User login
Bazex Syndrome (Paraneoplastic Acrokeratosis)
Psoriasiform dermatitis seen with Bazex syndrome may involve the nose and the helices of the ears in addition to the palms and soles. In most reported cases, the appearance of the characteristic psoriasiform lesions preceded the diagnosis of the associated underlying malignancy. Skin scrapings for potassium hydroxide and fungal cultures should be performed, and skin biopsy of keratotic plaques is recommended to exclude psoriasis.
Case Report
A 70-year-old man with no personal or family history of psoriasis or other skin diseases developed psoriasiform dermatitis of the fingers, toes, and helices of the ears over a period of 3 months. He reported a history of cigarette smoking (1 pack per day) with significant consumption of alcoholic beverages over a period of 30 years. Results from a review of systems revealed progressive hoarseness and dysphagia, with a recent history of a 15-lb weight loss. On physical examination, psoriasiform plaques were seen on the palms and soles, as well as on the helices of the ears (Figure 1) and the tip and dorsum of the nose. There was a yellowish discoloration and dystrophy of all the fingernails and toenails (Figure 2). Results from potassium hydroxide preparations from scrapings of the palms and soles were negative, and fungal culture did not grow any pathogenic fungi.
Six weeks later, the patient developed bilateral cervical lymphadenopathy. Otolaryngologic examination consisted of direct laryngoscopy; imaging studies including magnetic resonance imaging and computed tomography scans; and laryngeal biopsy, which revealed a stage IV squamous cell carcinoma (SCC) confined to the head and neck area. Although the patient did not return for follow-up, management of the laryngeal SCC with surgery and postoperative chemotherapy completely cleared his skin and nail lesions without adjunct dermatologic treatments.
PLEASE REFER TO THE PDF TO VIEW THE FIGURES
Comment
Bazex syndrome (paraneoplastic acrokeratosis) was first described as a clinical entity by Gougerot and Rupp1 more than 40 years prior to the coining of the disease's current widely used eponym of Bazex syndrome. In 1922, Gougerot and Grupper1 described a patient with hyperkeratotic lesions on the nose, ears, palms, and soles in conjunction with an SCC on the tongue. Years later, Bazex and colleagues2 described a patient with an SCC of the pyriform fossa and an associated psoriasiform dermatosis. Since that report, more than 110 cases of Bazex syndrome have been reported, most of which describe the condition as a cutaneous paraneoplastic syndrome characterized by psoriasiform lesions associated with an underlying malignancy of the upper aerodigestive tract (oropharynx, larynx, or esophagus), most often of the SCC subtype.3-7
Bazex syndrome can be classified among the cutaneous paraneoplastic disorders that also include acanthosis nigricans maligna, erythema gyratum, necrolytic migratory erythema, and hypertrichosis lanuginosa acquisita.7 The cutaneous manifestations of Bazex syndrome are paraneoplastic in that the developing skin changes coevolve with an underlying malignancy; these cutaneous hallmarks of the syndrome do not, however, represent metastatic extensions of this malignancy. On the contrary, they may actually serve as harbingers of future oncologic progression.
The cutaneous changes observed in Bazex syndrome have been classified into 3 stages.3 In the first stage, psoriasiform changes of the fingers, toes, auricular helix, and nose are noted. In addition, the earliest stage of the syndrome is characterized by nail changes, including horizontal and vertical ridging, subungual hyperkeratosis, yellow discoloration, and nail dystrophy. During this stage, the primary tumor is considered asymptomatic. The second stage is primarily typified by proximal extension of the cutaneous changes observed in the first stage to involve the dorsum of the hands and feet, as well as the malar regions of the face. Local symptoms secondary to growth of the primary tumor also may surface during this stage. The third stage in the course of the syndrome is defined by progressive centripetal extension of the cutaneous disease process to affect regions of the arms and legs (nails, hands, elbows, knees, and feet), scalp, and trunk.3-7 Other cutaneous changes that have been reported include hyperpigmentation, particularly in individuals with darker skin pigmentation,6 and development of bullous lesions.5,8,9
Based on the initial dermatologic manifestations of Bazex syndrome, it is not surprising that the condition is often misdiagnosed as psoriasis or chronic dermatitis. Indeed, histopathologic examination of skin lesions in the syndrome is nonspecific and may mimic psoriasis or other more common dermatoses, demonstrating hyperkeratosis, parakeratosis, acanthosis, vacuolar degeneration of keratinocytes, and/or perivascular lymphohistiocytic infiltrate.6,7,10 One potential distinguishing feature of Bazex syndrome, however, is specific psoriasiform involvement of the helix of the ear, as opposed to the entire ear, as would be more commonly expected in psoriasis.7 The tip of the nose also is involved in Bazex syndrome, which is an unusual location for psoriasis.
Extensive reviews of the literature reporting cases of Bazex syndrome demonstrate that most patients have been Caucasian, male, of French descent, and older than 40 years.6,7 SCCs have accounted for nearly 60% of tumors found in patients with this syndrome, and adenocarcinomas have accounted for less than 10% of malignancies. Furthermore, the majority of the neoplasms have involved the oropharynx and larynx.7 These neoplasms may be silent and only present with lymph node metastases. Less commonly, primary tumors may occur in the lungs and esophagus. Rare cases of neoplasms of the prostate, liver, stomach, thymus, uterus, vulva, and lymphoid tissues also have been reported.11 Numerous cases have been described in which the primary tumor could not be identified, and affected patients were diagnosed on the basis of metastases to cervical lymph nodes. In the vast majority of reported cases, the appearance of the characteristic psoriasiform lesions preceded the diagnosis of the associated malignancy.6,7 Finally, the skin lesions either markedly improved or completely resolved in the great preponderance of patients in whom the underlying malignancy was either treated with chemotherapy and/or radiation therapy or surgical excision.6,7,10-12 This was true of the patient presented in this report.
The pathogenesis of Bazex syndrome remains a mystery, though several authors have suggested an autoimmune etiology based on the common histologic finding of inflammatory infiltrates along the basal cell layer of affected skin regions.5,8,9 The immune reaction may be humoral or cellular; the proposed mechanism states that cross reactivity between skin and tumor antigens may produce the characteristic cutaneous changes observed, because antitumor antibodies cross reacting with the epidermis or basement membrane zone could elicit an immunologic response resulting in basal cell layer damage.13,14 Several authors also have proposed that the tumors may produce a host of growth factors that collectively lead to hyperkeratotic skin changes.14,15
Ideal treatment of Bazex syndrome is eradication of the underlying malignancy. Unresectable or treatment-resistant tumors, however, pose a significant challenge for the clinician. Numerous studies have been conducted demonstrating equivocal efficacies of various standard dermatological therapies in the treatment of skin lesions occurring in this syndrome. Unfortunately, in the vast majority of patients, such treatment options as topical tar, topical and systemic corticosteroids, UVB irradiation, antifungals, and antibiotics have proven to be of little use.6,7 Gill and colleagues9 have reported that oral psoralen–UVA phototherapy may offer some promise of effective treatment in these patients. However, larger studies are required to further investigate the therapeutic benefits of this treatment option. Although the management of treatment-resistant cutaneous lesions in Bazex syndrome may prove problematic, it is clear that the clinician must be astute in recognizing this disease process in its earlier stages to identify and effectively treat any underlying malignancy as expeditiously as possible.
Acknowledgment—The authors wish to thank Dr. Eric Ehrsam for his assistance with the preparation of this manuscript.
References
- Gougerot H, Grupper C. Dermatose érythémato-squameuse avec hyperkératose palmoplantaire, porectasies digitales et cancer de la langue latent. Paris Méd. 1922;43:234-237.
- Bazex A, Salvador R, Dupré A, et al. Syndrome paranéoplasique à type d'hyperkératose des extrémités. Guérison après le traitment del'épthélioma laryngé [letter]. Bull Soc Fr Dermatol Syphiligr. 1965;72:182.
- Bazex A, Griffiths A. Acrokeratosis paraneoplastica: a new cutaneous marker of malignancy. Br J Dermatol. 1980;103:801-805.
- O'Brien TJ. Bazex syndrome (acrokeratosis paraneoplastica). Australas J Dermatol. 1995;36:91-93.
- Bolognia JL, Brewer YP, Cooper DL. Bazex syndrome (acrokeratosis paraneoplastica): an analytic review. Medicine (Baltimore). 1991;70:269-280.
- Bolognia JL. Bazex syndrome: acrokeratosis paraneoplastica. Semin Dermatol. 1995;14:84-89.
- Sarkar B, Knecht R, Sarkar C, et al. Bazex syndrome (acrokeratosis paraneoplastica). Eur Arch Otorhinolaryngol. 1998;255:205-210.
- Handfield-Jones SE, Matthews CAN, Ellis JP, et al. Acrokeratosis paraneoplastica of Bazex. J R Soc Med. 1992;85:548-550.
- Gill D, Fergin P, Kelly J. Bullous lesions in Bazex syndrome and successful treatment with oral psoralen phototherapy. Australas J Dermatol. 2001;42:278-280.
- Wareing MJ, Vaughan-Jones SA, McGibbon DH. Acrokeratosis paraneoplastica: Bazex syndrome. J Laryngol Otol. 1996;110:899-900.
- Buxtorf K, Hübscher E, Panizzon R. Bazex syndrome. Dermatology. 2001;202:350-352.
- Hsu YS, Lien GS, Lai HH, et al. Acrokeratosis paraneoplastica (Bazex syndrome) with adenocarcinoma of the colon: report of a case and review of the literature. J Gastroenterol. 2000;35:460-464.
- Pecora AL, Landsman L, Imgrund SP, et al. Acrokeratosis paraneoplastica: report of a case and review of the literature. Arch Dermatol. 1983;119:820-826.
- Jean LB, Yvelise PB, Dennis LC. Bazex syndrome (acrokeratosis paraneoplastica): an analytic review. Medicine. 1991;70:269-280.
- Politi Y, Ophir J, Brenner S. Cutaneous paraneoplastic syndromes. Acta Derm Venereol (Stockh). 1993;73:161-170.
Psoriasiform dermatitis seen with Bazex syndrome may involve the nose and the helices of the ears in addition to the palms and soles. In most reported cases, the appearance of the characteristic psoriasiform lesions preceded the diagnosis of the associated underlying malignancy. Skin scrapings for potassium hydroxide and fungal cultures should be performed, and skin biopsy of keratotic plaques is recommended to exclude psoriasis.
Case Report
A 70-year-old man with no personal or family history of psoriasis or other skin diseases developed psoriasiform dermatitis of the fingers, toes, and helices of the ears over a period of 3 months. He reported a history of cigarette smoking (1 pack per day) with significant consumption of alcoholic beverages over a period of 30 years. Results from a review of systems revealed progressive hoarseness and dysphagia, with a recent history of a 15-lb weight loss. On physical examination, psoriasiform plaques were seen on the palms and soles, as well as on the helices of the ears (Figure 1) and the tip and dorsum of the nose. There was a yellowish discoloration and dystrophy of all the fingernails and toenails (Figure 2). Results from potassium hydroxide preparations from scrapings of the palms and soles were negative, and fungal culture did not grow any pathogenic fungi.
Six weeks later, the patient developed bilateral cervical lymphadenopathy. Otolaryngologic examination consisted of direct laryngoscopy; imaging studies including magnetic resonance imaging and computed tomography scans; and laryngeal biopsy, which revealed a stage IV squamous cell carcinoma (SCC) confined to the head and neck area. Although the patient did not return for follow-up, management of the laryngeal SCC with surgery and postoperative chemotherapy completely cleared his skin and nail lesions without adjunct dermatologic treatments.
PLEASE REFER TO THE PDF TO VIEW THE FIGURES
Comment
Bazex syndrome (paraneoplastic acrokeratosis) was first described as a clinical entity by Gougerot and Rupp1 more than 40 years prior to the coining of the disease's current widely used eponym of Bazex syndrome. In 1922, Gougerot and Grupper1 described a patient with hyperkeratotic lesions on the nose, ears, palms, and soles in conjunction with an SCC on the tongue. Years later, Bazex and colleagues2 described a patient with an SCC of the pyriform fossa and an associated psoriasiform dermatosis. Since that report, more than 110 cases of Bazex syndrome have been reported, most of which describe the condition as a cutaneous paraneoplastic syndrome characterized by psoriasiform lesions associated with an underlying malignancy of the upper aerodigestive tract (oropharynx, larynx, or esophagus), most often of the SCC subtype.3-7
Bazex syndrome can be classified among the cutaneous paraneoplastic disorders that also include acanthosis nigricans maligna, erythema gyratum, necrolytic migratory erythema, and hypertrichosis lanuginosa acquisita.7 The cutaneous manifestations of Bazex syndrome are paraneoplastic in that the developing skin changes coevolve with an underlying malignancy; these cutaneous hallmarks of the syndrome do not, however, represent metastatic extensions of this malignancy. On the contrary, they may actually serve as harbingers of future oncologic progression.
The cutaneous changes observed in Bazex syndrome have been classified into 3 stages.3 In the first stage, psoriasiform changes of the fingers, toes, auricular helix, and nose are noted. In addition, the earliest stage of the syndrome is characterized by nail changes, including horizontal and vertical ridging, subungual hyperkeratosis, yellow discoloration, and nail dystrophy. During this stage, the primary tumor is considered asymptomatic. The second stage is primarily typified by proximal extension of the cutaneous changes observed in the first stage to involve the dorsum of the hands and feet, as well as the malar regions of the face. Local symptoms secondary to growth of the primary tumor also may surface during this stage. The third stage in the course of the syndrome is defined by progressive centripetal extension of the cutaneous disease process to affect regions of the arms and legs (nails, hands, elbows, knees, and feet), scalp, and trunk.3-7 Other cutaneous changes that have been reported include hyperpigmentation, particularly in individuals with darker skin pigmentation,6 and development of bullous lesions.5,8,9
Based on the initial dermatologic manifestations of Bazex syndrome, it is not surprising that the condition is often misdiagnosed as psoriasis or chronic dermatitis. Indeed, histopathologic examination of skin lesions in the syndrome is nonspecific and may mimic psoriasis or other more common dermatoses, demonstrating hyperkeratosis, parakeratosis, acanthosis, vacuolar degeneration of keratinocytes, and/or perivascular lymphohistiocytic infiltrate.6,7,10 One potential distinguishing feature of Bazex syndrome, however, is specific psoriasiform involvement of the helix of the ear, as opposed to the entire ear, as would be more commonly expected in psoriasis.7 The tip of the nose also is involved in Bazex syndrome, which is an unusual location for psoriasis.
Extensive reviews of the literature reporting cases of Bazex syndrome demonstrate that most patients have been Caucasian, male, of French descent, and older than 40 years.6,7 SCCs have accounted for nearly 60% of tumors found in patients with this syndrome, and adenocarcinomas have accounted for less than 10% of malignancies. Furthermore, the majority of the neoplasms have involved the oropharynx and larynx.7 These neoplasms may be silent and only present with lymph node metastases. Less commonly, primary tumors may occur in the lungs and esophagus. Rare cases of neoplasms of the prostate, liver, stomach, thymus, uterus, vulva, and lymphoid tissues also have been reported.11 Numerous cases have been described in which the primary tumor could not be identified, and affected patients were diagnosed on the basis of metastases to cervical lymph nodes. In the vast majority of reported cases, the appearance of the characteristic psoriasiform lesions preceded the diagnosis of the associated malignancy.6,7 Finally, the skin lesions either markedly improved or completely resolved in the great preponderance of patients in whom the underlying malignancy was either treated with chemotherapy and/or radiation therapy or surgical excision.6,7,10-12 This was true of the patient presented in this report.
The pathogenesis of Bazex syndrome remains a mystery, though several authors have suggested an autoimmune etiology based on the common histologic finding of inflammatory infiltrates along the basal cell layer of affected skin regions.5,8,9 The immune reaction may be humoral or cellular; the proposed mechanism states that cross reactivity between skin and tumor antigens may produce the characteristic cutaneous changes observed, because antitumor antibodies cross reacting with the epidermis or basement membrane zone could elicit an immunologic response resulting in basal cell layer damage.13,14 Several authors also have proposed that the tumors may produce a host of growth factors that collectively lead to hyperkeratotic skin changes.14,15
Ideal treatment of Bazex syndrome is eradication of the underlying malignancy. Unresectable or treatment-resistant tumors, however, pose a significant challenge for the clinician. Numerous studies have been conducted demonstrating equivocal efficacies of various standard dermatological therapies in the treatment of skin lesions occurring in this syndrome. Unfortunately, in the vast majority of patients, such treatment options as topical tar, topical and systemic corticosteroids, UVB irradiation, antifungals, and antibiotics have proven to be of little use.6,7 Gill and colleagues9 have reported that oral psoralen–UVA phototherapy may offer some promise of effective treatment in these patients. However, larger studies are required to further investigate the therapeutic benefits of this treatment option. Although the management of treatment-resistant cutaneous lesions in Bazex syndrome may prove problematic, it is clear that the clinician must be astute in recognizing this disease process in its earlier stages to identify and effectively treat any underlying malignancy as expeditiously as possible.
Acknowledgment—The authors wish to thank Dr. Eric Ehrsam for his assistance with the preparation of this manuscript.
Psoriasiform dermatitis seen with Bazex syndrome may involve the nose and the helices of the ears in addition to the palms and soles. In most reported cases, the appearance of the characteristic psoriasiform lesions preceded the diagnosis of the associated underlying malignancy. Skin scrapings for potassium hydroxide and fungal cultures should be performed, and skin biopsy of keratotic plaques is recommended to exclude psoriasis.
Case Report
A 70-year-old man with no personal or family history of psoriasis or other skin diseases developed psoriasiform dermatitis of the fingers, toes, and helices of the ears over a period of 3 months. He reported a history of cigarette smoking (1 pack per day) with significant consumption of alcoholic beverages over a period of 30 years. Results from a review of systems revealed progressive hoarseness and dysphagia, with a recent history of a 15-lb weight loss. On physical examination, psoriasiform plaques were seen on the palms and soles, as well as on the helices of the ears (Figure 1) and the tip and dorsum of the nose. There was a yellowish discoloration and dystrophy of all the fingernails and toenails (Figure 2). Results from potassium hydroxide preparations from scrapings of the palms and soles were negative, and fungal culture did not grow any pathogenic fungi.
Six weeks later, the patient developed bilateral cervical lymphadenopathy. Otolaryngologic examination consisted of direct laryngoscopy; imaging studies including magnetic resonance imaging and computed tomography scans; and laryngeal biopsy, which revealed a stage IV squamous cell carcinoma (SCC) confined to the head and neck area. Although the patient did not return for follow-up, management of the laryngeal SCC with surgery and postoperative chemotherapy completely cleared his skin and nail lesions without adjunct dermatologic treatments.
PLEASE REFER TO THE PDF TO VIEW THE FIGURES
Comment
Bazex syndrome (paraneoplastic acrokeratosis) was first described as a clinical entity by Gougerot and Rupp1 more than 40 years prior to the coining of the disease's current widely used eponym of Bazex syndrome. In 1922, Gougerot and Grupper1 described a patient with hyperkeratotic lesions on the nose, ears, palms, and soles in conjunction with an SCC on the tongue. Years later, Bazex and colleagues2 described a patient with an SCC of the pyriform fossa and an associated psoriasiform dermatosis. Since that report, more than 110 cases of Bazex syndrome have been reported, most of which describe the condition as a cutaneous paraneoplastic syndrome characterized by psoriasiform lesions associated with an underlying malignancy of the upper aerodigestive tract (oropharynx, larynx, or esophagus), most often of the SCC subtype.3-7
Bazex syndrome can be classified among the cutaneous paraneoplastic disorders that also include acanthosis nigricans maligna, erythema gyratum, necrolytic migratory erythema, and hypertrichosis lanuginosa acquisita.7 The cutaneous manifestations of Bazex syndrome are paraneoplastic in that the developing skin changes coevolve with an underlying malignancy; these cutaneous hallmarks of the syndrome do not, however, represent metastatic extensions of this malignancy. On the contrary, they may actually serve as harbingers of future oncologic progression.
The cutaneous changes observed in Bazex syndrome have been classified into 3 stages.3 In the first stage, psoriasiform changes of the fingers, toes, auricular helix, and nose are noted. In addition, the earliest stage of the syndrome is characterized by nail changes, including horizontal and vertical ridging, subungual hyperkeratosis, yellow discoloration, and nail dystrophy. During this stage, the primary tumor is considered asymptomatic. The second stage is primarily typified by proximal extension of the cutaneous changes observed in the first stage to involve the dorsum of the hands and feet, as well as the malar regions of the face. Local symptoms secondary to growth of the primary tumor also may surface during this stage. The third stage in the course of the syndrome is defined by progressive centripetal extension of the cutaneous disease process to affect regions of the arms and legs (nails, hands, elbows, knees, and feet), scalp, and trunk.3-7 Other cutaneous changes that have been reported include hyperpigmentation, particularly in individuals with darker skin pigmentation,6 and development of bullous lesions.5,8,9
Based on the initial dermatologic manifestations of Bazex syndrome, it is not surprising that the condition is often misdiagnosed as psoriasis or chronic dermatitis. Indeed, histopathologic examination of skin lesions in the syndrome is nonspecific and may mimic psoriasis or other more common dermatoses, demonstrating hyperkeratosis, parakeratosis, acanthosis, vacuolar degeneration of keratinocytes, and/or perivascular lymphohistiocytic infiltrate.6,7,10 One potential distinguishing feature of Bazex syndrome, however, is specific psoriasiform involvement of the helix of the ear, as opposed to the entire ear, as would be more commonly expected in psoriasis.7 The tip of the nose also is involved in Bazex syndrome, which is an unusual location for psoriasis.
Extensive reviews of the literature reporting cases of Bazex syndrome demonstrate that most patients have been Caucasian, male, of French descent, and older than 40 years.6,7 SCCs have accounted for nearly 60% of tumors found in patients with this syndrome, and adenocarcinomas have accounted for less than 10% of malignancies. Furthermore, the majority of the neoplasms have involved the oropharynx and larynx.7 These neoplasms may be silent and only present with lymph node metastases. Less commonly, primary tumors may occur in the lungs and esophagus. Rare cases of neoplasms of the prostate, liver, stomach, thymus, uterus, vulva, and lymphoid tissues also have been reported.11 Numerous cases have been described in which the primary tumor could not be identified, and affected patients were diagnosed on the basis of metastases to cervical lymph nodes. In the vast majority of reported cases, the appearance of the characteristic psoriasiform lesions preceded the diagnosis of the associated malignancy.6,7 Finally, the skin lesions either markedly improved or completely resolved in the great preponderance of patients in whom the underlying malignancy was either treated with chemotherapy and/or radiation therapy or surgical excision.6,7,10-12 This was true of the patient presented in this report.
The pathogenesis of Bazex syndrome remains a mystery, though several authors have suggested an autoimmune etiology based on the common histologic finding of inflammatory infiltrates along the basal cell layer of affected skin regions.5,8,9 The immune reaction may be humoral or cellular; the proposed mechanism states that cross reactivity between skin and tumor antigens may produce the characteristic cutaneous changes observed, because antitumor antibodies cross reacting with the epidermis or basement membrane zone could elicit an immunologic response resulting in basal cell layer damage.13,14 Several authors also have proposed that the tumors may produce a host of growth factors that collectively lead to hyperkeratotic skin changes.14,15
Ideal treatment of Bazex syndrome is eradication of the underlying malignancy. Unresectable or treatment-resistant tumors, however, pose a significant challenge for the clinician. Numerous studies have been conducted demonstrating equivocal efficacies of various standard dermatological therapies in the treatment of skin lesions occurring in this syndrome. Unfortunately, in the vast majority of patients, such treatment options as topical tar, topical and systemic corticosteroids, UVB irradiation, antifungals, and antibiotics have proven to be of little use.6,7 Gill and colleagues9 have reported that oral psoralen–UVA phototherapy may offer some promise of effective treatment in these patients. However, larger studies are required to further investigate the therapeutic benefits of this treatment option. Although the management of treatment-resistant cutaneous lesions in Bazex syndrome may prove problematic, it is clear that the clinician must be astute in recognizing this disease process in its earlier stages to identify and effectively treat any underlying malignancy as expeditiously as possible.
Acknowledgment—The authors wish to thank Dr. Eric Ehrsam for his assistance with the preparation of this manuscript.
References
- Gougerot H, Grupper C. Dermatose érythémato-squameuse avec hyperkératose palmoplantaire, porectasies digitales et cancer de la langue latent. Paris Méd. 1922;43:234-237.
- Bazex A, Salvador R, Dupré A, et al. Syndrome paranéoplasique à type d'hyperkératose des extrémités. Guérison après le traitment del'épthélioma laryngé [letter]. Bull Soc Fr Dermatol Syphiligr. 1965;72:182.
- Bazex A, Griffiths A. Acrokeratosis paraneoplastica: a new cutaneous marker of malignancy. Br J Dermatol. 1980;103:801-805.
- O'Brien TJ. Bazex syndrome (acrokeratosis paraneoplastica). Australas J Dermatol. 1995;36:91-93.
- Bolognia JL, Brewer YP, Cooper DL. Bazex syndrome (acrokeratosis paraneoplastica): an analytic review. Medicine (Baltimore). 1991;70:269-280.
- Bolognia JL. Bazex syndrome: acrokeratosis paraneoplastica. Semin Dermatol. 1995;14:84-89.
- Sarkar B, Knecht R, Sarkar C, et al. Bazex syndrome (acrokeratosis paraneoplastica). Eur Arch Otorhinolaryngol. 1998;255:205-210.
- Handfield-Jones SE, Matthews CAN, Ellis JP, et al. Acrokeratosis paraneoplastica of Bazex. J R Soc Med. 1992;85:548-550.
- Gill D, Fergin P, Kelly J. Bullous lesions in Bazex syndrome and successful treatment with oral psoralen phototherapy. Australas J Dermatol. 2001;42:278-280.
- Wareing MJ, Vaughan-Jones SA, McGibbon DH. Acrokeratosis paraneoplastica: Bazex syndrome. J Laryngol Otol. 1996;110:899-900.
- Buxtorf K, Hübscher E, Panizzon R. Bazex syndrome. Dermatology. 2001;202:350-352.
- Hsu YS, Lien GS, Lai HH, et al. Acrokeratosis paraneoplastica (Bazex syndrome) with adenocarcinoma of the colon: report of a case and review of the literature. J Gastroenterol. 2000;35:460-464.
- Pecora AL, Landsman L, Imgrund SP, et al. Acrokeratosis paraneoplastica: report of a case and review of the literature. Arch Dermatol. 1983;119:820-826.
- Jean LB, Yvelise PB, Dennis LC. Bazex syndrome (acrokeratosis paraneoplastica): an analytic review. Medicine. 1991;70:269-280.
- Politi Y, Ophir J, Brenner S. Cutaneous paraneoplastic syndromes. Acta Derm Venereol (Stockh). 1993;73:161-170.
References
- Gougerot H, Grupper C. Dermatose érythémato-squameuse avec hyperkératose palmoplantaire, porectasies digitales et cancer de la langue latent. Paris Méd. 1922;43:234-237.
- Bazex A, Salvador R, Dupré A, et al. Syndrome paranéoplasique à type d'hyperkératose des extrémités. Guérison après le traitment del'épthélioma laryngé [letter]. Bull Soc Fr Dermatol Syphiligr. 1965;72:182.
- Bazex A, Griffiths A. Acrokeratosis paraneoplastica: a new cutaneous marker of malignancy. Br J Dermatol. 1980;103:801-805.
- O'Brien TJ. Bazex syndrome (acrokeratosis paraneoplastica). Australas J Dermatol. 1995;36:91-93.
- Bolognia JL, Brewer YP, Cooper DL. Bazex syndrome (acrokeratosis paraneoplastica): an analytic review. Medicine (Baltimore). 1991;70:269-280.
- Bolognia JL. Bazex syndrome: acrokeratosis paraneoplastica. Semin Dermatol. 1995;14:84-89.
- Sarkar B, Knecht R, Sarkar C, et al. Bazex syndrome (acrokeratosis paraneoplastica). Eur Arch Otorhinolaryngol. 1998;255:205-210.
- Handfield-Jones SE, Matthews CAN, Ellis JP, et al. Acrokeratosis paraneoplastica of Bazex. J R Soc Med. 1992;85:548-550.
- Gill D, Fergin P, Kelly J. Bullous lesions in Bazex syndrome and successful treatment with oral psoralen phototherapy. Australas J Dermatol. 2001;42:278-280.
- Wareing MJ, Vaughan-Jones SA, McGibbon DH. Acrokeratosis paraneoplastica: Bazex syndrome. J Laryngol Otol. 1996;110:899-900.
- Buxtorf K, Hübscher E, Panizzon R. Bazex syndrome. Dermatology. 2001;202:350-352.
- Hsu YS, Lien GS, Lai HH, et al. Acrokeratosis paraneoplastica (Bazex syndrome) with adenocarcinoma of the colon: report of a case and review of the literature. J Gastroenterol. 2000;35:460-464.
- Pecora AL, Landsman L, Imgrund SP, et al. Acrokeratosis paraneoplastica: report of a case and review of the literature. Arch Dermatol. 1983;119:820-826.
- Jean LB, Yvelise PB, Dennis LC. Bazex syndrome (acrokeratosis paraneoplastica): an analytic review. Medicine. 1991;70:269-280.
- Politi Y, Ophir J, Brenner S. Cutaneous paraneoplastic syndromes. Acta Derm Venereol (Stockh). 1993;73:161-170.
Back from the Peace Corps— with skin lesions from South America
A 25-year-old woman visited her family doctor for treatment of skin lesions on her left leg, which had developed over the previous 8 weeks. The patient had just returned to the US after working with the Peace Corps in the Amazon rain forest for 3 months. During that time, she slept outside in the jungle without any skin protection—no mosquito netting, no insect repellent, no protective clothing on her extremities. She reported no recent history of trauma, burns, or chemical exposure to the leg, and she had been healthy prior to this skin condition. She reported that she was taking no medications, and was not using alcohol or tobacco.
The lesions started with the development of small pustules on her left leg and ankle. Over the next 3 weeks, these grew into larger, ulcerated lesions. The ulcers persisted for 5 more weeks despite multiple attempts at local topical treatments in Ecuador. No pain or itching were associated with the lesions.
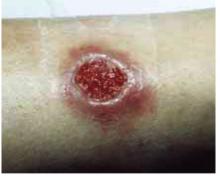
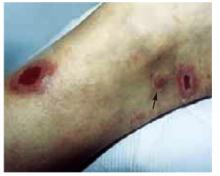
The physical examination revealed a generally healthy woman, remarkable only for the 2 skin ulcers on her left lower leg and ankle (Figure 1 and Figure 2). The patient was afebrile, and had no lymphadenopathy or hepatosplenomegaly.
FIGURE 1
Cutaneous ulceration with indurated border.
Two active ulcers with small healed satellite lesion near the medial malleolus (see arrow).
How would you treat this condition?
Differential diagnosis of tropical skin ulcers
The woman’s family physician used the Internet to determine that the differential diagnosis of tropical skin ulcers includes localized cutaneous leishmaniasis, foreign body reaction, impetigo, pyoderma gangrenosum, bacterial or deep fungal infection, atypical mycobacterial infection, sarcoidosis, squamous and basal cell carcinoma, and superinfected insect bites. The combination of the patient’s history of potential exposure, clinical presentation, and epidemiology of leishmaniasis within the region in which she was working supports a presumptive diagnosis of localized cutaneous leishmaniasis.
Leishmaniasis
Leishmaniasis is comprised of several diverse diseases with varying degrees of severity, ranging from spontaneously healing skin ulcers to a disfiguring mucocutaneous disease, and even to fatal visceral illness. Endemic worldwide except Australia, Oceania, and Antarctica, leishmaniasis is found in the tropics and subtropics.
Approximately 2 million new cases occur each year, with markedly increasing incidence in several parts of the world resulting from international travel, immigration, military deployment, and human immunodeficiency virus coinfection.1
An estimated 10% of the world’s population is at risk for contracting leishmaniasis. Most cases reported in the US are in travelers to countries where the disease is endemic, although sporadic cases have been reported in Texas and Oklahoma.2
Characteristics
Leishmaniasis is a vector-borne disease, caused by obligate intracellular protozoa of the genus Leishmaniaand transmitted by the bite of infected female sandflies. The disease may take several forms, including cutaneous, mucocutaneous, and visceral leishmaniasis, depending on the particular Leishmaniaspecies and host response.
Localized cutaneous leishmaniasis is characterized clinically by the appearance of skin lesions at the site of the sandfly bite, which typically present as inflammatory papules before progressing to nodules and ulcers. Local lymphadenopathy may occur. Sores may be painless or painful.
Both cutaneous and mucocutaneous forms generally yield normal laboratory values, although complete blood counts may show mild anemia, leukopenia, or thrombocytopenia.3 US travelers may consult numerous physicians before leishmaniasis is diagnosed. The median time from recognition of skin lesions to drug treatment is 112 days.4
Lab results
The patient’s complete blood count with differential, electrolytes, creatinine, liver function tests, and electrocardiogram were within normal limits. An infectious disease consultant suggested direct microscopic visualization of skin biopsies, dermal scrapings, and needle aspirates using Giemsa and Leishman stains, but these failed to reveal any parasitic organisms. It was thought these results were most likely false negatives.
In the best situation, the diagnosis of leishmaniasis is confirmed by isolating, visualizing, and culturing the parasite from infected tissue. Dermal scrapings or needle aspirates may reveal Leishmania amastigotes using a Giemsa stain. Early in the course of localized disease, Leishmania organisms may be numerous and found readily within the cytoplasm of macrophages. However, biopsy specimens from old lesions (>6 months), partially or incompletely treated, are frequently negative.
In vitro culture of the parasite from tissue samples using Nicolle-Noy-McNeal medium is often obtained to aid in diagnosis and to identify Leishmania species. With successful culture, the parasite can be sent to the Centers for Disease Control for speciation. New, rapid tests for leishmaniasis are being developed.
Treatment
Although 90% of skin lesions caused by cutaneous leishmaniasis heal spontaneously to form atrophic scars, the infectious disease consultant recommended treatment to prevent development of disfiguring mucocutaneous disease (level of evidence [LOE]=5)—otherwise, immunity may not be complete and skin ulcers can recur.
Experts believe it is important to treat cutaneous leishmaniasis if a species known or suspected of being capable of converting to the mucocutaneous disease form—eg, New World L braziliensis—is present. These lesions may appear months or even years after the initial exposure, and can be refractory to further treatment. However, surgical excision usually is not recommended because of the risk of relapse and further cosmetic disfigurement.3
Yearly follow-up to evaluate for recurrence or evolution of mucocutaneous leishmaniasis is crucial; early treatment of this form of the disease is more efficacious and can yield more favorable outcomes by limiting potential facial involvement (LOE=5).
Meglumine antimonate
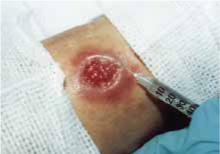
The patient’s initial treatment included gluteal injections of meglumine antimonate at a dosage of 20 mg antimony/kg/d intramuscularly for a total course of 21 days (LOE=5). In addition, one tenth of each dose was injected directly into skin lesions under the peripheral margins, as shown in Figure 3 (LOE=5).
Side effects. The patient’s side effects from the meglumine antimonate therapy included insomnia, lightheadedness, increased fatigue, pain at the gluteal injection sites, bone aches, painful splenomegaly, and left leg myoclonic spasms. These signs and symptoms resolved shortly after completion of the course of therapy with no apparent sequelae. Monitoring cardiac, renal, and hepatic function before and during treatment is important, as meglumine antimonate has significant potential to cause renal and hepatic toxicity.
Outcome
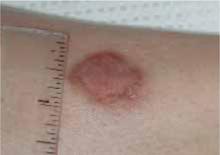
The patient’s skin lesions began to show signs of healing after a few days of treatment. Healed lesions can be seen in Figure 4 Of course, we don’t know that these lesions would not have healed without treatment.
Follow-up diagnostic tests including electrocardiograms, liver function tests, and renal function showed normal values at the conclusion of the 21-day course of therapy.
Fluconazole studied
Since this patient was treated, a Saudi Arabian study was published on use of oral fluconazole 200 mg/d for 6 weeks in patients with cutaneous leishmaniasis (L major).5 At the 3-month followup, healing of lesions was complete for 79% of patients in the fluconazole group and 34% patients in the placebo group (LOE=2b).
The toxicity of this treatment is much lower than the antimonials; we should be looking for further evidence of its benefits in other countries with other species of Leishmania.
FIGURE 3
Perilesional infiltration of leishmaniasis lesion using meglumine antimonate.
FIGURE 4
Healed cutaneous leishmaniasis lesions on left leg.
Preventing sandfly bites
Leishmaniasis is preventable by avoiding contact with the vector—the sandfly—while living or traveling in endemic areas. Sandflies are most active from dusk to dawn. Though relatively poor fliers, they are small enough to fit through standard mosquito netting and make no audible noise. Effective prevention may be achieved by avoiding nighttime outdoor activities, using topical insecticides (eg, N, N diethyl-m-toluamide [DEET]) on exposed skin surfaces, using insecticide-impregnated clothing (permethrin stays in material for many washings), using fine-mesh mosquito netting, and sleeping with a fan.3
In this time of the globalization of infectious diseases, family doctors in the US should be aware of the tropical diseases that our patients may bring to their offices.
1. Roberts LJ, Handman E, Foote SJ. Science, medicine and the future: leishmaniasis. BMJ 2000;321:801-804.
2. Melby PC, Kreutzer RD, McMahon-Pratt D, Gam AA, Neva FA. Cutaneous leishmaniasis: review of 59 cases seen at the National Institutes of Health. Clin Infect Dis 1992;15:924-937.
3. Kenner JR, Kaugh YC. Leishmaniasis. eMedicine J. 2001 2(11). Available at http://www.emedicine.com/derm/topic219.htm Accessed on May 29, 2003.
4. Herwaldt BL, Stokes SL, Juranek DD. American cutaneous leishmaniasis in U.S. travelers. Ann Intern Med 1993;118:779-784.
5. Alrajhi AA, Ibrahim EA, De Vol EB, Khairat M, Faris RM, Maguire JH. Fluconazole for the treatment of cutaneous leishmaniasis caused by Leishmania major. N Engl J Med 2002;346:891-895.
A 25-year-old woman visited her family doctor for treatment of skin lesions on her left leg, which had developed over the previous 8 weeks. The patient had just returned to the US after working with the Peace Corps in the Amazon rain forest for 3 months. During that time, she slept outside in the jungle without any skin protection—no mosquito netting, no insect repellent, no protective clothing on her extremities. She reported no recent history of trauma, burns, or chemical exposure to the leg, and she had been healthy prior to this skin condition. She reported that she was taking no medications, and was not using alcohol or tobacco.
The lesions started with the development of small pustules on her left leg and ankle. Over the next 3 weeks, these grew into larger, ulcerated lesions. The ulcers persisted for 5 more weeks despite multiple attempts at local topical treatments in Ecuador. No pain or itching were associated with the lesions.
The physical examination revealed a generally healthy woman, remarkable only for the 2 skin ulcers on her left lower leg and ankle (Figure 1 and Figure 2). The patient was afebrile, and had no lymphadenopathy or hepatosplenomegaly.
FIGURE 1
Cutaneous ulceration with indurated border.
Two active ulcers with small healed satellite lesion near the medial malleolus (see arrow).
How would you treat this condition?
Differential diagnosis of tropical skin ulcers
The woman’s family physician used the Internet to determine that the differential diagnosis of tropical skin ulcers includes localized cutaneous leishmaniasis, foreign body reaction, impetigo, pyoderma gangrenosum, bacterial or deep fungal infection, atypical mycobacterial infection, sarcoidosis, squamous and basal cell carcinoma, and superinfected insect bites. The combination of the patient’s history of potential exposure, clinical presentation, and epidemiology of leishmaniasis within the region in which she was working supports a presumptive diagnosis of localized cutaneous leishmaniasis.
Leishmaniasis
Leishmaniasis is comprised of several diverse diseases with varying degrees of severity, ranging from spontaneously healing skin ulcers to a disfiguring mucocutaneous disease, and even to fatal visceral illness. Endemic worldwide except Australia, Oceania, and Antarctica, leishmaniasis is found in the tropics and subtropics.
Approximately 2 million new cases occur each year, with markedly increasing incidence in several parts of the world resulting from international travel, immigration, military deployment, and human immunodeficiency virus coinfection.1
An estimated 10% of the world’s population is at risk for contracting leishmaniasis. Most cases reported in the US are in travelers to countries where the disease is endemic, although sporadic cases have been reported in Texas and Oklahoma.2
Characteristics
Leishmaniasis is a vector-borne disease, caused by obligate intracellular protozoa of the genus Leishmaniaand transmitted by the bite of infected female sandflies. The disease may take several forms, including cutaneous, mucocutaneous, and visceral leishmaniasis, depending on the particular Leishmaniaspecies and host response.
Localized cutaneous leishmaniasis is characterized clinically by the appearance of skin lesions at the site of the sandfly bite, which typically present as inflammatory papules before progressing to nodules and ulcers. Local lymphadenopathy may occur. Sores may be painless or painful.
Both cutaneous and mucocutaneous forms generally yield normal laboratory values, although complete blood counts may show mild anemia, leukopenia, or thrombocytopenia.3 US travelers may consult numerous physicians before leishmaniasis is diagnosed. The median time from recognition of skin lesions to drug treatment is 112 days.4
Lab results
The patient’s complete blood count with differential, electrolytes, creatinine, liver function tests, and electrocardiogram were within normal limits. An infectious disease consultant suggested direct microscopic visualization of skin biopsies, dermal scrapings, and needle aspirates using Giemsa and Leishman stains, but these failed to reveal any parasitic organisms. It was thought these results were most likely false negatives.
In the best situation, the diagnosis of leishmaniasis is confirmed by isolating, visualizing, and culturing the parasite from infected tissue. Dermal scrapings or needle aspirates may reveal Leishmania amastigotes using a Giemsa stain. Early in the course of localized disease, Leishmania organisms may be numerous and found readily within the cytoplasm of macrophages. However, biopsy specimens from old lesions (>6 months), partially or incompletely treated, are frequently negative.
In vitro culture of the parasite from tissue samples using Nicolle-Noy-McNeal medium is often obtained to aid in diagnosis and to identify Leishmania species. With successful culture, the parasite can be sent to the Centers for Disease Control for speciation. New, rapid tests for leishmaniasis are being developed.
Treatment
Although 90% of skin lesions caused by cutaneous leishmaniasis heal spontaneously to form atrophic scars, the infectious disease consultant recommended treatment to prevent development of disfiguring mucocutaneous disease (level of evidence [LOE]=5)—otherwise, immunity may not be complete and skin ulcers can recur.
Experts believe it is important to treat cutaneous leishmaniasis if a species known or suspected of being capable of converting to the mucocutaneous disease form—eg, New World L braziliensis—is present. These lesions may appear months or even years after the initial exposure, and can be refractory to further treatment. However, surgical excision usually is not recommended because of the risk of relapse and further cosmetic disfigurement.3
Yearly follow-up to evaluate for recurrence or evolution of mucocutaneous leishmaniasis is crucial; early treatment of this form of the disease is more efficacious and can yield more favorable outcomes by limiting potential facial involvement (LOE=5).
Meglumine antimonate
The patient’s initial treatment included gluteal injections of meglumine antimonate at a dosage of 20 mg antimony/kg/d intramuscularly for a total course of 21 days (LOE=5). In addition, one tenth of each dose was injected directly into skin lesions under the peripheral margins, as shown in Figure 3 (LOE=5).
Side effects. The patient’s side effects from the meglumine antimonate therapy included insomnia, lightheadedness, increased fatigue, pain at the gluteal injection sites, bone aches, painful splenomegaly, and left leg myoclonic spasms. These signs and symptoms resolved shortly after completion of the course of therapy with no apparent sequelae. Monitoring cardiac, renal, and hepatic function before and during treatment is important, as meglumine antimonate has significant potential to cause renal and hepatic toxicity.
Outcome
The patient’s skin lesions began to show signs of healing after a few days of treatment. Healed lesions can be seen in Figure 4 Of course, we don’t know that these lesions would not have healed without treatment.
Follow-up diagnostic tests including electrocardiograms, liver function tests, and renal function showed normal values at the conclusion of the 21-day course of therapy.
Fluconazole studied
Since this patient was treated, a Saudi Arabian study was published on use of oral fluconazole 200 mg/d for 6 weeks in patients with cutaneous leishmaniasis (L major).5 At the 3-month followup, healing of lesions was complete for 79% of patients in the fluconazole group and 34% patients in the placebo group (LOE=2b).
The toxicity of this treatment is much lower than the antimonials; we should be looking for further evidence of its benefits in other countries with other species of Leishmania.
FIGURE 3
Perilesional infiltration of leishmaniasis lesion using meglumine antimonate.
FIGURE 4
Healed cutaneous leishmaniasis lesions on left leg.
Preventing sandfly bites
Leishmaniasis is preventable by avoiding contact with the vector—the sandfly—while living or traveling in endemic areas. Sandflies are most active from dusk to dawn. Though relatively poor fliers, they are small enough to fit through standard mosquito netting and make no audible noise. Effective prevention may be achieved by avoiding nighttime outdoor activities, using topical insecticides (eg, N, N diethyl-m-toluamide [DEET]) on exposed skin surfaces, using insecticide-impregnated clothing (permethrin stays in material for many washings), using fine-mesh mosquito netting, and sleeping with a fan.3
In this time of the globalization of infectious diseases, family doctors in the US should be aware of the tropical diseases that our patients may bring to their offices.
A 25-year-old woman visited her family doctor for treatment of skin lesions on her left leg, which had developed over the previous 8 weeks. The patient had just returned to the US after working with the Peace Corps in the Amazon rain forest for 3 months. During that time, she slept outside in the jungle without any skin protection—no mosquito netting, no insect repellent, no protective clothing on her extremities. She reported no recent history of trauma, burns, or chemical exposure to the leg, and she had been healthy prior to this skin condition. She reported that she was taking no medications, and was not using alcohol or tobacco.
The lesions started with the development of small pustules on her left leg and ankle. Over the next 3 weeks, these grew into larger, ulcerated lesions. The ulcers persisted for 5 more weeks despite multiple attempts at local topical treatments in Ecuador. No pain or itching were associated with the lesions.
The physical examination revealed a generally healthy woman, remarkable only for the 2 skin ulcers on her left lower leg and ankle (Figure 1 and Figure 2). The patient was afebrile, and had no lymphadenopathy or hepatosplenomegaly.
FIGURE 1
Cutaneous ulceration with indurated border.
Two active ulcers with small healed satellite lesion near the medial malleolus (see arrow).
How would you treat this condition?
Differential diagnosis of tropical skin ulcers
The woman’s family physician used the Internet to determine that the differential diagnosis of tropical skin ulcers includes localized cutaneous leishmaniasis, foreign body reaction, impetigo, pyoderma gangrenosum, bacterial or deep fungal infection, atypical mycobacterial infection, sarcoidosis, squamous and basal cell carcinoma, and superinfected insect bites. The combination of the patient’s history of potential exposure, clinical presentation, and epidemiology of leishmaniasis within the region in which she was working supports a presumptive diagnosis of localized cutaneous leishmaniasis.
Leishmaniasis
Leishmaniasis is comprised of several diverse diseases with varying degrees of severity, ranging from spontaneously healing skin ulcers to a disfiguring mucocutaneous disease, and even to fatal visceral illness. Endemic worldwide except Australia, Oceania, and Antarctica, leishmaniasis is found in the tropics and subtropics.
Approximately 2 million new cases occur each year, with markedly increasing incidence in several parts of the world resulting from international travel, immigration, military deployment, and human immunodeficiency virus coinfection.1
An estimated 10% of the world’s population is at risk for contracting leishmaniasis. Most cases reported in the US are in travelers to countries where the disease is endemic, although sporadic cases have been reported in Texas and Oklahoma.2
Characteristics
Leishmaniasis is a vector-borne disease, caused by obligate intracellular protozoa of the genus Leishmaniaand transmitted by the bite of infected female sandflies. The disease may take several forms, including cutaneous, mucocutaneous, and visceral leishmaniasis, depending on the particular Leishmaniaspecies and host response.
Localized cutaneous leishmaniasis is characterized clinically by the appearance of skin lesions at the site of the sandfly bite, which typically present as inflammatory papules before progressing to nodules and ulcers. Local lymphadenopathy may occur. Sores may be painless or painful.
Both cutaneous and mucocutaneous forms generally yield normal laboratory values, although complete blood counts may show mild anemia, leukopenia, or thrombocytopenia.3 US travelers may consult numerous physicians before leishmaniasis is diagnosed. The median time from recognition of skin lesions to drug treatment is 112 days.4
Lab results
The patient’s complete blood count with differential, electrolytes, creatinine, liver function tests, and electrocardiogram were within normal limits. An infectious disease consultant suggested direct microscopic visualization of skin biopsies, dermal scrapings, and needle aspirates using Giemsa and Leishman stains, but these failed to reveal any parasitic organisms. It was thought these results were most likely false negatives.
In the best situation, the diagnosis of leishmaniasis is confirmed by isolating, visualizing, and culturing the parasite from infected tissue. Dermal scrapings or needle aspirates may reveal Leishmania amastigotes using a Giemsa stain. Early in the course of localized disease, Leishmania organisms may be numerous and found readily within the cytoplasm of macrophages. However, biopsy specimens from old lesions (>6 months), partially or incompletely treated, are frequently negative.
In vitro culture of the parasite from tissue samples using Nicolle-Noy-McNeal medium is often obtained to aid in diagnosis and to identify Leishmania species. With successful culture, the parasite can be sent to the Centers for Disease Control for speciation. New, rapid tests for leishmaniasis are being developed.
Treatment
Although 90% of skin lesions caused by cutaneous leishmaniasis heal spontaneously to form atrophic scars, the infectious disease consultant recommended treatment to prevent development of disfiguring mucocutaneous disease (level of evidence [LOE]=5)—otherwise, immunity may not be complete and skin ulcers can recur.
Experts believe it is important to treat cutaneous leishmaniasis if a species known or suspected of being capable of converting to the mucocutaneous disease form—eg, New World L braziliensis—is present. These lesions may appear months or even years after the initial exposure, and can be refractory to further treatment. However, surgical excision usually is not recommended because of the risk of relapse and further cosmetic disfigurement.3
Yearly follow-up to evaluate for recurrence or evolution of mucocutaneous leishmaniasis is crucial; early treatment of this form of the disease is more efficacious and can yield more favorable outcomes by limiting potential facial involvement (LOE=5).
Meglumine antimonate
The patient’s initial treatment included gluteal injections of meglumine antimonate at a dosage of 20 mg antimony/kg/d intramuscularly for a total course of 21 days (LOE=5). In addition, one tenth of each dose was injected directly into skin lesions under the peripheral margins, as shown in Figure 3 (LOE=5).
Side effects. The patient’s side effects from the meglumine antimonate therapy included insomnia, lightheadedness, increased fatigue, pain at the gluteal injection sites, bone aches, painful splenomegaly, and left leg myoclonic spasms. These signs and symptoms resolved shortly after completion of the course of therapy with no apparent sequelae. Monitoring cardiac, renal, and hepatic function before and during treatment is important, as meglumine antimonate has significant potential to cause renal and hepatic toxicity.
Outcome
The patient’s skin lesions began to show signs of healing after a few days of treatment. Healed lesions can be seen in Figure 4 Of course, we don’t know that these lesions would not have healed without treatment.
Follow-up diagnostic tests including electrocardiograms, liver function tests, and renal function showed normal values at the conclusion of the 21-day course of therapy.
Fluconazole studied
Since this patient was treated, a Saudi Arabian study was published on use of oral fluconazole 200 mg/d for 6 weeks in patients with cutaneous leishmaniasis (L major).5 At the 3-month followup, healing of lesions was complete for 79% of patients in the fluconazole group and 34% patients in the placebo group (LOE=2b).
The toxicity of this treatment is much lower than the antimonials; we should be looking for further evidence of its benefits in other countries with other species of Leishmania.
FIGURE 3
Perilesional infiltration of leishmaniasis lesion using meglumine antimonate.
FIGURE 4
Healed cutaneous leishmaniasis lesions on left leg.
Preventing sandfly bites
Leishmaniasis is preventable by avoiding contact with the vector—the sandfly—while living or traveling in endemic areas. Sandflies are most active from dusk to dawn. Though relatively poor fliers, they are small enough to fit through standard mosquito netting and make no audible noise. Effective prevention may be achieved by avoiding nighttime outdoor activities, using topical insecticides (eg, N, N diethyl-m-toluamide [DEET]) on exposed skin surfaces, using insecticide-impregnated clothing (permethrin stays in material for many washings), using fine-mesh mosquito netting, and sleeping with a fan.3
In this time of the globalization of infectious diseases, family doctors in the US should be aware of the tropical diseases that our patients may bring to their offices.
1. Roberts LJ, Handman E, Foote SJ. Science, medicine and the future: leishmaniasis. BMJ 2000;321:801-804.
2. Melby PC, Kreutzer RD, McMahon-Pratt D, Gam AA, Neva FA. Cutaneous leishmaniasis: review of 59 cases seen at the National Institutes of Health. Clin Infect Dis 1992;15:924-937.
3. Kenner JR, Kaugh YC. Leishmaniasis. eMedicine J. 2001 2(11). Available at http://www.emedicine.com/derm/topic219.htm Accessed on May 29, 2003.
4. Herwaldt BL, Stokes SL, Juranek DD. American cutaneous leishmaniasis in U.S. travelers. Ann Intern Med 1993;118:779-784.
5. Alrajhi AA, Ibrahim EA, De Vol EB, Khairat M, Faris RM, Maguire JH. Fluconazole for the treatment of cutaneous leishmaniasis caused by Leishmania major. N Engl J Med 2002;346:891-895.
1. Roberts LJ, Handman E, Foote SJ. Science, medicine and the future: leishmaniasis. BMJ 2000;321:801-804.
2. Melby PC, Kreutzer RD, McMahon-Pratt D, Gam AA, Neva FA. Cutaneous leishmaniasis: review of 59 cases seen at the National Institutes of Health. Clin Infect Dis 1992;15:924-937.
3. Kenner JR, Kaugh YC. Leishmaniasis. eMedicine J. 2001 2(11). Available at http://www.emedicine.com/derm/topic219.htm Accessed on May 29, 2003.
4. Herwaldt BL, Stokes SL, Juranek DD. American cutaneous leishmaniasis in U.S. travelers. Ann Intern Med 1993;118:779-784.
5. Alrajhi AA, Ibrahim EA, De Vol EB, Khairat M, Faris RM, Maguire JH. Fluconazole for the treatment of cutaneous leishmaniasis caused by Leishmania major. N Engl J Med 2002;346:891-895.
Can a bedside blood test predict death or myocardial infarction (MI) in patients with chest pain?
BACKGROUND: Emergency departments triage more than 5 million patients with chest pain each year. Cardiac blood tests are used to identify patients at higher risk for MI or death. The bedside instrument in this study is a new approach to the immediate risk stratification of patients with symptoms suggestive of myocardial ischemia.
POPULATION STUDIED: The investigators enrolled 1005 patients older than 18 years with possible myocardial ischemia who presented to the emergency room of 6 US hospitals. Patients were excluded if electrocardiography showed ST-segment elevation or left bundle branch block. The average patient age was 51years; 51% were women; 14% had a previous MI; 21% had diabetes; 53% had hypertension; and 38% were current cigarette smokers. The researchers were able to provide complete results for 95% of the patients.
STUDY DESIGN AND VALIDITY: This was a prospective study looking at the prognostic value of a bedside instrument measuring cardiac enzymes at the point of care. Blood samples were obtained from patients at baseline and at 3 and 6 hours. If the patient was hospitalized, samples were obtained at 9, 12, and 16 to 24 hours. These were analyzed by the Dade-Behring Stratus CS STAT near-patient instrument, which assays myoglobin; creatine kinase, myocardial bound (CK-MB); and troponin I (cTnI) from a blood sample in 15 to 20 minutes. Two multimarker strategies (MMS) were defined: MMS-1 included all 3 markers, and MMS-2 included only CK-MB and cTnI. A strategy was considered positive if any of the markers was positive. The CS STAT assay was compared with the CK-MB result of the local hospital laboratory. All patients underwent both local laboratory testing and bedside testing, but treating physicians used only local CK-MB results in making management decisions. This study was well designed for comparing the prognostic value of the CS STAT values with conventional CK-MB values. However, a more useful outcome would be the utility of this bedside instrument in the diagnosis of MI. Also, whether such an instrument would lead to clinically significant changes in prognosis outside of special chest pain units is unclear.
OUTCOMES MEASURED: The rate of MI or death at 30 days was determined for patients with either a positive or negative MMS or CK-MB test result. Additional assessments included time from arrival to a positive test result and the relation of MMS status to the rates of MI, death, and revascularization at 30 days.
RESULTS: Testing at baseline (on initial evaluation in the emergency department) predicted death or MI within 30 days in 19% of patients with a positive MMS-1, 22% with a positive MMS-2, and 13% with a positive CK-MB result. Conversely, only 3% with a negative MMS-1 or MMS-2 and 6% with a negative CK-MB result died or had a MI at 30 days. With serial testing 55% of persons with an abnormal CK-MB result had a MI at 30 days, while serial MMS testing did not discriminate any better than the single test. The bedside 3-marker strategy was superior in predicting 30-day mortality; all 3 patients who died had a positive MMS-1 at baseline, while only 1 of 3 had an abnormal CK-MB on serial testing. An abnormal CK-MB result predicted revascularization within 30-days better than bedside testing using both single and serial measurements. After emergency department arrival, positive test results were obtained more quickly using the bedside instrument (MMS-1=2.5 hours, MMS-2=2.8 hours, LL=3.4 hours; P=.0001).
A single bedside multimarker test (myoglobin, troponpin I, and CK-MB) is more likely than a single CK-MB result to determine risk for MI or death within 30 days after an episode of chest pain. Serial CK-MB testing outperformed both single and serial MMS testing in predicting 30-day MI, need for revascularization, and combined outcome of MI or death. This single bedside test may be a useful adjunct to standard tests in identifying patients at higher risk for MI or death. However, it should not replace serial CK-MB testing in the diagnosis of MI.
BACKGROUND: Emergency departments triage more than 5 million patients with chest pain each year. Cardiac blood tests are used to identify patients at higher risk for MI or death. The bedside instrument in this study is a new approach to the immediate risk stratification of patients with symptoms suggestive of myocardial ischemia.
POPULATION STUDIED: The investigators enrolled 1005 patients older than 18 years with possible myocardial ischemia who presented to the emergency room of 6 US hospitals. Patients were excluded if electrocardiography showed ST-segment elevation or left bundle branch block. The average patient age was 51years; 51% were women; 14% had a previous MI; 21% had diabetes; 53% had hypertension; and 38% were current cigarette smokers. The researchers were able to provide complete results for 95% of the patients.
STUDY DESIGN AND VALIDITY: This was a prospective study looking at the prognostic value of a bedside instrument measuring cardiac enzymes at the point of care. Blood samples were obtained from patients at baseline and at 3 and 6 hours. If the patient was hospitalized, samples were obtained at 9, 12, and 16 to 24 hours. These were analyzed by the Dade-Behring Stratus CS STAT near-patient instrument, which assays myoglobin; creatine kinase, myocardial bound (CK-MB); and troponin I (cTnI) from a blood sample in 15 to 20 minutes. Two multimarker strategies (MMS) were defined: MMS-1 included all 3 markers, and MMS-2 included only CK-MB and cTnI. A strategy was considered positive if any of the markers was positive. The CS STAT assay was compared with the CK-MB result of the local hospital laboratory. All patients underwent both local laboratory testing and bedside testing, but treating physicians used only local CK-MB results in making management decisions. This study was well designed for comparing the prognostic value of the CS STAT values with conventional CK-MB values. However, a more useful outcome would be the utility of this bedside instrument in the diagnosis of MI. Also, whether such an instrument would lead to clinically significant changes in prognosis outside of special chest pain units is unclear.
OUTCOMES MEASURED: The rate of MI or death at 30 days was determined for patients with either a positive or negative MMS or CK-MB test result. Additional assessments included time from arrival to a positive test result and the relation of MMS status to the rates of MI, death, and revascularization at 30 days.
RESULTS: Testing at baseline (on initial evaluation in the emergency department) predicted death or MI within 30 days in 19% of patients with a positive MMS-1, 22% with a positive MMS-2, and 13% with a positive CK-MB result. Conversely, only 3% with a negative MMS-1 or MMS-2 and 6% with a negative CK-MB result died or had a MI at 30 days. With serial testing 55% of persons with an abnormal CK-MB result had a MI at 30 days, while serial MMS testing did not discriminate any better than the single test. The bedside 3-marker strategy was superior in predicting 30-day mortality; all 3 patients who died had a positive MMS-1 at baseline, while only 1 of 3 had an abnormal CK-MB on serial testing. An abnormal CK-MB result predicted revascularization within 30-days better than bedside testing using both single and serial measurements. After emergency department arrival, positive test results were obtained more quickly using the bedside instrument (MMS-1=2.5 hours, MMS-2=2.8 hours, LL=3.4 hours; P=.0001).
A single bedside multimarker test (myoglobin, troponpin I, and CK-MB) is more likely than a single CK-MB result to determine risk for MI or death within 30 days after an episode of chest pain. Serial CK-MB testing outperformed both single and serial MMS testing in predicting 30-day MI, need for revascularization, and combined outcome of MI or death. This single bedside test may be a useful adjunct to standard tests in identifying patients at higher risk for MI or death. However, it should not replace serial CK-MB testing in the diagnosis of MI.
BACKGROUND: Emergency departments triage more than 5 million patients with chest pain each year. Cardiac blood tests are used to identify patients at higher risk for MI or death. The bedside instrument in this study is a new approach to the immediate risk stratification of patients with symptoms suggestive of myocardial ischemia.
POPULATION STUDIED: The investigators enrolled 1005 patients older than 18 years with possible myocardial ischemia who presented to the emergency room of 6 US hospitals. Patients were excluded if electrocardiography showed ST-segment elevation or left bundle branch block. The average patient age was 51years; 51% were women; 14% had a previous MI; 21% had diabetes; 53% had hypertension; and 38% were current cigarette smokers. The researchers were able to provide complete results for 95% of the patients.
STUDY DESIGN AND VALIDITY: This was a prospective study looking at the prognostic value of a bedside instrument measuring cardiac enzymes at the point of care. Blood samples were obtained from patients at baseline and at 3 and 6 hours. If the patient was hospitalized, samples were obtained at 9, 12, and 16 to 24 hours. These were analyzed by the Dade-Behring Stratus CS STAT near-patient instrument, which assays myoglobin; creatine kinase, myocardial bound (CK-MB); and troponin I (cTnI) from a blood sample in 15 to 20 minutes. Two multimarker strategies (MMS) were defined: MMS-1 included all 3 markers, and MMS-2 included only CK-MB and cTnI. A strategy was considered positive if any of the markers was positive. The CS STAT assay was compared with the CK-MB result of the local hospital laboratory. All patients underwent both local laboratory testing and bedside testing, but treating physicians used only local CK-MB results in making management decisions. This study was well designed for comparing the prognostic value of the CS STAT values with conventional CK-MB values. However, a more useful outcome would be the utility of this bedside instrument in the diagnosis of MI. Also, whether such an instrument would lead to clinically significant changes in prognosis outside of special chest pain units is unclear.
OUTCOMES MEASURED: The rate of MI or death at 30 days was determined for patients with either a positive or negative MMS or CK-MB test result. Additional assessments included time from arrival to a positive test result and the relation of MMS status to the rates of MI, death, and revascularization at 30 days.
RESULTS: Testing at baseline (on initial evaluation in the emergency department) predicted death or MI within 30 days in 19% of patients with a positive MMS-1, 22% with a positive MMS-2, and 13% with a positive CK-MB result. Conversely, only 3% with a negative MMS-1 or MMS-2 and 6% with a negative CK-MB result died or had a MI at 30 days. With serial testing 55% of persons with an abnormal CK-MB result had a MI at 30 days, while serial MMS testing did not discriminate any better than the single test. The bedside 3-marker strategy was superior in predicting 30-day mortality; all 3 patients who died had a positive MMS-1 at baseline, while only 1 of 3 had an abnormal CK-MB on serial testing. An abnormal CK-MB result predicted revascularization within 30-days better than bedside testing using both single and serial measurements. After emergency department arrival, positive test results were obtained more quickly using the bedside instrument (MMS-1=2.5 hours, MMS-2=2.8 hours, LL=3.4 hours; P=.0001).
A single bedside multimarker test (myoglobin, troponpin I, and CK-MB) is more likely than a single CK-MB result to determine risk for MI or death within 30 days after an episode of chest pain. Serial CK-MB testing outperformed both single and serial MMS testing in predicting 30-day MI, need for revascularization, and combined outcome of MI or death. This single bedside test may be a useful adjunct to standard tests in identifying patients at higher risk for MI or death. However, it should not replace serial CK-MB testing in the diagnosis of MI.