User login
Slow-growing lesion on eyebrow
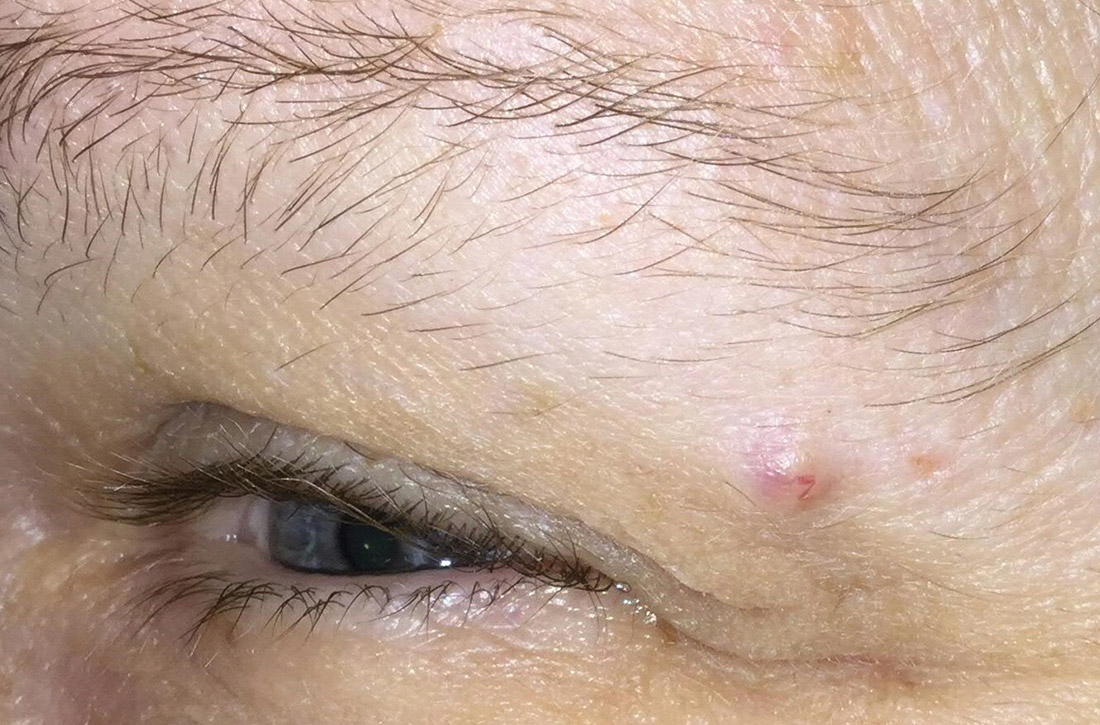
A 51-year-old woman presented to the family medicine clinic for evaluation of a slightly tender skin lesion on her left eyebrow. The lesion had been slowly growing for a year.
The patient’s family history included multiple family members with colon or breast cancer and other relatives with pancreatic and prostate cancer. A colonoscopy performed a year earlier on the patient was negative. The patient’s past medical history included hypertension, major depressive disorder, hyperlipidemia, and venous insufficiency. She also had a colon polyp history.
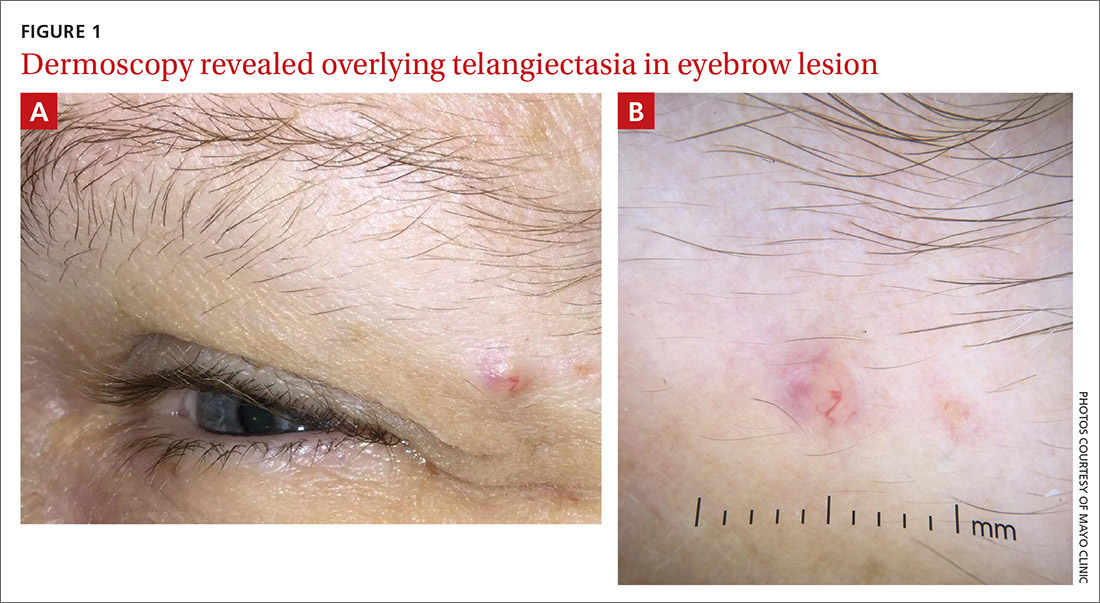
Physical examination of the eyebrow showed a 3-mm papule that was firm on palpation. Dermoscopy of the lesion revealed a yellow papule with
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Sebaceous carcinoma
A rapid teledermatology consultation helped us to determine that this was a sebaceous lesion, but its location and the overlying telangiectasia raised concerns for malignancy. After shared decision-making with the patient, she agreed to proceed with a biopsy. We first made an incision into the lesion, which was hard, demonstrating that it was not cystic. A shave biopsy was then completed. The dermatopathology findings showed clear-cell change consisting of bubbly or foamy cytoplasm, with scalloping of the nuclei, which is characteristic of a sebaceous origin. There were tumor cells that were enlarged with pleomorphism, multiple nucleoli, and scattered mitotic figures. These findings pointed to a diagnosis of sebaceous carcinoma.
Sebaceous carcinomas most commonly manifest on the eyelids. They can originate from the Meibomian glands as well as from pilosebaceous glands at other sites on the body.1 They are rare, accounting for only 1% to 5% of eyelid malignancies, and occur in approximately 2 per 1 million people.1 Tumors can invade locally and metastasize, particularly to surrounding lymph nodes. Periocular pathology may sometimes lead to misdiagnosis, which contributes to a mortality rate that has been reported as high as 20%.1 Suspicion for malignancy may arise due to ulceration, bleeding, pain, or rapid growth.
A lesson in considering the full differential
While sebaceous lesions on the eyelid and eyebrow are often benign, this case underscored the importance of considering the more worrisome elements in the differential. The differential diagnosis for lesions in the area of the eye include the following:
Sebaceous hyperplasia is a common condition (typically among older patients) in which sebaceous glands increase in size and number.2 The classic clinical feature is yellow or skin-colored papules. The lesions typically manifest on the face—particularly on the forehead. They are benign and often have a central umbilication.2
Sebaceous adenomas are benign tumors that may manifest as tan, skin-colored, pink, or yellow papules or nodules.2 The lesions are usually asymptomatic, small, and slow growing.2
Continue to: Basal and squamous cell carcinomas
Basal and squamous cell carcinomas. Basal cell carcinomas often feature translucent lesions on areas of the skin that are exposed to sunlight. These lesions often have slightly rolled border edges or overlying branching telangiectasia and may be nodular.3 Squamous cell carcinomas often feature scaled, reddened patches that may become tender and ulcerate.4
Hordeolums and chalazions. A hordeolum (or stye) is a painful, acute, localized swelling of the eyelid.5 These often develop externally at the lid margin from infection of the follicle. A chalazion is characterized by a persistent, nontender mass that results from small, noninfectious obstruction of the Meibomian glands with secondary granulomatous inflammation.5
Dermoscopy can (and did) help with the Dx
Dermoscopy can help confirm whether a lesion has a sebaceous origin because it would show yellow globules with “crown vessel” telangiectasias that classically do not cross midline.6 Unfortunately, the findings of yellow globules and dermal vessels do not adequately differentiate benign from malignant lesions.6 Carcinomas can manifest in an undifferentiated way early in their course.
Sebaceous carcinomas can be associated with the autosomal dominant Muir-Torre syndrome, a subset of the Lynch syndrome.7,8 Colorectal and genitourinary carcinomas are the most common internal malignancies seen in patients with Muir-Torre syndrome.9
Patients benefit from Mohs surgery
Treatment outcomes for sebaceous carcinoma appear to be improved by Mohs surgery. In a recent review of 1265 patients with early-stage sebaceous carcinomas, Su et al found that 234 patients who were treated with Mohs surgery had improved overall survival, compared with 1031 who were treated with surgical excision.10
Continue to: Our patient
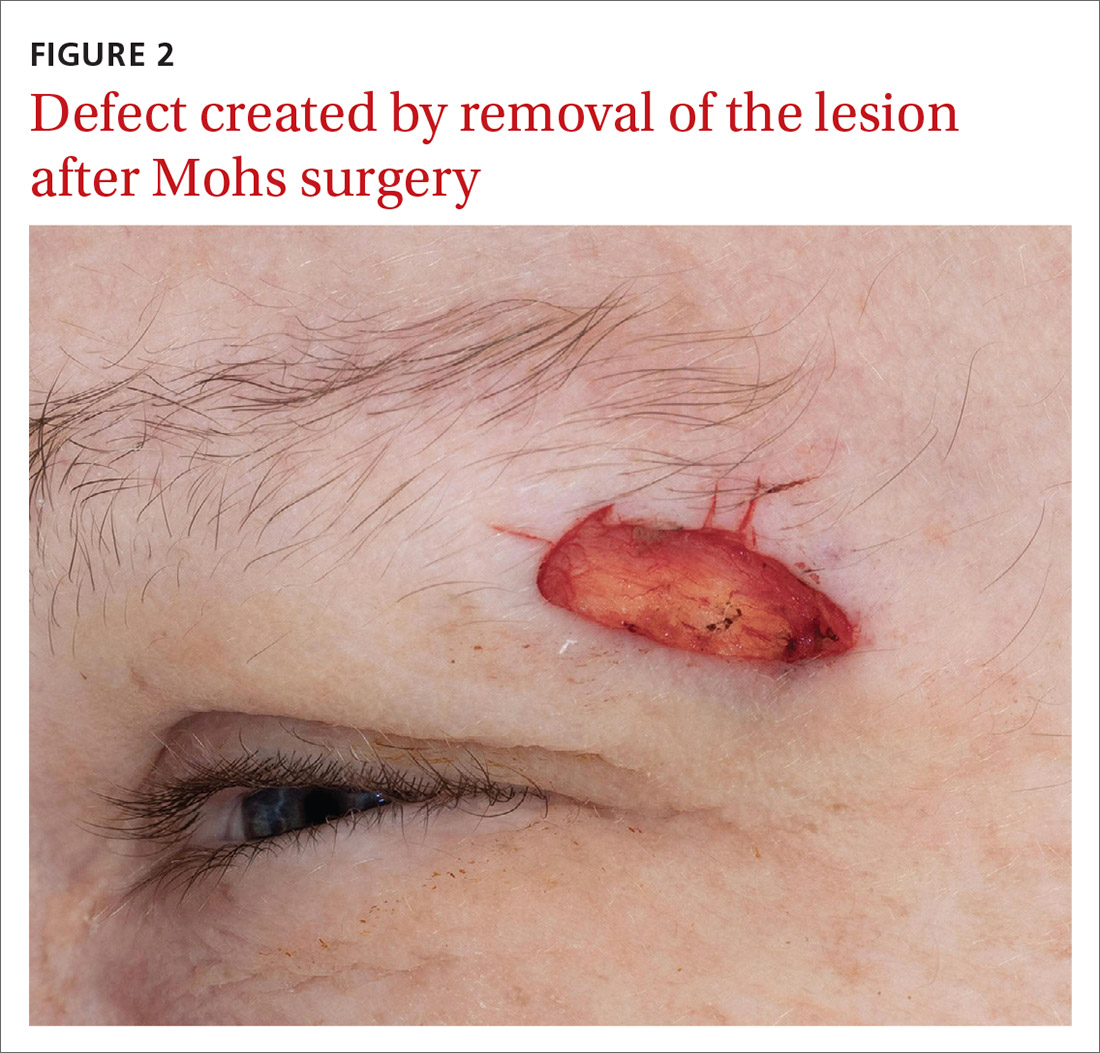
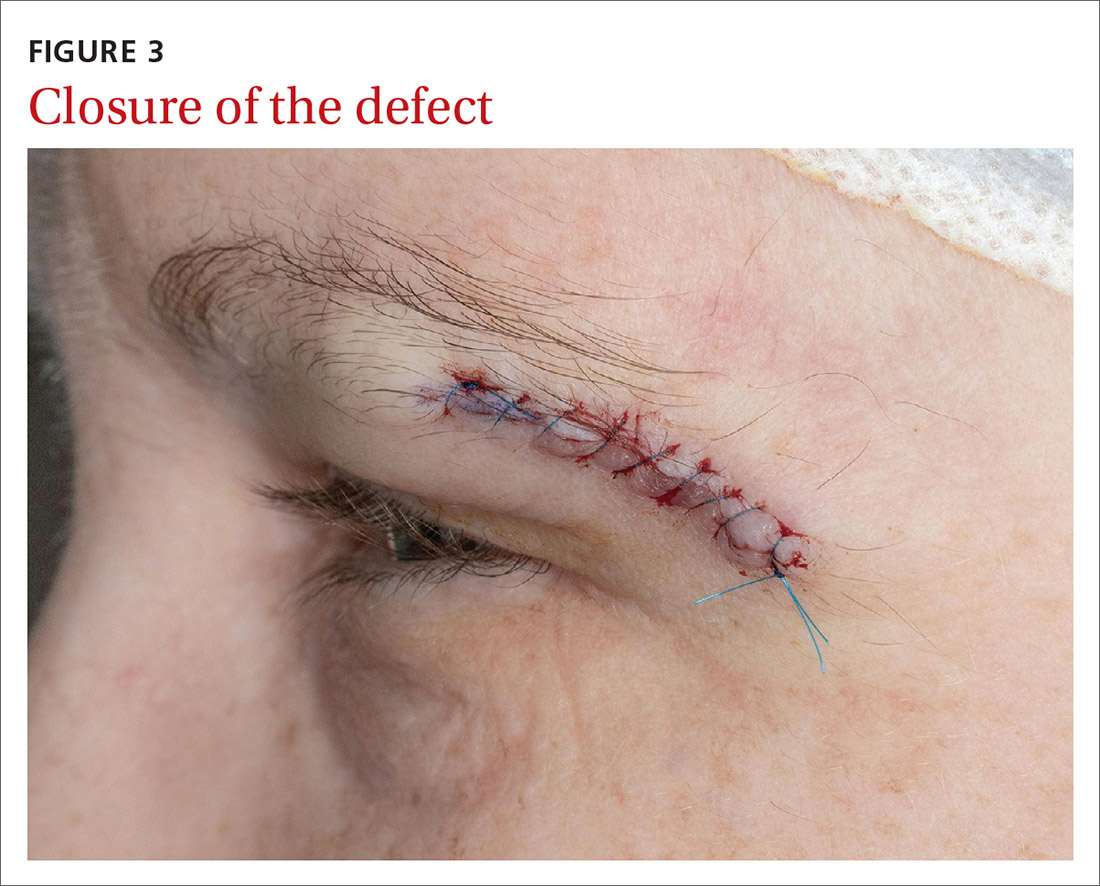
Our patient was referred to a Mohs surgeon who removed the lesion (FIGURES 2 and 3). Given the overall small tumor size, a sentinel lymph node biopsy was not necessary. Because of the patient’s family history, which was suggestive of a genetic predisposition to cancer, she requested a clinical genetics consultation for definitive testing. She went on to pursue genetic testing, which came back negative for Lynch syndrome genes.
The dermatologist recommended yearly skin examination for 5 years for the patient.
1. Kahana A, Pribila HT, Nelson CC, et al. Sebaceous cell carcinoma. In: Levin LA, Albert DM, eds. Ocular Disease: Mechanisms and Management. Saunders/Elsevier; 2010:396-407.
2. Iacobelli J, Harvey NT, Wood BA. Sebaceous lesions of the skin. Pathology. 2017;49:688-697.
3. Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
4. Smith H, Patel A. When to suspect a non-melanoma skin cancer. BMJ. 2020;368:m692.
5. Sun MT, Huang S, Huilgol SC, et al. Eyelid lesions in general practice. Aust J Gen Pract. 2019;48:509-514.
6. Kim NH, Zell DS, Kolm I, et al. The dermoscopic differential diagnosis of yellow lobularlike structures. Arch Dermatol. 2008;144:962.
7. EG, Bell AJY, Barlow KA. Multiple primary carcinomata of the colon, duodenum, and larynx associated with kerato-acanthomata of the face. Br J Surg. 1967;54:191-195.
8. Torre D. Multiple sebaceous tumors. Arch Dermatol. 1968;98:549-55.
9. Cohen PR, Kohn SR, Kurzrock R. Association of sebaceous gland tumors and internal malignancy: the Muir-Torre syndrome. Am J Med. 1991;90:606-613.
10. Su C, Nguyen KA, Bai HX, et al. Comparison of Mohs surgery and surgical excision in the treatment of localized sebaceous carcinoma. Dermatol Surg. 2019;45:1125-1135.
A 51-year-old woman presented to the family medicine clinic for evaluation of a slightly tender skin lesion on her left eyebrow. The lesion had been slowly growing for a year.
The patient’s family history included multiple family members with colon or breast cancer and other relatives with pancreatic and prostate cancer. A colonoscopy performed a year earlier on the patient was negative. The patient’s past medical history included hypertension, major depressive disorder, hyperlipidemia, and venous insufficiency. She also had a colon polyp history.
Physical examination of the eyebrow showed a 3-mm papule that was firm on palpation. Dermoscopy of the lesion revealed a yellow papule with

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Sebaceous carcinoma
A rapid teledermatology consultation helped us to determine that this was a sebaceous lesion, but its location and the overlying telangiectasia raised concerns for malignancy. After shared decision-making with the patient, she agreed to proceed with a biopsy. We first made an incision into the lesion, which was hard, demonstrating that it was not cystic. A shave biopsy was then completed. The dermatopathology findings showed clear-cell change consisting of bubbly or foamy cytoplasm, with scalloping of the nuclei, which is characteristic of a sebaceous origin. There were tumor cells that were enlarged with pleomorphism, multiple nucleoli, and scattered mitotic figures. These findings pointed to a diagnosis of sebaceous carcinoma.
Sebaceous carcinomas most commonly manifest on the eyelids. They can originate from the Meibomian glands as well as from pilosebaceous glands at other sites on the body.1 They are rare, accounting for only 1% to 5% of eyelid malignancies, and occur in approximately 2 per 1 million people.1 Tumors can invade locally and metastasize, particularly to surrounding lymph nodes. Periocular pathology may sometimes lead to misdiagnosis, which contributes to a mortality rate that has been reported as high as 20%.1 Suspicion for malignancy may arise due to ulceration, bleeding, pain, or rapid growth.
A lesson in considering the full differential
While sebaceous lesions on the eyelid and eyebrow are often benign, this case underscored the importance of considering the more worrisome elements in the differential. The differential diagnosis for lesions in the area of the eye include the following:
Sebaceous hyperplasia is a common condition (typically among older patients) in which sebaceous glands increase in size and number.2 The classic clinical feature is yellow or skin-colored papules. The lesions typically manifest on the face—particularly on the forehead. They are benign and often have a central umbilication.2
Sebaceous adenomas are benign tumors that may manifest as tan, skin-colored, pink, or yellow papules or nodules.2 The lesions are usually asymptomatic, small, and slow growing.2
Continue to: Basal and squamous cell carcinomas
Basal and squamous cell carcinomas. Basal cell carcinomas often feature translucent lesions on areas of the skin that are exposed to sunlight. These lesions often have slightly rolled border edges or overlying branching telangiectasia and may be nodular.3 Squamous cell carcinomas often feature scaled, reddened patches that may become tender and ulcerate.4
Hordeolums and chalazions. A hordeolum (or stye) is a painful, acute, localized swelling of the eyelid.5 These often develop externally at the lid margin from infection of the follicle. A chalazion is characterized by a persistent, nontender mass that results from small, noninfectious obstruction of the Meibomian glands with secondary granulomatous inflammation.5
Dermoscopy can (and did) help with the Dx
Dermoscopy can help confirm whether a lesion has a sebaceous origin because it would show yellow globules with “crown vessel” telangiectasias that classically do not cross midline.6 Unfortunately, the findings of yellow globules and dermal vessels do not adequately differentiate benign from malignant lesions.6 Carcinomas can manifest in an undifferentiated way early in their course.
Sebaceous carcinomas can be associated with the autosomal dominant Muir-Torre syndrome, a subset of the Lynch syndrome.7,8 Colorectal and genitourinary carcinomas are the most common internal malignancies seen in patients with Muir-Torre syndrome.9
Patients benefit from Mohs surgery
Treatment outcomes for sebaceous carcinoma appear to be improved by Mohs surgery. In a recent review of 1265 patients with early-stage sebaceous carcinomas, Su et al found that 234 patients who were treated with Mohs surgery had improved overall survival, compared with 1031 who were treated with surgical excision.10
Continue to: Our patient
Our patient was referred to a Mohs surgeon who removed the lesion (FIGURES 2 and 3). Given the overall small tumor size, a sentinel lymph node biopsy was not necessary. Because of the patient’s family history, which was suggestive of a genetic predisposition to cancer, she requested a clinical genetics consultation for definitive testing. She went on to pursue genetic testing, which came back negative for Lynch syndrome genes.

The dermatologist recommended yearly skin examination for 5 years for the patient.

A 51-year-old woman presented to the family medicine clinic for evaluation of a slightly tender skin lesion on her left eyebrow. The lesion had been slowly growing for a year.
The patient’s family history included multiple family members with colon or breast cancer and other relatives with pancreatic and prostate cancer. A colonoscopy performed a year earlier on the patient was negative. The patient’s past medical history included hypertension, major depressive disorder, hyperlipidemia, and venous insufficiency. She also had a colon polyp history.
Physical examination of the eyebrow showed a 3-mm papule that was firm on palpation. Dermoscopy of the lesion revealed a yellow papule with

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Sebaceous carcinoma
A rapid teledermatology consultation helped us to determine that this was a sebaceous lesion, but its location and the overlying telangiectasia raised concerns for malignancy. After shared decision-making with the patient, she agreed to proceed with a biopsy. We first made an incision into the lesion, which was hard, demonstrating that it was not cystic. A shave biopsy was then completed. The dermatopathology findings showed clear-cell change consisting of bubbly or foamy cytoplasm, with scalloping of the nuclei, which is characteristic of a sebaceous origin. There were tumor cells that were enlarged with pleomorphism, multiple nucleoli, and scattered mitotic figures. These findings pointed to a diagnosis of sebaceous carcinoma.
Sebaceous carcinomas most commonly manifest on the eyelids. They can originate from the Meibomian glands as well as from pilosebaceous glands at other sites on the body.1 They are rare, accounting for only 1% to 5% of eyelid malignancies, and occur in approximately 2 per 1 million people.1 Tumors can invade locally and metastasize, particularly to surrounding lymph nodes. Periocular pathology may sometimes lead to misdiagnosis, which contributes to a mortality rate that has been reported as high as 20%.1 Suspicion for malignancy may arise due to ulceration, bleeding, pain, or rapid growth.
A lesson in considering the full differential
While sebaceous lesions on the eyelid and eyebrow are often benign, this case underscored the importance of considering the more worrisome elements in the differential. The differential diagnosis for lesions in the area of the eye include the following:
Sebaceous hyperplasia is a common condition (typically among older patients) in which sebaceous glands increase in size and number.2 The classic clinical feature is yellow or skin-colored papules. The lesions typically manifest on the face—particularly on the forehead. They are benign and often have a central umbilication.2
Sebaceous adenomas are benign tumors that may manifest as tan, skin-colored, pink, or yellow papules or nodules.2 The lesions are usually asymptomatic, small, and slow growing.2
Continue to: Basal and squamous cell carcinomas
Basal and squamous cell carcinomas. Basal cell carcinomas often feature translucent lesions on areas of the skin that are exposed to sunlight. These lesions often have slightly rolled border edges or overlying branching telangiectasia and may be nodular.3 Squamous cell carcinomas often feature scaled, reddened patches that may become tender and ulcerate.4
Hordeolums and chalazions. A hordeolum (or stye) is a painful, acute, localized swelling of the eyelid.5 These often develop externally at the lid margin from infection of the follicle. A chalazion is characterized by a persistent, nontender mass that results from small, noninfectious obstruction of the Meibomian glands with secondary granulomatous inflammation.5
Dermoscopy can (and did) help with the Dx
Dermoscopy can help confirm whether a lesion has a sebaceous origin because it would show yellow globules with “crown vessel” telangiectasias that classically do not cross midline.6 Unfortunately, the findings of yellow globules and dermal vessels do not adequately differentiate benign from malignant lesions.6 Carcinomas can manifest in an undifferentiated way early in their course.
Sebaceous carcinomas can be associated with the autosomal dominant Muir-Torre syndrome, a subset of the Lynch syndrome.7,8 Colorectal and genitourinary carcinomas are the most common internal malignancies seen in patients with Muir-Torre syndrome.9
Patients benefit from Mohs surgery
Treatment outcomes for sebaceous carcinoma appear to be improved by Mohs surgery. In a recent review of 1265 patients with early-stage sebaceous carcinomas, Su et al found that 234 patients who were treated with Mohs surgery had improved overall survival, compared with 1031 who were treated with surgical excision.10
Continue to: Our patient
Our patient was referred to a Mohs surgeon who removed the lesion (FIGURES 2 and 3). Given the overall small tumor size, a sentinel lymph node biopsy was not necessary. Because of the patient’s family history, which was suggestive of a genetic predisposition to cancer, she requested a clinical genetics consultation for definitive testing. She went on to pursue genetic testing, which came back negative for Lynch syndrome genes.

The dermatologist recommended yearly skin examination for 5 years for the patient.

1. Kahana A, Pribila HT, Nelson CC, et al. Sebaceous cell carcinoma. In: Levin LA, Albert DM, eds. Ocular Disease: Mechanisms and Management. Saunders/Elsevier; 2010:396-407.
2. Iacobelli J, Harvey NT, Wood BA. Sebaceous lesions of the skin. Pathology. 2017;49:688-697.
3. Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
4. Smith H, Patel A. When to suspect a non-melanoma skin cancer. BMJ. 2020;368:m692.
5. Sun MT, Huang S, Huilgol SC, et al. Eyelid lesions in general practice. Aust J Gen Pract. 2019;48:509-514.
6. Kim NH, Zell DS, Kolm I, et al. The dermoscopic differential diagnosis of yellow lobularlike structures. Arch Dermatol. 2008;144:962.
7. EG, Bell AJY, Barlow KA. Multiple primary carcinomata of the colon, duodenum, and larynx associated with kerato-acanthomata of the face. Br J Surg. 1967;54:191-195.
8. Torre D. Multiple sebaceous tumors. Arch Dermatol. 1968;98:549-55.
9. Cohen PR, Kohn SR, Kurzrock R. Association of sebaceous gland tumors and internal malignancy: the Muir-Torre syndrome. Am J Med. 1991;90:606-613.
10. Su C, Nguyen KA, Bai HX, et al. Comparison of Mohs surgery and surgical excision in the treatment of localized sebaceous carcinoma. Dermatol Surg. 2019;45:1125-1135.
1. Kahana A, Pribila HT, Nelson CC, et al. Sebaceous cell carcinoma. In: Levin LA, Albert DM, eds. Ocular Disease: Mechanisms and Management. Saunders/Elsevier; 2010:396-407.
2. Iacobelli J, Harvey NT, Wood BA. Sebaceous lesions of the skin. Pathology. 2017;49:688-697.
3. Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
4. Smith H, Patel A. When to suspect a non-melanoma skin cancer. BMJ. 2020;368:m692.
5. Sun MT, Huang S, Huilgol SC, et al. Eyelid lesions in general practice. Aust J Gen Pract. 2019;48:509-514.
6. Kim NH, Zell DS, Kolm I, et al. The dermoscopic differential diagnosis of yellow lobularlike structures. Arch Dermatol. 2008;144:962.
7. EG, Bell AJY, Barlow KA. Multiple primary carcinomata of the colon, duodenum, and larynx associated with kerato-acanthomata of the face. Br J Surg. 1967;54:191-195.
8. Torre D. Multiple sebaceous tumors. Arch Dermatol. 1968;98:549-55.
9. Cohen PR, Kohn SR, Kurzrock R. Association of sebaceous gland tumors and internal malignancy: the Muir-Torre syndrome. Am J Med. 1991;90:606-613.
10. Su C, Nguyen KA, Bai HX, et al. Comparison of Mohs surgery and surgical excision in the treatment of localized sebaceous carcinoma. Dermatol Surg. 2019;45:1125-1135.
The benefits of a standardized approach to opioid prescribing
ABSTRACT
Purpose The “opioid epidemic” in the United States has received increasing attention over the past few years. Most drug overdose deaths involve an opioid, and prescription opioid deaths have quadrupled since 1999. We sought to improve patient safety and adhere to clinical guidelines by standardizing opioid prescribing in our practice.
Methods We implemented a standardized approach to opioid prescribing based on Arizona Department of Health Services guidelines. All of our providers received instruction on Arizona’s Controlled Substance Prescription Monitoring Program (AZCSPMP) database and were encouraged to use it online. Our goal was for patients to have quarterly office visits, complete random urine drug screens, and sign a controlled substance agreement (CSA). The CSA acknowledged their understanding of the risks and benefits of opioid therapy as well as our updated prescribing policies.
Results Three-hundred fifty-eight of our practice’s patients were receiving chronic opioid therapy. All providers enrolled in AZCSPMP and used it for patient care. We increased rates of signed CSAs from 4.5% to 43.6%, and urine drug screening from 0.8% to 20.1%. For 325 patients remaining in the practice after our interventions, a postintervention chart review demonstrated a statistically significant discontinuation of opioid therapy (71/325, 21.8%; 95% confidence interval, 17.4%-26.7%).
Conclusion Implementation of a standardized opioid prescribing process resulted in discontinuation of therapy for some patients. Rates increased for signed CSAs and completed random urine drug screening. Future process interventions may improve patient and provider adherence. All primary care physicians should examine their prescribing processes to enhance the safety of opioid therapy.
[polldaddy:10370177]
The US opioid epidemic has received increased attention both nationally and at the state level over the past 2 years. This attention is warranted given the significant societal burden of opioid misuse, abuse, and overdose. Most drug overdose deaths (> 6/10) involve an opioid.1 Deaths from prescription opioids have quadrupled since 1999 in the United States.2 Arizona, the state in which we practice, ranked sixth highest in the nation for drug overdose deaths and had the fifth highest opioid prescribing rate in 2011.3 In response to the growing epidemic, the Centers for Disease Control and Prevention (CDC) released guidelines in 2016 for prescribing and monitoring opioids for chronic pain.4
Chronic nonterminal pain (CNTP) remains a significant cause of human suffering and is more prevalent in the United States than cancer, diabetes, and heart disease combined.5 The increased use of opioids since 1999 to ease CNTP has not reduced Americans’ reports of pain overall.6,7 Given the growing opioid epidemic and disease burden of CNTP, we embarked on a quality improvement (QI) project to safely prescribe and refill opioid medications in the Department of Family Medicine at the Mayo Clinic Arizona.
METHODS
This project received an exemption from internal review board evaluation as a QI intervention. We used a team-based approach to address standardization of opioid prescribing and monitoring within our practice. The team included physicians (MD/DO), nurses (LPN/RN), and allied health staff (MA), operations and administrative personnel, and information technology (IT) support. We did not involve patients in the initial design of our project. With future quality efforts in this area, we plan to involve patients in design processes.
Continue to: We began by identifying...
We began by identifying the scope of the problem, establishing criteria to search the electronic medical record (EMR) and identify appropriate patients. Chronic pain is often defined as pain lasting more than 3 months. Chronic opioid therapy (COT) has been defined as opioid use lasting longer than 3 months.8 Working with our IT colleagues, we defined COT patients as those with 3 or more prescriptions for opioids in the past year or those who received ≥ 30 pills a month (ie, patients who received 180 pills with 2 prescriptions written for the year). This definition gave us the ability to query our EMR to determine which patients were on COT, and we prepared lists of patients by primary care provider (FIGURE). Providers reviewed the lists to ensure these individuals were in fact on COT for CNTP. The number of patients identified after EMR query and provider review was 358, comprising 2.6% of 14,000 empaneled patients.
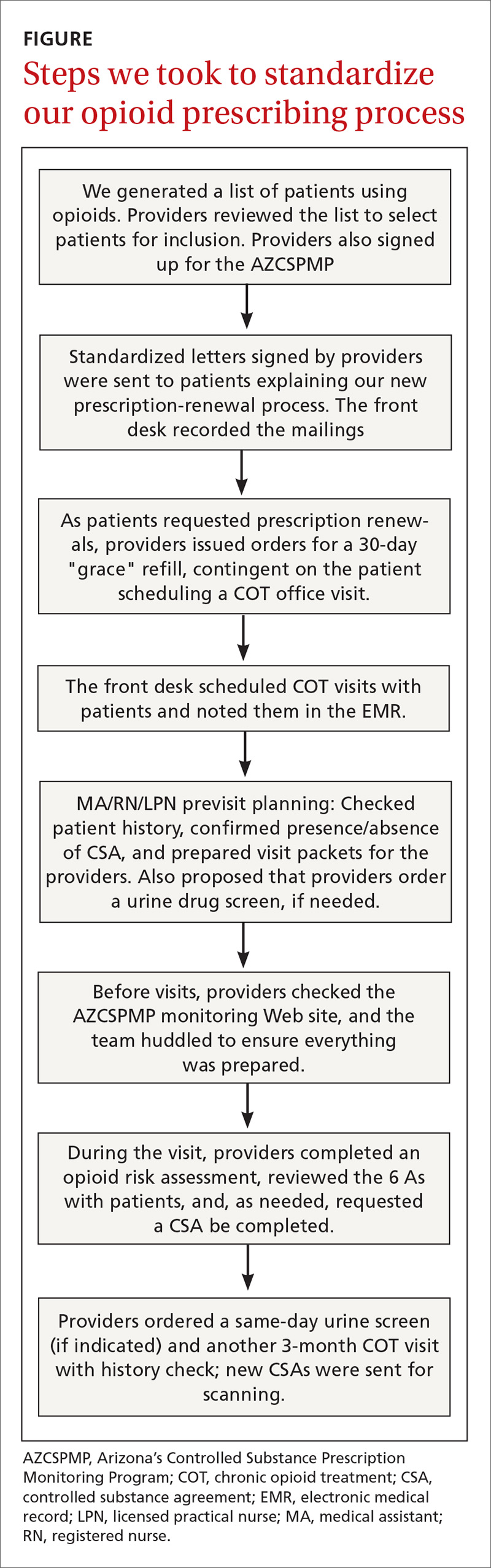
We based our interventions on the Arizona Department of Health Services 2014 opioid prescribing guidelines.3 The Arizona guidelines used existing national and state opioid prescribing guidelines along with clinical practice guidelines. Our study began prior to the 2016 CDC guidelines, so they were not used in this study. Our practice guidelines recommended that all 23 of our providers (MDs, DOs, and NPs) sign up for Arizona’s Controlled Substance Prescription Monitoring Program (AZCSPMP). We asked each patient to sign a controlled substance agreement (CSA), acknowledging their awareness of our proposed processes and the discussion of opioid therapy. Patients were expected to have face-to-face visits with providers at least quarterly and to complete a random urine drug screen at least annually. Patients were not incentivized to complete the process. We placed reminder calls for appointments just as we do for regular appointments.
Providers were asked to complete the Opioid Risk Tool9 with the patient at the initial visit, discuss the risks, benefits, and alternatives of long-term use of opioid medication, and review the 6 As (analgesia, activity, aberrant drug related behavior, adverse effects, affect, and adjunctive treatments). On the day before each patient visit, providers were reminded by a note in the EMR schedule to check AZCSPMP. Initial appointment times would be 30 minutes and follow-up appointments would be scheduled for 15 minutes if only addressing COT.
The QI project was introduced at an all-staff meeting in October 2015 that included providers, allied health staff, front desk personnel, and administrative staff, with the goal of beginning our COT process in November. We mailed letters to COT patients describing our new guidelines and asking them to call to schedule an appointment. If patients on COT came into the office for an alternate appointment and had not yet been seen for a COT visit, providers were encouraged to complete the COT process at that time.
We created a standard order set in the EMR for initial and follow-up visits and for the urine drug screen. We also added an interactive form to the EMR allowing providers to electronically complete the Opioid Risk Tool, and to confirm CSA completion and AZCSPMP review. We developed a database that would query the EMR for patient office visit frequency, CSA completion, and urine drug screen collection. We also placed paper copies of forms in exam rooms with a laminated instruction sheet reviewing the process steps and the 6 As.
Continue to: Soft rollout was...
Soft rollout was November 1, 2015, to assist in working through the process before full rollout. We asked providers to complete the full process on at least 1 patient during this period. This run-through would help ensure that allied health staff who room the patients would have the CSA and Opioid Risk Tool already in the chart before the visit. Full rollout was January 2, 2016. Every 2 to 4 weeks after the full rollout, regular email reminders were sent to providers about the project process and allowed for any feedback about issues that arose.
We provided regular updates and discussed the process at department meetings monthly. Quarterly data were reviewed and discussed for the first year of implementation. Providers and staff completed a chart review for each COT patient at project completion, to determine whether opioids had been decreased (in dosage) or discontinued, a nonopioid medicine had been initiated to augment pain control, or whether patients had died or left the practice.
Statistical analysis
We summarized binary data as counts and proportions and compared them using the chi square test. We summarized discrete data by their mean and standard deviation. To analyze binary variables measured repeatedly in time, we used the logistic generalized estimating equation (GEE) with an autoregressive (AR-1) correlation structure. We computed 95% confidence intervals (CIs)for odds ratios using the empirical or “sandwich” standard error estimates. For discrete variables representing counts, we used the negative binomial regression model.
For count data, a Poisson model is typically used; in our case the variance was considerably larger than the mean, exceeding the Poisson-model requirement that they not be significantly different if not exactly the same. This implies that the data are “over dispersed” or more variable than a Poisson model is thought to be able to model accurately. We therefore used a negative binomial model, which is regarded as the better model in this situation. The 95% CIs for the estimate resulting from the negative binomial regression model were computed using the profile-likelihood.10 All GEEs were clustered on patients (n = 358). We used SAS version 9.3 (Cary, NC) for all analyses.

RESULTS
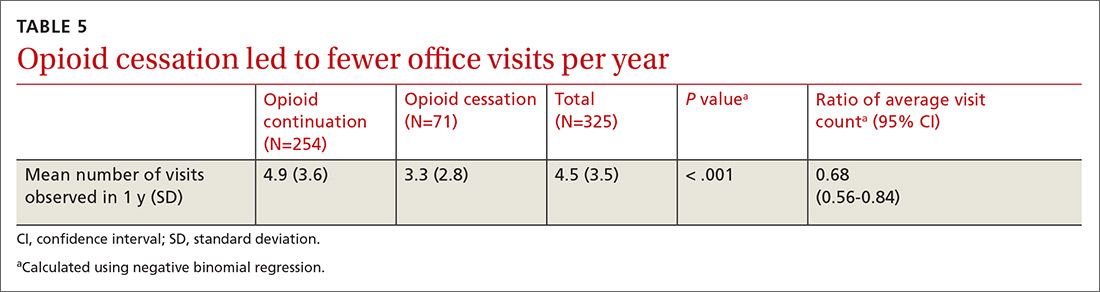
All providers enrolled for AZCSPMP. CSA completion increased from 16 (4.5%) at baseline to 156 (43.6%) after intervention (P < .001). Patients completed a urine drug screen more frequently as well, from 3 (0.8%) to 72 (20.1%) (P < .001) (TABLES 1 and 2). No statistically significant change was noted in the frequency of office visits.

Continue to: We excluded 33 patients...
We excluded 33 patients from the post-intervention chart review (TABLE 3). Twenty-seven had left the practice and 6 had died, leaving 325 patients included in the post-intervention chart review.
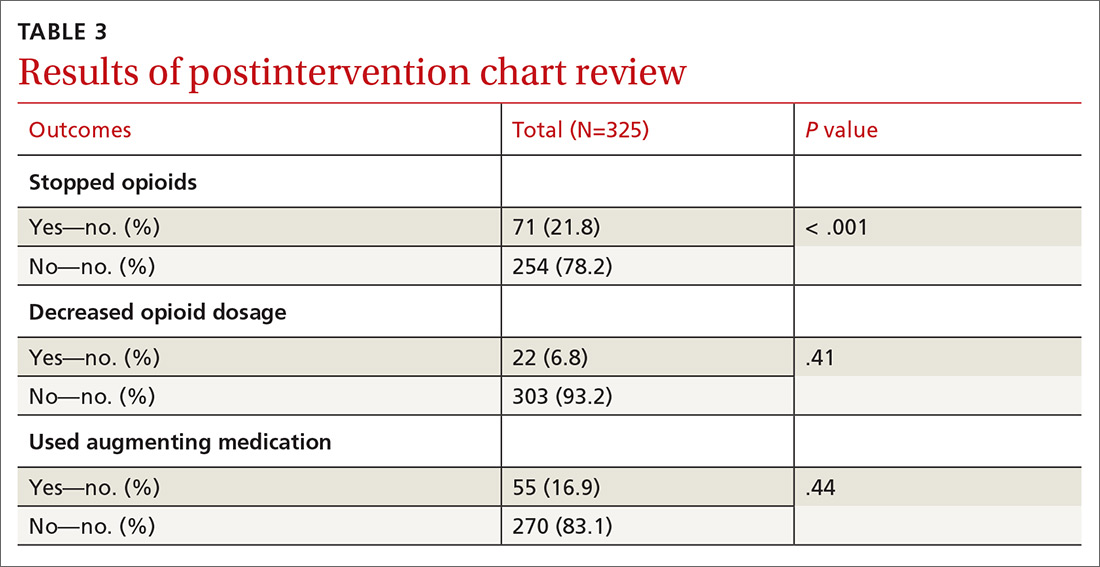
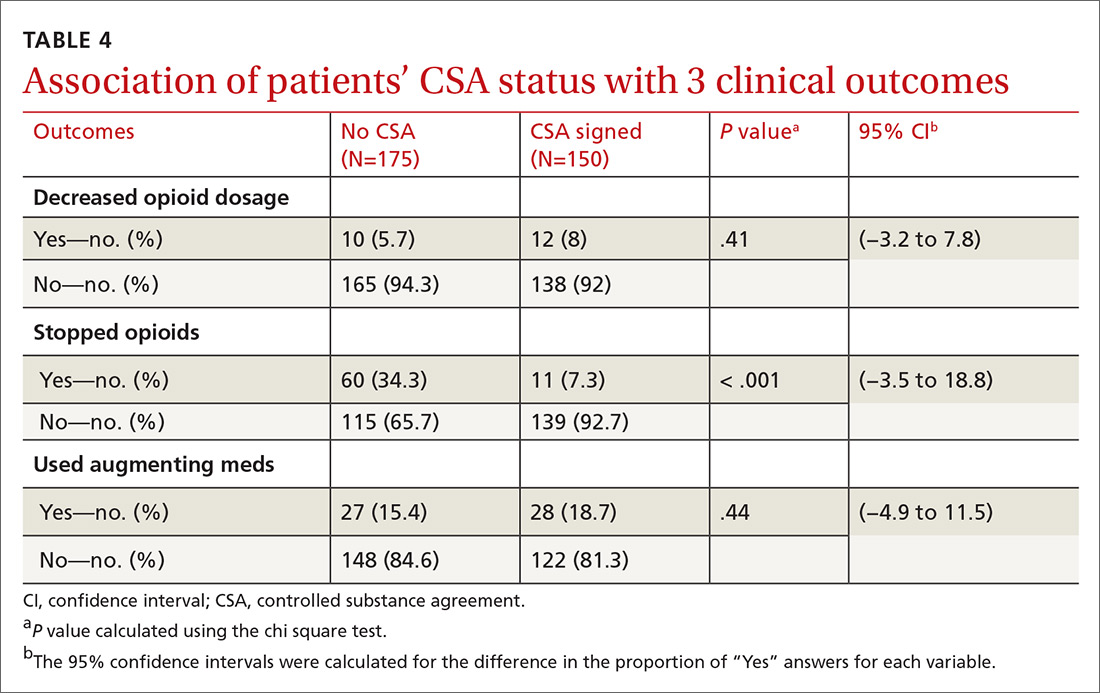
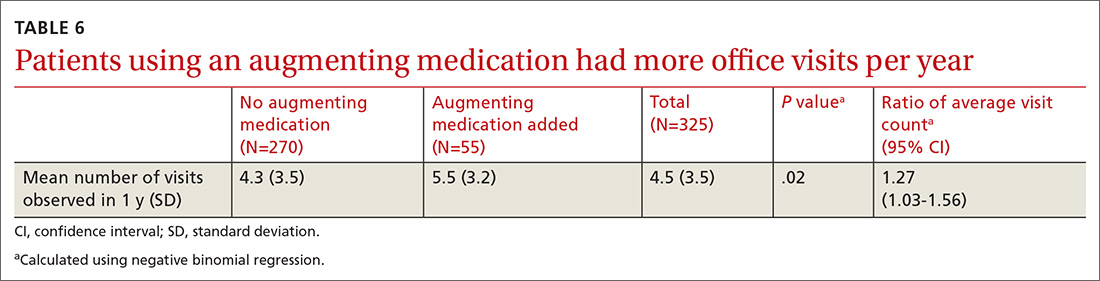
There was a statistically significant association between patients who discontinued opioids and those who neglected to sign a CSA (P < .001) (TABLE 4). We tested for associations between office visit frequency and process step completion. There was a nonsignificant trend between increased frequency of office visits and opioid dose reduction. Patients who stopped opioids had fewer office visits (TABLE 5), while patients who had initiated a medication to augment pain relief had more frequent office visits (TABLE 6).
DISCUSSION
Our interventions to improve the quality of our COT processes were moderately successful. We achieved statistically significant increases in our rates of CSA completion and in urine drug screening. However, these increases were not as clinically impactful as we had hoped. Improvements in both patient and provider adherence are needed. We plan to engage allied health staff more fully to assist with adherence and thereby improve quality. This study was not intended to obtain patient-oriented outcomes, such as decreased pain and improved function. The study was designed to improve patient safety and to standardize a process for prescribing and monitoring patients on COT. In the future we plan to look at patient outcomes and expand our focus to patients on high-dose opioids and those on combination therapy with benzodiazepines.
We believe the most impactful process steps were our letters sent to COT patients describing our updated, standardized prescribing process, and the ensuing provider-patient discussion to review the risks, benefits, and alternatives to opioid therapy. This frank discussion of treatment options resulted in more than 1 in 5 patients electing to discontinue COT.
There was an association between opioid discontinuation and patients not signing the CSA. This may have been due to patients deciding to discontinue opioids at the initiation review with providers after they received their letter. Therefore, signing the agreement was no longer necessary.
Continue to: We noted that some patients...
We noted that some patients elected to begin a new, nonopioid medication intended to augment their pain relief. However, they did not decrease their use of opioid medicines. We did not collect pain rating scale scores to determine whether the addition of augmenting medicines provided a reduction in pain perception.
Close monitoring of COT patients with frequent office visits may have had an impact on their care. We noted an association between more frequent visits and initiation of pain augmentation medicines. There was also a nonsignificant trend between office visit frequency and dose reduction. These are topics we may re-examine in our practice over time. There was no change in office visit frequency with our intervention, likely a result of these patients having frequent office visits for multiple comorbid medical conditions at baseline.
Evidence of similar benefits in primary care practices that standardized their opioid prescribing guidelines for patients on COT11 illustrates the importance of such a process for ensuring patient safety and decreasing opioid dosage and use.
Limitations to our project are that we did not measure functional changes and quality-of-life scores for patients. We also did not note the opioid dosages for individuals who chose to stop using opioids.
Looking forward. Based on our experience, patient notification with discussion of COT risks, benefits, and alternatives, as well as implementation of a process to monitor COT, appear to be related to patients’ decisions to discontinue COT. Our new standard process did show QI in the process steps but remained suboptimal to our expectations of clinical impact. More frequent office visits may impact patient decisions to reduce opioid dose and to add an augmenting pain medication. We plan to increase the involvement and responsibilities of our allied health staff in our processes to improve rates of adherence and the overall quality of how we manage patients on chronic opioid therapy.
CORRESPONDENCE
David Patchett, DO, Mayo Clinic, 13400 East Shea Blvd, Scottsdale, AZ 85259; [email protected]
1. Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths — United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65:1445-1452.
2. CDC. Opioid data analysis and resources. https://www.cdc.gov/drugoverdose/data/analysis.html. Published December 19, 2018. Accessed May 27, 2019.
3. Arizona Department of Health Services. Arizona opioid prescribing guidelines. https://www.azdhs.gov/documents/audiences/clinicians/clinical-guidelines-recommendations/prescribing-guidelines/az-opiod-prescribing-guidelines.pdf. Published November 2014. Accessed May 27, 2019.
4. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
5. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011.
6. Chang H, Daubresse M, Kruszewski S, et al. Prevalence and treatment of pain in EDs in the United States, 2000 to 2010. Am J Emerg Med. 2014;32:421-431.
7. Daubresse M, Chang H, Yu Y, et al. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000 - 2010. Med Care. 2013;51:870-878.
8. Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162:276-286.
9. Webster LR, Webster RM. Predicting aberrant behaviors in opioid‐treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6:432-442.
10. Hilbe JM. Negative Binomial Regression. Cambridge, United Kingdom: Cambridge University Press; 2013.
11. Liebschutz JM, Xuan Z, Shanahan CW, et al. Improving adherence to long-term opioid therapy guidelines to reduce opioid misuse in primary care: a cluster-randomized clinical trial. JAMA Intern Med. 2017;177:1265-1272.
ABSTRACT
Purpose The “opioid epidemic” in the United States has received increasing attention over the past few years. Most drug overdose deaths involve an opioid, and prescription opioid deaths have quadrupled since 1999. We sought to improve patient safety and adhere to clinical guidelines by standardizing opioid prescribing in our practice.
Methods We implemented a standardized approach to opioid prescribing based on Arizona Department of Health Services guidelines. All of our providers received instruction on Arizona’s Controlled Substance Prescription Monitoring Program (AZCSPMP) database and were encouraged to use it online. Our goal was for patients to have quarterly office visits, complete random urine drug screens, and sign a controlled substance agreement (CSA). The CSA acknowledged their understanding of the risks and benefits of opioid therapy as well as our updated prescribing policies.
Results Three-hundred fifty-eight of our practice’s patients were receiving chronic opioid therapy. All providers enrolled in AZCSPMP and used it for patient care. We increased rates of signed CSAs from 4.5% to 43.6%, and urine drug screening from 0.8% to 20.1%. For 325 patients remaining in the practice after our interventions, a postintervention chart review demonstrated a statistically significant discontinuation of opioid therapy (71/325, 21.8%; 95% confidence interval, 17.4%-26.7%).
Conclusion Implementation of a standardized opioid prescribing process resulted in discontinuation of therapy for some patients. Rates increased for signed CSAs and completed random urine drug screening. Future process interventions may improve patient and provider adherence. All primary care physicians should examine their prescribing processes to enhance the safety of opioid therapy.
[polldaddy:10370177]
The US opioid epidemic has received increased attention both nationally and at the state level over the past 2 years. This attention is warranted given the significant societal burden of opioid misuse, abuse, and overdose. Most drug overdose deaths (> 6/10) involve an opioid.1 Deaths from prescription opioids have quadrupled since 1999 in the United States.2 Arizona, the state in which we practice, ranked sixth highest in the nation for drug overdose deaths and had the fifth highest opioid prescribing rate in 2011.3 In response to the growing epidemic, the Centers for Disease Control and Prevention (CDC) released guidelines in 2016 for prescribing and monitoring opioids for chronic pain.4
Chronic nonterminal pain (CNTP) remains a significant cause of human suffering and is more prevalent in the United States than cancer, diabetes, and heart disease combined.5 The increased use of opioids since 1999 to ease CNTP has not reduced Americans’ reports of pain overall.6,7 Given the growing opioid epidemic and disease burden of CNTP, we embarked on a quality improvement (QI) project to safely prescribe and refill opioid medications in the Department of Family Medicine at the Mayo Clinic Arizona.
METHODS
This project received an exemption from internal review board evaluation as a QI intervention. We used a team-based approach to address standardization of opioid prescribing and monitoring within our practice. The team included physicians (MD/DO), nurses (LPN/RN), and allied health staff (MA), operations and administrative personnel, and information technology (IT) support. We did not involve patients in the initial design of our project. With future quality efforts in this area, we plan to involve patients in design processes.
Continue to: We began by identifying...
We began by identifying the scope of the problem, establishing criteria to search the electronic medical record (EMR) and identify appropriate patients. Chronic pain is often defined as pain lasting more than 3 months. Chronic opioid therapy (COT) has been defined as opioid use lasting longer than 3 months.8 Working with our IT colleagues, we defined COT patients as those with 3 or more prescriptions for opioids in the past year or those who received ≥ 30 pills a month (ie, patients who received 180 pills with 2 prescriptions written for the year). This definition gave us the ability to query our EMR to determine which patients were on COT, and we prepared lists of patients by primary care provider (FIGURE). Providers reviewed the lists to ensure these individuals were in fact on COT for CNTP. The number of patients identified after EMR query and provider review was 358, comprising 2.6% of 14,000 empaneled patients.

We based our interventions on the Arizona Department of Health Services 2014 opioid prescribing guidelines.3 The Arizona guidelines used existing national and state opioid prescribing guidelines along with clinical practice guidelines. Our study began prior to the 2016 CDC guidelines, so they were not used in this study. Our practice guidelines recommended that all 23 of our providers (MDs, DOs, and NPs) sign up for Arizona’s Controlled Substance Prescription Monitoring Program (AZCSPMP). We asked each patient to sign a controlled substance agreement (CSA), acknowledging their awareness of our proposed processes and the discussion of opioid therapy. Patients were expected to have face-to-face visits with providers at least quarterly and to complete a random urine drug screen at least annually. Patients were not incentivized to complete the process. We placed reminder calls for appointments just as we do for regular appointments.
Providers were asked to complete the Opioid Risk Tool9 with the patient at the initial visit, discuss the risks, benefits, and alternatives of long-term use of opioid medication, and review the 6 As (analgesia, activity, aberrant drug related behavior, adverse effects, affect, and adjunctive treatments). On the day before each patient visit, providers were reminded by a note in the EMR schedule to check AZCSPMP. Initial appointment times would be 30 minutes and follow-up appointments would be scheduled for 15 minutes if only addressing COT.
The QI project was introduced at an all-staff meeting in October 2015 that included providers, allied health staff, front desk personnel, and administrative staff, with the goal of beginning our COT process in November. We mailed letters to COT patients describing our new guidelines and asking them to call to schedule an appointment. If patients on COT came into the office for an alternate appointment and had not yet been seen for a COT visit, providers were encouraged to complete the COT process at that time.
We created a standard order set in the EMR for initial and follow-up visits and for the urine drug screen. We also added an interactive form to the EMR allowing providers to electronically complete the Opioid Risk Tool, and to confirm CSA completion and AZCSPMP review. We developed a database that would query the EMR for patient office visit frequency, CSA completion, and urine drug screen collection. We also placed paper copies of forms in exam rooms with a laminated instruction sheet reviewing the process steps and the 6 As.
Continue to: Soft rollout was...
Soft rollout was November 1, 2015, to assist in working through the process before full rollout. We asked providers to complete the full process on at least 1 patient during this period. This run-through would help ensure that allied health staff who room the patients would have the CSA and Opioid Risk Tool already in the chart before the visit. Full rollout was January 2, 2016. Every 2 to 4 weeks after the full rollout, regular email reminders were sent to providers about the project process and allowed for any feedback about issues that arose.
We provided regular updates and discussed the process at department meetings monthly. Quarterly data were reviewed and discussed for the first year of implementation. Providers and staff completed a chart review for each COT patient at project completion, to determine whether opioids had been decreased (in dosage) or discontinued, a nonopioid medicine had been initiated to augment pain control, or whether patients had died or left the practice.
Statistical analysis
We summarized binary data as counts and proportions and compared them using the chi square test. We summarized discrete data by their mean and standard deviation. To analyze binary variables measured repeatedly in time, we used the logistic generalized estimating equation (GEE) with an autoregressive (AR-1) correlation structure. We computed 95% confidence intervals (CIs)for odds ratios using the empirical or “sandwich” standard error estimates. For discrete variables representing counts, we used the negative binomial regression model.
For count data, a Poisson model is typically used; in our case the variance was considerably larger than the mean, exceeding the Poisson-model requirement that they not be significantly different if not exactly the same. This implies that the data are “over dispersed” or more variable than a Poisson model is thought to be able to model accurately. We therefore used a negative binomial model, which is regarded as the better model in this situation. The 95% CIs for the estimate resulting from the negative binomial regression model were computed using the profile-likelihood.10 All GEEs were clustered on patients (n = 358). We used SAS version 9.3 (Cary, NC) for all analyses.

RESULTS
All providers enrolled for AZCSPMP. CSA completion increased from 16 (4.5%) at baseline to 156 (43.6%) after intervention (P < .001). Patients completed a urine drug screen more frequently as well, from 3 (0.8%) to 72 (20.1%) (P < .001) (TABLES 1 and 2). No statistically significant change was noted in the frequency of office visits.

Continue to: We excluded 33 patients...
We excluded 33 patients from the post-intervention chart review (TABLE 3). Twenty-seven had left the practice and 6 had died, leaving 325 patients included in the post-intervention chart review.

There was a statistically significant association between patients who discontinued opioids and those who neglected to sign a CSA (P < .001) (TABLE 4). We tested for associations between office visit frequency and process step completion. There was a nonsignificant trend between increased frequency of office visits and opioid dose reduction. Patients who stopped opioids had fewer office visits (TABLE 5), while patients who had initiated a medication to augment pain relief had more frequent office visits (TABLE 6).

DISCUSSION
Our interventions to improve the quality of our COT processes were moderately successful. We achieved statistically significant increases in our rates of CSA completion and in urine drug screening. However, these increases were not as clinically impactful as we had hoped. Improvements in both patient and provider adherence are needed. We plan to engage allied health staff more fully to assist with adherence and thereby improve quality. This study was not intended to obtain patient-oriented outcomes, such as decreased pain and improved function. The study was designed to improve patient safety and to standardize a process for prescribing and monitoring patients on COT. In the future we plan to look at patient outcomes and expand our focus to patients on high-dose opioids and those on combination therapy with benzodiazepines.

We believe the most impactful process steps were our letters sent to COT patients describing our updated, standardized prescribing process, and the ensuing provider-patient discussion to review the risks, benefits, and alternatives to opioid therapy. This frank discussion of treatment options resulted in more than 1 in 5 patients electing to discontinue COT.

There was an association between opioid discontinuation and patients not signing the CSA. This may have been due to patients deciding to discontinue opioids at the initiation review with providers after they received their letter. Therefore, signing the agreement was no longer necessary.
Continue to: We noted that some patients...
We noted that some patients elected to begin a new, nonopioid medication intended to augment their pain relief. However, they did not decrease their use of opioid medicines. We did not collect pain rating scale scores to determine whether the addition of augmenting medicines provided a reduction in pain perception.
Close monitoring of COT patients with frequent office visits may have had an impact on their care. We noted an association between more frequent visits and initiation of pain augmentation medicines. There was also a nonsignificant trend between office visit frequency and dose reduction. These are topics we may re-examine in our practice over time. There was no change in office visit frequency with our intervention, likely a result of these patients having frequent office visits for multiple comorbid medical conditions at baseline.
Evidence of similar benefits in primary care practices that standardized their opioid prescribing guidelines for patients on COT11 illustrates the importance of such a process for ensuring patient safety and decreasing opioid dosage and use.
Limitations to our project are that we did not measure functional changes and quality-of-life scores for patients. We also did not note the opioid dosages for individuals who chose to stop using opioids.
Looking forward. Based on our experience, patient notification with discussion of COT risks, benefits, and alternatives, as well as implementation of a process to monitor COT, appear to be related to patients’ decisions to discontinue COT. Our new standard process did show QI in the process steps but remained suboptimal to our expectations of clinical impact. More frequent office visits may impact patient decisions to reduce opioid dose and to add an augmenting pain medication. We plan to increase the involvement and responsibilities of our allied health staff in our processes to improve rates of adherence and the overall quality of how we manage patients on chronic opioid therapy.
CORRESPONDENCE
David Patchett, DO, Mayo Clinic, 13400 East Shea Blvd, Scottsdale, AZ 85259; [email protected]
ABSTRACT
Purpose The “opioid epidemic” in the United States has received increasing attention over the past few years. Most drug overdose deaths involve an opioid, and prescription opioid deaths have quadrupled since 1999. We sought to improve patient safety and adhere to clinical guidelines by standardizing opioid prescribing in our practice.
Methods We implemented a standardized approach to opioid prescribing based on Arizona Department of Health Services guidelines. All of our providers received instruction on Arizona’s Controlled Substance Prescription Monitoring Program (AZCSPMP) database and were encouraged to use it online. Our goal was for patients to have quarterly office visits, complete random urine drug screens, and sign a controlled substance agreement (CSA). The CSA acknowledged their understanding of the risks and benefits of opioid therapy as well as our updated prescribing policies.
Results Three-hundred fifty-eight of our practice’s patients were receiving chronic opioid therapy. All providers enrolled in AZCSPMP and used it for patient care. We increased rates of signed CSAs from 4.5% to 43.6%, and urine drug screening from 0.8% to 20.1%. For 325 patients remaining in the practice after our interventions, a postintervention chart review demonstrated a statistically significant discontinuation of opioid therapy (71/325, 21.8%; 95% confidence interval, 17.4%-26.7%).
Conclusion Implementation of a standardized opioid prescribing process resulted in discontinuation of therapy for some patients. Rates increased for signed CSAs and completed random urine drug screening. Future process interventions may improve patient and provider adherence. All primary care physicians should examine their prescribing processes to enhance the safety of opioid therapy.
[polldaddy:10370177]
The US opioid epidemic has received increased attention both nationally and at the state level over the past 2 years. This attention is warranted given the significant societal burden of opioid misuse, abuse, and overdose. Most drug overdose deaths (> 6/10) involve an opioid.1 Deaths from prescription opioids have quadrupled since 1999 in the United States.2 Arizona, the state in which we practice, ranked sixth highest in the nation for drug overdose deaths and had the fifth highest opioid prescribing rate in 2011.3 In response to the growing epidemic, the Centers for Disease Control and Prevention (CDC) released guidelines in 2016 for prescribing and monitoring opioids for chronic pain.4
Chronic nonterminal pain (CNTP) remains a significant cause of human suffering and is more prevalent in the United States than cancer, diabetes, and heart disease combined.5 The increased use of opioids since 1999 to ease CNTP has not reduced Americans’ reports of pain overall.6,7 Given the growing opioid epidemic and disease burden of CNTP, we embarked on a quality improvement (QI) project to safely prescribe and refill opioid medications in the Department of Family Medicine at the Mayo Clinic Arizona.
METHODS
This project received an exemption from internal review board evaluation as a QI intervention. We used a team-based approach to address standardization of opioid prescribing and monitoring within our practice. The team included physicians (MD/DO), nurses (LPN/RN), and allied health staff (MA), operations and administrative personnel, and information technology (IT) support. We did not involve patients in the initial design of our project. With future quality efforts in this area, we plan to involve patients in design processes.
Continue to: We began by identifying...
We began by identifying the scope of the problem, establishing criteria to search the electronic medical record (EMR) and identify appropriate patients. Chronic pain is often defined as pain lasting more than 3 months. Chronic opioid therapy (COT) has been defined as opioid use lasting longer than 3 months.8 Working with our IT colleagues, we defined COT patients as those with 3 or more prescriptions for opioids in the past year or those who received ≥ 30 pills a month (ie, patients who received 180 pills with 2 prescriptions written for the year). This definition gave us the ability to query our EMR to determine which patients were on COT, and we prepared lists of patients by primary care provider (FIGURE). Providers reviewed the lists to ensure these individuals were in fact on COT for CNTP. The number of patients identified after EMR query and provider review was 358, comprising 2.6% of 14,000 empaneled patients.

We based our interventions on the Arizona Department of Health Services 2014 opioid prescribing guidelines.3 The Arizona guidelines used existing national and state opioid prescribing guidelines along with clinical practice guidelines. Our study began prior to the 2016 CDC guidelines, so they were not used in this study. Our practice guidelines recommended that all 23 of our providers (MDs, DOs, and NPs) sign up for Arizona’s Controlled Substance Prescription Monitoring Program (AZCSPMP). We asked each patient to sign a controlled substance agreement (CSA), acknowledging their awareness of our proposed processes and the discussion of opioid therapy. Patients were expected to have face-to-face visits with providers at least quarterly and to complete a random urine drug screen at least annually. Patients were not incentivized to complete the process. We placed reminder calls for appointments just as we do for regular appointments.
Providers were asked to complete the Opioid Risk Tool9 with the patient at the initial visit, discuss the risks, benefits, and alternatives of long-term use of opioid medication, and review the 6 As (analgesia, activity, aberrant drug related behavior, adverse effects, affect, and adjunctive treatments). On the day before each patient visit, providers were reminded by a note in the EMR schedule to check AZCSPMP. Initial appointment times would be 30 minutes and follow-up appointments would be scheduled for 15 minutes if only addressing COT.
The QI project was introduced at an all-staff meeting in October 2015 that included providers, allied health staff, front desk personnel, and administrative staff, with the goal of beginning our COT process in November. We mailed letters to COT patients describing our new guidelines and asking them to call to schedule an appointment. If patients on COT came into the office for an alternate appointment and had not yet been seen for a COT visit, providers were encouraged to complete the COT process at that time.
We created a standard order set in the EMR for initial and follow-up visits and for the urine drug screen. We also added an interactive form to the EMR allowing providers to electronically complete the Opioid Risk Tool, and to confirm CSA completion and AZCSPMP review. We developed a database that would query the EMR for patient office visit frequency, CSA completion, and urine drug screen collection. We also placed paper copies of forms in exam rooms with a laminated instruction sheet reviewing the process steps and the 6 As.
Continue to: Soft rollout was...
Soft rollout was November 1, 2015, to assist in working through the process before full rollout. We asked providers to complete the full process on at least 1 patient during this period. This run-through would help ensure that allied health staff who room the patients would have the CSA and Opioid Risk Tool already in the chart before the visit. Full rollout was January 2, 2016. Every 2 to 4 weeks after the full rollout, regular email reminders were sent to providers about the project process and allowed for any feedback about issues that arose.
We provided regular updates and discussed the process at department meetings monthly. Quarterly data were reviewed and discussed for the first year of implementation. Providers and staff completed a chart review for each COT patient at project completion, to determine whether opioids had been decreased (in dosage) or discontinued, a nonopioid medicine had been initiated to augment pain control, or whether patients had died or left the practice.
Statistical analysis
We summarized binary data as counts and proportions and compared them using the chi square test. We summarized discrete data by their mean and standard deviation. To analyze binary variables measured repeatedly in time, we used the logistic generalized estimating equation (GEE) with an autoregressive (AR-1) correlation structure. We computed 95% confidence intervals (CIs)for odds ratios using the empirical or “sandwich” standard error estimates. For discrete variables representing counts, we used the negative binomial regression model.
For count data, a Poisson model is typically used; in our case the variance was considerably larger than the mean, exceeding the Poisson-model requirement that they not be significantly different if not exactly the same. This implies that the data are “over dispersed” or more variable than a Poisson model is thought to be able to model accurately. We therefore used a negative binomial model, which is regarded as the better model in this situation. The 95% CIs for the estimate resulting from the negative binomial regression model were computed using the profile-likelihood.10 All GEEs were clustered on patients (n = 358). We used SAS version 9.3 (Cary, NC) for all analyses.

RESULTS
All providers enrolled for AZCSPMP. CSA completion increased from 16 (4.5%) at baseline to 156 (43.6%) after intervention (P < .001). Patients completed a urine drug screen more frequently as well, from 3 (0.8%) to 72 (20.1%) (P < .001) (TABLES 1 and 2). No statistically significant change was noted in the frequency of office visits.

Continue to: We excluded 33 patients...
We excluded 33 patients from the post-intervention chart review (TABLE 3). Twenty-seven had left the practice and 6 had died, leaving 325 patients included in the post-intervention chart review.

There was a statistically significant association between patients who discontinued opioids and those who neglected to sign a CSA (P < .001) (TABLE 4). We tested for associations between office visit frequency and process step completion. There was a nonsignificant trend between increased frequency of office visits and opioid dose reduction. Patients who stopped opioids had fewer office visits (TABLE 5), while patients who had initiated a medication to augment pain relief had more frequent office visits (TABLE 6).

DISCUSSION
Our interventions to improve the quality of our COT processes were moderately successful. We achieved statistically significant increases in our rates of CSA completion and in urine drug screening. However, these increases were not as clinically impactful as we had hoped. Improvements in both patient and provider adherence are needed. We plan to engage allied health staff more fully to assist with adherence and thereby improve quality. This study was not intended to obtain patient-oriented outcomes, such as decreased pain and improved function. The study was designed to improve patient safety and to standardize a process for prescribing and monitoring patients on COT. In the future we plan to look at patient outcomes and expand our focus to patients on high-dose opioids and those on combination therapy with benzodiazepines.

We believe the most impactful process steps were our letters sent to COT patients describing our updated, standardized prescribing process, and the ensuing provider-patient discussion to review the risks, benefits, and alternatives to opioid therapy. This frank discussion of treatment options resulted in more than 1 in 5 patients electing to discontinue COT.

There was an association between opioid discontinuation and patients not signing the CSA. This may have been due to patients deciding to discontinue opioids at the initiation review with providers after they received their letter. Therefore, signing the agreement was no longer necessary.
Continue to: We noted that some patients...
We noted that some patients elected to begin a new, nonopioid medication intended to augment their pain relief. However, they did not decrease their use of opioid medicines. We did not collect pain rating scale scores to determine whether the addition of augmenting medicines provided a reduction in pain perception.
Close monitoring of COT patients with frequent office visits may have had an impact on their care. We noted an association between more frequent visits and initiation of pain augmentation medicines. There was also a nonsignificant trend between office visit frequency and dose reduction. These are topics we may re-examine in our practice over time. There was no change in office visit frequency with our intervention, likely a result of these patients having frequent office visits for multiple comorbid medical conditions at baseline.
Evidence of similar benefits in primary care practices that standardized their opioid prescribing guidelines for patients on COT11 illustrates the importance of such a process for ensuring patient safety and decreasing opioid dosage and use.
Limitations to our project are that we did not measure functional changes and quality-of-life scores for patients. We also did not note the opioid dosages for individuals who chose to stop using opioids.
Looking forward. Based on our experience, patient notification with discussion of COT risks, benefits, and alternatives, as well as implementation of a process to monitor COT, appear to be related to patients’ decisions to discontinue COT. Our new standard process did show QI in the process steps but remained suboptimal to our expectations of clinical impact. More frequent office visits may impact patient decisions to reduce opioid dose and to add an augmenting pain medication. We plan to increase the involvement and responsibilities of our allied health staff in our processes to improve rates of adherence and the overall quality of how we manage patients on chronic opioid therapy.
CORRESPONDENCE
David Patchett, DO, Mayo Clinic, 13400 East Shea Blvd, Scottsdale, AZ 85259; [email protected]
1. Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths — United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65:1445-1452.
2. CDC. Opioid data analysis and resources. https://www.cdc.gov/drugoverdose/data/analysis.html. Published December 19, 2018. Accessed May 27, 2019.
3. Arizona Department of Health Services. Arizona opioid prescribing guidelines. https://www.azdhs.gov/documents/audiences/clinicians/clinical-guidelines-recommendations/prescribing-guidelines/az-opiod-prescribing-guidelines.pdf. Published November 2014. Accessed May 27, 2019.
4. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
5. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011.
6. Chang H, Daubresse M, Kruszewski S, et al. Prevalence and treatment of pain in EDs in the United States, 2000 to 2010. Am J Emerg Med. 2014;32:421-431.
7. Daubresse M, Chang H, Yu Y, et al. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000 - 2010. Med Care. 2013;51:870-878.
8. Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162:276-286.
9. Webster LR, Webster RM. Predicting aberrant behaviors in opioid‐treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6:432-442.
10. Hilbe JM. Negative Binomial Regression. Cambridge, United Kingdom: Cambridge University Press; 2013.
11. Liebschutz JM, Xuan Z, Shanahan CW, et al. Improving adherence to long-term opioid therapy guidelines to reduce opioid misuse in primary care: a cluster-randomized clinical trial. JAMA Intern Med. 2017;177:1265-1272.
1. Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths — United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65:1445-1452.
2. CDC. Opioid data analysis and resources. https://www.cdc.gov/drugoverdose/data/analysis.html. Published December 19, 2018. Accessed May 27, 2019.
3. Arizona Department of Health Services. Arizona opioid prescribing guidelines. https://www.azdhs.gov/documents/audiences/clinicians/clinical-guidelines-recommendations/prescribing-guidelines/az-opiod-prescribing-guidelines.pdf. Published November 2014. Accessed May 27, 2019.
4. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
5. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011.
6. Chang H, Daubresse M, Kruszewski S, et al. Prevalence and treatment of pain in EDs in the United States, 2000 to 2010. Am J Emerg Med. 2014;32:421-431.
7. Daubresse M, Chang H, Yu Y, et al. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000 - 2010. Med Care. 2013;51:870-878.
8. Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162:276-286.
9. Webster LR, Webster RM. Predicting aberrant behaviors in opioid‐treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6:432-442.
10. Hilbe JM. Negative Binomial Regression. Cambridge, United Kingdom: Cambridge University Press; 2013.
11. Liebschutz JM, Xuan Z, Shanahan CW, et al. Improving adherence to long-term opioid therapy guidelines to reduce opioid misuse in primary care: a cluster-randomized clinical trial. JAMA Intern Med. 2017;177:1265-1272.
Obstructive sleep apnea: A better Dx model for primary care
ABSTRACT
Purpose To derive a predictive model for obstructive sleep apnea (OSA) in primary care practice, using home-based overnight oximetry results to refine posttest probability (PTP) of disease after initial risk stratification with the Sleep Apnea Clinical Score (SACS).
Methods We performed secondary analyses on data from a SACS validation cohort, to compare the diagnostic accuracy of 3 overnight oximetry measurements (oxygen desaturation index [ODI], mean saturation, and minimum saturation) in predicting OSA. Receiver operator characteristics (ROC) were computed for each measurement independently and sequentially after risk stratifying with SACS. We examined the implications of oximetry results for OSA PTP for participants categorized as intermediate risk (SACS 6-14; 66/191 participants [35%]; OSA probability 41%). We calculated positive likelihood ratios (LR) for multiple ODI results and determined which ones allowed recalibration to high- or low-risk PTP.
Results Among the 3 oximetry findings, ODI best predicted OSA (area under the curve [AUC], 0.88; 95% confidence interval [CI], 0.83-0.93). An ODI ≥8.4 (likelihood ratio [LR], 4.19; 95% CI, 2.87-6.10) created a PTP of 77%, while an ODI of 0 to <8.4 (LR, 0.19, 95% CI, 0.12-0.33) created a 14% PTP. Sequential application of SACS and ODI results yielded an AUC result of 0.90 (95% CI, 0.85-0.95).
Conclusions SACS risk stratification provides an advantage over clinical gestalt. In those at intermediate risk, ODI results provide a simple and clinically useful way to further refine diagnostic prediction. Sequential use of SACS and selectively employed overnight oximetry may limit unnecessary polysomnography. Oximetry testing should be avoided in patients deemed low or high risk by SACS, as positive results do not substantially recalibrate risk.
Obstructive sleep apnea (OSA) is a prevalent and underdiagnosed condition. The National Sleep Foundation estimates that 18 million Americans have OSA.1 Primary care practice may be the best setting in which to identify OSA, as many of our patients have conditions frequently associated with apnea (eg, hypertension, obesity, diabetes, arrhythmia, and neurologic illness). Up to a third of patients in primary care practice may be at increased risk.2,3
Clinical guidelines of the American Academy of Sleep Medicine (AASM) recommend obtaining a sleep history to evaluate for possible OSA in 3 instances: as part of a routine health maintenance examination, during evaluation of specific complaints associated with OSA (eg, snoring, apnea, daytime sleepiness), and during comprehensive evaluations for individuals with high-risk conditions (ie, obesity, congestive heart failure, refractory hypertension, diabetes, stroke history).4
The American College of Physicians (ACP) Clinical Practice Guideline suggests assessing individuals who have unexplained daytime sleepiness.5 The ACP considers this assessment “High-Value Care,” as “evidence shows that before diagnosis, patients with OSA have higher rates of health care use, more frequent and longer hospital stays, and higher health care costs than after diagnosis.”5
Continue to: We recently validated the diagnostic accuracy...
We recently validated the diagnostic accuracy of the Sleep Apnea Clinical Score (SACS) for use in a primary care patient population suspected of having OSA.6 SACS uses historical and clinical data to derive a score that identifies a patient’s risk level.7 However, as an alternative to the 2 levels described in Flemons’ SACS,7 we propose creating 3 risk strata (FIGURE 17,8). We believe that patients at high risk (SACS ≥15) should be encouraged to undergo sleep evaluations as their posttest probability (PTP) of OSA is 75% to 80%. Individuals at low risk (SACS ≤5; PTP <20%) could receive lifestyle advice and simple clinical interventions that decrease symptoms (eg, weight loss, increased physical activity, sleeping on one’s side). For low-risk patients, clinical observation and reevaluation could take place over time with their primary care provider, without additional testing or referral to specialists.
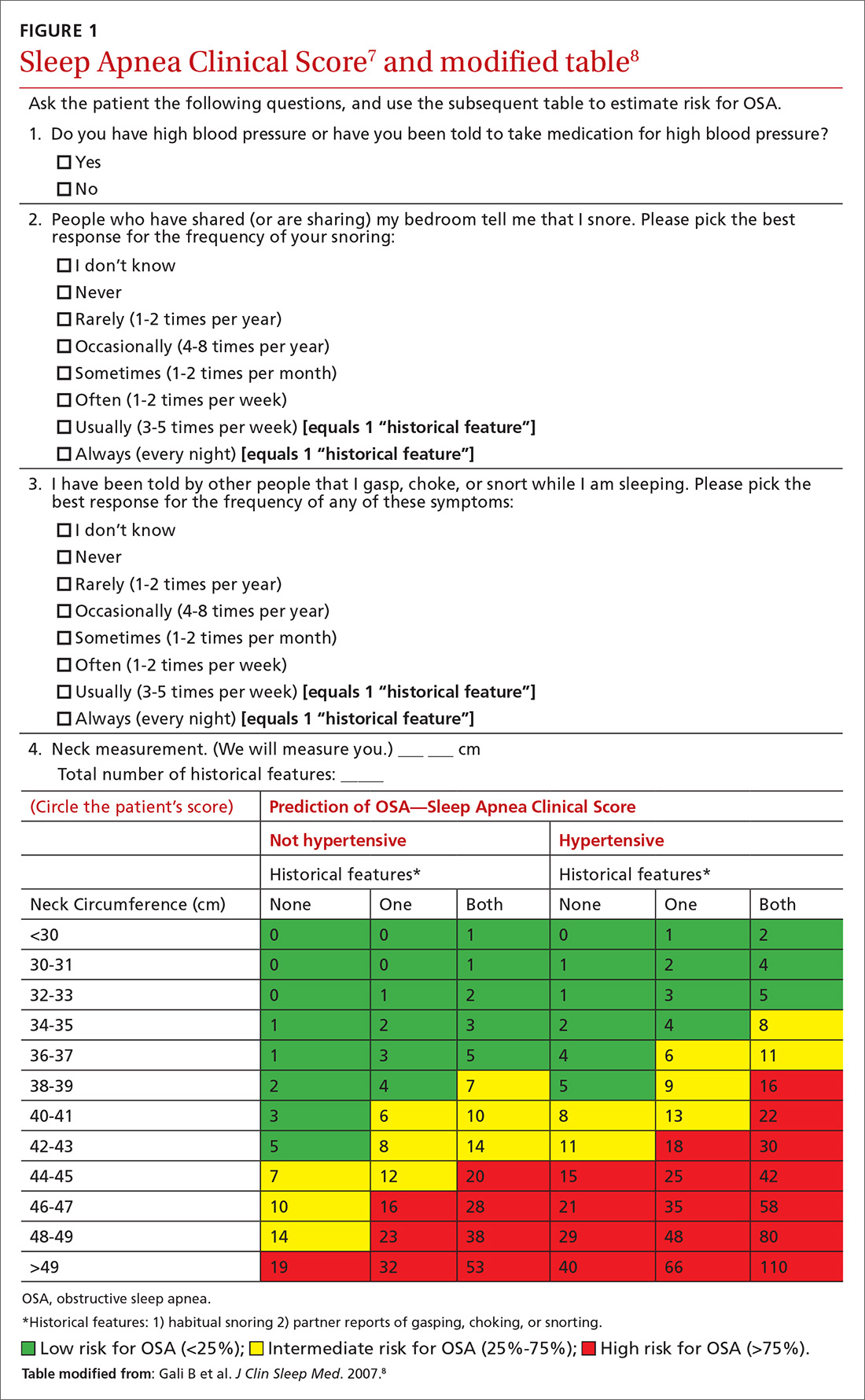
What about patients at intermediate risk? Many patients suspected of having OSA will be assigned to intermediate risk (SACS 6-14), and their PTP of OSA remains at 40% to 45%, the pre-test level most commonly encountered in suspected OSA. As polysomnography is a limited and expensive clinical resource, intermediate-risk patients would benefit from recalibration of their SACS-based risk assessment using an additional surrogate test such as home-based overnight oximetry. Our internal OSA practice guidelines recommend referral for sleep medicine consultation when oximetry results are abnormal—specifically, an oxygen desaturation index (ODI) of ≥5, a mean saturation less than 89%, and a minimum saturation of 75% or less.
Our objectives in this study were to compare the diagnostic implications of these 3 measurements from home-based overnight oximetry reports and use the most relevant result to derive a predictive model further refining PTP of OSA in a primary care patient population first stratified to intermediate risk by SACS.
METHODS
Subjects
We performed secondary analyses on data obtained from our SACS validation cohort.6 In brief, these were patients suspected of having OSA based on the presence of signs, symptoms, or associated risk factors. One hundred ninety-one patients completed all assessments. Sixty-six of 191 patients (35%) were categorized as intermediate risk (SACS 6-14; OSA probability 41% [27/66]).
Data collection and analyses
Participants completed home-based overnight oximetry using Nonin Model 2500 oximeters (Nonin Medical Inc., Plymouth, Minn). We transferred oximetry results from the sleep lab database to a statistical program for analyses of ODI, mean saturation, and minimal saturation. ODI was defined as the number of 4% drops in saturation from baseline divided by the number of hours of recording time. Although the AASM states that a diagnosis of OSA is confirmed if the number of obstructive events is more than 15 per hour or more than 5 per hour in a patient who reports related symptoms,4 we defined OSA as an apnea-hypopnea index (AHI) of >10 based on polysomnography (as this was the threshold used in the derivation cohort for SACS).7 We demonstrated the predictive ability of SACS at various AHI definitions of OSA in our validation cohort.6 The use of SACS in our validation cohort showed a statistically similar ability to predict OSA at both an AHI of 10 and 20, compared with the derivation cohort.
Continue to: We entered additional information...
We entered additional information reported directly by patients and obtained from their sleep studies into a REDCap database and transferred that to our statistical program. We used descriptive statistics to determine ranges and central tendencies of oximetry results. Receiver operator characteristic (ROC) analyses described the predictive abilities for each oximetry result individually and in serial application with prior SACS determinations. For comparison, we used the area under the ROC curve (AUC) from logistic regression to model the probability of OSA.
We calculated positive likelihood ratios (LR) and 95% confidence intervals (CI) to determine the degree of oximetry abnormality that would recalibrate risk either to a high PTP of OSA (>75%) or a low PTP (<25%). We sorted intermediate-risk SACS scores into quintiles based on ODI results to compare the resulting PTPs of OSA. We applied the PTP of OSA from our previous work (using the SACS score to compute the LR) as the new PTP, estimated the LR based on ODI, and computed an updated PTP of OSA. We also used ROC analysis to determine the optimal cutoff value of the ODI.
Finally, in accordance with our internal clinical practice recommendations, we examined the predictive ability of a “positive” ODI result of ≥5 to recalibrate risk prediction for OSA for patients in the low-risk group. We performed analyses using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
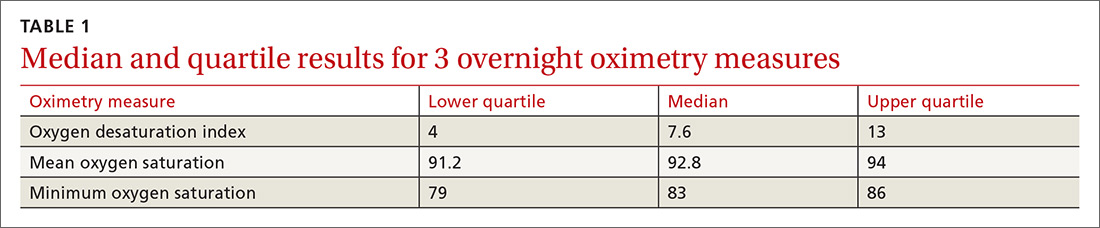
One hundred ninety-one subjects completed assessments. The median and quartile results for ODI, mean saturation, and minimum saturation are found in TABLE 1. TABLE 2 shows the distribution of patients with positive oximetry results. An ODI of 5 or greater was the most frequent abnormal result (135/191; 70.7%).
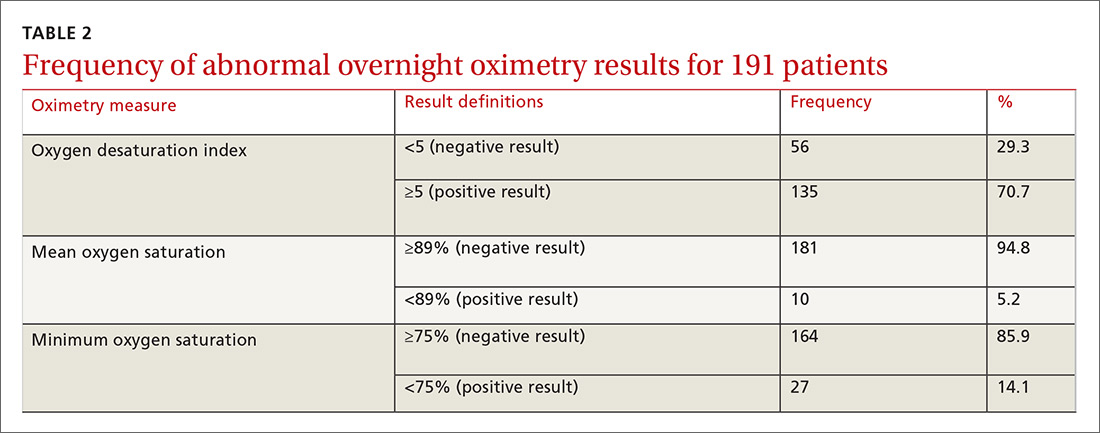
We used the AUC to measure the comparative abilities of SACS and the 3 overnight oximetry results in predicting OSA (TABLE 3). ODI results demonstrated the best ability to predict OSA, compared with polysomnography as the relative gold standard (AUC, 0.88; 95% confidence interval [CI], 0.83-0.93). Serial application of SACS and ODI yielded even better diagnostic results (AUC, 0.90; 95% CI, 0.85-0.95).
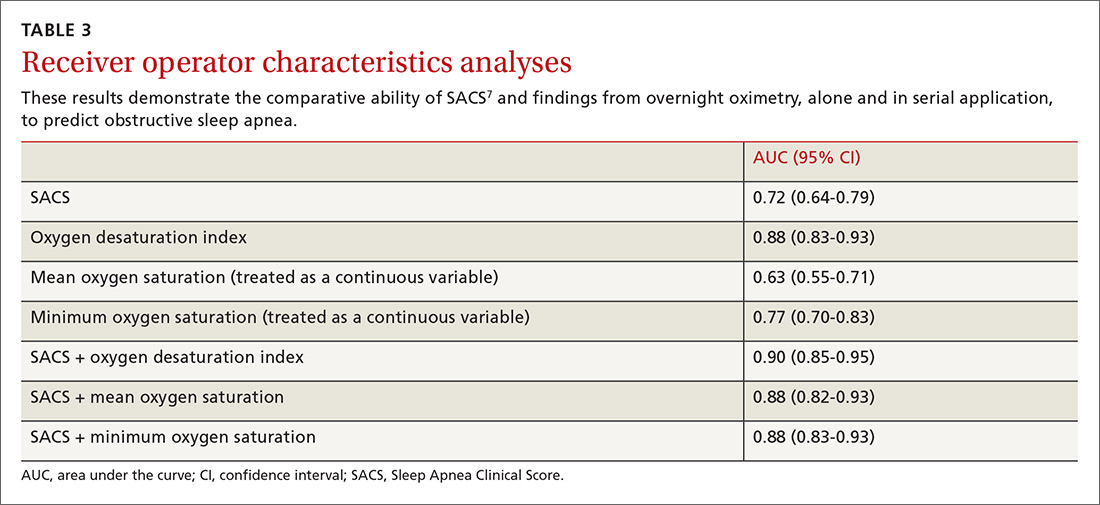
Continue to: As ODI was found to be the strongest predictor of OSA...
As ODI was found to be the strongest predictor of OSA, we grouped these results in quintiles and calculated positive LRs. TABLE 4 shows their effect on PTP of disease among patients with intermediate risk. An ODI result >10 effected an upward recalibration of disease probability (LR, 2.33; 95% CI, 1.27-4.26). The optimal cutoff of ODI to discriminate between those with and without OSA was determined by ROC analysis. An ODI greater than 8.4 created a PTP of disease of approximately 73% to 77%.
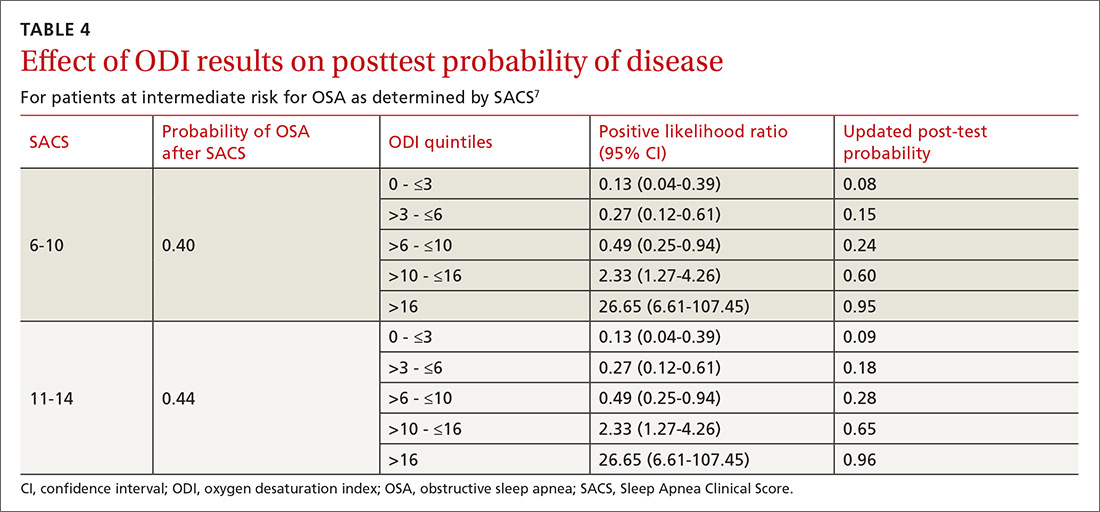
Our internal clinical guidelines recommend referring patients with an ODI of 5 or greater for sleep medicine consultation. We examined the ability of this ODI result to recalibrate disease suspicion for a patient at low risk (SACS ≤5). The LR for ODI of 5 or greater is 2.1, but this only results in a recalibration of risk from 24% pretest probability in our validation cohort to 41% PTP (95% CI, 33-49). This low cutoff for a positive test creates false-positive results more than 40% of the time due to low specificity (0.58). This is insufficient to change the suspicion of disease, resulting only in a shift to intermediate OSA risk.
DISCUSSION
Among 3 different oximetry measurements, an ODI ≥10 best predicts OSA, both independently and when used sequentially after the SACS. ODI was by far the most frequent abnormality on oximetry in our cohort, thereby increasing its utility in clinical decision making. For those subjects at intermediate risk, a cutoff of 10 for the ODI result may be a simple and clinically effective way to recalibrate risk and aid in making referral decisions. (This may also be simpler and more easily remembered by clinicians than the 8.4 ODI results from the ROC analyses.)
Assessment is inadequate without a clinical prediction rule. Unfortunately, providers cannot simply rely on clinical gestalt in diagnosing OSA. In their derivation cohort, Flemens et al examined the LRs created by SACS and by clinician prediction based on history and physical exam.7 The SACS LRs ranged from 5.17 to 0.25, a 20-fold range. This reflected superior diagnostic information compared with subjective physician impression, where LRs ranged from 3.7 to 0.52, a seven-fold range. Myers et al prepared a meta-analysis of 4 different trials that examined physicians’ ability to predict OSA.9 Despite the researchers’ use of experienced sleep medicine doctors, the overall diagnostic accuracy of clinical impression was modest (summary positive LR, 1.7; 95% CI, 1.5-2; I2 = 0%; summary negative LR, 0.67; 95% CI, 0.60-0.74; I2 = 10%; sensitivity, 58%; specificity, 67%). This is similar to reliance on a single clinical sign or symptom to predict OSA.
Wise use of oximetry augments SACS calculation. To limit unnecessary oximetry testing in low- and high-risk groups and to avoid polysomnography in cases of a low PTP of disease, we advocate limiting oximetry testing to individuals in the SACS intermediate-risk group (FIGURE 2) wherein ODI results can potentially recalibrate risk assessment up or down. (Those in the high- risk group should be referred to a sleep medicine specialist.) Our institutional recommendation of using an ODI result of ≥5 as a threshold to increase suspicion of disease requires a caveat for the low-risk group. “Positive” results at that low diagnostic threshold are frequently false.
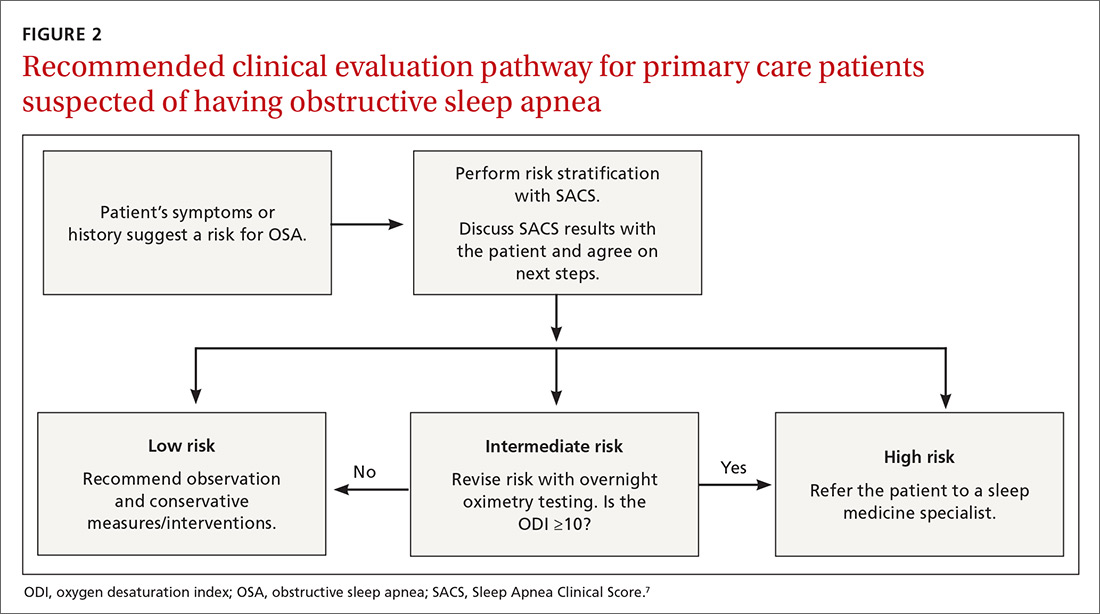
Continue to: Multiple benefits of SACS
Multiple benefits of SACS. We believe using the SACS calculation during clinical encounters with patients potentially at risk for OSA would increase diagnostic accuracy. Performing risk stratification with SACS should not be an undue burden on providers, and the increased time spent with patients has its own benefits, including helping them better understand their risk. Using this standardized process—augmented, as needed, with overnight ODI assessment—might also encourage more patients to follow through on subsequent recommendations, as their risk is further quantified objectively. Lastly, unnecessary testing with polysomnography could be avoided.
Limitations of our study. This study’s findings were derived from a patient population in a single institution. Replication of the findings from other settings would be helpful.
Looking forward. It is yet unclear if clinicians will embrace these strategies in real-world primary care practice. We have designed an implementation-and-dissemination trial to assess whether family physicians will use the SACS clinical predication rule in everyday practice and whether our evidence-based recommendations about overnight oximetry will be followed. Underlying our suggested clinical evaluation pathway (FIGURE 2) is the belief that there is value gained from sharing the decision-making process with patients. Although we provide new evidence that informs these conversations, the patient’s values and preferences are important when determining the best direction to proceed in the evaluation for suspected OSA. These recommendations are intended to aid, not replace, good clinical judgment.
Home-based sleep testing has become more widely available, is convenient for patients, and is less expensive than lab-based polysomnography. Our study did not directly address the appropriate circumstances for home studies in clinical evaluation. We rely on the expertise of our sleep medicine colleagues to determine which patients are appropriate candidates for home-based studies.
The AASM states that “portable monitors (PM) for the diagnosis of OSA should be [used] only in conjunction with a comprehensive sleep evaluation. Clinical sleep evaluations using PM must be supervised by a practitioner with board certification in sleep medicine or an individual who fulfills the eligibility criteria for the sleep medicine certification examination.”4 Additionally, the group recommends that PM “may be used in the unattended setting as an alternative to polysomnography for the diagnosis of OSA in patients with a high pretest probability of moderate to severe OSA and no comorbid sleep disorder or major comorbid medical disorders.”4
Continue to: GRANT SUPPORT
GRANT SUPPORT
The use of the REDCap database is supported by grant UL1 TR000135. This work was supported by a Mayo Foundation CR-20 grant awarded to Dr. Mookadam as Principal investigator and Dr. Grover as Coinvestigator.
Statistical analyses were supported, in part, by the Department of Family Medicine, Mayo Clinic, Scottsdale, Ariz.
CORRESPONDENCE
Michael Grover, DO, Mayo Clinic Thunderbird Primary Care Center-Family Medicine, 13737 N 92nd Street, Scottsdale, AZ 85260; [email protected]
1. National Sleep Foundation. Sleep apnea. https://sleepfoundation.org/sleep-disorders-problems/sleep-apnea. Accessed September 14, 2018.
2. Grover M, Mookadam M, Armas D, et al. Identifying patients at risk for obstructive sleep apnea in a primary care practice. J Am Board Fam Med. 2011;24:152-160.
3. Mold JW, Quattlebaum C, Schinnerer E, et al. Identification by primary care clinicians of patients with obstructive sleep apnea: a practice-based research network (PBRN) study. J Am Board Fam Med. 2011;24:138-145.
4. Epstein LJ, Kristo D, Strollo PJ, Jr., et al; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263-276.
5. Qaseem A, Dallas P, Owens DK, et al. Diagnosis of obstructive sleep apnea in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2014;161:210-220.
6. Grover M, Mookadam M, Chang Y-H, et al. Validating the Sleep Apnea Clinical Score for use in primary care populations. Mayo Clin Proc. 2016;91:469-476.
7. Flemons WW, Whitelaw WA, Brant R, et al. Likelihood ratios for a sleep apnea clinical prediction rule. Am J Respir Crit Care Med. 1994;150:1279-1285.
8. Gali B, Whalen FX, Gay PC, et al. Management plan to reduce risks in perioperative care of patients with presumed obstructive sleep apnea syndrome. J Clin Sleep Med. 2007;3:582-588.
9. Myers KA, Mrkobrada M, Simel DL. Does this patient have obstructive sleep apnea?: The rational clinical examination systematic review. JAMA. 2013;310(7):731-741.
ABSTRACT
Purpose To derive a predictive model for obstructive sleep apnea (OSA) in primary care practice, using home-based overnight oximetry results to refine posttest probability (PTP) of disease after initial risk stratification with the Sleep Apnea Clinical Score (SACS).
Methods We performed secondary analyses on data from a SACS validation cohort, to compare the diagnostic accuracy of 3 overnight oximetry measurements (oxygen desaturation index [ODI], mean saturation, and minimum saturation) in predicting OSA. Receiver operator characteristics (ROC) were computed for each measurement independently and sequentially after risk stratifying with SACS. We examined the implications of oximetry results for OSA PTP for participants categorized as intermediate risk (SACS 6-14; 66/191 participants [35%]; OSA probability 41%). We calculated positive likelihood ratios (LR) for multiple ODI results and determined which ones allowed recalibration to high- or low-risk PTP.
Results Among the 3 oximetry findings, ODI best predicted OSA (area under the curve [AUC], 0.88; 95% confidence interval [CI], 0.83-0.93). An ODI ≥8.4 (likelihood ratio [LR], 4.19; 95% CI, 2.87-6.10) created a PTP of 77%, while an ODI of 0 to <8.4 (LR, 0.19, 95% CI, 0.12-0.33) created a 14% PTP. Sequential application of SACS and ODI results yielded an AUC result of 0.90 (95% CI, 0.85-0.95).
Conclusions SACS risk stratification provides an advantage over clinical gestalt. In those at intermediate risk, ODI results provide a simple and clinically useful way to further refine diagnostic prediction. Sequential use of SACS and selectively employed overnight oximetry may limit unnecessary polysomnography. Oximetry testing should be avoided in patients deemed low or high risk by SACS, as positive results do not substantially recalibrate risk.
Obstructive sleep apnea (OSA) is a prevalent and underdiagnosed condition. The National Sleep Foundation estimates that 18 million Americans have OSA.1 Primary care practice may be the best setting in which to identify OSA, as many of our patients have conditions frequently associated with apnea (eg, hypertension, obesity, diabetes, arrhythmia, and neurologic illness). Up to a third of patients in primary care practice may be at increased risk.2,3
Clinical guidelines of the American Academy of Sleep Medicine (AASM) recommend obtaining a sleep history to evaluate for possible OSA in 3 instances: as part of a routine health maintenance examination, during evaluation of specific complaints associated with OSA (eg, snoring, apnea, daytime sleepiness), and during comprehensive evaluations for individuals with high-risk conditions (ie, obesity, congestive heart failure, refractory hypertension, diabetes, stroke history).4
The American College of Physicians (ACP) Clinical Practice Guideline suggests assessing individuals who have unexplained daytime sleepiness.5 The ACP considers this assessment “High-Value Care,” as “evidence shows that before diagnosis, patients with OSA have higher rates of health care use, more frequent and longer hospital stays, and higher health care costs than after diagnosis.”5
Continue to: We recently validated the diagnostic accuracy...
We recently validated the diagnostic accuracy of the Sleep Apnea Clinical Score (SACS) for use in a primary care patient population suspected of having OSA.6 SACS uses historical and clinical data to derive a score that identifies a patient’s risk level.7 However, as an alternative to the 2 levels described in Flemons’ SACS,7 we propose creating 3 risk strata (FIGURE 17,8). We believe that patients at high risk (SACS ≥15) should be encouraged to undergo sleep evaluations as their posttest probability (PTP) of OSA is 75% to 80%. Individuals at low risk (SACS ≤5; PTP <20%) could receive lifestyle advice and simple clinical interventions that decrease symptoms (eg, weight loss, increased physical activity, sleeping on one’s side). For low-risk patients, clinical observation and reevaluation could take place over time with their primary care provider, without additional testing or referral to specialists.

What about patients at intermediate risk? Many patients suspected of having OSA will be assigned to intermediate risk (SACS 6-14), and their PTP of OSA remains at 40% to 45%, the pre-test level most commonly encountered in suspected OSA. As polysomnography is a limited and expensive clinical resource, intermediate-risk patients would benefit from recalibration of their SACS-based risk assessment using an additional surrogate test such as home-based overnight oximetry. Our internal OSA practice guidelines recommend referral for sleep medicine consultation when oximetry results are abnormal—specifically, an oxygen desaturation index (ODI) of ≥5, a mean saturation less than 89%, and a minimum saturation of 75% or less.
Our objectives in this study were to compare the diagnostic implications of these 3 measurements from home-based overnight oximetry reports and use the most relevant result to derive a predictive model further refining PTP of OSA in a primary care patient population first stratified to intermediate risk by SACS.
METHODS
Subjects
We performed secondary analyses on data obtained from our SACS validation cohort.6 In brief, these were patients suspected of having OSA based on the presence of signs, symptoms, or associated risk factors. One hundred ninety-one patients completed all assessments. Sixty-six of 191 patients (35%) were categorized as intermediate risk (SACS 6-14; OSA probability 41% [27/66]).
Data collection and analyses
Participants completed home-based overnight oximetry using Nonin Model 2500 oximeters (Nonin Medical Inc., Plymouth, Minn). We transferred oximetry results from the sleep lab database to a statistical program for analyses of ODI, mean saturation, and minimal saturation. ODI was defined as the number of 4% drops in saturation from baseline divided by the number of hours of recording time. Although the AASM states that a diagnosis of OSA is confirmed if the number of obstructive events is more than 15 per hour or more than 5 per hour in a patient who reports related symptoms,4 we defined OSA as an apnea-hypopnea index (AHI) of >10 based on polysomnography (as this was the threshold used in the derivation cohort for SACS).7 We demonstrated the predictive ability of SACS at various AHI definitions of OSA in our validation cohort.6 The use of SACS in our validation cohort showed a statistically similar ability to predict OSA at both an AHI of 10 and 20, compared with the derivation cohort.
Continue to: We entered additional information...
We entered additional information reported directly by patients and obtained from their sleep studies into a REDCap database and transferred that to our statistical program. We used descriptive statistics to determine ranges and central tendencies of oximetry results. Receiver operator characteristic (ROC) analyses described the predictive abilities for each oximetry result individually and in serial application with prior SACS determinations. For comparison, we used the area under the ROC curve (AUC) from logistic regression to model the probability of OSA.
We calculated positive likelihood ratios (LR) and 95% confidence intervals (CI) to determine the degree of oximetry abnormality that would recalibrate risk either to a high PTP of OSA (>75%) or a low PTP (<25%). We sorted intermediate-risk SACS scores into quintiles based on ODI results to compare the resulting PTPs of OSA. We applied the PTP of OSA from our previous work (using the SACS score to compute the LR) as the new PTP, estimated the LR based on ODI, and computed an updated PTP of OSA. We also used ROC analysis to determine the optimal cutoff value of the ODI.
Finally, in accordance with our internal clinical practice recommendations, we examined the predictive ability of a “positive” ODI result of ≥5 to recalibrate risk prediction for OSA for patients in the low-risk group. We performed analyses using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS

One hundred ninety-one subjects completed assessments. The median and quartile results for ODI, mean saturation, and minimum saturation are found in TABLE 1. TABLE 2 shows the distribution of patients with positive oximetry results. An ODI of 5 or greater was the most frequent abnormal result (135/191; 70.7%).

We used the AUC to measure the comparative abilities of SACS and the 3 overnight oximetry results in predicting OSA (TABLE 3). ODI results demonstrated the best ability to predict OSA, compared with polysomnography as the relative gold standard (AUC, 0.88; 95% confidence interval [CI], 0.83-0.93). Serial application of SACS and ODI yielded even better diagnostic results (AUC, 0.90; 95% CI, 0.85-0.95).

Continue to: As ODI was found to be the strongest predictor of OSA...
As ODI was found to be the strongest predictor of OSA, we grouped these results in quintiles and calculated positive LRs. TABLE 4 shows their effect on PTP of disease among patients with intermediate risk. An ODI result >10 effected an upward recalibration of disease probability (LR, 2.33; 95% CI, 1.27-4.26). The optimal cutoff of ODI to discriminate between those with and without OSA was determined by ROC analysis. An ODI greater than 8.4 created a PTP of disease of approximately 73% to 77%.

Our internal clinical guidelines recommend referring patients with an ODI of 5 or greater for sleep medicine consultation. We examined the ability of this ODI result to recalibrate disease suspicion for a patient at low risk (SACS ≤5). The LR for ODI of 5 or greater is 2.1, but this only results in a recalibration of risk from 24% pretest probability in our validation cohort to 41% PTP (95% CI, 33-49). This low cutoff for a positive test creates false-positive results more than 40% of the time due to low specificity (0.58). This is insufficient to change the suspicion of disease, resulting only in a shift to intermediate OSA risk.
DISCUSSION
Among 3 different oximetry measurements, an ODI ≥10 best predicts OSA, both independently and when used sequentially after the SACS. ODI was by far the most frequent abnormality on oximetry in our cohort, thereby increasing its utility in clinical decision making. For those subjects at intermediate risk, a cutoff of 10 for the ODI result may be a simple and clinically effective way to recalibrate risk and aid in making referral decisions. (This may also be simpler and more easily remembered by clinicians than the 8.4 ODI results from the ROC analyses.)
Assessment is inadequate without a clinical prediction rule. Unfortunately, providers cannot simply rely on clinical gestalt in diagnosing OSA. In their derivation cohort, Flemens et al examined the LRs created by SACS and by clinician prediction based on history and physical exam.7 The SACS LRs ranged from 5.17 to 0.25, a 20-fold range. This reflected superior diagnostic information compared with subjective physician impression, where LRs ranged from 3.7 to 0.52, a seven-fold range. Myers et al prepared a meta-analysis of 4 different trials that examined physicians’ ability to predict OSA.9 Despite the researchers’ use of experienced sleep medicine doctors, the overall diagnostic accuracy of clinical impression was modest (summary positive LR, 1.7; 95% CI, 1.5-2; I2 = 0%; summary negative LR, 0.67; 95% CI, 0.60-0.74; I2 = 10%; sensitivity, 58%; specificity, 67%). This is similar to reliance on a single clinical sign or symptom to predict OSA.
Wise use of oximetry augments SACS calculation. To limit unnecessary oximetry testing in low- and high-risk groups and to avoid polysomnography in cases of a low PTP of disease, we advocate limiting oximetry testing to individuals in the SACS intermediate-risk group (FIGURE 2) wherein ODI results can potentially recalibrate risk assessment up or down. (Those in the high- risk group should be referred to a sleep medicine specialist.) Our institutional recommendation of using an ODI result of ≥5 as a threshold to increase suspicion of disease requires a caveat for the low-risk group. “Positive” results at that low diagnostic threshold are frequently false.

Continue to: Multiple benefits of SACS
Multiple benefits of SACS. We believe using the SACS calculation during clinical encounters with patients potentially at risk for OSA would increase diagnostic accuracy. Performing risk stratification with SACS should not be an undue burden on providers, and the increased time spent with patients has its own benefits, including helping them better understand their risk. Using this standardized process—augmented, as needed, with overnight ODI assessment—might also encourage more patients to follow through on subsequent recommendations, as their risk is further quantified objectively. Lastly, unnecessary testing with polysomnography could be avoided.
Limitations of our study. This study’s findings were derived from a patient population in a single institution. Replication of the findings from other settings would be helpful.
Looking forward. It is yet unclear if clinicians will embrace these strategies in real-world primary care practice. We have designed an implementation-and-dissemination trial to assess whether family physicians will use the SACS clinical predication rule in everyday practice and whether our evidence-based recommendations about overnight oximetry will be followed. Underlying our suggested clinical evaluation pathway (FIGURE 2) is the belief that there is value gained from sharing the decision-making process with patients. Although we provide new evidence that informs these conversations, the patient’s values and preferences are important when determining the best direction to proceed in the evaluation for suspected OSA. These recommendations are intended to aid, not replace, good clinical judgment.
Home-based sleep testing has become more widely available, is convenient for patients, and is less expensive than lab-based polysomnography. Our study did not directly address the appropriate circumstances for home studies in clinical evaluation. We rely on the expertise of our sleep medicine colleagues to determine which patients are appropriate candidates for home-based studies.
The AASM states that “portable monitors (PM) for the diagnosis of OSA should be [used] only in conjunction with a comprehensive sleep evaluation. Clinical sleep evaluations using PM must be supervised by a practitioner with board certification in sleep medicine or an individual who fulfills the eligibility criteria for the sleep medicine certification examination.”4 Additionally, the group recommends that PM “may be used in the unattended setting as an alternative to polysomnography for the diagnosis of OSA in patients with a high pretest probability of moderate to severe OSA and no comorbid sleep disorder or major comorbid medical disorders.”4
Continue to: GRANT SUPPORT
GRANT SUPPORT
The use of the REDCap database is supported by grant UL1 TR000135. This work was supported by a Mayo Foundation CR-20 grant awarded to Dr. Mookadam as Principal investigator and Dr. Grover as Coinvestigator.
Statistical analyses were supported, in part, by the Department of Family Medicine, Mayo Clinic, Scottsdale, Ariz.
CORRESPONDENCE
Michael Grover, DO, Mayo Clinic Thunderbird Primary Care Center-Family Medicine, 13737 N 92nd Street, Scottsdale, AZ 85260; [email protected]
ABSTRACT
Purpose To derive a predictive model for obstructive sleep apnea (OSA) in primary care practice, using home-based overnight oximetry results to refine posttest probability (PTP) of disease after initial risk stratification with the Sleep Apnea Clinical Score (SACS).
Methods We performed secondary analyses on data from a SACS validation cohort, to compare the diagnostic accuracy of 3 overnight oximetry measurements (oxygen desaturation index [ODI], mean saturation, and minimum saturation) in predicting OSA. Receiver operator characteristics (ROC) were computed for each measurement independently and sequentially after risk stratifying with SACS. We examined the implications of oximetry results for OSA PTP for participants categorized as intermediate risk (SACS 6-14; 66/191 participants [35%]; OSA probability 41%). We calculated positive likelihood ratios (LR) for multiple ODI results and determined which ones allowed recalibration to high- or low-risk PTP.
Results Among the 3 oximetry findings, ODI best predicted OSA (area under the curve [AUC], 0.88; 95% confidence interval [CI], 0.83-0.93). An ODI ≥8.4 (likelihood ratio [LR], 4.19; 95% CI, 2.87-6.10) created a PTP of 77%, while an ODI of 0 to <8.4 (LR, 0.19, 95% CI, 0.12-0.33) created a 14% PTP. Sequential application of SACS and ODI results yielded an AUC result of 0.90 (95% CI, 0.85-0.95).
Conclusions SACS risk stratification provides an advantage over clinical gestalt. In those at intermediate risk, ODI results provide a simple and clinically useful way to further refine diagnostic prediction. Sequential use of SACS and selectively employed overnight oximetry may limit unnecessary polysomnography. Oximetry testing should be avoided in patients deemed low or high risk by SACS, as positive results do not substantially recalibrate risk.
Obstructive sleep apnea (OSA) is a prevalent and underdiagnosed condition. The National Sleep Foundation estimates that 18 million Americans have OSA.1 Primary care practice may be the best setting in which to identify OSA, as many of our patients have conditions frequently associated with apnea (eg, hypertension, obesity, diabetes, arrhythmia, and neurologic illness). Up to a third of patients in primary care practice may be at increased risk.2,3
Clinical guidelines of the American Academy of Sleep Medicine (AASM) recommend obtaining a sleep history to evaluate for possible OSA in 3 instances: as part of a routine health maintenance examination, during evaluation of specific complaints associated with OSA (eg, snoring, apnea, daytime sleepiness), and during comprehensive evaluations for individuals with high-risk conditions (ie, obesity, congestive heart failure, refractory hypertension, diabetes, stroke history).4
The American College of Physicians (ACP) Clinical Practice Guideline suggests assessing individuals who have unexplained daytime sleepiness.5 The ACP considers this assessment “High-Value Care,” as “evidence shows that before diagnosis, patients with OSA have higher rates of health care use, more frequent and longer hospital stays, and higher health care costs than after diagnosis.”5
Continue to: We recently validated the diagnostic accuracy...
We recently validated the diagnostic accuracy of the Sleep Apnea Clinical Score (SACS) for use in a primary care patient population suspected of having OSA.6 SACS uses historical and clinical data to derive a score that identifies a patient’s risk level.7 However, as an alternative to the 2 levels described in Flemons’ SACS,7 we propose creating 3 risk strata (FIGURE 17,8). We believe that patients at high risk (SACS ≥15) should be encouraged to undergo sleep evaluations as their posttest probability (PTP) of OSA is 75% to 80%. Individuals at low risk (SACS ≤5; PTP <20%) could receive lifestyle advice and simple clinical interventions that decrease symptoms (eg, weight loss, increased physical activity, sleeping on one’s side). For low-risk patients, clinical observation and reevaluation could take place over time with their primary care provider, without additional testing or referral to specialists.

What about patients at intermediate risk? Many patients suspected of having OSA will be assigned to intermediate risk (SACS 6-14), and their PTP of OSA remains at 40% to 45%, the pre-test level most commonly encountered in suspected OSA. As polysomnography is a limited and expensive clinical resource, intermediate-risk patients would benefit from recalibration of their SACS-based risk assessment using an additional surrogate test such as home-based overnight oximetry. Our internal OSA practice guidelines recommend referral for sleep medicine consultation when oximetry results are abnormal—specifically, an oxygen desaturation index (ODI) of ≥5, a mean saturation less than 89%, and a minimum saturation of 75% or less.
Our objectives in this study were to compare the diagnostic implications of these 3 measurements from home-based overnight oximetry reports and use the most relevant result to derive a predictive model further refining PTP of OSA in a primary care patient population first stratified to intermediate risk by SACS.
METHODS
Subjects
We performed secondary analyses on data obtained from our SACS validation cohort.6 In brief, these were patients suspected of having OSA based on the presence of signs, symptoms, or associated risk factors. One hundred ninety-one patients completed all assessments. Sixty-six of 191 patients (35%) were categorized as intermediate risk (SACS 6-14; OSA probability 41% [27/66]).
Data collection and analyses
Participants completed home-based overnight oximetry using Nonin Model 2500 oximeters (Nonin Medical Inc., Plymouth, Minn). We transferred oximetry results from the sleep lab database to a statistical program for analyses of ODI, mean saturation, and minimal saturation. ODI was defined as the number of 4% drops in saturation from baseline divided by the number of hours of recording time. Although the AASM states that a diagnosis of OSA is confirmed if the number of obstructive events is more than 15 per hour or more than 5 per hour in a patient who reports related symptoms,4 we defined OSA as an apnea-hypopnea index (AHI) of >10 based on polysomnography (as this was the threshold used in the derivation cohort for SACS).7 We demonstrated the predictive ability of SACS at various AHI definitions of OSA in our validation cohort.6 The use of SACS in our validation cohort showed a statistically similar ability to predict OSA at both an AHI of 10 and 20, compared with the derivation cohort.
Continue to: We entered additional information...
We entered additional information reported directly by patients and obtained from their sleep studies into a REDCap database and transferred that to our statistical program. We used descriptive statistics to determine ranges and central tendencies of oximetry results. Receiver operator characteristic (ROC) analyses described the predictive abilities for each oximetry result individually and in serial application with prior SACS determinations. For comparison, we used the area under the ROC curve (AUC) from logistic regression to model the probability of OSA.
We calculated positive likelihood ratios (LR) and 95% confidence intervals (CI) to determine the degree of oximetry abnormality that would recalibrate risk either to a high PTP of OSA (>75%) or a low PTP (<25%). We sorted intermediate-risk SACS scores into quintiles based on ODI results to compare the resulting PTPs of OSA. We applied the PTP of OSA from our previous work (using the SACS score to compute the LR) as the new PTP, estimated the LR based on ODI, and computed an updated PTP of OSA. We also used ROC analysis to determine the optimal cutoff value of the ODI.
Finally, in accordance with our internal clinical practice recommendations, we examined the predictive ability of a “positive” ODI result of ≥5 to recalibrate risk prediction for OSA for patients in the low-risk group. We performed analyses using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS

One hundred ninety-one subjects completed assessments. The median and quartile results for ODI, mean saturation, and minimum saturation are found in TABLE 1. TABLE 2 shows the distribution of patients with positive oximetry results. An ODI of 5 or greater was the most frequent abnormal result (135/191; 70.7%).

We used the AUC to measure the comparative abilities of SACS and the 3 overnight oximetry results in predicting OSA (TABLE 3). ODI results demonstrated the best ability to predict OSA, compared with polysomnography as the relative gold standard (AUC, 0.88; 95% confidence interval [CI], 0.83-0.93). Serial application of SACS and ODI yielded even better diagnostic results (AUC, 0.90; 95% CI, 0.85-0.95).

Continue to: As ODI was found to be the strongest predictor of OSA...
As ODI was found to be the strongest predictor of OSA, we grouped these results in quintiles and calculated positive LRs. TABLE 4 shows their effect on PTP of disease among patients with intermediate risk. An ODI result >10 effected an upward recalibration of disease probability (LR, 2.33; 95% CI, 1.27-4.26). The optimal cutoff of ODI to discriminate between those with and without OSA was determined by ROC analysis. An ODI greater than 8.4 created a PTP of disease of approximately 73% to 77%.

Our internal clinical guidelines recommend referring patients with an ODI of 5 or greater for sleep medicine consultation. We examined the ability of this ODI result to recalibrate disease suspicion for a patient at low risk (SACS ≤5). The LR for ODI of 5 or greater is 2.1, but this only results in a recalibration of risk from 24% pretest probability in our validation cohort to 41% PTP (95% CI, 33-49). This low cutoff for a positive test creates false-positive results more than 40% of the time due to low specificity (0.58). This is insufficient to change the suspicion of disease, resulting only in a shift to intermediate OSA risk.
DISCUSSION
Among 3 different oximetry measurements, an ODI ≥10 best predicts OSA, both independently and when used sequentially after the SACS. ODI was by far the most frequent abnormality on oximetry in our cohort, thereby increasing its utility in clinical decision making. For those subjects at intermediate risk, a cutoff of 10 for the ODI result may be a simple and clinically effective way to recalibrate risk and aid in making referral decisions. (This may also be simpler and more easily remembered by clinicians than the 8.4 ODI results from the ROC analyses.)
Assessment is inadequate without a clinical prediction rule. Unfortunately, providers cannot simply rely on clinical gestalt in diagnosing OSA. In their derivation cohort, Flemens et al examined the LRs created by SACS and by clinician prediction based on history and physical exam.7 The SACS LRs ranged from 5.17 to 0.25, a 20-fold range. This reflected superior diagnostic information compared with subjective physician impression, where LRs ranged from 3.7 to 0.52, a seven-fold range. Myers et al prepared a meta-analysis of 4 different trials that examined physicians’ ability to predict OSA.9 Despite the researchers’ use of experienced sleep medicine doctors, the overall diagnostic accuracy of clinical impression was modest (summary positive LR, 1.7; 95% CI, 1.5-2; I2 = 0%; summary negative LR, 0.67; 95% CI, 0.60-0.74; I2 = 10%; sensitivity, 58%; specificity, 67%). This is similar to reliance on a single clinical sign or symptom to predict OSA.
Wise use of oximetry augments SACS calculation. To limit unnecessary oximetry testing in low- and high-risk groups and to avoid polysomnography in cases of a low PTP of disease, we advocate limiting oximetry testing to individuals in the SACS intermediate-risk group (FIGURE 2) wherein ODI results can potentially recalibrate risk assessment up or down. (Those in the high- risk group should be referred to a sleep medicine specialist.) Our institutional recommendation of using an ODI result of ≥5 as a threshold to increase suspicion of disease requires a caveat for the low-risk group. “Positive” results at that low diagnostic threshold are frequently false.

Continue to: Multiple benefits of SACS
Multiple benefits of SACS. We believe using the SACS calculation during clinical encounters with patients potentially at risk for OSA would increase diagnostic accuracy. Performing risk stratification with SACS should not be an undue burden on providers, and the increased time spent with patients has its own benefits, including helping them better understand their risk. Using this standardized process—augmented, as needed, with overnight ODI assessment—might also encourage more patients to follow through on subsequent recommendations, as their risk is further quantified objectively. Lastly, unnecessary testing with polysomnography could be avoided.
Limitations of our study. This study’s findings were derived from a patient population in a single institution. Replication of the findings from other settings would be helpful.
Looking forward. It is yet unclear if clinicians will embrace these strategies in real-world primary care practice. We have designed an implementation-and-dissemination trial to assess whether family physicians will use the SACS clinical predication rule in everyday practice and whether our evidence-based recommendations about overnight oximetry will be followed. Underlying our suggested clinical evaluation pathway (FIGURE 2) is the belief that there is value gained from sharing the decision-making process with patients. Although we provide new evidence that informs these conversations, the patient’s values and preferences are important when determining the best direction to proceed in the evaluation for suspected OSA. These recommendations are intended to aid, not replace, good clinical judgment.
Home-based sleep testing has become more widely available, is convenient for patients, and is less expensive than lab-based polysomnography. Our study did not directly address the appropriate circumstances for home studies in clinical evaluation. We rely on the expertise of our sleep medicine colleagues to determine which patients are appropriate candidates for home-based studies.
The AASM states that “portable monitors (PM) for the diagnosis of OSA should be [used] only in conjunction with a comprehensive sleep evaluation. Clinical sleep evaluations using PM must be supervised by a practitioner with board certification in sleep medicine or an individual who fulfills the eligibility criteria for the sleep medicine certification examination.”4 Additionally, the group recommends that PM “may be used in the unattended setting as an alternative to polysomnography for the diagnosis of OSA in patients with a high pretest probability of moderate to severe OSA and no comorbid sleep disorder or major comorbid medical disorders.”4
Continue to: GRANT SUPPORT
GRANT SUPPORT
The use of the REDCap database is supported by grant UL1 TR000135. This work was supported by a Mayo Foundation CR-20 grant awarded to Dr. Mookadam as Principal investigator and Dr. Grover as Coinvestigator.
Statistical analyses were supported, in part, by the Department of Family Medicine, Mayo Clinic, Scottsdale, Ariz.
CORRESPONDENCE
Michael Grover, DO, Mayo Clinic Thunderbird Primary Care Center-Family Medicine, 13737 N 92nd Street, Scottsdale, AZ 85260; [email protected]
1. National Sleep Foundation. Sleep apnea. https://sleepfoundation.org/sleep-disorders-problems/sleep-apnea. Accessed September 14, 2018.
2. Grover M, Mookadam M, Armas D, et al. Identifying patients at risk for obstructive sleep apnea in a primary care practice. J Am Board Fam Med. 2011;24:152-160.
3. Mold JW, Quattlebaum C, Schinnerer E, et al. Identification by primary care clinicians of patients with obstructive sleep apnea: a practice-based research network (PBRN) study. J Am Board Fam Med. 2011;24:138-145.
4. Epstein LJ, Kristo D, Strollo PJ, Jr., et al; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263-276.
5. Qaseem A, Dallas P, Owens DK, et al. Diagnosis of obstructive sleep apnea in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2014;161:210-220.
6. Grover M, Mookadam M, Chang Y-H, et al. Validating the Sleep Apnea Clinical Score for use in primary care populations. Mayo Clin Proc. 2016;91:469-476.
7. Flemons WW, Whitelaw WA, Brant R, et al. Likelihood ratios for a sleep apnea clinical prediction rule. Am J Respir Crit Care Med. 1994;150:1279-1285.
8. Gali B, Whalen FX, Gay PC, et al. Management plan to reduce risks in perioperative care of patients with presumed obstructive sleep apnea syndrome. J Clin Sleep Med. 2007;3:582-588.
9. Myers KA, Mrkobrada M, Simel DL. Does this patient have obstructive sleep apnea?: The rational clinical examination systematic review. JAMA. 2013;310(7):731-741.
1. National Sleep Foundation. Sleep apnea. https://sleepfoundation.org/sleep-disorders-problems/sleep-apnea. Accessed September 14, 2018.
2. Grover M, Mookadam M, Armas D, et al. Identifying patients at risk for obstructive sleep apnea in a primary care practice. J Am Board Fam Med. 2011;24:152-160.
3. Mold JW, Quattlebaum C, Schinnerer E, et al. Identification by primary care clinicians of patients with obstructive sleep apnea: a practice-based research network (PBRN) study. J Am Board Fam Med. 2011;24:138-145.
4. Epstein LJ, Kristo D, Strollo PJ, Jr., et al; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263-276.
5. Qaseem A, Dallas P, Owens DK, et al. Diagnosis of obstructive sleep apnea in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2014;161:210-220.
6. Grover M, Mookadam M, Chang Y-H, et al. Validating the Sleep Apnea Clinical Score for use in primary care populations. Mayo Clin Proc. 2016;91:469-476.
7. Flemons WW, Whitelaw WA, Brant R, et al. Likelihood ratios for a sleep apnea clinical prediction rule. Am J Respir Crit Care Med. 1994;150:1279-1285.
8. Gali B, Whalen FX, Gay PC, et al. Management plan to reduce risks in perioperative care of patients with presumed obstructive sleep apnea syndrome. J Clin Sleep Med. 2007;3:582-588.
9. Myers KA, Mrkobrada M, Simel DL. Does this patient have obstructive sleep apnea?: The rational clinical examination systematic review. JAMA. 2013;310(7):731-741.
How should you treat the newly diagnosed hypertensive patient?
IT DEPENDS ON THE PATIENT’S RISK FACTORS, physical condition, and preferences. All hypertensive patients can potentially benefit from lifestyle interventions, including weight reduction, aerobic physical activity, the dietary approaches to stop hypertension (DASH) diet, and moderation of alcohol use (strength of recommendation [SOR]: A, systematic reviews).
Although lifestyle interventions are effective for some patients, they haven’t been proven to provide long-term control and don’t lower blood pressure as much as medications (SOR: B, systematic review of inconsistent randomized controlled trial [RCT]). For specific high-risk patients, pharmacologic therapy is recommended at the time of diagnosis (SOR: C, expert opinion).
When considering lifestyle changes and medication, it’s important to assess patient preferences as well as overall cardiovascular risks, presence of target organ damage, and clinical cardiovascular disease, because lifestyle modification and medication can both affect quality of life (SOR: C, expert opinion).
Evidence summary
The prevalence of hypertension is increasing. Twenty-seven percent of adult Americans are hypertensive; 31% have prehypertension (TABLE).1 Among adults older than 50 years, the risk of developing high blood pressure approaches 90% if they live to age 80 or older.2 Cardiovascular risk rises along with blood pressure readings. Blood pressure values in the range of 130/85 to 139/89 mm Hg are associated with a more than 2-fold increase in cardiovascular disease risk compared with values below 120/80 mm Hg.2
Even small reductions in blood pressure, when applied to the population as a whole, produce significant improvements in patient-oriented outcomes. A drop in systolic blood pressure of 3 mm Hg can decrease stroke mortality by 8% and coronary artery disease by 5%.2
Lifestyle interventions for treating hypertension
Lifestyle interventions for hypertensive patients include:
- Striving to maintain or achieve an ideal body weight (body mass index of 18-25).2 However, even modest weight reductions in overweight and obese hypertensive patients significantly lower blood pressure and overall cardiovascular risk. A 10-kg decrease in body weight can lower systolic blood pressure by 5 to 20 mm Hg.2
- Adopting the DASH diet.1 Consuming a diet rich in fruits, vegetables, and lowfat dairy products and limiting saturated and total fat intake can reduce systolic blood pressure by 2 to 8 mm Hg.
- Engaging in regular physical activity for at least 30 minutes most days of the week. This regimen has been shown to decrease systolic blood pressure by 4 to 9 mm Hg.2
- Limiting daily alcohol intake, if the patient drinks, to 2 servings for men and 1 for women and lower-weight individuals.2 Restricting alcohol consumption may lower systolic blood pressure by 2 to 4 mm Hg.
Randomized controlled trials have consistently demonstrated that patients who combine multiple lifestyle interventions achieve the greatest benefits.3-5 Success obviously requires patient motivation. Health care providers need to continually assess motivation and encourage adherence.
Most patients need medication, too
Lifestyle changes alone haven’t been shown to achieve the same long-term reductions in blood pressure as medication.5 Although some motivated patients can control their blood pressure solely by adjusting their lifestyle, few succeed in reaching and maintaining blood pressure goals. Continued attention to lifestyle should be encouraged both to control blood pressure and reduce overall cardiovascular risk, but most patients with hypertension need medication.
The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC7) doesn’t specify whether to offer a trial of therapeutic lifestyle change before starting medication.2 Clinicians should negotiate interventions based on each patient’s preferences, risk factors, and the presence or absence of clinical cardiovascular disease or target organ damage. Lifestyle changes and medication both can affect quality of life. Immediate pharmacologic treatment with 2 medications has been recommended in addition to lifestyle interventions for patients with stage 2 hypertension (TABLE).2
TABLE
JNC7 classifications of blood pressure in adults
| Blood pressure classification | Blood pressure (mm Hg) | Recommended follow-up |
|---|---|---|
| Normal | Systolic <120 AND diastolic <80 | 2 y |
| Prehypertension | Systolic 120-139 OR diastolic 80-89 | 1 y |
| Stage 1 hypertension | Systolic 140-159 OR diastolic 90-99 | 2 mo |
| Stage 2 hypertension | Systolic ≥160 OR diastolic ≥100 | 1 mo* |
| JNC7, Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. *For people with higher values (>180/110 mm Hg), evaluate and treat immediately or within 1 week, depending on clinical situation. Source: Chobanian AV, et al. Hypertension. 2003.2 | ||
Recommendations
The European Society of Hypertension provides recommendations for the duration of lifestyle interventions before trying medication. The recommendations are based on a complex scheme of overall cardiovascular risk assessment that takes into account traditional Framingham risks and other factors (such as obesity, C-reactive protein, and micro albuminuria), as well as the stage of hypertension.6 The Society recommends starting drug therapy immediately in people with blood pressure >180/110 mm Hg. This blood pressure threshold drops in patients with increasing numbers of risk factors. For patients with lower, but still elevated, blood pressure, the recommendations call for “lifestyle changes for several months, then drug treatment if BP is uncontrolled.”
For patients with diabetes, the American Diabetes Association (ADA) recommends a blood pressure goal of <130/80 mm Hg and drug therapy in addition to lifestyle and behavioral therapy for patients with systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg.7 Like the JNC7, the ADA notes that a combination of medications is often required to achieve blood pressure targets. The ADA recommendations also state that patients with diabetes and a systolic blood pressure of 130 to 139 mm Hg or a diastolic blood pressure of 80 to 89 mm Hg should pursue lifestyle and behavioral interventions alone for a maximum of 3 months, then start drug therapy if they don’t achieve their blood pressure goals.
The American Heart Association and American College of Cardiology offer evidence-based guidelines for secondary prevention in patients with atherosclerosis.8 They set blood pressure goals of <140/90 mm Hg for all patients and <130/80 mm Hg for patients with diabetes or chronic kidney disease. All patients are encouraged to initiate or maintain lifestyle modifications. If a patient’s blood pressure is ≥140/90 mm Hg (>130/80 mm Hg for patients with chronic kidney disease or diabetes), medications should be titrated to goal, beginning with beta-blockers or angiotensin-converting enzyme inhibitors.
1. Appel LJ, Brands MW, Daniels SR, et al. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension. 2006;47:296-308.
2. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206-1252.
3. Burke V, Beilin LJ, Cutt HE, et al. Effects of a lifestyle programme on ambulatory blood pressure and drug dosage in treated hypertensive patients: a randomized controlled trial. J Hypertens. 2005;23:1241-1249.
4. Elmer PJ, Obarzanek E, Vollmer WM, et al. Effects of comprehensive lifestyle modification on diet, weight, physical fitness, and blood pressure control: 18-month results of a randomized trial. Ann Intern Med. 2006;144:485-495.
5. Nicolson DJ, Dickinson HO, Campbell F, et al. Lifestyle interventions or drugs for patients with essential hypertension: a systematic review. J Hypertens. 2004;22:2043-2048.
6. European Society of Hypertension-European Society of Cardiology Task Force on the Management of Arterial Hypertension. 2007 ESH-ESC practice guidelines for the management of arterial hypertension. J Hypertens. 2007;25:1751-1762.
7. American Diabetes Association. Standards of medical care in diabetes 2010. Diabetes Care. 2010;33(suppl 1):S11-S61.Available at: http://care.diabetesjournals.org/content/33/Supplement_1. Accessed March 1, 2010.
8. American Hospital Association, American College of Cardiology. AHA-ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update. Circulation. 2006;113:2363-2372.
IT DEPENDS ON THE PATIENT’S RISK FACTORS, physical condition, and preferences. All hypertensive patients can potentially benefit from lifestyle interventions, including weight reduction, aerobic physical activity, the dietary approaches to stop hypertension (DASH) diet, and moderation of alcohol use (strength of recommendation [SOR]: A, systematic reviews).
Although lifestyle interventions are effective for some patients, they haven’t been proven to provide long-term control and don’t lower blood pressure as much as medications (SOR: B, systematic review of inconsistent randomized controlled trial [RCT]). For specific high-risk patients, pharmacologic therapy is recommended at the time of diagnosis (SOR: C, expert opinion).
When considering lifestyle changes and medication, it’s important to assess patient preferences as well as overall cardiovascular risks, presence of target organ damage, and clinical cardiovascular disease, because lifestyle modification and medication can both affect quality of life (SOR: C, expert opinion).
Evidence summary
The prevalence of hypertension is increasing. Twenty-seven percent of adult Americans are hypertensive; 31% have prehypertension (TABLE).1 Among adults older than 50 years, the risk of developing high blood pressure approaches 90% if they live to age 80 or older.2 Cardiovascular risk rises along with blood pressure readings. Blood pressure values in the range of 130/85 to 139/89 mm Hg are associated with a more than 2-fold increase in cardiovascular disease risk compared with values below 120/80 mm Hg.2
Even small reductions in blood pressure, when applied to the population as a whole, produce significant improvements in patient-oriented outcomes. A drop in systolic blood pressure of 3 mm Hg can decrease stroke mortality by 8% and coronary artery disease by 5%.2
Lifestyle interventions for treating hypertension
Lifestyle interventions for hypertensive patients include:
- Striving to maintain or achieve an ideal body weight (body mass index of 18-25).2 However, even modest weight reductions in overweight and obese hypertensive patients significantly lower blood pressure and overall cardiovascular risk. A 10-kg decrease in body weight can lower systolic blood pressure by 5 to 20 mm Hg.2
- Adopting the DASH diet.1 Consuming a diet rich in fruits, vegetables, and lowfat dairy products and limiting saturated and total fat intake can reduce systolic blood pressure by 2 to 8 mm Hg.
- Engaging in regular physical activity for at least 30 minutes most days of the week. This regimen has been shown to decrease systolic blood pressure by 4 to 9 mm Hg.2
- Limiting daily alcohol intake, if the patient drinks, to 2 servings for men and 1 for women and lower-weight individuals.2 Restricting alcohol consumption may lower systolic blood pressure by 2 to 4 mm Hg.
Randomized controlled trials have consistently demonstrated that patients who combine multiple lifestyle interventions achieve the greatest benefits.3-5 Success obviously requires patient motivation. Health care providers need to continually assess motivation and encourage adherence.
Most patients need medication, too
Lifestyle changes alone haven’t been shown to achieve the same long-term reductions in blood pressure as medication.5 Although some motivated patients can control their blood pressure solely by adjusting their lifestyle, few succeed in reaching and maintaining blood pressure goals. Continued attention to lifestyle should be encouraged both to control blood pressure and reduce overall cardiovascular risk, but most patients with hypertension need medication.
The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC7) doesn’t specify whether to offer a trial of therapeutic lifestyle change before starting medication.2 Clinicians should negotiate interventions based on each patient’s preferences, risk factors, and the presence or absence of clinical cardiovascular disease or target organ damage. Lifestyle changes and medication both can affect quality of life. Immediate pharmacologic treatment with 2 medications has been recommended in addition to lifestyle interventions for patients with stage 2 hypertension (TABLE).2
TABLE
JNC7 classifications of blood pressure in adults
| Blood pressure classification | Blood pressure (mm Hg) | Recommended follow-up |
|---|---|---|
| Normal | Systolic <120 AND diastolic <80 | 2 y |
| Prehypertension | Systolic 120-139 OR diastolic 80-89 | 1 y |
| Stage 1 hypertension | Systolic 140-159 OR diastolic 90-99 | 2 mo |
| Stage 2 hypertension | Systolic ≥160 OR diastolic ≥100 | 1 mo* |
| JNC7, Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. *For people with higher values (>180/110 mm Hg), evaluate and treat immediately or within 1 week, depending on clinical situation. Source: Chobanian AV, et al. Hypertension. 2003.2 | ||
Recommendations
The European Society of Hypertension provides recommendations for the duration of lifestyle interventions before trying medication. The recommendations are based on a complex scheme of overall cardiovascular risk assessment that takes into account traditional Framingham risks and other factors (such as obesity, C-reactive protein, and micro albuminuria), as well as the stage of hypertension.6 The Society recommends starting drug therapy immediately in people with blood pressure >180/110 mm Hg. This blood pressure threshold drops in patients with increasing numbers of risk factors. For patients with lower, but still elevated, blood pressure, the recommendations call for “lifestyle changes for several months, then drug treatment if BP is uncontrolled.”
For patients with diabetes, the American Diabetes Association (ADA) recommends a blood pressure goal of <130/80 mm Hg and drug therapy in addition to lifestyle and behavioral therapy for patients with systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg.7 Like the JNC7, the ADA notes that a combination of medications is often required to achieve blood pressure targets. The ADA recommendations also state that patients with diabetes and a systolic blood pressure of 130 to 139 mm Hg or a diastolic blood pressure of 80 to 89 mm Hg should pursue lifestyle and behavioral interventions alone for a maximum of 3 months, then start drug therapy if they don’t achieve their blood pressure goals.
The American Heart Association and American College of Cardiology offer evidence-based guidelines for secondary prevention in patients with atherosclerosis.8 They set blood pressure goals of <140/90 mm Hg for all patients and <130/80 mm Hg for patients with diabetes or chronic kidney disease. All patients are encouraged to initiate or maintain lifestyle modifications. If a patient’s blood pressure is ≥140/90 mm Hg (>130/80 mm Hg for patients with chronic kidney disease or diabetes), medications should be titrated to goal, beginning with beta-blockers or angiotensin-converting enzyme inhibitors.
IT DEPENDS ON THE PATIENT’S RISK FACTORS, physical condition, and preferences. All hypertensive patients can potentially benefit from lifestyle interventions, including weight reduction, aerobic physical activity, the dietary approaches to stop hypertension (DASH) diet, and moderation of alcohol use (strength of recommendation [SOR]: A, systematic reviews).
Although lifestyle interventions are effective for some patients, they haven’t been proven to provide long-term control and don’t lower blood pressure as much as medications (SOR: B, systematic review of inconsistent randomized controlled trial [RCT]). For specific high-risk patients, pharmacologic therapy is recommended at the time of diagnosis (SOR: C, expert opinion).
When considering lifestyle changes and medication, it’s important to assess patient preferences as well as overall cardiovascular risks, presence of target organ damage, and clinical cardiovascular disease, because lifestyle modification and medication can both affect quality of life (SOR: C, expert opinion).
Evidence summary
The prevalence of hypertension is increasing. Twenty-seven percent of adult Americans are hypertensive; 31% have prehypertension (TABLE).1 Among adults older than 50 years, the risk of developing high blood pressure approaches 90% if they live to age 80 or older.2 Cardiovascular risk rises along with blood pressure readings. Blood pressure values in the range of 130/85 to 139/89 mm Hg are associated with a more than 2-fold increase in cardiovascular disease risk compared with values below 120/80 mm Hg.2
Even small reductions in blood pressure, when applied to the population as a whole, produce significant improvements in patient-oriented outcomes. A drop in systolic blood pressure of 3 mm Hg can decrease stroke mortality by 8% and coronary artery disease by 5%.2
Lifestyle interventions for treating hypertension
Lifestyle interventions for hypertensive patients include:
- Striving to maintain or achieve an ideal body weight (body mass index of 18-25).2 However, even modest weight reductions in overweight and obese hypertensive patients significantly lower blood pressure and overall cardiovascular risk. A 10-kg decrease in body weight can lower systolic blood pressure by 5 to 20 mm Hg.2
- Adopting the DASH diet.1 Consuming a diet rich in fruits, vegetables, and lowfat dairy products and limiting saturated and total fat intake can reduce systolic blood pressure by 2 to 8 mm Hg.
- Engaging in regular physical activity for at least 30 minutes most days of the week. This regimen has been shown to decrease systolic blood pressure by 4 to 9 mm Hg.2
- Limiting daily alcohol intake, if the patient drinks, to 2 servings for men and 1 for women and lower-weight individuals.2 Restricting alcohol consumption may lower systolic blood pressure by 2 to 4 mm Hg.
Randomized controlled trials have consistently demonstrated that patients who combine multiple lifestyle interventions achieve the greatest benefits.3-5 Success obviously requires patient motivation. Health care providers need to continually assess motivation and encourage adherence.
Most patients need medication, too
Lifestyle changes alone haven’t been shown to achieve the same long-term reductions in blood pressure as medication.5 Although some motivated patients can control their blood pressure solely by adjusting their lifestyle, few succeed in reaching and maintaining blood pressure goals. Continued attention to lifestyle should be encouraged both to control blood pressure and reduce overall cardiovascular risk, but most patients with hypertension need medication.
The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC7) doesn’t specify whether to offer a trial of therapeutic lifestyle change before starting medication.2 Clinicians should negotiate interventions based on each patient’s preferences, risk factors, and the presence or absence of clinical cardiovascular disease or target organ damage. Lifestyle changes and medication both can affect quality of life. Immediate pharmacologic treatment with 2 medications has been recommended in addition to lifestyle interventions for patients with stage 2 hypertension (TABLE).2
TABLE
JNC7 classifications of blood pressure in adults
| Blood pressure classification | Blood pressure (mm Hg) | Recommended follow-up |
|---|---|---|
| Normal | Systolic <120 AND diastolic <80 | 2 y |
| Prehypertension | Systolic 120-139 OR diastolic 80-89 | 1 y |
| Stage 1 hypertension | Systolic 140-159 OR diastolic 90-99 | 2 mo |
| Stage 2 hypertension | Systolic ≥160 OR diastolic ≥100 | 1 mo* |
| JNC7, Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. *For people with higher values (>180/110 mm Hg), evaluate and treat immediately or within 1 week, depending on clinical situation. Source: Chobanian AV, et al. Hypertension. 2003.2 | ||
Recommendations
The European Society of Hypertension provides recommendations for the duration of lifestyle interventions before trying medication. The recommendations are based on a complex scheme of overall cardiovascular risk assessment that takes into account traditional Framingham risks and other factors (such as obesity, C-reactive protein, and micro albuminuria), as well as the stage of hypertension.6 The Society recommends starting drug therapy immediately in people with blood pressure >180/110 mm Hg. This blood pressure threshold drops in patients with increasing numbers of risk factors. For patients with lower, but still elevated, blood pressure, the recommendations call for “lifestyle changes for several months, then drug treatment if BP is uncontrolled.”
For patients with diabetes, the American Diabetes Association (ADA) recommends a blood pressure goal of <130/80 mm Hg and drug therapy in addition to lifestyle and behavioral therapy for patients with systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg.7 Like the JNC7, the ADA notes that a combination of medications is often required to achieve blood pressure targets. The ADA recommendations also state that patients with diabetes and a systolic blood pressure of 130 to 139 mm Hg or a diastolic blood pressure of 80 to 89 mm Hg should pursue lifestyle and behavioral interventions alone for a maximum of 3 months, then start drug therapy if they don’t achieve their blood pressure goals.
The American Heart Association and American College of Cardiology offer evidence-based guidelines for secondary prevention in patients with atherosclerosis.8 They set blood pressure goals of <140/90 mm Hg for all patients and <130/80 mm Hg for patients with diabetes or chronic kidney disease. All patients are encouraged to initiate or maintain lifestyle modifications. If a patient’s blood pressure is ≥140/90 mm Hg (>130/80 mm Hg for patients with chronic kidney disease or diabetes), medications should be titrated to goal, beginning with beta-blockers or angiotensin-converting enzyme inhibitors.
1. Appel LJ, Brands MW, Daniels SR, et al. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension. 2006;47:296-308.
2. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206-1252.
3. Burke V, Beilin LJ, Cutt HE, et al. Effects of a lifestyle programme on ambulatory blood pressure and drug dosage in treated hypertensive patients: a randomized controlled trial. J Hypertens. 2005;23:1241-1249.
4. Elmer PJ, Obarzanek E, Vollmer WM, et al. Effects of comprehensive lifestyle modification on diet, weight, physical fitness, and blood pressure control: 18-month results of a randomized trial. Ann Intern Med. 2006;144:485-495.
5. Nicolson DJ, Dickinson HO, Campbell F, et al. Lifestyle interventions or drugs for patients with essential hypertension: a systematic review. J Hypertens. 2004;22:2043-2048.
6. European Society of Hypertension-European Society of Cardiology Task Force on the Management of Arterial Hypertension. 2007 ESH-ESC practice guidelines for the management of arterial hypertension. J Hypertens. 2007;25:1751-1762.
7. American Diabetes Association. Standards of medical care in diabetes 2010. Diabetes Care. 2010;33(suppl 1):S11-S61.Available at: http://care.diabetesjournals.org/content/33/Supplement_1. Accessed March 1, 2010.
8. American Hospital Association, American College of Cardiology. AHA-ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update. Circulation. 2006;113:2363-2372.
1. Appel LJ, Brands MW, Daniels SR, et al. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension. 2006;47:296-308.
2. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206-1252.
3. Burke V, Beilin LJ, Cutt HE, et al. Effects of a lifestyle programme on ambulatory blood pressure and drug dosage in treated hypertensive patients: a randomized controlled trial. J Hypertens. 2005;23:1241-1249.
4. Elmer PJ, Obarzanek E, Vollmer WM, et al. Effects of comprehensive lifestyle modification on diet, weight, physical fitness, and blood pressure control: 18-month results of a randomized trial. Ann Intern Med. 2006;144:485-495.
5. Nicolson DJ, Dickinson HO, Campbell F, et al. Lifestyle interventions or drugs for patients with essential hypertension: a systematic review. J Hypertens. 2004;22:2043-2048.
6. European Society of Hypertension-European Society of Cardiology Task Force on the Management of Arterial Hypertension. 2007 ESH-ESC practice guidelines for the management of arterial hypertension. J Hypertens. 2007;25:1751-1762.
7. American Diabetes Association. Standards of medical care in diabetes 2010. Diabetes Care. 2010;33(suppl 1):S11-S61.Available at: http://care.diabetesjournals.org/content/33/Supplement_1. Accessed March 1, 2010.
8. American Hospital Association, American College of Cardiology. AHA-ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update. Circulation. 2006;113:2363-2372.
Evidence-based answers from the Family Physicians Inquiries Network
What steps can reduce morbidity and mortality caused by hip fractures?
Surgery within 24 hours of hip fracture is a critical step in reducing complications, and may decrease mortality compared with conservative care (strength of recommendation [sor]: B, cohort studies). Give patients heparin at the time of admission to prevent venous thromboembolism (VTE) (SOR: A, systematic reviews of RCTs). Anticoagulation should be continued in some form for 10 days or until the patient is fully ambulatory (SOR: A). Patients should also get prophylactic antibiotics in the 2 hours before surgery (SOR: A, meta-analysis of RCT). reduce the risk of postoperative delirium by avoiding certain medications, minimizing sleep disturbances, and providing adequate analgesia (SOR: B, systematic review of cohort studies). Aggressive pain control should also be top of mind—higher pain scores are associated with longer hospital stays, delayed ambulation, and long-term functional impairment (SOR: C, extrapolation from a single cohort study).
Ensure that proper treatment continues after discharge
Mary M. Stephens, MD, MPH
East Tennessee State University, Kingsport
As an FP working in a hospital, I am often asked to consult on cases of hip fracture. It’s important to maximize the patient’s condition quickly in order for the orthopedist to be able to proceed with surgical repair within the first 24 hours of the injury.
Many hospitals have anticoagulation protocols or standing orders for postop hip fracture management, which should make VTE prevention almost automatic. However, it’s important to ensure that these orders are initiated preoperatively if surgery is delayed, and that treatment gets continued for the appropriate length of time—even after the patient is discharged to their home or to a facility for rehabilitation.
As physicians, we worry about the short-term mortality from VTE and pulmonary embolism, but delirium can be devastating for family members to watch, and carries its own morbidities. I talk to the patient and family preoperatively or immediately postoperatively about this risk so they can be prepared.
Evidence summary
Although most patients undergo surgery for hip fracture, family physicians often serve as consultants for perioperative management and rehabilitation. Several interventions have been studied that influence outcomes for hip fracture patients.
Surgical interventions: Timing is critical
Most ambulatory, medically stable patients elect to have surgical repair of their fractures. A meta-analysis found few randomized trials comparing operative with nonoperative therapy; it concluded that surgical treatment seems to be associated with a reduced length of hospital stay and improved rehabilitation.1
The timing of surgery appears to be an important variable, particularly whether patients should undergo surgery within 24 hours of the fracture. Some studies found decreased mortality with earlier surgical intervention,2 while others have not.3 A 2006 observational study4 found that delay in operating was associated with an increased risk of death in the hospital, even after adjusting for comorbidities. For all deaths in the hospital, the odds ratio [OR] for delaying more than 1 day, relative to 1 day or less, was 1.27 (95% confidence interval [CI], 1.23–1.32). Despite the inconsistency regarding mortality rates, complication rates (such as decubitus ulcers) do increase with a delay in surgery.3
Unfractionated vs LMW heparin
After a hip fracture, patients are at very high risk for VTE. For untreated patients, the rate of deep vein thromboses (DVT) may be as high as 50%, with an associated fatal pulmonary embolism rate as high as 7.5%.5
The effectiveness of unfractionated and low-molecular-weight heparin was evaluated in a 2002 Cochrane systematic review.6 While evidence was insufficient to recommend 1 agent over another, both were found to significantly decrease the incidence of lower-extremity DVT over placebo (for unfractionated heparin, relative risk [RR]=0.59 [95% CI, 0.49–0.72]; for low-molecular-weight heparin, RR=0.60 [95% CI, 0.50–0.71]). Number needed to treat [NNT] with either agent was 7.
Reduce infections with antibiotic prophylaxis
Antibiotic prophylaxis has been supported by a Cochrane review, which concluded that single-dose antibiotic prophylaxis before surgery significantly reduced the risk of deep wound infections (RR=0.40; 95% CI, 0.24–0.67; NNT=55), as well as superficial wound, urinary, and respiratory tract infections.7
Patients should receive antibiotics less than 2 hours before surgery to reduce the risk of infection.8 Classen et al found that patients treated less than 2 hours before surgery had a 0.6% rate of infection (10/1208), compared with a 3.85% rate for those treated 2 to 24 hours ahead (14/369) (NNT=31).8 It is unclear whether multiple-dose therapy provides additional benefit when administered over the first 24 to 36 hours after surgery9 (OR=0.60; 95% CI, 0.18–2.02). First- or second-generation cephalosporins were used in most studies.
Delirium: A common but avoidable complication
Delirium is a common complication seen after hip fracture, affecting approximately 10% to 16% of patients.10,11 Delirium may increase the duration of hospitalization, and may be associated with an increased mortality at 1 year.12 Delirium can be avoided by looking at each patient’s risk factors.13 Studies suggest avoiding use of meperidine, benzodiazepines, and medications with anticholinergic side effects.11,13 Sleep deprivation, delayed mobility, and inadequate pain control are also associated with the development of delirium.13
One study showed that prophylaxis with haloperidol for hip fracture patients did not decrease the incidence of postoperative delirium but did reduce its duration and severity.14 Haloperidol prophylaxis was also associated with shorter hospital stays. Treatment with haloperidol or risperidone for the agitation of postoperative delirium has been recommended when behavioral interventions fail.13
Pain control improves recovery
Providing adequate analgesia is of the utmost importance. In a 2003 prospective cohort study, patients without sufficient analgesia had an increased risk of poor functional recovery and longer hospitalization.15 In another cohort study, those patients whose pain was inadequately controlled also had an increased risk for delirium (RR=9.0; 95% CI, 1.8–45.2).11 Meperidine use increased the risk for delirium compared with other opioid analgesics (RR=2.4; 95% CI, 1.3–4.5).11
Recommendations of others
The American College of Physicians provides a comprehensive evidence-based guideline for the management of hip fracture patients in their PIER series (Physicians’ Information and Education Resource) (TABLE).16
The American College of Chest Physicians has published evidence-based guidelines for the prevention of VTE.5 For patients undergoing hip fracture surgery, they recommend routine use of fondaparinux, low-molecular-weight heparin at high-risk dosing, adjusted-dose warfarin (at a target international normalized ratio [INR] of 2.5, range 2.0–3.0), or unfractionated heparin. They recommend against routine use of aspirin alone. If surgery must be delayed, physicians should initiate prophylaxis with unfractionated or low-molecular-weight heparin at the time of hospital admission. Anticoagulation should routinely continue for 10 days after surgery or until the patient is ambulatory. If anticoagulation is contraindicated, mechanical prophylaxis of VTE with foot and calf pumping devices is recommended.5,6
TABLE
6 steps for managing hip fracture from the American College of Physicians
|
| Source: PIER: Physicians’ Information and education resource, American College of Physicians, 2006.16 |
1. Parker MJ, Handoll HH, Bhargara A. Conservative versus operative treatment for hip fractures. Cochrane Database Syst Rev. 2000;(4):CD000337.
2. Dorotka R, Schoechtner H, Buchinger W. The influence of immediate surgical treatment of proximal femoral fractures on mortality and quality of life. Operation within six hours of the fracture versus later than six hours. J Bone Joint Surg Br. 2003;85:1107-1113.
3. Grimes JP, Gregory PM, Noveck H, Butler MS, Carson JL. The effects of time-to-surgery on mortality and morbidity in patients following hip fracture. Am J Med. 2002;112:702.
4. Bottle A, Aylin P. mortality associated with delay in operation after hip fracture: observational study. BMJ. 2006;332:947-951.
5. Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):S338-S400.
6. Handoll HH, Farrar MJ, Mcbirnie J, Tytherleighstrong G, Milne AA, Gillespie WJ. Heparin, low molecular weight heparin and physical methods for preventing deep vein thrombosis and pulmonary embolism following surgery for hip fractures. Cochrane Database Syst Rev. 2002;(4):CD000305
7. Gillespie WJ, Walenkamp G. Antibiotic prophylaxis for surgery for proximal femoral and other closed long bone fractures. Cochrane Database Syst Rev. 2001;(1):CD000244.
8. Classen DC, Evans RS, Pestotnik SL, Horn SD, Menlove RL, Burke JP. The timing of prophylactic administration of antibiotics and the risk of surgical wound infection. N Engl J Med. 1992;326:281-286.
9. March L, Chamberlain A, Cameron I, et al. Prevention, Treatment, and Rehabilitation of Fractured Neck of Femur. report from the Northern sydney Area Fractured Neck of Femur Health outcomes Project. St. Leonards, Australia: Public Health Unit, Northern Sydney Area Health Service; 1996. Available at: www.mja.com.au/public/issues/iprs2/march/fnof.pdf. Accessed on october 11, 2007.
10. Brauer C, Morrison RS, Silberzweig SB, Siu AL. The cause of delirium in patients with hip fracture. Arch Intern Med. 2000;160:1856-1860.
11. Morrison RS, Magaziner J, Gilbert M, et al. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci. 2003;58:76-81.
12. Edelstein DM, Aharonoff GB, Karp A, Capla EL, Zuckerman JD, Koval KJ. Effect of postoperative delirium on outcome after hip fracture. Clin Orthop Relat Res. 2004;422:195-200.
13. Day H. Postoperative delirium. PIER: Physician’s Information and education resource, American College of Physicians. July 2006. Available at: pier.acponline.org/index.html. Accessed on October 11, 2007.
14. Kalisvaart KJ, de Jonghe JF, Bogaards MJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc. 2005;53:1658-1666.
15. Morrison RS, Magaziner J, Mclaughlin MA, et al. The impact of post-operative pain on outcomes following hip fracture. Pain. 2003;103:303-311.
16. Christmas C. Hip fracture. PIER: Physicians’ Information and Education Resource, American College of Physicians. updated July 2006. Available at: pier.acponline.org/index.html. october 11, 2007.
Surgery within 24 hours of hip fracture is a critical step in reducing complications, and may decrease mortality compared with conservative care (strength of recommendation [sor]: B, cohort studies). Give patients heparin at the time of admission to prevent venous thromboembolism (VTE) (SOR: A, systematic reviews of RCTs). Anticoagulation should be continued in some form for 10 days or until the patient is fully ambulatory (SOR: A). Patients should also get prophylactic antibiotics in the 2 hours before surgery (SOR: A, meta-analysis of RCT). reduce the risk of postoperative delirium by avoiding certain medications, minimizing sleep disturbances, and providing adequate analgesia (SOR: B, systematic review of cohort studies). Aggressive pain control should also be top of mind—higher pain scores are associated with longer hospital stays, delayed ambulation, and long-term functional impairment (SOR: C, extrapolation from a single cohort study).
Ensure that proper treatment continues after discharge
Mary M. Stephens, MD, MPH
East Tennessee State University, Kingsport
As an FP working in a hospital, I am often asked to consult on cases of hip fracture. It’s important to maximize the patient’s condition quickly in order for the orthopedist to be able to proceed with surgical repair within the first 24 hours of the injury.
Many hospitals have anticoagulation protocols or standing orders for postop hip fracture management, which should make VTE prevention almost automatic. However, it’s important to ensure that these orders are initiated preoperatively if surgery is delayed, and that treatment gets continued for the appropriate length of time—even after the patient is discharged to their home or to a facility for rehabilitation.
As physicians, we worry about the short-term mortality from VTE and pulmonary embolism, but delirium can be devastating for family members to watch, and carries its own morbidities. I talk to the patient and family preoperatively or immediately postoperatively about this risk so they can be prepared.
Evidence summary
Although most patients undergo surgery for hip fracture, family physicians often serve as consultants for perioperative management and rehabilitation. Several interventions have been studied that influence outcomes for hip fracture patients.
Surgical interventions: Timing is critical
Most ambulatory, medically stable patients elect to have surgical repair of their fractures. A meta-analysis found few randomized trials comparing operative with nonoperative therapy; it concluded that surgical treatment seems to be associated with a reduced length of hospital stay and improved rehabilitation.1
The timing of surgery appears to be an important variable, particularly whether patients should undergo surgery within 24 hours of the fracture. Some studies found decreased mortality with earlier surgical intervention,2 while others have not.3 A 2006 observational study4 found that delay in operating was associated with an increased risk of death in the hospital, even after adjusting for comorbidities. For all deaths in the hospital, the odds ratio [OR] for delaying more than 1 day, relative to 1 day or less, was 1.27 (95% confidence interval [CI], 1.23–1.32). Despite the inconsistency regarding mortality rates, complication rates (such as decubitus ulcers) do increase with a delay in surgery.3
Unfractionated vs LMW heparin
After a hip fracture, patients are at very high risk for VTE. For untreated patients, the rate of deep vein thromboses (DVT) may be as high as 50%, with an associated fatal pulmonary embolism rate as high as 7.5%.5
The effectiveness of unfractionated and low-molecular-weight heparin was evaluated in a 2002 Cochrane systematic review.6 While evidence was insufficient to recommend 1 agent over another, both were found to significantly decrease the incidence of lower-extremity DVT over placebo (for unfractionated heparin, relative risk [RR]=0.59 [95% CI, 0.49–0.72]; for low-molecular-weight heparin, RR=0.60 [95% CI, 0.50–0.71]). Number needed to treat [NNT] with either agent was 7.
Reduce infections with antibiotic prophylaxis
Antibiotic prophylaxis has been supported by a Cochrane review, which concluded that single-dose antibiotic prophylaxis before surgery significantly reduced the risk of deep wound infections (RR=0.40; 95% CI, 0.24–0.67; NNT=55), as well as superficial wound, urinary, and respiratory tract infections.7
Patients should receive antibiotics less than 2 hours before surgery to reduce the risk of infection.8 Classen et al found that patients treated less than 2 hours before surgery had a 0.6% rate of infection (10/1208), compared with a 3.85% rate for those treated 2 to 24 hours ahead (14/369) (NNT=31).8 It is unclear whether multiple-dose therapy provides additional benefit when administered over the first 24 to 36 hours after surgery9 (OR=0.60; 95% CI, 0.18–2.02). First- or second-generation cephalosporins were used in most studies.
Delirium: A common but avoidable complication
Delirium is a common complication seen after hip fracture, affecting approximately 10% to 16% of patients.10,11 Delirium may increase the duration of hospitalization, and may be associated with an increased mortality at 1 year.12 Delirium can be avoided by looking at each patient’s risk factors.13 Studies suggest avoiding use of meperidine, benzodiazepines, and medications with anticholinergic side effects.11,13 Sleep deprivation, delayed mobility, and inadequate pain control are also associated with the development of delirium.13
One study showed that prophylaxis with haloperidol for hip fracture patients did not decrease the incidence of postoperative delirium but did reduce its duration and severity.14 Haloperidol prophylaxis was also associated with shorter hospital stays. Treatment with haloperidol or risperidone for the agitation of postoperative delirium has been recommended when behavioral interventions fail.13
Pain control improves recovery
Providing adequate analgesia is of the utmost importance. In a 2003 prospective cohort study, patients without sufficient analgesia had an increased risk of poor functional recovery and longer hospitalization.15 In another cohort study, those patients whose pain was inadequately controlled also had an increased risk for delirium (RR=9.0; 95% CI, 1.8–45.2).11 Meperidine use increased the risk for delirium compared with other opioid analgesics (RR=2.4; 95% CI, 1.3–4.5).11
Recommendations of others
The American College of Physicians provides a comprehensive evidence-based guideline for the management of hip fracture patients in their PIER series (Physicians’ Information and Education Resource) (TABLE).16
The American College of Chest Physicians has published evidence-based guidelines for the prevention of VTE.5 For patients undergoing hip fracture surgery, they recommend routine use of fondaparinux, low-molecular-weight heparin at high-risk dosing, adjusted-dose warfarin (at a target international normalized ratio [INR] of 2.5, range 2.0–3.0), or unfractionated heparin. They recommend against routine use of aspirin alone. If surgery must be delayed, physicians should initiate prophylaxis with unfractionated or low-molecular-weight heparin at the time of hospital admission. Anticoagulation should routinely continue for 10 days after surgery or until the patient is ambulatory. If anticoagulation is contraindicated, mechanical prophylaxis of VTE with foot and calf pumping devices is recommended.5,6
TABLE
6 steps for managing hip fracture from the American College of Physicians
|
| Source: PIER: Physicians’ Information and education resource, American College of Physicians, 2006.16 |
Surgery within 24 hours of hip fracture is a critical step in reducing complications, and may decrease mortality compared with conservative care (strength of recommendation [sor]: B, cohort studies). Give patients heparin at the time of admission to prevent venous thromboembolism (VTE) (SOR: A, systematic reviews of RCTs). Anticoagulation should be continued in some form for 10 days or until the patient is fully ambulatory (SOR: A). Patients should also get prophylactic antibiotics in the 2 hours before surgery (SOR: A, meta-analysis of RCT). reduce the risk of postoperative delirium by avoiding certain medications, minimizing sleep disturbances, and providing adequate analgesia (SOR: B, systematic review of cohort studies). Aggressive pain control should also be top of mind—higher pain scores are associated with longer hospital stays, delayed ambulation, and long-term functional impairment (SOR: C, extrapolation from a single cohort study).
Ensure that proper treatment continues after discharge
Mary M. Stephens, MD, MPH
East Tennessee State University, Kingsport
As an FP working in a hospital, I am often asked to consult on cases of hip fracture. It’s important to maximize the patient’s condition quickly in order for the orthopedist to be able to proceed with surgical repair within the first 24 hours of the injury.
Many hospitals have anticoagulation protocols or standing orders for postop hip fracture management, which should make VTE prevention almost automatic. However, it’s important to ensure that these orders are initiated preoperatively if surgery is delayed, and that treatment gets continued for the appropriate length of time—even after the patient is discharged to their home or to a facility for rehabilitation.
As physicians, we worry about the short-term mortality from VTE and pulmonary embolism, but delirium can be devastating for family members to watch, and carries its own morbidities. I talk to the patient and family preoperatively or immediately postoperatively about this risk so they can be prepared.
Evidence summary
Although most patients undergo surgery for hip fracture, family physicians often serve as consultants for perioperative management and rehabilitation. Several interventions have been studied that influence outcomes for hip fracture patients.
Surgical interventions: Timing is critical
Most ambulatory, medically stable patients elect to have surgical repair of their fractures. A meta-analysis found few randomized trials comparing operative with nonoperative therapy; it concluded that surgical treatment seems to be associated with a reduced length of hospital stay and improved rehabilitation.1
The timing of surgery appears to be an important variable, particularly whether patients should undergo surgery within 24 hours of the fracture. Some studies found decreased mortality with earlier surgical intervention,2 while others have not.3 A 2006 observational study4 found that delay in operating was associated with an increased risk of death in the hospital, even after adjusting for comorbidities. For all deaths in the hospital, the odds ratio [OR] for delaying more than 1 day, relative to 1 day or less, was 1.27 (95% confidence interval [CI], 1.23–1.32). Despite the inconsistency regarding mortality rates, complication rates (such as decubitus ulcers) do increase with a delay in surgery.3
Unfractionated vs LMW heparin
After a hip fracture, patients are at very high risk for VTE. For untreated patients, the rate of deep vein thromboses (DVT) may be as high as 50%, with an associated fatal pulmonary embolism rate as high as 7.5%.5
The effectiveness of unfractionated and low-molecular-weight heparin was evaluated in a 2002 Cochrane systematic review.6 While evidence was insufficient to recommend 1 agent over another, both were found to significantly decrease the incidence of lower-extremity DVT over placebo (for unfractionated heparin, relative risk [RR]=0.59 [95% CI, 0.49–0.72]; for low-molecular-weight heparin, RR=0.60 [95% CI, 0.50–0.71]). Number needed to treat [NNT] with either agent was 7.
Reduce infections with antibiotic prophylaxis
Antibiotic prophylaxis has been supported by a Cochrane review, which concluded that single-dose antibiotic prophylaxis before surgery significantly reduced the risk of deep wound infections (RR=0.40; 95% CI, 0.24–0.67; NNT=55), as well as superficial wound, urinary, and respiratory tract infections.7
Patients should receive antibiotics less than 2 hours before surgery to reduce the risk of infection.8 Classen et al found that patients treated less than 2 hours before surgery had a 0.6% rate of infection (10/1208), compared with a 3.85% rate for those treated 2 to 24 hours ahead (14/369) (NNT=31).8 It is unclear whether multiple-dose therapy provides additional benefit when administered over the first 24 to 36 hours after surgery9 (OR=0.60; 95% CI, 0.18–2.02). First- or second-generation cephalosporins were used in most studies.
Delirium: A common but avoidable complication
Delirium is a common complication seen after hip fracture, affecting approximately 10% to 16% of patients.10,11 Delirium may increase the duration of hospitalization, and may be associated with an increased mortality at 1 year.12 Delirium can be avoided by looking at each patient’s risk factors.13 Studies suggest avoiding use of meperidine, benzodiazepines, and medications with anticholinergic side effects.11,13 Sleep deprivation, delayed mobility, and inadequate pain control are also associated with the development of delirium.13
One study showed that prophylaxis with haloperidol for hip fracture patients did not decrease the incidence of postoperative delirium but did reduce its duration and severity.14 Haloperidol prophylaxis was also associated with shorter hospital stays. Treatment with haloperidol or risperidone for the agitation of postoperative delirium has been recommended when behavioral interventions fail.13
Pain control improves recovery
Providing adequate analgesia is of the utmost importance. In a 2003 prospective cohort study, patients without sufficient analgesia had an increased risk of poor functional recovery and longer hospitalization.15 In another cohort study, those patients whose pain was inadequately controlled also had an increased risk for delirium (RR=9.0; 95% CI, 1.8–45.2).11 Meperidine use increased the risk for delirium compared with other opioid analgesics (RR=2.4; 95% CI, 1.3–4.5).11
Recommendations of others
The American College of Physicians provides a comprehensive evidence-based guideline for the management of hip fracture patients in their PIER series (Physicians’ Information and Education Resource) (TABLE).16
The American College of Chest Physicians has published evidence-based guidelines for the prevention of VTE.5 For patients undergoing hip fracture surgery, they recommend routine use of fondaparinux, low-molecular-weight heparin at high-risk dosing, adjusted-dose warfarin (at a target international normalized ratio [INR] of 2.5, range 2.0–3.0), or unfractionated heparin. They recommend against routine use of aspirin alone. If surgery must be delayed, physicians should initiate prophylaxis with unfractionated or low-molecular-weight heparin at the time of hospital admission. Anticoagulation should routinely continue for 10 days after surgery or until the patient is ambulatory. If anticoagulation is contraindicated, mechanical prophylaxis of VTE with foot and calf pumping devices is recommended.5,6
TABLE
6 steps for managing hip fracture from the American College of Physicians
|
| Source: PIER: Physicians’ Information and education resource, American College of Physicians, 2006.16 |
1. Parker MJ, Handoll HH, Bhargara A. Conservative versus operative treatment for hip fractures. Cochrane Database Syst Rev. 2000;(4):CD000337.
2. Dorotka R, Schoechtner H, Buchinger W. The influence of immediate surgical treatment of proximal femoral fractures on mortality and quality of life. Operation within six hours of the fracture versus later than six hours. J Bone Joint Surg Br. 2003;85:1107-1113.
3. Grimes JP, Gregory PM, Noveck H, Butler MS, Carson JL. The effects of time-to-surgery on mortality and morbidity in patients following hip fracture. Am J Med. 2002;112:702.
4. Bottle A, Aylin P. mortality associated with delay in operation after hip fracture: observational study. BMJ. 2006;332:947-951.
5. Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):S338-S400.
6. Handoll HH, Farrar MJ, Mcbirnie J, Tytherleighstrong G, Milne AA, Gillespie WJ. Heparin, low molecular weight heparin and physical methods for preventing deep vein thrombosis and pulmonary embolism following surgery for hip fractures. Cochrane Database Syst Rev. 2002;(4):CD000305
7. Gillespie WJ, Walenkamp G. Antibiotic prophylaxis for surgery for proximal femoral and other closed long bone fractures. Cochrane Database Syst Rev. 2001;(1):CD000244.
8. Classen DC, Evans RS, Pestotnik SL, Horn SD, Menlove RL, Burke JP. The timing of prophylactic administration of antibiotics and the risk of surgical wound infection. N Engl J Med. 1992;326:281-286.
9. March L, Chamberlain A, Cameron I, et al. Prevention, Treatment, and Rehabilitation of Fractured Neck of Femur. report from the Northern sydney Area Fractured Neck of Femur Health outcomes Project. St. Leonards, Australia: Public Health Unit, Northern Sydney Area Health Service; 1996. Available at: www.mja.com.au/public/issues/iprs2/march/fnof.pdf. Accessed on october 11, 2007.
10. Brauer C, Morrison RS, Silberzweig SB, Siu AL. The cause of delirium in patients with hip fracture. Arch Intern Med. 2000;160:1856-1860.
11. Morrison RS, Magaziner J, Gilbert M, et al. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci. 2003;58:76-81.
12. Edelstein DM, Aharonoff GB, Karp A, Capla EL, Zuckerman JD, Koval KJ. Effect of postoperative delirium on outcome after hip fracture. Clin Orthop Relat Res. 2004;422:195-200.
13. Day H. Postoperative delirium. PIER: Physician’s Information and education resource, American College of Physicians. July 2006. Available at: pier.acponline.org/index.html. Accessed on October 11, 2007.
14. Kalisvaart KJ, de Jonghe JF, Bogaards MJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc. 2005;53:1658-1666.
15. Morrison RS, Magaziner J, Mclaughlin MA, et al. The impact of post-operative pain on outcomes following hip fracture. Pain. 2003;103:303-311.
16. Christmas C. Hip fracture. PIER: Physicians’ Information and Education Resource, American College of Physicians. updated July 2006. Available at: pier.acponline.org/index.html. october 11, 2007.
1. Parker MJ, Handoll HH, Bhargara A. Conservative versus operative treatment for hip fractures. Cochrane Database Syst Rev. 2000;(4):CD000337.
2. Dorotka R, Schoechtner H, Buchinger W. The influence of immediate surgical treatment of proximal femoral fractures on mortality and quality of life. Operation within six hours of the fracture versus later than six hours. J Bone Joint Surg Br. 2003;85:1107-1113.
3. Grimes JP, Gregory PM, Noveck H, Butler MS, Carson JL. The effects of time-to-surgery on mortality and morbidity in patients following hip fracture. Am J Med. 2002;112:702.
4. Bottle A, Aylin P. mortality associated with delay in operation after hip fracture: observational study. BMJ. 2006;332:947-951.
5. Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):S338-S400.
6. Handoll HH, Farrar MJ, Mcbirnie J, Tytherleighstrong G, Milne AA, Gillespie WJ. Heparin, low molecular weight heparin and physical methods for preventing deep vein thrombosis and pulmonary embolism following surgery for hip fractures. Cochrane Database Syst Rev. 2002;(4):CD000305
7. Gillespie WJ, Walenkamp G. Antibiotic prophylaxis for surgery for proximal femoral and other closed long bone fractures. Cochrane Database Syst Rev. 2001;(1):CD000244.
8. Classen DC, Evans RS, Pestotnik SL, Horn SD, Menlove RL, Burke JP. The timing of prophylactic administration of antibiotics and the risk of surgical wound infection. N Engl J Med. 1992;326:281-286.
9. March L, Chamberlain A, Cameron I, et al. Prevention, Treatment, and Rehabilitation of Fractured Neck of Femur. report from the Northern sydney Area Fractured Neck of Femur Health outcomes Project. St. Leonards, Australia: Public Health Unit, Northern Sydney Area Health Service; 1996. Available at: www.mja.com.au/public/issues/iprs2/march/fnof.pdf. Accessed on october 11, 2007.
10. Brauer C, Morrison RS, Silberzweig SB, Siu AL. The cause of delirium in patients with hip fracture. Arch Intern Med. 2000;160:1856-1860.
11. Morrison RS, Magaziner J, Gilbert M, et al. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci. 2003;58:76-81.
12. Edelstein DM, Aharonoff GB, Karp A, Capla EL, Zuckerman JD, Koval KJ. Effect of postoperative delirium on outcome after hip fracture. Clin Orthop Relat Res. 2004;422:195-200.
13. Day H. Postoperative delirium. PIER: Physician’s Information and education resource, American College of Physicians. July 2006. Available at: pier.acponline.org/index.html. Accessed on October 11, 2007.
14. Kalisvaart KJ, de Jonghe JF, Bogaards MJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc. 2005;53:1658-1666.
15. Morrison RS, Magaziner J, Mclaughlin MA, et al. The impact of post-operative pain on outcomes following hip fracture. Pain. 2003;103:303-311.
16. Christmas C. Hip fracture. PIER: Physicians’ Information and Education Resource, American College of Physicians. updated July 2006. Available at: pier.acponline.org/index.html. october 11, 2007.
Evidence-based answers from the Family Physicians Inquiries Network
Should patients receive 23-valent pneumococcal vaccination more than once?
No patient-oriented evidence supports pneumococcal revaccination of any patient (high-risk or otherwise). Antibody levels may be augmented by revaccination; however, the clinical efficacy of revaccination, even among high-risk patients, is unknown. Revaccination is recommended by the Advisory Committee on Immunization Practices (ACIP) in certain circumstances. (strength of recommendation [SOR]: C, expert opinion based on physiology/bench research). Revaccination once appears to be safe, especially if provided 5 years or more after primary vaccination (SOR: B, based upon consistent results of cohort studies and nonrandomized prospective trials).
Until research proves otherwise, keep revaccinating high-risk patients
Richard Kim, MD
Glendale, Ariz
It is unfortunate that we lack any clinical data for or against pneumococcal revaccination. As a result, we may disagree on the correct revaccination schedule. Several questions arise from this uncertainty. First, do increasing antibody levels increase or prolong immunity? Second, how do we weigh the estimated numbers needed to treat (NNT) versus the numbers needed to harm (NNH)? And third, considering the prevalence and severity of the disease, should we err on the side of immunizing more rather than less? Since booster shots seem to be safe (implying a high NNH), the ACIP guideline is probably a good compromise between increasing antibody titers (theoretical benefit), and lack of efficacy data. Until we have further information to the contrary, I’m going to continue to revaccinate high-risk patients.
Evidence summary
Streptococcus pneumoniae is the most common cause of community-acquired pneumonia and the second most common cause of bacterial meningitis in the United States.1 An estimated 40,000 people die annually in the US from pneumococcal infections.1 Even with antibiotic treatment and intensive care unit support, the mortality of patients with pneumococcal bacteremia approaches 25% to 30%.1
Because of the importance of this pathogen, it has been the focus of several trials to demonstrate the efficacy of the primary vaccination. To date, 7 meta-analyses have been completed to assess the efficacy of pneumococcal vaccine in adults, with varying results.2-7 Most recently, a Cochrane Review (updated in 2005)8 concluded that “the combined results from randomized studies fail to show that the polysaccharide vaccine is effective in preventing either pneumonia or death.” However, they did recognize that the nonrandomized studies have consistently shown that the polysaccharide vaccine is effective in reducing the more specific outcome of invasive pneumococcal disease (bacteremia and meningitis).
Multiple studies have used measurement of antibody levels to assess the response of patients to the vaccine and for justification of the need for revaccination. However, measurement of antibody levels to pneumococcal serotypes is difficult, inexact, and is only a surrogate marker for the immune status of a patient, which also relies on the overall function of their immune system. Although pneumococcal vaccine is most highly recommended in patients with chronic disease or immunodeficiency, these patients have a poorer initial response rate and a faster decline in antibody levels than younger, immunocompetent recipients of the vaccine.1,9
A study of pneumococcal strains cultured from hospitalized patients demonstrated a duration of protection against pneumococcal infection that was much longer than that predicted by the shorter duration of antibody levels. The vaccine’s ability to reduce infection (due to serotypes included in the vaccine) lasted for at least 9 years and overall efficacy for preventing infection caused by the serotypes included in the vaccine was 57%.1
Revaccination is safe; particularly when performed more than 5 years after the initial vaccination. Injection site reactions are more common and more severe in revaccinated persons (rising from 3% to 15% in the immunocompetent patient).10 Revaccination, however, does not result in increased rates of hospitalization, and few severe reactions have been reported.11
No randomized or prospective trials regarding the clinical efficacy of revaccination have been completed. However, when reviewing the studies of antibody response, several summary conclusions can be made. Among those who were nonresponders to the initial vaccination, revaccination (even repeated revaccination) is not effective in stimulating any significant antibody response.12-14 Among those who responded to the primary vaccination, revaccination can stimulate a second antibody response—albeit to lower levels and with less duration than after the initial vaccination.13,15-17 Among those who do respond to revaccination, antibody levels can rapidly decline to undetectable levels in a matter of months, and they may or may not retain protection against disease over time.14,15 It appears that revaccination recommendations have been based on the safety of the vaccination, concern for patients at risk and reduced antibody levels, rather than on proven clinical utility.
Recommendations from others
The Advisory Committee on Immunization Practices (ACIP) advises that the vaccine be used in “persons with diseases and other conditions predisposing to the development of bacteremic pneumococcal pneumonia” and that “revaccination should not be done at intervals less than five years.” They state "the value of vaccination on the basis of advanced age is not clear at this time.” (Further details may be found at: www.aafp.org/PreBuilt/agecharts_adultimmunization.pdf.)
The American Academy of Family Physicians, American College of Physicians, the American College of Obstetricians and Gynecologists and the American Diabetes Association follow the ACIP recommendations.
1. Butler JC, Breiman RF, Campbell JF, Lipman HB, Broome CV, Facklam RR. Pneumococcal polysaccharide vaccine efficacy: An Evaluation of current recommendations. JAMA 1993;270:1826-1831.
2. Fine MJ, Smith MA, Carson CA, et al. Efficacy of pneumococcal vaccination in adults: A meta-analysis of randomized controlled trials. Arch Intern Med 1994;154:2666-2677.
3. Hutchinson BG, Oxman AD, Shannon HS, Lloyd S, Altmayer CA, Thomas K. Clinical effectiveness of pneumococcal vaccine. Meta analysis. Can Fam Physician 1999;45:2381-2393.
4. Cornu C, Yzebe D, Leophonte P, Gaillat J, Boissel JP, Cucherat M. Efficacy of pneumococcal polysaccharide vaccine in immunocompetent adults: a meta-analysis of randomized trials. Vaccine 2001;19:4780-4790.
5. Moore RA, Wiffen PJ, Lipsky BA. Are the pneumococcal polysaccharide vaccines effective? Meta-analysis of the prospective trials. BMC Fam Pract 2000;1:1.-
6. Puig-Barbera J, Belenguer Varea A, Goterris Pinto M, Brines Benlliure MJ. Pneumococcal vaccine effectiveness in the elderly. Systematic review and meta-analysis. Aten Primaria 2002;30:269-281.
7. Watson I, Wilson BJ, Waugh N. Pneumococcal polysaccharide vaccine: a systematic review of clinical effectiveness in adults. Vaccine 2002;20:2166-2173.
8. Dear KB, Holden J, Andrews R, Tatham D. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev 2003;(4):CD000422.-
9. Whitney CG, Schaffner W, Butler JC. Rethinking recommendations for use of pneumococcal vaccines in adults. Clin Infect Dis 2001;33:662-675.
10. Jackson LA, Benson P, Snellar VP, et al. Safety of revaccination with pneumococcal polysaccharide vaccine. JAMA 1999;281:243-248.
11. Snow R, Babish JD, McBean AM. Is there any connection between a second pneumonia shot and hospitalization among Medicare beneficiaries? Public Health Rep 1995;110:720-725.
12. Petrasch S, Kuhnemund O, Reinacher A, et al. Antibody responses of splenectomized patients with non-Hodgkin’s lymphoma to immunization with polyvalent pneumococcal vaccines. Clin Diagn Lab Immunol 1997;4:635-638.
13. Rodriguez-Barradas MC, Groover JE, Lacke CE, et al. IgG antibody to pneumococcal capsular polysaccharide in human immunodeficiency virus-infected subjects: persistence of antibody in responders, revaccination in nonresponders, and relationship of immunoglobulin allotype to response. J Infect Dis 1996;173:1347-1353.
14. Cherif H, Landgren O, Konradsen HB, Kalin M, Bjorkholm M. Poor antibody response to pneumococcal polysaccharide vaccination suggests increased susceptibility to pneumococcal infection in splenectomized patients with hematological diseases. Vaccine. 2006;24(1):75-81.
15. Lackner TE, Hamilton RG, Hill JJ, Davey C, Guay DR. Pneumococcal polysaccharide revaccination: immunoglobulin g seroconversion, persistence, and safety in frail, chronically ill older subjects. J Am Geriatr Soc 2003;51:240-245.
16. Landgren O, Bjorkholm M, Konradsen HB, et al. A prospective study on antibody response to repeated vaccinations with pneumococcal capsular polysaccharide in splenectomized individuals with special reference to Hodgkin’s lymphoma. J Intern Med 2004;255:664-673.
17. Torling J, Hedlund J, Konradsen HB, Ortqvist A. Revaccination with the 23-valent pneumococcal polysaccharide vaccine in middle-aged and elderly persons previously treated for pneumonia. Vaccine 2003;22:96-103.
No patient-oriented evidence supports pneumococcal revaccination of any patient (high-risk or otherwise). Antibody levels may be augmented by revaccination; however, the clinical efficacy of revaccination, even among high-risk patients, is unknown. Revaccination is recommended by the Advisory Committee on Immunization Practices (ACIP) in certain circumstances. (strength of recommendation [SOR]: C, expert opinion based on physiology/bench research). Revaccination once appears to be safe, especially if provided 5 years or more after primary vaccination (SOR: B, based upon consistent results of cohort studies and nonrandomized prospective trials).
Until research proves otherwise, keep revaccinating high-risk patients
Richard Kim, MD
Glendale, Ariz
It is unfortunate that we lack any clinical data for or against pneumococcal revaccination. As a result, we may disagree on the correct revaccination schedule. Several questions arise from this uncertainty. First, do increasing antibody levels increase or prolong immunity? Second, how do we weigh the estimated numbers needed to treat (NNT) versus the numbers needed to harm (NNH)? And third, considering the prevalence and severity of the disease, should we err on the side of immunizing more rather than less? Since booster shots seem to be safe (implying a high NNH), the ACIP guideline is probably a good compromise between increasing antibody titers (theoretical benefit), and lack of efficacy data. Until we have further information to the contrary, I’m going to continue to revaccinate high-risk patients.
Evidence summary
Streptococcus pneumoniae is the most common cause of community-acquired pneumonia and the second most common cause of bacterial meningitis in the United States.1 An estimated 40,000 people die annually in the US from pneumococcal infections.1 Even with antibiotic treatment and intensive care unit support, the mortality of patients with pneumococcal bacteremia approaches 25% to 30%.1
Because of the importance of this pathogen, it has been the focus of several trials to demonstrate the efficacy of the primary vaccination. To date, 7 meta-analyses have been completed to assess the efficacy of pneumococcal vaccine in adults, with varying results.2-7 Most recently, a Cochrane Review (updated in 2005)8 concluded that “the combined results from randomized studies fail to show that the polysaccharide vaccine is effective in preventing either pneumonia or death.” However, they did recognize that the nonrandomized studies have consistently shown that the polysaccharide vaccine is effective in reducing the more specific outcome of invasive pneumococcal disease (bacteremia and meningitis).
Multiple studies have used measurement of antibody levels to assess the response of patients to the vaccine and for justification of the need for revaccination. However, measurement of antibody levels to pneumococcal serotypes is difficult, inexact, and is only a surrogate marker for the immune status of a patient, which also relies on the overall function of their immune system. Although pneumococcal vaccine is most highly recommended in patients with chronic disease or immunodeficiency, these patients have a poorer initial response rate and a faster decline in antibody levels than younger, immunocompetent recipients of the vaccine.1,9
A study of pneumococcal strains cultured from hospitalized patients demonstrated a duration of protection against pneumococcal infection that was much longer than that predicted by the shorter duration of antibody levels. The vaccine’s ability to reduce infection (due to serotypes included in the vaccine) lasted for at least 9 years and overall efficacy for preventing infection caused by the serotypes included in the vaccine was 57%.1
Revaccination is safe; particularly when performed more than 5 years after the initial vaccination. Injection site reactions are more common and more severe in revaccinated persons (rising from 3% to 15% in the immunocompetent patient).10 Revaccination, however, does not result in increased rates of hospitalization, and few severe reactions have been reported.11
No randomized or prospective trials regarding the clinical efficacy of revaccination have been completed. However, when reviewing the studies of antibody response, several summary conclusions can be made. Among those who were nonresponders to the initial vaccination, revaccination (even repeated revaccination) is not effective in stimulating any significant antibody response.12-14 Among those who responded to the primary vaccination, revaccination can stimulate a second antibody response—albeit to lower levels and with less duration than after the initial vaccination.13,15-17 Among those who do respond to revaccination, antibody levels can rapidly decline to undetectable levels in a matter of months, and they may or may not retain protection against disease over time.14,15 It appears that revaccination recommendations have been based on the safety of the vaccination, concern for patients at risk and reduced antibody levels, rather than on proven clinical utility.
Recommendations from others
The Advisory Committee on Immunization Practices (ACIP) advises that the vaccine be used in “persons with diseases and other conditions predisposing to the development of bacteremic pneumococcal pneumonia” and that “revaccination should not be done at intervals less than five years.” They state "the value of vaccination on the basis of advanced age is not clear at this time.” (Further details may be found at: www.aafp.org/PreBuilt/agecharts_adultimmunization.pdf.)
The American Academy of Family Physicians, American College of Physicians, the American College of Obstetricians and Gynecologists and the American Diabetes Association follow the ACIP recommendations.
No patient-oriented evidence supports pneumococcal revaccination of any patient (high-risk or otherwise). Antibody levels may be augmented by revaccination; however, the clinical efficacy of revaccination, even among high-risk patients, is unknown. Revaccination is recommended by the Advisory Committee on Immunization Practices (ACIP) in certain circumstances. (strength of recommendation [SOR]: C, expert opinion based on physiology/bench research). Revaccination once appears to be safe, especially if provided 5 years or more after primary vaccination (SOR: B, based upon consistent results of cohort studies and nonrandomized prospective trials).
Until research proves otherwise, keep revaccinating high-risk patients
Richard Kim, MD
Glendale, Ariz
It is unfortunate that we lack any clinical data for or against pneumococcal revaccination. As a result, we may disagree on the correct revaccination schedule. Several questions arise from this uncertainty. First, do increasing antibody levels increase or prolong immunity? Second, how do we weigh the estimated numbers needed to treat (NNT) versus the numbers needed to harm (NNH)? And third, considering the prevalence and severity of the disease, should we err on the side of immunizing more rather than less? Since booster shots seem to be safe (implying a high NNH), the ACIP guideline is probably a good compromise between increasing antibody titers (theoretical benefit), and lack of efficacy data. Until we have further information to the contrary, I’m going to continue to revaccinate high-risk patients.
Evidence summary
Streptococcus pneumoniae is the most common cause of community-acquired pneumonia and the second most common cause of bacterial meningitis in the United States.1 An estimated 40,000 people die annually in the US from pneumococcal infections.1 Even with antibiotic treatment and intensive care unit support, the mortality of patients with pneumococcal bacteremia approaches 25% to 30%.1
Because of the importance of this pathogen, it has been the focus of several trials to demonstrate the efficacy of the primary vaccination. To date, 7 meta-analyses have been completed to assess the efficacy of pneumococcal vaccine in adults, with varying results.2-7 Most recently, a Cochrane Review (updated in 2005)8 concluded that “the combined results from randomized studies fail to show that the polysaccharide vaccine is effective in preventing either pneumonia or death.” However, they did recognize that the nonrandomized studies have consistently shown that the polysaccharide vaccine is effective in reducing the more specific outcome of invasive pneumococcal disease (bacteremia and meningitis).
Multiple studies have used measurement of antibody levels to assess the response of patients to the vaccine and for justification of the need for revaccination. However, measurement of antibody levels to pneumococcal serotypes is difficult, inexact, and is only a surrogate marker for the immune status of a patient, which also relies on the overall function of their immune system. Although pneumococcal vaccine is most highly recommended in patients with chronic disease or immunodeficiency, these patients have a poorer initial response rate and a faster decline in antibody levels than younger, immunocompetent recipients of the vaccine.1,9
A study of pneumococcal strains cultured from hospitalized patients demonstrated a duration of protection against pneumococcal infection that was much longer than that predicted by the shorter duration of antibody levels. The vaccine’s ability to reduce infection (due to serotypes included in the vaccine) lasted for at least 9 years and overall efficacy for preventing infection caused by the serotypes included in the vaccine was 57%.1
Revaccination is safe; particularly when performed more than 5 years after the initial vaccination. Injection site reactions are more common and more severe in revaccinated persons (rising from 3% to 15% in the immunocompetent patient).10 Revaccination, however, does not result in increased rates of hospitalization, and few severe reactions have been reported.11
No randomized or prospective trials regarding the clinical efficacy of revaccination have been completed. However, when reviewing the studies of antibody response, several summary conclusions can be made. Among those who were nonresponders to the initial vaccination, revaccination (even repeated revaccination) is not effective in stimulating any significant antibody response.12-14 Among those who responded to the primary vaccination, revaccination can stimulate a second antibody response—albeit to lower levels and with less duration than after the initial vaccination.13,15-17 Among those who do respond to revaccination, antibody levels can rapidly decline to undetectable levels in a matter of months, and they may or may not retain protection against disease over time.14,15 It appears that revaccination recommendations have been based on the safety of the vaccination, concern for patients at risk and reduced antibody levels, rather than on proven clinical utility.
Recommendations from others
The Advisory Committee on Immunization Practices (ACIP) advises that the vaccine be used in “persons with diseases and other conditions predisposing to the development of bacteremic pneumococcal pneumonia” and that “revaccination should not be done at intervals less than five years.” They state "the value of vaccination on the basis of advanced age is not clear at this time.” (Further details may be found at: www.aafp.org/PreBuilt/agecharts_adultimmunization.pdf.)
The American Academy of Family Physicians, American College of Physicians, the American College of Obstetricians and Gynecologists and the American Diabetes Association follow the ACIP recommendations.
1. Butler JC, Breiman RF, Campbell JF, Lipman HB, Broome CV, Facklam RR. Pneumococcal polysaccharide vaccine efficacy: An Evaluation of current recommendations. JAMA 1993;270:1826-1831.
2. Fine MJ, Smith MA, Carson CA, et al. Efficacy of pneumococcal vaccination in adults: A meta-analysis of randomized controlled trials. Arch Intern Med 1994;154:2666-2677.
3. Hutchinson BG, Oxman AD, Shannon HS, Lloyd S, Altmayer CA, Thomas K. Clinical effectiveness of pneumococcal vaccine. Meta analysis. Can Fam Physician 1999;45:2381-2393.
4. Cornu C, Yzebe D, Leophonte P, Gaillat J, Boissel JP, Cucherat M. Efficacy of pneumococcal polysaccharide vaccine in immunocompetent adults: a meta-analysis of randomized trials. Vaccine 2001;19:4780-4790.
5. Moore RA, Wiffen PJ, Lipsky BA. Are the pneumococcal polysaccharide vaccines effective? Meta-analysis of the prospective trials. BMC Fam Pract 2000;1:1.-
6. Puig-Barbera J, Belenguer Varea A, Goterris Pinto M, Brines Benlliure MJ. Pneumococcal vaccine effectiveness in the elderly. Systematic review and meta-analysis. Aten Primaria 2002;30:269-281.
7. Watson I, Wilson BJ, Waugh N. Pneumococcal polysaccharide vaccine: a systematic review of clinical effectiveness in adults. Vaccine 2002;20:2166-2173.
8. Dear KB, Holden J, Andrews R, Tatham D. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev 2003;(4):CD000422.-
9. Whitney CG, Schaffner W, Butler JC. Rethinking recommendations for use of pneumococcal vaccines in adults. Clin Infect Dis 2001;33:662-675.
10. Jackson LA, Benson P, Snellar VP, et al. Safety of revaccination with pneumococcal polysaccharide vaccine. JAMA 1999;281:243-248.
11. Snow R, Babish JD, McBean AM. Is there any connection between a second pneumonia shot and hospitalization among Medicare beneficiaries? Public Health Rep 1995;110:720-725.
12. Petrasch S, Kuhnemund O, Reinacher A, et al. Antibody responses of splenectomized patients with non-Hodgkin’s lymphoma to immunization with polyvalent pneumococcal vaccines. Clin Diagn Lab Immunol 1997;4:635-638.
13. Rodriguez-Barradas MC, Groover JE, Lacke CE, et al. IgG antibody to pneumococcal capsular polysaccharide in human immunodeficiency virus-infected subjects: persistence of antibody in responders, revaccination in nonresponders, and relationship of immunoglobulin allotype to response. J Infect Dis 1996;173:1347-1353.
14. Cherif H, Landgren O, Konradsen HB, Kalin M, Bjorkholm M. Poor antibody response to pneumococcal polysaccharide vaccination suggests increased susceptibility to pneumococcal infection in splenectomized patients with hematological diseases. Vaccine. 2006;24(1):75-81.
15. Lackner TE, Hamilton RG, Hill JJ, Davey C, Guay DR. Pneumococcal polysaccharide revaccination: immunoglobulin g seroconversion, persistence, and safety in frail, chronically ill older subjects. J Am Geriatr Soc 2003;51:240-245.
16. Landgren O, Bjorkholm M, Konradsen HB, et al. A prospective study on antibody response to repeated vaccinations with pneumococcal capsular polysaccharide in splenectomized individuals with special reference to Hodgkin’s lymphoma. J Intern Med 2004;255:664-673.
17. Torling J, Hedlund J, Konradsen HB, Ortqvist A. Revaccination with the 23-valent pneumococcal polysaccharide vaccine in middle-aged and elderly persons previously treated for pneumonia. Vaccine 2003;22:96-103.
1. Butler JC, Breiman RF, Campbell JF, Lipman HB, Broome CV, Facklam RR. Pneumococcal polysaccharide vaccine efficacy: An Evaluation of current recommendations. JAMA 1993;270:1826-1831.
2. Fine MJ, Smith MA, Carson CA, et al. Efficacy of pneumococcal vaccination in adults: A meta-analysis of randomized controlled trials. Arch Intern Med 1994;154:2666-2677.
3. Hutchinson BG, Oxman AD, Shannon HS, Lloyd S, Altmayer CA, Thomas K. Clinical effectiveness of pneumococcal vaccine. Meta analysis. Can Fam Physician 1999;45:2381-2393.
4. Cornu C, Yzebe D, Leophonte P, Gaillat J, Boissel JP, Cucherat M. Efficacy of pneumococcal polysaccharide vaccine in immunocompetent adults: a meta-analysis of randomized trials. Vaccine 2001;19:4780-4790.
5. Moore RA, Wiffen PJ, Lipsky BA. Are the pneumococcal polysaccharide vaccines effective? Meta-analysis of the prospective trials. BMC Fam Pract 2000;1:1.-
6. Puig-Barbera J, Belenguer Varea A, Goterris Pinto M, Brines Benlliure MJ. Pneumococcal vaccine effectiveness in the elderly. Systematic review and meta-analysis. Aten Primaria 2002;30:269-281.
7. Watson I, Wilson BJ, Waugh N. Pneumococcal polysaccharide vaccine: a systematic review of clinical effectiveness in adults. Vaccine 2002;20:2166-2173.
8. Dear KB, Holden J, Andrews R, Tatham D. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev 2003;(4):CD000422.-
9. Whitney CG, Schaffner W, Butler JC. Rethinking recommendations for use of pneumococcal vaccines in adults. Clin Infect Dis 2001;33:662-675.
10. Jackson LA, Benson P, Snellar VP, et al. Safety of revaccination with pneumococcal polysaccharide vaccine. JAMA 1999;281:243-248.
11. Snow R, Babish JD, McBean AM. Is there any connection between a second pneumonia shot and hospitalization among Medicare beneficiaries? Public Health Rep 1995;110:720-725.
12. Petrasch S, Kuhnemund O, Reinacher A, et al. Antibody responses of splenectomized patients with non-Hodgkin’s lymphoma to immunization with polyvalent pneumococcal vaccines. Clin Diagn Lab Immunol 1997;4:635-638.
13. Rodriguez-Barradas MC, Groover JE, Lacke CE, et al. IgG antibody to pneumococcal capsular polysaccharide in human immunodeficiency virus-infected subjects: persistence of antibody in responders, revaccination in nonresponders, and relationship of immunoglobulin allotype to response. J Infect Dis 1996;173:1347-1353.
14. Cherif H, Landgren O, Konradsen HB, Kalin M, Bjorkholm M. Poor antibody response to pneumococcal polysaccharide vaccination suggests increased susceptibility to pneumococcal infection in splenectomized patients with hematological diseases. Vaccine. 2006;24(1):75-81.
15. Lackner TE, Hamilton RG, Hill JJ, Davey C, Guay DR. Pneumococcal polysaccharide revaccination: immunoglobulin g seroconversion, persistence, and safety in frail, chronically ill older subjects. J Am Geriatr Soc 2003;51:240-245.
16. Landgren O, Bjorkholm M, Konradsen HB, et al. A prospective study on antibody response to repeated vaccinations with pneumococcal capsular polysaccharide in splenectomized individuals with special reference to Hodgkin’s lymphoma. J Intern Med 2004;255:664-673.
17. Torling J, Hedlund J, Konradsen HB, Ortqvist A. Revaccination with the 23-valent pneumococcal polysaccharide vaccine in middle-aged and elderly persons previously treated for pneumonia. Vaccine 2003;22:96-103.
Evidence-based answers from the Family Physicians Inquiries Network
How should patients with Barrett’s esophagus be monitored?
Some patients who have been diagnosed with Barrett’s esophagus will develop dysplasia and, in some cases, esophageal carcinoma (strength of recommendation [SOR]: A, based on consistent cohort studies). Endoscopic surveillance is recommended for all patients with Barrett’s esophagus as it is superior to other methods for detecting esophageal cancer (SOR: B, based on systematic review). The degree of dysplasia noted on biopsy specimens correlates with the risk of esophageal carcinoma development and should guide the frequency of subsequent evaluations (SOR: B, based on consistent cohort studies). The optimal frequency of endoscopy has yet to be determined in any randomized trial.
Recommendations from the 2002 American College of Gastroenterology (ACG) Practice Guideline provide guidance as to the frequency of endoscopy surveillance but were not based on an explicit systematic review of the literature (SOR: C, based on expert opinion; see TABLE 1).
Reduced monitoring for most patients with Barrett’s esophagus appears safe
Paul Crawford, MD
USAF–Eglin Family Practice Residency, Eglin Air Force Base, Fla
Family physicians have long been at the mercy of expert opinion when considering how to monitor patients with Barrett’s esophagus. This review of the evidence clearly shows that the days of yearly EGD for all Barrett’s esophagus patients are over.
Unlike other conditions—such as cervical dysplasia, where monitoring and therapies to remove dysplasia are proven to save lives—Barrett’s esophagus progresses slowly and unpredictably. Thus, until technological advances allow identification of higher risk Barrett’s esophagus patients, an EGD every 3 years for those without dysplasia seems to be a reasonable monitoring interval. Perhaps most importantly, family physicians can reassure Barrett’s esophagus patients in the community that they are likely to live a normal lifespan and die of something other than esophageal cancer.
Evidence summary
Barrett’s esophagus has been defined as “a change in the esophageal epithelium of any length that can be recognized at endoscopy and is confirmed to have intestinal metaplasia by biopsy of the tubular esophagus.”1 Intestinal metaplasia is a premalignant lesion for adenocarcinoma of the esophagus. Surveillance by serial endoscopy with biopsy has been recommended in an effort to find high-grade dysplasia or carcinoma in an early, asymptomatic, and potentially curable stage.1-4 Approximately 75% of patients involved in a Barrett’s esophagus surveillance program will present with resectable tumors, compared with only 25% of those not receiving surveillance.4
A recent systematic review assessing screening tools for esophageal carcinoma found standard endoscopy to be superior (90%–100% sensitivity) to other less invasive methods such as questionnaire (60%–70%), and fecal occult blood testing (20%).4 Additional endoscopy tools such as brush and balloon cytology increased the cost of surveillance without any improvement in diagnostic yield.
The degree of dysplasia on esophageal biopsy in Barrett’s esophagus patients is currently the best indicator of risk of progression to esophageal carcinoma. The data reviewed by the ACG for the practice guideline was drawn from several prospective studies and one available registry. In sum, a total of 783 Barrett’s esophagus patients were followed for a mean of 2.9 to 7.3 years. Esophageal carcinoma developed in 2% of patients with no dysplasia, 7% of patients with low-grade dysplasia (LGD) and 22% of patients with high-grade dysplasia (HGD).1 The ACG recommendations regarding frequency of esophagogastroduodenoscopy (EGD) were not based on an explicit critical appraisal of the literature. Recent cohort studies are consistent with recommendations for graded surveillance frequency. A randomized clinical trial to determine optimal endoscopic frequency and benefit has not been reported.
Several concerns have been raised regarding the utility of degree of dysplasia in determining optimal frequency of endoscopic surveillance. First, the progression of esophageal lesions over time is unpredictable. Skacel et al5 reported a series of 34 patients with LGD at initial pathologic examination. On subsequent surveillance endoscopy with repeat biopsy, 73% no longer demonstrated dysplasia. Such patients can be allowed to return to having surveillance every 3 years.
In addition to the non-linear progression of dysplasia, inter-rater reliability of the interpretation of pathology specimens varies substantially. Adequate reliability has been demonstrated among pathologists assigning results to 2 categories (either no dysplasia and LGD or HGD and carcinoma) (κ=0.7). Assignment to four distinct pathologic grades, however, was not reliable (κ=0.46, where 1.0 is complete agreement).1 In order to make a diagnosis of HGD or carcinoma, interpretation must be independently confirmed by 2 expert pathologists.1-3
Recommendations for frequent endoscopic surveillance are also weakened by the overall low rate of mortality from esophageal carcinoma noted in Barrett’s esophagus patients. A recent population based study demonstrated that there was no difference in overall mortality in those with a Barrett’s esophagus diagnosis compared with the general population.6 An increased risk of death from esophageal carcinoma was seen in patients with Barrett’s esophagus (4.7% seen in Barrett’s esophagus patients compared with 0.8% predicted in the general population; P<.05). The overall increased effect on mortality, however, was relatively small. Esophageal carcinoma accounted for less then 5% of deaths in Barrett’s esophagus patients reported during the study’s 6-year follow-up period.
Data from prospective studies published after 2002 may better predict prognosis for Barrett’s esophagus patients.6-9 Even lower rates of progression to esophageal carcinoma (<0.5% a year or <1/220 patient-years) have been reported in these studies drawing from the general population rather than referred patients, likely stemming from differences in gender mix, patient age, and risk factors.
In addition to grade of dysplasia, the length of the dysplastic Barrett’s esophagus segment is emerging as a potentially predictive risk factor. While the ACG cautions that esophageal cancer has been reported in patients with so-called “short segment” Barrett’s esophagus (SSBE) (≤3 cm),1 recent prospective studies have shown an increased risk of carcinoma development with long segment Barrett’s esophagus (LSBE).7-9 Weston et al7 reported a 2.4% progression rate to HGD or esophageal carcinoma with SSBE and no dysplasia compared with 6.8% with LSBE (P=.002). If patients had LGD, the rate of progression to esophageal carcinoma with SSBE was 5.3% and jumped to 35% in patients with LSBE (P<.001). Conio et al8 reported that 4 of 5 cases of esophageal carcinoma noted through surveillance had LSBE. Hage et al9 reported a significantly increased risk of progression to HGD or esophageal carcinoma with long segment disease (P<.02).
While currently still considered investigational, DNA content flow cytometry may be a future tool used in risk stratification. Reid et al10 report a 5-year cumulative risk of esophageal carcinoma of 1.7% in Barrett’s esophagus patients with negative, low-grade or indefinite grades of dysplasia. Subsequent application of flow cytometry allowed for further stratification of these low-risk patients. Those with neither aneuploidy nor an increased 4N had a 5-year cumulative risk of cancer of 0% while the risk for those with abnormalities on cytometry increased to 28% (relative risk=19; P<.001).
TABLE
Grade of dysplasia and recommendations for Barrett’s esophagus surveillance as proposed by the ACG
| DYSPLASIA | DOCUMENTATION | FOLLOW-UP ENDOSCOPY |
|---|---|---|
| None | Two EGDs with biopsy | 3 years |
| Low-grade | Highest grade on repeat | 1 year until no dysplasia |
| High-grade | Repeat EGD with biopsy to rule out cancer/document high-grade dysplasia; expert pathologist confirmation | Focal: every 3 months |
| Multifocal: intervention | ||
| Mucosal irregularity: EMR | ||
| ACG, American College of Gastroenterology; EGD, esophagogastroduodenoscopy; EMR, endoscopic mucosal resection. Intervention: ie, esophagectomy. Ablative therapies only in the setting of a clinical trial or for those unable to tolerate surgery. | ||
Recommendations from others
The French Society of Digestive Endoscopy has published guidelines on monitoring Barrett’s esophagus.3 Their recommendations differ only slightly from the ACG in advocating a slightly increased frequency of EGD surveillance based on degree of dysplasia, and utilizing the length of the dysplastic segment in decision-making. Neither the American Academy of Family Physicians nor the US Preventive Services Task Force make any specific recommendations about Barrett’s esophagus surveillance.
1. Sampliner RE. Updated guidelines for the diagnosis, surveillance, and therapy of Barrett’s esophagus. Am J Gastroenterol 2002;97:1888-1895.
2. Management of Barrett’s esophagus. The Society for Surgery of the Alimentary Tract (SSAT), American Gastroenterological Association (AGA), American Society for Gastrointestinal Endoscopy (ASGE) Consensus Panel. J Gastrointest Surg 2000;4:115-116.
3. Boyer J, Robaszkiewicz M. Guidelines of the French Society of Digestive Endoscopy: Monitoring of Barrett’s esophagus. The Council of the French Society of Digestive Endoscopy. Endoscopy 2000;32:498-499.
4. Gerson LB, Triadafilopoulos G. Screening for esophageal adenocarcinoma: an evidence-based approach. Am J Med 2002;113:499-505.
5. Skacel M, Petras RE, Gramlich TL, et al. The diagnosis of low grade dysplasia in Barrett’s esophagus and its implications for disease progression. Am J Gastroenterol 2000;95:3383-3387.
6. Anderson LA, Murray LJ, Murphy SJ, et al. Mortality in Barrett’s oesophagus: results from a population based study. Gut 2003;52:1081-1084.
7. Weston AP, Sharma P, Mathur S, et al. Risk stratification of barrett’s esophagus: updated prospective multivariate analysis. Am J Gastroenterol 2004;99:1657-1666.
8. Conio M, Blanchi S, Laertosa G, et al. Long-term endoscopic surveillance of patients with Barrett’s esophagus. Incidence of dysplasia and adenocarcinoma: a prospective study. Am J Gastroenterol 2003;98:1931-1939.
9. Hage M, Siersema PD, van Dekken H, Steyerber EW, Dees J, Kuipers EJ. Oesophageal cancer incidence and mortality in patients with long-segment Barrett’s oesophagus after a mean follow-up of 12.7 years. Scand J Gastroenterol 2004;39:1175-1179.
10. Reid BJ, Levine DS, Longton G, Blount P, Rabinovitch PS. Predictors of progression to cancer in Barrett’s esophagus: baseline histology and flow cytometry identify low- and high-risk patient subsets. Am J Gastroenterol 2000;95:1669-1676.
Some patients who have been diagnosed with Barrett’s esophagus will develop dysplasia and, in some cases, esophageal carcinoma (strength of recommendation [SOR]: A, based on consistent cohort studies). Endoscopic surveillance is recommended for all patients with Barrett’s esophagus as it is superior to other methods for detecting esophageal cancer (SOR: B, based on systematic review). The degree of dysplasia noted on biopsy specimens correlates with the risk of esophageal carcinoma development and should guide the frequency of subsequent evaluations (SOR: B, based on consistent cohort studies). The optimal frequency of endoscopy has yet to be determined in any randomized trial.
Recommendations from the 2002 American College of Gastroenterology (ACG) Practice Guideline provide guidance as to the frequency of endoscopy surveillance but were not based on an explicit systematic review of the literature (SOR: C, based on expert opinion; see TABLE 1).
Reduced monitoring for most patients with Barrett’s esophagus appears safe
Paul Crawford, MD
USAF–Eglin Family Practice Residency, Eglin Air Force Base, Fla
Family physicians have long been at the mercy of expert opinion when considering how to monitor patients with Barrett’s esophagus. This review of the evidence clearly shows that the days of yearly EGD for all Barrett’s esophagus patients are over.
Unlike other conditions—such as cervical dysplasia, where monitoring and therapies to remove dysplasia are proven to save lives—Barrett’s esophagus progresses slowly and unpredictably. Thus, until technological advances allow identification of higher risk Barrett’s esophagus patients, an EGD every 3 years for those without dysplasia seems to be a reasonable monitoring interval. Perhaps most importantly, family physicians can reassure Barrett’s esophagus patients in the community that they are likely to live a normal lifespan and die of something other than esophageal cancer.
Evidence summary
Barrett’s esophagus has been defined as “a change in the esophageal epithelium of any length that can be recognized at endoscopy and is confirmed to have intestinal metaplasia by biopsy of the tubular esophagus.”1 Intestinal metaplasia is a premalignant lesion for adenocarcinoma of the esophagus. Surveillance by serial endoscopy with biopsy has been recommended in an effort to find high-grade dysplasia or carcinoma in an early, asymptomatic, and potentially curable stage.1-4 Approximately 75% of patients involved in a Barrett’s esophagus surveillance program will present with resectable tumors, compared with only 25% of those not receiving surveillance.4
A recent systematic review assessing screening tools for esophageal carcinoma found standard endoscopy to be superior (90%–100% sensitivity) to other less invasive methods such as questionnaire (60%–70%), and fecal occult blood testing (20%).4 Additional endoscopy tools such as brush and balloon cytology increased the cost of surveillance without any improvement in diagnostic yield.
The degree of dysplasia on esophageal biopsy in Barrett’s esophagus patients is currently the best indicator of risk of progression to esophageal carcinoma. The data reviewed by the ACG for the practice guideline was drawn from several prospective studies and one available registry. In sum, a total of 783 Barrett’s esophagus patients were followed for a mean of 2.9 to 7.3 years. Esophageal carcinoma developed in 2% of patients with no dysplasia, 7% of patients with low-grade dysplasia (LGD) and 22% of patients with high-grade dysplasia (HGD).1 The ACG recommendations regarding frequency of esophagogastroduodenoscopy (EGD) were not based on an explicit critical appraisal of the literature. Recent cohort studies are consistent with recommendations for graded surveillance frequency. A randomized clinical trial to determine optimal endoscopic frequency and benefit has not been reported.
Several concerns have been raised regarding the utility of degree of dysplasia in determining optimal frequency of endoscopic surveillance. First, the progression of esophageal lesions over time is unpredictable. Skacel et al5 reported a series of 34 patients with LGD at initial pathologic examination. On subsequent surveillance endoscopy with repeat biopsy, 73% no longer demonstrated dysplasia. Such patients can be allowed to return to having surveillance every 3 years.
In addition to the non-linear progression of dysplasia, inter-rater reliability of the interpretation of pathology specimens varies substantially. Adequate reliability has been demonstrated among pathologists assigning results to 2 categories (either no dysplasia and LGD or HGD and carcinoma) (κ=0.7). Assignment to four distinct pathologic grades, however, was not reliable (κ=0.46, where 1.0 is complete agreement).1 In order to make a diagnosis of HGD or carcinoma, interpretation must be independently confirmed by 2 expert pathologists.1-3
Recommendations for frequent endoscopic surveillance are also weakened by the overall low rate of mortality from esophageal carcinoma noted in Barrett’s esophagus patients. A recent population based study demonstrated that there was no difference in overall mortality in those with a Barrett’s esophagus diagnosis compared with the general population.6 An increased risk of death from esophageal carcinoma was seen in patients with Barrett’s esophagus (4.7% seen in Barrett’s esophagus patients compared with 0.8% predicted in the general population; P<.05). The overall increased effect on mortality, however, was relatively small. Esophageal carcinoma accounted for less then 5% of deaths in Barrett’s esophagus patients reported during the study’s 6-year follow-up period.
Data from prospective studies published after 2002 may better predict prognosis for Barrett’s esophagus patients.6-9 Even lower rates of progression to esophageal carcinoma (<0.5% a year or <1/220 patient-years) have been reported in these studies drawing from the general population rather than referred patients, likely stemming from differences in gender mix, patient age, and risk factors.
In addition to grade of dysplasia, the length of the dysplastic Barrett’s esophagus segment is emerging as a potentially predictive risk factor. While the ACG cautions that esophageal cancer has been reported in patients with so-called “short segment” Barrett’s esophagus (SSBE) (≤3 cm),1 recent prospective studies have shown an increased risk of carcinoma development with long segment Barrett’s esophagus (LSBE).7-9 Weston et al7 reported a 2.4% progression rate to HGD or esophageal carcinoma with SSBE and no dysplasia compared with 6.8% with LSBE (P=.002). If patients had LGD, the rate of progression to esophageal carcinoma with SSBE was 5.3% and jumped to 35% in patients with LSBE (P<.001). Conio et al8 reported that 4 of 5 cases of esophageal carcinoma noted through surveillance had LSBE. Hage et al9 reported a significantly increased risk of progression to HGD or esophageal carcinoma with long segment disease (P<.02).
While currently still considered investigational, DNA content flow cytometry may be a future tool used in risk stratification. Reid et al10 report a 5-year cumulative risk of esophageal carcinoma of 1.7% in Barrett’s esophagus patients with negative, low-grade or indefinite grades of dysplasia. Subsequent application of flow cytometry allowed for further stratification of these low-risk patients. Those with neither aneuploidy nor an increased 4N had a 5-year cumulative risk of cancer of 0% while the risk for those with abnormalities on cytometry increased to 28% (relative risk=19; P<.001).
TABLE
Grade of dysplasia and recommendations for Barrett’s esophagus surveillance as proposed by the ACG
| DYSPLASIA | DOCUMENTATION | FOLLOW-UP ENDOSCOPY |
|---|---|---|
| None | Two EGDs with biopsy | 3 years |
| Low-grade | Highest grade on repeat | 1 year until no dysplasia |
| High-grade | Repeat EGD with biopsy to rule out cancer/document high-grade dysplasia; expert pathologist confirmation | Focal: every 3 months |
| Multifocal: intervention | ||
| Mucosal irregularity: EMR | ||
| ACG, American College of Gastroenterology; EGD, esophagogastroduodenoscopy; EMR, endoscopic mucosal resection. Intervention: ie, esophagectomy. Ablative therapies only in the setting of a clinical trial or for those unable to tolerate surgery. | ||
Recommendations from others
The French Society of Digestive Endoscopy has published guidelines on monitoring Barrett’s esophagus.3 Their recommendations differ only slightly from the ACG in advocating a slightly increased frequency of EGD surveillance based on degree of dysplasia, and utilizing the length of the dysplastic segment in decision-making. Neither the American Academy of Family Physicians nor the US Preventive Services Task Force make any specific recommendations about Barrett’s esophagus surveillance.
Some patients who have been diagnosed with Barrett’s esophagus will develop dysplasia and, in some cases, esophageal carcinoma (strength of recommendation [SOR]: A, based on consistent cohort studies). Endoscopic surveillance is recommended for all patients with Barrett’s esophagus as it is superior to other methods for detecting esophageal cancer (SOR: B, based on systematic review). The degree of dysplasia noted on biopsy specimens correlates with the risk of esophageal carcinoma development and should guide the frequency of subsequent evaluations (SOR: B, based on consistent cohort studies). The optimal frequency of endoscopy has yet to be determined in any randomized trial.
Recommendations from the 2002 American College of Gastroenterology (ACG) Practice Guideline provide guidance as to the frequency of endoscopy surveillance but were not based on an explicit systematic review of the literature (SOR: C, based on expert opinion; see TABLE 1).
Reduced monitoring for most patients with Barrett’s esophagus appears safe
Paul Crawford, MD
USAF–Eglin Family Practice Residency, Eglin Air Force Base, Fla
Family physicians have long been at the mercy of expert opinion when considering how to monitor patients with Barrett’s esophagus. This review of the evidence clearly shows that the days of yearly EGD for all Barrett’s esophagus patients are over.
Unlike other conditions—such as cervical dysplasia, where monitoring and therapies to remove dysplasia are proven to save lives—Barrett’s esophagus progresses slowly and unpredictably. Thus, until technological advances allow identification of higher risk Barrett’s esophagus patients, an EGD every 3 years for those without dysplasia seems to be a reasonable monitoring interval. Perhaps most importantly, family physicians can reassure Barrett’s esophagus patients in the community that they are likely to live a normal lifespan and die of something other than esophageal cancer.
Evidence summary
Barrett’s esophagus has been defined as “a change in the esophageal epithelium of any length that can be recognized at endoscopy and is confirmed to have intestinal metaplasia by biopsy of the tubular esophagus.”1 Intestinal metaplasia is a premalignant lesion for adenocarcinoma of the esophagus. Surveillance by serial endoscopy with biopsy has been recommended in an effort to find high-grade dysplasia or carcinoma in an early, asymptomatic, and potentially curable stage.1-4 Approximately 75% of patients involved in a Barrett’s esophagus surveillance program will present with resectable tumors, compared with only 25% of those not receiving surveillance.4
A recent systematic review assessing screening tools for esophageal carcinoma found standard endoscopy to be superior (90%–100% sensitivity) to other less invasive methods such as questionnaire (60%–70%), and fecal occult blood testing (20%).4 Additional endoscopy tools such as brush and balloon cytology increased the cost of surveillance without any improvement in diagnostic yield.
The degree of dysplasia on esophageal biopsy in Barrett’s esophagus patients is currently the best indicator of risk of progression to esophageal carcinoma. The data reviewed by the ACG for the practice guideline was drawn from several prospective studies and one available registry. In sum, a total of 783 Barrett’s esophagus patients were followed for a mean of 2.9 to 7.3 years. Esophageal carcinoma developed in 2% of patients with no dysplasia, 7% of patients with low-grade dysplasia (LGD) and 22% of patients with high-grade dysplasia (HGD).1 The ACG recommendations regarding frequency of esophagogastroduodenoscopy (EGD) were not based on an explicit critical appraisal of the literature. Recent cohort studies are consistent with recommendations for graded surveillance frequency. A randomized clinical trial to determine optimal endoscopic frequency and benefit has not been reported.
Several concerns have been raised regarding the utility of degree of dysplasia in determining optimal frequency of endoscopic surveillance. First, the progression of esophageal lesions over time is unpredictable. Skacel et al5 reported a series of 34 patients with LGD at initial pathologic examination. On subsequent surveillance endoscopy with repeat biopsy, 73% no longer demonstrated dysplasia. Such patients can be allowed to return to having surveillance every 3 years.
In addition to the non-linear progression of dysplasia, inter-rater reliability of the interpretation of pathology specimens varies substantially. Adequate reliability has been demonstrated among pathologists assigning results to 2 categories (either no dysplasia and LGD or HGD and carcinoma) (κ=0.7). Assignment to four distinct pathologic grades, however, was not reliable (κ=0.46, where 1.0 is complete agreement).1 In order to make a diagnosis of HGD or carcinoma, interpretation must be independently confirmed by 2 expert pathologists.1-3
Recommendations for frequent endoscopic surveillance are also weakened by the overall low rate of mortality from esophageal carcinoma noted in Barrett’s esophagus patients. A recent population based study demonstrated that there was no difference in overall mortality in those with a Barrett’s esophagus diagnosis compared with the general population.6 An increased risk of death from esophageal carcinoma was seen in patients with Barrett’s esophagus (4.7% seen in Barrett’s esophagus patients compared with 0.8% predicted in the general population; P<.05). The overall increased effect on mortality, however, was relatively small. Esophageal carcinoma accounted for less then 5% of deaths in Barrett’s esophagus patients reported during the study’s 6-year follow-up period.
Data from prospective studies published after 2002 may better predict prognosis for Barrett’s esophagus patients.6-9 Even lower rates of progression to esophageal carcinoma (<0.5% a year or <1/220 patient-years) have been reported in these studies drawing from the general population rather than referred patients, likely stemming from differences in gender mix, patient age, and risk factors.
In addition to grade of dysplasia, the length of the dysplastic Barrett’s esophagus segment is emerging as a potentially predictive risk factor. While the ACG cautions that esophageal cancer has been reported in patients with so-called “short segment” Barrett’s esophagus (SSBE) (≤3 cm),1 recent prospective studies have shown an increased risk of carcinoma development with long segment Barrett’s esophagus (LSBE).7-9 Weston et al7 reported a 2.4% progression rate to HGD or esophageal carcinoma with SSBE and no dysplasia compared with 6.8% with LSBE (P=.002). If patients had LGD, the rate of progression to esophageal carcinoma with SSBE was 5.3% and jumped to 35% in patients with LSBE (P<.001). Conio et al8 reported that 4 of 5 cases of esophageal carcinoma noted through surveillance had LSBE. Hage et al9 reported a significantly increased risk of progression to HGD or esophageal carcinoma with long segment disease (P<.02).
While currently still considered investigational, DNA content flow cytometry may be a future tool used in risk stratification. Reid et al10 report a 5-year cumulative risk of esophageal carcinoma of 1.7% in Barrett’s esophagus patients with negative, low-grade or indefinite grades of dysplasia. Subsequent application of flow cytometry allowed for further stratification of these low-risk patients. Those with neither aneuploidy nor an increased 4N had a 5-year cumulative risk of cancer of 0% while the risk for those with abnormalities on cytometry increased to 28% (relative risk=19; P<.001).
TABLE
Grade of dysplasia and recommendations for Barrett’s esophagus surveillance as proposed by the ACG
| DYSPLASIA | DOCUMENTATION | FOLLOW-UP ENDOSCOPY |
|---|---|---|
| None | Two EGDs with biopsy | 3 years |
| Low-grade | Highest grade on repeat | 1 year until no dysplasia |
| High-grade | Repeat EGD with biopsy to rule out cancer/document high-grade dysplasia; expert pathologist confirmation | Focal: every 3 months |
| Multifocal: intervention | ||
| Mucosal irregularity: EMR | ||
| ACG, American College of Gastroenterology; EGD, esophagogastroduodenoscopy; EMR, endoscopic mucosal resection. Intervention: ie, esophagectomy. Ablative therapies only in the setting of a clinical trial or for those unable to tolerate surgery. | ||
Recommendations from others
The French Society of Digestive Endoscopy has published guidelines on monitoring Barrett’s esophagus.3 Their recommendations differ only slightly from the ACG in advocating a slightly increased frequency of EGD surveillance based on degree of dysplasia, and utilizing the length of the dysplastic segment in decision-making. Neither the American Academy of Family Physicians nor the US Preventive Services Task Force make any specific recommendations about Barrett’s esophagus surveillance.
1. Sampliner RE. Updated guidelines for the diagnosis, surveillance, and therapy of Barrett’s esophagus. Am J Gastroenterol 2002;97:1888-1895.
2. Management of Barrett’s esophagus. The Society for Surgery of the Alimentary Tract (SSAT), American Gastroenterological Association (AGA), American Society for Gastrointestinal Endoscopy (ASGE) Consensus Panel. J Gastrointest Surg 2000;4:115-116.
3. Boyer J, Robaszkiewicz M. Guidelines of the French Society of Digestive Endoscopy: Monitoring of Barrett’s esophagus. The Council of the French Society of Digestive Endoscopy. Endoscopy 2000;32:498-499.
4. Gerson LB, Triadafilopoulos G. Screening for esophageal adenocarcinoma: an evidence-based approach. Am J Med 2002;113:499-505.
5. Skacel M, Petras RE, Gramlich TL, et al. The diagnosis of low grade dysplasia in Barrett’s esophagus and its implications for disease progression. Am J Gastroenterol 2000;95:3383-3387.
6. Anderson LA, Murray LJ, Murphy SJ, et al. Mortality in Barrett’s oesophagus: results from a population based study. Gut 2003;52:1081-1084.
7. Weston AP, Sharma P, Mathur S, et al. Risk stratification of barrett’s esophagus: updated prospective multivariate analysis. Am J Gastroenterol 2004;99:1657-1666.
8. Conio M, Blanchi S, Laertosa G, et al. Long-term endoscopic surveillance of patients with Barrett’s esophagus. Incidence of dysplasia and adenocarcinoma: a prospective study. Am J Gastroenterol 2003;98:1931-1939.
9. Hage M, Siersema PD, van Dekken H, Steyerber EW, Dees J, Kuipers EJ. Oesophageal cancer incidence and mortality in patients with long-segment Barrett’s oesophagus after a mean follow-up of 12.7 years. Scand J Gastroenterol 2004;39:1175-1179.
10. Reid BJ, Levine DS, Longton G, Blount P, Rabinovitch PS. Predictors of progression to cancer in Barrett’s esophagus: baseline histology and flow cytometry identify low- and high-risk patient subsets. Am J Gastroenterol 2000;95:1669-1676.
1. Sampliner RE. Updated guidelines for the diagnosis, surveillance, and therapy of Barrett’s esophagus. Am J Gastroenterol 2002;97:1888-1895.
2. Management of Barrett’s esophagus. The Society for Surgery of the Alimentary Tract (SSAT), American Gastroenterological Association (AGA), American Society for Gastrointestinal Endoscopy (ASGE) Consensus Panel. J Gastrointest Surg 2000;4:115-116.
3. Boyer J, Robaszkiewicz M. Guidelines of the French Society of Digestive Endoscopy: Monitoring of Barrett’s esophagus. The Council of the French Society of Digestive Endoscopy. Endoscopy 2000;32:498-499.
4. Gerson LB, Triadafilopoulos G. Screening for esophageal adenocarcinoma: an evidence-based approach. Am J Med 2002;113:499-505.
5. Skacel M, Petras RE, Gramlich TL, et al. The diagnosis of low grade dysplasia in Barrett’s esophagus and its implications for disease progression. Am J Gastroenterol 2000;95:3383-3387.
6. Anderson LA, Murray LJ, Murphy SJ, et al. Mortality in Barrett’s oesophagus: results from a population based study. Gut 2003;52:1081-1084.
7. Weston AP, Sharma P, Mathur S, et al. Risk stratification of barrett’s esophagus: updated prospective multivariate analysis. Am J Gastroenterol 2004;99:1657-1666.
8. Conio M, Blanchi S, Laertosa G, et al. Long-term endoscopic surveillance of patients with Barrett’s esophagus. Incidence of dysplasia and adenocarcinoma: a prospective study. Am J Gastroenterol 2003;98:1931-1939.
9. Hage M, Siersema PD, van Dekken H, Steyerber EW, Dees J, Kuipers EJ. Oesophageal cancer incidence and mortality in patients with long-segment Barrett’s oesophagus after a mean follow-up of 12.7 years. Scand J Gastroenterol 2004;39:1175-1179.
10. Reid BJ, Levine DS, Longton G, Blount P, Rabinovitch PS. Predictors of progression to cancer in Barrett’s esophagus: baseline histology and flow cytometry identify low- and high-risk patient subsets. Am J Gastroenterol 2000;95:1669-1676.
Evidence-based answers from the Family Physicians Inquiries Network