User login
Advisory Committee on Immunization Practices (ACIP)
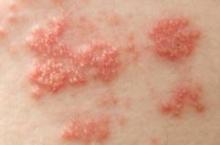
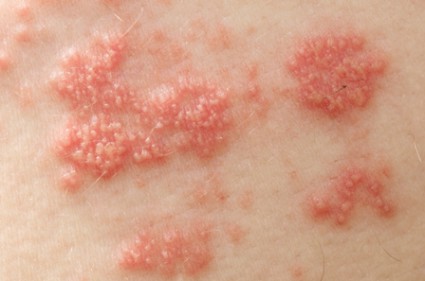
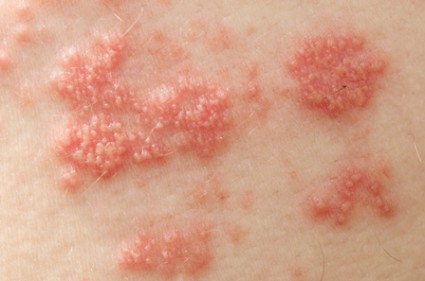
Shingles Vaccine Most Effective When Given to Patients in Their 60s
Because efficacy of the shingles vaccine appears to wane over time, it should not routinely be given to people in their 50s, according to information presented at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
"At this time, there is insufficient evidence supporting long-term protection of the vaccine, so if people younger than 60 [years] are vaccinated, they might not be protected when chance of disease is highest," said Dr. Jeff Duchin, chair of the ACIP’s herpes zoster working group. "We will continue to monitor data as they becomes available, but at this point we are reaffirming that vaccination be done in those age 60 [years] and older."
The committee made its recommendation after reviewing newly released data from Merck’s Long-Term Persistence Study for shingles. It found that, 10 years after vaccination, the overall incidence rate of shingles had increased from about 4/1,000 person-years to 11/1,000 person-years. The observed incidence rate of shingles in historical controls is about 13 cases/1,000 person-years.
Dr. Janie Parrino, director of vaccine clinical research at Merck Research Laboratories, presented results of the company’s latest study of the vaccine’s extended efficacy. The shingles Long-Term Persistence Study is an 8-year follow-up of the Shingles Prevention Study, in which subjects were randomized to Merck’s Zostavax vaccine or placebo. It showed that the vaccine significantly reduced the incidence of herpes zoster and postherpetic neuralgia (PHN) as well as the shingles burden of illness, in adults older than 60 years.
A short-term follow-up study appeared last year; it added 4 more years of follow-up data on more than 14,000 of the original cohort.
It found a gradual decrease in vaccine efficacy by 6-7 years after vaccination. "Analysis of vaccine efficacy in each year after vaccination for all outcomes showed a decrease after year 1, with a further decline thereafter," the investigators said. "Vaccine efficacy was statistically significant for the incidence of herpes zoster and herpes zoster burden of illness through year 5 after vaccination, but vaccine efficacy is uncertain beyond that point."
The long-term study presented at the Atlanta meeting involved up to 12 years of data on almost 7,000 people in the original cohort. It demonstrated a continuous time-bound waning of efficacy.
In addition to the increasing annual incidence of shingles, the study found an increasing incidence of PHN, from less than 1/1,000 person-years during the year of vaccination to about 1/1,000 person years by year 10. However, the incidence among placebo subjects and historical controls was more than 2/1,000 person years by that time. The shingles burden of illness (a measure of acute pain) also increased as time passed, Dr. Parrino noted.
Taking into account both clinical efficacy and cost, Ismael Ortega-Sanchez, Ph.D., a health economist with the CDC, concluded that the shingles vaccine is most effective when given to people in their 60s and 70s.
Vaccinating from age 50-59 years would prevent about 20,000 shingles cases annually. But vaccinating in the 60s would prevent 26,000 and, in the 70s, 21,000. The trend was similar for cases of PHN: prevention of 1,000 cases for vaccinating in the 50s, 4,000 for vaccinating in the 60s, and 8,000 for vaccinating in the 70s.
Other outcomes – emergency visits, ambulatory visits, hospitalizations, length of hospital stay, and prescriptions – also increased significantly over the 3 decades.
Savings tracked clinical outcomes in a value assessment model of 6 million people: 1 million vaccinated and unvaccinated in each of the three age cohorts.
Compared with not vaccinating, vaccinating those in their 50s would save $16.8 million annually. Vaccinating during the 60s would save about $24.3 million, and during the 70s, $31.9 million.
And, because younger people are likely to have more systemic reactions, they cost more to vaccinate. A model that accounted for the cost of the vaccine, administration, and treating local and systemic reactions, put the total price tag at $193 million for those in their 50s, and $190 million for each of the two older groups.
Finally, the number needed to treat to prevent one case of shingles, PHN, or non-PHN complication case was higher in those in their 50s than in those in their 60s or 70s (one shingles case – 51, 38, and 47 respectively; one PHN case – 988, 247, and 124).
"This isn’t intended to tell people not to get the vaccine early, but to make them aware of the risks and benefits, given that the greatest risk of the disease is later in life," Dr. Duchin said. Since there are no data on booster shingles vaccines, he said "we only have one shot at this and we need to focus our efforts on those at the greatest risk."
Because efficacy of the shingles vaccine appears to wane over time, it should not routinely be given to people in their 50s, according to information presented at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
"At this time, there is insufficient evidence supporting long-term protection of the vaccine, so if people younger than 60 [years] are vaccinated, they might not be protected when chance of disease is highest," said Dr. Jeff Duchin, chair of the ACIP’s herpes zoster working group. "We will continue to monitor data as they becomes available, but at this point we are reaffirming that vaccination be done in those age 60 [years] and older."
The committee made its recommendation after reviewing newly released data from Merck’s Long-Term Persistence Study for shingles. It found that, 10 years after vaccination, the overall incidence rate of shingles had increased from about 4/1,000 person-years to 11/1,000 person-years. The observed incidence rate of shingles in historical controls is about 13 cases/1,000 person-years.
Dr. Janie Parrino, director of vaccine clinical research at Merck Research Laboratories, presented results of the company’s latest study of the vaccine’s extended efficacy. The shingles Long-Term Persistence Study is an 8-year follow-up of the Shingles Prevention Study, in which subjects were randomized to Merck’s Zostavax vaccine or placebo. It showed that the vaccine significantly reduced the incidence of herpes zoster and postherpetic neuralgia (PHN) as well as the shingles burden of illness, in adults older than 60 years.
A short-term follow-up study appeared last year; it added 4 more years of follow-up data on more than 14,000 of the original cohort.
It found a gradual decrease in vaccine efficacy by 6-7 years after vaccination. "Analysis of vaccine efficacy in each year after vaccination for all outcomes showed a decrease after year 1, with a further decline thereafter," the investigators said. "Vaccine efficacy was statistically significant for the incidence of herpes zoster and herpes zoster burden of illness through year 5 after vaccination, but vaccine efficacy is uncertain beyond that point."
The long-term study presented at the Atlanta meeting involved up to 12 years of data on almost 7,000 people in the original cohort. It demonstrated a continuous time-bound waning of efficacy.
In addition to the increasing annual incidence of shingles, the study found an increasing incidence of PHN, from less than 1/1,000 person-years during the year of vaccination to about 1/1,000 person years by year 10. However, the incidence among placebo subjects and historical controls was more than 2/1,000 person years by that time. The shingles burden of illness (a measure of acute pain) also increased as time passed, Dr. Parrino noted.
Taking into account both clinical efficacy and cost, Ismael Ortega-Sanchez, Ph.D., a health economist with the CDC, concluded that the shingles vaccine is most effective when given to people in their 60s and 70s.
Vaccinating from age 50-59 years would prevent about 20,000 shingles cases annually. But vaccinating in the 60s would prevent 26,000 and, in the 70s, 21,000. The trend was similar for cases of PHN: prevention of 1,000 cases for vaccinating in the 50s, 4,000 for vaccinating in the 60s, and 8,000 for vaccinating in the 70s.
Other outcomes – emergency visits, ambulatory visits, hospitalizations, length of hospital stay, and prescriptions – also increased significantly over the 3 decades.
Savings tracked clinical outcomes in a value assessment model of 6 million people: 1 million vaccinated and unvaccinated in each of the three age cohorts.
Compared with not vaccinating, vaccinating those in their 50s would save $16.8 million annually. Vaccinating during the 60s would save about $24.3 million, and during the 70s, $31.9 million.
And, because younger people are likely to have more systemic reactions, they cost more to vaccinate. A model that accounted for the cost of the vaccine, administration, and treating local and systemic reactions, put the total price tag at $193 million for those in their 50s, and $190 million for each of the two older groups.
Finally, the number needed to treat to prevent one case of shingles, PHN, or non-PHN complication case was higher in those in their 50s than in those in their 60s or 70s (one shingles case – 51, 38, and 47 respectively; one PHN case – 988, 247, and 124).
"This isn’t intended to tell people not to get the vaccine early, but to make them aware of the risks and benefits, given that the greatest risk of the disease is later in life," Dr. Duchin said. Since there are no data on booster shingles vaccines, he said "we only have one shot at this and we need to focus our efforts on those at the greatest risk."
Because efficacy of the shingles vaccine appears to wane over time, it should not routinely be given to people in their 50s, according to information presented at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
"At this time, there is insufficient evidence supporting long-term protection of the vaccine, so if people younger than 60 [years] are vaccinated, they might not be protected when chance of disease is highest," said Dr. Jeff Duchin, chair of the ACIP’s herpes zoster working group. "We will continue to monitor data as they becomes available, but at this point we are reaffirming that vaccination be done in those age 60 [years] and older."
The committee made its recommendation after reviewing newly released data from Merck’s Long-Term Persistence Study for shingles. It found that, 10 years after vaccination, the overall incidence rate of shingles had increased from about 4/1,000 person-years to 11/1,000 person-years. The observed incidence rate of shingles in historical controls is about 13 cases/1,000 person-years.
Dr. Janie Parrino, director of vaccine clinical research at Merck Research Laboratories, presented results of the company’s latest study of the vaccine’s extended efficacy. The shingles Long-Term Persistence Study is an 8-year follow-up of the Shingles Prevention Study, in which subjects were randomized to Merck’s Zostavax vaccine or placebo. It showed that the vaccine significantly reduced the incidence of herpes zoster and postherpetic neuralgia (PHN) as well as the shingles burden of illness, in adults older than 60 years.
A short-term follow-up study appeared last year; it added 4 more years of follow-up data on more than 14,000 of the original cohort.
It found a gradual decrease in vaccine efficacy by 6-7 years after vaccination. "Analysis of vaccine efficacy in each year after vaccination for all outcomes showed a decrease after year 1, with a further decline thereafter," the investigators said. "Vaccine efficacy was statistically significant for the incidence of herpes zoster and herpes zoster burden of illness through year 5 after vaccination, but vaccine efficacy is uncertain beyond that point."
The long-term study presented at the Atlanta meeting involved up to 12 years of data on almost 7,000 people in the original cohort. It demonstrated a continuous time-bound waning of efficacy.
In addition to the increasing annual incidence of shingles, the study found an increasing incidence of PHN, from less than 1/1,000 person-years during the year of vaccination to about 1/1,000 person years by year 10. However, the incidence among placebo subjects and historical controls was more than 2/1,000 person years by that time. The shingles burden of illness (a measure of acute pain) also increased as time passed, Dr. Parrino noted.
Taking into account both clinical efficacy and cost, Ismael Ortega-Sanchez, Ph.D., a health economist with the CDC, concluded that the shingles vaccine is most effective when given to people in their 60s and 70s.
Vaccinating from age 50-59 years would prevent about 20,000 shingles cases annually. But vaccinating in the 60s would prevent 26,000 and, in the 70s, 21,000. The trend was similar for cases of PHN: prevention of 1,000 cases for vaccinating in the 50s, 4,000 for vaccinating in the 60s, and 8,000 for vaccinating in the 70s.
Other outcomes – emergency visits, ambulatory visits, hospitalizations, length of hospital stay, and prescriptions – also increased significantly over the 3 decades.
Savings tracked clinical outcomes in a value assessment model of 6 million people: 1 million vaccinated and unvaccinated in each of the three age cohorts.
Compared with not vaccinating, vaccinating those in their 50s would save $16.8 million annually. Vaccinating during the 60s would save about $24.3 million, and during the 70s, $31.9 million.
And, because younger people are likely to have more systemic reactions, they cost more to vaccinate. A model that accounted for the cost of the vaccine, administration, and treating local and systemic reactions, put the total price tag at $193 million for those in their 50s, and $190 million for each of the two older groups.
Finally, the number needed to treat to prevent one case of shingles, PHN, or non-PHN complication case was higher in those in their 50s than in those in their 60s or 70s (one shingles case – 51, 38, and 47 respectively; one PHN case – 988, 247, and 124).
"This isn’t intended to tell people not to get the vaccine early, but to make them aware of the risks and benefits, given that the greatest risk of the disease is later in life," Dr. Duchin said. Since there are no data on booster shingles vaccines, he said "we only have one shot at this and we need to focus our efforts on those at the greatest risk."
FROM ACIP
Shingles vaccine most effective and cost effective when given to patients in their 60s
Because efficacy of the shingles vaccine appears to wane over time, it should not routinely be given to people in their 50s, according to information presented at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
"At this time, there is insufficient evidence supporting long-term protection of the vaccine, so if people younger than 60 [years] are vaccinated, they might not be protected when chance of disease is highest," said Dr. Jeff Duchin, chair of the ACIP’s herpes zoster working group. "We will continue to monitor data as they becomes available, but at this point we are reaffirming that vaccination be done in those age 60 [years] and older."
The committee made its recommendation after reviewing newly released data from Merck’s Long-Term Persistence Study for shingles. It found that, 10 years after vaccination, the overall incidence rate of shingles had increased from about 4/1,000 person-years to 11/1,000 person-years. The observed incidence rate of shingles in historical controls is about 13 cases/1,000 person-years.
Dr. Janie Parrino, director of vaccine clinical research at Merck Research Laboratories, presented results of the company’s latest study of the vaccine’s extended efficacy. The shingles Long-Term Persistence Study is an 8-year follow-up of the Shingles Prevention Study, in which subjects were randomized to Merck’s Zostavax vaccine or placebo. It showed that the vaccine significantly reduced the incidence of herpes zoster and postherpetic neuralgia (PHN) as well as the shingles burden of illness, in adults older than 60 years.
A short-term follow-up study appeared last year; it added 4 more years of follow-up data on more than 14,000 of the original cohort.
It found a gradual decrease in vaccine efficacy by 6-7 years after vaccination. "Analysis of vaccine efficacy in each year after vaccination for all outcomes showed a decrease after year 1, with a further decline thereafter," the investigators said. "Vaccine efficacy was statistically significant for the incidence of herpes zoster and herpes zoster burden of illness through year 5 after vaccination, but vaccine efficacy is uncertain beyond that point."
The long-term study presented at the Atlanta meeting involved up to 12 years of data on almost 7,000 people in the original cohort. It demonstrated a continuous time-bound waning of efficacy.
In addition to the increasing annual incidence of shingles, the study found an increasing incidence of PHN, from less than 1/1,000 person-years during the year of vaccination to about 1/1,000 person years by year 10. However, the incidence among placebo subjects and historical controls was more than 2/1,000 person years by that time. The shingles burden of illness (a measure of acute pain) also increased as time passed, Dr. Parrino noted.
Taking into account both clinical efficacy and cost, Ismael Ortega-Sanchez, Ph.D., a health economist with the CDC, concluded that the shingles vaccine is most effective when given to people in their 60s and 70s.
Vaccinating from age 50-59 years would prevent about 20,000 shingles cases annually. But vaccinating in the 60s would prevent 26,000 and, in the 70s, 21,000. The trend was similar for cases of PHN: prevention of 1,000 cases for vaccinating in the 50s, 4,000 for vaccinating in the 60s, and 8,000 for vaccinating in the 70s.
Other outcomes – emergency visits, ambulatory visits, hospitalizations, length of hospital stay, and prescriptions – also increased significantly over the 3 decades.
Savings tracked clinical outcomes in a value assessment model of 6 million people: 1 million vaccinated and unvaccinated in each of the three age cohorts.
Compared with not vaccinating, vaccinating those in their 50s would save $16.8 million annually. Vaccinating during the 60s would save about $24.3 million, and during the 70s, $31.9 million.
And, because younger people are likely to have more systemic reactions, they cost more to vaccinate. A model that accounted for the cost of the vaccine, administration, and treating local and systemic reactions, put the total price tag at $193 million for those in their 50s, and $190 million for each of the two older groups.
Finally, the number needed to treat to prevent one case of shingles, PHN, or non-PHN complication case was higher in those in their 50s than in those in their 60s or 70s (one shingles case – 51, 38, and 47 respectively; one PHN case – 988, 247, and 124).
"This isn’t intended to tell people not to get the vaccine early, but to make them aware of the risks and benefits, given that the greatest risk of the disease is later in life," Dr. Duchin said. Since there are no data on booster shingles vaccines, he said "we only have one shot at this and we need to focus our efforts on those at the greatest risk."
Because efficacy of the shingles vaccine appears to wane over time, it should not routinely be given to people in their 50s, according to information presented at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
"At this time, there is insufficient evidence supporting long-term protection of the vaccine, so if people younger than 60 [years] are vaccinated, they might not be protected when chance of disease is highest," said Dr. Jeff Duchin, chair of the ACIP’s herpes zoster working group. "We will continue to monitor data as they becomes available, but at this point we are reaffirming that vaccination be done in those age 60 [years] and older."
The committee made its recommendation after reviewing newly released data from Merck’s Long-Term Persistence Study for shingles. It found that, 10 years after vaccination, the overall incidence rate of shingles had increased from about 4/1,000 person-years to 11/1,000 person-years. The observed incidence rate of shingles in historical controls is about 13 cases/1,000 person-years.
Dr. Janie Parrino, director of vaccine clinical research at Merck Research Laboratories, presented results of the company’s latest study of the vaccine’s extended efficacy. The shingles Long-Term Persistence Study is an 8-year follow-up of the Shingles Prevention Study, in which subjects were randomized to Merck’s Zostavax vaccine or placebo. It showed that the vaccine significantly reduced the incidence of herpes zoster and postherpetic neuralgia (PHN) as well as the shingles burden of illness, in adults older than 60 years.
A short-term follow-up study appeared last year; it added 4 more years of follow-up data on more than 14,000 of the original cohort.
It found a gradual decrease in vaccine efficacy by 6-7 years after vaccination. "Analysis of vaccine efficacy in each year after vaccination for all outcomes showed a decrease after year 1, with a further decline thereafter," the investigators said. "Vaccine efficacy was statistically significant for the incidence of herpes zoster and herpes zoster burden of illness through year 5 after vaccination, but vaccine efficacy is uncertain beyond that point."
The long-term study presented at the Atlanta meeting involved up to 12 years of data on almost 7,000 people in the original cohort. It demonstrated a continuous time-bound waning of efficacy.
In addition to the increasing annual incidence of shingles, the study found an increasing incidence of PHN, from less than 1/1,000 person-years during the year of vaccination to about 1/1,000 person years by year 10. However, the incidence among placebo subjects and historical controls was more than 2/1,000 person years by that time. The shingles burden of illness (a measure of acute pain) also increased as time passed, Dr. Parrino noted.
Taking into account both clinical efficacy and cost, Ismael Ortega-Sanchez, Ph.D., a health economist with the CDC, concluded that the shingles vaccine is most effective when given to people in their 60s and 70s.
Vaccinating from age 50-59 years would prevent about 20,000 shingles cases annually. But vaccinating in the 60s would prevent 26,000 and, in the 70s, 21,000. The trend was similar for cases of PHN: prevention of 1,000 cases for vaccinating in the 50s, 4,000 for vaccinating in the 60s, and 8,000 for vaccinating in the 70s.
Other outcomes – emergency visits, ambulatory visits, hospitalizations, length of hospital stay, and prescriptions – also increased significantly over the 3 decades.
Savings tracked clinical outcomes in a value assessment model of 6 million people: 1 million vaccinated and unvaccinated in each of the three age cohorts.
Compared with not vaccinating, vaccinating those in their 50s would save $16.8 million annually. Vaccinating during the 60s would save about $24.3 million, and during the 70s, $31.9 million.
And, because younger people are likely to have more systemic reactions, they cost more to vaccinate. A model that accounted for the cost of the vaccine, administration, and treating local and systemic reactions, put the total price tag at $193 million for those in their 50s, and $190 million for each of the two older groups.
Finally, the number needed to treat to prevent one case of shingles, PHN, or non-PHN complication case was higher in those in their 50s than in those in their 60s or 70s (one shingles case – 51, 38, and 47 respectively; one PHN case – 988, 247, and 124).
"This isn’t intended to tell people not to get the vaccine early, but to make them aware of the risks and benefits, given that the greatest risk of the disease is later in life," Dr. Duchin said. Since there are no data on booster shingles vaccines, he said "we only have one shot at this and we need to focus our efforts on those at the greatest risk."
Because efficacy of the shingles vaccine appears to wane over time, it should not routinely be given to people in their 50s, according to information presented at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
"At this time, there is insufficient evidence supporting long-term protection of the vaccine, so if people younger than 60 [years] are vaccinated, they might not be protected when chance of disease is highest," said Dr. Jeff Duchin, chair of the ACIP’s herpes zoster working group. "We will continue to monitor data as they becomes available, but at this point we are reaffirming that vaccination be done in those age 60 [years] and older."
The committee made its recommendation after reviewing newly released data from Merck’s Long-Term Persistence Study for shingles. It found that, 10 years after vaccination, the overall incidence rate of shingles had increased from about 4/1,000 person-years to 11/1,000 person-years. The observed incidence rate of shingles in historical controls is about 13 cases/1,000 person-years.
Dr. Janie Parrino, director of vaccine clinical research at Merck Research Laboratories, presented results of the company’s latest study of the vaccine’s extended efficacy. The shingles Long-Term Persistence Study is an 8-year follow-up of the Shingles Prevention Study, in which subjects were randomized to Merck’s Zostavax vaccine or placebo. It showed that the vaccine significantly reduced the incidence of herpes zoster and postherpetic neuralgia (PHN) as well as the shingles burden of illness, in adults older than 60 years.
A short-term follow-up study appeared last year; it added 4 more years of follow-up data on more than 14,000 of the original cohort.
It found a gradual decrease in vaccine efficacy by 6-7 years after vaccination. "Analysis of vaccine efficacy in each year after vaccination for all outcomes showed a decrease after year 1, with a further decline thereafter," the investigators said. "Vaccine efficacy was statistically significant for the incidence of herpes zoster and herpes zoster burden of illness through year 5 after vaccination, but vaccine efficacy is uncertain beyond that point."
The long-term study presented at the Atlanta meeting involved up to 12 years of data on almost 7,000 people in the original cohort. It demonstrated a continuous time-bound waning of efficacy.
In addition to the increasing annual incidence of shingles, the study found an increasing incidence of PHN, from less than 1/1,000 person-years during the year of vaccination to about 1/1,000 person years by year 10. However, the incidence among placebo subjects and historical controls was more than 2/1,000 person years by that time. The shingles burden of illness (a measure of acute pain) also increased as time passed, Dr. Parrino noted.
Taking into account both clinical efficacy and cost, Ismael Ortega-Sanchez, Ph.D., a health economist with the CDC, concluded that the shingles vaccine is most effective when given to people in their 60s and 70s.
Vaccinating from age 50-59 years would prevent about 20,000 shingles cases annually. But vaccinating in the 60s would prevent 26,000 and, in the 70s, 21,000. The trend was similar for cases of PHN: prevention of 1,000 cases for vaccinating in the 50s, 4,000 for vaccinating in the 60s, and 8,000 for vaccinating in the 70s.
Other outcomes – emergency visits, ambulatory visits, hospitalizations, length of hospital stay, and prescriptions – also increased significantly over the 3 decades.
Savings tracked clinical outcomes in a value assessment model of 6 million people: 1 million vaccinated and unvaccinated in each of the three age cohorts.
Compared with not vaccinating, vaccinating those in their 50s would save $16.8 million annually. Vaccinating during the 60s would save about $24.3 million, and during the 70s, $31.9 million.
And, because younger people are likely to have more systemic reactions, they cost more to vaccinate. A model that accounted for the cost of the vaccine, administration, and treating local and systemic reactions, put the total price tag at $193 million for those in their 50s, and $190 million for each of the two older groups.
Finally, the number needed to treat to prevent one case of shingles, PHN, or non-PHN complication case was higher in those in their 50s than in those in their 60s or 70s (one shingles case – 51, 38, and 47 respectively; one PHN case – 988, 247, and 124).
"This isn’t intended to tell people not to get the vaccine early, but to make them aware of the risks and benefits, given that the greatest risk of the disease is later in life," Dr. Duchin said. Since there are no data on booster shingles vaccines, he said "we only have one shot at this and we need to focus our efforts on those at the greatest risk."
FROM ACIP
New HPV vaccine promises to prevent 85% of invasive cervical cancer
An investigational 9-valent human papillomavirus vaccine could prevent up to 85% of invasive cervical cancer, according to a spokesman for Merck, the company developing the product.
The vaccine (V503) adds five new HPV strains to the four already included in the current quadrivalent vaccine. The addition of HPV strains 31/33/45/52/58 should protect against the 30% of cervical cancers that are not caused by HPV 16 and 18, Dr Alain Luxembourg said at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices in Atlanta.
"The 9-valent could increase coverage from 50% to 85% of high-grade lesions – which matches the best screening program – and increase the prevention of cervical intraepithelial neoplasia," said Dr. Luxembourg of Merck. "It also provides substantial additional coverage for cervical dysplasia, which will have a lot of potential impact."
The new vaccine has the same aluminum salt adjuvant as the quadrivalent and would be administered on the same three-dose schedule (initial injection followed by others 2 months and 6 months later). It would still be targeted at girls, women, boys, and men aged 9-26 years. In a press statement, Merck said it will submit a Biologics License Application to the Food and Drug Administration by the end of this year. Merck will be seeking a full transition from the quadrivalent vaccine.
The pivotal trial, along with five other phase III safety and efficacy studies, will form the basis of the FDA application, Dr. Luxembourg said at the meeting in Atlanta. All have been completed; two are still in study extension periods. The pivotal trial results will be released next month at a meeting of the European research Organization for Genital Infection and Neoplasia, he noted.
The pivotal trial comprised 14,000 girls and women aged 9-26 years. Because it would be unethical for a control group to take a placebo, all of the subjects received either V503 or the existing quadrivalent vaccine. The main endpoints were noninferior immunogenicity to the four types included in both vaccines. For the newly included strains, the main endpoints were superior efficacy compared with both the quadrivalent vaccine and controls included in the quadrivalent vaccine trials. This was determined by comparing the rates of invasive cervical and vaginal cancer caused by the new types; the rates of cervical, vulvar, and vaginal disease of any grade; 6- and 12-month persistent infections; and PAP abnormalities. The follow-up period is 4.5 years.
Dr. Luxembourg is an employee of Merck.
An investigational 9-valent human papillomavirus vaccine could prevent up to 85% of invasive cervical cancer, according to a spokesman for Merck, the company developing the product.
The vaccine (V503) adds five new HPV strains to the four already included in the current quadrivalent vaccine. The addition of HPV strains 31/33/45/52/58 should protect against the 30% of cervical cancers that are not caused by HPV 16 and 18, Dr Alain Luxembourg said at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices in Atlanta.
"The 9-valent could increase coverage from 50% to 85% of high-grade lesions – which matches the best screening program – and increase the prevention of cervical intraepithelial neoplasia," said Dr. Luxembourg of Merck. "It also provides substantial additional coverage for cervical dysplasia, which will have a lot of potential impact."
The new vaccine has the same aluminum salt adjuvant as the quadrivalent and would be administered on the same three-dose schedule (initial injection followed by others 2 months and 6 months later). It would still be targeted at girls, women, boys, and men aged 9-26 years. In a press statement, Merck said it will submit a Biologics License Application to the Food and Drug Administration by the end of this year. Merck will be seeking a full transition from the quadrivalent vaccine.
The pivotal trial, along with five other phase III safety and efficacy studies, will form the basis of the FDA application, Dr. Luxembourg said at the meeting in Atlanta. All have been completed; two are still in study extension periods. The pivotal trial results will be released next month at a meeting of the European research Organization for Genital Infection and Neoplasia, he noted.
The pivotal trial comprised 14,000 girls and women aged 9-26 years. Because it would be unethical for a control group to take a placebo, all of the subjects received either V503 or the existing quadrivalent vaccine. The main endpoints were noninferior immunogenicity to the four types included in both vaccines. For the newly included strains, the main endpoints were superior efficacy compared with both the quadrivalent vaccine and controls included in the quadrivalent vaccine trials. This was determined by comparing the rates of invasive cervical and vaginal cancer caused by the new types; the rates of cervical, vulvar, and vaginal disease of any grade; 6- and 12-month persistent infections; and PAP abnormalities. The follow-up period is 4.5 years.
Dr. Luxembourg is an employee of Merck.
An investigational 9-valent human papillomavirus vaccine could prevent up to 85% of invasive cervical cancer, according to a spokesman for Merck, the company developing the product.
The vaccine (V503) adds five new HPV strains to the four already included in the current quadrivalent vaccine. The addition of HPV strains 31/33/45/52/58 should protect against the 30% of cervical cancers that are not caused by HPV 16 and 18, Dr Alain Luxembourg said at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices in Atlanta.
"The 9-valent could increase coverage from 50% to 85% of high-grade lesions – which matches the best screening program – and increase the prevention of cervical intraepithelial neoplasia," said Dr. Luxembourg of Merck. "It also provides substantial additional coverage for cervical dysplasia, which will have a lot of potential impact."
The new vaccine has the same aluminum salt adjuvant as the quadrivalent and would be administered on the same three-dose schedule (initial injection followed by others 2 months and 6 months later). It would still be targeted at girls, women, boys, and men aged 9-26 years. In a press statement, Merck said it will submit a Biologics License Application to the Food and Drug Administration by the end of this year. Merck will be seeking a full transition from the quadrivalent vaccine.
The pivotal trial, along with five other phase III safety and efficacy studies, will form the basis of the FDA application, Dr. Luxembourg said at the meeting in Atlanta. All have been completed; two are still in study extension periods. The pivotal trial results will be released next month at a meeting of the European research Organization for Genital Infection and Neoplasia, he noted.
The pivotal trial comprised 14,000 girls and women aged 9-26 years. Because it would be unethical for a control group to take a placebo, all of the subjects received either V503 or the existing quadrivalent vaccine. The main endpoints were noninferior immunogenicity to the four types included in both vaccines. For the newly included strains, the main endpoints were superior efficacy compared with both the quadrivalent vaccine and controls included in the quadrivalent vaccine trials. This was determined by comparing the rates of invasive cervical and vaginal cancer caused by the new types; the rates of cervical, vulvar, and vaginal disease of any grade; 6- and 12-month persistent infections; and PAP abnormalities. The follow-up period is 4.5 years.
Dr. Luxembourg is an employee of Merck.
FROM ACIP
High-dose flu vaccine protects seniors better than regular dose
A high-dose influenza vaccine for elderly patients provided 24% more protection against the disease than did the standard-dose vaccine in a randomized postlicensure study.
Switching seniors to the higher-dose formulation could prevent as many as five cases of flu per 1,000 people aged 65 years and older each year, Dr. David Greenberg said at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP).
Fluzone High-Dose vaccine (Sanofi Pasteur) is a trivalent, inactivated, split-virus influenza vaccine that contains 16 mcg hemagglutinin per dose of each included strain (aH1N1, B, and aH3N2). This is four times more antigen than in the standard Fluzone (15 mcg/dose). The high-dose formulation was developed to induce better antibody responses in adults aged 65 years or older, in an attempt to provide better protection and avert some of the disease burden that accompanies influenza in older people.
"Older adults represent about 13% of the U.S. population, but account for 63% of the hospitalizations for influenzalike illness, and more than 80% of influenza-related deaths," Dr. Greenberg said.
The Food and Drug Administration approved the high-dose vaccine on its accelerated approval pathway in late 2009. A prelicensure phase III study was conducted in 3,600 elderly adults. The high-dose vaccine stimulated significantly more protective antibody responses against all three strains than did the corresponding regular-dose vaccine; the high-dose vaccine met the FDA superiority requirement for both A strains. The response was stable across age, sex, and the presence of comorbid conditions.
"Last year, however, only an estimated 19% of vaccinated seniors got the high-dose vaccine, largely because policy groups and providers have been waiting for the results of this postlicensure trial," Dr. Greenberg said. He reported these results – most of which came in just last week – at the meeting in Atlanta.
The postlicensure study comprised more than 32,000 persons aged 65 years and older. They were enrolled at 126 sites in the United States and Canada. The trial spanned two flu seasons (2011-2012 and 2012-2013). Participants were randomized to either one dose of the high concentration vaccine or one dose of the regular vaccine.
Over both seasons, the high-dose vaccine was an average of 24% more effective in preventing influenza-like illness from types A and B combined than the regular-dose vaccine.
That benefit was more pronounced in older subjects, Dr. Greenberg noted. Among those aged 65-74 years, the relative efficacy was almost 20%; among those aged 75 years and older, the relative efficacy was 32%. The benefit held whether the illness was defined as lab confirmed (24%) or culture confirmed (23%).
The high-dose vaccine significantly reduced the risk of pneumonia associated with laboratory-confirmed influenza by up to 53%. The risk of cardiorespiratory illness within 30 days of flu onset dropped by almost 30%, while the risk of flu-related 30-day hospital admissions fell by about 40%.
Safety outcomes were good when compared with the regular-dose vaccine, Dr. Greenberg said. Serious adverse events occurred in 8% of the high dose group and 9% of the regular-dose group.
Sanofi Pasteur will continue to analyze the study data, Dr. Greenberg said. The company intends to submit a clinical study report to the FDA’s Center for Biologics Evaluation and Research by the first quarter of next year. Sanofi will also seek a revision of the prescribing information supporting the vaccine’s clinical superiority to the regular-dose vaccine.
Dr. Greenberg is the senior director of U.S. scientific and medical affairs for Sanofi Pasteur.
A high-dose influenza vaccine for elderly patients provided 24% more protection against the disease than did the standard-dose vaccine in a randomized postlicensure study.
Switching seniors to the higher-dose formulation could prevent as many as five cases of flu per 1,000 people aged 65 years and older each year, Dr. David Greenberg said at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP).
Fluzone High-Dose vaccine (Sanofi Pasteur) is a trivalent, inactivated, split-virus influenza vaccine that contains 16 mcg hemagglutinin per dose of each included strain (aH1N1, B, and aH3N2). This is four times more antigen than in the standard Fluzone (15 mcg/dose). The high-dose formulation was developed to induce better antibody responses in adults aged 65 years or older, in an attempt to provide better protection and avert some of the disease burden that accompanies influenza in older people.
"Older adults represent about 13% of the U.S. population, but account for 63% of the hospitalizations for influenzalike illness, and more than 80% of influenza-related deaths," Dr. Greenberg said.
The Food and Drug Administration approved the high-dose vaccine on its accelerated approval pathway in late 2009. A prelicensure phase III study was conducted in 3,600 elderly adults. The high-dose vaccine stimulated significantly more protective antibody responses against all three strains than did the corresponding regular-dose vaccine; the high-dose vaccine met the FDA superiority requirement for both A strains. The response was stable across age, sex, and the presence of comorbid conditions.
"Last year, however, only an estimated 19% of vaccinated seniors got the high-dose vaccine, largely because policy groups and providers have been waiting for the results of this postlicensure trial," Dr. Greenberg said. He reported these results – most of which came in just last week – at the meeting in Atlanta.
The postlicensure study comprised more than 32,000 persons aged 65 years and older. They were enrolled at 126 sites in the United States and Canada. The trial spanned two flu seasons (2011-2012 and 2012-2013). Participants were randomized to either one dose of the high concentration vaccine or one dose of the regular vaccine.
Over both seasons, the high-dose vaccine was an average of 24% more effective in preventing influenza-like illness from types A and B combined than the regular-dose vaccine.
That benefit was more pronounced in older subjects, Dr. Greenberg noted. Among those aged 65-74 years, the relative efficacy was almost 20%; among those aged 75 years and older, the relative efficacy was 32%. The benefit held whether the illness was defined as lab confirmed (24%) or culture confirmed (23%).
The high-dose vaccine significantly reduced the risk of pneumonia associated with laboratory-confirmed influenza by up to 53%. The risk of cardiorespiratory illness within 30 days of flu onset dropped by almost 30%, while the risk of flu-related 30-day hospital admissions fell by about 40%.
Safety outcomes were good when compared with the regular-dose vaccine, Dr. Greenberg said. Serious adverse events occurred in 8% of the high dose group and 9% of the regular-dose group.
Sanofi Pasteur will continue to analyze the study data, Dr. Greenberg said. The company intends to submit a clinical study report to the FDA’s Center for Biologics Evaluation and Research by the first quarter of next year. Sanofi will also seek a revision of the prescribing information supporting the vaccine’s clinical superiority to the regular-dose vaccine.
Dr. Greenberg is the senior director of U.S. scientific and medical affairs for Sanofi Pasteur.
A high-dose influenza vaccine for elderly patients provided 24% more protection against the disease than did the standard-dose vaccine in a randomized postlicensure study.
Switching seniors to the higher-dose formulation could prevent as many as five cases of flu per 1,000 people aged 65 years and older each year, Dr. David Greenberg said at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP).
Fluzone High-Dose vaccine (Sanofi Pasteur) is a trivalent, inactivated, split-virus influenza vaccine that contains 16 mcg hemagglutinin per dose of each included strain (aH1N1, B, and aH3N2). This is four times more antigen than in the standard Fluzone (15 mcg/dose). The high-dose formulation was developed to induce better antibody responses in adults aged 65 years or older, in an attempt to provide better protection and avert some of the disease burden that accompanies influenza in older people.
"Older adults represent about 13% of the U.S. population, but account for 63% of the hospitalizations for influenzalike illness, and more than 80% of influenza-related deaths," Dr. Greenberg said.
The Food and Drug Administration approved the high-dose vaccine on its accelerated approval pathway in late 2009. A prelicensure phase III study was conducted in 3,600 elderly adults. The high-dose vaccine stimulated significantly more protective antibody responses against all three strains than did the corresponding regular-dose vaccine; the high-dose vaccine met the FDA superiority requirement for both A strains. The response was stable across age, sex, and the presence of comorbid conditions.
"Last year, however, only an estimated 19% of vaccinated seniors got the high-dose vaccine, largely because policy groups and providers have been waiting for the results of this postlicensure trial," Dr. Greenberg said. He reported these results – most of which came in just last week – at the meeting in Atlanta.
The postlicensure study comprised more than 32,000 persons aged 65 years and older. They were enrolled at 126 sites in the United States and Canada. The trial spanned two flu seasons (2011-2012 and 2012-2013). Participants were randomized to either one dose of the high concentration vaccine or one dose of the regular vaccine.
Over both seasons, the high-dose vaccine was an average of 24% more effective in preventing influenza-like illness from types A and B combined than the regular-dose vaccine.
That benefit was more pronounced in older subjects, Dr. Greenberg noted. Among those aged 65-74 years, the relative efficacy was almost 20%; among those aged 75 years and older, the relative efficacy was 32%. The benefit held whether the illness was defined as lab confirmed (24%) or culture confirmed (23%).
The high-dose vaccine significantly reduced the risk of pneumonia associated with laboratory-confirmed influenza by up to 53%. The risk of cardiorespiratory illness within 30 days of flu onset dropped by almost 30%, while the risk of flu-related 30-day hospital admissions fell by about 40%.
Safety outcomes were good when compared with the regular-dose vaccine, Dr. Greenberg said. Serious adverse events occurred in 8% of the high dose group and 9% of the regular-dose group.
Sanofi Pasteur will continue to analyze the study data, Dr. Greenberg said. The company intends to submit a clinical study report to the FDA’s Center for Biologics Evaluation and Research by the first quarter of next year. Sanofi will also seek a revision of the prescribing information supporting the vaccine’s clinical superiority to the regular-dose vaccine.
Dr. Greenberg is the senior director of U.S. scientific and medical affairs for Sanofi Pasteur.
FROM ACIP
Major finding: A high-dose influenza vaccine was 24% more effective in people aged 65 years and older than was the corresponding regular-dose vaccine.
Data source: The randomized, postlicensure study included more than 13,000 subjects.
Disclosures: Dr. David Greenberg is the senior director of U.S. scientific and medical affairs for Sanofi Pasteur.
Meningococcal Vaccine for At-risk Infants
The quadrivalent meningococcal vaccine MenACWY-CRM, or Menveo, may now be given to infants aged 2-23 months who are considered at high risk for meningococcal disease or who travel to areas where it is hyperendemic or epidemic, according to the recommendations of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
High-risk children are those with component complement deficiency, HIV infection, or anatomic or functional asplenia. Children in an outbreak of a vaccine-preventable meningitis serogroup also should receive the series. About 5,000 infants per year fit into these categories.
The Advisory Committee on Immunization Practices (ACIP) approved the decision by a vote of 13-1, with one abstention. The decision aligns ACIP’s recommendation with the recently expanded indication for the vaccine, which protects against serotypes A, C, W-135, and Y. In August, the Food and Drug Administration approved the vaccine for infants and toddlers older than 2 months.
However, because of the extremely low incidence of meningococcal disease in children in the United States, the committee did not recommend it for all healthy children. "Very few children are considered at increased risk, so a routine recommendation would prevent just a very low number of cases," said Jessica MacNeil, an epidemiologist with the CDC.
The FDA licensing of MenACWY-CRM and ACIP’s recommendation give pediatricians a third option for infant meningococcal vaccination. HibMenCY-TT (MenHibrix) and MenACWY-D (Menactra) are also approved for some of these same indications. However, Ms. MacNeil noted, Menactra cannot be used in children with asplenia, and MenHibrix can’t be used for travel or as a booster dose.
The committee based its decision on three phase III safety and efficacy trials. These studies comprised nearly 11,000 infants – 9,000 of whom had the four-dose series and 2,000 of whom had a two-dose series, said Dr. Peter Dull of Novartis Vaccines.
The studies confirmed an average 90% efficacy against all four serotypes after the 12-month dose. By 40 months of age, efficacy had waned somewhat: 10% of infants had a seroresponse to type A; 34%, to type C; 76%, to type W-135; and 67%, to type Y. Of the strains included in the vaccine, types C, Y, and W-135 cause 75% of meningococcal disease in those aged 11 years and older.
Dr. Dull said that Novartis is following a cohort that received the vaccine as infants; next year, 60-month immunogenicity data will be available.
After adjustment for some confounders, the vaccine did not significantly interact with the immunogenic potential of other recommended childhood vaccines. Novartis studies also found it safe. Up to 60% of children had mild to moderate injection site reactions. The incidence of systemic reactions was no different from that for routine vaccines alone.
There were 11 serious adverse events possibly related to the vaccine among the 5,000 children included in the safety analyses. These included acute encephalomyelitis, cellulitis, complex partial seizure, epilepsy, febrile seizure, and Kawasaki disease. Ten deaths occurred, but were not considered vaccine related.
The recommended dosing schedule will be:
• Aged 2-6 months: Four doses at 2, 4, 6, and 12 months.
• Aged 7-23 months: Two doses with the second dose given in the second year of life.
• Aged 2-11 years: One or two doses.
Boosters should be given to those who remain at risk, beginning at 3 years after the primary series and then every 5 years thereafter.
The committee also agreed, by a vote of 14 with one abstention, to include MenACWY-CRM in the Vaccines for Children program.
One member who voted disclosed that her institution receives research funding from drug companies. None of the other voting members had any relevant financial disclosures.
The quadrivalent meningococcal vaccine MenACWY-CRM, or Menveo, may now be given to infants aged 2-23 months who are considered at high risk for meningococcal disease or who travel to areas where it is hyperendemic or epidemic, according to the recommendations of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
High-risk children are those with component complement deficiency, HIV infection, or anatomic or functional asplenia. Children in an outbreak of a vaccine-preventable meningitis serogroup also should receive the series. About 5,000 infants per year fit into these categories.
The Advisory Committee on Immunization Practices (ACIP) approved the decision by a vote of 13-1, with one abstention. The decision aligns ACIP’s recommendation with the recently expanded indication for the vaccine, which protects against serotypes A, C, W-135, and Y. In August, the Food and Drug Administration approved the vaccine for infants and toddlers older than 2 months.
However, because of the extremely low incidence of meningococcal disease in children in the United States, the committee did not recommend it for all healthy children. "Very few children are considered at increased risk, so a routine recommendation would prevent just a very low number of cases," said Jessica MacNeil, an epidemiologist with the CDC.
The FDA licensing of MenACWY-CRM and ACIP’s recommendation give pediatricians a third option for infant meningococcal vaccination. HibMenCY-TT (MenHibrix) and MenACWY-D (Menactra) are also approved for some of these same indications. However, Ms. MacNeil noted, Menactra cannot be used in children with asplenia, and MenHibrix can’t be used for travel or as a booster dose.
The committee based its decision on three phase III safety and efficacy trials. These studies comprised nearly 11,000 infants – 9,000 of whom had the four-dose series and 2,000 of whom had a two-dose series, said Dr. Peter Dull of Novartis Vaccines.
The studies confirmed an average 90% efficacy against all four serotypes after the 12-month dose. By 40 months of age, efficacy had waned somewhat: 10% of infants had a seroresponse to type A; 34%, to type C; 76%, to type W-135; and 67%, to type Y. Of the strains included in the vaccine, types C, Y, and W-135 cause 75% of meningococcal disease in those aged 11 years and older.
Dr. Dull said that Novartis is following a cohort that received the vaccine as infants; next year, 60-month immunogenicity data will be available.
After adjustment for some confounders, the vaccine did not significantly interact with the immunogenic potential of other recommended childhood vaccines. Novartis studies also found it safe. Up to 60% of children had mild to moderate injection site reactions. The incidence of systemic reactions was no different from that for routine vaccines alone.
There were 11 serious adverse events possibly related to the vaccine among the 5,000 children included in the safety analyses. These included acute encephalomyelitis, cellulitis, complex partial seizure, epilepsy, febrile seizure, and Kawasaki disease. Ten deaths occurred, but were not considered vaccine related.
The recommended dosing schedule will be:
• Aged 2-6 months: Four doses at 2, 4, 6, and 12 months.
• Aged 7-23 months: Two doses with the second dose given in the second year of life.
• Aged 2-11 years: One or two doses.
Boosters should be given to those who remain at risk, beginning at 3 years after the primary series and then every 5 years thereafter.
The committee also agreed, by a vote of 14 with one abstention, to include MenACWY-CRM in the Vaccines for Children program.
One member who voted disclosed that her institution receives research funding from drug companies. None of the other voting members had any relevant financial disclosures.
The quadrivalent meningococcal vaccine MenACWY-CRM, or Menveo, may now be given to infants aged 2-23 months who are considered at high risk for meningococcal disease or who travel to areas where it is hyperendemic or epidemic, according to the recommendations of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
High-risk children are those with component complement deficiency, HIV infection, or anatomic or functional asplenia. Children in an outbreak of a vaccine-preventable meningitis serogroup also should receive the series. About 5,000 infants per year fit into these categories.
The Advisory Committee on Immunization Practices (ACIP) approved the decision by a vote of 13-1, with one abstention. The decision aligns ACIP’s recommendation with the recently expanded indication for the vaccine, which protects against serotypes A, C, W-135, and Y. In August, the Food and Drug Administration approved the vaccine for infants and toddlers older than 2 months.
However, because of the extremely low incidence of meningococcal disease in children in the United States, the committee did not recommend it for all healthy children. "Very few children are considered at increased risk, so a routine recommendation would prevent just a very low number of cases," said Jessica MacNeil, an epidemiologist with the CDC.
The FDA licensing of MenACWY-CRM and ACIP’s recommendation give pediatricians a third option for infant meningococcal vaccination. HibMenCY-TT (MenHibrix) and MenACWY-D (Menactra) are also approved for some of these same indications. However, Ms. MacNeil noted, Menactra cannot be used in children with asplenia, and MenHibrix can’t be used for travel or as a booster dose.
The committee based its decision on three phase III safety and efficacy trials. These studies comprised nearly 11,000 infants – 9,000 of whom had the four-dose series and 2,000 of whom had a two-dose series, said Dr. Peter Dull of Novartis Vaccines.
The studies confirmed an average 90% efficacy against all four serotypes after the 12-month dose. By 40 months of age, efficacy had waned somewhat: 10% of infants had a seroresponse to type A; 34%, to type C; 76%, to type W-135; and 67%, to type Y. Of the strains included in the vaccine, types C, Y, and W-135 cause 75% of meningococcal disease in those aged 11 years and older.
Dr. Dull said that Novartis is following a cohort that received the vaccine as infants; next year, 60-month immunogenicity data will be available.
After adjustment for some confounders, the vaccine did not significantly interact with the immunogenic potential of other recommended childhood vaccines. Novartis studies also found it safe. Up to 60% of children had mild to moderate injection site reactions. The incidence of systemic reactions was no different from that for routine vaccines alone.
There were 11 serious adverse events possibly related to the vaccine among the 5,000 children included in the safety analyses. These included acute encephalomyelitis, cellulitis, complex partial seizure, epilepsy, febrile seizure, and Kawasaki disease. Ten deaths occurred, but were not considered vaccine related.
The recommended dosing schedule will be:
• Aged 2-6 months: Four doses at 2, 4, 6, and 12 months.
• Aged 7-23 months: Two doses with the second dose given in the second year of life.
• Aged 2-11 years: One or two doses.
Boosters should be given to those who remain at risk, beginning at 3 years after the primary series and then every 5 years thereafter.
The committee also agreed, by a vote of 14 with one abstention, to include MenACWY-CRM in the Vaccines for Children program.
One member who voted disclosed that her institution receives research funding from drug companies. None of the other voting members had any relevant financial disclosures.
AT ACIP
ACIP recommends meningococcal vaccine for at-risk infants
The quadrivalent meningococcal vaccine MenACWY-CRM, or Menveo, may now be given to infants aged 2-23 months who are considered at high risk for meningococcal disease or who travel to areas where it is hyperendemic or epidemic, according to the recommendations of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
High-risk children are those with component complement deficiency, HIV infection, or anatomic or functional asplenia. Children in an outbreak of a vaccine-preventable meningitis serogroup also should receive the series. About 5,000 infants per year fit into these categories.
The Advisory Committee on Immunization Practices (ACIP) approved the decision by a vote of 13-1, with one abstention. The decision aligns ACIP’s recommendation with the recently expanded indication for the vaccine, which protects against serotypes A, C, W-135, and Y. In August, the Food and Drug Administration approved the vaccine for infants and toddlers older than 2 months.
However, because of the extremely low incidence of meningococcal disease in children in the United States, the committee did not recommend it for all healthy children. "Very few children are considered at increased risk, so a routine recommendation would prevent just a very low number of cases," said Jessica MacNeil, an epidemiologist with the CDC.
The FDA licensing of MenACWY-CRM and ACIP’s recommendation give pediatricians a third option for infant meningococcal vaccination. HibMenCY-TT (MenHibrix) and MenACWY-D (Menactra) are also approved for some of these same indications. However, Ms. MacNeil noted, Menactra cannot be used in children with asplenia, and MenHibrix can’t be used for travel or as a booster dose.
The committee based its decision on three phase III safety and efficacy trials. These studies comprised nearly 11,000 infants – 9,000 of whom had the four-dose series and 2,000 of whom had a two-dose series, said Dr. Peter Dull of Novartis Vaccines.
The studies confirmed an average 90% efficacy against all four serotypes after the 12-month dose. By 40 months of age, efficacy had waned somewhat: 10% of infants had a seroresponse to type A; 34%, to type C; 76%, to type W-135; and 67%, to type Y. Of the strains included in the vaccine, types C, Y, and W-135 cause 75% of meningococcal disease in those aged 11 years and older.
Dr. Dull said that Novartis is following a cohort that received the vaccine as infants; next year, 60-month immunogenicity data will be available.
After adjustment for some confounders, the vaccine did not significantly interact with the immunogenic potential of other recommended childhood vaccines. Novartis studies also found it safe. Up to 60% of children had mild to moderate injection site reactions. The incidence of systemic reactions was no different from that for routine vaccines alone.
There were 11 serious adverse events possibly related to the vaccine among the 5,000 children included in the safety analyses. These included acute encephalomyelitis, cellulitis, complex partial seizure, epilepsy, febrile seizure, and Kawasaki disease. Ten deaths occurred, but were not considered vaccine related.
The recommended dosing schedule will be:
• Aged 2-6 months: Four doses at 2, 4, 6, and 12 months.
• Aged 7-23 months: Two doses with the second dose given in the second year of life.
• Aged 2-11 years: One or two doses.
Boosters should be given to those who remain at risk, beginning at 3 years after the primary series and then every 5 years thereafter.
The committee also agreed, by a vote of 14 with one abstention, to include MenACWY-CRM in the Vaccines for Children program.
One member who voted disclosed that her institution receives research funding from drug companies. None of the other voting members had any relevant financial disclosures.
The quadrivalent meningococcal vaccine MenACWY-CRM, or Menveo, may now be given to infants aged 2-23 months who are considered at high risk for meningococcal disease or who travel to areas where it is hyperendemic or epidemic, according to the recommendations of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
High-risk children are those with component complement deficiency, HIV infection, or anatomic or functional asplenia. Children in an outbreak of a vaccine-preventable meningitis serogroup also should receive the series. About 5,000 infants per year fit into these categories.
The Advisory Committee on Immunization Practices (ACIP) approved the decision by a vote of 13-1, with one abstention. The decision aligns ACIP’s recommendation with the recently expanded indication for the vaccine, which protects against serotypes A, C, W-135, and Y. In August, the Food and Drug Administration approved the vaccine for infants and toddlers older than 2 months.
However, because of the extremely low incidence of meningococcal disease in children in the United States, the committee did not recommend it for all healthy children. "Very few children are considered at increased risk, so a routine recommendation would prevent just a very low number of cases," said Jessica MacNeil, an epidemiologist with the CDC.
The FDA licensing of MenACWY-CRM and ACIP’s recommendation give pediatricians a third option for infant meningococcal vaccination. HibMenCY-TT (MenHibrix) and MenACWY-D (Menactra) are also approved for some of these same indications. However, Ms. MacNeil noted, Menactra cannot be used in children with asplenia, and MenHibrix can’t be used for travel or as a booster dose.
The committee based its decision on three phase III safety and efficacy trials. These studies comprised nearly 11,000 infants – 9,000 of whom had the four-dose series and 2,000 of whom had a two-dose series, said Dr. Peter Dull of Novartis Vaccines.
The studies confirmed an average 90% efficacy against all four serotypes after the 12-month dose. By 40 months of age, efficacy had waned somewhat: 10% of infants had a seroresponse to type A; 34%, to type C; 76%, to type W-135; and 67%, to type Y. Of the strains included in the vaccine, types C, Y, and W-135 cause 75% of meningococcal disease in those aged 11 years and older.
Dr. Dull said that Novartis is following a cohort that received the vaccine as infants; next year, 60-month immunogenicity data will be available.
After adjustment for some confounders, the vaccine did not significantly interact with the immunogenic potential of other recommended childhood vaccines. Novartis studies also found it safe. Up to 60% of children had mild to moderate injection site reactions. The incidence of systemic reactions was no different from that for routine vaccines alone.
There were 11 serious adverse events possibly related to the vaccine among the 5,000 children included in the safety analyses. These included acute encephalomyelitis, cellulitis, complex partial seizure, epilepsy, febrile seizure, and Kawasaki disease. Ten deaths occurred, but were not considered vaccine related.
The recommended dosing schedule will be:
• Aged 2-6 months: Four doses at 2, 4, 6, and 12 months.
• Aged 7-23 months: Two doses with the second dose given in the second year of life.
• Aged 2-11 years: One or two doses.
Boosters should be given to those who remain at risk, beginning at 3 years after the primary series and then every 5 years thereafter.
The committee also agreed, by a vote of 14 with one abstention, to include MenACWY-CRM in the Vaccines for Children program.
One member who voted disclosed that her institution receives research funding from drug companies. None of the other voting members had any relevant financial disclosures.
The quadrivalent meningococcal vaccine MenACWY-CRM, or Menveo, may now be given to infants aged 2-23 months who are considered at high risk for meningococcal disease or who travel to areas where it is hyperendemic or epidemic, according to the recommendations of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
High-risk children are those with component complement deficiency, HIV infection, or anatomic or functional asplenia. Children in an outbreak of a vaccine-preventable meningitis serogroup also should receive the series. About 5,000 infants per year fit into these categories.
The Advisory Committee on Immunization Practices (ACIP) approved the decision by a vote of 13-1, with one abstention. The decision aligns ACIP’s recommendation with the recently expanded indication for the vaccine, which protects against serotypes A, C, W-135, and Y. In August, the Food and Drug Administration approved the vaccine for infants and toddlers older than 2 months.
However, because of the extremely low incidence of meningococcal disease in children in the United States, the committee did not recommend it for all healthy children. "Very few children are considered at increased risk, so a routine recommendation would prevent just a very low number of cases," said Jessica MacNeil, an epidemiologist with the CDC.
The FDA licensing of MenACWY-CRM and ACIP’s recommendation give pediatricians a third option for infant meningococcal vaccination. HibMenCY-TT (MenHibrix) and MenACWY-D (Menactra) are also approved for some of these same indications. However, Ms. MacNeil noted, Menactra cannot be used in children with asplenia, and MenHibrix can’t be used for travel or as a booster dose.
The committee based its decision on three phase III safety and efficacy trials. These studies comprised nearly 11,000 infants – 9,000 of whom had the four-dose series and 2,000 of whom had a two-dose series, said Dr. Peter Dull of Novartis Vaccines.
The studies confirmed an average 90% efficacy against all four serotypes after the 12-month dose. By 40 months of age, efficacy had waned somewhat: 10% of infants had a seroresponse to type A; 34%, to type C; 76%, to type W-135; and 67%, to type Y. Of the strains included in the vaccine, types C, Y, and W-135 cause 75% of meningococcal disease in those aged 11 years and older.
Dr. Dull said that Novartis is following a cohort that received the vaccine as infants; next year, 60-month immunogenicity data will be available.
After adjustment for some confounders, the vaccine did not significantly interact with the immunogenic potential of other recommended childhood vaccines. Novartis studies also found it safe. Up to 60% of children had mild to moderate injection site reactions. The incidence of systemic reactions was no different from that for routine vaccines alone.
There were 11 serious adverse events possibly related to the vaccine among the 5,000 children included in the safety analyses. These included acute encephalomyelitis, cellulitis, complex partial seizure, epilepsy, febrile seizure, and Kawasaki disease. Ten deaths occurred, but were not considered vaccine related.
The recommended dosing schedule will be:
• Aged 2-6 months: Four doses at 2, 4, 6, and 12 months.
• Aged 7-23 months: Two doses with the second dose given in the second year of life.
• Aged 2-11 years: One or two doses.
Boosters should be given to those who remain at risk, beginning at 3 years after the primary series and then every 5 years thereafter.
The committee also agreed, by a vote of 14 with one abstention, to include MenACWY-CRM in the Vaccines for Children program.
One member who voted disclosed that her institution receives research funding from drug companies. None of the other voting members had any relevant financial disclosures.
AT ACIP