User login
Oxygen Therapies and Clinical Outcomes for Patients Hospitalized With COVID-19: First Surge vs Second Surge
From Lahey Hospital and Medical Center, Burlington, MA (Drs. Liesching and Lei), and Tufts University School of Medicine, Boston, MA (Dr. Liesching)
ABSTRACT
Objective: To compare the utilization of oxygen therapies and clinical outcomes of patients admitted for COVID-19 during the second surge of the pandemic to that of patients admitted during the first surge.
Design: Observational study using a registry database.
Setting: Three hospitals (791 inpatient beds and 76 intensive care unit [ICU] beds) within the Beth Israel Lahey Health system in Massachusetts.
Participants: We included 3183 patients with COVID-19 admitted to hospitals.
Measurements: Baseline data included demographics and comorbidities. Treatments included low-flow supplemental oxygen (2-6 L/min), high-flow oxygen via nasal cannula, and invasive mechanical ventilation. Outcomes included ICU admission, length of stay, ventilator days, and mortality.
Results: A total of 3183 patients were included: 1586 during the first surge and 1597 during the second surge. Compared to the first surge, patients admitted during the second surge had a similar rate of receiving low-flow supplemental oxygen (65.8% vs 64.1%, P = .3), a higher rate of receiving high-flow nasal cannula (15.4% vs 10.8%, P = .0001), and a lower ventilation rate (5.6% vs 9.7%, P < .0001). The outcomes during the second surge were better than those during the first surge: lower ICU admission rate (8.1% vs 12.7%, P < .0001), shorter length of hospital stay (5 vs 6 days, P < .0001), fewer ventilator days (10 vs 16, P = .01), and lower mortality (8.3% vs 19.2%, P < .0001). Among ventilated patients, those who received high-flow nasal cannula had lower mortality.
Conclusion: Compared to the first surge of the COVID-19 pandemic, patients admitted during the second surge had similar likelihood of receiving low-flow supplemental oxygen, were more likely to receive high-flow nasal cannula, were less likely to be ventilated, and had better outcomes.
Keywords: supplemental oxygen, high-flow nasal cannula, ventilator.
The respiratory system receives the major impact of SARS-CoV-2 virus, and hypoxemia has been the predominant diagnosis for patients hospitalized with COVID-19.1,2 During the initial stage of the pandemic, oxygen therapies and mechanical ventilation were the only choices for these patients.3-6 Standard-of-care treatment for patients with COVID-19 during the initial surge included oxygen therapies and mechanical ventilation for hypoxemia and medications for comorbidities and COVID-19–associated sequelae, such as multi-organ dysfunction and failure. A report from New York during the first surge (May 2020) showed that among 5700 hospitalized patients with COVID-19, 27.8% received supplemental oxygen and 12.2% received invasive mechanical ventilation.7 High-flow nasal cannula (HFNC) oxygen delivery has been utilized widely throughout the pandemic due to its superiority over other noninvasive respiratory support techniques.8-12 Mechanical ventilation is always necessary for critically ill patients with acute respiratory distress syndrome. However, ventilator scarcity has become a bottleneck in caring for severely ill patients with COVID-19 during the pandemic.13
The clinical outcomes of hospitalized COVID-19 patients include a high intubation rate, long length of hospital and intensive care unit (ICU) stay, and high mortality.14,15 As the pandemic evolved, new medications, including remdesivir, hydroxychloroquine, lopinavir, or interferon β-1a, were used in addition to the standard of care, but these did not result in significantly different mortality from standard of care.16 Steroids are becoming foundational to the treatment of severe COVID-19 pneumonia, but evidence from high-quality randomized controlled clinical trials is lacking.17
During the first surge from March to May 2020, Massachusetts had the third highest number of COVID-19 cases among states in the United States.18 In early 2021, COVID-19 cases were climbing close to the peak of the second surge in Massachusetts. In this study, we compared utilization of low-flow supplemental oxygen, HFNC, and mechanical ventilation and clinical outcomes of patients admitted to 3 hospitals in Massachusetts during the second surge of the pandemic to that of patients admitted during the first surge.
Methods
Setting
Beth Israel Lahey Health is a system of academic and teaching hospitals with primary care and specialty care providers. We included 3 centers within the Beth Israel Lahey Health system in Massachusetts: Lahey Hospital and Medical Center, with 335 inpatient hospital beds and 52 critical care beds; Beverly Hospital, with 227 beds and 14 critical care beds; and Winchester Hospital, with 229 beds and 10 ICU beds.
Participants
We included patients admitted to the 3 hospitals with COVID-19 as a primary or secondary diagnosis during the first surge of the pandemic (March 1, 2020 to June 15, 2020) and the second surge (November 15, 2020 to January 27, 2021). The timeframe of the first surge was defined as the window between the start date and the end date of data collection. During the time window of the first surge, 1586 patients were included. The start time of the second surge was defined as the date when the data collection was restarted; the end date was set when the number of patients (1597) accumulated was close to the number of patients in the first surge (1586), so that the two groups had similar sample size.
Study Design
A data registry of COVID-19 patients was created by our institution, and the data were prospectively collected starting in March 2020. We retrospectively extracted data on the following from the registry database for this observational study: demographics and baseline comorbidities; the use of low-flow supplemental oxygen, HFNC, and invasive mechanical ventilator; and ICU admission, length of hospital stay, length of ICU stay, and hospital discharge disposition. Start and end times for each oxygen therapy were not entered in the registry. Data about other oxygen therapies, such as noninvasive positive-pressure ventilation, were not collected in the registry database, and therefore were not included in the analysis.
Statistical Analysis
Continuous variables (eg, age) were tested for data distribution normality using the Shapiro-Wilk test. Normally distributed data were tested using unpaired t-tests and displayed as mean (SD). The skewed data were tested using the Wilcoxon rank sum test and displayed as median (interquartile range [IQR]). The categorical variables were compared using chi-square test. Comparisons with P ≤ .05 were considered significantly different. Statistical analysis for this study was generated using Statistical Analysis Software (SAS), version 9.4 for Windows (SAS Institute Inc.).
Results
Baseline Characteristics
We included 3183 patients: 1586 admitted during the first surge and 1597 admitted during the second surge. Baseline characteristics of patients with COVID-19 admitted during the first and second surges are shown in Table 1. Patients admitted during the second surge were older (73 years vs 71 years, P = .01) and had higher rates of hypertension (64.8% vs 59.6%, P = .003) and asthma (12.9% vs 10.7%, P = .049) but a lower rate of interstitial lung disease (3.3% vs 7.7%, P < .001). Sequential organ failure assessment scores at admission and the rates of other comorbidities were not significantly different between the 2 surges.

Oxygen Therapies
The number of patients who were hospitalized and received low-flow supplemental oxygen, and/or HFNC, and/or ventilator in the first surge and the second surge is shown in the Figure. Of all patients included, 2067 (64.9%) received low-flow supplemental oxygen; of these, 374 (18.1%) subsequently received HFNC, and 85 (22.7%) of these subsequently received mechanical ventilation. Of all 3183 patients, 417 (13.1%) received HFNC; 43 of these patients received HFNC without receiving low-flow supplemental oxygen, and 98 (23.5%) subsequently received mechanical ventilation. Out of all 3183 patients, 244 (7.7%) received mechanical ventilation; 98 (40.2%) of these received HFNC while the remaining 146 (59.8%) did not. At the beginning of the first surge, the ratio of patients who received invasive mechanical ventilation to patients who received HFNC was close to 1:1 (10/10); the ratio decreased to 6:10 in May and June 2020. At the beginning of the second surge, the ratio was 8:10 and then decreased to 3:10 in December 2020 and January 2021.

As shown in Table 2, the proportion of patients who received low-flow supplemental oxygen during the second surge was similar to that during the first surge (65.8% vs 64.1%, P = .3). Patients admitted during the second surge were more likely to receive HFNC than patients admitted during the first surge (15.4% vs 10.8%, P = .0001). Patients admitted during the second surge were less likely to be ventilated than the patients admitted during the first surge (5.6% vs 9.7%, P < .0001).

Clinical Outcomes
As shown in Table 3, second surge outcomes were much better than first surge outcomes: the ICU admission rate was lower (8.1% vs 12.7%, P < .0001); patients were more likely to be discharged to home (60.2% vs 47.4%, P < .0001), had a shorter length of hospital stay (5 vs 6 days, P < .0001), and had fewer ventilator days (10 vs 16, P = .01); and mortality was lower (8.3% vs 19.2%, P < .0001). There was a trend that length of ICU stay was shorter during the second surge than during the first surge (7 days vs 9 days, P = .09).

As noted (Figure), the ratio of patients who received invasive mechanical ventilation to patients who received HFNC was decreasing during both the first surge and the second surge. To further analyze the relation between ventilator and HFNC, we performed a subgroup analysis for 244 ventilated patients during both surges to compare outcomes between patients who received HFNC and those who did not receive HFNC (Table 4). Ninety-eight (40%) patients received HFNC. Ventilated patients who received HFNC had lower mortality than those patients who did not receive HFNC (31.6% vs 48%, P = .01), but had a longer length of hospital stay (29 days vs 14 days, P < .0001), longer length of ICU stay (17 days vs 9 days, P < .0001), and a higher number of ventilator days (16 vs 11, P = .001).

Discussion
Our study compared the baseline patient characteristics; utilization of low-flow supplemental oxygen therapy, HFNC, and mechanical ventilation; and clinical outcomes between the first surge (n = 1586) and the second surge (n = 1597) of the COVID-19 pandemic. During both surges, about two-thirds of admitted patients received low-flow supplemental oxygen. A higher proportion of the admitted patients received HFNC during the second surge than during the first surge, while the intubation rate was lower during the second surge than during the first surge.
Reported low-flow supplemental oxygen use ranged from 28% to 63% depending on the cohort characteristics and location during the first surge.6,7,19 A report from New York during the first surge (March 1 to April 4, 2020) showed that among 5700 hospitalized patients with COVID-19, 27.8% received low-flow supplemental oxygen.7 HFNC is recommended in guidelines on management of patients with acute respiratory failure due to COVID-19.20 In our study, HFNC was utilized in a higher proportion of patients admitted for COVID-19 during the second surge (15.5% vs 10.8%, P = .0001). During the early pandemic period in Wuhan, China, 11% to 21% of admitted COVID-19 patients received HFNC.21,22 Utilization of HFNC in New York during the first surge (March to May 2020) varied from 5% to 14.3% of patients admitted with COVID-19.23,24 Our subgroup analysis of the ventilated patients showed that patients who received HFNC had lower mortality than those who did not (31.6% vs 48.0%, P = .011). Comparably, a report from Paris, France, showed that among patients admitted to ICUs for acute hypoxemic respiratory failure, those who received HFNC had lower mortality at day 60 than those who did not (21% vs 31%, P = .052).25 Our recent analysis showed that patients treated with HFNC prior to mechanical ventilation had lower mortality than those treated with only conventional oxygen (30% vs 52%, P = .05).26 In this subgroup analysis, we could not determine if HFNC treatment was administered before or after ventilation because HFNC was entered as dichotomous data (“Yes” or “No”) in the registry database. We merely showed the beneficial effect of HFNC on reducing mortality for ventilated COVID-19 patients, but did not mean to focus on how and when to apply HFNC.
We observed that the patients admitted during the second surge were less likely to be ventilated than the patients admitted during the first surge (5.6% vs 9.7%, P < .0001). During the first surge in New York, among 5700 patients admitted with COVID-19, 12.2% received invasive mechanical ventilation.7 In another report, also from New York during the first surge, 26.1% of 2015 hospitalized COVID-19 patients received mechanical ventilation.27 In our study, the ventilation rate of 9.7% during the first surge was lower.
Outcomes during the second surge were better than during the first surge, including ICU admission rate, hospital and ICU length of stay, ventilator days, and mortality. The mortality was 19.2% during the first surge vs 8.3% during the second surge (P < .0001). The mortality of 19.2% was lower than the 30.6% mortality reported for 2015 hospitalized COVID-19 patients in New York during the first surge.27 A retrospective study showed that early administration of remdesivir was associated with reduced ICU admission, ventilation use, and mortality.28 The RECOVERY clinical trial showed that dexamethasone improved mortality for COVID-19 patients who received respiratory support, but not for patients who did not receive any respiratory support.29 Perhaps some, if not all, of the improvement in ICU admission and mortality during the second surge was attributed to the new medications, such as antivirals and steroids.
The length of hospital stay for patients with moderate to severe COVID-19 varied from 4 to 53 days at different locations of the world, as shown in a meta-analysis by Rees and colleagues.30 Our results showing a length of stay of 6 days during the first surge and 5 days during the second surge fell into the shorter end of this range. In a retrospective analysis of 1643 adults with severe COVID-19 admitted to hospitals in New York City between March 9, 2020 and April 23, 2020, median hospital length of stay was 7 (IQR, 3-14) days.31 For the ventilated patients in our study, the length of stay of 14 days (did not receive HFNC) and 29 days (received HFNC) was much longer. This longer length of stay might be attributed to the patients in our study being older and having more severe comorbidities.
The main purpose of this study was to compare the oxygen therapies and outcomes between 2 surges. It is difficult to associate the clinical outcomes with the oxygen therapies because new therapies and medications were available after the first surge. It was not possible to adjust the outcomes with confounders (other therapies and medications) because the registry data did not include the new therapies and medications.
A strength of this study was that we included a large, balanced number of patients in the first surge and the second surge. We did not plan the sample size in both groups as we could not predict the number of admissions. We set the end date of data collection for analysis as the time when the number of patients admitted during the second surge was similar to the number of patients admitted during the first surge. A limitation was that the registry database was created by the institution and was not designed solely for this study. The data for oxygen therapies were limited to low-flow supplemental oxygen, HFNC, and invasive mechanical ventilation; data for noninvasive ventilation were not included.
Conclusion
At our centers, during the second surge of COVID-19 pandemic, patients hospitalized with COVID-19 infection were more likely to receive HFNC but less likely to be ventilated. Compared to the first surge, the hospitalized patients with COVID-19 infection had a lower ICU admission rate, shorter length of hospital stay, fewer ventilator days, and lower mortality. For ventilated patients, those who received HFNC had lower mortality than those who did not.
Corresponding author: Timothy N. Liesching, MD, 41 Mall Road, Burlington, MA 01805; [email protected]
Disclosures: None reported.
doi:10.12788/jcom.0086
1. Xie J, Covassin N, Fan Z, et al. Association between hypoxemia and mortality in patients with COVID-19. Mayo Clin Proc. 2020;95(6):1138-1147. doi:10.1016/j.mayocp.2020.04.006
2. Asleh R, Asher E, Yagel O, et al. Predictors of hypoxemia and related adverse outcomes in patients hospitalized with COVID-19: a double-center retrospective study. J Clin Med. 2021;10(16):3581. doi:10.3390/jcm10163581
3. Choi KJ, Hong HL, Kim EJ. Association between oxygen saturation/fraction of inhaled oxygen and mortality in patients with COVID-19 associated pneumonia requiring oxygen therapy. Tuberc Respir Dis (Seoul). 2021;84(2):125-133. doi:10.4046/trd.2020.0126
4. Dixit SB. Role of noninvasive oxygen therapy strategies in COVID-19 patients: Where are we going? Indian J Crit Care Med. 2020;24(10):897-898. doi:10.5005/jp-journals-10071-23625
5. Gonzalez-Castro A, Fajardo Campoverde A, Medina A, et al. Non-invasive mechanical ventilation and high-flow oxygen therapy in the COVID-19 pandemic: the value of a draw. Med Intensiva (Engl Ed). 2021;45(5):320-321. doi:10.1016/j.medine.2021.04.001
6. Pan W, Li J, Ou Y, et al. Clinical outcome of standardized oxygen therapy nursing strategy in COVID-19. Ann Palliat Med. 2020;9(4):2171-2177. doi:10.21037/apm-20-1272
7. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. doi:10.1001/jama.2020.6775
8. He G, Han Y, Fang Q, et al. Clinical experience of high-flow nasal cannula oxygen therapy in severe COVID-19 patients. Article in Chinese. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49(2):232-239. doi:10.3785/j.issn.1008-9292.2020.03.13
9. Lalla U, Allwood BW, Louw EH, et al. The utility of high-flow nasal cannula oxygen therapy in the management of respiratory failure secondary to COVID-19 pneumonia. S Afr Med J. 2020;110(6):12941.
10. Zhang TT, Dai B, Wang W. Should the high-flow nasal oxygen therapy be used or avoided in COVID-19? J Transl Int Med. 2020;8(2):57-58. doi:10.2478/jtim-2020-0018
11. Agarwal A, Basmaji J, Muttalib F, et al. High-flow nasal cannula for acute hypoxemic respiratory failure in patients with COVID-19: systematic reviews of effectiveness and its risks of aerosolization, dispersion, and infection transmission. Can J Anaesth. 2020;67(9):1217-1248. doi:10.1007/s12630-020-01740-2
12. Geng S, Mei Q, Zhu C, et al. High flow nasal cannula is a good treatment option for COVID-19. Heart Lung. 2020;49(5):444-445. doi:10.1016/j.hrtlng.2020.03.018
13. Feinstein MM, Niforatos JD, Hyun I, et al. Considerations for ventilator triage during the COVID-19 pandemic. Lancet Respir Med. 2020;8(6):e53. doi:10.1016/S2213-2600(20)30192-2
14. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242. doi:10.1001/jama.2020.2648
15. Rojas-Marte G, Hashmi AT, Khalid M, et al. Outcomes in patients with COVID-19 disease and high oxygen requirements. J Clin Med Res. 2021;13(1):26-37. doi:10.14740/jocmr4405
16. Zhang R, Mylonakis E. In inpatients with COVID-19, none of remdesivir, hydroxychloroquine, lopinavir, or interferon β-1a differed from standard care for in-hospital mortality. Ann Intern Med. 2021;174(2):JC17. doi:10.7326/ACPJ202102160-017
17. Rello J, Waterer GW, Bourdiol A, Roquilly A. COVID-19, steroids and other immunomodulators: The jigsaw is not complete. Anaesth Crit Care Pain Med. 2020;39(6):699-701. doi:10.1016/j.accpm.2020.10.011
18. Dargin J, Stempek S, Lei Y, Gray Jr. A, Liesching T. The effect of a tiered provider staffing model on patient outcomes during the coronavirus disease 2019 pandemic: A single-center observational study. Int J Crit Illn Inj Sci. 2021;11(3). doi:10.4103/ijciis.ijciis_37_21
19. Ni YN, Wang T, Liang BM, Liang ZA. The independent factors associated with oxygen therapy in COVID-19 patients under 65 years old. PLoS One. 2021;16(1):e0245690. doi:10.1371/journal.pone.0245690
20. Alhazzani W, Moller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med. 2020;48(6):e440-e469. doi:10.1097/CCM.0000000000004363
21. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. doi:10.1001/jama.2020.1585
22. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. doi:10.1016/S0140-6736(20)30566-3
23. Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. doi:10.1136/bmj.m1996
24. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2
25. Demoule A, Vieillard Baron A, Darmon M, et al. High-flow nasal cannula in critically ill patients with severe COVID-19. Am J Respir Crit Care Med. 2020;202(7):1039-1042. doi:10.1164/rccm.202005-2007LE
26. Hansen CK, Stempek S, Liesching T, Lei Y, Dargin J. Characteristics and outcomes of patients receiving high flow nasal cannula therapy prior to mechanical ventilation in COVID-19 respiratory failure: a prospective observational study. Int J Crit Illn Inj Sci. 2021;11(2):56-60. doi:10.4103/IJCIIS.IJCIIS_181_20
27. van Gerwen M, Alsen M, Little C, et al. Risk factors and outcomes of COVID-19 in New York City; a retrospective cohort study. J Med Virol. 2021;93(2):907-915. doi:10.1002/jmv.26337
28. Hussain Alsayed HA, Saheb Sharif-Askari F, Saheb Sharif-Askari N, Hussain AAS, Hamid Q, Halwani R. Early administration of remdesivir to COVID-19 patients associates with higher recovery rate and lower need for ICU admission: A retrospective cohort study. PLoS One. 2021;16(10):e0258643. doi:10.1371/journal.pone.0258643
29. RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693-704. doi:10.1056/NEJMoa2021436
30. Rees EM, Nightingale ES, Jafari Y, et al. COVID-19 length of hospital stay: a systematic review and data synthesis. BMC Med. 2020;18(1):270. doi:10.1186/s12916-020-01726-3
31. Anderson M, Bach P, Baldwin MR. Hospital length of stay for severe COVID-19: implications for Remdesivir’s value. medRxiv. 2020;2020.08.10.20171637. doi:10.1101/2020.08.10.20171637
From Lahey Hospital and Medical Center, Burlington, MA (Drs. Liesching and Lei), and Tufts University School of Medicine, Boston, MA (Dr. Liesching)
ABSTRACT
Objective: To compare the utilization of oxygen therapies and clinical outcomes of patients admitted for COVID-19 during the second surge of the pandemic to that of patients admitted during the first surge.
Design: Observational study using a registry database.
Setting: Three hospitals (791 inpatient beds and 76 intensive care unit [ICU] beds) within the Beth Israel Lahey Health system in Massachusetts.
Participants: We included 3183 patients with COVID-19 admitted to hospitals.
Measurements: Baseline data included demographics and comorbidities. Treatments included low-flow supplemental oxygen (2-6 L/min), high-flow oxygen via nasal cannula, and invasive mechanical ventilation. Outcomes included ICU admission, length of stay, ventilator days, and mortality.
Results: A total of 3183 patients were included: 1586 during the first surge and 1597 during the second surge. Compared to the first surge, patients admitted during the second surge had a similar rate of receiving low-flow supplemental oxygen (65.8% vs 64.1%, P = .3), a higher rate of receiving high-flow nasal cannula (15.4% vs 10.8%, P = .0001), and a lower ventilation rate (5.6% vs 9.7%, P < .0001). The outcomes during the second surge were better than those during the first surge: lower ICU admission rate (8.1% vs 12.7%, P < .0001), shorter length of hospital stay (5 vs 6 days, P < .0001), fewer ventilator days (10 vs 16, P = .01), and lower mortality (8.3% vs 19.2%, P < .0001). Among ventilated patients, those who received high-flow nasal cannula had lower mortality.
Conclusion: Compared to the first surge of the COVID-19 pandemic, patients admitted during the second surge had similar likelihood of receiving low-flow supplemental oxygen, were more likely to receive high-flow nasal cannula, were less likely to be ventilated, and had better outcomes.
Keywords: supplemental oxygen, high-flow nasal cannula, ventilator.
The respiratory system receives the major impact of SARS-CoV-2 virus, and hypoxemia has been the predominant diagnosis for patients hospitalized with COVID-19.1,2 During the initial stage of the pandemic, oxygen therapies and mechanical ventilation were the only choices for these patients.3-6 Standard-of-care treatment for patients with COVID-19 during the initial surge included oxygen therapies and mechanical ventilation for hypoxemia and medications for comorbidities and COVID-19–associated sequelae, such as multi-organ dysfunction and failure. A report from New York during the first surge (May 2020) showed that among 5700 hospitalized patients with COVID-19, 27.8% received supplemental oxygen and 12.2% received invasive mechanical ventilation.7 High-flow nasal cannula (HFNC) oxygen delivery has been utilized widely throughout the pandemic due to its superiority over other noninvasive respiratory support techniques.8-12 Mechanical ventilation is always necessary for critically ill patients with acute respiratory distress syndrome. However, ventilator scarcity has become a bottleneck in caring for severely ill patients with COVID-19 during the pandemic.13
The clinical outcomes of hospitalized COVID-19 patients include a high intubation rate, long length of hospital and intensive care unit (ICU) stay, and high mortality.14,15 As the pandemic evolved, new medications, including remdesivir, hydroxychloroquine, lopinavir, or interferon β-1a, were used in addition to the standard of care, but these did not result in significantly different mortality from standard of care.16 Steroids are becoming foundational to the treatment of severe COVID-19 pneumonia, but evidence from high-quality randomized controlled clinical trials is lacking.17
During the first surge from March to May 2020, Massachusetts had the third highest number of COVID-19 cases among states in the United States.18 In early 2021, COVID-19 cases were climbing close to the peak of the second surge in Massachusetts. In this study, we compared utilization of low-flow supplemental oxygen, HFNC, and mechanical ventilation and clinical outcomes of patients admitted to 3 hospitals in Massachusetts during the second surge of the pandemic to that of patients admitted during the first surge.
Methods
Setting
Beth Israel Lahey Health is a system of academic and teaching hospitals with primary care and specialty care providers. We included 3 centers within the Beth Israel Lahey Health system in Massachusetts: Lahey Hospital and Medical Center, with 335 inpatient hospital beds and 52 critical care beds; Beverly Hospital, with 227 beds and 14 critical care beds; and Winchester Hospital, with 229 beds and 10 ICU beds.
Participants
We included patients admitted to the 3 hospitals with COVID-19 as a primary or secondary diagnosis during the first surge of the pandemic (March 1, 2020 to June 15, 2020) and the second surge (November 15, 2020 to January 27, 2021). The timeframe of the first surge was defined as the window between the start date and the end date of data collection. During the time window of the first surge, 1586 patients were included. The start time of the second surge was defined as the date when the data collection was restarted; the end date was set when the number of patients (1597) accumulated was close to the number of patients in the first surge (1586), so that the two groups had similar sample size.
Study Design
A data registry of COVID-19 patients was created by our institution, and the data were prospectively collected starting in March 2020. We retrospectively extracted data on the following from the registry database for this observational study: demographics and baseline comorbidities; the use of low-flow supplemental oxygen, HFNC, and invasive mechanical ventilator; and ICU admission, length of hospital stay, length of ICU stay, and hospital discharge disposition. Start and end times for each oxygen therapy were not entered in the registry. Data about other oxygen therapies, such as noninvasive positive-pressure ventilation, were not collected in the registry database, and therefore were not included in the analysis.
Statistical Analysis
Continuous variables (eg, age) were tested for data distribution normality using the Shapiro-Wilk test. Normally distributed data were tested using unpaired t-tests and displayed as mean (SD). The skewed data were tested using the Wilcoxon rank sum test and displayed as median (interquartile range [IQR]). The categorical variables were compared using chi-square test. Comparisons with P ≤ .05 were considered significantly different. Statistical analysis for this study was generated using Statistical Analysis Software (SAS), version 9.4 for Windows (SAS Institute Inc.).
Results
Baseline Characteristics
We included 3183 patients: 1586 admitted during the first surge and 1597 admitted during the second surge. Baseline characteristics of patients with COVID-19 admitted during the first and second surges are shown in Table 1. Patients admitted during the second surge were older (73 years vs 71 years, P = .01) and had higher rates of hypertension (64.8% vs 59.6%, P = .003) and asthma (12.9% vs 10.7%, P = .049) but a lower rate of interstitial lung disease (3.3% vs 7.7%, P < .001). Sequential organ failure assessment scores at admission and the rates of other comorbidities were not significantly different between the 2 surges.

Oxygen Therapies
The number of patients who were hospitalized and received low-flow supplemental oxygen, and/or HFNC, and/or ventilator in the first surge and the second surge is shown in the Figure. Of all patients included, 2067 (64.9%) received low-flow supplemental oxygen; of these, 374 (18.1%) subsequently received HFNC, and 85 (22.7%) of these subsequently received mechanical ventilation. Of all 3183 patients, 417 (13.1%) received HFNC; 43 of these patients received HFNC without receiving low-flow supplemental oxygen, and 98 (23.5%) subsequently received mechanical ventilation. Out of all 3183 patients, 244 (7.7%) received mechanical ventilation; 98 (40.2%) of these received HFNC while the remaining 146 (59.8%) did not. At the beginning of the first surge, the ratio of patients who received invasive mechanical ventilation to patients who received HFNC was close to 1:1 (10/10); the ratio decreased to 6:10 in May and June 2020. At the beginning of the second surge, the ratio was 8:10 and then decreased to 3:10 in December 2020 and January 2021.

As shown in Table 2, the proportion of patients who received low-flow supplemental oxygen during the second surge was similar to that during the first surge (65.8% vs 64.1%, P = .3). Patients admitted during the second surge were more likely to receive HFNC than patients admitted during the first surge (15.4% vs 10.8%, P = .0001). Patients admitted during the second surge were less likely to be ventilated than the patients admitted during the first surge (5.6% vs 9.7%, P < .0001).

Clinical Outcomes
As shown in Table 3, second surge outcomes were much better than first surge outcomes: the ICU admission rate was lower (8.1% vs 12.7%, P < .0001); patients were more likely to be discharged to home (60.2% vs 47.4%, P < .0001), had a shorter length of hospital stay (5 vs 6 days, P < .0001), and had fewer ventilator days (10 vs 16, P = .01); and mortality was lower (8.3% vs 19.2%, P < .0001). There was a trend that length of ICU stay was shorter during the second surge than during the first surge (7 days vs 9 days, P = .09).

As noted (Figure), the ratio of patients who received invasive mechanical ventilation to patients who received HFNC was decreasing during both the first surge and the second surge. To further analyze the relation between ventilator and HFNC, we performed a subgroup analysis for 244 ventilated patients during both surges to compare outcomes between patients who received HFNC and those who did not receive HFNC (Table 4). Ninety-eight (40%) patients received HFNC. Ventilated patients who received HFNC had lower mortality than those patients who did not receive HFNC (31.6% vs 48%, P = .01), but had a longer length of hospital stay (29 days vs 14 days, P < .0001), longer length of ICU stay (17 days vs 9 days, P < .0001), and a higher number of ventilator days (16 vs 11, P = .001).

Discussion
Our study compared the baseline patient characteristics; utilization of low-flow supplemental oxygen therapy, HFNC, and mechanical ventilation; and clinical outcomes between the first surge (n = 1586) and the second surge (n = 1597) of the COVID-19 pandemic. During both surges, about two-thirds of admitted patients received low-flow supplemental oxygen. A higher proportion of the admitted patients received HFNC during the second surge than during the first surge, while the intubation rate was lower during the second surge than during the first surge.
Reported low-flow supplemental oxygen use ranged from 28% to 63% depending on the cohort characteristics and location during the first surge.6,7,19 A report from New York during the first surge (March 1 to April 4, 2020) showed that among 5700 hospitalized patients with COVID-19, 27.8% received low-flow supplemental oxygen.7 HFNC is recommended in guidelines on management of patients with acute respiratory failure due to COVID-19.20 In our study, HFNC was utilized in a higher proportion of patients admitted for COVID-19 during the second surge (15.5% vs 10.8%, P = .0001). During the early pandemic period in Wuhan, China, 11% to 21% of admitted COVID-19 patients received HFNC.21,22 Utilization of HFNC in New York during the first surge (March to May 2020) varied from 5% to 14.3% of patients admitted with COVID-19.23,24 Our subgroup analysis of the ventilated patients showed that patients who received HFNC had lower mortality than those who did not (31.6% vs 48.0%, P = .011). Comparably, a report from Paris, France, showed that among patients admitted to ICUs for acute hypoxemic respiratory failure, those who received HFNC had lower mortality at day 60 than those who did not (21% vs 31%, P = .052).25 Our recent analysis showed that patients treated with HFNC prior to mechanical ventilation had lower mortality than those treated with only conventional oxygen (30% vs 52%, P = .05).26 In this subgroup analysis, we could not determine if HFNC treatment was administered before or after ventilation because HFNC was entered as dichotomous data (“Yes” or “No”) in the registry database. We merely showed the beneficial effect of HFNC on reducing mortality for ventilated COVID-19 patients, but did not mean to focus on how and when to apply HFNC.
We observed that the patients admitted during the second surge were less likely to be ventilated than the patients admitted during the first surge (5.6% vs 9.7%, P < .0001). During the first surge in New York, among 5700 patients admitted with COVID-19, 12.2% received invasive mechanical ventilation.7 In another report, also from New York during the first surge, 26.1% of 2015 hospitalized COVID-19 patients received mechanical ventilation.27 In our study, the ventilation rate of 9.7% during the first surge was lower.
Outcomes during the second surge were better than during the first surge, including ICU admission rate, hospital and ICU length of stay, ventilator days, and mortality. The mortality was 19.2% during the first surge vs 8.3% during the second surge (P < .0001). The mortality of 19.2% was lower than the 30.6% mortality reported for 2015 hospitalized COVID-19 patients in New York during the first surge.27 A retrospective study showed that early administration of remdesivir was associated with reduced ICU admission, ventilation use, and mortality.28 The RECOVERY clinical trial showed that dexamethasone improved mortality for COVID-19 patients who received respiratory support, but not for patients who did not receive any respiratory support.29 Perhaps some, if not all, of the improvement in ICU admission and mortality during the second surge was attributed to the new medications, such as antivirals and steroids.
The length of hospital stay for patients with moderate to severe COVID-19 varied from 4 to 53 days at different locations of the world, as shown in a meta-analysis by Rees and colleagues.30 Our results showing a length of stay of 6 days during the first surge and 5 days during the second surge fell into the shorter end of this range. In a retrospective analysis of 1643 adults with severe COVID-19 admitted to hospitals in New York City between March 9, 2020 and April 23, 2020, median hospital length of stay was 7 (IQR, 3-14) days.31 For the ventilated patients in our study, the length of stay of 14 days (did not receive HFNC) and 29 days (received HFNC) was much longer. This longer length of stay might be attributed to the patients in our study being older and having more severe comorbidities.
The main purpose of this study was to compare the oxygen therapies and outcomes between 2 surges. It is difficult to associate the clinical outcomes with the oxygen therapies because new therapies and medications were available after the first surge. It was not possible to adjust the outcomes with confounders (other therapies and medications) because the registry data did not include the new therapies and medications.
A strength of this study was that we included a large, balanced number of patients in the first surge and the second surge. We did not plan the sample size in both groups as we could not predict the number of admissions. We set the end date of data collection for analysis as the time when the number of patients admitted during the second surge was similar to the number of patients admitted during the first surge. A limitation was that the registry database was created by the institution and was not designed solely for this study. The data for oxygen therapies were limited to low-flow supplemental oxygen, HFNC, and invasive mechanical ventilation; data for noninvasive ventilation were not included.
Conclusion
At our centers, during the second surge of COVID-19 pandemic, patients hospitalized with COVID-19 infection were more likely to receive HFNC but less likely to be ventilated. Compared to the first surge, the hospitalized patients with COVID-19 infection had a lower ICU admission rate, shorter length of hospital stay, fewer ventilator days, and lower mortality. For ventilated patients, those who received HFNC had lower mortality than those who did not.
Corresponding author: Timothy N. Liesching, MD, 41 Mall Road, Burlington, MA 01805; [email protected]
Disclosures: None reported.
doi:10.12788/jcom.0086
From Lahey Hospital and Medical Center, Burlington, MA (Drs. Liesching and Lei), and Tufts University School of Medicine, Boston, MA (Dr. Liesching)
ABSTRACT
Objective: To compare the utilization of oxygen therapies and clinical outcomes of patients admitted for COVID-19 during the second surge of the pandemic to that of patients admitted during the first surge.
Design: Observational study using a registry database.
Setting: Three hospitals (791 inpatient beds and 76 intensive care unit [ICU] beds) within the Beth Israel Lahey Health system in Massachusetts.
Participants: We included 3183 patients with COVID-19 admitted to hospitals.
Measurements: Baseline data included demographics and comorbidities. Treatments included low-flow supplemental oxygen (2-6 L/min), high-flow oxygen via nasal cannula, and invasive mechanical ventilation. Outcomes included ICU admission, length of stay, ventilator days, and mortality.
Results: A total of 3183 patients were included: 1586 during the first surge and 1597 during the second surge. Compared to the first surge, patients admitted during the second surge had a similar rate of receiving low-flow supplemental oxygen (65.8% vs 64.1%, P = .3), a higher rate of receiving high-flow nasal cannula (15.4% vs 10.8%, P = .0001), and a lower ventilation rate (5.6% vs 9.7%, P < .0001). The outcomes during the second surge were better than those during the first surge: lower ICU admission rate (8.1% vs 12.7%, P < .0001), shorter length of hospital stay (5 vs 6 days, P < .0001), fewer ventilator days (10 vs 16, P = .01), and lower mortality (8.3% vs 19.2%, P < .0001). Among ventilated patients, those who received high-flow nasal cannula had lower mortality.
Conclusion: Compared to the first surge of the COVID-19 pandemic, patients admitted during the second surge had similar likelihood of receiving low-flow supplemental oxygen, were more likely to receive high-flow nasal cannula, were less likely to be ventilated, and had better outcomes.
Keywords: supplemental oxygen, high-flow nasal cannula, ventilator.
The respiratory system receives the major impact of SARS-CoV-2 virus, and hypoxemia has been the predominant diagnosis for patients hospitalized with COVID-19.1,2 During the initial stage of the pandemic, oxygen therapies and mechanical ventilation were the only choices for these patients.3-6 Standard-of-care treatment for patients with COVID-19 during the initial surge included oxygen therapies and mechanical ventilation for hypoxemia and medications for comorbidities and COVID-19–associated sequelae, such as multi-organ dysfunction and failure. A report from New York during the first surge (May 2020) showed that among 5700 hospitalized patients with COVID-19, 27.8% received supplemental oxygen and 12.2% received invasive mechanical ventilation.7 High-flow nasal cannula (HFNC) oxygen delivery has been utilized widely throughout the pandemic due to its superiority over other noninvasive respiratory support techniques.8-12 Mechanical ventilation is always necessary for critically ill patients with acute respiratory distress syndrome. However, ventilator scarcity has become a bottleneck in caring for severely ill patients with COVID-19 during the pandemic.13
The clinical outcomes of hospitalized COVID-19 patients include a high intubation rate, long length of hospital and intensive care unit (ICU) stay, and high mortality.14,15 As the pandemic evolved, new medications, including remdesivir, hydroxychloroquine, lopinavir, or interferon β-1a, were used in addition to the standard of care, but these did not result in significantly different mortality from standard of care.16 Steroids are becoming foundational to the treatment of severe COVID-19 pneumonia, but evidence from high-quality randomized controlled clinical trials is lacking.17
During the first surge from March to May 2020, Massachusetts had the third highest number of COVID-19 cases among states in the United States.18 In early 2021, COVID-19 cases were climbing close to the peak of the second surge in Massachusetts. In this study, we compared utilization of low-flow supplemental oxygen, HFNC, and mechanical ventilation and clinical outcomes of patients admitted to 3 hospitals in Massachusetts during the second surge of the pandemic to that of patients admitted during the first surge.
Methods
Setting
Beth Israel Lahey Health is a system of academic and teaching hospitals with primary care and specialty care providers. We included 3 centers within the Beth Israel Lahey Health system in Massachusetts: Lahey Hospital and Medical Center, with 335 inpatient hospital beds and 52 critical care beds; Beverly Hospital, with 227 beds and 14 critical care beds; and Winchester Hospital, with 229 beds and 10 ICU beds.
Participants
We included patients admitted to the 3 hospitals with COVID-19 as a primary or secondary diagnosis during the first surge of the pandemic (March 1, 2020 to June 15, 2020) and the second surge (November 15, 2020 to January 27, 2021). The timeframe of the first surge was defined as the window between the start date and the end date of data collection. During the time window of the first surge, 1586 patients were included. The start time of the second surge was defined as the date when the data collection was restarted; the end date was set when the number of patients (1597) accumulated was close to the number of patients in the first surge (1586), so that the two groups had similar sample size.
Study Design
A data registry of COVID-19 patients was created by our institution, and the data were prospectively collected starting in March 2020. We retrospectively extracted data on the following from the registry database for this observational study: demographics and baseline comorbidities; the use of low-flow supplemental oxygen, HFNC, and invasive mechanical ventilator; and ICU admission, length of hospital stay, length of ICU stay, and hospital discharge disposition. Start and end times for each oxygen therapy were not entered in the registry. Data about other oxygen therapies, such as noninvasive positive-pressure ventilation, were not collected in the registry database, and therefore were not included in the analysis.
Statistical Analysis
Continuous variables (eg, age) were tested for data distribution normality using the Shapiro-Wilk test. Normally distributed data were tested using unpaired t-tests and displayed as mean (SD). The skewed data were tested using the Wilcoxon rank sum test and displayed as median (interquartile range [IQR]). The categorical variables were compared using chi-square test. Comparisons with P ≤ .05 were considered significantly different. Statistical analysis for this study was generated using Statistical Analysis Software (SAS), version 9.4 for Windows (SAS Institute Inc.).
Results
Baseline Characteristics
We included 3183 patients: 1586 admitted during the first surge and 1597 admitted during the second surge. Baseline characteristics of patients with COVID-19 admitted during the first and second surges are shown in Table 1. Patients admitted during the second surge were older (73 years vs 71 years, P = .01) and had higher rates of hypertension (64.8% vs 59.6%, P = .003) and asthma (12.9% vs 10.7%, P = .049) but a lower rate of interstitial lung disease (3.3% vs 7.7%, P < .001). Sequential organ failure assessment scores at admission and the rates of other comorbidities were not significantly different between the 2 surges.

Oxygen Therapies
The number of patients who were hospitalized and received low-flow supplemental oxygen, and/or HFNC, and/or ventilator in the first surge and the second surge is shown in the Figure. Of all patients included, 2067 (64.9%) received low-flow supplemental oxygen; of these, 374 (18.1%) subsequently received HFNC, and 85 (22.7%) of these subsequently received mechanical ventilation. Of all 3183 patients, 417 (13.1%) received HFNC; 43 of these patients received HFNC without receiving low-flow supplemental oxygen, and 98 (23.5%) subsequently received mechanical ventilation. Out of all 3183 patients, 244 (7.7%) received mechanical ventilation; 98 (40.2%) of these received HFNC while the remaining 146 (59.8%) did not. At the beginning of the first surge, the ratio of patients who received invasive mechanical ventilation to patients who received HFNC was close to 1:1 (10/10); the ratio decreased to 6:10 in May and June 2020. At the beginning of the second surge, the ratio was 8:10 and then decreased to 3:10 in December 2020 and January 2021.

As shown in Table 2, the proportion of patients who received low-flow supplemental oxygen during the second surge was similar to that during the first surge (65.8% vs 64.1%, P = .3). Patients admitted during the second surge were more likely to receive HFNC than patients admitted during the first surge (15.4% vs 10.8%, P = .0001). Patients admitted during the second surge were less likely to be ventilated than the patients admitted during the first surge (5.6% vs 9.7%, P < .0001).

Clinical Outcomes
As shown in Table 3, second surge outcomes were much better than first surge outcomes: the ICU admission rate was lower (8.1% vs 12.7%, P < .0001); patients were more likely to be discharged to home (60.2% vs 47.4%, P < .0001), had a shorter length of hospital stay (5 vs 6 days, P < .0001), and had fewer ventilator days (10 vs 16, P = .01); and mortality was lower (8.3% vs 19.2%, P < .0001). There was a trend that length of ICU stay was shorter during the second surge than during the first surge (7 days vs 9 days, P = .09).

As noted (Figure), the ratio of patients who received invasive mechanical ventilation to patients who received HFNC was decreasing during both the first surge and the second surge. To further analyze the relation between ventilator and HFNC, we performed a subgroup analysis for 244 ventilated patients during both surges to compare outcomes between patients who received HFNC and those who did not receive HFNC (Table 4). Ninety-eight (40%) patients received HFNC. Ventilated patients who received HFNC had lower mortality than those patients who did not receive HFNC (31.6% vs 48%, P = .01), but had a longer length of hospital stay (29 days vs 14 days, P < .0001), longer length of ICU stay (17 days vs 9 days, P < .0001), and a higher number of ventilator days (16 vs 11, P = .001).

Discussion
Our study compared the baseline patient characteristics; utilization of low-flow supplemental oxygen therapy, HFNC, and mechanical ventilation; and clinical outcomes between the first surge (n = 1586) and the second surge (n = 1597) of the COVID-19 pandemic. During both surges, about two-thirds of admitted patients received low-flow supplemental oxygen. A higher proportion of the admitted patients received HFNC during the second surge than during the first surge, while the intubation rate was lower during the second surge than during the first surge.
Reported low-flow supplemental oxygen use ranged from 28% to 63% depending on the cohort characteristics and location during the first surge.6,7,19 A report from New York during the first surge (March 1 to April 4, 2020) showed that among 5700 hospitalized patients with COVID-19, 27.8% received low-flow supplemental oxygen.7 HFNC is recommended in guidelines on management of patients with acute respiratory failure due to COVID-19.20 In our study, HFNC was utilized in a higher proportion of patients admitted for COVID-19 during the second surge (15.5% vs 10.8%, P = .0001). During the early pandemic period in Wuhan, China, 11% to 21% of admitted COVID-19 patients received HFNC.21,22 Utilization of HFNC in New York during the first surge (March to May 2020) varied from 5% to 14.3% of patients admitted with COVID-19.23,24 Our subgroup analysis of the ventilated patients showed that patients who received HFNC had lower mortality than those who did not (31.6% vs 48.0%, P = .011). Comparably, a report from Paris, France, showed that among patients admitted to ICUs for acute hypoxemic respiratory failure, those who received HFNC had lower mortality at day 60 than those who did not (21% vs 31%, P = .052).25 Our recent analysis showed that patients treated with HFNC prior to mechanical ventilation had lower mortality than those treated with only conventional oxygen (30% vs 52%, P = .05).26 In this subgroup analysis, we could not determine if HFNC treatment was administered before or after ventilation because HFNC was entered as dichotomous data (“Yes” or “No”) in the registry database. We merely showed the beneficial effect of HFNC on reducing mortality for ventilated COVID-19 patients, but did not mean to focus on how and when to apply HFNC.
We observed that the patients admitted during the second surge were less likely to be ventilated than the patients admitted during the first surge (5.6% vs 9.7%, P < .0001). During the first surge in New York, among 5700 patients admitted with COVID-19, 12.2% received invasive mechanical ventilation.7 In another report, also from New York during the first surge, 26.1% of 2015 hospitalized COVID-19 patients received mechanical ventilation.27 In our study, the ventilation rate of 9.7% during the first surge was lower.
Outcomes during the second surge were better than during the first surge, including ICU admission rate, hospital and ICU length of stay, ventilator days, and mortality. The mortality was 19.2% during the first surge vs 8.3% during the second surge (P < .0001). The mortality of 19.2% was lower than the 30.6% mortality reported for 2015 hospitalized COVID-19 patients in New York during the first surge.27 A retrospective study showed that early administration of remdesivir was associated with reduced ICU admission, ventilation use, and mortality.28 The RECOVERY clinical trial showed that dexamethasone improved mortality for COVID-19 patients who received respiratory support, but not for patients who did not receive any respiratory support.29 Perhaps some, if not all, of the improvement in ICU admission and mortality during the second surge was attributed to the new medications, such as antivirals and steroids.
The length of hospital stay for patients with moderate to severe COVID-19 varied from 4 to 53 days at different locations of the world, as shown in a meta-analysis by Rees and colleagues.30 Our results showing a length of stay of 6 days during the first surge and 5 days during the second surge fell into the shorter end of this range. In a retrospective analysis of 1643 adults with severe COVID-19 admitted to hospitals in New York City between March 9, 2020 and April 23, 2020, median hospital length of stay was 7 (IQR, 3-14) days.31 For the ventilated patients in our study, the length of stay of 14 days (did not receive HFNC) and 29 days (received HFNC) was much longer. This longer length of stay might be attributed to the patients in our study being older and having more severe comorbidities.
The main purpose of this study was to compare the oxygen therapies and outcomes between 2 surges. It is difficult to associate the clinical outcomes with the oxygen therapies because new therapies and medications were available after the first surge. It was not possible to adjust the outcomes with confounders (other therapies and medications) because the registry data did not include the new therapies and medications.
A strength of this study was that we included a large, balanced number of patients in the first surge and the second surge. We did not plan the sample size in both groups as we could not predict the number of admissions. We set the end date of data collection for analysis as the time when the number of patients admitted during the second surge was similar to the number of patients admitted during the first surge. A limitation was that the registry database was created by the institution and was not designed solely for this study. The data for oxygen therapies were limited to low-flow supplemental oxygen, HFNC, and invasive mechanical ventilation; data for noninvasive ventilation were not included.
Conclusion
At our centers, during the second surge of COVID-19 pandemic, patients hospitalized with COVID-19 infection were more likely to receive HFNC but less likely to be ventilated. Compared to the first surge, the hospitalized patients with COVID-19 infection had a lower ICU admission rate, shorter length of hospital stay, fewer ventilator days, and lower mortality. For ventilated patients, those who received HFNC had lower mortality than those who did not.
Corresponding author: Timothy N. Liesching, MD, 41 Mall Road, Burlington, MA 01805; [email protected]
Disclosures: None reported.
doi:10.12788/jcom.0086
1. Xie J, Covassin N, Fan Z, et al. Association between hypoxemia and mortality in patients with COVID-19. Mayo Clin Proc. 2020;95(6):1138-1147. doi:10.1016/j.mayocp.2020.04.006
2. Asleh R, Asher E, Yagel O, et al. Predictors of hypoxemia and related adverse outcomes in patients hospitalized with COVID-19: a double-center retrospective study. J Clin Med. 2021;10(16):3581. doi:10.3390/jcm10163581
3. Choi KJ, Hong HL, Kim EJ. Association between oxygen saturation/fraction of inhaled oxygen and mortality in patients with COVID-19 associated pneumonia requiring oxygen therapy. Tuberc Respir Dis (Seoul). 2021;84(2):125-133. doi:10.4046/trd.2020.0126
4. Dixit SB. Role of noninvasive oxygen therapy strategies in COVID-19 patients: Where are we going? Indian J Crit Care Med. 2020;24(10):897-898. doi:10.5005/jp-journals-10071-23625
5. Gonzalez-Castro A, Fajardo Campoverde A, Medina A, et al. Non-invasive mechanical ventilation and high-flow oxygen therapy in the COVID-19 pandemic: the value of a draw. Med Intensiva (Engl Ed). 2021;45(5):320-321. doi:10.1016/j.medine.2021.04.001
6. Pan W, Li J, Ou Y, et al. Clinical outcome of standardized oxygen therapy nursing strategy in COVID-19. Ann Palliat Med. 2020;9(4):2171-2177. doi:10.21037/apm-20-1272
7. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. doi:10.1001/jama.2020.6775
8. He G, Han Y, Fang Q, et al. Clinical experience of high-flow nasal cannula oxygen therapy in severe COVID-19 patients. Article in Chinese. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49(2):232-239. doi:10.3785/j.issn.1008-9292.2020.03.13
9. Lalla U, Allwood BW, Louw EH, et al. The utility of high-flow nasal cannula oxygen therapy in the management of respiratory failure secondary to COVID-19 pneumonia. S Afr Med J. 2020;110(6):12941.
10. Zhang TT, Dai B, Wang W. Should the high-flow nasal oxygen therapy be used or avoided in COVID-19? J Transl Int Med. 2020;8(2):57-58. doi:10.2478/jtim-2020-0018
11. Agarwal A, Basmaji J, Muttalib F, et al. High-flow nasal cannula for acute hypoxemic respiratory failure in patients with COVID-19: systematic reviews of effectiveness and its risks of aerosolization, dispersion, and infection transmission. Can J Anaesth. 2020;67(9):1217-1248. doi:10.1007/s12630-020-01740-2
12. Geng S, Mei Q, Zhu C, et al. High flow nasal cannula is a good treatment option for COVID-19. Heart Lung. 2020;49(5):444-445. doi:10.1016/j.hrtlng.2020.03.018
13. Feinstein MM, Niforatos JD, Hyun I, et al. Considerations for ventilator triage during the COVID-19 pandemic. Lancet Respir Med. 2020;8(6):e53. doi:10.1016/S2213-2600(20)30192-2
14. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242. doi:10.1001/jama.2020.2648
15. Rojas-Marte G, Hashmi AT, Khalid M, et al. Outcomes in patients with COVID-19 disease and high oxygen requirements. J Clin Med Res. 2021;13(1):26-37. doi:10.14740/jocmr4405
16. Zhang R, Mylonakis E. In inpatients with COVID-19, none of remdesivir, hydroxychloroquine, lopinavir, or interferon β-1a differed from standard care for in-hospital mortality. Ann Intern Med. 2021;174(2):JC17. doi:10.7326/ACPJ202102160-017
17. Rello J, Waterer GW, Bourdiol A, Roquilly A. COVID-19, steroids and other immunomodulators: The jigsaw is not complete. Anaesth Crit Care Pain Med. 2020;39(6):699-701. doi:10.1016/j.accpm.2020.10.011
18. Dargin J, Stempek S, Lei Y, Gray Jr. A, Liesching T. The effect of a tiered provider staffing model on patient outcomes during the coronavirus disease 2019 pandemic: A single-center observational study. Int J Crit Illn Inj Sci. 2021;11(3). doi:10.4103/ijciis.ijciis_37_21
19. Ni YN, Wang T, Liang BM, Liang ZA. The independent factors associated with oxygen therapy in COVID-19 patients under 65 years old. PLoS One. 2021;16(1):e0245690. doi:10.1371/journal.pone.0245690
20. Alhazzani W, Moller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med. 2020;48(6):e440-e469. doi:10.1097/CCM.0000000000004363
21. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. doi:10.1001/jama.2020.1585
22. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. doi:10.1016/S0140-6736(20)30566-3
23. Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. doi:10.1136/bmj.m1996
24. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2
25. Demoule A, Vieillard Baron A, Darmon M, et al. High-flow nasal cannula in critically ill patients with severe COVID-19. Am J Respir Crit Care Med. 2020;202(7):1039-1042. doi:10.1164/rccm.202005-2007LE
26. Hansen CK, Stempek S, Liesching T, Lei Y, Dargin J. Characteristics and outcomes of patients receiving high flow nasal cannula therapy prior to mechanical ventilation in COVID-19 respiratory failure: a prospective observational study. Int J Crit Illn Inj Sci. 2021;11(2):56-60. doi:10.4103/IJCIIS.IJCIIS_181_20
27. van Gerwen M, Alsen M, Little C, et al. Risk factors and outcomes of COVID-19 in New York City; a retrospective cohort study. J Med Virol. 2021;93(2):907-915. doi:10.1002/jmv.26337
28. Hussain Alsayed HA, Saheb Sharif-Askari F, Saheb Sharif-Askari N, Hussain AAS, Hamid Q, Halwani R. Early administration of remdesivir to COVID-19 patients associates with higher recovery rate and lower need for ICU admission: A retrospective cohort study. PLoS One. 2021;16(10):e0258643. doi:10.1371/journal.pone.0258643
29. RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693-704. doi:10.1056/NEJMoa2021436
30. Rees EM, Nightingale ES, Jafari Y, et al. COVID-19 length of hospital stay: a systematic review and data synthesis. BMC Med. 2020;18(1):270. doi:10.1186/s12916-020-01726-3
31. Anderson M, Bach P, Baldwin MR. Hospital length of stay for severe COVID-19: implications for Remdesivir’s value. medRxiv. 2020;2020.08.10.20171637. doi:10.1101/2020.08.10.20171637
1. Xie J, Covassin N, Fan Z, et al. Association between hypoxemia and mortality in patients with COVID-19. Mayo Clin Proc. 2020;95(6):1138-1147. doi:10.1016/j.mayocp.2020.04.006
2. Asleh R, Asher E, Yagel O, et al. Predictors of hypoxemia and related adverse outcomes in patients hospitalized with COVID-19: a double-center retrospective study. J Clin Med. 2021;10(16):3581. doi:10.3390/jcm10163581
3. Choi KJ, Hong HL, Kim EJ. Association between oxygen saturation/fraction of inhaled oxygen and mortality in patients with COVID-19 associated pneumonia requiring oxygen therapy. Tuberc Respir Dis (Seoul). 2021;84(2):125-133. doi:10.4046/trd.2020.0126
4. Dixit SB. Role of noninvasive oxygen therapy strategies in COVID-19 patients: Where are we going? Indian J Crit Care Med. 2020;24(10):897-898. doi:10.5005/jp-journals-10071-23625
5. Gonzalez-Castro A, Fajardo Campoverde A, Medina A, et al. Non-invasive mechanical ventilation and high-flow oxygen therapy in the COVID-19 pandemic: the value of a draw. Med Intensiva (Engl Ed). 2021;45(5):320-321. doi:10.1016/j.medine.2021.04.001
6. Pan W, Li J, Ou Y, et al. Clinical outcome of standardized oxygen therapy nursing strategy in COVID-19. Ann Palliat Med. 2020;9(4):2171-2177. doi:10.21037/apm-20-1272
7. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. doi:10.1001/jama.2020.6775
8. He G, Han Y, Fang Q, et al. Clinical experience of high-flow nasal cannula oxygen therapy in severe COVID-19 patients. Article in Chinese. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49(2):232-239. doi:10.3785/j.issn.1008-9292.2020.03.13
9. Lalla U, Allwood BW, Louw EH, et al. The utility of high-flow nasal cannula oxygen therapy in the management of respiratory failure secondary to COVID-19 pneumonia. S Afr Med J. 2020;110(6):12941.
10. Zhang TT, Dai B, Wang W. Should the high-flow nasal oxygen therapy be used or avoided in COVID-19? J Transl Int Med. 2020;8(2):57-58. doi:10.2478/jtim-2020-0018
11. Agarwal A, Basmaji J, Muttalib F, et al. High-flow nasal cannula for acute hypoxemic respiratory failure in patients with COVID-19: systematic reviews of effectiveness and its risks of aerosolization, dispersion, and infection transmission. Can J Anaesth. 2020;67(9):1217-1248. doi:10.1007/s12630-020-01740-2
12. Geng S, Mei Q, Zhu C, et al. High flow nasal cannula is a good treatment option for COVID-19. Heart Lung. 2020;49(5):444-445. doi:10.1016/j.hrtlng.2020.03.018
13. Feinstein MM, Niforatos JD, Hyun I, et al. Considerations for ventilator triage during the COVID-19 pandemic. Lancet Respir Med. 2020;8(6):e53. doi:10.1016/S2213-2600(20)30192-2
14. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242. doi:10.1001/jama.2020.2648
15. Rojas-Marte G, Hashmi AT, Khalid M, et al. Outcomes in patients with COVID-19 disease and high oxygen requirements. J Clin Med Res. 2021;13(1):26-37. doi:10.14740/jocmr4405
16. Zhang R, Mylonakis E. In inpatients with COVID-19, none of remdesivir, hydroxychloroquine, lopinavir, or interferon β-1a differed from standard care for in-hospital mortality. Ann Intern Med. 2021;174(2):JC17. doi:10.7326/ACPJ202102160-017
17. Rello J, Waterer GW, Bourdiol A, Roquilly A. COVID-19, steroids and other immunomodulators: The jigsaw is not complete. Anaesth Crit Care Pain Med. 2020;39(6):699-701. doi:10.1016/j.accpm.2020.10.011
18. Dargin J, Stempek S, Lei Y, Gray Jr. A, Liesching T. The effect of a tiered provider staffing model on patient outcomes during the coronavirus disease 2019 pandemic: A single-center observational study. Int J Crit Illn Inj Sci. 2021;11(3). doi:10.4103/ijciis.ijciis_37_21
19. Ni YN, Wang T, Liang BM, Liang ZA. The independent factors associated with oxygen therapy in COVID-19 patients under 65 years old. PLoS One. 2021;16(1):e0245690. doi:10.1371/journal.pone.0245690
20. Alhazzani W, Moller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med. 2020;48(6):e440-e469. doi:10.1097/CCM.0000000000004363
21. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. doi:10.1001/jama.2020.1585
22. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. doi:10.1016/S0140-6736(20)30566-3
23. Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. doi:10.1136/bmj.m1996
24. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2
25. Demoule A, Vieillard Baron A, Darmon M, et al. High-flow nasal cannula in critically ill patients with severe COVID-19. Am J Respir Crit Care Med. 2020;202(7):1039-1042. doi:10.1164/rccm.202005-2007LE
26. Hansen CK, Stempek S, Liesching T, Lei Y, Dargin J. Characteristics and outcomes of patients receiving high flow nasal cannula therapy prior to mechanical ventilation in COVID-19 respiratory failure: a prospective observational study. Int J Crit Illn Inj Sci. 2021;11(2):56-60. doi:10.4103/IJCIIS.IJCIIS_181_20
27. van Gerwen M, Alsen M, Little C, et al. Risk factors and outcomes of COVID-19 in New York City; a retrospective cohort study. J Med Virol. 2021;93(2):907-915. doi:10.1002/jmv.26337
28. Hussain Alsayed HA, Saheb Sharif-Askari F, Saheb Sharif-Askari N, Hussain AAS, Hamid Q, Halwani R. Early administration of remdesivir to COVID-19 patients associates with higher recovery rate and lower need for ICU admission: A retrospective cohort study. PLoS One. 2021;16(10):e0258643. doi:10.1371/journal.pone.0258643
29. RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693-704. doi:10.1056/NEJMoa2021436
30. Rees EM, Nightingale ES, Jafari Y, et al. COVID-19 length of hospital stay: a systematic review and data synthesis. BMC Med. 2020;18(1):270. doi:10.1186/s12916-020-01726-3
31. Anderson M, Bach P, Baldwin MR. Hospital length of stay for severe COVID-19: implications for Remdesivir’s value. medRxiv. 2020;2020.08.10.20171637. doi:10.1101/2020.08.10.20171637
Care after MET‐Implemented DNR Orders
Approximately 15% to 20% of inpatients develop significant adverse events, which are often preceded by a change in the patient's condition.[1, 2] Many hospitals utilize medical emergency teams (METs) to deliver prompt care for deteriorating patients. METs have an emerging role in end‐of‐life care; up to 35% of patients who die in the hospital with a do not resuscitate (DNR) order have a MET activation during the admission.[3] Thus, METs are well positioned to discuss preferences for cardiopulmonary resuscitation in a population at high risk for cardiac arrest.[4]
MET activations involve a change in code status in approximately 3% to 10% of cases.[5, 6, 7, 8] However, previous studies primarily involved hospitals in Australia, making the results difficult to generalize to other countries.[3, 6, 7] There may be important cultural differences in patient and clinician attitudes toward end‐of‐life care among different countries.[9] In addition, little is known about METs and hospital resources utilized in patients with MET‐implemented changes in code status. Anecdotal evidence suggests that MET activations addressing code status are more time consuming.[3] Conversely, by identifying patients unlikely to benefit from restorative care, METs may facilitate end‐of‐life comfort care, thereby reducing unplanned intensive care unit (ICU) admissions and hospital length of stay. Finally, preliminary evidence suggests that METs may improve the quality of end‐of‐life care.[10] However, the use of end‐of‐life resources, including inpatient palliative care consultation and hospice care following MET activation has not been well studied.
The purpose of our study was to examine the role of a US MET in end‐of‐life care. First, we assessed the proportion of MET calls that resulted in a new DNR order in a US hospital and compared this to previous reports in other countries. We also examined MET and hospital resource utilization in MET activations involving changes in code status by evaluating the duration of MET activations, the need for telemetry or ICU transfer, and hospital length of stay following MET activation. Finally, we explored the quality of end‐of‐life care in patients with MET‐implemented DNR orders by assessing the utilization of inpatient palliative care consultation and hospice care compared to patients with a preexisting DNR order.
MATERIALS AND METHODS
We conducted this study at Lahey Clinic, a 350‐bed academic, tertiary care center. The institutional review board approved this study and waived the need for informed consent. We performed a retrospective review of a prospectively collected MET registry. We included consecutive, adult (>18 years old) inpatient MET activations and excluded nonhospitalized patients. Data were recorded in an intranet registry at the time of the event by the MET nurse, including preexisting code status (full code or DNR), any change to the patient's code status (full code to DNR or DNR to full code) during the MET event, date of the event, disposition after the event (no transfer, transferred to ICU, or transfer to telemetry), and a description of MET interventions. The primary reason for the event was also recorded, including cardiovascular (systolic blood pressure <90 mm Hg, pulse <40 or >130 bpm), respiratory (respiratory rate <10 or >24 breaths per minute, need for noninvasive positive pressure ventilation, oxygen saturation <90%), neurologic (loss of consciousness, change in mental status, seizure, or suspected stroke), or clinical deterioration causing staff to become worried. MET activation occurs at our institution by utilizing a text paging system that records the date and time of the call, along with the patient's location. The event start time was recorded from the paging system. We considered the event stop time to be the time at which the patient is transferred to a different level of care (ie, ICU) or the MET members leave the bedside and transfer care back to the primary service. MET nurses recorded the duration of MET activation in the intranet database immediately following the activation. Data were collected from the medical record, including age, gender, race, medical insurance, religion, admission source (home, assisted living, rehabilitation, or other hospital), admission team (internal medicine or surgery), admission date and time, discharge or death date and time, disposition at discharge (home, rehabilitation, death with or without a DNR order, or hospice care), and admission diagnosis. We categorized patients discharged to a hospice bridge program as being discharged with hospice care. We also recorded whether or not code status was discussed at the time of admission. Our hospital policy requires that an advanced directives order (either full code or DNR) must be placed on all patients at the time of admission. However, we only considered a code status discussion to have occurred if there was explicit documentation of a code status discussion in the medical record at the time of hospital admission. Data from the time of hospital admission were collected to calculate a Charlson Comorbidity Index, a well‐validated predictor of mortality that uses a weighted sum of 17 medical conditions, with scores ranging from 0 to 37.[11] Higher scores indicate a greater burden of illness. We also recorded whether or not the inpatient palliative care service was consulted following MET activation by examining the medical record for a consult note. The inpatient palliative care service at our institution was implemented in 2005 and receives over 700 new inpatient consults per year. The most common reason for consultation is to assist the primary service with discussing goals of care and code status.
Our MET was established in 2005 and responds to approximately 30 to 40 events per 1000 admissions. There are 4 members on our MET, including a critical care nurse, a nursing supervisor, a respiratory therapist, and a team leader who, depending on a predetermined schedule, is either an attending hospital medicine physician, an attending critical care physician, a critical care training physician, or a critical care physician assistant.
The data were transferred from the intranet registry to an Excel (Microsoft Corp., Redmond, WA) spreadsheet for statistical analysis. The primary outcome was the proportion of activations resulting in a MET‐implemented DNR order. Secondary outcomes included the duration of MET activation, need for transfer to telemetry or the ICU, hospital length of stay following MET activation (time from the end of the MET activation to hospital discharge or death), and the frequency with which inpatient palliative care consultation and outpatient hospice care were utilized. For repeat MET activations in a single patient, we considered each MET activation as a separate event as the code status could potentially change more than once. We used SAS version 9.2 for Windows (SAS Institute Inc., Cary, NC) statistical analysis software for data analysis. The 2 method was used for categorical variables, and either a 2‐sample t test or Wilcoxon rank sum test was utilized for continuous variables. A P value 0.05 was considered significant.
RESULTS
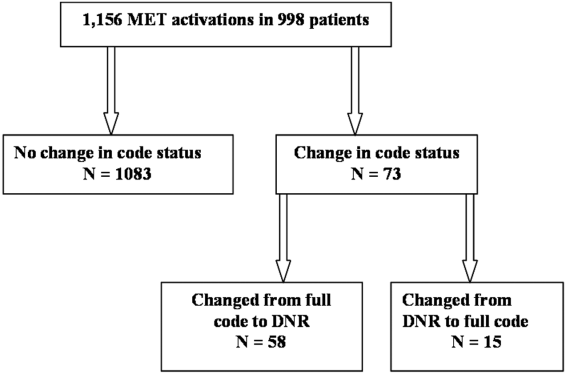
We observed 1156 MET activations in 998 patients. The mean age was 67 years and 57% (565/998) were male (Table 1). The mean Charlson Comorbidity Index was 5.4. Most patients were admitted from home (76%, 760/998), to a medical service (72%, 720/998), and to a teaching service (73%, 732/998). Sepsis (11%, 109/998) and trauma (11%, 105/998) were the most common admission diagnoses. A cardiovascular abnormality was the most common (35%, 399/1156) reason for activation. A code status discussion was documented on admission in 44% (440/998) of all patients.
| Variable | Total, n=998 | No Change in Code Status During MET Activation, n=926 | MET‐Implemented Change in Code Status, n=72a | P Valueb | DNR Prior to MET Activation, n=100 | MET‐Implemented DNR Order, n=58 | P Valuec |
|---|---|---|---|---|---|---|---|
| |||||||
| Gender, male | 565 (56.6) | 527 (56.9) | 38 (52.8) | 0.50 | 52 (52.0) | 34 (58.6) | 0.42 |
| Age, yr, meanSTD | 6717 | 6717 | 7115 | 0.08 | 8111 | 7016 | <0.0001 |
| Race | |||||||
| Caucasian | 927 (92.9) | 859 (92.8) | 68(94.4) | 0.59 | 99 (99) | 54 (93.1) | 0.04 |
| Minorityd | 71 (7.1) | 67 (7.2) | 4(5.6) | 1 (1.0) | 4 (6.9) | ||
| Insurance | |||||||
| Medicare | 694 (69.5) | 635 (68.6) | 59 (81.9) | 0.02 | 89 (89.0) | 45 (77.6) | 0.05 |
| Private | 244 (24.5) | 235 (25.4) | 9 (12.5) | 0.01 | 10 (10.0) | 9 (15.5) | 0.30 |
| Medicaid | 41 (4.1) | 39 (4.2) | 2 (2.8) | 0.55 | 1 (1.0) | 2 (3.5) | 0.27 |
| None | 18 (1.8) | 17 (1.8) | 1 (1.4) | 0.78 | 0 (0) | 1 (1.7) | 0.19 |
| Religion | |||||||
| Christian | 748 (75.0) | 691 (74.6) | 57 (79.2) | 0.39 | 82 (82.0) | 46 (79.3) | 0.68 |
| None specified | 226 (22.7) | 213 (23.0) | 13 (18.1) | 0.33 | 14 (14.0) | 10 (17.2) | 0.58 |
| Other religions | 24 (2.4) | 22 (2.4) | 2 (2.7) | 0.83 | 4 (4.0) | 2 (3.5) | 0.86 |
| Admission diagnosis | |||||||
| Sepsis | 109 (10.9) | 100 (10.8) | 9 (12.5) | 0.66 | 11 (11.0) | 8 (13.8) | 0.60 |
| Trauma/fall | 105 (10.5) | 100 (10.8) | 5 (6.9) | 0.30 | 6 (6.0) | 5 (8.6) | 0.53 |
| Malignancy related | 79 (7.9) | 73 (7.9) | 6 (8.3) | 0.89 | 7 (7.0) | 4 (6.9) | 0.98 |
| Stroke | 47 (4.7) | 43 (4.6) | 4 (5.6) | 0.73 | 6 (6.0) | 4 (6.9) | 0.82 |
| Pneumonia | 46 (4.6) | 41 (4.4) | 5 (6.9) | 0.33 | 11 (11.0) | 3 (5.2) | 0.21 |
| Altered mental status | 43 (4.3) | 40 (4.3) | 3 (4.2) | 0.95 | 5 (5.0) | 3 (5.2) | 0.96 |
| Myocardial infarct | 42 (4.2) | 40 (4.3) | 2 (2.8) | 0.53 | 1 (1.0) | 1 (1.7) | 0.69 |
| Respiratory failure | 40 (4.0) | 36 (3.9) | 4 (5.6) | 0.49 | 2 (2.0) | 3 (5.2) | 0.27 |
| Arrhythmia | 37 (3.7) | 33 (3.6) | 4 (5.6) | 0.39 | 2 (2.0) | 3 (5.2) | 0.27 |
| Heart failure | 35 (3.5) | 33 (3.6) | 2 (2.8) | 0.72 | 10 (10.0) | 2 (3.5) | 0.13 |
| Other | 415 (41.6) | 387 (41.8) | 28 (38.9) | 0.63 | 39 (39.0) | 22 (37.9) | 0.89 |
| Admission type | |||||||
| Medical | 720 (72.1) | 662 (71.5) | 58 (80.6) | 0.10 | 91 (91.0) | 46 (79.3) | 0.04 |
| Surgical | 278 (27.9) | 264 (28.5) | 14 (19.4) | 9 (9.0) | 12 (20.7) | ||
| Admission source | |||||||
| Home | 760 (76.2) | 706 (76.2) | 54 (75.0) | 0.81 | 60 (60.0) | 44 (75.9) | 0.04 |
| Assisted Living | 29 (2.9) | 28(3.0) | 1 (1.4) | 0.43 | 9 (9.0) | 1 (1.7) | 0.07 |
| Nursing Home | 69 (6.9) | 65(7.0) | 4 (5.6) | 0.64 | 19 (19.0) | 1 (1.7) | <0.01 |
| Outside hospital | 139 (13.9) | 126(13.6) | 13 (18.1) | 0.29 | 12 (12.0) | 12 (20.7) | 0.14 |
| Other | 1 (0.1) | 0(0) | 1 (0.1) | 0.78 | 0 (0) | 0 | |
| Teaching service | 732 (73.4) | 678 (73.2) | 54 (75.0) | 0.74 | 84 (84.0) | 41 (70.7) | 0.05 |
| Code status discussed on admission | 440(44.1) | 397 (42.9) | 43 (59.7) | 0.01 | 70 (70.0) | 32 (55.2) | 0.06 |
| CCI, meanSTDe | 5.43.0 | 5.43.0 | 5.83.0 | 0.21 | 7.72.4 | 5.73.0 | <0.001 |
| MI | 226 (22.7) | 210 (22.7) | 16 (22.2) | 0.93 | 36 (36.0) | 13 (22.4) | 0.08 |
| Heart failure | 138 (13.8) | 127 (13.7) | 11 (15.3) | 0.71 | 28 (28.0) | 8 (13.8) | 0.04 |
| PVD | 90 (9.0) | 85 (9.2) | 5 (6.9) | 0.52 | 14 (14.0) | 4(6.9) | 0.18 |
| Stroke | 131 (13.1) | 121 (13.1) | 10 (13.9) | 0.84 | 30 (30.0) | 9 (15.5) | 0.04 |
| Dementia | 58 (5.8) | 51(5.5) | 7 (9.7) | 0.14 | 19 (19.0) | 5 (8.6) | 0.08 |
| COPD | 173 (17.3) | 161 (17.4) | 12 (16.7) | 0.88 | 23 (23.0) | 8 (13.8) | 0.16 |
| CTD | 58 (5.8) | 56 (6.1) | 2 (2.8) | 0.25 | 6 (6.0) | 2 (3.5) | 0.48 |
| Peptic ulcer disease | 26 (2.6) | 25 (2.7) | 1 (1.4) | 0.50 | 2 (2.0) | 1 (1.7) | 0.90 |
| Mild liver disease | 33 (3.3) | 32 (3.5) | 1 (1.4) | 0.34 | 1 (1.0) | 1 (1.7) | 0.69 |
| DM | 213 (21.3) | 194 (21.0) | 19 (16.4) | 0.28 | 19 (19.0) | 15 (25.9) | 0.31 |
| Hemiplegia | 18 (1.8) | 17 (1.8) | 1 (1.4) | 0.78 | 2 (2.0) | 1 (1.7) | 0.90 |
| Renal disease | 131 (13.1) | 119 (12.9) | 12 (16.7) | 0.36 | 21 (21.0) | 9 (15.5) | 0.40 |
| DM+organ damage | 68 (6.8) | 64 (6.9) | 4 (5.6) | 0.66 | 7 (7.0) | 3 (5.2) | 0.65 |
| Any tumor | 188 (18.8) | 173 (18.7) | 15 (20.8) | 0.65 | 25 (25.0) | 14 (24.1) | 0.90 |
| Lymphoma | 21 (2.1) | 20 (2.2) | 1 (1.4) | 0.66 | 2 (2.0) | 1 (1.7) | 0.90 |
| Leukemia | 20 (2.0) | 18 (1.9) | 2 (2.8) | 0.63 | 1 (1.0) | 0 (0.0) | 0.45 |
| Moderate/severe liver disease | 45 (4.5) | 39 (4.2) | 6 (8.3) | 0.10 | 0 (0.0) | 6 (10.3) | 0.001 |
| Metastatic tumor | 61 (6.1) | 51 (5.5) | 10 (13.9) | 0.004 | 10 (10.0) | 8 (13.8) | 0.47 |
| AIDS | 4 (0.4) | 3 (0.3) | 1 (1.4) | 0.17 | 0 (0.0) | 1 (1.7) | 0.19 |
MET activation resulted in a DNR order in 5% (58/1156) of cases (Figure 1). In activations involving a change in code status, 21% (15/73) were changed from DNR to full code. When compared to patients with a preexisting DNR order, patients with a MET‐implemented DNR order where younger (70 vs 81 years, P<0.0001), more commonly admitted from home (60% vs 44%, P=0.04), less frequently from a nursing home (1% vs 9%, P<0.01), and had a lower Charlson index (5.7 vs 7.7, P<0.001) (Table 1). Moderate to severe liver disease was more common in patients with a MET‐implemented DNR order (10% vs 0%, P=0.001). Admission diagnoses were similar between patients with a preexisting DNR and a MET‐implemented DNR order (Table 1).
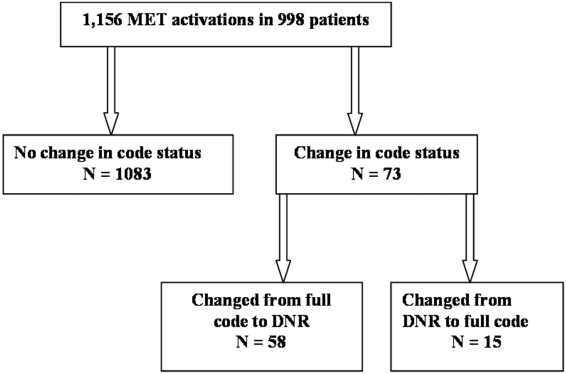
The median time spent on activations with a change in code status was significantly longer than activations without a change (66 vs 60 minutes, P=0.05). The rates of telemetry (6% vs 3%, P=0.24) and ICU transfer (40% vs 41%, P=0.8) were similar between patients with a change in code status and patients without a change (Table 2). Patients with a MET‐implemented DNR order were more frequently transferred to the ICU than patients with a preexisting DNR order (36% vs 17%, P<0.01). The median hospital length of stay following MET activation was shorter in patients with a change in code status compared to patients with no change (3 vs 5 days, P<0.0001).
| Variable | Total, N=1,156 | No Change in Code Status During MET Activation, n=1,083 | MET‐Implemented Change in Code Status, n=73a | PValuec | DNR Prior to MET Activation, n=115 | MET‐Implemented DNR Order, n=58 | P Valued |
|---|---|---|---|---|---|---|---|
| |||||||
| Reason for callb | |||||||
| Cardiovascular | 399 (34.5) | 379 (35.0) | 20 (27.4) | 0.19 | 39 (33.9) | 19 (32.8) | 0.88 |
| Respiratory | 319 (27.6) | 295 (27.2) | 2 (32.9) | 0.30 | 40 (34.8) | 18 (31.0) | 0.62 |
| Neurologic | 215 (18.6) | 196 (18.1) | 19 (26.0) | 0.09 | 21 (18.3) | 15 (25.9) | 0.25 |
| Other | 323 (27.9) | 303 (28.0) | 20 (27.4) | 0.92 | 22 (19.1) | 15 (25.9) | 0.31 |
| MET resources, call duration, min, median (IQR) | 60 (4090) | 60 (4090) | 66 (43100) | 0.05 | 50 (3075) | 67 (50100) | <0.001 |
| Hospital resources | |||||||
| Tele transfer | 68 (5.9) | 663 (6.1) | 2 (2.7) | 0.24 | 3 (2.6) | 2(3.5) | 0.76 |
| ICU transfer | 459 (39.7) | 429 (39.6) | 30 (41.1) | 0.8 | 19 (16.5) | 21 (36.2) | <0.01 |
| LOS after MET activation, d, median (IQR) | 5.2 (0.2510.7) | 5 (5.411.0) | 2.8 (0.66.7) | <0.0001 | 3.8 (1.56.5) | 2.9 (0.56.5) | 0.06 |
| End‐of‐life care | n=191e | n=157 | n=34 | n=41 | n=26 | ||
| Palliative care | 31 (16.2) | 27 (17.2) | 4 (11.8) | 0.44 | 8 (19.5) | 3 (11.5) | 0.39 |
| CMO orders | 159 (83.3) | 127 (80.9) | 32 (94.1) | 0.06 | 33 (80.5) | 25 (96.2) | 0.07 |
| Died full code | 10 (5.2) | 10 (6.4) | 0 (0) | 0.13 | 2 (4.9) | 0 (0) | 0.25 |
| Died DNR | 155 (81.2) | 123 (78.3) | 32 (94.1) | 0.03 | 27 (65.9) | 25 (96.2) | 0.004 |
| Hospice | 26 (13.6) | 24 (15.3) | 2 (5.9) | 0.15 | 12 (29.3) | 1 (3.9) | 0.01 |
The inpatient mortality was 17% (165/998). Most patients who died had the focus of care changed to comfort measures only (88%, 146/165). When examining the group of patients who died in the hospital with comfort care, we found that 58% (92/159) were transferred to the ICU following the MET call, 5% (8/159) were changed to comfort care during the MET call, and 18% (29/159) had a palliative care consult. We also observed that 16% (25/159) patients who died with comfort care were made DNR during MET activation. The inpatient mortality was significantly higher in patients with a change in code status compared to patients with no change in code status (44% vs 14%, 133/926, P<0.0001). Patients with a MET‐implemented DNR order had a higher inpatient mortality than patients with a preexisting DNR (43% vs 27%, P=0.04). Twenty‐five patients with a MET‐implemented DNR order died in the hospital. When examining a subgroup of patients who required end‐of‐life care (died or discharged from the hospital with hospice), we found patients with a MET‐implemented DNR order were less likely to be discharged with hospice care than patients with a preexisting DNR (4% vs 29%, P=0.01). There was no difference in the use of inpatient palliative care consultation at the end of life in patients with a preexisting DNR versus MET‐implemented DNR order (20% vs 12%, P=0.39). Patients with a MET‐implemented DNR order also had a significantly shorter median time from implementation of comfort care orders to death or discharge with hospice compared to patients with a preexisting DNR order (7 hours, interquartile range [IQR], 416 hours vs 22 hours, IQR 939 hours).
DISCUSSION
We observed a MET‐implemented DNR order in 5% of activations. Little is known about the role of METs in end‐of‐life discussions in the United States, and past experience has primarily come from Australian hospitals. Important differences in end‐of‐life care exist among different countries, particularly with regard to placing limitations on treatment.[9] Our observed rate is similar to the 3% to 10% rate of MET‐implemented DNR orders in previous reports worldwide.[3, 5, 8, 12, 13] Recent data from the United States suggest that METs initiate a DNR order in 28% of cases.[14] However, most DNR orders in that study were placed in the ICU days to weeks after MET activation, likely accounting for the high DNR rate. Our data add to the growing body of evidence that METs play an important role in end‐of‐life discussions among different countries throughout the world, including the United States.
To our knowledge, no prior study has evaluated the impact of code status discussions on MET resource utilization. Our MET spent 6 minutes longer on activations involving a change in code status when compared to activations with no changes made to code status. Presumably, some of this time was spent discussing goals of care. In our opinion, the additional time spent on these activations was invaluable, particularly when considering MET‐initiated end‐of‐life discussions may have prevented several unwanted resuscitations (25 patients died with a MET‐implemented DNR order). Interestingly, less than half of patients had code status discussions at the time of hospital admission. This finding suggests that clinicians could be more vigilant about discussing preferences for resuscitation at the time of admission in patients at risk for clinical deterioration. We suspect that in some cases code status discussions may have occurred between the patient and the primary service later in the patient's hospitalization, which were not captured in our study.
Surprisingly, when examining the use of hospital resources, we found no difference in the rate of unplanned ICU transfer in patients with a change in code status. In fact, we observed a higher rate of ICU transfer in patients with a MET‐implemented DNR order compared to those with a preexisting DNR order (36% vs 17%). These results were at odds with our hypothesis of a lower rate of ICU transfer in patients with MET‐implemented limitations in care. When compared to patients with a preexisting DNR order, patients with a MET‐implemented DNR order were younger, more commonly admitted from home, and had a lower Charlson index. Despite evidence of a lower burden of chronic illness and younger age, patients with a MET‐implemented DNR order had higher inpatient mortality than patients with a preexisting DNR order (43% vs 27%), suggesting an acute and rapidly progressive disease process. These observations may have compelled the MET to advocate for aggressive ICU‐level care in patients with a MET‐implemented DNR order. Another possible explanation for the relatively high rate of ICU transfer is that the MET is, in part, led by ICU staff. Thus, our MET may have made the decision to transfer the patient to the ICU and then subsequently initiated end‐of‐life discussions only after taking ownership of the patient. Furthermore, almost 20% of MET‐implemented changes to code status involved reversing status from DNR to full code. These data suggest that METs are not merely serving as a resource to review code status, but rather providing intensive treatment for acutely ill patients and simultaneously initiating end‐of‐life discussions in a population with a high inpatient mortality rate. The practice pattern observed in our study of transferring patients to the ICU for a trial of intensive therapy at the end of life is consistent with the overall trend in the United States for increased inpatient treatment intensity at the end of life.[14, 15, 16]
Our data suggest that the increased use of ICU resources in patients with a MET‐implemented DNR may be balanced by a shorter hospital length of stay following MET activation. In a multicenter observational study, Jones et al. found hospital length of stay to be similar in patients with and without a MET‐implemented limitation of medical therapy.[3] The authors did not examine length of stay specifically in patients with a DNR order, but rather examined patients with any limitation in medical therapy, including not for ICU admission. We suspect that the shorter length of stay following MET activation in our study was related to the fact that patients with a change in code status had a significantly higher inpatient mortality.
We observed several interesting findings with regard to end‐of‐life care following MET‐implemented DNR orders. First, the inpatient mortality in this population was remarkably high at 43%, compared to 27% in patients with a preexisting DNR order. Interestingly, there was no difference in the rate of palliative care consultation between the 2 groups despite the fact that all 25 patients who died following a MET‐implemented DNR order did so with a comfort measures only order. We also found that patients with a preexisting DNR also had a higher rate of discharge with hospice compared to patients with a MET‐implemented DNR order (29% vs 4%). Thus, our data suggest that inpatient palliative care consultation and hospice services are not resources that are routinely utilized in patients with MET‐initiated DNR orders. It may be the case that the acuity and severity of illness or patient preferences may have precluded the possibility of discharging some patients in our study home with hospice care or implementing comfort care earlier in the hospital course. Patients with MET‐implemented DNR orders were younger, had fewer comorbidities, and died sooner after comfort care orders were written. The overall rate of comfort care provided to patients who died was high at 88%. We have an inpatient comfort measures only order set at our hospital, which may account for the large proportion of patients receiving comfort care at the end of life. In addition, this order set may also help to improve the quality of end‐of‐life care and thus limit the need for palliative care consultation to some extent. However, we found that patients with a MET‐implemented DNR order had a shorter time from comfort care orders to death than patients with a preexisting DNR order. This finding suggests that patients with MET‐implemented DNR orders may have had comfort care implemented relatively late in the course of illness and had less‐than‐optimal end‐of‐life care. Vazquez et al. reported improved quality of end‐of‐life care after implementation of a MET.[10] However, an inpatient palliative care service was not available in that study, and it is not clear whether or not a comfort care order set was available. Evidence suggests that utilization of palliative care resources improves end‐of‐life care in the ICU.[17, 18, 19] We found that more than half of patients who died with comfort care in the hospital did so after being transferred to the ICU for a trial of aggressive care, suggesting that this population may have benefited from more involvement of our palliative care service. In summary, our data on end‐of‐life care following MET activation suggest that the METs are able to take advantage of an opportunity to identify patients who would not want resuscitation efforts because of personal preferences or futility of treatments. However, our surrogate measures of the quality of end‐of‐life care suggest that patients with MET‐implemented DNR orders may benefit from coordinated care with inpatient palliative care services, timelier implementation of comfort care orders, and possibly increased referrals for hospice care to help improve the quality of end‐of‐life care in this population.
Our study is subject to a number of limitations. This was a single‐center study, making the results difficult to generalize. The retrospective nature of our study makes it subject to the limitations inherent in this study design, including bias and confounding. The duration of MET activation was difficult to accurately and objectively measure and is subject to reporting bias. The event stop time, in particular, was subjectively measured by the MET nurse and is difficult to accurately assess, because MET members occasionally leave the bedside and return to reevaluate the response to therapy. We tried to account for this by clearly defining the MET stop time as the point at which MET members leave the bedside and transfer care back to the primary service or physically transfer the patient to a higher level of care. It also bears mentioning that the nurses performing data entry were not aware of the study hypothesis at the time of data entry. Despite including over 1100 MET calls in our analysis, the number of patients with a MET‐implemented DNR order was relatively small, which may have limited our ability to detect differences among subgroups during our analysis. We also did not document the clinical circumstances surrounding the MET‐implemented DNR order. Although we hypothesized that these patients had a higher mortality due to a higher acuity of illness, we were unable to support this hypothesis with the data available in our retrospective study. We did not record which providers were involved in code status discussions and the exact amount time spent on these discussions, making it difficult to accurately quantify the MET resources utilized on the calls. Our MET works closely with the patient's primary service, and it is possible that some of the changes to code status were implemented by the primary service and not MET providers. The patient's primary service may have a preexisting relationship with the patient and would be in a better position to discuss goals of care than MET providers who have had no prior relationship with the patient. However, even in this case, the clinical deterioration prompting MET activation was likely the event that triggered end‐of‐life discussions. Prospective studies would be helpful not only to identify the individuals involved in code status discussions during MET activations, but also to objectively measure the time spent on such discussions. Finally, our study population consisted primarily of Caucasian patients. Preferences for end‐of‐life care may differ among socioeconomic and ethnic groups, thus limiting the generalizability of our study findings.[20, 21]
In conclusion, we found the rate of MET‐implemented DNR orders in the United States to be similar to that of previous reports in other countries. MET events involving a change in code status are associated with increased utilization of MET and ICU resources, but a shorter hospital length of stay. Despite a high inpatient mortality rate, patients with a MET‐implemented DNR had a relatively low utilization of end‐of‐life resources, including palliative care and home hospice services. Coordinated care between METs and palliative care may help to improve of end‐of‐life care in patients with a change in code status following MET activation.
Acknowledgements
The authors acknowledge the hard work and dedication provided by Elizabeth Spellman during the data collection process.
Disclosures: This work was performed at Lahey Hospital and Medical Center. The authors report no conflicts of interest.
- , , , et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348(26):2635–2645.
- , , , et al. Antecedents to hospital deaths. Intern Med J. 2001;31(6):343–348.
- , , , et al. The role of the medical emergency team in end‐of‐life care: a multicenter, prospective, observational study. Crit Care Med. 2012;40(1):98–103.
- , , . Rapid‐response teams. N Engl J Med. 2011;365(2):139–146.
- , , , et al. The impact of Rapid Response System on delayed emergency team activation patient characteristics and outcomes—a follow‐up study. Resuscitation. 2010;81(1):31–35.
- , , , et al. Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial. Lancet. 2005;365(9477):2091–2097.
- , , , et al. Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study. BMJ. 2002;324(7334):387–390.
- , , , et al. The Medical Emergency Team System and not‐for‐resuscitation orders: results from the MERIT study. Resuscitation. 2008;79(3):391–397.
- . Cultural differences in end‐of‐life care. Crit Care Med. 2001;29(2 Suppl):N52–N55.
- , , , et al. Enhanced end‐of‐life care associated with deploying a rapid response team: a pilot study. J Hosp Med. 2009;4(7):449–452.
- , , , et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- , , , et al. The medical emergency team and end‐of‐life care: a pilot study. Crit Care Resusc. 2007;9(2):151–156.
- , , , et al. A retrospective cohort study of the effect of medical emergency teams on documentation of advance care directives. Crit Care Resusc. 2011;13(3):167–174.
- , , , et al. The medical emergency team a call: a sentinel event that triggers goals of care discussion. Crit Care Med. 2014;42(2):322–327.
- , , , et al. Change in end‐of‐life care for Medicare beneficiaries: site of death, place of care, and healthcare transitions in 2000, 2005, and 2009. JAMA. 2013;309(5):470–477.
- , , , et al. Trends in inpatient treatment intensity among Medicare beneficiaries at the end of life. Health Serv Res. 2004;39(2):363–375.
- , , , et al. Proactive palliative care in the medical intensive care unit: effects on length of stay for selected high‐risk patients. Crit Care Med. 2007;35(6):1530–1535.
- , . Impact of a proactive approach to improve end‐of‐life care in a medical ICU. Chest. 2003;123(1):266–271.
- , , , et al. In their own words: patients and families define high‐quality palliative care in the intensive care unit. Crit Care Med. 2010;38(3):808–818.
- , , , et al. The influence of race/ethnicity and socioeconomic status on end‐of‐life care in the ICU. Chest. 2011;139(5):1025–1033.
- , , , et al. Racial and ethnic differences in advance care planning among patients with cancer: impact of terminal illness acknowledgment, religiousness, and treatment preferences. J Clin Oncol. 2008;26(25):4131–4137.
Approximately 15% to 20% of inpatients develop significant adverse events, which are often preceded by a change in the patient's condition.[1, 2] Many hospitals utilize medical emergency teams (METs) to deliver prompt care for deteriorating patients. METs have an emerging role in end‐of‐life care; up to 35% of patients who die in the hospital with a do not resuscitate (DNR) order have a MET activation during the admission.[3] Thus, METs are well positioned to discuss preferences for cardiopulmonary resuscitation in a population at high risk for cardiac arrest.[4]
MET activations involve a change in code status in approximately 3% to 10% of cases.[5, 6, 7, 8] However, previous studies primarily involved hospitals in Australia, making the results difficult to generalize to other countries.[3, 6, 7] There may be important cultural differences in patient and clinician attitudes toward end‐of‐life care among different countries.[9] In addition, little is known about METs and hospital resources utilized in patients with MET‐implemented changes in code status. Anecdotal evidence suggests that MET activations addressing code status are more time consuming.[3] Conversely, by identifying patients unlikely to benefit from restorative care, METs may facilitate end‐of‐life comfort care, thereby reducing unplanned intensive care unit (ICU) admissions and hospital length of stay. Finally, preliminary evidence suggests that METs may improve the quality of end‐of‐life care.[10] However, the use of end‐of‐life resources, including inpatient palliative care consultation and hospice care following MET activation has not been well studied.
The purpose of our study was to examine the role of a US MET in end‐of‐life care. First, we assessed the proportion of MET calls that resulted in a new DNR order in a US hospital and compared this to previous reports in other countries. We also examined MET and hospital resource utilization in MET activations involving changes in code status by evaluating the duration of MET activations, the need for telemetry or ICU transfer, and hospital length of stay following MET activation. Finally, we explored the quality of end‐of‐life care in patients with MET‐implemented DNR orders by assessing the utilization of inpatient palliative care consultation and hospice care compared to patients with a preexisting DNR order.
MATERIALS AND METHODS
We conducted this study at Lahey Clinic, a 350‐bed academic, tertiary care center. The institutional review board approved this study and waived the need for informed consent. We performed a retrospective review of a prospectively collected MET registry. We included consecutive, adult (>18 years old) inpatient MET activations and excluded nonhospitalized patients. Data were recorded in an intranet registry at the time of the event by the MET nurse, including preexisting code status (full code or DNR), any change to the patient's code status (full code to DNR or DNR to full code) during the MET event, date of the event, disposition after the event (no transfer, transferred to ICU, or transfer to telemetry), and a description of MET interventions. The primary reason for the event was also recorded, including cardiovascular (systolic blood pressure <90 mm Hg, pulse <40 or >130 bpm), respiratory (respiratory rate <10 or >24 breaths per minute, need for noninvasive positive pressure ventilation, oxygen saturation <90%), neurologic (loss of consciousness, change in mental status, seizure, or suspected stroke), or clinical deterioration causing staff to become worried. MET activation occurs at our institution by utilizing a text paging system that records the date and time of the call, along with the patient's location. The event start time was recorded from the paging system. We considered the event stop time to be the time at which the patient is transferred to a different level of care (ie, ICU) or the MET members leave the bedside and transfer care back to the primary service. MET nurses recorded the duration of MET activation in the intranet database immediately following the activation. Data were collected from the medical record, including age, gender, race, medical insurance, religion, admission source (home, assisted living, rehabilitation, or other hospital), admission team (internal medicine or surgery), admission date and time, discharge or death date and time, disposition at discharge (home, rehabilitation, death with or without a DNR order, or hospice care), and admission diagnosis. We categorized patients discharged to a hospice bridge program as being discharged with hospice care. We also recorded whether or not code status was discussed at the time of admission. Our hospital policy requires that an advanced directives order (either full code or DNR) must be placed on all patients at the time of admission. However, we only considered a code status discussion to have occurred if there was explicit documentation of a code status discussion in the medical record at the time of hospital admission. Data from the time of hospital admission were collected to calculate a Charlson Comorbidity Index, a well‐validated predictor of mortality that uses a weighted sum of 17 medical conditions, with scores ranging from 0 to 37.[11] Higher scores indicate a greater burden of illness. We also recorded whether or not the inpatient palliative care service was consulted following MET activation by examining the medical record for a consult note. The inpatient palliative care service at our institution was implemented in 2005 and receives over 700 new inpatient consults per year. The most common reason for consultation is to assist the primary service with discussing goals of care and code status.
Our MET was established in 2005 and responds to approximately 30 to 40 events per 1000 admissions. There are 4 members on our MET, including a critical care nurse, a nursing supervisor, a respiratory therapist, and a team leader who, depending on a predetermined schedule, is either an attending hospital medicine physician, an attending critical care physician, a critical care training physician, or a critical care physician assistant.
The data were transferred from the intranet registry to an Excel (Microsoft Corp., Redmond, WA) spreadsheet for statistical analysis. The primary outcome was the proportion of activations resulting in a MET‐implemented DNR order. Secondary outcomes included the duration of MET activation, need for transfer to telemetry or the ICU, hospital length of stay following MET activation (time from the end of the MET activation to hospital discharge or death), and the frequency with which inpatient palliative care consultation and outpatient hospice care were utilized. For repeat MET activations in a single patient, we considered each MET activation as a separate event as the code status could potentially change more than once. We used SAS version 9.2 for Windows (SAS Institute Inc., Cary, NC) statistical analysis software for data analysis. The 2 method was used for categorical variables, and either a 2‐sample t test or Wilcoxon rank sum test was utilized for continuous variables. A P value 0.05 was considered significant.
RESULTS
We observed 1156 MET activations in 998 patients. The mean age was 67 years and 57% (565/998) were male (Table 1). The mean Charlson Comorbidity Index was 5.4. Most patients were admitted from home (76%, 760/998), to a medical service (72%, 720/998), and to a teaching service (73%, 732/998). Sepsis (11%, 109/998) and trauma (11%, 105/998) were the most common admission diagnoses. A cardiovascular abnormality was the most common (35%, 399/1156) reason for activation. A code status discussion was documented on admission in 44% (440/998) of all patients.
| Variable | Total, n=998 | No Change in Code Status During MET Activation, n=926 | MET‐Implemented Change in Code Status, n=72a | P Valueb | DNR Prior to MET Activation, n=100 | MET‐Implemented DNR Order, n=58 | P Valuec |
|---|---|---|---|---|---|---|---|
| |||||||
| Gender, male | 565 (56.6) | 527 (56.9) | 38 (52.8) | 0.50 | 52 (52.0) | 34 (58.6) | 0.42 |
| Age, yr, meanSTD | 6717 | 6717 | 7115 | 0.08 | 8111 | 7016 | <0.0001 |
| Race | |||||||
| Caucasian | 927 (92.9) | 859 (92.8) | 68(94.4) | 0.59 | 99 (99) | 54 (93.1) | 0.04 |
| Minorityd | 71 (7.1) | 67 (7.2) | 4(5.6) | 1 (1.0) | 4 (6.9) | ||
| Insurance | |||||||
| Medicare | 694 (69.5) | 635 (68.6) | 59 (81.9) | 0.02 | 89 (89.0) | 45 (77.6) | 0.05 |
| Private | 244 (24.5) | 235 (25.4) | 9 (12.5) | 0.01 | 10 (10.0) | 9 (15.5) | 0.30 |
| Medicaid | 41 (4.1) | 39 (4.2) | 2 (2.8) | 0.55 | 1 (1.0) | 2 (3.5) | 0.27 |
| None | 18 (1.8) | 17 (1.8) | 1 (1.4) | 0.78 | 0 (0) | 1 (1.7) | 0.19 |
| Religion | |||||||
| Christian | 748 (75.0) | 691 (74.6) | 57 (79.2) | 0.39 | 82 (82.0) | 46 (79.3) | 0.68 |
| None specified | 226 (22.7) | 213 (23.0) | 13 (18.1) | 0.33 | 14 (14.0) | 10 (17.2) | 0.58 |
| Other religions | 24 (2.4) | 22 (2.4) | 2 (2.7) | 0.83 | 4 (4.0) | 2 (3.5) | 0.86 |
| Admission diagnosis | |||||||
| Sepsis | 109 (10.9) | 100 (10.8) | 9 (12.5) | 0.66 | 11 (11.0) | 8 (13.8) | 0.60 |
| Trauma/fall | 105 (10.5) | 100 (10.8) | 5 (6.9) | 0.30 | 6 (6.0) | 5 (8.6) | 0.53 |
| Malignancy related | 79 (7.9) | 73 (7.9) | 6 (8.3) | 0.89 | 7 (7.0) | 4 (6.9) | 0.98 |
| Stroke | 47 (4.7) | 43 (4.6) | 4 (5.6) | 0.73 | 6 (6.0) | 4 (6.9) | 0.82 |
| Pneumonia | 46 (4.6) | 41 (4.4) | 5 (6.9) | 0.33 | 11 (11.0) | 3 (5.2) | 0.21 |
| Altered mental status | 43 (4.3) | 40 (4.3) | 3 (4.2) | 0.95 | 5 (5.0) | 3 (5.2) | 0.96 |
| Myocardial infarct | 42 (4.2) | 40 (4.3) | 2 (2.8) | 0.53 | 1 (1.0) | 1 (1.7) | 0.69 |
| Respiratory failure | 40 (4.0) | 36 (3.9) | 4 (5.6) | 0.49 | 2 (2.0) | 3 (5.2) | 0.27 |
| Arrhythmia | 37 (3.7) | 33 (3.6) | 4 (5.6) | 0.39 | 2 (2.0) | 3 (5.2) | 0.27 |
| Heart failure | 35 (3.5) | 33 (3.6) | 2 (2.8) | 0.72 | 10 (10.0) | 2 (3.5) | 0.13 |
| Other | 415 (41.6) | 387 (41.8) | 28 (38.9) | 0.63 | 39 (39.0) | 22 (37.9) | 0.89 |
| Admission type | |||||||
| Medical | 720 (72.1) | 662 (71.5) | 58 (80.6) | 0.10 | 91 (91.0) | 46 (79.3) | 0.04 |
| Surgical | 278 (27.9) | 264 (28.5) | 14 (19.4) | 9 (9.0) | 12 (20.7) | ||
| Admission source | |||||||
| Home | 760 (76.2) | 706 (76.2) | 54 (75.0) | 0.81 | 60 (60.0) | 44 (75.9) | 0.04 |
| Assisted Living | 29 (2.9) | 28(3.0) | 1 (1.4) | 0.43 | 9 (9.0) | 1 (1.7) | 0.07 |
| Nursing Home | 69 (6.9) | 65(7.0) | 4 (5.6) | 0.64 | 19 (19.0) | 1 (1.7) | <0.01 |
| Outside hospital | 139 (13.9) | 126(13.6) | 13 (18.1) | 0.29 | 12 (12.0) | 12 (20.7) | 0.14 |
| Other | 1 (0.1) | 0(0) | 1 (0.1) | 0.78 | 0 (0) | 0 | |
| Teaching service | 732 (73.4) | 678 (73.2) | 54 (75.0) | 0.74 | 84 (84.0) | 41 (70.7) | 0.05 |
| Code status discussed on admission | 440(44.1) | 397 (42.9) | 43 (59.7) | 0.01 | 70 (70.0) | 32 (55.2) | 0.06 |
| CCI, meanSTDe | 5.43.0 | 5.43.0 | 5.83.0 | 0.21 | 7.72.4 | 5.73.0 | <0.001 |
| MI | 226 (22.7) | 210 (22.7) | 16 (22.2) | 0.93 | 36 (36.0) | 13 (22.4) | 0.08 |
| Heart failure | 138 (13.8) | 127 (13.7) | 11 (15.3) | 0.71 | 28 (28.0) | 8 (13.8) | 0.04 |
| PVD | 90 (9.0) | 85 (9.2) | 5 (6.9) | 0.52 | 14 (14.0) | 4(6.9) | 0.18 |
| Stroke | 131 (13.1) | 121 (13.1) | 10 (13.9) | 0.84 | 30 (30.0) | 9 (15.5) | 0.04 |
| Dementia | 58 (5.8) | 51(5.5) | 7 (9.7) | 0.14 | 19 (19.0) | 5 (8.6) | 0.08 |
| COPD | 173 (17.3) | 161 (17.4) | 12 (16.7) | 0.88 | 23 (23.0) | 8 (13.8) | 0.16 |
| CTD | 58 (5.8) | 56 (6.1) | 2 (2.8) | 0.25 | 6 (6.0) | 2 (3.5) | 0.48 |
| Peptic ulcer disease | 26 (2.6) | 25 (2.7) | 1 (1.4) | 0.50 | 2 (2.0) | 1 (1.7) | 0.90 |
| Mild liver disease | 33 (3.3) | 32 (3.5) | 1 (1.4) | 0.34 | 1 (1.0) | 1 (1.7) | 0.69 |
| DM | 213 (21.3) | 194 (21.0) | 19 (16.4) | 0.28 | 19 (19.0) | 15 (25.9) | 0.31 |
| Hemiplegia | 18 (1.8) | 17 (1.8) | 1 (1.4) | 0.78 | 2 (2.0) | 1 (1.7) | 0.90 |
| Renal disease | 131 (13.1) | 119 (12.9) | 12 (16.7) | 0.36 | 21 (21.0) | 9 (15.5) | 0.40 |
| DM+organ damage | 68 (6.8) | 64 (6.9) | 4 (5.6) | 0.66 | 7 (7.0) | 3 (5.2) | 0.65 |
| Any tumor | 188 (18.8) | 173 (18.7) | 15 (20.8) | 0.65 | 25 (25.0) | 14 (24.1) | 0.90 |
| Lymphoma | 21 (2.1) | 20 (2.2) | 1 (1.4) | 0.66 | 2 (2.0) | 1 (1.7) | 0.90 |
| Leukemia | 20 (2.0) | 18 (1.9) | 2 (2.8) | 0.63 | 1 (1.0) | 0 (0.0) | 0.45 |
| Moderate/severe liver disease | 45 (4.5) | 39 (4.2) | 6 (8.3) | 0.10 | 0 (0.0) | 6 (10.3) | 0.001 |
| Metastatic tumor | 61 (6.1) | 51 (5.5) | 10 (13.9) | 0.004 | 10 (10.0) | 8 (13.8) | 0.47 |
| AIDS | 4 (0.4) | 3 (0.3) | 1 (1.4) | 0.17 | 0 (0.0) | 1 (1.7) | 0.19 |
MET activation resulted in a DNR order in 5% (58/1156) of cases (Figure 1). In activations involving a change in code status, 21% (15/73) were changed from DNR to full code. When compared to patients with a preexisting DNR order, patients with a MET‐implemented DNR order where younger (70 vs 81 years, P<0.0001), more commonly admitted from home (60% vs 44%, P=0.04), less frequently from a nursing home (1% vs 9%, P<0.01), and had a lower Charlson index (5.7 vs 7.7, P<0.001) (Table 1). Moderate to severe liver disease was more common in patients with a MET‐implemented DNR order (10% vs 0%, P=0.001). Admission diagnoses were similar between patients with a preexisting DNR and a MET‐implemented DNR order (Table 1).

The median time spent on activations with a change in code status was significantly longer than activations without a change (66 vs 60 minutes, P=0.05). The rates of telemetry (6% vs 3%, P=0.24) and ICU transfer (40% vs 41%, P=0.8) were similar between patients with a change in code status and patients without a change (Table 2). Patients with a MET‐implemented DNR order were more frequently transferred to the ICU than patients with a preexisting DNR order (36% vs 17%, P<0.01). The median hospital length of stay following MET activation was shorter in patients with a change in code status compared to patients with no change (3 vs 5 days, P<0.0001).
| Variable | Total, N=1,156 | No Change in Code Status During MET Activation, n=1,083 | MET‐Implemented Change in Code Status, n=73a | PValuec | DNR Prior to MET Activation, n=115 | MET‐Implemented DNR Order, n=58 | P Valued |
|---|---|---|---|---|---|---|---|
| |||||||
| Reason for callb | |||||||
| Cardiovascular | 399 (34.5) | 379 (35.0) | 20 (27.4) | 0.19 | 39 (33.9) | 19 (32.8) | 0.88 |
| Respiratory | 319 (27.6) | 295 (27.2) | 2 (32.9) | 0.30 | 40 (34.8) | 18 (31.0) | 0.62 |
| Neurologic | 215 (18.6) | 196 (18.1) | 19 (26.0) | 0.09 | 21 (18.3) | 15 (25.9) | 0.25 |
| Other | 323 (27.9) | 303 (28.0) | 20 (27.4) | 0.92 | 22 (19.1) | 15 (25.9) | 0.31 |
| MET resources, call duration, min, median (IQR) | 60 (4090) | 60 (4090) | 66 (43100) | 0.05 | 50 (3075) | 67 (50100) | <0.001 |
| Hospital resources | |||||||
| Tele transfer | 68 (5.9) | 663 (6.1) | 2 (2.7) | 0.24 | 3 (2.6) | 2(3.5) | 0.76 |
| ICU transfer | 459 (39.7) | 429 (39.6) | 30 (41.1) | 0.8 | 19 (16.5) | 21 (36.2) | <0.01 |
| LOS after MET activation, d, median (IQR) | 5.2 (0.2510.7) | 5 (5.411.0) | 2.8 (0.66.7) | <0.0001 | 3.8 (1.56.5) | 2.9 (0.56.5) | 0.06 |
| End‐of‐life care | n=191e | n=157 | n=34 | n=41 | n=26 | ||
| Palliative care | 31 (16.2) | 27 (17.2) | 4 (11.8) | 0.44 | 8 (19.5) | 3 (11.5) | 0.39 |
| CMO orders | 159 (83.3) | 127 (80.9) | 32 (94.1) | 0.06 | 33 (80.5) | 25 (96.2) | 0.07 |
| Died full code | 10 (5.2) | 10 (6.4) | 0 (0) | 0.13 | 2 (4.9) | 0 (0) | 0.25 |
| Died DNR | 155 (81.2) | 123 (78.3) | 32 (94.1) | 0.03 | 27 (65.9) | 25 (96.2) | 0.004 |
| Hospice | 26 (13.6) | 24 (15.3) | 2 (5.9) | 0.15 | 12 (29.3) | 1 (3.9) | 0.01 |
The inpatient mortality was 17% (165/998). Most patients who died had the focus of care changed to comfort measures only (88%, 146/165). When examining the group of patients who died in the hospital with comfort care, we found that 58% (92/159) were transferred to the ICU following the MET call, 5% (8/159) were changed to comfort care during the MET call, and 18% (29/159) had a palliative care consult. We also observed that 16% (25/159) patients who died with comfort care were made DNR during MET activation. The inpatient mortality was significantly higher in patients with a change in code status compared to patients with no change in code status (44% vs 14%, 133/926, P<0.0001). Patients with a MET‐implemented DNR order had a higher inpatient mortality than patients with a preexisting DNR (43% vs 27%, P=0.04). Twenty‐five patients with a MET‐implemented DNR order died in the hospital. When examining a subgroup of patients who required end‐of‐life care (died or discharged from the hospital with hospice), we found patients with a MET‐implemented DNR order were less likely to be discharged with hospice care than patients with a preexisting DNR (4% vs 29%, P=0.01). There was no difference in the use of inpatient palliative care consultation at the end of life in patients with a preexisting DNR versus MET‐implemented DNR order (20% vs 12%, P=0.39). Patients with a MET‐implemented DNR order also had a significantly shorter median time from implementation of comfort care orders to death or discharge with hospice compared to patients with a preexisting DNR order (7 hours, interquartile range [IQR], 416 hours vs 22 hours, IQR 939 hours).
DISCUSSION
We observed a MET‐implemented DNR order in 5% of activations. Little is known about the role of METs in end‐of‐life discussions in the United States, and past experience has primarily come from Australian hospitals. Important differences in end‐of‐life care exist among different countries, particularly with regard to placing limitations on treatment.[9] Our observed rate is similar to the 3% to 10% rate of MET‐implemented DNR orders in previous reports worldwide.[3, 5, 8, 12, 13] Recent data from the United States suggest that METs initiate a DNR order in 28% of cases.[14] However, most DNR orders in that study were placed in the ICU days to weeks after MET activation, likely accounting for the high DNR rate. Our data add to the growing body of evidence that METs play an important role in end‐of‐life discussions among different countries throughout the world, including the United States.
To our knowledge, no prior study has evaluated the impact of code status discussions on MET resource utilization. Our MET spent 6 minutes longer on activations involving a change in code status when compared to activations with no changes made to code status. Presumably, some of this time was spent discussing goals of care. In our opinion, the additional time spent on these activations was invaluable, particularly when considering MET‐initiated end‐of‐life discussions may have prevented several unwanted resuscitations (25 patients died with a MET‐implemented DNR order). Interestingly, less than half of patients had code status discussions at the time of hospital admission. This finding suggests that clinicians could be more vigilant about discussing preferences for resuscitation at the time of admission in patients at risk for clinical deterioration. We suspect that in some cases code status discussions may have occurred between the patient and the primary service later in the patient's hospitalization, which were not captured in our study.
Surprisingly, when examining the use of hospital resources, we found no difference in the rate of unplanned ICU transfer in patients with a change in code status. In fact, we observed a higher rate of ICU transfer in patients with a MET‐implemented DNR order compared to those with a preexisting DNR order (36% vs 17%). These results were at odds with our hypothesis of a lower rate of ICU transfer in patients with MET‐implemented limitations in care. When compared to patients with a preexisting DNR order, patients with a MET‐implemented DNR order were younger, more commonly admitted from home, and had a lower Charlson index. Despite evidence of a lower burden of chronic illness and younger age, patients with a MET‐implemented DNR order had higher inpatient mortality than patients with a preexisting DNR order (43% vs 27%), suggesting an acute and rapidly progressive disease process. These observations may have compelled the MET to advocate for aggressive ICU‐level care in patients with a MET‐implemented DNR order. Another possible explanation for the relatively high rate of ICU transfer is that the MET is, in part, led by ICU staff. Thus, our MET may have made the decision to transfer the patient to the ICU and then subsequently initiated end‐of‐life discussions only after taking ownership of the patient. Furthermore, almost 20% of MET‐implemented changes to code status involved reversing status from DNR to full code. These data suggest that METs are not merely serving as a resource to review code status, but rather providing intensive treatment for acutely ill patients and simultaneously initiating end‐of‐life discussions in a population with a high inpatient mortality rate. The practice pattern observed in our study of transferring patients to the ICU for a trial of intensive therapy at the end of life is consistent with the overall trend in the United States for increased inpatient treatment intensity at the end of life.[14, 15, 16]
Our data suggest that the increased use of ICU resources in patients with a MET‐implemented DNR may be balanced by a shorter hospital length of stay following MET activation. In a multicenter observational study, Jones et al. found hospital length of stay to be similar in patients with and without a MET‐implemented limitation of medical therapy.[3] The authors did not examine length of stay specifically in patients with a DNR order, but rather examined patients with any limitation in medical therapy, including not for ICU admission. We suspect that the shorter length of stay following MET activation in our study was related to the fact that patients with a change in code status had a significantly higher inpatient mortality.
We observed several interesting findings with regard to end‐of‐life care following MET‐implemented DNR orders. First, the inpatient mortality in this population was remarkably high at 43%, compared to 27% in patients with a preexisting DNR order. Interestingly, there was no difference in the rate of palliative care consultation between the 2 groups despite the fact that all 25 patients who died following a MET‐implemented DNR order did so with a comfort measures only order. We also found that patients with a preexisting DNR also had a higher rate of discharge with hospice compared to patients with a MET‐implemented DNR order (29% vs 4%). Thus, our data suggest that inpatient palliative care consultation and hospice services are not resources that are routinely utilized in patients with MET‐initiated DNR orders. It may be the case that the acuity and severity of illness or patient preferences may have precluded the possibility of discharging some patients in our study home with hospice care or implementing comfort care earlier in the hospital course. Patients with MET‐implemented DNR orders were younger, had fewer comorbidities, and died sooner after comfort care orders were written. The overall rate of comfort care provided to patients who died was high at 88%. We have an inpatient comfort measures only order set at our hospital, which may account for the large proportion of patients receiving comfort care at the end of life. In addition, this order set may also help to improve the quality of end‐of‐life care and thus limit the need for palliative care consultation to some extent. However, we found that patients with a MET‐implemented DNR order had a shorter time from comfort care orders to death than patients with a preexisting DNR order. This finding suggests that patients with MET‐implemented DNR orders may have had comfort care implemented relatively late in the course of illness and had less‐than‐optimal end‐of‐life care. Vazquez et al. reported improved quality of end‐of‐life care after implementation of a MET.[10] However, an inpatient palliative care service was not available in that study, and it is not clear whether or not a comfort care order set was available. Evidence suggests that utilization of palliative care resources improves end‐of‐life care in the ICU.[17, 18, 19] We found that more than half of patients who died with comfort care in the hospital did so after being transferred to the ICU for a trial of aggressive care, suggesting that this population may have benefited from more involvement of our palliative care service. In summary, our data on end‐of‐life care following MET activation suggest that the METs are able to take advantage of an opportunity to identify patients who would not want resuscitation efforts because of personal preferences or futility of treatments. However, our surrogate measures of the quality of end‐of‐life care suggest that patients with MET‐implemented DNR orders may benefit from coordinated care with inpatient palliative care services, timelier implementation of comfort care orders, and possibly increased referrals for hospice care to help improve the quality of end‐of‐life care in this population.
Our study is subject to a number of limitations. This was a single‐center study, making the results difficult to generalize. The retrospective nature of our study makes it subject to the limitations inherent in this study design, including bias and confounding. The duration of MET activation was difficult to accurately and objectively measure and is subject to reporting bias. The event stop time, in particular, was subjectively measured by the MET nurse and is difficult to accurately assess, because MET members occasionally leave the bedside and return to reevaluate the response to therapy. We tried to account for this by clearly defining the MET stop time as the point at which MET members leave the bedside and transfer care back to the primary service or physically transfer the patient to a higher level of care. It also bears mentioning that the nurses performing data entry were not aware of the study hypothesis at the time of data entry. Despite including over 1100 MET calls in our analysis, the number of patients with a MET‐implemented DNR order was relatively small, which may have limited our ability to detect differences among subgroups during our analysis. We also did not document the clinical circumstances surrounding the MET‐implemented DNR order. Although we hypothesized that these patients had a higher mortality due to a higher acuity of illness, we were unable to support this hypothesis with the data available in our retrospective study. We did not record which providers were involved in code status discussions and the exact amount time spent on these discussions, making it difficult to accurately quantify the MET resources utilized on the calls. Our MET works closely with the patient's primary service, and it is possible that some of the changes to code status were implemented by the primary service and not MET providers. The patient's primary service may have a preexisting relationship with the patient and would be in a better position to discuss goals of care than MET providers who have had no prior relationship with the patient. However, even in this case, the clinical deterioration prompting MET activation was likely the event that triggered end‐of‐life discussions. Prospective studies would be helpful not only to identify the individuals involved in code status discussions during MET activations, but also to objectively measure the time spent on such discussions. Finally, our study population consisted primarily of Caucasian patients. Preferences for end‐of‐life care may differ among socioeconomic and ethnic groups, thus limiting the generalizability of our study findings.[20, 21]
In conclusion, we found the rate of MET‐implemented DNR orders in the United States to be similar to that of previous reports in other countries. MET events involving a change in code status are associated with increased utilization of MET and ICU resources, but a shorter hospital length of stay. Despite a high inpatient mortality rate, patients with a MET‐implemented DNR had a relatively low utilization of end‐of‐life resources, including palliative care and home hospice services. Coordinated care between METs and palliative care may help to improve of end‐of‐life care in patients with a change in code status following MET activation.
Acknowledgements
The authors acknowledge the hard work and dedication provided by Elizabeth Spellman during the data collection process.
Disclosures: This work was performed at Lahey Hospital and Medical Center. The authors report no conflicts of interest.
Approximately 15% to 20% of inpatients develop significant adverse events, which are often preceded by a change in the patient's condition.[1, 2] Many hospitals utilize medical emergency teams (METs) to deliver prompt care for deteriorating patients. METs have an emerging role in end‐of‐life care; up to 35% of patients who die in the hospital with a do not resuscitate (DNR) order have a MET activation during the admission.[3] Thus, METs are well positioned to discuss preferences for cardiopulmonary resuscitation in a population at high risk for cardiac arrest.[4]
MET activations involve a change in code status in approximately 3% to 10% of cases.[5, 6, 7, 8] However, previous studies primarily involved hospitals in Australia, making the results difficult to generalize to other countries.[3, 6, 7] There may be important cultural differences in patient and clinician attitudes toward end‐of‐life care among different countries.[9] In addition, little is known about METs and hospital resources utilized in patients with MET‐implemented changes in code status. Anecdotal evidence suggests that MET activations addressing code status are more time consuming.[3] Conversely, by identifying patients unlikely to benefit from restorative care, METs may facilitate end‐of‐life comfort care, thereby reducing unplanned intensive care unit (ICU) admissions and hospital length of stay. Finally, preliminary evidence suggests that METs may improve the quality of end‐of‐life care.[10] However, the use of end‐of‐life resources, including inpatient palliative care consultation and hospice care following MET activation has not been well studied.
The purpose of our study was to examine the role of a US MET in end‐of‐life care. First, we assessed the proportion of MET calls that resulted in a new DNR order in a US hospital and compared this to previous reports in other countries. We also examined MET and hospital resource utilization in MET activations involving changes in code status by evaluating the duration of MET activations, the need for telemetry or ICU transfer, and hospital length of stay following MET activation. Finally, we explored the quality of end‐of‐life care in patients with MET‐implemented DNR orders by assessing the utilization of inpatient palliative care consultation and hospice care compared to patients with a preexisting DNR order.
MATERIALS AND METHODS
We conducted this study at Lahey Clinic, a 350‐bed academic, tertiary care center. The institutional review board approved this study and waived the need for informed consent. We performed a retrospective review of a prospectively collected MET registry. We included consecutive, adult (>18 years old) inpatient MET activations and excluded nonhospitalized patients. Data were recorded in an intranet registry at the time of the event by the MET nurse, including preexisting code status (full code or DNR), any change to the patient's code status (full code to DNR or DNR to full code) during the MET event, date of the event, disposition after the event (no transfer, transferred to ICU, or transfer to telemetry), and a description of MET interventions. The primary reason for the event was also recorded, including cardiovascular (systolic blood pressure <90 mm Hg, pulse <40 or >130 bpm), respiratory (respiratory rate <10 or >24 breaths per minute, need for noninvasive positive pressure ventilation, oxygen saturation <90%), neurologic (loss of consciousness, change in mental status, seizure, or suspected stroke), or clinical deterioration causing staff to become worried. MET activation occurs at our institution by utilizing a text paging system that records the date and time of the call, along with the patient's location. The event start time was recorded from the paging system. We considered the event stop time to be the time at which the patient is transferred to a different level of care (ie, ICU) or the MET members leave the bedside and transfer care back to the primary service. MET nurses recorded the duration of MET activation in the intranet database immediately following the activation. Data were collected from the medical record, including age, gender, race, medical insurance, religion, admission source (home, assisted living, rehabilitation, or other hospital), admission team (internal medicine or surgery), admission date and time, discharge or death date and time, disposition at discharge (home, rehabilitation, death with or without a DNR order, or hospice care), and admission diagnosis. We categorized patients discharged to a hospice bridge program as being discharged with hospice care. We also recorded whether or not code status was discussed at the time of admission. Our hospital policy requires that an advanced directives order (either full code or DNR) must be placed on all patients at the time of admission. However, we only considered a code status discussion to have occurred if there was explicit documentation of a code status discussion in the medical record at the time of hospital admission. Data from the time of hospital admission were collected to calculate a Charlson Comorbidity Index, a well‐validated predictor of mortality that uses a weighted sum of 17 medical conditions, with scores ranging from 0 to 37.[11] Higher scores indicate a greater burden of illness. We also recorded whether or not the inpatient palliative care service was consulted following MET activation by examining the medical record for a consult note. The inpatient palliative care service at our institution was implemented in 2005 and receives over 700 new inpatient consults per year. The most common reason for consultation is to assist the primary service with discussing goals of care and code status.
Our MET was established in 2005 and responds to approximately 30 to 40 events per 1000 admissions. There are 4 members on our MET, including a critical care nurse, a nursing supervisor, a respiratory therapist, and a team leader who, depending on a predetermined schedule, is either an attending hospital medicine physician, an attending critical care physician, a critical care training physician, or a critical care physician assistant.
The data were transferred from the intranet registry to an Excel (Microsoft Corp., Redmond, WA) spreadsheet for statistical analysis. The primary outcome was the proportion of activations resulting in a MET‐implemented DNR order. Secondary outcomes included the duration of MET activation, need for transfer to telemetry or the ICU, hospital length of stay following MET activation (time from the end of the MET activation to hospital discharge or death), and the frequency with which inpatient palliative care consultation and outpatient hospice care were utilized. For repeat MET activations in a single patient, we considered each MET activation as a separate event as the code status could potentially change more than once. We used SAS version 9.2 for Windows (SAS Institute Inc., Cary, NC) statistical analysis software for data analysis. The 2 method was used for categorical variables, and either a 2‐sample t test or Wilcoxon rank sum test was utilized for continuous variables. A P value 0.05 was considered significant.
RESULTS
We observed 1156 MET activations in 998 patients. The mean age was 67 years and 57% (565/998) were male (Table 1). The mean Charlson Comorbidity Index was 5.4. Most patients were admitted from home (76%, 760/998), to a medical service (72%, 720/998), and to a teaching service (73%, 732/998). Sepsis (11%, 109/998) and trauma (11%, 105/998) were the most common admission diagnoses. A cardiovascular abnormality was the most common (35%, 399/1156) reason for activation. A code status discussion was documented on admission in 44% (440/998) of all patients.
| Variable | Total, n=998 | No Change in Code Status During MET Activation, n=926 | MET‐Implemented Change in Code Status, n=72a | P Valueb | DNR Prior to MET Activation, n=100 | MET‐Implemented DNR Order, n=58 | P Valuec |
|---|---|---|---|---|---|---|---|
| |||||||
| Gender, male | 565 (56.6) | 527 (56.9) | 38 (52.8) | 0.50 | 52 (52.0) | 34 (58.6) | 0.42 |
| Age, yr, meanSTD | 6717 | 6717 | 7115 | 0.08 | 8111 | 7016 | <0.0001 |
| Race | |||||||
| Caucasian | 927 (92.9) | 859 (92.8) | 68(94.4) | 0.59 | 99 (99) | 54 (93.1) | 0.04 |
| Minorityd | 71 (7.1) | 67 (7.2) | 4(5.6) | 1 (1.0) | 4 (6.9) | ||
| Insurance | |||||||
| Medicare | 694 (69.5) | 635 (68.6) | 59 (81.9) | 0.02 | 89 (89.0) | 45 (77.6) | 0.05 |
| Private | 244 (24.5) | 235 (25.4) | 9 (12.5) | 0.01 | 10 (10.0) | 9 (15.5) | 0.30 |
| Medicaid | 41 (4.1) | 39 (4.2) | 2 (2.8) | 0.55 | 1 (1.0) | 2 (3.5) | 0.27 |
| None | 18 (1.8) | 17 (1.8) | 1 (1.4) | 0.78 | 0 (0) | 1 (1.7) | 0.19 |
| Religion | |||||||
| Christian | 748 (75.0) | 691 (74.6) | 57 (79.2) | 0.39 | 82 (82.0) | 46 (79.3) | 0.68 |
| None specified | 226 (22.7) | 213 (23.0) | 13 (18.1) | 0.33 | 14 (14.0) | 10 (17.2) | 0.58 |
| Other religions | 24 (2.4) | 22 (2.4) | 2 (2.7) | 0.83 | 4 (4.0) | 2 (3.5) | 0.86 |
| Admission diagnosis | |||||||
| Sepsis | 109 (10.9) | 100 (10.8) | 9 (12.5) | 0.66 | 11 (11.0) | 8 (13.8) | 0.60 |
| Trauma/fall | 105 (10.5) | 100 (10.8) | 5 (6.9) | 0.30 | 6 (6.0) | 5 (8.6) | 0.53 |
| Malignancy related | 79 (7.9) | 73 (7.9) | 6 (8.3) | 0.89 | 7 (7.0) | 4 (6.9) | 0.98 |
| Stroke | 47 (4.7) | 43 (4.6) | 4 (5.6) | 0.73 | 6 (6.0) | 4 (6.9) | 0.82 |
| Pneumonia | 46 (4.6) | 41 (4.4) | 5 (6.9) | 0.33 | 11 (11.0) | 3 (5.2) | 0.21 |
| Altered mental status | 43 (4.3) | 40 (4.3) | 3 (4.2) | 0.95 | 5 (5.0) | 3 (5.2) | 0.96 |
| Myocardial infarct | 42 (4.2) | 40 (4.3) | 2 (2.8) | 0.53 | 1 (1.0) | 1 (1.7) | 0.69 |
| Respiratory failure | 40 (4.0) | 36 (3.9) | 4 (5.6) | 0.49 | 2 (2.0) | 3 (5.2) | 0.27 |
| Arrhythmia | 37 (3.7) | 33 (3.6) | 4 (5.6) | 0.39 | 2 (2.0) | 3 (5.2) | 0.27 |
| Heart failure | 35 (3.5) | 33 (3.6) | 2 (2.8) | 0.72 | 10 (10.0) | 2 (3.5) | 0.13 |
| Other | 415 (41.6) | 387 (41.8) | 28 (38.9) | 0.63 | 39 (39.0) | 22 (37.9) | 0.89 |
| Admission type | |||||||
| Medical | 720 (72.1) | 662 (71.5) | 58 (80.6) | 0.10 | 91 (91.0) | 46 (79.3) | 0.04 |
| Surgical | 278 (27.9) | 264 (28.5) | 14 (19.4) | 9 (9.0) | 12 (20.7) | ||
| Admission source | |||||||
| Home | 760 (76.2) | 706 (76.2) | 54 (75.0) | 0.81 | 60 (60.0) | 44 (75.9) | 0.04 |
| Assisted Living | 29 (2.9) | 28(3.0) | 1 (1.4) | 0.43 | 9 (9.0) | 1 (1.7) | 0.07 |
| Nursing Home | 69 (6.9) | 65(7.0) | 4 (5.6) | 0.64 | 19 (19.0) | 1 (1.7) | <0.01 |
| Outside hospital | 139 (13.9) | 126(13.6) | 13 (18.1) | 0.29 | 12 (12.0) | 12 (20.7) | 0.14 |
| Other | 1 (0.1) | 0(0) | 1 (0.1) | 0.78 | 0 (0) | 0 | |
| Teaching service | 732 (73.4) | 678 (73.2) | 54 (75.0) | 0.74 | 84 (84.0) | 41 (70.7) | 0.05 |
| Code status discussed on admission | 440(44.1) | 397 (42.9) | 43 (59.7) | 0.01 | 70 (70.0) | 32 (55.2) | 0.06 |
| CCI, meanSTDe | 5.43.0 | 5.43.0 | 5.83.0 | 0.21 | 7.72.4 | 5.73.0 | <0.001 |
| MI | 226 (22.7) | 210 (22.7) | 16 (22.2) | 0.93 | 36 (36.0) | 13 (22.4) | 0.08 |
| Heart failure | 138 (13.8) | 127 (13.7) | 11 (15.3) | 0.71 | 28 (28.0) | 8 (13.8) | 0.04 |
| PVD | 90 (9.0) | 85 (9.2) | 5 (6.9) | 0.52 | 14 (14.0) | 4(6.9) | 0.18 |
| Stroke | 131 (13.1) | 121 (13.1) | 10 (13.9) | 0.84 | 30 (30.0) | 9 (15.5) | 0.04 |
| Dementia | 58 (5.8) | 51(5.5) | 7 (9.7) | 0.14 | 19 (19.0) | 5 (8.6) | 0.08 |
| COPD | 173 (17.3) | 161 (17.4) | 12 (16.7) | 0.88 | 23 (23.0) | 8 (13.8) | 0.16 |
| CTD | 58 (5.8) | 56 (6.1) | 2 (2.8) | 0.25 | 6 (6.0) | 2 (3.5) | 0.48 |
| Peptic ulcer disease | 26 (2.6) | 25 (2.7) | 1 (1.4) | 0.50 | 2 (2.0) | 1 (1.7) | 0.90 |
| Mild liver disease | 33 (3.3) | 32 (3.5) | 1 (1.4) | 0.34 | 1 (1.0) | 1 (1.7) | 0.69 |
| DM | 213 (21.3) | 194 (21.0) | 19 (16.4) | 0.28 | 19 (19.0) | 15 (25.9) | 0.31 |
| Hemiplegia | 18 (1.8) | 17 (1.8) | 1 (1.4) | 0.78 | 2 (2.0) | 1 (1.7) | 0.90 |
| Renal disease | 131 (13.1) | 119 (12.9) | 12 (16.7) | 0.36 | 21 (21.0) | 9 (15.5) | 0.40 |
| DM+organ damage | 68 (6.8) | 64 (6.9) | 4 (5.6) | 0.66 | 7 (7.0) | 3 (5.2) | 0.65 |
| Any tumor | 188 (18.8) | 173 (18.7) | 15 (20.8) | 0.65 | 25 (25.0) | 14 (24.1) | 0.90 |
| Lymphoma | 21 (2.1) | 20 (2.2) | 1 (1.4) | 0.66 | 2 (2.0) | 1 (1.7) | 0.90 |
| Leukemia | 20 (2.0) | 18 (1.9) | 2 (2.8) | 0.63 | 1 (1.0) | 0 (0.0) | 0.45 |
| Moderate/severe liver disease | 45 (4.5) | 39 (4.2) | 6 (8.3) | 0.10 | 0 (0.0) | 6 (10.3) | 0.001 |
| Metastatic tumor | 61 (6.1) | 51 (5.5) | 10 (13.9) | 0.004 | 10 (10.0) | 8 (13.8) | 0.47 |
| AIDS | 4 (0.4) | 3 (0.3) | 1 (1.4) | 0.17 | 0 (0.0) | 1 (1.7) | 0.19 |
MET activation resulted in a DNR order in 5% (58/1156) of cases (Figure 1). In activations involving a change in code status, 21% (15/73) were changed from DNR to full code. When compared to patients with a preexisting DNR order, patients with a MET‐implemented DNR order where younger (70 vs 81 years, P<0.0001), more commonly admitted from home (60% vs 44%, P=0.04), less frequently from a nursing home (1% vs 9%, P<0.01), and had a lower Charlson index (5.7 vs 7.7, P<0.001) (Table 1). Moderate to severe liver disease was more common in patients with a MET‐implemented DNR order (10% vs 0%, P=0.001). Admission diagnoses were similar between patients with a preexisting DNR and a MET‐implemented DNR order (Table 1).

The median time spent on activations with a change in code status was significantly longer than activations without a change (66 vs 60 minutes, P=0.05). The rates of telemetry (6% vs 3%, P=0.24) and ICU transfer (40% vs 41%, P=0.8) were similar between patients with a change in code status and patients without a change (Table 2). Patients with a MET‐implemented DNR order were more frequently transferred to the ICU than patients with a preexisting DNR order (36% vs 17%, P<0.01). The median hospital length of stay following MET activation was shorter in patients with a change in code status compared to patients with no change (3 vs 5 days, P<0.0001).
| Variable | Total, N=1,156 | No Change in Code Status During MET Activation, n=1,083 | MET‐Implemented Change in Code Status, n=73a | PValuec | DNR Prior to MET Activation, n=115 | MET‐Implemented DNR Order, n=58 | P Valued |
|---|---|---|---|---|---|---|---|
| |||||||
| Reason for callb | |||||||
| Cardiovascular | 399 (34.5) | 379 (35.0) | 20 (27.4) | 0.19 | 39 (33.9) | 19 (32.8) | 0.88 |
| Respiratory | 319 (27.6) | 295 (27.2) | 2 (32.9) | 0.30 | 40 (34.8) | 18 (31.0) | 0.62 |
| Neurologic | 215 (18.6) | 196 (18.1) | 19 (26.0) | 0.09 | 21 (18.3) | 15 (25.9) | 0.25 |
| Other | 323 (27.9) | 303 (28.0) | 20 (27.4) | 0.92 | 22 (19.1) | 15 (25.9) | 0.31 |
| MET resources, call duration, min, median (IQR) | 60 (4090) | 60 (4090) | 66 (43100) | 0.05 | 50 (3075) | 67 (50100) | <0.001 |
| Hospital resources | |||||||
| Tele transfer | 68 (5.9) | 663 (6.1) | 2 (2.7) | 0.24 | 3 (2.6) | 2(3.5) | 0.76 |
| ICU transfer | 459 (39.7) | 429 (39.6) | 30 (41.1) | 0.8 | 19 (16.5) | 21 (36.2) | <0.01 |
| LOS after MET activation, d, median (IQR) | 5.2 (0.2510.7) | 5 (5.411.0) | 2.8 (0.66.7) | <0.0001 | 3.8 (1.56.5) | 2.9 (0.56.5) | 0.06 |
| End‐of‐life care | n=191e | n=157 | n=34 | n=41 | n=26 | ||
| Palliative care | 31 (16.2) | 27 (17.2) | 4 (11.8) | 0.44 | 8 (19.5) | 3 (11.5) | 0.39 |
| CMO orders | 159 (83.3) | 127 (80.9) | 32 (94.1) | 0.06 | 33 (80.5) | 25 (96.2) | 0.07 |
| Died full code | 10 (5.2) | 10 (6.4) | 0 (0) | 0.13 | 2 (4.9) | 0 (0) | 0.25 |
| Died DNR | 155 (81.2) | 123 (78.3) | 32 (94.1) | 0.03 | 27 (65.9) | 25 (96.2) | 0.004 |
| Hospice | 26 (13.6) | 24 (15.3) | 2 (5.9) | 0.15 | 12 (29.3) | 1 (3.9) | 0.01 |
The inpatient mortality was 17% (165/998). Most patients who died had the focus of care changed to comfort measures only (88%, 146/165). When examining the group of patients who died in the hospital with comfort care, we found that 58% (92/159) were transferred to the ICU following the MET call, 5% (8/159) were changed to comfort care during the MET call, and 18% (29/159) had a palliative care consult. We also observed that 16% (25/159) patients who died with comfort care were made DNR during MET activation. The inpatient mortality was significantly higher in patients with a change in code status compared to patients with no change in code status (44% vs 14%, 133/926, P<0.0001). Patients with a MET‐implemented DNR order had a higher inpatient mortality than patients with a preexisting DNR (43% vs 27%, P=0.04). Twenty‐five patients with a MET‐implemented DNR order died in the hospital. When examining a subgroup of patients who required end‐of‐life care (died or discharged from the hospital with hospice), we found patients with a MET‐implemented DNR order were less likely to be discharged with hospice care than patients with a preexisting DNR (4% vs 29%, P=0.01). There was no difference in the use of inpatient palliative care consultation at the end of life in patients with a preexisting DNR versus MET‐implemented DNR order (20% vs 12%, P=0.39). Patients with a MET‐implemented DNR order also had a significantly shorter median time from implementation of comfort care orders to death or discharge with hospice compared to patients with a preexisting DNR order (7 hours, interquartile range [IQR], 416 hours vs 22 hours, IQR 939 hours).
DISCUSSION
We observed a MET‐implemented DNR order in 5% of activations. Little is known about the role of METs in end‐of‐life discussions in the United States, and past experience has primarily come from Australian hospitals. Important differences in end‐of‐life care exist among different countries, particularly with regard to placing limitations on treatment.[9] Our observed rate is similar to the 3% to 10% rate of MET‐implemented DNR orders in previous reports worldwide.[3, 5, 8, 12, 13] Recent data from the United States suggest that METs initiate a DNR order in 28% of cases.[14] However, most DNR orders in that study were placed in the ICU days to weeks after MET activation, likely accounting for the high DNR rate. Our data add to the growing body of evidence that METs play an important role in end‐of‐life discussions among different countries throughout the world, including the United States.
To our knowledge, no prior study has evaluated the impact of code status discussions on MET resource utilization. Our MET spent 6 minutes longer on activations involving a change in code status when compared to activations with no changes made to code status. Presumably, some of this time was spent discussing goals of care. In our opinion, the additional time spent on these activations was invaluable, particularly when considering MET‐initiated end‐of‐life discussions may have prevented several unwanted resuscitations (25 patients died with a MET‐implemented DNR order). Interestingly, less than half of patients had code status discussions at the time of hospital admission. This finding suggests that clinicians could be more vigilant about discussing preferences for resuscitation at the time of admission in patients at risk for clinical deterioration. We suspect that in some cases code status discussions may have occurred between the patient and the primary service later in the patient's hospitalization, which were not captured in our study.
Surprisingly, when examining the use of hospital resources, we found no difference in the rate of unplanned ICU transfer in patients with a change in code status. In fact, we observed a higher rate of ICU transfer in patients with a MET‐implemented DNR order compared to those with a preexisting DNR order (36% vs 17%). These results were at odds with our hypothesis of a lower rate of ICU transfer in patients with MET‐implemented limitations in care. When compared to patients with a preexisting DNR order, patients with a MET‐implemented DNR order were younger, more commonly admitted from home, and had a lower Charlson index. Despite evidence of a lower burden of chronic illness and younger age, patients with a MET‐implemented DNR order had higher inpatient mortality than patients with a preexisting DNR order (43% vs 27%), suggesting an acute and rapidly progressive disease process. These observations may have compelled the MET to advocate for aggressive ICU‐level care in patients with a MET‐implemented DNR order. Another possible explanation for the relatively high rate of ICU transfer is that the MET is, in part, led by ICU staff. Thus, our MET may have made the decision to transfer the patient to the ICU and then subsequently initiated end‐of‐life discussions only after taking ownership of the patient. Furthermore, almost 20% of MET‐implemented changes to code status involved reversing status from DNR to full code. These data suggest that METs are not merely serving as a resource to review code status, but rather providing intensive treatment for acutely ill patients and simultaneously initiating end‐of‐life discussions in a population with a high inpatient mortality rate. The practice pattern observed in our study of transferring patients to the ICU for a trial of intensive therapy at the end of life is consistent with the overall trend in the United States for increased inpatient treatment intensity at the end of life.[14, 15, 16]
Our data suggest that the increased use of ICU resources in patients with a MET‐implemented DNR may be balanced by a shorter hospital length of stay following MET activation. In a multicenter observational study, Jones et al. found hospital length of stay to be similar in patients with and without a MET‐implemented limitation of medical therapy.[3] The authors did not examine length of stay specifically in patients with a DNR order, but rather examined patients with any limitation in medical therapy, including not for ICU admission. We suspect that the shorter length of stay following MET activation in our study was related to the fact that patients with a change in code status had a significantly higher inpatient mortality.
We observed several interesting findings with regard to end‐of‐life care following MET‐implemented DNR orders. First, the inpatient mortality in this population was remarkably high at 43%, compared to 27% in patients with a preexisting DNR order. Interestingly, there was no difference in the rate of palliative care consultation between the 2 groups despite the fact that all 25 patients who died following a MET‐implemented DNR order did so with a comfort measures only order. We also found that patients with a preexisting DNR also had a higher rate of discharge with hospice compared to patients with a MET‐implemented DNR order (29% vs 4%). Thus, our data suggest that inpatient palliative care consultation and hospice services are not resources that are routinely utilized in patients with MET‐initiated DNR orders. It may be the case that the acuity and severity of illness or patient preferences may have precluded the possibility of discharging some patients in our study home with hospice care or implementing comfort care earlier in the hospital course. Patients with MET‐implemented DNR orders were younger, had fewer comorbidities, and died sooner after comfort care orders were written. The overall rate of comfort care provided to patients who died was high at 88%. We have an inpatient comfort measures only order set at our hospital, which may account for the large proportion of patients receiving comfort care at the end of life. In addition, this order set may also help to improve the quality of end‐of‐life care and thus limit the need for palliative care consultation to some extent. However, we found that patients with a MET‐implemented DNR order had a shorter time from comfort care orders to death than patients with a preexisting DNR order. This finding suggests that patients with MET‐implemented DNR orders may have had comfort care implemented relatively late in the course of illness and had less‐than‐optimal end‐of‐life care. Vazquez et al. reported improved quality of end‐of‐life care after implementation of a MET.[10] However, an inpatient palliative care service was not available in that study, and it is not clear whether or not a comfort care order set was available. Evidence suggests that utilization of palliative care resources improves end‐of‐life care in the ICU.[17, 18, 19] We found that more than half of patients who died with comfort care in the hospital did so after being transferred to the ICU for a trial of aggressive care, suggesting that this population may have benefited from more involvement of our palliative care service. In summary, our data on end‐of‐life care following MET activation suggest that the METs are able to take advantage of an opportunity to identify patients who would not want resuscitation efforts because of personal preferences or futility of treatments. However, our surrogate measures of the quality of end‐of‐life care suggest that patients with MET‐implemented DNR orders may benefit from coordinated care with inpatient palliative care services, timelier implementation of comfort care orders, and possibly increased referrals for hospice care to help improve the quality of end‐of‐life care in this population.
Our study is subject to a number of limitations. This was a single‐center study, making the results difficult to generalize. The retrospective nature of our study makes it subject to the limitations inherent in this study design, including bias and confounding. The duration of MET activation was difficult to accurately and objectively measure and is subject to reporting bias. The event stop time, in particular, was subjectively measured by the MET nurse and is difficult to accurately assess, because MET members occasionally leave the bedside and return to reevaluate the response to therapy. We tried to account for this by clearly defining the MET stop time as the point at which MET members leave the bedside and transfer care back to the primary service or physically transfer the patient to a higher level of care. It also bears mentioning that the nurses performing data entry were not aware of the study hypothesis at the time of data entry. Despite including over 1100 MET calls in our analysis, the number of patients with a MET‐implemented DNR order was relatively small, which may have limited our ability to detect differences among subgroups during our analysis. We also did not document the clinical circumstances surrounding the MET‐implemented DNR order. Although we hypothesized that these patients had a higher mortality due to a higher acuity of illness, we were unable to support this hypothesis with the data available in our retrospective study. We did not record which providers were involved in code status discussions and the exact amount time spent on these discussions, making it difficult to accurately quantify the MET resources utilized on the calls. Our MET works closely with the patient's primary service, and it is possible that some of the changes to code status were implemented by the primary service and not MET providers. The patient's primary service may have a preexisting relationship with the patient and would be in a better position to discuss goals of care than MET providers who have had no prior relationship with the patient. However, even in this case, the clinical deterioration prompting MET activation was likely the event that triggered end‐of‐life discussions. Prospective studies would be helpful not only to identify the individuals involved in code status discussions during MET activations, but also to objectively measure the time spent on such discussions. Finally, our study population consisted primarily of Caucasian patients. Preferences for end‐of‐life care may differ among socioeconomic and ethnic groups, thus limiting the generalizability of our study findings.[20, 21]
In conclusion, we found the rate of MET‐implemented DNR orders in the United States to be similar to that of previous reports in other countries. MET events involving a change in code status are associated with increased utilization of MET and ICU resources, but a shorter hospital length of stay. Despite a high inpatient mortality rate, patients with a MET‐implemented DNR had a relatively low utilization of end‐of‐life resources, including palliative care and home hospice services. Coordinated care between METs and palliative care may help to improve of end‐of‐life care in patients with a change in code status following MET activation.
Acknowledgements
The authors acknowledge the hard work and dedication provided by Elizabeth Spellman during the data collection process.
Disclosures: This work was performed at Lahey Hospital and Medical Center. The authors report no conflicts of interest.
- , , , et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348(26):2635–2645.
- , , , et al. Antecedents to hospital deaths. Intern Med J. 2001;31(6):343–348.
- , , , et al. The role of the medical emergency team in end‐of‐life care: a multicenter, prospective, observational study. Crit Care Med. 2012;40(1):98–103.
- , , . Rapid‐response teams. N Engl J Med. 2011;365(2):139–146.
- , , , et al. The impact of Rapid Response System on delayed emergency team activation patient characteristics and outcomes—a follow‐up study. Resuscitation. 2010;81(1):31–35.
- , , , et al. Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial. Lancet. 2005;365(9477):2091–2097.
- , , , et al. Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study. BMJ. 2002;324(7334):387–390.
- , , , et al. The Medical Emergency Team System and not‐for‐resuscitation orders: results from the MERIT study. Resuscitation. 2008;79(3):391–397.
- . Cultural differences in end‐of‐life care. Crit Care Med. 2001;29(2 Suppl):N52–N55.
- , , , et al. Enhanced end‐of‐life care associated with deploying a rapid response team: a pilot study. J Hosp Med. 2009;4(7):449–452.
- , , , et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- , , , et al. The medical emergency team and end‐of‐life care: a pilot study. Crit Care Resusc. 2007;9(2):151–156.
- , , , et al. A retrospective cohort study of the effect of medical emergency teams on documentation of advance care directives. Crit Care Resusc. 2011;13(3):167–174.
- , , , et al. The medical emergency team a call: a sentinel event that triggers goals of care discussion. Crit Care Med. 2014;42(2):322–327.
- , , , et al. Change in end‐of‐life care for Medicare beneficiaries: site of death, place of care, and healthcare transitions in 2000, 2005, and 2009. JAMA. 2013;309(5):470–477.
- , , , et al. Trends in inpatient treatment intensity among Medicare beneficiaries at the end of life. Health Serv Res. 2004;39(2):363–375.
- , , , et al. Proactive palliative care in the medical intensive care unit: effects on length of stay for selected high‐risk patients. Crit Care Med. 2007;35(6):1530–1535.
- , . Impact of a proactive approach to improve end‐of‐life care in a medical ICU. Chest. 2003;123(1):266–271.
- , , , et al. In their own words: patients and families define high‐quality palliative care in the intensive care unit. Crit Care Med. 2010;38(3):808–818.
- , , , et al. The influence of race/ethnicity and socioeconomic status on end‐of‐life care in the ICU. Chest. 2011;139(5):1025–1033.
- , , , et al. Racial and ethnic differences in advance care planning among patients with cancer: impact of terminal illness acknowledgment, religiousness, and treatment preferences. J Clin Oncol. 2008;26(25):4131–4137.
- , , , et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348(26):2635–2645.
- , , , et al. Antecedents to hospital deaths. Intern Med J. 2001;31(6):343–348.
- , , , et al. The role of the medical emergency team in end‐of‐life care: a multicenter, prospective, observational study. Crit Care Med. 2012;40(1):98–103.
- , , . Rapid‐response teams. N Engl J Med. 2011;365(2):139–146.
- , , , et al. The impact of Rapid Response System on delayed emergency team activation patient characteristics and outcomes—a follow‐up study. Resuscitation. 2010;81(1):31–35.
- , , , et al. Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial. Lancet. 2005;365(9477):2091–2097.
- , , , et al. Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study. BMJ. 2002;324(7334):387–390.
- , , , et al. The Medical Emergency Team System and not‐for‐resuscitation orders: results from the MERIT study. Resuscitation. 2008;79(3):391–397.
- . Cultural differences in end‐of‐life care. Crit Care Med. 2001;29(2 Suppl):N52–N55.
- , , , et al. Enhanced end‐of‐life care associated with deploying a rapid response team: a pilot study. J Hosp Med. 2009;4(7):449–452.
- , , , et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- , , , et al. The medical emergency team and end‐of‐life care: a pilot study. Crit Care Resusc. 2007;9(2):151–156.
- , , , et al. A retrospective cohort study of the effect of medical emergency teams on documentation of advance care directives. Crit Care Resusc. 2011;13(3):167–174.
- , , , et al. The medical emergency team a call: a sentinel event that triggers goals of care discussion. Crit Care Med. 2014;42(2):322–327.
- , , , et al. Change in end‐of‐life care for Medicare beneficiaries: site of death, place of care, and healthcare transitions in 2000, 2005, and 2009. JAMA. 2013;309(5):470–477.
- , , , et al. Trends in inpatient treatment intensity among Medicare beneficiaries at the end of life. Health Serv Res. 2004;39(2):363–375.
- , , , et al. Proactive palliative care in the medical intensive care unit: effects on length of stay for selected high‐risk patients. Crit Care Med. 2007;35(6):1530–1535.
- , . Impact of a proactive approach to improve end‐of‐life care in a medical ICU. Chest. 2003;123(1):266–271.
- , , , et al. In their own words: patients and families define high‐quality palliative care in the intensive care unit. Crit Care Med. 2010;38(3):808–818.
- , , , et al. The influence of race/ethnicity and socioeconomic status on end‐of‐life care in the ICU. Chest. 2011;139(5):1025–1033.
- , , , et al. Racial and ethnic differences in advance care planning among patients with cancer: impact of terminal illness acknowledgment, religiousness, and treatment preferences. J Clin Oncol. 2008;26(25):4131–4137.
© 2014 Society of Hospital Medicine