User login
Conservative or surgical management for that shoulder dislocation?
The shoulder, or glenohumeral joint, is the most commonly dislocated large joint; dislocation occurs at a rate of 23.9 per 100,000 person/years.1,2 There are 2 types of dislocation: traumatic anterior dislocation, which accounts for roughly 90% of dislocations, and posterior dislocation (10%).3 Anterior dislocation typically occurs when the patient’s shoulder is forcefully abducted and externally rotated.
The diagnosis is made after review of the history and mechanism of injury and performance of a complete physical exam with imaging studies—the most critical component of diagnosis.4 Standard radiographs (anteroposterior, axillary, and scapular Y) can confirm the presence of a dislocation; once the diagnosis is confirmed, closed reduction of the joint should be performed.1 (Methods of reduction are beyond the scope of this article but have been recently reviewed.5)
Risk for recurrence drives management choices
Following an initial shoulder dislocation, the risk of recurrence is high.6,7 Rates vary based on age, pathology after dislocation, activity level, type of immobilization, and whether surgery was performed. Overall, age is the strongest predictor of recurrence: 72% of patients ages 12 to 22 years, 56% of those ages 23 to 29 years, and 27% of those older than 30 years experience recurrence.6 Patients who have recurrent dislocations are at risk for arthropathy, fear of instability, and worsening surgical outcomes.6
Reducing the risk of a recurrent shoulder dislocation has been the focus of intense study. Proponents of surgical stabilization argue that surgery—rather than a trial of conservative treatment—is best when you consider the high risk of recurrence in young athletes (the population primarily studied), the soft-tissue and bony damage caused by recurrent instability, and the predictable improvement in quality of life following surgery.
In a recent systematic review and meta-analysis, there was evidence that, for first-time traumatic shoulder dislocations, early surgery led to fewer repeat shoulder dislocations (number needed to treat [NNT] = 2-4.7). However, a significant number of patients primarily treated nonoperatively did not experience a repeat shoulder dislocation within 2 years.2
The conflicting results from randomized trials comparing operative intervention to conservative management have led surgeons and physicians in other specialties to take different approaches to the management of shoulder dislocation.2 In this review, we aim to summarize considerations for conservative vs surgical management and provide clinical guidance for primary care physicians.
When to try conservative management
Although the initial treatment after a traumatic anterior shoulder dislocation has been debated, a recent meta-analysis of randomized controlled trials showed that at least half of first-time dislocations are successfully treated with conservative management.2 Management can include immobilization for comfort and/or physical therapy. Age will play a role, as mentioned earlier; in general, patients older than 30 have a significant decrease in recurrence rate and are good candidates for conservative therapy.6 It should be noted that much of the research with regard to management of shoulder dislocations has been done in an athletic population.
Continue to: Immobilization may benefit some
Immobilization may benefit some
Recent evidence has determined that the duration of immobilization in internal rotation does not impact recurrent instability.8,9 In patients older than 30, the rate of repeat dislocation is lower, and early mobilization after 1 week is advocated to avoid joint stiffness and minimize the risk of adhesive capsulitis.10
Arm position during immobilization remains controversial.11 In a classic study by Itoi et al, immobilization for 3 weeks in internal rotation vs 10° of external rotation was associated with a recurrence rate of 42% vs 26%, respectively.12 In this study, immobilization in 10° of external rotation was especially beneficial for patients ages 30 years or younger.12
Cadaveric and magnetic resonance imaging (MRI) studies have shown external rotation may improve the odds of labral tear healing by positioning the damaged and intact parts of the glenoid labrum in closer proximity.13 While this is theoretically plausible, a recent Cochrane review found insufficient evidence to determine whether immobilization in external rotation has any benefits beyond those offered by internal rotation.14 A recent systematic review and meta-analysis found that immobilization in external rotation vs internal rotation after a first-time traumatic shoulder dislocation did not change outcomes.2 With that said, most would prefer to immobilize in the internal rotation position for ease.
More research is needed. A Cochrane review highlighted the need for continued research.14 Additionally, most of the available randomized controlled trials to date have consisted of young men, with the majority of dislocations related to sports activities. Women, nonathletes, and older patients have been understudied to date; extrapolating current research to those groups of patients may not be appropriate and should be a focus for future research.2
Physical therapy: The conservative standard of care
Rehabilitation after glenohumeral joint dislocation is the current standard of care in conservative management to reduce the risk for repeat dislocation.15 Depending on the specific characteristics of the instability pattern, the approach may be adapted to the patient. A recent review focused on the following 4 key points: (1) restoration of rotator cuff strength, focusing on the eccentric capacity of the external rotators, (2) normalization of rotational range of motion with particular focus on internal range of motion, (3) optimization of the flexibility and muscle performance of the scapular muscles, and (4) increasing the functional sport-specific load on the shoulder girdle.
Continue to: A common approach to the care of...
A common approach to the care of a patient after a glenohumeral joint dislocation is to place the patient’s shoulder in a sling for comfort, with permitted pain-free isometric exercise along with passive and assisted elevation up to 100°.16 This is followed by a nonaggressive rehabilitation protocol for 2 months until full recovery, which includes progressive range of motion, strength, proprioception, and return to functional activities.16
More aggressive return-to-play protocols with accelerated timelines and functional progression have been studied, including in a multicenter observational study that followed 45 contact intercollegiate athletes prospectively after in-season anterior glenohumeral instability. Thirty-three of 45 (73%) athletes returned to sport for either all or part of the season after a median 5 days lost from competition, with 12 athletes (27%) successfully completing the season without recurrence. Athletes with a subluxation event were 5.3 times more likely to return to sport during the same season, compared with those with dislocations.17
Dynamic bracing may also allow for a safe and quicker return to sport in athletes18 but recently was shown to not impact recurrent dislocation risk.19
Return to play should be based on subjective assessment as well as objective measurements of range of motion, strength, and dynamic function.15 Patients who continue to have significant weakness and pain at 2 to 3 weeks post injury despite physical therapy should be re-evaluated with an MRI for concomitant rotator cuff tears and need for surgical referral.20
When to consider surgical intervention
In a recent meta-analysis, recurrent dislocation and instability occurred at a rate of 52.9% following nonsurgical treatment.2 The decision to perform surgical intervention is typically made following failure of conservative management. Other considerations include age, gender, bone loss, and cartilage defect.21,22 Age younger than 30 years, participation in competition, contact sports, and male gender have been associated with an increased risk of recurrence.23-25 For this reason, obtaining an MRI at time of first dislocation can help facilitate surgical decisions if the patient is at high risk for surgical need.26
Continue to: An increasing number...
An increasing number of dislocations portends a poor outcome with nonoperative treatment. Kao et al demonstrated a second dislocation leads to another dislocation in 19.6% of cases, while 44.3% of those with a third dislocation event will sustain another dislocation.24 Surgery should be considered for patients with recurrent instability events to prevent persistent instability and decrease the amount of bone loss that can occur with repetitive dislocations.
What are the surgical options?
Several surgical options exist to remedy the unstable shoulder. Procedures can range from an arthroscopic repair to an open stabilization combined with structural bone graft to replace a bone defect caused by repetitive dislocations.
Arthroscopic techniques have become the mainstay of treatment and account for 71% of stabilization procedures performed.21 These techniques cause less pain in the early postoperative period and provide for a faster return to work.27 Arthroscopy has the additional advantage of allowing for complete visualization of the glenohumeral joint to identify and address concomitant pathology, such as intra-articular loose bodies or rotator cuff tears.
Open repair was the mainstay of treatment prior to development of arthroscopic techniques. Some surgeons still prefer this method—especially in high-risk groups—because of a lower risk of recurrent disloca-tion.28 Open techniques often involve detachment and repair of the upper subscapularis tendon and are more likely to produce long-term losses in external rotation range of motion.28
Which one is appropriate for your patient? The decision to pursue an open or arthroscopic procedure and to augment with bone graft depends on the amount of glenoid and humeral head bone loss, patient activity level, risk of recurrent dislocation, and surgeon preference.
Continue to: For the nonathletic population...
For the nonathletic population, the timing of injury is less critical and surgery is typically recommended after conservative treatment has failed. In an athletic population, the timing of injury is a necessary consideration. An injury midseason may be “rehabbed” in hopes of returning to play. Individuals with injuries occurring at the end of a season, who are unable to regain desired function, and/or with peri-articular fractures or associated full-thickness rotator cuff tears may benefit from sooner surgical intervention.21
Owens et al have described appropriate surgical indications and recommendations for an in-season athlete.21 In this particular algorithm, the authors suggest obtaining an MRI for decision making, but this is specific to in-season athletes wishing to return to play. In general, an MRI is not always indicated for patients who wish to receive conservative therapy but would be indicated for surgical considerations. The algorithm otherwise uses bone and soft-tissue injury, recurrent instability, and timing in the season to help determine management.21
Outcomes: Surgery has advantages …
Recurrence rates following surgical intervention are considerably lower than with conservative management, especially among young, active individuals. A recent systematic review by Donohue et al demonstrated recurrent instability rates following surgical intervention as low as 2.4%.29 One study comparing the outcome of arthroscopic repair vs conservative management showed that the risk of postoperative instability was reduced by 20% compared to other treatments.7 Furthermore, early surgical fixation can improve quality of life, produce better functional outcomes, decrease time away from activity, increase patient satisfaction, and slow the development of glenohumeral osteoarthritis produced from recurrent instability.2,7
Complications. Surgery does carry inherent risks of infection, anesthesia effects, surgical complications, and surgical failure. Recurrent instability is the most common complication following surgical shoulder stabilization. Rates of recurrent instability after surgical stabilization depend on patient age, activity level, and amount of bone loss: males younger than 18 years who participate in contact competitive sports and have significant bone loss are more likely to have recurrent dislocation after surgery.23 The type of surgical procedure selected may decrease this risk.
While the open procedures decrease risk of postoperative instability, these surgeries can pose a significant risk of complications. Major complications for specific open techniques have been reported in up to 30% of patients30 and are associated with lower levels of surgeon experience.31 While the healing of bones and ligaments is always a concern, 1 of the most feared complications following stabilization surgery is iatrogenic nerve injury. Because of the axillary nerve’s close proximity to the inferior glenoid, this nerve can be injured without meticulous care and can result in paralysis of the deltoid muscle. This injury poses a major impediment to normal shoulder function. Some procedures may cause nerve injuries in up to 10% of patients, although most injuries are transient.32
Continue to: Bottom line
Bottom line
Due to the void of evidence-based guidelines for conservative vs surgical management of primary shoulder dislocation, it would be prudent to have a risk-benefit discussion with patients regarding treatment options.
Patients older than 30 years and those with uncomplicated injuries are best suited for conservative management of primary shoulder dislocations. Immobilization is debated and may not change outcomes, but a progressive rehabilitative program after the initial acute injury is helpful. Risk factors for failing conservative management include recurrent dislocation, subsequent arthropathy, and additional concomitant bone or soft-tissue injuries.
Patients younger than 30 years who have complicated injuries with bone or cartilage loss, rotator cuff tears, or recurrent instability, and highly physically active individuals are best suited for surgical management. Shoulder arthroscopy has become the mainstay of surgical treatment for shoulder dislocations. Outcomes are favorable and dislocation recurrence is low after surgical repair. Surgery does carry its own inherent risks of infection, anesthesia effects, complications during surgery, and surgical failure leading to recurrent instability.
Cayce Onks, DO, MS, ATC, Penn State Hershey, Milton S. Hershey Medical Center, Penn State College of Medicine, Family and Community Medicine H154, 500 University Drive, PO Box 850, Hershey, PA 17033-0850; [email protected]
1. Lin K, James E, Spitzer E, et al. Pediatric and adolescent anterior shoulder instability: clinical management of first time dislocators. Curr Opin Pediatr. 2018;30:49-56.
2. Kavaja L, Lähdeoja T, Malmivaara A, et al. Treatment after traumatic shoulder dislocation: a systematic review with a network meta-analysis. Br J Sports Med. 2018;52:1498-1506.
3. Brelin A, Dickens JF. Posterior shoulder instability. Sports Med Arthrosc Rev. 2017;25:136-143.
4. Galvin JW, Ernat JJ, Waterman BR, et al. The epidemiology and natural history of anterior shoulder dislocation. Curr Rev Musculoskelet Med. 2017;10:411-424.
5. Rozzi SL, Anderson JM, Doberstein ST, et al. National Athletic Trainers’ Association position statement: immediate management of appendicular joint dislocations. J Athl Train. 2018;53:1117-1128.
6. Hovelius L, Saeboe M. Arthropathy after primary anterior shoulder dislocation: 223 shoulders prospectively followed up for twenty-five years. J Shoulder Elbow Surg. 2009;18:339-347.
7. Polyzois I, Dattani R, Gupta R, et al. Traumatic first time shoulder dislocation: surgery vs non-operative treatment. Arch Bone Jt Surg. 2016;4:104-108.
8. Cox CL, Kuhn JE. Operative versus nonoperative treatment of acute shoulder dislocation in the athlete. Curr Sports Med Rep. 2008;7:263-268.
9. Kuhn JE. Treating the initial anterior shoulder dislocation—an evidence-based medicine approach. Sports Med Arthrosc Rev. 2006;14:192-198.
10. Smith TO. Immobilization following traumatic anterior glenohumeral joint dislocation: a literature review. Injury. 2006;37:228-237.
11. Liavaag S, Brox JI, Pripp AH, et al. Immobilization in external rotation after primary shoulder dislocation did not reduce the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am. 2011;93:897-904.
12. Itoi E, Hatakeyama Y, Sato T, et al. Immobilization in external rotation after shoulder dislocation reduces the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am. 2007;89:2124-2131.
13. Miller BS, Sonnabend DH, Hatrick C, et al. Should acute anterior dislocations of the shoulder be immobilized in external rotation? A cadaveric study. J Shoulder Elbow Surg. 2004;13:589-592.
14. Hanchard NCA, Goodchild LM, Kottam L. Conservative management following closed reduction of traumatic anterior dislocation of the shoulder. Cochrane Database Syst Rev. 2014;(4):CD004962.
15. Cools AM, Borms D, Castelein B, et al. Evidence-based rehabilitation of athletes with glenohumeral instability. Knee Surg Sports Traumatol Arthrosc. 2016;24:382-389.
16. Lafuente JLA, Marco SM, Pequerul JMG. Controversies in the management of the first time shoulder dislocation. Open Orthop J. 2017;11:1001-1010.
17. Dickens JF, Owens BD, Cameron KL, et al. Return to play and recurrent instability after in-season anterior shoulder instability: a prospective multicenter study. Am J Sports Med. 2014;42:2842-2850.
18. Conti M, Garofalo R, Castagna A, et al. Dynamic brace is a good option to treat first anterior shoulder dislocation in season. Musculoskelet Surg. 2017;101(suppl 2):169-173.
19. Shanley E, Thigpen C, Brooks J, et al. Return to sport as an outcome measure for shoulder instability. Am J Sports Med. 2019;47:1062-1067.
20. Gombera MM, Sekiya JK. Rotator cuff tear and glenohumeral instability. Clin Orthop Relat Res. 2014;472:2448-2456.
21. Owens BD, Dickens JF, Kilcoyne KG, et al. Management of mid-season traumatic anterior shoulder instability in athletes. J Am Acad Orthop Surg. 2012;20:518-526.
22. Ozturk BY, Maak TG, Fabricant P, et al. Return to sports after arthroscopic anterior stabilization in patients aged younger than 25 years. Arthroscopy. 2013;29:1922-1931.
23. Balg F, Boileau P. The instability severity index score. A simple preoperative score to select patients for arthroscopic or open shoulder stabilisation. J Bone Joint Surg Br. 2007;89:1470-1477.
24. Kao J-T, Chang C-L, Su W-R, et al. Incidence of recurrence after shoulder dislocation: a nationwide database study. J Shoulder Elbow Surg. 2018;27:1519-1525.
25. Porcillini G, Campi F, Pegreffi F, et al. Predisposing factors for recurrent shoulder dislocation after arthroscopic treatment. J Bone Joint Surg Am. 2009;91:2537-2542.
26. Magee T. 3T MRI of the shoulder: is MR arthrography necessary? AJR Am J Roentgenol. 2009;192:86-92.
27. Green MR, Christensen KP. Arthroscopic versus open Bankart procedures: a comparison of early morbidity and complications. Arthroscopy. 1993;9:371-374.
28. Khatri K, Arora H, Chaudhary S, et al. Meta-analysis of randomized controlled trials involving anterior shoulder instability. Open Orthop J. 2018;12:411-418.
29. Donohue MA, Owens BD, Dickens JF. Return to play following anterior shoulder dislocations and stabilization surgery. Clin Sports Med. 2016;35:545-561.
30. Griesser MJ, Harris JD, McCoy BW, et al. Complications and re-operations after Bristow-Latarjet shoulder stabilization: a systematic review. J Shoulder Elbow Surg. 2013;22:286-292.
31. Ekhtiari S, Horner NS, Bedi A, et al. The learning curve for the Latarjet procedure: a systematic review. Orthop J Sports Med. 2018;6:2325967118786930.
32. Shah AA, Butler RB, Romanowski J, et al. Short-term complications of the Latarjet procedure. J Bone Joint Surg Am. 2012;94:495-501.
The shoulder, or glenohumeral joint, is the most commonly dislocated large joint; dislocation occurs at a rate of 23.9 per 100,000 person/years.1,2 There are 2 types of dislocation: traumatic anterior dislocation, which accounts for roughly 90% of dislocations, and posterior dislocation (10%).3 Anterior dislocation typically occurs when the patient’s shoulder is forcefully abducted and externally rotated.
The diagnosis is made after review of the history and mechanism of injury and performance of a complete physical exam with imaging studies—the most critical component of diagnosis.4 Standard radiographs (anteroposterior, axillary, and scapular Y) can confirm the presence of a dislocation; once the diagnosis is confirmed, closed reduction of the joint should be performed.1 (Methods of reduction are beyond the scope of this article but have been recently reviewed.5)
Risk for recurrence drives management choices
Following an initial shoulder dislocation, the risk of recurrence is high.6,7 Rates vary based on age, pathology after dislocation, activity level, type of immobilization, and whether surgery was performed. Overall, age is the strongest predictor of recurrence: 72% of patients ages 12 to 22 years, 56% of those ages 23 to 29 years, and 27% of those older than 30 years experience recurrence.6 Patients who have recurrent dislocations are at risk for arthropathy, fear of instability, and worsening surgical outcomes.6
Reducing the risk of a recurrent shoulder dislocation has been the focus of intense study. Proponents of surgical stabilization argue that surgery—rather than a trial of conservative treatment—is best when you consider the high risk of recurrence in young athletes (the population primarily studied), the soft-tissue and bony damage caused by recurrent instability, and the predictable improvement in quality of life following surgery.
In a recent systematic review and meta-analysis, there was evidence that, for first-time traumatic shoulder dislocations, early surgery led to fewer repeat shoulder dislocations (number needed to treat [NNT] = 2-4.7). However, a significant number of patients primarily treated nonoperatively did not experience a repeat shoulder dislocation within 2 years.2
The conflicting results from randomized trials comparing operative intervention to conservative management have led surgeons and physicians in other specialties to take different approaches to the management of shoulder dislocation.2 In this review, we aim to summarize considerations for conservative vs surgical management and provide clinical guidance for primary care physicians.
When to try conservative management
Although the initial treatment after a traumatic anterior shoulder dislocation has been debated, a recent meta-analysis of randomized controlled trials showed that at least half of first-time dislocations are successfully treated with conservative management.2 Management can include immobilization for comfort and/or physical therapy. Age will play a role, as mentioned earlier; in general, patients older than 30 have a significant decrease in recurrence rate and are good candidates for conservative therapy.6 It should be noted that much of the research with regard to management of shoulder dislocations has been done in an athletic population.
Continue to: Immobilization may benefit some
Immobilization may benefit some
Recent evidence has determined that the duration of immobilization in internal rotation does not impact recurrent instability.8,9 In patients older than 30, the rate of repeat dislocation is lower, and early mobilization after 1 week is advocated to avoid joint stiffness and minimize the risk of adhesive capsulitis.10
Arm position during immobilization remains controversial.11 In a classic study by Itoi et al, immobilization for 3 weeks in internal rotation vs 10° of external rotation was associated with a recurrence rate of 42% vs 26%, respectively.12 In this study, immobilization in 10° of external rotation was especially beneficial for patients ages 30 years or younger.12
Cadaveric and magnetic resonance imaging (MRI) studies have shown external rotation may improve the odds of labral tear healing by positioning the damaged and intact parts of the glenoid labrum in closer proximity.13 While this is theoretically plausible, a recent Cochrane review found insufficient evidence to determine whether immobilization in external rotation has any benefits beyond those offered by internal rotation.14 A recent systematic review and meta-analysis found that immobilization in external rotation vs internal rotation after a first-time traumatic shoulder dislocation did not change outcomes.2 With that said, most would prefer to immobilize in the internal rotation position for ease.
More research is needed. A Cochrane review highlighted the need for continued research.14 Additionally, most of the available randomized controlled trials to date have consisted of young men, with the majority of dislocations related to sports activities. Women, nonathletes, and older patients have been understudied to date; extrapolating current research to those groups of patients may not be appropriate and should be a focus for future research.2
Physical therapy: The conservative standard of care
Rehabilitation after glenohumeral joint dislocation is the current standard of care in conservative management to reduce the risk for repeat dislocation.15 Depending on the specific characteristics of the instability pattern, the approach may be adapted to the patient. A recent review focused on the following 4 key points: (1) restoration of rotator cuff strength, focusing on the eccentric capacity of the external rotators, (2) normalization of rotational range of motion with particular focus on internal range of motion, (3) optimization of the flexibility and muscle performance of the scapular muscles, and (4) increasing the functional sport-specific load on the shoulder girdle.
Continue to: A common approach to the care of...
A common approach to the care of a patient after a glenohumeral joint dislocation is to place the patient’s shoulder in a sling for comfort, with permitted pain-free isometric exercise along with passive and assisted elevation up to 100°.16 This is followed by a nonaggressive rehabilitation protocol for 2 months until full recovery, which includes progressive range of motion, strength, proprioception, and return to functional activities.16
More aggressive return-to-play protocols with accelerated timelines and functional progression have been studied, including in a multicenter observational study that followed 45 contact intercollegiate athletes prospectively after in-season anterior glenohumeral instability. Thirty-three of 45 (73%) athletes returned to sport for either all or part of the season after a median 5 days lost from competition, with 12 athletes (27%) successfully completing the season without recurrence. Athletes with a subluxation event were 5.3 times more likely to return to sport during the same season, compared with those with dislocations.17
Dynamic bracing may also allow for a safe and quicker return to sport in athletes18 but recently was shown to not impact recurrent dislocation risk.19
Return to play should be based on subjective assessment as well as objective measurements of range of motion, strength, and dynamic function.15 Patients who continue to have significant weakness and pain at 2 to 3 weeks post injury despite physical therapy should be re-evaluated with an MRI for concomitant rotator cuff tears and need for surgical referral.20
When to consider surgical intervention
In a recent meta-analysis, recurrent dislocation and instability occurred at a rate of 52.9% following nonsurgical treatment.2 The decision to perform surgical intervention is typically made following failure of conservative management. Other considerations include age, gender, bone loss, and cartilage defect.21,22 Age younger than 30 years, participation in competition, contact sports, and male gender have been associated with an increased risk of recurrence.23-25 For this reason, obtaining an MRI at time of first dislocation can help facilitate surgical decisions if the patient is at high risk for surgical need.26
Continue to: An increasing number...
An increasing number of dislocations portends a poor outcome with nonoperative treatment. Kao et al demonstrated a second dislocation leads to another dislocation in 19.6% of cases, while 44.3% of those with a third dislocation event will sustain another dislocation.24 Surgery should be considered for patients with recurrent instability events to prevent persistent instability and decrease the amount of bone loss that can occur with repetitive dislocations.
What are the surgical options?
Several surgical options exist to remedy the unstable shoulder. Procedures can range from an arthroscopic repair to an open stabilization combined with structural bone graft to replace a bone defect caused by repetitive dislocations.
Arthroscopic techniques have become the mainstay of treatment and account for 71% of stabilization procedures performed.21 These techniques cause less pain in the early postoperative period and provide for a faster return to work.27 Arthroscopy has the additional advantage of allowing for complete visualization of the glenohumeral joint to identify and address concomitant pathology, such as intra-articular loose bodies or rotator cuff tears.
Open repair was the mainstay of treatment prior to development of arthroscopic techniques. Some surgeons still prefer this method—especially in high-risk groups—because of a lower risk of recurrent disloca-tion.28 Open techniques often involve detachment and repair of the upper subscapularis tendon and are more likely to produce long-term losses in external rotation range of motion.28
Which one is appropriate for your patient? The decision to pursue an open or arthroscopic procedure and to augment with bone graft depends on the amount of glenoid and humeral head bone loss, patient activity level, risk of recurrent dislocation, and surgeon preference.
Continue to: For the nonathletic population...
For the nonathletic population, the timing of injury is less critical and surgery is typically recommended after conservative treatment has failed. In an athletic population, the timing of injury is a necessary consideration. An injury midseason may be “rehabbed” in hopes of returning to play. Individuals with injuries occurring at the end of a season, who are unable to regain desired function, and/or with peri-articular fractures or associated full-thickness rotator cuff tears may benefit from sooner surgical intervention.21
Owens et al have described appropriate surgical indications and recommendations for an in-season athlete.21 In this particular algorithm, the authors suggest obtaining an MRI for decision making, but this is specific to in-season athletes wishing to return to play. In general, an MRI is not always indicated for patients who wish to receive conservative therapy but would be indicated for surgical considerations. The algorithm otherwise uses bone and soft-tissue injury, recurrent instability, and timing in the season to help determine management.21
Outcomes: Surgery has advantages …
Recurrence rates following surgical intervention are considerably lower than with conservative management, especially among young, active individuals. A recent systematic review by Donohue et al demonstrated recurrent instability rates following surgical intervention as low as 2.4%.29 One study comparing the outcome of arthroscopic repair vs conservative management showed that the risk of postoperative instability was reduced by 20% compared to other treatments.7 Furthermore, early surgical fixation can improve quality of life, produce better functional outcomes, decrease time away from activity, increase patient satisfaction, and slow the development of glenohumeral osteoarthritis produced from recurrent instability.2,7
Complications. Surgery does carry inherent risks of infection, anesthesia effects, surgical complications, and surgical failure. Recurrent instability is the most common complication following surgical shoulder stabilization. Rates of recurrent instability after surgical stabilization depend on patient age, activity level, and amount of bone loss: males younger than 18 years who participate in contact competitive sports and have significant bone loss are more likely to have recurrent dislocation after surgery.23 The type of surgical procedure selected may decrease this risk.
While the open procedures decrease risk of postoperative instability, these surgeries can pose a significant risk of complications. Major complications for specific open techniques have been reported in up to 30% of patients30 and are associated with lower levels of surgeon experience.31 While the healing of bones and ligaments is always a concern, 1 of the most feared complications following stabilization surgery is iatrogenic nerve injury. Because of the axillary nerve’s close proximity to the inferior glenoid, this nerve can be injured without meticulous care and can result in paralysis of the deltoid muscle. This injury poses a major impediment to normal shoulder function. Some procedures may cause nerve injuries in up to 10% of patients, although most injuries are transient.32
Continue to: Bottom line
Bottom line
Due to the void of evidence-based guidelines for conservative vs surgical management of primary shoulder dislocation, it would be prudent to have a risk-benefit discussion with patients regarding treatment options.
Patients older than 30 years and those with uncomplicated injuries are best suited for conservative management of primary shoulder dislocations. Immobilization is debated and may not change outcomes, but a progressive rehabilitative program after the initial acute injury is helpful. Risk factors for failing conservative management include recurrent dislocation, subsequent arthropathy, and additional concomitant bone or soft-tissue injuries.
Patients younger than 30 years who have complicated injuries with bone or cartilage loss, rotator cuff tears, or recurrent instability, and highly physically active individuals are best suited for surgical management. Shoulder arthroscopy has become the mainstay of surgical treatment for shoulder dislocations. Outcomes are favorable and dislocation recurrence is low after surgical repair. Surgery does carry its own inherent risks of infection, anesthesia effects, complications during surgery, and surgical failure leading to recurrent instability.
Cayce Onks, DO, MS, ATC, Penn State Hershey, Milton S. Hershey Medical Center, Penn State College of Medicine, Family and Community Medicine H154, 500 University Drive, PO Box 850, Hershey, PA 17033-0850; [email protected]
The shoulder, or glenohumeral joint, is the most commonly dislocated large joint; dislocation occurs at a rate of 23.9 per 100,000 person/years.1,2 There are 2 types of dislocation: traumatic anterior dislocation, which accounts for roughly 90% of dislocations, and posterior dislocation (10%).3 Anterior dislocation typically occurs when the patient’s shoulder is forcefully abducted and externally rotated.
The diagnosis is made after review of the history and mechanism of injury and performance of a complete physical exam with imaging studies—the most critical component of diagnosis.4 Standard radiographs (anteroposterior, axillary, and scapular Y) can confirm the presence of a dislocation; once the diagnosis is confirmed, closed reduction of the joint should be performed.1 (Methods of reduction are beyond the scope of this article but have been recently reviewed.5)
Risk for recurrence drives management choices
Following an initial shoulder dislocation, the risk of recurrence is high.6,7 Rates vary based on age, pathology after dislocation, activity level, type of immobilization, and whether surgery was performed. Overall, age is the strongest predictor of recurrence: 72% of patients ages 12 to 22 years, 56% of those ages 23 to 29 years, and 27% of those older than 30 years experience recurrence.6 Patients who have recurrent dislocations are at risk for arthropathy, fear of instability, and worsening surgical outcomes.6
Reducing the risk of a recurrent shoulder dislocation has been the focus of intense study. Proponents of surgical stabilization argue that surgery—rather than a trial of conservative treatment—is best when you consider the high risk of recurrence in young athletes (the population primarily studied), the soft-tissue and bony damage caused by recurrent instability, and the predictable improvement in quality of life following surgery.
In a recent systematic review and meta-analysis, there was evidence that, for first-time traumatic shoulder dislocations, early surgery led to fewer repeat shoulder dislocations (number needed to treat [NNT] = 2-4.7). However, a significant number of patients primarily treated nonoperatively did not experience a repeat shoulder dislocation within 2 years.2
The conflicting results from randomized trials comparing operative intervention to conservative management have led surgeons and physicians in other specialties to take different approaches to the management of shoulder dislocation.2 In this review, we aim to summarize considerations for conservative vs surgical management and provide clinical guidance for primary care physicians.
When to try conservative management
Although the initial treatment after a traumatic anterior shoulder dislocation has been debated, a recent meta-analysis of randomized controlled trials showed that at least half of first-time dislocations are successfully treated with conservative management.2 Management can include immobilization for comfort and/or physical therapy. Age will play a role, as mentioned earlier; in general, patients older than 30 have a significant decrease in recurrence rate and are good candidates for conservative therapy.6 It should be noted that much of the research with regard to management of shoulder dislocations has been done in an athletic population.
Continue to: Immobilization may benefit some
Immobilization may benefit some
Recent evidence has determined that the duration of immobilization in internal rotation does not impact recurrent instability.8,9 In patients older than 30, the rate of repeat dislocation is lower, and early mobilization after 1 week is advocated to avoid joint stiffness and minimize the risk of adhesive capsulitis.10
Arm position during immobilization remains controversial.11 In a classic study by Itoi et al, immobilization for 3 weeks in internal rotation vs 10° of external rotation was associated with a recurrence rate of 42% vs 26%, respectively.12 In this study, immobilization in 10° of external rotation was especially beneficial for patients ages 30 years or younger.12
Cadaveric and magnetic resonance imaging (MRI) studies have shown external rotation may improve the odds of labral tear healing by positioning the damaged and intact parts of the glenoid labrum in closer proximity.13 While this is theoretically plausible, a recent Cochrane review found insufficient evidence to determine whether immobilization in external rotation has any benefits beyond those offered by internal rotation.14 A recent systematic review and meta-analysis found that immobilization in external rotation vs internal rotation after a first-time traumatic shoulder dislocation did not change outcomes.2 With that said, most would prefer to immobilize in the internal rotation position for ease.
More research is needed. A Cochrane review highlighted the need for continued research.14 Additionally, most of the available randomized controlled trials to date have consisted of young men, with the majority of dislocations related to sports activities. Women, nonathletes, and older patients have been understudied to date; extrapolating current research to those groups of patients may not be appropriate and should be a focus for future research.2
Physical therapy: The conservative standard of care
Rehabilitation after glenohumeral joint dislocation is the current standard of care in conservative management to reduce the risk for repeat dislocation.15 Depending on the specific characteristics of the instability pattern, the approach may be adapted to the patient. A recent review focused on the following 4 key points: (1) restoration of rotator cuff strength, focusing on the eccentric capacity of the external rotators, (2) normalization of rotational range of motion with particular focus on internal range of motion, (3) optimization of the flexibility and muscle performance of the scapular muscles, and (4) increasing the functional sport-specific load on the shoulder girdle.
Continue to: A common approach to the care of...
A common approach to the care of a patient after a glenohumeral joint dislocation is to place the patient’s shoulder in a sling for comfort, with permitted pain-free isometric exercise along with passive and assisted elevation up to 100°.16 This is followed by a nonaggressive rehabilitation protocol for 2 months until full recovery, which includes progressive range of motion, strength, proprioception, and return to functional activities.16
More aggressive return-to-play protocols with accelerated timelines and functional progression have been studied, including in a multicenter observational study that followed 45 contact intercollegiate athletes prospectively after in-season anterior glenohumeral instability. Thirty-three of 45 (73%) athletes returned to sport for either all or part of the season after a median 5 days lost from competition, with 12 athletes (27%) successfully completing the season without recurrence. Athletes with a subluxation event were 5.3 times more likely to return to sport during the same season, compared with those with dislocations.17
Dynamic bracing may also allow for a safe and quicker return to sport in athletes18 but recently was shown to not impact recurrent dislocation risk.19
Return to play should be based on subjective assessment as well as objective measurements of range of motion, strength, and dynamic function.15 Patients who continue to have significant weakness and pain at 2 to 3 weeks post injury despite physical therapy should be re-evaluated with an MRI for concomitant rotator cuff tears and need for surgical referral.20
When to consider surgical intervention
In a recent meta-analysis, recurrent dislocation and instability occurred at a rate of 52.9% following nonsurgical treatment.2 The decision to perform surgical intervention is typically made following failure of conservative management. Other considerations include age, gender, bone loss, and cartilage defect.21,22 Age younger than 30 years, participation in competition, contact sports, and male gender have been associated with an increased risk of recurrence.23-25 For this reason, obtaining an MRI at time of first dislocation can help facilitate surgical decisions if the patient is at high risk for surgical need.26
Continue to: An increasing number...
An increasing number of dislocations portends a poor outcome with nonoperative treatment. Kao et al demonstrated a second dislocation leads to another dislocation in 19.6% of cases, while 44.3% of those with a third dislocation event will sustain another dislocation.24 Surgery should be considered for patients with recurrent instability events to prevent persistent instability and decrease the amount of bone loss that can occur with repetitive dislocations.
What are the surgical options?
Several surgical options exist to remedy the unstable shoulder. Procedures can range from an arthroscopic repair to an open stabilization combined with structural bone graft to replace a bone defect caused by repetitive dislocations.
Arthroscopic techniques have become the mainstay of treatment and account for 71% of stabilization procedures performed.21 These techniques cause less pain in the early postoperative period and provide for a faster return to work.27 Arthroscopy has the additional advantage of allowing for complete visualization of the glenohumeral joint to identify and address concomitant pathology, such as intra-articular loose bodies or rotator cuff tears.
Open repair was the mainstay of treatment prior to development of arthroscopic techniques. Some surgeons still prefer this method—especially in high-risk groups—because of a lower risk of recurrent disloca-tion.28 Open techniques often involve detachment and repair of the upper subscapularis tendon and are more likely to produce long-term losses in external rotation range of motion.28
Which one is appropriate for your patient? The decision to pursue an open or arthroscopic procedure and to augment with bone graft depends on the amount of glenoid and humeral head bone loss, patient activity level, risk of recurrent dislocation, and surgeon preference.
Continue to: For the nonathletic population...
For the nonathletic population, the timing of injury is less critical and surgery is typically recommended after conservative treatment has failed. In an athletic population, the timing of injury is a necessary consideration. An injury midseason may be “rehabbed” in hopes of returning to play. Individuals with injuries occurring at the end of a season, who are unable to regain desired function, and/or with peri-articular fractures or associated full-thickness rotator cuff tears may benefit from sooner surgical intervention.21
Owens et al have described appropriate surgical indications and recommendations for an in-season athlete.21 In this particular algorithm, the authors suggest obtaining an MRI for decision making, but this is specific to in-season athletes wishing to return to play. In general, an MRI is not always indicated for patients who wish to receive conservative therapy but would be indicated for surgical considerations. The algorithm otherwise uses bone and soft-tissue injury, recurrent instability, and timing in the season to help determine management.21
Outcomes: Surgery has advantages …
Recurrence rates following surgical intervention are considerably lower than with conservative management, especially among young, active individuals. A recent systematic review by Donohue et al demonstrated recurrent instability rates following surgical intervention as low as 2.4%.29 One study comparing the outcome of arthroscopic repair vs conservative management showed that the risk of postoperative instability was reduced by 20% compared to other treatments.7 Furthermore, early surgical fixation can improve quality of life, produce better functional outcomes, decrease time away from activity, increase patient satisfaction, and slow the development of glenohumeral osteoarthritis produced from recurrent instability.2,7
Complications. Surgery does carry inherent risks of infection, anesthesia effects, surgical complications, and surgical failure. Recurrent instability is the most common complication following surgical shoulder stabilization. Rates of recurrent instability after surgical stabilization depend on patient age, activity level, and amount of bone loss: males younger than 18 years who participate in contact competitive sports and have significant bone loss are more likely to have recurrent dislocation after surgery.23 The type of surgical procedure selected may decrease this risk.
While the open procedures decrease risk of postoperative instability, these surgeries can pose a significant risk of complications. Major complications for specific open techniques have been reported in up to 30% of patients30 and are associated with lower levels of surgeon experience.31 While the healing of bones and ligaments is always a concern, 1 of the most feared complications following stabilization surgery is iatrogenic nerve injury. Because of the axillary nerve’s close proximity to the inferior glenoid, this nerve can be injured without meticulous care and can result in paralysis of the deltoid muscle. This injury poses a major impediment to normal shoulder function. Some procedures may cause nerve injuries in up to 10% of patients, although most injuries are transient.32
Continue to: Bottom line
Bottom line
Due to the void of evidence-based guidelines for conservative vs surgical management of primary shoulder dislocation, it would be prudent to have a risk-benefit discussion with patients regarding treatment options.
Patients older than 30 years and those with uncomplicated injuries are best suited for conservative management of primary shoulder dislocations. Immobilization is debated and may not change outcomes, but a progressive rehabilitative program after the initial acute injury is helpful. Risk factors for failing conservative management include recurrent dislocation, subsequent arthropathy, and additional concomitant bone or soft-tissue injuries.
Patients younger than 30 years who have complicated injuries with bone or cartilage loss, rotator cuff tears, or recurrent instability, and highly physically active individuals are best suited for surgical management. Shoulder arthroscopy has become the mainstay of surgical treatment for shoulder dislocations. Outcomes are favorable and dislocation recurrence is low after surgical repair. Surgery does carry its own inherent risks of infection, anesthesia effects, complications during surgery, and surgical failure leading to recurrent instability.
Cayce Onks, DO, MS, ATC, Penn State Hershey, Milton S. Hershey Medical Center, Penn State College of Medicine, Family and Community Medicine H154, 500 University Drive, PO Box 850, Hershey, PA 17033-0850; [email protected]
1. Lin K, James E, Spitzer E, et al. Pediatric and adolescent anterior shoulder instability: clinical management of first time dislocators. Curr Opin Pediatr. 2018;30:49-56.
2. Kavaja L, Lähdeoja T, Malmivaara A, et al. Treatment after traumatic shoulder dislocation: a systematic review with a network meta-analysis. Br J Sports Med. 2018;52:1498-1506.
3. Brelin A, Dickens JF. Posterior shoulder instability. Sports Med Arthrosc Rev. 2017;25:136-143.
4. Galvin JW, Ernat JJ, Waterman BR, et al. The epidemiology and natural history of anterior shoulder dislocation. Curr Rev Musculoskelet Med. 2017;10:411-424.
5. Rozzi SL, Anderson JM, Doberstein ST, et al. National Athletic Trainers’ Association position statement: immediate management of appendicular joint dislocations. J Athl Train. 2018;53:1117-1128.
6. Hovelius L, Saeboe M. Arthropathy after primary anterior shoulder dislocation: 223 shoulders prospectively followed up for twenty-five years. J Shoulder Elbow Surg. 2009;18:339-347.
7. Polyzois I, Dattani R, Gupta R, et al. Traumatic first time shoulder dislocation: surgery vs non-operative treatment. Arch Bone Jt Surg. 2016;4:104-108.
8. Cox CL, Kuhn JE. Operative versus nonoperative treatment of acute shoulder dislocation in the athlete. Curr Sports Med Rep. 2008;7:263-268.
9. Kuhn JE. Treating the initial anterior shoulder dislocation—an evidence-based medicine approach. Sports Med Arthrosc Rev. 2006;14:192-198.
10. Smith TO. Immobilization following traumatic anterior glenohumeral joint dislocation: a literature review. Injury. 2006;37:228-237.
11. Liavaag S, Brox JI, Pripp AH, et al. Immobilization in external rotation after primary shoulder dislocation did not reduce the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am. 2011;93:897-904.
12. Itoi E, Hatakeyama Y, Sato T, et al. Immobilization in external rotation after shoulder dislocation reduces the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am. 2007;89:2124-2131.
13. Miller BS, Sonnabend DH, Hatrick C, et al. Should acute anterior dislocations of the shoulder be immobilized in external rotation? A cadaveric study. J Shoulder Elbow Surg. 2004;13:589-592.
14. Hanchard NCA, Goodchild LM, Kottam L. Conservative management following closed reduction of traumatic anterior dislocation of the shoulder. Cochrane Database Syst Rev. 2014;(4):CD004962.
15. Cools AM, Borms D, Castelein B, et al. Evidence-based rehabilitation of athletes with glenohumeral instability. Knee Surg Sports Traumatol Arthrosc. 2016;24:382-389.
16. Lafuente JLA, Marco SM, Pequerul JMG. Controversies in the management of the first time shoulder dislocation. Open Orthop J. 2017;11:1001-1010.
17. Dickens JF, Owens BD, Cameron KL, et al. Return to play and recurrent instability after in-season anterior shoulder instability: a prospective multicenter study. Am J Sports Med. 2014;42:2842-2850.
18. Conti M, Garofalo R, Castagna A, et al. Dynamic brace is a good option to treat first anterior shoulder dislocation in season. Musculoskelet Surg. 2017;101(suppl 2):169-173.
19. Shanley E, Thigpen C, Brooks J, et al. Return to sport as an outcome measure for shoulder instability. Am J Sports Med. 2019;47:1062-1067.
20. Gombera MM, Sekiya JK. Rotator cuff tear and glenohumeral instability. Clin Orthop Relat Res. 2014;472:2448-2456.
21. Owens BD, Dickens JF, Kilcoyne KG, et al. Management of mid-season traumatic anterior shoulder instability in athletes. J Am Acad Orthop Surg. 2012;20:518-526.
22. Ozturk BY, Maak TG, Fabricant P, et al. Return to sports after arthroscopic anterior stabilization in patients aged younger than 25 years. Arthroscopy. 2013;29:1922-1931.
23. Balg F, Boileau P. The instability severity index score. A simple preoperative score to select patients for arthroscopic or open shoulder stabilisation. J Bone Joint Surg Br. 2007;89:1470-1477.
24. Kao J-T, Chang C-L, Su W-R, et al. Incidence of recurrence after shoulder dislocation: a nationwide database study. J Shoulder Elbow Surg. 2018;27:1519-1525.
25. Porcillini G, Campi F, Pegreffi F, et al. Predisposing factors for recurrent shoulder dislocation after arthroscopic treatment. J Bone Joint Surg Am. 2009;91:2537-2542.
26. Magee T. 3T MRI of the shoulder: is MR arthrography necessary? AJR Am J Roentgenol. 2009;192:86-92.
27. Green MR, Christensen KP. Arthroscopic versus open Bankart procedures: a comparison of early morbidity and complications. Arthroscopy. 1993;9:371-374.
28. Khatri K, Arora H, Chaudhary S, et al. Meta-analysis of randomized controlled trials involving anterior shoulder instability. Open Orthop J. 2018;12:411-418.
29. Donohue MA, Owens BD, Dickens JF. Return to play following anterior shoulder dislocations and stabilization surgery. Clin Sports Med. 2016;35:545-561.
30. Griesser MJ, Harris JD, McCoy BW, et al. Complications and re-operations after Bristow-Latarjet shoulder stabilization: a systematic review. J Shoulder Elbow Surg. 2013;22:286-292.
31. Ekhtiari S, Horner NS, Bedi A, et al. The learning curve for the Latarjet procedure: a systematic review. Orthop J Sports Med. 2018;6:2325967118786930.
32. Shah AA, Butler RB, Romanowski J, et al. Short-term complications of the Latarjet procedure. J Bone Joint Surg Am. 2012;94:495-501.
1. Lin K, James E, Spitzer E, et al. Pediatric and adolescent anterior shoulder instability: clinical management of first time dislocators. Curr Opin Pediatr. 2018;30:49-56.
2. Kavaja L, Lähdeoja T, Malmivaara A, et al. Treatment after traumatic shoulder dislocation: a systematic review with a network meta-analysis. Br J Sports Med. 2018;52:1498-1506.
3. Brelin A, Dickens JF. Posterior shoulder instability. Sports Med Arthrosc Rev. 2017;25:136-143.
4. Galvin JW, Ernat JJ, Waterman BR, et al. The epidemiology and natural history of anterior shoulder dislocation. Curr Rev Musculoskelet Med. 2017;10:411-424.
5. Rozzi SL, Anderson JM, Doberstein ST, et al. National Athletic Trainers’ Association position statement: immediate management of appendicular joint dislocations. J Athl Train. 2018;53:1117-1128.
6. Hovelius L, Saeboe M. Arthropathy after primary anterior shoulder dislocation: 223 shoulders prospectively followed up for twenty-five years. J Shoulder Elbow Surg. 2009;18:339-347.
7. Polyzois I, Dattani R, Gupta R, et al. Traumatic first time shoulder dislocation: surgery vs non-operative treatment. Arch Bone Jt Surg. 2016;4:104-108.
8. Cox CL, Kuhn JE. Operative versus nonoperative treatment of acute shoulder dislocation in the athlete. Curr Sports Med Rep. 2008;7:263-268.
9. Kuhn JE. Treating the initial anterior shoulder dislocation—an evidence-based medicine approach. Sports Med Arthrosc Rev. 2006;14:192-198.
10. Smith TO. Immobilization following traumatic anterior glenohumeral joint dislocation: a literature review. Injury. 2006;37:228-237.
11. Liavaag S, Brox JI, Pripp AH, et al. Immobilization in external rotation after primary shoulder dislocation did not reduce the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am. 2011;93:897-904.
12. Itoi E, Hatakeyama Y, Sato T, et al. Immobilization in external rotation after shoulder dislocation reduces the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am. 2007;89:2124-2131.
13. Miller BS, Sonnabend DH, Hatrick C, et al. Should acute anterior dislocations of the shoulder be immobilized in external rotation? A cadaveric study. J Shoulder Elbow Surg. 2004;13:589-592.
14. Hanchard NCA, Goodchild LM, Kottam L. Conservative management following closed reduction of traumatic anterior dislocation of the shoulder. Cochrane Database Syst Rev. 2014;(4):CD004962.
15. Cools AM, Borms D, Castelein B, et al. Evidence-based rehabilitation of athletes with glenohumeral instability. Knee Surg Sports Traumatol Arthrosc. 2016;24:382-389.
16. Lafuente JLA, Marco SM, Pequerul JMG. Controversies in the management of the first time shoulder dislocation. Open Orthop J. 2017;11:1001-1010.
17. Dickens JF, Owens BD, Cameron KL, et al. Return to play and recurrent instability after in-season anterior shoulder instability: a prospective multicenter study. Am J Sports Med. 2014;42:2842-2850.
18. Conti M, Garofalo R, Castagna A, et al. Dynamic brace is a good option to treat first anterior shoulder dislocation in season. Musculoskelet Surg. 2017;101(suppl 2):169-173.
19. Shanley E, Thigpen C, Brooks J, et al. Return to sport as an outcome measure for shoulder instability. Am J Sports Med. 2019;47:1062-1067.
20. Gombera MM, Sekiya JK. Rotator cuff tear and glenohumeral instability. Clin Orthop Relat Res. 2014;472:2448-2456.
21. Owens BD, Dickens JF, Kilcoyne KG, et al. Management of mid-season traumatic anterior shoulder instability in athletes. J Am Acad Orthop Surg. 2012;20:518-526.
22. Ozturk BY, Maak TG, Fabricant P, et al. Return to sports after arthroscopic anterior stabilization in patients aged younger than 25 years. Arthroscopy. 2013;29:1922-1931.
23. Balg F, Boileau P. The instability severity index score. A simple preoperative score to select patients for arthroscopic or open shoulder stabilisation. J Bone Joint Surg Br. 2007;89:1470-1477.
24. Kao J-T, Chang C-L, Su W-R, et al. Incidence of recurrence after shoulder dislocation: a nationwide database study. J Shoulder Elbow Surg. 2018;27:1519-1525.
25. Porcillini G, Campi F, Pegreffi F, et al. Predisposing factors for recurrent shoulder dislocation after arthroscopic treatment. J Bone Joint Surg Am. 2009;91:2537-2542.
26. Magee T. 3T MRI of the shoulder: is MR arthrography necessary? AJR Am J Roentgenol. 2009;192:86-92.
27. Green MR, Christensen KP. Arthroscopic versus open Bankart procedures: a comparison of early morbidity and complications. Arthroscopy. 1993;9:371-374.
28. Khatri K, Arora H, Chaudhary S, et al. Meta-analysis of randomized controlled trials involving anterior shoulder instability. Open Orthop J. 2018;12:411-418.
29. Donohue MA, Owens BD, Dickens JF. Return to play following anterior shoulder dislocations and stabilization surgery. Clin Sports Med. 2016;35:545-561.
30. Griesser MJ, Harris JD, McCoy BW, et al. Complications and re-operations after Bristow-Latarjet shoulder stabilization: a systematic review. J Shoulder Elbow Surg. 2013;22:286-292.
31. Ekhtiari S, Horner NS, Bedi A, et al. The learning curve for the Latarjet procedure: a systematic review. Orthop J Sports Med. 2018;6:2325967118786930.
32. Shah AA, Butler RB, Romanowski J, et al. Short-term complications of the Latarjet procedure. J Bone Joint Surg Am. 2012;94:495-501.
PRACTICE RECOMMENDATIONS
› Start with conservative management of shoulder dislocation in patients older than 30 years and those with uncomplicated injuries. B
› Discourage strict immobilization; its utility is debated and it may not change outcomes. B
› Recommend a progressive rehabilitative program after the initial acute shoulder injury. B
› Consider surgical management for patients younger than 30 years who have complicated injuries with bone or cartilage loss, rotator cuff tears, or recurrent instability or for the highly physically active individual. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Low back pain in youth: Recognizing red flags
Low back pain in not uncommon in children and adolescents.1-3 Although the prevalence of low back pain in children < 7 years is low, it increases with age, with studies reporting lifetime prevalence at age 12 years between 16% and 18% and rates as high as 66% by 16 years of age.4,5 Although children and adolescents usually have pain that is transient and benign without a defined cause, structural causes of low back pain should be considered in school-aged children with pain that persists for > 3 to 6 weeks. 4 The most common structural causes of adolescent low back pain are reviewed here.
Etiology: A mixed bag
Back pain in school-aged children is most commonly due to muscular strain, overuse, or poor posture. The pain is often transient in nature and responds to rest and postural education.4,6 A herniated disc is an uncommon finding in younger school-aged children, but incidence increases slightly among older adolescents, particularly those who are active in collision sports and/or weight-lifting.7,8 Pain caused by a herniated disc often radiates along the distribution of the sciatic nerve and worsens during lumbar flexion.
Spondylolysis and spondylolisthesis are important causes of back pain in children. Spondylolysis is defined as a defect or abnormality of the pars interarticularis and surrounding lamina and pedicle. Spondylolisthesis, which is less common, is defined as the translation or “slippage” of one vertebral segment in relation to the next caudal segment. These conditions commonly occur as a result of repetitive stress.
In a prospective study of adolescents < 19 years with low back pain for > 2 weeks, the prevalence of spondylolysis was 39.7%.9 Adolescent athletes with symptomatic low back pain are more likely to have spondylolysis than nonathletes (32% vs 2%, respectively).2,10 Pain is often made worse by extension of the spine. Spondylolysis and spondylolisthesis can be congenital or acquired, and both can be asymptomatic. Children and teens who are athletes are at higher risk for symptomatic spondylolysis and spondylolisthesis.10-12 This is especially true for those involved in gymnastics, dance, football, and/or volleyball, where a repetitive load is placed onto an extended spine.
Idiopathic scoliosis is an abnormal lateral curvature of the spine that usually develops during adolescence and worsens with growth. Historically, painful scoliosis was considered rare, but more recently researchers determined that children with scoliosis have a higher rate of pain compared to their peers.13,14 School-aged children with scoliosis were found to be at 2 times the risk of low back pain compared to those without scoliosis.13 It is important to identify scoliosis in adolescents so that progression can be monitored.
Screening for scoliosis in primary care is somewhat controversial. The US Preventive Services Task Force (USPSTF) finds insufficient evidence for screening asymptomatic adolescents for scoliosis.15 This recommendation is based on the fact that there is little evidence on the effect of screening on long-term outcomes. Screening may also lead to unnecessary radiation. Conversely, a position statement released by the Scoliosis Research Society, the Pediatric Orthopedic Society of North America, the American Association of Orthopedic Surgeons, and the American Academy of Pediatrics recommends scoliosis screening during routine pediatric office visits.16 Screening for girls is recommended at ages 10 and 12 years, and for boys, once between ages 13 and 14 years. The statement highlights evidence showing that focused screening by appropriate personnel has value in detecting a clinically significant curve (> 20°).
Scheuermann disease is a rare cause of back pain in children that usually develops during adolescence and results in increasing thoracic kyphosis. An autosomal dominant mutation plays a role in this disease of the growth cartilage endplate; repetitive strain on the growth cartilage is also a contributing factor.17,18 An atypical variant manifests with kyphosis in the thoracolumbar region.17
Continue to: Other causes of low back pain
Other causes of low back pain—including inflammatory arthritis, infection (eg, discitis), and tumor—are rare in children but must always be considered, especially in the setting of persistent symptoms.4,19-21 More on the features of these conditions is listed in TABLE 1.1-7,13-15,17-30

History: Focus on onset, timing, and duration of symptoms
As with adults, obtaining a history that includes the onset, timing, and duration of symptoms is key in the evaluation of low back pain in children, as is obtaining a history of the patient’s activities; sports that repetitively load the lumbar spine in an extended position increase the risk of injury.10

Specific risk factors for low back pain in children and adolescents are poorly understood.4,9,31 Pain can be associated with trauma, or it can have a more progressive or insidious onset. Generally, pain that is present for up to 6 weeks and is intermittent or improving has a self-limited course. Pain that persists beyond 3 to 6 weeks or is worsening is more likely to have an anatomical cause that needs further evaluation.2,3,10,21
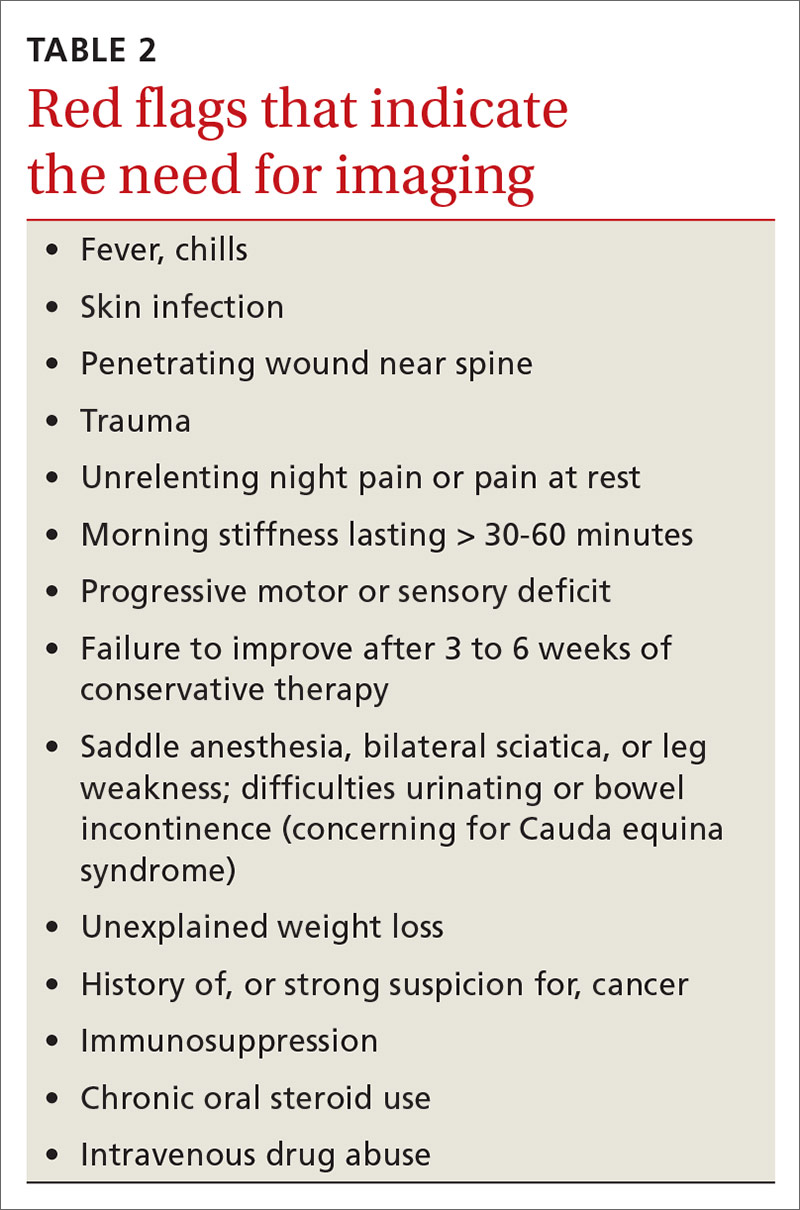
Identifying exacerbating and alleviating factors can provide useful information. Pain that is worse with lumbar flexion is more likely to come from muscular strain or disc pathology. Pain with extension is more likely due to a structural cause such as spondylolysis/spondylolisthesis, scoliosis, or Scheuermann disease.2,4,10,17,18,21 See TABLE 2 for red flag symptoms that indicate the need for imaging and further work-up.
The physical exam: Visualize, assess range of motion, and reproduce pain
The physical examination of any patient with low back pain should include direct visualization and inspection of the back, spine, and pelvis; palpation of the spine and paraspinal regions; assessment of lumbar range of motion and of the lumbar nerve roots, including tests of sensation, strength, and deep tendon reflexes; and an evaluation of the patient’s posture, which can provide clues to underlying causes of pain.
Continue to: Increased thoracic kyphosis...
Increased thoracic kyphosis that is not reversible is concerning for Scheuermann disease.9,17,18 A significant elevation in one shoulder or side of the pelvis can be indicative of scoliosis. Increased lumbar lordosis may predispose a patient to spondylolysis.
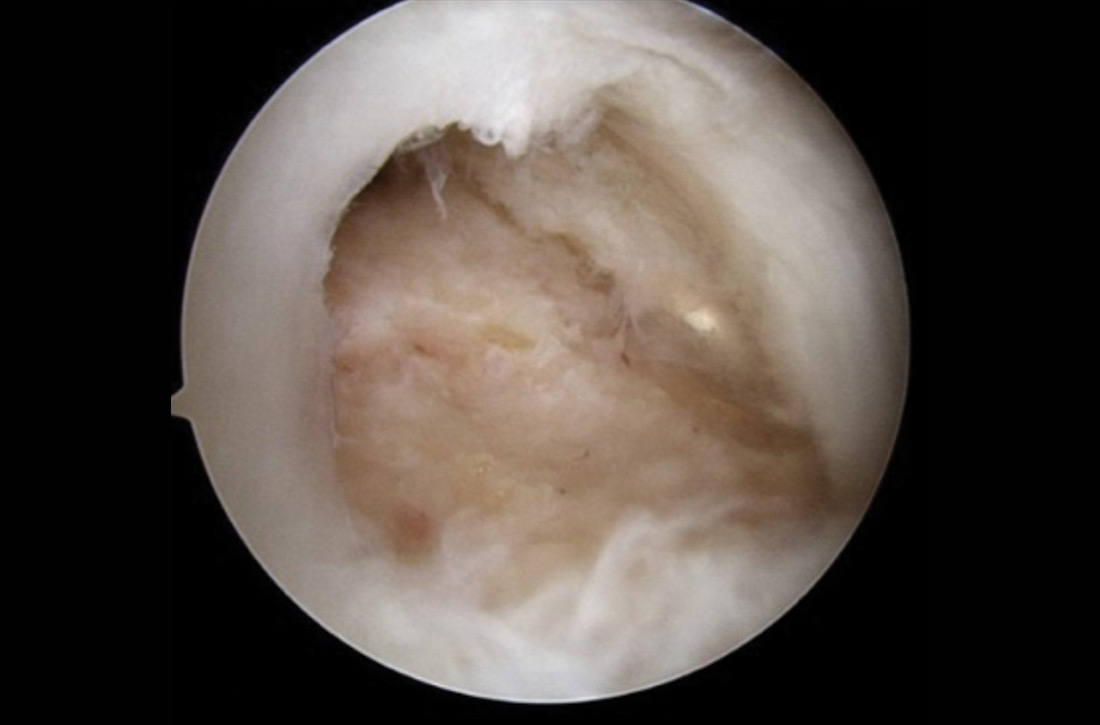
In patients with spondylolysis, lumbar extension will usually reproduce pain, which is often unilateral. Hyperextension in a single-leg stance, commonly known as the Stork test, is positive for unilateral spondylolysis when it reproduces pain on the ipsilateral side. The sensitivity of the Stork test for unilateral spondylolysis is approximately 50%.32 (For more information on the Stork test, see www.physio-pedia.com/Stork_test.)
Pain reproduced with lumbar flexion is less concerning for bony pathology and is most often related to soft-tissue strain. Lumbar flexion with concomitant radicular pain is associated with disc pathology.8 Pain with a straight-leg raise is also associated with disk pathology, especially if raising the contralateral leg increases pain.8
Using a scoliometer. Evaluate the flexed spine for the presence of asymmetry, which can indicate scoliosis.33 If asymmetry is present, use a scoliometer to determine the degree of asymmetry. Zero to 5° is considered clinically insignificant; monitor and reevaluate these patients at subsequent visits.34,35 Ten degrees or more of asymmetry with a scoliometer should prompt you to order radiographs.35,36 A smartphone-based scoliometer for iPhones was evaluated in 1 study and was shown to have reasonable reliability and validity for clinical use.37
Deformity of the lower extremities. Because low back pain may be caused by biomechanical or structural deformity of the lower extremities, examine the flexibility of the hip flexors, gluteal musculature, hamstrings, and the iliotibial band.38 In addition, evaluate for leg-length discrepancy and lower-extremity malalignment, such as femoral anteversion, tibial torsion, or pes planus.
Continue to: Imaging
Imaging: Know when it’s needed
Although imaging of the lumbar spine is often unnecessary in the presence of acute low back pain in children, always consider imaging in the setting of bony tenderness, pain that wakes a patient from sleep, and in the setting of other red flag symptoms (see TABLE 2). Low back pain in children that is reproducible with lumbar extension is concerning for spondylolysis or spondylolisthesis. If the pain with extension persists beyond 3 to 6 weeks, order imaging starting with radiographs.2,39
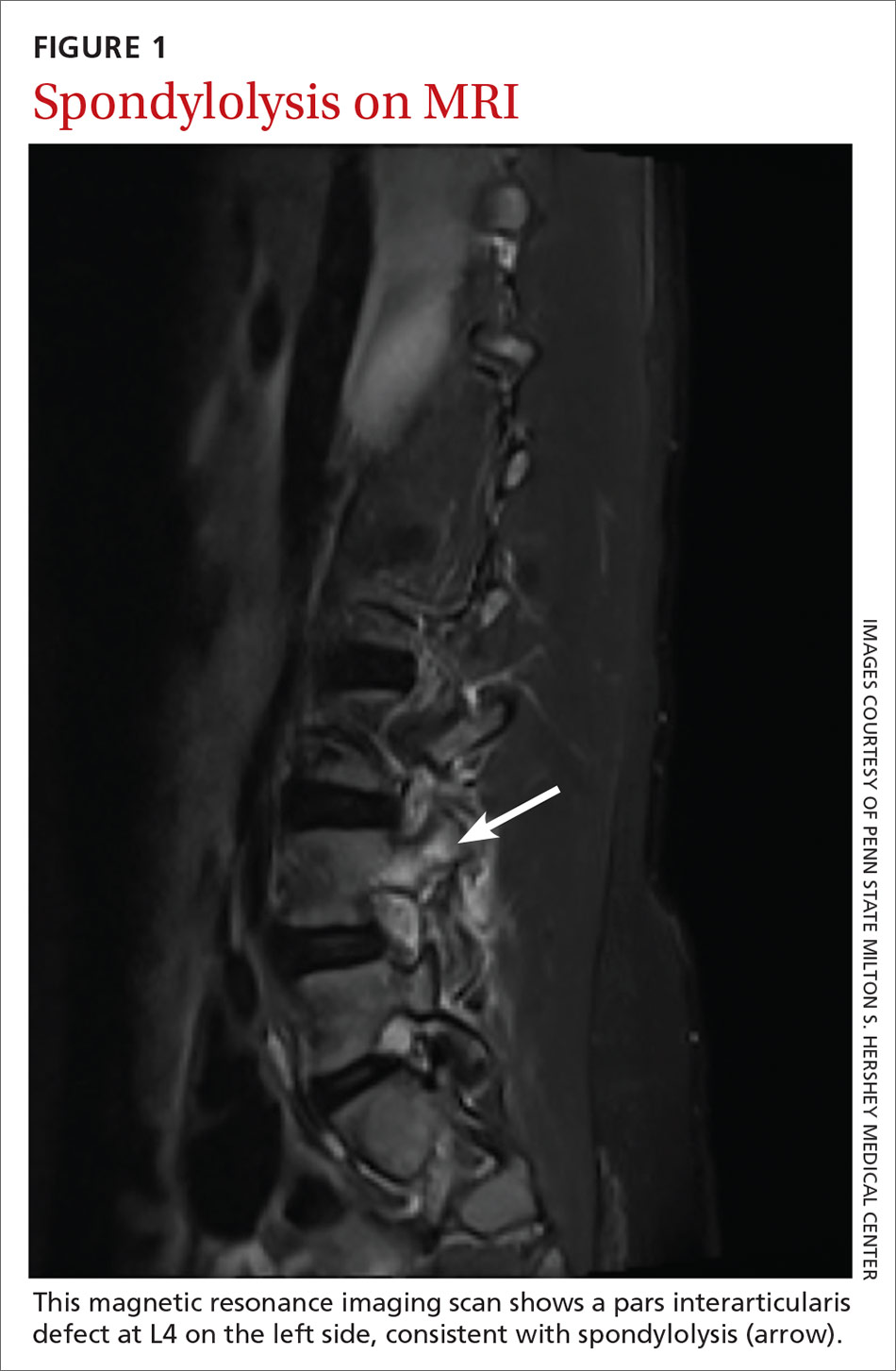
Traditionally, 4 views of the spine—anteroposterior (AP), lateral, and oblique (one right and one left)—were obtained, but recent evidence indicates that 2 views (AP and lateral) have similar sensitivity and specificity to 4 views with significantly reduced radiation exposure.2,39 Because the sensitivity of plain films is relatively low, consider more advanced imaging if spondylolysis or spondylolisthesis is strongly suspected. Recent studies indicate that magnetic resonance imaging (MRI) may be as effective as computed tomography (CT) or bone scan and has the advantage of lower radiation (FIGURE 1).2,22
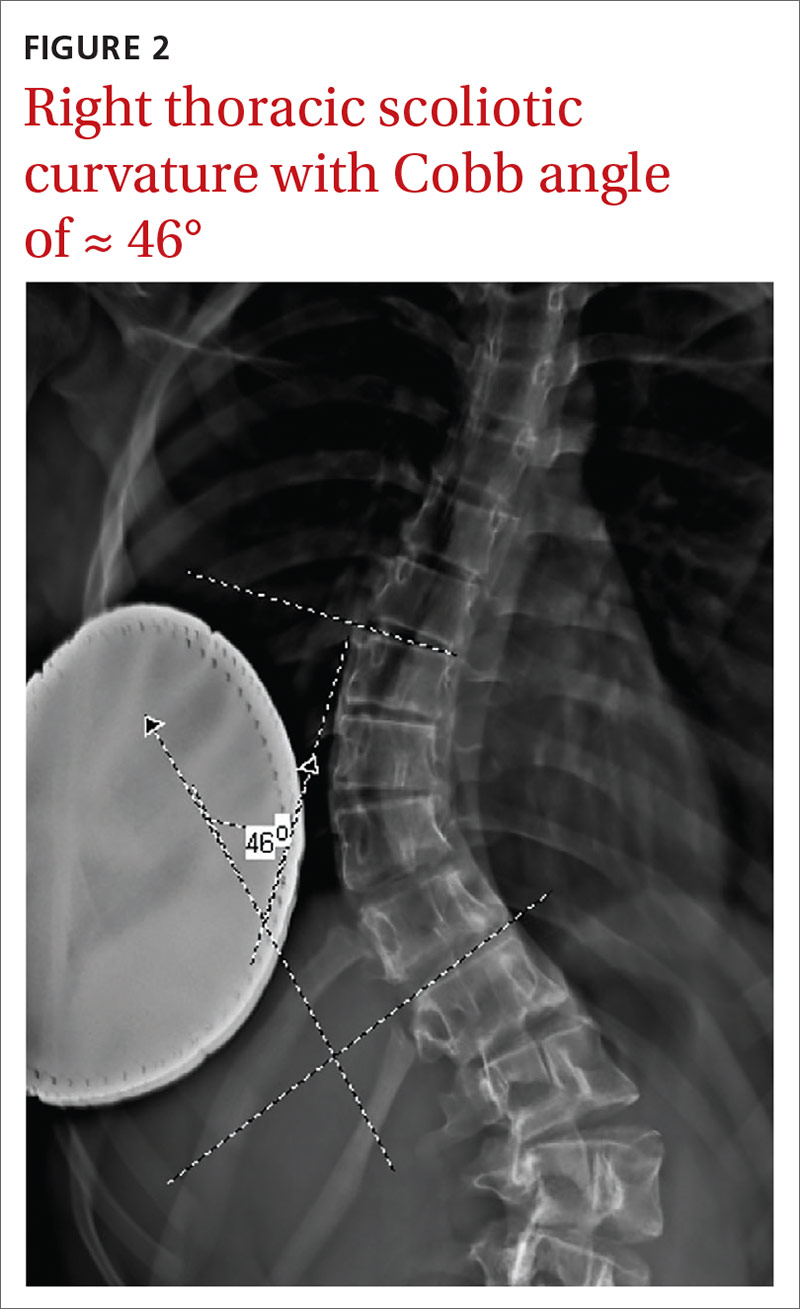
Similarly, order radiographs if there is > 10° of asymmetry noted on physical exam using a scoliometer.15,23 Calculate the Cobb angle to determine the severity of scoliosis. Refer patients with angles ≥ 20° to a pediatric orthopedist for monitoring of progression and consideration of bracing (FIGURE 2).23,34 For patients with curvatures between 10° and 19°, repeat imaging every 6 to 12 months. Because scoliosis is a risk factor for spondylolysis, evaluate radiographs in the setting of painful scoliosis for the presence of a spondylolysis.34,35
If excessive kyphosis is noted on exam, order radiographs to evaluate for Scheuermann disease. Classic imaging findings include Schmorl nodes, vertebral endplate changes, and anterior wedging (FIGURE 3).17,18
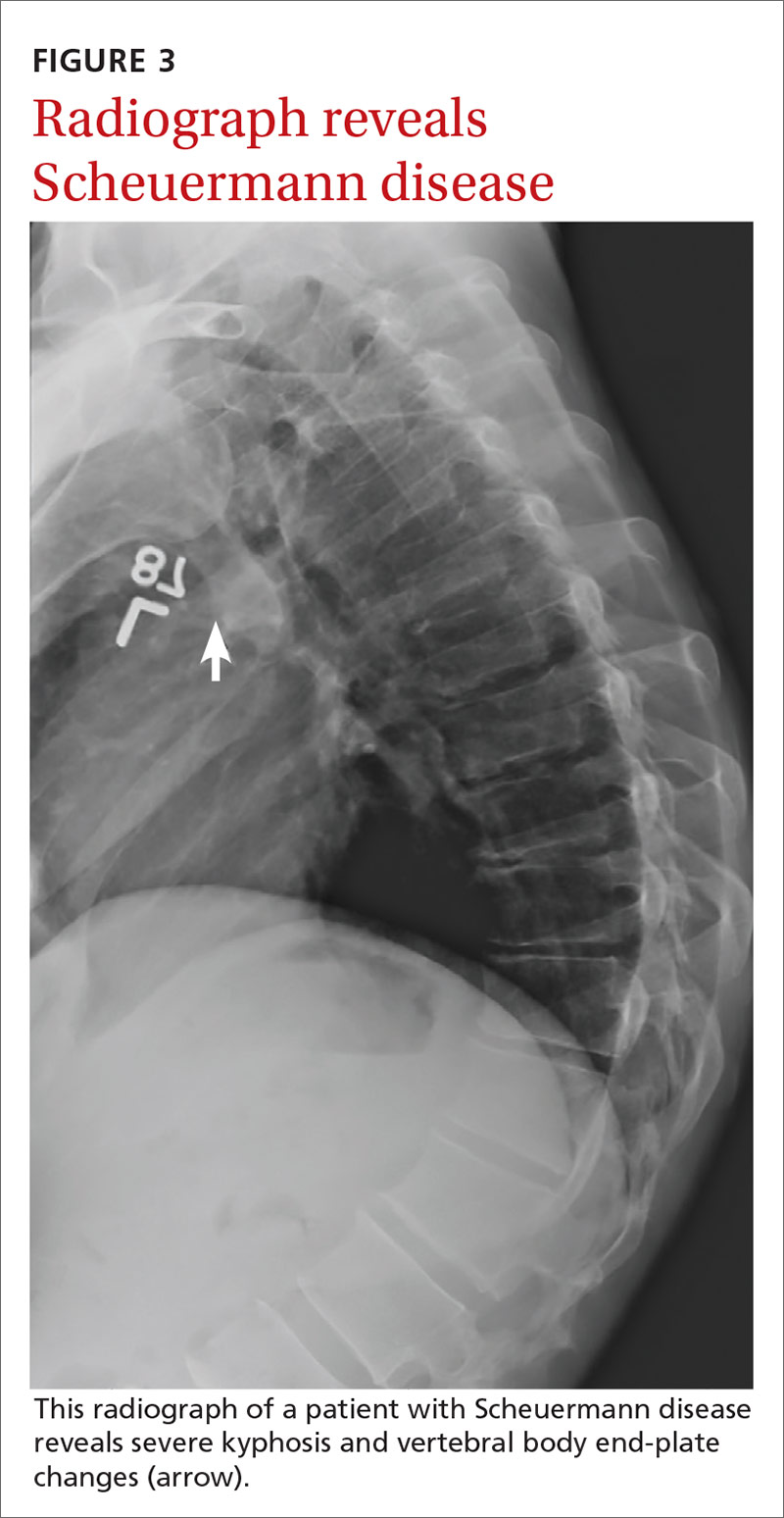
In the absence of the above concerns, defer imaging of the lumbar spine until after adequate rest and rehabilitation have been attempted.
Continue to: Treatment typically involves restor physical therapy
Treatment typically involves restor physical therapy
Most cases of low back pain in children and adolescents are benign and self-limited. Many children with low back pain can be treated with relative rest from the offending activity. For children with more persistent pain, physical therapy (PT) is often indicated. Similar to that for adults, there is little evidence for specific PT programs to help children with low back pain. Rehabilitation should be individualized based on the condition being treated.
Medications. There have been no high-quality studies on the benefit of medications to treat low back pain in children. Studies have shown nonsteroidal anti-inflammatory drugs (NSAIDs) have value in adults, and they are likely safe for use in children,40 but the risk of opiate abuse is significantly increased in adolescents who have been prescribed opiate pain medication prior to 12th grade.41
Lumbar disc herniation. Although still relatively rare, lumbar disc herniation is more common in older children and adolescents than in younger children and is treated similarly to that in adults.8 Range-of-motion exercise to restore lumbar motion is often first-line treatment. Research has shown that exercises that strengthen the abdominal or “core” musculature help prevent the return of low back pain.24,25
In the case of spondylolysis or spondylolisthesis, rest from activity is generally required for a minimum of 4 to 6 weeks. Rehabilitation in the form of range of motion, especially into the lumbar extension, and spinal stabilization exercises are effective for both reducing pain and restoring range-of-motion and strength.42 Have patients avoid heavy backpacks, which can reproduce pain. Children often benefit from leaving a second set of schoolbooks at home. For most patients with spondylolysis, conservative treatment with rehabilitation is equal to or better than surgical intervention in returning the patient to his/her pre-injury activity level.26,43,44 When returning athletes to their sport, aggressive PT, defined as rest for < 10 weeks prior to initiating PT, is superior to delaying PT beyond 10 weeks of rest.27
Idiopathic scoliosis. Much of the literature on the treatment of scoliosis is focused on limiting progression of the scoliotic curvature. Researchers thought that more severe curves were associated with more severe pain, but a recent systematic review showed that back pain can occur in patients with even small curvatures.28 Treatment for patients with smaller degrees of curvature is similar to that for mechanical low back pain. PT may have a role in the treatment of scoliosis, but there is little evidence in the literature of its effectiveness.
Continue to: A Cochrane review showed...
A Cochrane review showed that PT and exercise-based treatments had no effect on back pain or disability in patients with scoliosis.29 And outpatient PT alone, in the absence of bracing, does not arrest progression of the scoliotic curvature.35 One trial did demonstrate that an intensive inpatient treatment program of 4 to 6 weeks for patients with curvature of at least 40° reduced progression of curvature compared to an untreated control group at 1 year.34 The outcomes of functional mobility and pain were not measured. Follow-up data on curve progression beyond 1 year are not available. Unfortunately, intensive inpatient treatment is not readily available or cost-effective for most patients with scoliosis.
Scheuermann disease. The mainstay of treatment for mild Scheuermann disease is advising the patient to avoid repetitive loading of the spine. Patients should avoid sports such as competitive weight-lifting, gymnastics, and football. Lower impact athletics are encouraged. Refer patients with pain to PT to address posture and core stabilization. Patients with severe kyphosis may require surgery.17,18
Bracing: Rarely helpful for low back pain
The use of lumbar braces or corsets is rarely helpful for low back pain in children. Bracing in the setting of spondylolysis is controversial.One study indicated that bracing in combination with activity restriction and lumbar extension exercise is superior to activity restriction and lumbar flexion exercises alone.43 But a meta-analysis did not demonstrate a significant difference in recovery when bracing was added.44 Bracing may help to reduce pain initially in patients with spondylolysis who have pain at rest. Bracing is not recommended for patients with pain that abates with activity modification.
Scoliosis and Scheuermann kyphosis. Treatment of adolescent idiopathic scoliosis usually consists of observation and periodic reevaluation. Bracing is a mainstay of the nonsurgical management of scoliosis and is appropriate for curves of 20° to 40°; studies have reported successful control of curve progression in > 70% of patients.36 According to 1 study, the number of cases of scoliosis needed to treat with bracing to prevent 1 surgery is 3.30 Surgery is often indicated for patients with curvatures > 40°, although this is also debated.33
Bracing is used rarely for Scheuermann kyphosis but may be helpful in more severe or painful cases.17
CORRESPONDENCE
Shawn F. Phillips, MD, MSPT, 500 University Drive H154, Hershey, PA, 17033; [email protected].
1. MacDonald J, Stuart E, Rodenberg R. Musculoskeletal low back pain in school-aged children: a review. JAMA Pediatr. 2017;171:280-287.
2. Tofte JN CarlLee TL, Holte AJ, et al. Imaging pediatric spondylolysis: a systematic review. Spine. 2017;42:777-782.
3. Sakai T, Sairyo K, Suzue N, et al. Incidence and etiology of lumbar spondylolysis: review of the literature. J Orthop Sci. 2010;15:281-288.
4. Calvo-Muñoz I, Gómez-Conesa A, Sánchez-Meca J. Prevalence of low back pain in children and adolescents: a meta-analysis. BMC Pediatrics. 2013;13:14.
5. Bernstein RM, Cozen H. Evaluation of back pain in children and adolescents. Am Fam Physician. 2007;76:1669-1676.
6. Taxter AJ, Chauvin NA, Weiss PF. Diagnosis and treatment of low back pain in the pediatric population. Phys Sportsmed. 2014;42:94-104.
7. Haus BM, Micheli LJ. Back pain in the pediatric and adolescent athlete. Clin Sports Med. 2012;31:423-440.
8. Lavelle WF, Bianco A, Mason R, et al. Pediatric disk herniation. J Am Acad Orthop Surg. 2011;19:649-656.
9. Taimela S, Kujala UM, Salminen JJ, et al. The prevalence of low back pain among children and adolescents: a nationwide, cohort-based questionnaire survey in Finland. Spine. 1997;22:1132-1136.
10. Schroeder GD, LaBella CR, Mendoza M, et al. The role of intense athletic activity on structural lumbar abnormalities in adolescent patients with symptomatic low back pain. Eur Spine J. 2016;25:2842-2848.
11. Waicus KM, Smith BW. Back injuries in the pediatric athlete. Curr Sports Med Rep. 2002;1:52-58.
12. Daniels JM, Pontius G, El-Amin S, et al. Evaluation of low back pain in athletes. Sports Health. 2011;3:336-345.
13. Sato T, Hirano T, Ito T, et al. Back pain in adolescents with idiopathic scoliosis: epidemiological study for 43,630 pupils in Niigata City, Japan. Eur Spine J. 2011;20:274-279.
14. Smorgick Y, Mirovsky Y, Baker KC, et al. Predictors of back pain in adolescent idiopathic scoliosis surgical candidates. J Pediatr Orthop. 2013;33:289-292.
15. US Preventive Services Task Force. Screening for Adolescent Idiopathic Scoliosis. US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:165-172.
16. Hresko MT, Talwalkar VR, Schwend RM. Position statement–Screening for the early detection of idiopathic scoliosis in adolescents. SRS/POSNA/AAOS/AAP Position Statement. 2015. www.srs.org/about-srs/news-and-announcements/position-statement---screening-for-the-early-detection-for-idiopathic-scoliosis-in-adolescents. Accessed September 30, 2020.
17. Palazzo C, Sailhan F, Revel M. Scheuermann’s disease: an update. Joint Bone Spine. 2014;81:209-214.
18. Ali RM, Green DW, Patel TC. Scheuermann’s kyphosis. Curr Opin Pediatr. 1999;11:70-75.
19. de Moraes Barros Fucs PM, Meves R, Yamada HH, et al. Spinal infections in children: a review. Int Orthop. 2012;36:387-395.
20. Joaquim AF, Ghizoni E, Valadares MG, et al. Spinal tumors in children. Revista da Associação Médica Brasileira. 2017;63:459-465.
21. Weiss PF, Colbert RA. Juvenile spondyloarthritis: a distinct form of juvenile arthritis. Pediatr Clin North Am. 2018;65:675-690.
22. Rush JK, Astur N, Scott S, et al. Use of magnetic resonance imaging in the evaluation of spondylolysis. J Pediatr Orthop. 2015;35:271-275.
23. Janicki JA, Alman B. Scoliosis: review of diagnosis and treatment. Pediatr Child Health. 2007;12:771-776.
24. O’Sullivan PB, Phyty GD, Twomey LT, et al. Evaluation of specific stabilizing exercise in the treatment of chronic low back pain with radiologic diagnosis of spondylolysis or spondylolisthesis. Spine.1997;22:2959-2967.
25. Inani SB, Selkar SP. Effect of core stabilization exercises versus conventional exercises on pain and functional status in patients with non-specific low back pain: a randomized clinical trial. J Back Musculoskelet Rehabil. 2013;26:37-43.
26. Garet M, Reiman MP, Mathers J, et al. Nonoperative treatment in lumbar spondylolysis and spondylolisthesis: a systematic review. Sports Health. 2013;5:225-232.
27. Selhorst M, Fischer A, Graft K, et al. Timing of physical therapy referral in adolescent athletes with acute spondylolysis: a retrospective chart review. Clin J Sport Med. 2017;27:296-301.
28. Théroux J, Stomski N, Hodgetts CJ, et al. Prevalence of low back pain in adolescents with idiopathic scoliosis: a systematic review. Chiropr Man Ther. 2017;25:10.
29. Romano M, Minozzi S, Zaina F, et al. Exercises for adolescent idiopathic scoliosis: a Cochrane systematic review. Spine (Phila Pa 1976). 2013;38:E883-E893.
30. Sanders JO, Newton PO, Browne RH, et al. Bracing for idiopathic scoliosis: how many patients require treatment to prevent one surgery? J Bone Joint Surg Am. 2014;96:649-653.
31. Hill JJ, Keating JL. Risk factors for the first episode of low back pain in children are infrequently validated across samples and conditions: a systematic review. J Physiother. 2010;56:237-244.
32. Grødahl LHJ, Fawcett L, Nazareth M, et al. Diagnostic utility of patient history and physical examination data to detect spondylolysis and spondylolisthesis in athletes with low back pain: a systematic review. Man Ther. 2016;24:7-17.
33. Asher MA, Burton DC. Adolescent idiopathic scoliosis: natural history and long term treatment effects. Scoliosis. 2006;1:2.
34. Weiss HR, Weiss G, Petermann F. Incidence of curvature progression in idiopathic scoliosis patients treated with scoliosis inpatient rehabilitation (SIR): an age- and sex-matched controlled study. Pediatr Rehabil. 2003;6:23-30.
35. Gomez JA, Hresko MT, Glotzbecker MP. Nonsurgical management of adolescent idiopathic scoliosis. J Am Acad Orthop Surg. 2016;24:555-564.
36. Weinstein SL, Dolan LA, Wright JG, et al. Effects of bracing in adolescents with idiopathic scoliosis. N Engl J Med. 2013;369:1512-1521.
37. Balg F, Juteau M, Theoret C, et al. Validity and reliability of the iPhone to measure rib hump in scoliosis. J Pediatr Orthop. 2014;34:774-779.
38. Auerbach JD, Ahn J, Zgonis MH, et al. Streamlining the evaluation of low back pain in children. Clin Orthop Relatl Res. 2008;466:1971-1977.
39. Beck NA, Miller R, Baldwin K, et al. Do oblique views add value in the diagnosis of spondylolysis in adolescents? J Bone Joint Surg Am. 2013;95:e65.
40. Roelofs PD, Deyo RA, Koes BW, et al. Nonsteroidal anti-inflammatory drugs for low back pain: an updated Cochrane review. Spine (Phila Pa 1976). 2008;33:1766-1774.
41. Miech R, Johnston L, O’Malley PM, et al. Prescription opioids in adolescence and future opioid misuse. Pediatrics. 2015;136:e1169-e1177.
42. Hu S, Tribus C, Diab M, et al. Spondylolysis and spondylolisthesis. J Bone Joint Surg. 2008;90:655-671.
43. Panteliadis P, Nagra NS, Edwards KL, et al. Athletic population with spondylolysis: review of outcomes following surgical repair or conservative management. Global Spine J. 2016;6:615-625.
44. Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults: a meta-analysis of observational studies. J Pediatr Orthop. 2009;29:146-156.
Low back pain in not uncommon in children and adolescents.1-3 Although the prevalence of low back pain in children < 7 years is low, it increases with age, with studies reporting lifetime prevalence at age 12 years between 16% and 18% and rates as high as 66% by 16 years of age.4,5 Although children and adolescents usually have pain that is transient and benign without a defined cause, structural causes of low back pain should be considered in school-aged children with pain that persists for > 3 to 6 weeks. 4 The most common structural causes of adolescent low back pain are reviewed here.
Etiology: A mixed bag
Back pain in school-aged children is most commonly due to muscular strain, overuse, or poor posture. The pain is often transient in nature and responds to rest and postural education.4,6 A herniated disc is an uncommon finding in younger school-aged children, but incidence increases slightly among older adolescents, particularly those who are active in collision sports and/or weight-lifting.7,8 Pain caused by a herniated disc often radiates along the distribution of the sciatic nerve and worsens during lumbar flexion.
Spondylolysis and spondylolisthesis are important causes of back pain in children. Spondylolysis is defined as a defect or abnormality of the pars interarticularis and surrounding lamina and pedicle. Spondylolisthesis, which is less common, is defined as the translation or “slippage” of one vertebral segment in relation to the next caudal segment. These conditions commonly occur as a result of repetitive stress.
In a prospective study of adolescents < 19 years with low back pain for > 2 weeks, the prevalence of spondylolysis was 39.7%.9 Adolescent athletes with symptomatic low back pain are more likely to have spondylolysis than nonathletes (32% vs 2%, respectively).2,10 Pain is often made worse by extension of the spine. Spondylolysis and spondylolisthesis can be congenital or acquired, and both can be asymptomatic. Children and teens who are athletes are at higher risk for symptomatic spondylolysis and spondylolisthesis.10-12 This is especially true for those involved in gymnastics, dance, football, and/or volleyball, where a repetitive load is placed onto an extended spine.
Idiopathic scoliosis is an abnormal lateral curvature of the spine that usually develops during adolescence and worsens with growth. Historically, painful scoliosis was considered rare, but more recently researchers determined that children with scoliosis have a higher rate of pain compared to their peers.13,14 School-aged children with scoliosis were found to be at 2 times the risk of low back pain compared to those without scoliosis.13 It is important to identify scoliosis in adolescents so that progression can be monitored.
Screening for scoliosis in primary care is somewhat controversial. The US Preventive Services Task Force (USPSTF) finds insufficient evidence for screening asymptomatic adolescents for scoliosis.15 This recommendation is based on the fact that there is little evidence on the effect of screening on long-term outcomes. Screening may also lead to unnecessary radiation. Conversely, a position statement released by the Scoliosis Research Society, the Pediatric Orthopedic Society of North America, the American Association of Orthopedic Surgeons, and the American Academy of Pediatrics recommends scoliosis screening during routine pediatric office visits.16 Screening for girls is recommended at ages 10 and 12 years, and for boys, once between ages 13 and 14 years. The statement highlights evidence showing that focused screening by appropriate personnel has value in detecting a clinically significant curve (> 20°).
Scheuermann disease is a rare cause of back pain in children that usually develops during adolescence and results in increasing thoracic kyphosis. An autosomal dominant mutation plays a role in this disease of the growth cartilage endplate; repetitive strain on the growth cartilage is also a contributing factor.17,18 An atypical variant manifests with kyphosis in the thoracolumbar region.17
Continue to: Other causes of low back pain
Other causes of low back pain—including inflammatory arthritis, infection (eg, discitis), and tumor—are rare in children but must always be considered, especially in the setting of persistent symptoms.4,19-21 More on the features of these conditions is listed in TABLE 1.1-7,13-15,17-30

History: Focus on onset, timing, and duration of symptoms
As with adults, obtaining a history that includes the onset, timing, and duration of symptoms is key in the evaluation of low back pain in children, as is obtaining a history of the patient’s activities; sports that repetitively load the lumbar spine in an extended position increase the risk of injury.10

Specific risk factors for low back pain in children and adolescents are poorly understood.4,9,31 Pain can be associated with trauma, or it can have a more progressive or insidious onset. Generally, pain that is present for up to 6 weeks and is intermittent or improving has a self-limited course. Pain that persists beyond 3 to 6 weeks or is worsening is more likely to have an anatomical cause that needs further evaluation.2,3,10,21
Identifying exacerbating and alleviating factors can provide useful information. Pain that is worse with lumbar flexion is more likely to come from muscular strain or disc pathology. Pain with extension is more likely due to a structural cause such as spondylolysis/spondylolisthesis, scoliosis, or Scheuermann disease.2,4,10,17,18,21 See TABLE 2 for red flag symptoms that indicate the need for imaging and further work-up.

The physical exam: Visualize, assess range of motion, and reproduce pain
The physical examination of any patient with low back pain should include direct visualization and inspection of the back, spine, and pelvis; palpation of the spine and paraspinal regions; assessment of lumbar range of motion and of the lumbar nerve roots, including tests of sensation, strength, and deep tendon reflexes; and an evaluation of the patient’s posture, which can provide clues to underlying causes of pain.
Continue to: Increased thoracic kyphosis...
Increased thoracic kyphosis that is not reversible is concerning for Scheuermann disease.9,17,18 A significant elevation in one shoulder or side of the pelvis can be indicative of scoliosis. Increased lumbar lordosis may predispose a patient to spondylolysis.
In patients with spondylolysis, lumbar extension will usually reproduce pain, which is often unilateral. Hyperextension in a single-leg stance, commonly known as the Stork test, is positive for unilateral spondylolysis when it reproduces pain on the ipsilateral side. The sensitivity of the Stork test for unilateral spondylolysis is approximately 50%.32 (For more information on the Stork test, see www.physio-pedia.com/Stork_test.)
Pain reproduced with lumbar flexion is less concerning for bony pathology and is most often related to soft-tissue strain. Lumbar flexion with concomitant radicular pain is associated with disc pathology.8 Pain with a straight-leg raise is also associated with disk pathology, especially if raising the contralateral leg increases pain.8
Using a scoliometer. Evaluate the flexed spine for the presence of asymmetry, which can indicate scoliosis.33 If asymmetry is present, use a scoliometer to determine the degree of asymmetry. Zero to 5° is considered clinically insignificant; monitor and reevaluate these patients at subsequent visits.34,35 Ten degrees or more of asymmetry with a scoliometer should prompt you to order radiographs.35,36 A smartphone-based scoliometer for iPhones was evaluated in 1 study and was shown to have reasonable reliability and validity for clinical use.37
Deformity of the lower extremities. Because low back pain may be caused by biomechanical or structural deformity of the lower extremities, examine the flexibility of the hip flexors, gluteal musculature, hamstrings, and the iliotibial band.38 In addition, evaluate for leg-length discrepancy and lower-extremity malalignment, such as femoral anteversion, tibial torsion, or pes planus.
Continue to: Imaging
Imaging: Know when it’s needed
Although imaging of the lumbar spine is often unnecessary in the presence of acute low back pain in children, always consider imaging in the setting of bony tenderness, pain that wakes a patient from sleep, and in the setting of other red flag symptoms (see TABLE 2). Low back pain in children that is reproducible with lumbar extension is concerning for spondylolysis or spondylolisthesis. If the pain with extension persists beyond 3 to 6 weeks, order imaging starting with radiographs.2,39
Traditionally, 4 views of the spine—anteroposterior (AP), lateral, and oblique (one right and one left)—were obtained, but recent evidence indicates that 2 views (AP and lateral) have similar sensitivity and specificity to 4 views with significantly reduced radiation exposure.2,39 Because the sensitivity of plain films is relatively low, consider more advanced imaging if spondylolysis or spondylolisthesis is strongly suspected. Recent studies indicate that magnetic resonance imaging (MRI) may be as effective as computed tomography (CT) or bone scan and has the advantage of lower radiation (FIGURE 1).2,22

Similarly, order radiographs if there is > 10° of asymmetry noted on physical exam using a scoliometer.15,23 Calculate the Cobb angle to determine the severity of scoliosis. Refer patients with angles ≥ 20° to a pediatric orthopedist for monitoring of progression and consideration of bracing (FIGURE 2).23,34 For patients with curvatures between 10° and 19°, repeat imaging every 6 to 12 months. Because scoliosis is a risk factor for spondylolysis, evaluate radiographs in the setting of painful scoliosis for the presence of a spondylolysis.34,35

If excessive kyphosis is noted on exam, order radiographs to evaluate for Scheuermann disease. Classic imaging findings include Schmorl nodes, vertebral endplate changes, and anterior wedging (FIGURE 3).17,18

In the absence of the above concerns, defer imaging of the lumbar spine until after adequate rest and rehabilitation have been attempted.
Continue to: Treatment typically involves restor physical therapy
Treatment typically involves restor physical therapy
Most cases of low back pain in children and adolescents are benign and self-limited. Many children with low back pain can be treated with relative rest from the offending activity. For children with more persistent pain, physical therapy (PT) is often indicated. Similar to that for adults, there is little evidence for specific PT programs to help children with low back pain. Rehabilitation should be individualized based on the condition being treated.
Medications. There have been no high-quality studies on the benefit of medications to treat low back pain in children. Studies have shown nonsteroidal anti-inflammatory drugs (NSAIDs) have value in adults, and they are likely safe for use in children,40 but the risk of opiate abuse is significantly increased in adolescents who have been prescribed opiate pain medication prior to 12th grade.41
Lumbar disc herniation. Although still relatively rare, lumbar disc herniation is more common in older children and adolescents than in younger children and is treated similarly to that in adults.8 Range-of-motion exercise to restore lumbar motion is often first-line treatment. Research has shown that exercises that strengthen the abdominal or “core” musculature help prevent the return of low back pain.24,25
In the case of spondylolysis or spondylolisthesis, rest from activity is generally required for a minimum of 4 to 6 weeks. Rehabilitation in the form of range of motion, especially into the lumbar extension, and spinal stabilization exercises are effective for both reducing pain and restoring range-of-motion and strength.42 Have patients avoid heavy backpacks, which can reproduce pain. Children often benefit from leaving a second set of schoolbooks at home. For most patients with spondylolysis, conservative treatment with rehabilitation is equal to or better than surgical intervention in returning the patient to his/her pre-injury activity level.26,43,44 When returning athletes to their sport, aggressive PT, defined as rest for < 10 weeks prior to initiating PT, is superior to delaying PT beyond 10 weeks of rest.27
Idiopathic scoliosis. Much of the literature on the treatment of scoliosis is focused on limiting progression of the scoliotic curvature. Researchers thought that more severe curves were associated with more severe pain, but a recent systematic review showed that back pain can occur in patients with even small curvatures.28 Treatment for patients with smaller degrees of curvature is similar to that for mechanical low back pain. PT may have a role in the treatment of scoliosis, but there is little evidence in the literature of its effectiveness.
Continue to: A Cochrane review showed...
A Cochrane review showed that PT and exercise-based treatments had no effect on back pain or disability in patients with scoliosis.29 And outpatient PT alone, in the absence of bracing, does not arrest progression of the scoliotic curvature.35 One trial did demonstrate that an intensive inpatient treatment program of 4 to 6 weeks for patients with curvature of at least 40° reduced progression of curvature compared to an untreated control group at 1 year.34 The outcomes of functional mobility and pain were not measured. Follow-up data on curve progression beyond 1 year are not available. Unfortunately, intensive inpatient treatment is not readily available or cost-effective for most patients with scoliosis.
Scheuermann disease. The mainstay of treatment for mild Scheuermann disease is advising the patient to avoid repetitive loading of the spine. Patients should avoid sports such as competitive weight-lifting, gymnastics, and football. Lower impact athletics are encouraged. Refer patients with pain to PT to address posture and core stabilization. Patients with severe kyphosis may require surgery.17,18
Bracing: Rarely helpful for low back pain
The use of lumbar braces or corsets is rarely helpful for low back pain in children. Bracing in the setting of spondylolysis is controversial.One study indicated that bracing in combination with activity restriction and lumbar extension exercise is superior to activity restriction and lumbar flexion exercises alone.43 But a meta-analysis did not demonstrate a significant difference in recovery when bracing was added.44 Bracing may help to reduce pain initially in patients with spondylolysis who have pain at rest. Bracing is not recommended for patients with pain that abates with activity modification.
Scoliosis and Scheuermann kyphosis. Treatment of adolescent idiopathic scoliosis usually consists of observation and periodic reevaluation. Bracing is a mainstay of the nonsurgical management of scoliosis and is appropriate for curves of 20° to 40°; studies have reported successful control of curve progression in > 70% of patients.36 According to 1 study, the number of cases of scoliosis needed to treat with bracing to prevent 1 surgery is 3.30 Surgery is often indicated for patients with curvatures > 40°, although this is also debated.33
Bracing is used rarely for Scheuermann kyphosis but may be helpful in more severe or painful cases.17
CORRESPONDENCE
Shawn F. Phillips, MD, MSPT, 500 University Drive H154, Hershey, PA, 17033; [email protected].
Low back pain in not uncommon in children and adolescents.1-3 Although the prevalence of low back pain in children < 7 years is low, it increases with age, with studies reporting lifetime prevalence at age 12 years between 16% and 18% and rates as high as 66% by 16 years of age.4,5 Although children and adolescents usually have pain that is transient and benign without a defined cause, structural causes of low back pain should be considered in school-aged children with pain that persists for > 3 to 6 weeks. 4 The most common structural causes of adolescent low back pain are reviewed here.
Etiology: A mixed bag
Back pain in school-aged children is most commonly due to muscular strain, overuse, or poor posture. The pain is often transient in nature and responds to rest and postural education.4,6 A herniated disc is an uncommon finding in younger school-aged children, but incidence increases slightly among older adolescents, particularly those who are active in collision sports and/or weight-lifting.7,8 Pain caused by a herniated disc often radiates along the distribution of the sciatic nerve and worsens during lumbar flexion.
Spondylolysis and spondylolisthesis are important causes of back pain in children. Spondylolysis is defined as a defect or abnormality of the pars interarticularis and surrounding lamina and pedicle. Spondylolisthesis, which is less common, is defined as the translation or “slippage” of one vertebral segment in relation to the next caudal segment. These conditions commonly occur as a result of repetitive stress.
In a prospective study of adolescents < 19 years with low back pain for > 2 weeks, the prevalence of spondylolysis was 39.7%.9 Adolescent athletes with symptomatic low back pain are more likely to have spondylolysis than nonathletes (32% vs 2%, respectively).2,10 Pain is often made worse by extension of the spine. Spondylolysis and spondylolisthesis can be congenital or acquired, and both can be asymptomatic. Children and teens who are athletes are at higher risk for symptomatic spondylolysis and spondylolisthesis.10-12 This is especially true for those involved in gymnastics, dance, football, and/or volleyball, where a repetitive load is placed onto an extended spine.
Idiopathic scoliosis is an abnormal lateral curvature of the spine that usually develops during adolescence and worsens with growth. Historically, painful scoliosis was considered rare, but more recently researchers determined that children with scoliosis have a higher rate of pain compared to their peers.13,14 School-aged children with scoliosis were found to be at 2 times the risk of low back pain compared to those without scoliosis.13 It is important to identify scoliosis in adolescents so that progression can be monitored.
Screening for scoliosis in primary care is somewhat controversial. The US Preventive Services Task Force (USPSTF) finds insufficient evidence for screening asymptomatic adolescents for scoliosis.15 This recommendation is based on the fact that there is little evidence on the effect of screening on long-term outcomes. Screening may also lead to unnecessary radiation. Conversely, a position statement released by the Scoliosis Research Society, the Pediatric Orthopedic Society of North America, the American Association of Orthopedic Surgeons, and the American Academy of Pediatrics recommends scoliosis screening during routine pediatric office visits.16 Screening for girls is recommended at ages 10 and 12 years, and for boys, once between ages 13 and 14 years. The statement highlights evidence showing that focused screening by appropriate personnel has value in detecting a clinically significant curve (> 20°).
Scheuermann disease is a rare cause of back pain in children that usually develops during adolescence and results in increasing thoracic kyphosis. An autosomal dominant mutation plays a role in this disease of the growth cartilage endplate; repetitive strain on the growth cartilage is also a contributing factor.17,18 An atypical variant manifests with kyphosis in the thoracolumbar region.17
Continue to: Other causes of low back pain
Other causes of low back pain—including inflammatory arthritis, infection (eg, discitis), and tumor—are rare in children but must always be considered, especially in the setting of persistent symptoms.4,19-21 More on the features of these conditions is listed in TABLE 1.1-7,13-15,17-30

History: Focus on onset, timing, and duration of symptoms
As with adults, obtaining a history that includes the onset, timing, and duration of symptoms is key in the evaluation of low back pain in children, as is obtaining a history of the patient’s activities; sports that repetitively load the lumbar spine in an extended position increase the risk of injury.10

Specific risk factors for low back pain in children and adolescents are poorly understood.4,9,31 Pain can be associated with trauma, or it can have a more progressive or insidious onset. Generally, pain that is present for up to 6 weeks and is intermittent or improving has a self-limited course. Pain that persists beyond 3 to 6 weeks or is worsening is more likely to have an anatomical cause that needs further evaluation.2,3,10,21
Identifying exacerbating and alleviating factors can provide useful information. Pain that is worse with lumbar flexion is more likely to come from muscular strain or disc pathology. Pain with extension is more likely due to a structural cause such as spondylolysis/spondylolisthesis, scoliosis, or Scheuermann disease.2,4,10,17,18,21 See TABLE 2 for red flag symptoms that indicate the need for imaging and further work-up.

The physical exam: Visualize, assess range of motion, and reproduce pain
The physical examination of any patient with low back pain should include direct visualization and inspection of the back, spine, and pelvis; palpation of the spine and paraspinal regions; assessment of lumbar range of motion and of the lumbar nerve roots, including tests of sensation, strength, and deep tendon reflexes; and an evaluation of the patient’s posture, which can provide clues to underlying causes of pain.
Continue to: Increased thoracic kyphosis...
Increased thoracic kyphosis that is not reversible is concerning for Scheuermann disease.9,17,18 A significant elevation in one shoulder or side of the pelvis can be indicative of scoliosis. Increased lumbar lordosis may predispose a patient to spondylolysis.
In patients with spondylolysis, lumbar extension will usually reproduce pain, which is often unilateral. Hyperextension in a single-leg stance, commonly known as the Stork test, is positive for unilateral spondylolysis when it reproduces pain on the ipsilateral side. The sensitivity of the Stork test for unilateral spondylolysis is approximately 50%.32 (For more information on the Stork test, see www.physio-pedia.com/Stork_test.)
Pain reproduced with lumbar flexion is less concerning for bony pathology and is most often related to soft-tissue strain. Lumbar flexion with concomitant radicular pain is associated with disc pathology.8 Pain with a straight-leg raise is also associated with disk pathology, especially if raising the contralateral leg increases pain.8
Using a scoliometer. Evaluate the flexed spine for the presence of asymmetry, which can indicate scoliosis.33 If asymmetry is present, use a scoliometer to determine the degree of asymmetry. Zero to 5° is considered clinically insignificant; monitor and reevaluate these patients at subsequent visits.34,35 Ten degrees or more of asymmetry with a scoliometer should prompt you to order radiographs.35,36 A smartphone-based scoliometer for iPhones was evaluated in 1 study and was shown to have reasonable reliability and validity for clinical use.37
Deformity of the lower extremities. Because low back pain may be caused by biomechanical or structural deformity of the lower extremities, examine the flexibility of the hip flexors, gluteal musculature, hamstrings, and the iliotibial band.38 In addition, evaluate for leg-length discrepancy and lower-extremity malalignment, such as femoral anteversion, tibial torsion, or pes planus.
Continue to: Imaging
Imaging: Know when it’s needed
Although imaging of the lumbar spine is often unnecessary in the presence of acute low back pain in children, always consider imaging in the setting of bony tenderness, pain that wakes a patient from sleep, and in the setting of other red flag symptoms (see TABLE 2). Low back pain in children that is reproducible with lumbar extension is concerning for spondylolysis or spondylolisthesis. If the pain with extension persists beyond 3 to 6 weeks, order imaging starting with radiographs.2,39
Traditionally, 4 views of the spine—anteroposterior (AP), lateral, and oblique (one right and one left)—were obtained, but recent evidence indicates that 2 views (AP and lateral) have similar sensitivity and specificity to 4 views with significantly reduced radiation exposure.2,39 Because the sensitivity of plain films is relatively low, consider more advanced imaging if spondylolysis or spondylolisthesis is strongly suspected. Recent studies indicate that magnetic resonance imaging (MRI) may be as effective as computed tomography (CT) or bone scan and has the advantage of lower radiation (FIGURE 1).2,22

Similarly, order radiographs if there is > 10° of asymmetry noted on physical exam using a scoliometer.15,23 Calculate the Cobb angle to determine the severity of scoliosis. Refer patients with angles ≥ 20° to a pediatric orthopedist for monitoring of progression and consideration of bracing (FIGURE 2).23,34 For patients with curvatures between 10° and 19°, repeat imaging every 6 to 12 months. Because scoliosis is a risk factor for spondylolysis, evaluate radiographs in the setting of painful scoliosis for the presence of a spondylolysis.34,35

If excessive kyphosis is noted on exam, order radiographs to evaluate for Scheuermann disease. Classic imaging findings include Schmorl nodes, vertebral endplate changes, and anterior wedging (FIGURE 3).17,18

In the absence of the above concerns, defer imaging of the lumbar spine until after adequate rest and rehabilitation have been attempted.
Continue to: Treatment typically involves restor physical therapy
Treatment typically involves restor physical therapy
Most cases of low back pain in children and adolescents are benign and self-limited. Many children with low back pain can be treated with relative rest from the offending activity. For children with more persistent pain, physical therapy (PT) is often indicated. Similar to that for adults, there is little evidence for specific PT programs to help children with low back pain. Rehabilitation should be individualized based on the condition being treated.
Medications. There have been no high-quality studies on the benefit of medications to treat low back pain in children. Studies have shown nonsteroidal anti-inflammatory drugs (NSAIDs) have value in adults, and they are likely safe for use in children,40 but the risk of opiate abuse is significantly increased in adolescents who have been prescribed opiate pain medication prior to 12th grade.41
Lumbar disc herniation. Although still relatively rare, lumbar disc herniation is more common in older children and adolescents than in younger children and is treated similarly to that in adults.8 Range-of-motion exercise to restore lumbar motion is often first-line treatment. Research has shown that exercises that strengthen the abdominal or “core” musculature help prevent the return of low back pain.24,25
In the case of spondylolysis or spondylolisthesis, rest from activity is generally required for a minimum of 4 to 6 weeks. Rehabilitation in the form of range of motion, especially into the lumbar extension, and spinal stabilization exercises are effective for both reducing pain and restoring range-of-motion and strength.42 Have patients avoid heavy backpacks, which can reproduce pain. Children often benefit from leaving a second set of schoolbooks at home. For most patients with spondylolysis, conservative treatment with rehabilitation is equal to or better than surgical intervention in returning the patient to his/her pre-injury activity level.26,43,44 When returning athletes to their sport, aggressive PT, defined as rest for < 10 weeks prior to initiating PT, is superior to delaying PT beyond 10 weeks of rest.27
Idiopathic scoliosis. Much of the literature on the treatment of scoliosis is focused on limiting progression of the scoliotic curvature. Researchers thought that more severe curves were associated with more severe pain, but a recent systematic review showed that back pain can occur in patients with even small curvatures.28 Treatment for patients with smaller degrees of curvature is similar to that for mechanical low back pain. PT may have a role in the treatment of scoliosis, but there is little evidence in the literature of its effectiveness.
Continue to: A Cochrane review showed...
A Cochrane review showed that PT and exercise-based treatments had no effect on back pain or disability in patients with scoliosis.29 And outpatient PT alone, in the absence of bracing, does not arrest progression of the scoliotic curvature.35 One trial did demonstrate that an intensive inpatient treatment program of 4 to 6 weeks for patients with curvature of at least 40° reduced progression of curvature compared to an untreated control group at 1 year.34 The outcomes of functional mobility and pain were not measured. Follow-up data on curve progression beyond 1 year are not available. Unfortunately, intensive inpatient treatment is not readily available or cost-effective for most patients with scoliosis.
Scheuermann disease. The mainstay of treatment for mild Scheuermann disease is advising the patient to avoid repetitive loading of the spine. Patients should avoid sports such as competitive weight-lifting, gymnastics, and football. Lower impact athletics are encouraged. Refer patients with pain to PT to address posture and core stabilization. Patients with severe kyphosis may require surgery.17,18
Bracing: Rarely helpful for low back pain
The use of lumbar braces or corsets is rarely helpful for low back pain in children. Bracing in the setting of spondylolysis is controversial.One study indicated that bracing in combination with activity restriction and lumbar extension exercise is superior to activity restriction and lumbar flexion exercises alone.43 But a meta-analysis did not demonstrate a significant difference in recovery when bracing was added.44 Bracing may help to reduce pain initially in patients with spondylolysis who have pain at rest. Bracing is not recommended for patients with pain that abates with activity modification.
Scoliosis and Scheuermann kyphosis. Treatment of adolescent idiopathic scoliosis usually consists of observation and periodic reevaluation. Bracing is a mainstay of the nonsurgical management of scoliosis and is appropriate for curves of 20° to 40°; studies have reported successful control of curve progression in > 70% of patients.36 According to 1 study, the number of cases of scoliosis needed to treat with bracing to prevent 1 surgery is 3.30 Surgery is often indicated for patients with curvatures > 40°, although this is also debated.33
Bracing is used rarely for Scheuermann kyphosis but may be helpful in more severe or painful cases.17
CORRESPONDENCE
Shawn F. Phillips, MD, MSPT, 500 University Drive H154, Hershey, PA, 17033; [email protected].
1. MacDonald J, Stuart E, Rodenberg R. Musculoskeletal low back pain in school-aged children: a review. JAMA Pediatr. 2017;171:280-287.
2. Tofte JN CarlLee TL, Holte AJ, et al. Imaging pediatric spondylolysis: a systematic review. Spine. 2017;42:777-782.
3. Sakai T, Sairyo K, Suzue N, et al. Incidence and etiology of lumbar spondylolysis: review of the literature. J Orthop Sci. 2010;15:281-288.
4. Calvo-Muñoz I, Gómez-Conesa A, Sánchez-Meca J. Prevalence of low back pain in children and adolescents: a meta-analysis. BMC Pediatrics. 2013;13:14.
5. Bernstein RM, Cozen H. Evaluation of back pain in children and adolescents. Am Fam Physician. 2007;76:1669-1676.
6. Taxter AJ, Chauvin NA, Weiss PF. Diagnosis and treatment of low back pain in the pediatric population. Phys Sportsmed. 2014;42:94-104.
7. Haus BM, Micheli LJ. Back pain in the pediatric and adolescent athlete. Clin Sports Med. 2012;31:423-440.
8. Lavelle WF, Bianco A, Mason R, et al. Pediatric disk herniation. J Am Acad Orthop Surg. 2011;19:649-656.
9. Taimela S, Kujala UM, Salminen JJ, et al. The prevalence of low back pain among children and adolescents: a nationwide, cohort-based questionnaire survey in Finland. Spine. 1997;22:1132-1136.
10. Schroeder GD, LaBella CR, Mendoza M, et al. The role of intense athletic activity on structural lumbar abnormalities in adolescent patients with symptomatic low back pain. Eur Spine J. 2016;25:2842-2848.
11. Waicus KM, Smith BW. Back injuries in the pediatric athlete. Curr Sports Med Rep. 2002;1:52-58.
12. Daniels JM, Pontius G, El-Amin S, et al. Evaluation of low back pain in athletes. Sports Health. 2011;3:336-345.
13. Sato T, Hirano T, Ito T, et al. Back pain in adolescents with idiopathic scoliosis: epidemiological study for 43,630 pupils in Niigata City, Japan. Eur Spine J. 2011;20:274-279.
14. Smorgick Y, Mirovsky Y, Baker KC, et al. Predictors of back pain in adolescent idiopathic scoliosis surgical candidates. J Pediatr Orthop. 2013;33:289-292.
15. US Preventive Services Task Force. Screening for Adolescent Idiopathic Scoliosis. US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:165-172.
16. Hresko MT, Talwalkar VR, Schwend RM. Position statement–Screening for the early detection of idiopathic scoliosis in adolescents. SRS/POSNA/AAOS/AAP Position Statement. 2015. www.srs.org/about-srs/news-and-announcements/position-statement---screening-for-the-early-detection-for-idiopathic-scoliosis-in-adolescents. Accessed September 30, 2020.
17. Palazzo C, Sailhan F, Revel M. Scheuermann’s disease: an update. Joint Bone Spine. 2014;81:209-214.
18. Ali RM, Green DW, Patel TC. Scheuermann’s kyphosis. Curr Opin Pediatr. 1999;11:70-75.
19. de Moraes Barros Fucs PM, Meves R, Yamada HH, et al. Spinal infections in children: a review. Int Orthop. 2012;36:387-395.
20. Joaquim AF, Ghizoni E, Valadares MG, et al. Spinal tumors in children. Revista da Associação Médica Brasileira. 2017;63:459-465.
21. Weiss PF, Colbert RA. Juvenile spondyloarthritis: a distinct form of juvenile arthritis. Pediatr Clin North Am. 2018;65:675-690.
22. Rush JK, Astur N, Scott S, et al. Use of magnetic resonance imaging in the evaluation of spondylolysis. J Pediatr Orthop. 2015;35:271-275.
23. Janicki JA, Alman B. Scoliosis: review of diagnosis and treatment. Pediatr Child Health. 2007;12:771-776.
24. O’Sullivan PB, Phyty GD, Twomey LT, et al. Evaluation of specific stabilizing exercise in the treatment of chronic low back pain with radiologic diagnosis of spondylolysis or spondylolisthesis. Spine.1997;22:2959-2967.
25. Inani SB, Selkar SP. Effect of core stabilization exercises versus conventional exercises on pain and functional status in patients with non-specific low back pain: a randomized clinical trial. J Back Musculoskelet Rehabil. 2013;26:37-43.
26. Garet M, Reiman MP, Mathers J, et al. Nonoperative treatment in lumbar spondylolysis and spondylolisthesis: a systematic review. Sports Health. 2013;5:225-232.
27. Selhorst M, Fischer A, Graft K, et al. Timing of physical therapy referral in adolescent athletes with acute spondylolysis: a retrospective chart review. Clin J Sport Med. 2017;27:296-301.
28. Théroux J, Stomski N, Hodgetts CJ, et al. Prevalence of low back pain in adolescents with idiopathic scoliosis: a systematic review. Chiropr Man Ther. 2017;25:10.
29. Romano M, Minozzi S, Zaina F, et al. Exercises for adolescent idiopathic scoliosis: a Cochrane systematic review. Spine (Phila Pa 1976). 2013;38:E883-E893.
30. Sanders JO, Newton PO, Browne RH, et al. Bracing for idiopathic scoliosis: how many patients require treatment to prevent one surgery? J Bone Joint Surg Am. 2014;96:649-653.
31. Hill JJ, Keating JL. Risk factors for the first episode of low back pain in children are infrequently validated across samples and conditions: a systematic review. J Physiother. 2010;56:237-244.
32. Grødahl LHJ, Fawcett L, Nazareth M, et al. Diagnostic utility of patient history and physical examination data to detect spondylolysis and spondylolisthesis in athletes with low back pain: a systematic review. Man Ther. 2016;24:7-17.
33. Asher MA, Burton DC. Adolescent idiopathic scoliosis: natural history and long term treatment effects. Scoliosis. 2006;1:2.
34. Weiss HR, Weiss G, Petermann F. Incidence of curvature progression in idiopathic scoliosis patients treated with scoliosis inpatient rehabilitation (SIR): an age- and sex-matched controlled study. Pediatr Rehabil. 2003;6:23-30.
35. Gomez JA, Hresko MT, Glotzbecker MP. Nonsurgical management of adolescent idiopathic scoliosis. J Am Acad Orthop Surg. 2016;24:555-564.
36. Weinstein SL, Dolan LA, Wright JG, et al. Effects of bracing in adolescents with idiopathic scoliosis. N Engl J Med. 2013;369:1512-1521.
37. Balg F, Juteau M, Theoret C, et al. Validity and reliability of the iPhone to measure rib hump in scoliosis. J Pediatr Orthop. 2014;34:774-779.
38. Auerbach JD, Ahn J, Zgonis MH, et al. Streamlining the evaluation of low back pain in children. Clin Orthop Relatl Res. 2008;466:1971-1977.
39. Beck NA, Miller R, Baldwin K, et al. Do oblique views add value in the diagnosis of spondylolysis in adolescents? J Bone Joint Surg Am. 2013;95:e65.
40. Roelofs PD, Deyo RA, Koes BW, et al. Nonsteroidal anti-inflammatory drugs for low back pain: an updated Cochrane review. Spine (Phila Pa 1976). 2008;33:1766-1774.
41. Miech R, Johnston L, O’Malley PM, et al. Prescription opioids in adolescence and future opioid misuse. Pediatrics. 2015;136:e1169-e1177.
42. Hu S, Tribus C, Diab M, et al. Spondylolysis and spondylolisthesis. J Bone Joint Surg. 2008;90:655-671.
43. Panteliadis P, Nagra NS, Edwards KL, et al. Athletic population with spondylolysis: review of outcomes following surgical repair or conservative management. Global Spine J. 2016;6:615-625.
44. Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults: a meta-analysis of observational studies. J Pediatr Orthop. 2009;29:146-156.
1. MacDonald J, Stuart E, Rodenberg R. Musculoskeletal low back pain in school-aged children: a review. JAMA Pediatr. 2017;171:280-287.
2. Tofte JN CarlLee TL, Holte AJ, et al. Imaging pediatric spondylolysis: a systematic review. Spine. 2017;42:777-782.
3. Sakai T, Sairyo K, Suzue N, et al. Incidence and etiology of lumbar spondylolysis: review of the literature. J Orthop Sci. 2010;15:281-288.
4. Calvo-Muñoz I, Gómez-Conesa A, Sánchez-Meca J. Prevalence of low back pain in children and adolescents: a meta-analysis. BMC Pediatrics. 2013;13:14.
5. Bernstein RM, Cozen H. Evaluation of back pain in children and adolescents. Am Fam Physician. 2007;76:1669-1676.
6. Taxter AJ, Chauvin NA, Weiss PF. Diagnosis and treatment of low back pain in the pediatric population. Phys Sportsmed. 2014;42:94-104.
7. Haus BM, Micheli LJ. Back pain in the pediatric and adolescent athlete. Clin Sports Med. 2012;31:423-440.
8. Lavelle WF, Bianco A, Mason R, et al. Pediatric disk herniation. J Am Acad Orthop Surg. 2011;19:649-656.
9. Taimela S, Kujala UM, Salminen JJ, et al. The prevalence of low back pain among children and adolescents: a nationwide, cohort-based questionnaire survey in Finland. Spine. 1997;22:1132-1136.
10. Schroeder GD, LaBella CR, Mendoza M, et al. The role of intense athletic activity on structural lumbar abnormalities in adolescent patients with symptomatic low back pain. Eur Spine J. 2016;25:2842-2848.
11. Waicus KM, Smith BW. Back injuries in the pediatric athlete. Curr Sports Med Rep. 2002;1:52-58.
12. Daniels JM, Pontius G, El-Amin S, et al. Evaluation of low back pain in athletes. Sports Health. 2011;3:336-345.
13. Sato T, Hirano T, Ito T, et al. Back pain in adolescents with idiopathic scoliosis: epidemiological study for 43,630 pupils in Niigata City, Japan. Eur Spine J. 2011;20:274-279.
14. Smorgick Y, Mirovsky Y, Baker KC, et al. Predictors of back pain in adolescent idiopathic scoliosis surgical candidates. J Pediatr Orthop. 2013;33:289-292.
15. US Preventive Services Task Force. Screening for Adolescent Idiopathic Scoliosis. US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:165-172.
16. Hresko MT, Talwalkar VR, Schwend RM. Position statement–Screening for the early detection of idiopathic scoliosis in adolescents. SRS/POSNA/AAOS/AAP Position Statement. 2015. www.srs.org/about-srs/news-and-announcements/position-statement---screening-for-the-early-detection-for-idiopathic-scoliosis-in-adolescents. Accessed September 30, 2020.
17. Palazzo C, Sailhan F, Revel M. Scheuermann’s disease: an update. Joint Bone Spine. 2014;81:209-214.
18. Ali RM, Green DW, Patel TC. Scheuermann’s kyphosis. Curr Opin Pediatr. 1999;11:70-75.
19. de Moraes Barros Fucs PM, Meves R, Yamada HH, et al. Spinal infections in children: a review. Int Orthop. 2012;36:387-395.
20. Joaquim AF, Ghizoni E, Valadares MG, et al. Spinal tumors in children. Revista da Associação Médica Brasileira. 2017;63:459-465.
21. Weiss PF, Colbert RA. Juvenile spondyloarthritis: a distinct form of juvenile arthritis. Pediatr Clin North Am. 2018;65:675-690.
22. Rush JK, Astur N, Scott S, et al. Use of magnetic resonance imaging in the evaluation of spondylolysis. J Pediatr Orthop. 2015;35:271-275.
23. Janicki JA, Alman B. Scoliosis: review of diagnosis and treatment. Pediatr Child Health. 2007;12:771-776.
24. O’Sullivan PB, Phyty GD, Twomey LT, et al. Evaluation of specific stabilizing exercise in the treatment of chronic low back pain with radiologic diagnosis of spondylolysis or spondylolisthesis. Spine.1997;22:2959-2967.
25. Inani SB, Selkar SP. Effect of core stabilization exercises versus conventional exercises on pain and functional status in patients with non-specific low back pain: a randomized clinical trial. J Back Musculoskelet Rehabil. 2013;26:37-43.
26. Garet M, Reiman MP, Mathers J, et al. Nonoperative treatment in lumbar spondylolysis and spondylolisthesis: a systematic review. Sports Health. 2013;5:225-232.
27. Selhorst M, Fischer A, Graft K, et al. Timing of physical therapy referral in adolescent athletes with acute spondylolysis: a retrospective chart review. Clin J Sport Med. 2017;27:296-301.
28. Théroux J, Stomski N, Hodgetts CJ, et al. Prevalence of low back pain in adolescents with idiopathic scoliosis: a systematic review. Chiropr Man Ther. 2017;25:10.
29. Romano M, Minozzi S, Zaina F, et al. Exercises for adolescent idiopathic scoliosis: a Cochrane systematic review. Spine (Phila Pa 1976). 2013;38:E883-E893.
30. Sanders JO, Newton PO, Browne RH, et al. Bracing for idiopathic scoliosis: how many patients require treatment to prevent one surgery? J Bone Joint Surg Am. 2014;96:649-653.
31. Hill JJ, Keating JL. Risk factors for the first episode of low back pain in children are infrequently validated across samples and conditions: a systematic review. J Physiother. 2010;56:237-244.
32. Grødahl LHJ, Fawcett L, Nazareth M, et al. Diagnostic utility of patient history and physical examination data to detect spondylolysis and spondylolisthesis in athletes with low back pain: a systematic review. Man Ther. 2016;24:7-17.
33. Asher MA, Burton DC. Adolescent idiopathic scoliosis: natural history and long term treatment effects. Scoliosis. 2006;1:2.
34. Weiss HR, Weiss G, Petermann F. Incidence of curvature progression in idiopathic scoliosis patients treated with scoliosis inpatient rehabilitation (SIR): an age- and sex-matched controlled study. Pediatr Rehabil. 2003;6:23-30.
35. Gomez JA, Hresko MT, Glotzbecker MP. Nonsurgical management of adolescent idiopathic scoliosis. J Am Acad Orthop Surg. 2016;24:555-564.
36. Weinstein SL, Dolan LA, Wright JG, et al. Effects of bracing in adolescents with idiopathic scoliosis. N Engl J Med. 2013;369:1512-1521.
37. Balg F, Juteau M, Theoret C, et al. Validity and reliability of the iPhone to measure rib hump in scoliosis. J Pediatr Orthop. 2014;34:774-779.
38. Auerbach JD, Ahn J, Zgonis MH, et al. Streamlining the evaluation of low back pain in children. Clin Orthop Relatl Res. 2008;466:1971-1977.
39. Beck NA, Miller R, Baldwin K, et al. Do oblique views add value in the diagnosis of spondylolysis in adolescents? J Bone Joint Surg Am. 2013;95:e65.
40. Roelofs PD, Deyo RA, Koes BW, et al. Nonsteroidal anti-inflammatory drugs for low back pain: an updated Cochrane review. Spine (Phila Pa 1976). 2008;33:1766-1774.
41. Miech R, Johnston L, O’Malley PM, et al. Prescription opioids in adolescence and future opioid misuse. Pediatrics. 2015;136:e1169-e1177.
42. Hu S, Tribus C, Diab M, et al. Spondylolysis and spondylolisthesis. J Bone Joint Surg. 2008;90:655-671.
43. Panteliadis P, Nagra NS, Edwards KL, et al. Athletic population with spondylolysis: review of outcomes following surgical repair or conservative management. Global Spine J. 2016;6:615-625.
44. Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults: a meta-analysis of observational studies. J Pediatr Orthop. 2009;29:146-156.
PRACTICE RECOMMENDATIONS
› Be aware that low back pain is rare in children < 7 years but increases in incidence as children near adolescence. A
› Consider imaging in the setting of bony tenderness, pain that awakens the patient from sleep, or in the presence of other “red flag” symptoms. A
› Consider spondylolysis and spondylolisthesis in adolescent athletes with low back pain lasting longer than 3 to 6 weeks. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Conservative care or surgery for rotator cuff tears?
Rotator cuff disease accounts for as many as 65% of shoulder-related visits to physicians’ offices,1 yet the natural course of rotator cuff tears is still not well understood.2 Treatment options are controversial because both conservative and surgical management have been successful. Physical therapy is a durable and reliable treatment option, but there are concerns about long-term progression of the tear.3 Surgical arthroscopic techniques, which result in less morbidity than open surgery, have improved overall surgical care; as such, the rate of rotator cuff procedures has increased significantly.4
Our goal in this article is to provide clinical guidance to the primary care provider. We review management options for rotator cuff injury; summarize considerations for proceeding with conservative or surgical management; and discuss surgical risks and complications.
Conservative management: Who is most likely to benefit?
The choice of treatment for rotator cuff injury depends on a host of variables, including shoulder dominance, duration of symptoms, type of tear (partial or full), age, demands (activity level, occupation, sport), and comorbidities (diabetes, tobacco use). Treatment goals include resolution of pain, normalized range of motion and strength, and restored arm and shoulder function.5
Initial nonoperative management is indicated in patients who
- have a partial-thickness tear (a notable exception is young patients with traumatic injury),6
- have lower functional demands and moderate symptoms, or
- refuse surgery.7
Patients who respond to nonoperative management will, typically, do so within 6 to 12 weeks.5,8
Few randomized, controlled trials have compared conservative and surgical management of rotator cuff tears; furthermore, the findings of these studies have been mixed. Nonoperative management has been shown to be the favored initial treatment for isolated, symptomatic, nontraumatic, supraspinatus tears in older patients.9 In a recent study,10 5-year outcomes were examined in a prospective cohort enrolled in a rotator cuff treatment program: Approximately 75% of patients remained successfully treated with nonoperative management, and clinical outcomes of the operative and nonoperative groups were not significantly different at 5-year follow-up. Investigators concluded that nonoperative treatment is effective for many patients who have a chronic, full-thickness rotator cuff tear.
In a study investigating the treatment of degenerative rotator cuff tear, patients were randomly treated using an operative or nonoperative protocol. No differences in functional outcomes were observed at 1 year after treatment; however, surgical treatment significantly improved subjective parameters of pain and disability.11 A similar study suggested statistically significant improvement in outcomes for patients managed operatively, compared with those treated nonoperatively, but differences in shoulder outcome and the visual analog pain score were small and failed to meet thresholds considered clinically significant. Larger studies, with longer follow-up, are required to determine whether clinical differences between these types of treatment become more evident over time.12
Continue to: A look at nonoperative options and outcomes
A look at nonoperative options and outcomes
Surveillance. Rotator cuff disease of the supraspinatus tendon often results from a degenerative process that progresses to partial and, eventually, full-thickness tearing.8 Once a tear develops, progression is difficult to predict. Many rotator cuff tears grow larger over time; this progression is commonly associated with new or increased pain and weakness, or both. Although asymptomatic progression of a tear is uncommon, many patients—and physicians—are apprehensive about proceeding with nonoperative treatment for a full-thickness tear.8
To diminish such fears, surveillance can include regular assessment of shoulder motion and strength, with consideration of repeat imaging until surgery is performed or the patient is no longer a surgical candidate or interested in surgical treatment.7 Patients and providers need to remain vigilant because tears that are initially graded as repairable can become irreparable if the tendon retracts or there is fatty infiltration of the muscle belly. Results of secondary surgical repair following failed prolonged nonoperative treatment tend to be inferior to results seen in patients who undergo primary tendon repair.7
Analgesics. Simple analgesics, such as acetaminophen, are a low-risk first-line option for pain relief; however, there are limited data on the efficacy of acetaminophen in rotator cuff disease. A topical or oral nonsteroidal anti-inflammatory drug (NSAID), or both, can be considered, but potential contraindications, such as gastrointestinal, renal, and cardiovascular risks, should be monitored.13 Avoid opioids, given the potential for abuse, except during the immediate postoperative period.5
Glucocorticoid injection. Injection of a glucocorticoid drug into the subacromial space should be considered in patients whose pain interferes with sleep, limits activities of daily living, or hinders the ability to participate in physical therapy.5 A recent systematic review demonstrated that NSAIDs and glucocorticoids brought similar pain relief and active abduction at 4 to 6 weeks, but that glucocorticoids were significantly better at achieving remission of symptoms.14 There are no data comparing glucocorticoid preparations (ie, different glucocorticoids or anesthetics, dosages, volumes), and ultrasound guidance does not appear to be necessary for short-term pain relief.15 Note: Repeated injection has been shown to decrease the durability of surgically repaired tendons16; if a patient is a candidate for surgery, repeat injections should be carefully considered—and avoided if possible.
Physical therapy. The goals of physical therapy are activity modification, stretching the shoulder capsule, and strengthening the surrounding musculature (periscapular, rotator cuff, and deltoid). Patients advance through 3 phases of recovery: shoulder mobility, strengthening, and function (ie, joint reactivation to improve shoulder proprioception and coordination).
Continue to: A recent meta-analysis...
A recent meta-analysis17 found comparative evidence on treating rotator cuff tears with physical therapy to be inconclusive. At 1-year follow-up, there was no clinically significant difference between surgery and active physical therapy in either improving the Constant Shoulder Score (an assessment of function) or reducing pain caused by a rotator cuff tear. Therefore, the authors proposed, given the low risk of harm, a conservative approach should be the initial treatment modality for a tear.
A Cochrane review18 examined 60 eligible trials, in which the mean age of patients was 51 years and the mean duration of symptoms, 11 months. Overall, the review concluded that the effects of manual therapy and exercise might be similar to those of glucocorticoid injection and arthroscopic subacromial decompression. The authors noted that this conclusion is based on low-quality evidence, with only 1 study in the review that compared the combination of manual therapy and exercise to placebo.
Other conservative options. Ultrasound, topical nitroglycerin, topical lidocaine, glucocorticoid iontophoresis, transcutaneous electrical nerve stimulation, massage, acupuncture, extracorporeal shockwave therapy, hyaluronic acid, and platelet-rich plasma have been used to treat rotator cuff disease. These modalities require further study, however, to determine their effectiveness for this indication.7,19
Who is a candidate for surgical management?
Although nonoperative treatment is preferred for rotator cuff tendinitis or tendinosis and partial-thickness tears, appropriate management of full-thickness tears is debatable.20 Some surgeons advocate early operative intervention of repairable full-thickness tears to prevent further progression and reduce the risk of long-term dysfunction.
The decision to pursue operative repair depends on
- patient characteristics (age, activity level, comorbidities),
- patient function (amount of disability caused by the tear),
- characteristics of the tear (length, depth, retraction), and
- chronicity of the tear (acuity).
Continue to: TABLE 1...
TABLE 121,22 highlights variables that influence the decision to proceed, or not to proceed, with operative intervention. Because enlargement of a tear usually exacerbates symptoms,23 patients with a tear who are successfully managed nonoperatively should be counseled on the potential of the tear to progress.
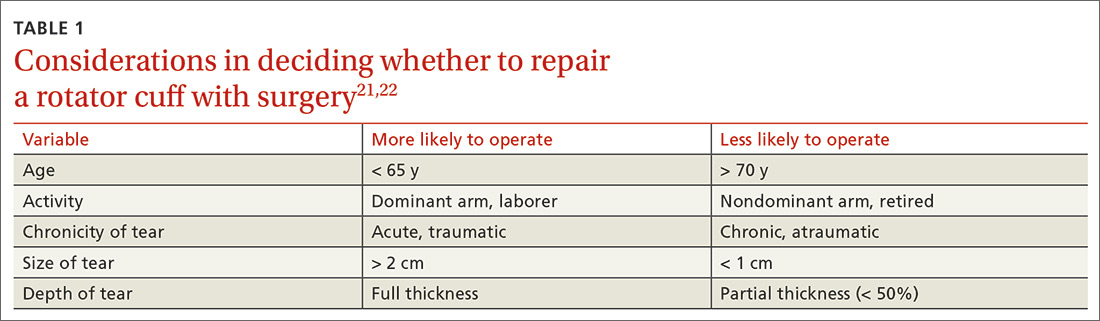
What are the surgical options?
Little clinical evidence favors one exposure technique over another. This equivalency has been demonstrated by a systematic review of randomized controlled trials comparing arthroscopic and mini-open rotator cuff repair, which showed no difference in function, pain, or range of motion.24 That conclusion notwithstanding, arthroscopic repair is increasingly popular because it results in less pain, initially, and faster return to work.20
There is controversy among surgeons regarding the choice of fixation technique: Tendons can be secured using 1 or 2 rows of anchors (FIGURE). Advocates of single-row repair cite shorter surgical time, decreased cost, and equivalent outcomes; surgeons who favor double-row, or so-called transosseous-equivalent, repair claim that it provides better restoration of normal anatomy and biomechanical superiority.25,26
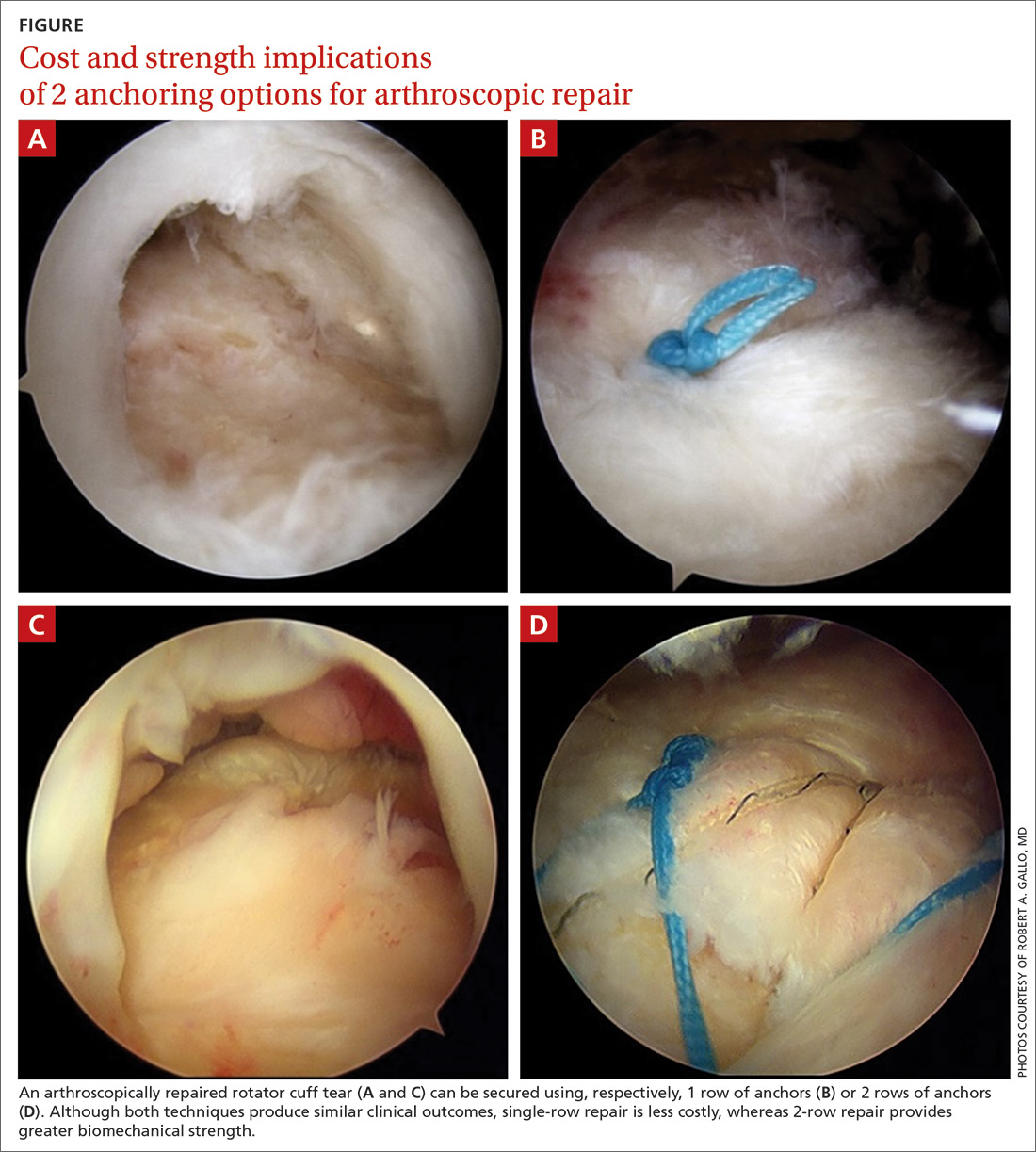
Regardless of technique, most patients are immobilized for 4 to 6 weeks postoperatively.27 Physical therapy usually commences within the first week or 2 postop, limited to passive motion for 6 to 12 weeks. Active motion and strengthening of rotator-cuff muscles often is initiated by 3 months postop, although this phase is sometimes delayed because of concern over slow tendon healing. Typically, patients make a full return to sports and manual work at 6 months postop. Patients experience most symptomatic improvement during the first 6 months following surgery, although functional gains can be realized for as long as 2 years after surgery.28
Most torn rotator cuffs can be fixed back to the greater tuberosity, but some chronic, massive, retracted tears lack the mobility to be repaired, or re-tear shortly after repair. Over time, the humeral head in a rotator cuff–deficient shoulder can migrate superiorly to abut the undersurface of the acromion, which can lead to significant glenohumeral osteoarthritis. To prevent or remedy elevation of the humeral head, salvage procedures—debridement, partial repair, spanning graft, tendon transfer, superior capsule reconstruction, balloon arthroplasty, reverse total shoulder replacement—can be used to alleviate pain and restore function. These procedures have significant limitations, however, and usually provide less favorable outcomes than standard repair.29-35
Continue to: Surgical outcomes
Surgical outcomes
Pain, function, and patient satisfaction outcomes following rotator cuff repair are generally favorable: 90% of patients are “happy” 6 months postop.28 Younger populations often have traumatic rotator cuff tears; they generally are interested in returning to sporting activities following their injury. Nearly 85% of younger patients who undergo rotator cuff repair return to sports, and 65.9% return to an equivalent level of play.36
Variables associated with an unfavorable outcome include increasing age, smoking, increased size of the tear, poor tendon quality, hyperlipidemia, workers’ compensation status, fatty infiltration of muscle, obesity, diabetes, and additional procedures to the biceps tendon and acromioclavicular joint performed at the time of rotator cuff repair.37-39 Interestingly, a study concluded that, if a patient expects a good surgical outcome, they are more likely to go on to report a favorable outcome—suggesting that a patient’s expectations might influence their actual outcome.40
Risks and complications
Although rotator cuff surgery has much lower morbidity than other orthopedic surgeries, it is not without risk of complications. If re-tears are excluded, postop complications have been reported in approximately 10% of patients.41 Common complications and their anticipated rate of occurrence are listed in TABLE 2.42-49
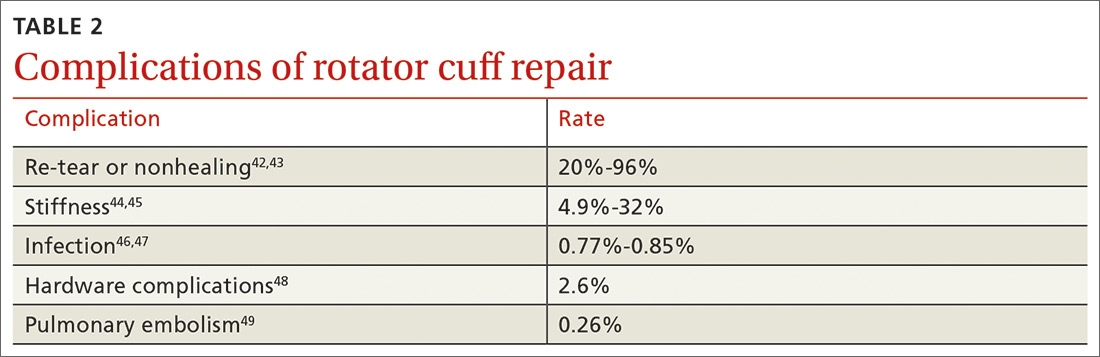
Re-tear of the surgically repaired tendon is the most common postop complication. Published re-tear rates range from 20% to 96%42,43 and generally correlate with initial tear size: A small tear is twice as likely to heal as a massive tear.50 That large range—a span of 76%—results from using a variety of methods to measure re-tear and might not have clinical meaning. A meta-analysis that examined more than 8000 shoulder surgeries reported an overall re-tear rate of 26.6%; however, both patients whose tendons healed and those who re-tore demonstrated clinical improvement.51 In a separate study, patients reported improvement in pain, function, range of motion, and satisfaction regardless of the integrity of the tendon; however, significant improvement in strength was seen only in those whose repair had healed.52
Postop stiffness is more common with arthroscopic repair than with open surgery, and with smaller rather than larger tears.53 Patient variables associated with an increased risk of postop adhesive capsulitis include workers’ compensation insurance, age < 50 years, and preoperative calcific tendonitis or adhesive capsulitis.53 Stiffness generally responds to physical therapy and rarely requires surgical lysis of adhesions or capsular release.
Continue to: Significant injury...
Significant injury to the deltoid muscle has become increasingly uncommon with the advancement of arthroscopic surgery. In traditional open surgery, detachment of the deltoid (and subsequent repair) is required to improve visualization; however, doing so can lead to atrophy and muscle rupture and dehiscence. Deltoid damage occurs in ≤ 60% of open surgeries but is negligible in arthroscopic and mini-open repairs, which involve splitting deltoid fibers to gain exposure of the underlying rotator cuff.54
SIDEBAR
Key takeaways in the management of rotator cuff injury
- Chronic, nontraumatic, and partial-thickness tears respond well to conservative management as first-line treatment. Poor surgical candidates should also be offered a trial of conservative therapy.
- Consider referral for surgical consultation if the patient does not respond to conservative therapy in 6 to 12 weeks; also, patients who have a full-thickness tear and young patients with traumatic injury should be referred for surgical consultation.
- Arthroscopy has become the preferred approach to rotator cuff repair because it is associated with less pain, fewer complications, and faster recovery.
- Patients should be counseled that recovery from surgical repair of a torn rotator cuff takes, on average, 6 months. Some massive or retracted rotator cuff injuries require more extensive procedures that increase healing time.
- Overall, patients are “happy” with rotator cuff repair at 6 months; clinical complications are uncommon, making surgery a suitable option in appropriately selected patients.
CORRESPONDENCE
Cayce Onks, DO, MS, ATC, Penn State Health Milton S. Hershey Medical Center, Penn State College of Medicine, Family and Community Medicine H154, 500 University Drive, PO Box 850, Hershey, PA 17033-0850; [email protected].
1. Vecchio P, Kavanagh R, Hazleman BL, et al. Shoulder pain in a community-based rheumatology clinic. Br J Rheumatol. 1995;34:440-442.
2. Eljabu W, Klinger HM, von Knoch M. The natural history of rotator cuff tears: a systematic review. Arch Orthop Trauma Surg. 2015;135:1055-1061.
3. Dunn WR, Kuhn JE, Sanders R, et al; MOON Shoulder Group. 2013 Neer Award: predictors of failure of nonoperative treatment of chronic, symptomatic, full-thickness rotator cuff tears. J Shoulder Elbow Surg. 2016;25:1303-1311.
4. Colvin AC, Egorova N, Harrison AK, et al. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94:227-233.
5. Whittle S, Buchbinder R. In the clinic. Rotator cuff disease. Ann Intern Med. 2015;162:ITC1-ITC15.
6. Lazarides AL, Alentorn-Geli E, Choi JHJ, et al. Rotator cuff tears in young patients: a different disease than rotator cuff tears in elderly patients. J Shoulder Elbow Surg. 2015;24:1834-1843.
7. Petri M, Ettinger M, Brand S, et al. Non-operative management of rotator cuff tears. Open Orthop J. 2016;10:349-356.
8. Schmidt CC, Jarrett CD, Brown BT. Management of rotator cuff tears. J Hand Surg Am. 2015;40:399-408.
9. Kukkonen J, Joukainen A, Lehtinen J, et al. Treatment of nontraumatic rotator cuff tears: a randomized controlled trial with two years of clinical and imaging follow-up. J Bone Joint Surg Am. 2015;97:1729-1737.
10. Boorman RS, More KD, Hollinshead RM, et al. What happens to patients when we do not repair their cuff tears? Five-year rotator cuff quality-of-life index outcomes following nonoperative treatment of patients with full-thickness rotator cuff tears. J Shoulder Elbow Surg. 2018;27:444-448.
11. Lambers Heerspink FO, van Raay JJ, Koorevaar RCT, et al. Comparing surgical repair with conservative treatment for degenerative rotator cuff tears: a randomized controlled trial. J Shoulder Elbow Surg. 2015;24:1274-1281.
12. Piper CC, Hughes AJ, Ma Y, et al. Operative versus nonoperative treatment for the management of full-thickness rotator cuff tears: a systematic review and meta-analysis. J Shoulder Elbow Surg. 2018;27:572-576.
13. Boudreault J, Desmeules F, Roy J-S, et al. The efficacy of oral non-steroidal anti-inflammatory drugs for rotator cuff tendinopathy: a systematic review and meta-analysis. J Rehabil Med. 2014;46:294-306.
14. Zheng X-Q, Li K, Wei Y-D, et al. Nonsteroidal anti-inflammatory drugs versus corticosteroid for treatment of shoulder pain: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2014;95:1824-1831.
15. Bloom JE, Rischin A, Johnston RV, et al. Image-guided versus blind glucocorticoid injection for shoulder pain. Cochrane Database Syst Rev. 2012;(8):CD009147.
16. Wiggins ME, Fadale PD, Ehrlich MG, et al. Effects of local injection of corticosteroids on the healing of ligaments. A follow-up report. J Bone Joint Surg Am. 1995;77:1682-1691.
17. Ryösä A, Laimi K, Äärimaa V, et al. Surgery or conservative treatment for rotator cuff tear: a meta-analysis. Disabil Rehabil. 2017;39:1357-1363.
18. Page MJ, Green S, McBain B, et al. Manual therapy and exercise for rotator cuff disease. Cochrane Database Syst Rev. 2016;(6):CD012224.
19. Page MJ, Green S, Mrocki MA, et al. Electrotherapy modalities for rotator cuff disease. Cochrane Database Syst Rev. 2016;(6):CD012225.
20. Acevedo DC, Paxton ES, Williams GR, et al. A survey of expert opinion regarding rotator cuff repair. J Bone Joint Surg Am. 2014;96:e123.
21. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94:163-167.
22. Thorpe A, Hurworth M, O’Sullivan P, et al. Rotator cuff disease: opinion regarding surgical criteria and likely outcome. ANZ J Surg. 2017;87:291-295.
23. Mall NA, Kim HM, Keener JD, et al. Symptomatic progression of asymptomatic rotator cuff tears: a prospective study of clinical and sonographic variables. J Bone Joint Surg Am. 2010;92:2623-2633.
24. Ji X, Bi C, Wang F, et al. Arthroscopic versus mini-open rotator cuff repair: an up-to-date meta-analysis of randomized controlled trials. Arthroscopy. 2015;31:118-124.
25. Duquin TR, Buyea C, Bisson LJ. Which method of rotator cuff repair leads to the highest rate of structural healing? A systematic review. Am J Sports Med. 2010;38:835-841.
26. Choi S, Kim MK, Kim GM, et al. Factors associated with clinical and structural outcomes after arthroscopic rotator cuff repair with a suture bridge technique in medium, large, and massive tears. J Shoulder Elbow Surg. 2014;23:1675-1681.
27. Shen C, Tang Z-H, Hu J-Z, et al. Does immobilization after arthroscopic rotator cuff repair increase tendon healing? A systematic review and meta-analysis. Arch Orthop Trauma Surg. 2014;134:1279-1285.
28. Gulotta LV, Nho SJ, Dodson CC, et al; . Prospective evaluation of arthroscopic rotator cuff repairs at 5 years: part I. Functional outcomes and radiographic healing rates. J Shoulder Elbow Surg. 2011;20:934-940.
29. Liem D, Lengers N, Dedy N, et al. Arthroscopic debridement of massive irreparable rotator cuff tears. Arthroscopy. 2008;24:743-748.
30. Weber SC. Partial rotator cuff repair in massive rotator cuff tears: long-term follow-up. J Shoulder Elbow Surg. 2017;26:e171.
31. Lewington MR, Ferguson DP, Smith TD, et al. Graft utilization in the bridging reconstruction of irreparable rotator cuff tears: a systematic review. Am J Sports Med. 2017;45:3149-3157.
32. Longo UG, Franceschetti E, Petrillo S, et al. Latissimus dorsi tendon transfer for massive irreparable rotator cuff tears: a systematic review. Sports Med Arthrosc Rev. 2011;19:428-437.
33. Noyes MP, Denard PJ. Arthroscopic superior capsular reconstruction: indications and outcomes. Oper Tech Sports Med. 2018;26:29-34.
34. Piekaar RSM, Bouman ICE, van Kampen PM, et al. Early promising outcome following arthroscopic implantation of the subacromial balloon spacer for treating massive rotator cuff tear. Musculoskeletal Surg. 2018;102:247-255.
35. Ek ETH, Neukom L, Catanzaro S, et al. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg. 2013;22:1199-1208.
36. Klouche S, Lefevre N, Herman S, et al. Return to sport after rotator cuff tear repair: a systematic review and meta-analysis. Am J Sports Med. 2016;44:1877-1887.
37. Garcia GH, Liu JN, Wong A, et al. Hyperlipidemia increases the risk of retear after arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2017;26:2086-2090.
38. Khair MM, Lehman J, Tsouris N, et al. A systematic review of preoperative fatty infiltration and rotator cuff outcomes. HSS J. 2016;12:170-176.
39. Lambers Heerspink FO, Dorrestijn O, van Raay JJAM, et al. Specific patient-related prognostic factors for rotator cuff repair: a systematic review. J Shoulder Elbow Surg. 2014;23:1073-1080.
40. Henn RF 3rd, Kang L, Tashjian RZ, et al. Patients’ preoperative expectations predict the outcome of rotator cuff repair. J Bone Joint Surg Am. 2007;89:1913-1919.
41. Mansat P, Cofield RH, Kersten TE, et al. Complications of rotator cuff repair. Orthop Clin North Am. 1997;28:205-213.
42. Boileau P, Brassart N, Watkinson DJ, et al. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87:1229-1240.
43. Galatz LM, Ball CM, Teefey SA, et al. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86:219-224.
44. Aydin N, Kocaoglu B, Guven O. Single-row versus double-row arthroscopic rotator cuff repair in small- to medium-sized tears. J Shoulder Elbow Surg. 2010;19:722-725.
45. Peltz CD, Dourte LM, Kuntz AF, et al. The effect of postoperative passive motion on rotator cuff healing in a rat model. J Bone Joint Surg Am. 2009;91:2421-2429.
46. Vopat BG, Lee BJ, DeStefano S, et al. Risk factors for infection after rotator cuff repair. Arthroscopy. 2016;32:428-434.
47. Pauzenberger L, Grieb A, Hexel M, et al. Infections following arthroscopic rotator cuff repair: incidence, risk factors, and prophylaxis. Knee Surg Sports Traumatol Arthrosc. 2017;25:595-601.
48. Randelli P, Spennacchio P, Ragone V, et al. Complications associated with arthroscopic rotator cuff repair: a literature review. Musculoskelet Surg. 2012;96:9-16.
49. Hoxie SC, Sperling JW, Cofield RH. Pulmonary embolism following rotator cuff repair. Int J Shoulder Surg. 2008;2:49-51.
50. Wu XL, Briggs L, Murrell GAC. Intraoperative determinants of rotator cuff repair integrity: an analysis of 500 consecutive repairs. Am J Sports Med. 2012;40:2771-2776.
51. McElvany MD, McGoldrick E, Gee AO, et al. Rotator cuff repair: published evidence on factors associated with repair integrity and clinical outcome. Am J Sports Med. 2015;43:491-500.
52. Yoo JH, Cho NS, Rhee YG. Effect of postoperative repair integrity on health-related quality of life after rotator cuff repair: healed versus retear group. Am J Sports Med. 2013;41;2637-2644.
53. Huberty DP, Schoolfield JD, Brady PC, et al. Incidence and treatment of postoperative stiffness following arthroscopic rotator cuff repair. Arthroscopy. 2009;25:880-890.
54. Cho NS, Cha SW, Rhee YG. Alterations of the deltoid muscle after open versus arthroscopic rotator cuff repair. Am J Sports Med. 2015;43:2927-2934.
Rotator cuff disease accounts for as many as 65% of shoulder-related visits to physicians’ offices,1 yet the natural course of rotator cuff tears is still not well understood.2 Treatment options are controversial because both conservative and surgical management have been successful. Physical therapy is a durable and reliable treatment option, but there are concerns about long-term progression of the tear.3 Surgical arthroscopic techniques, which result in less morbidity than open surgery, have improved overall surgical care; as such, the rate of rotator cuff procedures has increased significantly.4
Our goal in this article is to provide clinical guidance to the primary care provider. We review management options for rotator cuff injury; summarize considerations for proceeding with conservative or surgical management; and discuss surgical risks and complications.
Conservative management: Who is most likely to benefit?
The choice of treatment for rotator cuff injury depends on a host of variables, including shoulder dominance, duration of symptoms, type of tear (partial or full), age, demands (activity level, occupation, sport), and comorbidities (diabetes, tobacco use). Treatment goals include resolution of pain, normalized range of motion and strength, and restored arm and shoulder function.5
Initial nonoperative management is indicated in patients who
- have a partial-thickness tear (a notable exception is young patients with traumatic injury),6
- have lower functional demands and moderate symptoms, or
- refuse surgery.7
Patients who respond to nonoperative management will, typically, do so within 6 to 12 weeks.5,8
Few randomized, controlled trials have compared conservative and surgical management of rotator cuff tears; furthermore, the findings of these studies have been mixed. Nonoperative management has been shown to be the favored initial treatment for isolated, symptomatic, nontraumatic, supraspinatus tears in older patients.9 In a recent study,10 5-year outcomes were examined in a prospective cohort enrolled in a rotator cuff treatment program: Approximately 75% of patients remained successfully treated with nonoperative management, and clinical outcomes of the operative and nonoperative groups were not significantly different at 5-year follow-up. Investigators concluded that nonoperative treatment is effective for many patients who have a chronic, full-thickness rotator cuff tear.
In a study investigating the treatment of degenerative rotator cuff tear, patients were randomly treated using an operative or nonoperative protocol. No differences in functional outcomes were observed at 1 year after treatment; however, surgical treatment significantly improved subjective parameters of pain and disability.11 A similar study suggested statistically significant improvement in outcomes for patients managed operatively, compared with those treated nonoperatively, but differences in shoulder outcome and the visual analog pain score were small and failed to meet thresholds considered clinically significant. Larger studies, with longer follow-up, are required to determine whether clinical differences between these types of treatment become more evident over time.12
Continue to: A look at nonoperative options and outcomes
A look at nonoperative options and outcomes
Surveillance. Rotator cuff disease of the supraspinatus tendon often results from a degenerative process that progresses to partial and, eventually, full-thickness tearing.8 Once a tear develops, progression is difficult to predict. Many rotator cuff tears grow larger over time; this progression is commonly associated with new or increased pain and weakness, or both. Although asymptomatic progression of a tear is uncommon, many patients—and physicians—are apprehensive about proceeding with nonoperative treatment for a full-thickness tear.8
To diminish such fears, surveillance can include regular assessment of shoulder motion and strength, with consideration of repeat imaging until surgery is performed or the patient is no longer a surgical candidate or interested in surgical treatment.7 Patients and providers need to remain vigilant because tears that are initially graded as repairable can become irreparable if the tendon retracts or there is fatty infiltration of the muscle belly. Results of secondary surgical repair following failed prolonged nonoperative treatment tend to be inferior to results seen in patients who undergo primary tendon repair.7
Analgesics. Simple analgesics, such as acetaminophen, are a low-risk first-line option for pain relief; however, there are limited data on the efficacy of acetaminophen in rotator cuff disease. A topical or oral nonsteroidal anti-inflammatory drug (NSAID), or both, can be considered, but potential contraindications, such as gastrointestinal, renal, and cardiovascular risks, should be monitored.13 Avoid opioids, given the potential for abuse, except during the immediate postoperative period.5
Glucocorticoid injection. Injection of a glucocorticoid drug into the subacromial space should be considered in patients whose pain interferes with sleep, limits activities of daily living, or hinders the ability to participate in physical therapy.5 A recent systematic review demonstrated that NSAIDs and glucocorticoids brought similar pain relief and active abduction at 4 to 6 weeks, but that glucocorticoids were significantly better at achieving remission of symptoms.14 There are no data comparing glucocorticoid preparations (ie, different glucocorticoids or anesthetics, dosages, volumes), and ultrasound guidance does not appear to be necessary for short-term pain relief.15 Note: Repeated injection has been shown to decrease the durability of surgically repaired tendons16; if a patient is a candidate for surgery, repeat injections should be carefully considered—and avoided if possible.
Physical therapy. The goals of physical therapy are activity modification, stretching the shoulder capsule, and strengthening the surrounding musculature (periscapular, rotator cuff, and deltoid). Patients advance through 3 phases of recovery: shoulder mobility, strengthening, and function (ie, joint reactivation to improve shoulder proprioception and coordination).
Continue to: A recent meta-analysis...
A recent meta-analysis17 found comparative evidence on treating rotator cuff tears with physical therapy to be inconclusive. At 1-year follow-up, there was no clinically significant difference between surgery and active physical therapy in either improving the Constant Shoulder Score (an assessment of function) or reducing pain caused by a rotator cuff tear. Therefore, the authors proposed, given the low risk of harm, a conservative approach should be the initial treatment modality for a tear.
A Cochrane review18 examined 60 eligible trials, in which the mean age of patients was 51 years and the mean duration of symptoms, 11 months. Overall, the review concluded that the effects of manual therapy and exercise might be similar to those of glucocorticoid injection and arthroscopic subacromial decompression. The authors noted that this conclusion is based on low-quality evidence, with only 1 study in the review that compared the combination of manual therapy and exercise to placebo.
Other conservative options. Ultrasound, topical nitroglycerin, topical lidocaine, glucocorticoid iontophoresis, transcutaneous electrical nerve stimulation, massage, acupuncture, extracorporeal shockwave therapy, hyaluronic acid, and platelet-rich plasma have been used to treat rotator cuff disease. These modalities require further study, however, to determine their effectiveness for this indication.7,19
Who is a candidate for surgical management?
Although nonoperative treatment is preferred for rotator cuff tendinitis or tendinosis and partial-thickness tears, appropriate management of full-thickness tears is debatable.20 Some surgeons advocate early operative intervention of repairable full-thickness tears to prevent further progression and reduce the risk of long-term dysfunction.
The decision to pursue operative repair depends on
- patient characteristics (age, activity level, comorbidities),
- patient function (amount of disability caused by the tear),
- characteristics of the tear (length, depth, retraction), and
- chronicity of the tear (acuity).
Continue to: TABLE 1...
TABLE 121,22 highlights variables that influence the decision to proceed, or not to proceed, with operative intervention. Because enlargement of a tear usually exacerbates symptoms,23 patients with a tear who are successfully managed nonoperatively should be counseled on the potential of the tear to progress.

What are the surgical options?
Little clinical evidence favors one exposure technique over another. This equivalency has been demonstrated by a systematic review of randomized controlled trials comparing arthroscopic and mini-open rotator cuff repair, which showed no difference in function, pain, or range of motion.24 That conclusion notwithstanding, arthroscopic repair is increasingly popular because it results in less pain, initially, and faster return to work.20
There is controversy among surgeons regarding the choice of fixation technique: Tendons can be secured using 1 or 2 rows of anchors (FIGURE). Advocates of single-row repair cite shorter surgical time, decreased cost, and equivalent outcomes; surgeons who favor double-row, or so-called transosseous-equivalent, repair claim that it provides better restoration of normal anatomy and biomechanical superiority.25,26

Regardless of technique, most patients are immobilized for 4 to 6 weeks postoperatively.27 Physical therapy usually commences within the first week or 2 postop, limited to passive motion for 6 to 12 weeks. Active motion and strengthening of rotator-cuff muscles often is initiated by 3 months postop, although this phase is sometimes delayed because of concern over slow tendon healing. Typically, patients make a full return to sports and manual work at 6 months postop. Patients experience most symptomatic improvement during the first 6 months following surgery, although functional gains can be realized for as long as 2 years after surgery.28
Most torn rotator cuffs can be fixed back to the greater tuberosity, but some chronic, massive, retracted tears lack the mobility to be repaired, or re-tear shortly after repair. Over time, the humeral head in a rotator cuff–deficient shoulder can migrate superiorly to abut the undersurface of the acromion, which can lead to significant glenohumeral osteoarthritis. To prevent or remedy elevation of the humeral head, salvage procedures—debridement, partial repair, spanning graft, tendon transfer, superior capsule reconstruction, balloon arthroplasty, reverse total shoulder replacement—can be used to alleviate pain and restore function. These procedures have significant limitations, however, and usually provide less favorable outcomes than standard repair.29-35
Continue to: Surgical outcomes
Surgical outcomes
Pain, function, and patient satisfaction outcomes following rotator cuff repair are generally favorable: 90% of patients are “happy” 6 months postop.28 Younger populations often have traumatic rotator cuff tears; they generally are interested in returning to sporting activities following their injury. Nearly 85% of younger patients who undergo rotator cuff repair return to sports, and 65.9% return to an equivalent level of play.36
Variables associated with an unfavorable outcome include increasing age, smoking, increased size of the tear, poor tendon quality, hyperlipidemia, workers’ compensation status, fatty infiltration of muscle, obesity, diabetes, and additional procedures to the biceps tendon and acromioclavicular joint performed at the time of rotator cuff repair.37-39 Interestingly, a study concluded that, if a patient expects a good surgical outcome, they are more likely to go on to report a favorable outcome—suggesting that a patient’s expectations might influence their actual outcome.40
Risks and complications
Although rotator cuff surgery has much lower morbidity than other orthopedic surgeries, it is not without risk of complications. If re-tears are excluded, postop complications have been reported in approximately 10% of patients.41 Common complications and their anticipated rate of occurrence are listed in TABLE 2.42-49

Re-tear of the surgically repaired tendon is the most common postop complication. Published re-tear rates range from 20% to 96%42,43 and generally correlate with initial tear size: A small tear is twice as likely to heal as a massive tear.50 That large range—a span of 76%—results from using a variety of methods to measure re-tear and might not have clinical meaning. A meta-analysis that examined more than 8000 shoulder surgeries reported an overall re-tear rate of 26.6%; however, both patients whose tendons healed and those who re-tore demonstrated clinical improvement.51 In a separate study, patients reported improvement in pain, function, range of motion, and satisfaction regardless of the integrity of the tendon; however, significant improvement in strength was seen only in those whose repair had healed.52
Postop stiffness is more common with arthroscopic repair than with open surgery, and with smaller rather than larger tears.53 Patient variables associated with an increased risk of postop adhesive capsulitis include workers’ compensation insurance, age < 50 years, and preoperative calcific tendonitis or adhesive capsulitis.53 Stiffness generally responds to physical therapy and rarely requires surgical lysis of adhesions or capsular release.
Continue to: Significant injury...
Significant injury to the deltoid muscle has become increasingly uncommon with the advancement of arthroscopic surgery. In traditional open surgery, detachment of the deltoid (and subsequent repair) is required to improve visualization; however, doing so can lead to atrophy and muscle rupture and dehiscence. Deltoid damage occurs in ≤ 60% of open surgeries but is negligible in arthroscopic and mini-open repairs, which involve splitting deltoid fibers to gain exposure of the underlying rotator cuff.54
SIDEBAR
Key takeaways in the management of rotator cuff injury
- Chronic, nontraumatic, and partial-thickness tears respond well to conservative management as first-line treatment. Poor surgical candidates should also be offered a trial of conservative therapy.
- Consider referral for surgical consultation if the patient does not respond to conservative therapy in 6 to 12 weeks; also, patients who have a full-thickness tear and young patients with traumatic injury should be referred for surgical consultation.
- Arthroscopy has become the preferred approach to rotator cuff repair because it is associated with less pain, fewer complications, and faster recovery.
- Patients should be counseled that recovery from surgical repair of a torn rotator cuff takes, on average, 6 months. Some massive or retracted rotator cuff injuries require more extensive procedures that increase healing time.
- Overall, patients are “happy” with rotator cuff repair at 6 months; clinical complications are uncommon, making surgery a suitable option in appropriately selected patients.
CORRESPONDENCE
Cayce Onks, DO, MS, ATC, Penn State Health Milton S. Hershey Medical Center, Penn State College of Medicine, Family and Community Medicine H154, 500 University Drive, PO Box 850, Hershey, PA 17033-0850; [email protected].
Rotator cuff disease accounts for as many as 65% of shoulder-related visits to physicians’ offices,1 yet the natural course of rotator cuff tears is still not well understood.2 Treatment options are controversial because both conservative and surgical management have been successful. Physical therapy is a durable and reliable treatment option, but there are concerns about long-term progression of the tear.3 Surgical arthroscopic techniques, which result in less morbidity than open surgery, have improved overall surgical care; as such, the rate of rotator cuff procedures has increased significantly.4
Our goal in this article is to provide clinical guidance to the primary care provider. We review management options for rotator cuff injury; summarize considerations for proceeding with conservative or surgical management; and discuss surgical risks and complications.
Conservative management: Who is most likely to benefit?
The choice of treatment for rotator cuff injury depends on a host of variables, including shoulder dominance, duration of symptoms, type of tear (partial or full), age, demands (activity level, occupation, sport), and comorbidities (diabetes, tobacco use). Treatment goals include resolution of pain, normalized range of motion and strength, and restored arm and shoulder function.5
Initial nonoperative management is indicated in patients who
- have a partial-thickness tear (a notable exception is young patients with traumatic injury),6
- have lower functional demands and moderate symptoms, or
- refuse surgery.7
Patients who respond to nonoperative management will, typically, do so within 6 to 12 weeks.5,8
Few randomized, controlled trials have compared conservative and surgical management of rotator cuff tears; furthermore, the findings of these studies have been mixed. Nonoperative management has been shown to be the favored initial treatment for isolated, symptomatic, nontraumatic, supraspinatus tears in older patients.9 In a recent study,10 5-year outcomes were examined in a prospective cohort enrolled in a rotator cuff treatment program: Approximately 75% of patients remained successfully treated with nonoperative management, and clinical outcomes of the operative and nonoperative groups were not significantly different at 5-year follow-up. Investigators concluded that nonoperative treatment is effective for many patients who have a chronic, full-thickness rotator cuff tear.
In a study investigating the treatment of degenerative rotator cuff tear, patients were randomly treated using an operative or nonoperative protocol. No differences in functional outcomes were observed at 1 year after treatment; however, surgical treatment significantly improved subjective parameters of pain and disability.11 A similar study suggested statistically significant improvement in outcomes for patients managed operatively, compared with those treated nonoperatively, but differences in shoulder outcome and the visual analog pain score were small and failed to meet thresholds considered clinically significant. Larger studies, with longer follow-up, are required to determine whether clinical differences between these types of treatment become more evident over time.12
Continue to: A look at nonoperative options and outcomes
A look at nonoperative options and outcomes
Surveillance. Rotator cuff disease of the supraspinatus tendon often results from a degenerative process that progresses to partial and, eventually, full-thickness tearing.8 Once a tear develops, progression is difficult to predict. Many rotator cuff tears grow larger over time; this progression is commonly associated with new or increased pain and weakness, or both. Although asymptomatic progression of a tear is uncommon, many patients—and physicians—are apprehensive about proceeding with nonoperative treatment for a full-thickness tear.8
To diminish such fears, surveillance can include regular assessment of shoulder motion and strength, with consideration of repeat imaging until surgery is performed or the patient is no longer a surgical candidate or interested in surgical treatment.7 Patients and providers need to remain vigilant because tears that are initially graded as repairable can become irreparable if the tendon retracts or there is fatty infiltration of the muscle belly. Results of secondary surgical repair following failed prolonged nonoperative treatment tend to be inferior to results seen in patients who undergo primary tendon repair.7
Analgesics. Simple analgesics, such as acetaminophen, are a low-risk first-line option for pain relief; however, there are limited data on the efficacy of acetaminophen in rotator cuff disease. A topical or oral nonsteroidal anti-inflammatory drug (NSAID), or both, can be considered, but potential contraindications, such as gastrointestinal, renal, and cardiovascular risks, should be monitored.13 Avoid opioids, given the potential for abuse, except during the immediate postoperative period.5
Glucocorticoid injection. Injection of a glucocorticoid drug into the subacromial space should be considered in patients whose pain interferes with sleep, limits activities of daily living, or hinders the ability to participate in physical therapy.5 A recent systematic review demonstrated that NSAIDs and glucocorticoids brought similar pain relief and active abduction at 4 to 6 weeks, but that glucocorticoids were significantly better at achieving remission of symptoms.14 There are no data comparing glucocorticoid preparations (ie, different glucocorticoids or anesthetics, dosages, volumes), and ultrasound guidance does not appear to be necessary for short-term pain relief.15 Note: Repeated injection has been shown to decrease the durability of surgically repaired tendons16; if a patient is a candidate for surgery, repeat injections should be carefully considered—and avoided if possible.
Physical therapy. The goals of physical therapy are activity modification, stretching the shoulder capsule, and strengthening the surrounding musculature (periscapular, rotator cuff, and deltoid). Patients advance through 3 phases of recovery: shoulder mobility, strengthening, and function (ie, joint reactivation to improve shoulder proprioception and coordination).
Continue to: A recent meta-analysis...
A recent meta-analysis17 found comparative evidence on treating rotator cuff tears with physical therapy to be inconclusive. At 1-year follow-up, there was no clinically significant difference between surgery and active physical therapy in either improving the Constant Shoulder Score (an assessment of function) or reducing pain caused by a rotator cuff tear. Therefore, the authors proposed, given the low risk of harm, a conservative approach should be the initial treatment modality for a tear.
A Cochrane review18 examined 60 eligible trials, in which the mean age of patients was 51 years and the mean duration of symptoms, 11 months. Overall, the review concluded that the effects of manual therapy and exercise might be similar to those of glucocorticoid injection and arthroscopic subacromial decompression. The authors noted that this conclusion is based on low-quality evidence, with only 1 study in the review that compared the combination of manual therapy and exercise to placebo.
Other conservative options. Ultrasound, topical nitroglycerin, topical lidocaine, glucocorticoid iontophoresis, transcutaneous electrical nerve stimulation, massage, acupuncture, extracorporeal shockwave therapy, hyaluronic acid, and platelet-rich plasma have been used to treat rotator cuff disease. These modalities require further study, however, to determine their effectiveness for this indication.7,19
Who is a candidate for surgical management?
Although nonoperative treatment is preferred for rotator cuff tendinitis or tendinosis and partial-thickness tears, appropriate management of full-thickness tears is debatable.20 Some surgeons advocate early operative intervention of repairable full-thickness tears to prevent further progression and reduce the risk of long-term dysfunction.
The decision to pursue operative repair depends on
- patient characteristics (age, activity level, comorbidities),
- patient function (amount of disability caused by the tear),
- characteristics of the tear (length, depth, retraction), and
- chronicity of the tear (acuity).
Continue to: TABLE 1...
TABLE 121,22 highlights variables that influence the decision to proceed, or not to proceed, with operative intervention. Because enlargement of a tear usually exacerbates symptoms,23 patients with a tear who are successfully managed nonoperatively should be counseled on the potential of the tear to progress.

What are the surgical options?
Little clinical evidence favors one exposure technique over another. This equivalency has been demonstrated by a systematic review of randomized controlled trials comparing arthroscopic and mini-open rotator cuff repair, which showed no difference in function, pain, or range of motion.24 That conclusion notwithstanding, arthroscopic repair is increasingly popular because it results in less pain, initially, and faster return to work.20
There is controversy among surgeons regarding the choice of fixation technique: Tendons can be secured using 1 or 2 rows of anchors (FIGURE). Advocates of single-row repair cite shorter surgical time, decreased cost, and equivalent outcomes; surgeons who favor double-row, or so-called transosseous-equivalent, repair claim that it provides better restoration of normal anatomy and biomechanical superiority.25,26

Regardless of technique, most patients are immobilized for 4 to 6 weeks postoperatively.27 Physical therapy usually commences within the first week or 2 postop, limited to passive motion for 6 to 12 weeks. Active motion and strengthening of rotator-cuff muscles often is initiated by 3 months postop, although this phase is sometimes delayed because of concern over slow tendon healing. Typically, patients make a full return to sports and manual work at 6 months postop. Patients experience most symptomatic improvement during the first 6 months following surgery, although functional gains can be realized for as long as 2 years after surgery.28
Most torn rotator cuffs can be fixed back to the greater tuberosity, but some chronic, massive, retracted tears lack the mobility to be repaired, or re-tear shortly after repair. Over time, the humeral head in a rotator cuff–deficient shoulder can migrate superiorly to abut the undersurface of the acromion, which can lead to significant glenohumeral osteoarthritis. To prevent or remedy elevation of the humeral head, salvage procedures—debridement, partial repair, spanning graft, tendon transfer, superior capsule reconstruction, balloon arthroplasty, reverse total shoulder replacement—can be used to alleviate pain and restore function. These procedures have significant limitations, however, and usually provide less favorable outcomes than standard repair.29-35
Continue to: Surgical outcomes
Surgical outcomes
Pain, function, and patient satisfaction outcomes following rotator cuff repair are generally favorable: 90% of patients are “happy” 6 months postop.28 Younger populations often have traumatic rotator cuff tears; they generally are interested in returning to sporting activities following their injury. Nearly 85% of younger patients who undergo rotator cuff repair return to sports, and 65.9% return to an equivalent level of play.36
Variables associated with an unfavorable outcome include increasing age, smoking, increased size of the tear, poor tendon quality, hyperlipidemia, workers’ compensation status, fatty infiltration of muscle, obesity, diabetes, and additional procedures to the biceps tendon and acromioclavicular joint performed at the time of rotator cuff repair.37-39 Interestingly, a study concluded that, if a patient expects a good surgical outcome, they are more likely to go on to report a favorable outcome—suggesting that a patient’s expectations might influence their actual outcome.40
Risks and complications
Although rotator cuff surgery has much lower morbidity than other orthopedic surgeries, it is not without risk of complications. If re-tears are excluded, postop complications have been reported in approximately 10% of patients.41 Common complications and their anticipated rate of occurrence are listed in TABLE 2.42-49

Re-tear of the surgically repaired tendon is the most common postop complication. Published re-tear rates range from 20% to 96%42,43 and generally correlate with initial tear size: A small tear is twice as likely to heal as a massive tear.50 That large range—a span of 76%—results from using a variety of methods to measure re-tear and might not have clinical meaning. A meta-analysis that examined more than 8000 shoulder surgeries reported an overall re-tear rate of 26.6%; however, both patients whose tendons healed and those who re-tore demonstrated clinical improvement.51 In a separate study, patients reported improvement in pain, function, range of motion, and satisfaction regardless of the integrity of the tendon; however, significant improvement in strength was seen only in those whose repair had healed.52
Postop stiffness is more common with arthroscopic repair than with open surgery, and with smaller rather than larger tears.53 Patient variables associated with an increased risk of postop adhesive capsulitis include workers’ compensation insurance, age < 50 years, and preoperative calcific tendonitis or adhesive capsulitis.53 Stiffness generally responds to physical therapy and rarely requires surgical lysis of adhesions or capsular release.
Continue to: Significant injury...
Significant injury to the deltoid muscle has become increasingly uncommon with the advancement of arthroscopic surgery. In traditional open surgery, detachment of the deltoid (and subsequent repair) is required to improve visualization; however, doing so can lead to atrophy and muscle rupture and dehiscence. Deltoid damage occurs in ≤ 60% of open surgeries but is negligible in arthroscopic and mini-open repairs, which involve splitting deltoid fibers to gain exposure of the underlying rotator cuff.54
SIDEBAR
Key takeaways in the management of rotator cuff injury
- Chronic, nontraumatic, and partial-thickness tears respond well to conservative management as first-line treatment. Poor surgical candidates should also be offered a trial of conservative therapy.
- Consider referral for surgical consultation if the patient does not respond to conservative therapy in 6 to 12 weeks; also, patients who have a full-thickness tear and young patients with traumatic injury should be referred for surgical consultation.
- Arthroscopy has become the preferred approach to rotator cuff repair because it is associated with less pain, fewer complications, and faster recovery.
- Patients should be counseled that recovery from surgical repair of a torn rotator cuff takes, on average, 6 months. Some massive or retracted rotator cuff injuries require more extensive procedures that increase healing time.
- Overall, patients are “happy” with rotator cuff repair at 6 months; clinical complications are uncommon, making surgery a suitable option in appropriately selected patients.
CORRESPONDENCE
Cayce Onks, DO, MS, ATC, Penn State Health Milton S. Hershey Medical Center, Penn State College of Medicine, Family and Community Medicine H154, 500 University Drive, PO Box 850, Hershey, PA 17033-0850; [email protected].
1. Vecchio P, Kavanagh R, Hazleman BL, et al. Shoulder pain in a community-based rheumatology clinic. Br J Rheumatol. 1995;34:440-442.
2. Eljabu W, Klinger HM, von Knoch M. The natural history of rotator cuff tears: a systematic review. Arch Orthop Trauma Surg. 2015;135:1055-1061.
3. Dunn WR, Kuhn JE, Sanders R, et al; MOON Shoulder Group. 2013 Neer Award: predictors of failure of nonoperative treatment of chronic, symptomatic, full-thickness rotator cuff tears. J Shoulder Elbow Surg. 2016;25:1303-1311.
4. Colvin AC, Egorova N, Harrison AK, et al. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94:227-233.
5. Whittle S, Buchbinder R. In the clinic. Rotator cuff disease. Ann Intern Med. 2015;162:ITC1-ITC15.
6. Lazarides AL, Alentorn-Geli E, Choi JHJ, et al. Rotator cuff tears in young patients: a different disease than rotator cuff tears in elderly patients. J Shoulder Elbow Surg. 2015;24:1834-1843.
7. Petri M, Ettinger M, Brand S, et al. Non-operative management of rotator cuff tears. Open Orthop J. 2016;10:349-356.
8. Schmidt CC, Jarrett CD, Brown BT. Management of rotator cuff tears. J Hand Surg Am. 2015;40:399-408.
9. Kukkonen J, Joukainen A, Lehtinen J, et al. Treatment of nontraumatic rotator cuff tears: a randomized controlled trial with two years of clinical and imaging follow-up. J Bone Joint Surg Am. 2015;97:1729-1737.
10. Boorman RS, More KD, Hollinshead RM, et al. What happens to patients when we do not repair their cuff tears? Five-year rotator cuff quality-of-life index outcomes following nonoperative treatment of patients with full-thickness rotator cuff tears. J Shoulder Elbow Surg. 2018;27:444-448.
11. Lambers Heerspink FO, van Raay JJ, Koorevaar RCT, et al. Comparing surgical repair with conservative treatment for degenerative rotator cuff tears: a randomized controlled trial. J Shoulder Elbow Surg. 2015;24:1274-1281.
12. Piper CC, Hughes AJ, Ma Y, et al. Operative versus nonoperative treatment for the management of full-thickness rotator cuff tears: a systematic review and meta-analysis. J Shoulder Elbow Surg. 2018;27:572-576.
13. Boudreault J, Desmeules F, Roy J-S, et al. The efficacy of oral non-steroidal anti-inflammatory drugs for rotator cuff tendinopathy: a systematic review and meta-analysis. J Rehabil Med. 2014;46:294-306.
14. Zheng X-Q, Li K, Wei Y-D, et al. Nonsteroidal anti-inflammatory drugs versus corticosteroid for treatment of shoulder pain: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2014;95:1824-1831.
15. Bloom JE, Rischin A, Johnston RV, et al. Image-guided versus blind glucocorticoid injection for shoulder pain. Cochrane Database Syst Rev. 2012;(8):CD009147.
16. Wiggins ME, Fadale PD, Ehrlich MG, et al. Effects of local injection of corticosteroids on the healing of ligaments. A follow-up report. J Bone Joint Surg Am. 1995;77:1682-1691.
17. Ryösä A, Laimi K, Äärimaa V, et al. Surgery or conservative treatment for rotator cuff tear: a meta-analysis. Disabil Rehabil. 2017;39:1357-1363.
18. Page MJ, Green S, McBain B, et al. Manual therapy and exercise for rotator cuff disease. Cochrane Database Syst Rev. 2016;(6):CD012224.
19. Page MJ, Green S, Mrocki MA, et al. Electrotherapy modalities for rotator cuff disease. Cochrane Database Syst Rev. 2016;(6):CD012225.
20. Acevedo DC, Paxton ES, Williams GR, et al. A survey of expert opinion regarding rotator cuff repair. J Bone Joint Surg Am. 2014;96:e123.
21. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94:163-167.
22. Thorpe A, Hurworth M, O’Sullivan P, et al. Rotator cuff disease: opinion regarding surgical criteria and likely outcome. ANZ J Surg. 2017;87:291-295.
23. Mall NA, Kim HM, Keener JD, et al. Symptomatic progression of asymptomatic rotator cuff tears: a prospective study of clinical and sonographic variables. J Bone Joint Surg Am. 2010;92:2623-2633.
24. Ji X, Bi C, Wang F, et al. Arthroscopic versus mini-open rotator cuff repair: an up-to-date meta-analysis of randomized controlled trials. Arthroscopy. 2015;31:118-124.
25. Duquin TR, Buyea C, Bisson LJ. Which method of rotator cuff repair leads to the highest rate of structural healing? A systematic review. Am J Sports Med. 2010;38:835-841.
26. Choi S, Kim MK, Kim GM, et al. Factors associated with clinical and structural outcomes after arthroscopic rotator cuff repair with a suture bridge technique in medium, large, and massive tears. J Shoulder Elbow Surg. 2014;23:1675-1681.
27. Shen C, Tang Z-H, Hu J-Z, et al. Does immobilization after arthroscopic rotator cuff repair increase tendon healing? A systematic review and meta-analysis. Arch Orthop Trauma Surg. 2014;134:1279-1285.
28. Gulotta LV, Nho SJ, Dodson CC, et al; . Prospective evaluation of arthroscopic rotator cuff repairs at 5 years: part I. Functional outcomes and radiographic healing rates. J Shoulder Elbow Surg. 2011;20:934-940.
29. Liem D, Lengers N, Dedy N, et al. Arthroscopic debridement of massive irreparable rotator cuff tears. Arthroscopy. 2008;24:743-748.
30. Weber SC. Partial rotator cuff repair in massive rotator cuff tears: long-term follow-up. J Shoulder Elbow Surg. 2017;26:e171.
31. Lewington MR, Ferguson DP, Smith TD, et al. Graft utilization in the bridging reconstruction of irreparable rotator cuff tears: a systematic review. Am J Sports Med. 2017;45:3149-3157.
32. Longo UG, Franceschetti E, Petrillo S, et al. Latissimus dorsi tendon transfer for massive irreparable rotator cuff tears: a systematic review. Sports Med Arthrosc Rev. 2011;19:428-437.
33. Noyes MP, Denard PJ. Arthroscopic superior capsular reconstruction: indications and outcomes. Oper Tech Sports Med. 2018;26:29-34.
34. Piekaar RSM, Bouman ICE, van Kampen PM, et al. Early promising outcome following arthroscopic implantation of the subacromial balloon spacer for treating massive rotator cuff tear. Musculoskeletal Surg. 2018;102:247-255.
35. Ek ETH, Neukom L, Catanzaro S, et al. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg. 2013;22:1199-1208.
36. Klouche S, Lefevre N, Herman S, et al. Return to sport after rotator cuff tear repair: a systematic review and meta-analysis. Am J Sports Med. 2016;44:1877-1887.
37. Garcia GH, Liu JN, Wong A, et al. Hyperlipidemia increases the risk of retear after arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2017;26:2086-2090.
38. Khair MM, Lehman J, Tsouris N, et al. A systematic review of preoperative fatty infiltration and rotator cuff outcomes. HSS J. 2016;12:170-176.
39. Lambers Heerspink FO, Dorrestijn O, van Raay JJAM, et al. Specific patient-related prognostic factors for rotator cuff repair: a systematic review. J Shoulder Elbow Surg. 2014;23:1073-1080.
40. Henn RF 3rd, Kang L, Tashjian RZ, et al. Patients’ preoperative expectations predict the outcome of rotator cuff repair. J Bone Joint Surg Am. 2007;89:1913-1919.
41. Mansat P, Cofield RH, Kersten TE, et al. Complications of rotator cuff repair. Orthop Clin North Am. 1997;28:205-213.
42. Boileau P, Brassart N, Watkinson DJ, et al. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87:1229-1240.
43. Galatz LM, Ball CM, Teefey SA, et al. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86:219-224.
44. Aydin N, Kocaoglu B, Guven O. Single-row versus double-row arthroscopic rotator cuff repair in small- to medium-sized tears. J Shoulder Elbow Surg. 2010;19:722-725.
45. Peltz CD, Dourte LM, Kuntz AF, et al. The effect of postoperative passive motion on rotator cuff healing in a rat model. J Bone Joint Surg Am. 2009;91:2421-2429.
46. Vopat BG, Lee BJ, DeStefano S, et al. Risk factors for infection after rotator cuff repair. Arthroscopy. 2016;32:428-434.
47. Pauzenberger L, Grieb A, Hexel M, et al. Infections following arthroscopic rotator cuff repair: incidence, risk factors, and prophylaxis. Knee Surg Sports Traumatol Arthrosc. 2017;25:595-601.
48. Randelli P, Spennacchio P, Ragone V, et al. Complications associated with arthroscopic rotator cuff repair: a literature review. Musculoskelet Surg. 2012;96:9-16.
49. Hoxie SC, Sperling JW, Cofield RH. Pulmonary embolism following rotator cuff repair. Int J Shoulder Surg. 2008;2:49-51.
50. Wu XL, Briggs L, Murrell GAC. Intraoperative determinants of rotator cuff repair integrity: an analysis of 500 consecutive repairs. Am J Sports Med. 2012;40:2771-2776.
51. McElvany MD, McGoldrick E, Gee AO, et al. Rotator cuff repair: published evidence on factors associated with repair integrity and clinical outcome. Am J Sports Med. 2015;43:491-500.
52. Yoo JH, Cho NS, Rhee YG. Effect of postoperative repair integrity on health-related quality of life after rotator cuff repair: healed versus retear group. Am J Sports Med. 2013;41;2637-2644.
53. Huberty DP, Schoolfield JD, Brady PC, et al. Incidence and treatment of postoperative stiffness following arthroscopic rotator cuff repair. Arthroscopy. 2009;25:880-890.
54. Cho NS, Cha SW, Rhee YG. Alterations of the deltoid muscle after open versus arthroscopic rotator cuff repair. Am J Sports Med. 2015;43:2927-2934.
1. Vecchio P, Kavanagh R, Hazleman BL, et al. Shoulder pain in a community-based rheumatology clinic. Br J Rheumatol. 1995;34:440-442.
2. Eljabu W, Klinger HM, von Knoch M. The natural history of rotator cuff tears: a systematic review. Arch Orthop Trauma Surg. 2015;135:1055-1061.
3. Dunn WR, Kuhn JE, Sanders R, et al; MOON Shoulder Group. 2013 Neer Award: predictors of failure of nonoperative treatment of chronic, symptomatic, full-thickness rotator cuff tears. J Shoulder Elbow Surg. 2016;25:1303-1311.
4. Colvin AC, Egorova N, Harrison AK, et al. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94:227-233.
5. Whittle S, Buchbinder R. In the clinic. Rotator cuff disease. Ann Intern Med. 2015;162:ITC1-ITC15.
6. Lazarides AL, Alentorn-Geli E, Choi JHJ, et al. Rotator cuff tears in young patients: a different disease than rotator cuff tears in elderly patients. J Shoulder Elbow Surg. 2015;24:1834-1843.
7. Petri M, Ettinger M, Brand S, et al. Non-operative management of rotator cuff tears. Open Orthop J. 2016;10:349-356.
8. Schmidt CC, Jarrett CD, Brown BT. Management of rotator cuff tears. J Hand Surg Am. 2015;40:399-408.
9. Kukkonen J, Joukainen A, Lehtinen J, et al. Treatment of nontraumatic rotator cuff tears: a randomized controlled trial with two years of clinical and imaging follow-up. J Bone Joint Surg Am. 2015;97:1729-1737.
10. Boorman RS, More KD, Hollinshead RM, et al. What happens to patients when we do not repair their cuff tears? Five-year rotator cuff quality-of-life index outcomes following nonoperative treatment of patients with full-thickness rotator cuff tears. J Shoulder Elbow Surg. 2018;27:444-448.
11. Lambers Heerspink FO, van Raay JJ, Koorevaar RCT, et al. Comparing surgical repair with conservative treatment for degenerative rotator cuff tears: a randomized controlled trial. J Shoulder Elbow Surg. 2015;24:1274-1281.
12. Piper CC, Hughes AJ, Ma Y, et al. Operative versus nonoperative treatment for the management of full-thickness rotator cuff tears: a systematic review and meta-analysis. J Shoulder Elbow Surg. 2018;27:572-576.
13. Boudreault J, Desmeules F, Roy J-S, et al. The efficacy of oral non-steroidal anti-inflammatory drugs for rotator cuff tendinopathy: a systematic review and meta-analysis. J Rehabil Med. 2014;46:294-306.
14. Zheng X-Q, Li K, Wei Y-D, et al. Nonsteroidal anti-inflammatory drugs versus corticosteroid for treatment of shoulder pain: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2014;95:1824-1831.
15. Bloom JE, Rischin A, Johnston RV, et al. Image-guided versus blind glucocorticoid injection for shoulder pain. Cochrane Database Syst Rev. 2012;(8):CD009147.
16. Wiggins ME, Fadale PD, Ehrlich MG, et al. Effects of local injection of corticosteroids on the healing of ligaments. A follow-up report. J Bone Joint Surg Am. 1995;77:1682-1691.
17. Ryösä A, Laimi K, Äärimaa V, et al. Surgery or conservative treatment for rotator cuff tear: a meta-analysis. Disabil Rehabil. 2017;39:1357-1363.
18. Page MJ, Green S, McBain B, et al. Manual therapy and exercise for rotator cuff disease. Cochrane Database Syst Rev. 2016;(6):CD012224.
19. Page MJ, Green S, Mrocki MA, et al. Electrotherapy modalities for rotator cuff disease. Cochrane Database Syst Rev. 2016;(6):CD012225.
20. Acevedo DC, Paxton ES, Williams GR, et al. A survey of expert opinion regarding rotator cuff repair. J Bone Joint Surg Am. 2014;96:e123.
21. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94:163-167.
22. Thorpe A, Hurworth M, O’Sullivan P, et al. Rotator cuff disease: opinion regarding surgical criteria and likely outcome. ANZ J Surg. 2017;87:291-295.
23. Mall NA, Kim HM, Keener JD, et al. Symptomatic progression of asymptomatic rotator cuff tears: a prospective study of clinical and sonographic variables. J Bone Joint Surg Am. 2010;92:2623-2633.
24. Ji X, Bi C, Wang F, et al. Arthroscopic versus mini-open rotator cuff repair: an up-to-date meta-analysis of randomized controlled trials. Arthroscopy. 2015;31:118-124.
25. Duquin TR, Buyea C, Bisson LJ. Which method of rotator cuff repair leads to the highest rate of structural healing? A systematic review. Am J Sports Med. 2010;38:835-841.
26. Choi S, Kim MK, Kim GM, et al. Factors associated with clinical and structural outcomes after arthroscopic rotator cuff repair with a suture bridge technique in medium, large, and massive tears. J Shoulder Elbow Surg. 2014;23:1675-1681.
27. Shen C, Tang Z-H, Hu J-Z, et al. Does immobilization after arthroscopic rotator cuff repair increase tendon healing? A systematic review and meta-analysis. Arch Orthop Trauma Surg. 2014;134:1279-1285.
28. Gulotta LV, Nho SJ, Dodson CC, et al; . Prospective evaluation of arthroscopic rotator cuff repairs at 5 years: part I. Functional outcomes and radiographic healing rates. J Shoulder Elbow Surg. 2011;20:934-940.
29. Liem D, Lengers N, Dedy N, et al. Arthroscopic debridement of massive irreparable rotator cuff tears. Arthroscopy. 2008;24:743-748.
30. Weber SC. Partial rotator cuff repair in massive rotator cuff tears: long-term follow-up. J Shoulder Elbow Surg. 2017;26:e171.
31. Lewington MR, Ferguson DP, Smith TD, et al. Graft utilization in the bridging reconstruction of irreparable rotator cuff tears: a systematic review. Am J Sports Med. 2017;45:3149-3157.
32. Longo UG, Franceschetti E, Petrillo S, et al. Latissimus dorsi tendon transfer for massive irreparable rotator cuff tears: a systematic review. Sports Med Arthrosc Rev. 2011;19:428-437.
33. Noyes MP, Denard PJ. Arthroscopic superior capsular reconstruction: indications and outcomes. Oper Tech Sports Med. 2018;26:29-34.
34. Piekaar RSM, Bouman ICE, van Kampen PM, et al. Early promising outcome following arthroscopic implantation of the subacromial balloon spacer for treating massive rotator cuff tear. Musculoskeletal Surg. 2018;102:247-255.
35. Ek ETH, Neukom L, Catanzaro S, et al. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg. 2013;22:1199-1208.
36. Klouche S, Lefevre N, Herman S, et al. Return to sport after rotator cuff tear repair: a systematic review and meta-analysis. Am J Sports Med. 2016;44:1877-1887.
37. Garcia GH, Liu JN, Wong A, et al. Hyperlipidemia increases the risk of retear after arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2017;26:2086-2090.
38. Khair MM, Lehman J, Tsouris N, et al. A systematic review of preoperative fatty infiltration and rotator cuff outcomes. HSS J. 2016;12:170-176.
39. Lambers Heerspink FO, Dorrestijn O, van Raay JJAM, et al. Specific patient-related prognostic factors for rotator cuff repair: a systematic review. J Shoulder Elbow Surg. 2014;23:1073-1080.
40. Henn RF 3rd, Kang L, Tashjian RZ, et al. Patients’ preoperative expectations predict the outcome of rotator cuff repair. J Bone Joint Surg Am. 2007;89:1913-1919.
41. Mansat P, Cofield RH, Kersten TE, et al. Complications of rotator cuff repair. Orthop Clin North Am. 1997;28:205-213.
42. Boileau P, Brassart N, Watkinson DJ, et al. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87:1229-1240.
43. Galatz LM, Ball CM, Teefey SA, et al. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86:219-224.
44. Aydin N, Kocaoglu B, Guven O. Single-row versus double-row arthroscopic rotator cuff repair in small- to medium-sized tears. J Shoulder Elbow Surg. 2010;19:722-725.
45. Peltz CD, Dourte LM, Kuntz AF, et al. The effect of postoperative passive motion on rotator cuff healing in a rat model. J Bone Joint Surg Am. 2009;91:2421-2429.
46. Vopat BG, Lee BJ, DeStefano S, et al. Risk factors for infection after rotator cuff repair. Arthroscopy. 2016;32:428-434.
47. Pauzenberger L, Grieb A, Hexel M, et al. Infections following arthroscopic rotator cuff repair: incidence, risk factors, and prophylaxis. Knee Surg Sports Traumatol Arthrosc. 2017;25:595-601.
48. Randelli P, Spennacchio P, Ragone V, et al. Complications associated with arthroscopic rotator cuff repair: a literature review. Musculoskelet Surg. 2012;96:9-16.
49. Hoxie SC, Sperling JW, Cofield RH. Pulmonary embolism following rotator cuff repair. Int J Shoulder Surg. 2008;2:49-51.
50. Wu XL, Briggs L, Murrell GAC. Intraoperative determinants of rotator cuff repair integrity: an analysis of 500 consecutive repairs. Am J Sports Med. 2012;40:2771-2776.
51. McElvany MD, McGoldrick E, Gee AO, et al. Rotator cuff repair: published evidence on factors associated with repair integrity and clinical outcome. Am J Sports Med. 2015;43:491-500.
52. Yoo JH, Cho NS, Rhee YG. Effect of postoperative repair integrity on health-related quality of life after rotator cuff repair: healed versus retear group. Am J Sports Med. 2013;41;2637-2644.
53. Huberty DP, Schoolfield JD, Brady PC, et al. Incidence and treatment of postoperative stiffness following arthroscopic rotator cuff repair. Arthroscopy. 2009;25:880-890.
54. Cho NS, Cha SW, Rhee YG. Alterations of the deltoid muscle after open versus arthroscopic rotator cuff repair. Am J Sports Med. 2015;43:2927-2934.
PRACTICE RECOMMENDATIONS
› Offer a trial of conservative management to patients with chronic, nontraumatic, or partial-thickness rotator cuff injury and to those who are poor surgical candidates. B
› Counsel patients that the rate of surgical complications is low and outcomes are favorable in properly selected patients for operative repair of rotator cuff tear. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series