg Depression Rating Scale), it was associated with significantly more adverse events. The tDCS group reported more tinnitus, more nervousness, and more itching, tingling, burning, and skin redness at the electrode sites than did the other study groups. The escitalopram group reported higher rates of sleepiness and obstipation than did the other study groups.
One concerning finding was that two patients in the tDCS group developed new-onset mania during treatment, Dr. Brunoni and his associates noted.
“Future studies of tDCS could investigate different total doses of electrical stimulation in patients with major depressive disorder,” they wrote.
This trial was supported by the Fundacão de Amparo à Pesquisa do Estado de São Paulo, the Brain and Behavior Research Foundation, the Sao Paulo State Foundation, the National Council for Scientific and Technological Development, the Associacao Beneficente Alzira Denise Hertzog de Silva, and the Brazilian Coordination for the Improvement of Higher Education Personnel. Soterix Medical supplied the tDCS devices, and Libbs Laboratory supplied the escitalopram used in this study free of charge. Dr. Brunoni reported ties to Soterix Medical, Libbs Laboratory, and Delta Medical. His associates reported ties to numerous industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Transcranial direct-current stimulation did not show noninferiority to escitalopram for major depressive disorder in a single-center trial.
Major finding: The primary outcome – decrease in mean depression score on the 17-item Hamilton Depression Rating Scale – was 9.0 points with tDCS, 11.3 points with escitalopram, and 5.8 points with placebo.
Data source: A single-center randomized double-blind placebo-controlled noninferiority trial involving 245 adults with moderate to severe major depression.
Disclosures: This trial was funded by the Fundacão de Amparo à Pesquisa do Estado de São Paulo, the Brain and Behavior Research Foundation, the São Paulo State Foundation, the National Council for Scientific and Technological Development, the Associacao Beneficente Alzira Denise Hertzog de Silva, and the Brazilian Coordination for the Improvement of Higher Education Personnel. Soterix Medical supplied the tDCS devices, and Libbs Laboratory supplied the escitalopram used in this study free of charge. Dr. Brunoni reported ties to Soterix Medical, Libbs Laboratory, and Delta Medical. His associates reported ties to numerous industry sources.