User login
Management of Psoriasis With Topicals: Applying the 2020 AAD-NPF Guidelines of Care to Clinical Practice
Psoriasis is a chronic inflammatory skin disease characterized by erythematous scaly plaques that can invoke substantial pain, pruritus, and quality-of-life disturbance in patients. Topical therapies are the most commonly used medications for treating psoriasis, with one study (N = 128,308) showing that more than 85% of patients with psoriasis were managed solely with topical medications. 1 For patients with mild to moderate psoriasis, topical agents alone may be able to control disease completely. For those with more severe disease, topical agents are used adjunctively with systemic or biologic agents to optimize disease control in localized areas.
The American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) published guidelines in 2020 for managing psoriasis with topical agents in adults.2 This review presents the most up-to-date clinical recommendations for topical agent use in adult patients with psoriasis and elaborates on each drug’s pharmacologic and safety profile. Specifically, evidence-based treatment recommendations for topical steroids, calcineurin inhibitors (CNIs), vitamin D analogues, retinoids (tazarotene), emollients, keratolytics (salicylic acid), anthracenes (anthralin), and keratoplastics (coal tar) will be addressed (Table 1). Recommendations for combination therapy with other treatment modalities including UVB light therapy, biologics, and systemic nonbiologic agents also will be discussed.


Selecting a Topical Agent Based on Disease Localization
When treating patients with psoriasis with topical therapies, clinicians should take into consideration drug potency, as it determines how effective a treatment will be in penetrating the skin barrier. Plaque characteristics, such as distribution (localized vs widespread), anatomical localization (flexural, scalp, palms/soles/nails), size (large vs small), and thickness (thick vs thin), not only influence treatment effectiveness but also the incidence of drug-related adverse events. Furthermore, preferred topical therapies are tailored to each patient based on disease characteristics and activity. Coal tar and anthralin have been used less frequently than other topical therapies for psoriasis because of their undesirable side-effect profiles (Table 1).3
Face and Intertriginous Regions—The face and intertriginous areas are sensitive because skin tends to be thin in these regions. Emollients are recommended for disease in these locations given their safety and flexibility in use for most areas. Conversely, anthralin should be avoided on the face, intertriginous areas, and even highly visible locations because of the potential for skin staining. Low-potency corticosteroids also have utility in psoriasis distributed on the face and intertriginous regions. Additionally, application of steroids around the eyes should be cautioned because topical steroids can induce ocular complications such as glaucoma and cataracts in rare circumstances.4
Off-label use of CNIs for psoriasis on the face and intertriginous areas also is effective. Currently, there is a level B recommendation for off-label use of 0.1% tacrolimus for up to 8 weeks for inverse psoriasis or psoriasis on the face. Off-label use of pimecrolimus for 4 to 8 weeks also can be considered for inverse psoriasis. Combination therapy consisting of hydrocortisone with calcipotriol ointment is another effective regimen.5 One study also suggested that use of crisaborole for 4 to 8 weeks in intertriginous psoriasis can be effective and well tolerated.6
Scalp—The vehicle of medication administration is especially important in hair-bearing areas such as the scalp, as these areas are challenging for medication application and patient adherence. Thus, patient preferences for the vehicle must be considered. Several studies have been conducted to assess preference for various vehicles in scalp psoriasis. A foam or solution may be preferable to ointments, gels, or creams.7 Gels may be preferred over ointments.8 There is a level A recommendation supporting the use of class 1 to 7 topical steroids for a minimum of 4 weeks as initial and maintenance treatment of scalp psoriasis. The highest level of evidence (level A) also supports the use of calcipotriol foam or combination therapy of calcipotriol–betamethasone dipropionate gel for 4 to 12 weeks as treatment of mild to moderate scalp psoriasis.
Nails—Several options for topical medications have been recommended for the treatment of nail psoriasis. Currently, there is a level B recommendation for the use of tazarotene for the treatment of nail psoriasis. Another effective regimen is combination therapy with vitamin D analogues and betamethasone dipropionate.9 Topical steroid use for nail psoriasis should be limited to 12 weeks because of the risk for bone atrophy with chronic steroid use.
Palmoplantar—The palms and soles have a thicker epidermal layer than other areas of the body. As a result, class 1 corticosteroids can be used for palmoplantar psoriasis for more than 4 weeks with vigilant monitoring for adverse effects such as skin atrophy, tachyphylaxis, or tinea infection. Tazarotene also has been shown to be helpful in treating palmoplantar psoriasis.
Resistant Disease—Intralesional steroids are beneficial treatment options for recalcitrant psoriasis in glabrous areas, as well as for palmoplantar, nail, and scalp psoriasis. Up to 10 mg/mL of triamcinolone acetonide used every 3 to 4 weeks is an effective regimen.10Pregnancy/Breastfeeding—Women of childbearing potential have additional safety precautions that should be considered during medication selection. Emollients have been shown to be safe during pregnancy and lactation. Currently, there is little known about CNI use during pregnancy. During lactation, CNIs can be used by breastfeeding mothers in most areas, excluding the breasts. Evaluation of the safety of anthralin and vitamin D analogues during pregnancy and lactation have not been studied. For these agents, dermatologists need to use their clinical judgment to weigh the risks and benefits of medication, particularly in patients requiring occlusion, higher medication doses, or treatment over a large surface area. Salicylic acid should be used with caution in pregnant and breastfeeding mothers because it is a pregnancy category C drug. Lower-potency corticosteroids may be used with caution during pregnancy and breastfeeding. More potent corticosteroids and coal tar, however, should be avoided. Similarly, tazarotene use is contraindicated in pregnancy. According to the US Food and Drug Administration labels for all forms of topical tazarotene, a pregnancy test must be obtained 2 weeks prior to tazarotene treatment initiation in women of childbearing potential because of the risk for serious fetal malformations and toxicity.
Recommendations, Risks, and Benefits of Topical Therapy for the Management of Psoriasis
Topical Corticosteroids—Topical corticosteroids (TCs) are widely used for inflammatory skin conditions and are available in a variety of strengths (Table 2). They are thought to exert their action by regulating the gene transcription of proinflammatory mediators. For psoriasis, steroids are recommended for 2 to 4 weeks, depending on disease severity. Although potent and superpotent steroids are more effective than mild- to moderate-strength TCs, use of lower-potency TCs may be warranted depending on disease distribution and localization.11 For treatment of psoriasis with no involvement of the intertriginous areas, use of class 1 to 5 TCs for up to 4 weeks is recommended.

For moderate to severe psoriasis with 20% or less body surface area (BSA) affected, combination therapy consisting of mometasone and salicylic acid has been shown to be more effective than mometasone alone.12,13 There currently is a level A recommendation for the use of combination therapy with class 1 TCs and etanercept for 12 weeks in patients with moderate to severe psoriasis who require both systemic and topical therapies for disease control. Similarly, combination therapy with infliximab and high-potency TCs has a level B recommendation to enhance efficacy for the treatment of moderate to severe psoriasis.14 High-quality studies on the use of TCs with anti–IL-12/IL-23, anti–IL-23, and anti–IL-17 currently are unavailable, but the combination is not expected to be unsafe.14,15 Combination therapy of betamethasone dipropionate ointment and low-dose cyclosporine is an alternative regimen with a level B recommendation.
The most common adverse effects with use of TCs are skin thinning and atrophy, telangiectasia, and striae (Table 1). With clinical improvement of disease, it is recommended that clinicians taper TCs to prevent rebound effect. To decrease TC-related adverse effects, clinicians should use combination therapy with steroid-sparing agents for disease maintenance, transition to lower-potency corticosteroids, or use intermittent steroid therapy. Systemic effects of TC use include hypothalamic-pituitary-adrenal axis suppression, Cushing syndrome, and osteonecrosis of the femoral head.16-18 These systemic effects with TC use are rare unless treatment is for disease involving greater than 20% BSA or occlusion for more than 4 weeks.
Calcineurin Inhibitors—Calcineurin inhibitors inhibit calcineurin phosphorylation and T-cell activation, subsequently decreasing the expression of proinflammatory cytokines. Currently, they are not approved by the US Food and Drug Administration to treat psoriasis but have demonstrated efficacy in randomized control trials (RCTs) for facial and intertriginous psoriasis. In RCTs, 71% of patients using pimecrolimus cream 0.1% twice daily for 8 weeks achieved an investigator global assessment score of clear (0) or almost clear (1) compared with 21% of placebo-treated patients (N=57).19 Other trials have shown that 65% of patients receiving tacrolimus ointment 0.1% for 8 weeks achieved an investigator global assessment score of 0 or 1 compared with 31% of placebo-treated patients (N=167).20 Because of their efficacy in RCTs, CNIs commonly are used off label to treat psoriasis.
The most common adverse effects with CNI use are burning, pruritus, and flushing with alcohol ingestion (Table 1). Additionally, CNIs have a black box warning that use may increase the risk for malignancy, but this risk has not been demonstrated with topical use in humans.21Vitamin D Analogues—The class of vitamin D analogues—calcipotriol/calcipotriene and calcitriol—frequently are used to treat psoriasis. Vitamin D analogues exert their beneficial effects by inhibiting keratinocyte proliferation and enhancing keratinocyte differentiation. They also are ideal for long-term use (up to 52 weeks) in mild to moderate psoriasis and can be used in combination with class 2 and 3 TCs. There is a level A recommendation that supports the use of combination therapy with calcipotriol and TCs for the treatment of mild to moderate psoriasis.
For severe psoriasis, many studies have investigated the efficacy of combination therapy with vitamin D analogues and systemic treatments. Combination therapy with calcipotriol and methotrexate or calcipotriol and acitretin are effective treatment regimens with level A recommendations. Calcipotriol–betamethasone dipropionate ointment in combination with low-dose cyclosporine is an alternative option with a level B recommendation. Because vitamin D analogues are inactivated by UVA and UVB radiation, clinicians should advise their patients to use vitamin D analogues after receiving UVB phototherapy.22
Common adverse effects of vitamin D analogues include burning, pruritus, erythema, and dryness (Table 1). Hypercalcemia and parathyroid hormone suppression are extremely rare unless treatment occurs over a large surface area (>30% BSA) or the patient has concurrent renal disease or impairments in calcium metabolism.
Tazarotene—Tazarotene is a topical retinoid that acts by decreasing keratinocyte proliferation, facilitating keratinocyte differentiation, and inhibiting inflammation. Patients with mild to moderate psoriasis are recommended to receive tazarotene treatment for 8 to 12 weeks. In several RCTs, tazarotene gel 0.1% and tazarotene cream 0.1% and 0.05% achieved treatment success in treating plaque psoriasis.23,24
For increased efficacy, clinicians can recommend combination therapy with tazarotene and a TC. Combination therapy with tazarotene and a mid- or high-potency TC for 8 to 16 weeks has been shown to be more effective than treatment with tazarotene alone.25 Thus, there is a level A recommendation for use of this combination to treat mild to moderate psoriasis. Agents used in combination therapy work synergistically to decrease the length of treatment and increase the duration of remission. The frequency of adverse effects, such as irritation from tazarotene and skin atrophy from TCs, also are reduced.26 Combination therapy with tazarotene and narrowband UVB (NB-UVB) is another effective option that requires less UV radiation than NB-UVB alone because of the synergistic effects of both treatment modalities.27 Clinicians should counsel patients on the adverse effects of tazarotene, which include local irritation, burning, pruritus, and erythema (Table 1).
Emollients—Emollients are nonmedicated moisturizers that decrease the amount of transepidermal water loss. There is a level B recommendation for use of emollients and TCs in combination for 4 to 8 weeks to treat psoriasis. In fact, combination therapy with mometasone and emollients has demonstrated greater improvement in symptoms of palmoplantar psoriasis (ie, erythema, desquamation, infiltration, BSA involvement) than mometasone alone.28 Emollients are safe options that can be used on all areas of the body and during pregnancy and lactation. Although adverse effects of emollients are rare, clinicians should counsel patients on the risk for contact dermatitis if specific allergies to ingredients/fragrances exist (Table 1).
Salicylic Acid—Salicylic acid is a topical keratolytic that can be used to treat psoriatic plaques. Use of salicylic acid for 8 to 16 weeks has been shown to be effective for mild to moderate psoriasis. Combination therapy of salicylic acid and TCs in patients with 20% or less BSA affected is a safe and effective option with a level B recommendation. Combination therapy with salicylic acid and calcipotriene, however, should be avoided because calcipotriene is inactivated by salicylic acid. It also is recommended that salicylic acid application follow phototherapy when both treatment modalities are used in combination.29,30 Clinicians should be cautious about using salicylic acid in patients with renal or hepatic disease because of the increased risk for salicylate toxicity (Table 1).
Anthralin—Anthralin is a synthetic hydrocarbon derivative that has been shown to reduce inflammation and normalize keratinocyte proliferation through an unknown mechanism. It is recommended that patients with mild to moderate psoriasis receive anthralin treatment for 8 to 12 weeks, with a maximum application time of 2 hours per day. Combination therapy of excimer laser and anthralin has been shown to be more effective in treating psoriasis than anthralin alone.31 Therefore, clinicians have the option of including excimer laser therapy for additional disease control. Anthralin should be avoided on the face, flexural regions, and highly visible areas because of potential skin staining (Table 1). Other adverse effects include application-site burning and erythema.
Coal Tar—Coal tar is a heterogenous mixture of aromatic hydrocarbons that is an effective treatment of psoriasis because of its inherent anti-inflammatory and keratoplastic properties. There is high-quality evidence supporting a level A recommendation for coal tar use in mild to moderate psoriasis. Combination therapy with NB-UVB and coal tar (also known as Goeckerman therapy) is a recommended treatment option with a quicker onset of action and improved outcomes compared with NB-UVB therapy alone.32,33 Adverse events of coal tar include application-site irritation, folliculitis, contact dermatitis, phototoxicity, and skin pigmentation (Table 1).
Conclusion
Topical medications are versatile treatment options that can be utilized as monotherapy or adjunct therapy for mild to severe psoriasis. Benefits of topical agents include minimal required monitoring, few contraindications, and direct localized effect on plaques. Therefore, side effects with topical agent use rarely are systemic. Medication interactions are less of a concern with topical therapies; thus, they have better safety profiles compared with systemic therapies. This clinical review summarizes the recently published evidence-based guidelines from the AAD and NPF on the use of topical agents in psoriasis and may be a useful guiding framework for clinicians in their everyday practice.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study. J Med Econ. 2018:1-9.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470.
- Svendsen MT, Jeyabalan J, Andersen KE, et al. Worldwide utilization of topical remedies in treatment of psoriasis: a systematic review. J Dermatolog Treat. 2017;28:374-383.
- Day A, Abramson AK, Patel M, et al. The spectrum of oculocutaneous disease: part II. neoplastic and drug-related causes of oculocutaneous disease. J Am Acad Dermatol. 2014;70:821.e821-819.
- Choi JW, Choi JW, Kwon IH, et al. High-concentration (20 μg g-¹) tacalcitol ointment in the treatment of facial psoriasis: an 8-week open-label clinical trial. Br J Dermatol. 2010;162:1359-1364.
- Hashim PW, Chima M, Kim HJ, et al. Crisaborole 2% ointment for the treatment of intertriginous, anogenital, and facial psoriasis: a double-blind, randomized, vehicle-controlled trial. J Am Acad Dermatol. 2020;82:360-365.
- Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
- Iversen L, Jakobsen HB. Patient preferences for topical psoriasis treatments are diverse and difficult to predict. Dermatol Ther. 2016;6:273-285.
- Clobex Package insert. Galderma Laboratories, LP; 2012.
- Kenalog-10 Injection. Package insert. Bristol-Myers Squibb Company; 2018.
- Mason J, Mason AR, Cork MJ. Topical preparations for the treatment of psoriasis: a systematic review. Br J Dermatol. 2002;146:351-364.
- Koo J, Cuffie CA, Tanner DJ, et al. Mometasone furoate 0.1%-salicylic acid 5% ointment versus mometasone furoate 0.1% ointment in the treatment of moderate-to-severe psoriasis: a multicenter study. Clin Ther. 1998;20:283-291.
- Tiplica GS, Salavastru CM. Mometasone furoate 0.1% and salicylic acid 5% vs. mometasone furoate 0.1% as sequential local therapy in psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23:905-912.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Strober BE, Bissonnette R, Fiorentino D, et al. Comparative effectiveness of biologic agents for the treatment of psoriasis in a real-world setting: results from a large, prospective, observational study (Psoriasis Longitudinal Assessment and Registry [PSOLAR]). J Am Acad Dermatol. 2016;74:851-861.e854.
- Castela E, Archier E, Devaux S, et al. Topical corticosteroids in plaque psoriasis: a systematic review of risk of adrenal axis suppression and skin atrophy. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):47-51.
- Takahashi H, Tsuji H, Honma M, et al. Femoral head osteonecrosis after long-term topical corticosteroid treatment in a psoriasis patient. J Dermatol. 2012;39:887-888.
- el Maghraoui A, Tabache F, Bezza A, et al. Femoral head osteonecrosis after topical corticosteroid therapy. Clin Exp Rheumatol. 2001;19:233.
- Gribetz C, Ling M, Lebwohl M, et al. Pimecrolimus cream 1% in the treatment of intertriginous psoriasis: a double-blind, randomized study. J Am Acad Dermatol. 2004;51:731-738.
- Lebwohl M, Freeman AK, Chapman MS, et al. Tacrolimus ointment is effective for facial and intertriginous psoriasis. J Am Acad Dermatol. 2004;51:723-730.
- Paller AS, Fölster-Holst R, Chen SC, et al. No evidence of increased cancer incidence in children using topical tacrolimus for atopic dermatitis. J Am Acad Dermatol. 2020;83:375-381.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Lebwohl M, Ast E, Callen JP, et al. Once-daily tazarotene gel versus twice-daily fluocinonide cream in the treatment of plaque psoriasis. J Am Acad Dermatol. 1998;38:705-711.
- Weinstein GD, Koo JY, Krueger GG, et al. Tazarotene cream in the treatment of psoriasis: two multicenter, double-blind, randomized, vehicle-controlled studies of the safety and efficacy of tazarotene creams 0.05% and 0.1% applied once daily for 12 weeks. J Am Acad Dermatol. 2003;48:760-767.
- Lebwohl M, Lombardi K, Tan MH. Duration of improvement in psoriasis after treatment with tazarotene 0.1% gel plus clobetasol propionate 0.05% ointment: comparison of maintenance treatments. Int J Dermatol. 2001;40:64-66.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Koo JY, Lowe NJ, Lew-Kaya DA, et al. Tazarotene plus UVB phototherapy in the treatment of psoriasis. J Am Acad Dermatol. 2000;43:821-828.
- Cassano N, Mantegazza R, Battaglini S, et al. Adjuvant role of a new emollient cream in patients with palmar and/or plantar psoriasis: a pilot randomized open-label study. G Ital Dermatol Venereol. 2010;145:789-792.
- Kristensen B, Kristensen O. Topical salicylic acid interferes with UVB therapy for psoriasis. Acta Derm Venereol. 1991;71:37-40.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Rogalski C, Grunewald S, Schetschorke M, et al. Treatment of plaque-type psoriasis with the 308 nm excimer laser in combination with dithranol or calcipotriol. Int J Hyperthermia. 2012;28:184-190.
- Bagel J. LCD plus NB-UVB reduces time to improvement of psoriasis vs. NB-UVB alone. J Drugs Dermatol. 2009;8:351-357.
- Abdallah MA, El-Khateeb EA, Abdel-Rahman SH. The influence of psoriatic plaques pretreatment with crude coal tar vs. petrolatum on the efficacy of narrow-band ultraviolet B: a half-vs.-half intra-individual double-blinded comparative study. Photodermatol Photoimmunol Photomed. 2011;27:226-230.
Psoriasis is a chronic inflammatory skin disease characterized by erythematous scaly plaques that can invoke substantial pain, pruritus, and quality-of-life disturbance in patients. Topical therapies are the most commonly used medications for treating psoriasis, with one study (N = 128,308) showing that more than 85% of patients with psoriasis were managed solely with topical medications. 1 For patients with mild to moderate psoriasis, topical agents alone may be able to control disease completely. For those with more severe disease, topical agents are used adjunctively with systemic or biologic agents to optimize disease control in localized areas.
The American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) published guidelines in 2020 for managing psoriasis with topical agents in adults.2 This review presents the most up-to-date clinical recommendations for topical agent use in adult patients with psoriasis and elaborates on each drug’s pharmacologic and safety profile. Specifically, evidence-based treatment recommendations for topical steroids, calcineurin inhibitors (CNIs), vitamin D analogues, retinoids (tazarotene), emollients, keratolytics (salicylic acid), anthracenes (anthralin), and keratoplastics (coal tar) will be addressed (Table 1). Recommendations for combination therapy with other treatment modalities including UVB light therapy, biologics, and systemic nonbiologic agents also will be discussed.


Selecting a Topical Agent Based on Disease Localization
When treating patients with psoriasis with topical therapies, clinicians should take into consideration drug potency, as it determines how effective a treatment will be in penetrating the skin barrier. Plaque characteristics, such as distribution (localized vs widespread), anatomical localization (flexural, scalp, palms/soles/nails), size (large vs small), and thickness (thick vs thin), not only influence treatment effectiveness but also the incidence of drug-related adverse events. Furthermore, preferred topical therapies are tailored to each patient based on disease characteristics and activity. Coal tar and anthralin have been used less frequently than other topical therapies for psoriasis because of their undesirable side-effect profiles (Table 1).3
Face and Intertriginous Regions—The face and intertriginous areas are sensitive because skin tends to be thin in these regions. Emollients are recommended for disease in these locations given their safety and flexibility in use for most areas. Conversely, anthralin should be avoided on the face, intertriginous areas, and even highly visible locations because of the potential for skin staining. Low-potency corticosteroids also have utility in psoriasis distributed on the face and intertriginous regions. Additionally, application of steroids around the eyes should be cautioned because topical steroids can induce ocular complications such as glaucoma and cataracts in rare circumstances.4
Off-label use of CNIs for psoriasis on the face and intertriginous areas also is effective. Currently, there is a level B recommendation for off-label use of 0.1% tacrolimus for up to 8 weeks for inverse psoriasis or psoriasis on the face. Off-label use of pimecrolimus for 4 to 8 weeks also can be considered for inverse psoriasis. Combination therapy consisting of hydrocortisone with calcipotriol ointment is another effective regimen.5 One study also suggested that use of crisaborole for 4 to 8 weeks in intertriginous psoriasis can be effective and well tolerated.6
Scalp—The vehicle of medication administration is especially important in hair-bearing areas such as the scalp, as these areas are challenging for medication application and patient adherence. Thus, patient preferences for the vehicle must be considered. Several studies have been conducted to assess preference for various vehicles in scalp psoriasis. A foam or solution may be preferable to ointments, gels, or creams.7 Gels may be preferred over ointments.8 There is a level A recommendation supporting the use of class 1 to 7 topical steroids for a minimum of 4 weeks as initial and maintenance treatment of scalp psoriasis. The highest level of evidence (level A) also supports the use of calcipotriol foam or combination therapy of calcipotriol–betamethasone dipropionate gel for 4 to 12 weeks as treatment of mild to moderate scalp psoriasis.
Nails—Several options for topical medications have been recommended for the treatment of nail psoriasis. Currently, there is a level B recommendation for the use of tazarotene for the treatment of nail psoriasis. Another effective regimen is combination therapy with vitamin D analogues and betamethasone dipropionate.9 Topical steroid use for nail psoriasis should be limited to 12 weeks because of the risk for bone atrophy with chronic steroid use.
Palmoplantar—The palms and soles have a thicker epidermal layer than other areas of the body. As a result, class 1 corticosteroids can be used for palmoplantar psoriasis for more than 4 weeks with vigilant monitoring for adverse effects such as skin atrophy, tachyphylaxis, or tinea infection. Tazarotene also has been shown to be helpful in treating palmoplantar psoriasis.
Resistant Disease—Intralesional steroids are beneficial treatment options for recalcitrant psoriasis in glabrous areas, as well as for palmoplantar, nail, and scalp psoriasis. Up to 10 mg/mL of triamcinolone acetonide used every 3 to 4 weeks is an effective regimen.10Pregnancy/Breastfeeding—Women of childbearing potential have additional safety precautions that should be considered during medication selection. Emollients have been shown to be safe during pregnancy and lactation. Currently, there is little known about CNI use during pregnancy. During lactation, CNIs can be used by breastfeeding mothers in most areas, excluding the breasts. Evaluation of the safety of anthralin and vitamin D analogues during pregnancy and lactation have not been studied. For these agents, dermatologists need to use their clinical judgment to weigh the risks and benefits of medication, particularly in patients requiring occlusion, higher medication doses, or treatment over a large surface area. Salicylic acid should be used with caution in pregnant and breastfeeding mothers because it is a pregnancy category C drug. Lower-potency corticosteroids may be used with caution during pregnancy and breastfeeding. More potent corticosteroids and coal tar, however, should be avoided. Similarly, tazarotene use is contraindicated in pregnancy. According to the US Food and Drug Administration labels for all forms of topical tazarotene, a pregnancy test must be obtained 2 weeks prior to tazarotene treatment initiation in women of childbearing potential because of the risk for serious fetal malformations and toxicity.
Recommendations, Risks, and Benefits of Topical Therapy for the Management of Psoriasis
Topical Corticosteroids—Topical corticosteroids (TCs) are widely used for inflammatory skin conditions and are available in a variety of strengths (Table 2). They are thought to exert their action by regulating the gene transcription of proinflammatory mediators. For psoriasis, steroids are recommended for 2 to 4 weeks, depending on disease severity. Although potent and superpotent steroids are more effective than mild- to moderate-strength TCs, use of lower-potency TCs may be warranted depending on disease distribution and localization.11 For treatment of psoriasis with no involvement of the intertriginous areas, use of class 1 to 5 TCs for up to 4 weeks is recommended.

For moderate to severe psoriasis with 20% or less body surface area (BSA) affected, combination therapy consisting of mometasone and salicylic acid has been shown to be more effective than mometasone alone.12,13 There currently is a level A recommendation for the use of combination therapy with class 1 TCs and etanercept for 12 weeks in patients with moderate to severe psoriasis who require both systemic and topical therapies for disease control. Similarly, combination therapy with infliximab and high-potency TCs has a level B recommendation to enhance efficacy for the treatment of moderate to severe psoriasis.14 High-quality studies on the use of TCs with anti–IL-12/IL-23, anti–IL-23, and anti–IL-17 currently are unavailable, but the combination is not expected to be unsafe.14,15 Combination therapy of betamethasone dipropionate ointment and low-dose cyclosporine is an alternative regimen with a level B recommendation.
The most common adverse effects with use of TCs are skin thinning and atrophy, telangiectasia, and striae (Table 1). With clinical improvement of disease, it is recommended that clinicians taper TCs to prevent rebound effect. To decrease TC-related adverse effects, clinicians should use combination therapy with steroid-sparing agents for disease maintenance, transition to lower-potency corticosteroids, or use intermittent steroid therapy. Systemic effects of TC use include hypothalamic-pituitary-adrenal axis suppression, Cushing syndrome, and osteonecrosis of the femoral head.16-18 These systemic effects with TC use are rare unless treatment is for disease involving greater than 20% BSA or occlusion for more than 4 weeks.
Calcineurin Inhibitors—Calcineurin inhibitors inhibit calcineurin phosphorylation and T-cell activation, subsequently decreasing the expression of proinflammatory cytokines. Currently, they are not approved by the US Food and Drug Administration to treat psoriasis but have demonstrated efficacy in randomized control trials (RCTs) for facial and intertriginous psoriasis. In RCTs, 71% of patients using pimecrolimus cream 0.1% twice daily for 8 weeks achieved an investigator global assessment score of clear (0) or almost clear (1) compared with 21% of placebo-treated patients (N=57).19 Other trials have shown that 65% of patients receiving tacrolimus ointment 0.1% for 8 weeks achieved an investigator global assessment score of 0 or 1 compared with 31% of placebo-treated patients (N=167).20 Because of their efficacy in RCTs, CNIs commonly are used off label to treat psoriasis.
The most common adverse effects with CNI use are burning, pruritus, and flushing with alcohol ingestion (Table 1). Additionally, CNIs have a black box warning that use may increase the risk for malignancy, but this risk has not been demonstrated with topical use in humans.21Vitamin D Analogues—The class of vitamin D analogues—calcipotriol/calcipotriene and calcitriol—frequently are used to treat psoriasis. Vitamin D analogues exert their beneficial effects by inhibiting keratinocyte proliferation and enhancing keratinocyte differentiation. They also are ideal for long-term use (up to 52 weeks) in mild to moderate psoriasis and can be used in combination with class 2 and 3 TCs. There is a level A recommendation that supports the use of combination therapy with calcipotriol and TCs for the treatment of mild to moderate psoriasis.
For severe psoriasis, many studies have investigated the efficacy of combination therapy with vitamin D analogues and systemic treatments. Combination therapy with calcipotriol and methotrexate or calcipotriol and acitretin are effective treatment regimens with level A recommendations. Calcipotriol–betamethasone dipropionate ointment in combination with low-dose cyclosporine is an alternative option with a level B recommendation. Because vitamin D analogues are inactivated by UVA and UVB radiation, clinicians should advise their patients to use vitamin D analogues after receiving UVB phototherapy.22
Common adverse effects of vitamin D analogues include burning, pruritus, erythema, and dryness (Table 1). Hypercalcemia and parathyroid hormone suppression are extremely rare unless treatment occurs over a large surface area (>30% BSA) or the patient has concurrent renal disease or impairments in calcium metabolism.
Tazarotene—Tazarotene is a topical retinoid that acts by decreasing keratinocyte proliferation, facilitating keratinocyte differentiation, and inhibiting inflammation. Patients with mild to moderate psoriasis are recommended to receive tazarotene treatment for 8 to 12 weeks. In several RCTs, tazarotene gel 0.1% and tazarotene cream 0.1% and 0.05% achieved treatment success in treating plaque psoriasis.23,24
For increased efficacy, clinicians can recommend combination therapy with tazarotene and a TC. Combination therapy with tazarotene and a mid- or high-potency TC for 8 to 16 weeks has been shown to be more effective than treatment with tazarotene alone.25 Thus, there is a level A recommendation for use of this combination to treat mild to moderate psoriasis. Agents used in combination therapy work synergistically to decrease the length of treatment and increase the duration of remission. The frequency of adverse effects, such as irritation from tazarotene and skin atrophy from TCs, also are reduced.26 Combination therapy with tazarotene and narrowband UVB (NB-UVB) is another effective option that requires less UV radiation than NB-UVB alone because of the synergistic effects of both treatment modalities.27 Clinicians should counsel patients on the adverse effects of tazarotene, which include local irritation, burning, pruritus, and erythema (Table 1).
Emollients—Emollients are nonmedicated moisturizers that decrease the amount of transepidermal water loss. There is a level B recommendation for use of emollients and TCs in combination for 4 to 8 weeks to treat psoriasis. In fact, combination therapy with mometasone and emollients has demonstrated greater improvement in symptoms of palmoplantar psoriasis (ie, erythema, desquamation, infiltration, BSA involvement) than mometasone alone.28 Emollients are safe options that can be used on all areas of the body and during pregnancy and lactation. Although adverse effects of emollients are rare, clinicians should counsel patients on the risk for contact dermatitis if specific allergies to ingredients/fragrances exist (Table 1).
Salicylic Acid—Salicylic acid is a topical keratolytic that can be used to treat psoriatic plaques. Use of salicylic acid for 8 to 16 weeks has been shown to be effective for mild to moderate psoriasis. Combination therapy of salicylic acid and TCs in patients with 20% or less BSA affected is a safe and effective option with a level B recommendation. Combination therapy with salicylic acid and calcipotriene, however, should be avoided because calcipotriene is inactivated by salicylic acid. It also is recommended that salicylic acid application follow phototherapy when both treatment modalities are used in combination.29,30 Clinicians should be cautious about using salicylic acid in patients with renal or hepatic disease because of the increased risk for salicylate toxicity (Table 1).
Anthralin—Anthralin is a synthetic hydrocarbon derivative that has been shown to reduce inflammation and normalize keratinocyte proliferation through an unknown mechanism. It is recommended that patients with mild to moderate psoriasis receive anthralin treatment for 8 to 12 weeks, with a maximum application time of 2 hours per day. Combination therapy of excimer laser and anthralin has been shown to be more effective in treating psoriasis than anthralin alone.31 Therefore, clinicians have the option of including excimer laser therapy for additional disease control. Anthralin should be avoided on the face, flexural regions, and highly visible areas because of potential skin staining (Table 1). Other adverse effects include application-site burning and erythema.
Coal Tar—Coal tar is a heterogenous mixture of aromatic hydrocarbons that is an effective treatment of psoriasis because of its inherent anti-inflammatory and keratoplastic properties. There is high-quality evidence supporting a level A recommendation for coal tar use in mild to moderate psoriasis. Combination therapy with NB-UVB and coal tar (also known as Goeckerman therapy) is a recommended treatment option with a quicker onset of action and improved outcomes compared with NB-UVB therapy alone.32,33 Adverse events of coal tar include application-site irritation, folliculitis, contact dermatitis, phototoxicity, and skin pigmentation (Table 1).
Conclusion
Topical medications are versatile treatment options that can be utilized as monotherapy or adjunct therapy for mild to severe psoriasis. Benefits of topical agents include minimal required monitoring, few contraindications, and direct localized effect on plaques. Therefore, side effects with topical agent use rarely are systemic. Medication interactions are less of a concern with topical therapies; thus, they have better safety profiles compared with systemic therapies. This clinical review summarizes the recently published evidence-based guidelines from the AAD and NPF on the use of topical agents in psoriasis and may be a useful guiding framework for clinicians in their everyday practice.
Psoriasis is a chronic inflammatory skin disease characterized by erythematous scaly plaques that can invoke substantial pain, pruritus, and quality-of-life disturbance in patients. Topical therapies are the most commonly used medications for treating psoriasis, with one study (N = 128,308) showing that more than 85% of patients with psoriasis were managed solely with topical medications. 1 For patients with mild to moderate psoriasis, topical agents alone may be able to control disease completely. For those with more severe disease, topical agents are used adjunctively with systemic or biologic agents to optimize disease control in localized areas.
The American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) published guidelines in 2020 for managing psoriasis with topical agents in adults.2 This review presents the most up-to-date clinical recommendations for topical agent use in adult patients with psoriasis and elaborates on each drug’s pharmacologic and safety profile. Specifically, evidence-based treatment recommendations for topical steroids, calcineurin inhibitors (CNIs), vitamin D analogues, retinoids (tazarotene), emollients, keratolytics (salicylic acid), anthracenes (anthralin), and keratoplastics (coal tar) will be addressed (Table 1). Recommendations for combination therapy with other treatment modalities including UVB light therapy, biologics, and systemic nonbiologic agents also will be discussed.


Selecting a Topical Agent Based on Disease Localization
When treating patients with psoriasis with topical therapies, clinicians should take into consideration drug potency, as it determines how effective a treatment will be in penetrating the skin barrier. Plaque characteristics, such as distribution (localized vs widespread), anatomical localization (flexural, scalp, palms/soles/nails), size (large vs small), and thickness (thick vs thin), not only influence treatment effectiveness but also the incidence of drug-related adverse events. Furthermore, preferred topical therapies are tailored to each patient based on disease characteristics and activity. Coal tar and anthralin have been used less frequently than other topical therapies for psoriasis because of their undesirable side-effect profiles (Table 1).3
Face and Intertriginous Regions—The face and intertriginous areas are sensitive because skin tends to be thin in these regions. Emollients are recommended for disease in these locations given their safety and flexibility in use for most areas. Conversely, anthralin should be avoided on the face, intertriginous areas, and even highly visible locations because of the potential for skin staining. Low-potency corticosteroids also have utility in psoriasis distributed on the face and intertriginous regions. Additionally, application of steroids around the eyes should be cautioned because topical steroids can induce ocular complications such as glaucoma and cataracts in rare circumstances.4
Off-label use of CNIs for psoriasis on the face and intertriginous areas also is effective. Currently, there is a level B recommendation for off-label use of 0.1% tacrolimus for up to 8 weeks for inverse psoriasis or psoriasis on the face. Off-label use of pimecrolimus for 4 to 8 weeks also can be considered for inverse psoriasis. Combination therapy consisting of hydrocortisone with calcipotriol ointment is another effective regimen.5 One study also suggested that use of crisaborole for 4 to 8 weeks in intertriginous psoriasis can be effective and well tolerated.6
Scalp—The vehicle of medication administration is especially important in hair-bearing areas such as the scalp, as these areas are challenging for medication application and patient adherence. Thus, patient preferences for the vehicle must be considered. Several studies have been conducted to assess preference for various vehicles in scalp psoriasis. A foam or solution may be preferable to ointments, gels, or creams.7 Gels may be preferred over ointments.8 There is a level A recommendation supporting the use of class 1 to 7 topical steroids for a minimum of 4 weeks as initial and maintenance treatment of scalp psoriasis. The highest level of evidence (level A) also supports the use of calcipotriol foam or combination therapy of calcipotriol–betamethasone dipropionate gel for 4 to 12 weeks as treatment of mild to moderate scalp psoriasis.
Nails—Several options for topical medications have been recommended for the treatment of nail psoriasis. Currently, there is a level B recommendation for the use of tazarotene for the treatment of nail psoriasis. Another effective regimen is combination therapy with vitamin D analogues and betamethasone dipropionate.9 Topical steroid use for nail psoriasis should be limited to 12 weeks because of the risk for bone atrophy with chronic steroid use.
Palmoplantar—The palms and soles have a thicker epidermal layer than other areas of the body. As a result, class 1 corticosteroids can be used for palmoplantar psoriasis for more than 4 weeks with vigilant monitoring for adverse effects such as skin atrophy, tachyphylaxis, or tinea infection. Tazarotene also has been shown to be helpful in treating palmoplantar psoriasis.
Resistant Disease—Intralesional steroids are beneficial treatment options for recalcitrant psoriasis in glabrous areas, as well as for palmoplantar, nail, and scalp psoriasis. Up to 10 mg/mL of triamcinolone acetonide used every 3 to 4 weeks is an effective regimen.10Pregnancy/Breastfeeding—Women of childbearing potential have additional safety precautions that should be considered during medication selection. Emollients have been shown to be safe during pregnancy and lactation. Currently, there is little known about CNI use during pregnancy. During lactation, CNIs can be used by breastfeeding mothers in most areas, excluding the breasts. Evaluation of the safety of anthralin and vitamin D analogues during pregnancy and lactation have not been studied. For these agents, dermatologists need to use their clinical judgment to weigh the risks and benefits of medication, particularly in patients requiring occlusion, higher medication doses, or treatment over a large surface area. Salicylic acid should be used with caution in pregnant and breastfeeding mothers because it is a pregnancy category C drug. Lower-potency corticosteroids may be used with caution during pregnancy and breastfeeding. More potent corticosteroids and coal tar, however, should be avoided. Similarly, tazarotene use is contraindicated in pregnancy. According to the US Food and Drug Administration labels for all forms of topical tazarotene, a pregnancy test must be obtained 2 weeks prior to tazarotene treatment initiation in women of childbearing potential because of the risk for serious fetal malformations and toxicity.
Recommendations, Risks, and Benefits of Topical Therapy for the Management of Psoriasis
Topical Corticosteroids—Topical corticosteroids (TCs) are widely used for inflammatory skin conditions and are available in a variety of strengths (Table 2). They are thought to exert their action by regulating the gene transcription of proinflammatory mediators. For psoriasis, steroids are recommended for 2 to 4 weeks, depending on disease severity. Although potent and superpotent steroids are more effective than mild- to moderate-strength TCs, use of lower-potency TCs may be warranted depending on disease distribution and localization.11 For treatment of psoriasis with no involvement of the intertriginous areas, use of class 1 to 5 TCs for up to 4 weeks is recommended.

For moderate to severe psoriasis with 20% or less body surface area (BSA) affected, combination therapy consisting of mometasone and salicylic acid has been shown to be more effective than mometasone alone.12,13 There currently is a level A recommendation for the use of combination therapy with class 1 TCs and etanercept for 12 weeks in patients with moderate to severe psoriasis who require both systemic and topical therapies for disease control. Similarly, combination therapy with infliximab and high-potency TCs has a level B recommendation to enhance efficacy for the treatment of moderate to severe psoriasis.14 High-quality studies on the use of TCs with anti–IL-12/IL-23, anti–IL-23, and anti–IL-17 currently are unavailable, but the combination is not expected to be unsafe.14,15 Combination therapy of betamethasone dipropionate ointment and low-dose cyclosporine is an alternative regimen with a level B recommendation.
The most common adverse effects with use of TCs are skin thinning and atrophy, telangiectasia, and striae (Table 1). With clinical improvement of disease, it is recommended that clinicians taper TCs to prevent rebound effect. To decrease TC-related adverse effects, clinicians should use combination therapy with steroid-sparing agents for disease maintenance, transition to lower-potency corticosteroids, or use intermittent steroid therapy. Systemic effects of TC use include hypothalamic-pituitary-adrenal axis suppression, Cushing syndrome, and osteonecrosis of the femoral head.16-18 These systemic effects with TC use are rare unless treatment is for disease involving greater than 20% BSA or occlusion for more than 4 weeks.
Calcineurin Inhibitors—Calcineurin inhibitors inhibit calcineurin phosphorylation and T-cell activation, subsequently decreasing the expression of proinflammatory cytokines. Currently, they are not approved by the US Food and Drug Administration to treat psoriasis but have demonstrated efficacy in randomized control trials (RCTs) for facial and intertriginous psoriasis. In RCTs, 71% of patients using pimecrolimus cream 0.1% twice daily for 8 weeks achieved an investigator global assessment score of clear (0) or almost clear (1) compared with 21% of placebo-treated patients (N=57).19 Other trials have shown that 65% of patients receiving tacrolimus ointment 0.1% for 8 weeks achieved an investigator global assessment score of 0 or 1 compared with 31% of placebo-treated patients (N=167).20 Because of their efficacy in RCTs, CNIs commonly are used off label to treat psoriasis.
The most common adverse effects with CNI use are burning, pruritus, and flushing with alcohol ingestion (Table 1). Additionally, CNIs have a black box warning that use may increase the risk for malignancy, but this risk has not been demonstrated with topical use in humans.21Vitamin D Analogues—The class of vitamin D analogues—calcipotriol/calcipotriene and calcitriol—frequently are used to treat psoriasis. Vitamin D analogues exert their beneficial effects by inhibiting keratinocyte proliferation and enhancing keratinocyte differentiation. They also are ideal for long-term use (up to 52 weeks) in mild to moderate psoriasis and can be used in combination with class 2 and 3 TCs. There is a level A recommendation that supports the use of combination therapy with calcipotriol and TCs for the treatment of mild to moderate psoriasis.
For severe psoriasis, many studies have investigated the efficacy of combination therapy with vitamin D analogues and systemic treatments. Combination therapy with calcipotriol and methotrexate or calcipotriol and acitretin are effective treatment regimens with level A recommendations. Calcipotriol–betamethasone dipropionate ointment in combination with low-dose cyclosporine is an alternative option with a level B recommendation. Because vitamin D analogues are inactivated by UVA and UVB radiation, clinicians should advise their patients to use vitamin D analogues after receiving UVB phototherapy.22
Common adverse effects of vitamin D analogues include burning, pruritus, erythema, and dryness (Table 1). Hypercalcemia and parathyroid hormone suppression are extremely rare unless treatment occurs over a large surface area (>30% BSA) or the patient has concurrent renal disease or impairments in calcium metabolism.
Tazarotene—Tazarotene is a topical retinoid that acts by decreasing keratinocyte proliferation, facilitating keratinocyte differentiation, and inhibiting inflammation. Patients with mild to moderate psoriasis are recommended to receive tazarotene treatment for 8 to 12 weeks. In several RCTs, tazarotene gel 0.1% and tazarotene cream 0.1% and 0.05% achieved treatment success in treating plaque psoriasis.23,24
For increased efficacy, clinicians can recommend combination therapy with tazarotene and a TC. Combination therapy with tazarotene and a mid- or high-potency TC for 8 to 16 weeks has been shown to be more effective than treatment with tazarotene alone.25 Thus, there is a level A recommendation for use of this combination to treat mild to moderate psoriasis. Agents used in combination therapy work synergistically to decrease the length of treatment and increase the duration of remission. The frequency of adverse effects, such as irritation from tazarotene and skin atrophy from TCs, also are reduced.26 Combination therapy with tazarotene and narrowband UVB (NB-UVB) is another effective option that requires less UV radiation than NB-UVB alone because of the synergistic effects of both treatment modalities.27 Clinicians should counsel patients on the adverse effects of tazarotene, which include local irritation, burning, pruritus, and erythema (Table 1).
Emollients—Emollients are nonmedicated moisturizers that decrease the amount of transepidermal water loss. There is a level B recommendation for use of emollients and TCs in combination for 4 to 8 weeks to treat psoriasis. In fact, combination therapy with mometasone and emollients has demonstrated greater improvement in symptoms of palmoplantar psoriasis (ie, erythema, desquamation, infiltration, BSA involvement) than mometasone alone.28 Emollients are safe options that can be used on all areas of the body and during pregnancy and lactation. Although adverse effects of emollients are rare, clinicians should counsel patients on the risk for contact dermatitis if specific allergies to ingredients/fragrances exist (Table 1).
Salicylic Acid—Salicylic acid is a topical keratolytic that can be used to treat psoriatic plaques. Use of salicylic acid for 8 to 16 weeks has been shown to be effective for mild to moderate psoriasis. Combination therapy of salicylic acid and TCs in patients with 20% or less BSA affected is a safe and effective option with a level B recommendation. Combination therapy with salicylic acid and calcipotriene, however, should be avoided because calcipotriene is inactivated by salicylic acid. It also is recommended that salicylic acid application follow phototherapy when both treatment modalities are used in combination.29,30 Clinicians should be cautious about using salicylic acid in patients with renal or hepatic disease because of the increased risk for salicylate toxicity (Table 1).
Anthralin—Anthralin is a synthetic hydrocarbon derivative that has been shown to reduce inflammation and normalize keratinocyte proliferation through an unknown mechanism. It is recommended that patients with mild to moderate psoriasis receive anthralin treatment for 8 to 12 weeks, with a maximum application time of 2 hours per day. Combination therapy of excimer laser and anthralin has been shown to be more effective in treating psoriasis than anthralin alone.31 Therefore, clinicians have the option of including excimer laser therapy for additional disease control. Anthralin should be avoided on the face, flexural regions, and highly visible areas because of potential skin staining (Table 1). Other adverse effects include application-site burning and erythema.
Coal Tar—Coal tar is a heterogenous mixture of aromatic hydrocarbons that is an effective treatment of psoriasis because of its inherent anti-inflammatory and keratoplastic properties. There is high-quality evidence supporting a level A recommendation for coal tar use in mild to moderate psoriasis. Combination therapy with NB-UVB and coal tar (also known as Goeckerman therapy) is a recommended treatment option with a quicker onset of action and improved outcomes compared with NB-UVB therapy alone.32,33 Adverse events of coal tar include application-site irritation, folliculitis, contact dermatitis, phototoxicity, and skin pigmentation (Table 1).
Conclusion
Topical medications are versatile treatment options that can be utilized as monotherapy or adjunct therapy for mild to severe psoriasis. Benefits of topical agents include minimal required monitoring, few contraindications, and direct localized effect on plaques. Therefore, side effects with topical agent use rarely are systemic. Medication interactions are less of a concern with topical therapies; thus, they have better safety profiles compared with systemic therapies. This clinical review summarizes the recently published evidence-based guidelines from the AAD and NPF on the use of topical agents in psoriasis and may be a useful guiding framework for clinicians in their everyday practice.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study. J Med Econ. 2018:1-9.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470.
- Svendsen MT, Jeyabalan J, Andersen KE, et al. Worldwide utilization of topical remedies in treatment of psoriasis: a systematic review. J Dermatolog Treat. 2017;28:374-383.
- Day A, Abramson AK, Patel M, et al. The spectrum of oculocutaneous disease: part II. neoplastic and drug-related causes of oculocutaneous disease. J Am Acad Dermatol. 2014;70:821.e821-819.
- Choi JW, Choi JW, Kwon IH, et al. High-concentration (20 μg g-¹) tacalcitol ointment in the treatment of facial psoriasis: an 8-week open-label clinical trial. Br J Dermatol. 2010;162:1359-1364.
- Hashim PW, Chima M, Kim HJ, et al. Crisaborole 2% ointment for the treatment of intertriginous, anogenital, and facial psoriasis: a double-blind, randomized, vehicle-controlled trial. J Am Acad Dermatol. 2020;82:360-365.
- Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
- Iversen L, Jakobsen HB. Patient preferences for topical psoriasis treatments are diverse and difficult to predict. Dermatol Ther. 2016;6:273-285.
- Clobex Package insert. Galderma Laboratories, LP; 2012.
- Kenalog-10 Injection. Package insert. Bristol-Myers Squibb Company; 2018.
- Mason J, Mason AR, Cork MJ. Topical preparations for the treatment of psoriasis: a systematic review. Br J Dermatol. 2002;146:351-364.
- Koo J, Cuffie CA, Tanner DJ, et al. Mometasone furoate 0.1%-salicylic acid 5% ointment versus mometasone furoate 0.1% ointment in the treatment of moderate-to-severe psoriasis: a multicenter study. Clin Ther. 1998;20:283-291.
- Tiplica GS, Salavastru CM. Mometasone furoate 0.1% and salicylic acid 5% vs. mometasone furoate 0.1% as sequential local therapy in psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23:905-912.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Strober BE, Bissonnette R, Fiorentino D, et al. Comparative effectiveness of biologic agents for the treatment of psoriasis in a real-world setting: results from a large, prospective, observational study (Psoriasis Longitudinal Assessment and Registry [PSOLAR]). J Am Acad Dermatol. 2016;74:851-861.e854.
- Castela E, Archier E, Devaux S, et al. Topical corticosteroids in plaque psoriasis: a systematic review of risk of adrenal axis suppression and skin atrophy. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):47-51.
- Takahashi H, Tsuji H, Honma M, et al. Femoral head osteonecrosis after long-term topical corticosteroid treatment in a psoriasis patient. J Dermatol. 2012;39:887-888.
- el Maghraoui A, Tabache F, Bezza A, et al. Femoral head osteonecrosis after topical corticosteroid therapy. Clin Exp Rheumatol. 2001;19:233.
- Gribetz C, Ling M, Lebwohl M, et al. Pimecrolimus cream 1% in the treatment of intertriginous psoriasis: a double-blind, randomized study. J Am Acad Dermatol. 2004;51:731-738.
- Lebwohl M, Freeman AK, Chapman MS, et al. Tacrolimus ointment is effective for facial and intertriginous psoriasis. J Am Acad Dermatol. 2004;51:723-730.
- Paller AS, Fölster-Holst R, Chen SC, et al. No evidence of increased cancer incidence in children using topical tacrolimus for atopic dermatitis. J Am Acad Dermatol. 2020;83:375-381.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Lebwohl M, Ast E, Callen JP, et al. Once-daily tazarotene gel versus twice-daily fluocinonide cream in the treatment of plaque psoriasis. J Am Acad Dermatol. 1998;38:705-711.
- Weinstein GD, Koo JY, Krueger GG, et al. Tazarotene cream in the treatment of psoriasis: two multicenter, double-blind, randomized, vehicle-controlled studies of the safety and efficacy of tazarotene creams 0.05% and 0.1% applied once daily for 12 weeks. J Am Acad Dermatol. 2003;48:760-767.
- Lebwohl M, Lombardi K, Tan MH. Duration of improvement in psoriasis after treatment with tazarotene 0.1% gel plus clobetasol propionate 0.05% ointment: comparison of maintenance treatments. Int J Dermatol. 2001;40:64-66.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Koo JY, Lowe NJ, Lew-Kaya DA, et al. Tazarotene plus UVB phototherapy in the treatment of psoriasis. J Am Acad Dermatol. 2000;43:821-828.
- Cassano N, Mantegazza R, Battaglini S, et al. Adjuvant role of a new emollient cream in patients with palmar and/or plantar psoriasis: a pilot randomized open-label study. G Ital Dermatol Venereol. 2010;145:789-792.
- Kristensen B, Kristensen O. Topical salicylic acid interferes with UVB therapy for psoriasis. Acta Derm Venereol. 1991;71:37-40.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Rogalski C, Grunewald S, Schetschorke M, et al. Treatment of plaque-type psoriasis with the 308 nm excimer laser in combination with dithranol or calcipotriol. Int J Hyperthermia. 2012;28:184-190.
- Bagel J. LCD plus NB-UVB reduces time to improvement of psoriasis vs. NB-UVB alone. J Drugs Dermatol. 2009;8:351-357.
- Abdallah MA, El-Khateeb EA, Abdel-Rahman SH. The influence of psoriatic plaques pretreatment with crude coal tar vs. petrolatum on the efficacy of narrow-band ultraviolet B: a half-vs.-half intra-individual double-blinded comparative study. Photodermatol Photoimmunol Photomed. 2011;27:226-230.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study. J Med Econ. 2018:1-9.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470.
- Svendsen MT, Jeyabalan J, Andersen KE, et al. Worldwide utilization of topical remedies in treatment of psoriasis: a systematic review. J Dermatolog Treat. 2017;28:374-383.
- Day A, Abramson AK, Patel M, et al. The spectrum of oculocutaneous disease: part II. neoplastic and drug-related causes of oculocutaneous disease. J Am Acad Dermatol. 2014;70:821.e821-819.
- Choi JW, Choi JW, Kwon IH, et al. High-concentration (20 μg g-¹) tacalcitol ointment in the treatment of facial psoriasis: an 8-week open-label clinical trial. Br J Dermatol. 2010;162:1359-1364.
- Hashim PW, Chima M, Kim HJ, et al. Crisaborole 2% ointment for the treatment of intertriginous, anogenital, and facial psoriasis: a double-blind, randomized, vehicle-controlled trial. J Am Acad Dermatol. 2020;82:360-365.
- Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
- Iversen L, Jakobsen HB. Patient preferences for topical psoriasis treatments are diverse and difficult to predict. Dermatol Ther. 2016;6:273-285.
- Clobex Package insert. Galderma Laboratories, LP; 2012.
- Kenalog-10 Injection. Package insert. Bristol-Myers Squibb Company; 2018.
- Mason J, Mason AR, Cork MJ. Topical preparations for the treatment of psoriasis: a systematic review. Br J Dermatol. 2002;146:351-364.
- Koo J, Cuffie CA, Tanner DJ, et al. Mometasone furoate 0.1%-salicylic acid 5% ointment versus mometasone furoate 0.1% ointment in the treatment of moderate-to-severe psoriasis: a multicenter study. Clin Ther. 1998;20:283-291.
- Tiplica GS, Salavastru CM. Mometasone furoate 0.1% and salicylic acid 5% vs. mometasone furoate 0.1% as sequential local therapy in psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23:905-912.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Strober BE, Bissonnette R, Fiorentino D, et al. Comparative effectiveness of biologic agents for the treatment of psoriasis in a real-world setting: results from a large, prospective, observational study (Psoriasis Longitudinal Assessment and Registry [PSOLAR]). J Am Acad Dermatol. 2016;74:851-861.e854.
- Castela E, Archier E, Devaux S, et al. Topical corticosteroids in plaque psoriasis: a systematic review of risk of adrenal axis suppression and skin atrophy. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):47-51.
- Takahashi H, Tsuji H, Honma M, et al. Femoral head osteonecrosis after long-term topical corticosteroid treatment in a psoriasis patient. J Dermatol. 2012;39:887-888.
- el Maghraoui A, Tabache F, Bezza A, et al. Femoral head osteonecrosis after topical corticosteroid therapy. Clin Exp Rheumatol. 2001;19:233.
- Gribetz C, Ling M, Lebwohl M, et al. Pimecrolimus cream 1% in the treatment of intertriginous psoriasis: a double-blind, randomized study. J Am Acad Dermatol. 2004;51:731-738.
- Lebwohl M, Freeman AK, Chapman MS, et al. Tacrolimus ointment is effective for facial and intertriginous psoriasis. J Am Acad Dermatol. 2004;51:723-730.
- Paller AS, Fölster-Holst R, Chen SC, et al. No evidence of increased cancer incidence in children using topical tacrolimus for atopic dermatitis. J Am Acad Dermatol. 2020;83:375-381.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Lebwohl M, Ast E, Callen JP, et al. Once-daily tazarotene gel versus twice-daily fluocinonide cream in the treatment of plaque psoriasis. J Am Acad Dermatol. 1998;38:705-711.
- Weinstein GD, Koo JY, Krueger GG, et al. Tazarotene cream in the treatment of psoriasis: two multicenter, double-blind, randomized, vehicle-controlled studies of the safety and efficacy of tazarotene creams 0.05% and 0.1% applied once daily for 12 weeks. J Am Acad Dermatol. 2003;48:760-767.
- Lebwohl M, Lombardi K, Tan MH. Duration of improvement in psoriasis after treatment with tazarotene 0.1% gel plus clobetasol propionate 0.05% ointment: comparison of maintenance treatments. Int J Dermatol. 2001;40:64-66.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Koo JY, Lowe NJ, Lew-Kaya DA, et al. Tazarotene plus UVB phototherapy in the treatment of psoriasis. J Am Acad Dermatol. 2000;43:821-828.
- Cassano N, Mantegazza R, Battaglini S, et al. Adjuvant role of a new emollient cream in patients with palmar and/or plantar psoriasis: a pilot randomized open-label study. G Ital Dermatol Venereol. 2010;145:789-792.
- Kristensen B, Kristensen O. Topical salicylic acid interferes with UVB therapy for psoriasis. Acta Derm Venereol. 1991;71:37-40.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Rogalski C, Grunewald S, Schetschorke M, et al. Treatment of plaque-type psoriasis with the 308 nm excimer laser in combination with dithranol or calcipotriol. Int J Hyperthermia. 2012;28:184-190.
- Bagel J. LCD plus NB-UVB reduces time to improvement of psoriasis vs. NB-UVB alone. J Drugs Dermatol. 2009;8:351-357.
- Abdallah MA, El-Khateeb EA, Abdel-Rahman SH. The influence of psoriatic plaques pretreatment with crude coal tar vs. petrolatum on the efficacy of narrow-band ultraviolet B: a half-vs.-half intra-individual double-blinded comparative study. Photodermatol Photoimmunol Photomed. 2011;27:226-230.
Practice Points
- Topical medications collectively represent the most common form of psoriasis treatment. Depending on disease severity and distribution, topical agents can be used as monotherapy or adjunct therapy, offering the benefit of localized treatment without systemic side effects.
- Dermatologists should base the selection of an appropriate topical medication on factors including adverse effects, potency, vehicle, and anatomic localization of disease.
Translating the 2019 AAD-NPF Guidelines of Care for Psoriasis With Attention to Comorbidities
Psoriasis is a chronic and relapsing systemic inflammatory disease that predisposes patients to a host of other conditions. It is believed that these widespread effects are due to chronic inflammation and cytokine activation, which affect multiple body processes and lead to the development of various comorbidities that need to be proactively managed.
In April 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released recommendation guidelines for managing psoriasis in adults with an emphasis on common disease comorbidities, including psoriatic arthritis (PsA), cardiovascular disease (CVD), inflammatory bowel disease (IBD), metabolic syndrome, and mood disorders. Psychosocial wellness, mental health, and quality of life (QOL) measures in relation to psoriatic disease also were discussed.1
The AAD-NPF guidelines address current screening, monitoring, education, and treatment recommendations for the management of psoriatic comorbidities. The Table and eTable summarize the screening recommendations. These guidelines aim to assist dermatologists with comprehensive disease management by addressing potential extracutaneous manifestations of psoriasis in adults.

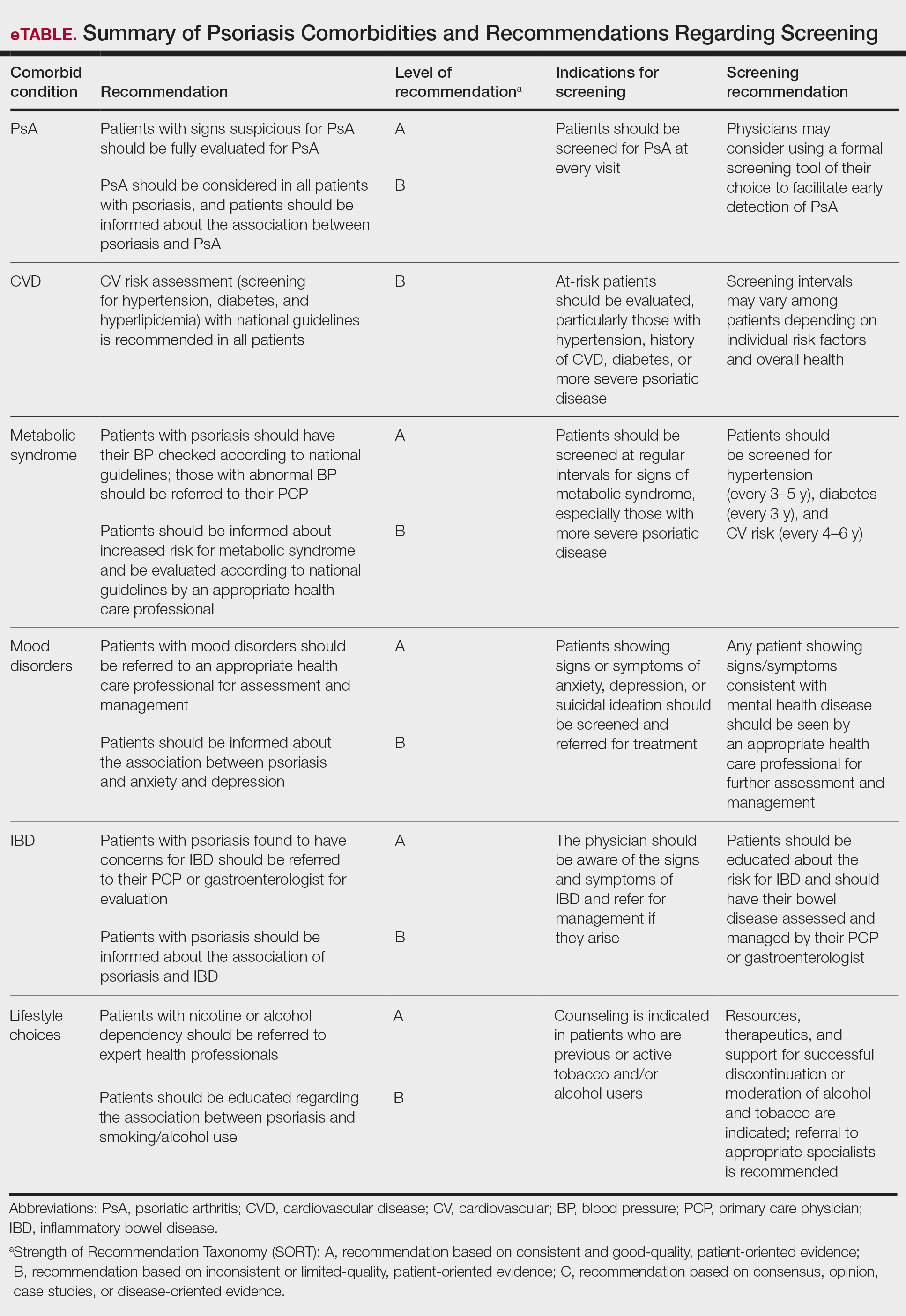
Screening and Risk Assessment
Patients with psoriasis should receive a thorough history and physical examination to assess disease severity and risk for potential comorbidities. Patients with greater disease severity—as measured by body surface area (BSA) involvement and type of therapy required—have a greater risk for other disease-related comorbidities, specifically metabolic syndrome, renal disease, chronic obstructive pulmonary disease (COPD), obstructive sleep apnea, uveitis, IBD, malignancy, and increased mortality.2 Because the likelihood of comorbidities is greatest with severe disease, more frequent monitoring is recommended for these patients.
Psoriatic Arthritis
Patients with psoriasis need to be evaluated for PsA at every visit. Patients presenting with signs and symptoms suspicious for PsA—joint swelling, peripheral joint involvement, and joint inflammation—warrant further evaluation and consultation. Early detection and treatment of PsA is essential for preventing unnecessary suffering and progressive joint destruction.3
There are several PsA screening questionnaires currently available, including the Psoriatic Arthritis Screening Evaluation, Psoriasis Epidemiology Screening Tool, and Toronto Psoriatic Arthritis Screen. No significant differences in sensitivity and specificity were found among these questionnaires when using the Classification Criteria for Psoriatic Arthritis as the gold standard. All 3 questionnaires—the Psoriatic Arthritis Screening Evaluation and the Psoriasis Epidemiology Screening Tool were developed for use in dermatology and rheumatology clinics, and the Toronto Psoriatic Arthritis Screen was developed for use in the primary care setting—were found to be effective in dermatology/rheumatology clinics and primary care clinics, respectively.3 False-positive results predominantly were seen in patients with degenerative joint disease or osteoarthritis. Dermatologists should conduct a thorough physical examination to distinguish PsA from degenerative joint disease. Imaging and laboratory tests to evaluate for signs of systemic inflammation (erythrocyte sedimentation rate, C-reactive protein) also can be helpful in distinguishing the 2 conditions; however, these metrics have not been shown to contribute to PsA diagnosis.1 Full rheumatologic consultation is warranted in challenging cases.
Cardiovascular Disease
Primary care physicians (PCPs) are recommended to screen patients for CVD risk factors using height, weight, blood pressure, blood glucose, hemoglobin A1C, lipid levels, abdominal circumference, and body mass index (BMI). Lifestyle modifications such as smoking cessation, exercise, and dietary changes are encouraged to achieve and maintain a normal BMI.
Dermatologists also need to give special consideration to comorbidities when selecting medications and/or therapies for disease management. Patients on TNF inhibitors have a lower risk for MI compared with patients using topical medications, phototherapy, and other oral agents.10 Additionally, patients on TNF inhibitors have a lower risk for occurrence of major adverse cardiovascular events compared with patients treated with methotrexate or phototherapy.11,12
Metabolic Syndrome
Numerous studies have demonstrated an association between psoriasis and metabolic syndrome. Patients with increased BSA involvement and
The association between psoriasis and weight loss has been analyzed in several studies. Weight loss, particularly in obese patients, has been shown to improve psoriasis severity, as measured by psoriasis area and severity index score and QOL measures.15 Another study found that gastric bypass was associated with a significant risk reduction in the development of psoriasis (P=.004) and the disease prognosis (P=.02 for severe psoriasis; P=.01 for PsA).16 Therefore, patients with moderate to severe psoriasis are recommended to have their obesity status determined according to national guidelines. For patients with a BMI above 40 kg/m2 and standard weight-loss measures fail, bariatric surgery is recommended. Additionally, the impact of psoriasis medications on weight has been studied. Apremilast has been associated with weight loss, whereas etanercept and infliximab have been linked to weight gain.17,18
An association between psoriasis and hypertension also has been demonstrated by studies, especially among patients with severe disease. Therefore, patients with moderate to severe psoriasis are recommended to have their blood pressure evaluated according to national guidelines, and those with a blood pressure of 140/90 mm Hg or higher should be referred to their PCP for assessment and treatment. Current evidence does not support restrictions on antihypertensive medications in patients with psoriasis. Physicians should be aware of the potential for cyclosporine to induce hypertension, which should be treated, specifically with amlodipine.19
Many studies have demonstrated an association between psoriasis and dyslipidemia, though the results are somewhat conflicting. In 2018, the American Heart Association and the American College of Cardiology deemed psoriasis as an atherosclerotic CVD risk-enhancing condition, favoring early initiation of statin therapy. Because dyslipidemia plays a prominent role in atherosclerosis and CVD, patients with moderate to severe psoriasis are recommended to undergo periodic screening with lipid tests (eg, fasting total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides).20 Patients with elevated fasting triglycerides or low-density lipoprotein cholesterol should be referred to their PCP for further management. Certain psoriasis medications also have been linked to dyslipidemia. Acitretin and cyclosporine are known to adversely affect lipid levels, so patients treated with either agent should undergo routine monitoring of serum lipid levels.
Psoriasis is strongly associated with diabetes mellitus. Because of the increased risk for diabetes in patients with severe disease, regular monitoring of fasting blood glucose and/or hemoglobin A1C levels in patients with moderate to severe psoriasis is recommended. Patients who meet criteria for prediabetes or diabetes should be referred to their PCP for further assessment and management.21,22
Mood Disorders
Psoriasis affects QOL and can have a major impact on patients’ interpersonal relationships. Studies have shown an association between psoriasis and mood disorders, specifically depression and anxiety. Unfortunately, patients with mood disorders are less likely to seek intervention for their skin disease, which poses a tremendous treatment barrier. Dermatologists should regularly monitor patients for psychiatric symptoms so that resources and treatments can be offered.
Certain psoriasis therapies have been shown to help alleviate associated depression and anxiety. Improvements in Beck Depression Inventory and Hamilton Depression Rating Scale scores were seen with etanercept.23 Adalimumab and ustekinumab showed improvement in Dermatology Life Quality Index compared with placebo.24,25 Patients receiving Goeckerman treatment also had improvement in anxiety and depression scores compared with conventional therapy.26 Biologic medications had the largest impact on improving depression symptoms compared with conventional systemic therapy and phototherapy.27 The recommendations support use of biologics and the Goeckerman regimen for the concomitant treatment of mood disorders and psoriasis.
Renal Disease
Studies have supported an association between psoriasis and chronic kidney disease (CKD), independent of risk factors including vascular disease, hypertension, and diabetes. The prevalence of moderate to advanced CKD also has been found to be directly related to increasing BSA affected by psoriasis.28 Patients should receive testing of blood urea nitrogen, creatinine, and urine microalbumin levels to assess for occult renal disease. In addition, physicians should be cautious when prescribing nephrotoxic drugs (nonsteroidal anti-inflammatory drugs and cyclosporine) and renally excreted agents (methotrexate and apremilast) because of the risk for underlying renal disease in patients with psoriasis. If newly acquired renal disease is suspected, physicians should withhold the offending agents. Patients with psoriasis with CKD are recommended to follow up with their PCP or nephrologist for evaluation and management.
Pulmonary Disease
Psoriasis also has an independent association with COPD. Patients with psoriasis have a higher likelihood of developing COPD (hazard ratio, 2.35; 95% CI, 1.42-3.89; P<.01) than controls.29 The prevalence of COPD also was found to correlate with psoriasis severity. Dermatologists should educate patients about the association between smoking and psoriasis as well as advise patients to discontinue smoking to reduce their risk for developing COPD and cancer.
Patients with psoriasis also are at an increased risk for obstructive sleep apnea. Obstructive sleep apnea should be considered in patients with risk factors including snoring, obesity, hypertension, or diabetes.
Inflammatory Bowel Disease
Patients with psoriasis have an increased risk for developing IBD. The prevalence ratios of both Crohn disease (2.49) and ulcerative colitis (1.64) are increased in patients with psoriasis relative to patients without psoriasis.30 Physicians need to be aware of the association between psoriasis and IBD and the effect that their coexistence may have on treatment choice for patients.
Adalimumab and infliximab are approved for the treatment of IBD, and certolizumab and ustekinumab are approved for Crohn disease. Use of TNF inhibitors in patients with IBD may cause psoriasiform lesions to develop.31 Nonetheless, treatment should be individualized and psoriasiform lesions treated with standard psoriasis measures. Psoriasis patients with IBD are recommended to avoid IL-17–inhibitor therapy, given its potential to worsen IBD flares.
Malignancy
Psoriasis patients aged 0 to 79 years have a greater overall risk for malignancy compared with patients without psoriasis.32 Patients with psoriasis have an increased risk for respiratory tract cancer, upper aerodigestive tract cancer, urinary tract cancer, and non-Hodgkin lymphoma.33 A mild association exists between PsA and lymphoma, nonmelanoma skin cancer (NMSC), and lung cancer.34 More severe psoriasis is associated with greater risk for lymphoma and NMSC. Dermatologists are recommended to educate patients on their risk for certain malignancies and to refer patients to specialists upon suspicion of malignancy.
Risk for malignancy has been shown to be affected by psoriasis treatments. Patients treated with UVB have reduced overall cancer rates for all age groups (hazard ratio, 0.52; P=.3), while those treated with psoralen plus UVA have an increased incidence of
Lifestyle Choices and QOL
A crucial aspect of successful psoriasis management is patient education. The strongest recommendations support lifestyle changes, such as smoking cessation and limitation of alcohol use. A tactful discussion regarding substance use, work productivity, interpersonal relationships, and sexual function can address substantial effects of psoriasis on QOL so that support and resources can be provided.
Final Thoughts
Management of psoriasis is multifaceted and involves screening, education, monitoring, and collaboration with PCPs and specialists. Regular follow-up with a dermatologist and PCP is strongly recommended for patients with psoriasis given the systemic nature of the disease. The 2019 AAD-NPF recommendations provide important information for dermatologists to coordinate care for complicated psoriasis cases, but clinical judgment is paramount when making medical decisions. The consideration of comorbidities is critical for developing a comprehensive treatment approach, and this approach will lead to better health outcomes and improved QOL for patients with psoriasis.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143:1493-1499.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209.
- Lerman JB, Joshi AA, Chaturvedi A, et al. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. 2017;136:263-276.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Dunlay SM, Weston SA, Redfield MM, et al. Tumor necrosis factor-alpha and mortality in heart failure: a community study. Circulation. 2008;118:625-631.
- Russell SD, Saval MA, Robbins JL, et al. New York Heart Association functional class predicts exercise parameters in the current era. Am Heart J. 2009;158(4 suppl):S24-S30.
- Wu JJ, Poon K-YT, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403-414.
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132:556-562.
- Jensen P, Zachariae C, Christensen R, et al. Effect of weight loss on the severity of psoriasis: a randomized clinical study. JAMA Dermatol. 2013;149:795-801.
- Egeberg A, Sørensen JA, Gislason GH, et al. Incidence and prognosis of psoriasis and psoriatic arthritis in patients undergoing bariatric surgery. JAMA Surg. 2017;152:344-349.
- Crowley J, Thaçi D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for ≥156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.e1. doi:10.1016/j.jaad.2017.01.052
- Gisondi P, Del Giglio M, Di Francesco V, et al. Weight loss improves the response of obese patients with moderate-to-severe chronic plaque psoriasis to low-dose cyclosporine therapy: a randomized, controlled, investigator-blinded clinical trial. Am J Clin Nutr. 2008;88:1242-1247.
- Leenen FHH, Coletta E, Davies RA. Prevention of renal dysfunction and hypertension by amlodipine after heart transplant. Am J Cardiol. 2007;100:531-535.
- Goff DC Jr, Lloyd-Jones DM, Bennet G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation. 2014;129(suppl 2):S49-S73.
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14-S80.
- Ratner RE, Diabetes Prevention Program Research Group. An update on the diabetes prevention program. Endocr Pract. 2006;12(suppl 1):20-24.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
- Kimball AB, Edson-Heredia E, Zhu B, et al. Understanding the relationship between pruritus severity and work productivity in patients with moderate-to-severe psoriasis: sleep problems are a mediating factor. J Drugs Dermatol. 2016;15:183-188.
- Langley RG, Tsai T-F, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Chern E, Yau D, Ho J-C, et al. Positive effect of modified Goeckerman regimen on quality of life and psychosocial distress in moderate and severe psoriasis. Acta Derm Venereol. 2011;91:447-451.
- Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
- Wan J, Wang S, Haynes K, et al. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ. 2013;347:f5961. doi:10.1136/bmj.f5961
- Chiang Y-Y, Lin H-W. Association between psoriasis and chronic obstructive pulmonary disease: a population-based study in Taiwan. J Eur Acad Dermatol Venereol. 2012;26:59-65.
- Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn’s disease. J Eur Acad Dermatol Venereol. 2009;23:561-565.
- Denadai R, Teixeira FV, Saad-Hossne R. The onset of psoriasis during the treatment of inflammatory bowel diseases with infliximab: should biological therapy be suspended? Arq Gastroenterol. 2012;49:172-176.
- Chen Y-J, Wu C-Y, Chen T-J, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in Taiwan. J Am Acad Dermatol. 2011;65:84-91.
- Pouplard C, Brenaut E, Horreau C, et al. Risk of cancer in psoriasis: a systematic review and meta-analysis of epidemiological studies. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):36-46.
- Chiesa Fuxench ZC, Shin DB, Ogdie Beatty A, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in the health improvement network. JAMA Dermatol. 2016;152:282-290.
- Burmester GR, Panaccione R, Gordon KB, et al. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis. 2013;72:517-524.
- Dommasch ED, Abuabara K, Shin DB, et al. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2011;64:1035-1050.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
Psoriasis is a chronic and relapsing systemic inflammatory disease that predisposes patients to a host of other conditions. It is believed that these widespread effects are due to chronic inflammation and cytokine activation, which affect multiple body processes and lead to the development of various comorbidities that need to be proactively managed.
In April 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released recommendation guidelines for managing psoriasis in adults with an emphasis on common disease comorbidities, including psoriatic arthritis (PsA), cardiovascular disease (CVD), inflammatory bowel disease (IBD), metabolic syndrome, and mood disorders. Psychosocial wellness, mental health, and quality of life (QOL) measures in relation to psoriatic disease also were discussed.1
The AAD-NPF guidelines address current screening, monitoring, education, and treatment recommendations for the management of psoriatic comorbidities. The Table and eTable summarize the screening recommendations. These guidelines aim to assist dermatologists with comprehensive disease management by addressing potential extracutaneous manifestations of psoriasis in adults.


Screening and Risk Assessment
Patients with psoriasis should receive a thorough history and physical examination to assess disease severity and risk for potential comorbidities. Patients with greater disease severity—as measured by body surface area (BSA) involvement and type of therapy required—have a greater risk for other disease-related comorbidities, specifically metabolic syndrome, renal disease, chronic obstructive pulmonary disease (COPD), obstructive sleep apnea, uveitis, IBD, malignancy, and increased mortality.2 Because the likelihood of comorbidities is greatest with severe disease, more frequent monitoring is recommended for these patients.
Psoriatic Arthritis
Patients with psoriasis need to be evaluated for PsA at every visit. Patients presenting with signs and symptoms suspicious for PsA—joint swelling, peripheral joint involvement, and joint inflammation—warrant further evaluation and consultation. Early detection and treatment of PsA is essential for preventing unnecessary suffering and progressive joint destruction.3
There are several PsA screening questionnaires currently available, including the Psoriatic Arthritis Screening Evaluation, Psoriasis Epidemiology Screening Tool, and Toronto Psoriatic Arthritis Screen. No significant differences in sensitivity and specificity were found among these questionnaires when using the Classification Criteria for Psoriatic Arthritis as the gold standard. All 3 questionnaires—the Psoriatic Arthritis Screening Evaluation and the Psoriasis Epidemiology Screening Tool were developed for use in dermatology and rheumatology clinics, and the Toronto Psoriatic Arthritis Screen was developed for use in the primary care setting—were found to be effective in dermatology/rheumatology clinics and primary care clinics, respectively.3 False-positive results predominantly were seen in patients with degenerative joint disease or osteoarthritis. Dermatologists should conduct a thorough physical examination to distinguish PsA from degenerative joint disease. Imaging and laboratory tests to evaluate for signs of systemic inflammation (erythrocyte sedimentation rate, C-reactive protein) also can be helpful in distinguishing the 2 conditions; however, these metrics have not been shown to contribute to PsA diagnosis.1 Full rheumatologic consultation is warranted in challenging cases.
Cardiovascular Disease
Primary care physicians (PCPs) are recommended to screen patients for CVD risk factors using height, weight, blood pressure, blood glucose, hemoglobin A1C, lipid levels, abdominal circumference, and body mass index (BMI). Lifestyle modifications such as smoking cessation, exercise, and dietary changes are encouraged to achieve and maintain a normal BMI.
Dermatologists also need to give special consideration to comorbidities when selecting medications and/or therapies for disease management. Patients on TNF inhibitors have a lower risk for MI compared with patients using topical medications, phototherapy, and other oral agents.10 Additionally, patients on TNF inhibitors have a lower risk for occurrence of major adverse cardiovascular events compared with patients treated with methotrexate or phototherapy.11,12
Metabolic Syndrome
Numerous studies have demonstrated an association between psoriasis and metabolic syndrome. Patients with increased BSA involvement and
The association between psoriasis and weight loss has been analyzed in several studies. Weight loss, particularly in obese patients, has been shown to improve psoriasis severity, as measured by psoriasis area and severity index score and QOL measures.15 Another study found that gastric bypass was associated with a significant risk reduction in the development of psoriasis (P=.004) and the disease prognosis (P=.02 for severe psoriasis; P=.01 for PsA).16 Therefore, patients with moderate to severe psoriasis are recommended to have their obesity status determined according to national guidelines. For patients with a BMI above 40 kg/m2 and standard weight-loss measures fail, bariatric surgery is recommended. Additionally, the impact of psoriasis medications on weight has been studied. Apremilast has been associated with weight loss, whereas etanercept and infliximab have been linked to weight gain.17,18
An association between psoriasis and hypertension also has been demonstrated by studies, especially among patients with severe disease. Therefore, patients with moderate to severe psoriasis are recommended to have their blood pressure evaluated according to national guidelines, and those with a blood pressure of 140/90 mm Hg or higher should be referred to their PCP for assessment and treatment. Current evidence does not support restrictions on antihypertensive medications in patients with psoriasis. Physicians should be aware of the potential for cyclosporine to induce hypertension, which should be treated, specifically with amlodipine.19
Many studies have demonstrated an association between psoriasis and dyslipidemia, though the results are somewhat conflicting. In 2018, the American Heart Association and the American College of Cardiology deemed psoriasis as an atherosclerotic CVD risk-enhancing condition, favoring early initiation of statin therapy. Because dyslipidemia plays a prominent role in atherosclerosis and CVD, patients with moderate to severe psoriasis are recommended to undergo periodic screening with lipid tests (eg, fasting total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides).20 Patients with elevated fasting triglycerides or low-density lipoprotein cholesterol should be referred to their PCP for further management. Certain psoriasis medications also have been linked to dyslipidemia. Acitretin and cyclosporine are known to adversely affect lipid levels, so patients treated with either agent should undergo routine monitoring of serum lipid levels.
Psoriasis is strongly associated with diabetes mellitus. Because of the increased risk for diabetes in patients with severe disease, regular monitoring of fasting blood glucose and/or hemoglobin A1C levels in patients with moderate to severe psoriasis is recommended. Patients who meet criteria for prediabetes or diabetes should be referred to their PCP for further assessment and management.21,22
Mood Disorders
Psoriasis affects QOL and can have a major impact on patients’ interpersonal relationships. Studies have shown an association between psoriasis and mood disorders, specifically depression and anxiety. Unfortunately, patients with mood disorders are less likely to seek intervention for their skin disease, which poses a tremendous treatment barrier. Dermatologists should regularly monitor patients for psychiatric symptoms so that resources and treatments can be offered.
Certain psoriasis therapies have been shown to help alleviate associated depression and anxiety. Improvements in Beck Depression Inventory and Hamilton Depression Rating Scale scores were seen with etanercept.23 Adalimumab and ustekinumab showed improvement in Dermatology Life Quality Index compared with placebo.24,25 Patients receiving Goeckerman treatment also had improvement in anxiety and depression scores compared with conventional therapy.26 Biologic medications had the largest impact on improving depression symptoms compared with conventional systemic therapy and phototherapy.27 The recommendations support use of biologics and the Goeckerman regimen for the concomitant treatment of mood disorders and psoriasis.
Renal Disease
Studies have supported an association between psoriasis and chronic kidney disease (CKD), independent of risk factors including vascular disease, hypertension, and diabetes. The prevalence of moderate to advanced CKD also has been found to be directly related to increasing BSA affected by psoriasis.28 Patients should receive testing of blood urea nitrogen, creatinine, and urine microalbumin levels to assess for occult renal disease. In addition, physicians should be cautious when prescribing nephrotoxic drugs (nonsteroidal anti-inflammatory drugs and cyclosporine) and renally excreted agents (methotrexate and apremilast) because of the risk for underlying renal disease in patients with psoriasis. If newly acquired renal disease is suspected, physicians should withhold the offending agents. Patients with psoriasis with CKD are recommended to follow up with their PCP or nephrologist for evaluation and management.
Pulmonary Disease
Psoriasis also has an independent association with COPD. Patients with psoriasis have a higher likelihood of developing COPD (hazard ratio, 2.35; 95% CI, 1.42-3.89; P<.01) than controls.29 The prevalence of COPD also was found to correlate with psoriasis severity. Dermatologists should educate patients about the association between smoking and psoriasis as well as advise patients to discontinue smoking to reduce their risk for developing COPD and cancer.
Patients with psoriasis also are at an increased risk for obstructive sleep apnea. Obstructive sleep apnea should be considered in patients with risk factors including snoring, obesity, hypertension, or diabetes.
Inflammatory Bowel Disease
Patients with psoriasis have an increased risk for developing IBD. The prevalence ratios of both Crohn disease (2.49) and ulcerative colitis (1.64) are increased in patients with psoriasis relative to patients without psoriasis.30 Physicians need to be aware of the association between psoriasis and IBD and the effect that their coexistence may have on treatment choice for patients.
Adalimumab and infliximab are approved for the treatment of IBD, and certolizumab and ustekinumab are approved for Crohn disease. Use of TNF inhibitors in patients with IBD may cause psoriasiform lesions to develop.31 Nonetheless, treatment should be individualized and psoriasiform lesions treated with standard psoriasis measures. Psoriasis patients with IBD are recommended to avoid IL-17–inhibitor therapy, given its potential to worsen IBD flares.
Malignancy
Psoriasis patients aged 0 to 79 years have a greater overall risk for malignancy compared with patients without psoriasis.32 Patients with psoriasis have an increased risk for respiratory tract cancer, upper aerodigestive tract cancer, urinary tract cancer, and non-Hodgkin lymphoma.33 A mild association exists between PsA and lymphoma, nonmelanoma skin cancer (NMSC), and lung cancer.34 More severe psoriasis is associated with greater risk for lymphoma and NMSC. Dermatologists are recommended to educate patients on their risk for certain malignancies and to refer patients to specialists upon suspicion of malignancy.
Risk for malignancy has been shown to be affected by psoriasis treatments. Patients treated with UVB have reduced overall cancer rates for all age groups (hazard ratio, 0.52; P=.3), while those treated with psoralen plus UVA have an increased incidence of
Lifestyle Choices and QOL
A crucial aspect of successful psoriasis management is patient education. The strongest recommendations support lifestyle changes, such as smoking cessation and limitation of alcohol use. A tactful discussion regarding substance use, work productivity, interpersonal relationships, and sexual function can address substantial effects of psoriasis on QOL so that support and resources can be provided.
Final Thoughts
Management of psoriasis is multifaceted and involves screening, education, monitoring, and collaboration with PCPs and specialists. Regular follow-up with a dermatologist and PCP is strongly recommended for patients with psoriasis given the systemic nature of the disease. The 2019 AAD-NPF recommendations provide important information for dermatologists to coordinate care for complicated psoriasis cases, but clinical judgment is paramount when making medical decisions. The consideration of comorbidities is critical for developing a comprehensive treatment approach, and this approach will lead to better health outcomes and improved QOL for patients with psoriasis.
Psoriasis is a chronic and relapsing systemic inflammatory disease that predisposes patients to a host of other conditions. It is believed that these widespread effects are due to chronic inflammation and cytokine activation, which affect multiple body processes and lead to the development of various comorbidities that need to be proactively managed.
In April 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released recommendation guidelines for managing psoriasis in adults with an emphasis on common disease comorbidities, including psoriatic arthritis (PsA), cardiovascular disease (CVD), inflammatory bowel disease (IBD), metabolic syndrome, and mood disorders. Psychosocial wellness, mental health, and quality of life (QOL) measures in relation to psoriatic disease also were discussed.1
The AAD-NPF guidelines address current screening, monitoring, education, and treatment recommendations for the management of psoriatic comorbidities. The Table and eTable summarize the screening recommendations. These guidelines aim to assist dermatologists with comprehensive disease management by addressing potential extracutaneous manifestations of psoriasis in adults.


Screening and Risk Assessment
Patients with psoriasis should receive a thorough history and physical examination to assess disease severity and risk for potential comorbidities. Patients with greater disease severity—as measured by body surface area (BSA) involvement and type of therapy required—have a greater risk for other disease-related comorbidities, specifically metabolic syndrome, renal disease, chronic obstructive pulmonary disease (COPD), obstructive sleep apnea, uveitis, IBD, malignancy, and increased mortality.2 Because the likelihood of comorbidities is greatest with severe disease, more frequent monitoring is recommended for these patients.
Psoriatic Arthritis
Patients with psoriasis need to be evaluated for PsA at every visit. Patients presenting with signs and symptoms suspicious for PsA—joint swelling, peripheral joint involvement, and joint inflammation—warrant further evaluation and consultation. Early detection and treatment of PsA is essential for preventing unnecessary suffering and progressive joint destruction.3
There are several PsA screening questionnaires currently available, including the Psoriatic Arthritis Screening Evaluation, Psoriasis Epidemiology Screening Tool, and Toronto Psoriatic Arthritis Screen. No significant differences in sensitivity and specificity were found among these questionnaires when using the Classification Criteria for Psoriatic Arthritis as the gold standard. All 3 questionnaires—the Psoriatic Arthritis Screening Evaluation and the Psoriasis Epidemiology Screening Tool were developed for use in dermatology and rheumatology clinics, and the Toronto Psoriatic Arthritis Screen was developed for use in the primary care setting—were found to be effective in dermatology/rheumatology clinics and primary care clinics, respectively.3 False-positive results predominantly were seen in patients with degenerative joint disease or osteoarthritis. Dermatologists should conduct a thorough physical examination to distinguish PsA from degenerative joint disease. Imaging and laboratory tests to evaluate for signs of systemic inflammation (erythrocyte sedimentation rate, C-reactive protein) also can be helpful in distinguishing the 2 conditions; however, these metrics have not been shown to contribute to PsA diagnosis.1 Full rheumatologic consultation is warranted in challenging cases.
Cardiovascular Disease
Primary care physicians (PCPs) are recommended to screen patients for CVD risk factors using height, weight, blood pressure, blood glucose, hemoglobin A1C, lipid levels, abdominal circumference, and body mass index (BMI). Lifestyle modifications such as smoking cessation, exercise, and dietary changes are encouraged to achieve and maintain a normal BMI.
Dermatologists also need to give special consideration to comorbidities when selecting medications and/or therapies for disease management. Patients on TNF inhibitors have a lower risk for MI compared with patients using topical medications, phototherapy, and other oral agents.10 Additionally, patients on TNF inhibitors have a lower risk for occurrence of major adverse cardiovascular events compared with patients treated with methotrexate or phototherapy.11,12
Metabolic Syndrome
Numerous studies have demonstrated an association between psoriasis and metabolic syndrome. Patients with increased BSA involvement and
The association between psoriasis and weight loss has been analyzed in several studies. Weight loss, particularly in obese patients, has been shown to improve psoriasis severity, as measured by psoriasis area and severity index score and QOL measures.15 Another study found that gastric bypass was associated with a significant risk reduction in the development of psoriasis (P=.004) and the disease prognosis (P=.02 for severe psoriasis; P=.01 for PsA).16 Therefore, patients with moderate to severe psoriasis are recommended to have their obesity status determined according to national guidelines. For patients with a BMI above 40 kg/m2 and standard weight-loss measures fail, bariatric surgery is recommended. Additionally, the impact of psoriasis medications on weight has been studied. Apremilast has been associated with weight loss, whereas etanercept and infliximab have been linked to weight gain.17,18
An association between psoriasis and hypertension also has been demonstrated by studies, especially among patients with severe disease. Therefore, patients with moderate to severe psoriasis are recommended to have their blood pressure evaluated according to national guidelines, and those with a blood pressure of 140/90 mm Hg or higher should be referred to their PCP for assessment and treatment. Current evidence does not support restrictions on antihypertensive medications in patients with psoriasis. Physicians should be aware of the potential for cyclosporine to induce hypertension, which should be treated, specifically with amlodipine.19
Many studies have demonstrated an association between psoriasis and dyslipidemia, though the results are somewhat conflicting. In 2018, the American Heart Association and the American College of Cardiology deemed psoriasis as an atherosclerotic CVD risk-enhancing condition, favoring early initiation of statin therapy. Because dyslipidemia plays a prominent role in atherosclerosis and CVD, patients with moderate to severe psoriasis are recommended to undergo periodic screening with lipid tests (eg, fasting total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides).20 Patients with elevated fasting triglycerides or low-density lipoprotein cholesterol should be referred to their PCP for further management. Certain psoriasis medications also have been linked to dyslipidemia. Acitretin and cyclosporine are known to adversely affect lipid levels, so patients treated with either agent should undergo routine monitoring of serum lipid levels.
Psoriasis is strongly associated with diabetes mellitus. Because of the increased risk for diabetes in patients with severe disease, regular monitoring of fasting blood glucose and/or hemoglobin A1C levels in patients with moderate to severe psoriasis is recommended. Patients who meet criteria for prediabetes or diabetes should be referred to their PCP for further assessment and management.21,22
Mood Disorders
Psoriasis affects QOL and can have a major impact on patients’ interpersonal relationships. Studies have shown an association between psoriasis and mood disorders, specifically depression and anxiety. Unfortunately, patients with mood disorders are less likely to seek intervention for their skin disease, which poses a tremendous treatment barrier. Dermatologists should regularly monitor patients for psychiatric symptoms so that resources and treatments can be offered.
Certain psoriasis therapies have been shown to help alleviate associated depression and anxiety. Improvements in Beck Depression Inventory and Hamilton Depression Rating Scale scores were seen with etanercept.23 Adalimumab and ustekinumab showed improvement in Dermatology Life Quality Index compared with placebo.24,25 Patients receiving Goeckerman treatment also had improvement in anxiety and depression scores compared with conventional therapy.26 Biologic medications had the largest impact on improving depression symptoms compared with conventional systemic therapy and phototherapy.27 The recommendations support use of biologics and the Goeckerman regimen for the concomitant treatment of mood disorders and psoriasis.
Renal Disease
Studies have supported an association between psoriasis and chronic kidney disease (CKD), independent of risk factors including vascular disease, hypertension, and diabetes. The prevalence of moderate to advanced CKD also has been found to be directly related to increasing BSA affected by psoriasis.28 Patients should receive testing of blood urea nitrogen, creatinine, and urine microalbumin levels to assess for occult renal disease. In addition, physicians should be cautious when prescribing nephrotoxic drugs (nonsteroidal anti-inflammatory drugs and cyclosporine) and renally excreted agents (methotrexate and apremilast) because of the risk for underlying renal disease in patients with psoriasis. If newly acquired renal disease is suspected, physicians should withhold the offending agents. Patients with psoriasis with CKD are recommended to follow up with their PCP or nephrologist for evaluation and management.
Pulmonary Disease
Psoriasis also has an independent association with COPD. Patients with psoriasis have a higher likelihood of developing COPD (hazard ratio, 2.35; 95% CI, 1.42-3.89; P<.01) than controls.29 The prevalence of COPD also was found to correlate with psoriasis severity. Dermatologists should educate patients about the association between smoking and psoriasis as well as advise patients to discontinue smoking to reduce their risk for developing COPD and cancer.
Patients with psoriasis also are at an increased risk for obstructive sleep apnea. Obstructive sleep apnea should be considered in patients with risk factors including snoring, obesity, hypertension, or diabetes.
Inflammatory Bowel Disease
Patients with psoriasis have an increased risk for developing IBD. The prevalence ratios of both Crohn disease (2.49) and ulcerative colitis (1.64) are increased in patients with psoriasis relative to patients without psoriasis.30 Physicians need to be aware of the association between psoriasis and IBD and the effect that their coexistence may have on treatment choice for patients.
Adalimumab and infliximab are approved for the treatment of IBD, and certolizumab and ustekinumab are approved for Crohn disease. Use of TNF inhibitors in patients with IBD may cause psoriasiform lesions to develop.31 Nonetheless, treatment should be individualized and psoriasiform lesions treated with standard psoriasis measures. Psoriasis patients with IBD are recommended to avoid IL-17–inhibitor therapy, given its potential to worsen IBD flares.
Malignancy
Psoriasis patients aged 0 to 79 years have a greater overall risk for malignancy compared with patients without psoriasis.32 Patients with psoriasis have an increased risk for respiratory tract cancer, upper aerodigestive tract cancer, urinary tract cancer, and non-Hodgkin lymphoma.33 A mild association exists between PsA and lymphoma, nonmelanoma skin cancer (NMSC), and lung cancer.34 More severe psoriasis is associated with greater risk for lymphoma and NMSC. Dermatologists are recommended to educate patients on their risk for certain malignancies and to refer patients to specialists upon suspicion of malignancy.
Risk for malignancy has been shown to be affected by psoriasis treatments. Patients treated with UVB have reduced overall cancer rates for all age groups (hazard ratio, 0.52; P=.3), while those treated with psoralen plus UVA have an increased incidence of
Lifestyle Choices and QOL
A crucial aspect of successful psoriasis management is patient education. The strongest recommendations support lifestyle changes, such as smoking cessation and limitation of alcohol use. A tactful discussion regarding substance use, work productivity, interpersonal relationships, and sexual function can address substantial effects of psoriasis on QOL so that support and resources can be provided.
Final Thoughts
Management of psoriasis is multifaceted and involves screening, education, monitoring, and collaboration with PCPs and specialists. Regular follow-up with a dermatologist and PCP is strongly recommended for patients with psoriasis given the systemic nature of the disease. The 2019 AAD-NPF recommendations provide important information for dermatologists to coordinate care for complicated psoriasis cases, but clinical judgment is paramount when making medical decisions. The consideration of comorbidities is critical for developing a comprehensive treatment approach, and this approach will lead to better health outcomes and improved QOL for patients with psoriasis.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143:1493-1499.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209.
- Lerman JB, Joshi AA, Chaturvedi A, et al. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. 2017;136:263-276.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Dunlay SM, Weston SA, Redfield MM, et al. Tumor necrosis factor-alpha and mortality in heart failure: a community study. Circulation. 2008;118:625-631.
- Russell SD, Saval MA, Robbins JL, et al. New York Heart Association functional class predicts exercise parameters in the current era. Am Heart J. 2009;158(4 suppl):S24-S30.
- Wu JJ, Poon K-YT, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403-414.
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132:556-562.
- Jensen P, Zachariae C, Christensen R, et al. Effect of weight loss on the severity of psoriasis: a randomized clinical study. JAMA Dermatol. 2013;149:795-801.
- Egeberg A, Sørensen JA, Gislason GH, et al. Incidence and prognosis of psoriasis and psoriatic arthritis in patients undergoing bariatric surgery. JAMA Surg. 2017;152:344-349.
- Crowley J, Thaçi D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for ≥156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.e1. doi:10.1016/j.jaad.2017.01.052
- Gisondi P, Del Giglio M, Di Francesco V, et al. Weight loss improves the response of obese patients with moderate-to-severe chronic plaque psoriasis to low-dose cyclosporine therapy: a randomized, controlled, investigator-blinded clinical trial. Am J Clin Nutr. 2008;88:1242-1247.
- Leenen FHH, Coletta E, Davies RA. Prevention of renal dysfunction and hypertension by amlodipine after heart transplant. Am J Cardiol. 2007;100:531-535.
- Goff DC Jr, Lloyd-Jones DM, Bennet G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation. 2014;129(suppl 2):S49-S73.
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14-S80.
- Ratner RE, Diabetes Prevention Program Research Group. An update on the diabetes prevention program. Endocr Pract. 2006;12(suppl 1):20-24.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
- Kimball AB, Edson-Heredia E, Zhu B, et al. Understanding the relationship between pruritus severity and work productivity in patients with moderate-to-severe psoriasis: sleep problems are a mediating factor. J Drugs Dermatol. 2016;15:183-188.
- Langley RG, Tsai T-F, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Chern E, Yau D, Ho J-C, et al. Positive effect of modified Goeckerman regimen on quality of life and psychosocial distress in moderate and severe psoriasis. Acta Derm Venereol. 2011;91:447-451.
- Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
- Wan J, Wang S, Haynes K, et al. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ. 2013;347:f5961. doi:10.1136/bmj.f5961
- Chiang Y-Y, Lin H-W. Association between psoriasis and chronic obstructive pulmonary disease: a population-based study in Taiwan. J Eur Acad Dermatol Venereol. 2012;26:59-65.
- Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn’s disease. J Eur Acad Dermatol Venereol. 2009;23:561-565.
- Denadai R, Teixeira FV, Saad-Hossne R. The onset of psoriasis during the treatment of inflammatory bowel diseases with infliximab: should biological therapy be suspended? Arq Gastroenterol. 2012;49:172-176.
- Chen Y-J, Wu C-Y, Chen T-J, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in Taiwan. J Am Acad Dermatol. 2011;65:84-91.
- Pouplard C, Brenaut E, Horreau C, et al. Risk of cancer in psoriasis: a systematic review and meta-analysis of epidemiological studies. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):36-46.
- Chiesa Fuxench ZC, Shin DB, Ogdie Beatty A, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in the health improvement network. JAMA Dermatol. 2016;152:282-290.
- Burmester GR, Panaccione R, Gordon KB, et al. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis. 2013;72:517-524.
- Dommasch ED, Abuabara K, Shin DB, et al. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2011;64:1035-1050.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143:1493-1499.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209.
- Lerman JB, Joshi AA, Chaturvedi A, et al. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. 2017;136:263-276.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Dunlay SM, Weston SA, Redfield MM, et al. Tumor necrosis factor-alpha and mortality in heart failure: a community study. Circulation. 2008;118:625-631.
- Russell SD, Saval MA, Robbins JL, et al. New York Heart Association functional class predicts exercise parameters in the current era. Am Heart J. 2009;158(4 suppl):S24-S30.
- Wu JJ, Poon K-YT, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403-414.
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132:556-562.
- Jensen P, Zachariae C, Christensen R, et al. Effect of weight loss on the severity of psoriasis: a randomized clinical study. JAMA Dermatol. 2013;149:795-801.
- Egeberg A, Sørensen JA, Gislason GH, et al. Incidence and prognosis of psoriasis and psoriatic arthritis in patients undergoing bariatric surgery. JAMA Surg. 2017;152:344-349.
- Crowley J, Thaçi D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for ≥156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.e1. doi:10.1016/j.jaad.2017.01.052
- Gisondi P, Del Giglio M, Di Francesco V, et al. Weight loss improves the response of obese patients with moderate-to-severe chronic plaque psoriasis to low-dose cyclosporine therapy: a randomized, controlled, investigator-blinded clinical trial. Am J Clin Nutr. 2008;88:1242-1247.
- Leenen FHH, Coletta E, Davies RA. Prevention of renal dysfunction and hypertension by amlodipine after heart transplant. Am J Cardiol. 2007;100:531-535.
- Goff DC Jr, Lloyd-Jones DM, Bennet G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation. 2014;129(suppl 2):S49-S73.
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14-S80.
- Ratner RE, Diabetes Prevention Program Research Group. An update on the diabetes prevention program. Endocr Pract. 2006;12(suppl 1):20-24.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
- Kimball AB, Edson-Heredia E, Zhu B, et al. Understanding the relationship between pruritus severity and work productivity in patients with moderate-to-severe psoriasis: sleep problems are a mediating factor. J Drugs Dermatol. 2016;15:183-188.
- Langley RG, Tsai T-F, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Chern E, Yau D, Ho J-C, et al. Positive effect of modified Goeckerman regimen on quality of life and psychosocial distress in moderate and severe psoriasis. Acta Derm Venereol. 2011;91:447-451.
- Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
- Wan J, Wang S, Haynes K, et al. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ. 2013;347:f5961. doi:10.1136/bmj.f5961
- Chiang Y-Y, Lin H-W. Association between psoriasis and chronic obstructive pulmonary disease: a population-based study in Taiwan. J Eur Acad Dermatol Venereol. 2012;26:59-65.
- Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn’s disease. J Eur Acad Dermatol Venereol. 2009;23:561-565.
- Denadai R, Teixeira FV, Saad-Hossne R. The onset of psoriasis during the treatment of inflammatory bowel diseases with infliximab: should biological therapy be suspended? Arq Gastroenterol. 2012;49:172-176.
- Chen Y-J, Wu C-Y, Chen T-J, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in Taiwan. J Am Acad Dermatol. 2011;65:84-91.
- Pouplard C, Brenaut E, Horreau C, et al. Risk of cancer in psoriasis: a systematic review and meta-analysis of epidemiological studies. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):36-46.
- Chiesa Fuxench ZC, Shin DB, Ogdie Beatty A, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in the health improvement network. JAMA Dermatol. 2016;152:282-290.
- Burmester GR, Panaccione R, Gordon KB, et al. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis. 2013;72:517-524.
- Dommasch ED, Abuabara K, Shin DB, et al. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2011;64:1035-1050.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
Practice Points
- Educating patients about psoriasis and its extracutaneous manifestations, available treatment options, and the impact of lifestyle choices is advised to maximize their patient’s disease awareness and to promote a collaborative physician-patient partnership.
- Physicians are strongly recommended to screen patients with psoriasis for the presence of disease comorbidities to ensure comprehensive management of their disease.
- Managing psoriasis as a multisystem inflammatory disorder requires the combined effort of dermatologists and other specialists to prevent and treat disease comorbidities and enhance patients’ quality of life.
Translating the 2020 AAD-NPF Guidelines of Care for the Management of Psoriasis With Systemic Nonbiologics to Clinical Practice
Psoriasis is a chronic relapsing skin condition characterized by keratinocyte hyperproliferation and a chronic inflammatory cascade. Therefore, controlling inflammatory responses with systemic medications is beneficial in managing psoriatic lesions and their accompanying symptoms, especially in disease inadequately controlled by topicals. Ease of drug administration and treatment availability are benefits that systemic nonbiologic therapies may have over biologic therapies.
In 2020, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) published guidelines for managing psoriasis in adults with systemic nonbiologic therapies.1 Dosing, efficacy, toxicity, drug-related interactions, and contraindications are addressed alongside evidence-based treatment recommendations. This review addresses current recommendations for systemic nonbiologics in psoriasis with a focus on the treatments approved by the US Food and Drug Administration (FDA): acitretin, apremilast, cyclosporine, and methotrexate (eTable). Fumaric acid esters and tofacitinib are FDA approved for psoriatic arthritis but not for plaque psoriasis. Additional long-term safety analyses of tofacitinib for plaque psoriasis were requested by the FDA. Dimethyl fumarate is approved by the European Medicines Agency for treatment of psoriasis and is among the first-line systemic treatments used in Germany.2
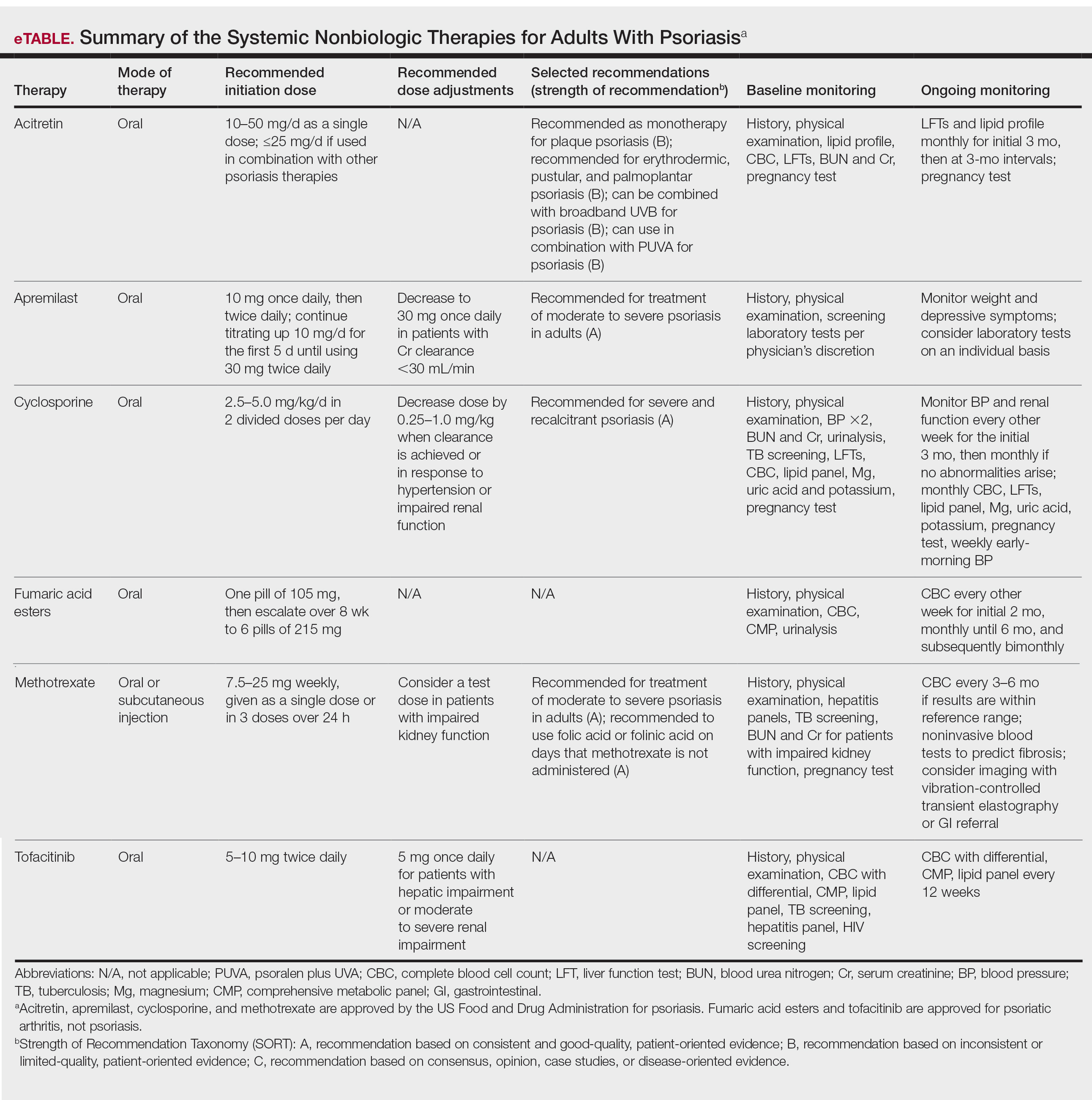
Selecting a Systemic Nonbiologic Agent
Methotrexate and apremilast have a strength level A recommendation for treating moderate to severe psoriasis in adults. However, methotrexate is less effective than biologic agents, including adalimumab and infliximab, for cutaneous psoriasis. Methotrexate is believed to improve psoriasis because of its direct immunosuppressive effect and inhibition of lymphoid cell proliferation. It typically is administered orally but can be administered subcutaneously for decreased gastrointestinal (GI) adverse effects. Compliance with close laboratory monitoring and lifestyle modifications, such as contraceptive use (because of teratogenicity) and alcohol cessation (because of the risk of liver damage) are essential in patients using methotrexate.
Apremilast, the most recently FDA-approved oral systemic medication for psoriasis, inhibits phosphodiesterase 4, subsequently decreasing inflammatory responses involving helper T cells TH1 and TH17 as well as type 1 interferon pathways. Apremilast is particularly effective in treating psoriasis with scalp and palmoplantar involvement.3 Additionally, it has an encouraging safety profile and is favorable in patients with multiple comorbidities.
Among the 4 oral agents, cyclosporine has the quickest onset of effect and has a strength level A recommendation for treating severe and recalcitrant psoriasis. Because of its high-risk profile, it is recommended for short periods of time, acute flares, or during transitions to safer long-term treatment. Patients with multiple comorbidities should avoid cyclosporine as a treatment option.
Acitretin, an FDA-approved oral retinoid, is an optimal treatment option for immunosuppressed patients or patients with HIV on antiretroviral therapy because it is not immunosuppressive.4 Unlike cyclosporine, acitretin is less helpful for acute flares because it takes 3 to 6 months to reach peak therapeutic response for treating plaque psoriasis. Similar to cyclosporine, acitretin can be recommended for severe psoriatic variants of erythrodermic, generalized pustular, and palmoplantar psoriasis. Acitretin has been reported to be more effective and have a more rapid onset of action in erythrodermic and pustular psoriasis than in plaque psoriasis.5
Patient Comorbidities
Psoriatic arthritis (PsA) is a common comorbidity that affects treatment choice. Patients with coexisting PsA could be treated with apremilast, as it is approved for both psoriasis and PsA. In a phase 3 randomized, controlled trial, American College of Rheumatology (ACR) 20 response at weeks 16 and 52 was achieved by significantly more patients on apremilast at 20 mg twice daily (BID)(P=.0166) or 30 mg BID (P=.0001) than placebo.6 Although not FDA approved for PsA, methotrexate has been shown to improve concomitant PsA of the peripheral joints in patients with psoriasis. Furthermore, a trial of methotrexate has shown considerable improvements in PsA symptoms in patients with psoriasis—a 62.7% decrease in proportion of patients with dactylitis, 25.7% decrease in enthesitis, and improvements in ACR outcomes (ACR20 in 40.8%, ACR50 in 18.8%, and ACR70 in 8.6%, with 22.4% achieving minimal disease activity).7
Prior to starting a systemic medication for psoriasis, it is necessary to discuss effects on pregnancy and fertility. Pregnancy is an absolute contraindication for methotrexate and acitretin use because of the drugs’ teratogenicity. Fetal death and fetal abnormalities have been reported with methotrexate use in pregnant women.8 Bone, central nervous system, auditory, ocular, and cardiovascular fetal abnormalities have been reported with maternal acitretin use.9 Breastfeeding also is an absolute contraindication for methotrexate use, as methotrexate passes into breastmilk in small quantities. Patients taking acitretin also are strongly discouraged from nursing because of the long half-life (168 days) of etretinate, a reverse metabolism product of acitretin that is increased in the presence of alcohol. Women should wait 3 months after discontinuing methotrexate for complete drug clearance before conceiving compared to 3 years in women who have discontinued acitretin.8,10 Men also are recommended to wait 3 months after discontinuing methotrexate before attempting to conceive, as its effect on male spermatogenesis and teratogenicity is unclear. Acitretin has no documented teratogenic effect in men. For women planning to become pregnant, apremilast and cyclosporine can be continued throughout pregnancy on an individual basis. The benefit of apremilast should be weighed against its potential risk to the fetus. There is no evidence of teratogenicity of apremilast at doses of 20 mg/kg daily.11 Current research regarding cyclosporine use in pregnancy only exists in transplant patients and has revealed higher rates of prematurity and lower birth weight without teratogenic effects.10,12 The risks and benefits of continuing cyclosporine while nursing should be evaluated, as cyclosporine (and ethanol-methanol components used in some formulations) is detectable in breast milk.
Drug Contraindications
Hypersensitivity to a specific systemic nonbiologic medication is a contraindication to its use and is an absolute contraindication for methotrexate. Other absolute contraindications to methotrexate are pregnancy and nursing, alcoholism, alcoholic liver disease, chronic liver disease, immunodeficiency, and cytopenia. Contraindications to acitretin include pregnancy, severely impaired liver and kidney function, and chronic abnormally elevated lipid levels. There are no additional contraindications for apremilast, but patients must be informed of the risk for depression before initiating therapy. Cyclosporine is contraindicated in patients with prior psoralen plus UVA (PUVA) treatment or radiation therapy, abnormal renal function, uncontrolled hypertension, uncontrolled and active infections, and a history of systemic malignancy. Live vaccines should be avoided in patients on cyclosporine, and caution is advised when cyclosporine is prescribed for patients with poorly controlled diabetes.
Pretreatment Screening
Because of drug interactions, a detailed medication history is essential prior to starting any systemic medication for psoriasis. Apremilast and cyclosporine are metabolized by cytochrome P450 and therefore are more susceptible to drug-related interactions. Cyclosporine use can affect levels of other medications that are metabolized by cytochrome P450, such as statins, calcium channel blockers, and warfarin. Similarly, acitretin’s metabolism is affected by drugs that interfere with cytochrome P450. Additionally, screening laboratory tests are needed before initiating systemic nonbiologic agents for psoriasis, with the exception of apremilast.
Prior to initiating methotrexate treatment, patients may require tuberculosis (TB), hepatitis B, and hepatitis C screening tests, depending on their risk factors. A baseline liver fibrosis assessment is recommended because of the potential of hepatotoxicity in patients receiving methotrexate. Noninvasive serology tests utilized to evaluate the presence of pre-existing liver disease include Fibrosis-4, FibroMeter, FibroSure, and Hepascore. Patients with impaired renal function have an increased predisposition to methotrexate-induced hematologic toxicity. Thus, it is necessary to administer a test dose of methotrexate in these patients followed by a complete blood cell count (CBC) 5 to 7 days later. An unremarkable CBC after the test dose suggests the absence of myelosuppression, and methotrexate dosage can be increased weekly. Patients on methotrexate also must receive folate supplementation to reduce the risk for adverse effects during treatment.
Patients considering cyclosporine must undergo screening for family and personal history of renal disease. Prior to initiating treatment, patients require 2 blood pressure measurements, hepatitis screening, TB screening, urinalysis, serum creatinine (Cr), blood urea nitrogen (BUN), CBC, potassium and magnesium levels, uric acid levels, lipid profile, bilirubin, and liver function tests (LFTs). A pregnancy test also is warranted for women of childbearing potential (WOCP).
Patients receiving acitretin should receive screening laboratory tests consisting of fasting cholesterol and triglycerides, CBC, renal function tests, LFTs, and a pregnancy test, if applicable.
After baseline evaluations, the selected oral systemic can be initiated using specific dosing regimens to ensure optimal drug efficacy and reduce incidence of adverse effects (eTable).
Monitoring During Active Treatment
Physicians need to counsel patients on potential adverse effects of their medications. Because of its relatively safe profile among the systemic nonbiologic agents, apremilast requires the least monitoring during treatment. There is no required routine laboratory monitoring for patients using apremilast, though testing may be pursued at the clinician’s discretion. However, weight should be regularly measured in patients on apremilast. In a phase 3 clinical trial of patients with psoriasis, 12% of patients on apremilast experienced a 5% to 10% weight loss compared to 5% of patients on placebo.11,13 Thus, it is recommended that physicians consider discontinuing apremilast in patients with a weight loss of more than 5% from baseline, especially if it may lead to other unfavorable health effects. Because depression is reported among 1% of patients on apremilast, close monitoring for new or worsening symptoms of depression should be performed during treatment.11,13 To avoid common GI side effects, apremilast is initiated at 10 mg/d and is increased by 10 mg/d over the first 5 days to a final dose of 30 mg BID. Elderly patients in particular should be cautioned about the risk of dehydration associated with GI side effects. Patients with severe renal impairment (Cr clearance, <30 mL/min) should use apremilast at a dosage of 30 mg once daily.
For patients on methotrexate, laboratory monitoring is essential after each dose increase. It also is important for physicians to obtain regular blood work to assess for hematologic abnormalities and hepatoxicity. Patients with risk factors such as renal insufficiency, increased age, hypoalbuminemia, alcohol abuse and alcoholic liver disease, and methotrexate dosing errors, as well as those prone to drug-related interactions, must be monitored closely for pancytopenia.14,15 The protocol for screening for methotrexate-induced hepatotoxicity during treatment depends on patient risk factors. Risk factors for hepatoxicity include history of or current alcohol abuse, abnormal LFTs, personal or family history of liver disease, diabetes, obesity, use of other hepatotoxic drugs, and hyperlipidemia.16 In patients without blood work abnormalities, CBC and LFTs can be performed every 3 to 6 months. Patients with abnormally elevated LFTs require repeat blood work every 2 to 4 weeks. Persistent elevations in LFTs require further evaluation by a GI specialist. After a cumulative dose of 3.5 to 4 g, patients should receive a GI referral and further studies (such as vibration-controlled transient elastography or liver biopsy) to assess for liver fibrosis. Patients with signs of stage 3 liver fibrosis are recommended to discontinue methotrexate and switch to another medication for psoriasis. For patients with impaired renal function, periodic BUN and Cr monitoring are needed. Common adverse effects of methotrexate include diarrhea, nausea, and anorexia, which can be mitigated by taking methotrexate with food or lowering the dosage.8 Patients on methotrexate should be monitored for rare but potential risks of infection and reactivation of latent TB, hepatitis, and lymphoma. To reduce the incidence of methotrexate toxicity from drug interactions, a review of current medications at each follow-up visit is recommended.
Nephrotoxicity and hypertension are the most common adverse effects of cyclosporine. It is important to monitor BUN and Cr biweekly for the initial 3 months, then at monthly intervals if there are no persistent abnormalities. Patients also must receive monthly CBC, potassium and magnesium levels, uric acid levels, lipid panel, serum bilirubin, and LFTs to monitor for adverse effects.17 Physicians should obtain regular pregnancy tests in WOCP. Weekly monitoring of early-morning blood pressure is recommended for patients on cyclosporine to detect early cyclosporine-induced nephrotoxicity. Hypertension on 2 separate occasions warrants a reduction in cyclosporine dosage or an addition of a calcium channel blocker for blood pressure control. Dose reduction also should be performed in patients with an increase in Cr above baseline greater than 25%.17 If Cr level is persistently elevated or if blood pressure does not normalize to lower than 140/90 after dose reduction, cyclosporine should be immediately discontinued. Patients on cyclosporine for more than a year warrant an annual estimation of glomerular filtration rate because of irreversible kidney damage associated with long-term use. A systematic review of patients treated with cyclosporine for more than 2 years found that at least 50% of patients experienced a 30% increase in Cr above baseline.18
Patients taking acitretin should be monitored for hyperlipidemia, the most common laboratory abnormality seen in 25% to 50% of patients.19 Fasting lipid panel and LFTs should be performed monthly for the initial 3 months on acitretin, then at 3-month intervals. Lifestyle changes should be encouraged to reduce hyperlipidemia, and fibrates may be given to treat elevated triglyceride levels, the most common type of hyperlipidemia seen with acitretin. Acitretin-induced toxic hepatitis is a rare occurrence that warrants immediate discontinuation of the medication.20 Monthly pregnancy tests must be performed in WOCP.
Combination Therapy
For apremilast, there is anecdotal evidence supporting its use in conjunction with phototherapy or biologics in some cases, but no high-quality data.21 On the other hand, using combination therapy with other systemic therapies can reduce adverse effects and decrease the amount of medication needed to achieve psoriasis clearance. Methotrexate used with etanercept, for example, has been more effective than methotrexate monotherapy in treating psoriasis, which has been attributed to a methotrexate-mediated reduction in the production of antidrug antibodies.22,23
Methotrexate, cyclosporine, and acitretin have synergistic effects when used with phototherapy. Narrowband UVB (NB-UVB) phototherapy combined with methotrexate is more effective in clearing psoriasis than methotrexate or NB-UVB phototherapy alone. Similarly, acitretin and PUVA combination therapy is more effective than acitretin or PUVA phototherapy alone. Combination regimens of acitretin and broadband UVB phototherapy, acitretin and NB-UVB phototherapy, and acitretin and PUVA phototherapy also have been more effective than individual modalities alone. Combination therapy reduces the cumulative doses of both therapies and reduces the frequency and duration of phototherapy needed for psoriatic clearance.24 In acitretin combination therapy with UVB phototherapy, the recommended regimen is 2 weeks of acitretin monotherapy followed by UVB phototherapy. For patients with an inadequate response to UVB phototherapy, the UVB dose can be reduced by 30% to 50%, and acitretin 25 mg/d can be added to phototherapy treatment. Acitretin-UVB combination therapy has been shown to reduce the risk of UVB-induced erythema seen in UVB monotherapy. Similarly, the risk of squamous cell carcinoma is reduced in acitretin-PUVA combination therapy compared to PUVA monotherapy.25
The timing of phototherapy in combination with systemic nonbiologic agents is critical. Phototherapy used simultaneously with cyclosporine is contraindicated owing to increased risk of photocarcinogenesis, whereas phototherapy used in sequence with cyclosporine is well tolerated and effective. Furthermore, cyclosporine 3 mg/kg/d for 4 weeks followed by a rapid cyclosporine taper and initiation of NB-UVB phototherapy demonstrated resolution of psoriasis with fewer NB-UVB treatments and less UVB exposure than NB-UVB therapy alone.26
Final Thoughts
The FDA-approved systemic nonbiologic agents are accessible and effective treatment options for adults with widespread or inadequately controlled psoriasis. Selecting the ideal therapy requires careful consideration of medication toxicity, contraindications, monitoring requirements, and patient comorbidities. The AAD-NPF guidelines guide dermatologists in prescribing systemic nonbiologic treatments in adults with psoriasis. Utilizing these recommendations in combination with clinician judgment will help patients achieve safe and optimal psoriasis clearance.
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486.
- Mrowietz U, Barker J, Boehncke WH, et al. Clinical use of dimethyl fumarate in moderate-to-severe plaque-type psoriasis: a European expert consensus. J Eur Acad Dermatol Venereol. 2018;32(suppl 3):3-14.
- Van Voorhees AS, Gold LS, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate to severe plaque psoriasis of the scalp: results of a phase 3b, multicenter, randomized, placebo-controlled, double-blind study. J Am Acad Dermatol. 2020;83:96-103.
- Buccheri L, Katchen BR, Karter AJ, et al. Acitretin therapy is effective for psoriasis associated with human immunodeficiency virus infection. Arch Dermatol. 1997;133:711-715.
- Ormerod AD, Campalani E, Goodfield MJD. British Association of Dermatologists guidelines on the efficacy and use of acitretin in dermatology. Br J Dermatol. 2010;162:952-963.
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Longterm (52-week) results of a phase III randomized, controlled trial of apremilast in patients with psoriatic arthritis. J Rheumatol. 2015;42:479-488.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Antares Pharma, Inc. Otrexup PFS (methotrexate) [package insert]. US Food and Drug Administration website. Revised June 2019. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/204824s009lbl.pdf
- David M, Hodak E, Lowe NJ. Adverse effects of retinoids. Med Toxicol Adverse Drug Exp. 1988;3:273-288.
- Stiefel Laboratories, Inc. Soriatane (acitretin) [package insert]. US Food and Drug Administration website. Revised September 2017. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/019821s028lbl.pdf
- Celgene Corporation. Otezla (apremilast) [package insert]. US Food and Drug Administration website. Revised March 2014. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205437s000lbl.pdf
- Ghanem ME, El-Baghdadi LA, Badawy AM, et al. Pregnancy outcome after renal allograft transplantation: 15 years experience. Eur J Obstet Gynecol Reprod Biol. 2005;121:178-181.
- Zerilli T, Ocheretyaner E. Apremilast (Otezla): A new oral treatment for adults with psoriasis and psoriatic arthritis. P T. 2015;40:495-500.
- Kivity S, Zafrir Y, Loebstein R, et al. Clinical characteristics and risk factors for low dose methotrexate toxicity: a cohort of 28 patients. Autoimmun Rev. 2014;13:1109-1113.
- Boffa MJ, Chalmers RJ. Methotrexate for psoriasis. Clin Exp Dermatol. 1996;21:399-408.
- Rosenberg P, Urwitz H, Johannesson A, et al. Psoriasis patients with diabetes type 2 are at high risk of developing liver fibrosis during methotrexate treatment. J Hepatol. 2007;46:1111-1118.
- Novartis Pharmaceuticals Corporation. Sandimmune (cyclosporine) [package insert]. US Food and Drug Administration website. Published 2015. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/050573s041,050574s051,050625s055lbl.pdf
- Maza A, Montaudie H, Sbidian E, et al. Oral cyclosporin in psoriasis: a systematic review on treatment modalities, risk of kidney toxicity and evidence for use in non-plaque psoriasis. J Eur Acad Dermatol Venereol. 2011;25(suppl 2):19-27.
- Yamauchi PS, Rizk D, Kormilli T, et al. Systemic retinoids. In: Weinstein GD, Gottlieb AB, eds. Therapy of Moderate-to-Severe Psoriasis. Marcel Dekker; 2003:137-150.
- van Ditzhuijsen TJ, van Haelst UJ, van Dooren-Greebe RJ, et al. Severe hepatotoxic reaction with progression to cirrhosis after use of a novel retinoid (acitretin). J Hepatol. 1990;11:185-188.
- AbuHilal M, Walsh S, Shear N. Use of apremilast in combination with other therapies for treatment of chronic plaque psoriasis: a retrospective study. J Cutan Med Surg. 2016;20:313-316.
- Gottlieb AB, Langley RG, Strober BE, et al. A randomized, double-blind, placebo-controlled study to evaluate the addition of methotrexate to etanercept in patients with moderate to severe plaque psoriasis. Br J Dermatol. 2012;167:649-657.
- Cronstein BN. Methotrexate BAFFles anti-drug antibodies. Nat Rev Rheumatol. 2018;14:505-506.
- Lebwohl M, Drake L, Menter A, et al. Consensus conference: acitretin in combination with UVB or PUVA in the treatment of psoriasis. J Am Acad Dermatol. 2001;45:544-553.
- Nijsten TE, Stern RS. Oral retinoid use reduces cutaneous squamous cell carcinoma risk in patients with psoriasis treated with psoralen-UVA: a nested cohort study. J Am Acad Dermatol. 2003;49:644-650.
- Calzavara-Pinton P, Leone G, Venturini M, et al. A comparative non randomized study of narrow-band (NB) (312 +/- 2 nm) UVB phototherapy versus sequential therapy with oral administration of low-dose cyclosporin A and NB-UVB phototherapy in patients with severe psoriasis vulgaris. Eur J Dermatol. 2005;15:470-473.
Psoriasis is a chronic relapsing skin condition characterized by keratinocyte hyperproliferation and a chronic inflammatory cascade. Therefore, controlling inflammatory responses with systemic medications is beneficial in managing psoriatic lesions and their accompanying symptoms, especially in disease inadequately controlled by topicals. Ease of drug administration and treatment availability are benefits that systemic nonbiologic therapies may have over biologic therapies.
In 2020, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) published guidelines for managing psoriasis in adults with systemic nonbiologic therapies.1 Dosing, efficacy, toxicity, drug-related interactions, and contraindications are addressed alongside evidence-based treatment recommendations. This review addresses current recommendations for systemic nonbiologics in psoriasis with a focus on the treatments approved by the US Food and Drug Administration (FDA): acitretin, apremilast, cyclosporine, and methotrexate (eTable). Fumaric acid esters and tofacitinib are FDA approved for psoriatic arthritis but not for plaque psoriasis. Additional long-term safety analyses of tofacitinib for plaque psoriasis were requested by the FDA. Dimethyl fumarate is approved by the European Medicines Agency for treatment of psoriasis and is among the first-line systemic treatments used in Germany.2

Selecting a Systemic Nonbiologic Agent
Methotrexate and apremilast have a strength level A recommendation for treating moderate to severe psoriasis in adults. However, methotrexate is less effective than biologic agents, including adalimumab and infliximab, for cutaneous psoriasis. Methotrexate is believed to improve psoriasis because of its direct immunosuppressive effect and inhibition of lymphoid cell proliferation. It typically is administered orally but can be administered subcutaneously for decreased gastrointestinal (GI) adverse effects. Compliance with close laboratory monitoring and lifestyle modifications, such as contraceptive use (because of teratogenicity) and alcohol cessation (because of the risk of liver damage) are essential in patients using methotrexate.
Apremilast, the most recently FDA-approved oral systemic medication for psoriasis, inhibits phosphodiesterase 4, subsequently decreasing inflammatory responses involving helper T cells TH1 and TH17 as well as type 1 interferon pathways. Apremilast is particularly effective in treating psoriasis with scalp and palmoplantar involvement.3 Additionally, it has an encouraging safety profile and is favorable in patients with multiple comorbidities.
Among the 4 oral agents, cyclosporine has the quickest onset of effect and has a strength level A recommendation for treating severe and recalcitrant psoriasis. Because of its high-risk profile, it is recommended for short periods of time, acute flares, or during transitions to safer long-term treatment. Patients with multiple comorbidities should avoid cyclosporine as a treatment option.
Acitretin, an FDA-approved oral retinoid, is an optimal treatment option for immunosuppressed patients or patients with HIV on antiretroviral therapy because it is not immunosuppressive.4 Unlike cyclosporine, acitretin is less helpful for acute flares because it takes 3 to 6 months to reach peak therapeutic response for treating plaque psoriasis. Similar to cyclosporine, acitretin can be recommended for severe psoriatic variants of erythrodermic, generalized pustular, and palmoplantar psoriasis. Acitretin has been reported to be more effective and have a more rapid onset of action in erythrodermic and pustular psoriasis than in plaque psoriasis.5
Patient Comorbidities
Psoriatic arthritis (PsA) is a common comorbidity that affects treatment choice. Patients with coexisting PsA could be treated with apremilast, as it is approved for both psoriasis and PsA. In a phase 3 randomized, controlled trial, American College of Rheumatology (ACR) 20 response at weeks 16 and 52 was achieved by significantly more patients on apremilast at 20 mg twice daily (BID)(P=.0166) or 30 mg BID (P=.0001) than placebo.6 Although not FDA approved for PsA, methotrexate has been shown to improve concomitant PsA of the peripheral joints in patients with psoriasis. Furthermore, a trial of methotrexate has shown considerable improvements in PsA symptoms in patients with psoriasis—a 62.7% decrease in proportion of patients with dactylitis, 25.7% decrease in enthesitis, and improvements in ACR outcomes (ACR20 in 40.8%, ACR50 in 18.8%, and ACR70 in 8.6%, with 22.4% achieving minimal disease activity).7
Prior to starting a systemic medication for psoriasis, it is necessary to discuss effects on pregnancy and fertility. Pregnancy is an absolute contraindication for methotrexate and acitretin use because of the drugs’ teratogenicity. Fetal death and fetal abnormalities have been reported with methotrexate use in pregnant women.8 Bone, central nervous system, auditory, ocular, and cardiovascular fetal abnormalities have been reported with maternal acitretin use.9 Breastfeeding also is an absolute contraindication for methotrexate use, as methotrexate passes into breastmilk in small quantities. Patients taking acitretin also are strongly discouraged from nursing because of the long half-life (168 days) of etretinate, a reverse metabolism product of acitretin that is increased in the presence of alcohol. Women should wait 3 months after discontinuing methotrexate for complete drug clearance before conceiving compared to 3 years in women who have discontinued acitretin.8,10 Men also are recommended to wait 3 months after discontinuing methotrexate before attempting to conceive, as its effect on male spermatogenesis and teratogenicity is unclear. Acitretin has no documented teratogenic effect in men. For women planning to become pregnant, apremilast and cyclosporine can be continued throughout pregnancy on an individual basis. The benefit of apremilast should be weighed against its potential risk to the fetus. There is no evidence of teratogenicity of apremilast at doses of 20 mg/kg daily.11 Current research regarding cyclosporine use in pregnancy only exists in transplant patients and has revealed higher rates of prematurity and lower birth weight without teratogenic effects.10,12 The risks and benefits of continuing cyclosporine while nursing should be evaluated, as cyclosporine (and ethanol-methanol components used in some formulations) is detectable in breast milk.
Drug Contraindications
Hypersensitivity to a specific systemic nonbiologic medication is a contraindication to its use and is an absolute contraindication for methotrexate. Other absolute contraindications to methotrexate are pregnancy and nursing, alcoholism, alcoholic liver disease, chronic liver disease, immunodeficiency, and cytopenia. Contraindications to acitretin include pregnancy, severely impaired liver and kidney function, and chronic abnormally elevated lipid levels. There are no additional contraindications for apremilast, but patients must be informed of the risk for depression before initiating therapy. Cyclosporine is contraindicated in patients with prior psoralen plus UVA (PUVA) treatment or radiation therapy, abnormal renal function, uncontrolled hypertension, uncontrolled and active infections, and a history of systemic malignancy. Live vaccines should be avoided in patients on cyclosporine, and caution is advised when cyclosporine is prescribed for patients with poorly controlled diabetes.
Pretreatment Screening
Because of drug interactions, a detailed medication history is essential prior to starting any systemic medication for psoriasis. Apremilast and cyclosporine are metabolized by cytochrome P450 and therefore are more susceptible to drug-related interactions. Cyclosporine use can affect levels of other medications that are metabolized by cytochrome P450, such as statins, calcium channel blockers, and warfarin. Similarly, acitretin’s metabolism is affected by drugs that interfere with cytochrome P450. Additionally, screening laboratory tests are needed before initiating systemic nonbiologic agents for psoriasis, with the exception of apremilast.
Prior to initiating methotrexate treatment, patients may require tuberculosis (TB), hepatitis B, and hepatitis C screening tests, depending on their risk factors. A baseline liver fibrosis assessment is recommended because of the potential of hepatotoxicity in patients receiving methotrexate. Noninvasive serology tests utilized to evaluate the presence of pre-existing liver disease include Fibrosis-4, FibroMeter, FibroSure, and Hepascore. Patients with impaired renal function have an increased predisposition to methotrexate-induced hematologic toxicity. Thus, it is necessary to administer a test dose of methotrexate in these patients followed by a complete blood cell count (CBC) 5 to 7 days later. An unremarkable CBC after the test dose suggests the absence of myelosuppression, and methotrexate dosage can be increased weekly. Patients on methotrexate also must receive folate supplementation to reduce the risk for adverse effects during treatment.
Patients considering cyclosporine must undergo screening for family and personal history of renal disease. Prior to initiating treatment, patients require 2 blood pressure measurements, hepatitis screening, TB screening, urinalysis, serum creatinine (Cr), blood urea nitrogen (BUN), CBC, potassium and magnesium levels, uric acid levels, lipid profile, bilirubin, and liver function tests (LFTs). A pregnancy test also is warranted for women of childbearing potential (WOCP).
Patients receiving acitretin should receive screening laboratory tests consisting of fasting cholesterol and triglycerides, CBC, renal function tests, LFTs, and a pregnancy test, if applicable.
After baseline evaluations, the selected oral systemic can be initiated using specific dosing regimens to ensure optimal drug efficacy and reduce incidence of adverse effects (eTable).
Monitoring During Active Treatment
Physicians need to counsel patients on potential adverse effects of their medications. Because of its relatively safe profile among the systemic nonbiologic agents, apremilast requires the least monitoring during treatment. There is no required routine laboratory monitoring for patients using apremilast, though testing may be pursued at the clinician’s discretion. However, weight should be regularly measured in patients on apremilast. In a phase 3 clinical trial of patients with psoriasis, 12% of patients on apremilast experienced a 5% to 10% weight loss compared to 5% of patients on placebo.11,13 Thus, it is recommended that physicians consider discontinuing apremilast in patients with a weight loss of more than 5% from baseline, especially if it may lead to other unfavorable health effects. Because depression is reported among 1% of patients on apremilast, close monitoring for new or worsening symptoms of depression should be performed during treatment.11,13 To avoid common GI side effects, apremilast is initiated at 10 mg/d and is increased by 10 mg/d over the first 5 days to a final dose of 30 mg BID. Elderly patients in particular should be cautioned about the risk of dehydration associated with GI side effects. Patients with severe renal impairment (Cr clearance, <30 mL/min) should use apremilast at a dosage of 30 mg once daily.
For patients on methotrexate, laboratory monitoring is essential after each dose increase. It also is important for physicians to obtain regular blood work to assess for hematologic abnormalities and hepatoxicity. Patients with risk factors such as renal insufficiency, increased age, hypoalbuminemia, alcohol abuse and alcoholic liver disease, and methotrexate dosing errors, as well as those prone to drug-related interactions, must be monitored closely for pancytopenia.14,15 The protocol for screening for methotrexate-induced hepatotoxicity during treatment depends on patient risk factors. Risk factors for hepatoxicity include history of or current alcohol abuse, abnormal LFTs, personal or family history of liver disease, diabetes, obesity, use of other hepatotoxic drugs, and hyperlipidemia.16 In patients without blood work abnormalities, CBC and LFTs can be performed every 3 to 6 months. Patients with abnormally elevated LFTs require repeat blood work every 2 to 4 weeks. Persistent elevations in LFTs require further evaluation by a GI specialist. After a cumulative dose of 3.5 to 4 g, patients should receive a GI referral and further studies (such as vibration-controlled transient elastography or liver biopsy) to assess for liver fibrosis. Patients with signs of stage 3 liver fibrosis are recommended to discontinue methotrexate and switch to another medication for psoriasis. For patients with impaired renal function, periodic BUN and Cr monitoring are needed. Common adverse effects of methotrexate include diarrhea, nausea, and anorexia, which can be mitigated by taking methotrexate with food or lowering the dosage.8 Patients on methotrexate should be monitored for rare but potential risks of infection and reactivation of latent TB, hepatitis, and lymphoma. To reduce the incidence of methotrexate toxicity from drug interactions, a review of current medications at each follow-up visit is recommended.
Nephrotoxicity and hypertension are the most common adverse effects of cyclosporine. It is important to monitor BUN and Cr biweekly for the initial 3 months, then at monthly intervals if there are no persistent abnormalities. Patients also must receive monthly CBC, potassium and magnesium levels, uric acid levels, lipid panel, serum bilirubin, and LFTs to monitor for adverse effects.17 Physicians should obtain regular pregnancy tests in WOCP. Weekly monitoring of early-morning blood pressure is recommended for patients on cyclosporine to detect early cyclosporine-induced nephrotoxicity. Hypertension on 2 separate occasions warrants a reduction in cyclosporine dosage or an addition of a calcium channel blocker for blood pressure control. Dose reduction also should be performed in patients with an increase in Cr above baseline greater than 25%.17 If Cr level is persistently elevated or if blood pressure does not normalize to lower than 140/90 after dose reduction, cyclosporine should be immediately discontinued. Patients on cyclosporine for more than a year warrant an annual estimation of glomerular filtration rate because of irreversible kidney damage associated with long-term use. A systematic review of patients treated with cyclosporine for more than 2 years found that at least 50% of patients experienced a 30% increase in Cr above baseline.18
Patients taking acitretin should be monitored for hyperlipidemia, the most common laboratory abnormality seen in 25% to 50% of patients.19 Fasting lipid panel and LFTs should be performed monthly for the initial 3 months on acitretin, then at 3-month intervals. Lifestyle changes should be encouraged to reduce hyperlipidemia, and fibrates may be given to treat elevated triglyceride levels, the most common type of hyperlipidemia seen with acitretin. Acitretin-induced toxic hepatitis is a rare occurrence that warrants immediate discontinuation of the medication.20 Monthly pregnancy tests must be performed in WOCP.
Combination Therapy
For apremilast, there is anecdotal evidence supporting its use in conjunction with phototherapy or biologics in some cases, but no high-quality data.21 On the other hand, using combination therapy with other systemic therapies can reduce adverse effects and decrease the amount of medication needed to achieve psoriasis clearance. Methotrexate used with etanercept, for example, has been more effective than methotrexate monotherapy in treating psoriasis, which has been attributed to a methotrexate-mediated reduction in the production of antidrug antibodies.22,23
Methotrexate, cyclosporine, and acitretin have synergistic effects when used with phototherapy. Narrowband UVB (NB-UVB) phototherapy combined with methotrexate is more effective in clearing psoriasis than methotrexate or NB-UVB phototherapy alone. Similarly, acitretin and PUVA combination therapy is more effective than acitretin or PUVA phototherapy alone. Combination regimens of acitretin and broadband UVB phototherapy, acitretin and NB-UVB phototherapy, and acitretin and PUVA phototherapy also have been more effective than individual modalities alone. Combination therapy reduces the cumulative doses of both therapies and reduces the frequency and duration of phototherapy needed for psoriatic clearance.24 In acitretin combination therapy with UVB phototherapy, the recommended regimen is 2 weeks of acitretin monotherapy followed by UVB phototherapy. For patients with an inadequate response to UVB phototherapy, the UVB dose can be reduced by 30% to 50%, and acitretin 25 mg/d can be added to phototherapy treatment. Acitretin-UVB combination therapy has been shown to reduce the risk of UVB-induced erythema seen in UVB monotherapy. Similarly, the risk of squamous cell carcinoma is reduced in acitretin-PUVA combination therapy compared to PUVA monotherapy.25
The timing of phototherapy in combination with systemic nonbiologic agents is critical. Phototherapy used simultaneously with cyclosporine is contraindicated owing to increased risk of photocarcinogenesis, whereas phototherapy used in sequence with cyclosporine is well tolerated and effective. Furthermore, cyclosporine 3 mg/kg/d for 4 weeks followed by a rapid cyclosporine taper and initiation of NB-UVB phototherapy demonstrated resolution of psoriasis with fewer NB-UVB treatments and less UVB exposure than NB-UVB therapy alone.26
Final Thoughts
The FDA-approved systemic nonbiologic agents are accessible and effective treatment options for adults with widespread or inadequately controlled psoriasis. Selecting the ideal therapy requires careful consideration of medication toxicity, contraindications, monitoring requirements, and patient comorbidities. The AAD-NPF guidelines guide dermatologists in prescribing systemic nonbiologic treatments in adults with psoriasis. Utilizing these recommendations in combination with clinician judgment will help patients achieve safe and optimal psoriasis clearance.
Psoriasis is a chronic relapsing skin condition characterized by keratinocyte hyperproliferation and a chronic inflammatory cascade. Therefore, controlling inflammatory responses with systemic medications is beneficial in managing psoriatic lesions and their accompanying symptoms, especially in disease inadequately controlled by topicals. Ease of drug administration and treatment availability are benefits that systemic nonbiologic therapies may have over biologic therapies.
In 2020, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) published guidelines for managing psoriasis in adults with systemic nonbiologic therapies.1 Dosing, efficacy, toxicity, drug-related interactions, and contraindications are addressed alongside evidence-based treatment recommendations. This review addresses current recommendations for systemic nonbiologics in psoriasis with a focus on the treatments approved by the US Food and Drug Administration (FDA): acitretin, apremilast, cyclosporine, and methotrexate (eTable). Fumaric acid esters and tofacitinib are FDA approved for psoriatic arthritis but not for plaque psoriasis. Additional long-term safety analyses of tofacitinib for plaque psoriasis were requested by the FDA. Dimethyl fumarate is approved by the European Medicines Agency for treatment of psoriasis and is among the first-line systemic treatments used in Germany.2

Selecting a Systemic Nonbiologic Agent
Methotrexate and apremilast have a strength level A recommendation for treating moderate to severe psoriasis in adults. However, methotrexate is less effective than biologic agents, including adalimumab and infliximab, for cutaneous psoriasis. Methotrexate is believed to improve psoriasis because of its direct immunosuppressive effect and inhibition of lymphoid cell proliferation. It typically is administered orally but can be administered subcutaneously for decreased gastrointestinal (GI) adverse effects. Compliance with close laboratory monitoring and lifestyle modifications, such as contraceptive use (because of teratogenicity) and alcohol cessation (because of the risk of liver damage) are essential in patients using methotrexate.
Apremilast, the most recently FDA-approved oral systemic medication for psoriasis, inhibits phosphodiesterase 4, subsequently decreasing inflammatory responses involving helper T cells TH1 and TH17 as well as type 1 interferon pathways. Apremilast is particularly effective in treating psoriasis with scalp and palmoplantar involvement.3 Additionally, it has an encouraging safety profile and is favorable in patients with multiple comorbidities.
Among the 4 oral agents, cyclosporine has the quickest onset of effect and has a strength level A recommendation for treating severe and recalcitrant psoriasis. Because of its high-risk profile, it is recommended for short periods of time, acute flares, or during transitions to safer long-term treatment. Patients with multiple comorbidities should avoid cyclosporine as a treatment option.
Acitretin, an FDA-approved oral retinoid, is an optimal treatment option for immunosuppressed patients or patients with HIV on antiretroviral therapy because it is not immunosuppressive.4 Unlike cyclosporine, acitretin is less helpful for acute flares because it takes 3 to 6 months to reach peak therapeutic response for treating plaque psoriasis. Similar to cyclosporine, acitretin can be recommended for severe psoriatic variants of erythrodermic, generalized pustular, and palmoplantar psoriasis. Acitretin has been reported to be more effective and have a more rapid onset of action in erythrodermic and pustular psoriasis than in plaque psoriasis.5
Patient Comorbidities
Psoriatic arthritis (PsA) is a common comorbidity that affects treatment choice. Patients with coexisting PsA could be treated with apremilast, as it is approved for both psoriasis and PsA. In a phase 3 randomized, controlled trial, American College of Rheumatology (ACR) 20 response at weeks 16 and 52 was achieved by significantly more patients on apremilast at 20 mg twice daily (BID)(P=.0166) or 30 mg BID (P=.0001) than placebo.6 Although not FDA approved for PsA, methotrexate has been shown to improve concomitant PsA of the peripheral joints in patients with psoriasis. Furthermore, a trial of methotrexate has shown considerable improvements in PsA symptoms in patients with psoriasis—a 62.7% decrease in proportion of patients with dactylitis, 25.7% decrease in enthesitis, and improvements in ACR outcomes (ACR20 in 40.8%, ACR50 in 18.8%, and ACR70 in 8.6%, with 22.4% achieving minimal disease activity).7
Prior to starting a systemic medication for psoriasis, it is necessary to discuss effects on pregnancy and fertility. Pregnancy is an absolute contraindication for methotrexate and acitretin use because of the drugs’ teratogenicity. Fetal death and fetal abnormalities have been reported with methotrexate use in pregnant women.8 Bone, central nervous system, auditory, ocular, and cardiovascular fetal abnormalities have been reported with maternal acitretin use.9 Breastfeeding also is an absolute contraindication for methotrexate use, as methotrexate passes into breastmilk in small quantities. Patients taking acitretin also are strongly discouraged from nursing because of the long half-life (168 days) of etretinate, a reverse metabolism product of acitretin that is increased in the presence of alcohol. Women should wait 3 months after discontinuing methotrexate for complete drug clearance before conceiving compared to 3 years in women who have discontinued acitretin.8,10 Men also are recommended to wait 3 months after discontinuing methotrexate before attempting to conceive, as its effect on male spermatogenesis and teratogenicity is unclear. Acitretin has no documented teratogenic effect in men. For women planning to become pregnant, apremilast and cyclosporine can be continued throughout pregnancy on an individual basis. The benefit of apremilast should be weighed against its potential risk to the fetus. There is no evidence of teratogenicity of apremilast at doses of 20 mg/kg daily.11 Current research regarding cyclosporine use in pregnancy only exists in transplant patients and has revealed higher rates of prematurity and lower birth weight without teratogenic effects.10,12 The risks and benefits of continuing cyclosporine while nursing should be evaluated, as cyclosporine (and ethanol-methanol components used in some formulations) is detectable in breast milk.
Drug Contraindications
Hypersensitivity to a specific systemic nonbiologic medication is a contraindication to its use and is an absolute contraindication for methotrexate. Other absolute contraindications to methotrexate are pregnancy and nursing, alcoholism, alcoholic liver disease, chronic liver disease, immunodeficiency, and cytopenia. Contraindications to acitretin include pregnancy, severely impaired liver and kidney function, and chronic abnormally elevated lipid levels. There are no additional contraindications for apremilast, but patients must be informed of the risk for depression before initiating therapy. Cyclosporine is contraindicated in patients with prior psoralen plus UVA (PUVA) treatment or radiation therapy, abnormal renal function, uncontrolled hypertension, uncontrolled and active infections, and a history of systemic malignancy. Live vaccines should be avoided in patients on cyclosporine, and caution is advised when cyclosporine is prescribed for patients with poorly controlled diabetes.
Pretreatment Screening
Because of drug interactions, a detailed medication history is essential prior to starting any systemic medication for psoriasis. Apremilast and cyclosporine are metabolized by cytochrome P450 and therefore are more susceptible to drug-related interactions. Cyclosporine use can affect levels of other medications that are metabolized by cytochrome P450, such as statins, calcium channel blockers, and warfarin. Similarly, acitretin’s metabolism is affected by drugs that interfere with cytochrome P450. Additionally, screening laboratory tests are needed before initiating systemic nonbiologic agents for psoriasis, with the exception of apremilast.
Prior to initiating methotrexate treatment, patients may require tuberculosis (TB), hepatitis B, and hepatitis C screening tests, depending on their risk factors. A baseline liver fibrosis assessment is recommended because of the potential of hepatotoxicity in patients receiving methotrexate. Noninvasive serology tests utilized to evaluate the presence of pre-existing liver disease include Fibrosis-4, FibroMeter, FibroSure, and Hepascore. Patients with impaired renal function have an increased predisposition to methotrexate-induced hematologic toxicity. Thus, it is necessary to administer a test dose of methotrexate in these patients followed by a complete blood cell count (CBC) 5 to 7 days later. An unremarkable CBC after the test dose suggests the absence of myelosuppression, and methotrexate dosage can be increased weekly. Patients on methotrexate also must receive folate supplementation to reduce the risk for adverse effects during treatment.
Patients considering cyclosporine must undergo screening for family and personal history of renal disease. Prior to initiating treatment, patients require 2 blood pressure measurements, hepatitis screening, TB screening, urinalysis, serum creatinine (Cr), blood urea nitrogen (BUN), CBC, potassium and magnesium levels, uric acid levels, lipid profile, bilirubin, and liver function tests (LFTs). A pregnancy test also is warranted for women of childbearing potential (WOCP).
Patients receiving acitretin should receive screening laboratory tests consisting of fasting cholesterol and triglycerides, CBC, renal function tests, LFTs, and a pregnancy test, if applicable.
After baseline evaluations, the selected oral systemic can be initiated using specific dosing regimens to ensure optimal drug efficacy and reduce incidence of adverse effects (eTable).
Monitoring During Active Treatment
Physicians need to counsel patients on potential adverse effects of their medications. Because of its relatively safe profile among the systemic nonbiologic agents, apremilast requires the least monitoring during treatment. There is no required routine laboratory monitoring for patients using apremilast, though testing may be pursued at the clinician’s discretion. However, weight should be regularly measured in patients on apremilast. In a phase 3 clinical trial of patients with psoriasis, 12% of patients on apremilast experienced a 5% to 10% weight loss compared to 5% of patients on placebo.11,13 Thus, it is recommended that physicians consider discontinuing apremilast in patients with a weight loss of more than 5% from baseline, especially if it may lead to other unfavorable health effects. Because depression is reported among 1% of patients on apremilast, close monitoring for new or worsening symptoms of depression should be performed during treatment.11,13 To avoid common GI side effects, apremilast is initiated at 10 mg/d and is increased by 10 mg/d over the first 5 days to a final dose of 30 mg BID. Elderly patients in particular should be cautioned about the risk of dehydration associated with GI side effects. Patients with severe renal impairment (Cr clearance, <30 mL/min) should use apremilast at a dosage of 30 mg once daily.
For patients on methotrexate, laboratory monitoring is essential after each dose increase. It also is important for physicians to obtain regular blood work to assess for hematologic abnormalities and hepatoxicity. Patients with risk factors such as renal insufficiency, increased age, hypoalbuminemia, alcohol abuse and alcoholic liver disease, and methotrexate dosing errors, as well as those prone to drug-related interactions, must be monitored closely for pancytopenia.14,15 The protocol for screening for methotrexate-induced hepatotoxicity during treatment depends on patient risk factors. Risk factors for hepatoxicity include history of or current alcohol abuse, abnormal LFTs, personal or family history of liver disease, diabetes, obesity, use of other hepatotoxic drugs, and hyperlipidemia.16 In patients without blood work abnormalities, CBC and LFTs can be performed every 3 to 6 months. Patients with abnormally elevated LFTs require repeat blood work every 2 to 4 weeks. Persistent elevations in LFTs require further evaluation by a GI specialist. After a cumulative dose of 3.5 to 4 g, patients should receive a GI referral and further studies (such as vibration-controlled transient elastography or liver biopsy) to assess for liver fibrosis. Patients with signs of stage 3 liver fibrosis are recommended to discontinue methotrexate and switch to another medication for psoriasis. For patients with impaired renal function, periodic BUN and Cr monitoring are needed. Common adverse effects of methotrexate include diarrhea, nausea, and anorexia, which can be mitigated by taking methotrexate with food or lowering the dosage.8 Patients on methotrexate should be monitored for rare but potential risks of infection and reactivation of latent TB, hepatitis, and lymphoma. To reduce the incidence of methotrexate toxicity from drug interactions, a review of current medications at each follow-up visit is recommended.
Nephrotoxicity and hypertension are the most common adverse effects of cyclosporine. It is important to monitor BUN and Cr biweekly for the initial 3 months, then at monthly intervals if there are no persistent abnormalities. Patients also must receive monthly CBC, potassium and magnesium levels, uric acid levels, lipid panel, serum bilirubin, and LFTs to monitor for adverse effects.17 Physicians should obtain regular pregnancy tests in WOCP. Weekly monitoring of early-morning blood pressure is recommended for patients on cyclosporine to detect early cyclosporine-induced nephrotoxicity. Hypertension on 2 separate occasions warrants a reduction in cyclosporine dosage or an addition of a calcium channel blocker for blood pressure control. Dose reduction also should be performed in patients with an increase in Cr above baseline greater than 25%.17 If Cr level is persistently elevated or if blood pressure does not normalize to lower than 140/90 after dose reduction, cyclosporine should be immediately discontinued. Patients on cyclosporine for more than a year warrant an annual estimation of glomerular filtration rate because of irreversible kidney damage associated with long-term use. A systematic review of patients treated with cyclosporine for more than 2 years found that at least 50% of patients experienced a 30% increase in Cr above baseline.18
Patients taking acitretin should be monitored for hyperlipidemia, the most common laboratory abnormality seen in 25% to 50% of patients.19 Fasting lipid panel and LFTs should be performed monthly for the initial 3 months on acitretin, then at 3-month intervals. Lifestyle changes should be encouraged to reduce hyperlipidemia, and fibrates may be given to treat elevated triglyceride levels, the most common type of hyperlipidemia seen with acitretin. Acitretin-induced toxic hepatitis is a rare occurrence that warrants immediate discontinuation of the medication.20 Monthly pregnancy tests must be performed in WOCP.
Combination Therapy
For apremilast, there is anecdotal evidence supporting its use in conjunction with phototherapy or biologics in some cases, but no high-quality data.21 On the other hand, using combination therapy with other systemic therapies can reduce adverse effects and decrease the amount of medication needed to achieve psoriasis clearance. Methotrexate used with etanercept, for example, has been more effective than methotrexate monotherapy in treating psoriasis, which has been attributed to a methotrexate-mediated reduction in the production of antidrug antibodies.22,23
Methotrexate, cyclosporine, and acitretin have synergistic effects when used with phototherapy. Narrowband UVB (NB-UVB) phototherapy combined with methotrexate is more effective in clearing psoriasis than methotrexate or NB-UVB phototherapy alone. Similarly, acitretin and PUVA combination therapy is more effective than acitretin or PUVA phototherapy alone. Combination regimens of acitretin and broadband UVB phototherapy, acitretin and NB-UVB phototherapy, and acitretin and PUVA phototherapy also have been more effective than individual modalities alone. Combination therapy reduces the cumulative doses of both therapies and reduces the frequency and duration of phototherapy needed for psoriatic clearance.24 In acitretin combination therapy with UVB phototherapy, the recommended regimen is 2 weeks of acitretin monotherapy followed by UVB phototherapy. For patients with an inadequate response to UVB phototherapy, the UVB dose can be reduced by 30% to 50%, and acitretin 25 mg/d can be added to phototherapy treatment. Acitretin-UVB combination therapy has been shown to reduce the risk of UVB-induced erythema seen in UVB monotherapy. Similarly, the risk of squamous cell carcinoma is reduced in acitretin-PUVA combination therapy compared to PUVA monotherapy.25
The timing of phototherapy in combination with systemic nonbiologic agents is critical. Phototherapy used simultaneously with cyclosporine is contraindicated owing to increased risk of photocarcinogenesis, whereas phototherapy used in sequence with cyclosporine is well tolerated and effective. Furthermore, cyclosporine 3 mg/kg/d for 4 weeks followed by a rapid cyclosporine taper and initiation of NB-UVB phototherapy demonstrated resolution of psoriasis with fewer NB-UVB treatments and less UVB exposure than NB-UVB therapy alone.26
Final Thoughts
The FDA-approved systemic nonbiologic agents are accessible and effective treatment options for adults with widespread or inadequately controlled psoriasis. Selecting the ideal therapy requires careful consideration of medication toxicity, contraindications, monitoring requirements, and patient comorbidities. The AAD-NPF guidelines guide dermatologists in prescribing systemic nonbiologic treatments in adults with psoriasis. Utilizing these recommendations in combination with clinician judgment will help patients achieve safe and optimal psoriasis clearance.
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486.
- Mrowietz U, Barker J, Boehncke WH, et al. Clinical use of dimethyl fumarate in moderate-to-severe plaque-type psoriasis: a European expert consensus. J Eur Acad Dermatol Venereol. 2018;32(suppl 3):3-14.
- Van Voorhees AS, Gold LS, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate to severe plaque psoriasis of the scalp: results of a phase 3b, multicenter, randomized, placebo-controlled, double-blind study. J Am Acad Dermatol. 2020;83:96-103.
- Buccheri L, Katchen BR, Karter AJ, et al. Acitretin therapy is effective for psoriasis associated with human immunodeficiency virus infection. Arch Dermatol. 1997;133:711-715.
- Ormerod AD, Campalani E, Goodfield MJD. British Association of Dermatologists guidelines on the efficacy and use of acitretin in dermatology. Br J Dermatol. 2010;162:952-963.
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Longterm (52-week) results of a phase III randomized, controlled trial of apremilast in patients with psoriatic arthritis. J Rheumatol. 2015;42:479-488.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Antares Pharma, Inc. Otrexup PFS (methotrexate) [package insert]. US Food and Drug Administration website. Revised June 2019. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/204824s009lbl.pdf
- David M, Hodak E, Lowe NJ. Adverse effects of retinoids. Med Toxicol Adverse Drug Exp. 1988;3:273-288.
- Stiefel Laboratories, Inc. Soriatane (acitretin) [package insert]. US Food and Drug Administration website. Revised September 2017. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/019821s028lbl.pdf
- Celgene Corporation. Otezla (apremilast) [package insert]. US Food and Drug Administration website. Revised March 2014. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205437s000lbl.pdf
- Ghanem ME, El-Baghdadi LA, Badawy AM, et al. Pregnancy outcome after renal allograft transplantation: 15 years experience. Eur J Obstet Gynecol Reprod Biol. 2005;121:178-181.
- Zerilli T, Ocheretyaner E. Apremilast (Otezla): A new oral treatment for adults with psoriasis and psoriatic arthritis. P T. 2015;40:495-500.
- Kivity S, Zafrir Y, Loebstein R, et al. Clinical characteristics and risk factors for low dose methotrexate toxicity: a cohort of 28 patients. Autoimmun Rev. 2014;13:1109-1113.
- Boffa MJ, Chalmers RJ. Methotrexate for psoriasis. Clin Exp Dermatol. 1996;21:399-408.
- Rosenberg P, Urwitz H, Johannesson A, et al. Psoriasis patients with diabetes type 2 are at high risk of developing liver fibrosis during methotrexate treatment. J Hepatol. 2007;46:1111-1118.
- Novartis Pharmaceuticals Corporation. Sandimmune (cyclosporine) [package insert]. US Food and Drug Administration website. Published 2015. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/050573s041,050574s051,050625s055lbl.pdf
- Maza A, Montaudie H, Sbidian E, et al. Oral cyclosporin in psoriasis: a systematic review on treatment modalities, risk of kidney toxicity and evidence for use in non-plaque psoriasis. J Eur Acad Dermatol Venereol. 2011;25(suppl 2):19-27.
- Yamauchi PS, Rizk D, Kormilli T, et al. Systemic retinoids. In: Weinstein GD, Gottlieb AB, eds. Therapy of Moderate-to-Severe Psoriasis. Marcel Dekker; 2003:137-150.
- van Ditzhuijsen TJ, van Haelst UJ, van Dooren-Greebe RJ, et al. Severe hepatotoxic reaction with progression to cirrhosis after use of a novel retinoid (acitretin). J Hepatol. 1990;11:185-188.
- AbuHilal M, Walsh S, Shear N. Use of apremilast in combination with other therapies for treatment of chronic plaque psoriasis: a retrospective study. J Cutan Med Surg. 2016;20:313-316.
- Gottlieb AB, Langley RG, Strober BE, et al. A randomized, double-blind, placebo-controlled study to evaluate the addition of methotrexate to etanercept in patients with moderate to severe plaque psoriasis. Br J Dermatol. 2012;167:649-657.
- Cronstein BN. Methotrexate BAFFles anti-drug antibodies. Nat Rev Rheumatol. 2018;14:505-506.
- Lebwohl M, Drake L, Menter A, et al. Consensus conference: acitretin in combination with UVB or PUVA in the treatment of psoriasis. J Am Acad Dermatol. 2001;45:544-553.
- Nijsten TE, Stern RS. Oral retinoid use reduces cutaneous squamous cell carcinoma risk in patients with psoriasis treated with psoralen-UVA: a nested cohort study. J Am Acad Dermatol. 2003;49:644-650.
- Calzavara-Pinton P, Leone G, Venturini M, et al. A comparative non randomized study of narrow-band (NB) (312 +/- 2 nm) UVB phototherapy versus sequential therapy with oral administration of low-dose cyclosporin A and NB-UVB phototherapy in patients with severe psoriasis vulgaris. Eur J Dermatol. 2005;15:470-473.
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486.
- Mrowietz U, Barker J, Boehncke WH, et al. Clinical use of dimethyl fumarate in moderate-to-severe plaque-type psoriasis: a European expert consensus. J Eur Acad Dermatol Venereol. 2018;32(suppl 3):3-14.
- Van Voorhees AS, Gold LS, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate to severe plaque psoriasis of the scalp: results of a phase 3b, multicenter, randomized, placebo-controlled, double-blind study. J Am Acad Dermatol. 2020;83:96-103.
- Buccheri L, Katchen BR, Karter AJ, et al. Acitretin therapy is effective for psoriasis associated with human immunodeficiency virus infection. Arch Dermatol. 1997;133:711-715.
- Ormerod AD, Campalani E, Goodfield MJD. British Association of Dermatologists guidelines on the efficacy and use of acitretin in dermatology. Br J Dermatol. 2010;162:952-963.
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Longterm (52-week) results of a phase III randomized, controlled trial of apremilast in patients with psoriatic arthritis. J Rheumatol. 2015;42:479-488.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Antares Pharma, Inc. Otrexup PFS (methotrexate) [package insert]. US Food and Drug Administration website. Revised June 2019. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/204824s009lbl.pdf
- David M, Hodak E, Lowe NJ. Adverse effects of retinoids. Med Toxicol Adverse Drug Exp. 1988;3:273-288.
- Stiefel Laboratories, Inc. Soriatane (acitretin) [package insert]. US Food and Drug Administration website. Revised September 2017. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/019821s028lbl.pdf
- Celgene Corporation. Otezla (apremilast) [package insert]. US Food and Drug Administration website. Revised March 2014. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205437s000lbl.pdf
- Ghanem ME, El-Baghdadi LA, Badawy AM, et al. Pregnancy outcome after renal allograft transplantation: 15 years experience. Eur J Obstet Gynecol Reprod Biol. 2005;121:178-181.
- Zerilli T, Ocheretyaner E. Apremilast (Otezla): A new oral treatment for adults with psoriasis and psoriatic arthritis. P T. 2015;40:495-500.
- Kivity S, Zafrir Y, Loebstein R, et al. Clinical characteristics and risk factors for low dose methotrexate toxicity: a cohort of 28 patients. Autoimmun Rev. 2014;13:1109-1113.
- Boffa MJ, Chalmers RJ. Methotrexate for psoriasis. Clin Exp Dermatol. 1996;21:399-408.
- Rosenberg P, Urwitz H, Johannesson A, et al. Psoriasis patients with diabetes type 2 are at high risk of developing liver fibrosis during methotrexate treatment. J Hepatol. 2007;46:1111-1118.
- Novartis Pharmaceuticals Corporation. Sandimmune (cyclosporine) [package insert]. US Food and Drug Administration website. Published 2015. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/050573s041,050574s051,050625s055lbl.pdf
- Maza A, Montaudie H, Sbidian E, et al. Oral cyclosporin in psoriasis: a systematic review on treatment modalities, risk of kidney toxicity and evidence for use in non-plaque psoriasis. J Eur Acad Dermatol Venereol. 2011;25(suppl 2):19-27.
- Yamauchi PS, Rizk D, Kormilli T, et al. Systemic retinoids. In: Weinstein GD, Gottlieb AB, eds. Therapy of Moderate-to-Severe Psoriasis. Marcel Dekker; 2003:137-150.
- van Ditzhuijsen TJ, van Haelst UJ, van Dooren-Greebe RJ, et al. Severe hepatotoxic reaction with progression to cirrhosis after use of a novel retinoid (acitretin). J Hepatol. 1990;11:185-188.
- AbuHilal M, Walsh S, Shear N. Use of apremilast in combination with other therapies for treatment of chronic plaque psoriasis: a retrospective study. J Cutan Med Surg. 2016;20:313-316.
- Gottlieb AB, Langley RG, Strober BE, et al. A randomized, double-blind, placebo-controlled study to evaluate the addition of methotrexate to etanercept in patients with moderate to severe plaque psoriasis. Br J Dermatol. 2012;167:649-657.
- Cronstein BN. Methotrexate BAFFles anti-drug antibodies. Nat Rev Rheumatol. 2018;14:505-506.
- Lebwohl M, Drake L, Menter A, et al. Consensus conference: acitretin in combination with UVB or PUVA in the treatment of psoriasis. J Am Acad Dermatol. 2001;45:544-553.
- Nijsten TE, Stern RS. Oral retinoid use reduces cutaneous squamous cell carcinoma risk in patients with psoriasis treated with psoralen-UVA: a nested cohort study. J Am Acad Dermatol. 2003;49:644-650.
- Calzavara-Pinton P, Leone G, Venturini M, et al. A comparative non randomized study of narrow-band (NB) (312 +/- 2 nm) UVB phototherapy versus sequential therapy with oral administration of low-dose cyclosporin A and NB-UVB phototherapy in patients with severe psoriasis vulgaris. Eur J Dermatol. 2005;15:470-473.
Practice Points
- Systemic nonbiologic therapies are effective treatments for adults with psoriasis. The benefits of these treatments include ease of administration and the ability to control widespread disease.
- When selecting a therapy, a thorough evaluation of patient characteristics and commitment to lifestyle adjustments is necessary, including careful consideration in women of childbearing potential and those with plans of starting a family.
- Regular drug monitoring and patient follow-up is crucial to ensure safe dosing adjustments and to mitigate potential adverse effects.
Dermatologists as Social Media Contributors During the COVID-19 Pandemic
On December 31, 2019, cases of a severe pneumonia in patients in Wuhan, Hubei Province, China, were reported to the World Health Organization.1,2 The novel coronavirus—severe acute respiratory syndrome coronavirus 2—was identified, and the coronavirus disease 2019 (COVID-19) became a public health emergency of international concern.1 In March 2020, the World Health Organization officially characterized COVID-19 as a pandemic.3 As of October 2020, more than 42.3 million cases and 1.1 million deaths from COVID-19 have been confirmed worldwide.4
As more understanding of severe acute respiratory syndrome coronavirus 2 develops, various cutaneous manifestations of COVID-19 are being uncovered.5 The most common cutaneous manifestations of COVID-19 reported in the literature are maculopapular or morbilliform exanthem (36.1% of cutaneous manifestations), papulovesicular rash (34.7%), painful acral red purple papules (15.3%), urticaria (9.7%), livedo reticularis lesions (2.8%), and petechiae (1.4%).5
Interestingly, a series of unique cases was identified in April 2020 by a group of dermatologists in Spain. Most patients were children (median age, 13 years) or young adults (median age, 31 years; average age, 36 years; adult age range, 18–91 years).1 Reporting on a representative sample of 6 patients in that series, the group noted that lesions, initially reddish, papular, and resembling chilblains (pernio), progressively became purpuric and flattened in the course of 1 week. Although the lesions presented with some referred discomfort or pain with palpation, they were not highly symptomatic, and no signs of ischemia or Raynaud syndrome were identified. Over time, lesions self-resolved without intervention. Most patients also did not present with what are considered classic COVID-19 signs or symptoms. Only the oldest patient (aged 91 years) presented with a notable respiratory condition; the remaining patients generally were in good health.1 Dermatologists in Italy, France, and the United States also have witnessed these COVID-19–associated cutaneous manifestations.
Scientific understanding of COVID-19 and its associated dermatologic symptoms is evolving. Attention has turned to social media to inform and provide possible health solutions during this unprecedented medical crisis.6 Strict physical distancing measures have made patients and providers alike reliant on global digital social networks, such as Instagram, Twitter, and Facebook, to facilitate information sharing about COVID-19.7 The abundance of nonexpert advice and misinformation on social media makes communication of unbiased expert information difficult.8,9 Furthermore, there is a need for dermatologists to provide medical information to patients regarding COVID-19, such as dermatologic manifestations, and clear guidance on immunobiologic or systemic medications during this unprecedented time.9
In recent years, dermatologists have established a growing presence on social media, with many recognized as social media influencers with the ability to affect patients’ health-related behavior.10 Social media frequently has been used by patients to solicit advice regarding skin concerns.9,10 Many individuals, in fact, never see a physician after consulting social media for medical concerns or professional advice.9
In addition, as of March 2020, more than 61% of health care workers were found to use social media as a source of COVID-19–related information.11 Therefore, dermatologists should utilize social media as a platform to share evidence-based information with the public and other health care workers.
Through social media, dermatologists can post high-quality images with clear descriptions to fully characterize skin manifestations in patients with COVID-19. The process of capturing and posting images to the virtual world using a smartphone allows practitioners to gain advice from peers and consultants, share findings with colleagues, and inform the public.12 Social media posts of many deidentified clinical images of rashes in COVID-19–infected patients already have enabled rapid recognition of skin signs by dermatologists.13
Social media sites also are resources where organizations can post updated, evidence-based findings from academic journals. For example, the American Academy of Dermatology and its official journal, the Journal of the American Academy of Dermatology, had more than 22,000 and 27,000 Instagram followers, respectively, as of a March 2020 analysis.14 Recent online forums and social media posts contain accessible, graphical, patient-friendly images and information on evidence-based treatments for skin disease during the COVID-19 pandemic.13
We should consider initiatives that empower dermatologists to use social media to post unbiased, evidence-based information regarding manifestations of COVID-19 and guidelines for treatment of skin disease during this medical crisis. We hope that dermatologists will help lead the global response to the COVID-19 pandemic and contribute to the evolving knowledge base by characterizing COVID-19–related rashes, understanding their implications, and determining the best evidence for treatment.
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743.
- Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA. 2020;323:709-710.
- World Health Organization. Coronavirus disease (COVID-19) Situation Report – 133. WHO Website. June 1, 2020. www.who.int/docs/default-source/coronaviruse/situation-reports/20200601-covid-19-sitrep-133.pdf?sfvrsn=9a56f2ac_4. Accessed October 14, 2020.
- COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at John Hopkins University. John Hopkins Coronavirus Resource Center website. https://coronavirus.jhu.edu/map.html. Accessed October 24, 2020.
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatolog Sci. 2020;98:75-81.
- Kapoor A, Guha S, Kanti Das M, et al. Digital healthcare: the only solution for better healthcare during COVID-19 pandemic? Indian Heart J. 2020;72:61-64.
- Limaye RJ, Sauer M, Ali J, et al. Building trust while influencing online COVID-19 content in the social media world. Lancet Digit Health. 2020;2:E277-E278.
- Chawla S. COVID-19: challenges and opportunities for dermatology response. J Dermatolog Treat. 2020;31:326-326.
- Schoenberg E, Shalabi D, Wang JV, et al. Public social media consultations for dermatologic conditions: an online survey. Dermatol Online J. 2020;26:6.
- DeBord LC, Patel V, Braun TL, et al. Social media in dermatology: clinical relevance, academic value, and trends across platforms. J Dermatolog Treat. 2019;30:511-518.
- Bhagavathula AS, Aldhaleei WA, Rahmani J, et al. Knowledge and perceptions of COVID-19 among health care workers: cross-sectional study. JMIR Public Health Surveill. 2020;6:E19160.
- Ashique KT, Kaliyadan F, Aurangabadkar SJ. Clinical photography in dermatology using smartphones: an overview. Indian Dermatol Online J. 2015;6:158-163.
- Madigan LM, Micheletti RG, Shinkai K. How dermatologists can learn and contribute at the leading edge of the COVID-19 global pandemic. JAMA Dermatol. 2020;156:733-734.
- Guzman AK, Barbieri JS. Response to: “Dermatologists in social media: a study on top influencers, posts, and user engagement” [published online April 20, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.03.118.
On December 31, 2019, cases of a severe pneumonia in patients in Wuhan, Hubei Province, China, were reported to the World Health Organization.1,2 The novel coronavirus—severe acute respiratory syndrome coronavirus 2—was identified, and the coronavirus disease 2019 (COVID-19) became a public health emergency of international concern.1 In March 2020, the World Health Organization officially characterized COVID-19 as a pandemic.3 As of October 2020, more than 42.3 million cases and 1.1 million deaths from COVID-19 have been confirmed worldwide.4
As more understanding of severe acute respiratory syndrome coronavirus 2 develops, various cutaneous manifestations of COVID-19 are being uncovered.5 The most common cutaneous manifestations of COVID-19 reported in the literature are maculopapular or morbilliform exanthem (36.1% of cutaneous manifestations), papulovesicular rash (34.7%), painful acral red purple papules (15.3%), urticaria (9.7%), livedo reticularis lesions (2.8%), and petechiae (1.4%).5
Interestingly, a series of unique cases was identified in April 2020 by a group of dermatologists in Spain. Most patients were children (median age, 13 years) or young adults (median age, 31 years; average age, 36 years; adult age range, 18–91 years).1 Reporting on a representative sample of 6 patients in that series, the group noted that lesions, initially reddish, papular, and resembling chilblains (pernio), progressively became purpuric and flattened in the course of 1 week. Although the lesions presented with some referred discomfort or pain with palpation, they were not highly symptomatic, and no signs of ischemia or Raynaud syndrome were identified. Over time, lesions self-resolved without intervention. Most patients also did not present with what are considered classic COVID-19 signs or symptoms. Only the oldest patient (aged 91 years) presented with a notable respiratory condition; the remaining patients generally were in good health.1 Dermatologists in Italy, France, and the United States also have witnessed these COVID-19–associated cutaneous manifestations.
Scientific understanding of COVID-19 and its associated dermatologic symptoms is evolving. Attention has turned to social media to inform and provide possible health solutions during this unprecedented medical crisis.6 Strict physical distancing measures have made patients and providers alike reliant on global digital social networks, such as Instagram, Twitter, and Facebook, to facilitate information sharing about COVID-19.7 The abundance of nonexpert advice and misinformation on social media makes communication of unbiased expert information difficult.8,9 Furthermore, there is a need for dermatologists to provide medical information to patients regarding COVID-19, such as dermatologic manifestations, and clear guidance on immunobiologic or systemic medications during this unprecedented time.9
In recent years, dermatologists have established a growing presence on social media, with many recognized as social media influencers with the ability to affect patients’ health-related behavior.10 Social media frequently has been used by patients to solicit advice regarding skin concerns.9,10 Many individuals, in fact, never see a physician after consulting social media for medical concerns or professional advice.9
In addition, as of March 2020, more than 61% of health care workers were found to use social media as a source of COVID-19–related information.11 Therefore, dermatologists should utilize social media as a platform to share evidence-based information with the public and other health care workers.
Through social media, dermatologists can post high-quality images with clear descriptions to fully characterize skin manifestations in patients with COVID-19. The process of capturing and posting images to the virtual world using a smartphone allows practitioners to gain advice from peers and consultants, share findings with colleagues, and inform the public.12 Social media posts of many deidentified clinical images of rashes in COVID-19–infected patients already have enabled rapid recognition of skin signs by dermatologists.13
Social media sites also are resources where organizations can post updated, evidence-based findings from academic journals. For example, the American Academy of Dermatology and its official journal, the Journal of the American Academy of Dermatology, had more than 22,000 and 27,000 Instagram followers, respectively, as of a March 2020 analysis.14 Recent online forums and social media posts contain accessible, graphical, patient-friendly images and information on evidence-based treatments for skin disease during the COVID-19 pandemic.13
We should consider initiatives that empower dermatologists to use social media to post unbiased, evidence-based information regarding manifestations of COVID-19 and guidelines for treatment of skin disease during this medical crisis. We hope that dermatologists will help lead the global response to the COVID-19 pandemic and contribute to the evolving knowledge base by characterizing COVID-19–related rashes, understanding their implications, and determining the best evidence for treatment.
On December 31, 2019, cases of a severe pneumonia in patients in Wuhan, Hubei Province, China, were reported to the World Health Organization.1,2 The novel coronavirus—severe acute respiratory syndrome coronavirus 2—was identified, and the coronavirus disease 2019 (COVID-19) became a public health emergency of international concern.1 In March 2020, the World Health Organization officially characterized COVID-19 as a pandemic.3 As of October 2020, more than 42.3 million cases and 1.1 million deaths from COVID-19 have been confirmed worldwide.4
As more understanding of severe acute respiratory syndrome coronavirus 2 develops, various cutaneous manifestations of COVID-19 are being uncovered.5 The most common cutaneous manifestations of COVID-19 reported in the literature are maculopapular or morbilliform exanthem (36.1% of cutaneous manifestations), papulovesicular rash (34.7%), painful acral red purple papules (15.3%), urticaria (9.7%), livedo reticularis lesions (2.8%), and petechiae (1.4%).5
Interestingly, a series of unique cases was identified in April 2020 by a group of dermatologists in Spain. Most patients were children (median age, 13 years) or young adults (median age, 31 years; average age, 36 years; adult age range, 18–91 years).1 Reporting on a representative sample of 6 patients in that series, the group noted that lesions, initially reddish, papular, and resembling chilblains (pernio), progressively became purpuric and flattened in the course of 1 week. Although the lesions presented with some referred discomfort or pain with palpation, they were not highly symptomatic, and no signs of ischemia or Raynaud syndrome were identified. Over time, lesions self-resolved without intervention. Most patients also did not present with what are considered classic COVID-19 signs or symptoms. Only the oldest patient (aged 91 years) presented with a notable respiratory condition; the remaining patients generally were in good health.1 Dermatologists in Italy, France, and the United States also have witnessed these COVID-19–associated cutaneous manifestations.
Scientific understanding of COVID-19 and its associated dermatologic symptoms is evolving. Attention has turned to social media to inform and provide possible health solutions during this unprecedented medical crisis.6 Strict physical distancing measures have made patients and providers alike reliant on global digital social networks, such as Instagram, Twitter, and Facebook, to facilitate information sharing about COVID-19.7 The abundance of nonexpert advice and misinformation on social media makes communication of unbiased expert information difficult.8,9 Furthermore, there is a need for dermatologists to provide medical information to patients regarding COVID-19, such as dermatologic manifestations, and clear guidance on immunobiologic or systemic medications during this unprecedented time.9
In recent years, dermatologists have established a growing presence on social media, with many recognized as social media influencers with the ability to affect patients’ health-related behavior.10 Social media frequently has been used by patients to solicit advice regarding skin concerns.9,10 Many individuals, in fact, never see a physician after consulting social media for medical concerns or professional advice.9
In addition, as of March 2020, more than 61% of health care workers were found to use social media as a source of COVID-19–related information.11 Therefore, dermatologists should utilize social media as a platform to share evidence-based information with the public and other health care workers.
Through social media, dermatologists can post high-quality images with clear descriptions to fully characterize skin manifestations in patients with COVID-19. The process of capturing and posting images to the virtual world using a smartphone allows practitioners to gain advice from peers and consultants, share findings with colleagues, and inform the public.12 Social media posts of many deidentified clinical images of rashes in COVID-19–infected patients already have enabled rapid recognition of skin signs by dermatologists.13
Social media sites also are resources where organizations can post updated, evidence-based findings from academic journals. For example, the American Academy of Dermatology and its official journal, the Journal of the American Academy of Dermatology, had more than 22,000 and 27,000 Instagram followers, respectively, as of a March 2020 analysis.14 Recent online forums and social media posts contain accessible, graphical, patient-friendly images and information on evidence-based treatments for skin disease during the COVID-19 pandemic.13
We should consider initiatives that empower dermatologists to use social media to post unbiased, evidence-based information regarding manifestations of COVID-19 and guidelines for treatment of skin disease during this medical crisis. We hope that dermatologists will help lead the global response to the COVID-19 pandemic and contribute to the evolving knowledge base by characterizing COVID-19–related rashes, understanding their implications, and determining the best evidence for treatment.
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743.
- Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA. 2020;323:709-710.
- World Health Organization. Coronavirus disease (COVID-19) Situation Report – 133. WHO Website. June 1, 2020. www.who.int/docs/default-source/coronaviruse/situation-reports/20200601-covid-19-sitrep-133.pdf?sfvrsn=9a56f2ac_4. Accessed October 14, 2020.
- COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at John Hopkins University. John Hopkins Coronavirus Resource Center website. https://coronavirus.jhu.edu/map.html. Accessed October 24, 2020.
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatolog Sci. 2020;98:75-81.
- Kapoor A, Guha S, Kanti Das M, et al. Digital healthcare: the only solution for better healthcare during COVID-19 pandemic? Indian Heart J. 2020;72:61-64.
- Limaye RJ, Sauer M, Ali J, et al. Building trust while influencing online COVID-19 content in the social media world. Lancet Digit Health. 2020;2:E277-E278.
- Chawla S. COVID-19: challenges and opportunities for dermatology response. J Dermatolog Treat. 2020;31:326-326.
- Schoenberg E, Shalabi D, Wang JV, et al. Public social media consultations for dermatologic conditions: an online survey. Dermatol Online J. 2020;26:6.
- DeBord LC, Patel V, Braun TL, et al. Social media in dermatology: clinical relevance, academic value, and trends across platforms. J Dermatolog Treat. 2019;30:511-518.
- Bhagavathula AS, Aldhaleei WA, Rahmani J, et al. Knowledge and perceptions of COVID-19 among health care workers: cross-sectional study. JMIR Public Health Surveill. 2020;6:E19160.
- Ashique KT, Kaliyadan F, Aurangabadkar SJ. Clinical photography in dermatology using smartphones: an overview. Indian Dermatol Online J. 2015;6:158-163.
- Madigan LM, Micheletti RG, Shinkai K. How dermatologists can learn and contribute at the leading edge of the COVID-19 global pandemic. JAMA Dermatol. 2020;156:733-734.
- Guzman AK, Barbieri JS. Response to: “Dermatologists in social media: a study on top influencers, posts, and user engagement” [published online April 20, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.03.118.
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743.
- Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA. 2020;323:709-710.
- World Health Organization. Coronavirus disease (COVID-19) Situation Report – 133. WHO Website. June 1, 2020. www.who.int/docs/default-source/coronaviruse/situation-reports/20200601-covid-19-sitrep-133.pdf?sfvrsn=9a56f2ac_4. Accessed October 14, 2020.
- COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at John Hopkins University. John Hopkins Coronavirus Resource Center website. https://coronavirus.jhu.edu/map.html. Accessed October 24, 2020.
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatolog Sci. 2020;98:75-81.
- Kapoor A, Guha S, Kanti Das M, et al. Digital healthcare: the only solution for better healthcare during COVID-19 pandemic? Indian Heart J. 2020;72:61-64.
- Limaye RJ, Sauer M, Ali J, et al. Building trust while influencing online COVID-19 content in the social media world. Lancet Digit Health. 2020;2:E277-E278.
- Chawla S. COVID-19: challenges and opportunities for dermatology response. J Dermatolog Treat. 2020;31:326-326.
- Schoenberg E, Shalabi D, Wang JV, et al. Public social media consultations for dermatologic conditions: an online survey. Dermatol Online J. 2020;26:6.
- DeBord LC, Patel V, Braun TL, et al. Social media in dermatology: clinical relevance, academic value, and trends across platforms. J Dermatolog Treat. 2019;30:511-518.
- Bhagavathula AS, Aldhaleei WA, Rahmani J, et al. Knowledge and perceptions of COVID-19 among health care workers: cross-sectional study. JMIR Public Health Surveill. 2020;6:E19160.
- Ashique KT, Kaliyadan F, Aurangabadkar SJ. Clinical photography in dermatology using smartphones: an overview. Indian Dermatol Online J. 2015;6:158-163.
- Madigan LM, Micheletti RG, Shinkai K. How dermatologists can learn and contribute at the leading edge of the COVID-19 global pandemic. JAMA Dermatol. 2020;156:733-734.
- Guzman AK, Barbieri JS. Response to: “Dermatologists in social media: a study on top influencers, posts, and user engagement” [published online April 20, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.03.118.
Practice Points
- With the coronavirus disease 2019 (COVID-19) pandemic, strict physical distancing measures have made patients and providers alike reliant on global digital social networks such as Instagram, Twitter, and Facebook to facilitate information sharing about COVID-19.
- Dermatologists should utilize social media as a platform to post unbiased, evidence-based information regarding manifestations of COVID-19 and guidelines for treatment of skin disease during the global pandemic.
