User login
Impact of 3 Months of Supervised Exercise on Function by Arthritis Status
Impact of 3 Months of Supervised Exercise on Function by Arthritis Status
About half of US adults aged ≥ 65 years report arthritis, and of those, 44% have an arthritis-attributable activity limitation.1,2 Arthritis is a significant health issue for veterans, with veterans reporting higher rates of disability compared with the civilian population.3
Osteoarthritis (OA) is the most common type of arthritis.4 Among individuals aged ≥ 40 years, the incidence of OA is nearly twice as high among veterans compared with civilians and is a leading cause of separation from military service and disability.5,6 OA pain and disability have been shown to be associated with increases in health care and medication use, including opioids, nonsteroidal anti-inflammatory medications, and muscle relaxants.7,8 Because OA is chronic and has no cure, safe and effective management strategies—such as exercise— are critical to minimize pain and maintain physical function.9
Exercise can reduce pain and disability associated with OA and is a first-line recommendation in guidelines for the treatment of knee and hip OA.9 Given the limited exercise and high levels of physical inactivity among veterans with OA, there is a need to identify opportunities that support veterans with OA engaging in regular exercise.
Gerofit, an outpatient clinical exercise program available at 30 Veterans Health Administration (VHA) sites, may provide an opportunity for older veterans with arthritis to engage in exercise.10 Gerofit is specifically designed for veterans aged ≥ 65 years. It is not disease-specific and supports older veterans with multiple chronic conditions, including OA. Veterans aged ≥ 65 years with a referral from a VA clinician are eligible for Gerofit. Those who are unable to perform activities of daily living; unable to independently function without assistance; have a history of unstable angina, proliferative diabetic retinopathy, oxygen dependence, volatile behavioral issues, or are unable to work successfully in a group environment/setting; experience active substance abuse, homelessness, or uncontrolled incontinence; and have open wounds that cannot be appropriately dressed are excluded from Gerofit. Exercise sessions are held 3 times per week and last from 60 to 90 minutes. Sessions are supervised by Gerofit staff and include personalized exercise prescriptions based on functional assessments. Exercise prescriptions include aerobic, resistance, and balance/flexibility components and are modified by the Gerofit program staff as needed. Gerofit adopts a functional fitness approach and includes individual progression as appropriate according to evidence-based guidelines, using the Borg ratings of perceived exertion. 11 Assessments are performed at baseline, 3 months, 6 months, and annually thereafter. Clinical staff conduct all assessments, including physical function testing, and record them in a database. Assessments are reviewed with the veteran to chart progress and identify future goals or needs. Veterans perform personalized self-paced exercises in the Gerofit group setting. Exercise prescriptions are continuously modified to meet individualized needs and goals. Veterans may participate continuously with no end date.
Participation in supervised exercise is associated with improved physical function and individuals with arthritis can improve function even though their baseline functional status is lower than individuals without arthritis. 12 In this analysis, we examine the impact of exercise on the status and location of arthritis (upper body, lower body, or both). Lower body arthritis is more common than upper body arthritis and lower extremity function is associated with increased ability to perform activities of daily living, resulting in independence among older adults.13,14 We also include upper body strength measures to capture important functional movements such as reaching and pulling.15 Among those who participate in Gerofit, the greatest gains in physical function occur during the initial 3 months, which tend to be sustained over 12 months.16 For this reason, this study focused on the initial 3 months of the program.
Older adults with arthritis may have pain and functional limitations that exceed those of the general older adult population. Exercise programs for older adults that do not specifically target arthritis but are able to improve physical function among those with arthritis could potentially increase access to exercise for older adults living with arthritis. Therefore, the purpose of this study was to determine whether change in physical function with participation in Gerofit for 3 months varies by arthritis status, including no arthritis, any arthritis, lower body arthritis, or both upper and lower body arthritis compared with no arthritis.
Methods
This is a secondary analysis of previously collected data from 10 VHA Gerofit sites (Ann Arbor, Baltimore, Greater Los Angeles, Canandaigua, Cincinnati, Miami, Honolulu, Denver, Durham, and Pittsburgh) from 2002 to 2019. Implementation data regarding the consistency of the program delivery at Gerofit expansion sites have been previously published.16 Although the delivery of Gerofit transitioned to telehealth due to COVID-19, data for this analysis were collected from in-person exercise sessions prior to the pandemic.17 Data were collected for clinical purposes. This project was part of the Gerofit quality improvement initiative and was reviewed and approved by the Durham Institutional Review Board as quality improvement.
Participants in Gerofit who completed baseline and 3-month assessments were included to analyze the effects of exercise on physical function. At each of the time points, physical functional assessments included: (1) usual gait speed (> 10 meters [m/s], or 10- meter walk test [10MWT]); (2) lower body strength (chair stands [number completed in 30 seconds]); (3) upper body strength (number of arm curls [5-lb for females/8-lb for males] completed in 30 seconds); and (4) 6-minute walk distance [6MWD] in meters to measure aerobic endurance). These measures have been validated in older adults.18-21 Arm curls were added to the physical function assessments after the 10MWT, chair stands, and 6MWD; therefore, fewer participants had data for this measure. Participants self-reported at baseline on 45 common medical conditions, including arthritis or rheumatism (both upper body and lower body were offered as choices). Self-reporting has been shown to be an acceptable method of identifying arthritis in adults.22
Descriptive statistics at baseline were calculated for all participants. One-way analysis of variance and X2 tests were used to determine differences in baseline characteristics across arthritis status. The primary outcomes were changes in physical function measures from baseline to 3 months by arthritis status. Arthritis status was defined as: any arthritis, which includes individuals who reported upper body arthritis, lower body arthritis, or both; and arthritis status individuals reporting either upper body arthritis, lower body arthritis, or both. Categories of arthritis for arthritis status were mutually exclusive. Two separate linear models were constructed for each of the 4 physical function measures, with change from baseline to 3 months as the outcome (dependent variable) and arthritis status, age, and body mass index (BMI) as predictors (independent variables). The first model compared any arthritis with no arthritis and the second model compared arthritis status (both upper and lower body arthritis vs lower body arthritis) with no arthritis. These models were used to obtain mean changes and 95% CIs in physical function and to test for differences in the change in physical function measures by arthritis status. Statistical analyses were performed using R software, version 4.0.3.
Results
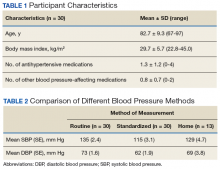
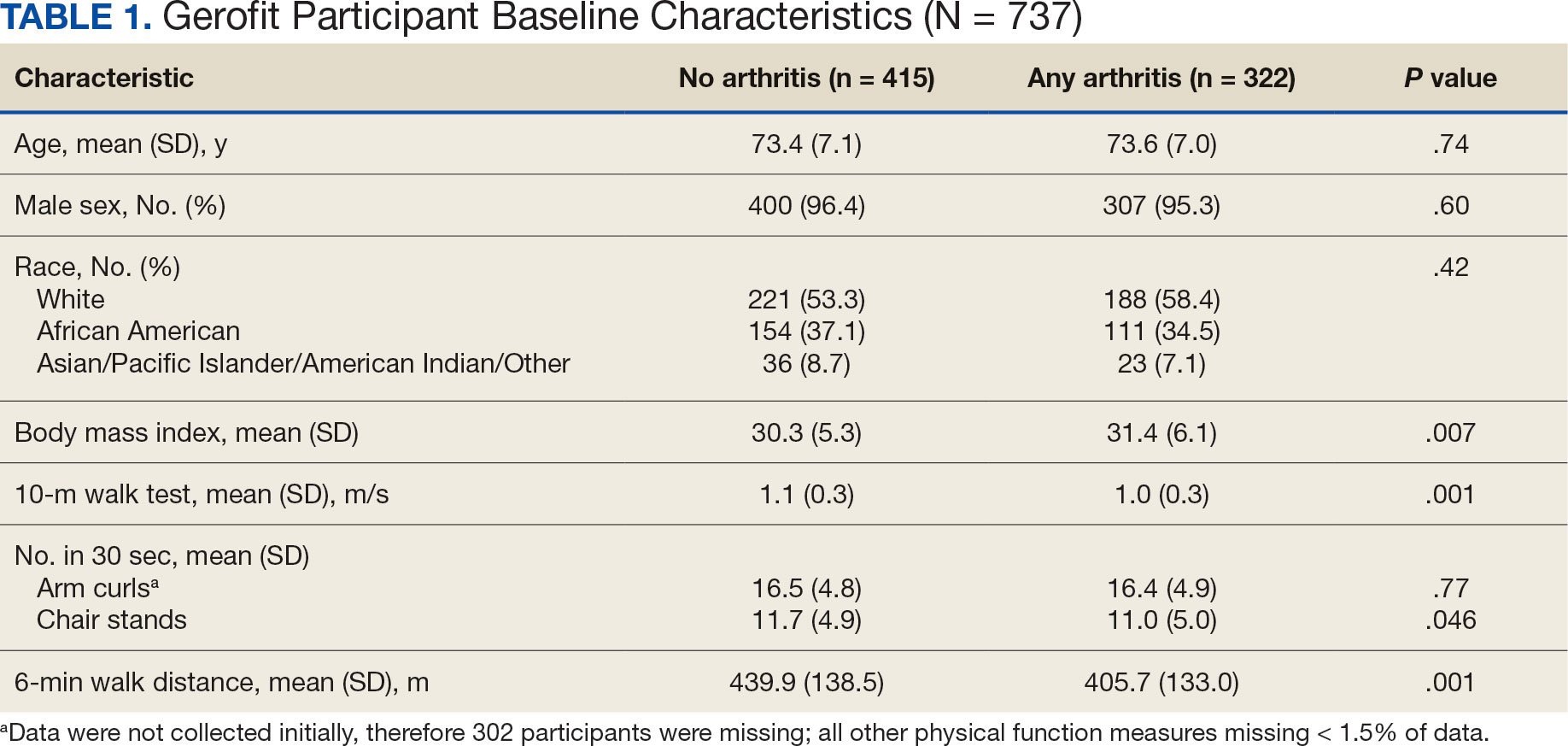
Baseline and 3-month data were available for 737 Gerofit participants and included in the analysis. The mean (SD) age was 73.5 (7.1) years. A total of 707 participants were male (95.9%) and 322 (43.6%) reported some arthritis, with arthritis in both the upper and lower body being reported by 168 participants (52.2%) (Table 1). There were no differences in age, sex, or race for those with any arthritis compared with those with no arthritis, but BMI was significantly higher in those reporting any arthritis compared with no arthritis. For the baseline functional measures, statistically significant differences were observed between those with no arthritis and those reporting any arthritis for the 10MWT (P = .001), chair stands (P = .046), and 6MWD (P = .001), but not for arm curls (P = .77), with those with no arthritis performing better.
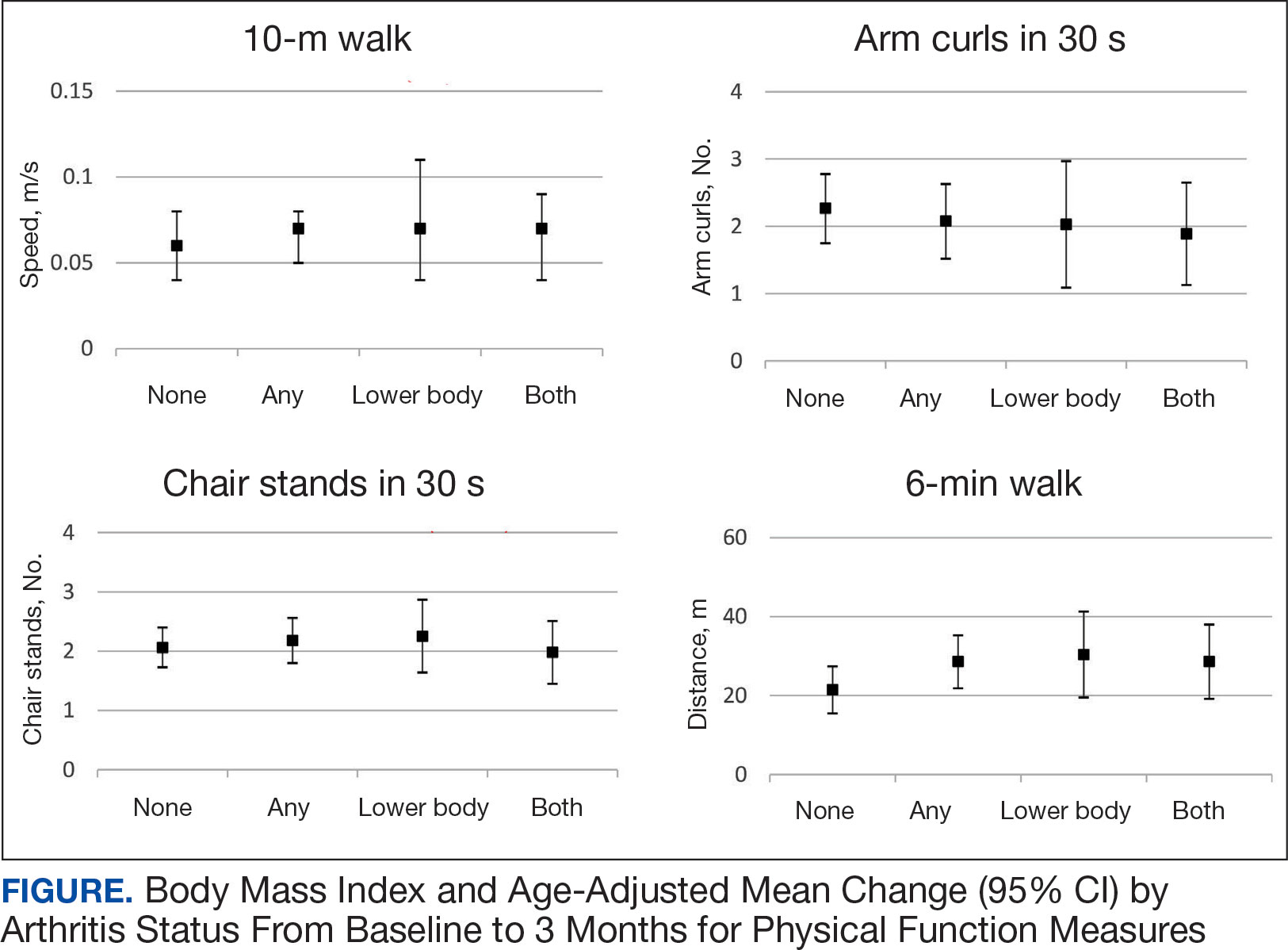
All 4 arthritis status groups showed improvements in each of the physical function measures over 3 months. For the 10MWT the mean change (95% CI) in gait speed (m/s) was 0.06 (0.04-0.08) for patients with no arthritis, 0.07 (0.05- 0.08) for any arthritis, 0.07 (0.04-0.11) for lower body arthritis, and 0.07 (0.04- 0.09) for both lower and upper body arthritis. For the number of arm curls in 30 seconds the mean change (95% CI) was 2.3 (1.8-2.8) for patients with no arthritis, 2.1 (1.5-2.6) for any arthritis, 2.0 (1.1-3.0) for lower body arthritis, and 1.9 (1.1-2.7) for both lower and upper body arthritis. For the number of chair stands in 30 seconds the mean change (95% CI) was 2.1 (1.7-2.4) for patients with no arthritis, 2.2 (1.8-2.6) for any arthritis, 2.3 (1.6-2.9), for lower body arthritis, and 2.0 (1.5-2.5) for both lower and upper body arthritis. For the 6MWD distance in meters the mean change (95% CI) was 21.5 (15.5-27.4) for patients with no arthritis, 28.6 (21.9-35.3) for any arthritis, 30.4 (19.5-41.3) for lower body arthritis, and 28.6 (19.2-38.0) for both lower and upper body arthritis (Figure).
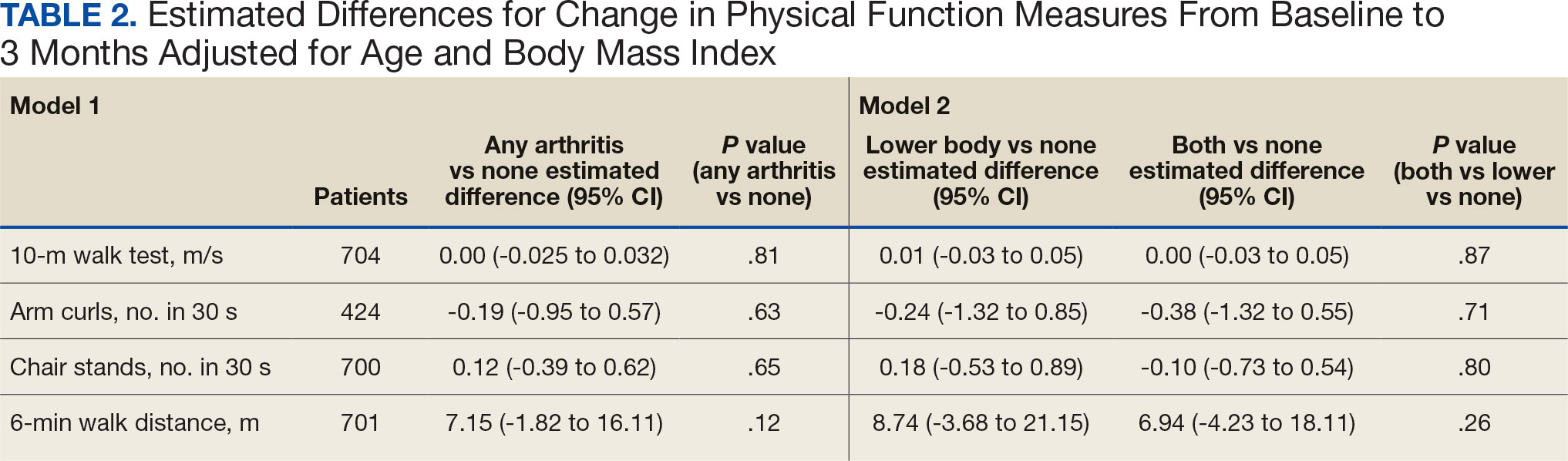
We used 2 models to measure the change from baseline to 3 months for each of the arthritis groups. Model 1 compared any arthritis vs no arthritis and model 2 compared lower body arthritis and both upper and lower body arthritis vs no arthritis for each physical function measure (Table 2). There were no statistically significant differences in 3-month change in physical function for any of the physical function measures between arthritis groups after adjusting for age and BMI.
Discussion
Participation in Gerofit was associated with functional gains among all participants over 3 months, regardless of arthritis status. Older veterans reporting any arthritis had significantly lower physical function scores upon enrollment into Gerofit compared with those veterans reporting no arthritis. However, compared with individuals who reported no arthritis, individuals who reported arthritis (any arthritis, lower body arthritis only, or both lower and upper body arthritis) experienced similar improvements (ie, no statistically significant differences in mean change from baseline to follow-up among those with and without arthritis). This study suggests that progressive, multicomponent exercise programs for older adults may be beneficial for those with arthritis.
Involvement of multiple sites of arthritis is associated with moderate to severe functional limitations as well as lower healthrelated quality of life.23 While it has been found that individuals with arthritis can improve function with supervised exercise, even though their baseline functional status is lower than individuals without arthritis, it was not clear whether individuals with multiple joint involvement also would benefit.12 The results of this study suggest that these individuals can improve across various domains of physical function despite variation in arthritis location and status. As incidence of arthritis increases with age, targeting older adults for exercise programs such as Gerofit may improve functional limitations and health-related quality of life associated with arthritis.2
We evaluated physical function using multiple measures to assess upper (arm curls) and lower (chair stands, 10MWT) extremity physical function and aerobic endurance (6MWD). Participants in this study reached clinically meaningful changes with 3 months of participation in Gerofit for most of the physical function measures. Gerofit participants had a mean gait speed improvement of 0.05 to 0.07 m/s compared with 0.10 to 0.30 m/s, which was reported previously. 24,25 In this study, nearly all groups achieved the clinically important improvements in the chair stand in 30 seconds (2.0 to 2.6) and the 6MWD (21.8 to 59.1 m) that have been reported in the literature.24-26
The Osteoarthritis Research Society International recommends the chair stand and 6MWD performance-based tests for individuals with hip and knee arthritis because they align with patient-reported outcomes and represent the types of activities relevant to this population.27 The findings of this study suggest that improvement in these physical function measures with participation in exercise align with data from arthritis-specific exercise programs designed for wide implementation. Hughes and colleagues reported improvements in the 6MWD after the 8-week Fit and Strong exercise intervention, which included walking and lower body resistance training.28 The Arthritis Foundation’s Walk With Ease program is a 6-week walking program that has shown improvements in chair stands and gait speed.29 Another Arthritis Foundation program, People with Arthritis Can Exercise, is an 8-week course consisting of a variety of resistance, aerobic, and balance activities. This program has been associated with increases in chair stands but not gait speed or 6MWD.30,31
This study found that participation in a VHA outpatient clinical supervised exercise program results in improvements in physical function that can be realized by older adults regardless of arthritis burden. Gerofit programs typically require 1.5 to 2.0 dedicated full-time equivalent employees to run the program effectively and additional administrative support, depending on size of the program.32 The cost savings generated by the program include reductions in hospitalization rates, emergency department visits, days in hospital, and medication use and provide a compelling argument for the program’s financial viability to health care systems through long-term savings and improved health outcomes for older adults.33-36
While evidenced-based arthritis programs exist, this study illustrates that an exercise program without a focus on arthritis also improves physical function, potentially reducing the risk of disability related to arthritis. The clinical implication for these findings is that arthritis-specific exercise programs may not be needed to achieve functional improvements in individuals with arthritis. This is critical for under-resourced or exercise- limited health care systems or communities. Therefore, if exercise programming is limited, or arthritis-specific programs and interventions are not available, nonspecific exercise programs will also be beneficial to individuals with arthritis. Thus, individuals with arthritis should be encouraged to participate in any available exercise programming to achieve improvements in physical function. In addition, many older adults have multiple comorbidities, most of which improve with participation in exercise. 37 Disease-specific exercise programs can offer tailored exercises and coaching related to common barriers in participation, such as joint pain for arthritis.31 It is unclear whether these additional programmatic components are associated with greater improvements in outcomes, such as physical function. More research is needed to explore the benefits of disease-specific tailored exercise programs compared with general exercise programs.
Strengths and Limitations
This study demonstrated the effect of participation in a clinical, supervised exercise program in a real-world setting. It suggests that even exercise programs not specifically targeted for arthritis populations can improve physical function among those with arthritis.
As a VHA clinical supervised exercise program, Gerofit may not be generalizable to all older adults or other exercise programs. In addition, this analysis only included a veteran population that was > 95% male and may not be generalizable to other populations. Arthritis status was defined by self-report and not verified in the health record. However, this approach has been shown to be acceptable in this setting and the most common type of arthritis in this population (OA) is a painful musculoskeletal condition associated with functional limitations.4,22,38,39 Self-reported arthritis or rheumatism is associated with functional limitations.1 Therefore, it is unlikely that the results would differ for physician-diagnosed or radiographically defined OA. Additionally, the study did not have data on the total number of joints with arthritis or arthritis severity but rather used upper body, lower body, and both upper and lower body arthritis as a proxy for arthritis status. While our models were adjusted for age and BMI, 2 known confounding factors for the association between arthritis and physical function, there are other potential confounding factors that were not included in the models. 40,41 Finally, this study only included individuals with completed baseline and 3-month follow-up assessments, and the individuals who participated for longer or shorter periods may have had different physical function outcomes than individuals included in this study.
Conclusions
Participation in 3 months VHA Gerofit outpatient supervised exercise programs can improve physical function for all older adults, regardless of arthritis status. These programs may increase access to exercise programming that is beneficial for common conditions affecting older adults, such as arthritis.
- Centers for Disease Control and Prevention. Prevalence and most common causes of disability among adults- -United States, 2005. MMWR Morb Mortal Wkly Rep. 2009;58:421-426.
- Theis KA, Murphy LB, Guglielmo D, et al. Prevalence of arthritis and arthritis-attributable activity limitation—United States, 2016–2018. MMWR Morb Mortal Wkly Rep. 2021;70:1401-1407. doi:10.15585/mmwr.mm7040a2
- Murphy LB, Helmick CG, Allen KD, et al. Arthritis among veterans—United States, 2011–2013. MMWR Morb Mortal Wkly Rep. 2014;63:999-1003.
- Park J, Mendy A, Vieira ER. Various types of arthritis in the United States: prevalence and age-related trends from 1999 to 2014. Am J Public Health. 2018;108:256-258.
- Cameron KL, Hsiao MS, Owens BD, Burks R, Svoboda SJ. Incidence of physician-diagnosed osteoarthritis among active duty United States military service members. Arthritis Rheum. 2011;63:2974-2982. doi:10.1002/art.30498
- Patzkowski JC, Rivera JC, Ficke JR, Wenke JC. The changing face of disability in the US Army: the Operation Enduring Freedom and Operation Iraqi Freedom effect. J Am Acad Orthop Surg. 2012;20(suppl 1):S23-S30. doi:10.5435/JAAOS-20-08-S23
- Rivera JC, Amuan ME, Morris RM, Johnson AE, Pugh MJ. Arthritis, comorbidities, and care utilization in veterans of Operations Enduring and Iraqi Freedom. J Orthop Res. 2017;35:682-687. doi:10.1002/jor.23323
- Singh JA, Nelson DB, Fink HA, Nichol KL. Health-related quality of life predicts future health care utilization and mortality in veterans with self-reported physician-diagnosed arthritis: the Veterans Arthritis Quality of Life Study. Semin Arthritis Rheum. 2005;34:755- 765. doi:10.1016/j.semarthrit.2004.08.001
- Nelson AE, Allen KD, Golightly YM, Goode AP, Jordan JM. A systematic review of recommendations and guidelines for the management of osteoarthritis: the Chronic Osteoarthritis Management Initiative of the U.S. Bone and Joint Initiative. Semin Arthritis Rheum. 2014;43:701-712. doi:10.1016/j.semarthrit.2013.11.012
- Morey MC, Crowley GM, Robbins MS, Cowper PA, Sullivan RJ Jr. The Gerofit Program: a VA innovation. South Med J. 1994;87:S83-S87.
- Chen MJ, Fan X, Moe ST. Criterion-related validity of the Borg ratings of perceived exertion scale in healthy individuals: a meta-analysis. J Sports Sci. 2002;20:873-899. doi:10.1080/026404102320761787
- Morey MC, Pieper CF, Sullivan RJ Jr, Crowley GM, Cowper PA, Robbins MS. Five-year performance trends for older exercisers: a hierarchical model of endurance, strength, and flexibility. J Am Geriatr Soc. 1996;44:1226-1231. doi:10.1111/j.1532-5415.1996.tb01374.x
- Allen KD, Gol ight ly YM. State of the evidence. Curr Opin Rheumatol. 2015;27:276-283. doi:10.1097/BOR.0000000000000161
- den Ouden MEM, Schuurmans MJ, Arts IEMA, van der Schouw YT. Association between physical performance characteristics and independence in activities of daily living in middle-aged and elderly men. Geriatr Gerontol Int. 2013;13:274-280. doi:10.1111/j.1447-0594.2012.00890.x
- Daly M, Vidt ME, Eggebeen JD, et al. Upper extremity muscle volumes and functional strength after resistance training in older adults. J Aging Phys Act. 2013;21:186-207. doi:10.1123/japa.21.2.186
- Morey MC, Lee CC, Castle S, et al. Should structured exercise be promoted as a model of care? Dissemination of the Department of Veterans Affairs Gerofit Program. J Am Geriatr Soc. 2018;66:1009-1016. doi:10.1111/jgs.15276
- Jennings SC, Manning KM, Bettger JP, et al. Rapid transition to telehealth group exercise and functional assessments in response to COVID-19. Gerontol Geriatr Med. 2020;6:2333721420980313. doi:10.1177/ 2333721420980313
- Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314-322. doi:10.1046/j.1532-5415.2003.51104.x
- Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community residing older adults. Res Q Exerc Sport. 1999;70:113- 119. doi:10.1080/02701367.1999.10608028
- Rikli RE, Jones CJ. Development and validation of a functional fitness test for community-residing older adults. J Aging Phys Act. 1999;7:129-161. doi:10.1123/japa.7.2.129
- Harada ND, Chiu V, Stewart AL. Mobility-related function in older adults: assessment with a 6-minute walk test. Arch Phys Med Rehabil. 1999;80:837-841. doi:10.1016/s0003-9993(99)90236-8
- Peeters GGME, Alshurafa M, Schaap L, de Vet HCW. Diagnostic accuracy of self-reported arthritis in the general adult population is acceptable. J Clin Epidemiol. 2015;68:452-459. doi:10.1016/j.jclinepi.2014.09.019
- Cuperus N, Vliet Vlieland TPM, Mahler EAM, Kersten CC, Hoogeboom TJ, van den Ende CHM. The clinical burden of generalized osteoarthritis represented by self-reported health-related quality of life and activity limitations: a cross-sectional study. Rheumatol Int. 2015;35:871-877. doi:10.1007/s00296-014-3149-1
- Coleman G, Dobson F, Hinman RS, Bennell K, White DK. Measures of physical performance. Arthritis Care Res (Hoboken). 2020;72(suppl 10):452-485. doi:10.1002/acr.24373
- Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743-749. doi:10.1111/j.1532-5415.2006.00701.x
- Wright AA, Cook CE, Baxter GD, Dockerty JD, Abbott JH. A comparison of 3 methodological approaches to defining major clinically important improvement of 4 performance measures in patients with hip osteoarthritis. J Orthop Sports Phys Ther. 2011;41:319-327. doi:10.2519/jospt.2011.3515
- Dobson F, Hinman R, Roos EM, et al. OARSI recommended performance-based tests to assess physical function in people diagnosed with hip or knee osteoarthritis. Osteoarthritis Cartilage. 2013;21:1042- 1052. doi:10.1016/j.joca.2013.05.002
- Hughes SL, Seymour RB, Campbell R, Pollak N, Huber G, Sharma L. Impact of the fit and strong intervention on older adults with osteoarthritis. Gerontologist. 2004;44:217-228. doi:10.1093/geront/44.2.217
- Callahan LF, Shreffler JH, Altpeter M, et al. Evaluation of group and self-directed formats of the Arthritis Foundation's Walk With Ease Program. Arthritis Care Res (Hoboken). 2011;63:1098-1107. doi:10.1002/acr.20490
- Boutaugh ML. Arthritis Foundation community-based physical activity programs: effectiveness and implementation issues. Arthritis Rheum. 2003;49:463-470. doi:10.1002/art.11050
- Callahan LF, Mielenz T, Freburger J, et al. A randomized controlled trial of the People with Arthritis Can Exercise Program: symptoms, function, physical activity, and psychosocial outcomes. Arthritis Rheum. 2008;59:92-101. doi:10.1002/art.23239
- Hall KS, Jennings SC, Pearson MP. Outpatient care models: the Gerofit model of care for exercise promotion in older adults. In: Malone ML, Boltz M, Macias Tejada J, White H, eds. Geriatrics Models of Care. Springer; 2024:205-213. doi:10.1007/978-3-031-56204-4_21
- Pepin MJ, Valencia WM, Bettger JP, et al. Impact of supervised exercise on one-year medication use in older veterans with multiple morbidities. Gerontol Geriatr Med. 2020;6:2333721420956751. doi:10.1177/ 2333721420956751
- Abbate L, Li J, Veazie P, et al. Does Gerofit exercise reduce veterans’ use of emergency department and inpatient care? Innov Aging. 2020;4(suppl 1):771. doi:10.1093/geroni/igaa057.2786
- Morey MC, Pieper CF, Crowley GM, Sullivan RJ Jr, Puglisi CM. Exercise adherence and 10-year mortality in chronically ill older adults. J Am Geriatr Soc. 2002;50:1929-1933. doi:10.1046/j.1532-5415.2002.50602.x
- Manning KM, Hall KS, Sloane R, et al. Longitudinal analysis of physical function in older adults: the effects of physical inactivity and exercise training. Aging Cell. 2024;23:e13987. doi:10.1111/acel.13987
- Bean JF, Vora A, Frontera WR. Benefits of exercise for community-dwelling older adults. Arch Phys Med Rehabil. 2004;85(7 suppl 3):S31-S42; quiz S3-S4. doi:10.1016/j.apmr.2004.03.010
- Covinsky KE, Lindquist K, Dunlop DD, Yelin E. Pain, functional limitations, and aging. J Am Geriatr Soc. 2009; 57:1556-1561. doi:10.1111/j.1532-5415.2009.02388.x
- Katz JN, Wright EA, Baron JA, Losina E. Development and validation of an index of musculoskeletal functional limitations. BMC Musculoskelet Disord. 2009;10:62. doi:10.1186/1471-2474-10-62
- Allen KD, Thoma LM, Golightly YM. Epidemiology of osteoarthritis. Osteoarthritis Cartilage. 2022;30:184-195. doi:10.1016/j.joca.2021.04.020
- Riebe D, Blissmer BJ, Greaney ML, Ewing Garber C, Lees FD, Clark PG. The relationship between obesity, physical activity, and physical function in older adults. J Aging Health. 2009;21:1159-1178. doi:10.1177/0898264309350076
About half of US adults aged ≥ 65 years report arthritis, and of those, 44% have an arthritis-attributable activity limitation.1,2 Arthritis is a significant health issue for veterans, with veterans reporting higher rates of disability compared with the civilian population.3
Osteoarthritis (OA) is the most common type of arthritis.4 Among individuals aged ≥ 40 years, the incidence of OA is nearly twice as high among veterans compared with civilians and is a leading cause of separation from military service and disability.5,6 OA pain and disability have been shown to be associated with increases in health care and medication use, including opioids, nonsteroidal anti-inflammatory medications, and muscle relaxants.7,8 Because OA is chronic and has no cure, safe and effective management strategies—such as exercise— are critical to minimize pain and maintain physical function.9
Exercise can reduce pain and disability associated with OA and is a first-line recommendation in guidelines for the treatment of knee and hip OA.9 Given the limited exercise and high levels of physical inactivity among veterans with OA, there is a need to identify opportunities that support veterans with OA engaging in regular exercise.
Gerofit, an outpatient clinical exercise program available at 30 Veterans Health Administration (VHA) sites, may provide an opportunity for older veterans with arthritis to engage in exercise.10 Gerofit is specifically designed for veterans aged ≥ 65 years. It is not disease-specific and supports older veterans with multiple chronic conditions, including OA. Veterans aged ≥ 65 years with a referral from a VA clinician are eligible for Gerofit. Those who are unable to perform activities of daily living; unable to independently function without assistance; have a history of unstable angina, proliferative diabetic retinopathy, oxygen dependence, volatile behavioral issues, or are unable to work successfully in a group environment/setting; experience active substance abuse, homelessness, or uncontrolled incontinence; and have open wounds that cannot be appropriately dressed are excluded from Gerofit. Exercise sessions are held 3 times per week and last from 60 to 90 minutes. Sessions are supervised by Gerofit staff and include personalized exercise prescriptions based on functional assessments. Exercise prescriptions include aerobic, resistance, and balance/flexibility components and are modified by the Gerofit program staff as needed. Gerofit adopts a functional fitness approach and includes individual progression as appropriate according to evidence-based guidelines, using the Borg ratings of perceived exertion. 11 Assessments are performed at baseline, 3 months, 6 months, and annually thereafter. Clinical staff conduct all assessments, including physical function testing, and record them in a database. Assessments are reviewed with the veteran to chart progress and identify future goals or needs. Veterans perform personalized self-paced exercises in the Gerofit group setting. Exercise prescriptions are continuously modified to meet individualized needs and goals. Veterans may participate continuously with no end date.
Participation in supervised exercise is associated with improved physical function and individuals with arthritis can improve function even though their baseline functional status is lower than individuals without arthritis. 12 In this analysis, we examine the impact of exercise on the status and location of arthritis (upper body, lower body, or both). Lower body arthritis is more common than upper body arthritis and lower extremity function is associated with increased ability to perform activities of daily living, resulting in independence among older adults.13,14 We also include upper body strength measures to capture important functional movements such as reaching and pulling.15 Among those who participate in Gerofit, the greatest gains in physical function occur during the initial 3 months, which tend to be sustained over 12 months.16 For this reason, this study focused on the initial 3 months of the program.
Older adults with arthritis may have pain and functional limitations that exceed those of the general older adult population. Exercise programs for older adults that do not specifically target arthritis but are able to improve physical function among those with arthritis could potentially increase access to exercise for older adults living with arthritis. Therefore, the purpose of this study was to determine whether change in physical function with participation in Gerofit for 3 months varies by arthritis status, including no arthritis, any arthritis, lower body arthritis, or both upper and lower body arthritis compared with no arthritis.
Methods
This is a secondary analysis of previously collected data from 10 VHA Gerofit sites (Ann Arbor, Baltimore, Greater Los Angeles, Canandaigua, Cincinnati, Miami, Honolulu, Denver, Durham, and Pittsburgh) from 2002 to 2019. Implementation data regarding the consistency of the program delivery at Gerofit expansion sites have been previously published.16 Although the delivery of Gerofit transitioned to telehealth due to COVID-19, data for this analysis were collected from in-person exercise sessions prior to the pandemic.17 Data were collected for clinical purposes. This project was part of the Gerofit quality improvement initiative and was reviewed and approved by the Durham Institutional Review Board as quality improvement.
Participants in Gerofit who completed baseline and 3-month assessments were included to analyze the effects of exercise on physical function. At each of the time points, physical functional assessments included: (1) usual gait speed (> 10 meters [m/s], or 10- meter walk test [10MWT]); (2) lower body strength (chair stands [number completed in 30 seconds]); (3) upper body strength (number of arm curls [5-lb for females/8-lb for males] completed in 30 seconds); and (4) 6-minute walk distance [6MWD] in meters to measure aerobic endurance). These measures have been validated in older adults.18-21 Arm curls were added to the physical function assessments after the 10MWT, chair stands, and 6MWD; therefore, fewer participants had data for this measure. Participants self-reported at baseline on 45 common medical conditions, including arthritis or rheumatism (both upper body and lower body were offered as choices). Self-reporting has been shown to be an acceptable method of identifying arthritis in adults.22
Descriptive statistics at baseline were calculated for all participants. One-way analysis of variance and X2 tests were used to determine differences in baseline characteristics across arthritis status. The primary outcomes were changes in physical function measures from baseline to 3 months by arthritis status. Arthritis status was defined as: any arthritis, which includes individuals who reported upper body arthritis, lower body arthritis, or both; and arthritis status individuals reporting either upper body arthritis, lower body arthritis, or both. Categories of arthritis for arthritis status were mutually exclusive. Two separate linear models were constructed for each of the 4 physical function measures, with change from baseline to 3 months as the outcome (dependent variable) and arthritis status, age, and body mass index (BMI) as predictors (independent variables). The first model compared any arthritis with no arthritis and the second model compared arthritis status (both upper and lower body arthritis vs lower body arthritis) with no arthritis. These models were used to obtain mean changes and 95% CIs in physical function and to test for differences in the change in physical function measures by arthritis status. Statistical analyses were performed using R software, version 4.0.3.
Results
Baseline and 3-month data were available for 737 Gerofit participants and included in the analysis. The mean (SD) age was 73.5 (7.1) years. A total of 707 participants were male (95.9%) and 322 (43.6%) reported some arthritis, with arthritis in both the upper and lower body being reported by 168 participants (52.2%) (Table 1). There were no differences in age, sex, or race for those with any arthritis compared with those with no arthritis, but BMI was significantly higher in those reporting any arthritis compared with no arthritis. For the baseline functional measures, statistically significant differences were observed between those with no arthritis and those reporting any arthritis for the 10MWT (P = .001), chair stands (P = .046), and 6MWD (P = .001), but not for arm curls (P = .77), with those with no arthritis performing better.

All 4 arthritis status groups showed improvements in each of the physical function measures over 3 months. For the 10MWT the mean change (95% CI) in gait speed (m/s) was 0.06 (0.04-0.08) for patients with no arthritis, 0.07 (0.05- 0.08) for any arthritis, 0.07 (0.04-0.11) for lower body arthritis, and 0.07 (0.04- 0.09) for both lower and upper body arthritis. For the number of arm curls in 30 seconds the mean change (95% CI) was 2.3 (1.8-2.8) for patients with no arthritis, 2.1 (1.5-2.6) for any arthritis, 2.0 (1.1-3.0) for lower body arthritis, and 1.9 (1.1-2.7) for both lower and upper body arthritis. For the number of chair stands in 30 seconds the mean change (95% CI) was 2.1 (1.7-2.4) for patients with no arthritis, 2.2 (1.8-2.6) for any arthritis, 2.3 (1.6-2.9), for lower body arthritis, and 2.0 (1.5-2.5) for both lower and upper body arthritis. For the 6MWD distance in meters the mean change (95% CI) was 21.5 (15.5-27.4) for patients with no arthritis, 28.6 (21.9-35.3) for any arthritis, 30.4 (19.5-41.3) for lower body arthritis, and 28.6 (19.2-38.0) for both lower and upper body arthritis (Figure).

We used 2 models to measure the change from baseline to 3 months for each of the arthritis groups. Model 1 compared any arthritis vs no arthritis and model 2 compared lower body arthritis and both upper and lower body arthritis vs no arthritis for each physical function measure (Table 2). There were no statistically significant differences in 3-month change in physical function for any of the physical function measures between arthritis groups after adjusting for age and BMI.

Discussion
Participation in Gerofit was associated with functional gains among all participants over 3 months, regardless of arthritis status. Older veterans reporting any arthritis had significantly lower physical function scores upon enrollment into Gerofit compared with those veterans reporting no arthritis. However, compared with individuals who reported no arthritis, individuals who reported arthritis (any arthritis, lower body arthritis only, or both lower and upper body arthritis) experienced similar improvements (ie, no statistically significant differences in mean change from baseline to follow-up among those with and without arthritis). This study suggests that progressive, multicomponent exercise programs for older adults may be beneficial for those with arthritis.
Involvement of multiple sites of arthritis is associated with moderate to severe functional limitations as well as lower healthrelated quality of life.23 While it has been found that individuals with arthritis can improve function with supervised exercise, even though their baseline functional status is lower than individuals without arthritis, it was not clear whether individuals with multiple joint involvement also would benefit.12 The results of this study suggest that these individuals can improve across various domains of physical function despite variation in arthritis location and status. As incidence of arthritis increases with age, targeting older adults for exercise programs such as Gerofit may improve functional limitations and health-related quality of life associated with arthritis.2
We evaluated physical function using multiple measures to assess upper (arm curls) and lower (chair stands, 10MWT) extremity physical function and aerobic endurance (6MWD). Participants in this study reached clinically meaningful changes with 3 months of participation in Gerofit for most of the physical function measures. Gerofit participants had a mean gait speed improvement of 0.05 to 0.07 m/s compared with 0.10 to 0.30 m/s, which was reported previously. 24,25 In this study, nearly all groups achieved the clinically important improvements in the chair stand in 30 seconds (2.0 to 2.6) and the 6MWD (21.8 to 59.1 m) that have been reported in the literature.24-26
The Osteoarthritis Research Society International recommends the chair stand and 6MWD performance-based tests for individuals with hip and knee arthritis because they align with patient-reported outcomes and represent the types of activities relevant to this population.27 The findings of this study suggest that improvement in these physical function measures with participation in exercise align with data from arthritis-specific exercise programs designed for wide implementation. Hughes and colleagues reported improvements in the 6MWD after the 8-week Fit and Strong exercise intervention, which included walking and lower body resistance training.28 The Arthritis Foundation’s Walk With Ease program is a 6-week walking program that has shown improvements in chair stands and gait speed.29 Another Arthritis Foundation program, People with Arthritis Can Exercise, is an 8-week course consisting of a variety of resistance, aerobic, and balance activities. This program has been associated with increases in chair stands but not gait speed or 6MWD.30,31
This study found that participation in a VHA outpatient clinical supervised exercise program results in improvements in physical function that can be realized by older adults regardless of arthritis burden. Gerofit programs typically require 1.5 to 2.0 dedicated full-time equivalent employees to run the program effectively and additional administrative support, depending on size of the program.32 The cost savings generated by the program include reductions in hospitalization rates, emergency department visits, days in hospital, and medication use and provide a compelling argument for the program’s financial viability to health care systems through long-term savings and improved health outcomes for older adults.33-36
While evidenced-based arthritis programs exist, this study illustrates that an exercise program without a focus on arthritis also improves physical function, potentially reducing the risk of disability related to arthritis. The clinical implication for these findings is that arthritis-specific exercise programs may not be needed to achieve functional improvements in individuals with arthritis. This is critical for under-resourced or exercise- limited health care systems or communities. Therefore, if exercise programming is limited, or arthritis-specific programs and interventions are not available, nonspecific exercise programs will also be beneficial to individuals with arthritis. Thus, individuals with arthritis should be encouraged to participate in any available exercise programming to achieve improvements in physical function. In addition, many older adults have multiple comorbidities, most of which improve with participation in exercise. 37 Disease-specific exercise programs can offer tailored exercises and coaching related to common barriers in participation, such as joint pain for arthritis.31 It is unclear whether these additional programmatic components are associated with greater improvements in outcomes, such as physical function. More research is needed to explore the benefits of disease-specific tailored exercise programs compared with general exercise programs.
Strengths and Limitations
This study demonstrated the effect of participation in a clinical, supervised exercise program in a real-world setting. It suggests that even exercise programs not specifically targeted for arthritis populations can improve physical function among those with arthritis.
As a VHA clinical supervised exercise program, Gerofit may not be generalizable to all older adults or other exercise programs. In addition, this analysis only included a veteran population that was > 95% male and may not be generalizable to other populations. Arthritis status was defined by self-report and not verified in the health record. However, this approach has been shown to be acceptable in this setting and the most common type of arthritis in this population (OA) is a painful musculoskeletal condition associated with functional limitations.4,22,38,39 Self-reported arthritis or rheumatism is associated with functional limitations.1 Therefore, it is unlikely that the results would differ for physician-diagnosed or radiographically defined OA. Additionally, the study did not have data on the total number of joints with arthritis or arthritis severity but rather used upper body, lower body, and both upper and lower body arthritis as a proxy for arthritis status. While our models were adjusted for age and BMI, 2 known confounding factors for the association between arthritis and physical function, there are other potential confounding factors that were not included in the models. 40,41 Finally, this study only included individuals with completed baseline and 3-month follow-up assessments, and the individuals who participated for longer or shorter periods may have had different physical function outcomes than individuals included in this study.
Conclusions
Participation in 3 months VHA Gerofit outpatient supervised exercise programs can improve physical function for all older adults, regardless of arthritis status. These programs may increase access to exercise programming that is beneficial for common conditions affecting older adults, such as arthritis.
About half of US adults aged ≥ 65 years report arthritis, and of those, 44% have an arthritis-attributable activity limitation.1,2 Arthritis is a significant health issue for veterans, with veterans reporting higher rates of disability compared with the civilian population.3
Osteoarthritis (OA) is the most common type of arthritis.4 Among individuals aged ≥ 40 years, the incidence of OA is nearly twice as high among veterans compared with civilians and is a leading cause of separation from military service and disability.5,6 OA pain and disability have been shown to be associated with increases in health care and medication use, including opioids, nonsteroidal anti-inflammatory medications, and muscle relaxants.7,8 Because OA is chronic and has no cure, safe and effective management strategies—such as exercise— are critical to minimize pain and maintain physical function.9
Exercise can reduce pain and disability associated with OA and is a first-line recommendation in guidelines for the treatment of knee and hip OA.9 Given the limited exercise and high levels of physical inactivity among veterans with OA, there is a need to identify opportunities that support veterans with OA engaging in regular exercise.
Gerofit, an outpatient clinical exercise program available at 30 Veterans Health Administration (VHA) sites, may provide an opportunity for older veterans with arthritis to engage in exercise.10 Gerofit is specifically designed for veterans aged ≥ 65 years. It is not disease-specific and supports older veterans with multiple chronic conditions, including OA. Veterans aged ≥ 65 years with a referral from a VA clinician are eligible for Gerofit. Those who are unable to perform activities of daily living; unable to independently function without assistance; have a history of unstable angina, proliferative diabetic retinopathy, oxygen dependence, volatile behavioral issues, or are unable to work successfully in a group environment/setting; experience active substance abuse, homelessness, or uncontrolled incontinence; and have open wounds that cannot be appropriately dressed are excluded from Gerofit. Exercise sessions are held 3 times per week and last from 60 to 90 minutes. Sessions are supervised by Gerofit staff and include personalized exercise prescriptions based on functional assessments. Exercise prescriptions include aerobic, resistance, and balance/flexibility components and are modified by the Gerofit program staff as needed. Gerofit adopts a functional fitness approach and includes individual progression as appropriate according to evidence-based guidelines, using the Borg ratings of perceived exertion. 11 Assessments are performed at baseline, 3 months, 6 months, and annually thereafter. Clinical staff conduct all assessments, including physical function testing, and record them in a database. Assessments are reviewed with the veteran to chart progress and identify future goals or needs. Veterans perform personalized self-paced exercises in the Gerofit group setting. Exercise prescriptions are continuously modified to meet individualized needs and goals. Veterans may participate continuously with no end date.
Participation in supervised exercise is associated with improved physical function and individuals with arthritis can improve function even though their baseline functional status is lower than individuals without arthritis. 12 In this analysis, we examine the impact of exercise on the status and location of arthritis (upper body, lower body, or both). Lower body arthritis is more common than upper body arthritis and lower extremity function is associated with increased ability to perform activities of daily living, resulting in independence among older adults.13,14 We also include upper body strength measures to capture important functional movements such as reaching and pulling.15 Among those who participate in Gerofit, the greatest gains in physical function occur during the initial 3 months, which tend to be sustained over 12 months.16 For this reason, this study focused on the initial 3 months of the program.
Older adults with arthritis may have pain and functional limitations that exceed those of the general older adult population. Exercise programs for older adults that do not specifically target arthritis but are able to improve physical function among those with arthritis could potentially increase access to exercise for older adults living with arthritis. Therefore, the purpose of this study was to determine whether change in physical function with participation in Gerofit for 3 months varies by arthritis status, including no arthritis, any arthritis, lower body arthritis, or both upper and lower body arthritis compared with no arthritis.
Methods
This is a secondary analysis of previously collected data from 10 VHA Gerofit sites (Ann Arbor, Baltimore, Greater Los Angeles, Canandaigua, Cincinnati, Miami, Honolulu, Denver, Durham, and Pittsburgh) from 2002 to 2019. Implementation data regarding the consistency of the program delivery at Gerofit expansion sites have been previously published.16 Although the delivery of Gerofit transitioned to telehealth due to COVID-19, data for this analysis were collected from in-person exercise sessions prior to the pandemic.17 Data were collected for clinical purposes. This project was part of the Gerofit quality improvement initiative and was reviewed and approved by the Durham Institutional Review Board as quality improvement.
Participants in Gerofit who completed baseline and 3-month assessments were included to analyze the effects of exercise on physical function. At each of the time points, physical functional assessments included: (1) usual gait speed (> 10 meters [m/s], or 10- meter walk test [10MWT]); (2) lower body strength (chair stands [number completed in 30 seconds]); (3) upper body strength (number of arm curls [5-lb for females/8-lb for males] completed in 30 seconds); and (4) 6-minute walk distance [6MWD] in meters to measure aerobic endurance). These measures have been validated in older adults.18-21 Arm curls were added to the physical function assessments after the 10MWT, chair stands, and 6MWD; therefore, fewer participants had data for this measure. Participants self-reported at baseline on 45 common medical conditions, including arthritis or rheumatism (both upper body and lower body were offered as choices). Self-reporting has been shown to be an acceptable method of identifying arthritis in adults.22
Descriptive statistics at baseline were calculated for all participants. One-way analysis of variance and X2 tests were used to determine differences in baseline characteristics across arthritis status. The primary outcomes were changes in physical function measures from baseline to 3 months by arthritis status. Arthritis status was defined as: any arthritis, which includes individuals who reported upper body arthritis, lower body arthritis, or both; and arthritis status individuals reporting either upper body arthritis, lower body arthritis, or both. Categories of arthritis for arthritis status were mutually exclusive. Two separate linear models were constructed for each of the 4 physical function measures, with change from baseline to 3 months as the outcome (dependent variable) and arthritis status, age, and body mass index (BMI) as predictors (independent variables). The first model compared any arthritis with no arthritis and the second model compared arthritis status (both upper and lower body arthritis vs lower body arthritis) with no arthritis. These models were used to obtain mean changes and 95% CIs in physical function and to test for differences in the change in physical function measures by arthritis status. Statistical analyses were performed using R software, version 4.0.3.
Results
Baseline and 3-month data were available for 737 Gerofit participants and included in the analysis. The mean (SD) age was 73.5 (7.1) years. A total of 707 participants were male (95.9%) and 322 (43.6%) reported some arthritis, with arthritis in both the upper and lower body being reported by 168 participants (52.2%) (Table 1). There were no differences in age, sex, or race for those with any arthritis compared with those with no arthritis, but BMI was significantly higher in those reporting any arthritis compared with no arthritis. For the baseline functional measures, statistically significant differences were observed between those with no arthritis and those reporting any arthritis for the 10MWT (P = .001), chair stands (P = .046), and 6MWD (P = .001), but not for arm curls (P = .77), with those with no arthritis performing better.

All 4 arthritis status groups showed improvements in each of the physical function measures over 3 months. For the 10MWT the mean change (95% CI) in gait speed (m/s) was 0.06 (0.04-0.08) for patients with no arthritis, 0.07 (0.05- 0.08) for any arthritis, 0.07 (0.04-0.11) for lower body arthritis, and 0.07 (0.04- 0.09) for both lower and upper body arthritis. For the number of arm curls in 30 seconds the mean change (95% CI) was 2.3 (1.8-2.8) for patients with no arthritis, 2.1 (1.5-2.6) for any arthritis, 2.0 (1.1-3.0) for lower body arthritis, and 1.9 (1.1-2.7) for both lower and upper body arthritis. For the number of chair stands in 30 seconds the mean change (95% CI) was 2.1 (1.7-2.4) for patients with no arthritis, 2.2 (1.8-2.6) for any arthritis, 2.3 (1.6-2.9), for lower body arthritis, and 2.0 (1.5-2.5) for both lower and upper body arthritis. For the 6MWD distance in meters the mean change (95% CI) was 21.5 (15.5-27.4) for patients with no arthritis, 28.6 (21.9-35.3) for any arthritis, 30.4 (19.5-41.3) for lower body arthritis, and 28.6 (19.2-38.0) for both lower and upper body arthritis (Figure).

We used 2 models to measure the change from baseline to 3 months for each of the arthritis groups. Model 1 compared any arthritis vs no arthritis and model 2 compared lower body arthritis and both upper and lower body arthritis vs no arthritis for each physical function measure (Table 2). There were no statistically significant differences in 3-month change in physical function for any of the physical function measures between arthritis groups after adjusting for age and BMI.

Discussion
Participation in Gerofit was associated with functional gains among all participants over 3 months, regardless of arthritis status. Older veterans reporting any arthritis had significantly lower physical function scores upon enrollment into Gerofit compared with those veterans reporting no arthritis. However, compared with individuals who reported no arthritis, individuals who reported arthritis (any arthritis, lower body arthritis only, or both lower and upper body arthritis) experienced similar improvements (ie, no statistically significant differences in mean change from baseline to follow-up among those with and without arthritis). This study suggests that progressive, multicomponent exercise programs for older adults may be beneficial for those with arthritis.
Involvement of multiple sites of arthritis is associated with moderate to severe functional limitations as well as lower healthrelated quality of life.23 While it has been found that individuals with arthritis can improve function with supervised exercise, even though their baseline functional status is lower than individuals without arthritis, it was not clear whether individuals with multiple joint involvement also would benefit.12 The results of this study suggest that these individuals can improve across various domains of physical function despite variation in arthritis location and status. As incidence of arthritis increases with age, targeting older adults for exercise programs such as Gerofit may improve functional limitations and health-related quality of life associated with arthritis.2
We evaluated physical function using multiple measures to assess upper (arm curls) and lower (chair stands, 10MWT) extremity physical function and aerobic endurance (6MWD). Participants in this study reached clinically meaningful changes with 3 months of participation in Gerofit for most of the physical function measures. Gerofit participants had a mean gait speed improvement of 0.05 to 0.07 m/s compared with 0.10 to 0.30 m/s, which was reported previously. 24,25 In this study, nearly all groups achieved the clinically important improvements in the chair stand in 30 seconds (2.0 to 2.6) and the 6MWD (21.8 to 59.1 m) that have been reported in the literature.24-26
The Osteoarthritis Research Society International recommends the chair stand and 6MWD performance-based tests for individuals with hip and knee arthritis because they align with patient-reported outcomes and represent the types of activities relevant to this population.27 The findings of this study suggest that improvement in these physical function measures with participation in exercise align with data from arthritis-specific exercise programs designed for wide implementation. Hughes and colleagues reported improvements in the 6MWD after the 8-week Fit and Strong exercise intervention, which included walking and lower body resistance training.28 The Arthritis Foundation’s Walk With Ease program is a 6-week walking program that has shown improvements in chair stands and gait speed.29 Another Arthritis Foundation program, People with Arthritis Can Exercise, is an 8-week course consisting of a variety of resistance, aerobic, and balance activities. This program has been associated with increases in chair stands but not gait speed or 6MWD.30,31
This study found that participation in a VHA outpatient clinical supervised exercise program results in improvements in physical function that can be realized by older adults regardless of arthritis burden. Gerofit programs typically require 1.5 to 2.0 dedicated full-time equivalent employees to run the program effectively and additional administrative support, depending on size of the program.32 The cost savings generated by the program include reductions in hospitalization rates, emergency department visits, days in hospital, and medication use and provide a compelling argument for the program’s financial viability to health care systems through long-term savings and improved health outcomes for older adults.33-36
While evidenced-based arthritis programs exist, this study illustrates that an exercise program without a focus on arthritis also improves physical function, potentially reducing the risk of disability related to arthritis. The clinical implication for these findings is that arthritis-specific exercise programs may not be needed to achieve functional improvements in individuals with arthritis. This is critical for under-resourced or exercise- limited health care systems or communities. Therefore, if exercise programming is limited, or arthritis-specific programs and interventions are not available, nonspecific exercise programs will also be beneficial to individuals with arthritis. Thus, individuals with arthritis should be encouraged to participate in any available exercise programming to achieve improvements in physical function. In addition, many older adults have multiple comorbidities, most of which improve with participation in exercise. 37 Disease-specific exercise programs can offer tailored exercises and coaching related to common barriers in participation, such as joint pain for arthritis.31 It is unclear whether these additional programmatic components are associated with greater improvements in outcomes, such as physical function. More research is needed to explore the benefits of disease-specific tailored exercise programs compared with general exercise programs.
Strengths and Limitations
This study demonstrated the effect of participation in a clinical, supervised exercise program in a real-world setting. It suggests that even exercise programs not specifically targeted for arthritis populations can improve physical function among those with arthritis.
As a VHA clinical supervised exercise program, Gerofit may not be generalizable to all older adults or other exercise programs. In addition, this analysis only included a veteran population that was > 95% male and may not be generalizable to other populations. Arthritis status was defined by self-report and not verified in the health record. However, this approach has been shown to be acceptable in this setting and the most common type of arthritis in this population (OA) is a painful musculoskeletal condition associated with functional limitations.4,22,38,39 Self-reported arthritis or rheumatism is associated with functional limitations.1 Therefore, it is unlikely that the results would differ for physician-diagnosed or radiographically defined OA. Additionally, the study did not have data on the total number of joints with arthritis or arthritis severity but rather used upper body, lower body, and both upper and lower body arthritis as a proxy for arthritis status. While our models were adjusted for age and BMI, 2 known confounding factors for the association between arthritis and physical function, there are other potential confounding factors that were not included in the models. 40,41 Finally, this study only included individuals with completed baseline and 3-month follow-up assessments, and the individuals who participated for longer or shorter periods may have had different physical function outcomes than individuals included in this study.
Conclusions
Participation in 3 months VHA Gerofit outpatient supervised exercise programs can improve physical function for all older adults, regardless of arthritis status. These programs may increase access to exercise programming that is beneficial for common conditions affecting older adults, such as arthritis.
- Centers for Disease Control and Prevention. Prevalence and most common causes of disability among adults- -United States, 2005. MMWR Morb Mortal Wkly Rep. 2009;58:421-426.
- Theis KA, Murphy LB, Guglielmo D, et al. Prevalence of arthritis and arthritis-attributable activity limitation—United States, 2016–2018. MMWR Morb Mortal Wkly Rep. 2021;70:1401-1407. doi:10.15585/mmwr.mm7040a2
- Murphy LB, Helmick CG, Allen KD, et al. Arthritis among veterans—United States, 2011–2013. MMWR Morb Mortal Wkly Rep. 2014;63:999-1003.
- Park J, Mendy A, Vieira ER. Various types of arthritis in the United States: prevalence and age-related trends from 1999 to 2014. Am J Public Health. 2018;108:256-258.
- Cameron KL, Hsiao MS, Owens BD, Burks R, Svoboda SJ. Incidence of physician-diagnosed osteoarthritis among active duty United States military service members. Arthritis Rheum. 2011;63:2974-2982. doi:10.1002/art.30498
- Patzkowski JC, Rivera JC, Ficke JR, Wenke JC. The changing face of disability in the US Army: the Operation Enduring Freedom and Operation Iraqi Freedom effect. J Am Acad Orthop Surg. 2012;20(suppl 1):S23-S30. doi:10.5435/JAAOS-20-08-S23
- Rivera JC, Amuan ME, Morris RM, Johnson AE, Pugh MJ. Arthritis, comorbidities, and care utilization in veterans of Operations Enduring and Iraqi Freedom. J Orthop Res. 2017;35:682-687. doi:10.1002/jor.23323
- Singh JA, Nelson DB, Fink HA, Nichol KL. Health-related quality of life predicts future health care utilization and mortality in veterans with self-reported physician-diagnosed arthritis: the Veterans Arthritis Quality of Life Study. Semin Arthritis Rheum. 2005;34:755- 765. doi:10.1016/j.semarthrit.2004.08.001
- Nelson AE, Allen KD, Golightly YM, Goode AP, Jordan JM. A systematic review of recommendations and guidelines for the management of osteoarthritis: the Chronic Osteoarthritis Management Initiative of the U.S. Bone and Joint Initiative. Semin Arthritis Rheum. 2014;43:701-712. doi:10.1016/j.semarthrit.2013.11.012
- Morey MC, Crowley GM, Robbins MS, Cowper PA, Sullivan RJ Jr. The Gerofit Program: a VA innovation. South Med J. 1994;87:S83-S87.
- Chen MJ, Fan X, Moe ST. Criterion-related validity of the Borg ratings of perceived exertion scale in healthy individuals: a meta-analysis. J Sports Sci. 2002;20:873-899. doi:10.1080/026404102320761787
- Morey MC, Pieper CF, Sullivan RJ Jr, Crowley GM, Cowper PA, Robbins MS. Five-year performance trends for older exercisers: a hierarchical model of endurance, strength, and flexibility. J Am Geriatr Soc. 1996;44:1226-1231. doi:10.1111/j.1532-5415.1996.tb01374.x
- Allen KD, Gol ight ly YM. State of the evidence. Curr Opin Rheumatol. 2015;27:276-283. doi:10.1097/BOR.0000000000000161
- den Ouden MEM, Schuurmans MJ, Arts IEMA, van der Schouw YT. Association between physical performance characteristics and independence in activities of daily living in middle-aged and elderly men. Geriatr Gerontol Int. 2013;13:274-280. doi:10.1111/j.1447-0594.2012.00890.x
- Daly M, Vidt ME, Eggebeen JD, et al. Upper extremity muscle volumes and functional strength after resistance training in older adults. J Aging Phys Act. 2013;21:186-207. doi:10.1123/japa.21.2.186
- Morey MC, Lee CC, Castle S, et al. Should structured exercise be promoted as a model of care? Dissemination of the Department of Veterans Affairs Gerofit Program. J Am Geriatr Soc. 2018;66:1009-1016. doi:10.1111/jgs.15276
- Jennings SC, Manning KM, Bettger JP, et al. Rapid transition to telehealth group exercise and functional assessments in response to COVID-19. Gerontol Geriatr Med. 2020;6:2333721420980313. doi:10.1177/ 2333721420980313
- Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314-322. doi:10.1046/j.1532-5415.2003.51104.x
- Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community residing older adults. Res Q Exerc Sport. 1999;70:113- 119. doi:10.1080/02701367.1999.10608028
- Rikli RE, Jones CJ. Development and validation of a functional fitness test for community-residing older adults. J Aging Phys Act. 1999;7:129-161. doi:10.1123/japa.7.2.129
- Harada ND, Chiu V, Stewart AL. Mobility-related function in older adults: assessment with a 6-minute walk test. Arch Phys Med Rehabil. 1999;80:837-841. doi:10.1016/s0003-9993(99)90236-8
- Peeters GGME, Alshurafa M, Schaap L, de Vet HCW. Diagnostic accuracy of self-reported arthritis in the general adult population is acceptable. J Clin Epidemiol. 2015;68:452-459. doi:10.1016/j.jclinepi.2014.09.019
- Cuperus N, Vliet Vlieland TPM, Mahler EAM, Kersten CC, Hoogeboom TJ, van den Ende CHM. The clinical burden of generalized osteoarthritis represented by self-reported health-related quality of life and activity limitations: a cross-sectional study. Rheumatol Int. 2015;35:871-877. doi:10.1007/s00296-014-3149-1
- Coleman G, Dobson F, Hinman RS, Bennell K, White DK. Measures of physical performance. Arthritis Care Res (Hoboken). 2020;72(suppl 10):452-485. doi:10.1002/acr.24373
- Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743-749. doi:10.1111/j.1532-5415.2006.00701.x
- Wright AA, Cook CE, Baxter GD, Dockerty JD, Abbott JH. A comparison of 3 methodological approaches to defining major clinically important improvement of 4 performance measures in patients with hip osteoarthritis. J Orthop Sports Phys Ther. 2011;41:319-327. doi:10.2519/jospt.2011.3515
- Dobson F, Hinman R, Roos EM, et al. OARSI recommended performance-based tests to assess physical function in people diagnosed with hip or knee osteoarthritis. Osteoarthritis Cartilage. 2013;21:1042- 1052. doi:10.1016/j.joca.2013.05.002
- Hughes SL, Seymour RB, Campbell R, Pollak N, Huber G, Sharma L. Impact of the fit and strong intervention on older adults with osteoarthritis. Gerontologist. 2004;44:217-228. doi:10.1093/geront/44.2.217
- Callahan LF, Shreffler JH, Altpeter M, et al. Evaluation of group and self-directed formats of the Arthritis Foundation's Walk With Ease Program. Arthritis Care Res (Hoboken). 2011;63:1098-1107. doi:10.1002/acr.20490
- Boutaugh ML. Arthritis Foundation community-based physical activity programs: effectiveness and implementation issues. Arthritis Rheum. 2003;49:463-470. doi:10.1002/art.11050
- Callahan LF, Mielenz T, Freburger J, et al. A randomized controlled trial of the People with Arthritis Can Exercise Program: symptoms, function, physical activity, and psychosocial outcomes. Arthritis Rheum. 2008;59:92-101. doi:10.1002/art.23239
- Hall KS, Jennings SC, Pearson MP. Outpatient care models: the Gerofit model of care for exercise promotion in older adults. In: Malone ML, Boltz M, Macias Tejada J, White H, eds. Geriatrics Models of Care. Springer; 2024:205-213. doi:10.1007/978-3-031-56204-4_21
- Pepin MJ, Valencia WM, Bettger JP, et al. Impact of supervised exercise on one-year medication use in older veterans with multiple morbidities. Gerontol Geriatr Med. 2020;6:2333721420956751. doi:10.1177/ 2333721420956751
- Abbate L, Li J, Veazie P, et al. Does Gerofit exercise reduce veterans’ use of emergency department and inpatient care? Innov Aging. 2020;4(suppl 1):771. doi:10.1093/geroni/igaa057.2786
- Morey MC, Pieper CF, Crowley GM, Sullivan RJ Jr, Puglisi CM. Exercise adherence and 10-year mortality in chronically ill older adults. J Am Geriatr Soc. 2002;50:1929-1933. doi:10.1046/j.1532-5415.2002.50602.x
- Manning KM, Hall KS, Sloane R, et al. Longitudinal analysis of physical function in older adults: the effects of physical inactivity and exercise training. Aging Cell. 2024;23:e13987. doi:10.1111/acel.13987
- Bean JF, Vora A, Frontera WR. Benefits of exercise for community-dwelling older adults. Arch Phys Med Rehabil. 2004;85(7 suppl 3):S31-S42; quiz S3-S4. doi:10.1016/j.apmr.2004.03.010
- Covinsky KE, Lindquist K, Dunlop DD, Yelin E. Pain, functional limitations, and aging. J Am Geriatr Soc. 2009; 57:1556-1561. doi:10.1111/j.1532-5415.2009.02388.x
- Katz JN, Wright EA, Baron JA, Losina E. Development and validation of an index of musculoskeletal functional limitations. BMC Musculoskelet Disord. 2009;10:62. doi:10.1186/1471-2474-10-62
- Allen KD, Thoma LM, Golightly YM. Epidemiology of osteoarthritis. Osteoarthritis Cartilage. 2022;30:184-195. doi:10.1016/j.joca.2021.04.020
- Riebe D, Blissmer BJ, Greaney ML, Ewing Garber C, Lees FD, Clark PG. The relationship between obesity, physical activity, and physical function in older adults. J Aging Health. 2009;21:1159-1178. doi:10.1177/0898264309350076
- Centers for Disease Control and Prevention. Prevalence and most common causes of disability among adults- -United States, 2005. MMWR Morb Mortal Wkly Rep. 2009;58:421-426.
- Theis KA, Murphy LB, Guglielmo D, et al. Prevalence of arthritis and arthritis-attributable activity limitation—United States, 2016–2018. MMWR Morb Mortal Wkly Rep. 2021;70:1401-1407. doi:10.15585/mmwr.mm7040a2
- Murphy LB, Helmick CG, Allen KD, et al. Arthritis among veterans—United States, 2011–2013. MMWR Morb Mortal Wkly Rep. 2014;63:999-1003.
- Park J, Mendy A, Vieira ER. Various types of arthritis in the United States: prevalence and age-related trends from 1999 to 2014. Am J Public Health. 2018;108:256-258.
- Cameron KL, Hsiao MS, Owens BD, Burks R, Svoboda SJ. Incidence of physician-diagnosed osteoarthritis among active duty United States military service members. Arthritis Rheum. 2011;63:2974-2982. doi:10.1002/art.30498
- Patzkowski JC, Rivera JC, Ficke JR, Wenke JC. The changing face of disability in the US Army: the Operation Enduring Freedom and Operation Iraqi Freedom effect. J Am Acad Orthop Surg. 2012;20(suppl 1):S23-S30. doi:10.5435/JAAOS-20-08-S23
- Rivera JC, Amuan ME, Morris RM, Johnson AE, Pugh MJ. Arthritis, comorbidities, and care utilization in veterans of Operations Enduring and Iraqi Freedom. J Orthop Res. 2017;35:682-687. doi:10.1002/jor.23323
- Singh JA, Nelson DB, Fink HA, Nichol KL. Health-related quality of life predicts future health care utilization and mortality in veterans with self-reported physician-diagnosed arthritis: the Veterans Arthritis Quality of Life Study. Semin Arthritis Rheum. 2005;34:755- 765. doi:10.1016/j.semarthrit.2004.08.001
- Nelson AE, Allen KD, Golightly YM, Goode AP, Jordan JM. A systematic review of recommendations and guidelines for the management of osteoarthritis: the Chronic Osteoarthritis Management Initiative of the U.S. Bone and Joint Initiative. Semin Arthritis Rheum. 2014;43:701-712. doi:10.1016/j.semarthrit.2013.11.012
- Morey MC, Crowley GM, Robbins MS, Cowper PA, Sullivan RJ Jr. The Gerofit Program: a VA innovation. South Med J. 1994;87:S83-S87.
- Chen MJ, Fan X, Moe ST. Criterion-related validity of the Borg ratings of perceived exertion scale in healthy individuals: a meta-analysis. J Sports Sci. 2002;20:873-899. doi:10.1080/026404102320761787
- Morey MC, Pieper CF, Sullivan RJ Jr, Crowley GM, Cowper PA, Robbins MS. Five-year performance trends for older exercisers: a hierarchical model of endurance, strength, and flexibility. J Am Geriatr Soc. 1996;44:1226-1231. doi:10.1111/j.1532-5415.1996.tb01374.x
- Allen KD, Gol ight ly YM. State of the evidence. Curr Opin Rheumatol. 2015;27:276-283. doi:10.1097/BOR.0000000000000161
- den Ouden MEM, Schuurmans MJ, Arts IEMA, van der Schouw YT. Association between physical performance characteristics and independence in activities of daily living in middle-aged and elderly men. Geriatr Gerontol Int. 2013;13:274-280. doi:10.1111/j.1447-0594.2012.00890.x
- Daly M, Vidt ME, Eggebeen JD, et al. Upper extremity muscle volumes and functional strength after resistance training in older adults. J Aging Phys Act. 2013;21:186-207. doi:10.1123/japa.21.2.186
- Morey MC, Lee CC, Castle S, et al. Should structured exercise be promoted as a model of care? Dissemination of the Department of Veterans Affairs Gerofit Program. J Am Geriatr Soc. 2018;66:1009-1016. doi:10.1111/jgs.15276
- Jennings SC, Manning KM, Bettger JP, et al. Rapid transition to telehealth group exercise and functional assessments in response to COVID-19. Gerontol Geriatr Med. 2020;6:2333721420980313. doi:10.1177/ 2333721420980313
- Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314-322. doi:10.1046/j.1532-5415.2003.51104.x
- Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community residing older adults. Res Q Exerc Sport. 1999;70:113- 119. doi:10.1080/02701367.1999.10608028
- Rikli RE, Jones CJ. Development and validation of a functional fitness test for community-residing older adults. J Aging Phys Act. 1999;7:129-161. doi:10.1123/japa.7.2.129
- Harada ND, Chiu V, Stewart AL. Mobility-related function in older adults: assessment with a 6-minute walk test. Arch Phys Med Rehabil. 1999;80:837-841. doi:10.1016/s0003-9993(99)90236-8
- Peeters GGME, Alshurafa M, Schaap L, de Vet HCW. Diagnostic accuracy of self-reported arthritis in the general adult population is acceptable. J Clin Epidemiol. 2015;68:452-459. doi:10.1016/j.jclinepi.2014.09.019
- Cuperus N, Vliet Vlieland TPM, Mahler EAM, Kersten CC, Hoogeboom TJ, van den Ende CHM. The clinical burden of generalized osteoarthritis represented by self-reported health-related quality of life and activity limitations: a cross-sectional study. Rheumatol Int. 2015;35:871-877. doi:10.1007/s00296-014-3149-1
- Coleman G, Dobson F, Hinman RS, Bennell K, White DK. Measures of physical performance. Arthritis Care Res (Hoboken). 2020;72(suppl 10):452-485. doi:10.1002/acr.24373
- Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743-749. doi:10.1111/j.1532-5415.2006.00701.x
- Wright AA, Cook CE, Baxter GD, Dockerty JD, Abbott JH. A comparison of 3 methodological approaches to defining major clinically important improvement of 4 performance measures in patients with hip osteoarthritis. J Orthop Sports Phys Ther. 2011;41:319-327. doi:10.2519/jospt.2011.3515
- Dobson F, Hinman R, Roos EM, et al. OARSI recommended performance-based tests to assess physical function in people diagnosed with hip or knee osteoarthritis. Osteoarthritis Cartilage. 2013;21:1042- 1052. doi:10.1016/j.joca.2013.05.002
- Hughes SL, Seymour RB, Campbell R, Pollak N, Huber G, Sharma L. Impact of the fit and strong intervention on older adults with osteoarthritis. Gerontologist. 2004;44:217-228. doi:10.1093/geront/44.2.217
- Callahan LF, Shreffler JH, Altpeter M, et al. Evaluation of group and self-directed formats of the Arthritis Foundation's Walk With Ease Program. Arthritis Care Res (Hoboken). 2011;63:1098-1107. doi:10.1002/acr.20490
- Boutaugh ML. Arthritis Foundation community-based physical activity programs: effectiveness and implementation issues. Arthritis Rheum. 2003;49:463-470. doi:10.1002/art.11050
- Callahan LF, Mielenz T, Freburger J, et al. A randomized controlled trial of the People with Arthritis Can Exercise Program: symptoms, function, physical activity, and psychosocial outcomes. Arthritis Rheum. 2008;59:92-101. doi:10.1002/art.23239
- Hall KS, Jennings SC, Pearson MP. Outpatient care models: the Gerofit model of care for exercise promotion in older adults. In: Malone ML, Boltz M, Macias Tejada J, White H, eds. Geriatrics Models of Care. Springer; 2024:205-213. doi:10.1007/978-3-031-56204-4_21
- Pepin MJ, Valencia WM, Bettger JP, et al. Impact of supervised exercise on one-year medication use in older veterans with multiple morbidities. Gerontol Geriatr Med. 2020;6:2333721420956751. doi:10.1177/ 2333721420956751
- Abbate L, Li J, Veazie P, et al. Does Gerofit exercise reduce veterans’ use of emergency department and inpatient care? Innov Aging. 2020;4(suppl 1):771. doi:10.1093/geroni/igaa057.2786
- Morey MC, Pieper CF, Crowley GM, Sullivan RJ Jr, Puglisi CM. Exercise adherence and 10-year mortality in chronically ill older adults. J Am Geriatr Soc. 2002;50:1929-1933. doi:10.1046/j.1532-5415.2002.50602.x
- Manning KM, Hall KS, Sloane R, et al. Longitudinal analysis of physical function in older adults: the effects of physical inactivity and exercise training. Aging Cell. 2024;23:e13987. doi:10.1111/acel.13987
- Bean JF, Vora A, Frontera WR. Benefits of exercise for community-dwelling older adults. Arch Phys Med Rehabil. 2004;85(7 suppl 3):S31-S42; quiz S3-S4. doi:10.1016/j.apmr.2004.03.010
- Covinsky KE, Lindquist K, Dunlop DD, Yelin E. Pain, functional limitations, and aging. J Am Geriatr Soc. 2009; 57:1556-1561. doi:10.1111/j.1532-5415.2009.02388.x
- Katz JN, Wright EA, Baron JA, Losina E. Development and validation of an index of musculoskeletal functional limitations. BMC Musculoskelet Disord. 2009;10:62. doi:10.1186/1471-2474-10-62
- Allen KD, Thoma LM, Golightly YM. Epidemiology of osteoarthritis. Osteoarthritis Cartilage. 2022;30:184-195. doi:10.1016/j.joca.2021.04.020
- Riebe D, Blissmer BJ, Greaney ML, Ewing Garber C, Lees FD, Clark PG. The relationship between obesity, physical activity, and physical function in older adults. J Aging Health. 2009;21:1159-1178. doi:10.1177/0898264309350076
Impact of 3 Months of Supervised Exercise on Function by Arthritis Status
Impact of 3 Months of Supervised Exercise on Function by Arthritis Status
Setting and Method of Measurement Affect Blood Pressure Readings in Older Veterans
Seventy-five percent of adults aged >75 years have hypertension.1-3 According to the Joint National Commission 8 (JNC 8), the recommended target blood pressure (BP) is < 150/80 mm Hg for adults aged > 60 years.4 In 2016 the Systolic Blood Pressure Intervention Trial (SPRINT) suggested that more aggressive BP control with a goal of < 120/80 mm Hg reduced rates of cardiovascular disease and lowered the risk of death in adults aged > 50 years with hypertension.5 It is anticipated that as a result of the landmark SPRINT results, clinicians may attempt to treat hypertension more intensely in older patients with an increased risk of adverse consequences if BPs are not appropriately measured.
There is a standardized protocol for BP measurement, but these recommendations typically are not followed in routine office visits.6,7 Some studies have noted that home BP measurement may be more accurate than office measurement.8 However, clinicians may not always trust the accuracy of home BP readings, and many patients are not adherent with home measurement. As a result, physicians usually manage hypertension in older patients based on office readings, though it is likely that most office measurements do not follow protocol on proper measurement. Office measurements have been noted to be inaccurate with high likelihood of overestimating or underestimating BP control.9
Office BP measurements demonstrate poor correlation with home measurements and have not been shown to be as good of a predictor for target organ damage or long-term cardiovascular outcomes compared with that of home measurements.10,11 Although there have been studies comparing home and office BP measurements and comparing office and ambulatory BP measurement, no literature has been found that reports on the difference between routine office and standardized measurement of BP.9,12-14
This study seeks to identify the magnitude of difference among BP measured according to a standardized protocol, routine clinical, and home BP. The authors hypothesized that there would be a significant, clinically relevant difference among the 3 BP measurement methods, especially between the routine office and standardized office measurements. This study has implications for implementing intensive treatment of hypertension based on office measurements.
Methods
Participants included 30 male veterans aged > 65 years who were actively participating in the Gerofit program at the VA Greater Los Angeles Healthcare System (VAGLAHS). The Gerofit program is a model clinical demonstration exercise and health promotion program targeting older and veterans at risk for falls or institutionalization. Gerofit was established in 1987 at the Durham VA Health System and successfully implemented in 2014 at VAGLAHS. Supervised exercise is offered 3 times per week and consists of individually tailored exercises aimed at reducing functional deficits that are identified and monitored by an initial and quarterly functional assessment. Blood pressures are checked routinely once a week as a part of the program. Gerofit was reviewed and approved by the institutional review board at VAGLAHS as a quality improvement/quality assurance project.
Data
Routine office and standardized protocol measurements were obtained by a single CasMED 740 (Branford, CT) automated BP machine and were conducted separately on different days. The CasMED 740 machine was not otherwise calibrated; however, a one-time correlation was performed between the CasMED 740 and the home BP monitor for each participant, when it was brought to VAGLAHS. Two measurements were made with the CasMed 740 automated BP machine on the arm that gave the higher BP reading throughout the standardized and routine protocol. Two subsequent measurements were made with the participant’s home automated BP cuff. Averages for the CasMED 740 and the home BP monitoring device were compared and assessed for significance by paired t test. No rest was scheduled prior to the first measurement, but there was a 1-minute rest after each subsequent measurement.
Mean values (SD) were used for participant characteristics and mean values (standard error [SE]) were used for BP measurements. Data were analyzed using Microsoft Excel (Redmond, WA) and GraphPad Prism version 7.03 (San Diego, CA). T tests were used for analysis of home BP measurements due to low sample size. Values of P < .05 were considered to be statistically significant.
Routine office protocol. Automated BP was measured to mimic routine office visits. Upon arrival, participants sat down, and the BP cuff was placed around their arm. Any rest before a measurement was incidental and not intentionally structured. Appropriate cuff size was determined by visual estimation of arm circumference. Only 1 measurement was made unless BP was > 150/90 mm Hg, in which case a repeat measurement was made after 2 to 4 minutes of rest. The BP was then determined based on the average of 2 or more readings. The BPs were recorded by hand in a weekly log. Participants had at least 12 weeks of BP readings measured by the routine method, and these BPs were averaged over 12 weeks to yield their average routine measured BP.
Standardized protocol. Automated BP was measured according to the 2015 USPSTF Guidelines and Look AHEAD trial protocol.7,15 A participant’s arm circumference was measured, and appropriate cuff size was determined. The participant rested quietly in a chair for at least 5 minutes with feet flat on the floor and back supported. The cuff was snugly placed 2 to 3 cm above the antecubital fossa, and the arm was supported at the level of the right atrium during the measurement. Blood pressure was determined using the mean of 4 automated cuff readings, 2 on each arm, taken 1 minute apart. Participants did not necessarily have their BP measured by the standardized method immediately following the routine method but all measurements were performed during the same 12-week time period.
Home blood pressure protocol. Participants were given instructions according to the American Heart Association (AHA) recommendations for measuring home BP. Patients were instructed to use a calibrated, automated arm BP cuff. Home BP machines were not provided in advance, and each individual’s BP machine was not calibrated. They also were instructed to rest at least 5 minutes before measuring their BP. The mean home BP was determined by the cumulative average of 3 readings in the morning and evening, taken 1 minute between each reading, for a total of 6 readings/d. Participants recorded home BPs for 2 weeks before submitting their readings. Each participant affirmed clear understanding of how to measure BP by correctly demonstrating placement of the cuff 1 time under supervision.
Results
Thirty veterans aged > 65 years participated in the study. The average age (SD) was 82.7 (9.3) years. The average BMI (SD) was overweight at 29.7 kg/m2 (5.7). Most (87.6%) of the study participants had been diagnosed with hypertension prior to the study, and no new diagnoses were made as a result of the study.
Both systolic BP (SBP) and diastolic BPs (DBP) measured by the standardized method were significantly lower than those by the routine method (P < .01 and P < .01, respectively) (Figure 1).
To determine the accuracy of the home BP monitors, the average routine VAGLAHS BP measure was compared with home BP results. For SBPs, there was a significant correlation coefficient of 0.91 (P < .01).
Discussion
The present study demonstrated that standardized measurements of BP were lower than that of the routine method used in most office settings. These results suggest that there could be a risk of overtreatment for some patients those of whose results are higher than the SPRINT BP target of < 120/80 mm Hg. Clinicians might be treating BPs that are elevated due to improper measurement, which can lead to deleterious consequences in older adults, such as syncope and falls.16
Each participant exhibited a significantly lower BP reading with the standardized method than the routine method. The 20-point decrease in SBP and 10-point decrease in DBP are clinically significant. The routine method of measurement was intended to simulate BP measurement in outpatient settings. There is usually little time structured for rest, and because the protocol established by the AHA and other professional organizations is time consuming, it usually is not strictly followed. With guidelines proposed by JNC 8 and new findings from SPRINT, the method of BP readings should be reviewed in all clinical settings.
While changes in BP management are not necessarily immediate, the differences in recommendations proposed by SPRINT and JNC 8 can lead to confusion regarding how intensely to treat BP. These recommendations guide clinical practice, but clinicians’ best judgment ultimately determines BP management. Physicians who utilize routine office measurements likely rely on BP readings that are higher on average than are readings done under proper conditions. This leads to the prospect of overtreatment, where physicians attempt to control hypertension too aggressively, potentially leading to orthostatic hypotension, syncope, and increased risk for falls.16 With findings from SPRINT recommending even lower BPs than that by JNC 8, overtreatment risk becomes especially relevant. While BP protocol was strictly followed in SPRINT, some clinicians may not necessarily follow the same fastidious protocol.
The average differences between the home and standardized BPs were not statistically significant possibly due to the small sample size in the home BP measurements; however, the difference might represent some clinical relevance. There was a 15-point difference in SBP results between home (129 mm Hg) and standardized (115 mm Hg) measures. There also was a difference in DBP between home (69 mm Hg) and standardized (62 mm Hg) results. The close correlation between both home and BPs measured in VAGLAHS demonstrated that any difference was not due to variability in the measurement devices. Previous studies have demonstrated that home BPs are better indicators of cardiovascular risk than office BP.8
Despite lack of statistical significance, home BPs were lower than routine, which suggests that they still may be more reliable than routine office measurements. Definitive conclusions regarding the accuracy of the home BPs in the present study cannot be drawn due to the small sample size (n = 13). Further exploration with comparisons to ambulatory BP monitoring could yield more information on accuracy of home BP monitoring.
In this study’s cohort of older veterans, the average BMI was between 25 and 30 (overweight), which is a risk factor for hypertension.17 Every participant with hypertension was taking at least 1 antihypertensive medication and being actively managed. In this study, the authors accounted for other medications that may affect BP, such as α blockers used in patients with benign prostatic hyperplasia.18 These could have potential elevating or lowering effects on BP measurements.
An issue in this study was the lack of adherence to home BP monitoring. Many patients forgot to bring in their records or to measure their BPs at home. The difficulties highlight real-life issues. Clinicians often request that patients monitor their BP at home, but few may actually remember, let alone keep diligent records. There are many barriers between measuring and reporting home BPs, which may prevent the usefulness of monitoring BP at home.
Limitations
There were several limitations to the study. There was no specific protocol for the routine method of BP measurement, as it was intended to simulate the haphazard nature of office measurements. However, this approach limits its reproducibility. For home BP monitoring, it would have been ideal to provide the same calibrated, automated BP device to each participant. This study of older veterans may not be applicable to the general population. Finally, the relatively small number of participants in the study (n = 30) may have limited power in drawing definitive conclusions.
Future Directions
For future studies, comparing the standardized method to ambulatory BP monitoring would provide more information on accuracy. In addition, the authors would like to evaluate the effect of exercise on BP measurements in the different settings: home, standardized, and routine methods.
1. Mozaffarian D, Benjamin EJ, Go AS, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29-e322.
2. Benjamin EJ, Blaha MJ, Chiuve SE, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146-e603.
3. Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011-2012. NCHS Data Brief. 2013;(133):1-8.
4. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520.
5. Williamson JD, Supiano MA, Applegate WB, et al; SPRINT Research Group. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥ 75 years: a randomized clinical trial. JAMA. 2016;315(24):2673-2682.
6. Pickering TG, Hall JE, Appel LJ, et al; Council on High Blood Pressure Research Professional and Public Education Subcommittee, American Heart Association. Recommendations for blood pressure measurement in humans: an AHA scientific statement from the Council on High Blood Pressure Research Professional and Public Education Subcommittee. J Clin Hypertens (Greenwich). 2005;7(2):102-109.
7. Siu AL; U.S. Preventive Services Task Force. Screening for high blood pressure in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163(10):778-786.
8. Niiranen TJ, Hänninen MR, Johansson J, Reunanen A, Jula AM. Home-measured blood pressure is a stronger predictor of cardiovascular risk than office blood pressure: the Finn-Home study. Hypertension. 2010;55(6):1346-1351.
9. Reino-Gonzalez S, Pita-Fernández S, Seoane-Pillado T, López-Calviño B, Pértega Díaz S. How in-office and ambulatory BP monitoring compare: a systematic review and meta-analysis. J Fam Pract. 2017;66(1):E5-E12.
10. Cohen JB, Cohen DL. Integrating out-of-office blood pressure in the diagnosis and management of hypertension. Curr Cardiol Rep. 2016;18(11):112.
11. Fuchs SC, Mello RB, Fuchs FC. Home blood pressure monitoring is better predictor of cardiovascular disease and target organ damage than office blood pressure: a systematic review and meta-analysis. Curr Cardiol Rep. 2013;15(11):413.
12. Imai Y, Obara T, Asamaya K, Ohkubo T. The reason why home blood pressure measurements are preferred over clinic or ambulatory blood pressure in Japan. Hypertens Res. 2013;36(8):661-672.
13. Bliziotis IA, Destounis A, Stergiou GS. Home versus ambulatory and office blood pressure in predicting target organ damage in hypertension: a systematic review and meta-analysis. J Hypertens. 2012;30(7):1289-1299.
14. Yang Y, Xu JZ, Wang Y, Gao PJ. Ambulatory versus clinic blood pressure in predicting overall subclinical target organ damage progression in essential hypertensive patients: a 3-year follow-up study. Blood Press Monit. 2016;21(6):319-326.
15. Espeland MA, Probstfield J, Hire D, et al; Look AHEAD Research Group; ACCORD Study Group. Systolic blood pressure control among individuals with type 2 diabetes: a comparative effectiveness analysis of three interventions. Am J Hypertens. 2015;28(8):995-1009.
16. Weiss J, Freeman M, Low A, et al. Benefits and harms of intensive blood pressure treatment in adults aged 60 years or older: a systematic review and meta-analysis. Ann Intern Med. 2017;166(6):419-429.
17. Nagai M, Ohkubo T, Murakami Y, et al; NIPPON DATA80/90/2010 Research Group. Secular trends of the impact of overweight and obesity on hypertension in Japan, 1980-2010. Hypertens Res. 2015;38(11):790-795.
18. Press Y, Punchik B, Freud T. Orthostatic hypotension and drug therapy in patients at an outpatient comprehensive geriatric assessment unit. J Hypertens. 2016;34(2):351-358.
Seventy-five percent of adults aged >75 years have hypertension.1-3 According to the Joint National Commission 8 (JNC 8), the recommended target blood pressure (BP) is < 150/80 mm Hg for adults aged > 60 years.4 In 2016 the Systolic Blood Pressure Intervention Trial (SPRINT) suggested that more aggressive BP control with a goal of < 120/80 mm Hg reduced rates of cardiovascular disease and lowered the risk of death in adults aged > 50 years with hypertension.5 It is anticipated that as a result of the landmark SPRINT results, clinicians may attempt to treat hypertension more intensely in older patients with an increased risk of adverse consequences if BPs are not appropriately measured.
There is a standardized protocol for BP measurement, but these recommendations typically are not followed in routine office visits.6,7 Some studies have noted that home BP measurement may be more accurate than office measurement.8 However, clinicians may not always trust the accuracy of home BP readings, and many patients are not adherent with home measurement. As a result, physicians usually manage hypertension in older patients based on office readings, though it is likely that most office measurements do not follow protocol on proper measurement. Office measurements have been noted to be inaccurate with high likelihood of overestimating or underestimating BP control.9
Office BP measurements demonstrate poor correlation with home measurements and have not been shown to be as good of a predictor for target organ damage or long-term cardiovascular outcomes compared with that of home measurements.10,11 Although there have been studies comparing home and office BP measurements and comparing office and ambulatory BP measurement, no literature has been found that reports on the difference between routine office and standardized measurement of BP.9,12-14
This study seeks to identify the magnitude of difference among BP measured according to a standardized protocol, routine clinical, and home BP. The authors hypothesized that there would be a significant, clinically relevant difference among the 3 BP measurement methods, especially between the routine office and standardized office measurements. This study has implications for implementing intensive treatment of hypertension based on office measurements.
Methods
Participants included 30 male veterans aged > 65 years who were actively participating in the Gerofit program at the VA Greater Los Angeles Healthcare System (VAGLAHS). The Gerofit program is a model clinical demonstration exercise and health promotion program targeting older and veterans at risk for falls or institutionalization. Gerofit was established in 1987 at the Durham VA Health System and successfully implemented in 2014 at VAGLAHS. Supervised exercise is offered 3 times per week and consists of individually tailored exercises aimed at reducing functional deficits that are identified and monitored by an initial and quarterly functional assessment. Blood pressures are checked routinely once a week as a part of the program. Gerofit was reviewed and approved by the institutional review board at VAGLAHS as a quality improvement/quality assurance project.
Data
Routine office and standardized protocol measurements were obtained by a single CasMED 740 (Branford, CT) automated BP machine and were conducted separately on different days. The CasMED 740 machine was not otherwise calibrated; however, a one-time correlation was performed between the CasMED 740 and the home BP monitor for each participant, when it was brought to VAGLAHS. Two measurements were made with the CasMed 740 automated BP machine on the arm that gave the higher BP reading throughout the standardized and routine protocol. Two subsequent measurements were made with the participant’s home automated BP cuff. Averages for the CasMED 740 and the home BP monitoring device were compared and assessed for significance by paired t test. No rest was scheduled prior to the first measurement, but there was a 1-minute rest after each subsequent measurement.
Mean values (SD) were used for participant characteristics and mean values (standard error [SE]) were used for BP measurements. Data were analyzed using Microsoft Excel (Redmond, WA) and GraphPad Prism version 7.03 (San Diego, CA). T tests were used for analysis of home BP measurements due to low sample size. Values of P < .05 were considered to be statistically significant.
Routine office protocol. Automated BP was measured to mimic routine office visits. Upon arrival, participants sat down, and the BP cuff was placed around their arm. Any rest before a measurement was incidental and not intentionally structured. Appropriate cuff size was determined by visual estimation of arm circumference. Only 1 measurement was made unless BP was > 150/90 mm Hg, in which case a repeat measurement was made after 2 to 4 minutes of rest. The BP was then determined based on the average of 2 or more readings. The BPs were recorded by hand in a weekly log. Participants had at least 12 weeks of BP readings measured by the routine method, and these BPs were averaged over 12 weeks to yield their average routine measured BP.
Standardized protocol. Automated BP was measured according to the 2015 USPSTF Guidelines and Look AHEAD trial protocol.7,15 A participant’s arm circumference was measured, and appropriate cuff size was determined. The participant rested quietly in a chair for at least 5 minutes with feet flat on the floor and back supported. The cuff was snugly placed 2 to 3 cm above the antecubital fossa, and the arm was supported at the level of the right atrium during the measurement. Blood pressure was determined using the mean of 4 automated cuff readings, 2 on each arm, taken 1 minute apart. Participants did not necessarily have their BP measured by the standardized method immediately following the routine method but all measurements were performed during the same 12-week time period.
Home blood pressure protocol. Participants were given instructions according to the American Heart Association (AHA) recommendations for measuring home BP. Patients were instructed to use a calibrated, automated arm BP cuff. Home BP machines were not provided in advance, and each individual’s BP machine was not calibrated. They also were instructed to rest at least 5 minutes before measuring their BP. The mean home BP was determined by the cumulative average of 3 readings in the morning and evening, taken 1 minute between each reading, for a total of 6 readings/d. Participants recorded home BPs for 2 weeks before submitting their readings. Each participant affirmed clear understanding of how to measure BP by correctly demonstrating placement of the cuff 1 time under supervision.
Results
Thirty veterans aged > 65 years participated in the study. The average age (SD) was 82.7 (9.3) years. The average BMI (SD) was overweight at 29.7 kg/m2 (5.7). Most (87.6%) of the study participants had been diagnosed with hypertension prior to the study, and no new diagnoses were made as a result of the study.
Both systolic BP (SBP) and diastolic BPs (DBP) measured by the standardized method were significantly lower than those by the routine method (P < .01 and P < .01, respectively) (Figure 1).
To determine the accuracy of the home BP monitors, the average routine VAGLAHS BP measure was compared with home BP results. For SBPs, there was a significant correlation coefficient of 0.91 (P < .01).
Discussion
The present study demonstrated that standardized measurements of BP were lower than that of the routine method used in most office settings. These results suggest that there could be a risk of overtreatment for some patients those of whose results are higher than the SPRINT BP target of < 120/80 mm Hg. Clinicians might be treating BPs that are elevated due to improper measurement, which can lead to deleterious consequences in older adults, such as syncope and falls.16
Each participant exhibited a significantly lower BP reading with the standardized method than the routine method. The 20-point decrease in SBP and 10-point decrease in DBP are clinically significant. The routine method of measurement was intended to simulate BP measurement in outpatient settings. There is usually little time structured for rest, and because the protocol established by the AHA and other professional organizations is time consuming, it usually is not strictly followed. With guidelines proposed by JNC 8 and new findings from SPRINT, the method of BP readings should be reviewed in all clinical settings.
While changes in BP management are not necessarily immediate, the differences in recommendations proposed by SPRINT and JNC 8 can lead to confusion regarding how intensely to treat BP. These recommendations guide clinical practice, but clinicians’ best judgment ultimately determines BP management. Physicians who utilize routine office measurements likely rely on BP readings that are higher on average than are readings done under proper conditions. This leads to the prospect of overtreatment, where physicians attempt to control hypertension too aggressively, potentially leading to orthostatic hypotension, syncope, and increased risk for falls.16 With findings from SPRINT recommending even lower BPs than that by JNC 8, overtreatment risk becomes especially relevant. While BP protocol was strictly followed in SPRINT, some clinicians may not necessarily follow the same fastidious protocol.
The average differences between the home and standardized BPs were not statistically significant possibly due to the small sample size in the home BP measurements; however, the difference might represent some clinical relevance. There was a 15-point difference in SBP results between home (129 mm Hg) and standardized (115 mm Hg) measures. There also was a difference in DBP between home (69 mm Hg) and standardized (62 mm Hg) results. The close correlation between both home and BPs measured in VAGLAHS demonstrated that any difference was not due to variability in the measurement devices. Previous studies have demonstrated that home BPs are better indicators of cardiovascular risk than office BP.8
Despite lack of statistical significance, home BPs were lower than routine, which suggests that they still may be more reliable than routine office measurements. Definitive conclusions regarding the accuracy of the home BPs in the present study cannot be drawn due to the small sample size (n = 13). Further exploration with comparisons to ambulatory BP monitoring could yield more information on accuracy of home BP monitoring.
In this study’s cohort of older veterans, the average BMI was between 25 and 30 (overweight), which is a risk factor for hypertension.17 Every participant with hypertension was taking at least 1 antihypertensive medication and being actively managed. In this study, the authors accounted for other medications that may affect BP, such as α blockers used in patients with benign prostatic hyperplasia.18 These could have potential elevating or lowering effects on BP measurements.
An issue in this study was the lack of adherence to home BP monitoring. Many patients forgot to bring in their records or to measure their BPs at home. The difficulties highlight real-life issues. Clinicians often request that patients monitor their BP at home, but few may actually remember, let alone keep diligent records. There are many barriers between measuring and reporting home BPs, which may prevent the usefulness of monitoring BP at home.
Limitations
There were several limitations to the study. There was no specific protocol for the routine method of BP measurement, as it was intended to simulate the haphazard nature of office measurements. However, this approach limits its reproducibility. For home BP monitoring, it would have been ideal to provide the same calibrated, automated BP device to each participant. This study of older veterans may not be applicable to the general population. Finally, the relatively small number of participants in the study (n = 30) may have limited power in drawing definitive conclusions.
Future Directions
For future studies, comparing the standardized method to ambulatory BP monitoring would provide more information on accuracy. In addition, the authors would like to evaluate the effect of exercise on BP measurements in the different settings: home, standardized, and routine methods.
Seventy-five percent of adults aged >75 years have hypertension.1-3 According to the Joint National Commission 8 (JNC 8), the recommended target blood pressure (BP) is < 150/80 mm Hg for adults aged > 60 years.4 In 2016 the Systolic Blood Pressure Intervention Trial (SPRINT) suggested that more aggressive BP control with a goal of < 120/80 mm Hg reduced rates of cardiovascular disease and lowered the risk of death in adults aged > 50 years with hypertension.5 It is anticipated that as a result of the landmark SPRINT results, clinicians may attempt to treat hypertension more intensely in older patients with an increased risk of adverse consequences if BPs are not appropriately measured.
There is a standardized protocol for BP measurement, but these recommendations typically are not followed in routine office visits.6,7 Some studies have noted that home BP measurement may be more accurate than office measurement.8 However, clinicians may not always trust the accuracy of home BP readings, and many patients are not adherent with home measurement. As a result, physicians usually manage hypertension in older patients based on office readings, though it is likely that most office measurements do not follow protocol on proper measurement. Office measurements have been noted to be inaccurate with high likelihood of overestimating or underestimating BP control.9
Office BP measurements demonstrate poor correlation with home measurements and have not been shown to be as good of a predictor for target organ damage or long-term cardiovascular outcomes compared with that of home measurements.10,11 Although there have been studies comparing home and office BP measurements and comparing office and ambulatory BP measurement, no literature has been found that reports on the difference between routine office and standardized measurement of BP.9,12-14
This study seeks to identify the magnitude of difference among BP measured according to a standardized protocol, routine clinical, and home BP. The authors hypothesized that there would be a significant, clinically relevant difference among the 3 BP measurement methods, especially between the routine office and standardized office measurements. This study has implications for implementing intensive treatment of hypertension based on office measurements.
Methods
Participants included 30 male veterans aged > 65 years who were actively participating in the Gerofit program at the VA Greater Los Angeles Healthcare System (VAGLAHS). The Gerofit program is a model clinical demonstration exercise and health promotion program targeting older and veterans at risk for falls or institutionalization. Gerofit was established in 1987 at the Durham VA Health System and successfully implemented in 2014 at VAGLAHS. Supervised exercise is offered 3 times per week and consists of individually tailored exercises aimed at reducing functional deficits that are identified and monitored by an initial and quarterly functional assessment. Blood pressures are checked routinely once a week as a part of the program. Gerofit was reviewed and approved by the institutional review board at VAGLAHS as a quality improvement/quality assurance project.
Data
Routine office and standardized protocol measurements were obtained by a single CasMED 740 (Branford, CT) automated BP machine and were conducted separately on different days. The CasMED 740 machine was not otherwise calibrated; however, a one-time correlation was performed between the CasMED 740 and the home BP monitor for each participant, when it was brought to VAGLAHS. Two measurements were made with the CasMed 740 automated BP machine on the arm that gave the higher BP reading throughout the standardized and routine protocol. Two subsequent measurements were made with the participant’s home automated BP cuff. Averages for the CasMED 740 and the home BP monitoring device were compared and assessed for significance by paired t test. No rest was scheduled prior to the first measurement, but there was a 1-minute rest after each subsequent measurement.
Mean values (SD) were used for participant characteristics and mean values (standard error [SE]) were used for BP measurements. Data were analyzed using Microsoft Excel (Redmond, WA) and GraphPad Prism version 7.03 (San Diego, CA). T tests were used for analysis of home BP measurements due to low sample size. Values of P < .05 were considered to be statistically significant.
Routine office protocol. Automated BP was measured to mimic routine office visits. Upon arrival, participants sat down, and the BP cuff was placed around their arm. Any rest before a measurement was incidental and not intentionally structured. Appropriate cuff size was determined by visual estimation of arm circumference. Only 1 measurement was made unless BP was > 150/90 mm Hg, in which case a repeat measurement was made after 2 to 4 minutes of rest. The BP was then determined based on the average of 2 or more readings. The BPs were recorded by hand in a weekly log. Participants had at least 12 weeks of BP readings measured by the routine method, and these BPs were averaged over 12 weeks to yield their average routine measured BP.
Standardized protocol. Automated BP was measured according to the 2015 USPSTF Guidelines and Look AHEAD trial protocol.7,15 A participant’s arm circumference was measured, and appropriate cuff size was determined. The participant rested quietly in a chair for at least 5 minutes with feet flat on the floor and back supported. The cuff was snugly placed 2 to 3 cm above the antecubital fossa, and the arm was supported at the level of the right atrium during the measurement. Blood pressure was determined using the mean of 4 automated cuff readings, 2 on each arm, taken 1 minute apart. Participants did not necessarily have their BP measured by the standardized method immediately following the routine method but all measurements were performed during the same 12-week time period.
Home blood pressure protocol. Participants were given instructions according to the American Heart Association (AHA) recommendations for measuring home BP. Patients were instructed to use a calibrated, automated arm BP cuff. Home BP machines were not provided in advance, and each individual’s BP machine was not calibrated. They also were instructed to rest at least 5 minutes before measuring their BP. The mean home BP was determined by the cumulative average of 3 readings in the morning and evening, taken 1 minute between each reading, for a total of 6 readings/d. Participants recorded home BPs for 2 weeks before submitting their readings. Each participant affirmed clear understanding of how to measure BP by correctly demonstrating placement of the cuff 1 time under supervision.
Results
Thirty veterans aged > 65 years participated in the study. The average age (SD) was 82.7 (9.3) years. The average BMI (SD) was overweight at 29.7 kg/m2 (5.7). Most (87.6%) of the study participants had been diagnosed with hypertension prior to the study, and no new diagnoses were made as a result of the study.
Both systolic BP (SBP) and diastolic BPs (DBP) measured by the standardized method were significantly lower than those by the routine method (P < .01 and P < .01, respectively) (Figure 1).
To determine the accuracy of the home BP monitors, the average routine VAGLAHS BP measure was compared with home BP results. For SBPs, there was a significant correlation coefficient of 0.91 (P < .01).
Discussion
The present study demonstrated that standardized measurements of BP were lower than that of the routine method used in most office settings. These results suggest that there could be a risk of overtreatment for some patients those of whose results are higher than the SPRINT BP target of < 120/80 mm Hg. Clinicians might be treating BPs that are elevated due to improper measurement, which can lead to deleterious consequences in older adults, such as syncope and falls.16
Each participant exhibited a significantly lower BP reading with the standardized method than the routine method. The 20-point decrease in SBP and 10-point decrease in DBP are clinically significant. The routine method of measurement was intended to simulate BP measurement in outpatient settings. There is usually little time structured for rest, and because the protocol established by the AHA and other professional organizations is time consuming, it usually is not strictly followed. With guidelines proposed by JNC 8 and new findings from SPRINT, the method of BP readings should be reviewed in all clinical settings.
While changes in BP management are not necessarily immediate, the differences in recommendations proposed by SPRINT and JNC 8 can lead to confusion regarding how intensely to treat BP. These recommendations guide clinical practice, but clinicians’ best judgment ultimately determines BP management. Physicians who utilize routine office measurements likely rely on BP readings that are higher on average than are readings done under proper conditions. This leads to the prospect of overtreatment, where physicians attempt to control hypertension too aggressively, potentially leading to orthostatic hypotension, syncope, and increased risk for falls.16 With findings from SPRINT recommending even lower BPs than that by JNC 8, overtreatment risk becomes especially relevant. While BP protocol was strictly followed in SPRINT, some clinicians may not necessarily follow the same fastidious protocol.
The average differences between the home and standardized BPs were not statistically significant possibly due to the small sample size in the home BP measurements; however, the difference might represent some clinical relevance. There was a 15-point difference in SBP results between home (129 mm Hg) and standardized (115 mm Hg) measures. There also was a difference in DBP between home (69 mm Hg) and standardized (62 mm Hg) results. The close correlation between both home and BPs measured in VAGLAHS demonstrated that any difference was not due to variability in the measurement devices. Previous studies have demonstrated that home BPs are better indicators of cardiovascular risk than office BP.8
Despite lack of statistical significance, home BPs were lower than routine, which suggests that they still may be more reliable than routine office measurements. Definitive conclusions regarding the accuracy of the home BPs in the present study cannot be drawn due to the small sample size (n = 13). Further exploration with comparisons to ambulatory BP monitoring could yield more information on accuracy of home BP monitoring.
In this study’s cohort of older veterans, the average BMI was between 25 and 30 (overweight), which is a risk factor for hypertension.17 Every participant with hypertension was taking at least 1 antihypertensive medication and being actively managed. In this study, the authors accounted for other medications that may affect BP, such as α blockers used in patients with benign prostatic hyperplasia.18 These could have potential elevating or lowering effects on BP measurements.
An issue in this study was the lack of adherence to home BP monitoring. Many patients forgot to bring in their records or to measure their BPs at home. The difficulties highlight real-life issues. Clinicians often request that patients monitor their BP at home, but few may actually remember, let alone keep diligent records. There are many barriers between measuring and reporting home BPs, which may prevent the usefulness of monitoring BP at home.
Limitations
There were several limitations to the study. There was no specific protocol for the routine method of BP measurement, as it was intended to simulate the haphazard nature of office measurements. However, this approach limits its reproducibility. For home BP monitoring, it would have been ideal to provide the same calibrated, automated BP device to each participant. This study of older veterans may not be applicable to the general population. Finally, the relatively small number of participants in the study (n = 30) may have limited power in drawing definitive conclusions.
Future Directions
For future studies, comparing the standardized method to ambulatory BP monitoring would provide more information on accuracy. In addition, the authors would like to evaluate the effect of exercise on BP measurements in the different settings: home, standardized, and routine methods.
1. Mozaffarian D, Benjamin EJ, Go AS, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29-e322.
2. Benjamin EJ, Blaha MJ, Chiuve SE, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146-e603.
3. Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011-2012. NCHS Data Brief. 2013;(133):1-8.
4. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520.
5. Williamson JD, Supiano MA, Applegate WB, et al; SPRINT Research Group. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥ 75 years: a randomized clinical trial. JAMA. 2016;315(24):2673-2682.
6. Pickering TG, Hall JE, Appel LJ, et al; Council on High Blood Pressure Research Professional and Public Education Subcommittee, American Heart Association. Recommendations for blood pressure measurement in humans: an AHA scientific statement from the Council on High Blood Pressure Research Professional and Public Education Subcommittee. J Clin Hypertens (Greenwich). 2005;7(2):102-109.
7. Siu AL; U.S. Preventive Services Task Force. Screening for high blood pressure in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163(10):778-786.
8. Niiranen TJ, Hänninen MR, Johansson J, Reunanen A, Jula AM. Home-measured blood pressure is a stronger predictor of cardiovascular risk than office blood pressure: the Finn-Home study. Hypertension. 2010;55(6):1346-1351.
9. Reino-Gonzalez S, Pita-Fernández S, Seoane-Pillado T, López-Calviño B, Pértega Díaz S. How in-office and ambulatory BP monitoring compare: a systematic review and meta-analysis. J Fam Pract. 2017;66(1):E5-E12.
10. Cohen JB, Cohen DL. Integrating out-of-office blood pressure in the diagnosis and management of hypertension. Curr Cardiol Rep. 2016;18(11):112.
11. Fuchs SC, Mello RB, Fuchs FC. Home blood pressure monitoring is better predictor of cardiovascular disease and target organ damage than office blood pressure: a systematic review and meta-analysis. Curr Cardiol Rep. 2013;15(11):413.
12. Imai Y, Obara T, Asamaya K, Ohkubo T. The reason why home blood pressure measurements are preferred over clinic or ambulatory blood pressure in Japan. Hypertens Res. 2013;36(8):661-672.
13. Bliziotis IA, Destounis A, Stergiou GS. Home versus ambulatory and office blood pressure in predicting target organ damage in hypertension: a systematic review and meta-analysis. J Hypertens. 2012;30(7):1289-1299.
14. Yang Y, Xu JZ, Wang Y, Gao PJ. Ambulatory versus clinic blood pressure in predicting overall subclinical target organ damage progression in essential hypertensive patients: a 3-year follow-up study. Blood Press Monit. 2016;21(6):319-326.
15. Espeland MA, Probstfield J, Hire D, et al; Look AHEAD Research Group; ACCORD Study Group. Systolic blood pressure control among individuals with type 2 diabetes: a comparative effectiveness analysis of three interventions. Am J Hypertens. 2015;28(8):995-1009.
16. Weiss J, Freeman M, Low A, et al. Benefits and harms of intensive blood pressure treatment in adults aged 60 years or older: a systematic review and meta-analysis. Ann Intern Med. 2017;166(6):419-429.
17. Nagai M, Ohkubo T, Murakami Y, et al; NIPPON DATA80/90/2010 Research Group. Secular trends of the impact of overweight and obesity on hypertension in Japan, 1980-2010. Hypertens Res. 2015;38(11):790-795.
18. Press Y, Punchik B, Freud T. Orthostatic hypotension and drug therapy in patients at an outpatient comprehensive geriatric assessment unit. J Hypertens. 2016;34(2):351-358.
1. Mozaffarian D, Benjamin EJ, Go AS, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29-e322.
2. Benjamin EJ, Blaha MJ, Chiuve SE, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146-e603.
3. Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011-2012. NCHS Data Brief. 2013;(133):1-8.
4. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520.
5. Williamson JD, Supiano MA, Applegate WB, et al; SPRINT Research Group. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥ 75 years: a randomized clinical trial. JAMA. 2016;315(24):2673-2682.
6. Pickering TG, Hall JE, Appel LJ, et al; Council on High Blood Pressure Research Professional and Public Education Subcommittee, American Heart Association. Recommendations for blood pressure measurement in humans: an AHA scientific statement from the Council on High Blood Pressure Research Professional and Public Education Subcommittee. J Clin Hypertens (Greenwich). 2005;7(2):102-109.
7. Siu AL; U.S. Preventive Services Task Force. Screening for high blood pressure in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163(10):778-786.
8. Niiranen TJ, Hänninen MR, Johansson J, Reunanen A, Jula AM. Home-measured blood pressure is a stronger predictor of cardiovascular risk than office blood pressure: the Finn-Home study. Hypertension. 2010;55(6):1346-1351.
9. Reino-Gonzalez S, Pita-Fernández S, Seoane-Pillado T, López-Calviño B, Pértega Díaz S. How in-office and ambulatory BP monitoring compare: a systematic review and meta-analysis. J Fam Pract. 2017;66(1):E5-E12.
10. Cohen JB, Cohen DL. Integrating out-of-office blood pressure in the diagnosis and management of hypertension. Curr Cardiol Rep. 2016;18(11):112.
11. Fuchs SC, Mello RB, Fuchs FC. Home blood pressure monitoring is better predictor of cardiovascular disease and target organ damage than office blood pressure: a systematic review and meta-analysis. Curr Cardiol Rep. 2013;15(11):413.
12. Imai Y, Obara T, Asamaya K, Ohkubo T. The reason why home blood pressure measurements are preferred over clinic or ambulatory blood pressure in Japan. Hypertens Res. 2013;36(8):661-672.
13. Bliziotis IA, Destounis A, Stergiou GS. Home versus ambulatory and office blood pressure in predicting target organ damage in hypertension: a systematic review and meta-analysis. J Hypertens. 2012;30(7):1289-1299.
14. Yang Y, Xu JZ, Wang Y, Gao PJ. Ambulatory versus clinic blood pressure in predicting overall subclinical target organ damage progression in essential hypertensive patients: a 3-year follow-up study. Blood Press Monit. 2016;21(6):319-326.
15. Espeland MA, Probstfield J, Hire D, et al; Look AHEAD Research Group; ACCORD Study Group. Systolic blood pressure control among individuals with type 2 diabetes: a comparative effectiveness analysis of three interventions. Am J Hypertens. 2015;28(8):995-1009.
16. Weiss J, Freeman M, Low A, et al. Benefits and harms of intensive blood pressure treatment in adults aged 60 years or older: a systematic review and meta-analysis. Ann Intern Med. 2017;166(6):419-429.
17. Nagai M, Ohkubo T, Murakami Y, et al; NIPPON DATA80/90/2010 Research Group. Secular trends of the impact of overweight and obesity on hypertension in Japan, 1980-2010. Hypertens Res. 2015;38(11):790-795.
18. Press Y, Punchik B, Freud T. Orthostatic hypotension and drug therapy in patients at an outpatient comprehensive geriatric assessment unit. J Hypertens. 2016;34(2):351-358.