User login
Heartburn or heart attack? A mimic of MI
A 71-year-old man with a history of hypertension, 4 prior myocardial infarctions (MIs), and well-compensated ischemic cardiomyopathy presented to the emergency department after 2 episodes of sharp pain in the left upper abdomen and chest. The episodes lasted 1 to 2 minutes and were not relieved by rest. Their location was similar to that of the pain he experienced with his MIs. He could not identify any exacerbating or ameliorating factors. The pain had resolved without specific therapy before he arrived.
He reported polydipsia and constipation over the past 2 weeks and generalized muscle weakness and acute exacerbations of chronic back pain in the past 2 days. Neither he nor a friend who accompanied him noticed any confusion. He had been taking as many as 15 calcium carbonate tablets a day for 6 weeks to self-treat dyspepsia refractory to once-daily ranitidine, and hydrochlorothiazide for his hypertension for 3 weeks.
FURTHER EVALUATION, CARDIOLOGY CONSULT
On physical examination, he had diffuse weakness, dry mucous membranes, and an irregular heart rhythm.
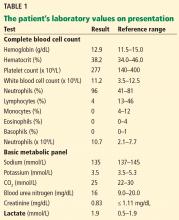
Laboratory testing showed the following:
- Troponin I 0.11 ng/mL (reference range ≤ 0.04); repeated, it was 0.12 ng/mL
- Serum creatinine 3.4 mg/dL (0.44–1.27) (9 months earlier it had been 0.99 mg/dL)
- Serum calcium 17.3 mg/dL (8.6–10.5)
- Parathyroid hormone 9 pg/mL (12–88)
- Serum bicarbonate 33 mmol/L (24–32); 2 weeks earlier, it had been 27 mmol/L.
DIAGNOSIS: MILK-ALKALI SYNDROME
The diagnosis, based on the presentation and the results of the workup, was milk-alkali syndrome complicated by recent hydrochlorothiazide use. This syndrome consists of the triad of hypercalcemia, metabolic alkalosis, and acute kidney injury, all due to excessive ingestion of calcium and alkali, usually calcium carbonate.
His hydrochlorothiazide and calcium carbonate were discontinued. He was given intravenous normal saline and subcutaneous calcitonin, and his serum calcium level came down to 11.5 mg/dL within the next 24 hours. His dyspepsia was treated with pantoprazole.
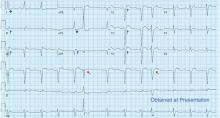
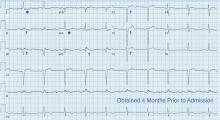
The patient had no further episodes of chest pain, and the cardiology consult team again recommended against coronary angiography. Repeat ECG after the hypercalcemia resolved showed results identical to those 4 months before his admission. Two months later, his serum calcium level was 9.4 mg/dL and his creatinine level was 1.24 mg/dL.
A MIMIC OF STEMI
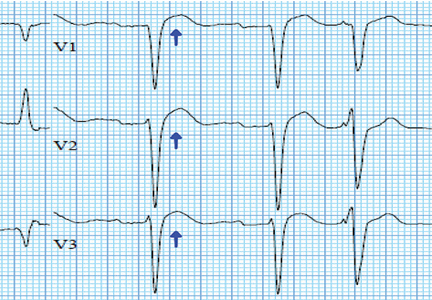
In numerous reported cases, these electrocardiographic findings coupled with chest pain led to misdiagnosis of STEMI.1–3 While STEMI and occasionally hypercalcemia can cause ST elevation, hypercalcemia causes a significant shortening of the corrected QT interval that is not associated with STEMI.4,5
Ultimately, the diagnosis of MI involves clinical, laboratory, and ECG findings, and if a strong clinical suspicion for myocardial ischemia exists, STEMI cannot reliably be distinguished from hypercalcemia by ECG alone. It is nonetheless important to be aware of this complication of hypercalcemia to avoid unnecessary cardiac interventions.
- Ashizawa N, Arakawa S, Koide Y, Toda G, Seto S, Yano K. Hypercalcemia due to vitamin D intoxication with clinical features mimicking acute myocardial infarction. Intern Med 2003; 42:340–344.
- Nishi SP, Barbagelata NA, Atar S, Birnbaum Y, Tuero E. Hypercalcemia-induced ST-segment elevation mimicking acute myocardial infarction. J Electrocardiol 2006; 39:298–300.
- Turnham S, Kilickap M, Kilinc S. ST segment elevation mimicking acute myocardial infarction in hypercalcemia. Heart 2005; 91:999.
- Nierenberg DW, Ransil BJ. Q-aTc interval as a clinical indicator of hypercalcemia. Am J Cardiol 1979; 44:243–248.
- Ahmed R, Hashiba K. Reliability of QT intervals as indicators of clinical hypercalcemia. Clin Cardiol 1988; 11:395–400.
A 71-year-old man with a history of hypertension, 4 prior myocardial infarctions (MIs), and well-compensated ischemic cardiomyopathy presented to the emergency department after 2 episodes of sharp pain in the left upper abdomen and chest. The episodes lasted 1 to 2 minutes and were not relieved by rest. Their location was similar to that of the pain he experienced with his MIs. He could not identify any exacerbating or ameliorating factors. The pain had resolved without specific therapy before he arrived.
He reported polydipsia and constipation over the past 2 weeks and generalized muscle weakness and acute exacerbations of chronic back pain in the past 2 days. Neither he nor a friend who accompanied him noticed any confusion. He had been taking as many as 15 calcium carbonate tablets a day for 6 weeks to self-treat dyspepsia refractory to once-daily ranitidine, and hydrochlorothiazide for his hypertension for 3 weeks.
FURTHER EVALUATION, CARDIOLOGY CONSULT
On physical examination, he had diffuse weakness, dry mucous membranes, and an irregular heart rhythm.
Laboratory testing showed the following:
- Troponin I 0.11 ng/mL (reference range ≤ 0.04); repeated, it was 0.12 ng/mL
- Serum creatinine 3.4 mg/dL (0.44–1.27) (9 months earlier it had been 0.99 mg/dL)
- Serum calcium 17.3 mg/dL (8.6–10.5)
- Parathyroid hormone 9 pg/mL (12–88)
- Serum bicarbonate 33 mmol/L (24–32); 2 weeks earlier, it had been 27 mmol/L.
DIAGNOSIS: MILK-ALKALI SYNDROME
The diagnosis, based on the presentation and the results of the workup, was milk-alkali syndrome complicated by recent hydrochlorothiazide use. This syndrome consists of the triad of hypercalcemia, metabolic alkalosis, and acute kidney injury, all due to excessive ingestion of calcium and alkali, usually calcium carbonate.
His hydrochlorothiazide and calcium carbonate were discontinued. He was given intravenous normal saline and subcutaneous calcitonin, and his serum calcium level came down to 11.5 mg/dL within the next 24 hours. His dyspepsia was treated with pantoprazole.
The patient had no further episodes of chest pain, and the cardiology consult team again recommended against coronary angiography. Repeat ECG after the hypercalcemia resolved showed results identical to those 4 months before his admission. Two months later, his serum calcium level was 9.4 mg/dL and his creatinine level was 1.24 mg/dL.
A MIMIC OF STEMI
In numerous reported cases, these electrocardiographic findings coupled with chest pain led to misdiagnosis of STEMI.1–3 While STEMI and occasionally hypercalcemia can cause ST elevation, hypercalcemia causes a significant shortening of the corrected QT interval that is not associated with STEMI.4,5
Ultimately, the diagnosis of MI involves clinical, laboratory, and ECG findings, and if a strong clinical suspicion for myocardial ischemia exists, STEMI cannot reliably be distinguished from hypercalcemia by ECG alone. It is nonetheless important to be aware of this complication of hypercalcemia to avoid unnecessary cardiac interventions.
A 71-year-old man with a history of hypertension, 4 prior myocardial infarctions (MIs), and well-compensated ischemic cardiomyopathy presented to the emergency department after 2 episodes of sharp pain in the left upper abdomen and chest. The episodes lasted 1 to 2 minutes and were not relieved by rest. Their location was similar to that of the pain he experienced with his MIs. He could not identify any exacerbating or ameliorating factors. The pain had resolved without specific therapy before he arrived.
He reported polydipsia and constipation over the past 2 weeks and generalized muscle weakness and acute exacerbations of chronic back pain in the past 2 days. Neither he nor a friend who accompanied him noticed any confusion. He had been taking as many as 15 calcium carbonate tablets a day for 6 weeks to self-treat dyspepsia refractory to once-daily ranitidine, and hydrochlorothiazide for his hypertension for 3 weeks.
FURTHER EVALUATION, CARDIOLOGY CONSULT
On physical examination, he had diffuse weakness, dry mucous membranes, and an irregular heart rhythm.
Laboratory testing showed the following:
- Troponin I 0.11 ng/mL (reference range ≤ 0.04); repeated, it was 0.12 ng/mL
- Serum creatinine 3.4 mg/dL (0.44–1.27) (9 months earlier it had been 0.99 mg/dL)
- Serum calcium 17.3 mg/dL (8.6–10.5)
- Parathyroid hormone 9 pg/mL (12–88)
- Serum bicarbonate 33 mmol/L (24–32); 2 weeks earlier, it had been 27 mmol/L.
DIAGNOSIS: MILK-ALKALI SYNDROME
The diagnosis, based on the presentation and the results of the workup, was milk-alkali syndrome complicated by recent hydrochlorothiazide use. This syndrome consists of the triad of hypercalcemia, metabolic alkalosis, and acute kidney injury, all due to excessive ingestion of calcium and alkali, usually calcium carbonate.
His hydrochlorothiazide and calcium carbonate were discontinued. He was given intravenous normal saline and subcutaneous calcitonin, and his serum calcium level came down to 11.5 mg/dL within the next 24 hours. His dyspepsia was treated with pantoprazole.
The patient had no further episodes of chest pain, and the cardiology consult team again recommended against coronary angiography. Repeat ECG after the hypercalcemia resolved showed results identical to those 4 months before his admission. Two months later, his serum calcium level was 9.4 mg/dL and his creatinine level was 1.24 mg/dL.
A MIMIC OF STEMI
In numerous reported cases, these electrocardiographic findings coupled with chest pain led to misdiagnosis of STEMI.1–3 While STEMI and occasionally hypercalcemia can cause ST elevation, hypercalcemia causes a significant shortening of the corrected QT interval that is not associated with STEMI.4,5
Ultimately, the diagnosis of MI involves clinical, laboratory, and ECG findings, and if a strong clinical suspicion for myocardial ischemia exists, STEMI cannot reliably be distinguished from hypercalcemia by ECG alone. It is nonetheless important to be aware of this complication of hypercalcemia to avoid unnecessary cardiac interventions.
- Ashizawa N, Arakawa S, Koide Y, Toda G, Seto S, Yano K. Hypercalcemia due to vitamin D intoxication with clinical features mimicking acute myocardial infarction. Intern Med 2003; 42:340–344.
- Nishi SP, Barbagelata NA, Atar S, Birnbaum Y, Tuero E. Hypercalcemia-induced ST-segment elevation mimicking acute myocardial infarction. J Electrocardiol 2006; 39:298–300.
- Turnham S, Kilickap M, Kilinc S. ST segment elevation mimicking acute myocardial infarction in hypercalcemia. Heart 2005; 91:999.
- Nierenberg DW, Ransil BJ. Q-aTc interval as a clinical indicator of hypercalcemia. Am J Cardiol 1979; 44:243–248.
- Ahmed R, Hashiba K. Reliability of QT intervals as indicators of clinical hypercalcemia. Clin Cardiol 1988; 11:395–400.
- Ashizawa N, Arakawa S, Koide Y, Toda G, Seto S, Yano K. Hypercalcemia due to vitamin D intoxication with clinical features mimicking acute myocardial infarction. Intern Med 2003; 42:340–344.
- Nishi SP, Barbagelata NA, Atar S, Birnbaum Y, Tuero E. Hypercalcemia-induced ST-segment elevation mimicking acute myocardial infarction. J Electrocardiol 2006; 39:298–300.
- Turnham S, Kilickap M, Kilinc S. ST segment elevation mimicking acute myocardial infarction in hypercalcemia. Heart 2005; 91:999.
- Nierenberg DW, Ransil BJ. Q-aTc interval as a clinical indicator of hypercalcemia. Am J Cardiol 1979; 44:243–248.
- Ahmed R, Hashiba K. Reliability of QT intervals as indicators of clinical hypercalcemia. Clin Cardiol 1988; 11:395–400.
Man’s best friend, fatal in the end
A previously healthy 59-year-old woman with a remote history of splenectomy following a motor vehicle accident presented to the emergency department with a chief complaint of fever. She had been in her usual state of health until the day before, when she developed chills and fever, with temperatures as high as 39.4°C (102.9°F). She also began to have nausea, vomiting, and diffuse body weakness and had to be brought to the emergency department in a wheelchair. She denied upper-respiratory or urinary symptoms, headache, stiff neck, recent travel, or sick contacts.
She had sustained a minor dog bite on her right hand 2 days before, but she denied swelling, erythema, or exudate. The dog, a family pet, was up to date on all of its vaccinations, including rabies.
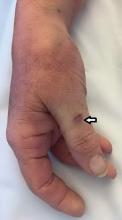
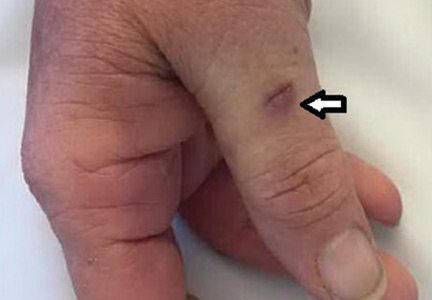
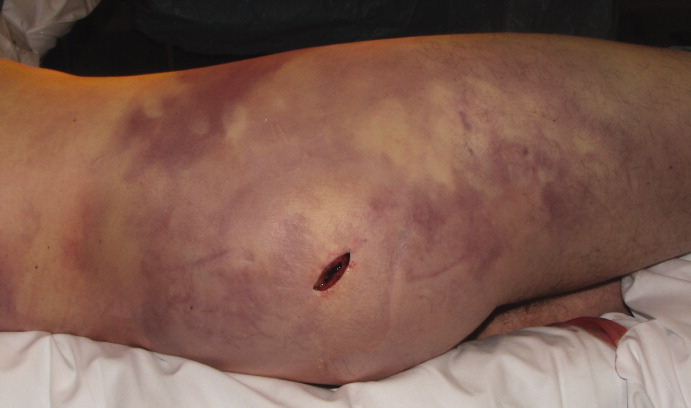
Her temperature was 39.3°C (102.7°F), heart rate 121 beats per minute, and blood pressure 113/71 mm Hg. She had a clean, nonerythematous, healing, 1-cm laceration on her right thumb (Figure 1).
Initial laboratory values (Table 1) and a radiograph of her right thumb were unremarkable.
FEVER IN ASPLENIC PATIENTS
1. What is the appropriate next step in this patient’s management?
- Discharge her from the emergency department and have her follow up with her primary care physician within 48 hours
- Admit her for observation and defer antibiotic therapy
- Admit her and start empiric antibiotic therapy
- Admit but wait for culture results to come back before starting antibiotic therapy
The patient’s history of splenectomy and presentation with fever raise the concern that she may be going into sepsis. In addition to fever, patients with sepsis may present with flulike symptoms such as myalgias, headache, vomiting, diarrhea, and abdominal pain.1
Sepsis in asplenic patients, also known as overwhelming postsplenectomy infection, can have a sudden onset and fulminant course, with a mortality rate as high as 50%.2 It is important to recognize those who are susceptible, including patients without a spleen from splenectomy or congenital asplenia, as well as those with functional asplenia from diseases such as sickle cell disease. Without the spleen, the immune system cannot clear immunoglobulin G-coated bacteria and encapsulated bacteria that are not opsonized by antibodies or complement.3
Any asplenic patient presenting with fever or other symptoms of systemic infection warrants immediate antibiotic treatment, without delay for cultures or further testing.1
CASE CONTINUED: RAPID DETERIORATION
With no clear source of infection, the patient’s clinical presentation was presumed to be due to a viral infection, and antibiotics were deferred. She was admitted to the hospital for observation.
By the next morning, her mental status had declined. Her temperature at that time was 39.6°C (103.2°F), heart rate 115 per minute, and blood pressure 113/74 mm Hg. Her skin became mottled, and her lactate level increased from 1.9 mmol/L to 4.9 mmol/L (reference range 0.5–1.9 mmol/L) within 9 hours and continued to climb (Table 2).
EMPIRIC ANTIBIOTICS IN ASPLENIC SEPSIS
2. Which first-line antibiotics should have been started on initial presentation?
- Intravenous vancomycin and intravenous ceftriaxone
- Intravenous vancomycin and intravenous metronidazole
- Oral levofloxacin
- Oral amoxicillin
At initial presentation to the hospital, the most appropriate regimen for this patient would have been vancomycin and ceftriaxone or cefepime in meningitis-level (ie, high) doses.2,4
Due to impaired immunity, asplenic patients are highly susceptible to encapsulated gram-positive organisms such as Streptococcus pneumoniae and gram-negative organisms such as Haemophilus influenzae, Neisseria meningitidis, and Capnocytophaga canimorsus. These organisms are all susceptible to ceftriaxone, with the exception of methicillin-resistant S pneumoniae, which is best covered with vancomycin.1 Patients with beta-lactam hypersensitivity can be treated with moxifloxacin instead.4,5
Vancomycin and metronidazole alone would not be adequate. Oral levofloxacin or amoxicillin would be appropriate initial treatment if the patient did not have access to a hospital within 2 hours. Ideally, the patient would have had one of these medications on hand and taken it at the first sign of fever.4
CASE CONTINUED: TRANSFER TO ICU
The patient was empirically started on vancomycin and ceftriaxone and transferred to the intensive care unit. She required intubation for airway protection. She became hypotensive despite receiving intravenous fluids and multiple vasopressors. She continued to rapidly decline and developed lactic acidosis, which resulted in a severe anion gap metabolic acidosis with respiratory compensation. Her course was further complicated by disseminated intravascular coagulation, acute kidney failure, and ischemic hepatitis (“shock liver”) (Table 2).
CAUSES OF SEPSIS IN ASPLENIC PATIENTS
3. The patient’s septic shock is likely the result of which bacterial pathogen?
- S pneumoniae
- H influenzae
- C canimorsus
- N meningitidis
Encapsulated organisms including S pneumoniae, H influenzae, and N meningitidis account for almost 70% of infections in postsplenectomy patients, including those with overwhelming postsplenectomy infection.6 S pneumoniae is the most common culprit. However, the patient’s history of a recent dog bite suggests that the most likely cause was C canimorsus.
C canimorsus is a gram-negative bacillus commonly associated with exposure to dogs or cats through saliva, scratches, or bites.7,8 Even a seemingly small, benign-appearing wound, as seen in this case, can be a portal of entry for this organism. About 84 cases leading to fulminant sepsis were reported in the United States from 1990 to 2014.9 Patients infected with this organism can progress to fulminant sepsis with multiorgan failure with disseminated intravascular coagulation, anuria, and hypotension.10–12
CASE CONCLUDED
The patient died 40 hours after admission. Her blood cultures grew a slow-growing gram-negative rod within 2 days, subsequently identified as C canimorsus.
4. What is the best strategy for prevention of sepsis in an asplenic patient?
- Vaccinate against S pneumoniae (with PCV13 and PPSV23), H influenzae type b, and N meningitidis
- Prescribe antibiotics that the patient can take in case of fever
- Both of the above
- Prescribe lifelong daily antibiotic prophylaxis
- All of the above
Asplenic patients should receive pneumococcal, H influenzae type b, and meningococcal vaccines.13 Invasive bacterial infections, particularly with encapsulated organisms, occur 10 to 50 times more often in this population than in a healthy population and can be fatal.13 These vaccines have been shown to reduce the rate of life-threatening infections. Patients should receive the vaccines at least 2 weeks before an elective splenectomy or 2 weeks after a nonelective splenectomy.2
For the pneumococcal vaccines, PCV13 should be given first, followed by PPSV23 at least 8 weeks later. If the patient has already received PCV13, PPSV23 should be given at least 2 weeks after splenectomy. A second dose of PPSV23 should be given 5 years later.
The H influenzae type b vaccine should be administered if not already given.
For the meningococcal vaccine, the two-dose series should be administered with an interval of 8 to 12 weeks between doses. A booster meningococcal dose should be given every 5 years.
The patient should also receive the flu vaccine annually.2,14
Patients should also be given antibiotics (typically an antibiotic with activity against S pneumoniae, such as amoxicillin or levofloxacin) to carry with them. They should be told to take them if fever or chills develop and they cannot see a physician within 2 hours.2
Daily antibiotic prophylaxis with penicillin is typically given to patients younger than age 5, as studies have shown benefit in reducing pneumococcal sepsis. In adults, some experts recommend daily antibiotic prophylaxis for 1 year after splenectomy.2 However, there is a lack of data and expert consensus to recommend lifelong daily antibiotic prophylaxis for all asplenic patients. Thus, it is not recommended in adults unless the patient is immunocompromised or is a survivor of pneumococcal sepsis.4
KEY POINTS
- In an asplenic patient, fever can be an early sign of sepsis, which can have a rapid and fulminant course.
- Asplenic patients are particularly susceptible to infection by encapsulated organisms such as S pneumoniae, H influenzae, N meningitidis, and C canimorsus due to impaired immunity.
- If an asplenic patient has been exposed to a dog bite, scratch, or saliva, one should suspect C canimorsus.
- Asplenic patients who present with fever should be treated immediately with intravenous vancomycin and ceftriaxone without delay for laboratory tests or imaging.
- To help prevent fulminant sepsis, asplenic patients should receive vaccines (pneumococcal, meningococcal, and H influenzae type b) as well as a prescription for antibiotics (levofloxacin) to be used if they develop fever and cannot see a physician within 2 hours.
- Brigden ML. Detection, education and management of the asplenic or hyposplenic patient. Am Fam Physician 2001; 63:499–508.
- Rubin LG, Schaffner W. Clinical practice. Care of the asplenic patient. N Engl J Med 2014; 371:349–356.
- Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet 2011; 378:86–97.
- Brigden ML, Pattullo AL. Prevention and management of overwhelming postsplenectomy infection—an update. Crit Care Med 1999; 27:836–842.
- Lynch AM, Kapila R. Overwhelming postsplenectomy infection. Infect Dis Clin North Am 1996; 10:693–707.
- Kuchar E, Miskiewicz K, Karlikowska M. A review of guidance on immunization in persons with defective or deficient splenic function. Br J Haematol 2015; 171:683–694.
- Le Moal G, Landron C, Grollier G, Robert R, Burucoa C. Meningitis due to Capnocytophaga canimorsus after receipt of a dog bite: case report and review of the literature. Clin Infect Dis 2003; 36:e42–e46.
- Lion C, Escande F, Burdin JC. Capnocytophaga canimorsus infections in human: review of the literature and cases report. Eur J Epidemiol 1996; 12:521–533.
- Butler T. Capnocytophaga canimorsus: an emerging cause of sepsis, meningitis, and post-splenectomy infection after dog bites. Eur J Clin Microbiol Infect Dis 2015; 34:1271–1280.
- Pers C, Gahrn-Hansen B, Frederiksen W. Capnocytophaga canimorsus septicemia in Denmark, 1982-1995: review of 39 cases. Clin Infect Dis 1996; 23:71–75.
- Chiappa V, Chang CY, Sellas MI, Pierce VM, Kradin RL. Case records of the Massachusetts General Hospital. Case 10-2014. A 45-year-old man with a rash. N Engl J Med 2014; 370:1238–1248.
- Martone WJ, Zuehl RW, Minson GE, Scheld WM. Postsplenectomy sepsis with DF-2: report of a case with isolation of the organism from the patient’s dog. Ann Intern Med 1980; 93:457–458.
- Centers for Disease Control and Prevention (CDC). Asplenia and adult vaccination. www.cdc.gov/vaccines/adults/rec-vac/health-conditions/asplenia.html. Accessed January 6, 2017.
- Rubin LG, Levin MJ, Ljungman P, et al; Infectious Diseases Society of America. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014; 58:309–318.
A previously healthy 59-year-old woman with a remote history of splenectomy following a motor vehicle accident presented to the emergency department with a chief complaint of fever. She had been in her usual state of health until the day before, when she developed chills and fever, with temperatures as high as 39.4°C (102.9°F). She also began to have nausea, vomiting, and diffuse body weakness and had to be brought to the emergency department in a wheelchair. She denied upper-respiratory or urinary symptoms, headache, stiff neck, recent travel, or sick contacts.
She had sustained a minor dog bite on her right hand 2 days before, but she denied swelling, erythema, or exudate. The dog, a family pet, was up to date on all of its vaccinations, including rabies.
Her temperature was 39.3°C (102.7°F), heart rate 121 beats per minute, and blood pressure 113/71 mm Hg. She had a clean, nonerythematous, healing, 1-cm laceration on her right thumb (Figure 1).
Initial laboratory values (Table 1) and a radiograph of her right thumb were unremarkable.
FEVER IN ASPLENIC PATIENTS
1. What is the appropriate next step in this patient’s management?
- Discharge her from the emergency department and have her follow up with her primary care physician within 48 hours
- Admit her for observation and defer antibiotic therapy
- Admit her and start empiric antibiotic therapy
- Admit but wait for culture results to come back before starting antibiotic therapy
The patient’s history of splenectomy and presentation with fever raise the concern that she may be going into sepsis. In addition to fever, patients with sepsis may present with flulike symptoms such as myalgias, headache, vomiting, diarrhea, and abdominal pain.1
Sepsis in asplenic patients, also known as overwhelming postsplenectomy infection, can have a sudden onset and fulminant course, with a mortality rate as high as 50%.2 It is important to recognize those who are susceptible, including patients without a spleen from splenectomy or congenital asplenia, as well as those with functional asplenia from diseases such as sickle cell disease. Without the spleen, the immune system cannot clear immunoglobulin G-coated bacteria and encapsulated bacteria that are not opsonized by antibodies or complement.3
Any asplenic patient presenting with fever or other symptoms of systemic infection warrants immediate antibiotic treatment, without delay for cultures or further testing.1
CASE CONTINUED: RAPID DETERIORATION
With no clear source of infection, the patient’s clinical presentation was presumed to be due to a viral infection, and antibiotics were deferred. She was admitted to the hospital for observation.
By the next morning, her mental status had declined. Her temperature at that time was 39.6°C (103.2°F), heart rate 115 per minute, and blood pressure 113/74 mm Hg. Her skin became mottled, and her lactate level increased from 1.9 mmol/L to 4.9 mmol/L (reference range 0.5–1.9 mmol/L) within 9 hours and continued to climb (Table 2).
EMPIRIC ANTIBIOTICS IN ASPLENIC SEPSIS
2. Which first-line antibiotics should have been started on initial presentation?
- Intravenous vancomycin and intravenous ceftriaxone
- Intravenous vancomycin and intravenous metronidazole
- Oral levofloxacin
- Oral amoxicillin
At initial presentation to the hospital, the most appropriate regimen for this patient would have been vancomycin and ceftriaxone or cefepime in meningitis-level (ie, high) doses.2,4
Due to impaired immunity, asplenic patients are highly susceptible to encapsulated gram-positive organisms such as Streptococcus pneumoniae and gram-negative organisms such as Haemophilus influenzae, Neisseria meningitidis, and Capnocytophaga canimorsus. These organisms are all susceptible to ceftriaxone, with the exception of methicillin-resistant S pneumoniae, which is best covered with vancomycin.1 Patients with beta-lactam hypersensitivity can be treated with moxifloxacin instead.4,5
Vancomycin and metronidazole alone would not be adequate. Oral levofloxacin or amoxicillin would be appropriate initial treatment if the patient did not have access to a hospital within 2 hours. Ideally, the patient would have had one of these medications on hand and taken it at the first sign of fever.4
CASE CONTINUED: TRANSFER TO ICU
The patient was empirically started on vancomycin and ceftriaxone and transferred to the intensive care unit. She required intubation for airway protection. She became hypotensive despite receiving intravenous fluids and multiple vasopressors. She continued to rapidly decline and developed lactic acidosis, which resulted in a severe anion gap metabolic acidosis with respiratory compensation. Her course was further complicated by disseminated intravascular coagulation, acute kidney failure, and ischemic hepatitis (“shock liver”) (Table 2).
CAUSES OF SEPSIS IN ASPLENIC PATIENTS
3. The patient’s septic shock is likely the result of which bacterial pathogen?
- S pneumoniae
- H influenzae
- C canimorsus
- N meningitidis
Encapsulated organisms including S pneumoniae, H influenzae, and N meningitidis account for almost 70% of infections in postsplenectomy patients, including those with overwhelming postsplenectomy infection.6 S pneumoniae is the most common culprit. However, the patient’s history of a recent dog bite suggests that the most likely cause was C canimorsus.
C canimorsus is a gram-negative bacillus commonly associated with exposure to dogs or cats through saliva, scratches, or bites.7,8 Even a seemingly small, benign-appearing wound, as seen in this case, can be a portal of entry for this organism. About 84 cases leading to fulminant sepsis were reported in the United States from 1990 to 2014.9 Patients infected with this organism can progress to fulminant sepsis with multiorgan failure with disseminated intravascular coagulation, anuria, and hypotension.10–12
CASE CONCLUDED
The patient died 40 hours after admission. Her blood cultures grew a slow-growing gram-negative rod within 2 days, subsequently identified as C canimorsus.
4. What is the best strategy for prevention of sepsis in an asplenic patient?
- Vaccinate against S pneumoniae (with PCV13 and PPSV23), H influenzae type b, and N meningitidis
- Prescribe antibiotics that the patient can take in case of fever
- Both of the above
- Prescribe lifelong daily antibiotic prophylaxis
- All of the above
Asplenic patients should receive pneumococcal, H influenzae type b, and meningococcal vaccines.13 Invasive bacterial infections, particularly with encapsulated organisms, occur 10 to 50 times more often in this population than in a healthy population and can be fatal.13 These vaccines have been shown to reduce the rate of life-threatening infections. Patients should receive the vaccines at least 2 weeks before an elective splenectomy or 2 weeks after a nonelective splenectomy.2
For the pneumococcal vaccines, PCV13 should be given first, followed by PPSV23 at least 8 weeks later. If the patient has already received PCV13, PPSV23 should be given at least 2 weeks after splenectomy. A second dose of PPSV23 should be given 5 years later.
The H influenzae type b vaccine should be administered if not already given.
For the meningococcal vaccine, the two-dose series should be administered with an interval of 8 to 12 weeks between doses. A booster meningococcal dose should be given every 5 years.
The patient should also receive the flu vaccine annually.2,14
Patients should also be given antibiotics (typically an antibiotic with activity against S pneumoniae, such as amoxicillin or levofloxacin) to carry with them. They should be told to take them if fever or chills develop and they cannot see a physician within 2 hours.2
Daily antibiotic prophylaxis with penicillin is typically given to patients younger than age 5, as studies have shown benefit in reducing pneumococcal sepsis. In adults, some experts recommend daily antibiotic prophylaxis for 1 year after splenectomy.2 However, there is a lack of data and expert consensus to recommend lifelong daily antibiotic prophylaxis for all asplenic patients. Thus, it is not recommended in adults unless the patient is immunocompromised or is a survivor of pneumococcal sepsis.4
KEY POINTS
- In an asplenic patient, fever can be an early sign of sepsis, which can have a rapid and fulminant course.
- Asplenic patients are particularly susceptible to infection by encapsulated organisms such as S pneumoniae, H influenzae, N meningitidis, and C canimorsus due to impaired immunity.
- If an asplenic patient has been exposed to a dog bite, scratch, or saliva, one should suspect C canimorsus.
- Asplenic patients who present with fever should be treated immediately with intravenous vancomycin and ceftriaxone without delay for laboratory tests or imaging.
- To help prevent fulminant sepsis, asplenic patients should receive vaccines (pneumococcal, meningococcal, and H influenzae type b) as well as a prescription for antibiotics (levofloxacin) to be used if they develop fever and cannot see a physician within 2 hours.
A previously healthy 59-year-old woman with a remote history of splenectomy following a motor vehicle accident presented to the emergency department with a chief complaint of fever. She had been in her usual state of health until the day before, when she developed chills and fever, with temperatures as high as 39.4°C (102.9°F). She also began to have nausea, vomiting, and diffuse body weakness and had to be brought to the emergency department in a wheelchair. She denied upper-respiratory or urinary symptoms, headache, stiff neck, recent travel, or sick contacts.
She had sustained a minor dog bite on her right hand 2 days before, but she denied swelling, erythema, or exudate. The dog, a family pet, was up to date on all of its vaccinations, including rabies.
Her temperature was 39.3°C (102.7°F), heart rate 121 beats per minute, and blood pressure 113/71 mm Hg. She had a clean, nonerythematous, healing, 1-cm laceration on her right thumb (Figure 1).
Initial laboratory values (Table 1) and a radiograph of her right thumb were unremarkable.
FEVER IN ASPLENIC PATIENTS
1. What is the appropriate next step in this patient’s management?
- Discharge her from the emergency department and have her follow up with her primary care physician within 48 hours
- Admit her for observation and defer antibiotic therapy
- Admit her and start empiric antibiotic therapy
- Admit but wait for culture results to come back before starting antibiotic therapy
The patient’s history of splenectomy and presentation with fever raise the concern that she may be going into sepsis. In addition to fever, patients with sepsis may present with flulike symptoms such as myalgias, headache, vomiting, diarrhea, and abdominal pain.1
Sepsis in asplenic patients, also known as overwhelming postsplenectomy infection, can have a sudden onset and fulminant course, with a mortality rate as high as 50%.2 It is important to recognize those who are susceptible, including patients without a spleen from splenectomy or congenital asplenia, as well as those with functional asplenia from diseases such as sickle cell disease. Without the spleen, the immune system cannot clear immunoglobulin G-coated bacteria and encapsulated bacteria that are not opsonized by antibodies or complement.3
Any asplenic patient presenting with fever or other symptoms of systemic infection warrants immediate antibiotic treatment, without delay for cultures or further testing.1
CASE CONTINUED: RAPID DETERIORATION
With no clear source of infection, the patient’s clinical presentation was presumed to be due to a viral infection, and antibiotics were deferred. She was admitted to the hospital for observation.
By the next morning, her mental status had declined. Her temperature at that time was 39.6°C (103.2°F), heart rate 115 per minute, and blood pressure 113/74 mm Hg. Her skin became mottled, and her lactate level increased from 1.9 mmol/L to 4.9 mmol/L (reference range 0.5–1.9 mmol/L) within 9 hours and continued to climb (Table 2).
EMPIRIC ANTIBIOTICS IN ASPLENIC SEPSIS
2. Which first-line antibiotics should have been started on initial presentation?
- Intravenous vancomycin and intravenous ceftriaxone
- Intravenous vancomycin and intravenous metronidazole
- Oral levofloxacin
- Oral amoxicillin
At initial presentation to the hospital, the most appropriate regimen for this patient would have been vancomycin and ceftriaxone or cefepime in meningitis-level (ie, high) doses.2,4
Due to impaired immunity, asplenic patients are highly susceptible to encapsulated gram-positive organisms such as Streptococcus pneumoniae and gram-negative organisms such as Haemophilus influenzae, Neisseria meningitidis, and Capnocytophaga canimorsus. These organisms are all susceptible to ceftriaxone, with the exception of methicillin-resistant S pneumoniae, which is best covered with vancomycin.1 Patients with beta-lactam hypersensitivity can be treated with moxifloxacin instead.4,5
Vancomycin and metronidazole alone would not be adequate. Oral levofloxacin or amoxicillin would be appropriate initial treatment if the patient did not have access to a hospital within 2 hours. Ideally, the patient would have had one of these medications on hand and taken it at the first sign of fever.4
CASE CONTINUED: TRANSFER TO ICU
The patient was empirically started on vancomycin and ceftriaxone and transferred to the intensive care unit. She required intubation for airway protection. She became hypotensive despite receiving intravenous fluids and multiple vasopressors. She continued to rapidly decline and developed lactic acidosis, which resulted in a severe anion gap metabolic acidosis with respiratory compensation. Her course was further complicated by disseminated intravascular coagulation, acute kidney failure, and ischemic hepatitis (“shock liver”) (Table 2).
CAUSES OF SEPSIS IN ASPLENIC PATIENTS
3. The patient’s septic shock is likely the result of which bacterial pathogen?
- S pneumoniae
- H influenzae
- C canimorsus
- N meningitidis
Encapsulated organisms including S pneumoniae, H influenzae, and N meningitidis account for almost 70% of infections in postsplenectomy patients, including those with overwhelming postsplenectomy infection.6 S pneumoniae is the most common culprit. However, the patient’s history of a recent dog bite suggests that the most likely cause was C canimorsus.
C canimorsus is a gram-negative bacillus commonly associated with exposure to dogs or cats through saliva, scratches, or bites.7,8 Even a seemingly small, benign-appearing wound, as seen in this case, can be a portal of entry for this organism. About 84 cases leading to fulminant sepsis were reported in the United States from 1990 to 2014.9 Patients infected with this organism can progress to fulminant sepsis with multiorgan failure with disseminated intravascular coagulation, anuria, and hypotension.10–12
CASE CONCLUDED
The patient died 40 hours after admission. Her blood cultures grew a slow-growing gram-negative rod within 2 days, subsequently identified as C canimorsus.
4. What is the best strategy for prevention of sepsis in an asplenic patient?
- Vaccinate against S pneumoniae (with PCV13 and PPSV23), H influenzae type b, and N meningitidis
- Prescribe antibiotics that the patient can take in case of fever
- Both of the above
- Prescribe lifelong daily antibiotic prophylaxis
- All of the above
Asplenic patients should receive pneumococcal, H influenzae type b, and meningococcal vaccines.13 Invasive bacterial infections, particularly with encapsulated organisms, occur 10 to 50 times more often in this population than in a healthy population and can be fatal.13 These vaccines have been shown to reduce the rate of life-threatening infections. Patients should receive the vaccines at least 2 weeks before an elective splenectomy or 2 weeks after a nonelective splenectomy.2
For the pneumococcal vaccines, PCV13 should be given first, followed by PPSV23 at least 8 weeks later. If the patient has already received PCV13, PPSV23 should be given at least 2 weeks after splenectomy. A second dose of PPSV23 should be given 5 years later.
The H influenzae type b vaccine should be administered if not already given.
For the meningococcal vaccine, the two-dose series should be administered with an interval of 8 to 12 weeks between doses. A booster meningococcal dose should be given every 5 years.
The patient should also receive the flu vaccine annually.2,14
Patients should also be given antibiotics (typically an antibiotic with activity against S pneumoniae, such as amoxicillin or levofloxacin) to carry with them. They should be told to take them if fever or chills develop and they cannot see a physician within 2 hours.2
Daily antibiotic prophylaxis with penicillin is typically given to patients younger than age 5, as studies have shown benefit in reducing pneumococcal sepsis. In adults, some experts recommend daily antibiotic prophylaxis for 1 year after splenectomy.2 However, there is a lack of data and expert consensus to recommend lifelong daily antibiotic prophylaxis for all asplenic patients. Thus, it is not recommended in adults unless the patient is immunocompromised or is a survivor of pneumococcal sepsis.4
KEY POINTS
- In an asplenic patient, fever can be an early sign of sepsis, which can have a rapid and fulminant course.
- Asplenic patients are particularly susceptible to infection by encapsulated organisms such as S pneumoniae, H influenzae, N meningitidis, and C canimorsus due to impaired immunity.
- If an asplenic patient has been exposed to a dog bite, scratch, or saliva, one should suspect C canimorsus.
- Asplenic patients who present with fever should be treated immediately with intravenous vancomycin and ceftriaxone without delay for laboratory tests or imaging.
- To help prevent fulminant sepsis, asplenic patients should receive vaccines (pneumococcal, meningococcal, and H influenzae type b) as well as a prescription for antibiotics (levofloxacin) to be used if they develop fever and cannot see a physician within 2 hours.
- Brigden ML. Detection, education and management of the asplenic or hyposplenic patient. Am Fam Physician 2001; 63:499–508.
- Rubin LG, Schaffner W. Clinical practice. Care of the asplenic patient. N Engl J Med 2014; 371:349–356.
- Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet 2011; 378:86–97.
- Brigden ML, Pattullo AL. Prevention and management of overwhelming postsplenectomy infection—an update. Crit Care Med 1999; 27:836–842.
- Lynch AM, Kapila R. Overwhelming postsplenectomy infection. Infect Dis Clin North Am 1996; 10:693–707.
- Kuchar E, Miskiewicz K, Karlikowska M. A review of guidance on immunization in persons with defective or deficient splenic function. Br J Haematol 2015; 171:683–694.
- Le Moal G, Landron C, Grollier G, Robert R, Burucoa C. Meningitis due to Capnocytophaga canimorsus after receipt of a dog bite: case report and review of the literature. Clin Infect Dis 2003; 36:e42–e46.
- Lion C, Escande F, Burdin JC. Capnocytophaga canimorsus infections in human: review of the literature and cases report. Eur J Epidemiol 1996; 12:521–533.
- Butler T. Capnocytophaga canimorsus: an emerging cause of sepsis, meningitis, and post-splenectomy infection after dog bites. Eur J Clin Microbiol Infect Dis 2015; 34:1271–1280.
- Pers C, Gahrn-Hansen B, Frederiksen W. Capnocytophaga canimorsus septicemia in Denmark, 1982-1995: review of 39 cases. Clin Infect Dis 1996; 23:71–75.
- Chiappa V, Chang CY, Sellas MI, Pierce VM, Kradin RL. Case records of the Massachusetts General Hospital. Case 10-2014. A 45-year-old man with a rash. N Engl J Med 2014; 370:1238–1248.
- Martone WJ, Zuehl RW, Minson GE, Scheld WM. Postsplenectomy sepsis with DF-2: report of a case with isolation of the organism from the patient’s dog. Ann Intern Med 1980; 93:457–458.
- Centers for Disease Control and Prevention (CDC). Asplenia and adult vaccination. www.cdc.gov/vaccines/adults/rec-vac/health-conditions/asplenia.html. Accessed January 6, 2017.
- Rubin LG, Levin MJ, Ljungman P, et al; Infectious Diseases Society of America. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014; 58:309–318.
- Brigden ML. Detection, education and management of the asplenic or hyposplenic patient. Am Fam Physician 2001; 63:499–508.
- Rubin LG, Schaffner W. Clinical practice. Care of the asplenic patient. N Engl J Med 2014; 371:349–356.
- Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet 2011; 378:86–97.
- Brigden ML, Pattullo AL. Prevention and management of overwhelming postsplenectomy infection—an update. Crit Care Med 1999; 27:836–842.
- Lynch AM, Kapila R. Overwhelming postsplenectomy infection. Infect Dis Clin North Am 1996; 10:693–707.
- Kuchar E, Miskiewicz K, Karlikowska M. A review of guidance on immunization in persons with defective or deficient splenic function. Br J Haematol 2015; 171:683–694.
- Le Moal G, Landron C, Grollier G, Robert R, Burucoa C. Meningitis due to Capnocytophaga canimorsus after receipt of a dog bite: case report and review of the literature. Clin Infect Dis 2003; 36:e42–e46.
- Lion C, Escande F, Burdin JC. Capnocytophaga canimorsus infections in human: review of the literature and cases report. Eur J Epidemiol 1996; 12:521–533.
- Butler T. Capnocytophaga canimorsus: an emerging cause of sepsis, meningitis, and post-splenectomy infection after dog bites. Eur J Clin Microbiol Infect Dis 2015; 34:1271–1280.
- Pers C, Gahrn-Hansen B, Frederiksen W. Capnocytophaga canimorsus septicemia in Denmark, 1982-1995: review of 39 cases. Clin Infect Dis 1996; 23:71–75.
- Chiappa V, Chang CY, Sellas MI, Pierce VM, Kradin RL. Case records of the Massachusetts General Hospital. Case 10-2014. A 45-year-old man with a rash. N Engl J Med 2014; 370:1238–1248.
- Martone WJ, Zuehl RW, Minson GE, Scheld WM. Postsplenectomy sepsis with DF-2: report of a case with isolation of the organism from the patient’s dog. Ann Intern Med 1980; 93:457–458.
- Centers for Disease Control and Prevention (CDC). Asplenia and adult vaccination. www.cdc.gov/vaccines/adults/rec-vac/health-conditions/asplenia.html. Accessed January 6, 2017.
- Rubin LG, Levin MJ, Ljungman P, et al; Infectious Diseases Society of America. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014; 58:309–318.
Retroperitoneal cyst hemorrhage in polycystic kidney disease
A 59-year-old man with autosomal dominant polycystic kidney disease (ADPKD), end-stage renal disease on hemodialysis, hypertension, and diverticulosis presented with acute pain in the left lower abdomen. The pain began 4 days previously, was dull and nonradiating, was relieved partially with hydrocodone-acetaminophen, and had no clear exacerbating factors. Two days before presentation, he developed a fever with chills. He reported no recent dysuria, diarrhea, hematuria, hematochezia, or melena. He had not been taking anticoagulants or nonsteroidal anti-inflammatory drugs, and he had no history of heavy lifting or trauma.
His temperature was 38.5˚C (101.3˚F), blood pressure 141/60 mm Hg (normal for this patient). On examination, his left lower quadrant was tender with voluntary guarding. Also present was a reducible ventral hernia, which was not new.
His hemoglobin level was 10.6 g/dL (reference range 13.0–17.0), which had dropped from a previous value of 13.7 g/dL.
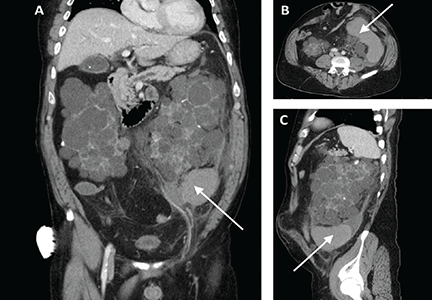
Computed tomography of the abdomen and pelvis revealed a ruptured retroperitoneal hemorrhagic cyst (Figure 1) in the inferior aspect of the left kidney extending into the fascia of Gerota.
Since his vital signs were stable, he was managed supportively during his hospitalization with intravenous fluids, serial hemoglobin checks, and analgesia. He was eventually discharged home in good condition.
CYST HEMORRHAGE IN POLYCYSTIC KIDNEY DISEASE
ADPKD is a relatively common, inherited systemic disease that leads to cyst formation, primarily in the kidneys but also in the liver (94%), seminal vesicles (40%), pancreas (9%), arachnoid membrane (8%), and spinal meningeal area (2%).1
In addition to cyst formation in multiple organs, ADPKD can have extrarenal manifestations such as connective-tissue abnormalities (including mitral valve prolapse) (25%), abdominal hernia (10%), and intracranial aneurysm (8%).1 Management of extrarenal complications of ADPKD is discussed in detail elsewhere.2
The estimated prevalence of ADPKD is 1 of every 400 to 1,000 live births. However, given that ADPKD is often clinically silent, it is diagnosed during the lifetime of fewer than half of people who have it.3
Most ADPKD cases are caused by mutations in either the PKD1 or PKD2 gene.4,5 Although the mechanism of cyst formation in ADPKD is still unclear, it is known that PKD1 and PKD2 encode proteins called polycystin-1 and polycystin-2, respectively. Polycystin-1 is a membrane protein found in renal tubular epithelia, hepatic bile ductules, and pancreatic ducts. Polycystin-2 is involved in cell calcium signaling and has been identified in the renal distal tubules, collecting duct, and thick ascending limb. Mutations in PKD1 and PKD2 are thought to contribute to cyst formation, with PKD1 mutations associated with earlier onset and more severe development of renal and extrarenal cysts.
Cyst hemorrhage
Hemorrhage of renal cysts is a well-known complication, occurring in up to 70% of patients with ADPKD.6 Renal cyst hemorrhage often presents clinically as flank pain with point tenderness or hematuria, or both. Flank pain results from hemorrhage into a cyst with consequent distention of the renal capsule, whereas hematuria results from rupture of a cyst into the collecting system.
Spontaneous nonfatal retroperitoneal cyst hemorrhage, as in our patient, is rare. Indeed, in one series reviewing the abdominal computed tomographic findings of 66 patients with ADPKD, only 2 patients (3%) had perinephric hematomas in the absence of recent trauma.6
Management of cyst hemorrhage is primarily conservative. Pain associated with cyst hemorrhage is managed conservatively with bed rest, intravenous hydration, and analgesics (but not nonsteroidal anti-inflammatory drugs).
Hematuria is also managed conservatively with bedrest and intravenous hydration, and most episodes of hematuria are self-limiting and last 2 to 7 days. However, if excessive bleeding occurs, the patient may be at risk of urinary tract obstruction from clot formation. If obstruction occurs and persists beyond 2 weeks, then ureteral stenting may be necessary. In rare cases of prolonged, severe bleeding with extensive subcapsular or retroperitoneal hematomas, patients require hospitalization, transfusion, or percutaneous transcatheter embolization of the renal artery. If such efforts are not successful, surgery, including nephrectomy, may be required to control the hemorrhage.2
Other causes of abdominal pain
In addition to renal cyst hemorrhage, the differential diagnosis of abdominal pain in a patient with ADPKD includes cyst enlargement causing stretching of the renal capsule or traction on the renal pedicle, cyst infection, nephrolithiasis, pyelonephritis, and rarely, tumors including renal cell carcinoma.
Unlike cyst rupture and hemorrhage, which are associated with point tenderness, cyst infection often manifests as diffuse, usually unilateral flank pain with associated fever, nausea, malaise, and leukocytosis. Our patient had none of these except for fever, which can also occur in cyst hemorrhage.
Nephrolithiasis occurs in up to 35% of patients with ADPKD,7 but no kidney stones were seen on computed tomography in our patient.
Pyelonephritis was unlikely in our patient, given that he had no significant white blood cells in his urinalysis and no leukocytosis.
Abdominal and pelvic imaging did not reveal any tumors in our patient.
- Pirson Y. Extrarenal manifestations of autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis 2010; 17:173–180.
- Harris PC, Torres VE. Polycystic kidney disease, autosomal dominant. In: Pagon RA, Adam MP, Bird TD, et al, editors. GeneReviews. Seattle, WA: University of Washington, Seattle; 1993–2014.
- Grantham JJ. Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med 2008; 359:1477–1485.
- Peters DJ, Spruit L, Saris JJ, et al. Chromosome 4 localization of a second gene for autosomal dominant polycystic kidney disease. Nat Genet 1993; 5:359–362.
- Rossetti S, Consugar MB, Chapman AB, et al; CRISP Consortium. Comprehensive molecular diagnostics in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 2007; 18:2143–2160.
- Levine E, Grantham JJ. Perinephric hemorrhage in autosomal dominant polycystic kidney disease: CT and MR findings. J Comput Assist Tomogr 1987; 11:108–111.
- Delaney VB, Adler S, Bruns FJ, Licinia M, Segel DP, Fraley DS. Autosomal dominant polycystic kidney disease: presentation, complications, and prognosis. Am J Kidney Dis 1985; 5:104–111.
A 59-year-old man with autosomal dominant polycystic kidney disease (ADPKD), end-stage renal disease on hemodialysis, hypertension, and diverticulosis presented with acute pain in the left lower abdomen. The pain began 4 days previously, was dull and nonradiating, was relieved partially with hydrocodone-acetaminophen, and had no clear exacerbating factors. Two days before presentation, he developed a fever with chills. He reported no recent dysuria, diarrhea, hematuria, hematochezia, or melena. He had not been taking anticoagulants or nonsteroidal anti-inflammatory drugs, and he had no history of heavy lifting or trauma.
His temperature was 38.5˚C (101.3˚F), blood pressure 141/60 mm Hg (normal for this patient). On examination, his left lower quadrant was tender with voluntary guarding. Also present was a reducible ventral hernia, which was not new.
His hemoglobin level was 10.6 g/dL (reference range 13.0–17.0), which had dropped from a previous value of 13.7 g/dL.
Computed tomography of the abdomen and pelvis revealed a ruptured retroperitoneal hemorrhagic cyst (Figure 1) in the inferior aspect of the left kidney extending into the fascia of Gerota.
Since his vital signs were stable, he was managed supportively during his hospitalization with intravenous fluids, serial hemoglobin checks, and analgesia. He was eventually discharged home in good condition.
CYST HEMORRHAGE IN POLYCYSTIC KIDNEY DISEASE
ADPKD is a relatively common, inherited systemic disease that leads to cyst formation, primarily in the kidneys but also in the liver (94%), seminal vesicles (40%), pancreas (9%), arachnoid membrane (8%), and spinal meningeal area (2%).1
In addition to cyst formation in multiple organs, ADPKD can have extrarenal manifestations such as connective-tissue abnormalities (including mitral valve prolapse) (25%), abdominal hernia (10%), and intracranial aneurysm (8%).1 Management of extrarenal complications of ADPKD is discussed in detail elsewhere.2
The estimated prevalence of ADPKD is 1 of every 400 to 1,000 live births. However, given that ADPKD is often clinically silent, it is diagnosed during the lifetime of fewer than half of people who have it.3
Most ADPKD cases are caused by mutations in either the PKD1 or PKD2 gene.4,5 Although the mechanism of cyst formation in ADPKD is still unclear, it is known that PKD1 and PKD2 encode proteins called polycystin-1 and polycystin-2, respectively. Polycystin-1 is a membrane protein found in renal tubular epithelia, hepatic bile ductules, and pancreatic ducts. Polycystin-2 is involved in cell calcium signaling and has been identified in the renal distal tubules, collecting duct, and thick ascending limb. Mutations in PKD1 and PKD2 are thought to contribute to cyst formation, with PKD1 mutations associated with earlier onset and more severe development of renal and extrarenal cysts.
Cyst hemorrhage
Hemorrhage of renal cysts is a well-known complication, occurring in up to 70% of patients with ADPKD.6 Renal cyst hemorrhage often presents clinically as flank pain with point tenderness or hematuria, or both. Flank pain results from hemorrhage into a cyst with consequent distention of the renal capsule, whereas hematuria results from rupture of a cyst into the collecting system.
Spontaneous nonfatal retroperitoneal cyst hemorrhage, as in our patient, is rare. Indeed, in one series reviewing the abdominal computed tomographic findings of 66 patients with ADPKD, only 2 patients (3%) had perinephric hematomas in the absence of recent trauma.6
Management of cyst hemorrhage is primarily conservative. Pain associated with cyst hemorrhage is managed conservatively with bed rest, intravenous hydration, and analgesics (but not nonsteroidal anti-inflammatory drugs).
Hematuria is also managed conservatively with bedrest and intravenous hydration, and most episodes of hematuria are self-limiting and last 2 to 7 days. However, if excessive bleeding occurs, the patient may be at risk of urinary tract obstruction from clot formation. If obstruction occurs and persists beyond 2 weeks, then ureteral stenting may be necessary. In rare cases of prolonged, severe bleeding with extensive subcapsular or retroperitoneal hematomas, patients require hospitalization, transfusion, or percutaneous transcatheter embolization of the renal artery. If such efforts are not successful, surgery, including nephrectomy, may be required to control the hemorrhage.2
Other causes of abdominal pain
In addition to renal cyst hemorrhage, the differential diagnosis of abdominal pain in a patient with ADPKD includes cyst enlargement causing stretching of the renal capsule or traction on the renal pedicle, cyst infection, nephrolithiasis, pyelonephritis, and rarely, tumors including renal cell carcinoma.
Unlike cyst rupture and hemorrhage, which are associated with point tenderness, cyst infection often manifests as diffuse, usually unilateral flank pain with associated fever, nausea, malaise, and leukocytosis. Our patient had none of these except for fever, which can also occur in cyst hemorrhage.
Nephrolithiasis occurs in up to 35% of patients with ADPKD,7 but no kidney stones were seen on computed tomography in our patient.
Pyelonephritis was unlikely in our patient, given that he had no significant white blood cells in his urinalysis and no leukocytosis.
Abdominal and pelvic imaging did not reveal any tumors in our patient.
A 59-year-old man with autosomal dominant polycystic kidney disease (ADPKD), end-stage renal disease on hemodialysis, hypertension, and diverticulosis presented with acute pain in the left lower abdomen. The pain began 4 days previously, was dull and nonradiating, was relieved partially with hydrocodone-acetaminophen, and had no clear exacerbating factors. Two days before presentation, he developed a fever with chills. He reported no recent dysuria, diarrhea, hematuria, hematochezia, or melena. He had not been taking anticoagulants or nonsteroidal anti-inflammatory drugs, and he had no history of heavy lifting or trauma.
His temperature was 38.5˚C (101.3˚F), blood pressure 141/60 mm Hg (normal for this patient). On examination, his left lower quadrant was tender with voluntary guarding. Also present was a reducible ventral hernia, which was not new.
His hemoglobin level was 10.6 g/dL (reference range 13.0–17.0), which had dropped from a previous value of 13.7 g/dL.
Computed tomography of the abdomen and pelvis revealed a ruptured retroperitoneal hemorrhagic cyst (Figure 1) in the inferior aspect of the left kidney extending into the fascia of Gerota.
Since his vital signs were stable, he was managed supportively during his hospitalization with intravenous fluids, serial hemoglobin checks, and analgesia. He was eventually discharged home in good condition.
CYST HEMORRHAGE IN POLYCYSTIC KIDNEY DISEASE
ADPKD is a relatively common, inherited systemic disease that leads to cyst formation, primarily in the kidneys but also in the liver (94%), seminal vesicles (40%), pancreas (9%), arachnoid membrane (8%), and spinal meningeal area (2%).1
In addition to cyst formation in multiple organs, ADPKD can have extrarenal manifestations such as connective-tissue abnormalities (including mitral valve prolapse) (25%), abdominal hernia (10%), and intracranial aneurysm (8%).1 Management of extrarenal complications of ADPKD is discussed in detail elsewhere.2
The estimated prevalence of ADPKD is 1 of every 400 to 1,000 live births. However, given that ADPKD is often clinically silent, it is diagnosed during the lifetime of fewer than half of people who have it.3
Most ADPKD cases are caused by mutations in either the PKD1 or PKD2 gene.4,5 Although the mechanism of cyst formation in ADPKD is still unclear, it is known that PKD1 and PKD2 encode proteins called polycystin-1 and polycystin-2, respectively. Polycystin-1 is a membrane protein found in renal tubular epithelia, hepatic bile ductules, and pancreatic ducts. Polycystin-2 is involved in cell calcium signaling and has been identified in the renal distal tubules, collecting duct, and thick ascending limb. Mutations in PKD1 and PKD2 are thought to contribute to cyst formation, with PKD1 mutations associated with earlier onset and more severe development of renal and extrarenal cysts.
Cyst hemorrhage
Hemorrhage of renal cysts is a well-known complication, occurring in up to 70% of patients with ADPKD.6 Renal cyst hemorrhage often presents clinically as flank pain with point tenderness or hematuria, or both. Flank pain results from hemorrhage into a cyst with consequent distention of the renal capsule, whereas hematuria results from rupture of a cyst into the collecting system.
Spontaneous nonfatal retroperitoneal cyst hemorrhage, as in our patient, is rare. Indeed, in one series reviewing the abdominal computed tomographic findings of 66 patients with ADPKD, only 2 patients (3%) had perinephric hematomas in the absence of recent trauma.6
Management of cyst hemorrhage is primarily conservative. Pain associated with cyst hemorrhage is managed conservatively with bed rest, intravenous hydration, and analgesics (but not nonsteroidal anti-inflammatory drugs).
Hematuria is also managed conservatively with bedrest and intravenous hydration, and most episodes of hematuria are self-limiting and last 2 to 7 days. However, if excessive bleeding occurs, the patient may be at risk of urinary tract obstruction from clot formation. If obstruction occurs and persists beyond 2 weeks, then ureteral stenting may be necessary. In rare cases of prolonged, severe bleeding with extensive subcapsular or retroperitoneal hematomas, patients require hospitalization, transfusion, or percutaneous transcatheter embolization of the renal artery. If such efforts are not successful, surgery, including nephrectomy, may be required to control the hemorrhage.2
Other causes of abdominal pain
In addition to renal cyst hemorrhage, the differential diagnosis of abdominal pain in a patient with ADPKD includes cyst enlargement causing stretching of the renal capsule or traction on the renal pedicle, cyst infection, nephrolithiasis, pyelonephritis, and rarely, tumors including renal cell carcinoma.
Unlike cyst rupture and hemorrhage, which are associated with point tenderness, cyst infection often manifests as diffuse, usually unilateral flank pain with associated fever, nausea, malaise, and leukocytosis. Our patient had none of these except for fever, which can also occur in cyst hemorrhage.
Nephrolithiasis occurs in up to 35% of patients with ADPKD,7 but no kidney stones were seen on computed tomography in our patient.
Pyelonephritis was unlikely in our patient, given that he had no significant white blood cells in his urinalysis and no leukocytosis.
Abdominal and pelvic imaging did not reveal any tumors in our patient.
- Pirson Y. Extrarenal manifestations of autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis 2010; 17:173–180.
- Harris PC, Torres VE. Polycystic kidney disease, autosomal dominant. In: Pagon RA, Adam MP, Bird TD, et al, editors. GeneReviews. Seattle, WA: University of Washington, Seattle; 1993–2014.
- Grantham JJ. Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med 2008; 359:1477–1485.
- Peters DJ, Spruit L, Saris JJ, et al. Chromosome 4 localization of a second gene for autosomal dominant polycystic kidney disease. Nat Genet 1993; 5:359–362.
- Rossetti S, Consugar MB, Chapman AB, et al; CRISP Consortium. Comprehensive molecular diagnostics in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 2007; 18:2143–2160.
- Levine E, Grantham JJ. Perinephric hemorrhage in autosomal dominant polycystic kidney disease: CT and MR findings. J Comput Assist Tomogr 1987; 11:108–111.
- Delaney VB, Adler S, Bruns FJ, Licinia M, Segel DP, Fraley DS. Autosomal dominant polycystic kidney disease: presentation, complications, and prognosis. Am J Kidney Dis 1985; 5:104–111.
- Pirson Y. Extrarenal manifestations of autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis 2010; 17:173–180.
- Harris PC, Torres VE. Polycystic kidney disease, autosomal dominant. In: Pagon RA, Adam MP, Bird TD, et al, editors. GeneReviews. Seattle, WA: University of Washington, Seattle; 1993–2014.
- Grantham JJ. Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med 2008; 359:1477–1485.
- Peters DJ, Spruit L, Saris JJ, et al. Chromosome 4 localization of a second gene for autosomal dominant polycystic kidney disease. Nat Genet 1993; 5:359–362.
- Rossetti S, Consugar MB, Chapman AB, et al; CRISP Consortium. Comprehensive molecular diagnostics in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 2007; 18:2143–2160.
- Levine E, Grantham JJ. Perinephric hemorrhage in autosomal dominant polycystic kidney disease: CT and MR findings. J Comput Assist Tomogr 1987; 11:108–111.
- Delaney VB, Adler S, Bruns FJ, Licinia M, Segel DP, Fraley DS. Autosomal dominant polycystic kidney disease: presentation, complications, and prognosis. Am J Kidney Dis 1985; 5:104–111.
Double trouble: Simultaneous complications of therapeutic thoracentesis
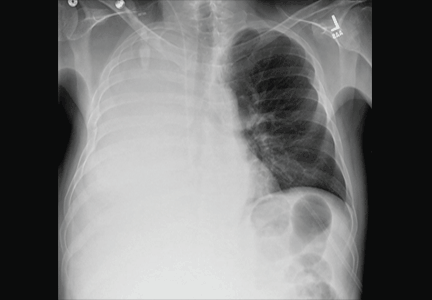
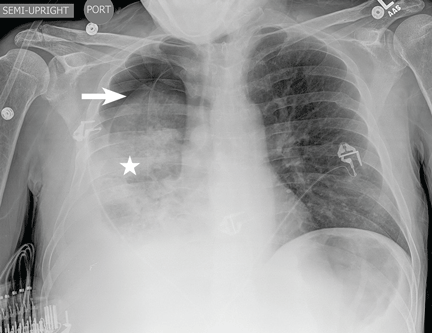
A 51-year-old man with end-stage liver disease from alcohol abuse presented with worsening dyspnea on exertion. He had a history of ascites requiring diuretic therapy and intermittent paracentesis, as well as symptomatic hepatic hydrothorax requiring thoracentesis. Chest radiography showed a large right hydrothorax (Figure 1).
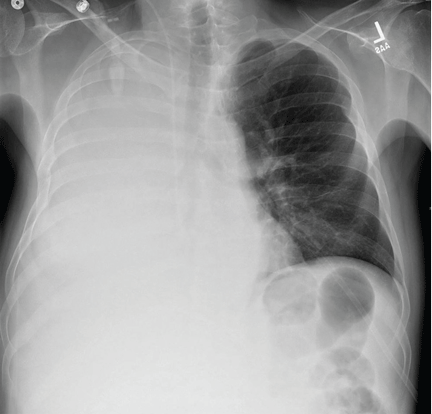
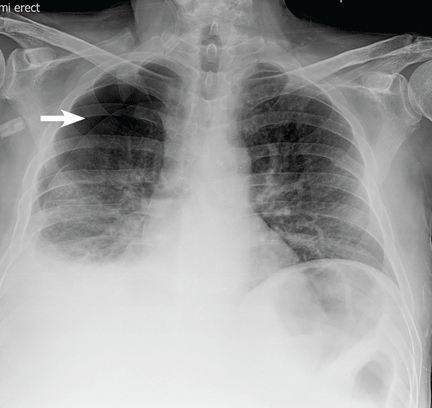
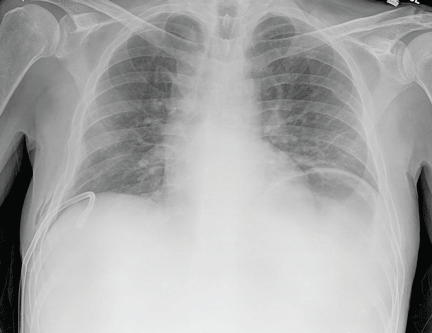
The patient underwent high-volume thoracentesis, and 3.2 L of clear fluid was removed. Chest radiography after the procedure revealed a right-sided pneumothorax (Figure 2, arrow). The patient was mildly short of breath and was treated with high-flow oxygen. Later the same day, his shortness of breath worsened, and repeat chest radiography showed an unchanged pneumothorax that was now complicated by reexpansion pulmonary edema after thoracentesis (Figure 3, star). The reexpansion pulmonary edema resolved by the following day, and the pneumothorax resolved after placement of a pig-tail catheter into the pleural space (Figure 4).
Iatrogenic pneumothorax after thoracentesis occurs in 6% of cases.1 Iatrogenic reexpansion pulmonary edema after thoracentesis occurs in fewer than 1% of cases.2,3 Simultaneous pneumothorax and reexpansion pulmonary edema arising from the same procedure appears to be extremely rare.
- Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med 2010; 170:332–339.
- Ragozzino MW, Greene R. Bilateral reexpansion pulmonary edema following unilateral pleurocentesis. Chest 1991; 99:506–508.
- Dias OM, Teixeira LR, Vargas FS. Reexpansion pulmonary edema after therapeutic thoracentesis. Clinics (Sao Paulo) 2010; 65:1387–1389.
A 51-year-old man with end-stage liver disease from alcohol abuse presented with worsening dyspnea on exertion. He had a history of ascites requiring diuretic therapy and intermittent paracentesis, as well as symptomatic hepatic hydrothorax requiring thoracentesis. Chest radiography showed a large right hydrothorax (Figure 1).

The patient underwent high-volume thoracentesis, and 3.2 L of clear fluid was removed. Chest radiography after the procedure revealed a right-sided pneumothorax (Figure 2, arrow). The patient was mildly short of breath and was treated with high-flow oxygen. Later the same day, his shortness of breath worsened, and repeat chest radiography showed an unchanged pneumothorax that was now complicated by reexpansion pulmonary edema after thoracentesis (Figure 3, star). The reexpansion pulmonary edema resolved by the following day, and the pneumothorax resolved after placement of a pig-tail catheter into the pleural space (Figure 4).
Iatrogenic pneumothorax after thoracentesis occurs in 6% of cases.1 Iatrogenic reexpansion pulmonary edema after thoracentesis occurs in fewer than 1% of cases.2,3 Simultaneous pneumothorax and reexpansion pulmonary edema arising from the same procedure appears to be extremely rare.



A 51-year-old man with end-stage liver disease from alcohol abuse presented with worsening dyspnea on exertion. He had a history of ascites requiring diuretic therapy and intermittent paracentesis, as well as symptomatic hepatic hydrothorax requiring thoracentesis. Chest radiography showed a large right hydrothorax (Figure 1).

The patient underwent high-volume thoracentesis, and 3.2 L of clear fluid was removed. Chest radiography after the procedure revealed a right-sided pneumothorax (Figure 2, arrow). The patient was mildly short of breath and was treated with high-flow oxygen. Later the same day, his shortness of breath worsened, and repeat chest radiography showed an unchanged pneumothorax that was now complicated by reexpansion pulmonary edema after thoracentesis (Figure 3, star). The reexpansion pulmonary edema resolved by the following day, and the pneumothorax resolved after placement of a pig-tail catheter into the pleural space (Figure 4).
Iatrogenic pneumothorax after thoracentesis occurs in 6% of cases.1 Iatrogenic reexpansion pulmonary edema after thoracentesis occurs in fewer than 1% of cases.2,3 Simultaneous pneumothorax and reexpansion pulmonary edema arising from the same procedure appears to be extremely rare.



- Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med 2010; 170:332–339.
- Ragozzino MW, Greene R. Bilateral reexpansion pulmonary edema following unilateral pleurocentesis. Chest 1991; 99:506–508.
- Dias OM, Teixeira LR, Vargas FS. Reexpansion pulmonary edema after therapeutic thoracentesis. Clinics (Sao Paulo) 2010; 65:1387–1389.
- Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med 2010; 170:332–339.
- Ragozzino MW, Greene R. Bilateral reexpansion pulmonary edema following unilateral pleurocentesis. Chest 1991; 99:506–508.
- Dias OM, Teixeira LR, Vargas FS. Reexpansion pulmonary edema after therapeutic thoracentesis. Clinics (Sao Paulo) 2010; 65:1387–1389.
Sore throat, odynophagia, hoarseness, and a muffled, high-pitched voice
A 55-year-old man presents to the emergency department with a sore throat, odynophagia, hoarseness, and a high-pitched muffled voice for 1 day. He is otherwise healthy, with no fever, chills, or exposure to sick contacts.
Q: Which is the most likely diagnosis?
- Retropharyngeal abscess
- Croup
- Streptococcal pharyngitis (“strep throat”)
- Acute epiglottitis
- Viral upper-respiratory infection
A: The correct answer is acute epiglottitis.
Acute epiglottitis is a cellulitis of the epiglottis and adjacent tissues that, without treatment, can progress to life-threatening airway obstruction.
In children, this entity most often occurs between ages 2 and 4. Previously, it was mainly associated with Haemophilus influenzae type B infection, but since the start of vaccination for this organism, epiglottitis has become less common.
In recent years, Streptococcus pneumoniae and beta-hemolytic streptococci have become the main culprits in epiglottitis in children. In adults, common organisms include H influenzae, S pneumoniae, Staphylococcus aureus, and betahemolytic streptococci. Epiglottitis may also be caused by herpes simplex, varicella-zoster, and influenza viruses. Noninfectious causes include ingestion of a foreign body and thermal injury.
Common signs and symptoms of epiglottitis include sore throat, a high-pitched and muffled voice, labored breathing, and respiratory distress.1 Since airway obstruction can be remarkably precipitous, it is important to obtain early consultation with an otolaryngologist for laryngoscopy and visualization of the erythematous, swollen epiglottis. Signs of severe upper-airway obstruction may be absent until late in the disease process, making early diagnosis crucial.
On physical examination, the oropharynx will often appear benign. Currently, the preferred diagnostic test for epiglottitis is visualization by laryngoscopy, which has close to 100% sensitivity and specificity.2 By comparison, lateral neck radiography has poor sensitivity (38% to 88%) and specificity (78%).3,4 Bedside ultrasonography has been reported as capable of revealing the “alphabet P sign” in the longitudinal view through the thyrohyoid membrane.5
At triage, if clinical suspicion of total airway obstruction is high, the patient may be taken directly to the operating room for visualization of the epiglottis and, if necessary, for endotracheal intubation. When the risk is considered low or moderate, lateral neck radiography may be helpful,6 as it will sometimes reveal a swollen epiglottis (ie, “thumb sign”), thickened arytenoepiglottic folds, and obliteration of the epiglottic vallecula. If the radiograph is normal and clinical suspicion is still present, laryngoscopy can be done.
For low-risk, acute epiglottitis, closely monitoring the patient in an intensive care unit is recommended. For severe, high-risk cases, securing the airway is of the utmost importance. In addition to securing the airway, empiric antimicrobial therapy—such as with cefotaxime or ceftriaxone plus clindamycin or vancomycin—is often warranted in both low-risk and high-risk cases. The use of corticosteroids in epiglottitis has not been studied in a randomized, controlled trial, and, though common, it remains controversial.7 While undergoing treatment, patients should be closely monitored for respiratory compromise.
HOW OUR PATIENT WAS MANAGED
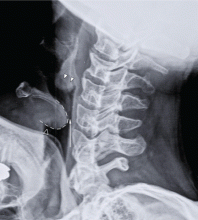
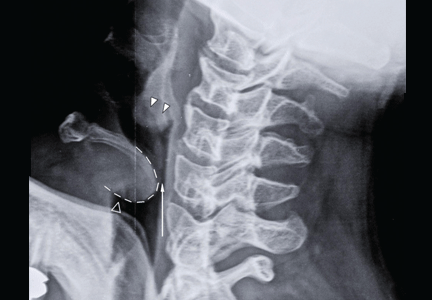
Our patient had a high-pitched, muffled voice and prominent, tender anterior cervical lymph nodes. No drooling or stridor was noted. The oropharynx appeared normal. Lateral soft-tissue radiography of the neck (Figure 1) revealed several key findings:
- A swollen epiglottis (ie, the “thumb sign”) consistent with acute epiglottitis and a narrowed airway
- Swollen arytenoids
- A shallow V-shaped epiglottic vallecula.
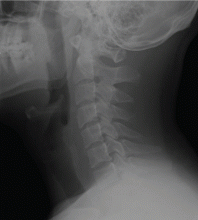
For comparison, Figure 2 shows a normal lateral soft-tissue radiograph of the neck from a different patient.
Our patient was referred to an otolaryngologist, who noted a severely inflamed epiglottis on laryngoscopy.
The patient was given intravenous vancomycin, ceftriaxone, and high-dose corticosteroids and was admitted to the intensive care unit. After his symptoms improved, repeat laryngoscopy revealed markedly diminished inflammation. He was discharged home with an additional course of oral antibiotics.
- Alcaide ML, Bisno AL. Pharyngitis and epiglottitis. Infect Dis Clin North Am 2007; 21:449–469.
- Cheung CS, Man SY, Graham CA, et al. Adult epiglottitis: 6 years experience in a university teaching hospital in Hong Kong. Eur J Emerg Med 2009; 16:221–226.
- Stankiewicz JA, Bowes AK. Croup and epiglottitis: a radiologic study. Laryngoscope 1985; 95:1159–1160.
- Solomon P, Weisbrod M, Irish JC, Gullane PJ. Adult epiglottitis: the Toronto Hospital experience. J Otolaryngol 1998; 27:332–336.
- Hung TY, Li S, Chen PS, et al. Bedside ultrasonography as a safe and effective tool to diagnose acute epiglottitis. Am J Emerg Med 2011; 29:359.e1–359.e3.
- Ragosta KG, Orr R, Detweiler MJ. Revisiting epiglottitis: a protocol—the value of lateral neck radiographs. J Am Osteopath Assoc 1997; 97:227–229.
- Glynn F, Fenton JE. Diagnosis and management of supraglottitis (epiglottitis). Curr Infect Dis Rep 2008; 10:200–204.
A 55-year-old man presents to the emergency department with a sore throat, odynophagia, hoarseness, and a high-pitched muffled voice for 1 day. He is otherwise healthy, with no fever, chills, or exposure to sick contacts.
Q: Which is the most likely diagnosis?
- Retropharyngeal abscess
- Croup
- Streptococcal pharyngitis (“strep throat”)
- Acute epiglottitis
- Viral upper-respiratory infection
A: The correct answer is acute epiglottitis.
Acute epiglottitis is a cellulitis of the epiglottis and adjacent tissues that, without treatment, can progress to life-threatening airway obstruction.
In children, this entity most often occurs between ages 2 and 4. Previously, it was mainly associated with Haemophilus influenzae type B infection, but since the start of vaccination for this organism, epiglottitis has become less common.
In recent years, Streptococcus pneumoniae and beta-hemolytic streptococci have become the main culprits in epiglottitis in children. In adults, common organisms include H influenzae, S pneumoniae, Staphylococcus aureus, and betahemolytic streptococci. Epiglottitis may also be caused by herpes simplex, varicella-zoster, and influenza viruses. Noninfectious causes include ingestion of a foreign body and thermal injury.
Common signs and symptoms of epiglottitis include sore throat, a high-pitched and muffled voice, labored breathing, and respiratory distress.1 Since airway obstruction can be remarkably precipitous, it is important to obtain early consultation with an otolaryngologist for laryngoscopy and visualization of the erythematous, swollen epiglottis. Signs of severe upper-airway obstruction may be absent until late in the disease process, making early diagnosis crucial.
On physical examination, the oropharynx will often appear benign. Currently, the preferred diagnostic test for epiglottitis is visualization by laryngoscopy, which has close to 100% sensitivity and specificity.2 By comparison, lateral neck radiography has poor sensitivity (38% to 88%) and specificity (78%).3,4 Bedside ultrasonography has been reported as capable of revealing the “alphabet P sign” in the longitudinal view through the thyrohyoid membrane.5
At triage, if clinical suspicion of total airway obstruction is high, the patient may be taken directly to the operating room for visualization of the epiglottis and, if necessary, for endotracheal intubation. When the risk is considered low or moderate, lateral neck radiography may be helpful,6 as it will sometimes reveal a swollen epiglottis (ie, “thumb sign”), thickened arytenoepiglottic folds, and obliteration of the epiglottic vallecula. If the radiograph is normal and clinical suspicion is still present, laryngoscopy can be done.
For low-risk, acute epiglottitis, closely monitoring the patient in an intensive care unit is recommended. For severe, high-risk cases, securing the airway is of the utmost importance. In addition to securing the airway, empiric antimicrobial therapy—such as with cefotaxime or ceftriaxone plus clindamycin or vancomycin—is often warranted in both low-risk and high-risk cases. The use of corticosteroids in epiglottitis has not been studied in a randomized, controlled trial, and, though common, it remains controversial.7 While undergoing treatment, patients should be closely monitored for respiratory compromise.
HOW OUR PATIENT WAS MANAGED
Our patient had a high-pitched, muffled voice and prominent, tender anterior cervical lymph nodes. No drooling or stridor was noted. The oropharynx appeared normal. Lateral soft-tissue radiography of the neck (Figure 1) revealed several key findings:
- A swollen epiglottis (ie, the “thumb sign”) consistent with acute epiglottitis and a narrowed airway
- Swollen arytenoids
- A shallow V-shaped epiglottic vallecula.
For comparison, Figure 2 shows a normal lateral soft-tissue radiograph of the neck from a different patient.
Our patient was referred to an otolaryngologist, who noted a severely inflamed epiglottis on laryngoscopy.
The patient was given intravenous vancomycin, ceftriaxone, and high-dose corticosteroids and was admitted to the intensive care unit. After his symptoms improved, repeat laryngoscopy revealed markedly diminished inflammation. He was discharged home with an additional course of oral antibiotics.
A 55-year-old man presents to the emergency department with a sore throat, odynophagia, hoarseness, and a high-pitched muffled voice for 1 day. He is otherwise healthy, with no fever, chills, or exposure to sick contacts.
Q: Which is the most likely diagnosis?
- Retropharyngeal abscess
- Croup
- Streptococcal pharyngitis (“strep throat”)
- Acute epiglottitis
- Viral upper-respiratory infection
A: The correct answer is acute epiglottitis.
Acute epiglottitis is a cellulitis of the epiglottis and adjacent tissues that, without treatment, can progress to life-threatening airway obstruction.
In children, this entity most often occurs between ages 2 and 4. Previously, it was mainly associated with Haemophilus influenzae type B infection, but since the start of vaccination for this organism, epiglottitis has become less common.
In recent years, Streptococcus pneumoniae and beta-hemolytic streptococci have become the main culprits in epiglottitis in children. In adults, common organisms include H influenzae, S pneumoniae, Staphylococcus aureus, and betahemolytic streptococci. Epiglottitis may also be caused by herpes simplex, varicella-zoster, and influenza viruses. Noninfectious causes include ingestion of a foreign body and thermal injury.
Common signs and symptoms of epiglottitis include sore throat, a high-pitched and muffled voice, labored breathing, and respiratory distress.1 Since airway obstruction can be remarkably precipitous, it is important to obtain early consultation with an otolaryngologist for laryngoscopy and visualization of the erythematous, swollen epiglottis. Signs of severe upper-airway obstruction may be absent until late in the disease process, making early diagnosis crucial.
On physical examination, the oropharynx will often appear benign. Currently, the preferred diagnostic test for epiglottitis is visualization by laryngoscopy, which has close to 100% sensitivity and specificity.2 By comparison, lateral neck radiography has poor sensitivity (38% to 88%) and specificity (78%).3,4 Bedside ultrasonography has been reported as capable of revealing the “alphabet P sign” in the longitudinal view through the thyrohyoid membrane.5
At triage, if clinical suspicion of total airway obstruction is high, the patient may be taken directly to the operating room for visualization of the epiglottis and, if necessary, for endotracheal intubation. When the risk is considered low or moderate, lateral neck radiography may be helpful,6 as it will sometimes reveal a swollen epiglottis (ie, “thumb sign”), thickened arytenoepiglottic folds, and obliteration of the epiglottic vallecula. If the radiograph is normal and clinical suspicion is still present, laryngoscopy can be done.
For low-risk, acute epiglottitis, closely monitoring the patient in an intensive care unit is recommended. For severe, high-risk cases, securing the airway is of the utmost importance. In addition to securing the airway, empiric antimicrobial therapy—such as with cefotaxime or ceftriaxone plus clindamycin or vancomycin—is often warranted in both low-risk and high-risk cases. The use of corticosteroids in epiglottitis has not been studied in a randomized, controlled trial, and, though common, it remains controversial.7 While undergoing treatment, patients should be closely monitored for respiratory compromise.
HOW OUR PATIENT WAS MANAGED
Our patient had a high-pitched, muffled voice and prominent, tender anterior cervical lymph nodes. No drooling or stridor was noted. The oropharynx appeared normal. Lateral soft-tissue radiography of the neck (Figure 1) revealed several key findings:
- A swollen epiglottis (ie, the “thumb sign”) consistent with acute epiglottitis and a narrowed airway
- Swollen arytenoids
- A shallow V-shaped epiglottic vallecula.
For comparison, Figure 2 shows a normal lateral soft-tissue radiograph of the neck from a different patient.
Our patient was referred to an otolaryngologist, who noted a severely inflamed epiglottis on laryngoscopy.
The patient was given intravenous vancomycin, ceftriaxone, and high-dose corticosteroids and was admitted to the intensive care unit. After his symptoms improved, repeat laryngoscopy revealed markedly diminished inflammation. He was discharged home with an additional course of oral antibiotics.
- Alcaide ML, Bisno AL. Pharyngitis and epiglottitis. Infect Dis Clin North Am 2007; 21:449–469.
- Cheung CS, Man SY, Graham CA, et al. Adult epiglottitis: 6 years experience in a university teaching hospital in Hong Kong. Eur J Emerg Med 2009; 16:221–226.
- Stankiewicz JA, Bowes AK. Croup and epiglottitis: a radiologic study. Laryngoscope 1985; 95:1159–1160.
- Solomon P, Weisbrod M, Irish JC, Gullane PJ. Adult epiglottitis: the Toronto Hospital experience. J Otolaryngol 1998; 27:332–336.
- Hung TY, Li S, Chen PS, et al. Bedside ultrasonography as a safe and effective tool to diagnose acute epiglottitis. Am J Emerg Med 2011; 29:359.e1–359.e3.
- Ragosta KG, Orr R, Detweiler MJ. Revisiting epiglottitis: a protocol—the value of lateral neck radiographs. J Am Osteopath Assoc 1997; 97:227–229.
- Glynn F, Fenton JE. Diagnosis and management of supraglottitis (epiglottitis). Curr Infect Dis Rep 2008; 10:200–204.
- Alcaide ML, Bisno AL. Pharyngitis and epiglottitis. Infect Dis Clin North Am 2007; 21:449–469.
- Cheung CS, Man SY, Graham CA, et al. Adult epiglottitis: 6 years experience in a university teaching hospital in Hong Kong. Eur J Emerg Med 2009; 16:221–226.
- Stankiewicz JA, Bowes AK. Croup and epiglottitis: a radiologic study. Laryngoscope 1985; 95:1159–1160.
- Solomon P, Weisbrod M, Irish JC, Gullane PJ. Adult epiglottitis: the Toronto Hospital experience. J Otolaryngol 1998; 27:332–336.
- Hung TY, Li S, Chen PS, et al. Bedside ultrasonography as a safe and effective tool to diagnose acute epiglottitis. Am J Emerg Med 2011; 29:359.e1–359.e3.
- Ragosta KG, Orr R, Detweiler MJ. Revisiting epiglottitis: a protocol—the value of lateral neck radiographs. J Am Osteopath Assoc 1997; 97:227–229.
- Glynn F, Fenton JE. Diagnosis and management of supraglottitis (epiglottitis). Curr Infect Dis Rep 2008; 10:200–204.
A fall to remember
A healthy 44‐year‐old man presented to the emergency room with buttock pain, 2 days after falling from a 4‐foot table onto a cement floor, striking his right buttock. On presentation he was in moderate distress, with heart rate = 130 beats per minute (bpm) and blood pressure = 80/40 mm Hg. There was a 2‐cm area of erythema on the right flank, and mild tenderness of the right buttock. There was no evidence of skin anesthesia or crepitance. Initial laboratory results were notable for creatinine = 1.5 mg/dl, creatine phosphokinase = 11,500 U/L, lactate = 6.1 mmo/L, and white blood cell count = 10 k/UL, with 93% neutrophils. Plain radiography of the hip and spine were negative for fractures and soft tissue gas. Despite receiving multiple doses of narcotic analgesia, the patient continued to complain of severe pain. Within 2 hours of presentation, the right flank erythema extended proximally up the back and distally to the right thigh (Figure 1). The patient was taken to the operating room for surgical exploration and was diagnosed with necrotizing fasciitis extending from his posterior neck to the right popliteal fossa (Figures 2 and 3). Intraoperative cultures grew Group A streptococcus, confirming a diagnosis of Type II necrotizing fasciitis. The patient required multiple debridements and subsequent reconstructive procedures. After more than 3 months in the hospital and acute rehabilitation, he returned home.
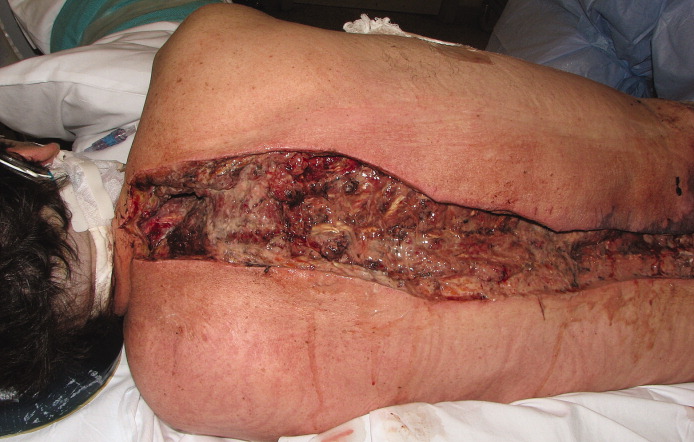
Necrotizing fasciitis is a rapidly progressive infection that spreads along fascial planes, causing necrosis of subcutaneous tissues. Type II necrotizing fasciitis is a monomicrobial infection and occurs in healthy patients, with blunt trauma being a known precipitant. The classic finding in necrotizing fasciitis is pain out of proportion to the physical examination. Additionally, patients will typically present with septic shock and end‐organ dysfunction. Diagnosis requires a strong index of suspicion and surgical exploration is necessary for diagnosis and treatment.
A healthy 44‐year‐old man presented to the emergency room with buttock pain, 2 days after falling from a 4‐foot table onto a cement floor, striking his right buttock. On presentation he was in moderate distress, with heart rate = 130 beats per minute (bpm) and blood pressure = 80/40 mm Hg. There was a 2‐cm area of erythema on the right flank, and mild tenderness of the right buttock. There was no evidence of skin anesthesia or crepitance. Initial laboratory results were notable for creatinine = 1.5 mg/dl, creatine phosphokinase = 11,500 U/L, lactate = 6.1 mmo/L, and white blood cell count = 10 k/UL, with 93% neutrophils. Plain radiography of the hip and spine were negative for fractures and soft tissue gas. Despite receiving multiple doses of narcotic analgesia, the patient continued to complain of severe pain. Within 2 hours of presentation, the right flank erythema extended proximally up the back and distally to the right thigh (Figure 1). The patient was taken to the operating room for surgical exploration and was diagnosed with necrotizing fasciitis extending from his posterior neck to the right popliteal fossa (Figures 2 and 3). Intraoperative cultures grew Group A streptococcus, confirming a diagnosis of Type II necrotizing fasciitis. The patient required multiple debridements and subsequent reconstructive procedures. After more than 3 months in the hospital and acute rehabilitation, he returned home.



Necrotizing fasciitis is a rapidly progressive infection that spreads along fascial planes, causing necrosis of subcutaneous tissues. Type II necrotizing fasciitis is a monomicrobial infection and occurs in healthy patients, with blunt trauma being a known precipitant. The classic finding in necrotizing fasciitis is pain out of proportion to the physical examination. Additionally, patients will typically present with septic shock and end‐organ dysfunction. Diagnosis requires a strong index of suspicion and surgical exploration is necessary for diagnosis and treatment.
A healthy 44‐year‐old man presented to the emergency room with buttock pain, 2 days after falling from a 4‐foot table onto a cement floor, striking his right buttock. On presentation he was in moderate distress, with heart rate = 130 beats per minute (bpm) and blood pressure = 80/40 mm Hg. There was a 2‐cm area of erythema on the right flank, and mild tenderness of the right buttock. There was no evidence of skin anesthesia or crepitance. Initial laboratory results were notable for creatinine = 1.5 mg/dl, creatine phosphokinase = 11,500 U/L, lactate = 6.1 mmo/L, and white blood cell count = 10 k/UL, with 93% neutrophils. Plain radiography of the hip and spine were negative for fractures and soft tissue gas. Despite receiving multiple doses of narcotic analgesia, the patient continued to complain of severe pain. Within 2 hours of presentation, the right flank erythema extended proximally up the back and distally to the right thigh (Figure 1). The patient was taken to the operating room for surgical exploration and was diagnosed with necrotizing fasciitis extending from his posterior neck to the right popliteal fossa (Figures 2 and 3). Intraoperative cultures grew Group A streptococcus, confirming a diagnosis of Type II necrotizing fasciitis. The patient required multiple debridements and subsequent reconstructive procedures. After more than 3 months in the hospital and acute rehabilitation, he returned home.



Necrotizing fasciitis is a rapidly progressive infection that spreads along fascial planes, causing necrosis of subcutaneous tissues. Type II necrotizing fasciitis is a monomicrobial infection and occurs in healthy patients, with blunt trauma being a known precipitant. The classic finding in necrotizing fasciitis is pain out of proportion to the physical examination. Additionally, patients will typically present with septic shock and end‐organ dysfunction. Diagnosis requires a strong index of suspicion and surgical exploration is necessary for diagnosis and treatment.
Drumstick Digits
A 42‐year‐old man with chronic kidney disease and a history of childhood repair of Tetralogy of Fallot was admitted with pneumonia. Examination of his extremities revealed clubbing of his fingers (Figure 1) and toes (Figure 2).
Clubbing may be primary, known as pachydermoperiostosis, or secondary, due to a variety of neoplastic, pulmonary, cardiac, gastrointestinal, and infectious diseases.1 Examination reveals softening of the nail bed with loss of the normal angle between the nail and the proximal nail fold, an increase in the nail fold convexity, and thickening of the distal phalange with eventual hyperextensibility of the distal interphalangeal joint. Diagnosis is based on various criteria, such as the profile angle (Lovibond's angle) or distal phalangeal to interphalangeal depth ratio. The loss of the normal diamond‐shaped window created by placing the back surfaces of terminal phalanges of similar fingers together, also known as Schamroth's sign, was noted by Dr. Leo Schamroth when he developed endocarditis and is one of the few eponyms named after both a physician and the patient in whom it was found (Figure 3).2 Recent literature suggests that vascular endothelial growth factor (VEGF), a platelet‐derived factor induced by hypoxia, may play a role in digital clubbing.3 Processes that alter normal pulmonary circulation disrupt fragmentation of megakaryocytes in the lung into platelets. Consequently, whole megakaryocytes enter the systemic circulation and become impacted in the peripheral capillaries, where they cause stromal hypoxia and release of platelet‐derived growth factor and VEGF, leading to the vascular hyperplasia that underlies clubbing.

- ,,.Clubbing: an update on diagnosis, differential diagnosis, pathophysiology, and clinical relevance.J Am Acad Dermatol.2005;52:1020–1028.
- .A unique eponymous sign of finger clubbing (Schamroth sign) that is named not only after a physician who described it but also after the patient who happened to be the physician himself.Am J Cardiol.2005;96:1614–1615.
- .Exploring the cause of the most ancient clinical sign of medicine: finger clubbing.Semin Arthritis Rheum.2007;36:380–385.
A 42‐year‐old man with chronic kidney disease and a history of childhood repair of Tetralogy of Fallot was admitted with pneumonia. Examination of his extremities revealed clubbing of his fingers (Figure 1) and toes (Figure 2).


Clubbing may be primary, known as pachydermoperiostosis, or secondary, due to a variety of neoplastic, pulmonary, cardiac, gastrointestinal, and infectious diseases.1 Examination reveals softening of the nail bed with loss of the normal angle between the nail and the proximal nail fold, an increase in the nail fold convexity, and thickening of the distal phalange with eventual hyperextensibility of the distal interphalangeal joint. Diagnosis is based on various criteria, such as the profile angle (Lovibond's angle) or distal phalangeal to interphalangeal depth ratio. The loss of the normal diamond‐shaped window created by placing the back surfaces of terminal phalanges of similar fingers together, also known as Schamroth's sign, was noted by Dr. Leo Schamroth when he developed endocarditis and is one of the few eponyms named after both a physician and the patient in whom it was found (Figure 3).2 Recent literature suggests that vascular endothelial growth factor (VEGF), a platelet‐derived factor induced by hypoxia, may play a role in digital clubbing.3 Processes that alter normal pulmonary circulation disrupt fragmentation of megakaryocytes in the lung into platelets. Consequently, whole megakaryocytes enter the systemic circulation and become impacted in the peripheral capillaries, where they cause stromal hypoxia and release of platelet‐derived growth factor and VEGF, leading to the vascular hyperplasia that underlies clubbing.

A 42‐year‐old man with chronic kidney disease and a history of childhood repair of Tetralogy of Fallot was admitted with pneumonia. Examination of his extremities revealed clubbing of his fingers (Figure 1) and toes (Figure 2).


Clubbing may be primary, known as pachydermoperiostosis, or secondary, due to a variety of neoplastic, pulmonary, cardiac, gastrointestinal, and infectious diseases.1 Examination reveals softening of the nail bed with loss of the normal angle between the nail and the proximal nail fold, an increase in the nail fold convexity, and thickening of the distal phalange with eventual hyperextensibility of the distal interphalangeal joint. Diagnosis is based on various criteria, such as the profile angle (Lovibond's angle) or distal phalangeal to interphalangeal depth ratio. The loss of the normal diamond‐shaped window created by placing the back surfaces of terminal phalanges of similar fingers together, also known as Schamroth's sign, was noted by Dr. Leo Schamroth when he developed endocarditis and is one of the few eponyms named after both a physician and the patient in whom it was found (Figure 3).2 Recent literature suggests that vascular endothelial growth factor (VEGF), a platelet‐derived factor induced by hypoxia, may play a role in digital clubbing.3 Processes that alter normal pulmonary circulation disrupt fragmentation of megakaryocytes in the lung into platelets. Consequently, whole megakaryocytes enter the systemic circulation and become impacted in the peripheral capillaries, where they cause stromal hypoxia and release of platelet‐derived growth factor and VEGF, leading to the vascular hyperplasia that underlies clubbing.

- ,,.Clubbing: an update on diagnosis, differential diagnosis, pathophysiology, and clinical relevance.J Am Acad Dermatol.2005;52:1020–1028.
- .A unique eponymous sign of finger clubbing (Schamroth sign) that is named not only after a physician who described it but also after the patient who happened to be the physician himself.Am J Cardiol.2005;96:1614–1615.
- .Exploring the cause of the most ancient clinical sign of medicine: finger clubbing.Semin Arthritis Rheum.2007;36:380–385.
- ,,.Clubbing: an update on diagnosis, differential diagnosis, pathophysiology, and clinical relevance.J Am Acad Dermatol.2005;52:1020–1028.
- .A unique eponymous sign of finger clubbing (Schamroth sign) that is named not only after a physician who described it but also after the patient who happened to be the physician himself.Am J Cardiol.2005;96:1614–1615.
- .Exploring the cause of the most ancient clinical sign of medicine: finger clubbing.Semin Arthritis Rheum.2007;36:380–385.
Introducing Hospital Images Dx—A call for submissions
Of the many skills a hospitalist nurtures and develops, seeing and processing visual images is paramount. From the moment we enter the hospital each day, we look at patient faces, trying to fathom their levels of pain, of illness, and of response to therapy. We look at their rashes, facial droops, surgical wounds, and neck vein elevation. When we are done visually scrutinizing our patients, we inspect their rhythm strips or electrocardiograms and then move on to their X‐rays and advanced imaging.
Although we are, in a sense, slaves to the images before us, we also enjoy medicine for the challenge that these images providethere is always something novel to see. For the experienced clinician, a deep sense of satisfaction perfuses our limbic system with the quick recognition of the delta wave of Wolf‐Parkinson‐White on an EKG or the first vesicle of a zoster outbreak in a patient with initially unexplained cutaneous pain. What we recognize easily tends to come from having seen something beforefor better or worse, we depend on pattern recognition.
Unfortunately, we are all busier than we like and receive more journals than we have time to read. To make JHM even more germane and stimulating, we are initiating Hospital Images Dx. The main goal of Hospital Images Dx will be to show interesting images that a hospitalist might encounter, both the common and the obscure. Images, whether subtle or awe‐inspiring, should generally be able to speak the proverbial thousand words. To supplement those thousand words spoken by the submitted image, accompanying text will be limited to 250 words. The text should give a brief clinical summary of the patient's problem, relevant adjunct data, and 1 or 2 succinct teaching points related to the image. Our goal, simply stated, is for the reader to walk away after reading Hospital Images Dx with an image and a couple of key teaching points stored away for a rainy day.
We anticipate a substantial number of exciting submissions to Hospital Images Dx. Health care providers clearly get excited about the things they see as well as about the recordsthe imagesthat document both mundane and unusual encounters with a patient or a patient's data. We hope to tap into this enthusiasm and to teach a few things along the way. The editors look forward to receiving your Hospital Images Dx submission soon!
Of the many skills a hospitalist nurtures and develops, seeing and processing visual images is paramount. From the moment we enter the hospital each day, we look at patient faces, trying to fathom their levels of pain, of illness, and of response to therapy. We look at their rashes, facial droops, surgical wounds, and neck vein elevation. When we are done visually scrutinizing our patients, we inspect their rhythm strips or electrocardiograms and then move on to their X‐rays and advanced imaging.
Although we are, in a sense, slaves to the images before us, we also enjoy medicine for the challenge that these images providethere is always something novel to see. For the experienced clinician, a deep sense of satisfaction perfuses our limbic system with the quick recognition of the delta wave of Wolf‐Parkinson‐White on an EKG or the first vesicle of a zoster outbreak in a patient with initially unexplained cutaneous pain. What we recognize easily tends to come from having seen something beforefor better or worse, we depend on pattern recognition.
Unfortunately, we are all busier than we like and receive more journals than we have time to read. To make JHM even more germane and stimulating, we are initiating Hospital Images Dx. The main goal of Hospital Images Dx will be to show interesting images that a hospitalist might encounter, both the common and the obscure. Images, whether subtle or awe‐inspiring, should generally be able to speak the proverbial thousand words. To supplement those thousand words spoken by the submitted image, accompanying text will be limited to 250 words. The text should give a brief clinical summary of the patient's problem, relevant adjunct data, and 1 or 2 succinct teaching points related to the image. Our goal, simply stated, is for the reader to walk away after reading Hospital Images Dx with an image and a couple of key teaching points stored away for a rainy day.
We anticipate a substantial number of exciting submissions to Hospital Images Dx. Health care providers clearly get excited about the things they see as well as about the recordsthe imagesthat document both mundane and unusual encounters with a patient or a patient's data. We hope to tap into this enthusiasm and to teach a few things along the way. The editors look forward to receiving your Hospital Images Dx submission soon!
Of the many skills a hospitalist nurtures and develops, seeing and processing visual images is paramount. From the moment we enter the hospital each day, we look at patient faces, trying to fathom their levels of pain, of illness, and of response to therapy. We look at their rashes, facial droops, surgical wounds, and neck vein elevation. When we are done visually scrutinizing our patients, we inspect their rhythm strips or electrocardiograms and then move on to their X‐rays and advanced imaging.
Although we are, in a sense, slaves to the images before us, we also enjoy medicine for the challenge that these images providethere is always something novel to see. For the experienced clinician, a deep sense of satisfaction perfuses our limbic system with the quick recognition of the delta wave of Wolf‐Parkinson‐White on an EKG or the first vesicle of a zoster outbreak in a patient with initially unexplained cutaneous pain. What we recognize easily tends to come from having seen something beforefor better or worse, we depend on pattern recognition.
Unfortunately, we are all busier than we like and receive more journals than we have time to read. To make JHM even more germane and stimulating, we are initiating Hospital Images Dx. The main goal of Hospital Images Dx will be to show interesting images that a hospitalist might encounter, both the common and the obscure. Images, whether subtle or awe‐inspiring, should generally be able to speak the proverbial thousand words. To supplement those thousand words spoken by the submitted image, accompanying text will be limited to 250 words. The text should give a brief clinical summary of the patient's problem, relevant adjunct data, and 1 or 2 succinct teaching points related to the image. Our goal, simply stated, is for the reader to walk away after reading Hospital Images Dx with an image and a couple of key teaching points stored away for a rainy day.
We anticipate a substantial number of exciting submissions to Hospital Images Dx. Health care providers clearly get excited about the things they see as well as about the recordsthe imagesthat document both mundane and unusual encounters with a patient or a patient's data. We hope to tap into this enthusiasm and to teach a few things along the way. The editors look forward to receiving your Hospital Images Dx submission soon!