User login
Management of Cardiovascular Disease Risk in Rheumatoid Arthritis
From the Division of Rh
Abstract
- Objective: To review the management of traditional and nontraditional CVD cardiovascular disease risk factors in rheumatoid arthritis (RA).
- Methods: Literature review of the management of CVD risk in RA.
- Results: Because of the increased risk of CVD events and CVD mortality among RA patients, aggressive management of CVD risk is essential. Providers should follow national guidelines for the management of traditional CVD risk factors, including dyslipidemia, hypertension, and diabetes mellitus. Similar efforts are needed in counseling on lifestyle modifications, including smoking cessation, regular exercise, and maintaining a healthy body weight. Because higher RA disease activity is also linked with CVD risk, aggressive treatment of RA to a target of low disease activity or remission is critical. Furthermore, the selection of potentially “cardioprotective” agents such as methotrexate and tumor necrosis factor inhibitors, while limiting use of nonsteroidal anti-inflammatory drugs and glucocorticoids, are strategies that could be employed by rheumatologists to help mitigate CVD risk in their patients with RA.
- Conclusion: Routine assessment of CVD risk, management of traditional CVD risk factors, counseling on healthy lifestyle habits, and aggressive treatment of RA are essential to minimize CVD risk in this population.
Keywords: rheumatoid arthritis; cardiovascular disease; cardiovascular risk assessment; cardiovascular risk management.
Editor’s note: This article is part 2 of a 2-part article. “Assessment of Cardiovascular Disease Risk in Rheumatoid Arthritis” was published in the January/February 2019 issue.
Rheumatoid arthritis (RA) is a systemic autoimmune condition that contributes to an increased risk for cardiovascular disease (CVD) among affected patients. In persons with RA, the risk of incident CVD and CVD mortality are increased by approximately 50% compared with the general population.1,2 To minimize CVD risk in this population, providers must routinely assess for CVD risk factors3 and aggressively manage both traditional and nontraditional CVD risk factors.
Managing Traditional Risk Factors
As in the general population, identification and management of traditional CVD risk factors are crucial to minimize CVD risk in the RA population. A prospective study of 201 RA patients demonstrated that traditional CVD risk factors were in fact more predictive of endothelial dysfunction and carotid atherosclerosis than were disease-related inflammatory markers in RA.4 Management of traditional risk factors is detailed in the following sections, and recommendations for managing all traditional CVD risk factors are summarized in the Table.
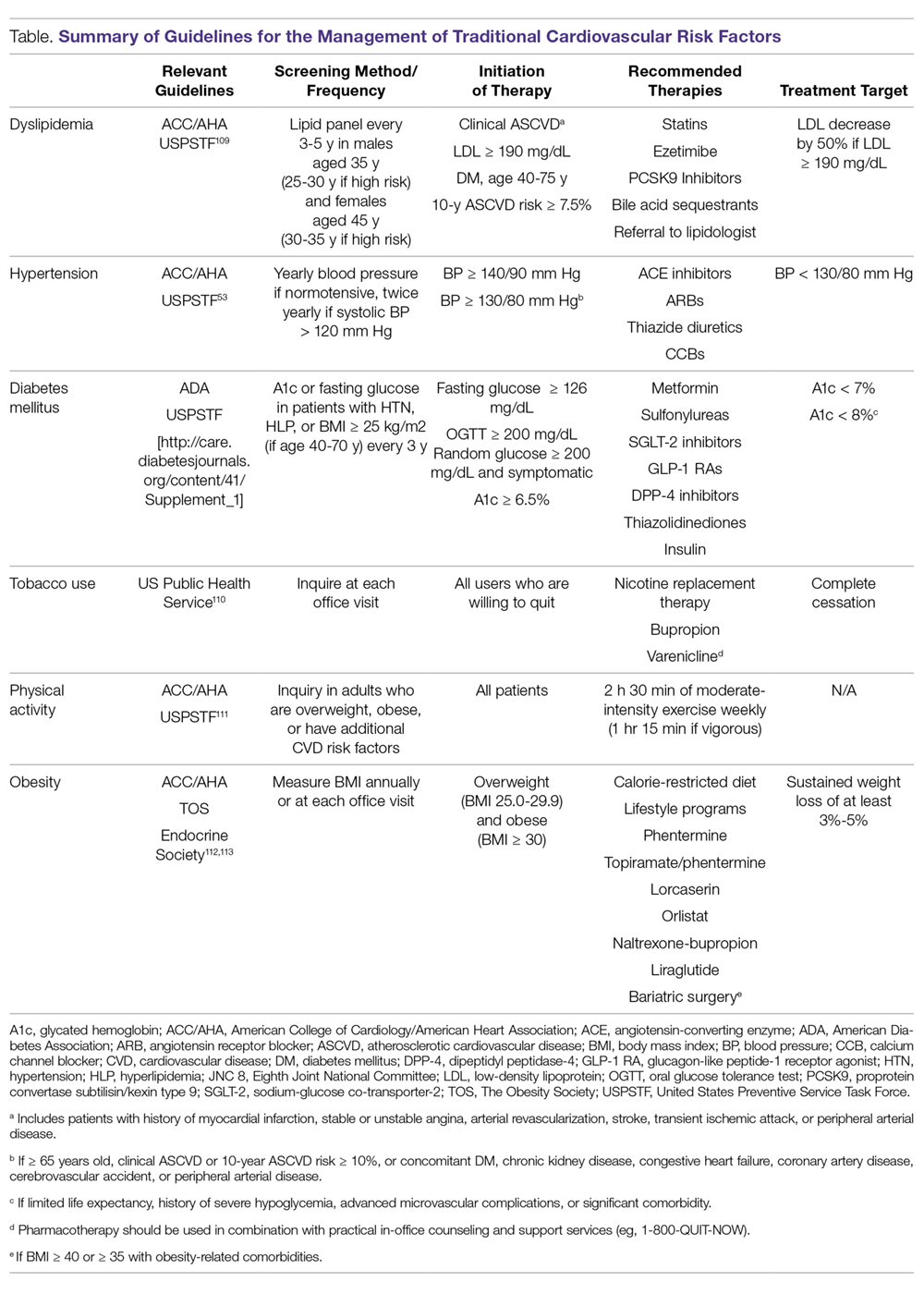
Dyslipidemia
The role of dyslipidemia in atherogenesis is well established, and as a result, lipid levels are nearly universally included in CVD risk stratification tools. However, the interpretation of lipid levels in the context of RA is challenging because of the effects of systemic inflammation on their absolute values. Compared to the general population, patients with RA have lower total cholesterol (TC) and low-density lipoprotein (LDL) levels independent of lipid-lowering therapy.5,6 Despite this, RA patients are at increased risk for CVD. There is even some evidence to suggest a “lipid paradox” in RA, whereby lower TC (< 4 mmol/L) and LDL levels suggest an increased risk of CVD.7,8 In contrast to LDL, higher levels of high-density lipoprotein (HDL) are typically associated with reduced CVD risk, as in the general population.8,9 Interestingly, in a cohort of 16,085 RA patients and 48,499 age- and sex-matched controls, there was no significant difference in the relationship between LDL and CVD risk, suggesting that quantitative lipid levels alone may not entirely explain the CVD mortality gap in RA.9 As such, there is substantial interest in lipoprotein function within the context of CVD risk in RA. Recent investigations have identified impaired HDL function, with reduced cholesterol efflux capacity and antioxidant properties, as well as increased scavenger receptor expression and foam cell formation, in patients with RA.10,11 More research is needed to elucidate how these alterations affect CVD morbidity and mortality and how their measurement could be integrated into improved CVD risk assessment.
Meta-analyses of randomized controlled trials have estimated that lipid-lowering therapy with HMG-CoA reductase inhibitors (statins) reduces the risk of CVD by 25% to 30%; as such, statin therapy has become the standard of care for reduction of CVD risk in the general population.12 Benefits for primary prevention of CVD in RA have also been observed; statin therapy was associated with a reduced risk of CVD events (hazard ratio [HR], 0.45; 95% confidence interval [CI], 0.20-0.98) and all-cause mortality (HR, 0.43; 95% CI, 0.20-0.92) in a population-based cohort study.13 Statins appear to have similar lipid-lowering effects and result in similar CVD risk reduction when used for primary or secondary prevention in RA patients compared to non-RA controls.14-16 Additionally, anti-inflammatory properties of statins may act in synergy with disease-modifying antirheumatic drugs (DMARDs) to improve RA disease activity. In a small study of RA patients, statin therapy improved subjective and objective markers of RA disease activity in conjunction with methotrexate.17
While statins provide robust reduction in CVD risk, some individuals cannot tolerate statin therapy or do not achieve goal LDL levels with statin therapy. Select non-statin LDL-cholesterol-lowering agents have shown promise for reducing CVD events in the general population.18 Ezetimibe, which inhibits cholesterol absorption in the small intestine, very modestly reduced CVD events when added to atorvastatin (relative risk [RR], 0.94; 95% CI, 0.89-0.99) in a double-blind randomized controlled trial.19 Novel monoclonal antibodies to proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibit the internalization of surface LDL receptors, promoting LDL clearance. Two PCSK-9 inhibitors, alirocumab and evolocumab, were approved by the US Food and Drug Administration (FDA) after randomized controlled trials demonstrated their efficacy in lowering LDL by approximately 60% and reducing CVD events by approximately 15% in patients on maximum-tolerated statin therapy.20-22 To date, non-statin LDL-cholesterol-lowering agents have been subject to limited study in RA.23
Identification and management of dyslipidemia offers an opportunity for substantial CVD risk reduction at the RA population level. Unfortunately, current rates of lipid screening are inadequate in this high-risk group. In a study of 3298 Medicare patients with RA, less than half of RA patients with an indication underwent appropriate lipid screening.24 Additionally, statins are often underutilized for both primary and secondary prevention in RA patients. Only 27% of RA patients meeting National Cholesterol Education Program Adult Treatment Panel III criteria were initiated on statin therapy in a population-based cohort study.25 Among patients discharged after a first myocardial infarction (MI), the odds of receiving lipid-lowering therapy were 31% lower for RA patients (odds ratio [OR], 0.69; 95% CI, 0.58-0.82).26 Similar to the general population, adherence to statins in RA patients appears to be poor.27-30 This raises particular concern considering that a population-based cohort study of RA patients demonstrated a 67% increased risk of MI associated with statin discontinuation, regardless of prior MI status.27 Providers—rheumatologists, primary care providers, and cardiologists alike—need to remain vigilant in efforts to assess CVD risk to identify patients who will benefit from lipid-lowering therapy and to emphasize the importance to patients of statin adherence. Novel models of health-care delivery, health technologies, and patient engagement in care may prove useful for improving lipid screening and management in RA.
Tobacco Use
Cigarette smoking is a shared risk factor for both CVD and RA. Large cohort studies have identified a dose-dependent increased risk of incident RA, particularly seropositive RA, among smokers.31-34 Tobacco smoking has also been associated with increased levels of inflammation and RA disease activity.35 The consequences of tobacco use in the general population are staggering. Among individuals over the age of 30 years, tobacco use is responsible for 12% of all deaths and 10% of all CVD deaths.36 Similar findings are observed in RA; a recent meta-analysis estimated there is a 50% increased risk of CVD events in RA related to smoking tobacco.37 In the general population, smoking cessation markedly lowers CVD risk, and over time CVD risk may approach that of nonsmokers.38,39 Thus, regular counseling and interventions to facilitate smoking cessation are critical to reducing CVD risk in RA patients. RA-specific smoking cessation programs have been proposed, but have yet to outperform standard smoking cessation programs.40
Diabetes Mellitus
It is estimated that almost 10% of the US population has diabetes mellitus (DM), which in isolation portends substantial CVD risk.41 There is an increased prevalence of DM in RA, perhaps owing to factors such as physical inactivity and chronic glucocorticoid use, though a higher level of RA disease activity itself has been associated with increased insulin resistance.42-45 In a cohort of 100 RA patients who were neither obese nor diabetic, RA patients had significantly higher fasting blood glucose and insulin levels than age- and sex-matched controls. These findings were even more pronounced in RA patients with higher levels of disease activity.44 Similar to the general population, DM is associated with poor CVD outcomes in RA.37 Therefore, both appropriate management of diabetes and control of RA disease activity are vitally important to minimize CVD risk related to DM.
Hypertension
Though not a universal finding, there may be an increased prevalence of hypertension in RA patients.31,46 Nonsteroidal anti-inflammatory drug (NSAID) and glucocorticoid use may play a role in the development of hypertension, while DMARDs appear to exert a less substantial effect on blood pressure.47,48 At least one study found that DMARD initiation (particularly for methotrexate and hydroxychloroquine) was associated with significant, albeit small, declines in both systolic and diastolic blood pressure over the first 6 months of treatment.49
Despite its potentially higher prevalence in this population, hypertension is both underdiagnosed and undertreated in RA patients.24,50-52 This is an important deficiency to target because, as in the general population, hypertension is associated with an increased risk of MI (RR, 1.84; 95% CI, 1.38-2.46) and composite CVD outcomes (RR, 2.24; 95% CI, 1.42-3.06) in RA.37 Thresholds for initiation and escalation of antihypertensive therapy are not specific to the RA population; thus, diagnosis and management of hypertension should be informed by the American College of Cardiology/American Heart Association guidelines, treating those with in-office blood pressures exceeding 140/90 mm Hg (> 130/80 mm Hg if aged > 65 years or with concomitant CVD, DM, chronic kidney disease, or 10-year atherosclerotic cardiovascular disease risk > 10%), typically with angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, or thiazide diuretics as comorbidities may dictate or allow.53 Also, the use of NSAIDs and glucocorticoids should be minimized, particularly in those with concomitant hypertension.
Physical Activity
Likely due to factors such as articular pain and stiffness, as well as physical limitations, RA patients are more sedentary than the general population.54,55 In a study of objectively assessed sedentary behavior in RA patients, greater average sedentary time per day and greater number of sedentary bouts (> 20 min) were associated with increased 10-year risk of CVD as assessed by the QRISK2.56 Conversely, the beneficial effects of exercise are well documented. Light to moderate physical activity has been associated with improved cardiovascular outcomes, greater physical function, higher levels of HDL, as well as reduced systemic inflammation and disease activity, and improved endothelial function in RA patients.57-61 While there has been concern that physical activity may result in accelerated joint damage, even high-intensity exercise was shown to be safe without causing significant progression of joint damage.58
Obesity, Weight Loss, and Diet
While obesity is clearly associated with CVD risk in the general population, this relationship is much more complex in RA, as underweight RA patients are also at higher risk for CVD and CVD-related mortality.62-64 One potential explanation for this finding is that pathological weight loss resulting in an underweight body mass index (BMI) is an independent predictor of CVD. In a study of US Veterans with RA, higher rates of weight loss (> 3 kg/m2/year) were associated with increased CVD mortality (HR, 2.27; 95% CI, 1.61-3.19) independent of BMI.65 Systemic inflammation in RA can lead to “rheumatoid cachexia,” characterized by decreased muscle mass, increased adiposity, and increased CVD risk despite a normal or potentially decreased BMI.66 Practitioners should be mindful of not only current body weight, but also patients’ weight trajectories when counseling on lifestyle practices such as healthy diet and regular exercise in RA patients. For obese individuals with RA, healthy weight loss should be encouraged. Interestingly, bariatric surgery in RA patients may improve RA disease activity in addition to its known effects on body weight and DM.67
Counseling on healthy diet with a focus on limiting foods high in saturated- and trans-fatty acids and high glycemic index foods, and increasing consumption of fruits, vegetables, and mono-unsaturated fatty acids is a well-accepted and common practice to help minimize CVD risk in the general population.68 No studies to date have investigated the effect of specific diets on CVD risk in RA patients, and thus we recommend adherence to general population recommendations.
Managing RA-related CVD Risk Factors
Disease Activity
In addition to traditional risk factors, several studies have identified associations between the level of RA disease activity and risk of CVD. In a cohort of US Veterans with RA, CVD-related mortality increased in a dose-dependent manner with higher disease activity categories. In stark contrast, the CVD mortality rates of those in remission paralleled the rates from the general population (standardized mortality ratio [SMR], 0.68; 95% CI, 0.37-1.27).69 In a separate cohort of 1157 RA patients without prior CVD, achieving low disease activity was associated with a lower risk of incident CVD events (HR, 0.65; 95% CI, 0.43-0.99).70 Additionally, high disease activity has been associated with surrogate markers of CVD and other CVD risk factors including NT-proBNP and systolic blood pressure.71,72 While no randomized controlled trial data is available to inform this recommendation, observational data suggest RA should be aggressively treated (ideally to achieve and maintain remission or low disease activity) to minimize CVD risk. While keeping this treatment goal in mind, the differential effects of specific RA therapies on CVD must also be considered.
Glucocorticoids and NSAIDs
With the expanding repertoire of DMARDs available and more aggressive treatment approaches, the role of glucocorticoids and NSAIDs in RA treatment is decreasing over time. While their efficacy for improving pain and stiffness is well established, concern regarding their contribution to CVD risk in RA patients is warranted.
Glucocorticoids are known to have detrimental effects on traditional CVD risk factors such as hypertension, insulin resistance, and dyslipidemia in the general population, as well as in RA patients.73,74 In a meta-analysis of predominantly observational studies of RA patients, glucocorticoid use was associated with an increased risk of CVD events (RR, 1.47; 95% CI, 1.34-1.60), including MI, congestive heart failure (CHF), and cerebrovascular accident (CVA).75 Evidence is conflicting in regards to a clear dose threshold that leads to increased CVD risk with glucocorticoids, though higher doses are associated with greater risk.76-81 As RA patients requiring glucocorticoids typically have higher disease activity, confounding by indication remains a complicating factor in assessing the relative contributions of glucocorticoid use and RA disease activity to elevated CVD risk in many analyses.
The increased CVD risk with NSAID use is not specific to RA and has been well established in the general population.82-84 In the previously mentioned meta-analysis, an increased overall risk of CVD events was observed with NSAID use in RA (RR, 1.18; 95% CI, 1.01-1.38). It should be noted that cyclo-oxygenase 2 (COX-2) inhibitors, in particular rofecoxib (now removed from the market), appeared to drive the majority of this risk (RR, 1.36; 95% CI, 1.10-1.67 in COX-2 inhibitors and RR 1.08, 95% CI, 0.94-1.24 in nonselective NSAIDs), suggesting a potential differential risk among NSAIDs.75 While naproxen has been thought to carry the lowest risk of CVD based on initial studies, this has not been universally observed, including in a recent randomized controlled trial of more than 24,000 RA and osteoarthritis patients.82,85,86
Providers should use the lowest possible dose and duration of glucocorticoids and NSAIDs to achieve symptom relief, with continual efforts to taper or discontinue. Candidates for glucocorticoid and NSAID therapy should be selected carefully, and use of these therapies should be avoided in those with prior CVD or at high risk for CVD based on traditional CVD risk factors. Most importantly, providers should focus on utilizing DMARDs for the management of RA, which more effectively treat RA as well as reduce CVD risk.
Methotrexate
Methotrexate (MTX), a mainstay in the treatment of RA, is a conventional DMARD observed to improve overall survival and mitigate CVD risk in multiple RA cohorts.75,87,88 In a recent meta-analysis comprised of 236,525 RA patients and 5410 CVD events, MTX use was associated with a 28% reduction in overall CVD events across 8 studies (RR, 0.72; 95% CI, 0.57-0.91), substantiating similar findings in a prior meta-analysis.75,88 MTX use was specifically associated with a decreased risk of MI (RR, 0.81; 95% CI, 0.68-0.96). Case-control and cohort studies have cited a 20% to 50% reduced risk of CHF with MTX use.89,90 The potential cardioprotective effect of MTX appears to be both multifactorial and complex, likely mediated through both direct and indirect mechanisms. MTX directly promotes anti-atherogenic lipoprotein function, improves endothelial function, and scavenges free radicals.91,92 Indirectly, MTX likely reduces CVD risk by effectively reducing RA disease activity. Based on these and other data, MTX remains the cornerstone of DMARD therapy in RA patients when targeting CVD risk reduction.
Hydroxychloroquine
Emerging evidence suggests that hydroxychloroquine (HCQ), an antimalarial most often utilized in combination with alternative DMARDs in RA, prevents DM and has beneficial effects on lipid profiles. A recent meta-analysis compiled 3 homogenous observational studies that investigated the effect of HCQ on incident DM. RA patients ever exposed to HCQ had a 40% lower incidence of DM (HR, 0.59; 95% CI, 0.49-0.70).93 Increased duration of HCQ use was shown to further reduce risk of incident DM.94 The aforementioned meta-analysis also pooled 5 studies investigating the effect of HCQ on lipid profiles, with favorable mean differences in TC (–9.82 mg/dL), LDL (–10.61 mg/dL), HDL (4.13 mg/dL), and triglycerides (–19.15 mg/dL) in HCQ users compared to non-users.93 Given these favorable changes to traditional CVD risk factors, it is not surprising that in a retrospective study of 1266 RA patients without prior CVD, HCQ was associated with significantly lower risk of incident CVD. While external validation of these findings is needed, HCQ is an attractive conventional DMARD to be used in RA for CVD risk reduction. Moreover, its combination with MTX and sulfasalazine also shows promise for CVD risk reduction.95,96
TNF Inhibitors
Tumor necrosis factor (TNF) inhibitors are often the initial biologic DMARD therapy used in RA patients not responding to conventional DMARDs. In the previously described meta-analysis, TNF inhibitors were associated with similar reductions in CVD events as MTX (RR, 0.70; 95% CI, 0.54-0.90).75 Of note, there was a trend toward reduced risk of CHF (RR, 0.75; 95% CI, 0.49-1.15) in this same meta-analysis, an area of concern with TNF inhibitor use due to a prior randomized controlled trial demonstrating worsening clinical status in patients with existing moderate-to-severe CHF treated with high-dose infliximab.97 Current RA treatment guidelines recommend avoiding TNF inhibitor use in individuals with CHF.98
Aside from the risk of CHF exacerbation, TNF inhibitors appear to be cardioprotective. Similar to MTX, the mechanism by which TNF inhibition reduces cardiovascular risk is complex and likely due to both direct and indirect mechanisms. Substantial research has been conducted on the effect of TNF inhibition on lipids, with a recent meta-analysis demonstrating increases in HDL and TC, with stable LDL and atherogenic index over treatment follow-up.99 A subsequent meta-analysis not limited to RA patients yielded similar results.100 In addition to quantitative lipid changes, alteration of lipoprotein function, improvement in myocardial function, reduced aortic stiffness, improved blood pressure, and reduced RA disease activity may also be responsible for cardioprotective benefits of these agents.101,102
Non-TNF Biologic and Traditional Synthetic DMARDs
Tocilizumab, an IL-6 inhibitor, can potently increase LDL levels, but it does not appear to increase the risk of CVD events and may actually promote more favorable anti-atherogenic lipoprotein function.103-106 Although these quantitative lipid changes received significant attention in the wake of early reports detailing this effect, similar lipid changes appear to accompany other DMARDs including TNF inhibitors and tofacitinib.107 There have been few studies evaluating the risk of CVD with other non-TNF inhibitor biologic DMARDs and traditional synthetic DMARDs, warranting future study.
Conclusion
To mitigate the increased risk of CVD in RA, primary care and subspecialty providers alike must be aware of this heightened risk in RA, perform frequent assessments of CVD risk,3 and aggressively manage both traditional and nontraditional CVD risk factors. The differential roles in this effort may not be clear; thus, we have proposed a co-management strategy detailed in the Figure. Clear communication between providers is of the utmost importance to ensure effective management of CVD risk.
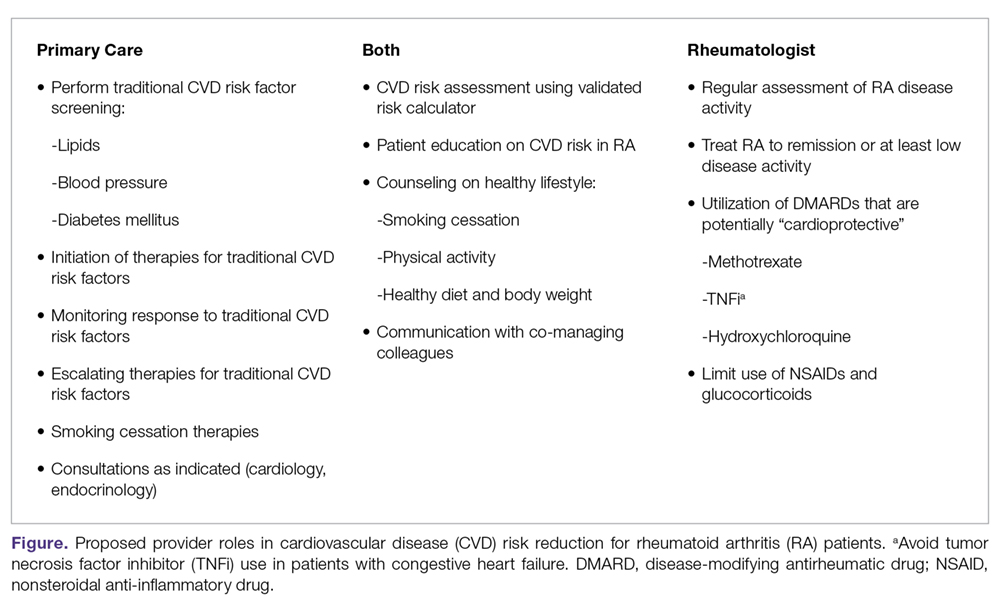
Given limited evidence for RA-specific CVD risk assessments and traditional risk factor treatment targets, management should follow pertinent national guidelines. The importance of lifestyle counseling should not be overlooked, with a focus on smoking cessation, healthy diet and body weight, and regular aerobic exercise. Finally, rheumatologists should aggressively manage RA using a treat-to-target approach, minimize the use of glucocorticoids and NSAIDs, and preferentially select DMARDs that have been associated with lower CVD risk. Through this comprehensive approach, recent trends of improved CVD outcomes in RA will hopefully become more widespread.108
Corresponding author: Bryant R. England, MD; 986270 Nebraska Medical Center, Omaha, NE 68198-6270; [email protected].
Financial disclosures: Dr. England is supported by UNMC Internal Medicine Scientist Development Award, UNMC Physician-Scientist Training Program, the UNMC Mentored Scholars Program, and the Rheumatology Research Foundation Scientist Development Award. Dr. Mikuls is supported by a VA Merit Award (CX000896) and grants from the National Institutes of Health: National Institute of General Medical Sciences (U54GM115458), National Institute on Alcohol Abuse and Alcoholism (R25AA020818), and National Institute of Arthritis and Musculoskeletal and Skin Diseases (2P50AR60772).
1. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
2. Avina-Zubieta JA, Thomas J, Sadatsafavi M, et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann Rheum Dis. 2012;71:1524-1529.
3. Johnson TM, Mikuls TR, England BR. Assessment of cardiovascular risk in rheumatoid arthritis. J Clin Outcomes Manage. 2019;26:41-47.
4. Sandoo A, Chanchlani N, Hodson J, et al. Classical cardiovascular disease risk factors associate with vascular function and morphology in rheumatoid arthritis: A six-year prospective study. Arthritis Res Ther. 2013;15:R203.
5. Myasoedova E, Crowson CS, Kremers HM, et al. Total cholesterol and LDL levels decrease before rheumatoid arthritis. Ann Rheum Dis. 2010;69:1310-1314.
6. Liao KP, Cai T, Gainer VS, et al. Lipid and lipoprotein levels and trend in rheumatoid arthritis compared to the general population. Arthritis Care Res (Hoboken). 2013;65:2046-2050.
7. Myasoedova E, Crowson CS, Kremers HM, et al. Lipid paradox in rheumatoid arthritis: The impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis. 2011;70:482-487.
8. Zhang J, Chen L, Delzell E, et al. Republished: The association between inflammatory markers, serum lipids and the risk of cardiovascular events in patients with rheumatoid arthritis. Postgrad Med J. 2014;90:722-729.
9. Liao KP, Liu J, Lu B, et al. Association between lipid levels and major adverse cardiovascular events in rheumatoid arthritis compared to non-rheumatoid arthritis patients. Arthritis Rheumatol. 2015;67:2004-2010.
10. Charles-Schoeman C, Lee YY, Grijalva V, et al. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann Rheum Dis. 2012;71:1157-1162.
11. Voloshyna I, Modayil S, Littlefield MJ, et al. Plasma from rheumatoid arthritis patients promotes pro-atherogenic cholesterol transport gene expression in THP-1 human macrophages. Exp Biol Med (Maywood). 2013 238:1192-1197.
12. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;(1):CD004816.
13. Sheng X, Murphy MJ, Macdonald TM, Wei L. Effectiveness of statins on total cholesterol and cardiovascular disease and all-cause mortality in osteoarthritis and rheumatoid arthritis. J Rheumatol. 2012;39:32-40.
14. An J, Alemao E, Reynolds K, et al. Cardiovascular outcomes associated with lowering low-density lipoprotein cholesterol in rheumatoid arthritis and matched nonrheumatoid arthritis. J Rheumatol. 2016;43:1989-1996.
15. Semb AG, Holme I, Kvien TK, Pedersen TR. Intensive lipid lowering in patients with rheumatoid arthritis and previous myocardial infarction: An explorative analysis from the incremental decrease in endpoints through aggressive lipid lowering (IDEAL) trial. Rheumatology (Oxford). 2011;50:324-329.
16. Semb AG, Kvien TK, DeMicco DA, et al. Effect of intensive lipid-lowering therapy on cardiovascular outcome in patients with and those without inflammatory joint disease. Arthritis Rheum. 2012;64:2836-2846.
17. El-Barbary AM, Hussein MS, Rageh EM, et al. Effect of atorvastatin on inflammation and modification of vascular risk factors in rheumatoid arthritis. J Rheumatol. 2011;38:229-235.
18. Writing Committee, Lloyd-Jones DM, Morris PB, et al. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: A report of the American college of cardiology task force on clinical expert consensus documents. J Am Coll Cardiol. 2016;68:92-125.
19. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
20. Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509.
21. Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499.
22. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
23. Maki-Petaja KM, Booth AD, Hall FC, et al. Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. J Am Coll Cardiol. 2007;50:852-858.
24. Bartels CM, Kind AJ, Everett C, et al. Low frequency of primary lipid screening among medicare patients with rheumatoid arthritis. Arthritis Rheum. 2011;63:1221-1230.
25. Akkara Veetil BM, Myasoedova E, Matteson EL, et al. Use of lipid-lowering agents in rheumatoid arthritis: A population-based cohort study. J Rheumatol. 2013;40:1082-1088.
26. Lindhardsen J, Ahlehoff O, Gislason GH, et al. Initiation and adherence to secondary prevention pharmacotherapy after myocardial infarction in patients with rheumatoid arthritis: A nationwide cohort study. Ann Rheum Dis. 2012;71:1496-1501.
27. De Vera MA, Choi H, Abrahamowicz M, et al. Statin discontinuation and risk of acute myocardial infarction in patients with rheumatoid arthritis: A population-based cohort study. Ann Rheum Dis. 2011;70:1020-1024.
28. Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: A cohort study. Ann Intern Med. 2013;158:526-534.
29. Zhang H, Plutzky J, Shubina M, Turchin A. Continued statin prescriptions after adverse reactions and patient outcomes: A cohort study. Ann Intern Med. 2017;167:221-227.
30. Lemstra M, Blackburn D, Crawley A, Fung R. Proportion and risk indicators of nonadherence to statin therapy: A meta-analysis. Can J Cardiol. 2012;28:574-580.
31. Boyer JF, Gourraud PA, Cantagrel A, et al. Traditional cardiovascular risk factors in rheumatoid arthritis: A meta-analysis. Joint Bone Spine. 2011;78:179-183.
32. Bergstrom U, Jacobsson LT, Nilsson JA, et al. Pulmonary dysfunction, smoking, socioeconomic status and the risk of developing rheumatoid arthritis. Rheumatology (Oxford). 2011;50:2005-2013.
33. Costenbader KH, Feskanich D, Mandl LA, Karlson EW. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am J Med. 2006;119:503.e1,503.e9.
34. Klareskog L, Stolt P, Lundberg K, et al. A new model for an etiology of rheumatoid arthritis: Smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54:38-46.
35. Sokolove J, Wagner CA, Lahey LJ, et al. Increased inflammation and disease activity among current cigarette smokers with rheumatoid arthritis: A cross-sectional analysis of US veterans. Rheumatology (Oxford). 2016;55:1969-1977.
36. World Health Organization. WHO Global Report: Mortality Attributable to Tobacco. Geneva, World Health Organization, 2012.
37. Baghdadi LR, Woodman RJ, Shanahan EM, Mangoni AA. The impact of traditional cardiovascular risk factors on cardiovascular outcomes in patients with rheumatoid arthritis: A systematic review and meta-analysis. PLoS One. 2015;10:e0117952.
38. Centers for Disease Control and Prevention; National Center for Chronic Disease Prevention and Health Promotion. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention; 2010. 6, Cardiovascular Diseases. Available from: https://ncbi.nlm.nih.gov/books/NBK53012/
39. Mons U, Muezzinler A, Gellert C, et al. Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: Meta-analysis of individual participant data from prospective cohort studies of the CHANCES consortium. BMJ. 2015;350:h1551.
40. Aimer P, Treharne GJ, Stebbings S, Frampton C, Cameron V, Kirby S, et al. Efficacy of a rheumatoid arthritis-specific smoking cessation program: A randomized controlled pilot trial. Arthritis Care Res (Hoboken). 2017;69:28-37.
41. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2017.
42. Jiang P, Li H, Li X. Diabetes mellitus risk factors in rheumatoid arthritis: A systematic review and meta-analysis. Clin Exp Rheumatol. 2015;33:115-121.
43. Shahin D, Eltoraby E, Mesbah A, Houssen M. Insulin resistance in early untreated rheumatoid arthritis patients. Clin Biochem. 2010;43:661-335.
44. Arias de la Rosa I, Escudero-Contreras A, Rodriguez-Cuenca S, et al. Defective glucose and lipid metabolism in rheumatoid arthritis is determined by chronic inflammation in metabolic tissues. J Intern Med. 2018;84(1):61-77.
45. Wilson JC, Sarsour K, Gale S, et al. Incidence and risk of glucocorticoid-associated adverse effects in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2018 Jun 1. doi: 10.1002/acr.23611.
46. Chung CP, Giles JT, Petri M, et al. Prevalence of traditional modifiable cardiovascular risk factors in patients with rheumatoid arthritis: Comparison with control subjects from the multi-ethnic study of atherosclerosis. Semin Arthritis Rheum. 2012;41:535-544.
47. Goodwin JE, Geller DS. Glucocorticoid-induced hypertension. Pediatr Nephrol. 2012;27:1059-1066.
48. Snowden S, Nelson R. The effects of nonsteroidal anti-inflammatory drugs on blood pressure in hypertensive patients. Cardiol Rev. 2011;19:184-191.
49. Baker JF, Sauer B, Teng CC, et al. Initiation of disease-modifying therapies in rheumatoid arthritis is associated with changes in blood pressure. J Clin Rheumatol. 2018;24:203-209.
50. Panoulas VF, Douglas KM, Milionis HJ, et al. Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology (Oxford). 2007;46:1477-1482.
51. Protogerou AD, Panagiotakos DB, Zampeli E, et al. Arterial hypertension assessed “out-of-office” in a contemporary cohort of rheumatoid arthritis patients free of cardiovascular disease is characterized by high prevalence, low awareness, poor control and increased vascular damage-associated “white coat” phenomenon. Arthritis Res Ther. 2013;15:R142.
52. van Breukelen-van der Stoep DF, van Zeben D, Klop B, et al. Marked underdiagnosis and undertreatment of hypertension and hypercholesterolaemia in rheumatoid arthritis. Rheumatology (Oxford). 2016;55:1210-1216.
53. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71:e127-248.
54. Lee J, Dunlop D, Ehrlich-Jones L, et al. Public health impact of risk factors for physical inactivity in adults with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:488-493.
55. Sokka T, Hakkinen A, Kautiainen H, et al. Physical inactivity in patients with rheumatoid arthritis: Data from twenty-one countries in a cross-sectional, international study. Arthritis Rheum. 2008;59:42-50.
56. Fenton SAM, Veldhuijzen van Zanten JJCS, Kitas GD, et al. Sedentary behaviour is associated with increased long-term cardiovascular risk in patients with rheumatoid arthritis independently of moderate-to-vigorous physical activity. BMC Musculoskelet Disord. 2017;18:131,017-1473-9.
57. Byram KW, Oeser AM, Linton MF, et al. Exercise is associated with increased small HDL particle concentration and decreased vascular stiffness in rheumatoid arthritis. J Clin Rheumatol. 2018 May 25. 9.
58. de Jong Z, Munneke M, Zwinderman AH, et al. Is a long-term high-intensity exercise program effective and safe in patients with rheumatoid arthritis? results of a randomized controlled trial. Arthritis Rheum. 2003;48:2415-2424.
59. Stavropoulos-Kalinoglou A, Metsios GS, Veldhuijzen van Zanten JJ, et al. Individualised aerobic and resistance exercise training improves cardiorespiratory fitness and reduces cardiovascular risk in patients with rheumatoid arthritis. Ann Rheum Dis. 2013;72:1819-1825.
60. Khoja SS, Almeida GJ, Chester Wasko M, et al. Association of light-intensity physical activity with lower cardiovascular disease risk burden in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:424-431.
61. Metsios GS, Koutedakis Y, Veldhuijzen van Zanten JJ, et al. Cardiorespiratory fitness levels and their association with cardiovascular profile in patients with rheumatoid arthritis: A cross-sectional study. Rheumatology (Oxford). 2015;54:2215-2220.
62. Escalante A, Haas RW, del Rincon I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: Role of comorbidity and systemic inflammation. Arch Intern Med. 2005;165:1624-1629.
63. Kremers HM, Nicola PJ, Crowson CS, et al. Prognostic importance of low body mass index in relation to cardiovascular mortality in rheumatoid arthritis. Arthritis Rheum. 2004;50:3450-3457.
64. Wolfe F, Michaud K. Effect of body mass index on mortality and clinical status in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:1471-1479.
65. England BR, Baker JF, Sayles H, et al. Body mass index, weight loss, and cause-specific mortality in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2018;70:11-18.
66. Dessein PH, Solomon A, Hollan I. Metabolic abnormalities in patients with inflammatory rheumatic diseases. Best Pract Res Clin Rheumatol. 2016;30:901-915.
67. Sparks JA, Halperin F, Karlson JC, et al. Impact of bariatric surgery on patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2015;67:1619-1626.
68. Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 2009;169:659-669.
69. England BR, Sayles H, Michaud K, et al. Cause-specific mortality in male US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:36-45.
70. Arts EE, Fransen J, Den Broeder AA, et al. Low disease activity (DAS28≤3.2) reduces the risk of first cardiovascular event in rheumatoid arthritis: a time-dependent Cox regression analysis in a large cohort study. Ann Rheum Dis. 2017;76(10):1693-1699.
71. Provan SA, Semb AG, Hisdal J, et al. Remission is the goal for cardiovascular risk management in patients with rheumatoid arthritis: A cross-sectional comparative study. Ann Rheum Dis. 2011;70:812-817.
72. Klarenbeek NB, van der Kooij SM, Huizinga TJ, et al. Blood pressure changes in patients with recent-onset rheumatoid arthritis treated with four different treatment strategies: A post hoc analysis from the BeSt trial. Ann Rheum Dis. 2010;69:1342-1345.
73. Hafstrom I, Rohani M, Deneberg S, et al. Effects of low-dose prednisolone on endothelial function, atherosclerosis, and traditional risk factors for atherosclerosis in patients with rheumatoid arthritis—a randomized study. J Rheumatol. 2007;34:1810-1816.
74. Hoes JN, van der Goes MC, van Raalte DH, et al. Glucose tolerance, insulin sensitivity and beta-cell function in patients with rheumatoid arthritis treated with or without low-to-medium dose glucocorticoids. Ann Rheum Dis. 2011;70:1887-1894.
75. Roubille C. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: A systematic review and meta-analysis. Ann Rheum Dis. 2003;74:480-489.
76. Ajeganova S, Svensson B, Hafstrom I, BARFOT Study Group. Low-dose prednisolone treatment of early rheumatoid arthritis and late cardiovascular outcome and survival: 10-year follow-up of a 2-year randomised trial. BMJ Open. 2014;4:e004259,2013-004259.
77. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
78. del Rincon I, Battafarano DF, Restrepo JF, et al. Glucocorticoid dose thresholds associated with all-cause and cardiovascular mortality in rheumatoid arthritis. Arthritis Rheumatol. 2014;66:264-272.
79. Davis JM,3rd, Maradit Kremers H, Crowson CS, et al. Glucocorticoids and cardiovascular events in rheumatoid arthritis: A population-based cohort study. Arthritis Rheum. 2007;56:820-830.
80. Zhang J, Xie F, Yun H, et al. Comparative effects of biologics on cardiovascular risk among older patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1813-1818.
81. Greenberg JD, Kremer JM, Curtis JR, et al. Tumour necrosis factor antagonist use and associated risk reduction of cardiovascular events among patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70:576-582.
82. Lindhardsen J, Gislason GH, Jacobsen S, et al. Non-steroidal anti-inflammatory drugs and risk of cardiovascular disease in patients with rheumatoid arthritis: A nationwide cohort study. Ann Rheum Dis. 2014;73:1515-1521.
83. Schjerning Olsen AM, Fosbol EL, Lindhardsen J, et al. Duration of treatment with nonsteroidal anti-inflammatory drugs and impact on risk of death and recurrent myocardial infarction in patients with prior myocardial infarction: A nationwide cohort study. Circulation. 2011;123:2226-2235.
84. Gislason GH, Rasmussen JN, Abildstrom SZ, et al. Increased mortality and cardiovascular morbidity associated with use of nonsteroidal anti-inflammatory drugs in chronic heart failure. Arch Intern Med. 2009;169:141-149.
85. Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: Network meta-analysis. BMJ. 2011;342:c7086.
86. Nissen SE, Yeomans ND, Solomon DH, et al. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N Engl J Med. 2016;375:2519-2529.
87. Wasko MC, Dasgupta A, Hubert Het al. Propensity-adjusted association of methotrexate with overall survival in rheumatoid arthritis. Arthritis Rheum. 2013;65:334-342.
88. Micha R, Imamura F, Wyler von Ballmoos M, et al. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol. 2011;108:1362-1370.
89. Bernatsky S, Hudson M, Suissa S. Anti-rheumatic drug use and risk of hospitalization for congestive heart failure in rheumatoid arthritis. Rheumatology (Oxford). 2005;44:677-680.
90. Myasoedova E, Crowson CS, Nicola PJ, et al. The influence of rheumatoid arthritis disease characteristics on heart failure. J Rheumatol. 2011;38:1601-1606.
91. Ronda N, Greco D, Adorni MP, et al. Newly identified antiatherosclerotic activity of methotrexate and adalimumab: Complementary effects on lipoprotein function and macrophage cholesterol metabolism. Arthritis Rheumatol. 2015;67:1155-1164.
92. Zimmerman MC, Clemens DL, Duryee MJ, et al. Direct antioxidant properties of methotrexate: Inhibition of malondialdehyde-acetaldehyde-protein adduct formation and superoxide scavenging. Redox Biol. 2017;13:588-593.
93. Rempenault C, Combe B, Barnetche T, et al. Metabolic and cardiovascular benefits of hydroxychloroquine in patients with rheumatoid arthritis: A systematic review and meta-analysis. Ann Rheum Dis. 2018;77:98-103.
94. Wasko MC, Hubert HB, Lingala VB, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. 2007;298:187-193.
95. Charles-Schoeman C, Wang X, Lee YY, et al. Association of triple therapy with improvement in cholesterol profiles over two-year followup in the treatment of early aggressive rheumatoid arthritis trial. Arthritis Rheumatol. 2016;68:577-586.
96. Charles-Schoeman C, Yin Lee Y, Shahbazian A, et al. Improvement of high-density lipoprotein function in patients with early rheumatoid arthritis treated with methotrexate monotherapy or combination therapies in a randomized controlled trial. Arthritis Rheumatol. 2017;69:46-57.
97. Chung ES, Packer M, Lo KH, , Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: Results of the anti-TNF therapy against congestive heart failure (ATTACH) trial. Circulation. 2003;107:3133-3140.
98. Singh JA, Saag KG, Bridges SL, Jr, et al. 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1-26.
99. Daien CI, Duny Y, Barnetche Tet al. Effect of TNF inhibitors on lipid profile in rheumatoid arthritis: A systematic review with meta-analysis. Ann Rheum Dis. 2012;71:862-868.
100. Di Minno MN, Ambrosino P, Peluso R, et al. Lipid profile changes in patients with rheumatic diseases receiving a treatment with TNF-alpha blockers: A meta-analysis of prospective studies. Ann Med. 2014;46:73-83.
101. Popa C, van Tits LJ, Barrera P, et al. Anti-inflammatory therapy with tumour necrosis factor alpha inhibitors improves high-density lipoprotein cholesterol antioxidative capacity in rheumatoid arthritis patients. Ann Rheum Dis. 2009;68:868-872.
102. O’Neill F, Charakida M, Topham E, et al. Anti-inflammatory treatment improves high-density lipoprotein function in rheumatoid arthritis. Heart. 2017;103:766-773.
103. Nishimoto N, Ito K, Takagi N. Safety and efficacy profiles of tocilizumab monotherapy in Japanese patients with rheumatoid arthritis: Meta-analysis of six initial trials and five long-term extensions. Mod Rheumatol. 2010;20:222-232.
104. Rao VU, Pavlov A, Klearman M, et al. An evaluation of risk factors for major adverse cardiovascular events during tocilizumab therapy. Arthritis Rheumatol. 2015;67:372-380.
105. Gabay C, McInnes IB, Kavanaugh A, et al. Comparison of lipid and lipid-associated cardiovascular risk marker changes after treatment with tocilizumab or adalimumab in patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1806-1812.
106. McInnes IB, Thompson L, Giles JT, et al. Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann Rheum Dis. 2015;74:694-702.
107. Souto A, Salgado E, Maneiro JR, et al. Lipid profile changes in patients with chronic inflammatory arthritis treated with biologic agents and tofacitinib in randomized clinical trials: A systematic review and meta-analysis. Arthritis Rheumatol. 2015;67:117-127.
108. Myasoedova E, Gabriel SE, Matteson EL, et al. Decreased cardiovascular mortality in patients with incident rheumatoid arthritis (RA) in recent years: Dawn of a new era in cardiovascular disease in RA? J Rheumatol. 2017;44:732-739.
109. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2889-2934.
110. Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. public health service report. Am J Prev Med. 2008;35:158-176.
111. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American college of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2960-2984.
112. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342-362.
113. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American college of cardiology/American heart association task force on practice guidelines and the obesity society. J Am Coll Cardiol. 2014;63:2985-3023.
From the Division of Rh
Abstract
- Objective: To review the management of traditional and nontraditional CVD cardiovascular disease risk factors in rheumatoid arthritis (RA).
- Methods: Literature review of the management of CVD risk in RA.
- Results: Because of the increased risk of CVD events and CVD mortality among RA patients, aggressive management of CVD risk is essential. Providers should follow national guidelines for the management of traditional CVD risk factors, including dyslipidemia, hypertension, and diabetes mellitus. Similar efforts are needed in counseling on lifestyle modifications, including smoking cessation, regular exercise, and maintaining a healthy body weight. Because higher RA disease activity is also linked with CVD risk, aggressive treatment of RA to a target of low disease activity or remission is critical. Furthermore, the selection of potentially “cardioprotective” agents such as methotrexate and tumor necrosis factor inhibitors, while limiting use of nonsteroidal anti-inflammatory drugs and glucocorticoids, are strategies that could be employed by rheumatologists to help mitigate CVD risk in their patients with RA.
- Conclusion: Routine assessment of CVD risk, management of traditional CVD risk factors, counseling on healthy lifestyle habits, and aggressive treatment of RA are essential to minimize CVD risk in this population.
Keywords: rheumatoid arthritis; cardiovascular disease; cardiovascular risk assessment; cardiovascular risk management.
Editor’s note: This article is part 2 of a 2-part article. “Assessment of Cardiovascular Disease Risk in Rheumatoid Arthritis” was published in the January/February 2019 issue.
Rheumatoid arthritis (RA) is a systemic autoimmune condition that contributes to an increased risk for cardiovascular disease (CVD) among affected patients. In persons with RA, the risk of incident CVD and CVD mortality are increased by approximately 50% compared with the general population.1,2 To minimize CVD risk in this population, providers must routinely assess for CVD risk factors3 and aggressively manage both traditional and nontraditional CVD risk factors.
Managing Traditional Risk Factors
As in the general population, identification and management of traditional CVD risk factors are crucial to minimize CVD risk in the RA population. A prospective study of 201 RA patients demonstrated that traditional CVD risk factors were in fact more predictive of endothelial dysfunction and carotid atherosclerosis than were disease-related inflammatory markers in RA.4 Management of traditional risk factors is detailed in the following sections, and recommendations for managing all traditional CVD risk factors are summarized in the Table.

Dyslipidemia
The role of dyslipidemia in atherogenesis is well established, and as a result, lipid levels are nearly universally included in CVD risk stratification tools. However, the interpretation of lipid levels in the context of RA is challenging because of the effects of systemic inflammation on their absolute values. Compared to the general population, patients with RA have lower total cholesterol (TC) and low-density lipoprotein (LDL) levels independent of lipid-lowering therapy.5,6 Despite this, RA patients are at increased risk for CVD. There is even some evidence to suggest a “lipid paradox” in RA, whereby lower TC (< 4 mmol/L) and LDL levels suggest an increased risk of CVD.7,8 In contrast to LDL, higher levels of high-density lipoprotein (HDL) are typically associated with reduced CVD risk, as in the general population.8,9 Interestingly, in a cohort of 16,085 RA patients and 48,499 age- and sex-matched controls, there was no significant difference in the relationship between LDL and CVD risk, suggesting that quantitative lipid levels alone may not entirely explain the CVD mortality gap in RA.9 As such, there is substantial interest in lipoprotein function within the context of CVD risk in RA. Recent investigations have identified impaired HDL function, with reduced cholesterol efflux capacity and antioxidant properties, as well as increased scavenger receptor expression and foam cell formation, in patients with RA.10,11 More research is needed to elucidate how these alterations affect CVD morbidity and mortality and how their measurement could be integrated into improved CVD risk assessment.
Meta-analyses of randomized controlled trials have estimated that lipid-lowering therapy with HMG-CoA reductase inhibitors (statins) reduces the risk of CVD by 25% to 30%; as such, statin therapy has become the standard of care for reduction of CVD risk in the general population.12 Benefits for primary prevention of CVD in RA have also been observed; statin therapy was associated with a reduced risk of CVD events (hazard ratio [HR], 0.45; 95% confidence interval [CI], 0.20-0.98) and all-cause mortality (HR, 0.43; 95% CI, 0.20-0.92) in a population-based cohort study.13 Statins appear to have similar lipid-lowering effects and result in similar CVD risk reduction when used for primary or secondary prevention in RA patients compared to non-RA controls.14-16 Additionally, anti-inflammatory properties of statins may act in synergy with disease-modifying antirheumatic drugs (DMARDs) to improve RA disease activity. In a small study of RA patients, statin therapy improved subjective and objective markers of RA disease activity in conjunction with methotrexate.17
While statins provide robust reduction in CVD risk, some individuals cannot tolerate statin therapy or do not achieve goal LDL levels with statin therapy. Select non-statin LDL-cholesterol-lowering agents have shown promise for reducing CVD events in the general population.18 Ezetimibe, which inhibits cholesterol absorption in the small intestine, very modestly reduced CVD events when added to atorvastatin (relative risk [RR], 0.94; 95% CI, 0.89-0.99) in a double-blind randomized controlled trial.19 Novel monoclonal antibodies to proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibit the internalization of surface LDL receptors, promoting LDL clearance. Two PCSK-9 inhibitors, alirocumab and evolocumab, were approved by the US Food and Drug Administration (FDA) after randomized controlled trials demonstrated their efficacy in lowering LDL by approximately 60% and reducing CVD events by approximately 15% in patients on maximum-tolerated statin therapy.20-22 To date, non-statin LDL-cholesterol-lowering agents have been subject to limited study in RA.23
Identification and management of dyslipidemia offers an opportunity for substantial CVD risk reduction at the RA population level. Unfortunately, current rates of lipid screening are inadequate in this high-risk group. In a study of 3298 Medicare patients with RA, less than half of RA patients with an indication underwent appropriate lipid screening.24 Additionally, statins are often underutilized for both primary and secondary prevention in RA patients. Only 27% of RA patients meeting National Cholesterol Education Program Adult Treatment Panel III criteria were initiated on statin therapy in a population-based cohort study.25 Among patients discharged after a first myocardial infarction (MI), the odds of receiving lipid-lowering therapy were 31% lower for RA patients (odds ratio [OR], 0.69; 95% CI, 0.58-0.82).26 Similar to the general population, adherence to statins in RA patients appears to be poor.27-30 This raises particular concern considering that a population-based cohort study of RA patients demonstrated a 67% increased risk of MI associated with statin discontinuation, regardless of prior MI status.27 Providers—rheumatologists, primary care providers, and cardiologists alike—need to remain vigilant in efforts to assess CVD risk to identify patients who will benefit from lipid-lowering therapy and to emphasize the importance to patients of statin adherence. Novel models of health-care delivery, health technologies, and patient engagement in care may prove useful for improving lipid screening and management in RA.
Tobacco Use
Cigarette smoking is a shared risk factor for both CVD and RA. Large cohort studies have identified a dose-dependent increased risk of incident RA, particularly seropositive RA, among smokers.31-34 Tobacco smoking has also been associated with increased levels of inflammation and RA disease activity.35 The consequences of tobacco use in the general population are staggering. Among individuals over the age of 30 years, tobacco use is responsible for 12% of all deaths and 10% of all CVD deaths.36 Similar findings are observed in RA; a recent meta-analysis estimated there is a 50% increased risk of CVD events in RA related to smoking tobacco.37 In the general population, smoking cessation markedly lowers CVD risk, and over time CVD risk may approach that of nonsmokers.38,39 Thus, regular counseling and interventions to facilitate smoking cessation are critical to reducing CVD risk in RA patients. RA-specific smoking cessation programs have been proposed, but have yet to outperform standard smoking cessation programs.40
Diabetes Mellitus
It is estimated that almost 10% of the US population has diabetes mellitus (DM), which in isolation portends substantial CVD risk.41 There is an increased prevalence of DM in RA, perhaps owing to factors such as physical inactivity and chronic glucocorticoid use, though a higher level of RA disease activity itself has been associated with increased insulin resistance.42-45 In a cohort of 100 RA patients who were neither obese nor diabetic, RA patients had significantly higher fasting blood glucose and insulin levels than age- and sex-matched controls. These findings were even more pronounced in RA patients with higher levels of disease activity.44 Similar to the general population, DM is associated with poor CVD outcomes in RA.37 Therefore, both appropriate management of diabetes and control of RA disease activity are vitally important to minimize CVD risk related to DM.
Hypertension
Though not a universal finding, there may be an increased prevalence of hypertension in RA patients.31,46 Nonsteroidal anti-inflammatory drug (NSAID) and glucocorticoid use may play a role in the development of hypertension, while DMARDs appear to exert a less substantial effect on blood pressure.47,48 At least one study found that DMARD initiation (particularly for methotrexate and hydroxychloroquine) was associated with significant, albeit small, declines in both systolic and diastolic blood pressure over the first 6 months of treatment.49
Despite its potentially higher prevalence in this population, hypertension is both underdiagnosed and undertreated in RA patients.24,50-52 This is an important deficiency to target because, as in the general population, hypertension is associated with an increased risk of MI (RR, 1.84; 95% CI, 1.38-2.46) and composite CVD outcomes (RR, 2.24; 95% CI, 1.42-3.06) in RA.37 Thresholds for initiation and escalation of antihypertensive therapy are not specific to the RA population; thus, diagnosis and management of hypertension should be informed by the American College of Cardiology/American Heart Association guidelines, treating those with in-office blood pressures exceeding 140/90 mm Hg (> 130/80 mm Hg if aged > 65 years or with concomitant CVD, DM, chronic kidney disease, or 10-year atherosclerotic cardiovascular disease risk > 10%), typically with angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, or thiazide diuretics as comorbidities may dictate or allow.53 Also, the use of NSAIDs and glucocorticoids should be minimized, particularly in those with concomitant hypertension.
Physical Activity
Likely due to factors such as articular pain and stiffness, as well as physical limitations, RA patients are more sedentary than the general population.54,55 In a study of objectively assessed sedentary behavior in RA patients, greater average sedentary time per day and greater number of sedentary bouts (> 20 min) were associated with increased 10-year risk of CVD as assessed by the QRISK2.56 Conversely, the beneficial effects of exercise are well documented. Light to moderate physical activity has been associated with improved cardiovascular outcomes, greater physical function, higher levels of HDL, as well as reduced systemic inflammation and disease activity, and improved endothelial function in RA patients.57-61 While there has been concern that physical activity may result in accelerated joint damage, even high-intensity exercise was shown to be safe without causing significant progression of joint damage.58
Obesity, Weight Loss, and Diet
While obesity is clearly associated with CVD risk in the general population, this relationship is much more complex in RA, as underweight RA patients are also at higher risk for CVD and CVD-related mortality.62-64 One potential explanation for this finding is that pathological weight loss resulting in an underweight body mass index (BMI) is an independent predictor of CVD. In a study of US Veterans with RA, higher rates of weight loss (> 3 kg/m2/year) were associated with increased CVD mortality (HR, 2.27; 95% CI, 1.61-3.19) independent of BMI.65 Systemic inflammation in RA can lead to “rheumatoid cachexia,” characterized by decreased muscle mass, increased adiposity, and increased CVD risk despite a normal or potentially decreased BMI.66 Practitioners should be mindful of not only current body weight, but also patients’ weight trajectories when counseling on lifestyle practices such as healthy diet and regular exercise in RA patients. For obese individuals with RA, healthy weight loss should be encouraged. Interestingly, bariatric surgery in RA patients may improve RA disease activity in addition to its known effects on body weight and DM.67
Counseling on healthy diet with a focus on limiting foods high in saturated- and trans-fatty acids and high glycemic index foods, and increasing consumption of fruits, vegetables, and mono-unsaturated fatty acids is a well-accepted and common practice to help minimize CVD risk in the general population.68 No studies to date have investigated the effect of specific diets on CVD risk in RA patients, and thus we recommend adherence to general population recommendations.
Managing RA-related CVD Risk Factors
Disease Activity
In addition to traditional risk factors, several studies have identified associations between the level of RA disease activity and risk of CVD. In a cohort of US Veterans with RA, CVD-related mortality increased in a dose-dependent manner with higher disease activity categories. In stark contrast, the CVD mortality rates of those in remission paralleled the rates from the general population (standardized mortality ratio [SMR], 0.68; 95% CI, 0.37-1.27).69 In a separate cohort of 1157 RA patients without prior CVD, achieving low disease activity was associated with a lower risk of incident CVD events (HR, 0.65; 95% CI, 0.43-0.99).70 Additionally, high disease activity has been associated with surrogate markers of CVD and other CVD risk factors including NT-proBNP and systolic blood pressure.71,72 While no randomized controlled trial data is available to inform this recommendation, observational data suggest RA should be aggressively treated (ideally to achieve and maintain remission or low disease activity) to minimize CVD risk. While keeping this treatment goal in mind, the differential effects of specific RA therapies on CVD must also be considered.
Glucocorticoids and NSAIDs
With the expanding repertoire of DMARDs available and more aggressive treatment approaches, the role of glucocorticoids and NSAIDs in RA treatment is decreasing over time. While their efficacy for improving pain and stiffness is well established, concern regarding their contribution to CVD risk in RA patients is warranted.
Glucocorticoids are known to have detrimental effects on traditional CVD risk factors such as hypertension, insulin resistance, and dyslipidemia in the general population, as well as in RA patients.73,74 In a meta-analysis of predominantly observational studies of RA patients, glucocorticoid use was associated with an increased risk of CVD events (RR, 1.47; 95% CI, 1.34-1.60), including MI, congestive heart failure (CHF), and cerebrovascular accident (CVA).75 Evidence is conflicting in regards to a clear dose threshold that leads to increased CVD risk with glucocorticoids, though higher doses are associated with greater risk.76-81 As RA patients requiring glucocorticoids typically have higher disease activity, confounding by indication remains a complicating factor in assessing the relative contributions of glucocorticoid use and RA disease activity to elevated CVD risk in many analyses.
The increased CVD risk with NSAID use is not specific to RA and has been well established in the general population.82-84 In the previously mentioned meta-analysis, an increased overall risk of CVD events was observed with NSAID use in RA (RR, 1.18; 95% CI, 1.01-1.38). It should be noted that cyclo-oxygenase 2 (COX-2) inhibitors, in particular rofecoxib (now removed from the market), appeared to drive the majority of this risk (RR, 1.36; 95% CI, 1.10-1.67 in COX-2 inhibitors and RR 1.08, 95% CI, 0.94-1.24 in nonselective NSAIDs), suggesting a potential differential risk among NSAIDs.75 While naproxen has been thought to carry the lowest risk of CVD based on initial studies, this has not been universally observed, including in a recent randomized controlled trial of more than 24,000 RA and osteoarthritis patients.82,85,86
Providers should use the lowest possible dose and duration of glucocorticoids and NSAIDs to achieve symptom relief, with continual efforts to taper or discontinue. Candidates for glucocorticoid and NSAID therapy should be selected carefully, and use of these therapies should be avoided in those with prior CVD or at high risk for CVD based on traditional CVD risk factors. Most importantly, providers should focus on utilizing DMARDs for the management of RA, which more effectively treat RA as well as reduce CVD risk.
Methotrexate
Methotrexate (MTX), a mainstay in the treatment of RA, is a conventional DMARD observed to improve overall survival and mitigate CVD risk in multiple RA cohorts.75,87,88 In a recent meta-analysis comprised of 236,525 RA patients and 5410 CVD events, MTX use was associated with a 28% reduction in overall CVD events across 8 studies (RR, 0.72; 95% CI, 0.57-0.91), substantiating similar findings in a prior meta-analysis.75,88 MTX use was specifically associated with a decreased risk of MI (RR, 0.81; 95% CI, 0.68-0.96). Case-control and cohort studies have cited a 20% to 50% reduced risk of CHF with MTX use.89,90 The potential cardioprotective effect of MTX appears to be both multifactorial and complex, likely mediated through both direct and indirect mechanisms. MTX directly promotes anti-atherogenic lipoprotein function, improves endothelial function, and scavenges free radicals.91,92 Indirectly, MTX likely reduces CVD risk by effectively reducing RA disease activity. Based on these and other data, MTX remains the cornerstone of DMARD therapy in RA patients when targeting CVD risk reduction.
Hydroxychloroquine
Emerging evidence suggests that hydroxychloroquine (HCQ), an antimalarial most often utilized in combination with alternative DMARDs in RA, prevents DM and has beneficial effects on lipid profiles. A recent meta-analysis compiled 3 homogenous observational studies that investigated the effect of HCQ on incident DM. RA patients ever exposed to HCQ had a 40% lower incidence of DM (HR, 0.59; 95% CI, 0.49-0.70).93 Increased duration of HCQ use was shown to further reduce risk of incident DM.94 The aforementioned meta-analysis also pooled 5 studies investigating the effect of HCQ on lipid profiles, with favorable mean differences in TC (–9.82 mg/dL), LDL (–10.61 mg/dL), HDL (4.13 mg/dL), and triglycerides (–19.15 mg/dL) in HCQ users compared to non-users.93 Given these favorable changes to traditional CVD risk factors, it is not surprising that in a retrospective study of 1266 RA patients without prior CVD, HCQ was associated with significantly lower risk of incident CVD. While external validation of these findings is needed, HCQ is an attractive conventional DMARD to be used in RA for CVD risk reduction. Moreover, its combination with MTX and sulfasalazine also shows promise for CVD risk reduction.95,96
TNF Inhibitors
Tumor necrosis factor (TNF) inhibitors are often the initial biologic DMARD therapy used in RA patients not responding to conventional DMARDs. In the previously described meta-analysis, TNF inhibitors were associated with similar reductions in CVD events as MTX (RR, 0.70; 95% CI, 0.54-0.90).75 Of note, there was a trend toward reduced risk of CHF (RR, 0.75; 95% CI, 0.49-1.15) in this same meta-analysis, an area of concern with TNF inhibitor use due to a prior randomized controlled trial demonstrating worsening clinical status in patients with existing moderate-to-severe CHF treated with high-dose infliximab.97 Current RA treatment guidelines recommend avoiding TNF inhibitor use in individuals with CHF.98
Aside from the risk of CHF exacerbation, TNF inhibitors appear to be cardioprotective. Similar to MTX, the mechanism by which TNF inhibition reduces cardiovascular risk is complex and likely due to both direct and indirect mechanisms. Substantial research has been conducted on the effect of TNF inhibition on lipids, with a recent meta-analysis demonstrating increases in HDL and TC, with stable LDL and atherogenic index over treatment follow-up.99 A subsequent meta-analysis not limited to RA patients yielded similar results.100 In addition to quantitative lipid changes, alteration of lipoprotein function, improvement in myocardial function, reduced aortic stiffness, improved blood pressure, and reduced RA disease activity may also be responsible for cardioprotective benefits of these agents.101,102
Non-TNF Biologic and Traditional Synthetic DMARDs
Tocilizumab, an IL-6 inhibitor, can potently increase LDL levels, but it does not appear to increase the risk of CVD events and may actually promote more favorable anti-atherogenic lipoprotein function.103-106 Although these quantitative lipid changes received significant attention in the wake of early reports detailing this effect, similar lipid changes appear to accompany other DMARDs including TNF inhibitors and tofacitinib.107 There have been few studies evaluating the risk of CVD with other non-TNF inhibitor biologic DMARDs and traditional synthetic DMARDs, warranting future study.
Conclusion
To mitigate the increased risk of CVD in RA, primary care and subspecialty providers alike must be aware of this heightened risk in RA, perform frequent assessments of CVD risk,3 and aggressively manage both traditional and nontraditional CVD risk factors. The differential roles in this effort may not be clear; thus, we have proposed a co-management strategy detailed in the Figure. Clear communication between providers is of the utmost importance to ensure effective management of CVD risk.

Given limited evidence for RA-specific CVD risk assessments and traditional risk factor treatment targets, management should follow pertinent national guidelines. The importance of lifestyle counseling should not be overlooked, with a focus on smoking cessation, healthy diet and body weight, and regular aerobic exercise. Finally, rheumatologists should aggressively manage RA using a treat-to-target approach, minimize the use of glucocorticoids and NSAIDs, and preferentially select DMARDs that have been associated with lower CVD risk. Through this comprehensive approach, recent trends of improved CVD outcomes in RA will hopefully become more widespread.108
Corresponding author: Bryant R. England, MD; 986270 Nebraska Medical Center, Omaha, NE 68198-6270; [email protected].
Financial disclosures: Dr. England is supported by UNMC Internal Medicine Scientist Development Award, UNMC Physician-Scientist Training Program, the UNMC Mentored Scholars Program, and the Rheumatology Research Foundation Scientist Development Award. Dr. Mikuls is supported by a VA Merit Award (CX000896) and grants from the National Institutes of Health: National Institute of General Medical Sciences (U54GM115458), National Institute on Alcohol Abuse and Alcoholism (R25AA020818), and National Institute of Arthritis and Musculoskeletal and Skin Diseases (2P50AR60772).
From the Division of Rh
Abstract
- Objective: To review the management of traditional and nontraditional CVD cardiovascular disease risk factors in rheumatoid arthritis (RA).
- Methods: Literature review of the management of CVD risk in RA.
- Results: Because of the increased risk of CVD events and CVD mortality among RA patients, aggressive management of CVD risk is essential. Providers should follow national guidelines for the management of traditional CVD risk factors, including dyslipidemia, hypertension, and diabetes mellitus. Similar efforts are needed in counseling on lifestyle modifications, including smoking cessation, regular exercise, and maintaining a healthy body weight. Because higher RA disease activity is also linked with CVD risk, aggressive treatment of RA to a target of low disease activity or remission is critical. Furthermore, the selection of potentially “cardioprotective” agents such as methotrexate and tumor necrosis factor inhibitors, while limiting use of nonsteroidal anti-inflammatory drugs and glucocorticoids, are strategies that could be employed by rheumatologists to help mitigate CVD risk in their patients with RA.
- Conclusion: Routine assessment of CVD risk, management of traditional CVD risk factors, counseling on healthy lifestyle habits, and aggressive treatment of RA are essential to minimize CVD risk in this population.
Keywords: rheumatoid arthritis; cardiovascular disease; cardiovascular risk assessment; cardiovascular risk management.
Editor’s note: This article is part 2 of a 2-part article. “Assessment of Cardiovascular Disease Risk in Rheumatoid Arthritis” was published in the January/February 2019 issue.
Rheumatoid arthritis (RA) is a systemic autoimmune condition that contributes to an increased risk for cardiovascular disease (CVD) among affected patients. In persons with RA, the risk of incident CVD and CVD mortality are increased by approximately 50% compared with the general population.1,2 To minimize CVD risk in this population, providers must routinely assess for CVD risk factors3 and aggressively manage both traditional and nontraditional CVD risk factors.
Managing Traditional Risk Factors
As in the general population, identification and management of traditional CVD risk factors are crucial to minimize CVD risk in the RA population. A prospective study of 201 RA patients demonstrated that traditional CVD risk factors were in fact more predictive of endothelial dysfunction and carotid atherosclerosis than were disease-related inflammatory markers in RA.4 Management of traditional risk factors is detailed in the following sections, and recommendations for managing all traditional CVD risk factors are summarized in the Table.

Dyslipidemia
The role of dyslipidemia in atherogenesis is well established, and as a result, lipid levels are nearly universally included in CVD risk stratification tools. However, the interpretation of lipid levels in the context of RA is challenging because of the effects of systemic inflammation on their absolute values. Compared to the general population, patients with RA have lower total cholesterol (TC) and low-density lipoprotein (LDL) levels independent of lipid-lowering therapy.5,6 Despite this, RA patients are at increased risk for CVD. There is even some evidence to suggest a “lipid paradox” in RA, whereby lower TC (< 4 mmol/L) and LDL levels suggest an increased risk of CVD.7,8 In contrast to LDL, higher levels of high-density lipoprotein (HDL) are typically associated with reduced CVD risk, as in the general population.8,9 Interestingly, in a cohort of 16,085 RA patients and 48,499 age- and sex-matched controls, there was no significant difference in the relationship between LDL and CVD risk, suggesting that quantitative lipid levels alone may not entirely explain the CVD mortality gap in RA.9 As such, there is substantial interest in lipoprotein function within the context of CVD risk in RA. Recent investigations have identified impaired HDL function, with reduced cholesterol efflux capacity and antioxidant properties, as well as increased scavenger receptor expression and foam cell formation, in patients with RA.10,11 More research is needed to elucidate how these alterations affect CVD morbidity and mortality and how their measurement could be integrated into improved CVD risk assessment.
Meta-analyses of randomized controlled trials have estimated that lipid-lowering therapy with HMG-CoA reductase inhibitors (statins) reduces the risk of CVD by 25% to 30%; as such, statin therapy has become the standard of care for reduction of CVD risk in the general population.12 Benefits for primary prevention of CVD in RA have also been observed; statin therapy was associated with a reduced risk of CVD events (hazard ratio [HR], 0.45; 95% confidence interval [CI], 0.20-0.98) and all-cause mortality (HR, 0.43; 95% CI, 0.20-0.92) in a population-based cohort study.13 Statins appear to have similar lipid-lowering effects and result in similar CVD risk reduction when used for primary or secondary prevention in RA patients compared to non-RA controls.14-16 Additionally, anti-inflammatory properties of statins may act in synergy with disease-modifying antirheumatic drugs (DMARDs) to improve RA disease activity. In a small study of RA patients, statin therapy improved subjective and objective markers of RA disease activity in conjunction with methotrexate.17
While statins provide robust reduction in CVD risk, some individuals cannot tolerate statin therapy or do not achieve goal LDL levels with statin therapy. Select non-statin LDL-cholesterol-lowering agents have shown promise for reducing CVD events in the general population.18 Ezetimibe, which inhibits cholesterol absorption in the small intestine, very modestly reduced CVD events when added to atorvastatin (relative risk [RR], 0.94; 95% CI, 0.89-0.99) in a double-blind randomized controlled trial.19 Novel monoclonal antibodies to proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibit the internalization of surface LDL receptors, promoting LDL clearance. Two PCSK-9 inhibitors, alirocumab and evolocumab, were approved by the US Food and Drug Administration (FDA) after randomized controlled trials demonstrated their efficacy in lowering LDL by approximately 60% and reducing CVD events by approximately 15% in patients on maximum-tolerated statin therapy.20-22 To date, non-statin LDL-cholesterol-lowering agents have been subject to limited study in RA.23
Identification and management of dyslipidemia offers an opportunity for substantial CVD risk reduction at the RA population level. Unfortunately, current rates of lipid screening are inadequate in this high-risk group. In a study of 3298 Medicare patients with RA, less than half of RA patients with an indication underwent appropriate lipid screening.24 Additionally, statins are often underutilized for both primary and secondary prevention in RA patients. Only 27% of RA patients meeting National Cholesterol Education Program Adult Treatment Panel III criteria were initiated on statin therapy in a population-based cohort study.25 Among patients discharged after a first myocardial infarction (MI), the odds of receiving lipid-lowering therapy were 31% lower for RA patients (odds ratio [OR], 0.69; 95% CI, 0.58-0.82).26 Similar to the general population, adherence to statins in RA patients appears to be poor.27-30 This raises particular concern considering that a population-based cohort study of RA patients demonstrated a 67% increased risk of MI associated with statin discontinuation, regardless of prior MI status.27 Providers—rheumatologists, primary care providers, and cardiologists alike—need to remain vigilant in efforts to assess CVD risk to identify patients who will benefit from lipid-lowering therapy and to emphasize the importance to patients of statin adherence. Novel models of health-care delivery, health technologies, and patient engagement in care may prove useful for improving lipid screening and management in RA.
Tobacco Use
Cigarette smoking is a shared risk factor for both CVD and RA. Large cohort studies have identified a dose-dependent increased risk of incident RA, particularly seropositive RA, among smokers.31-34 Tobacco smoking has also been associated with increased levels of inflammation and RA disease activity.35 The consequences of tobacco use in the general population are staggering. Among individuals over the age of 30 years, tobacco use is responsible for 12% of all deaths and 10% of all CVD deaths.36 Similar findings are observed in RA; a recent meta-analysis estimated there is a 50% increased risk of CVD events in RA related to smoking tobacco.37 In the general population, smoking cessation markedly lowers CVD risk, and over time CVD risk may approach that of nonsmokers.38,39 Thus, regular counseling and interventions to facilitate smoking cessation are critical to reducing CVD risk in RA patients. RA-specific smoking cessation programs have been proposed, but have yet to outperform standard smoking cessation programs.40
Diabetes Mellitus
It is estimated that almost 10% of the US population has diabetes mellitus (DM), which in isolation portends substantial CVD risk.41 There is an increased prevalence of DM in RA, perhaps owing to factors such as physical inactivity and chronic glucocorticoid use, though a higher level of RA disease activity itself has been associated with increased insulin resistance.42-45 In a cohort of 100 RA patients who were neither obese nor diabetic, RA patients had significantly higher fasting blood glucose and insulin levels than age- and sex-matched controls. These findings were even more pronounced in RA patients with higher levels of disease activity.44 Similar to the general population, DM is associated with poor CVD outcomes in RA.37 Therefore, both appropriate management of diabetes and control of RA disease activity are vitally important to minimize CVD risk related to DM.
Hypertension
Though not a universal finding, there may be an increased prevalence of hypertension in RA patients.31,46 Nonsteroidal anti-inflammatory drug (NSAID) and glucocorticoid use may play a role in the development of hypertension, while DMARDs appear to exert a less substantial effect on blood pressure.47,48 At least one study found that DMARD initiation (particularly for methotrexate and hydroxychloroquine) was associated with significant, albeit small, declines in both systolic and diastolic blood pressure over the first 6 months of treatment.49
Despite its potentially higher prevalence in this population, hypertension is both underdiagnosed and undertreated in RA patients.24,50-52 This is an important deficiency to target because, as in the general population, hypertension is associated with an increased risk of MI (RR, 1.84; 95% CI, 1.38-2.46) and composite CVD outcomes (RR, 2.24; 95% CI, 1.42-3.06) in RA.37 Thresholds for initiation and escalation of antihypertensive therapy are not specific to the RA population; thus, diagnosis and management of hypertension should be informed by the American College of Cardiology/American Heart Association guidelines, treating those with in-office blood pressures exceeding 140/90 mm Hg (> 130/80 mm Hg if aged > 65 years or with concomitant CVD, DM, chronic kidney disease, or 10-year atherosclerotic cardiovascular disease risk > 10%), typically with angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, or thiazide diuretics as comorbidities may dictate or allow.53 Also, the use of NSAIDs and glucocorticoids should be minimized, particularly in those with concomitant hypertension.
Physical Activity
Likely due to factors such as articular pain and stiffness, as well as physical limitations, RA patients are more sedentary than the general population.54,55 In a study of objectively assessed sedentary behavior in RA patients, greater average sedentary time per day and greater number of sedentary bouts (> 20 min) were associated with increased 10-year risk of CVD as assessed by the QRISK2.56 Conversely, the beneficial effects of exercise are well documented. Light to moderate physical activity has been associated with improved cardiovascular outcomes, greater physical function, higher levels of HDL, as well as reduced systemic inflammation and disease activity, and improved endothelial function in RA patients.57-61 While there has been concern that physical activity may result in accelerated joint damage, even high-intensity exercise was shown to be safe without causing significant progression of joint damage.58
Obesity, Weight Loss, and Diet
While obesity is clearly associated with CVD risk in the general population, this relationship is much more complex in RA, as underweight RA patients are also at higher risk for CVD and CVD-related mortality.62-64 One potential explanation for this finding is that pathological weight loss resulting in an underweight body mass index (BMI) is an independent predictor of CVD. In a study of US Veterans with RA, higher rates of weight loss (> 3 kg/m2/year) were associated with increased CVD mortality (HR, 2.27; 95% CI, 1.61-3.19) independent of BMI.65 Systemic inflammation in RA can lead to “rheumatoid cachexia,” characterized by decreased muscle mass, increased adiposity, and increased CVD risk despite a normal or potentially decreased BMI.66 Practitioners should be mindful of not only current body weight, but also patients’ weight trajectories when counseling on lifestyle practices such as healthy diet and regular exercise in RA patients. For obese individuals with RA, healthy weight loss should be encouraged. Interestingly, bariatric surgery in RA patients may improve RA disease activity in addition to its known effects on body weight and DM.67
Counseling on healthy diet with a focus on limiting foods high in saturated- and trans-fatty acids and high glycemic index foods, and increasing consumption of fruits, vegetables, and mono-unsaturated fatty acids is a well-accepted and common practice to help minimize CVD risk in the general population.68 No studies to date have investigated the effect of specific diets on CVD risk in RA patients, and thus we recommend adherence to general population recommendations.
Managing RA-related CVD Risk Factors
Disease Activity
In addition to traditional risk factors, several studies have identified associations between the level of RA disease activity and risk of CVD. In a cohort of US Veterans with RA, CVD-related mortality increased in a dose-dependent manner with higher disease activity categories. In stark contrast, the CVD mortality rates of those in remission paralleled the rates from the general population (standardized mortality ratio [SMR], 0.68; 95% CI, 0.37-1.27).69 In a separate cohort of 1157 RA patients without prior CVD, achieving low disease activity was associated with a lower risk of incident CVD events (HR, 0.65; 95% CI, 0.43-0.99).70 Additionally, high disease activity has been associated with surrogate markers of CVD and other CVD risk factors including NT-proBNP and systolic blood pressure.71,72 While no randomized controlled trial data is available to inform this recommendation, observational data suggest RA should be aggressively treated (ideally to achieve and maintain remission or low disease activity) to minimize CVD risk. While keeping this treatment goal in mind, the differential effects of specific RA therapies on CVD must also be considered.
Glucocorticoids and NSAIDs
With the expanding repertoire of DMARDs available and more aggressive treatment approaches, the role of glucocorticoids and NSAIDs in RA treatment is decreasing over time. While their efficacy for improving pain and stiffness is well established, concern regarding their contribution to CVD risk in RA patients is warranted.
Glucocorticoids are known to have detrimental effects on traditional CVD risk factors such as hypertension, insulin resistance, and dyslipidemia in the general population, as well as in RA patients.73,74 In a meta-analysis of predominantly observational studies of RA patients, glucocorticoid use was associated with an increased risk of CVD events (RR, 1.47; 95% CI, 1.34-1.60), including MI, congestive heart failure (CHF), and cerebrovascular accident (CVA).75 Evidence is conflicting in regards to a clear dose threshold that leads to increased CVD risk with glucocorticoids, though higher doses are associated with greater risk.76-81 As RA patients requiring glucocorticoids typically have higher disease activity, confounding by indication remains a complicating factor in assessing the relative contributions of glucocorticoid use and RA disease activity to elevated CVD risk in many analyses.
The increased CVD risk with NSAID use is not specific to RA and has been well established in the general population.82-84 In the previously mentioned meta-analysis, an increased overall risk of CVD events was observed with NSAID use in RA (RR, 1.18; 95% CI, 1.01-1.38). It should be noted that cyclo-oxygenase 2 (COX-2) inhibitors, in particular rofecoxib (now removed from the market), appeared to drive the majority of this risk (RR, 1.36; 95% CI, 1.10-1.67 in COX-2 inhibitors and RR 1.08, 95% CI, 0.94-1.24 in nonselective NSAIDs), suggesting a potential differential risk among NSAIDs.75 While naproxen has been thought to carry the lowest risk of CVD based on initial studies, this has not been universally observed, including in a recent randomized controlled trial of more than 24,000 RA and osteoarthritis patients.82,85,86
Providers should use the lowest possible dose and duration of glucocorticoids and NSAIDs to achieve symptom relief, with continual efforts to taper or discontinue. Candidates for glucocorticoid and NSAID therapy should be selected carefully, and use of these therapies should be avoided in those with prior CVD or at high risk for CVD based on traditional CVD risk factors. Most importantly, providers should focus on utilizing DMARDs for the management of RA, which more effectively treat RA as well as reduce CVD risk.
Methotrexate
Methotrexate (MTX), a mainstay in the treatment of RA, is a conventional DMARD observed to improve overall survival and mitigate CVD risk in multiple RA cohorts.75,87,88 In a recent meta-analysis comprised of 236,525 RA patients and 5410 CVD events, MTX use was associated with a 28% reduction in overall CVD events across 8 studies (RR, 0.72; 95% CI, 0.57-0.91), substantiating similar findings in a prior meta-analysis.75,88 MTX use was specifically associated with a decreased risk of MI (RR, 0.81; 95% CI, 0.68-0.96). Case-control and cohort studies have cited a 20% to 50% reduced risk of CHF with MTX use.89,90 The potential cardioprotective effect of MTX appears to be both multifactorial and complex, likely mediated through both direct and indirect mechanisms. MTX directly promotes anti-atherogenic lipoprotein function, improves endothelial function, and scavenges free radicals.91,92 Indirectly, MTX likely reduces CVD risk by effectively reducing RA disease activity. Based on these and other data, MTX remains the cornerstone of DMARD therapy in RA patients when targeting CVD risk reduction.
Hydroxychloroquine
Emerging evidence suggests that hydroxychloroquine (HCQ), an antimalarial most often utilized in combination with alternative DMARDs in RA, prevents DM and has beneficial effects on lipid profiles. A recent meta-analysis compiled 3 homogenous observational studies that investigated the effect of HCQ on incident DM. RA patients ever exposed to HCQ had a 40% lower incidence of DM (HR, 0.59; 95% CI, 0.49-0.70).93 Increased duration of HCQ use was shown to further reduce risk of incident DM.94 The aforementioned meta-analysis also pooled 5 studies investigating the effect of HCQ on lipid profiles, with favorable mean differences in TC (–9.82 mg/dL), LDL (–10.61 mg/dL), HDL (4.13 mg/dL), and triglycerides (–19.15 mg/dL) in HCQ users compared to non-users.93 Given these favorable changes to traditional CVD risk factors, it is not surprising that in a retrospective study of 1266 RA patients without prior CVD, HCQ was associated with significantly lower risk of incident CVD. While external validation of these findings is needed, HCQ is an attractive conventional DMARD to be used in RA for CVD risk reduction. Moreover, its combination with MTX and sulfasalazine also shows promise for CVD risk reduction.95,96
TNF Inhibitors
Tumor necrosis factor (TNF) inhibitors are often the initial biologic DMARD therapy used in RA patients not responding to conventional DMARDs. In the previously described meta-analysis, TNF inhibitors were associated with similar reductions in CVD events as MTX (RR, 0.70; 95% CI, 0.54-0.90).75 Of note, there was a trend toward reduced risk of CHF (RR, 0.75; 95% CI, 0.49-1.15) in this same meta-analysis, an area of concern with TNF inhibitor use due to a prior randomized controlled trial demonstrating worsening clinical status in patients with existing moderate-to-severe CHF treated with high-dose infliximab.97 Current RA treatment guidelines recommend avoiding TNF inhibitor use in individuals with CHF.98
Aside from the risk of CHF exacerbation, TNF inhibitors appear to be cardioprotective. Similar to MTX, the mechanism by which TNF inhibition reduces cardiovascular risk is complex and likely due to both direct and indirect mechanisms. Substantial research has been conducted on the effect of TNF inhibition on lipids, with a recent meta-analysis demonstrating increases in HDL and TC, with stable LDL and atherogenic index over treatment follow-up.99 A subsequent meta-analysis not limited to RA patients yielded similar results.100 In addition to quantitative lipid changes, alteration of lipoprotein function, improvement in myocardial function, reduced aortic stiffness, improved blood pressure, and reduced RA disease activity may also be responsible for cardioprotective benefits of these agents.101,102
Non-TNF Biologic and Traditional Synthetic DMARDs
Tocilizumab, an IL-6 inhibitor, can potently increase LDL levels, but it does not appear to increase the risk of CVD events and may actually promote more favorable anti-atherogenic lipoprotein function.103-106 Although these quantitative lipid changes received significant attention in the wake of early reports detailing this effect, similar lipid changes appear to accompany other DMARDs including TNF inhibitors and tofacitinib.107 There have been few studies evaluating the risk of CVD with other non-TNF inhibitor biologic DMARDs and traditional synthetic DMARDs, warranting future study.
Conclusion
To mitigate the increased risk of CVD in RA, primary care and subspecialty providers alike must be aware of this heightened risk in RA, perform frequent assessments of CVD risk,3 and aggressively manage both traditional and nontraditional CVD risk factors. The differential roles in this effort may not be clear; thus, we have proposed a co-management strategy detailed in the Figure. Clear communication between providers is of the utmost importance to ensure effective management of CVD risk.

Given limited evidence for RA-specific CVD risk assessments and traditional risk factor treatment targets, management should follow pertinent national guidelines. The importance of lifestyle counseling should not be overlooked, with a focus on smoking cessation, healthy diet and body weight, and regular aerobic exercise. Finally, rheumatologists should aggressively manage RA using a treat-to-target approach, minimize the use of glucocorticoids and NSAIDs, and preferentially select DMARDs that have been associated with lower CVD risk. Through this comprehensive approach, recent trends of improved CVD outcomes in RA will hopefully become more widespread.108
Corresponding author: Bryant R. England, MD; 986270 Nebraska Medical Center, Omaha, NE 68198-6270; [email protected].
Financial disclosures: Dr. England is supported by UNMC Internal Medicine Scientist Development Award, UNMC Physician-Scientist Training Program, the UNMC Mentored Scholars Program, and the Rheumatology Research Foundation Scientist Development Award. Dr. Mikuls is supported by a VA Merit Award (CX000896) and grants from the National Institutes of Health: National Institute of General Medical Sciences (U54GM115458), National Institute on Alcohol Abuse and Alcoholism (R25AA020818), and National Institute of Arthritis and Musculoskeletal and Skin Diseases (2P50AR60772).
1. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
2. Avina-Zubieta JA, Thomas J, Sadatsafavi M, et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann Rheum Dis. 2012;71:1524-1529.
3. Johnson TM, Mikuls TR, England BR. Assessment of cardiovascular risk in rheumatoid arthritis. J Clin Outcomes Manage. 2019;26:41-47.
4. Sandoo A, Chanchlani N, Hodson J, et al. Classical cardiovascular disease risk factors associate with vascular function and morphology in rheumatoid arthritis: A six-year prospective study. Arthritis Res Ther. 2013;15:R203.
5. Myasoedova E, Crowson CS, Kremers HM, et al. Total cholesterol and LDL levels decrease before rheumatoid arthritis. Ann Rheum Dis. 2010;69:1310-1314.
6. Liao KP, Cai T, Gainer VS, et al. Lipid and lipoprotein levels and trend in rheumatoid arthritis compared to the general population. Arthritis Care Res (Hoboken). 2013;65:2046-2050.
7. Myasoedova E, Crowson CS, Kremers HM, et al. Lipid paradox in rheumatoid arthritis: The impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis. 2011;70:482-487.
8. Zhang J, Chen L, Delzell E, et al. Republished: The association between inflammatory markers, serum lipids and the risk of cardiovascular events in patients with rheumatoid arthritis. Postgrad Med J. 2014;90:722-729.
9. Liao KP, Liu J, Lu B, et al. Association between lipid levels and major adverse cardiovascular events in rheumatoid arthritis compared to non-rheumatoid arthritis patients. Arthritis Rheumatol. 2015;67:2004-2010.
10. Charles-Schoeman C, Lee YY, Grijalva V, et al. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann Rheum Dis. 2012;71:1157-1162.
11. Voloshyna I, Modayil S, Littlefield MJ, et al. Plasma from rheumatoid arthritis patients promotes pro-atherogenic cholesterol transport gene expression in THP-1 human macrophages. Exp Biol Med (Maywood). 2013 238:1192-1197.
12. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;(1):CD004816.
13. Sheng X, Murphy MJ, Macdonald TM, Wei L. Effectiveness of statins on total cholesterol and cardiovascular disease and all-cause mortality in osteoarthritis and rheumatoid arthritis. J Rheumatol. 2012;39:32-40.
14. An J, Alemao E, Reynolds K, et al. Cardiovascular outcomes associated with lowering low-density lipoprotein cholesterol in rheumatoid arthritis and matched nonrheumatoid arthritis. J Rheumatol. 2016;43:1989-1996.
15. Semb AG, Holme I, Kvien TK, Pedersen TR. Intensive lipid lowering in patients with rheumatoid arthritis and previous myocardial infarction: An explorative analysis from the incremental decrease in endpoints through aggressive lipid lowering (IDEAL) trial. Rheumatology (Oxford). 2011;50:324-329.
16. Semb AG, Kvien TK, DeMicco DA, et al. Effect of intensive lipid-lowering therapy on cardiovascular outcome in patients with and those without inflammatory joint disease. Arthritis Rheum. 2012;64:2836-2846.
17. El-Barbary AM, Hussein MS, Rageh EM, et al. Effect of atorvastatin on inflammation and modification of vascular risk factors in rheumatoid arthritis. J Rheumatol. 2011;38:229-235.
18. Writing Committee, Lloyd-Jones DM, Morris PB, et al. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: A report of the American college of cardiology task force on clinical expert consensus documents. J Am Coll Cardiol. 2016;68:92-125.
19. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
20. Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509.
21. Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499.
22. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
23. Maki-Petaja KM, Booth AD, Hall FC, et al. Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. J Am Coll Cardiol. 2007;50:852-858.
24. Bartels CM, Kind AJ, Everett C, et al. Low frequency of primary lipid screening among medicare patients with rheumatoid arthritis. Arthritis Rheum. 2011;63:1221-1230.
25. Akkara Veetil BM, Myasoedova E, Matteson EL, et al. Use of lipid-lowering agents in rheumatoid arthritis: A population-based cohort study. J Rheumatol. 2013;40:1082-1088.
26. Lindhardsen J, Ahlehoff O, Gislason GH, et al. Initiation and adherence to secondary prevention pharmacotherapy after myocardial infarction in patients with rheumatoid arthritis: A nationwide cohort study. Ann Rheum Dis. 2012;71:1496-1501.
27. De Vera MA, Choi H, Abrahamowicz M, et al. Statin discontinuation and risk of acute myocardial infarction in patients with rheumatoid arthritis: A population-based cohort study. Ann Rheum Dis. 2011;70:1020-1024.
28. Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: A cohort study. Ann Intern Med. 2013;158:526-534.
29. Zhang H, Plutzky J, Shubina M, Turchin A. Continued statin prescriptions after adverse reactions and patient outcomes: A cohort study. Ann Intern Med. 2017;167:221-227.
30. Lemstra M, Blackburn D, Crawley A, Fung R. Proportion and risk indicators of nonadherence to statin therapy: A meta-analysis. Can J Cardiol. 2012;28:574-580.
31. Boyer JF, Gourraud PA, Cantagrel A, et al. Traditional cardiovascular risk factors in rheumatoid arthritis: A meta-analysis. Joint Bone Spine. 2011;78:179-183.
32. Bergstrom U, Jacobsson LT, Nilsson JA, et al. Pulmonary dysfunction, smoking, socioeconomic status and the risk of developing rheumatoid arthritis. Rheumatology (Oxford). 2011;50:2005-2013.
33. Costenbader KH, Feskanich D, Mandl LA, Karlson EW. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am J Med. 2006;119:503.e1,503.e9.
34. Klareskog L, Stolt P, Lundberg K, et al. A new model for an etiology of rheumatoid arthritis: Smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54:38-46.
35. Sokolove J, Wagner CA, Lahey LJ, et al. Increased inflammation and disease activity among current cigarette smokers with rheumatoid arthritis: A cross-sectional analysis of US veterans. Rheumatology (Oxford). 2016;55:1969-1977.
36. World Health Organization. WHO Global Report: Mortality Attributable to Tobacco. Geneva, World Health Organization, 2012.
37. Baghdadi LR, Woodman RJ, Shanahan EM, Mangoni AA. The impact of traditional cardiovascular risk factors on cardiovascular outcomes in patients with rheumatoid arthritis: A systematic review and meta-analysis. PLoS One. 2015;10:e0117952.
38. Centers for Disease Control and Prevention; National Center for Chronic Disease Prevention and Health Promotion. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention; 2010. 6, Cardiovascular Diseases. Available from: https://ncbi.nlm.nih.gov/books/NBK53012/
39. Mons U, Muezzinler A, Gellert C, et al. Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: Meta-analysis of individual participant data from prospective cohort studies of the CHANCES consortium. BMJ. 2015;350:h1551.
40. Aimer P, Treharne GJ, Stebbings S, Frampton C, Cameron V, Kirby S, et al. Efficacy of a rheumatoid arthritis-specific smoking cessation program: A randomized controlled pilot trial. Arthritis Care Res (Hoboken). 2017;69:28-37.
41. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2017.
42. Jiang P, Li H, Li X. Diabetes mellitus risk factors in rheumatoid arthritis: A systematic review and meta-analysis. Clin Exp Rheumatol. 2015;33:115-121.
43. Shahin D, Eltoraby E, Mesbah A, Houssen M. Insulin resistance in early untreated rheumatoid arthritis patients. Clin Biochem. 2010;43:661-335.
44. Arias de la Rosa I, Escudero-Contreras A, Rodriguez-Cuenca S, et al. Defective glucose and lipid metabolism in rheumatoid arthritis is determined by chronic inflammation in metabolic tissues. J Intern Med. 2018;84(1):61-77.
45. Wilson JC, Sarsour K, Gale S, et al. Incidence and risk of glucocorticoid-associated adverse effects in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2018 Jun 1. doi: 10.1002/acr.23611.
46. Chung CP, Giles JT, Petri M, et al. Prevalence of traditional modifiable cardiovascular risk factors in patients with rheumatoid arthritis: Comparison with control subjects from the multi-ethnic study of atherosclerosis. Semin Arthritis Rheum. 2012;41:535-544.
47. Goodwin JE, Geller DS. Glucocorticoid-induced hypertension. Pediatr Nephrol. 2012;27:1059-1066.
48. Snowden S, Nelson R. The effects of nonsteroidal anti-inflammatory drugs on blood pressure in hypertensive patients. Cardiol Rev. 2011;19:184-191.
49. Baker JF, Sauer B, Teng CC, et al. Initiation of disease-modifying therapies in rheumatoid arthritis is associated with changes in blood pressure. J Clin Rheumatol. 2018;24:203-209.
50. Panoulas VF, Douglas KM, Milionis HJ, et al. Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology (Oxford). 2007;46:1477-1482.
51. Protogerou AD, Panagiotakos DB, Zampeli E, et al. Arterial hypertension assessed “out-of-office” in a contemporary cohort of rheumatoid arthritis patients free of cardiovascular disease is characterized by high prevalence, low awareness, poor control and increased vascular damage-associated “white coat” phenomenon. Arthritis Res Ther. 2013;15:R142.
52. van Breukelen-van der Stoep DF, van Zeben D, Klop B, et al. Marked underdiagnosis and undertreatment of hypertension and hypercholesterolaemia in rheumatoid arthritis. Rheumatology (Oxford). 2016;55:1210-1216.
53. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71:e127-248.
54. Lee J, Dunlop D, Ehrlich-Jones L, et al. Public health impact of risk factors for physical inactivity in adults with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:488-493.
55. Sokka T, Hakkinen A, Kautiainen H, et al. Physical inactivity in patients with rheumatoid arthritis: Data from twenty-one countries in a cross-sectional, international study. Arthritis Rheum. 2008;59:42-50.
56. Fenton SAM, Veldhuijzen van Zanten JJCS, Kitas GD, et al. Sedentary behaviour is associated with increased long-term cardiovascular risk in patients with rheumatoid arthritis independently of moderate-to-vigorous physical activity. BMC Musculoskelet Disord. 2017;18:131,017-1473-9.
57. Byram KW, Oeser AM, Linton MF, et al. Exercise is associated with increased small HDL particle concentration and decreased vascular stiffness in rheumatoid arthritis. J Clin Rheumatol. 2018 May 25. 9.
58. de Jong Z, Munneke M, Zwinderman AH, et al. Is a long-term high-intensity exercise program effective and safe in patients with rheumatoid arthritis? results of a randomized controlled trial. Arthritis Rheum. 2003;48:2415-2424.
59. Stavropoulos-Kalinoglou A, Metsios GS, Veldhuijzen van Zanten JJ, et al. Individualised aerobic and resistance exercise training improves cardiorespiratory fitness and reduces cardiovascular risk in patients with rheumatoid arthritis. Ann Rheum Dis. 2013;72:1819-1825.
60. Khoja SS, Almeida GJ, Chester Wasko M, et al. Association of light-intensity physical activity with lower cardiovascular disease risk burden in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:424-431.
61. Metsios GS, Koutedakis Y, Veldhuijzen van Zanten JJ, et al. Cardiorespiratory fitness levels and their association with cardiovascular profile in patients with rheumatoid arthritis: A cross-sectional study. Rheumatology (Oxford). 2015;54:2215-2220.
62. Escalante A, Haas RW, del Rincon I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: Role of comorbidity and systemic inflammation. Arch Intern Med. 2005;165:1624-1629.
63. Kremers HM, Nicola PJ, Crowson CS, et al. Prognostic importance of low body mass index in relation to cardiovascular mortality in rheumatoid arthritis. Arthritis Rheum. 2004;50:3450-3457.
64. Wolfe F, Michaud K. Effect of body mass index on mortality and clinical status in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:1471-1479.
65. England BR, Baker JF, Sayles H, et al. Body mass index, weight loss, and cause-specific mortality in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2018;70:11-18.
66. Dessein PH, Solomon A, Hollan I. Metabolic abnormalities in patients with inflammatory rheumatic diseases. Best Pract Res Clin Rheumatol. 2016;30:901-915.
67. Sparks JA, Halperin F, Karlson JC, et al. Impact of bariatric surgery on patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2015;67:1619-1626.
68. Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 2009;169:659-669.
69. England BR, Sayles H, Michaud K, et al. Cause-specific mortality in male US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:36-45.
70. Arts EE, Fransen J, Den Broeder AA, et al. Low disease activity (DAS28≤3.2) reduces the risk of first cardiovascular event in rheumatoid arthritis: a time-dependent Cox regression analysis in a large cohort study. Ann Rheum Dis. 2017;76(10):1693-1699.
71. Provan SA, Semb AG, Hisdal J, et al. Remission is the goal for cardiovascular risk management in patients with rheumatoid arthritis: A cross-sectional comparative study. Ann Rheum Dis. 2011;70:812-817.
72. Klarenbeek NB, van der Kooij SM, Huizinga TJ, et al. Blood pressure changes in patients with recent-onset rheumatoid arthritis treated with four different treatment strategies: A post hoc analysis from the BeSt trial. Ann Rheum Dis. 2010;69:1342-1345.
73. Hafstrom I, Rohani M, Deneberg S, et al. Effects of low-dose prednisolone on endothelial function, atherosclerosis, and traditional risk factors for atherosclerosis in patients with rheumatoid arthritis—a randomized study. J Rheumatol. 2007;34:1810-1816.
74. Hoes JN, van der Goes MC, van Raalte DH, et al. Glucose tolerance, insulin sensitivity and beta-cell function in patients with rheumatoid arthritis treated with or without low-to-medium dose glucocorticoids. Ann Rheum Dis. 2011;70:1887-1894.
75. Roubille C. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: A systematic review and meta-analysis. Ann Rheum Dis. 2003;74:480-489.
76. Ajeganova S, Svensson B, Hafstrom I, BARFOT Study Group. Low-dose prednisolone treatment of early rheumatoid arthritis and late cardiovascular outcome and survival: 10-year follow-up of a 2-year randomised trial. BMJ Open. 2014;4:e004259,2013-004259.
77. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
78. del Rincon I, Battafarano DF, Restrepo JF, et al. Glucocorticoid dose thresholds associated with all-cause and cardiovascular mortality in rheumatoid arthritis. Arthritis Rheumatol. 2014;66:264-272.
79. Davis JM,3rd, Maradit Kremers H, Crowson CS, et al. Glucocorticoids and cardiovascular events in rheumatoid arthritis: A population-based cohort study. Arthritis Rheum. 2007;56:820-830.
80. Zhang J, Xie F, Yun H, et al. Comparative effects of biologics on cardiovascular risk among older patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1813-1818.
81. Greenberg JD, Kremer JM, Curtis JR, et al. Tumour necrosis factor antagonist use and associated risk reduction of cardiovascular events among patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70:576-582.
82. Lindhardsen J, Gislason GH, Jacobsen S, et al. Non-steroidal anti-inflammatory drugs and risk of cardiovascular disease in patients with rheumatoid arthritis: A nationwide cohort study. Ann Rheum Dis. 2014;73:1515-1521.
83. Schjerning Olsen AM, Fosbol EL, Lindhardsen J, et al. Duration of treatment with nonsteroidal anti-inflammatory drugs and impact on risk of death and recurrent myocardial infarction in patients with prior myocardial infarction: A nationwide cohort study. Circulation. 2011;123:2226-2235.
84. Gislason GH, Rasmussen JN, Abildstrom SZ, et al. Increased mortality and cardiovascular morbidity associated with use of nonsteroidal anti-inflammatory drugs in chronic heart failure. Arch Intern Med. 2009;169:141-149.
85. Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: Network meta-analysis. BMJ. 2011;342:c7086.
86. Nissen SE, Yeomans ND, Solomon DH, et al. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N Engl J Med. 2016;375:2519-2529.
87. Wasko MC, Dasgupta A, Hubert Het al. Propensity-adjusted association of methotrexate with overall survival in rheumatoid arthritis. Arthritis Rheum. 2013;65:334-342.
88. Micha R, Imamura F, Wyler von Ballmoos M, et al. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol. 2011;108:1362-1370.
89. Bernatsky S, Hudson M, Suissa S. Anti-rheumatic drug use and risk of hospitalization for congestive heart failure in rheumatoid arthritis. Rheumatology (Oxford). 2005;44:677-680.
90. Myasoedova E, Crowson CS, Nicola PJ, et al. The influence of rheumatoid arthritis disease characteristics on heart failure. J Rheumatol. 2011;38:1601-1606.
91. Ronda N, Greco D, Adorni MP, et al. Newly identified antiatherosclerotic activity of methotrexate and adalimumab: Complementary effects on lipoprotein function and macrophage cholesterol metabolism. Arthritis Rheumatol. 2015;67:1155-1164.
92. Zimmerman MC, Clemens DL, Duryee MJ, et al. Direct antioxidant properties of methotrexate: Inhibition of malondialdehyde-acetaldehyde-protein adduct formation and superoxide scavenging. Redox Biol. 2017;13:588-593.
93. Rempenault C, Combe B, Barnetche T, et al. Metabolic and cardiovascular benefits of hydroxychloroquine in patients with rheumatoid arthritis: A systematic review and meta-analysis. Ann Rheum Dis. 2018;77:98-103.
94. Wasko MC, Hubert HB, Lingala VB, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. 2007;298:187-193.
95. Charles-Schoeman C, Wang X, Lee YY, et al. Association of triple therapy with improvement in cholesterol profiles over two-year followup in the treatment of early aggressive rheumatoid arthritis trial. Arthritis Rheumatol. 2016;68:577-586.
96. Charles-Schoeman C, Yin Lee Y, Shahbazian A, et al. Improvement of high-density lipoprotein function in patients with early rheumatoid arthritis treated with methotrexate monotherapy or combination therapies in a randomized controlled trial. Arthritis Rheumatol. 2017;69:46-57.
97. Chung ES, Packer M, Lo KH, , Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: Results of the anti-TNF therapy against congestive heart failure (ATTACH) trial. Circulation. 2003;107:3133-3140.
98. Singh JA, Saag KG, Bridges SL, Jr, et al. 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1-26.
99. Daien CI, Duny Y, Barnetche Tet al. Effect of TNF inhibitors on lipid profile in rheumatoid arthritis: A systematic review with meta-analysis. Ann Rheum Dis. 2012;71:862-868.
100. Di Minno MN, Ambrosino P, Peluso R, et al. Lipid profile changes in patients with rheumatic diseases receiving a treatment with TNF-alpha blockers: A meta-analysis of prospective studies. Ann Med. 2014;46:73-83.
101. Popa C, van Tits LJ, Barrera P, et al. Anti-inflammatory therapy with tumour necrosis factor alpha inhibitors improves high-density lipoprotein cholesterol antioxidative capacity in rheumatoid arthritis patients. Ann Rheum Dis. 2009;68:868-872.
102. O’Neill F, Charakida M, Topham E, et al. Anti-inflammatory treatment improves high-density lipoprotein function in rheumatoid arthritis. Heart. 2017;103:766-773.
103. Nishimoto N, Ito K, Takagi N. Safety and efficacy profiles of tocilizumab monotherapy in Japanese patients with rheumatoid arthritis: Meta-analysis of six initial trials and five long-term extensions. Mod Rheumatol. 2010;20:222-232.
104. Rao VU, Pavlov A, Klearman M, et al. An evaluation of risk factors for major adverse cardiovascular events during tocilizumab therapy. Arthritis Rheumatol. 2015;67:372-380.
105. Gabay C, McInnes IB, Kavanaugh A, et al. Comparison of lipid and lipid-associated cardiovascular risk marker changes after treatment with tocilizumab or adalimumab in patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1806-1812.
106. McInnes IB, Thompson L, Giles JT, et al. Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann Rheum Dis. 2015;74:694-702.
107. Souto A, Salgado E, Maneiro JR, et al. Lipid profile changes in patients with chronic inflammatory arthritis treated with biologic agents and tofacitinib in randomized clinical trials: A systematic review and meta-analysis. Arthritis Rheumatol. 2015;67:117-127.
108. Myasoedova E, Gabriel SE, Matteson EL, et al. Decreased cardiovascular mortality in patients with incident rheumatoid arthritis (RA) in recent years: Dawn of a new era in cardiovascular disease in RA? J Rheumatol. 2017;44:732-739.
109. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2889-2934.
110. Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. public health service report. Am J Prev Med. 2008;35:158-176.
111. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American college of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2960-2984.
112. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342-362.
113. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American college of cardiology/American heart association task force on practice guidelines and the obesity society. J Am Coll Cardiol. 2014;63:2985-3023.
1. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
2. Avina-Zubieta JA, Thomas J, Sadatsafavi M, et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann Rheum Dis. 2012;71:1524-1529.
3. Johnson TM, Mikuls TR, England BR. Assessment of cardiovascular risk in rheumatoid arthritis. J Clin Outcomes Manage. 2019;26:41-47.
4. Sandoo A, Chanchlani N, Hodson J, et al. Classical cardiovascular disease risk factors associate with vascular function and morphology in rheumatoid arthritis: A six-year prospective study. Arthritis Res Ther. 2013;15:R203.
5. Myasoedova E, Crowson CS, Kremers HM, et al. Total cholesterol and LDL levels decrease before rheumatoid arthritis. Ann Rheum Dis. 2010;69:1310-1314.
6. Liao KP, Cai T, Gainer VS, et al. Lipid and lipoprotein levels and trend in rheumatoid arthritis compared to the general population. Arthritis Care Res (Hoboken). 2013;65:2046-2050.
7. Myasoedova E, Crowson CS, Kremers HM, et al. Lipid paradox in rheumatoid arthritis: The impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis. 2011;70:482-487.
8. Zhang J, Chen L, Delzell E, et al. Republished: The association between inflammatory markers, serum lipids and the risk of cardiovascular events in patients with rheumatoid arthritis. Postgrad Med J. 2014;90:722-729.
9. Liao KP, Liu J, Lu B, et al. Association between lipid levels and major adverse cardiovascular events in rheumatoid arthritis compared to non-rheumatoid arthritis patients. Arthritis Rheumatol. 2015;67:2004-2010.
10. Charles-Schoeman C, Lee YY, Grijalva V, et al. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann Rheum Dis. 2012;71:1157-1162.
11. Voloshyna I, Modayil S, Littlefield MJ, et al. Plasma from rheumatoid arthritis patients promotes pro-atherogenic cholesterol transport gene expression in THP-1 human macrophages. Exp Biol Med (Maywood). 2013 238:1192-1197.
12. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;(1):CD004816.
13. Sheng X, Murphy MJ, Macdonald TM, Wei L. Effectiveness of statins on total cholesterol and cardiovascular disease and all-cause mortality in osteoarthritis and rheumatoid arthritis. J Rheumatol. 2012;39:32-40.
14. An J, Alemao E, Reynolds K, et al. Cardiovascular outcomes associated with lowering low-density lipoprotein cholesterol in rheumatoid arthritis and matched nonrheumatoid arthritis. J Rheumatol. 2016;43:1989-1996.
15. Semb AG, Holme I, Kvien TK, Pedersen TR. Intensive lipid lowering in patients with rheumatoid arthritis and previous myocardial infarction: An explorative analysis from the incremental decrease in endpoints through aggressive lipid lowering (IDEAL) trial. Rheumatology (Oxford). 2011;50:324-329.
16. Semb AG, Kvien TK, DeMicco DA, et al. Effect of intensive lipid-lowering therapy on cardiovascular outcome in patients with and those without inflammatory joint disease. Arthritis Rheum. 2012;64:2836-2846.
17. El-Barbary AM, Hussein MS, Rageh EM, et al. Effect of atorvastatin on inflammation and modification of vascular risk factors in rheumatoid arthritis. J Rheumatol. 2011;38:229-235.
18. Writing Committee, Lloyd-Jones DM, Morris PB, et al. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: A report of the American college of cardiology task force on clinical expert consensus documents. J Am Coll Cardiol. 2016;68:92-125.
19. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
20. Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509.
21. Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499.
22. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
23. Maki-Petaja KM, Booth AD, Hall FC, et al. Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. J Am Coll Cardiol. 2007;50:852-858.
24. Bartels CM, Kind AJ, Everett C, et al. Low frequency of primary lipid screening among medicare patients with rheumatoid arthritis. Arthritis Rheum. 2011;63:1221-1230.
25. Akkara Veetil BM, Myasoedova E, Matteson EL, et al. Use of lipid-lowering agents in rheumatoid arthritis: A population-based cohort study. J Rheumatol. 2013;40:1082-1088.
26. Lindhardsen J, Ahlehoff O, Gislason GH, et al. Initiation and adherence to secondary prevention pharmacotherapy after myocardial infarction in patients with rheumatoid arthritis: A nationwide cohort study. Ann Rheum Dis. 2012;71:1496-1501.
27. De Vera MA, Choi H, Abrahamowicz M, et al. Statin discontinuation and risk of acute myocardial infarction in patients with rheumatoid arthritis: A population-based cohort study. Ann Rheum Dis. 2011;70:1020-1024.
28. Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: A cohort study. Ann Intern Med. 2013;158:526-534.
29. Zhang H, Plutzky J, Shubina M, Turchin A. Continued statin prescriptions after adverse reactions and patient outcomes: A cohort study. Ann Intern Med. 2017;167:221-227.
30. Lemstra M, Blackburn D, Crawley A, Fung R. Proportion and risk indicators of nonadherence to statin therapy: A meta-analysis. Can J Cardiol. 2012;28:574-580.
31. Boyer JF, Gourraud PA, Cantagrel A, et al. Traditional cardiovascular risk factors in rheumatoid arthritis: A meta-analysis. Joint Bone Spine. 2011;78:179-183.
32. Bergstrom U, Jacobsson LT, Nilsson JA, et al. Pulmonary dysfunction, smoking, socioeconomic status and the risk of developing rheumatoid arthritis. Rheumatology (Oxford). 2011;50:2005-2013.
33. Costenbader KH, Feskanich D, Mandl LA, Karlson EW. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am J Med. 2006;119:503.e1,503.e9.
34. Klareskog L, Stolt P, Lundberg K, et al. A new model for an etiology of rheumatoid arthritis: Smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54:38-46.
35. Sokolove J, Wagner CA, Lahey LJ, et al. Increased inflammation and disease activity among current cigarette smokers with rheumatoid arthritis: A cross-sectional analysis of US veterans. Rheumatology (Oxford). 2016;55:1969-1977.
36. World Health Organization. WHO Global Report: Mortality Attributable to Tobacco. Geneva, World Health Organization, 2012.
37. Baghdadi LR, Woodman RJ, Shanahan EM, Mangoni AA. The impact of traditional cardiovascular risk factors on cardiovascular outcomes in patients with rheumatoid arthritis: A systematic review and meta-analysis. PLoS One. 2015;10:e0117952.
38. Centers for Disease Control and Prevention; National Center for Chronic Disease Prevention and Health Promotion. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention; 2010. 6, Cardiovascular Diseases. Available from: https://ncbi.nlm.nih.gov/books/NBK53012/
39. Mons U, Muezzinler A, Gellert C, et al. Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: Meta-analysis of individual participant data from prospective cohort studies of the CHANCES consortium. BMJ. 2015;350:h1551.
40. Aimer P, Treharne GJ, Stebbings S, Frampton C, Cameron V, Kirby S, et al. Efficacy of a rheumatoid arthritis-specific smoking cessation program: A randomized controlled pilot trial. Arthritis Care Res (Hoboken). 2017;69:28-37.
41. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2017.
42. Jiang P, Li H, Li X. Diabetes mellitus risk factors in rheumatoid arthritis: A systematic review and meta-analysis. Clin Exp Rheumatol. 2015;33:115-121.
43. Shahin D, Eltoraby E, Mesbah A, Houssen M. Insulin resistance in early untreated rheumatoid arthritis patients. Clin Biochem. 2010;43:661-335.
44. Arias de la Rosa I, Escudero-Contreras A, Rodriguez-Cuenca S, et al. Defective glucose and lipid metabolism in rheumatoid arthritis is determined by chronic inflammation in metabolic tissues. J Intern Med. 2018;84(1):61-77.
45. Wilson JC, Sarsour K, Gale S, et al. Incidence and risk of glucocorticoid-associated adverse effects in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2018 Jun 1. doi: 10.1002/acr.23611.
46. Chung CP, Giles JT, Petri M, et al. Prevalence of traditional modifiable cardiovascular risk factors in patients with rheumatoid arthritis: Comparison with control subjects from the multi-ethnic study of atherosclerosis. Semin Arthritis Rheum. 2012;41:535-544.
47. Goodwin JE, Geller DS. Glucocorticoid-induced hypertension. Pediatr Nephrol. 2012;27:1059-1066.
48. Snowden S, Nelson R. The effects of nonsteroidal anti-inflammatory drugs on blood pressure in hypertensive patients. Cardiol Rev. 2011;19:184-191.
49. Baker JF, Sauer B, Teng CC, et al. Initiation of disease-modifying therapies in rheumatoid arthritis is associated with changes in blood pressure. J Clin Rheumatol. 2018;24:203-209.
50. Panoulas VF, Douglas KM, Milionis HJ, et al. Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology (Oxford). 2007;46:1477-1482.
51. Protogerou AD, Panagiotakos DB, Zampeli E, et al. Arterial hypertension assessed “out-of-office” in a contemporary cohort of rheumatoid arthritis patients free of cardiovascular disease is characterized by high prevalence, low awareness, poor control and increased vascular damage-associated “white coat” phenomenon. Arthritis Res Ther. 2013;15:R142.
52. van Breukelen-van der Stoep DF, van Zeben D, Klop B, et al. Marked underdiagnosis and undertreatment of hypertension and hypercholesterolaemia in rheumatoid arthritis. Rheumatology (Oxford). 2016;55:1210-1216.
53. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71:e127-248.
54. Lee J, Dunlop D, Ehrlich-Jones L, et al. Public health impact of risk factors for physical inactivity in adults with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:488-493.
55. Sokka T, Hakkinen A, Kautiainen H, et al. Physical inactivity in patients with rheumatoid arthritis: Data from twenty-one countries in a cross-sectional, international study. Arthritis Rheum. 2008;59:42-50.
56. Fenton SAM, Veldhuijzen van Zanten JJCS, Kitas GD, et al. Sedentary behaviour is associated with increased long-term cardiovascular risk in patients with rheumatoid arthritis independently of moderate-to-vigorous physical activity. BMC Musculoskelet Disord. 2017;18:131,017-1473-9.
57. Byram KW, Oeser AM, Linton MF, et al. Exercise is associated with increased small HDL particle concentration and decreased vascular stiffness in rheumatoid arthritis. J Clin Rheumatol. 2018 May 25. 9.
58. de Jong Z, Munneke M, Zwinderman AH, et al. Is a long-term high-intensity exercise program effective and safe in patients with rheumatoid arthritis? results of a randomized controlled trial. Arthritis Rheum. 2003;48:2415-2424.
59. Stavropoulos-Kalinoglou A, Metsios GS, Veldhuijzen van Zanten JJ, et al. Individualised aerobic and resistance exercise training improves cardiorespiratory fitness and reduces cardiovascular risk in patients with rheumatoid arthritis. Ann Rheum Dis. 2013;72:1819-1825.
60. Khoja SS, Almeida GJ, Chester Wasko M, et al. Association of light-intensity physical activity with lower cardiovascular disease risk burden in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:424-431.
61. Metsios GS, Koutedakis Y, Veldhuijzen van Zanten JJ, et al. Cardiorespiratory fitness levels and their association with cardiovascular profile in patients with rheumatoid arthritis: A cross-sectional study. Rheumatology (Oxford). 2015;54:2215-2220.
62. Escalante A, Haas RW, del Rincon I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: Role of comorbidity and systemic inflammation. Arch Intern Med. 2005;165:1624-1629.
63. Kremers HM, Nicola PJ, Crowson CS, et al. Prognostic importance of low body mass index in relation to cardiovascular mortality in rheumatoid arthritis. Arthritis Rheum. 2004;50:3450-3457.
64. Wolfe F, Michaud K. Effect of body mass index on mortality and clinical status in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:1471-1479.
65. England BR, Baker JF, Sayles H, et al. Body mass index, weight loss, and cause-specific mortality in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2018;70:11-18.
66. Dessein PH, Solomon A, Hollan I. Metabolic abnormalities in patients with inflammatory rheumatic diseases. Best Pract Res Clin Rheumatol. 2016;30:901-915.
67. Sparks JA, Halperin F, Karlson JC, et al. Impact of bariatric surgery on patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2015;67:1619-1626.
68. Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 2009;169:659-669.
69. England BR, Sayles H, Michaud K, et al. Cause-specific mortality in male US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:36-45.
70. Arts EE, Fransen J, Den Broeder AA, et al. Low disease activity (DAS28≤3.2) reduces the risk of first cardiovascular event in rheumatoid arthritis: a time-dependent Cox regression analysis in a large cohort study. Ann Rheum Dis. 2017;76(10):1693-1699.
71. Provan SA, Semb AG, Hisdal J, et al. Remission is the goal for cardiovascular risk management in patients with rheumatoid arthritis: A cross-sectional comparative study. Ann Rheum Dis. 2011;70:812-817.
72. Klarenbeek NB, van der Kooij SM, Huizinga TJ, et al. Blood pressure changes in patients with recent-onset rheumatoid arthritis treated with four different treatment strategies: A post hoc analysis from the BeSt trial. Ann Rheum Dis. 2010;69:1342-1345.
73. Hafstrom I, Rohani M, Deneberg S, et al. Effects of low-dose prednisolone on endothelial function, atherosclerosis, and traditional risk factors for atherosclerosis in patients with rheumatoid arthritis—a randomized study. J Rheumatol. 2007;34:1810-1816.
74. Hoes JN, van der Goes MC, van Raalte DH, et al. Glucose tolerance, insulin sensitivity and beta-cell function in patients with rheumatoid arthritis treated with or without low-to-medium dose glucocorticoids. Ann Rheum Dis. 2011;70:1887-1894.
75. Roubille C. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: A systematic review and meta-analysis. Ann Rheum Dis. 2003;74:480-489.
76. Ajeganova S, Svensson B, Hafstrom I, BARFOT Study Group. Low-dose prednisolone treatment of early rheumatoid arthritis and late cardiovascular outcome and survival: 10-year follow-up of a 2-year randomised trial. BMJ Open. 2014;4:e004259,2013-004259.
77. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
78. del Rincon I, Battafarano DF, Restrepo JF, et al. Glucocorticoid dose thresholds associated with all-cause and cardiovascular mortality in rheumatoid arthritis. Arthritis Rheumatol. 2014;66:264-272.
79. Davis JM,3rd, Maradit Kremers H, Crowson CS, et al. Glucocorticoids and cardiovascular events in rheumatoid arthritis: A population-based cohort study. Arthritis Rheum. 2007;56:820-830.
80. Zhang J, Xie F, Yun H, et al. Comparative effects of biologics on cardiovascular risk among older patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1813-1818.
81. Greenberg JD, Kremer JM, Curtis JR, et al. Tumour necrosis factor antagonist use and associated risk reduction of cardiovascular events among patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70:576-582.
82. Lindhardsen J, Gislason GH, Jacobsen S, et al. Non-steroidal anti-inflammatory drugs and risk of cardiovascular disease in patients with rheumatoid arthritis: A nationwide cohort study. Ann Rheum Dis. 2014;73:1515-1521.
83. Schjerning Olsen AM, Fosbol EL, Lindhardsen J, et al. Duration of treatment with nonsteroidal anti-inflammatory drugs and impact on risk of death and recurrent myocardial infarction in patients with prior myocardial infarction: A nationwide cohort study. Circulation. 2011;123:2226-2235.
84. Gislason GH, Rasmussen JN, Abildstrom SZ, et al. Increased mortality and cardiovascular morbidity associated with use of nonsteroidal anti-inflammatory drugs in chronic heart failure. Arch Intern Med. 2009;169:141-149.
85. Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: Network meta-analysis. BMJ. 2011;342:c7086.
86. Nissen SE, Yeomans ND, Solomon DH, et al. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N Engl J Med. 2016;375:2519-2529.
87. Wasko MC, Dasgupta A, Hubert Het al. Propensity-adjusted association of methotrexate with overall survival in rheumatoid arthritis. Arthritis Rheum. 2013;65:334-342.
88. Micha R, Imamura F, Wyler von Ballmoos M, et al. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol. 2011;108:1362-1370.
89. Bernatsky S, Hudson M, Suissa S. Anti-rheumatic drug use and risk of hospitalization for congestive heart failure in rheumatoid arthritis. Rheumatology (Oxford). 2005;44:677-680.
90. Myasoedova E, Crowson CS, Nicola PJ, et al. The influence of rheumatoid arthritis disease characteristics on heart failure. J Rheumatol. 2011;38:1601-1606.
91. Ronda N, Greco D, Adorni MP, et al. Newly identified antiatherosclerotic activity of methotrexate and adalimumab: Complementary effects on lipoprotein function and macrophage cholesterol metabolism. Arthritis Rheumatol. 2015;67:1155-1164.
92. Zimmerman MC, Clemens DL, Duryee MJ, et al. Direct antioxidant properties of methotrexate: Inhibition of malondialdehyde-acetaldehyde-protein adduct formation and superoxide scavenging. Redox Biol. 2017;13:588-593.
93. Rempenault C, Combe B, Barnetche T, et al. Metabolic and cardiovascular benefits of hydroxychloroquine in patients with rheumatoid arthritis: A systematic review and meta-analysis. Ann Rheum Dis. 2018;77:98-103.
94. Wasko MC, Hubert HB, Lingala VB, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. 2007;298:187-193.
95. Charles-Schoeman C, Wang X, Lee YY, et al. Association of triple therapy with improvement in cholesterol profiles over two-year followup in the treatment of early aggressive rheumatoid arthritis trial. Arthritis Rheumatol. 2016;68:577-586.
96. Charles-Schoeman C, Yin Lee Y, Shahbazian A, et al. Improvement of high-density lipoprotein function in patients with early rheumatoid arthritis treated with methotrexate monotherapy or combination therapies in a randomized controlled trial. Arthritis Rheumatol. 2017;69:46-57.
97. Chung ES, Packer M, Lo KH, , Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: Results of the anti-TNF therapy against congestive heart failure (ATTACH) trial. Circulation. 2003;107:3133-3140.
98. Singh JA, Saag KG, Bridges SL, Jr, et al. 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1-26.
99. Daien CI, Duny Y, Barnetche Tet al. Effect of TNF inhibitors on lipid profile in rheumatoid arthritis: A systematic review with meta-analysis. Ann Rheum Dis. 2012;71:862-868.
100. Di Minno MN, Ambrosino P, Peluso R, et al. Lipid profile changes in patients with rheumatic diseases receiving a treatment with TNF-alpha blockers: A meta-analysis of prospective studies. Ann Med. 2014;46:73-83.
101. Popa C, van Tits LJ, Barrera P, et al. Anti-inflammatory therapy with tumour necrosis factor alpha inhibitors improves high-density lipoprotein cholesterol antioxidative capacity in rheumatoid arthritis patients. Ann Rheum Dis. 2009;68:868-872.
102. O’Neill F, Charakida M, Topham E, et al. Anti-inflammatory treatment improves high-density lipoprotein function in rheumatoid arthritis. Heart. 2017;103:766-773.
103. Nishimoto N, Ito K, Takagi N. Safety and efficacy profiles of tocilizumab monotherapy in Japanese patients with rheumatoid arthritis: Meta-analysis of six initial trials and five long-term extensions. Mod Rheumatol. 2010;20:222-232.
104. Rao VU, Pavlov A, Klearman M, et al. An evaluation of risk factors for major adverse cardiovascular events during tocilizumab therapy. Arthritis Rheumatol. 2015;67:372-380.
105. Gabay C, McInnes IB, Kavanaugh A, et al. Comparison of lipid and lipid-associated cardiovascular risk marker changes after treatment with tocilizumab or adalimumab in patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1806-1812.
106. McInnes IB, Thompson L, Giles JT, et al. Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann Rheum Dis. 2015;74:694-702.
107. Souto A, Salgado E, Maneiro JR, et al. Lipid profile changes in patients with chronic inflammatory arthritis treated with biologic agents and tofacitinib in randomized clinical trials: A systematic review and meta-analysis. Arthritis Rheumatol. 2015;67:117-127.
108. Myasoedova E, Gabriel SE, Matteson EL, et al. Decreased cardiovascular mortality in patients with incident rheumatoid arthritis (RA) in recent years: Dawn of a new era in cardiovascular disease in RA? J Rheumatol. 2017;44:732-739.
109. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2889-2934.
110. Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. public health service report. Am J Prev Med. 2008;35:158-176.
111. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American college of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2960-2984.
112. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342-362.
113. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American college of cardiology/American heart association task force on practice guidelines and the obesity society. J Am Coll Cardiol. 2014;63:2985-3023.
Assessment of Cardiovascular Disease Risk in Rheumatoid Arthritis
From the Division of Rheumatology & Immunology, University of Nebraska Medical Center, and Veterans Affairs Nebraska-Western Iowa Health Care System, Omaha, NE.
Abstract
- Objective: To review cardiovascular disease (CVD) risk assessment in patients with rheumatoid arthritis (RA).
- Methods: Literature review of the assessment of CVD risk in RA.
- Results: CVD is the leading cause of death among RA patients.
Because of the increased risk of CVD events and CVD mortality in patients with RA, regular assessment of CVD risk and aggressive management of CVD risk in these patients is crucial. CVD risk estimation typically centers on the use of well-established CVD risk calculators. Most CVD risk scores from the general population do not contain RA-related factors predictive of CVD but have had more extensive performance testing, while novel RA-derived CVD risk scores that incorporate RA-related factors have had limited external validity testing. Neither set of risk scores incorporates novel imaging modalities or serum biomarkers, which are most likely to be helpful among individuals at intermediate risk. - Conclusion: Primary care and rheumatology providers must be aware of the increased risk of CVD in RA, a risk that approaches that of diabetic patients.
Routine assessment of CVD risk is an essential first step in minimizing CVD risk in this population. Until the performance of RA-specific CVD risk scores can be better established, we recommend the use of nationally endorsed CVD risk scores, with the frequency of reassessment based on CVD risk.
Keywords: rheumatoid arthritis; cardiovascular disease; cardiovascular risk assessment.
Editor’s note: This article is part 1 of a 2-part article. “Management of Cardiovascular Disease Risk in Rheumatoid Arthritis” was published in the March/April 2019 issue.
Rheumatoid arthritis (RA) is a chronic, autoimmune inflammatory arthritis affecting up to 1% of the US population that can lead to joint damage, functional disability, and reduced quality of life.1 In addition to articular involvement, systemic inflammation accompanying RA may lead to extra-articular manifestations and increase the risk of premature death.2 Cardiovascular disease (CVD), accounting for nearly half of all deaths among RA patients, is now recognized as a critical extra-articular manifestation of RA.2,3 As such, assessment and management of CVD risk is essential to the comprehensive care of the RA patient. This article reviews the approach to assessing CVD risk in patients with RA; the management of both traditional and RA-specific risk factors is discussed in a separate article.
Scope of the Problem
In a large meta-analysis of observational studies that included more than 111,000 patients with RA, CVD-related mortality rates were 1.5 times higher among RA patients than among general population controls.4 The risk of overall CVD, including nonfatal events, is similar; a separate meta-analysis of observational studies that included more than 41,000 patients with RA calculated a pooled relative risk for incident CVD of 1.48.5 Individual analyses identified heightened risk of acute coronary syndrome (ACS), cerebrovascular accident, and congestive heart failure (CHF).5 Perhaps more illustrative of the magnitude of the problem, the risk of CVD in RA approaches that observed among individuals with diabetes mellitus.6,7
Coronary artery disease (CAD) accounts for a significant portion of the CVD risk in RA, but its presentation may be atypical in RA patients. RA patients are at higher risk of suffering unrecognized myocardial infarction (MI) and sudden cardiac death.8 The reasons for silent ischemia in RA are not fully known, but have been hypothesized to include imbalances of inflammatory cytokines, alterations in pain sensitization, or the female predominance of RA (with women more often presenting with atypical symptoms of myocardial ischemia).9 Alarmingly, a retrospective chart review study reported that RA patients admitted for an acute MI were less likely to receive appropriate reperfusion therapy as well as secondary prevention with beta-blockers and lipid-lowering agents.10 Even with appropriate therapy, long-term outcomes such as mortality and recurrent ischemic events are more likely to occur in RA patients after acute MI.11-13
Independent of ischemic heart disease, RA patients are at increased risk of CHF.14-16 RA patients are at particular risk for CHF with preserved ejection fraction,17 which may be a result of systemic inflammation causing left ventricular stiffening.18,19 Similar to CAD, patients with RA are less likely to present with typical CHF symptoms, are less likely to receive guideline-concordant care, and have higher mortality rates following presentation with CHF.17
Although accounting for a lower proportion of the excess CVD morbidity and mortality in RA, the risk of noncardiac vascular disease is also increased in RA patients. Large meta-analyses have identified positive associations between RA with both ischemic (odds ratio [OR], 1.64 [95% confidence interval {CI}, 1.32-2.05]) and hemorrhagic (OR, 1.68 [95% CI, 1.11-2.53]) stroke.20 Similarly, RA patients appear to have an approximately twofold higher risk of venous thromboembolic events.21 Less frequently studied than other forms of CVD, peripheral arterial disease may be increased in RA patients independent of other CVD and CVD risk factors.22,23
Assessing CVD Risk in RA
CVD Risk Scores
In order to identify patients who may benefit from primary prevention interventions, such as lipid-lowering therapy, CVD risk estimation typically centers on the use of well-established CVD risk calculators (Table). CVD risk scores such as the Framingham Risk Score (FRS), Systematic Coronary Risk Evaluation (SCORE), and American College of Cardiology/ American Heart Association (ACC/AHA) Pooled Cohort Equation incorporate traditional CVD risk factors, including age, sex, smoking status, blood pressure, lipid levels, and presence of diabetes mellitus.24,25 However, CVD risk in RA patients appears to be inadequately explained by traditional CVD risk factors,26 with disease activity and inflammation being associated with higher CVD risk. Recognizing that inflammation may contribute to CVD risk even among non-RA patients, the Reynolds Risk Score includes high-sensitivity C-reactive protein (hsCRP) in its calculation.27 In contrast to more robust performance in the general population, these well-established CVD risk scores have had variable predictive potential of incident CVD in RA patients.28-30
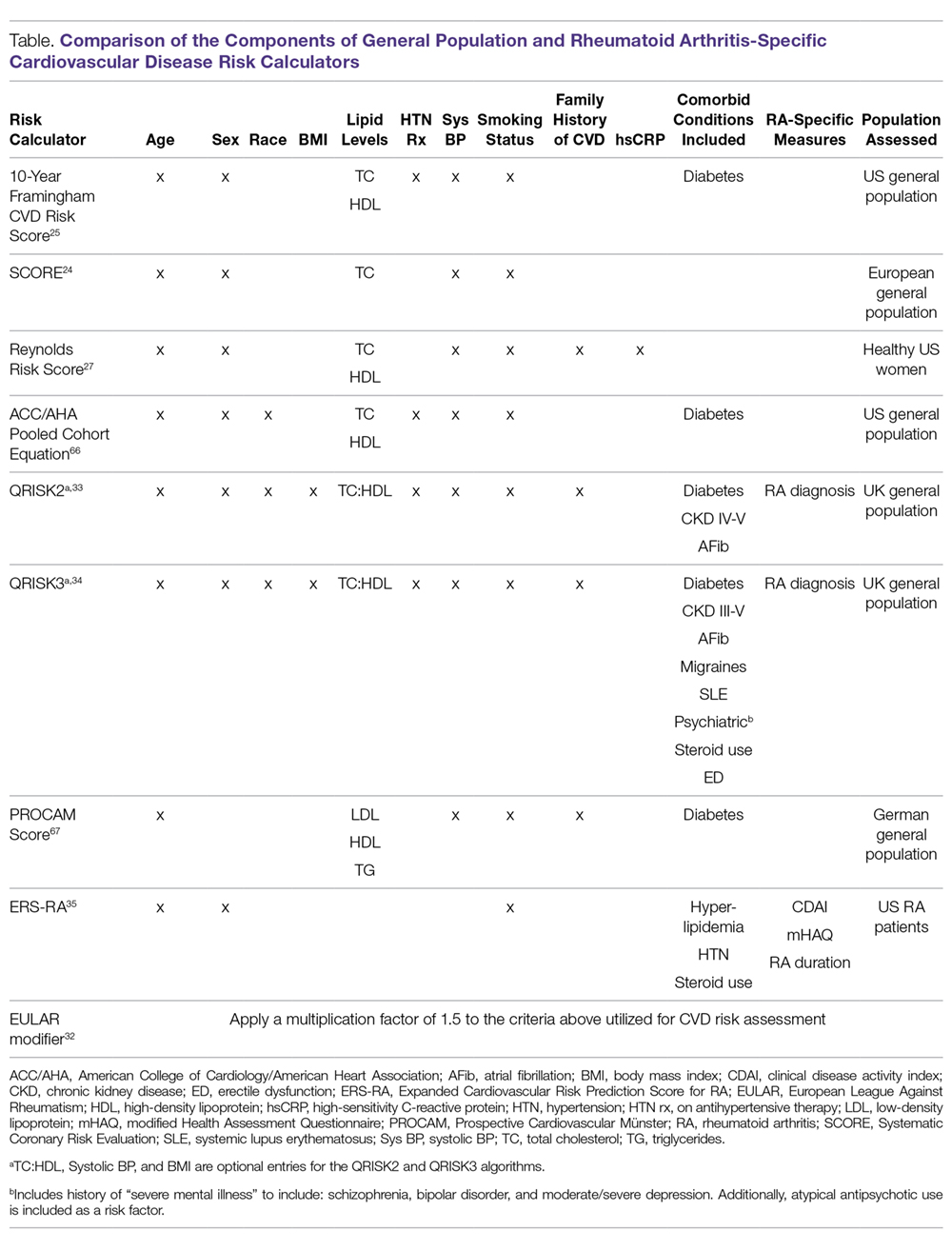
Several models, or adaptations to existing models, have been proposed to improve CVD risk assessment in RA populations (Table). In 2009, the European League Against Rheumatism (EULAR) task force suggested using a correction factor of 1.5 with traditional CVD risk models in RA patients with 2 of the following criteria: disease duration exceeding 10 years, rheumatoid factor or anti-cyclic citrullinated peptide (CCP) antibody positivity, or extra-articular manifestations of RA.31 An update to these recommendations in 2015 continued to propose the use of a 1.5 correction factor, but suggested applying this to all RA patients.32 QRISK2, a modification to QRISK1 which was developed to predict CVD in the UK general population, includes the diagnosis of RA as a risk factor, and in early validation efforts more accurately discriminated patients in the general population at increased risk of CVD compared to the FRS.33 Additional disease-specific risk factors such as systemic lupus, steroid use, severe mental illness, and steroid and atypical antipsychotic use were incorporated in the QRISK3 algorithm, with model performance similar to the QRISK2.34 The Expanded Cardiovascular Risk Prediction Score for RA (ERS-RA) was specifically developed to assess CVD risk in RA patients by including RA disease activity, level of physical disability, RA disease duration, and prednisone use.35 Despite efforts to develop “RA-specific” risk scores, these have not consistently outperformed traditional CVD risk calculators.36-38 In one study involving more than 1700 RA patients, the ERS-RA performed similarly to the FRS and Reynolds Risk Score, with a net reclassification index of just 2.3% versus the FRS.36
Imaging Modalities
Imaging modalities may assist in characterizing the increased risk of CVD in RA and the subclinical CVD manifestations that occur. For example, RA patients were shown to have more prevalent and unstable coronary plaque, higher carotid intima media thickness, and impaired myocardial function with computed tomography (CT) angiography and carotid ultrasound.39,40 However, studies harnessing noninvasive imaging to augment CVD risk assessment in RA patients are limited.
Carotid ultrasound has been the most extensively studied imaging modality for CVD risk assessment in RA. In a cohort of 599 RA patients with no history of ACS, rates of ACS were nearly 4 times higher in RA patients with bilateral carotid plaque on carotid ultrasound, and the association with ACS was independent of other traditional and RA-related risk factors.41 Presence of bilateral carotid plaques was similarly associated with an increased risk of overall CVD events (hazard ratio [HR], 3.34 [95% CI, 1.21-9.22]), ACS alone (HR, 6.31 [95% CI, 1.27-31.40]), and a lower mean CVD event-free survival (13.9 versus 15.2 years, P = 0.01) in a separate inception cohort of 105 RA patients with no prior history of CVD.42 The most useful application of carotid ultrasound may be in conjunction with clinical CVD risk models. Use of carotid ultrasound improved CVD risk stratification among RA patients who were considered at moderate risk by the EULAR-modified SCORE calculator.43 Beyond carotid ultrasound, measurement of arterial stiffness through ultrasound could also aid in CVD risk stratification. Aortic pulse wave velocity and augmentation index, measures of arterial stiffness, are predictive of CVD in the general population as well as RA patients and improve with reduction in RA disease activity.44,45 Peripheral arterial stiffness (brachial-ankle elasticity index) is impaired in RA patients and predictive of CVD morbidity and mortality in the general population.46,47
CT coronary angiography and coronary artery calcium (CAC) scores are reliable measures of coronary artery atherosclerosis and have been validated for CVD risk assessment in the general population.48-52 While the association between RA and CT-related findings of atherosclerosis is well established, assessment of CT-mediated evaluation as a prognostic tool for CVD in RA is limited. In one cohort study, CAC predicted higher rates of CVD events in Chinese patients with RA and systemic lupus erythematosus in a pooled analysis, although results were limited by low event rates and the absence of RA-only subanalyses.53
While the aforementioned imaging modalities have focused on enhancing the identification of atherosclerosis, echocardiography or cardiac magnetic resonance imaging (MRI) may be useful for detecting subclinical structural and/or functional abnormalities that predispose to CHF. Structural abnormalities including increased left ventricular mass and hypertrophy are more prevalent in RA patients and predict incident CHF in the general population.54-56 MRI measures of myocardial inflammation, including T1 mapping and extracellular volume, are associated with higher mortality rates and also appear to be elevated in RA patients.57,58 Whether identification of these imaging findings influences the cost-effective clinical management of RA patients needs further study.
Biomarkers
Serum biomarkers, such as the anti-CCP antibody, have become crucial to the evaluation of patients suspected to have RA. With the growing understanding of the role pro-inflammatory mediators play in CVD pathogenesis and the relative ease with which they can be measured, serum biomarkers have potential to inform CVD risk assessment. In the general population, hsCRP concentrations are predictive of CVD and are included in the Reynolds Risk Score.27 In RA, CRP concentrations are typically much higher than those observed among individuals in the general population solely at increased CVD risk, yet elevated levels remain predictive of CVD death independent of RA disease activity and traditional CVD risk factors.59 Several additional cytokines, chemokines, and adhesion molecules have been associated with surrogate markers of CVD in RA patients, although further study is needed to elucidate thresholds that signify increased CVD risk in a population characterized by the presence of systemic inflammation.60
Cardiac biomarkers used frequently in the general population may be useful to assess CVD risk in RA patients. N-terminal-pro brain natriuretic peptide (NT-pro BNP) is a biomarker typically used to evaluate CHF severity, but it may also predict long-term mortality in patients with coronary heart disease.61,62 Circulating NT-pro BNP concentrations are elevated in RA independent of prevalent CHF and may serve as a useful tool to identify subclinical cardiac disease in RA patients.63 High-sensitivity cardiac troponin I (HS-cTnI) assays are capable of detecting levels of cardiac troponin below the threshold typically used to diagnose ACS. HS-cTnI levels are increased in RA patients independent of additional CVD risk factors, and elevated levels (> 1.5 pg/mL) were associated with more severe CT angiography findings of coronary plaque as well as increased risk of CVD events.64,65
Clinical Application
A fully validated algorithm for CVD risk assessment in RA is lacking. Most CVD risk scores from the general population do not contain RA-related factors predictive of CVD but have had more extensive performance testing. In contrast, novel RA-derived CVD risk scores incorporate RA-related factors, but have had limited external validity testing. Additionally, RA-derived risk scores are less likely to be utilized and adopted by primary care providers and cardiologists involved in RA patients’ care. Neither set of risk scores incorporates novel imaging modalities or serum biomarkers, which are most likely to be helpful among individuals at intermediate risk. Therefore, until the performance of RA-specific CVD risk scores can be better established, we recommend the use of nationally endorsed CVD risk scores, with the frequency of reassessment based on CVD risk.
Conclusion
RA patients are at increased risk of CVD and CVD-related mortality relative to the general population. The disproportionate CVD burden seen in RA appears to be multifactorial, owing to the complex effects of systemic inflammation, endothelial dysfunction, and pro-atherogenic lipoprotein modifications. Additionally, many traditional CVD risk factors are more prevalent and suboptimally managed in RA patients. To mitigate the increased risk of CVD in RA, primary care and subspecialty providers alike must be aware of this heightened risk in RA, perform frequent assessment of CVD risk, and
Corresponding author: Bryant R. England, MD; 986270 Nebraska Medical Center, Omaha, NE 68198-6270; [email protected].
Financial disclosures: Dr. England is supported by UNMC Internal Medicine Scientist Development Award, UNMC Physician-Scientist Training Program, the UNMC Mentored Scholars Program, and the Rheumatology Research Foundation Scientist Development Award. Dr. Mikuls is supported by a VA Merit Award (CX000896) and grants from the National Institutes of Health: National Institute of General Medical Sciences (U54GM115458), National Institute on Alcohol Abuse and Alcoholism (R25AA020818), and National Institute of Arthritis and Musculoskeletal and Skin Diseases (2P50AR60772).
1. Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the united states. part I. Arthritis Rheum. 2008;58:15-25.
2. England BR, Sayles H, Michaud K, et al. Cause-specific mortality in male US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:36-45.
3. Sokka T, Abelson B, Pincus T. Mortality in rheumatoid arthritis: 2008 update. Clin Exp Rheumatol. 2008;26:S35-61.
4. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
5. Avina-Zubieta JA, Thomas J, Sadatsafavi M, et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann Rheum Dis. 2012;71:1524-1529.
6. van Halm VP, Peters MJ, Voskuyl AE, et al. Rheumatoid arthritis versus diabetes as a risk factor for cardiovascular disease: A cross-sectional study, the CARRE investigation. Ann Rheum Dis. 2009;68:1395-1400.
7. Peters MJ, van Halm VP, Voskuyl AE, et al. Does rheumatoid arthritis equal diabetes mellitus as an independent risk factor for cardiovascular disease? A prospective study. Arthritis Rheum. 2009;61:1571-1579.
8. Maradit-Kremers H, Crowson CS, Nicola PJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: A population-based cohort study. Arthritis Rheum. 2005;52:402-411.
9. Cardiovascular disease in women--often silent and fatal. Lancet. 2011;378:200,6736(11)61108-61112.
10. Van Doornum S, Brand C, Sundararajan V, et al. Rheumatoid arthritis patients receive less frequent acute reperfusion and secondary prevention therapy after myocardial infarction compared with the general population. Arthritis Res Ther. 2010;12:R183.
11. Sodergren A, Stegmayr B, Lundberg V, et al. Increased incidence of and impaired prognosis after acute myocardial infarction among patients with seropositive rheumatoid arthritis. Ann Rheum Dis. 2007;66:263-266.
12. Douglas KM, Pace AV, Treharne GJ, et al. Excess recurrent cardiac events in rheumatoid arthritis patients with acute coronary syndrome. Ann Rheum Dis. 2006;65:348-353.
13. McCoy SS, Crowson CS, Maradit-Kremers H, et al. Long-term outcomes and treatment after myocardial infarction in patients with rheumatoid arthritis. J Rheumatol. 2013;40:605-610.
14. Mantel A, Holmqvist M, Andersson DC, et al. Association between rheumatoid arthritis and risk of ischemic and nonischemic heart failure. J Am Coll Cardiol. 2017;69:1275-1285.
15. Crowson CS, Nicola PJ, Kremers HM, et al. How much of the increased incidence of heart failure in rheumatoid arthritis is attributable to traditional cardiovascular risk factors and ischemic heart disease? Arthritis Rheum. 2005;52:3039-3044.
16. Nicola PJ, Maradit-Kremers H, Roger VL, et al. The risk of congestive heart failure in rheumatoid arthritis: A population-based study over 46 years. Arthritis Rheum. 2005;52:412-420.
17. Davis JM,3rd, Roger VL, Crowson CS, et al. The presentation and outcome of heart failure in patients with rheumatoid arthritis differs from that in the general population. Arthritis Rheum. 2008;58:2603-2611.
18. Arslan S, Bozkurt E, Sari RA, Erol MK. Diastolic function abnormalities in active rheumatoid arthritis evaluation by conventional doppler and tissue doppler: Relation with duration of disease. Clin Rheumatol. 2006;25:294-299.
19. Liang KP, Myasoedova E, Crowson CS, et al. Increased prevalence of diastolic dysfunction in rheumatoid arthritis. Ann Rheum Dis. 2010;69:1665-1670.
20. Wiseman SJ, Ralston SH, Wardlaw JM. Cerebrovascular disease in rheumatic diseases: A systematic review and meta-analysis. Stroke. 2016;47:943-950.
21. Ungprasert P, Srivali N, Spanuchart I, et al. Risk of venous thromboembolism in patients with rheumatoid arthritis: A systematic review and meta-analysis. Clin Rheumatol. 2014;33:297-304.
22. Stamatelopoulos KS, Kitas GD, Papamichael CM, et al. Subclinical peripheral arterial disease in rheumatoid arthritis. Atherosclerosis. 2010;212:305-309.
23. Chuang YW, Yu MC, Lin CL, et al. Risk of peripheral arterial occlusive disease in patients with rheumatoid arthritis. A nationwide population-based cohort study. Thromb Haemost. 2016;115:439-445.
24. Conroy RM, Pyorala K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in europe: The SCORE project. Eur Heart J. 2003;24:987-1003.
25. D’Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: The Framingham heart study. Circulation. 2008;117:743-753.
26. del Rincon ID, Williams K, Stern MP, et al. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44:2737-2745.
27. Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: The Reynolds Risk Score. JAMA. 2007;297:611-619.
28. Arts EE, Popa C, Den Broeder AA, et al. Performance of four current risk algorithms in predicting cardiovascular events in patients with early rheumatoid arthritis. Ann Rheum Dis. 2015;74:668-674.
29. Crowson CS, Matteson EL, Roger VL, et al. Usefulness of risk scores to estimate the risk of cardiovascular disease in patients with rheumatoid arthritis. Am J Cardiol. 2012;110:420-424.
30. Kawai VK, Chung CP, Solus JF, et al. The ability of the 2013 American College of Cardiology/American Heart Association cardiovascular risk score to identify rheumatoid arthritis patients with high coronary artery calcification scores. Arthritis Rheumatol. 2015;67:381-385.
31. Peters MJ, Symmons DP, McCarey D, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69:325-331.
32. Agca R, Heslinga SC, Rollefstad S, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis. 2017;76:17-28.
33. Hippisley-Cox J, Coupland C, Vinogradova Y, et al. Predicting cardiovascular risk in England and Wales: Prospective derivation and validation of QRISK2. BMJ. 2008;336:1475-1482.
34. Hippisley-Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: Prospective cohort study. BMJ. 2017;357:j2099.
35. Solomon DH, Greenberg J, Curtis JR, et al. Derivation and internal validation of an expanded cardiovascular risk prediction score for rheumatoid arthritis: A consortium of rheumatology researchers of north america registry study. Arthritis Rheumatol. 2015;67:1995-2003.
36. Crowson CS, Gabriel SE, Semb AG, et al. Rheumatoid arthritis-specific cardiovascular risk scores are not superior to general risk scores: A validation analysis of patients from seven countries. Rheumatology (Oxford). 2017;56:1102-1110.
37. Alemao E, Cawston H, Bourhis F, et al. Comparison of cardiovascular risk algorithms in patients with vs without rheumatoid arthritis and the role of C-reactive protein in predicting cardiovascular outcomes in rheumatoid arthritis. Rheumatology (Oxford). 2017;56:777-786.
38. Crowson CS, Rollefstad S, Kitas GD, et al. Challenges of developing a cardiovascular risk calculator for patients with rheumatoid arthritis. PLoS One. 2017;12: e0174656.
39. Karpouzas GA, Malpeso J, Choi TY, et al. Prevalence, extent and composition of coronary plaque in patients with rheumatoid arthritis without symptoms or prior diagnosis of coronary artery disease. Ann Rheum Dis. 2014;73:1797-1804.
40. van Sijl AM, Peters MJ, Knol DK, et al. Carotid intima media thickness in rheumatoid arthritis as compared to control subjects: A meta-analysis. Semin Arthritis Rheum. 2011;40:3893-97.
41. Evans MR, Escalante A, Battafarano DF, et al. Carotid atherosclerosis predicts incident acute coronary syndromes in rheumatoid arthritis. Arthritis Rheum. 2011;63:1211-1220.
42. Ajeganova S, de Faire U, Jogestrand T, et al. Carotid atherosclerosis, disease measures, oxidized low-density lipoproteins, and atheroprotective natural antibodies for cardiovascular disease in early rheumatoid arthritis--an inception cohort study. J Rheumatol. 2012;39:1146-1154.
43. Corrales A, Gonzalez-Juanatey C, Peiro ME, et al. Carotid ultrasound is useful for the cardiovascular risk stratification of patients with rheumatoid arthritis: Results of a population-based study. Ann Rheum Dis. 2014;73:722-727.
44. Ikdahl E, Rollefstad S, Wibetoe G, et al. Predictive value of arterial stiffness and subclinical carotid atherosclerosis for cardiovascular disease in patients with rheumatoid arthritis. J Rheumatol. 2016;43:1622-1630.
45. Provan SA, Semb AG, Hisdal J, et al. Remission is the goal for cardiovascular risk management in patients with rheumatoid arthritis: A cross-sectional comparative study. Ann Rheum Dis. 2011;70:812-817.
46. Vlachopoulos C, Aznaouridis K, Terentes-Printzios D, et al. Prediction of cardiovascular events and all-cause mortality with brachial-ankle elasticity index: A systematic review and meta-analysis. Hypertension. 2012;60:556-562.
47. Ambrosino P, Tasso M, Lupoli R, et al. Non-invasive assessment of arterial stiffness in patients with rheumatoid arthritis: A systematic review and meta-analysis of literature studies. Ann Med. 2015;47:457-467.
48. Rumberger JA, Simons DB, Fitzpatrick LA, et al. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157-2162.
49. Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336-1345.
50. Task Force Members, Montalescot G, Sechtem U, et al. 2013 ESC guidelines on the management of stable coronary artery disease: The task force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949-3003.
51. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2935-2959.
52. Hou ZH, Lu B, Gao Y, et al. Prognostic value of coronary CT angiography and calcium score for major adverse cardiac events in outpatients. JACC Cardiovasc Imaging. 2012;5:990-999.
53. Yiu KH, Mok MY, Wang S, et al. Prognostic role of coronary calcification in patients with rheumatoid arthritis and systemic lupus erythematosus. Clin Exp Rheumatol. 2012;30:345-350.
54. Wright K, Crowson CS, Gabriel SE. Cardiovascular comorbidity in rheumatic diseases: A focus on heart failure. Heart Fail Clin. 2014;10:339-352.
55. Rudominer RL, Roman MJ, Devereux RB, et al. Independent association of rheumatoid arthritis with increased left ventricular mass but not with reduced ejection fraction. Arthritis Rheum. 2009;60:22-29.
56. Bluemke DA, Kronmal RA, Lima JA, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: The MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148-2155.
57. Ntusi NAB, Piechnik SK, Francis JM, et al. Diffuse myocardial fibrosis and inflammation in rheumatoid arthritis: Insights from CMR T1 mapping. JACC Cardiovasc Imaging. 2015;8:526-536.
58. Wong TC, Piehler K, Meier CG, et al. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation. 2012;126:1206-1216.
59. Goodson NJ, Symmons DP, Scott DG, et al. Baseline levels of C-reactive protein and prediction of death from cardiovascular disease in patients with inflammatory polyarthritis: A ten-year followup study of a primary care-based inception cohort. Arthritis Rheum. 2005;52:2293-2299.
60. Kozera L, Andrews J, Morgan AW. Cardiovascular risk and rheumatoid arthritis--the next step: Differentiating true soluble biomarkers of cardiovascular risk from surrogate measures of inflammation. Rheumatology (Oxford). 2011;50:1944-1954.
61. Cardarelli R, Lumicao TG Jr. B-type natriuretic peptide: A review of its diagnostic, prognostic, and therapeutic monitoring value in heart failure for primary care physicians. J Am Board Fam Pract. 2003;16:327-333.
62. Kragelund C, Gronning B, Kober L, et al. N-terminal pro-B-type natriuretic peptide and long-term mortality in stable coronary heart disease. N Engl J Med. 2005;352:666-675.
63. Harney SM, Timperley J, Daly C, et al. Brain natriuretic peptide is a potentially useful screening tool for the detection of cardiovascular disease in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65:136.
64. Bradham WS, Bian A, Oeser A, et al. High-sensitivity cardiac troponin-I is elevated in patients with rheumatoid arthritis, independent of cardiovascular risk factors and inflammation. PLoS One. 2012;7:e38930.
65. Karpouzas GA, Estis J, Rezaeian P, et al. High-sensitivity cardiac troponin I is a biomarker for occult coronary plaque burden and cardiovascular events in patients with rheumatoid arthritis. Rheumatology (Oxford). 2018;57:1080-1088.
66. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2889-2934.
67. Assmann G, Cullen P, Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular munster (PROCAM) study. Circulation. 2002;105:310-315.
From the Division of Rheumatology & Immunology, University of Nebraska Medical Center, and Veterans Affairs Nebraska-Western Iowa Health Care System, Omaha, NE.
Abstract
- Objective: To review cardiovascular disease (CVD) risk assessment in patients with rheumatoid arthritis (RA).
- Methods: Literature review of the assessment of CVD risk in RA.
- Results: CVD is the leading cause of death among RA patients.
Because of the increased risk of CVD events and CVD mortality in patients with RA, regular assessment of CVD risk and aggressive management of CVD risk in these patients is crucial. CVD risk estimation typically centers on the use of well-established CVD risk calculators. Most CVD risk scores from the general population do not contain RA-related factors predictive of CVD but have had more extensive performance testing, while novel RA-derived CVD risk scores that incorporate RA-related factors have had limited external validity testing. Neither set of risk scores incorporates novel imaging modalities or serum biomarkers, which are most likely to be helpful among individuals at intermediate risk. - Conclusion: Primary care and rheumatology providers must be aware of the increased risk of CVD in RA, a risk that approaches that of diabetic patients.
Routine assessment of CVD risk is an essential first step in minimizing CVD risk in this population. Until the performance of RA-specific CVD risk scores can be better established, we recommend the use of nationally endorsed CVD risk scores, with the frequency of reassessment based on CVD risk.
Keywords: rheumatoid arthritis; cardiovascular disease; cardiovascular risk assessment.
Editor’s note: This article is part 1 of a 2-part article. “Management of Cardiovascular Disease Risk in Rheumatoid Arthritis” was published in the March/April 2019 issue.
Rheumatoid arthritis (RA) is a chronic, autoimmune inflammatory arthritis affecting up to 1% of the US population that can lead to joint damage, functional disability, and reduced quality of life.1 In addition to articular involvement, systemic inflammation accompanying RA may lead to extra-articular manifestations and increase the risk of premature death.2 Cardiovascular disease (CVD), accounting for nearly half of all deaths among RA patients, is now recognized as a critical extra-articular manifestation of RA.2,3 As such, assessment and management of CVD risk is essential to the comprehensive care of the RA patient. This article reviews the approach to assessing CVD risk in patients with RA; the management of both traditional and RA-specific risk factors is discussed in a separate article.
Scope of the Problem
In a large meta-analysis of observational studies that included more than 111,000 patients with RA, CVD-related mortality rates were 1.5 times higher among RA patients than among general population controls.4 The risk of overall CVD, including nonfatal events, is similar; a separate meta-analysis of observational studies that included more than 41,000 patients with RA calculated a pooled relative risk for incident CVD of 1.48.5 Individual analyses identified heightened risk of acute coronary syndrome (ACS), cerebrovascular accident, and congestive heart failure (CHF).5 Perhaps more illustrative of the magnitude of the problem, the risk of CVD in RA approaches that observed among individuals with diabetes mellitus.6,7
Coronary artery disease (CAD) accounts for a significant portion of the CVD risk in RA, but its presentation may be atypical in RA patients. RA patients are at higher risk of suffering unrecognized myocardial infarction (MI) and sudden cardiac death.8 The reasons for silent ischemia in RA are not fully known, but have been hypothesized to include imbalances of inflammatory cytokines, alterations in pain sensitization, or the female predominance of RA (with women more often presenting with atypical symptoms of myocardial ischemia).9 Alarmingly, a retrospective chart review study reported that RA patients admitted for an acute MI were less likely to receive appropriate reperfusion therapy as well as secondary prevention with beta-blockers and lipid-lowering agents.10 Even with appropriate therapy, long-term outcomes such as mortality and recurrent ischemic events are more likely to occur in RA patients after acute MI.11-13
Independent of ischemic heart disease, RA patients are at increased risk of CHF.14-16 RA patients are at particular risk for CHF with preserved ejection fraction,17 which may be a result of systemic inflammation causing left ventricular stiffening.18,19 Similar to CAD, patients with RA are less likely to present with typical CHF symptoms, are less likely to receive guideline-concordant care, and have higher mortality rates following presentation with CHF.17
Although accounting for a lower proportion of the excess CVD morbidity and mortality in RA, the risk of noncardiac vascular disease is also increased in RA patients. Large meta-analyses have identified positive associations between RA with both ischemic (odds ratio [OR], 1.64 [95% confidence interval {CI}, 1.32-2.05]) and hemorrhagic (OR, 1.68 [95% CI, 1.11-2.53]) stroke.20 Similarly, RA patients appear to have an approximately twofold higher risk of venous thromboembolic events.21 Less frequently studied than other forms of CVD, peripheral arterial disease may be increased in RA patients independent of other CVD and CVD risk factors.22,23
Assessing CVD Risk in RA
CVD Risk Scores
In order to identify patients who may benefit from primary prevention interventions, such as lipid-lowering therapy, CVD risk estimation typically centers on the use of well-established CVD risk calculators (Table). CVD risk scores such as the Framingham Risk Score (FRS), Systematic Coronary Risk Evaluation (SCORE), and American College of Cardiology/ American Heart Association (ACC/AHA) Pooled Cohort Equation incorporate traditional CVD risk factors, including age, sex, smoking status, blood pressure, lipid levels, and presence of diabetes mellitus.24,25 However, CVD risk in RA patients appears to be inadequately explained by traditional CVD risk factors,26 with disease activity and inflammation being associated with higher CVD risk. Recognizing that inflammation may contribute to CVD risk even among non-RA patients, the Reynolds Risk Score includes high-sensitivity C-reactive protein (hsCRP) in its calculation.27 In contrast to more robust performance in the general population, these well-established CVD risk scores have had variable predictive potential of incident CVD in RA patients.28-30

Several models, or adaptations to existing models, have been proposed to improve CVD risk assessment in RA populations (Table). In 2009, the European League Against Rheumatism (EULAR) task force suggested using a correction factor of 1.5 with traditional CVD risk models in RA patients with 2 of the following criteria: disease duration exceeding 10 years, rheumatoid factor or anti-cyclic citrullinated peptide (CCP) antibody positivity, or extra-articular manifestations of RA.31 An update to these recommendations in 2015 continued to propose the use of a 1.5 correction factor, but suggested applying this to all RA patients.32 QRISK2, a modification to QRISK1 which was developed to predict CVD in the UK general population, includes the diagnosis of RA as a risk factor, and in early validation efforts more accurately discriminated patients in the general population at increased risk of CVD compared to the FRS.33 Additional disease-specific risk factors such as systemic lupus, steroid use, severe mental illness, and steroid and atypical antipsychotic use were incorporated in the QRISK3 algorithm, with model performance similar to the QRISK2.34 The Expanded Cardiovascular Risk Prediction Score for RA (ERS-RA) was specifically developed to assess CVD risk in RA patients by including RA disease activity, level of physical disability, RA disease duration, and prednisone use.35 Despite efforts to develop “RA-specific” risk scores, these have not consistently outperformed traditional CVD risk calculators.36-38 In one study involving more than 1700 RA patients, the ERS-RA performed similarly to the FRS and Reynolds Risk Score, with a net reclassification index of just 2.3% versus the FRS.36
Imaging Modalities
Imaging modalities may assist in characterizing the increased risk of CVD in RA and the subclinical CVD manifestations that occur. For example, RA patients were shown to have more prevalent and unstable coronary plaque, higher carotid intima media thickness, and impaired myocardial function with computed tomography (CT) angiography and carotid ultrasound.39,40 However, studies harnessing noninvasive imaging to augment CVD risk assessment in RA patients are limited.
Carotid ultrasound has been the most extensively studied imaging modality for CVD risk assessment in RA. In a cohort of 599 RA patients with no history of ACS, rates of ACS were nearly 4 times higher in RA patients with bilateral carotid plaque on carotid ultrasound, and the association with ACS was independent of other traditional and RA-related risk factors.41 Presence of bilateral carotid plaques was similarly associated with an increased risk of overall CVD events (hazard ratio [HR], 3.34 [95% CI, 1.21-9.22]), ACS alone (HR, 6.31 [95% CI, 1.27-31.40]), and a lower mean CVD event-free survival (13.9 versus 15.2 years, P = 0.01) in a separate inception cohort of 105 RA patients with no prior history of CVD.42 The most useful application of carotid ultrasound may be in conjunction with clinical CVD risk models. Use of carotid ultrasound improved CVD risk stratification among RA patients who were considered at moderate risk by the EULAR-modified SCORE calculator.43 Beyond carotid ultrasound, measurement of arterial stiffness through ultrasound could also aid in CVD risk stratification. Aortic pulse wave velocity and augmentation index, measures of arterial stiffness, are predictive of CVD in the general population as well as RA patients and improve with reduction in RA disease activity.44,45 Peripheral arterial stiffness (brachial-ankle elasticity index) is impaired in RA patients and predictive of CVD morbidity and mortality in the general population.46,47
CT coronary angiography and coronary artery calcium (CAC) scores are reliable measures of coronary artery atherosclerosis and have been validated for CVD risk assessment in the general population.48-52 While the association between RA and CT-related findings of atherosclerosis is well established, assessment of CT-mediated evaluation as a prognostic tool for CVD in RA is limited. In one cohort study, CAC predicted higher rates of CVD events in Chinese patients with RA and systemic lupus erythematosus in a pooled analysis, although results were limited by low event rates and the absence of RA-only subanalyses.53
While the aforementioned imaging modalities have focused on enhancing the identification of atherosclerosis, echocardiography or cardiac magnetic resonance imaging (MRI) may be useful for detecting subclinical structural and/or functional abnormalities that predispose to CHF. Structural abnormalities including increased left ventricular mass and hypertrophy are more prevalent in RA patients and predict incident CHF in the general population.54-56 MRI measures of myocardial inflammation, including T1 mapping and extracellular volume, are associated with higher mortality rates and also appear to be elevated in RA patients.57,58 Whether identification of these imaging findings influences the cost-effective clinical management of RA patients needs further study.
Biomarkers
Serum biomarkers, such as the anti-CCP antibody, have become crucial to the evaluation of patients suspected to have RA. With the growing understanding of the role pro-inflammatory mediators play in CVD pathogenesis and the relative ease with which they can be measured, serum biomarkers have potential to inform CVD risk assessment. In the general population, hsCRP concentrations are predictive of CVD and are included in the Reynolds Risk Score.27 In RA, CRP concentrations are typically much higher than those observed among individuals in the general population solely at increased CVD risk, yet elevated levels remain predictive of CVD death independent of RA disease activity and traditional CVD risk factors.59 Several additional cytokines, chemokines, and adhesion molecules have been associated with surrogate markers of CVD in RA patients, although further study is needed to elucidate thresholds that signify increased CVD risk in a population characterized by the presence of systemic inflammation.60
Cardiac biomarkers used frequently in the general population may be useful to assess CVD risk in RA patients. N-terminal-pro brain natriuretic peptide (NT-pro BNP) is a biomarker typically used to evaluate CHF severity, but it may also predict long-term mortality in patients with coronary heart disease.61,62 Circulating NT-pro BNP concentrations are elevated in RA independent of prevalent CHF and may serve as a useful tool to identify subclinical cardiac disease in RA patients.63 High-sensitivity cardiac troponin I (HS-cTnI) assays are capable of detecting levels of cardiac troponin below the threshold typically used to diagnose ACS. HS-cTnI levels are increased in RA patients independent of additional CVD risk factors, and elevated levels (> 1.5 pg/mL) were associated with more severe CT angiography findings of coronary plaque as well as increased risk of CVD events.64,65
Clinical Application
A fully validated algorithm for CVD risk assessment in RA is lacking. Most CVD risk scores from the general population do not contain RA-related factors predictive of CVD but have had more extensive performance testing. In contrast, novel RA-derived CVD risk scores incorporate RA-related factors, but have had limited external validity testing. Additionally, RA-derived risk scores are less likely to be utilized and adopted by primary care providers and cardiologists involved in RA patients’ care. Neither set of risk scores incorporates novel imaging modalities or serum biomarkers, which are most likely to be helpful among individuals at intermediate risk. Therefore, until the performance of RA-specific CVD risk scores can be better established, we recommend the use of nationally endorsed CVD risk scores, with the frequency of reassessment based on CVD risk.
Conclusion
RA patients are at increased risk of CVD and CVD-related mortality relative to the general population. The disproportionate CVD burden seen in RA appears to be multifactorial, owing to the complex effects of systemic inflammation, endothelial dysfunction, and pro-atherogenic lipoprotein modifications. Additionally, many traditional CVD risk factors are more prevalent and suboptimally managed in RA patients. To mitigate the increased risk of CVD in RA, primary care and subspecialty providers alike must be aware of this heightened risk in RA, perform frequent assessment of CVD risk, and
Corresponding author: Bryant R. England, MD; 986270 Nebraska Medical Center, Omaha, NE 68198-6270; [email protected].
Financial disclosures: Dr. England is supported by UNMC Internal Medicine Scientist Development Award, UNMC Physician-Scientist Training Program, the UNMC Mentored Scholars Program, and the Rheumatology Research Foundation Scientist Development Award. Dr. Mikuls is supported by a VA Merit Award (CX000896) and grants from the National Institutes of Health: National Institute of General Medical Sciences (U54GM115458), National Institute on Alcohol Abuse and Alcoholism (R25AA020818), and National Institute of Arthritis and Musculoskeletal and Skin Diseases (2P50AR60772).
From the Division of Rheumatology & Immunology, University of Nebraska Medical Center, and Veterans Affairs Nebraska-Western Iowa Health Care System, Omaha, NE.
Abstract
- Objective: To review cardiovascular disease (CVD) risk assessment in patients with rheumatoid arthritis (RA).
- Methods: Literature review of the assessment of CVD risk in RA.
- Results: CVD is the leading cause of death among RA patients.
Because of the increased risk of CVD events and CVD mortality in patients with RA, regular assessment of CVD risk and aggressive management of CVD risk in these patients is crucial. CVD risk estimation typically centers on the use of well-established CVD risk calculators. Most CVD risk scores from the general population do not contain RA-related factors predictive of CVD but have had more extensive performance testing, while novel RA-derived CVD risk scores that incorporate RA-related factors have had limited external validity testing. Neither set of risk scores incorporates novel imaging modalities or serum biomarkers, which are most likely to be helpful among individuals at intermediate risk. - Conclusion: Primary care and rheumatology providers must be aware of the increased risk of CVD in RA, a risk that approaches that of diabetic patients.
Routine assessment of CVD risk is an essential first step in minimizing CVD risk in this population. Until the performance of RA-specific CVD risk scores can be better established, we recommend the use of nationally endorsed CVD risk scores, with the frequency of reassessment based on CVD risk.
Keywords: rheumatoid arthritis; cardiovascular disease; cardiovascular risk assessment.
Editor’s note: This article is part 1 of a 2-part article. “Management of Cardiovascular Disease Risk in Rheumatoid Arthritis” was published in the March/April 2019 issue.
Rheumatoid arthritis (RA) is a chronic, autoimmune inflammatory arthritis affecting up to 1% of the US population that can lead to joint damage, functional disability, and reduced quality of life.1 In addition to articular involvement, systemic inflammation accompanying RA may lead to extra-articular manifestations and increase the risk of premature death.2 Cardiovascular disease (CVD), accounting for nearly half of all deaths among RA patients, is now recognized as a critical extra-articular manifestation of RA.2,3 As such, assessment and management of CVD risk is essential to the comprehensive care of the RA patient. This article reviews the approach to assessing CVD risk in patients with RA; the management of both traditional and RA-specific risk factors is discussed in a separate article.
Scope of the Problem
In a large meta-analysis of observational studies that included more than 111,000 patients with RA, CVD-related mortality rates were 1.5 times higher among RA patients than among general population controls.4 The risk of overall CVD, including nonfatal events, is similar; a separate meta-analysis of observational studies that included more than 41,000 patients with RA calculated a pooled relative risk for incident CVD of 1.48.5 Individual analyses identified heightened risk of acute coronary syndrome (ACS), cerebrovascular accident, and congestive heart failure (CHF).5 Perhaps more illustrative of the magnitude of the problem, the risk of CVD in RA approaches that observed among individuals with diabetes mellitus.6,7
Coronary artery disease (CAD) accounts for a significant portion of the CVD risk in RA, but its presentation may be atypical in RA patients. RA patients are at higher risk of suffering unrecognized myocardial infarction (MI) and sudden cardiac death.8 The reasons for silent ischemia in RA are not fully known, but have been hypothesized to include imbalances of inflammatory cytokines, alterations in pain sensitization, or the female predominance of RA (with women more often presenting with atypical symptoms of myocardial ischemia).9 Alarmingly, a retrospective chart review study reported that RA patients admitted for an acute MI were less likely to receive appropriate reperfusion therapy as well as secondary prevention with beta-blockers and lipid-lowering agents.10 Even with appropriate therapy, long-term outcomes such as mortality and recurrent ischemic events are more likely to occur in RA patients after acute MI.11-13
Independent of ischemic heart disease, RA patients are at increased risk of CHF.14-16 RA patients are at particular risk for CHF with preserved ejection fraction,17 which may be a result of systemic inflammation causing left ventricular stiffening.18,19 Similar to CAD, patients with RA are less likely to present with typical CHF symptoms, are less likely to receive guideline-concordant care, and have higher mortality rates following presentation with CHF.17
Although accounting for a lower proportion of the excess CVD morbidity and mortality in RA, the risk of noncardiac vascular disease is also increased in RA patients. Large meta-analyses have identified positive associations between RA with both ischemic (odds ratio [OR], 1.64 [95% confidence interval {CI}, 1.32-2.05]) and hemorrhagic (OR, 1.68 [95% CI, 1.11-2.53]) stroke.20 Similarly, RA patients appear to have an approximately twofold higher risk of venous thromboembolic events.21 Less frequently studied than other forms of CVD, peripheral arterial disease may be increased in RA patients independent of other CVD and CVD risk factors.22,23
Assessing CVD Risk in RA
CVD Risk Scores
In order to identify patients who may benefit from primary prevention interventions, such as lipid-lowering therapy, CVD risk estimation typically centers on the use of well-established CVD risk calculators (Table). CVD risk scores such as the Framingham Risk Score (FRS), Systematic Coronary Risk Evaluation (SCORE), and American College of Cardiology/ American Heart Association (ACC/AHA) Pooled Cohort Equation incorporate traditional CVD risk factors, including age, sex, smoking status, blood pressure, lipid levels, and presence of diabetes mellitus.24,25 However, CVD risk in RA patients appears to be inadequately explained by traditional CVD risk factors,26 with disease activity and inflammation being associated with higher CVD risk. Recognizing that inflammation may contribute to CVD risk even among non-RA patients, the Reynolds Risk Score includes high-sensitivity C-reactive protein (hsCRP) in its calculation.27 In contrast to more robust performance in the general population, these well-established CVD risk scores have had variable predictive potential of incident CVD in RA patients.28-30

Several models, or adaptations to existing models, have been proposed to improve CVD risk assessment in RA populations (Table). In 2009, the European League Against Rheumatism (EULAR) task force suggested using a correction factor of 1.5 with traditional CVD risk models in RA patients with 2 of the following criteria: disease duration exceeding 10 years, rheumatoid factor or anti-cyclic citrullinated peptide (CCP) antibody positivity, or extra-articular manifestations of RA.31 An update to these recommendations in 2015 continued to propose the use of a 1.5 correction factor, but suggested applying this to all RA patients.32 QRISK2, a modification to QRISK1 which was developed to predict CVD in the UK general population, includes the diagnosis of RA as a risk factor, and in early validation efforts more accurately discriminated patients in the general population at increased risk of CVD compared to the FRS.33 Additional disease-specific risk factors such as systemic lupus, steroid use, severe mental illness, and steroid and atypical antipsychotic use were incorporated in the QRISK3 algorithm, with model performance similar to the QRISK2.34 The Expanded Cardiovascular Risk Prediction Score for RA (ERS-RA) was specifically developed to assess CVD risk in RA patients by including RA disease activity, level of physical disability, RA disease duration, and prednisone use.35 Despite efforts to develop “RA-specific” risk scores, these have not consistently outperformed traditional CVD risk calculators.36-38 In one study involving more than 1700 RA patients, the ERS-RA performed similarly to the FRS and Reynolds Risk Score, with a net reclassification index of just 2.3% versus the FRS.36
Imaging Modalities
Imaging modalities may assist in characterizing the increased risk of CVD in RA and the subclinical CVD manifestations that occur. For example, RA patients were shown to have more prevalent and unstable coronary plaque, higher carotid intima media thickness, and impaired myocardial function with computed tomography (CT) angiography and carotid ultrasound.39,40 However, studies harnessing noninvasive imaging to augment CVD risk assessment in RA patients are limited.
Carotid ultrasound has been the most extensively studied imaging modality for CVD risk assessment in RA. In a cohort of 599 RA patients with no history of ACS, rates of ACS were nearly 4 times higher in RA patients with bilateral carotid plaque on carotid ultrasound, and the association with ACS was independent of other traditional and RA-related risk factors.41 Presence of bilateral carotid plaques was similarly associated with an increased risk of overall CVD events (hazard ratio [HR], 3.34 [95% CI, 1.21-9.22]), ACS alone (HR, 6.31 [95% CI, 1.27-31.40]), and a lower mean CVD event-free survival (13.9 versus 15.2 years, P = 0.01) in a separate inception cohort of 105 RA patients with no prior history of CVD.42 The most useful application of carotid ultrasound may be in conjunction with clinical CVD risk models. Use of carotid ultrasound improved CVD risk stratification among RA patients who were considered at moderate risk by the EULAR-modified SCORE calculator.43 Beyond carotid ultrasound, measurement of arterial stiffness through ultrasound could also aid in CVD risk stratification. Aortic pulse wave velocity and augmentation index, measures of arterial stiffness, are predictive of CVD in the general population as well as RA patients and improve with reduction in RA disease activity.44,45 Peripheral arterial stiffness (brachial-ankle elasticity index) is impaired in RA patients and predictive of CVD morbidity and mortality in the general population.46,47
CT coronary angiography and coronary artery calcium (CAC) scores are reliable measures of coronary artery atherosclerosis and have been validated for CVD risk assessment in the general population.48-52 While the association between RA and CT-related findings of atherosclerosis is well established, assessment of CT-mediated evaluation as a prognostic tool for CVD in RA is limited. In one cohort study, CAC predicted higher rates of CVD events in Chinese patients with RA and systemic lupus erythematosus in a pooled analysis, although results were limited by low event rates and the absence of RA-only subanalyses.53
While the aforementioned imaging modalities have focused on enhancing the identification of atherosclerosis, echocardiography or cardiac magnetic resonance imaging (MRI) may be useful for detecting subclinical structural and/or functional abnormalities that predispose to CHF. Structural abnormalities including increased left ventricular mass and hypertrophy are more prevalent in RA patients and predict incident CHF in the general population.54-56 MRI measures of myocardial inflammation, including T1 mapping and extracellular volume, are associated with higher mortality rates and also appear to be elevated in RA patients.57,58 Whether identification of these imaging findings influences the cost-effective clinical management of RA patients needs further study.
Biomarkers
Serum biomarkers, such as the anti-CCP antibody, have become crucial to the evaluation of patients suspected to have RA. With the growing understanding of the role pro-inflammatory mediators play in CVD pathogenesis and the relative ease with which they can be measured, serum biomarkers have potential to inform CVD risk assessment. In the general population, hsCRP concentrations are predictive of CVD and are included in the Reynolds Risk Score.27 In RA, CRP concentrations are typically much higher than those observed among individuals in the general population solely at increased CVD risk, yet elevated levels remain predictive of CVD death independent of RA disease activity and traditional CVD risk factors.59 Several additional cytokines, chemokines, and adhesion molecules have been associated with surrogate markers of CVD in RA patients, although further study is needed to elucidate thresholds that signify increased CVD risk in a population characterized by the presence of systemic inflammation.60
Cardiac biomarkers used frequently in the general population may be useful to assess CVD risk in RA patients. N-terminal-pro brain natriuretic peptide (NT-pro BNP) is a biomarker typically used to evaluate CHF severity, but it may also predict long-term mortality in patients with coronary heart disease.61,62 Circulating NT-pro BNP concentrations are elevated in RA independent of prevalent CHF and may serve as a useful tool to identify subclinical cardiac disease in RA patients.63 High-sensitivity cardiac troponin I (HS-cTnI) assays are capable of detecting levels of cardiac troponin below the threshold typically used to diagnose ACS. HS-cTnI levels are increased in RA patients independent of additional CVD risk factors, and elevated levels (> 1.5 pg/mL) were associated with more severe CT angiography findings of coronary plaque as well as increased risk of CVD events.64,65
Clinical Application
A fully validated algorithm for CVD risk assessment in RA is lacking. Most CVD risk scores from the general population do not contain RA-related factors predictive of CVD but have had more extensive performance testing. In contrast, novel RA-derived CVD risk scores incorporate RA-related factors, but have had limited external validity testing. Additionally, RA-derived risk scores are less likely to be utilized and adopted by primary care providers and cardiologists involved in RA patients’ care. Neither set of risk scores incorporates novel imaging modalities or serum biomarkers, which are most likely to be helpful among individuals at intermediate risk. Therefore, until the performance of RA-specific CVD risk scores can be better established, we recommend the use of nationally endorsed CVD risk scores, with the frequency of reassessment based on CVD risk.
Conclusion
RA patients are at increased risk of CVD and CVD-related mortality relative to the general population. The disproportionate CVD burden seen in RA appears to be multifactorial, owing to the complex effects of systemic inflammation, endothelial dysfunction, and pro-atherogenic lipoprotein modifications. Additionally, many traditional CVD risk factors are more prevalent and suboptimally managed in RA patients. To mitigate the increased risk of CVD in RA, primary care and subspecialty providers alike must be aware of this heightened risk in RA, perform frequent assessment of CVD risk, and
Corresponding author: Bryant R. England, MD; 986270 Nebraska Medical Center, Omaha, NE 68198-6270; [email protected].
Financial disclosures: Dr. England is supported by UNMC Internal Medicine Scientist Development Award, UNMC Physician-Scientist Training Program, the UNMC Mentored Scholars Program, and the Rheumatology Research Foundation Scientist Development Award. Dr. Mikuls is supported by a VA Merit Award (CX000896) and grants from the National Institutes of Health: National Institute of General Medical Sciences (U54GM115458), National Institute on Alcohol Abuse and Alcoholism (R25AA020818), and National Institute of Arthritis and Musculoskeletal and Skin Diseases (2P50AR60772).
1. Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the united states. part I. Arthritis Rheum. 2008;58:15-25.
2. England BR, Sayles H, Michaud K, et al. Cause-specific mortality in male US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:36-45.
3. Sokka T, Abelson B, Pincus T. Mortality in rheumatoid arthritis: 2008 update. Clin Exp Rheumatol. 2008;26:S35-61.
4. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
5. Avina-Zubieta JA, Thomas J, Sadatsafavi M, et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann Rheum Dis. 2012;71:1524-1529.
6. van Halm VP, Peters MJ, Voskuyl AE, et al. Rheumatoid arthritis versus diabetes as a risk factor for cardiovascular disease: A cross-sectional study, the CARRE investigation. Ann Rheum Dis. 2009;68:1395-1400.
7. Peters MJ, van Halm VP, Voskuyl AE, et al. Does rheumatoid arthritis equal diabetes mellitus as an independent risk factor for cardiovascular disease? A prospective study. Arthritis Rheum. 2009;61:1571-1579.
8. Maradit-Kremers H, Crowson CS, Nicola PJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: A population-based cohort study. Arthritis Rheum. 2005;52:402-411.
9. Cardiovascular disease in women--often silent and fatal. Lancet. 2011;378:200,6736(11)61108-61112.
10. Van Doornum S, Brand C, Sundararajan V, et al. Rheumatoid arthritis patients receive less frequent acute reperfusion and secondary prevention therapy after myocardial infarction compared with the general population. Arthritis Res Ther. 2010;12:R183.
11. Sodergren A, Stegmayr B, Lundberg V, et al. Increased incidence of and impaired prognosis after acute myocardial infarction among patients with seropositive rheumatoid arthritis. Ann Rheum Dis. 2007;66:263-266.
12. Douglas KM, Pace AV, Treharne GJ, et al. Excess recurrent cardiac events in rheumatoid arthritis patients with acute coronary syndrome. Ann Rheum Dis. 2006;65:348-353.
13. McCoy SS, Crowson CS, Maradit-Kremers H, et al. Long-term outcomes and treatment after myocardial infarction in patients with rheumatoid arthritis. J Rheumatol. 2013;40:605-610.
14. Mantel A, Holmqvist M, Andersson DC, et al. Association between rheumatoid arthritis and risk of ischemic and nonischemic heart failure. J Am Coll Cardiol. 2017;69:1275-1285.
15. Crowson CS, Nicola PJ, Kremers HM, et al. How much of the increased incidence of heart failure in rheumatoid arthritis is attributable to traditional cardiovascular risk factors and ischemic heart disease? Arthritis Rheum. 2005;52:3039-3044.
16. Nicola PJ, Maradit-Kremers H, Roger VL, et al. The risk of congestive heart failure in rheumatoid arthritis: A population-based study over 46 years. Arthritis Rheum. 2005;52:412-420.
17. Davis JM,3rd, Roger VL, Crowson CS, et al. The presentation and outcome of heart failure in patients with rheumatoid arthritis differs from that in the general population. Arthritis Rheum. 2008;58:2603-2611.
18. Arslan S, Bozkurt E, Sari RA, Erol MK. Diastolic function abnormalities in active rheumatoid arthritis evaluation by conventional doppler and tissue doppler: Relation with duration of disease. Clin Rheumatol. 2006;25:294-299.
19. Liang KP, Myasoedova E, Crowson CS, et al. Increased prevalence of diastolic dysfunction in rheumatoid arthritis. Ann Rheum Dis. 2010;69:1665-1670.
20. Wiseman SJ, Ralston SH, Wardlaw JM. Cerebrovascular disease in rheumatic diseases: A systematic review and meta-analysis. Stroke. 2016;47:943-950.
21. Ungprasert P, Srivali N, Spanuchart I, et al. Risk of venous thromboembolism in patients with rheumatoid arthritis: A systematic review and meta-analysis. Clin Rheumatol. 2014;33:297-304.
22. Stamatelopoulos KS, Kitas GD, Papamichael CM, et al. Subclinical peripheral arterial disease in rheumatoid arthritis. Atherosclerosis. 2010;212:305-309.
23. Chuang YW, Yu MC, Lin CL, et al. Risk of peripheral arterial occlusive disease in patients with rheumatoid arthritis. A nationwide population-based cohort study. Thromb Haemost. 2016;115:439-445.
24. Conroy RM, Pyorala K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in europe: The SCORE project. Eur Heart J. 2003;24:987-1003.
25. D’Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: The Framingham heart study. Circulation. 2008;117:743-753.
26. del Rincon ID, Williams K, Stern MP, et al. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44:2737-2745.
27. Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: The Reynolds Risk Score. JAMA. 2007;297:611-619.
28. Arts EE, Popa C, Den Broeder AA, et al. Performance of four current risk algorithms in predicting cardiovascular events in patients with early rheumatoid arthritis. Ann Rheum Dis. 2015;74:668-674.
29. Crowson CS, Matteson EL, Roger VL, et al. Usefulness of risk scores to estimate the risk of cardiovascular disease in patients with rheumatoid arthritis. Am J Cardiol. 2012;110:420-424.
30. Kawai VK, Chung CP, Solus JF, et al. The ability of the 2013 American College of Cardiology/American Heart Association cardiovascular risk score to identify rheumatoid arthritis patients with high coronary artery calcification scores. Arthritis Rheumatol. 2015;67:381-385.
31. Peters MJ, Symmons DP, McCarey D, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69:325-331.
32. Agca R, Heslinga SC, Rollefstad S, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis. 2017;76:17-28.
33. Hippisley-Cox J, Coupland C, Vinogradova Y, et al. Predicting cardiovascular risk in England and Wales: Prospective derivation and validation of QRISK2. BMJ. 2008;336:1475-1482.
34. Hippisley-Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: Prospective cohort study. BMJ. 2017;357:j2099.
35. Solomon DH, Greenberg J, Curtis JR, et al. Derivation and internal validation of an expanded cardiovascular risk prediction score for rheumatoid arthritis: A consortium of rheumatology researchers of north america registry study. Arthritis Rheumatol. 2015;67:1995-2003.
36. Crowson CS, Gabriel SE, Semb AG, et al. Rheumatoid arthritis-specific cardiovascular risk scores are not superior to general risk scores: A validation analysis of patients from seven countries. Rheumatology (Oxford). 2017;56:1102-1110.
37. Alemao E, Cawston H, Bourhis F, et al. Comparison of cardiovascular risk algorithms in patients with vs without rheumatoid arthritis and the role of C-reactive protein in predicting cardiovascular outcomes in rheumatoid arthritis. Rheumatology (Oxford). 2017;56:777-786.
38. Crowson CS, Rollefstad S, Kitas GD, et al. Challenges of developing a cardiovascular risk calculator for patients with rheumatoid arthritis. PLoS One. 2017;12: e0174656.
39. Karpouzas GA, Malpeso J, Choi TY, et al. Prevalence, extent and composition of coronary plaque in patients with rheumatoid arthritis without symptoms or prior diagnosis of coronary artery disease. Ann Rheum Dis. 2014;73:1797-1804.
40. van Sijl AM, Peters MJ, Knol DK, et al. Carotid intima media thickness in rheumatoid arthritis as compared to control subjects: A meta-analysis. Semin Arthritis Rheum. 2011;40:3893-97.
41. Evans MR, Escalante A, Battafarano DF, et al. Carotid atherosclerosis predicts incident acute coronary syndromes in rheumatoid arthritis. Arthritis Rheum. 2011;63:1211-1220.
42. Ajeganova S, de Faire U, Jogestrand T, et al. Carotid atherosclerosis, disease measures, oxidized low-density lipoproteins, and atheroprotective natural antibodies for cardiovascular disease in early rheumatoid arthritis--an inception cohort study. J Rheumatol. 2012;39:1146-1154.
43. Corrales A, Gonzalez-Juanatey C, Peiro ME, et al. Carotid ultrasound is useful for the cardiovascular risk stratification of patients with rheumatoid arthritis: Results of a population-based study. Ann Rheum Dis. 2014;73:722-727.
44. Ikdahl E, Rollefstad S, Wibetoe G, et al. Predictive value of arterial stiffness and subclinical carotid atherosclerosis for cardiovascular disease in patients with rheumatoid arthritis. J Rheumatol. 2016;43:1622-1630.
45. Provan SA, Semb AG, Hisdal J, et al. Remission is the goal for cardiovascular risk management in patients with rheumatoid arthritis: A cross-sectional comparative study. Ann Rheum Dis. 2011;70:812-817.
46. Vlachopoulos C, Aznaouridis K, Terentes-Printzios D, et al. Prediction of cardiovascular events and all-cause mortality with brachial-ankle elasticity index: A systematic review and meta-analysis. Hypertension. 2012;60:556-562.
47. Ambrosino P, Tasso M, Lupoli R, et al. Non-invasive assessment of arterial stiffness in patients with rheumatoid arthritis: A systematic review and meta-analysis of literature studies. Ann Med. 2015;47:457-467.
48. Rumberger JA, Simons DB, Fitzpatrick LA, et al. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157-2162.
49. Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336-1345.
50. Task Force Members, Montalescot G, Sechtem U, et al. 2013 ESC guidelines on the management of stable coronary artery disease: The task force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949-3003.
51. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2935-2959.
52. Hou ZH, Lu B, Gao Y, et al. Prognostic value of coronary CT angiography and calcium score for major adverse cardiac events in outpatients. JACC Cardiovasc Imaging. 2012;5:990-999.
53. Yiu KH, Mok MY, Wang S, et al. Prognostic role of coronary calcification in patients with rheumatoid arthritis and systemic lupus erythematosus. Clin Exp Rheumatol. 2012;30:345-350.
54. Wright K, Crowson CS, Gabriel SE. Cardiovascular comorbidity in rheumatic diseases: A focus on heart failure. Heart Fail Clin. 2014;10:339-352.
55. Rudominer RL, Roman MJ, Devereux RB, et al. Independent association of rheumatoid arthritis with increased left ventricular mass but not with reduced ejection fraction. Arthritis Rheum. 2009;60:22-29.
56. Bluemke DA, Kronmal RA, Lima JA, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: The MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148-2155.
57. Ntusi NAB, Piechnik SK, Francis JM, et al. Diffuse myocardial fibrosis and inflammation in rheumatoid arthritis: Insights from CMR T1 mapping. JACC Cardiovasc Imaging. 2015;8:526-536.
58. Wong TC, Piehler K, Meier CG, et al. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation. 2012;126:1206-1216.
59. Goodson NJ, Symmons DP, Scott DG, et al. Baseline levels of C-reactive protein and prediction of death from cardiovascular disease in patients with inflammatory polyarthritis: A ten-year followup study of a primary care-based inception cohort. Arthritis Rheum. 2005;52:2293-2299.
60. Kozera L, Andrews J, Morgan AW. Cardiovascular risk and rheumatoid arthritis--the next step: Differentiating true soluble biomarkers of cardiovascular risk from surrogate measures of inflammation. Rheumatology (Oxford). 2011;50:1944-1954.
61. Cardarelli R, Lumicao TG Jr. B-type natriuretic peptide: A review of its diagnostic, prognostic, and therapeutic monitoring value in heart failure for primary care physicians. J Am Board Fam Pract. 2003;16:327-333.
62. Kragelund C, Gronning B, Kober L, et al. N-terminal pro-B-type natriuretic peptide and long-term mortality in stable coronary heart disease. N Engl J Med. 2005;352:666-675.
63. Harney SM, Timperley J, Daly C, et al. Brain natriuretic peptide is a potentially useful screening tool for the detection of cardiovascular disease in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65:136.
64. Bradham WS, Bian A, Oeser A, et al. High-sensitivity cardiac troponin-I is elevated in patients with rheumatoid arthritis, independent of cardiovascular risk factors and inflammation. PLoS One. 2012;7:e38930.
65. Karpouzas GA, Estis J, Rezaeian P, et al. High-sensitivity cardiac troponin I is a biomarker for occult coronary plaque burden and cardiovascular events in patients with rheumatoid arthritis. Rheumatology (Oxford). 2018;57:1080-1088.
66. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2889-2934.
67. Assmann G, Cullen P, Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular munster (PROCAM) study. Circulation. 2002;105:310-315.
1. Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the united states. part I. Arthritis Rheum. 2008;58:15-25.
2. England BR, Sayles H, Michaud K, et al. Cause-specific mortality in male US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:36-45.
3. Sokka T, Abelson B, Pincus T. Mortality in rheumatoid arthritis: 2008 update. Clin Exp Rheumatol. 2008;26:S35-61.
4. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
5. Avina-Zubieta JA, Thomas J, Sadatsafavi M, et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann Rheum Dis. 2012;71:1524-1529.
6. van Halm VP, Peters MJ, Voskuyl AE, et al. Rheumatoid arthritis versus diabetes as a risk factor for cardiovascular disease: A cross-sectional study, the CARRE investigation. Ann Rheum Dis. 2009;68:1395-1400.
7. Peters MJ, van Halm VP, Voskuyl AE, et al. Does rheumatoid arthritis equal diabetes mellitus as an independent risk factor for cardiovascular disease? A prospective study. Arthritis Rheum. 2009;61:1571-1579.
8. Maradit-Kremers H, Crowson CS, Nicola PJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: A population-based cohort study. Arthritis Rheum. 2005;52:402-411.
9. Cardiovascular disease in women--often silent and fatal. Lancet. 2011;378:200,6736(11)61108-61112.
10. Van Doornum S, Brand C, Sundararajan V, et al. Rheumatoid arthritis patients receive less frequent acute reperfusion and secondary prevention therapy after myocardial infarction compared with the general population. Arthritis Res Ther. 2010;12:R183.
11. Sodergren A, Stegmayr B, Lundberg V, et al. Increased incidence of and impaired prognosis after acute myocardial infarction among patients with seropositive rheumatoid arthritis. Ann Rheum Dis. 2007;66:263-266.
12. Douglas KM, Pace AV, Treharne GJ, et al. Excess recurrent cardiac events in rheumatoid arthritis patients with acute coronary syndrome. Ann Rheum Dis. 2006;65:348-353.
13. McCoy SS, Crowson CS, Maradit-Kremers H, et al. Long-term outcomes and treatment after myocardial infarction in patients with rheumatoid arthritis. J Rheumatol. 2013;40:605-610.
14. Mantel A, Holmqvist M, Andersson DC, et al. Association between rheumatoid arthritis and risk of ischemic and nonischemic heart failure. J Am Coll Cardiol. 2017;69:1275-1285.
15. Crowson CS, Nicola PJ, Kremers HM, et al. How much of the increased incidence of heart failure in rheumatoid arthritis is attributable to traditional cardiovascular risk factors and ischemic heart disease? Arthritis Rheum. 2005;52:3039-3044.
16. Nicola PJ, Maradit-Kremers H, Roger VL, et al. The risk of congestive heart failure in rheumatoid arthritis: A population-based study over 46 years. Arthritis Rheum. 2005;52:412-420.
17. Davis JM,3rd, Roger VL, Crowson CS, et al. The presentation and outcome of heart failure in patients with rheumatoid arthritis differs from that in the general population. Arthritis Rheum. 2008;58:2603-2611.
18. Arslan S, Bozkurt E, Sari RA, Erol MK. Diastolic function abnormalities in active rheumatoid arthritis evaluation by conventional doppler and tissue doppler: Relation with duration of disease. Clin Rheumatol. 2006;25:294-299.
19. Liang KP, Myasoedova E, Crowson CS, et al. Increased prevalence of diastolic dysfunction in rheumatoid arthritis. Ann Rheum Dis. 2010;69:1665-1670.
20. Wiseman SJ, Ralston SH, Wardlaw JM. Cerebrovascular disease in rheumatic diseases: A systematic review and meta-analysis. Stroke. 2016;47:943-950.
21. Ungprasert P, Srivali N, Spanuchart I, et al. Risk of venous thromboembolism in patients with rheumatoid arthritis: A systematic review and meta-analysis. Clin Rheumatol. 2014;33:297-304.
22. Stamatelopoulos KS, Kitas GD, Papamichael CM, et al. Subclinical peripheral arterial disease in rheumatoid arthritis. Atherosclerosis. 2010;212:305-309.
23. Chuang YW, Yu MC, Lin CL, et al. Risk of peripheral arterial occlusive disease in patients with rheumatoid arthritis. A nationwide population-based cohort study. Thromb Haemost. 2016;115:439-445.
24. Conroy RM, Pyorala K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in europe: The SCORE project. Eur Heart J. 2003;24:987-1003.
25. D’Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: The Framingham heart study. Circulation. 2008;117:743-753.
26. del Rincon ID, Williams K, Stern MP, et al. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44:2737-2745.
27. Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: The Reynolds Risk Score. JAMA. 2007;297:611-619.
28. Arts EE, Popa C, Den Broeder AA, et al. Performance of four current risk algorithms in predicting cardiovascular events in patients with early rheumatoid arthritis. Ann Rheum Dis. 2015;74:668-674.
29. Crowson CS, Matteson EL, Roger VL, et al. Usefulness of risk scores to estimate the risk of cardiovascular disease in patients with rheumatoid arthritis. Am J Cardiol. 2012;110:420-424.
30. Kawai VK, Chung CP, Solus JF, et al. The ability of the 2013 American College of Cardiology/American Heart Association cardiovascular risk score to identify rheumatoid arthritis patients with high coronary artery calcification scores. Arthritis Rheumatol. 2015;67:381-385.
31. Peters MJ, Symmons DP, McCarey D, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69:325-331.
32. Agca R, Heslinga SC, Rollefstad S, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis. 2017;76:17-28.
33. Hippisley-Cox J, Coupland C, Vinogradova Y, et al. Predicting cardiovascular risk in England and Wales: Prospective derivation and validation of QRISK2. BMJ. 2008;336:1475-1482.
34. Hippisley-Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: Prospective cohort study. BMJ. 2017;357:j2099.
35. Solomon DH, Greenberg J, Curtis JR, et al. Derivation and internal validation of an expanded cardiovascular risk prediction score for rheumatoid arthritis: A consortium of rheumatology researchers of north america registry study. Arthritis Rheumatol. 2015;67:1995-2003.
36. Crowson CS, Gabriel SE, Semb AG, et al. Rheumatoid arthritis-specific cardiovascular risk scores are not superior to general risk scores: A validation analysis of patients from seven countries. Rheumatology (Oxford). 2017;56:1102-1110.
37. Alemao E, Cawston H, Bourhis F, et al. Comparison of cardiovascular risk algorithms in patients with vs without rheumatoid arthritis and the role of C-reactive protein in predicting cardiovascular outcomes in rheumatoid arthritis. Rheumatology (Oxford). 2017;56:777-786.
38. Crowson CS, Rollefstad S, Kitas GD, et al. Challenges of developing a cardiovascular risk calculator for patients with rheumatoid arthritis. PLoS One. 2017;12: e0174656.
39. Karpouzas GA, Malpeso J, Choi TY, et al. Prevalence, extent and composition of coronary plaque in patients with rheumatoid arthritis without symptoms or prior diagnosis of coronary artery disease. Ann Rheum Dis. 2014;73:1797-1804.
40. van Sijl AM, Peters MJ, Knol DK, et al. Carotid intima media thickness in rheumatoid arthritis as compared to control subjects: A meta-analysis. Semin Arthritis Rheum. 2011;40:3893-97.
41. Evans MR, Escalante A, Battafarano DF, et al. Carotid atherosclerosis predicts incident acute coronary syndromes in rheumatoid arthritis. Arthritis Rheum. 2011;63:1211-1220.
42. Ajeganova S, de Faire U, Jogestrand T, et al. Carotid atherosclerosis, disease measures, oxidized low-density lipoproteins, and atheroprotective natural antibodies for cardiovascular disease in early rheumatoid arthritis--an inception cohort study. J Rheumatol. 2012;39:1146-1154.
43. Corrales A, Gonzalez-Juanatey C, Peiro ME, et al. Carotid ultrasound is useful for the cardiovascular risk stratification of patients with rheumatoid arthritis: Results of a population-based study. Ann Rheum Dis. 2014;73:722-727.
44. Ikdahl E, Rollefstad S, Wibetoe G, et al. Predictive value of arterial stiffness and subclinical carotid atherosclerosis for cardiovascular disease in patients with rheumatoid arthritis. J Rheumatol. 2016;43:1622-1630.
45. Provan SA, Semb AG, Hisdal J, et al. Remission is the goal for cardiovascular risk management in patients with rheumatoid arthritis: A cross-sectional comparative study. Ann Rheum Dis. 2011;70:812-817.
46. Vlachopoulos C, Aznaouridis K, Terentes-Printzios D, et al. Prediction of cardiovascular events and all-cause mortality with brachial-ankle elasticity index: A systematic review and meta-analysis. Hypertension. 2012;60:556-562.
47. Ambrosino P, Tasso M, Lupoli R, et al. Non-invasive assessment of arterial stiffness in patients with rheumatoid arthritis: A systematic review and meta-analysis of literature studies. Ann Med. 2015;47:457-467.
48. Rumberger JA, Simons DB, Fitzpatrick LA, et al. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157-2162.
49. Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336-1345.
50. Task Force Members, Montalescot G, Sechtem U, et al. 2013 ESC guidelines on the management of stable coronary artery disease: The task force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949-3003.
51. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2935-2959.
52. Hou ZH, Lu B, Gao Y, et al. Prognostic value of coronary CT angiography and calcium score for major adverse cardiac events in outpatients. JACC Cardiovasc Imaging. 2012;5:990-999.
53. Yiu KH, Mok MY, Wang S, et al. Prognostic role of coronary calcification in patients with rheumatoid arthritis and systemic lupus erythematosus. Clin Exp Rheumatol. 2012;30:345-350.
54. Wright K, Crowson CS, Gabriel SE. Cardiovascular comorbidity in rheumatic diseases: A focus on heart failure. Heart Fail Clin. 2014;10:339-352.
55. Rudominer RL, Roman MJ, Devereux RB, et al. Independent association of rheumatoid arthritis with increased left ventricular mass but not with reduced ejection fraction. Arthritis Rheum. 2009;60:22-29.
56. Bluemke DA, Kronmal RA, Lima JA, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: The MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148-2155.
57. Ntusi NAB, Piechnik SK, Francis JM, et al. Diffuse myocardial fibrosis and inflammation in rheumatoid arthritis: Insights from CMR T1 mapping. JACC Cardiovasc Imaging. 2015;8:526-536.
58. Wong TC, Piehler K, Meier CG, et al. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation. 2012;126:1206-1216.
59. Goodson NJ, Symmons DP, Scott DG, et al. Baseline levels of C-reactive protein and prediction of death from cardiovascular disease in patients with inflammatory polyarthritis: A ten-year followup study of a primary care-based inception cohort. Arthritis Rheum. 2005;52:2293-2299.
60. Kozera L, Andrews J, Morgan AW. Cardiovascular risk and rheumatoid arthritis--the next step: Differentiating true soluble biomarkers of cardiovascular risk from surrogate measures of inflammation. Rheumatology (Oxford). 2011;50:1944-1954.
61. Cardarelli R, Lumicao TG Jr. B-type natriuretic peptide: A review of its diagnostic, prognostic, and therapeutic monitoring value in heart failure for primary care physicians. J Am Board Fam Pract. 2003;16:327-333.
62. Kragelund C, Gronning B, Kober L, et al. N-terminal pro-B-type natriuretic peptide and long-term mortality in stable coronary heart disease. N Engl J Med. 2005;352:666-675.
63. Harney SM, Timperley J, Daly C, et al. Brain natriuretic peptide is a potentially useful screening tool for the detection of cardiovascular disease in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65:136.
64. Bradham WS, Bian A, Oeser A, et al. High-sensitivity cardiac troponin-I is elevated in patients with rheumatoid arthritis, independent of cardiovascular risk factors and inflammation. PLoS One. 2012;7:e38930.
65. Karpouzas GA, Estis J, Rezaeian P, et al. High-sensitivity cardiac troponin I is a biomarker for occult coronary plaque burden and cardiovascular events in patients with rheumatoid arthritis. Rheumatology (Oxford). 2018;57:1080-1088.
66. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2889-2934.
67. Assmann G, Cullen P, Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular munster (PROCAM) study. Circulation. 2002;105:310-315.
Treatment Options for Acute Gout
Gout is an extremely painful arthritis initiated by innate immune responses to monosodium urate crystals that accumulate in affected joints and surrounding tissues. As a result, gout is characterized by painful arthritis flares followed by intervening periods of disease quiescence. Over time, gout can lead to chronic pain, disability, and tophi. Nearly 10% of those aged > 65 years report having gout. The overall prevalence in the U.S. population approaches 4%.1
Gout treatment has 2 overarching goals: alleviating the pain and inflammation caused by acute gout attacks and long-term management that is focused on lowering serum urate (sUA) levels to reduce the risk of future attacks. Alleviating the pain and inflammation of an acute attack is often complicated by patient characteristics, namely, other chronic health conditions that frequently accompany gout, such as diabetes mellitus (DM), chronic kidney disease (CKD), hypertension, and cardiovascular disease (CVD).
Patients with gout tend to be older and have multiple comorbidities that require the use of many medications.2 Because the VA patient population tends to be older, acute gout and attendant complications of treatment are an important consideration for VA health care providers (HCPs).
Recently, the American College of Rheumatology (ACR) released management recommendations for gout, including those for the treatment of acute gout.3 The ACR recommends 3 first-line therapies, but limited guidance is provided for deciding among therapies. This article briefly reviews the relevant ACR recommendations and details important comorbidity and concomitant medication considerations in the treatment of acute gout.
Acute Gout Characteristics
Acute gout attacks are characterized by a rapid onset and escalation with joint pain typically peaking within 24 hours of attack onset. An acute attack often begins to remit after 5 to 12 days without intervention, but complete resolution may take longer in some patients.4 In one study, at 24 hours after attack onset, 16% of patients on placebo had > 50% reduction in pain compared with 70% that had no recovery at all.5 By 48 hours, one-third of patients on placebo achieved a 50% reduction in pain.6
Treatment Recommendations
Therapy for acute gout attacks aims to reduce pain and promote a full, early resolution. The ACR recommends pharmacologic therapy as first-line treatment with adjunctive topical ice and rest as needed.3 Typically, monotherapy is appropriate if the individual is experiencing mild-to-moderate pain affecting ≤ 2 joints of any size. Severe pain or attacks affecting multiple joints may benefit from initial combination therapy. Three first-line therapies are available: nonsteroidal anti-inflammatory drugs (NSAIDs) or cyclooxygenase-2 (COX-2) inhibitors, colchicine, or systemic glucocorticoids (Figure 2).
Few studies compare the efficacy of first-line therapeutic categories. There are no clinical trials directly comparing colchicine with NSAIDs or colchicine with glucocorticoids. No difference in mean reduction of pain and no differences in adverse events (AEs) were shown in a trial that compared glucocorticoids with NSAIDs.8 Thus, without further study, treatment choices made by HCPs are often guided by factors other than the existence of robust evidence.
Treatment with NSAIDs or COX-2 inhibitors should be initiated at the approved dose and continued until the gout attack has completely resolved. In one study, 73% of patients had pain reduction of ≥ 50% when taking NSAIDs relative to only 27% of patients on placebo.8 All available NSAIDs are considered effective, but only 3 NSAIDs are specifically approved for treatment of acute gout (naproxen, indomethacin, and sulindac). There is no evidence supporting one NSAID as being more effective than another; evidence fails to show a meaningful difference.8 Limited evidence indicates that selective COX-2 inhibitors, including celexocib, have similar efficacy as nonselective NSAIDs but may have fewer AEs, driven in part by fewer gastrointestinal (GI) events (6% vs 16% for GI events).8
Colchicine has long been used as prophylaxis for acute gout attacks and has been endorsed for the treatment of acute attacks. Recent evidence suggests that colchicine initially dosed at 1.2 mg followed by a single 0.6-mg dose 1 hour later is as effective with fewer AEs compared with a traditional regimen of 1.2 mg followed by 0.6 mg every hour for up to 6 hours.5 About 40% of patients have 50% pain reduction within 24 hours and a 40% absolute risk reduction in AEs on this low-dose regimen. The efficacy of colchicine relative to other therapies is poorly defined, especially for patients presenting longer after attack onset. The ACR guidelines recommend colchicine only if treatment is initiated within 36 hours of attack onset, but this is based solely on expert consensus. Likewise, the above trial for low-dose colchicine did not provide information about dosing beyond the first 6 hours, leaving little guidance for follow-up treatment of residual pain beyond the 32 hours reported.5 Traditionally, one 0.6-mg dose is provided every 12 to 24 hours.3
Systemic glucocorticoids are also commonly used in treating acute gout.9 There was a small pain reduction benefit for prednisolone, but the difference was not clinically significant in one clinical trial comparing oral prednisolone 30 mg daily for 5 days vs a combination of indomethacin for 5 days and an initial intramuscular injection of diclofenac 75 mg.10 The prednisolone group also had fewer patients with AEs, including abdominal pain (0% vs 30%) and GI bleeding (0% vs 11%). The lower incidence of short-term AEs may be a primary benefit of systemic glucocorticoids.11
Intra-articular glucocorticoids are not suggested first-line therapies but are commonly used by rheumatologists.9 In an uncontrolled study conducted by Fernández and colleagues, intra-articular glucocorticoid injections helped to quickly resolve 20 out of 20 crystal-proven gout attacks.12 However, no randomized controlled trials have examined this approach. Although seemingly efficacious, other considerations are important for this modality. Intra-articular glucocorticoids may not be preferred for polyarticular attacks or attacks in difficult-to-aspirate joints. Additionally, intra-articular glucocorticoids have been anecdotally associated with rebound attacks (ie, attacks that occur shortly after resolution without other interventions). However, the Fernández study had no such attacks occur among participants.12 Finally, septic arthritis must be ruled out as in any case of acute onset monoarticular arthritis.
Biologic agents targeting interleukin-1(IL-1) are not currently approved for gout, although there is burgeoning data suggesting that this strategy may have substantial merit.13 Additionally, there is limited evidence that adrenocorticotropic hormone (ACTH) may provide rapid pain relief when other available therapies are ineffective or contraindicated. However, ACTH studies have not provided robust trial designs, and drug costs remain substantial, thus limiting the widespread use of ACTH in acute gout.14,15 Anti-IL-1 agents and ACTH may both be considered as second-line options if first-line therapies are contraindicated or fail. Careful consideration should be given to AE profiles, patient preferences, and cost.
Comorbidities
Acute gout care, especially in the context of comorbidities, has been identified as a critical treatment concern by an international panel of rheumatologists as part of the 3e (Evidence, Expertise, Exchange) Initiative.16 However, regular clinical trial exclusion criteria have limited data necessary to guide treatment when comorbidities are present. Therefore, studies of acute gout treatment in the context of disease comorbidity represents a major unmet need in understanding and optimizing gout care.
Chronic Kidney Disease
Chronic kidney disease is common in gout; 20% of patients with gout have an estimated glomerular filtration rate (eGFR) of < 30 mL/min.2 Thus, CKD is an important consideration when deciding the best treatment for acute gout. The ACR recommendations do not provide specific guidance on NSAID use in CKD but suggest the potential option of tapering the dose as pain begins to resolve. There is mixed evidence that NSAIDs accelerate CKD progression with the best evidence for high-dose NSAID use.17 When prescribing the concomitant use of NSAIDs with other medications affecting kidney function, HCPs should consider CKD.
For colchicine, current labeling and evidence indicate that no dose adjustments are needed for stage 3 or better CKD (eGFR ≥ 60 mL/min) even among the elderly.18,19 Although labeling indicates that a single unadjusted dose (0.6 mg) can be given once every 2 weeks for those with severe CKD (eGFR < 30 mL/min) or for those who are on dialysis, alternative therapies should be considered, as AEs increase with decreasing renal function.19 Colchicine should not be used in those with eGFR < 10 mL/min.20 All patients who have CKD and are treated with colchicine should be informed of the AEs and closely observed for signs of toxicity, including blood dyscrasias, neuromyopathy, emesis, or diarrhea.
Considering the potential complications for NSAIDs and colchicine, patients with CKD may be good candidates for glucocorticoid therapy, administered either systemically or as an intra-articular injection. Alternatively, second-line agents such as ACTH or IL-1 inhibition may be considered in such patients.
Hypertension
Hypertension is one of the most common comorbidities among patients with gout. It is important for HCP consideration when deciding treatment. Poorly controlled hypertension is a contraindication for both NSAIDs and systemic glucocorticoids. Patients with hypertension in the absence of significant renal impairment may be good candidates for colchicine.
Diabetes and Hyperlipidemia
Glucocorticoids should be avoided if possible in the setting of inadequately controlled type 2 DM (T2DM) or hyperlipidemia. Glucocorticoids exacerbate insulin resistance and stimulate glucose secretion from the liver. This can create substantial and sometimes dangerous fluctuations in circulating glucose concentrations. Additionally, glucocorticoids may increase serum triglycerides and low-density lipoprotein levels. Thus, patients with T2DM or hyperlipidemia may be good candidates for alternative treatments, such as colchicine or NSAIDs.
Cardiovascular Disease
Cardiovascular disease risk has been shown to increase with the use of COX-2 inhibitors. This risk may be present for all NSAIDs. Current FDA labeling suggests limiting NSAID and COX-2 inhibitor use in patients with a history of myocardial infarction (MI), congestive heart failure, or stroke. Given the potential impact on cardiovascular risk factors, including hypertension, T2DM, and hyperlipidemia, glucocorticoids may not be ideal for patients with known CVD or those at high risk.
Recent evidence has shown that colchicine use is associated with a lower risk of MI among patients with gout.21 These results, in addition to a proposed dual role of IL-1 in both gout and CVD, suggest that either colchicine or IL-1 inhibitors may be rational agents in the treatment of acute gout in the context of CVD.22
Hepatic Impairment and GI Bleeding
Patients with cirrhosis should avoid NSAID use due to the potential increased bleeding risk from underlying coagulopathy. Additionally, colchicine clearance may be reduced in patients with severe liver impairment, mandating close surveillance when this agent is used. If hepatic impairment is mild to moderate, judicious use of any of the first-line therapies may be appropriate.
Patients with GI bleeding or a history of peptic ulcer disease should avoid NSAID use because of increased bleeding risk. If an NSAID is used, proton pump inhibitors decrease the risk of NSAID-associated mucosal damage.
Drug Interactions
Colchicine is metabolized by the cytochrome P450 3A4 enzyme (CYP3A4) and is a substrate for P-glycoprotein (P-gp). Therefore, concomitant use of colchicine with potent inhibitors of CYP3A4 or P-gp should be avoided when possible. These agents include macrolide antibiotics (clarithromyocin), calcium channel blockers (verapamil and diltiazem), and cyclosporine (commonly used in transplant patients who are at high risk for gout). New evidence-based dosing recommendations indicate that no dose reduction is required with azithromyocin.23
Nonsteroidal anti-inflammatory drugs are contraindicated with the concomitant use of angiotensin-converting enzyme (ACE) inhibitors and/or diuretics. Prostaglandin production is decreased while using NSAIDs, resulting in increased constriction of afferent renal arterioles and decreased glomerular filtration pressure. This physiologic effect of NSAIDs can be exacerbated when used in combination with ACE inhibitors or diuretics, both of which can also reduce glomerular filtration pressures. Combination therapy with either ACE inhibitors or diuretics increases the risk for NSAID-mediated acute kidney injury. Additionally, NSAID use should be avoided in patients taking anticoagulants such as warfarin or heparin due to increased bleeding risk.
Diagnosis
Diagnosis is a key component of proper treatment of acute gout. A gout diagnosis is usually made based on clinical signs and symptoms, including sudden onset of pain that peaks within 24 hours, past history of acute self-limited attacks of arthritis, first MTP involvement, and an elevated sUA. However, not all these factors must be present. In a study conducted by Janssens and colleagues, these factors plus additional demographics had a sensitivity of 90% and specificity of 65% when compared with crystal diagnosis.24 However, a normal sUA level does not exclude gout as a diagnosis due to the uricosuric effect of the inflammatory process.25 In fact, one observational cohort recorded an average sUA decrease from baseline of 2 mg/dL during an acute gout attack.26
Alternative diagnoses, including septic arthritis, should be considered, particularly in the context of treatment failure (< 50% reduction in pain) within the first 24 to 48 hours. A definitive diagnosis is made by identifying negatively birefringent crystals in the synovial fluid of the affected joint (using polarized microscopy) with negative cultures. In the absence of crystal confirmation, there is an emerging role for imaging in gout diagnosis, including the use of ultrasound and dual-energy computed tomography.27
Long-term Treatment Considerations
During treatment for acute gout attacks, urate-lowering therapy that was initiated before the attack should not be discontinued.28 There is no evidence to suggest that current urate lowering has any AEs during attacks. However, removing treatment may increase sUA levels, precipitating attacks in other joints by “destabilizing” crystals still present. Current recommendations also state that urate-lowering therapy may be started during an attack despite traditionally being deferred until the attack has resolved.28 In a randomized trial comparing a group starting allopurinol 300 mg during an attack vs a placebo group (with all patients receiving anti-inflammatory treatment for the acute attack), there was no difference in pain outcomes.29 Regardless of the chosen timing, lowering and maintaining sUA ≤ 6.0 mg/dL is the primary method for minimizing long-term risk of gout attacks.28
Health care providers should discuss with patients the likely need for indefinite urate-lowering therapy while noting that attacks related to therapy initiation are relatively common.30 Current guidelines recommend starting urate-lowering therapy in low doses (≤ 100 mg/d for allopurinol) and titrating to achieve and maintain the target sUA level. Along with the judicious use of anti-inflammatory prophylaxis, this may minimize attacks related to therapy initiation.3,28 By lowering and maintaining sUA below the target level, monosodium urate crystals will dissolve, thereby eliminating the major inciting factor of acute attacks.
Other day-to-day triggers such as alcohol, meat or seafood consumption, and dehydration exist for some patients with gout. Patients should be informed of these inciting factors, as they could potentially be avoided, reducing the risk of future gout attacks. It is important to recognize, however, that dietary or behavioral interventions have generally yielded only modest sUA reductions. For the majority of patients, therefore, reduction and maintenance of sUA ≤ 6.0 mg/dL requires pharmacologic intervention.
Conclusions
Gout attacks should be treated immediately with pharmacologic treatment when contraindications are absent. First-line treatment options include NSAIDs, colchicine, and systemic glucocorticoids. Use of these modalities can be complicated because of comorbidity and concomitant medication use that is prevalent among patients with gout. Comorbidities commonly limiting treatment choice include hypertension (NSAIDs, glucocorticoids), CKD (NSAIDS, colchicine), CVD (NSAIDs, COX-2 inhibitors, glucocorticoids), T2DM (glucocorticoids), and liver disease (NSAIDs, colchicine). Careful consideration must be given to these comorbidities and contraindications as well as patient preferences.
1. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63(10):3136-3141.
2. Zhu Y, Pandya BJ, Choi HK. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007-2008. Am J Med. 2012;125(7):679-687.e1.
3. Khanna D, Khanna PP, Fitzgerald JD, et al; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64(10):1447-1461.
4. Bellamy N, Downie WW, Buchanan WW. Observations on spontaneous improvement in patients with podagra: implications for therapeutic trials of non-steroidal anti-inflammatory drugs. Br J Clin Pharmacol. 1987;24(1):33-36.
5. Terkeltaub RA, Furst DE, Bennett K, Kook KA, Crockett RS, Davis MW. High versus low dosing of oral colchicine for early acute gout flare: twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. Arthritis Rheum. 2010;62(4):1060-1068.
6. Ahern MJ, Reid C, Gordon TP, McCredie M, Brooks PM, Jones M. Does colchicine work? The results of the first controlled study in acute gout. Aust N Z J Med. 1987;17(3):301-304.
7. Puig JG, Michán AD, Jiménez ML, et al. Female gout. Clinical spectrum and uric acid metabolism. Arch Intern Med. 1991;151(4):726-732.
8. van Durme CM, Wechalekar MD, Buchbinder R, Schlesinger N, van der Heijde D, Landewé RB. Non-steroidal anti-inflammatory drugs for acute gout. Cochrane Database Syst Rev. 2014;9:CD010120.
9. Schlesinger N, Moore DF, Sun JD, Schumacher HR Jr. A survey of current evaluation and treatment of gout. J Rheumatol. 2006;33(10):2050-2052.
10. Man CY, Cheung IT, Cameron PA, Rainer TH. Comparison of oral prednisolone/paracetamol and oral indomethacin/paracetamol combination therapy in the treatment of acute goutlike arthritis: a double-blind, randomized, controlled trial. Ann Emerg Med. 2007;49(5):670-677.
11. Janssens HJ, Lucassen PL, Van de Laar FA, Janssen M, Van de Lisdonk EH. Systemic corticosteroids for acute gout. Cochrane Database Syst Rev. 2008;(2):CD005521.
12. Fernández C, Noguera R, González JA, Pascual E. Treatment of acute attacks of gout with a small dose of intraarticular triamcinolone acetonide. J Rheumatol. 1999;26(10):2285-2286.
13. Burns CM, Wortmann RL. Gout therapeutics: new drugs for an old disease. Lancet. 2011;377(9760):165-177.
14. Axelrod D, Preston S. Comparison of parenteral adrenocorticotropic hormone with oral indomethacin in the treatment of acute gout. Arthritis Rheum. 1988;31(6):803-805.
15. Ritter J, Kerr LD, Valeriano-Marcet J, Spiera H. ACTH revisited: effective treatment for acute crystal induced synovitis in patients with multiple medical problems. J Rheumatol. 1994;21(4):696-699.
16. Sivera F, Andrés M, Carmona L, et al. Multinational evidence-based recommendations for the diagnosis and management of gout: integrating systematic literature review and expert opinion of a broad panel of rheumatologists in the 3e initiative. Ann Rheum Dis. 2014;73(2):328-335.
17. Nderitu P, Doos L, Jones PW, Davies SJ, Kadam UT. Non-steroidal anti-inflammatory drugs and chronic kidney disease progression: a systematic review. Fam Pract. 2013;30(3):247-255.
18. Wason S, Faulkner RD, Davis MW. Are dosing adjustments required for colchicine in the elderly compared with younger patients? Adv Ther. 2012;29(6):551-561.
19. Wason S, Mount D, Faulkner R. Single-dose, open-label study of the differences in pharmacokinetics of colchicine in subjects with renal impairment, including end-stage renal disease. Clin Drug Investig. 2014;34(12):845-855.
20. Hanlon JT, Aspinall SL, Semla TP, et al. Consensus guidelines for oral dosing of primarily renally cleared medications in older adults. J Am Geriatr Soc. 2009;57(2):335-340.
21. Crittenden DB, Lehmann RA, Schneck L, et al. Colchicine use is associated with decreased prevalence of myocardial infarction in patients with gout. J Rheumatol. 2012;39(7):1458-1464.
22. Esser N, Paquot N, Scheen AJ. Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Expert Opin Investig Drugs. 2015;24(3):283-307.
23. Terkeltaub RA, Furst DE, Digiacinto JL, Kook KA, Davis MW. Novel evidence-based colchicine dose-reduction algorithm to predict and prevent colchicine toxicity in the presence of cytochrome P450 3A4/P-glycoprotein inhibitors. Arthritis Rheum. 2011;63(8):2226-2237.
24. Janssens HJ, Fransen J, van de Lisdonk EH, van Riel PL, van Weel C, Janssen M. A diagnostic rule for acute gouty arthritis in primary care without joint fluid analysis. Arch Intern Med. 2010;170(13):1120-1126.
25. Urano W, Yamanaka H, Tsutani H, et al. The inflammatory process in the mechanism of decreased serum uric acid concentrations during acute gouty arthritis. J Rheumatol. 2002;29(9):1950-1953.
26. Logan JA, Morrison E, McGill PE. Serum uric acid in acute gout. Ann Rheum Dis. 1997;56(11):696-697.
27. Ogdie A, Taylor WJ, Weatherall M, et al. Imaging modalities for the classification of gout: systematic literature review and meta-analysis. Ann Rheum Dis. 2015; 74(10):1868-1874.
28. Khanna D, Fitzgerald JD, Khanna PP, et al; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012;64(10):1431-1446.
29. Taylor TH, Mecchella JN, Larson RJ, Kerin KD, Mackenzie TA. Initiation of allopurinol at first medical contact for acute attacks of gout: a randomized clinical trial. Am J Med. 2012;125(11):1126-11134.e7.
30. Becker MA, MacDonald PA, Hunt BJ, Lademacher C, Joseph-Ridge N. Determinants of the clinical outcomes of gout during the first year of urate-lowering therapy. Nucleosides Nucleotides Nucleic Acids. 2008;27(6):585-591.
Gout is an extremely painful arthritis initiated by innate immune responses to monosodium urate crystals that accumulate in affected joints and surrounding tissues. As a result, gout is characterized by painful arthritis flares followed by intervening periods of disease quiescence. Over time, gout can lead to chronic pain, disability, and tophi. Nearly 10% of those aged > 65 years report having gout. The overall prevalence in the U.S. population approaches 4%.1
Gout treatment has 2 overarching goals: alleviating the pain and inflammation caused by acute gout attacks and long-term management that is focused on lowering serum urate (sUA) levels to reduce the risk of future attacks. Alleviating the pain and inflammation of an acute attack is often complicated by patient characteristics, namely, other chronic health conditions that frequently accompany gout, such as diabetes mellitus (DM), chronic kidney disease (CKD), hypertension, and cardiovascular disease (CVD).
Patients with gout tend to be older and have multiple comorbidities that require the use of many medications.2 Because the VA patient population tends to be older, acute gout and attendant complications of treatment are an important consideration for VA health care providers (HCPs).
Recently, the American College of Rheumatology (ACR) released management recommendations for gout, including those for the treatment of acute gout.3 The ACR recommends 3 first-line therapies, but limited guidance is provided for deciding among therapies. This article briefly reviews the relevant ACR recommendations and details important comorbidity and concomitant medication considerations in the treatment of acute gout.
Acute Gout Characteristics
Acute gout attacks are characterized by a rapid onset and escalation with joint pain typically peaking within 24 hours of attack onset. An acute attack often begins to remit after 5 to 12 days without intervention, but complete resolution may take longer in some patients.4 In one study, at 24 hours after attack onset, 16% of patients on placebo had > 50% reduction in pain compared with 70% that had no recovery at all.5 By 48 hours, one-third of patients on placebo achieved a 50% reduction in pain.6
Treatment Recommendations
Therapy for acute gout attacks aims to reduce pain and promote a full, early resolution. The ACR recommends pharmacologic therapy as first-line treatment with adjunctive topical ice and rest as needed.3 Typically, monotherapy is appropriate if the individual is experiencing mild-to-moderate pain affecting ≤ 2 joints of any size. Severe pain or attacks affecting multiple joints may benefit from initial combination therapy. Three first-line therapies are available: nonsteroidal anti-inflammatory drugs (NSAIDs) or cyclooxygenase-2 (COX-2) inhibitors, colchicine, or systemic glucocorticoids (Figure 2).
Few studies compare the efficacy of first-line therapeutic categories. There are no clinical trials directly comparing colchicine with NSAIDs or colchicine with glucocorticoids. No difference in mean reduction of pain and no differences in adverse events (AEs) were shown in a trial that compared glucocorticoids with NSAIDs.8 Thus, without further study, treatment choices made by HCPs are often guided by factors other than the existence of robust evidence.
Treatment with NSAIDs or COX-2 inhibitors should be initiated at the approved dose and continued until the gout attack has completely resolved. In one study, 73% of patients had pain reduction of ≥ 50% when taking NSAIDs relative to only 27% of patients on placebo.8 All available NSAIDs are considered effective, but only 3 NSAIDs are specifically approved for treatment of acute gout (naproxen, indomethacin, and sulindac). There is no evidence supporting one NSAID as being more effective than another; evidence fails to show a meaningful difference.8 Limited evidence indicates that selective COX-2 inhibitors, including celexocib, have similar efficacy as nonselective NSAIDs but may have fewer AEs, driven in part by fewer gastrointestinal (GI) events (6% vs 16% for GI events).8
Colchicine has long been used as prophylaxis for acute gout attacks and has been endorsed for the treatment of acute attacks. Recent evidence suggests that colchicine initially dosed at 1.2 mg followed by a single 0.6-mg dose 1 hour later is as effective with fewer AEs compared with a traditional regimen of 1.2 mg followed by 0.6 mg every hour for up to 6 hours.5 About 40% of patients have 50% pain reduction within 24 hours and a 40% absolute risk reduction in AEs on this low-dose regimen. The efficacy of colchicine relative to other therapies is poorly defined, especially for patients presenting longer after attack onset. The ACR guidelines recommend colchicine only if treatment is initiated within 36 hours of attack onset, but this is based solely on expert consensus. Likewise, the above trial for low-dose colchicine did not provide information about dosing beyond the first 6 hours, leaving little guidance for follow-up treatment of residual pain beyond the 32 hours reported.5 Traditionally, one 0.6-mg dose is provided every 12 to 24 hours.3
Systemic glucocorticoids are also commonly used in treating acute gout.9 There was a small pain reduction benefit for prednisolone, but the difference was not clinically significant in one clinical trial comparing oral prednisolone 30 mg daily for 5 days vs a combination of indomethacin for 5 days and an initial intramuscular injection of diclofenac 75 mg.10 The prednisolone group also had fewer patients with AEs, including abdominal pain (0% vs 30%) and GI bleeding (0% vs 11%). The lower incidence of short-term AEs may be a primary benefit of systemic glucocorticoids.11
Intra-articular glucocorticoids are not suggested first-line therapies but are commonly used by rheumatologists.9 In an uncontrolled study conducted by Fernández and colleagues, intra-articular glucocorticoid injections helped to quickly resolve 20 out of 20 crystal-proven gout attacks.12 However, no randomized controlled trials have examined this approach. Although seemingly efficacious, other considerations are important for this modality. Intra-articular glucocorticoids may not be preferred for polyarticular attacks or attacks in difficult-to-aspirate joints. Additionally, intra-articular glucocorticoids have been anecdotally associated with rebound attacks (ie, attacks that occur shortly after resolution without other interventions). However, the Fernández study had no such attacks occur among participants.12 Finally, septic arthritis must be ruled out as in any case of acute onset monoarticular arthritis.
Biologic agents targeting interleukin-1(IL-1) are not currently approved for gout, although there is burgeoning data suggesting that this strategy may have substantial merit.13 Additionally, there is limited evidence that adrenocorticotropic hormone (ACTH) may provide rapid pain relief when other available therapies are ineffective or contraindicated. However, ACTH studies have not provided robust trial designs, and drug costs remain substantial, thus limiting the widespread use of ACTH in acute gout.14,15 Anti-IL-1 agents and ACTH may both be considered as second-line options if first-line therapies are contraindicated or fail. Careful consideration should be given to AE profiles, patient preferences, and cost.
Comorbidities
Acute gout care, especially in the context of comorbidities, has been identified as a critical treatment concern by an international panel of rheumatologists as part of the 3e (Evidence, Expertise, Exchange) Initiative.16 However, regular clinical trial exclusion criteria have limited data necessary to guide treatment when comorbidities are present. Therefore, studies of acute gout treatment in the context of disease comorbidity represents a major unmet need in understanding and optimizing gout care.
Chronic Kidney Disease
Chronic kidney disease is common in gout; 20% of patients with gout have an estimated glomerular filtration rate (eGFR) of < 30 mL/min.2 Thus, CKD is an important consideration when deciding the best treatment for acute gout. The ACR recommendations do not provide specific guidance on NSAID use in CKD but suggest the potential option of tapering the dose as pain begins to resolve. There is mixed evidence that NSAIDs accelerate CKD progression with the best evidence for high-dose NSAID use.17 When prescribing the concomitant use of NSAIDs with other medications affecting kidney function, HCPs should consider CKD.
For colchicine, current labeling and evidence indicate that no dose adjustments are needed for stage 3 or better CKD (eGFR ≥ 60 mL/min) even among the elderly.18,19 Although labeling indicates that a single unadjusted dose (0.6 mg) can be given once every 2 weeks for those with severe CKD (eGFR < 30 mL/min) or for those who are on dialysis, alternative therapies should be considered, as AEs increase with decreasing renal function.19 Colchicine should not be used in those with eGFR < 10 mL/min.20 All patients who have CKD and are treated with colchicine should be informed of the AEs and closely observed for signs of toxicity, including blood dyscrasias, neuromyopathy, emesis, or diarrhea.
Considering the potential complications for NSAIDs and colchicine, patients with CKD may be good candidates for glucocorticoid therapy, administered either systemically or as an intra-articular injection. Alternatively, second-line agents such as ACTH or IL-1 inhibition may be considered in such patients.
Hypertension
Hypertension is one of the most common comorbidities among patients with gout. It is important for HCP consideration when deciding treatment. Poorly controlled hypertension is a contraindication for both NSAIDs and systemic glucocorticoids. Patients with hypertension in the absence of significant renal impairment may be good candidates for colchicine.
Diabetes and Hyperlipidemia
Glucocorticoids should be avoided if possible in the setting of inadequately controlled type 2 DM (T2DM) or hyperlipidemia. Glucocorticoids exacerbate insulin resistance and stimulate glucose secretion from the liver. This can create substantial and sometimes dangerous fluctuations in circulating glucose concentrations. Additionally, glucocorticoids may increase serum triglycerides and low-density lipoprotein levels. Thus, patients with T2DM or hyperlipidemia may be good candidates for alternative treatments, such as colchicine or NSAIDs.
Cardiovascular Disease
Cardiovascular disease risk has been shown to increase with the use of COX-2 inhibitors. This risk may be present for all NSAIDs. Current FDA labeling suggests limiting NSAID and COX-2 inhibitor use in patients with a history of myocardial infarction (MI), congestive heart failure, or stroke. Given the potential impact on cardiovascular risk factors, including hypertension, T2DM, and hyperlipidemia, glucocorticoids may not be ideal for patients with known CVD or those at high risk.
Recent evidence has shown that colchicine use is associated with a lower risk of MI among patients with gout.21 These results, in addition to a proposed dual role of IL-1 in both gout and CVD, suggest that either colchicine or IL-1 inhibitors may be rational agents in the treatment of acute gout in the context of CVD.22
Hepatic Impairment and GI Bleeding
Patients with cirrhosis should avoid NSAID use due to the potential increased bleeding risk from underlying coagulopathy. Additionally, colchicine clearance may be reduced in patients with severe liver impairment, mandating close surveillance when this agent is used. If hepatic impairment is mild to moderate, judicious use of any of the first-line therapies may be appropriate.
Patients with GI bleeding or a history of peptic ulcer disease should avoid NSAID use because of increased bleeding risk. If an NSAID is used, proton pump inhibitors decrease the risk of NSAID-associated mucosal damage.
Drug Interactions
Colchicine is metabolized by the cytochrome P450 3A4 enzyme (CYP3A4) and is a substrate for P-glycoprotein (P-gp). Therefore, concomitant use of colchicine with potent inhibitors of CYP3A4 or P-gp should be avoided when possible. These agents include macrolide antibiotics (clarithromyocin), calcium channel blockers (verapamil and diltiazem), and cyclosporine (commonly used in transplant patients who are at high risk for gout). New evidence-based dosing recommendations indicate that no dose reduction is required with azithromyocin.23
Nonsteroidal anti-inflammatory drugs are contraindicated with the concomitant use of angiotensin-converting enzyme (ACE) inhibitors and/or diuretics. Prostaglandin production is decreased while using NSAIDs, resulting in increased constriction of afferent renal arterioles and decreased glomerular filtration pressure. This physiologic effect of NSAIDs can be exacerbated when used in combination with ACE inhibitors or diuretics, both of which can also reduce glomerular filtration pressures. Combination therapy with either ACE inhibitors or diuretics increases the risk for NSAID-mediated acute kidney injury. Additionally, NSAID use should be avoided in patients taking anticoagulants such as warfarin or heparin due to increased bleeding risk.
Diagnosis
Diagnosis is a key component of proper treatment of acute gout. A gout diagnosis is usually made based on clinical signs and symptoms, including sudden onset of pain that peaks within 24 hours, past history of acute self-limited attacks of arthritis, first MTP involvement, and an elevated sUA. However, not all these factors must be present. In a study conducted by Janssens and colleagues, these factors plus additional demographics had a sensitivity of 90% and specificity of 65% when compared with crystal diagnosis.24 However, a normal sUA level does not exclude gout as a diagnosis due to the uricosuric effect of the inflammatory process.25 In fact, one observational cohort recorded an average sUA decrease from baseline of 2 mg/dL during an acute gout attack.26
Alternative diagnoses, including septic arthritis, should be considered, particularly in the context of treatment failure (< 50% reduction in pain) within the first 24 to 48 hours. A definitive diagnosis is made by identifying negatively birefringent crystals in the synovial fluid of the affected joint (using polarized microscopy) with negative cultures. In the absence of crystal confirmation, there is an emerging role for imaging in gout diagnosis, including the use of ultrasound and dual-energy computed tomography.27
Long-term Treatment Considerations
During treatment for acute gout attacks, urate-lowering therapy that was initiated before the attack should not be discontinued.28 There is no evidence to suggest that current urate lowering has any AEs during attacks. However, removing treatment may increase sUA levels, precipitating attacks in other joints by “destabilizing” crystals still present. Current recommendations also state that urate-lowering therapy may be started during an attack despite traditionally being deferred until the attack has resolved.28 In a randomized trial comparing a group starting allopurinol 300 mg during an attack vs a placebo group (with all patients receiving anti-inflammatory treatment for the acute attack), there was no difference in pain outcomes.29 Regardless of the chosen timing, lowering and maintaining sUA ≤ 6.0 mg/dL is the primary method for minimizing long-term risk of gout attacks.28
Health care providers should discuss with patients the likely need for indefinite urate-lowering therapy while noting that attacks related to therapy initiation are relatively common.30 Current guidelines recommend starting urate-lowering therapy in low doses (≤ 100 mg/d for allopurinol) and titrating to achieve and maintain the target sUA level. Along with the judicious use of anti-inflammatory prophylaxis, this may minimize attacks related to therapy initiation.3,28 By lowering and maintaining sUA below the target level, monosodium urate crystals will dissolve, thereby eliminating the major inciting factor of acute attacks.
Other day-to-day triggers such as alcohol, meat or seafood consumption, and dehydration exist for some patients with gout. Patients should be informed of these inciting factors, as they could potentially be avoided, reducing the risk of future gout attacks. It is important to recognize, however, that dietary or behavioral interventions have generally yielded only modest sUA reductions. For the majority of patients, therefore, reduction and maintenance of sUA ≤ 6.0 mg/dL requires pharmacologic intervention.
Conclusions
Gout attacks should be treated immediately with pharmacologic treatment when contraindications are absent. First-line treatment options include NSAIDs, colchicine, and systemic glucocorticoids. Use of these modalities can be complicated because of comorbidity and concomitant medication use that is prevalent among patients with gout. Comorbidities commonly limiting treatment choice include hypertension (NSAIDs, glucocorticoids), CKD (NSAIDS, colchicine), CVD (NSAIDs, COX-2 inhibitors, glucocorticoids), T2DM (glucocorticoids), and liver disease (NSAIDs, colchicine). Careful consideration must be given to these comorbidities and contraindications as well as patient preferences.
Gout is an extremely painful arthritis initiated by innate immune responses to monosodium urate crystals that accumulate in affected joints and surrounding tissues. As a result, gout is characterized by painful arthritis flares followed by intervening periods of disease quiescence. Over time, gout can lead to chronic pain, disability, and tophi. Nearly 10% of those aged > 65 years report having gout. The overall prevalence in the U.S. population approaches 4%.1
Gout treatment has 2 overarching goals: alleviating the pain and inflammation caused by acute gout attacks and long-term management that is focused on lowering serum urate (sUA) levels to reduce the risk of future attacks. Alleviating the pain and inflammation of an acute attack is often complicated by patient characteristics, namely, other chronic health conditions that frequently accompany gout, such as diabetes mellitus (DM), chronic kidney disease (CKD), hypertension, and cardiovascular disease (CVD).
Patients with gout tend to be older and have multiple comorbidities that require the use of many medications.2 Because the VA patient population tends to be older, acute gout and attendant complications of treatment are an important consideration for VA health care providers (HCPs).
Recently, the American College of Rheumatology (ACR) released management recommendations for gout, including those for the treatment of acute gout.3 The ACR recommends 3 first-line therapies, but limited guidance is provided for deciding among therapies. This article briefly reviews the relevant ACR recommendations and details important comorbidity and concomitant medication considerations in the treatment of acute gout.
Acute Gout Characteristics
Acute gout attacks are characterized by a rapid onset and escalation with joint pain typically peaking within 24 hours of attack onset. An acute attack often begins to remit after 5 to 12 days without intervention, but complete resolution may take longer in some patients.4 In one study, at 24 hours after attack onset, 16% of patients on placebo had > 50% reduction in pain compared with 70% that had no recovery at all.5 By 48 hours, one-third of patients on placebo achieved a 50% reduction in pain.6
Treatment Recommendations
Therapy for acute gout attacks aims to reduce pain and promote a full, early resolution. The ACR recommends pharmacologic therapy as first-line treatment with adjunctive topical ice and rest as needed.3 Typically, monotherapy is appropriate if the individual is experiencing mild-to-moderate pain affecting ≤ 2 joints of any size. Severe pain or attacks affecting multiple joints may benefit from initial combination therapy. Three first-line therapies are available: nonsteroidal anti-inflammatory drugs (NSAIDs) or cyclooxygenase-2 (COX-2) inhibitors, colchicine, or systemic glucocorticoids (Figure 2).
Few studies compare the efficacy of first-line therapeutic categories. There are no clinical trials directly comparing colchicine with NSAIDs or colchicine with glucocorticoids. No difference in mean reduction of pain and no differences in adverse events (AEs) were shown in a trial that compared glucocorticoids with NSAIDs.8 Thus, without further study, treatment choices made by HCPs are often guided by factors other than the existence of robust evidence.
Treatment with NSAIDs or COX-2 inhibitors should be initiated at the approved dose and continued until the gout attack has completely resolved. In one study, 73% of patients had pain reduction of ≥ 50% when taking NSAIDs relative to only 27% of patients on placebo.8 All available NSAIDs are considered effective, but only 3 NSAIDs are specifically approved for treatment of acute gout (naproxen, indomethacin, and sulindac). There is no evidence supporting one NSAID as being more effective than another; evidence fails to show a meaningful difference.8 Limited evidence indicates that selective COX-2 inhibitors, including celexocib, have similar efficacy as nonselective NSAIDs but may have fewer AEs, driven in part by fewer gastrointestinal (GI) events (6% vs 16% for GI events).8
Colchicine has long been used as prophylaxis for acute gout attacks and has been endorsed for the treatment of acute attacks. Recent evidence suggests that colchicine initially dosed at 1.2 mg followed by a single 0.6-mg dose 1 hour later is as effective with fewer AEs compared with a traditional regimen of 1.2 mg followed by 0.6 mg every hour for up to 6 hours.5 About 40% of patients have 50% pain reduction within 24 hours and a 40% absolute risk reduction in AEs on this low-dose regimen. The efficacy of colchicine relative to other therapies is poorly defined, especially for patients presenting longer after attack onset. The ACR guidelines recommend colchicine only if treatment is initiated within 36 hours of attack onset, but this is based solely on expert consensus. Likewise, the above trial for low-dose colchicine did not provide information about dosing beyond the first 6 hours, leaving little guidance for follow-up treatment of residual pain beyond the 32 hours reported.5 Traditionally, one 0.6-mg dose is provided every 12 to 24 hours.3
Systemic glucocorticoids are also commonly used in treating acute gout.9 There was a small pain reduction benefit for prednisolone, but the difference was not clinically significant in one clinical trial comparing oral prednisolone 30 mg daily for 5 days vs a combination of indomethacin for 5 days and an initial intramuscular injection of diclofenac 75 mg.10 The prednisolone group also had fewer patients with AEs, including abdominal pain (0% vs 30%) and GI bleeding (0% vs 11%). The lower incidence of short-term AEs may be a primary benefit of systemic glucocorticoids.11
Intra-articular glucocorticoids are not suggested first-line therapies but are commonly used by rheumatologists.9 In an uncontrolled study conducted by Fernández and colleagues, intra-articular glucocorticoid injections helped to quickly resolve 20 out of 20 crystal-proven gout attacks.12 However, no randomized controlled trials have examined this approach. Although seemingly efficacious, other considerations are important for this modality. Intra-articular glucocorticoids may not be preferred for polyarticular attacks or attacks in difficult-to-aspirate joints. Additionally, intra-articular glucocorticoids have been anecdotally associated with rebound attacks (ie, attacks that occur shortly after resolution without other interventions). However, the Fernández study had no such attacks occur among participants.12 Finally, septic arthritis must be ruled out as in any case of acute onset monoarticular arthritis.
Biologic agents targeting interleukin-1(IL-1) are not currently approved for gout, although there is burgeoning data suggesting that this strategy may have substantial merit.13 Additionally, there is limited evidence that adrenocorticotropic hormone (ACTH) may provide rapid pain relief when other available therapies are ineffective or contraindicated. However, ACTH studies have not provided robust trial designs, and drug costs remain substantial, thus limiting the widespread use of ACTH in acute gout.14,15 Anti-IL-1 agents and ACTH may both be considered as second-line options if first-line therapies are contraindicated or fail. Careful consideration should be given to AE profiles, patient preferences, and cost.
Comorbidities
Acute gout care, especially in the context of comorbidities, has been identified as a critical treatment concern by an international panel of rheumatologists as part of the 3e (Evidence, Expertise, Exchange) Initiative.16 However, regular clinical trial exclusion criteria have limited data necessary to guide treatment when comorbidities are present. Therefore, studies of acute gout treatment in the context of disease comorbidity represents a major unmet need in understanding and optimizing gout care.
Chronic Kidney Disease
Chronic kidney disease is common in gout; 20% of patients with gout have an estimated glomerular filtration rate (eGFR) of < 30 mL/min.2 Thus, CKD is an important consideration when deciding the best treatment for acute gout. The ACR recommendations do not provide specific guidance on NSAID use in CKD but suggest the potential option of tapering the dose as pain begins to resolve. There is mixed evidence that NSAIDs accelerate CKD progression with the best evidence for high-dose NSAID use.17 When prescribing the concomitant use of NSAIDs with other medications affecting kidney function, HCPs should consider CKD.
For colchicine, current labeling and evidence indicate that no dose adjustments are needed for stage 3 or better CKD (eGFR ≥ 60 mL/min) even among the elderly.18,19 Although labeling indicates that a single unadjusted dose (0.6 mg) can be given once every 2 weeks for those with severe CKD (eGFR < 30 mL/min) or for those who are on dialysis, alternative therapies should be considered, as AEs increase with decreasing renal function.19 Colchicine should not be used in those with eGFR < 10 mL/min.20 All patients who have CKD and are treated with colchicine should be informed of the AEs and closely observed for signs of toxicity, including blood dyscrasias, neuromyopathy, emesis, or diarrhea.
Considering the potential complications for NSAIDs and colchicine, patients with CKD may be good candidates for glucocorticoid therapy, administered either systemically or as an intra-articular injection. Alternatively, second-line agents such as ACTH or IL-1 inhibition may be considered in such patients.
Hypertension
Hypertension is one of the most common comorbidities among patients with gout. It is important for HCP consideration when deciding treatment. Poorly controlled hypertension is a contraindication for both NSAIDs and systemic glucocorticoids. Patients with hypertension in the absence of significant renal impairment may be good candidates for colchicine.
Diabetes and Hyperlipidemia
Glucocorticoids should be avoided if possible in the setting of inadequately controlled type 2 DM (T2DM) or hyperlipidemia. Glucocorticoids exacerbate insulin resistance and stimulate glucose secretion from the liver. This can create substantial and sometimes dangerous fluctuations in circulating glucose concentrations. Additionally, glucocorticoids may increase serum triglycerides and low-density lipoprotein levels. Thus, patients with T2DM or hyperlipidemia may be good candidates for alternative treatments, such as colchicine or NSAIDs.
Cardiovascular Disease
Cardiovascular disease risk has been shown to increase with the use of COX-2 inhibitors. This risk may be present for all NSAIDs. Current FDA labeling suggests limiting NSAID and COX-2 inhibitor use in patients with a history of myocardial infarction (MI), congestive heart failure, or stroke. Given the potential impact on cardiovascular risk factors, including hypertension, T2DM, and hyperlipidemia, glucocorticoids may not be ideal for patients with known CVD or those at high risk.
Recent evidence has shown that colchicine use is associated with a lower risk of MI among patients with gout.21 These results, in addition to a proposed dual role of IL-1 in both gout and CVD, suggest that either colchicine or IL-1 inhibitors may be rational agents in the treatment of acute gout in the context of CVD.22
Hepatic Impairment and GI Bleeding
Patients with cirrhosis should avoid NSAID use due to the potential increased bleeding risk from underlying coagulopathy. Additionally, colchicine clearance may be reduced in patients with severe liver impairment, mandating close surveillance when this agent is used. If hepatic impairment is mild to moderate, judicious use of any of the first-line therapies may be appropriate.
Patients with GI bleeding or a history of peptic ulcer disease should avoid NSAID use because of increased bleeding risk. If an NSAID is used, proton pump inhibitors decrease the risk of NSAID-associated mucosal damage.
Drug Interactions
Colchicine is metabolized by the cytochrome P450 3A4 enzyme (CYP3A4) and is a substrate for P-glycoprotein (P-gp). Therefore, concomitant use of colchicine with potent inhibitors of CYP3A4 or P-gp should be avoided when possible. These agents include macrolide antibiotics (clarithromyocin), calcium channel blockers (verapamil and diltiazem), and cyclosporine (commonly used in transplant patients who are at high risk for gout). New evidence-based dosing recommendations indicate that no dose reduction is required with azithromyocin.23
Nonsteroidal anti-inflammatory drugs are contraindicated with the concomitant use of angiotensin-converting enzyme (ACE) inhibitors and/or diuretics. Prostaglandin production is decreased while using NSAIDs, resulting in increased constriction of afferent renal arterioles and decreased glomerular filtration pressure. This physiologic effect of NSAIDs can be exacerbated when used in combination with ACE inhibitors or diuretics, both of which can also reduce glomerular filtration pressures. Combination therapy with either ACE inhibitors or diuretics increases the risk for NSAID-mediated acute kidney injury. Additionally, NSAID use should be avoided in patients taking anticoagulants such as warfarin or heparin due to increased bleeding risk.
Diagnosis
Diagnosis is a key component of proper treatment of acute gout. A gout diagnosis is usually made based on clinical signs and symptoms, including sudden onset of pain that peaks within 24 hours, past history of acute self-limited attacks of arthritis, first MTP involvement, and an elevated sUA. However, not all these factors must be present. In a study conducted by Janssens and colleagues, these factors plus additional demographics had a sensitivity of 90% and specificity of 65% when compared with crystal diagnosis.24 However, a normal sUA level does not exclude gout as a diagnosis due to the uricosuric effect of the inflammatory process.25 In fact, one observational cohort recorded an average sUA decrease from baseline of 2 mg/dL during an acute gout attack.26
Alternative diagnoses, including septic arthritis, should be considered, particularly in the context of treatment failure (< 50% reduction in pain) within the first 24 to 48 hours. A definitive diagnosis is made by identifying negatively birefringent crystals in the synovial fluid of the affected joint (using polarized microscopy) with negative cultures. In the absence of crystal confirmation, there is an emerging role for imaging in gout diagnosis, including the use of ultrasound and dual-energy computed tomography.27
Long-term Treatment Considerations
During treatment for acute gout attacks, urate-lowering therapy that was initiated before the attack should not be discontinued.28 There is no evidence to suggest that current urate lowering has any AEs during attacks. However, removing treatment may increase sUA levels, precipitating attacks in other joints by “destabilizing” crystals still present. Current recommendations also state that urate-lowering therapy may be started during an attack despite traditionally being deferred until the attack has resolved.28 In a randomized trial comparing a group starting allopurinol 300 mg during an attack vs a placebo group (with all patients receiving anti-inflammatory treatment for the acute attack), there was no difference in pain outcomes.29 Regardless of the chosen timing, lowering and maintaining sUA ≤ 6.0 mg/dL is the primary method for minimizing long-term risk of gout attacks.28
Health care providers should discuss with patients the likely need for indefinite urate-lowering therapy while noting that attacks related to therapy initiation are relatively common.30 Current guidelines recommend starting urate-lowering therapy in low doses (≤ 100 mg/d for allopurinol) and titrating to achieve and maintain the target sUA level. Along with the judicious use of anti-inflammatory prophylaxis, this may minimize attacks related to therapy initiation.3,28 By lowering and maintaining sUA below the target level, monosodium urate crystals will dissolve, thereby eliminating the major inciting factor of acute attacks.
Other day-to-day triggers such as alcohol, meat or seafood consumption, and dehydration exist for some patients with gout. Patients should be informed of these inciting factors, as they could potentially be avoided, reducing the risk of future gout attacks. It is important to recognize, however, that dietary or behavioral interventions have generally yielded only modest sUA reductions. For the majority of patients, therefore, reduction and maintenance of sUA ≤ 6.0 mg/dL requires pharmacologic intervention.
Conclusions
Gout attacks should be treated immediately with pharmacologic treatment when contraindications are absent. First-line treatment options include NSAIDs, colchicine, and systemic glucocorticoids. Use of these modalities can be complicated because of comorbidity and concomitant medication use that is prevalent among patients with gout. Comorbidities commonly limiting treatment choice include hypertension (NSAIDs, glucocorticoids), CKD (NSAIDS, colchicine), CVD (NSAIDs, COX-2 inhibitors, glucocorticoids), T2DM (glucocorticoids), and liver disease (NSAIDs, colchicine). Careful consideration must be given to these comorbidities and contraindications as well as patient preferences.
1. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63(10):3136-3141.
2. Zhu Y, Pandya BJ, Choi HK. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007-2008. Am J Med. 2012;125(7):679-687.e1.
3. Khanna D, Khanna PP, Fitzgerald JD, et al; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64(10):1447-1461.
4. Bellamy N, Downie WW, Buchanan WW. Observations on spontaneous improvement in patients with podagra: implications for therapeutic trials of non-steroidal anti-inflammatory drugs. Br J Clin Pharmacol. 1987;24(1):33-36.
5. Terkeltaub RA, Furst DE, Bennett K, Kook KA, Crockett RS, Davis MW. High versus low dosing of oral colchicine for early acute gout flare: twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. Arthritis Rheum. 2010;62(4):1060-1068.
6. Ahern MJ, Reid C, Gordon TP, McCredie M, Brooks PM, Jones M. Does colchicine work? The results of the first controlled study in acute gout. Aust N Z J Med. 1987;17(3):301-304.
7. Puig JG, Michán AD, Jiménez ML, et al. Female gout. Clinical spectrum and uric acid metabolism. Arch Intern Med. 1991;151(4):726-732.
8. van Durme CM, Wechalekar MD, Buchbinder R, Schlesinger N, van der Heijde D, Landewé RB. Non-steroidal anti-inflammatory drugs for acute gout. Cochrane Database Syst Rev. 2014;9:CD010120.
9. Schlesinger N, Moore DF, Sun JD, Schumacher HR Jr. A survey of current evaluation and treatment of gout. J Rheumatol. 2006;33(10):2050-2052.
10. Man CY, Cheung IT, Cameron PA, Rainer TH. Comparison of oral prednisolone/paracetamol and oral indomethacin/paracetamol combination therapy in the treatment of acute goutlike arthritis: a double-blind, randomized, controlled trial. Ann Emerg Med. 2007;49(5):670-677.
11. Janssens HJ, Lucassen PL, Van de Laar FA, Janssen M, Van de Lisdonk EH. Systemic corticosteroids for acute gout. Cochrane Database Syst Rev. 2008;(2):CD005521.
12. Fernández C, Noguera R, González JA, Pascual E. Treatment of acute attacks of gout with a small dose of intraarticular triamcinolone acetonide. J Rheumatol. 1999;26(10):2285-2286.
13. Burns CM, Wortmann RL. Gout therapeutics: new drugs for an old disease. Lancet. 2011;377(9760):165-177.
14. Axelrod D, Preston S. Comparison of parenteral adrenocorticotropic hormone with oral indomethacin in the treatment of acute gout. Arthritis Rheum. 1988;31(6):803-805.
15. Ritter J, Kerr LD, Valeriano-Marcet J, Spiera H. ACTH revisited: effective treatment for acute crystal induced synovitis in patients with multiple medical problems. J Rheumatol. 1994;21(4):696-699.
16. Sivera F, Andrés M, Carmona L, et al. Multinational evidence-based recommendations for the diagnosis and management of gout: integrating systematic literature review and expert opinion of a broad panel of rheumatologists in the 3e initiative. Ann Rheum Dis. 2014;73(2):328-335.
17. Nderitu P, Doos L, Jones PW, Davies SJ, Kadam UT. Non-steroidal anti-inflammatory drugs and chronic kidney disease progression: a systematic review. Fam Pract. 2013;30(3):247-255.
18. Wason S, Faulkner RD, Davis MW. Are dosing adjustments required for colchicine in the elderly compared with younger patients? Adv Ther. 2012;29(6):551-561.
19. Wason S, Mount D, Faulkner R. Single-dose, open-label study of the differences in pharmacokinetics of colchicine in subjects with renal impairment, including end-stage renal disease. Clin Drug Investig. 2014;34(12):845-855.
20. Hanlon JT, Aspinall SL, Semla TP, et al. Consensus guidelines for oral dosing of primarily renally cleared medications in older adults. J Am Geriatr Soc. 2009;57(2):335-340.
21. Crittenden DB, Lehmann RA, Schneck L, et al. Colchicine use is associated with decreased prevalence of myocardial infarction in patients with gout. J Rheumatol. 2012;39(7):1458-1464.
22. Esser N, Paquot N, Scheen AJ. Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Expert Opin Investig Drugs. 2015;24(3):283-307.
23. Terkeltaub RA, Furst DE, Digiacinto JL, Kook KA, Davis MW. Novel evidence-based colchicine dose-reduction algorithm to predict and prevent colchicine toxicity in the presence of cytochrome P450 3A4/P-glycoprotein inhibitors. Arthritis Rheum. 2011;63(8):2226-2237.
24. Janssens HJ, Fransen J, van de Lisdonk EH, van Riel PL, van Weel C, Janssen M. A diagnostic rule for acute gouty arthritis in primary care without joint fluid analysis. Arch Intern Med. 2010;170(13):1120-1126.
25. Urano W, Yamanaka H, Tsutani H, et al. The inflammatory process in the mechanism of decreased serum uric acid concentrations during acute gouty arthritis. J Rheumatol. 2002;29(9):1950-1953.
26. Logan JA, Morrison E, McGill PE. Serum uric acid in acute gout. Ann Rheum Dis. 1997;56(11):696-697.
27. Ogdie A, Taylor WJ, Weatherall M, et al. Imaging modalities for the classification of gout: systematic literature review and meta-analysis. Ann Rheum Dis. 2015; 74(10):1868-1874.
28. Khanna D, Fitzgerald JD, Khanna PP, et al; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012;64(10):1431-1446.
29. Taylor TH, Mecchella JN, Larson RJ, Kerin KD, Mackenzie TA. Initiation of allopurinol at first medical contact for acute attacks of gout: a randomized clinical trial. Am J Med. 2012;125(11):1126-11134.e7.
30. Becker MA, MacDonald PA, Hunt BJ, Lademacher C, Joseph-Ridge N. Determinants of the clinical outcomes of gout during the first year of urate-lowering therapy. Nucleosides Nucleotides Nucleic Acids. 2008;27(6):585-591.
1. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63(10):3136-3141.
2. Zhu Y, Pandya BJ, Choi HK. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007-2008. Am J Med. 2012;125(7):679-687.e1.
3. Khanna D, Khanna PP, Fitzgerald JD, et al; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64(10):1447-1461.
4. Bellamy N, Downie WW, Buchanan WW. Observations on spontaneous improvement in patients with podagra: implications for therapeutic trials of non-steroidal anti-inflammatory drugs. Br J Clin Pharmacol. 1987;24(1):33-36.
5. Terkeltaub RA, Furst DE, Bennett K, Kook KA, Crockett RS, Davis MW. High versus low dosing of oral colchicine for early acute gout flare: twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. Arthritis Rheum. 2010;62(4):1060-1068.
6. Ahern MJ, Reid C, Gordon TP, McCredie M, Brooks PM, Jones M. Does colchicine work? The results of the first controlled study in acute gout. Aust N Z J Med. 1987;17(3):301-304.
7. Puig JG, Michán AD, Jiménez ML, et al. Female gout. Clinical spectrum and uric acid metabolism. Arch Intern Med. 1991;151(4):726-732.
8. van Durme CM, Wechalekar MD, Buchbinder R, Schlesinger N, van der Heijde D, Landewé RB. Non-steroidal anti-inflammatory drugs for acute gout. Cochrane Database Syst Rev. 2014;9:CD010120.
9. Schlesinger N, Moore DF, Sun JD, Schumacher HR Jr. A survey of current evaluation and treatment of gout. J Rheumatol. 2006;33(10):2050-2052.
10. Man CY, Cheung IT, Cameron PA, Rainer TH. Comparison of oral prednisolone/paracetamol and oral indomethacin/paracetamol combination therapy in the treatment of acute goutlike arthritis: a double-blind, randomized, controlled trial. Ann Emerg Med. 2007;49(5):670-677.
11. Janssens HJ, Lucassen PL, Van de Laar FA, Janssen M, Van de Lisdonk EH. Systemic corticosteroids for acute gout. Cochrane Database Syst Rev. 2008;(2):CD005521.
12. Fernández C, Noguera R, González JA, Pascual E. Treatment of acute attacks of gout with a small dose of intraarticular triamcinolone acetonide. J Rheumatol. 1999;26(10):2285-2286.
13. Burns CM, Wortmann RL. Gout therapeutics: new drugs for an old disease. Lancet. 2011;377(9760):165-177.
14. Axelrod D, Preston S. Comparison of parenteral adrenocorticotropic hormone with oral indomethacin in the treatment of acute gout. Arthritis Rheum. 1988;31(6):803-805.
15. Ritter J, Kerr LD, Valeriano-Marcet J, Spiera H. ACTH revisited: effective treatment for acute crystal induced synovitis in patients with multiple medical problems. J Rheumatol. 1994;21(4):696-699.
16. Sivera F, Andrés M, Carmona L, et al. Multinational evidence-based recommendations for the diagnosis and management of gout: integrating systematic literature review and expert opinion of a broad panel of rheumatologists in the 3e initiative. Ann Rheum Dis. 2014;73(2):328-335.
17. Nderitu P, Doos L, Jones PW, Davies SJ, Kadam UT. Non-steroidal anti-inflammatory drugs and chronic kidney disease progression: a systematic review. Fam Pract. 2013;30(3):247-255.
18. Wason S, Faulkner RD, Davis MW. Are dosing adjustments required for colchicine in the elderly compared with younger patients? Adv Ther. 2012;29(6):551-561.
19. Wason S, Mount D, Faulkner R. Single-dose, open-label study of the differences in pharmacokinetics of colchicine in subjects with renal impairment, including end-stage renal disease. Clin Drug Investig. 2014;34(12):845-855.
20. Hanlon JT, Aspinall SL, Semla TP, et al. Consensus guidelines for oral dosing of primarily renally cleared medications in older adults. J Am Geriatr Soc. 2009;57(2):335-340.
21. Crittenden DB, Lehmann RA, Schneck L, et al. Colchicine use is associated with decreased prevalence of myocardial infarction in patients with gout. J Rheumatol. 2012;39(7):1458-1464.
22. Esser N, Paquot N, Scheen AJ. Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Expert Opin Investig Drugs. 2015;24(3):283-307.
23. Terkeltaub RA, Furst DE, Digiacinto JL, Kook KA, Davis MW. Novel evidence-based colchicine dose-reduction algorithm to predict and prevent colchicine toxicity in the presence of cytochrome P450 3A4/P-glycoprotein inhibitors. Arthritis Rheum. 2011;63(8):2226-2237.
24. Janssens HJ, Fransen J, van de Lisdonk EH, van Riel PL, van Weel C, Janssen M. A diagnostic rule for acute gouty arthritis in primary care without joint fluid analysis. Arch Intern Med. 2010;170(13):1120-1126.
25. Urano W, Yamanaka H, Tsutani H, et al. The inflammatory process in the mechanism of decreased serum uric acid concentrations during acute gouty arthritis. J Rheumatol. 2002;29(9):1950-1953.
26. Logan JA, Morrison E, McGill PE. Serum uric acid in acute gout. Ann Rheum Dis. 1997;56(11):696-697.
27. Ogdie A, Taylor WJ, Weatherall M, et al. Imaging modalities for the classification of gout: systematic literature review and meta-analysis. Ann Rheum Dis. 2015; 74(10):1868-1874.
28. Khanna D, Fitzgerald JD, Khanna PP, et al; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012;64(10):1431-1446.
29. Taylor TH, Mecchella JN, Larson RJ, Kerin KD, Mackenzie TA. Initiation of allopurinol at first medical contact for acute attacks of gout: a randomized clinical trial. Am J Med. 2012;125(11):1126-11134.e7.
30. Becker MA, MacDonald PA, Hunt BJ, Lademacher C, Joseph-Ridge N. Determinants of the clinical outcomes of gout during the first year of urate-lowering therapy. Nucleosides Nucleotides Nucleic Acids. 2008;27(6):585-591.
Insights and Implications of the VA Rheumatoid Arthritis Registry
Rheumatoid arthritis (RA) is a systemic autoimmune disease that manifests primarily in the joints, leading to substantial morbidity, reduced survival, and enormous health care costs. As a result, RA exerts a major impact on patients and health care systems. U.S. military veterans and active-duty personnel have traditionally been underrepresented in RA research, likely due in part to the challenges posed by conducting investigations across federal facilities or the common refrain that such populations are not generalizable to the demographic groups (eg, younger women) most prone to develop RA.
Although RA is 3 to 4 times more common in women than in men (the latter comprising about 90% of the U.S. veteran population), its relevance to the VA health system has grown with the increase in women veterans. Well-defined risk factors for RA, such as cigarette smoking, are highly prevalent in these populations, as are comorbid conditions that frequently complicate its disease course, most notably cardiovascular disease.1 Men with RA, a disease demographic common in the VA, seem to experience a more severe disease arthritis course than do women with RA and more commonly have extra-articular manifestations, which are known to contribute to worse outcomes.2 Yet, data from predominantly male RA cohorts are sparse.
To address this gap in RA research, the VA Rheumatoid Arthritis Registry (VARA) was established in 2002 with its first patient enrolled in early 2003. Since its early inception, the registry has served as a research resource not only for VA investigators, but also for their collaborators, the VA health system, and U.S. veteran patients. This report reviews the resources available in VARA, the important insights gained in these efforts, and implications for both patients and health systems providing care. Future directions and opportunities for VARA and other disease registries are provided.
Registry Background
The VARA is a prospective, observational, multicenter study that includes VAMCs in 12 cities (Birmingham, Alabama; Brooklyn, New York; Dallas, Texas; Denver, Colorado; Jackson, Mississippi; Iowa City, Iowa; Little Rock, Arkansas; Omaha, Nebraska; Portland, Oregon; Philadelphia, Pennsylvania; Salt Lake City, Utah; and Washington, DC). In addition to support from VA research, this multicenter effort has been supported by the VA Office of Research Development, the National Institutes of Health, industry, and nonprofit foundations. The VARA serves as a repository linking banked serum, plasma, and DNA samples with an array of patient-level information, including sociodemographics, medical history, medications, comorbid conditions, longitudinal disease activity measures, and other variables (eFigure).
Clinical data are entered by investigators during routine rheumatologic care, facilitated by the use of standardized patient note templates in the VA Computerized Patient Record System, semi-automated data abstraction, and a secure intranet-based platform. With regulatory approvals, including approval of the VARA Scientific and Ethics Advisory Committee (SEAC), registry data are accessed using the VA Informatics and Computer Infrastructure (VINCI), allowing for secure linkage with detailed administrative data, including medication dispensing, diagnostic and procedural codes, and vital status.
The VARA includes > 2,200 veteran patients, all having provided informed consent, aged ≥ 18 years at disease onset, and satisfying the American College of Rheumatology (ACR) classification criteria for RA (Table 1). Serum, plasma, and DNA samples are collected at enrollment and banked in a central biorepository housed at the Nebraska Western-Iowa VA Health Care System in Omaha. In addition to providing ethical and scientific review, the VARA SEAC also provides oversight for biospecimen access. Upon receipt of specimens, the central biobank performs standardized laboratory assays on serum, including C-reactive protein (CRP), rheumatoid factor (RF), and anticyclic citrullinated (anti-CCP) antibody. These data are made available for all future investigations.
Vara Research Insights
The VARA has served as a valuable resource for a wide scope of clinical and clinical-translational research, ranging from studies of disease outcomes and their determinants, genetic and environmental risk factors, the validation of biomarkers, and health care resource utilization, among others (Table 2).
Mortality and Morbidity
The VARA researchers observed a more than 2-fold increase in mortality risk among men with RA compared with age-matched men without RA in the general U.S. population (standardized mortality ratio [SMR] 2.1; 95% confidence interval, 1.8-2.5), a risk that seems to be higher than that observed in other RA cohorts.3 Of the variables associated with mortality in this group, several potentially modifiable factors can be identified, including high erythrocyte sedimentation rate (ESR); elevated Disease Activity Score (DAS)-28 (a composite measure of disease activity including assessments of 28 joints); prednisone use; and low body weight. Patients with a body mass index < 20 kg/m2 (considered underweight) had an SMR > 5.0. Based on more recent VARA evaluations, this association seems to be driven primarily by prior weight loss rather than absolute body weight.4
Related: Methotrexate: Finding the Right Starting Dose
In contrast to oral prednisone use, which is associated with increased mortality risk, the use of methotrexate (MTX), the most commonly prescribed disease-modifying drug in RA, was associated with about a 40% reduction in all-cause mortality.3 This finding was consistent with data from other groups demonstrating that MTX use, alone or in combination with other treatments, is associated with substantial reductions in RA-related mortality, a benefit that seems to result from a robust cardioprotective effect in this population.5 Indeed, prior examinations of a VARA subpopulation revealed high rates of major acute coronary events during observation, a risk that was higher with increased disease activity.1 Studies are now underway in non-RA patients to examine the effectiveness of MTX in secondary cardiovascular disease prevention.
Although not associated with a reduced mortality risk in a previous study, hydroxychloroquine (HCQ) seems to be associated with favorable changes in lipid profiles.3 The VARA participants using HCQ were far more likely to achieve target lipid goals than were participants not using HCQ, including total cholesterol to high-density lipoprotein cholesterol (HDL-C) ratio and HDL-C to low-density lipoprotein cholesterol ratio.6 Importantly, these lipid changes appeared soon after HCQ initiation but were lost within 1 year of discontinuation. These results, coupled with data from separate groups suggesting that HCQ may also improve insulin resistance and even prevent the onset of diabetes, suggest that HCQ could play an important adjuvant treatment role by reducing cardiovascular morbidity in RA.7
Measurement Pitfalls
Proposed best practices in RA management increasingly call for the adoption of a “treat-to-target” approach, with the goal of achieving and maintaining patients in a state of low disease activity or remission.8 Although this strategy receives broad endorsement, its routine implementation is limited in the absence of a single universally accepted method for quantifying disease activity or assessing treatment response in the clinical setting. Indeed, several different measures of RA disease activity have been proposed, including at least 1 that was developed by VARA investigators.9
In a prior study, only poor to modest agreement was found among various proposed measures of treatment response and similar differences among the many proposed definitions of clinical remission.9-11 Moreover, important limitations with the validity and reliability of the patient global health assessment in clinical practice was observed. This reflected, at least in part, the contributions of many non-RA factors to its value.12 This is important, because the patient global health assessment is common to several composite disease activity measures, including remission criteria published by both the ACR and European League Against Rheumatism.13
RA Risk Factors
As part of a large collaborative consortium, VARA has been instrumental in studies examining risk factors for developing RA. These efforts have included reports of novel genetic risk factors in addition to others highlighting the importance of both gene-gene and gene-environment interactions in disease susceptibility.14-16 Among existing literature, these reports inform future efforts to further the understanding of RA pathogenesis in addition to those working to identify methods of risk stratification and disease prevention.
Disease Activity and Severity
The VARA has served as an important resource for studies examining biomarkers and other predictive factors in RA. In addition to serving as important diagnostic tools in the clinic, a recent report highlighted the potential synergistic role of RF and anti-CCP antibody in promoting disease inflammation.17 In this study, patients who were positive for both autoantibodies had much higher disease activity compared with sero-negative patients or individuals with just 1 positive autoantibody. Likewise, patients who were positive for both RF and anti-CCP had higher serum concentrations of CRP and several proinflammatory cytokines than did patients who were sero-negative or who had only 1 positive autoantibody.
In vitro studies done in parallel corroborated these observations, demonstrating for the first time that anticitrullinated protein antibody (ACPA)-containing immune complexes stimulated macrophage production of cytokines, which was further enhanced in the presence of RF. Other biomarkers investigated have included 25-hydroxy vitamin D, soluble forms of CD14 and autoantibodies to deiminated histones, neutrophil extracellular traps, and citrullinated heat shock protein.18-21
Related: The Golden Era of Treatment in Rheumatology
Of high relevance to the VA, VARA has demonstrated robust associations of treatment noncompliance, posttraumatic stress disorder (PTSD), and cigarette smoking with worse RA outcomes.22-24 In a longitudinal study of about 1,500 VARA enrollees, PTSD was independently associated with higher pain levels, tender joint counts, and self-reported disability in addition to worse patient global well-being.23 In contrast, PTSD demonstrated no associations with measures more commonly attributed to ongoing inflammation, including swollen joint counts, ESR, or DAS-28 scores. In addition to demonstrating associations of PTSD with a more severe RA course, these findings suggest that the higher disease burden observed in patients with comorbid PTSD may be attributable to noninflammatory factors that may call for management strategies beyond disease-modifying therapies.
Cigarette smoking is a well-known risk factor for RA, and emerging data, including preliminary results from VARA, suggest that smoking may render a detrimental impact on outcomes.25 Current or former smoking (observed in about 4 of 5 VARA enrollees) is associated with higher ACPA and RF levels, relevant because these autoantibodies are predictive of worse long-term outcomes, including the accrual of joint damage.24-26 Disease activity of VARA participants, measured with multiple clinical measures and an array of proinflammatory cytokines, was higher among current smokers and significantly lower in former smokers, with the former smoking group demonstrating disease activity levels approaching that of never smokers.24 In addition to its benefit in other chronic health conditions, these results suggest that smoking cessation may be a viable approach in ameliorating the systemic inflammatory effects of RA.
Health Care Use
The economic and societal burden posed by RA is enormous and growing. A large proportion of this growth relates to the near exponential increase in direct treatment costs accompanying the emergence of highly effective biologic therapies. Capitalizing on direct links between the VARA and administrative databases maintained in VINCI (eFigure), a recent investigation focused on the use of agents targeting tumor necrosis factor (TNF).27 These efforts have shown that among the 3 most commonly prescribed TNF inhibitors, persistence on initial treatment is similar over time, although important differences exist across agents in the frequency with which patients with RA undergo dose escalation. Recognizing that several reports have demonstrated their cost-effectiveness in RA, annual VA costs for a course of anti-TNF therapy approximated $13,000 to $17,000 per patient treated, and higher costs did not seem to translate into improved patient outcomes.27
Future Directions
Several recent initiatives have been undertaken within the VARA with the goal of expanding the breadth and depth of research that it supports. Ongoing efforts will link VARA with data from the National Death Index, allowing for examinations of cause-specific mortality. Given the high frequency of VA beneficiaries receiving dual care outside the VA system, future links with datasets, such as those from Medicare, will be essential to assure a more optimal capture of relevant health outcomes. Indeed, in recent surveys, almost 1 in 2 VARA participants reported the receipt of dual care, which was most common in those aged > 65 years or receiving prior joint replacement surgery (Pascale Schwab, MD, written communication, April 1, 2015).
Efforts are underway to add other well-annotated specimens to the biorepository, such as synovial fluid and tissues obtained during routine care. The VARA investigators, under regulatory approvals, have begun to collect serum samples longitudinally to complement the prospective disease activity assessments already in place. Other efforts will include the full adoption of standardized patient note templates and transitioning data entry from a decentralized and semi-automated process to one that is centralized and fully automated. This change will reduce the resources required for site investigators and study personnel.
Other Rheumatic Disease Registries
The VA health care system is the largest integrated health system in the U.S. and as such, represents an ideal setting for the investigation of chronic health conditions and patient outcomes. The assets and potential of this system have been at least partially borne out in VARA over the past decade and now extend to other rheumatic disease registries in the VA, including those focused on spondyloarthritis (PULSAR) and gout (Crystal registry). Together, these registries are poised to provide valuable information about these rheumatic conditions and will continue to serve as models for patient registries from other medical disciplines in the VA and elsewhere.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Banerjee S, Compton AP, Hooker RS, et al. Cardiovascular outcomes in male veterans with rheumatoid arthritis. Am J Cardiol. 2008;101(8):1201-1205.
2. Weyand CM, Schmidt D, Wagner U, Goronzy JJ. The influence of sex on the phenotype of rheumatoid arthritis. Arthritis Rheum. 1998;41(5):817-822.
3. Mikuls TR, Fay BT, Michaud K, et al. Associations of disease activity and treatments with mortality in men with rheumatoid arthritis: results from the VARA registry. Rheumatol (Oxford). 2011;50(1):101-109.
4. Baker JF, Billig E, Cannon GW, Caplan L, Majithia V, Mikuls TR. Weight loss and risk of death in rheumatoid arthritis [abstract 1391]. Arthritis Rheumatol. 2014;66(suppl 10):S613-S614.
5. Choi HK, Hernán MA, Seeger JD, Robins JM, Wolfe F. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. 2002;359(9313):1173-1177.
6. Kerr G, Aujero M, Richards J, et al. Associations of hydroxychloroquine use with lipid profiles in rheumatoid arthritis: pharmacologic implications. Arthritis Care Res (Hoboken). 2014;66(11):1619-1626.
7. Wasko MC, Hubert HB, Lingala VB, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. 2007;298(2):187-193.
8. Saag KG, Teng GG, Patkar NM, et al; American College of Rheumatology. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59(6):762-784.
9. Michaud K, Mikuls TR, Call SE, et al. Poor to modest agreement between rheumatoid arthritis response measures in clinical practice. Clin Exp Rheumatol. 2009;27(4):633-640.
10. Shahouri SH, Michaud K, Mikuls TR, et al. Remission of rheumatoid arthritis in clinical practice: application of the American College of Rheumatology/European League Against Rheumatism 2011 remission criteria. Arthritis Rheum. 2011;63(11):3204-3215.
11. Shaver TS, Anderson JD, Weidensaul DN, et al. The problem of rheumatoid arthritis disease activity and remission in clinical practice. J Rheumatol. 2008;35(6):1015-1022.
12. Masri KR, Shaver TS, Shahouri SH, et al. Validity and reliability problems with patient global as a component of the ACR/EULAR remission criteria as used in clinical practice. J Rheumatol. 2012;39(6):1139-1145.
13. Aletaha D, Landewe R, Karonitsch T, et al. Reporting disease activity in clinical trials of patients with rheumatoid arthritis: EULAR/ACR collaborative recommendations. Ann Rheum Dis. 2008;67(10):1360-1364.
14. Gregersen PK, Amos CI, Lee AT, et al. REL, encoding a member of the NF-kappaB family of transcription factors, is a newly defined risk locus for rheumatoid arthritis. Nat Genet. 2009;41(7):820-823.
15. Briggs FB, Ramsay PP, Madden E, et al. Supervised machine learning and logistic regression identifies novel epistatic risk factors with PTPN22 for rheumatoid arthritis. Genes Immun. 2010;11(3):199-208.
16. Mikuls TR, Gould KA, Bynoté KK, et al. Anticitrullinated protein antibody (ACPA) in rheumatoid arthritis: influence of an interaction between HLA-DRB1 shared epitope and a deletion polymorphism in glutathione S-transferase in a cross-sectional study. Arthritis Res Ther. 2010;12(6):R213.
17. Sokolove J, Johnson DS, Lahey LJ, et al. Rheumatoid factor as a potentiator of anti-citrullinated protein antibody-mediated inflammation in rheumatoid arthritis. Arthritis Rheumatol. 2014;66(4):813-821.
18. Kerr GS, Sabahi I, Richards JS, et al. Prevalence of vitamin D insufficiency/deficiency in rheumatoid arthritis and associations with disease severity and activity. J Rheumatol. 2011;38(1):53-59.
19. Mikuls TR, LeVan TD, Sayles H, et al. Soluble CD14 and CD14 polymorphisms in rheumatoid arthritis. J Rheumatol. 2011;38(12):2509-2516.
20. Dwivedi N, Upadhyay J, Neeli I, et al. Felty’s syndrome autoantibodies bind to deiminated histones and neutrophil extracellular chromatin traps. Arthritis Rheum. 2012;64(4):982-992.
21. Harlow L, Rosas IO, Gochuico BR, et al. Identification of citrullinated hsp90 isoforms as novel autoantigens in rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheum. 2013;65(4):869-879.
22. Cannon GW, Mikuls TR, Hayden CL, et al. Merging Veterans Affairs rheumatoid arthritis registry and pharmacy data to assess methotrexate adherence and disease activity in clinical practice. Arthritis Care Res (Hoboken). 2011;63(12):1680-1690.
23. Mikuls TR, Padala PR, Sayles HR, et al. Prospective study of posttraumatic stress disorder and disease activity outcomes in US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2013;65(2):227-234.
24. Sokolove J, Sayles H, Wagner CA, et al. Smoking status is associated with inflammatory cytokine profile and disease activity: decreased inflammation and disease improvement with smoking cessation? [abstract 348]. Arthritis Rheumatol. 2014;66(suppl 10):S146.
25. Criswell LA, Merlino LA, Cerhan JR, et al. Cigarette smoking and the risk of rheumatoid arthritis among postmenopausal women: results from the Iowa Women’s Health Study. Am J Med. 2002;112(6):465-471.
26. Hecht C, Englbrecht M, Rech J, et al. Additive effect of anti-citrullinated protein antibodies and rheumatoid factor on bone erosions in patients with RA [published online ahead of print August 12, 2014]. Ann Rheum Dis. doi: 10.1136/annrheumdis -2014-205428.
27. Cannon GW, DuVall SL, Haroldsen CL, et al. Persistence and dose escalation of tumor necrosis factor inhibitors in US veterans with rheumatoid arthritis. J Rheumatol. 2014;41(10):1935-1943.
28. Curtis JR, Baddley JW, Yang S, et al. Derivation and preliminary validation of an administrative claims-based algorithm for the effectiveness of medications for rheumatoid arthritis. Arthritis Res Ther. 2011;13(5):R155.
29. Caplan L, Davis LA, Bright CM, et al. Body mass index and the rheumatoid arthritis swollen joint count: an observational study. Arthritis Care Res (Hoboken). 2013;65(1):101-106.
30. Davis LA, Whitfield E, Cannon GW, et al. Association of rheumatoid arthritis susceptibility gene with lipid profiles in patients with rheumatoid arthritis. Rheumatology (Oxford). 2014;53(6):1014-1021.
31. Mikuls TR, Kazi S, Cipher D, et al. The association of race and ethnicity with disease expression in male US veterans with rheumatoid arthritis. J Rheumatol. 2007;34(7):1480-1484.
32. Miriovsky BJ, Michaud K, Thiele GM, et al. Anti-CCP antibody and rheumatoid factor concentrations predict greater disease activity in men with rheumatoid arthritis. Ann Rheum Dis. 2010;69(7):1292-1297.
33. Oei HB, Hooker RS, Cipher DJ, Reimold A. High rates of stopping or switching biological medications in veterans with rheumatoid arthritis. Clin Exp Rheumatol. 2009;27(6):926-934.
34. Richards JS, Peng J, Amdur RL, et al. Dual-energy X-ray absorptiometry and evaluation of the osteoporosis self-assessment tool in men with rheumatoid arthritis. J Clin Densitom. 2009;12(4):434-440.
35. Richards JS, Cannon GW, Hayden CL, et al. Adherence with bisphosphonate therapy in US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64(12):1864-1870.
Rheumatoid arthritis (RA) is a systemic autoimmune disease that manifests primarily in the joints, leading to substantial morbidity, reduced survival, and enormous health care costs. As a result, RA exerts a major impact on patients and health care systems. U.S. military veterans and active-duty personnel have traditionally been underrepresented in RA research, likely due in part to the challenges posed by conducting investigations across federal facilities or the common refrain that such populations are not generalizable to the demographic groups (eg, younger women) most prone to develop RA.
Although RA is 3 to 4 times more common in women than in men (the latter comprising about 90% of the U.S. veteran population), its relevance to the VA health system has grown with the increase in women veterans. Well-defined risk factors for RA, such as cigarette smoking, are highly prevalent in these populations, as are comorbid conditions that frequently complicate its disease course, most notably cardiovascular disease.1 Men with RA, a disease demographic common in the VA, seem to experience a more severe disease arthritis course than do women with RA and more commonly have extra-articular manifestations, which are known to contribute to worse outcomes.2 Yet, data from predominantly male RA cohorts are sparse.
To address this gap in RA research, the VA Rheumatoid Arthritis Registry (VARA) was established in 2002 with its first patient enrolled in early 2003. Since its early inception, the registry has served as a research resource not only for VA investigators, but also for their collaborators, the VA health system, and U.S. veteran patients. This report reviews the resources available in VARA, the important insights gained in these efforts, and implications for both patients and health systems providing care. Future directions and opportunities for VARA and other disease registries are provided.
Registry Background
The VARA is a prospective, observational, multicenter study that includes VAMCs in 12 cities (Birmingham, Alabama; Brooklyn, New York; Dallas, Texas; Denver, Colorado; Jackson, Mississippi; Iowa City, Iowa; Little Rock, Arkansas; Omaha, Nebraska; Portland, Oregon; Philadelphia, Pennsylvania; Salt Lake City, Utah; and Washington, DC). In addition to support from VA research, this multicenter effort has been supported by the VA Office of Research Development, the National Institutes of Health, industry, and nonprofit foundations. The VARA serves as a repository linking banked serum, plasma, and DNA samples with an array of patient-level information, including sociodemographics, medical history, medications, comorbid conditions, longitudinal disease activity measures, and other variables (eFigure).
Clinical data are entered by investigators during routine rheumatologic care, facilitated by the use of standardized patient note templates in the VA Computerized Patient Record System, semi-automated data abstraction, and a secure intranet-based platform. With regulatory approvals, including approval of the VARA Scientific and Ethics Advisory Committee (SEAC), registry data are accessed using the VA Informatics and Computer Infrastructure (VINCI), allowing for secure linkage with detailed administrative data, including medication dispensing, diagnostic and procedural codes, and vital status.
The VARA includes > 2,200 veteran patients, all having provided informed consent, aged ≥ 18 years at disease onset, and satisfying the American College of Rheumatology (ACR) classification criteria for RA (Table 1). Serum, plasma, and DNA samples are collected at enrollment and banked in a central biorepository housed at the Nebraska Western-Iowa VA Health Care System in Omaha. In addition to providing ethical and scientific review, the VARA SEAC also provides oversight for biospecimen access. Upon receipt of specimens, the central biobank performs standardized laboratory assays on serum, including C-reactive protein (CRP), rheumatoid factor (RF), and anticyclic citrullinated (anti-CCP) antibody. These data are made available for all future investigations.
Vara Research Insights
The VARA has served as a valuable resource for a wide scope of clinical and clinical-translational research, ranging from studies of disease outcomes and their determinants, genetic and environmental risk factors, the validation of biomarkers, and health care resource utilization, among others (Table 2).
Mortality and Morbidity
The VARA researchers observed a more than 2-fold increase in mortality risk among men with RA compared with age-matched men without RA in the general U.S. population (standardized mortality ratio [SMR] 2.1; 95% confidence interval, 1.8-2.5), a risk that seems to be higher than that observed in other RA cohorts.3 Of the variables associated with mortality in this group, several potentially modifiable factors can be identified, including high erythrocyte sedimentation rate (ESR); elevated Disease Activity Score (DAS)-28 (a composite measure of disease activity including assessments of 28 joints); prednisone use; and low body weight. Patients with a body mass index < 20 kg/m2 (considered underweight) had an SMR > 5.0. Based on more recent VARA evaluations, this association seems to be driven primarily by prior weight loss rather than absolute body weight.4
Related: Methotrexate: Finding the Right Starting Dose
In contrast to oral prednisone use, which is associated with increased mortality risk, the use of methotrexate (MTX), the most commonly prescribed disease-modifying drug in RA, was associated with about a 40% reduction in all-cause mortality.3 This finding was consistent with data from other groups demonstrating that MTX use, alone or in combination with other treatments, is associated with substantial reductions in RA-related mortality, a benefit that seems to result from a robust cardioprotective effect in this population.5 Indeed, prior examinations of a VARA subpopulation revealed high rates of major acute coronary events during observation, a risk that was higher with increased disease activity.1 Studies are now underway in non-RA patients to examine the effectiveness of MTX in secondary cardiovascular disease prevention.
Although not associated with a reduced mortality risk in a previous study, hydroxychloroquine (HCQ) seems to be associated with favorable changes in lipid profiles.3 The VARA participants using HCQ were far more likely to achieve target lipid goals than were participants not using HCQ, including total cholesterol to high-density lipoprotein cholesterol (HDL-C) ratio and HDL-C to low-density lipoprotein cholesterol ratio.6 Importantly, these lipid changes appeared soon after HCQ initiation but were lost within 1 year of discontinuation. These results, coupled with data from separate groups suggesting that HCQ may also improve insulin resistance and even prevent the onset of diabetes, suggest that HCQ could play an important adjuvant treatment role by reducing cardiovascular morbidity in RA.7
Measurement Pitfalls
Proposed best practices in RA management increasingly call for the adoption of a “treat-to-target” approach, with the goal of achieving and maintaining patients in a state of low disease activity or remission.8 Although this strategy receives broad endorsement, its routine implementation is limited in the absence of a single universally accepted method for quantifying disease activity or assessing treatment response in the clinical setting. Indeed, several different measures of RA disease activity have been proposed, including at least 1 that was developed by VARA investigators.9
In a prior study, only poor to modest agreement was found among various proposed measures of treatment response and similar differences among the many proposed definitions of clinical remission.9-11 Moreover, important limitations with the validity and reliability of the patient global health assessment in clinical practice was observed. This reflected, at least in part, the contributions of many non-RA factors to its value.12 This is important, because the patient global health assessment is common to several composite disease activity measures, including remission criteria published by both the ACR and European League Against Rheumatism.13
RA Risk Factors
As part of a large collaborative consortium, VARA has been instrumental in studies examining risk factors for developing RA. These efforts have included reports of novel genetic risk factors in addition to others highlighting the importance of both gene-gene and gene-environment interactions in disease susceptibility.14-16 Among existing literature, these reports inform future efforts to further the understanding of RA pathogenesis in addition to those working to identify methods of risk stratification and disease prevention.
Disease Activity and Severity
The VARA has served as an important resource for studies examining biomarkers and other predictive factors in RA. In addition to serving as important diagnostic tools in the clinic, a recent report highlighted the potential synergistic role of RF and anti-CCP antibody in promoting disease inflammation.17 In this study, patients who were positive for both autoantibodies had much higher disease activity compared with sero-negative patients or individuals with just 1 positive autoantibody. Likewise, patients who were positive for both RF and anti-CCP had higher serum concentrations of CRP and several proinflammatory cytokines than did patients who were sero-negative or who had only 1 positive autoantibody.
In vitro studies done in parallel corroborated these observations, demonstrating for the first time that anticitrullinated protein antibody (ACPA)-containing immune complexes stimulated macrophage production of cytokines, which was further enhanced in the presence of RF. Other biomarkers investigated have included 25-hydroxy vitamin D, soluble forms of CD14 and autoantibodies to deiminated histones, neutrophil extracellular traps, and citrullinated heat shock protein.18-21
Related: The Golden Era of Treatment in Rheumatology
Of high relevance to the VA, VARA has demonstrated robust associations of treatment noncompliance, posttraumatic stress disorder (PTSD), and cigarette smoking with worse RA outcomes.22-24 In a longitudinal study of about 1,500 VARA enrollees, PTSD was independently associated with higher pain levels, tender joint counts, and self-reported disability in addition to worse patient global well-being.23 In contrast, PTSD demonstrated no associations with measures more commonly attributed to ongoing inflammation, including swollen joint counts, ESR, or DAS-28 scores. In addition to demonstrating associations of PTSD with a more severe RA course, these findings suggest that the higher disease burden observed in patients with comorbid PTSD may be attributable to noninflammatory factors that may call for management strategies beyond disease-modifying therapies.
Cigarette smoking is a well-known risk factor for RA, and emerging data, including preliminary results from VARA, suggest that smoking may render a detrimental impact on outcomes.25 Current or former smoking (observed in about 4 of 5 VARA enrollees) is associated with higher ACPA and RF levels, relevant because these autoantibodies are predictive of worse long-term outcomes, including the accrual of joint damage.24-26 Disease activity of VARA participants, measured with multiple clinical measures and an array of proinflammatory cytokines, was higher among current smokers and significantly lower in former smokers, with the former smoking group demonstrating disease activity levels approaching that of never smokers.24 In addition to its benefit in other chronic health conditions, these results suggest that smoking cessation may be a viable approach in ameliorating the systemic inflammatory effects of RA.
Health Care Use
The economic and societal burden posed by RA is enormous and growing. A large proportion of this growth relates to the near exponential increase in direct treatment costs accompanying the emergence of highly effective biologic therapies. Capitalizing on direct links between the VARA and administrative databases maintained in VINCI (eFigure), a recent investigation focused on the use of agents targeting tumor necrosis factor (TNF).27 These efforts have shown that among the 3 most commonly prescribed TNF inhibitors, persistence on initial treatment is similar over time, although important differences exist across agents in the frequency with which patients with RA undergo dose escalation. Recognizing that several reports have demonstrated their cost-effectiveness in RA, annual VA costs for a course of anti-TNF therapy approximated $13,000 to $17,000 per patient treated, and higher costs did not seem to translate into improved patient outcomes.27
Future Directions
Several recent initiatives have been undertaken within the VARA with the goal of expanding the breadth and depth of research that it supports. Ongoing efforts will link VARA with data from the National Death Index, allowing for examinations of cause-specific mortality. Given the high frequency of VA beneficiaries receiving dual care outside the VA system, future links with datasets, such as those from Medicare, will be essential to assure a more optimal capture of relevant health outcomes. Indeed, in recent surveys, almost 1 in 2 VARA participants reported the receipt of dual care, which was most common in those aged > 65 years or receiving prior joint replacement surgery (Pascale Schwab, MD, written communication, April 1, 2015).
Efforts are underway to add other well-annotated specimens to the biorepository, such as synovial fluid and tissues obtained during routine care. The VARA investigators, under regulatory approvals, have begun to collect serum samples longitudinally to complement the prospective disease activity assessments already in place. Other efforts will include the full adoption of standardized patient note templates and transitioning data entry from a decentralized and semi-automated process to one that is centralized and fully automated. This change will reduce the resources required for site investigators and study personnel.
Other Rheumatic Disease Registries
The VA health care system is the largest integrated health system in the U.S. and as such, represents an ideal setting for the investigation of chronic health conditions and patient outcomes. The assets and potential of this system have been at least partially borne out in VARA over the past decade and now extend to other rheumatic disease registries in the VA, including those focused on spondyloarthritis (PULSAR) and gout (Crystal registry). Together, these registries are poised to provide valuable information about these rheumatic conditions and will continue to serve as models for patient registries from other medical disciplines in the VA and elsewhere.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Rheumatoid arthritis (RA) is a systemic autoimmune disease that manifests primarily in the joints, leading to substantial morbidity, reduced survival, and enormous health care costs. As a result, RA exerts a major impact on patients and health care systems. U.S. military veterans and active-duty personnel have traditionally been underrepresented in RA research, likely due in part to the challenges posed by conducting investigations across federal facilities or the common refrain that such populations are not generalizable to the demographic groups (eg, younger women) most prone to develop RA.
Although RA is 3 to 4 times more common in women than in men (the latter comprising about 90% of the U.S. veteran population), its relevance to the VA health system has grown with the increase in women veterans. Well-defined risk factors for RA, such as cigarette smoking, are highly prevalent in these populations, as are comorbid conditions that frequently complicate its disease course, most notably cardiovascular disease.1 Men with RA, a disease demographic common in the VA, seem to experience a more severe disease arthritis course than do women with RA and more commonly have extra-articular manifestations, which are known to contribute to worse outcomes.2 Yet, data from predominantly male RA cohorts are sparse.
To address this gap in RA research, the VA Rheumatoid Arthritis Registry (VARA) was established in 2002 with its first patient enrolled in early 2003. Since its early inception, the registry has served as a research resource not only for VA investigators, but also for their collaborators, the VA health system, and U.S. veteran patients. This report reviews the resources available in VARA, the important insights gained in these efforts, and implications for both patients and health systems providing care. Future directions and opportunities for VARA and other disease registries are provided.
Registry Background
The VARA is a prospective, observational, multicenter study that includes VAMCs in 12 cities (Birmingham, Alabama; Brooklyn, New York; Dallas, Texas; Denver, Colorado; Jackson, Mississippi; Iowa City, Iowa; Little Rock, Arkansas; Omaha, Nebraska; Portland, Oregon; Philadelphia, Pennsylvania; Salt Lake City, Utah; and Washington, DC). In addition to support from VA research, this multicenter effort has been supported by the VA Office of Research Development, the National Institutes of Health, industry, and nonprofit foundations. The VARA serves as a repository linking banked serum, plasma, and DNA samples with an array of patient-level information, including sociodemographics, medical history, medications, comorbid conditions, longitudinal disease activity measures, and other variables (eFigure).
Clinical data are entered by investigators during routine rheumatologic care, facilitated by the use of standardized patient note templates in the VA Computerized Patient Record System, semi-automated data abstraction, and a secure intranet-based platform. With regulatory approvals, including approval of the VARA Scientific and Ethics Advisory Committee (SEAC), registry data are accessed using the VA Informatics and Computer Infrastructure (VINCI), allowing for secure linkage with detailed administrative data, including medication dispensing, diagnostic and procedural codes, and vital status.
The VARA includes > 2,200 veteran patients, all having provided informed consent, aged ≥ 18 years at disease onset, and satisfying the American College of Rheumatology (ACR) classification criteria for RA (Table 1). Serum, plasma, and DNA samples are collected at enrollment and banked in a central biorepository housed at the Nebraska Western-Iowa VA Health Care System in Omaha. In addition to providing ethical and scientific review, the VARA SEAC also provides oversight for biospecimen access. Upon receipt of specimens, the central biobank performs standardized laboratory assays on serum, including C-reactive protein (CRP), rheumatoid factor (RF), and anticyclic citrullinated (anti-CCP) antibody. These data are made available for all future investigations.
Vara Research Insights
The VARA has served as a valuable resource for a wide scope of clinical and clinical-translational research, ranging from studies of disease outcomes and their determinants, genetic and environmental risk factors, the validation of biomarkers, and health care resource utilization, among others (Table 2).
Mortality and Morbidity
The VARA researchers observed a more than 2-fold increase in mortality risk among men with RA compared with age-matched men without RA in the general U.S. population (standardized mortality ratio [SMR] 2.1; 95% confidence interval, 1.8-2.5), a risk that seems to be higher than that observed in other RA cohorts.3 Of the variables associated with mortality in this group, several potentially modifiable factors can be identified, including high erythrocyte sedimentation rate (ESR); elevated Disease Activity Score (DAS)-28 (a composite measure of disease activity including assessments of 28 joints); prednisone use; and low body weight. Patients with a body mass index < 20 kg/m2 (considered underweight) had an SMR > 5.0. Based on more recent VARA evaluations, this association seems to be driven primarily by prior weight loss rather than absolute body weight.4
Related: Methotrexate: Finding the Right Starting Dose
In contrast to oral prednisone use, which is associated with increased mortality risk, the use of methotrexate (MTX), the most commonly prescribed disease-modifying drug in RA, was associated with about a 40% reduction in all-cause mortality.3 This finding was consistent with data from other groups demonstrating that MTX use, alone or in combination with other treatments, is associated with substantial reductions in RA-related mortality, a benefit that seems to result from a robust cardioprotective effect in this population.5 Indeed, prior examinations of a VARA subpopulation revealed high rates of major acute coronary events during observation, a risk that was higher with increased disease activity.1 Studies are now underway in non-RA patients to examine the effectiveness of MTX in secondary cardiovascular disease prevention.
Although not associated with a reduced mortality risk in a previous study, hydroxychloroquine (HCQ) seems to be associated with favorable changes in lipid profiles.3 The VARA participants using HCQ were far more likely to achieve target lipid goals than were participants not using HCQ, including total cholesterol to high-density lipoprotein cholesterol (HDL-C) ratio and HDL-C to low-density lipoprotein cholesterol ratio.6 Importantly, these lipid changes appeared soon after HCQ initiation but were lost within 1 year of discontinuation. These results, coupled with data from separate groups suggesting that HCQ may also improve insulin resistance and even prevent the onset of diabetes, suggest that HCQ could play an important adjuvant treatment role by reducing cardiovascular morbidity in RA.7
Measurement Pitfalls
Proposed best practices in RA management increasingly call for the adoption of a “treat-to-target” approach, with the goal of achieving and maintaining patients in a state of low disease activity or remission.8 Although this strategy receives broad endorsement, its routine implementation is limited in the absence of a single universally accepted method for quantifying disease activity or assessing treatment response in the clinical setting. Indeed, several different measures of RA disease activity have been proposed, including at least 1 that was developed by VARA investigators.9
In a prior study, only poor to modest agreement was found among various proposed measures of treatment response and similar differences among the many proposed definitions of clinical remission.9-11 Moreover, important limitations with the validity and reliability of the patient global health assessment in clinical practice was observed. This reflected, at least in part, the contributions of many non-RA factors to its value.12 This is important, because the patient global health assessment is common to several composite disease activity measures, including remission criteria published by both the ACR and European League Against Rheumatism.13
RA Risk Factors
As part of a large collaborative consortium, VARA has been instrumental in studies examining risk factors for developing RA. These efforts have included reports of novel genetic risk factors in addition to others highlighting the importance of both gene-gene and gene-environment interactions in disease susceptibility.14-16 Among existing literature, these reports inform future efforts to further the understanding of RA pathogenesis in addition to those working to identify methods of risk stratification and disease prevention.
Disease Activity and Severity
The VARA has served as an important resource for studies examining biomarkers and other predictive factors in RA. In addition to serving as important diagnostic tools in the clinic, a recent report highlighted the potential synergistic role of RF and anti-CCP antibody in promoting disease inflammation.17 In this study, patients who were positive for both autoantibodies had much higher disease activity compared with sero-negative patients or individuals with just 1 positive autoantibody. Likewise, patients who were positive for both RF and anti-CCP had higher serum concentrations of CRP and several proinflammatory cytokines than did patients who were sero-negative or who had only 1 positive autoantibody.
In vitro studies done in parallel corroborated these observations, demonstrating for the first time that anticitrullinated protein antibody (ACPA)-containing immune complexes stimulated macrophage production of cytokines, which was further enhanced in the presence of RF. Other biomarkers investigated have included 25-hydroxy vitamin D, soluble forms of CD14 and autoantibodies to deiminated histones, neutrophil extracellular traps, and citrullinated heat shock protein.18-21
Related: The Golden Era of Treatment in Rheumatology
Of high relevance to the VA, VARA has demonstrated robust associations of treatment noncompliance, posttraumatic stress disorder (PTSD), and cigarette smoking with worse RA outcomes.22-24 In a longitudinal study of about 1,500 VARA enrollees, PTSD was independently associated with higher pain levels, tender joint counts, and self-reported disability in addition to worse patient global well-being.23 In contrast, PTSD demonstrated no associations with measures more commonly attributed to ongoing inflammation, including swollen joint counts, ESR, or DAS-28 scores. In addition to demonstrating associations of PTSD with a more severe RA course, these findings suggest that the higher disease burden observed in patients with comorbid PTSD may be attributable to noninflammatory factors that may call for management strategies beyond disease-modifying therapies.
Cigarette smoking is a well-known risk factor for RA, and emerging data, including preliminary results from VARA, suggest that smoking may render a detrimental impact on outcomes.25 Current or former smoking (observed in about 4 of 5 VARA enrollees) is associated with higher ACPA and RF levels, relevant because these autoantibodies are predictive of worse long-term outcomes, including the accrual of joint damage.24-26 Disease activity of VARA participants, measured with multiple clinical measures and an array of proinflammatory cytokines, was higher among current smokers and significantly lower in former smokers, with the former smoking group demonstrating disease activity levels approaching that of never smokers.24 In addition to its benefit in other chronic health conditions, these results suggest that smoking cessation may be a viable approach in ameliorating the systemic inflammatory effects of RA.
Health Care Use
The economic and societal burden posed by RA is enormous and growing. A large proportion of this growth relates to the near exponential increase in direct treatment costs accompanying the emergence of highly effective biologic therapies. Capitalizing on direct links between the VARA and administrative databases maintained in VINCI (eFigure), a recent investigation focused on the use of agents targeting tumor necrosis factor (TNF).27 These efforts have shown that among the 3 most commonly prescribed TNF inhibitors, persistence on initial treatment is similar over time, although important differences exist across agents in the frequency with which patients with RA undergo dose escalation. Recognizing that several reports have demonstrated their cost-effectiveness in RA, annual VA costs for a course of anti-TNF therapy approximated $13,000 to $17,000 per patient treated, and higher costs did not seem to translate into improved patient outcomes.27
Future Directions
Several recent initiatives have been undertaken within the VARA with the goal of expanding the breadth and depth of research that it supports. Ongoing efforts will link VARA with data from the National Death Index, allowing for examinations of cause-specific mortality. Given the high frequency of VA beneficiaries receiving dual care outside the VA system, future links with datasets, such as those from Medicare, will be essential to assure a more optimal capture of relevant health outcomes. Indeed, in recent surveys, almost 1 in 2 VARA participants reported the receipt of dual care, which was most common in those aged > 65 years or receiving prior joint replacement surgery (Pascale Schwab, MD, written communication, April 1, 2015).
Efforts are underway to add other well-annotated specimens to the biorepository, such as synovial fluid and tissues obtained during routine care. The VARA investigators, under regulatory approvals, have begun to collect serum samples longitudinally to complement the prospective disease activity assessments already in place. Other efforts will include the full adoption of standardized patient note templates and transitioning data entry from a decentralized and semi-automated process to one that is centralized and fully automated. This change will reduce the resources required for site investigators and study personnel.
Other Rheumatic Disease Registries
The VA health care system is the largest integrated health system in the U.S. and as such, represents an ideal setting for the investigation of chronic health conditions and patient outcomes. The assets and potential of this system have been at least partially borne out in VARA over the past decade and now extend to other rheumatic disease registries in the VA, including those focused on spondyloarthritis (PULSAR) and gout (Crystal registry). Together, these registries are poised to provide valuable information about these rheumatic conditions and will continue to serve as models for patient registries from other medical disciplines in the VA and elsewhere.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Banerjee S, Compton AP, Hooker RS, et al. Cardiovascular outcomes in male veterans with rheumatoid arthritis. Am J Cardiol. 2008;101(8):1201-1205.
2. Weyand CM, Schmidt D, Wagner U, Goronzy JJ. The influence of sex on the phenotype of rheumatoid arthritis. Arthritis Rheum. 1998;41(5):817-822.
3. Mikuls TR, Fay BT, Michaud K, et al. Associations of disease activity and treatments with mortality in men with rheumatoid arthritis: results from the VARA registry. Rheumatol (Oxford). 2011;50(1):101-109.
4. Baker JF, Billig E, Cannon GW, Caplan L, Majithia V, Mikuls TR. Weight loss and risk of death in rheumatoid arthritis [abstract 1391]. Arthritis Rheumatol. 2014;66(suppl 10):S613-S614.
5. Choi HK, Hernán MA, Seeger JD, Robins JM, Wolfe F. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. 2002;359(9313):1173-1177.
6. Kerr G, Aujero M, Richards J, et al. Associations of hydroxychloroquine use with lipid profiles in rheumatoid arthritis: pharmacologic implications. Arthritis Care Res (Hoboken). 2014;66(11):1619-1626.
7. Wasko MC, Hubert HB, Lingala VB, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. 2007;298(2):187-193.
8. Saag KG, Teng GG, Patkar NM, et al; American College of Rheumatology. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59(6):762-784.
9. Michaud K, Mikuls TR, Call SE, et al. Poor to modest agreement between rheumatoid arthritis response measures in clinical practice. Clin Exp Rheumatol. 2009;27(4):633-640.
10. Shahouri SH, Michaud K, Mikuls TR, et al. Remission of rheumatoid arthritis in clinical practice: application of the American College of Rheumatology/European League Against Rheumatism 2011 remission criteria. Arthritis Rheum. 2011;63(11):3204-3215.
11. Shaver TS, Anderson JD, Weidensaul DN, et al. The problem of rheumatoid arthritis disease activity and remission in clinical practice. J Rheumatol. 2008;35(6):1015-1022.
12. Masri KR, Shaver TS, Shahouri SH, et al. Validity and reliability problems with patient global as a component of the ACR/EULAR remission criteria as used in clinical practice. J Rheumatol. 2012;39(6):1139-1145.
13. Aletaha D, Landewe R, Karonitsch T, et al. Reporting disease activity in clinical trials of patients with rheumatoid arthritis: EULAR/ACR collaborative recommendations. Ann Rheum Dis. 2008;67(10):1360-1364.
14. Gregersen PK, Amos CI, Lee AT, et al. REL, encoding a member of the NF-kappaB family of transcription factors, is a newly defined risk locus for rheumatoid arthritis. Nat Genet. 2009;41(7):820-823.
15. Briggs FB, Ramsay PP, Madden E, et al. Supervised machine learning and logistic regression identifies novel epistatic risk factors with PTPN22 for rheumatoid arthritis. Genes Immun. 2010;11(3):199-208.
16. Mikuls TR, Gould KA, Bynoté KK, et al. Anticitrullinated protein antibody (ACPA) in rheumatoid arthritis: influence of an interaction between HLA-DRB1 shared epitope and a deletion polymorphism in glutathione S-transferase in a cross-sectional study. Arthritis Res Ther. 2010;12(6):R213.
17. Sokolove J, Johnson DS, Lahey LJ, et al. Rheumatoid factor as a potentiator of anti-citrullinated protein antibody-mediated inflammation in rheumatoid arthritis. Arthritis Rheumatol. 2014;66(4):813-821.
18. Kerr GS, Sabahi I, Richards JS, et al. Prevalence of vitamin D insufficiency/deficiency in rheumatoid arthritis and associations with disease severity and activity. J Rheumatol. 2011;38(1):53-59.
19. Mikuls TR, LeVan TD, Sayles H, et al. Soluble CD14 and CD14 polymorphisms in rheumatoid arthritis. J Rheumatol. 2011;38(12):2509-2516.
20. Dwivedi N, Upadhyay J, Neeli I, et al. Felty’s syndrome autoantibodies bind to deiminated histones and neutrophil extracellular chromatin traps. Arthritis Rheum. 2012;64(4):982-992.
21. Harlow L, Rosas IO, Gochuico BR, et al. Identification of citrullinated hsp90 isoforms as novel autoantigens in rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheum. 2013;65(4):869-879.
22. Cannon GW, Mikuls TR, Hayden CL, et al. Merging Veterans Affairs rheumatoid arthritis registry and pharmacy data to assess methotrexate adherence and disease activity in clinical practice. Arthritis Care Res (Hoboken). 2011;63(12):1680-1690.
23. Mikuls TR, Padala PR, Sayles HR, et al. Prospective study of posttraumatic stress disorder and disease activity outcomes in US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2013;65(2):227-234.
24. Sokolove J, Sayles H, Wagner CA, et al. Smoking status is associated with inflammatory cytokine profile and disease activity: decreased inflammation and disease improvement with smoking cessation? [abstract 348]. Arthritis Rheumatol. 2014;66(suppl 10):S146.
25. Criswell LA, Merlino LA, Cerhan JR, et al. Cigarette smoking and the risk of rheumatoid arthritis among postmenopausal women: results from the Iowa Women’s Health Study. Am J Med. 2002;112(6):465-471.
26. Hecht C, Englbrecht M, Rech J, et al. Additive effect of anti-citrullinated protein antibodies and rheumatoid factor on bone erosions in patients with RA [published online ahead of print August 12, 2014]. Ann Rheum Dis. doi: 10.1136/annrheumdis -2014-205428.
27. Cannon GW, DuVall SL, Haroldsen CL, et al. Persistence and dose escalation of tumor necrosis factor inhibitors in US veterans with rheumatoid arthritis. J Rheumatol. 2014;41(10):1935-1943.
28. Curtis JR, Baddley JW, Yang S, et al. Derivation and preliminary validation of an administrative claims-based algorithm for the effectiveness of medications for rheumatoid arthritis. Arthritis Res Ther. 2011;13(5):R155.
29. Caplan L, Davis LA, Bright CM, et al. Body mass index and the rheumatoid arthritis swollen joint count: an observational study. Arthritis Care Res (Hoboken). 2013;65(1):101-106.
30. Davis LA, Whitfield E, Cannon GW, et al. Association of rheumatoid arthritis susceptibility gene with lipid profiles in patients with rheumatoid arthritis. Rheumatology (Oxford). 2014;53(6):1014-1021.
31. Mikuls TR, Kazi S, Cipher D, et al. The association of race and ethnicity with disease expression in male US veterans with rheumatoid arthritis. J Rheumatol. 2007;34(7):1480-1484.
32. Miriovsky BJ, Michaud K, Thiele GM, et al. Anti-CCP antibody and rheumatoid factor concentrations predict greater disease activity in men with rheumatoid arthritis. Ann Rheum Dis. 2010;69(7):1292-1297.
33. Oei HB, Hooker RS, Cipher DJ, Reimold A. High rates of stopping or switching biological medications in veterans with rheumatoid arthritis. Clin Exp Rheumatol. 2009;27(6):926-934.
34. Richards JS, Peng J, Amdur RL, et al. Dual-energy X-ray absorptiometry and evaluation of the osteoporosis self-assessment tool in men with rheumatoid arthritis. J Clin Densitom. 2009;12(4):434-440.
35. Richards JS, Cannon GW, Hayden CL, et al. Adherence with bisphosphonate therapy in US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64(12):1864-1870.
1. Banerjee S, Compton AP, Hooker RS, et al. Cardiovascular outcomes in male veterans with rheumatoid arthritis. Am J Cardiol. 2008;101(8):1201-1205.
2. Weyand CM, Schmidt D, Wagner U, Goronzy JJ. The influence of sex on the phenotype of rheumatoid arthritis. Arthritis Rheum. 1998;41(5):817-822.
3. Mikuls TR, Fay BT, Michaud K, et al. Associations of disease activity and treatments with mortality in men with rheumatoid arthritis: results from the VARA registry. Rheumatol (Oxford). 2011;50(1):101-109.
4. Baker JF, Billig E, Cannon GW, Caplan L, Majithia V, Mikuls TR. Weight loss and risk of death in rheumatoid arthritis [abstract 1391]. Arthritis Rheumatol. 2014;66(suppl 10):S613-S614.
5. Choi HK, Hernán MA, Seeger JD, Robins JM, Wolfe F. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. 2002;359(9313):1173-1177.
6. Kerr G, Aujero M, Richards J, et al. Associations of hydroxychloroquine use with lipid profiles in rheumatoid arthritis: pharmacologic implications. Arthritis Care Res (Hoboken). 2014;66(11):1619-1626.
7. Wasko MC, Hubert HB, Lingala VB, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. 2007;298(2):187-193.
8. Saag KG, Teng GG, Patkar NM, et al; American College of Rheumatology. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59(6):762-784.
9. Michaud K, Mikuls TR, Call SE, et al. Poor to modest agreement between rheumatoid arthritis response measures in clinical practice. Clin Exp Rheumatol. 2009;27(4):633-640.
10. Shahouri SH, Michaud K, Mikuls TR, et al. Remission of rheumatoid arthritis in clinical practice: application of the American College of Rheumatology/European League Against Rheumatism 2011 remission criteria. Arthritis Rheum. 2011;63(11):3204-3215.
11. Shaver TS, Anderson JD, Weidensaul DN, et al. The problem of rheumatoid arthritis disease activity and remission in clinical practice. J Rheumatol. 2008;35(6):1015-1022.
12. Masri KR, Shaver TS, Shahouri SH, et al. Validity and reliability problems with patient global as a component of the ACR/EULAR remission criteria as used in clinical practice. J Rheumatol. 2012;39(6):1139-1145.
13. Aletaha D, Landewe R, Karonitsch T, et al. Reporting disease activity in clinical trials of patients with rheumatoid arthritis: EULAR/ACR collaborative recommendations. Ann Rheum Dis. 2008;67(10):1360-1364.
14. Gregersen PK, Amos CI, Lee AT, et al. REL, encoding a member of the NF-kappaB family of transcription factors, is a newly defined risk locus for rheumatoid arthritis. Nat Genet. 2009;41(7):820-823.
15. Briggs FB, Ramsay PP, Madden E, et al. Supervised machine learning and logistic regression identifies novel epistatic risk factors with PTPN22 for rheumatoid arthritis. Genes Immun. 2010;11(3):199-208.
16. Mikuls TR, Gould KA, Bynoté KK, et al. Anticitrullinated protein antibody (ACPA) in rheumatoid arthritis: influence of an interaction between HLA-DRB1 shared epitope and a deletion polymorphism in glutathione S-transferase in a cross-sectional study. Arthritis Res Ther. 2010;12(6):R213.
17. Sokolove J, Johnson DS, Lahey LJ, et al. Rheumatoid factor as a potentiator of anti-citrullinated protein antibody-mediated inflammation in rheumatoid arthritis. Arthritis Rheumatol. 2014;66(4):813-821.
18. Kerr GS, Sabahi I, Richards JS, et al. Prevalence of vitamin D insufficiency/deficiency in rheumatoid arthritis and associations with disease severity and activity. J Rheumatol. 2011;38(1):53-59.
19. Mikuls TR, LeVan TD, Sayles H, et al. Soluble CD14 and CD14 polymorphisms in rheumatoid arthritis. J Rheumatol. 2011;38(12):2509-2516.
20. Dwivedi N, Upadhyay J, Neeli I, et al. Felty’s syndrome autoantibodies bind to deiminated histones and neutrophil extracellular chromatin traps. Arthritis Rheum. 2012;64(4):982-992.
21. Harlow L, Rosas IO, Gochuico BR, et al. Identification of citrullinated hsp90 isoforms as novel autoantigens in rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheum. 2013;65(4):869-879.
22. Cannon GW, Mikuls TR, Hayden CL, et al. Merging Veterans Affairs rheumatoid arthritis registry and pharmacy data to assess methotrexate adherence and disease activity in clinical practice. Arthritis Care Res (Hoboken). 2011;63(12):1680-1690.
23. Mikuls TR, Padala PR, Sayles HR, et al. Prospective study of posttraumatic stress disorder and disease activity outcomes in US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2013;65(2):227-234.
24. Sokolove J, Sayles H, Wagner CA, et al. Smoking status is associated with inflammatory cytokine profile and disease activity: decreased inflammation and disease improvement with smoking cessation? [abstract 348]. Arthritis Rheumatol. 2014;66(suppl 10):S146.
25. Criswell LA, Merlino LA, Cerhan JR, et al. Cigarette smoking and the risk of rheumatoid arthritis among postmenopausal women: results from the Iowa Women’s Health Study. Am J Med. 2002;112(6):465-471.
26. Hecht C, Englbrecht M, Rech J, et al. Additive effect of anti-citrullinated protein antibodies and rheumatoid factor on bone erosions in patients with RA [published online ahead of print August 12, 2014]. Ann Rheum Dis. doi: 10.1136/annrheumdis -2014-205428.
27. Cannon GW, DuVall SL, Haroldsen CL, et al. Persistence and dose escalation of tumor necrosis factor inhibitors in US veterans with rheumatoid arthritis. J Rheumatol. 2014;41(10):1935-1943.
28. Curtis JR, Baddley JW, Yang S, et al. Derivation and preliminary validation of an administrative claims-based algorithm for the effectiveness of medications for rheumatoid arthritis. Arthritis Res Ther. 2011;13(5):R155.
29. Caplan L, Davis LA, Bright CM, et al. Body mass index and the rheumatoid arthritis swollen joint count: an observational study. Arthritis Care Res (Hoboken). 2013;65(1):101-106.
30. Davis LA, Whitfield E, Cannon GW, et al. Association of rheumatoid arthritis susceptibility gene with lipid profiles in patients with rheumatoid arthritis. Rheumatology (Oxford). 2014;53(6):1014-1021.
31. Mikuls TR, Kazi S, Cipher D, et al. The association of race and ethnicity with disease expression in male US veterans with rheumatoid arthritis. J Rheumatol. 2007;34(7):1480-1484.
32. Miriovsky BJ, Michaud K, Thiele GM, et al. Anti-CCP antibody and rheumatoid factor concentrations predict greater disease activity in men with rheumatoid arthritis. Ann Rheum Dis. 2010;69(7):1292-1297.
33. Oei HB, Hooker RS, Cipher DJ, Reimold A. High rates of stopping or switching biological medications in veterans with rheumatoid arthritis. Clin Exp Rheumatol. 2009;27(6):926-934.
34. Richards JS, Peng J, Amdur RL, et al. Dual-energy X-ray absorptiometry and evaluation of the osteoporosis self-assessment tool in men with rheumatoid arthritis. J Clin Densitom. 2009;12(4):434-440.
35. Richards JS, Cannon GW, Hayden CL, et al. Adherence with bisphosphonate therapy in US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64(12):1864-1870.