User login
Mandibular Device Comparable to CPAP to Reduce BP in Hypertension, OSA
Use of a mandibular advancement device (MAD) proved non-inferior to guideline-recommended continuous positive airway pressure (CPAP) to reduce blood pressure in patients with hypertension and obstructive sleep apnea (OSA), in a randomized trial.
“These findings suggest that MAD could be considered an alternative to CPAP for optimizing blood pressure control in OSA patients with hypertension and high cardiovascular risk,” the researchers conclude.
“Looking at the totality of evidence available in the literature, it is still reasonable to say that CPAP is the first-line treatment until we have more data on the MAD,” said Ronald Lee Chi-Hang, MD, professor of medicine at Yong Loo Lin School of Medicine, National University of Singapore, who presented the results.
“However, for patients who truly cannot tolerate or accept using a CPAP, we should be more open-minded in looking for an alternative therapy such as a MAD, which based on our study, numerically had a better blood pressure reduction in patients compared with a CPAP,” said Dr. Chi-Hang, who is also a senior consultant in the Department of Cardiology at Singapore’s National University Heart Centre.
The results were presented April 6 at the American College of Cardiology Scientific Sessions 2024 and published online simultaneously in the Journal of the American College of Cardiology
Oral Appliance
OSA is increasingly recognized as “an underdiagnosed and modifiable cause of hypertension,” the researchers note in their report. “Patients with OSA develop recurrent collapse of the upper airway during sleep, resulting in hypoxemia, sympathetic hyperactivity, and BP surges.”
Current guidelines recommend screening and treatment of OSA in patients with hypertension, and CPAP is considered first-line therapy, they note.
“Despite being effective, unfortunately, many patients decline to use a CPAP or find it challenging to stick to the therapy,” Dr. Chi-Hang said, particularly those without daytime sleepiness.
MADs are oral appliances that work by advancing the mandible about 5 to 10 mm during sleep, he said. They provide an alternative to OSA patients and have been shown to improve daytime sleepiness and quality of life, “and in general, is better accepted and tolerated than CPAP.”
However, early studies are small, with short follow up, included patients with and without hypertension, and didn’t specify BP reduction as the primary outcome.
The CRESCENT trial was an investigator-initiated, randomized, non-inferiority trial that aimed to compare the relative effectiveness of MAD vs CPAP in reducing 24-hour ambulatory blood pressure in patients with moderate-to-severe OSA, hypertension and high cardiovascular risk. The prespecified margin for non-inferiority was 1.5 mm Hg.
A total of 321 participants were recruited at three public hospitals for polysomnography. All were older than age 40 years, had hypertension, and were at increased cardiovascular risk. Of these, 220 with moderate-to-severe OSA, defined as an apnea–hypopnea index (AHI) of ≥ 15 events/hour, were randomly assigned to either MAD or CPAP treatment.
The primary outcome was the difference between the 24-hour mean arterial BP at baseline and 6 months. The median age was 61 years, most patients (85.5%) were male, and all were Chinese. All had essential hypertension and were on one or more antihypertensive medications. Hypertension was relatively well controlled at baseline.
At 6 months, 24-hour mean arterial BP decreased by 2.5 mm Hg in the MAD group (P = .003) compared to no change from baseline in the CPAP group (P = .374).
The between-group difference was -1.6 mm Hg (95% CI, -3.51 to 0.24, non-inferiority P < .001).
There was a larger between-group reduction in all secondary ambulatory BP parameters in the MAD versus the CPAP group, with the most pronounced effects seen in the asleep BP parameters.
Both the MAD and CPAP significantly improved daytime sleepiness, with no between-group differences (P =.384). There were no between-group differences in cardiovascular biomarkers.
During the presentation, panel discussant Julie B. Damp, MD, associate professor of medicine at Vanderbilt Health in Nashville, Tennessee, called CRESCENT “a really interesting study, and I think it has a lot of information to add [regarding] what we know about this comparison in the literature, because this is a big study and it also followed these patients for longer than we’ve seen in some of the previous studies.”
Dr. Damp asked, however, about how these results might be extrapolated to other populations, since the vast majority of participants were male.
Dr. Chi-Hang pointed out that most OSA studies include mostly male patients, but noted that particularly in Asian culture, female patients may be more conservative in seeking treatment for problems with snoring, poor quality of sleep, or extensive daytime sleepiness. “Therefore, lots of times, even in clinical practice, we see that over 80 or 90% of patients are male patients,” he said.
Dr. Damp followed up by asking about the differential effectiveness of CPAP vs MAD. “Just in thinking about these two therapies, there is some evidence that the mandibular devices are potentially less effective on some of the sleep apnea-specific measures, so how much of this do you think is an issue of a better vs a not better treatment as opposed to an issue truly of compliance and what patients are able to tolerate?”
Dr. Chi-Hang agreed that in terms of reducing the AHI, CPAP is more effective than MAD. “In fact, in our data, the residual AHI was 10 for the MAD group and 2 for the CPAP group. Clearly, CPAP is more effective,” he said. “But the problem we are facing in this area is the value of AHI as an index is being questioned.”
AHI considers only the number of events, without taking into account the duration or the depth of the apnea, he said. “AHI is simply not an ideal index to document the disease severity,” or the impact on cardiovascular outcomes.
A Tailored Approach
In an editorial accompanying the JACC publication, Michele Emdin, MD, PhD, Francesco Gentile, MD, and Alberto Giannoni, MD, PhD, all from the Health Science Interdisciplinary Center, Scuola Superiore Sant’ Anna, and Fondazione Toscana Gabriele Monasterio, in Pisa, Italy, commend the researchers for designing and conducting “such a pragmatic and informative trial, which confirms and extends previous findings.”
They also discuss the compliance vs effectiveness issue, pointing out that although CPAP appeared to be more effective in reducing apnea burden, there was higher adherence to MAD — with 57% using the device 6 or more hours per night, vs 23% for CPAP — which might have offset the greater reduction in apnea burden and resulted in the reduction in blood pressure seen in the trial.
“Addressing poor adherence to OSA treatments seems therefore necessary, particularly in the case of less symptomatic patients, who often have a lower perception of the related risks,” they write.
“Currently, a tailored approach seems reasonable, based on updated evidence, considering: a) the differential effects of CPAP or MAD on OSA, blood pressure; b) the treatment feasibility; c) the individual baseline demographic and clinical characteristics, including the presence of resistant hypertension; and d) compliance with the therapeutic tool and patient’s preferences,” the editorialists conclude.
The study was funded by the Singapore Ministry of Health. The authors and editorialists report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Use of a mandibular advancement device (MAD) proved non-inferior to guideline-recommended continuous positive airway pressure (CPAP) to reduce blood pressure in patients with hypertension and obstructive sleep apnea (OSA), in a randomized trial.
“These findings suggest that MAD could be considered an alternative to CPAP for optimizing blood pressure control in OSA patients with hypertension and high cardiovascular risk,” the researchers conclude.
“Looking at the totality of evidence available in the literature, it is still reasonable to say that CPAP is the first-line treatment until we have more data on the MAD,” said Ronald Lee Chi-Hang, MD, professor of medicine at Yong Loo Lin School of Medicine, National University of Singapore, who presented the results.
“However, for patients who truly cannot tolerate or accept using a CPAP, we should be more open-minded in looking for an alternative therapy such as a MAD, which based on our study, numerically had a better blood pressure reduction in patients compared with a CPAP,” said Dr. Chi-Hang, who is also a senior consultant in the Department of Cardiology at Singapore’s National University Heart Centre.
The results were presented April 6 at the American College of Cardiology Scientific Sessions 2024 and published online simultaneously in the Journal of the American College of Cardiology
Oral Appliance
OSA is increasingly recognized as “an underdiagnosed and modifiable cause of hypertension,” the researchers note in their report. “Patients with OSA develop recurrent collapse of the upper airway during sleep, resulting in hypoxemia, sympathetic hyperactivity, and BP surges.”
Current guidelines recommend screening and treatment of OSA in patients with hypertension, and CPAP is considered first-line therapy, they note.
“Despite being effective, unfortunately, many patients decline to use a CPAP or find it challenging to stick to the therapy,” Dr. Chi-Hang said, particularly those without daytime sleepiness.
MADs are oral appliances that work by advancing the mandible about 5 to 10 mm during sleep, he said. They provide an alternative to OSA patients and have been shown to improve daytime sleepiness and quality of life, “and in general, is better accepted and tolerated than CPAP.”
However, early studies are small, with short follow up, included patients with and without hypertension, and didn’t specify BP reduction as the primary outcome.
The CRESCENT trial was an investigator-initiated, randomized, non-inferiority trial that aimed to compare the relative effectiveness of MAD vs CPAP in reducing 24-hour ambulatory blood pressure in patients with moderate-to-severe OSA, hypertension and high cardiovascular risk. The prespecified margin for non-inferiority was 1.5 mm Hg.
A total of 321 participants were recruited at three public hospitals for polysomnography. All were older than age 40 years, had hypertension, and were at increased cardiovascular risk. Of these, 220 with moderate-to-severe OSA, defined as an apnea–hypopnea index (AHI) of ≥ 15 events/hour, were randomly assigned to either MAD or CPAP treatment.
The primary outcome was the difference between the 24-hour mean arterial BP at baseline and 6 months. The median age was 61 years, most patients (85.5%) were male, and all were Chinese. All had essential hypertension and were on one or more antihypertensive medications. Hypertension was relatively well controlled at baseline.
At 6 months, 24-hour mean arterial BP decreased by 2.5 mm Hg in the MAD group (P = .003) compared to no change from baseline in the CPAP group (P = .374).
The between-group difference was -1.6 mm Hg (95% CI, -3.51 to 0.24, non-inferiority P < .001).
There was a larger between-group reduction in all secondary ambulatory BP parameters in the MAD versus the CPAP group, with the most pronounced effects seen in the asleep BP parameters.
Both the MAD and CPAP significantly improved daytime sleepiness, with no between-group differences (P =.384). There were no between-group differences in cardiovascular biomarkers.
During the presentation, panel discussant Julie B. Damp, MD, associate professor of medicine at Vanderbilt Health in Nashville, Tennessee, called CRESCENT “a really interesting study, and I think it has a lot of information to add [regarding] what we know about this comparison in the literature, because this is a big study and it also followed these patients for longer than we’ve seen in some of the previous studies.”
Dr. Damp asked, however, about how these results might be extrapolated to other populations, since the vast majority of participants were male.
Dr. Chi-Hang pointed out that most OSA studies include mostly male patients, but noted that particularly in Asian culture, female patients may be more conservative in seeking treatment for problems with snoring, poor quality of sleep, or extensive daytime sleepiness. “Therefore, lots of times, even in clinical practice, we see that over 80 or 90% of patients are male patients,” he said.
Dr. Damp followed up by asking about the differential effectiveness of CPAP vs MAD. “Just in thinking about these two therapies, there is some evidence that the mandibular devices are potentially less effective on some of the sleep apnea-specific measures, so how much of this do you think is an issue of a better vs a not better treatment as opposed to an issue truly of compliance and what patients are able to tolerate?”
Dr. Chi-Hang agreed that in terms of reducing the AHI, CPAP is more effective than MAD. “In fact, in our data, the residual AHI was 10 for the MAD group and 2 for the CPAP group. Clearly, CPAP is more effective,” he said. “But the problem we are facing in this area is the value of AHI as an index is being questioned.”
AHI considers only the number of events, without taking into account the duration or the depth of the apnea, he said. “AHI is simply not an ideal index to document the disease severity,” or the impact on cardiovascular outcomes.
A Tailored Approach
In an editorial accompanying the JACC publication, Michele Emdin, MD, PhD, Francesco Gentile, MD, and Alberto Giannoni, MD, PhD, all from the Health Science Interdisciplinary Center, Scuola Superiore Sant’ Anna, and Fondazione Toscana Gabriele Monasterio, in Pisa, Italy, commend the researchers for designing and conducting “such a pragmatic and informative trial, which confirms and extends previous findings.”
They also discuss the compliance vs effectiveness issue, pointing out that although CPAP appeared to be more effective in reducing apnea burden, there was higher adherence to MAD — with 57% using the device 6 or more hours per night, vs 23% for CPAP — which might have offset the greater reduction in apnea burden and resulted in the reduction in blood pressure seen in the trial.
“Addressing poor adherence to OSA treatments seems therefore necessary, particularly in the case of less symptomatic patients, who often have a lower perception of the related risks,” they write.
“Currently, a tailored approach seems reasonable, based on updated evidence, considering: a) the differential effects of CPAP or MAD on OSA, blood pressure; b) the treatment feasibility; c) the individual baseline demographic and clinical characteristics, including the presence of resistant hypertension; and d) compliance with the therapeutic tool and patient’s preferences,” the editorialists conclude.
The study was funded by the Singapore Ministry of Health. The authors and editorialists report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Use of a mandibular advancement device (MAD) proved non-inferior to guideline-recommended continuous positive airway pressure (CPAP) to reduce blood pressure in patients with hypertension and obstructive sleep apnea (OSA), in a randomized trial.
“These findings suggest that MAD could be considered an alternative to CPAP for optimizing blood pressure control in OSA patients with hypertension and high cardiovascular risk,” the researchers conclude.
“Looking at the totality of evidence available in the literature, it is still reasonable to say that CPAP is the first-line treatment until we have more data on the MAD,” said Ronald Lee Chi-Hang, MD, professor of medicine at Yong Loo Lin School of Medicine, National University of Singapore, who presented the results.
“However, for patients who truly cannot tolerate or accept using a CPAP, we should be more open-minded in looking for an alternative therapy such as a MAD, which based on our study, numerically had a better blood pressure reduction in patients compared with a CPAP,” said Dr. Chi-Hang, who is also a senior consultant in the Department of Cardiology at Singapore’s National University Heart Centre.
The results were presented April 6 at the American College of Cardiology Scientific Sessions 2024 and published online simultaneously in the Journal of the American College of Cardiology
Oral Appliance
OSA is increasingly recognized as “an underdiagnosed and modifiable cause of hypertension,” the researchers note in their report. “Patients with OSA develop recurrent collapse of the upper airway during sleep, resulting in hypoxemia, sympathetic hyperactivity, and BP surges.”
Current guidelines recommend screening and treatment of OSA in patients with hypertension, and CPAP is considered first-line therapy, they note.
“Despite being effective, unfortunately, many patients decline to use a CPAP or find it challenging to stick to the therapy,” Dr. Chi-Hang said, particularly those without daytime sleepiness.
MADs are oral appliances that work by advancing the mandible about 5 to 10 mm during sleep, he said. They provide an alternative to OSA patients and have been shown to improve daytime sleepiness and quality of life, “and in general, is better accepted and tolerated than CPAP.”
However, early studies are small, with short follow up, included patients with and without hypertension, and didn’t specify BP reduction as the primary outcome.
The CRESCENT trial was an investigator-initiated, randomized, non-inferiority trial that aimed to compare the relative effectiveness of MAD vs CPAP in reducing 24-hour ambulatory blood pressure in patients with moderate-to-severe OSA, hypertension and high cardiovascular risk. The prespecified margin for non-inferiority was 1.5 mm Hg.
A total of 321 participants were recruited at three public hospitals for polysomnography. All were older than age 40 years, had hypertension, and were at increased cardiovascular risk. Of these, 220 with moderate-to-severe OSA, defined as an apnea–hypopnea index (AHI) of ≥ 15 events/hour, were randomly assigned to either MAD or CPAP treatment.
The primary outcome was the difference between the 24-hour mean arterial BP at baseline and 6 months. The median age was 61 years, most patients (85.5%) were male, and all were Chinese. All had essential hypertension and were on one or more antihypertensive medications. Hypertension was relatively well controlled at baseline.
At 6 months, 24-hour mean arterial BP decreased by 2.5 mm Hg in the MAD group (P = .003) compared to no change from baseline in the CPAP group (P = .374).
The between-group difference was -1.6 mm Hg (95% CI, -3.51 to 0.24, non-inferiority P < .001).
There was a larger between-group reduction in all secondary ambulatory BP parameters in the MAD versus the CPAP group, with the most pronounced effects seen in the asleep BP parameters.
Both the MAD and CPAP significantly improved daytime sleepiness, with no between-group differences (P =.384). There were no between-group differences in cardiovascular biomarkers.
During the presentation, panel discussant Julie B. Damp, MD, associate professor of medicine at Vanderbilt Health in Nashville, Tennessee, called CRESCENT “a really interesting study, and I think it has a lot of information to add [regarding] what we know about this comparison in the literature, because this is a big study and it also followed these patients for longer than we’ve seen in some of the previous studies.”
Dr. Damp asked, however, about how these results might be extrapolated to other populations, since the vast majority of participants were male.
Dr. Chi-Hang pointed out that most OSA studies include mostly male patients, but noted that particularly in Asian culture, female patients may be more conservative in seeking treatment for problems with snoring, poor quality of sleep, or extensive daytime sleepiness. “Therefore, lots of times, even in clinical practice, we see that over 80 or 90% of patients are male patients,” he said.
Dr. Damp followed up by asking about the differential effectiveness of CPAP vs MAD. “Just in thinking about these two therapies, there is some evidence that the mandibular devices are potentially less effective on some of the sleep apnea-specific measures, so how much of this do you think is an issue of a better vs a not better treatment as opposed to an issue truly of compliance and what patients are able to tolerate?”
Dr. Chi-Hang agreed that in terms of reducing the AHI, CPAP is more effective than MAD. “In fact, in our data, the residual AHI was 10 for the MAD group and 2 for the CPAP group. Clearly, CPAP is more effective,” he said. “But the problem we are facing in this area is the value of AHI as an index is being questioned.”
AHI considers only the number of events, without taking into account the duration or the depth of the apnea, he said. “AHI is simply not an ideal index to document the disease severity,” or the impact on cardiovascular outcomes.
A Tailored Approach
In an editorial accompanying the JACC publication, Michele Emdin, MD, PhD, Francesco Gentile, MD, and Alberto Giannoni, MD, PhD, all from the Health Science Interdisciplinary Center, Scuola Superiore Sant’ Anna, and Fondazione Toscana Gabriele Monasterio, in Pisa, Italy, commend the researchers for designing and conducting “such a pragmatic and informative trial, which confirms and extends previous findings.”
They also discuss the compliance vs effectiveness issue, pointing out that although CPAP appeared to be more effective in reducing apnea burden, there was higher adherence to MAD — with 57% using the device 6 or more hours per night, vs 23% for CPAP — which might have offset the greater reduction in apnea burden and resulted in the reduction in blood pressure seen in the trial.
“Addressing poor adherence to OSA treatments seems therefore necessary, particularly in the case of less symptomatic patients, who often have a lower perception of the related risks,” they write.
“Currently, a tailored approach seems reasonable, based on updated evidence, considering: a) the differential effects of CPAP or MAD on OSA, blood pressure; b) the treatment feasibility; c) the individual baseline demographic and clinical characteristics, including the presence of resistant hypertension; and d) compliance with the therapeutic tool and patient’s preferences,” the editorialists conclude.
The study was funded by the Singapore Ministry of Health. The authors and editorialists report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Blood pressure lowering reduces dementia risk
Results of a trial using an intensive, 4-year program aimed at blood pressure lowering showed that intervention reduced not only blood pressure, but also significantly reduced the risk of total dementia over that period.
and cognitive impairment no dementia (CIND), a secondary outcome, was also significantly reduced by 16%.
“Blood pressure reduction is effective in reducing the risk of dementia in patients with hypertension,” concluded Jiang He, MD, PhD, professor of epidemiology and medicine and director of Tulane University’s Translational Science Institute, New Orleans. “This proven, effective intervention should be widely scaled up to reduce the global burden of dementia.”
He presented these results from the China Rural Hypertension Control Project (CRHCP) at the annual scientific sessions of the American Heart Association.
Target organ damage
Keith Ferdinand, MD, also from Tulane University, commented on the findings during a press conference at the meeting, noting that the result “opens our opportunity to recognize that the target organ damage of hypertension also now includes dementia.”
The researchers were able to “rigorously lower blood pressure from 157 to 127.6 in the intervention, 155 to 147 in the controls – 22 mg Hg – and if you look at the P values for all the various outcomes, they were very robust,” Dr. Ferdinand said.
Another interesting feature about the strategy used in this trial is that “this was true team-based care,” he pointed out. The trained interventionists in the study, called village doctors, collaborated with primary care physicians and initiated medications. “They stayed on a simple treatment protocol, and they were able to assist patients to ensure they had free medications, health coaching for lifestyle, home blood pressure measurement, and ensuring adherence.”
So, Dr. Ferdinand added, “one of the questions is whether this is a model we can use in other places around the globe, in places with low resources, and in the United States in disadvantaged populations.”
Public health priority
It’s estimated that the global number of those living with dementia will increase from 57.4 million in 2019 to 152.8 million by 2050, Dr. He said. “In the absence of curative treatment, the primary prevention of dementia through risk factor reduction, such as blood pressure lowering, becomes a public health priority.”
Previous randomized trials have lacked sample size and duration but have reported a nonsignificant reduction in dementia associated with antihypertensive treatment in patients with hypertension or a history of stroke, Dr. He noted.
This new trial aimed to test the effectiveness of intensive BP intervention to reduce the risk of all-cause dementia and cognitive impairment over a 48-month intervention period versus usual care.
It was an open-label, blinded-endpoint, cluster-randomized trial, and included 33,995 individual patients from 325 villages in China, aged 40 years and older, with untreated hypertension. The villages were randomly assigned to an intervention group or usual care, stratified by province, county, and township.
Patients were eligible if they had mean untreated systolic BP greater than 140 mm Hg and/or diastolic BP greater than 90 mm Hg or mean treated systolic BP of greater than 130 and/or diastolic greater than 80 mm Hg. Patients with a history of cardiovascular disease, chronic kidney disease, or diabetes and a mean systolic BP greater than 130 mm Hg and/or diastolic BP greater than 80 mm Hg from six measures on two different days were also eligible.
All were enrolled in the China New Rural Cooperative Medical Scheme, which covers 99% of rural residents for health care services, Dr. He noted.
The intervention was a simple stepped-care protocol for hypertension treatment, aimed at achieving a target systolic BP of less than 130 mm Hg and diastolic of less than 80 mm Hg.
Village doctors started and titrated antihypertensive treatment based on a protocol and were able to deliver discounted and free medications to patients. They also did health coaching on lifestyle modification and adherence to medication, and instructed patients on home BP monitoring.
Patients were provided training, supervision, and consultation by primary care physicians and hypertension specialists.
At the month 48 follow-up visit, the participants were assessed by neurologists who were blinded to randomization assignments. Neurologists did a variety of tests and assessments including collecting data on the patient’s medical and psychiatric history and risk factors for dementia, as well as neurologic assessment using the Mini-Mental State Examination, the Functional Activities Questionnaire, and the Quick Dementia Rating System.
The primary outcome was all-cause dementia, defined according to recommendations from the National Institute on Aging–Alzheimer’s Association work groups on diagnostic guidelines for Alzheimer’s disease.
Secondary outcomes included CIND, a composite outcome of dementia or CIND, and a composite of dementia or deaths.
The final diagnosis of all-cause dementia or CIND was made by an expert adjudication panel blinded to the intervention assignment.
At 48 months, 91.3% of patients completed the follow-up for clinical outcomes. Participants were an average of 63 years of age, 61% were female, and 23% had less than a primary school education, Dr. He noted.
The net group differences in systolic and diastolic BP reduction were 22 and 9.3 mm Hg, respectively (P < .0001).
Significant differences were also seen between the groups in the primary outcome of all-cause dementia, as well as secondary outcomes of CIND, dementia or cognitive impairment, or dementia or deaths.
Serious adverse events were more common in the usual care group, and there was no difference between groups in the occurrence of falls or syncope.
The effect was consistent across subgroups, Dr. He said, including age, sex, education, cigarette smoking, body mass index, systolic BP, and fasting plasma glucose at baseline.
First definitive evidence
Invited discussant for the trial, Daniel W. Jones, MD, University of Mississippi Medical Center, Jackson, and past president of the AHA, pointed out that previous results from CRHCP on cardiovascular outcomes, reported earlier in 2023 in The Lancet, showed that, similar to results of the large SPRINT trial, lowering systolic BP to a goal of less than 130 mm Hg reduced a composite endpoint of MI, stroke, heart failure requiring hospitalization, and cardiovascular disease death over the 36-month follow-up.
The SPRINT findings also suggested a possible reduction in dementia, Dr. Jones said.
Now, in these new CRHCP results, “there was a clear benefit for intensive BP control in reducing risk for dementia and cognitive dysfunction,” he said. “This is, importantly, the first definitive evidence of dementia risk reduction demonstrated in a randomized controlled clinical trial. This outcome supports observational data that shows a strong relationship between BP and dementia.”
Since it is the first of its kind though, replication of the results will be important, he noted.
The study also showed that the intervention, using minimally trained village doctors, sustained BP control for 48 months. “This model could be used in any setting with modifications, including in the United States,” Dr. Jones said.
The study was supported by the Ministry of Science and Technology of China; U.S. investigators did not receive financial support from this study. The researchers and Dr. Jones disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Results of a trial using an intensive, 4-year program aimed at blood pressure lowering showed that intervention reduced not only blood pressure, but also significantly reduced the risk of total dementia over that period.
and cognitive impairment no dementia (CIND), a secondary outcome, was also significantly reduced by 16%.
“Blood pressure reduction is effective in reducing the risk of dementia in patients with hypertension,” concluded Jiang He, MD, PhD, professor of epidemiology and medicine and director of Tulane University’s Translational Science Institute, New Orleans. “This proven, effective intervention should be widely scaled up to reduce the global burden of dementia.”
He presented these results from the China Rural Hypertension Control Project (CRHCP) at the annual scientific sessions of the American Heart Association.
Target organ damage
Keith Ferdinand, MD, also from Tulane University, commented on the findings during a press conference at the meeting, noting that the result “opens our opportunity to recognize that the target organ damage of hypertension also now includes dementia.”
The researchers were able to “rigorously lower blood pressure from 157 to 127.6 in the intervention, 155 to 147 in the controls – 22 mg Hg – and if you look at the P values for all the various outcomes, they were very robust,” Dr. Ferdinand said.
Another interesting feature about the strategy used in this trial is that “this was true team-based care,” he pointed out. The trained interventionists in the study, called village doctors, collaborated with primary care physicians and initiated medications. “They stayed on a simple treatment protocol, and they were able to assist patients to ensure they had free medications, health coaching for lifestyle, home blood pressure measurement, and ensuring adherence.”
So, Dr. Ferdinand added, “one of the questions is whether this is a model we can use in other places around the globe, in places with low resources, and in the United States in disadvantaged populations.”
Public health priority
It’s estimated that the global number of those living with dementia will increase from 57.4 million in 2019 to 152.8 million by 2050, Dr. He said. “In the absence of curative treatment, the primary prevention of dementia through risk factor reduction, such as blood pressure lowering, becomes a public health priority.”
Previous randomized trials have lacked sample size and duration but have reported a nonsignificant reduction in dementia associated with antihypertensive treatment in patients with hypertension or a history of stroke, Dr. He noted.
This new trial aimed to test the effectiveness of intensive BP intervention to reduce the risk of all-cause dementia and cognitive impairment over a 48-month intervention period versus usual care.
It was an open-label, blinded-endpoint, cluster-randomized trial, and included 33,995 individual patients from 325 villages in China, aged 40 years and older, with untreated hypertension. The villages were randomly assigned to an intervention group or usual care, stratified by province, county, and township.
Patients were eligible if they had mean untreated systolic BP greater than 140 mm Hg and/or diastolic BP greater than 90 mm Hg or mean treated systolic BP of greater than 130 and/or diastolic greater than 80 mm Hg. Patients with a history of cardiovascular disease, chronic kidney disease, or diabetes and a mean systolic BP greater than 130 mm Hg and/or diastolic BP greater than 80 mm Hg from six measures on two different days were also eligible.
All were enrolled in the China New Rural Cooperative Medical Scheme, which covers 99% of rural residents for health care services, Dr. He noted.
The intervention was a simple stepped-care protocol for hypertension treatment, aimed at achieving a target systolic BP of less than 130 mm Hg and diastolic of less than 80 mm Hg.
Village doctors started and titrated antihypertensive treatment based on a protocol and were able to deliver discounted and free medications to patients. They also did health coaching on lifestyle modification and adherence to medication, and instructed patients on home BP monitoring.
Patients were provided training, supervision, and consultation by primary care physicians and hypertension specialists.
At the month 48 follow-up visit, the participants were assessed by neurologists who were blinded to randomization assignments. Neurologists did a variety of tests and assessments including collecting data on the patient’s medical and psychiatric history and risk factors for dementia, as well as neurologic assessment using the Mini-Mental State Examination, the Functional Activities Questionnaire, and the Quick Dementia Rating System.
The primary outcome was all-cause dementia, defined according to recommendations from the National Institute on Aging–Alzheimer’s Association work groups on diagnostic guidelines for Alzheimer’s disease.
Secondary outcomes included CIND, a composite outcome of dementia or CIND, and a composite of dementia or deaths.
The final diagnosis of all-cause dementia or CIND was made by an expert adjudication panel blinded to the intervention assignment.
At 48 months, 91.3% of patients completed the follow-up for clinical outcomes. Participants were an average of 63 years of age, 61% were female, and 23% had less than a primary school education, Dr. He noted.
The net group differences in systolic and diastolic BP reduction were 22 and 9.3 mm Hg, respectively (P < .0001).
Significant differences were also seen between the groups in the primary outcome of all-cause dementia, as well as secondary outcomes of CIND, dementia or cognitive impairment, or dementia or deaths.
Serious adverse events were more common in the usual care group, and there was no difference between groups in the occurrence of falls or syncope.
The effect was consistent across subgroups, Dr. He said, including age, sex, education, cigarette smoking, body mass index, systolic BP, and fasting plasma glucose at baseline.
First definitive evidence
Invited discussant for the trial, Daniel W. Jones, MD, University of Mississippi Medical Center, Jackson, and past president of the AHA, pointed out that previous results from CRHCP on cardiovascular outcomes, reported earlier in 2023 in The Lancet, showed that, similar to results of the large SPRINT trial, lowering systolic BP to a goal of less than 130 mm Hg reduced a composite endpoint of MI, stroke, heart failure requiring hospitalization, and cardiovascular disease death over the 36-month follow-up.
The SPRINT findings also suggested a possible reduction in dementia, Dr. Jones said.
Now, in these new CRHCP results, “there was a clear benefit for intensive BP control in reducing risk for dementia and cognitive dysfunction,” he said. “This is, importantly, the first definitive evidence of dementia risk reduction demonstrated in a randomized controlled clinical trial. This outcome supports observational data that shows a strong relationship between BP and dementia.”
Since it is the first of its kind though, replication of the results will be important, he noted.
The study also showed that the intervention, using minimally trained village doctors, sustained BP control for 48 months. “This model could be used in any setting with modifications, including in the United States,” Dr. Jones said.
The study was supported by the Ministry of Science and Technology of China; U.S. investigators did not receive financial support from this study. The researchers and Dr. Jones disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Results of a trial using an intensive, 4-year program aimed at blood pressure lowering showed that intervention reduced not only blood pressure, but also significantly reduced the risk of total dementia over that period.
and cognitive impairment no dementia (CIND), a secondary outcome, was also significantly reduced by 16%.
“Blood pressure reduction is effective in reducing the risk of dementia in patients with hypertension,” concluded Jiang He, MD, PhD, professor of epidemiology and medicine and director of Tulane University’s Translational Science Institute, New Orleans. “This proven, effective intervention should be widely scaled up to reduce the global burden of dementia.”
He presented these results from the China Rural Hypertension Control Project (CRHCP) at the annual scientific sessions of the American Heart Association.
Target organ damage
Keith Ferdinand, MD, also from Tulane University, commented on the findings during a press conference at the meeting, noting that the result “opens our opportunity to recognize that the target organ damage of hypertension also now includes dementia.”
The researchers were able to “rigorously lower blood pressure from 157 to 127.6 in the intervention, 155 to 147 in the controls – 22 mg Hg – and if you look at the P values for all the various outcomes, they were very robust,” Dr. Ferdinand said.
Another interesting feature about the strategy used in this trial is that “this was true team-based care,” he pointed out. The trained interventionists in the study, called village doctors, collaborated with primary care physicians and initiated medications. “They stayed on a simple treatment protocol, and they were able to assist patients to ensure they had free medications, health coaching for lifestyle, home blood pressure measurement, and ensuring adherence.”
So, Dr. Ferdinand added, “one of the questions is whether this is a model we can use in other places around the globe, in places with low resources, and in the United States in disadvantaged populations.”
Public health priority
It’s estimated that the global number of those living with dementia will increase from 57.4 million in 2019 to 152.8 million by 2050, Dr. He said. “In the absence of curative treatment, the primary prevention of dementia through risk factor reduction, such as blood pressure lowering, becomes a public health priority.”
Previous randomized trials have lacked sample size and duration but have reported a nonsignificant reduction in dementia associated with antihypertensive treatment in patients with hypertension or a history of stroke, Dr. He noted.
This new trial aimed to test the effectiveness of intensive BP intervention to reduce the risk of all-cause dementia and cognitive impairment over a 48-month intervention period versus usual care.
It was an open-label, blinded-endpoint, cluster-randomized trial, and included 33,995 individual patients from 325 villages in China, aged 40 years and older, with untreated hypertension. The villages were randomly assigned to an intervention group or usual care, stratified by province, county, and township.
Patients were eligible if they had mean untreated systolic BP greater than 140 mm Hg and/or diastolic BP greater than 90 mm Hg or mean treated systolic BP of greater than 130 and/or diastolic greater than 80 mm Hg. Patients with a history of cardiovascular disease, chronic kidney disease, or diabetes and a mean systolic BP greater than 130 mm Hg and/or diastolic BP greater than 80 mm Hg from six measures on two different days were also eligible.
All were enrolled in the China New Rural Cooperative Medical Scheme, which covers 99% of rural residents for health care services, Dr. He noted.
The intervention was a simple stepped-care protocol for hypertension treatment, aimed at achieving a target systolic BP of less than 130 mm Hg and diastolic of less than 80 mm Hg.
Village doctors started and titrated antihypertensive treatment based on a protocol and were able to deliver discounted and free medications to patients. They also did health coaching on lifestyle modification and adherence to medication, and instructed patients on home BP monitoring.
Patients were provided training, supervision, and consultation by primary care physicians and hypertension specialists.
At the month 48 follow-up visit, the participants were assessed by neurologists who were blinded to randomization assignments. Neurologists did a variety of tests and assessments including collecting data on the patient’s medical and psychiatric history and risk factors for dementia, as well as neurologic assessment using the Mini-Mental State Examination, the Functional Activities Questionnaire, and the Quick Dementia Rating System.
The primary outcome was all-cause dementia, defined according to recommendations from the National Institute on Aging–Alzheimer’s Association work groups on diagnostic guidelines for Alzheimer’s disease.
Secondary outcomes included CIND, a composite outcome of dementia or CIND, and a composite of dementia or deaths.
The final diagnosis of all-cause dementia or CIND was made by an expert adjudication panel blinded to the intervention assignment.
At 48 months, 91.3% of patients completed the follow-up for clinical outcomes. Participants were an average of 63 years of age, 61% were female, and 23% had less than a primary school education, Dr. He noted.
The net group differences in systolic and diastolic BP reduction were 22 and 9.3 mm Hg, respectively (P < .0001).
Significant differences were also seen between the groups in the primary outcome of all-cause dementia, as well as secondary outcomes of CIND, dementia or cognitive impairment, or dementia or deaths.
Serious adverse events were more common in the usual care group, and there was no difference between groups in the occurrence of falls or syncope.
The effect was consistent across subgroups, Dr. He said, including age, sex, education, cigarette smoking, body mass index, systolic BP, and fasting plasma glucose at baseline.
First definitive evidence
Invited discussant for the trial, Daniel W. Jones, MD, University of Mississippi Medical Center, Jackson, and past president of the AHA, pointed out that previous results from CRHCP on cardiovascular outcomes, reported earlier in 2023 in The Lancet, showed that, similar to results of the large SPRINT trial, lowering systolic BP to a goal of less than 130 mm Hg reduced a composite endpoint of MI, stroke, heart failure requiring hospitalization, and cardiovascular disease death over the 36-month follow-up.
The SPRINT findings also suggested a possible reduction in dementia, Dr. Jones said.
Now, in these new CRHCP results, “there was a clear benefit for intensive BP control in reducing risk for dementia and cognitive dysfunction,” he said. “This is, importantly, the first definitive evidence of dementia risk reduction demonstrated in a randomized controlled clinical trial. This outcome supports observational data that shows a strong relationship between BP and dementia.”
Since it is the first of its kind though, replication of the results will be important, he noted.
The study also showed that the intervention, using minimally trained village doctors, sustained BP control for 48 months. “This model could be used in any setting with modifications, including in the United States,” Dr. Jones said.
The study was supported by the Ministry of Science and Technology of China; U.S. investigators did not receive financial support from this study. The researchers and Dr. Jones disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM AHA 2023
FDA OKs Paradise Renal Denervation system for hypertension
Recor Medical and parent company Otsuka Medical Devices have announced.
Approval follows a positive review by the FDA’s Circulatory Systems Device panel in August that deemed the system both safe and effective in lowering blood pressure for adults with uncontrolled hypertension who may be inadequately responsive to, or who are intolerant of, antihypertensive medications.
Data supporting approval were provided by the RADIANCE program, the pivotal RADIANCE II trial, as well as RADIANCE-HTN SOLO and RADIANCE-HTN TRIO. RADIANCE II and RADIANCE-HTN SOLO studied patients with mild to moderate hypertension in an “off-meds” setting, and RADIANCE-HTN TRIO enrolled patients with resistant hypertension on standardized triple antihypertensive therapy.
Renal denervation is intended as an adjunctive treatment option when lifestyle changes and medication have not resulted in adequate blood pressure control, the statement notes. It works by denervating the sympathetic nerves surrounding the renal arteries, reducing the overactivity that can lead to hypertension.
The system delivers two to three doses of 360-degree ultrasound energy, lasting 7 seconds each, through each of the main renal arteries to the surrounding nerves. This particular system is water-cooled to protect the renal artery wall, the statement adds.
“Given the significant blood pressure reductions seen in the ultrasound renal denervation trials, the Paradise Ultrasound Renal Denervation system offers a much-needed advancement in our currently available options to control hypertension,” site principal investigator Naomi Fisher, MD, associate professor of medicine, Harvard Medical School, and director of hypertension service and hypertension innovation, division of endocrinology, diabetes, and hypertension at Brigham and Women’s Hospital, Boston, said in the statement.
Ultrasound renal denervation “has proven efficacy in patients with truly resistant hypertension, a population for whom medication therapy often fails. It is also effective in patients with mild to moderate hypertension who cannot tolerate enough medication to control their blood pressure,” Dr. Fisher added.
The Paradise ultrasound renal denervation system previously received CE mark and has been successfully introduced in Europe and is an investigational device in Japan, the companies note.
A second renal denervation system, the Symplicity Spyral Renal Denervation System (Medtronic) underwent FDA panel review the day after the Paradise system review in August, and although the panel voted unanimously that the Symplicity system is safe, they were split on whether or not it was efficacious. A final decision on approval by the FDA of that system is still pending.
A version of this article first appeared in Medscape.com.
Recor Medical and parent company Otsuka Medical Devices have announced.
Approval follows a positive review by the FDA’s Circulatory Systems Device panel in August that deemed the system both safe and effective in lowering blood pressure for adults with uncontrolled hypertension who may be inadequately responsive to, or who are intolerant of, antihypertensive medications.
Data supporting approval were provided by the RADIANCE program, the pivotal RADIANCE II trial, as well as RADIANCE-HTN SOLO and RADIANCE-HTN TRIO. RADIANCE II and RADIANCE-HTN SOLO studied patients with mild to moderate hypertension in an “off-meds” setting, and RADIANCE-HTN TRIO enrolled patients with resistant hypertension on standardized triple antihypertensive therapy.
Renal denervation is intended as an adjunctive treatment option when lifestyle changes and medication have not resulted in adequate blood pressure control, the statement notes. It works by denervating the sympathetic nerves surrounding the renal arteries, reducing the overactivity that can lead to hypertension.
The system delivers two to three doses of 360-degree ultrasound energy, lasting 7 seconds each, through each of the main renal arteries to the surrounding nerves. This particular system is water-cooled to protect the renal artery wall, the statement adds.
“Given the significant blood pressure reductions seen in the ultrasound renal denervation trials, the Paradise Ultrasound Renal Denervation system offers a much-needed advancement in our currently available options to control hypertension,” site principal investigator Naomi Fisher, MD, associate professor of medicine, Harvard Medical School, and director of hypertension service and hypertension innovation, division of endocrinology, diabetes, and hypertension at Brigham and Women’s Hospital, Boston, said in the statement.
Ultrasound renal denervation “has proven efficacy in patients with truly resistant hypertension, a population for whom medication therapy often fails. It is also effective in patients with mild to moderate hypertension who cannot tolerate enough medication to control their blood pressure,” Dr. Fisher added.
The Paradise ultrasound renal denervation system previously received CE mark and has been successfully introduced in Europe and is an investigational device in Japan, the companies note.
A second renal denervation system, the Symplicity Spyral Renal Denervation System (Medtronic) underwent FDA panel review the day after the Paradise system review in August, and although the panel voted unanimously that the Symplicity system is safe, they were split on whether or not it was efficacious. A final decision on approval by the FDA of that system is still pending.
A version of this article first appeared in Medscape.com.
Recor Medical and parent company Otsuka Medical Devices have announced.
Approval follows a positive review by the FDA’s Circulatory Systems Device panel in August that deemed the system both safe and effective in lowering blood pressure for adults with uncontrolled hypertension who may be inadequately responsive to, or who are intolerant of, antihypertensive medications.
Data supporting approval were provided by the RADIANCE program, the pivotal RADIANCE II trial, as well as RADIANCE-HTN SOLO and RADIANCE-HTN TRIO. RADIANCE II and RADIANCE-HTN SOLO studied patients with mild to moderate hypertension in an “off-meds” setting, and RADIANCE-HTN TRIO enrolled patients with resistant hypertension on standardized triple antihypertensive therapy.
Renal denervation is intended as an adjunctive treatment option when lifestyle changes and medication have not resulted in adequate blood pressure control, the statement notes. It works by denervating the sympathetic nerves surrounding the renal arteries, reducing the overactivity that can lead to hypertension.
The system delivers two to three doses of 360-degree ultrasound energy, lasting 7 seconds each, through each of the main renal arteries to the surrounding nerves. This particular system is water-cooled to protect the renal artery wall, the statement adds.
“Given the significant blood pressure reductions seen in the ultrasound renal denervation trials, the Paradise Ultrasound Renal Denervation system offers a much-needed advancement in our currently available options to control hypertension,” site principal investigator Naomi Fisher, MD, associate professor of medicine, Harvard Medical School, and director of hypertension service and hypertension innovation, division of endocrinology, diabetes, and hypertension at Brigham and Women’s Hospital, Boston, said in the statement.
Ultrasound renal denervation “has proven efficacy in patients with truly resistant hypertension, a population for whom medication therapy often fails. It is also effective in patients with mild to moderate hypertension who cannot tolerate enough medication to control their blood pressure,” Dr. Fisher added.
The Paradise ultrasound renal denervation system previously received CE mark and has been successfully introduced in Europe and is an investigational device in Japan, the companies note.
A second renal denervation system, the Symplicity Spyral Renal Denervation System (Medtronic) underwent FDA panel review the day after the Paradise system review in August, and although the panel voted unanimously that the Symplicity system is safe, they were split on whether or not it was efficacious. A final decision on approval by the FDA of that system is still pending.
A version of this article first appeared in Medscape.com.
Steady VKA therapy beats switch to NOAC in frail AFib patients: FRAIL-AF
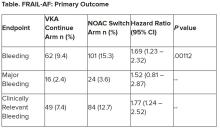
Switching frail patients with atrial fibrillation (AFib) from anticoagulation therapy with vitamin K antagonists (VKAs) to a novel oral anticoagulant (NOAC) resulted in more bleeding without any reduction in thromboembolic complications or all-cause mortality, randomized trial results show.
The study, FRAIL-AF, is the first randomized NOAC trial to exclusively include frail older patients, said lead author Linda P.T. Joosten, MD, Julius Center for Health Sciences and Primary Care in Utrecht, the Netherlands, and these unexpected findings provide evidence that goes beyond what is currently available.
“Data from the FRAIL-AF trial showed that switching from a VKA to a NOAC should not be considered without a clear indication in frail older patients with AF[ib], as switching to a NOAC leads to 69% more bleeding,” she concluded, without any benefit on secondary clinical endpoints, including thromboembolic events and all-cause mortality.
“The results turned out different than we expected,” Dr. Joosten said. “The hypothesis of this superiority trial was that switching from VKA therapy to a NOAC would result in less bleeding. However, we observed the opposite. After the interim analysis, the data and safety monitoring board advised to stop inclusion because switching from a VKA to a NOAC was clearly contraindicated with a hazard ratio of 1.69 and a highly significant P value of .001.”
Results of FRAIL-AF were presented at the annual congress of the European Society of Cardiology and published online in the journal Circulation.
Session moderator Renate B. Schnabel, MD, interventional cardiologist with University Heart & Vascular Center Hamburg (Germany), congratulated the researchers on these “astonishing” data.
“The thing I want to emphasize here is that, in the absence of randomized controlled trial data, we should be very cautious in extrapolating data from the landmark trials to populations not enrolled in those, and to rely on observational data only,” Dr. Schnabel told Dr. Joosten. “We need randomized controlled trials that sometimes give astonishing results.”
Frailty a clinical syndrome
Frailty is “a lot more than just aging, multiple comorbidities and polypharmacy,” Dr. Joosten explained. “It’s really a clinical syndrome, with people with a high biological vulnerability, dependency on significant others, and a reduced capacity to resist stressors, all leading to a reduced homeostatic reserve.”
Frailty is common in the community, with a prevalence of about 12%, she noted, “and even more important, AF[ib] in frail older people is very common, with a prevalence of 18%. And “without any doubt, we have to adequately anticoagulate frail AF[ib] patients, as they have a high stroke risk, with an incidence of 12.4% per year,” Dr. Joosten noted, compared with 3.9% per year among nonfrail AFib patients.
NOACs are preferred over VKAs in nonfrail AFib patients, after four major trials, RE-LY with dabigatran, ROCKET-AF with rivaroxaban, ARISTOTLE with apixaban, and ENGAGE-AF with edoxaban, showed that NOAC treatment resulted in less major bleeding while stroke risk was comparable with treatment with warfarin, she noted.
The 2023 European Heart Rhythm Association consensus document on management of arrhythmias in frailty syndrome concludes that the advantages of NOACs relative to VKAs are “likely consistent” in frail and nonfrail AFib patients, but the level of evidence is low.
So it’s unknown if NOACs are preferred over VKAs in frail AFib patients, “and it’s even more questionable whether patients on VKAs should switch to NOAC therapy,” Dr. Joosten said.
This new trial aimed to answer the question of whether switching frail AFib patients currently managed on a VKA to a NOAC would reduce bleeding. FRAIL-AF was a pragmatic, multicenter, open-label, randomized, controlled superiority trial.
Older AFib patients were deemed frail if they were aged 75 years or older and had a score of 3 or more on the validated Groningen Frailty Indicator (GFI). Patients with a glomerular filtration rate of less than 30 mL/min per 1.73 m2 or with valvular AFib were excluded.
Eligible patients were then assigned randomly to switch from their international normalized ratio (INR)–guided VKA treatment with either 1 mg acenocoumarol or 3 mg phenprocoumon, to a NOAC, or to continue VKA treatment. They were followed for 12 months for the primary outcome – major bleeding or clinically relevant nonmajor bleeding complication, whichever came first – accounting for death as a competing risk.
A total of 1,330 patients were randomly assigned between January 2018 and June 2022. Their mean age was 83 years, and they had a median GFI of 4. After randomization, 6 patients in the switch-to-NOAC arm, and 1 in the continue-VKA arm were found to have exclusion criteria, so in the end, 662 patients were switched from a VKA to NOAC, while 661 continued on VKA therapy. The choice of NOAC was made by the treating physician.
Major bleeding was defined as a fatal bleeding; bleeding in a critical area or organ; bleeding leading to transfusion; and/or bleeding leading to a fall in hemoglobin level of 2 g/dL (1.24 mmol/L) or more. Nonmajor bleeding was bleeding not considered major but requiring face-to-face consultation, hospitalization or increased level of care, or medical intervention.
After a prespecified futility analysis planned after 163 primary outcome events, the trial was halted when it was seen that there were 101 primary outcome events in the switch arm compared to 62 in the continue arm, Dr. Joosten said. The difference appeared to be driven by clinically relevant nonmajor bleeding.
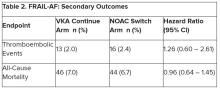
Secondary outcomes of thromboembolic events and all-cause mortality were similar between the groups.
Completely different patients
Discussant at the meeting for the presentation was Isabelle C. Van Gelder, MD, University Medical Centre Groningen (the Netherlands). She said the results are important and relevant because it “provides data on an important gap of knowledge in our AF[ib] guidelines, and a note for all the cardiologists – this study was not done in the hospital. This trial was done in general practitioner practices, so that’s important to consider.”
Comparing FRAIL-AF patients with those of the four previous NOAC trials, “you see that enormous difference in age,” with an average age of 83 years versus 70-73 years in those trials. “These are completely different patients than have been included previously,” she said.
That GFI score of 4 or more includes patients on four or more different types of medication, as well as memory complaints, an inability to walk around the house, and problems with vision or hearing.
The finding of a 69% increase in bleeding with NOACs in FRAIL-AF was “completely unexpected, and I think that we as cardiologists and as NOAC believers did not expect it at all, but it is as clear as it is.” The curves don’t diverge immediately, but rather after 3 months or thereafter, “so it has nothing to do with the switching process. So why did it occur?”
The Netherlands has dedicated thrombosis services that might improve time in therapeutic range for VKA patients, but there is no real difference in TTRs in FRAIL-AF versus the other NOAC trials, Dr. Van Gelder noted.
The most likely suspect in her view is frailty itself, in particular the tendency for patients to be on a high number of medications. A previous study showed, for example, that polypharmacy could be used as a proxy for the effect of frailty on bleeding risk; patients on 10 or more medications had a higher risk for bleeding on treatment with rivaroxaban versus those on 4 or fewer medications.
“Therefore, in my view, why was there such a high risk of bleeding? It’s because these are other patients than we are normally used to treat, we as cardiologists,” although general practitioners see these patients all the time. “It’s all about frailty.”
NOACs are still relatively new drugs, with possible unknown interactions, she added. Because of their frailty and polypharmacy, these patients may benefit from INR control, Dr. Van Gelder speculated. “Therefore, I agree with them that we should be careful; if such old, frail patients survive on VKA, do not change medications and do not switch!”
The study was supported by the Dutch government with additional and unrestricted educational grants from Boehringer Ingelheim, BMS-Pfizer, Bayer, and Daiichi Sankyo. Dr. Joosten reported no relevant financial relationships. Dr. Van Gelder reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Switching frail patients with atrial fibrillation (AFib) from anticoagulation therapy with vitamin K antagonists (VKAs) to a novel oral anticoagulant (NOAC) resulted in more bleeding without any reduction in thromboembolic complications or all-cause mortality, randomized trial results show.
The study, FRAIL-AF, is the first randomized NOAC trial to exclusively include frail older patients, said lead author Linda P.T. Joosten, MD, Julius Center for Health Sciences and Primary Care in Utrecht, the Netherlands, and these unexpected findings provide evidence that goes beyond what is currently available.
“Data from the FRAIL-AF trial showed that switching from a VKA to a NOAC should not be considered without a clear indication in frail older patients with AF[ib], as switching to a NOAC leads to 69% more bleeding,” she concluded, without any benefit on secondary clinical endpoints, including thromboembolic events and all-cause mortality.
“The results turned out different than we expected,” Dr. Joosten said. “The hypothesis of this superiority trial was that switching from VKA therapy to a NOAC would result in less bleeding. However, we observed the opposite. After the interim analysis, the data and safety monitoring board advised to stop inclusion because switching from a VKA to a NOAC was clearly contraindicated with a hazard ratio of 1.69 and a highly significant P value of .001.”
Results of FRAIL-AF were presented at the annual congress of the European Society of Cardiology and published online in the journal Circulation.
Session moderator Renate B. Schnabel, MD, interventional cardiologist with University Heart & Vascular Center Hamburg (Germany), congratulated the researchers on these “astonishing” data.
“The thing I want to emphasize here is that, in the absence of randomized controlled trial data, we should be very cautious in extrapolating data from the landmark trials to populations not enrolled in those, and to rely on observational data only,” Dr. Schnabel told Dr. Joosten. “We need randomized controlled trials that sometimes give astonishing results.”
Frailty a clinical syndrome
Frailty is “a lot more than just aging, multiple comorbidities and polypharmacy,” Dr. Joosten explained. “It’s really a clinical syndrome, with people with a high biological vulnerability, dependency on significant others, and a reduced capacity to resist stressors, all leading to a reduced homeostatic reserve.”
Frailty is common in the community, with a prevalence of about 12%, she noted, “and even more important, AF[ib] in frail older people is very common, with a prevalence of 18%. And “without any doubt, we have to adequately anticoagulate frail AF[ib] patients, as they have a high stroke risk, with an incidence of 12.4% per year,” Dr. Joosten noted, compared with 3.9% per year among nonfrail AFib patients.
NOACs are preferred over VKAs in nonfrail AFib patients, after four major trials, RE-LY with dabigatran, ROCKET-AF with rivaroxaban, ARISTOTLE with apixaban, and ENGAGE-AF with edoxaban, showed that NOAC treatment resulted in less major bleeding while stroke risk was comparable with treatment with warfarin, she noted.
The 2023 European Heart Rhythm Association consensus document on management of arrhythmias in frailty syndrome concludes that the advantages of NOACs relative to VKAs are “likely consistent” in frail and nonfrail AFib patients, but the level of evidence is low.
So it’s unknown if NOACs are preferred over VKAs in frail AFib patients, “and it’s even more questionable whether patients on VKAs should switch to NOAC therapy,” Dr. Joosten said.
This new trial aimed to answer the question of whether switching frail AFib patients currently managed on a VKA to a NOAC would reduce bleeding. FRAIL-AF was a pragmatic, multicenter, open-label, randomized, controlled superiority trial.
Older AFib patients were deemed frail if they were aged 75 years or older and had a score of 3 or more on the validated Groningen Frailty Indicator (GFI). Patients with a glomerular filtration rate of less than 30 mL/min per 1.73 m2 or with valvular AFib were excluded.
Eligible patients were then assigned randomly to switch from their international normalized ratio (INR)–guided VKA treatment with either 1 mg acenocoumarol or 3 mg phenprocoumon, to a NOAC, or to continue VKA treatment. They were followed for 12 months for the primary outcome – major bleeding or clinically relevant nonmajor bleeding complication, whichever came first – accounting for death as a competing risk.
A total of 1,330 patients were randomly assigned between January 2018 and June 2022. Their mean age was 83 years, and they had a median GFI of 4. After randomization, 6 patients in the switch-to-NOAC arm, and 1 in the continue-VKA arm were found to have exclusion criteria, so in the end, 662 patients were switched from a VKA to NOAC, while 661 continued on VKA therapy. The choice of NOAC was made by the treating physician.
Major bleeding was defined as a fatal bleeding; bleeding in a critical area or organ; bleeding leading to transfusion; and/or bleeding leading to a fall in hemoglobin level of 2 g/dL (1.24 mmol/L) or more. Nonmajor bleeding was bleeding not considered major but requiring face-to-face consultation, hospitalization or increased level of care, or medical intervention.
After a prespecified futility analysis planned after 163 primary outcome events, the trial was halted when it was seen that there were 101 primary outcome events in the switch arm compared to 62 in the continue arm, Dr. Joosten said. The difference appeared to be driven by clinically relevant nonmajor bleeding.
Secondary outcomes of thromboembolic events and all-cause mortality were similar between the groups.
Completely different patients
Discussant at the meeting for the presentation was Isabelle C. Van Gelder, MD, University Medical Centre Groningen (the Netherlands). She said the results are important and relevant because it “provides data on an important gap of knowledge in our AF[ib] guidelines, and a note for all the cardiologists – this study was not done in the hospital. This trial was done in general practitioner practices, so that’s important to consider.”
Comparing FRAIL-AF patients with those of the four previous NOAC trials, “you see that enormous difference in age,” with an average age of 83 years versus 70-73 years in those trials. “These are completely different patients than have been included previously,” she said.
That GFI score of 4 or more includes patients on four or more different types of medication, as well as memory complaints, an inability to walk around the house, and problems with vision or hearing.
The finding of a 69% increase in bleeding with NOACs in FRAIL-AF was “completely unexpected, and I think that we as cardiologists and as NOAC believers did not expect it at all, but it is as clear as it is.” The curves don’t diverge immediately, but rather after 3 months or thereafter, “so it has nothing to do with the switching process. So why did it occur?”
The Netherlands has dedicated thrombosis services that might improve time in therapeutic range for VKA patients, but there is no real difference in TTRs in FRAIL-AF versus the other NOAC trials, Dr. Van Gelder noted.
The most likely suspect in her view is frailty itself, in particular the tendency for patients to be on a high number of medications. A previous study showed, for example, that polypharmacy could be used as a proxy for the effect of frailty on bleeding risk; patients on 10 or more medications had a higher risk for bleeding on treatment with rivaroxaban versus those on 4 or fewer medications.
“Therefore, in my view, why was there such a high risk of bleeding? It’s because these are other patients than we are normally used to treat, we as cardiologists,” although general practitioners see these patients all the time. “It’s all about frailty.”
NOACs are still relatively new drugs, with possible unknown interactions, she added. Because of their frailty and polypharmacy, these patients may benefit from INR control, Dr. Van Gelder speculated. “Therefore, I agree with them that we should be careful; if such old, frail patients survive on VKA, do not change medications and do not switch!”
The study was supported by the Dutch government with additional and unrestricted educational grants from Boehringer Ingelheim, BMS-Pfizer, Bayer, and Daiichi Sankyo. Dr. Joosten reported no relevant financial relationships. Dr. Van Gelder reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Switching frail patients with atrial fibrillation (AFib) from anticoagulation therapy with vitamin K antagonists (VKAs) to a novel oral anticoagulant (NOAC) resulted in more bleeding without any reduction in thromboembolic complications or all-cause mortality, randomized trial results show.
The study, FRAIL-AF, is the first randomized NOAC trial to exclusively include frail older patients, said lead author Linda P.T. Joosten, MD, Julius Center for Health Sciences and Primary Care in Utrecht, the Netherlands, and these unexpected findings provide evidence that goes beyond what is currently available.
“Data from the FRAIL-AF trial showed that switching from a VKA to a NOAC should not be considered without a clear indication in frail older patients with AF[ib], as switching to a NOAC leads to 69% more bleeding,” she concluded, without any benefit on secondary clinical endpoints, including thromboembolic events and all-cause mortality.
“The results turned out different than we expected,” Dr. Joosten said. “The hypothesis of this superiority trial was that switching from VKA therapy to a NOAC would result in less bleeding. However, we observed the opposite. After the interim analysis, the data and safety monitoring board advised to stop inclusion because switching from a VKA to a NOAC was clearly contraindicated with a hazard ratio of 1.69 and a highly significant P value of .001.”
Results of FRAIL-AF were presented at the annual congress of the European Society of Cardiology and published online in the journal Circulation.
Session moderator Renate B. Schnabel, MD, interventional cardiologist with University Heart & Vascular Center Hamburg (Germany), congratulated the researchers on these “astonishing” data.
“The thing I want to emphasize here is that, in the absence of randomized controlled trial data, we should be very cautious in extrapolating data from the landmark trials to populations not enrolled in those, and to rely on observational data only,” Dr. Schnabel told Dr. Joosten. “We need randomized controlled trials that sometimes give astonishing results.”
Frailty a clinical syndrome
Frailty is “a lot more than just aging, multiple comorbidities and polypharmacy,” Dr. Joosten explained. “It’s really a clinical syndrome, with people with a high biological vulnerability, dependency on significant others, and a reduced capacity to resist stressors, all leading to a reduced homeostatic reserve.”
Frailty is common in the community, with a prevalence of about 12%, she noted, “and even more important, AF[ib] in frail older people is very common, with a prevalence of 18%. And “without any doubt, we have to adequately anticoagulate frail AF[ib] patients, as they have a high stroke risk, with an incidence of 12.4% per year,” Dr. Joosten noted, compared with 3.9% per year among nonfrail AFib patients.
NOACs are preferred over VKAs in nonfrail AFib patients, after four major trials, RE-LY with dabigatran, ROCKET-AF with rivaroxaban, ARISTOTLE with apixaban, and ENGAGE-AF with edoxaban, showed that NOAC treatment resulted in less major bleeding while stroke risk was comparable with treatment with warfarin, she noted.
The 2023 European Heart Rhythm Association consensus document on management of arrhythmias in frailty syndrome concludes that the advantages of NOACs relative to VKAs are “likely consistent” in frail and nonfrail AFib patients, but the level of evidence is low.
So it’s unknown if NOACs are preferred over VKAs in frail AFib patients, “and it’s even more questionable whether patients on VKAs should switch to NOAC therapy,” Dr. Joosten said.
This new trial aimed to answer the question of whether switching frail AFib patients currently managed on a VKA to a NOAC would reduce bleeding. FRAIL-AF was a pragmatic, multicenter, open-label, randomized, controlled superiority trial.
Older AFib patients were deemed frail if they were aged 75 years or older and had a score of 3 or more on the validated Groningen Frailty Indicator (GFI). Patients with a glomerular filtration rate of less than 30 mL/min per 1.73 m2 or with valvular AFib were excluded.
Eligible patients were then assigned randomly to switch from their international normalized ratio (INR)–guided VKA treatment with either 1 mg acenocoumarol or 3 mg phenprocoumon, to a NOAC, or to continue VKA treatment. They were followed for 12 months for the primary outcome – major bleeding or clinically relevant nonmajor bleeding complication, whichever came first – accounting for death as a competing risk.
A total of 1,330 patients were randomly assigned between January 2018 and June 2022. Their mean age was 83 years, and they had a median GFI of 4. After randomization, 6 patients in the switch-to-NOAC arm, and 1 in the continue-VKA arm were found to have exclusion criteria, so in the end, 662 patients were switched from a VKA to NOAC, while 661 continued on VKA therapy. The choice of NOAC was made by the treating physician.
Major bleeding was defined as a fatal bleeding; bleeding in a critical area or organ; bleeding leading to transfusion; and/or bleeding leading to a fall in hemoglobin level of 2 g/dL (1.24 mmol/L) or more. Nonmajor bleeding was bleeding not considered major but requiring face-to-face consultation, hospitalization or increased level of care, or medical intervention.
After a prespecified futility analysis planned after 163 primary outcome events, the trial was halted when it was seen that there were 101 primary outcome events in the switch arm compared to 62 in the continue arm, Dr. Joosten said. The difference appeared to be driven by clinically relevant nonmajor bleeding.
Secondary outcomes of thromboembolic events and all-cause mortality were similar between the groups.
Completely different patients
Discussant at the meeting for the presentation was Isabelle C. Van Gelder, MD, University Medical Centre Groningen (the Netherlands). She said the results are important and relevant because it “provides data on an important gap of knowledge in our AF[ib] guidelines, and a note for all the cardiologists – this study was not done in the hospital. This trial was done in general practitioner practices, so that’s important to consider.”
Comparing FRAIL-AF patients with those of the four previous NOAC trials, “you see that enormous difference in age,” with an average age of 83 years versus 70-73 years in those trials. “These are completely different patients than have been included previously,” she said.
That GFI score of 4 or more includes patients on four or more different types of medication, as well as memory complaints, an inability to walk around the house, and problems with vision or hearing.
The finding of a 69% increase in bleeding with NOACs in FRAIL-AF was “completely unexpected, and I think that we as cardiologists and as NOAC believers did not expect it at all, but it is as clear as it is.” The curves don’t diverge immediately, but rather after 3 months or thereafter, “so it has nothing to do with the switching process. So why did it occur?”
The Netherlands has dedicated thrombosis services that might improve time in therapeutic range for VKA patients, but there is no real difference in TTRs in FRAIL-AF versus the other NOAC trials, Dr. Van Gelder noted.
The most likely suspect in her view is frailty itself, in particular the tendency for patients to be on a high number of medications. A previous study showed, for example, that polypharmacy could be used as a proxy for the effect of frailty on bleeding risk; patients on 10 or more medications had a higher risk for bleeding on treatment with rivaroxaban versus those on 4 or fewer medications.
“Therefore, in my view, why was there such a high risk of bleeding? It’s because these are other patients than we are normally used to treat, we as cardiologists,” although general practitioners see these patients all the time. “It’s all about frailty.”
NOACs are still relatively new drugs, with possible unknown interactions, she added. Because of their frailty and polypharmacy, these patients may benefit from INR control, Dr. Van Gelder speculated. “Therefore, I agree with them that we should be careful; if such old, frail patients survive on VKA, do not change medications and do not switch!”
The study was supported by the Dutch government with additional and unrestricted educational grants from Boehringer Ingelheim, BMS-Pfizer, Bayer, and Daiichi Sankyo. Dr. Joosten reported no relevant financial relationships. Dr. Van Gelder reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE ESC CONGRESS 2023
No reduction in AFib after noncardiac surgery with colchicine: COP-AF
Trends were seen with reductions in events, but these did not reach significance. However, benefit was seen in a post-hoc analysis looking at a composite of both of those endpoints, the researchers note, as well as a composite of vascular death, nonfatal MINS, nonfatal stroke, and clinically important perioperative AFib, the researchers report.
“We interpret that as there is a trend that is promising, a trend that needs to be further explored,” lead author David Conen, MD, Population Health Research Institute, Hamilton, Ont., said in an interview. “We think that further studies are needed to tease out which patients can benefit from colchicine and in what setting it can be used.”
Treatment was safe, with no effect on the risk for sepsis or infection, but it did cause an increase in noninfectious diarrhea. “These events were mostly benign and did not increase length of stay, and only one patient was readmitted because of diarrhea,” Dr. Conen noted.
Results of the COP-AF trial were presented at the annual congress of the European Society of Cardiology, Amsterdam, and published online in The Lancet .
Inflammation and perioperative AFib
AFib and MINS are common complications in patients undergoing major thoracic surgery, Dr. Conen explained. The literature suggests AFib occurs in about 10% and MINS in about 20% of these patients, “and patients with these complications have a much higher risk of additional complications, such as stroke or MI [myocardial infarction],” Dr. Conen said.
Both disorders are associated with high levels of inflammatory biomarkers, so they set out to test colchicine, a well-known anti-inflammatory drug used in higher doses to treat common clinical disorders, such as gout and pericarditis. Small, randomized trials had shown it reduced the incidence of perioperative AFib after cardiac surgery, he noted.
Low-dose colchicine (LoDoCo, Agepha Pharma) was recently approved by the U.S. Food and Drug Administration to reduce the risk for MI, stroke, coronary revascularization, or death in patients with established atherosclerotic disease or multiple risk factors for cardiovascular disease. It was approved on the basis of the LoDoCo 2 trial in patients with stable coronary artery disease and the COLCOT trial in patients with recent MI.
COP-AF was a randomized trial, conducted at 45 sites in 11 countries, and enrolled 3,209 patients aged 55 years or older (51.6% male) undergoing major noncardiac thoracic surgery. Patients were excluded if they had previous AFib, had any contraindications to colchicine, or required colchicine on a clinical basis.
Patients were randomly assigned 1:1 to receive oral colchicine at a dose of 0.5 mg twice daily (1,608 patients) or placebo (1,601 patients). Treatment was begun within 4 hours before surgery and continued for 10 days. Health care providers and patients, as well as data collectors and adjudicators, were blinded to treatment assignment.
The co-primary outcomes were clinically important perioperative AFib or MINS over 14 days of follow-up. The trial was originally looking only at clinically important AFib, Dr. Conen noted, but after the publication of LoDoCo 2 and COLCOT, “MINS was added as an independent co-primary outcome,” requiring more patients to achieve adequate power.
The main safety outcomes were a composite of sepsis or infection, along with noninfectious diarrhea.
Clinically important AFib was defined as AFib that results in angina, heart failure, or symptomatic hypotension or required treatment with a rate-controlling drug, antiarrhythmic drug, or electrical cardioversion. “This definition was chosen because of its prognostic relevance, and to avoid adding short, asymptomatic AFib episodes of uncertain clinical relevance to the primary outcome,” Dr. Conen said during his presentation.
MINS was defined as an MI or any postoperative troponin elevation that was judged by an adjudication panel to be of ischemic origin.
At 14 days, there was no significant difference between groups on either of the co-primary end points.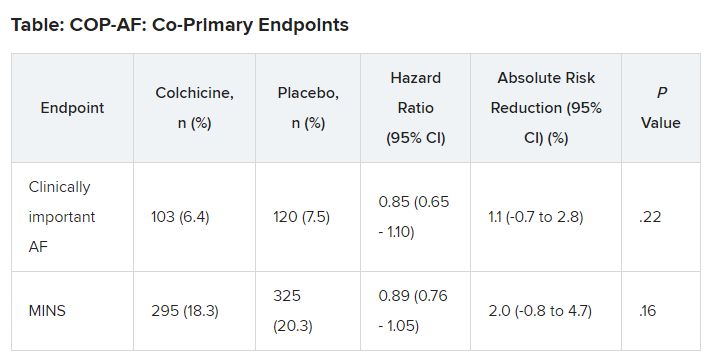
No significant differences but positive trends were similarly seen in secondary outcomes of a composite of all-cause death, nonfatal MINS, and nonfatal stroke; the composite of all-cause death, nonfatal MI, and nonfatal stroke; MINS not fulfilling the fourth universal definition of MI; or MI.
There were no differences in time to chest tube removal, days in hospital, nights in the step-down unit, or nights in the intensive care unit.
In terms of safety, there was no difference between groups on sepsis or infection, which occurred in 6.4% of patients in the colchicine group and 5.2% of those in the placebo group (hazard ratio, 1.24; 95% confidence interval, 0.93-1.66).
Noninfectious diarrhea was more common with colchicine, with 134 events (8.3%) versus 38 with placebo (2.4%), for an HR of 3.64 (95% CI, 2.54-5.22).
“In two post hoc analyses, colchicine significantly reduced the composite of the two co-primary outcomes,” Dr. Conen noted in his presentation. Clinically important perioperative AFib or MINS occurred in 22.4% in the colchicine group and 25.9% in the placebo group (HR, 0.84; 95% CI, 0.73-0.97; P = .02).
“Colchicine also significantly reduced the composite of vascular mortality, nonfatal MINS, nonfatal stroke, and clinically important AFib,” he said; 22.6% of patients in the colchicine group had one of these events versus 26.4% of those in the placebo group (HR, 0.83; 95% CI, 0.72-0.96; P = .01).
The researchers also reported significant interactions on both co-primary outcomes for the type of incision, “suggesting that stronger and statistically significant effects among patients undergoing thoracoscopic surgery as opposed to nonthoracoscopic surgery,” Dr. Conen said.
Patients undergoing thoracoscopic surgery treated with colchicine had a reduced risk for clinically important AFib (n = 2,397; HR, 0.53; 95% CI, 0.36-0.77), but colchicine treatment increased the risk in patients having open surgery (n = 784; HR, 1.59; 95% CI, 1.07-2.35; P for interaction < .0001).
There was a beneficial effect on MINS with colchicine among patients undergoing thoracoscopic surgery (HR, 0.80; 95% CI, 0.66-0.98), but no effect was seen among those having open surgery (HR, 1.15; 95% CI, 0.87-1.53; P for interaction = .041).
Low-risk patients
Jean-Claude Tardif, MD, Montreal Heart Institute and Université de Montréal, was the invited discussant for the COP-AF presentation and congratulated the researchers on “a job well done.”
He made the point that the risk for perioperative AFib has decreased substantially with the greater use of thoracoscopic rather than open surgical approaches. The population of this trial was relatively young, with an average age of 68 years; the presence of concomitant CVD was low, at about 9%; by design, patients with previous AFib were excluded; and only about 20% of patients had surgery with an open approach.
“So that population of patients were probably at relatively low risk of atrial fibrillation, and sure enough, the incidence of perioperative AFib in that population at 7.5% was lower than the assumed rate in the statistical powering of the study at 9%,” Dr. Tardif noted.
The post-hoc analyses showed a “nominally significant effect on the composite of MINS and AFib; however, that combination is fairly difficult to justify given the different pathophysiology and clinical consequences of both outcomes,” he pointed out.
The incidence of postoperative MI as a secondary outcome was low, less than 1%, as was the incidence of postoperative stroke in that study, Dr. Tardif added. “Given the link between presence of blood in the pericardium as a trigger for AFib, it would be interesting to know the incidence of perioperative pericarditis in COP-AF.”
In conclusion, he said, “when trying to put these results into the bigger picture of colchicine in cardiovascular disease, I believe we need large, well-powered clinical trials to determine the value of colchicine to reduce the risk of AFib after cardiac surgery and after catheter ablation,” Dr. Tardif said.
“We all know that colchicine represents the first line of therapy for the treatment of acute and recurrent pericarditis, and finally, low-dose colchicine, at a lower dose than was used in COP-AF, 0.5 mg once daily, is the first anti-inflammatory agent approved by both U.S. FDA and Health Canada to reduce the risk of atherothombotic events in patients with ASCVD [atherosclerotic cardiovascular disease], I believe offering a new pillar of treatment for the prevention of ischemic events in such patients.”
Session co-moderator Franz Weidinger, MD, Landstrasse Clinic, Vienna, Austria, called the COP-AF results “very important” but also noted that they show “the challenge of doing well-powered randomized trials these days when we have patients so well treated for a wide array of cardiovascular disease.”
The study was supported by the Canadian Institutes of Health Research (CIHR); Accelerating Clinical Trials Consortium; Innovation Fund of the Alternative Funding Plan for the Academic Health Sciences Centres of Ontario; Population Health Research Institute; Hamilton Health Sciences; Division of Cardiology at McMaster University, Canada; Hanela Foundation, Switzerland; and General Research Fund, Research Grants Council, Hong Kong. Dr. Conen reports receiving research grants from CIHR, speaker fees from Servier outside the current study, and advisory board fees from Roche Diagnostics and Trimedics outside the current study.
A version of this article appeared on Medscape.com.
Trends were seen with reductions in events, but these did not reach significance. However, benefit was seen in a post-hoc analysis looking at a composite of both of those endpoints, the researchers note, as well as a composite of vascular death, nonfatal MINS, nonfatal stroke, and clinically important perioperative AFib, the researchers report.
“We interpret that as there is a trend that is promising, a trend that needs to be further explored,” lead author David Conen, MD, Population Health Research Institute, Hamilton, Ont., said in an interview. “We think that further studies are needed to tease out which patients can benefit from colchicine and in what setting it can be used.”
Treatment was safe, with no effect on the risk for sepsis or infection, but it did cause an increase in noninfectious diarrhea. “These events were mostly benign and did not increase length of stay, and only one patient was readmitted because of diarrhea,” Dr. Conen noted.
Results of the COP-AF trial were presented at the annual congress of the European Society of Cardiology, Amsterdam, and published online in The Lancet .
Inflammation and perioperative AFib
AFib and MINS are common complications in patients undergoing major thoracic surgery, Dr. Conen explained. The literature suggests AFib occurs in about 10% and MINS in about 20% of these patients, “and patients with these complications have a much higher risk of additional complications, such as stroke or MI [myocardial infarction],” Dr. Conen said.
Both disorders are associated with high levels of inflammatory biomarkers, so they set out to test colchicine, a well-known anti-inflammatory drug used in higher doses to treat common clinical disorders, such as gout and pericarditis. Small, randomized trials had shown it reduced the incidence of perioperative AFib after cardiac surgery, he noted.
Low-dose colchicine (LoDoCo, Agepha Pharma) was recently approved by the U.S. Food and Drug Administration to reduce the risk for MI, stroke, coronary revascularization, or death in patients with established atherosclerotic disease or multiple risk factors for cardiovascular disease. It was approved on the basis of the LoDoCo 2 trial in patients with stable coronary artery disease and the COLCOT trial in patients with recent MI.
COP-AF was a randomized trial, conducted at 45 sites in 11 countries, and enrolled 3,209 patients aged 55 years or older (51.6% male) undergoing major noncardiac thoracic surgery. Patients were excluded if they had previous AFib, had any contraindications to colchicine, or required colchicine on a clinical basis.
Patients were randomly assigned 1:1 to receive oral colchicine at a dose of 0.5 mg twice daily (1,608 patients) or placebo (1,601 patients). Treatment was begun within 4 hours before surgery and continued for 10 days. Health care providers and patients, as well as data collectors and adjudicators, were blinded to treatment assignment.
The co-primary outcomes were clinically important perioperative AFib or MINS over 14 days of follow-up. The trial was originally looking only at clinically important AFib, Dr. Conen noted, but after the publication of LoDoCo 2 and COLCOT, “MINS was added as an independent co-primary outcome,” requiring more patients to achieve adequate power.
The main safety outcomes were a composite of sepsis or infection, along with noninfectious diarrhea.
Clinically important AFib was defined as AFib that results in angina, heart failure, or symptomatic hypotension or required treatment with a rate-controlling drug, antiarrhythmic drug, or electrical cardioversion. “This definition was chosen because of its prognostic relevance, and to avoid adding short, asymptomatic AFib episodes of uncertain clinical relevance to the primary outcome,” Dr. Conen said during his presentation.
MINS was defined as an MI or any postoperative troponin elevation that was judged by an adjudication panel to be of ischemic origin.
At 14 days, there was no significant difference between groups on either of the co-primary end points.
No significant differences but positive trends were similarly seen in secondary outcomes of a composite of all-cause death, nonfatal MINS, and nonfatal stroke; the composite of all-cause death, nonfatal MI, and nonfatal stroke; MINS not fulfilling the fourth universal definition of MI; or MI.
There were no differences in time to chest tube removal, days in hospital, nights in the step-down unit, or nights in the intensive care unit.
In terms of safety, there was no difference between groups on sepsis or infection, which occurred in 6.4% of patients in the colchicine group and 5.2% of those in the placebo group (hazard ratio, 1.24; 95% confidence interval, 0.93-1.66).
Noninfectious diarrhea was more common with colchicine, with 134 events (8.3%) versus 38 with placebo (2.4%), for an HR of 3.64 (95% CI, 2.54-5.22).
“In two post hoc analyses, colchicine significantly reduced the composite of the two co-primary outcomes,” Dr. Conen noted in his presentation. Clinically important perioperative AFib or MINS occurred in 22.4% in the colchicine group and 25.9% in the placebo group (HR, 0.84; 95% CI, 0.73-0.97; P = .02).
“Colchicine also significantly reduced the composite of vascular mortality, nonfatal MINS, nonfatal stroke, and clinically important AFib,” he said; 22.6% of patients in the colchicine group had one of these events versus 26.4% of those in the placebo group (HR, 0.83; 95% CI, 0.72-0.96; P = .01).
The researchers also reported significant interactions on both co-primary outcomes for the type of incision, “suggesting that stronger and statistically significant effects among patients undergoing thoracoscopic surgery as opposed to nonthoracoscopic surgery,” Dr. Conen said.
Patients undergoing thoracoscopic surgery treated with colchicine had a reduced risk for clinically important AFib (n = 2,397; HR, 0.53; 95% CI, 0.36-0.77), but colchicine treatment increased the risk in patients having open surgery (n = 784; HR, 1.59; 95% CI, 1.07-2.35; P for interaction < .0001).
There was a beneficial effect on MINS with colchicine among patients undergoing thoracoscopic surgery (HR, 0.80; 95% CI, 0.66-0.98), but no effect was seen among those having open surgery (HR, 1.15; 95% CI, 0.87-1.53; P for interaction = .041).
Low-risk patients
Jean-Claude Tardif, MD, Montreal Heart Institute and Université de Montréal, was the invited discussant for the COP-AF presentation and congratulated the researchers on “a job well done.”
He made the point that the risk for perioperative AFib has decreased substantially with the greater use of thoracoscopic rather than open surgical approaches. The population of this trial was relatively young, with an average age of 68 years; the presence of concomitant CVD was low, at about 9%; by design, patients with previous AFib were excluded; and only about 20% of patients had surgery with an open approach.
“So that population of patients were probably at relatively low risk of atrial fibrillation, and sure enough, the incidence of perioperative AFib in that population at 7.5% was lower than the assumed rate in the statistical powering of the study at 9%,” Dr. Tardif noted.
The post-hoc analyses showed a “nominally significant effect on the composite of MINS and AFib; however, that combination is fairly difficult to justify given the different pathophysiology and clinical consequences of both outcomes,” he pointed out.
The incidence of postoperative MI as a secondary outcome was low, less than 1%, as was the incidence of postoperative stroke in that study, Dr. Tardif added. “Given the link between presence of blood in the pericardium as a trigger for AFib, it would be interesting to know the incidence of perioperative pericarditis in COP-AF.”
In conclusion, he said, “when trying to put these results into the bigger picture of colchicine in cardiovascular disease, I believe we need large, well-powered clinical trials to determine the value of colchicine to reduce the risk of AFib after cardiac surgery and after catheter ablation,” Dr. Tardif said.
“We all know that colchicine represents the first line of therapy for the treatment of acute and recurrent pericarditis, and finally, low-dose colchicine, at a lower dose than was used in COP-AF, 0.5 mg once daily, is the first anti-inflammatory agent approved by both U.S. FDA and Health Canada to reduce the risk of atherothombotic events in patients with ASCVD [atherosclerotic cardiovascular disease], I believe offering a new pillar of treatment for the prevention of ischemic events in such patients.”
Session co-moderator Franz Weidinger, MD, Landstrasse Clinic, Vienna, Austria, called the COP-AF results “very important” but also noted that they show “the challenge of doing well-powered randomized trials these days when we have patients so well treated for a wide array of cardiovascular disease.”
The study was supported by the Canadian Institutes of Health Research (CIHR); Accelerating Clinical Trials Consortium; Innovation Fund of the Alternative Funding Plan for the Academic Health Sciences Centres of Ontario; Population Health Research Institute; Hamilton Health Sciences; Division of Cardiology at McMaster University, Canada; Hanela Foundation, Switzerland; and General Research Fund, Research Grants Council, Hong Kong. Dr. Conen reports receiving research grants from CIHR, speaker fees from Servier outside the current study, and advisory board fees from Roche Diagnostics and Trimedics outside the current study.
A version of this article appeared on Medscape.com.
Trends were seen with reductions in events, but these did not reach significance. However, benefit was seen in a post-hoc analysis looking at a composite of both of those endpoints, the researchers note, as well as a composite of vascular death, nonfatal MINS, nonfatal stroke, and clinically important perioperative AFib, the researchers report.
“We interpret that as there is a trend that is promising, a trend that needs to be further explored,” lead author David Conen, MD, Population Health Research Institute, Hamilton, Ont., said in an interview. “We think that further studies are needed to tease out which patients can benefit from colchicine and in what setting it can be used.”
Treatment was safe, with no effect on the risk for sepsis or infection, but it did cause an increase in noninfectious diarrhea. “These events were mostly benign and did not increase length of stay, and only one patient was readmitted because of diarrhea,” Dr. Conen noted.
Results of the COP-AF trial were presented at the annual congress of the European Society of Cardiology, Amsterdam, and published online in The Lancet .
Inflammation and perioperative AFib
AFib and MINS are common complications in patients undergoing major thoracic surgery, Dr. Conen explained. The literature suggests AFib occurs in about 10% and MINS in about 20% of these patients, “and patients with these complications have a much higher risk of additional complications, such as stroke or MI [myocardial infarction],” Dr. Conen said.
Both disorders are associated with high levels of inflammatory biomarkers, so they set out to test colchicine, a well-known anti-inflammatory drug used in higher doses to treat common clinical disorders, such as gout and pericarditis. Small, randomized trials had shown it reduced the incidence of perioperative AFib after cardiac surgery, he noted.
Low-dose colchicine (LoDoCo, Agepha Pharma) was recently approved by the U.S. Food and Drug Administration to reduce the risk for MI, stroke, coronary revascularization, or death in patients with established atherosclerotic disease or multiple risk factors for cardiovascular disease. It was approved on the basis of the LoDoCo 2 trial in patients with stable coronary artery disease and the COLCOT trial in patients with recent MI.
COP-AF was a randomized trial, conducted at 45 sites in 11 countries, and enrolled 3,209 patients aged 55 years or older (51.6% male) undergoing major noncardiac thoracic surgery. Patients were excluded if they had previous AFib, had any contraindications to colchicine, or required colchicine on a clinical basis.
Patients were randomly assigned 1:1 to receive oral colchicine at a dose of 0.5 mg twice daily (1,608 patients) or placebo (1,601 patients). Treatment was begun within 4 hours before surgery and continued for 10 days. Health care providers and patients, as well as data collectors and adjudicators, were blinded to treatment assignment.
The co-primary outcomes were clinically important perioperative AFib or MINS over 14 days of follow-up. The trial was originally looking only at clinically important AFib, Dr. Conen noted, but after the publication of LoDoCo 2 and COLCOT, “MINS was added as an independent co-primary outcome,” requiring more patients to achieve adequate power.
The main safety outcomes were a composite of sepsis or infection, along with noninfectious diarrhea.
Clinically important AFib was defined as AFib that results in angina, heart failure, or symptomatic hypotension or required treatment with a rate-controlling drug, antiarrhythmic drug, or electrical cardioversion. “This definition was chosen because of its prognostic relevance, and to avoid adding short, asymptomatic AFib episodes of uncertain clinical relevance to the primary outcome,” Dr. Conen said during his presentation.
MINS was defined as an MI or any postoperative troponin elevation that was judged by an adjudication panel to be of ischemic origin.
At 14 days, there was no significant difference between groups on either of the co-primary end points.
No significant differences but positive trends were similarly seen in secondary outcomes of a composite of all-cause death, nonfatal MINS, and nonfatal stroke; the composite of all-cause death, nonfatal MI, and nonfatal stroke; MINS not fulfilling the fourth universal definition of MI; or MI.
There were no differences in time to chest tube removal, days in hospital, nights in the step-down unit, or nights in the intensive care unit.
In terms of safety, there was no difference between groups on sepsis or infection, which occurred in 6.4% of patients in the colchicine group and 5.2% of those in the placebo group (hazard ratio, 1.24; 95% confidence interval, 0.93-1.66).
Noninfectious diarrhea was more common with colchicine, with 134 events (8.3%) versus 38 with placebo (2.4%), for an HR of 3.64 (95% CI, 2.54-5.22).
“In two post hoc analyses, colchicine significantly reduced the composite of the two co-primary outcomes,” Dr. Conen noted in his presentation. Clinically important perioperative AFib or MINS occurred in 22.4% in the colchicine group and 25.9% in the placebo group (HR, 0.84; 95% CI, 0.73-0.97; P = .02).
“Colchicine also significantly reduced the composite of vascular mortality, nonfatal MINS, nonfatal stroke, and clinically important AFib,” he said; 22.6% of patients in the colchicine group had one of these events versus 26.4% of those in the placebo group (HR, 0.83; 95% CI, 0.72-0.96; P = .01).
The researchers also reported significant interactions on both co-primary outcomes for the type of incision, “suggesting that stronger and statistically significant effects among patients undergoing thoracoscopic surgery as opposed to nonthoracoscopic surgery,” Dr. Conen said.
Patients undergoing thoracoscopic surgery treated with colchicine had a reduced risk for clinically important AFib (n = 2,397; HR, 0.53; 95% CI, 0.36-0.77), but colchicine treatment increased the risk in patients having open surgery (n = 784; HR, 1.59; 95% CI, 1.07-2.35; P for interaction < .0001).
There was a beneficial effect on MINS with colchicine among patients undergoing thoracoscopic surgery (HR, 0.80; 95% CI, 0.66-0.98), but no effect was seen among those having open surgery (HR, 1.15; 95% CI, 0.87-1.53; P for interaction = .041).
Low-risk patients
Jean-Claude Tardif, MD, Montreal Heart Institute and Université de Montréal, was the invited discussant for the COP-AF presentation and congratulated the researchers on “a job well done.”
He made the point that the risk for perioperative AFib has decreased substantially with the greater use of thoracoscopic rather than open surgical approaches. The population of this trial was relatively young, with an average age of 68 years; the presence of concomitant CVD was low, at about 9%; by design, patients with previous AFib were excluded; and only about 20% of patients had surgery with an open approach.
“So that population of patients were probably at relatively low risk of atrial fibrillation, and sure enough, the incidence of perioperative AFib in that population at 7.5% was lower than the assumed rate in the statistical powering of the study at 9%,” Dr. Tardif noted.
The post-hoc analyses showed a “nominally significant effect on the composite of MINS and AFib; however, that combination is fairly difficult to justify given the different pathophysiology and clinical consequences of both outcomes,” he pointed out.
The incidence of postoperative MI as a secondary outcome was low, less than 1%, as was the incidence of postoperative stroke in that study, Dr. Tardif added. “Given the link between presence of blood in the pericardium as a trigger for AFib, it would be interesting to know the incidence of perioperative pericarditis in COP-AF.”
In conclusion, he said, “when trying to put these results into the bigger picture of colchicine in cardiovascular disease, I believe we need large, well-powered clinical trials to determine the value of colchicine to reduce the risk of AFib after cardiac surgery and after catheter ablation,” Dr. Tardif said.
“We all know that colchicine represents the first line of therapy for the treatment of acute and recurrent pericarditis, and finally, low-dose colchicine, at a lower dose than was used in COP-AF, 0.5 mg once daily, is the first anti-inflammatory agent approved by both U.S. FDA and Health Canada to reduce the risk of atherothombotic events in patients with ASCVD [atherosclerotic cardiovascular disease], I believe offering a new pillar of treatment for the prevention of ischemic events in such patients.”
Session co-moderator Franz Weidinger, MD, Landstrasse Clinic, Vienna, Austria, called the COP-AF results “very important” but also noted that they show “the challenge of doing well-powered randomized trials these days when we have patients so well treated for a wide array of cardiovascular disease.”
The study was supported by the Canadian Institutes of Health Research (CIHR); Accelerating Clinical Trials Consortium; Innovation Fund of the Alternative Funding Plan for the Academic Health Sciences Centres of Ontario; Population Health Research Institute; Hamilton Health Sciences; Division of Cardiology at McMaster University, Canada; Hanela Foundation, Switzerland; and General Research Fund, Research Grants Council, Hong Kong. Dr. Conen reports receiving research grants from CIHR, speaker fees from Servier outside the current study, and advisory board fees from Roche Diagnostics and Trimedics outside the current study.
A version of this article appeared on Medscape.com.
FROM ESC CONGRESS 2023
No benefit of routine stress test POST-PCI in high-risk patients
New randomized trial results show no benefit in clinical outcomes from active surveillance using functional testing over usual care among high-risk patients with previous percutaneous coronary intervention (PCI).
At 2 years, there was no difference in a composite outcome of death from any cause, MI, or hospitalization for unstable angina between patients who had routine functional testing at 1 year and patients receiving standard care in the POST-PCI trial.
“Our trial does not support active surveillance with routine functional testing for follow-up strategy in high-risk patients who undergo PCI,” first author Duk-Woo Park, MD, division of cardiology, Asan Medical Center, University of Ulsan, Seoul, South Korea, said in an interview.
The researchers said their results should be interpreted in the context of previous findings from the ISCHEMIA trial that showed no difference in death or ischemic events with an initial invasive versus an initial conservative approach in patients with stable coronary artery disease and moderate to severe ischemia on stress testing.
“Both the ISCHEMIA and POST-PCI trials show the benefits of a ‘less is more’ concept (i.e., if more invasive strategies or testing are performed less frequently, it will result in better patient outcomes),” the authors wrote. Although characteristics of the patients in these trials “were quite different, a more invasive therapeutic approach (in the ISCHEMIA trial) as well as a more aggressive follow-up approach (in the POST-PCI trial) did not provide an additional treatment effect beyond a conservative strategy on the basis of guideline-directed medical therapy.”
Results were presented at the annual congress of the European Society of Cardiology and published online simultaneously in the New England Journal of Medicine.
‘Compelling new evidence’
In an editorial accompanying the publication, Jacqueline E. Tamis-Holland, MD, Icahn School of Medicine at Mount Sinai, Mount Sinai Morningside Hospital, New York, also agreed that this new result “builds on the findings” from the ISCHEMIA trial. “Collectively, these trials highlight the lack of benefit of routine stress testing in asymptomatic patients.”
Dr. Tamis-Holland pointed out that many of the deaths in this trial occurred before the 1-year stress test, possibly related to stent thrombosis, and therefore would not have been prevented by routine testing at 1 year. And overall, event rates were “quite low, and most likely reflect adherence to guideline recommendations” in the trial. For example, 99% of patients were receiving statins, and 74% of the procedures used intravascular imaging for the PCI procedures, “a much greater proportion of use than most centers in the United States,” she noted.
“The POST-PCI trial provides compelling new evidence for a future class III recommendation for routine surveillance testing after PCI,” Dr. Tamis-Holland concluded “Until then, we must refrain from prescribing surveillance stress testing to our patients after PCI, in the absence of other clinical signs or symptoms suggestive of stent failure.”
Commenting on the results, B. Hadley Wilson, MD, executive vice chair of the Sanger Heart & Vascular Institute/Atrium Health, clinical professor of medicine at University of North Carolina at Chapel Hill, and vice president of the American College of Cardiology, said that for decades it’s been thought that patients who had high-risk PCI needed to be followed more closely for potential future events.
“And it actually turned out there was no difference in outcomes between the groups,” he said in an interview.
“So, I think it’s a good study – well conducted, good numbers – that answers the question that routine functional stress testing, even for high-risk PCI patients, is not effective or cost effective or beneficial on a yearly basis,” he said. “I think it will help frame care that patients will just be followed with best medical therapy and then if they have recurrence of symptoms they would be considered for further evaluation, either with stress testing or angiography.”
High-risk characteristics
Current guidelines do not advocate the use of routine stress testing after revascularization, the authors wrote in their paper. “However, surveillance with the use of imaging-based stress testing may be considered in high-risk patients at 6 months after a revascularization procedure (class IIb recommendation), and routine imaging-based stress testing may be considered at 1 year after PCI and more than 5 years after CABG [coronary artery bypass graft] (class IIb recommendation).”
But in real-world clinical practice, Dr. Park said, “follow-up strategy for patients who underwent PCI or CABG is still undetermined.” Particularly, “it could be more problematic in high-risk PCI patients with high-risk anatomical or clinical characteristics. Thus, we performed this POST-PCI trial comparing routine stress testing follow-up strategy versus standard-care follow-up strategy in high-risk PCI patients.”
The researchers randomly assigned 1,706 patients with high-risk anatomical or clinical characteristics who had undergone PCI to a follow-up strategy of routine functional testing, including nuclear stress testing, exercise electrocardiography, or stress echocardiography at 1 year, or to standard care alone.
High-risk anatomical features included left main or bifurcation disease; restenotic or long, diffuse lesions; or bypass graft disease. High-risk clinical characteristics included diabetes mellitus, chronic kidney disease, or enzyme-positive acute coronary syndrome.
Mean age of the patients was 64.7 years; 21.0% had left main disease, 43.5% had bifurcation disease, 69.8% had multivessel disease, 70.1% had diffuse long lesions, 38.7% had diabetes, and 96.4% had been treated with drug-eluting stents.
At 2 years, a primary-outcome event had occurred in 46 of 849 patients (Kaplan-Meier estimate, 5.5%) in the functional-testing group and in 51 of 857 (Kaplan-Meier estimate, 6.0%) in the standard-care group (hazard ratio, 0.90; 95% confidence interval, 0.61-1.35; P = .62). There were no between-group differences in the components of the primary outcome.
Secondary endpoints included invasive coronary angiography or repeat revascularization. At 2 years, 12.3% of the patients in the functional-testing group and 9.3% in the standard-care group had undergone invasive coronary angiography (difference, 2.99 percentage points; 95% CI, −0.01 to 5.99 percentage points), and 8.1% and 5.8% of patients, respectively, had a repeat revascularization procedure (difference, 2.23 percentage points; 95% CI, −0.22 to 4.68 percentage points).
Positive results on stress tests were more common with nuclear imaging than with exercise ECG or stress echocardiography, the authors noted. Subsequent coronary angiography and repeat revascularization were more common in patients with positive results on nuclear stress imaging and exercise ECG than in those with discordant results between nuclear imaging and exercise ECG.
POST-PCI was funded by the CardioVascular Research Foundation and Daewoong Pharmaceutical Company. Dr. Park reported grants from the Cardiovascular Research Foundation and Daewoong Pharmaceutical Company. Dr. Tamis-Holland reported “other” funding from Pfizer outside the submitted work. Dr. Wilson reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
New randomized trial results show no benefit in clinical outcomes from active surveillance using functional testing over usual care among high-risk patients with previous percutaneous coronary intervention (PCI).
At 2 years, there was no difference in a composite outcome of death from any cause, MI, or hospitalization for unstable angina between patients who had routine functional testing at 1 year and patients receiving standard care in the POST-PCI trial.
“Our trial does not support active surveillance with routine functional testing for follow-up strategy in high-risk patients who undergo PCI,” first author Duk-Woo Park, MD, division of cardiology, Asan Medical Center, University of Ulsan, Seoul, South Korea, said in an interview.
The researchers said their results should be interpreted in the context of previous findings from the ISCHEMIA trial that showed no difference in death or ischemic events with an initial invasive versus an initial conservative approach in patients with stable coronary artery disease and moderate to severe ischemia on stress testing.
“Both the ISCHEMIA and POST-PCI trials show the benefits of a ‘less is more’ concept (i.e., if more invasive strategies or testing are performed less frequently, it will result in better patient outcomes),” the authors wrote. Although characteristics of the patients in these trials “were quite different, a more invasive therapeutic approach (in the ISCHEMIA trial) as well as a more aggressive follow-up approach (in the POST-PCI trial) did not provide an additional treatment effect beyond a conservative strategy on the basis of guideline-directed medical therapy.”
Results were presented at the annual congress of the European Society of Cardiology and published online simultaneously in the New England Journal of Medicine.
‘Compelling new evidence’
In an editorial accompanying the publication, Jacqueline E. Tamis-Holland, MD, Icahn School of Medicine at Mount Sinai, Mount Sinai Morningside Hospital, New York, also agreed that this new result “builds on the findings” from the ISCHEMIA trial. “Collectively, these trials highlight the lack of benefit of routine stress testing in asymptomatic patients.”
Dr. Tamis-Holland pointed out that many of the deaths in this trial occurred before the 1-year stress test, possibly related to stent thrombosis, and therefore would not have been prevented by routine testing at 1 year. And overall, event rates were “quite low, and most likely reflect adherence to guideline recommendations” in the trial. For example, 99% of patients were receiving statins, and 74% of the procedures used intravascular imaging for the PCI procedures, “a much greater proportion of use than most centers in the United States,” she noted.
“The POST-PCI trial provides compelling new evidence for a future class III recommendation for routine surveillance testing after PCI,” Dr. Tamis-Holland concluded “Until then, we must refrain from prescribing surveillance stress testing to our patients after PCI, in the absence of other clinical signs or symptoms suggestive of stent failure.”
Commenting on the results, B. Hadley Wilson, MD, executive vice chair of the Sanger Heart & Vascular Institute/Atrium Health, clinical professor of medicine at University of North Carolina at Chapel Hill, and vice president of the American College of Cardiology, said that for decades it’s been thought that patients who had high-risk PCI needed to be followed more closely for potential future events.
“And it actually turned out there was no difference in outcomes between the groups,” he said in an interview.
“So, I think it’s a good study – well conducted, good numbers – that answers the question that routine functional stress testing, even for high-risk PCI patients, is not effective or cost effective or beneficial on a yearly basis,” he said. “I think it will help frame care that patients will just be followed with best medical therapy and then if they have recurrence of symptoms they would be considered for further evaluation, either with stress testing or angiography.”
High-risk characteristics
Current guidelines do not advocate the use of routine stress testing after revascularization, the authors wrote in their paper. “However, surveillance with the use of imaging-based stress testing may be considered in high-risk patients at 6 months after a revascularization procedure (class IIb recommendation), and routine imaging-based stress testing may be considered at 1 year after PCI and more than 5 years after CABG [coronary artery bypass graft] (class IIb recommendation).”
But in real-world clinical practice, Dr. Park said, “follow-up strategy for patients who underwent PCI or CABG is still undetermined.” Particularly, “it could be more problematic in high-risk PCI patients with high-risk anatomical or clinical characteristics. Thus, we performed this POST-PCI trial comparing routine stress testing follow-up strategy versus standard-care follow-up strategy in high-risk PCI patients.”
The researchers randomly assigned 1,706 patients with high-risk anatomical or clinical characteristics who had undergone PCI to a follow-up strategy of routine functional testing, including nuclear stress testing, exercise electrocardiography, or stress echocardiography at 1 year, or to standard care alone.
High-risk anatomical features included left main or bifurcation disease; restenotic or long, diffuse lesions; or bypass graft disease. High-risk clinical characteristics included diabetes mellitus, chronic kidney disease, or enzyme-positive acute coronary syndrome.
Mean age of the patients was 64.7 years; 21.0% had left main disease, 43.5% had bifurcation disease, 69.8% had multivessel disease, 70.1% had diffuse long lesions, 38.7% had diabetes, and 96.4% had been treated with drug-eluting stents.
At 2 years, a primary-outcome event had occurred in 46 of 849 patients (Kaplan-Meier estimate, 5.5%) in the functional-testing group and in 51 of 857 (Kaplan-Meier estimate, 6.0%) in the standard-care group (hazard ratio, 0.90; 95% confidence interval, 0.61-1.35; P = .62). There were no between-group differences in the components of the primary outcome.
Secondary endpoints included invasive coronary angiography or repeat revascularization. At 2 years, 12.3% of the patients in the functional-testing group and 9.3% in the standard-care group had undergone invasive coronary angiography (difference, 2.99 percentage points; 95% CI, −0.01 to 5.99 percentage points), and 8.1% and 5.8% of patients, respectively, had a repeat revascularization procedure (difference, 2.23 percentage points; 95% CI, −0.22 to 4.68 percentage points).
Positive results on stress tests were more common with nuclear imaging than with exercise ECG or stress echocardiography, the authors noted. Subsequent coronary angiography and repeat revascularization were more common in patients with positive results on nuclear stress imaging and exercise ECG than in those with discordant results between nuclear imaging and exercise ECG.
POST-PCI was funded by the CardioVascular Research Foundation and Daewoong Pharmaceutical Company. Dr. Park reported grants from the Cardiovascular Research Foundation and Daewoong Pharmaceutical Company. Dr. Tamis-Holland reported “other” funding from Pfizer outside the submitted work. Dr. Wilson reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
New randomized trial results show no benefit in clinical outcomes from active surveillance using functional testing over usual care among high-risk patients with previous percutaneous coronary intervention (PCI).
At 2 years, there was no difference in a composite outcome of death from any cause, MI, or hospitalization for unstable angina between patients who had routine functional testing at 1 year and patients receiving standard care in the POST-PCI trial.
“Our trial does not support active surveillance with routine functional testing for follow-up strategy in high-risk patients who undergo PCI,” first author Duk-Woo Park, MD, division of cardiology, Asan Medical Center, University of Ulsan, Seoul, South Korea, said in an interview.
The researchers said their results should be interpreted in the context of previous findings from the ISCHEMIA trial that showed no difference in death or ischemic events with an initial invasive versus an initial conservative approach in patients with stable coronary artery disease and moderate to severe ischemia on stress testing.
“Both the ISCHEMIA and POST-PCI trials show the benefits of a ‘less is more’ concept (i.e., if more invasive strategies or testing are performed less frequently, it will result in better patient outcomes),” the authors wrote. Although characteristics of the patients in these trials “were quite different, a more invasive therapeutic approach (in the ISCHEMIA trial) as well as a more aggressive follow-up approach (in the POST-PCI trial) did not provide an additional treatment effect beyond a conservative strategy on the basis of guideline-directed medical therapy.”
Results were presented at the annual congress of the European Society of Cardiology and published online simultaneously in the New England Journal of Medicine.
‘Compelling new evidence’
In an editorial accompanying the publication, Jacqueline E. Tamis-Holland, MD, Icahn School of Medicine at Mount Sinai, Mount Sinai Morningside Hospital, New York, also agreed that this new result “builds on the findings” from the ISCHEMIA trial. “Collectively, these trials highlight the lack of benefit of routine stress testing in asymptomatic patients.”
Dr. Tamis-Holland pointed out that many of the deaths in this trial occurred before the 1-year stress test, possibly related to stent thrombosis, and therefore would not have been prevented by routine testing at 1 year. And overall, event rates were “quite low, and most likely reflect adherence to guideline recommendations” in the trial. For example, 99% of patients were receiving statins, and 74% of the procedures used intravascular imaging for the PCI procedures, “a much greater proportion of use than most centers in the United States,” she noted.
“The POST-PCI trial provides compelling new evidence for a future class III recommendation for routine surveillance testing after PCI,” Dr. Tamis-Holland concluded “Until then, we must refrain from prescribing surveillance stress testing to our patients after PCI, in the absence of other clinical signs or symptoms suggestive of stent failure.”
Commenting on the results, B. Hadley Wilson, MD, executive vice chair of the Sanger Heart & Vascular Institute/Atrium Health, clinical professor of medicine at University of North Carolina at Chapel Hill, and vice president of the American College of Cardiology, said that for decades it’s been thought that patients who had high-risk PCI needed to be followed more closely for potential future events.
“And it actually turned out there was no difference in outcomes between the groups,” he said in an interview.
“So, I think it’s a good study – well conducted, good numbers – that answers the question that routine functional stress testing, even for high-risk PCI patients, is not effective or cost effective or beneficial on a yearly basis,” he said. “I think it will help frame care that patients will just be followed with best medical therapy and then if they have recurrence of symptoms they would be considered for further evaluation, either with stress testing or angiography.”
High-risk characteristics
Current guidelines do not advocate the use of routine stress testing after revascularization, the authors wrote in their paper. “However, surveillance with the use of imaging-based stress testing may be considered in high-risk patients at 6 months after a revascularization procedure (class IIb recommendation), and routine imaging-based stress testing may be considered at 1 year after PCI and more than 5 years after CABG [coronary artery bypass graft] (class IIb recommendation).”
But in real-world clinical practice, Dr. Park said, “follow-up strategy for patients who underwent PCI or CABG is still undetermined.” Particularly, “it could be more problematic in high-risk PCI patients with high-risk anatomical or clinical characteristics. Thus, we performed this POST-PCI trial comparing routine stress testing follow-up strategy versus standard-care follow-up strategy in high-risk PCI patients.”
The researchers randomly assigned 1,706 patients with high-risk anatomical or clinical characteristics who had undergone PCI to a follow-up strategy of routine functional testing, including nuclear stress testing, exercise electrocardiography, or stress echocardiography at 1 year, or to standard care alone.
High-risk anatomical features included left main or bifurcation disease; restenotic or long, diffuse lesions; or bypass graft disease. High-risk clinical characteristics included diabetes mellitus, chronic kidney disease, or enzyme-positive acute coronary syndrome.
Mean age of the patients was 64.7 years; 21.0% had left main disease, 43.5% had bifurcation disease, 69.8% had multivessel disease, 70.1% had diffuse long lesions, 38.7% had diabetes, and 96.4% had been treated with drug-eluting stents.
At 2 years, a primary-outcome event had occurred in 46 of 849 patients (Kaplan-Meier estimate, 5.5%) in the functional-testing group and in 51 of 857 (Kaplan-Meier estimate, 6.0%) in the standard-care group (hazard ratio, 0.90; 95% confidence interval, 0.61-1.35; P = .62). There were no between-group differences in the components of the primary outcome.
Secondary endpoints included invasive coronary angiography or repeat revascularization. At 2 years, 12.3% of the patients in the functional-testing group and 9.3% in the standard-care group had undergone invasive coronary angiography (difference, 2.99 percentage points; 95% CI, −0.01 to 5.99 percentage points), and 8.1% and 5.8% of patients, respectively, had a repeat revascularization procedure (difference, 2.23 percentage points; 95% CI, −0.22 to 4.68 percentage points).
Positive results on stress tests were more common with nuclear imaging than with exercise ECG or stress echocardiography, the authors noted. Subsequent coronary angiography and repeat revascularization were more common in patients with positive results on nuclear stress imaging and exercise ECG than in those with discordant results between nuclear imaging and exercise ECG.
POST-PCI was funded by the CardioVascular Research Foundation and Daewoong Pharmaceutical Company. Dr. Park reported grants from the Cardiovascular Research Foundation and Daewoong Pharmaceutical Company. Dr. Tamis-Holland reported “other” funding from Pfizer outside the submitted work. Dr. Wilson reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2022
ALL-HEART: No benefit of allopurinol in ischemic heart disease
Allopurinol, a drug commonly used to treat gout, provided no benefit in terms of reducing cardiovascular (CV) events in patients with ischemic heart disease, new randomized trial results show.
Treatment of these patients without gout with 600 mg of allopurinol daily had no effect on composite primary endpoint outcomes, including nonfatal MI, nonfatal stroke, or CV death.
“ALL-HEART is the first large, prospective, randomized trial of the effect of allopurinol on major cardiovascular outcomes in patients with ischemic heart disease and provides robust evidence on the role of allopurinol in these patients,” principal investigator Isla Shelagh Mackenzie, MBChB (Honors), PhD, University of Dundee (Scotland), concluded at a press conference.
Their results suggest allopurinol should not be recommended for secondary prevention of events in this group, Dr. Mackenzie said. Although it remains an important treatment for gout, she added, “other avenues for treatment of ischemic heart disease should be explored in future.”
Results of the ALL-HEART (Allopurinol and Cardiovascular Outcomes in Ischemic Heart Disease) trial were presented at the annual congress of the European Society of Cardiology.
Gout treatment
Allopurinol is a xanthine oxidase inhibitor and acts by reducing serum uric acid levels and oxidative stress. Treatment is generally well tolerated, Dr. Mackenzie noted in her presentation, but some patients develop a rash, which can in some cases be serious or even fatal, progressing to Stevens-Johnson syndrome or toxic epidermal necrolysis, “particularly in certain ethnicities.” If rash develops, the advice is to stop treatment immediately.
“The importance of serum uric acid levels in cardiovascular disease is controversial, and there have been different reports over the years of how important they may be,” Dr. Mackenzie explained.
Observational studies have shown variable results, whereas intervention trials, most with fewer than 100 participants, have suggested potential improvements in factors such as blood pressure, endothelial function, left ventricular hypertrophy, or carotid intima-media thickness. Some have reported benefits in acute coronary syndrome and coronary artery bypass grafting, but others have not, she said. A previous study by their own group suggested an improvement in chest pain and exercise time in patients with chronic stable angina and documented coronary artery disease (CAD).
“So, until now, there have been no large prospective randomized trials of the effects of allopurinol on major cardiovascular outcomes in patients with ischemic heart disease,” Dr. Mackenzie said, and this was the aim of ALL-HEART.
ALL-HEART was a prospective, randomized, open-label, blinded-endpoint, multicenter trial. Patients with ischemia heart disease but no history of gout were recruited from 424 general practices across the United Kingdom, starting in February 2014 and with follow-up ending in September 2021. Participants were randomly assigned 1:1 to receive 600 mg of allopurinol daily or usual care.
“It was a decentralized trial, so the follow-up was largely remote after the first 6 weeks, and that included using record linkage data collected from centralized NHS [National Health Service] databases for hospitalizations and deaths in Scotland and England,” she said. The average follow-up was 4.8 years.
During that time, 258 (9.0%) participants in the allopurinol group and 76 (2.6%) in usual care withdrew from follow-up. By the end of the trial, 57.4% of patients in the allopurinol arm withdrew from randomized treatment.
Mean serum uric acid levels dropped from 0.34 mmol/L at baseline to 0.18 mmol/L at 6 weeks of treatment, “so we can see that the treatment was effective at lowering uric acid,” she noted.
In total, there were 5,721 patients in the final intention-to-treat analysis, and 639 patients had a first primary event.
For the primary outcome of nonfatal MI, nonfatal stroke, and cardiovascular death, there was no difference between the groups, the researchers reported, with a hazard ratio of 1.04 (95% confidence interval, 0.89-1.21; P = .65). Similarly, in secondary analyses, there were no differences in any of the component endpoints making up the primary outcome (nonfatal MI: HR, 0.97; 95% CI, 0.78-1.21; P = .81; nonfatal stroke: HR, 1.20; 95% CI, 0.89-1.60; P = .23; cardiovascular death: HR, 1.10; 95% CI, 0.85-1.43; P = .48), or in all-cause mortality (HR, 1.02; 95% CI, 0.87-1.20; P = .77), between the two groups, Dr. Mackenzie noted, “so a definitively neutral trial all round.”
In addition, no differences were seen in prespecified subgroups, including age, sex, estimated glomerular filtration rate, or diabetes, MI, heart failure, peripheral arterial disease, stroke, and stroke or transient ischemic attack at baseline.
There were also no significant effects on quality of life outcomes. Cost-effectiveness analyses are ongoing, although no differences are expected there, Dr. Mackenzie noted.
In terms of safety, incident cancers and all-cause mortality did not differ between groups. Serious adverse events were also similar between groups, Dr. Mackenzie said, “and there were no fatal treatment-related SAEs [serious adverse events] in the study.”
Another negative antioxidant trial
Invited discussant for the presentation, Leslie Cho, MD, of the Cleveland Clinic said that ALL-HEART, while an excellent trial with a pragmatic design, constitutes yet another negative antioxidant trial.
She pointed to three problems with this study and antioxidant trials in general. “First, the problem is with the antioxidant,” a xanthine oxidase inhibitor. “Xanthine oxidase is not a major trigger of oxidative stress. In a field of major players,” including nitric oxide, uncoupled endothelial nitric oxide synthase, and mitochondria myeloperoxidase, Dr. Cho said, “xanthine oxidase is a minor player.”
“Moreover, 57% of the patients stopped taking allopurinol, and rightfully so,” she said. Patients were receiving optimal medical therapies, many of which are also antioxidants, including statins, ACE inhibitors, angiotensin receptor blockers, and beta-blockers.
Second, the patient population was older, with an average age of 72 years. “This makes the ALL-HEART study a chronic angina study, chronic CAD study, one of the oldest modern day CAD trials. If you look at LoDoCo or ISCHEMIA trials, the average age is 63.” Patients also had established disease, many with previous revascularization.
The final issue seen with this trial, and all antioxidant trials, is that patient selection is not based on oxidative stress or antioxidant level. “The antioxidant trials have been disappointing at best. There is clear and convincing evidence that oxidative stress is involved in the pathogenesis of atherosclerosis, and yet study after study of antioxidant trials have been negative,” she said.
“Currently, there is no reliable measurement of global level of oxidative stress,” Dr. Cho noted. “Moreover, dose response was not tested, and if we cannot test the baseline antioxidant stress level of patients, we also cannot measure the effect of treatment on the global oxidative stress.”
So, “is there no hope for antioxidant trials?” she asked. Three factors will be required for future success, she said. “No. 1, selecting the right patient at the right time. No. 2, a reliable biomarker to measure oxidative stress to guide who should get therapy, and if the therapy is working. And lastly, targeted therapies that work on major triggers of oxidative stress.”
Also commenting on the results, B. Hadley Wilson, MD, executive vice chair of the Sanger Heart & Vascular Institute/Atrium Health, clinical professor of medicine at the University of North Carolina at Chapel Hill, and vice president of the American College of Cardiology, called ALL-HEART “an important and interesting study.”
“For years, cardiologists and others have been interested in allopurinol as an anti-inflammatory, xanthine oxidase inhibitor ... to prevent coronary ischemic events,” he said in an interview.
But this was a well-designed, well-conducted study, and “unfortunately there was no improvement in the primary outcome, no reduction in major cardiovascular events like myocardial infarction or stroke or cardiovascular death,” Dr. Wilson said. “So, it’s a bit of a disappointment that it’s not there as an important medication to help us with these patients with ischemic heart disease, but it’s also an important question answered — that we need to look at treatments for ischemic heart disease other than allopurinol.”
The trial was supported by the National Institute for Health and Care Research Health Technology Assessment Program in the United Kingdom. Dr. Mackenzie reported research contracts to her institution from NIHR HTA for this work, and other disclosures related to other work. Dr. Cho and Dr. Wilson reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Allopurinol, a drug commonly used to treat gout, provided no benefit in terms of reducing cardiovascular (CV) events in patients with ischemic heart disease, new randomized trial results show.
Treatment of these patients without gout with 600 mg of allopurinol daily had no effect on composite primary endpoint outcomes, including nonfatal MI, nonfatal stroke, or CV death.
“ALL-HEART is the first large, prospective, randomized trial of the effect of allopurinol on major cardiovascular outcomes in patients with ischemic heart disease and provides robust evidence on the role of allopurinol in these patients,” principal investigator Isla Shelagh Mackenzie, MBChB (Honors), PhD, University of Dundee (Scotland), concluded at a press conference.
Their results suggest allopurinol should not be recommended for secondary prevention of events in this group, Dr. Mackenzie said. Although it remains an important treatment for gout, she added, “other avenues for treatment of ischemic heart disease should be explored in future.”
Results of the ALL-HEART (Allopurinol and Cardiovascular Outcomes in Ischemic Heart Disease) trial were presented at the annual congress of the European Society of Cardiology.
Gout treatment
Allopurinol is a xanthine oxidase inhibitor and acts by reducing serum uric acid levels and oxidative stress. Treatment is generally well tolerated, Dr. Mackenzie noted in her presentation, but some patients develop a rash, which can in some cases be serious or even fatal, progressing to Stevens-Johnson syndrome or toxic epidermal necrolysis, “particularly in certain ethnicities.” If rash develops, the advice is to stop treatment immediately.
“The importance of serum uric acid levels in cardiovascular disease is controversial, and there have been different reports over the years of how important they may be,” Dr. Mackenzie explained.
Observational studies have shown variable results, whereas intervention trials, most with fewer than 100 participants, have suggested potential improvements in factors such as blood pressure, endothelial function, left ventricular hypertrophy, or carotid intima-media thickness. Some have reported benefits in acute coronary syndrome and coronary artery bypass grafting, but others have not, she said. A previous study by their own group suggested an improvement in chest pain and exercise time in patients with chronic stable angina and documented coronary artery disease (CAD).
“So, until now, there have been no large prospective randomized trials of the effects of allopurinol on major cardiovascular outcomes in patients with ischemic heart disease,” Dr. Mackenzie said, and this was the aim of ALL-HEART.
ALL-HEART was a prospective, randomized, open-label, blinded-endpoint, multicenter trial. Patients with ischemia heart disease but no history of gout were recruited from 424 general practices across the United Kingdom, starting in February 2014 and with follow-up ending in September 2021. Participants were randomly assigned 1:1 to receive 600 mg of allopurinol daily or usual care.
“It was a decentralized trial, so the follow-up was largely remote after the first 6 weeks, and that included using record linkage data collected from centralized NHS [National Health Service] databases for hospitalizations and deaths in Scotland and England,” she said. The average follow-up was 4.8 years.
During that time, 258 (9.0%) participants in the allopurinol group and 76 (2.6%) in usual care withdrew from follow-up. By the end of the trial, 57.4% of patients in the allopurinol arm withdrew from randomized treatment.
Mean serum uric acid levels dropped from 0.34 mmol/L at baseline to 0.18 mmol/L at 6 weeks of treatment, “so we can see that the treatment was effective at lowering uric acid,” she noted.
In total, there were 5,721 patients in the final intention-to-treat analysis, and 639 patients had a first primary event.
For the primary outcome of nonfatal MI, nonfatal stroke, and cardiovascular death, there was no difference between the groups, the researchers reported, with a hazard ratio of 1.04 (95% confidence interval, 0.89-1.21; P = .65). Similarly, in secondary analyses, there were no differences in any of the component endpoints making up the primary outcome (nonfatal MI: HR, 0.97; 95% CI, 0.78-1.21; P = .81; nonfatal stroke: HR, 1.20; 95% CI, 0.89-1.60; P = .23; cardiovascular death: HR, 1.10; 95% CI, 0.85-1.43; P = .48), or in all-cause mortality (HR, 1.02; 95% CI, 0.87-1.20; P = .77), between the two groups, Dr. Mackenzie noted, “so a definitively neutral trial all round.”
In addition, no differences were seen in prespecified subgroups, including age, sex, estimated glomerular filtration rate, or diabetes, MI, heart failure, peripheral arterial disease, stroke, and stroke or transient ischemic attack at baseline.
There were also no significant effects on quality of life outcomes. Cost-effectiveness analyses are ongoing, although no differences are expected there, Dr. Mackenzie noted.
In terms of safety, incident cancers and all-cause mortality did not differ between groups. Serious adverse events were also similar between groups, Dr. Mackenzie said, “and there were no fatal treatment-related SAEs [serious adverse events] in the study.”
Another negative antioxidant trial
Invited discussant for the presentation, Leslie Cho, MD, of the Cleveland Clinic said that ALL-HEART, while an excellent trial with a pragmatic design, constitutes yet another negative antioxidant trial.
She pointed to three problems with this study and antioxidant trials in general. “First, the problem is with the antioxidant,” a xanthine oxidase inhibitor. “Xanthine oxidase is not a major trigger of oxidative stress. In a field of major players,” including nitric oxide, uncoupled endothelial nitric oxide synthase, and mitochondria myeloperoxidase, Dr. Cho said, “xanthine oxidase is a minor player.”
“Moreover, 57% of the patients stopped taking allopurinol, and rightfully so,” she said. Patients were receiving optimal medical therapies, many of which are also antioxidants, including statins, ACE inhibitors, angiotensin receptor blockers, and beta-blockers.
Second, the patient population was older, with an average age of 72 years. “This makes the ALL-HEART study a chronic angina study, chronic CAD study, one of the oldest modern day CAD trials. If you look at LoDoCo or ISCHEMIA trials, the average age is 63.” Patients also had established disease, many with previous revascularization.
The final issue seen with this trial, and all antioxidant trials, is that patient selection is not based on oxidative stress or antioxidant level. “The antioxidant trials have been disappointing at best. There is clear and convincing evidence that oxidative stress is involved in the pathogenesis of atherosclerosis, and yet study after study of antioxidant trials have been negative,” she said.
“Currently, there is no reliable measurement of global level of oxidative stress,” Dr. Cho noted. “Moreover, dose response was not tested, and if we cannot test the baseline antioxidant stress level of patients, we also cannot measure the effect of treatment on the global oxidative stress.”
So, “is there no hope for antioxidant trials?” she asked. Three factors will be required for future success, she said. “No. 1, selecting the right patient at the right time. No. 2, a reliable biomarker to measure oxidative stress to guide who should get therapy, and if the therapy is working. And lastly, targeted therapies that work on major triggers of oxidative stress.”
Also commenting on the results, B. Hadley Wilson, MD, executive vice chair of the Sanger Heart & Vascular Institute/Atrium Health, clinical professor of medicine at the University of North Carolina at Chapel Hill, and vice president of the American College of Cardiology, called ALL-HEART “an important and interesting study.”
“For years, cardiologists and others have been interested in allopurinol as an anti-inflammatory, xanthine oxidase inhibitor ... to prevent coronary ischemic events,” he said in an interview.
But this was a well-designed, well-conducted study, and “unfortunately there was no improvement in the primary outcome, no reduction in major cardiovascular events like myocardial infarction or stroke or cardiovascular death,” Dr. Wilson said. “So, it’s a bit of a disappointment that it’s not there as an important medication to help us with these patients with ischemic heart disease, but it’s also an important question answered — that we need to look at treatments for ischemic heart disease other than allopurinol.”
The trial was supported by the National Institute for Health and Care Research Health Technology Assessment Program in the United Kingdom. Dr. Mackenzie reported research contracts to her institution from NIHR HTA for this work, and other disclosures related to other work. Dr. Cho and Dr. Wilson reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Allopurinol, a drug commonly used to treat gout, provided no benefit in terms of reducing cardiovascular (CV) events in patients with ischemic heart disease, new randomized trial results show.
Treatment of these patients without gout with 600 mg of allopurinol daily had no effect on composite primary endpoint outcomes, including nonfatal MI, nonfatal stroke, or CV death.
“ALL-HEART is the first large, prospective, randomized trial of the effect of allopurinol on major cardiovascular outcomes in patients with ischemic heart disease and provides robust evidence on the role of allopurinol in these patients,” principal investigator Isla Shelagh Mackenzie, MBChB (Honors), PhD, University of Dundee (Scotland), concluded at a press conference.
Their results suggest allopurinol should not be recommended for secondary prevention of events in this group, Dr. Mackenzie said. Although it remains an important treatment for gout, she added, “other avenues for treatment of ischemic heart disease should be explored in future.”
Results of the ALL-HEART (Allopurinol and Cardiovascular Outcomes in Ischemic Heart Disease) trial were presented at the annual congress of the European Society of Cardiology.
Gout treatment
Allopurinol is a xanthine oxidase inhibitor and acts by reducing serum uric acid levels and oxidative stress. Treatment is generally well tolerated, Dr. Mackenzie noted in her presentation, but some patients develop a rash, which can in some cases be serious or even fatal, progressing to Stevens-Johnson syndrome or toxic epidermal necrolysis, “particularly in certain ethnicities.” If rash develops, the advice is to stop treatment immediately.
“The importance of serum uric acid levels in cardiovascular disease is controversial, and there have been different reports over the years of how important they may be,” Dr. Mackenzie explained.
Observational studies have shown variable results, whereas intervention trials, most with fewer than 100 participants, have suggested potential improvements in factors such as blood pressure, endothelial function, left ventricular hypertrophy, or carotid intima-media thickness. Some have reported benefits in acute coronary syndrome and coronary artery bypass grafting, but others have not, she said. A previous study by their own group suggested an improvement in chest pain and exercise time in patients with chronic stable angina and documented coronary artery disease (CAD).
“So, until now, there have been no large prospective randomized trials of the effects of allopurinol on major cardiovascular outcomes in patients with ischemic heart disease,” Dr. Mackenzie said, and this was the aim of ALL-HEART.
ALL-HEART was a prospective, randomized, open-label, blinded-endpoint, multicenter trial. Patients with ischemia heart disease but no history of gout were recruited from 424 general practices across the United Kingdom, starting in February 2014 and with follow-up ending in September 2021. Participants were randomly assigned 1:1 to receive 600 mg of allopurinol daily or usual care.
“It was a decentralized trial, so the follow-up was largely remote after the first 6 weeks, and that included using record linkage data collected from centralized NHS [National Health Service] databases for hospitalizations and deaths in Scotland and England,” she said. The average follow-up was 4.8 years.
During that time, 258 (9.0%) participants in the allopurinol group and 76 (2.6%) in usual care withdrew from follow-up. By the end of the trial, 57.4% of patients in the allopurinol arm withdrew from randomized treatment.
Mean serum uric acid levels dropped from 0.34 mmol/L at baseline to 0.18 mmol/L at 6 weeks of treatment, “so we can see that the treatment was effective at lowering uric acid,” she noted.
In total, there were 5,721 patients in the final intention-to-treat analysis, and 639 patients had a first primary event.
For the primary outcome of nonfatal MI, nonfatal stroke, and cardiovascular death, there was no difference between the groups, the researchers reported, with a hazard ratio of 1.04 (95% confidence interval, 0.89-1.21; P = .65). Similarly, in secondary analyses, there were no differences in any of the component endpoints making up the primary outcome (nonfatal MI: HR, 0.97; 95% CI, 0.78-1.21; P = .81; nonfatal stroke: HR, 1.20; 95% CI, 0.89-1.60; P = .23; cardiovascular death: HR, 1.10; 95% CI, 0.85-1.43; P = .48), or in all-cause mortality (HR, 1.02; 95% CI, 0.87-1.20; P = .77), between the two groups, Dr. Mackenzie noted, “so a definitively neutral trial all round.”
In addition, no differences were seen in prespecified subgroups, including age, sex, estimated glomerular filtration rate, or diabetes, MI, heart failure, peripheral arterial disease, stroke, and stroke or transient ischemic attack at baseline.
There were also no significant effects on quality of life outcomes. Cost-effectiveness analyses are ongoing, although no differences are expected there, Dr. Mackenzie noted.
In terms of safety, incident cancers and all-cause mortality did not differ between groups. Serious adverse events were also similar between groups, Dr. Mackenzie said, “and there were no fatal treatment-related SAEs [serious adverse events] in the study.”
Another negative antioxidant trial
Invited discussant for the presentation, Leslie Cho, MD, of the Cleveland Clinic said that ALL-HEART, while an excellent trial with a pragmatic design, constitutes yet another negative antioxidant trial.
She pointed to three problems with this study and antioxidant trials in general. “First, the problem is with the antioxidant,” a xanthine oxidase inhibitor. “Xanthine oxidase is not a major trigger of oxidative stress. In a field of major players,” including nitric oxide, uncoupled endothelial nitric oxide synthase, and mitochondria myeloperoxidase, Dr. Cho said, “xanthine oxidase is a minor player.”
“Moreover, 57% of the patients stopped taking allopurinol, and rightfully so,” she said. Patients were receiving optimal medical therapies, many of which are also antioxidants, including statins, ACE inhibitors, angiotensin receptor blockers, and beta-blockers.
Second, the patient population was older, with an average age of 72 years. “This makes the ALL-HEART study a chronic angina study, chronic CAD study, one of the oldest modern day CAD trials. If you look at LoDoCo or ISCHEMIA trials, the average age is 63.” Patients also had established disease, many with previous revascularization.
The final issue seen with this trial, and all antioxidant trials, is that patient selection is not based on oxidative stress or antioxidant level. “The antioxidant trials have been disappointing at best. There is clear and convincing evidence that oxidative stress is involved in the pathogenesis of atherosclerosis, and yet study after study of antioxidant trials have been negative,” she said.
“Currently, there is no reliable measurement of global level of oxidative stress,” Dr. Cho noted. “Moreover, dose response was not tested, and if we cannot test the baseline antioxidant stress level of patients, we also cannot measure the effect of treatment on the global oxidative stress.”
So, “is there no hope for antioxidant trials?” she asked. Three factors will be required for future success, she said. “No. 1, selecting the right patient at the right time. No. 2, a reliable biomarker to measure oxidative stress to guide who should get therapy, and if the therapy is working. And lastly, targeted therapies that work on major triggers of oxidative stress.”
Also commenting on the results, B. Hadley Wilson, MD, executive vice chair of the Sanger Heart & Vascular Institute/Atrium Health, clinical professor of medicine at the University of North Carolina at Chapel Hill, and vice president of the American College of Cardiology, called ALL-HEART “an important and interesting study.”
“For years, cardiologists and others have been interested in allopurinol as an anti-inflammatory, xanthine oxidase inhibitor ... to prevent coronary ischemic events,” he said in an interview.
But this was a well-designed, well-conducted study, and “unfortunately there was no improvement in the primary outcome, no reduction in major cardiovascular events like myocardial infarction or stroke or cardiovascular death,” Dr. Wilson said. “So, it’s a bit of a disappointment that it’s not there as an important medication to help us with these patients with ischemic heart disease, but it’s also an important question answered — that we need to look at treatments for ischemic heart disease other than allopurinol.”
The trial was supported by the National Institute for Health and Care Research Health Technology Assessment Program in the United Kingdom. Dr. Mackenzie reported research contracts to her institution from NIHR HTA for this work, and other disclosures related to other work. Dr. Cho and Dr. Wilson reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2022
Angiography can wait for cardiac arrest without ST-elevation
A protocol of immediate angiography provided no mortality benefit over a strategy or delayed or more selective angiography among patients resuscitated from out-of-hospital cardiac arrest and without ST-segment elevation, new randomized results show.
“Among patients with resuscitated out-of-hospital cardiac arrest of possible cardiac origin, with shockable and nonshockable arrest rhythm and no ST-elevation, a strategy of immediate, unselected coronary angiography was not found to be beneficial over a delayed and selective approach with regard to the 30-day risk of all-cause death,” concluded principal investigator Steffen Desch, MD, University of Leipzig (Germany) Heart Center.
The results support previous results of the Coronary Angiography after Cardiac Arrest (COACT) trial, in patients with shockable rhythms, which also showed no differences in clinical outcomes between immediate and delayed coronary angiography at both 90 days and 1 year, he noted.
“What the clinicians wanted to know is, is it really necessary to get up at 3 a.m. in the morning to perform a coronary angiography on these patients, and that’s certainly out,” Dr. Desch said in an interview. “So, there’s really no room for this strategy anymore. You can take your time and wait a day or 2.”
These findings, from the TOMAHAWK trial, were presented Aug. 29 at the annual congress of the European Society of Cardiology and simultaneously published online in the New England Journal of Medicine.
Larger group without ST-segment elevation
Prognosis after out-of-hospital cardiac arrest is extremely poor, with an overall survival rate of less than 10%, Dr. Desch noted. “Actually, only 20% make it to the hospital; the vast majority of these patients die out in the field, so there’s really a great need in improving treatment.”
Acute coronary syndrome accounts for up to 60% of out-of-hospital arrests in which a cardiac cause has been identified, the authors wrote in their report. ST-segment elevation on postresuscitation electrocardiography “has good positive predictive value” for acute coronary lesions triggering the arrest, but in the far larger subgroup of patients without ST-segment elevation, “the spectrum of underlying causes is considerably broader and includes both cardiac and noncardiac causes.”
In patients with myocardial infarction, early revascularization would prevent negative consequences of myocardial injury, but unselected early coronary angiography would put patients not having an MI at unnecessary risk for procedural complications or delay in the diagnosis of the actual cause of their arrest, they noted.
In this trial, the researchers randomly assigned 554 patients from 31 sites in Germany and Denmark who were successfully resuscitated after cardiac arrest of possible cardiac origin to immediate transfer for coronary angiography or to initial intensive care assessment with delayed or selective angiography after a minimum delay of at least 1 day.
In the end, the average delay in this arm was 2 days, Dr. Desch noted. If the clinical course indicated that a coronary cause was unlikely, angiography might not be performed at all in this group.
No patient had ST-segment elevation on postresuscitation electrocardiography. The primary endpoint was death from any cause at 30 days; secondary end points were death from any cause or severe neurologic deficit at 30 days.
Results showed that 95% of patients in the immediate angiography group actually underwent the procedure, compared with 62% of those in the delayed group, a finding that was “logical” given the study design, he said.
At 30 days, 54% of patients in the immediate angiography group and 46% in the delayed group had died, a nonsignificant difference (P = .06). Because the researchers had performed an interim analysis, Dr. Desch explained, the final P value for significance in this trial was not .05, but rather .034, to account for multiple comparisons.
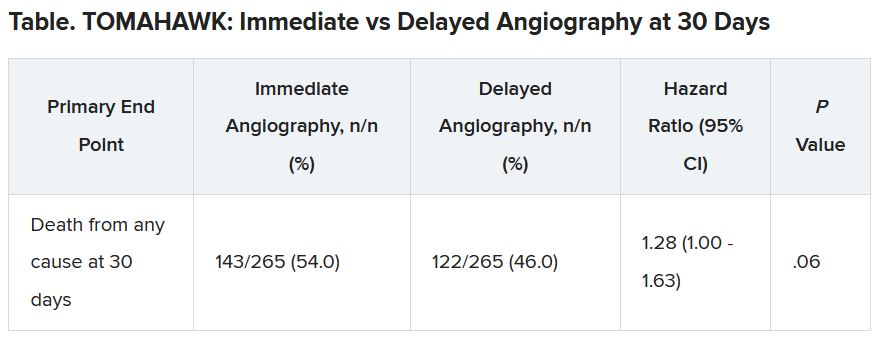
The secondary end point of death from any cause or severe neurologic deficit at 30 days “was actually nominally significant in favor of the delayed group,” he said. “So, this is not corrected for multiple testing, it’s just a hypothesis that’s in the room, but it’s certainly worthy of discussion that the immediate strategy might actually cause harm.”
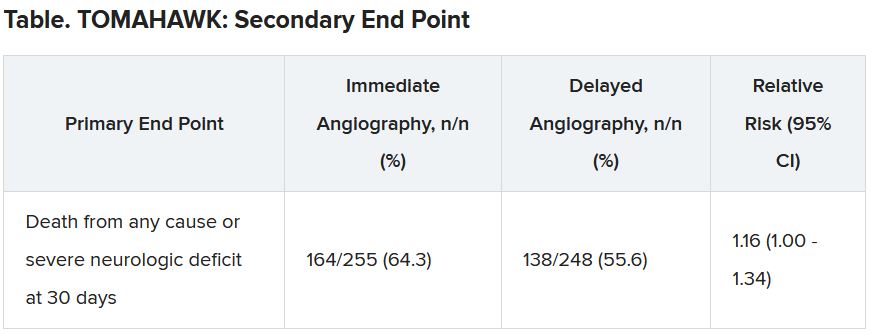
There was no difference between the groups in peak release of myocardial enzymes, or any other safety end points, including bleeding, stroke, or renal failure, Dr. Desch said.
Further analyses showed no large differences between subgroups, including age, diabetes, first monitored rhythm, confirmed MI as the trigger of the arrest, sex, and the time from cardiac arrest to the return of spontaneous circulation, he noted.
Opportunity to minimize harm
Discussant for the results during the presentation was Susanna Price, MBBS, PhD, Royal Brompton Hospital, London.
Dr. Price concluded: “What this means for me, is it gives me information that’s useful regarding the opportunity to minimize harm, which is a lot of what critical care is about, so we don’t necessarily now have to move these patients very acutely when they’ve just come in through the ED [emergency department]. It has implications for resource utilization, but also implications for mobilizing patients around the hospital during COVID-19.”
It’s also important to note that coronary angiography was still carried out in certain patients, “so we still have to have that dialogue with our interventional cardiologists for certain patients who may need to go to the cath lab, and what it should now allow us to do is give appropriate focus to how to manage these patients when they come in to the ED or to our ICUs [intensive care units],” she said.
Dr. Price added, though, that perhaps “the most important slide” in the presentation was that showing 90% of these patients had a witnessed cardiac arrest, “and yet a third of these patients, 168 of them, had no bystander CPR at all.”
She pointed to the “chain of survival” after cardiac arrest, of which Charles D. Deakin, MD, University Hospital Southampton (England), wrote that “not all links are equal.”
“Early recognition and calling for help, early CPR, early defibrillation where appropriate are very, very important, and we need to be addressing all of these, as well as what happens in the cath lab and after admission,” Dr. Price said.
This research was funded by the German Center for Cardiovascular Research. Dr. Desch and Dr. Price reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
A protocol of immediate angiography provided no mortality benefit over a strategy or delayed or more selective angiography among patients resuscitated from out-of-hospital cardiac arrest and without ST-segment elevation, new randomized results show.
“Among patients with resuscitated out-of-hospital cardiac arrest of possible cardiac origin, with shockable and nonshockable arrest rhythm and no ST-elevation, a strategy of immediate, unselected coronary angiography was not found to be beneficial over a delayed and selective approach with regard to the 30-day risk of all-cause death,” concluded principal investigator Steffen Desch, MD, University of Leipzig (Germany) Heart Center.
The results support previous results of the Coronary Angiography after Cardiac Arrest (COACT) trial, in patients with shockable rhythms, which also showed no differences in clinical outcomes between immediate and delayed coronary angiography at both 90 days and 1 year, he noted.
“What the clinicians wanted to know is, is it really necessary to get up at 3 a.m. in the morning to perform a coronary angiography on these patients, and that’s certainly out,” Dr. Desch said in an interview. “So, there’s really no room for this strategy anymore. You can take your time and wait a day or 2.”
These findings, from the TOMAHAWK trial, were presented Aug. 29 at the annual congress of the European Society of Cardiology and simultaneously published online in the New England Journal of Medicine.
Larger group without ST-segment elevation
Prognosis after out-of-hospital cardiac arrest is extremely poor, with an overall survival rate of less than 10%, Dr. Desch noted. “Actually, only 20% make it to the hospital; the vast majority of these patients die out in the field, so there’s really a great need in improving treatment.”
Acute coronary syndrome accounts for up to 60% of out-of-hospital arrests in which a cardiac cause has been identified, the authors wrote in their report. ST-segment elevation on postresuscitation electrocardiography “has good positive predictive value” for acute coronary lesions triggering the arrest, but in the far larger subgroup of patients without ST-segment elevation, “the spectrum of underlying causes is considerably broader and includes both cardiac and noncardiac causes.”
In patients with myocardial infarction, early revascularization would prevent negative consequences of myocardial injury, but unselected early coronary angiography would put patients not having an MI at unnecessary risk for procedural complications or delay in the diagnosis of the actual cause of their arrest, they noted.
In this trial, the researchers randomly assigned 554 patients from 31 sites in Germany and Denmark who were successfully resuscitated after cardiac arrest of possible cardiac origin to immediate transfer for coronary angiography or to initial intensive care assessment with delayed or selective angiography after a minimum delay of at least 1 day.
In the end, the average delay in this arm was 2 days, Dr. Desch noted. If the clinical course indicated that a coronary cause was unlikely, angiography might not be performed at all in this group.
No patient had ST-segment elevation on postresuscitation electrocardiography. The primary endpoint was death from any cause at 30 days; secondary end points were death from any cause or severe neurologic deficit at 30 days.
Results showed that 95% of patients in the immediate angiography group actually underwent the procedure, compared with 62% of those in the delayed group, a finding that was “logical” given the study design, he said.
At 30 days, 54% of patients in the immediate angiography group and 46% in the delayed group had died, a nonsignificant difference (P = .06). Because the researchers had performed an interim analysis, Dr. Desch explained, the final P value for significance in this trial was not .05, but rather .034, to account for multiple comparisons.

The secondary end point of death from any cause or severe neurologic deficit at 30 days “was actually nominally significant in favor of the delayed group,” he said. “So, this is not corrected for multiple testing, it’s just a hypothesis that’s in the room, but it’s certainly worthy of discussion that the immediate strategy might actually cause harm.”

There was no difference between the groups in peak release of myocardial enzymes, or any other safety end points, including bleeding, stroke, or renal failure, Dr. Desch said.
Further analyses showed no large differences between subgroups, including age, diabetes, first monitored rhythm, confirmed MI as the trigger of the arrest, sex, and the time from cardiac arrest to the return of spontaneous circulation, he noted.
Opportunity to minimize harm
Discussant for the results during the presentation was Susanna Price, MBBS, PhD, Royal Brompton Hospital, London.
Dr. Price concluded: “What this means for me, is it gives me information that’s useful regarding the opportunity to minimize harm, which is a lot of what critical care is about, so we don’t necessarily now have to move these patients very acutely when they’ve just come in through the ED [emergency department]. It has implications for resource utilization, but also implications for mobilizing patients around the hospital during COVID-19.”
It’s also important to note that coronary angiography was still carried out in certain patients, “so we still have to have that dialogue with our interventional cardiologists for certain patients who may need to go to the cath lab, and what it should now allow us to do is give appropriate focus to how to manage these patients when they come in to the ED or to our ICUs [intensive care units],” she said.
Dr. Price added, though, that perhaps “the most important slide” in the presentation was that showing 90% of these patients had a witnessed cardiac arrest, “and yet a third of these patients, 168 of them, had no bystander CPR at all.”
She pointed to the “chain of survival” after cardiac arrest, of which Charles D. Deakin, MD, University Hospital Southampton (England), wrote that “not all links are equal.”
“Early recognition and calling for help, early CPR, early defibrillation where appropriate are very, very important, and we need to be addressing all of these, as well as what happens in the cath lab and after admission,” Dr. Price said.
This research was funded by the German Center for Cardiovascular Research. Dr. Desch and Dr. Price reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
A protocol of immediate angiography provided no mortality benefit over a strategy or delayed or more selective angiography among patients resuscitated from out-of-hospital cardiac arrest and without ST-segment elevation, new randomized results show.
“Among patients with resuscitated out-of-hospital cardiac arrest of possible cardiac origin, with shockable and nonshockable arrest rhythm and no ST-elevation, a strategy of immediate, unselected coronary angiography was not found to be beneficial over a delayed and selective approach with regard to the 30-day risk of all-cause death,” concluded principal investigator Steffen Desch, MD, University of Leipzig (Germany) Heart Center.
The results support previous results of the Coronary Angiography after Cardiac Arrest (COACT) trial, in patients with shockable rhythms, which also showed no differences in clinical outcomes between immediate and delayed coronary angiography at both 90 days and 1 year, he noted.
“What the clinicians wanted to know is, is it really necessary to get up at 3 a.m. in the morning to perform a coronary angiography on these patients, and that’s certainly out,” Dr. Desch said in an interview. “So, there’s really no room for this strategy anymore. You can take your time and wait a day or 2.”
These findings, from the TOMAHAWK trial, were presented Aug. 29 at the annual congress of the European Society of Cardiology and simultaneously published online in the New England Journal of Medicine.
Larger group without ST-segment elevation
Prognosis after out-of-hospital cardiac arrest is extremely poor, with an overall survival rate of less than 10%, Dr. Desch noted. “Actually, only 20% make it to the hospital; the vast majority of these patients die out in the field, so there’s really a great need in improving treatment.”
Acute coronary syndrome accounts for up to 60% of out-of-hospital arrests in which a cardiac cause has been identified, the authors wrote in their report. ST-segment elevation on postresuscitation electrocardiography “has good positive predictive value” for acute coronary lesions triggering the arrest, but in the far larger subgroup of patients without ST-segment elevation, “the spectrum of underlying causes is considerably broader and includes both cardiac and noncardiac causes.”
In patients with myocardial infarction, early revascularization would prevent negative consequences of myocardial injury, but unselected early coronary angiography would put patients not having an MI at unnecessary risk for procedural complications or delay in the diagnosis of the actual cause of their arrest, they noted.
In this trial, the researchers randomly assigned 554 patients from 31 sites in Germany and Denmark who were successfully resuscitated after cardiac arrest of possible cardiac origin to immediate transfer for coronary angiography or to initial intensive care assessment with delayed or selective angiography after a minimum delay of at least 1 day.
In the end, the average delay in this arm was 2 days, Dr. Desch noted. If the clinical course indicated that a coronary cause was unlikely, angiography might not be performed at all in this group.
No patient had ST-segment elevation on postresuscitation electrocardiography. The primary endpoint was death from any cause at 30 days; secondary end points were death from any cause or severe neurologic deficit at 30 days.
Results showed that 95% of patients in the immediate angiography group actually underwent the procedure, compared with 62% of those in the delayed group, a finding that was “logical” given the study design, he said.
At 30 days, 54% of patients in the immediate angiography group and 46% in the delayed group had died, a nonsignificant difference (P = .06). Because the researchers had performed an interim analysis, Dr. Desch explained, the final P value for significance in this trial was not .05, but rather .034, to account for multiple comparisons.

The secondary end point of death from any cause or severe neurologic deficit at 30 days “was actually nominally significant in favor of the delayed group,” he said. “So, this is not corrected for multiple testing, it’s just a hypothesis that’s in the room, but it’s certainly worthy of discussion that the immediate strategy might actually cause harm.”

There was no difference between the groups in peak release of myocardial enzymes, or any other safety end points, including bleeding, stroke, or renal failure, Dr. Desch said.
Further analyses showed no large differences between subgroups, including age, diabetes, first monitored rhythm, confirmed MI as the trigger of the arrest, sex, and the time from cardiac arrest to the return of spontaneous circulation, he noted.
Opportunity to minimize harm
Discussant for the results during the presentation was Susanna Price, MBBS, PhD, Royal Brompton Hospital, London.
Dr. Price concluded: “What this means for me, is it gives me information that’s useful regarding the opportunity to minimize harm, which is a lot of what critical care is about, so we don’t necessarily now have to move these patients very acutely when they’ve just come in through the ED [emergency department]. It has implications for resource utilization, but also implications for mobilizing patients around the hospital during COVID-19.”
It’s also important to note that coronary angiography was still carried out in certain patients, “so we still have to have that dialogue with our interventional cardiologists for certain patients who may need to go to the cath lab, and what it should now allow us to do is give appropriate focus to how to manage these patients when they come in to the ED or to our ICUs [intensive care units],” she said.
Dr. Price added, though, that perhaps “the most important slide” in the presentation was that showing 90% of these patients had a witnessed cardiac arrest, “and yet a third of these patients, 168 of them, had no bystander CPR at all.”
She pointed to the “chain of survival” after cardiac arrest, of which Charles D. Deakin, MD, University Hospital Southampton (England), wrote that “not all links are equal.”
“Early recognition and calling for help, early CPR, early defibrillation where appropriate are very, very important, and we need to be addressing all of these, as well as what happens in the cath lab and after admission,” Dr. Price said.
This research was funded by the German Center for Cardiovascular Research. Dr. Desch and Dr. Price reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FDA approves dapagliflozin (Farxiga) for chronic kidney disease
The Food and Drug Administration has approved dapagliflozin (Farxiga, AstraZeneca) to reduce the risk for kidney function decline, kidney failure, cardiovascular death, and hospitalization for heart failure in adult patients with chronic kidney disease (CKD) at risk for disease progression.
“Chronic kidney disease is an important public health issue, and there is a significant unmet need for therapies that slow disease progression and improve outcomes,” said Aliza Thompson, MD, deputy director of the division of cardiology and nephrology at the FDA’s Center for Drug Evaluation and Research. “Today’s approval of Farxiga for the treatment of chronic kidney disease is an important step forward in helping people living with kidney disease.”
Dapagliflozin was approved in 2014 to improve glycemic control in patients with diabetes mellitus, and approval was expanded in 2020 to include treatment of patients with heart failure and reduced ejection fraction, based on results of the DAPA-HF trial.
This new approval in chronic kidney disease was based on results of the DAPA-CKD trial that was stopped early in March 2020 because of efficacy of the treatment.
DAPA-CKD randomly assigned 4,304 patients with CKD but without diabetes to receive either dapagliflozin or placebo. The full study results, reported at the 2020 annual congress of the European Society of Cardiology and simultaneously published in the New England Journal of Medicine, showed that, during a median of 2.4 years, treatment with dapagliflozin led to a significant 31% relative reduction, compared with placebo in the study’s primary outcome, a composite that included at least a 50% drop in estimated glomerular filtration rate, compared with baseline, end-stage kidney disease, kidney transplant, renal death, or cardiovascular death.
Dapagliflozin treatment also cut all-cause mortality by a statistically significant relative reduction of 31%, and another secondary-endpoint analysis showed a statistically significant 29% relative reduction in the rate of cardiovascular death or heart failure hospitalization.
“Farxiga was not studied, nor is expected to be effective, in treating chronic kidney disease among patients with autosomal dominant or recessive polycystic (characterized by multiple cysts) kidney disease or among patients who require or have recently used immunosuppressive therapy to treat kidney disease,” the FDA statement noted.
Dapagliflozin should not be used by patients with a history of serious hypersensitivity reactions to this medication, or who are on dialysis, the agency added. “Serious, life-threatening cases of Fournier’s Gangrene have occurred in patients with diabetes taking Farxiga.”
Patients should consider taking a lower dose of insulin or insulin secretagogue to reduce hypoglycemic risk if they are also taking dapagliflozin. Treatment can also cause dehydration, serious urinary tract infections, genital yeast infections, and metabolic acidosis, the announcement said. “Patients should be assessed for their volume status and kidney function before starting Farxiga.”
Dapagliflozin previously received Fast Track, Breakthrough Therapy, and Priority Review designations for this new indication.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved dapagliflozin (Farxiga, AstraZeneca) to reduce the risk for kidney function decline, kidney failure, cardiovascular death, and hospitalization for heart failure in adult patients with chronic kidney disease (CKD) at risk for disease progression.
“Chronic kidney disease is an important public health issue, and there is a significant unmet need for therapies that slow disease progression and improve outcomes,” said Aliza Thompson, MD, deputy director of the division of cardiology and nephrology at the FDA’s Center for Drug Evaluation and Research. “Today’s approval of Farxiga for the treatment of chronic kidney disease is an important step forward in helping people living with kidney disease.”
Dapagliflozin was approved in 2014 to improve glycemic control in patients with diabetes mellitus, and approval was expanded in 2020 to include treatment of patients with heart failure and reduced ejection fraction, based on results of the DAPA-HF trial.
This new approval in chronic kidney disease was based on results of the DAPA-CKD trial that was stopped early in March 2020 because of efficacy of the treatment.
DAPA-CKD randomly assigned 4,304 patients with CKD but without diabetes to receive either dapagliflozin or placebo. The full study results, reported at the 2020 annual congress of the European Society of Cardiology and simultaneously published in the New England Journal of Medicine, showed that, during a median of 2.4 years, treatment with dapagliflozin led to a significant 31% relative reduction, compared with placebo in the study’s primary outcome, a composite that included at least a 50% drop in estimated glomerular filtration rate, compared with baseline, end-stage kidney disease, kidney transplant, renal death, or cardiovascular death.
Dapagliflozin treatment also cut all-cause mortality by a statistically significant relative reduction of 31%, and another secondary-endpoint analysis showed a statistically significant 29% relative reduction in the rate of cardiovascular death or heart failure hospitalization.
“Farxiga was not studied, nor is expected to be effective, in treating chronic kidney disease among patients with autosomal dominant or recessive polycystic (characterized by multiple cysts) kidney disease or among patients who require or have recently used immunosuppressive therapy to treat kidney disease,” the FDA statement noted.
Dapagliflozin should not be used by patients with a history of serious hypersensitivity reactions to this medication, or who are on dialysis, the agency added. “Serious, life-threatening cases of Fournier’s Gangrene have occurred in patients with diabetes taking Farxiga.”
Patients should consider taking a lower dose of insulin or insulin secretagogue to reduce hypoglycemic risk if they are also taking dapagliflozin. Treatment can also cause dehydration, serious urinary tract infections, genital yeast infections, and metabolic acidosis, the announcement said. “Patients should be assessed for their volume status and kidney function before starting Farxiga.”
Dapagliflozin previously received Fast Track, Breakthrough Therapy, and Priority Review designations for this new indication.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved dapagliflozin (Farxiga, AstraZeneca) to reduce the risk for kidney function decline, kidney failure, cardiovascular death, and hospitalization for heart failure in adult patients with chronic kidney disease (CKD) at risk for disease progression.
“Chronic kidney disease is an important public health issue, and there is a significant unmet need for therapies that slow disease progression and improve outcomes,” said Aliza Thompson, MD, deputy director of the division of cardiology and nephrology at the FDA’s Center for Drug Evaluation and Research. “Today’s approval of Farxiga for the treatment of chronic kidney disease is an important step forward in helping people living with kidney disease.”
Dapagliflozin was approved in 2014 to improve glycemic control in patients with diabetes mellitus, and approval was expanded in 2020 to include treatment of patients with heart failure and reduced ejection fraction, based on results of the DAPA-HF trial.
This new approval in chronic kidney disease was based on results of the DAPA-CKD trial that was stopped early in March 2020 because of efficacy of the treatment.
DAPA-CKD randomly assigned 4,304 patients with CKD but without diabetes to receive either dapagliflozin or placebo. The full study results, reported at the 2020 annual congress of the European Society of Cardiology and simultaneously published in the New England Journal of Medicine, showed that, during a median of 2.4 years, treatment with dapagliflozin led to a significant 31% relative reduction, compared with placebo in the study’s primary outcome, a composite that included at least a 50% drop in estimated glomerular filtration rate, compared with baseline, end-stage kidney disease, kidney transplant, renal death, or cardiovascular death.
Dapagliflozin treatment also cut all-cause mortality by a statistically significant relative reduction of 31%, and another secondary-endpoint analysis showed a statistically significant 29% relative reduction in the rate of cardiovascular death or heart failure hospitalization.
“Farxiga was not studied, nor is expected to be effective, in treating chronic kidney disease among patients with autosomal dominant or recessive polycystic (characterized by multiple cysts) kidney disease or among patients who require or have recently used immunosuppressive therapy to treat kidney disease,” the FDA statement noted.
Dapagliflozin should not be used by patients with a history of serious hypersensitivity reactions to this medication, or who are on dialysis, the agency added. “Serious, life-threatening cases of Fournier’s Gangrene have occurred in patients with diabetes taking Farxiga.”
Patients should consider taking a lower dose of insulin or insulin secretagogue to reduce hypoglycemic risk if they are also taking dapagliflozin. Treatment can also cause dehydration, serious urinary tract infections, genital yeast infections, and metabolic acidosis, the announcement said. “Patients should be assessed for their volume status and kidney function before starting Farxiga.”
Dapagliflozin previously received Fast Track, Breakthrough Therapy, and Priority Review designations for this new indication.
A version of this article first appeared on Medscape.com.
ACC issues guidance on cardiac implications of coronavirus
The American College of Cardiology on Feb. 13, 2020, released a clinical bulletin that aims to address cardiac implications of the current epidemic of the novel coronavirus, now known as COVID-19.
The bulletin, reviewed and approved by the college’s Science and Quality Oversight Committee, “provides background on the epidemic, which was first reported in late December 2019, and looks at early cardiac implications from case reports,” the ACC noted in a press release. “It also provides information on the potential cardiac implications from analog viral respiratory pandemics and offers early clinical guidance given current COVID-19 uncertainty.”
The document looks at some early cardiac implications of the infection. For example, early case reports suggest patients with underlying conditions are at higher risk of complications or mortality from the virus, with up to 50% of hospitalized patients having a chronic medical illness, the authors wrote.
About 40% of hospitalized patients confirmed to have the virus have cardiovascular or cerebrovascular disease, they noted.
In a recent case report on 138 hospitalized COVID-19 patients, they noted, 19.6% developed acute respiratory distress syndrome, 16.7% developed arrhythmia, 8.7% developed shock, 7.2% developed acute cardiac injury, and 3.6% developed acute kidney injury. “Rates of complication were universally higher for ICU patients,” they wrote.
“The first reported death was a 61-year-old male, with a long history of smoking, who succumbed to acute respiratory distress, heart failure, and cardiac arrest,” the document noted. “Early, unpublished first-hand reports suggest at least some patients develop myocarditis.”
Stressing the current uncertainty about the virus, the bulletin provides the following clinical guidance:
- COVID-19 is spread through droplets and can live for substantial periods outside the body; containment and prevention using standard public health and personal strategies for preventing the spread of communicable disease remains the priority.
- In geographies with active COVID-19 transmission (mainly China), it is reasonable to advise patients with underlying cardiovascular disease of the potential increased risk and to encourage additional, reasonable precautions.
- Older adults are less likely to present with fever, thus close assessment for other symptoms such as cough or shortness of breath is warranted.
- Some experts have suggested that the rigorous use of guideline-directed, plaque-stabilizing agents could offer additional protection to cardiovascular disease (CVD) patients during a widespread outbreak (statins, beta-blockers, ACE inhibitors, acetylsalicylic acid); however, such therapies should be tailored to individual patients.
- It is important for patients with CVD to remain current with vaccinations, including the pneumococcal vaccine, given the increased risk of secondary bacterial infection; it would also be prudent to receive vaccination to prevent another source of fever which could be initially confused with coronavirus infection.
- It may be reasonable to triage COVID-19 patients according to the presence of underlying cardiovascular, respiratory, renal, and other chronic diseases for prioritized treatment.
- Providers are cautioned that classic symptoms and presentation of acute MI may be overshadowed in the context of coronavirus, resulting in underdiagnosis.
- For CVD patients in geographies without widespread COVID-19, emphasis should remain on the threat from influenza, the importance of vaccination and frequent handwashing, and continued adherence to all guideline-directed therapy for underlying chronic conditions.
- COVID-19 is a fast-moving epidemic with an uncertain clinical profile; providers should be prepared for guidance to shift as more information becomes available.
The full clinical update is available here.
This article first appeared on Medscape.com.
The American College of Cardiology on Feb. 13, 2020, released a clinical bulletin that aims to address cardiac implications of the current epidemic of the novel coronavirus, now known as COVID-19.
The bulletin, reviewed and approved by the college’s Science and Quality Oversight Committee, “provides background on the epidemic, which was first reported in late December 2019, and looks at early cardiac implications from case reports,” the ACC noted in a press release. “It also provides information on the potential cardiac implications from analog viral respiratory pandemics and offers early clinical guidance given current COVID-19 uncertainty.”
The document looks at some early cardiac implications of the infection. For example, early case reports suggest patients with underlying conditions are at higher risk of complications or mortality from the virus, with up to 50% of hospitalized patients having a chronic medical illness, the authors wrote.
About 40% of hospitalized patients confirmed to have the virus have cardiovascular or cerebrovascular disease, they noted.
In a recent case report on 138 hospitalized COVID-19 patients, they noted, 19.6% developed acute respiratory distress syndrome, 16.7% developed arrhythmia, 8.7% developed shock, 7.2% developed acute cardiac injury, and 3.6% developed acute kidney injury. “Rates of complication were universally higher for ICU patients,” they wrote.
“The first reported death was a 61-year-old male, with a long history of smoking, who succumbed to acute respiratory distress, heart failure, and cardiac arrest,” the document noted. “Early, unpublished first-hand reports suggest at least some patients develop myocarditis.”
Stressing the current uncertainty about the virus, the bulletin provides the following clinical guidance:
- COVID-19 is spread through droplets and can live for substantial periods outside the body; containment and prevention using standard public health and personal strategies for preventing the spread of communicable disease remains the priority.
- In geographies with active COVID-19 transmission (mainly China), it is reasonable to advise patients with underlying cardiovascular disease of the potential increased risk and to encourage additional, reasonable precautions.
- Older adults are less likely to present with fever, thus close assessment for other symptoms such as cough or shortness of breath is warranted.
- Some experts have suggested that the rigorous use of guideline-directed, plaque-stabilizing agents could offer additional protection to cardiovascular disease (CVD) patients during a widespread outbreak (statins, beta-blockers, ACE inhibitors, acetylsalicylic acid); however, such therapies should be tailored to individual patients.
- It is important for patients with CVD to remain current with vaccinations, including the pneumococcal vaccine, given the increased risk of secondary bacterial infection; it would also be prudent to receive vaccination to prevent another source of fever which could be initially confused with coronavirus infection.
- It may be reasonable to triage COVID-19 patients according to the presence of underlying cardiovascular, respiratory, renal, and other chronic diseases for prioritized treatment.
- Providers are cautioned that classic symptoms and presentation of acute MI may be overshadowed in the context of coronavirus, resulting in underdiagnosis.
- For CVD patients in geographies without widespread COVID-19, emphasis should remain on the threat from influenza, the importance of vaccination and frequent handwashing, and continued adherence to all guideline-directed therapy for underlying chronic conditions.
- COVID-19 is a fast-moving epidemic with an uncertain clinical profile; providers should be prepared for guidance to shift as more information becomes available.
The full clinical update is available here.
This article first appeared on Medscape.com.
The American College of Cardiology on Feb. 13, 2020, released a clinical bulletin that aims to address cardiac implications of the current epidemic of the novel coronavirus, now known as COVID-19.
The bulletin, reviewed and approved by the college’s Science and Quality Oversight Committee, “provides background on the epidemic, which was first reported in late December 2019, and looks at early cardiac implications from case reports,” the ACC noted in a press release. “It also provides information on the potential cardiac implications from analog viral respiratory pandemics and offers early clinical guidance given current COVID-19 uncertainty.”
The document looks at some early cardiac implications of the infection. For example, early case reports suggest patients with underlying conditions are at higher risk of complications or mortality from the virus, with up to 50% of hospitalized patients having a chronic medical illness, the authors wrote.
About 40% of hospitalized patients confirmed to have the virus have cardiovascular or cerebrovascular disease, they noted.
In a recent case report on 138 hospitalized COVID-19 patients, they noted, 19.6% developed acute respiratory distress syndrome, 16.7% developed arrhythmia, 8.7% developed shock, 7.2% developed acute cardiac injury, and 3.6% developed acute kidney injury. “Rates of complication were universally higher for ICU patients,” they wrote.
“The first reported death was a 61-year-old male, with a long history of smoking, who succumbed to acute respiratory distress, heart failure, and cardiac arrest,” the document noted. “Early, unpublished first-hand reports suggest at least some patients develop myocarditis.”
Stressing the current uncertainty about the virus, the bulletin provides the following clinical guidance:
- COVID-19 is spread through droplets and can live for substantial periods outside the body; containment and prevention using standard public health and personal strategies for preventing the spread of communicable disease remains the priority.
- In geographies with active COVID-19 transmission (mainly China), it is reasonable to advise patients with underlying cardiovascular disease of the potential increased risk and to encourage additional, reasonable precautions.
- Older adults are less likely to present with fever, thus close assessment for other symptoms such as cough or shortness of breath is warranted.
- Some experts have suggested that the rigorous use of guideline-directed, plaque-stabilizing agents could offer additional protection to cardiovascular disease (CVD) patients during a widespread outbreak (statins, beta-blockers, ACE inhibitors, acetylsalicylic acid); however, such therapies should be tailored to individual patients.
- It is important for patients with CVD to remain current with vaccinations, including the pneumococcal vaccine, given the increased risk of secondary bacterial infection; it would also be prudent to receive vaccination to prevent another source of fever which could be initially confused with coronavirus infection.
- It may be reasonable to triage COVID-19 patients according to the presence of underlying cardiovascular, respiratory, renal, and other chronic diseases for prioritized treatment.
- Providers are cautioned that classic symptoms and presentation of acute MI may be overshadowed in the context of coronavirus, resulting in underdiagnosis.
- For CVD patients in geographies without widespread COVID-19, emphasis should remain on the threat from influenza, the importance of vaccination and frequent handwashing, and continued adherence to all guideline-directed therapy for underlying chronic conditions.
- COVID-19 is a fast-moving epidemic with an uncertain clinical profile; providers should be prepared for guidance to shift as more information becomes available.
The full clinical update is available here.
This article first appeared on Medscape.com.