User login
Gallstones: Watch and wait, or intervene?
The prevalence of gallstones is approximately 10% to 15% of the adult US population.1,2 Most cases are asymptomatic, as gallstones are usually discovered incidentally during routine imaging for other abdominal conditions, and only about 20% of patients with asymptomatic gallstones develop clinically significant complications.2,3
Nevertheless, gallstones carry significant healthcare costs. In 2004, the median inpatient cost for any gallstone-related disease was $11,584, with an overall annual cost of $6.2 billion.4,5
Laparoscopic cholecystectomy is the standard treatment for symptomatic cholelithiasis. For asymptomatic cholelithasis, the usual approach is expectant management (“watch and wait”), but prophylactic cholecystectomy may be an option in certain patients at high risk.
CHEMICAL COMPOSITION
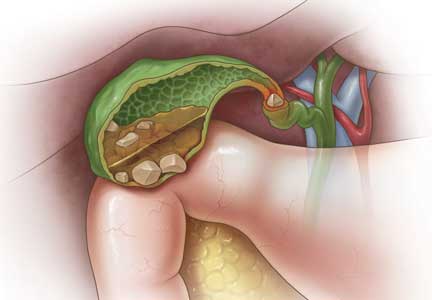
Gallstones can be classified into 2 main categories based on their predominant chemical composition: cholesterol or pigment.
Cholesterol gallstones
About 75% of gallstones are composed of cholesterol.3,4 In the past, this type of stone was thought to be caused by gallbladder inflammation, bile stasis, and absorption of bile salts from damaged mucosa. However, it is now known that cholesterol gallstones are the result of biliary supersaturation caused by cholesterol hypersecretion into the gallbladder, gallbladder hypomotility, accelerated cholesterol nucleation and crystallization, and mucin gel accumulation.
Pigment gallstones
Black pigment gallstones account for 10% to 15% of all gallstones.6 They are caused by chronic hemolysis in association with supersaturation of bile with calcium hydrogen bilirubinate, along with deposition of calcium carbonate, phosphate, and inorganic salts.7
Brown pigment stones, accounting for 5% to 10% of all gallstones,6 are caused by infection in the obstructed bile ducts, where bacteria that produce beta-glucuronidase, phospholipase, and slime contribute to formation of the stone.8,9
RISK FACTORS FOR GALLSTONES
Age. After age 40, the risk increases dramatically, with an incidence 4 times higher for those ages 40 to 69 than in younger people.10
Female sex. Women of reproductive age are 4 times more likely to develop gallstones than men, but this gap narrows after menopause.11 The higher risk is attributed to female sex hormones, pregnancy, and oral contraceptive use. Estrogen decreases secretion of bile salts and increases secretion of cholesterol into the gallbladder, which leads to cholesterol supersaturation. Progesterone acts synergistically by causing hypomobility of the gallbladder, which in turn leads to bile stasis.12,13
Ethnicity. The risk is higher in Mexican Americans and Native Americans than in other ethnic groups.14
Rapid weight loss, such as after bariatric surgery, occurs from decreased caloric intake and promotes bile stasis, while lipolysis increases cholesterol mobilization and secretion into the gallbladder. This creates an environment conducive to bile supersaturation with cholesterol, leading to gallstone formation.
Chronic hemolytic disorders carry an increased risk of developing calcium bilirubinate stones due to increased excretion of bilirubin during hemolysis.
Obesity and diabetes mellitus are both attributed to insulin resistance. Obesity also increases bile stasis and cholesterol saturation.
CLINICAL PRESENTATION OF GALLSTONES (CHOLELITHIASIS)
Most patients with gallstones (cholelithiasis) experience no symptoms. Their gallstones are often discovered incidentally during imaging tests for unrelated or unexplained abdominal symptoms. Most patients with asymptomatic gallstones remain symptom-free, while about 20% develop gallstone-related symptoms.2,3
Abdominal pain is the most common symptom. The phrase biliary colic—suggesting pain that is fluctuating in nature—appears ubiquitously in the medical literature, but it does not correctly characterize the pain associated with gallstones.
Most patients with gallstone symptoms describe a constant and often severe pain in the right upper abdomen, epigastrium, or both, often persisting for 30 to 120 minutes. Symptoms are frequently reported in the epigastrium when only visceral pain fibers are stimulated due to gallbladder distention. This is usually called midline pain; however, pain occurs in the back and right shoulder in up to 60% of patients, with involvement of somatic fibers.15,16 Gallstone pain is not relieved by change of position or passage of stool or gas.
Onset of symptoms more than an hour after eating or in the late evening or at night also very strongly suggests biliary pain. Patients with a history of biliary pain are more likely to experience it again, with a 69% chance of developing recurrent pain within 2 years.17
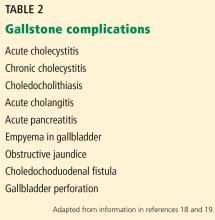
GALLSTONE-RELATED COMPLICATIONS
Acute gallbladder inflammation (cholecystitis)
Gallbladder inflammation (cholecystitis) is the most common complication, occurring in up to 10% of symptomatic cases. Many patients with acute cholecystitis present with right upper quadrant pain that may be accompanied by anorexia, nausea, or vomiting. Inspiratory arrest on deep palpation of the right upper quadrant (Murphy sign) has a specificity of 79% to 96% for acute cholecystitis.20 Markers of systemic inflammation such as fever, elevated white blood cell count, and elevated C-reactive protein are highly suggestive of acute cholecystitis.20,21
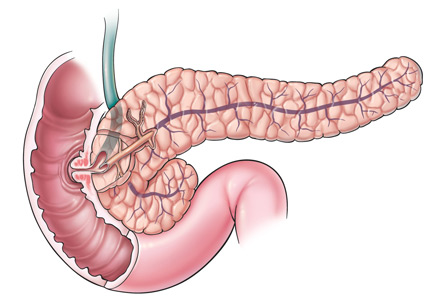
Bile duct stones (choledocholithiasis)
Bile duct stones (choledocholithiasis) are detected in 3.4% to 12% of patients with gallstones.22,23 Most stones in the common bile duct migrate there from the gallbladder via the cystic duct. Less commonly, primary duct stones form in the duct due to biliary stasis. Removing the gallbladder does not completely eliminate the risk of bile duct stones, as stones can remain or recur after surgery.
Bile duct stones can obstruct the common bile duct, which disrupts normal bile flow and leads to jaundice. Other symptoms may include pruritus, right upper quadrant pain, nausea, and vomiting. Serum levels of bilirubin, aspartate aminotransferase, alanine aminotransferase (ALT), and alkaline phosphatase are usually high.24
Acute bacterial infection (cholangitis)
Acute bacterial infection of the biliary system (cholangitis) is usually associated with obstruction of the common bile duct. Common symptoms of acute cholangitis include right upper quadrant pain, fever, and jaundice (Charcot triad), and these are present in about 50% to 75% of cases.21 In severe cases, patients can develop altered mental status and septicemic shock in addition to the Charcot triad, a condition called the Reynold pentad. White blood cell counts and serum levels of C-reactive protein, bilirubin, aminotransferases, and alkaline phosphatase are usually elevated.21
Pancreatitis
Approximately 4% to 8% of patients with gallstones develop inflammation of the pancreas (pancreatitis).25 The diagnosis of acute pancreatitis requires at least 2 of the following:26,27
- Abdominal pain (typically epigastric, often radiating to the back)
- Amylase or lipase levels at least 3 times above the normal limit
- Imaging findings that suggest acute pancreatitis.
Gallstone-related pancreatitis should be considered if the ALT level is greater than 150 U/mL, which has a 97% specificity for gallstone-related pancreatitis.28
ABDOMINAL ULTRASONOGRAPHY FOR DIAGNOSIS
Transabdominal ultrasonography, with a sensitivity of 84% to 89% and a specificity of up to 99%, is the test of choice for detecting gallstones.29 The characteristic findings of acute cholecystitis on ultrasonography include enlargement of the gallbladder, thickening of the gallbladder wall, presence of pericholecystic fluid, and tenderness elicited by the ultrasound probe over the gallbladder (sonographic Murphy sign).
Scintigraphy as a second test
Acute cholecystitis is primarily a clinical diagnosis and typically does not require additional imaging beyond ultrasonography. When there is discordance between clinical and ultrasonographic findings, the most accurate second imaging test is scintigraphy of the biliary tract, usually performed with technetium-labeled hydroxy iminodiacetic acid. Given intravenously, the radionuclide is rapidly taken up by the liver and then secreted into the bile. In acute cholecystitis, the cystic duct is functionally occluded and the isotope does not enter the gallbladder, creating an imaging void compared with a normal appearance.
Scintigraphy is more sensitive than abdominal ultrasonography, with a sensitivity of up to 97% vs 81% to 88%, respectively.29,30 The tests have about equal specificity.
Even though scintigraphy is more sensitive, abdominal ultrasonography is often the initial test for patients with suspected acute cholecystitis because it is more widely available, takes less time, does not involve radiation exposure, and can assess for the presence or absence of gallstones and dilation of the intra- and extrahepatic bile ducts.
Looking for stones in the common bile duct
When acute cholangitis due to choledocholithiasis is suspected, abdominal ultrasonography is a prudent initial test to look for gallstones or biliary dilation suggesting obstruction by stones in the common bile duct. Abdominal ultrasonography has only a 22% to 55% sensitivity for visualizing stones in the common bile duct, but it has a 77% to 87% sensitivity for detecting common bile duct dilation, a surrogate marker of stones.31
The normal bile duct diameter ranges from 3 to 6 mm, although mild dilation is often seen in older patients or after cholecystectomy or Roux-en-Y gastric bypass surgery.32,33 Bile duct dilation of up to 10 mm can be considered normal in patients after cholecystectomy.34 A normal-appearing bile duct on ultrasonography has a negative predictive value of 95% for excluding common bile duct stones.31
Endoscopic ultrasonography (EUS), magnetic resonance cholangiopancreatography (MRCP), and endoscopic retrograde cholangiopancreatography (ERCP) have similar sensitivity (89%–94%, 85%–92%, and 89%–93%, respectively) and specificity (94%–95%, 93%–97%, and 100%, respectively) for detecting common bile duct stones.35–37 EUS is superior to MRCP in detecting stones smaller than 6 mm.38
ERCP should be reserved for managing rather than diagnosing common bile duct stones because of the risk of pancreatitis and perforation. Patients undergoing cholecystectomy who are suspected of having choledocholithiasis may undergo intraoperative cholangiography or laparoscopic common bile duct ultrasonography.
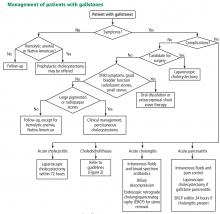
WATCH AND WAIT, OR INTERVENE?
Asymptomatic gallstones
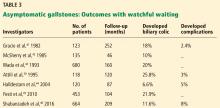
Standard treatment for these patients is expectant management. Cholecystectomy is not recommended for patients with asymptomatic gallstones.47 Nevertheless, some patients may benefit from prophylactic cholecystectomy. We and others48 suggest considering cholecystectomy in the following patients.
Patients with chronic hemolytic anemia (including children with sickle cell anemia and spherocytosis). These patients have a higher risk of developing calcium bilirubinate stones, and cholecystectomy has improved outcomes.49 It should be noted that most of these data come from pediatric populations and have been extrapolated to adults.
Native Americans, who have a higher risk of gallbladder cancer if they have gallstones.2,50
Conversely, calcification of the gallbladder wall (“porcelain gallbladder”) is no longer considered an absolute indication for cholecystectomy. This condition was thought to be associated with a high rate of gallbladder carcinoma, but analyses of larger, more recent data sets found much smaller risks.51,52 Further, cholecystectomy in these patients was found to be associated with high rates of postoperative complications. Thus, prophylactic cholecystectomy is no longer recommended in asymptomatic cases of porcelain gallbladder.
In addition, concomitant cholecystectomy in patients undergoing bariatric surgery is no longer considered the therapeutic standard. Historically, cholecystectomy was performed in these patients because of the increased risk of gallstones associated with rapid weight loss after surgery. However, research now weighs against concomitant cholecystectomy with bariatric surgery and most other abdominal surgeries for asymptomatic gallstones.53
Laparoscopic surgery for symptomatic gallstones
For patients experiencing acute cholecystitis, laparoscopic cholecystectomy within 72 hours is recommended.48 There were safety concerns regarding higher rates of morbidity and conversion from laparoscopic to open cholecystectomy in patients who underwent surgery before the acute cholecystitis episode had settled. However, a large meta-analysis found no significant difference between early and delayed laparoscopic cholecystectomy in bile duct injury or conversion rates.54 Further, early cholecystectomy—defined as within 1 week of symptom onset—has been found to reduce gallstone-related complications, shorten hospital stays, and lower costs.55–57 If the patient cannot undergo surgery, percutaneous cholecystotomy or novel endoscopic gallbladder drainage interventions can be used.
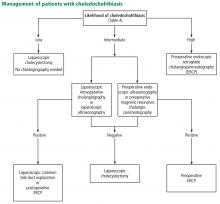
Several variables predict the presence of bile duct stones in patients who have symptoms (Table 4). Based on these predictors, the ASGE classifies the probabilities as low (< 10%), intermediate (10% to 50%), and high (> 50%)31:
- High-risk patients should undergo preoperative ERCP and stone extraction if needed
- Intermediate-risk patients should undergo preoperative imaging with EUS or MRCP or intraoperative bile duct evaluation, depending on the availability, costs, and local expertise.
Patients with associated cholangitis should be given intravenous fluids and broad-spectrum antibiotics. Biliary decompression should be done as early as possible to decrease the risk of morbidity and mortality. For acute cholangitis, ERCP is the treatment of choice.25
Patients with acute gallstone pancreatitis should receive conservative management with intravenous isotonic solutions and pain control, followed by laparoscopic cholecystectomy.48
The timing of laparoscopic cholecystectomy in acute gallstone pancreatitis has been debated. Studies conducted during the era of open cholecystectomy reported similar or worse outcomes if cholecystectomy was done sooner rather than later.
However, in 1999, Uhl et al58 reported that 48 of 77 patients admitted with acute gallstone pancreatitis were able to undergo laparoscopic cholecystectomy during the same admission. Success rates were 85% (30 of 35 patients) in those with mild disease and 62% (8 of 13 patients) in those with severe disease. They concluded laparoscopic cholecystectomy could be safely performed within 7 days in patients with mild disease, whereas in severe disease at least 3 weeks should elapse because of the risk of infection.
In a randomized trial published in 2010, Aboulian et al59 reported that hospital length of stay (the primary end point) was shorter in 25 patients who underwent laparoscopic cholecystectomy early (within 48 hours of admission) than in 25 patients who underwent surgery after abdominal pain had resolved and laboratory enzymes showed a normalizing trend, 3.5 vs 5.8 days (P = .0016). Rates of perioperative complications and need for conversion to open surgery were similar between the 2 groups.
If there is associated cholangitis, patients should also be given broad-spectrum antibiotics and should undergo ERCP within 24 hours of admission.25–27
SUMMARY
Gallstones are common in US adults. Abdominal ultrasonography is the diagnostic imaging test of choice to detect gallbladder stones and assess for findings suggestive of acute cholecystitis and dilation of the common bile duct. Fortunately, most gallstones are asymptomatic and can usually be managed expectantly. In patients who have symptoms or have gallstone complications, laparoscopic cholecystectomy is the standard of care.
- Schirmer BD, Winters KL, Edlich RF. Cholelithiasis and cholecystitis. J Long Term Eff Med Implants 2005; 15(3):329–338. doi:10.1615/JLongTermEffMedImplants.v15.i3.90
- Stinton LM, Shaffer EA. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liver 2012; 6(2):172–187. doi:10.5009/gnl.2012.6.2.172
- Lee JY, Keane MG, Pereira S. Diagnosis and treatment of gallstone disease. Practitioner 2015; 259(1783):15–19.
- Russo MW, Wei JT, Thiny MT, et al. Digestive and liver diseases statistics, 2004. Gastroenterology 2004; 126(5):1448–1453. doi:10.1053/j.gastro.2004.01.025
- Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part I: overall and upper gastrointestinal diseases. Gastroenterology 2009; 136(2):376–386. doi:10.1053/j.gastro.2008.12.015
- Cariati A. Gallstone classification in Western countries. Indian J Surg 2015; 77(suppl 2):376–380. doi.org/10.1007/s12262-013-0847-y
- Carey MC. Pathogenesis of gallstones. Am J Surg 1993; 165(4):410–419. doi:10.1016/S0002-9610(05)80932-8
- Lammert F, Gurusamy K, Ko CW, et al. Gallstones. Nat Rev Dis Primers 2016; 2:16024. doi:10.1038/nrdp.2016.24
- Stewart L, Oesterle AL, Erdan I, Griffiss JM, Way LW. Pathogenesis of pigment gallstones in Western societies: the central role of bacteria. J Gastrointest Surg 2002; 6(6):891–904.
- Barbara L, Sama C, Morselli Labate AM, et al. A population study on the prevalence of gallstone disease: the Sirmione Study. Hepatology 1987; 7(5):913–917. doi:10.1002/hep.1840070520
- Sood S, Winn T, Ibrahim S, et al. Natural history of asymptomatic gallstones: differential behaviour in male and female subjects. Med J Malaysia 2015; 70(6):341–345.
- Maringhini A, Ciambra M, Baccelliere P, et al. Biliary sludge and gallstones in pregnancy: incidence, risk factors, and natural history. Ann Intern Med 1993; 119(2):116–120. doi:10.7326/0003-4819-119-2-199307150-00004
- Etminan M, Delaney JA, Bressler B, Brophy JM. Oral contraceptives and the risk of gallbladder disease: a comparative safety study. CMAJ 2011; 183(8):899–904. doi:10.1503/cmaj.110161
- Everhart JE, Khare M, Hill M, Maurer KR. Prevalence and ethnic differences in gallbladder disease in the United States. Gastroenterology 1999; 117(3):632–639.
- Festi D, Sottili S, Colecchia A, et al. Clinical manifestations of gallstone disease: evidence from the multicenter Italian study on cholelithiasis (MICOL). Hepatology 1999; 30(4):839–846. doi:10.1002/hep.510300401
- Berhane T, Vetrhus M, Hausken T, Olafsson S, Sondenaa K. Pain attacks in non-complicated and complicated gallstone disease have a characteristic pattern and are accompanied by dyspepsia in most patients: the results of a prospective study. Scand J Gastroenterol 2006; 41(1):93–101. doi:10.1080/00365520510023990
- Thistle JL, Cleary PA, Lachin JM, Tyor MP, Hersh T. The natural history of cholelithiasis: the National Cooperative Gallstone Study. Ann Intern Med 1984; 101(2):171–175. doi:10.7326/0003-4819-101-2-171
- Friedman GD. Natural history of asymptomatic and symptomatic gallstones. Am J Surg 1993; 165(4):399–404. doi:0.1016/S0002-9610(05)80930-4
- Friedman GD, Raviola CA, Fireman B. Prognosis of gallstones with mild or no symptoms: 25 years of follow-up in a health maintenance organization. J Clin Epidemiol 1989; 42(2):127–136. doi:10.1016/0895-4356(89)90086-3
- Hirota M, Takada T, Kawarada Y, et al. Diagnostic criteria and severity assessment of acute cholecystitis: Tokyo guidelines. J Hepatobiliary Pancreat Surg 2007; 14(1):78–82. doi:10.1007/s00534-006-1159-4
- Miura F, Takada T, Kawarada Y, et al. Flowcharts for the diagnosis and treatment of acute cholangitis and cholecystitis: Tokyo guidelines. J Hepatobiliary Pancreat Surg 2007; 14(1):27–34. doi:10.1007/s00534-006-1153-x
- Koo KP, Traverso LW. Do preoperative indicators predict the presence of common bile duct stones during laparoscopic cholecystectomy? Am J Surg 1996; 171(5):495–499. doi:10.1016/S0002-9610(97)89611-0
- Collins C, Maguire D, Ireland A, Fitzgerald E, O’Sullivan GC. A prospective study of common bile duct calculi in patients undergoing laparoscopic cholecystectomy: natural history of choledocholithiasis revisited. Ann Surg 2004; 239(1):28–33. doi:10.1097/01.sla.0000103069.00170.9c
- Costi R, Gnocchi A, Di Mario F, Sarli L. Diagnosis and management of choledocholithiasis in the golden age of imaging, endoscopy and laparoscopy. World J Gastroenterol 2014; 20(37):13382–13401. doi:10.3748/wjg.v20.i37.13382
- European Association for the Study of the Liver (EASL). EASL Clinical Practice Guidelines on the prevention, diagnosis and treatment of gallstones. J Hepatol 2016; 65(1):146–181. doi:10.1016/j.jhep.2016.03.005
- Greenberg JA, Hsu J, Bawazeer M, et al. Clinical practice guideline: management of acute pancreatitis. Can J Surg 2016; 59 (2):128–140. doi:10.1503/cjs.015015
- Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol 2013; 108(9):1400–1416. doi:10.1038/ajg.2013.218
- Moolla Z, Anderson F, Thomson SR. Use of amylase and alanine transaminase to predict acute gallstone pancreatitis in a population with high HIV prevalence. World J Surg 2013; 37(1):156–161. doi:10.1007/s00268-012-1801-z
- Shea JA, Berlin JA, Escarce JJ, et al. Revised estimates of diagnostic test sensitivity and specificity in suspected biliary tract disease. Arch Intern Med 1994; 154(22):2573–2581. doi:10.1001/archinte.1994.00420220069008
- Kiewiet JJ, Leeuwenburgh MM, Bipat S, et al. A systematic review and meta-analysis of diagnostic performance of imaging in acute cholecystitis. Radiology 2012; 264(3):708–720. doi:10.1148/radiol.12111561
- ASGE Standards of Practice Committee; Maple JT, Ben-Menachem T, Anderson MA, et al. The role of endoscopy in the evaluation of suspected choledocholithiasis. Gastrointest Endosc 2010; 71(1):1–9. doi:10.1016/j.gie.2009.09.041
- Bachar GN, Cohen M, Belenky A, Atar E, Gideon S. Effect of aging on the adult extrahepatic bile duct: a sonographic study. J Ultrasound Med 2003; 22(9):879–885. doi:10.7863/jum.2003.22.9.879
- El-Hayek K, Timratana P, Meranda J, Shimizu H, Eldar S, Chand B. Post Roux-en-Y gastric bypass biliary dilation: natural process or significant entity? J Gastrointest Surg 2012; 16(12):2185–2189. doi:10.1007/s11605-012-2058-4
- Park SM, Kim WS, Bae IH, et al. Common bile duct dilatation after cholecystectomy: a one-year prospective study. J Korean Surg Soc 2012; 83(2):97–101. doi:10.4174/jkss.2012.83.2.97
- Tse F, Liu L, Barkun AN, Armstrong D, Moayyedi P. EUS: a meta-analysis of test performance in suspected choledocholithiasis. Gastrointest Endosc 2008; 67(2):235–244. doi:10.1016/j.gie.2007.09.047
- Verma D, Kapadia A, Eisen GM, Adler DG. EUS vs MRCP for detection of choledocholithiasis. Gastrointest Endosc 2006; 64(2):248–254. doi:10.1016/j.gie.2005.12.038
- Tseng LJ, Jao YT, Mo LR, Lin RC. Over-the-wire US catheter probe as an adjunct to ERCP in the detection of choledocholithiasis. Gastrointest Endosc 2001; 54(6):720–723. doi:10.1067/mge.2001.119255
- Kondo S, Isayama H, Akahane M, et al. Detection of common bile duct stones: comparison between endoscopic ultrasonography, magnetic resonance cholangiography, and helical-computed-tomographic cholangiography. Eur J Radiol 2005; 54(2):271–275. doi:10.1016/j.ejrad.2004.07.007
- Attili AF, De Santis A, Capri R, Repice AM, Maselli S. The natural history of gallstones: the GREPCO experience. The GREPCO Group. Hepatology 1995; 21(3):656–660. doi:10.1016/0270-9139(95)90514-6
- Sakorafas GH, Milingos D, Peros G. Asymptomatic cholelithiasis: is cholecystectomy really needed? A critical reappraisal 15 years after the introduction of laparoscopic cholecystectomy. Dig Dis Sci 2007; 52(5):1313–1325. doi:10.1007/s10620-006-9107-3
- Gracie WA, Ransohoff DF. The natural history of silent gallstones: the innocent gallstone is not a myth. N Engl J Med 1982; 307(13):798–800. doi:10.1056/NEJM198209233071305
- McSherry CK, Ferstenberg H, Calhoun WF, Lahman E, Virshup M. The natural history of diagnosed gallstone disease in symptomatic and asymptomatic patients. Ann Surg 1985; 202(1):59–63. doi:10.1097/00000658-198507000-00009
- Wada K, Wada K, Imamura T. Natural course of asymptomatic gallstone disease. Nihon Rinsho 1993; 51(7):1737–1743. Japanese.
- Halldestam I, Enell EL, Kullman E, Borch K. Development of symptoms and complications in individuals with asymptomatic gallstones. Br J Surg 2004; 91(6):734–738. doi:10.1002/bjs.4547
- Festi D, Reggiani ML, Attili AF, et al. Natural history of gallstone disease: expectant management or active treatment? Results from a population-based cohort study. J Gastroenterol Hepatol 2010; 25(4):719–724. doi:10.1111/j.1440-1746.2009.06146.x
- Shabanzadeh DM, Sorensen LT, Jorgensen T. A prediction rule for risk stratification of incidentally discovered gallstones: results from a large cohort study. Gastroenterology 2016; 150(1):156–167e1. doi:10.1053/j.gastro.2015.09.002
- Overby DW, Apelgren KN, Richardson W, Fanelli R; Society of American Gastrointestinal and Endoscopic Surgeons. SAGES guidelines for the clinical application of laparoscopic biliary tract surgery. Surg Endosc 2010; 24(10):2368–2386. doi:10.1007/s00464-010-1268-7
- Abraham S, Rivero HG, Erlikh IV, Griffith LF, Kondamudi VK. Surgical and nonsurgical management of gallstones. Am Fam Physician 2014; 89(10):795–802.
- Currò G,, Iapichino G, Lorenzini C, Palmeri R, Cucinotta E. Laparoscopic cholecystectomy in children with chronic hemolytic anemia. Is the outcome related to the timing of the procedure? Surg Endosc 2006; 20(2):252–255. doi:10.1007/s00464-005-0318-z
- Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol 2014; 6:99–109. doi:10.2147/CLEP.S37357
- Chen GL, Akmal Y, DiFronzo AL, Vuong B, O’Connor V. Porcelain gallbladder: no longer an indication for prophylactic cholecystectomy. Am Surg 2015; 81(10):936–940.
- Schnelldorfer T. Porcelain gallbladder: a benign process or concern for malignancy? J Gastrointest Surg 2013; 17(6):1161–1168. doi:10.1007/s11605-013-2170-0
- Warschkow R, Tarantino I, Ukegjini K, et al. Concomitant cholecystectomy during laparoscopic Roux-en-Y gastric bypass in obese patients is not justified: a meta-analysis. Obes Surg 2013; 23(3)3979–408. doi:10.1007/s11695-012-0852-4
- Gurusamy K, Samraj K, Gluud C, Wilson E, Davidson BR. Meta-analysis of randomized controlled trials on the safety and effectiveness of early versus delayed laparoscopic cholecystectomy for acute cholecystitis. Br J Surg 2010; 97(2):141–150. doi:10.1002/bjs.6870
- Papi C, Catarci M, D’Ambrosio L, et al. Timing of cholecystectomy for acute calculous cholecystitis: a meta-analysis. Am J Gastroenterol 2004; 99(1):147–155. doi:10.1046/j.1572-0241.2003.04002.x
- Gurusamy KS, Davidson C, Gluud C, Davidson BR. Early versus delayed laparoscopic cholecystectomy for people with acute cholecystitis. Cochrane Database Syst Rev 2013; 6:CD005440. doi:10.1002/14651858
- Menahem B, Mulliri A, Fohlen A, Guittet L, Alves A, Lubrano J. Delayed laparoscopic cholecystectomy increases the total hospital stay compared to an early laparoscopic cholecystectomy after acute cholecystitis: an updated meta-analysis of randomized controlled trials. HPB (Oxford) 2015; 17(10):857–862. doi:10.1111/hpb.12449
- Uhl W, Müller CA, Krähenbühl L, Schmid SW, Schölzel S, Büchler MW. Acute gallstone pancreatitis: timing of laparoscopic cholecystectomy in mild and severe disease. Surg Endosc 1999; 13(11):1070–1076. doi:10.1007/s004649901175
- Aboulian A, Chan T, Yaghoubian A, et al. Early cholecystectomy safely decreases hospital stay in patients with mild gallstone pancreatitis: a randomized prospective study. Ann Surg 2010(4): 251:615–619. doi:10.1097/SLA.0b013e3181c38f1f
The prevalence of gallstones is approximately 10% to 15% of the adult US population.1,2 Most cases are asymptomatic, as gallstones are usually discovered incidentally during routine imaging for other abdominal conditions, and only about 20% of patients with asymptomatic gallstones develop clinically significant complications.2,3
Nevertheless, gallstones carry significant healthcare costs. In 2004, the median inpatient cost for any gallstone-related disease was $11,584, with an overall annual cost of $6.2 billion.4,5
Laparoscopic cholecystectomy is the standard treatment for symptomatic cholelithiasis. For asymptomatic cholelithasis, the usual approach is expectant management (“watch and wait”), but prophylactic cholecystectomy may be an option in certain patients at high risk.
CHEMICAL COMPOSITION
Gallstones can be classified into 2 main categories based on their predominant chemical composition: cholesterol or pigment.
Cholesterol gallstones
About 75% of gallstones are composed of cholesterol.3,4 In the past, this type of stone was thought to be caused by gallbladder inflammation, bile stasis, and absorption of bile salts from damaged mucosa. However, it is now known that cholesterol gallstones are the result of biliary supersaturation caused by cholesterol hypersecretion into the gallbladder, gallbladder hypomotility, accelerated cholesterol nucleation and crystallization, and mucin gel accumulation.
Pigment gallstones
Black pigment gallstones account for 10% to 15% of all gallstones.6 They are caused by chronic hemolysis in association with supersaturation of bile with calcium hydrogen bilirubinate, along with deposition of calcium carbonate, phosphate, and inorganic salts.7
Brown pigment stones, accounting for 5% to 10% of all gallstones,6 are caused by infection in the obstructed bile ducts, where bacteria that produce beta-glucuronidase, phospholipase, and slime contribute to formation of the stone.8,9
RISK FACTORS FOR GALLSTONES
Age. After age 40, the risk increases dramatically, with an incidence 4 times higher for those ages 40 to 69 than in younger people.10
Female sex. Women of reproductive age are 4 times more likely to develop gallstones than men, but this gap narrows after menopause.11 The higher risk is attributed to female sex hormones, pregnancy, and oral contraceptive use. Estrogen decreases secretion of bile salts and increases secretion of cholesterol into the gallbladder, which leads to cholesterol supersaturation. Progesterone acts synergistically by causing hypomobility of the gallbladder, which in turn leads to bile stasis.12,13
Ethnicity. The risk is higher in Mexican Americans and Native Americans than in other ethnic groups.14
Rapid weight loss, such as after bariatric surgery, occurs from decreased caloric intake and promotes bile stasis, while lipolysis increases cholesterol mobilization and secretion into the gallbladder. This creates an environment conducive to bile supersaturation with cholesterol, leading to gallstone formation.
Chronic hemolytic disorders carry an increased risk of developing calcium bilirubinate stones due to increased excretion of bilirubin during hemolysis.
Obesity and diabetes mellitus are both attributed to insulin resistance. Obesity also increases bile stasis and cholesterol saturation.
CLINICAL PRESENTATION OF GALLSTONES (CHOLELITHIASIS)
Most patients with gallstones (cholelithiasis) experience no symptoms. Their gallstones are often discovered incidentally during imaging tests for unrelated or unexplained abdominal symptoms. Most patients with asymptomatic gallstones remain symptom-free, while about 20% develop gallstone-related symptoms.2,3
Abdominal pain is the most common symptom. The phrase biliary colic—suggesting pain that is fluctuating in nature—appears ubiquitously in the medical literature, but it does not correctly characterize the pain associated with gallstones.
Most patients with gallstone symptoms describe a constant and often severe pain in the right upper abdomen, epigastrium, or both, often persisting for 30 to 120 minutes. Symptoms are frequently reported in the epigastrium when only visceral pain fibers are stimulated due to gallbladder distention. This is usually called midline pain; however, pain occurs in the back and right shoulder in up to 60% of patients, with involvement of somatic fibers.15,16 Gallstone pain is not relieved by change of position or passage of stool or gas.
Onset of symptoms more than an hour after eating or in the late evening or at night also very strongly suggests biliary pain. Patients with a history of biliary pain are more likely to experience it again, with a 69% chance of developing recurrent pain within 2 years.17
GALLSTONE-RELATED COMPLICATIONS
Acute gallbladder inflammation (cholecystitis)
Gallbladder inflammation (cholecystitis) is the most common complication, occurring in up to 10% of symptomatic cases. Many patients with acute cholecystitis present with right upper quadrant pain that may be accompanied by anorexia, nausea, or vomiting. Inspiratory arrest on deep palpation of the right upper quadrant (Murphy sign) has a specificity of 79% to 96% for acute cholecystitis.20 Markers of systemic inflammation such as fever, elevated white blood cell count, and elevated C-reactive protein are highly suggestive of acute cholecystitis.20,21
Bile duct stones (choledocholithiasis)
Bile duct stones (choledocholithiasis) are detected in 3.4% to 12% of patients with gallstones.22,23 Most stones in the common bile duct migrate there from the gallbladder via the cystic duct. Less commonly, primary duct stones form in the duct due to biliary stasis. Removing the gallbladder does not completely eliminate the risk of bile duct stones, as stones can remain or recur after surgery.
Bile duct stones can obstruct the common bile duct, which disrupts normal bile flow and leads to jaundice. Other symptoms may include pruritus, right upper quadrant pain, nausea, and vomiting. Serum levels of bilirubin, aspartate aminotransferase, alanine aminotransferase (ALT), and alkaline phosphatase are usually high.24
Acute bacterial infection (cholangitis)
Acute bacterial infection of the biliary system (cholangitis) is usually associated with obstruction of the common bile duct. Common symptoms of acute cholangitis include right upper quadrant pain, fever, and jaundice (Charcot triad), and these are present in about 50% to 75% of cases.21 In severe cases, patients can develop altered mental status and septicemic shock in addition to the Charcot triad, a condition called the Reynold pentad. White blood cell counts and serum levels of C-reactive protein, bilirubin, aminotransferases, and alkaline phosphatase are usually elevated.21
Pancreatitis
Approximately 4% to 8% of patients with gallstones develop inflammation of the pancreas (pancreatitis).25 The diagnosis of acute pancreatitis requires at least 2 of the following:26,27
- Abdominal pain (typically epigastric, often radiating to the back)
- Amylase or lipase levels at least 3 times above the normal limit
- Imaging findings that suggest acute pancreatitis.
Gallstone-related pancreatitis should be considered if the ALT level is greater than 150 U/mL, which has a 97% specificity for gallstone-related pancreatitis.28
ABDOMINAL ULTRASONOGRAPHY FOR DIAGNOSIS
Transabdominal ultrasonography, with a sensitivity of 84% to 89% and a specificity of up to 99%, is the test of choice for detecting gallstones.29 The characteristic findings of acute cholecystitis on ultrasonography include enlargement of the gallbladder, thickening of the gallbladder wall, presence of pericholecystic fluid, and tenderness elicited by the ultrasound probe over the gallbladder (sonographic Murphy sign).
Scintigraphy as a second test
Acute cholecystitis is primarily a clinical diagnosis and typically does not require additional imaging beyond ultrasonography. When there is discordance between clinical and ultrasonographic findings, the most accurate second imaging test is scintigraphy of the biliary tract, usually performed with technetium-labeled hydroxy iminodiacetic acid. Given intravenously, the radionuclide is rapidly taken up by the liver and then secreted into the bile. In acute cholecystitis, the cystic duct is functionally occluded and the isotope does not enter the gallbladder, creating an imaging void compared with a normal appearance.
Scintigraphy is more sensitive than abdominal ultrasonography, with a sensitivity of up to 97% vs 81% to 88%, respectively.29,30 The tests have about equal specificity.
Even though scintigraphy is more sensitive, abdominal ultrasonography is often the initial test for patients with suspected acute cholecystitis because it is more widely available, takes less time, does not involve radiation exposure, and can assess for the presence or absence of gallstones and dilation of the intra- and extrahepatic bile ducts.
Looking for stones in the common bile duct
When acute cholangitis due to choledocholithiasis is suspected, abdominal ultrasonography is a prudent initial test to look for gallstones or biliary dilation suggesting obstruction by stones in the common bile duct. Abdominal ultrasonography has only a 22% to 55% sensitivity for visualizing stones in the common bile duct, but it has a 77% to 87% sensitivity for detecting common bile duct dilation, a surrogate marker of stones.31
The normal bile duct diameter ranges from 3 to 6 mm, although mild dilation is often seen in older patients or after cholecystectomy or Roux-en-Y gastric bypass surgery.32,33 Bile duct dilation of up to 10 mm can be considered normal in patients after cholecystectomy.34 A normal-appearing bile duct on ultrasonography has a negative predictive value of 95% for excluding common bile duct stones.31
Endoscopic ultrasonography (EUS), magnetic resonance cholangiopancreatography (MRCP), and endoscopic retrograde cholangiopancreatography (ERCP) have similar sensitivity (89%–94%, 85%–92%, and 89%–93%, respectively) and specificity (94%–95%, 93%–97%, and 100%, respectively) for detecting common bile duct stones.35–37 EUS is superior to MRCP in detecting stones smaller than 6 mm.38
ERCP should be reserved for managing rather than diagnosing common bile duct stones because of the risk of pancreatitis and perforation. Patients undergoing cholecystectomy who are suspected of having choledocholithiasis may undergo intraoperative cholangiography or laparoscopic common bile duct ultrasonography.
WATCH AND WAIT, OR INTERVENE?
Asymptomatic gallstones
Standard treatment for these patients is expectant management. Cholecystectomy is not recommended for patients with asymptomatic gallstones.47 Nevertheless, some patients may benefit from prophylactic cholecystectomy. We and others48 suggest considering cholecystectomy in the following patients.
Patients with chronic hemolytic anemia (including children with sickle cell anemia and spherocytosis). These patients have a higher risk of developing calcium bilirubinate stones, and cholecystectomy has improved outcomes.49 It should be noted that most of these data come from pediatric populations and have been extrapolated to adults.
Native Americans, who have a higher risk of gallbladder cancer if they have gallstones.2,50
Conversely, calcification of the gallbladder wall (“porcelain gallbladder”) is no longer considered an absolute indication for cholecystectomy. This condition was thought to be associated with a high rate of gallbladder carcinoma, but analyses of larger, more recent data sets found much smaller risks.51,52 Further, cholecystectomy in these patients was found to be associated with high rates of postoperative complications. Thus, prophylactic cholecystectomy is no longer recommended in asymptomatic cases of porcelain gallbladder.
In addition, concomitant cholecystectomy in patients undergoing bariatric surgery is no longer considered the therapeutic standard. Historically, cholecystectomy was performed in these patients because of the increased risk of gallstones associated with rapid weight loss after surgery. However, research now weighs against concomitant cholecystectomy with bariatric surgery and most other abdominal surgeries for asymptomatic gallstones.53
Laparoscopic surgery for symptomatic gallstones
For patients experiencing acute cholecystitis, laparoscopic cholecystectomy within 72 hours is recommended.48 There were safety concerns regarding higher rates of morbidity and conversion from laparoscopic to open cholecystectomy in patients who underwent surgery before the acute cholecystitis episode had settled. However, a large meta-analysis found no significant difference between early and delayed laparoscopic cholecystectomy in bile duct injury or conversion rates.54 Further, early cholecystectomy—defined as within 1 week of symptom onset—has been found to reduce gallstone-related complications, shorten hospital stays, and lower costs.55–57 If the patient cannot undergo surgery, percutaneous cholecystotomy or novel endoscopic gallbladder drainage interventions can be used.
Several variables predict the presence of bile duct stones in patients who have symptoms (Table 4). Based on these predictors, the ASGE classifies the probabilities as low (< 10%), intermediate (10% to 50%), and high (> 50%)31:
- High-risk patients should undergo preoperative ERCP and stone extraction if needed
- Intermediate-risk patients should undergo preoperative imaging with EUS or MRCP or intraoperative bile duct evaluation, depending on the availability, costs, and local expertise.
Patients with associated cholangitis should be given intravenous fluids and broad-spectrum antibiotics. Biliary decompression should be done as early as possible to decrease the risk of morbidity and mortality. For acute cholangitis, ERCP is the treatment of choice.25
Patients with acute gallstone pancreatitis should receive conservative management with intravenous isotonic solutions and pain control, followed by laparoscopic cholecystectomy.48
The timing of laparoscopic cholecystectomy in acute gallstone pancreatitis has been debated. Studies conducted during the era of open cholecystectomy reported similar or worse outcomes if cholecystectomy was done sooner rather than later.
However, in 1999, Uhl et al58 reported that 48 of 77 patients admitted with acute gallstone pancreatitis were able to undergo laparoscopic cholecystectomy during the same admission. Success rates were 85% (30 of 35 patients) in those with mild disease and 62% (8 of 13 patients) in those with severe disease. They concluded laparoscopic cholecystectomy could be safely performed within 7 days in patients with mild disease, whereas in severe disease at least 3 weeks should elapse because of the risk of infection.
In a randomized trial published in 2010, Aboulian et al59 reported that hospital length of stay (the primary end point) was shorter in 25 patients who underwent laparoscopic cholecystectomy early (within 48 hours of admission) than in 25 patients who underwent surgery after abdominal pain had resolved and laboratory enzymes showed a normalizing trend, 3.5 vs 5.8 days (P = .0016). Rates of perioperative complications and need for conversion to open surgery were similar between the 2 groups.
If there is associated cholangitis, patients should also be given broad-spectrum antibiotics and should undergo ERCP within 24 hours of admission.25–27
SUMMARY
Gallstones are common in US adults. Abdominal ultrasonography is the diagnostic imaging test of choice to detect gallbladder stones and assess for findings suggestive of acute cholecystitis and dilation of the common bile duct. Fortunately, most gallstones are asymptomatic and can usually be managed expectantly. In patients who have symptoms or have gallstone complications, laparoscopic cholecystectomy is the standard of care.
The prevalence of gallstones is approximately 10% to 15% of the adult US population.1,2 Most cases are asymptomatic, as gallstones are usually discovered incidentally during routine imaging for other abdominal conditions, and only about 20% of patients with asymptomatic gallstones develop clinically significant complications.2,3
Nevertheless, gallstones carry significant healthcare costs. In 2004, the median inpatient cost for any gallstone-related disease was $11,584, with an overall annual cost of $6.2 billion.4,5
Laparoscopic cholecystectomy is the standard treatment for symptomatic cholelithiasis. For asymptomatic cholelithasis, the usual approach is expectant management (“watch and wait”), but prophylactic cholecystectomy may be an option in certain patients at high risk.
CHEMICAL COMPOSITION
Gallstones can be classified into 2 main categories based on their predominant chemical composition: cholesterol or pigment.
Cholesterol gallstones
About 75% of gallstones are composed of cholesterol.3,4 In the past, this type of stone was thought to be caused by gallbladder inflammation, bile stasis, and absorption of bile salts from damaged mucosa. However, it is now known that cholesterol gallstones are the result of biliary supersaturation caused by cholesterol hypersecretion into the gallbladder, gallbladder hypomotility, accelerated cholesterol nucleation and crystallization, and mucin gel accumulation.
Pigment gallstones
Black pigment gallstones account for 10% to 15% of all gallstones.6 They are caused by chronic hemolysis in association with supersaturation of bile with calcium hydrogen bilirubinate, along with deposition of calcium carbonate, phosphate, and inorganic salts.7
Brown pigment stones, accounting for 5% to 10% of all gallstones,6 are caused by infection in the obstructed bile ducts, where bacteria that produce beta-glucuronidase, phospholipase, and slime contribute to formation of the stone.8,9
RISK FACTORS FOR GALLSTONES
Age. After age 40, the risk increases dramatically, with an incidence 4 times higher for those ages 40 to 69 than in younger people.10
Female sex. Women of reproductive age are 4 times more likely to develop gallstones than men, but this gap narrows after menopause.11 The higher risk is attributed to female sex hormones, pregnancy, and oral contraceptive use. Estrogen decreases secretion of bile salts and increases secretion of cholesterol into the gallbladder, which leads to cholesterol supersaturation. Progesterone acts synergistically by causing hypomobility of the gallbladder, which in turn leads to bile stasis.12,13
Ethnicity. The risk is higher in Mexican Americans and Native Americans than in other ethnic groups.14
Rapid weight loss, such as after bariatric surgery, occurs from decreased caloric intake and promotes bile stasis, while lipolysis increases cholesterol mobilization and secretion into the gallbladder. This creates an environment conducive to bile supersaturation with cholesterol, leading to gallstone formation.
Chronic hemolytic disorders carry an increased risk of developing calcium bilirubinate stones due to increased excretion of bilirubin during hemolysis.
Obesity and diabetes mellitus are both attributed to insulin resistance. Obesity also increases bile stasis and cholesterol saturation.
CLINICAL PRESENTATION OF GALLSTONES (CHOLELITHIASIS)
Most patients with gallstones (cholelithiasis) experience no symptoms. Their gallstones are often discovered incidentally during imaging tests for unrelated or unexplained abdominal symptoms. Most patients with asymptomatic gallstones remain symptom-free, while about 20% develop gallstone-related symptoms.2,3
Abdominal pain is the most common symptom. The phrase biliary colic—suggesting pain that is fluctuating in nature—appears ubiquitously in the medical literature, but it does not correctly characterize the pain associated with gallstones.
Most patients with gallstone symptoms describe a constant and often severe pain in the right upper abdomen, epigastrium, or both, often persisting for 30 to 120 minutes. Symptoms are frequently reported in the epigastrium when only visceral pain fibers are stimulated due to gallbladder distention. This is usually called midline pain; however, pain occurs in the back and right shoulder in up to 60% of patients, with involvement of somatic fibers.15,16 Gallstone pain is not relieved by change of position or passage of stool or gas.
Onset of symptoms more than an hour after eating or in the late evening or at night also very strongly suggests biliary pain. Patients with a history of biliary pain are more likely to experience it again, with a 69% chance of developing recurrent pain within 2 years.17
GALLSTONE-RELATED COMPLICATIONS
Acute gallbladder inflammation (cholecystitis)
Gallbladder inflammation (cholecystitis) is the most common complication, occurring in up to 10% of symptomatic cases. Many patients with acute cholecystitis present with right upper quadrant pain that may be accompanied by anorexia, nausea, or vomiting. Inspiratory arrest on deep palpation of the right upper quadrant (Murphy sign) has a specificity of 79% to 96% for acute cholecystitis.20 Markers of systemic inflammation such as fever, elevated white blood cell count, and elevated C-reactive protein are highly suggestive of acute cholecystitis.20,21
Bile duct stones (choledocholithiasis)
Bile duct stones (choledocholithiasis) are detected in 3.4% to 12% of patients with gallstones.22,23 Most stones in the common bile duct migrate there from the gallbladder via the cystic duct. Less commonly, primary duct stones form in the duct due to biliary stasis. Removing the gallbladder does not completely eliminate the risk of bile duct stones, as stones can remain or recur after surgery.
Bile duct stones can obstruct the common bile duct, which disrupts normal bile flow and leads to jaundice. Other symptoms may include pruritus, right upper quadrant pain, nausea, and vomiting. Serum levels of bilirubin, aspartate aminotransferase, alanine aminotransferase (ALT), and alkaline phosphatase are usually high.24
Acute bacterial infection (cholangitis)
Acute bacterial infection of the biliary system (cholangitis) is usually associated with obstruction of the common bile duct. Common symptoms of acute cholangitis include right upper quadrant pain, fever, and jaundice (Charcot triad), and these are present in about 50% to 75% of cases.21 In severe cases, patients can develop altered mental status and septicemic shock in addition to the Charcot triad, a condition called the Reynold pentad. White blood cell counts and serum levels of C-reactive protein, bilirubin, aminotransferases, and alkaline phosphatase are usually elevated.21
Pancreatitis
Approximately 4% to 8% of patients with gallstones develop inflammation of the pancreas (pancreatitis).25 The diagnosis of acute pancreatitis requires at least 2 of the following:26,27
- Abdominal pain (typically epigastric, often radiating to the back)
- Amylase or lipase levels at least 3 times above the normal limit
- Imaging findings that suggest acute pancreatitis.
Gallstone-related pancreatitis should be considered if the ALT level is greater than 150 U/mL, which has a 97% specificity for gallstone-related pancreatitis.28
ABDOMINAL ULTRASONOGRAPHY FOR DIAGNOSIS
Transabdominal ultrasonography, with a sensitivity of 84% to 89% and a specificity of up to 99%, is the test of choice for detecting gallstones.29 The characteristic findings of acute cholecystitis on ultrasonography include enlargement of the gallbladder, thickening of the gallbladder wall, presence of pericholecystic fluid, and tenderness elicited by the ultrasound probe over the gallbladder (sonographic Murphy sign).
Scintigraphy as a second test
Acute cholecystitis is primarily a clinical diagnosis and typically does not require additional imaging beyond ultrasonography. When there is discordance between clinical and ultrasonographic findings, the most accurate second imaging test is scintigraphy of the biliary tract, usually performed with technetium-labeled hydroxy iminodiacetic acid. Given intravenously, the radionuclide is rapidly taken up by the liver and then secreted into the bile. In acute cholecystitis, the cystic duct is functionally occluded and the isotope does not enter the gallbladder, creating an imaging void compared with a normal appearance.
Scintigraphy is more sensitive than abdominal ultrasonography, with a sensitivity of up to 97% vs 81% to 88%, respectively.29,30 The tests have about equal specificity.
Even though scintigraphy is more sensitive, abdominal ultrasonography is often the initial test for patients with suspected acute cholecystitis because it is more widely available, takes less time, does not involve radiation exposure, and can assess for the presence or absence of gallstones and dilation of the intra- and extrahepatic bile ducts.
Looking for stones in the common bile duct
When acute cholangitis due to choledocholithiasis is suspected, abdominal ultrasonography is a prudent initial test to look for gallstones or biliary dilation suggesting obstruction by stones in the common bile duct. Abdominal ultrasonography has only a 22% to 55% sensitivity for visualizing stones in the common bile duct, but it has a 77% to 87% sensitivity for detecting common bile duct dilation, a surrogate marker of stones.31
The normal bile duct diameter ranges from 3 to 6 mm, although mild dilation is often seen in older patients or after cholecystectomy or Roux-en-Y gastric bypass surgery.32,33 Bile duct dilation of up to 10 mm can be considered normal in patients after cholecystectomy.34 A normal-appearing bile duct on ultrasonography has a negative predictive value of 95% for excluding common bile duct stones.31
Endoscopic ultrasonography (EUS), magnetic resonance cholangiopancreatography (MRCP), and endoscopic retrograde cholangiopancreatography (ERCP) have similar sensitivity (89%–94%, 85%–92%, and 89%–93%, respectively) and specificity (94%–95%, 93%–97%, and 100%, respectively) for detecting common bile duct stones.35–37 EUS is superior to MRCP in detecting stones smaller than 6 mm.38
ERCP should be reserved for managing rather than diagnosing common bile duct stones because of the risk of pancreatitis and perforation. Patients undergoing cholecystectomy who are suspected of having choledocholithiasis may undergo intraoperative cholangiography or laparoscopic common bile duct ultrasonography.
WATCH AND WAIT, OR INTERVENE?
Asymptomatic gallstones
Standard treatment for these patients is expectant management. Cholecystectomy is not recommended for patients with asymptomatic gallstones.47 Nevertheless, some patients may benefit from prophylactic cholecystectomy. We and others48 suggest considering cholecystectomy in the following patients.
Patients with chronic hemolytic anemia (including children with sickle cell anemia and spherocytosis). These patients have a higher risk of developing calcium bilirubinate stones, and cholecystectomy has improved outcomes.49 It should be noted that most of these data come from pediatric populations and have been extrapolated to adults.
Native Americans, who have a higher risk of gallbladder cancer if they have gallstones.2,50
Conversely, calcification of the gallbladder wall (“porcelain gallbladder”) is no longer considered an absolute indication for cholecystectomy. This condition was thought to be associated with a high rate of gallbladder carcinoma, but analyses of larger, more recent data sets found much smaller risks.51,52 Further, cholecystectomy in these patients was found to be associated with high rates of postoperative complications. Thus, prophylactic cholecystectomy is no longer recommended in asymptomatic cases of porcelain gallbladder.
In addition, concomitant cholecystectomy in patients undergoing bariatric surgery is no longer considered the therapeutic standard. Historically, cholecystectomy was performed in these patients because of the increased risk of gallstones associated with rapid weight loss after surgery. However, research now weighs against concomitant cholecystectomy with bariatric surgery and most other abdominal surgeries for asymptomatic gallstones.53
Laparoscopic surgery for symptomatic gallstones
For patients experiencing acute cholecystitis, laparoscopic cholecystectomy within 72 hours is recommended.48 There were safety concerns regarding higher rates of morbidity and conversion from laparoscopic to open cholecystectomy in patients who underwent surgery before the acute cholecystitis episode had settled. However, a large meta-analysis found no significant difference between early and delayed laparoscopic cholecystectomy in bile duct injury or conversion rates.54 Further, early cholecystectomy—defined as within 1 week of symptom onset—has been found to reduce gallstone-related complications, shorten hospital stays, and lower costs.55–57 If the patient cannot undergo surgery, percutaneous cholecystotomy or novel endoscopic gallbladder drainage interventions can be used.
Several variables predict the presence of bile duct stones in patients who have symptoms (Table 4). Based on these predictors, the ASGE classifies the probabilities as low (< 10%), intermediate (10% to 50%), and high (> 50%)31:
- High-risk patients should undergo preoperative ERCP and stone extraction if needed
- Intermediate-risk patients should undergo preoperative imaging with EUS or MRCP or intraoperative bile duct evaluation, depending on the availability, costs, and local expertise.
Patients with associated cholangitis should be given intravenous fluids and broad-spectrum antibiotics. Biliary decompression should be done as early as possible to decrease the risk of morbidity and mortality. For acute cholangitis, ERCP is the treatment of choice.25
Patients with acute gallstone pancreatitis should receive conservative management with intravenous isotonic solutions and pain control, followed by laparoscopic cholecystectomy.48
The timing of laparoscopic cholecystectomy in acute gallstone pancreatitis has been debated. Studies conducted during the era of open cholecystectomy reported similar or worse outcomes if cholecystectomy was done sooner rather than later.
However, in 1999, Uhl et al58 reported that 48 of 77 patients admitted with acute gallstone pancreatitis were able to undergo laparoscopic cholecystectomy during the same admission. Success rates were 85% (30 of 35 patients) in those with mild disease and 62% (8 of 13 patients) in those with severe disease. They concluded laparoscopic cholecystectomy could be safely performed within 7 days in patients with mild disease, whereas in severe disease at least 3 weeks should elapse because of the risk of infection.
In a randomized trial published in 2010, Aboulian et al59 reported that hospital length of stay (the primary end point) was shorter in 25 patients who underwent laparoscopic cholecystectomy early (within 48 hours of admission) than in 25 patients who underwent surgery after abdominal pain had resolved and laboratory enzymes showed a normalizing trend, 3.5 vs 5.8 days (P = .0016). Rates of perioperative complications and need for conversion to open surgery were similar between the 2 groups.
If there is associated cholangitis, patients should also be given broad-spectrum antibiotics and should undergo ERCP within 24 hours of admission.25–27
SUMMARY
Gallstones are common in US adults. Abdominal ultrasonography is the diagnostic imaging test of choice to detect gallbladder stones and assess for findings suggestive of acute cholecystitis and dilation of the common bile duct. Fortunately, most gallstones are asymptomatic and can usually be managed expectantly. In patients who have symptoms or have gallstone complications, laparoscopic cholecystectomy is the standard of care.
- Schirmer BD, Winters KL, Edlich RF. Cholelithiasis and cholecystitis. J Long Term Eff Med Implants 2005; 15(3):329–338. doi:10.1615/JLongTermEffMedImplants.v15.i3.90
- Stinton LM, Shaffer EA. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liver 2012; 6(2):172–187. doi:10.5009/gnl.2012.6.2.172
- Lee JY, Keane MG, Pereira S. Diagnosis and treatment of gallstone disease. Practitioner 2015; 259(1783):15–19.
- Russo MW, Wei JT, Thiny MT, et al. Digestive and liver diseases statistics, 2004. Gastroenterology 2004; 126(5):1448–1453. doi:10.1053/j.gastro.2004.01.025
- Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part I: overall and upper gastrointestinal diseases. Gastroenterology 2009; 136(2):376–386. doi:10.1053/j.gastro.2008.12.015
- Cariati A. Gallstone classification in Western countries. Indian J Surg 2015; 77(suppl 2):376–380. doi.org/10.1007/s12262-013-0847-y
- Carey MC. Pathogenesis of gallstones. Am J Surg 1993; 165(4):410–419. doi:10.1016/S0002-9610(05)80932-8
- Lammert F, Gurusamy K, Ko CW, et al. Gallstones. Nat Rev Dis Primers 2016; 2:16024. doi:10.1038/nrdp.2016.24
- Stewart L, Oesterle AL, Erdan I, Griffiss JM, Way LW. Pathogenesis of pigment gallstones in Western societies: the central role of bacteria. J Gastrointest Surg 2002; 6(6):891–904.
- Barbara L, Sama C, Morselli Labate AM, et al. A population study on the prevalence of gallstone disease: the Sirmione Study. Hepatology 1987; 7(5):913–917. doi:10.1002/hep.1840070520
- Sood S, Winn T, Ibrahim S, et al. Natural history of asymptomatic gallstones: differential behaviour in male and female subjects. Med J Malaysia 2015; 70(6):341–345.
- Maringhini A, Ciambra M, Baccelliere P, et al. Biliary sludge and gallstones in pregnancy: incidence, risk factors, and natural history. Ann Intern Med 1993; 119(2):116–120. doi:10.7326/0003-4819-119-2-199307150-00004
- Etminan M, Delaney JA, Bressler B, Brophy JM. Oral contraceptives and the risk of gallbladder disease: a comparative safety study. CMAJ 2011; 183(8):899–904. doi:10.1503/cmaj.110161
- Everhart JE, Khare M, Hill M, Maurer KR. Prevalence and ethnic differences in gallbladder disease in the United States. Gastroenterology 1999; 117(3):632–639.
- Festi D, Sottili S, Colecchia A, et al. Clinical manifestations of gallstone disease: evidence from the multicenter Italian study on cholelithiasis (MICOL). Hepatology 1999; 30(4):839–846. doi:10.1002/hep.510300401
- Berhane T, Vetrhus M, Hausken T, Olafsson S, Sondenaa K. Pain attacks in non-complicated and complicated gallstone disease have a characteristic pattern and are accompanied by dyspepsia in most patients: the results of a prospective study. Scand J Gastroenterol 2006; 41(1):93–101. doi:10.1080/00365520510023990
- Thistle JL, Cleary PA, Lachin JM, Tyor MP, Hersh T. The natural history of cholelithiasis: the National Cooperative Gallstone Study. Ann Intern Med 1984; 101(2):171–175. doi:10.7326/0003-4819-101-2-171
- Friedman GD. Natural history of asymptomatic and symptomatic gallstones. Am J Surg 1993; 165(4):399–404. doi:0.1016/S0002-9610(05)80930-4
- Friedman GD, Raviola CA, Fireman B. Prognosis of gallstones with mild or no symptoms: 25 years of follow-up in a health maintenance organization. J Clin Epidemiol 1989; 42(2):127–136. doi:10.1016/0895-4356(89)90086-3
- Hirota M, Takada T, Kawarada Y, et al. Diagnostic criteria and severity assessment of acute cholecystitis: Tokyo guidelines. J Hepatobiliary Pancreat Surg 2007; 14(1):78–82. doi:10.1007/s00534-006-1159-4
- Miura F, Takada T, Kawarada Y, et al. Flowcharts for the diagnosis and treatment of acute cholangitis and cholecystitis: Tokyo guidelines. J Hepatobiliary Pancreat Surg 2007; 14(1):27–34. doi:10.1007/s00534-006-1153-x
- Koo KP, Traverso LW. Do preoperative indicators predict the presence of common bile duct stones during laparoscopic cholecystectomy? Am J Surg 1996; 171(5):495–499. doi:10.1016/S0002-9610(97)89611-0
- Collins C, Maguire D, Ireland A, Fitzgerald E, O’Sullivan GC. A prospective study of common bile duct calculi in patients undergoing laparoscopic cholecystectomy: natural history of choledocholithiasis revisited. Ann Surg 2004; 239(1):28–33. doi:10.1097/01.sla.0000103069.00170.9c
- Costi R, Gnocchi A, Di Mario F, Sarli L. Diagnosis and management of choledocholithiasis in the golden age of imaging, endoscopy and laparoscopy. World J Gastroenterol 2014; 20(37):13382–13401. doi:10.3748/wjg.v20.i37.13382
- European Association for the Study of the Liver (EASL). EASL Clinical Practice Guidelines on the prevention, diagnosis and treatment of gallstones. J Hepatol 2016; 65(1):146–181. doi:10.1016/j.jhep.2016.03.005
- Greenberg JA, Hsu J, Bawazeer M, et al. Clinical practice guideline: management of acute pancreatitis. Can J Surg 2016; 59 (2):128–140. doi:10.1503/cjs.015015
- Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol 2013; 108(9):1400–1416. doi:10.1038/ajg.2013.218
- Moolla Z, Anderson F, Thomson SR. Use of amylase and alanine transaminase to predict acute gallstone pancreatitis in a population with high HIV prevalence. World J Surg 2013; 37(1):156–161. doi:10.1007/s00268-012-1801-z
- Shea JA, Berlin JA, Escarce JJ, et al. Revised estimates of diagnostic test sensitivity and specificity in suspected biliary tract disease. Arch Intern Med 1994; 154(22):2573–2581. doi:10.1001/archinte.1994.00420220069008
- Kiewiet JJ, Leeuwenburgh MM, Bipat S, et al. A systematic review and meta-analysis of diagnostic performance of imaging in acute cholecystitis. Radiology 2012; 264(3):708–720. doi:10.1148/radiol.12111561
- ASGE Standards of Practice Committee; Maple JT, Ben-Menachem T, Anderson MA, et al. The role of endoscopy in the evaluation of suspected choledocholithiasis. Gastrointest Endosc 2010; 71(1):1–9. doi:10.1016/j.gie.2009.09.041
- Bachar GN, Cohen M, Belenky A, Atar E, Gideon S. Effect of aging on the adult extrahepatic bile duct: a sonographic study. J Ultrasound Med 2003; 22(9):879–885. doi:10.7863/jum.2003.22.9.879
- El-Hayek K, Timratana P, Meranda J, Shimizu H, Eldar S, Chand B. Post Roux-en-Y gastric bypass biliary dilation: natural process or significant entity? J Gastrointest Surg 2012; 16(12):2185–2189. doi:10.1007/s11605-012-2058-4
- Park SM, Kim WS, Bae IH, et al. Common bile duct dilatation after cholecystectomy: a one-year prospective study. J Korean Surg Soc 2012; 83(2):97–101. doi:10.4174/jkss.2012.83.2.97
- Tse F, Liu L, Barkun AN, Armstrong D, Moayyedi P. EUS: a meta-analysis of test performance in suspected choledocholithiasis. Gastrointest Endosc 2008; 67(2):235–244. doi:10.1016/j.gie.2007.09.047
- Verma D, Kapadia A, Eisen GM, Adler DG. EUS vs MRCP for detection of choledocholithiasis. Gastrointest Endosc 2006; 64(2):248–254. doi:10.1016/j.gie.2005.12.038
- Tseng LJ, Jao YT, Mo LR, Lin RC. Over-the-wire US catheter probe as an adjunct to ERCP in the detection of choledocholithiasis. Gastrointest Endosc 2001; 54(6):720–723. doi:10.1067/mge.2001.119255
- Kondo S, Isayama H, Akahane M, et al. Detection of common bile duct stones: comparison between endoscopic ultrasonography, magnetic resonance cholangiography, and helical-computed-tomographic cholangiography. Eur J Radiol 2005; 54(2):271–275. doi:10.1016/j.ejrad.2004.07.007
- Attili AF, De Santis A, Capri R, Repice AM, Maselli S. The natural history of gallstones: the GREPCO experience. The GREPCO Group. Hepatology 1995; 21(3):656–660. doi:10.1016/0270-9139(95)90514-6
- Sakorafas GH, Milingos D, Peros G. Asymptomatic cholelithiasis: is cholecystectomy really needed? A critical reappraisal 15 years after the introduction of laparoscopic cholecystectomy. Dig Dis Sci 2007; 52(5):1313–1325. doi:10.1007/s10620-006-9107-3
- Gracie WA, Ransohoff DF. The natural history of silent gallstones: the innocent gallstone is not a myth. N Engl J Med 1982; 307(13):798–800. doi:10.1056/NEJM198209233071305
- McSherry CK, Ferstenberg H, Calhoun WF, Lahman E, Virshup M. The natural history of diagnosed gallstone disease in symptomatic and asymptomatic patients. Ann Surg 1985; 202(1):59–63. doi:10.1097/00000658-198507000-00009
- Wada K, Wada K, Imamura T. Natural course of asymptomatic gallstone disease. Nihon Rinsho 1993; 51(7):1737–1743. Japanese.
- Halldestam I, Enell EL, Kullman E, Borch K. Development of symptoms and complications in individuals with asymptomatic gallstones. Br J Surg 2004; 91(6):734–738. doi:10.1002/bjs.4547
- Festi D, Reggiani ML, Attili AF, et al. Natural history of gallstone disease: expectant management or active treatment? Results from a population-based cohort study. J Gastroenterol Hepatol 2010; 25(4):719–724. doi:10.1111/j.1440-1746.2009.06146.x
- Shabanzadeh DM, Sorensen LT, Jorgensen T. A prediction rule for risk stratification of incidentally discovered gallstones: results from a large cohort study. Gastroenterology 2016; 150(1):156–167e1. doi:10.1053/j.gastro.2015.09.002
- Overby DW, Apelgren KN, Richardson W, Fanelli R; Society of American Gastrointestinal and Endoscopic Surgeons. SAGES guidelines for the clinical application of laparoscopic biliary tract surgery. Surg Endosc 2010; 24(10):2368–2386. doi:10.1007/s00464-010-1268-7
- Abraham S, Rivero HG, Erlikh IV, Griffith LF, Kondamudi VK. Surgical and nonsurgical management of gallstones. Am Fam Physician 2014; 89(10):795–802.
- Currò G,, Iapichino G, Lorenzini C, Palmeri R, Cucinotta E. Laparoscopic cholecystectomy in children with chronic hemolytic anemia. Is the outcome related to the timing of the procedure? Surg Endosc 2006; 20(2):252–255. doi:10.1007/s00464-005-0318-z
- Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol 2014; 6:99–109. doi:10.2147/CLEP.S37357
- Chen GL, Akmal Y, DiFronzo AL, Vuong B, O’Connor V. Porcelain gallbladder: no longer an indication for prophylactic cholecystectomy. Am Surg 2015; 81(10):936–940.
- Schnelldorfer T. Porcelain gallbladder: a benign process or concern for malignancy? J Gastrointest Surg 2013; 17(6):1161–1168. doi:10.1007/s11605-013-2170-0
- Warschkow R, Tarantino I, Ukegjini K, et al. Concomitant cholecystectomy during laparoscopic Roux-en-Y gastric bypass in obese patients is not justified: a meta-analysis. Obes Surg 2013; 23(3)3979–408. doi:10.1007/s11695-012-0852-4
- Gurusamy K, Samraj K, Gluud C, Wilson E, Davidson BR. Meta-analysis of randomized controlled trials on the safety and effectiveness of early versus delayed laparoscopic cholecystectomy for acute cholecystitis. Br J Surg 2010; 97(2):141–150. doi:10.1002/bjs.6870
- Papi C, Catarci M, D’Ambrosio L, et al. Timing of cholecystectomy for acute calculous cholecystitis: a meta-analysis. Am J Gastroenterol 2004; 99(1):147–155. doi:10.1046/j.1572-0241.2003.04002.x
- Gurusamy KS, Davidson C, Gluud C, Davidson BR. Early versus delayed laparoscopic cholecystectomy for people with acute cholecystitis. Cochrane Database Syst Rev 2013; 6:CD005440. doi:10.1002/14651858
- Menahem B, Mulliri A, Fohlen A, Guittet L, Alves A, Lubrano J. Delayed laparoscopic cholecystectomy increases the total hospital stay compared to an early laparoscopic cholecystectomy after acute cholecystitis: an updated meta-analysis of randomized controlled trials. HPB (Oxford) 2015; 17(10):857–862. doi:10.1111/hpb.12449
- Uhl W, Müller CA, Krähenbühl L, Schmid SW, Schölzel S, Büchler MW. Acute gallstone pancreatitis: timing of laparoscopic cholecystectomy in mild and severe disease. Surg Endosc 1999; 13(11):1070–1076. doi:10.1007/s004649901175
- Aboulian A, Chan T, Yaghoubian A, et al. Early cholecystectomy safely decreases hospital stay in patients with mild gallstone pancreatitis: a randomized prospective study. Ann Surg 2010(4): 251:615–619. doi:10.1097/SLA.0b013e3181c38f1f
- Schirmer BD, Winters KL, Edlich RF. Cholelithiasis and cholecystitis. J Long Term Eff Med Implants 2005; 15(3):329–338. doi:10.1615/JLongTermEffMedImplants.v15.i3.90
- Stinton LM, Shaffer EA. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liver 2012; 6(2):172–187. doi:10.5009/gnl.2012.6.2.172
- Lee JY, Keane MG, Pereira S. Diagnosis and treatment of gallstone disease. Practitioner 2015; 259(1783):15–19.
- Russo MW, Wei JT, Thiny MT, et al. Digestive and liver diseases statistics, 2004. Gastroenterology 2004; 126(5):1448–1453. doi:10.1053/j.gastro.2004.01.025
- Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part I: overall and upper gastrointestinal diseases. Gastroenterology 2009; 136(2):376–386. doi:10.1053/j.gastro.2008.12.015
- Cariati A. Gallstone classification in Western countries. Indian J Surg 2015; 77(suppl 2):376–380. doi.org/10.1007/s12262-013-0847-y
- Carey MC. Pathogenesis of gallstones. Am J Surg 1993; 165(4):410–419. doi:10.1016/S0002-9610(05)80932-8
- Lammert F, Gurusamy K, Ko CW, et al. Gallstones. Nat Rev Dis Primers 2016; 2:16024. doi:10.1038/nrdp.2016.24
- Stewart L, Oesterle AL, Erdan I, Griffiss JM, Way LW. Pathogenesis of pigment gallstones in Western societies: the central role of bacteria. J Gastrointest Surg 2002; 6(6):891–904.
- Barbara L, Sama C, Morselli Labate AM, et al. A population study on the prevalence of gallstone disease: the Sirmione Study. Hepatology 1987; 7(5):913–917. doi:10.1002/hep.1840070520
- Sood S, Winn T, Ibrahim S, et al. Natural history of asymptomatic gallstones: differential behaviour in male and female subjects. Med J Malaysia 2015; 70(6):341–345.
- Maringhini A, Ciambra M, Baccelliere P, et al. Biliary sludge and gallstones in pregnancy: incidence, risk factors, and natural history. Ann Intern Med 1993; 119(2):116–120. doi:10.7326/0003-4819-119-2-199307150-00004
- Etminan M, Delaney JA, Bressler B, Brophy JM. Oral contraceptives and the risk of gallbladder disease: a comparative safety study. CMAJ 2011; 183(8):899–904. doi:10.1503/cmaj.110161
- Everhart JE, Khare M, Hill M, Maurer KR. Prevalence and ethnic differences in gallbladder disease in the United States. Gastroenterology 1999; 117(3):632–639.
- Festi D, Sottili S, Colecchia A, et al. Clinical manifestations of gallstone disease: evidence from the multicenter Italian study on cholelithiasis (MICOL). Hepatology 1999; 30(4):839–846. doi:10.1002/hep.510300401
- Berhane T, Vetrhus M, Hausken T, Olafsson S, Sondenaa K. Pain attacks in non-complicated and complicated gallstone disease have a characteristic pattern and are accompanied by dyspepsia in most patients: the results of a prospective study. Scand J Gastroenterol 2006; 41(1):93–101. doi:10.1080/00365520510023990
- Thistle JL, Cleary PA, Lachin JM, Tyor MP, Hersh T. The natural history of cholelithiasis: the National Cooperative Gallstone Study. Ann Intern Med 1984; 101(2):171–175. doi:10.7326/0003-4819-101-2-171
- Friedman GD. Natural history of asymptomatic and symptomatic gallstones. Am J Surg 1993; 165(4):399–404. doi:0.1016/S0002-9610(05)80930-4
- Friedman GD, Raviola CA, Fireman B. Prognosis of gallstones with mild or no symptoms: 25 years of follow-up in a health maintenance organization. J Clin Epidemiol 1989; 42(2):127–136. doi:10.1016/0895-4356(89)90086-3
- Hirota M, Takada T, Kawarada Y, et al. Diagnostic criteria and severity assessment of acute cholecystitis: Tokyo guidelines. J Hepatobiliary Pancreat Surg 2007; 14(1):78–82. doi:10.1007/s00534-006-1159-4
- Miura F, Takada T, Kawarada Y, et al. Flowcharts for the diagnosis and treatment of acute cholangitis and cholecystitis: Tokyo guidelines. J Hepatobiliary Pancreat Surg 2007; 14(1):27–34. doi:10.1007/s00534-006-1153-x
- Koo KP, Traverso LW. Do preoperative indicators predict the presence of common bile duct stones during laparoscopic cholecystectomy? Am J Surg 1996; 171(5):495–499. doi:10.1016/S0002-9610(97)89611-0
- Collins C, Maguire D, Ireland A, Fitzgerald E, O’Sullivan GC. A prospective study of common bile duct calculi in patients undergoing laparoscopic cholecystectomy: natural history of choledocholithiasis revisited. Ann Surg 2004; 239(1):28–33. doi:10.1097/01.sla.0000103069.00170.9c
- Costi R, Gnocchi A, Di Mario F, Sarli L. Diagnosis and management of choledocholithiasis in the golden age of imaging, endoscopy and laparoscopy. World J Gastroenterol 2014; 20(37):13382–13401. doi:10.3748/wjg.v20.i37.13382
- European Association for the Study of the Liver (EASL). EASL Clinical Practice Guidelines on the prevention, diagnosis and treatment of gallstones. J Hepatol 2016; 65(1):146–181. doi:10.1016/j.jhep.2016.03.005
- Greenberg JA, Hsu J, Bawazeer M, et al. Clinical practice guideline: management of acute pancreatitis. Can J Surg 2016; 59 (2):128–140. doi:10.1503/cjs.015015
- Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol 2013; 108(9):1400–1416. doi:10.1038/ajg.2013.218
- Moolla Z, Anderson F, Thomson SR. Use of amylase and alanine transaminase to predict acute gallstone pancreatitis in a population with high HIV prevalence. World J Surg 2013; 37(1):156–161. doi:10.1007/s00268-012-1801-z
- Shea JA, Berlin JA, Escarce JJ, et al. Revised estimates of diagnostic test sensitivity and specificity in suspected biliary tract disease. Arch Intern Med 1994; 154(22):2573–2581. doi:10.1001/archinte.1994.00420220069008
- Kiewiet JJ, Leeuwenburgh MM, Bipat S, et al. A systematic review and meta-analysis of diagnostic performance of imaging in acute cholecystitis. Radiology 2012; 264(3):708–720. doi:10.1148/radiol.12111561
- ASGE Standards of Practice Committee; Maple JT, Ben-Menachem T, Anderson MA, et al. The role of endoscopy in the evaluation of suspected choledocholithiasis. Gastrointest Endosc 2010; 71(1):1–9. doi:10.1016/j.gie.2009.09.041
- Bachar GN, Cohen M, Belenky A, Atar E, Gideon S. Effect of aging on the adult extrahepatic bile duct: a sonographic study. J Ultrasound Med 2003; 22(9):879–885. doi:10.7863/jum.2003.22.9.879
- El-Hayek K, Timratana P, Meranda J, Shimizu H, Eldar S, Chand B. Post Roux-en-Y gastric bypass biliary dilation: natural process or significant entity? J Gastrointest Surg 2012; 16(12):2185–2189. doi:10.1007/s11605-012-2058-4
- Park SM, Kim WS, Bae IH, et al. Common bile duct dilatation after cholecystectomy: a one-year prospective study. J Korean Surg Soc 2012; 83(2):97–101. doi:10.4174/jkss.2012.83.2.97
- Tse F, Liu L, Barkun AN, Armstrong D, Moayyedi P. EUS: a meta-analysis of test performance in suspected choledocholithiasis. Gastrointest Endosc 2008; 67(2):235–244. doi:10.1016/j.gie.2007.09.047
- Verma D, Kapadia A, Eisen GM, Adler DG. EUS vs MRCP for detection of choledocholithiasis. Gastrointest Endosc 2006; 64(2):248–254. doi:10.1016/j.gie.2005.12.038
- Tseng LJ, Jao YT, Mo LR, Lin RC. Over-the-wire US catheter probe as an adjunct to ERCP in the detection of choledocholithiasis. Gastrointest Endosc 2001; 54(6):720–723. doi:10.1067/mge.2001.119255
- Kondo S, Isayama H, Akahane M, et al. Detection of common bile duct stones: comparison between endoscopic ultrasonography, magnetic resonance cholangiography, and helical-computed-tomographic cholangiography. Eur J Radiol 2005; 54(2):271–275. doi:10.1016/j.ejrad.2004.07.007
- Attili AF, De Santis A, Capri R, Repice AM, Maselli S. The natural history of gallstones: the GREPCO experience. The GREPCO Group. Hepatology 1995; 21(3):656–660. doi:10.1016/0270-9139(95)90514-6
- Sakorafas GH, Milingos D, Peros G. Asymptomatic cholelithiasis: is cholecystectomy really needed? A critical reappraisal 15 years after the introduction of laparoscopic cholecystectomy. Dig Dis Sci 2007; 52(5):1313–1325. doi:10.1007/s10620-006-9107-3
- Gracie WA, Ransohoff DF. The natural history of silent gallstones: the innocent gallstone is not a myth. N Engl J Med 1982; 307(13):798–800. doi:10.1056/NEJM198209233071305
- McSherry CK, Ferstenberg H, Calhoun WF, Lahman E, Virshup M. The natural history of diagnosed gallstone disease in symptomatic and asymptomatic patients. Ann Surg 1985; 202(1):59–63. doi:10.1097/00000658-198507000-00009
- Wada K, Wada K, Imamura T. Natural course of asymptomatic gallstone disease. Nihon Rinsho 1993; 51(7):1737–1743. Japanese.
- Halldestam I, Enell EL, Kullman E, Borch K. Development of symptoms and complications in individuals with asymptomatic gallstones. Br J Surg 2004; 91(6):734–738. doi:10.1002/bjs.4547
- Festi D, Reggiani ML, Attili AF, et al. Natural history of gallstone disease: expectant management or active treatment? Results from a population-based cohort study. J Gastroenterol Hepatol 2010; 25(4):719–724. doi:10.1111/j.1440-1746.2009.06146.x
- Shabanzadeh DM, Sorensen LT, Jorgensen T. A prediction rule for risk stratification of incidentally discovered gallstones: results from a large cohort study. Gastroenterology 2016; 150(1):156–167e1. doi:10.1053/j.gastro.2015.09.002
- Overby DW, Apelgren KN, Richardson W, Fanelli R; Society of American Gastrointestinal and Endoscopic Surgeons. SAGES guidelines for the clinical application of laparoscopic biliary tract surgery. Surg Endosc 2010; 24(10):2368–2386. doi:10.1007/s00464-010-1268-7
- Abraham S, Rivero HG, Erlikh IV, Griffith LF, Kondamudi VK. Surgical and nonsurgical management of gallstones. Am Fam Physician 2014; 89(10):795–802.
- Currò G,, Iapichino G, Lorenzini C, Palmeri R, Cucinotta E. Laparoscopic cholecystectomy in children with chronic hemolytic anemia. Is the outcome related to the timing of the procedure? Surg Endosc 2006; 20(2):252–255. doi:10.1007/s00464-005-0318-z
- Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol 2014; 6:99–109. doi:10.2147/CLEP.S37357
- Chen GL, Akmal Y, DiFronzo AL, Vuong B, O’Connor V. Porcelain gallbladder: no longer an indication for prophylactic cholecystectomy. Am Surg 2015; 81(10):936–940.
- Schnelldorfer T. Porcelain gallbladder: a benign process or concern for malignancy? J Gastrointest Surg 2013; 17(6):1161–1168. doi:10.1007/s11605-013-2170-0
- Warschkow R, Tarantino I, Ukegjini K, et al. Concomitant cholecystectomy during laparoscopic Roux-en-Y gastric bypass in obese patients is not justified: a meta-analysis. Obes Surg 2013; 23(3)3979–408. doi:10.1007/s11695-012-0852-4
- Gurusamy K, Samraj K, Gluud C, Wilson E, Davidson BR. Meta-analysis of randomized controlled trials on the safety and effectiveness of early versus delayed laparoscopic cholecystectomy for acute cholecystitis. Br J Surg 2010; 97(2):141–150. doi:10.1002/bjs.6870
- Papi C, Catarci M, D’Ambrosio L, et al. Timing of cholecystectomy for acute calculous cholecystitis: a meta-analysis. Am J Gastroenterol 2004; 99(1):147–155. doi:10.1046/j.1572-0241.2003.04002.x
- Gurusamy KS, Davidson C, Gluud C, Davidson BR. Early versus delayed laparoscopic cholecystectomy for people with acute cholecystitis. Cochrane Database Syst Rev 2013; 6:CD005440. doi:10.1002/14651858
- Menahem B, Mulliri A, Fohlen A, Guittet L, Alves A, Lubrano J. Delayed laparoscopic cholecystectomy increases the total hospital stay compared to an early laparoscopic cholecystectomy after acute cholecystitis: an updated meta-analysis of randomized controlled trials. HPB (Oxford) 2015; 17(10):857–862. doi:10.1111/hpb.12449
- Uhl W, Müller CA, Krähenbühl L, Schmid SW, Schölzel S, Büchler MW. Acute gallstone pancreatitis: timing of laparoscopic cholecystectomy in mild and severe disease. Surg Endosc 1999; 13(11):1070–1076. doi:10.1007/s004649901175
- Aboulian A, Chan T, Yaghoubian A, et al. Early cholecystectomy safely decreases hospital stay in patients with mild gallstone pancreatitis: a randomized prospective study. Ann Surg 2010(4): 251:615–619. doi:10.1097/SLA.0b013e3181c38f1f
KEY POINTS
- Abdominal pain is the primary symptom associated with gallstones.
- Abdominal ultrasonography is the diagnostic test of choice to detect gallstones and assess for findings suggestive of acute cholecystitis and dilation of the common bile duct.
- First-line therapy for asymptomatic gallstones is expectant management.
- First-line therapy for symptomatic gallstones is cholecystectomy.
Total pancreatectomy and islet cell autotransplantation: Definitive treatment for chronic pancreatitis
For some patients with chronic pancreatitis, the best option is to remove the entire pancreas. This does not necessarily doom the patient to diabetes mellitus, because we can harvest the islet cells and reinsert them so that, lodged in the liver, they can continue making insulin. However, this approach is underemphasized in the general medical literature and is likely underutilized in the United States.
Here, we discuss chronic pancreatitis, the indications for and contraindications to this procedure, its outcomes, and the management of patients who undergo it.
CHRONIC PANCREATITIS IS PROGRESSIVE AND PAINFUL
Chronic pancreatitis is a progressive condition characterized by chronic inflammation, irreversible fibrosis, and scarring, resulting in loss of both exocrine and endocrine tissue.
According to a National Institutes of Health database, pancreatitis is the seventh most common digestive disease diagnosis on hospitalization, with annual healthcare costs exceeding $3 billion.1 Although data are scarce, by some estimates the incidence of chronic pancreatitis ranges from 4 to 14 per 100,000 person-years, and the prevalence ranges from 26.4 to 52 per 100,000.2–4 Moreover, a meta-analysis5 found that acute pancreatitis progresses to chronic pancreatitis in 10% of patients who have a first episode of acute pancreatitis and in 36% who have recurrent episodes.
Historically, alcoholism was and still is the most common cause of chronic pancreatitis, contributing to 60% to 90% of cases in Western countries.6,7 However, cases due to nonalcoholic causes have been increasing, and in more than one-fourth of patients, no identifiable cause is found.6,8 Smoking is an independent risk factor.6,8,9 Some cases can be linked to genetic abnormalities, particularly in children.10
The clinical manifestations of chronic pancreatitis include exocrine pancreatic insufficiency (leading to malnutrition and steatorrhea), endocrine insufficiency (causing diabetes mellitus), and intractable pain.11 Pain is the predominant clinical symptom early in the disease and is often debilitating and difficult to manage. Uncontrolled pain has a devastating impact on quality of life and may become complicated by narcotic dependence.
The pain of chronic pancreatitis is often multifactorial, with mechanisms that include increased intraductal pressure from obstruction of the pancreatic duct, pancreatic ischemia, neuronal injury, and neuroimmune interactions between neuronal processes and chronic inflammation.12
Treatment: Medical and surgical
In chronic pancreatitis, the aim of treatment is to alleviate deficiencies of exocrine and endocrine function and mitigate the pain. Patients who smoke or drink alcohol should be strongly encouraged to quit.
Loss of exocrine function is mainly managed with oral pancreatic enzyme supplements, and diabetes control is often attained with insulin therapy.13 Besides helping digestion, pancreatic enzyme therapy in the form of nonenteric tablets may also reduce pain and pancreatitis attacks.14 The mechanism may be by degrading cholecystokinin-releasing factor in the duodenum, lowering cholecystokinin levels and thereby reducing pain.12
Nonnarcotic analgesics are often the first line of therapy for pain management, but many patients need narcotic analgesics. Along with narcotics, adjunctive agents such as tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, selective serotonin reuptake inhibitors, and gabapentinoids have been used to treat chronic pancreatitis pain, but with limited success.15
In patients for whom medical pain management has failed, one can consider another option, such as nerve block, neurolysis, or endoscopic or surgical therapy. Neuromodulators are often prescribed by pain clinics.15 Percutaneous and endoscopic celiac ganglion blocks can be an option but rarely achieve substantial or permanent pain relief, and the induced transient responses (on average 2 to 4 months) often cannot be repeated.14–17
Surgical options to relieve pain try to preserve pancreatic function and vary depending on the degree of severity and nature of pancreatic damage. In broad terms, the surgical procedures can be divided into two types:
- Drainage procedures (eg, pseudocyst drainage; minimally invasive endoscopic duct drainage via sphincterotomy or stent placement, or both; pancreaticojejunostomy)
- Resectional procedures (eg, distal pancreatectomy, isolated head resection, pancreaticoduodenectomy, Whipple procedure, total pancreatectomy).
In carefully selected patients, total pancreatectomy can be offered to remove the cause of the pain.18 This procedure is most often performed in patients who have small-duct disease or a genetic cause or for whom other surgical procedures have failed.11
HISTORY OF THE PROCEDURE
Islet cell transplantation grew out of visionary work by Paul Lacy and David Scharp at the University of Washington at Seattle, whose research focused on isolating and transplanting islet cells in rodent models. The topic has been reviewed by Jahansouz et al.19 In the 1970s, experiments in pancreatectomized dogs showed that transplanting unpurified pancreatic islet tissue that was dispersed by collagenase digestion into the spleen or portal vein could prevent diabetes.20,21 In 1974, the first human trials of transplanting islet cells were conducted, using isolated islets from cadaveric donors to treat diabetes.19
In the past, pancreatectomy was performed to treat painful chronic pancreatitis, but it was viewed as undesirable because removing the gland would inevitably cause insulin-dependent diabetes.22 That changed in 1977 at the University of Minnesota, with the first reported islet cell autotransplant after pancreatectomy. The patient remained pain-free and insulin-independent long-term.23 This seminal case showed that endocrine function could be preserved by autotransplant of islets prepared from the excised pancreas.24
In 1992, Pyzdrowski et al25 reported that intrahepatic transplant of as few as 265,000 islets was enough to prevent the need for insulin therapy. Since this technique was first described, there have been many advances, and now more than 30 centers worldwide do it.
PRIMARY INDICATION: INTRACTABLE PAIN
Interest has been growing in using total pancreatectomy and islet autotransplant to treat recurrent acute pancreatitis, chronic pancreatitis, and hereditary pancreatitis. The rationale is that removing the offending tissue eliminates pancreatitis, pain, and cancer risk, while preserving and replacing the islet cells prevents the development of brittle diabetes with loss of insulin and glucagon.26
Proposed criteria for total pancreatectomy and islet autotransplant
Bellin et al14 proposed five criteria for patient selection for this procedure based on imaging studies, pancreatic function tests, and histopathology to detect pancreatic fibrosis. Patients must fulfill all five of the following criteria:
Criterion 1. Diagnosis of chronic pancreatitis, based on chronic abdominal pain lasting more than 6 months with either at least one of the following:
- Pancreatic calcifications on computed tomography
- At least two of the following: four or more of nine criteria on endoscopic ultrasonography described by Catalano et al,27 a compatible ductal or parenchymal abnormality on secretin magnetic resonance cholangiopancreatography; abnormal endoscopic pancreatic function test (peak HCO2 ≤ 80 mmol/L)
- Histopathologically confirmed diagnosis of chronic pancreatitis
- Compatible clinical history and documented hereditary pancreatitis (PRSS1 gene mutation)
OR
- History of recurrent acute pancreatitis (more than one episode of characteristic pain associated with imaging diagnostic of acute pancreatitis or elevated serum amylase or lipase > 3 times the upper limit of normal).
Criterion 2. At least one of the following:
- Daily narcotic dependence
- Pain resulting in impaired quality of life, which may include inability to attend school, recurrent hospitalizations, or inability to participate in usual age-appropriate activities.
Criterion 3. Complete evaluation with no reversible cause of pancreatitis present or untreated.
Criterion 4. Failure to respond to maximal medical and endoscopic therapy.
Criterion 5. Adequate islet cell function (nondiabetic or C-peptide-positive). Patients with C-peptide-negative diabetes meeting criteria 1 to 4 are candidates for total pancreatectomy alone.
The primary goal is to treat intractable pain and improve quality of life in selected patients with chronic pancreatitis or recurrent acute pancreatitis when endoscopic and prior surgical therapies have failed, and whose impairment due to pain is substantial enough to accept the risk of postoperative insulin-dependent diabetes and lifelong commitment to pancreatic enzyme replacement therapy.15,26 Patients with a known genetic cause of chronic pancreatitis should be offered special consideration for the procedure, as their disease is unlikely to remit.
CONTRAINDICATIONS
Total pancreatectomy and islet autotransplant should not be performed in patients with active alcoholism, illicit drug use, or untreated or poorly controlled psychiatric illnesses that could impair the patient’s ability to adhere to a complicated postoperative medical regimen.
A poor support network may be a relative contraindication in view of the cost and complexity of diabetic and pancreatic enzyme replacement therapy.18,26
Islet cell autotransplant is contraindicated in patients with conditions such as C-peptide-negative or type 1 diabetes or a history of portal vein thrombosis, portal hypertension, significant liver disease, high-risk cardiopulmonary disease, or pancreatic cancer (Table 1).26
WHEN TO CONSIDER REFERRAL FOR THIS PROCEDURE
The choice of total pancreatectomy and islet autotransplant vs conventional surgery must be individualized on the basis of each patient’s anatomy, comorbidities, symptom burden, presence or degree of diabetes, and rate of disease progression. The most important factors to consider in determining the need for and timing of this procedure are the patient’s pain, narcotic requirements, and impaired ability to function.26
Sooner rather than later?
An argument can be made for performing this procedure sooner in the course of the disease rather than later when all else has failed. First, prolonged pain can result in central sensitization, in which the threshold for perceiving pain is lowered by damage to the nociceptive neurons from repeated stimulation and inflammation.28
Further, prolonged opioid therapy can lead to opioid-induced hyperalgesia, which may also render patients more sensitive to pain and aggravate their preexisting pain.26,28
In addition, although operative drainage procedures and partial resections are often considered the gold standard for chronic pancreatitis management, patients who undergo partial pancreatectomy or lateral pancreaticojejunostomy (Puestow procedure) have fewer islet cells left to harvest (about 50% fewer) if they subsequently undergo total pancreatectomy and islet cell autotransplant.22,26
Therefore, performing this procedure earlier may help the patient avoid chronic pain syndromes and complications of chronic opioid use, including hyperalgesia, and give the best chance of harvesting enough islet cells to prevent or minimize diabetes afterward.11
REMOVING THE PANCREAS, RETURNING THE ISLET CELLS
During this procedure, the blood supply to the pancreas must be preserved until just before its removal to minimize warm ischemia of the islet cells.18,29 Although there are several surgical variations, a pylorus-preserving total pancreatectomy with duodenectomy is typically performed, usually with splenectomy to preserve perfusion to the body and tail.30
The resected pancreas is taken to the islet isolation laboratory. There, the pancreatic duct is cannulated to fill the organ with a cold collagenase solution, followed by gentle mechanical dispersion using the semiautomated Ricordi method,31 which separates the islet cells from the exocrine tissue.32
The number of islet cells is quantified as islet equivalents; 1 islet equivalent is equal to the volume of an islet with a diameter of 150 µm. Islet equivalents per kilogram of body weight is the unit commonly used to report the graft amount transplanted.33
After digestion, the islet cells can be purified or partially purified by a gradient separation method using a Cobe 2991 cell processor (Terumo Corporation, Tokyo, Japan),34 or can be transplanted as an unpurified preparation. In islet cell autotransplant for chronic pancreatitis, purification is not always necessary due to the small tissue volume extracted from the often atrophic and fibrotic pancreas.32 The decision to purify depends on the postdigest tissue volume; usually, a tissue volume greater than 0.25 mL/kg body weight is an indication to at least partially purify.18,35
The final preparation is returned to the operating room, and after heparin is given, the islets are infused into the portal system using a stump of the splenic vein, or alternatively through direct puncture of the portal vein or cannulation of the umbilical vein.32,36 If the portal vein pressure reaches 25 cm H2O, the infusion is stopped and the remaining islets can be placed in the peritoneal cavity or elsewhere.18 Transplant of the islets into the liver or peritoneum allows the islets to secrete insulin into the hepatic portal circulation, which is the route used by the native pancreas.22
CONTROLLING GLUCOSE DURING AND AFTER THE PROCEDURE
Animal studies have shown that hyperglycemia impairs islet revascularization,37 and glucose toxicity may cause dysfunction and structural lesions of the transplanted islets.11,38
Therefore, during and after the procedure, most centers maintain euglycemia by an intravenous insulin infusion and subsequently move to subcutaneous insulin when the patient starts eating again. Some centers continue insulin at discharge and gradually taper it over months, even in patients who can possibly achieve euglycemia without it.
OUTCOMES
Many institutions have reported their clinical outcomes in terms of pain relief, islet function, glycemic control, and improvement of quality of life. The largest series have been from the University of Minnesota, Leicester General Hospital, the University of Cincinnati, and the Medical University of South Carolina.
Insulin independence is common but wanes with time
The ability to achieve insulin independence after islet autotransplant appears to be related to the number of islets transplanted, with the best results when more than 2,000 or 3,000 islet equivalents/kg are transplanted.39,40
Sutherland et al18 reported that of 409 patients who underwent islet cell autotransplant at the University of Minnesota (the largest series reported to date), 30% were insulin-independent at 3 years, 33% had partial graft function (defined by positive C-peptide), and 82% achieved a mean hemoglobin A1c of less than 7%. However, in the subset who received more than 5,000 islet equivalents/kg, nearly three-fourths of patients were insulin-independent at 3 years.
The Leicester General Hospital group presented long-term data on 46 patients who underwent total pancreatectomy and islet cell autotransplant. Twelve of the 46 had shown periods of insulin independence for a median of 16.5 months, and 5 remained insulin-free at the time of the publication.41 Over the 10 years of follow-up, insulin requirements and hemoglobin A1c increased notably. However, all of the patients tested C-peptide-positive, suggesting long-lasting graft function.
Most recently, the University of Cincinnati group reported long-term data on 116 patients. The insulin independence rate was 38% at 1 year, decreasing to 27% at 5 years. The number of patients with partial graft function was 38% at 1 year and 35% at 5 years.42
Thus, all three institutions confirmed that the autotransplanted islets continue to secrete insulin long-term, but that function decreases over time.
Pancreatectomy reduces pain
Multiple studies have shown that total pancreatectomy reduces pain in patients with chronic pancreatitis. Ahmad et al43 reported a marked reduction in narcotic use (mean morphine equivalents 206 mg/day before surgery, compared with 90 mg after), and a 58% reduction in pain as demonstrated by narcotic independence.
In the University of Minnesota series, 85% of the 409 patients had less pain at 2 years, and 59% were able to stop taking narcotics.18
The University of Cincinnati group reported a narcotic independence rate of 55% at 1 year, which continued to improve to 73% at 5 years.42
Although the source of pain is removed, pain persists or recurs in 10% to 20% of patients after total pancreatectomy and islet cell autotransplant, showing that the pathogenesis of pain is complex, and some uncertainty exists about it.26
Quality of life
Reports evaluating health-related quality of life after total pancreatectomy and islet autotransplant are limited.
The University of Cincinnati group reported the long-term outcomes of quality of life as measured by the Short Form 36 Health Survey.42 Ninety-two percent of patients reported overall improvement in their health at 1 year, and 85% continued to report improved health more than 5 years after the surgery.
In a series of 20 patients, 79% to 90% reported improvements in the seven various domains of the Pain Disability Index. In addition, 60% showed improvement in depression and 70% showed improvement in anxiety. The greatest improvements were in those who had not undergone prior pancreatic surgery, who were younger, and in those with higher levels of preoperative pain.30
Similarly, in a series of 74 patients, the Medical University of South Carolina group reported significant improvement in physical and mental health components of the Short Form 12 Health Survey and an associated decrease in daily narcotic requirements. Moreover, the need to start or increase the dose of insulin after the surgery was not associated with a lower quality of life.44
OFF-SITE ISLET CELL ISOLATION
Despite the positive outcomes in terms of pain relief and insulin independence in many patients after total pancreatectomy and islet cell autotransplant, few medical centers have an on-site islet-processing facility. Since the mid-1990s, a few centers have been able to circumvent this limitation by working with off-site islet cell isolation laboratories.45,46
The University of California, Los Angeles, first reported on a series of nine patients who received autologous islet cells after near-total or total pancreatectomy using a remote islet cell isolation facility, with results comparable to those of other large institutions.45
Similarly, the procedure has been performed at Cleveland Clinic since 2007 with the collaboration of an off-site islet cell isolation laboratory at the University of Pittsburgh. A cohort study from this collaboration published in 2015 showed that in 36 patients (mean follow-up 28 months, range 3–26 months), 33% were insulin-independent, with a C-peptide-positive rate of 70%. This is the largest cohort to date from a center utilizing an off-site islet isolation facility.47
In view of the positive outcomes at these centers, lack of a local islet-processing facility should no longer be a barrier to total pancreatectomy and islet cell autotransplant.
PATIENT CARE AFTER THE PROCEDURE
A multidisciplinary team is an essential component of the postoperative management of patients who undergo total pancreatectomy and islet cell autotransplant.
For patients who had been receiving narcotics for a long time before surgery or who were receiving frequent doses, an experienced pain management physician should be involved in the patient’s postoperative care.
Because islet function can wane over time, testing for diabetes should be done at least annually for the rest of the patient’s life and should include fasting plasma glucose, hemoglobin A1c, and C-peptide, along with self-monitored blood glucose.26
All patients who have surgically induced exocrine insufficiency are at risk of malabsorption and fat-soluble vitamin deficiencies.48 Hence, lifelong pancreatic enzyme replacement therapy is mandatory. Nutritional monitoring should include assessment of steatorrhea, body composition, and fat-soluble vitamin levels (vitamins A, D, and E) at least every year.26 Patients with chronic pancreatitis are at increased risk for low bone density from malabsorption of vitamin D and calcium; therefore, it is recommended that a dual-energy x-ray absorptiometry bone density scan be obtained.26,49
Patients who undergo splenectomy as part of their procedure will require appropriate precautions and ongoing vaccinations as recommended by the US Centers for Disease Control and Prevention.26,50,51
WHAT TO EXPECT FOR THE FUTURE
The National Institute of Diabetes and Digestive and Kidney Diseases has reviewed the potential future research directions for total pancreatectomy and islet autotransplant.15
Patient selection remains challenging despite the availability of criteria15 and guidelines.26 More research is needed to better assess preoperative beta-cell function and to predict postoperative outcomes. Mixed meal-tolerance testing is adopted by most clinical centers to predict posttransplant beta-cell function. The use of arginine instead of glucagon in a stimulation test for insulin and C-peptide response has been validated and may allow more accurate assessment.52,53
Another targeted area of research is the advancement of safety and metabolic outcomes. Techniques to minimize warm ischemic time and complications are being evaluated. Islet isolation methods that yield more islets, reduce beta-cell apoptosis, and can isolate islets from glands with malignancy should be further investigated.54 Further, enhanced islet infusion methods that achieve lower portal venous pressures and minimize portal vein thrombosis are needed.
Unfortunately, the function of transplanted islet grafts declines over time. This phenomenon is at least partially attributed to the immediate blood-mediated inflammatory response,55,56 along with islet hypoxia,57 leading to islet apoptosis. Research on different strategies is expanding our knowledge in islet engraftment and posttransplant beta-cell apoptosis, with the expectation that the transplanted islet lifespan will increase. Alternative transplant sites with low inflammatory reaction, such as the omental pouch,58 muscle,59 and bone marrow,60 have shown encouraging data. Other approaches, such as adjuvant anti-inflammatory agents and heparinization, have been proposed.15
Research into complications is also of clinical importance. There is growing attention to hypoglycemia unrelated to exogenous insulin use in posttransplant patients. One hypothesis is that glucagon secretion, a counterregulatory response to hypoglycemia, is defective if the islet cells are transplanted into the liver, and that implanting them into another site may avoid this effect.61
- Everhart JE. Pancreatitis. In: Everhart JE, editor. The Burden of Digestive Diseases in the United States. US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of
- Diabetes and Digestive and Kidney Diseases. Washington, DC: US Government Printing Office; 2008. www.niddk.nih.gov/about-niddk/strategic-plans-reports/Pages/burden-digestive-diseases-in-united-states-report.aspx. Accessed May 10, 2016.
- Yadav D, Timmons L, Benson JT, Dierkhising RA, Chari ST. Incidence, prevalence, and survival of chronic pancreatitis: a population-based study. Am J Gastroenterol 2011; 106:2192–2199.
- Lévy P, Barthet M, Mollard BR, Amouretti M, Marion-Audibert AM, Dyard F. Estimation of the prevalence and incidence of chronic pancreatitis and its complications. Gastroenterol Clin Biol 2006; 30:838–844.
- Hirota M, Shimosegawa T, Masamune A, et al; Research Committee of Intractable Pancreatic Diseases. The seventh nationwide epidemiological survey for chronic pancreatitis in Japan: clinical significance of smoking habit in Japanese patients. Pancreatology 2014; 14:490–496.
- Sankaran SJ, Xiao AY, Wu LM, Windsor JA, Forsmark CE, Petrov MS. Frequency of progression from acute to chronic pancreatitis and risk factors: a meta-analysis. Gastroenterology 2015; 149:1490–1500.e1.
- Coté GA, Yadav D, Slivka A, et al; North American Pancreatitis Study Group. Alcohol and smoking as risk factors in an epidemiology study of patients with chronic pancreatitis. Clin Gastroenterol Hepatol 2011; 9:266–273.
- Muniraj T, Aslanian HR, Farrell J, Jamidar PA. Chronic pancreatitis, a comprehensive review and update. Part I: epidemiology, etiology, risk factors, genetics, pathophysiology, and clinical features. Dis Mon 2014; 60:530–550.
- Frulloni L, Gabbrielli A, Pezzilli R, et al; PanCroInfAISP Study Group. Chronic pancreatitis: report from a multicenter Italian survey (PanCroInfAISP) on 893 patients. Dig Liver Dis 2009; 41:311–317.
- Talamini G, Bassi C, Falconi M, et al. Alcohol and smoking as risk factors in chronic pancreatitis and pancreatic cancer. Dig Dis Sci 1999; 44:1303–1311.
- Schwarzenberg SJ, Bellin M, Husain SZ, et al. Pediatric chronic pancreatitis is associated with genetic risk factors and substantial disease burden. J Pediatr 2015; 166:890–896.e1.
- Blondet JJ, Carlson AM, Kobayashi T, et al. The role of total pancreatectomy and islet autotransplantation for chronic pancreatitis. Surg Clin North Am 2007; 87:1477–1501.
- Lieb JG 2nd, Forsmark CE. Review article: pain and chronic pancreatitis. Aliment Pharmacol Ther 2009; 29:706–719.
- Lin YK, Johnston PC, Arce K, Hatipoglu BA. Chronic pancreatitis and diabetes mellitus. Curr Treat Options Gastroenterol 2015; 13:319–331.
- Bellin MD, Gelrud A, Arreaza-Rubin G, et al. Total pancreatectomy with islet autotransplantation: summary of a National Institute of Diabetes and Digestive and Kidney diseases workshop. Pancreas 2014; 43:1163–1171.
- Muniraj T, Aslanian HR, Farrell J, Jamidar PA. Chronic pancreatitis, a comprehensive review and update. Part II: diagnosis, complications, and management. Dis Mon 2015; 61:5–37.
- Warshaw AL, Banks PA, Fernández-Del Castillo C. AGA technical review: treatment of pain in chronic pancreatitis. Gastroenterology 1998; 115:765–776.
- Chauhan S, Forsmark CE. Pain management in chronic pancreatitis: a treatment algorithm. Best Pract Res Clin Gastroenterol 2010; 24:323–335.
- Sutherland DE, Radosevich DM, Bellin MD, et al. Total pancreatectomy and islet autotransplantation for chronic pancreatitis. J Am Coll Surg 2012; 214:409–426.
- Jahansouz C, Jahansouz C, Kumer SC, Brayman KL. Evolution of beta-cell replacement therapy in diabetes mellitus: islet cell transplantation. J Transplant 2011; 2011:247959.
- Kretschmer GJ, Sutherland DE, Matas AJ, Cain TL, Najarian JS. Autotransplantation of pancreatic islets without separation of exocrine and endocrine tissue in totally pancreatectomized dogs. Surgery 1977; 82:74–81.
- Kretschmer GJ, Sutherland DR, Matas AJ, Payne WD, Najarian JS. Autotransplantation of pancreatic fragments to the portal vein and spleen of totally pancreatectomized dogs: a comparative evaluation. Ann Surg 1978; 187:79–86.
- Bellin MD, Sutherland DE, Robertson RP. Pancreatectomy and autologous islet transplantation for painful chronic pancreatitis: indications and outcomes. Hosp Pract (1995) 2012; 40:80–87.
- Najarian JS, Sutherland DE, Baumgartner D, et al. Total or near total pancreatectomy and islet autotransplantation for treatment of chronic pancreatitis. Ann Surg 1980; 192:526–542.
- Sutherland DE, Matas AJ, Najarian JS. Pancreatic islet cell transplantation. Surg Clin North Am 1978; 58:365–382.
- Pyzdrowski KL, Kendall DM, Halter JB, Nakhleh RE, Sutherland DE, Robertson RP. Preserved insulin secretion and insulin independence in recipients of islet autografts. N Engl J Med 1992; 327:220–226.
- Bellin MD, Freeman ML, Gelrud A, et al. Total pancreatectomy and islet autotransplantation in chronic pancreatitis: recommendations from PancreasFest. Pancreatology 2014; 14:27–35.
- Catalano MF, Sahai A, Levy M, et al. EUS-based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointest Endosc 2009; 69:1251–1261.
- Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology 2006; 104:570–587.
- Bramis K, Gordon-Weeks AN, Friend PJ, et al. Systematic review of total pancreatectomy and islet autotransplantation for chronic pancreatitis. Br J Surg 2012; 99:761–766.
- Walsh RM, Saavedra JR, Lentz G, et al. Improved quality of life following total pancreatectomy and auto-islet transplantation for chronic pancreatitis. J Gastrointest Surg 2012; 16:1469–1477.
- Ricordi C, Lacy PE, Scharp DW. Automated islet isolation from human pancreas. Diabetes 1989; 38(suppl 1):140–142.
- Witkowski P, Savari O, Matthews JB. Islet autotransplantation and total pancreatectomy. Adv Surg 2014; 48:223–233.
- Bellin MD, Beilman GJ, Dunn TB, et al. Islet autotransplantation to preserve beta cell mass in selected patients with chronic pancreatitis and diabetes mellitus undergoing total pancreatectomy. Pancreas 2013; 42:317–321.
- Anazawa T, Matsumoto S, Yonekawa Y, et al. Prediction of pancreatic tissue densities by an analytical test gradient system before purification maximizes human islet recovery for islet autotransplantation/allotransplantation. Transplantation 2011; 91:508–514.
- Lake SP, Bassett PD, Larkins A, et al. Large-scale purification of human islets utilizing discontinuous albumin gradient on IBM 2991 cell separator. Diabetes 1989; 38(suppl 1):143–145.
- Bellin MD, Freeman ML, Schwarzenberg SJ, et al. Quality of life improves for pediatric patients after total pancreatectomy and islet autotransplant for chronic pancreatitis. Clin Gastroenterol Hepatol 2011; 9:793–799.
- Andersson A, Korsgren O, Jansson L. Intraportally transplanted pancreatic islets revascularized from hepatic arterial system. Diabetes 1989; 38(suppl 1):192–195.
- Leahy JL, Bonner-Weir S, Weir GC. Beta-cell dysfunction induced by chronic hyperglycemia. Current ideas on mechanism of impaired glucose-induced insulin secretion. Diabetes Care 1992; 15:442–455.
- Bellin MD, Carlson AM, Kobayashi T, et al. Outcome after pancreatectomy and islet autotransplantation in a pediatric population. J Pediatr Gastroenterol Nutr 2008; 47:37–44.
- White SA, Davies JE, Pollard C, et al. Pancreas resection and islet autotransplantation for end-stage chronic pancreatitis. Ann Surg 2001; 233:423–431.
- Webb MA, Illouz SC, Pollard CA, et al. Islet auto transplantation following total pancreatectomy: a long-term assessment of graft function. Pancreas 2008; 37:282–287.
- Wilson GC, Sutton JM, Abbott DE, et al. Long-term outcomes after total pancreatectomy and islet cell autotransplantation: is it a durable operation? Ann Surg 2014; 260:659–667.
- Ahmad SA, Lowy AM, Wray CJ, et al. Factors associated with insulin and narcotic independence after islet autotransplantation in patients with severe chronic pancreatitis. J Am Coll Surg 2005; 201:680–687.
- Dorlon M, Owczarski S, Wang H, Adams D, Morgan K. Increase in postoperative insulin requirements does not lead to decreased quality of life after total pancreatectomy with islet cell autotransplantation for chronic pancreatitis. Am Surg 2013; 79:676–680.
- Tai DS, Shen N, Szot GL, et al. Autologous islet transplantation with remote islet isolation after pancreas resection for chronic pancreatitis. JAMA Surg 2015; 150:118–124.
- Rabkin JM, Olyaei AJ, Orloff SL, et al. Distant processing of pancreas islets for autotransplantation following total pancreatectomy. Am J Surg 1999; 177:423–427.
- Johnston PC, Lin YK, Walsh RM, et al. Factors associated with islet yield and insulin independence after total pancreatectomy and islet cell autotransplantation in patients with chronic pancreatitis utilizing off-site islet isolation: Cleveland Clinic experience. J Clin Endocrinol Metab 2015; 100:1765–1770.
- Dresler CM, Fortner JG, McDermott K, Bajorunas DR. Metabolic consequences of (regional) total pancreatectomy. Ann Surg 1991; 214:131–140.
- Duggan SN, O’Sullivan M, Hamilton S, Feehan SM, Ridgway PF, Conlon KC. Patients with chronic pancreatitis are at increased risk for osteoporosis. Pancreas 2012; 41:1119–1124.
- Rubin LG, Levin MJ, Ljungman P, et al; Infectious Diseases Society of America. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014; 58:e44–e100.
- Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet 2011; 378:86–97.
- Robertson RP, Raymond RH, Lee DS, et al; Beta Cell Project Team of the Foundation for the NIH Biomarkers Consortium. Arginine is preferred to glucagon for stimulation testing of beta-cell function. Am J Physiol Endocrinol Metab 2014; 307:E720–E727.
- Robertson RP, Bogachus LD, Oseid E, et al. Assessment of beta-cell mass and alpha- and beta-cell survival and function by arginine stimulation in human autologous islet recipients. Diabetes 2015; 64:565–572.
- Balzano G, Piemonti L. Autologous islet transplantation in patients requiring pancreatectomy for neoplasm. Curr Diab Rep 2014; 14:512.
- Naziruddin B, Iwahashi S, Kanak MA, Takita M, Itoh T, Levy MF. Evidence for instant blood-mediated inflammatory reaction in clinical autologous islet transplantation. Am J Transplant 2014; 14:428–437.
- Abdelli S, Ansite J, Roduit R, et al. Intracellular stress signaling pathways activated during human islet preparation and following acute cytokine exposure. Diabetes 2004; 53:2815–2823.
- Olsson R, Olerud J, Pettersson U, Carlsson PO. Increased numbers of low-oxygenated pancreatic islets after intraportal islet transplantation. Diabetes 2011; 60:2350–2353.
- Berman DM, O’Neil JJ, Coffey LC, et al. Long-term survival of nonhuman primate islets implanted in an omental pouch on a biodegradable scaffold. Am J Transplant 2009; 9:91–104.
- Sterkers A, Hubert T, Gmyr V, et al. Islet survival and function following intramuscular autotransplantation in the minipig. Am J Transplant 2013; 13:891–898.
- Maffi P, Balzano G, Ponzoni M, et al. Autologous pancreatic islet transplantation in human bone marrow. Diabetes 2013; 62:3523–3531.
- Bellin MD, Parazzoli S, Oseid E, et al. Defective glucagon secretion during hypoglycemia after intrahepatic but not nonhepatic islet autotransplantation. Am J Transplant 2014; 14:1880–1886.
For some patients with chronic pancreatitis, the best option is to remove the entire pancreas. This does not necessarily doom the patient to diabetes mellitus, because we can harvest the islet cells and reinsert them so that, lodged in the liver, they can continue making insulin. However, this approach is underemphasized in the general medical literature and is likely underutilized in the United States.
Here, we discuss chronic pancreatitis, the indications for and contraindications to this procedure, its outcomes, and the management of patients who undergo it.
CHRONIC PANCREATITIS IS PROGRESSIVE AND PAINFUL
Chronic pancreatitis is a progressive condition characterized by chronic inflammation, irreversible fibrosis, and scarring, resulting in loss of both exocrine and endocrine tissue.
According to a National Institutes of Health database, pancreatitis is the seventh most common digestive disease diagnosis on hospitalization, with annual healthcare costs exceeding $3 billion.1 Although data are scarce, by some estimates the incidence of chronic pancreatitis ranges from 4 to 14 per 100,000 person-years, and the prevalence ranges from 26.4 to 52 per 100,000.2–4 Moreover, a meta-analysis5 found that acute pancreatitis progresses to chronic pancreatitis in 10% of patients who have a first episode of acute pancreatitis and in 36% who have recurrent episodes.
Historically, alcoholism was and still is the most common cause of chronic pancreatitis, contributing to 60% to 90% of cases in Western countries.6,7 However, cases due to nonalcoholic causes have been increasing, and in more than one-fourth of patients, no identifiable cause is found.6,8 Smoking is an independent risk factor.6,8,9 Some cases can be linked to genetic abnormalities, particularly in children.10
The clinical manifestations of chronic pancreatitis include exocrine pancreatic insufficiency (leading to malnutrition and steatorrhea), endocrine insufficiency (causing diabetes mellitus), and intractable pain.11 Pain is the predominant clinical symptom early in the disease and is often debilitating and difficult to manage. Uncontrolled pain has a devastating impact on quality of life and may become complicated by narcotic dependence.
The pain of chronic pancreatitis is often multifactorial, with mechanisms that include increased intraductal pressure from obstruction of the pancreatic duct, pancreatic ischemia, neuronal injury, and neuroimmune interactions between neuronal processes and chronic inflammation.12
Treatment: Medical and surgical
In chronic pancreatitis, the aim of treatment is to alleviate deficiencies of exocrine and endocrine function and mitigate the pain. Patients who smoke or drink alcohol should be strongly encouraged to quit.
Loss of exocrine function is mainly managed with oral pancreatic enzyme supplements, and diabetes control is often attained with insulin therapy.13 Besides helping digestion, pancreatic enzyme therapy in the form of nonenteric tablets may also reduce pain and pancreatitis attacks.14 The mechanism may be by degrading cholecystokinin-releasing factor in the duodenum, lowering cholecystokinin levels and thereby reducing pain.12
Nonnarcotic analgesics are often the first line of therapy for pain management, but many patients need narcotic analgesics. Along with narcotics, adjunctive agents such as tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, selective serotonin reuptake inhibitors, and gabapentinoids have been used to treat chronic pancreatitis pain, but with limited success.15
In patients for whom medical pain management has failed, one can consider another option, such as nerve block, neurolysis, or endoscopic or surgical therapy. Neuromodulators are often prescribed by pain clinics.15 Percutaneous and endoscopic celiac ganglion blocks can be an option but rarely achieve substantial or permanent pain relief, and the induced transient responses (on average 2 to 4 months) often cannot be repeated.14–17
Surgical options to relieve pain try to preserve pancreatic function and vary depending on the degree of severity and nature of pancreatic damage. In broad terms, the surgical procedures can be divided into two types:
- Drainage procedures (eg, pseudocyst drainage; minimally invasive endoscopic duct drainage via sphincterotomy or stent placement, or both; pancreaticojejunostomy)
- Resectional procedures (eg, distal pancreatectomy, isolated head resection, pancreaticoduodenectomy, Whipple procedure, total pancreatectomy).
In carefully selected patients, total pancreatectomy can be offered to remove the cause of the pain.18 This procedure is most often performed in patients who have small-duct disease or a genetic cause or for whom other surgical procedures have failed.11
HISTORY OF THE PROCEDURE
Islet cell transplantation grew out of visionary work by Paul Lacy and David Scharp at the University of Washington at Seattle, whose research focused on isolating and transplanting islet cells in rodent models. The topic has been reviewed by Jahansouz et al.19 In the 1970s, experiments in pancreatectomized dogs showed that transplanting unpurified pancreatic islet tissue that was dispersed by collagenase digestion into the spleen or portal vein could prevent diabetes.20,21 In 1974, the first human trials of transplanting islet cells were conducted, using isolated islets from cadaveric donors to treat diabetes.19
In the past, pancreatectomy was performed to treat painful chronic pancreatitis, but it was viewed as undesirable because removing the gland would inevitably cause insulin-dependent diabetes.22 That changed in 1977 at the University of Minnesota, with the first reported islet cell autotransplant after pancreatectomy. The patient remained pain-free and insulin-independent long-term.23 This seminal case showed that endocrine function could be preserved by autotransplant of islets prepared from the excised pancreas.24
In 1992, Pyzdrowski et al25 reported that intrahepatic transplant of as few as 265,000 islets was enough to prevent the need for insulin therapy. Since this technique was first described, there have been many advances, and now more than 30 centers worldwide do it.
PRIMARY INDICATION: INTRACTABLE PAIN
Interest has been growing in using total pancreatectomy and islet autotransplant to treat recurrent acute pancreatitis, chronic pancreatitis, and hereditary pancreatitis. The rationale is that removing the offending tissue eliminates pancreatitis, pain, and cancer risk, while preserving and replacing the islet cells prevents the development of brittle diabetes with loss of insulin and glucagon.26
Proposed criteria for total pancreatectomy and islet autotransplant
Bellin et al14 proposed five criteria for patient selection for this procedure based on imaging studies, pancreatic function tests, and histopathology to detect pancreatic fibrosis. Patients must fulfill all five of the following criteria:
Criterion 1. Diagnosis of chronic pancreatitis, based on chronic abdominal pain lasting more than 6 months with either at least one of the following:
- Pancreatic calcifications on computed tomography
- At least two of the following: four or more of nine criteria on endoscopic ultrasonography described by Catalano et al,27 a compatible ductal or parenchymal abnormality on secretin magnetic resonance cholangiopancreatography; abnormal endoscopic pancreatic function test (peak HCO2 ≤ 80 mmol/L)
- Histopathologically confirmed diagnosis of chronic pancreatitis
- Compatible clinical history and documented hereditary pancreatitis (PRSS1 gene mutation)
OR
- History of recurrent acute pancreatitis (more than one episode of characteristic pain associated with imaging diagnostic of acute pancreatitis or elevated serum amylase or lipase > 3 times the upper limit of normal).
Criterion 2. At least one of the following:
- Daily narcotic dependence
- Pain resulting in impaired quality of life, which may include inability to attend school, recurrent hospitalizations, or inability to participate in usual age-appropriate activities.
Criterion 3. Complete evaluation with no reversible cause of pancreatitis present or untreated.
Criterion 4. Failure to respond to maximal medical and endoscopic therapy.
Criterion 5. Adequate islet cell function (nondiabetic or C-peptide-positive). Patients with C-peptide-negative diabetes meeting criteria 1 to 4 are candidates for total pancreatectomy alone.
The primary goal is to treat intractable pain and improve quality of life in selected patients with chronic pancreatitis or recurrent acute pancreatitis when endoscopic and prior surgical therapies have failed, and whose impairment due to pain is substantial enough to accept the risk of postoperative insulin-dependent diabetes and lifelong commitment to pancreatic enzyme replacement therapy.15,26 Patients with a known genetic cause of chronic pancreatitis should be offered special consideration for the procedure, as their disease is unlikely to remit.
CONTRAINDICATIONS
Total pancreatectomy and islet autotransplant should not be performed in patients with active alcoholism, illicit drug use, or untreated or poorly controlled psychiatric illnesses that could impair the patient’s ability to adhere to a complicated postoperative medical regimen.
A poor support network may be a relative contraindication in view of the cost and complexity of diabetic and pancreatic enzyme replacement therapy.18,26
Islet cell autotransplant is contraindicated in patients with conditions such as C-peptide-negative or type 1 diabetes or a history of portal vein thrombosis, portal hypertension, significant liver disease, high-risk cardiopulmonary disease, or pancreatic cancer (Table 1).26
WHEN TO CONSIDER REFERRAL FOR THIS PROCEDURE
The choice of total pancreatectomy and islet autotransplant vs conventional surgery must be individualized on the basis of each patient’s anatomy, comorbidities, symptom burden, presence or degree of diabetes, and rate of disease progression. The most important factors to consider in determining the need for and timing of this procedure are the patient’s pain, narcotic requirements, and impaired ability to function.26
Sooner rather than later?
An argument can be made for performing this procedure sooner in the course of the disease rather than later when all else has failed. First, prolonged pain can result in central sensitization, in which the threshold for perceiving pain is lowered by damage to the nociceptive neurons from repeated stimulation and inflammation.28
Further, prolonged opioid therapy can lead to opioid-induced hyperalgesia, which may also render patients more sensitive to pain and aggravate their preexisting pain.26,28
In addition, although operative drainage procedures and partial resections are often considered the gold standard for chronic pancreatitis management, patients who undergo partial pancreatectomy or lateral pancreaticojejunostomy (Puestow procedure) have fewer islet cells left to harvest (about 50% fewer) if they subsequently undergo total pancreatectomy and islet cell autotransplant.22,26
Therefore, performing this procedure earlier may help the patient avoid chronic pain syndromes and complications of chronic opioid use, including hyperalgesia, and give the best chance of harvesting enough islet cells to prevent or minimize diabetes afterward.11
REMOVING THE PANCREAS, RETURNING THE ISLET CELLS
During this procedure, the blood supply to the pancreas must be preserved until just before its removal to minimize warm ischemia of the islet cells.18,29 Although there are several surgical variations, a pylorus-preserving total pancreatectomy with duodenectomy is typically performed, usually with splenectomy to preserve perfusion to the body and tail.30
The resected pancreas is taken to the islet isolation laboratory. There, the pancreatic duct is cannulated to fill the organ with a cold collagenase solution, followed by gentle mechanical dispersion using the semiautomated Ricordi method,31 which separates the islet cells from the exocrine tissue.32
The number of islet cells is quantified as islet equivalents; 1 islet equivalent is equal to the volume of an islet with a diameter of 150 µm. Islet equivalents per kilogram of body weight is the unit commonly used to report the graft amount transplanted.33
After digestion, the islet cells can be purified or partially purified by a gradient separation method using a Cobe 2991 cell processor (Terumo Corporation, Tokyo, Japan),34 or can be transplanted as an unpurified preparation. In islet cell autotransplant for chronic pancreatitis, purification is not always necessary due to the small tissue volume extracted from the often atrophic and fibrotic pancreas.32 The decision to purify depends on the postdigest tissue volume; usually, a tissue volume greater than 0.25 mL/kg body weight is an indication to at least partially purify.18,35
The final preparation is returned to the operating room, and after heparin is given, the islets are infused into the portal system using a stump of the splenic vein, or alternatively through direct puncture of the portal vein or cannulation of the umbilical vein.32,36 If the portal vein pressure reaches 25 cm H2O, the infusion is stopped and the remaining islets can be placed in the peritoneal cavity or elsewhere.18 Transplant of the islets into the liver or peritoneum allows the islets to secrete insulin into the hepatic portal circulation, which is the route used by the native pancreas.22
CONTROLLING GLUCOSE DURING AND AFTER THE PROCEDURE
Animal studies have shown that hyperglycemia impairs islet revascularization,37 and glucose toxicity may cause dysfunction and structural lesions of the transplanted islets.11,38
Therefore, during and after the procedure, most centers maintain euglycemia by an intravenous insulin infusion and subsequently move to subcutaneous insulin when the patient starts eating again. Some centers continue insulin at discharge and gradually taper it over months, even in patients who can possibly achieve euglycemia without it.
OUTCOMES
Many institutions have reported their clinical outcomes in terms of pain relief, islet function, glycemic control, and improvement of quality of life. The largest series have been from the University of Minnesota, Leicester General Hospital, the University of Cincinnati, and the Medical University of South Carolina.
Insulin independence is common but wanes with time
The ability to achieve insulin independence after islet autotransplant appears to be related to the number of islets transplanted, with the best results when more than 2,000 or 3,000 islet equivalents/kg are transplanted.39,40
Sutherland et al18 reported that of 409 patients who underwent islet cell autotransplant at the University of Minnesota (the largest series reported to date), 30% were insulin-independent at 3 years, 33% had partial graft function (defined by positive C-peptide), and 82% achieved a mean hemoglobin A1c of less than 7%. However, in the subset who received more than 5,000 islet equivalents/kg, nearly three-fourths of patients were insulin-independent at 3 years.
The Leicester General Hospital group presented long-term data on 46 patients who underwent total pancreatectomy and islet cell autotransplant. Twelve of the 46 had shown periods of insulin independence for a median of 16.5 months, and 5 remained insulin-free at the time of the publication.41 Over the 10 years of follow-up, insulin requirements and hemoglobin A1c increased notably. However, all of the patients tested C-peptide-positive, suggesting long-lasting graft function.
Most recently, the University of Cincinnati group reported long-term data on 116 patients. The insulin independence rate was 38% at 1 year, decreasing to 27% at 5 years. The number of patients with partial graft function was 38% at 1 year and 35% at 5 years.42
Thus, all three institutions confirmed that the autotransplanted islets continue to secrete insulin long-term, but that function decreases over time.
Pancreatectomy reduces pain
Multiple studies have shown that total pancreatectomy reduces pain in patients with chronic pancreatitis. Ahmad et al43 reported a marked reduction in narcotic use (mean morphine equivalents 206 mg/day before surgery, compared with 90 mg after), and a 58% reduction in pain as demonstrated by narcotic independence.
In the University of Minnesota series, 85% of the 409 patients had less pain at 2 years, and 59% were able to stop taking narcotics.18
The University of Cincinnati group reported a narcotic independence rate of 55% at 1 year, which continued to improve to 73% at 5 years.42
Although the source of pain is removed, pain persists or recurs in 10% to 20% of patients after total pancreatectomy and islet cell autotransplant, showing that the pathogenesis of pain is complex, and some uncertainty exists about it.26
Quality of life
Reports evaluating health-related quality of life after total pancreatectomy and islet autotransplant are limited.
The University of Cincinnati group reported the long-term outcomes of quality of life as measured by the Short Form 36 Health Survey.42 Ninety-two percent of patients reported overall improvement in their health at 1 year, and 85% continued to report improved health more than 5 years after the surgery.
In a series of 20 patients, 79% to 90% reported improvements in the seven various domains of the Pain Disability Index. In addition, 60% showed improvement in depression and 70% showed improvement in anxiety. The greatest improvements were in those who had not undergone prior pancreatic surgery, who were younger, and in those with higher levels of preoperative pain.30
Similarly, in a series of 74 patients, the Medical University of South Carolina group reported significant improvement in physical and mental health components of the Short Form 12 Health Survey and an associated decrease in daily narcotic requirements. Moreover, the need to start or increase the dose of insulin after the surgery was not associated with a lower quality of life.44
OFF-SITE ISLET CELL ISOLATION
Despite the positive outcomes in terms of pain relief and insulin independence in many patients after total pancreatectomy and islet cell autotransplant, few medical centers have an on-site islet-processing facility. Since the mid-1990s, a few centers have been able to circumvent this limitation by working with off-site islet cell isolation laboratories.45,46
The University of California, Los Angeles, first reported on a series of nine patients who received autologous islet cells after near-total or total pancreatectomy using a remote islet cell isolation facility, with results comparable to those of other large institutions.45
Similarly, the procedure has been performed at Cleveland Clinic since 2007 with the collaboration of an off-site islet cell isolation laboratory at the University of Pittsburgh. A cohort study from this collaboration published in 2015 showed that in 36 patients (mean follow-up 28 months, range 3–26 months), 33% were insulin-independent, with a C-peptide-positive rate of 70%. This is the largest cohort to date from a center utilizing an off-site islet isolation facility.47
In view of the positive outcomes at these centers, lack of a local islet-processing facility should no longer be a barrier to total pancreatectomy and islet cell autotransplant.
PATIENT CARE AFTER THE PROCEDURE
A multidisciplinary team is an essential component of the postoperative management of patients who undergo total pancreatectomy and islet cell autotransplant.
For patients who had been receiving narcotics for a long time before surgery or who were receiving frequent doses, an experienced pain management physician should be involved in the patient’s postoperative care.
Because islet function can wane over time, testing for diabetes should be done at least annually for the rest of the patient’s life and should include fasting plasma glucose, hemoglobin A1c, and C-peptide, along with self-monitored blood glucose.26
All patients who have surgically induced exocrine insufficiency are at risk of malabsorption and fat-soluble vitamin deficiencies.48 Hence, lifelong pancreatic enzyme replacement therapy is mandatory. Nutritional monitoring should include assessment of steatorrhea, body composition, and fat-soluble vitamin levels (vitamins A, D, and E) at least every year.26 Patients with chronic pancreatitis are at increased risk for low bone density from malabsorption of vitamin D and calcium; therefore, it is recommended that a dual-energy x-ray absorptiometry bone density scan be obtained.26,49
Patients who undergo splenectomy as part of their procedure will require appropriate precautions and ongoing vaccinations as recommended by the US Centers for Disease Control and Prevention.26,50,51
WHAT TO EXPECT FOR THE FUTURE
The National Institute of Diabetes and Digestive and Kidney Diseases has reviewed the potential future research directions for total pancreatectomy and islet autotransplant.15
Patient selection remains challenging despite the availability of criteria15 and guidelines.26 More research is needed to better assess preoperative beta-cell function and to predict postoperative outcomes. Mixed meal-tolerance testing is adopted by most clinical centers to predict posttransplant beta-cell function. The use of arginine instead of glucagon in a stimulation test for insulin and C-peptide response has been validated and may allow more accurate assessment.52,53
Another targeted area of research is the advancement of safety and metabolic outcomes. Techniques to minimize warm ischemic time and complications are being evaluated. Islet isolation methods that yield more islets, reduce beta-cell apoptosis, and can isolate islets from glands with malignancy should be further investigated.54 Further, enhanced islet infusion methods that achieve lower portal venous pressures and minimize portal vein thrombosis are needed.
Unfortunately, the function of transplanted islet grafts declines over time. This phenomenon is at least partially attributed to the immediate blood-mediated inflammatory response,55,56 along with islet hypoxia,57 leading to islet apoptosis. Research on different strategies is expanding our knowledge in islet engraftment and posttransplant beta-cell apoptosis, with the expectation that the transplanted islet lifespan will increase. Alternative transplant sites with low inflammatory reaction, such as the omental pouch,58 muscle,59 and bone marrow,60 have shown encouraging data. Other approaches, such as adjuvant anti-inflammatory agents and heparinization, have been proposed.15
Research into complications is also of clinical importance. There is growing attention to hypoglycemia unrelated to exogenous insulin use in posttransplant patients. One hypothesis is that glucagon secretion, a counterregulatory response to hypoglycemia, is defective if the islet cells are transplanted into the liver, and that implanting them into another site may avoid this effect.61
For some patients with chronic pancreatitis, the best option is to remove the entire pancreas. This does not necessarily doom the patient to diabetes mellitus, because we can harvest the islet cells and reinsert them so that, lodged in the liver, they can continue making insulin. However, this approach is underemphasized in the general medical literature and is likely underutilized in the United States.
Here, we discuss chronic pancreatitis, the indications for and contraindications to this procedure, its outcomes, and the management of patients who undergo it.
CHRONIC PANCREATITIS IS PROGRESSIVE AND PAINFUL
Chronic pancreatitis is a progressive condition characterized by chronic inflammation, irreversible fibrosis, and scarring, resulting in loss of both exocrine and endocrine tissue.
According to a National Institutes of Health database, pancreatitis is the seventh most common digestive disease diagnosis on hospitalization, with annual healthcare costs exceeding $3 billion.1 Although data are scarce, by some estimates the incidence of chronic pancreatitis ranges from 4 to 14 per 100,000 person-years, and the prevalence ranges from 26.4 to 52 per 100,000.2–4 Moreover, a meta-analysis5 found that acute pancreatitis progresses to chronic pancreatitis in 10% of patients who have a first episode of acute pancreatitis and in 36% who have recurrent episodes.
Historically, alcoholism was and still is the most common cause of chronic pancreatitis, contributing to 60% to 90% of cases in Western countries.6,7 However, cases due to nonalcoholic causes have been increasing, and in more than one-fourth of patients, no identifiable cause is found.6,8 Smoking is an independent risk factor.6,8,9 Some cases can be linked to genetic abnormalities, particularly in children.10
The clinical manifestations of chronic pancreatitis include exocrine pancreatic insufficiency (leading to malnutrition and steatorrhea), endocrine insufficiency (causing diabetes mellitus), and intractable pain.11 Pain is the predominant clinical symptom early in the disease and is often debilitating and difficult to manage. Uncontrolled pain has a devastating impact on quality of life and may become complicated by narcotic dependence.
The pain of chronic pancreatitis is often multifactorial, with mechanisms that include increased intraductal pressure from obstruction of the pancreatic duct, pancreatic ischemia, neuronal injury, and neuroimmune interactions between neuronal processes and chronic inflammation.12
Treatment: Medical and surgical
In chronic pancreatitis, the aim of treatment is to alleviate deficiencies of exocrine and endocrine function and mitigate the pain. Patients who smoke or drink alcohol should be strongly encouraged to quit.
Loss of exocrine function is mainly managed with oral pancreatic enzyme supplements, and diabetes control is often attained with insulin therapy.13 Besides helping digestion, pancreatic enzyme therapy in the form of nonenteric tablets may also reduce pain and pancreatitis attacks.14 The mechanism may be by degrading cholecystokinin-releasing factor in the duodenum, lowering cholecystokinin levels and thereby reducing pain.12
Nonnarcotic analgesics are often the first line of therapy for pain management, but many patients need narcotic analgesics. Along with narcotics, adjunctive agents such as tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, selective serotonin reuptake inhibitors, and gabapentinoids have been used to treat chronic pancreatitis pain, but with limited success.15
In patients for whom medical pain management has failed, one can consider another option, such as nerve block, neurolysis, or endoscopic or surgical therapy. Neuromodulators are often prescribed by pain clinics.15 Percutaneous and endoscopic celiac ganglion blocks can be an option but rarely achieve substantial or permanent pain relief, and the induced transient responses (on average 2 to 4 months) often cannot be repeated.14–17
Surgical options to relieve pain try to preserve pancreatic function and vary depending on the degree of severity and nature of pancreatic damage. In broad terms, the surgical procedures can be divided into two types:
- Drainage procedures (eg, pseudocyst drainage; minimally invasive endoscopic duct drainage via sphincterotomy or stent placement, or both; pancreaticojejunostomy)
- Resectional procedures (eg, distal pancreatectomy, isolated head resection, pancreaticoduodenectomy, Whipple procedure, total pancreatectomy).
In carefully selected patients, total pancreatectomy can be offered to remove the cause of the pain.18 This procedure is most often performed in patients who have small-duct disease or a genetic cause or for whom other surgical procedures have failed.11
HISTORY OF THE PROCEDURE
Islet cell transplantation grew out of visionary work by Paul Lacy and David Scharp at the University of Washington at Seattle, whose research focused on isolating and transplanting islet cells in rodent models. The topic has been reviewed by Jahansouz et al.19 In the 1970s, experiments in pancreatectomized dogs showed that transplanting unpurified pancreatic islet tissue that was dispersed by collagenase digestion into the spleen or portal vein could prevent diabetes.20,21 In 1974, the first human trials of transplanting islet cells were conducted, using isolated islets from cadaveric donors to treat diabetes.19
In the past, pancreatectomy was performed to treat painful chronic pancreatitis, but it was viewed as undesirable because removing the gland would inevitably cause insulin-dependent diabetes.22 That changed in 1977 at the University of Minnesota, with the first reported islet cell autotransplant after pancreatectomy. The patient remained pain-free and insulin-independent long-term.23 This seminal case showed that endocrine function could be preserved by autotransplant of islets prepared from the excised pancreas.24
In 1992, Pyzdrowski et al25 reported that intrahepatic transplant of as few as 265,000 islets was enough to prevent the need for insulin therapy. Since this technique was first described, there have been many advances, and now more than 30 centers worldwide do it.
PRIMARY INDICATION: INTRACTABLE PAIN
Interest has been growing in using total pancreatectomy and islet autotransplant to treat recurrent acute pancreatitis, chronic pancreatitis, and hereditary pancreatitis. The rationale is that removing the offending tissue eliminates pancreatitis, pain, and cancer risk, while preserving and replacing the islet cells prevents the development of brittle diabetes with loss of insulin and glucagon.26
Proposed criteria for total pancreatectomy and islet autotransplant
Bellin et al14 proposed five criteria for patient selection for this procedure based on imaging studies, pancreatic function tests, and histopathology to detect pancreatic fibrosis. Patients must fulfill all five of the following criteria:
Criterion 1. Diagnosis of chronic pancreatitis, based on chronic abdominal pain lasting more than 6 months with either at least one of the following:
- Pancreatic calcifications on computed tomography
- At least two of the following: four or more of nine criteria on endoscopic ultrasonography described by Catalano et al,27 a compatible ductal or parenchymal abnormality on secretin magnetic resonance cholangiopancreatography; abnormal endoscopic pancreatic function test (peak HCO2 ≤ 80 mmol/L)
- Histopathologically confirmed diagnosis of chronic pancreatitis
- Compatible clinical history and documented hereditary pancreatitis (PRSS1 gene mutation)
OR
- History of recurrent acute pancreatitis (more than one episode of characteristic pain associated with imaging diagnostic of acute pancreatitis or elevated serum amylase or lipase > 3 times the upper limit of normal).
Criterion 2. At least one of the following:
- Daily narcotic dependence
- Pain resulting in impaired quality of life, which may include inability to attend school, recurrent hospitalizations, or inability to participate in usual age-appropriate activities.
Criterion 3. Complete evaluation with no reversible cause of pancreatitis present or untreated.
Criterion 4. Failure to respond to maximal medical and endoscopic therapy.
Criterion 5. Adequate islet cell function (nondiabetic or C-peptide-positive). Patients with C-peptide-negative diabetes meeting criteria 1 to 4 are candidates for total pancreatectomy alone.
The primary goal is to treat intractable pain and improve quality of life in selected patients with chronic pancreatitis or recurrent acute pancreatitis when endoscopic and prior surgical therapies have failed, and whose impairment due to pain is substantial enough to accept the risk of postoperative insulin-dependent diabetes and lifelong commitment to pancreatic enzyme replacement therapy.15,26 Patients with a known genetic cause of chronic pancreatitis should be offered special consideration for the procedure, as their disease is unlikely to remit.
CONTRAINDICATIONS
Total pancreatectomy and islet autotransplant should not be performed in patients with active alcoholism, illicit drug use, or untreated or poorly controlled psychiatric illnesses that could impair the patient’s ability to adhere to a complicated postoperative medical regimen.
A poor support network may be a relative contraindication in view of the cost and complexity of diabetic and pancreatic enzyme replacement therapy.18,26
Islet cell autotransplant is contraindicated in patients with conditions such as C-peptide-negative or type 1 diabetes or a history of portal vein thrombosis, portal hypertension, significant liver disease, high-risk cardiopulmonary disease, or pancreatic cancer (Table 1).26
WHEN TO CONSIDER REFERRAL FOR THIS PROCEDURE
The choice of total pancreatectomy and islet autotransplant vs conventional surgery must be individualized on the basis of each patient’s anatomy, comorbidities, symptom burden, presence or degree of diabetes, and rate of disease progression. The most important factors to consider in determining the need for and timing of this procedure are the patient’s pain, narcotic requirements, and impaired ability to function.26
Sooner rather than later?
An argument can be made for performing this procedure sooner in the course of the disease rather than later when all else has failed. First, prolonged pain can result in central sensitization, in which the threshold for perceiving pain is lowered by damage to the nociceptive neurons from repeated stimulation and inflammation.28
Further, prolonged opioid therapy can lead to opioid-induced hyperalgesia, which may also render patients more sensitive to pain and aggravate their preexisting pain.26,28
In addition, although operative drainage procedures and partial resections are often considered the gold standard for chronic pancreatitis management, patients who undergo partial pancreatectomy or lateral pancreaticojejunostomy (Puestow procedure) have fewer islet cells left to harvest (about 50% fewer) if they subsequently undergo total pancreatectomy and islet cell autotransplant.22,26
Therefore, performing this procedure earlier may help the patient avoid chronic pain syndromes and complications of chronic opioid use, including hyperalgesia, and give the best chance of harvesting enough islet cells to prevent or minimize diabetes afterward.11
REMOVING THE PANCREAS, RETURNING THE ISLET CELLS
During this procedure, the blood supply to the pancreas must be preserved until just before its removal to minimize warm ischemia of the islet cells.18,29 Although there are several surgical variations, a pylorus-preserving total pancreatectomy with duodenectomy is typically performed, usually with splenectomy to preserve perfusion to the body and tail.30
The resected pancreas is taken to the islet isolation laboratory. There, the pancreatic duct is cannulated to fill the organ with a cold collagenase solution, followed by gentle mechanical dispersion using the semiautomated Ricordi method,31 which separates the islet cells from the exocrine tissue.32
The number of islet cells is quantified as islet equivalents; 1 islet equivalent is equal to the volume of an islet with a diameter of 150 µm. Islet equivalents per kilogram of body weight is the unit commonly used to report the graft amount transplanted.33
After digestion, the islet cells can be purified or partially purified by a gradient separation method using a Cobe 2991 cell processor (Terumo Corporation, Tokyo, Japan),34 or can be transplanted as an unpurified preparation. In islet cell autotransplant for chronic pancreatitis, purification is not always necessary due to the small tissue volume extracted from the often atrophic and fibrotic pancreas.32 The decision to purify depends on the postdigest tissue volume; usually, a tissue volume greater than 0.25 mL/kg body weight is an indication to at least partially purify.18,35
The final preparation is returned to the operating room, and after heparin is given, the islets are infused into the portal system using a stump of the splenic vein, or alternatively through direct puncture of the portal vein or cannulation of the umbilical vein.32,36 If the portal vein pressure reaches 25 cm H2O, the infusion is stopped and the remaining islets can be placed in the peritoneal cavity or elsewhere.18 Transplant of the islets into the liver or peritoneum allows the islets to secrete insulin into the hepatic portal circulation, which is the route used by the native pancreas.22
CONTROLLING GLUCOSE DURING AND AFTER THE PROCEDURE
Animal studies have shown that hyperglycemia impairs islet revascularization,37 and glucose toxicity may cause dysfunction and structural lesions of the transplanted islets.11,38
Therefore, during and after the procedure, most centers maintain euglycemia by an intravenous insulin infusion and subsequently move to subcutaneous insulin when the patient starts eating again. Some centers continue insulin at discharge and gradually taper it over months, even in patients who can possibly achieve euglycemia without it.
OUTCOMES
Many institutions have reported their clinical outcomes in terms of pain relief, islet function, glycemic control, and improvement of quality of life. The largest series have been from the University of Minnesota, Leicester General Hospital, the University of Cincinnati, and the Medical University of South Carolina.
Insulin independence is common but wanes with time
The ability to achieve insulin independence after islet autotransplant appears to be related to the number of islets transplanted, with the best results when more than 2,000 or 3,000 islet equivalents/kg are transplanted.39,40
Sutherland et al18 reported that of 409 patients who underwent islet cell autotransplant at the University of Minnesota (the largest series reported to date), 30% were insulin-independent at 3 years, 33% had partial graft function (defined by positive C-peptide), and 82% achieved a mean hemoglobin A1c of less than 7%. However, in the subset who received more than 5,000 islet equivalents/kg, nearly three-fourths of patients were insulin-independent at 3 years.
The Leicester General Hospital group presented long-term data on 46 patients who underwent total pancreatectomy and islet cell autotransplant. Twelve of the 46 had shown periods of insulin independence for a median of 16.5 months, and 5 remained insulin-free at the time of the publication.41 Over the 10 years of follow-up, insulin requirements and hemoglobin A1c increased notably. However, all of the patients tested C-peptide-positive, suggesting long-lasting graft function.
Most recently, the University of Cincinnati group reported long-term data on 116 patients. The insulin independence rate was 38% at 1 year, decreasing to 27% at 5 years. The number of patients with partial graft function was 38% at 1 year and 35% at 5 years.42
Thus, all three institutions confirmed that the autotransplanted islets continue to secrete insulin long-term, but that function decreases over time.
Pancreatectomy reduces pain
Multiple studies have shown that total pancreatectomy reduces pain in patients with chronic pancreatitis. Ahmad et al43 reported a marked reduction in narcotic use (mean morphine equivalents 206 mg/day before surgery, compared with 90 mg after), and a 58% reduction in pain as demonstrated by narcotic independence.
In the University of Minnesota series, 85% of the 409 patients had less pain at 2 years, and 59% were able to stop taking narcotics.18
The University of Cincinnati group reported a narcotic independence rate of 55% at 1 year, which continued to improve to 73% at 5 years.42
Although the source of pain is removed, pain persists or recurs in 10% to 20% of patients after total pancreatectomy and islet cell autotransplant, showing that the pathogenesis of pain is complex, and some uncertainty exists about it.26
Quality of life
Reports evaluating health-related quality of life after total pancreatectomy and islet autotransplant are limited.
The University of Cincinnati group reported the long-term outcomes of quality of life as measured by the Short Form 36 Health Survey.42 Ninety-two percent of patients reported overall improvement in their health at 1 year, and 85% continued to report improved health more than 5 years after the surgery.
In a series of 20 patients, 79% to 90% reported improvements in the seven various domains of the Pain Disability Index. In addition, 60% showed improvement in depression and 70% showed improvement in anxiety. The greatest improvements were in those who had not undergone prior pancreatic surgery, who were younger, and in those with higher levels of preoperative pain.30
Similarly, in a series of 74 patients, the Medical University of South Carolina group reported significant improvement in physical and mental health components of the Short Form 12 Health Survey and an associated decrease in daily narcotic requirements. Moreover, the need to start or increase the dose of insulin after the surgery was not associated with a lower quality of life.44
OFF-SITE ISLET CELL ISOLATION
Despite the positive outcomes in terms of pain relief and insulin independence in many patients after total pancreatectomy and islet cell autotransplant, few medical centers have an on-site islet-processing facility. Since the mid-1990s, a few centers have been able to circumvent this limitation by working with off-site islet cell isolation laboratories.45,46
The University of California, Los Angeles, first reported on a series of nine patients who received autologous islet cells after near-total or total pancreatectomy using a remote islet cell isolation facility, with results comparable to those of other large institutions.45
Similarly, the procedure has been performed at Cleveland Clinic since 2007 with the collaboration of an off-site islet cell isolation laboratory at the University of Pittsburgh. A cohort study from this collaboration published in 2015 showed that in 36 patients (mean follow-up 28 months, range 3–26 months), 33% were insulin-independent, with a C-peptide-positive rate of 70%. This is the largest cohort to date from a center utilizing an off-site islet isolation facility.47
In view of the positive outcomes at these centers, lack of a local islet-processing facility should no longer be a barrier to total pancreatectomy and islet cell autotransplant.
PATIENT CARE AFTER THE PROCEDURE
A multidisciplinary team is an essential component of the postoperative management of patients who undergo total pancreatectomy and islet cell autotransplant.
For patients who had been receiving narcotics for a long time before surgery or who were receiving frequent doses, an experienced pain management physician should be involved in the patient’s postoperative care.
Because islet function can wane over time, testing for diabetes should be done at least annually for the rest of the patient’s life and should include fasting plasma glucose, hemoglobin A1c, and C-peptide, along with self-monitored blood glucose.26
All patients who have surgically induced exocrine insufficiency are at risk of malabsorption and fat-soluble vitamin deficiencies.48 Hence, lifelong pancreatic enzyme replacement therapy is mandatory. Nutritional monitoring should include assessment of steatorrhea, body composition, and fat-soluble vitamin levels (vitamins A, D, and E) at least every year.26 Patients with chronic pancreatitis are at increased risk for low bone density from malabsorption of vitamin D and calcium; therefore, it is recommended that a dual-energy x-ray absorptiometry bone density scan be obtained.26,49
Patients who undergo splenectomy as part of their procedure will require appropriate precautions and ongoing vaccinations as recommended by the US Centers for Disease Control and Prevention.26,50,51
WHAT TO EXPECT FOR THE FUTURE
The National Institute of Diabetes and Digestive and Kidney Diseases has reviewed the potential future research directions for total pancreatectomy and islet autotransplant.15
Patient selection remains challenging despite the availability of criteria15 and guidelines.26 More research is needed to better assess preoperative beta-cell function and to predict postoperative outcomes. Mixed meal-tolerance testing is adopted by most clinical centers to predict posttransplant beta-cell function. The use of arginine instead of glucagon in a stimulation test for insulin and C-peptide response has been validated and may allow more accurate assessment.52,53
Another targeted area of research is the advancement of safety and metabolic outcomes. Techniques to minimize warm ischemic time and complications are being evaluated. Islet isolation methods that yield more islets, reduce beta-cell apoptosis, and can isolate islets from glands with malignancy should be further investigated.54 Further, enhanced islet infusion methods that achieve lower portal venous pressures and minimize portal vein thrombosis are needed.
Unfortunately, the function of transplanted islet grafts declines over time. This phenomenon is at least partially attributed to the immediate blood-mediated inflammatory response,55,56 along with islet hypoxia,57 leading to islet apoptosis. Research on different strategies is expanding our knowledge in islet engraftment and posttransplant beta-cell apoptosis, with the expectation that the transplanted islet lifespan will increase. Alternative transplant sites with low inflammatory reaction, such as the omental pouch,58 muscle,59 and bone marrow,60 have shown encouraging data. Other approaches, such as adjuvant anti-inflammatory agents and heparinization, have been proposed.15
Research into complications is also of clinical importance. There is growing attention to hypoglycemia unrelated to exogenous insulin use in posttransplant patients. One hypothesis is that glucagon secretion, a counterregulatory response to hypoglycemia, is defective if the islet cells are transplanted into the liver, and that implanting them into another site may avoid this effect.61
- Everhart JE. Pancreatitis. In: Everhart JE, editor. The Burden of Digestive Diseases in the United States. US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of
- Diabetes and Digestive and Kidney Diseases. Washington, DC: US Government Printing Office; 2008. www.niddk.nih.gov/about-niddk/strategic-plans-reports/Pages/burden-digestive-diseases-in-united-states-report.aspx. Accessed May 10, 2016.
- Yadav D, Timmons L, Benson JT, Dierkhising RA, Chari ST. Incidence, prevalence, and survival of chronic pancreatitis: a population-based study. Am J Gastroenterol 2011; 106:2192–2199.
- Lévy P, Barthet M, Mollard BR, Amouretti M, Marion-Audibert AM, Dyard F. Estimation of the prevalence and incidence of chronic pancreatitis and its complications. Gastroenterol Clin Biol 2006; 30:838–844.
- Hirota M, Shimosegawa T, Masamune A, et al; Research Committee of Intractable Pancreatic Diseases. The seventh nationwide epidemiological survey for chronic pancreatitis in Japan: clinical significance of smoking habit in Japanese patients. Pancreatology 2014; 14:490–496.
- Sankaran SJ, Xiao AY, Wu LM, Windsor JA, Forsmark CE, Petrov MS. Frequency of progression from acute to chronic pancreatitis and risk factors: a meta-analysis. Gastroenterology 2015; 149:1490–1500.e1.
- Coté GA, Yadav D, Slivka A, et al; North American Pancreatitis Study Group. Alcohol and smoking as risk factors in an epidemiology study of patients with chronic pancreatitis. Clin Gastroenterol Hepatol 2011; 9:266–273.
- Muniraj T, Aslanian HR, Farrell J, Jamidar PA. Chronic pancreatitis, a comprehensive review and update. Part I: epidemiology, etiology, risk factors, genetics, pathophysiology, and clinical features. Dis Mon 2014; 60:530–550.
- Frulloni L, Gabbrielli A, Pezzilli R, et al; PanCroInfAISP Study Group. Chronic pancreatitis: report from a multicenter Italian survey (PanCroInfAISP) on 893 patients. Dig Liver Dis 2009; 41:311–317.
- Talamini G, Bassi C, Falconi M, et al. Alcohol and smoking as risk factors in chronic pancreatitis and pancreatic cancer. Dig Dis Sci 1999; 44:1303–1311.
- Schwarzenberg SJ, Bellin M, Husain SZ, et al. Pediatric chronic pancreatitis is associated with genetic risk factors and substantial disease burden. J Pediatr 2015; 166:890–896.e1.
- Blondet JJ, Carlson AM, Kobayashi T, et al. The role of total pancreatectomy and islet autotransplantation for chronic pancreatitis. Surg Clin North Am 2007; 87:1477–1501.
- Lieb JG 2nd, Forsmark CE. Review article: pain and chronic pancreatitis. Aliment Pharmacol Ther 2009; 29:706–719.
- Lin YK, Johnston PC, Arce K, Hatipoglu BA. Chronic pancreatitis and diabetes mellitus. Curr Treat Options Gastroenterol 2015; 13:319–331.
- Bellin MD, Gelrud A, Arreaza-Rubin G, et al. Total pancreatectomy with islet autotransplantation: summary of a National Institute of Diabetes and Digestive and Kidney diseases workshop. Pancreas 2014; 43:1163–1171.
- Muniraj T, Aslanian HR, Farrell J, Jamidar PA. Chronic pancreatitis, a comprehensive review and update. Part II: diagnosis, complications, and management. Dis Mon 2015; 61:5–37.
- Warshaw AL, Banks PA, Fernández-Del Castillo C. AGA technical review: treatment of pain in chronic pancreatitis. Gastroenterology 1998; 115:765–776.
- Chauhan S, Forsmark CE. Pain management in chronic pancreatitis: a treatment algorithm. Best Pract Res Clin Gastroenterol 2010; 24:323–335.
- Sutherland DE, Radosevich DM, Bellin MD, et al. Total pancreatectomy and islet autotransplantation for chronic pancreatitis. J Am Coll Surg 2012; 214:409–426.
- Jahansouz C, Jahansouz C, Kumer SC, Brayman KL. Evolution of beta-cell replacement therapy in diabetes mellitus: islet cell transplantation. J Transplant 2011; 2011:247959.
- Kretschmer GJ, Sutherland DE, Matas AJ, Cain TL, Najarian JS. Autotransplantation of pancreatic islets without separation of exocrine and endocrine tissue in totally pancreatectomized dogs. Surgery 1977; 82:74–81.
- Kretschmer GJ, Sutherland DR, Matas AJ, Payne WD, Najarian JS. Autotransplantation of pancreatic fragments to the portal vein and spleen of totally pancreatectomized dogs: a comparative evaluation. Ann Surg 1978; 187:79–86.
- Bellin MD, Sutherland DE, Robertson RP. Pancreatectomy and autologous islet transplantation for painful chronic pancreatitis: indications and outcomes. Hosp Pract (1995) 2012; 40:80–87.
- Najarian JS, Sutherland DE, Baumgartner D, et al. Total or near total pancreatectomy and islet autotransplantation for treatment of chronic pancreatitis. Ann Surg 1980; 192:526–542.
- Sutherland DE, Matas AJ, Najarian JS. Pancreatic islet cell transplantation. Surg Clin North Am 1978; 58:365–382.
- Pyzdrowski KL, Kendall DM, Halter JB, Nakhleh RE, Sutherland DE, Robertson RP. Preserved insulin secretion and insulin independence in recipients of islet autografts. N Engl J Med 1992; 327:220–226.
- Bellin MD, Freeman ML, Gelrud A, et al. Total pancreatectomy and islet autotransplantation in chronic pancreatitis: recommendations from PancreasFest. Pancreatology 2014; 14:27–35.
- Catalano MF, Sahai A, Levy M, et al. EUS-based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointest Endosc 2009; 69:1251–1261.
- Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology 2006; 104:570–587.
- Bramis K, Gordon-Weeks AN, Friend PJ, et al. Systematic review of total pancreatectomy and islet autotransplantation for chronic pancreatitis. Br J Surg 2012; 99:761–766.
- Walsh RM, Saavedra JR, Lentz G, et al. Improved quality of life following total pancreatectomy and auto-islet transplantation for chronic pancreatitis. J Gastrointest Surg 2012; 16:1469–1477.
- Ricordi C, Lacy PE, Scharp DW. Automated islet isolation from human pancreas. Diabetes 1989; 38(suppl 1):140–142.
- Witkowski P, Savari O, Matthews JB. Islet autotransplantation and total pancreatectomy. Adv Surg 2014; 48:223–233.
- Bellin MD, Beilman GJ, Dunn TB, et al. Islet autotransplantation to preserve beta cell mass in selected patients with chronic pancreatitis and diabetes mellitus undergoing total pancreatectomy. Pancreas 2013; 42:317–321.
- Anazawa T, Matsumoto S, Yonekawa Y, et al. Prediction of pancreatic tissue densities by an analytical test gradient system before purification maximizes human islet recovery for islet autotransplantation/allotransplantation. Transplantation 2011; 91:508–514.
- Lake SP, Bassett PD, Larkins A, et al. Large-scale purification of human islets utilizing discontinuous albumin gradient on IBM 2991 cell separator. Diabetes 1989; 38(suppl 1):143–145.
- Bellin MD, Freeman ML, Schwarzenberg SJ, et al. Quality of life improves for pediatric patients after total pancreatectomy and islet autotransplant for chronic pancreatitis. Clin Gastroenterol Hepatol 2011; 9:793–799.
- Andersson A, Korsgren O, Jansson L. Intraportally transplanted pancreatic islets revascularized from hepatic arterial system. Diabetes 1989; 38(suppl 1):192–195.
- Leahy JL, Bonner-Weir S, Weir GC. Beta-cell dysfunction induced by chronic hyperglycemia. Current ideas on mechanism of impaired glucose-induced insulin secretion. Diabetes Care 1992; 15:442–455.
- Bellin MD, Carlson AM, Kobayashi T, et al. Outcome after pancreatectomy and islet autotransplantation in a pediatric population. J Pediatr Gastroenterol Nutr 2008; 47:37–44.
- White SA, Davies JE, Pollard C, et al. Pancreas resection and islet autotransplantation for end-stage chronic pancreatitis. Ann Surg 2001; 233:423–431.
- Webb MA, Illouz SC, Pollard CA, et al. Islet auto transplantation following total pancreatectomy: a long-term assessment of graft function. Pancreas 2008; 37:282–287.
- Wilson GC, Sutton JM, Abbott DE, et al. Long-term outcomes after total pancreatectomy and islet cell autotransplantation: is it a durable operation? Ann Surg 2014; 260:659–667.
- Ahmad SA, Lowy AM, Wray CJ, et al. Factors associated with insulin and narcotic independence after islet autotransplantation in patients with severe chronic pancreatitis. J Am Coll Surg 2005; 201:680–687.
- Dorlon M, Owczarski S, Wang H, Adams D, Morgan K. Increase in postoperative insulin requirements does not lead to decreased quality of life after total pancreatectomy with islet cell autotransplantation for chronic pancreatitis. Am Surg 2013; 79:676–680.
- Tai DS, Shen N, Szot GL, et al. Autologous islet transplantation with remote islet isolation after pancreas resection for chronic pancreatitis. JAMA Surg 2015; 150:118–124.
- Rabkin JM, Olyaei AJ, Orloff SL, et al. Distant processing of pancreas islets for autotransplantation following total pancreatectomy. Am J Surg 1999; 177:423–427.
- Johnston PC, Lin YK, Walsh RM, et al. Factors associated with islet yield and insulin independence after total pancreatectomy and islet cell autotransplantation in patients with chronic pancreatitis utilizing off-site islet isolation: Cleveland Clinic experience. J Clin Endocrinol Metab 2015; 100:1765–1770.
- Dresler CM, Fortner JG, McDermott K, Bajorunas DR. Metabolic consequences of (regional) total pancreatectomy. Ann Surg 1991; 214:131–140.
- Duggan SN, O’Sullivan M, Hamilton S, Feehan SM, Ridgway PF, Conlon KC. Patients with chronic pancreatitis are at increased risk for osteoporosis. Pancreas 2012; 41:1119–1124.
- Rubin LG, Levin MJ, Ljungman P, et al; Infectious Diseases Society of America. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014; 58:e44–e100.
- Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet 2011; 378:86–97.
- Robertson RP, Raymond RH, Lee DS, et al; Beta Cell Project Team of the Foundation for the NIH Biomarkers Consortium. Arginine is preferred to glucagon for stimulation testing of beta-cell function. Am J Physiol Endocrinol Metab 2014; 307:E720–E727.
- Robertson RP, Bogachus LD, Oseid E, et al. Assessment of beta-cell mass and alpha- and beta-cell survival and function by arginine stimulation in human autologous islet recipients. Diabetes 2015; 64:565–572.
- Balzano G, Piemonti L. Autologous islet transplantation in patients requiring pancreatectomy for neoplasm. Curr Diab Rep 2014; 14:512.
- Naziruddin B, Iwahashi S, Kanak MA, Takita M, Itoh T, Levy MF. Evidence for instant blood-mediated inflammatory reaction in clinical autologous islet transplantation. Am J Transplant 2014; 14:428–437.
- Abdelli S, Ansite J, Roduit R, et al. Intracellular stress signaling pathways activated during human islet preparation and following acute cytokine exposure. Diabetes 2004; 53:2815–2823.
- Olsson R, Olerud J, Pettersson U, Carlsson PO. Increased numbers of low-oxygenated pancreatic islets after intraportal islet transplantation. Diabetes 2011; 60:2350–2353.
- Berman DM, O’Neil JJ, Coffey LC, et al. Long-term survival of nonhuman primate islets implanted in an omental pouch on a biodegradable scaffold. Am J Transplant 2009; 9:91–104.
- Sterkers A, Hubert T, Gmyr V, et al. Islet survival and function following intramuscular autotransplantation in the minipig. Am J Transplant 2013; 13:891–898.
- Maffi P, Balzano G, Ponzoni M, et al. Autologous pancreatic islet transplantation in human bone marrow. Diabetes 2013; 62:3523–3531.
- Bellin MD, Parazzoli S, Oseid E, et al. Defective glucagon secretion during hypoglycemia after intrahepatic but not nonhepatic islet autotransplantation. Am J Transplant 2014; 14:1880–1886.
- Everhart JE. Pancreatitis. In: Everhart JE, editor. The Burden of Digestive Diseases in the United States. US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of
- Diabetes and Digestive and Kidney Diseases. Washington, DC: US Government Printing Office; 2008. www.niddk.nih.gov/about-niddk/strategic-plans-reports/Pages/burden-digestive-diseases-in-united-states-report.aspx. Accessed May 10, 2016.
- Yadav D, Timmons L, Benson JT, Dierkhising RA, Chari ST. Incidence, prevalence, and survival of chronic pancreatitis: a population-based study. Am J Gastroenterol 2011; 106:2192–2199.
- Lévy P, Barthet M, Mollard BR, Amouretti M, Marion-Audibert AM, Dyard F. Estimation of the prevalence and incidence of chronic pancreatitis and its complications. Gastroenterol Clin Biol 2006; 30:838–844.
- Hirota M, Shimosegawa T, Masamune A, et al; Research Committee of Intractable Pancreatic Diseases. The seventh nationwide epidemiological survey for chronic pancreatitis in Japan: clinical significance of smoking habit in Japanese patients. Pancreatology 2014; 14:490–496.
- Sankaran SJ, Xiao AY, Wu LM, Windsor JA, Forsmark CE, Petrov MS. Frequency of progression from acute to chronic pancreatitis and risk factors: a meta-analysis. Gastroenterology 2015; 149:1490–1500.e1.
- Coté GA, Yadav D, Slivka A, et al; North American Pancreatitis Study Group. Alcohol and smoking as risk factors in an epidemiology study of patients with chronic pancreatitis. Clin Gastroenterol Hepatol 2011; 9:266–273.
- Muniraj T, Aslanian HR, Farrell J, Jamidar PA. Chronic pancreatitis, a comprehensive review and update. Part I: epidemiology, etiology, risk factors, genetics, pathophysiology, and clinical features. Dis Mon 2014; 60:530–550.
- Frulloni L, Gabbrielli A, Pezzilli R, et al; PanCroInfAISP Study Group. Chronic pancreatitis: report from a multicenter Italian survey (PanCroInfAISP) on 893 patients. Dig Liver Dis 2009; 41:311–317.
- Talamini G, Bassi C, Falconi M, et al. Alcohol and smoking as risk factors in chronic pancreatitis and pancreatic cancer. Dig Dis Sci 1999; 44:1303–1311.
- Schwarzenberg SJ, Bellin M, Husain SZ, et al. Pediatric chronic pancreatitis is associated with genetic risk factors and substantial disease burden. J Pediatr 2015; 166:890–896.e1.
- Blondet JJ, Carlson AM, Kobayashi T, et al. The role of total pancreatectomy and islet autotransplantation for chronic pancreatitis. Surg Clin North Am 2007; 87:1477–1501.
- Lieb JG 2nd, Forsmark CE. Review article: pain and chronic pancreatitis. Aliment Pharmacol Ther 2009; 29:706–719.
- Lin YK, Johnston PC, Arce K, Hatipoglu BA. Chronic pancreatitis and diabetes mellitus. Curr Treat Options Gastroenterol 2015; 13:319–331.
- Bellin MD, Gelrud A, Arreaza-Rubin G, et al. Total pancreatectomy with islet autotransplantation: summary of a National Institute of Diabetes and Digestive and Kidney diseases workshop. Pancreas 2014; 43:1163–1171.
- Muniraj T, Aslanian HR, Farrell J, Jamidar PA. Chronic pancreatitis, a comprehensive review and update. Part II: diagnosis, complications, and management. Dis Mon 2015; 61:5–37.
- Warshaw AL, Banks PA, Fernández-Del Castillo C. AGA technical review: treatment of pain in chronic pancreatitis. Gastroenterology 1998; 115:765–776.
- Chauhan S, Forsmark CE. Pain management in chronic pancreatitis: a treatment algorithm. Best Pract Res Clin Gastroenterol 2010; 24:323–335.
- Sutherland DE, Radosevich DM, Bellin MD, et al. Total pancreatectomy and islet autotransplantation for chronic pancreatitis. J Am Coll Surg 2012; 214:409–426.
- Jahansouz C, Jahansouz C, Kumer SC, Brayman KL. Evolution of beta-cell replacement therapy in diabetes mellitus: islet cell transplantation. J Transplant 2011; 2011:247959.
- Kretschmer GJ, Sutherland DE, Matas AJ, Cain TL, Najarian JS. Autotransplantation of pancreatic islets without separation of exocrine and endocrine tissue in totally pancreatectomized dogs. Surgery 1977; 82:74–81.
- Kretschmer GJ, Sutherland DR, Matas AJ, Payne WD, Najarian JS. Autotransplantation of pancreatic fragments to the portal vein and spleen of totally pancreatectomized dogs: a comparative evaluation. Ann Surg 1978; 187:79–86.
- Bellin MD, Sutherland DE, Robertson RP. Pancreatectomy and autologous islet transplantation for painful chronic pancreatitis: indications and outcomes. Hosp Pract (1995) 2012; 40:80–87.
- Najarian JS, Sutherland DE, Baumgartner D, et al. Total or near total pancreatectomy and islet autotransplantation for treatment of chronic pancreatitis. Ann Surg 1980; 192:526–542.
- Sutherland DE, Matas AJ, Najarian JS. Pancreatic islet cell transplantation. Surg Clin North Am 1978; 58:365–382.
- Pyzdrowski KL, Kendall DM, Halter JB, Nakhleh RE, Sutherland DE, Robertson RP. Preserved insulin secretion and insulin independence in recipients of islet autografts. N Engl J Med 1992; 327:220–226.
- Bellin MD, Freeman ML, Gelrud A, et al. Total pancreatectomy and islet autotransplantation in chronic pancreatitis: recommendations from PancreasFest. Pancreatology 2014; 14:27–35.
- Catalano MF, Sahai A, Levy M, et al. EUS-based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointest Endosc 2009; 69:1251–1261.
- Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology 2006; 104:570–587.
- Bramis K, Gordon-Weeks AN, Friend PJ, et al. Systematic review of total pancreatectomy and islet autotransplantation for chronic pancreatitis. Br J Surg 2012; 99:761–766.
- Walsh RM, Saavedra JR, Lentz G, et al. Improved quality of life following total pancreatectomy and auto-islet transplantation for chronic pancreatitis. J Gastrointest Surg 2012; 16:1469–1477.
- Ricordi C, Lacy PE, Scharp DW. Automated islet isolation from human pancreas. Diabetes 1989; 38(suppl 1):140–142.
- Witkowski P, Savari O, Matthews JB. Islet autotransplantation and total pancreatectomy. Adv Surg 2014; 48:223–233.
- Bellin MD, Beilman GJ, Dunn TB, et al. Islet autotransplantation to preserve beta cell mass in selected patients with chronic pancreatitis and diabetes mellitus undergoing total pancreatectomy. Pancreas 2013; 42:317–321.
- Anazawa T, Matsumoto S, Yonekawa Y, et al. Prediction of pancreatic tissue densities by an analytical test gradient system before purification maximizes human islet recovery for islet autotransplantation/allotransplantation. Transplantation 2011; 91:508–514.
- Lake SP, Bassett PD, Larkins A, et al. Large-scale purification of human islets utilizing discontinuous albumin gradient on IBM 2991 cell separator. Diabetes 1989; 38(suppl 1):143–145.
- Bellin MD, Freeman ML, Schwarzenberg SJ, et al. Quality of life improves for pediatric patients after total pancreatectomy and islet autotransplant for chronic pancreatitis. Clin Gastroenterol Hepatol 2011; 9:793–799.
- Andersson A, Korsgren O, Jansson L. Intraportally transplanted pancreatic islets revascularized from hepatic arterial system. Diabetes 1989; 38(suppl 1):192–195.
- Leahy JL, Bonner-Weir S, Weir GC. Beta-cell dysfunction induced by chronic hyperglycemia. Current ideas on mechanism of impaired glucose-induced insulin secretion. Diabetes Care 1992; 15:442–455.
- Bellin MD, Carlson AM, Kobayashi T, et al. Outcome after pancreatectomy and islet autotransplantation in a pediatric population. J Pediatr Gastroenterol Nutr 2008; 47:37–44.
- White SA, Davies JE, Pollard C, et al. Pancreas resection and islet autotransplantation for end-stage chronic pancreatitis. Ann Surg 2001; 233:423–431.
- Webb MA, Illouz SC, Pollard CA, et al. Islet auto transplantation following total pancreatectomy: a long-term assessment of graft function. Pancreas 2008; 37:282–287.
- Wilson GC, Sutton JM, Abbott DE, et al. Long-term outcomes after total pancreatectomy and islet cell autotransplantation: is it a durable operation? Ann Surg 2014; 260:659–667.
- Ahmad SA, Lowy AM, Wray CJ, et al. Factors associated with insulin and narcotic independence after islet autotransplantation in patients with severe chronic pancreatitis. J Am Coll Surg 2005; 201:680–687.
- Dorlon M, Owczarski S, Wang H, Adams D, Morgan K. Increase in postoperative insulin requirements does not lead to decreased quality of life after total pancreatectomy with islet cell autotransplantation for chronic pancreatitis. Am Surg 2013; 79:676–680.
- Tai DS, Shen N, Szot GL, et al. Autologous islet transplantation with remote islet isolation after pancreas resection for chronic pancreatitis. JAMA Surg 2015; 150:118–124.
- Rabkin JM, Olyaei AJ, Orloff SL, et al. Distant processing of pancreas islets for autotransplantation following total pancreatectomy. Am J Surg 1999; 177:423–427.
- Johnston PC, Lin YK, Walsh RM, et al. Factors associated with islet yield and insulin independence after total pancreatectomy and islet cell autotransplantation in patients with chronic pancreatitis utilizing off-site islet isolation: Cleveland Clinic experience. J Clin Endocrinol Metab 2015; 100:1765–1770.
- Dresler CM, Fortner JG, McDermott K, Bajorunas DR. Metabolic consequences of (regional) total pancreatectomy. Ann Surg 1991; 214:131–140.
- Duggan SN, O’Sullivan M, Hamilton S, Feehan SM, Ridgway PF, Conlon KC. Patients with chronic pancreatitis are at increased risk for osteoporosis. Pancreas 2012; 41:1119–1124.
- Rubin LG, Levin MJ, Ljungman P, et al; Infectious Diseases Society of America. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014; 58:e44–e100.
- Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet 2011; 378:86–97.
- Robertson RP, Raymond RH, Lee DS, et al; Beta Cell Project Team of the Foundation for the NIH Biomarkers Consortium. Arginine is preferred to glucagon for stimulation testing of beta-cell function. Am J Physiol Endocrinol Metab 2014; 307:E720–E727.
- Robertson RP, Bogachus LD, Oseid E, et al. Assessment of beta-cell mass and alpha- and beta-cell survival and function by arginine stimulation in human autologous islet recipients. Diabetes 2015; 64:565–572.
- Balzano G, Piemonti L. Autologous islet transplantation in patients requiring pancreatectomy for neoplasm. Curr Diab Rep 2014; 14:512.
- Naziruddin B, Iwahashi S, Kanak MA, Takita M, Itoh T, Levy MF. Evidence for instant blood-mediated inflammatory reaction in clinical autologous islet transplantation. Am J Transplant 2014; 14:428–437.
- Abdelli S, Ansite J, Roduit R, et al. Intracellular stress signaling pathways activated during human islet preparation and following acute cytokine exposure. Diabetes 2004; 53:2815–2823.
- Olsson R, Olerud J, Pettersson U, Carlsson PO. Increased numbers of low-oxygenated pancreatic islets after intraportal islet transplantation. Diabetes 2011; 60:2350–2353.
- Berman DM, O’Neil JJ, Coffey LC, et al. Long-term survival of nonhuman primate islets implanted in an omental pouch on a biodegradable scaffold. Am J Transplant 2009; 9:91–104.
- Sterkers A, Hubert T, Gmyr V, et al. Islet survival and function following intramuscular autotransplantation in the minipig. Am J Transplant 2013; 13:891–898.
- Maffi P, Balzano G, Ponzoni M, et al. Autologous pancreatic islet transplantation in human bone marrow. Diabetes 2013; 62:3523–3531.
- Bellin MD, Parazzoli S, Oseid E, et al. Defective glucagon secretion during hypoglycemia after intrahepatic but not nonhepatic islet autotransplantation. Am J Transplant 2014; 14:1880–1886.
KEY POINTS
- Chronic pancreatitis is caused by inflammation and results in progressive, irreversible loss of both exocrine and endocrine function.
- Total pancreatectomy with islet cell autotransplant is a definitive treatment for chronic pancreatitis, with most patients reporting less pain and better quality of life.
- Patients who have undergone this procedure need lifelong pancreatic enzyme replacement therapy along with surveillance for and treatment of diabetes.
- Research in this field is expanding our knowledge, from altered physiology to patient selection to improvement in islet yield and survival.