User login
AAD: Serial Screening Pays Off in Early Detection of Nodular Melanoma
NEW ORLEANS - Serial screening of patients at high risk for melanoma is effective for detecting lesions, and appears to have prevented the development of aggressive advanced nodular melanoma.
For the past 19 years, Dr. Ronald N. Shore has been conducting serial screening on patients at high risk for melanoma, as many as 1,100 patients per year. He reported finding no metastases, recurrences, or deaths during this time period.
In addition, only one of his patients developed nodular melanoma, a phenomenon that Dr. Shore, who is in private practice in Rockville, Md., attributes to serial screening. The one patient who developed nodular melanoma failed to return for screening for 27 months.
Dr. Shore said that he believes the total absence of nodular melanoma for the first 17 years of his program is best explained by the program's detection of previously unrecognized early melanomas that he suspects have the potential to become nodular lesions.
He presented his most recent findings at the annual meeting of the American Academy of Dermatology.
He described finding what he termed "papular erythematous melanomas," which are distinct, amelanotic, red or pink, symmetrical lesions that closely resemble some of the recently described early nodular melanomas. The lesions were distinguished by their smaller size, lack of rapid growth, and radial growth phase histopathology.
The absence of classic ABCDE features may be another reason why the lesions had not been recognized, he said.
These findings raise the possibility that these lesions are recognizable precursors of nodular melanoma, he said. If the theory proves correct, "it reveals a way we can prevent development of nodular melanoma and by doing so, save lives," he added.
Dr. Shore noted that two prior studies have also reported 100% survival of screened patients at increased risk for melanoma: one led by Dr. Darrell S. Rigel, a professor of dermatology and dermatologic surgery at New York University Medical Center (Cancer 1989;63:386-9), and another more recent study led by Dr. Stephen Wang of the Memorial Sloan-Kettering Cancer Center in New York (J. Am. Acad. Dermatol. 2004;50:15-20). Dr. Shore pointed out that the studies share two common features: the use of serial screening, and performance of thorough examinations by dermatologists.
Analysis of 5 years of study data showed that 10 new cases of melanoma were detected in serially screened patients. The greatest Breslow depth was 0.15 mm; the lesions were all in radial growth phase; 70% were in men aged older than 50 years; and only 10% of the lesions were detected by patients.
The latter finding is one that Dr. Shore emphasized.
"Neither random screenings nor patient self-examinations can provide anywhere near the efficacy provided by serial professional examinations in the office setting," Dr. Shore said.
"Patients are very symptom oriented," he said, but early melanomas are mostly asymptomatic. In addition, "our records clearly show that some patients – especially men over 50 – are very poor at self-examination."
The study classified high-risk patients as those with significant past sun exposure and damage, especially multiple or severe sunburns; numerous or dysplastic nevi; actinic keratoses; any skin cancer; and family history of melanoma, especially in multiple blood relatives.
His practice has implemented a recall system, in which high-risk patients get reminders for their 6-month visit and continued follow-up reminders if they do not schedule an appointment.
Dr. Shore's findings are scheduled to appear in an upcoming issue of the Journal of Drugs in Dermatology.
He said that he had no financial interests relevant to his study.
NEW ORLEANS - Serial screening of patients at high risk for melanoma is effective for detecting lesions, and appears to have prevented the development of aggressive advanced nodular melanoma.
For the past 19 years, Dr. Ronald N. Shore has been conducting serial screening on patients at high risk for melanoma, as many as 1,100 patients per year. He reported finding no metastases, recurrences, or deaths during this time period.
In addition, only one of his patients developed nodular melanoma, a phenomenon that Dr. Shore, who is in private practice in Rockville, Md., attributes to serial screening. The one patient who developed nodular melanoma failed to return for screening for 27 months.
Dr. Shore said that he believes the total absence of nodular melanoma for the first 17 years of his program is best explained by the program's detection of previously unrecognized early melanomas that he suspects have the potential to become nodular lesions.
He presented his most recent findings at the annual meeting of the American Academy of Dermatology.
He described finding what he termed "papular erythematous melanomas," which are distinct, amelanotic, red or pink, symmetrical lesions that closely resemble some of the recently described early nodular melanomas. The lesions were distinguished by their smaller size, lack of rapid growth, and radial growth phase histopathology.
The absence of classic ABCDE features may be another reason why the lesions had not been recognized, he said.
These findings raise the possibility that these lesions are recognizable precursors of nodular melanoma, he said. If the theory proves correct, "it reveals a way we can prevent development of nodular melanoma and by doing so, save lives," he added.
Dr. Shore noted that two prior studies have also reported 100% survival of screened patients at increased risk for melanoma: one led by Dr. Darrell S. Rigel, a professor of dermatology and dermatologic surgery at New York University Medical Center (Cancer 1989;63:386-9), and another more recent study led by Dr. Stephen Wang of the Memorial Sloan-Kettering Cancer Center in New York (J. Am. Acad. Dermatol. 2004;50:15-20). Dr. Shore pointed out that the studies share two common features: the use of serial screening, and performance of thorough examinations by dermatologists.
Analysis of 5 years of study data showed that 10 new cases of melanoma were detected in serially screened patients. The greatest Breslow depth was 0.15 mm; the lesions were all in radial growth phase; 70% were in men aged older than 50 years; and only 10% of the lesions were detected by patients.
The latter finding is one that Dr. Shore emphasized.
"Neither random screenings nor patient self-examinations can provide anywhere near the efficacy provided by serial professional examinations in the office setting," Dr. Shore said.
"Patients are very symptom oriented," he said, but early melanomas are mostly asymptomatic. In addition, "our records clearly show that some patients – especially men over 50 – are very poor at self-examination."
The study classified high-risk patients as those with significant past sun exposure and damage, especially multiple or severe sunburns; numerous or dysplastic nevi; actinic keratoses; any skin cancer; and family history of melanoma, especially in multiple blood relatives.
His practice has implemented a recall system, in which high-risk patients get reminders for their 6-month visit and continued follow-up reminders if they do not schedule an appointment.
Dr. Shore's findings are scheduled to appear in an upcoming issue of the Journal of Drugs in Dermatology.
He said that he had no financial interests relevant to his study.
NEW ORLEANS - Serial screening of patients at high risk for melanoma is effective for detecting lesions, and appears to have prevented the development of aggressive advanced nodular melanoma.
For the past 19 years, Dr. Ronald N. Shore has been conducting serial screening on patients at high risk for melanoma, as many as 1,100 patients per year. He reported finding no metastases, recurrences, or deaths during this time period.
In addition, only one of his patients developed nodular melanoma, a phenomenon that Dr. Shore, who is in private practice in Rockville, Md., attributes to serial screening. The one patient who developed nodular melanoma failed to return for screening for 27 months.
Dr. Shore said that he believes the total absence of nodular melanoma for the first 17 years of his program is best explained by the program's detection of previously unrecognized early melanomas that he suspects have the potential to become nodular lesions.
He presented his most recent findings at the annual meeting of the American Academy of Dermatology.
He described finding what he termed "papular erythematous melanomas," which are distinct, amelanotic, red or pink, symmetrical lesions that closely resemble some of the recently described early nodular melanomas. The lesions were distinguished by their smaller size, lack of rapid growth, and radial growth phase histopathology.
The absence of classic ABCDE features may be another reason why the lesions had not been recognized, he said.
These findings raise the possibility that these lesions are recognizable precursors of nodular melanoma, he said. If the theory proves correct, "it reveals a way we can prevent development of nodular melanoma and by doing so, save lives," he added.
Dr. Shore noted that two prior studies have also reported 100% survival of screened patients at increased risk for melanoma: one led by Dr. Darrell S. Rigel, a professor of dermatology and dermatologic surgery at New York University Medical Center (Cancer 1989;63:386-9), and another more recent study led by Dr. Stephen Wang of the Memorial Sloan-Kettering Cancer Center in New York (J. Am. Acad. Dermatol. 2004;50:15-20). Dr. Shore pointed out that the studies share two common features: the use of serial screening, and performance of thorough examinations by dermatologists.
Analysis of 5 years of study data showed that 10 new cases of melanoma were detected in serially screened patients. The greatest Breslow depth was 0.15 mm; the lesions were all in radial growth phase; 70% were in men aged older than 50 years; and only 10% of the lesions were detected by patients.
The latter finding is one that Dr. Shore emphasized.
"Neither random screenings nor patient self-examinations can provide anywhere near the efficacy provided by serial professional examinations in the office setting," Dr. Shore said.
"Patients are very symptom oriented," he said, but early melanomas are mostly asymptomatic. In addition, "our records clearly show that some patients – especially men over 50 – are very poor at self-examination."
The study classified high-risk patients as those with significant past sun exposure and damage, especially multiple or severe sunburns; numerous or dysplastic nevi; actinic keratoses; any skin cancer; and family history of melanoma, especially in multiple blood relatives.
His practice has implemented a recall system, in which high-risk patients get reminders for their 6-month visit and continued follow-up reminders if they do not schedule an appointment.
Dr. Shore's findings are scheduled to appear in an upcoming issue of the Journal of Drugs in Dermatology.
He said that he had no financial interests relevant to his study.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF DERMATOLOGY
Serial Screening Program Detects Nodular Melanomas
NEW ORLEANS – Serial screening of patients at high risk for melanoma is highly effective for detecting lesions, and appears to have prevented the development of aggressive advanced nodular melanoma.
For the past 19 years, Dr. Ronald N. Shore has been conducting serial screening on patients at high risk for melanoma, as many as 1,100 patients per year. He reported finding no metastases, recurrences, or deaths during this time period.
In addition, none but one of his patients developed nodular melanoma, a phenomenon that Dr. Shore, who is in private practice in Rockville, Md., attributes to serial screening. The one patient who developed nodular melanoma failed to return for screening for 27 months.
Dr. Shore said that he believes the total absence of nodular melanoma for the first 17 years of his program is best explained by the program’s detection of previously unrecognized early melanomas that he suspects have the potential to become nodular lesions.
He presented his most recent findings at the annual meeting of the American Academy of Dermatology.
He described finding what he termed "papular erythematous melanomas," which are distinct, amelanotic, red or pink, symmetrical lesions that closely resemble some of the recently described early nodular melanomas. The lesions were distinguished by their smaller size, lack of rapid growth, and radial growth phase histopathology.
The absence of classic ABCDE features may be another reason why the lesions had not been recognized, he said.
These findings raise the possibility that these lesions are recognizable precursors of nodular melanoma, he said. If the theory proves correct, "it reveals a way we can prevent development of nodular melanoma and by doing so, save lives," he added.
Dr. Shore noted that two prior studies have also reported 100% survival of screened patients at increased risk for melanoma: one led by Dr. Darrell S. Rigel, a professor of dermatology and dermatologic surgery at New York University Medical Center (Cancer 1989;63:386-9), and another more recent study led by Dr. Stephen Wang of the Memorial Sloan-Kettering Cancer Center in New York (J. Am. Acad. Dermatol. 2004;50:15-20). Dr. Shore pointed out that the studies share two common features: the use of serial screening, and performance of thorough examinations by dermatologists.
Analysis of 5 years of study data showed that 10 new cases of melanoma were detected in serially screened patients. The greatest Breslow depth was 0.15 mm; the lesions were all in radial growth phase; 70% were in men aged older than 50 years; and only 10% of the lesions were detected by patients.
The latter finding is one that Dr. Shore emphasized.
"Neither random screenings nor patient self-examinations can provide anywhere near the efficacy provided by serial professional examinations in the office setting," Dr. Shore said.
"Patients are very symptom oriented," he said, but early melanomas are mostly asymptomatic. In addition, "our records clearly show that some patients – especially men over 50 – are very poor at self-examination."
The study classified high-risk patients as those with significant past sun exposure and damage, especially multiple or severe sunburns; numerous or dysplastic nevi; actinic keratoses; any skin cancer; and family history of melanoma, especially in multiple blood relatives.
His practice has implemented a recall system, in which high-risk patients get reminders for their 6-month visit and continued follow-up reminders if they do not schedule an appointment.
Dr. Shore’s findings are scheduled to appear in an upcoming issue of the Journal of Drugs in Dermatology.
He said that he had no financial interests relevant to his study.
NEW ORLEANS – Serial screening of patients at high risk for melanoma is highly effective for detecting lesions, and appears to have prevented the development of aggressive advanced nodular melanoma.
For the past 19 years, Dr. Ronald N. Shore has been conducting serial screening on patients at high risk for melanoma, as many as 1,100 patients per year. He reported finding no metastases, recurrences, or deaths during this time period.
In addition, none but one of his patients developed nodular melanoma, a phenomenon that Dr. Shore, who is in private practice in Rockville, Md., attributes to serial screening. The one patient who developed nodular melanoma failed to return for screening for 27 months.
Dr. Shore said that he believes the total absence of nodular melanoma for the first 17 years of his program is best explained by the program’s detection of previously unrecognized early melanomas that he suspects have the potential to become nodular lesions.
He presented his most recent findings at the annual meeting of the American Academy of Dermatology.
He described finding what he termed "papular erythematous melanomas," which are distinct, amelanotic, red or pink, symmetrical lesions that closely resemble some of the recently described early nodular melanomas. The lesions were distinguished by their smaller size, lack of rapid growth, and radial growth phase histopathology.
The absence of classic ABCDE features may be another reason why the lesions had not been recognized, he said.
These findings raise the possibility that these lesions are recognizable precursors of nodular melanoma, he said. If the theory proves correct, "it reveals a way we can prevent development of nodular melanoma and by doing so, save lives," he added.
Dr. Shore noted that two prior studies have also reported 100% survival of screened patients at increased risk for melanoma: one led by Dr. Darrell S. Rigel, a professor of dermatology and dermatologic surgery at New York University Medical Center (Cancer 1989;63:386-9), and another more recent study led by Dr. Stephen Wang of the Memorial Sloan-Kettering Cancer Center in New York (J. Am. Acad. Dermatol. 2004;50:15-20). Dr. Shore pointed out that the studies share two common features: the use of serial screening, and performance of thorough examinations by dermatologists.
Analysis of 5 years of study data showed that 10 new cases of melanoma were detected in serially screened patients. The greatest Breslow depth was 0.15 mm; the lesions were all in radial growth phase; 70% were in men aged older than 50 years; and only 10% of the lesions were detected by patients.
The latter finding is one that Dr. Shore emphasized.
"Neither random screenings nor patient self-examinations can provide anywhere near the efficacy provided by serial professional examinations in the office setting," Dr. Shore said.
"Patients are very symptom oriented," he said, but early melanomas are mostly asymptomatic. In addition, "our records clearly show that some patients – especially men over 50 – are very poor at self-examination."
The study classified high-risk patients as those with significant past sun exposure and damage, especially multiple or severe sunburns; numerous or dysplastic nevi; actinic keratoses; any skin cancer; and family history of melanoma, especially in multiple blood relatives.
His practice has implemented a recall system, in which high-risk patients get reminders for their 6-month visit and continued follow-up reminders if they do not schedule an appointment.
Dr. Shore’s findings are scheduled to appear in an upcoming issue of the Journal of Drugs in Dermatology.
He said that he had no financial interests relevant to his study.
NEW ORLEANS – Serial screening of patients at high risk for melanoma is highly effective for detecting lesions, and appears to have prevented the development of aggressive advanced nodular melanoma.
For the past 19 years, Dr. Ronald N. Shore has been conducting serial screening on patients at high risk for melanoma, as many as 1,100 patients per year. He reported finding no metastases, recurrences, or deaths during this time period.
In addition, none but one of his patients developed nodular melanoma, a phenomenon that Dr. Shore, who is in private practice in Rockville, Md., attributes to serial screening. The one patient who developed nodular melanoma failed to return for screening for 27 months.
Dr. Shore said that he believes the total absence of nodular melanoma for the first 17 years of his program is best explained by the program’s detection of previously unrecognized early melanomas that he suspects have the potential to become nodular lesions.
He presented his most recent findings at the annual meeting of the American Academy of Dermatology.
He described finding what he termed "papular erythematous melanomas," which are distinct, amelanotic, red or pink, symmetrical lesions that closely resemble some of the recently described early nodular melanomas. The lesions were distinguished by their smaller size, lack of rapid growth, and radial growth phase histopathology.
The absence of classic ABCDE features may be another reason why the lesions had not been recognized, he said.
These findings raise the possibility that these lesions are recognizable precursors of nodular melanoma, he said. If the theory proves correct, "it reveals a way we can prevent development of nodular melanoma and by doing so, save lives," he added.
Dr. Shore noted that two prior studies have also reported 100% survival of screened patients at increased risk for melanoma: one led by Dr. Darrell S. Rigel, a professor of dermatology and dermatologic surgery at New York University Medical Center (Cancer 1989;63:386-9), and another more recent study led by Dr. Stephen Wang of the Memorial Sloan-Kettering Cancer Center in New York (J. Am. Acad. Dermatol. 2004;50:15-20). Dr. Shore pointed out that the studies share two common features: the use of serial screening, and performance of thorough examinations by dermatologists.
Analysis of 5 years of study data showed that 10 new cases of melanoma were detected in serially screened patients. The greatest Breslow depth was 0.15 mm; the lesions were all in radial growth phase; 70% were in men aged older than 50 years; and only 10% of the lesions were detected by patients.
The latter finding is one that Dr. Shore emphasized.
"Neither random screenings nor patient self-examinations can provide anywhere near the efficacy provided by serial professional examinations in the office setting," Dr. Shore said.
"Patients are very symptom oriented," he said, but early melanomas are mostly asymptomatic. In addition, "our records clearly show that some patients – especially men over 50 – are very poor at self-examination."
The study classified high-risk patients as those with significant past sun exposure and damage, especially multiple or severe sunburns; numerous or dysplastic nevi; actinic keratoses; any skin cancer; and family history of melanoma, especially in multiple blood relatives.
His practice has implemented a recall system, in which high-risk patients get reminders for their 6-month visit and continued follow-up reminders if they do not schedule an appointment.
Dr. Shore’s findings are scheduled to appear in an upcoming issue of the Journal of Drugs in Dermatology.
He said that he had no financial interests relevant to his study.
FROM THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF DERMATOLOGY
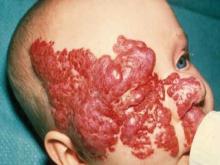
AAD: Questions Remain About Treating Hemangiomas With Propranolol
NEW ORLEANS - A blood pressure medication that has been in use for decades also could be an effective treatment for hemangiomas, the most common benign growths in children.
"People talk about products of the year, people of the year, and most handsome and most beautiful, but this is the drug of the year for [those of us] who are taking care of children," said Dr. Sheila Fallon Friedlander, professor of clinical pediatrics and medicine at the University of California, San Diego, and section chief of pediatric dermatology at Rady Children's Hospital in San Diego.
The beta-blocker is propranolol, which Dr. Friedlander called a "not-so-new kid on the block." For nearly 5 decades propranolol has been used to treat high blood pressure in adults and children. But over the past 2 years evidence has emerged showing that propranolol can be an effective treatment for hemangiomas.
The drug's effect on the infantile growths was a serendipitous discovery by observant French physicians who treated two infants for hemangiomas with corticosteroids and administered propranolol for other medical conditions (N. Engl. J. Med. 2008;358:2649-51). Within 1 day of administration of propranolol, the hemangioma changed in color and softened. The improvements continued even after corticosteroids were tapered and discontinued, and no regrowth was noted. Since then, other studies, including a follow-up by the French researchers, have shown that propranolol can rapidly shorten the natural course of hemangiomas.
The treatment comes with a caution, said Dr. Friedlander. Despite positive results and a good safety profile, there are still many unknowns, such as the proper dosage and length of treatment. The mechanism of the drug is not known.
Dr. Friedlander is participating in a large multinational study investigating propranolol. The patients are monitored closely during the study period, because improper use of the drug could lead to serious conditions such as low blood sugar, changes of heart beat rhythm, changes in blood pressure, and wheezing, she said.
Dermatologists should consider using the drug when the hemangioma is functionally deforming, causing pain, or creating problems with vision or airway. But Dr. Friedlander suggested referral to a dermatologist who has experience using the drug. "We have some great new treatments with great effects, but they have to be used very carefully."
Roughly 10% of children have hemangiomas, many of which are benign and disappear within a few months. But some turn into very serious lesions, leading to blindness, obstruction of the airway, and painful genital ulcers.
A study of more than 1,000 children (Pediatrics 2006;118:882-7) showed that 24% of children with hemangiomas developed complications, and 38% needed treatment.
Steroids, laser therapy, and surgery are among the most commonly used treatments. The topical beta-blocker ointment timolol could be an alternative to propranolol, and it has shown some promise, according to Dr. Friedlander, but the results so far haven't been as impressive as those achieved with propranolol.
Dr. Friedlander is a clinical investigator for a hemangioma study sponsored by Pierre Fabre.
NEW ORLEANS - A blood pressure medication that has been in use for decades also could be an effective treatment for hemangiomas, the most common benign growths in children.
"People talk about products of the year, people of the year, and most handsome and most beautiful, but this is the drug of the year for [those of us] who are taking care of children," said Dr. Sheila Fallon Friedlander, professor of clinical pediatrics and medicine at the University of California, San Diego, and section chief of pediatric dermatology at Rady Children's Hospital in San Diego.
The beta-blocker is propranolol, which Dr. Friedlander called a "not-so-new kid on the block." For nearly 5 decades propranolol has been used to treat high blood pressure in adults and children. But over the past 2 years evidence has emerged showing that propranolol can be an effective treatment for hemangiomas.
The drug's effect on the infantile growths was a serendipitous discovery by observant French physicians who treated two infants for hemangiomas with corticosteroids and administered propranolol for other medical conditions (N. Engl. J. Med. 2008;358:2649-51). Within 1 day of administration of propranolol, the hemangioma changed in color and softened. The improvements continued even after corticosteroids were tapered and discontinued, and no regrowth was noted. Since then, other studies, including a follow-up by the French researchers, have shown that propranolol can rapidly shorten the natural course of hemangiomas.
The treatment comes with a caution, said Dr. Friedlander. Despite positive results and a good safety profile, there are still many unknowns, such as the proper dosage and length of treatment. The mechanism of the drug is not known.
Dr. Friedlander is participating in a large multinational study investigating propranolol. The patients are monitored closely during the study period, because improper use of the drug could lead to serious conditions such as low blood sugar, changes of heart beat rhythm, changes in blood pressure, and wheezing, she said.
Dermatologists should consider using the drug when the hemangioma is functionally deforming, causing pain, or creating problems with vision or airway. But Dr. Friedlander suggested referral to a dermatologist who has experience using the drug. "We have some great new treatments with great effects, but they have to be used very carefully."
Roughly 10% of children have hemangiomas, many of which are benign and disappear within a few months. But some turn into very serious lesions, leading to blindness, obstruction of the airway, and painful genital ulcers.
A study of more than 1,000 children (Pediatrics 2006;118:882-7) showed that 24% of children with hemangiomas developed complications, and 38% needed treatment.
Steroids, laser therapy, and surgery are among the most commonly used treatments. The topical beta-blocker ointment timolol could be an alternative to propranolol, and it has shown some promise, according to Dr. Friedlander, but the results so far haven't been as impressive as those achieved with propranolol.
Dr. Friedlander is a clinical investigator for a hemangioma study sponsored by Pierre Fabre.
NEW ORLEANS - A blood pressure medication that has been in use for decades also could be an effective treatment for hemangiomas, the most common benign growths in children.
"People talk about products of the year, people of the year, and most handsome and most beautiful, but this is the drug of the year for [those of us] who are taking care of children," said Dr. Sheila Fallon Friedlander, professor of clinical pediatrics and medicine at the University of California, San Diego, and section chief of pediatric dermatology at Rady Children's Hospital in San Diego.
The beta-blocker is propranolol, which Dr. Friedlander called a "not-so-new kid on the block." For nearly 5 decades propranolol has been used to treat high blood pressure in adults and children. But over the past 2 years evidence has emerged showing that propranolol can be an effective treatment for hemangiomas.
The drug's effect on the infantile growths was a serendipitous discovery by observant French physicians who treated two infants for hemangiomas with corticosteroids and administered propranolol for other medical conditions (N. Engl. J. Med. 2008;358:2649-51). Within 1 day of administration of propranolol, the hemangioma changed in color and softened. The improvements continued even after corticosteroids were tapered and discontinued, and no regrowth was noted. Since then, other studies, including a follow-up by the French researchers, have shown that propranolol can rapidly shorten the natural course of hemangiomas.
The treatment comes with a caution, said Dr. Friedlander. Despite positive results and a good safety profile, there are still many unknowns, such as the proper dosage and length of treatment. The mechanism of the drug is not known.
Dr. Friedlander is participating in a large multinational study investigating propranolol. The patients are monitored closely during the study period, because improper use of the drug could lead to serious conditions such as low blood sugar, changes of heart beat rhythm, changes in blood pressure, and wheezing, she said.
Dermatologists should consider using the drug when the hemangioma is functionally deforming, causing pain, or creating problems with vision or airway. But Dr. Friedlander suggested referral to a dermatologist who has experience using the drug. "We have some great new treatments with great effects, but they have to be used very carefully."
Roughly 10% of children have hemangiomas, many of which are benign and disappear within a few months. But some turn into very serious lesions, leading to blindness, obstruction of the airway, and painful genital ulcers.
A study of more than 1,000 children (Pediatrics 2006;118:882-7) showed that 24% of children with hemangiomas developed complications, and 38% needed treatment.
Steroids, laser therapy, and surgery are among the most commonly used treatments. The topical beta-blocker ointment timolol could be an alternative to propranolol, and it has shown some promise, according to Dr. Friedlander, but the results so far haven't been as impressive as those achieved with propranolol.
Dr. Friedlander is a clinical investigator for a hemangioma study sponsored by Pierre Fabre.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF DERMATOLOGY
Bariatric Surgery Improved Knee OA Symptoms
Major Finding: At 6 months after bariatric surgery, patients had a 20% drop from baseline BMI and their average serum level of procollagen type II N-terminal propeptide (PIIANP), a marker of cartilage synthesis, had risen 32%, while their average serum level of cartilage oligomeric matrix protein (COMP) had decreased by 36%.
Data Source: Baseline and 6-month follow-up measures in 36 women and 8 men who underwent bariatric surgery for obesity (average baseline BMI of 50.7) and had moderate to severe knee osteoarthritis.
Disclosures: The study was funded by Assistance Publique-Hôpitaux de Paris, Direction of Clinical Research, which promoted and supported the clinical investigation, a grant from the European community, and the Association Rhumatisme et Travail (Hôpital Lariboisière, Paris). The authors reported no financial conflicts of interest.
In obese patients with knee osteoarthritis, significant weight loss after bariatric surgery reduced pain and stiffness, decreased low-grade inflammation, and changed cartilage turnover, according to a small study.
In addition to the well-known relationship between obesity and onset of knee osteoarthritis (OA), several studies have now shown that the association goes beyond the increase in mechanical load on the tibiofemoral cartilage. “Adipose tissue may act as an endocrine organ, releasing several proinflammatory mediators and adipokines in blood that may participate in cartilage alteration in obese patients,” according to Dr. Pascal Richette of Hôpital Lariboisière and coauthors. “Trials that have assessed the efficacy of surgically induced massive weight loss on knee OA symptoms are scarce and have not specifically included patients with well-defined radiographic evidence of knee OA, as in our study.” (Ann. Rheum. Dis. 2011;70:139-44).
The authors studied 44 obese patients (36 women) with a baseline body mass index of 50.7 before surgery and moderate to severe knee OA. The patients underwent laparoscopic Roux-en-Y gastric bypass surgery or laparoscopic adjustable gastric banding. Patient data were collected before and 6 months after surgery.
At 6 months, patients had a 20% drop from baseline BMIs. Their VAS (visual acuity scores) decreased from 50 mm to 24.5 mm and their scores on the WOMAC (Western Ontario MacMaster) Questionnaire improved. Significant decreases were seen in average serum levels of interleukin-6 (IL-6), which declined by 26%, and of high-sensitivity C-reactive protein (hsCRP), which dropped 46%. Also, weight loss was associated with changes in adipokine levels: Mean serum leptin concentration was decreased by 48%, and serum level of adiponectin was increased by 21%, the authors reported.
The average serum level of procollagen type II N-terminal propeptide (PIIANP), a marker of cartilage synthesis, rose 32%, while the serum level of cartilage oligomeric matrix protein (COMP) decreased by 36%. “These results are the first to suggest a benefit of weight loss on both cartilage anabolism and catabolism,” the authors wrote.
The researchers found a significant correlation between IL-6 level and WOMAC Questionnaire scores as well as between urinary type II collagen helical peptide (helix-II) and hsCRP. Variation in COMP concentration was significantly correlated with changes in VAS pain scores and WOMAC stiffness score, the authors wrote, adding, “Our findings extend the results of recent work showing a significant association of IL-6 circulating levels and the prevalence and incidence of knee OA.”
Major Finding: At 6 months after bariatric surgery, patients had a 20% drop from baseline BMI and their average serum level of procollagen type II N-terminal propeptide (PIIANP), a marker of cartilage synthesis, had risen 32%, while their average serum level of cartilage oligomeric matrix protein (COMP) had decreased by 36%.
Data Source: Baseline and 6-month follow-up measures in 36 women and 8 men who underwent bariatric surgery for obesity (average baseline BMI of 50.7) and had moderate to severe knee osteoarthritis.
Disclosures: The study was funded by Assistance Publique-Hôpitaux de Paris, Direction of Clinical Research, which promoted and supported the clinical investigation, a grant from the European community, and the Association Rhumatisme et Travail (Hôpital Lariboisière, Paris). The authors reported no financial conflicts of interest.
In obese patients with knee osteoarthritis, significant weight loss after bariatric surgery reduced pain and stiffness, decreased low-grade inflammation, and changed cartilage turnover, according to a small study.
In addition to the well-known relationship between obesity and onset of knee osteoarthritis (OA), several studies have now shown that the association goes beyond the increase in mechanical load on the tibiofemoral cartilage. “Adipose tissue may act as an endocrine organ, releasing several proinflammatory mediators and adipokines in blood that may participate in cartilage alteration in obese patients,” according to Dr. Pascal Richette of Hôpital Lariboisière and coauthors. “Trials that have assessed the efficacy of surgically induced massive weight loss on knee OA symptoms are scarce and have not specifically included patients with well-defined radiographic evidence of knee OA, as in our study.” (Ann. Rheum. Dis. 2011;70:139-44).
The authors studied 44 obese patients (36 women) with a baseline body mass index of 50.7 before surgery and moderate to severe knee OA. The patients underwent laparoscopic Roux-en-Y gastric bypass surgery or laparoscopic adjustable gastric banding. Patient data were collected before and 6 months after surgery.
At 6 months, patients had a 20% drop from baseline BMIs. Their VAS (visual acuity scores) decreased from 50 mm to 24.5 mm and their scores on the WOMAC (Western Ontario MacMaster) Questionnaire improved. Significant decreases were seen in average serum levels of interleukin-6 (IL-6), which declined by 26%, and of high-sensitivity C-reactive protein (hsCRP), which dropped 46%. Also, weight loss was associated with changes in adipokine levels: Mean serum leptin concentration was decreased by 48%, and serum level of adiponectin was increased by 21%, the authors reported.
The average serum level of procollagen type II N-terminal propeptide (PIIANP), a marker of cartilage synthesis, rose 32%, while the serum level of cartilage oligomeric matrix protein (COMP) decreased by 36%. “These results are the first to suggest a benefit of weight loss on both cartilage anabolism and catabolism,” the authors wrote.
The researchers found a significant correlation between IL-6 level and WOMAC Questionnaire scores as well as between urinary type II collagen helical peptide (helix-II) and hsCRP. Variation in COMP concentration was significantly correlated with changes in VAS pain scores and WOMAC stiffness score, the authors wrote, adding, “Our findings extend the results of recent work showing a significant association of IL-6 circulating levels and the prevalence and incidence of knee OA.”
Major Finding: At 6 months after bariatric surgery, patients had a 20% drop from baseline BMI and their average serum level of procollagen type II N-terminal propeptide (PIIANP), a marker of cartilage synthesis, had risen 32%, while their average serum level of cartilage oligomeric matrix protein (COMP) had decreased by 36%.
Data Source: Baseline and 6-month follow-up measures in 36 women and 8 men who underwent bariatric surgery for obesity (average baseline BMI of 50.7) and had moderate to severe knee osteoarthritis.
Disclosures: The study was funded by Assistance Publique-Hôpitaux de Paris, Direction of Clinical Research, which promoted and supported the clinical investigation, a grant from the European community, and the Association Rhumatisme et Travail (Hôpital Lariboisière, Paris). The authors reported no financial conflicts of interest.
In obese patients with knee osteoarthritis, significant weight loss after bariatric surgery reduced pain and stiffness, decreased low-grade inflammation, and changed cartilage turnover, according to a small study.
In addition to the well-known relationship between obesity and onset of knee osteoarthritis (OA), several studies have now shown that the association goes beyond the increase in mechanical load on the tibiofemoral cartilage. “Adipose tissue may act as an endocrine organ, releasing several proinflammatory mediators and adipokines in blood that may participate in cartilage alteration in obese patients,” according to Dr. Pascal Richette of Hôpital Lariboisière and coauthors. “Trials that have assessed the efficacy of surgically induced massive weight loss on knee OA symptoms are scarce and have not specifically included patients with well-defined radiographic evidence of knee OA, as in our study.” (Ann. Rheum. Dis. 2011;70:139-44).
The authors studied 44 obese patients (36 women) with a baseline body mass index of 50.7 before surgery and moderate to severe knee OA. The patients underwent laparoscopic Roux-en-Y gastric bypass surgery or laparoscopic adjustable gastric banding. Patient data were collected before and 6 months after surgery.
At 6 months, patients had a 20% drop from baseline BMIs. Their VAS (visual acuity scores) decreased from 50 mm to 24.5 mm and their scores on the WOMAC (Western Ontario MacMaster) Questionnaire improved. Significant decreases were seen in average serum levels of interleukin-6 (IL-6), which declined by 26%, and of high-sensitivity C-reactive protein (hsCRP), which dropped 46%. Also, weight loss was associated with changes in adipokine levels: Mean serum leptin concentration was decreased by 48%, and serum level of adiponectin was increased by 21%, the authors reported.
The average serum level of procollagen type II N-terminal propeptide (PIIANP), a marker of cartilage synthesis, rose 32%, while the serum level of cartilage oligomeric matrix protein (COMP) decreased by 36%. “These results are the first to suggest a benefit of weight loss on both cartilage anabolism and catabolism,” the authors wrote.
The researchers found a significant correlation between IL-6 level and WOMAC Questionnaire scores as well as between urinary type II collagen helical peptide (helix-II) and hsCRP. Variation in COMP concentration was significantly correlated with changes in VAS pain scores and WOMAC stiffness score, the authors wrote, adding, “Our findings extend the results of recent work showing a significant association of IL-6 circulating levels and the prevalence and incidence of knee OA.”
From Annals of the Rheumatic Diseases
Web Site Aims to Help Improve OR Safety
It's a simple idea, but it could help save millions of lives: a Web site helping hospitals and surgeons worldwide improve surgical outcomes by making a commitment to implement proven protocols in their operating rooms, to share ideas, and to receive feedback on what works best.
Called ORReady, the grass roots project is the brainchild of Dr. Paul Alan Wetter, founder and chairman of the Society of Laparoendoscopic Surgeons.
Inspired by the humble beginnings of Facebook and the power of collaboration in the Human Genome Project, Dr. Wetter decided that his idea – a global effort to improve surgical outcomes – would be just as feasible, because “smart doctors around the world can get together and do it.” No bureaucracy. No big dollar budget.
Launched in early 2010, the project is still in its infancy, and the Web site (www.orready.com
“There are many examples of people who have really improved outcomes in surgery with increased use of safety measures,” said Dr. Wetter, who is an ob.gyn. in South Miami, Fla., and an internationally recognized leader in the field of minimally invasive surgery.
He hopes that by sharing OR safety information, there will be at least a 2%-3% improvement in outcomes. That's six million lives saved worldwide each year. He hopes that hospitals, medical societies, and surgical centers worldwide will sign onto this effort within the coming years.
He admits that this is a lofty goal. But he also believes that the increasing emphasis on improving patient safety will help the initiative take off. Add to that the power of technology and collaboration: “[The] world is becoming a small place and information is disseminated quickly,” said Dr. Wetter, who is also clinical professor emeritus at the University of Miami.
ORReady is a nonprofit project and is run by members and institutions that have volunteered their time and resources. The Web site follows the Creative Commons guidelines. “We encourage you to copy and use any materials that will help improve surgical outcome and create centers of merit in surgery and MIS [minimally invasive surgery].” It encourages hospitals and departments to download and sign an “Outcome Commitment Letter,” to choose from a set of protocols on the Web site that suit their operating rooms, and to register as an ORReady center of merit.
The guidelines suggest several main steps for surgeons and their teams: “Slow Down for Warm Up and Check Lists; Stop for Time Out before you Go.” A stoplight on the site sums up the message.
Soon, participants can register with an open-access database that can be used for sharing data, doing research to improve outcomes, and providing feedback. The school of biological and health systems engineering at Arizona State University, Tempe, has offered to help create the database.
“Information goes in; information comes out. We want to know about that [information] and we want to distribute it. Each hospital then can choose what works for them,” he said.
Dr. Wetter said that so far, he has approached a handful of institutions in the United States and abroad and has received a unanimously positive response.
“Using the power of the Internet to get ideas in one place is a great idea,” said Dr. Darrell A. Campbell Jr., the chief medical officer of the University of Michigan Medical System, who is not involved in the project. “I can support that wholeheartedly.”
His only concern, he said, was that procedures and processes that worked for one institution might now apply to another; more research and hands-on evaluation might be needed before the process can be implemented.
The project recently won its first award. The Society of Laparoendoscopic Surgeons won the 2011 Alliance for Continuing Medical Education Great Idea Award in the Medical Specialty Societies Member section for introducing ORReady as a way to encourage surgical facilities to improve CME for improved surgical outcomes.
“We're looking for things that are best practices, are innovative, and that other people may want to replicate, adapt, [and] consider using,” Jann Balmer, Ph.D., president of the Alliance, said in an interview.
“It's very exciting to do this and see this great enthusiasm,” said Dr. Wetter. “For almost any doctor, the main concern is the safety of their patients.”
Dr. Wetter is now focusing on spreading the word and making more surgeons and hospitals aware of and involved in ORReady. “The more people that know about this, the more successful it's going to be.”
He hopes to see his project make an impact within the next few years.
It's a simple idea, but it could help save millions of lives: a Web site helping hospitals and surgeons worldwide improve surgical outcomes by making a commitment to implement proven protocols in their operating rooms, to share ideas, and to receive feedback on what works best.
Called ORReady, the grass roots project is the brainchild of Dr. Paul Alan Wetter, founder and chairman of the Society of Laparoendoscopic Surgeons.
Inspired by the humble beginnings of Facebook and the power of collaboration in the Human Genome Project, Dr. Wetter decided that his idea – a global effort to improve surgical outcomes – would be just as feasible, because “smart doctors around the world can get together and do it.” No bureaucracy. No big dollar budget.
Launched in early 2010, the project is still in its infancy, and the Web site (www.orready.com
“There are many examples of people who have really improved outcomes in surgery with increased use of safety measures,” said Dr. Wetter, who is an ob.gyn. in South Miami, Fla., and an internationally recognized leader in the field of minimally invasive surgery.
He hopes that by sharing OR safety information, there will be at least a 2%-3% improvement in outcomes. That's six million lives saved worldwide each year. He hopes that hospitals, medical societies, and surgical centers worldwide will sign onto this effort within the coming years.
He admits that this is a lofty goal. But he also believes that the increasing emphasis on improving patient safety will help the initiative take off. Add to that the power of technology and collaboration: “[The] world is becoming a small place and information is disseminated quickly,” said Dr. Wetter, who is also clinical professor emeritus at the University of Miami.
ORReady is a nonprofit project and is run by members and institutions that have volunteered their time and resources. The Web site follows the Creative Commons guidelines. “We encourage you to copy and use any materials that will help improve surgical outcome and create centers of merit in surgery and MIS [minimally invasive surgery].” It encourages hospitals and departments to download and sign an “Outcome Commitment Letter,” to choose from a set of protocols on the Web site that suit their operating rooms, and to register as an ORReady center of merit.
The guidelines suggest several main steps for surgeons and their teams: “Slow Down for Warm Up and Check Lists; Stop for Time Out before you Go.” A stoplight on the site sums up the message.
Soon, participants can register with an open-access database that can be used for sharing data, doing research to improve outcomes, and providing feedback. The school of biological and health systems engineering at Arizona State University, Tempe, has offered to help create the database.
“Information goes in; information comes out. We want to know about that [information] and we want to distribute it. Each hospital then can choose what works for them,” he said.
Dr. Wetter said that so far, he has approached a handful of institutions in the United States and abroad and has received a unanimously positive response.
“Using the power of the Internet to get ideas in one place is a great idea,” said Dr. Darrell A. Campbell Jr., the chief medical officer of the University of Michigan Medical System, who is not involved in the project. “I can support that wholeheartedly.”
His only concern, he said, was that procedures and processes that worked for one institution might now apply to another; more research and hands-on evaluation might be needed before the process can be implemented.
The project recently won its first award. The Society of Laparoendoscopic Surgeons won the 2011 Alliance for Continuing Medical Education Great Idea Award in the Medical Specialty Societies Member section for introducing ORReady as a way to encourage surgical facilities to improve CME for improved surgical outcomes.
“We're looking for things that are best practices, are innovative, and that other people may want to replicate, adapt, [and] consider using,” Jann Balmer, Ph.D., president of the Alliance, said in an interview.
“It's very exciting to do this and see this great enthusiasm,” said Dr. Wetter. “For almost any doctor, the main concern is the safety of their patients.”
Dr. Wetter is now focusing on spreading the word and making more surgeons and hospitals aware of and involved in ORReady. “The more people that know about this, the more successful it's going to be.”
He hopes to see his project make an impact within the next few years.
It's a simple idea, but it could help save millions of lives: a Web site helping hospitals and surgeons worldwide improve surgical outcomes by making a commitment to implement proven protocols in their operating rooms, to share ideas, and to receive feedback on what works best.
Called ORReady, the grass roots project is the brainchild of Dr. Paul Alan Wetter, founder and chairman of the Society of Laparoendoscopic Surgeons.
Inspired by the humble beginnings of Facebook and the power of collaboration in the Human Genome Project, Dr. Wetter decided that his idea – a global effort to improve surgical outcomes – would be just as feasible, because “smart doctors around the world can get together and do it.” No bureaucracy. No big dollar budget.
Launched in early 2010, the project is still in its infancy, and the Web site (www.orready.com
“There are many examples of people who have really improved outcomes in surgery with increased use of safety measures,” said Dr. Wetter, who is an ob.gyn. in South Miami, Fla., and an internationally recognized leader in the field of minimally invasive surgery.
He hopes that by sharing OR safety information, there will be at least a 2%-3% improvement in outcomes. That's six million lives saved worldwide each year. He hopes that hospitals, medical societies, and surgical centers worldwide will sign onto this effort within the coming years.
He admits that this is a lofty goal. But he also believes that the increasing emphasis on improving patient safety will help the initiative take off. Add to that the power of technology and collaboration: “[The] world is becoming a small place and information is disseminated quickly,” said Dr. Wetter, who is also clinical professor emeritus at the University of Miami.
ORReady is a nonprofit project and is run by members and institutions that have volunteered their time and resources. The Web site follows the Creative Commons guidelines. “We encourage you to copy and use any materials that will help improve surgical outcome and create centers of merit in surgery and MIS [minimally invasive surgery].” It encourages hospitals and departments to download and sign an “Outcome Commitment Letter,” to choose from a set of protocols on the Web site that suit their operating rooms, and to register as an ORReady center of merit.
The guidelines suggest several main steps for surgeons and their teams: “Slow Down for Warm Up and Check Lists; Stop for Time Out before you Go.” A stoplight on the site sums up the message.
Soon, participants can register with an open-access database that can be used for sharing data, doing research to improve outcomes, and providing feedback. The school of biological and health systems engineering at Arizona State University, Tempe, has offered to help create the database.
“Information goes in; information comes out. We want to know about that [information] and we want to distribute it. Each hospital then can choose what works for them,” he said.
Dr. Wetter said that so far, he has approached a handful of institutions in the United States and abroad and has received a unanimously positive response.
“Using the power of the Internet to get ideas in one place is a great idea,” said Dr. Darrell A. Campbell Jr., the chief medical officer of the University of Michigan Medical System, who is not involved in the project. “I can support that wholeheartedly.”
His only concern, he said, was that procedures and processes that worked for one institution might now apply to another; more research and hands-on evaluation might be needed before the process can be implemented.
The project recently won its first award. The Society of Laparoendoscopic Surgeons won the 2011 Alliance for Continuing Medical Education Great Idea Award in the Medical Specialty Societies Member section for introducing ORReady as a way to encourage surgical facilities to improve CME for improved surgical outcomes.
“We're looking for things that are best practices, are innovative, and that other people may want to replicate, adapt, [and] consider using,” Jann Balmer, Ph.D., president of the Alliance, said in an interview.
“It's very exciting to do this and see this great enthusiasm,” said Dr. Wetter. “For almost any doctor, the main concern is the safety of their patients.”
Dr. Wetter is now focusing on spreading the word and making more surgeons and hospitals aware of and involved in ORReady. “The more people that know about this, the more successful it's going to be.”
He hopes to see his project make an impact within the next few years.
Policy & Practice : Want more health reform news? Subscribe to our podcast – search “Policy & Practice” in the iTunes store
New Public Health Goals Set
The nation's updated public health objectives, Healthy People 2020, will include dementias and sleep disorders as focus areas for the first time. “Our challenge and opportunity is to avoid preventable diseases from occurring in the first place,” said Health and Human Services Secretary Kathleen Sebelius in announcing the update. HHS set 10-year goals of raising public knowledge about sleep disorders, such as that they can increase heart disease and stroke risks, and sleep-disorder treatments. The document also calls for reductions in the morbidity and costs associated with Alzheimer's disease and other dementias, and improvements in the quality of life for people with these diseases.
O2 Denied for Headaches
The home use of oxygen to treat cluster headaches will not be covered under Medicare Part A or B except in an approved prospective clinical trial. There is not enough evidence that unsupervised use of oxygen works against the condition, according to a decision by the Centers for Medicare and Medicaid Services. The agency considered coverage after requests from the American Academy of Neurology and American Headache Society. The decision does not affect home use of oxygen already approved for other conditions.
Multiple Sclerosis Disparities
There are significant differences in symptoms and treatments of multiple sclerosis between whites, African Americans, and Hispanic Americans, according to a study published in the journal Ethnicity & Disease. Using data from the North American Research Committee on Multiple Sclerosis, the researchers showed that a larger proportion of African Americans with the disease has never been treated by a neurologist specializing in MS. Hispanic Americans with MS were more likely than whites or African Americans to have received no mental health care. African Americans are youngest when MS is diagnosed, Hispanic Americans next, and whites the oldest. The authors said that more research is needed into such differences, especially given the growing population of Hispanic Americans.
VA Issues Notice on Agent Orange
Hairy cell leukemia and other chronic B-cell leukemias, Parkinson's disease, and ischemic heart disease are now officially the only categories of disease the Department of Veterans Affairs acknowledges to have proven associations and presumptions of service connection with the use of Agent Orange and other herbicides during the Vietnam War. The VA posted that notice in the Federal Register in late December, and attributed its content to “careful review of the findings of the [National Academy of Sciences] Report, Veterans and Agent Orange Update 2008.” The “presumption of service connection” permits veterans to claim VA treatment of diseases without proving a link to their military service. The Agent Orange Act of 1991 directed the VA to work with the National Academy of Sciences to evaluate possible evidence of associations between exposure to herbicides used during the Vietnam War and suspected diseases.
Guilty of Inflated Prices
Three drug makers – Abbott Laboratories, B. Braun Medical, and Roxane Laboratories – have agreed to pay $421 million to settle the government's claim that they inflated wholesale prices of their drugs to get higher reimbursement from Medicare and Medicaid. The Department of Justice said that the government had paid “millions of claims of far greater amounts than it would have if Abbott, B. Braun, and Roxane had reported truthful prices.” Roxane was charged with reporting false prices for azathioprine, diclofenac sodium, furosemide, hydromorphone, ipratropium bromide, Oramorph SR, Roxanol, and Roxicodone. The Abbott products were dextrose solutions, sodium chloride solutions, sterile water, vancomycin, and erythromycin. B. Braun was alleged to have inflated prices for 49 products. The case was brought to light by a whistle-blower in Florida, who is to receive nearly $90 million, according to the government statement.
M.D. Heads Ways and Means Panel
Dr. Charles Boustany Jr., a Republican House member from Louisiana and a former cardiothoracic surgeon, has been named to head the Ways and Means Oversight and Investigations Subcommittee. In a statementhtfter his appointment, Rep. Boustany made no secret of his desire to take on the Affordable Care Act in his subcommittee. “As we begin to undo the damage caused by President Obama's health care law, I plan to hold IRS officials accountable to the taxpayers and press them on how this law will be implemented,” he said. “I also plan to work with the Government Accountability Office and other watchdog groups to identify existing programs … that warrant review and improvements to save taxpayer dollars and increase efficiency,” he said. Medicare and Medicaid are among the programs within the Ways and Means Committee's jurisdiction.
New Public Health Goals Set
The nation's updated public health objectives, Healthy People 2020, will include dementias and sleep disorders as focus areas for the first time. “Our challenge and opportunity is to avoid preventable diseases from occurring in the first place,” said Health and Human Services Secretary Kathleen Sebelius in announcing the update. HHS set 10-year goals of raising public knowledge about sleep disorders, such as that they can increase heart disease and stroke risks, and sleep-disorder treatments. The document also calls for reductions in the morbidity and costs associated with Alzheimer's disease and other dementias, and improvements in the quality of life for people with these diseases.
O2 Denied for Headaches
The home use of oxygen to treat cluster headaches will not be covered under Medicare Part A or B except in an approved prospective clinical trial. There is not enough evidence that unsupervised use of oxygen works against the condition, according to a decision by the Centers for Medicare and Medicaid Services. The agency considered coverage after requests from the American Academy of Neurology and American Headache Society. The decision does not affect home use of oxygen already approved for other conditions.
Multiple Sclerosis Disparities
There are significant differences in symptoms and treatments of multiple sclerosis between whites, African Americans, and Hispanic Americans, according to a study published in the journal Ethnicity & Disease. Using data from the North American Research Committee on Multiple Sclerosis, the researchers showed that a larger proportion of African Americans with the disease has never been treated by a neurologist specializing in MS. Hispanic Americans with MS were more likely than whites or African Americans to have received no mental health care. African Americans are youngest when MS is diagnosed, Hispanic Americans next, and whites the oldest. The authors said that more research is needed into such differences, especially given the growing population of Hispanic Americans.
VA Issues Notice on Agent Orange
Hairy cell leukemia and other chronic B-cell leukemias, Parkinson's disease, and ischemic heart disease are now officially the only categories of disease the Department of Veterans Affairs acknowledges to have proven associations and presumptions of service connection with the use of Agent Orange and other herbicides during the Vietnam War. The VA posted that notice in the Federal Register in late December, and attributed its content to “careful review of the findings of the [National Academy of Sciences] Report, Veterans and Agent Orange Update 2008.” The “presumption of service connection” permits veterans to claim VA treatment of diseases without proving a link to their military service. The Agent Orange Act of 1991 directed the VA to work with the National Academy of Sciences to evaluate possible evidence of associations between exposure to herbicides used during the Vietnam War and suspected diseases.
Guilty of Inflated Prices
Three drug makers – Abbott Laboratories, B. Braun Medical, and Roxane Laboratories – have agreed to pay $421 million to settle the government's claim that they inflated wholesale prices of their drugs to get higher reimbursement from Medicare and Medicaid. The Department of Justice said that the government had paid “millions of claims of far greater amounts than it would have if Abbott, B. Braun, and Roxane had reported truthful prices.” Roxane was charged with reporting false prices for azathioprine, diclofenac sodium, furosemide, hydromorphone, ipratropium bromide, Oramorph SR, Roxanol, and Roxicodone. The Abbott products were dextrose solutions, sodium chloride solutions, sterile water, vancomycin, and erythromycin. B. Braun was alleged to have inflated prices for 49 products. The case was brought to light by a whistle-blower in Florida, who is to receive nearly $90 million, according to the government statement.
M.D. Heads Ways and Means Panel
Dr. Charles Boustany Jr., a Republican House member from Louisiana and a former cardiothoracic surgeon, has been named to head the Ways and Means Oversight and Investigations Subcommittee. In a statementhtfter his appointment, Rep. Boustany made no secret of his desire to take on the Affordable Care Act in his subcommittee. “As we begin to undo the damage caused by President Obama's health care law, I plan to hold IRS officials accountable to the taxpayers and press them on how this law will be implemented,” he said. “I also plan to work with the Government Accountability Office and other watchdog groups to identify existing programs … that warrant review and improvements to save taxpayer dollars and increase efficiency,” he said. Medicare and Medicaid are among the programs within the Ways and Means Committee's jurisdiction.
New Public Health Goals Set
The nation's updated public health objectives, Healthy People 2020, will include dementias and sleep disorders as focus areas for the first time. “Our challenge and opportunity is to avoid preventable diseases from occurring in the first place,” said Health and Human Services Secretary Kathleen Sebelius in announcing the update. HHS set 10-year goals of raising public knowledge about sleep disorders, such as that they can increase heart disease and stroke risks, and sleep-disorder treatments. The document also calls for reductions in the morbidity and costs associated with Alzheimer's disease and other dementias, and improvements in the quality of life for people with these diseases.
O2 Denied for Headaches
The home use of oxygen to treat cluster headaches will not be covered under Medicare Part A or B except in an approved prospective clinical trial. There is not enough evidence that unsupervised use of oxygen works against the condition, according to a decision by the Centers for Medicare and Medicaid Services. The agency considered coverage after requests from the American Academy of Neurology and American Headache Society. The decision does not affect home use of oxygen already approved for other conditions.
Multiple Sclerosis Disparities
There are significant differences in symptoms and treatments of multiple sclerosis between whites, African Americans, and Hispanic Americans, according to a study published in the journal Ethnicity & Disease. Using data from the North American Research Committee on Multiple Sclerosis, the researchers showed that a larger proportion of African Americans with the disease has never been treated by a neurologist specializing in MS. Hispanic Americans with MS were more likely than whites or African Americans to have received no mental health care. African Americans are youngest when MS is diagnosed, Hispanic Americans next, and whites the oldest. The authors said that more research is needed into such differences, especially given the growing population of Hispanic Americans.
VA Issues Notice on Agent Orange
Hairy cell leukemia and other chronic B-cell leukemias, Parkinson's disease, and ischemic heart disease are now officially the only categories of disease the Department of Veterans Affairs acknowledges to have proven associations and presumptions of service connection with the use of Agent Orange and other herbicides during the Vietnam War. The VA posted that notice in the Federal Register in late December, and attributed its content to “careful review of the findings of the [National Academy of Sciences] Report, Veterans and Agent Orange Update 2008.” The “presumption of service connection” permits veterans to claim VA treatment of diseases without proving a link to their military service. The Agent Orange Act of 1991 directed the VA to work with the National Academy of Sciences to evaluate possible evidence of associations between exposure to herbicides used during the Vietnam War and suspected diseases.
Guilty of Inflated Prices
Three drug makers – Abbott Laboratories, B. Braun Medical, and Roxane Laboratories – have agreed to pay $421 million to settle the government's claim that they inflated wholesale prices of their drugs to get higher reimbursement from Medicare and Medicaid. The Department of Justice said that the government had paid “millions of claims of far greater amounts than it would have if Abbott, B. Braun, and Roxane had reported truthful prices.” Roxane was charged with reporting false prices for azathioprine, diclofenac sodium, furosemide, hydromorphone, ipratropium bromide, Oramorph SR, Roxanol, and Roxicodone. The Abbott products were dextrose solutions, sodium chloride solutions, sterile water, vancomycin, and erythromycin. B. Braun was alleged to have inflated prices for 49 products. The case was brought to light by a whistle-blower in Florida, who is to receive nearly $90 million, according to the government statement.
M.D. Heads Ways and Means Panel
Dr. Charles Boustany Jr., a Republican House member from Louisiana and a former cardiothoracic surgeon, has been named to head the Ways and Means Oversight and Investigations Subcommittee. In a statementhtfter his appointment, Rep. Boustany made no secret of his desire to take on the Affordable Care Act in his subcommittee. “As we begin to undo the damage caused by President Obama's health care law, I plan to hold IRS officials accountable to the taxpayers and press them on how this law will be implemented,” he said. “I also plan to work with the Government Accountability Office and other watchdog groups to identify existing programs … that warrant review and improvements to save taxpayer dollars and increase efficiency,” he said. Medicare and Medicaid are among the programs within the Ways and Means Committee's jurisdiction.
Feds Want Public Disclosure, Justification of Insurance Costs
In an effort to control rising health insurance rates and to bring transparency to the market, the federal government has proposed rules requiring insurers to publicly disclose and justify large rate increases.
Starting in 2011, proposed rate increases of 10% or higher will be publicly disclosed and reviewed to determine if the rate increase is reasonable, according to proposed regulations announced by Health and Human Services Secretary Kathleen Sebelius in December. The effort will be conducted in collaboration with the states.
The initial threshold for review is set at 10% in 2011, Ms. Sebelius said; however, starting in 2012, the states will set their own thresholds based on data and trends they gather. If a state is unable to do so, the proposed rule allows the HHS to do so.
Beginning in 2014, states will be able to exclude from the new health insurance exchanges any health plans that show a pattern of excessive or unjustified premium increases.
Ms. Sebelius said that the states will have the responsibility to keep insurance rates in check, and that the federal government is “not going to be sitting on state commissioners' shoulders and question what it is that they're doing.”
Over the past decade, the average health insurance premiums for family coverage have risen 131%, according to the HHS. Some states such as Connecticut and Rhode Island already have the power to review and reject excessive rate increases but not all do and some lack the legal authority or resources to do so.
“The proposed rate review policy will empower consumers, promote competition, encourage insurers to do more to control health care costs and discourage insurers from charging premiums which are unjustified,” Jay Angoff, director of the HHS Office of Consumer Information and Insurance Oversight, said in a statement.
The Affordable Care Act makes $250 million available to states to take action against insurers seeking unreasonable rate hikes, and so far $46 million has been awarded to 45 states and the District of Columbia for improving oversight of health insurance rate increases, according to the HHS. The proposed regulations also will work in conjunction with medical loss ratio regulations, which were released in November.
In a statement, Karen Ignani, president and CEO of the insurance trade group America's Health Insurance Plans, said, “While the proposed rule gives consideration to the impact of rising medical costs, it also establishes a threshold for review that is incomplete because it does not adequately factor in all of the components that determine premiums, including the cost of new benefit mandates and the impact of younger and healthier people dropping coverage. Premium review must consider the unique circumstances of small employers that are struggling to afford coverage for their employees, and of the individual market in which people move in and out of coverage depending on whether they anticipate needing medical services.”
She added, “It is also important to remember that the new federal law already caps health plans' administrative costs and profits. We welcome the opportunity to submit comments on this proposed rule.”
The proposed rule was published in the Federal Register on Dec. 23 and is open for public comment until Feb. 22. Comments can be filed at www.regulations.gov
For more information, visit www.hhs.gov/ociio/initiative/index.html
The federal government isn't going to sit on commissioners' shoulders and question what they're doing.
Source MS. SEBELIUS
In an effort to control rising health insurance rates and to bring transparency to the market, the federal government has proposed rules requiring insurers to publicly disclose and justify large rate increases.
Starting in 2011, proposed rate increases of 10% or higher will be publicly disclosed and reviewed to determine if the rate increase is reasonable, according to proposed regulations announced by Health and Human Services Secretary Kathleen Sebelius in December. The effort will be conducted in collaboration with the states.
The initial threshold for review is set at 10% in 2011, Ms. Sebelius said; however, starting in 2012, the states will set their own thresholds based on data and trends they gather. If a state is unable to do so, the proposed rule allows the HHS to do so.
Beginning in 2014, states will be able to exclude from the new health insurance exchanges any health plans that show a pattern of excessive or unjustified premium increases.
Ms. Sebelius said that the states will have the responsibility to keep insurance rates in check, and that the federal government is “not going to be sitting on state commissioners' shoulders and question what it is that they're doing.”
Over the past decade, the average health insurance premiums for family coverage have risen 131%, according to the HHS. Some states such as Connecticut and Rhode Island already have the power to review and reject excessive rate increases but not all do and some lack the legal authority or resources to do so.
“The proposed rate review policy will empower consumers, promote competition, encourage insurers to do more to control health care costs and discourage insurers from charging premiums which are unjustified,” Jay Angoff, director of the HHS Office of Consumer Information and Insurance Oversight, said in a statement.
The Affordable Care Act makes $250 million available to states to take action against insurers seeking unreasonable rate hikes, and so far $46 million has been awarded to 45 states and the District of Columbia for improving oversight of health insurance rate increases, according to the HHS. The proposed regulations also will work in conjunction with medical loss ratio regulations, which were released in November.
In a statement, Karen Ignani, president and CEO of the insurance trade group America's Health Insurance Plans, said, “While the proposed rule gives consideration to the impact of rising medical costs, it also establishes a threshold for review that is incomplete because it does not adequately factor in all of the components that determine premiums, including the cost of new benefit mandates and the impact of younger and healthier people dropping coverage. Premium review must consider the unique circumstances of small employers that are struggling to afford coverage for their employees, and of the individual market in which people move in and out of coverage depending on whether they anticipate needing medical services.”
She added, “It is also important to remember that the new federal law already caps health plans' administrative costs and profits. We welcome the opportunity to submit comments on this proposed rule.”
The proposed rule was published in the Federal Register on Dec. 23 and is open for public comment until Feb. 22. Comments can be filed at www.regulations.gov
For more information, visit www.hhs.gov/ociio/initiative/index.html
The federal government isn't going to sit on commissioners' shoulders and question what they're doing.
Source MS. SEBELIUS
In an effort to control rising health insurance rates and to bring transparency to the market, the federal government has proposed rules requiring insurers to publicly disclose and justify large rate increases.
Starting in 2011, proposed rate increases of 10% or higher will be publicly disclosed and reviewed to determine if the rate increase is reasonable, according to proposed regulations announced by Health and Human Services Secretary Kathleen Sebelius in December. The effort will be conducted in collaboration with the states.
The initial threshold for review is set at 10% in 2011, Ms. Sebelius said; however, starting in 2012, the states will set their own thresholds based on data and trends they gather. If a state is unable to do so, the proposed rule allows the HHS to do so.
Beginning in 2014, states will be able to exclude from the new health insurance exchanges any health plans that show a pattern of excessive or unjustified premium increases.
Ms. Sebelius said that the states will have the responsibility to keep insurance rates in check, and that the federal government is “not going to be sitting on state commissioners' shoulders and question what it is that they're doing.”
Over the past decade, the average health insurance premiums for family coverage have risen 131%, according to the HHS. Some states such as Connecticut and Rhode Island already have the power to review and reject excessive rate increases but not all do and some lack the legal authority or resources to do so.
“The proposed rate review policy will empower consumers, promote competition, encourage insurers to do more to control health care costs and discourage insurers from charging premiums which are unjustified,” Jay Angoff, director of the HHS Office of Consumer Information and Insurance Oversight, said in a statement.
The Affordable Care Act makes $250 million available to states to take action against insurers seeking unreasonable rate hikes, and so far $46 million has been awarded to 45 states and the District of Columbia for improving oversight of health insurance rate increases, according to the HHS. The proposed regulations also will work in conjunction with medical loss ratio regulations, which were released in November.
In a statement, Karen Ignani, president and CEO of the insurance trade group America's Health Insurance Plans, said, “While the proposed rule gives consideration to the impact of rising medical costs, it also establishes a threshold for review that is incomplete because it does not adequately factor in all of the components that determine premiums, including the cost of new benefit mandates and the impact of younger and healthier people dropping coverage. Premium review must consider the unique circumstances of small employers that are struggling to afford coverage for their employees, and of the individual market in which people move in and out of coverage depending on whether they anticipate needing medical services.”
She added, “It is also important to remember that the new federal law already caps health plans' administrative costs and profits. We welcome the opportunity to submit comments on this proposed rule.”
The proposed rule was published in the Federal Register on Dec. 23 and is open for public comment until Feb. 22. Comments can be filed at www.regulations.gov
For more information, visit www.hhs.gov/ociio/initiative/index.html
The federal government isn't going to sit on commissioners' shoulders and question what they're doing.
Source MS. SEBELIUS
Feds Want Insurance Costs to Be Transparent : The policy 'will empower consumers,' discourage insurers from charging unjustified premiums.
In an effort to control rising health insurance rates and to bring transparency to the market, the federal government has proposed rules requiring insurers to publicly disclose and justify large rate increases.
Starting this year, proposed rate increases of 10% or higher will be publicly disclosed and reviewed to determine if the rate increase is reasonable, according to proposed regulations announced by Health and Human Services Secretary Kathleen Sebelius. The effort will be conducted in collaboration with the states.
The initial threshold for review is set at 10% in this year, Ms. Sebelius said; however, starting in 2012, the states will set their own thresholds based on data and trends they gather. If a state is unable to do so, the proposed rule allows the HHS to do so.
Beginning in 2014, states will be able to exclude from the new health insurance exchanges any health plans that show a pattern of excessive or unjustified premium increases.
Ms. Sebelius said that the states will have the responsibility to keep insurance rates in check, and that the federal government is “not going to be sitting on state commissioners' shoulders and question what it is that they're doing.”
Over the past decade, the average health insurance premiums for family coverage have risen 131%, according to the HHS. Some states such as Connecticut and Rhode Island already have the power to review and reject excessive rate increases but not all do and some lack the legal authority or resources to do so.
“The proposed rate review policy will empower consumers, promote competition, encourage insurers to do more to control health care costs and discourage insurers from charging premiums which are unjustified,” Jay Angoff, director of the HHS Office of Consumer Information and Insurance Oversight, said in a statement.
The Affordable Care Act makes $250 million available to states to take action against insurers seeking unreasonable rate hikes, and so far $46 million has been awarded to 45 states and the District of Columbia for improving oversight of health insurance rate increases, according to the HHS. The proposed regulations also will work in conjunction with medical loss ratio regulations, which were released in November.
In a statement, Karen Ignani, president and CEO of the insurance trade group America's Health Insurance Plans, said, “While the proposed rule gives consideration to the impact of rising medical costs, it also establishes a threshold for review that is incomplete because it does not adequately factor in all of the components that determine premiums, including the cost of new benefit mandates and the impact of younger and healthier people dropping coverage.
Premium review must consider the unique circumstances of small employers that are struggling to afford coverage for their employees, and of the individual market in which people move in and out of coverage depending on whether they anticipate needing medical services.” She added, “It is also important to remember that the new federal law already caps health plans' administrative costs and profits. We welcome the opportunity to submit comments on this proposed rule.”
The proposed rule was published in the Federal Register Dec. 23 and is open for public comment until Feb. 22. Comments can be filed at www.regulations.gov
For more information, visit www.hhs.gov/ociio/initiative/index.html
Starting this year, proposed rate increases of 10% or higher will be publicly disclosed and reviewed.
Source MS. SEBELIUS
In an effort to control rising health insurance rates and to bring transparency to the market, the federal government has proposed rules requiring insurers to publicly disclose and justify large rate increases.
Starting this year, proposed rate increases of 10% or higher will be publicly disclosed and reviewed to determine if the rate increase is reasonable, according to proposed regulations announced by Health and Human Services Secretary Kathleen Sebelius. The effort will be conducted in collaboration with the states.
The initial threshold for review is set at 10% in this year, Ms. Sebelius said; however, starting in 2012, the states will set their own thresholds based on data and trends they gather. If a state is unable to do so, the proposed rule allows the HHS to do so.
Beginning in 2014, states will be able to exclude from the new health insurance exchanges any health plans that show a pattern of excessive or unjustified premium increases.
Ms. Sebelius said that the states will have the responsibility to keep insurance rates in check, and that the federal government is “not going to be sitting on state commissioners' shoulders and question what it is that they're doing.”
Over the past decade, the average health insurance premiums for family coverage have risen 131%, according to the HHS. Some states such as Connecticut and Rhode Island already have the power to review and reject excessive rate increases but not all do and some lack the legal authority or resources to do so.
“The proposed rate review policy will empower consumers, promote competition, encourage insurers to do more to control health care costs and discourage insurers from charging premiums which are unjustified,” Jay Angoff, director of the HHS Office of Consumer Information and Insurance Oversight, said in a statement.
The Affordable Care Act makes $250 million available to states to take action against insurers seeking unreasonable rate hikes, and so far $46 million has been awarded to 45 states and the District of Columbia for improving oversight of health insurance rate increases, according to the HHS. The proposed regulations also will work in conjunction with medical loss ratio regulations, which were released in November.
In a statement, Karen Ignani, president and CEO of the insurance trade group America's Health Insurance Plans, said, “While the proposed rule gives consideration to the impact of rising medical costs, it also establishes a threshold for review that is incomplete because it does not adequately factor in all of the components that determine premiums, including the cost of new benefit mandates and the impact of younger and healthier people dropping coverage.
Premium review must consider the unique circumstances of small employers that are struggling to afford coverage for their employees, and of the individual market in which people move in and out of coverage depending on whether they anticipate needing medical services.” She added, “It is also important to remember that the new federal law already caps health plans' administrative costs and profits. We welcome the opportunity to submit comments on this proposed rule.”
The proposed rule was published in the Federal Register Dec. 23 and is open for public comment until Feb. 22. Comments can be filed at www.regulations.gov
For more information, visit www.hhs.gov/ociio/initiative/index.html
Starting this year, proposed rate increases of 10% or higher will be publicly disclosed and reviewed.
Source MS. SEBELIUS
In an effort to control rising health insurance rates and to bring transparency to the market, the federal government has proposed rules requiring insurers to publicly disclose and justify large rate increases.
Starting this year, proposed rate increases of 10% or higher will be publicly disclosed and reviewed to determine if the rate increase is reasonable, according to proposed regulations announced by Health and Human Services Secretary Kathleen Sebelius. The effort will be conducted in collaboration with the states.
The initial threshold for review is set at 10% in this year, Ms. Sebelius said; however, starting in 2012, the states will set their own thresholds based on data and trends they gather. If a state is unable to do so, the proposed rule allows the HHS to do so.
Beginning in 2014, states will be able to exclude from the new health insurance exchanges any health plans that show a pattern of excessive or unjustified premium increases.
Ms. Sebelius said that the states will have the responsibility to keep insurance rates in check, and that the federal government is “not going to be sitting on state commissioners' shoulders and question what it is that they're doing.”
Over the past decade, the average health insurance premiums for family coverage have risen 131%, according to the HHS. Some states such as Connecticut and Rhode Island already have the power to review and reject excessive rate increases but not all do and some lack the legal authority or resources to do so.
“The proposed rate review policy will empower consumers, promote competition, encourage insurers to do more to control health care costs and discourage insurers from charging premiums which are unjustified,” Jay Angoff, director of the HHS Office of Consumer Information and Insurance Oversight, said in a statement.
The Affordable Care Act makes $250 million available to states to take action against insurers seeking unreasonable rate hikes, and so far $46 million has been awarded to 45 states and the District of Columbia for improving oversight of health insurance rate increases, according to the HHS. The proposed regulations also will work in conjunction with medical loss ratio regulations, which were released in November.
In a statement, Karen Ignani, president and CEO of the insurance trade group America's Health Insurance Plans, said, “While the proposed rule gives consideration to the impact of rising medical costs, it also establishes a threshold for review that is incomplete because it does not adequately factor in all of the components that determine premiums, including the cost of new benefit mandates and the impact of younger and healthier people dropping coverage.
Premium review must consider the unique circumstances of small employers that are struggling to afford coverage for their employees, and of the individual market in which people move in and out of coverage depending on whether they anticipate needing medical services.” She added, “It is also important to remember that the new federal law already caps health plans' administrative costs and profits. We welcome the opportunity to submit comments on this proposed rule.”
The proposed rule was published in the Federal Register Dec. 23 and is open for public comment until Feb. 22. Comments can be filed at www.regulations.gov
For more information, visit www.hhs.gov/ociio/initiative/index.html
Starting this year, proposed rate increases of 10% or higher will be publicly disclosed and reviewed.
Source MS. SEBELIUS
Bariatric Surgery Improved Knee OA, Metabolism
In obese patients with knee osteoarthritis, significant weight loss after bariatric surgery reduced pain and stiffness, decreased low-grade inflammation, and changed cartilage turnover, according to a study published in the journal.
In addition to the well-known relationship between obesity and onset of knee osteoarthritis (OA), several studies have now shown that the association goes beyond the increase in mechanical load on the tibiofemoral cartilage. “Adipose tissue may act as an endocrine organ, releasing several proinflammatory mediators and adipokines in blood that may participate in cartilage alteration in obese patients,” according to Dr. Pascal Richette of Hôpital Lariboisière and coauthors. The authors added, “Trials that have assessed the efficacy of surgically induced massive weight loss on knee OA symptoms are scarce and have not specifically included patients with well-defined radiographic evidence of knee OA, as in our study” (Ann. Rheum. Dis. 2011;70:139-44).
The authors studied 44 obese patients (36 women) with a baseline body mass index of 50.7 before surgery and moderate to severe knee OA. The patients underwent laparoscopic Roux-en-Y gastric bypass surgery or laparoscopic adjustable gastric banding. Patient data were collected before and 6 months after the surgery. At 6 months, patients had a 20% drop from baseline BMIs. Their VAS (visual acuity scores) decreased from 50 mm to 24.5 mm and their scores on the WOMAC (Western Ontario MacMaster) Questionnaire improved.
Significant decreases were seen in average serum levels of interleukin-6 (IL-6), which declined by 26%, and of high-sensitivity C-reactive protein (hsCRP), which dropped 46%. Also, weight loss was associated with changes in adipokine levels: Mean serum leptin concentration was decreased by 48% and serum level of adiponectin was increased by 21%, the authors reported.
The average serum level of procollagen type II N-terminal propeptide (PIIANP), a marker of cartilage synthesis, rose 32%, while the serum level of cartilage oligomeric matrix protein (COMP) decreased by 36%. “These results are the first to suggest a benefit of weight loss on both cartilage anabolism and catabolism,” the authors wrote.
The researchers found a significant correlation between IL-6 level and WOMAC Questionnaire scores as well as between urinary type II collagen helical peptide (helix-II) and hsCRP. Variation in COMP concentration was significantly correlated with changes in VAS pain scores and WOMAC stiffness score, the authors wrote, adding, “Our findings extend the results of recent work showing a significant association of IL-6 circulating levels and the prevalence and incidence of knee OA.”
In obese patients with knee osteoarthritis, significant weight loss after bariatric surgery reduced pain and stiffness, decreased low-grade inflammation, and changed cartilage turnover, according to a study published in the journal.
In addition to the well-known relationship between obesity and onset of knee osteoarthritis (OA), several studies have now shown that the association goes beyond the increase in mechanical load on the tibiofemoral cartilage. “Adipose tissue may act as an endocrine organ, releasing several proinflammatory mediators and adipokines in blood that may participate in cartilage alteration in obese patients,” according to Dr. Pascal Richette of Hôpital Lariboisière and coauthors. The authors added, “Trials that have assessed the efficacy of surgically induced massive weight loss on knee OA symptoms are scarce and have not specifically included patients with well-defined radiographic evidence of knee OA, as in our study” (Ann. Rheum. Dis. 2011;70:139-44).
The authors studied 44 obese patients (36 women) with a baseline body mass index of 50.7 before surgery and moderate to severe knee OA. The patients underwent laparoscopic Roux-en-Y gastric bypass surgery or laparoscopic adjustable gastric banding. Patient data were collected before and 6 months after the surgery. At 6 months, patients had a 20% drop from baseline BMIs. Their VAS (visual acuity scores) decreased from 50 mm to 24.5 mm and their scores on the WOMAC (Western Ontario MacMaster) Questionnaire improved.
Significant decreases were seen in average serum levels of interleukin-6 (IL-6), which declined by 26%, and of high-sensitivity C-reactive protein (hsCRP), which dropped 46%. Also, weight loss was associated with changes in adipokine levels: Mean serum leptin concentration was decreased by 48% and serum level of adiponectin was increased by 21%, the authors reported.
The average serum level of procollagen type II N-terminal propeptide (PIIANP), a marker of cartilage synthesis, rose 32%, while the serum level of cartilage oligomeric matrix protein (COMP) decreased by 36%. “These results are the first to suggest a benefit of weight loss on both cartilage anabolism and catabolism,” the authors wrote.
The researchers found a significant correlation between IL-6 level and WOMAC Questionnaire scores as well as between urinary type II collagen helical peptide (helix-II) and hsCRP. Variation in COMP concentration was significantly correlated with changes in VAS pain scores and WOMAC stiffness score, the authors wrote, adding, “Our findings extend the results of recent work showing a significant association of IL-6 circulating levels and the prevalence and incidence of knee OA.”
In obese patients with knee osteoarthritis, significant weight loss after bariatric surgery reduced pain and stiffness, decreased low-grade inflammation, and changed cartilage turnover, according to a study published in the journal.
In addition to the well-known relationship between obesity and onset of knee osteoarthritis (OA), several studies have now shown that the association goes beyond the increase in mechanical load on the tibiofemoral cartilage. “Adipose tissue may act as an endocrine organ, releasing several proinflammatory mediators and adipokines in blood that may participate in cartilage alteration in obese patients,” according to Dr. Pascal Richette of Hôpital Lariboisière and coauthors. The authors added, “Trials that have assessed the efficacy of surgically induced massive weight loss on knee OA symptoms are scarce and have not specifically included patients with well-defined radiographic evidence of knee OA, as in our study” (Ann. Rheum. Dis. 2011;70:139-44).
The authors studied 44 obese patients (36 women) with a baseline body mass index of 50.7 before surgery and moderate to severe knee OA. The patients underwent laparoscopic Roux-en-Y gastric bypass surgery or laparoscopic adjustable gastric banding. Patient data were collected before and 6 months after the surgery. At 6 months, patients had a 20% drop from baseline BMIs. Their VAS (visual acuity scores) decreased from 50 mm to 24.5 mm and their scores on the WOMAC (Western Ontario MacMaster) Questionnaire improved.
Significant decreases were seen in average serum levels of interleukin-6 (IL-6), which declined by 26%, and of high-sensitivity C-reactive protein (hsCRP), which dropped 46%. Also, weight loss was associated with changes in adipokine levels: Mean serum leptin concentration was decreased by 48% and serum level of adiponectin was increased by 21%, the authors reported.
The average serum level of procollagen type II N-terminal propeptide (PIIANP), a marker of cartilage synthesis, rose 32%, while the serum level of cartilage oligomeric matrix protein (COMP) decreased by 36%. “These results are the first to suggest a benefit of weight loss on both cartilage anabolism and catabolism,” the authors wrote.
The researchers found a significant correlation between IL-6 level and WOMAC Questionnaire scores as well as between urinary type II collagen helical peptide (helix-II) and hsCRP. Variation in COMP concentration was significantly correlated with changes in VAS pain scores and WOMAC stiffness score, the authors wrote, adding, “Our findings extend the results of recent work showing a significant association of IL-6 circulating levels and the prevalence and incidence of knee OA.”
Feds Want Insurance Costs to Be Transparent
In an effort to control rising health insurance rates and to bring transparency to the market, the federal government has proposed rules requiring insurers to publicly disclose and justify large rate increases.
Starting this year, proposed rate increases of 10% or higher will be publicly disclosed and reviewed to determine if the rate increase is reasonable, according to proposed regulations announced by Health and Human Services Secretary Kathleen Sebelius. The effort will be conducted in collaboration with the states.
The initial threshold for review is set at 10% for 2011, Ms. Sebelius said; however, starting in 2012, the states will set their own thresholds based on data and trends they gather. If a state is unable to do so, the proposed rule allows the HHS to do so.
Beginning in 2014, states will be able to exclude from the new health insurance exchanges any health plans that show a pattern of excessive or unjustified premium increases.
Ms. Sebelius said that the states will have the responsibility to keep insurance rates in check, and that the federal government is “not going to be sitting on state commissioners' shoulders and question what it is that they're doing.”
Over the past decade, the average health insurance premiums for family coverage have risen 131%, according to the HHS. Some states such as Connecticut and Rhode Island already have the power to review and reject excessive rate increases, but not all do and some lack the legal authority or resources to do so.
“The proposed rate review policy will empower consumers, promote competition, encourage insurers to do more to control health care costs and discourage insurers from charging premiums which are unjustified,” Jay Angoff, director of the HHS Office of Consumer Information and Insurance Oversight, said in a statement.
The Affordable Care Act makes $250 million available to states to take action against insurers seeking unreasonable rate hikes, and so far $46 million has been awarded to 45 states and the District of Columbia for improving oversight of health insurance rate increases, according to the HHS. The proposed regulations also will work in conjunction with medical loss ratio regulations, which were released in November.
In a statement, Karen Ignani, president and CEO of the insurance trade group America's Health Insurance Plans, said, “While the proposed rule gives consideration to the impact of rising medical costs, it also establishes a threshold for review that is incomplete because it does not adequately factor in all of the components that determine premiums, including the cost of new benefit mandates and the impact of younger and healthier people dropping coverage.
“Premium review must consider the unique circumstances of small employers who are struggling to afford coverage for their employees, and of the individual market in which people move in and out of coverage depending on whether they anticipate needing medical services.”
She added, “It is also important to remember that the new federal law already caps health plans' administrative costs and profits. We welcome the opportunity to submit comments on this proposed rule.”
The proposed rule was published in the Federal Register on Dec. 23 and is open for public comment until Feb. 22. Comments can be filed at www.regulations.govwww.hhs.gov/ociio/initiative/index.html
Starting this year, proposed rate increases of 10% or higher will be publicly disclosed and reviewed.
Source MS. SEBELIUS
In an effort to control rising health insurance rates and to bring transparency to the market, the federal government has proposed rules requiring insurers to publicly disclose and justify large rate increases.
Starting this year, proposed rate increases of 10% or higher will be publicly disclosed and reviewed to determine if the rate increase is reasonable, according to proposed regulations announced by Health and Human Services Secretary Kathleen Sebelius. The effort will be conducted in collaboration with the states.
The initial threshold for review is set at 10% for 2011, Ms. Sebelius said; however, starting in 2012, the states will set their own thresholds based on data and trends they gather. If a state is unable to do so, the proposed rule allows the HHS to do so.
Beginning in 2014, states will be able to exclude from the new health insurance exchanges any health plans that show a pattern of excessive or unjustified premium increases.
Ms. Sebelius said that the states will have the responsibility to keep insurance rates in check, and that the federal government is “not going to be sitting on state commissioners' shoulders and question what it is that they're doing.”
Over the past decade, the average health insurance premiums for family coverage have risen 131%, according to the HHS. Some states such as Connecticut and Rhode Island already have the power to review and reject excessive rate increases, but not all do and some lack the legal authority or resources to do so.
“The proposed rate review policy will empower consumers, promote competition, encourage insurers to do more to control health care costs and discourage insurers from charging premiums which are unjustified,” Jay Angoff, director of the HHS Office of Consumer Information and Insurance Oversight, said in a statement.
The Affordable Care Act makes $250 million available to states to take action against insurers seeking unreasonable rate hikes, and so far $46 million has been awarded to 45 states and the District of Columbia for improving oversight of health insurance rate increases, according to the HHS. The proposed regulations also will work in conjunction with medical loss ratio regulations, which were released in November.
In a statement, Karen Ignani, president and CEO of the insurance trade group America's Health Insurance Plans, said, “While the proposed rule gives consideration to the impact of rising medical costs, it also establishes a threshold for review that is incomplete because it does not adequately factor in all of the components that determine premiums, including the cost of new benefit mandates and the impact of younger and healthier people dropping coverage.
“Premium review must consider the unique circumstances of small employers who are struggling to afford coverage for their employees, and of the individual market in which people move in and out of coverage depending on whether they anticipate needing medical services.”
She added, “It is also important to remember that the new federal law already caps health plans' administrative costs and profits. We welcome the opportunity to submit comments on this proposed rule.”
The proposed rule was published in the Federal Register on Dec. 23 and is open for public comment until Feb. 22. Comments can be filed at www.regulations.govwww.hhs.gov/ociio/initiative/index.html
Starting this year, proposed rate increases of 10% or higher will be publicly disclosed and reviewed.
Source MS. SEBELIUS
In an effort to control rising health insurance rates and to bring transparency to the market, the federal government has proposed rules requiring insurers to publicly disclose and justify large rate increases.
Starting this year, proposed rate increases of 10% or higher will be publicly disclosed and reviewed to determine if the rate increase is reasonable, according to proposed regulations announced by Health and Human Services Secretary Kathleen Sebelius. The effort will be conducted in collaboration with the states.
The initial threshold for review is set at 10% for 2011, Ms. Sebelius said; however, starting in 2012, the states will set their own thresholds based on data and trends they gather. If a state is unable to do so, the proposed rule allows the HHS to do so.
Beginning in 2014, states will be able to exclude from the new health insurance exchanges any health plans that show a pattern of excessive or unjustified premium increases.
Ms. Sebelius said that the states will have the responsibility to keep insurance rates in check, and that the federal government is “not going to be sitting on state commissioners' shoulders and question what it is that they're doing.”
Over the past decade, the average health insurance premiums for family coverage have risen 131%, according to the HHS. Some states such as Connecticut and Rhode Island already have the power to review and reject excessive rate increases, but not all do and some lack the legal authority or resources to do so.
“The proposed rate review policy will empower consumers, promote competition, encourage insurers to do more to control health care costs and discourage insurers from charging premiums which are unjustified,” Jay Angoff, director of the HHS Office of Consumer Information and Insurance Oversight, said in a statement.
The Affordable Care Act makes $250 million available to states to take action against insurers seeking unreasonable rate hikes, and so far $46 million has been awarded to 45 states and the District of Columbia for improving oversight of health insurance rate increases, according to the HHS. The proposed regulations also will work in conjunction with medical loss ratio regulations, which were released in November.
In a statement, Karen Ignani, president and CEO of the insurance trade group America's Health Insurance Plans, said, “While the proposed rule gives consideration to the impact of rising medical costs, it also establishes a threshold for review that is incomplete because it does not adequately factor in all of the components that determine premiums, including the cost of new benefit mandates and the impact of younger and healthier people dropping coverage.
“Premium review must consider the unique circumstances of small employers who are struggling to afford coverage for their employees, and of the individual market in which people move in and out of coverage depending on whether they anticipate needing medical services.”
She added, “It is also important to remember that the new federal law already caps health plans' administrative costs and profits. We welcome the opportunity to submit comments on this proposed rule.”
The proposed rule was published in the Federal Register on Dec. 23 and is open for public comment until Feb. 22. Comments can be filed at www.regulations.govwww.hhs.gov/ociio/initiative/index.html
Starting this year, proposed rate increases of 10% or higher will be publicly disclosed and reviewed.
Source MS. SEBELIUS