User login
Heart on the right may sometimes be ‘right’
A 76-year-old man presented to the emergency department with right-sided exertional chest pain radiating to the right shoulder and arm associated with shortness of breath. His vital signs were normal. On clinical examination, the cardiac apex was palpated on the right side, 9 cm from the midsternal line in the fifth intercostal space.
A standard left-sided 12-lead electrocardiogram (ECG) showed right-axis deviation and inverted P, QRS, and T waves in leads I and aVL (Figure 1). Although these changes are also seen when the right and left arm electrode wires are transposed, the precordial lead morphology in such a situation would usually be normal. In our patient, the precordial leads showed the absence or even slight reversal of R-wave progression, a feature indicative of dextrocardia.1,2
In patients with dextrocardia, right-sided hookup of the electrodes is usually necessary for proper interpretation of the ECG. When this was done in our patient, the ECG showed a normal cardiac axis, a negative QRS complex in lead aVR, a positive P wave and other complexes in lead I, and normal R-wave progression in the precordial leads—findings suggestive of dextrocardia (Figure 2).
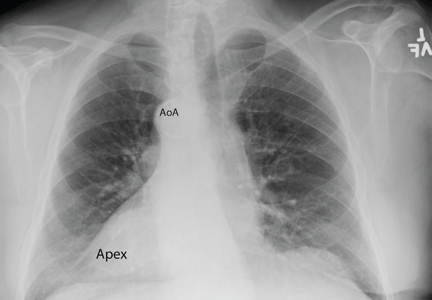
Chest radiography showed a right-sided cardiac silhouette (Figure 3), and computed tomography of the abdomen (Figure 4) revealed the liver positioned on the left side and the spleen on the right, confirming the diagnosis of situs inversus totalis. The ECG showed dextrocardia, but no other abnormalities. The patient eventually underwent coronary angiography, which showed nonobstructive coronary artery disease.
DEXTROCARDIA, OTHER CONGENITAL CARDIOVASCULAR MALFORMATIONS
Dextrocardia was first described in early 17th century.1 Situs solitus is the normal position of the heart and viscera, whereas situs inversus is a mirror-image anatomic arrangement of the organs. Situs inversus with dextrocardia, also called situs inversus totalis, is a rare condition (with a prevalence of 1 in 8,000) in which the heart and descending aorta are on the right and the thoracic and abdominal viscera are usually mirror images of the normal morphology.1,3,4 A mirror-image sinus node lies at the junction of the left superior vena cava and the left-sided (morphologic right) atrium.1 People with situs inversus with dextrocardia are usually asymptomatic and have a normal life expectancy.1,2 Situs inversus with levocardia is a rare condition in which the heart is in the normal position but the viscera are in the dextro-position. This anomaly has a prevalence of 1 in 22,000.5
Atrial situs almost always corresponds to visceral situs. However, when the alignment of the atria and viscera is inconsistent and situs cannot be determined clearly because of the malpositioning of organs, the condition is called “situs ambiguous.” This is very rare, with a prevalence of 1 in 40,000.6
Risk factors
The cause of congenital cardiovascular malformations such as these is not known, but risk factors include positive family history, maternal diabetes, and cocaine use in the first trimester.7
The prevalence of congenital heart disease in patients with situs inversus with dextrocardia is low and ranges from 2% to 5%. This is in contrast to situs solitus with dextrocardia (isolated dextrocardia), which is almost always associated with cardiovascular anomalies.2,4 Kartagener syndrome—the triad of situs inversus, sinusitis, and bronchiectasis—occurs in 25% of people with situs inversus with dextrocardia.4 Situs inversus with levocardia is also frequently associated with cardiac anomalies.5
The major features of dextrocardia on ECG are:
- Negative P wave, QRS complex, and T wave in lead I
- Positive QRS complex in aVR
- Right-axis deviation
- Reversal of R-wave progression in the precordial leads.
Ventricular activation and repolarization are reversed, resulting in a negative QRS complex and an inverted T wave in lead I. The absence of R-wave progression in the precordial leads helps differentiate mirror-image dextrocardia from erroneously reversed limb-electrode placement, which shows normal R-wave progression from V1 to V6 while showing similar features to those seen in dextrocardia in the limb leads.2 In right-sided hookup, the limb electrodes are reversed, and the chest electrodes are recorded from the right precordium.
CORONARY INTERVENTIONS REQUIRE SPECIAL CONSIDERATION
In patients with dextrocardia, coronary interventions can be challenging because of the mirror-image position of the coronary ostia and the aortic arch.8 These patients also need careful imaging, consideration of other associated congenital cardiac abnormalities, and detailed planning before cardiac surgery, including coronary artery bypass grafting.9
Patients with dextrocardia may present with cardiac symptoms localized to the right side of the body and have confusing clinical and diagnostic findings. Keeping dextrocardia and other such anomalies in mind can prevent delay in appropriately directed interventions. In a patient such as ours, the heart on the right side of the chest may indeed be “right.” Still, diagnostic tests to look for disorders encountered with dextrocardia may be necessary.
- Perloff JK. The cardiac malpositions. Am J Cardiol 2011; 108:1352–1361.
- Tanawuttiwat T, Vasaiwala S, Dia M. ECG image of the month. Mirror mirror. Am J Med 2010; 123:34–36.
- Douard R, Feldman A, Bargy F, Loric S, Delmas V. Anomalies of lateralization in man: a case of total situs in-versus. Surg Radiol Anat 2000; 22:293–297.
- Maldjian PD, Saric M. Approach to dextrocardia in adults: review. AJR Am J Roentgenol 2007; 188(suppl 6):S39–S49.
- Gindes L, Hegesh J, Barkai G, Jacobson JM, Achiron R. Isolated levocardia: prenatal diagnosis, clinical im-portance, and literature review. J Ultrasound Med 2007; 26:361–365.
- Abut E, Arman A, Güveli H, et al. Malposition of internal organs: a case of situs ambiguous anomaly in an adult. Turk J Gastroenterol 2003; 14:151–155.
- Kuehl KS, Loffredo C. Risk factors for heart disease associated with abnormal sidedness. Teratology 2002; 66:242–248.
- Aksoy S, Cam N, Gurkan U, Altay S, Bozbay M, Agirbasli M. Primary percutaneous intervention: for acute myo-cardial infarction in a patient with dextrocardia and situs inversus. Tex Heart Inst J 2012; 39:140–141.
- Murtuza B, Gupta P, Goli G, Lall KS. Coronary revascularization in adults with dextrocardia: surgical implications of the anatomic variants. Tex Heart Inst J 2010; 37:633–640.
A 76-year-old man presented to the emergency department with right-sided exertional chest pain radiating to the right shoulder and arm associated with shortness of breath. His vital signs were normal. On clinical examination, the cardiac apex was palpated on the right side, 9 cm from the midsternal line in the fifth intercostal space.
A standard left-sided 12-lead electrocardiogram (ECG) showed right-axis deviation and inverted P, QRS, and T waves in leads I and aVL (Figure 1). Although these changes are also seen when the right and left arm electrode wires are transposed, the precordial lead morphology in such a situation would usually be normal. In our patient, the precordial leads showed the absence or even slight reversal of R-wave progression, a feature indicative of dextrocardia.1,2
In patients with dextrocardia, right-sided hookup of the electrodes is usually necessary for proper interpretation of the ECG. When this was done in our patient, the ECG showed a normal cardiac axis, a negative QRS complex in lead aVR, a positive P wave and other complexes in lead I, and normal R-wave progression in the precordial leads—findings suggestive of dextrocardia (Figure 2).
Chest radiography showed a right-sided cardiac silhouette (Figure 3), and computed tomography of the abdomen (Figure 4) revealed the liver positioned on the left side and the spleen on the right, confirming the diagnosis of situs inversus totalis. The ECG showed dextrocardia, but no other abnormalities. The patient eventually underwent coronary angiography, which showed nonobstructive coronary artery disease.
DEXTROCARDIA, OTHER CONGENITAL CARDIOVASCULAR MALFORMATIONS
Dextrocardia was first described in early 17th century.1 Situs solitus is the normal position of the heart and viscera, whereas situs inversus is a mirror-image anatomic arrangement of the organs. Situs inversus with dextrocardia, also called situs inversus totalis, is a rare condition (with a prevalence of 1 in 8,000) in which the heart and descending aorta are on the right and the thoracic and abdominal viscera are usually mirror images of the normal morphology.1,3,4 A mirror-image sinus node lies at the junction of the left superior vena cava and the left-sided (morphologic right) atrium.1 People with situs inversus with dextrocardia are usually asymptomatic and have a normal life expectancy.1,2 Situs inversus with levocardia is a rare condition in which the heart is in the normal position but the viscera are in the dextro-position. This anomaly has a prevalence of 1 in 22,000.5
Atrial situs almost always corresponds to visceral situs. However, when the alignment of the atria and viscera is inconsistent and situs cannot be determined clearly because of the malpositioning of organs, the condition is called “situs ambiguous.” This is very rare, with a prevalence of 1 in 40,000.6
Risk factors
The cause of congenital cardiovascular malformations such as these is not known, but risk factors include positive family history, maternal diabetes, and cocaine use in the first trimester.7
The prevalence of congenital heart disease in patients with situs inversus with dextrocardia is low and ranges from 2% to 5%. This is in contrast to situs solitus with dextrocardia (isolated dextrocardia), which is almost always associated with cardiovascular anomalies.2,4 Kartagener syndrome—the triad of situs inversus, sinusitis, and bronchiectasis—occurs in 25% of people with situs inversus with dextrocardia.4 Situs inversus with levocardia is also frequently associated with cardiac anomalies.5
The major features of dextrocardia on ECG are:
- Negative P wave, QRS complex, and T wave in lead I
- Positive QRS complex in aVR
- Right-axis deviation
- Reversal of R-wave progression in the precordial leads.
Ventricular activation and repolarization are reversed, resulting in a negative QRS complex and an inverted T wave in lead I. The absence of R-wave progression in the precordial leads helps differentiate mirror-image dextrocardia from erroneously reversed limb-electrode placement, which shows normal R-wave progression from V1 to V6 while showing similar features to those seen in dextrocardia in the limb leads.2 In right-sided hookup, the limb electrodes are reversed, and the chest electrodes are recorded from the right precordium.
CORONARY INTERVENTIONS REQUIRE SPECIAL CONSIDERATION
In patients with dextrocardia, coronary interventions can be challenging because of the mirror-image position of the coronary ostia and the aortic arch.8 These patients also need careful imaging, consideration of other associated congenital cardiac abnormalities, and detailed planning before cardiac surgery, including coronary artery bypass grafting.9
Patients with dextrocardia may present with cardiac symptoms localized to the right side of the body and have confusing clinical and diagnostic findings. Keeping dextrocardia and other such anomalies in mind can prevent delay in appropriately directed interventions. In a patient such as ours, the heart on the right side of the chest may indeed be “right.” Still, diagnostic tests to look for disorders encountered with dextrocardia may be necessary.
A 76-year-old man presented to the emergency department with right-sided exertional chest pain radiating to the right shoulder and arm associated with shortness of breath. His vital signs were normal. On clinical examination, the cardiac apex was palpated on the right side, 9 cm from the midsternal line in the fifth intercostal space.
A standard left-sided 12-lead electrocardiogram (ECG) showed right-axis deviation and inverted P, QRS, and T waves in leads I and aVL (Figure 1). Although these changes are also seen when the right and left arm electrode wires are transposed, the precordial lead morphology in such a situation would usually be normal. In our patient, the precordial leads showed the absence or even slight reversal of R-wave progression, a feature indicative of dextrocardia.1,2
In patients with dextrocardia, right-sided hookup of the electrodes is usually necessary for proper interpretation of the ECG. When this was done in our patient, the ECG showed a normal cardiac axis, a negative QRS complex in lead aVR, a positive P wave and other complexes in lead I, and normal R-wave progression in the precordial leads—findings suggestive of dextrocardia (Figure 2).
Chest radiography showed a right-sided cardiac silhouette (Figure 3), and computed tomography of the abdomen (Figure 4) revealed the liver positioned on the left side and the spleen on the right, confirming the diagnosis of situs inversus totalis. The ECG showed dextrocardia, but no other abnormalities. The patient eventually underwent coronary angiography, which showed nonobstructive coronary artery disease.
DEXTROCARDIA, OTHER CONGENITAL CARDIOVASCULAR MALFORMATIONS
Dextrocardia was first described in early 17th century.1 Situs solitus is the normal position of the heart and viscera, whereas situs inversus is a mirror-image anatomic arrangement of the organs. Situs inversus with dextrocardia, also called situs inversus totalis, is a rare condition (with a prevalence of 1 in 8,000) in which the heart and descending aorta are on the right and the thoracic and abdominal viscera are usually mirror images of the normal morphology.1,3,4 A mirror-image sinus node lies at the junction of the left superior vena cava and the left-sided (morphologic right) atrium.1 People with situs inversus with dextrocardia are usually asymptomatic and have a normal life expectancy.1,2 Situs inversus with levocardia is a rare condition in which the heart is in the normal position but the viscera are in the dextro-position. This anomaly has a prevalence of 1 in 22,000.5
Atrial situs almost always corresponds to visceral situs. However, when the alignment of the atria and viscera is inconsistent and situs cannot be determined clearly because of the malpositioning of organs, the condition is called “situs ambiguous.” This is very rare, with a prevalence of 1 in 40,000.6
Risk factors
The cause of congenital cardiovascular malformations such as these is not known, but risk factors include positive family history, maternal diabetes, and cocaine use in the first trimester.7
The prevalence of congenital heart disease in patients with situs inversus with dextrocardia is low and ranges from 2% to 5%. This is in contrast to situs solitus with dextrocardia (isolated dextrocardia), which is almost always associated with cardiovascular anomalies.2,4 Kartagener syndrome—the triad of situs inversus, sinusitis, and bronchiectasis—occurs in 25% of people with situs inversus with dextrocardia.4 Situs inversus with levocardia is also frequently associated with cardiac anomalies.5
The major features of dextrocardia on ECG are:
- Negative P wave, QRS complex, and T wave in lead I
- Positive QRS complex in aVR
- Right-axis deviation
- Reversal of R-wave progression in the precordial leads.
Ventricular activation and repolarization are reversed, resulting in a negative QRS complex and an inverted T wave in lead I. The absence of R-wave progression in the precordial leads helps differentiate mirror-image dextrocardia from erroneously reversed limb-electrode placement, which shows normal R-wave progression from V1 to V6 while showing similar features to those seen in dextrocardia in the limb leads.2 In right-sided hookup, the limb electrodes are reversed, and the chest electrodes are recorded from the right precordium.
CORONARY INTERVENTIONS REQUIRE SPECIAL CONSIDERATION
In patients with dextrocardia, coronary interventions can be challenging because of the mirror-image position of the coronary ostia and the aortic arch.8 These patients also need careful imaging, consideration of other associated congenital cardiac abnormalities, and detailed planning before cardiac surgery, including coronary artery bypass grafting.9
Patients with dextrocardia may present with cardiac symptoms localized to the right side of the body and have confusing clinical and diagnostic findings. Keeping dextrocardia and other such anomalies in mind can prevent delay in appropriately directed interventions. In a patient such as ours, the heart on the right side of the chest may indeed be “right.” Still, diagnostic tests to look for disorders encountered with dextrocardia may be necessary.
- Perloff JK. The cardiac malpositions. Am J Cardiol 2011; 108:1352–1361.
- Tanawuttiwat T, Vasaiwala S, Dia M. ECG image of the month. Mirror mirror. Am J Med 2010; 123:34–36.
- Douard R, Feldman A, Bargy F, Loric S, Delmas V. Anomalies of lateralization in man: a case of total situs in-versus. Surg Radiol Anat 2000; 22:293–297.
- Maldjian PD, Saric M. Approach to dextrocardia in adults: review. AJR Am J Roentgenol 2007; 188(suppl 6):S39–S49.
- Gindes L, Hegesh J, Barkai G, Jacobson JM, Achiron R. Isolated levocardia: prenatal diagnosis, clinical im-portance, and literature review. J Ultrasound Med 2007; 26:361–365.
- Abut E, Arman A, Güveli H, et al. Malposition of internal organs: a case of situs ambiguous anomaly in an adult. Turk J Gastroenterol 2003; 14:151–155.
- Kuehl KS, Loffredo C. Risk factors for heart disease associated with abnormal sidedness. Teratology 2002; 66:242–248.
- Aksoy S, Cam N, Gurkan U, Altay S, Bozbay M, Agirbasli M. Primary percutaneous intervention: for acute myo-cardial infarction in a patient with dextrocardia and situs inversus. Tex Heart Inst J 2012; 39:140–141.
- Murtuza B, Gupta P, Goli G, Lall KS. Coronary revascularization in adults with dextrocardia: surgical implications of the anatomic variants. Tex Heart Inst J 2010; 37:633–640.
- Perloff JK. The cardiac malpositions. Am J Cardiol 2011; 108:1352–1361.
- Tanawuttiwat T, Vasaiwala S, Dia M. ECG image of the month. Mirror mirror. Am J Med 2010; 123:34–36.
- Douard R, Feldman A, Bargy F, Loric S, Delmas V. Anomalies of lateralization in man: a case of total situs in-versus. Surg Radiol Anat 2000; 22:293–297.
- Maldjian PD, Saric M. Approach to dextrocardia in adults: review. AJR Am J Roentgenol 2007; 188(suppl 6):S39–S49.
- Gindes L, Hegesh J, Barkai G, Jacobson JM, Achiron R. Isolated levocardia: prenatal diagnosis, clinical im-portance, and literature review. J Ultrasound Med 2007; 26:361–365.
- Abut E, Arman A, Güveli H, et al. Malposition of internal organs: a case of situs ambiguous anomaly in an adult. Turk J Gastroenterol 2003; 14:151–155.
- Kuehl KS, Loffredo C. Risk factors for heart disease associated with abnormal sidedness. Teratology 2002; 66:242–248.
- Aksoy S, Cam N, Gurkan U, Altay S, Bozbay M, Agirbasli M. Primary percutaneous intervention: for acute myo-cardial infarction in a patient with dextrocardia and situs inversus. Tex Heart Inst J 2012; 39:140–141.
- Murtuza B, Gupta P, Goli G, Lall KS. Coronary revascularization in adults with dextrocardia: surgical implications of the anatomic variants. Tex Heart Inst J 2010; 37:633–640.
Should all patients have a resting 12-lead ECG before elective noncardiac surgery?
A 55-year-old lawyer with hypertension well controlled on lisinopril and amlodipine is scheduled for elective hernia repair under general anesthesia. His surgeon has referred him for a preoperative evaluation. He has never smoked, runs 4 miles on days off from work, and enjoys long hiking trips. On examination, his body mass index is 26 kg/m2 and his blood pressure is 130/78 mm Hg; his cardiac examination and the rest of the clinical examination are unremarkable. He asks if he should have an electrocardiogram (ECG) as a part of his workup.
A preoperative ECG is not routinely recommended in all asymptomatic patients undergoing noncardiac surgery.
Consider obtaining an ECG in patients planning to undergo a high-risk surgical procedure, especially if they have one or more clinical risk factors for coronary artery disease, and in patients undergoing elevated-cardiac-risk surgery who are known to have coronary artery disease, chronic heart failure, peripheral arterial disease, or cerebrovascular disease. However, a preoperative ECG is not routinely recommended for patients perceived to be at low cardiac risk who are planning to undergo low-risk surgery. In those patients it could delay surgery unnecessarily, cause further unnecessary testing, drive up costs, and increase patient anxiety.
Here we discuss the perioperative cardiac risk based on type of surgery and patient characteristics and summarize the current guidelines and recommendations on obtaining a preoperative 12-lead ECG in patients undergoing noncardiac surgery.
RISK DEPENDS ON TYPE OF SURGERY AND PATIENT FACTORS
Physicians, including primary care physicians, hospitalists, cardiologists, and anesthesiologists, are routinely asked to evaluate patients before surgical procedures. The purpose of the preoperative evaluation is to optimize existing medical conditions, to identify undiagnosed conditions that can increase risk of perioperative morbidity and death, and to suggest strategies to mitigate risk.1,2
Cardiac risk is multifactorial, and risk factors for postoperative adverse cardiac events include the type of surgery and patient factors.1,3
Cardiac risk based on type of surgery
Low-risk procedures are those in which the risk of a perioperative major adverse cardiac event is less than 1%.1,4 Examples:
- Ambulatory surgery
- Breast or plastic surgery
- Cataract surgery
- Endoscopic procedures.
Elevated-risk procedures are those in which the risk is 1% or higher. Examples:
- Intraperitoneal surgery
- Intrathoracic surgery
- Carotid endarterectomy
- Head and neck surgery
- Orthopedic surgery
- Prostate surgery
- Aortic surgery
- Major vascular surgery
- Peripheral arterial surgery.
Cardiac risk based on patient factors
The 2014 American College of Cardiology and American Heart Association (ACC/AHA) perioperative guidelines list a number of clinical risk factors for perioperative cardiac morbidity and death.1 These include coronary artery disease, chronic heart failure, clinically suspected moderate or greater degrees of valvular heart disease, arrhythmias, conduction disorders, pulmonary vascular disease, and adult congenital heart disease.
Patients with these conditions and patients with unstable coronary syndromes warrant preoperative ECGs and sometimes even urgent interventions before any nonemergency surgery, provided such interventions would affect decision-making and perioperative care.1
The risk of perioperative cardiac morbidity and death can be calculated using either the Revised Cardiac Risk Index scoring system or the American College of Surgeons National Surgical Quality Improvement Program calculator.157 The former is fairly simple, validated, and accepted, while the latter requires use of online calculators (eg, www.surgicalriskcalculator.com/miorcardiacarrest, www.riskcalculator.facs.org).
The Revised Cardiac Risk Index has six clinical predictors of major perioperative cardiac events:
- History of cerebrovascular disease
- Prior or current compensated congestive heart failure
- History of coronary artery disease
- Insulin-dependent diabetes mellitus
- Renal insufficiency, defined as a serum creatinine level of 2 mg/dL or higher
- Patient undergoing suprainguinal vascular, intraperitoneal, or intrathoracic surgery.
A patient who has 0 or 1 of these predictors would have a low risk of a major adverse cardiac event, whereas a patient with 2 or more would have elevated risk. These risk factors must be taken into consideration to determine the need, if any, for a preoperative ECG.
What an ECG can tell us
Abnormalities such as left ventricular hypertrophy, ST-segment depression, and pathologic Q waves on a preoperative ECG in a patient undergoing an elevated-risk surgical procedure may predict adverse perioperative cardiac events.3,6
In a retrospective study of 23,036 patients, Noordzij et al7 found that in patients undergoing elevated-risk surgery, those with an abnormal preoperative ECG had a higher incidence of cardiovascular death than those with a normal ECG. However, a preoperative ECG was obtained only in patients with established coronary artery disease or risk factors for cardiovascular disease. Hence, although an abnormal ECG in such patients undergoing elevated-risk surgery was predictive of adverse postoperative cardiac outcomes, we cannot say that the same would apply to patients without these characteristics undergoing elevated-risk surgery.
In a prospective observational study of patients with known coronary artery disease undergoing major noncardiac surgery, a preoperative ECG was found to contain prognostic information and was predictive of long-term outcome independent of clinical findings and perioperative ischemia.8
CURRENT GUIDELINES AND RECOMMENDATIONS
Several guidelines address whether to order a preoperative ECG but are mostly based on low-level evidence and expert opinion.1,2,6,9
Current guidelines recommend obtaining a preoperative ECG in patients with known coronary, peripheral arterial, or cerebrovascular disease.1,6,9
Obesity and associated comorbidities such as coronary artery disease, heart failure, systemic hypertension, and sleep apnea can predispose to increased perioperative complications. A preoperative 12-lead ECG is reasonable in morbidly obese patients (body mass index ≥ 40 kg/m2) and in obese patients (body mass index ≥ 30 kg/m2) with at least one risk factor for coronary artery disease or poor exercise tolerance, or both.10
Liu et al11 looked at the predictive value of a preoperative 12-lead ECG in 513 elderly patients (age ≥ 70) undergoing noncardiac surgery and found that electrocardiographic abnormalities were not predictive of adverse cardiac outcomes. In this study, although electrocardiographic abnormalities were common (noted in 75% of the patients), they were nonspecific and less useful in predicting postoperative cardiac complications than was the presence of comorbidities.11 Age alone as a cutoff for obtaining a preoperative ECG is not predictive of postoperative outcomes and a preoperative ECG is not warranted in all elderly patients. This is also reflected in current ACC/AHA guidelines on perioperative cardiovascular evaluation1 and is a change from prior ACC/AHA guidelines when age was used as a criterion for preoperative ECGs.12
Current guidelines do not recommend getting a preoperative ECG in asymptomatic patients undergoing low-cardiac-risk surgery.1,4,9
Although the ideal time for ordering an ECG before a planned surgery is unknown, obtaining one within 90 days before the surgery is considered adequate in stable patients in whom an ECG is indicated.1
BACK TO OUR PATIENT
On the basis of current evidence, our patient does not need a preoperative ECG, as it is unlikely to alter his perioperative management and instead may delay his surgery unnecessarily if any nonspecific changes prompt further cardiac workup.
CLINICAL BOTTOM LINE
Although frequently ordered in clinical practice, preoperative electrocardiography has a limited role in predicting postoperative outcome and should be ordered only in the appropriate clinical setting.1 Moreover, there is little evidence that outcomes are better if we obtain an ECG before surgery. The clinician should consider patient factors and the type of surgery before ordering diagnostic tests, including electrocardiography.
In asymptomatic patients undergoing nonemergent surgery:
- It is reasonable to obtain a preoperative ECG in patients with known coronary artery disease, significant arrhythmia, peripheral arterial disease, cerebrovascular disease, chronic heart failure, or other significant structural heart disease undergoing elevated-cardiac-risk surgery.
- Do not order a preoperative ECG in asymptomatic patients undergoing low-risk surgery.
- Obtaining a preoperative ECG is reasonable in morbidly obese patients and in obese patients with one or more risk factors for coronary artery disease, or poor exercise tolerance, undergoing high-risk surgery.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. J Am Coll Cardiol 2014; Jul 29. 10.1016/j.jacc.2014.07.944. [Epub ahead of print]
- Feely MA, Collins CS, Daniels PR, Kebede EB, Jatoi A, Mauck KF. Preoperative testing before noncardiac surgery: guidelines and recommendations. Am Fam Physician 2013; 87:414–418.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Task Force for Preoperative Cardiac Risk Assessment and Perioperative Cardiac Management in Non-cardiac Surgery; European Society of Cardiology (ESC); Poldermans D, Bax JJ, Boersma E, et al. Guidelines for pre-operative cardiac risk assessment and perioperative cardiac management in non-cardiac surgery. Eur Heart J 2009; 30:2769–2812.
- Bilimoria KY, Liu Y, Paruch JL, Zhou L, Kmiecik TE, Ko CY, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013; 217:833–842.
- Landesberg G, Einav S, Christopherson R, et al. Perioperative ischemia and cardiac complications in major vascular surgery: importance of the preoperative twelve-lead electrocardiogram. J Vasc Surg 1997; 26:570–578.
- Noordzij PG, Boersma E, Bax JJ, et al. Prognostic value of routine preoperative electrocardiography in patients undergoing noncardiac surgery. Am J Cardiol 2006; 97:1103–1106.
- Jeger RV, Probst C, Arsenic R, et al. Long-term prognostic value of the preoperative 12-lead electrocardiogram before major noncardiac surgery in coronary artery disease. Am Heart J 2006; 151:508–513.
- Committee on Standards and Practice Parameters; Apfelbaum JL, Connis RT, Nickinovich DG, Pasternak LR, Arens JF, Caplan RA, et al. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology 2012; 116:522–538.
- Poirier P, Alpert MA, Fleisher LA, et al. Cardiovascular evaluation and management of severely obese patients undergoing surgery: a science advisory from the American Heart Association. Circulation 2009; 120:86–95.
- Liu LL, Dzankic S, Leung JM. Preoperative electrocardiogram abnormalities do not predict postoperative cardiac complications in geriatric surgical patients. J Am Geriatr Soc 2002; 50:1186–1191.
- Eagle KA, Berger PB, Calkins H, et al; American College of Cardiology; American Heart Association. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary. J Am Coll Cardiol 2002; 39:542–553.
A 55-year-old lawyer with hypertension well controlled on lisinopril and amlodipine is scheduled for elective hernia repair under general anesthesia. His surgeon has referred him for a preoperative evaluation. He has never smoked, runs 4 miles on days off from work, and enjoys long hiking trips. On examination, his body mass index is 26 kg/m2 and his blood pressure is 130/78 mm Hg; his cardiac examination and the rest of the clinical examination are unremarkable. He asks if he should have an electrocardiogram (ECG) as a part of his workup.
A preoperative ECG is not routinely recommended in all asymptomatic patients undergoing noncardiac surgery.
Consider obtaining an ECG in patients planning to undergo a high-risk surgical procedure, especially if they have one or more clinical risk factors for coronary artery disease, and in patients undergoing elevated-cardiac-risk surgery who are known to have coronary artery disease, chronic heart failure, peripheral arterial disease, or cerebrovascular disease. However, a preoperative ECG is not routinely recommended for patients perceived to be at low cardiac risk who are planning to undergo low-risk surgery. In those patients it could delay surgery unnecessarily, cause further unnecessary testing, drive up costs, and increase patient anxiety.
Here we discuss the perioperative cardiac risk based on type of surgery and patient characteristics and summarize the current guidelines and recommendations on obtaining a preoperative 12-lead ECG in patients undergoing noncardiac surgery.
RISK DEPENDS ON TYPE OF SURGERY AND PATIENT FACTORS
Physicians, including primary care physicians, hospitalists, cardiologists, and anesthesiologists, are routinely asked to evaluate patients before surgical procedures. The purpose of the preoperative evaluation is to optimize existing medical conditions, to identify undiagnosed conditions that can increase risk of perioperative morbidity and death, and to suggest strategies to mitigate risk.1,2
Cardiac risk is multifactorial, and risk factors for postoperative adverse cardiac events include the type of surgery and patient factors.1,3
Cardiac risk based on type of surgery
Low-risk procedures are those in which the risk of a perioperative major adverse cardiac event is less than 1%.1,4 Examples:
- Ambulatory surgery
- Breast or plastic surgery
- Cataract surgery
- Endoscopic procedures.
Elevated-risk procedures are those in which the risk is 1% or higher. Examples:
- Intraperitoneal surgery
- Intrathoracic surgery
- Carotid endarterectomy
- Head and neck surgery
- Orthopedic surgery
- Prostate surgery
- Aortic surgery
- Major vascular surgery
- Peripheral arterial surgery.
Cardiac risk based on patient factors
The 2014 American College of Cardiology and American Heart Association (ACC/AHA) perioperative guidelines list a number of clinical risk factors for perioperative cardiac morbidity and death.1 These include coronary artery disease, chronic heart failure, clinically suspected moderate or greater degrees of valvular heart disease, arrhythmias, conduction disorders, pulmonary vascular disease, and adult congenital heart disease.
Patients with these conditions and patients with unstable coronary syndromes warrant preoperative ECGs and sometimes even urgent interventions before any nonemergency surgery, provided such interventions would affect decision-making and perioperative care.1
The risk of perioperative cardiac morbidity and death can be calculated using either the Revised Cardiac Risk Index scoring system or the American College of Surgeons National Surgical Quality Improvement Program calculator.157 The former is fairly simple, validated, and accepted, while the latter requires use of online calculators (eg, www.surgicalriskcalculator.com/miorcardiacarrest, www.riskcalculator.facs.org).
The Revised Cardiac Risk Index has six clinical predictors of major perioperative cardiac events:
- History of cerebrovascular disease
- Prior or current compensated congestive heart failure
- History of coronary artery disease
- Insulin-dependent diabetes mellitus
- Renal insufficiency, defined as a serum creatinine level of 2 mg/dL or higher
- Patient undergoing suprainguinal vascular, intraperitoneal, or intrathoracic surgery.
A patient who has 0 or 1 of these predictors would have a low risk of a major adverse cardiac event, whereas a patient with 2 or more would have elevated risk. These risk factors must be taken into consideration to determine the need, if any, for a preoperative ECG.
What an ECG can tell us
Abnormalities such as left ventricular hypertrophy, ST-segment depression, and pathologic Q waves on a preoperative ECG in a patient undergoing an elevated-risk surgical procedure may predict adverse perioperative cardiac events.3,6
In a retrospective study of 23,036 patients, Noordzij et al7 found that in patients undergoing elevated-risk surgery, those with an abnormal preoperative ECG had a higher incidence of cardiovascular death than those with a normal ECG. However, a preoperative ECG was obtained only in patients with established coronary artery disease or risk factors for cardiovascular disease. Hence, although an abnormal ECG in such patients undergoing elevated-risk surgery was predictive of adverse postoperative cardiac outcomes, we cannot say that the same would apply to patients without these characteristics undergoing elevated-risk surgery.
In a prospective observational study of patients with known coronary artery disease undergoing major noncardiac surgery, a preoperative ECG was found to contain prognostic information and was predictive of long-term outcome independent of clinical findings and perioperative ischemia.8
CURRENT GUIDELINES AND RECOMMENDATIONS
Several guidelines address whether to order a preoperative ECG but are mostly based on low-level evidence and expert opinion.1,2,6,9
Current guidelines recommend obtaining a preoperative ECG in patients with known coronary, peripheral arterial, or cerebrovascular disease.1,6,9
Obesity and associated comorbidities such as coronary artery disease, heart failure, systemic hypertension, and sleep apnea can predispose to increased perioperative complications. A preoperative 12-lead ECG is reasonable in morbidly obese patients (body mass index ≥ 40 kg/m2) and in obese patients (body mass index ≥ 30 kg/m2) with at least one risk factor for coronary artery disease or poor exercise tolerance, or both.10
Liu et al11 looked at the predictive value of a preoperative 12-lead ECG in 513 elderly patients (age ≥ 70) undergoing noncardiac surgery and found that electrocardiographic abnormalities were not predictive of adverse cardiac outcomes. In this study, although electrocardiographic abnormalities were common (noted in 75% of the patients), they were nonspecific and less useful in predicting postoperative cardiac complications than was the presence of comorbidities.11 Age alone as a cutoff for obtaining a preoperative ECG is not predictive of postoperative outcomes and a preoperative ECG is not warranted in all elderly patients. This is also reflected in current ACC/AHA guidelines on perioperative cardiovascular evaluation1 and is a change from prior ACC/AHA guidelines when age was used as a criterion for preoperative ECGs.12
Current guidelines do not recommend getting a preoperative ECG in asymptomatic patients undergoing low-cardiac-risk surgery.1,4,9
Although the ideal time for ordering an ECG before a planned surgery is unknown, obtaining one within 90 days before the surgery is considered adequate in stable patients in whom an ECG is indicated.1
BACK TO OUR PATIENT
On the basis of current evidence, our patient does not need a preoperative ECG, as it is unlikely to alter his perioperative management and instead may delay his surgery unnecessarily if any nonspecific changes prompt further cardiac workup.
CLINICAL BOTTOM LINE
Although frequently ordered in clinical practice, preoperative electrocardiography has a limited role in predicting postoperative outcome and should be ordered only in the appropriate clinical setting.1 Moreover, there is little evidence that outcomes are better if we obtain an ECG before surgery. The clinician should consider patient factors and the type of surgery before ordering diagnostic tests, including electrocardiography.
In asymptomatic patients undergoing nonemergent surgery:
- It is reasonable to obtain a preoperative ECG in patients with known coronary artery disease, significant arrhythmia, peripheral arterial disease, cerebrovascular disease, chronic heart failure, or other significant structural heart disease undergoing elevated-cardiac-risk surgery.
- Do not order a preoperative ECG in asymptomatic patients undergoing low-risk surgery.
- Obtaining a preoperative ECG is reasonable in morbidly obese patients and in obese patients with one or more risk factors for coronary artery disease, or poor exercise tolerance, undergoing high-risk surgery.
A 55-year-old lawyer with hypertension well controlled on lisinopril and amlodipine is scheduled for elective hernia repair under general anesthesia. His surgeon has referred him for a preoperative evaluation. He has never smoked, runs 4 miles on days off from work, and enjoys long hiking trips. On examination, his body mass index is 26 kg/m2 and his blood pressure is 130/78 mm Hg; his cardiac examination and the rest of the clinical examination are unremarkable. He asks if he should have an electrocardiogram (ECG) as a part of his workup.
A preoperative ECG is not routinely recommended in all asymptomatic patients undergoing noncardiac surgery.
Consider obtaining an ECG in patients planning to undergo a high-risk surgical procedure, especially if they have one or more clinical risk factors for coronary artery disease, and in patients undergoing elevated-cardiac-risk surgery who are known to have coronary artery disease, chronic heart failure, peripheral arterial disease, or cerebrovascular disease. However, a preoperative ECG is not routinely recommended for patients perceived to be at low cardiac risk who are planning to undergo low-risk surgery. In those patients it could delay surgery unnecessarily, cause further unnecessary testing, drive up costs, and increase patient anxiety.
Here we discuss the perioperative cardiac risk based on type of surgery and patient characteristics and summarize the current guidelines and recommendations on obtaining a preoperative 12-lead ECG in patients undergoing noncardiac surgery.
RISK DEPENDS ON TYPE OF SURGERY AND PATIENT FACTORS
Physicians, including primary care physicians, hospitalists, cardiologists, and anesthesiologists, are routinely asked to evaluate patients before surgical procedures. The purpose of the preoperative evaluation is to optimize existing medical conditions, to identify undiagnosed conditions that can increase risk of perioperative morbidity and death, and to suggest strategies to mitigate risk.1,2
Cardiac risk is multifactorial, and risk factors for postoperative adverse cardiac events include the type of surgery and patient factors.1,3
Cardiac risk based on type of surgery
Low-risk procedures are those in which the risk of a perioperative major adverse cardiac event is less than 1%.1,4 Examples:
- Ambulatory surgery
- Breast or plastic surgery
- Cataract surgery
- Endoscopic procedures.
Elevated-risk procedures are those in which the risk is 1% or higher. Examples:
- Intraperitoneal surgery
- Intrathoracic surgery
- Carotid endarterectomy
- Head and neck surgery
- Orthopedic surgery
- Prostate surgery
- Aortic surgery
- Major vascular surgery
- Peripheral arterial surgery.
Cardiac risk based on patient factors
The 2014 American College of Cardiology and American Heart Association (ACC/AHA) perioperative guidelines list a number of clinical risk factors for perioperative cardiac morbidity and death.1 These include coronary artery disease, chronic heart failure, clinically suspected moderate or greater degrees of valvular heart disease, arrhythmias, conduction disorders, pulmonary vascular disease, and adult congenital heart disease.
Patients with these conditions and patients with unstable coronary syndromes warrant preoperative ECGs and sometimes even urgent interventions before any nonemergency surgery, provided such interventions would affect decision-making and perioperative care.1
The risk of perioperative cardiac morbidity and death can be calculated using either the Revised Cardiac Risk Index scoring system or the American College of Surgeons National Surgical Quality Improvement Program calculator.157 The former is fairly simple, validated, and accepted, while the latter requires use of online calculators (eg, www.surgicalriskcalculator.com/miorcardiacarrest, www.riskcalculator.facs.org).
The Revised Cardiac Risk Index has six clinical predictors of major perioperative cardiac events:
- History of cerebrovascular disease
- Prior or current compensated congestive heart failure
- History of coronary artery disease
- Insulin-dependent diabetes mellitus
- Renal insufficiency, defined as a serum creatinine level of 2 mg/dL or higher
- Patient undergoing suprainguinal vascular, intraperitoneal, or intrathoracic surgery.
A patient who has 0 or 1 of these predictors would have a low risk of a major adverse cardiac event, whereas a patient with 2 or more would have elevated risk. These risk factors must be taken into consideration to determine the need, if any, for a preoperative ECG.
What an ECG can tell us
Abnormalities such as left ventricular hypertrophy, ST-segment depression, and pathologic Q waves on a preoperative ECG in a patient undergoing an elevated-risk surgical procedure may predict adverse perioperative cardiac events.3,6
In a retrospective study of 23,036 patients, Noordzij et al7 found that in patients undergoing elevated-risk surgery, those with an abnormal preoperative ECG had a higher incidence of cardiovascular death than those with a normal ECG. However, a preoperative ECG was obtained only in patients with established coronary artery disease or risk factors for cardiovascular disease. Hence, although an abnormal ECG in such patients undergoing elevated-risk surgery was predictive of adverse postoperative cardiac outcomes, we cannot say that the same would apply to patients without these characteristics undergoing elevated-risk surgery.
In a prospective observational study of patients with known coronary artery disease undergoing major noncardiac surgery, a preoperative ECG was found to contain prognostic information and was predictive of long-term outcome independent of clinical findings and perioperative ischemia.8
CURRENT GUIDELINES AND RECOMMENDATIONS
Several guidelines address whether to order a preoperative ECG but are mostly based on low-level evidence and expert opinion.1,2,6,9
Current guidelines recommend obtaining a preoperative ECG in patients with known coronary, peripheral arterial, or cerebrovascular disease.1,6,9
Obesity and associated comorbidities such as coronary artery disease, heart failure, systemic hypertension, and sleep apnea can predispose to increased perioperative complications. A preoperative 12-lead ECG is reasonable in morbidly obese patients (body mass index ≥ 40 kg/m2) and in obese patients (body mass index ≥ 30 kg/m2) with at least one risk factor for coronary artery disease or poor exercise tolerance, or both.10
Liu et al11 looked at the predictive value of a preoperative 12-lead ECG in 513 elderly patients (age ≥ 70) undergoing noncardiac surgery and found that electrocardiographic abnormalities were not predictive of adverse cardiac outcomes. In this study, although electrocardiographic abnormalities were common (noted in 75% of the patients), they were nonspecific and less useful in predicting postoperative cardiac complications than was the presence of comorbidities.11 Age alone as a cutoff for obtaining a preoperative ECG is not predictive of postoperative outcomes and a preoperative ECG is not warranted in all elderly patients. This is also reflected in current ACC/AHA guidelines on perioperative cardiovascular evaluation1 and is a change from prior ACC/AHA guidelines when age was used as a criterion for preoperative ECGs.12
Current guidelines do not recommend getting a preoperative ECG in asymptomatic patients undergoing low-cardiac-risk surgery.1,4,9
Although the ideal time for ordering an ECG before a planned surgery is unknown, obtaining one within 90 days before the surgery is considered adequate in stable patients in whom an ECG is indicated.1
BACK TO OUR PATIENT
On the basis of current evidence, our patient does not need a preoperative ECG, as it is unlikely to alter his perioperative management and instead may delay his surgery unnecessarily if any nonspecific changes prompt further cardiac workup.
CLINICAL BOTTOM LINE
Although frequently ordered in clinical practice, preoperative electrocardiography has a limited role in predicting postoperative outcome and should be ordered only in the appropriate clinical setting.1 Moreover, there is little evidence that outcomes are better if we obtain an ECG before surgery. The clinician should consider patient factors and the type of surgery before ordering diagnostic tests, including electrocardiography.
In asymptomatic patients undergoing nonemergent surgery:
- It is reasonable to obtain a preoperative ECG in patients with known coronary artery disease, significant arrhythmia, peripheral arterial disease, cerebrovascular disease, chronic heart failure, or other significant structural heart disease undergoing elevated-cardiac-risk surgery.
- Do not order a preoperative ECG in asymptomatic patients undergoing low-risk surgery.
- Obtaining a preoperative ECG is reasonable in morbidly obese patients and in obese patients with one or more risk factors for coronary artery disease, or poor exercise tolerance, undergoing high-risk surgery.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. J Am Coll Cardiol 2014; Jul 29. 10.1016/j.jacc.2014.07.944. [Epub ahead of print]
- Feely MA, Collins CS, Daniels PR, Kebede EB, Jatoi A, Mauck KF. Preoperative testing before noncardiac surgery: guidelines and recommendations. Am Fam Physician 2013; 87:414–418.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Task Force for Preoperative Cardiac Risk Assessment and Perioperative Cardiac Management in Non-cardiac Surgery; European Society of Cardiology (ESC); Poldermans D, Bax JJ, Boersma E, et al. Guidelines for pre-operative cardiac risk assessment and perioperative cardiac management in non-cardiac surgery. Eur Heart J 2009; 30:2769–2812.
- Bilimoria KY, Liu Y, Paruch JL, Zhou L, Kmiecik TE, Ko CY, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013; 217:833–842.
- Landesberg G, Einav S, Christopherson R, et al. Perioperative ischemia and cardiac complications in major vascular surgery: importance of the preoperative twelve-lead electrocardiogram. J Vasc Surg 1997; 26:570–578.
- Noordzij PG, Boersma E, Bax JJ, et al. Prognostic value of routine preoperative electrocardiography in patients undergoing noncardiac surgery. Am J Cardiol 2006; 97:1103–1106.
- Jeger RV, Probst C, Arsenic R, et al. Long-term prognostic value of the preoperative 12-lead electrocardiogram before major noncardiac surgery in coronary artery disease. Am Heart J 2006; 151:508–513.
- Committee on Standards and Practice Parameters; Apfelbaum JL, Connis RT, Nickinovich DG, Pasternak LR, Arens JF, Caplan RA, et al. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology 2012; 116:522–538.
- Poirier P, Alpert MA, Fleisher LA, et al. Cardiovascular evaluation and management of severely obese patients undergoing surgery: a science advisory from the American Heart Association. Circulation 2009; 120:86–95.
- Liu LL, Dzankic S, Leung JM. Preoperative electrocardiogram abnormalities do not predict postoperative cardiac complications in geriatric surgical patients. J Am Geriatr Soc 2002; 50:1186–1191.
- Eagle KA, Berger PB, Calkins H, et al; American College of Cardiology; American Heart Association. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary. J Am Coll Cardiol 2002; 39:542–553.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. J Am Coll Cardiol 2014; Jul 29. 10.1016/j.jacc.2014.07.944. [Epub ahead of print]
- Feely MA, Collins CS, Daniels PR, Kebede EB, Jatoi A, Mauck KF. Preoperative testing before noncardiac surgery: guidelines and recommendations. Am Fam Physician 2013; 87:414–418.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Task Force for Preoperative Cardiac Risk Assessment and Perioperative Cardiac Management in Non-cardiac Surgery; European Society of Cardiology (ESC); Poldermans D, Bax JJ, Boersma E, et al. Guidelines for pre-operative cardiac risk assessment and perioperative cardiac management in non-cardiac surgery. Eur Heart J 2009; 30:2769–2812.
- Bilimoria KY, Liu Y, Paruch JL, Zhou L, Kmiecik TE, Ko CY, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013; 217:833–842.
- Landesberg G, Einav S, Christopherson R, et al. Perioperative ischemia and cardiac complications in major vascular surgery: importance of the preoperative twelve-lead electrocardiogram. J Vasc Surg 1997; 26:570–578.
- Noordzij PG, Boersma E, Bax JJ, et al. Prognostic value of routine preoperative electrocardiography in patients undergoing noncardiac surgery. Am J Cardiol 2006; 97:1103–1106.
- Jeger RV, Probst C, Arsenic R, et al. Long-term prognostic value of the preoperative 12-lead electrocardiogram before major noncardiac surgery in coronary artery disease. Am Heart J 2006; 151:508–513.
- Committee on Standards and Practice Parameters; Apfelbaum JL, Connis RT, Nickinovich DG, Pasternak LR, Arens JF, Caplan RA, et al. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology 2012; 116:522–538.
- Poirier P, Alpert MA, Fleisher LA, et al. Cardiovascular evaluation and management of severely obese patients undergoing surgery: a science advisory from the American Heart Association. Circulation 2009; 120:86–95.
- Liu LL, Dzankic S, Leung JM. Preoperative electrocardiogram abnormalities do not predict postoperative cardiac complications in geriatric surgical patients. J Am Geriatr Soc 2002; 50:1186–1191.
- Eagle KA, Berger PB, Calkins H, et al; American College of Cardiology; American Heart Association. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary. J Am Coll Cardiol 2002; 39:542–553.
Can an ARB be given to patients who have had angioedema on an ACE inhibitor?
Current evidence suggests no absolute contraindication to angiotensin receptor blockers (ARBs) in patients who have had angioedema attributable to an angiotensin-converting enzyme (ACE) inhibitor. However, since ARBs can also cause angioedema, they should be prescribed with extreme caution after a thorough risk-benefit analysis and after educating the patient to watch for signs of angioedema while taking the drug.
A GROWING PROBLEM
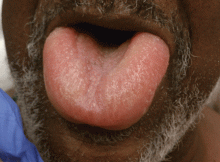
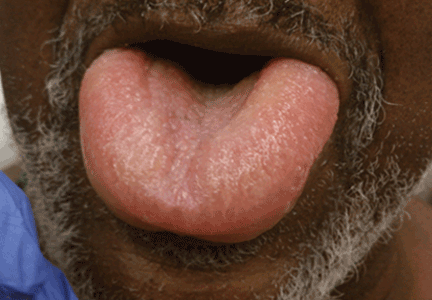
Angioedema is a potentially life-threatening swelling of the skin and subcutaneous tissues, often affecting the lips and tongue (Figure 1), and in some cases interfering with breathing and requiring tracheostomy.1 The incidence rate of angioedema in patients taking ACE inhibitors ranges from 0.1% to 0.7%.2–4 Although this rate may seem low, the widespread and growing use of ACE inhibitors and ARBs in patients with diabetes, diabetic nephropathy, and congestive heart failure5 makes angioedema fairly common in clinical practice.
ACE inhibitor-induced angioedema most commonly occurs within days of initiating therapy, but it also may occur weeks, months, or even years after the start of treatment.1 Patients who are over age 65, black, or female are at higher risk, as are renal transplant recipients taking mTOR inhibitors such as sirolimus. Diabetes appears to be associated with a lower risk.4,6,7 This adverse reaction to ACE inhibitors is thought to be a class side effect, and the future use of this class of drugs would be contraindicated.8,9
ACE inhibitors cause angioedema by direct interference with the degradation of bradykinin, thereby increasing bradykinin levels and potentiating its biologic effect, leading to increased vascular permeability, inflammation, and activation of nociceptors.8
EVIDENCE TO SUPPORT THE USE OF ARBs
ACE inhibitors and ARBs both block the renin-angiotensin-aldosterone pathway and confer similar advantages in patients with congestive heart failure, renal failure, and diabetes. But since ARBs directly inhibit the angiotensin receptor and do not interfere with bradykinin degradation, how they cause angioedema is unclear, and clinicians have questioned whether these agents might be used safely in patients who have had angioedema on an ACE inhibitor.
In a large meta-analysis of randomized clinical trials, Makani et al2 investigated the risk of angioedema with ARB use in 35,479 patients and compared this with other commonly used antihypertensive drugs. The weighted incidence of angioedema was 0.30% with an ACE inhibitor, 0.11% with an ARB, and 0.07% with placebo.2 In seven trials included in this study that compared ARBs with placebo, there was no significant difference in the risk of angioedema. Even in such a large study, the event rate was small, making definite conclusions difficult.
In a retrospective observational study of 4 million patients by Toh et al,3 patients on beta-blockers were used as a reference, and propensity scoring was used to estimate the hazard ratio of angioedema separately for drugs targeting the renin-angiotensin-aldosterone system, including ACE inhibitors and ARBs. The risk of angioedema, as measured by the cumulative incidence and incidence rate, was highest for ACE inhibitors and was similar between ARBs and beta-blockers. The risk of serious angioedema was three times higher with ACE inhibitors than with beta-blockers, and there was no higher risk of serious angioedema with ARBs than with beta-blockers.3
Looking specifically at the use of ARBs in patients who developed angioedema on an ACE inhibitor, Haymore et al10 performed a meta-analysis evaluating only three studies that showed the estimated risk of angioedema with an ARB was between 3.5% and 9.4% in patients with a history of ACE inhibitor-induced angioedema. Later, when the results of the Telmisartan Randomised Assessment Study in ACE Intolerant Subjects With Cardiovascular Disease trial11 were published, the previous meta-analysis was updated12: the risk of angioedema with an ARB was only 2.5% (95% confidence interval 0%–6.6%), and there was no statistically significant difference in the odds (odds ratio 1.1; 95% confidence interval 0.07–17) of angioedema between ARBs and placebo.10,12 Again, these results should be interpreted with caution, as only two patients in the ARB (telmisartan) group and three patients in the placebo group developed angioedema.
In another review, Beavers et al13 advised that the prescribing practitioner should carefully perform a risk-benefit analysis before substituting an ARB in patients with ACE inhibitor-induced angioedema. They concluded that an ARB could be considered in patients who are likely to have a large clinical benefit from an ARB, such as those with heart failure. They also suggested that angioedema related to ARBs was less severe and occurred earlier than with that linked to ACE inhibitors.
No large clinical trial has yet been specifically designed to address the use of ARBs in patients with a history of ACE inhibitor-induced angioedema. The package insert for the ARB losartan mentions that the risk of this adverse reaction might be higher in patients who have had angioedema on an ACE inhibitor. However, the issue of recurrent angioedema is not further addressed for this or other commonly used ARBs.
GENERAL RECOMMENDATIONS
The mechanisms of ARB-induced angioedema are yet unknown. However, studies have shown that the incidence of angioedema while on an ARB is low and is probably comparable to that of placebo.2,3,12–14 And since ARBs share many of the cardiac and renal protective effects of ACE inhibitors, ARBs may be beneficial for patients who discontinue an ACE inhibitor because of adverse effects including angioedema.9,15,16 Based on the discussion above, there is no clear evidence to suggest that ARBs are contraindicated in such patients, especially if there is a compelling indication for an ARB.
The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI) guidelines on hypertension in chronic kidney disease recommend caution when substituting an ARB for an ACE inhibitor after angioedema.15 The joint guidelines of the American College of Cardiology and American Heart Association (ACC/AHA) for the diagnosis and management of heart failure in adults advise “extreme caution.”9,16
The risks and benefits of ARB therapy in this setting should be analyzed by the prescribing physician and discussed with the patient. The patient should be closely monitored for the recurrence of angioedema and should be given a clear plan of action should symptoms recur.
OUR ADVICE
In patients with ACE inhibitor-induced angioedema, we recommend the following:
- Determine that the patient truly has one of the evidence-based, compelling indications for an ARB. Carefully weigh the risks and benefits for the individual patient, and discuss the risk of angioedema based on age, race, sex, and medical history, and the availability of immediate medical care should angioedema occur.
- If there is an evidence-based indication for an ARB that outweighs the risk of angioedema, an ARB may be started with caution.
- Specifically discuss with the patient the possibility of recurrence of angioedema while on an ARB, and provide instructions on how to proceed if this should occur.
- Kaplan AP, Greaves MW. Angioedema. J Am Acad Dermatol 2005; 53:373–388.
- Makani H, Messerli FH, Romero J, et al. Meta-analysis of randomized trials of angioedema as an adverse event of renin-angiotensin system inhibitors. Am J Cardiol 2012; 110:383–391.
- Toh S, Reichman ME, Houstoun M, et al. Comparative risk for angioedema associated with the use of drugs that target the renin-angiotensin-aldosterone system. Arch Intern Med 2012; 172:1582–1589.
- Kostis JB, Kim HJ, Rusnak J, et al. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med 2005; 165:1637–1642.
- Taylor AA, Siragy H, Nesbitt S. Angiotensin receptor blockers: pharmacology, efficacy, and safety. J Clin Hypertens (Greenwich) 2011; 13:677–686.
- Duerr M, Glander P, Diekmann F, Dragun D, Neumayer HH, Budde K. Increased incidence of angioedema with ACE inhibitors in combination with mTOR inhibitors in kidney transplant recipients. Clin J Am Soc Nephrol 2010; 5:703–708.
- Byrd JB, Adam A, Brown NJ. Angiotensin-converting enzyme inhibitor-associated angioedema. Immunol Allergy Clin North Am 2006; 26:725–737.
- Inomata N. Recent advances in drug-induced angioedema. Allergol Int 2012; 61:545–557.
- Hunt SA, Abraham WT, Chin MH, et al; American College of Cardiology Foundation; American Heart Association. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol 2009; 53:e1–e90.
- Haymore BR, Yoon J, Mikita CP, Klote MM, DeZee KJ. Risk of angioedema with angiotensin receptor blockers in patients with prior angioedema associated with angiotensin-converting enzyme inhibitors: a meta-analysis. Ann Allergy Asthma Immunol 2008; 101:495–499.
- Telmisartan Randomised Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease (TRANSCEND) Investigators. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet 2008; 372:1174–1183.
- Haymore BR, DeZee KJ. Use of angiotensin receptor blockers after angioedema with an angiotensin-converting enzyme inhibitor. Ann Allergy Asthma Immunol 2009; 103:83–84.
- Beavers CJ, Dunn SP, Macaulay TE. The role of angiotensin receptor blockers in patients with angiotensin-converting enzyme inhibitor-induced angioedema. Ann Pharmacother 2011; 45:520–524.
- Caldeira D, David C, Sampaio C. Tolerability of angiotensin-receptor blockers in patients with intolerance to angiotensin-converting enzyme inhibitors: a systematic review and meta-analysis. Am J Cardiovasc Drugs 2012; 12:263–277.
- Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 2004; 43(suppl 1):S1–S290.
- Smith SC, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association. J Am Coll Cardiol 2011; 58:2432–2446.
Current evidence suggests no absolute contraindication to angiotensin receptor blockers (ARBs) in patients who have had angioedema attributable to an angiotensin-converting enzyme (ACE) inhibitor. However, since ARBs can also cause angioedema, they should be prescribed with extreme caution after a thorough risk-benefit analysis and after educating the patient to watch for signs of angioedema while taking the drug.
A GROWING PROBLEM
Angioedema is a potentially life-threatening swelling of the skin and subcutaneous tissues, often affecting the lips and tongue (Figure 1), and in some cases interfering with breathing and requiring tracheostomy.1 The incidence rate of angioedema in patients taking ACE inhibitors ranges from 0.1% to 0.7%.2–4 Although this rate may seem low, the widespread and growing use of ACE inhibitors and ARBs in patients with diabetes, diabetic nephropathy, and congestive heart failure5 makes angioedema fairly common in clinical practice.
ACE inhibitor-induced angioedema most commonly occurs within days of initiating therapy, but it also may occur weeks, months, or even years after the start of treatment.1 Patients who are over age 65, black, or female are at higher risk, as are renal transplant recipients taking mTOR inhibitors such as sirolimus. Diabetes appears to be associated with a lower risk.4,6,7 This adverse reaction to ACE inhibitors is thought to be a class side effect, and the future use of this class of drugs would be contraindicated.8,9
ACE inhibitors cause angioedema by direct interference with the degradation of bradykinin, thereby increasing bradykinin levels and potentiating its biologic effect, leading to increased vascular permeability, inflammation, and activation of nociceptors.8
EVIDENCE TO SUPPORT THE USE OF ARBs
ACE inhibitors and ARBs both block the renin-angiotensin-aldosterone pathway and confer similar advantages in patients with congestive heart failure, renal failure, and diabetes. But since ARBs directly inhibit the angiotensin receptor and do not interfere with bradykinin degradation, how they cause angioedema is unclear, and clinicians have questioned whether these agents might be used safely in patients who have had angioedema on an ACE inhibitor.
In a large meta-analysis of randomized clinical trials, Makani et al2 investigated the risk of angioedema with ARB use in 35,479 patients and compared this with other commonly used antihypertensive drugs. The weighted incidence of angioedema was 0.30% with an ACE inhibitor, 0.11% with an ARB, and 0.07% with placebo.2 In seven trials included in this study that compared ARBs with placebo, there was no significant difference in the risk of angioedema. Even in such a large study, the event rate was small, making definite conclusions difficult.
In a retrospective observational study of 4 million patients by Toh et al,3 patients on beta-blockers were used as a reference, and propensity scoring was used to estimate the hazard ratio of angioedema separately for drugs targeting the renin-angiotensin-aldosterone system, including ACE inhibitors and ARBs. The risk of angioedema, as measured by the cumulative incidence and incidence rate, was highest for ACE inhibitors and was similar between ARBs and beta-blockers. The risk of serious angioedema was three times higher with ACE inhibitors than with beta-blockers, and there was no higher risk of serious angioedema with ARBs than with beta-blockers.3
Looking specifically at the use of ARBs in patients who developed angioedema on an ACE inhibitor, Haymore et al10 performed a meta-analysis evaluating only three studies that showed the estimated risk of angioedema with an ARB was between 3.5% and 9.4% in patients with a history of ACE inhibitor-induced angioedema. Later, when the results of the Telmisartan Randomised Assessment Study in ACE Intolerant Subjects With Cardiovascular Disease trial11 were published, the previous meta-analysis was updated12: the risk of angioedema with an ARB was only 2.5% (95% confidence interval 0%–6.6%), and there was no statistically significant difference in the odds (odds ratio 1.1; 95% confidence interval 0.07–17) of angioedema between ARBs and placebo.10,12 Again, these results should be interpreted with caution, as only two patients in the ARB (telmisartan) group and three patients in the placebo group developed angioedema.
In another review, Beavers et al13 advised that the prescribing practitioner should carefully perform a risk-benefit analysis before substituting an ARB in patients with ACE inhibitor-induced angioedema. They concluded that an ARB could be considered in patients who are likely to have a large clinical benefit from an ARB, such as those with heart failure. They also suggested that angioedema related to ARBs was less severe and occurred earlier than with that linked to ACE inhibitors.
No large clinical trial has yet been specifically designed to address the use of ARBs in patients with a history of ACE inhibitor-induced angioedema. The package insert for the ARB losartan mentions that the risk of this adverse reaction might be higher in patients who have had angioedema on an ACE inhibitor. However, the issue of recurrent angioedema is not further addressed for this or other commonly used ARBs.
GENERAL RECOMMENDATIONS
The mechanisms of ARB-induced angioedema are yet unknown. However, studies have shown that the incidence of angioedema while on an ARB is low and is probably comparable to that of placebo.2,3,12–14 And since ARBs share many of the cardiac and renal protective effects of ACE inhibitors, ARBs may be beneficial for patients who discontinue an ACE inhibitor because of adverse effects including angioedema.9,15,16 Based on the discussion above, there is no clear evidence to suggest that ARBs are contraindicated in such patients, especially if there is a compelling indication for an ARB.
The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI) guidelines on hypertension in chronic kidney disease recommend caution when substituting an ARB for an ACE inhibitor after angioedema.15 The joint guidelines of the American College of Cardiology and American Heart Association (ACC/AHA) for the diagnosis and management of heart failure in adults advise “extreme caution.”9,16
The risks and benefits of ARB therapy in this setting should be analyzed by the prescribing physician and discussed with the patient. The patient should be closely monitored for the recurrence of angioedema and should be given a clear plan of action should symptoms recur.
OUR ADVICE
In patients with ACE inhibitor-induced angioedema, we recommend the following:
- Determine that the patient truly has one of the evidence-based, compelling indications for an ARB. Carefully weigh the risks and benefits for the individual patient, and discuss the risk of angioedema based on age, race, sex, and medical history, and the availability of immediate medical care should angioedema occur.
- If there is an evidence-based indication for an ARB that outweighs the risk of angioedema, an ARB may be started with caution.
- Specifically discuss with the patient the possibility of recurrence of angioedema while on an ARB, and provide instructions on how to proceed if this should occur.
Current evidence suggests no absolute contraindication to angiotensin receptor blockers (ARBs) in patients who have had angioedema attributable to an angiotensin-converting enzyme (ACE) inhibitor. However, since ARBs can also cause angioedema, they should be prescribed with extreme caution after a thorough risk-benefit analysis and after educating the patient to watch for signs of angioedema while taking the drug.
A GROWING PROBLEM
Angioedema is a potentially life-threatening swelling of the skin and subcutaneous tissues, often affecting the lips and tongue (Figure 1), and in some cases interfering with breathing and requiring tracheostomy.1 The incidence rate of angioedema in patients taking ACE inhibitors ranges from 0.1% to 0.7%.2–4 Although this rate may seem low, the widespread and growing use of ACE inhibitors and ARBs in patients with diabetes, diabetic nephropathy, and congestive heart failure5 makes angioedema fairly common in clinical practice.
ACE inhibitor-induced angioedema most commonly occurs within days of initiating therapy, but it also may occur weeks, months, or even years after the start of treatment.1 Patients who are over age 65, black, or female are at higher risk, as are renal transplant recipients taking mTOR inhibitors such as sirolimus. Diabetes appears to be associated with a lower risk.4,6,7 This adverse reaction to ACE inhibitors is thought to be a class side effect, and the future use of this class of drugs would be contraindicated.8,9
ACE inhibitors cause angioedema by direct interference with the degradation of bradykinin, thereby increasing bradykinin levels and potentiating its biologic effect, leading to increased vascular permeability, inflammation, and activation of nociceptors.8
EVIDENCE TO SUPPORT THE USE OF ARBs
ACE inhibitors and ARBs both block the renin-angiotensin-aldosterone pathway and confer similar advantages in patients with congestive heart failure, renal failure, and diabetes. But since ARBs directly inhibit the angiotensin receptor and do not interfere with bradykinin degradation, how they cause angioedema is unclear, and clinicians have questioned whether these agents might be used safely in patients who have had angioedema on an ACE inhibitor.
In a large meta-analysis of randomized clinical trials, Makani et al2 investigated the risk of angioedema with ARB use in 35,479 patients and compared this with other commonly used antihypertensive drugs. The weighted incidence of angioedema was 0.30% with an ACE inhibitor, 0.11% with an ARB, and 0.07% with placebo.2 In seven trials included in this study that compared ARBs with placebo, there was no significant difference in the risk of angioedema. Even in such a large study, the event rate was small, making definite conclusions difficult.
In a retrospective observational study of 4 million patients by Toh et al,3 patients on beta-blockers were used as a reference, and propensity scoring was used to estimate the hazard ratio of angioedema separately for drugs targeting the renin-angiotensin-aldosterone system, including ACE inhibitors and ARBs. The risk of angioedema, as measured by the cumulative incidence and incidence rate, was highest for ACE inhibitors and was similar between ARBs and beta-blockers. The risk of serious angioedema was three times higher with ACE inhibitors than with beta-blockers, and there was no higher risk of serious angioedema with ARBs than with beta-blockers.3
Looking specifically at the use of ARBs in patients who developed angioedema on an ACE inhibitor, Haymore et al10 performed a meta-analysis evaluating only three studies that showed the estimated risk of angioedema with an ARB was between 3.5% and 9.4% in patients with a history of ACE inhibitor-induced angioedema. Later, when the results of the Telmisartan Randomised Assessment Study in ACE Intolerant Subjects With Cardiovascular Disease trial11 were published, the previous meta-analysis was updated12: the risk of angioedema with an ARB was only 2.5% (95% confidence interval 0%–6.6%), and there was no statistically significant difference in the odds (odds ratio 1.1; 95% confidence interval 0.07–17) of angioedema between ARBs and placebo.10,12 Again, these results should be interpreted with caution, as only two patients in the ARB (telmisartan) group and three patients in the placebo group developed angioedema.
In another review, Beavers et al13 advised that the prescribing practitioner should carefully perform a risk-benefit analysis before substituting an ARB in patients with ACE inhibitor-induced angioedema. They concluded that an ARB could be considered in patients who are likely to have a large clinical benefit from an ARB, such as those with heart failure. They also suggested that angioedema related to ARBs was less severe and occurred earlier than with that linked to ACE inhibitors.
No large clinical trial has yet been specifically designed to address the use of ARBs in patients with a history of ACE inhibitor-induced angioedema. The package insert for the ARB losartan mentions that the risk of this adverse reaction might be higher in patients who have had angioedema on an ACE inhibitor. However, the issue of recurrent angioedema is not further addressed for this or other commonly used ARBs.
GENERAL RECOMMENDATIONS
The mechanisms of ARB-induced angioedema are yet unknown. However, studies have shown that the incidence of angioedema while on an ARB is low and is probably comparable to that of placebo.2,3,12–14 And since ARBs share many of the cardiac and renal protective effects of ACE inhibitors, ARBs may be beneficial for patients who discontinue an ACE inhibitor because of adverse effects including angioedema.9,15,16 Based on the discussion above, there is no clear evidence to suggest that ARBs are contraindicated in such patients, especially if there is a compelling indication for an ARB.
The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI) guidelines on hypertension in chronic kidney disease recommend caution when substituting an ARB for an ACE inhibitor after angioedema.15 The joint guidelines of the American College of Cardiology and American Heart Association (ACC/AHA) for the diagnosis and management of heart failure in adults advise “extreme caution.”9,16
The risks and benefits of ARB therapy in this setting should be analyzed by the prescribing physician and discussed with the patient. The patient should be closely monitored for the recurrence of angioedema and should be given a clear plan of action should symptoms recur.
OUR ADVICE
In patients with ACE inhibitor-induced angioedema, we recommend the following:
- Determine that the patient truly has one of the evidence-based, compelling indications for an ARB. Carefully weigh the risks and benefits for the individual patient, and discuss the risk of angioedema based on age, race, sex, and medical history, and the availability of immediate medical care should angioedema occur.
- If there is an evidence-based indication for an ARB that outweighs the risk of angioedema, an ARB may be started with caution.
- Specifically discuss with the patient the possibility of recurrence of angioedema while on an ARB, and provide instructions on how to proceed if this should occur.
- Kaplan AP, Greaves MW. Angioedema. J Am Acad Dermatol 2005; 53:373–388.
- Makani H, Messerli FH, Romero J, et al. Meta-analysis of randomized trials of angioedema as an adverse event of renin-angiotensin system inhibitors. Am J Cardiol 2012; 110:383–391.
- Toh S, Reichman ME, Houstoun M, et al. Comparative risk for angioedema associated with the use of drugs that target the renin-angiotensin-aldosterone system. Arch Intern Med 2012; 172:1582–1589.
- Kostis JB, Kim HJ, Rusnak J, et al. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med 2005; 165:1637–1642.
- Taylor AA, Siragy H, Nesbitt S. Angiotensin receptor blockers: pharmacology, efficacy, and safety. J Clin Hypertens (Greenwich) 2011; 13:677–686.
- Duerr M, Glander P, Diekmann F, Dragun D, Neumayer HH, Budde K. Increased incidence of angioedema with ACE inhibitors in combination with mTOR inhibitors in kidney transplant recipients. Clin J Am Soc Nephrol 2010; 5:703–708.
- Byrd JB, Adam A, Brown NJ. Angiotensin-converting enzyme inhibitor-associated angioedema. Immunol Allergy Clin North Am 2006; 26:725–737.
- Inomata N. Recent advances in drug-induced angioedema. Allergol Int 2012; 61:545–557.
- Hunt SA, Abraham WT, Chin MH, et al; American College of Cardiology Foundation; American Heart Association. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol 2009; 53:e1–e90.
- Haymore BR, Yoon J, Mikita CP, Klote MM, DeZee KJ. Risk of angioedema with angiotensin receptor blockers in patients with prior angioedema associated with angiotensin-converting enzyme inhibitors: a meta-analysis. Ann Allergy Asthma Immunol 2008; 101:495–499.
- Telmisartan Randomised Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease (TRANSCEND) Investigators. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet 2008; 372:1174–1183.
- Haymore BR, DeZee KJ. Use of angiotensin receptor blockers after angioedema with an angiotensin-converting enzyme inhibitor. Ann Allergy Asthma Immunol 2009; 103:83–84.
- Beavers CJ, Dunn SP, Macaulay TE. The role of angiotensin receptor blockers in patients with angiotensin-converting enzyme inhibitor-induced angioedema. Ann Pharmacother 2011; 45:520–524.
- Caldeira D, David C, Sampaio C. Tolerability of angiotensin-receptor blockers in patients with intolerance to angiotensin-converting enzyme inhibitors: a systematic review and meta-analysis. Am J Cardiovasc Drugs 2012; 12:263–277.
- Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 2004; 43(suppl 1):S1–S290.
- Smith SC, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association. J Am Coll Cardiol 2011; 58:2432–2446.
- Kaplan AP, Greaves MW. Angioedema. J Am Acad Dermatol 2005; 53:373–388.
- Makani H, Messerli FH, Romero J, et al. Meta-analysis of randomized trials of angioedema as an adverse event of renin-angiotensin system inhibitors. Am J Cardiol 2012; 110:383–391.
- Toh S, Reichman ME, Houstoun M, et al. Comparative risk for angioedema associated with the use of drugs that target the renin-angiotensin-aldosterone system. Arch Intern Med 2012; 172:1582–1589.
- Kostis JB, Kim HJ, Rusnak J, et al. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med 2005; 165:1637–1642.
- Taylor AA, Siragy H, Nesbitt S. Angiotensin receptor blockers: pharmacology, efficacy, and safety. J Clin Hypertens (Greenwich) 2011; 13:677–686.
- Duerr M, Glander P, Diekmann F, Dragun D, Neumayer HH, Budde K. Increased incidence of angioedema with ACE inhibitors in combination with mTOR inhibitors in kidney transplant recipients. Clin J Am Soc Nephrol 2010; 5:703–708.
- Byrd JB, Adam A, Brown NJ. Angiotensin-converting enzyme inhibitor-associated angioedema. Immunol Allergy Clin North Am 2006; 26:725–737.
- Inomata N. Recent advances in drug-induced angioedema. Allergol Int 2012; 61:545–557.
- Hunt SA, Abraham WT, Chin MH, et al; American College of Cardiology Foundation; American Heart Association. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol 2009; 53:e1–e90.
- Haymore BR, Yoon J, Mikita CP, Klote MM, DeZee KJ. Risk of angioedema with angiotensin receptor blockers in patients with prior angioedema associated with angiotensin-converting enzyme inhibitors: a meta-analysis. Ann Allergy Asthma Immunol 2008; 101:495–499.
- Telmisartan Randomised Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease (TRANSCEND) Investigators. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet 2008; 372:1174–1183.
- Haymore BR, DeZee KJ. Use of angiotensin receptor blockers after angioedema with an angiotensin-converting enzyme inhibitor. Ann Allergy Asthma Immunol 2009; 103:83–84.
- Beavers CJ, Dunn SP, Macaulay TE. The role of angiotensin receptor blockers in patients with angiotensin-converting enzyme inhibitor-induced angioedema. Ann Pharmacother 2011; 45:520–524.
- Caldeira D, David C, Sampaio C. Tolerability of angiotensin-receptor blockers in patients with intolerance to angiotensin-converting enzyme inhibitors: a systematic review and meta-analysis. Am J Cardiovasc Drugs 2012; 12:263–277.
- Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 2004; 43(suppl 1):S1–S290.
- Smith SC, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association. J Am Coll Cardiol 2011; 58:2432–2446.