User login
Hyaluronidase for Skin Necrosis Induced by Amiodarone
To the Editor:
Amiodarone is an oral or intravenous (IV) drug commonly used to treat supraventricular and ventricular arrhythmia as well as atrial fibrillation.1 Adverse drug reactions associated with the use of amiodarone include pulmonary, gastrointestinal, thyroid, ocular, neurologic, and cutaneous reactions.1 Long-term use of amiodarone—typically more than 4 months—can lead to slate-gray skin discoloration and photosensitivity, both of which can be reversed with drug withdrawal.2,3 Phlebitis also has been described in less than 3% of patients who receive peripheral IV administration of amiodarone.4
Amiodarone-induced skin necrosis due to extravasation is a rare complication of this antiarrhythmic medication, with only 3 reported cases in the literature according to a PubMed search of articles indexed for MEDLINE using the search terms amiodarone and skin and (necrosis or ischemia or extravasation or reaction).5–7 Although hyaluronidase is a known therapy for extravasation of fluids, including parenteral nutrition and chemotherapy, its use for the treatment of extravasation from amiodarone is not well documented.6 We report a case of skin necrosis of the left dorsal forearm and the left dorsal and ventral hand following infusion of amiodarone through a peripheral IV line, which was treated with injections of hyaluronidase.
A 77-year-old man was admitted to the emergency department for sepsis secondary to cholangitis in the setting of an obstructive gallbladder stone. His medical history was notable for multivessel coronary artery disease and atrial flutter treated with ablation. One day after admission, endoscopic retrograde cholangiopancreatography was attempted and aborted due to atrial fibrillation with rapid ventricular response. A second endoscopic retrograde cholangiopancreatography attempt was made 4 days later, during which the patient underwent cardiac arrest. During this event, amiodarone was administered in a 200-mL solution (1.8 mg/mL) in 5% dextrose through a peripheral IV line in the left forearm. The patient was stabilized and transferred to the intensive care unit.
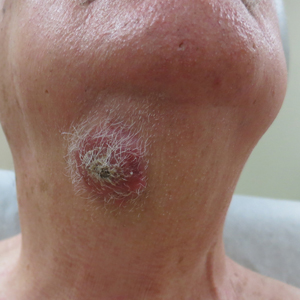
Twenty-four hours after amiodarone administration, erythema was noted on the left dorsal forearm. Within hours, the digits of the hand became a dark, dusky color, which spread to involve the forearm. Surgical debridement was not deemed necessary; the left arm was elevated, and warm compresses were applied regularly. Within the next week, the skin of the left hand and dorsal forearm had progressively worsened and took on a well-demarcated, dusky blue hue surrounded by an erythematous border involving the proximal forearm and upper arm (Figure 1A). The skin was fragile and had overlying bullae (Figure 1B).
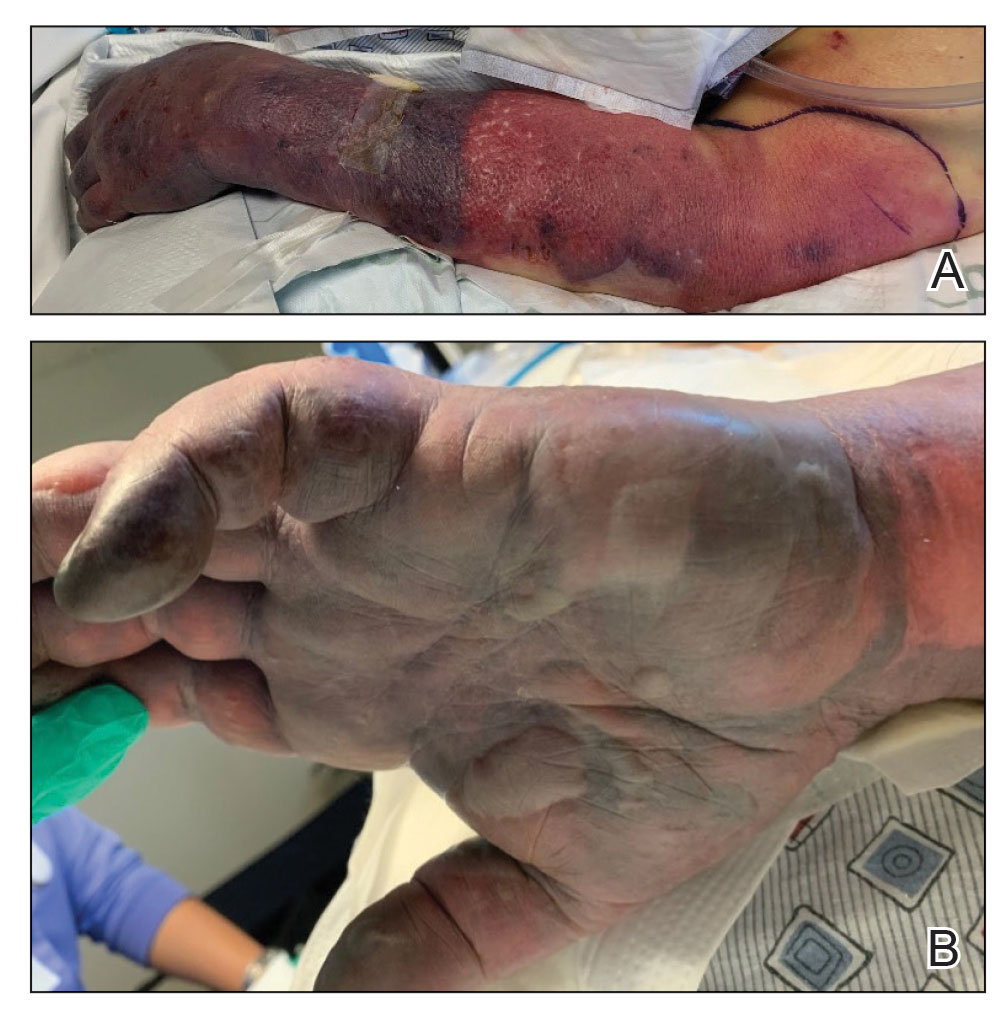
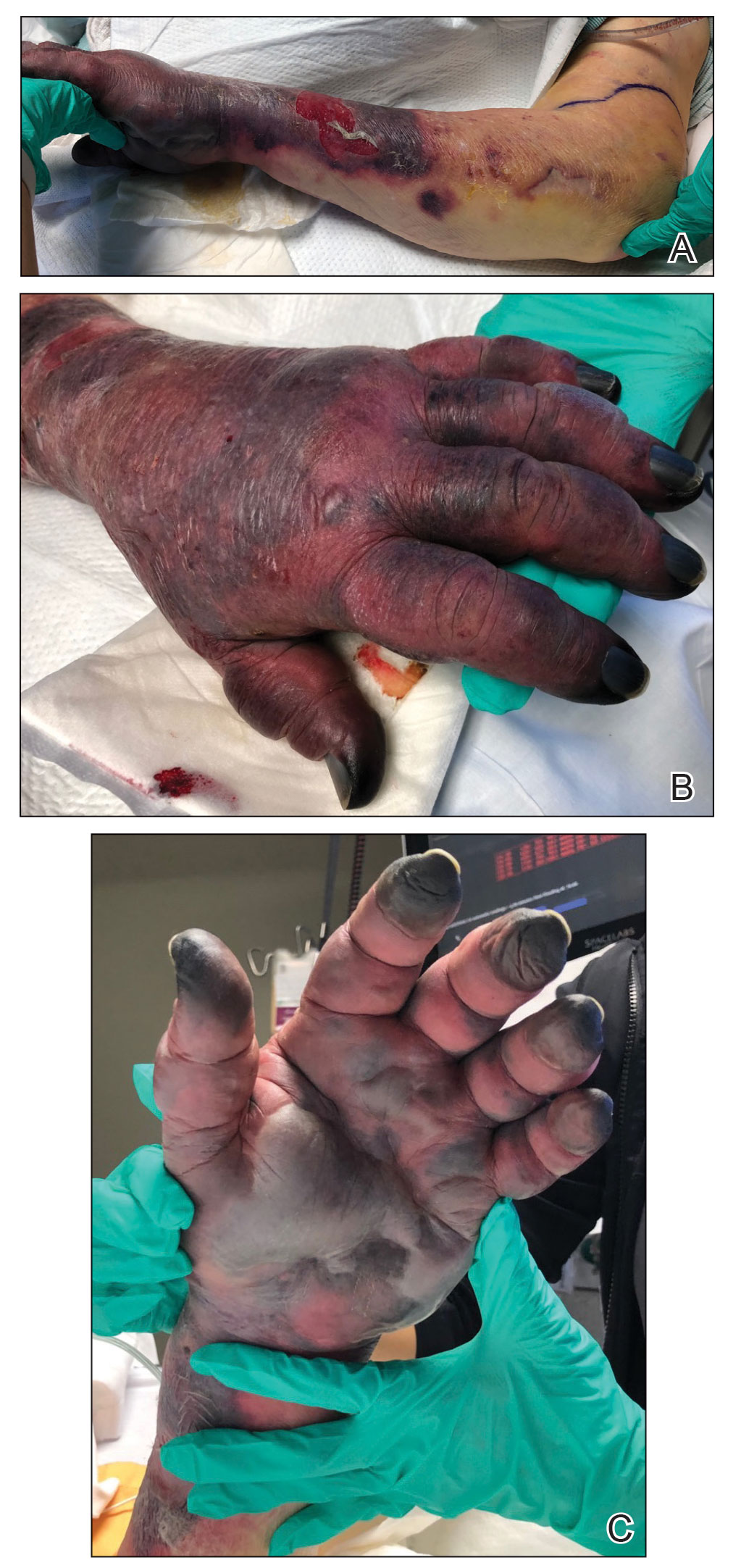
Hyaluronidase (1000 U) was injected into the surrounding areas of erythema, which resolved from the left proximal forearm to the elbow within 2 days after injection (Figure 2). The dusky violaceous patches were persistent, and the necrotic bullae were unchanged. Hyaluronidase (1000 U) was injected into necrotic skin of the left dorsal forearm and dorsal and ventral hand. No improvement was noted on subsequent evaluations of this area. While still an inpatient, he received wound care and twice-daily Doppler ultrasounds in the areas of necrosis. The patient lost sensation in the left hand with increased soft tissue necrosis and developed an eschar on the left dorsal forearm. Due to the progressive loss of function and necrosis, a partial forearm amputation was performed that healed well, and the patient experienced improvement in range of motion of the left upper extremity.
Well-known adverse reactions of amiodarone treatment include pulmonary fibrosis, hepatic dysfunction, hypothyroidism and hyperthyroidism, peripheral neuropathy, and corneal deposits.1 Cutaneous adverse reactions include photosensitivity (phototoxic and photoallergic reactions), hyperpigmentation, pseudoporphyria, and linear IgA bullous dermatosis. Less commonly, it also can cause urticaria, pruritus, erythema nodosum, purpura, and toxic epidermal necrolysis.3 Amiodarone-induced skin necrosis is rare, first described by Russell and Saltissi5 in 2006 in a 60-year-old man who developed dark discoloration and edema of the forearm 24 hours after initiation of an amiodarone peripheral IV. The patient was treated with hot or cold packs and steroid cream per the pharmaceutical company’s recommendations; however, patient outcomes were not discussed.5 A 77-year-old man who received subcutaneous amiodarone due to misplaced vascular access developed edema and bullae of the forearm followed by tissue necrosis, resulting in notably reduced mobility.6 Fox et al7 described a 60-year-old man who developed atrial fibrillation after emergent spinal fusion and laminectomy. He received intradermal hyaluronidase administration within 24 hours of developing severe pain from extravasation induced by amiodarone with no adverse outcomes and full recovery.7
There are numerous properties of amiodarone that may have resulted in the skin necrosis seen in these cases. The acidic pH (3.5–4.5) of amiodarone can contribute to coagulative necrosis, cellular desiccation, eschar formation, and edema.8 It also can contain additives such as polysorbate and benzyl alcohol, which may contribute to the drug’s vesicant properties.9
Current recommendations for IV administration of amiodarone include delivery through a central vein with high concentrations (>2 mg/mL) because peripheral infusion is slower and may cause phlebitis.4 In-line filters also may be a potential method of preventing phlebitis with peripheral IV administration of amiodarone.10 Extravasation of amiodarone can be treated nonpharmacologically with limb elevation and warm compresses, as these methods may promote vasodilation and enhance drug removal.5-7 However, when extravasation leads to progressive erythema and skin necrosis or is refractory to these therapies, intradermal injection of hyaluronidase should be considered. Hyaluronidase mediates the degradation of hyaluronic acid in the extracellular matrix, allowing for increased permeability of injected fluids into tissues and diluting the concentration of toxins at the site of exposure.9,11 It has been used to treat extravasation of fluids such as parenteral nutrition, electrolyte infusion, antibiotics, aminophylline, mannitol, and chemotherapy.11 Although hyaluronidase has been recognized as therapeutic for extravasation, there is no established consistent dosing or proper technique. In the setting of infiltration of chemotherapy, doses of hyaluronidase ranging from 150 to 1500 U/mL can be subcutaneously or intradermally injected into the site within 1 hour of extravasation. Side effects of using hyaluronidase are rare, including local pruritus, allergic reactions, urticaria, and angioedema.12
The patient described by Fox et al7 who fully recovered from amiodarone extravasation after hyaluronidase injections likely benefited from quick intervention, as he received amiodarone within 24 hours of the care team identifying initial erythema. Although our patient did have improvement of the areas of erythema on the forearm, evidence of skin and subcutaneous tissue necrosis on the left hand and proximal forearm was already apparent and not reversible, most likely caused by late intervention of intradermal hyaluronidase almost a week after the extravasation event. It is important to identify amiodarone as the source of extravasation and administer intradermal hyaluronidase in a timely fashion for extravasation refractory to conventional measurements to prevent progression to severe tissue damage.
Our case draws attention to the risk for skin necrosis with peripheral IV administration of amiodarone. Interventions include limb elevation, warm compresses, and consideration of intradermal hyaluronidase within 24 hours of extravasation, as this may reduce the severity of subsequent tissue damage with minimal side effects.
- Epstein AE, Olshansky B, Naccarelli GV, et al. Practical management guide for clinicians who treat patients with amiodarone. Am J Med. 2016;129:468-475. doi:10.1016/j.amjmed.2015.08.039
- Harris L, McKenna WJ, Rowland E, et al. Side effects of long-term amiodarone therapy. Circulation. 1983;67:45-51. doi:10.1161/01.cir.67.1.45
- Jaworski K, Walecka I, Rudnicka L, et al. Cutaneous adverse reactions of amiodarone. Med Sci Monit. 2014;20:2369-2372. doi:10.12659/MSM.890881
- Kowey Peter R, Marinchak Roger A, Rials Seth J, et al. Intravenous amiodarone. J Am Coll Cardiol. 1997;29:1190-1198. doi:10.1016/S0735-1097(97)00069-7
- Russell SJ, Saltissi S. Amiodarone induced skin necrosis. Heart. 2006;92:1395. doi:10.1136/hrt.2005.086157
- Grove EL. Skin necrosis and consequences of accidental subcutaneous administration of amiodarone. Ugeskr Laeger. 2015;177:V66928.
- Fox AN, Villanueva R, Miller JL. Management of amiodarone extravasation with intradermal hyaluronidase. Am J Health Syst Pharm. 2017;74:1545-1548. doi:10.2146/ajhp160737
- Reynolds PM, MacLaren R, Mueller SW, et al. Management of extravasation injuries: a focused evaluation of noncytotoxic medications. Pharmacotherapy. 2014;34:617-632. doi:https://doi.org/10.1002/phar.1396
- Le A, Patel S. Extravasation of noncytotoxic drugs: a review of the literature. Ann Pharmacother. 2014;48:870-886. doi:10.1177/1060028014527820
- Slim AM, Roth JE, Duffy B, et al. The incidence of phlebitis with intravenous amiodarone at guideline dose recommendations. Mil Med. 2007;172:1279-1283.
- Girish KS, Kemparaju K. The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life Sci. 2007;80:1921-1943. doi:10.1016/j.lfs.2007.02.037
- Jung H. Hyaluronidase: an overview of its properties, applications, and side effects. Arch Plast Surg. 2020;47:297-300. doi:10.5999/aps.2020.00752
To the Editor:
Amiodarone is an oral or intravenous (IV) drug commonly used to treat supraventricular and ventricular arrhythmia as well as atrial fibrillation.1 Adverse drug reactions associated with the use of amiodarone include pulmonary, gastrointestinal, thyroid, ocular, neurologic, and cutaneous reactions.1 Long-term use of amiodarone—typically more than 4 months—can lead to slate-gray skin discoloration and photosensitivity, both of which can be reversed with drug withdrawal.2,3 Phlebitis also has been described in less than 3% of patients who receive peripheral IV administration of amiodarone.4
Amiodarone-induced skin necrosis due to extravasation is a rare complication of this antiarrhythmic medication, with only 3 reported cases in the literature according to a PubMed search of articles indexed for MEDLINE using the search terms amiodarone and skin and (necrosis or ischemia or extravasation or reaction).5–7 Although hyaluronidase is a known therapy for extravasation of fluids, including parenteral nutrition and chemotherapy, its use for the treatment of extravasation from amiodarone is not well documented.6 We report a case of skin necrosis of the left dorsal forearm and the left dorsal and ventral hand following infusion of amiodarone through a peripheral IV line, which was treated with injections of hyaluronidase.
A 77-year-old man was admitted to the emergency department for sepsis secondary to cholangitis in the setting of an obstructive gallbladder stone. His medical history was notable for multivessel coronary artery disease and atrial flutter treated with ablation. One day after admission, endoscopic retrograde cholangiopancreatography was attempted and aborted due to atrial fibrillation with rapid ventricular response. A second endoscopic retrograde cholangiopancreatography attempt was made 4 days later, during which the patient underwent cardiac arrest. During this event, amiodarone was administered in a 200-mL solution (1.8 mg/mL) in 5% dextrose through a peripheral IV line in the left forearm. The patient was stabilized and transferred to the intensive care unit.
Twenty-four hours after amiodarone administration, erythema was noted on the left dorsal forearm. Within hours, the digits of the hand became a dark, dusky color, which spread to involve the forearm. Surgical debridement was not deemed necessary; the left arm was elevated, and warm compresses were applied regularly. Within the next week, the skin of the left hand and dorsal forearm had progressively worsened and took on a well-demarcated, dusky blue hue surrounded by an erythematous border involving the proximal forearm and upper arm (Figure 1A). The skin was fragile and had overlying bullae (Figure 1B).

Hyaluronidase (1000 U) was injected into the surrounding areas of erythema, which resolved from the left proximal forearm to the elbow within 2 days after injection (Figure 2). The dusky violaceous patches were persistent, and the necrotic bullae were unchanged. Hyaluronidase (1000 U) was injected into necrotic skin of the left dorsal forearm and dorsal and ventral hand. No improvement was noted on subsequent evaluations of this area. While still an inpatient, he received wound care and twice-daily Doppler ultrasounds in the areas of necrosis. The patient lost sensation in the left hand with increased soft tissue necrosis and developed an eschar on the left dorsal forearm. Due to the progressive loss of function and necrosis, a partial forearm amputation was performed that healed well, and the patient experienced improvement in range of motion of the left upper extremity.

Well-known adverse reactions of amiodarone treatment include pulmonary fibrosis, hepatic dysfunction, hypothyroidism and hyperthyroidism, peripheral neuropathy, and corneal deposits.1 Cutaneous adverse reactions include photosensitivity (phototoxic and photoallergic reactions), hyperpigmentation, pseudoporphyria, and linear IgA bullous dermatosis. Less commonly, it also can cause urticaria, pruritus, erythema nodosum, purpura, and toxic epidermal necrolysis.3 Amiodarone-induced skin necrosis is rare, first described by Russell and Saltissi5 in 2006 in a 60-year-old man who developed dark discoloration and edema of the forearm 24 hours after initiation of an amiodarone peripheral IV. The patient was treated with hot or cold packs and steroid cream per the pharmaceutical company’s recommendations; however, patient outcomes were not discussed.5 A 77-year-old man who received subcutaneous amiodarone due to misplaced vascular access developed edema and bullae of the forearm followed by tissue necrosis, resulting in notably reduced mobility.6 Fox et al7 described a 60-year-old man who developed atrial fibrillation after emergent spinal fusion and laminectomy. He received intradermal hyaluronidase administration within 24 hours of developing severe pain from extravasation induced by amiodarone with no adverse outcomes and full recovery.7
There are numerous properties of amiodarone that may have resulted in the skin necrosis seen in these cases. The acidic pH (3.5–4.5) of amiodarone can contribute to coagulative necrosis, cellular desiccation, eschar formation, and edema.8 It also can contain additives such as polysorbate and benzyl alcohol, which may contribute to the drug’s vesicant properties.9
Current recommendations for IV administration of amiodarone include delivery through a central vein with high concentrations (>2 mg/mL) because peripheral infusion is slower and may cause phlebitis.4 In-line filters also may be a potential method of preventing phlebitis with peripheral IV administration of amiodarone.10 Extravasation of amiodarone can be treated nonpharmacologically with limb elevation and warm compresses, as these methods may promote vasodilation and enhance drug removal.5-7 However, when extravasation leads to progressive erythema and skin necrosis or is refractory to these therapies, intradermal injection of hyaluronidase should be considered. Hyaluronidase mediates the degradation of hyaluronic acid in the extracellular matrix, allowing for increased permeability of injected fluids into tissues and diluting the concentration of toxins at the site of exposure.9,11 It has been used to treat extravasation of fluids such as parenteral nutrition, electrolyte infusion, antibiotics, aminophylline, mannitol, and chemotherapy.11 Although hyaluronidase has been recognized as therapeutic for extravasation, there is no established consistent dosing or proper technique. In the setting of infiltration of chemotherapy, doses of hyaluronidase ranging from 150 to 1500 U/mL can be subcutaneously or intradermally injected into the site within 1 hour of extravasation. Side effects of using hyaluronidase are rare, including local pruritus, allergic reactions, urticaria, and angioedema.12
The patient described by Fox et al7 who fully recovered from amiodarone extravasation after hyaluronidase injections likely benefited from quick intervention, as he received amiodarone within 24 hours of the care team identifying initial erythema. Although our patient did have improvement of the areas of erythema on the forearm, evidence of skin and subcutaneous tissue necrosis on the left hand and proximal forearm was already apparent and not reversible, most likely caused by late intervention of intradermal hyaluronidase almost a week after the extravasation event. It is important to identify amiodarone as the source of extravasation and administer intradermal hyaluronidase in a timely fashion for extravasation refractory to conventional measurements to prevent progression to severe tissue damage.
Our case draws attention to the risk for skin necrosis with peripheral IV administration of amiodarone. Interventions include limb elevation, warm compresses, and consideration of intradermal hyaluronidase within 24 hours of extravasation, as this may reduce the severity of subsequent tissue damage with minimal side effects.
To the Editor:
Amiodarone is an oral or intravenous (IV) drug commonly used to treat supraventricular and ventricular arrhythmia as well as atrial fibrillation.1 Adverse drug reactions associated with the use of amiodarone include pulmonary, gastrointestinal, thyroid, ocular, neurologic, and cutaneous reactions.1 Long-term use of amiodarone—typically more than 4 months—can lead to slate-gray skin discoloration and photosensitivity, both of which can be reversed with drug withdrawal.2,3 Phlebitis also has been described in less than 3% of patients who receive peripheral IV administration of amiodarone.4
Amiodarone-induced skin necrosis due to extravasation is a rare complication of this antiarrhythmic medication, with only 3 reported cases in the literature according to a PubMed search of articles indexed for MEDLINE using the search terms amiodarone and skin and (necrosis or ischemia or extravasation or reaction).5–7 Although hyaluronidase is a known therapy for extravasation of fluids, including parenteral nutrition and chemotherapy, its use for the treatment of extravasation from amiodarone is not well documented.6 We report a case of skin necrosis of the left dorsal forearm and the left dorsal and ventral hand following infusion of amiodarone through a peripheral IV line, which was treated with injections of hyaluronidase.
A 77-year-old man was admitted to the emergency department for sepsis secondary to cholangitis in the setting of an obstructive gallbladder stone. His medical history was notable for multivessel coronary artery disease and atrial flutter treated with ablation. One day after admission, endoscopic retrograde cholangiopancreatography was attempted and aborted due to atrial fibrillation with rapid ventricular response. A second endoscopic retrograde cholangiopancreatography attempt was made 4 days later, during which the patient underwent cardiac arrest. During this event, amiodarone was administered in a 200-mL solution (1.8 mg/mL) in 5% dextrose through a peripheral IV line in the left forearm. The patient was stabilized and transferred to the intensive care unit.
Twenty-four hours after amiodarone administration, erythema was noted on the left dorsal forearm. Within hours, the digits of the hand became a dark, dusky color, which spread to involve the forearm. Surgical debridement was not deemed necessary; the left arm was elevated, and warm compresses were applied regularly. Within the next week, the skin of the left hand and dorsal forearm had progressively worsened and took on a well-demarcated, dusky blue hue surrounded by an erythematous border involving the proximal forearm and upper arm (Figure 1A). The skin was fragile and had overlying bullae (Figure 1B).

Hyaluronidase (1000 U) was injected into the surrounding areas of erythema, which resolved from the left proximal forearm to the elbow within 2 days after injection (Figure 2). The dusky violaceous patches were persistent, and the necrotic bullae were unchanged. Hyaluronidase (1000 U) was injected into necrotic skin of the left dorsal forearm and dorsal and ventral hand. No improvement was noted on subsequent evaluations of this area. While still an inpatient, he received wound care and twice-daily Doppler ultrasounds in the areas of necrosis. The patient lost sensation in the left hand with increased soft tissue necrosis and developed an eschar on the left dorsal forearm. Due to the progressive loss of function and necrosis, a partial forearm amputation was performed that healed well, and the patient experienced improvement in range of motion of the left upper extremity.

Well-known adverse reactions of amiodarone treatment include pulmonary fibrosis, hepatic dysfunction, hypothyroidism and hyperthyroidism, peripheral neuropathy, and corneal deposits.1 Cutaneous adverse reactions include photosensitivity (phototoxic and photoallergic reactions), hyperpigmentation, pseudoporphyria, and linear IgA bullous dermatosis. Less commonly, it also can cause urticaria, pruritus, erythema nodosum, purpura, and toxic epidermal necrolysis.3 Amiodarone-induced skin necrosis is rare, first described by Russell and Saltissi5 in 2006 in a 60-year-old man who developed dark discoloration and edema of the forearm 24 hours after initiation of an amiodarone peripheral IV. The patient was treated with hot or cold packs and steroid cream per the pharmaceutical company’s recommendations; however, patient outcomes were not discussed.5 A 77-year-old man who received subcutaneous amiodarone due to misplaced vascular access developed edema and bullae of the forearm followed by tissue necrosis, resulting in notably reduced mobility.6 Fox et al7 described a 60-year-old man who developed atrial fibrillation after emergent spinal fusion and laminectomy. He received intradermal hyaluronidase administration within 24 hours of developing severe pain from extravasation induced by amiodarone with no adverse outcomes and full recovery.7
There are numerous properties of amiodarone that may have resulted in the skin necrosis seen in these cases. The acidic pH (3.5–4.5) of amiodarone can contribute to coagulative necrosis, cellular desiccation, eschar formation, and edema.8 It also can contain additives such as polysorbate and benzyl alcohol, which may contribute to the drug’s vesicant properties.9
Current recommendations for IV administration of amiodarone include delivery through a central vein with high concentrations (>2 mg/mL) because peripheral infusion is slower and may cause phlebitis.4 In-line filters also may be a potential method of preventing phlebitis with peripheral IV administration of amiodarone.10 Extravasation of amiodarone can be treated nonpharmacologically with limb elevation and warm compresses, as these methods may promote vasodilation and enhance drug removal.5-7 However, when extravasation leads to progressive erythema and skin necrosis or is refractory to these therapies, intradermal injection of hyaluronidase should be considered. Hyaluronidase mediates the degradation of hyaluronic acid in the extracellular matrix, allowing for increased permeability of injected fluids into tissues and diluting the concentration of toxins at the site of exposure.9,11 It has been used to treat extravasation of fluids such as parenteral nutrition, electrolyte infusion, antibiotics, aminophylline, mannitol, and chemotherapy.11 Although hyaluronidase has been recognized as therapeutic for extravasation, there is no established consistent dosing or proper technique. In the setting of infiltration of chemotherapy, doses of hyaluronidase ranging from 150 to 1500 U/mL can be subcutaneously or intradermally injected into the site within 1 hour of extravasation. Side effects of using hyaluronidase are rare, including local pruritus, allergic reactions, urticaria, and angioedema.12
The patient described by Fox et al7 who fully recovered from amiodarone extravasation after hyaluronidase injections likely benefited from quick intervention, as he received amiodarone within 24 hours of the care team identifying initial erythema. Although our patient did have improvement of the areas of erythema on the forearm, evidence of skin and subcutaneous tissue necrosis on the left hand and proximal forearm was already apparent and not reversible, most likely caused by late intervention of intradermal hyaluronidase almost a week after the extravasation event. It is important to identify amiodarone as the source of extravasation and administer intradermal hyaluronidase in a timely fashion for extravasation refractory to conventional measurements to prevent progression to severe tissue damage.
Our case draws attention to the risk for skin necrosis with peripheral IV administration of amiodarone. Interventions include limb elevation, warm compresses, and consideration of intradermal hyaluronidase within 24 hours of extravasation, as this may reduce the severity of subsequent tissue damage with minimal side effects.
- Epstein AE, Olshansky B, Naccarelli GV, et al. Practical management guide for clinicians who treat patients with amiodarone. Am J Med. 2016;129:468-475. doi:10.1016/j.amjmed.2015.08.039
- Harris L, McKenna WJ, Rowland E, et al. Side effects of long-term amiodarone therapy. Circulation. 1983;67:45-51. doi:10.1161/01.cir.67.1.45
- Jaworski K, Walecka I, Rudnicka L, et al. Cutaneous adverse reactions of amiodarone. Med Sci Monit. 2014;20:2369-2372. doi:10.12659/MSM.890881
- Kowey Peter R, Marinchak Roger A, Rials Seth J, et al. Intravenous amiodarone. J Am Coll Cardiol. 1997;29:1190-1198. doi:10.1016/S0735-1097(97)00069-7
- Russell SJ, Saltissi S. Amiodarone induced skin necrosis. Heart. 2006;92:1395. doi:10.1136/hrt.2005.086157
- Grove EL. Skin necrosis and consequences of accidental subcutaneous administration of amiodarone. Ugeskr Laeger. 2015;177:V66928.
- Fox AN, Villanueva R, Miller JL. Management of amiodarone extravasation with intradermal hyaluronidase. Am J Health Syst Pharm. 2017;74:1545-1548. doi:10.2146/ajhp160737
- Reynolds PM, MacLaren R, Mueller SW, et al. Management of extravasation injuries: a focused evaluation of noncytotoxic medications. Pharmacotherapy. 2014;34:617-632. doi:https://doi.org/10.1002/phar.1396
- Le A, Patel S. Extravasation of noncytotoxic drugs: a review of the literature. Ann Pharmacother. 2014;48:870-886. doi:10.1177/1060028014527820
- Slim AM, Roth JE, Duffy B, et al. The incidence of phlebitis with intravenous amiodarone at guideline dose recommendations. Mil Med. 2007;172:1279-1283.
- Girish KS, Kemparaju K. The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life Sci. 2007;80:1921-1943. doi:10.1016/j.lfs.2007.02.037
- Jung H. Hyaluronidase: an overview of its properties, applications, and side effects. Arch Plast Surg. 2020;47:297-300. doi:10.5999/aps.2020.00752
- Epstein AE, Olshansky B, Naccarelli GV, et al. Practical management guide for clinicians who treat patients with amiodarone. Am J Med. 2016;129:468-475. doi:10.1016/j.amjmed.2015.08.039
- Harris L, McKenna WJ, Rowland E, et al. Side effects of long-term amiodarone therapy. Circulation. 1983;67:45-51. doi:10.1161/01.cir.67.1.45
- Jaworski K, Walecka I, Rudnicka L, et al. Cutaneous adverse reactions of amiodarone. Med Sci Monit. 2014;20:2369-2372. doi:10.12659/MSM.890881
- Kowey Peter R, Marinchak Roger A, Rials Seth J, et al. Intravenous amiodarone. J Am Coll Cardiol. 1997;29:1190-1198. doi:10.1016/S0735-1097(97)00069-7
- Russell SJ, Saltissi S. Amiodarone induced skin necrosis. Heart. 2006;92:1395. doi:10.1136/hrt.2005.086157
- Grove EL. Skin necrosis and consequences of accidental subcutaneous administration of amiodarone. Ugeskr Laeger. 2015;177:V66928.
- Fox AN, Villanueva R, Miller JL. Management of amiodarone extravasation with intradermal hyaluronidase. Am J Health Syst Pharm. 2017;74:1545-1548. doi:10.2146/ajhp160737
- Reynolds PM, MacLaren R, Mueller SW, et al. Management of extravasation injuries: a focused evaluation of noncytotoxic medications. Pharmacotherapy. 2014;34:617-632. doi:https://doi.org/10.1002/phar.1396
- Le A, Patel S. Extravasation of noncytotoxic drugs: a review of the literature. Ann Pharmacother. 2014;48:870-886. doi:10.1177/1060028014527820
- Slim AM, Roth JE, Duffy B, et al. The incidence of phlebitis with intravenous amiodarone at guideline dose recommendations. Mil Med. 2007;172:1279-1283.
- Girish KS, Kemparaju K. The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life Sci. 2007;80:1921-1943. doi:10.1016/j.lfs.2007.02.037
- Jung H. Hyaluronidase: an overview of its properties, applications, and side effects. Arch Plast Surg. 2020;47:297-300. doi:10.5999/aps.2020.00752
Practice Points
- Intravenous amiodarone administered peripherally can induce skin extravasation, leading to necrosis.
- Dermatologists should be aware that early intervention with intradermal hyaluronidase may reduce the severity of tissue damage caused by amiodarone-induced skin necrosis.
Comment on “Distribution of Skin-Type Diversity in Photographs in AAD Online Educational Modules”
To the Editor:
We read with great interest the article by Chu et al1 (Cutis. 2021;107:157-159) and commend them for noting the underrepresentation of skin of color (SOC) in the American Academy of Dermatology (AAD) Basic Dermatology Curriculum. The AAD Basic Dermatology Curriculum represents one introductory resource that is ubiquitously utilized by medical students. Herein, we add an analysis of the representation of SOC in the following resources that also comprise the first exposure medical students have to dermatology: Dermatology Clinics Clinical Advisor articles (https://www.clinicaladvisor.com/home/dermatology/dermatology-clinics/), Learn Derm Module (LDM) by VisualDx (https://www.visualdx.com/learnderm/), Lookingbill and Marks’ Principles of Dermatology (6th ed)(LB&M),2 and DermNet NZ (https://dermnetnz.org/). We performed a focused search of the DermNet NZ database for images of the following common dermatologic conditions: acne, rosacea, alopecia, urticaria, arthropod bites, blistering diseases (bullous pemphigoid and pemphigus vulgaris), connective tissue diseases (dermatomyositis and lupus), inflammatory conditions (atopic dermatitis, contact dermatitis, and psoriasis), keloids, benign and malignant neoplasms (nevi, seborrheic keratosis, actinic keratosis, basal and squamous cell carcinomas, and melanoma including acral melanoma), bacterial skin infections (impetigo, erysipelas, cellulitis, staphylococcal scalded skin syndrome, and syphilis), fungal infections (dermatophyte infections), and viral skin infections (herpes, molluscum contagiosum, varicella-zoster virus, and warts). We classified images as light (Fitzpatrick phototypes I–IV) or dark (Fitzpatrick phototypes V or VI). We excluded images without visible background skin (eg, images of oral mucosa, genitalia, nails, palms and soles, dermoscopic images, histopathologic images).
We found the representation of SOC in the resources we selected to be as follows: Dermatology Clinics Clinical Advisor articles (70/367 or 19%); LDM (26/150 or 17%); LB&M (52/374 or 14%); DermNet NZ (11/310 or 4%). Representation of SOC in common dermatologic conditions such as actinic keratosis, alopecia, rosacea, urticaria, and warts was entirely absent across all resources. Other common skin diseases were represented in only one of the resources we analyzed: acne (represented only in LB&M, where only 3/11 images of acne were depicted in SOC); contact dermatitis (represented only in LB&M, where only 1/6 images of contact dermatitis were depicted in SOC); psoriasis (represented only on DermNet NZ, where only 2/25 images of psoriasis were depicted in SOC); seborrheic keratosis (represented only in LB&M, where 1/2 images of seborrheic keratosis were depicted in SOC). Furthermore, none of the resources we analyzed depicted malignancy (basal cell carcinoma, squamous cell carcinoma, and melanoma) in SOC. Although the poor representation of SOC in malignancies can be explained by the predilection of skin cancer for light skin, other dermatologic conditions that are more common in SOC also were poorly represented in these resources in SOC: acral melanoma, not represented in any of the resources we analyzed; subacute cutaneous lupus erythematosus and systemic lupus erythematosus, also not represented in any of the resources we analyzed; keloids, represented only in LB&M.
Although no study has investigated the true prevalence of Fitzpatrick phototypes in the United States, He et al3 demonstrated the prevalence of Fitzpatrick phototypes V and VI to be 25.0% and 18.8%, respectively, in an ethnically diverse study of 3386 participants. Indeed, the representation of SOC in the resources we analyzed falls short of this plausible estimate of SOC in an increasingly diverse US population.
Our work adds to the growing body of literature exposing the deficiencies in SOC representation in dermatology. As Lester et al4 noted, such poor representation of SOC is deleterious not just to patients, who may be misdiagnosed, but also more generally to the integrity of the field of dermatology. Moreover, our study, which analyzes introductory resources referenced by the junior medical student, highlights a potential danger of poor SOC representation for trainees—limited exposure to SOC may leave medical students unprepared to recognize lesions in SOC during clerkships and residency. Furthermore, we note an additional concern with minimal SOC representation in online modules such as the AAD and LDM module as well as online databases such as DermNet NZ; images from these resources may be used as training sets for machine learning (ML) software (indeed, DermNet NZ has been used as a training set for ML programs5). However, if data sets with poor representation of SOC are used to train ML algorithms, then ML software may be unable to recognize lesions in SOC.6 Thus, inadequate representation of SOC in online modules and databases may exacerbate existing inequities in dermatology.
To address the paucity of SOC representation, students can be directed to resources devoted to depicting SOC; however, as discussed eloquently by Chu et al,1 an attempt to update existing resources also must be made. The senior author in our study (S.J.K.) embraced such an approach, updating the dermatology lectures given to medical students to include more images of SOC. Such a top-down approach may represent a major step in dismantling the systemic biases that pervade dermatology.
A limitation of our analysis was use of the Fitzpatrick scale, which was conceived as a phenotypic scale to assess cutaneous responses to UV irradiation.7 Although it is the most commonly used scale to describe race/ethnicity and/or constitute skin color, it is not possible to include all non-White skin types and classify strictly under this umbrella term.
References
1. Chu B, Fathy R, Onyekaba G, et al. Distribution of skin-type diversity in photographs in AAD online educational modules. Cutis. 2021;107:157-159. doi:10.12788/cutis.0196
2. Marks JG Jr, Miller JJ. Lookingbill and Marks’ Principles of Dermatology. 6th ed. Saunders Elsevier; 2018.
3. He SY, McCulloch CE, Boscardin WJ, et al. Self-reported pigmentary phenotypes and race are significant but incomplete predictors of Fitzpatrick skin phototype in an ethnically diverse population. J Am Acad Dermatol. 2014;71:731-737. doi:10.1016/j.jaad.2014.05.023
4. Lester JC, Taylor SC, Chren M-M. Under‐representation of skin of colour in dermatology images: not just an educational issue. Br J Dermatol. 2019;180:1521-1522. doi:10.1111/bjd.17608
5. Aggarwal P. Data augmentation in dermatology image recognition using machine learning. Skin Res Technol. 2019;25:815-820. doi:10.1111/srt.12726
6. Adamson AS, Smith A. Machine learning and health care disparities in dermatology. JAMA Dermatol. 2018;154:1247-1248. doi:10.1001/jamadermatol.2018.2348
7. Ware OR, Dawson JE, Shinohara MM, et al. Racial limitations of Fitzpatrick skin type. Cutis. 2020;105:77-80.
Authors’ Response
We thank Mr. Joshi and Dr. Kim for their reply to our article and their added contribution to the literature on inadequate representation of skin of color (SOC) in dermatology educational materials. In recent years, multiple analyses have reviewed textbooks and popular online resources for SOC representation.1 These resources encompass all levels of education—from the laypatient to the medical student, and to residency and beyond—demonstrating the significant challenges to overcome.
In addition, as Mr. Joshi and Dr. Kim state, the potential for these inadequately representative resources to serve as training data for prediction and classification tools adds further urgency to the broader task at hand, as we do not wish to perpetuate disparities. Several tools already exist, including Derm Assist, a recent Google-produced tool that suggests a list of diagnoses from patient-provided images.2 Although Derm Assist has been marked as a CE Class I (low risk) medical device in the European Union, the original research it is built on relied on training data with low representation of darker skin types (2.7% Fitzpatrick V and 0% Fitzpatrick VI),3 drawing concern for its generalizability.
These concerns about SOC representation are not new; dermatology advocates, scholars, and organizations such as the Skin of Color Society have been working to address these deficiencies for many years, contributing to education (including writing of resources and textbooks) and academic research. This work continues today. For instance, Lester et al4 described best practices for clinical photography in SOC; this guidance was not yet published at the time of our original submission. Not only should dermatology strive for increased quantity of representation but also quality. This metric is particularly important if the images are intended not just for education but also for use as training data for prediction and classification tools.
Examples of more recent actions at the organizational level include the American Academy of Dermatology (AAD) announcing a 3-year plan to promote diversity, equity, and inclusion5 and VisualDx establishing #ProjectIMPACT, a collaboration to reduce health care biases in SOC.6 In the AAD 3-year plan, one goal is to “[i]ncrease use of images reflecting full spectrum of skin types and highlight topics on skin of color, health disparities, and cultural competency across all AAD education.”5 Although not specifically mentioned, we hope that the AAD has included updating the Basic Dermatology Curriculum, given its inadequate SOC representation, as part of its short-term goals. The greater recognition of these issues through more prevalent analyses published in leading dermatology journals is encouraging, and we hope both that improvements can be successfully implemented and that future studies will reveal improvements in representation.
Brian Chu, BS; Ramie Fathy, AB; Ginikanwa Onyekaba, BS; Jules B. Lipoff, MD
From the Perelman School of Medicine, University of Pennsylvania, Philadelphia. Dr. Lipoff is from the Department of Dermatology and the Leonard Davis Institute of Health Economics.
The authors report no conflict of interest.
Correspondence: Jules B. Lipoff, MD, Department of Dermatology, University of Pennsylvania, Penn Medicine University City, 3737 Market St, Ste 1100, Philadelphia, PA 19104 ([email protected]).
References
1. Perlman KL, Williams NM, Egbeto IA, et al. Skin of color lacks representation in medical student resources: a cross-sectional study. Int J Womens Dermatol. 2021;7:195-196. doi:10.1016/j.ijwd.2020.12.018
2. Bui P, Liu Y. Using AI to help find answers to common skin conditions. Published May 18, 2021. Accessed June 12, 2021. https://blog.google/technology/health/ai-dermatology-preview-io-2021
3. Liu Y, Jain A, Eng C, et al. A deep learning system for differential diagnosis of skin diseases. Nature Medicine. 2020;26:900-908. doi:10.1038/s41591-020-0842-3
4. Lester JC, Clark L, Linos E, et al. Clinical photography in skin of colour: tips and best practices. Br J Dermatol. 2021;184:1177-1179. doi:10.1111/bjd.19811
5. American Academy of Dermatology Association. Diversity in dermatology: diversity committee approved plan 2021-2023. Published January 26, 2021. Accessed June 24, 2021. https://assets.ctfassets.net/1ny4yoiyrqia/xQgnCE6ji5skUlcZQHS2b/65f0a9072811e11afcc33d043e02cd4d/DEI_Plan.pdf
6. VisualDx. #ProjectIMPACT. Accessed June 24, 2021. https://www.visualdx.com/projectimpact/
To the Editor:
We read with great interest the article by Chu et al1 (Cutis. 2021;107:157-159) and commend them for noting the underrepresentation of skin of color (SOC) in the American Academy of Dermatology (AAD) Basic Dermatology Curriculum. The AAD Basic Dermatology Curriculum represents one introductory resource that is ubiquitously utilized by medical students. Herein, we add an analysis of the representation of SOC in the following resources that also comprise the first exposure medical students have to dermatology: Dermatology Clinics Clinical Advisor articles (https://www.clinicaladvisor.com/home/dermatology/dermatology-clinics/), Learn Derm Module (LDM) by VisualDx (https://www.visualdx.com/learnderm/), Lookingbill and Marks’ Principles of Dermatology (6th ed)(LB&M),2 and DermNet NZ (https://dermnetnz.org/). We performed a focused search of the DermNet NZ database for images of the following common dermatologic conditions: acne, rosacea, alopecia, urticaria, arthropod bites, blistering diseases (bullous pemphigoid and pemphigus vulgaris), connective tissue diseases (dermatomyositis and lupus), inflammatory conditions (atopic dermatitis, contact dermatitis, and psoriasis), keloids, benign and malignant neoplasms (nevi, seborrheic keratosis, actinic keratosis, basal and squamous cell carcinomas, and melanoma including acral melanoma), bacterial skin infections (impetigo, erysipelas, cellulitis, staphylococcal scalded skin syndrome, and syphilis), fungal infections (dermatophyte infections), and viral skin infections (herpes, molluscum contagiosum, varicella-zoster virus, and warts). We classified images as light (Fitzpatrick phototypes I–IV) or dark (Fitzpatrick phototypes V or VI). We excluded images without visible background skin (eg, images of oral mucosa, genitalia, nails, palms and soles, dermoscopic images, histopathologic images).
We found the representation of SOC in the resources we selected to be as follows: Dermatology Clinics Clinical Advisor articles (70/367 or 19%); LDM (26/150 or 17%); LB&M (52/374 or 14%); DermNet NZ (11/310 or 4%). Representation of SOC in common dermatologic conditions such as actinic keratosis, alopecia, rosacea, urticaria, and warts was entirely absent across all resources. Other common skin diseases were represented in only one of the resources we analyzed: acne (represented only in LB&M, where only 3/11 images of acne were depicted in SOC); contact dermatitis (represented only in LB&M, where only 1/6 images of contact dermatitis were depicted in SOC); psoriasis (represented only on DermNet NZ, where only 2/25 images of psoriasis were depicted in SOC); seborrheic keratosis (represented only in LB&M, where 1/2 images of seborrheic keratosis were depicted in SOC). Furthermore, none of the resources we analyzed depicted malignancy (basal cell carcinoma, squamous cell carcinoma, and melanoma) in SOC. Although the poor representation of SOC in malignancies can be explained by the predilection of skin cancer for light skin, other dermatologic conditions that are more common in SOC also were poorly represented in these resources in SOC: acral melanoma, not represented in any of the resources we analyzed; subacute cutaneous lupus erythematosus and systemic lupus erythematosus, also not represented in any of the resources we analyzed; keloids, represented only in LB&M.
Although no study has investigated the true prevalence of Fitzpatrick phototypes in the United States, He et al3 demonstrated the prevalence of Fitzpatrick phototypes V and VI to be 25.0% and 18.8%, respectively, in an ethnically diverse study of 3386 participants. Indeed, the representation of SOC in the resources we analyzed falls short of this plausible estimate of SOC in an increasingly diverse US population.
Our work adds to the growing body of literature exposing the deficiencies in SOC representation in dermatology. As Lester et al4 noted, such poor representation of SOC is deleterious not just to patients, who may be misdiagnosed, but also more generally to the integrity of the field of dermatology. Moreover, our study, which analyzes introductory resources referenced by the junior medical student, highlights a potential danger of poor SOC representation for trainees—limited exposure to SOC may leave medical students unprepared to recognize lesions in SOC during clerkships and residency. Furthermore, we note an additional concern with minimal SOC representation in online modules such as the AAD and LDM module as well as online databases such as DermNet NZ; images from these resources may be used as training sets for machine learning (ML) software (indeed, DermNet NZ has been used as a training set for ML programs5). However, if data sets with poor representation of SOC are used to train ML algorithms, then ML software may be unable to recognize lesions in SOC.6 Thus, inadequate representation of SOC in online modules and databases may exacerbate existing inequities in dermatology.
To address the paucity of SOC representation, students can be directed to resources devoted to depicting SOC; however, as discussed eloquently by Chu et al,1 an attempt to update existing resources also must be made. The senior author in our study (S.J.K.) embraced such an approach, updating the dermatology lectures given to medical students to include more images of SOC. Such a top-down approach may represent a major step in dismantling the systemic biases that pervade dermatology.
A limitation of our analysis was use of the Fitzpatrick scale, which was conceived as a phenotypic scale to assess cutaneous responses to UV irradiation.7 Although it is the most commonly used scale to describe race/ethnicity and/or constitute skin color, it is not possible to include all non-White skin types and classify strictly under this umbrella term.
References
1. Chu B, Fathy R, Onyekaba G, et al. Distribution of skin-type diversity in photographs in AAD online educational modules. Cutis. 2021;107:157-159. doi:10.12788/cutis.0196
2. Marks JG Jr, Miller JJ. Lookingbill and Marks’ Principles of Dermatology. 6th ed. Saunders Elsevier; 2018.
3. He SY, McCulloch CE, Boscardin WJ, et al. Self-reported pigmentary phenotypes and race are significant but incomplete predictors of Fitzpatrick skin phototype in an ethnically diverse population. J Am Acad Dermatol. 2014;71:731-737. doi:10.1016/j.jaad.2014.05.023
4. Lester JC, Taylor SC, Chren M-M. Under‐representation of skin of colour in dermatology images: not just an educational issue. Br J Dermatol. 2019;180:1521-1522. doi:10.1111/bjd.17608
5. Aggarwal P. Data augmentation in dermatology image recognition using machine learning. Skin Res Technol. 2019;25:815-820. doi:10.1111/srt.12726
6. Adamson AS, Smith A. Machine learning and health care disparities in dermatology. JAMA Dermatol. 2018;154:1247-1248. doi:10.1001/jamadermatol.2018.2348
7. Ware OR, Dawson JE, Shinohara MM, et al. Racial limitations of Fitzpatrick skin type. Cutis. 2020;105:77-80.
Authors’ Response
We thank Mr. Joshi and Dr. Kim for their reply to our article and their added contribution to the literature on inadequate representation of skin of color (SOC) in dermatology educational materials. In recent years, multiple analyses have reviewed textbooks and popular online resources for SOC representation.1 These resources encompass all levels of education—from the laypatient to the medical student, and to residency and beyond—demonstrating the significant challenges to overcome.
In addition, as Mr. Joshi and Dr. Kim state, the potential for these inadequately representative resources to serve as training data for prediction and classification tools adds further urgency to the broader task at hand, as we do not wish to perpetuate disparities. Several tools already exist, including Derm Assist, a recent Google-produced tool that suggests a list of diagnoses from patient-provided images.2 Although Derm Assist has been marked as a CE Class I (low risk) medical device in the European Union, the original research it is built on relied on training data with low representation of darker skin types (2.7% Fitzpatrick V and 0% Fitzpatrick VI),3 drawing concern for its generalizability.
These concerns about SOC representation are not new; dermatology advocates, scholars, and organizations such as the Skin of Color Society have been working to address these deficiencies for many years, contributing to education (including writing of resources and textbooks) and academic research. This work continues today. For instance, Lester et al4 described best practices for clinical photography in SOC; this guidance was not yet published at the time of our original submission. Not only should dermatology strive for increased quantity of representation but also quality. This metric is particularly important if the images are intended not just for education but also for use as training data for prediction and classification tools.
Examples of more recent actions at the organizational level include the American Academy of Dermatology (AAD) announcing a 3-year plan to promote diversity, equity, and inclusion5 and VisualDx establishing #ProjectIMPACT, a collaboration to reduce health care biases in SOC.6 In the AAD 3-year plan, one goal is to “[i]ncrease use of images reflecting full spectrum of skin types and highlight topics on skin of color, health disparities, and cultural competency across all AAD education.”5 Although not specifically mentioned, we hope that the AAD has included updating the Basic Dermatology Curriculum, given its inadequate SOC representation, as part of its short-term goals. The greater recognition of these issues through more prevalent analyses published in leading dermatology journals is encouraging, and we hope both that improvements can be successfully implemented and that future studies will reveal improvements in representation.
Brian Chu, BS; Ramie Fathy, AB; Ginikanwa Onyekaba, BS; Jules B. Lipoff, MD
From the Perelman School of Medicine, University of Pennsylvania, Philadelphia. Dr. Lipoff is from the Department of Dermatology and the Leonard Davis Institute of Health Economics.
The authors report no conflict of interest.
Correspondence: Jules B. Lipoff, MD, Department of Dermatology, University of Pennsylvania, Penn Medicine University City, 3737 Market St, Ste 1100, Philadelphia, PA 19104 ([email protected]).
References
1. Perlman KL, Williams NM, Egbeto IA, et al. Skin of color lacks representation in medical student resources: a cross-sectional study. Int J Womens Dermatol. 2021;7:195-196. doi:10.1016/j.ijwd.2020.12.018
2. Bui P, Liu Y. Using AI to help find answers to common skin conditions. Published May 18, 2021. Accessed June 12, 2021. https://blog.google/technology/health/ai-dermatology-preview-io-2021
3. Liu Y, Jain A, Eng C, et al. A deep learning system for differential diagnosis of skin diseases. Nature Medicine. 2020;26:900-908. doi:10.1038/s41591-020-0842-3
4. Lester JC, Clark L, Linos E, et al. Clinical photography in skin of colour: tips and best practices. Br J Dermatol. 2021;184:1177-1179. doi:10.1111/bjd.19811
5. American Academy of Dermatology Association. Diversity in dermatology: diversity committee approved plan 2021-2023. Published January 26, 2021. Accessed June 24, 2021. https://assets.ctfassets.net/1ny4yoiyrqia/xQgnCE6ji5skUlcZQHS2b/65f0a9072811e11afcc33d043e02cd4d/DEI_Plan.pdf
6. VisualDx. #ProjectIMPACT. Accessed June 24, 2021. https://www.visualdx.com/projectimpact/
To the Editor:
We read with great interest the article by Chu et al1 (Cutis. 2021;107:157-159) and commend them for noting the underrepresentation of skin of color (SOC) in the American Academy of Dermatology (AAD) Basic Dermatology Curriculum. The AAD Basic Dermatology Curriculum represents one introductory resource that is ubiquitously utilized by medical students. Herein, we add an analysis of the representation of SOC in the following resources that also comprise the first exposure medical students have to dermatology: Dermatology Clinics Clinical Advisor articles (https://www.clinicaladvisor.com/home/dermatology/dermatology-clinics/), Learn Derm Module (LDM) by VisualDx (https://www.visualdx.com/learnderm/), Lookingbill and Marks’ Principles of Dermatology (6th ed)(LB&M),2 and DermNet NZ (https://dermnetnz.org/). We performed a focused search of the DermNet NZ database for images of the following common dermatologic conditions: acne, rosacea, alopecia, urticaria, arthropod bites, blistering diseases (bullous pemphigoid and pemphigus vulgaris), connective tissue diseases (dermatomyositis and lupus), inflammatory conditions (atopic dermatitis, contact dermatitis, and psoriasis), keloids, benign and malignant neoplasms (nevi, seborrheic keratosis, actinic keratosis, basal and squamous cell carcinomas, and melanoma including acral melanoma), bacterial skin infections (impetigo, erysipelas, cellulitis, staphylococcal scalded skin syndrome, and syphilis), fungal infections (dermatophyte infections), and viral skin infections (herpes, molluscum contagiosum, varicella-zoster virus, and warts). We classified images as light (Fitzpatrick phototypes I–IV) or dark (Fitzpatrick phototypes V or VI). We excluded images without visible background skin (eg, images of oral mucosa, genitalia, nails, palms and soles, dermoscopic images, histopathologic images).
We found the representation of SOC in the resources we selected to be as follows: Dermatology Clinics Clinical Advisor articles (70/367 or 19%); LDM (26/150 or 17%); LB&M (52/374 or 14%); DermNet NZ (11/310 or 4%). Representation of SOC in common dermatologic conditions such as actinic keratosis, alopecia, rosacea, urticaria, and warts was entirely absent across all resources. Other common skin diseases were represented in only one of the resources we analyzed: acne (represented only in LB&M, where only 3/11 images of acne were depicted in SOC); contact dermatitis (represented only in LB&M, where only 1/6 images of contact dermatitis were depicted in SOC); psoriasis (represented only on DermNet NZ, where only 2/25 images of psoriasis were depicted in SOC); seborrheic keratosis (represented only in LB&M, where 1/2 images of seborrheic keratosis were depicted in SOC). Furthermore, none of the resources we analyzed depicted malignancy (basal cell carcinoma, squamous cell carcinoma, and melanoma) in SOC. Although the poor representation of SOC in malignancies can be explained by the predilection of skin cancer for light skin, other dermatologic conditions that are more common in SOC also were poorly represented in these resources in SOC: acral melanoma, not represented in any of the resources we analyzed; subacute cutaneous lupus erythematosus and systemic lupus erythematosus, also not represented in any of the resources we analyzed; keloids, represented only in LB&M.
Although no study has investigated the true prevalence of Fitzpatrick phototypes in the United States, He et al3 demonstrated the prevalence of Fitzpatrick phototypes V and VI to be 25.0% and 18.8%, respectively, in an ethnically diverse study of 3386 participants. Indeed, the representation of SOC in the resources we analyzed falls short of this plausible estimate of SOC in an increasingly diverse US population.
Our work adds to the growing body of literature exposing the deficiencies in SOC representation in dermatology. As Lester et al4 noted, such poor representation of SOC is deleterious not just to patients, who may be misdiagnosed, but also more generally to the integrity of the field of dermatology. Moreover, our study, which analyzes introductory resources referenced by the junior medical student, highlights a potential danger of poor SOC representation for trainees—limited exposure to SOC may leave medical students unprepared to recognize lesions in SOC during clerkships and residency. Furthermore, we note an additional concern with minimal SOC representation in online modules such as the AAD and LDM module as well as online databases such as DermNet NZ; images from these resources may be used as training sets for machine learning (ML) software (indeed, DermNet NZ has been used as a training set for ML programs5). However, if data sets with poor representation of SOC are used to train ML algorithms, then ML software may be unable to recognize lesions in SOC.6 Thus, inadequate representation of SOC in online modules and databases may exacerbate existing inequities in dermatology.
To address the paucity of SOC representation, students can be directed to resources devoted to depicting SOC; however, as discussed eloquently by Chu et al,1 an attempt to update existing resources also must be made. The senior author in our study (S.J.K.) embraced such an approach, updating the dermatology lectures given to medical students to include more images of SOC. Such a top-down approach may represent a major step in dismantling the systemic biases that pervade dermatology.
A limitation of our analysis was use of the Fitzpatrick scale, which was conceived as a phenotypic scale to assess cutaneous responses to UV irradiation.7 Although it is the most commonly used scale to describe race/ethnicity and/or constitute skin color, it is not possible to include all non-White skin types and classify strictly under this umbrella term.
References
1. Chu B, Fathy R, Onyekaba G, et al. Distribution of skin-type diversity in photographs in AAD online educational modules. Cutis. 2021;107:157-159. doi:10.12788/cutis.0196
2. Marks JG Jr, Miller JJ. Lookingbill and Marks’ Principles of Dermatology. 6th ed. Saunders Elsevier; 2018.
3. He SY, McCulloch CE, Boscardin WJ, et al. Self-reported pigmentary phenotypes and race are significant but incomplete predictors of Fitzpatrick skin phototype in an ethnically diverse population. J Am Acad Dermatol. 2014;71:731-737. doi:10.1016/j.jaad.2014.05.023
4. Lester JC, Taylor SC, Chren M-M. Under‐representation of skin of colour in dermatology images: not just an educational issue. Br J Dermatol. 2019;180:1521-1522. doi:10.1111/bjd.17608
5. Aggarwal P. Data augmentation in dermatology image recognition using machine learning. Skin Res Technol. 2019;25:815-820. doi:10.1111/srt.12726
6. Adamson AS, Smith A. Machine learning and health care disparities in dermatology. JAMA Dermatol. 2018;154:1247-1248. doi:10.1001/jamadermatol.2018.2348
7. Ware OR, Dawson JE, Shinohara MM, et al. Racial limitations of Fitzpatrick skin type. Cutis. 2020;105:77-80.
Authors’ Response
We thank Mr. Joshi and Dr. Kim for their reply to our article and their added contribution to the literature on inadequate representation of skin of color (SOC) in dermatology educational materials. In recent years, multiple analyses have reviewed textbooks and popular online resources for SOC representation.1 These resources encompass all levels of education—from the laypatient to the medical student, and to residency and beyond—demonstrating the significant challenges to overcome.
In addition, as Mr. Joshi and Dr. Kim state, the potential for these inadequately representative resources to serve as training data for prediction and classification tools adds further urgency to the broader task at hand, as we do not wish to perpetuate disparities. Several tools already exist, including Derm Assist, a recent Google-produced tool that suggests a list of diagnoses from patient-provided images.2 Although Derm Assist has been marked as a CE Class I (low risk) medical device in the European Union, the original research it is built on relied on training data with low representation of darker skin types (2.7% Fitzpatrick V and 0% Fitzpatrick VI),3 drawing concern for its generalizability.
These concerns about SOC representation are not new; dermatology advocates, scholars, and organizations such as the Skin of Color Society have been working to address these deficiencies for many years, contributing to education (including writing of resources and textbooks) and academic research. This work continues today. For instance, Lester et al4 described best practices for clinical photography in SOC; this guidance was not yet published at the time of our original submission. Not only should dermatology strive for increased quantity of representation but also quality. This metric is particularly important if the images are intended not just for education but also for use as training data for prediction and classification tools.
Examples of more recent actions at the organizational level include the American Academy of Dermatology (AAD) announcing a 3-year plan to promote diversity, equity, and inclusion5 and VisualDx establishing #ProjectIMPACT, a collaboration to reduce health care biases in SOC.6 In the AAD 3-year plan, one goal is to “[i]ncrease use of images reflecting full spectrum of skin types and highlight topics on skin of color, health disparities, and cultural competency across all AAD education.”5 Although not specifically mentioned, we hope that the AAD has included updating the Basic Dermatology Curriculum, given its inadequate SOC representation, as part of its short-term goals. The greater recognition of these issues through more prevalent analyses published in leading dermatology journals is encouraging, and we hope both that improvements can be successfully implemented and that future studies will reveal improvements in representation.
Brian Chu, BS; Ramie Fathy, AB; Ginikanwa Onyekaba, BS; Jules B. Lipoff, MD
From the Perelman School of Medicine, University of Pennsylvania, Philadelphia. Dr. Lipoff is from the Department of Dermatology and the Leonard Davis Institute of Health Economics.
The authors report no conflict of interest.
Correspondence: Jules B. Lipoff, MD, Department of Dermatology, University of Pennsylvania, Penn Medicine University City, 3737 Market St, Ste 1100, Philadelphia, PA 19104 ([email protected]).
References
1. Perlman KL, Williams NM, Egbeto IA, et al. Skin of color lacks representation in medical student resources: a cross-sectional study. Int J Womens Dermatol. 2021;7:195-196. doi:10.1016/j.ijwd.2020.12.018
2. Bui P, Liu Y. Using AI to help find answers to common skin conditions. Published May 18, 2021. Accessed June 12, 2021. https://blog.google/technology/health/ai-dermatology-preview-io-2021
3. Liu Y, Jain A, Eng C, et al. A deep learning system for differential diagnosis of skin diseases. Nature Medicine. 2020;26:900-908. doi:10.1038/s41591-020-0842-3
4. Lester JC, Clark L, Linos E, et al. Clinical photography in skin of colour: tips and best practices. Br J Dermatol. 2021;184:1177-1179. doi:10.1111/bjd.19811
5. American Academy of Dermatology Association. Diversity in dermatology: diversity committee approved plan 2021-2023. Published January 26, 2021. Accessed June 24, 2021. https://assets.ctfassets.net/1ny4yoiyrqia/xQgnCE6ji5skUlcZQHS2b/65f0a9072811e11afcc33d043e02cd4d/DEI_Plan.pdf
6. VisualDx. #ProjectIMPACT. Accessed June 24, 2021. https://www.visualdx.com/projectimpact/
Ocular Complications of Atopic Dermatitis
Atopic dermatitis (AD) is a chronic inflammatory skin condition with a lifetime prevalence of 15% to 20% in industrialized countries.1 It affects both children and adults and is predominantly characterized by a waxing and waning course of eczematous skin lesions and pruritus. In recent years, there is increasing recognition that AD can present with extracutaneous findings. Large-scale epidemiologic studies have reported a notably higher prevalence of ophthalmic complications in the AD population compared to the general population, in a severity-dependent manner.2,3 Potential complications include blepharitis, keratoconjunctivitis, keratoconus, glaucoma, cataracts, retinal detachment, ophthalmic herpes simplex virus infections, and dupilumab-associated ocular complications.
The etiology of each ocular complication in the context of AD is complex and likely multifactorial. Intrinsic immune dysregulation, physical trauma from eye rubbing, AD medication side effects, and genetics all have been speculated to play a role.2 Some of these ocular complications have a chronic course, while others present with sudden onset of symptoms; many of them can result in visual impairment if undiagnosed or left untreated. This article reviews several of the most common ocular comorbidities associated with AD. We discuss the clinical presentation, pathophysiology, and management strategies for each condition.
Blepharitis
Blepharitis, an inflammatory condition of the eyelids, is estimated to affect more than 6% of patients with AD compared to less than 1% of the general population.2 Blepharitis can be classified as anterior or posterior, based on the anatomic location of the affected region relative to the lash margin. Affected individuals may experience pruritus and irritation of the eyelids, tearing, a foreign body or burning sensation, crusting of the eyelids, and photophobia.4 Anterior blepharitis commonly is due to staphylococcal disease, and posterior blepharitis is secondary to structural changes and obstruction of meibomian gland orifices.
Although the pathophysiology is not well defined, xerosis in atopic patients is accompanied by barrier disruption and transepidermal water loss, which promote eyelid skin inflammation.
The mainstay of therapy for atopic blepharitis consists of conventional lid hygiene regimens, such as warm compresses and gentle scrubbing of the lid margins to remove crust and debris, which can be done with nonprescription cleansers, pads, and baby shampoos. Acute exacerbations may require topical antibiotics (ie, erythromycin or bacitracin applied to the lid margins once daily), topical calcineurin inhibitors (ie, cyclosporine ophthalmic emulsion 0.05%), or low-potency topical corticosteroids (ie, fluorometholone 0.1% or loteprednol etabonate 0.5% ophthalmic suspensions).5 Due to potential side effects of medications, especially topical corticosteroids, patients should be referred to ophthalmologists for definitive diagnosis and treatment.
Keratoconjunctivitis
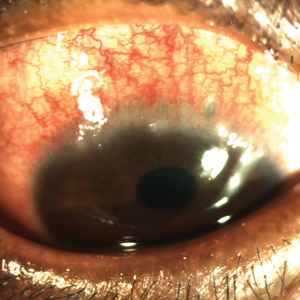
Atopic keratoconjunctivitis (AKC) is a noninfectious inflammatory condition of the cornea and conjunctiva that occurs in an estimated 25% to 42% of patients with AD.6,7 It frequently presents in late adolescence and has a peak incidence between 30 and 50 years of age.8 The symptoms of AKC include ocular pruritus, redness, ropy mucoid discharge, burning discomfort, photophobia, and blurring of vision. Corneal involvement can progress to corneal neovascularization and punctate or macroepithelial erosions and ulcerations, which increase the risk for corneal scarring and visual impairment.7
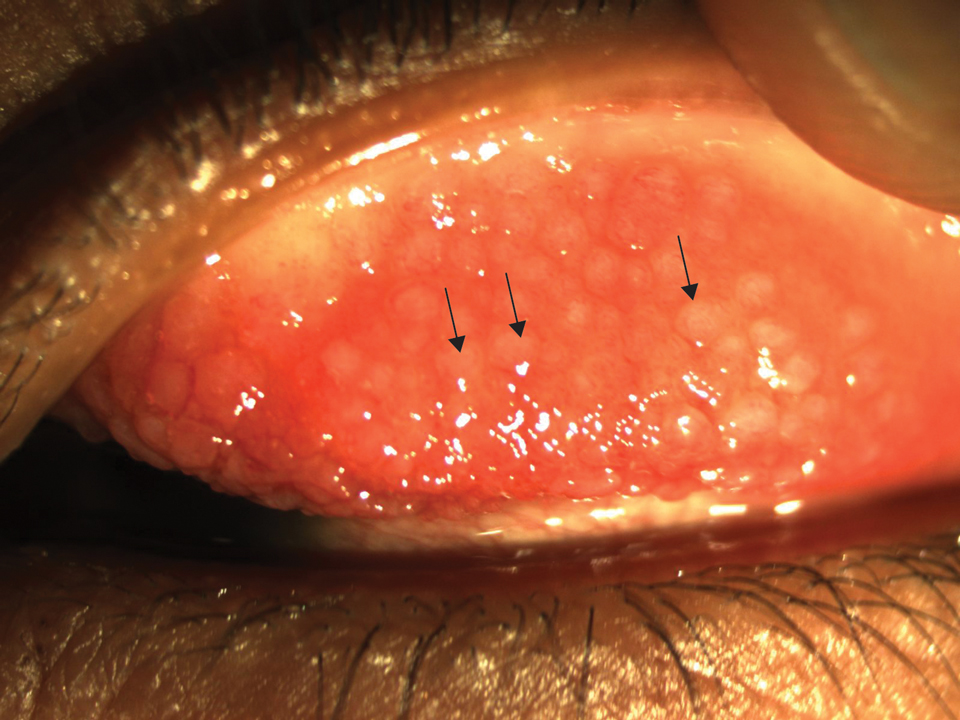
Keratoconjunctivitis is a complex inflammatory disease characterized by infiltration of the conjunctival epithelium by eosinophils, mast cells, and lymphocytes. On examination, patients frequently are found to have concurrent AD of the periorbital skin as well as papillary hypertrophy of the tarsal conjunctiva with accompanying fibrosis, which can lead to entropion (turning inward of the lid margins and lashes) in severe cases.7 Ophthalmic evaluation is strongly recommended for patients with AKC to control symptoms, to limit exacerbations, and to prevent sight-threatening inflammation leading to vision loss. Treatment can be challenging given the chronicity of the condition and may require multiple treatment arms. Conservative measures include cool compresses and treatment with ophthalmic eye drops containing antihistamines (ie, ketotifen 0.025% [available over-the-counter]) and mast cell stabilizers (ie, olopatadine ophthalmic solution 0.1%).8 Atopic keratoconjunctivitis exacerbations may require short-term use of topical steroids or calcineurin inhibitors, or systemic equivalents for refractory cases.6 Long-term maintenance therapy typically consists of proper eye hygiene and steroid-sparing agents that reduce ocular inflammation, such as topical cyclosporine and tacrolimus, neither of which are associated with increased intraocular pressure (IOP)(Figure 1).8 Cornea disease resulting from chronic conjunctival/lid microtrauma can be managed with soft or scleral contact lenses.

Keratoconus
Keratoconus is a noninflammatory ocular disorder characterized by progressive thinning and conelike protrusion of the cornea. The corneal topographic changes result in high irregular astigmatism and reduced visual acuity, which can manifest as image blurring or distortion (Figure 2).2,9 Multiple case series and controlled studies have reported a positive association between keratoconus and a history of atopic disease.10,11
The precise etiology of keratoconus in the context of AD is unclear and likely is multifactorial. Habitual eye rubbing from periocular pruritus and discomfort has been reported to be a notable contributor to keratoconus.12 In addition, intrinsic inflammation and imbalance of cytokines and proteases also may contribute to development of keratoconus.13
Keratoconus is a progressive condition that can severely impact vision, making it critical to diagnose patients before irreversible vision loss occurs. Individuals with risk factors, such as AD of the eyelids, history of eye rubbing, or family history of keratoconus, should be advised to receive routine vision screening for worsening astigmatism, especially during the first few decades of life when keratoconus progresses rapidly.
The conservative management for early keratoconus includes glasses and gas-permeable contact lenses for correction of visual acuity and astigmatism. For advanced keratoconus, scleral lenses often are prescribed. These large-diameter, gas-permeable lenses are designed to rest on the sclera and arch over the entire cornea.9 Alternatively, corneal collagen cross-linking is a newer technique that utilizes riboflavin and UVA irradiation to strengthen the corneal tissue. It has proven to be safe and effective in slowing or stopping the progression of keratoconus, particularly when treated at the early stage, and received US Food and Drug Administration approval in 2016.9
Glaucoma
Glaucoma is a well-known complication of AD and can lead to irreversible ocular hypertension and optic nerve damage. Corticosteroid use is a major risk factor for glaucoma, and the rise in IOP is thought to be due to increased aqueous outflow resistance.14
Multiple case reports have linked glaucoma to long-term use of potent topical corticosteroids in the facial and palpebral regions, which has been attributed to direct steroid contact and absorption by ocular tissues, as glaucoma rarely occurs with topical steroid application elsewhere on the body.15-17 Systemic steroids (ie, prednisolone) taken for more than 8 weeks also have been associated with a marked rise in IOP.18
Certain risk factors may predispose a steroid user to increased IOP, including existing open-angle glaucoma, diabetes mellitus, collagen disease, and high myopia.15,19 Steroid responders and younger individuals also demonstrate increased sensitivity to steroids.20
Given that glaucoma often is asymptomatic until advanced stages, early detection is the key for proper intervention. Periodic glaucoma screening by an ophthalmologist would be appropriate for known steroid responders, as well as patients with a prolonged history of topical steroid application in the palpebral region and systemic steroid use, family history of glaucoma, or known ocular pathology.21 Furthermore, patients with concurrent glaucoma and AD should be jointly managed by dermatology and ophthalmology, and systemic and topical corticosteroid use should be minimized in favor of alterative agents such as calcineurin inhibitors.22
In addition to steroid-induced glaucoma, intrinsic atopic glaucoma recently has been proposed as a clinical entity and is characterized by increased inflammatory cytokines—IL-8 and CCL2—in the aqueous humor and abnormal accumulation of fibers in corneoscleral meshwork.23
Cataracts
Cataracts are estimated to affect 8% to 25% of patients with AD.21,24 Unlike age-related cataracts, cataracts associated with AD are observed in adolescents and young adults in addition to the older population. The progression of lenticular opacity can rapidly occur and has been reported to coincide with AD flares.25,26
Patients with AD typically present with anterior or posterior subcapsular cataracts instead of nuclear and cortical cataracts, which are more common in the general population.27,28 Anterior subcapsular cataracts are more specific to AD, whereas posterior subcapsular cataracts are associated with both prolonged corticosteroid use and AD.26 Children generally are more sensitive to steroids than adults and may develop cataracts more rapidly and at lower concentrations.29
The pathophysiology of cataract formation and progression in the context of AD is multifactorial. Cataract patients with AD have compromised blood-retinal barrier integrity as well as increased oxidative damage in the lens.30,31 Genetics and blunt trauma from eye rubbing are thought to play a role, and the latter has been associated with faster progression of cataracts.28 In contrast, corticosteroid-induced cataracts likely are caused by transcriptional changes and disrupted osmotic balance in the lens fibers, which can lead to fiber rupture and lens opacification.26,32 Systemic corticosteroids show the strongest association with cataract development, but inhaled and topical steroids also have been implicated.26
Although cataracts can be surgically corrected, prevention is critical. Patients with early-onset periorbital AD, prolonged use of topical or systemic corticosteroids, and family history of cataracts should be routinely screened. Anterior and posterior subcapsular cataracts are diagnosed with red reflex examinations that can be readily performed by the primary care physician or ophthalmologist.33 Atopic dermatitis patients with cataracts should be advised to use calcineurin inhibitors and alternative treatments in place of corticosteroids.
Retinal Detachment
Retinal detachment (RD) is a serious complication of AD that can present in individuals younger than 35 years. The incidence of RD in patients with AD has been estimated to be 4% to 8%.34 Retinal detachment manifests with visual disturbances such as flashing lights, shadows, visual field defect, and blurring of vision, but also may occur in the absence of vision changes.35,36
Across multiple case series, patients who developed RD were consistently found to have AD in the facial or periorbital region and a history of chronic eye rubbing. Multiple patients also presented with concurrent proliferative vitreoretinopathy, lens subluxation, and/or cataracts.35,37 The mechanism for RD has been attributed to ocular contusion from vigorous eye rubbing, as fundus findings between traumatic and AD-associated RD are similarly characterized by tractional breaks in the retina at vitreous base borders.37
Avoidance of eye rubbing and optimized treatment of facial AD may help prevent RD in patients with AD. Furthermore, all patients with symptoms of RD should be immediately referred to ophthalmology for surgical repair.
Herpetic Ocular Disease
Ocular herpes simplex virus infections cause ocular pain and are associated with notable visual morbidity, as recurrences can result in irreversible corneal scarring and neovascularization. Two retrospective case-control studies independently reported that individuals with a history of AD are at greater risk for herpetic ocular disease compared to age-matched controls.38,39 Furthermore, atopic disease is associated with higher recurrence rates and slower regeneration of the corneal epithelium.40
These findings suggest that AD patients with a history of recurrent herpetic ocular diseases should be closely monitored and treated with antiviral prophylaxis and/or topical corticosteroids, depending on the type of keratitis (epithelial or stromal).40 Furthermore, active ocular herpetic infections warrant urgent referral to an ophthalmologist.
Dupilumab-Associated Ocular Complications
Dupilumab, a monoclonal antibody that blocks IL-4 and IL-13 signaling, is the first biologic therapy to be approved for treatment of moderate to severe AD. Prior clinical trials have described a higher incidence of anterior conjunctivitis in dupilumab-treated AD patients (5%–28%) compared to placebo (2%–11%).41 Of note, the incidence may be as high as 70%, as reported in a recent case series.42 Interestingly, independent trials assessing dupilumab treatment in asthma, nasal polyposis, and eosinophilic esophagitis patients did not observe a higher incidence of conjunctivitis in dupilumab-treated patients compared to placebo, suggesting an AD-specific mechanism.43
Prominent features of dupilumab-associated conjunctivitis include hyperemia of the conjunctiva and limbus, in addition to ocular symptoms such as tearing, burning, and bilateral decrease in visual acuity. Marked reduction of conjunctival goblet cells has been reported.44 In addition to conjunctivitis, blepharitis also has been reported during dupilumab treatment.45
Standardized treatment guidelines for dupilumab-associated ocular complications have not yet been established. Surprisingly, antihistamine eye drops appear to be inefficacious in the treatment of dupilumab-associated conjunctivitis.41 However, the condition has been successfully managed with topical steroids (fluorometholone ophthalmic suspension 0.1%) and tacrolimus ointment 0.03%.41 Lifitegrast, an anti-inflammatory agent approved for chronic dry eye, also has been suggested as a treatment option for patients refractory to topical steroids.45 Alternatively, cessation of dupilumab could be considered in AD patients who experience severe ocular complications. Atopic dermatitis patients taking dupilumab who have any concerning signs for ocular complications should be referred to an ophthalmologist for further diagnosis and management.
Conclusion
Practicing dermatologists likely will encounter patients with concurrent AD and ocular complications. Although eye examinations are not routinely performed in the care of AD patients, dermatologists can proactively inquire about ocular symptoms and monitor patients longitudinally. Early diagnosis and treatment of these ocular conditions can prevent vision loss in these patients. Furthermore, symptomatic control of AD and careful consideration of the side-effect profiles of medications can potentially reduce the incidence of ocular complications in individuals with AD.
Patients with visual concerns or risk factors, such as a history of vigorous eye rubbing or chronic corticosteroid use, should be jointly managed with an ophthalmologist for optimized care. Moreover, acute exacerbations of ocular symptoms and visual deterioration warrant urgent referral to ophthalmology.
- Weidinger S, Beck LA, Bieber T, et al. Atopic dermatitis. Nat Rev Dis Primers. 2018;4:1.
- Govind K, Whang K, Khanna R, et al. Atopic dermatitis is associated with increased prevalence of multiple ocular comorbidities. J Allergy Clin Immunol Pract. 2019;7:298-299.
- Thyssen JP, Toft PB, Halling-Overgaard AS, et al. Incidence, prevalence, and risk of selected ocular disease in adults with atopic dermatitis. J Am Acad Dermatol. 2017;77:280-286.e281.
- Putnam CM. Diagnosis and management of blepharitis: an optometrist’s perspective. Clin Optom (Auckl). 2016;8:71-78.
- Amescua G, Akpek EK, Farid M, et al. Blepharitis Preferred Practice Pattern®. Ophthalmology. 2019;126:P56-P93.
- Bielory B, Bielory L. Atopic dermatitis and keratoconjunctivitis. Immunol Allergy Clin North Am. 2010;30:323-336.
- Guglielmetti S, Dart JK, Calder V. Atopic keratoconjunctivitis and atopic dermatitis. Curr Opin Allergy Clin Immunol. 2010;10:478-485.
- Chen JJ, Applebaum DS, Sun GS, et al. Atopic keratoconjunctivitis: a review. J Am Acad Dermatol. 2014;70:569-575.
- Andreanos KD, Hashemi K, Petrelli M, et al. Keratoconus treatment algorithm. Ophthalmol Ther. 2017;6:245-262.
- Rahi A, Davies P, Ruben M, et al. Keratoconus and coexisting atopic disease. Br J Ophthalmol. 1977;61:761-764.
- Gasset AR, Hinson WA, Frias JL. Keratoconus and atopic diseases. Ann Ophthalmol. 1978;10:991-994.
- Bawazeer AM, Hodge WG, Lorimer B. Atopy and keratoconus: a multivariate analysis. Br J Ophthalmol. 2000;84:834-836.
- Galvis V, Sherwin T, Tello A, et al. Keratoconus: an inflammatory disorder? Eye (Lond). 2015;29:843-859.
- Clark AF, Wordinger RJ. The role of steroids in outflow resistance. Exp Eye Res. 2009;88:752-759.
- Daniel BS, Orchard D. Ocular side-effects of topical corticosteroids: what a dermatologist needs to know. Australas J Dermatol. 2015;56:164-169.
- Garrott HM, Walland MJ. Glaucoma from topical corticosteroids to the eyelids. Clin Exp Ophthalmol. 2004;32:224-226.
- Aggarwal RK, Potamitis T, Chong NH, et al. Extensive visual loss with topical facial steroids. Eye (Lond). 1993;7(pt 5):664-666.
- Mandapati JS, Metta AK. Intraocular pressure variation in patients on long-term corticosteroids. Indian Dermatol Online J. 2011;2:67-69.
- Jones R 3rd, Rhee DJ. Corticosteroid-induced ocular hypertension and glaucoma: a brief review and update of the literature. Curr Opin Ophthalmol. 2006;17:163-167.
- Tamagawa-Mineoka R, Yasuoka N, Ueta M, et al. Influence of topical steroids on intraocular pressure in patients with atopic dermatitis. Allergol Int. 2018;67:388-391.
- Bercovitch L. Screening for ocular complications in atopic dermatitis. Arch Dermatol. 2011;147:588-589.
- Abramovits W, Hung P, Tong KB. Efficacy and economics of topical calcineurin inhibitors for the treatment of atopic dermatitis. Am J Clin Dermatol. 2006;7:213-222.
- Takakuwa K, Hamanaka T, Mori K, et al. Atopic glaucoma: clinical and pathophysiological analysis. J Glaucoma. 2015;24:662-668.
- Haeck IM, Rouwen TJ, Timmer-de Mik L, et al. Topical corticosteroids in atopic dermatitis and the risk of glaucoma and cataracts. J Am Acad Dermatol. 2011;64:275-281.
- Amemiya T, Matsuda H, Uehara M. Ocular findings in atopic dermatitis with special reference to the clinical features of atopic cataract. Ophthalmologica. 1980;180:129-132.
- Tatham A. Atopic dermatitis, cutaneous steroids and cataracts in children: two case reports. J Med Case Rep. 2008;2:124.
- Chew M, Chiang PP, Zheng Y, et al. The impact of cataract, cataract types, and cataract grades on vision-specific functioning using Rasch analysis. Am J Ophthalmol. 2012;154:29-38.
- Nagaki Y, Hayasaka S, Kadoi C. Cataract progression in patients with atopic dermatitis. J Cataract Refract Surg. 1999;25:96-99.
- Kaye LD, Kalenak JW, Price RL, et al. Ocular implications of long-term prednisone therapy in children. J Pediatr Ophthalmol Strabismus. 1993;30:142-144.
- Matsuo T, Saito H, Matsuo N. Cataract and aqueous flare levels in patients with atopic dermatitis. Am J Ophthalmol. 1997;124:36-39.
- Namazi MR, Handjani F, Amirahmadi M. Increased oxidative activity from hydrogen peroxide may be the cause of the predisposition to cataracts among patients with atopic dermatitis. Med Hypotheses. 2006;66:863-864.
- James ER. The etiology of steroid cataract. J Ocul Pharmacol Ther. 2007;23:403-420.
- Lambert SR, Teng JMC. Assessing whether the cataracts associated with atopic dermatitis are associated with steroids or inflammatory factors. JAMA Ophthalmol. 2018;136:918-919.
- Sasoh M, Mizutani H, Matsubara H, et al. Incidence of retinal detachment associated with atopic dermatitis in Japan: review of cases from 1992 to 2011. Clin Ophthalmol. 2015;9:1129-1134.
- Yoneda K, Okamoto H, Wada Y, et al. Atopic retinal detachment. report of four cases and a review of the literature. Br J Dermatol. 1995;133:586-591.
- Gnana Jothi V, McGimpsey S, Sharkey JA, et al. Retinal detachment repair and cataract surgery in patients with atopic dermatitis. Eye (Lond). 2017;31:1296-1301.
- Oka C, Ideta H, Nagasaki H, et al. Retinal detachment with atopic dermatitis similar to traumatic retinal detachment. Ophthalmology. 1994;101:1050-1054.
- Prabriputaloong T, Margolis TP, Lietman TM, et al. Atopic disease and herpes simplex eye disease: a population-based case-control study. Am J Ophthalmol. 2006;142:745-749.
- Borkar DS, Gonzales JA, Tham VM, et al. Association between atopy and herpetic eye disease: results from the pacific ocular inflammation study. JAMA Ophthalmol. 2014;132:326-331.
- Rezende RA, Hammersmith K, Bisol T, et al. Comparative study of ocular herpes simplex virus in patients with and without self-reported atopy. Am J Ophthalmol. 2006;141:1120-1125.
- Wollenberg A, Ariens L, Thurau S, et al. Conjunctivitis occurring in atopic dermatitis patients treated with dupilumab-clinical characteristics and treatment. J Allergy Clin Immunol Pract. 2018;6:1778-1780.e1.
- Ivert LU, Wahlgren CF, Ivert L, et al. Eye complications during dupilumab treatment for severe atopic dermatitis. Acta Derm Venereol. 2019;99:375-378.
- Akinlade B, Guttman-Yassky E, de Bruin-Weller M, et al. Conjunctivitis in dupilumab clinical trials [published online March 9, 2019]. Br J Dermatol. doi:10.1111/bjd.17869.
- Bakker DS, Ariens LFM, van Luijk C, et al. Goblet cell scarcity and conjunctival inflammation during treatment with dupilumab in patients with atopic dermatitis. Br J Dermatol. 2019;180:1248-1249.
- Zirwas MJ, Wulff K, Beckman K. Lifitegrast add-on treatment for dupilumab-induced ocular surface disease (DIOSD): a novel case report. JAAD Case Rep. 2019;5:34-36.
Atopic dermatitis (AD) is a chronic inflammatory skin condition with a lifetime prevalence of 15% to 20% in industrialized countries.1 It affects both children and adults and is predominantly characterized by a waxing and waning course of eczematous skin lesions and pruritus. In recent years, there is increasing recognition that AD can present with extracutaneous findings. Large-scale epidemiologic studies have reported a notably higher prevalence of ophthalmic complications in the AD population compared to the general population, in a severity-dependent manner.2,3 Potential complications include blepharitis, keratoconjunctivitis, keratoconus, glaucoma, cataracts, retinal detachment, ophthalmic herpes simplex virus infections, and dupilumab-associated ocular complications.
The etiology of each ocular complication in the context of AD is complex and likely multifactorial. Intrinsic immune dysregulation, physical trauma from eye rubbing, AD medication side effects, and genetics all have been speculated to play a role.2 Some of these ocular complications have a chronic course, while others present with sudden onset of symptoms; many of them can result in visual impairment if undiagnosed or left untreated. This article reviews several of the most common ocular comorbidities associated with AD. We discuss the clinical presentation, pathophysiology, and management strategies for each condition.
Blepharitis
Blepharitis, an inflammatory condition of the eyelids, is estimated to affect more than 6% of patients with AD compared to less than 1% of the general population.2 Blepharitis can be classified as anterior or posterior, based on the anatomic location of the affected region relative to the lash margin. Affected individuals may experience pruritus and irritation of the eyelids, tearing, a foreign body or burning sensation, crusting of the eyelids, and photophobia.4 Anterior blepharitis commonly is due to staphylococcal disease, and posterior blepharitis is secondary to structural changes and obstruction of meibomian gland orifices.
Although the pathophysiology is not well defined, xerosis in atopic patients is accompanied by barrier disruption and transepidermal water loss, which promote eyelid skin inflammation.
The mainstay of therapy for atopic blepharitis consists of conventional lid hygiene regimens, such as warm compresses and gentle scrubbing of the lid margins to remove crust and debris, which can be done with nonprescription cleansers, pads, and baby shampoos. Acute exacerbations may require topical antibiotics (ie, erythromycin or bacitracin applied to the lid margins once daily), topical calcineurin inhibitors (ie, cyclosporine ophthalmic emulsion 0.05%), or low-potency topical corticosteroids (ie, fluorometholone 0.1% or loteprednol etabonate 0.5% ophthalmic suspensions).5 Due to potential side effects of medications, especially topical corticosteroids, patients should be referred to ophthalmologists for definitive diagnosis and treatment.
Keratoconjunctivitis
Atopic keratoconjunctivitis (AKC) is a noninfectious inflammatory condition of the cornea and conjunctiva that occurs in an estimated 25% to 42% of patients with AD.6,7 It frequently presents in late adolescence and has a peak incidence between 30 and 50 years of age.8 The symptoms of AKC include ocular pruritus, redness, ropy mucoid discharge, burning discomfort, photophobia, and blurring of vision. Corneal involvement can progress to corneal neovascularization and punctate or macroepithelial erosions and ulcerations, which increase the risk for corneal scarring and visual impairment.7
Keratoconjunctivitis is a complex inflammatory disease characterized by infiltration of the conjunctival epithelium by eosinophils, mast cells, and lymphocytes. On examination, patients frequently are found to have concurrent AD of the periorbital skin as well as papillary hypertrophy of the tarsal conjunctiva with accompanying fibrosis, which can lead to entropion (turning inward of the lid margins and lashes) in severe cases.7 Ophthalmic evaluation is strongly recommended for patients with AKC to control symptoms, to limit exacerbations, and to prevent sight-threatening inflammation leading to vision loss. Treatment can be challenging given the chronicity of the condition and may require multiple treatment arms. Conservative measures include cool compresses and treatment with ophthalmic eye drops containing antihistamines (ie, ketotifen 0.025% [available over-the-counter]) and mast cell stabilizers (ie, olopatadine ophthalmic solution 0.1%).8 Atopic keratoconjunctivitis exacerbations may require short-term use of topical steroids or calcineurin inhibitors, or systemic equivalents for refractory cases.6 Long-term maintenance therapy typically consists of proper eye hygiene and steroid-sparing agents that reduce ocular inflammation, such as topical cyclosporine and tacrolimus, neither of which are associated with increased intraocular pressure (IOP)(Figure 1).8 Cornea disease resulting from chronic conjunctival/lid microtrauma can be managed with soft or scleral contact lenses.

Keratoconus
Keratoconus is a noninflammatory ocular disorder characterized by progressive thinning and conelike protrusion of the cornea. The corneal topographic changes result in high irregular astigmatism and reduced visual acuity, which can manifest as image blurring or distortion (Figure 2).2,9 Multiple case series and controlled studies have reported a positive association between keratoconus and a history of atopic disease.10,11

The precise etiology of keratoconus in the context of AD is unclear and likely is multifactorial. Habitual eye rubbing from periocular pruritus and discomfort has been reported to be a notable contributor to keratoconus.12 In addition, intrinsic inflammation and imbalance of cytokines and proteases also may contribute to development of keratoconus.13
Keratoconus is a progressive condition that can severely impact vision, making it critical to diagnose patients before irreversible vision loss occurs. Individuals with risk factors, such as AD of the eyelids, history of eye rubbing, or family history of keratoconus, should be advised to receive routine vision screening for worsening astigmatism, especially during the first few decades of life when keratoconus progresses rapidly.
The conservative management for early keratoconus includes glasses and gas-permeable contact lenses for correction of visual acuity and astigmatism. For advanced keratoconus, scleral lenses often are prescribed. These large-diameter, gas-permeable lenses are designed to rest on the sclera and arch over the entire cornea.9 Alternatively, corneal collagen cross-linking is a newer technique that utilizes riboflavin and UVA irradiation to strengthen the corneal tissue. It has proven to be safe and effective in slowing or stopping the progression of keratoconus, particularly when treated at the early stage, and received US Food and Drug Administration approval in 2016.9
Glaucoma
Glaucoma is a well-known complication of AD and can lead to irreversible ocular hypertension and optic nerve damage. Corticosteroid use is a major risk factor for glaucoma, and the rise in IOP is thought to be due to increased aqueous outflow resistance.14
Multiple case reports have linked glaucoma to long-term use of potent topical corticosteroids in the facial and palpebral regions, which has been attributed to direct steroid contact and absorption by ocular tissues, as glaucoma rarely occurs with topical steroid application elsewhere on the body.15-17 Systemic steroids (ie, prednisolone) taken for more than 8 weeks also have been associated with a marked rise in IOP.18
Certain risk factors may predispose a steroid user to increased IOP, including existing open-angle glaucoma, diabetes mellitus, collagen disease, and high myopia.15,19 Steroid responders and younger individuals also demonstrate increased sensitivity to steroids.20
Given that glaucoma often is asymptomatic until advanced stages, early detection is the key for proper intervention. Periodic glaucoma screening by an ophthalmologist would be appropriate for known steroid responders, as well as patients with a prolonged history of topical steroid application in the palpebral region and systemic steroid use, family history of glaucoma, or known ocular pathology.21 Furthermore, patients with concurrent glaucoma and AD should be jointly managed by dermatology and ophthalmology, and systemic and topical corticosteroid use should be minimized in favor of alterative agents such as calcineurin inhibitors.22
In addition to steroid-induced glaucoma, intrinsic atopic glaucoma recently has been proposed as a clinical entity and is characterized by increased inflammatory cytokines—IL-8 and CCL2—in the aqueous humor and abnormal accumulation of fibers in corneoscleral meshwork.23
Cataracts
Cataracts are estimated to affect 8% to 25% of patients with AD.21,24 Unlike age-related cataracts, cataracts associated with AD are observed in adolescents and young adults in addition to the older population. The progression of lenticular opacity can rapidly occur and has been reported to coincide with AD flares.25,26
Patients with AD typically present with anterior or posterior subcapsular cataracts instead of nuclear and cortical cataracts, which are more common in the general population.27,28 Anterior subcapsular cataracts are more specific to AD, whereas posterior subcapsular cataracts are associated with both prolonged corticosteroid use and AD.26 Children generally are more sensitive to steroids than adults and may develop cataracts more rapidly and at lower concentrations.29
The pathophysiology of cataract formation and progression in the context of AD is multifactorial. Cataract patients with AD have compromised blood-retinal barrier integrity as well as increased oxidative damage in the lens.30,31 Genetics and blunt trauma from eye rubbing are thought to play a role, and the latter has been associated with faster progression of cataracts.28 In contrast, corticosteroid-induced cataracts likely are caused by transcriptional changes and disrupted osmotic balance in the lens fibers, which can lead to fiber rupture and lens opacification.26,32 Systemic corticosteroids show the strongest association with cataract development, but inhaled and topical steroids also have been implicated.26
Although cataracts can be surgically corrected, prevention is critical. Patients with early-onset periorbital AD, prolonged use of topical or systemic corticosteroids, and family history of cataracts should be routinely screened. Anterior and posterior subcapsular cataracts are diagnosed with red reflex examinations that can be readily performed by the primary care physician or ophthalmologist.33 Atopic dermatitis patients with cataracts should be advised to use calcineurin inhibitors and alternative treatments in place of corticosteroids.
Retinal Detachment
Retinal detachment (RD) is a serious complication of AD that can present in individuals younger than 35 years. The incidence of RD in patients with AD has been estimated to be 4% to 8%.34 Retinal detachment manifests with visual disturbances such as flashing lights, shadows, visual field defect, and blurring of vision, but also may occur in the absence of vision changes.35,36
Across multiple case series, patients who developed RD were consistently found to have AD in the facial or periorbital region and a history of chronic eye rubbing. Multiple patients also presented with concurrent proliferative vitreoretinopathy, lens subluxation, and/or cataracts.35,37 The mechanism for RD has been attributed to ocular contusion from vigorous eye rubbing, as fundus findings between traumatic and AD-associated RD are similarly characterized by tractional breaks in the retina at vitreous base borders.37
Avoidance of eye rubbing and optimized treatment of facial AD may help prevent RD in patients with AD. Furthermore, all patients with symptoms of RD should be immediately referred to ophthalmology for surgical repair.
Herpetic Ocular Disease
Ocular herpes simplex virus infections cause ocular pain and are associated with notable visual morbidity, as recurrences can result in irreversible corneal scarring and neovascularization. Two retrospective case-control studies independently reported that individuals with a history of AD are at greater risk for herpetic ocular disease compared to age-matched controls.38,39 Furthermore, atopic disease is associated with higher recurrence rates and slower regeneration of the corneal epithelium.40
These findings suggest that AD patients with a history of recurrent herpetic ocular diseases should be closely monitored and treated with antiviral prophylaxis and/or topical corticosteroids, depending on the type of keratitis (epithelial or stromal).40 Furthermore, active ocular herpetic infections warrant urgent referral to an ophthalmologist.
Dupilumab-Associated Ocular Complications
Dupilumab, a monoclonal antibody that blocks IL-4 and IL-13 signaling, is the first biologic therapy to be approved for treatment of moderate to severe AD. Prior clinical trials have described a higher incidence of anterior conjunctivitis in dupilumab-treated AD patients (5%–28%) compared to placebo (2%–11%).41 Of note, the incidence may be as high as 70%, as reported in a recent case series.42 Interestingly, independent trials assessing dupilumab treatment in asthma, nasal polyposis, and eosinophilic esophagitis patients did not observe a higher incidence of conjunctivitis in dupilumab-treated patients compared to placebo, suggesting an AD-specific mechanism.43
Prominent features of dupilumab-associated conjunctivitis include hyperemia of the conjunctiva and limbus, in addition to ocular symptoms such as tearing, burning, and bilateral decrease in visual acuity. Marked reduction of conjunctival goblet cells has been reported.44 In addition to conjunctivitis, blepharitis also has been reported during dupilumab treatment.45
Standardized treatment guidelines for dupilumab-associated ocular complications have not yet been established. Surprisingly, antihistamine eye drops appear to be inefficacious in the treatment of dupilumab-associated conjunctivitis.41 However, the condition has been successfully managed with topical steroids (fluorometholone ophthalmic suspension 0.1%) and tacrolimus ointment 0.03%.41 Lifitegrast, an anti-inflammatory agent approved for chronic dry eye, also has been suggested as a treatment option for patients refractory to topical steroids.45 Alternatively, cessation of dupilumab could be considered in AD patients who experience severe ocular complications. Atopic dermatitis patients taking dupilumab who have any concerning signs for ocular complications should be referred to an ophthalmologist for further diagnosis and management.
Conclusion
Practicing dermatologists likely will encounter patients with concurrent AD and ocular complications. Although eye examinations are not routinely performed in the care of AD patients, dermatologists can proactively inquire about ocular symptoms and monitor patients longitudinally. Early diagnosis and treatment of these ocular conditions can prevent vision loss in these patients. Furthermore, symptomatic control of AD and careful consideration of the side-effect profiles of medications can potentially reduce the incidence of ocular complications in individuals with AD.
Patients with visual concerns or risk factors, such as a history of vigorous eye rubbing or chronic corticosteroid use, should be jointly managed with an ophthalmologist for optimized care. Moreover, acute exacerbations of ocular symptoms and visual deterioration warrant urgent referral to ophthalmology.
Atopic dermatitis (AD) is a chronic inflammatory skin condition with a lifetime prevalence of 15% to 20% in industrialized countries.1 It affects both children and adults and is predominantly characterized by a waxing and waning course of eczematous skin lesions and pruritus. In recent years, there is increasing recognition that AD can present with extracutaneous findings. Large-scale epidemiologic studies have reported a notably higher prevalence of ophthalmic complications in the AD population compared to the general population, in a severity-dependent manner.2,3 Potential complications include blepharitis, keratoconjunctivitis, keratoconus, glaucoma, cataracts, retinal detachment, ophthalmic herpes simplex virus infections, and dupilumab-associated ocular complications.
The etiology of each ocular complication in the context of AD is complex and likely multifactorial. Intrinsic immune dysregulation, physical trauma from eye rubbing, AD medication side effects, and genetics all have been speculated to play a role.2 Some of these ocular complications have a chronic course, while others present with sudden onset of symptoms; many of them can result in visual impairment if undiagnosed or left untreated. This article reviews several of the most common ocular comorbidities associated with AD. We discuss the clinical presentation, pathophysiology, and management strategies for each condition.
Blepharitis
Blepharitis, an inflammatory condition of the eyelids, is estimated to affect more than 6% of patients with AD compared to less than 1% of the general population.2 Blepharitis can be classified as anterior or posterior, based on the anatomic location of the affected region relative to the lash margin. Affected individuals may experience pruritus and irritation of the eyelids, tearing, a foreign body or burning sensation, crusting of the eyelids, and photophobia.4 Anterior blepharitis commonly is due to staphylococcal disease, and posterior blepharitis is secondary to structural changes and obstruction of meibomian gland orifices.
Although the pathophysiology is not well defined, xerosis in atopic patients is accompanied by barrier disruption and transepidermal water loss, which promote eyelid skin inflammation.
The mainstay of therapy for atopic blepharitis consists of conventional lid hygiene regimens, such as warm compresses and gentle scrubbing of the lid margins to remove crust and debris, which can be done with nonprescription cleansers, pads, and baby shampoos. Acute exacerbations may require topical antibiotics (ie, erythromycin or bacitracin applied to the lid margins once daily), topical calcineurin inhibitors (ie, cyclosporine ophthalmic emulsion 0.05%), or low-potency topical corticosteroids (ie, fluorometholone 0.1% or loteprednol etabonate 0.5% ophthalmic suspensions).5 Due to potential side effects of medications, especially topical corticosteroids, patients should be referred to ophthalmologists for definitive diagnosis and treatment.
Keratoconjunctivitis
Atopic keratoconjunctivitis (AKC) is a noninfectious inflammatory condition of the cornea and conjunctiva that occurs in an estimated 25% to 42% of patients with AD.6,7 It frequently presents in late adolescence and has a peak incidence between 30 and 50 years of age.8 The symptoms of AKC include ocular pruritus, redness, ropy mucoid discharge, burning discomfort, photophobia, and blurring of vision. Corneal involvement can progress to corneal neovascularization and punctate or macroepithelial erosions and ulcerations, which increase the risk for corneal scarring and visual impairment.7
Keratoconjunctivitis is a complex inflammatory disease characterized by infiltration of the conjunctival epithelium by eosinophils, mast cells, and lymphocytes. On examination, patients frequently are found to have concurrent AD of the periorbital skin as well as papillary hypertrophy of the tarsal conjunctiva with accompanying fibrosis, which can lead to entropion (turning inward of the lid margins and lashes) in severe cases.7 Ophthalmic evaluation is strongly recommended for patients with AKC to control symptoms, to limit exacerbations, and to prevent sight-threatening inflammation leading to vision loss. Treatment can be challenging given the chronicity of the condition and may require multiple treatment arms. Conservative measures include cool compresses and treatment with ophthalmic eye drops containing antihistamines (ie, ketotifen 0.025% [available over-the-counter]) and mast cell stabilizers (ie, olopatadine ophthalmic solution 0.1%).8 Atopic keratoconjunctivitis exacerbations may require short-term use of topical steroids or calcineurin inhibitors, or systemic equivalents for refractory cases.6 Long-term maintenance therapy typically consists of proper eye hygiene and steroid-sparing agents that reduce ocular inflammation, such as topical cyclosporine and tacrolimus, neither of which are associated with increased intraocular pressure (IOP)(Figure 1).8 Cornea disease resulting from chronic conjunctival/lid microtrauma can be managed with soft or scleral contact lenses.

Keratoconus
Keratoconus is a noninflammatory ocular disorder characterized by progressive thinning and conelike protrusion of the cornea. The corneal topographic changes result in high irregular astigmatism and reduced visual acuity, which can manifest as image blurring or distortion (Figure 2).2,9 Multiple case series and controlled studies have reported a positive association between keratoconus and a history of atopic disease.10,11

The precise etiology of keratoconus in the context of AD is unclear and likely is multifactorial. Habitual eye rubbing from periocular pruritus and discomfort has been reported to be a notable contributor to keratoconus.12 In addition, intrinsic inflammation and imbalance of cytokines and proteases also may contribute to development of keratoconus.13
Keratoconus is a progressive condition that can severely impact vision, making it critical to diagnose patients before irreversible vision loss occurs. Individuals with risk factors, such as AD of the eyelids, history of eye rubbing, or family history of keratoconus, should be advised to receive routine vision screening for worsening astigmatism, especially during the first few decades of life when keratoconus progresses rapidly.
The conservative management for early keratoconus includes glasses and gas-permeable contact lenses for correction of visual acuity and astigmatism. For advanced keratoconus, scleral lenses often are prescribed. These large-diameter, gas-permeable lenses are designed to rest on the sclera and arch over the entire cornea.9 Alternatively, corneal collagen cross-linking is a newer technique that utilizes riboflavin and UVA irradiation to strengthen the corneal tissue. It has proven to be safe and effective in slowing or stopping the progression of keratoconus, particularly when treated at the early stage, and received US Food and Drug Administration approval in 2016.9
Glaucoma
Glaucoma is a well-known complication of AD and can lead to irreversible ocular hypertension and optic nerve damage. Corticosteroid use is a major risk factor for glaucoma, and the rise in IOP is thought to be due to increased aqueous outflow resistance.14
Multiple case reports have linked glaucoma to long-term use of potent topical corticosteroids in the facial and palpebral regions, which has been attributed to direct steroid contact and absorption by ocular tissues, as glaucoma rarely occurs with topical steroid application elsewhere on the body.15-17 Systemic steroids (ie, prednisolone) taken for more than 8 weeks also have been associated with a marked rise in IOP.18
Certain risk factors may predispose a steroid user to increased IOP, including existing open-angle glaucoma, diabetes mellitus, collagen disease, and high myopia.15,19 Steroid responders and younger individuals also demonstrate increased sensitivity to steroids.20
Given that glaucoma often is asymptomatic until advanced stages, early detection is the key for proper intervention. Periodic glaucoma screening by an ophthalmologist would be appropriate for known steroid responders, as well as patients with a prolonged history of topical steroid application in the palpebral region and systemic steroid use, family history of glaucoma, or known ocular pathology.21 Furthermore, patients with concurrent glaucoma and AD should be jointly managed by dermatology and ophthalmology, and systemic and topical corticosteroid use should be minimized in favor of alterative agents such as calcineurin inhibitors.22
In addition to steroid-induced glaucoma, intrinsic atopic glaucoma recently has been proposed as a clinical entity and is characterized by increased inflammatory cytokines—IL-8 and CCL2—in the aqueous humor and abnormal accumulation of fibers in corneoscleral meshwork.23
Cataracts
Cataracts are estimated to affect 8% to 25% of patients with AD.21,24 Unlike age-related cataracts, cataracts associated with AD are observed in adolescents and young adults in addition to the older population. The progression of lenticular opacity can rapidly occur and has been reported to coincide with AD flares.25,26
Patients with AD typically present with anterior or posterior subcapsular cataracts instead of nuclear and cortical cataracts, which are more common in the general population.27,28 Anterior subcapsular cataracts are more specific to AD, whereas posterior subcapsular cataracts are associated with both prolonged corticosteroid use and AD.26 Children generally are more sensitive to steroids than adults and may develop cataracts more rapidly and at lower concentrations.29
The pathophysiology of cataract formation and progression in the context of AD is multifactorial. Cataract patients with AD have compromised blood-retinal barrier integrity as well as increased oxidative damage in the lens.30,31 Genetics and blunt trauma from eye rubbing are thought to play a role, and the latter has been associated with faster progression of cataracts.28 In contrast, corticosteroid-induced cataracts likely are caused by transcriptional changes and disrupted osmotic balance in the lens fibers, which can lead to fiber rupture and lens opacification.26,32 Systemic corticosteroids show the strongest association with cataract development, but inhaled and topical steroids also have been implicated.26
Although cataracts can be surgically corrected, prevention is critical. Patients with early-onset periorbital AD, prolonged use of topical or systemic corticosteroids, and family history of cataracts should be routinely screened. Anterior and posterior subcapsular cataracts are diagnosed with red reflex examinations that can be readily performed by the primary care physician or ophthalmologist.33 Atopic dermatitis patients with cataracts should be advised to use calcineurin inhibitors and alternative treatments in place of corticosteroids.
Retinal Detachment
Retinal detachment (RD) is a serious complication of AD that can present in individuals younger than 35 years. The incidence of RD in patients with AD has been estimated to be 4% to 8%.34 Retinal detachment manifests with visual disturbances such as flashing lights, shadows, visual field defect, and blurring of vision, but also may occur in the absence of vision changes.35,36
Across multiple case series, patients who developed RD were consistently found to have AD in the facial or periorbital region and a history of chronic eye rubbing. Multiple patients also presented with concurrent proliferative vitreoretinopathy, lens subluxation, and/or cataracts.35,37 The mechanism for RD has been attributed to ocular contusion from vigorous eye rubbing, as fundus findings between traumatic and AD-associated RD are similarly characterized by tractional breaks in the retina at vitreous base borders.37
Avoidance of eye rubbing and optimized treatment of facial AD may help prevent RD in patients with AD. Furthermore, all patients with symptoms of RD should be immediately referred to ophthalmology for surgical repair.
Herpetic Ocular Disease
Ocular herpes simplex virus infections cause ocular pain and are associated with notable visual morbidity, as recurrences can result in irreversible corneal scarring and neovascularization. Two retrospective case-control studies independently reported that individuals with a history of AD are at greater risk for herpetic ocular disease compared to age-matched controls.38,39 Furthermore, atopic disease is associated with higher recurrence rates and slower regeneration of the corneal epithelium.40
These findings suggest that AD patients with a history of recurrent herpetic ocular diseases should be closely monitored and treated with antiviral prophylaxis and/or topical corticosteroids, depending on the type of keratitis (epithelial or stromal).40 Furthermore, active ocular herpetic infections warrant urgent referral to an ophthalmologist.
Dupilumab-Associated Ocular Complications
Dupilumab, a monoclonal antibody that blocks IL-4 and IL-13 signaling, is the first biologic therapy to be approved for treatment of moderate to severe AD. Prior clinical trials have described a higher incidence of anterior conjunctivitis in dupilumab-treated AD patients (5%–28%) compared to placebo (2%–11%).41 Of note, the incidence may be as high as 70%, as reported in a recent case series.42 Interestingly, independent trials assessing dupilumab treatment in asthma, nasal polyposis, and eosinophilic esophagitis patients did not observe a higher incidence of conjunctivitis in dupilumab-treated patients compared to placebo, suggesting an AD-specific mechanism.43
Prominent features of dupilumab-associated conjunctivitis include hyperemia of the conjunctiva and limbus, in addition to ocular symptoms such as tearing, burning, and bilateral decrease in visual acuity. Marked reduction of conjunctival goblet cells has been reported.44 In addition to conjunctivitis, blepharitis also has been reported during dupilumab treatment.45
Standardized treatment guidelines for dupilumab-associated ocular complications have not yet been established. Surprisingly, antihistamine eye drops appear to be inefficacious in the treatment of dupilumab-associated conjunctivitis.41 However, the condition has been successfully managed with topical steroids (fluorometholone ophthalmic suspension 0.1%) and tacrolimus ointment 0.03%.41 Lifitegrast, an anti-inflammatory agent approved for chronic dry eye, also has been suggested as a treatment option for patients refractory to topical steroids.45 Alternatively, cessation of dupilumab could be considered in AD patients who experience severe ocular complications. Atopic dermatitis patients taking dupilumab who have any concerning signs for ocular complications should be referred to an ophthalmologist for further diagnosis and management.
Conclusion
Practicing dermatologists likely will encounter patients with concurrent AD and ocular complications. Although eye examinations are not routinely performed in the care of AD patients, dermatologists can proactively inquire about ocular symptoms and monitor patients longitudinally. Early diagnosis and treatment of these ocular conditions can prevent vision loss in these patients. Furthermore, symptomatic control of AD and careful consideration of the side-effect profiles of medications can potentially reduce the incidence of ocular complications in individuals with AD.
Patients with visual concerns or risk factors, such as a history of vigorous eye rubbing or chronic corticosteroid use, should be jointly managed with an ophthalmologist for optimized care. Moreover, acute exacerbations of ocular symptoms and visual deterioration warrant urgent referral to ophthalmology.
- Weidinger S, Beck LA, Bieber T, et al. Atopic dermatitis. Nat Rev Dis Primers. 2018;4:1.
- Govind K, Whang K, Khanna R, et al. Atopic dermatitis is associated with increased prevalence of multiple ocular comorbidities. J Allergy Clin Immunol Pract. 2019;7:298-299.
- Thyssen JP, Toft PB, Halling-Overgaard AS, et al. Incidence, prevalence, and risk of selected ocular disease in adults with atopic dermatitis. J Am Acad Dermatol. 2017;77:280-286.e281.
- Putnam CM. Diagnosis and management of blepharitis: an optometrist’s perspective. Clin Optom (Auckl). 2016;8:71-78.
- Amescua G, Akpek EK, Farid M, et al. Blepharitis Preferred Practice Pattern®. Ophthalmology. 2019;126:P56-P93.
- Bielory B, Bielory L. Atopic dermatitis and keratoconjunctivitis. Immunol Allergy Clin North Am. 2010;30:323-336.
- Guglielmetti S, Dart JK, Calder V. Atopic keratoconjunctivitis and atopic dermatitis. Curr Opin Allergy Clin Immunol. 2010;10:478-485.
- Chen JJ, Applebaum DS, Sun GS, et al. Atopic keratoconjunctivitis: a review. J Am Acad Dermatol. 2014;70:569-575.
- Andreanos KD, Hashemi K, Petrelli M, et al. Keratoconus treatment algorithm. Ophthalmol Ther. 2017;6:245-262.
- Rahi A, Davies P, Ruben M, et al. Keratoconus and coexisting atopic disease. Br J Ophthalmol. 1977;61:761-764.
- Gasset AR, Hinson WA, Frias JL. Keratoconus and atopic diseases. Ann Ophthalmol. 1978;10:991-994.
- Bawazeer AM, Hodge WG, Lorimer B. Atopy and keratoconus: a multivariate analysis. Br J Ophthalmol. 2000;84:834-836.
- Galvis V, Sherwin T, Tello A, et al. Keratoconus: an inflammatory disorder? Eye (Lond). 2015;29:843-859.
- Clark AF, Wordinger RJ. The role of steroids in outflow resistance. Exp Eye Res. 2009;88:752-759.
- Daniel BS, Orchard D. Ocular side-effects of topical corticosteroids: what a dermatologist needs to know. Australas J Dermatol. 2015;56:164-169.
- Garrott HM, Walland MJ. Glaucoma from topical corticosteroids to the eyelids. Clin Exp Ophthalmol. 2004;32:224-226.
- Aggarwal RK, Potamitis T, Chong NH, et al. Extensive visual loss with topical facial steroids. Eye (Lond). 1993;7(pt 5):664-666.
- Mandapati JS, Metta AK. Intraocular pressure variation in patients on long-term corticosteroids. Indian Dermatol Online J. 2011;2:67-69.
- Jones R 3rd, Rhee DJ. Corticosteroid-induced ocular hypertension and glaucoma: a brief review and update of the literature. Curr Opin Ophthalmol. 2006;17:163-167.
- Tamagawa-Mineoka R, Yasuoka N, Ueta M, et al. Influence of topical steroids on intraocular pressure in patients with atopic dermatitis. Allergol Int. 2018;67:388-391.
- Bercovitch L. Screening for ocular complications in atopic dermatitis. Arch Dermatol. 2011;147:588-589.
- Abramovits W, Hung P, Tong KB. Efficacy and economics of topical calcineurin inhibitors for the treatment of atopic dermatitis. Am J Clin Dermatol. 2006;7:213-222.
- Takakuwa K, Hamanaka T, Mori K, et al. Atopic glaucoma: clinical and pathophysiological analysis. J Glaucoma. 2015;24:662-668.
- Haeck IM, Rouwen TJ, Timmer-de Mik L, et al. Topical corticosteroids in atopic dermatitis and the risk of glaucoma and cataracts. J Am Acad Dermatol. 2011;64:275-281.
- Amemiya T, Matsuda H, Uehara M. Ocular findings in atopic dermatitis with special reference to the clinical features of atopic cataract. Ophthalmologica. 1980;180:129-132.
- Tatham A. Atopic dermatitis, cutaneous steroids and cataracts in children: two case reports. J Med Case Rep. 2008;2:124.
- Chew M, Chiang PP, Zheng Y, et al. The impact of cataract, cataract types, and cataract grades on vision-specific functioning using Rasch analysis. Am J Ophthalmol. 2012;154:29-38.
- Nagaki Y, Hayasaka S, Kadoi C. Cataract progression in patients with atopic dermatitis. J Cataract Refract Surg. 1999;25:96-99.
- Kaye LD, Kalenak JW, Price RL, et al. Ocular implications of long-term prednisone therapy in children. J Pediatr Ophthalmol Strabismus. 1993;30:142-144.
- Matsuo T, Saito H, Matsuo N. Cataract and aqueous flare levels in patients with atopic dermatitis. Am J Ophthalmol. 1997;124:36-39.
- Namazi MR, Handjani F, Amirahmadi M. Increased oxidative activity from hydrogen peroxide may be the cause of the predisposition to cataracts among patients with atopic dermatitis. Med Hypotheses. 2006;66:863-864.
- James ER. The etiology of steroid cataract. J Ocul Pharmacol Ther. 2007;23:403-420.
- Lambert SR, Teng JMC. Assessing whether the cataracts associated with atopic dermatitis are associated with steroids or inflammatory factors. JAMA Ophthalmol. 2018;136:918-919.
- Sasoh M, Mizutani H, Matsubara H, et al. Incidence of retinal detachment associated with atopic dermatitis in Japan: review of cases from 1992 to 2011. Clin Ophthalmol. 2015;9:1129-1134.
- Yoneda K, Okamoto H, Wada Y, et al. Atopic retinal detachment. report of four cases and a review of the literature. Br J Dermatol. 1995;133:586-591.
- Gnana Jothi V, McGimpsey S, Sharkey JA, et al. Retinal detachment repair and cataract surgery in patients with atopic dermatitis. Eye (Lond). 2017;31:1296-1301.
- Oka C, Ideta H, Nagasaki H, et al. Retinal detachment with atopic dermatitis similar to traumatic retinal detachment. Ophthalmology. 1994;101:1050-1054.
- Prabriputaloong T, Margolis TP, Lietman TM, et al. Atopic disease and herpes simplex eye disease: a population-based case-control study. Am J Ophthalmol. 2006;142:745-749.
- Borkar DS, Gonzales JA, Tham VM, et al. Association between atopy and herpetic eye disease: results from the pacific ocular inflammation study. JAMA Ophthalmol. 2014;132:326-331.
- Rezende RA, Hammersmith K, Bisol T, et al. Comparative study of ocular herpes simplex virus in patients with and without self-reported atopy. Am J Ophthalmol. 2006;141:1120-1125.
- Wollenberg A, Ariens L, Thurau S, et al. Conjunctivitis occurring in atopic dermatitis patients treated with dupilumab-clinical characteristics and treatment. J Allergy Clin Immunol Pract. 2018;6:1778-1780.e1.
- Ivert LU, Wahlgren CF, Ivert L, et al. Eye complications during dupilumab treatment for severe atopic dermatitis. Acta Derm Venereol. 2019;99:375-378.
- Akinlade B, Guttman-Yassky E, de Bruin-Weller M, et al. Conjunctivitis in dupilumab clinical trials [published online March 9, 2019]. Br J Dermatol. doi:10.1111/bjd.17869.
- Bakker DS, Ariens LFM, van Luijk C, et al. Goblet cell scarcity and conjunctival inflammation during treatment with dupilumab in patients with atopic dermatitis. Br J Dermatol. 2019;180:1248-1249.
- Zirwas MJ, Wulff K, Beckman K. Lifitegrast add-on treatment for dupilumab-induced ocular surface disease (DIOSD): a novel case report. JAAD Case Rep. 2019;5:34-36.
- Weidinger S, Beck LA, Bieber T, et al. Atopic dermatitis. Nat Rev Dis Primers. 2018;4:1.
- Govind K, Whang K, Khanna R, et al. Atopic dermatitis is associated with increased prevalence of multiple ocular comorbidities. J Allergy Clin Immunol Pract. 2019;7:298-299.
- Thyssen JP, Toft PB, Halling-Overgaard AS, et al. Incidence, prevalence, and risk of selected ocular disease in adults with atopic dermatitis. J Am Acad Dermatol. 2017;77:280-286.e281.
- Putnam CM. Diagnosis and management of blepharitis: an optometrist’s perspective. Clin Optom (Auckl). 2016;8:71-78.
- Amescua G, Akpek EK, Farid M, et al. Blepharitis Preferred Practice Pattern®. Ophthalmology. 2019;126:P56-P93.
- Bielory B, Bielory L. Atopic dermatitis and keratoconjunctivitis. Immunol Allergy Clin North Am. 2010;30:323-336.
- Guglielmetti S, Dart JK, Calder V. Atopic keratoconjunctivitis and atopic dermatitis. Curr Opin Allergy Clin Immunol. 2010;10:478-485.
- Chen JJ, Applebaum DS, Sun GS, et al. Atopic keratoconjunctivitis: a review. J Am Acad Dermatol. 2014;70:569-575.
- Andreanos KD, Hashemi K, Petrelli M, et al. Keratoconus treatment algorithm. Ophthalmol Ther. 2017;6:245-262.
- Rahi A, Davies P, Ruben M, et al. Keratoconus and coexisting atopic disease. Br J Ophthalmol. 1977;61:761-764.
- Gasset AR, Hinson WA, Frias JL. Keratoconus and atopic diseases. Ann Ophthalmol. 1978;10:991-994.
- Bawazeer AM, Hodge WG, Lorimer B. Atopy and keratoconus: a multivariate analysis. Br J Ophthalmol. 2000;84:834-836.
- Galvis V, Sherwin T, Tello A, et al. Keratoconus: an inflammatory disorder? Eye (Lond). 2015;29:843-859.
- Clark AF, Wordinger RJ. The role of steroids in outflow resistance. Exp Eye Res. 2009;88:752-759.
- Daniel BS, Orchard D. Ocular side-effects of topical corticosteroids: what a dermatologist needs to know. Australas J Dermatol. 2015;56:164-169.
- Garrott HM, Walland MJ. Glaucoma from topical corticosteroids to the eyelids. Clin Exp Ophthalmol. 2004;32:224-226.
- Aggarwal RK, Potamitis T, Chong NH, et al. Extensive visual loss with topical facial steroids. Eye (Lond). 1993;7(pt 5):664-666.
- Mandapati JS, Metta AK. Intraocular pressure variation in patients on long-term corticosteroids. Indian Dermatol Online J. 2011;2:67-69.
- Jones R 3rd, Rhee DJ. Corticosteroid-induced ocular hypertension and glaucoma: a brief review and update of the literature. Curr Opin Ophthalmol. 2006;17:163-167.
- Tamagawa-Mineoka R, Yasuoka N, Ueta M, et al. Influence of topical steroids on intraocular pressure in patients with atopic dermatitis. Allergol Int. 2018;67:388-391.
- Bercovitch L. Screening for ocular complications in atopic dermatitis. Arch Dermatol. 2011;147:588-589.
- Abramovits W, Hung P, Tong KB. Efficacy and economics of topical calcineurin inhibitors for the treatment of atopic dermatitis. Am J Clin Dermatol. 2006;7:213-222.
- Takakuwa K, Hamanaka T, Mori K, et al. Atopic glaucoma: clinical and pathophysiological analysis. J Glaucoma. 2015;24:662-668.
- Haeck IM, Rouwen TJ, Timmer-de Mik L, et al. Topical corticosteroids in atopic dermatitis and the risk of glaucoma and cataracts. J Am Acad Dermatol. 2011;64:275-281.
- Amemiya T, Matsuda H, Uehara M. Ocular findings in atopic dermatitis with special reference to the clinical features of atopic cataract. Ophthalmologica. 1980;180:129-132.
- Tatham A. Atopic dermatitis, cutaneous steroids and cataracts in children: two case reports. J Med Case Rep. 2008;2:124.
- Chew M, Chiang PP, Zheng Y, et al. The impact of cataract, cataract types, and cataract grades on vision-specific functioning using Rasch analysis. Am J Ophthalmol. 2012;154:29-38.
- Nagaki Y, Hayasaka S, Kadoi C. Cataract progression in patients with atopic dermatitis. J Cataract Refract Surg. 1999;25:96-99.
- Kaye LD, Kalenak JW, Price RL, et al. Ocular implications of long-term prednisone therapy in children. J Pediatr Ophthalmol Strabismus. 1993;30:142-144.
- Matsuo T, Saito H, Matsuo N. Cataract and aqueous flare levels in patients with atopic dermatitis. Am J Ophthalmol. 1997;124:36-39.
- Namazi MR, Handjani F, Amirahmadi M. Increased oxidative activity from hydrogen peroxide may be the cause of the predisposition to cataracts among patients with atopic dermatitis. Med Hypotheses. 2006;66:863-864.
- James ER. The etiology of steroid cataract. J Ocul Pharmacol Ther. 2007;23:403-420.
- Lambert SR, Teng JMC. Assessing whether the cataracts associated with atopic dermatitis are associated with steroids or inflammatory factors. JAMA Ophthalmol. 2018;136:918-919.
- Sasoh M, Mizutani H, Matsubara H, et al. Incidence of retinal detachment associated with atopic dermatitis in Japan: review of cases from 1992 to 2011. Clin Ophthalmol. 2015;9:1129-1134.
- Yoneda K, Okamoto H, Wada Y, et al. Atopic retinal detachment. report of four cases and a review of the literature. Br J Dermatol. 1995;133:586-591.
- Gnana Jothi V, McGimpsey S, Sharkey JA, et al. Retinal detachment repair and cataract surgery in patients with atopic dermatitis. Eye (Lond). 2017;31:1296-1301.
- Oka C, Ideta H, Nagasaki H, et al. Retinal detachment with atopic dermatitis similar to traumatic retinal detachment. Ophthalmology. 1994;101:1050-1054.
- Prabriputaloong T, Margolis TP, Lietman TM, et al. Atopic disease and herpes simplex eye disease: a population-based case-control study. Am J Ophthalmol. 2006;142:745-749.
- Borkar DS, Gonzales JA, Tham VM, et al. Association between atopy and herpetic eye disease: results from the pacific ocular inflammation study. JAMA Ophthalmol. 2014;132:326-331.
- Rezende RA, Hammersmith K, Bisol T, et al. Comparative study of ocular herpes simplex virus in patients with and without self-reported atopy. Am J Ophthalmol. 2006;141:1120-1125.
- Wollenberg A, Ariens L, Thurau S, et al. Conjunctivitis occurring in atopic dermatitis patients treated with dupilumab-clinical characteristics and treatment. J Allergy Clin Immunol Pract. 2018;6:1778-1780.e1.
- Ivert LU, Wahlgren CF, Ivert L, et al. Eye complications during dupilumab treatment for severe atopic dermatitis. Acta Derm Venereol. 2019;99:375-378.
- Akinlade B, Guttman-Yassky E, de Bruin-Weller M, et al. Conjunctivitis in dupilumab clinical trials [published online March 9, 2019]. Br J Dermatol. doi:10.1111/bjd.17869.
- Bakker DS, Ariens LFM, van Luijk C, et al. Goblet cell scarcity and conjunctival inflammation during treatment with dupilumab in patients with atopic dermatitis. Br J Dermatol. 2019;180:1248-1249.
- Zirwas MJ, Wulff K, Beckman K. Lifitegrast add-on treatment for dupilumab-induced ocular surface disease (DIOSD): a novel case report. JAAD Case Rep. 2019;5:34-36.
Practice Points
- Atopic dermatitis (AD) is associated with various ocular comorbidities that can result in permanent vision loss if untreated.
- Timely recognition of ocular complications in AD patients is critical, and dermatologists should proactively inquire about ocular symptoms in the review of systems.
- Patients with ocular symptoms should be jointly managed with ophthalmology.