User login
Clinical Characteristics and Outcomes of Non-ICU Hospitalization for COVID-19 in a Nonepicenter, Centrally Monitored Healthcare System
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of coronavirus disease 2019 (COVID-19), is associated with a wide range of illness severity and community prevalence, with an estimated 20% to 30% of patients requiring hospitalization.1,2 Outcome studies of hospitalized patients to date have focused on epicenter healthcare systems operating at surge-level bed capacity in resource-limited settings with mortality exceeding 20% among patients with a discharge disposition3,4 and have had a publication bias toward those suffering critical illness.5-7 Generalizability of these results to nonepicenter hospital systems is unclear given potential differences in triage practices and resource availability according to disease prevalence, with nonepicenter systems that are operating below capacity potentially able to accommodate the needs of most, if not all patients, requiring inpatient level care. Clinical outcomes associated with non–critically ill patients in nonepicenter regions remain poorly characterized yet highly relevant because these will ultimately apply to most US and global healthcare environments.
Nonepicenter healthcare systems must anticipate disease requirements for noncritically ill patients hospitalized with COVID-19 in order to appropriately allocate resources, including monitoring services like continuous pulse oximetry and cardiac telemetry. Data regarding the incidence of in-hospital respiratory and cardiovascular complications, including arrhythmias, among non–intensive care unit (non-ICU) hospitalized patients with COVID-19 are limited, with little granularity in terms of associated variables.7-11 Further data are needed to guide prioritization of valuable non-ICU continuous monitoring resources to the highest-risk patients in order to minimize consumption of personal protective equipment, reduce healthcare worker exposure, and ensure adequate availability for the expansion of prepandemic services.
Projections indicate that COVID-19 incidence may persist in the coming months.11-13 As nonessential hospital operations simultaneously resume, planning for resource allocation for patients with COVID-19 must be incorporated into broader systems of care. Further data are needed to help hospitals anticipate resource needs during this transition, especially by most systems that are caring for COVID-19 patients in nonepicenter environments. Therefore, we conducted a retrospective study of a large, multihospital, nonepicenter health system equipped with centralized continuous monitoring services in order to describe the detailed clinical course, resource utilization, and risk factors for adverse events in patients with COVID-19 initially admitted to the non-ICU setting.
METHODS
Central Monitoring Unit
The central monitoring unit (CMU) provides standardized and continuous off-site secondary monitoring of cardiac telemetry and pulse oximetry for non-ICU patients within Cleveland Clinic hospitals (Ohio, Florida), with direct communication to bedside nursing and inpatient emergency response teams for clinically significant cardiac arrhythmias, respiratory events, and vital sign changes according to standardized indications, as previously reported.14 Clinical variables of interest, including electrocardiographic and vital sign data, are collected and periodically analyzed within a central registry for quality assurance, risk stratification, and resource allocation. The data registry carries Institutional Review Board approval for retrospective analysis and deidentified outcomes reporting with consent form waiver.
Study Design and Data Collection
All patients positive for SARS-CoV-2 infection by nasopharyngeal polymerase chain reaction assay (Applied Biosystems) admitted from the emergency department to a non-ICU bed at a CMU hospital on or after March 13, 2020, and subsequently discharged on or before May 1, 2020, were identified. Retrospective review of the electronic medical record was performed, with follow-up continued through hospital discharge. Data were collected on patient demographics, clinical characteristics including admission laboratories and chest x-ray findings (abnormal defined as presence of an infiltrate/opacity consistent with airspace disease), continuous monitoring utilization, respiratory support, medication treatment, ICU transfer, and final hospital disposition. In addition, prospective recordings of cardiac arrhythmias that prompted CMU notification of bedside nursing were reviewed.
The primary outcome was a composite of death, ICU transfer, or increased oxygen requirement defined as escalation from simple nasal cannula to either high-flow nasal cannula (HFNC), noninvasive ventilation (NIV) consisting of continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BiPAP), or mechanical ventilation. In accordance with published guidelines, patients were treated with supplemental oxygen to maintain peripheral oxygen saturation between 92% and 96%.15
Of note, based on the validated performance of high sensitivity troponin primarily for the diagnosis of acute myocardial infarction in patients presenting to the emergency department with chest pain, our system reserves its use for this context and prefers conventional (fourth generation) troponin T testing for inpatients. Therefore, conventional troponin T values are reported in this study.
Statistical Analyses
Continuous variables are expressed as mean ± standard deviation or median (interquartile range), and categorical variables are expressed as absolute numbers with percentages. Independent samples t and Mann-Whitney U tests were used to compare continuous variables, as appropriate, and chi-square testing was used to compare categorical variables. Clinical variables satisfying an a priori two-tailed threshold of P < .05 were retained for multivariable logistic regression analysis. Variables retaining P < .05 in multivariable modeling were considered statistically significant. Analyses were performed using SPSS software, Version 23 (SPSS Inc).
RESULTS
Baseline Characteristics
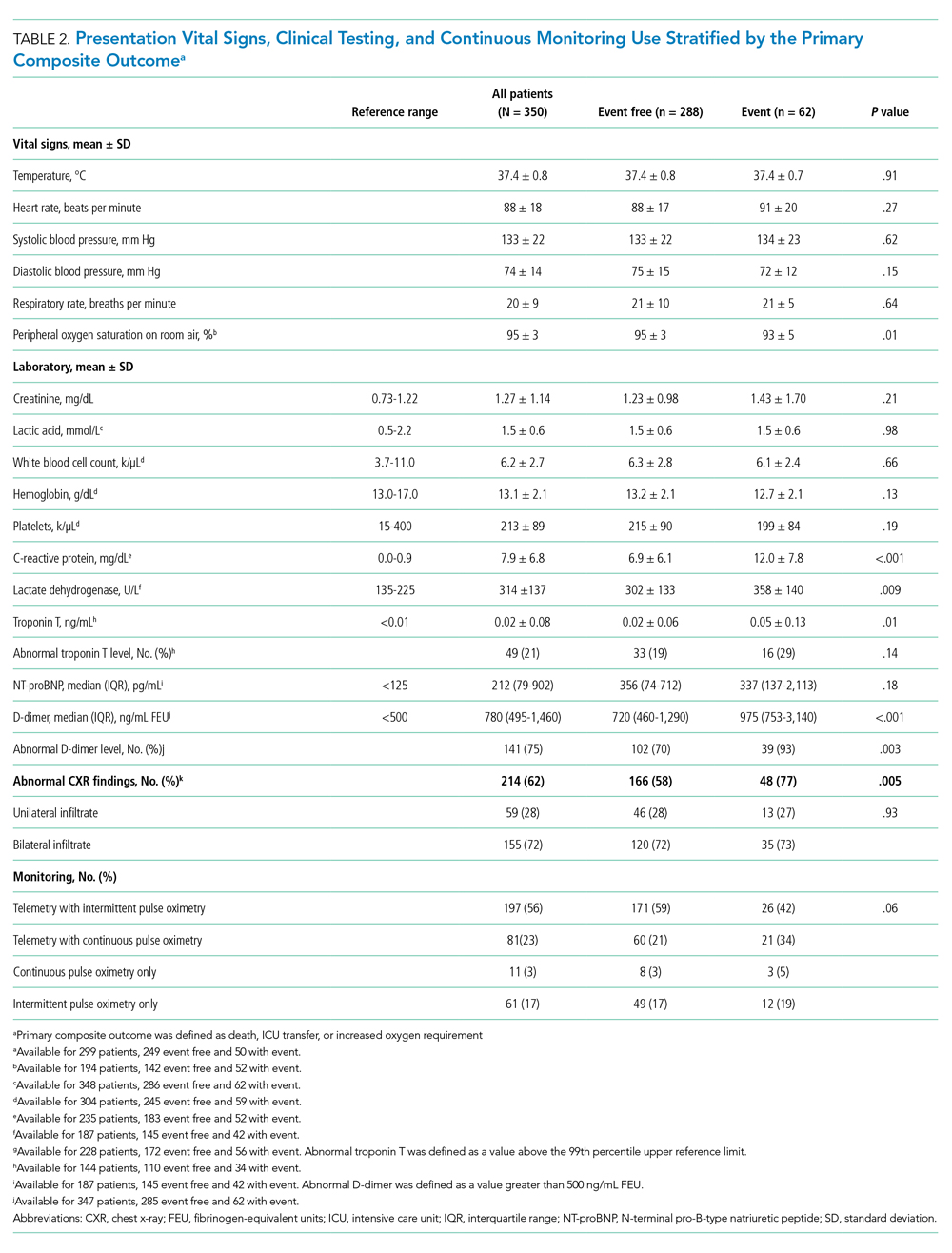
Between March 13, 2020, and May 1, 2020, a total of 350 patients admitted from the emergency department to a non-ICU inpatient bed had a final hospital disposition. Baseline characteristics, medication treatments, and continuous monitoring utilization are shown in Table 1 and Table 2. The average age was 64 ± 16 years, more than half of patients were male (n = 194; 55%), and most patients had at least one underlying comorbidity (n = 297; 85%), the most common being hypertension (n = 230; 66%), diabetes mellitus (n = 113; 32%), and current or prior tobacco use (n = 99; 28%). The presenting syndrome most frequently included subjective fever (n = 191; 55%), cough (n = 191; 55%), or dyspnea (n = 180; 51%).
Continuous Monitoring Use
Continuous monitoring was used in most patients (n = 289; 83%), including telemetry with intermittent pulse oximetry (n = 197; 56%), telemetry with continuous pulse oximetry (n = 81; 23%), or continuous pulse oximetry alone (n = 11; 3%). Among telemetry-monitored patients (n = 278; 79%), the most frequent indication was for a noncardiac disease state (n = 187; 67%), while indications for known cardiac arrhythmia (n = 74; 27%), heart failure (n = 10; 4%), or coronary artery disease (n = 7; 2%) were less common.
Oxygen Requirements and Cardiac Arrhythmias
The maximum level of respiratory support required by each patient is shown in Appendix Figure 1A. A total of 256 patients (73%) required 3 L/min or less of supplemental oxygen by nasal cannula, 45 (13%) required more than 3 L/min of supplemental oxygen by nasal cannula, 19 (5%) required HFNC, 8 (2%) required NIV, and 22 patients (6%) required mechanical ventilation. Among patients requiring HFNC or NIV, there were 13 (48%) who remained in a non-ICU bed, while the remaining 14 patients (52%) were transferred to the ICU.
Cardiac arrhythmias were detected in 39 (14%) of the 278 telemetry-monitored patients (Appendix Figure 1B). Clinical arrhythmias consisted of supraventricular tachycardia (SVT) in 17 patients (6%), nonsustained monomorphic ventricular tachycardia (VT) in 15 patients (5%), and a prolonged pause or severe bradyarrhythmia in 12 patients (4%). There were no cases of sustained monomorphic VT, polymorphic VT (including torsades de pointes), or ventricular fibrillation. All supraventricular tachycardias, nonsustained monomorphic VTs, and bradyarrhythmias/pauses were managed medically in the non-ICU setting, with the exception of one patient who was transferred to the ICU for a primary indication of atrial fibrillation with rapid ventricular response, which was treated with amiodarone. No patient with supraventricular tachycardia required emergent cardioversion, and no patient with a bradyarrhythmia or pause required temporary or permanent pacemaker implantation.
The detection of any arrhythmia was more common in patients with a history of cardiac arrhythmia (n = 18/41 vs 21/237; 44% vs 9%; P < .001), congestive heart failure (n = 11/36 vs 28/242; 31% vs 12%; P = .002), coronary artery disease (n = 12/49 vs 27/229; 24% vs 12%; P = .02), hypertension (n = 33/190 vs 6/88; 17% vs 7%; P = .02), and an abnormal admission troponin level (n = 13/40 vs 19/142; 33% vs 13%; P = .005). Notably, of the 39 patients with cardiac arrhythmias, 35 (90%) had either an abnormal admission troponin level or a history of cardiac arrhythmia, congestive heart failure, coronary artery disease, or hypertension. Of the 17 patients with SVT episodes, 13 (76%) had a known history of atrial fibrillation. Among patients who had a cardiac arrhythmia vs those who did not, there were no differences in levels of C-reactive protein (CRP; 7.3 ± 6.2 mg/dL vs. 7.8 ± 6.8 mg/dL, P = .63) or lactate dehydrogenase (LDH; 281 ± 89 U/L vs. 318 ± 142 U/L; P = .17). Approximately half of patients were treated with hydroxychloroquine (n = 185; 53%) or azithromycin (n = 182; 52%); 41% were treated with both (n = 142), with no observed association between any arrhythmia type and treatment with one or both medications (P > .05 for all comparisons).
Discharge Disposition and Adverse Outcomes
After an average length of stay of 6.1 ± 5.9 days, final hospital disposition included discharge to home (n = 278; 79%), discharge to subacute facility (n = 40; 11%), discharge to hospice (n = 8; 2%), death (n = 22, 6%), or release against medical advice (n = 2; 1%) (Figure). The primary composite outcome occurred in 62 patients (18%), including 22 deaths (6%), 48 ICU transfers (14%), and 49 patients with increased oxygen requirements (14%). Only two deaths occurred in the absence of an increased oxygen requirement or ICU transfer.

Increased oxygen requirement was the indication for ICU transfer in 37 of 48 patients (77%), with 22 patients (46%) requiring mechanical ventilation. Of the 48 patients requiring ICU transfer, 14 (29%) died, including 10 of the 22 patients (45%) treated with mechanical ventilation. Of the 302 patients who remained in the non-ICU setting, 8 (3%) died and 8 (3%) were discharged to hospice.
In univariable analyses, the primary composite outcome was more common among older patients (event vs event free, 72 ± 13 years vs 63 ± 16 years; P < .001); it was also more common in patients with congestive heart failure (n = 14/62 vs 28/288; 23% vs 10%; P = .005), chronic obstructive pulmonary disease (n = 9/62 vs 19/288; 15% vs 7%; P = .04), lower body mass index (29 ± 5 kg/m2 vs 31 ± 7 kg/m2; P = .006), lower peripheral oxygen saturation on room air (93% ± 5% vs 95% ± 3%; P = .005), higher CRP level (12.0 ± 7.8 mg/dL vs 6.9 ± 6.1 mg/dL; P < .001), higher LDH level (358 ± 140 U/L vs 302 ± 133 U/L; P = .009), higher troponin level (0.05 ± 0.13 ng/dL vs 0.02 ± 0.06 ng/dL; P = .01), abnormal D-dimer level (n = 39/42 vs 102/145; 93% vs 70%; P = .003), and abnormal chest x-ray findings (n = 48/62 vs 166/285; 77% vs 58%; P = .005) (Table 1 and Table 2). After multivariable adjustment, CRP level (odds ratio [OR], 1.09 per 1 mg/dL increase; 95% CI, 1.01-1.18; P = .04) and LDH level (OR, 1.006 per 1 U/L increase; 95% CI, 1.001-1.012; P = .03) remained significantly associated with the composite adverse outcome (Table 3). The rate of death, ICU transfer, or increased oxygen requirement was sixfold higher in patients with a CRP level in the fourth quartile (≥11.0 mg/dL) than it was among those in the first quartile (≤ 2.6 mg/dL) (P < .001 for trend), and it was fivefold higher in patients with an LDH level in the fourth quartile (≥ 354 U/L) than it was among those in the first quartile (≤ 232 U/L) (P = .001 for trend) (Appendix Figure 2). No patient with a CRP level in the reference range (≤ 0.9 mg/dL) experienced the composite adverse event, compared to three patients (n = 3/49, 6.1%) within the reference range for LDH level (≤ 225 U/L), all of whom had an elevated CRP.

DISCUSSION
In this study of 350 patients initially admitted to a non-ICU hospital bed within a large, nonepicenter healthcare system, the primary outcome of death, ICU transfer, or increased oxygen requirement occurred in 18% of patients and was independently associated with higher admission CRP and LDH levels on multivariable analysis. Most patients (73%) required 3 L/min or less of supplemental oxygen, while 14% of patients required escalation to HFNC, NIV, or mechanical ventilation. Despite frequent telemetry use (79%), cardiac arrhythmias were uncommon (14%), including no life-threatening ventricular arrhythmias. Clinical deterioration requiring ICU transfer occurred in 14% of patients, most often for an indication of increased oxygen requirement (77%). In-hospital mortality was 6% for the entire cohort, 29% for patients requiring ICU transfer, and 3% for patients who remained in the non-ICU setting.
Nonepicenter, Non-ICU Mortality
This study offers an assessment of clinical outcomes in patients with COVID-19 hospitalized in a non-ICU, nonepicenter healthcare system operating below capacity. Although such systems account for most institutions caring for patients with COVID-19, this population has been underrepresented in the literature, which has focused on epicenter hospitals and critically ill patients.3-7 Existing epicenter estimates of in-hospital mortality for patients not requiring ICU-level care range from 6% in Northern California2 to at least 10% in New York, New York,3 and 11% in Wuhan, China.4 The corresponding non-ICU in-hospital mortality in our study was only 3%, supporting the vital role of social distancing in reducing COVID-19 mortality by facilitating care delivery in a non–resource limited hospital setting.
Oxygen Requirements and Cardiac Arrhythmias in Non-ICU Patients
Beyond nonepicenter mortality estimates, this study is the first to provide a detailed characterization of the clinical course and resource usage among patients with COVID-19 admitted to the non-ICU setting. Given the predicted persistence of SARS-CoV-2 spread,11-13 this information is crucial to healthcare systems that must anticipate resource requirements, such as respiratory support and continuous monitoring equipment, for the care of hospitalized patients with COVID-19. Such informed planning takes on even greater importance as prepandemic hospital services resume.
While most patients (73%) with COVID-19 admitted to a non-ICU bed required peak supplemental oxygen of 3 L/min or less, a relevant proportion (14%) developed a need for HFNC, NIV, or mechanical ventilation. Furthermore, among telemetry-monitored patients (79%), cardiac arrhythmias were uncommon (14%), and nearly all (90%) occurred in patients with either a positive troponin or known history of cardiac disease. There were no life-threatening ventricular arrhythmias associated with frequent use of hydroxychloroquine (53%) and azithromycin (52%).
These telemetry findings expand upon a smaller study of non-ICU patients receiving either hydroxychloroquine or azithromycin, in which no life-threatening ventricular tachyarrhythmias were detected.8 A separate study reported a 5.9% incidence of malignant ventricular tachyarrhythmias in hospitalized patients with COVID-19,10 but this study did not stratify arrhythmias by illness severity, and a high frequency of critical illness is suggested by the mechanical ventilation rate of 24%, thereby limiting comparison with our non-ICU telemetry findings.
CRP and LDH Levels as Predictors of Adverse Outcomes
This study supports the utility of obtaining CRP and LDH levels for risk stratification at the time of non-ICU hospital admission. In multivariable analysis, higher CRP and LDH levels were significantly associated with the composite adverse outcome. The adverse event rates was increased sixfold between patients with a CRP in the fourth quartile (≥ 11.0 mg/dL, 36%) and those in the first quartile (≤ 2.6 mg/dL, 5.3%), and it was fivefold higher in patients with an LDH level in the fourth quartile (≥ 354 U/L, 34%) compared with those in the first quartile (≤ 232 U/L, 7%).
These findings are consistent with prior studies that have associated elevated inflammatory markers with poor prognosis and death.7,9,16 In some cases, COVID-19 may manifest similar to a cytokine storm syndrome, which highlights the importance of inflammation-associated tissue injury and leads to widespread interest in the use of immunosuppressive medications.17,18 Several studies also have demonstrated an association between LDH level and severe illness,4,7,19 although this is the first to specifically demonstrate its association with clinical decompensation in the non-ICU hospitalized population. Given that SARS-CoV-2 can infect multiple organs,20,21 there is biological plausibility for the use of LDH levels as a nonspecific marker of tissue injury for early identification of more severe infection.
Notably, while elevated troponin levels have been strongly associated with the need for mechanical ventilation and with death, this has primarily been established using either high-sensitivity troponin assays at the time of admission22 or using peak conventional troponin levels during hospitalization.10 In this study, while abnormal conventional troponin levels at the time of non-ICU admission were not significantly associated with the primary outcome in multivariable analysis, absolute troponin values were significantly higher in univariable analysis. Incomplete troponin sampling and the lack of routine high-sensitivity troponin assay use may explain the lack of more robust troponin significance in this study.
Implications for Non-ICU Continuous Monitoring Resource Allocation
Prioritization of non-ICU continuous monitoring resources among patients with COVID-19 has numerous benefits, including reduced consumption of personal protective equipment, fewer healthcare worker exposures, and adequate availability of continuous monitoring for the expansion of prepandemic hospital services. While individualized clinical discretion is still required, the results of this study can be used as a guide for the allocation of continuous pulse oximetry and cardiac telemetry. Patients with a normal presenting CRP level and/or LDH level had a low incidence of clinical decompensation, which suggests that such patients could be monitored with intermittent rather than continuous pulse oximetry. Furthermore, cardiac telemetry could be reserved for patients with a history of cardiac comorbidities or abnormal troponin levels because such patients accounted for 90% of cardiac arrhythmias in this study.
Limitations
This study was limited to a single health system, and it lacks a direct comparison to nonhospitalized patients and those directly admitted to the ICU. Triage practices and thresholds for hospitalization may differ across institutions and regions, thereby limiting the generalizability of our study. Additional limitations include the lack of selected admission laboratories for all patients, as well as the lack of telemetry monitoring in all patients. However, any resulting selection bias may be more likely to attenuate the magnitude of observed effects given that additional testing and increased telemetry use may be expected in patients who are felt to be higher risk by routine clinical assessment.
CONCLUSION
In this study of non–critically ill patients hospitalized within a nonepicenter health system, the development of more severe illness or death was significantly associated with higher levels of CRP and LDH on admission. Clinical decompensation was driven largely by respiratory complications, while cardiac arrhythmias were rare. Overall, the non-ICU mortality rate was at least half of that reported in epicenter regions. Altogether, these findings provide valuable information for resource allocation planning while nonepicenter health systems continue caring for patients with COVID-19 as they also resume prepandemic operations.
1. Bialek S, Boundy E, Bowen V, et al; CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343-346. https://doi.org/10.15585/mmwr.mm6912e2
2. Myers LC, Parodi SM, Escobar GJ, Liu VX. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. JAMA. 2020;323(21):2195-2198. https://doi.org/10.1001/jama.2020.7202
3. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. Published online April 22, 2020. https://doi.org/10.1001/jama.2020.6775
4. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. https://doi.org/10.1016/s0140-6736(20)30566-3
5. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA. 2020;323(16):1612-1614. https://doi.org/10.1001/jama.2020.4326
6. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1574-1581. https://doi.org/10.1001/jama.2020.5394
7. Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. https://doi.org/10.1001/jama.2020.1585
8. Chang D, Saleh M, Gabriels J, et al. Inpatient use of ambulatory telemetry monitors for COVID-19 patients treated with hydroxychloroquine and/or azithromycin. J Am Coll Cardiol. 2020;75(23):2992-2993. https://doi.org/10.1016/j.jacc.2020.04.032
9. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. https://doi.org/10.1016/s0140-6736(20)30183-5
10. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):1-8. https://doi.org/10.1001/jamacardio.2020.1017
11. Centers for Disease Control and Prevention COVID-19 Forecasts. Accessed May 19, 2020. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/forecasting-us.html
12. Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368(6493):860-868. https://doi.org/10.1126/science.abb5793
13. Baker RE, Yang W, Vecchi GA, Metcalf CJE, Grenfell BT. Susceptible supply limits the role of climate in the early SARS-CoV-2 pandemic. Science. 2020;369(6501):315-319. https://doi.org/10.1126/science.abc2535
14. Cantillon DJ, Loy M, Burkle A, et al. Association between off-site central monitoring using standardized cardiac telemetry and clinical outcomes among non-critically ill patients. JAMA. 2016;316(5):519-524. https://doi.org/10.1001/jama.2016.10258
15. Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med. 2020;48(6):e440-e469. https://doi.org/10.1097/ccm.0000000000004363
16. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. https://doi.org/10.1056/nejmoa2002032
17. Mehta P, McAuley DF, Brown M, et al; HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033-1034. https://doi.org/10.1016/s0140-6736(20)30628-0
18. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. Published online April 13, 2020. https://doi.org/10.1001/jama.2020.6019
19. Liang W, Liang H, Ou L, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020;180(8):1-9. https://doi.org/10.1001/jamainternmed.2020.2033
20. Puelles VG, Lütgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383(6):590-592. https://doi.org/10.1056/nejmc2011400
21. Zhou J, Li C, Liu X, et al. Infection of bat and human intestinal organoids by SARS-CoV-2. Nat Med. 2020;26(7):1077-1083. https://doi.org/10.1038/s41591-020-0912-6
22. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802-810. https://doi.org/10.1001/jamacardio.2020.0950
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of coronavirus disease 2019 (COVID-19), is associated with a wide range of illness severity and community prevalence, with an estimated 20% to 30% of patients requiring hospitalization.1,2 Outcome studies of hospitalized patients to date have focused on epicenter healthcare systems operating at surge-level bed capacity in resource-limited settings with mortality exceeding 20% among patients with a discharge disposition3,4 and have had a publication bias toward those suffering critical illness.5-7 Generalizability of these results to nonepicenter hospital systems is unclear given potential differences in triage practices and resource availability according to disease prevalence, with nonepicenter systems that are operating below capacity potentially able to accommodate the needs of most, if not all patients, requiring inpatient level care. Clinical outcomes associated with non–critically ill patients in nonepicenter regions remain poorly characterized yet highly relevant because these will ultimately apply to most US and global healthcare environments.
Nonepicenter healthcare systems must anticipate disease requirements for noncritically ill patients hospitalized with COVID-19 in order to appropriately allocate resources, including monitoring services like continuous pulse oximetry and cardiac telemetry. Data regarding the incidence of in-hospital respiratory and cardiovascular complications, including arrhythmias, among non–intensive care unit (non-ICU) hospitalized patients with COVID-19 are limited, with little granularity in terms of associated variables.7-11 Further data are needed to guide prioritization of valuable non-ICU continuous monitoring resources to the highest-risk patients in order to minimize consumption of personal protective equipment, reduce healthcare worker exposure, and ensure adequate availability for the expansion of prepandemic services.
Projections indicate that COVID-19 incidence may persist in the coming months.11-13 As nonessential hospital operations simultaneously resume, planning for resource allocation for patients with COVID-19 must be incorporated into broader systems of care. Further data are needed to help hospitals anticipate resource needs during this transition, especially by most systems that are caring for COVID-19 patients in nonepicenter environments. Therefore, we conducted a retrospective study of a large, multihospital, nonepicenter health system equipped with centralized continuous monitoring services in order to describe the detailed clinical course, resource utilization, and risk factors for adverse events in patients with COVID-19 initially admitted to the non-ICU setting.
METHODS
Central Monitoring Unit
The central monitoring unit (CMU) provides standardized and continuous off-site secondary monitoring of cardiac telemetry and pulse oximetry for non-ICU patients within Cleveland Clinic hospitals (Ohio, Florida), with direct communication to bedside nursing and inpatient emergency response teams for clinically significant cardiac arrhythmias, respiratory events, and vital sign changes according to standardized indications, as previously reported.14 Clinical variables of interest, including electrocardiographic and vital sign data, are collected and periodically analyzed within a central registry for quality assurance, risk stratification, and resource allocation. The data registry carries Institutional Review Board approval for retrospective analysis and deidentified outcomes reporting with consent form waiver.
Study Design and Data Collection
All patients positive for SARS-CoV-2 infection by nasopharyngeal polymerase chain reaction assay (Applied Biosystems) admitted from the emergency department to a non-ICU bed at a CMU hospital on or after March 13, 2020, and subsequently discharged on or before May 1, 2020, were identified. Retrospective review of the electronic medical record was performed, with follow-up continued through hospital discharge. Data were collected on patient demographics, clinical characteristics including admission laboratories and chest x-ray findings (abnormal defined as presence of an infiltrate/opacity consistent with airspace disease), continuous monitoring utilization, respiratory support, medication treatment, ICU transfer, and final hospital disposition. In addition, prospective recordings of cardiac arrhythmias that prompted CMU notification of bedside nursing were reviewed.
The primary outcome was a composite of death, ICU transfer, or increased oxygen requirement defined as escalation from simple nasal cannula to either high-flow nasal cannula (HFNC), noninvasive ventilation (NIV) consisting of continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BiPAP), or mechanical ventilation. In accordance with published guidelines, patients were treated with supplemental oxygen to maintain peripheral oxygen saturation between 92% and 96%.15
Of note, based on the validated performance of high sensitivity troponin primarily for the diagnosis of acute myocardial infarction in patients presenting to the emergency department with chest pain, our system reserves its use for this context and prefers conventional (fourth generation) troponin T testing for inpatients. Therefore, conventional troponin T values are reported in this study.
Statistical Analyses
Continuous variables are expressed as mean ± standard deviation or median (interquartile range), and categorical variables are expressed as absolute numbers with percentages. Independent samples t and Mann-Whitney U tests were used to compare continuous variables, as appropriate, and chi-square testing was used to compare categorical variables. Clinical variables satisfying an a priori two-tailed threshold of P < .05 were retained for multivariable logistic regression analysis. Variables retaining P < .05 in multivariable modeling were considered statistically significant. Analyses were performed using SPSS software, Version 23 (SPSS Inc).
RESULTS
Baseline Characteristics
Between March 13, 2020, and May 1, 2020, a total of 350 patients admitted from the emergency department to a non-ICU inpatient bed had a final hospital disposition. Baseline characteristics, medication treatments, and continuous monitoring utilization are shown in Table 1 and Table 2. The average age was 64 ± 16 years, more than half of patients were male (n = 194; 55%), and most patients had at least one underlying comorbidity (n = 297; 85%), the most common being hypertension (n = 230; 66%), diabetes mellitus (n = 113; 32%), and current or prior tobacco use (n = 99; 28%). The presenting syndrome most frequently included subjective fever (n = 191; 55%), cough (n = 191; 55%), or dyspnea (n = 180; 51%).

Continuous Monitoring Use
Continuous monitoring was used in most patients (n = 289; 83%), including telemetry with intermittent pulse oximetry (n = 197; 56%), telemetry with continuous pulse oximetry (n = 81; 23%), or continuous pulse oximetry alone (n = 11; 3%). Among telemetry-monitored patients (n = 278; 79%), the most frequent indication was for a noncardiac disease state (n = 187; 67%), while indications for known cardiac arrhythmia (n = 74; 27%), heart failure (n = 10; 4%), or coronary artery disease (n = 7; 2%) were less common.

Oxygen Requirements and Cardiac Arrhythmias
The maximum level of respiratory support required by each patient is shown in Appendix Figure 1A. A total of 256 patients (73%) required 3 L/min or less of supplemental oxygen by nasal cannula, 45 (13%) required more than 3 L/min of supplemental oxygen by nasal cannula, 19 (5%) required HFNC, 8 (2%) required NIV, and 22 patients (6%) required mechanical ventilation. Among patients requiring HFNC or NIV, there were 13 (48%) who remained in a non-ICU bed, while the remaining 14 patients (52%) were transferred to the ICU.
Cardiac arrhythmias were detected in 39 (14%) of the 278 telemetry-monitored patients (Appendix Figure 1B). Clinical arrhythmias consisted of supraventricular tachycardia (SVT) in 17 patients (6%), nonsustained monomorphic ventricular tachycardia (VT) in 15 patients (5%), and a prolonged pause or severe bradyarrhythmia in 12 patients (4%). There were no cases of sustained monomorphic VT, polymorphic VT (including torsades de pointes), or ventricular fibrillation. All supraventricular tachycardias, nonsustained monomorphic VTs, and bradyarrhythmias/pauses were managed medically in the non-ICU setting, with the exception of one patient who was transferred to the ICU for a primary indication of atrial fibrillation with rapid ventricular response, which was treated with amiodarone. No patient with supraventricular tachycardia required emergent cardioversion, and no patient with a bradyarrhythmia or pause required temporary or permanent pacemaker implantation.
The detection of any arrhythmia was more common in patients with a history of cardiac arrhythmia (n = 18/41 vs 21/237; 44% vs 9%; P < .001), congestive heart failure (n = 11/36 vs 28/242; 31% vs 12%; P = .002), coronary artery disease (n = 12/49 vs 27/229; 24% vs 12%; P = .02), hypertension (n = 33/190 vs 6/88; 17% vs 7%; P = .02), and an abnormal admission troponin level (n = 13/40 vs 19/142; 33% vs 13%; P = .005). Notably, of the 39 patients with cardiac arrhythmias, 35 (90%) had either an abnormal admission troponin level or a history of cardiac arrhythmia, congestive heart failure, coronary artery disease, or hypertension. Of the 17 patients with SVT episodes, 13 (76%) had a known history of atrial fibrillation. Among patients who had a cardiac arrhythmia vs those who did not, there were no differences in levels of C-reactive protein (CRP; 7.3 ± 6.2 mg/dL vs. 7.8 ± 6.8 mg/dL, P = .63) or lactate dehydrogenase (LDH; 281 ± 89 U/L vs. 318 ± 142 U/L; P = .17). Approximately half of patients were treated with hydroxychloroquine (n = 185; 53%) or azithromycin (n = 182; 52%); 41% were treated with both (n = 142), with no observed association between any arrhythmia type and treatment with one or both medications (P > .05 for all comparisons).
Discharge Disposition and Adverse Outcomes
After an average length of stay of 6.1 ± 5.9 days, final hospital disposition included discharge to home (n = 278; 79%), discharge to subacute facility (n = 40; 11%), discharge to hospice (n = 8; 2%), death (n = 22, 6%), or release against medical advice (n = 2; 1%) (Figure). The primary composite outcome occurred in 62 patients (18%), including 22 deaths (6%), 48 ICU transfers (14%), and 49 patients with increased oxygen requirements (14%). Only two deaths occurred in the absence of an increased oxygen requirement or ICU transfer.

Increased oxygen requirement was the indication for ICU transfer in 37 of 48 patients (77%), with 22 patients (46%) requiring mechanical ventilation. Of the 48 patients requiring ICU transfer, 14 (29%) died, including 10 of the 22 patients (45%) treated with mechanical ventilation. Of the 302 patients who remained in the non-ICU setting, 8 (3%) died and 8 (3%) were discharged to hospice.
In univariable analyses, the primary composite outcome was more common among older patients (event vs event free, 72 ± 13 years vs 63 ± 16 years; P < .001); it was also more common in patients with congestive heart failure (n = 14/62 vs 28/288; 23% vs 10%; P = .005), chronic obstructive pulmonary disease (n = 9/62 vs 19/288; 15% vs 7%; P = .04), lower body mass index (29 ± 5 kg/m2 vs 31 ± 7 kg/m2; P = .006), lower peripheral oxygen saturation on room air (93% ± 5% vs 95% ± 3%; P = .005), higher CRP level (12.0 ± 7.8 mg/dL vs 6.9 ± 6.1 mg/dL; P < .001), higher LDH level (358 ± 140 U/L vs 302 ± 133 U/L; P = .009), higher troponin level (0.05 ± 0.13 ng/dL vs 0.02 ± 0.06 ng/dL; P = .01), abnormal D-dimer level (n = 39/42 vs 102/145; 93% vs 70%; P = .003), and abnormal chest x-ray findings (n = 48/62 vs 166/285; 77% vs 58%; P = .005) (Table 1 and Table 2). After multivariable adjustment, CRP level (odds ratio [OR], 1.09 per 1 mg/dL increase; 95% CI, 1.01-1.18; P = .04) and LDH level (OR, 1.006 per 1 U/L increase; 95% CI, 1.001-1.012; P = .03) remained significantly associated with the composite adverse outcome (Table 3). The rate of death, ICU transfer, or increased oxygen requirement was sixfold higher in patients with a CRP level in the fourth quartile (≥11.0 mg/dL) than it was among those in the first quartile (≤ 2.6 mg/dL) (P < .001 for trend), and it was fivefold higher in patients with an LDH level in the fourth quartile (≥ 354 U/L) than it was among those in the first quartile (≤ 232 U/L) (P = .001 for trend) (Appendix Figure 2). No patient with a CRP level in the reference range (≤ 0.9 mg/dL) experienced the composite adverse event, compared to three patients (n = 3/49, 6.1%) within the reference range for LDH level (≤ 225 U/L), all of whom had an elevated CRP.

DISCUSSION
In this study of 350 patients initially admitted to a non-ICU hospital bed within a large, nonepicenter healthcare system, the primary outcome of death, ICU transfer, or increased oxygen requirement occurred in 18% of patients and was independently associated with higher admission CRP and LDH levels on multivariable analysis. Most patients (73%) required 3 L/min or less of supplemental oxygen, while 14% of patients required escalation to HFNC, NIV, or mechanical ventilation. Despite frequent telemetry use (79%), cardiac arrhythmias were uncommon (14%), including no life-threatening ventricular arrhythmias. Clinical deterioration requiring ICU transfer occurred in 14% of patients, most often for an indication of increased oxygen requirement (77%). In-hospital mortality was 6% for the entire cohort, 29% for patients requiring ICU transfer, and 3% for patients who remained in the non-ICU setting.
Nonepicenter, Non-ICU Mortality
This study offers an assessment of clinical outcomes in patients with COVID-19 hospitalized in a non-ICU, nonepicenter healthcare system operating below capacity. Although such systems account for most institutions caring for patients with COVID-19, this population has been underrepresented in the literature, which has focused on epicenter hospitals and critically ill patients.3-7 Existing epicenter estimates of in-hospital mortality for patients not requiring ICU-level care range from 6% in Northern California2 to at least 10% in New York, New York,3 and 11% in Wuhan, China.4 The corresponding non-ICU in-hospital mortality in our study was only 3%, supporting the vital role of social distancing in reducing COVID-19 mortality by facilitating care delivery in a non–resource limited hospital setting.
Oxygen Requirements and Cardiac Arrhythmias in Non-ICU Patients
Beyond nonepicenter mortality estimates, this study is the first to provide a detailed characterization of the clinical course and resource usage among patients with COVID-19 admitted to the non-ICU setting. Given the predicted persistence of SARS-CoV-2 spread,11-13 this information is crucial to healthcare systems that must anticipate resource requirements, such as respiratory support and continuous monitoring equipment, for the care of hospitalized patients with COVID-19. Such informed planning takes on even greater importance as prepandemic hospital services resume.
While most patients (73%) with COVID-19 admitted to a non-ICU bed required peak supplemental oxygen of 3 L/min or less, a relevant proportion (14%) developed a need for HFNC, NIV, or mechanical ventilation. Furthermore, among telemetry-monitored patients (79%), cardiac arrhythmias were uncommon (14%), and nearly all (90%) occurred in patients with either a positive troponin or known history of cardiac disease. There were no life-threatening ventricular arrhythmias associated with frequent use of hydroxychloroquine (53%) and azithromycin (52%).
These telemetry findings expand upon a smaller study of non-ICU patients receiving either hydroxychloroquine or azithromycin, in which no life-threatening ventricular tachyarrhythmias were detected.8 A separate study reported a 5.9% incidence of malignant ventricular tachyarrhythmias in hospitalized patients with COVID-19,10 but this study did not stratify arrhythmias by illness severity, and a high frequency of critical illness is suggested by the mechanical ventilation rate of 24%, thereby limiting comparison with our non-ICU telemetry findings.
CRP and LDH Levels as Predictors of Adverse Outcomes
This study supports the utility of obtaining CRP and LDH levels for risk stratification at the time of non-ICU hospital admission. In multivariable analysis, higher CRP and LDH levels were significantly associated with the composite adverse outcome. The adverse event rates was increased sixfold between patients with a CRP in the fourth quartile (≥ 11.0 mg/dL, 36%) and those in the first quartile (≤ 2.6 mg/dL, 5.3%), and it was fivefold higher in patients with an LDH level in the fourth quartile (≥ 354 U/L, 34%) compared with those in the first quartile (≤ 232 U/L, 7%).
These findings are consistent with prior studies that have associated elevated inflammatory markers with poor prognosis and death.7,9,16 In some cases, COVID-19 may manifest similar to a cytokine storm syndrome, which highlights the importance of inflammation-associated tissue injury and leads to widespread interest in the use of immunosuppressive medications.17,18 Several studies also have demonstrated an association between LDH level and severe illness,4,7,19 although this is the first to specifically demonstrate its association with clinical decompensation in the non-ICU hospitalized population. Given that SARS-CoV-2 can infect multiple organs,20,21 there is biological plausibility for the use of LDH levels as a nonspecific marker of tissue injury for early identification of more severe infection.
Notably, while elevated troponin levels have been strongly associated with the need for mechanical ventilation and with death, this has primarily been established using either high-sensitivity troponin assays at the time of admission22 or using peak conventional troponin levels during hospitalization.10 In this study, while abnormal conventional troponin levels at the time of non-ICU admission were not significantly associated with the primary outcome in multivariable analysis, absolute troponin values were significantly higher in univariable analysis. Incomplete troponin sampling and the lack of routine high-sensitivity troponin assay use may explain the lack of more robust troponin significance in this study.
Implications for Non-ICU Continuous Monitoring Resource Allocation
Prioritization of non-ICU continuous monitoring resources among patients with COVID-19 has numerous benefits, including reduced consumption of personal protective equipment, fewer healthcare worker exposures, and adequate availability of continuous monitoring for the expansion of prepandemic hospital services. While individualized clinical discretion is still required, the results of this study can be used as a guide for the allocation of continuous pulse oximetry and cardiac telemetry. Patients with a normal presenting CRP level and/or LDH level had a low incidence of clinical decompensation, which suggests that such patients could be monitored with intermittent rather than continuous pulse oximetry. Furthermore, cardiac telemetry could be reserved for patients with a history of cardiac comorbidities or abnormal troponin levels because such patients accounted for 90% of cardiac arrhythmias in this study.
Limitations
This study was limited to a single health system, and it lacks a direct comparison to nonhospitalized patients and those directly admitted to the ICU. Triage practices and thresholds for hospitalization may differ across institutions and regions, thereby limiting the generalizability of our study. Additional limitations include the lack of selected admission laboratories for all patients, as well as the lack of telemetry monitoring in all patients. However, any resulting selection bias may be more likely to attenuate the magnitude of observed effects given that additional testing and increased telemetry use may be expected in patients who are felt to be higher risk by routine clinical assessment.
CONCLUSION
In this study of non–critically ill patients hospitalized within a nonepicenter health system, the development of more severe illness or death was significantly associated with higher levels of CRP and LDH on admission. Clinical decompensation was driven largely by respiratory complications, while cardiac arrhythmias were rare. Overall, the non-ICU mortality rate was at least half of that reported in epicenter regions. Altogether, these findings provide valuable information for resource allocation planning while nonepicenter health systems continue caring for patients with COVID-19 as they also resume prepandemic operations.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of coronavirus disease 2019 (COVID-19), is associated with a wide range of illness severity and community prevalence, with an estimated 20% to 30% of patients requiring hospitalization.1,2 Outcome studies of hospitalized patients to date have focused on epicenter healthcare systems operating at surge-level bed capacity in resource-limited settings with mortality exceeding 20% among patients with a discharge disposition3,4 and have had a publication bias toward those suffering critical illness.5-7 Generalizability of these results to nonepicenter hospital systems is unclear given potential differences in triage practices and resource availability according to disease prevalence, with nonepicenter systems that are operating below capacity potentially able to accommodate the needs of most, if not all patients, requiring inpatient level care. Clinical outcomes associated with non–critically ill patients in nonepicenter regions remain poorly characterized yet highly relevant because these will ultimately apply to most US and global healthcare environments.
Nonepicenter healthcare systems must anticipate disease requirements for noncritically ill patients hospitalized with COVID-19 in order to appropriately allocate resources, including monitoring services like continuous pulse oximetry and cardiac telemetry. Data regarding the incidence of in-hospital respiratory and cardiovascular complications, including arrhythmias, among non–intensive care unit (non-ICU) hospitalized patients with COVID-19 are limited, with little granularity in terms of associated variables.7-11 Further data are needed to guide prioritization of valuable non-ICU continuous monitoring resources to the highest-risk patients in order to minimize consumption of personal protective equipment, reduce healthcare worker exposure, and ensure adequate availability for the expansion of prepandemic services.
Projections indicate that COVID-19 incidence may persist in the coming months.11-13 As nonessential hospital operations simultaneously resume, planning for resource allocation for patients with COVID-19 must be incorporated into broader systems of care. Further data are needed to help hospitals anticipate resource needs during this transition, especially by most systems that are caring for COVID-19 patients in nonepicenter environments. Therefore, we conducted a retrospective study of a large, multihospital, nonepicenter health system equipped with centralized continuous monitoring services in order to describe the detailed clinical course, resource utilization, and risk factors for adverse events in patients with COVID-19 initially admitted to the non-ICU setting.
METHODS
Central Monitoring Unit
The central monitoring unit (CMU) provides standardized and continuous off-site secondary monitoring of cardiac telemetry and pulse oximetry for non-ICU patients within Cleveland Clinic hospitals (Ohio, Florida), with direct communication to bedside nursing and inpatient emergency response teams for clinically significant cardiac arrhythmias, respiratory events, and vital sign changes according to standardized indications, as previously reported.14 Clinical variables of interest, including electrocardiographic and vital sign data, are collected and periodically analyzed within a central registry for quality assurance, risk stratification, and resource allocation. The data registry carries Institutional Review Board approval for retrospective analysis and deidentified outcomes reporting with consent form waiver.
Study Design and Data Collection
All patients positive for SARS-CoV-2 infection by nasopharyngeal polymerase chain reaction assay (Applied Biosystems) admitted from the emergency department to a non-ICU bed at a CMU hospital on or after March 13, 2020, and subsequently discharged on or before May 1, 2020, were identified. Retrospective review of the electronic medical record was performed, with follow-up continued through hospital discharge. Data were collected on patient demographics, clinical characteristics including admission laboratories and chest x-ray findings (abnormal defined as presence of an infiltrate/opacity consistent with airspace disease), continuous monitoring utilization, respiratory support, medication treatment, ICU transfer, and final hospital disposition. In addition, prospective recordings of cardiac arrhythmias that prompted CMU notification of bedside nursing were reviewed.
The primary outcome was a composite of death, ICU transfer, or increased oxygen requirement defined as escalation from simple nasal cannula to either high-flow nasal cannula (HFNC), noninvasive ventilation (NIV) consisting of continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BiPAP), or mechanical ventilation. In accordance with published guidelines, patients were treated with supplemental oxygen to maintain peripheral oxygen saturation between 92% and 96%.15
Of note, based on the validated performance of high sensitivity troponin primarily for the diagnosis of acute myocardial infarction in patients presenting to the emergency department with chest pain, our system reserves its use for this context and prefers conventional (fourth generation) troponin T testing for inpatients. Therefore, conventional troponin T values are reported in this study.
Statistical Analyses
Continuous variables are expressed as mean ± standard deviation or median (interquartile range), and categorical variables are expressed as absolute numbers with percentages. Independent samples t and Mann-Whitney U tests were used to compare continuous variables, as appropriate, and chi-square testing was used to compare categorical variables. Clinical variables satisfying an a priori two-tailed threshold of P < .05 were retained for multivariable logistic regression analysis. Variables retaining P < .05 in multivariable modeling were considered statistically significant. Analyses were performed using SPSS software, Version 23 (SPSS Inc).
RESULTS
Baseline Characteristics
Between March 13, 2020, and May 1, 2020, a total of 350 patients admitted from the emergency department to a non-ICU inpatient bed had a final hospital disposition. Baseline characteristics, medication treatments, and continuous monitoring utilization are shown in Table 1 and Table 2. The average age was 64 ± 16 years, more than half of patients were male (n = 194; 55%), and most patients had at least one underlying comorbidity (n = 297; 85%), the most common being hypertension (n = 230; 66%), diabetes mellitus (n = 113; 32%), and current or prior tobacco use (n = 99; 28%). The presenting syndrome most frequently included subjective fever (n = 191; 55%), cough (n = 191; 55%), or dyspnea (n = 180; 51%).

Continuous Monitoring Use
Continuous monitoring was used in most patients (n = 289; 83%), including telemetry with intermittent pulse oximetry (n = 197; 56%), telemetry with continuous pulse oximetry (n = 81; 23%), or continuous pulse oximetry alone (n = 11; 3%). Among telemetry-monitored patients (n = 278; 79%), the most frequent indication was for a noncardiac disease state (n = 187; 67%), while indications for known cardiac arrhythmia (n = 74; 27%), heart failure (n = 10; 4%), or coronary artery disease (n = 7; 2%) were less common.

Oxygen Requirements and Cardiac Arrhythmias
The maximum level of respiratory support required by each patient is shown in Appendix Figure 1A. A total of 256 patients (73%) required 3 L/min or less of supplemental oxygen by nasal cannula, 45 (13%) required more than 3 L/min of supplemental oxygen by nasal cannula, 19 (5%) required HFNC, 8 (2%) required NIV, and 22 patients (6%) required mechanical ventilation. Among patients requiring HFNC or NIV, there were 13 (48%) who remained in a non-ICU bed, while the remaining 14 patients (52%) were transferred to the ICU.
Cardiac arrhythmias were detected in 39 (14%) of the 278 telemetry-monitored patients (Appendix Figure 1B). Clinical arrhythmias consisted of supraventricular tachycardia (SVT) in 17 patients (6%), nonsustained monomorphic ventricular tachycardia (VT) in 15 patients (5%), and a prolonged pause or severe bradyarrhythmia in 12 patients (4%). There were no cases of sustained monomorphic VT, polymorphic VT (including torsades de pointes), or ventricular fibrillation. All supraventricular tachycardias, nonsustained monomorphic VTs, and bradyarrhythmias/pauses were managed medically in the non-ICU setting, with the exception of one patient who was transferred to the ICU for a primary indication of atrial fibrillation with rapid ventricular response, which was treated with amiodarone. No patient with supraventricular tachycardia required emergent cardioversion, and no patient with a bradyarrhythmia or pause required temporary or permanent pacemaker implantation.
The detection of any arrhythmia was more common in patients with a history of cardiac arrhythmia (n = 18/41 vs 21/237; 44% vs 9%; P < .001), congestive heart failure (n = 11/36 vs 28/242; 31% vs 12%; P = .002), coronary artery disease (n = 12/49 vs 27/229; 24% vs 12%; P = .02), hypertension (n = 33/190 vs 6/88; 17% vs 7%; P = .02), and an abnormal admission troponin level (n = 13/40 vs 19/142; 33% vs 13%; P = .005). Notably, of the 39 patients with cardiac arrhythmias, 35 (90%) had either an abnormal admission troponin level or a history of cardiac arrhythmia, congestive heart failure, coronary artery disease, or hypertension. Of the 17 patients with SVT episodes, 13 (76%) had a known history of atrial fibrillation. Among patients who had a cardiac arrhythmia vs those who did not, there were no differences in levels of C-reactive protein (CRP; 7.3 ± 6.2 mg/dL vs. 7.8 ± 6.8 mg/dL, P = .63) or lactate dehydrogenase (LDH; 281 ± 89 U/L vs. 318 ± 142 U/L; P = .17). Approximately half of patients were treated with hydroxychloroquine (n = 185; 53%) or azithromycin (n = 182; 52%); 41% were treated with both (n = 142), with no observed association between any arrhythmia type and treatment with one or both medications (P > .05 for all comparisons).
Discharge Disposition and Adverse Outcomes
After an average length of stay of 6.1 ± 5.9 days, final hospital disposition included discharge to home (n = 278; 79%), discharge to subacute facility (n = 40; 11%), discharge to hospice (n = 8; 2%), death (n = 22, 6%), or release against medical advice (n = 2; 1%) (Figure). The primary composite outcome occurred in 62 patients (18%), including 22 deaths (6%), 48 ICU transfers (14%), and 49 patients with increased oxygen requirements (14%). Only two deaths occurred in the absence of an increased oxygen requirement or ICU transfer.

Increased oxygen requirement was the indication for ICU transfer in 37 of 48 patients (77%), with 22 patients (46%) requiring mechanical ventilation. Of the 48 patients requiring ICU transfer, 14 (29%) died, including 10 of the 22 patients (45%) treated with mechanical ventilation. Of the 302 patients who remained in the non-ICU setting, 8 (3%) died and 8 (3%) were discharged to hospice.
In univariable analyses, the primary composite outcome was more common among older patients (event vs event free, 72 ± 13 years vs 63 ± 16 years; P < .001); it was also more common in patients with congestive heart failure (n = 14/62 vs 28/288; 23% vs 10%; P = .005), chronic obstructive pulmonary disease (n = 9/62 vs 19/288; 15% vs 7%; P = .04), lower body mass index (29 ± 5 kg/m2 vs 31 ± 7 kg/m2; P = .006), lower peripheral oxygen saturation on room air (93% ± 5% vs 95% ± 3%; P = .005), higher CRP level (12.0 ± 7.8 mg/dL vs 6.9 ± 6.1 mg/dL; P < .001), higher LDH level (358 ± 140 U/L vs 302 ± 133 U/L; P = .009), higher troponin level (0.05 ± 0.13 ng/dL vs 0.02 ± 0.06 ng/dL; P = .01), abnormal D-dimer level (n = 39/42 vs 102/145; 93% vs 70%; P = .003), and abnormal chest x-ray findings (n = 48/62 vs 166/285; 77% vs 58%; P = .005) (Table 1 and Table 2). After multivariable adjustment, CRP level (odds ratio [OR], 1.09 per 1 mg/dL increase; 95% CI, 1.01-1.18; P = .04) and LDH level (OR, 1.006 per 1 U/L increase; 95% CI, 1.001-1.012; P = .03) remained significantly associated with the composite adverse outcome (Table 3). The rate of death, ICU transfer, or increased oxygen requirement was sixfold higher in patients with a CRP level in the fourth quartile (≥11.0 mg/dL) than it was among those in the first quartile (≤ 2.6 mg/dL) (P < .001 for trend), and it was fivefold higher in patients with an LDH level in the fourth quartile (≥ 354 U/L) than it was among those in the first quartile (≤ 232 U/L) (P = .001 for trend) (Appendix Figure 2). No patient with a CRP level in the reference range (≤ 0.9 mg/dL) experienced the composite adverse event, compared to three patients (n = 3/49, 6.1%) within the reference range for LDH level (≤ 225 U/L), all of whom had an elevated CRP.

DISCUSSION
In this study of 350 patients initially admitted to a non-ICU hospital bed within a large, nonepicenter healthcare system, the primary outcome of death, ICU transfer, or increased oxygen requirement occurred in 18% of patients and was independently associated with higher admission CRP and LDH levels on multivariable analysis. Most patients (73%) required 3 L/min or less of supplemental oxygen, while 14% of patients required escalation to HFNC, NIV, or mechanical ventilation. Despite frequent telemetry use (79%), cardiac arrhythmias were uncommon (14%), including no life-threatening ventricular arrhythmias. Clinical deterioration requiring ICU transfer occurred in 14% of patients, most often for an indication of increased oxygen requirement (77%). In-hospital mortality was 6% for the entire cohort, 29% for patients requiring ICU transfer, and 3% for patients who remained in the non-ICU setting.
Nonepicenter, Non-ICU Mortality
This study offers an assessment of clinical outcomes in patients with COVID-19 hospitalized in a non-ICU, nonepicenter healthcare system operating below capacity. Although such systems account for most institutions caring for patients with COVID-19, this population has been underrepresented in the literature, which has focused on epicenter hospitals and critically ill patients.3-7 Existing epicenter estimates of in-hospital mortality for patients not requiring ICU-level care range from 6% in Northern California2 to at least 10% in New York, New York,3 and 11% in Wuhan, China.4 The corresponding non-ICU in-hospital mortality in our study was only 3%, supporting the vital role of social distancing in reducing COVID-19 mortality by facilitating care delivery in a non–resource limited hospital setting.
Oxygen Requirements and Cardiac Arrhythmias in Non-ICU Patients
Beyond nonepicenter mortality estimates, this study is the first to provide a detailed characterization of the clinical course and resource usage among patients with COVID-19 admitted to the non-ICU setting. Given the predicted persistence of SARS-CoV-2 spread,11-13 this information is crucial to healthcare systems that must anticipate resource requirements, such as respiratory support and continuous monitoring equipment, for the care of hospitalized patients with COVID-19. Such informed planning takes on even greater importance as prepandemic hospital services resume.
While most patients (73%) with COVID-19 admitted to a non-ICU bed required peak supplemental oxygen of 3 L/min or less, a relevant proportion (14%) developed a need for HFNC, NIV, or mechanical ventilation. Furthermore, among telemetry-monitored patients (79%), cardiac arrhythmias were uncommon (14%), and nearly all (90%) occurred in patients with either a positive troponin or known history of cardiac disease. There were no life-threatening ventricular arrhythmias associated with frequent use of hydroxychloroquine (53%) and azithromycin (52%).
These telemetry findings expand upon a smaller study of non-ICU patients receiving either hydroxychloroquine or azithromycin, in which no life-threatening ventricular tachyarrhythmias were detected.8 A separate study reported a 5.9% incidence of malignant ventricular tachyarrhythmias in hospitalized patients with COVID-19,10 but this study did not stratify arrhythmias by illness severity, and a high frequency of critical illness is suggested by the mechanical ventilation rate of 24%, thereby limiting comparison with our non-ICU telemetry findings.
CRP and LDH Levels as Predictors of Adverse Outcomes
This study supports the utility of obtaining CRP and LDH levels for risk stratification at the time of non-ICU hospital admission. In multivariable analysis, higher CRP and LDH levels were significantly associated with the composite adverse outcome. The adverse event rates was increased sixfold between patients with a CRP in the fourth quartile (≥ 11.0 mg/dL, 36%) and those in the first quartile (≤ 2.6 mg/dL, 5.3%), and it was fivefold higher in patients with an LDH level in the fourth quartile (≥ 354 U/L, 34%) compared with those in the first quartile (≤ 232 U/L, 7%).
These findings are consistent with prior studies that have associated elevated inflammatory markers with poor prognosis and death.7,9,16 In some cases, COVID-19 may manifest similar to a cytokine storm syndrome, which highlights the importance of inflammation-associated tissue injury and leads to widespread interest in the use of immunosuppressive medications.17,18 Several studies also have demonstrated an association between LDH level and severe illness,4,7,19 although this is the first to specifically demonstrate its association with clinical decompensation in the non-ICU hospitalized population. Given that SARS-CoV-2 can infect multiple organs,20,21 there is biological plausibility for the use of LDH levels as a nonspecific marker of tissue injury for early identification of more severe infection.
Notably, while elevated troponin levels have been strongly associated with the need for mechanical ventilation and with death, this has primarily been established using either high-sensitivity troponin assays at the time of admission22 or using peak conventional troponin levels during hospitalization.10 In this study, while abnormal conventional troponin levels at the time of non-ICU admission were not significantly associated with the primary outcome in multivariable analysis, absolute troponin values were significantly higher in univariable analysis. Incomplete troponin sampling and the lack of routine high-sensitivity troponin assay use may explain the lack of more robust troponin significance in this study.
Implications for Non-ICU Continuous Monitoring Resource Allocation
Prioritization of non-ICU continuous monitoring resources among patients with COVID-19 has numerous benefits, including reduced consumption of personal protective equipment, fewer healthcare worker exposures, and adequate availability of continuous monitoring for the expansion of prepandemic hospital services. While individualized clinical discretion is still required, the results of this study can be used as a guide for the allocation of continuous pulse oximetry and cardiac telemetry. Patients with a normal presenting CRP level and/or LDH level had a low incidence of clinical decompensation, which suggests that such patients could be monitored with intermittent rather than continuous pulse oximetry. Furthermore, cardiac telemetry could be reserved for patients with a history of cardiac comorbidities or abnormal troponin levels because such patients accounted for 90% of cardiac arrhythmias in this study.
Limitations
This study was limited to a single health system, and it lacks a direct comparison to nonhospitalized patients and those directly admitted to the ICU. Triage practices and thresholds for hospitalization may differ across institutions and regions, thereby limiting the generalizability of our study. Additional limitations include the lack of selected admission laboratories for all patients, as well as the lack of telemetry monitoring in all patients. However, any resulting selection bias may be more likely to attenuate the magnitude of observed effects given that additional testing and increased telemetry use may be expected in patients who are felt to be higher risk by routine clinical assessment.
CONCLUSION
In this study of non–critically ill patients hospitalized within a nonepicenter health system, the development of more severe illness or death was significantly associated with higher levels of CRP and LDH on admission. Clinical decompensation was driven largely by respiratory complications, while cardiac arrhythmias were rare. Overall, the non-ICU mortality rate was at least half of that reported in epicenter regions. Altogether, these findings provide valuable information for resource allocation planning while nonepicenter health systems continue caring for patients with COVID-19 as they also resume prepandemic operations.
1. Bialek S, Boundy E, Bowen V, et al; CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343-346. https://doi.org/10.15585/mmwr.mm6912e2
2. Myers LC, Parodi SM, Escobar GJ, Liu VX. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. JAMA. 2020;323(21):2195-2198. https://doi.org/10.1001/jama.2020.7202
3. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. Published online April 22, 2020. https://doi.org/10.1001/jama.2020.6775
4. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. https://doi.org/10.1016/s0140-6736(20)30566-3
5. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA. 2020;323(16):1612-1614. https://doi.org/10.1001/jama.2020.4326
6. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1574-1581. https://doi.org/10.1001/jama.2020.5394
7. Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. https://doi.org/10.1001/jama.2020.1585
8. Chang D, Saleh M, Gabriels J, et al. Inpatient use of ambulatory telemetry monitors for COVID-19 patients treated with hydroxychloroquine and/or azithromycin. J Am Coll Cardiol. 2020;75(23):2992-2993. https://doi.org/10.1016/j.jacc.2020.04.032
9. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. https://doi.org/10.1016/s0140-6736(20)30183-5
10. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):1-8. https://doi.org/10.1001/jamacardio.2020.1017
11. Centers for Disease Control and Prevention COVID-19 Forecasts. Accessed May 19, 2020. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/forecasting-us.html
12. Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368(6493):860-868. https://doi.org/10.1126/science.abb5793
13. Baker RE, Yang W, Vecchi GA, Metcalf CJE, Grenfell BT. Susceptible supply limits the role of climate in the early SARS-CoV-2 pandemic. Science. 2020;369(6501):315-319. https://doi.org/10.1126/science.abc2535
14. Cantillon DJ, Loy M, Burkle A, et al. Association between off-site central monitoring using standardized cardiac telemetry and clinical outcomes among non-critically ill patients. JAMA. 2016;316(5):519-524. https://doi.org/10.1001/jama.2016.10258
15. Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med. 2020;48(6):e440-e469. https://doi.org/10.1097/ccm.0000000000004363
16. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. https://doi.org/10.1056/nejmoa2002032
17. Mehta P, McAuley DF, Brown M, et al; HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033-1034. https://doi.org/10.1016/s0140-6736(20)30628-0
18. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. Published online April 13, 2020. https://doi.org/10.1001/jama.2020.6019
19. Liang W, Liang H, Ou L, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020;180(8):1-9. https://doi.org/10.1001/jamainternmed.2020.2033
20. Puelles VG, Lütgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383(6):590-592. https://doi.org/10.1056/nejmc2011400
21. Zhou J, Li C, Liu X, et al. Infection of bat and human intestinal organoids by SARS-CoV-2. Nat Med. 2020;26(7):1077-1083. https://doi.org/10.1038/s41591-020-0912-6
22. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802-810. https://doi.org/10.1001/jamacardio.2020.0950
1. Bialek S, Boundy E, Bowen V, et al; CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343-346. https://doi.org/10.15585/mmwr.mm6912e2
2. Myers LC, Parodi SM, Escobar GJ, Liu VX. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. JAMA. 2020;323(21):2195-2198. https://doi.org/10.1001/jama.2020.7202
3. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. Published online April 22, 2020. https://doi.org/10.1001/jama.2020.6775
4. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. https://doi.org/10.1016/s0140-6736(20)30566-3
5. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA. 2020;323(16):1612-1614. https://doi.org/10.1001/jama.2020.4326
6. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1574-1581. https://doi.org/10.1001/jama.2020.5394
7. Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. https://doi.org/10.1001/jama.2020.1585
8. Chang D, Saleh M, Gabriels J, et al. Inpatient use of ambulatory telemetry monitors for COVID-19 patients treated with hydroxychloroquine and/or azithromycin. J Am Coll Cardiol. 2020;75(23):2992-2993. https://doi.org/10.1016/j.jacc.2020.04.032
9. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. https://doi.org/10.1016/s0140-6736(20)30183-5
10. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):1-8. https://doi.org/10.1001/jamacardio.2020.1017
11. Centers for Disease Control and Prevention COVID-19 Forecasts. Accessed May 19, 2020. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/forecasting-us.html
12. Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368(6493):860-868. https://doi.org/10.1126/science.abb5793
13. Baker RE, Yang W, Vecchi GA, Metcalf CJE, Grenfell BT. Susceptible supply limits the role of climate in the early SARS-CoV-2 pandemic. Science. 2020;369(6501):315-319. https://doi.org/10.1126/science.abc2535
14. Cantillon DJ, Loy M, Burkle A, et al. Association between off-site central monitoring using standardized cardiac telemetry and clinical outcomes among non-critically ill patients. JAMA. 2016;316(5):519-524. https://doi.org/10.1001/jama.2016.10258
15. Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med. 2020;48(6):e440-e469. https://doi.org/10.1097/ccm.0000000000004363
16. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. https://doi.org/10.1056/nejmoa2002032
17. Mehta P, McAuley DF, Brown M, et al; HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033-1034. https://doi.org/10.1016/s0140-6736(20)30628-0
18. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. Published online April 13, 2020. https://doi.org/10.1001/jama.2020.6019
19. Liang W, Liang H, Ou L, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020;180(8):1-9. https://doi.org/10.1001/jamainternmed.2020.2033
20. Puelles VG, Lütgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383(6):590-592. https://doi.org/10.1056/nejmc2011400
21. Zhou J, Li C, Liu X, et al. Infection of bat and human intestinal organoids by SARS-CoV-2. Nat Med. 2020;26(7):1077-1083. https://doi.org/10.1038/s41591-020-0912-6
22. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802-810. https://doi.org/10.1001/jamacardio.2020.0950
© 2021 Society of Hospital Medicine
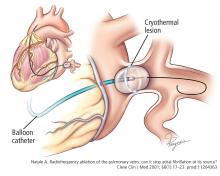
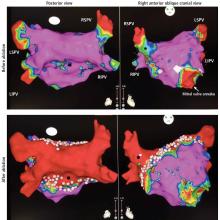
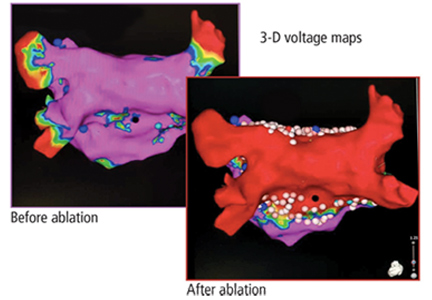
Ablation of atrial fibrillation: Facts for the referring physician
A 64-year-old man with hypertension but without known structural heart disease presents for a second opinion on management of his atrial fibrillation. The condition was first diagnosed at age 38, when he experienced palpitations and shortness of breath on exertion; at times he also experienced decreased endurance and fatigue without overt palpitations. At first, these episodes occurred about twice a year, and the patient was managed with a beta-blocker for rate control and an oral anticoagulant.
Over the past 10 years, the episodes have become more frequent and longer-lasting and have required frequent cardioversions. He was given flecainide for rhythm control but continued to have frequent episodes, and so about 1 year ago he was switched to amiodarone, which controlled his rhythm better. However, after reading about side effects of amiodarone, he decided to seek a second opinion.
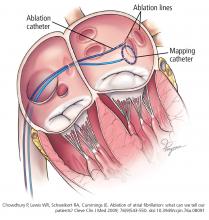
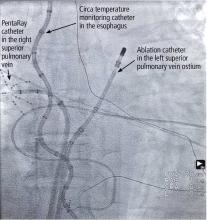
He was evaluated by our team and eventually underwent radiofrequency ablation. During the procedure, he was noted to have diffuse scarring and fibrosis of his left atrium, and afterward he continued to require antiarrhythmic drugs to maintain sinus rhythm.
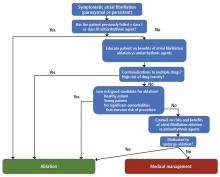
Should he have been referred sooner? What factors should primary care physicians consider when referring a patient with atrial fibrillation for ablation?
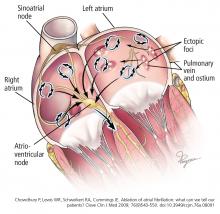
THE EPIDEMIC OF ATRIAL FIBRILLATION
Atrial fibrillation is a large and growing public health problem. In 2010, it was estimated to affect 2.7 to 6.1 million people in the United States, and with the rapid aging of our population, its prevalence is expected to rise to between 5.6 and 12 million by 2050.1–3 It is associated with significant morbidity, poor quality of life, and increased risk of death, heart failure, stroke, and cognitive impairment.
The number of new cases per year has increased over the years despite research and preventive measures, which may reflect aging of the population and increased survival rates in patients with cardiovascular or comorbid conditions.1,4
Thus, atrial fibrillation is one of the most common cardiovascular conditions encountered by primary care physicians and cardiologists, putting them at the forefront of its management. Proper treatment in its early stages and referral to a specialist for advanced management may alter its natural history and improve clinical outcomes.
HOW DOES ATRIAL FIBRILLATION ARISE AND PERSIST?
Much is still unknown about the pathogenesis of atrial fibrillation, but considerable progress has been made in the past few decades, opening the door for clinical ablative strategies.
Multiple wavelet hypothesis
Until the late 1980s, the most widely accepted conceptual mechanism of atrial fibrillation was the multiple wavelet hypothesis developed by Moe et al.5 According to this hypothesis, atrial fibrillation begins with multiple independent wavelets occurring simultaneously and spreading randomly throughout both atria, and it persists if there are a minimum number of coexisting wavelets, increased atrial mass, and heterogeneous conduction delays across the atrial tissue.
The surgical maze procedure, in which a series of incisions arranged in a maze-like pattern is created in the left atrium, was predicated on this model. The theory was that these surgical lesions would compartmentalize the atria into discrete electrical segments and thereby reduce the number of circulating random wavelets.6,7
However, experimental and clinical studies suggest that although randomly propagating wavelets can contribute to maintaining atrial fibrillation, focal triggers are noted in most cases.
Focal triggers
In 1997, Jaïs et al8 observed that atrial fibrillation is often triggered by a rapidly firing ectopic focus and that ablation of that focus can eliminate it. These ectopic foci are often found at or near the ostia of the pulmonary veins or near the superior vena cava.8,9 It is now well established that ectopic foci in the pulmonary veins are crucial triggers that initiate atrial fibrillation.
Trigger-and-substrate theory
The substrate for maintaining atrial fibrillation consists of an abnormal left atrium with heterogeneous fibrosis (scarring) and conduction delays. Any heart disease that increases left atrial pressure could lead to atrial dilation and remodeling, which could be substrates for atrial fibrillation. Extensive atrial remodeling and scarring are associated with progression and persistence of atrial fibrillation and make rhythm control more challenging.
Atrial fibrillation begets atrial fibrillation
As shown in the case above, over time, paroxysmal atrial fibrillation often progresses to persistent and long-standing atrial fibrillation if not aggressively managed initially.
In 1972, Davies and Pomerance10 performed 100 autopsies and found that the people who had had atrial fibrillation for longer than 1 month had lost muscle mass in the sinus node and internodal tract, and their atria were dilated. The study introduced the concept that atrial fibrillation itself causes pathologic changes in the atrium.
Wijffels et al,11 in an experiment in goats, showed that atrial fibrillation produced by rapid bursts of atrial pacing was initially paroxysmal. However, as they continued to induce atrial fibrillation over and over again, it lasted progressively longer until it would persist for more than 24 hours. Thus, in a relatively short time, the atria went from supporting paroxysmal fibrillation to supporting persistent fibrillation.
Atrial fibrillation leads to electrophysiologic and anatomic remodeling in the atrium, which leads to a shorter action potential duration and a shorter refractory period. This in turn makes it easier for atrial fibrillation to persist.12
Because atrial fibrillation tends to progress, intervening early may improve its outcomes. Early ablation has been shown to improve the chances of staying in sinus rhythm in both paroxysmal and persistent atrial fibrillation.13–15
CATHETER ABLATION OF ATRIAL FIBRILLATION
The goal of ablation is to prevent atrial fibrillation by eliminating the trigger that initiates it, altering the arrhythmogenic substrate, or both.
Pulmonary vein isolation
The most common ablation strategy is to electrically isolate the pulmonary veins by creating circumferential lesions around their antra. This creates a nonconducting rim of scar tissue, electrically disconnecting the pulmonary veins from the atrium.
Ablation outside of the pulmonary veins
Because recurrence rates are high in patients with persistent atrial fibrillation who undergo pulmonary vein ablation alone, the search continues for adjunctive strategies to improve outcomes. Although these strategies have a sound rationale based on experimental data and anecdotal evidence in humans, they have not yet been convincingly shown to be helpful in large clinical studies. Nonetheless, it is possible that more extensive substrate ablation—atrial “debulking”—could improve outcomes by reducing the amount of tissue that can fibrillate.
Linear ablation. Creating lines of ablation (as in the maze procedure) isolates different segments of the left atrium. Often, these lines are created along the roof of the left atrium between the right and left upper pulmonary veins and from the mitral valve to the left inferior pulmonary vein. The benefit of linear ablation has not been proven, and gaps in such lines may introduce atrial flutter.
Triggers not in the pulmonary veins. Common sites of nonpulmonary vein triggers include the posterior wall of the left atrium, the superior vena cava, the coronary sinus, and along the ligament of Marshall. Provocative maneuvers such as isoproterenol infusion can help find those triggers, which can then be ablated. A limitation is that there is no protocol proven to reproducibly elicit triggers.
Complex fractionated atrial electrograms are areas in the atrium with highly fractionated, low voltage potentials. They may be critical sites of substrate for atrial fibrillation, and many electrophysiologists target them in patients with persistent atrial fibrillation. But despite initial enthusiasm, doing so has not resulted in better outcomes in persistent atrial fibrillation.
Rotors. Animal studies have shown that atrial fibrillation can be triggered or maintained by localized sources of organized reentrant circuits (rotors) or focal impulses. Recent studies have shown that these electrical rotors and focal sources could potentially be mapped and ablated in humans. But positive results in initial reports have not been reproduced, and this remains an area of controversy.
Our practice. We isolate the pulmonary veins with antral ablations, ablate the posterior wall, and extend the ablation toward the septum and inferior to the right pulmonary veins, with good long-term outcomes.14 The rationale behind ablating the posterior wall is that it shares embryologic origins with the pulmonary veins and may be a common source of triggers in atrial fibrillation.
We do not routinely create empiric ablation lines in the left or right atrium unless the patient has atrial flutter. Empiric ablation lines have not been convincingly shown to provide additional benefit compared with our extensive ablation approach, which involves the posterior wall. Empiric ablation of the appendage or coronary sinus is typically reserved for repeat ablation in patients with recurrent persistent atrial fibrillation.
RATIONALE FOR TREATING ATRIAL FIBRILLATION WITH ABLATION
To control symptoms
At this time, the primary aim of atrial fibrillation ablation is to reduce symptoms and improve quality of life. In theory, ablation could also decrease the risk of stroke, heart failure, and death. However, these outcomes have not been systematically evaluated in any large randomized controlled trial.
To control rhythm and improve survival
Randomized controlled trials of rhythm vs rate control of atrial fibrillation16–18 have failed to demonstrate that restoring sinus rhythm is associated with better survival. All of these trials used antiarrhythmic drugs for rhythm control. However, nonrandomized studies19,20 showed that maintaining sinus rhythm is associated with a significant reduction in mortality rates, whereas the use of antiarrhythmic drugs increased mortality risk.
This suggests that the beneficial effect of restoring sinus rhythm may be offset by adverse effects of antiarrhythmic drugs, and if rhythm control could be achieved by a method other than antiarrhythmic drug therapy, it may be superior to rate control. On the other hand, these data may be affected by residual confounding. This topic deserves further research, but maintaining sinus rhythm is typically preferred whenever possible.
Discontinuing anticoagulation is not a goal at this time
Retrospective studies have reported a low risk of stroke in patients who discontinue anticoagulation several months after undergoing atrial fibrillation ablation.21–23 However, atrial fibrillation can recur, and risk of stroke increases with age.
Therefore, guidelines24 still recommend continuing anticoagulation after ablation. Generally, we do not offer ablation with a goal of discontinuing anticoagulation. That said, stopping anticoagulation may be considered after long-term suppression of paroxysmal atrial fibrillation on a case-by-case basis in patients deemed to be at low risk. Left atrial appendage closure devices may eventually allow concomitant atrial fibrillation ablation and closure of the appendage, so that anticoagulation could then be stopped. This remains a topic of investigation.
Who should be considered for ablation?
There are no absolute age or comorbidity contraindications to ablation. Everyone who has atrial fibrillation deserves, in our opinion, a referral to the electrophysiology clinic.
PROCEDURAL CONSIDERATIONS
Atrial fibrillation ablation is most often performed by electrophysiologists using a minimally invasive endovascular approach. The patient can be under either moderate sedation or general anesthesia; we prefer general anesthesia for patient comfort, safety, and efficacy.
We use an electrogram-based technique to target and eliminate electrical potentials and ensure continuity of ablation sets, with additional guidance by 3-dimensional cardiac mapping systems and intracardiac echocardiography. We also use contact force-sensing catheters to ensure catheter-tissue contact during ablation and to avoid excessive contact, which may enhance the safety of the procedure.
Energy: Hot or cold
Two types of energy can be used for ablation:
Radiofrequency energy (low voltage, high frequency—30 kHz to 1.5 mHz) is delivered to the endocardial surface via a point-source catheter. The radiofrequency energy produces controlled, focal thermal ablation.
In a randomized trial,25 these ablation technologies were shown to be equivalent for preventing recurrences of atrial fibrillation. We use both in our practice. The choice depends primarily on the planned ablation set, given that balloon cryoablation can achieve antral isolation of the pulmonary veins but allows little or no substrate modification.
Improved ablation technology
Contact force-sensing catheters. Radiofrequency ablation catheters are now equipped with a pressure sensor at the tip that measures how hard the catheter is pressing on the heart wall.26,27 In our experience, this has improved the outcomes of ablation procedures, primarily in persistent atrial fibrillation.28
Complications of ablation
Although catheter ablation for atrial fibrillation is safe, it is still one of the most complex electrophysiologic procedures. Improvements in technology and techniques and accumulated experience over the past 15 years have made ablation safer, especially in tertiary care centers. But adverse outcomes are more frequent in low-volume centers.29
Minor procedural complications include pericarditis, complications at the site of vascular access, and anesthesia-related complications. While they do not affect the long-term outcome for the patient, they may increase hospital length of stay and cause temporary inconvenience.
Major complications include cardiac perforation and tamponade, periprocedural stroke, pulmonary vein stenosis, atrioesophageal fistula, phrenic nerve paralysis, major bleeding, myocardial infarction, and death. In a worldwide survey published in 2005, when atrial fibrillation ablation was still novel, the rate of major complications was 6%.30 By 2010, this had declined to 4.5%,31 and the rates of major complications may be significantly lower in more experienced centers.29 In our practice, in 2015, the rate of major complications was 1.3% (unpublished data).
Outcomes of catheter ablation
Clinical outcomes depend on many factors including the type of atrial fibrillation (paroxysmal vs nonparoxysmal), overall health of the atria (atrial size and scarring), patient age and comorbidities, and most importantly, the center’s and operator’s experience.
In randomized controlled trials comparing ablation and antiarrhythmic drug therapy, the efficacy of ablation in maintaining sinus rhythm has been in the range of 66% to 86% vs 16% to 22% for drug therapy,32,33 but these trials have been predominantly in middle-aged white men with paroxysmal atrial fibrillation. These trials also showed that catheter ablation reduced symptoms and improved quality of life. Ablation is less effective in persistent than in paroxysmal atrial fibrillation.34
In a long-term study from our group,14 660 (79.4%) of 831 patients who underwent ablation in 2005 were arrhythmia-free and not on antiarrhythmic drug therapy after a total of 1,019 ablations (an average of 1.2 ablations per patient) at a median of 55 months; 125 patients (15%, 41 with more than 1 ablation) continued to have atrial arrhythmia, controlled with drugs in 87 patients (69.6%). Only 38 patients (4.6%) continued to have drug-resistant atrial fibrillation and were treated with rate control with negative dromotropic agents.
Recent evidence
The largest randomized controlled trial of catheter ablation vs drug therapy for atrial fibrillation (Catheter Ablation Versus Antiarrhythmic Drug Therapy for Atrial Fibrillation [CABANA]) was completed recently, and the results were presented at a national meeting, although they have not yet been published in a peer-reviewed journal.35
A total of 2,204 patients with atrial fibrillation (42.4% paroxysmal, 47.3% persistent, and 10.3% long-standing persistent) were randomized to either ablation or drug therapy. Median follow-up was 4 years. The crossover rate was high—9.2% of those randomized to ablation did not undergo it, and 27.5% of those randomized to drug therapy underwent ablation.
The incidence of the primary end point (a composite of death, disabling stroke, serious bleeding, and cardiac arrest) was not significantly different between the 2 groups in the intention-to-treat analysis; however, given the high crossover rates, the as-treated and per-protocol analyses become important, and as-treated and per-protocol analyses revealed a significant benefit of ablation compared with drug therapy. The hazard ratio (HR) for the primary composite outcome was 0.67 (P = .006) on as-treated analysis and 0.73 (P = .05) on per-protocol analysis. The HR for all-cause mortality was 0.60 (P = .005) on as-treated analysis.
PERIPROCEDURAL CONSIDERATIONS
Periprocedural anticoagulation
The risk of thromboembolism is increased during, immediately following, and for several weeks to months after atrial fibrillation ablation.36,37
During the procedure, the risk is related to transseptal sheath placement, electrode catheters in the left atrium, and char formation on ablation catheters. These risks are mitigated with proper and careful sheath and catheter manipulation, maintenance of bubble-free irrigation through lines and sheaths, use of irrigated catheters, and initiation of heparin before transseptal access. Heparin is also infused during the procedure, with close monitoring of activated clotting time.
Postprocedurally, the transiently increased clotting risk could be due to damaged endothelium from the ablation itself and stunning of atrial tissue, which results in impaired contraction. Damaged endothelium improves as the tissue heals, and the stunning resolves by electrical reverse remodeling with sinus rhythm maintenance.
In view of these risks, the referring physician and electrophysiologist must pay careful attention to anticoagulation before and after ablation.
Before the procedure. It is safe to continue anticoagulation uninterrupted through the procedure.38,39 If the patient is on warfarin, we want the international normalized ratio to be in the therapeutic range when we perform atrial fibrillation ablation, and the patient takes his or her usual dose on the day of the procedure. If taking a direct oral anticoagulant, patients typically skip a dose the day before ablation and again on the morning of the procedure, and resume taking it immediately afterward while in the anesthesia recovery room.
During the procedure, we start heparin before transseptal puncture, adjust it to achieve an activated clotting time of 300 to 400 seconds, and keep it in this range as long as there are sheaths or catheters in the left atrium.
After the procedure. The current guidelines24 recommend that oral anticoagulation be continued without interruption for at least 2 months after the procedure, and in most cases indefinitely, depending on age and comorbidities. The decision to stop anticoagulation after 2 months is typically based on the stroke risk as assessed by the CHA2DS2-VASc score (www.chadsvasc.org) and not on the success of the ablation procedure.
ANTIARRHYTHMIC DRUGS AFTER THE PROCEDURE
Some patients actually experience more atrial fibrillation in the first weeks to months after the procedure. The mechanism in this setting may be different from that causing the arrhythmia in the first place. The causes of early recurrence of atrial arrhythmias include postablation inflammation, temporary autonomic imbalance, and delay of atrial radiofrequency lesion formation.40,41 These arrhythmias may completely resolve as the ablation lesions heal and scars mature.
It has been hypothesized that short-term use of antiarrhythmic drugs after atrial fibrillation ablation is effective in preventing arrhythmias because it alters atrial electrophysiologic characteristics induced by the above transient factors. A recent systematic review of 6 clinical trials showed that short-term use of antiarrhythmic drugs reduces the risk of early arrhythmia recurrence but does not reduce recurrence in the long term.42
In terms of outcomes, any arrhythmias that occur in the first 3 months do not necessarily affect long-term success. This is referred to as the “blanking period.” However, generally speaking, it is preferable to maintain sinus rhythm during that time to avoid further anatomic or electrical left atrial adverse remodeling. In many situations, patients continue taking the same antiarrhythmic agent or start on antiarrhythmic therapy in the first few months after ablation.43,44
The mechanisms of late recurrence of atrial arrhythmias after ablation are thought to be different from those in early recurrence. Late recurrence has been ascribed to incomplete pulmonary vein isolation, recovery of pulmonary vein-left atrium connections, or recovery of any other lines of ablation created in the procedure.45,46 For late recurrence of atrial arrhythmia, studies and guidelines suggest that repeat ablation may be an option.24,47
PRACTICAL CONSIDERATIONS FOR PROCEDURAL PLANNING
Before the procedure, some electrophysiologists use cardiac computed tomography or magnetic resonance imaging to evaluate the pulmonary vein anatomy. This helps in planning and in selecting the appropriate tools for the procedure.
The patient is asked to fast on the day of the procedure. The procedure can take 3 to 6 hours, depending on the patient’s anatomy and the operator’s technique and experience. It can be performed with the patient under general anesthesia or conscious sedation. Currently, we use general anesthesia most of the time to maximize patient comfort.
After the procedure, our patients must stay in bed for 4 hours and stay overnight for observation. If no complications arise, they are discharged the next day.
- Go AS. The epidemiology of atrial fibrillation in elderly persons: the tip of the iceberg. Am J Geriatr Cardiol 2005; 14(2):56–61. pmid:15785146
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285(18):2370–2375. pmid:11343485
- Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006; 114(2):119–125. doi:10.1161/CIRCULATIONAHA.105.595140
- Piccini JP, Hammill BG, Sinner MF, et al. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993–2007. Circ Cardiovasc Qual Outcomes 2012; 5(1):85–93. doi:10.1161/CIRCOUTCOMES.111.962688
- Moe GK, Rheinboldt WC, Abildskov JA. A computer model of atrial fibrillation. Am Heart J 1964; 67:200–220. pmid:14118488
- Cox JL, Schuessler RB, Boineau JP. The surgical treatment of atrial fibrillation. I. Summary of the current concepts of the mechanisms of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg 1991; 101(3):402–405. pmid:1999933
- Cox JL, Schuessler RB, D’Agostino HJ Jr, et al. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg 1991; 101(4):569–583. pmid:2008095
- Jaïs P, Haïssaguerre M, Shah DC, et al. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation 1997; 95(3):572–576. pmid:9024141
- Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998; 339(10):659–666. doi:10.1056/NEJM199809033391003
- Davies MJ, Pomerance A. Pathology of atrial fibrillation in man. Br Heart J 1972; 34(5):520–525. pmid:5031645
- Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 1995; 92(7):1954–1968. pmid:7671380
- Nattel S. New ideas about atrial fibrillation 50 years on. Nature 2002; 415(6868):219–226. doi:10.1038/415219a
- Medi C, Sparks PB, Morton JB, et al. Pulmonary vein antral isolation for paroxysmal atrial fibrillation: results from long-term follow-up. J Cardiovasc Electrophysiol 2011; 22(2):137–141. doi:10.1111/j.1540-8167.2010.01885.x
- Hussein AA, Saliba WI, Martin DO, et al. Natural history and long-term outcomes of ablated atrial fibrillation. Circ Arrhythm Electrophysiol 2011; 4(3):271–278. doi:10.1161/CIRCEP.111.962100
- Hussein AA, Saliba WI, Barakat A, et al. Radiofrequency ablation of persistent atrial fibrillation: diagnosis-to-ablation time, markers of pathways of atrial remodeling, and outcomes. Circ Arrhythm Electrophysiol 2016; 9(1):e003669. doi:10.1161/CIRCEP.115.003669
- Carlsson J, Miketic S, Windeler J, et al. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. J Am Coll Cardiol 2003; 41(10):1690–1696. pmid:12767648
- Van Gelder IC, Hagens VE, Bosker HA, et al; Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study Group. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med 2002; 347(23):1834–1840. doi:10.1056/NEJMoa021375
- Wyse DG, Waldo AL, DiMarco JP, et al; Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002; 347(23):1825–1833. doi:10.1056/NEJMoa021328
- Hagens VE, Crijns HJ, Van Veldhuisen DJ, et al; RAte Control versus Electrical cardioversion for persistent atrial fibrillation study group. Rate control versus rhythm control for patients with persistent atrial fibrillation with mild to moderate heart failure: results from the RAte Control versus Electrical cardioversion (RACE) study. Am Heart J 2005; 149(6):1106–111. doi:10.1016/j.ahj.2004.11.030
- Pedersen OD, Bagger H, Keller N, Marchant B, Køber L, Torp-Pedersen C. Efficacy of dofetilide in the treatment of atrial fibrillation-flutter in patients with reduced left ventricular function: a Danish investigations of arrhythmia and mortality on dofetilide (diamond) substudy. Circulation 2001; 104(3):292–296. pmid:11457747
- Guiot A, Jongnarangsin K, Chugh A, et al. Anticoagulant therapy and risk of cerebrovascular events after catheter ablation of atrial fibrillation in the elderly. J Cardiovasc Electrophysiol 2012; 23(1):36–43. doi:10.1111/j.1540-8167.2011.02141.x
- Oral H, Chugh A, Ozaydin M, et al. Risk of thromboembolic events after percutaneous left atrial radiofrequency ablation of atrial fibrillation. Circulation 2006; 114(8):759–765. doi:10.1161/CIRCULATIONAHA.106.641225
- Themistoclakis S, Corrado A, Marchlinski FE, et al. The risk of thromboembolism and need for oral anticoagulation after successful atrial fibrillation ablation. J Am Coll Cardiol 2010; 55(8):735–743. doi:10.1016/j.jacc.2009.11.039
- Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. J Arrhythm 2017; 33(5):369–409. doi:10.1016/j.joa.2017.08.001
- Kuck KH, Brugada J, Fürnkranz A, et al; FIRE AND ICE Investigators. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016; 374(23):2235–2245. doi:10.1056/NEJMoa1602014
- Reddy VY, Dukkipati SR, Neuzil P, et al. Randomized, controlled trial of the safety and effectiveness of a contact force-sensing irrigated catheter for ablation of paroxysmal atrial fibrillation: results of the TactiCath Contact Force Ablation Catheter Study for Atrial Fibrillation (TOCCASTAR) Study. Circulation 2015; 132(10):907–915. doi:10.1161/CIRCULATIONAHA.114.014092
- Natale A, Reddy VY, Monir G, et al. Paroxysmal AF catheter ablation with a contact force sensing catheter: results of the prospective, multicenter SMART-AF trial. J Am Coll Cardiol 2014; 64(7):647–656. doi:10.1016/j.jacc.2014.04.072
- Hussein AA, Barakat AF, Saliba WI, et al. Persistent atrial fibrillation ablation with or without contact force sensing. J Cardiovasc Electrophysiol 2017; 28(5):483–488. doi:10.1111/jce.13179
- Deshmukh A, Patel NJ, Pant I, et al. In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: analysis of 93,801 procedures. Circulation 2013; 128(19):2104–2112. doi:10.1161/CIRCULATIONAHA.113.003862
- Cappato R, Calkins H, Chen SA, et al. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation 2005; 111(9):1100–1105. doi:10.1161/01.CIR.0000157153.30978.67
- Cappato R, Calkins H, Chen SA, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 2010; 3(1):32–38. doi:10.1161/CIRCEP.109.859116
- Wazni OM, Marrouche NF, Martin DO, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA 2005; 293(21):2634–2640. doi:10.1001/jama.293.21.2634
- Jaïs P, Cauchemez B, Macle L, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation 2008; 118(24):2498–2505. doi:10.1161/CIRCULATIONAHA.108.772582
- Brooks AG, Stiles MK, Laborderie J, et al. Outcomes of long-standing persistent atrial fibrillation ablation: a systematic review. Heart Rhythm 2010; 7(6):835–846. doi:10.1016/j.hrthm.2010.01.017
- Packer DL, Lee KL, Mark DB, Robb RA. Catheter Ablation versus Antiarrhythmic Drug Therapy for Atrial Fibrillation Trial, CABANA. http://cabanatrial.org/. Accessed September 10, 2018.
- Scherr D, Sharma K, Dalal D, et al. Incidence and predictors of periprocedural cerebrovascular accident in patients undergoing catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2009; 20(12):1357–1363. doi:10.1111/j.1540-8167.2009.01540.x
- Wazni OM, Rossillo A, Marrouche NF, et al. Embolic events and char formation during pulmonary vein isolation in patients with atrial fibrillation: impact of different anticoagulation regimens and importance of intracardiac echo imaging. J Cardiovasc Electrophysiol 2005; 16(6):576–581. doi:10.1111/j.1540-8167.2005.40480.x
- Hussein AA, Martin DO, Saliba W, et al. Radiofrequency ablation of atrial fibrillation under therapeutic international normalized ratio: a safe and efficacious periprocedural anticoagulation strategy. Heart Rhythm 2009; 6(10):1425–1429. doi:10.1016/j.hrthm.2009.07.007
- Bassiouny M, Saliba W, Rickard J, et al. Use of dabigatran for periprocedural anticoagulation in patients undergoing catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol 2013; 6(3):460–466. doi:10.1161/CIRCEP.113.000320
- Koyama T, Tada H, Sekiguchi Y, et al. Prevention of atrial fibrillation recurrence with corticosteroids after radiofrequency catheter ablation: a randomized controlled trial. J Am Coll Cardiol 2010; 56(18):1463–1472. doi:10.1016/j.jacc.2010.04.057
- Oral H, Knight BP, Ozaydin M, et al. Clinical significance of early recurrences of atrial fibrillation after pulmonary vein isolation. J Am Coll Cardiol 2002; 40(1):100–104. pmid:12103262
- Chen W, Liu H, Ling Z, et al. Efficacy of short-term antiarrhythmic drugs use after catheter ablation of atrial fibrillation—a systematic review with meta-analyses and trial sequential analyses of randomized controlled trials. PLoS One 2016; 11(5):e0156121. doi:10.1371/journal.pone.0156121
- Leong-Sit P, Roux JF, Zado E, et al. Antiarrhythmics after ablation of atrial fibrillation (5A Study): six-month follow-up study. Circ Arrhythm Electrophysiol 2011; 4(1):11–14. doi:10.1161/CIRCEP.110.955393
- Roux JF, Zado E, Callans DJ, et al. Antiarrhythmics after ablation of atrial fibrillation (5A Study). Circulation 2009; 120(12):1036–1040. doi:10.1161/CIRCULATIONAHA.108.839639
- Sotomi Y, Inoue K, Ito N, et al. Cause of very late recurrence of atrial fibrillation or flutter after catheter ablation for atrial fibrillation. Am J Cardiol 2013; 111(4):552–556. doi:10.1016/j.amjcard.2012.10.040
- Lee SH, Tai CT, Hsieh MH, et al. Predictors of early and late recurrence of atrial fibrillation after catheter ablation of paroxysmal atrial fibrillation. J Interv Card Electrophysiol. 2004 Jun;10(3):221-6. doi:10.1023/B:JICE.0000026915.02503.92
- Zhang XD, Gu J, Jiang WF, et al. Optimal rhythm-control strategy for recurrent atrial tachycardia after catheter ablation of persistent atrial fibrillation: a randomized clinical trial. Eur Heart J 2014; 35(20):1327–1334. doi:10.1093/eurheartj/ehu017
A 64-year-old man with hypertension but without known structural heart disease presents for a second opinion on management of his atrial fibrillation. The condition was first diagnosed at age 38, when he experienced palpitations and shortness of breath on exertion; at times he also experienced decreased endurance and fatigue without overt palpitations. At first, these episodes occurred about twice a year, and the patient was managed with a beta-blocker for rate control and an oral anticoagulant.
Over the past 10 years, the episodes have become more frequent and longer-lasting and have required frequent cardioversions. He was given flecainide for rhythm control but continued to have frequent episodes, and so about 1 year ago he was switched to amiodarone, which controlled his rhythm better. However, after reading about side effects of amiodarone, he decided to seek a second opinion.
He was evaluated by our team and eventually underwent radiofrequency ablation. During the procedure, he was noted to have diffuse scarring and fibrosis of his left atrium, and afterward he continued to require antiarrhythmic drugs to maintain sinus rhythm.
Should he have been referred sooner? What factors should primary care physicians consider when referring a patient with atrial fibrillation for ablation?
THE EPIDEMIC OF ATRIAL FIBRILLATION
Atrial fibrillation is a large and growing public health problem. In 2010, it was estimated to affect 2.7 to 6.1 million people in the United States, and with the rapid aging of our population, its prevalence is expected to rise to between 5.6 and 12 million by 2050.1–3 It is associated with significant morbidity, poor quality of life, and increased risk of death, heart failure, stroke, and cognitive impairment.
The number of new cases per year has increased over the years despite research and preventive measures, which may reflect aging of the population and increased survival rates in patients with cardiovascular or comorbid conditions.1,4
Thus, atrial fibrillation is one of the most common cardiovascular conditions encountered by primary care physicians and cardiologists, putting them at the forefront of its management. Proper treatment in its early stages and referral to a specialist for advanced management may alter its natural history and improve clinical outcomes.
HOW DOES ATRIAL FIBRILLATION ARISE AND PERSIST?
Much is still unknown about the pathogenesis of atrial fibrillation, but considerable progress has been made in the past few decades, opening the door for clinical ablative strategies.
Multiple wavelet hypothesis
Until the late 1980s, the most widely accepted conceptual mechanism of atrial fibrillation was the multiple wavelet hypothesis developed by Moe et al.5 According to this hypothesis, atrial fibrillation begins with multiple independent wavelets occurring simultaneously and spreading randomly throughout both atria, and it persists if there are a minimum number of coexisting wavelets, increased atrial mass, and heterogeneous conduction delays across the atrial tissue.
The surgical maze procedure, in which a series of incisions arranged in a maze-like pattern is created in the left atrium, was predicated on this model. The theory was that these surgical lesions would compartmentalize the atria into discrete electrical segments and thereby reduce the number of circulating random wavelets.6,7
However, experimental and clinical studies suggest that although randomly propagating wavelets can contribute to maintaining atrial fibrillation, focal triggers are noted in most cases.
Focal triggers
In 1997, Jaïs et al8 observed that atrial fibrillation is often triggered by a rapidly firing ectopic focus and that ablation of that focus can eliminate it. These ectopic foci are often found at or near the ostia of the pulmonary veins or near the superior vena cava.8,9 It is now well established that ectopic foci in the pulmonary veins are crucial triggers that initiate atrial fibrillation.
Trigger-and-substrate theory
The substrate for maintaining atrial fibrillation consists of an abnormal left atrium with heterogeneous fibrosis (scarring) and conduction delays. Any heart disease that increases left atrial pressure could lead to atrial dilation and remodeling, which could be substrates for atrial fibrillation. Extensive atrial remodeling and scarring are associated with progression and persistence of atrial fibrillation and make rhythm control more challenging.
Atrial fibrillation begets atrial fibrillation
As shown in the case above, over time, paroxysmal atrial fibrillation often progresses to persistent and long-standing atrial fibrillation if not aggressively managed initially.
In 1972, Davies and Pomerance10 performed 100 autopsies and found that the people who had had atrial fibrillation for longer than 1 month had lost muscle mass in the sinus node and internodal tract, and their atria were dilated. The study introduced the concept that atrial fibrillation itself causes pathologic changes in the atrium.
Wijffels et al,11 in an experiment in goats, showed that atrial fibrillation produced by rapid bursts of atrial pacing was initially paroxysmal. However, as they continued to induce atrial fibrillation over and over again, it lasted progressively longer until it would persist for more than 24 hours. Thus, in a relatively short time, the atria went from supporting paroxysmal fibrillation to supporting persistent fibrillation.
Atrial fibrillation leads to electrophysiologic and anatomic remodeling in the atrium, which leads to a shorter action potential duration and a shorter refractory period. This in turn makes it easier for atrial fibrillation to persist.12
Because atrial fibrillation tends to progress, intervening early may improve its outcomes. Early ablation has been shown to improve the chances of staying in sinus rhythm in both paroxysmal and persistent atrial fibrillation.13–15
CATHETER ABLATION OF ATRIAL FIBRILLATION
The goal of ablation is to prevent atrial fibrillation by eliminating the trigger that initiates it, altering the arrhythmogenic substrate, or both.
Pulmonary vein isolation
The most common ablation strategy is to electrically isolate the pulmonary veins by creating circumferential lesions around their antra. This creates a nonconducting rim of scar tissue, electrically disconnecting the pulmonary veins from the atrium.
Ablation outside of the pulmonary veins
Because recurrence rates are high in patients with persistent atrial fibrillation who undergo pulmonary vein ablation alone, the search continues for adjunctive strategies to improve outcomes. Although these strategies have a sound rationale based on experimental data and anecdotal evidence in humans, they have not yet been convincingly shown to be helpful in large clinical studies. Nonetheless, it is possible that more extensive substrate ablation—atrial “debulking”—could improve outcomes by reducing the amount of tissue that can fibrillate.
Linear ablation. Creating lines of ablation (as in the maze procedure) isolates different segments of the left atrium. Often, these lines are created along the roof of the left atrium between the right and left upper pulmonary veins and from the mitral valve to the left inferior pulmonary vein. The benefit of linear ablation has not been proven, and gaps in such lines may introduce atrial flutter.
Triggers not in the pulmonary veins. Common sites of nonpulmonary vein triggers include the posterior wall of the left atrium, the superior vena cava, the coronary sinus, and along the ligament of Marshall. Provocative maneuvers such as isoproterenol infusion can help find those triggers, which can then be ablated. A limitation is that there is no protocol proven to reproducibly elicit triggers.
Complex fractionated atrial electrograms are areas in the atrium with highly fractionated, low voltage potentials. They may be critical sites of substrate for atrial fibrillation, and many electrophysiologists target them in patients with persistent atrial fibrillation. But despite initial enthusiasm, doing so has not resulted in better outcomes in persistent atrial fibrillation.
Rotors. Animal studies have shown that atrial fibrillation can be triggered or maintained by localized sources of organized reentrant circuits (rotors) or focal impulses. Recent studies have shown that these electrical rotors and focal sources could potentially be mapped and ablated in humans. But positive results in initial reports have not been reproduced, and this remains an area of controversy.
Our practice. We isolate the pulmonary veins with antral ablations, ablate the posterior wall, and extend the ablation toward the septum and inferior to the right pulmonary veins, with good long-term outcomes.14 The rationale behind ablating the posterior wall is that it shares embryologic origins with the pulmonary veins and may be a common source of triggers in atrial fibrillation.
We do not routinely create empiric ablation lines in the left or right atrium unless the patient has atrial flutter. Empiric ablation lines have not been convincingly shown to provide additional benefit compared with our extensive ablation approach, which involves the posterior wall. Empiric ablation of the appendage or coronary sinus is typically reserved for repeat ablation in patients with recurrent persistent atrial fibrillation.
RATIONALE FOR TREATING ATRIAL FIBRILLATION WITH ABLATION
To control symptoms
At this time, the primary aim of atrial fibrillation ablation is to reduce symptoms and improve quality of life. In theory, ablation could also decrease the risk of stroke, heart failure, and death. However, these outcomes have not been systematically evaluated in any large randomized controlled trial.
To control rhythm and improve survival
Randomized controlled trials of rhythm vs rate control of atrial fibrillation16–18 have failed to demonstrate that restoring sinus rhythm is associated with better survival. All of these trials used antiarrhythmic drugs for rhythm control. However, nonrandomized studies19,20 showed that maintaining sinus rhythm is associated with a significant reduction in mortality rates, whereas the use of antiarrhythmic drugs increased mortality risk.
This suggests that the beneficial effect of restoring sinus rhythm may be offset by adverse effects of antiarrhythmic drugs, and if rhythm control could be achieved by a method other than antiarrhythmic drug therapy, it may be superior to rate control. On the other hand, these data may be affected by residual confounding. This topic deserves further research, but maintaining sinus rhythm is typically preferred whenever possible.
Discontinuing anticoagulation is not a goal at this time
Retrospective studies have reported a low risk of stroke in patients who discontinue anticoagulation several months after undergoing atrial fibrillation ablation.21–23 However, atrial fibrillation can recur, and risk of stroke increases with age.
Therefore, guidelines24 still recommend continuing anticoagulation after ablation. Generally, we do not offer ablation with a goal of discontinuing anticoagulation. That said, stopping anticoagulation may be considered after long-term suppression of paroxysmal atrial fibrillation on a case-by-case basis in patients deemed to be at low risk. Left atrial appendage closure devices may eventually allow concomitant atrial fibrillation ablation and closure of the appendage, so that anticoagulation could then be stopped. This remains a topic of investigation.
Who should be considered for ablation?
There are no absolute age or comorbidity contraindications to ablation. Everyone who has atrial fibrillation deserves, in our opinion, a referral to the electrophysiology clinic.
PROCEDURAL CONSIDERATIONS
Atrial fibrillation ablation is most often performed by electrophysiologists using a minimally invasive endovascular approach. The patient can be under either moderate sedation or general anesthesia; we prefer general anesthesia for patient comfort, safety, and efficacy.
We use an electrogram-based technique to target and eliminate electrical potentials and ensure continuity of ablation sets, with additional guidance by 3-dimensional cardiac mapping systems and intracardiac echocardiography. We also use contact force-sensing catheters to ensure catheter-tissue contact during ablation and to avoid excessive contact, which may enhance the safety of the procedure.
Energy: Hot or cold
Two types of energy can be used for ablation:
Radiofrequency energy (low voltage, high frequency—30 kHz to 1.5 mHz) is delivered to the endocardial surface via a point-source catheter. The radiofrequency energy produces controlled, focal thermal ablation.
In a randomized trial,25 these ablation technologies were shown to be equivalent for preventing recurrences of atrial fibrillation. We use both in our practice. The choice depends primarily on the planned ablation set, given that balloon cryoablation can achieve antral isolation of the pulmonary veins but allows little or no substrate modification.
Improved ablation technology
Contact force-sensing catheters. Radiofrequency ablation catheters are now equipped with a pressure sensor at the tip that measures how hard the catheter is pressing on the heart wall.26,27 In our experience, this has improved the outcomes of ablation procedures, primarily in persistent atrial fibrillation.28
Complications of ablation
Although catheter ablation for atrial fibrillation is safe, it is still one of the most complex electrophysiologic procedures. Improvements in technology and techniques and accumulated experience over the past 15 years have made ablation safer, especially in tertiary care centers. But adverse outcomes are more frequent in low-volume centers.29
Minor procedural complications include pericarditis, complications at the site of vascular access, and anesthesia-related complications. While they do not affect the long-term outcome for the patient, they may increase hospital length of stay and cause temporary inconvenience.
Major complications include cardiac perforation and tamponade, periprocedural stroke, pulmonary vein stenosis, atrioesophageal fistula, phrenic nerve paralysis, major bleeding, myocardial infarction, and death. In a worldwide survey published in 2005, when atrial fibrillation ablation was still novel, the rate of major complications was 6%.30 By 2010, this had declined to 4.5%,31 and the rates of major complications may be significantly lower in more experienced centers.29 In our practice, in 2015, the rate of major complications was 1.3% (unpublished data).
Outcomes of catheter ablation
Clinical outcomes depend on many factors including the type of atrial fibrillation (paroxysmal vs nonparoxysmal), overall health of the atria (atrial size and scarring), patient age and comorbidities, and most importantly, the center’s and operator’s experience.
In randomized controlled trials comparing ablation and antiarrhythmic drug therapy, the efficacy of ablation in maintaining sinus rhythm has been in the range of 66% to 86% vs 16% to 22% for drug therapy,32,33 but these trials have been predominantly in middle-aged white men with paroxysmal atrial fibrillation. These trials also showed that catheter ablation reduced symptoms and improved quality of life. Ablation is less effective in persistent than in paroxysmal atrial fibrillation.34
In a long-term study from our group,14 660 (79.4%) of 831 patients who underwent ablation in 2005 were arrhythmia-free and not on antiarrhythmic drug therapy after a total of 1,019 ablations (an average of 1.2 ablations per patient) at a median of 55 months; 125 patients (15%, 41 with more than 1 ablation) continued to have atrial arrhythmia, controlled with drugs in 87 patients (69.6%). Only 38 patients (4.6%) continued to have drug-resistant atrial fibrillation and were treated with rate control with negative dromotropic agents.
Recent evidence
The largest randomized controlled trial of catheter ablation vs drug therapy for atrial fibrillation (Catheter Ablation Versus Antiarrhythmic Drug Therapy for Atrial Fibrillation [CABANA]) was completed recently, and the results were presented at a national meeting, although they have not yet been published in a peer-reviewed journal.35
A total of 2,204 patients with atrial fibrillation (42.4% paroxysmal, 47.3% persistent, and 10.3% long-standing persistent) were randomized to either ablation or drug therapy. Median follow-up was 4 years. The crossover rate was high—9.2% of those randomized to ablation did not undergo it, and 27.5% of those randomized to drug therapy underwent ablation.
The incidence of the primary end point (a composite of death, disabling stroke, serious bleeding, and cardiac arrest) was not significantly different between the 2 groups in the intention-to-treat analysis; however, given the high crossover rates, the as-treated and per-protocol analyses become important, and as-treated and per-protocol analyses revealed a significant benefit of ablation compared with drug therapy. The hazard ratio (HR) for the primary composite outcome was 0.67 (P = .006) on as-treated analysis and 0.73 (P = .05) on per-protocol analysis. The HR for all-cause mortality was 0.60 (P = .005) on as-treated analysis.
PERIPROCEDURAL CONSIDERATIONS
Periprocedural anticoagulation
The risk of thromboembolism is increased during, immediately following, and for several weeks to months after atrial fibrillation ablation.36,37
During the procedure, the risk is related to transseptal sheath placement, electrode catheters in the left atrium, and char formation on ablation catheters. These risks are mitigated with proper and careful sheath and catheter manipulation, maintenance of bubble-free irrigation through lines and sheaths, use of irrigated catheters, and initiation of heparin before transseptal access. Heparin is also infused during the procedure, with close monitoring of activated clotting time.
Postprocedurally, the transiently increased clotting risk could be due to damaged endothelium from the ablation itself and stunning of atrial tissue, which results in impaired contraction. Damaged endothelium improves as the tissue heals, and the stunning resolves by electrical reverse remodeling with sinus rhythm maintenance.
In view of these risks, the referring physician and electrophysiologist must pay careful attention to anticoagulation before and after ablation.
Before the procedure. It is safe to continue anticoagulation uninterrupted through the procedure.38,39 If the patient is on warfarin, we want the international normalized ratio to be in the therapeutic range when we perform atrial fibrillation ablation, and the patient takes his or her usual dose on the day of the procedure. If taking a direct oral anticoagulant, patients typically skip a dose the day before ablation and again on the morning of the procedure, and resume taking it immediately afterward while in the anesthesia recovery room.
During the procedure, we start heparin before transseptal puncture, adjust it to achieve an activated clotting time of 300 to 400 seconds, and keep it in this range as long as there are sheaths or catheters in the left atrium.
After the procedure. The current guidelines24 recommend that oral anticoagulation be continued without interruption for at least 2 months after the procedure, and in most cases indefinitely, depending on age and comorbidities. The decision to stop anticoagulation after 2 months is typically based on the stroke risk as assessed by the CHA2DS2-VASc score (www.chadsvasc.org) and not on the success of the ablation procedure.
ANTIARRHYTHMIC DRUGS AFTER THE PROCEDURE
Some patients actually experience more atrial fibrillation in the first weeks to months after the procedure. The mechanism in this setting may be different from that causing the arrhythmia in the first place. The causes of early recurrence of atrial arrhythmias include postablation inflammation, temporary autonomic imbalance, and delay of atrial radiofrequency lesion formation.40,41 These arrhythmias may completely resolve as the ablation lesions heal and scars mature.
It has been hypothesized that short-term use of antiarrhythmic drugs after atrial fibrillation ablation is effective in preventing arrhythmias because it alters atrial electrophysiologic characteristics induced by the above transient factors. A recent systematic review of 6 clinical trials showed that short-term use of antiarrhythmic drugs reduces the risk of early arrhythmia recurrence but does not reduce recurrence in the long term.42
In terms of outcomes, any arrhythmias that occur in the first 3 months do not necessarily affect long-term success. This is referred to as the “blanking period.” However, generally speaking, it is preferable to maintain sinus rhythm during that time to avoid further anatomic or electrical left atrial adverse remodeling. In many situations, patients continue taking the same antiarrhythmic agent or start on antiarrhythmic therapy in the first few months after ablation.43,44
The mechanisms of late recurrence of atrial arrhythmias after ablation are thought to be different from those in early recurrence. Late recurrence has been ascribed to incomplete pulmonary vein isolation, recovery of pulmonary vein-left atrium connections, or recovery of any other lines of ablation created in the procedure.45,46 For late recurrence of atrial arrhythmia, studies and guidelines suggest that repeat ablation may be an option.24,47
PRACTICAL CONSIDERATIONS FOR PROCEDURAL PLANNING
Before the procedure, some electrophysiologists use cardiac computed tomography or magnetic resonance imaging to evaluate the pulmonary vein anatomy. This helps in planning and in selecting the appropriate tools for the procedure.
The patient is asked to fast on the day of the procedure. The procedure can take 3 to 6 hours, depending on the patient’s anatomy and the operator’s technique and experience. It can be performed with the patient under general anesthesia or conscious sedation. Currently, we use general anesthesia most of the time to maximize patient comfort.
After the procedure, our patients must stay in bed for 4 hours and stay overnight for observation. If no complications arise, they are discharged the next day.
A 64-year-old man with hypertension but without known structural heart disease presents for a second opinion on management of his atrial fibrillation. The condition was first diagnosed at age 38, when he experienced palpitations and shortness of breath on exertion; at times he also experienced decreased endurance and fatigue without overt palpitations. At first, these episodes occurred about twice a year, and the patient was managed with a beta-blocker for rate control and an oral anticoagulant.
Over the past 10 years, the episodes have become more frequent and longer-lasting and have required frequent cardioversions. He was given flecainide for rhythm control but continued to have frequent episodes, and so about 1 year ago he was switched to amiodarone, which controlled his rhythm better. However, after reading about side effects of amiodarone, he decided to seek a second opinion.
He was evaluated by our team and eventually underwent radiofrequency ablation. During the procedure, he was noted to have diffuse scarring and fibrosis of his left atrium, and afterward he continued to require antiarrhythmic drugs to maintain sinus rhythm.
Should he have been referred sooner? What factors should primary care physicians consider when referring a patient with atrial fibrillation for ablation?
THE EPIDEMIC OF ATRIAL FIBRILLATION
Atrial fibrillation is a large and growing public health problem. In 2010, it was estimated to affect 2.7 to 6.1 million people in the United States, and with the rapid aging of our population, its prevalence is expected to rise to between 5.6 and 12 million by 2050.1–3 It is associated with significant morbidity, poor quality of life, and increased risk of death, heart failure, stroke, and cognitive impairment.
The number of new cases per year has increased over the years despite research and preventive measures, which may reflect aging of the population and increased survival rates in patients with cardiovascular or comorbid conditions.1,4
Thus, atrial fibrillation is one of the most common cardiovascular conditions encountered by primary care physicians and cardiologists, putting them at the forefront of its management. Proper treatment in its early stages and referral to a specialist for advanced management may alter its natural history and improve clinical outcomes.
HOW DOES ATRIAL FIBRILLATION ARISE AND PERSIST?
Much is still unknown about the pathogenesis of atrial fibrillation, but considerable progress has been made in the past few decades, opening the door for clinical ablative strategies.
Multiple wavelet hypothesis
Until the late 1980s, the most widely accepted conceptual mechanism of atrial fibrillation was the multiple wavelet hypothesis developed by Moe et al.5 According to this hypothesis, atrial fibrillation begins with multiple independent wavelets occurring simultaneously and spreading randomly throughout both atria, and it persists if there are a minimum number of coexisting wavelets, increased atrial mass, and heterogeneous conduction delays across the atrial tissue.
The surgical maze procedure, in which a series of incisions arranged in a maze-like pattern is created in the left atrium, was predicated on this model. The theory was that these surgical lesions would compartmentalize the atria into discrete electrical segments and thereby reduce the number of circulating random wavelets.6,7
However, experimental and clinical studies suggest that although randomly propagating wavelets can contribute to maintaining atrial fibrillation, focal triggers are noted in most cases.
Focal triggers
In 1997, Jaïs et al8 observed that atrial fibrillation is often triggered by a rapidly firing ectopic focus and that ablation of that focus can eliminate it. These ectopic foci are often found at or near the ostia of the pulmonary veins or near the superior vena cava.8,9 It is now well established that ectopic foci in the pulmonary veins are crucial triggers that initiate atrial fibrillation.
Trigger-and-substrate theory
The substrate for maintaining atrial fibrillation consists of an abnormal left atrium with heterogeneous fibrosis (scarring) and conduction delays. Any heart disease that increases left atrial pressure could lead to atrial dilation and remodeling, which could be substrates for atrial fibrillation. Extensive atrial remodeling and scarring are associated with progression and persistence of atrial fibrillation and make rhythm control more challenging.
Atrial fibrillation begets atrial fibrillation
As shown in the case above, over time, paroxysmal atrial fibrillation often progresses to persistent and long-standing atrial fibrillation if not aggressively managed initially.
In 1972, Davies and Pomerance10 performed 100 autopsies and found that the people who had had atrial fibrillation for longer than 1 month had lost muscle mass in the sinus node and internodal tract, and their atria were dilated. The study introduced the concept that atrial fibrillation itself causes pathologic changes in the atrium.
Wijffels et al,11 in an experiment in goats, showed that atrial fibrillation produced by rapid bursts of atrial pacing was initially paroxysmal. However, as they continued to induce atrial fibrillation over and over again, it lasted progressively longer until it would persist for more than 24 hours. Thus, in a relatively short time, the atria went from supporting paroxysmal fibrillation to supporting persistent fibrillation.
Atrial fibrillation leads to electrophysiologic and anatomic remodeling in the atrium, which leads to a shorter action potential duration and a shorter refractory period. This in turn makes it easier for atrial fibrillation to persist.12
Because atrial fibrillation tends to progress, intervening early may improve its outcomes. Early ablation has been shown to improve the chances of staying in sinus rhythm in both paroxysmal and persistent atrial fibrillation.13–15
CATHETER ABLATION OF ATRIAL FIBRILLATION
The goal of ablation is to prevent atrial fibrillation by eliminating the trigger that initiates it, altering the arrhythmogenic substrate, or both.
Pulmonary vein isolation
The most common ablation strategy is to electrically isolate the pulmonary veins by creating circumferential lesions around their antra. This creates a nonconducting rim of scar tissue, electrically disconnecting the pulmonary veins from the atrium.
Ablation outside of the pulmonary veins
Because recurrence rates are high in patients with persistent atrial fibrillation who undergo pulmonary vein ablation alone, the search continues for adjunctive strategies to improve outcomes. Although these strategies have a sound rationale based on experimental data and anecdotal evidence in humans, they have not yet been convincingly shown to be helpful in large clinical studies. Nonetheless, it is possible that more extensive substrate ablation—atrial “debulking”—could improve outcomes by reducing the amount of tissue that can fibrillate.
Linear ablation. Creating lines of ablation (as in the maze procedure) isolates different segments of the left atrium. Often, these lines are created along the roof of the left atrium between the right and left upper pulmonary veins and from the mitral valve to the left inferior pulmonary vein. The benefit of linear ablation has not been proven, and gaps in such lines may introduce atrial flutter.
Triggers not in the pulmonary veins. Common sites of nonpulmonary vein triggers include the posterior wall of the left atrium, the superior vena cava, the coronary sinus, and along the ligament of Marshall. Provocative maneuvers such as isoproterenol infusion can help find those triggers, which can then be ablated. A limitation is that there is no protocol proven to reproducibly elicit triggers.
Complex fractionated atrial electrograms are areas in the atrium with highly fractionated, low voltage potentials. They may be critical sites of substrate for atrial fibrillation, and many electrophysiologists target them in patients with persistent atrial fibrillation. But despite initial enthusiasm, doing so has not resulted in better outcomes in persistent atrial fibrillation.
Rotors. Animal studies have shown that atrial fibrillation can be triggered or maintained by localized sources of organized reentrant circuits (rotors) or focal impulses. Recent studies have shown that these electrical rotors and focal sources could potentially be mapped and ablated in humans. But positive results in initial reports have not been reproduced, and this remains an area of controversy.
Our practice. We isolate the pulmonary veins with antral ablations, ablate the posterior wall, and extend the ablation toward the septum and inferior to the right pulmonary veins, with good long-term outcomes.14 The rationale behind ablating the posterior wall is that it shares embryologic origins with the pulmonary veins and may be a common source of triggers in atrial fibrillation.
We do not routinely create empiric ablation lines in the left or right atrium unless the patient has atrial flutter. Empiric ablation lines have not been convincingly shown to provide additional benefit compared with our extensive ablation approach, which involves the posterior wall. Empiric ablation of the appendage or coronary sinus is typically reserved for repeat ablation in patients with recurrent persistent atrial fibrillation.
RATIONALE FOR TREATING ATRIAL FIBRILLATION WITH ABLATION
To control symptoms
At this time, the primary aim of atrial fibrillation ablation is to reduce symptoms and improve quality of life. In theory, ablation could also decrease the risk of stroke, heart failure, and death. However, these outcomes have not been systematically evaluated in any large randomized controlled trial.
To control rhythm and improve survival
Randomized controlled trials of rhythm vs rate control of atrial fibrillation16–18 have failed to demonstrate that restoring sinus rhythm is associated with better survival. All of these trials used antiarrhythmic drugs for rhythm control. However, nonrandomized studies19,20 showed that maintaining sinus rhythm is associated with a significant reduction in mortality rates, whereas the use of antiarrhythmic drugs increased mortality risk.
This suggests that the beneficial effect of restoring sinus rhythm may be offset by adverse effects of antiarrhythmic drugs, and if rhythm control could be achieved by a method other than antiarrhythmic drug therapy, it may be superior to rate control. On the other hand, these data may be affected by residual confounding. This topic deserves further research, but maintaining sinus rhythm is typically preferred whenever possible.
Discontinuing anticoagulation is not a goal at this time
Retrospective studies have reported a low risk of stroke in patients who discontinue anticoagulation several months after undergoing atrial fibrillation ablation.21–23 However, atrial fibrillation can recur, and risk of stroke increases with age.
Therefore, guidelines24 still recommend continuing anticoagulation after ablation. Generally, we do not offer ablation with a goal of discontinuing anticoagulation. That said, stopping anticoagulation may be considered after long-term suppression of paroxysmal atrial fibrillation on a case-by-case basis in patients deemed to be at low risk. Left atrial appendage closure devices may eventually allow concomitant atrial fibrillation ablation and closure of the appendage, so that anticoagulation could then be stopped. This remains a topic of investigation.
Who should be considered for ablation?
There are no absolute age or comorbidity contraindications to ablation. Everyone who has atrial fibrillation deserves, in our opinion, a referral to the electrophysiology clinic.
PROCEDURAL CONSIDERATIONS
Atrial fibrillation ablation is most often performed by electrophysiologists using a minimally invasive endovascular approach. The patient can be under either moderate sedation or general anesthesia; we prefer general anesthesia for patient comfort, safety, and efficacy.
We use an electrogram-based technique to target and eliminate electrical potentials and ensure continuity of ablation sets, with additional guidance by 3-dimensional cardiac mapping systems and intracardiac echocardiography. We also use contact force-sensing catheters to ensure catheter-tissue contact during ablation and to avoid excessive contact, which may enhance the safety of the procedure.
Energy: Hot or cold
Two types of energy can be used for ablation:
Radiofrequency energy (low voltage, high frequency—30 kHz to 1.5 mHz) is delivered to the endocardial surface via a point-source catheter. The radiofrequency energy produces controlled, focal thermal ablation.
In a randomized trial,25 these ablation technologies were shown to be equivalent for preventing recurrences of atrial fibrillation. We use both in our practice. The choice depends primarily on the planned ablation set, given that balloon cryoablation can achieve antral isolation of the pulmonary veins but allows little or no substrate modification.
Improved ablation technology
Contact force-sensing catheters. Radiofrequency ablation catheters are now equipped with a pressure sensor at the tip that measures how hard the catheter is pressing on the heart wall.26,27 In our experience, this has improved the outcomes of ablation procedures, primarily in persistent atrial fibrillation.28
Complications of ablation
Although catheter ablation for atrial fibrillation is safe, it is still one of the most complex electrophysiologic procedures. Improvements in technology and techniques and accumulated experience over the past 15 years have made ablation safer, especially in tertiary care centers. But adverse outcomes are more frequent in low-volume centers.29
Minor procedural complications include pericarditis, complications at the site of vascular access, and anesthesia-related complications. While they do not affect the long-term outcome for the patient, they may increase hospital length of stay and cause temporary inconvenience.
Major complications include cardiac perforation and tamponade, periprocedural stroke, pulmonary vein stenosis, atrioesophageal fistula, phrenic nerve paralysis, major bleeding, myocardial infarction, and death. In a worldwide survey published in 2005, when atrial fibrillation ablation was still novel, the rate of major complications was 6%.30 By 2010, this had declined to 4.5%,31 and the rates of major complications may be significantly lower in more experienced centers.29 In our practice, in 2015, the rate of major complications was 1.3% (unpublished data).
Outcomes of catheter ablation
Clinical outcomes depend on many factors including the type of atrial fibrillation (paroxysmal vs nonparoxysmal), overall health of the atria (atrial size and scarring), patient age and comorbidities, and most importantly, the center’s and operator’s experience.
In randomized controlled trials comparing ablation and antiarrhythmic drug therapy, the efficacy of ablation in maintaining sinus rhythm has been in the range of 66% to 86% vs 16% to 22% for drug therapy,32,33 but these trials have been predominantly in middle-aged white men with paroxysmal atrial fibrillation. These trials also showed that catheter ablation reduced symptoms and improved quality of life. Ablation is less effective in persistent than in paroxysmal atrial fibrillation.34
In a long-term study from our group,14 660 (79.4%) of 831 patients who underwent ablation in 2005 were arrhythmia-free and not on antiarrhythmic drug therapy after a total of 1,019 ablations (an average of 1.2 ablations per patient) at a median of 55 months; 125 patients (15%, 41 with more than 1 ablation) continued to have atrial arrhythmia, controlled with drugs in 87 patients (69.6%). Only 38 patients (4.6%) continued to have drug-resistant atrial fibrillation and were treated with rate control with negative dromotropic agents.
Recent evidence
The largest randomized controlled trial of catheter ablation vs drug therapy for atrial fibrillation (Catheter Ablation Versus Antiarrhythmic Drug Therapy for Atrial Fibrillation [CABANA]) was completed recently, and the results were presented at a national meeting, although they have not yet been published in a peer-reviewed journal.35
A total of 2,204 patients with atrial fibrillation (42.4% paroxysmal, 47.3% persistent, and 10.3% long-standing persistent) were randomized to either ablation or drug therapy. Median follow-up was 4 years. The crossover rate was high—9.2% of those randomized to ablation did not undergo it, and 27.5% of those randomized to drug therapy underwent ablation.
The incidence of the primary end point (a composite of death, disabling stroke, serious bleeding, and cardiac arrest) was not significantly different between the 2 groups in the intention-to-treat analysis; however, given the high crossover rates, the as-treated and per-protocol analyses become important, and as-treated and per-protocol analyses revealed a significant benefit of ablation compared with drug therapy. The hazard ratio (HR) for the primary composite outcome was 0.67 (P = .006) on as-treated analysis and 0.73 (P = .05) on per-protocol analysis. The HR for all-cause mortality was 0.60 (P = .005) on as-treated analysis.
PERIPROCEDURAL CONSIDERATIONS
Periprocedural anticoagulation
The risk of thromboembolism is increased during, immediately following, and for several weeks to months after atrial fibrillation ablation.36,37
During the procedure, the risk is related to transseptal sheath placement, electrode catheters in the left atrium, and char formation on ablation catheters. These risks are mitigated with proper and careful sheath and catheter manipulation, maintenance of bubble-free irrigation through lines and sheaths, use of irrigated catheters, and initiation of heparin before transseptal access. Heparin is also infused during the procedure, with close monitoring of activated clotting time.
Postprocedurally, the transiently increased clotting risk could be due to damaged endothelium from the ablation itself and stunning of atrial tissue, which results in impaired contraction. Damaged endothelium improves as the tissue heals, and the stunning resolves by electrical reverse remodeling with sinus rhythm maintenance.
In view of these risks, the referring physician and electrophysiologist must pay careful attention to anticoagulation before and after ablation.
Before the procedure. It is safe to continue anticoagulation uninterrupted through the procedure.38,39 If the patient is on warfarin, we want the international normalized ratio to be in the therapeutic range when we perform atrial fibrillation ablation, and the patient takes his or her usual dose on the day of the procedure. If taking a direct oral anticoagulant, patients typically skip a dose the day before ablation and again on the morning of the procedure, and resume taking it immediately afterward while in the anesthesia recovery room.
During the procedure, we start heparin before transseptal puncture, adjust it to achieve an activated clotting time of 300 to 400 seconds, and keep it in this range as long as there are sheaths or catheters in the left atrium.
After the procedure. The current guidelines24 recommend that oral anticoagulation be continued without interruption for at least 2 months after the procedure, and in most cases indefinitely, depending on age and comorbidities. The decision to stop anticoagulation after 2 months is typically based on the stroke risk as assessed by the CHA2DS2-VASc score (www.chadsvasc.org) and not on the success of the ablation procedure.
ANTIARRHYTHMIC DRUGS AFTER THE PROCEDURE
Some patients actually experience more atrial fibrillation in the first weeks to months after the procedure. The mechanism in this setting may be different from that causing the arrhythmia in the first place. The causes of early recurrence of atrial arrhythmias include postablation inflammation, temporary autonomic imbalance, and delay of atrial radiofrequency lesion formation.40,41 These arrhythmias may completely resolve as the ablation lesions heal and scars mature.
It has been hypothesized that short-term use of antiarrhythmic drugs after atrial fibrillation ablation is effective in preventing arrhythmias because it alters atrial electrophysiologic characteristics induced by the above transient factors. A recent systematic review of 6 clinical trials showed that short-term use of antiarrhythmic drugs reduces the risk of early arrhythmia recurrence but does not reduce recurrence in the long term.42
In terms of outcomes, any arrhythmias that occur in the first 3 months do not necessarily affect long-term success. This is referred to as the “blanking period.” However, generally speaking, it is preferable to maintain sinus rhythm during that time to avoid further anatomic or electrical left atrial adverse remodeling. In many situations, patients continue taking the same antiarrhythmic agent or start on antiarrhythmic therapy in the first few months after ablation.43,44
The mechanisms of late recurrence of atrial arrhythmias after ablation are thought to be different from those in early recurrence. Late recurrence has been ascribed to incomplete pulmonary vein isolation, recovery of pulmonary vein-left atrium connections, or recovery of any other lines of ablation created in the procedure.45,46 For late recurrence of atrial arrhythmia, studies and guidelines suggest that repeat ablation may be an option.24,47
PRACTICAL CONSIDERATIONS FOR PROCEDURAL PLANNING
Before the procedure, some electrophysiologists use cardiac computed tomography or magnetic resonance imaging to evaluate the pulmonary vein anatomy. This helps in planning and in selecting the appropriate tools for the procedure.
The patient is asked to fast on the day of the procedure. The procedure can take 3 to 6 hours, depending on the patient’s anatomy and the operator’s technique and experience. It can be performed with the patient under general anesthesia or conscious sedation. Currently, we use general anesthesia most of the time to maximize patient comfort.
After the procedure, our patients must stay in bed for 4 hours and stay overnight for observation. If no complications arise, they are discharged the next day.
- Go AS. The epidemiology of atrial fibrillation in elderly persons: the tip of the iceberg. Am J Geriatr Cardiol 2005; 14(2):56–61. pmid:15785146
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285(18):2370–2375. pmid:11343485
- Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006; 114(2):119–125. doi:10.1161/CIRCULATIONAHA.105.595140
- Piccini JP, Hammill BG, Sinner MF, et al. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993–2007. Circ Cardiovasc Qual Outcomes 2012; 5(1):85–93. doi:10.1161/CIRCOUTCOMES.111.962688
- Moe GK, Rheinboldt WC, Abildskov JA. A computer model of atrial fibrillation. Am Heart J 1964; 67:200–220. pmid:14118488
- Cox JL, Schuessler RB, Boineau JP. The surgical treatment of atrial fibrillation. I. Summary of the current concepts of the mechanisms of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg 1991; 101(3):402–405. pmid:1999933
- Cox JL, Schuessler RB, D’Agostino HJ Jr, et al. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg 1991; 101(4):569–583. pmid:2008095
- Jaïs P, Haïssaguerre M, Shah DC, et al. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation 1997; 95(3):572–576. pmid:9024141
- Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998; 339(10):659–666. doi:10.1056/NEJM199809033391003
- Davies MJ, Pomerance A. Pathology of atrial fibrillation in man. Br Heart J 1972; 34(5):520–525. pmid:5031645
- Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 1995; 92(7):1954–1968. pmid:7671380
- Nattel S. New ideas about atrial fibrillation 50 years on. Nature 2002; 415(6868):219–226. doi:10.1038/415219a
- Medi C, Sparks PB, Morton JB, et al. Pulmonary vein antral isolation for paroxysmal atrial fibrillation: results from long-term follow-up. J Cardiovasc Electrophysiol 2011; 22(2):137–141. doi:10.1111/j.1540-8167.2010.01885.x
- Hussein AA, Saliba WI, Martin DO, et al. Natural history and long-term outcomes of ablated atrial fibrillation. Circ Arrhythm Electrophysiol 2011; 4(3):271–278. doi:10.1161/CIRCEP.111.962100
- Hussein AA, Saliba WI, Barakat A, et al. Radiofrequency ablation of persistent atrial fibrillation: diagnosis-to-ablation time, markers of pathways of atrial remodeling, and outcomes. Circ Arrhythm Electrophysiol 2016; 9(1):e003669. doi:10.1161/CIRCEP.115.003669
- Carlsson J, Miketic S, Windeler J, et al. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. J Am Coll Cardiol 2003; 41(10):1690–1696. pmid:12767648
- Van Gelder IC, Hagens VE, Bosker HA, et al; Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study Group. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med 2002; 347(23):1834–1840. doi:10.1056/NEJMoa021375
- Wyse DG, Waldo AL, DiMarco JP, et al; Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002; 347(23):1825–1833. doi:10.1056/NEJMoa021328
- Hagens VE, Crijns HJ, Van Veldhuisen DJ, et al; RAte Control versus Electrical cardioversion for persistent atrial fibrillation study group. Rate control versus rhythm control for patients with persistent atrial fibrillation with mild to moderate heart failure: results from the RAte Control versus Electrical cardioversion (RACE) study. Am Heart J 2005; 149(6):1106–111. doi:10.1016/j.ahj.2004.11.030
- Pedersen OD, Bagger H, Keller N, Marchant B, Køber L, Torp-Pedersen C. Efficacy of dofetilide in the treatment of atrial fibrillation-flutter in patients with reduced left ventricular function: a Danish investigations of arrhythmia and mortality on dofetilide (diamond) substudy. Circulation 2001; 104(3):292–296. pmid:11457747
- Guiot A, Jongnarangsin K, Chugh A, et al. Anticoagulant therapy and risk of cerebrovascular events after catheter ablation of atrial fibrillation in the elderly. J Cardiovasc Electrophysiol 2012; 23(1):36–43. doi:10.1111/j.1540-8167.2011.02141.x
- Oral H, Chugh A, Ozaydin M, et al. Risk of thromboembolic events after percutaneous left atrial radiofrequency ablation of atrial fibrillation. Circulation 2006; 114(8):759–765. doi:10.1161/CIRCULATIONAHA.106.641225
- Themistoclakis S, Corrado A, Marchlinski FE, et al. The risk of thromboembolism and need for oral anticoagulation after successful atrial fibrillation ablation. J Am Coll Cardiol 2010; 55(8):735–743. doi:10.1016/j.jacc.2009.11.039
- Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. J Arrhythm 2017; 33(5):369–409. doi:10.1016/j.joa.2017.08.001
- Kuck KH, Brugada J, Fürnkranz A, et al; FIRE AND ICE Investigators. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016; 374(23):2235–2245. doi:10.1056/NEJMoa1602014
- Reddy VY, Dukkipati SR, Neuzil P, et al. Randomized, controlled trial of the safety and effectiveness of a contact force-sensing irrigated catheter for ablation of paroxysmal atrial fibrillation: results of the TactiCath Contact Force Ablation Catheter Study for Atrial Fibrillation (TOCCASTAR) Study. Circulation 2015; 132(10):907–915. doi:10.1161/CIRCULATIONAHA.114.014092
- Natale A, Reddy VY, Monir G, et al. Paroxysmal AF catheter ablation with a contact force sensing catheter: results of the prospective, multicenter SMART-AF trial. J Am Coll Cardiol 2014; 64(7):647–656. doi:10.1016/j.jacc.2014.04.072
- Hussein AA, Barakat AF, Saliba WI, et al. Persistent atrial fibrillation ablation with or without contact force sensing. J Cardiovasc Electrophysiol 2017; 28(5):483–488. doi:10.1111/jce.13179
- Deshmukh A, Patel NJ, Pant I, et al. In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: analysis of 93,801 procedures. Circulation 2013; 128(19):2104–2112. doi:10.1161/CIRCULATIONAHA.113.003862
- Cappato R, Calkins H, Chen SA, et al. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation 2005; 111(9):1100–1105. doi:10.1161/01.CIR.0000157153.30978.67
- Cappato R, Calkins H, Chen SA, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 2010; 3(1):32–38. doi:10.1161/CIRCEP.109.859116
- Wazni OM, Marrouche NF, Martin DO, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA 2005; 293(21):2634–2640. doi:10.1001/jama.293.21.2634
- Jaïs P, Cauchemez B, Macle L, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation 2008; 118(24):2498–2505. doi:10.1161/CIRCULATIONAHA.108.772582
- Brooks AG, Stiles MK, Laborderie J, et al. Outcomes of long-standing persistent atrial fibrillation ablation: a systematic review. Heart Rhythm 2010; 7(6):835–846. doi:10.1016/j.hrthm.2010.01.017
- Packer DL, Lee KL, Mark DB, Robb RA. Catheter Ablation versus Antiarrhythmic Drug Therapy for Atrial Fibrillation Trial, CABANA. http://cabanatrial.org/. Accessed September 10, 2018.
- Scherr D, Sharma K, Dalal D, et al. Incidence and predictors of periprocedural cerebrovascular accident in patients undergoing catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2009; 20(12):1357–1363. doi:10.1111/j.1540-8167.2009.01540.x
- Wazni OM, Rossillo A, Marrouche NF, et al. Embolic events and char formation during pulmonary vein isolation in patients with atrial fibrillation: impact of different anticoagulation regimens and importance of intracardiac echo imaging. J Cardiovasc Electrophysiol 2005; 16(6):576–581. doi:10.1111/j.1540-8167.2005.40480.x
- Hussein AA, Martin DO, Saliba W, et al. Radiofrequency ablation of atrial fibrillation under therapeutic international normalized ratio: a safe and efficacious periprocedural anticoagulation strategy. Heart Rhythm 2009; 6(10):1425–1429. doi:10.1016/j.hrthm.2009.07.007
- Bassiouny M, Saliba W, Rickard J, et al. Use of dabigatran for periprocedural anticoagulation in patients undergoing catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol 2013; 6(3):460–466. doi:10.1161/CIRCEP.113.000320
- Koyama T, Tada H, Sekiguchi Y, et al. Prevention of atrial fibrillation recurrence with corticosteroids after radiofrequency catheter ablation: a randomized controlled trial. J Am Coll Cardiol 2010; 56(18):1463–1472. doi:10.1016/j.jacc.2010.04.057
- Oral H, Knight BP, Ozaydin M, et al. Clinical significance of early recurrences of atrial fibrillation after pulmonary vein isolation. J Am Coll Cardiol 2002; 40(1):100–104. pmid:12103262
- Chen W, Liu H, Ling Z, et al. Efficacy of short-term antiarrhythmic drugs use after catheter ablation of atrial fibrillation—a systematic review with meta-analyses and trial sequential analyses of randomized controlled trials. PLoS One 2016; 11(5):e0156121. doi:10.1371/journal.pone.0156121
- Leong-Sit P, Roux JF, Zado E, et al. Antiarrhythmics after ablation of atrial fibrillation (5A Study): six-month follow-up study. Circ Arrhythm Electrophysiol 2011; 4(1):11–14. doi:10.1161/CIRCEP.110.955393
- Roux JF, Zado E, Callans DJ, et al. Antiarrhythmics after ablation of atrial fibrillation (5A Study). Circulation 2009; 120(12):1036–1040. doi:10.1161/CIRCULATIONAHA.108.839639
- Sotomi Y, Inoue K, Ito N, et al. Cause of very late recurrence of atrial fibrillation or flutter after catheter ablation for atrial fibrillation. Am J Cardiol 2013; 111(4):552–556. doi:10.1016/j.amjcard.2012.10.040
- Lee SH, Tai CT, Hsieh MH, et al. Predictors of early and late recurrence of atrial fibrillation after catheter ablation of paroxysmal atrial fibrillation. J Interv Card Electrophysiol. 2004 Jun;10(3):221-6. doi:10.1023/B:JICE.0000026915.02503.92
- Zhang XD, Gu J, Jiang WF, et al. Optimal rhythm-control strategy for recurrent atrial tachycardia after catheter ablation of persistent atrial fibrillation: a randomized clinical trial. Eur Heart J 2014; 35(20):1327–1334. doi:10.1093/eurheartj/ehu017
- Go AS. The epidemiology of atrial fibrillation in elderly persons: the tip of the iceberg. Am J Geriatr Cardiol 2005; 14(2):56–61. pmid:15785146
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285(18):2370–2375. pmid:11343485
- Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006; 114(2):119–125. doi:10.1161/CIRCULATIONAHA.105.595140
- Piccini JP, Hammill BG, Sinner MF, et al. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993–2007. Circ Cardiovasc Qual Outcomes 2012; 5(1):85–93. doi:10.1161/CIRCOUTCOMES.111.962688
- Moe GK, Rheinboldt WC, Abildskov JA. A computer model of atrial fibrillation. Am Heart J 1964; 67:200–220. pmid:14118488
- Cox JL, Schuessler RB, Boineau JP. The surgical treatment of atrial fibrillation. I. Summary of the current concepts of the mechanisms of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg 1991; 101(3):402–405. pmid:1999933
- Cox JL, Schuessler RB, D’Agostino HJ Jr, et al. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg 1991; 101(4):569–583. pmid:2008095
- Jaïs P, Haïssaguerre M, Shah DC, et al. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation 1997; 95(3):572–576. pmid:9024141
- Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998; 339(10):659–666. doi:10.1056/NEJM199809033391003
- Davies MJ, Pomerance A. Pathology of atrial fibrillation in man. Br Heart J 1972; 34(5):520–525. pmid:5031645
- Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 1995; 92(7):1954–1968. pmid:7671380
- Nattel S. New ideas about atrial fibrillation 50 years on. Nature 2002; 415(6868):219–226. doi:10.1038/415219a
- Medi C, Sparks PB, Morton JB, et al. Pulmonary vein antral isolation for paroxysmal atrial fibrillation: results from long-term follow-up. J Cardiovasc Electrophysiol 2011; 22(2):137–141. doi:10.1111/j.1540-8167.2010.01885.x
- Hussein AA, Saliba WI, Martin DO, et al. Natural history and long-term outcomes of ablated atrial fibrillation. Circ Arrhythm Electrophysiol 2011; 4(3):271–278. doi:10.1161/CIRCEP.111.962100
- Hussein AA, Saliba WI, Barakat A, et al. Radiofrequency ablation of persistent atrial fibrillation: diagnosis-to-ablation time, markers of pathways of atrial remodeling, and outcomes. Circ Arrhythm Electrophysiol 2016; 9(1):e003669. doi:10.1161/CIRCEP.115.003669
- Carlsson J, Miketic S, Windeler J, et al. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. J Am Coll Cardiol 2003; 41(10):1690–1696. pmid:12767648
- Van Gelder IC, Hagens VE, Bosker HA, et al; Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study Group. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med 2002; 347(23):1834–1840. doi:10.1056/NEJMoa021375
- Wyse DG, Waldo AL, DiMarco JP, et al; Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002; 347(23):1825–1833. doi:10.1056/NEJMoa021328
- Hagens VE, Crijns HJ, Van Veldhuisen DJ, et al; RAte Control versus Electrical cardioversion for persistent atrial fibrillation study group. Rate control versus rhythm control for patients with persistent atrial fibrillation with mild to moderate heart failure: results from the RAte Control versus Electrical cardioversion (RACE) study. Am Heart J 2005; 149(6):1106–111. doi:10.1016/j.ahj.2004.11.030
- Pedersen OD, Bagger H, Keller N, Marchant B, Køber L, Torp-Pedersen C. Efficacy of dofetilide in the treatment of atrial fibrillation-flutter in patients with reduced left ventricular function: a Danish investigations of arrhythmia and mortality on dofetilide (diamond) substudy. Circulation 2001; 104(3):292–296. pmid:11457747
- Guiot A, Jongnarangsin K, Chugh A, et al. Anticoagulant therapy and risk of cerebrovascular events after catheter ablation of atrial fibrillation in the elderly. J Cardiovasc Electrophysiol 2012; 23(1):36–43. doi:10.1111/j.1540-8167.2011.02141.x
- Oral H, Chugh A, Ozaydin M, et al. Risk of thromboembolic events after percutaneous left atrial radiofrequency ablation of atrial fibrillation. Circulation 2006; 114(8):759–765. doi:10.1161/CIRCULATIONAHA.106.641225
- Themistoclakis S, Corrado A, Marchlinski FE, et al. The risk of thromboembolism and need for oral anticoagulation after successful atrial fibrillation ablation. J Am Coll Cardiol 2010; 55(8):735–743. doi:10.1016/j.jacc.2009.11.039
- Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. J Arrhythm 2017; 33(5):369–409. doi:10.1016/j.joa.2017.08.001
- Kuck KH, Brugada J, Fürnkranz A, et al; FIRE AND ICE Investigators. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016; 374(23):2235–2245. doi:10.1056/NEJMoa1602014
- Reddy VY, Dukkipati SR, Neuzil P, et al. Randomized, controlled trial of the safety and effectiveness of a contact force-sensing irrigated catheter for ablation of paroxysmal atrial fibrillation: results of the TactiCath Contact Force Ablation Catheter Study for Atrial Fibrillation (TOCCASTAR) Study. Circulation 2015; 132(10):907–915. doi:10.1161/CIRCULATIONAHA.114.014092
- Natale A, Reddy VY, Monir G, et al. Paroxysmal AF catheter ablation with a contact force sensing catheter: results of the prospective, multicenter SMART-AF trial. J Am Coll Cardiol 2014; 64(7):647–656. doi:10.1016/j.jacc.2014.04.072
- Hussein AA, Barakat AF, Saliba WI, et al. Persistent atrial fibrillation ablation with or without contact force sensing. J Cardiovasc Electrophysiol 2017; 28(5):483–488. doi:10.1111/jce.13179
- Deshmukh A, Patel NJ, Pant I, et al. In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: analysis of 93,801 procedures. Circulation 2013; 128(19):2104–2112. doi:10.1161/CIRCULATIONAHA.113.003862
- Cappato R, Calkins H, Chen SA, et al. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation 2005; 111(9):1100–1105. doi:10.1161/01.CIR.0000157153.30978.67
- Cappato R, Calkins H, Chen SA, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 2010; 3(1):32–38. doi:10.1161/CIRCEP.109.859116
- Wazni OM, Marrouche NF, Martin DO, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA 2005; 293(21):2634–2640. doi:10.1001/jama.293.21.2634
- Jaïs P, Cauchemez B, Macle L, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation 2008; 118(24):2498–2505. doi:10.1161/CIRCULATIONAHA.108.772582
- Brooks AG, Stiles MK, Laborderie J, et al. Outcomes of long-standing persistent atrial fibrillation ablation: a systematic review. Heart Rhythm 2010; 7(6):835–846. doi:10.1016/j.hrthm.2010.01.017
- Packer DL, Lee KL, Mark DB, Robb RA. Catheter Ablation versus Antiarrhythmic Drug Therapy for Atrial Fibrillation Trial, CABANA. http://cabanatrial.org/. Accessed September 10, 2018.
- Scherr D, Sharma K, Dalal D, et al. Incidence and predictors of periprocedural cerebrovascular accident in patients undergoing catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2009; 20(12):1357–1363. doi:10.1111/j.1540-8167.2009.01540.x
- Wazni OM, Rossillo A, Marrouche NF, et al. Embolic events and char formation during pulmonary vein isolation in patients with atrial fibrillation: impact of different anticoagulation regimens and importance of intracardiac echo imaging. J Cardiovasc Electrophysiol 2005; 16(6):576–581. doi:10.1111/j.1540-8167.2005.40480.x
- Hussein AA, Martin DO, Saliba W, et al. Radiofrequency ablation of atrial fibrillation under therapeutic international normalized ratio: a safe and efficacious periprocedural anticoagulation strategy. Heart Rhythm 2009; 6(10):1425–1429. doi:10.1016/j.hrthm.2009.07.007
- Bassiouny M, Saliba W, Rickard J, et al. Use of dabigatran for periprocedural anticoagulation in patients undergoing catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol 2013; 6(3):460–466. doi:10.1161/CIRCEP.113.000320
- Koyama T, Tada H, Sekiguchi Y, et al. Prevention of atrial fibrillation recurrence with corticosteroids after radiofrequency catheter ablation: a randomized controlled trial. J Am Coll Cardiol 2010; 56(18):1463–1472. doi:10.1016/j.jacc.2010.04.057
- Oral H, Knight BP, Ozaydin M, et al. Clinical significance of early recurrences of atrial fibrillation after pulmonary vein isolation. J Am Coll Cardiol 2002; 40(1):100–104. pmid:12103262
- Chen W, Liu H, Ling Z, et al. Efficacy of short-term antiarrhythmic drugs use after catheter ablation of atrial fibrillation—a systematic review with meta-analyses and trial sequential analyses of randomized controlled trials. PLoS One 2016; 11(5):e0156121. doi:10.1371/journal.pone.0156121
- Leong-Sit P, Roux JF, Zado E, et al. Antiarrhythmics after ablation of atrial fibrillation (5A Study): six-month follow-up study. Circ Arrhythm Electrophysiol 2011; 4(1):11–14. doi:10.1161/CIRCEP.110.955393
- Roux JF, Zado E, Callans DJ, et al. Antiarrhythmics after ablation of atrial fibrillation (5A Study). Circulation 2009; 120(12):1036–1040. doi:10.1161/CIRCULATIONAHA.108.839639
- Sotomi Y, Inoue K, Ito N, et al. Cause of very late recurrence of atrial fibrillation or flutter after catheter ablation for atrial fibrillation. Am J Cardiol 2013; 111(4):552–556. doi:10.1016/j.amjcard.2012.10.040
- Lee SH, Tai CT, Hsieh MH, et al. Predictors of early and late recurrence of atrial fibrillation after catheter ablation of paroxysmal atrial fibrillation. J Interv Card Electrophysiol. 2004 Jun;10(3):221-6. doi:10.1023/B:JICE.0000026915.02503.92
- Zhang XD, Gu J, Jiang WF, et al. Optimal rhythm-control strategy for recurrent atrial tachycardia after catheter ablation of persistent atrial fibrillation: a randomized clinical trial. Eur Heart J 2014; 35(20):1327–1334. doi:10.1093/eurheartj/ehu017
KEY POINTS
- Atrial fibrillation is increasing in prevalence with the aging of the US population and is associated with worsening quality of life and increased risk of stroke, heart failure, and death.
- Atrial fibrillation results in adverse atrial remodeling and fibrosis, eventually leading to persistence of the arrhythmia and making rhythm control difficult.
- Catheter ablation has evolved to be a safe procedure with technologic advancements, especially in experienced tertiary care centers.
- The primary aim of atrial fibrillation ablation is to reduce symptoms and improve quality of life. In theory, it could also decrease the risk of stroke, heart failure, and death, but these outcomes have not been systematically evaluated in a large randomized controlled trial.
Evolving strategies to prevent stroke and thromboembolism in nonvalvular atrial fibrillation
Atrial fibrillation (AF), the most common cardiac arrhythmia, has become a major public health problem. In the United States, the prevalence of AF was estimated at 2.7 to 6.1 million in 2010, and it is expected to rise to between 5.6 and 12 million by 2050.1 The arrhythmia is associated with impaired quality of life and increased morbidity and mortality.1,2 Stroke remains the most devastating consequence of AF.
The clinical management of patients with AF typically targets two main goals: prevention of stroke or thromboembolism and control of symptoms. This article addresses the evolving pharmacologic and nonpharmacologic strategies in stroke prevention in nonvalvular AF; reviews clinical trials evaluating medical and procedural strategies, including the novel oral anticoagulants and left atrial appendage (LAA) exclusion devices; and assesses the impact of these novel strategies on clinical practice.
RISK OF STROKE AND THROMBOEMBOLISM IN NONVALVULAR AF
Stroke occurrence from AF is primarily caused by thrombi formation in the left atrium, most commonly in the LAA. It is important to recognize that the cardiovascular risk factors for AF are also risk factors for atheroembolism; therefore, specific risk factor management is as important as anticoagulation when addressing stroke risk.
The incidence of all-cause stroke in patients with AF is 5%, and it is believed that AF causes approximately 15% of all strokes in the United States.1 This risk appears to be more significant in older patients who are more vulnerable to ischemic strokes. Estimates are that AF independently increases the risk of stroke by fivefold throughout all ages, with a steep increase in percentage of strokes attributed to AF from 1.5% at ages 50 to 59 to 23.5% at ages 80 to 89.1 Importantly, the clinical course of ischemic stroke associated with AF is often more severe than for strokes of other causes,3 further emphasizing the need for stroke prevention.
Assessment of stroke risk/thromboembolism
Multiple risk estimation scores have been developed based on epidemiologic data. Until recently, the CHADS2 score4 was the most commonly used, but it has been superseded by the CHA2DS2-VASc score.5 The point system for this scoring system is shown in Table 1. In contrast with CHADS2, this updated system assigns 2 points for age over 75 years and accounts for stroke risk in the relatively younger group of patients (age 65–75) and in females, neither of whom were included in CHADS2. The CHA2DS2-VASc score ranges between 0 and 9 with a respective estimated stroke risk of 0 to 15.2% per year. Note that for females who are younger than 65 years, no points are given for sex. The major advantage of the CHA2DS2-VASc score over the CHADS2 score is that it is more accurate for lower-risk categories. It has been adopted in most of the recent guidelines that address stroke risk in AF.
In clinical practice, practitioners use these scores to define three primary stroke risk categories: low, intermediate, or high. In our practice, we use a 2% per year cut-off to identify high-risk patients in whom the risk of stroke significantly outweighs the risk of bleeding on anticoagulants. In general, patients with a CHA2DS2-VASc score equal to or greater than 2 have a greater than 2% stroke risk per year and are most likely to benefit from antithrombotic therapies.
In male patients with a CHA2DS2-VASc score of 0 and in most patients with a score of 1, the stroke risk is less than 1% per year. These patients are not likely to derive benefit from anticoagulant therapy. They are usually approached on a case-by-case basis with careful assessment of bleeding risk and discussion of risks and benefits of anticoagulant strategies.
Assessment of bleeding risk
Any general approach to thromboembolism risk assessment in patients with AF should include an analysis that weighs the benefits of anticoagulant therapies against the risks of bleeding. Although no precise tools exist to predict bleeding risk, the HAS-BLED score is increasingly used.6 This score assigns 1 point to each of the following:
- systolic blood pressure greater than 160 mm Hg
- abnormal renal function
- abnormal liver function
- age older than 65
- prior cerebrovascular event
- prior bleeding
- history of labile international normalized ratios (INR)
- alcohol intake (> 8 U/week)
- drug use, especially antiplatelet agents or nonsteroidal anti-inflammatory drugs (NSAIDs).
In general, a HAS-BLED score of 3 or greater indicates increased 1-year risk of intracranial bleed, bleeding requiring hospitalization, drop in hemoglobin of at least 2 g/dL, or need for transfusion.
One problem with the bleeding risk scores is that they were derived from studies that included bleeding events of differing severity. Most bleeding events do not lead to death or severe disability with the exception of intracranial bleeding, which is, therefore, the primary concern when assessing bleeding risk.
The estimated bleeding risk with anticoagulant therapy ranges from 0.2% to 0.4% per year but could be much higher in patients with prior severe bleeding, intracranial hemorrhage, thrombocytopenia, coagulopathies, recent surgery, or ongoing bleeding, aortic dissection, malignant hypertension, and in those receiving a combination of anticoagulant and antiplatelet agents.
MEDICAL THERAPIES TO PREVENT STROKE AND THROMBOEMBOLISM IN AF
In general, anticoagulation reduces the risk of ischemic stroke and thromboembolic events by approximately two-thirds, regardless of baseline risk. Anticoagulant options have increased substantially in the past few years with the introduction of novel oral anticoagulants, including the direct thrombin-inhibitor dabigatran and the factor Xa inhibitors rivaroxaban, apixaban, and edoxaban.
Warfarin
Warfarin has been used for decades for stroke prevention. It remains the only acceptable anticoagulant in patients with valvular AF. Multiple randomized clinical trials have assessed the efficacy of warfarin for stroke prevention in patients with nonvalvular AF.7 These trials demonstrated that warfarin significantly reduces stroke risk, stroke severity, and 30-day mortality compared with no anticoagulant therapy.7,8
Although warfarin is one of the most efficacious drugs to prevent stroke in AF, it has several key limitations. The most important is the need for dose adjustment to keep the INR in a narrow window (2.0 to 3.0) in which net clinical benefit is achieved without increased bleeding risk. The need for continuous monitoring is an inconvenience to patients and often leads to drug discontinuation and nonadherence. A meta-analysis found that patients are only in the therapeutic INR about half of the time.9 Importantly, the time spent in therapeutic INR range cor- relates significantly with the reduction in stroke risk.10 Furthermore, patients who spend less than 40% of the time in the therapeutic INR range are at a higher stroke risk than those not taking warfarin.10
Another limitation with the medication is the dietary restriction on intake of vitamin K-rich green vegetables, which are emphasized as healthy food choices especially in patients with heart disease. Higher warfarin doses are required in patients who consume greens and salads. It is important that patients be consistent in their intake of vitamin K-rich foods to avoid labile INRs, a difficult task for most patients.
Finally, there are several drugs that might interact with warfarin and potentially interfere with its safety or efficacy. These drugs include amiodarone, statins including simvastatin and rosuvastatin (not atorvastatin or pravastatin), fibrates (fenofibrate, gemfibrozil), antibiotics (sulfamethoxazole/trimethoprim, metronidazole), and azole antifungals (fluconazole, miconazole, voriconazole). The use of drugs that induce the cytochrome P450 enzyme CYP2C9, such as rifampin, decrease warfarin effectiveness by reducing INR values. Other non-CYP2C9-dependent drug interactions exist as well.
Aspirin monotherapy or in combination with other agents
Aspirin monotherapy or aspirin plus clopidogrel both increase the risk of bleeding without appreciable benefit, and, as such, their use for stroke prevention in patients with AF is not well supported. The combination of aspirin plus low-dose warfarin was assessed in the SPAF-III trial11 that randomized AF patients at stroke risk to either aspirin plus low-dose warfarin or to dose-adjusted warfarin to target a therapeutic INR. In this trial, patients on aspirin plus low-dose warfarin had significantly higher morbidity and mortality than patients who took adjusted-dose warfarin alone. Thus, the combination of low-dose warfarin plus aspirin should not be used for stroke prevention in AF.
In contrast, the combination of aspirin plus full anticoagulation with warfarin has not been well studied. Limited post-hoc data from the SPORTIF trials suggest, however, that this combination does not reduce the risk of stroke or thromboembolism more than warfarin alone.12
Novel oral anticoagulants
Dabigatran. The value of dabigatran for prevention of stroke or thromboembolism in AF was tested in the RE-LY trial (Randomized Evaluation of Long-Term Anticoagulation Therapy).13 In this trial, 18,113 patients with AF at high risk for stroke were randomized to dabigatran (110 mg or 150 mg twice daily) or adjusted-dose warfarin (INR target 2.0–3.0). By intention-to-treat analysis, dabigatran 150 mg was superior to warfarin for stroke prevention. Importantly, the risk of intracranial or life-threatening bleeding was significantly lower for both dabigatran doses compared with warfarin. Of note, gastrointestinal bleeding was more common with dabigatran 150 mg than warfarin; rates were similar for dabigatran 110 mg versus warfarin.
Rivaroxaban. This factor-Xa inhibitor was assessed in ROCKET-AF (Rivaroxaban Once-daily oral direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation).14 In this trial, 14,264 patients with AF and at risk for stroke were randomized to rivaroxaban (20 mg once daily) or warfarin (INR target 2.5). In the warfarin arm, INR was in the therapeutic range only 55% of the time. Results showed rivaroxaban was noninferior, but not superior, to warfarin for the prevention of stroke or systemic thromboembolism, the primary end points. From a safety standpoint, the overall bleeding rates were similar in the treatment arms with less life-threatening (fatal or intracranial) hemorrhage events with rivaroxaban.
Apixaban. This factor-Xa inhibitor was tested for stroke prevention in AF in two separate clinical trials.15,16 In the AVERROES trial (Apixaban Versus Acetylsalicylic Acid to Prevent Strokes),15 5,599 patients with AF deemed “unsuitable” for warfarin were randomized to apixaban (5 mg twice daily) or aspirin (81–324 mg daily). The trial was terminated early due to superiority of apixaban in achieving the primary end point: occurrence of stroke or systemic embolism. Importantly, the risk of major bleeding appeared to be similar with apixaban versus aspirin.
The ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial16 randomized 18,201 patients with AF and at least one additional risk factor for stroke to either apixaban 5 mg or warfarin (target INR 2.0–3.0). In this trial, apixaban was superior to warfarin for the prevention of stroke or systemic embolism, the primary efficacy end point. There also appeared to be a mortality benefit with apixaban versus warfarin. Importantly, the risk of major and intracranial bleeding, the primary safety outcomes, occurred at lower rates in the apixaban group.
All three of these oral anticoagulants require dose adjustment in patients with renal insufficiency and are contraindicated in patients with end-stage renal failure. They are not indicated in patients with valvular heart disease or a mechanical heart valves.
NONPHARMACOLOGIC INTERVENTIONS
Most atrial thrombi in patients with nonvalvular AF form in the LAA. Nonpharmacologic interventions have been developed to block the LAA to reduce the risk of stroke. These are especially valuable options for patients who are not candidates for chronic anticoagulation. In patients undergoing mitral valve surgery,17 ligation to close the LAA has become a standard practice at experienced centers. The introduction of less invasive catheter-based interventions to occlude the LAA has provided additional options.
Watchman device
The Watchman implant, a closure device that blocks the LAA, was recently approved by the US Food and Drug Administration (FDA) for stroke prevention in patients with nonvalvular AF who are at increased risk for stroke based on a CHA2DS2-VASc score of 2 or greater; candidates also must have an appropriate rationale for nonpharmacologic therapy. The expandable-cage device is surgically delivered into the LAA (Figure 1), which subsequently endothelializes and isolates the LAA. (Video 1 shows the delivery sheath positioned at the os of the appendage with a confirmatory contrast appendogram. Video 2 shows the delivery of the device into the appendage.) Therapeutic warfarin is required for a minimum of 45 days after implant followed by aspirin and clopidogrel for 6 months and then aspirin alone.
This device was initially tested in the PROTECT AF (Watchman Left Atrial Appendage System for Embolic PROTECTion in Patients with Atrial Fibrillation) trial, a noninferiority trial that randomized patients to either device implant or warfarin.18 Device implant was successful in 91% of patients in whom it was attempted. Overall, the study showed noninferiority of the device to warfarin in terms of the primary efficacy standpoint, which included stroke, systemic embolism, and cardiovascular death; however, this came at the expense of higher incidence of procedure-related complications, which seemed to be dependent on a learning curve with the device.
In the PREVAIL (Evaluation of the Watchman LAA Closure Device in Patients with Atrial Fibrillation Versus Long-Term Warfarin Therapy) trial,19 although noninferiority was not achieved, the event rates were low and the safety of the procedure was much improved. This was also demonstrated in registry data, which showed improved safety with device implantation with increased operator experience.
Of note, both of these trials included only patients who were eligible for warfarin. Another nonrandomized study20 assessed the use of this device in patients with a contraindication to long-term anticoagulation. Results showed a very low incidence of stroke, which was lower than CHADS2-matched controls taking either aspirin or clopidogrel. The FDA has approved this device for stroke prevention in AF.
Lariat system
The Lariat system is another percutaneous system for occlusion of the LAA. This device, which requires both atrial transseptal and epicardial access, ligates the appendage from the pericardial space. While some small studies have shown it safe and efficacious in patients with AF who cannot take anticoagulation,21 other reports have not been as encouraging.22 The device has been approved by the FDA for “soft tissue approximation”; it is not approved for stroke prevention.
SUMMARY
The most common serious complications of AF are stroke and thromboembolism. Medical and interventional therapies have been developed to prevent these complications. In patients with an estimated thromboembolic risk of greater than 1% to 2% per year, anticoagulation is warranted to reduce that risk. Warfarin remains one of the most studied and useful medications for this purpose, but its use is limited by the need for frequent monitoring, multiple drug interactions, dietary restrictions, and, most importantly, by the difficulty of consistently maintaining therapeutic INRs.
The recently introduced novel oral anticoagulants have been found to be at least noninferior to warfarin and for some agents to be superior to warfarin for the prevention of stroke and thromboembolism. Their main advantage is that they do not require monitoring and have fewer drug interactions and dietary restrictions. Most important, there appear to be fewer major and life-threatening bleeding events than with warfarin. However, use of these novel agents could be limited by patients’ renal function, which needs to be assessed when these agents are being considered. Another limitation with these agents (versus warfarin) is that patients are not therapeutically anticoagulated when they miss a dose, whereas missing a single dose of warfarin may not have the same effect.
In our practice, we have transitioned to use of these novel oral anticoagulants whenever possible using an approach that assesses the individual patient’s risks for both stroke and thromboembolism as well as the risk for bleeding. In patients who are at increased risk of bleeding but at risk of stroke in AF, percutaneous occlusion of the LAA using the Watchman device is offered. This device has been shown to be noninferior to warfarin for stroke prevention and may provide a survival benefit due to the reduction of life-threatening bleeding, which is an inherent risk with anticoagulants. One caveat is that there is a residual risk of stroke after undergoing LAA occlusion because the same risk factors for stroke in AF contribute to stroke from atherothrombosis and atheroembolism; also, thrombi can form in the body of the left atrium.
Clinical decision-making is often challenging in patients with AF who are at risk of stroke and bleeding. In fact, these risk factors often overlap. In our practice, we have established a multidisciplinary clinic for stroke prevention in AF that involves cardiologists, cardiac electrophysiologists, neurologists, gastroenterologists, and vascular medicine specialists. This model allows a multidisciplinary assessment of patients’ individual risks and ultimately facilitates clinical decision-making in terms of strategies to prevent stroke and thromboembolism in AF.
- Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 2012; 125:e2–e220.
- January CT, Wann LS, Alpert JS, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64(21):e1–76.
- Dulli DA, Stanko H, Levine RL. Atrial fibrillation is associated with severe acute ischemic stroke. Neuroepidemiology 2003; 22:118–123.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the national registry of atrial fibrillation. JAMA 2001; 285:2864–2870.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010; 137:263-272.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007; 146:857–867.
- Johnsen SP, Svendsen ML, Hansen ML, Brandes A, Mehnert F, Husted SE. Preadmission oral anticoagulant treatment and clinical outcome among patients hospitalized with acute stroke and atrial fibrillation: a nationwide study. Stroke 2014; 45:168-175.
- Baker WL, Cios DA, Sander SD, Coleman CI. Meta-analysis to assess the quality of warfarin control in atrial fibrillation patients in the united states. J Manage Care Pharm 2009; 15:244-252.
- Morgan CL, McEwan P, Tukiendorf A, Robinson PA, Clemens A, Plumb JM. Warfarin treatment in patients with atrial fibrillation: observing outcomes associated with varying levels of INR control. Thromb Res 2009; 124:37–41.
- Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: Stroke prevention in atrial fibrillation III randomised clinical trial. Lancet 1996; 348(9028):633–638.
- Flaker GC, Gruber M, Connolly SJ, et al. Risks and benefits of combining aspirin with anticoagulant therapy in patients with atrial fibrillation: an exploratory analysis of stroke prevention using an oral thrombin inhibitor in atrial fibrillation (SPORTIF) trials. Am Heart J 2006; 152:967–973.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al, and the RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011; 364:806–817.
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Garcia-Fernandez MA, Perez-David E, Quiles J, et al. Role of left atrial appendage obliteration in stroke reduction in patients with mitral valve prosthesis: a transesophageal echocardiographic study. J Am Coll Cardiol 2003; 42:1253–1258.
- Holmes DR, Reddy VY, Turi ZG, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 2009; 374(9689):534–542.
- Holmes DR Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: The PREVAIL trial. J Am Coll Cardiol 2014; 64:1–12.
- Reddy VY, Mobius-Winkler S, Miller MA, et al. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA plavix feasibility study with watchman left atrial appendage closure technology). J Am Coll Cardiol 2013; 61:2551–2556.
- Bartus K, Han FT, Bednarek J, et al. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol 2013; 62:108–118.
- Chatterjee S, Herrmann HC, Wilensky RL, et al. Safety and procedural success of left atrial appendage exclusion with the Lariat device: a systematic review of published reports and analytic review of the FDA MAUDE database. JAMA Intern Med 2015; 175(7):1104–9.
Atrial fibrillation (AF), the most common cardiac arrhythmia, has become a major public health problem. In the United States, the prevalence of AF was estimated at 2.7 to 6.1 million in 2010, and it is expected to rise to between 5.6 and 12 million by 2050.1 The arrhythmia is associated with impaired quality of life and increased morbidity and mortality.1,2 Stroke remains the most devastating consequence of AF.
The clinical management of patients with AF typically targets two main goals: prevention of stroke or thromboembolism and control of symptoms. This article addresses the evolving pharmacologic and nonpharmacologic strategies in stroke prevention in nonvalvular AF; reviews clinical trials evaluating medical and procedural strategies, including the novel oral anticoagulants and left atrial appendage (LAA) exclusion devices; and assesses the impact of these novel strategies on clinical practice.
RISK OF STROKE AND THROMBOEMBOLISM IN NONVALVULAR AF
Stroke occurrence from AF is primarily caused by thrombi formation in the left atrium, most commonly in the LAA. It is important to recognize that the cardiovascular risk factors for AF are also risk factors for atheroembolism; therefore, specific risk factor management is as important as anticoagulation when addressing stroke risk.
The incidence of all-cause stroke in patients with AF is 5%, and it is believed that AF causes approximately 15% of all strokes in the United States.1 This risk appears to be more significant in older patients who are more vulnerable to ischemic strokes. Estimates are that AF independently increases the risk of stroke by fivefold throughout all ages, with a steep increase in percentage of strokes attributed to AF from 1.5% at ages 50 to 59 to 23.5% at ages 80 to 89.1 Importantly, the clinical course of ischemic stroke associated with AF is often more severe than for strokes of other causes,3 further emphasizing the need for stroke prevention.
Assessment of stroke risk/thromboembolism
Multiple risk estimation scores have been developed based on epidemiologic data. Until recently, the CHADS2 score4 was the most commonly used, but it has been superseded by the CHA2DS2-VASc score.5 The point system for this scoring system is shown in Table 1. In contrast with CHADS2, this updated system assigns 2 points for age over 75 years and accounts for stroke risk in the relatively younger group of patients (age 65–75) and in females, neither of whom were included in CHADS2. The CHA2DS2-VASc score ranges between 0 and 9 with a respective estimated stroke risk of 0 to 15.2% per year. Note that for females who are younger than 65 years, no points are given for sex. The major advantage of the CHA2DS2-VASc score over the CHADS2 score is that it is more accurate for lower-risk categories. It has been adopted in most of the recent guidelines that address stroke risk in AF.
In clinical practice, practitioners use these scores to define three primary stroke risk categories: low, intermediate, or high. In our practice, we use a 2% per year cut-off to identify high-risk patients in whom the risk of stroke significantly outweighs the risk of bleeding on anticoagulants. In general, patients with a CHA2DS2-VASc score equal to or greater than 2 have a greater than 2% stroke risk per year and are most likely to benefit from antithrombotic therapies.
In male patients with a CHA2DS2-VASc score of 0 and in most patients with a score of 1, the stroke risk is less than 1% per year. These patients are not likely to derive benefit from anticoagulant therapy. They are usually approached on a case-by-case basis with careful assessment of bleeding risk and discussion of risks and benefits of anticoagulant strategies.
Assessment of bleeding risk
Any general approach to thromboembolism risk assessment in patients with AF should include an analysis that weighs the benefits of anticoagulant therapies against the risks of bleeding. Although no precise tools exist to predict bleeding risk, the HAS-BLED score is increasingly used.6 This score assigns 1 point to each of the following:
- systolic blood pressure greater than 160 mm Hg
- abnormal renal function
- abnormal liver function
- age older than 65
- prior cerebrovascular event
- prior bleeding
- history of labile international normalized ratios (INR)
- alcohol intake (> 8 U/week)
- drug use, especially antiplatelet agents or nonsteroidal anti-inflammatory drugs (NSAIDs).
In general, a HAS-BLED score of 3 or greater indicates increased 1-year risk of intracranial bleed, bleeding requiring hospitalization, drop in hemoglobin of at least 2 g/dL, or need for transfusion.
One problem with the bleeding risk scores is that they were derived from studies that included bleeding events of differing severity. Most bleeding events do not lead to death or severe disability with the exception of intracranial bleeding, which is, therefore, the primary concern when assessing bleeding risk.
The estimated bleeding risk with anticoagulant therapy ranges from 0.2% to 0.4% per year but could be much higher in patients with prior severe bleeding, intracranial hemorrhage, thrombocytopenia, coagulopathies, recent surgery, or ongoing bleeding, aortic dissection, malignant hypertension, and in those receiving a combination of anticoagulant and antiplatelet agents.
MEDICAL THERAPIES TO PREVENT STROKE AND THROMBOEMBOLISM IN AF
In general, anticoagulation reduces the risk of ischemic stroke and thromboembolic events by approximately two-thirds, regardless of baseline risk. Anticoagulant options have increased substantially in the past few years with the introduction of novel oral anticoagulants, including the direct thrombin-inhibitor dabigatran and the factor Xa inhibitors rivaroxaban, apixaban, and edoxaban.
Warfarin
Warfarin has been used for decades for stroke prevention. It remains the only acceptable anticoagulant in patients with valvular AF. Multiple randomized clinical trials have assessed the efficacy of warfarin for stroke prevention in patients with nonvalvular AF.7 These trials demonstrated that warfarin significantly reduces stroke risk, stroke severity, and 30-day mortality compared with no anticoagulant therapy.7,8
Although warfarin is one of the most efficacious drugs to prevent stroke in AF, it has several key limitations. The most important is the need for dose adjustment to keep the INR in a narrow window (2.0 to 3.0) in which net clinical benefit is achieved without increased bleeding risk. The need for continuous monitoring is an inconvenience to patients and often leads to drug discontinuation and nonadherence. A meta-analysis found that patients are only in the therapeutic INR about half of the time.9 Importantly, the time spent in therapeutic INR range cor- relates significantly with the reduction in stroke risk.10 Furthermore, patients who spend less than 40% of the time in the therapeutic INR range are at a higher stroke risk than those not taking warfarin.10
Another limitation with the medication is the dietary restriction on intake of vitamin K-rich green vegetables, which are emphasized as healthy food choices especially in patients with heart disease. Higher warfarin doses are required in patients who consume greens and salads. It is important that patients be consistent in their intake of vitamin K-rich foods to avoid labile INRs, a difficult task for most patients.
Finally, there are several drugs that might interact with warfarin and potentially interfere with its safety or efficacy. These drugs include amiodarone, statins including simvastatin and rosuvastatin (not atorvastatin or pravastatin), fibrates (fenofibrate, gemfibrozil), antibiotics (sulfamethoxazole/trimethoprim, metronidazole), and azole antifungals (fluconazole, miconazole, voriconazole). The use of drugs that induce the cytochrome P450 enzyme CYP2C9, such as rifampin, decrease warfarin effectiveness by reducing INR values. Other non-CYP2C9-dependent drug interactions exist as well.
Aspirin monotherapy or in combination with other agents
Aspirin monotherapy or aspirin plus clopidogrel both increase the risk of bleeding without appreciable benefit, and, as such, their use for stroke prevention in patients with AF is not well supported. The combination of aspirin plus low-dose warfarin was assessed in the SPAF-III trial11 that randomized AF patients at stroke risk to either aspirin plus low-dose warfarin or to dose-adjusted warfarin to target a therapeutic INR. In this trial, patients on aspirin plus low-dose warfarin had significantly higher morbidity and mortality than patients who took adjusted-dose warfarin alone. Thus, the combination of low-dose warfarin plus aspirin should not be used for stroke prevention in AF.
In contrast, the combination of aspirin plus full anticoagulation with warfarin has not been well studied. Limited post-hoc data from the SPORTIF trials suggest, however, that this combination does not reduce the risk of stroke or thromboembolism more than warfarin alone.12
Novel oral anticoagulants
Dabigatran. The value of dabigatran for prevention of stroke or thromboembolism in AF was tested in the RE-LY trial (Randomized Evaluation of Long-Term Anticoagulation Therapy).13 In this trial, 18,113 patients with AF at high risk for stroke were randomized to dabigatran (110 mg or 150 mg twice daily) or adjusted-dose warfarin (INR target 2.0–3.0). By intention-to-treat analysis, dabigatran 150 mg was superior to warfarin for stroke prevention. Importantly, the risk of intracranial or life-threatening bleeding was significantly lower for both dabigatran doses compared with warfarin. Of note, gastrointestinal bleeding was more common with dabigatran 150 mg than warfarin; rates were similar for dabigatran 110 mg versus warfarin.
Rivaroxaban. This factor-Xa inhibitor was assessed in ROCKET-AF (Rivaroxaban Once-daily oral direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation).14 In this trial, 14,264 patients with AF and at risk for stroke were randomized to rivaroxaban (20 mg once daily) or warfarin (INR target 2.5). In the warfarin arm, INR was in the therapeutic range only 55% of the time. Results showed rivaroxaban was noninferior, but not superior, to warfarin for the prevention of stroke or systemic thromboembolism, the primary end points. From a safety standpoint, the overall bleeding rates were similar in the treatment arms with less life-threatening (fatal or intracranial) hemorrhage events with rivaroxaban.
Apixaban. This factor-Xa inhibitor was tested for stroke prevention in AF in two separate clinical trials.15,16 In the AVERROES trial (Apixaban Versus Acetylsalicylic Acid to Prevent Strokes),15 5,599 patients with AF deemed “unsuitable” for warfarin were randomized to apixaban (5 mg twice daily) or aspirin (81–324 mg daily). The trial was terminated early due to superiority of apixaban in achieving the primary end point: occurrence of stroke or systemic embolism. Importantly, the risk of major bleeding appeared to be similar with apixaban versus aspirin.
The ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial16 randomized 18,201 patients with AF and at least one additional risk factor for stroke to either apixaban 5 mg or warfarin (target INR 2.0–3.0). In this trial, apixaban was superior to warfarin for the prevention of stroke or systemic embolism, the primary efficacy end point. There also appeared to be a mortality benefit with apixaban versus warfarin. Importantly, the risk of major and intracranial bleeding, the primary safety outcomes, occurred at lower rates in the apixaban group.
All three of these oral anticoagulants require dose adjustment in patients with renal insufficiency and are contraindicated in patients with end-stage renal failure. They are not indicated in patients with valvular heart disease or a mechanical heart valves.
NONPHARMACOLOGIC INTERVENTIONS
Most atrial thrombi in patients with nonvalvular AF form in the LAA. Nonpharmacologic interventions have been developed to block the LAA to reduce the risk of stroke. These are especially valuable options for patients who are not candidates for chronic anticoagulation. In patients undergoing mitral valve surgery,17 ligation to close the LAA has become a standard practice at experienced centers. The introduction of less invasive catheter-based interventions to occlude the LAA has provided additional options.
Watchman device
The Watchman implant, a closure device that blocks the LAA, was recently approved by the US Food and Drug Administration (FDA) for stroke prevention in patients with nonvalvular AF who are at increased risk for stroke based on a CHA2DS2-VASc score of 2 or greater; candidates also must have an appropriate rationale for nonpharmacologic therapy. The expandable-cage device is surgically delivered into the LAA (Figure 1), which subsequently endothelializes and isolates the LAA. (Video 1 shows the delivery sheath positioned at the os of the appendage with a confirmatory contrast appendogram. Video 2 shows the delivery of the device into the appendage.) Therapeutic warfarin is required for a minimum of 45 days after implant followed by aspirin and clopidogrel for 6 months and then aspirin alone.
This device was initially tested in the PROTECT AF (Watchman Left Atrial Appendage System for Embolic PROTECTion in Patients with Atrial Fibrillation) trial, a noninferiority trial that randomized patients to either device implant or warfarin.18 Device implant was successful in 91% of patients in whom it was attempted. Overall, the study showed noninferiority of the device to warfarin in terms of the primary efficacy standpoint, which included stroke, systemic embolism, and cardiovascular death; however, this came at the expense of higher incidence of procedure-related complications, which seemed to be dependent on a learning curve with the device.
In the PREVAIL (Evaluation of the Watchman LAA Closure Device in Patients with Atrial Fibrillation Versus Long-Term Warfarin Therapy) trial,19 although noninferiority was not achieved, the event rates were low and the safety of the procedure was much improved. This was also demonstrated in registry data, which showed improved safety with device implantation with increased operator experience.
Of note, both of these trials included only patients who were eligible for warfarin. Another nonrandomized study20 assessed the use of this device in patients with a contraindication to long-term anticoagulation. Results showed a very low incidence of stroke, which was lower than CHADS2-matched controls taking either aspirin or clopidogrel. The FDA has approved this device for stroke prevention in AF.
Lariat system
The Lariat system is another percutaneous system for occlusion of the LAA. This device, which requires both atrial transseptal and epicardial access, ligates the appendage from the pericardial space. While some small studies have shown it safe and efficacious in patients with AF who cannot take anticoagulation,21 other reports have not been as encouraging.22 The device has been approved by the FDA for “soft tissue approximation”; it is not approved for stroke prevention.
SUMMARY
The most common serious complications of AF are stroke and thromboembolism. Medical and interventional therapies have been developed to prevent these complications. In patients with an estimated thromboembolic risk of greater than 1% to 2% per year, anticoagulation is warranted to reduce that risk. Warfarin remains one of the most studied and useful medications for this purpose, but its use is limited by the need for frequent monitoring, multiple drug interactions, dietary restrictions, and, most importantly, by the difficulty of consistently maintaining therapeutic INRs.
The recently introduced novel oral anticoagulants have been found to be at least noninferior to warfarin and for some agents to be superior to warfarin for the prevention of stroke and thromboembolism. Their main advantage is that they do not require monitoring and have fewer drug interactions and dietary restrictions. Most important, there appear to be fewer major and life-threatening bleeding events than with warfarin. However, use of these novel agents could be limited by patients’ renal function, which needs to be assessed when these agents are being considered. Another limitation with these agents (versus warfarin) is that patients are not therapeutically anticoagulated when they miss a dose, whereas missing a single dose of warfarin may not have the same effect.
In our practice, we have transitioned to use of these novel oral anticoagulants whenever possible using an approach that assesses the individual patient’s risks for both stroke and thromboembolism as well as the risk for bleeding. In patients who are at increased risk of bleeding but at risk of stroke in AF, percutaneous occlusion of the LAA using the Watchman device is offered. This device has been shown to be noninferior to warfarin for stroke prevention and may provide a survival benefit due to the reduction of life-threatening bleeding, which is an inherent risk with anticoagulants. One caveat is that there is a residual risk of stroke after undergoing LAA occlusion because the same risk factors for stroke in AF contribute to stroke from atherothrombosis and atheroembolism; also, thrombi can form in the body of the left atrium.
Clinical decision-making is often challenging in patients with AF who are at risk of stroke and bleeding. In fact, these risk factors often overlap. In our practice, we have established a multidisciplinary clinic for stroke prevention in AF that involves cardiologists, cardiac electrophysiologists, neurologists, gastroenterologists, and vascular medicine specialists. This model allows a multidisciplinary assessment of patients’ individual risks and ultimately facilitates clinical decision-making in terms of strategies to prevent stroke and thromboembolism in AF.
Atrial fibrillation (AF), the most common cardiac arrhythmia, has become a major public health problem. In the United States, the prevalence of AF was estimated at 2.7 to 6.1 million in 2010, and it is expected to rise to between 5.6 and 12 million by 2050.1 The arrhythmia is associated with impaired quality of life and increased morbidity and mortality.1,2 Stroke remains the most devastating consequence of AF.
The clinical management of patients with AF typically targets two main goals: prevention of stroke or thromboembolism and control of symptoms. This article addresses the evolving pharmacologic and nonpharmacologic strategies in stroke prevention in nonvalvular AF; reviews clinical trials evaluating medical and procedural strategies, including the novel oral anticoagulants and left atrial appendage (LAA) exclusion devices; and assesses the impact of these novel strategies on clinical practice.
RISK OF STROKE AND THROMBOEMBOLISM IN NONVALVULAR AF
Stroke occurrence from AF is primarily caused by thrombi formation in the left atrium, most commonly in the LAA. It is important to recognize that the cardiovascular risk factors for AF are also risk factors for atheroembolism; therefore, specific risk factor management is as important as anticoagulation when addressing stroke risk.
The incidence of all-cause stroke in patients with AF is 5%, and it is believed that AF causes approximately 15% of all strokes in the United States.1 This risk appears to be more significant in older patients who are more vulnerable to ischemic strokes. Estimates are that AF independently increases the risk of stroke by fivefold throughout all ages, with a steep increase in percentage of strokes attributed to AF from 1.5% at ages 50 to 59 to 23.5% at ages 80 to 89.1 Importantly, the clinical course of ischemic stroke associated with AF is often more severe than for strokes of other causes,3 further emphasizing the need for stroke prevention.
Assessment of stroke risk/thromboembolism
Multiple risk estimation scores have been developed based on epidemiologic data. Until recently, the CHADS2 score4 was the most commonly used, but it has been superseded by the CHA2DS2-VASc score.5 The point system for this scoring system is shown in Table 1. In contrast with CHADS2, this updated system assigns 2 points for age over 75 years and accounts for stroke risk in the relatively younger group of patients (age 65–75) and in females, neither of whom were included in CHADS2. The CHA2DS2-VASc score ranges between 0 and 9 with a respective estimated stroke risk of 0 to 15.2% per year. Note that for females who are younger than 65 years, no points are given for sex. The major advantage of the CHA2DS2-VASc score over the CHADS2 score is that it is more accurate for lower-risk categories. It has been adopted in most of the recent guidelines that address stroke risk in AF.
In clinical practice, practitioners use these scores to define three primary stroke risk categories: low, intermediate, or high. In our practice, we use a 2% per year cut-off to identify high-risk patients in whom the risk of stroke significantly outweighs the risk of bleeding on anticoagulants. In general, patients with a CHA2DS2-VASc score equal to or greater than 2 have a greater than 2% stroke risk per year and are most likely to benefit from antithrombotic therapies.
In male patients with a CHA2DS2-VASc score of 0 and in most patients with a score of 1, the stroke risk is less than 1% per year. These patients are not likely to derive benefit from anticoagulant therapy. They are usually approached on a case-by-case basis with careful assessment of bleeding risk and discussion of risks and benefits of anticoagulant strategies.
Assessment of bleeding risk
Any general approach to thromboembolism risk assessment in patients with AF should include an analysis that weighs the benefits of anticoagulant therapies against the risks of bleeding. Although no precise tools exist to predict bleeding risk, the HAS-BLED score is increasingly used.6 This score assigns 1 point to each of the following:
- systolic blood pressure greater than 160 mm Hg
- abnormal renal function
- abnormal liver function
- age older than 65
- prior cerebrovascular event
- prior bleeding
- history of labile international normalized ratios (INR)
- alcohol intake (> 8 U/week)
- drug use, especially antiplatelet agents or nonsteroidal anti-inflammatory drugs (NSAIDs).
In general, a HAS-BLED score of 3 or greater indicates increased 1-year risk of intracranial bleed, bleeding requiring hospitalization, drop in hemoglobin of at least 2 g/dL, or need for transfusion.
One problem with the bleeding risk scores is that they were derived from studies that included bleeding events of differing severity. Most bleeding events do not lead to death or severe disability with the exception of intracranial bleeding, which is, therefore, the primary concern when assessing bleeding risk.
The estimated bleeding risk with anticoagulant therapy ranges from 0.2% to 0.4% per year but could be much higher in patients with prior severe bleeding, intracranial hemorrhage, thrombocytopenia, coagulopathies, recent surgery, or ongoing bleeding, aortic dissection, malignant hypertension, and in those receiving a combination of anticoagulant and antiplatelet agents.
MEDICAL THERAPIES TO PREVENT STROKE AND THROMBOEMBOLISM IN AF
In general, anticoagulation reduces the risk of ischemic stroke and thromboembolic events by approximately two-thirds, regardless of baseline risk. Anticoagulant options have increased substantially in the past few years with the introduction of novel oral anticoagulants, including the direct thrombin-inhibitor dabigatran and the factor Xa inhibitors rivaroxaban, apixaban, and edoxaban.
Warfarin
Warfarin has been used for decades for stroke prevention. It remains the only acceptable anticoagulant in patients with valvular AF. Multiple randomized clinical trials have assessed the efficacy of warfarin for stroke prevention in patients with nonvalvular AF.7 These trials demonstrated that warfarin significantly reduces stroke risk, stroke severity, and 30-day mortality compared with no anticoagulant therapy.7,8
Although warfarin is one of the most efficacious drugs to prevent stroke in AF, it has several key limitations. The most important is the need for dose adjustment to keep the INR in a narrow window (2.0 to 3.0) in which net clinical benefit is achieved without increased bleeding risk. The need for continuous monitoring is an inconvenience to patients and often leads to drug discontinuation and nonadherence. A meta-analysis found that patients are only in the therapeutic INR about half of the time.9 Importantly, the time spent in therapeutic INR range cor- relates significantly with the reduction in stroke risk.10 Furthermore, patients who spend less than 40% of the time in the therapeutic INR range are at a higher stroke risk than those not taking warfarin.10
Another limitation with the medication is the dietary restriction on intake of vitamin K-rich green vegetables, which are emphasized as healthy food choices especially in patients with heart disease. Higher warfarin doses are required in patients who consume greens and salads. It is important that patients be consistent in their intake of vitamin K-rich foods to avoid labile INRs, a difficult task for most patients.
Finally, there are several drugs that might interact with warfarin and potentially interfere with its safety or efficacy. These drugs include amiodarone, statins including simvastatin and rosuvastatin (not atorvastatin or pravastatin), fibrates (fenofibrate, gemfibrozil), antibiotics (sulfamethoxazole/trimethoprim, metronidazole), and azole antifungals (fluconazole, miconazole, voriconazole). The use of drugs that induce the cytochrome P450 enzyme CYP2C9, such as rifampin, decrease warfarin effectiveness by reducing INR values. Other non-CYP2C9-dependent drug interactions exist as well.
Aspirin monotherapy or in combination with other agents
Aspirin monotherapy or aspirin plus clopidogrel both increase the risk of bleeding without appreciable benefit, and, as such, their use for stroke prevention in patients with AF is not well supported. The combination of aspirin plus low-dose warfarin was assessed in the SPAF-III trial11 that randomized AF patients at stroke risk to either aspirin plus low-dose warfarin or to dose-adjusted warfarin to target a therapeutic INR. In this trial, patients on aspirin plus low-dose warfarin had significantly higher morbidity and mortality than patients who took adjusted-dose warfarin alone. Thus, the combination of low-dose warfarin plus aspirin should not be used for stroke prevention in AF.
In contrast, the combination of aspirin plus full anticoagulation with warfarin has not been well studied. Limited post-hoc data from the SPORTIF trials suggest, however, that this combination does not reduce the risk of stroke or thromboembolism more than warfarin alone.12
Novel oral anticoagulants
Dabigatran. The value of dabigatran for prevention of stroke or thromboembolism in AF was tested in the RE-LY trial (Randomized Evaluation of Long-Term Anticoagulation Therapy).13 In this trial, 18,113 patients with AF at high risk for stroke were randomized to dabigatran (110 mg or 150 mg twice daily) or adjusted-dose warfarin (INR target 2.0–3.0). By intention-to-treat analysis, dabigatran 150 mg was superior to warfarin for stroke prevention. Importantly, the risk of intracranial or life-threatening bleeding was significantly lower for both dabigatran doses compared with warfarin. Of note, gastrointestinal bleeding was more common with dabigatran 150 mg than warfarin; rates were similar for dabigatran 110 mg versus warfarin.
Rivaroxaban. This factor-Xa inhibitor was assessed in ROCKET-AF (Rivaroxaban Once-daily oral direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation).14 In this trial, 14,264 patients with AF and at risk for stroke were randomized to rivaroxaban (20 mg once daily) or warfarin (INR target 2.5). In the warfarin arm, INR was in the therapeutic range only 55% of the time. Results showed rivaroxaban was noninferior, but not superior, to warfarin for the prevention of stroke or systemic thromboembolism, the primary end points. From a safety standpoint, the overall bleeding rates were similar in the treatment arms with less life-threatening (fatal or intracranial) hemorrhage events with rivaroxaban.
Apixaban. This factor-Xa inhibitor was tested for stroke prevention in AF in two separate clinical trials.15,16 In the AVERROES trial (Apixaban Versus Acetylsalicylic Acid to Prevent Strokes),15 5,599 patients with AF deemed “unsuitable” for warfarin were randomized to apixaban (5 mg twice daily) or aspirin (81–324 mg daily). The trial was terminated early due to superiority of apixaban in achieving the primary end point: occurrence of stroke or systemic embolism. Importantly, the risk of major bleeding appeared to be similar with apixaban versus aspirin.
The ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial16 randomized 18,201 patients with AF and at least one additional risk factor for stroke to either apixaban 5 mg or warfarin (target INR 2.0–3.0). In this trial, apixaban was superior to warfarin for the prevention of stroke or systemic embolism, the primary efficacy end point. There also appeared to be a mortality benefit with apixaban versus warfarin. Importantly, the risk of major and intracranial bleeding, the primary safety outcomes, occurred at lower rates in the apixaban group.
All three of these oral anticoagulants require dose adjustment in patients with renal insufficiency and are contraindicated in patients with end-stage renal failure. They are not indicated in patients with valvular heart disease or a mechanical heart valves.
NONPHARMACOLOGIC INTERVENTIONS
Most atrial thrombi in patients with nonvalvular AF form in the LAA. Nonpharmacologic interventions have been developed to block the LAA to reduce the risk of stroke. These are especially valuable options for patients who are not candidates for chronic anticoagulation. In patients undergoing mitral valve surgery,17 ligation to close the LAA has become a standard practice at experienced centers. The introduction of less invasive catheter-based interventions to occlude the LAA has provided additional options.
Watchman device
The Watchman implant, a closure device that blocks the LAA, was recently approved by the US Food and Drug Administration (FDA) for stroke prevention in patients with nonvalvular AF who are at increased risk for stroke based on a CHA2DS2-VASc score of 2 or greater; candidates also must have an appropriate rationale for nonpharmacologic therapy. The expandable-cage device is surgically delivered into the LAA (Figure 1), which subsequently endothelializes and isolates the LAA. (Video 1 shows the delivery sheath positioned at the os of the appendage with a confirmatory contrast appendogram. Video 2 shows the delivery of the device into the appendage.) Therapeutic warfarin is required for a minimum of 45 days after implant followed by aspirin and clopidogrel for 6 months and then aspirin alone.
This device was initially tested in the PROTECT AF (Watchman Left Atrial Appendage System for Embolic PROTECTion in Patients with Atrial Fibrillation) trial, a noninferiority trial that randomized patients to either device implant or warfarin.18 Device implant was successful in 91% of patients in whom it was attempted. Overall, the study showed noninferiority of the device to warfarin in terms of the primary efficacy standpoint, which included stroke, systemic embolism, and cardiovascular death; however, this came at the expense of higher incidence of procedure-related complications, which seemed to be dependent on a learning curve with the device.
In the PREVAIL (Evaluation of the Watchman LAA Closure Device in Patients with Atrial Fibrillation Versus Long-Term Warfarin Therapy) trial,19 although noninferiority was not achieved, the event rates were low and the safety of the procedure was much improved. This was also demonstrated in registry data, which showed improved safety with device implantation with increased operator experience.
Of note, both of these trials included only patients who were eligible for warfarin. Another nonrandomized study20 assessed the use of this device in patients with a contraindication to long-term anticoagulation. Results showed a very low incidence of stroke, which was lower than CHADS2-matched controls taking either aspirin or clopidogrel. The FDA has approved this device for stroke prevention in AF.
Lariat system
The Lariat system is another percutaneous system for occlusion of the LAA. This device, which requires both atrial transseptal and epicardial access, ligates the appendage from the pericardial space. While some small studies have shown it safe and efficacious in patients with AF who cannot take anticoagulation,21 other reports have not been as encouraging.22 The device has been approved by the FDA for “soft tissue approximation”; it is not approved for stroke prevention.
SUMMARY
The most common serious complications of AF are stroke and thromboembolism. Medical and interventional therapies have been developed to prevent these complications. In patients with an estimated thromboembolic risk of greater than 1% to 2% per year, anticoagulation is warranted to reduce that risk. Warfarin remains one of the most studied and useful medications for this purpose, but its use is limited by the need for frequent monitoring, multiple drug interactions, dietary restrictions, and, most importantly, by the difficulty of consistently maintaining therapeutic INRs.
The recently introduced novel oral anticoagulants have been found to be at least noninferior to warfarin and for some agents to be superior to warfarin for the prevention of stroke and thromboembolism. Their main advantage is that they do not require monitoring and have fewer drug interactions and dietary restrictions. Most important, there appear to be fewer major and life-threatening bleeding events than with warfarin. However, use of these novel agents could be limited by patients’ renal function, which needs to be assessed when these agents are being considered. Another limitation with these agents (versus warfarin) is that patients are not therapeutically anticoagulated when they miss a dose, whereas missing a single dose of warfarin may not have the same effect.
In our practice, we have transitioned to use of these novel oral anticoagulants whenever possible using an approach that assesses the individual patient’s risks for both stroke and thromboembolism as well as the risk for bleeding. In patients who are at increased risk of bleeding but at risk of stroke in AF, percutaneous occlusion of the LAA using the Watchman device is offered. This device has been shown to be noninferior to warfarin for stroke prevention and may provide a survival benefit due to the reduction of life-threatening bleeding, which is an inherent risk with anticoagulants. One caveat is that there is a residual risk of stroke after undergoing LAA occlusion because the same risk factors for stroke in AF contribute to stroke from atherothrombosis and atheroembolism; also, thrombi can form in the body of the left atrium.
Clinical decision-making is often challenging in patients with AF who are at risk of stroke and bleeding. In fact, these risk factors often overlap. In our practice, we have established a multidisciplinary clinic for stroke prevention in AF that involves cardiologists, cardiac electrophysiologists, neurologists, gastroenterologists, and vascular medicine specialists. This model allows a multidisciplinary assessment of patients’ individual risks and ultimately facilitates clinical decision-making in terms of strategies to prevent stroke and thromboembolism in AF.
- Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 2012; 125:e2–e220.
- January CT, Wann LS, Alpert JS, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64(21):e1–76.
- Dulli DA, Stanko H, Levine RL. Atrial fibrillation is associated with severe acute ischemic stroke. Neuroepidemiology 2003; 22:118–123.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the national registry of atrial fibrillation. JAMA 2001; 285:2864–2870.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010; 137:263-272.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007; 146:857–867.
- Johnsen SP, Svendsen ML, Hansen ML, Brandes A, Mehnert F, Husted SE. Preadmission oral anticoagulant treatment and clinical outcome among patients hospitalized with acute stroke and atrial fibrillation: a nationwide study. Stroke 2014; 45:168-175.
- Baker WL, Cios DA, Sander SD, Coleman CI. Meta-analysis to assess the quality of warfarin control in atrial fibrillation patients in the united states. J Manage Care Pharm 2009; 15:244-252.
- Morgan CL, McEwan P, Tukiendorf A, Robinson PA, Clemens A, Plumb JM. Warfarin treatment in patients with atrial fibrillation: observing outcomes associated with varying levels of INR control. Thromb Res 2009; 124:37–41.
- Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: Stroke prevention in atrial fibrillation III randomised clinical trial. Lancet 1996; 348(9028):633–638.
- Flaker GC, Gruber M, Connolly SJ, et al. Risks and benefits of combining aspirin with anticoagulant therapy in patients with atrial fibrillation: an exploratory analysis of stroke prevention using an oral thrombin inhibitor in atrial fibrillation (SPORTIF) trials. Am Heart J 2006; 152:967–973.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al, and the RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011; 364:806–817.
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Garcia-Fernandez MA, Perez-David E, Quiles J, et al. Role of left atrial appendage obliteration in stroke reduction in patients with mitral valve prosthesis: a transesophageal echocardiographic study. J Am Coll Cardiol 2003; 42:1253–1258.
- Holmes DR, Reddy VY, Turi ZG, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 2009; 374(9689):534–542.
- Holmes DR Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: The PREVAIL trial. J Am Coll Cardiol 2014; 64:1–12.
- Reddy VY, Mobius-Winkler S, Miller MA, et al. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA plavix feasibility study with watchman left atrial appendage closure technology). J Am Coll Cardiol 2013; 61:2551–2556.
- Bartus K, Han FT, Bednarek J, et al. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol 2013; 62:108–118.
- Chatterjee S, Herrmann HC, Wilensky RL, et al. Safety and procedural success of left atrial appendage exclusion with the Lariat device: a systematic review of published reports and analytic review of the FDA MAUDE database. JAMA Intern Med 2015; 175(7):1104–9.
- Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 2012; 125:e2–e220.
- January CT, Wann LS, Alpert JS, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64(21):e1–76.
- Dulli DA, Stanko H, Levine RL. Atrial fibrillation is associated with severe acute ischemic stroke. Neuroepidemiology 2003; 22:118–123.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the national registry of atrial fibrillation. JAMA 2001; 285:2864–2870.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010; 137:263-272.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007; 146:857–867.
- Johnsen SP, Svendsen ML, Hansen ML, Brandes A, Mehnert F, Husted SE. Preadmission oral anticoagulant treatment and clinical outcome among patients hospitalized with acute stroke and atrial fibrillation: a nationwide study. Stroke 2014; 45:168-175.
- Baker WL, Cios DA, Sander SD, Coleman CI. Meta-analysis to assess the quality of warfarin control in atrial fibrillation patients in the united states. J Manage Care Pharm 2009; 15:244-252.
- Morgan CL, McEwan P, Tukiendorf A, Robinson PA, Clemens A, Plumb JM. Warfarin treatment in patients with atrial fibrillation: observing outcomes associated with varying levels of INR control. Thromb Res 2009; 124:37–41.
- Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: Stroke prevention in atrial fibrillation III randomised clinical trial. Lancet 1996; 348(9028):633–638.
- Flaker GC, Gruber M, Connolly SJ, et al. Risks and benefits of combining aspirin with anticoagulant therapy in patients with atrial fibrillation: an exploratory analysis of stroke prevention using an oral thrombin inhibitor in atrial fibrillation (SPORTIF) trials. Am Heart J 2006; 152:967–973.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al, and the RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011; 364:806–817.
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Garcia-Fernandez MA, Perez-David E, Quiles J, et al. Role of left atrial appendage obliteration in stroke reduction in patients with mitral valve prosthesis: a transesophageal echocardiographic study. J Am Coll Cardiol 2003; 42:1253–1258.
- Holmes DR, Reddy VY, Turi ZG, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 2009; 374(9689):534–542.
- Holmes DR Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: The PREVAIL trial. J Am Coll Cardiol 2014; 64:1–12.
- Reddy VY, Mobius-Winkler S, Miller MA, et al. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA plavix feasibility study with watchman left atrial appendage closure technology). J Am Coll Cardiol 2013; 61:2551–2556.
- Bartus K, Han FT, Bednarek J, et al. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol 2013; 62:108–118.
- Chatterjee S, Herrmann HC, Wilensky RL, et al. Safety and procedural success of left atrial appendage exclusion with the Lariat device: a systematic review of published reports and analytic review of the FDA MAUDE database. JAMA Intern Med 2015; 175(7):1104–9.
KEY POINTS
- Specific risk factor management is as important as anticoagulation when addressing stroke risk.
- The CHADS2 score has been superseded by the CHA2DS2-VASc score, which is more accurate for lower-risk categories.
- Anticoagulant options have increased substantially in the past few years with the introduction of novel oral anticoagulants, including the direct thrombin-inhibitor dabigatran and the factor Xa inhibitors rivaroxaban, apixaban, and edoxaban.
- Most atrial thrombi in patients with nonvalvular atrial fibrillation form in the left atrial appendage (LAA); nonpharmacologic interventions have been developed to block the LAA and reduce the risk of stroke.