User login
Cross-contamination of Pathology Specimens: A Cautionary Tale
Cross-contamination of pathology specimens is a rare but nonnegligible source of potential morbidity in clinical practice. Contaminant tissue fragments, colloquially referred to as floaters, typically are readily identifiable based on obvious cytomorphologic differences, especially if the tissues arise from different organs; however, one cannot rely on such distinctions in a pathology laboratory dedicated to a single organ system (eg, dermatopathology). The inability to identify quickly and confidently the presence of a contaminant puts the patient at risk for misdiagnosis, which can lead to unnecessary morbidity or even mortality in the case of cancer misdiagnosis. Studies that have been conducted to estimate the incidence of this type of error have suggested an overall incidence rate between approximately 1% and 3%.1,2 Awareness of this phenomenon and careful scrutiny when the histopathologic evidence diverges considerably from the clinical impression is critical for minimizing the negative outcomes that could result from the presence of contaminant tissue. We present a case in which cross-contamination of a pathology specimen led to an initial erroneous diagnosis of an aggressive cutaneous melanoma in a patient with a benign adnexal neoplasm.
Case Report
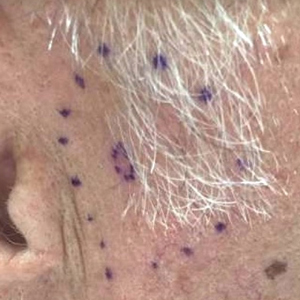
A 72-year-old man was referred to the Pigmented Lesion and Melanoma Program at Stanford University Medical Center and Cancer Institute (Palo Alto, California) for evaluation and treatment of a presumed stage IIB melanoma on the right preauricular cheek based on a shave biopsy that had been performed (<1 month prior) by his local dermatology provider and subsequently read by an affiliated out-of-state dermatopathology laboratory. Per the clinical history that was gathered at the current presentation, neither the patient nor his wife had noticed the lesion prior to his dermatology provider pointing it out on the day of the biopsy. Additionally, he denied associated pain, bleeding, or ulceration. According to outside medical records, the referring dermatology provider described the lesion as a 4-mm pink pearly papule with telangiectasia favoring a diagnosis of basal cell carcinoma, and a diagnostic shave biopsy was performed. On presentation to our clinic, physical examination of the right preauricular cheek revealed a 4×3-mm depressed erythematous scar with no evidence of residual pigmentation or nodularity (Figure 1). There was no clinically appreciable regional lymphadenopathy.
The original dermatopathology report indicated an invasive melanoma with the following pathologic characteristics: superficial spreading type, Breslow depth of at least 2.16 mm, ulceration, and a mitotic index of 8 mitotic figures/mm2 with transection of the invasive component at the peripheral and deep margins. There was no evidence of regression, perineural invasion, lymphovascular invasion, or microsatellites. Interestingly, the report indicated that there also was a basaloid proliferation with features of cylindroma in the same pathology slide adjacent to the aggressive invasive melanoma that was described. Given the complexity of cases referred to our academic center, the standard of care includes internal dermatopathology review of all outside pathology specimens. This review proved critical to this patient’s care in light of the considerable divergence of the initial pathologic diagnosis and the reported clinical features of the lesion.
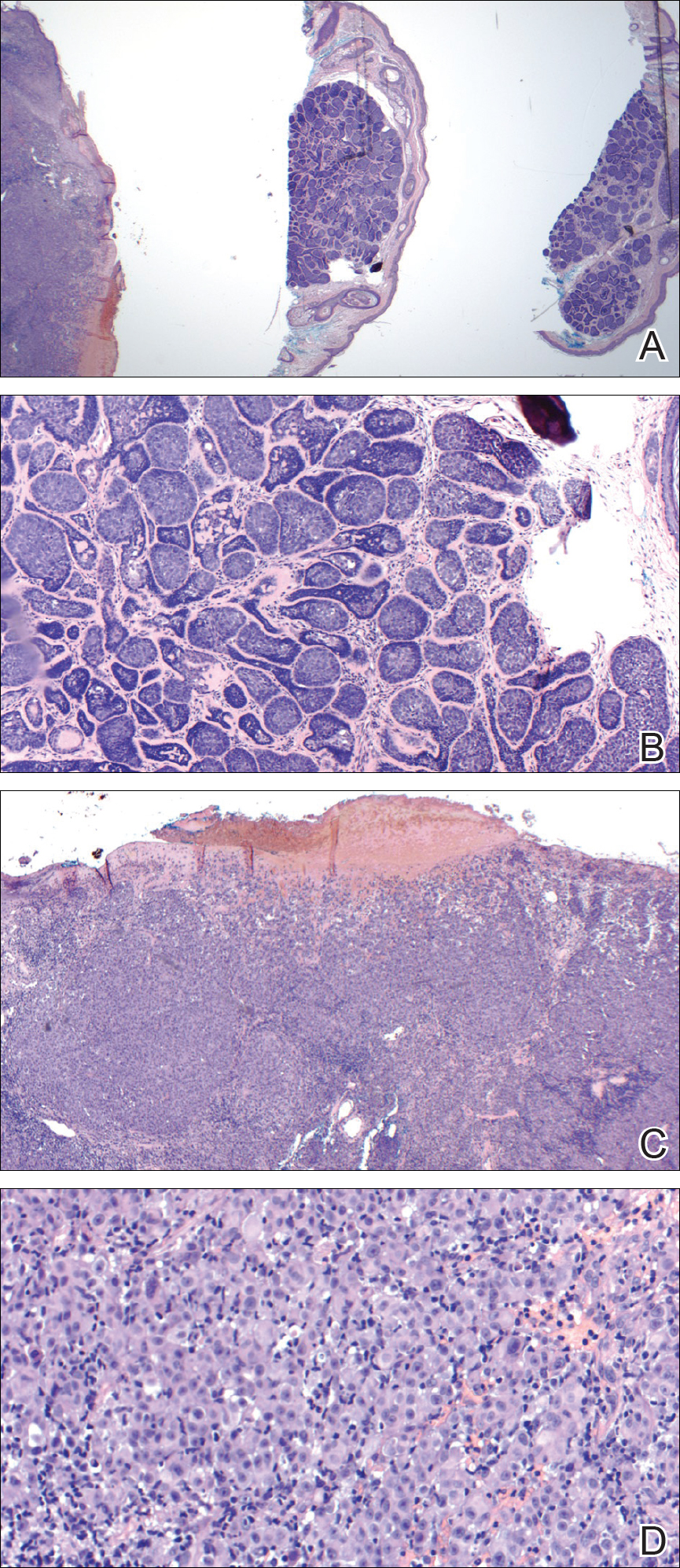
Internal review of the single pathology slide received from the referring provider showed a total of 4 sections, 3 of which are shown here (Figure 2A). Three sections, including the one not shown, were all consistent with a diagnosis of cylindroma and showed no evidence of a melanocytic proliferation (Figure 2B). However, the fourth section demonstrated marked morphologic dissimilarity compared to the other 3 sections. This outlier section showed a thick cutaneous melanoma with a Breslow depth of at least 2.1 mm, ulceration, a mitotic rate of 12 mitotic figures/mm2, and broad transection of the invasive component at the peripheral and deep margins (Figures 2C and 2D). Correlation with the gross description of tissue processing on the original pathology report indicating that the specimen had been trisected raised suspicion that the fourth and very dissimilar section could be a contaminant from another source that was incorporated into our patient’s histologic sections during processing. Taken together, these discrepancies made the diagnosis of cylindroma alone far more likely than cutaneous melanoma, but we needed conclusive evidence given the dramatic difference in prognosis and management between a cylindroma and an aggressive cutaneous melanoma.
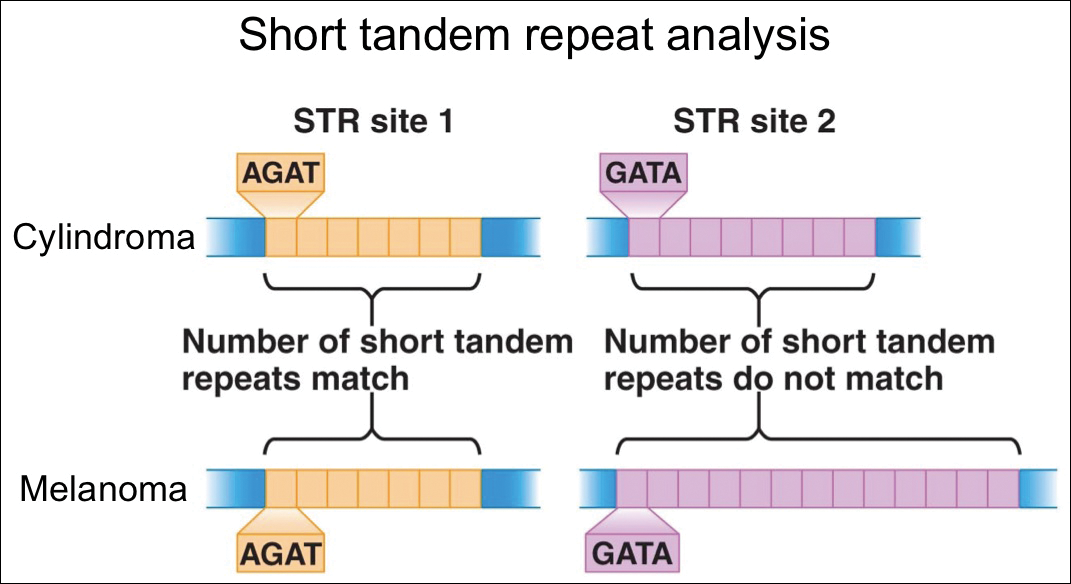
For further diagnostic clarification, we performed polymorphic short tandem repeat (STR) analysis, a well-described forensic pathology technique, to determine if the melanoma and cylindroma specimens derived from different patients, as we hypothesized. This analysis revealed differences in all but one DNA locus tested between the cylindroma specimen and the melanoma specimen, confirming our hypothesis (Figure 3). Subsequent discussion of the case with staff from the dermatopathology laboratory that processed this specimen provided further support for our suspicion that the invasive melanoma specimen was part of a case processed prior to our patient’s benign lesion. Therefore, the wide local excision for treatment of the suspected melanoma fortunately was canceled, and the patient did not require further treatment of the benign cylindroma. The patient expressed relief and gratitude for this critical clarification and change in management.
Comment
Shah et al3 reported a similar case in which a benign granuloma of the lung masqueraded as a squamous cell carcinoma due to histopathologic contamination. Although few similar cases have been described in the literature, the risk posed by such contamination is remarkable, regardless of whether it occurs during specimen grossing, embedding, sectioning, or staining.1,4,5 This risk is amplified in facilities that process specimens originating predominantly from a single organ system or tissue type, as is often the case in dedicated dermatopathology laboratories. In this scenario, it is unlikely that one could use the presence of tissues from 2 different organ systems on a single slide as a way of easily recognizing the presence of a contaminant and rectifying the error. Additionally, the presence of malignant cells in the contaminant further complicates the problem and requires an investigation that can conclusively distinguish the contaminant from the patient’s actual tissue.
In our case, our dermatology and dermatopathology teams partnered with our molecular pathology team to find a solution. Polymorphic STR analysis via polymerase chain reaction amplification is a sensitive method employed commonly in forensic DNA laboratories for determining whether a sample submitted as evidence belongs to a given suspect.6 Although much more commonly used in forensics, STR analysis does have known roles in clinical medicine, such as chimerism testing after bone marrow or allogeneic stem cell transplantation.7 Given the relatively short period of time it takes along with the convenience of commercially available kits, a high discriminative ability, and well-validated interpretation procedures, STR analysis is an excellent method for determining if a given tissue sample came from a given patient, which is what was needed in our case.
The combined clinical, histopathologic, and molecular data in our case allowed for confident clarification of our patient’s diagnosis, sparing him the morbidity of wide local excision on the face, sentinel lymph node biopsy, and emotional distress associated with a diagnosis of aggressive cutaneous melanoma. Our case highlights the critical importance of internal review of pathology specimens in ensuring proper diagnosis and management and reminds us that, though rare, accidental contamination during processing of pathology specimens is a potential adverse event that must be considered, especially when a pathologic finding diverges considerably from what is anticipated based on the patient’s history and physical examination.
Acknowledgment
The authors express gratitude to the patient described herein who graciously provided permission for us to publish his case and clinical photography.
- Gephardt GN, Zarbo RJ. Extraneous tissue in surgical pathology: a College of American Pathologists Q-Probes study of 275 laboratories. Arch Pathol Lab Med. 1996;120:1009-1014.
- Alam M, Shah AD, Ali S, et al. Floaters in Mohs micrographic surgery [published online June 27, 2013]. Dermatol Surg. 2013;39:1317-1322.
- Shah PA, Prat MP, Hostler DC. Benign granuloma masquerading as squamous cell carcinoma due to a “floater.” Hawaii J Med Public Health. 2017;76(11, suppl 2):19-21.
- Platt E, Sommer P, McDonald L, et al. Tissue floaters and contaminants in the histology laboratory. Arch Pathol Lab Med. 2009;133:973-978.
- Layfield LJ, Witt BL, Metzger KG, et al. Extraneous tissue: a potential source for diagnostic error in surgical pathology. Am J Clin Pathol. 2011;136:767-772.
- Butler JM. Forensic DNA testing. Cold Spring Harb Protoc. 2011;2011:1438-1450.
- Manasatienkij C, Ra-ngabpai C. Clinical application of forensic DNA analysis: a literature review. J Med Assoc Thai. 2012;95:1357-1363.
Cross-contamination of pathology specimens is a rare but nonnegligible source of potential morbidity in clinical practice. Contaminant tissue fragments, colloquially referred to as floaters, typically are readily identifiable based on obvious cytomorphologic differences, especially if the tissues arise from different organs; however, one cannot rely on such distinctions in a pathology laboratory dedicated to a single organ system (eg, dermatopathology). The inability to identify quickly and confidently the presence of a contaminant puts the patient at risk for misdiagnosis, which can lead to unnecessary morbidity or even mortality in the case of cancer misdiagnosis. Studies that have been conducted to estimate the incidence of this type of error have suggested an overall incidence rate between approximately 1% and 3%.1,2 Awareness of this phenomenon and careful scrutiny when the histopathologic evidence diverges considerably from the clinical impression is critical for minimizing the negative outcomes that could result from the presence of contaminant tissue. We present a case in which cross-contamination of a pathology specimen led to an initial erroneous diagnosis of an aggressive cutaneous melanoma in a patient with a benign adnexal neoplasm.
Case Report
A 72-year-old man was referred to the Pigmented Lesion and Melanoma Program at Stanford University Medical Center and Cancer Institute (Palo Alto, California) for evaluation and treatment of a presumed stage IIB melanoma on the right preauricular cheek based on a shave biopsy that had been performed (<1 month prior) by his local dermatology provider and subsequently read by an affiliated out-of-state dermatopathology laboratory. Per the clinical history that was gathered at the current presentation, neither the patient nor his wife had noticed the lesion prior to his dermatology provider pointing it out on the day of the biopsy. Additionally, he denied associated pain, bleeding, or ulceration. According to outside medical records, the referring dermatology provider described the lesion as a 4-mm pink pearly papule with telangiectasia favoring a diagnosis of basal cell carcinoma, and a diagnostic shave biopsy was performed. On presentation to our clinic, physical examination of the right preauricular cheek revealed a 4×3-mm depressed erythematous scar with no evidence of residual pigmentation or nodularity (Figure 1). There was no clinically appreciable regional lymphadenopathy.

The original dermatopathology report indicated an invasive melanoma with the following pathologic characteristics: superficial spreading type, Breslow depth of at least 2.16 mm, ulceration, and a mitotic index of 8 mitotic figures/mm2 with transection of the invasive component at the peripheral and deep margins. There was no evidence of regression, perineural invasion, lymphovascular invasion, or microsatellites. Interestingly, the report indicated that there also was a basaloid proliferation with features of cylindroma in the same pathology slide adjacent to the aggressive invasive melanoma that was described. Given the complexity of cases referred to our academic center, the standard of care includes internal dermatopathology review of all outside pathology specimens. This review proved critical to this patient’s care in light of the considerable divergence of the initial pathologic diagnosis and the reported clinical features of the lesion.
Internal review of the single pathology slide received from the referring provider showed a total of 4 sections, 3 of which are shown here (Figure 2A). Three sections, including the one not shown, were all consistent with a diagnosis of cylindroma and showed no evidence of a melanocytic proliferation (Figure 2B). However, the fourth section demonstrated marked morphologic dissimilarity compared to the other 3 sections. This outlier section showed a thick cutaneous melanoma with a Breslow depth of at least 2.1 mm, ulceration, a mitotic rate of 12 mitotic figures/mm2, and broad transection of the invasive component at the peripheral and deep margins (Figures 2C and 2D). Correlation with the gross description of tissue processing on the original pathology report indicating that the specimen had been trisected raised suspicion that the fourth and very dissimilar section could be a contaminant from another source that was incorporated into our patient’s histologic sections during processing. Taken together, these discrepancies made the diagnosis of cylindroma alone far more likely than cutaneous melanoma, but we needed conclusive evidence given the dramatic difference in prognosis and management between a cylindroma and an aggressive cutaneous melanoma.

For further diagnostic clarification, we performed polymorphic short tandem repeat (STR) analysis, a well-described forensic pathology technique, to determine if the melanoma and cylindroma specimens derived from different patients, as we hypothesized. This analysis revealed differences in all but one DNA locus tested between the cylindroma specimen and the melanoma specimen, confirming our hypothesis (Figure 3). Subsequent discussion of the case with staff from the dermatopathology laboratory that processed this specimen provided further support for our suspicion that the invasive melanoma specimen was part of a case processed prior to our patient’s benign lesion. Therefore, the wide local excision for treatment of the suspected melanoma fortunately was canceled, and the patient did not require further treatment of the benign cylindroma. The patient expressed relief and gratitude for this critical clarification and change in management.

Comment
Shah et al3 reported a similar case in which a benign granuloma of the lung masqueraded as a squamous cell carcinoma due to histopathologic contamination. Although few similar cases have been described in the literature, the risk posed by such contamination is remarkable, regardless of whether it occurs during specimen grossing, embedding, sectioning, or staining.1,4,5 This risk is amplified in facilities that process specimens originating predominantly from a single organ system or tissue type, as is often the case in dedicated dermatopathology laboratories. In this scenario, it is unlikely that one could use the presence of tissues from 2 different organ systems on a single slide as a way of easily recognizing the presence of a contaminant and rectifying the error. Additionally, the presence of malignant cells in the contaminant further complicates the problem and requires an investigation that can conclusively distinguish the contaminant from the patient’s actual tissue.
In our case, our dermatology and dermatopathology teams partnered with our molecular pathology team to find a solution. Polymorphic STR analysis via polymerase chain reaction amplification is a sensitive method employed commonly in forensic DNA laboratories for determining whether a sample submitted as evidence belongs to a given suspect.6 Although much more commonly used in forensics, STR analysis does have known roles in clinical medicine, such as chimerism testing after bone marrow or allogeneic stem cell transplantation.7 Given the relatively short period of time it takes along with the convenience of commercially available kits, a high discriminative ability, and well-validated interpretation procedures, STR analysis is an excellent method for determining if a given tissue sample came from a given patient, which is what was needed in our case.
The combined clinical, histopathologic, and molecular data in our case allowed for confident clarification of our patient’s diagnosis, sparing him the morbidity of wide local excision on the face, sentinel lymph node biopsy, and emotional distress associated with a diagnosis of aggressive cutaneous melanoma. Our case highlights the critical importance of internal review of pathology specimens in ensuring proper diagnosis and management and reminds us that, though rare, accidental contamination during processing of pathology specimens is a potential adverse event that must be considered, especially when a pathologic finding diverges considerably from what is anticipated based on the patient’s history and physical examination.
Acknowledgment
The authors express gratitude to the patient described herein who graciously provided permission for us to publish his case and clinical photography.
Cross-contamination of pathology specimens is a rare but nonnegligible source of potential morbidity in clinical practice. Contaminant tissue fragments, colloquially referred to as floaters, typically are readily identifiable based on obvious cytomorphologic differences, especially if the tissues arise from different organs; however, one cannot rely on such distinctions in a pathology laboratory dedicated to a single organ system (eg, dermatopathology). The inability to identify quickly and confidently the presence of a contaminant puts the patient at risk for misdiagnosis, which can lead to unnecessary morbidity or even mortality in the case of cancer misdiagnosis. Studies that have been conducted to estimate the incidence of this type of error have suggested an overall incidence rate between approximately 1% and 3%.1,2 Awareness of this phenomenon and careful scrutiny when the histopathologic evidence diverges considerably from the clinical impression is critical for minimizing the negative outcomes that could result from the presence of contaminant tissue. We present a case in which cross-contamination of a pathology specimen led to an initial erroneous diagnosis of an aggressive cutaneous melanoma in a patient with a benign adnexal neoplasm.
Case Report
A 72-year-old man was referred to the Pigmented Lesion and Melanoma Program at Stanford University Medical Center and Cancer Institute (Palo Alto, California) for evaluation and treatment of a presumed stage IIB melanoma on the right preauricular cheek based on a shave biopsy that had been performed (<1 month prior) by his local dermatology provider and subsequently read by an affiliated out-of-state dermatopathology laboratory. Per the clinical history that was gathered at the current presentation, neither the patient nor his wife had noticed the lesion prior to his dermatology provider pointing it out on the day of the biopsy. Additionally, he denied associated pain, bleeding, or ulceration. According to outside medical records, the referring dermatology provider described the lesion as a 4-mm pink pearly papule with telangiectasia favoring a diagnosis of basal cell carcinoma, and a diagnostic shave biopsy was performed. On presentation to our clinic, physical examination of the right preauricular cheek revealed a 4×3-mm depressed erythematous scar with no evidence of residual pigmentation or nodularity (Figure 1). There was no clinically appreciable regional lymphadenopathy.

The original dermatopathology report indicated an invasive melanoma with the following pathologic characteristics: superficial spreading type, Breslow depth of at least 2.16 mm, ulceration, and a mitotic index of 8 mitotic figures/mm2 with transection of the invasive component at the peripheral and deep margins. There was no evidence of regression, perineural invasion, lymphovascular invasion, or microsatellites. Interestingly, the report indicated that there also was a basaloid proliferation with features of cylindroma in the same pathology slide adjacent to the aggressive invasive melanoma that was described. Given the complexity of cases referred to our academic center, the standard of care includes internal dermatopathology review of all outside pathology specimens. This review proved critical to this patient’s care in light of the considerable divergence of the initial pathologic diagnosis and the reported clinical features of the lesion.
Internal review of the single pathology slide received from the referring provider showed a total of 4 sections, 3 of which are shown here (Figure 2A). Three sections, including the one not shown, were all consistent with a diagnosis of cylindroma and showed no evidence of a melanocytic proliferation (Figure 2B). However, the fourth section demonstrated marked morphologic dissimilarity compared to the other 3 sections. This outlier section showed a thick cutaneous melanoma with a Breslow depth of at least 2.1 mm, ulceration, a mitotic rate of 12 mitotic figures/mm2, and broad transection of the invasive component at the peripheral and deep margins (Figures 2C and 2D). Correlation with the gross description of tissue processing on the original pathology report indicating that the specimen had been trisected raised suspicion that the fourth and very dissimilar section could be a contaminant from another source that was incorporated into our patient’s histologic sections during processing. Taken together, these discrepancies made the diagnosis of cylindroma alone far more likely than cutaneous melanoma, but we needed conclusive evidence given the dramatic difference in prognosis and management between a cylindroma and an aggressive cutaneous melanoma.

For further diagnostic clarification, we performed polymorphic short tandem repeat (STR) analysis, a well-described forensic pathology technique, to determine if the melanoma and cylindroma specimens derived from different patients, as we hypothesized. This analysis revealed differences in all but one DNA locus tested between the cylindroma specimen and the melanoma specimen, confirming our hypothesis (Figure 3). Subsequent discussion of the case with staff from the dermatopathology laboratory that processed this specimen provided further support for our suspicion that the invasive melanoma specimen was part of a case processed prior to our patient’s benign lesion. Therefore, the wide local excision for treatment of the suspected melanoma fortunately was canceled, and the patient did not require further treatment of the benign cylindroma. The patient expressed relief and gratitude for this critical clarification and change in management.

Comment
Shah et al3 reported a similar case in which a benign granuloma of the lung masqueraded as a squamous cell carcinoma due to histopathologic contamination. Although few similar cases have been described in the literature, the risk posed by such contamination is remarkable, regardless of whether it occurs during specimen grossing, embedding, sectioning, or staining.1,4,5 This risk is amplified in facilities that process specimens originating predominantly from a single organ system or tissue type, as is often the case in dedicated dermatopathology laboratories. In this scenario, it is unlikely that one could use the presence of tissues from 2 different organ systems on a single slide as a way of easily recognizing the presence of a contaminant and rectifying the error. Additionally, the presence of malignant cells in the contaminant further complicates the problem and requires an investigation that can conclusively distinguish the contaminant from the patient’s actual tissue.
In our case, our dermatology and dermatopathology teams partnered with our molecular pathology team to find a solution. Polymorphic STR analysis via polymerase chain reaction amplification is a sensitive method employed commonly in forensic DNA laboratories for determining whether a sample submitted as evidence belongs to a given suspect.6 Although much more commonly used in forensics, STR analysis does have known roles in clinical medicine, such as chimerism testing after bone marrow or allogeneic stem cell transplantation.7 Given the relatively short period of time it takes along with the convenience of commercially available kits, a high discriminative ability, and well-validated interpretation procedures, STR analysis is an excellent method for determining if a given tissue sample came from a given patient, which is what was needed in our case.
The combined clinical, histopathologic, and molecular data in our case allowed for confident clarification of our patient’s diagnosis, sparing him the morbidity of wide local excision on the face, sentinel lymph node biopsy, and emotional distress associated with a diagnosis of aggressive cutaneous melanoma. Our case highlights the critical importance of internal review of pathology specimens in ensuring proper diagnosis and management and reminds us that, though rare, accidental contamination during processing of pathology specimens is a potential adverse event that must be considered, especially when a pathologic finding diverges considerably from what is anticipated based on the patient’s history and physical examination.
Acknowledgment
The authors express gratitude to the patient described herein who graciously provided permission for us to publish his case and clinical photography.
- Gephardt GN, Zarbo RJ. Extraneous tissue in surgical pathology: a College of American Pathologists Q-Probes study of 275 laboratories. Arch Pathol Lab Med. 1996;120:1009-1014.
- Alam M, Shah AD, Ali S, et al. Floaters in Mohs micrographic surgery [published online June 27, 2013]. Dermatol Surg. 2013;39:1317-1322.
- Shah PA, Prat MP, Hostler DC. Benign granuloma masquerading as squamous cell carcinoma due to a “floater.” Hawaii J Med Public Health. 2017;76(11, suppl 2):19-21.
- Platt E, Sommer P, McDonald L, et al. Tissue floaters and contaminants in the histology laboratory. Arch Pathol Lab Med. 2009;133:973-978.
- Layfield LJ, Witt BL, Metzger KG, et al. Extraneous tissue: a potential source for diagnostic error in surgical pathology. Am J Clin Pathol. 2011;136:767-772.
- Butler JM. Forensic DNA testing. Cold Spring Harb Protoc. 2011;2011:1438-1450.
- Manasatienkij C, Ra-ngabpai C. Clinical application of forensic DNA analysis: a literature review. J Med Assoc Thai. 2012;95:1357-1363.
- Gephardt GN, Zarbo RJ. Extraneous tissue in surgical pathology: a College of American Pathologists Q-Probes study of 275 laboratories. Arch Pathol Lab Med. 1996;120:1009-1014.
- Alam M, Shah AD, Ali S, et al. Floaters in Mohs micrographic surgery [published online June 27, 2013]. Dermatol Surg. 2013;39:1317-1322.
- Shah PA, Prat MP, Hostler DC. Benign granuloma masquerading as squamous cell carcinoma due to a “floater.” Hawaii J Med Public Health. 2017;76(11, suppl 2):19-21.
- Platt E, Sommer P, McDonald L, et al. Tissue floaters and contaminants in the histology laboratory. Arch Pathol Lab Med. 2009;133:973-978.
- Layfield LJ, Witt BL, Metzger KG, et al. Extraneous tissue: a potential source for diagnostic error in surgical pathology. Am J Clin Pathol. 2011;136:767-772.
- Butler JM. Forensic DNA testing. Cold Spring Harb Protoc. 2011;2011:1438-1450.
- Manasatienkij C, Ra-ngabpai C. Clinical application of forensic DNA analysis: a literature review. J Med Assoc Thai. 2012;95:1357-1363.
Resident Pearl
- Although cross-contamination of pathology specimens is rare, it does occur and can impact diagnosis and management if detected early.
Enhanced Radiation Dermatitis Associated With Concurrent Palliative Radiation and Vemurafenib Therapy
To the Editor:
Vemurafenib is a selective BRAF inhibitor that was approved by the US Food and Drug Administration (FDA) in August 2011 for the treatment of patients with unresectable or metastatic melanoma with the BRAF V600E mutation as detected by an approved test. Both malignant and nonmalignant cutaneous findings have been well documented in association with vemurafenib, including squamous cell carcinoma, keratoacanthomas, UVA photosensitivity, keratosis pilaris–like eruptions, seborrheic dermatitis, follicular plugging, follicular hyperkeratosis, and eruptive melanocytic nevi.1 As more patients with metastatic melanoma are treated with vemurafenib, the use of concomitant palliative or adjuvant radiation therapy with vemurafenib will inevitably occur in greater frequency. Therefore, it is critical to understand the potential cutaneous side effects of this combination.
A predisposition to enhanced radiation dermatitis has been well described with concurrent use of targeted chemotherapies such as the epidermal growth factor receptor inhibitor cetuximab with radiotherapy.2 We report a case of radiation dermatitis occurring shortly after initiating radiation therapy in a patient on vemurafenib.
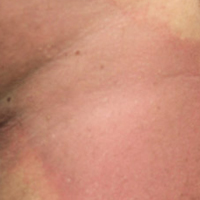
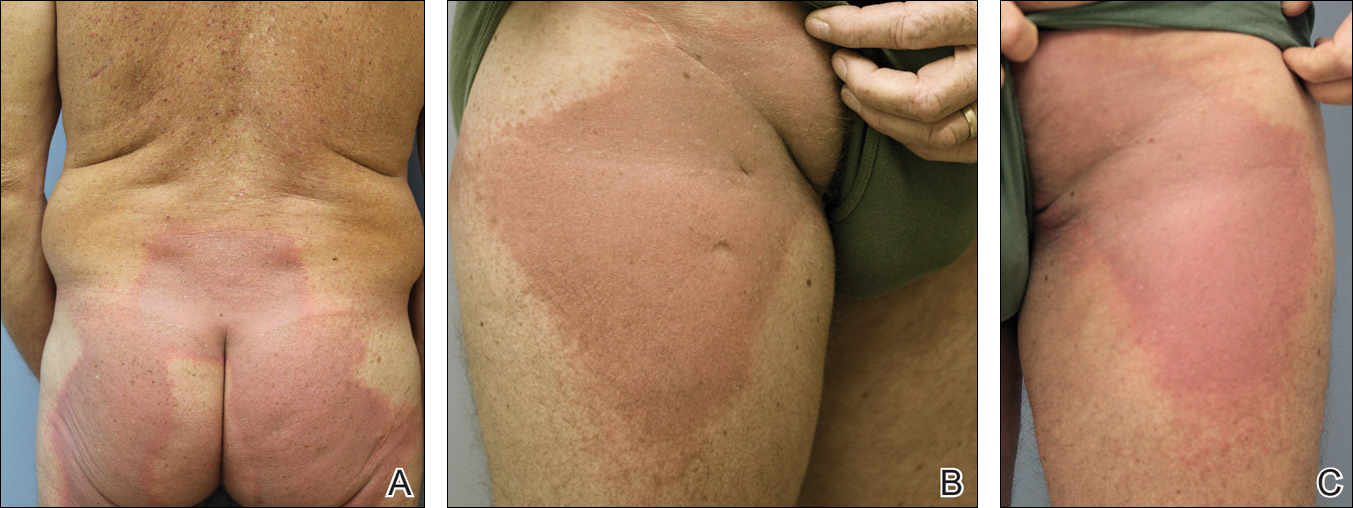
A 53-year-old man with initial stage IIIB melanoma, Breslow depth 2.2 mm with histologic ulceration, and a mitotic index of 2/mm2 on the right buttock underwent wide local excision and sentinel lymph node biopsy followed by complete lymph node dissection with a total of 2 of 10 positive lymph nodes. The patient subsequently underwent 1 year of adjuvant high-dose interferon therapy. Four years after his initial presentation he developed metastases to the lungs, pelvis, and both femurs. He was started on oral vemurafenib 960 mg twice daily. Due to painful bony metastases in the pelvis, the patient also was started on concurrent palliative radiation therapy to both femurs, L5 vertebra, and the sacrum 1 day after initiation of vemurafenib. Three days after initiation of radiation therapy at a cumulative radiation dose of 0.75 Gy, the patient developed severe, painful, well-demarcated, erythematous plaques in the anterior and posterior pelvic distribution overlying the radiation field (Figure 1) that subsequently evolved to eroded desquamative plaques with copious transudate. The patient also developed hyperkeratotic papules on the chest and thighs consistent with the keratosis pilaris–like eruptions associated with vemurafenib therapy.1 Five months later the patient developed worsening neurologic symptoms, and magnetic resonance imaging of the brain revealed multiple brain metastases. Given his disease progression, vemurafenib was discontinued. Ten days later, the patient underwent palliative whole-brain radiation therapy. He received a total dose of 3.25 Gy to the whole brain without any cutaneous sequelae.
The pathophysiology of radiation dermatitis is caused by a dose-dependent loss of basal and endothelial cells following irradiation.3 If surviving basal cells are able to repopulate the basal monolayer, normal skin barrier function is preserved. Dose tolerance is exceeded when cell loss without replacement occurs, resulting in necrosis and clinical evidence of radiation dermatitis, which is characterized by painful erythema or hyperpigmentation followed by desquamation and skin necrosis. In general, occurrence and severity of radiation dermatitis when radiation therapy is used alone in the absence of concurrent chemotherapy is dose dependent, with cutaneous evidence of radiation dermatitis occurring at doses ranging from as low as 2 Gy but most commonly 5 to 10 Gy.4 A report of radiation recall dermatitis in 2 patients who received vemurafenib after completing a full course of radiotherapy5 supports the hypothesis that vemurafenib is a radiosensitizing medication. Enhanced radiation dermatitis was reported in a single case of a patient on vemurafenib who developed radiation dermatitis after completing 3.25 Gy of radiation to the lumbar spine. Although this case likely depicted enhanced radiation dermatitis secondary to concurrent vemurafenib use, it was inconclusive whether vemurafenib contributed to the cutaneous effect, as the patient developed a cutaneous skin reaction 1 week after receiving a cumulative radiation dose of 3.25 Gy, a level at which radiation alone has been shown to cause skin toxicity.6 In our patient, cutaneous manifestations were noted 3 days after initiation of radiation treatment, at which point he had received a total radiation dose of 0.75 Gy, which is well below the threshold commonly recognized to cause radiation-induced skin toxicities. In addition, rechallenge in this patient with higher-dose radiotherapy while off of vemurafenib treatment led to no skin toxicity, despite the common side effects of whole-brain radiation therapy including radiation dermatitis and alopecia.7
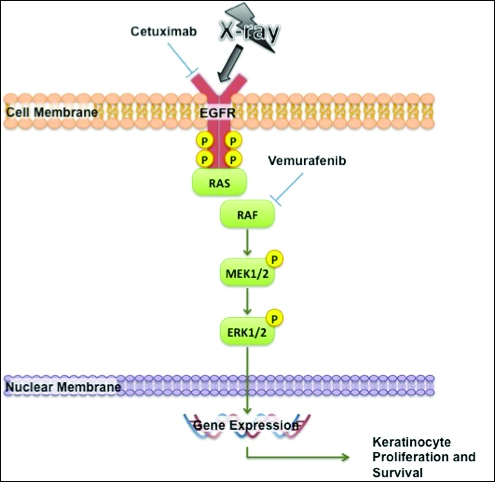
The exact mechanism of increased radiosensitivity caused by targeted chemotherapies such as cetuximab and vemurafenib is unclear. One possible explanation is that the drug interferes with the mitogen-activated protein kinase (MAPK) pathway, which plays a crucial role in controlling cell survival and regeneration following radiation exposure.8 Disruption of this signaling pathway through targeted therapies leads to impaired keratinocyte cell survival and recovery, and thus may enhance susceptibility to radiation-induced skin injury (Figure 2). In vivo studies have demonstrated that the epidermal growth factor receptor is activated following UV irradiation in human keratinocytes, leading to activation of the downstream MAPK signal transduction pathway required for cellular proliferation mediated via the RAF family of proteins.9,10 Further supporting the importance of this pathway in keratinocyte survival and recovery are findings that somatic deletion of BRAF in fibroblasts results in decreased growth factor–induced MAPK activation and enhanced apoptosis,8 whereas activated BRAF has been shown to exert protective effects against oxidative stress as well as tumorigenesis.11 The observation that mutant BRAF melanoma cells demonstrated increased radiosensitivity following BRAF inhibition with vemurafenib12 is consistent with our hypothesis that increased radiosensitivity occurs when signal transduction mediated by MAPK pathway is blocked, thereby inhibiting cell survival. As a result, radiation dermatitis is likely to occur more frequently and at a lower dose when signaling pathways upstream in the MAPK pathway required for keratinocyte regeneration, such as epidermal growth factor receptor and BRAF, are inhibited by targeted therapies. This hypothesis supports the observation that patients on medications that inhibit these signaling pathways, such as cetuximab and vemurafenib, develop enhanced sensitivity to both UV radiation and radiation therapy.
We report a case of enhanced radiation dermatitis occurring at a total dose of 0.75 Gy of radiotherapy, well below the threshold commonly recognized to cause radiation-induced skin toxicities. Our observation suggests that vemurafenib likely acts as a radiosensitizing agent that notably decreases the threshold for radiotherapy-related skin toxicities. Furthermore, the radiosensitizing effect of vemurafenib appears to be transient, as our patient showed no evidence of any skin reaction to subsequent radiation treatment soon after vemurafenib was discontinued. As more patients with metastatic melanoma are treated with vemurafenib, the combination of palliative or adjuvant radiation therapy with vemurafenib will likely be used more frequently. Caution should be exercised in patients on vemurafenib who receive concurrent radiotherapy, even at low radiation doses.
- Huang V, Hepper D, Anadkat M, et al. Cutaneous toxic effects associated with vemurafenib and inhibition of the BRAF pathway. Arch Dermatol. 2012;148:628-633.
- Studer G, Brown M, Dalgueiro E, et al. Grade 3/4 dermatitis in head and neck cancer patients treated with concurrent cetuximab and IMRT. Int J Radiat Oncol Biol Phys. 2011;81:110-117.
- Archambeau JO, Pezner R, Wasserman T. Pathophysiology of irradiated skin and breast. Int J Radiat Oncol Biol Phys. 1995;31:1171-1185.
- Balter S, Hopewell JW, Miller DL, et al. Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology. 2010;254:326-341.
- Boussemart L, Boivin C, Claveau J, et al. Vemurafenib and radiosensitization. JAMA Dermatol. 2013;149:855-857.
- Churilla TM, Chowdhry VK, Pan D, et al. Radiation-induced dermatitis with vemurafenib therapy. Pract Radiat Oncol. 2013;3:e195-e198.
- Anker CJ, Grossmann KF, Atkins MB, et al. Avoiding severe toxicity from combined BRAF inhibitor and radiation treatment: consensus guidelines from the Eastern Cooperative Oncology Group (ECOG). Int J Radiat Oncol Biol Phys. 2016;95:632-646.
- Dent P, Yacoub A, Fisher PB, et al. MAPK pathways in radiation responses. Oncogene. 2003;22:5885-5896.
- Cao C, Lus S, Jiang Q, et al. EGFR activation confers protections against UV-induced apoptosis in cultured mouse skin dendritic cells. Cell Signal. 2008;20:1830-1838.
- Xu Y, Shao Y, Zhou J, et al. Ultraviolet irradiation-induces epidermal growth factor receptor (EGFR) nuclear translocation in human keratinocytes. J Cell Biochem. 2009;107:873-880.
- Valerie K, Yacoub A, Hagan M, et al. Radiation-induced cell signaling: inside-out and outside-in. Mol Cancer Ther. 2007;6:789-801.
- Sambade M, Peters E, Thomas N, et al. Melanoma cells show a heterogeneous range of sensitivity to ionizing radiation and are radiosensitized by inhibition of B-RAF with PLX-4032. Radiother Oncol. 2011;98:394-399.
To the Editor:
Vemurafenib is a selective BRAF inhibitor that was approved by the US Food and Drug Administration (FDA) in August 2011 for the treatment of patients with unresectable or metastatic melanoma with the BRAF V600E mutation as detected by an approved test. Both malignant and nonmalignant cutaneous findings have been well documented in association with vemurafenib, including squamous cell carcinoma, keratoacanthomas, UVA photosensitivity, keratosis pilaris–like eruptions, seborrheic dermatitis, follicular plugging, follicular hyperkeratosis, and eruptive melanocytic nevi.1 As more patients with metastatic melanoma are treated with vemurafenib, the use of concomitant palliative or adjuvant radiation therapy with vemurafenib will inevitably occur in greater frequency. Therefore, it is critical to understand the potential cutaneous side effects of this combination.
A predisposition to enhanced radiation dermatitis has been well described with concurrent use of targeted chemotherapies such as the epidermal growth factor receptor inhibitor cetuximab with radiotherapy.2 We report a case of radiation dermatitis occurring shortly after initiating radiation therapy in a patient on vemurafenib.
A 53-year-old man with initial stage IIIB melanoma, Breslow depth 2.2 mm with histologic ulceration, and a mitotic index of 2/mm2 on the right buttock underwent wide local excision and sentinel lymph node biopsy followed by complete lymph node dissection with a total of 2 of 10 positive lymph nodes. The patient subsequently underwent 1 year of adjuvant high-dose interferon therapy. Four years after his initial presentation he developed metastases to the lungs, pelvis, and both femurs. He was started on oral vemurafenib 960 mg twice daily. Due to painful bony metastases in the pelvis, the patient also was started on concurrent palliative radiation therapy to both femurs, L5 vertebra, and the sacrum 1 day after initiation of vemurafenib. Three days after initiation of radiation therapy at a cumulative radiation dose of 0.75 Gy, the patient developed severe, painful, well-demarcated, erythematous plaques in the anterior and posterior pelvic distribution overlying the radiation field (Figure 1) that subsequently evolved to eroded desquamative plaques with copious transudate. The patient also developed hyperkeratotic papules on the chest and thighs consistent with the keratosis pilaris–like eruptions associated with vemurafenib therapy.1 Five months later the patient developed worsening neurologic symptoms, and magnetic resonance imaging of the brain revealed multiple brain metastases. Given his disease progression, vemurafenib was discontinued. Ten days later, the patient underwent palliative whole-brain radiation therapy. He received a total dose of 3.25 Gy to the whole brain without any cutaneous sequelae.

The pathophysiology of radiation dermatitis is caused by a dose-dependent loss of basal and endothelial cells following irradiation.3 If surviving basal cells are able to repopulate the basal monolayer, normal skin barrier function is preserved. Dose tolerance is exceeded when cell loss without replacement occurs, resulting in necrosis and clinical evidence of radiation dermatitis, which is characterized by painful erythema or hyperpigmentation followed by desquamation and skin necrosis. In general, occurrence and severity of radiation dermatitis when radiation therapy is used alone in the absence of concurrent chemotherapy is dose dependent, with cutaneous evidence of radiation dermatitis occurring at doses ranging from as low as 2 Gy but most commonly 5 to 10 Gy.4 A report of radiation recall dermatitis in 2 patients who received vemurafenib after completing a full course of radiotherapy5 supports the hypothesis that vemurafenib is a radiosensitizing medication. Enhanced radiation dermatitis was reported in a single case of a patient on vemurafenib who developed radiation dermatitis after completing 3.25 Gy of radiation to the lumbar spine. Although this case likely depicted enhanced radiation dermatitis secondary to concurrent vemurafenib use, it was inconclusive whether vemurafenib contributed to the cutaneous effect, as the patient developed a cutaneous skin reaction 1 week after receiving a cumulative radiation dose of 3.25 Gy, a level at which radiation alone has been shown to cause skin toxicity.6 In our patient, cutaneous manifestations were noted 3 days after initiation of radiation treatment, at which point he had received a total radiation dose of 0.75 Gy, which is well below the threshold commonly recognized to cause radiation-induced skin toxicities. In addition, rechallenge in this patient with higher-dose radiotherapy while off of vemurafenib treatment led to no skin toxicity, despite the common side effects of whole-brain radiation therapy including radiation dermatitis and alopecia.7
The exact mechanism of increased radiosensitivity caused by targeted chemotherapies such as cetuximab and vemurafenib is unclear. One possible explanation is that the drug interferes with the mitogen-activated protein kinase (MAPK) pathway, which plays a crucial role in controlling cell survival and regeneration following radiation exposure.8 Disruption of this signaling pathway through targeted therapies leads to impaired keratinocyte cell survival and recovery, and thus may enhance susceptibility to radiation-induced skin injury (Figure 2). In vivo studies have demonstrated that the epidermal growth factor receptor is activated following UV irradiation in human keratinocytes, leading to activation of the downstream MAPK signal transduction pathway required for cellular proliferation mediated via the RAF family of proteins.9,10 Further supporting the importance of this pathway in keratinocyte survival and recovery are findings that somatic deletion of BRAF in fibroblasts results in decreased growth factor–induced MAPK activation and enhanced apoptosis,8 whereas activated BRAF has been shown to exert protective effects against oxidative stress as well as tumorigenesis.11 The observation that mutant BRAF melanoma cells demonstrated increased radiosensitivity following BRAF inhibition with vemurafenib12 is consistent with our hypothesis that increased radiosensitivity occurs when signal transduction mediated by MAPK pathway is blocked, thereby inhibiting cell survival. As a result, radiation dermatitis is likely to occur more frequently and at a lower dose when signaling pathways upstream in the MAPK pathway required for keratinocyte regeneration, such as epidermal growth factor receptor and BRAF, are inhibited by targeted therapies. This hypothesis supports the observation that patients on medications that inhibit these signaling pathways, such as cetuximab and vemurafenib, develop enhanced sensitivity to both UV radiation and radiation therapy.

We report a case of enhanced radiation dermatitis occurring at a total dose of 0.75 Gy of radiotherapy, well below the threshold commonly recognized to cause radiation-induced skin toxicities. Our observation suggests that vemurafenib likely acts as a radiosensitizing agent that notably decreases the threshold for radiotherapy-related skin toxicities. Furthermore, the radiosensitizing effect of vemurafenib appears to be transient, as our patient showed no evidence of any skin reaction to subsequent radiation treatment soon after vemurafenib was discontinued. As more patients with metastatic melanoma are treated with vemurafenib, the combination of palliative or adjuvant radiation therapy with vemurafenib will likely be used more frequently. Caution should be exercised in patients on vemurafenib who receive concurrent radiotherapy, even at low radiation doses.
To the Editor:
Vemurafenib is a selective BRAF inhibitor that was approved by the US Food and Drug Administration (FDA) in August 2011 for the treatment of patients with unresectable or metastatic melanoma with the BRAF V600E mutation as detected by an approved test. Both malignant and nonmalignant cutaneous findings have been well documented in association with vemurafenib, including squamous cell carcinoma, keratoacanthomas, UVA photosensitivity, keratosis pilaris–like eruptions, seborrheic dermatitis, follicular plugging, follicular hyperkeratosis, and eruptive melanocytic nevi.1 As more patients with metastatic melanoma are treated with vemurafenib, the use of concomitant palliative or adjuvant radiation therapy with vemurafenib will inevitably occur in greater frequency. Therefore, it is critical to understand the potential cutaneous side effects of this combination.
A predisposition to enhanced radiation dermatitis has been well described with concurrent use of targeted chemotherapies such as the epidermal growth factor receptor inhibitor cetuximab with radiotherapy.2 We report a case of radiation dermatitis occurring shortly after initiating radiation therapy in a patient on vemurafenib.
A 53-year-old man with initial stage IIIB melanoma, Breslow depth 2.2 mm with histologic ulceration, and a mitotic index of 2/mm2 on the right buttock underwent wide local excision and sentinel lymph node biopsy followed by complete lymph node dissection with a total of 2 of 10 positive lymph nodes. The patient subsequently underwent 1 year of adjuvant high-dose interferon therapy. Four years after his initial presentation he developed metastases to the lungs, pelvis, and both femurs. He was started on oral vemurafenib 960 mg twice daily. Due to painful bony metastases in the pelvis, the patient also was started on concurrent palliative radiation therapy to both femurs, L5 vertebra, and the sacrum 1 day after initiation of vemurafenib. Three days after initiation of radiation therapy at a cumulative radiation dose of 0.75 Gy, the patient developed severe, painful, well-demarcated, erythematous plaques in the anterior and posterior pelvic distribution overlying the radiation field (Figure 1) that subsequently evolved to eroded desquamative plaques with copious transudate. The patient also developed hyperkeratotic papules on the chest and thighs consistent with the keratosis pilaris–like eruptions associated with vemurafenib therapy.1 Five months later the patient developed worsening neurologic symptoms, and magnetic resonance imaging of the brain revealed multiple brain metastases. Given his disease progression, vemurafenib was discontinued. Ten days later, the patient underwent palliative whole-brain radiation therapy. He received a total dose of 3.25 Gy to the whole brain without any cutaneous sequelae.

The pathophysiology of radiation dermatitis is caused by a dose-dependent loss of basal and endothelial cells following irradiation.3 If surviving basal cells are able to repopulate the basal monolayer, normal skin barrier function is preserved. Dose tolerance is exceeded when cell loss without replacement occurs, resulting in necrosis and clinical evidence of radiation dermatitis, which is characterized by painful erythema or hyperpigmentation followed by desquamation and skin necrosis. In general, occurrence and severity of radiation dermatitis when radiation therapy is used alone in the absence of concurrent chemotherapy is dose dependent, with cutaneous evidence of radiation dermatitis occurring at doses ranging from as low as 2 Gy but most commonly 5 to 10 Gy.4 A report of radiation recall dermatitis in 2 patients who received vemurafenib after completing a full course of radiotherapy5 supports the hypothesis that vemurafenib is a radiosensitizing medication. Enhanced radiation dermatitis was reported in a single case of a patient on vemurafenib who developed radiation dermatitis after completing 3.25 Gy of radiation to the lumbar spine. Although this case likely depicted enhanced radiation dermatitis secondary to concurrent vemurafenib use, it was inconclusive whether vemurafenib contributed to the cutaneous effect, as the patient developed a cutaneous skin reaction 1 week after receiving a cumulative radiation dose of 3.25 Gy, a level at which radiation alone has been shown to cause skin toxicity.6 In our patient, cutaneous manifestations were noted 3 days after initiation of radiation treatment, at which point he had received a total radiation dose of 0.75 Gy, which is well below the threshold commonly recognized to cause radiation-induced skin toxicities. In addition, rechallenge in this patient with higher-dose radiotherapy while off of vemurafenib treatment led to no skin toxicity, despite the common side effects of whole-brain radiation therapy including radiation dermatitis and alopecia.7
The exact mechanism of increased radiosensitivity caused by targeted chemotherapies such as cetuximab and vemurafenib is unclear. One possible explanation is that the drug interferes with the mitogen-activated protein kinase (MAPK) pathway, which plays a crucial role in controlling cell survival and regeneration following radiation exposure.8 Disruption of this signaling pathway through targeted therapies leads to impaired keratinocyte cell survival and recovery, and thus may enhance susceptibility to radiation-induced skin injury (Figure 2). In vivo studies have demonstrated that the epidermal growth factor receptor is activated following UV irradiation in human keratinocytes, leading to activation of the downstream MAPK signal transduction pathway required for cellular proliferation mediated via the RAF family of proteins.9,10 Further supporting the importance of this pathway in keratinocyte survival and recovery are findings that somatic deletion of BRAF in fibroblasts results in decreased growth factor–induced MAPK activation and enhanced apoptosis,8 whereas activated BRAF has been shown to exert protective effects against oxidative stress as well as tumorigenesis.11 The observation that mutant BRAF melanoma cells demonstrated increased radiosensitivity following BRAF inhibition with vemurafenib12 is consistent with our hypothesis that increased radiosensitivity occurs when signal transduction mediated by MAPK pathway is blocked, thereby inhibiting cell survival. As a result, radiation dermatitis is likely to occur more frequently and at a lower dose when signaling pathways upstream in the MAPK pathway required for keratinocyte regeneration, such as epidermal growth factor receptor and BRAF, are inhibited by targeted therapies. This hypothesis supports the observation that patients on medications that inhibit these signaling pathways, such as cetuximab and vemurafenib, develop enhanced sensitivity to both UV radiation and radiation therapy.

We report a case of enhanced radiation dermatitis occurring at a total dose of 0.75 Gy of radiotherapy, well below the threshold commonly recognized to cause radiation-induced skin toxicities. Our observation suggests that vemurafenib likely acts as a radiosensitizing agent that notably decreases the threshold for radiotherapy-related skin toxicities. Furthermore, the radiosensitizing effect of vemurafenib appears to be transient, as our patient showed no evidence of any skin reaction to subsequent radiation treatment soon after vemurafenib was discontinued. As more patients with metastatic melanoma are treated with vemurafenib, the combination of palliative or adjuvant radiation therapy with vemurafenib will likely be used more frequently. Caution should be exercised in patients on vemurafenib who receive concurrent radiotherapy, even at low radiation doses.
- Huang V, Hepper D, Anadkat M, et al. Cutaneous toxic effects associated with vemurafenib and inhibition of the BRAF pathway. Arch Dermatol. 2012;148:628-633.
- Studer G, Brown M, Dalgueiro E, et al. Grade 3/4 dermatitis in head and neck cancer patients treated with concurrent cetuximab and IMRT. Int J Radiat Oncol Biol Phys. 2011;81:110-117.
- Archambeau JO, Pezner R, Wasserman T. Pathophysiology of irradiated skin and breast. Int J Radiat Oncol Biol Phys. 1995;31:1171-1185.
- Balter S, Hopewell JW, Miller DL, et al. Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology. 2010;254:326-341.
- Boussemart L, Boivin C, Claveau J, et al. Vemurafenib and radiosensitization. JAMA Dermatol. 2013;149:855-857.
- Churilla TM, Chowdhry VK, Pan D, et al. Radiation-induced dermatitis with vemurafenib therapy. Pract Radiat Oncol. 2013;3:e195-e198.
- Anker CJ, Grossmann KF, Atkins MB, et al. Avoiding severe toxicity from combined BRAF inhibitor and radiation treatment: consensus guidelines from the Eastern Cooperative Oncology Group (ECOG). Int J Radiat Oncol Biol Phys. 2016;95:632-646.
- Dent P, Yacoub A, Fisher PB, et al. MAPK pathways in radiation responses. Oncogene. 2003;22:5885-5896.
- Cao C, Lus S, Jiang Q, et al. EGFR activation confers protections against UV-induced apoptosis in cultured mouse skin dendritic cells. Cell Signal. 2008;20:1830-1838.
- Xu Y, Shao Y, Zhou J, et al. Ultraviolet irradiation-induces epidermal growth factor receptor (EGFR) nuclear translocation in human keratinocytes. J Cell Biochem. 2009;107:873-880.
- Valerie K, Yacoub A, Hagan M, et al. Radiation-induced cell signaling: inside-out and outside-in. Mol Cancer Ther. 2007;6:789-801.
- Sambade M, Peters E, Thomas N, et al. Melanoma cells show a heterogeneous range of sensitivity to ionizing radiation and are radiosensitized by inhibition of B-RAF with PLX-4032. Radiother Oncol. 2011;98:394-399.
- Huang V, Hepper D, Anadkat M, et al. Cutaneous toxic effects associated with vemurafenib and inhibition of the BRAF pathway. Arch Dermatol. 2012;148:628-633.
- Studer G, Brown M, Dalgueiro E, et al. Grade 3/4 dermatitis in head and neck cancer patients treated with concurrent cetuximab and IMRT. Int J Radiat Oncol Biol Phys. 2011;81:110-117.
- Archambeau JO, Pezner R, Wasserman T. Pathophysiology of irradiated skin and breast. Int J Radiat Oncol Biol Phys. 1995;31:1171-1185.
- Balter S, Hopewell JW, Miller DL, et al. Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology. 2010;254:326-341.
- Boussemart L, Boivin C, Claveau J, et al. Vemurafenib and radiosensitization. JAMA Dermatol. 2013;149:855-857.
- Churilla TM, Chowdhry VK, Pan D, et al. Radiation-induced dermatitis with vemurafenib therapy. Pract Radiat Oncol. 2013;3:e195-e198.
- Anker CJ, Grossmann KF, Atkins MB, et al. Avoiding severe toxicity from combined BRAF inhibitor and radiation treatment: consensus guidelines from the Eastern Cooperative Oncology Group (ECOG). Int J Radiat Oncol Biol Phys. 2016;95:632-646.
- Dent P, Yacoub A, Fisher PB, et al. MAPK pathways in radiation responses. Oncogene. 2003;22:5885-5896.
- Cao C, Lus S, Jiang Q, et al. EGFR activation confers protections against UV-induced apoptosis in cultured mouse skin dendritic cells. Cell Signal. 2008;20:1830-1838.
- Xu Y, Shao Y, Zhou J, et al. Ultraviolet irradiation-induces epidermal growth factor receptor (EGFR) nuclear translocation in human keratinocytes. J Cell Biochem. 2009;107:873-880.
- Valerie K, Yacoub A, Hagan M, et al. Radiation-induced cell signaling: inside-out and outside-in. Mol Cancer Ther. 2007;6:789-801.
- Sambade M, Peters E, Thomas N, et al. Melanoma cells show a heterogeneous range of sensitivity to ionizing radiation and are radiosensitized by inhibition of B-RAF with PLX-4032. Radiother Oncol. 2011;98:394-399.
Practice Points
- Given the increased frequency of palliative and adjuvant radiation therapy in patients with metastatic melanoma, it is critical to understand the potential cutaneous side effects of vemurafenib when used in conjunction with radiotherapy.
- Clinicians should be aware of the increased risk for severe radiation dermatitis in patients on vemurafenib who are receiving concurrent palliative radiation therapy.
Update on Melanoma Guidelines: Report From the AAD Meeting
Melanoma was an important topic at multiple sessions of the 73rd Annual Meeting of the American Academy of Dermatology (AAD) in San Francisco, California. Dr. Susan M. Swetter reviews the AAD and National Comprehensive Cancer Network recommendations for biopsy of pigmented suspicious lesions. She also discusses when sentinel lymph node biopsies are recommended and factors that would indicate a patient needs a sentinel lymph node biopsy. Dr. Swetter also outlines surgical margins for melanoma and emphasizes that these are clinical margins taken at the time of surgery, not histologic margins. She concludes with a review of the melanoma subtype lentigo maligna.
Melanoma was an important topic at multiple sessions of the 73rd Annual Meeting of the American Academy of Dermatology (AAD) in San Francisco, California. Dr. Susan M. Swetter reviews the AAD and National Comprehensive Cancer Network recommendations for biopsy of pigmented suspicious lesions. She also discusses when sentinel lymph node biopsies are recommended and factors that would indicate a patient needs a sentinel lymph node biopsy. Dr. Swetter also outlines surgical margins for melanoma and emphasizes that these are clinical margins taken at the time of surgery, not histologic margins. She concludes with a review of the melanoma subtype lentigo maligna.
Melanoma was an important topic at multiple sessions of the 73rd Annual Meeting of the American Academy of Dermatology (AAD) in San Francisco, California. Dr. Susan M. Swetter reviews the AAD and National Comprehensive Cancer Network recommendations for biopsy of pigmented suspicious lesions. She also discusses when sentinel lymph node biopsies are recommended and factors that would indicate a patient needs a sentinel lymph node biopsy. Dr. Swetter also outlines surgical margins for melanoma and emphasizes that these are clinical margins taken at the time of surgery, not histologic margins. She concludes with a review of the melanoma subtype lentigo maligna.