User login
Treatments for COVID-19: Update for hospitalists
Most patients with COVID-19 will have a mild presentation and not require hospitalization or any treatment. Inpatient management revolves around the supportive management of the most common complications of severe COVID-19, which includes pneumonia, hypoxemic respiratory failure, acute respiratory distress syndrome (ARDS), and septic shock.
Currently, there is no clinically proven specific antiviral treatment for COVID-19. A few antivirals and treatment modalities have been studied and used, with the hope of decreasing mortality and improving recovery time for those with moderate to severe cases of COVID-19.
Remdesivir
The antiviral remdesivir was the second drug to receive emergency use authorization by the Food and Drug Administration for the treatment of suspected or laboratory-confirmed COVID-19 in adults and children hospitalized with severe disease. Severe disease is defined as patients with an oxygen saturation less than 94% on room air or requiring supplemental oxygen or requiring mechanical ventilation or requiring extracorporeal membrane oxygenation (ECMO).
Remdesivir is a nucleotide analogue that has shown in vitro antiviral activity against a range of RNA viruses. It acts by causing premature termination of viral RNA transcription. Remdesivir is administered intravenously and the recommended dose is 200 mg on day 1, followed by 100 mg daily for various time courses.
A few clinical studies have reported benefits of remdesivir rather than no remdesivir for treatment of severe COVID-19 in hospitalized patients. The Infectious Diseases Society of America (IDSA) recommends 5 days of remdesivir in patients with severe COVID-19 on noninvasive supplemental oxygen and 10 days treatment for those on mechanical ventilation and ECMO. In a randomized, uncontrolled, phase 3 trial, investigators compared 5-day (n = 200) versus 10-day (n = 197) courses of remdesivir in patients with severe COVID-19. Clinical data revealed no differences in outcomes in the two groups.
Common reported adverse effects of the drug include elevated alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) and gastrointestinal symptoms including nausea, vomiting, and hematochezia. There is insufficient data on using remdesivir in patients requiring dialysis.
Corticosteroids
Is dexamethasone effective for treating COVID-19? In the early days of the COVID-19 pandemic, corticosteroids were not recommended with the fear that, if started too soon, you could blunt the body’s natural defense system and that could allow the virus to thrive. Recent clinical data has shown clinical benefits and decreased mortality with the use of dexamethasone in patients with severe COVID-19 infection because glucocorticoids may modulate inflammation-mediated lung injury and reduce progression to respiratory failure and death.
The Recovery Trial was an open label study which used 6-mg once-daily doses of dexamethasone for up to 10 days or until hospital discharge if sooner. The study concluded that the use of dexamethasone for up to 10 days in hospitalized patients with severe COVID-19 resulted in lower 28-day mortality than usual care.
Dexamethasone is recommended in COVID-19 patients who require supplemental oxygen. If dexamethasone is not available, alternative forms of steroids – prednisone, methylprednisolone, or hydrocortisone – can be used. However, there is no clear evidence that the use of other steroids provides the same benefit as dexamethasone.
Both the IDSA and National Institutes of Health guidelines have recommended the use of steroids. However, clinicians should closely monitor the adverse effects like hyperglycemia, secondary infections, psychiatric effects, and avascular necrosis.
Convalescent plasma
Convalescent plasma is a blood product believed to provide passive antibody therapy through the transmission of neutralizing viral antibodies. Convalescent plasma has been used for decades for different viral infections including the treatment of H1N1 influenza virus, polio, chicken pox, measles, SARS-CoV-1, and MERS-CoV.
On Aug. 23, 2020, the FDA issued an emergency use authorization for investigational convalescent plasma for the treatment of COVID-19 in hospitalized patients. The FDA recommends neutralizing antibodies of at least 1:160. However, such assays have not been widely available and titers in plasma have often not been assessed prior to infusion.
There is no current standard recommended dosing. Most study protocols infuse 1-2 units of convalescent plasma for persons with COVID-19.
There is insufficient data to recommend either for or against the use of convalescent plasma for the treatment of COVID-19. Existing data suggest that, if a benefit exists, convalescent plasma is most useful when given early and with a high titer of neutralizing antibodies.
The adverse effects of convalescent plasma is very similar to the receipt of other blood products, including allergic reactions to the plasma, transfusion-associated circulatory overload (TACO), transfusion-related acute lung injury (TRALI), and acquisition of infections, though the latter is rare because of the rigorous screening process.
Tocilizumab
Tocilizumab is a recombinant humanized monoclonal antibody that binds to interleukin (IL)-6 receptors. Tocilizumab is currently FDA approved for the treatment of severe or life-threatening cytokine release syndrome that is associated with chimeric antigen–receptor (CAR) T-cell therapy and for the treatment of rheumatologic disorders.
The interest in using tocilizumab to treat persons with COVID-19 is based on the observations that a subset of patients with COVID-19 develop a severe inflammatory response that can result in cytokine storm resulting in ARDS, multiorgan failure, and potentially death. Very high levels of IL-6 have been observed in these individuals, thereby suggesting IL-6 may play a central role in the acute clinical decompensation seen with severe COVID-19.
The optimal dosing of tocilizumab in patients with COVID-19 is not known. The FDA recommends dosing of tocilizumab for cytokine release syndrome should not exceed 800 mg. There is limited data about the potential benefit of tocilizumab in patients with COVID-19. The COVACTA trial showed no difference between tocilizumab and placebo in regard to mortality. The time to hospital discharge was shorter in patients treated with tocilizumab; however, the difference was not statistically significant.
Reported adverse effects of tocilizumab include increase in ALT and AST, increased risk of serious infections (especially tuberculosis and invasive fungal infections), reactivation of hepatitis B virus, and rare reports of gastrointestinal perforation.
Hydroxychloroquine
Hydroxycholoroquine (HCQ) and its sister drug chloroquine, have been used for many decades as treatment for malaria and autoimmune diseases. HCQ gained widespread popularity in the early days of the COVID-19 pandemic when clinical studies showed that it had significant in vitro activity against SARS-CoV-2, which provided the rationale for its use in the treatment and prevention of COVID-19 infection.
It was the first drug that was authorized for emergency use by the FDA during the COVID-19 pandemic. However, On June 15, 2020, because of accumulating harmful data, the FDA revoked the emergency authorization use of HCQ as a COVID-19 treatment.
Randomized controlled trials showed that patients treated with HCQ experienced a longer hospital stay with increase in mortality rates and increased likelihood of being placed on mechanical ventilation. In addition, studies revealed an increase in QT prolongation in patients treated with HCQ, especially when coadministered with azithromycin, which can lead to torsades de pointes, ventricular tachycardia, and sudden cardiac death.
The IDSA and National Institutes of Health, both recommend against the use of hydroxychloroquine with or without azithromycin to treat COVID-19 because the harms outweigh the benefits, even if high quality RCTs were to become available in the future.
Other drugs
There have been experimental studies on other medications for the treatment of COVID-19, including losartan, amlodipine, ivermectin, famotidine, Anakinra, Bruton’s tyrosine kinase inhibitors such as ibrutinib, and Janus kinase inhibitors, such as tofacitinib. Additionally, a few supplements such as vitamin C, vitamin D, and zinc have been used in both inpatient and outpatient settings for COVID-19 treatment. Polyclonal antibodies are being investigated in phase 3 trials. However, the data is insufficient, and the effectiveness of these drugs is unknown. The COVID-19 treatment guidelines panel recommends against the use of these treatment modalities.
Dr Tiyouh is an infectious diseases physician at Keystone Health in Chambersburg, Pa. Dr. Tenneti completed medical school at Vydehi Institute of Medical Sciences and Research Centre in Karnataka, India, and is interested in pursuing internal medicine residency. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg Hospital and Waynesboro (Pa.) Hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson, and a member of the editorial advisory board for The Hospitalist.
Sources
Goldman JD et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N Engl J Med. 2020 May 27. doi: 10.1056/NEJMoa2015301.
Beigel JH et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020 Oct 8. doi: 10.1056/NEJMoa2007764
Wang Y et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020 May 16;395(10236):1569-78.
National Institutes of Health. COVID-19 Treatment Guidelines.
Infectious Diseases Society of America. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19.
Joyner et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest. 2020;130(9):4791-7.
Luo P et al. Tocilizumab treatment in COVID-19: A single center experience. J Med Virol. 2020 Jul;92(7):814-8.
Centers for Disease Control and Prevention. Healthcare Workers: Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19).
University of Washington. COVID-19 Treatments: Prescribing Information, Clinical Studies, and Slide Decks.
Most patients with COVID-19 will have a mild presentation and not require hospitalization or any treatment. Inpatient management revolves around the supportive management of the most common complications of severe COVID-19, which includes pneumonia, hypoxemic respiratory failure, acute respiratory distress syndrome (ARDS), and septic shock.
Currently, there is no clinically proven specific antiviral treatment for COVID-19. A few antivirals and treatment modalities have been studied and used, with the hope of decreasing mortality and improving recovery time for those with moderate to severe cases of COVID-19.
Remdesivir
The antiviral remdesivir was the second drug to receive emergency use authorization by the Food and Drug Administration for the treatment of suspected or laboratory-confirmed COVID-19 in adults and children hospitalized with severe disease. Severe disease is defined as patients with an oxygen saturation less than 94% on room air or requiring supplemental oxygen or requiring mechanical ventilation or requiring extracorporeal membrane oxygenation (ECMO).
Remdesivir is a nucleotide analogue that has shown in vitro antiviral activity against a range of RNA viruses. It acts by causing premature termination of viral RNA transcription. Remdesivir is administered intravenously and the recommended dose is 200 mg on day 1, followed by 100 mg daily for various time courses.
A few clinical studies have reported benefits of remdesivir rather than no remdesivir for treatment of severe COVID-19 in hospitalized patients. The Infectious Diseases Society of America (IDSA) recommends 5 days of remdesivir in patients with severe COVID-19 on noninvasive supplemental oxygen and 10 days treatment for those on mechanical ventilation and ECMO. In a randomized, uncontrolled, phase 3 trial, investigators compared 5-day (n = 200) versus 10-day (n = 197) courses of remdesivir in patients with severe COVID-19. Clinical data revealed no differences in outcomes in the two groups.
Common reported adverse effects of the drug include elevated alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) and gastrointestinal symptoms including nausea, vomiting, and hematochezia. There is insufficient data on using remdesivir in patients requiring dialysis.
Corticosteroids
Is dexamethasone effective for treating COVID-19? In the early days of the COVID-19 pandemic, corticosteroids were not recommended with the fear that, if started too soon, you could blunt the body’s natural defense system and that could allow the virus to thrive. Recent clinical data has shown clinical benefits and decreased mortality with the use of dexamethasone in patients with severe COVID-19 infection because glucocorticoids may modulate inflammation-mediated lung injury and reduce progression to respiratory failure and death.
The Recovery Trial was an open label study which used 6-mg once-daily doses of dexamethasone for up to 10 days or until hospital discharge if sooner. The study concluded that the use of dexamethasone for up to 10 days in hospitalized patients with severe COVID-19 resulted in lower 28-day mortality than usual care.
Dexamethasone is recommended in COVID-19 patients who require supplemental oxygen. If dexamethasone is not available, alternative forms of steroids – prednisone, methylprednisolone, or hydrocortisone – can be used. However, there is no clear evidence that the use of other steroids provides the same benefit as dexamethasone.
Both the IDSA and National Institutes of Health guidelines have recommended the use of steroids. However, clinicians should closely monitor the adverse effects like hyperglycemia, secondary infections, psychiatric effects, and avascular necrosis.
Convalescent plasma
Convalescent plasma is a blood product believed to provide passive antibody therapy through the transmission of neutralizing viral antibodies. Convalescent plasma has been used for decades for different viral infections including the treatment of H1N1 influenza virus, polio, chicken pox, measles, SARS-CoV-1, and MERS-CoV.
On Aug. 23, 2020, the FDA issued an emergency use authorization for investigational convalescent plasma for the treatment of COVID-19 in hospitalized patients. The FDA recommends neutralizing antibodies of at least 1:160. However, such assays have not been widely available and titers in plasma have often not been assessed prior to infusion.
There is no current standard recommended dosing. Most study protocols infuse 1-2 units of convalescent plasma for persons with COVID-19.
There is insufficient data to recommend either for or against the use of convalescent plasma for the treatment of COVID-19. Existing data suggest that, if a benefit exists, convalescent plasma is most useful when given early and with a high titer of neutralizing antibodies.
The adverse effects of convalescent plasma is very similar to the receipt of other blood products, including allergic reactions to the plasma, transfusion-associated circulatory overload (TACO), transfusion-related acute lung injury (TRALI), and acquisition of infections, though the latter is rare because of the rigorous screening process.
Tocilizumab
Tocilizumab is a recombinant humanized monoclonal antibody that binds to interleukin (IL)-6 receptors. Tocilizumab is currently FDA approved for the treatment of severe or life-threatening cytokine release syndrome that is associated with chimeric antigen–receptor (CAR) T-cell therapy and for the treatment of rheumatologic disorders.
The interest in using tocilizumab to treat persons with COVID-19 is based on the observations that a subset of patients with COVID-19 develop a severe inflammatory response that can result in cytokine storm resulting in ARDS, multiorgan failure, and potentially death. Very high levels of IL-6 have been observed in these individuals, thereby suggesting IL-6 may play a central role in the acute clinical decompensation seen with severe COVID-19.
The optimal dosing of tocilizumab in patients with COVID-19 is not known. The FDA recommends dosing of tocilizumab for cytokine release syndrome should not exceed 800 mg. There is limited data about the potential benefit of tocilizumab in patients with COVID-19. The COVACTA trial showed no difference between tocilizumab and placebo in regard to mortality. The time to hospital discharge was shorter in patients treated with tocilizumab; however, the difference was not statistically significant.
Reported adverse effects of tocilizumab include increase in ALT and AST, increased risk of serious infections (especially tuberculosis and invasive fungal infections), reactivation of hepatitis B virus, and rare reports of gastrointestinal perforation.
Hydroxychloroquine
Hydroxycholoroquine (HCQ) and its sister drug chloroquine, have been used for many decades as treatment for malaria and autoimmune diseases. HCQ gained widespread popularity in the early days of the COVID-19 pandemic when clinical studies showed that it had significant in vitro activity against SARS-CoV-2, which provided the rationale for its use in the treatment and prevention of COVID-19 infection.
It was the first drug that was authorized for emergency use by the FDA during the COVID-19 pandemic. However, On June 15, 2020, because of accumulating harmful data, the FDA revoked the emergency authorization use of HCQ as a COVID-19 treatment.
Randomized controlled trials showed that patients treated with HCQ experienced a longer hospital stay with increase in mortality rates and increased likelihood of being placed on mechanical ventilation. In addition, studies revealed an increase in QT prolongation in patients treated with HCQ, especially when coadministered with azithromycin, which can lead to torsades de pointes, ventricular tachycardia, and sudden cardiac death.
The IDSA and National Institutes of Health, both recommend against the use of hydroxychloroquine with or without azithromycin to treat COVID-19 because the harms outweigh the benefits, even if high quality RCTs were to become available in the future.
Other drugs
There have been experimental studies on other medications for the treatment of COVID-19, including losartan, amlodipine, ivermectin, famotidine, Anakinra, Bruton’s tyrosine kinase inhibitors such as ibrutinib, and Janus kinase inhibitors, such as tofacitinib. Additionally, a few supplements such as vitamin C, vitamin D, and zinc have been used in both inpatient and outpatient settings for COVID-19 treatment. Polyclonal antibodies are being investigated in phase 3 trials. However, the data is insufficient, and the effectiveness of these drugs is unknown. The COVID-19 treatment guidelines panel recommends against the use of these treatment modalities.
Dr Tiyouh is an infectious diseases physician at Keystone Health in Chambersburg, Pa. Dr. Tenneti completed medical school at Vydehi Institute of Medical Sciences and Research Centre in Karnataka, India, and is interested in pursuing internal medicine residency. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg Hospital and Waynesboro (Pa.) Hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson, and a member of the editorial advisory board for The Hospitalist.
Sources
Goldman JD et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N Engl J Med. 2020 May 27. doi: 10.1056/NEJMoa2015301.
Beigel JH et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020 Oct 8. doi: 10.1056/NEJMoa2007764
Wang Y et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020 May 16;395(10236):1569-78.
National Institutes of Health. COVID-19 Treatment Guidelines.
Infectious Diseases Society of America. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19.
Joyner et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest. 2020;130(9):4791-7.
Luo P et al. Tocilizumab treatment in COVID-19: A single center experience. J Med Virol. 2020 Jul;92(7):814-8.
Centers for Disease Control and Prevention. Healthcare Workers: Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19).
University of Washington. COVID-19 Treatments: Prescribing Information, Clinical Studies, and Slide Decks.
Most patients with COVID-19 will have a mild presentation and not require hospitalization or any treatment. Inpatient management revolves around the supportive management of the most common complications of severe COVID-19, which includes pneumonia, hypoxemic respiratory failure, acute respiratory distress syndrome (ARDS), and septic shock.
Currently, there is no clinically proven specific antiviral treatment for COVID-19. A few antivirals and treatment modalities have been studied and used, with the hope of decreasing mortality and improving recovery time for those with moderate to severe cases of COVID-19.
Remdesivir
The antiviral remdesivir was the second drug to receive emergency use authorization by the Food and Drug Administration for the treatment of suspected or laboratory-confirmed COVID-19 in adults and children hospitalized with severe disease. Severe disease is defined as patients with an oxygen saturation less than 94% on room air or requiring supplemental oxygen or requiring mechanical ventilation or requiring extracorporeal membrane oxygenation (ECMO).
Remdesivir is a nucleotide analogue that has shown in vitro antiviral activity against a range of RNA viruses. It acts by causing premature termination of viral RNA transcription. Remdesivir is administered intravenously and the recommended dose is 200 mg on day 1, followed by 100 mg daily for various time courses.
A few clinical studies have reported benefits of remdesivir rather than no remdesivir for treatment of severe COVID-19 in hospitalized patients. The Infectious Diseases Society of America (IDSA) recommends 5 days of remdesivir in patients with severe COVID-19 on noninvasive supplemental oxygen and 10 days treatment for those on mechanical ventilation and ECMO. In a randomized, uncontrolled, phase 3 trial, investigators compared 5-day (n = 200) versus 10-day (n = 197) courses of remdesivir in patients with severe COVID-19. Clinical data revealed no differences in outcomes in the two groups.
Common reported adverse effects of the drug include elevated alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) and gastrointestinal symptoms including nausea, vomiting, and hematochezia. There is insufficient data on using remdesivir in patients requiring dialysis.
Corticosteroids
Is dexamethasone effective for treating COVID-19? In the early days of the COVID-19 pandemic, corticosteroids were not recommended with the fear that, if started too soon, you could blunt the body’s natural defense system and that could allow the virus to thrive. Recent clinical data has shown clinical benefits and decreased mortality with the use of dexamethasone in patients with severe COVID-19 infection because glucocorticoids may modulate inflammation-mediated lung injury and reduce progression to respiratory failure and death.
The Recovery Trial was an open label study which used 6-mg once-daily doses of dexamethasone for up to 10 days or until hospital discharge if sooner. The study concluded that the use of dexamethasone for up to 10 days in hospitalized patients with severe COVID-19 resulted in lower 28-day mortality than usual care.
Dexamethasone is recommended in COVID-19 patients who require supplemental oxygen. If dexamethasone is not available, alternative forms of steroids – prednisone, methylprednisolone, or hydrocortisone – can be used. However, there is no clear evidence that the use of other steroids provides the same benefit as dexamethasone.
Both the IDSA and National Institutes of Health guidelines have recommended the use of steroids. However, clinicians should closely monitor the adverse effects like hyperglycemia, secondary infections, psychiatric effects, and avascular necrosis.
Convalescent plasma
Convalescent plasma is a blood product believed to provide passive antibody therapy through the transmission of neutralizing viral antibodies. Convalescent plasma has been used for decades for different viral infections including the treatment of H1N1 influenza virus, polio, chicken pox, measles, SARS-CoV-1, and MERS-CoV.
On Aug. 23, 2020, the FDA issued an emergency use authorization for investigational convalescent plasma for the treatment of COVID-19 in hospitalized patients. The FDA recommends neutralizing antibodies of at least 1:160. However, such assays have not been widely available and titers in plasma have often not been assessed prior to infusion.
There is no current standard recommended dosing. Most study protocols infuse 1-2 units of convalescent plasma for persons with COVID-19.
There is insufficient data to recommend either for or against the use of convalescent plasma for the treatment of COVID-19. Existing data suggest that, if a benefit exists, convalescent plasma is most useful when given early and with a high titer of neutralizing antibodies.
The adverse effects of convalescent plasma is very similar to the receipt of other blood products, including allergic reactions to the plasma, transfusion-associated circulatory overload (TACO), transfusion-related acute lung injury (TRALI), and acquisition of infections, though the latter is rare because of the rigorous screening process.
Tocilizumab
Tocilizumab is a recombinant humanized monoclonal antibody that binds to interleukin (IL)-6 receptors. Tocilizumab is currently FDA approved for the treatment of severe or life-threatening cytokine release syndrome that is associated with chimeric antigen–receptor (CAR) T-cell therapy and for the treatment of rheumatologic disorders.
The interest in using tocilizumab to treat persons with COVID-19 is based on the observations that a subset of patients with COVID-19 develop a severe inflammatory response that can result in cytokine storm resulting in ARDS, multiorgan failure, and potentially death. Very high levels of IL-6 have been observed in these individuals, thereby suggesting IL-6 may play a central role in the acute clinical decompensation seen with severe COVID-19.
The optimal dosing of tocilizumab in patients with COVID-19 is not known. The FDA recommends dosing of tocilizumab for cytokine release syndrome should not exceed 800 mg. There is limited data about the potential benefit of tocilizumab in patients with COVID-19. The COVACTA trial showed no difference between tocilizumab and placebo in regard to mortality. The time to hospital discharge was shorter in patients treated with tocilizumab; however, the difference was not statistically significant.
Reported adverse effects of tocilizumab include increase in ALT and AST, increased risk of serious infections (especially tuberculosis and invasive fungal infections), reactivation of hepatitis B virus, and rare reports of gastrointestinal perforation.
Hydroxychloroquine
Hydroxycholoroquine (HCQ) and its sister drug chloroquine, have been used for many decades as treatment for malaria and autoimmune diseases. HCQ gained widespread popularity in the early days of the COVID-19 pandemic when clinical studies showed that it had significant in vitro activity against SARS-CoV-2, which provided the rationale for its use in the treatment and prevention of COVID-19 infection.
It was the first drug that was authorized for emergency use by the FDA during the COVID-19 pandemic. However, On June 15, 2020, because of accumulating harmful data, the FDA revoked the emergency authorization use of HCQ as a COVID-19 treatment.
Randomized controlled trials showed that patients treated with HCQ experienced a longer hospital stay with increase in mortality rates and increased likelihood of being placed on mechanical ventilation. In addition, studies revealed an increase in QT prolongation in patients treated with HCQ, especially when coadministered with azithromycin, which can lead to torsades de pointes, ventricular tachycardia, and sudden cardiac death.
The IDSA and National Institutes of Health, both recommend against the use of hydroxychloroquine with or without azithromycin to treat COVID-19 because the harms outweigh the benefits, even if high quality RCTs were to become available in the future.
Other drugs
There have been experimental studies on other medications for the treatment of COVID-19, including losartan, amlodipine, ivermectin, famotidine, Anakinra, Bruton’s tyrosine kinase inhibitors such as ibrutinib, and Janus kinase inhibitors, such as tofacitinib. Additionally, a few supplements such as vitamin C, vitamin D, and zinc have been used in both inpatient and outpatient settings for COVID-19 treatment. Polyclonal antibodies are being investigated in phase 3 trials. However, the data is insufficient, and the effectiveness of these drugs is unknown. The COVID-19 treatment guidelines panel recommends against the use of these treatment modalities.
Dr Tiyouh is an infectious diseases physician at Keystone Health in Chambersburg, Pa. Dr. Tenneti completed medical school at Vydehi Institute of Medical Sciences and Research Centre in Karnataka, India, and is interested in pursuing internal medicine residency. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg Hospital and Waynesboro (Pa.) Hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson, and a member of the editorial advisory board for The Hospitalist.
Sources
Goldman JD et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N Engl J Med. 2020 May 27. doi: 10.1056/NEJMoa2015301.
Beigel JH et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020 Oct 8. doi: 10.1056/NEJMoa2007764
Wang Y et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020 May 16;395(10236):1569-78.
National Institutes of Health. COVID-19 Treatment Guidelines.
Infectious Diseases Society of America. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19.
Joyner et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest. 2020;130(9):4791-7.
Luo P et al. Tocilizumab treatment in COVID-19: A single center experience. J Med Virol. 2020 Jul;92(7):814-8.
Centers for Disease Control and Prevention. Healthcare Workers: Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19).
University of Washington. COVID-19 Treatments: Prescribing Information, Clinical Studies, and Slide Decks.
Maskomania: Masks and COVID-19
A comprehensive review
On April 3, the Centers for Disease Control and Prevention issued an advisory that the general public wear cloth face masks when outside, particularly those residing in areas with significant severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) community transmission.1 Recent research reveals several factors related to the nature of the virus as well as the epidemiologic spread of the illness that may have led to this decision.
However, controversy still prevails whether this recommendation will alleviate or aggravate disease progression. With many hospitals across America lacking sufficient personal protective equipment (PPE) and scrambling for supplies, universal masking may create more chaos, especially with certain states imposing monetary fines on individuals spotted outdoors without a mask. With new information being discovered each day about COVID-19, it is more imperative than ever to update existing strategies and formulate more effective methods to flatten the curve.
Airborne vs. droplet transmission
According to a scientific brief released by the World Health Organization, there have been studies with mixed evidence and opinions regarding the presence of COVID-19 ribonucleic acid (RNA) in air samples.2 In medRxiv, Santarpia et al., from the University of Nebraska Medical Center, Omaha, detected viral RNA in samples taken from beneath a patient’s bed and from a window ledge, both areas in which neither the patient nor health care personnel had any direct contact. They also found that 66.7% of air samples taken from a hospital hallway carried virus-containing particles.3 It is worth noting that certain aerosol-generating procedures (AGP) may increase the likelihood of airborne dissemination. Whether airborne transmission is a major mode of COVID-19 spread in the community and routine clinical settings (with no aerosol-generating procedures) is still a debatable question without a definitive answer.
We should consider the epidemiology of COVID-19 thus far in the pandemic to determine if transmission patterns are more consistent with that of other common respiratory viral pathogens or more consistent with that of the agents we classically consider to be transmitted by the airborne route (measles, varicella zoster virus, and Mycobacterium tuberculosis). The attack rates in various settings (household, health care, and the public) as well as the expected number of secondary cases from a single infected individual in a susceptible population (R0) are more consistent with those of a droplet spread pathogen.
For measles, the R0 is 12-18, and the secondary household attack rates are ≥ 90%. In case of the varicella zoster virus, the R0 is ~10, and the secondary household attack rate is 85%. The R0 for pulmonary tuberculosis is up to 10 (per year) and the secondary household attack rate has been reported to be >50%. With COVID-19, the R0 appears to be around 2.5-3 and secondary household attack rates are ~ 10% from data available so far, similar to that of influenza viruses. This discrepancy suggests that droplet transmission may be more likely. The dichotomy of airborne versus droplet mode of spread may be better described as a continuum, as pointed out in a recent article in the JAMA. Infectious droplets form turbulent gas clouds allowing the virus particles to travel further and remain in the air longer.4 The necessary precautions for an airborne illness should be chosen over droplet precautions, especially when there is concern for an AGP.
Universal masking: Risks and benefits
The idea of universal masking has been debated extensively since the initial stages of the COVID-19 pandemic. According to public health authorities, significant exposure is defined as “face-to-face contact within 6 feet with a patient with symptomatic COVID-19” in the range of a few minutes up to 30 minutes.5 The researchers wrote in the New England Journal of Medicine that the chance of catching COVID-19 from a passing interaction in a public space is therefore minimal, and it may seem unnecessary to wear a mask at all times in public.
As reported in Science, randomized clinical studies performed on other viruses in the past have shown no added protection conferred by wearing a mask, though small sample sizes and noncompliance are limiting factors to their validity.6 On the contrary, mask wearing has been enforced in many parts of Asia, including Hong Kong and Singapore with promising results.5 Leung et al. stated in The Lancet that the lack of proof that masks are effective should not rule them as ineffective. Also, universal masking would reduce the stigma around symptomatic individuals covering their faces. It has become a cultural phenomenon in many southeast Asian countries and has been cited as one of the reasons for relatively successful containment in Singapore, South Korea, and Taiwan. The most important benefit of universal masking is protection attained by preventing spread from asymptomatic, mildly symptomatic, and presymptomatic carriers.7
In a study in the New England Journal of Medicine that estimated viral loads during various stages of COVID-19, researchers found that asymptomatic patients had similar viral loads to symptomatic patients, thereby suggesting high potential for transmission.8 Furthermore, numerous cases are being reported concerning the spread of illness from asymptomatic carriers.9-12 In an outbreak at a skilled nursing facility in Washington outlined in MMWR, 13 of 23 residents with positive test results were asymptomatic at the time of testing, and of those, 3 never developed any symptoms.12
Many hospitals are now embracing the policy of universal masking. A mask is a critical component of the personal protective equipment (PPE) clinicians need when caring for symptomatic patients with respiratory viral infections, in conjunction with a gown, gloves, and eye protection. Masking in this context is already part of routine operations in most hospitals. There are two scenarios in which there may be possible benefits. One scenario is the lower likelihood of transmission from asymptomatic and minimally symptomatic health care workers with COVID-19 to other providers and patients. The other less plausible benefit of universal masking among health care workers is that it may provide some protection in the possibility of caring for an unrecognized COVID-19 patient. However, universal masking should be coupled with other favorable practices like temperature checks and symptom screening on a daily basis to avail the maximum benefit from masking. Despite varied opinions on the outcomes of universal masking, this measure helps improve health care workers’ safety, psychological well-being, trust in their hospital, and decreases anxiety of acquiring the illness.
Efficacy of various types of masks
With the possibility of airborne transmission of the virus, are cloth masks as recommended by the CDC truly helpful in preventing infection? A study in the Journal of Medical Virology demonstrates 99.98%, 97.14%, and 95.15% efficacy for N95, surgical, and homemade masks, respectively, in blocking the avian influenza virus (comparable to coronavirus in size and physical characteristics). The homemade mask was created using one layer of polyester cloth and a four-layered kitchen filter paper.13
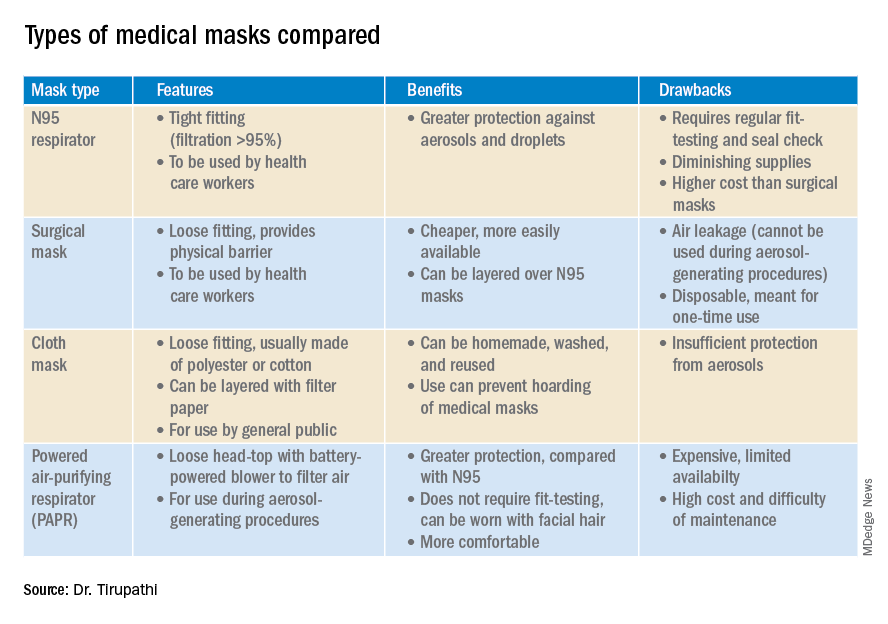
N95 masks (equivalent to FFP/P2 in European countries) are made of electrostatically charged polypropylene microfibers designed to filter particles measuring 100-300nm in diameter with 95% efficacy. A single SARS-CoV-2 molecule measures 125 nm approximately. N99 (FFP3) and N100 (P3) masks are also available, though not as widely used, with 99% and 99.7% efficacy respectively for the same size range. Though cloth masks are the clear-cut last resort for medical professionals, a few studies state no clinically proven difference in protection between surgical masks and N95 respirators.14,15 Even aerosolized droplets (< 5 mcm) were found to be blocked by surgical masks in a Nature Medicine study in which 4/10 subjects tested positive for coronavirus in exhaled breath samples without masks and 0/10 subjects with masks.16
On the contrary, an Annals of Internal Medicine study of four COVID-19 positive subjects that “neither surgical masks nor cloth masks effectively filtered SARS-CoV-2 during coughs of infected patients.” In fact, more contamination was found on the outer surface of the masks when compared to the inner surface, probably owing to the masks’ aerodynamic properties.17 Because of limitations present in the above-mentioned studies, further research is necessary to conclusively determine which types of masks are efficacious in preventing infection by the virus. In a scarcity of surgical masks and respirators for health care personnel, suboptimal masks can be of some use provided there is adherent use, minimal donning and doffing, and it is to be accompanied by adequate hand washing practices.14
In case of severe infections with high viral loads or patients undergoing aerosol-generating procedures, powered air-purifying respirators (PAPRs) also are advisable as they confer greater protection than N95 respirators, according to a study in the Annals of Work Exposures and Health. Despite being more comfortable for long-term use and accommodative of facial hair, their use is limited because of high cost and difficult maintenance.18 3-D printing also is being used to combat the current shortage of masks worldwide. However, a study from the International Journal of Oral & Maxillofacial Surgery reported that virologic testing for leakage between the two reusable components and contamination of the components themselves after one or multiple disinfection cycles is essential before application in real-life situations.19
Ongoing issues
WHO estimates a monthly requirement of nearly 90 million masks exclusively for health care workers to protect themselves against COVID-19.20 In spite of increasing the production rate by 40%, if the general public hoards masks and respirators, the results could be disastrous. Personal protective equipment is currently at 100 times the usual demand and 20 times the usual cost, with stocks backlogged by 4-6 months. The appropriate order of priority in distribution to health care professionals first, followed by those caring for infected patients is critical.20 In a survey conducted by the Association for Professionals in Infection Control and Epidemiology, results revealed that 48% of the U.S. health care facilities that responded were either out or nearly out of respirators as of March 25. 21
The gravest risk behind the universal masking policy is the likely depletion of medical resources.22 A possible solution to this issue could be to modify the policy to stagger the requirement based on the severity of community transmission in that area of residence. In the article appropriately titled “Rational use of face masks in the COVID-19 pandemic” published in The Lancet Respiratory Medicine, researchers described how the Chinese population was classified into moderate, low, and very-low risk of infection categories and advised to wear a surgical or disposable mask, disposable mask, and no mask respectively.23 This curbs widespread panic and eagerness by the general public to stock up on essential medical equipment when it may not even be necessary.
Reuse, extended use, and sterilization
Several studies have been conducted to identify the viability of the COVID-19 on various surfaces.24-25 The CDC and National Institute for Occupational Safety and Health (NIOSH) guidelines state that an N95 respirator can be used up to 8 hours with intermittent or continuous use, though this number is not fixed and heavily depends upon the extent of exposure, risk of contamination, and frequency of donning and doffing26,27. Though traditionally meant for single-time usage, after 8 hours, the mask can be decontaminated and reused. The CDC defines extended use as the “practice of wearing the same N95 respirator for repeated close-contact encounters with several patients, without removing the respirator between patient encounters.” Reuse is defined as “using the same N95 respirator for multiple encounters with patients but removing it (‘doffing’) after each encounter. The respirator is stored in between encounters to be put on again (‘donned’) prior to the next encounter with a patient.”
It has been established that extended use is more advisable than reuse given the lower risk of self-inoculation. Furthermore, health care professionals are urged to wear a cleanable face shield or disposable mask over the respirator to minimize contamination and practice diligent hand hygiene before and after handling the respirator. N95 respirators are to be discarded following aerosol-generating procedures or if they come in contact with blood, respiratory secretions, or bodily fluids. They should also be discarded in case of close contact with an infected patient or if they cause breathing difficulties to the wearer.27 This may not always be possible given the unprecedented shortage of PPE, hence decontamination techniques and repurposing are the need of the hour.
In Anesthesia & Analgesia, Naveen Nathan, MD, of Northwestern University, Chicago, recommends recycling four masks in a series, using one per day, keeping the mask in a dry, clean environment, and then repeating use of the first mask on the 5th day, the second on the 6th day, and so forth. This ensures clearance of the virus particles by the next use. Alternatively, respirators can be sterilized between uses by heating to 70º C (158º F) for 30 minutes. Liquid disinfectants such as alcohol and bleach as well as ultraviolet rays in sunlight tend to damage masks.28 Steam sterilization is the most commonly utilized technique in hospitals. Other methods, described by the N95/PPE Working Group, report include gamma irradiation at 20kGy (2MRad) for large-scale sterilization (though the facilities may not be widely available), vaporized hydrogen peroxide, ozone decontamination, ultraviolet germicidal irradiation, and ethylene oxide.29 Though a discussion on various considerations of decontamination techniques is out of the scope of this article, detailed guidelines have been published by the CDC30 and the COVID-19 Healthcare Coalition.30
Conclusion
A recent startling discovery reported on in Emerging Infectious Diseases suggests that the basic COVID-19 reproductive number (R0) is actually much higher than previously thought. Using expanded data, updated epidemiologic parameters, and the current outbreak dynamics in Wuhan, the team came to the conclusion that the R0 for the novel coronavirus is actually 5.7 (95% CI 3.8-8.9), compared with an initial estimate of 2.2-2.7.31 Concern for transmissibility demands heightened prevention strategies until more data evolves. The latest recommendation by the CDC regarding cloth masking in the public may help slow the progression of the pandemic. However, it is of paramount importance to keep in mind that masks alone are not enough to control the disease and must be coupled with other nonpharmacologic interventions such as social distancing, quarantining/isolation, and diligent hand hygiene.
Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Bharathidasan is a recent medical graduate from India with an interest in public health and community research; she plans to pursue residency training in the United States. Ms. Freshman is currently the regional director of infection prevention for WellSpan Health and has 35 years of experience in nursing. Dr. Palabindala is the medical director, utilization management and physician advisory services, at the University of Mississippi Medical Center, Jackson. He is an associate professor of medicine and academic hospitalist in the UMMC School of Medicine.
References
1. Centers for Disease Control and Prevention. Recommendation regarding the use of cloth face coverings.
2. World Health Organization. Modes of transmission of virus causing COVID-19 : implications for IPC precaution recommendations. Sci Br. 2020 Mar 29:1-3.
3. Santarpia JL et al. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. 2020 Mar 26. medRxiv. 2020;2020.03.23.20039446.
4. Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: Potential implications for reducing transmission of COVID-19. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4756.
5. Klompas M et al. Universal masking in hospitals in the Covid-19 era. N Engl J Med. 2020 Apr 1. doi: 10.1056/NEJMp2006372.
6. Servick K. Would everyone wearing face masks help us slow the pandemic? Science 2020 Mar 28. doi: 10.1126/science.abb9371.
7. Leung CC et al. Mass masking in the COVID-19 epidemic: People need guidance. Lancet 2020 Mar 21;395(10228):945. doi: 10.1016/S0140-6736(20)30520-1.
8. Zou L et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020 Mar 19;382(12):1177-9.
9. Pan X et al. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis. 2020 Apr;20(4):410-1.
10. Bai Y et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020 Feb 21;323(14):1406-7.
11. Wei WE et al. Presymptomatic transmission of SARS-CoV-2 – Singapore, Jan. 23–March 16, 2020. MMWR Morb Mortal Wkly Rep 2020;69:411-5.
12. Kimball A et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility – King County, Washington, March 2020. 2020 Apr 3. MMWR Morb Mortal Wkly Rep 2020;69:377-81.
13. Ma Q-X et al. Potential utilities of mask wearing and instant hand hygiene for fighting SARS-CoV-2. J Med Virol. 2020 Mar 31;10.1002/jmv.25805. doi: 10.1002/jmv.25805.
14. Abd-Elsayed A et al. Utility of substandard face mask options for health care workers during the COVID-19 pandemic. Anesth Analg. 2020 Mar 31;10.1213/ANE.0000000000004841. doi: 10.1213/ANE.0000000000004841.
15. Long Y et al. Effectiveness of N95 respirators versus surgical masks against influenza: A systematic review and meta-analysis. J Evid Based Med. 2020 Mar 13;10.1111/jebm.12381. doi: 10.1111/jebm.12381.
16. Leung NHL et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020 May;26(5):676-80.
17. Bae S et al. Effectiveness of surgical and cotton masks in blocking SARS-CoV-2: A controlled comparison in 4 patients. Ann Intern Med. 2020 Apr 6;M20-1342. doi: 10.7326/M20-1342.
18. Brosseau LM. Are powered air purifying respirators a solution for protecting healthcare workers from emerging aerosol-transmissible diseases? Ann Work Expo Health. 2020 Apr 30;64(4):339-41.
19. Swennen GRJ et al. Custom-made 3D-printed face masks in case of pandemic crisis situations with a lack of commercially available FFP2/3 masks. Int J Oral Maxillofac Surg. 2020 May;49(5):673-7.
20. Mahase E. Coronavirus: Global stocks of protective gear are depleted, with demand at “100 times” normal level, WHO warns. BMJ. 2020 Feb 10;368:m543. doi: 10.1136/bmj.m543.
21. National survey shows dire shortages of PPE, hand sanitizer across the U.S. 2020 Mar 27. Association for Professionals in Infection Control and Epidemiology (APIC) press briefing.
22. Wu HL et al. Facemask shortage and the novel coronavirus disease (COVID-19) outbreak: Reflections on public health measures. EClinicalMedicine. 2020 Apr 3:100329. doi: 10.1016/j.eclinm.2020.100329.
23. Feng S et al. Rational use of face masks in the COVID-19 pandemic. Lancet Respir Med. 2020 May;8(5):434-6.
24. Chin AWH et al. Stability of SARS-CoV-2 in different environmental. The Lancet Microbe. 2020 May 1;5247(20):2004973. doi. org/10.1016/S2666-5247(20)30003-3.
25. van Doremalen N et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020 Apr 16;382(16):1564-7.
26. NIOSH – Workplace Safety and Health Topics: Recommended guidance for extended use and limited reuse of n95 filtering facepiece respirators in healthcare settings.
27. Centers for Disease Control and Prevention. COVID-19 decontamination and reuse of filtering facepiece respirators. 2020 Apr 15.
28. Nathan N. Waste not, want not: The re-usability of N95 masks. Anesth Analg. 2020 Mar 31.doi: 10.1213/ane.0000000000004843.
29. European Centre for Disease Prevention and Control technical report. Cloth masks and mask sterilisation as options in case of shortage of surgical masks and respirators. 2020 Mar.
30. N95/PPE Working Group report. Evaluation of decontamination techniques for the reuse of N95 respirators. 2020 Apr 3;2:1-7.
31. Sanche Set al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020 Jul. doi. org/10.3201/eid2607.200282.
A comprehensive review
A comprehensive review
On April 3, the Centers for Disease Control and Prevention issued an advisory that the general public wear cloth face masks when outside, particularly those residing in areas with significant severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) community transmission.1 Recent research reveals several factors related to the nature of the virus as well as the epidemiologic spread of the illness that may have led to this decision.
However, controversy still prevails whether this recommendation will alleviate or aggravate disease progression. With many hospitals across America lacking sufficient personal protective equipment (PPE) and scrambling for supplies, universal masking may create more chaos, especially with certain states imposing monetary fines on individuals spotted outdoors without a mask. With new information being discovered each day about COVID-19, it is more imperative than ever to update existing strategies and formulate more effective methods to flatten the curve.
Airborne vs. droplet transmission
According to a scientific brief released by the World Health Organization, there have been studies with mixed evidence and opinions regarding the presence of COVID-19 ribonucleic acid (RNA) in air samples.2 In medRxiv, Santarpia et al., from the University of Nebraska Medical Center, Omaha, detected viral RNA in samples taken from beneath a patient’s bed and from a window ledge, both areas in which neither the patient nor health care personnel had any direct contact. They also found that 66.7% of air samples taken from a hospital hallway carried virus-containing particles.3 It is worth noting that certain aerosol-generating procedures (AGP) may increase the likelihood of airborne dissemination. Whether airborne transmission is a major mode of COVID-19 spread in the community and routine clinical settings (with no aerosol-generating procedures) is still a debatable question without a definitive answer.
We should consider the epidemiology of COVID-19 thus far in the pandemic to determine if transmission patterns are more consistent with that of other common respiratory viral pathogens or more consistent with that of the agents we classically consider to be transmitted by the airborne route (measles, varicella zoster virus, and Mycobacterium tuberculosis). The attack rates in various settings (household, health care, and the public) as well as the expected number of secondary cases from a single infected individual in a susceptible population (R0) are more consistent with those of a droplet spread pathogen.
For measles, the R0 is 12-18, and the secondary household attack rates are ≥ 90%. In case of the varicella zoster virus, the R0 is ~10, and the secondary household attack rate is 85%. The R0 for pulmonary tuberculosis is up to 10 (per year) and the secondary household attack rate has been reported to be >50%. With COVID-19, the R0 appears to be around 2.5-3 and secondary household attack rates are ~ 10% from data available so far, similar to that of influenza viruses. This discrepancy suggests that droplet transmission may be more likely. The dichotomy of airborne versus droplet mode of spread may be better described as a continuum, as pointed out in a recent article in the JAMA. Infectious droplets form turbulent gas clouds allowing the virus particles to travel further and remain in the air longer.4 The necessary precautions for an airborne illness should be chosen over droplet precautions, especially when there is concern for an AGP.
Universal masking: Risks and benefits
The idea of universal masking has been debated extensively since the initial stages of the COVID-19 pandemic. According to public health authorities, significant exposure is defined as “face-to-face contact within 6 feet with a patient with symptomatic COVID-19” in the range of a few minutes up to 30 minutes.5 The researchers wrote in the New England Journal of Medicine that the chance of catching COVID-19 from a passing interaction in a public space is therefore minimal, and it may seem unnecessary to wear a mask at all times in public.
As reported in Science, randomized clinical studies performed on other viruses in the past have shown no added protection conferred by wearing a mask, though small sample sizes and noncompliance are limiting factors to their validity.6 On the contrary, mask wearing has been enforced in many parts of Asia, including Hong Kong and Singapore with promising results.5 Leung et al. stated in The Lancet that the lack of proof that masks are effective should not rule them as ineffective. Also, universal masking would reduce the stigma around symptomatic individuals covering their faces. It has become a cultural phenomenon in many southeast Asian countries and has been cited as one of the reasons for relatively successful containment in Singapore, South Korea, and Taiwan. The most important benefit of universal masking is protection attained by preventing spread from asymptomatic, mildly symptomatic, and presymptomatic carriers.7
In a study in the New England Journal of Medicine that estimated viral loads during various stages of COVID-19, researchers found that asymptomatic patients had similar viral loads to symptomatic patients, thereby suggesting high potential for transmission.8 Furthermore, numerous cases are being reported concerning the spread of illness from asymptomatic carriers.9-12 In an outbreak at a skilled nursing facility in Washington outlined in MMWR, 13 of 23 residents with positive test results were asymptomatic at the time of testing, and of those, 3 never developed any symptoms.12
Many hospitals are now embracing the policy of universal masking. A mask is a critical component of the personal protective equipment (PPE) clinicians need when caring for symptomatic patients with respiratory viral infections, in conjunction with a gown, gloves, and eye protection. Masking in this context is already part of routine operations in most hospitals. There are two scenarios in which there may be possible benefits. One scenario is the lower likelihood of transmission from asymptomatic and minimally symptomatic health care workers with COVID-19 to other providers and patients. The other less plausible benefit of universal masking among health care workers is that it may provide some protection in the possibility of caring for an unrecognized COVID-19 patient. However, universal masking should be coupled with other favorable practices like temperature checks and symptom screening on a daily basis to avail the maximum benefit from masking. Despite varied opinions on the outcomes of universal masking, this measure helps improve health care workers’ safety, psychological well-being, trust in their hospital, and decreases anxiety of acquiring the illness.
Efficacy of various types of masks
With the possibility of airborne transmission of the virus, are cloth masks as recommended by the CDC truly helpful in preventing infection? A study in the Journal of Medical Virology demonstrates 99.98%, 97.14%, and 95.15% efficacy for N95, surgical, and homemade masks, respectively, in blocking the avian influenza virus (comparable to coronavirus in size and physical characteristics). The homemade mask was created using one layer of polyester cloth and a four-layered kitchen filter paper.13

N95 masks (equivalent to FFP/P2 in European countries) are made of electrostatically charged polypropylene microfibers designed to filter particles measuring 100-300nm in diameter with 95% efficacy. A single SARS-CoV-2 molecule measures 125 nm approximately. N99 (FFP3) and N100 (P3) masks are also available, though not as widely used, with 99% and 99.7% efficacy respectively for the same size range. Though cloth masks are the clear-cut last resort for medical professionals, a few studies state no clinically proven difference in protection between surgical masks and N95 respirators.14,15 Even aerosolized droplets (< 5 mcm) were found to be blocked by surgical masks in a Nature Medicine study in which 4/10 subjects tested positive for coronavirus in exhaled breath samples without masks and 0/10 subjects with masks.16
On the contrary, an Annals of Internal Medicine study of four COVID-19 positive subjects that “neither surgical masks nor cloth masks effectively filtered SARS-CoV-2 during coughs of infected patients.” In fact, more contamination was found on the outer surface of the masks when compared to the inner surface, probably owing to the masks’ aerodynamic properties.17 Because of limitations present in the above-mentioned studies, further research is necessary to conclusively determine which types of masks are efficacious in preventing infection by the virus. In a scarcity of surgical masks and respirators for health care personnel, suboptimal masks can be of some use provided there is adherent use, minimal donning and doffing, and it is to be accompanied by adequate hand washing practices.14
In case of severe infections with high viral loads or patients undergoing aerosol-generating procedures, powered air-purifying respirators (PAPRs) also are advisable as they confer greater protection than N95 respirators, according to a study in the Annals of Work Exposures and Health. Despite being more comfortable for long-term use and accommodative of facial hair, their use is limited because of high cost and difficult maintenance.18 3-D printing also is being used to combat the current shortage of masks worldwide. However, a study from the International Journal of Oral & Maxillofacial Surgery reported that virologic testing for leakage between the two reusable components and contamination of the components themselves after one or multiple disinfection cycles is essential before application in real-life situations.19
Ongoing issues
WHO estimates a monthly requirement of nearly 90 million masks exclusively for health care workers to protect themselves against COVID-19.20 In spite of increasing the production rate by 40%, if the general public hoards masks and respirators, the results could be disastrous. Personal protective equipment is currently at 100 times the usual demand and 20 times the usual cost, with stocks backlogged by 4-6 months. The appropriate order of priority in distribution to health care professionals first, followed by those caring for infected patients is critical.20 In a survey conducted by the Association for Professionals in Infection Control and Epidemiology, results revealed that 48% of the U.S. health care facilities that responded were either out or nearly out of respirators as of March 25. 21
The gravest risk behind the universal masking policy is the likely depletion of medical resources.22 A possible solution to this issue could be to modify the policy to stagger the requirement based on the severity of community transmission in that area of residence. In the article appropriately titled “Rational use of face masks in the COVID-19 pandemic” published in The Lancet Respiratory Medicine, researchers described how the Chinese population was classified into moderate, low, and very-low risk of infection categories and advised to wear a surgical or disposable mask, disposable mask, and no mask respectively.23 This curbs widespread panic and eagerness by the general public to stock up on essential medical equipment when it may not even be necessary.
Reuse, extended use, and sterilization
Several studies have been conducted to identify the viability of the COVID-19 on various surfaces.24-25 The CDC and National Institute for Occupational Safety and Health (NIOSH) guidelines state that an N95 respirator can be used up to 8 hours with intermittent or continuous use, though this number is not fixed and heavily depends upon the extent of exposure, risk of contamination, and frequency of donning and doffing26,27. Though traditionally meant for single-time usage, after 8 hours, the mask can be decontaminated and reused. The CDC defines extended use as the “practice of wearing the same N95 respirator for repeated close-contact encounters with several patients, without removing the respirator between patient encounters.” Reuse is defined as “using the same N95 respirator for multiple encounters with patients but removing it (‘doffing’) after each encounter. The respirator is stored in between encounters to be put on again (‘donned’) prior to the next encounter with a patient.”
It has been established that extended use is more advisable than reuse given the lower risk of self-inoculation. Furthermore, health care professionals are urged to wear a cleanable face shield or disposable mask over the respirator to minimize contamination and practice diligent hand hygiene before and after handling the respirator. N95 respirators are to be discarded following aerosol-generating procedures or if they come in contact with blood, respiratory secretions, or bodily fluids. They should also be discarded in case of close contact with an infected patient or if they cause breathing difficulties to the wearer.27 This may not always be possible given the unprecedented shortage of PPE, hence decontamination techniques and repurposing are the need of the hour.
In Anesthesia & Analgesia, Naveen Nathan, MD, of Northwestern University, Chicago, recommends recycling four masks in a series, using one per day, keeping the mask in a dry, clean environment, and then repeating use of the first mask on the 5th day, the second on the 6th day, and so forth. This ensures clearance of the virus particles by the next use. Alternatively, respirators can be sterilized between uses by heating to 70º C (158º F) for 30 minutes. Liquid disinfectants such as alcohol and bleach as well as ultraviolet rays in sunlight tend to damage masks.28 Steam sterilization is the most commonly utilized technique in hospitals. Other methods, described by the N95/PPE Working Group, report include gamma irradiation at 20kGy (2MRad) for large-scale sterilization (though the facilities may not be widely available), vaporized hydrogen peroxide, ozone decontamination, ultraviolet germicidal irradiation, and ethylene oxide.29 Though a discussion on various considerations of decontamination techniques is out of the scope of this article, detailed guidelines have been published by the CDC30 and the COVID-19 Healthcare Coalition.30
Conclusion
A recent startling discovery reported on in Emerging Infectious Diseases suggests that the basic COVID-19 reproductive number (R0) is actually much higher than previously thought. Using expanded data, updated epidemiologic parameters, and the current outbreak dynamics in Wuhan, the team came to the conclusion that the R0 for the novel coronavirus is actually 5.7 (95% CI 3.8-8.9), compared with an initial estimate of 2.2-2.7.31 Concern for transmissibility demands heightened prevention strategies until more data evolves. The latest recommendation by the CDC regarding cloth masking in the public may help slow the progression of the pandemic. However, it is of paramount importance to keep in mind that masks alone are not enough to control the disease and must be coupled with other nonpharmacologic interventions such as social distancing, quarantining/isolation, and diligent hand hygiene.
Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Bharathidasan is a recent medical graduate from India with an interest in public health and community research; she plans to pursue residency training in the United States. Ms. Freshman is currently the regional director of infection prevention for WellSpan Health and has 35 years of experience in nursing. Dr. Palabindala is the medical director, utilization management and physician advisory services, at the University of Mississippi Medical Center, Jackson. He is an associate professor of medicine and academic hospitalist in the UMMC School of Medicine.
References
1. Centers for Disease Control and Prevention. Recommendation regarding the use of cloth face coverings.
2. World Health Organization. Modes of transmission of virus causing COVID-19 : implications for IPC precaution recommendations. Sci Br. 2020 Mar 29:1-3.
3. Santarpia JL et al. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. 2020 Mar 26. medRxiv. 2020;2020.03.23.20039446.
4. Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: Potential implications for reducing transmission of COVID-19. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4756.
5. Klompas M et al. Universal masking in hospitals in the Covid-19 era. N Engl J Med. 2020 Apr 1. doi: 10.1056/NEJMp2006372.
6. Servick K. Would everyone wearing face masks help us slow the pandemic? Science 2020 Mar 28. doi: 10.1126/science.abb9371.
7. Leung CC et al. Mass masking in the COVID-19 epidemic: People need guidance. Lancet 2020 Mar 21;395(10228):945. doi: 10.1016/S0140-6736(20)30520-1.
8. Zou L et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020 Mar 19;382(12):1177-9.
9. Pan X et al. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis. 2020 Apr;20(4):410-1.
10. Bai Y et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020 Feb 21;323(14):1406-7.
11. Wei WE et al. Presymptomatic transmission of SARS-CoV-2 – Singapore, Jan. 23–March 16, 2020. MMWR Morb Mortal Wkly Rep 2020;69:411-5.
12. Kimball A et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility – King County, Washington, March 2020. 2020 Apr 3. MMWR Morb Mortal Wkly Rep 2020;69:377-81.
13. Ma Q-X et al. Potential utilities of mask wearing and instant hand hygiene for fighting SARS-CoV-2. J Med Virol. 2020 Mar 31;10.1002/jmv.25805. doi: 10.1002/jmv.25805.
14. Abd-Elsayed A et al. Utility of substandard face mask options for health care workers during the COVID-19 pandemic. Anesth Analg. 2020 Mar 31;10.1213/ANE.0000000000004841. doi: 10.1213/ANE.0000000000004841.
15. Long Y et al. Effectiveness of N95 respirators versus surgical masks against influenza: A systematic review and meta-analysis. J Evid Based Med. 2020 Mar 13;10.1111/jebm.12381. doi: 10.1111/jebm.12381.
16. Leung NHL et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020 May;26(5):676-80.
17. Bae S et al. Effectiveness of surgical and cotton masks in blocking SARS-CoV-2: A controlled comparison in 4 patients. Ann Intern Med. 2020 Apr 6;M20-1342. doi: 10.7326/M20-1342.
18. Brosseau LM. Are powered air purifying respirators a solution for protecting healthcare workers from emerging aerosol-transmissible diseases? Ann Work Expo Health. 2020 Apr 30;64(4):339-41.
19. Swennen GRJ et al. Custom-made 3D-printed face masks in case of pandemic crisis situations with a lack of commercially available FFP2/3 masks. Int J Oral Maxillofac Surg. 2020 May;49(5):673-7.
20. Mahase E. Coronavirus: Global stocks of protective gear are depleted, with demand at “100 times” normal level, WHO warns. BMJ. 2020 Feb 10;368:m543. doi: 10.1136/bmj.m543.
21. National survey shows dire shortages of PPE, hand sanitizer across the U.S. 2020 Mar 27. Association for Professionals in Infection Control and Epidemiology (APIC) press briefing.
22. Wu HL et al. Facemask shortage and the novel coronavirus disease (COVID-19) outbreak: Reflections on public health measures. EClinicalMedicine. 2020 Apr 3:100329. doi: 10.1016/j.eclinm.2020.100329.
23. Feng S et al. Rational use of face masks in the COVID-19 pandemic. Lancet Respir Med. 2020 May;8(5):434-6.
24. Chin AWH et al. Stability of SARS-CoV-2 in different environmental. The Lancet Microbe. 2020 May 1;5247(20):2004973. doi. org/10.1016/S2666-5247(20)30003-3.
25. van Doremalen N et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020 Apr 16;382(16):1564-7.
26. NIOSH – Workplace Safety and Health Topics: Recommended guidance for extended use and limited reuse of n95 filtering facepiece respirators in healthcare settings.
27. Centers for Disease Control and Prevention. COVID-19 decontamination and reuse of filtering facepiece respirators. 2020 Apr 15.
28. Nathan N. Waste not, want not: The re-usability of N95 masks. Anesth Analg. 2020 Mar 31.doi: 10.1213/ane.0000000000004843.
29. European Centre for Disease Prevention and Control technical report. Cloth masks and mask sterilisation as options in case of shortage of surgical masks and respirators. 2020 Mar.
30. N95/PPE Working Group report. Evaluation of decontamination techniques for the reuse of N95 respirators. 2020 Apr 3;2:1-7.
31. Sanche Set al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020 Jul. doi. org/10.3201/eid2607.200282.
On April 3, the Centers for Disease Control and Prevention issued an advisory that the general public wear cloth face masks when outside, particularly those residing in areas with significant severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) community transmission.1 Recent research reveals several factors related to the nature of the virus as well as the epidemiologic spread of the illness that may have led to this decision.
However, controversy still prevails whether this recommendation will alleviate or aggravate disease progression. With many hospitals across America lacking sufficient personal protective equipment (PPE) and scrambling for supplies, universal masking may create more chaos, especially with certain states imposing monetary fines on individuals spotted outdoors without a mask. With new information being discovered each day about COVID-19, it is more imperative than ever to update existing strategies and formulate more effective methods to flatten the curve.
Airborne vs. droplet transmission
According to a scientific brief released by the World Health Organization, there have been studies with mixed evidence and opinions regarding the presence of COVID-19 ribonucleic acid (RNA) in air samples.2 In medRxiv, Santarpia et al., from the University of Nebraska Medical Center, Omaha, detected viral RNA in samples taken from beneath a patient’s bed and from a window ledge, both areas in which neither the patient nor health care personnel had any direct contact. They also found that 66.7% of air samples taken from a hospital hallway carried virus-containing particles.3 It is worth noting that certain aerosol-generating procedures (AGP) may increase the likelihood of airborne dissemination. Whether airborne transmission is a major mode of COVID-19 spread in the community and routine clinical settings (with no aerosol-generating procedures) is still a debatable question without a definitive answer.
We should consider the epidemiology of COVID-19 thus far in the pandemic to determine if transmission patterns are more consistent with that of other common respiratory viral pathogens or more consistent with that of the agents we classically consider to be transmitted by the airborne route (measles, varicella zoster virus, and Mycobacterium tuberculosis). The attack rates in various settings (household, health care, and the public) as well as the expected number of secondary cases from a single infected individual in a susceptible population (R0) are more consistent with those of a droplet spread pathogen.
For measles, the R0 is 12-18, and the secondary household attack rates are ≥ 90%. In case of the varicella zoster virus, the R0 is ~10, and the secondary household attack rate is 85%. The R0 for pulmonary tuberculosis is up to 10 (per year) and the secondary household attack rate has been reported to be >50%. With COVID-19, the R0 appears to be around 2.5-3 and secondary household attack rates are ~ 10% from data available so far, similar to that of influenza viruses. This discrepancy suggests that droplet transmission may be more likely. The dichotomy of airborne versus droplet mode of spread may be better described as a continuum, as pointed out in a recent article in the JAMA. Infectious droplets form turbulent gas clouds allowing the virus particles to travel further and remain in the air longer.4 The necessary precautions for an airborne illness should be chosen over droplet precautions, especially when there is concern for an AGP.
Universal masking: Risks and benefits
The idea of universal masking has been debated extensively since the initial stages of the COVID-19 pandemic. According to public health authorities, significant exposure is defined as “face-to-face contact within 6 feet with a patient with symptomatic COVID-19” in the range of a few minutes up to 30 minutes.5 The researchers wrote in the New England Journal of Medicine that the chance of catching COVID-19 from a passing interaction in a public space is therefore minimal, and it may seem unnecessary to wear a mask at all times in public.
As reported in Science, randomized clinical studies performed on other viruses in the past have shown no added protection conferred by wearing a mask, though small sample sizes and noncompliance are limiting factors to their validity.6 On the contrary, mask wearing has been enforced in many parts of Asia, including Hong Kong and Singapore with promising results.5 Leung et al. stated in The Lancet that the lack of proof that masks are effective should not rule them as ineffective. Also, universal masking would reduce the stigma around symptomatic individuals covering their faces. It has become a cultural phenomenon in many southeast Asian countries and has been cited as one of the reasons for relatively successful containment in Singapore, South Korea, and Taiwan. The most important benefit of universal masking is protection attained by preventing spread from asymptomatic, mildly symptomatic, and presymptomatic carriers.7
In a study in the New England Journal of Medicine that estimated viral loads during various stages of COVID-19, researchers found that asymptomatic patients had similar viral loads to symptomatic patients, thereby suggesting high potential for transmission.8 Furthermore, numerous cases are being reported concerning the spread of illness from asymptomatic carriers.9-12 In an outbreak at a skilled nursing facility in Washington outlined in MMWR, 13 of 23 residents with positive test results were asymptomatic at the time of testing, and of those, 3 never developed any symptoms.12
Many hospitals are now embracing the policy of universal masking. A mask is a critical component of the personal protective equipment (PPE) clinicians need when caring for symptomatic patients with respiratory viral infections, in conjunction with a gown, gloves, and eye protection. Masking in this context is already part of routine operations in most hospitals. There are two scenarios in which there may be possible benefits. One scenario is the lower likelihood of transmission from asymptomatic and minimally symptomatic health care workers with COVID-19 to other providers and patients. The other less plausible benefit of universal masking among health care workers is that it may provide some protection in the possibility of caring for an unrecognized COVID-19 patient. However, universal masking should be coupled with other favorable practices like temperature checks and symptom screening on a daily basis to avail the maximum benefit from masking. Despite varied opinions on the outcomes of universal masking, this measure helps improve health care workers’ safety, psychological well-being, trust in their hospital, and decreases anxiety of acquiring the illness.
Efficacy of various types of masks
With the possibility of airborne transmission of the virus, are cloth masks as recommended by the CDC truly helpful in preventing infection? A study in the Journal of Medical Virology demonstrates 99.98%, 97.14%, and 95.15% efficacy for N95, surgical, and homemade masks, respectively, in blocking the avian influenza virus (comparable to coronavirus in size and physical characteristics). The homemade mask was created using one layer of polyester cloth and a four-layered kitchen filter paper.13

N95 masks (equivalent to FFP/P2 in European countries) are made of electrostatically charged polypropylene microfibers designed to filter particles measuring 100-300nm in diameter with 95% efficacy. A single SARS-CoV-2 molecule measures 125 nm approximately. N99 (FFP3) and N100 (P3) masks are also available, though not as widely used, with 99% and 99.7% efficacy respectively for the same size range. Though cloth masks are the clear-cut last resort for medical professionals, a few studies state no clinically proven difference in protection between surgical masks and N95 respirators.14,15 Even aerosolized droplets (< 5 mcm) were found to be blocked by surgical masks in a Nature Medicine study in which 4/10 subjects tested positive for coronavirus in exhaled breath samples without masks and 0/10 subjects with masks.16
On the contrary, an Annals of Internal Medicine study of four COVID-19 positive subjects that “neither surgical masks nor cloth masks effectively filtered SARS-CoV-2 during coughs of infected patients.” In fact, more contamination was found on the outer surface of the masks when compared to the inner surface, probably owing to the masks’ aerodynamic properties.17 Because of limitations present in the above-mentioned studies, further research is necessary to conclusively determine which types of masks are efficacious in preventing infection by the virus. In a scarcity of surgical masks and respirators for health care personnel, suboptimal masks can be of some use provided there is adherent use, minimal donning and doffing, and it is to be accompanied by adequate hand washing practices.14
In case of severe infections with high viral loads or patients undergoing aerosol-generating procedures, powered air-purifying respirators (PAPRs) also are advisable as they confer greater protection than N95 respirators, according to a study in the Annals of Work Exposures and Health. Despite being more comfortable for long-term use and accommodative of facial hair, their use is limited because of high cost and difficult maintenance.18 3-D printing also is being used to combat the current shortage of masks worldwide. However, a study from the International Journal of Oral & Maxillofacial Surgery reported that virologic testing for leakage between the two reusable components and contamination of the components themselves after one or multiple disinfection cycles is essential before application in real-life situations.19
Ongoing issues
WHO estimates a monthly requirement of nearly 90 million masks exclusively for health care workers to protect themselves against COVID-19.20 In spite of increasing the production rate by 40%, if the general public hoards masks and respirators, the results could be disastrous. Personal protective equipment is currently at 100 times the usual demand and 20 times the usual cost, with stocks backlogged by 4-6 months. The appropriate order of priority in distribution to health care professionals first, followed by those caring for infected patients is critical.20 In a survey conducted by the Association for Professionals in Infection Control and Epidemiology, results revealed that 48% of the U.S. health care facilities that responded were either out or nearly out of respirators as of March 25. 21
The gravest risk behind the universal masking policy is the likely depletion of medical resources.22 A possible solution to this issue could be to modify the policy to stagger the requirement based on the severity of community transmission in that area of residence. In the article appropriately titled “Rational use of face masks in the COVID-19 pandemic” published in The Lancet Respiratory Medicine, researchers described how the Chinese population was classified into moderate, low, and very-low risk of infection categories and advised to wear a surgical or disposable mask, disposable mask, and no mask respectively.23 This curbs widespread panic and eagerness by the general public to stock up on essential medical equipment when it may not even be necessary.
Reuse, extended use, and sterilization
Several studies have been conducted to identify the viability of the COVID-19 on various surfaces.24-25 The CDC and National Institute for Occupational Safety and Health (NIOSH) guidelines state that an N95 respirator can be used up to 8 hours with intermittent or continuous use, though this number is not fixed and heavily depends upon the extent of exposure, risk of contamination, and frequency of donning and doffing26,27. Though traditionally meant for single-time usage, after 8 hours, the mask can be decontaminated and reused. The CDC defines extended use as the “practice of wearing the same N95 respirator for repeated close-contact encounters with several patients, without removing the respirator between patient encounters.” Reuse is defined as “using the same N95 respirator for multiple encounters with patients but removing it (‘doffing’) after each encounter. The respirator is stored in between encounters to be put on again (‘donned’) prior to the next encounter with a patient.”
It has been established that extended use is more advisable than reuse given the lower risk of self-inoculation. Furthermore, health care professionals are urged to wear a cleanable face shield or disposable mask over the respirator to minimize contamination and practice diligent hand hygiene before and after handling the respirator. N95 respirators are to be discarded following aerosol-generating procedures or if they come in contact with blood, respiratory secretions, or bodily fluids. They should also be discarded in case of close contact with an infected patient or if they cause breathing difficulties to the wearer.27 This may not always be possible given the unprecedented shortage of PPE, hence decontamination techniques and repurposing are the need of the hour.
In Anesthesia & Analgesia, Naveen Nathan, MD, of Northwestern University, Chicago, recommends recycling four masks in a series, using one per day, keeping the mask in a dry, clean environment, and then repeating use of the first mask on the 5th day, the second on the 6th day, and so forth. This ensures clearance of the virus particles by the next use. Alternatively, respirators can be sterilized between uses by heating to 70º C (158º F) for 30 minutes. Liquid disinfectants such as alcohol and bleach as well as ultraviolet rays in sunlight tend to damage masks.28 Steam sterilization is the most commonly utilized technique in hospitals. Other methods, described by the N95/PPE Working Group, report include gamma irradiation at 20kGy (2MRad) for large-scale sterilization (though the facilities may not be widely available), vaporized hydrogen peroxide, ozone decontamination, ultraviolet germicidal irradiation, and ethylene oxide.29 Though a discussion on various considerations of decontamination techniques is out of the scope of this article, detailed guidelines have been published by the CDC30 and the COVID-19 Healthcare Coalition.30
Conclusion
A recent startling discovery reported on in Emerging Infectious Diseases suggests that the basic COVID-19 reproductive number (R0) is actually much higher than previously thought. Using expanded data, updated epidemiologic parameters, and the current outbreak dynamics in Wuhan, the team came to the conclusion that the R0 for the novel coronavirus is actually 5.7 (95% CI 3.8-8.9), compared with an initial estimate of 2.2-2.7.31 Concern for transmissibility demands heightened prevention strategies until more data evolves. The latest recommendation by the CDC regarding cloth masking in the public may help slow the progression of the pandemic. However, it is of paramount importance to keep in mind that masks alone are not enough to control the disease and must be coupled with other nonpharmacologic interventions such as social distancing, quarantining/isolation, and diligent hand hygiene.
Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Bharathidasan is a recent medical graduate from India with an interest in public health and community research; she plans to pursue residency training in the United States. Ms. Freshman is currently the regional director of infection prevention for WellSpan Health and has 35 years of experience in nursing. Dr. Palabindala is the medical director, utilization management and physician advisory services, at the University of Mississippi Medical Center, Jackson. He is an associate professor of medicine and academic hospitalist in the UMMC School of Medicine.
References
1. Centers for Disease Control and Prevention. Recommendation regarding the use of cloth face coverings.
2. World Health Organization. Modes of transmission of virus causing COVID-19 : implications for IPC precaution recommendations. Sci Br. 2020 Mar 29:1-3.
3. Santarpia JL et al. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. 2020 Mar 26. medRxiv. 2020;2020.03.23.20039446.
4. Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: Potential implications for reducing transmission of COVID-19. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4756.
5. Klompas M et al. Universal masking in hospitals in the Covid-19 era. N Engl J Med. 2020 Apr 1. doi: 10.1056/NEJMp2006372.
6. Servick K. Would everyone wearing face masks help us slow the pandemic? Science 2020 Mar 28. doi: 10.1126/science.abb9371.
7. Leung CC et al. Mass masking in the COVID-19 epidemic: People need guidance. Lancet 2020 Mar 21;395(10228):945. doi: 10.1016/S0140-6736(20)30520-1.
8. Zou L et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020 Mar 19;382(12):1177-9.
9. Pan X et al. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis. 2020 Apr;20(4):410-1.
10. Bai Y et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020 Feb 21;323(14):1406-7.
11. Wei WE et al. Presymptomatic transmission of SARS-CoV-2 – Singapore, Jan. 23–March 16, 2020. MMWR Morb Mortal Wkly Rep 2020;69:411-5.
12. Kimball A et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility – King County, Washington, March 2020. 2020 Apr 3. MMWR Morb Mortal Wkly Rep 2020;69:377-81.
13. Ma Q-X et al. Potential utilities of mask wearing and instant hand hygiene for fighting SARS-CoV-2. J Med Virol. 2020 Mar 31;10.1002/jmv.25805. doi: 10.1002/jmv.25805.
14. Abd-Elsayed A et al. Utility of substandard face mask options for health care workers during the COVID-19 pandemic. Anesth Analg. 2020 Mar 31;10.1213/ANE.0000000000004841. doi: 10.1213/ANE.0000000000004841.
15. Long Y et al. Effectiveness of N95 respirators versus surgical masks against influenza: A systematic review and meta-analysis. J Evid Based Med. 2020 Mar 13;10.1111/jebm.12381. doi: 10.1111/jebm.12381.
16. Leung NHL et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020 May;26(5):676-80.
17. Bae S et al. Effectiveness of surgical and cotton masks in blocking SARS-CoV-2: A controlled comparison in 4 patients. Ann Intern Med. 2020 Apr 6;M20-1342. doi: 10.7326/M20-1342.
18. Brosseau LM. Are powered air purifying respirators a solution for protecting healthcare workers from emerging aerosol-transmissible diseases? Ann Work Expo Health. 2020 Apr 30;64(4):339-41.
19. Swennen GRJ et al. Custom-made 3D-printed face masks in case of pandemic crisis situations with a lack of commercially available FFP2/3 masks. Int J Oral Maxillofac Surg. 2020 May;49(5):673-7.
20. Mahase E. Coronavirus: Global stocks of protective gear are depleted, with demand at “100 times” normal level, WHO warns. BMJ. 2020 Feb 10;368:m543. doi: 10.1136/bmj.m543.
21. National survey shows dire shortages of PPE, hand sanitizer across the U.S. 2020 Mar 27. Association for Professionals in Infection Control and Epidemiology (APIC) press briefing.
22. Wu HL et al. Facemask shortage and the novel coronavirus disease (COVID-19) outbreak: Reflections on public health measures. EClinicalMedicine. 2020 Apr 3:100329. doi: 10.1016/j.eclinm.2020.100329.
23. Feng S et al. Rational use of face masks in the COVID-19 pandemic. Lancet Respir Med. 2020 May;8(5):434-6.
24. Chin AWH et al. Stability of SARS-CoV-2 in different environmental. The Lancet Microbe. 2020 May 1;5247(20):2004973. doi. org/10.1016/S2666-5247(20)30003-3.
25. van Doremalen N et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020 Apr 16;382(16):1564-7.
26. NIOSH – Workplace Safety and Health Topics: Recommended guidance for extended use and limited reuse of n95 filtering facepiece respirators in healthcare settings.
27. Centers for Disease Control and Prevention. COVID-19 decontamination and reuse of filtering facepiece respirators. 2020 Apr 15.
28. Nathan N. Waste not, want not: The re-usability of N95 masks. Anesth Analg. 2020 Mar 31.doi: 10.1213/ane.0000000000004843.
29. European Centre for Disease Prevention and Control technical report. Cloth masks and mask sterilisation as options in case of shortage of surgical masks and respirators. 2020 Mar.
30. N95/PPE Working Group report. Evaluation of decontamination techniques for the reuse of N95 respirators. 2020 Apr 3;2:1-7.
31. Sanche Set al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020 Jul. doi. org/10.3201/eid2607.200282.
COVID-19–associated coagulopathy
Coronavirus disease 2019 (COVID-19) is a viral illness caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), currently causing a pandemic affecting many countries around the world, beginning in December 2019 and spreading rapidly on a global scale since. Globally, its burden has been increasing rapidly, with more than 1.2 million people testing positive for the illness and 123,000 people losing their lives, as per April 15th’s WHO COVID-19 Situation Report.1 These numbers are increasing with each passing day. Clinically, SARS-CoV-2 has a highly variable course, ranging from mild disease manifested as a self-limited illness (seen in younger and healthier patients) to severe pneumonia/ARDS and multiorgan failure with intravascular coagulopathy.2
In this article, we intend to investigate and establish a comprehensive review of COVID-19–associated coagulopathy mechanisms, laboratory findings, and current management guidelines put forth by various societies globally.
Mechanism of coagulopathy
COVID-19–associated coagulopathy has been shown to predispose to both arterial and venous thrombosis through excessive inflammation and hypoxia, leading to activation of the coagulation cascade and consumption of coagulation factors, resulting in microvascular thrombosis.3 Though the exact pathophysiology for the activation of this cascade is not known, the proposed mechanism has been: endothelial damage triggering platelet activation within the lung, leading to aggregation, thrombosis, and consumption of platelets in the lung.2,5,6
Fox et al. noted similar coagulopathy findings of four deceased COVID-19 patients. Autopsy results concluded that the dominant process was diffuse alveolar damage, notable CD4+ aggregates around thrombosed small vessels, significant associated hemorrhage, and thrombotic microangiopathy restricted to the lungs. The proposed mechanism was the activation of megakaryocytes, possibly native to the lung, with platelet aggregation, formation of platelet-rich clots, and fibrin deposition playing a major role.4
It has been noted that diabetic patients are at an increased risk of vascular events and hypercoagulability with COVID-19.7 COVID-19 can also cause livedo reticularis and acrocyanosis because of the microthrombosis in the cutaneous vasculature secondary to underlying coagulopathy, as reported in a case report of two U.S. patients with COVID-19.8
Clinical and laboratory abnormalities
A recent study reported from Netherlands by Klok et al. analyzed 184 ICU patients with COVID-19 pneumonia and concluded that the cumulative incidence of acute pulmonary embolism (PE), deep vein thrombosis (DVT), ischemic stroke, MI, or systemic arterial embolism was 31% (95% confidence interval, 20%-41%). PE was the most frequent thrombotic complication and was noted in 81% of patients. Coagulopathy, defined as spontaneous prolongation of prothrombin time (PT) > 3s or activated partial thromboplastin time (aPTT) > 5s, was reported as an independent predictor of thrombotic complications.3
Hematologic abnormalities that were noted in COVID-19 coagulopathy include: decreased platelet counts, decreased fibrinogen levels, elevated PT/INR, elevated partial thromboplastin time (PTT), and elevated d-dimer.9,10 In a retrospective analysis9 by Tang et al., 71.4% of nonsurvivors and 0.6% of survivors had met the criteria of disseminated intravascular coagulation (DIC) during their hospital stay. Nonsurvivors of COVID-19 had statistically significant elevation of d-dimer levels, FDP levels, PT, and aPTT, when compared to survivors (P < .05). The overall mortality in this study was reported as 11.5%.9 In addition, elevated d-dimer, fibrin and fibrinogen degradation product (FDP) levels and longer PT and aPTT were associated with poor prognosis.
Thus, d-dimer, PT, and platelet count should be measured in all patients who present with COVID-19 infection. We can also suggest that in patients with markedly elevated d-dimer (three- to fourfold increase), admission to hospital should be considered even in the absence of severe clinical symptoms.11
COVID-19 coagulopathy management
In a retrospective study9 of 449 patients with severe COVID-19 from Wuhan, China, by Tang et al., 99 patients mainly received low-weight molecular heparin (LMWH) for 7 days or longer. No difference in 28-day mortality was noted between heparin users and nonusers (30.3% vs. 29.7%; P = .910). A lower 28-day mortality rate was noted in heparin patients with sepsis-induced coagulopathy score of ≥4.0 (40.0% vs. 64.2%; P = .029) or a d-dimer level greater than sixfold of upper limit of normal, compared with nonusers of heparin.12
Another small study of seven COVID-19 patients with acroischemia in China demonstrated that administering LMWH was successful at decreasing the d-dimer and fibrinogen degradation product levels but noted no significant improvement in clinical symptoms.13
Recently, the International Society of Thrombosis and Hemostasis and American Society of Hematology published recommendations and guidelines regarding the recognition and management of coagulopathy in COVID-19.11 Prophylactic anticoagulation therapy with LMWH was recommended in all hospitalized patients with COVID-19, provided there was an absence of any contraindications (active bleeding, platelet count less than 25 x 109/L and fibrinogen less than 0.5 g/dL). Anticoagulation with LMWH was associated with better prognosis in severe COVID-19 patients and in COVID-19 patients with markedly elevated d-dimer, as it also has anti-inflammatory effects.12 This anti-inflammatory property of heparin has been documented in previous studies but the underlying mechanism is unknown and more research is required.14,15
Despite coagulopathy being noticed with cases of COVID-19, bleeding has been a rare finding in COVID-19 infections. If bleeding is noted, recommendations were made to keep platelet levels greater than 50 x109/L, fibrinogen less than 2.0 g/L, and INR [international normalized ratio] greater than 1.5.11 Mechanical thromboprophylaxis should be used when pharmacologic thromboprophylaxis is contraindicated.16
COVID-19 patients with new diagnoses of venous thromboembolism (VTE) or atrial fibrillation should be prescribed therapeutic anticoagulation. Patients who are already on anticoagulation for VTE or atrial fibrillation should continue their therapy unless the platelet count is less than 30-50x109/L or if the fibrinogen is less than 1.0 g/L.16
Conclusion
Coagulopathies associated with COVID-19 infections have been documented in several studies around the world, and it has been shown to be fatal in some cases. Despite documentation, the mechanism behind this coagulopathy is not well understood. Because of the potentially lethal complications associated with coagulopathies, early recognition and anticoagulation is imperative to improve clinical outcomes. These results are very preliminary: More studies are required to understand the role of anticoagulation and its effect on the morbidity and mortality associated with COVID-19–associated coagulopathy.
Dr. Yeruva is a board-certified hematologist/medical oncologist with WellSpan Health and clinical assistant professor of internal medicine, Penn State University, Hershey. Mr. Henderson is a third-year graduate-entry medical student at the Royal College of Surgeons in Ireland with interests in family medicine, dermatology, and tropical diseases. Dr. Al-Tawfiq is a consultant of internal medicine & infectious diseases, and the director of quality at Johns Hopkins Aramco Healthcare in Dhahran, Saudi Arabia, an adjunct associate professor of infectious diseases, molecular medicine and clinical pharmacology at Johns Hopkins University School of Medicine, and adjunct associate professor at Indiana University School of Medicine, Indianapolis. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals.
References
1. World Health Organization. Coronavirus disease (COVID-2019) situation reports.
2. Lippi G et al. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin Chim Acta. 2020 Mar 13. 506:145-8. doi: 10.1016/j.cca.2020.03.022.
3. Klok FA et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Throm Res. 2020;18(4):844-7. doi: 10.1016/j.thromres.2020.04.013.
4. Fox S et al. Pulmonary and cardiac pathology in Covid-19: The first autopsy series from New Orleans. MedRxiv. 2020 Apr 10. doi: 10.1101/2020.04.06.20050575.
5. Yang M et al. Thrombocytopenia in patients with severe acute respiratory syndrome (review). Hematology 2013 Sep 4. doi: 10.1080/1024533040002617.
6. Giannis D et al. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020 June. doi: 10.1016/j.jcv.2020.104362.
7. Guo W et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020 Mar 31. doi: 10.1002/dmrr.3319.
8. Manalo IF et al. A dermatologic manifestation of COVID-19: Transient livedo reticularis. J Am Acad Dermat. 2020 Apr. doi: 10.1016/j.jaad.2020.04.018.
9. Tang N et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020 Feb 19. doi: 10.1111/jth.14768, 18: 844-847.
10. Huang C et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020 Jan 24. doi: 10.1016/S0140-6736(20)30183-5.
11. Thachil J et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020 Mar 25. doi: 10.1111/JTH.14810.
12. Tang N et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020 Mar 27. doi: 10.1111/JTH.14817.
13. Zhang Y et al. Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia. Zhonghua Xue Ye Xue Za Zhi. 2020 Mar 28. doi: 10.3760/cma.j.issn.0253-2727.2020.0006.
14. Poterucha TJ et al. More than an anticoagulant: Do heparins have direct anti-inflammatory effects? Thromb Haemost. 2017. doi: 10.1160/TH16-08-0620.
15. Mousavi S et al. Anti-inflammatory effects of heparin and its derivatives: A systematic review. Adv Pharmacol Pharm Sci. 2015 May 12. doi: 10.1155/2015/507151.
16. Kreuziger L et al. COVID-19 and VTE/anticoagulation: Frequently asked questions. American Society of Hematology. 2020 Apr 17.
Coronavirus disease 2019 (COVID-19) is a viral illness caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), currently causing a pandemic affecting many countries around the world, beginning in December 2019 and spreading rapidly on a global scale since. Globally, its burden has been increasing rapidly, with more than 1.2 million people testing positive for the illness and 123,000 people losing their lives, as per April 15th’s WHO COVID-19 Situation Report.1 These numbers are increasing with each passing day. Clinically, SARS-CoV-2 has a highly variable course, ranging from mild disease manifested as a self-limited illness (seen in younger and healthier patients) to severe pneumonia/ARDS and multiorgan failure with intravascular coagulopathy.2
In this article, we intend to investigate and establish a comprehensive review of COVID-19–associated coagulopathy mechanisms, laboratory findings, and current management guidelines put forth by various societies globally.
Mechanism of coagulopathy
COVID-19–associated coagulopathy has been shown to predispose to both arterial and venous thrombosis through excessive inflammation and hypoxia, leading to activation of the coagulation cascade and consumption of coagulation factors, resulting in microvascular thrombosis.3 Though the exact pathophysiology for the activation of this cascade is not known, the proposed mechanism has been: endothelial damage triggering platelet activation within the lung, leading to aggregation, thrombosis, and consumption of platelets in the lung.2,5,6
Fox et al. noted similar coagulopathy findings of four deceased COVID-19 patients. Autopsy results concluded that the dominant process was diffuse alveolar damage, notable CD4+ aggregates around thrombosed small vessels, significant associated hemorrhage, and thrombotic microangiopathy restricted to the lungs. The proposed mechanism was the activation of megakaryocytes, possibly native to the lung, with platelet aggregation, formation of platelet-rich clots, and fibrin deposition playing a major role.4
It has been noted that diabetic patients are at an increased risk of vascular events and hypercoagulability with COVID-19.7 COVID-19 can also cause livedo reticularis and acrocyanosis because of the microthrombosis in the cutaneous vasculature secondary to underlying coagulopathy, as reported in a case report of two U.S. patients with COVID-19.8
Clinical and laboratory abnormalities
A recent study reported from Netherlands by Klok et al. analyzed 184 ICU patients with COVID-19 pneumonia and concluded that the cumulative incidence of acute pulmonary embolism (PE), deep vein thrombosis (DVT), ischemic stroke, MI, or systemic arterial embolism was 31% (95% confidence interval, 20%-41%). PE was the most frequent thrombotic complication and was noted in 81% of patients. Coagulopathy, defined as spontaneous prolongation of prothrombin time (PT) > 3s or activated partial thromboplastin time (aPTT) > 5s, was reported as an independent predictor of thrombotic complications.3
Hematologic abnormalities that were noted in COVID-19 coagulopathy include: decreased platelet counts, decreased fibrinogen levels, elevated PT/INR, elevated partial thromboplastin time (PTT), and elevated d-dimer.9,10 In a retrospective analysis9 by Tang et al., 71.4% of nonsurvivors and 0.6% of survivors had met the criteria of disseminated intravascular coagulation (DIC) during their hospital stay. Nonsurvivors of COVID-19 had statistically significant elevation of d-dimer levels, FDP levels, PT, and aPTT, when compared to survivors (P < .05). The overall mortality in this study was reported as 11.5%.9 In addition, elevated d-dimer, fibrin and fibrinogen degradation product (FDP) levels and longer PT and aPTT were associated with poor prognosis.
Thus, d-dimer, PT, and platelet count should be measured in all patients who present with COVID-19 infection. We can also suggest that in patients with markedly elevated d-dimer (three- to fourfold increase), admission to hospital should be considered even in the absence of severe clinical symptoms.11
COVID-19 coagulopathy management
In a retrospective study9 of 449 patients with severe COVID-19 from Wuhan, China, by Tang et al., 99 patients mainly received low-weight molecular heparin (LMWH) for 7 days or longer. No difference in 28-day mortality was noted between heparin users and nonusers (30.3% vs. 29.7%; P = .910). A lower 28-day mortality rate was noted in heparin patients with sepsis-induced coagulopathy score of ≥4.0 (40.0% vs. 64.2%; P = .029) or a d-dimer level greater than sixfold of upper limit of normal, compared with nonusers of heparin.12
Another small study of seven COVID-19 patients with acroischemia in China demonstrated that administering LMWH was successful at decreasing the d-dimer and fibrinogen degradation product levels but noted no significant improvement in clinical symptoms.13
Recently, the International Society of Thrombosis and Hemostasis and American Society of Hematology published recommendations and guidelines regarding the recognition and management of coagulopathy in COVID-19.11 Prophylactic anticoagulation therapy with LMWH was recommended in all hospitalized patients with COVID-19, provided there was an absence of any contraindications (active bleeding, platelet count less than 25 x 109/L and fibrinogen less than 0.5 g/dL). Anticoagulation with LMWH was associated with better prognosis in severe COVID-19 patients and in COVID-19 patients with markedly elevated d-dimer, as it also has anti-inflammatory effects.12 This anti-inflammatory property of heparin has been documented in previous studies but the underlying mechanism is unknown and more research is required.14,15
Despite coagulopathy being noticed with cases of COVID-19, bleeding has been a rare finding in COVID-19 infections. If bleeding is noted, recommendations were made to keep platelet levels greater than 50 x109/L, fibrinogen less than 2.0 g/L, and INR [international normalized ratio] greater than 1.5.11 Mechanical thromboprophylaxis should be used when pharmacologic thromboprophylaxis is contraindicated.16
COVID-19 patients with new diagnoses of venous thromboembolism (VTE) or atrial fibrillation should be prescribed therapeutic anticoagulation. Patients who are already on anticoagulation for VTE or atrial fibrillation should continue their therapy unless the platelet count is less than 30-50x109/L or if the fibrinogen is less than 1.0 g/L.16
Conclusion
Coagulopathies associated with COVID-19 infections have been documented in several studies around the world, and it has been shown to be fatal in some cases. Despite documentation, the mechanism behind this coagulopathy is not well understood. Because of the potentially lethal complications associated with coagulopathies, early recognition and anticoagulation is imperative to improve clinical outcomes. These results are very preliminary: More studies are required to understand the role of anticoagulation and its effect on the morbidity and mortality associated with COVID-19–associated coagulopathy.
Dr. Yeruva is a board-certified hematologist/medical oncologist with WellSpan Health and clinical assistant professor of internal medicine, Penn State University, Hershey. Mr. Henderson is a third-year graduate-entry medical student at the Royal College of Surgeons in Ireland with interests in family medicine, dermatology, and tropical diseases. Dr. Al-Tawfiq is a consultant of internal medicine & infectious diseases, and the director of quality at Johns Hopkins Aramco Healthcare in Dhahran, Saudi Arabia, an adjunct associate professor of infectious diseases, molecular medicine and clinical pharmacology at Johns Hopkins University School of Medicine, and adjunct associate professor at Indiana University School of Medicine, Indianapolis. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals.
References
1. World Health Organization. Coronavirus disease (COVID-2019) situation reports.
2. Lippi G et al. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin Chim Acta. 2020 Mar 13. 506:145-8. doi: 10.1016/j.cca.2020.03.022.
3. Klok FA et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Throm Res. 2020;18(4):844-7. doi: 10.1016/j.thromres.2020.04.013.
4. Fox S et al. Pulmonary and cardiac pathology in Covid-19: The first autopsy series from New Orleans. MedRxiv. 2020 Apr 10. doi: 10.1101/2020.04.06.20050575.
5. Yang M et al. Thrombocytopenia in patients with severe acute respiratory syndrome (review). Hematology 2013 Sep 4. doi: 10.1080/1024533040002617.
6. Giannis D et al. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020 June. doi: 10.1016/j.jcv.2020.104362.
7. Guo W et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020 Mar 31. doi: 10.1002/dmrr.3319.
8. Manalo IF et al. A dermatologic manifestation of COVID-19: Transient livedo reticularis. J Am Acad Dermat. 2020 Apr. doi: 10.1016/j.jaad.2020.04.018.
9. Tang N et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020 Feb 19. doi: 10.1111/jth.14768, 18: 844-847.
10. Huang C et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020 Jan 24. doi: 10.1016/S0140-6736(20)30183-5.
11. Thachil J et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020 Mar 25. doi: 10.1111/JTH.14810.
12. Tang N et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020 Mar 27. doi: 10.1111/JTH.14817.
13. Zhang Y et al. Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia. Zhonghua Xue Ye Xue Za Zhi. 2020 Mar 28. doi: 10.3760/cma.j.issn.0253-2727.2020.0006.
14. Poterucha TJ et al. More than an anticoagulant: Do heparins have direct anti-inflammatory effects? Thromb Haemost. 2017. doi: 10.1160/TH16-08-0620.
15. Mousavi S et al. Anti-inflammatory effects of heparin and its derivatives: A systematic review. Adv Pharmacol Pharm Sci. 2015 May 12. doi: 10.1155/2015/507151.
16. Kreuziger L et al. COVID-19 and VTE/anticoagulation: Frequently asked questions. American Society of Hematology. 2020 Apr 17.
Coronavirus disease 2019 (COVID-19) is a viral illness caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), currently causing a pandemic affecting many countries around the world, beginning in December 2019 and spreading rapidly on a global scale since. Globally, its burden has been increasing rapidly, with more than 1.2 million people testing positive for the illness and 123,000 people losing their lives, as per April 15th’s WHO COVID-19 Situation Report.1 These numbers are increasing with each passing day. Clinically, SARS-CoV-2 has a highly variable course, ranging from mild disease manifested as a self-limited illness (seen in younger and healthier patients) to severe pneumonia/ARDS and multiorgan failure with intravascular coagulopathy.2
In this article, we intend to investigate and establish a comprehensive review of COVID-19–associated coagulopathy mechanisms, laboratory findings, and current management guidelines put forth by various societies globally.
Mechanism of coagulopathy
COVID-19–associated coagulopathy has been shown to predispose to both arterial and venous thrombosis through excessive inflammation and hypoxia, leading to activation of the coagulation cascade and consumption of coagulation factors, resulting in microvascular thrombosis.3 Though the exact pathophysiology for the activation of this cascade is not known, the proposed mechanism has been: endothelial damage triggering platelet activation within the lung, leading to aggregation, thrombosis, and consumption of platelets in the lung.2,5,6
Fox et al. noted similar coagulopathy findings of four deceased COVID-19 patients. Autopsy results concluded that the dominant process was diffuse alveolar damage, notable CD4+ aggregates around thrombosed small vessels, significant associated hemorrhage, and thrombotic microangiopathy restricted to the lungs. The proposed mechanism was the activation of megakaryocytes, possibly native to the lung, with platelet aggregation, formation of platelet-rich clots, and fibrin deposition playing a major role.4
It has been noted that diabetic patients are at an increased risk of vascular events and hypercoagulability with COVID-19.7 COVID-19 can also cause livedo reticularis and acrocyanosis because of the microthrombosis in the cutaneous vasculature secondary to underlying coagulopathy, as reported in a case report of two U.S. patients with COVID-19.8
Clinical and laboratory abnormalities
A recent study reported from Netherlands by Klok et al. analyzed 184 ICU patients with COVID-19 pneumonia and concluded that the cumulative incidence of acute pulmonary embolism (PE), deep vein thrombosis (DVT), ischemic stroke, MI, or systemic arterial embolism was 31% (95% confidence interval, 20%-41%). PE was the most frequent thrombotic complication and was noted in 81% of patients. Coagulopathy, defined as spontaneous prolongation of prothrombin time (PT) > 3s or activated partial thromboplastin time (aPTT) > 5s, was reported as an independent predictor of thrombotic complications.3
Hematologic abnormalities that were noted in COVID-19 coagulopathy include: decreased platelet counts, decreased fibrinogen levels, elevated PT/INR, elevated partial thromboplastin time (PTT), and elevated d-dimer.9,10 In a retrospective analysis9 by Tang et al., 71.4% of nonsurvivors and 0.6% of survivors had met the criteria of disseminated intravascular coagulation (DIC) during their hospital stay. Nonsurvivors of COVID-19 had statistically significant elevation of d-dimer levels, FDP levels, PT, and aPTT, when compared to survivors (P < .05). The overall mortality in this study was reported as 11.5%.9 In addition, elevated d-dimer, fibrin and fibrinogen degradation product (FDP) levels and longer PT and aPTT were associated with poor prognosis.
Thus, d-dimer, PT, and platelet count should be measured in all patients who present with COVID-19 infection. We can also suggest that in patients with markedly elevated d-dimer (three- to fourfold increase), admission to hospital should be considered even in the absence of severe clinical symptoms.11
COVID-19 coagulopathy management
In a retrospective study9 of 449 patients with severe COVID-19 from Wuhan, China, by Tang et al., 99 patients mainly received low-weight molecular heparin (LMWH) for 7 days or longer. No difference in 28-day mortality was noted between heparin users and nonusers (30.3% vs. 29.7%; P = .910). A lower 28-day mortality rate was noted in heparin patients with sepsis-induced coagulopathy score of ≥4.0 (40.0% vs. 64.2%; P = .029) or a d-dimer level greater than sixfold of upper limit of normal, compared with nonusers of heparin.12
Another small study of seven COVID-19 patients with acroischemia in China demonstrated that administering LMWH was successful at decreasing the d-dimer and fibrinogen degradation product levels but noted no significant improvement in clinical symptoms.13
Recently, the International Society of Thrombosis and Hemostasis and American Society of Hematology published recommendations and guidelines regarding the recognition and management of coagulopathy in COVID-19.11 Prophylactic anticoagulation therapy with LMWH was recommended in all hospitalized patients with COVID-19, provided there was an absence of any contraindications (active bleeding, platelet count less than 25 x 109/L and fibrinogen less than 0.5 g/dL). Anticoagulation with LMWH was associated with better prognosis in severe COVID-19 patients and in COVID-19 patients with markedly elevated d-dimer, as it also has anti-inflammatory effects.12 This anti-inflammatory property of heparin has been documented in previous studies but the underlying mechanism is unknown and more research is required.14,15
Despite coagulopathy being noticed with cases of COVID-19, bleeding has been a rare finding in COVID-19 infections. If bleeding is noted, recommendations were made to keep platelet levels greater than 50 x109/L, fibrinogen less than 2.0 g/L, and INR [international normalized ratio] greater than 1.5.11 Mechanical thromboprophylaxis should be used when pharmacologic thromboprophylaxis is contraindicated.16
COVID-19 patients with new diagnoses of venous thromboembolism (VTE) or atrial fibrillation should be prescribed therapeutic anticoagulation. Patients who are already on anticoagulation for VTE or atrial fibrillation should continue their therapy unless the platelet count is less than 30-50x109/L or if the fibrinogen is less than 1.0 g/L.16
Conclusion
Coagulopathies associated with COVID-19 infections have been documented in several studies around the world, and it has been shown to be fatal in some cases. Despite documentation, the mechanism behind this coagulopathy is not well understood. Because of the potentially lethal complications associated with coagulopathies, early recognition and anticoagulation is imperative to improve clinical outcomes. These results are very preliminary: More studies are required to understand the role of anticoagulation and its effect on the morbidity and mortality associated with COVID-19–associated coagulopathy.
Dr. Yeruva is a board-certified hematologist/medical oncologist with WellSpan Health and clinical assistant professor of internal medicine, Penn State University, Hershey. Mr. Henderson is a third-year graduate-entry medical student at the Royal College of Surgeons in Ireland with interests in family medicine, dermatology, and tropical diseases. Dr. Al-Tawfiq is a consultant of internal medicine & infectious diseases, and the director of quality at Johns Hopkins Aramco Healthcare in Dhahran, Saudi Arabia, an adjunct associate professor of infectious diseases, molecular medicine and clinical pharmacology at Johns Hopkins University School of Medicine, and adjunct associate professor at Indiana University School of Medicine, Indianapolis. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals.
References
1. World Health Organization. Coronavirus disease (COVID-2019) situation reports.
2. Lippi G et al. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin Chim Acta. 2020 Mar 13. 506:145-8. doi: 10.1016/j.cca.2020.03.022.
3. Klok FA et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Throm Res. 2020;18(4):844-7. doi: 10.1016/j.thromres.2020.04.013.
4. Fox S et al. Pulmonary and cardiac pathology in Covid-19: The first autopsy series from New Orleans. MedRxiv. 2020 Apr 10. doi: 10.1101/2020.04.06.20050575.
5. Yang M et al. Thrombocytopenia in patients with severe acute respiratory syndrome (review). Hematology 2013 Sep 4. doi: 10.1080/1024533040002617.
6. Giannis D et al. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020 June. doi: 10.1016/j.jcv.2020.104362.
7. Guo W et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020 Mar 31. doi: 10.1002/dmrr.3319.
8. Manalo IF et al. A dermatologic manifestation of COVID-19: Transient livedo reticularis. J Am Acad Dermat. 2020 Apr. doi: 10.1016/j.jaad.2020.04.018.
9. Tang N et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020 Feb 19. doi: 10.1111/jth.14768, 18: 844-847.
10. Huang C et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020 Jan 24. doi: 10.1016/S0140-6736(20)30183-5.
11. Thachil J et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020 Mar 25. doi: 10.1111/JTH.14810.
12. Tang N et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020 Mar 27. doi: 10.1111/JTH.14817.
13. Zhang Y et al. Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia. Zhonghua Xue Ye Xue Za Zhi. 2020 Mar 28. doi: 10.3760/cma.j.issn.0253-2727.2020.0006.
14. Poterucha TJ et al. More than an anticoagulant: Do heparins have direct anti-inflammatory effects? Thromb Haemost. 2017. doi: 10.1160/TH16-08-0620.
15. Mousavi S et al. Anti-inflammatory effects of heparin and its derivatives: A systematic review. Adv Pharmacol Pharm Sci. 2015 May 12. doi: 10.1155/2015/507151.
16. Kreuziger L et al. COVID-19 and VTE/anticoagulation: Frequently asked questions. American Society of Hematology. 2020 Apr 17.
COVID-19: What are the major cardiovascular issues?
Acute viral myocarditis often confounds with ischemic injury
Frontline health care workers are facing escalating challenges with rapidly spreading coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.1 Hospitalists will often deal with various manifestations of acute cardiac injury, controversial withholding of ACE inhibitors (ACEI) or angiotensin receptor blockers (ARBs), arrhythmic toxicities from such drug therapies as hydroxychloroquine.
Presentation and cardiac risks from COVID-19
Patients with COVID-19 often have presented with noncardiac symptoms, usually a febrile illness associated with cough or shortness of breath. Recent reports from Italy and New York have suggested patients also can present with isolated cardiac involvement without any other symptoms that can portend a grim prognosis.2 Cardiac effects include myocarditis, acute coronary syndrome, malignant arrhythmias ultimately cardiogenic shock and cardiac arrest.3
The mortality rate correlates with older age, preexisting health conditions, and availability of medical resources. A recent meta-analysis including 53,000 COVID-19 patients found the most common comorbidities were hypertension (19%), diabetes (8 %) and cardiovascular disease (CVD) (3%).4 Half of the cases died from respiratory failure and one-third have died from concomitant respiratory and heart failure. Acute heart failure alone accounted for about 7% of cases.5
Overall mortality rate can be better understood with the largest case series to-date of COVID-19 in mainland China published by the Chinese Center for Disease Control and Prevention. The overall case-fatality rate was 2.3% (1,023 deaths among 44,672 confirmed cases), but the mortality reached 10.5% in patients with underlying CVD.6
Acute cardiac injuries in COVID-19
Acute cardiac injury (ACI) is defined as troponin elevation above the 99th percentile of the upper reference limit.7 A practical description of ACI in COVID-19 patients should also include broader definition with new abnormalities in ECG since not all patients with acute cardiac effects have developed troponin elevation.3 More recent reports showed up to 28% of hospitalized patients had a myocardial injury.3
It is not uncommon to see a patient with COVID-19 myocarditis as a mimicker of acute ST-elevation myocardial infarction (STEMI). The mechanism of ACI is unknown, though several hypotheses have been proposed based on case series and retrospective reviews. These include direct viral invasion into myocardial cells leading to myocarditis, oxygen demand-supply mismatch, acute coronary syndrome from plaque rupture, stress, or cytokine-mediated cardiomyopathy.3 The exact incidence of true MI from occlusive coronary disease in the COVID-19 population is yet unknown.
In some cases, troponin elevation may be a late manifestation of COVID-19. As coronavirus disease progressed slowly, a rapid rise of troponin was noted when patients developed acute respiratory failure after 10 days of illness. Among nonsurvivors, a steady rise in troponin was observed from day 4 through day 22.8
ACI is associated with ICU admission and mortality. Both troponin and BNP levels increased significantly during the course of hospitalization in those who ultimately died, but no such changes were evident in survivors.3 ACI was higher in nonsurvivors (59%) than in survivors (1%).8 ACI was higher in ICU patients (22%), compared with non-ICU patients (2%).9 Patients with CVD were more likely to exhibit elevation of troponin levels (54%), compared with patients without CVD (13%).3
Higher troponin levels and the presence of CVD are directly proportional to severe disease and death. Patients with elevated troponin developed more frequent complications including acute respiratory distress syndrome, malignant arrhythmias including ventricular tachycardia/ventricular fibrillation, acute coagulopathy, and acute kidney injury.3,8 Death was markedly higher in patients with elevated troponin, compared with normal levels: 60% versus 9%. Only 8% with no CVD and normal troponin died, whereas 69% of people with underlying CVD and elevated troponin died.3
The median duration from illness onset to death was 23 (8-41) days in the group with elevated troponin. Patients with CVD and escalation of troponin levels had the shortest survival of 1-5 days. The dynamic rise of cardiac biomarkers and increased incidence of malignant arrhythmias during the course of illness shows that myocardial injury played a greater role in the fatal outcome of COVID-19 than the presence of preexisting CVD itself.3
Management of acute cardiac issues in COVID-19
There are no established therapeutic options with randomized, clinical trials specific to the management of COVID-19 patients at this point. Standard supportive care and individualized treatment plan based on existing guidelines is probably the best approach. Disposition of cases and cardiac testing should be tailored, based on local protocols, availability of resources and expertise.10
There seems to be a consensus that baseline troponin levels should be obtained in all admitted patients. Repeat troponin levels can be obtained based on the severity of illness, for example, daily troponin checks are reasonable in ICU patients and every-other-day troponin testing may be reasonable in general inpatients. Routine troponin testing in minimally symptomatic or asymptomatic patients will likely not change any outcome.3,11,12
Daily ECG is reasonable in severe COVID-19. However, routine transthoracic ECGs are not reasonable, unless it will change further treatment plans. Transthoracic electrocardiograms (TTE) are reasonable in patients with significant troponin elevation, a decline in central venous oxygen saturation, new heart failure, shock, new persistent arrhythmias, or significant new ECG changes.12
Limited TTEs for a focused exam enough to answer the clinical question should be ordered to minimize the risk of viral exposure to the sonographers. Transesophageal echo will rarely be needed, and its use should be minimized to reduce direct contact exposure and because of anesthesia risks.13 Routine stress testing should not be ordered in active COVID-19 and should be deferred for outpatient evaluation, if clinically indicated, once the patient recovers from the infection.12
Myocarditis and pericarditis are potential manifestations of acute cardiac injury. Recent case reports have suggested evidence of myocarditis confirmed with cardiac MRI.11 Because of high fatality rates with cardiac involvement and no proven therapies yet, the role of routine advanced cardiac imaging such as cardiac CT, cardiac MRI, or cardiac biopsy is unclear.
Myocarditis can likely be caused either by the virus itself, or the body’s immune and inflammatory response (cytokine storm) to the virus.2,3 The use of anti-inflammatory drugs like colchicine, ibuprofen, steroids, or statins is not yet established.10,12 Drugs like remdesivir, lopinavir-ritonavir, hydroxychloroquine, chloroquine, and anti-interleukin-6 agents have been invariably used with some anecdotal success and randomized clinical trials for some of these drugs are presently undergoing.
Physicians may encounter situations to call a STEMI code or not in COVID-19 patients.2,11 Patients may have substernal pain, diffuse or regional ST elevations in ECG and reduced left ventricular dysfunction with regional wall motion abnormalities on ECG. These findings may be casued by myocarditis, acute type 1 MI, or stress-induced cardiomyopathy. Clinicians should make their judgment based on the overall pretest probability for type 1 MI, incorporating risk factor profiles and the presence of typical symptoms.
Treatment practice for questionable STEMI cases will likely vary across the country as we are learning more about the virus. Cath lab operators are at risk for COVID-19 infection through direct contact with patients. Few cardiologists were admitted after COVID-19 infections in the ICU at a New York hospital after they were involved in a acute MI case in a cath lab.14 Based on the Chinese experience, some have suggested the idea of lytic therapy first with follow-up cardiac CT to assess the recanalization of perfusion status, but at this point, this strategy remains controversial in the United States. In addition, if the patient has myocarditis instead, there will be a risk for pericardial effusion and hemorrhagic complications with lytic therapy.
Case examples
1. A 70-year-old male presents with fevers, chest pain, cough, shortness of breath. He has a history of metabolic syndrome and 30 pack-years of smoking. His ECG showed 1.5 mm ST elevation in inferior leads with reciprocal ST depressions in lateral leads, and his initial troponin is 2. Echocardiogram showed reduced left ventricle ejection fraction of 32% and inferior wall hypokinesis. He is suspected COVID-19 and his PCR result is pending. How would you manage this patient?
This patient presented with febrile illness and, but he had a very high pretest probability for obstructive coronary artery disease based on his age, male sex, and multiple risk factors. He may have a viral syndrome and it is a stressful situation for him. This may have precipitated plaque rupture causing acute MI.
Activating the STEMI pathway for emergent left heart catheterization is likely appropriate in this case. Coronary angiogram in this patient showed a 100% occluded mid-right coronary artery with a fresh thrombus. Delaying cardiac cath would have possibly led to malignant arrhythmias and death from ischemic injury. We need to be cognizant patients can die from non–COVID-related emergencies also.
2. An 18-year-old healthy male presents with cough and chest pain and has bilateral lung infiltrates. ECG showed anterolateral 2 mm ST elevations and no reciprocal ST changes. Stat TTE showed anterior wall hypokinesis and LV function 30% and his initial troponin are 0.6 (normal is < .05). The nasopharyngeal swab is sent out and his COVID result is pending. How would you manage this patient?
A young patient with no cardiovascular risk factors has a very low pretest probability for obstructive coronary disease and the likelihood of having a true ischemic MI is low even though he has significant new ST elevations. Especially with presumed COVID-19 and risk of virus exposure to the cath lab personnel, it will be prudent to manage this patient with supportive therapy including beta-blockers, ACEIs, etc. Repeat echo in 7 days before discharge showed improved LVEF 45%.
Controversy on ACEI/ARB
The SARS-CoV-2 virus enters via cell-entry receptor namely angiotensin-converting enzyme 2 (ACE2). SARS-CoV-2 is thought to have a higher affinity for ACE2 than other SARS-viruses.15
ACE2 is expressed in the heart, lungs, vasculature, and kidneys. ACEI and ARBs in animal models increase the expression of ACE2,16 though this has not been confirmed in human studies. This has led to the hypothesis that ACEI and ARBs might worsen myocarditis or precipitate the acute coronary syndrome. It has also been hypothesized that the upregulation of ACE2 is therapeutic in COVID-19 and that ARBs might be protective during infection.17
The increased ACE2 expression induced by ACEI or ARB would aggravate lung injury of patients with COVID-19. However, a previous study showed a beneficial effect of ACEI/ARB in patients admitted with viral pneumonia, as it significantly reduced the pulmonary inflammatory response and cytokine release caused by virus infection.18
Therefore, this remains an area of investigation and it is unclear how these medications affect patients with COVID-19. In a recent review, with a limited number of patients, the mortality of those treated with or without the use of ACEI/ARB did not show a significant difference in the outcome.3
Both American and European cardiology societies recommend against routine discontinuation of ACEI and ARBs in patients with COVID-19 because of risks of uncontrolled hypertension and heart failure, stroke, or heart attack.19 However, it will be reasonable to hold off in inpatients in cases of acute kidney injury, hypotension, shock, etc.12
Cardiac concern about hydroxychloroquine and chloroquine
Hydroxychloroquine (HCQ) is an antimalarial drug shown to have in vitro (but not yet in vivo) activity against diverse RNA viruses, including SARS-CoV-1.20 An expert consensus group from China suggests that chloroquine improved lung imaging and shortened disease course.21 HCQ was found to be more potent than chloroquine in inhibiting SARS-CoV-2 in vitro.22
Based on limited in vitro and anecdotal clinical data from other countries, the U.S. Food and Drug Administration recently authorized emergency use of chloroquine and HCQ in hopes of slowing the progression of the disease when a clinical trial is not available, or participation is not feasible for use of these drugs in hospitalized patients. However, with no clear benefit, there is a concern for possible risks with cardiac toxicity.
HCQ is known to cause cardiomyopathy in a dose-dependent manner over several years. Given the anticipated short duration in COVID-19, it is not an expected risk. QT-segment prolongation and torsades de pointes, especially if administered in combination with azithromycin, is possible even in short term use.23
Given above, frequent ECG monitoring is indicated for patients being treated with chloroquine or HCQ. All other QT-prolonging drugs should be discontinued. Continuous telemetry monitoring while under treatment is reasonable. HCQ should not be started if baseline QTc is > 500 msec and it should be stopped if the patient develops ventricular arrhythmias.12
Dr. Subedi is a noninvasive cardiologist for Wellspan Health System in Franklin and Cumberland counties in south central Pennsylvania. He is a clinical assistant professor of medicine at Penn State College of Medicine, Hershey, Pa. He is an active member of the critical care committee at Wellspan Chambersburg (Pa.) Hospital. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro Hospitals, all in Pennsylvania. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Areti is currently working as a hospitalist at Wellspan Chambersburg Hospital and is a member of the Wellspan pharmacy and therapeutics committee. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson.
Key points
- Acute cardiac injury or myocarditis is common among patients infected with COVID-19. Often, COVID myocarditis can mimic acute MI or stress cardiomyopathy and will present diagnostic and therapeutic challenges. On the other hand, isolated cardiac involvement can occur, even without symptoms and signs of interstitial pneumonia.
- A most important indicator of worse prediction is the degree of myocardial injury, regardless of preexisting conditions or underlying cardiovascular disease.
- Early recognition of cardiac involvement will be helpful in targeting more aggressive supportive therapies. Commonly available clinical tools like bloodwork, ECG, or echocardiogram should be adequate to diagnose carditis in most cases.
- Advanced cardiac imaging tests or cardiac biopsy are of uncertain benefits. Meticulous evaluation is needed for possible ischemic changes before taking the patient to the cardiac cath lab in order to reduce unnecessary virus exposure to the operators.
- ACEI/ARB should be continued in most cases in COVID patients based on cardiology societies’ recommendations.
- With the widespread use of antimalarial drugs like chloroquine or hydroxychloroquine, frequent ECG and continuous telemetry monitoring is reasonable to rule out ventricular arrhythmias like torsades.
- There is no specific treatment to date for acute cardiac injuries. Since there are no specific guidelines and information about the virus is rapidly changing, it will be prudent to follow common-sense approaches outlined by institutions like the Brigham and Women’s Hospital COVID-19 Critical Care clinical guidelines, which incorporate new clinical information on a daily basis ().
References
1. Rothan HA and Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020 May;109:102433. doi: 10.1016/j.jaut.2020.102433.
2. Kolata G. A heart attack? No, it was the coronavirus. New York Times 2020 Mar 27.
3. Guo T et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020 Mar 27. doi: 10.1001/jamacardio.2020.1017.
4. Zhao X et al. Incidence, clinical characteristics and prognostic factor of patients with COVID-19: a systematic review and meta-analysis. MedRxIV. 2020 Mar 20. doi: 10.1101/2020.03.17.20037572.
5. Ruan Q et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 Mar 3. doi: 10.1007/s00134-020-05991-x.
6. Wu Z and McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648.
7. Thygesen K et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018 Oct;72:2231-64.
8. Zhou F et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054-62.
9. Wang D et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 Feb 7. doi: 10.1001/jama.2020.1585.
10. CDC: Therapeutic options for patients with COVID-19. Updated April 13, 2020.
11. Inciardi RM et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020 Mar 27. doi: 10.1001/jamacardio.2020.1096.
12. Brigham and Women’s Hospital COVID-19 Critical Care Clinical Guidelines.
13. American Society of Echocardiography Statement on COVID-19. 2020 Apr 1.
14. A cardiologist in Brooklyn infected with COVID-19. @jigneshpatelMD. 2020 Mar 20.
15. Paules CI et al. Coronavirus infections – more than just the common cold. JAMA. 2020 Jan 23. doi: 10.1001/jama.2020.0757.
16. Zheng YY et al. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020 May;17(5):259-60.
17. Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 2020 Mar 4. doi: 10.1002/ddr.21656.
18. Henry C et al. Impact of angiotensin-converting enzyme inhibitors and statins on viral pneumonia. Proc (Bayl Univ Med Cent). 2018 Oct 26;31(4):419-23.
19. HFSA/ACC/AHA statement addresses concerns re: Using RAAS antagonists in COVID-19. 2020 Mar 17.
20. Touret F and de Lamballerie X. Of chloroquine and COVID-19. Antiviral Res. 2020 May;177:104762. doi: 10.1016/j.antiviral.2020.104762.
21. Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia. Chinese journal of tuberculosis and respiratory diseases. 2020 Mar 12;43(3):185-8.
22. Yao X et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020 Mar 9. doi: 10.1093/cid/ciaa237.
23. Devaux CA et al. New insights on the antiviral effects of chloroquine against coronavirus: What to expect for COVID-19? Int J Antimicrob Agents. 2020 Mar 12:105938. doi: 10.1016/j.ijantimicag.2020.105938.
Acute viral myocarditis often confounds with ischemic injury
Acute viral myocarditis often confounds with ischemic injury
Frontline health care workers are facing escalating challenges with rapidly spreading coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.1 Hospitalists will often deal with various manifestations of acute cardiac injury, controversial withholding of ACE inhibitors (ACEI) or angiotensin receptor blockers (ARBs), arrhythmic toxicities from such drug therapies as hydroxychloroquine.
Presentation and cardiac risks from COVID-19
Patients with COVID-19 often have presented with noncardiac symptoms, usually a febrile illness associated with cough or shortness of breath. Recent reports from Italy and New York have suggested patients also can present with isolated cardiac involvement without any other symptoms that can portend a grim prognosis.2 Cardiac effects include myocarditis, acute coronary syndrome, malignant arrhythmias ultimately cardiogenic shock and cardiac arrest.3
The mortality rate correlates with older age, preexisting health conditions, and availability of medical resources. A recent meta-analysis including 53,000 COVID-19 patients found the most common comorbidities were hypertension (19%), diabetes (8 %) and cardiovascular disease (CVD) (3%).4 Half of the cases died from respiratory failure and one-third have died from concomitant respiratory and heart failure. Acute heart failure alone accounted for about 7% of cases.5
Overall mortality rate can be better understood with the largest case series to-date of COVID-19 in mainland China published by the Chinese Center for Disease Control and Prevention. The overall case-fatality rate was 2.3% (1,023 deaths among 44,672 confirmed cases), but the mortality reached 10.5% in patients with underlying CVD.6
Acute cardiac injuries in COVID-19
Acute cardiac injury (ACI) is defined as troponin elevation above the 99th percentile of the upper reference limit.7 A practical description of ACI in COVID-19 patients should also include broader definition with new abnormalities in ECG since not all patients with acute cardiac effects have developed troponin elevation.3 More recent reports showed up to 28% of hospitalized patients had a myocardial injury.3
It is not uncommon to see a patient with COVID-19 myocarditis as a mimicker of acute ST-elevation myocardial infarction (STEMI). The mechanism of ACI is unknown, though several hypotheses have been proposed based on case series and retrospective reviews. These include direct viral invasion into myocardial cells leading to myocarditis, oxygen demand-supply mismatch, acute coronary syndrome from plaque rupture, stress, or cytokine-mediated cardiomyopathy.3 The exact incidence of true MI from occlusive coronary disease in the COVID-19 population is yet unknown.
In some cases, troponin elevation may be a late manifestation of COVID-19. As coronavirus disease progressed slowly, a rapid rise of troponin was noted when patients developed acute respiratory failure after 10 days of illness. Among nonsurvivors, a steady rise in troponin was observed from day 4 through day 22.8
ACI is associated with ICU admission and mortality. Both troponin and BNP levels increased significantly during the course of hospitalization in those who ultimately died, but no such changes were evident in survivors.3 ACI was higher in nonsurvivors (59%) than in survivors (1%).8 ACI was higher in ICU patients (22%), compared with non-ICU patients (2%).9 Patients with CVD were more likely to exhibit elevation of troponin levels (54%), compared with patients without CVD (13%).3
Higher troponin levels and the presence of CVD are directly proportional to severe disease and death. Patients with elevated troponin developed more frequent complications including acute respiratory distress syndrome, malignant arrhythmias including ventricular tachycardia/ventricular fibrillation, acute coagulopathy, and acute kidney injury.3,8 Death was markedly higher in patients with elevated troponin, compared with normal levels: 60% versus 9%. Only 8% with no CVD and normal troponin died, whereas 69% of people with underlying CVD and elevated troponin died.3
The median duration from illness onset to death was 23 (8-41) days in the group with elevated troponin. Patients with CVD and escalation of troponin levels had the shortest survival of 1-5 days. The dynamic rise of cardiac biomarkers and increased incidence of malignant arrhythmias during the course of illness shows that myocardial injury played a greater role in the fatal outcome of COVID-19 than the presence of preexisting CVD itself.3
Management of acute cardiac issues in COVID-19
There are no established therapeutic options with randomized, clinical trials specific to the management of COVID-19 patients at this point. Standard supportive care and individualized treatment plan based on existing guidelines is probably the best approach. Disposition of cases and cardiac testing should be tailored, based on local protocols, availability of resources and expertise.10
There seems to be a consensus that baseline troponin levels should be obtained in all admitted patients. Repeat troponin levels can be obtained based on the severity of illness, for example, daily troponin checks are reasonable in ICU patients and every-other-day troponin testing may be reasonable in general inpatients. Routine troponin testing in minimally symptomatic or asymptomatic patients will likely not change any outcome.3,11,12
Daily ECG is reasonable in severe COVID-19. However, routine transthoracic ECGs are not reasonable, unless it will change further treatment plans. Transthoracic electrocardiograms (TTE) are reasonable in patients with significant troponin elevation, a decline in central venous oxygen saturation, new heart failure, shock, new persistent arrhythmias, or significant new ECG changes.12
Limited TTEs for a focused exam enough to answer the clinical question should be ordered to minimize the risk of viral exposure to the sonographers. Transesophageal echo will rarely be needed, and its use should be minimized to reduce direct contact exposure and because of anesthesia risks.13 Routine stress testing should not be ordered in active COVID-19 and should be deferred for outpatient evaluation, if clinically indicated, once the patient recovers from the infection.12
Myocarditis and pericarditis are potential manifestations of acute cardiac injury. Recent case reports have suggested evidence of myocarditis confirmed with cardiac MRI.11 Because of high fatality rates with cardiac involvement and no proven therapies yet, the role of routine advanced cardiac imaging such as cardiac CT, cardiac MRI, or cardiac biopsy is unclear.
Myocarditis can likely be caused either by the virus itself, or the body’s immune and inflammatory response (cytokine storm) to the virus.2,3 The use of anti-inflammatory drugs like colchicine, ibuprofen, steroids, or statins is not yet established.10,12 Drugs like remdesivir, lopinavir-ritonavir, hydroxychloroquine, chloroquine, and anti-interleukin-6 agents have been invariably used with some anecdotal success and randomized clinical trials for some of these drugs are presently undergoing.
Physicians may encounter situations to call a STEMI code or not in COVID-19 patients.2,11 Patients may have substernal pain, diffuse or regional ST elevations in ECG and reduced left ventricular dysfunction with regional wall motion abnormalities on ECG. These findings may be casued by myocarditis, acute type 1 MI, or stress-induced cardiomyopathy. Clinicians should make their judgment based on the overall pretest probability for type 1 MI, incorporating risk factor profiles and the presence of typical symptoms.
Treatment practice for questionable STEMI cases will likely vary across the country as we are learning more about the virus. Cath lab operators are at risk for COVID-19 infection through direct contact with patients. Few cardiologists were admitted after COVID-19 infections in the ICU at a New York hospital after they were involved in a acute MI case in a cath lab.14 Based on the Chinese experience, some have suggested the idea of lytic therapy first with follow-up cardiac CT to assess the recanalization of perfusion status, but at this point, this strategy remains controversial in the United States. In addition, if the patient has myocarditis instead, there will be a risk for pericardial effusion and hemorrhagic complications with lytic therapy.
Case examples
1. A 70-year-old male presents with fevers, chest pain, cough, shortness of breath. He has a history of metabolic syndrome and 30 pack-years of smoking. His ECG showed 1.5 mm ST elevation in inferior leads with reciprocal ST depressions in lateral leads, and his initial troponin is 2. Echocardiogram showed reduced left ventricle ejection fraction of 32% and inferior wall hypokinesis. He is suspected COVID-19 and his PCR result is pending. How would you manage this patient?
This patient presented with febrile illness and, but he had a very high pretest probability for obstructive coronary artery disease based on his age, male sex, and multiple risk factors. He may have a viral syndrome and it is a stressful situation for him. This may have precipitated plaque rupture causing acute MI.
Activating the STEMI pathway for emergent left heart catheterization is likely appropriate in this case. Coronary angiogram in this patient showed a 100% occluded mid-right coronary artery with a fresh thrombus. Delaying cardiac cath would have possibly led to malignant arrhythmias and death from ischemic injury. We need to be cognizant patients can die from non–COVID-related emergencies also.
2. An 18-year-old healthy male presents with cough and chest pain and has bilateral lung infiltrates. ECG showed anterolateral 2 mm ST elevations and no reciprocal ST changes. Stat TTE showed anterior wall hypokinesis and LV function 30% and his initial troponin are 0.6 (normal is < .05). The nasopharyngeal swab is sent out and his COVID result is pending. How would you manage this patient?
A young patient with no cardiovascular risk factors has a very low pretest probability for obstructive coronary disease and the likelihood of having a true ischemic MI is low even though he has significant new ST elevations. Especially with presumed COVID-19 and risk of virus exposure to the cath lab personnel, it will be prudent to manage this patient with supportive therapy including beta-blockers, ACEIs, etc. Repeat echo in 7 days before discharge showed improved LVEF 45%.
Controversy on ACEI/ARB
The SARS-CoV-2 virus enters via cell-entry receptor namely angiotensin-converting enzyme 2 (ACE2). SARS-CoV-2 is thought to have a higher affinity for ACE2 than other SARS-viruses.15
ACE2 is expressed in the heart, lungs, vasculature, and kidneys. ACEI and ARBs in animal models increase the expression of ACE2,16 though this has not been confirmed in human studies. This has led to the hypothesis that ACEI and ARBs might worsen myocarditis or precipitate the acute coronary syndrome. It has also been hypothesized that the upregulation of ACE2 is therapeutic in COVID-19 and that ARBs might be protective during infection.17
The increased ACE2 expression induced by ACEI or ARB would aggravate lung injury of patients with COVID-19. However, a previous study showed a beneficial effect of ACEI/ARB in patients admitted with viral pneumonia, as it significantly reduced the pulmonary inflammatory response and cytokine release caused by virus infection.18
Therefore, this remains an area of investigation and it is unclear how these medications affect patients with COVID-19. In a recent review, with a limited number of patients, the mortality of those treated with or without the use of ACEI/ARB did not show a significant difference in the outcome.3
Both American and European cardiology societies recommend against routine discontinuation of ACEI and ARBs in patients with COVID-19 because of risks of uncontrolled hypertension and heart failure, stroke, or heart attack.19 However, it will be reasonable to hold off in inpatients in cases of acute kidney injury, hypotension, shock, etc.12
Cardiac concern about hydroxychloroquine and chloroquine
Hydroxychloroquine (HCQ) is an antimalarial drug shown to have in vitro (but not yet in vivo) activity against diverse RNA viruses, including SARS-CoV-1.20 An expert consensus group from China suggests that chloroquine improved lung imaging and shortened disease course.21 HCQ was found to be more potent than chloroquine in inhibiting SARS-CoV-2 in vitro.22
Based on limited in vitro and anecdotal clinical data from other countries, the U.S. Food and Drug Administration recently authorized emergency use of chloroquine and HCQ in hopes of slowing the progression of the disease when a clinical trial is not available, or participation is not feasible for use of these drugs in hospitalized patients. However, with no clear benefit, there is a concern for possible risks with cardiac toxicity.
HCQ is known to cause cardiomyopathy in a dose-dependent manner over several years. Given the anticipated short duration in COVID-19, it is not an expected risk. QT-segment prolongation and torsades de pointes, especially if administered in combination with azithromycin, is possible even in short term use.23
Given above, frequent ECG monitoring is indicated for patients being treated with chloroquine or HCQ. All other QT-prolonging drugs should be discontinued. Continuous telemetry monitoring while under treatment is reasonable. HCQ should not be started if baseline QTc is > 500 msec and it should be stopped if the patient develops ventricular arrhythmias.12
Dr. Subedi is a noninvasive cardiologist for Wellspan Health System in Franklin and Cumberland counties in south central Pennsylvania. He is a clinical assistant professor of medicine at Penn State College of Medicine, Hershey, Pa. He is an active member of the critical care committee at Wellspan Chambersburg (Pa.) Hospital. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro Hospitals, all in Pennsylvania. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Areti is currently working as a hospitalist at Wellspan Chambersburg Hospital and is a member of the Wellspan pharmacy and therapeutics committee. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson.
Key points
- Acute cardiac injury or myocarditis is common among patients infected with COVID-19. Often, COVID myocarditis can mimic acute MI or stress cardiomyopathy and will present diagnostic and therapeutic challenges. On the other hand, isolated cardiac involvement can occur, even without symptoms and signs of interstitial pneumonia.
- A most important indicator of worse prediction is the degree of myocardial injury, regardless of preexisting conditions or underlying cardiovascular disease.
- Early recognition of cardiac involvement will be helpful in targeting more aggressive supportive therapies. Commonly available clinical tools like bloodwork, ECG, or echocardiogram should be adequate to diagnose carditis in most cases.
- Advanced cardiac imaging tests or cardiac biopsy are of uncertain benefits. Meticulous evaluation is needed for possible ischemic changes before taking the patient to the cardiac cath lab in order to reduce unnecessary virus exposure to the operators.
- ACEI/ARB should be continued in most cases in COVID patients based on cardiology societies’ recommendations.
- With the widespread use of antimalarial drugs like chloroquine or hydroxychloroquine, frequent ECG and continuous telemetry monitoring is reasonable to rule out ventricular arrhythmias like torsades.
- There is no specific treatment to date for acute cardiac injuries. Since there are no specific guidelines and information about the virus is rapidly changing, it will be prudent to follow common-sense approaches outlined by institutions like the Brigham and Women’s Hospital COVID-19 Critical Care clinical guidelines, which incorporate new clinical information on a daily basis ().
References
1. Rothan HA and Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020 May;109:102433. doi: 10.1016/j.jaut.2020.102433.
2. Kolata G. A heart attack? No, it was the coronavirus. New York Times 2020 Mar 27.
3. Guo T et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020 Mar 27. doi: 10.1001/jamacardio.2020.1017.
4. Zhao X et al. Incidence, clinical characteristics and prognostic factor of patients with COVID-19: a systematic review and meta-analysis. MedRxIV. 2020 Mar 20. doi: 10.1101/2020.03.17.20037572.
5. Ruan Q et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 Mar 3. doi: 10.1007/s00134-020-05991-x.
6. Wu Z and McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648.
7. Thygesen K et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018 Oct;72:2231-64.
8. Zhou F et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054-62.
9. Wang D et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 Feb 7. doi: 10.1001/jama.2020.1585.
10. CDC: Therapeutic options for patients with COVID-19. Updated April 13, 2020.
11. Inciardi RM et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020 Mar 27. doi: 10.1001/jamacardio.2020.1096.
12. Brigham and Women’s Hospital COVID-19 Critical Care Clinical Guidelines.
13. American Society of Echocardiography Statement on COVID-19. 2020 Apr 1.
14. A cardiologist in Brooklyn infected with COVID-19. @jigneshpatelMD. 2020 Mar 20.
15. Paules CI et al. Coronavirus infections – more than just the common cold. JAMA. 2020 Jan 23. doi: 10.1001/jama.2020.0757.
16. Zheng YY et al. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020 May;17(5):259-60.
17. Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 2020 Mar 4. doi: 10.1002/ddr.21656.
18. Henry C et al. Impact of angiotensin-converting enzyme inhibitors and statins on viral pneumonia. Proc (Bayl Univ Med Cent). 2018 Oct 26;31(4):419-23.
19. HFSA/ACC/AHA statement addresses concerns re: Using RAAS antagonists in COVID-19. 2020 Mar 17.
20. Touret F and de Lamballerie X. Of chloroquine and COVID-19. Antiviral Res. 2020 May;177:104762. doi: 10.1016/j.antiviral.2020.104762.
21. Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia. Chinese journal of tuberculosis and respiratory diseases. 2020 Mar 12;43(3):185-8.
22. Yao X et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020 Mar 9. doi: 10.1093/cid/ciaa237.
23. Devaux CA et al. New insights on the antiviral effects of chloroquine against coronavirus: What to expect for COVID-19? Int J Antimicrob Agents. 2020 Mar 12:105938. doi: 10.1016/j.ijantimicag.2020.105938.
Frontline health care workers are facing escalating challenges with rapidly spreading coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.1 Hospitalists will often deal with various manifestations of acute cardiac injury, controversial withholding of ACE inhibitors (ACEI) or angiotensin receptor blockers (ARBs), arrhythmic toxicities from such drug therapies as hydroxychloroquine.
Presentation and cardiac risks from COVID-19
Patients with COVID-19 often have presented with noncardiac symptoms, usually a febrile illness associated with cough or shortness of breath. Recent reports from Italy and New York have suggested patients also can present with isolated cardiac involvement without any other symptoms that can portend a grim prognosis.2 Cardiac effects include myocarditis, acute coronary syndrome, malignant arrhythmias ultimately cardiogenic shock and cardiac arrest.3
The mortality rate correlates with older age, preexisting health conditions, and availability of medical resources. A recent meta-analysis including 53,000 COVID-19 patients found the most common comorbidities were hypertension (19%), diabetes (8 %) and cardiovascular disease (CVD) (3%).4 Half of the cases died from respiratory failure and one-third have died from concomitant respiratory and heart failure. Acute heart failure alone accounted for about 7% of cases.5
Overall mortality rate can be better understood with the largest case series to-date of COVID-19 in mainland China published by the Chinese Center for Disease Control and Prevention. The overall case-fatality rate was 2.3% (1,023 deaths among 44,672 confirmed cases), but the mortality reached 10.5% in patients with underlying CVD.6
Acute cardiac injuries in COVID-19
Acute cardiac injury (ACI) is defined as troponin elevation above the 99th percentile of the upper reference limit.7 A practical description of ACI in COVID-19 patients should also include broader definition with new abnormalities in ECG since not all patients with acute cardiac effects have developed troponin elevation.3 More recent reports showed up to 28% of hospitalized patients had a myocardial injury.3
It is not uncommon to see a patient with COVID-19 myocarditis as a mimicker of acute ST-elevation myocardial infarction (STEMI). The mechanism of ACI is unknown, though several hypotheses have been proposed based on case series and retrospective reviews. These include direct viral invasion into myocardial cells leading to myocarditis, oxygen demand-supply mismatch, acute coronary syndrome from plaque rupture, stress, or cytokine-mediated cardiomyopathy.3 The exact incidence of true MI from occlusive coronary disease in the COVID-19 population is yet unknown.
In some cases, troponin elevation may be a late manifestation of COVID-19. As coronavirus disease progressed slowly, a rapid rise of troponin was noted when patients developed acute respiratory failure after 10 days of illness. Among nonsurvivors, a steady rise in troponin was observed from day 4 through day 22.8
ACI is associated with ICU admission and mortality. Both troponin and BNP levels increased significantly during the course of hospitalization in those who ultimately died, but no such changes were evident in survivors.3 ACI was higher in nonsurvivors (59%) than in survivors (1%).8 ACI was higher in ICU patients (22%), compared with non-ICU patients (2%).9 Patients with CVD were more likely to exhibit elevation of troponin levels (54%), compared with patients without CVD (13%).3
Higher troponin levels and the presence of CVD are directly proportional to severe disease and death. Patients with elevated troponin developed more frequent complications including acute respiratory distress syndrome, malignant arrhythmias including ventricular tachycardia/ventricular fibrillation, acute coagulopathy, and acute kidney injury.3,8 Death was markedly higher in patients with elevated troponin, compared with normal levels: 60% versus 9%. Only 8% with no CVD and normal troponin died, whereas 69% of people with underlying CVD and elevated troponin died.3
The median duration from illness onset to death was 23 (8-41) days in the group with elevated troponin. Patients with CVD and escalation of troponin levels had the shortest survival of 1-5 days. The dynamic rise of cardiac biomarkers and increased incidence of malignant arrhythmias during the course of illness shows that myocardial injury played a greater role in the fatal outcome of COVID-19 than the presence of preexisting CVD itself.3
Management of acute cardiac issues in COVID-19
There are no established therapeutic options with randomized, clinical trials specific to the management of COVID-19 patients at this point. Standard supportive care and individualized treatment plan based on existing guidelines is probably the best approach. Disposition of cases and cardiac testing should be tailored, based on local protocols, availability of resources and expertise.10
There seems to be a consensus that baseline troponin levels should be obtained in all admitted patients. Repeat troponin levels can be obtained based on the severity of illness, for example, daily troponin checks are reasonable in ICU patients and every-other-day troponin testing may be reasonable in general inpatients. Routine troponin testing in minimally symptomatic or asymptomatic patients will likely not change any outcome.3,11,12
Daily ECG is reasonable in severe COVID-19. However, routine transthoracic ECGs are not reasonable, unless it will change further treatment plans. Transthoracic electrocardiograms (TTE) are reasonable in patients with significant troponin elevation, a decline in central venous oxygen saturation, new heart failure, shock, new persistent arrhythmias, or significant new ECG changes.12
Limited TTEs for a focused exam enough to answer the clinical question should be ordered to minimize the risk of viral exposure to the sonographers. Transesophageal echo will rarely be needed, and its use should be minimized to reduce direct contact exposure and because of anesthesia risks.13 Routine stress testing should not be ordered in active COVID-19 and should be deferred for outpatient evaluation, if clinically indicated, once the patient recovers from the infection.12
Myocarditis and pericarditis are potential manifestations of acute cardiac injury. Recent case reports have suggested evidence of myocarditis confirmed with cardiac MRI.11 Because of high fatality rates with cardiac involvement and no proven therapies yet, the role of routine advanced cardiac imaging such as cardiac CT, cardiac MRI, or cardiac biopsy is unclear.
Myocarditis can likely be caused either by the virus itself, or the body’s immune and inflammatory response (cytokine storm) to the virus.2,3 The use of anti-inflammatory drugs like colchicine, ibuprofen, steroids, or statins is not yet established.10,12 Drugs like remdesivir, lopinavir-ritonavir, hydroxychloroquine, chloroquine, and anti-interleukin-6 agents have been invariably used with some anecdotal success and randomized clinical trials for some of these drugs are presently undergoing.
Physicians may encounter situations to call a STEMI code or not in COVID-19 patients.2,11 Patients may have substernal pain, diffuse or regional ST elevations in ECG and reduced left ventricular dysfunction with regional wall motion abnormalities on ECG. These findings may be casued by myocarditis, acute type 1 MI, or stress-induced cardiomyopathy. Clinicians should make their judgment based on the overall pretest probability for type 1 MI, incorporating risk factor profiles and the presence of typical symptoms.
Treatment practice for questionable STEMI cases will likely vary across the country as we are learning more about the virus. Cath lab operators are at risk for COVID-19 infection through direct contact with patients. Few cardiologists were admitted after COVID-19 infections in the ICU at a New York hospital after they were involved in a acute MI case in a cath lab.14 Based on the Chinese experience, some have suggested the idea of lytic therapy first with follow-up cardiac CT to assess the recanalization of perfusion status, but at this point, this strategy remains controversial in the United States. In addition, if the patient has myocarditis instead, there will be a risk for pericardial effusion and hemorrhagic complications with lytic therapy.
Case examples
1. A 70-year-old male presents with fevers, chest pain, cough, shortness of breath. He has a history of metabolic syndrome and 30 pack-years of smoking. His ECG showed 1.5 mm ST elevation in inferior leads with reciprocal ST depressions in lateral leads, and his initial troponin is 2. Echocardiogram showed reduced left ventricle ejection fraction of 32% and inferior wall hypokinesis. He is suspected COVID-19 and his PCR result is pending. How would you manage this patient?
This patient presented with febrile illness and, but he had a very high pretest probability for obstructive coronary artery disease based on his age, male sex, and multiple risk factors. He may have a viral syndrome and it is a stressful situation for him. This may have precipitated plaque rupture causing acute MI.
Activating the STEMI pathway for emergent left heart catheterization is likely appropriate in this case. Coronary angiogram in this patient showed a 100% occluded mid-right coronary artery with a fresh thrombus. Delaying cardiac cath would have possibly led to malignant arrhythmias and death from ischemic injury. We need to be cognizant patients can die from non–COVID-related emergencies also.
2. An 18-year-old healthy male presents with cough and chest pain and has bilateral lung infiltrates. ECG showed anterolateral 2 mm ST elevations and no reciprocal ST changes. Stat TTE showed anterior wall hypokinesis and LV function 30% and his initial troponin are 0.6 (normal is < .05). The nasopharyngeal swab is sent out and his COVID result is pending. How would you manage this patient?
A young patient with no cardiovascular risk factors has a very low pretest probability for obstructive coronary disease and the likelihood of having a true ischemic MI is low even though he has significant new ST elevations. Especially with presumed COVID-19 and risk of virus exposure to the cath lab personnel, it will be prudent to manage this patient with supportive therapy including beta-blockers, ACEIs, etc. Repeat echo in 7 days before discharge showed improved LVEF 45%.
Controversy on ACEI/ARB
The SARS-CoV-2 virus enters via cell-entry receptor namely angiotensin-converting enzyme 2 (ACE2). SARS-CoV-2 is thought to have a higher affinity for ACE2 than other SARS-viruses.15
ACE2 is expressed in the heart, lungs, vasculature, and kidneys. ACEI and ARBs in animal models increase the expression of ACE2,16 though this has not been confirmed in human studies. This has led to the hypothesis that ACEI and ARBs might worsen myocarditis or precipitate the acute coronary syndrome. It has also been hypothesized that the upregulation of ACE2 is therapeutic in COVID-19 and that ARBs might be protective during infection.17
The increased ACE2 expression induced by ACEI or ARB would aggravate lung injury of patients with COVID-19. However, a previous study showed a beneficial effect of ACEI/ARB in patients admitted with viral pneumonia, as it significantly reduced the pulmonary inflammatory response and cytokine release caused by virus infection.18
Therefore, this remains an area of investigation and it is unclear how these medications affect patients with COVID-19. In a recent review, with a limited number of patients, the mortality of those treated with or without the use of ACEI/ARB did not show a significant difference in the outcome.3
Both American and European cardiology societies recommend against routine discontinuation of ACEI and ARBs in patients with COVID-19 because of risks of uncontrolled hypertension and heart failure, stroke, or heart attack.19 However, it will be reasonable to hold off in inpatients in cases of acute kidney injury, hypotension, shock, etc.12
Cardiac concern about hydroxychloroquine and chloroquine
Hydroxychloroquine (HCQ) is an antimalarial drug shown to have in vitro (but not yet in vivo) activity against diverse RNA viruses, including SARS-CoV-1.20 An expert consensus group from China suggests that chloroquine improved lung imaging and shortened disease course.21 HCQ was found to be more potent than chloroquine in inhibiting SARS-CoV-2 in vitro.22
Based on limited in vitro and anecdotal clinical data from other countries, the U.S. Food and Drug Administration recently authorized emergency use of chloroquine and HCQ in hopes of slowing the progression of the disease when a clinical trial is not available, or participation is not feasible for use of these drugs in hospitalized patients. However, with no clear benefit, there is a concern for possible risks with cardiac toxicity.
HCQ is known to cause cardiomyopathy in a dose-dependent manner over several years. Given the anticipated short duration in COVID-19, it is not an expected risk. QT-segment prolongation and torsades de pointes, especially if administered in combination with azithromycin, is possible even in short term use.23
Given above, frequent ECG monitoring is indicated for patients being treated with chloroquine or HCQ. All other QT-prolonging drugs should be discontinued. Continuous telemetry monitoring while under treatment is reasonable. HCQ should not be started if baseline QTc is > 500 msec and it should be stopped if the patient develops ventricular arrhythmias.12
Dr. Subedi is a noninvasive cardiologist for Wellspan Health System in Franklin and Cumberland counties in south central Pennsylvania. He is a clinical assistant professor of medicine at Penn State College of Medicine, Hershey, Pa. He is an active member of the critical care committee at Wellspan Chambersburg (Pa.) Hospital. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro Hospitals, all in Pennsylvania. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Areti is currently working as a hospitalist at Wellspan Chambersburg Hospital and is a member of the Wellspan pharmacy and therapeutics committee. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson.
Key points
- Acute cardiac injury or myocarditis is common among patients infected with COVID-19. Often, COVID myocarditis can mimic acute MI or stress cardiomyopathy and will present diagnostic and therapeutic challenges. On the other hand, isolated cardiac involvement can occur, even without symptoms and signs of interstitial pneumonia.
- A most important indicator of worse prediction is the degree of myocardial injury, regardless of preexisting conditions or underlying cardiovascular disease.
- Early recognition of cardiac involvement will be helpful in targeting more aggressive supportive therapies. Commonly available clinical tools like bloodwork, ECG, or echocardiogram should be adequate to diagnose carditis in most cases.
- Advanced cardiac imaging tests or cardiac biopsy are of uncertain benefits. Meticulous evaluation is needed for possible ischemic changes before taking the patient to the cardiac cath lab in order to reduce unnecessary virus exposure to the operators.
- ACEI/ARB should be continued in most cases in COVID patients based on cardiology societies’ recommendations.
- With the widespread use of antimalarial drugs like chloroquine or hydroxychloroquine, frequent ECG and continuous telemetry monitoring is reasonable to rule out ventricular arrhythmias like torsades.
- There is no specific treatment to date for acute cardiac injuries. Since there are no specific guidelines and information about the virus is rapidly changing, it will be prudent to follow common-sense approaches outlined by institutions like the Brigham and Women’s Hospital COVID-19 Critical Care clinical guidelines, which incorporate new clinical information on a daily basis ().
References
1. Rothan HA and Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020 May;109:102433. doi: 10.1016/j.jaut.2020.102433.
2. Kolata G. A heart attack? No, it was the coronavirus. New York Times 2020 Mar 27.
3. Guo T et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020 Mar 27. doi: 10.1001/jamacardio.2020.1017.
4. Zhao X et al. Incidence, clinical characteristics and prognostic factor of patients with COVID-19: a systematic review and meta-analysis. MedRxIV. 2020 Mar 20. doi: 10.1101/2020.03.17.20037572.
5. Ruan Q et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 Mar 3. doi: 10.1007/s00134-020-05991-x.
6. Wu Z and McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648.
7. Thygesen K et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018 Oct;72:2231-64.
8. Zhou F et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054-62.
9. Wang D et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 Feb 7. doi: 10.1001/jama.2020.1585.
10. CDC: Therapeutic options for patients with COVID-19. Updated April 13, 2020.
11. Inciardi RM et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020 Mar 27. doi: 10.1001/jamacardio.2020.1096.
12. Brigham and Women’s Hospital COVID-19 Critical Care Clinical Guidelines.
13. American Society of Echocardiography Statement on COVID-19. 2020 Apr 1.
14. A cardiologist in Brooklyn infected with COVID-19. @jigneshpatelMD. 2020 Mar 20.
15. Paules CI et al. Coronavirus infections – more than just the common cold. JAMA. 2020 Jan 23. doi: 10.1001/jama.2020.0757.
16. Zheng YY et al. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020 May;17(5):259-60.
17. Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 2020 Mar 4. doi: 10.1002/ddr.21656.
18. Henry C et al. Impact of angiotensin-converting enzyme inhibitors and statins on viral pneumonia. Proc (Bayl Univ Med Cent). 2018 Oct 26;31(4):419-23.
19. HFSA/ACC/AHA statement addresses concerns re: Using RAAS antagonists in COVID-19. 2020 Mar 17.
20. Touret F and de Lamballerie X. Of chloroquine and COVID-19. Antiviral Res. 2020 May;177:104762. doi: 10.1016/j.antiviral.2020.104762.
21. Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia. Chinese journal of tuberculosis and respiratory diseases. 2020 Mar 12;43(3):185-8.
22. Yao X et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020 Mar 9. doi: 10.1093/cid/ciaa237.
23. Devaux CA et al. New insights on the antiviral effects of chloroquine against coronavirus: What to expect for COVID-19? Int J Antimicrob Agents. 2020 Mar 12:105938. doi: 10.1016/j.ijantimicag.2020.105938.
COVID-19 in pediatric patients: What the hospitalist needs to know
Coronavirus disease (COVID-19) was declared a pandemic by the World Health Organization on March 11. This rapidly spreading disease is caused by the novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The infection has spread to more than 140 countries, including the United States. As of March 16, more than 170,400 people had tested positive for SARS-CoV-2 and more than 6,619 people have died across the globe.
The number of new COVID-19 cases appears to be decreasing in China, but the number of cases are rapidly increasing worldwide. Based on available data, primarily from China, children (aged 0-19 years) account for only about 2% of all cases. Despite the probable low virulence and incidence of infection in children, they could act as potential vectors and transmit infection to more vulnerable populations. As of March 16, approximately 3,823 cases and more than 67 deaths had been reported in the United States with few pediatric patients testing positive for the disease.
SARS-CoV2 transmission mainly occurs via respiratory route through close contact with infected individuals and through fomites. The incubation period ranges from 2-14 days with an average of about 5 days. Adult patients present with cough and fever, which may progress to lower respiratory tract symptoms, including shortness of breath. Approximately 10% of all patients develop severe disease and acute respiratory distress syndrome (ARDS), requiring mechanical ventilation.
COVID-19 carries a mortality rate of up to 3%, but has been significantly higher in the elderly population, and those with chronic health conditions. Available data so far shows that children are at lower risk and the severity of the disease has been milder compared to adults. The reasons for this are not clear at this time. As of March 16, there were no reported COVID-19 related deaths in children under age 9 years.
The pediatric population: Disease patterns and transmission
The epidemiology and spectrum of disease for COVID-19 is poorly understood in pediatrics because of the low number of reported pediatric cases and limited data available from these patients. Small numbers of reported cases in children has led some to believe that children are relatively immune to the infection by SARS-CoV-2. However, Oifang et al. found that children are equally as likely as adults to be infected.1
Liu et al. found that of 366 children admitted to a hospital in Wuhan with respiratory infections in January 2020, 1.6% (six patients) cases were positive for SARS-CoV-2.2 These six children were aged 1-7 years and had all been previously healthy; all six presented with cough and fever of 102.2° F or greater. Four of the children also had vomiting. Laboratory findings were notable for lymphopenia (six of six), leukopenia (four of six), and neutropenia (3/6) with mild to moderate elevation in C-reactive protein (6.8-58.8 mg/L). Five of six children had chest CT scans. One child’s CT scan showed “bilateral ground-glass opacities” (similar to what is reported in adults), three showed “bilateral patchy shadows,” and one was normal. One child (aged 3 years) was admitted to the ICU. All of the children were treated with supportive measures, empiric antibiotics, and antivirals (six of six received oseltamivir and four of six received ribavirin). All six children recovered completely and their median hospital stay was 7.5 days with a range of 5-13 days.
Xia et al. reviewed 20 children (aged 1 day to 14 years) admitted to a hospital in Wuhan during Jan. 23–Feb. 8.3 The study reported that fever and cough were the most common presenting symptoms (approximately 65%). Less common symptoms included rhinorrhea (15%), diarrhea (15%), vomiting (10%), and sore throat (5%). WBC count was normal in majority of children (70%) with leukopenia in 20% and leukocytosis in 10%. Lymphopenia was noted to be 35%. Elevated procalcitonin was noted in 80% of children, although the degree of elevation is unclear. In this study, 8 of 20 children were coinfected with other respiratory pathogens such as influenza, respiratory syncytial virus, mycoplasma, and cytomegalovirus. All children had chest CT scans. Ten of 20 children had bilateral pulmonary lesions, 6 of 20 had unilateral pulmonary lesions, 12 of 20 had ground-glass opacities and 10 of 20 had lung consolidations with halo signs.
Wei et al., retrospective chart review of nine infants admitted for COVID-19 found that all nine had at least one infected family member.4 This study reported that seven of nine were female infants, four of nine had fever, two had mild upper respiratory infection symptoms, and one had no symptoms. The study did report that two infants did not have any information available related to symptoms. None of the infants developed severe symptoms or required ICU admission.
The youngest patient to be diagnosed with COVID-19 was a newborn of less than 24 hours old from England, whose mother also tested positive for SARS-CoV-2. However, Chen et al. found no evidence of vertical transmission of the virus from infected pregnant women to their newborns.5
Although the risk of infection in children has been reported to be low, the infection has been shown to be particularly severe in adults with compromised immune systems and chronic health conditions. Thus immunocompromised children and those with chronic health conditions are thought to be at a higher risk for contracting the infection, with the probability for increased morbidity and mortality. Some of these risk groups include premature infants, young infants, immunocompromised children, and children with chronic health conditions like asthma, diabetes, and others. It is essential that caregivers, healthy siblings, and other family members are protected from contracting the infection in order to protect these vulnerable children. Given the high infectivity of SARS-CoV-2, the implications of infected children attending schools and daycares may be far reaching if there is delayed identification of the infection. For these reasons, it is important to closely monitor and promptly test children living with infected adults to prevent the spread. It may become necessary to close schools to mitigate transmission.
Schools and daycares should work with their local health departments and physicians in case of infected individuals in their community. In China, authorities closed schools and allowed students to receive virtual education from home, which may be a reasonable choice depending on resources.
Current challenges
Given the aggressive transmission of COVID-19, these numbers seem to be increasing exponentially with a significant impact on the life of the entire country. Therefore, we must focus on containing the spread and mitigating the transmission with a multimodality approach.
Some of the initial challenges faced by physicians in the United States were related to difficulty in access to testing in persons under investigation (PUI), which in turn resulted in a delay in diagnosis and infection control. At this time, the need is to increase surge testing capabilities across the country through a variety of innovative approaches including public-private partnerships with commercial labs through Emergency Use Authorization (EUA) issued by the Centers for Disease Control and Prevention and the Department of Health and Human Services. To minimize exposure to health care professionals, telemedicine and telehealth capabilities should be exploited. This will minimize the exposure to infected patients and reduce the need for already limited personal protective equipment (PPE). As the number of cases rise, hospitals should expect and prepare for a surge in COVID-19–related hospitalizations and health care utilization.
Conclusion
Various theories are being proposed as to why children are not experiencing severe disease with COVID-19. Children may have cross-protective immunity from infection with other coronaviruses. Children may not have the same exposures from work, travel, and caregiving that adults experience as they are typically exposed by someone in their home. At this time, not enough is known about clinical presentations in children as the situation continues to evolve across the globe.
Respiratory infections in children pose unique infection control challenges with respect to compliant hand hygiene, cough etiquette, and the use of PPE when indicated. There is also concern for persistent fecal shedding of virus in infected pediatric patients, which could be another mode of transmission.6 Children could, however, be very efficient vectors of COVID-19, similar to flu, and potentially spread the pathogen to very vulnerable populations leading to high morbidity and mortality. School closures are an effective social distancing measure needed to flatten the curve and avoid overwhelming the health care structure of the United States.
Dr. Konanki is a board-certified pediatrician doing inpatient work at Wellspan Chambersburg Hospital and outpatient work at Keystone Pediatrics in Chambersburg, Pa. He also serves as the physician member of the hospital’s Code Blue Jr. committee and as a member of Quality Metrics committee at Keystone Health. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson.
References
1. Bi Q et al. Epidemiology and transmission of COVID-19 in Shenzhen China: Analysis of 391 cases and 1,286 of their close contacts. medRxiv 2020.03.03.20028423.
2. Liu W et al. Detection of Covid-19 in children in early January 2020 in Wuhan, China. N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMc2003717.
3. Xia W et al. Clinical and CT features in pediatric patients with COVID‐19 infection: Different points from adults. Pediatr Pulmonol. 2020 Mar 5. doi: 10.1002/ppul.24718.
4. Wei M et al. Novel Coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. 2020 Feb. 14. doi: 10.1001/jama.2020.2131.
5. Huijun C et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet. 2020 Mar 7 395;10226:809-15.
6. Xu Y et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020 Mar 13. doi. org/10.1038/s41591-020-0817-4.
Coronavirus disease (COVID-19) was declared a pandemic by the World Health Organization on March 11. This rapidly spreading disease is caused by the novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The infection has spread to more than 140 countries, including the United States. As of March 16, more than 170,400 people had tested positive for SARS-CoV-2 and more than 6,619 people have died across the globe.
The number of new COVID-19 cases appears to be decreasing in China, but the number of cases are rapidly increasing worldwide. Based on available data, primarily from China, children (aged 0-19 years) account for only about 2% of all cases. Despite the probable low virulence and incidence of infection in children, they could act as potential vectors and transmit infection to more vulnerable populations. As of March 16, approximately 3,823 cases and more than 67 deaths had been reported in the United States with few pediatric patients testing positive for the disease.
SARS-CoV2 transmission mainly occurs via respiratory route through close contact with infected individuals and through fomites. The incubation period ranges from 2-14 days with an average of about 5 days. Adult patients present with cough and fever, which may progress to lower respiratory tract symptoms, including shortness of breath. Approximately 10% of all patients develop severe disease and acute respiratory distress syndrome (ARDS), requiring mechanical ventilation.
COVID-19 carries a mortality rate of up to 3%, but has been significantly higher in the elderly population, and those with chronic health conditions. Available data so far shows that children are at lower risk and the severity of the disease has been milder compared to adults. The reasons for this are not clear at this time. As of March 16, there were no reported COVID-19 related deaths in children under age 9 years.
The pediatric population: Disease patterns and transmission
The epidemiology and spectrum of disease for COVID-19 is poorly understood in pediatrics because of the low number of reported pediatric cases and limited data available from these patients. Small numbers of reported cases in children has led some to believe that children are relatively immune to the infection by SARS-CoV-2. However, Oifang et al. found that children are equally as likely as adults to be infected.1
Liu et al. found that of 366 children admitted to a hospital in Wuhan with respiratory infections in January 2020, 1.6% (six patients) cases were positive for SARS-CoV-2.2 These six children were aged 1-7 years and had all been previously healthy; all six presented with cough and fever of 102.2° F or greater. Four of the children also had vomiting. Laboratory findings were notable for lymphopenia (six of six), leukopenia (four of six), and neutropenia (3/6) with mild to moderate elevation in C-reactive protein (6.8-58.8 mg/L). Five of six children had chest CT scans. One child’s CT scan showed “bilateral ground-glass opacities” (similar to what is reported in adults), three showed “bilateral patchy shadows,” and one was normal. One child (aged 3 years) was admitted to the ICU. All of the children were treated with supportive measures, empiric antibiotics, and antivirals (six of six received oseltamivir and four of six received ribavirin). All six children recovered completely and their median hospital stay was 7.5 days with a range of 5-13 days.
Xia et al. reviewed 20 children (aged 1 day to 14 years) admitted to a hospital in Wuhan during Jan. 23–Feb. 8.3 The study reported that fever and cough were the most common presenting symptoms (approximately 65%). Less common symptoms included rhinorrhea (15%), diarrhea (15%), vomiting (10%), and sore throat (5%). WBC count was normal in majority of children (70%) with leukopenia in 20% and leukocytosis in 10%. Lymphopenia was noted to be 35%. Elevated procalcitonin was noted in 80% of children, although the degree of elevation is unclear. In this study, 8 of 20 children were coinfected with other respiratory pathogens such as influenza, respiratory syncytial virus, mycoplasma, and cytomegalovirus. All children had chest CT scans. Ten of 20 children had bilateral pulmonary lesions, 6 of 20 had unilateral pulmonary lesions, 12 of 20 had ground-glass opacities and 10 of 20 had lung consolidations with halo signs.
Wei et al., retrospective chart review of nine infants admitted for COVID-19 found that all nine had at least one infected family member.4 This study reported that seven of nine were female infants, four of nine had fever, two had mild upper respiratory infection symptoms, and one had no symptoms. The study did report that two infants did not have any information available related to symptoms. None of the infants developed severe symptoms or required ICU admission.
The youngest patient to be diagnosed with COVID-19 was a newborn of less than 24 hours old from England, whose mother also tested positive for SARS-CoV-2. However, Chen et al. found no evidence of vertical transmission of the virus from infected pregnant women to their newborns.5
Although the risk of infection in children has been reported to be low, the infection has been shown to be particularly severe in adults with compromised immune systems and chronic health conditions. Thus immunocompromised children and those with chronic health conditions are thought to be at a higher risk for contracting the infection, with the probability for increased morbidity and mortality. Some of these risk groups include premature infants, young infants, immunocompromised children, and children with chronic health conditions like asthma, diabetes, and others. It is essential that caregivers, healthy siblings, and other family members are protected from contracting the infection in order to protect these vulnerable children. Given the high infectivity of SARS-CoV-2, the implications of infected children attending schools and daycares may be far reaching if there is delayed identification of the infection. For these reasons, it is important to closely monitor and promptly test children living with infected adults to prevent the spread. It may become necessary to close schools to mitigate transmission.
Schools and daycares should work with their local health departments and physicians in case of infected individuals in their community. In China, authorities closed schools and allowed students to receive virtual education from home, which may be a reasonable choice depending on resources.
Current challenges
Given the aggressive transmission of COVID-19, these numbers seem to be increasing exponentially with a significant impact on the life of the entire country. Therefore, we must focus on containing the spread and mitigating the transmission with a multimodality approach.
Some of the initial challenges faced by physicians in the United States were related to difficulty in access to testing in persons under investigation (PUI), which in turn resulted in a delay in diagnosis and infection control. At this time, the need is to increase surge testing capabilities across the country through a variety of innovative approaches including public-private partnerships with commercial labs through Emergency Use Authorization (EUA) issued by the Centers for Disease Control and Prevention and the Department of Health and Human Services. To minimize exposure to health care professionals, telemedicine and telehealth capabilities should be exploited. This will minimize the exposure to infected patients and reduce the need for already limited personal protective equipment (PPE). As the number of cases rise, hospitals should expect and prepare for a surge in COVID-19–related hospitalizations and health care utilization.
Conclusion
Various theories are being proposed as to why children are not experiencing severe disease with COVID-19. Children may have cross-protective immunity from infection with other coronaviruses. Children may not have the same exposures from work, travel, and caregiving that adults experience as they are typically exposed by someone in their home. At this time, not enough is known about clinical presentations in children as the situation continues to evolve across the globe.
Respiratory infections in children pose unique infection control challenges with respect to compliant hand hygiene, cough etiquette, and the use of PPE when indicated. There is also concern for persistent fecal shedding of virus in infected pediatric patients, which could be another mode of transmission.6 Children could, however, be very efficient vectors of COVID-19, similar to flu, and potentially spread the pathogen to very vulnerable populations leading to high morbidity and mortality. School closures are an effective social distancing measure needed to flatten the curve and avoid overwhelming the health care structure of the United States.
Dr. Konanki is a board-certified pediatrician doing inpatient work at Wellspan Chambersburg Hospital and outpatient work at Keystone Pediatrics in Chambersburg, Pa. He also serves as the physician member of the hospital’s Code Blue Jr. committee and as a member of Quality Metrics committee at Keystone Health. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson.
References
1. Bi Q et al. Epidemiology and transmission of COVID-19 in Shenzhen China: Analysis of 391 cases and 1,286 of their close contacts. medRxiv 2020.03.03.20028423.
2. Liu W et al. Detection of Covid-19 in children in early January 2020 in Wuhan, China. N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMc2003717.
3. Xia W et al. Clinical and CT features in pediatric patients with COVID‐19 infection: Different points from adults. Pediatr Pulmonol. 2020 Mar 5. doi: 10.1002/ppul.24718.
4. Wei M et al. Novel Coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. 2020 Feb. 14. doi: 10.1001/jama.2020.2131.
5. Huijun C et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet. 2020 Mar 7 395;10226:809-15.
6. Xu Y et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020 Mar 13. doi. org/10.1038/s41591-020-0817-4.
Coronavirus disease (COVID-19) was declared a pandemic by the World Health Organization on March 11. This rapidly spreading disease is caused by the novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The infection has spread to more than 140 countries, including the United States. As of March 16, more than 170,400 people had tested positive for SARS-CoV-2 and more than 6,619 people have died across the globe.
The number of new COVID-19 cases appears to be decreasing in China, but the number of cases are rapidly increasing worldwide. Based on available data, primarily from China, children (aged 0-19 years) account for only about 2% of all cases. Despite the probable low virulence and incidence of infection in children, they could act as potential vectors and transmit infection to more vulnerable populations. As of March 16, approximately 3,823 cases and more than 67 deaths had been reported in the United States with few pediatric patients testing positive for the disease.
SARS-CoV2 transmission mainly occurs via respiratory route through close contact with infected individuals and through fomites. The incubation period ranges from 2-14 days with an average of about 5 days. Adult patients present with cough and fever, which may progress to lower respiratory tract symptoms, including shortness of breath. Approximately 10% of all patients develop severe disease and acute respiratory distress syndrome (ARDS), requiring mechanical ventilation.
COVID-19 carries a mortality rate of up to 3%, but has been significantly higher in the elderly population, and those with chronic health conditions. Available data so far shows that children are at lower risk and the severity of the disease has been milder compared to adults. The reasons for this are not clear at this time. As of March 16, there were no reported COVID-19 related deaths in children under age 9 years.
The pediatric population: Disease patterns and transmission
The epidemiology and spectrum of disease for COVID-19 is poorly understood in pediatrics because of the low number of reported pediatric cases and limited data available from these patients. Small numbers of reported cases in children has led some to believe that children are relatively immune to the infection by SARS-CoV-2. However, Oifang et al. found that children are equally as likely as adults to be infected.1
Liu et al. found that of 366 children admitted to a hospital in Wuhan with respiratory infections in January 2020, 1.6% (six patients) cases were positive for SARS-CoV-2.2 These six children were aged 1-7 years and had all been previously healthy; all six presented with cough and fever of 102.2° F or greater. Four of the children also had vomiting. Laboratory findings were notable for lymphopenia (six of six), leukopenia (four of six), and neutropenia (3/6) with mild to moderate elevation in C-reactive protein (6.8-58.8 mg/L). Five of six children had chest CT scans. One child’s CT scan showed “bilateral ground-glass opacities” (similar to what is reported in adults), three showed “bilateral patchy shadows,” and one was normal. One child (aged 3 years) was admitted to the ICU. All of the children were treated with supportive measures, empiric antibiotics, and antivirals (six of six received oseltamivir and four of six received ribavirin). All six children recovered completely and their median hospital stay was 7.5 days with a range of 5-13 days.
Xia et al. reviewed 20 children (aged 1 day to 14 years) admitted to a hospital in Wuhan during Jan. 23–Feb. 8.3 The study reported that fever and cough were the most common presenting symptoms (approximately 65%). Less common symptoms included rhinorrhea (15%), diarrhea (15%), vomiting (10%), and sore throat (5%). WBC count was normal in majority of children (70%) with leukopenia in 20% and leukocytosis in 10%. Lymphopenia was noted to be 35%. Elevated procalcitonin was noted in 80% of children, although the degree of elevation is unclear. In this study, 8 of 20 children were coinfected with other respiratory pathogens such as influenza, respiratory syncytial virus, mycoplasma, and cytomegalovirus. All children had chest CT scans. Ten of 20 children had bilateral pulmonary lesions, 6 of 20 had unilateral pulmonary lesions, 12 of 20 had ground-glass opacities and 10 of 20 had lung consolidations with halo signs.
Wei et al., retrospective chart review of nine infants admitted for COVID-19 found that all nine had at least one infected family member.4 This study reported that seven of nine were female infants, four of nine had fever, two had mild upper respiratory infection symptoms, and one had no symptoms. The study did report that two infants did not have any information available related to symptoms. None of the infants developed severe symptoms or required ICU admission.
The youngest patient to be diagnosed with COVID-19 was a newborn of less than 24 hours old from England, whose mother also tested positive for SARS-CoV-2. However, Chen et al. found no evidence of vertical transmission of the virus from infected pregnant women to their newborns.5
Although the risk of infection in children has been reported to be low, the infection has been shown to be particularly severe in adults with compromised immune systems and chronic health conditions. Thus immunocompromised children and those with chronic health conditions are thought to be at a higher risk for contracting the infection, with the probability for increased morbidity and mortality. Some of these risk groups include premature infants, young infants, immunocompromised children, and children with chronic health conditions like asthma, diabetes, and others. It is essential that caregivers, healthy siblings, and other family members are protected from contracting the infection in order to protect these vulnerable children. Given the high infectivity of SARS-CoV-2, the implications of infected children attending schools and daycares may be far reaching if there is delayed identification of the infection. For these reasons, it is important to closely monitor and promptly test children living with infected adults to prevent the spread. It may become necessary to close schools to mitigate transmission.
Schools and daycares should work with their local health departments and physicians in case of infected individuals in their community. In China, authorities closed schools and allowed students to receive virtual education from home, which may be a reasonable choice depending on resources.
Current challenges
Given the aggressive transmission of COVID-19, these numbers seem to be increasing exponentially with a significant impact on the life of the entire country. Therefore, we must focus on containing the spread and mitigating the transmission with a multimodality approach.
Some of the initial challenges faced by physicians in the United States were related to difficulty in access to testing in persons under investigation (PUI), which in turn resulted in a delay in diagnosis and infection control. At this time, the need is to increase surge testing capabilities across the country through a variety of innovative approaches including public-private partnerships with commercial labs through Emergency Use Authorization (EUA) issued by the Centers for Disease Control and Prevention and the Department of Health and Human Services. To minimize exposure to health care professionals, telemedicine and telehealth capabilities should be exploited. This will minimize the exposure to infected patients and reduce the need for already limited personal protective equipment (PPE). As the number of cases rise, hospitals should expect and prepare for a surge in COVID-19–related hospitalizations and health care utilization.
Conclusion
Various theories are being proposed as to why children are not experiencing severe disease with COVID-19. Children may have cross-protective immunity from infection with other coronaviruses. Children may not have the same exposures from work, travel, and caregiving that adults experience as they are typically exposed by someone in their home. At this time, not enough is known about clinical presentations in children as the situation continues to evolve across the globe.
Respiratory infections in children pose unique infection control challenges with respect to compliant hand hygiene, cough etiquette, and the use of PPE when indicated. There is also concern for persistent fecal shedding of virus in infected pediatric patients, which could be another mode of transmission.6 Children could, however, be very efficient vectors of COVID-19, similar to flu, and potentially spread the pathogen to very vulnerable populations leading to high morbidity and mortality. School closures are an effective social distancing measure needed to flatten the curve and avoid overwhelming the health care structure of the United States.
Dr. Konanki is a board-certified pediatrician doing inpatient work at Wellspan Chambersburg Hospital and outpatient work at Keystone Pediatrics in Chambersburg, Pa. He also serves as the physician member of the hospital’s Code Blue Jr. committee and as a member of Quality Metrics committee at Keystone Health. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson.
References
1. Bi Q et al. Epidemiology and transmission of COVID-19 in Shenzhen China: Analysis of 391 cases and 1,286 of their close contacts. medRxiv 2020.03.03.20028423.
2. Liu W et al. Detection of Covid-19 in children in early January 2020 in Wuhan, China. N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMc2003717.
3. Xia W et al. Clinical and CT features in pediatric patients with COVID‐19 infection: Different points from adults. Pediatr Pulmonol. 2020 Mar 5. doi: 10.1002/ppul.24718.
4. Wei M et al. Novel Coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. 2020 Feb. 14. doi: 10.1001/jama.2020.2131.
5. Huijun C et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet. 2020 Mar 7 395;10226:809-15.
6. Xu Y et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020 Mar 13. doi. org/10.1038/s41591-020-0817-4.
What to do if you encounter Candida auris
Closely monitor patients for treatment failure
Candida auris is an emerging, often multidrug-resistant yeast that causes invasive infections (such as bloodstream, intra-abdominal) and is transmitted in health care settings. It is difficult to diagnose using traditional yeast identification methods. C. auris also has been found in noninvasive body sites and can colonize a person without causing active infection and hence permitting transmission of the pathogen between patients. These sites include skin, urine, external ear, wounds, and respiratory specimens.
This fungus was first described in 2009 in an ear-discharge culture from a patient in Japan. The first clinical cases were described in South Korea in 2011. An unknown pathogen before 2009, C. auris caused 4%-8% of candidemia in Indian ICUs during 2011-2012 and 38% of candidemia in one Kenyan hospital during 2010-2013. It has now spread across Asia and Europe, only to arrive in the United States in 2016.
As of Aug. 31, 2017, a total of 153 clinical cases of C. auris infection have been reported to CDC from 10 U.S. states; most have occurred in New York and New Jersey. An additional 143 patients have been found to be colonized with C. auris. Based on epidemiologic and molecular information, including whole genome sequencing, the Centers for Disease Control and Prevention infers that most U.S. cases likely resulted from local transmission of C. auris following previous introduction from other countries in Asia.
The majority of infections within the United States have been in blood streams. The reported all-cause mortality from these infections has been up to 60%. Most C. auris isolates in the United States have been resistant to at least one antifungal, most commonly fluconazole, and patients have developed resistance to echinocandin drugs while on treatment. Amphotericin B resistance also has been seen in about 30% of isolates.
In response to global reports and a large outbreak in a specialty hospital in the United Kingdom, the CDC issued its first advisory and clinical alert to health care facilities in June 2016. It is essential for hospitalist physicians to be aware of this emerging pathogen and also of the interventions needed to curb its spread, given they are the frontline warriors in the fight against hospital-acquired infections.
The first step in controlling C. auris is identification. C. auris can be misidentified when using traditional biochemical methods. They are most commonly misidentified as Candida haemulonii. Currently, accurate identification for C. auris can be performed by Vitek MS and matrix-assisted laser desorption/ionization time-of-flight using research use–only databases. Hospitalists should be aware of the diagnostic instruments used in their hospital laboratories and their ability to detect C. auris. Clinical laboratories should request testing of suspect C. auris isolates from their state or regional public health laboratory or the CDC. Laboratories should also consider reviewing historical microbiology records for suspect isolates (e.g., C. haemulonii) to identify missed cases of C. auris.
All cultures positive for Candida should be further speciated and antifungal susceptibilities should be reported as per new Infectious Diseases Society of America guidelines for candidiasis from 2016. As many clinical laboratories do not determine the species of Candida from noninvasive sites, C. auris colonization may go unrecognized and lead to transmission. About 54% of recognized U.S. clinical cases have been identified from blood cultures. The remaining patients with positive C. auris cultures, including those with recent hospitalizations abroad, have had the organism isolated from other body sites, including skin wounds, urine, respiratory specimens, bile fluid, and ears. Determining the species of Candida for isolates from these noninvasive sites in certain situations may allow for more rapid identification of C. auris and allow for timely implementation of targeted infection control measures to reduce transmission.
Patients have been persistently colonized with C. auris, posing long-term risk of transmission. Currently, data on effective decolonization methods are lacking. Patients with suspected or confirmed C. auris infection should be placed in a single room if possible and standard and contact precautions should be initiated and thorough environmental cleaning and disinfection of the patient care area should be undertaken. Using an Environmental Protection Agency–registered antimicrobial product active against Clostridium difficile for routine and terminal disinfection is recommended.
Implement contact tracing and testing to identify other patients colonized with C. auris. Review past microbiology records (at least for the preceding 1 year) for suspect or confirmed cases of C. auris at the institution. Set up enhanced surveillance for C. auris in the laboratory setting.
Echinocandin drugs are the first-line treatment for most invasive Candida infections, making resistance to this class of antifungal drugs particularly concerning. As of Sept. 15, 2017, at least five patients in the United States had echinocandin-resistant isolates. In one patient, resistance to echinocandin drugs developed while being treated with echinocandins.
Based on these findings, CDC is concerned that echinocandin-resistant C. auris could become more common. Patients with C. auris infection should be closely monitored for treatment failure, as indicated by persistently positive clinical cultures (lasting more than 5 days). Consultation with an infectious disease specialist is highly recommended.
Dr. Tirupathi is medical director, infectious diseases/HIV at Keystone Health, and chair, infection prevention, at Summit Health, both in Chambersburg, Pa. He is clinical assistant professor of medicine at Penn State University, Hershey.
Closely monitor patients for treatment failure
Closely monitor patients for treatment failure
Candida auris is an emerging, often multidrug-resistant yeast that causes invasive infections (such as bloodstream, intra-abdominal) and is transmitted in health care settings. It is difficult to diagnose using traditional yeast identification methods. C. auris also has been found in noninvasive body sites and can colonize a person without causing active infection and hence permitting transmission of the pathogen between patients. These sites include skin, urine, external ear, wounds, and respiratory specimens.
This fungus was first described in 2009 in an ear-discharge culture from a patient in Japan. The first clinical cases were described in South Korea in 2011. An unknown pathogen before 2009, C. auris caused 4%-8% of candidemia in Indian ICUs during 2011-2012 and 38% of candidemia in one Kenyan hospital during 2010-2013. It has now spread across Asia and Europe, only to arrive in the United States in 2016.
As of Aug. 31, 2017, a total of 153 clinical cases of C. auris infection have been reported to CDC from 10 U.S. states; most have occurred in New York and New Jersey. An additional 143 patients have been found to be colonized with C. auris. Based on epidemiologic and molecular information, including whole genome sequencing, the Centers for Disease Control and Prevention infers that most U.S. cases likely resulted from local transmission of C. auris following previous introduction from other countries in Asia.
The majority of infections within the United States have been in blood streams. The reported all-cause mortality from these infections has been up to 60%. Most C. auris isolates in the United States have been resistant to at least one antifungal, most commonly fluconazole, and patients have developed resistance to echinocandin drugs while on treatment. Amphotericin B resistance also has been seen in about 30% of isolates.
In response to global reports and a large outbreak in a specialty hospital in the United Kingdom, the CDC issued its first advisory and clinical alert to health care facilities in June 2016. It is essential for hospitalist physicians to be aware of this emerging pathogen and also of the interventions needed to curb its spread, given they are the frontline warriors in the fight against hospital-acquired infections.
The first step in controlling C. auris is identification. C. auris can be misidentified when using traditional biochemical methods. They are most commonly misidentified as Candida haemulonii. Currently, accurate identification for C. auris can be performed by Vitek MS and matrix-assisted laser desorption/ionization time-of-flight using research use–only databases. Hospitalists should be aware of the diagnostic instruments used in their hospital laboratories and their ability to detect C. auris. Clinical laboratories should request testing of suspect C. auris isolates from their state or regional public health laboratory or the CDC. Laboratories should also consider reviewing historical microbiology records for suspect isolates (e.g., C. haemulonii) to identify missed cases of C. auris.
All cultures positive for Candida should be further speciated and antifungal susceptibilities should be reported as per new Infectious Diseases Society of America guidelines for candidiasis from 2016. As many clinical laboratories do not determine the species of Candida from noninvasive sites, C. auris colonization may go unrecognized and lead to transmission. About 54% of recognized U.S. clinical cases have been identified from blood cultures. The remaining patients with positive C. auris cultures, including those with recent hospitalizations abroad, have had the organism isolated from other body sites, including skin wounds, urine, respiratory specimens, bile fluid, and ears. Determining the species of Candida for isolates from these noninvasive sites in certain situations may allow for more rapid identification of C. auris and allow for timely implementation of targeted infection control measures to reduce transmission.
Patients have been persistently colonized with C. auris, posing long-term risk of transmission. Currently, data on effective decolonization methods are lacking. Patients with suspected or confirmed C. auris infection should be placed in a single room if possible and standard and contact precautions should be initiated and thorough environmental cleaning and disinfection of the patient care area should be undertaken. Using an Environmental Protection Agency–registered antimicrobial product active against Clostridium difficile for routine and terminal disinfection is recommended.
Implement contact tracing and testing to identify other patients colonized with C. auris. Review past microbiology records (at least for the preceding 1 year) for suspect or confirmed cases of C. auris at the institution. Set up enhanced surveillance for C. auris in the laboratory setting.
Echinocandin drugs are the first-line treatment for most invasive Candida infections, making resistance to this class of antifungal drugs particularly concerning. As of Sept. 15, 2017, at least five patients in the United States had echinocandin-resistant isolates. In one patient, resistance to echinocandin drugs developed while being treated with echinocandins.
Based on these findings, CDC is concerned that echinocandin-resistant C. auris could become more common. Patients with C. auris infection should be closely monitored for treatment failure, as indicated by persistently positive clinical cultures (lasting more than 5 days). Consultation with an infectious disease specialist is highly recommended.
Dr. Tirupathi is medical director, infectious diseases/HIV at Keystone Health, and chair, infection prevention, at Summit Health, both in Chambersburg, Pa. He is clinical assistant professor of medicine at Penn State University, Hershey.
Candida auris is an emerging, often multidrug-resistant yeast that causes invasive infections (such as bloodstream, intra-abdominal) and is transmitted in health care settings. It is difficult to diagnose using traditional yeast identification methods. C. auris also has been found in noninvasive body sites and can colonize a person without causing active infection and hence permitting transmission of the pathogen between patients. These sites include skin, urine, external ear, wounds, and respiratory specimens.
This fungus was first described in 2009 in an ear-discharge culture from a patient in Japan. The first clinical cases were described in South Korea in 2011. An unknown pathogen before 2009, C. auris caused 4%-8% of candidemia in Indian ICUs during 2011-2012 and 38% of candidemia in one Kenyan hospital during 2010-2013. It has now spread across Asia and Europe, only to arrive in the United States in 2016.
As of Aug. 31, 2017, a total of 153 clinical cases of C. auris infection have been reported to CDC from 10 U.S. states; most have occurred in New York and New Jersey. An additional 143 patients have been found to be colonized with C. auris. Based on epidemiologic and molecular information, including whole genome sequencing, the Centers for Disease Control and Prevention infers that most U.S. cases likely resulted from local transmission of C. auris following previous introduction from other countries in Asia.
The majority of infections within the United States have been in blood streams. The reported all-cause mortality from these infections has been up to 60%. Most C. auris isolates in the United States have been resistant to at least one antifungal, most commonly fluconazole, and patients have developed resistance to echinocandin drugs while on treatment. Amphotericin B resistance also has been seen in about 30% of isolates.
In response to global reports and a large outbreak in a specialty hospital in the United Kingdom, the CDC issued its first advisory and clinical alert to health care facilities in June 2016. It is essential for hospitalist physicians to be aware of this emerging pathogen and also of the interventions needed to curb its spread, given they are the frontline warriors in the fight against hospital-acquired infections.
The first step in controlling C. auris is identification. C. auris can be misidentified when using traditional biochemical methods. They are most commonly misidentified as Candida haemulonii. Currently, accurate identification for C. auris can be performed by Vitek MS and matrix-assisted laser desorption/ionization time-of-flight using research use–only databases. Hospitalists should be aware of the diagnostic instruments used in their hospital laboratories and their ability to detect C. auris. Clinical laboratories should request testing of suspect C. auris isolates from their state or regional public health laboratory or the CDC. Laboratories should also consider reviewing historical microbiology records for suspect isolates (e.g., C. haemulonii) to identify missed cases of C. auris.
All cultures positive for Candida should be further speciated and antifungal susceptibilities should be reported as per new Infectious Diseases Society of America guidelines for candidiasis from 2016. As many clinical laboratories do not determine the species of Candida from noninvasive sites, C. auris colonization may go unrecognized and lead to transmission. About 54% of recognized U.S. clinical cases have been identified from blood cultures. The remaining patients with positive C. auris cultures, including those with recent hospitalizations abroad, have had the organism isolated from other body sites, including skin wounds, urine, respiratory specimens, bile fluid, and ears. Determining the species of Candida for isolates from these noninvasive sites in certain situations may allow for more rapid identification of C. auris and allow for timely implementation of targeted infection control measures to reduce transmission.
Patients have been persistently colonized with C. auris, posing long-term risk of transmission. Currently, data on effective decolonization methods are lacking. Patients with suspected or confirmed C. auris infection should be placed in a single room if possible and standard and contact precautions should be initiated and thorough environmental cleaning and disinfection of the patient care area should be undertaken. Using an Environmental Protection Agency–registered antimicrobial product active against Clostridium difficile for routine and terminal disinfection is recommended.
Implement contact tracing and testing to identify other patients colonized with C. auris. Review past microbiology records (at least for the preceding 1 year) for suspect or confirmed cases of C. auris at the institution. Set up enhanced surveillance for C. auris in the laboratory setting.
Echinocandin drugs are the first-line treatment for most invasive Candida infections, making resistance to this class of antifungal drugs particularly concerning. As of Sept. 15, 2017, at least five patients in the United States had echinocandin-resistant isolates. In one patient, resistance to echinocandin drugs developed while being treated with echinocandins.
Based on these findings, CDC is concerned that echinocandin-resistant C. auris could become more common. Patients with C. auris infection should be closely monitored for treatment failure, as indicated by persistently positive clinical cultures (lasting more than 5 days). Consultation with an infectious disease specialist is highly recommended.
Dr. Tirupathi is medical director, infectious diseases/HIV at Keystone Health, and chair, infection prevention, at Summit Health, both in Chambersburg, Pa. He is clinical assistant professor of medicine at Penn State University, Hershey.















