User login
Cocaine-induced ecchymotic rash
A 50-year-old man presented with a painful rash over his extremities for the past 2 days (Figure 1). He said he had been in his usual state of health until the day he woke up with the rash. The rash was initially limited to his upper and lower extremities, but the next day he noticed similar lesions over his cheek and hard palate. He was a smoker and was known to have hepatitis C virus infection. He denied recent trauma, fever, or chills. He said he had snorted cocaine about 24 hours before the rash first appeared.
On examination, his vital signs were normal. He had an extensive retiform rash involving the upper and lower extremities, earlobes, right cheek, and hard palate. Otherwise, the physical examination was normal.
Initial laboratory evaluation showed:
- Hemoglobin 11.5 g/dL (reference range 14.0–17.5)
- White blood cell count 2.1 × 109/L (4.5–11.0)
- Platelet count 168 × 109/L (150–350)
- Absolute neutrophil count 0.9 × 109/L (≥ 1.5)
- Urine toxicology screen positive for cocaine.
He was admitted to the hospital and was started on intravenous vancomycin and piperacillin-sulbactam for a presumed infectious cause of the rash.
One day later, testing for myeloperoxidase-specific antineutrophil cytoplasmic antibodies (p-ANCA) was strongly positive. Skin biopsy revealed leukocytoclastic vasculitis with small-vessel thrombosis. These findings, along with the timing of the appearance of the rash after his cocaine use, led to a diagnosis of levamisole-adulterated cocaine-induced vasculitis.
LEVAMISOLE AND VASCULITIS
Levamisole used to be used as an antihelminthic and as an adjuvant chemotherapeutic agent, but it is also added to cocaine to increase its euphoric and psychotropic effects.1 It has been withdrawn from the market for human use because of toxic side effects including agranulocytosis, vasculitis, and autoantibody positivity.
Levamisole-adulterated cocaine has been known to induce ANCA-associated vasculitis.2 Symptoms of levamisole-induced vasculitis usually start a few hours to a few days after the last dose of cocaine. Almost all patients with this condition present with a characteristic retiform purpuric rash, which has a predilection for the ears, nose, cheeks, and extremities. It also causes neutropenia and agranulocytosis (as well as autoantibodies, including antinuclear antibodies and antiphospholipid antibodies).
The characteristic lesions tend to be in a stellate pattern with erythematous borders. They often but not always have a central necrotic area. The location of the rash and the fact that it resolves after discontinuation of the offending agent help distinguish this condition from other types of vasculitis. Usually, antibodies against myeloperoxidase are present.3
Leukopenia does not typically occur in patients with primary vasculitis syndromes. The literature is mixed on the presence or absence of specific ANCAs.
Inflammatory and noninflammatory vasculopathic disorders that can present similarly include disseminated intravascular coagulation, antiphospholipid syndrome, warfarin-induced skin necrosis, paroxysmal nocturnal hemoglobinuria, cryoglobulinemia, vasculitis, and calciphylaxis.
Treatment is mainly supportive, but emollients and steroids4 have been reported to alleviate the symptoms. Surgical debridement is rarely needed unless skin necrosis is extensive.
Levamisole-induced vasculitis is a diagnosis of exclusion but should be strongly considered when the rash is associated with recent cocaine use, retiform purpura, or bullae with skin involvement, leukopenia, and positive ANCA in high titers.3 If cocaine use is discontinued, the syndrome is expected to resolve.
- Tervaert JW, Stegeman CA. A difficult diagnosis. Lancet 2004; 364:1313–1314.
- Chang A, Osterloh J, Thomas J. Levamisole: a dangerous new cocaine adulterant. Clin Pharmacol Ther 2010; 88:408–411.
- Gross RL, Brucker J, Bahce-Altuntas A, et al. A novel cutaneous vasculitis syndrome induced by levamisole-contaminated cocaine. Clin Rheumatol 2011; 30:1385–1392.
- Carter MR, Amirhaeri S. p-ANCA-associated vasculitis caused by levamisole-adulterated cocaine: a case report. Case Rep Emerg Med 2013; 2013:878903.
A 50-year-old man presented with a painful rash over his extremities for the past 2 days (Figure 1). He said he had been in his usual state of health until the day he woke up with the rash. The rash was initially limited to his upper and lower extremities, but the next day he noticed similar lesions over his cheek and hard palate. He was a smoker and was known to have hepatitis C virus infection. He denied recent trauma, fever, or chills. He said he had snorted cocaine about 24 hours before the rash first appeared.
On examination, his vital signs were normal. He had an extensive retiform rash involving the upper and lower extremities, earlobes, right cheek, and hard palate. Otherwise, the physical examination was normal.
Initial laboratory evaluation showed:
- Hemoglobin 11.5 g/dL (reference range 14.0–17.5)
- White blood cell count 2.1 × 109/L (4.5–11.0)
- Platelet count 168 × 109/L (150–350)
- Absolute neutrophil count 0.9 × 109/L (≥ 1.5)
- Urine toxicology screen positive for cocaine.
He was admitted to the hospital and was started on intravenous vancomycin and piperacillin-sulbactam for a presumed infectious cause of the rash.
One day later, testing for myeloperoxidase-specific antineutrophil cytoplasmic antibodies (p-ANCA) was strongly positive. Skin biopsy revealed leukocytoclastic vasculitis with small-vessel thrombosis. These findings, along with the timing of the appearance of the rash after his cocaine use, led to a diagnosis of levamisole-adulterated cocaine-induced vasculitis.
LEVAMISOLE AND VASCULITIS
Levamisole used to be used as an antihelminthic and as an adjuvant chemotherapeutic agent, but it is also added to cocaine to increase its euphoric and psychotropic effects.1 It has been withdrawn from the market for human use because of toxic side effects including agranulocytosis, vasculitis, and autoantibody positivity.
Levamisole-adulterated cocaine has been known to induce ANCA-associated vasculitis.2 Symptoms of levamisole-induced vasculitis usually start a few hours to a few days after the last dose of cocaine. Almost all patients with this condition present with a characteristic retiform purpuric rash, which has a predilection for the ears, nose, cheeks, and extremities. It also causes neutropenia and agranulocytosis (as well as autoantibodies, including antinuclear antibodies and antiphospholipid antibodies).
The characteristic lesions tend to be in a stellate pattern with erythematous borders. They often but not always have a central necrotic area. The location of the rash and the fact that it resolves after discontinuation of the offending agent help distinguish this condition from other types of vasculitis. Usually, antibodies against myeloperoxidase are present.3
Leukopenia does not typically occur in patients with primary vasculitis syndromes. The literature is mixed on the presence or absence of specific ANCAs.
Inflammatory and noninflammatory vasculopathic disorders that can present similarly include disseminated intravascular coagulation, antiphospholipid syndrome, warfarin-induced skin necrosis, paroxysmal nocturnal hemoglobinuria, cryoglobulinemia, vasculitis, and calciphylaxis.
Treatment is mainly supportive, but emollients and steroids4 have been reported to alleviate the symptoms. Surgical debridement is rarely needed unless skin necrosis is extensive.
Levamisole-induced vasculitis is a diagnosis of exclusion but should be strongly considered when the rash is associated with recent cocaine use, retiform purpura, or bullae with skin involvement, leukopenia, and positive ANCA in high titers.3 If cocaine use is discontinued, the syndrome is expected to resolve.
A 50-year-old man presented with a painful rash over his extremities for the past 2 days (Figure 1). He said he had been in his usual state of health until the day he woke up with the rash. The rash was initially limited to his upper and lower extremities, but the next day he noticed similar lesions over his cheek and hard palate. He was a smoker and was known to have hepatitis C virus infection. He denied recent trauma, fever, or chills. He said he had snorted cocaine about 24 hours before the rash first appeared.
On examination, his vital signs were normal. He had an extensive retiform rash involving the upper and lower extremities, earlobes, right cheek, and hard palate. Otherwise, the physical examination was normal.
Initial laboratory evaluation showed:
- Hemoglobin 11.5 g/dL (reference range 14.0–17.5)
- White blood cell count 2.1 × 109/L (4.5–11.0)
- Platelet count 168 × 109/L (150–350)
- Absolute neutrophil count 0.9 × 109/L (≥ 1.5)
- Urine toxicology screen positive for cocaine.
He was admitted to the hospital and was started on intravenous vancomycin and piperacillin-sulbactam for a presumed infectious cause of the rash.
One day later, testing for myeloperoxidase-specific antineutrophil cytoplasmic antibodies (p-ANCA) was strongly positive. Skin biopsy revealed leukocytoclastic vasculitis with small-vessel thrombosis. These findings, along with the timing of the appearance of the rash after his cocaine use, led to a diagnosis of levamisole-adulterated cocaine-induced vasculitis.
LEVAMISOLE AND VASCULITIS
Levamisole used to be used as an antihelminthic and as an adjuvant chemotherapeutic agent, but it is also added to cocaine to increase its euphoric and psychotropic effects.1 It has been withdrawn from the market for human use because of toxic side effects including agranulocytosis, vasculitis, and autoantibody positivity.
Levamisole-adulterated cocaine has been known to induce ANCA-associated vasculitis.2 Symptoms of levamisole-induced vasculitis usually start a few hours to a few days after the last dose of cocaine. Almost all patients with this condition present with a characteristic retiform purpuric rash, which has a predilection for the ears, nose, cheeks, and extremities. It also causes neutropenia and agranulocytosis (as well as autoantibodies, including antinuclear antibodies and antiphospholipid antibodies).
The characteristic lesions tend to be in a stellate pattern with erythematous borders. They often but not always have a central necrotic area. The location of the rash and the fact that it resolves after discontinuation of the offending agent help distinguish this condition from other types of vasculitis. Usually, antibodies against myeloperoxidase are present.3
Leukopenia does not typically occur in patients with primary vasculitis syndromes. The literature is mixed on the presence or absence of specific ANCAs.
Inflammatory and noninflammatory vasculopathic disorders that can present similarly include disseminated intravascular coagulation, antiphospholipid syndrome, warfarin-induced skin necrosis, paroxysmal nocturnal hemoglobinuria, cryoglobulinemia, vasculitis, and calciphylaxis.
Treatment is mainly supportive, but emollients and steroids4 have been reported to alleviate the symptoms. Surgical debridement is rarely needed unless skin necrosis is extensive.
Levamisole-induced vasculitis is a diagnosis of exclusion but should be strongly considered when the rash is associated with recent cocaine use, retiform purpura, or bullae with skin involvement, leukopenia, and positive ANCA in high titers.3 If cocaine use is discontinued, the syndrome is expected to resolve.
- Tervaert JW, Stegeman CA. A difficult diagnosis. Lancet 2004; 364:1313–1314.
- Chang A, Osterloh J, Thomas J. Levamisole: a dangerous new cocaine adulterant. Clin Pharmacol Ther 2010; 88:408–411.
- Gross RL, Brucker J, Bahce-Altuntas A, et al. A novel cutaneous vasculitis syndrome induced by levamisole-contaminated cocaine. Clin Rheumatol 2011; 30:1385–1392.
- Carter MR, Amirhaeri S. p-ANCA-associated vasculitis caused by levamisole-adulterated cocaine: a case report. Case Rep Emerg Med 2013; 2013:878903.
- Tervaert JW, Stegeman CA. A difficult diagnosis. Lancet 2004; 364:1313–1314.
- Chang A, Osterloh J, Thomas J. Levamisole: a dangerous new cocaine adulterant. Clin Pharmacol Ther 2010; 88:408–411.
- Gross RL, Brucker J, Bahce-Altuntas A, et al. A novel cutaneous vasculitis syndrome induced by levamisole-contaminated cocaine. Clin Rheumatol 2011; 30:1385–1392.
- Carter MR, Amirhaeri S. p-ANCA-associated vasculitis caused by levamisole-adulterated cocaine: a case report. Case Rep Emerg Med 2013; 2013:878903.
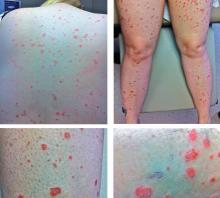
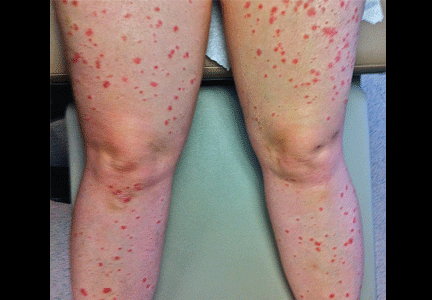
A rash after streptococcal infection
A previously healthy 39-year-old woman presented to the emergency department with 7 days of a gradually worsening rash. One week before the onset of the rash, her primary care physician had diagnosed streptococcal pharyngitis, for which she was treated with oral amoxicillin. She had no history of skin or joint problems and was not currently taking any medications.
She was afebrile and her vital signs were normal. She had mild pharyngeal erythema but no palpable cervical lymph nodes. The skin examination showed well demarcated, erythmatous papules 1 cm in diameter, with overlying scales over the entire body, sparing the palms and the soles of the feet (Figure 1).
Q: Which is the most likely diagnosis?
- Impetigo
- Drug reaction
- Guttate psoriasis
- Nummular eczema
- Pityriasis rosea
A: The most likely diagnosis is guttate psoriasis.
Guttate psoriasis is a relatively uncommon condition that affects less than 2% of patients with psoriasis, primarily children and young adults. It is strongly associated with recent or concomitant beta-hemolytic streptococcal infection.1 The rash usually develops 1 to 2 weeks after the streptococcal pharyngitis or upper respiratory tract infection. Other organisms involved in guttate psoriasis are Staphylococcus aureus, Candida, and viruses such as human papillomavirus, human immunodeficiency virus, and human endogenous retrovirus. 2 Several commonly used drugs are also implicated in psoriasiform eruptions, including beta-blockers, nonsteroidal anti-inflammatory drugs, angiotensin-converting enzyme inhibitors, lithium, metformin, and digoxin.
Acute onset of skin lesions caused by streptococcal infection can be either the first manifestation in a previously unaffected person or an acute exacerbation of long-standing psoriasis. Skin lesions are usually scaly, erythematous, and guttate (drop-shaped); they primarily involve the trunk but can spread to the rest of the body, sparing the palms and soles.
Throat culture should be done to confirm streptococcal infection. Titers of antistreptolysin O are elevated in more than half of patients with guttate psoriasis. Histopathologic examination can differentiate guttate psoriasis from other psoriasiform conditions, such as pityriasis rosea, secondary syphilis, and lichen simplex chronicus; however, the clinical appearance of the rash is so characteristic that biopsy is not usually needed to confirm the diagnosis.
Guttate psoriasis responds well to phototherapy with ultraviolet B radiation and medium-potency topical corticosteroids.3 And since streptococcal throat infection triggers the condition, it must also be treated for complete recovery.
CASE CONTINUED
Our patient was treated with topical steroid creams. Her rash improved slowly and had completely resolved in 6 weeks.
- England RJ, Strachan DR, Knight LC. Streptococcal tonsillitis and its association with psoriasis: a review. Clin Otolaryngol Allied Sci 1997; 22:532–535.
- Fry L, Baker BS. Triggering psoriasis: the role of infections and medications. Clin Dermatol 2007; 25:606–615.
- Thappa DM, Laxmisha C. Suit PUVA as an effective and safe modality of treatment in guttate psoriasis. J Eur Acad Dermatol Venereol 2006; 20:1146–1147.
A previously healthy 39-year-old woman presented to the emergency department with 7 days of a gradually worsening rash. One week before the onset of the rash, her primary care physician had diagnosed streptococcal pharyngitis, for which she was treated with oral amoxicillin. She had no history of skin or joint problems and was not currently taking any medications.
She was afebrile and her vital signs were normal. She had mild pharyngeal erythema but no palpable cervical lymph nodes. The skin examination showed well demarcated, erythmatous papules 1 cm in diameter, with overlying scales over the entire body, sparing the palms and the soles of the feet (Figure 1).
Q: Which is the most likely diagnosis?
- Impetigo
- Drug reaction
- Guttate psoriasis
- Nummular eczema
- Pityriasis rosea
A: The most likely diagnosis is guttate psoriasis.
Guttate psoriasis is a relatively uncommon condition that affects less than 2% of patients with psoriasis, primarily children and young adults. It is strongly associated with recent or concomitant beta-hemolytic streptococcal infection.1 The rash usually develops 1 to 2 weeks after the streptococcal pharyngitis or upper respiratory tract infection. Other organisms involved in guttate psoriasis are Staphylococcus aureus, Candida, and viruses such as human papillomavirus, human immunodeficiency virus, and human endogenous retrovirus. 2 Several commonly used drugs are also implicated in psoriasiform eruptions, including beta-blockers, nonsteroidal anti-inflammatory drugs, angiotensin-converting enzyme inhibitors, lithium, metformin, and digoxin.
Acute onset of skin lesions caused by streptococcal infection can be either the first manifestation in a previously unaffected person or an acute exacerbation of long-standing psoriasis. Skin lesions are usually scaly, erythematous, and guttate (drop-shaped); they primarily involve the trunk but can spread to the rest of the body, sparing the palms and soles.
Throat culture should be done to confirm streptococcal infection. Titers of antistreptolysin O are elevated in more than half of patients with guttate psoriasis. Histopathologic examination can differentiate guttate psoriasis from other psoriasiform conditions, such as pityriasis rosea, secondary syphilis, and lichen simplex chronicus; however, the clinical appearance of the rash is so characteristic that biopsy is not usually needed to confirm the diagnosis.
Guttate psoriasis responds well to phototherapy with ultraviolet B radiation and medium-potency topical corticosteroids.3 And since streptococcal throat infection triggers the condition, it must also be treated for complete recovery.
CASE CONTINUED
Our patient was treated with topical steroid creams. Her rash improved slowly and had completely resolved in 6 weeks.
A previously healthy 39-year-old woman presented to the emergency department with 7 days of a gradually worsening rash. One week before the onset of the rash, her primary care physician had diagnosed streptococcal pharyngitis, for which she was treated with oral amoxicillin. She had no history of skin or joint problems and was not currently taking any medications.
She was afebrile and her vital signs were normal. She had mild pharyngeal erythema but no palpable cervical lymph nodes. The skin examination showed well demarcated, erythmatous papules 1 cm in diameter, with overlying scales over the entire body, sparing the palms and the soles of the feet (Figure 1).
Q: Which is the most likely diagnosis?
- Impetigo
- Drug reaction
- Guttate psoriasis
- Nummular eczema
- Pityriasis rosea
A: The most likely diagnosis is guttate psoriasis.
Guttate psoriasis is a relatively uncommon condition that affects less than 2% of patients with psoriasis, primarily children and young adults. It is strongly associated with recent or concomitant beta-hemolytic streptococcal infection.1 The rash usually develops 1 to 2 weeks after the streptococcal pharyngitis or upper respiratory tract infection. Other organisms involved in guttate psoriasis are Staphylococcus aureus, Candida, and viruses such as human papillomavirus, human immunodeficiency virus, and human endogenous retrovirus. 2 Several commonly used drugs are also implicated in psoriasiform eruptions, including beta-blockers, nonsteroidal anti-inflammatory drugs, angiotensin-converting enzyme inhibitors, lithium, metformin, and digoxin.
Acute onset of skin lesions caused by streptococcal infection can be either the first manifestation in a previously unaffected person or an acute exacerbation of long-standing psoriasis. Skin lesions are usually scaly, erythematous, and guttate (drop-shaped); they primarily involve the trunk but can spread to the rest of the body, sparing the palms and soles.
Throat culture should be done to confirm streptococcal infection. Titers of antistreptolysin O are elevated in more than half of patients with guttate psoriasis. Histopathologic examination can differentiate guttate psoriasis from other psoriasiform conditions, such as pityriasis rosea, secondary syphilis, and lichen simplex chronicus; however, the clinical appearance of the rash is so characteristic that biopsy is not usually needed to confirm the diagnosis.
Guttate psoriasis responds well to phototherapy with ultraviolet B radiation and medium-potency topical corticosteroids.3 And since streptococcal throat infection triggers the condition, it must also be treated for complete recovery.
CASE CONTINUED
Our patient was treated with topical steroid creams. Her rash improved slowly and had completely resolved in 6 weeks.
- England RJ, Strachan DR, Knight LC. Streptococcal tonsillitis and its association with psoriasis: a review. Clin Otolaryngol Allied Sci 1997; 22:532–535.
- Fry L, Baker BS. Triggering psoriasis: the role of infections and medications. Clin Dermatol 2007; 25:606–615.
- Thappa DM, Laxmisha C. Suit PUVA as an effective and safe modality of treatment in guttate psoriasis. J Eur Acad Dermatol Venereol 2006; 20:1146–1147.
- England RJ, Strachan DR, Knight LC. Streptococcal tonsillitis and its association with psoriasis: a review. Clin Otolaryngol Allied Sci 1997; 22:532–535.
- Fry L, Baker BS. Triggering psoriasis: the role of infections and medications. Clin Dermatol 2007; 25:606–615.
- Thappa DM, Laxmisha C. Suit PUVA as an effective and safe modality of treatment in guttate psoriasis. J Eur Acad Dermatol Venereol 2006; 20:1146–1147.
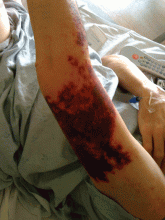
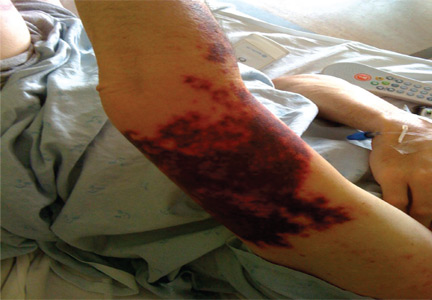
Purpuric lesion on the elbow
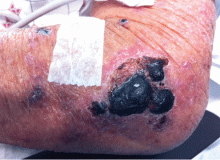
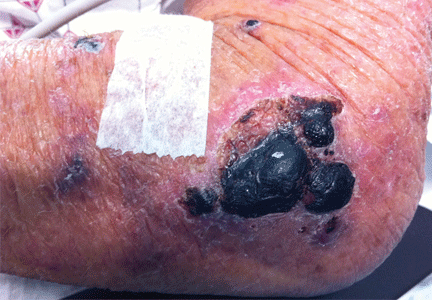
A 75-year-old man was admitted to the hospital with new-onset atrial fibrillation. He underwent rate control, and a heparin infusion was started. Warfarin (Coumadin) 10 mg was added on the second hospital day. Two days later, the heparin infusion was discontinued when the international normalized ratio (INR) was in the therapeutic range.
Q: Which is the most likely diagnosis?
- Pyoderma gangrenosum
- Cutaneous vasculitis
- Warfarin-induced skin necrosis
- Ecthyma gangrenosum
- Dermatitis herpetiformis
A: The most likely diagnosis is warfarin-induced skin necrosis, a rare paradoxical complication that occurs in 0.01% to 0.1% of patients receiving this drug.1 Microthrombosis leads to necrosis of the skin and subcutaneous tissues, arising within 2 to 10 days after the start of anticoagulation therapy, although in rare cases it can occur months to years later.2,3
The most common risk factors include the unopposed use of warfarin (ie, unopposed by heparin at the start of therapy), using higher doses of warfarin during the initiation of anticoagulation, and inadequate overlap with an effective parenteral anticoagulant. Patients with protein C or S deficiency, heparin-induced thrombocytopenia,4 resistance to activated protein C, antithrombin deficiency, and lupus anticoagulant have also been reported to be at risk.
The most common sites affected are areas with high subcutaneous fat content, such as the abdomen, thighs, breasts, and buttocks. Skin presentations can vary from dermal plaques to petechial lesions, which rapidly progress to well-demarcated, bluish-black, painful lesions and eventually to hemorrhagic bullae and necrosis.1
At the start of warfarin therapy, the levels of protein C and factor VII (with half-lives of 5 to 8 hours) fall faster than those of other vitamin-K-dependent factors (ie, factors II, IX, and X). This causes a transient imbalance in procoagulant and anticoagulant pathways favoring thrombosis of the microvasculature, with resulting necrosis. Patients with hereditary protein C deficiency are at higher risk.5
Histologic review of lesions often shows venous thrombosis and diffuse necrosis of the dermis and subcutaneous tissue.2
Promptly stopping the warfarin and choosing alternative anticoagulation may help prevent further progression of this condition. Wound care, debridement, and sometimes skin grafting may be necessary, depending on the extent of the lesions. A rechallenge with warfarin is often difficult, but cases have been reported in which treatment was resumed without adverse consequences.6 Avoiding large loading doses of warfarin, gradually increasing doses over an extended period (about 10 days),3 and starting warfarin with a heparin bridge for at least 5 days (which was not done in this patient) would prevent the condition.
Early recognition, differentiation, and diagnosis are essential to minimize morbidity and to prevent death.
CASE CONTINUED
Warfarin was discontinued once the patient developed the skin lesions. He received vitamin K and fresh frozen plasma to normalize his INR, and he was started on a heparin infusion, after which the lesions began to heal. The patient refused a skin biopsy. Platelet counts remained stable during his hospital course. Protein C levels were not checked, given his recent use of warfarin. He was started on dabigatran (Pradaxa) and was discharged a week later.
THE OTHER DIAGNOSTIC CHOICES
Pyoderma gangrenosum is an uncommon ulcerative skin condition often associated with autoimmune disease. It usually starts at the site of a minor injury, more commonly on the legs, and gradually progresses to a painful ulcer.
Cutaneous vasculitis is an inflammation of small blood vessels characterized by palpable purpura. The lesions can resemble urticaria, petechia, or erythema multiforme. It is commonly associated with infection, drug therapy, inflammatory disease, and malignancy.
Ecthyma gangrenosum is an infection of skin caused by Pseudomonas aeruginosa. Usually, it presents as hemorrhagic pustules or infarct-like areas with surrounding erythema that evolve into necrotic ulcers surrounded by erythema.
Dermatitis herpetiformis is a chronic skin condition, presenting with fluid-filled blisters and commonly involving the neck, back, scalp, and elbows. This condition is associated with celiac disease, and the lesions are extremely pruritic.
- Nazarian RM, Van Cott EM, Zembowicz A, Duncan LM. Warfarin-induced skin necrosis. J Am Acad Dermatol 2009; 61:325–332.
- Ward CT, Chavalitanonda N. Atypical warfarin-induced skin necrosis. Pharmacotherapy 2006; 26:1175–1179.
- Chan YC, Valenti D, Mansfield AO, Stansby G. Warfarin induced skin necrosis. Br J Surg 2000; 87:266–272.
- Warkentin TE, Sikov WM, Lillicrap DP. Multicentric warfarin-induced skin necrosis complicating heparin-induced thrombocytopenia. Am J Hematol 1999; 62:44–48.
- Ad-El DD, Meirovitz A, Weinberg A, et al. Warfarin skin necrosis: local and systemic factors. Br J Plast Surg 2000; 53:624–626.
- Jillella AP, Lutcher CL. Reinstituting warfarin in patients who develop warfarin skin necrosis. Am J Hematol 1996; 52:117–119.
A 75-year-old man was admitted to the hospital with new-onset atrial fibrillation. He underwent rate control, and a heparin infusion was started. Warfarin (Coumadin) 10 mg was added on the second hospital day. Two days later, the heparin infusion was discontinued when the international normalized ratio (INR) was in the therapeutic range.
Q: Which is the most likely diagnosis?
- Pyoderma gangrenosum
- Cutaneous vasculitis
- Warfarin-induced skin necrosis
- Ecthyma gangrenosum
- Dermatitis herpetiformis
A: The most likely diagnosis is warfarin-induced skin necrosis, a rare paradoxical complication that occurs in 0.01% to 0.1% of patients receiving this drug.1 Microthrombosis leads to necrosis of the skin and subcutaneous tissues, arising within 2 to 10 days after the start of anticoagulation therapy, although in rare cases it can occur months to years later.2,3
The most common risk factors include the unopposed use of warfarin (ie, unopposed by heparin at the start of therapy), using higher doses of warfarin during the initiation of anticoagulation, and inadequate overlap with an effective parenteral anticoagulant. Patients with protein C or S deficiency, heparin-induced thrombocytopenia,4 resistance to activated protein C, antithrombin deficiency, and lupus anticoagulant have also been reported to be at risk.
The most common sites affected are areas with high subcutaneous fat content, such as the abdomen, thighs, breasts, and buttocks. Skin presentations can vary from dermal plaques to petechial lesions, which rapidly progress to well-demarcated, bluish-black, painful lesions and eventually to hemorrhagic bullae and necrosis.1
At the start of warfarin therapy, the levels of protein C and factor VII (with half-lives of 5 to 8 hours) fall faster than those of other vitamin-K-dependent factors (ie, factors II, IX, and X). This causes a transient imbalance in procoagulant and anticoagulant pathways favoring thrombosis of the microvasculature, with resulting necrosis. Patients with hereditary protein C deficiency are at higher risk.5
Histologic review of lesions often shows venous thrombosis and diffuse necrosis of the dermis and subcutaneous tissue.2
Promptly stopping the warfarin and choosing alternative anticoagulation may help prevent further progression of this condition. Wound care, debridement, and sometimes skin grafting may be necessary, depending on the extent of the lesions. A rechallenge with warfarin is often difficult, but cases have been reported in which treatment was resumed without adverse consequences.6 Avoiding large loading doses of warfarin, gradually increasing doses over an extended period (about 10 days),3 and starting warfarin with a heparin bridge for at least 5 days (which was not done in this patient) would prevent the condition.
Early recognition, differentiation, and diagnosis are essential to minimize morbidity and to prevent death.
CASE CONTINUED
Warfarin was discontinued once the patient developed the skin lesions. He received vitamin K and fresh frozen plasma to normalize his INR, and he was started on a heparin infusion, after which the lesions began to heal. The patient refused a skin biopsy. Platelet counts remained stable during his hospital course. Protein C levels were not checked, given his recent use of warfarin. He was started on dabigatran (Pradaxa) and was discharged a week later.
THE OTHER DIAGNOSTIC CHOICES
Pyoderma gangrenosum is an uncommon ulcerative skin condition often associated with autoimmune disease. It usually starts at the site of a minor injury, more commonly on the legs, and gradually progresses to a painful ulcer.
Cutaneous vasculitis is an inflammation of small blood vessels characterized by palpable purpura. The lesions can resemble urticaria, petechia, or erythema multiforme. It is commonly associated with infection, drug therapy, inflammatory disease, and malignancy.
Ecthyma gangrenosum is an infection of skin caused by Pseudomonas aeruginosa. Usually, it presents as hemorrhagic pustules or infarct-like areas with surrounding erythema that evolve into necrotic ulcers surrounded by erythema.
Dermatitis herpetiformis is a chronic skin condition, presenting with fluid-filled blisters and commonly involving the neck, back, scalp, and elbows. This condition is associated with celiac disease, and the lesions are extremely pruritic.
A 75-year-old man was admitted to the hospital with new-onset atrial fibrillation. He underwent rate control, and a heparin infusion was started. Warfarin (Coumadin) 10 mg was added on the second hospital day. Two days later, the heparin infusion was discontinued when the international normalized ratio (INR) was in the therapeutic range.
Q: Which is the most likely diagnosis?
- Pyoderma gangrenosum
- Cutaneous vasculitis
- Warfarin-induced skin necrosis
- Ecthyma gangrenosum
- Dermatitis herpetiformis
A: The most likely diagnosis is warfarin-induced skin necrosis, a rare paradoxical complication that occurs in 0.01% to 0.1% of patients receiving this drug.1 Microthrombosis leads to necrosis of the skin and subcutaneous tissues, arising within 2 to 10 days after the start of anticoagulation therapy, although in rare cases it can occur months to years later.2,3
The most common risk factors include the unopposed use of warfarin (ie, unopposed by heparin at the start of therapy), using higher doses of warfarin during the initiation of anticoagulation, and inadequate overlap with an effective parenteral anticoagulant. Patients with protein C or S deficiency, heparin-induced thrombocytopenia,4 resistance to activated protein C, antithrombin deficiency, and lupus anticoagulant have also been reported to be at risk.
The most common sites affected are areas with high subcutaneous fat content, such as the abdomen, thighs, breasts, and buttocks. Skin presentations can vary from dermal plaques to petechial lesions, which rapidly progress to well-demarcated, bluish-black, painful lesions and eventually to hemorrhagic bullae and necrosis.1
At the start of warfarin therapy, the levels of protein C and factor VII (with half-lives of 5 to 8 hours) fall faster than those of other vitamin-K-dependent factors (ie, factors II, IX, and X). This causes a transient imbalance in procoagulant and anticoagulant pathways favoring thrombosis of the microvasculature, with resulting necrosis. Patients with hereditary protein C deficiency are at higher risk.5
Histologic review of lesions often shows venous thrombosis and diffuse necrosis of the dermis and subcutaneous tissue.2
Promptly stopping the warfarin and choosing alternative anticoagulation may help prevent further progression of this condition. Wound care, debridement, and sometimes skin grafting may be necessary, depending on the extent of the lesions. A rechallenge with warfarin is often difficult, but cases have been reported in which treatment was resumed without adverse consequences.6 Avoiding large loading doses of warfarin, gradually increasing doses over an extended period (about 10 days),3 and starting warfarin with a heparin bridge for at least 5 days (which was not done in this patient) would prevent the condition.
Early recognition, differentiation, and diagnosis are essential to minimize morbidity and to prevent death.
CASE CONTINUED
Warfarin was discontinued once the patient developed the skin lesions. He received vitamin K and fresh frozen plasma to normalize his INR, and he was started on a heparin infusion, after which the lesions began to heal. The patient refused a skin biopsy. Platelet counts remained stable during his hospital course. Protein C levels were not checked, given his recent use of warfarin. He was started on dabigatran (Pradaxa) and was discharged a week later.
THE OTHER DIAGNOSTIC CHOICES
Pyoderma gangrenosum is an uncommon ulcerative skin condition often associated with autoimmune disease. It usually starts at the site of a minor injury, more commonly on the legs, and gradually progresses to a painful ulcer.
Cutaneous vasculitis is an inflammation of small blood vessels characterized by palpable purpura. The lesions can resemble urticaria, petechia, or erythema multiforme. It is commonly associated with infection, drug therapy, inflammatory disease, and malignancy.
Ecthyma gangrenosum is an infection of skin caused by Pseudomonas aeruginosa. Usually, it presents as hemorrhagic pustules or infarct-like areas with surrounding erythema that evolve into necrotic ulcers surrounded by erythema.
Dermatitis herpetiformis is a chronic skin condition, presenting with fluid-filled blisters and commonly involving the neck, back, scalp, and elbows. This condition is associated with celiac disease, and the lesions are extremely pruritic.
- Nazarian RM, Van Cott EM, Zembowicz A, Duncan LM. Warfarin-induced skin necrosis. J Am Acad Dermatol 2009; 61:325–332.
- Ward CT, Chavalitanonda N. Atypical warfarin-induced skin necrosis. Pharmacotherapy 2006; 26:1175–1179.
- Chan YC, Valenti D, Mansfield AO, Stansby G. Warfarin induced skin necrosis. Br J Surg 2000; 87:266–272.
- Warkentin TE, Sikov WM, Lillicrap DP. Multicentric warfarin-induced skin necrosis complicating heparin-induced thrombocytopenia. Am J Hematol 1999; 62:44–48.
- Ad-El DD, Meirovitz A, Weinberg A, et al. Warfarin skin necrosis: local and systemic factors. Br J Plast Surg 2000; 53:624–626.
- Jillella AP, Lutcher CL. Reinstituting warfarin in patients who develop warfarin skin necrosis. Am J Hematol 1996; 52:117–119.
- Nazarian RM, Van Cott EM, Zembowicz A, Duncan LM. Warfarin-induced skin necrosis. J Am Acad Dermatol 2009; 61:325–332.
- Ward CT, Chavalitanonda N. Atypical warfarin-induced skin necrosis. Pharmacotherapy 2006; 26:1175–1179.
- Chan YC, Valenti D, Mansfield AO, Stansby G. Warfarin induced skin necrosis. Br J Surg 2000; 87:266–272.
- Warkentin TE, Sikov WM, Lillicrap DP. Multicentric warfarin-induced skin necrosis complicating heparin-induced thrombocytopenia. Am J Hematol 1999; 62:44–48.
- Ad-El DD, Meirovitz A, Weinberg A, et al. Warfarin skin necrosis: local and systemic factors. Br J Plast Surg 2000; 53:624–626.
- Jillella AP, Lutcher CL. Reinstituting warfarin in patients who develop warfarin skin necrosis. Am J Hematol 1996; 52:117–119.