User login
Aesthetic Dermatology Grabs More Headlines
WASHINGTON – When it comes to news coverage of dermatology, aesthetic issues get much more attention than oncologic, surgical, or medical topics, according to a recent analysis of the nation’s top newspapers.
"I found it surprising that cosmetic procedures were so strongly emphasized, with Botox by far the most commonly covered dermatology topic," Dr. Kristina Collins, a fellow in dermatologic surgery in the department of dermatology at Harvard Medical School, Boston, and Lahey Clinic, Burlington, Mass., said in an interview. "I think that many dermatologists find that patients or even colleagues in other specialties are not aware of the important medical diseases that the field of dermatology encompasses, and many people are under the false impression that most of a typical dermatologist’s time is dedicated to cosmetics."
In fact, according to Dr. Collins, national practice data shows that the average dermatologist spends only about 10% of his or her time on cosmetic procedures.
To conduct the study, Dr. Collins and her colleagues analyzed the contents of 1,669 dermatology-related articles gathered from the top 10 most widely circulated newspapers over a 10-year period ending on Jan. 1, 2011.
"I found it surprising that cosmetic procedures were so strongly emphasized, with Botox by far the most commonly covered dermatology topic," said Dr. Kristina Collins.
Cosmetic procedures received 32% of the coverage, followed by skin diseases and disorders at 24%, skin cancer/tanning/sun protection at 22%, skin care at 12%, and acne and hair loss at 5% each.
Comparing the percentage of cosmetic vs. noncosmetic articles, the New York Post took the top spot with 72% cosmetic articles. It was followed by the New York Daily News (57%) and USA Today (55%). The Philadelphia Inquirer and the Denver Post had the lowest percentage of cosmetic articles at 37% and 29%, respectively.
Botox topped the chart when the articles were analyzed by topic (105 articles), followed by lasers (64), popularity of procedures (63), and sun protection tips (61). Botox for hyperhidrosis, smallpox/vaccine complications, epidermolysis bullosa, and tanning laws and restrictions took the bottom spots with 15 articles each.
A handful of other studies have also arrived at the same conclusion, with one focusing on the iconic TV sitcom Seinfeld and its reference to dermatologists. "Selecting satire to portray an already misunderstood and unknown subject matter may perpetuate incorrect public beliefs and stereotypes about those with skin diseases and diminish cultural sensitivity towards people who have dermatologic conditions and their caregivers," the authors wrote. (Dermatol. Online J. 2010;16:1).
Dr. Collins said that, with the aging population and the cultural shift in beauty norms, "people are genuinely interested in some of the cosmetic procedures that are available and that interest, in turn, drives the news media." But, "Somehow as a profession, we need to find ways to make important skin health information compelling both to the media and their target audiences.
"All of the aspects of our field have a place in the news, whether we are talking about Botox or basal cell carcinoma. We owe it to our patients to try to get them vital health information any way we are able," she said in an interview.
Dr. Collins had no disclosures.
WASHINGTON – When it comes to news coverage of dermatology, aesthetic issues get much more attention than oncologic, surgical, or medical topics, according to a recent analysis of the nation’s top newspapers.
"I found it surprising that cosmetic procedures were so strongly emphasized, with Botox by far the most commonly covered dermatology topic," Dr. Kristina Collins, a fellow in dermatologic surgery in the department of dermatology at Harvard Medical School, Boston, and Lahey Clinic, Burlington, Mass., said in an interview. "I think that many dermatologists find that patients or even colleagues in other specialties are not aware of the important medical diseases that the field of dermatology encompasses, and many people are under the false impression that most of a typical dermatologist’s time is dedicated to cosmetics."
In fact, according to Dr. Collins, national practice data shows that the average dermatologist spends only about 10% of his or her time on cosmetic procedures.
To conduct the study, Dr. Collins and her colleagues analyzed the contents of 1,669 dermatology-related articles gathered from the top 10 most widely circulated newspapers over a 10-year period ending on Jan. 1, 2011.
"I found it surprising that cosmetic procedures were so strongly emphasized, with Botox by far the most commonly covered dermatology topic," said Dr. Kristina Collins.
Cosmetic procedures received 32% of the coverage, followed by skin diseases and disorders at 24%, skin cancer/tanning/sun protection at 22%, skin care at 12%, and acne and hair loss at 5% each.
Comparing the percentage of cosmetic vs. noncosmetic articles, the New York Post took the top spot with 72% cosmetic articles. It was followed by the New York Daily News (57%) and USA Today (55%). The Philadelphia Inquirer and the Denver Post had the lowest percentage of cosmetic articles at 37% and 29%, respectively.
Botox topped the chart when the articles were analyzed by topic (105 articles), followed by lasers (64), popularity of procedures (63), and sun protection tips (61). Botox for hyperhidrosis, smallpox/vaccine complications, epidermolysis bullosa, and tanning laws and restrictions took the bottom spots with 15 articles each.
A handful of other studies have also arrived at the same conclusion, with one focusing on the iconic TV sitcom Seinfeld and its reference to dermatologists. "Selecting satire to portray an already misunderstood and unknown subject matter may perpetuate incorrect public beliefs and stereotypes about those with skin diseases and diminish cultural sensitivity towards people who have dermatologic conditions and their caregivers," the authors wrote. (Dermatol. Online J. 2010;16:1).
Dr. Collins said that, with the aging population and the cultural shift in beauty norms, "people are genuinely interested in some of the cosmetic procedures that are available and that interest, in turn, drives the news media." But, "Somehow as a profession, we need to find ways to make important skin health information compelling both to the media and their target audiences.
"All of the aspects of our field have a place in the news, whether we are talking about Botox or basal cell carcinoma. We owe it to our patients to try to get them vital health information any way we are able," she said in an interview.
Dr. Collins had no disclosures.
WASHINGTON – When it comes to news coverage of dermatology, aesthetic issues get much more attention than oncologic, surgical, or medical topics, according to a recent analysis of the nation’s top newspapers.
"I found it surprising that cosmetic procedures were so strongly emphasized, with Botox by far the most commonly covered dermatology topic," Dr. Kristina Collins, a fellow in dermatologic surgery in the department of dermatology at Harvard Medical School, Boston, and Lahey Clinic, Burlington, Mass., said in an interview. "I think that many dermatologists find that patients or even colleagues in other specialties are not aware of the important medical diseases that the field of dermatology encompasses, and many people are under the false impression that most of a typical dermatologist’s time is dedicated to cosmetics."
In fact, according to Dr. Collins, national practice data shows that the average dermatologist spends only about 10% of his or her time on cosmetic procedures.
To conduct the study, Dr. Collins and her colleagues analyzed the contents of 1,669 dermatology-related articles gathered from the top 10 most widely circulated newspapers over a 10-year period ending on Jan. 1, 2011.
"I found it surprising that cosmetic procedures were so strongly emphasized, with Botox by far the most commonly covered dermatology topic," said Dr. Kristina Collins.
Cosmetic procedures received 32% of the coverage, followed by skin diseases and disorders at 24%, skin cancer/tanning/sun protection at 22%, skin care at 12%, and acne and hair loss at 5% each.
Comparing the percentage of cosmetic vs. noncosmetic articles, the New York Post took the top spot with 72% cosmetic articles. It was followed by the New York Daily News (57%) and USA Today (55%). The Philadelphia Inquirer and the Denver Post had the lowest percentage of cosmetic articles at 37% and 29%, respectively.
Botox topped the chart when the articles were analyzed by topic (105 articles), followed by lasers (64), popularity of procedures (63), and sun protection tips (61). Botox for hyperhidrosis, smallpox/vaccine complications, epidermolysis bullosa, and tanning laws and restrictions took the bottom spots with 15 articles each.
A handful of other studies have also arrived at the same conclusion, with one focusing on the iconic TV sitcom Seinfeld and its reference to dermatologists. "Selecting satire to portray an already misunderstood and unknown subject matter may perpetuate incorrect public beliefs and stereotypes about those with skin diseases and diminish cultural sensitivity towards people who have dermatologic conditions and their caregivers," the authors wrote. (Dermatol. Online J. 2010;16:1).
Dr. Collins said that, with the aging population and the cultural shift in beauty norms, "people are genuinely interested in some of the cosmetic procedures that are available and that interest, in turn, drives the news media." But, "Somehow as a profession, we need to find ways to make important skin health information compelling both to the media and their target audiences.
"All of the aspects of our field have a place in the news, whether we are talking about Botox or basal cell carcinoma. We owe it to our patients to try to get them vital health information any way we are able," she said in an interview.
Dr. Collins had no disclosures.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR DERMATOLOGIC SURGERY
Vitamin C Deficiency Ups Heart Risk
ORLANDO – Low vitamin C intake may worsen heart failure and put patients at a higher risk for cardiac events, according to a study conducted in three U.S. clinics.
The findings showed that patients who had low intakes of vitamin C were 2.4 times as likely to have higher levels of high-sensitivity C-reactive protein (hsCRP) – a marker for inflammation – compared with those who had high vitamin C intake. They also had shorter intervals without cardiac events.
In other words, "adequate intake of vitamin C was associated with longer survival in patients with heart failure," said Eun Kyeung Song, Ph.D., of the University of Ulsan (South Korea), who is the lead author of the study and a registered nurse.
The study, which is the first of its kind, was "provocative," and could lead to more investigations and larger studies to verify the findings, said Dr. Roger S. Blumenthal, director of preventive cardiology at Johns Hopkins University, Baltimore. He was not involved in the study.
"We generally attribute high CRP to increased weight in the midsection," said Dr. Blumenthal. "But this study suggests that it may be dietary inadequacies that may also increase inflammation and may be even a stronger predictor than just the CRP blood test by itself."
After taking a detailed, 4-day food diary, the authors measured the serum hsCRP of 212 patients at three outpatient heart failure clinics in Kentucky, Indiana, and Georgia during a 12-month follow-up.
Of the cohort, 82 patients (39%) had low vitamin C intake, as defined by the Institute of Medicine’s guidelines. In all, 98 (46%) had hsCRP greater than 3 mg/L, which is the cut-off point for being at high risk of having cardiac events. The patients with low vitamin C were 2.4 times more likely to have a high hsCRP level.
During the follow-up, 61 patients (29%) had cardiac events. After the authors controlled for age, sex, body mass index, New York Heart Association class, ejection fraction, comorbidities, total caloric intake, and medications, the results showed that the patients with low vitamin C levels were exactly twice as likely as those with adequate levels to have shorter cumulative cardiac event–free survival. Furthermore, those with hsCRP levels of at least 3 mg/L were 1.9 times as likely to have that outcome. Both differences were significant.
"Adequate intake of vitamin C was associated with longer survival in patients with heart failure."
"The data suggest that one possible mechanism by which vitamin C deficiency contributed to poor health outcomes is through inflammatory pathways in [heart failure] patients," they concluded.
"Diet is the best source of vitamin C," said Terry Lennie, Ph.D., one of the study authors and an associate dean at the University of Kentucky in Lexington. "Eating the recommended five servings of fruits and vegetables a day provides an adequate amount."
Dr. Blumenthal added that "sometimes we just talk to our patients about avoiding fast foods, and we don’t give as much positive reinforcement for things that are good for them to eat. ... We should go back to what our mothers and grandmothers told us, and try to have a more balanced diet and have more fruits and vegetables."
Dr. Lennie has received research grant from multiple extramural funders in the research area. Dr. Song and Dr. Blumenthal had no disclosures.
ORLANDO – Low vitamin C intake may worsen heart failure and put patients at a higher risk for cardiac events, according to a study conducted in three U.S. clinics.
The findings showed that patients who had low intakes of vitamin C were 2.4 times as likely to have higher levels of high-sensitivity C-reactive protein (hsCRP) – a marker for inflammation – compared with those who had high vitamin C intake. They also had shorter intervals without cardiac events.
In other words, "adequate intake of vitamin C was associated with longer survival in patients with heart failure," said Eun Kyeung Song, Ph.D., of the University of Ulsan (South Korea), who is the lead author of the study and a registered nurse.
The study, which is the first of its kind, was "provocative," and could lead to more investigations and larger studies to verify the findings, said Dr. Roger S. Blumenthal, director of preventive cardiology at Johns Hopkins University, Baltimore. He was not involved in the study.
"We generally attribute high CRP to increased weight in the midsection," said Dr. Blumenthal. "But this study suggests that it may be dietary inadequacies that may also increase inflammation and may be even a stronger predictor than just the CRP blood test by itself."
After taking a detailed, 4-day food diary, the authors measured the serum hsCRP of 212 patients at three outpatient heart failure clinics in Kentucky, Indiana, and Georgia during a 12-month follow-up.
Of the cohort, 82 patients (39%) had low vitamin C intake, as defined by the Institute of Medicine’s guidelines. In all, 98 (46%) had hsCRP greater than 3 mg/L, which is the cut-off point for being at high risk of having cardiac events. The patients with low vitamin C were 2.4 times more likely to have a high hsCRP level.
During the follow-up, 61 patients (29%) had cardiac events. After the authors controlled for age, sex, body mass index, New York Heart Association class, ejection fraction, comorbidities, total caloric intake, and medications, the results showed that the patients with low vitamin C levels were exactly twice as likely as those with adequate levels to have shorter cumulative cardiac event–free survival. Furthermore, those with hsCRP levels of at least 3 mg/L were 1.9 times as likely to have that outcome. Both differences were significant.
"Adequate intake of vitamin C was associated with longer survival in patients with heart failure."
"The data suggest that one possible mechanism by which vitamin C deficiency contributed to poor health outcomes is through inflammatory pathways in [heart failure] patients," they concluded.
"Diet is the best source of vitamin C," said Terry Lennie, Ph.D., one of the study authors and an associate dean at the University of Kentucky in Lexington. "Eating the recommended five servings of fruits and vegetables a day provides an adequate amount."
Dr. Blumenthal added that "sometimes we just talk to our patients about avoiding fast foods, and we don’t give as much positive reinforcement for things that are good for them to eat. ... We should go back to what our mothers and grandmothers told us, and try to have a more balanced diet and have more fruits and vegetables."
Dr. Lennie has received research grant from multiple extramural funders in the research area. Dr. Song and Dr. Blumenthal had no disclosures.
ORLANDO – Low vitamin C intake may worsen heart failure and put patients at a higher risk for cardiac events, according to a study conducted in three U.S. clinics.
The findings showed that patients who had low intakes of vitamin C were 2.4 times as likely to have higher levels of high-sensitivity C-reactive protein (hsCRP) – a marker for inflammation – compared with those who had high vitamin C intake. They also had shorter intervals without cardiac events.
In other words, "adequate intake of vitamin C was associated with longer survival in patients with heart failure," said Eun Kyeung Song, Ph.D., of the University of Ulsan (South Korea), who is the lead author of the study and a registered nurse.
The study, which is the first of its kind, was "provocative," and could lead to more investigations and larger studies to verify the findings, said Dr. Roger S. Blumenthal, director of preventive cardiology at Johns Hopkins University, Baltimore. He was not involved in the study.
"We generally attribute high CRP to increased weight in the midsection," said Dr. Blumenthal. "But this study suggests that it may be dietary inadequacies that may also increase inflammation and may be even a stronger predictor than just the CRP blood test by itself."
After taking a detailed, 4-day food diary, the authors measured the serum hsCRP of 212 patients at three outpatient heart failure clinics in Kentucky, Indiana, and Georgia during a 12-month follow-up.
Of the cohort, 82 patients (39%) had low vitamin C intake, as defined by the Institute of Medicine’s guidelines. In all, 98 (46%) had hsCRP greater than 3 mg/L, which is the cut-off point for being at high risk of having cardiac events. The patients with low vitamin C were 2.4 times more likely to have a high hsCRP level.
During the follow-up, 61 patients (29%) had cardiac events. After the authors controlled for age, sex, body mass index, New York Heart Association class, ejection fraction, comorbidities, total caloric intake, and medications, the results showed that the patients with low vitamin C levels were exactly twice as likely as those with adequate levels to have shorter cumulative cardiac event–free survival. Furthermore, those with hsCRP levels of at least 3 mg/L were 1.9 times as likely to have that outcome. Both differences were significant.
"Adequate intake of vitamin C was associated with longer survival in patients with heart failure."
"The data suggest that one possible mechanism by which vitamin C deficiency contributed to poor health outcomes is through inflammatory pathways in [heart failure] patients," they concluded.
"Diet is the best source of vitamin C," said Terry Lennie, Ph.D., one of the study authors and an associate dean at the University of Kentucky in Lexington. "Eating the recommended five servings of fruits and vegetables a day provides an adequate amount."
Dr. Blumenthal added that "sometimes we just talk to our patients about avoiding fast foods, and we don’t give as much positive reinforcement for things that are good for them to eat. ... We should go back to what our mothers and grandmothers told us, and try to have a more balanced diet and have more fruits and vegetables."
Dr. Lennie has received research grant from multiple extramural funders in the research area. Dr. Song and Dr. Blumenthal had no disclosures.
FROM THE ANNUAL SCIENTIFIC SESSIONS OF THE AMERICAN HEART ASSOCIATION
Major Finding: Patients who had low intakes of vitamin C were 2.4 times as likely to have higher levels of hsCRP – a marker for inflammation – compared with those who had high vitamin C intake. They also had shorter intervals without cardiac events.
Data Source: A study based on a detailed, 4-day food diary and measurement of serum hsCRP levels in 212 patients in three outpatient heart failure clinics during a 12-month follow-up.
Disclosures: Dr. Lennie has received research grants from multiple extramural funders in the research area. Dr. Song and Dr. Blumenthal had no disclosures.
SCD Screenings in Young Athletes Often Miss the Mark
ORLANDO – Although guidelines for assessing the risk of sudden cardiac death in high school athletes were first issued 15 years ago, less than 6% of physicians fully follow those guidelines, according to a survey conducted in Washington State.
Preparticipation physical evaluation is the main mechanism of screening for sudden cardiac death (SCD) in the United States, and is recognized by all national medical organizations, according to Dr. Nicolas Madsen, the survey’s lead author and a pediatric cardiology fellow at Seattle Children’s Hospital and the University of Washington School of Medicine.
The American Heart Association’s (AHA’s) guidelines, issued in 1996 and reaffirmed in 2007, recommend 12 elements for SCD screening of competitive athletes (Circulation 2007;115:1643-55). There are also websites such as ppesportsevaluation.org that provide screening forms for free.
Yet, according to the survey, 47% of physicians and only 6% of high school athletic directors were aware of the guidelines. And although 60% used a screening form, they incorporated only 8 of the 12 recommended elements. Not a single school surveyed was in complete compliance with the AHA guidelines, Dr. Madsen reported at the annual scientific sessions of the American Heart Association.
Lack of compliance with the guidelines was driven mainly by lack of knowledge, and not by location, experience, or physician specialty, said Dr. Madsen.
"New directions for provider education and policy requirements are needed to improve this implementation gap," he and his coauthors wrote.
Dr. Madsen had no disclosures. Dr. G. Paul Matherne (featured in video above) also had no disclosures.
ORLANDO – Although guidelines for assessing the risk of sudden cardiac death in high school athletes were first issued 15 years ago, less than 6% of physicians fully follow those guidelines, according to a survey conducted in Washington State.
Preparticipation physical evaluation is the main mechanism of screening for sudden cardiac death (SCD) in the United States, and is recognized by all national medical organizations, according to Dr. Nicolas Madsen, the survey’s lead author and a pediatric cardiology fellow at Seattle Children’s Hospital and the University of Washington School of Medicine.
The American Heart Association’s (AHA’s) guidelines, issued in 1996 and reaffirmed in 2007, recommend 12 elements for SCD screening of competitive athletes (Circulation 2007;115:1643-55). There are also websites such as ppesportsevaluation.org that provide screening forms for free.
Yet, according to the survey, 47% of physicians and only 6% of high school athletic directors were aware of the guidelines. And although 60% used a screening form, they incorporated only 8 of the 12 recommended elements. Not a single school surveyed was in complete compliance with the AHA guidelines, Dr. Madsen reported at the annual scientific sessions of the American Heart Association.
Lack of compliance with the guidelines was driven mainly by lack of knowledge, and not by location, experience, or physician specialty, said Dr. Madsen.
"New directions for provider education and policy requirements are needed to improve this implementation gap," he and his coauthors wrote.
Dr. Madsen had no disclosures. Dr. G. Paul Matherne (featured in video above) also had no disclosures.
ORLANDO – Although guidelines for assessing the risk of sudden cardiac death in high school athletes were first issued 15 years ago, less than 6% of physicians fully follow those guidelines, according to a survey conducted in Washington State.
Preparticipation physical evaluation is the main mechanism of screening for sudden cardiac death (SCD) in the United States, and is recognized by all national medical organizations, according to Dr. Nicolas Madsen, the survey’s lead author and a pediatric cardiology fellow at Seattle Children’s Hospital and the University of Washington School of Medicine.
The American Heart Association’s (AHA’s) guidelines, issued in 1996 and reaffirmed in 2007, recommend 12 elements for SCD screening of competitive athletes (Circulation 2007;115:1643-55). There are also websites such as ppesportsevaluation.org that provide screening forms for free.
Yet, according to the survey, 47% of physicians and only 6% of high school athletic directors were aware of the guidelines. And although 60% used a screening form, they incorporated only 8 of the 12 recommended elements. Not a single school surveyed was in complete compliance with the AHA guidelines, Dr. Madsen reported at the annual scientific sessions of the American Heart Association.
Lack of compliance with the guidelines was driven mainly by lack of knowledge, and not by location, experience, or physician specialty, said Dr. Madsen.
"New directions for provider education and policy requirements are needed to improve this implementation gap," he and his coauthors wrote.
Dr. Madsen had no disclosures. Dr. G. Paul Matherne (featured in video above) also had no disclosures.
FROM THE ANNUAL SCIENTIFIC SESSIONS OF THE AMERICAN HEART ASSOCIATION
NCAA Athletes Not Using Sunscreen
WASHINGTON – Despite high levels of sun exposure, nearly half of collegiate athletes don't use sunscreen, according to a recent survey.
The anonymous survey of 290 athletes from two universities – one on the East Coast and one on the West Coast – found that 96% of respondents believed sunscreen would help protect them from skin cancer. Yet, 43% of the athletes surveyed reported never using sunscreen, 31% reported only using sunscreen 1-3 days per week, 18% reported using sunscreen 4-6 days per week, and 8% reported using sunscreen every day of the week.
On average, the athletes reported spending 4 hours a day outdoors for 10 months a year, said Dr. Ashley Wysong, a resident in the department of dermatology at Stanford (Calif.) University.
With more than 400,000 NCAA collegiate athletes in universities across the nation, "An organized educational campaign by dermatologists, the NCAA, member universities, and individual coaches may be beneficial" in achieving better sun protection among college athletes, said Dr. Wysong, a former six-time NCAA All-American and national champion in middle distance running.
The survey, which included 13 outdoor sports, found that the percentage of athletes who wore sunscreen increased with the frequency of coaches or athletic administrators speaking with them about sun protection.
Survey respondents listed several reasons for not using sunscreen. The most common was forgetting to use it (63%), followed by inconvenience of use (41%), and "I like to be tan" (39%). Other reasons the athletes reported for not using sunscreen included: belief that they don’t burn (35%), the greasy feel of sunscreen (34%), and the time it takes for application (22%).
Not surprisingly, sunburns were common among the participants. Nearly 84% reported experiencing at least one sunburn in the past year, with 28% reporting four or more, and 10% reporting a blistering sunburn.
The majority of respondents were Fitzpatrick Skin Type I and II, but all skin types were represented in the study, the authors reported.
A few factors contributed to sunscreen use: living in the West Coast, female gender, number of sunburns in the past year, belief in the risk of skin cancer, knowing someone with skin cancer, and worry about wrinkles.
A study by researchers at the University of Cincinnati reached a similar conclusion, finding "a need for improved primary prevention of ultraviolet damage" among college athletes. The survey found that 85% of athletes at four universities reported no sunscreen use during the prior week, and only 7% reported using sunscreen at least three times during that time period (J. Am. Acad. Dermatol. 2005;53:237-41).
Dr. Wysong said that sun protective behavior extends beyond just the athletes themselves, because "they are role models for younger kids."
To follow-up their study, the authors are planning to hand out educational literature to freshmen athletes and conduct before and after questionnaires on sunscreen use.
Dr. Wysong reported having no conflicts of interest.
WASHINGTON – Despite high levels of sun exposure, nearly half of collegiate athletes don't use sunscreen, according to a recent survey.
The anonymous survey of 290 athletes from two universities – one on the East Coast and one on the West Coast – found that 96% of respondents believed sunscreen would help protect them from skin cancer. Yet, 43% of the athletes surveyed reported never using sunscreen, 31% reported only using sunscreen 1-3 days per week, 18% reported using sunscreen 4-6 days per week, and 8% reported using sunscreen every day of the week.
On average, the athletes reported spending 4 hours a day outdoors for 10 months a year, said Dr. Ashley Wysong, a resident in the department of dermatology at Stanford (Calif.) University.
With more than 400,000 NCAA collegiate athletes in universities across the nation, "An organized educational campaign by dermatologists, the NCAA, member universities, and individual coaches may be beneficial" in achieving better sun protection among college athletes, said Dr. Wysong, a former six-time NCAA All-American and national champion in middle distance running.
The survey, which included 13 outdoor sports, found that the percentage of athletes who wore sunscreen increased with the frequency of coaches or athletic administrators speaking with them about sun protection.
Survey respondents listed several reasons for not using sunscreen. The most common was forgetting to use it (63%), followed by inconvenience of use (41%), and "I like to be tan" (39%). Other reasons the athletes reported for not using sunscreen included: belief that they don’t burn (35%), the greasy feel of sunscreen (34%), and the time it takes for application (22%).
Not surprisingly, sunburns were common among the participants. Nearly 84% reported experiencing at least one sunburn in the past year, with 28% reporting four or more, and 10% reporting a blistering sunburn.
The majority of respondents were Fitzpatrick Skin Type I and II, but all skin types were represented in the study, the authors reported.
A few factors contributed to sunscreen use: living in the West Coast, female gender, number of sunburns in the past year, belief in the risk of skin cancer, knowing someone with skin cancer, and worry about wrinkles.
A study by researchers at the University of Cincinnati reached a similar conclusion, finding "a need for improved primary prevention of ultraviolet damage" among college athletes. The survey found that 85% of athletes at four universities reported no sunscreen use during the prior week, and only 7% reported using sunscreen at least three times during that time period (J. Am. Acad. Dermatol. 2005;53:237-41).
Dr. Wysong said that sun protective behavior extends beyond just the athletes themselves, because "they are role models for younger kids."
To follow-up their study, the authors are planning to hand out educational literature to freshmen athletes and conduct before and after questionnaires on sunscreen use.
Dr. Wysong reported having no conflicts of interest.
WASHINGTON – Despite high levels of sun exposure, nearly half of collegiate athletes don't use sunscreen, according to a recent survey.
The anonymous survey of 290 athletes from two universities – one on the East Coast and one on the West Coast – found that 96% of respondents believed sunscreen would help protect them from skin cancer. Yet, 43% of the athletes surveyed reported never using sunscreen, 31% reported only using sunscreen 1-3 days per week, 18% reported using sunscreen 4-6 days per week, and 8% reported using sunscreen every day of the week.
On average, the athletes reported spending 4 hours a day outdoors for 10 months a year, said Dr. Ashley Wysong, a resident in the department of dermatology at Stanford (Calif.) University.
With more than 400,000 NCAA collegiate athletes in universities across the nation, "An organized educational campaign by dermatologists, the NCAA, member universities, and individual coaches may be beneficial" in achieving better sun protection among college athletes, said Dr. Wysong, a former six-time NCAA All-American and national champion in middle distance running.
The survey, which included 13 outdoor sports, found that the percentage of athletes who wore sunscreen increased with the frequency of coaches or athletic administrators speaking with them about sun protection.
Survey respondents listed several reasons for not using sunscreen. The most common was forgetting to use it (63%), followed by inconvenience of use (41%), and "I like to be tan" (39%). Other reasons the athletes reported for not using sunscreen included: belief that they don’t burn (35%), the greasy feel of sunscreen (34%), and the time it takes for application (22%).
Not surprisingly, sunburns were common among the participants. Nearly 84% reported experiencing at least one sunburn in the past year, with 28% reporting four or more, and 10% reporting a blistering sunburn.
The majority of respondents were Fitzpatrick Skin Type I and II, but all skin types were represented in the study, the authors reported.
A few factors contributed to sunscreen use: living in the West Coast, female gender, number of sunburns in the past year, belief in the risk of skin cancer, knowing someone with skin cancer, and worry about wrinkles.
A study by researchers at the University of Cincinnati reached a similar conclusion, finding "a need for improved primary prevention of ultraviolet damage" among college athletes. The survey found that 85% of athletes at four universities reported no sunscreen use during the prior week, and only 7% reported using sunscreen at least three times during that time period (J. Am. Acad. Dermatol. 2005;53:237-41).
Dr. Wysong said that sun protective behavior extends beyond just the athletes themselves, because "they are role models for younger kids."
To follow-up their study, the authors are planning to hand out educational literature to freshmen athletes and conduct before and after questionnaires on sunscreen use.
Dr. Wysong reported having no conflicts of interest.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR DERMATOLOGIC SURGERY
Is Transcatheter Valve Replacement Transforming Cardiology?
The field of cardiology is undergoing a quiet and gradual revolution.
Emerging new technologies are bringing together subspecialties that have long been at odds with one another. Also arising is a new breed of specialists who have a new vision for cardiovascular medicine.
The procedure that lies at the heart of this movement is transcatheter valve therapy.
Although open valve replacement, a highly successful and durable treatment, remains the standard treatment for patients with severe aortic stenosis, patients too sick for surgery have few options: They are medically treated or undergo balloon aortic valvuloplasty.
But transcatheter valve replacement devices have the potential to improve the dismal landscape inoperable patients now face. Devices for transcatheter aortic valve replacement (TAVR) have been commercially available in Europe since 2007. Just this week, on Nov. 2, the Food and Drug Administration approved the first such device for use in the United States, and another is undergoing clinical trials.
"It’s a major breakthrough for [valve replacement,]" said Dr. William A. Zoghbi, the American College of Cardiology’s incoming president and the director of the Cardiovascular Imaging Institute at Methodist Hospital in Houston. "This was not present more than a decade ago, and now it’s becoming a reality. We had patients who had no other option."
What’s also significant about TAVR, experts say, is the mandated teamwork between cardiac surgeons and interventional cardiologists.
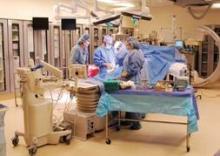
In dozens of hospitals and medical centers across the nation participating in either the Edwards Sapien valve trial or Medtronic’s CoreValve pivotal trial, cardiac surgeons, interventional cardiologists, and medical cardiologists gather almost weekly to discuss the best treatment options for patients. They perform the procedure side by side in hybrid operating rooms and learn new skills from each other.
The therapy can "change medicine forever," said Dr. Augusto D. Pichard, director of the cardiac catheterization lab at the Washington Hospital Center, which is a participant in Edwards Lifesciences’ PARTNER (Placement of Aortic Transcatheter Valve) trial of its Sapien valve (N. Engl. J. Med. 2010;363:1597-607).
"It’s proving that multidisciplinary medicine is indispensable," said Dr. Pichard, who is one of the trial’s principle investigators at the hospital.
Such multidisciplinary "Heart Teams" as required by the PARTNER trial, or Medtronic’s CoreValve trial, are not new to cardiology.
The SYNTAX trial, which compared the TAXUS drug-eluting stent with coronary artery bypass surgery, required interventional cardiologists and cardiac surgeons to review the angiography results together to decide the best treatment for patients.
Heart Teams also showed to be successful in the EVEREST II trial that compared percutaneous mitral valve repair using Abbott Vascular’s investigational MitraClip with conventional surgical repair or replacement (N. Engl. J. Med. 2011;364:1395-406).
"Such a Heart Team will be even more critical as the issue with structural heart disease become more complex, as the treatment expands to more centers, and as new technology is applied outside of the constraints of randomized clinical trials," wrote Dr. David R. Holmes Jr. of Mayo Clinic, Rochester, Minn., who is president of the ACC, and Dr. Michael J. Mack of Medical City Dallas Hospital, who is president of the Society of Thoracic Surgeons, in an expert consensus document on transcatheter valve therapy. (J. Am. Coll. Cardiol. 2011;58:445-55).
Transcatheter valve therapy is a "transformational technology," that is, "one that, when introduced, radically changes markets, creates wholly new markets, or could even eliminate existing markets for older technology," they wrote.
Having gotten a glimpse into the future through trials such as PARTNER, Dr. Mathew Williams, one of a new breed of so-called hybrid cardiovascular specialists, says it’s important to have dual training in the rapidly evolving field of invasive cardiovascular medicine.
Being a cardiac surgeon without knowing any "wire skills" is somewhat outdated. "You need to know both," said Dr. Mathew Williams.
Cardiac surgeons and interventional cardiologists "treat the same diseases and deal with the same patients. They just use different skill sets," said Dr. Williams, surgical director of Cardiovascular Transcatheter Therapies at New York–Presbyterian Hospital/Columbia University Medical Center.
He is among a handful of cardiologists who have recently trained as both cardiac surgeons and interventional cardiologists.
After completing his training in cardiac surgery, he completed a 1-year fellowship in interventional cardiology.
"I decided to do it because that’s where a lot of valve diseases are headed," said the 41-year-old in an interview. Being a cardiac surgeon without knowing any "wire skills" is somewhat outdated. "You need to know both."
In addition, "being trained in both areas will take out the financial and political battles," he said. "It will help you become the least biased decision maker."
The approval of the Edwards Sapien valve was based the results from PARTNER's Cohort B, comprising inoperable patients with severe aortic stenosis, the proportion of survival at 1 year was 50.3% for the 179 control patients and 69.3% for the 179 patients assigned to the Sapien device, a significant difference. The median survival in years was 0.97 in the control group and 2.18 in the treated group, also a significant difference. These results swayed the FDA’s Circulatory System Devices Panel to recommend the Sapien model 9000TFX, sizes 23 mm and 26 mm, for approval, despite a high neurologic event rate (stroke and transient ischemic heart attack). In anticipation of an FDA approval, the Centers for Medicare and Medicaid Services has already opened a national coverage determination analysis for TAVI and is expected to issue its decision in March 2012.
Meanwhile, inoperable aortic stenosis patients are being enrolled in the PARTNER II trial of the next-generation Sapien XT valve, which has already supplanted the first-generation Sapien in Europe.
But while Heart Teams are excited about the potential of stent-valve systems, they are aware of some apprehension, especially among some cardiovascular surgeons who have long been the gatekeepers for valve replacement surgeries.
"The rationale for having both the cardiologist and surgeon involved is that one acts as the check for the other. So that every time the cardiologist says I can stick one of those in there, it wouldn’t be easy. It takes judgment, precision, preparation, work-up and evaluation. It takes a very takes a very long time," said Dr. Alan H. Markowitz, director of the Heart Valve Center at the University Hospitals Case Medical Center, Cleveland. His center is participating in the Medtronic CoreValve U.S. Pivotal Trial. "Surgeons have to understand it, get involved in it, or it will pass them by."
Some experts outside the TAVR realm are skeptical that the interdisciplinary teams will change the trajectory of cardiology. "The collaboration that these cardiovascular specialists are enjoying is generated by the trial protocol itself. So whether this collegiality will spill over into cardiovascular science in a larger sense remains to be seen," commented Dr. Sidney Goldstein, professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. Regarding the merits of the new treatment, "TAVR represents an important step in treating high risk aortic patients. It does carry a significant risk of stroke, and its performance against standard AVR in less critically ill patients has not been determined."
The two valves in U.S. trials have more than one route of implantation. The Edwards Sapien valve can be implanted through a transfemoral or transapical route. The CoreValve routes include transfemoral, subclavian, and direct aortic access. A cardiac surgeon and interventional cardiologist collaborate and are present during all procedures.
There are still many unknowns, among them the durability of the devices or whether the patient criteria will expand beyond the "sickest of the sick," as it already has in Europe.
"This is just the very beginning," said Dr. Paul Joseph Corso, director of cardiac surgery at Washington Hospital Center and the co-principle investigator for the PARTNER trial. "We’re not going to have turf wars that will be to the detriment of the patients."
Dr. Holmes, Dr. Mack, and Dr. Zoghbi said they had no relevant disclosures. Dr. Williams, Dr. Corso, and Dr. Pichard are involved in the PARTNER trial; Dr. Pichard has received honoraria from Edwards Lifesciences as a proctor for percutaneous aortic valves. Dr. Markowitz is involved in the CoreValve trial.
The field of cardiology is undergoing a quiet and gradual revolution.
Emerging new technologies are bringing together subspecialties that have long been at odds with one another. Also arising is a new breed of specialists who have a new vision for cardiovascular medicine.
The procedure that lies at the heart of this movement is transcatheter valve therapy.
Although open valve replacement, a highly successful and durable treatment, remains the standard treatment for patients with severe aortic stenosis, patients too sick for surgery have few options: They are medically treated or undergo balloon aortic valvuloplasty.
But transcatheter valve replacement devices have the potential to improve the dismal landscape inoperable patients now face. Devices for transcatheter aortic valve replacement (TAVR) have been commercially available in Europe since 2007. Just this week, on Nov. 2, the Food and Drug Administration approved the first such device for use in the United States, and another is undergoing clinical trials.
"It’s a major breakthrough for [valve replacement,]" said Dr. William A. Zoghbi, the American College of Cardiology’s incoming president and the director of the Cardiovascular Imaging Institute at Methodist Hospital in Houston. "This was not present more than a decade ago, and now it’s becoming a reality. We had patients who had no other option."
What’s also significant about TAVR, experts say, is the mandated teamwork between cardiac surgeons and interventional cardiologists.
In dozens of hospitals and medical centers across the nation participating in either the Edwards Sapien valve trial or Medtronic’s CoreValve pivotal trial, cardiac surgeons, interventional cardiologists, and medical cardiologists gather almost weekly to discuss the best treatment options for patients. They perform the procedure side by side in hybrid operating rooms and learn new skills from each other.
The therapy can "change medicine forever," said Dr. Augusto D. Pichard, director of the cardiac catheterization lab at the Washington Hospital Center, which is a participant in Edwards Lifesciences’ PARTNER (Placement of Aortic Transcatheter Valve) trial of its Sapien valve (N. Engl. J. Med. 2010;363:1597-607).
"It’s proving that multidisciplinary medicine is indispensable," said Dr. Pichard, who is one of the trial’s principle investigators at the hospital.
Such multidisciplinary "Heart Teams" as required by the PARTNER trial, or Medtronic’s CoreValve trial, are not new to cardiology.
The SYNTAX trial, which compared the TAXUS drug-eluting stent with coronary artery bypass surgery, required interventional cardiologists and cardiac surgeons to review the angiography results together to decide the best treatment for patients.
Heart Teams also showed to be successful in the EVEREST II trial that compared percutaneous mitral valve repair using Abbott Vascular’s investigational MitraClip with conventional surgical repair or replacement (N. Engl. J. Med. 2011;364:1395-406).
"Such a Heart Team will be even more critical as the issue with structural heart disease become more complex, as the treatment expands to more centers, and as new technology is applied outside of the constraints of randomized clinical trials," wrote Dr. David R. Holmes Jr. of Mayo Clinic, Rochester, Minn., who is president of the ACC, and Dr. Michael J. Mack of Medical City Dallas Hospital, who is president of the Society of Thoracic Surgeons, in an expert consensus document on transcatheter valve therapy. (J. Am. Coll. Cardiol. 2011;58:445-55).
Transcatheter valve therapy is a "transformational technology," that is, "one that, when introduced, radically changes markets, creates wholly new markets, or could even eliminate existing markets for older technology," they wrote.
Having gotten a glimpse into the future through trials such as PARTNER, Dr. Mathew Williams, one of a new breed of so-called hybrid cardiovascular specialists, says it’s important to have dual training in the rapidly evolving field of invasive cardiovascular medicine.
Being a cardiac surgeon without knowing any "wire skills" is somewhat outdated. "You need to know both," said Dr. Mathew Williams.
Cardiac surgeons and interventional cardiologists "treat the same diseases and deal with the same patients. They just use different skill sets," said Dr. Williams, surgical director of Cardiovascular Transcatheter Therapies at New York–Presbyterian Hospital/Columbia University Medical Center.
He is among a handful of cardiologists who have recently trained as both cardiac surgeons and interventional cardiologists.
After completing his training in cardiac surgery, he completed a 1-year fellowship in interventional cardiology.
"I decided to do it because that’s where a lot of valve diseases are headed," said the 41-year-old in an interview. Being a cardiac surgeon without knowing any "wire skills" is somewhat outdated. "You need to know both."
In addition, "being trained in both areas will take out the financial and political battles," he said. "It will help you become the least biased decision maker."
The approval of the Edwards Sapien valve was based the results from PARTNER's Cohort B, comprising inoperable patients with severe aortic stenosis, the proportion of survival at 1 year was 50.3% for the 179 control patients and 69.3% for the 179 patients assigned to the Sapien device, a significant difference. The median survival in years was 0.97 in the control group and 2.18 in the treated group, also a significant difference. These results swayed the FDA’s Circulatory System Devices Panel to recommend the Sapien model 9000TFX, sizes 23 mm and 26 mm, for approval, despite a high neurologic event rate (stroke and transient ischemic heart attack). In anticipation of an FDA approval, the Centers for Medicare and Medicaid Services has already opened a national coverage determination analysis for TAVI and is expected to issue its decision in March 2012.
Meanwhile, inoperable aortic stenosis patients are being enrolled in the PARTNER II trial of the next-generation Sapien XT valve, which has already supplanted the first-generation Sapien in Europe.
But while Heart Teams are excited about the potential of stent-valve systems, they are aware of some apprehension, especially among some cardiovascular surgeons who have long been the gatekeepers for valve replacement surgeries.
"The rationale for having both the cardiologist and surgeon involved is that one acts as the check for the other. So that every time the cardiologist says I can stick one of those in there, it wouldn’t be easy. It takes judgment, precision, preparation, work-up and evaluation. It takes a very takes a very long time," said Dr. Alan H. Markowitz, director of the Heart Valve Center at the University Hospitals Case Medical Center, Cleveland. His center is participating in the Medtronic CoreValve U.S. Pivotal Trial. "Surgeons have to understand it, get involved in it, or it will pass them by."
Some experts outside the TAVR realm are skeptical that the interdisciplinary teams will change the trajectory of cardiology. "The collaboration that these cardiovascular specialists are enjoying is generated by the trial protocol itself. So whether this collegiality will spill over into cardiovascular science in a larger sense remains to be seen," commented Dr. Sidney Goldstein, professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. Regarding the merits of the new treatment, "TAVR represents an important step in treating high risk aortic patients. It does carry a significant risk of stroke, and its performance against standard AVR in less critically ill patients has not been determined."
The two valves in U.S. trials have more than one route of implantation. The Edwards Sapien valve can be implanted through a transfemoral or transapical route. The CoreValve routes include transfemoral, subclavian, and direct aortic access. A cardiac surgeon and interventional cardiologist collaborate and are present during all procedures.
There are still many unknowns, among them the durability of the devices or whether the patient criteria will expand beyond the "sickest of the sick," as it already has in Europe.
"This is just the very beginning," said Dr. Paul Joseph Corso, director of cardiac surgery at Washington Hospital Center and the co-principle investigator for the PARTNER trial. "We’re not going to have turf wars that will be to the detriment of the patients."
Dr. Holmes, Dr. Mack, and Dr. Zoghbi said they had no relevant disclosures. Dr. Williams, Dr. Corso, and Dr. Pichard are involved in the PARTNER trial; Dr. Pichard has received honoraria from Edwards Lifesciences as a proctor for percutaneous aortic valves. Dr. Markowitz is involved in the CoreValve trial.
The field of cardiology is undergoing a quiet and gradual revolution.
Emerging new technologies are bringing together subspecialties that have long been at odds with one another. Also arising is a new breed of specialists who have a new vision for cardiovascular medicine.
The procedure that lies at the heart of this movement is transcatheter valve therapy.
Although open valve replacement, a highly successful and durable treatment, remains the standard treatment for patients with severe aortic stenosis, patients too sick for surgery have few options: They are medically treated or undergo balloon aortic valvuloplasty.
But transcatheter valve replacement devices have the potential to improve the dismal landscape inoperable patients now face. Devices for transcatheter aortic valve replacement (TAVR) have been commercially available in Europe since 2007. Just this week, on Nov. 2, the Food and Drug Administration approved the first such device for use in the United States, and another is undergoing clinical trials.
"It’s a major breakthrough for [valve replacement,]" said Dr. William A. Zoghbi, the American College of Cardiology’s incoming president and the director of the Cardiovascular Imaging Institute at Methodist Hospital in Houston. "This was not present more than a decade ago, and now it’s becoming a reality. We had patients who had no other option."
What’s also significant about TAVR, experts say, is the mandated teamwork between cardiac surgeons and interventional cardiologists.
In dozens of hospitals and medical centers across the nation participating in either the Edwards Sapien valve trial or Medtronic’s CoreValve pivotal trial, cardiac surgeons, interventional cardiologists, and medical cardiologists gather almost weekly to discuss the best treatment options for patients. They perform the procedure side by side in hybrid operating rooms and learn new skills from each other.
The therapy can "change medicine forever," said Dr. Augusto D. Pichard, director of the cardiac catheterization lab at the Washington Hospital Center, which is a participant in Edwards Lifesciences’ PARTNER (Placement of Aortic Transcatheter Valve) trial of its Sapien valve (N. Engl. J. Med. 2010;363:1597-607).
"It’s proving that multidisciplinary medicine is indispensable," said Dr. Pichard, who is one of the trial’s principle investigators at the hospital.
Such multidisciplinary "Heart Teams" as required by the PARTNER trial, or Medtronic’s CoreValve trial, are not new to cardiology.
The SYNTAX trial, which compared the TAXUS drug-eluting stent with coronary artery bypass surgery, required interventional cardiologists and cardiac surgeons to review the angiography results together to decide the best treatment for patients.
Heart Teams also showed to be successful in the EVEREST II trial that compared percutaneous mitral valve repair using Abbott Vascular’s investigational MitraClip with conventional surgical repair or replacement (N. Engl. J. Med. 2011;364:1395-406).
"Such a Heart Team will be even more critical as the issue with structural heart disease become more complex, as the treatment expands to more centers, and as new technology is applied outside of the constraints of randomized clinical trials," wrote Dr. David R. Holmes Jr. of Mayo Clinic, Rochester, Minn., who is president of the ACC, and Dr. Michael J. Mack of Medical City Dallas Hospital, who is president of the Society of Thoracic Surgeons, in an expert consensus document on transcatheter valve therapy. (J. Am. Coll. Cardiol. 2011;58:445-55).
Transcatheter valve therapy is a "transformational technology," that is, "one that, when introduced, radically changes markets, creates wholly new markets, or could even eliminate existing markets for older technology," they wrote.
Having gotten a glimpse into the future through trials such as PARTNER, Dr. Mathew Williams, one of a new breed of so-called hybrid cardiovascular specialists, says it’s important to have dual training in the rapidly evolving field of invasive cardiovascular medicine.
Being a cardiac surgeon without knowing any "wire skills" is somewhat outdated. "You need to know both," said Dr. Mathew Williams.
Cardiac surgeons and interventional cardiologists "treat the same diseases and deal with the same patients. They just use different skill sets," said Dr. Williams, surgical director of Cardiovascular Transcatheter Therapies at New York–Presbyterian Hospital/Columbia University Medical Center.
He is among a handful of cardiologists who have recently trained as both cardiac surgeons and interventional cardiologists.
After completing his training in cardiac surgery, he completed a 1-year fellowship in interventional cardiology.
"I decided to do it because that’s where a lot of valve diseases are headed," said the 41-year-old in an interview. Being a cardiac surgeon without knowing any "wire skills" is somewhat outdated. "You need to know both."
In addition, "being trained in both areas will take out the financial and political battles," he said. "It will help you become the least biased decision maker."
The approval of the Edwards Sapien valve was based the results from PARTNER's Cohort B, comprising inoperable patients with severe aortic stenosis, the proportion of survival at 1 year was 50.3% for the 179 control patients and 69.3% for the 179 patients assigned to the Sapien device, a significant difference. The median survival in years was 0.97 in the control group and 2.18 in the treated group, also a significant difference. These results swayed the FDA’s Circulatory System Devices Panel to recommend the Sapien model 9000TFX, sizes 23 mm and 26 mm, for approval, despite a high neurologic event rate (stroke and transient ischemic heart attack). In anticipation of an FDA approval, the Centers for Medicare and Medicaid Services has already opened a national coverage determination analysis for TAVI and is expected to issue its decision in March 2012.
Meanwhile, inoperable aortic stenosis patients are being enrolled in the PARTNER II trial of the next-generation Sapien XT valve, which has already supplanted the first-generation Sapien in Europe.
But while Heart Teams are excited about the potential of stent-valve systems, they are aware of some apprehension, especially among some cardiovascular surgeons who have long been the gatekeepers for valve replacement surgeries.
"The rationale for having both the cardiologist and surgeon involved is that one acts as the check for the other. So that every time the cardiologist says I can stick one of those in there, it wouldn’t be easy. It takes judgment, precision, preparation, work-up and evaluation. It takes a very takes a very long time," said Dr. Alan H. Markowitz, director of the Heart Valve Center at the University Hospitals Case Medical Center, Cleveland. His center is participating in the Medtronic CoreValve U.S. Pivotal Trial. "Surgeons have to understand it, get involved in it, or it will pass them by."
Some experts outside the TAVR realm are skeptical that the interdisciplinary teams will change the trajectory of cardiology. "The collaboration that these cardiovascular specialists are enjoying is generated by the trial protocol itself. So whether this collegiality will spill over into cardiovascular science in a larger sense remains to be seen," commented Dr. Sidney Goldstein, professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. Regarding the merits of the new treatment, "TAVR represents an important step in treating high risk aortic patients. It does carry a significant risk of stroke, and its performance against standard AVR in less critically ill patients has not been determined."
The two valves in U.S. trials have more than one route of implantation. The Edwards Sapien valve can be implanted through a transfemoral or transapical route. The CoreValve routes include transfemoral, subclavian, and direct aortic access. A cardiac surgeon and interventional cardiologist collaborate and are present during all procedures.
There are still many unknowns, among them the durability of the devices or whether the patient criteria will expand beyond the "sickest of the sick," as it already has in Europe.
"This is just the very beginning," said Dr. Paul Joseph Corso, director of cardiac surgery at Washington Hospital Center and the co-principle investigator for the PARTNER trial. "We’re not going to have turf wars that will be to the detriment of the patients."
Dr. Holmes, Dr. Mack, and Dr. Zoghbi said they had no relevant disclosures. Dr. Williams, Dr. Corso, and Dr. Pichard are involved in the PARTNER trial; Dr. Pichard has received honoraria from Edwards Lifesciences as a proctor for percutaneous aortic valves. Dr. Markowitz is involved in the CoreValve trial.
FDA Approves First Percutanous Aortic Valve
The first artificial valve for percutaneous placement in stenotic aortic valves was approved today by the Food and Drug Administration.
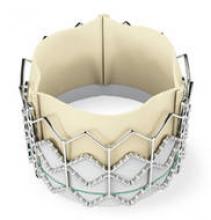
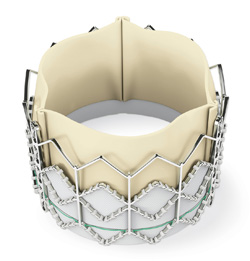
The Sapien transcatheter heart valve, made by Edwards Lifesciences, could be a treatment option for patients who have severe aortic stenosis and are deemed too sick to undergo surgical heart valve replacement.
The long-term durability of the valve is not known, and the information about restrictions on which centers are allowed to perform the procedure is yet to emerge.
In anticipation of the FDA approval, the Centers for Medicare and Medicaid Services opened a national coverage determination analysis for transcatheter valve replacement. CMS opened the coverage analysis in response to a joint request by the Society of Thoracic Surgeons and the American College of Cardiology. The groups argue that only cardiologists with appropriate training and credentials, working at specialized heart centers, should be performing transcatheter valve replacement.
So far, one of the clear requirements has been the presence and collaboration of interventional cardiologists and cardiac surgeons in patient selection as well as the procedure. The valve-stent system is placed in the patient’s beating heart by a multidisciplinary team at a hybrid operating room or a hybrid catheterization laboratory.
The device’s label was not available at the time of the FDA announcement. According to the agency’s statement, "The Sapien THV is approved for patients who are not eligible for open-heart surgery for replacement of their aortic valve and have a calcified aortic annulus. ... The product label advises that a heart surgeon should be involved in determining if the Sapien THV is an appropriate treatment for the patient. It is not approved for patients who can be treated by open-heart surgery. Patients who have congenital heart valve anomalies, have masses or an infection in their hearts, or cannot tolerate anticoagulation/antiplatelet therapy should not receive the Sapien THV."
Transcatheter aortic valve replacement, or TAVR, has been commercially available in Europe since 2007, and has been undergoing clinical trials in the United States. The first trial to launch in the United States was Edwards’ Placement of Aortic Transcatheter Valve Trial, or PARTNER.
Another trial is underway for Medtronic’s CoreValve.
The Sapien valve was approved on the basis of results from the Cohort B of PARTNER, which showed that TAVR improved patients’ quality of life and decreased mortality at 30 days and 1 year.
However, results also showed that patients who received TAVR had a 2.5-fold higher rate of strokes and an 8-fold higher rate of vascular and bleeding complications as compared to patients who did not receive the implant, according to an FDA statement.
Edwards is also pursuing an indication for patients with severe aortic stenosis who are at high risk for surgical complications (Cohort A of the PARTNER trial), on the basis of positive results released in March.
The first artificial valve for percutaneous placement in stenotic aortic valves was approved today by the Food and Drug Administration.
The Sapien transcatheter heart valve, made by Edwards Lifesciences, could be a treatment option for patients who have severe aortic stenosis and are deemed too sick to undergo surgical heart valve replacement.
The long-term durability of the valve is not known, and the information about restrictions on which centers are allowed to perform the procedure is yet to emerge.
In anticipation of the FDA approval, the Centers for Medicare and Medicaid Services opened a national coverage determination analysis for transcatheter valve replacement. CMS opened the coverage analysis in response to a joint request by the Society of Thoracic Surgeons and the American College of Cardiology. The groups argue that only cardiologists with appropriate training and credentials, working at specialized heart centers, should be performing transcatheter valve replacement.
So far, one of the clear requirements has been the presence and collaboration of interventional cardiologists and cardiac surgeons in patient selection as well as the procedure. The valve-stent system is placed in the patient’s beating heart by a multidisciplinary team at a hybrid operating room or a hybrid catheterization laboratory.
The device’s label was not available at the time of the FDA announcement. According to the agency’s statement, "The Sapien THV is approved for patients who are not eligible for open-heart surgery for replacement of their aortic valve and have a calcified aortic annulus. ... The product label advises that a heart surgeon should be involved in determining if the Sapien THV is an appropriate treatment for the patient. It is not approved for patients who can be treated by open-heart surgery. Patients who have congenital heart valve anomalies, have masses or an infection in their hearts, or cannot tolerate anticoagulation/antiplatelet therapy should not receive the Sapien THV."
Transcatheter aortic valve replacement, or TAVR, has been commercially available in Europe since 2007, and has been undergoing clinical trials in the United States. The first trial to launch in the United States was Edwards’ Placement of Aortic Transcatheter Valve Trial, or PARTNER.
Another trial is underway for Medtronic’s CoreValve.
The Sapien valve was approved on the basis of results from the Cohort B of PARTNER, which showed that TAVR improved patients’ quality of life and decreased mortality at 30 days and 1 year.
However, results also showed that patients who received TAVR had a 2.5-fold higher rate of strokes and an 8-fold higher rate of vascular and bleeding complications as compared to patients who did not receive the implant, according to an FDA statement.
Edwards is also pursuing an indication for patients with severe aortic stenosis who are at high risk for surgical complications (Cohort A of the PARTNER trial), on the basis of positive results released in March.
The first artificial valve for percutaneous placement in stenotic aortic valves was approved today by the Food and Drug Administration.
The Sapien transcatheter heart valve, made by Edwards Lifesciences, could be a treatment option for patients who have severe aortic stenosis and are deemed too sick to undergo surgical heart valve replacement.
The long-term durability of the valve is not known, and the information about restrictions on which centers are allowed to perform the procedure is yet to emerge.
In anticipation of the FDA approval, the Centers for Medicare and Medicaid Services opened a national coverage determination analysis for transcatheter valve replacement. CMS opened the coverage analysis in response to a joint request by the Society of Thoracic Surgeons and the American College of Cardiology. The groups argue that only cardiologists with appropriate training and credentials, working at specialized heart centers, should be performing transcatheter valve replacement.
So far, one of the clear requirements has been the presence and collaboration of interventional cardiologists and cardiac surgeons in patient selection as well as the procedure. The valve-stent system is placed in the patient’s beating heart by a multidisciplinary team at a hybrid operating room or a hybrid catheterization laboratory.
The device’s label was not available at the time of the FDA announcement. According to the agency’s statement, "The Sapien THV is approved for patients who are not eligible for open-heart surgery for replacement of their aortic valve and have a calcified aortic annulus. ... The product label advises that a heart surgeon should be involved in determining if the Sapien THV is an appropriate treatment for the patient. It is not approved for patients who can be treated by open-heart surgery. Patients who have congenital heart valve anomalies, have masses or an infection in their hearts, or cannot tolerate anticoagulation/antiplatelet therapy should not receive the Sapien THV."
Transcatheter aortic valve replacement, or TAVR, has been commercially available in Europe since 2007, and has been undergoing clinical trials in the United States. The first trial to launch in the United States was Edwards’ Placement of Aortic Transcatheter Valve Trial, or PARTNER.
Another trial is underway for Medtronic’s CoreValve.
The Sapien valve was approved on the basis of results from the Cohort B of PARTNER, which showed that TAVR improved patients’ quality of life and decreased mortality at 30 days and 1 year.
However, results also showed that patients who received TAVR had a 2.5-fold higher rate of strokes and an 8-fold higher rate of vascular and bleeding complications as compared to patients who did not receive the implant, according to an FDA statement.
Edwards is also pursuing an indication for patients with severe aortic stenosis who are at high risk for surgical complications (Cohort A of the PARTNER trial), on the basis of positive results released in March.
Policy & Practice : Want more health reform news? Subscribe to our podcast – search 'Policy & Practice' in the iTunes store
Neighborhoods Affect Health
Where people live directly affects their development of obesity and diabetes, according to a study in the New England Journal of Medicine. Researchers looked at 4,500 women who lived in low-income neighborhoods in the mid-1990s and tracked their weight and diabetes rates, by whether they stayed in those neighborhoods or used a government voucher to move to wealthier ones. Those who moved were less likely to be morbidly obese or have diabetes. The study has some limitations, and it doesn't prove cause and effect, the researchers acknowledged. But the results, with earlier research, “raise the possibility that clinical or public health interventions that ameliorate the effects of neighborhood environment on obesity and diabetes could generate substantial social benefits,” they concluded.
Shortage of Insulin Pens
The worldwide shortage of the insulin injection pen Apidra SoloSTAR, which began in late October, will continue until the first quarter of 2012, according to maker Sanofi. It blamed the problem on a “technical incident” in July at a factory in Frankfurt, Germany. “Our investigation and controls have confirmed that [the company's pens] currently on the market is not affected by the event,” according to a company statement. Sanofi has advised patients and providers to consider Apidra vials instead since the dose and timing of injections don't need to be changed. “Please use your professional judgment on the need for patient training and guidance on syringe use to facilitate administration,” the company advised.
Health by Text Messaging
The McKesson Foundation has granted more than $1 million to six groups to study the impact of mobile phones on health and their potential to promote healthy living. For instance, the Center for Connected Health will study the effect of text messaging on the clinical outcomes and physical activity of people with type 2 diabetes living in medically underserved areas. In previous work, the center has created texts conveying motivational and educational messages that coach the patients to increase physical activity. “Our experience with text messaging programs in underserved patient populations is demonstrating great potential for providing low-cost, accessible educational messaging to patients,” the center's director, Dr. Joseph C. Kvedar, said in a statement.
Wound-Care Product Cleared
The Food and Drug Administration has approved Cardium Therapeutics's collagen-based topical gel for management of diabetic foot ulcers and other dermal wounds. Called Excellagen, the product is a sterile, fibrillar, flowable bovine collagen gel. It will be initially marketed in syringes for professional use immediately after surgical debridement. The product will “address the large and rapidly growing advanced wound care market,” said company Chairman and CEO Christopher J. Reinhard in a statement.
Noninfectious Diseases Targeted
The United Nations has launched an “all-out attack” on “noncommunicable” diseases such as cancer and diabetes. Tobacco and alcohol use contributes to noncommunicable diseases, which cause 63% of all deaths worldwide, according to the U.N.'s announcement. At a 2-day meeting, the General Assembly adopted a declaration calling for price and tax measures to reduce alcohol consumption; new curbs on marketing unhealthy foods to children; and measures to increase healthy diets and physical exercise. The declaration also highlights the need for universal national health coverage, along with strengthened international cooperation to prevent deaths from noncommunicable diseases in developing countries.
Panel: Patients' Needs Overlooked
Even though most doctors realize that improving patient engagement can reduce costs and improve the quality of care, physicians still frequently overlook patients' needs and concerns, according to a report from the Institute of Medicine. For example, studies show that care improves when providers listen carefully to patients and their families, according to the report based on an April workshop. However, research has shown that physicians typically interrupt within 15 seconds when patients begin to raise their concerns. Patient engagement can be improved by providing patients with clear information on the benefits and downsides of potential treatments, the report said.
Nearly a Trillion Saved
The Generic Pharmaceutical Association said a new study shows generic drugs have saved the United States health care system $931 billion over the past decade. IMS Institute for Healthcare Informatics conducted the study for the industry association. It showed that in 2010, generic drug use saved $158 billion, the association said. Its president and CEO, Ralph Neas, said the analysis should prompt policy makers to increase use of generics. The report shows, for instance, that Medicaid could save $1.3 billion a year by increasing generic use from the current 70% of prescriptions to 72%.
Neighborhoods Affect Health
Where people live directly affects their development of obesity and diabetes, according to a study in the New England Journal of Medicine. Researchers looked at 4,500 women who lived in low-income neighborhoods in the mid-1990s and tracked their weight and diabetes rates, by whether they stayed in those neighborhoods or used a government voucher to move to wealthier ones. Those who moved were less likely to be morbidly obese or have diabetes. The study has some limitations, and it doesn't prove cause and effect, the researchers acknowledged. But the results, with earlier research, “raise the possibility that clinical or public health interventions that ameliorate the effects of neighborhood environment on obesity and diabetes could generate substantial social benefits,” they concluded.
Shortage of Insulin Pens
The worldwide shortage of the insulin injection pen Apidra SoloSTAR, which began in late October, will continue until the first quarter of 2012, according to maker Sanofi. It blamed the problem on a “technical incident” in July at a factory in Frankfurt, Germany. “Our investigation and controls have confirmed that [the company's pens] currently on the market is not affected by the event,” according to a company statement. Sanofi has advised patients and providers to consider Apidra vials instead since the dose and timing of injections don't need to be changed. “Please use your professional judgment on the need for patient training and guidance on syringe use to facilitate administration,” the company advised.
Health by Text Messaging
The McKesson Foundation has granted more than $1 million to six groups to study the impact of mobile phones on health and their potential to promote healthy living. For instance, the Center for Connected Health will study the effect of text messaging on the clinical outcomes and physical activity of people with type 2 diabetes living in medically underserved areas. In previous work, the center has created texts conveying motivational and educational messages that coach the patients to increase physical activity. “Our experience with text messaging programs in underserved patient populations is demonstrating great potential for providing low-cost, accessible educational messaging to patients,” the center's director, Dr. Joseph C. Kvedar, said in a statement.
Wound-Care Product Cleared
The Food and Drug Administration has approved Cardium Therapeutics's collagen-based topical gel for management of diabetic foot ulcers and other dermal wounds. Called Excellagen, the product is a sterile, fibrillar, flowable bovine collagen gel. It will be initially marketed in syringes for professional use immediately after surgical debridement. The product will “address the large and rapidly growing advanced wound care market,” said company Chairman and CEO Christopher J. Reinhard in a statement.
Noninfectious Diseases Targeted
The United Nations has launched an “all-out attack” on “noncommunicable” diseases such as cancer and diabetes. Tobacco and alcohol use contributes to noncommunicable diseases, which cause 63% of all deaths worldwide, according to the U.N.'s announcement. At a 2-day meeting, the General Assembly adopted a declaration calling for price and tax measures to reduce alcohol consumption; new curbs on marketing unhealthy foods to children; and measures to increase healthy diets and physical exercise. The declaration also highlights the need for universal national health coverage, along with strengthened international cooperation to prevent deaths from noncommunicable diseases in developing countries.
Panel: Patients' Needs Overlooked
Even though most doctors realize that improving patient engagement can reduce costs and improve the quality of care, physicians still frequently overlook patients' needs and concerns, according to a report from the Institute of Medicine. For example, studies show that care improves when providers listen carefully to patients and their families, according to the report based on an April workshop. However, research has shown that physicians typically interrupt within 15 seconds when patients begin to raise their concerns. Patient engagement can be improved by providing patients with clear information on the benefits and downsides of potential treatments, the report said.
Nearly a Trillion Saved
The Generic Pharmaceutical Association said a new study shows generic drugs have saved the United States health care system $931 billion over the past decade. IMS Institute for Healthcare Informatics conducted the study for the industry association. It showed that in 2010, generic drug use saved $158 billion, the association said. Its president and CEO, Ralph Neas, said the analysis should prompt policy makers to increase use of generics. The report shows, for instance, that Medicaid could save $1.3 billion a year by increasing generic use from the current 70% of prescriptions to 72%.
Neighborhoods Affect Health
Where people live directly affects their development of obesity and diabetes, according to a study in the New England Journal of Medicine. Researchers looked at 4,500 women who lived in low-income neighborhoods in the mid-1990s and tracked their weight and diabetes rates, by whether they stayed in those neighborhoods or used a government voucher to move to wealthier ones. Those who moved were less likely to be morbidly obese or have diabetes. The study has some limitations, and it doesn't prove cause and effect, the researchers acknowledged. But the results, with earlier research, “raise the possibility that clinical or public health interventions that ameliorate the effects of neighborhood environment on obesity and diabetes could generate substantial social benefits,” they concluded.
Shortage of Insulin Pens
The worldwide shortage of the insulin injection pen Apidra SoloSTAR, which began in late October, will continue until the first quarter of 2012, according to maker Sanofi. It blamed the problem on a “technical incident” in July at a factory in Frankfurt, Germany. “Our investigation and controls have confirmed that [the company's pens] currently on the market is not affected by the event,” according to a company statement. Sanofi has advised patients and providers to consider Apidra vials instead since the dose and timing of injections don't need to be changed. “Please use your professional judgment on the need for patient training and guidance on syringe use to facilitate administration,” the company advised.
Health by Text Messaging
The McKesson Foundation has granted more than $1 million to six groups to study the impact of mobile phones on health and their potential to promote healthy living. For instance, the Center for Connected Health will study the effect of text messaging on the clinical outcomes and physical activity of people with type 2 diabetes living in medically underserved areas. In previous work, the center has created texts conveying motivational and educational messages that coach the patients to increase physical activity. “Our experience with text messaging programs in underserved patient populations is demonstrating great potential for providing low-cost, accessible educational messaging to patients,” the center's director, Dr. Joseph C. Kvedar, said in a statement.
Wound-Care Product Cleared
The Food and Drug Administration has approved Cardium Therapeutics's collagen-based topical gel for management of diabetic foot ulcers and other dermal wounds. Called Excellagen, the product is a sterile, fibrillar, flowable bovine collagen gel. It will be initially marketed in syringes for professional use immediately after surgical debridement. The product will “address the large and rapidly growing advanced wound care market,” said company Chairman and CEO Christopher J. Reinhard in a statement.
Noninfectious Diseases Targeted
The United Nations has launched an “all-out attack” on “noncommunicable” diseases such as cancer and diabetes. Tobacco and alcohol use contributes to noncommunicable diseases, which cause 63% of all deaths worldwide, according to the U.N.'s announcement. At a 2-day meeting, the General Assembly adopted a declaration calling for price and tax measures to reduce alcohol consumption; new curbs on marketing unhealthy foods to children; and measures to increase healthy diets and physical exercise. The declaration also highlights the need for universal national health coverage, along with strengthened international cooperation to prevent deaths from noncommunicable diseases in developing countries.
Panel: Patients' Needs Overlooked
Even though most doctors realize that improving patient engagement can reduce costs and improve the quality of care, physicians still frequently overlook patients' needs and concerns, according to a report from the Institute of Medicine. For example, studies show that care improves when providers listen carefully to patients and their families, according to the report based on an April workshop. However, research has shown that physicians typically interrupt within 15 seconds when patients begin to raise their concerns. Patient engagement can be improved by providing patients with clear information on the benefits and downsides of potential treatments, the report said.
Nearly a Trillion Saved
The Generic Pharmaceutical Association said a new study shows generic drugs have saved the United States health care system $931 billion over the past decade. IMS Institute for Healthcare Informatics conducted the study for the industry association. It showed that in 2010, generic drug use saved $158 billion, the association said. Its president and CEO, Ralph Neas, said the analysis should prompt policy makers to increase use of generics. The report shows, for instance, that Medicaid could save $1.3 billion a year by increasing generic use from the current 70% of prescriptions to 72%.
Policy & Practice : Want more health reform news? Subscribe to our podcast – search 'Policy & Practice' in the iTunes store
Neurologist Is a 'Genius'
A California neurologist has won a MacArthur Award for his work on frontotemporal dementia. Dr. William Seeley, a clinician-researcher at the University of California, San Francisco, and his team studied von Economo neurons and mapped changes in cortical networks corresponding to clinical subtypes of the disorder. “Seeley's observations represent a key step toward identifying the molecular and cellular mechanisms underlying the initiation and spread of frontotemporal dementia and lay the groundwork for developing targeted treatments to halt its progression,” according to the foundation's statement, which notes that the disorder is second only to Alzheimer's disease as the main cause of progressive presenile dementia.
More Youth Brain Injuries
The number of visits to emergency departments by children and young adults with traumatic brain injures increased 62% between 2001 and 2009, according to the Centers for Disease Control and Prevention. That's a rise from 153,375 to 248,418 in people 19 years or younger. The agency attributed the increase to heightened awareness of concussions and other brain injuries. But the number of resulting hospitalizations did not change. Nearly 70% of the ED visits were among youth aged 10-19 years. The most common causes of the injuries among boys were football and bicycling. Soccer, bicycling, and basketball were the most common activities causing the injuries among girls. During the period, 6.5% of the total of 2,651,581 youth ED visits for sports and recreation injuries were traumatic brain injuries, the CDC estimated.
YouTube Carries Misinformation
Most popular YouTube videos about movement disorders such as Parkinson's disease and dystonias depict people who don't have a movement disorder, according to an analysis of 29 videos by seven neurologists. Many times, the origin of a depicted disorder was instead psychogenic, they said. More alarming to the reviewers was that the videos contained dubious advice about therapies, such as that dystonia could be relieved by wearing cotton clothes and avoiding radiation. “Physicians should caution their patients to be wary of relying on information from potentially unreliable Web sources, and should also help to make reliable medical information freely available to those with either organic or psychogenic disorders,” the authors wrote in a letter to the editor in the New England Journal of Medicine. They added that their review “highlights an underlying problem that affects virtually every medical specialty.”
Device Approved for ALS Patients
The Food and Drug Administration has approved an implantable device (NeuRx, Synapse Biomedical Inc.) for amyotrophic lateral sclerosis patients with chronic hypoventilation. The device stimulates the diaphragm with electrical impulses. “The availability of the NeuRx DPS device to appropriate ALS patients is a major advance in the treatment of the disease that will enhance survival and quality of life,” Dr. Rup Tandan of the University of Vermont's ALS Certified Center, said in a statement. About 1 in 10 of the 30,000 ALS patients in this country have both respiratory problems and intact phrenic nerves that could be stimulated, according to the ALS Association.
Neurologist Is a 'Genius'
A California neurologist has won a MacArthur Award for his work on frontotemporal dementia. Dr. William Seeley, a clinician-researcher at the University of California, San Francisco, and his team studied von Economo neurons and mapped changes in cortical networks corresponding to clinical subtypes of the disorder. “Seeley's observations represent a key step toward identifying the molecular and cellular mechanisms underlying the initiation and spread of frontotemporal dementia and lay the groundwork for developing targeted treatments to halt its progression,” according to the foundation's statement, which notes that the disorder is second only to Alzheimer's disease as the main cause of progressive presenile dementia.
More Youth Brain Injuries
The number of visits to emergency departments by children and young adults with traumatic brain injures increased 62% between 2001 and 2009, according to the Centers for Disease Control and Prevention. That's a rise from 153,375 to 248,418 in people 19 years or younger. The agency attributed the increase to heightened awareness of concussions and other brain injuries. But the number of resulting hospitalizations did not change. Nearly 70% of the ED visits were among youth aged 10-19 years. The most common causes of the injuries among boys were football and bicycling. Soccer, bicycling, and basketball were the most common activities causing the injuries among girls. During the period, 6.5% of the total of 2,651,581 youth ED visits for sports and recreation injuries were traumatic brain injuries, the CDC estimated.
YouTube Carries Misinformation
Most popular YouTube videos about movement disorders such as Parkinson's disease and dystonias depict people who don't have a movement disorder, according to an analysis of 29 videos by seven neurologists. Many times, the origin of a depicted disorder was instead psychogenic, they said. More alarming to the reviewers was that the videos contained dubious advice about therapies, such as that dystonia could be relieved by wearing cotton clothes and avoiding radiation. “Physicians should caution their patients to be wary of relying on information from potentially unreliable Web sources, and should also help to make reliable medical information freely available to those with either organic or psychogenic disorders,” the authors wrote in a letter to the editor in the New England Journal of Medicine. They added that their review “highlights an underlying problem that affects virtually every medical specialty.”
Device Approved for ALS Patients
The Food and Drug Administration has approved an implantable device (NeuRx, Synapse Biomedical Inc.) for amyotrophic lateral sclerosis patients with chronic hypoventilation. The device stimulates the diaphragm with electrical impulses. “The availability of the NeuRx DPS device to appropriate ALS patients is a major advance in the treatment of the disease that will enhance survival and quality of life,” Dr. Rup Tandan of the University of Vermont's ALS Certified Center, said in a statement. About 1 in 10 of the 30,000 ALS patients in this country have both respiratory problems and intact phrenic nerves that could be stimulated, according to the ALS Association.
Neurologist Is a 'Genius'
A California neurologist has won a MacArthur Award for his work on frontotemporal dementia. Dr. William Seeley, a clinician-researcher at the University of California, San Francisco, and his team studied von Economo neurons and mapped changes in cortical networks corresponding to clinical subtypes of the disorder. “Seeley's observations represent a key step toward identifying the molecular and cellular mechanisms underlying the initiation and spread of frontotemporal dementia and lay the groundwork for developing targeted treatments to halt its progression,” according to the foundation's statement, which notes that the disorder is second only to Alzheimer's disease as the main cause of progressive presenile dementia.
More Youth Brain Injuries
The number of visits to emergency departments by children and young adults with traumatic brain injures increased 62% between 2001 and 2009, according to the Centers for Disease Control and Prevention. That's a rise from 153,375 to 248,418 in people 19 years or younger. The agency attributed the increase to heightened awareness of concussions and other brain injuries. But the number of resulting hospitalizations did not change. Nearly 70% of the ED visits were among youth aged 10-19 years. The most common causes of the injuries among boys were football and bicycling. Soccer, bicycling, and basketball were the most common activities causing the injuries among girls. During the period, 6.5% of the total of 2,651,581 youth ED visits for sports and recreation injuries were traumatic brain injuries, the CDC estimated.
YouTube Carries Misinformation
Most popular YouTube videos about movement disorders such as Parkinson's disease and dystonias depict people who don't have a movement disorder, according to an analysis of 29 videos by seven neurologists. Many times, the origin of a depicted disorder was instead psychogenic, they said. More alarming to the reviewers was that the videos contained dubious advice about therapies, such as that dystonia could be relieved by wearing cotton clothes and avoiding radiation. “Physicians should caution their patients to be wary of relying on information from potentially unreliable Web sources, and should also help to make reliable medical information freely available to those with either organic or psychogenic disorders,” the authors wrote in a letter to the editor in the New England Journal of Medicine. They added that their review “highlights an underlying problem that affects virtually every medical specialty.”
Device Approved for ALS Patients
The Food and Drug Administration has approved an implantable device (NeuRx, Synapse Biomedical Inc.) for amyotrophic lateral sclerosis patients with chronic hypoventilation. The device stimulates the diaphragm with electrical impulses. “The availability of the NeuRx DPS device to appropriate ALS patients is a major advance in the treatment of the disease that will enhance survival and quality of life,” Dr. Rup Tandan of the University of Vermont's ALS Certified Center, said in a statement. About 1 in 10 of the 30,000 ALS patients in this country have both respiratory problems and intact phrenic nerves that could be stimulated, according to the ALS Association.
Younger Soldiers at Higher Risk of Osteoarthritis
The recent finding that active-duty soldiers are at a significantly elevated risk for osteoarthritis may have relevance for the civilian population.
Data from 10 years of military medical surveillance data showed that active-duty U.S. military service members who are 40 years of age or older are twice as likely to be diagnosed with osteoarthritis (OA) as their peers in the general population.
The study also found that, when researchers controlled for other factors, women had a 20% higher rate of OA compared with men; the incidence of OA in service members 40 years or older was almost 19 times higher than service members less than 20 years old; members of Army had the highest incidence rate of OA, followed by Air Force, Marine Corps, and Navy; and junior service members had the highest incidence rate of OA, followed by senior enlisted, senior officers and junior officers.
The study found 108,266 incident cases of physician-diagnosed OA in the military’s Defense Medical Surveillance System (DMSS) between 1999 and 2008. Among the study’s other findings were that the incidence of OA was most likely to be increased among soldiers who were women, black, somewhat older, in the Army rather than another branch of the military, or of enlisted rank (Arthritis Rheum. 2011;63:2974-82).
A number of factors may explain the increased incidence of OA among soldiers. Traumatic knee injuries are common among military service members, according to the study’s authors and studies by other groups (Am. J. Sports Med. 2010;38:1997-2004; Mil. Med. 2007;172:90-1; J. Bone Joint Surg. Am. 2003;85-A:1656-66; Am. J. Prev. Med. 2000;18(suppl 3):33-40).
This population is "very active, constantly traveling, training, attending sporting activities, so they’re engaged in high-demand activities," said Kenneth L. Cameron, Ph.D., the lead author of the study, and director of orthopedic research at the Keller Army Community Hospital, West Point, N.Y. Also, several studies of professional athletes, such as soccer players, have shown that knee and hip OA are common among them (Foot Ankle Spec. 2011 Sep 30. [Epub ahead of print]).
Osteoarthritis, the most common form of arthritis, is one of the leading causes of disability and medical discharge in the military, according to the authors. "The rates of OA can affect force readiness," said Dr. Cameron.
Dr. Cameron and his team queried 10 years of data in DMSS, which captures almost all medical visits for all four branches of military, by sex, race, age, branch of military service, and rank. They used International Classification of Disease, Ninth Revision (ICD-9) code 715 (osteoarthrosis and allied disorders.)
The results showed that between 1999 and 2008, there were 108,266 incident cases of OA, and 13,768,885 person-years of follow-up were documented. The overall incidence rate was 7.86 per 1,000 person-years. That is roughly an average of 10,827 incident cases of OA each year among 1,376,889 active duty personnel.
For comparison to the general population, the authors used Canadian studies by Dr. Jacek A. Kopec and colleagues (J. Rheumatol. 2007;34:386-93; Arthritis Rheum. 2008;59:929-34). The Kopec groups’ findings, when calculated for the comparable age group, showed an OA incidence rate of 7.19 per 1,000 person-years in the general population.
Dr. Cameron said the lack of comparable studies to his study groups is "likely because the U.S. general population does not have free and open access to healthcare like they do in Canada and active duty U.S. service members do through the Military Health System."
So using available data that were most comparable to the study’s design and criteria, the authors concluded that rates of OA were significantly higher in the military populations when compared with similar age groups in the general population.
Comparisons also showed that the incidence rate of OA in military service members in the 20-24 year age group was 26% higher than those in the general population.
Dr. Amanda Nelson noted in an interview that "[t]his study is a nice addition to the literature. ... It gives us an idea that, despite all of its caveats, members of the military are at risk for osteoarthritis at a younger age compared to the general population."
"So the question is, what do we do for younger people with osteoarthritis? Is there a way to slow progression? We don’t yet have a lot of proven treatments for osteoarthritis," said Dr. Nelson, of the Thurston Arthritis Research Center, University of North Carolina at Chapel Hill.
Epidemiological studies of OA in the general population have shown that old age, female gender, being overweight or obese, knee injury, repetitive use of joints, black race, muscle weakness, and genetics play a role in OA development (Clin. Geriatr. Med. 2010;26:355-69).
Meanwhile, no medications have proven effective in preventing OA, and research on cartilage repair is still developing.
Black race was also shown to be associated with higher incidence rate of OA, compared with white race and those in the "others" category.
A few studies, including several by Dr. Nelson’s group, have shown that blacks are more likely to have severe knee and hip OA. "This study confirms our findings," she said.
The authors cited several limitations, including potential for coding errors, potential for information bias due to misclassification of the outcome of interest, lack of incidence rates for specific sites, and definitions used for incident cases of OA (physician-diagnosed vs. patient self-report, radiographic criteria or combination of both.)
Despite its limitations, some experts believe that the study’s findings support those of previous reports on OA.
Dr. Thomas M. Link, professor of radiology and clinical director of musculoskeletal and quantitative imaging research at the University of California, San Francisco, said, "The key message is that prevention is more important than anything else."
Several programs such as RunSafe Healthy Runners Clinic at the University of California, San Francisco, try to reduce the odds of injury by making slight modifications in how the athletes run, and their work has proven effective, said Dr. Link.
"I think what we found in the study is consistent with what we expected. The next question is why that is, and what are the modifiable risk factors," said Dr. Cameron.
The authors reported no conflicts of interest.
The authors start with an incredible data bank. However, although this is one of the largest studies of its kind, it does have some significant limitations.
For one, the study compares its findings in a U.S. military population to Canadian epidemiologic data. In addition, the study relies solely on physician-diagnosed osteoarthritis without the benefit of having the diagnosis confirmed by clinical or radiographic criteria, like those of the American College of Rheumatology.
Perhaps most troubling is that the researchers do not specify how many cases of osteoarthritis occurred at each anatomic site (knee vs. hip vs. hand vs. other). Rather, they combined all the anatomic subsets of the disease under the ICD-9-DM classification code 715, which covers all osteoarthrosis and allied disorders. Because the researchers have lumped osteoarthritis at all sites together, I have trouble drawing any conclusion from the study.
Dr. Roy D. Altman is professor of medicine at the University of California, Los Angeles. He has no relevant conflicts of interest to disclose.
The authors start with an incredible data bank. However, although this is one of the largest studies of its kind, it does have some significant limitations.
For one, the study compares its findings in a U.S. military population to Canadian epidemiologic data. In addition, the study relies solely on physician-diagnosed osteoarthritis without the benefit of having the diagnosis confirmed by clinical or radiographic criteria, like those of the American College of Rheumatology.
Perhaps most troubling is that the researchers do not specify how many cases of osteoarthritis occurred at each anatomic site (knee vs. hip vs. hand vs. other). Rather, they combined all the anatomic subsets of the disease under the ICD-9-DM classification code 715, which covers all osteoarthrosis and allied disorders. Because the researchers have lumped osteoarthritis at all sites together, I have trouble drawing any conclusion from the study.
Dr. Roy D. Altman is professor of medicine at the University of California, Los Angeles. He has no relevant conflicts of interest to disclose.
The authors start with an incredible data bank. However, although this is one of the largest studies of its kind, it does have some significant limitations.
For one, the study compares its findings in a U.S. military population to Canadian epidemiologic data. In addition, the study relies solely on physician-diagnosed osteoarthritis without the benefit of having the diagnosis confirmed by clinical or radiographic criteria, like those of the American College of Rheumatology.
Perhaps most troubling is that the researchers do not specify how many cases of osteoarthritis occurred at each anatomic site (knee vs. hip vs. hand vs. other). Rather, they combined all the anatomic subsets of the disease under the ICD-9-DM classification code 715, which covers all osteoarthrosis and allied disorders. Because the researchers have lumped osteoarthritis at all sites together, I have trouble drawing any conclusion from the study.
Dr. Roy D. Altman is professor of medicine at the University of California, Los Angeles. He has no relevant conflicts of interest to disclose.
The recent finding that active-duty soldiers are at a significantly elevated risk for osteoarthritis may have relevance for the civilian population.
Data from 10 years of military medical surveillance data showed that active-duty U.S. military service members who are 40 years of age or older are twice as likely to be diagnosed with osteoarthritis (OA) as their peers in the general population.
The study also found that, when researchers controlled for other factors, women had a 20% higher rate of OA compared with men; the incidence of OA in service members 40 years or older was almost 19 times higher than service members less than 20 years old; members of Army had the highest incidence rate of OA, followed by Air Force, Marine Corps, and Navy; and junior service members had the highest incidence rate of OA, followed by senior enlisted, senior officers and junior officers.
The study found 108,266 incident cases of physician-diagnosed OA in the military’s Defense Medical Surveillance System (DMSS) between 1999 and 2008. Among the study’s other findings were that the incidence of OA was most likely to be increased among soldiers who were women, black, somewhat older, in the Army rather than another branch of the military, or of enlisted rank (Arthritis Rheum. 2011;63:2974-82).
A number of factors may explain the increased incidence of OA among soldiers. Traumatic knee injuries are common among military service members, according to the study’s authors and studies by other groups (Am. J. Sports Med. 2010;38:1997-2004; Mil. Med. 2007;172:90-1; J. Bone Joint Surg. Am. 2003;85-A:1656-66; Am. J. Prev. Med. 2000;18(suppl 3):33-40).
This population is "very active, constantly traveling, training, attending sporting activities, so they’re engaged in high-demand activities," said Kenneth L. Cameron, Ph.D., the lead author of the study, and director of orthopedic research at the Keller Army Community Hospital, West Point, N.Y. Also, several studies of professional athletes, such as soccer players, have shown that knee and hip OA are common among them (Foot Ankle Spec. 2011 Sep 30. [Epub ahead of print]).
Osteoarthritis, the most common form of arthritis, is one of the leading causes of disability and medical discharge in the military, according to the authors. "The rates of OA can affect force readiness," said Dr. Cameron.
Dr. Cameron and his team queried 10 years of data in DMSS, which captures almost all medical visits for all four branches of military, by sex, race, age, branch of military service, and rank. They used International Classification of Disease, Ninth Revision (ICD-9) code 715 (osteoarthrosis and allied disorders.)
The results showed that between 1999 and 2008, there were 108,266 incident cases of OA, and 13,768,885 person-years of follow-up were documented. The overall incidence rate was 7.86 per 1,000 person-years. That is roughly an average of 10,827 incident cases of OA each year among 1,376,889 active duty personnel.
For comparison to the general population, the authors used Canadian studies by Dr. Jacek A. Kopec and colleagues (J. Rheumatol. 2007;34:386-93; Arthritis Rheum. 2008;59:929-34). The Kopec groups’ findings, when calculated for the comparable age group, showed an OA incidence rate of 7.19 per 1,000 person-years in the general population.
Dr. Cameron said the lack of comparable studies to his study groups is "likely because the U.S. general population does not have free and open access to healthcare like they do in Canada and active duty U.S. service members do through the Military Health System."
So using available data that were most comparable to the study’s design and criteria, the authors concluded that rates of OA were significantly higher in the military populations when compared with similar age groups in the general population.
Comparisons also showed that the incidence rate of OA in military service members in the 20-24 year age group was 26% higher than those in the general population.
Dr. Amanda Nelson noted in an interview that "[t]his study is a nice addition to the literature. ... It gives us an idea that, despite all of its caveats, members of the military are at risk for osteoarthritis at a younger age compared to the general population."
"So the question is, what do we do for younger people with osteoarthritis? Is there a way to slow progression? We don’t yet have a lot of proven treatments for osteoarthritis," said Dr. Nelson, of the Thurston Arthritis Research Center, University of North Carolina at Chapel Hill.
Epidemiological studies of OA in the general population have shown that old age, female gender, being overweight or obese, knee injury, repetitive use of joints, black race, muscle weakness, and genetics play a role in OA development (Clin. Geriatr. Med. 2010;26:355-69).
Meanwhile, no medications have proven effective in preventing OA, and research on cartilage repair is still developing.
Black race was also shown to be associated with higher incidence rate of OA, compared with white race and those in the "others" category.
A few studies, including several by Dr. Nelson’s group, have shown that blacks are more likely to have severe knee and hip OA. "This study confirms our findings," she said.
The authors cited several limitations, including potential for coding errors, potential for information bias due to misclassification of the outcome of interest, lack of incidence rates for specific sites, and definitions used for incident cases of OA (physician-diagnosed vs. patient self-report, radiographic criteria or combination of both.)
Despite its limitations, some experts believe that the study’s findings support those of previous reports on OA.
Dr. Thomas M. Link, professor of radiology and clinical director of musculoskeletal and quantitative imaging research at the University of California, San Francisco, said, "The key message is that prevention is more important than anything else."
Several programs such as RunSafe Healthy Runners Clinic at the University of California, San Francisco, try to reduce the odds of injury by making slight modifications in how the athletes run, and their work has proven effective, said Dr. Link.
"I think what we found in the study is consistent with what we expected. The next question is why that is, and what are the modifiable risk factors," said Dr. Cameron.
The authors reported no conflicts of interest.
The recent finding that active-duty soldiers are at a significantly elevated risk for osteoarthritis may have relevance for the civilian population.
Data from 10 years of military medical surveillance data showed that active-duty U.S. military service members who are 40 years of age or older are twice as likely to be diagnosed with osteoarthritis (OA) as their peers in the general population.
The study also found that, when researchers controlled for other factors, women had a 20% higher rate of OA compared with men; the incidence of OA in service members 40 years or older was almost 19 times higher than service members less than 20 years old; members of Army had the highest incidence rate of OA, followed by Air Force, Marine Corps, and Navy; and junior service members had the highest incidence rate of OA, followed by senior enlisted, senior officers and junior officers.
The study found 108,266 incident cases of physician-diagnosed OA in the military’s Defense Medical Surveillance System (DMSS) between 1999 and 2008. Among the study’s other findings were that the incidence of OA was most likely to be increased among soldiers who were women, black, somewhat older, in the Army rather than another branch of the military, or of enlisted rank (Arthritis Rheum. 2011;63:2974-82).
A number of factors may explain the increased incidence of OA among soldiers. Traumatic knee injuries are common among military service members, according to the study’s authors and studies by other groups (Am. J. Sports Med. 2010;38:1997-2004; Mil. Med. 2007;172:90-1; J. Bone Joint Surg. Am. 2003;85-A:1656-66; Am. J. Prev. Med. 2000;18(suppl 3):33-40).
This population is "very active, constantly traveling, training, attending sporting activities, so they’re engaged in high-demand activities," said Kenneth L. Cameron, Ph.D., the lead author of the study, and director of orthopedic research at the Keller Army Community Hospital, West Point, N.Y. Also, several studies of professional athletes, such as soccer players, have shown that knee and hip OA are common among them (Foot Ankle Spec. 2011 Sep 30. [Epub ahead of print]).
Osteoarthritis, the most common form of arthritis, is one of the leading causes of disability and medical discharge in the military, according to the authors. "The rates of OA can affect force readiness," said Dr. Cameron.
Dr. Cameron and his team queried 10 years of data in DMSS, which captures almost all medical visits for all four branches of military, by sex, race, age, branch of military service, and rank. They used International Classification of Disease, Ninth Revision (ICD-9) code 715 (osteoarthrosis and allied disorders.)
The results showed that between 1999 and 2008, there were 108,266 incident cases of OA, and 13,768,885 person-years of follow-up were documented. The overall incidence rate was 7.86 per 1,000 person-years. That is roughly an average of 10,827 incident cases of OA each year among 1,376,889 active duty personnel.
For comparison to the general population, the authors used Canadian studies by Dr. Jacek A. Kopec and colleagues (J. Rheumatol. 2007;34:386-93; Arthritis Rheum. 2008;59:929-34). The Kopec groups’ findings, when calculated for the comparable age group, showed an OA incidence rate of 7.19 per 1,000 person-years in the general population.
Dr. Cameron said the lack of comparable studies to his study groups is "likely because the U.S. general population does not have free and open access to healthcare like they do in Canada and active duty U.S. service members do through the Military Health System."
So using available data that were most comparable to the study’s design and criteria, the authors concluded that rates of OA were significantly higher in the military populations when compared with similar age groups in the general population.
Comparisons also showed that the incidence rate of OA in military service members in the 20-24 year age group was 26% higher than those in the general population.
Dr. Amanda Nelson noted in an interview that "[t]his study is a nice addition to the literature. ... It gives us an idea that, despite all of its caveats, members of the military are at risk for osteoarthritis at a younger age compared to the general population."
"So the question is, what do we do for younger people with osteoarthritis? Is there a way to slow progression? We don’t yet have a lot of proven treatments for osteoarthritis," said Dr. Nelson, of the Thurston Arthritis Research Center, University of North Carolina at Chapel Hill.
Epidemiological studies of OA in the general population have shown that old age, female gender, being overweight or obese, knee injury, repetitive use of joints, black race, muscle weakness, and genetics play a role in OA development (Clin. Geriatr. Med. 2010;26:355-69).
Meanwhile, no medications have proven effective in preventing OA, and research on cartilage repair is still developing.
Black race was also shown to be associated with higher incidence rate of OA, compared with white race and those in the "others" category.
A few studies, including several by Dr. Nelson’s group, have shown that blacks are more likely to have severe knee and hip OA. "This study confirms our findings," she said.
The authors cited several limitations, including potential for coding errors, potential for information bias due to misclassification of the outcome of interest, lack of incidence rates for specific sites, and definitions used for incident cases of OA (physician-diagnosed vs. patient self-report, radiographic criteria or combination of both.)
Despite its limitations, some experts believe that the study’s findings support those of previous reports on OA.
Dr. Thomas M. Link, professor of radiology and clinical director of musculoskeletal and quantitative imaging research at the University of California, San Francisco, said, "The key message is that prevention is more important than anything else."
Several programs such as RunSafe Healthy Runners Clinic at the University of California, San Francisco, try to reduce the odds of injury by making slight modifications in how the athletes run, and their work has proven effective, said Dr. Link.
"I think what we found in the study is consistent with what we expected. The next question is why that is, and what are the modifiable risk factors," said Dr. Cameron.
The authors reported no conflicts of interest.
Policy & Practice : Want more health reform news? Subscribe to our podcast – search 'Policy & Practice' in the iTunes store
Bill Calls for Coordinated Care
The American Association of Clinical Endocrinologists is urging Congress to create a National Diabetes Clinical Care Commission to coordinate federal efforts against the disease. Three Republican representatives from Texas introduced a bill to create the commission and encourage public-private efforts to make sure people with prediabetes and diabetes get needed care. “With annual rates of diabetes and prediabetes continuing to skyrocket, we need to have an honest discussion about what we're doing as a nation, and what is working and what is not working,” Dr. Yehuda Handelsman, president of the association, said in a statement. Although 37 federal agencies have activities concerning the disease, the efforts aren't slowing the diabetes epidemic, according to Dr. Handelsman.
FDA Will Review Study
A Food and Drug Administration committee will review the results of Merck & Co.'s Study of Heart and Renal Protection, or SHARP, in a meeting Nov. 2. The 9,000-patient study is the first to show that a regimen of ezetimibe and simvastatin benefited patients with chronic kidney disease. Combining the two anticholesterol drugs reduced the relative risk of a major cardiovascular event by 16% vs. placebo in kidney patients with no history of heart disease. The Endocrinologic and Metabolic Drugs Advisory Committee will discuss Merck's application to relabel its ezetimibe-simvastatin combination (Vytorin) and ezetimibe (Zetia) in light of the SHARP results. This level of review of a “supplemental new drug application” is unusual, but areas of concern could include an absence of benefit in mortality or progression of renal disease, cancer risk, and questions about the study's end points.
Groups Fight Budget Cuts
As the Joint Select Committee on Deficit Reduction – the “Super Committee” – began its budget negotiations, the American Diabetes Association and other advocacy groups made their case against cuts to state Medicaid programs. Diabetes disproportionately affects low-income people, making Medicaid essential, according to the association. Currently, more than 950,000 people with diabetes are enrolled in Medicaid in Illinois, 550,000 in California, 350,000 in New York, and 260,000 in Texas, the diabetes association said in a report released by the Medicaid-advocacy group Families USA.
Companies Hit as Antigeneric
The Federal Trade Commission has issued a long-awaited report on the generic drug market and concluded that brand-name manufacturers have been actively discouraging copy-cat products. The brand-name makers often introduce their own generic versions to discourage generic-focused companies from entering the market when a patent runs out, the FTC said. The presence of an authorized generic tends to tamp down sales 40%–50% for a generic competitor, the agency said. FTC Chairman Jon Leibowitz said in a statement that “some brand companies may be using the threat of launching an authorized generic as a powerful inducement for generic companies to delay bringing their drugs to market.” During that delay, consumers have to continue to pay the high price of the brand-name drug, he said.
Patients Think Newer Is Better
Patients are more likely to choose newer drugs over older when they're not provided information about the products' safety and effectiveness, according to a study published in Archives of Internal Medicine. The researchers gave participants a choice between two fictitious drugs for heartburn and two for high cholesterol. More people chose a drug described as older if they were also told the newer drug may not be as safe and effective. But for the heartburn drug, most people who were not given that warning chose the newer drug. In their Internet survey, the researchers also found that 39% of respondents believed that the Food and Drug Administration approves only “extremely effective” drugs and 25% believed the FDA approves only drugs without serious side effects.
Bill Calls for Coordinated Care
The American Association of Clinical Endocrinologists is urging Congress to create a National Diabetes Clinical Care Commission to coordinate federal efforts against the disease. Three Republican representatives from Texas introduced a bill to create the commission and encourage public-private efforts to make sure people with prediabetes and diabetes get needed care. “With annual rates of diabetes and prediabetes continuing to skyrocket, we need to have an honest discussion about what we're doing as a nation, and what is working and what is not working,” Dr. Yehuda Handelsman, president of the association, said in a statement. Although 37 federal agencies have activities concerning the disease, the efforts aren't slowing the diabetes epidemic, according to Dr. Handelsman.
FDA Will Review Study
A Food and Drug Administration committee will review the results of Merck & Co.'s Study of Heart and Renal Protection, or SHARP, in a meeting Nov. 2. The 9,000-patient study is the first to show that a regimen of ezetimibe and simvastatin benefited patients with chronic kidney disease. Combining the two anticholesterol drugs reduced the relative risk of a major cardiovascular event by 16% vs. placebo in kidney patients with no history of heart disease. The Endocrinologic and Metabolic Drugs Advisory Committee will discuss Merck's application to relabel its ezetimibe-simvastatin combination (Vytorin) and ezetimibe (Zetia) in light of the SHARP results. This level of review of a “supplemental new drug application” is unusual, but areas of concern could include an absence of benefit in mortality or progression of renal disease, cancer risk, and questions about the study's end points.
Groups Fight Budget Cuts
As the Joint Select Committee on Deficit Reduction – the “Super Committee” – began its budget negotiations, the American Diabetes Association and other advocacy groups made their case against cuts to state Medicaid programs. Diabetes disproportionately affects low-income people, making Medicaid essential, according to the association. Currently, more than 950,000 people with diabetes are enrolled in Medicaid in Illinois, 550,000 in California, 350,000 in New York, and 260,000 in Texas, the diabetes association said in a report released by the Medicaid-advocacy group Families USA.
Companies Hit as Antigeneric
The Federal Trade Commission has issued a long-awaited report on the generic drug market and concluded that brand-name manufacturers have been actively discouraging copy-cat products. The brand-name makers often introduce their own generic versions to discourage generic-focused companies from entering the market when a patent runs out, the FTC said. The presence of an authorized generic tends to tamp down sales 40%–50% for a generic competitor, the agency said. FTC Chairman Jon Leibowitz said in a statement that “some brand companies may be using the threat of launching an authorized generic as a powerful inducement for generic companies to delay bringing their drugs to market.” During that delay, consumers have to continue to pay the high price of the brand-name drug, he said.
Patients Think Newer Is Better
Patients are more likely to choose newer drugs over older when they're not provided information about the products' safety and effectiveness, according to a study published in Archives of Internal Medicine. The researchers gave participants a choice between two fictitious drugs for heartburn and two for high cholesterol. More people chose a drug described as older if they were also told the newer drug may not be as safe and effective. But for the heartburn drug, most people who were not given that warning chose the newer drug. In their Internet survey, the researchers also found that 39% of respondents believed that the Food and Drug Administration approves only “extremely effective” drugs and 25% believed the FDA approves only drugs without serious side effects.
Bill Calls for Coordinated Care
The American Association of Clinical Endocrinologists is urging Congress to create a National Diabetes Clinical Care Commission to coordinate federal efforts against the disease. Three Republican representatives from Texas introduced a bill to create the commission and encourage public-private efforts to make sure people with prediabetes and diabetes get needed care. “With annual rates of diabetes and prediabetes continuing to skyrocket, we need to have an honest discussion about what we're doing as a nation, and what is working and what is not working,” Dr. Yehuda Handelsman, president of the association, said in a statement. Although 37 federal agencies have activities concerning the disease, the efforts aren't slowing the diabetes epidemic, according to Dr. Handelsman.
FDA Will Review Study
A Food and Drug Administration committee will review the results of Merck & Co.'s Study of Heart and Renal Protection, or SHARP, in a meeting Nov. 2. The 9,000-patient study is the first to show that a regimen of ezetimibe and simvastatin benefited patients with chronic kidney disease. Combining the two anticholesterol drugs reduced the relative risk of a major cardiovascular event by 16% vs. placebo in kidney patients with no history of heart disease. The Endocrinologic and Metabolic Drugs Advisory Committee will discuss Merck's application to relabel its ezetimibe-simvastatin combination (Vytorin) and ezetimibe (Zetia) in light of the SHARP results. This level of review of a “supplemental new drug application” is unusual, but areas of concern could include an absence of benefit in mortality or progression of renal disease, cancer risk, and questions about the study's end points.
Groups Fight Budget Cuts
As the Joint Select Committee on Deficit Reduction – the “Super Committee” – began its budget negotiations, the American Diabetes Association and other advocacy groups made their case against cuts to state Medicaid programs. Diabetes disproportionately affects low-income people, making Medicaid essential, according to the association. Currently, more than 950,000 people with diabetes are enrolled in Medicaid in Illinois, 550,000 in California, 350,000 in New York, and 260,000 in Texas, the diabetes association said in a report released by the Medicaid-advocacy group Families USA.
Companies Hit as Antigeneric
The Federal Trade Commission has issued a long-awaited report on the generic drug market and concluded that brand-name manufacturers have been actively discouraging copy-cat products. The brand-name makers often introduce their own generic versions to discourage generic-focused companies from entering the market when a patent runs out, the FTC said. The presence of an authorized generic tends to tamp down sales 40%–50% for a generic competitor, the agency said. FTC Chairman Jon Leibowitz said in a statement that “some brand companies may be using the threat of launching an authorized generic as a powerful inducement for generic companies to delay bringing their drugs to market.” During that delay, consumers have to continue to pay the high price of the brand-name drug, he said.
Patients Think Newer Is Better
Patients are more likely to choose newer drugs over older when they're not provided information about the products' safety and effectiveness, according to a study published in Archives of Internal Medicine. The researchers gave participants a choice between two fictitious drugs for heartburn and two for high cholesterol. More people chose a drug described as older if they were also told the newer drug may not be as safe and effective. But for the heartburn drug, most people who were not given that warning chose the newer drug. In their Internet survey, the researchers also found that 39% of respondents believed that the Food and Drug Administration approves only “extremely effective” drugs and 25% believed the FDA approves only drugs without serious side effects.