User login
Endoscopic Sleeve Gastroplasty is an Effective Treatment for Obesity in a Veteran With Metabolic and Psychiatric Comorbidities
Endoscopic Sleeve Gastroplasty is an Effective Treatment for Obesity in a Veteran With Metabolic and Psychiatric Comorbidities
Obesity is a growing worldwide epidemic with significant implications for individual health and public health care costs. It is also associated with several medical conditions, including diabetes, cardiovascular disease, cancer, and mental health disorders.1 Comprehensive lifestyle intervention is a first-line therapy for obesity consisting of dietary and exercise interventions. Despite initial success, long-term results and durability of weight loss with lifestyle modifications are limited. 2 Bariatric surgery, including sleeve gastrectomy and gastric bypass surgery, is a more invasive approach that is highly effective in weight loss. However, these operations are not reversible, and patients may not be eligible for or may not desire surgery. Overall, bariatric surgery is widely underutilized, with < 1% of eligible patients ultimately undergoing surgery.3,4
Endoscopic bariatric therapies are increasingly popular procedures that address the need for additional treatments for obesity among individuals who have not had success with lifestyle changes and are not surgical candidates. The most common procedure is the endoscopic sleeve gastroplasty (ESG), which applies full-thickness sutures in the stomach to reduce gastric volume, delay gastric emptying, and limit food intake while keeping the fundus intact compared with sleeve gastrectomy. This procedure is typically considered in patients with body mass index (BMI) ≥ 30, who do not qualify for or do not want traditional bariatric surgery. The literature supports robust outcomes after ESG, with studies demonstrating significant and sustained total body weight loss of up to 14% to 16% at 5 years and significant improvement in ≥ 1 metabolic comorbidities in 80% of patients.5,6 ESG adverse events (AEs) include abdominal pain, nausea, and vomiting that are typically self-limited to 1 week. Rarer but more serious AEs include bleeding, perforation, or infection, and occur in 2% of cases based on large trial data.5,7
Although the weight loss benefits of ESG are well established, to date, there are limited data on the effects of endoscopic bariatric therapies like ESG on mental health conditions. Here, we describe a case of a veteran with a history of mental health disorders that prevented him from completing bariatric surgery. The patient underwent ESG and had a successful clinical course.
CASE PRESENTATION
A 59-year-old male veteran with a medical history of class III obesity (42.4 BMI), obstructive sleep apnea, hypothyroidism, hypertension, type 2 diabetes mellitus, and a large ventral hernia was referred to the MOVE! (Management of Overweight/ Obese Veterans Everywhere!) multidisciplinary high-intensity weight loss program at the US Department of Veterans Affairs (VA) West Los Angeles VA Medical Center (WLAVAMC). His psychiatric history included generalized anxiety disorder, posttraumatic stress disorder (PTSD), and panic disorder, managed by the Psychiatry Service and treated with sertraline 25 mg daily, lorazepam 0.5 mg twice daily, and hydroxyzine 20 mg nightly. He had previously implemented lifestyle changes and attended MOVE! classes and nutrition coaching for 1 year but was unsuccessful in losing weight. He had also tried liraglutide 3 mg daily for weight loss but was unable to tolerate it and reported worsening medication-related anxiety.
The patient declined further weight loss pharmacotherapy and was referred to bariatric surgery. He was scheduled for a surgical sleeve gastrectomy. However, on the day he arrived at the hospital for surgery, he developed severe anxiety and had a panic attack, and it was canceled. Due to his mental health issues, he was no longer comfortable proceeding with surgery and was left without other options for obesity treatment. The veteran was extremely disappointed because the ventral hernia caused significant quality of life impairment, limited his ability to exercise, and caused him embarrassment in public settings. The hernia could not be surgically repaired until there was significant weight loss.
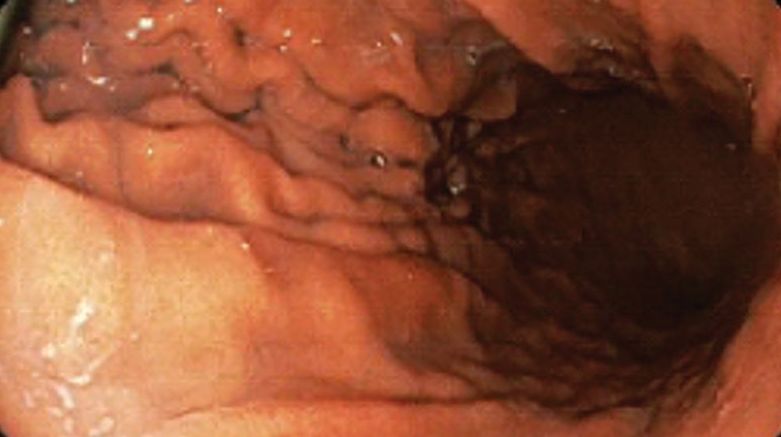
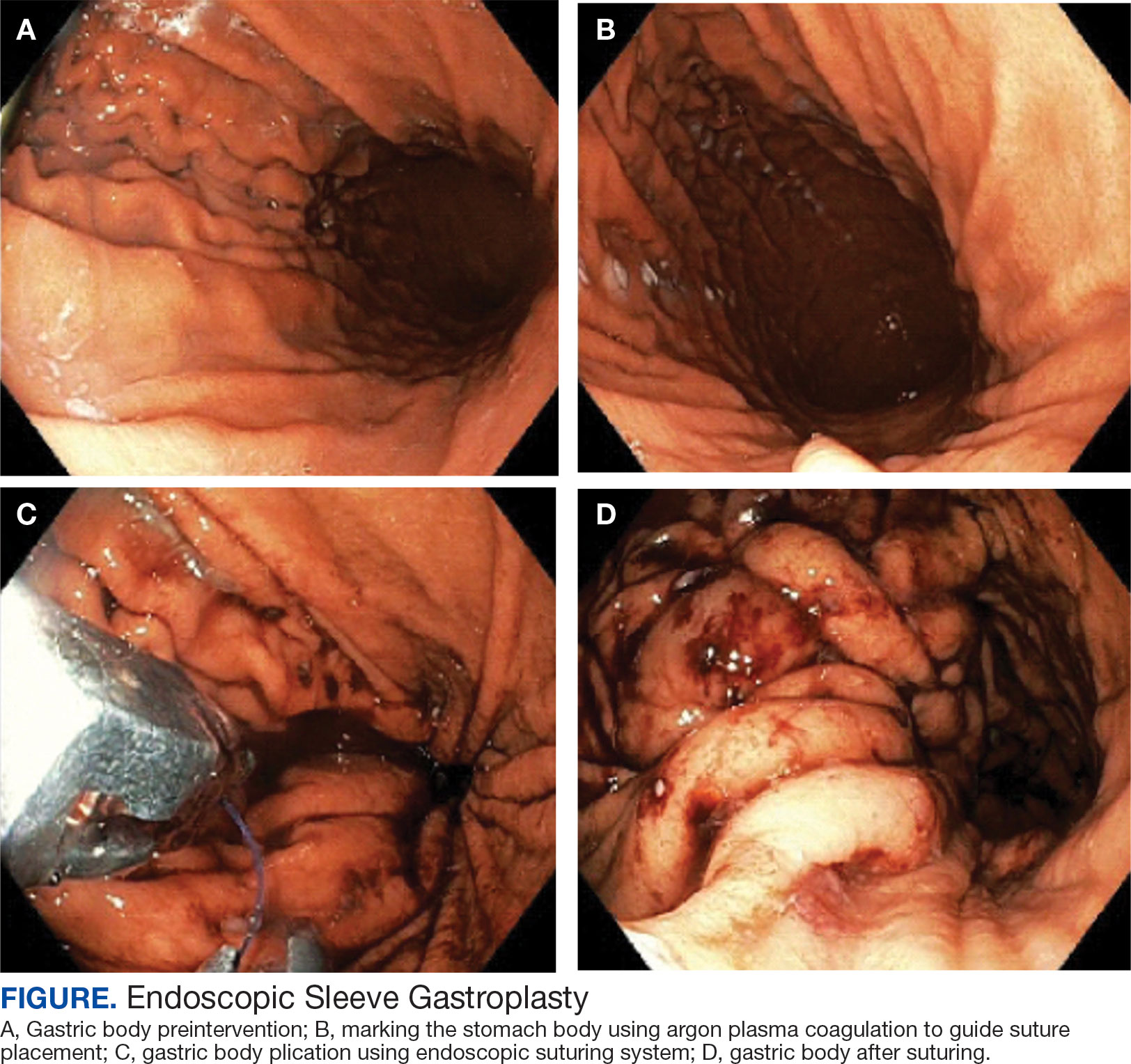
A bariatric endoscopy program within the Division of Gastroenterology was developed and implemented at the WLAVAMC in February 2023 in conjunction with MOVE! The patient was referred for consideration of an endoscopic weight loss procedure. He was determined to be a suitable candidate for ESG based on his BMI being > 40 and personal preference not to proceed with surgery to lose enough weight to qualify for hernia repair. The veteran underwent an endoscopy, which showed normal anatomy and gastric mucosa. ESG was performed in standard fashion (Figure).8 Three vertical lines were made using argon plasma coagulation from the incisura to 2 cm below the gastroesophageal junction along the anterior, posterior, and greater curvature of the stomach to mark the area for endoscopic suture placement. Starting at the incisura, 7 full-thickness sutures were placed to create a volume reduction plication, with preservation of the fundus. The patient did well postprocedure with no immediate or delayed AEs and was discharged home the same day.
Follow-up
The veteran followed a gradual dietary advancement from a clear liquid diet to pureed and soft texture food. The patient’s weight dropped from 359 lbs preprocedure to 304 lbs 6 months postprocedure, a total body weight loss (TWBL) of 15.3%. At 12 months the veteran weighed 299 lbs (16.7% TBWL). He also had notable improvements in metabolic parameters. His systolic blood pressure decreased from ≥ 140 mm Hg to 120 to 130 mm Hg and hemoglobin A1c dropped from 7.0% to 6.3%. Remarkably, his psychiatrist noted significant improvement in his overall mental health. The veteran reported complete cessation of panic attacks since the ESG, improvements in PTSD and anxiety, and was able to discontinue lorazepam and decrease his dose of sertraline to 12.5 mg daily. He reported feeling more energetic and goal-oriented with increased clarity of thought. Perhaps the most significant outcome was that after the 55-lb weight loss at 6 months, the patient was eligible to undergo ventral hernia surgical repair, which had previously contributed to shame and social isolation. This, in turn, improved his quality of life, allowed him to start walking again, up to 8 miles daily, and to feel comfortable again going out in public settings.
DISCUSSION
Bariatric surgeries are an effective method of achieving weight loss and improving obesity-related comorbidities. However, only a small percentage of individuals with obesity are candidates for bariatric surgery. Given the dramatic increase in the prevalence of obesity, other options are needed. Specifically, within the VA, an estimated 80% of veterans are overweight or obese, but only about 500 bariatric surgeries are performed annually.9 With the need for additional weight loss therapies, VA programs are starting to offer endoscopic bariatric procedures as an alternative option. This may be a desirable choice for patients with obesity (BMI > 30), with or without associated metabolic comorbidities, who need more aggressive intervention beyond dietary and lifestyle changes and are either not interested in or not eligible for bariatric surgery or weight loss medications.
Although there is evidence that metabolic comorbidities are associated with obesity, there has been less research on obesity and mental health comorbidities such as depression and anxiety. These psychiatric conditions may even be more common among patients seeking weight loss procedures and more prominent in certain groups such as veterans, which may ultimately exclude these patients from bariatric surgery.10 Prior studies suggest that bariatric surgery can reduce the severity of depression and, to a lesser extent, anxiety symptoms at 2 years following the initial surgery; however, there is limited literature describing the impact of weight loss procedure on panic disorders.11-14 We suspect that a weight loss procedure such as ESG may have indirectly improved the veteran’s mood disorder due to the weight loss it induced, increasing the ability to exercise, quality of sleep, and participation in public settings.
This case highlights a veteran who did not tolerate weight loss medication and had severe anxiety and PTSD that prevented him from going through with bariatric surgery. He then underwent an endoscopic weight loss procedure. The ESG helped him successfully achieve significant weight loss, increase his physical activity, reduce his anxiety and panic disorder, and overall, significantly improve his quality of life. More than 1 year after the procedure, the patient has sustained improvements in his psychiatric and emotional health along with durable weight loss, maintaining > 15% of his total weight lost. Additional studies are needed to further understand the prevalence and long-term outcomes of mental health comorbidities, as well as weight loss outcomes in this group of patients who undergo endoscopic bariatric procedures.
CONCLUSIONS
We describe a case of a veteran with severe obesity and significant psychiatric comorbidities that prevented him from undergoing bariatric surgery, who underwent an ESG. This procedure led to significant weight loss, improvement of metabolic parameters, reduction in anxiety and PTSD, and enhancement of his quality of life. This case emphasizes the unique advantages of ESG and supports the expansion of endoscopic bariatric programs in the VA.
- Ritchie SA, Connell JM. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cardiovasc Dis. 2007;17(4):319-326. doi:10.1016/j.numecd.2006.07.005
- Bray GA, Kim KK, Wilding JPH; World Obesity Federation. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev. 2017;18(7):715-723. doi:10.1111/obr.12551
- Imbus JR, Voils CI, Funk LM. Bariatric surgery barriers: a review using andersen’s model of health services use. Surg Obes Relat Dis. 2018;14(3):404-412. doi:10.1016/j.soard.2017.11.012
- Dawes AJ, Maggard-Gibbons M, Maher AR, et al. Mental health conditions among patients seeking and undergoing bariatric surgery: a meta-analysis. JAMA. 2016;315(2):150- 163. doi:10.1001/jama.2015.18118
- Abu Dayyeh BK, Bazerbachi F, Vargas EJ, et al.. Endoscopic sleeve gastroplasty for treatment of class 1 and 2 obesity (MERIT): a prospective, multicentre, randomised trial. Lancet. 2022;400(10350):441-451. doi:10.1016/S0140-6736(22)01280-6
- Matteo MV, Bove V, Ciasca G, et al. Success predictors of endoscopic sleeve gastroplasty. Obes Surg. 2024;34(5):1496-1504. doi:10.1007/s11695-024-07109-4
- Maselli DB, Hoff AC, Kucera A, et al. Endoscopic sleeve gastroplasty in class III obesity: efficacy, safety, and durability outcomes in 404 consecutive patients. World J Gastrointest Endosc. 2023;15(6):469-479. doi:10.4253/wjge.v15.i6.469
- Kumar N, Abu Dayyeh BK, Lopez-Nava Breviere G, et al. Endoscopic sutured gastroplasty: procedure evolution from first-in-man cases through current technique. Surg Endosc. 2018;32(4):2159-2164. doi:10.1007/s00464-017-5869-2
- Maggard-Gibbons M, Shekelle PG, Girgis MD, et al. Endoscopic Bariatric Interventions versus lifestyle interventions or surgery for weight loss in patients with obesity: a systematic review and meta-analysis. Department of Veterans Affairs (US); 2022. https://www.ncbi.nlm.nih.gov/books/NBK587943/
- Maggard Gibbons MA, Maher AM, Dawes AJ, et al. Psychological clearance for bariatric surgery: a systematic review. VA-ESP project #05-2262014.
- van Hout GC, Verschure SK, van Heck GL. Psychosocial predictors of success following bariatric surgery. Obes Surg. 2005;15(4):552-560. doi:10.1381/0960892053723484
- Hudson JI, Hiripi E, Pope HG Jr, Kessler RC. The prevalence and correlates of eating disorders in the national comorbidity survey replication. Biol Psychiatry. 2007;61(3):348-358. doi:10.1016/j.biopsych.2006.03.040
- Aylward L, Lilly C, Konsor M, et al. How soon do depression and anxiety symptoms improve after bariatric surgery?. Healthcare (Basel). 2023;11(6):862. doi:10.3390/healthcare11060862
- Law S, Dong S, Zhou F, Zheng D, Wang C, Dong Z. Bariatric surgery and mental health outcomes: an umbrella review. Front Endocrinol (Lausanne). 2023;14:1283621. doi:10.3389/fendo.2023.1283621
Obesity is a growing worldwide epidemic with significant implications for individual health and public health care costs. It is also associated with several medical conditions, including diabetes, cardiovascular disease, cancer, and mental health disorders.1 Comprehensive lifestyle intervention is a first-line therapy for obesity consisting of dietary and exercise interventions. Despite initial success, long-term results and durability of weight loss with lifestyle modifications are limited. 2 Bariatric surgery, including sleeve gastrectomy and gastric bypass surgery, is a more invasive approach that is highly effective in weight loss. However, these operations are not reversible, and patients may not be eligible for or may not desire surgery. Overall, bariatric surgery is widely underutilized, with < 1% of eligible patients ultimately undergoing surgery.3,4
Endoscopic bariatric therapies are increasingly popular procedures that address the need for additional treatments for obesity among individuals who have not had success with lifestyle changes and are not surgical candidates. The most common procedure is the endoscopic sleeve gastroplasty (ESG), which applies full-thickness sutures in the stomach to reduce gastric volume, delay gastric emptying, and limit food intake while keeping the fundus intact compared with sleeve gastrectomy. This procedure is typically considered in patients with body mass index (BMI) ≥ 30, who do not qualify for or do not want traditional bariatric surgery. The literature supports robust outcomes after ESG, with studies demonstrating significant and sustained total body weight loss of up to 14% to 16% at 5 years and significant improvement in ≥ 1 metabolic comorbidities in 80% of patients.5,6 ESG adverse events (AEs) include abdominal pain, nausea, and vomiting that are typically self-limited to 1 week. Rarer but more serious AEs include bleeding, perforation, or infection, and occur in 2% of cases based on large trial data.5,7
Although the weight loss benefits of ESG are well established, to date, there are limited data on the effects of endoscopic bariatric therapies like ESG on mental health conditions. Here, we describe a case of a veteran with a history of mental health disorders that prevented him from completing bariatric surgery. The patient underwent ESG and had a successful clinical course.
CASE PRESENTATION
A 59-year-old male veteran with a medical history of class III obesity (42.4 BMI), obstructive sleep apnea, hypothyroidism, hypertension, type 2 diabetes mellitus, and a large ventral hernia was referred to the MOVE! (Management of Overweight/ Obese Veterans Everywhere!) multidisciplinary high-intensity weight loss program at the US Department of Veterans Affairs (VA) West Los Angeles VA Medical Center (WLAVAMC). His psychiatric history included generalized anxiety disorder, posttraumatic stress disorder (PTSD), and panic disorder, managed by the Psychiatry Service and treated with sertraline 25 mg daily, lorazepam 0.5 mg twice daily, and hydroxyzine 20 mg nightly. He had previously implemented lifestyle changes and attended MOVE! classes and nutrition coaching for 1 year but was unsuccessful in losing weight. He had also tried liraglutide 3 mg daily for weight loss but was unable to tolerate it and reported worsening medication-related anxiety.
The patient declined further weight loss pharmacotherapy and was referred to bariatric surgery. He was scheduled for a surgical sleeve gastrectomy. However, on the day he arrived at the hospital for surgery, he developed severe anxiety and had a panic attack, and it was canceled. Due to his mental health issues, he was no longer comfortable proceeding with surgery and was left without other options for obesity treatment. The veteran was extremely disappointed because the ventral hernia caused significant quality of life impairment, limited his ability to exercise, and caused him embarrassment in public settings. The hernia could not be surgically repaired until there was significant weight loss.
A bariatric endoscopy program within the Division of Gastroenterology was developed and implemented at the WLAVAMC in February 2023 in conjunction with MOVE! The patient was referred for consideration of an endoscopic weight loss procedure. He was determined to be a suitable candidate for ESG based on his BMI being > 40 and personal preference not to proceed with surgery to lose enough weight to qualify for hernia repair. The veteran underwent an endoscopy, which showed normal anatomy and gastric mucosa. ESG was performed in standard fashion (Figure).8 Three vertical lines were made using argon plasma coagulation from the incisura to 2 cm below the gastroesophageal junction along the anterior, posterior, and greater curvature of the stomach to mark the area for endoscopic suture placement. Starting at the incisura, 7 full-thickness sutures were placed to create a volume reduction plication, with preservation of the fundus. The patient did well postprocedure with no immediate or delayed AEs and was discharged home the same day.

Follow-up
The veteran followed a gradual dietary advancement from a clear liquid diet to pureed and soft texture food. The patient’s weight dropped from 359 lbs preprocedure to 304 lbs 6 months postprocedure, a total body weight loss (TWBL) of 15.3%. At 12 months the veteran weighed 299 lbs (16.7% TBWL). He also had notable improvements in metabolic parameters. His systolic blood pressure decreased from ≥ 140 mm Hg to 120 to 130 mm Hg and hemoglobin A1c dropped from 7.0% to 6.3%. Remarkably, his psychiatrist noted significant improvement in his overall mental health. The veteran reported complete cessation of panic attacks since the ESG, improvements in PTSD and anxiety, and was able to discontinue lorazepam and decrease his dose of sertraline to 12.5 mg daily. He reported feeling more energetic and goal-oriented with increased clarity of thought. Perhaps the most significant outcome was that after the 55-lb weight loss at 6 months, the patient was eligible to undergo ventral hernia surgical repair, which had previously contributed to shame and social isolation. This, in turn, improved his quality of life, allowed him to start walking again, up to 8 miles daily, and to feel comfortable again going out in public settings.
DISCUSSION
Bariatric surgeries are an effective method of achieving weight loss and improving obesity-related comorbidities. However, only a small percentage of individuals with obesity are candidates for bariatric surgery. Given the dramatic increase in the prevalence of obesity, other options are needed. Specifically, within the VA, an estimated 80% of veterans are overweight or obese, but only about 500 bariatric surgeries are performed annually.9 With the need for additional weight loss therapies, VA programs are starting to offer endoscopic bariatric procedures as an alternative option. This may be a desirable choice for patients with obesity (BMI > 30), with or without associated metabolic comorbidities, who need more aggressive intervention beyond dietary and lifestyle changes and are either not interested in or not eligible for bariatric surgery or weight loss medications.
Although there is evidence that metabolic comorbidities are associated with obesity, there has been less research on obesity and mental health comorbidities such as depression and anxiety. These psychiatric conditions may even be more common among patients seeking weight loss procedures and more prominent in certain groups such as veterans, which may ultimately exclude these patients from bariatric surgery.10 Prior studies suggest that bariatric surgery can reduce the severity of depression and, to a lesser extent, anxiety symptoms at 2 years following the initial surgery; however, there is limited literature describing the impact of weight loss procedure on panic disorders.11-14 We suspect that a weight loss procedure such as ESG may have indirectly improved the veteran’s mood disorder due to the weight loss it induced, increasing the ability to exercise, quality of sleep, and participation in public settings.
This case highlights a veteran who did not tolerate weight loss medication and had severe anxiety and PTSD that prevented him from going through with bariatric surgery. He then underwent an endoscopic weight loss procedure. The ESG helped him successfully achieve significant weight loss, increase his physical activity, reduce his anxiety and panic disorder, and overall, significantly improve his quality of life. More than 1 year after the procedure, the patient has sustained improvements in his psychiatric and emotional health along with durable weight loss, maintaining > 15% of his total weight lost. Additional studies are needed to further understand the prevalence and long-term outcomes of mental health comorbidities, as well as weight loss outcomes in this group of patients who undergo endoscopic bariatric procedures.
CONCLUSIONS
We describe a case of a veteran with severe obesity and significant psychiatric comorbidities that prevented him from undergoing bariatric surgery, who underwent an ESG. This procedure led to significant weight loss, improvement of metabolic parameters, reduction in anxiety and PTSD, and enhancement of his quality of life. This case emphasizes the unique advantages of ESG and supports the expansion of endoscopic bariatric programs in the VA.
Obesity is a growing worldwide epidemic with significant implications for individual health and public health care costs. It is also associated with several medical conditions, including diabetes, cardiovascular disease, cancer, and mental health disorders.1 Comprehensive lifestyle intervention is a first-line therapy for obesity consisting of dietary and exercise interventions. Despite initial success, long-term results and durability of weight loss with lifestyle modifications are limited. 2 Bariatric surgery, including sleeve gastrectomy and gastric bypass surgery, is a more invasive approach that is highly effective in weight loss. However, these operations are not reversible, and patients may not be eligible for or may not desire surgery. Overall, bariatric surgery is widely underutilized, with < 1% of eligible patients ultimately undergoing surgery.3,4
Endoscopic bariatric therapies are increasingly popular procedures that address the need for additional treatments for obesity among individuals who have not had success with lifestyle changes and are not surgical candidates. The most common procedure is the endoscopic sleeve gastroplasty (ESG), which applies full-thickness sutures in the stomach to reduce gastric volume, delay gastric emptying, and limit food intake while keeping the fundus intact compared with sleeve gastrectomy. This procedure is typically considered in patients with body mass index (BMI) ≥ 30, who do not qualify for or do not want traditional bariatric surgery. The literature supports robust outcomes after ESG, with studies demonstrating significant and sustained total body weight loss of up to 14% to 16% at 5 years and significant improvement in ≥ 1 metabolic comorbidities in 80% of patients.5,6 ESG adverse events (AEs) include abdominal pain, nausea, and vomiting that are typically self-limited to 1 week. Rarer but more serious AEs include bleeding, perforation, or infection, and occur in 2% of cases based on large trial data.5,7
Although the weight loss benefits of ESG are well established, to date, there are limited data on the effects of endoscopic bariatric therapies like ESG on mental health conditions. Here, we describe a case of a veteran with a history of mental health disorders that prevented him from completing bariatric surgery. The patient underwent ESG and had a successful clinical course.
CASE PRESENTATION
A 59-year-old male veteran with a medical history of class III obesity (42.4 BMI), obstructive sleep apnea, hypothyroidism, hypertension, type 2 diabetes mellitus, and a large ventral hernia was referred to the MOVE! (Management of Overweight/ Obese Veterans Everywhere!) multidisciplinary high-intensity weight loss program at the US Department of Veterans Affairs (VA) West Los Angeles VA Medical Center (WLAVAMC). His psychiatric history included generalized anxiety disorder, posttraumatic stress disorder (PTSD), and panic disorder, managed by the Psychiatry Service and treated with sertraline 25 mg daily, lorazepam 0.5 mg twice daily, and hydroxyzine 20 mg nightly. He had previously implemented lifestyle changes and attended MOVE! classes and nutrition coaching for 1 year but was unsuccessful in losing weight. He had also tried liraglutide 3 mg daily for weight loss but was unable to tolerate it and reported worsening medication-related anxiety.
The patient declined further weight loss pharmacotherapy and was referred to bariatric surgery. He was scheduled for a surgical sleeve gastrectomy. However, on the day he arrived at the hospital for surgery, he developed severe anxiety and had a panic attack, and it was canceled. Due to his mental health issues, he was no longer comfortable proceeding with surgery and was left without other options for obesity treatment. The veteran was extremely disappointed because the ventral hernia caused significant quality of life impairment, limited his ability to exercise, and caused him embarrassment in public settings. The hernia could not be surgically repaired until there was significant weight loss.
A bariatric endoscopy program within the Division of Gastroenterology was developed and implemented at the WLAVAMC in February 2023 in conjunction with MOVE! The patient was referred for consideration of an endoscopic weight loss procedure. He was determined to be a suitable candidate for ESG based on his BMI being > 40 and personal preference not to proceed with surgery to lose enough weight to qualify for hernia repair. The veteran underwent an endoscopy, which showed normal anatomy and gastric mucosa. ESG was performed in standard fashion (Figure).8 Three vertical lines were made using argon plasma coagulation from the incisura to 2 cm below the gastroesophageal junction along the anterior, posterior, and greater curvature of the stomach to mark the area for endoscopic suture placement. Starting at the incisura, 7 full-thickness sutures were placed to create a volume reduction plication, with preservation of the fundus. The patient did well postprocedure with no immediate or delayed AEs and was discharged home the same day.

Follow-up
The veteran followed a gradual dietary advancement from a clear liquid diet to pureed and soft texture food. The patient’s weight dropped from 359 lbs preprocedure to 304 lbs 6 months postprocedure, a total body weight loss (TWBL) of 15.3%. At 12 months the veteran weighed 299 lbs (16.7% TBWL). He also had notable improvements in metabolic parameters. His systolic blood pressure decreased from ≥ 140 mm Hg to 120 to 130 mm Hg and hemoglobin A1c dropped from 7.0% to 6.3%. Remarkably, his psychiatrist noted significant improvement in his overall mental health. The veteran reported complete cessation of panic attacks since the ESG, improvements in PTSD and anxiety, and was able to discontinue lorazepam and decrease his dose of sertraline to 12.5 mg daily. He reported feeling more energetic and goal-oriented with increased clarity of thought. Perhaps the most significant outcome was that after the 55-lb weight loss at 6 months, the patient was eligible to undergo ventral hernia surgical repair, which had previously contributed to shame and social isolation. This, in turn, improved his quality of life, allowed him to start walking again, up to 8 miles daily, and to feel comfortable again going out in public settings.
DISCUSSION
Bariatric surgeries are an effective method of achieving weight loss and improving obesity-related comorbidities. However, only a small percentage of individuals with obesity are candidates for bariatric surgery. Given the dramatic increase in the prevalence of obesity, other options are needed. Specifically, within the VA, an estimated 80% of veterans are overweight or obese, but only about 500 bariatric surgeries are performed annually.9 With the need for additional weight loss therapies, VA programs are starting to offer endoscopic bariatric procedures as an alternative option. This may be a desirable choice for patients with obesity (BMI > 30), with or without associated metabolic comorbidities, who need more aggressive intervention beyond dietary and lifestyle changes and are either not interested in or not eligible for bariatric surgery or weight loss medications.
Although there is evidence that metabolic comorbidities are associated with obesity, there has been less research on obesity and mental health comorbidities such as depression and anxiety. These psychiatric conditions may even be more common among patients seeking weight loss procedures and more prominent in certain groups such as veterans, which may ultimately exclude these patients from bariatric surgery.10 Prior studies suggest that bariatric surgery can reduce the severity of depression and, to a lesser extent, anxiety symptoms at 2 years following the initial surgery; however, there is limited literature describing the impact of weight loss procedure on panic disorders.11-14 We suspect that a weight loss procedure such as ESG may have indirectly improved the veteran’s mood disorder due to the weight loss it induced, increasing the ability to exercise, quality of sleep, and participation in public settings.
This case highlights a veteran who did not tolerate weight loss medication and had severe anxiety and PTSD that prevented him from going through with bariatric surgery. He then underwent an endoscopic weight loss procedure. The ESG helped him successfully achieve significant weight loss, increase his physical activity, reduce his anxiety and panic disorder, and overall, significantly improve his quality of life. More than 1 year after the procedure, the patient has sustained improvements in his psychiatric and emotional health along with durable weight loss, maintaining > 15% of his total weight lost. Additional studies are needed to further understand the prevalence and long-term outcomes of mental health comorbidities, as well as weight loss outcomes in this group of patients who undergo endoscopic bariatric procedures.
CONCLUSIONS
We describe a case of a veteran with severe obesity and significant psychiatric comorbidities that prevented him from undergoing bariatric surgery, who underwent an ESG. This procedure led to significant weight loss, improvement of metabolic parameters, reduction in anxiety and PTSD, and enhancement of his quality of life. This case emphasizes the unique advantages of ESG and supports the expansion of endoscopic bariatric programs in the VA.
- Ritchie SA, Connell JM. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cardiovasc Dis. 2007;17(4):319-326. doi:10.1016/j.numecd.2006.07.005
- Bray GA, Kim KK, Wilding JPH; World Obesity Federation. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev. 2017;18(7):715-723. doi:10.1111/obr.12551
- Imbus JR, Voils CI, Funk LM. Bariatric surgery barriers: a review using andersen’s model of health services use. Surg Obes Relat Dis. 2018;14(3):404-412. doi:10.1016/j.soard.2017.11.012
- Dawes AJ, Maggard-Gibbons M, Maher AR, et al. Mental health conditions among patients seeking and undergoing bariatric surgery: a meta-analysis. JAMA. 2016;315(2):150- 163. doi:10.1001/jama.2015.18118
- Abu Dayyeh BK, Bazerbachi F, Vargas EJ, et al.. Endoscopic sleeve gastroplasty for treatment of class 1 and 2 obesity (MERIT): a prospective, multicentre, randomised trial. Lancet. 2022;400(10350):441-451. doi:10.1016/S0140-6736(22)01280-6
- Matteo MV, Bove V, Ciasca G, et al. Success predictors of endoscopic sleeve gastroplasty. Obes Surg. 2024;34(5):1496-1504. doi:10.1007/s11695-024-07109-4
- Maselli DB, Hoff AC, Kucera A, et al. Endoscopic sleeve gastroplasty in class III obesity: efficacy, safety, and durability outcomes in 404 consecutive patients. World J Gastrointest Endosc. 2023;15(6):469-479. doi:10.4253/wjge.v15.i6.469
- Kumar N, Abu Dayyeh BK, Lopez-Nava Breviere G, et al. Endoscopic sutured gastroplasty: procedure evolution from first-in-man cases through current technique. Surg Endosc. 2018;32(4):2159-2164. doi:10.1007/s00464-017-5869-2
- Maggard-Gibbons M, Shekelle PG, Girgis MD, et al. Endoscopic Bariatric Interventions versus lifestyle interventions or surgery for weight loss in patients with obesity: a systematic review and meta-analysis. Department of Veterans Affairs (US); 2022. https://www.ncbi.nlm.nih.gov/books/NBK587943/
- Maggard Gibbons MA, Maher AM, Dawes AJ, et al. Psychological clearance for bariatric surgery: a systematic review. VA-ESP project #05-2262014.
- van Hout GC, Verschure SK, van Heck GL. Psychosocial predictors of success following bariatric surgery. Obes Surg. 2005;15(4):552-560. doi:10.1381/0960892053723484
- Hudson JI, Hiripi E, Pope HG Jr, Kessler RC. The prevalence and correlates of eating disorders in the national comorbidity survey replication. Biol Psychiatry. 2007;61(3):348-358. doi:10.1016/j.biopsych.2006.03.040
- Aylward L, Lilly C, Konsor M, et al. How soon do depression and anxiety symptoms improve after bariatric surgery?. Healthcare (Basel). 2023;11(6):862. doi:10.3390/healthcare11060862
- Law S, Dong S, Zhou F, Zheng D, Wang C, Dong Z. Bariatric surgery and mental health outcomes: an umbrella review. Front Endocrinol (Lausanne). 2023;14:1283621. doi:10.3389/fendo.2023.1283621
- Ritchie SA, Connell JM. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cardiovasc Dis. 2007;17(4):319-326. doi:10.1016/j.numecd.2006.07.005
- Bray GA, Kim KK, Wilding JPH; World Obesity Federation. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev. 2017;18(7):715-723. doi:10.1111/obr.12551
- Imbus JR, Voils CI, Funk LM. Bariatric surgery barriers: a review using andersen’s model of health services use. Surg Obes Relat Dis. 2018;14(3):404-412. doi:10.1016/j.soard.2017.11.012
- Dawes AJ, Maggard-Gibbons M, Maher AR, et al. Mental health conditions among patients seeking and undergoing bariatric surgery: a meta-analysis. JAMA. 2016;315(2):150- 163. doi:10.1001/jama.2015.18118
- Abu Dayyeh BK, Bazerbachi F, Vargas EJ, et al.. Endoscopic sleeve gastroplasty for treatment of class 1 and 2 obesity (MERIT): a prospective, multicentre, randomised trial. Lancet. 2022;400(10350):441-451. doi:10.1016/S0140-6736(22)01280-6
- Matteo MV, Bove V, Ciasca G, et al. Success predictors of endoscopic sleeve gastroplasty. Obes Surg. 2024;34(5):1496-1504. doi:10.1007/s11695-024-07109-4
- Maselli DB, Hoff AC, Kucera A, et al. Endoscopic sleeve gastroplasty in class III obesity: efficacy, safety, and durability outcomes in 404 consecutive patients. World J Gastrointest Endosc. 2023;15(6):469-479. doi:10.4253/wjge.v15.i6.469
- Kumar N, Abu Dayyeh BK, Lopez-Nava Breviere G, et al. Endoscopic sutured gastroplasty: procedure evolution from first-in-man cases through current technique. Surg Endosc. 2018;32(4):2159-2164. doi:10.1007/s00464-017-5869-2
- Maggard-Gibbons M, Shekelle PG, Girgis MD, et al. Endoscopic Bariatric Interventions versus lifestyle interventions or surgery for weight loss in patients with obesity: a systematic review and meta-analysis. Department of Veterans Affairs (US); 2022. https://www.ncbi.nlm.nih.gov/books/NBK587943/
- Maggard Gibbons MA, Maher AM, Dawes AJ, et al. Psychological clearance for bariatric surgery: a systematic review. VA-ESP project #05-2262014.
- van Hout GC, Verschure SK, van Heck GL. Psychosocial predictors of success following bariatric surgery. Obes Surg. 2005;15(4):552-560. doi:10.1381/0960892053723484
- Hudson JI, Hiripi E, Pope HG Jr, Kessler RC. The prevalence and correlates of eating disorders in the national comorbidity survey replication. Biol Psychiatry. 2007;61(3):348-358. doi:10.1016/j.biopsych.2006.03.040
- Aylward L, Lilly C, Konsor M, et al. How soon do depression and anxiety symptoms improve after bariatric surgery?. Healthcare (Basel). 2023;11(6):862. doi:10.3390/healthcare11060862
- Law S, Dong S, Zhou F, Zheng D, Wang C, Dong Z. Bariatric surgery and mental health outcomes: an umbrella review. Front Endocrinol (Lausanne). 2023;14:1283621. doi:10.3389/fendo.2023.1283621
Endoscopic Sleeve Gastroplasty is an Effective Treatment for Obesity in a Veteran With Metabolic and Psychiatric Comorbidities
Endoscopic Sleeve Gastroplasty is an Effective Treatment for Obesity in a Veteran With Metabolic and Psychiatric Comorbidities
A Veteran Presenting With Symptomatic Postprandial Episodes
A Veteran Presenting With Symptomatic Postprandial Episodes
Idiopathic postprandial syndrome (IPP), initially termed reactive hypoglycemia, presents with hypoglycemic-like symptoms in the absence of biochemical hypoglycemia and remains a diagnosis of exclusion. Its pathophysiology is poorly understood. The diagnosis requires thorough evaluation of cardiac, metabolic, neurologic, and gastrointestinal causes, as well as Whipple triad criteria. Dietary modifications, including reduced carbohydrate intake, increased protein and fiber, and frequent small meals, remain the cornerstone of IPP management. Continuous glucose monitoring (CGM) may be a useful adjunct in correlating symptoms with glucose trends, but its role is still evolving.
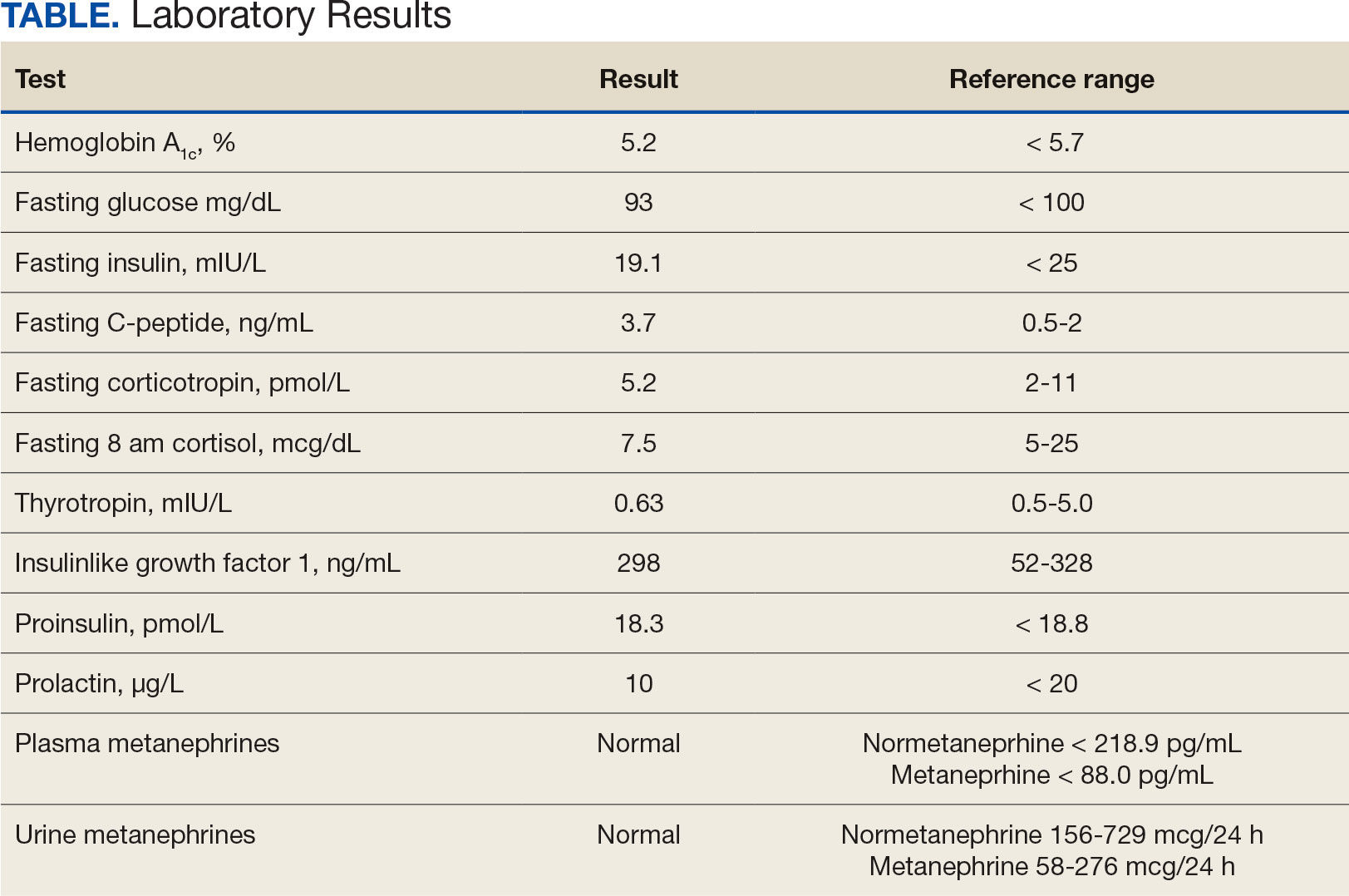
In the evaluation of patients with symptoms suggestive of hypoglycemia (Figure 1), patients should first be assessed for Whipple triad: symptoms consistent with hypoglycemia, blood glucose level < 55 mg/dL, and reversal of symptoms with glucose.1 Patients who meet Whipple triad criteria should be investigated to identify further etiologies of hypoglycemia. They may include insulinoma, medication-induced (insulin, sulfonylurea, meglitinide, or β blocker use), postbariatric surgery complications, noninsulinoma pancreatogenous hypoglycemia syndrome, ackee fruit consumption, or familial conditions.2 The presence of hypoglycemic symptoms in the postprandial or fasting state can provide valuable insights into underlying etiology.
Patients who do not meet Whipple triad criteria, but exhibit postprandial symptoms consistent with hypoglycemia, as in this case, present a diagnostic dilemma. IPP is defined as hypoglycemic symptoms occuring after carbohydrate ingestion without biochemical hypoglycemia. Initially termed reactive hypoglycemia, it was renamed in 1981to reflect the absence of low blood glucose levels.3
The understanding of this diagnosis has not significantly progressed since the 1980s. Its prevalence, incidence, risk factors, and societal burden remain unclear. IPP is a challenging diagnosis due to nonspecific symptoms that overlap with a myriad of conditions. These symptoms may include adrenergic symptoms such as diaphoresis, tremulousness, palpitations, anxiety, and hunger. Potentially severe neuroglycopenic symptoms, including weakness, dizziness, behavior changes, confusion, and coma, are not typically observed.4 Given that objective criteria are not well established, IPP remains a diagnosis of exclusion. It is imperative to rule out alternative etiologies, particularly cardiac, gastrointestinal, and neurologic causes.
CASE PRESENTATION
A male aged 41 years presented to primary care for evaluation of acute on chronic symptomatic postprandial episodes. He reported a history of symptomatic sinus bradycardia in the setting of sick sinus syndrome following dual-chamber pacemaker placement, posttraumatic stress disorder, and gastroesophageal reflux disease. He was a retired Navy sailor without any known occupational exposures who worked in the real estate industry. The patient reported feeling lightheaded, tremulous, and anxious most afternoons after lunch for several years. He also reported that meals heavy in carbohydrates exacerbated his symptoms, whereas skipping meals or lying down alleviated his symptoms. The patient also reported concomitant arm numbness, shortness of breath, palpitations, and nausea during these episodes. Review of systems was otherwise negative, including no weight changes, fever, chills, night sweats, chest pain, or syncope.
The patient’s medications included ferrous sulfate 325 mg once every other day, bupropion 200 mg once daily, metoprolol succinate 25 mg once daily, and as-needed lorazepam 1 mg once daily. The patient reported no current substance use but reported previous tobacco use 3 years prior (maximum 1 pack/week) and alcohol use 5 years prior (750 ml/day for 15 years). The patient did not exercise and typically ate oatmeal for breakfast, a sandwich or salad for lunch, and taquitos or salad for dinner, with snacks throughout the day. Notable family history included a maternal grandmother with colon cancer. The patient’s vital signs included a 36.8 °C temperature, heart rate 87 beats/min, 118/71 mm Hg blood pressure, oxygen saturation 98% on room air, 125.2 kg weight, and 38.5 body mass index. There were no orthostatic vital sign changes. A physical examination demonstrated obesity with an unremarkable cardiopulmonary and volume examination.
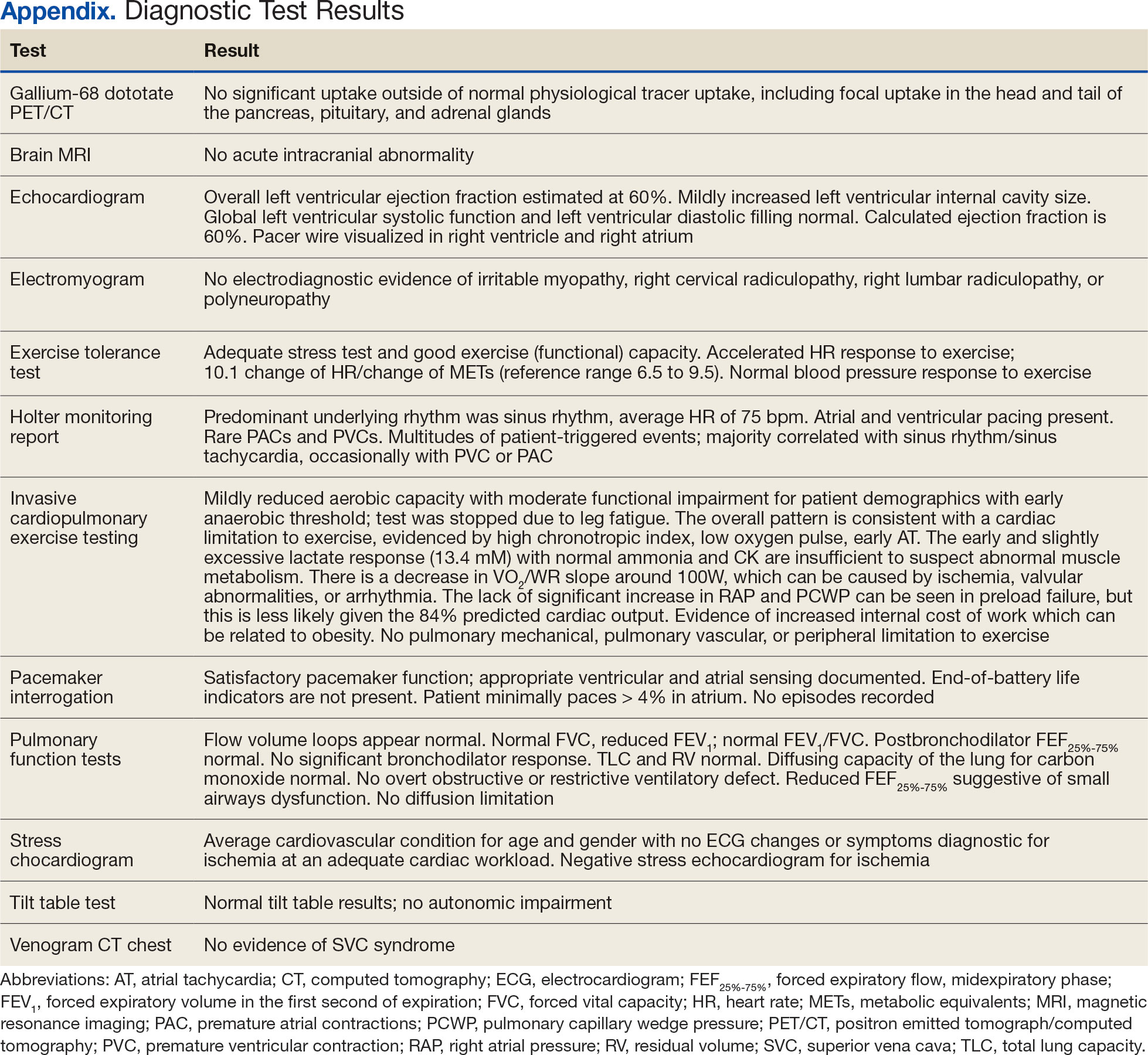
Additional testing included Gallium-68 dototate positron emission tomography/computed tomography, brain magnetic resonance imaging, echocardiogram, electromyogram, exercise tolerance test, Holter monitoring, invasive cardiopulmonary exercise testing, pacemaker interrogation, pulmonary function testing, stress echocardiogram, tilt table test, and venogram computed tomography of the chest, but the results were unremarkable (Appendix). His afternoon nonfasting glucose level was 138 mg/dL with a concurrent hemoglobin A1c of 5.2%. The patient had a fasting C-peptide level of 3.7 ng/mL (reference range 0.5-2.0 ng/mL), fasting insulin level 19.1 mIU/L (reference range < 25 mIU/L), and a fasting glucose level of 93 mg/dL (reference range 70-99 mg/dL). The patient’s urine 5-HIAA, plasma metanephrines, urine metanephrines, insulin-like growth factor 1, prolactin, corticotropin, fasting cortisol, and thyrotropin yielded results within reference ranges (Table). The veteran was prescribed a CGM, which demonstrated normal glucose levels (≥ 55 mg/dL) during symptomatic episodes (Figure 2).
The patient was diagnosed with IPP given normoglycemia, exclusion of alternative diagnoses, and symptomatic improvement with dietary changes. He was referred to a nutritionist for a high-protein, high-fiber, and low-carbohydrate diet.
DISCUSSION
Seemingly simple diagnostic tools can lead to diagnostic pitfalls. Home glucose monitoring with the use of a standard glucometer during an episode is the typical first step in identifying hypoglycemia, as it is both pragmatic and accurate, with a mean absolute relative difference (MARD) of about 10% in hypoglycemic ranges.5 While the advent of CGM provides real-time data and can reveal clinically relevant fluctuations, it reveals mild hypoglycemia (54 to 70 mg/dL) of no clinical significance in a large proportion of individuals.
Additionally, CGM is less accurate than glucometers with a MARD of about 20% in hypoglycemia ranges.6 CGM technology, however, is rapidly evolving and undergoing further investigation for hypoglycemia detection. Therefore, CGM may be considered in select patients as prospective study results are established; the newest CGMs have MARDs very similar to fingerstick blood glucose data.7,8 In the patient described in this case, CGM helped corroborate the diagnosis, given that symptomatic episodes correlated with lower glucose levels. Provocative testing with oral glucose tolerance testing can frequently result in false positive hypoglycemic readings and is not recommended.9 Supervised mixed meal testing can also be used, which entails monitoring after consuming a mixed macronutrient meal. The test concludes after hypoglycemic symptoms develop or 5 hours elapse, whichever occurs first.1
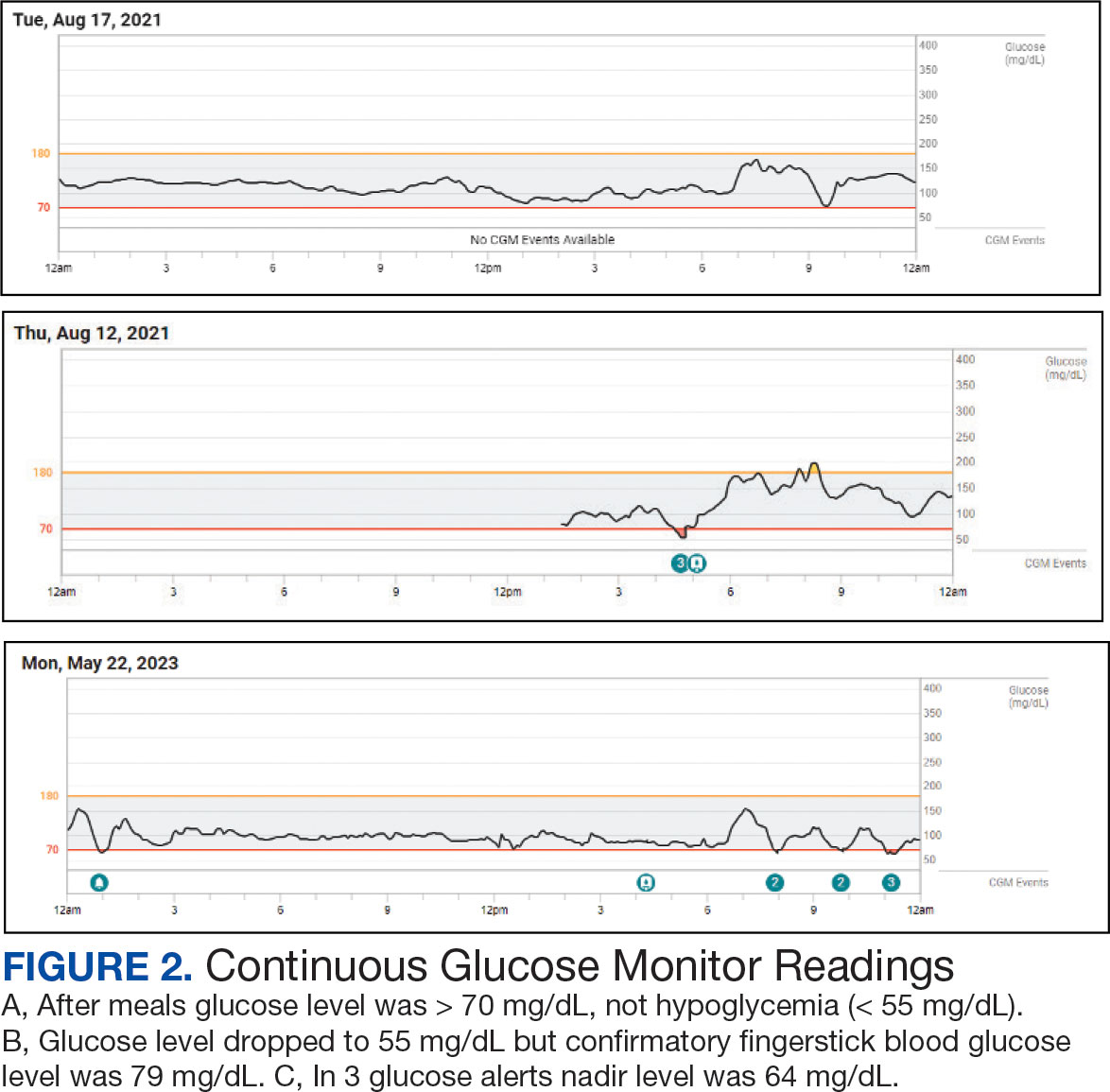
The pathophysiology of IPP is poorly understood. Proposed mechanisms include increased insulin sensitivity, increased adrenergic sensitivity, impaired glucagon regulation, emotional distress, insulin resistance, and increased glucagon-like peptide-1 production.10-13 Research suggests this may occur as pancreatic β cells fail in early type 2 diabetes mellitus, with diminished first-phase insulin release leading to an initial exuberant rise in blood glucose, an overshooting of the second phase of insulin secretion, and the feeling of the postprandial blood glucose falling, even though the final glucose level achieved is not truly low.13 There are contradictory studies in the literature demonstrating no association between insulin resistance and hypoglycemic symptoms.14 In 2022, Kosuda and colleagues looked at homeostatic model assessment for insulin resistance in patients with postprandial syndrome. They found that the patients were slightly insulin resistant but had normal or exaggerated insulin secretory capacity compared to an oral glucose load, whereas glucagon levels were robustly suppressed by a glucose load. The observed hormonal responses may result in the glycemic patterns and symptoms observed; further study is warranted to elucidate the mechanism.15
Dietary modification is the cornerstone treatment for postprandial syndrome, including reduced carbohydrate intake, increased protein and fiber intake, and more frequent and smaller meals. There is also evidence that a Mediterranean diet may be beneficial for managing hypoglycemic symptoms.16 Furthermore, α-glucosidase inhibitors, whose mechanism of action delays the digestion of carbohydrates, have demonstrated promise. This medication class has demonstrated significance in raising postprandial glucose levels and alleviating hypoglycemic symptoms in patients with true postprandial hypoglycemia.17
CONCLUSIONS
IPP is a benign diagnosis encompassing hypoglycemic symptoms without biochemical hypoglycemia. It is not a true hypoglycemic disorder. IPP is challenging to diagnose, given that it is an interpretation of exclusion, supported by symptom improvement with dietary changes (ie, reduced carbohydrate intake, increased protein and fiber intake, and more frequent and smaller meals). Supervised mixed meal testing or CGM can be used to assist with diagnosis. Even though CGM is undergoing further study in this patient population, it corroborated the diagnosis in the patient described in this case.
For hypoglycemic symptoms, physicians should first assess for evidence of Whipple triad to evaluate for true biochemical hypoglycemia. For true hypoglycemia (< 55 mg/dL), physicians may conduct an examination in conjunction with an endocrinologist. For normoglycemia (≥ 55 mg/dL), physicians should first exclude alternative etiologies (including cardiac and neurologic), and subsequently consider IPP.
Bansal N, Weinstock RS. Non-Diabetic Hypoglycemia. In: Feingold KR, Anawalt B, Blackman MR, et al, eds. Endotext. MDText.com, Inc.; 2000.
Service FJ. Hypoglycemic disorders. New Engl J Med. 1995;332(17):1144-1152.doi:10.1056/NEJM199504273321707
Charles MA, Hofeldt F, Shackelford A, et al. Comparison of oral glucose tolerance tests and mixed meals in patients with apparent idiopathic postabsorptive hypoglycemia: absence of hypoglycemia after meals. Diabetes. 1981;30(6):465-470.
Douillard C, Jannin A, Vantyghem MC. Rare causes of hypoglycemia in adults. Ann Endocrinol (Paris). 2020;81(2-3):110-117. doi:10.1016/j.ando.2020.04.003
Ekhlaspour L, Mondesir D, Lautsch N, et al. Comparative accuracy of 17 point-of-care glucose meters. J Diabetes Sci Technol. 2017;11(3):558-566. doi:10.1177/1932296816672237
Alitta Q, Grino M, Adjemout L, Langar A, Retornaz F, Oliver C. Overestimation of hypoglycemia diagnosis by FreeStyle Libre continuous glucose monitoring in long-term care home residents with diabetes. J Diabetes Sci Technol. 2018;12(3):727-728. doi:10.1177/1932296817747887
Mongraw-Chaffin M, Beavers DP, McClain DA. Hypoglycemic symptoms in the absence of diabetes: pilot evidence of clinical hypoglycemia in young women. J Clin Transl Endocrinol. 2019;18:100202. doi:10.1016/j.jcte.2019.100202
Shah VN, DuBose SN, Li Z, et al. Continuous glucose monitoring profiles in healthy nondiabetic participants: a multicenter prospective study. J Clin Endocrinol Metab. 2019;104(10):4356-4364. doi:10.1210/jc.2018-02763
Cryer PE, Axelrod L, Grossman AB, et al. Evaluation and management of adult hypoglycemic disorders: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2009;94(3):709-728. doi:10.1210/jc.2008-1410
Galati SJ, Rayfield EJ. Approach to the patient with postprandial hypoglycemia. Endocr Pract. 2014;20(4):331-340. doi:10.4158/EP13132.RA
Altuntas Y. Postprandial reactive hypoglycemia. Sisli Etfal Hastan Tip Bul. 2019;53(3):215-220.doi:10.14744/SEMB.2019.59455
HARRIS S. HYPERINSULINISM AND DYSINSULINISM. JAMA. 1924;83(10):729-733.doi:10.1001/jama.1924.02660100003002
Harris S. HYPERINSULINISM AND DYSINSULINISM (INSULOGENIC HYPOGLYCBMIA). Endocrinology. 1932;16(1):29-42. doi:10.1210/endo-16-1-29
Hall M, Walicka M, Panczyk M, Traczyk I. Metabolic parameters in patients with suspected reactive hypoglycemia. J Pers Med. 2021;11(4):276. doi:10.3390/jpm11040276
Kosuda M, Watanabe K, Koike M, et al. Glucagon response to glucose challenge in patients with idiopathic postprandial syndrome. J Nippon Med Sch. 2022;89(1):102-107. doi:10.1272/jnms.JNMS.2022_89-205
Hall M, Walicka M, Panczyk M, Traczyk I. Assessing long-term impact of dietary interventions on occurrence of symptoms consistent with hypoglycemia in patients without diabetes: a one-year follow-up study. Nutrients. 2022;14(3):497. doi:10.3390/nu14030497
Ozgen AG, Hamulu F, Bayraktar F, et al. Long-term treatment with acarbose for the treatment of reactive hypoglycemia. Eat Weight Disord. 1998;3(3):136-140. doi:10.1007/BF03340001
Idiopathic postprandial syndrome (IPP), initially termed reactive hypoglycemia, presents with hypoglycemic-like symptoms in the absence of biochemical hypoglycemia and remains a diagnosis of exclusion. Its pathophysiology is poorly understood. The diagnosis requires thorough evaluation of cardiac, metabolic, neurologic, and gastrointestinal causes, as well as Whipple triad criteria. Dietary modifications, including reduced carbohydrate intake, increased protein and fiber, and frequent small meals, remain the cornerstone of IPP management. Continuous glucose monitoring (CGM) may be a useful adjunct in correlating symptoms with glucose trends, but its role is still evolving.
In the evaluation of patients with symptoms suggestive of hypoglycemia (Figure 1), patients should first be assessed for Whipple triad: symptoms consistent with hypoglycemia, blood glucose level < 55 mg/dL, and reversal of symptoms with glucose.1 Patients who meet Whipple triad criteria should be investigated to identify further etiologies of hypoglycemia. They may include insulinoma, medication-induced (insulin, sulfonylurea, meglitinide, or β blocker use), postbariatric surgery complications, noninsulinoma pancreatogenous hypoglycemia syndrome, ackee fruit consumption, or familial conditions.2 The presence of hypoglycemic symptoms in the postprandial or fasting state can provide valuable insights into underlying etiology.
Patients who do not meet Whipple triad criteria, but exhibit postprandial symptoms consistent with hypoglycemia, as in this case, present a diagnostic dilemma. IPP is defined as hypoglycemic symptoms occuring after carbohydrate ingestion without biochemical hypoglycemia. Initially termed reactive hypoglycemia, it was renamed in 1981to reflect the absence of low blood glucose levels.3
The understanding of this diagnosis has not significantly progressed since the 1980s. Its prevalence, incidence, risk factors, and societal burden remain unclear. IPP is a challenging diagnosis due to nonspecific symptoms that overlap with a myriad of conditions. These symptoms may include adrenergic symptoms such as diaphoresis, tremulousness, palpitations, anxiety, and hunger. Potentially severe neuroglycopenic symptoms, including weakness, dizziness, behavior changes, confusion, and coma, are not typically observed.4 Given that objective criteria are not well established, IPP remains a diagnosis of exclusion. It is imperative to rule out alternative etiologies, particularly cardiac, gastrointestinal, and neurologic causes.
CASE PRESENTATION
A male aged 41 years presented to primary care for evaluation of acute on chronic symptomatic postprandial episodes. He reported a history of symptomatic sinus bradycardia in the setting of sick sinus syndrome following dual-chamber pacemaker placement, posttraumatic stress disorder, and gastroesophageal reflux disease. He was a retired Navy sailor without any known occupational exposures who worked in the real estate industry. The patient reported feeling lightheaded, tremulous, and anxious most afternoons after lunch for several years. He also reported that meals heavy in carbohydrates exacerbated his symptoms, whereas skipping meals or lying down alleviated his symptoms. The patient also reported concomitant arm numbness, shortness of breath, palpitations, and nausea during these episodes. Review of systems was otherwise negative, including no weight changes, fever, chills, night sweats, chest pain, or syncope.

The patient’s medications included ferrous sulfate 325 mg once every other day, bupropion 200 mg once daily, metoprolol succinate 25 mg once daily, and as-needed lorazepam 1 mg once daily. The patient reported no current substance use but reported previous tobacco use 3 years prior (maximum 1 pack/week) and alcohol use 5 years prior (750 ml/day for 15 years). The patient did not exercise and typically ate oatmeal for breakfast, a sandwich or salad for lunch, and taquitos or salad for dinner, with snacks throughout the day. Notable family history included a maternal grandmother with colon cancer. The patient’s vital signs included a 36.8 °C temperature, heart rate 87 beats/min, 118/71 mm Hg blood pressure, oxygen saturation 98% on room air, 125.2 kg weight, and 38.5 body mass index. There were no orthostatic vital sign changes. A physical examination demonstrated obesity with an unremarkable cardiopulmonary and volume examination.
Additional testing included Gallium-68 dototate positron emission tomography/computed tomography, brain magnetic resonance imaging, echocardiogram, electromyogram, exercise tolerance test, Holter monitoring, invasive cardiopulmonary exercise testing, pacemaker interrogation, pulmonary function testing, stress echocardiogram, tilt table test, and venogram computed tomography of the chest, but the results were unremarkable (Appendix). His afternoon nonfasting glucose level was 138 mg/dL with a concurrent hemoglobin A1c of 5.2%. The patient had a fasting C-peptide level of 3.7 ng/mL (reference range 0.5-2.0 ng/mL), fasting insulin level 19.1 mIU/L (reference range < 25 mIU/L), and a fasting glucose level of 93 mg/dL (reference range 70-99 mg/dL). The patient’s urine 5-HIAA, plasma metanephrines, urine metanephrines, insulin-like growth factor 1, prolactin, corticotropin, fasting cortisol, and thyrotropin yielded results within reference ranges (Table). The veteran was prescribed a CGM, which demonstrated normal glucose levels (≥ 55 mg/dL) during symptomatic episodes (Figure 2).

The patient was diagnosed with IPP given normoglycemia, exclusion of alternative diagnoses, and symptomatic improvement with dietary changes. He was referred to a nutritionist for a high-protein, high-fiber, and low-carbohydrate diet.
DISCUSSION
Seemingly simple diagnostic tools can lead to diagnostic pitfalls. Home glucose monitoring with the use of a standard glucometer during an episode is the typical first step in identifying hypoglycemia, as it is both pragmatic and accurate, with a mean absolute relative difference (MARD) of about 10% in hypoglycemic ranges.5 While the advent of CGM provides real-time data and can reveal clinically relevant fluctuations, it reveals mild hypoglycemia (54 to 70 mg/dL) of no clinical significance in a large proportion of individuals.
Additionally, CGM is less accurate than glucometers with a MARD of about 20% in hypoglycemia ranges.6 CGM technology, however, is rapidly evolving and undergoing further investigation for hypoglycemia detection. Therefore, CGM may be considered in select patients as prospective study results are established; the newest CGMs have MARDs very similar to fingerstick blood glucose data.7,8 In the patient described in this case, CGM helped corroborate the diagnosis, given that symptomatic episodes correlated with lower glucose levels. Provocative testing with oral glucose tolerance testing can frequently result in false positive hypoglycemic readings and is not recommended.9 Supervised mixed meal testing can also be used, which entails monitoring after consuming a mixed macronutrient meal. The test concludes after hypoglycemic symptoms develop or 5 hours elapse, whichever occurs first.1

The pathophysiology of IPP is poorly understood. Proposed mechanisms include increased insulin sensitivity, increased adrenergic sensitivity, impaired glucagon regulation, emotional distress, insulin resistance, and increased glucagon-like peptide-1 production.10-13 Research suggests this may occur as pancreatic β cells fail in early type 2 diabetes mellitus, with diminished first-phase insulin release leading to an initial exuberant rise in blood glucose, an overshooting of the second phase of insulin secretion, and the feeling of the postprandial blood glucose falling, even though the final glucose level achieved is not truly low.13 There are contradictory studies in the literature demonstrating no association between insulin resistance and hypoglycemic symptoms.14 In 2022, Kosuda and colleagues looked at homeostatic model assessment for insulin resistance in patients with postprandial syndrome. They found that the patients were slightly insulin resistant but had normal or exaggerated insulin secretory capacity compared to an oral glucose load, whereas glucagon levels were robustly suppressed by a glucose load. The observed hormonal responses may result in the glycemic patterns and symptoms observed; further study is warranted to elucidate the mechanism.15
Dietary modification is the cornerstone treatment for postprandial syndrome, including reduced carbohydrate intake, increased protein and fiber intake, and more frequent and smaller meals. There is also evidence that a Mediterranean diet may be beneficial for managing hypoglycemic symptoms.16 Furthermore, α-glucosidase inhibitors, whose mechanism of action delays the digestion of carbohydrates, have demonstrated promise. This medication class has demonstrated significance in raising postprandial glucose levels and alleviating hypoglycemic symptoms in patients with true postprandial hypoglycemia.17
CONCLUSIONS
IPP is a benign diagnosis encompassing hypoglycemic symptoms without biochemical hypoglycemia. It is not a true hypoglycemic disorder. IPP is challenging to diagnose, given that it is an interpretation of exclusion, supported by symptom improvement with dietary changes (ie, reduced carbohydrate intake, increased protein and fiber intake, and more frequent and smaller meals). Supervised mixed meal testing or CGM can be used to assist with diagnosis. Even though CGM is undergoing further study in this patient population, it corroborated the diagnosis in the patient described in this case.
For hypoglycemic symptoms, physicians should first assess for evidence of Whipple triad to evaluate for true biochemical hypoglycemia. For true hypoglycemia (< 55 mg/dL), physicians may conduct an examination in conjunction with an endocrinologist. For normoglycemia (≥ 55 mg/dL), physicians should first exclude alternative etiologies (including cardiac and neurologic), and subsequently consider IPP.

Idiopathic postprandial syndrome (IPP), initially termed reactive hypoglycemia, presents with hypoglycemic-like symptoms in the absence of biochemical hypoglycemia and remains a diagnosis of exclusion. Its pathophysiology is poorly understood. The diagnosis requires thorough evaluation of cardiac, metabolic, neurologic, and gastrointestinal causes, as well as Whipple triad criteria. Dietary modifications, including reduced carbohydrate intake, increased protein and fiber, and frequent small meals, remain the cornerstone of IPP management. Continuous glucose monitoring (CGM) may be a useful adjunct in correlating symptoms with glucose trends, but its role is still evolving.
In the evaluation of patients with symptoms suggestive of hypoglycemia (Figure 1), patients should first be assessed for Whipple triad: symptoms consistent with hypoglycemia, blood glucose level < 55 mg/dL, and reversal of symptoms with glucose.1 Patients who meet Whipple triad criteria should be investigated to identify further etiologies of hypoglycemia. They may include insulinoma, medication-induced (insulin, sulfonylurea, meglitinide, or β blocker use), postbariatric surgery complications, noninsulinoma pancreatogenous hypoglycemia syndrome, ackee fruit consumption, or familial conditions.2 The presence of hypoglycemic symptoms in the postprandial or fasting state can provide valuable insights into underlying etiology.
Patients who do not meet Whipple triad criteria, but exhibit postprandial symptoms consistent with hypoglycemia, as in this case, present a diagnostic dilemma. IPP is defined as hypoglycemic symptoms occuring after carbohydrate ingestion without biochemical hypoglycemia. Initially termed reactive hypoglycemia, it was renamed in 1981to reflect the absence of low blood glucose levels.3
The understanding of this diagnosis has not significantly progressed since the 1980s. Its prevalence, incidence, risk factors, and societal burden remain unclear. IPP is a challenging diagnosis due to nonspecific symptoms that overlap with a myriad of conditions. These symptoms may include adrenergic symptoms such as diaphoresis, tremulousness, palpitations, anxiety, and hunger. Potentially severe neuroglycopenic symptoms, including weakness, dizziness, behavior changes, confusion, and coma, are not typically observed.4 Given that objective criteria are not well established, IPP remains a diagnosis of exclusion. It is imperative to rule out alternative etiologies, particularly cardiac, gastrointestinal, and neurologic causes.
CASE PRESENTATION
A male aged 41 years presented to primary care for evaluation of acute on chronic symptomatic postprandial episodes. He reported a history of symptomatic sinus bradycardia in the setting of sick sinus syndrome following dual-chamber pacemaker placement, posttraumatic stress disorder, and gastroesophageal reflux disease. He was a retired Navy sailor without any known occupational exposures who worked in the real estate industry. The patient reported feeling lightheaded, tremulous, and anxious most afternoons after lunch for several years. He also reported that meals heavy in carbohydrates exacerbated his symptoms, whereas skipping meals or lying down alleviated his symptoms. The patient also reported concomitant arm numbness, shortness of breath, palpitations, and nausea during these episodes. Review of systems was otherwise negative, including no weight changes, fever, chills, night sweats, chest pain, or syncope.

The patient’s medications included ferrous sulfate 325 mg once every other day, bupropion 200 mg once daily, metoprolol succinate 25 mg once daily, and as-needed lorazepam 1 mg once daily. The patient reported no current substance use but reported previous tobacco use 3 years prior (maximum 1 pack/week) and alcohol use 5 years prior (750 ml/day for 15 years). The patient did not exercise and typically ate oatmeal for breakfast, a sandwich or salad for lunch, and taquitos or salad for dinner, with snacks throughout the day. Notable family history included a maternal grandmother with colon cancer. The patient’s vital signs included a 36.8 °C temperature, heart rate 87 beats/min, 118/71 mm Hg blood pressure, oxygen saturation 98% on room air, 125.2 kg weight, and 38.5 body mass index. There were no orthostatic vital sign changes. A physical examination demonstrated obesity with an unremarkable cardiopulmonary and volume examination.
Additional testing included Gallium-68 dototate positron emission tomography/computed tomography, brain magnetic resonance imaging, echocardiogram, electromyogram, exercise tolerance test, Holter monitoring, invasive cardiopulmonary exercise testing, pacemaker interrogation, pulmonary function testing, stress echocardiogram, tilt table test, and venogram computed tomography of the chest, but the results were unremarkable (Appendix). His afternoon nonfasting glucose level was 138 mg/dL with a concurrent hemoglobin A1c of 5.2%. The patient had a fasting C-peptide level of 3.7 ng/mL (reference range 0.5-2.0 ng/mL), fasting insulin level 19.1 mIU/L (reference range < 25 mIU/L), and a fasting glucose level of 93 mg/dL (reference range 70-99 mg/dL). The patient’s urine 5-HIAA, plasma metanephrines, urine metanephrines, insulin-like growth factor 1, prolactin, corticotropin, fasting cortisol, and thyrotropin yielded results within reference ranges (Table). The veteran was prescribed a CGM, which demonstrated normal glucose levels (≥ 55 mg/dL) during symptomatic episodes (Figure 2).

The patient was diagnosed with IPP given normoglycemia, exclusion of alternative diagnoses, and symptomatic improvement with dietary changes. He was referred to a nutritionist for a high-protein, high-fiber, and low-carbohydrate diet.
DISCUSSION
Seemingly simple diagnostic tools can lead to diagnostic pitfalls. Home glucose monitoring with the use of a standard glucometer during an episode is the typical first step in identifying hypoglycemia, as it is both pragmatic and accurate, with a mean absolute relative difference (MARD) of about 10% in hypoglycemic ranges.5 While the advent of CGM provides real-time data and can reveal clinically relevant fluctuations, it reveals mild hypoglycemia (54 to 70 mg/dL) of no clinical significance in a large proportion of individuals.
Additionally, CGM is less accurate than glucometers with a MARD of about 20% in hypoglycemia ranges.6 CGM technology, however, is rapidly evolving and undergoing further investigation for hypoglycemia detection. Therefore, CGM may be considered in select patients as prospective study results are established; the newest CGMs have MARDs very similar to fingerstick blood glucose data.7,8 In the patient described in this case, CGM helped corroborate the diagnosis, given that symptomatic episodes correlated with lower glucose levels. Provocative testing with oral glucose tolerance testing can frequently result in false positive hypoglycemic readings and is not recommended.9 Supervised mixed meal testing can also be used, which entails monitoring after consuming a mixed macronutrient meal. The test concludes after hypoglycemic symptoms develop or 5 hours elapse, whichever occurs first.1

The pathophysiology of IPP is poorly understood. Proposed mechanisms include increased insulin sensitivity, increased adrenergic sensitivity, impaired glucagon regulation, emotional distress, insulin resistance, and increased glucagon-like peptide-1 production.10-13 Research suggests this may occur as pancreatic β cells fail in early type 2 diabetes mellitus, with diminished first-phase insulin release leading to an initial exuberant rise in blood glucose, an overshooting of the second phase of insulin secretion, and the feeling of the postprandial blood glucose falling, even though the final glucose level achieved is not truly low.13 There are contradictory studies in the literature demonstrating no association between insulin resistance and hypoglycemic symptoms.14 In 2022, Kosuda and colleagues looked at homeostatic model assessment for insulin resistance in patients with postprandial syndrome. They found that the patients were slightly insulin resistant but had normal or exaggerated insulin secretory capacity compared to an oral glucose load, whereas glucagon levels were robustly suppressed by a glucose load. The observed hormonal responses may result in the glycemic patterns and symptoms observed; further study is warranted to elucidate the mechanism.15
Dietary modification is the cornerstone treatment for postprandial syndrome, including reduced carbohydrate intake, increased protein and fiber intake, and more frequent and smaller meals. There is also evidence that a Mediterranean diet may be beneficial for managing hypoglycemic symptoms.16 Furthermore, α-glucosidase inhibitors, whose mechanism of action delays the digestion of carbohydrates, have demonstrated promise. This medication class has demonstrated significance in raising postprandial glucose levels and alleviating hypoglycemic symptoms in patients with true postprandial hypoglycemia.17
CONCLUSIONS
IPP is a benign diagnosis encompassing hypoglycemic symptoms without biochemical hypoglycemia. It is not a true hypoglycemic disorder. IPP is challenging to diagnose, given that it is an interpretation of exclusion, supported by symptom improvement with dietary changes (ie, reduced carbohydrate intake, increased protein and fiber intake, and more frequent and smaller meals). Supervised mixed meal testing or CGM can be used to assist with diagnosis. Even though CGM is undergoing further study in this patient population, it corroborated the diagnosis in the patient described in this case.
For hypoglycemic symptoms, physicians should first assess for evidence of Whipple triad to evaluate for true biochemical hypoglycemia. For true hypoglycemia (< 55 mg/dL), physicians may conduct an examination in conjunction with an endocrinologist. For normoglycemia (≥ 55 mg/dL), physicians should first exclude alternative etiologies (including cardiac and neurologic), and subsequently consider IPP.

Bansal N, Weinstock RS. Non-Diabetic Hypoglycemia. In: Feingold KR, Anawalt B, Blackman MR, et al, eds. Endotext. MDText.com, Inc.; 2000.
Service FJ. Hypoglycemic disorders. New Engl J Med. 1995;332(17):1144-1152.doi:10.1056/NEJM199504273321707
Charles MA, Hofeldt F, Shackelford A, et al. Comparison of oral glucose tolerance tests and mixed meals in patients with apparent idiopathic postabsorptive hypoglycemia: absence of hypoglycemia after meals. Diabetes. 1981;30(6):465-470.
Douillard C, Jannin A, Vantyghem MC. Rare causes of hypoglycemia in adults. Ann Endocrinol (Paris). 2020;81(2-3):110-117. doi:10.1016/j.ando.2020.04.003
Ekhlaspour L, Mondesir D, Lautsch N, et al. Comparative accuracy of 17 point-of-care glucose meters. J Diabetes Sci Technol. 2017;11(3):558-566. doi:10.1177/1932296816672237
Alitta Q, Grino M, Adjemout L, Langar A, Retornaz F, Oliver C. Overestimation of hypoglycemia diagnosis by FreeStyle Libre continuous glucose monitoring in long-term care home residents with diabetes. J Diabetes Sci Technol. 2018;12(3):727-728. doi:10.1177/1932296817747887
Mongraw-Chaffin M, Beavers DP, McClain DA. Hypoglycemic symptoms in the absence of diabetes: pilot evidence of clinical hypoglycemia in young women. J Clin Transl Endocrinol. 2019;18:100202. doi:10.1016/j.jcte.2019.100202
Shah VN, DuBose SN, Li Z, et al. Continuous glucose monitoring profiles in healthy nondiabetic participants: a multicenter prospective study. J Clin Endocrinol Metab. 2019;104(10):4356-4364. doi:10.1210/jc.2018-02763
Cryer PE, Axelrod L, Grossman AB, et al. Evaluation and management of adult hypoglycemic disorders: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2009;94(3):709-728. doi:10.1210/jc.2008-1410
Galati SJ, Rayfield EJ. Approach to the patient with postprandial hypoglycemia. Endocr Pract. 2014;20(4):331-340. doi:10.4158/EP13132.RA
Altuntas Y. Postprandial reactive hypoglycemia. Sisli Etfal Hastan Tip Bul. 2019;53(3):215-220.doi:10.14744/SEMB.2019.59455
HARRIS S. HYPERINSULINISM AND DYSINSULINISM. JAMA. 1924;83(10):729-733.doi:10.1001/jama.1924.02660100003002
Harris S. HYPERINSULINISM AND DYSINSULINISM (INSULOGENIC HYPOGLYCBMIA). Endocrinology. 1932;16(1):29-42. doi:10.1210/endo-16-1-29
Hall M, Walicka M, Panczyk M, Traczyk I. Metabolic parameters in patients with suspected reactive hypoglycemia. J Pers Med. 2021;11(4):276. doi:10.3390/jpm11040276
Kosuda M, Watanabe K, Koike M, et al. Glucagon response to glucose challenge in patients with idiopathic postprandial syndrome. J Nippon Med Sch. 2022;89(1):102-107. doi:10.1272/jnms.JNMS.2022_89-205
Hall M, Walicka M, Panczyk M, Traczyk I. Assessing long-term impact of dietary interventions on occurrence of symptoms consistent with hypoglycemia in patients without diabetes: a one-year follow-up study. Nutrients. 2022;14(3):497. doi:10.3390/nu14030497
Ozgen AG, Hamulu F, Bayraktar F, et al. Long-term treatment with acarbose for the treatment of reactive hypoglycemia. Eat Weight Disord. 1998;3(3):136-140. doi:10.1007/BF03340001
Bansal N, Weinstock RS. Non-Diabetic Hypoglycemia. In: Feingold KR, Anawalt B, Blackman MR, et al, eds. Endotext. MDText.com, Inc.; 2000.
Service FJ. Hypoglycemic disorders. New Engl J Med. 1995;332(17):1144-1152.doi:10.1056/NEJM199504273321707
Charles MA, Hofeldt F, Shackelford A, et al. Comparison of oral glucose tolerance tests and mixed meals in patients with apparent idiopathic postabsorptive hypoglycemia: absence of hypoglycemia after meals. Diabetes. 1981;30(6):465-470.
Douillard C, Jannin A, Vantyghem MC. Rare causes of hypoglycemia in adults. Ann Endocrinol (Paris). 2020;81(2-3):110-117. doi:10.1016/j.ando.2020.04.003
Ekhlaspour L, Mondesir D, Lautsch N, et al. Comparative accuracy of 17 point-of-care glucose meters. J Diabetes Sci Technol. 2017;11(3):558-566. doi:10.1177/1932296816672237
Alitta Q, Grino M, Adjemout L, Langar A, Retornaz F, Oliver C. Overestimation of hypoglycemia diagnosis by FreeStyle Libre continuous glucose monitoring in long-term care home residents with diabetes. J Diabetes Sci Technol. 2018;12(3):727-728. doi:10.1177/1932296817747887
Mongraw-Chaffin M, Beavers DP, McClain DA. Hypoglycemic symptoms in the absence of diabetes: pilot evidence of clinical hypoglycemia in young women. J Clin Transl Endocrinol. 2019;18:100202. doi:10.1016/j.jcte.2019.100202
Shah VN, DuBose SN, Li Z, et al. Continuous glucose monitoring profiles in healthy nondiabetic participants: a multicenter prospective study. J Clin Endocrinol Metab. 2019;104(10):4356-4364. doi:10.1210/jc.2018-02763
Cryer PE, Axelrod L, Grossman AB, et al. Evaluation and management of adult hypoglycemic disorders: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2009;94(3):709-728. doi:10.1210/jc.2008-1410
Galati SJ, Rayfield EJ. Approach to the patient with postprandial hypoglycemia. Endocr Pract. 2014;20(4):331-340. doi:10.4158/EP13132.RA
Altuntas Y. Postprandial reactive hypoglycemia. Sisli Etfal Hastan Tip Bul. 2019;53(3):215-220.doi:10.14744/SEMB.2019.59455
HARRIS S. HYPERINSULINISM AND DYSINSULINISM. JAMA. 1924;83(10):729-733.doi:10.1001/jama.1924.02660100003002
Harris S. HYPERINSULINISM AND DYSINSULINISM (INSULOGENIC HYPOGLYCBMIA). Endocrinology. 1932;16(1):29-42. doi:10.1210/endo-16-1-29
Hall M, Walicka M, Panczyk M, Traczyk I. Metabolic parameters in patients with suspected reactive hypoglycemia. J Pers Med. 2021;11(4):276. doi:10.3390/jpm11040276
Kosuda M, Watanabe K, Koike M, et al. Glucagon response to glucose challenge in patients with idiopathic postprandial syndrome. J Nippon Med Sch. 2022;89(1):102-107. doi:10.1272/jnms.JNMS.2022_89-205
Hall M, Walicka M, Panczyk M, Traczyk I. Assessing long-term impact of dietary interventions on occurrence of symptoms consistent with hypoglycemia in patients without diabetes: a one-year follow-up study. Nutrients. 2022;14(3):497. doi:10.3390/nu14030497
Ozgen AG, Hamulu F, Bayraktar F, et al. Long-term treatment with acarbose for the treatment of reactive hypoglycemia. Eat Weight Disord. 1998;3(3):136-140. doi:10.1007/BF03340001
A Veteran Presenting With Symptomatic Postprandial Episodes
A Veteran Presenting With Symptomatic Postprandial Episodes
Pain, Anxiety, and Dementia: A Catastrophic Outcome
Advanced psychiatric illness and dementia create a wide range of barriers to health care. These patients are unable to provide reliable details with respect to their illness or even discuss basic features of their medical history, forcing providers to rely on contributions from caregiver reports and medical records. Confounding the limits on medical information, physical examinations are often abbreviated or completely refused because of the patient’s distrust, discomfort, or delusion. Over time, the involvement of consulting services may amplify the impact of these barriers as the need for diagnostic and therapeutic interventions emerge. Meanwhile, this delay in definitive management opens a window of risk for deterioration, in which patients cannot be relied on to report important clinical changes.
This case report describes a patient with significant cognitive dysfunction who developed a rare and devastating complication of a hematologic disorder. As the case illustrates, transferring a patient from the psychiatric ward to Internal Medicine (IM) can create unique diagnostic and management challenges.
CASE REPORT
A 64-year-old man developed hematochezia after having been hospitalized in a locked psychiatric ward for the preceding 6 months following a suicide attempt. The episode of hematochezia occurred while on anticoagulation treatment with warfarin for chronic lower extremity deep venous thrombosis (DVT), which prompted the IM consultation. The patient’s past medical history was notable for dementia, hypothyroidism, Crohn disease, and primary sclerosing cholangitis.
The IM Consult Service recommended holding anticoagulation therapy and reversing the coagulopathy with vitamin K. The patient’s stool returned hemoccult and toxin positive for Clostridium difficile (C difficile). The hematochezia was attributed to the infection with C difficile in the setting of anticoagulation. Oral metronidazole was started. Hemoglobin remained stable without further episodes of bleeding. Seven days after the episode of hematochezia, the patient experienced worsening generalized pain and new skin findings. He was transferred to the general medical ward for further management.
The patient’s medical records revealed early cognitive decline with recommendations for supervised residential care as early as age 59 years. An extensive neurocognitive assessment indicated a diagnosis of semantic dementia. He also had a history of recurrent DVT with anticoagulation therapy for > 10 years with no prior workup for a hypercoagulable state. A recent baseline mental status report described a childlike demeanor, profound global speech deficits with marked difficulty understanding even basic medical concepts (eg, the need for a peripheral intravenous catheter), and generalized anxiety disorder complicated by hyperesthesia. The patient frequently refused physical examinations and blood draws as a result. He devoted himself to simple puzzles of kittens and puppies.
Vital signs were normal as were the head and neck, pulmonary, cardiac, and abdominal examinations. The patient’s neurocognitive examination was remarkable for his dependence on instrumental activities of daily living, global aphasia, impaired short- and long-term recall, and poor judgment. He scored 21 out of 30 on a recent mini-mental state examination: failure to achieve 3-word recall; disorientation to month, season, hospital, and county; and an inability to write a sentence or identify a pen. Otherwise, he had fluent speech, facial symmetry, intact strength and sensation throughout, and normal reflexes.
A skin examination revealed diffuse tender subcutaneous lesions. The largest lesion was about 5 cm, located in the left anterolateral thigh. Smaller lesions of about 1 cm were noted in the abdominal wall, right thigh, and bilateral upper extremities. An exquisitely tender, well-demarcated 20-cm elliptical lesion with central necrosis and an erythematous border developed in the left axilla the following day (Figure 1A). Pain limited adduction of the left arm.
The initial laboratory evaluation demonstrated a stable hemoglobin level of 11.1 g/dL, a platelet count of 128 k/mL, and no leukocytosis. Electrolytes and renal indexes were normal. D-dimer and fibrin split products were > 10,000 ng/mL and 20 mg/mL, respectively. Fibrinogen level was 351 mg/dL. The prothrombin time and international normalized ratio were 15.1 seconds and 1.4, respectively. The activated partial thromboplastin time (aPTT) was measured at 40 seconds. High sensitivity C-reactive protein was 4.59. Recent head imaging included a brain magnetic resonance imaging (MRI) notable for enlarged sulci and ventricles with temporal predominance. Positron emission tomography (PET) brain imaging was significant for diffuse hypometabolism in bilateral parietal and temporal lobes with preservation of sensorimotor and occipital cortexes. There was no clear radiographic evidence of cerebral embolic phenomenon or focal cerebrovascular events.
Enoxaparin treatment was initiated for a suspected hypercoagulable state. Ceftriaxone was administered for a urinary tract infection (UTI). Despite premedication, the bedside biopsy of his necrotic skin lesion was aborted due to severe anxiety and generalized somatic pain. A surgical excisional biopsy was thus obtained under general anesthesia. Enoxaparin was held the night before and the morning of surgery. There were no immediate complications related to the biopsy, and malignancy was not seen on intraoperative frozen sections.
Generalized somatic pain persisted the morning after the surgical biopsy, but the patient remained clinically unchanged. An hour later, he was found unresponsive with no pulse. Despite extensive resuscitative efforts, the patient died.
Postmortem
There was a high index of suspicion for a hemostatic perturbation given the skin findings and recent manipulation of anticoagulation with a prior thrombotic event. The axillary lesion closely resembled warfarin-related skin necrosis. Management included enoxaparin with supportive care, pending definitive pathologic findings.
Postmortem examination confirmed diffuse multiorgan involvement similar to the process seen in the thigh biopsy. Ischemic injury secondary to small vessel microthrombi were evident in the skin, subcutaneous fat, large bowel, urinary bladder, and associated pericystic fat (Figure 1B). Interpretation of the surgical thigh biopsy became available after the patient died. It demonstrated infarcted fat with fat necrosis and hemorrhage (Figure 2).
The results of the laboratory investigations for thrombophilia also came back after the patient died. A potent lupus anticoagulant (LA) was demonstrated. It manifested primarily in the intrinsic pathway as a strongly positive LA-sensitive-aPTT (delta time = 20.5 seconds) assay with a weakly positive dilute Russell’s viper venom time assay. The antigenic specificity of the LA antibodies was not uncovered, as the plasma levels of both IgM and IgG anticardiolipin and anti-Β2-glycoprotein-I antibodies were within the reference range. Factor (F) II and FV genotyping revealed wild-type FV, and the prothrombin gene G20210A was without mutation.
Assays for plasma levels of protein S and antithrombin activity were also normal, which excluded deficiencies in these proteins. The assay for protein C activity was slightly decreased. This may have exacerbated the hemostatic imbalance caused by the LA, as the FVII level had normalized. However, the etiology of the protein C deficiency is not clear. Considerations include (1) a warfarin disequilibrium state due to the discontinuation of oral anticoagulation and institution of vitamin K therapy; (2) an epiphenomenon resulting from active thromboses; or (3) a possible hereditary protein C deficiency.
The definitive diagnosis of the catastrophic antiphospholipid antibody (APA) syndrome relies on multiorgan failure in < 1 week, histopathologic evidence of small vessel thrombosis, and a positive LA.1 The study patient fulfilled these criteria (Table).
DISCUSSION
Catastrophic progression of APA syndrome is an infrequent and devastating complication of this autoimmune disorder with a mortality rate of nearly 50%.1 Antiphospholipid antibody syndrome typically presents with thromboses of the larger vessels, and it more commonly affects the venous system. In contrast, diffuse small vessel thromboses underlie the pathogenesis of catastrophic APA syndrome (CAPS).2 This catastrophic progression occurs in < 1 out of 100 patients with the APA syndrome, more frequently in women (69%), and over an age range of 7 decades (mean 38 years).2 A case series analysis identified older age (aged > 36 years), history of systemic lupus erythematous, and broader organ involvement as prognostic indicators of a poor outcome. Better outcomes are associated with thrombocytopenia and anticoagulation treatment. However, gender did not influence mortality.3
Prevention is key to APA management, given the lack of efficacious treatment.2 Preventive measures are focused on avoiding triggers and aggressively treating those triggers that may arise. Possible triggers in this case included cessation of anticoagulation due to hematochezia and in anticipation of surgery, infection (C difficile colitis, suspected necrotic skin wound super infection, and a UTI), and biopsy-related trauma.
Initial clinical stability in this patient with abrupt decompensation along with pending laboratory and pathology results limited the opportunity for more aggressive therapeutic intervention for CAPS. Moreover, the relative sparing of the cardiopulmonary and renal systems contrasted with the more classical systemic involvement usually seen in CAPS. Second-line therapies for CAPS include plasma exchange and high-dose steroids.2 Third-line therapeutics include immunosuppressive agents, such as cyclophosphamide.2
The rapid decompensation, described on postoperative day 1, after a low-risk surgical biopsy highlights the importance of perioperative care in patients with this autoimmune condition. Following a review of surgical cases, Erkan and colleagues concluded that standard antithrombotic regimens for general and orthopedic surgery are likely to undertreat patients with APA syndrome.4 They recommend the following guidelines in place of standard antithrombotic management: preoperative platelet count > 100 k/µL, higher threshold before proceeding with surgery/interventional procedures, limiting intravascular manipulations, and minimizing periods without anticoagulation therapy.4
A case report of a 31-year-old female undergoing mitral valve replacement complicated postoperatively by CAPS-associated biventricular failure, despite preoperative transition of warfarin to unfractionated heparin, illustrates this significant perioperative risk.5 Evidence-based guidelines recommend holding enoxaparin 24 hours before surgery and 24 hours after invasive procedures in patients requiring bridging anticoagulation therapy.6
Treatment of the patient in this case was complicated by his cognitive impairment. Dementia is a less common but well-documented consequence of APA syndrome. A case review of 28 patients with the APA syndrome and dementia suggests an early onset of cognitive decline with a mean age of 49 years. There may be no clear preceding history of stroke in > 50% of patients.7 Interestingly, dementia followed initial manifestations of disease by an average of 3.5 years, even in some patients receiving anticoagulation therapy.7
A nuclear medicine study of 22 patients with APA syndrome and mild neuropsychiatric symptoms demonstrated a 73% incidence of cerebral hypoperfusion (55% diffuse and 18% local) based on PET imaging despite unremarkable MRI findings.8 Extended periods of hypoperfusion secondary to arterial thromboses in the temporal and parietal lobes may have been the primary etiology for dementia in this case. As such, the coexistence of neurologic abnormalities and a hypercoagulability state warrants a thorough diagnostic workup for similar disorders, despite the higher prevalence of dementia in advanced age.
Unfortunately, this patient’s cognitive disorder prevented a timely and less invasive bedside biopsy and required a surgical biopsy for which anticoagulation therapy was interrupted. A less invasive biopsy and timelier laboratory findings may have avoided triggers, including trauma from the surgical biopsy and interruptions in anticoagulation therapy, which may have contributed to the onset of CAPS.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Asherson RA, Cervera R, Piette J, et al. Catastrophic antiphospholipid syndrome: Clues to the pathogenesis from a series of 80 patients. Medicine (Baltimore). 2001;80(6):355-377.
2. Cervera R, Asherson RA. Multiorgan failure due to rapid occlusive vascular disease in antiphospholipid syndrome: The ‘catastrophic’ antiphospholipid syndrome. APLAR J Rheumatol. 2004;7(3):254-262.
3. Bayraktar UD, Erkan D, Bucciarelli S, Epinosa G, Asherson R; Catastrophic Antiphospholipid Syndrome Project Group. The clinical spectrum of catastrophic antiphospholipid syndrome in the absence and presence of lupus. J Rheumatol. 2007;34(2):346-352.
4. Erkan D, Leibowitz E, Berman J, Lockshin MD. Perioperative medical management of antiphospholipid syndrome: Hospital for special surgery experience, review of literature, and recommendations. J Rheumatol. 2002;29(4):843-849.
5. Dornan RIP. Acute postoperative biventricular failure associated with antiphospholipid antibody syndrome. Br J Anaesth. 2004;92(5):748-754.
6. Douketis JD, Berger PD, Dunn AS, et al; American College of Chest Physicians. The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(suppl 6):S299-S339.
7. Gómez-Puerta JA, Cervera R, Calvo LM, et al. Dementia associated with the antiphospolipid syndrome: Clinical and radiological characteristics of 30 patients. Rheumatology (Oxford). 2005;44(1):95-99.
8. Kao C-H, Lan J-L, Hsieh J-F, Ho Y-J, ChangLai S-P, Lee J-K, et al. Evaluation of regional cerebral blood flow with 99mTc-HMPAO in primary antiphospholipid antibody syndrome. J Nucl Med. 1999;40:1446-1450.
Advanced psychiatric illness and dementia create a wide range of barriers to health care. These patients are unable to provide reliable details with respect to their illness or even discuss basic features of their medical history, forcing providers to rely on contributions from caregiver reports and medical records. Confounding the limits on medical information, physical examinations are often abbreviated or completely refused because of the patient’s distrust, discomfort, or delusion. Over time, the involvement of consulting services may amplify the impact of these barriers as the need for diagnostic and therapeutic interventions emerge. Meanwhile, this delay in definitive management opens a window of risk for deterioration, in which patients cannot be relied on to report important clinical changes.
This case report describes a patient with significant cognitive dysfunction who developed a rare and devastating complication of a hematologic disorder. As the case illustrates, transferring a patient from the psychiatric ward to Internal Medicine (IM) can create unique diagnostic and management challenges.
CASE REPORT
A 64-year-old man developed hematochezia after having been hospitalized in a locked psychiatric ward for the preceding 6 months following a suicide attempt. The episode of hematochezia occurred while on anticoagulation treatment with warfarin for chronic lower extremity deep venous thrombosis (DVT), which prompted the IM consultation. The patient’s past medical history was notable for dementia, hypothyroidism, Crohn disease, and primary sclerosing cholangitis.
The IM Consult Service recommended holding anticoagulation therapy and reversing the coagulopathy with vitamin K. The patient’s stool returned hemoccult and toxin positive for Clostridium difficile (C difficile). The hematochezia was attributed to the infection with C difficile in the setting of anticoagulation. Oral metronidazole was started. Hemoglobin remained stable without further episodes of bleeding. Seven days after the episode of hematochezia, the patient experienced worsening generalized pain and new skin findings. He was transferred to the general medical ward for further management.
The patient’s medical records revealed early cognitive decline with recommendations for supervised residential care as early as age 59 years. An extensive neurocognitive assessment indicated a diagnosis of semantic dementia. He also had a history of recurrent DVT with anticoagulation therapy for > 10 years with no prior workup for a hypercoagulable state. A recent baseline mental status report described a childlike demeanor, profound global speech deficits with marked difficulty understanding even basic medical concepts (eg, the need for a peripheral intravenous catheter), and generalized anxiety disorder complicated by hyperesthesia. The patient frequently refused physical examinations and blood draws as a result. He devoted himself to simple puzzles of kittens and puppies.
Vital signs were normal as were the head and neck, pulmonary, cardiac, and abdominal examinations. The patient’s neurocognitive examination was remarkable for his dependence on instrumental activities of daily living, global aphasia, impaired short- and long-term recall, and poor judgment. He scored 21 out of 30 on a recent mini-mental state examination: failure to achieve 3-word recall; disorientation to month, season, hospital, and county; and an inability to write a sentence or identify a pen. Otherwise, he had fluent speech, facial symmetry, intact strength and sensation throughout, and normal reflexes.
A skin examination revealed diffuse tender subcutaneous lesions. The largest lesion was about 5 cm, located in the left anterolateral thigh. Smaller lesions of about 1 cm were noted in the abdominal wall, right thigh, and bilateral upper extremities. An exquisitely tender, well-demarcated 20-cm elliptical lesion with central necrosis and an erythematous border developed in the left axilla the following day (Figure 1A). Pain limited adduction of the left arm.
The initial laboratory evaluation demonstrated a stable hemoglobin level of 11.1 g/dL, a platelet count of 128 k/mL, and no leukocytosis. Electrolytes and renal indexes were normal. D-dimer and fibrin split products were > 10,000 ng/mL and 20 mg/mL, respectively. Fibrinogen level was 351 mg/dL. The prothrombin time and international normalized ratio were 15.1 seconds and 1.4, respectively. The activated partial thromboplastin time (aPTT) was measured at 40 seconds. High sensitivity C-reactive protein was 4.59. Recent head imaging included a brain magnetic resonance imaging (MRI) notable for enlarged sulci and ventricles with temporal predominance. Positron emission tomography (PET) brain imaging was significant for diffuse hypometabolism in bilateral parietal and temporal lobes with preservation of sensorimotor and occipital cortexes. There was no clear radiographic evidence of cerebral embolic phenomenon or focal cerebrovascular events.
Enoxaparin treatment was initiated for a suspected hypercoagulable state. Ceftriaxone was administered for a urinary tract infection (UTI). Despite premedication, the bedside biopsy of his necrotic skin lesion was aborted due to severe anxiety and generalized somatic pain. A surgical excisional biopsy was thus obtained under general anesthesia. Enoxaparin was held the night before and the morning of surgery. There were no immediate complications related to the biopsy, and malignancy was not seen on intraoperative frozen sections.
Generalized somatic pain persisted the morning after the surgical biopsy, but the patient remained clinically unchanged. An hour later, he was found unresponsive with no pulse. Despite extensive resuscitative efforts, the patient died.
Postmortem
There was a high index of suspicion for a hemostatic perturbation given the skin findings and recent manipulation of anticoagulation with a prior thrombotic event. The axillary lesion closely resembled warfarin-related skin necrosis. Management included enoxaparin with supportive care, pending definitive pathologic findings.
Postmortem examination confirmed diffuse multiorgan involvement similar to the process seen in the thigh biopsy. Ischemic injury secondary to small vessel microthrombi were evident in the skin, subcutaneous fat, large bowel, urinary bladder, and associated pericystic fat (Figure 1B). Interpretation of the surgical thigh biopsy became available after the patient died. It demonstrated infarcted fat with fat necrosis and hemorrhage (Figure 2).
The results of the laboratory investigations for thrombophilia also came back after the patient died. A potent lupus anticoagulant (LA) was demonstrated. It manifested primarily in the intrinsic pathway as a strongly positive LA-sensitive-aPTT (delta time = 20.5 seconds) assay with a weakly positive dilute Russell’s viper venom time assay. The antigenic specificity of the LA antibodies was not uncovered, as the plasma levels of both IgM and IgG anticardiolipin and anti-Β2-glycoprotein-I antibodies were within the reference range. Factor (F) II and FV genotyping revealed wild-type FV, and the prothrombin gene G20210A was without mutation.
Assays for plasma levels of protein S and antithrombin activity were also normal, which excluded deficiencies in these proteins. The assay for protein C activity was slightly decreased. This may have exacerbated the hemostatic imbalance caused by the LA, as the FVII level had normalized. However, the etiology of the protein C deficiency is not clear. Considerations include (1) a warfarin disequilibrium state due to the discontinuation of oral anticoagulation and institution of vitamin K therapy; (2) an epiphenomenon resulting from active thromboses; or (3) a possible hereditary protein C deficiency.
The definitive diagnosis of the catastrophic antiphospholipid antibody (APA) syndrome relies on multiorgan failure in < 1 week, histopathologic evidence of small vessel thrombosis, and a positive LA.1 The study patient fulfilled these criteria (Table).
DISCUSSION
Catastrophic progression of APA syndrome is an infrequent and devastating complication of this autoimmune disorder with a mortality rate of nearly 50%.1 Antiphospholipid antibody syndrome typically presents with thromboses of the larger vessels, and it more commonly affects the venous system. In contrast, diffuse small vessel thromboses underlie the pathogenesis of catastrophic APA syndrome (CAPS).2 This catastrophic progression occurs in < 1 out of 100 patients with the APA syndrome, more frequently in women (69%), and over an age range of 7 decades (mean 38 years).2 A case series analysis identified older age (aged > 36 years), history of systemic lupus erythematous, and broader organ involvement as prognostic indicators of a poor outcome. Better outcomes are associated with thrombocytopenia and anticoagulation treatment. However, gender did not influence mortality.3
Prevention is key to APA management, given the lack of efficacious treatment.2 Preventive measures are focused on avoiding triggers and aggressively treating those triggers that may arise. Possible triggers in this case included cessation of anticoagulation due to hematochezia and in anticipation of surgery, infection (C difficile colitis, suspected necrotic skin wound super infection, and a UTI), and biopsy-related trauma.
Initial clinical stability in this patient with abrupt decompensation along with pending laboratory and pathology results limited the opportunity for more aggressive therapeutic intervention for CAPS. Moreover, the relative sparing of the cardiopulmonary and renal systems contrasted with the more classical systemic involvement usually seen in CAPS. Second-line therapies for CAPS include plasma exchange and high-dose steroids.2 Third-line therapeutics include immunosuppressive agents, such as cyclophosphamide.2
The rapid decompensation, described on postoperative day 1, after a low-risk surgical biopsy highlights the importance of perioperative care in patients with this autoimmune condition. Following a review of surgical cases, Erkan and colleagues concluded that standard antithrombotic regimens for general and orthopedic surgery are likely to undertreat patients with APA syndrome.4 They recommend the following guidelines in place of standard antithrombotic management: preoperative platelet count > 100 k/µL, higher threshold before proceeding with surgery/interventional procedures, limiting intravascular manipulations, and minimizing periods without anticoagulation therapy.4
A case report of a 31-year-old female undergoing mitral valve replacement complicated postoperatively by CAPS-associated biventricular failure, despite preoperative transition of warfarin to unfractionated heparin, illustrates this significant perioperative risk.5 Evidence-based guidelines recommend holding enoxaparin 24 hours before surgery and 24 hours after invasive procedures in patients requiring bridging anticoagulation therapy.6
Treatment of the patient in this case was complicated by his cognitive impairment. Dementia is a less common but well-documented consequence of APA syndrome. A case review of 28 patients with the APA syndrome and dementia suggests an early onset of cognitive decline with a mean age of 49 years. There may be no clear preceding history of stroke in > 50% of patients.7 Interestingly, dementia followed initial manifestations of disease by an average of 3.5 years, even in some patients receiving anticoagulation therapy.7
A nuclear medicine study of 22 patients with APA syndrome and mild neuropsychiatric symptoms demonstrated a 73% incidence of cerebral hypoperfusion (55% diffuse and 18% local) based on PET imaging despite unremarkable MRI findings.8 Extended periods of hypoperfusion secondary to arterial thromboses in the temporal and parietal lobes may have been the primary etiology for dementia in this case. As such, the coexistence of neurologic abnormalities and a hypercoagulability state warrants a thorough diagnostic workup for similar disorders, despite the higher prevalence of dementia in advanced age.
Unfortunately, this patient’s cognitive disorder prevented a timely and less invasive bedside biopsy and required a surgical biopsy for which anticoagulation therapy was interrupted. A less invasive biopsy and timelier laboratory findings may have avoided triggers, including trauma from the surgical biopsy and interruptions in anticoagulation therapy, which may have contributed to the onset of CAPS.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Advanced psychiatric illness and dementia create a wide range of barriers to health care. These patients are unable to provide reliable details with respect to their illness or even discuss basic features of their medical history, forcing providers to rely on contributions from caregiver reports and medical records. Confounding the limits on medical information, physical examinations are often abbreviated or completely refused because of the patient’s distrust, discomfort, or delusion. Over time, the involvement of consulting services may amplify the impact of these barriers as the need for diagnostic and therapeutic interventions emerge. Meanwhile, this delay in definitive management opens a window of risk for deterioration, in which patients cannot be relied on to report important clinical changes.
This case report describes a patient with significant cognitive dysfunction who developed a rare and devastating complication of a hematologic disorder. As the case illustrates, transferring a patient from the psychiatric ward to Internal Medicine (IM) can create unique diagnostic and management challenges.
CASE REPORT
A 64-year-old man developed hematochezia after having been hospitalized in a locked psychiatric ward for the preceding 6 months following a suicide attempt. The episode of hematochezia occurred while on anticoagulation treatment with warfarin for chronic lower extremity deep venous thrombosis (DVT), which prompted the IM consultation. The patient’s past medical history was notable for dementia, hypothyroidism, Crohn disease, and primary sclerosing cholangitis.
The IM Consult Service recommended holding anticoagulation therapy and reversing the coagulopathy with vitamin K. The patient’s stool returned hemoccult and toxin positive for Clostridium difficile (C difficile). The hematochezia was attributed to the infection with C difficile in the setting of anticoagulation. Oral metronidazole was started. Hemoglobin remained stable without further episodes of bleeding. Seven days after the episode of hematochezia, the patient experienced worsening generalized pain and new skin findings. He was transferred to the general medical ward for further management.
The patient’s medical records revealed early cognitive decline with recommendations for supervised residential care as early as age 59 years. An extensive neurocognitive assessment indicated a diagnosis of semantic dementia. He also had a history of recurrent DVT with anticoagulation therapy for > 10 years with no prior workup for a hypercoagulable state. A recent baseline mental status report described a childlike demeanor, profound global speech deficits with marked difficulty understanding even basic medical concepts (eg, the need for a peripheral intravenous catheter), and generalized anxiety disorder complicated by hyperesthesia. The patient frequently refused physical examinations and blood draws as a result. He devoted himself to simple puzzles of kittens and puppies.
Vital signs were normal as were the head and neck, pulmonary, cardiac, and abdominal examinations. The patient’s neurocognitive examination was remarkable for his dependence on instrumental activities of daily living, global aphasia, impaired short- and long-term recall, and poor judgment. He scored 21 out of 30 on a recent mini-mental state examination: failure to achieve 3-word recall; disorientation to month, season, hospital, and county; and an inability to write a sentence or identify a pen. Otherwise, he had fluent speech, facial symmetry, intact strength and sensation throughout, and normal reflexes.
A skin examination revealed diffuse tender subcutaneous lesions. The largest lesion was about 5 cm, located in the left anterolateral thigh. Smaller lesions of about 1 cm were noted in the abdominal wall, right thigh, and bilateral upper extremities. An exquisitely tender, well-demarcated 20-cm elliptical lesion with central necrosis and an erythematous border developed in the left axilla the following day (Figure 1A). Pain limited adduction of the left arm.
The initial laboratory evaluation demonstrated a stable hemoglobin level of 11.1 g/dL, a platelet count of 128 k/mL, and no leukocytosis. Electrolytes and renal indexes were normal. D-dimer and fibrin split products were > 10,000 ng/mL and 20 mg/mL, respectively. Fibrinogen level was 351 mg/dL. The prothrombin time and international normalized ratio were 15.1 seconds and 1.4, respectively. The activated partial thromboplastin time (aPTT) was measured at 40 seconds. High sensitivity C-reactive protein was 4.59. Recent head imaging included a brain magnetic resonance imaging (MRI) notable for enlarged sulci and ventricles with temporal predominance. Positron emission tomography (PET) brain imaging was significant for diffuse hypometabolism in bilateral parietal and temporal lobes with preservation of sensorimotor and occipital cortexes. There was no clear radiographic evidence of cerebral embolic phenomenon or focal cerebrovascular events.
Enoxaparin treatment was initiated for a suspected hypercoagulable state. Ceftriaxone was administered for a urinary tract infection (UTI). Despite premedication, the bedside biopsy of his necrotic skin lesion was aborted due to severe anxiety and generalized somatic pain. A surgical excisional biopsy was thus obtained under general anesthesia. Enoxaparin was held the night before and the morning of surgery. There were no immediate complications related to the biopsy, and malignancy was not seen on intraoperative frozen sections.
Generalized somatic pain persisted the morning after the surgical biopsy, but the patient remained clinically unchanged. An hour later, he was found unresponsive with no pulse. Despite extensive resuscitative efforts, the patient died.
Postmortem
There was a high index of suspicion for a hemostatic perturbation given the skin findings and recent manipulation of anticoagulation with a prior thrombotic event. The axillary lesion closely resembled warfarin-related skin necrosis. Management included enoxaparin with supportive care, pending definitive pathologic findings.
Postmortem examination confirmed diffuse multiorgan involvement similar to the process seen in the thigh biopsy. Ischemic injury secondary to small vessel microthrombi were evident in the skin, subcutaneous fat, large bowel, urinary bladder, and associated pericystic fat (Figure 1B). Interpretation of the surgical thigh biopsy became available after the patient died. It demonstrated infarcted fat with fat necrosis and hemorrhage (Figure 2).
The results of the laboratory investigations for thrombophilia also came back after the patient died. A potent lupus anticoagulant (LA) was demonstrated. It manifested primarily in the intrinsic pathway as a strongly positive LA-sensitive-aPTT (delta time = 20.5 seconds) assay with a weakly positive dilute Russell’s viper venom time assay. The antigenic specificity of the LA antibodies was not uncovered, as the plasma levels of both IgM and IgG anticardiolipin and anti-Β2-glycoprotein-I antibodies were within the reference range. Factor (F) II and FV genotyping revealed wild-type FV, and the prothrombin gene G20210A was without mutation.
Assays for plasma levels of protein S and antithrombin activity were also normal, which excluded deficiencies in these proteins. The assay for protein C activity was slightly decreased. This may have exacerbated the hemostatic imbalance caused by the LA, as the FVII level had normalized. However, the etiology of the protein C deficiency is not clear. Considerations include (1) a warfarin disequilibrium state due to the discontinuation of oral anticoagulation and institution of vitamin K therapy; (2) an epiphenomenon resulting from active thromboses; or (3) a possible hereditary protein C deficiency.
The definitive diagnosis of the catastrophic antiphospholipid antibody (APA) syndrome relies on multiorgan failure in < 1 week, histopathologic evidence of small vessel thrombosis, and a positive LA.1 The study patient fulfilled these criteria (Table).
DISCUSSION
Catastrophic progression of APA syndrome is an infrequent and devastating complication of this autoimmune disorder with a mortality rate of nearly 50%.1 Antiphospholipid antibody syndrome typically presents with thromboses of the larger vessels, and it more commonly affects the venous system. In contrast, diffuse small vessel thromboses underlie the pathogenesis of catastrophic APA syndrome (CAPS).2 This catastrophic progression occurs in < 1 out of 100 patients with the APA syndrome, more frequently in women (69%), and over an age range of 7 decades (mean 38 years).2 A case series analysis identified older age (aged > 36 years), history of systemic lupus erythematous, and broader organ involvement as prognostic indicators of a poor outcome. Better outcomes are associated with thrombocytopenia and anticoagulation treatment. However, gender did not influence mortality.3
Prevention is key to APA management, given the lack of efficacious treatment.2 Preventive measures are focused on avoiding triggers and aggressively treating those triggers that may arise. Possible triggers in this case included cessation of anticoagulation due to hematochezia and in anticipation of surgery, infection (C difficile colitis, suspected necrotic skin wound super infection, and a UTI), and biopsy-related trauma.
Initial clinical stability in this patient with abrupt decompensation along with pending laboratory and pathology results limited the opportunity for more aggressive therapeutic intervention for CAPS. Moreover, the relative sparing of the cardiopulmonary and renal systems contrasted with the more classical systemic involvement usually seen in CAPS. Second-line therapies for CAPS include plasma exchange and high-dose steroids.2 Third-line therapeutics include immunosuppressive agents, such as cyclophosphamide.2
The rapid decompensation, described on postoperative day 1, after a low-risk surgical biopsy highlights the importance of perioperative care in patients with this autoimmune condition. Following a review of surgical cases, Erkan and colleagues concluded that standard antithrombotic regimens for general and orthopedic surgery are likely to undertreat patients with APA syndrome.4 They recommend the following guidelines in place of standard antithrombotic management: preoperative platelet count > 100 k/µL, higher threshold before proceeding with surgery/interventional procedures, limiting intravascular manipulations, and minimizing periods without anticoagulation therapy.4
A case report of a 31-year-old female undergoing mitral valve replacement complicated postoperatively by CAPS-associated biventricular failure, despite preoperative transition of warfarin to unfractionated heparin, illustrates this significant perioperative risk.5 Evidence-based guidelines recommend holding enoxaparin 24 hours before surgery and 24 hours after invasive procedures in patients requiring bridging anticoagulation therapy.6
Treatment of the patient in this case was complicated by his cognitive impairment. Dementia is a less common but well-documented consequence of APA syndrome. A case review of 28 patients with the APA syndrome and dementia suggests an early onset of cognitive decline with a mean age of 49 years. There may be no clear preceding history of stroke in > 50% of patients.7 Interestingly, dementia followed initial manifestations of disease by an average of 3.5 years, even in some patients receiving anticoagulation therapy.7
A nuclear medicine study of 22 patients with APA syndrome and mild neuropsychiatric symptoms demonstrated a 73% incidence of cerebral hypoperfusion (55% diffuse and 18% local) based on PET imaging despite unremarkable MRI findings.8 Extended periods of hypoperfusion secondary to arterial thromboses in the temporal and parietal lobes may have been the primary etiology for dementia in this case. As such, the coexistence of neurologic abnormalities and a hypercoagulability state warrants a thorough diagnostic workup for similar disorders, despite the higher prevalence of dementia in advanced age.
Unfortunately, this patient’s cognitive disorder prevented a timely and less invasive bedside biopsy and required a surgical biopsy for which anticoagulation therapy was interrupted. A less invasive biopsy and timelier laboratory findings may have avoided triggers, including trauma from the surgical biopsy and interruptions in anticoagulation therapy, which may have contributed to the onset of CAPS.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Asherson RA, Cervera R, Piette J, et al. Catastrophic antiphospholipid syndrome: Clues to the pathogenesis from a series of 80 patients. Medicine (Baltimore). 2001;80(6):355-377.
2. Cervera R, Asherson RA. Multiorgan failure due to rapid occlusive vascular disease in antiphospholipid syndrome: The ‘catastrophic’ antiphospholipid syndrome. APLAR J Rheumatol. 2004;7(3):254-262.
3. Bayraktar UD, Erkan D, Bucciarelli S, Epinosa G, Asherson R; Catastrophic Antiphospholipid Syndrome Project Group. The clinical spectrum of catastrophic antiphospholipid syndrome in the absence and presence of lupus. J Rheumatol. 2007;34(2):346-352.
4. Erkan D, Leibowitz E, Berman J, Lockshin MD. Perioperative medical management of antiphospholipid syndrome: Hospital for special surgery experience, review of literature, and recommendations. J Rheumatol. 2002;29(4):843-849.
5. Dornan RIP. Acute postoperative biventricular failure associated with antiphospholipid antibody syndrome. Br J Anaesth. 2004;92(5):748-754.
6. Douketis JD, Berger PD, Dunn AS, et al; American College of Chest Physicians. The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(suppl 6):S299-S339.
7. Gómez-Puerta JA, Cervera R, Calvo LM, et al. Dementia associated with the antiphospolipid syndrome: Clinical and radiological characteristics of 30 patients. Rheumatology (Oxford). 2005;44(1):95-99.
8. Kao C-H, Lan J-L, Hsieh J-F, Ho Y-J, ChangLai S-P, Lee J-K, et al. Evaluation of regional cerebral blood flow with 99mTc-HMPAO in primary antiphospholipid antibody syndrome. J Nucl Med. 1999;40:1446-1450.
1. Asherson RA, Cervera R, Piette J, et al. Catastrophic antiphospholipid syndrome: Clues to the pathogenesis from a series of 80 patients. Medicine (Baltimore). 2001;80(6):355-377.
2. Cervera R, Asherson RA. Multiorgan failure due to rapid occlusive vascular disease in antiphospholipid syndrome: The ‘catastrophic’ antiphospholipid syndrome. APLAR J Rheumatol. 2004;7(3):254-262.
3. Bayraktar UD, Erkan D, Bucciarelli S, Epinosa G, Asherson R; Catastrophic Antiphospholipid Syndrome Project Group. The clinical spectrum of catastrophic antiphospholipid syndrome in the absence and presence of lupus. J Rheumatol. 2007;34(2):346-352.
4. Erkan D, Leibowitz E, Berman J, Lockshin MD. Perioperative medical management of antiphospholipid syndrome: Hospital for special surgery experience, review of literature, and recommendations. J Rheumatol. 2002;29(4):843-849.
5. Dornan RIP. Acute postoperative biventricular failure associated with antiphospholipid antibody syndrome. Br J Anaesth. 2004;92(5):748-754.
6. Douketis JD, Berger PD, Dunn AS, et al; American College of Chest Physicians. The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(suppl 6):S299-S339.
7. Gómez-Puerta JA, Cervera R, Calvo LM, et al. Dementia associated with the antiphospolipid syndrome: Clinical and radiological characteristics of 30 patients. Rheumatology (Oxford). 2005;44(1):95-99.
8. Kao C-H, Lan J-L, Hsieh J-F, Ho Y-J, ChangLai S-P, Lee J-K, et al. Evaluation of regional cerebral blood flow with 99mTc-HMPAO in primary antiphospholipid antibody syndrome. J Nucl Med. 1999;40:1446-1450.