User login
Effectiveness of an Employee Skin Cancer Screening Program for Secondary Prevention
The incidence of skin cancer, along with its effects on patients and the economy, has continued to increase and therefore requires particular attention from dermatologists. UV light has been shown to be of etiopathologic importance in the development of various types of skin cancer.1-3 Studies have shown that there is a direct correlation between the incidence of skin cancer and average annual amounts of UV radiation exposure.3 Accordingly, in 2009 the International Agency for Research on Cancer classified UV light as carcinogenic to humans.4 Therefore, the general public must be made aware of the danger of exposure to UV radiation.
In Australia, government initiatives to educate the population on causes of skin cancer development and its relationship to UV radiation have already caused the public to change their way of thinking and to deal with sunlight in a conscious and responsible manner.5 A large proportion of the Australian population with light skin is at a particularly high risk for developing skin cancer due to intense exposure to UV radiation. Numerous campaigns in Germany and other countries have attempted to sensitize the public to this issue by emphasizing a reduction in UV exposure (primary prevention) or highlighting the importance of early diagnosis (secondary prevention).6,7
For a good prognosis, it is crucial that skin cancer, particularly melanoma, is discovered at an early or precancerous stage.8 For this reason, self-examination of the skin and skin cancer screening are important factors that can contribute to ensuring early and curative treatment.9-11 Since July 1, 2008, skin cancer screenings have been included in the preventative health care program by statutory health insurance providers in Germany. As part of this program, the cost of screening once every 2 years for individuals 35 years and older is covered by statutory health insurance.12 Several studies have shown a decline in the melanoma mortality rate since the introduction of skin cancer screening programs in Germany.11,13,14
Employee skin cancer screening programs are an important method of examining high numbers of individuals quickly and effectively. These programs have been carried out in Germany and other countries.15,16 Studies have shown that skin cancer screening carried out selectively on defined groups can be an effective form of secondary prevention, particularly for those who work outdoors.17
An employee skin cancer screening program was carried out as part of this study. The findings are interpreted and discussed in relation to other employee screening programs that have been reported as well as those introduced by statutory health insurance providers in Germany. The aim of this study was to determine the importance and effectiveness of employee skin cancer screening programs and the role they play in secondary prevention of skin cancer.
Methods
Study Population
Employees of a technical company in Bavaria, Germany, were offered a skin cancer screening program by the employer’s occupational health service and health insurance provider in collaboration with the Department of Dermatology at the University Hospital Erlangen (Erlangen, Germany). Skin examinations were performed exclusively by 5 trained dermatologists. Only direct employees of the company at 3 of its locations in the Erlangen area were eligible to participate. The total number of employees varied by location (1072–5126 employees). The majority of employees had a university education or had completed technical training. Family members and other individuals who were not members of the company were excluded. There were no further inclusion or exclusion criteria. Over a period of 13 days, 783 of 7823 total employees (10.0%) were examined and included in the study. The study was approved by the Responsible Ethics Commission of the Faculty of Medicine at Friedrich-Alexander-University Erlangen-Nürnberg, Germany.
Study Design
Employees signed a consent form for participation in the study and completed a standardized questionnaire. The questionnaire was based on surveys used in a prior study18 and collected information on current and prior skin lesions, prior dermatological screening, personal and family history of skin tumors, frequency of UV exposure, and type of UV protection used. For the question on measures taken for protection from UV radiation, possible answers included with sunscreen cream, with suitable sun-protective clothing, and by staying in the shade, or no measures were taken. In contrast to the other questions, multiple answers were accepted for this question. Answering no automatically excluded other possible answers. Participants also were asked to assess their own Fitzpatrick skin type19; the questionnaire included explanations of each skin type (I–IV).
The participants were then called in for examination by the dermatologist at 15-minute intervals. All clothing was removed and the skin was examined. Dermatoscopes were used for closer examination of suspicious skin lesions. The clinical results of the examinations were recorded on a standardized form.
An estimation of the number of melanocytic nevi—≤20, 21–49, or ≥50—was recorded for each patient. Suspicious skin lesions were assigned to one of the following categories: nevus requiring future checkup (Nc), nevus requiring excision (Ne), suspected malignant melanoma (MM), suspected squamous cell carcinoma, suspected basal cell carcinoma (BCC), suspected other skin tumor, and precancerous lesion. Fitzpatrick skin type also was assessed for all participants and recorded by the dermatologist carrying out the examination. Each participant was assigned to a risk group—low, moderate, or high risk—based on their individual risk for developing a skin tumor. Factors that were considered when determining participants’ risk for developing skin cancer included Fitzpatrick skin type, number of melanocytic nevi, personal and family history, leisure activities, UV protection used, and current clinical diagnosis of skin lesions.
After the skin examination, participants were informed of recommended treatment but were not given any additional dermatologic advice. Participants could arrange an appointment at the Department of Dermatology, University Hospital Erlangen, for the excision and histological analysis of the skin lesions. All recorded data were collected in a computerized spreadsheet program. When evaluating the questionnaires, questions that were not answered or were answered incorrectly (participant chose more than 1 answer) were ignored.
Statistical Analysis
Statistical analysis was carried out using SPSS software version 16.0. The majority of the data were nominal or ordinal. Metric data were checked for normal distribution using the Shapiro-Wilk test before carrying out parametric tests. Statistical tests were carried out using the χ2 test and the t test for independent samples. Non-nominal distributed data were checked using the Mann-Whitney U test. P<.05 was considered statistically significant in the exploratory data analysis.
Results
Of 783 employees included in the study, 288 (36.8%) were female and 495 (63.2%) were male (Table 1). In comparison with the total workforce, a significantly higher proportion of women than men took part in the cross-sectional study (P<.01). The average age (SD) was 42.3 (9.5) years (range, 18–64 years). Female participants (average age [SD], 39.8 [10.2] years) were significantly younger than male participants (average age [SD], 43.8 [8.8] years; P<.01). Forty-one percent of participants had a prior skin cancer screening. One percent of participants had a personal history of skin cancer, with 1 participant reporting a history of MM; 6.5% had a family history of skin cancer, of which 39.2% had a family history of MM.
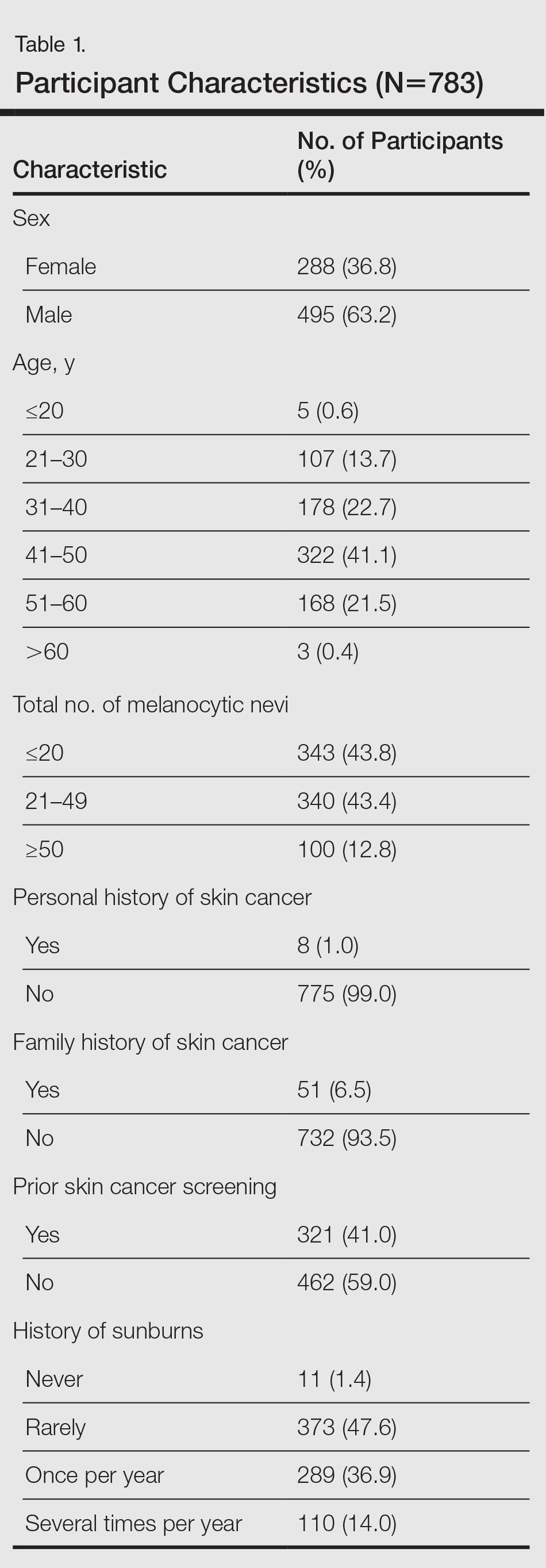
The results of the clinical examinations showed that 43.8% of participants had 20 or fewer melanocytic nevi, 43.4% had 21 to 49 melanocytic nevi, and 12.8% had 50 or more melanocytic nevi. Significantly more women than men had 20 or fewer melanocytic nevi (P<.05).
Approximately 92% of participants assessed themselves as having Fitzpatrick skin types II (35.2%) or III (56.7%), while only approximately 3.6% and 4.5% assessed themselves as having skin types I and IV, respectively. The results of the Fitzpatrick skin type assessments made by dermatologists were similar: 96.9% of participants were assessed as having Fitzpatrick skin types II (43.0%) and III (53.8%); approximately 1.9% and 1.3% were assessed as having Fitzpatrick skin types I and IV, respectively. Results showed that 80.2% of all participants assessed their skin type in the same way as the dermatologist; 13.5% assessed their skin type as darker and 6.3% (49/783) assessed it as lighter. A quantitative analysis of Fitzpatrick skin type and sex showed that significantly more male participants than female participants assessed their Fitzpatrick skin type darker than their actual skin type (P<.01).
Overall, 47.6% of participants reported having had sunburn rarely in the past, while 36.9% and 14.0% had experienced sunburn once per year and several times per year, respectively. Approximately 1.4% of participants reported never having a sunburn. More of the male participants made use of comprehensive sun protection using all methods listed (34.5%; P<.05) or a combination of sunscreen and sun-protective clothing (14.9%; P<.01) than the female participants who relied more frequently on sunscreen alone (29.5%; P<.01) or a combination of sunscreen and staying in the shade (29.5%; P<.01)
In general it was clear that sunscreen, either alone or in combination with other sun-protection methods, was used most frequently (88.0%); 58.0% protected themselves by staying in the shade, while 48.0% used suitable sun-protective clothing. Only 3.6% of participants did not protect themselves using any of the suggested methods.
A total of 661 categorized skin lesions were found in 377 participants. Of these lesions, 491 were Nc and 121 were Ne. Twenty-four of the skin lesions were suspected precancerous lesions, 13 were suspected BCC, 2 were suspected MM, and 10 were suspected other skin tumor (Table 2). Overall, male participants who were diagnosed with at least 1 skin lesion (average age, 44.0 years) were significantly older than the women (average age, 39.3 years)(P<.01). Similar findings were observed in participants with at least 1 Nc (men, 43.3 years; women, 38.7 years; P<.01) and at least 1 Ne (men, 44.2 years; women, 38.0 years; P<.05). With regard to the individual risk for developing skin cancer, 32.6% of participants were considered to be at low risk, 64.9% were at moderate risk, and 2.6% were at high risk.
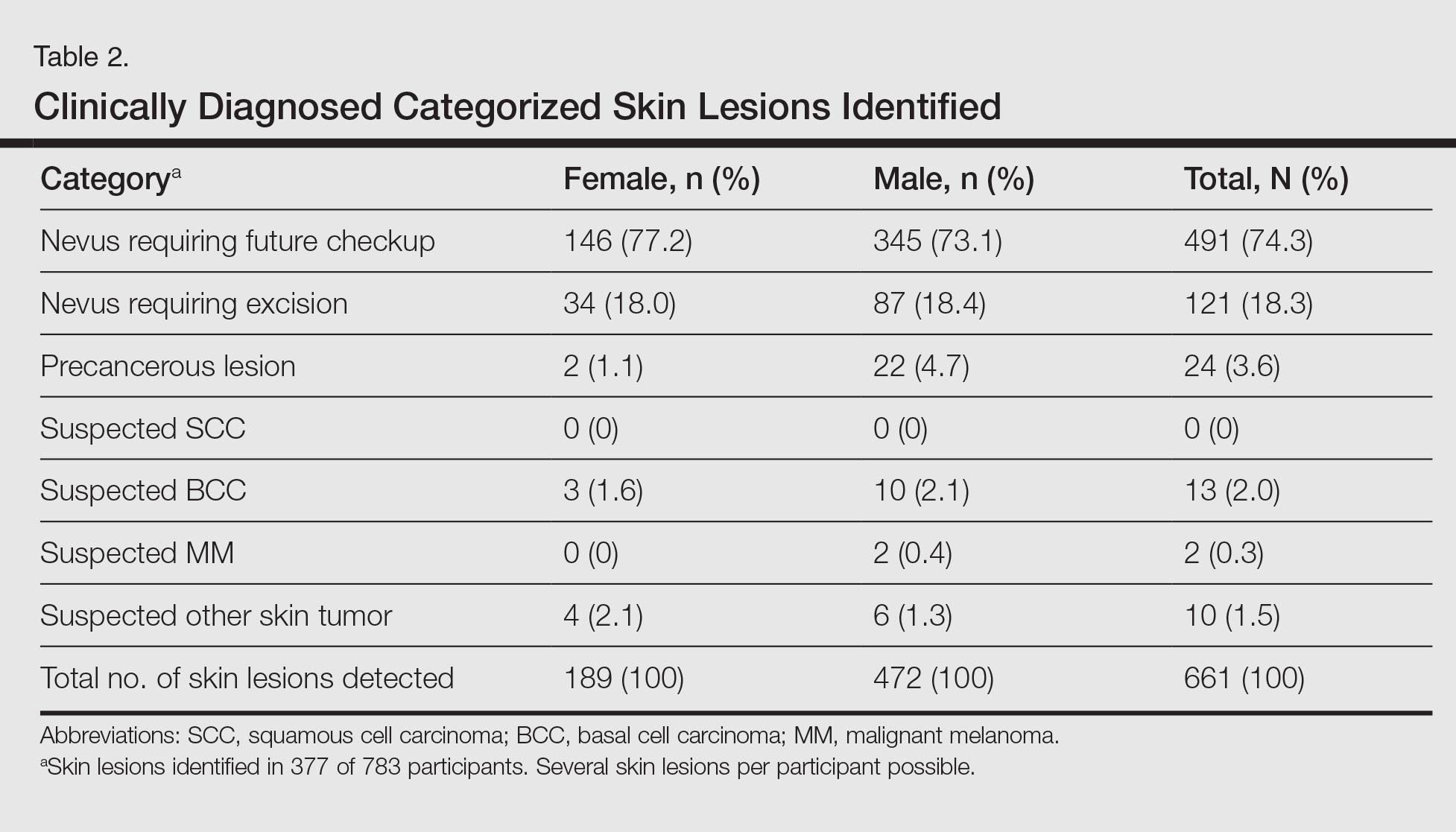
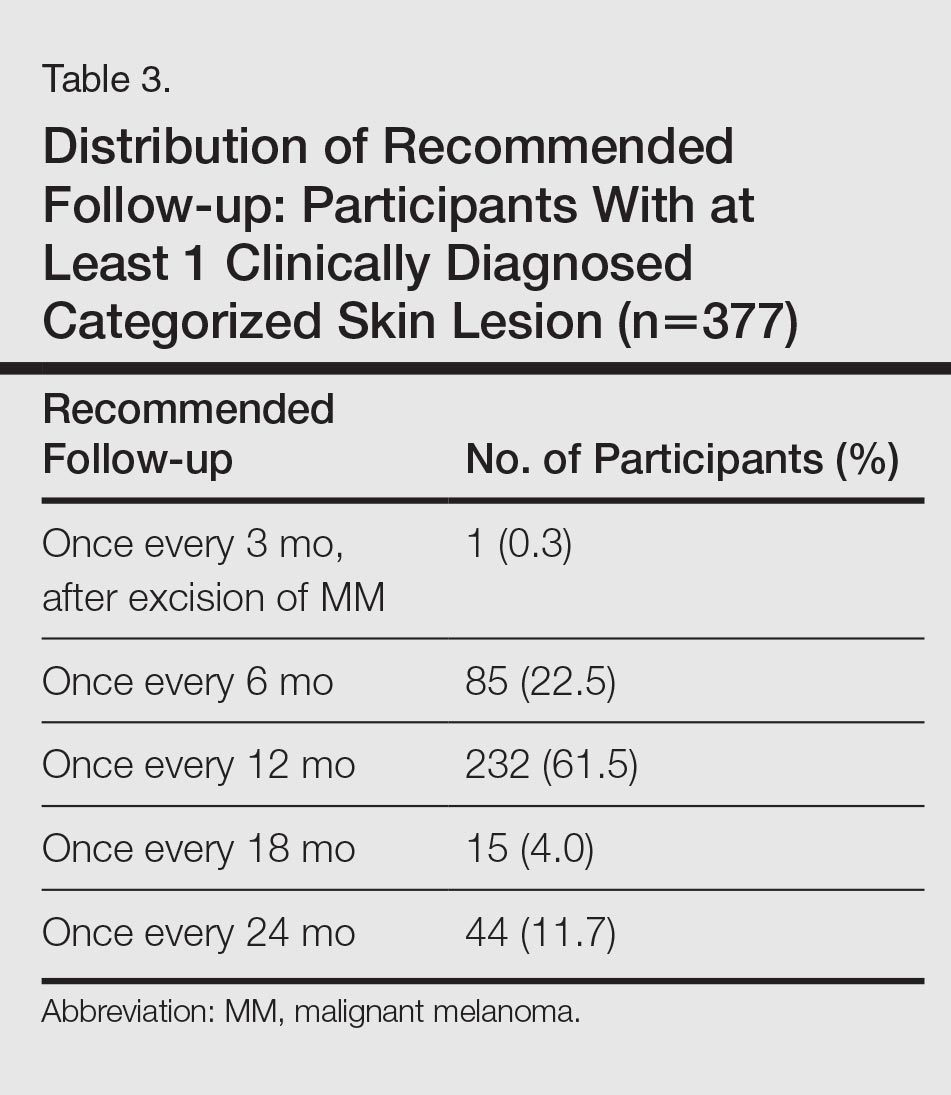
Approximately 61.5% of 377 participants who were diagnosed with at least 1 categorized skin lesion were advised to have a specific skin lesion checked by a dermatologist or to have a full examination for skin cancer once every 12 months. Furthermore, 22.5% were advised to follow-up biannually and 11.7% were advised to follow-up once every 2 years. Of the remaining participants who were advised to have follow-ups, 0.3% were advised to have a skin examination once every 3 months after having had MM, and 4.0% were advised to have follow-up once every 18 months. Overall, follow-up was recommended within 1 year in 84.4% of cases and within 1 to 2 years in 15.6% (Table 3).
Subsequent histological analysis of the excised tissue resulted in a diagnosis of only 21 clinically significant skin conditions. One case of Bowen disease and 1 case of BCC was confirmed. Histological analysis identified the remaining 19 excised skin lesions, which included the 2 suspected MMs, as dysplastic nevi.
Comment
The aim of this cross-sectional study was to examine the importance and effectiveness of employee skin cancer screening programs. In comparison with the total workforce, significantly more women took part than men. Female participants were significantly younger than male participants, which mirrors the findings of prior studies showing that screening programs reach women more frequently than men and that women who participate in screenings are also younger on average in comparison to men.7-13 Men and older individuals usually are underrepresented.7,13 The average age of participants in our study was 42.3 years, which is lower than in the SCREEN (Skin Cancer Research to Provide Evidence for Effectiveness of Screening in Northern Germany) study (average age, 49.7 years).13 The average age in our study also is likely to be lower than patients who undergo skin cancer screenings offered by statutory health insurance providers in Germany, which has a minimum age restriction of 35 years; however, it is comparable to the average age of participants in other employee screening programs and therefore represents the average age of individuals employed in Germany.15,16
The employee skin cancer screening program in this study generated a high level of interest, indicated by the fact that all available appointments had been booked just 36 hours after the screening was announced. Furthermore, there was a waiting list of approximately 300 employees who were not able to undergo a skin examination. For logistical reasons, the number of participants was limited to 10% of the workforce. The high level of interest is an indication of increased awareness of the importance of recognizing skin tumors early and the associated need for information as well as the need to undergo screening for skin cancer as a precaution. This observation also can be made with regard to the skin cancer screening introduced by statutory health insurance providers in Germany. Studies published by Augustin et al20 and Kornek et al21,22 confirm that skin cancer screenings have gained wide acceptance in Germany because they were introduced by statutory health insurance providers in 2008. The number of skin cancer screenings carried out by dermatologists in Germany also is increasing.20-22 Although approximately 19% of those eligible to participate took part in the SCREEN pilot project,13 approximately 31% of individuals who were eligible to participate took part in skin cancer screenings offered by statutory health insurance providers in Germany in 2012, and the percentage is rising.23 Two important factors affecting the high level of interest in the employee screening program used in our study were undoubtedly the advantages of the examination taking place during working hours and being held on the occupational health services’ premises in the workplace, which helped participants avoid the cost of travel and wait times associated with visiting a medical practice.
Of 783 participants included in this study, 377 displayed at least 1 categorized skin lesion; the majority were suspicious melanocytic nevi. This high incidence rate suggested that regular skin cancer screenings are useful, as it has been shown that there is a correlation between higher numbers of melanocytic nevi and increased risk for developing melanoma.24
In a study by Winkler et al,25 a skin cancer screening of 1658 bank and insurance employees found that 33.8% of those examined displayed at least 1 atypical melanocytic nevus and 27.2% displayed more than 50 melanocytic nevi (compared to 12.8% with ≥50 melanocytic nevi in the current study). The risk for developing skin cancer was classified as intermediate or high in 54.5% (compared to 67.5% at moderate or high risk in the current study).25 Therefore, the rate of suspicious skin lesions was lower in the population of the study by Winkler et al25 in comparison to the population of the current study. As the overall number of melanocytic nevi and the individual risk for skin cancer, however, was underestimated by the majority of the bank and insurance employees,25 employee skin cancer screening programs can be used as a potentially effective tool to make employees aware of the issue and sensitizing them to it. Employee screening in addition to a final diagnosis can contribute to ensuring suitable treatment is started. For example, in the large-scale employee screening published by Schaefer et al15 and Augustin et al,16 48,665 and 90,880 employees, respectively, were screened for inflammatory and noninflammatory skin diseases, and 19% and 27% of participants, respectively, were diagnosed with skin lesions that required treatment.
Participants in the current study were given no further treatment or advice. Recommendations were made that participants monitor suspicious skin lesions or have them removed. With regard to future screening, 84.4% of participants with at least 1 categorized skin lesion were advised to have a regular follow-up within 1 year, while 15.6% were advised to follow-up within 1 to 2 years. Therefore, a period of 2 years before the next checkup, the period between screenings offered by statutory health insurance providers in Germany,12 was considered too long for the majority of participants, according to the dermatologists involved with our study.
Conclusion
The high rate of suspicious skin lesions diagnosed demonstrated the effectiveness of skin cancer screenings organized in the workplace, which should be recommended for all employees, not only those who are at high risk for developing skin cancer due to the nature of their work, such as those who work outdoors. It should be noted that the study group examined in the current study was a homogeneous group of employees of a technical company only and is therefore relatively selective. Nevertheless, despite the comparatively selective and young participant group, these examinations provide evidence of the importance of skin cancer screening programs for a wider population.
Acknowledgments
The authors thank Heidi Seybold, MD; Petra Wörl, MD; Sybille Thoma-Uszynski, MD; and Jens Bussmann, MD (all from Erlangen, Germany), for their support and active assistance in the practical implementation of this study.
- Boniol M, Autier P, Boyle P, et al. Cutaneous melanoma attributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:e4757.
- Gilchrest BA, Eller MS, Geller AC, et al. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 1999;340:1341-1348.
- Rigel DS. Cutaneous ultraviolet exposure and its relationship to the development of skin cancer. J Am Acad Dermatol. 2008;58:129-132.
- El Ghissassi F, Baan R, Straif K, et al; WHO International Agency for Research on Cancer Monograph Working Group. A review of human carcinogens—part D: radiation. Lancet Oncol. 2009;10:751-752.
- MacLennan R, Green AC, McLeod GR, et al. Increasing incidence of cutaneous melanoma in Queensland, Australia. J Natl Cancer Inst. 1992;84:1427-1432.
- Heinzerling LM, Dummer R, Panizzon RG, et al. Prevention campaign against skin cancer. Dermatology. 2002;205:229-233.
- Stratigos A, Nikolaou V, Kedicoglou S, et al. Melanoma/skin cancer screening in a Mediterranean country: results of the Euromelanoma Screening Day Campaign in Greece. J Eur Acad Dermatol Venereol. 2007;21:56-62.
- Garbe C, Hauschild A, Volkenandt M, et al. Evidence and interdisciplinary consense-based German guidelines: diagnosis and surveillance of melanoma. Melanoma Res. 2007;17:393-399.
- Choudhury K, Volkmer B, Greinert R, et al. Effectiveness of skin cancer screening programmes. Br J Dermatol. 2012;167:94-98.
- Eisemann N, Waldmann A, Geller AC, et al. Non-melanoma skin cancer incidence and impact of skin cancer screening on incidence. J Invest Dermatol. 2014;134:43-50.
- Katalinic A, Waldmann A, Weinstock MA, et al. Does skin cancer screening save lives? an observational study comparing trends in melanoma mortality in regions with and without screening. Cancer. 2012;118:5395-5402.
- Bekanntmachung (1430 A) eines Beschlusses des Gemeinsamen Bundeausschusses über eine Änderung der Krebsfrüherkennungs-Richtlinien: Hautkrebs-Screening [press release]. Berlin, Germany: Bundesministerium für Gesundheit (Federal Ministry of Health, Germany); vom 15. November 2007.
- Breitbart EW, Waldmann A, Nolte S, et al. Systematic skin cancer screening in Northern Germany. J Am Acad Dermatol. 2012;66:201-211.
- Waldmann A, Nolte S, Weinstock MA, et al. Skin cancer screening participation and impact on melanoma incidence in Germany—an observational study on incidence trends in regions with and without population-based screening. Br J Cancer. 2012;106:970-974.
- Schaefer I, Rustenbach SJ, Zimmer L, et al. Prevalence of skin diseases in a cohort of 48,665 employees in Germany. Dermatology. 2008;217:169-172.
- Augustin M, Herberger K, Hintzen S, et al. Prevalence of skin lesions and need for treatment in a cohort of 90880 workers. Br J Dermatol. 2011;165:865-873.
- LeBlanc WG, Vidal L, Kirsner RS, et al. Reported skin cancer screening of US adult workers. J Am Acad Dermatol. 2008;59:55-63.
- Harbauer A, Binder M, Pehamberger H, et al. Validity of an unsupervised self-administered questionnaire for self-assessment of melanoma risk. Melanoma Res. 2003;13:537-542.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Augustin M, Stadler R, Reusch M, et al. Skin cancer screening in Germany—perception by the public. J Dtsch Dermatol Ges. 2012;10:42-49.
- Kornek T, Augustin M. Skin cancer prevention. J Dtsch Dermatol Ges. 2013;11:283-296.
- Kornek T, Schäfer I, Reusch M, et al. Routine skin cancer screening in Germany: four years of experience from the dermatologists’ perspective. Dermatology. 2012;225:289-293.
- Barmer GEK Arztreport 2014 [press release]. Berlin, Germany: Barmer GEK; February 4, 2014.
- Bauer J, Garbe C. Acquired melanocytic nevi as riskfactor for melanoma development. a comprehensive review of epidemiological data. Pigment Cell Res. 2003;16:297-306.
- Winkler A, Plugfelder A, Weide B, et al. Screening for skin cancer in bank and insurance employees: risk profile and correlation of self and physician’s assessment. Int J Dermatol. 2015;54:419-423.
The incidence of skin cancer, along with its effects on patients and the economy, has continued to increase and therefore requires particular attention from dermatologists. UV light has been shown to be of etiopathologic importance in the development of various types of skin cancer.1-3 Studies have shown that there is a direct correlation between the incidence of skin cancer and average annual amounts of UV radiation exposure.3 Accordingly, in 2009 the International Agency for Research on Cancer classified UV light as carcinogenic to humans.4 Therefore, the general public must be made aware of the danger of exposure to UV radiation.
In Australia, government initiatives to educate the population on causes of skin cancer development and its relationship to UV radiation have already caused the public to change their way of thinking and to deal with sunlight in a conscious and responsible manner.5 A large proportion of the Australian population with light skin is at a particularly high risk for developing skin cancer due to intense exposure to UV radiation. Numerous campaigns in Germany and other countries have attempted to sensitize the public to this issue by emphasizing a reduction in UV exposure (primary prevention) or highlighting the importance of early diagnosis (secondary prevention).6,7
For a good prognosis, it is crucial that skin cancer, particularly melanoma, is discovered at an early or precancerous stage.8 For this reason, self-examination of the skin and skin cancer screening are important factors that can contribute to ensuring early and curative treatment.9-11 Since July 1, 2008, skin cancer screenings have been included in the preventative health care program by statutory health insurance providers in Germany. As part of this program, the cost of screening once every 2 years for individuals 35 years and older is covered by statutory health insurance.12 Several studies have shown a decline in the melanoma mortality rate since the introduction of skin cancer screening programs in Germany.11,13,14
Employee skin cancer screening programs are an important method of examining high numbers of individuals quickly and effectively. These programs have been carried out in Germany and other countries.15,16 Studies have shown that skin cancer screening carried out selectively on defined groups can be an effective form of secondary prevention, particularly for those who work outdoors.17
An employee skin cancer screening program was carried out as part of this study. The findings are interpreted and discussed in relation to other employee screening programs that have been reported as well as those introduced by statutory health insurance providers in Germany. The aim of this study was to determine the importance and effectiveness of employee skin cancer screening programs and the role they play in secondary prevention of skin cancer.
Methods
Study Population
Employees of a technical company in Bavaria, Germany, were offered a skin cancer screening program by the employer’s occupational health service and health insurance provider in collaboration with the Department of Dermatology at the University Hospital Erlangen (Erlangen, Germany). Skin examinations were performed exclusively by 5 trained dermatologists. Only direct employees of the company at 3 of its locations in the Erlangen area were eligible to participate. The total number of employees varied by location (1072–5126 employees). The majority of employees had a university education or had completed technical training. Family members and other individuals who were not members of the company were excluded. There were no further inclusion or exclusion criteria. Over a period of 13 days, 783 of 7823 total employees (10.0%) were examined and included in the study. The study was approved by the Responsible Ethics Commission of the Faculty of Medicine at Friedrich-Alexander-University Erlangen-Nürnberg, Germany.
Study Design
Employees signed a consent form for participation in the study and completed a standardized questionnaire. The questionnaire was based on surveys used in a prior study18 and collected information on current and prior skin lesions, prior dermatological screening, personal and family history of skin tumors, frequency of UV exposure, and type of UV protection used. For the question on measures taken for protection from UV radiation, possible answers included with sunscreen cream, with suitable sun-protective clothing, and by staying in the shade, or no measures were taken. In contrast to the other questions, multiple answers were accepted for this question. Answering no automatically excluded other possible answers. Participants also were asked to assess their own Fitzpatrick skin type19; the questionnaire included explanations of each skin type (I–IV).
The participants were then called in for examination by the dermatologist at 15-minute intervals. All clothing was removed and the skin was examined. Dermatoscopes were used for closer examination of suspicious skin lesions. The clinical results of the examinations were recorded on a standardized form.
An estimation of the number of melanocytic nevi—≤20, 21–49, or ≥50—was recorded for each patient. Suspicious skin lesions were assigned to one of the following categories: nevus requiring future checkup (Nc), nevus requiring excision (Ne), suspected malignant melanoma (MM), suspected squamous cell carcinoma, suspected basal cell carcinoma (BCC), suspected other skin tumor, and precancerous lesion. Fitzpatrick skin type also was assessed for all participants and recorded by the dermatologist carrying out the examination. Each participant was assigned to a risk group—low, moderate, or high risk—based on their individual risk for developing a skin tumor. Factors that were considered when determining participants’ risk for developing skin cancer included Fitzpatrick skin type, number of melanocytic nevi, personal and family history, leisure activities, UV protection used, and current clinical diagnosis of skin lesions.
After the skin examination, participants were informed of recommended treatment but were not given any additional dermatologic advice. Participants could arrange an appointment at the Department of Dermatology, University Hospital Erlangen, for the excision and histological analysis of the skin lesions. All recorded data were collected in a computerized spreadsheet program. When evaluating the questionnaires, questions that were not answered or were answered incorrectly (participant chose more than 1 answer) were ignored.
Statistical Analysis
Statistical analysis was carried out using SPSS software version 16.0. The majority of the data were nominal or ordinal. Metric data were checked for normal distribution using the Shapiro-Wilk test before carrying out parametric tests. Statistical tests were carried out using the χ2 test and the t test for independent samples. Non-nominal distributed data were checked using the Mann-Whitney U test. P<.05 was considered statistically significant in the exploratory data analysis.
Results
Of 783 employees included in the study, 288 (36.8%) were female and 495 (63.2%) were male (Table 1). In comparison with the total workforce, a significantly higher proportion of women than men took part in the cross-sectional study (P<.01). The average age (SD) was 42.3 (9.5) years (range, 18–64 years). Female participants (average age [SD], 39.8 [10.2] years) were significantly younger than male participants (average age [SD], 43.8 [8.8] years; P<.01). Forty-one percent of participants had a prior skin cancer screening. One percent of participants had a personal history of skin cancer, with 1 participant reporting a history of MM; 6.5% had a family history of skin cancer, of which 39.2% had a family history of MM.
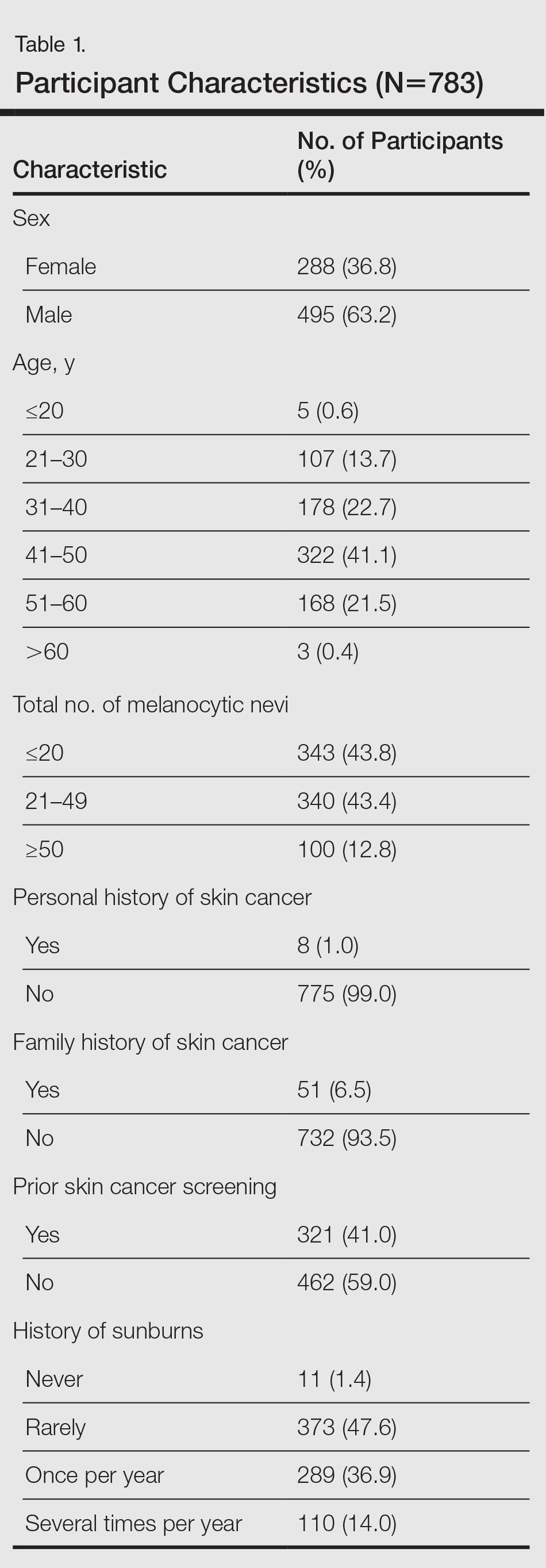
The results of the clinical examinations showed that 43.8% of participants had 20 or fewer melanocytic nevi, 43.4% had 21 to 49 melanocytic nevi, and 12.8% had 50 or more melanocytic nevi. Significantly more women than men had 20 or fewer melanocytic nevi (P<.05).
Approximately 92% of participants assessed themselves as having Fitzpatrick skin types II (35.2%) or III (56.7%), while only approximately 3.6% and 4.5% assessed themselves as having skin types I and IV, respectively. The results of the Fitzpatrick skin type assessments made by dermatologists were similar: 96.9% of participants were assessed as having Fitzpatrick skin types II (43.0%) and III (53.8%); approximately 1.9% and 1.3% were assessed as having Fitzpatrick skin types I and IV, respectively. Results showed that 80.2% of all participants assessed their skin type in the same way as the dermatologist; 13.5% assessed their skin type as darker and 6.3% (49/783) assessed it as lighter. A quantitative analysis of Fitzpatrick skin type and sex showed that significantly more male participants than female participants assessed their Fitzpatrick skin type darker than their actual skin type (P<.01).
Overall, 47.6% of participants reported having had sunburn rarely in the past, while 36.9% and 14.0% had experienced sunburn once per year and several times per year, respectively. Approximately 1.4% of participants reported never having a sunburn. More of the male participants made use of comprehensive sun protection using all methods listed (34.5%; P<.05) or a combination of sunscreen and sun-protective clothing (14.9%; P<.01) than the female participants who relied more frequently on sunscreen alone (29.5%; P<.01) or a combination of sunscreen and staying in the shade (29.5%; P<.01)
In general it was clear that sunscreen, either alone or in combination with other sun-protection methods, was used most frequently (88.0%); 58.0% protected themselves by staying in the shade, while 48.0% used suitable sun-protective clothing. Only 3.6% of participants did not protect themselves using any of the suggested methods.
A total of 661 categorized skin lesions were found in 377 participants. Of these lesions, 491 were Nc and 121 were Ne. Twenty-four of the skin lesions were suspected precancerous lesions, 13 were suspected BCC, 2 were suspected MM, and 10 were suspected other skin tumor (Table 2). Overall, male participants who were diagnosed with at least 1 skin lesion (average age, 44.0 years) were significantly older than the women (average age, 39.3 years)(P<.01). Similar findings were observed in participants with at least 1 Nc (men, 43.3 years; women, 38.7 years; P<.01) and at least 1 Ne (men, 44.2 years; women, 38.0 years; P<.05). With regard to the individual risk for developing skin cancer, 32.6% of participants were considered to be at low risk, 64.9% were at moderate risk, and 2.6% were at high risk.

Approximately 61.5% of 377 participants who were diagnosed with at least 1 categorized skin lesion were advised to have a specific skin lesion checked by a dermatologist or to have a full examination for skin cancer once every 12 months. Furthermore, 22.5% were advised to follow-up biannually and 11.7% were advised to follow-up once every 2 years. Of the remaining participants who were advised to have follow-ups, 0.3% were advised to have a skin examination once every 3 months after having had MM, and 4.0% were advised to have follow-up once every 18 months. Overall, follow-up was recommended within 1 year in 84.4% of cases and within 1 to 2 years in 15.6% (Table 3).

Subsequent histological analysis of the excised tissue resulted in a diagnosis of only 21 clinically significant skin conditions. One case of Bowen disease and 1 case of BCC was confirmed. Histological analysis identified the remaining 19 excised skin lesions, which included the 2 suspected MMs, as dysplastic nevi.
Comment
The aim of this cross-sectional study was to examine the importance and effectiveness of employee skin cancer screening programs. In comparison with the total workforce, significantly more women took part than men. Female participants were significantly younger than male participants, which mirrors the findings of prior studies showing that screening programs reach women more frequently than men and that women who participate in screenings are also younger on average in comparison to men.7-13 Men and older individuals usually are underrepresented.7,13 The average age of participants in our study was 42.3 years, which is lower than in the SCREEN (Skin Cancer Research to Provide Evidence for Effectiveness of Screening in Northern Germany) study (average age, 49.7 years).13 The average age in our study also is likely to be lower than patients who undergo skin cancer screenings offered by statutory health insurance providers in Germany, which has a minimum age restriction of 35 years; however, it is comparable to the average age of participants in other employee screening programs and therefore represents the average age of individuals employed in Germany.15,16
The employee skin cancer screening program in this study generated a high level of interest, indicated by the fact that all available appointments had been booked just 36 hours after the screening was announced. Furthermore, there was a waiting list of approximately 300 employees who were not able to undergo a skin examination. For logistical reasons, the number of participants was limited to 10% of the workforce. The high level of interest is an indication of increased awareness of the importance of recognizing skin tumors early and the associated need for information as well as the need to undergo screening for skin cancer as a precaution. This observation also can be made with regard to the skin cancer screening introduced by statutory health insurance providers in Germany. Studies published by Augustin et al20 and Kornek et al21,22 confirm that skin cancer screenings have gained wide acceptance in Germany because they were introduced by statutory health insurance providers in 2008. The number of skin cancer screenings carried out by dermatologists in Germany also is increasing.20-22 Although approximately 19% of those eligible to participate took part in the SCREEN pilot project,13 approximately 31% of individuals who were eligible to participate took part in skin cancer screenings offered by statutory health insurance providers in Germany in 2012, and the percentage is rising.23 Two important factors affecting the high level of interest in the employee screening program used in our study were undoubtedly the advantages of the examination taking place during working hours and being held on the occupational health services’ premises in the workplace, which helped participants avoid the cost of travel and wait times associated with visiting a medical practice.
Of 783 participants included in this study, 377 displayed at least 1 categorized skin lesion; the majority were suspicious melanocytic nevi. This high incidence rate suggested that regular skin cancer screenings are useful, as it has been shown that there is a correlation between higher numbers of melanocytic nevi and increased risk for developing melanoma.24
In a study by Winkler et al,25 a skin cancer screening of 1658 bank and insurance employees found that 33.8% of those examined displayed at least 1 atypical melanocytic nevus and 27.2% displayed more than 50 melanocytic nevi (compared to 12.8% with ≥50 melanocytic nevi in the current study). The risk for developing skin cancer was classified as intermediate or high in 54.5% (compared to 67.5% at moderate or high risk in the current study).25 Therefore, the rate of suspicious skin lesions was lower in the population of the study by Winkler et al25 in comparison to the population of the current study. As the overall number of melanocytic nevi and the individual risk for skin cancer, however, was underestimated by the majority of the bank and insurance employees,25 employee skin cancer screening programs can be used as a potentially effective tool to make employees aware of the issue and sensitizing them to it. Employee screening in addition to a final diagnosis can contribute to ensuring suitable treatment is started. For example, in the large-scale employee screening published by Schaefer et al15 and Augustin et al,16 48,665 and 90,880 employees, respectively, were screened for inflammatory and noninflammatory skin diseases, and 19% and 27% of participants, respectively, were diagnosed with skin lesions that required treatment.
Participants in the current study were given no further treatment or advice. Recommendations were made that participants monitor suspicious skin lesions or have them removed. With regard to future screening, 84.4% of participants with at least 1 categorized skin lesion were advised to have a regular follow-up within 1 year, while 15.6% were advised to follow-up within 1 to 2 years. Therefore, a period of 2 years before the next checkup, the period between screenings offered by statutory health insurance providers in Germany,12 was considered too long for the majority of participants, according to the dermatologists involved with our study.
Conclusion
The high rate of suspicious skin lesions diagnosed demonstrated the effectiveness of skin cancer screenings organized in the workplace, which should be recommended for all employees, not only those who are at high risk for developing skin cancer due to the nature of their work, such as those who work outdoors. It should be noted that the study group examined in the current study was a homogeneous group of employees of a technical company only and is therefore relatively selective. Nevertheless, despite the comparatively selective and young participant group, these examinations provide evidence of the importance of skin cancer screening programs for a wider population.
Acknowledgments
The authors thank Heidi Seybold, MD; Petra Wörl, MD; Sybille Thoma-Uszynski, MD; and Jens Bussmann, MD (all from Erlangen, Germany), for their support and active assistance in the practical implementation of this study.
The incidence of skin cancer, along with its effects on patients and the economy, has continued to increase and therefore requires particular attention from dermatologists. UV light has been shown to be of etiopathologic importance in the development of various types of skin cancer.1-3 Studies have shown that there is a direct correlation between the incidence of skin cancer and average annual amounts of UV radiation exposure.3 Accordingly, in 2009 the International Agency for Research on Cancer classified UV light as carcinogenic to humans.4 Therefore, the general public must be made aware of the danger of exposure to UV radiation.
In Australia, government initiatives to educate the population on causes of skin cancer development and its relationship to UV radiation have already caused the public to change their way of thinking and to deal with sunlight in a conscious and responsible manner.5 A large proportion of the Australian population with light skin is at a particularly high risk for developing skin cancer due to intense exposure to UV radiation. Numerous campaigns in Germany and other countries have attempted to sensitize the public to this issue by emphasizing a reduction in UV exposure (primary prevention) or highlighting the importance of early diagnosis (secondary prevention).6,7
For a good prognosis, it is crucial that skin cancer, particularly melanoma, is discovered at an early or precancerous stage.8 For this reason, self-examination of the skin and skin cancer screening are important factors that can contribute to ensuring early and curative treatment.9-11 Since July 1, 2008, skin cancer screenings have been included in the preventative health care program by statutory health insurance providers in Germany. As part of this program, the cost of screening once every 2 years for individuals 35 years and older is covered by statutory health insurance.12 Several studies have shown a decline in the melanoma mortality rate since the introduction of skin cancer screening programs in Germany.11,13,14
Employee skin cancer screening programs are an important method of examining high numbers of individuals quickly and effectively. These programs have been carried out in Germany and other countries.15,16 Studies have shown that skin cancer screening carried out selectively on defined groups can be an effective form of secondary prevention, particularly for those who work outdoors.17
An employee skin cancer screening program was carried out as part of this study. The findings are interpreted and discussed in relation to other employee screening programs that have been reported as well as those introduced by statutory health insurance providers in Germany. The aim of this study was to determine the importance and effectiveness of employee skin cancer screening programs and the role they play in secondary prevention of skin cancer.
Methods
Study Population
Employees of a technical company in Bavaria, Germany, were offered a skin cancer screening program by the employer’s occupational health service and health insurance provider in collaboration with the Department of Dermatology at the University Hospital Erlangen (Erlangen, Germany). Skin examinations were performed exclusively by 5 trained dermatologists. Only direct employees of the company at 3 of its locations in the Erlangen area were eligible to participate. The total number of employees varied by location (1072–5126 employees). The majority of employees had a university education or had completed technical training. Family members and other individuals who were not members of the company were excluded. There were no further inclusion or exclusion criteria. Over a period of 13 days, 783 of 7823 total employees (10.0%) were examined and included in the study. The study was approved by the Responsible Ethics Commission of the Faculty of Medicine at Friedrich-Alexander-University Erlangen-Nürnberg, Germany.
Study Design
Employees signed a consent form for participation in the study and completed a standardized questionnaire. The questionnaire was based on surveys used in a prior study18 and collected information on current and prior skin lesions, prior dermatological screening, personal and family history of skin tumors, frequency of UV exposure, and type of UV protection used. For the question on measures taken for protection from UV radiation, possible answers included with sunscreen cream, with suitable sun-protective clothing, and by staying in the shade, or no measures were taken. In contrast to the other questions, multiple answers were accepted for this question. Answering no automatically excluded other possible answers. Participants also were asked to assess their own Fitzpatrick skin type19; the questionnaire included explanations of each skin type (I–IV).
The participants were then called in for examination by the dermatologist at 15-minute intervals. All clothing was removed and the skin was examined. Dermatoscopes were used for closer examination of suspicious skin lesions. The clinical results of the examinations were recorded on a standardized form.
An estimation of the number of melanocytic nevi—≤20, 21–49, or ≥50—was recorded for each patient. Suspicious skin lesions were assigned to one of the following categories: nevus requiring future checkup (Nc), nevus requiring excision (Ne), suspected malignant melanoma (MM), suspected squamous cell carcinoma, suspected basal cell carcinoma (BCC), suspected other skin tumor, and precancerous lesion. Fitzpatrick skin type also was assessed for all participants and recorded by the dermatologist carrying out the examination. Each participant was assigned to a risk group—low, moderate, or high risk—based on their individual risk for developing a skin tumor. Factors that were considered when determining participants’ risk for developing skin cancer included Fitzpatrick skin type, number of melanocytic nevi, personal and family history, leisure activities, UV protection used, and current clinical diagnosis of skin lesions.
After the skin examination, participants were informed of recommended treatment but were not given any additional dermatologic advice. Participants could arrange an appointment at the Department of Dermatology, University Hospital Erlangen, for the excision and histological analysis of the skin lesions. All recorded data were collected in a computerized spreadsheet program. When evaluating the questionnaires, questions that were not answered or were answered incorrectly (participant chose more than 1 answer) were ignored.
Statistical Analysis
Statistical analysis was carried out using SPSS software version 16.0. The majority of the data were nominal or ordinal. Metric data were checked for normal distribution using the Shapiro-Wilk test before carrying out parametric tests. Statistical tests were carried out using the χ2 test and the t test for independent samples. Non-nominal distributed data were checked using the Mann-Whitney U test. P<.05 was considered statistically significant in the exploratory data analysis.
Results
Of 783 employees included in the study, 288 (36.8%) were female and 495 (63.2%) were male (Table 1). In comparison with the total workforce, a significantly higher proportion of women than men took part in the cross-sectional study (P<.01). The average age (SD) was 42.3 (9.5) years (range, 18–64 years). Female participants (average age [SD], 39.8 [10.2] years) were significantly younger than male participants (average age [SD], 43.8 [8.8] years; P<.01). Forty-one percent of participants had a prior skin cancer screening. One percent of participants had a personal history of skin cancer, with 1 participant reporting a history of MM; 6.5% had a family history of skin cancer, of which 39.2% had a family history of MM.
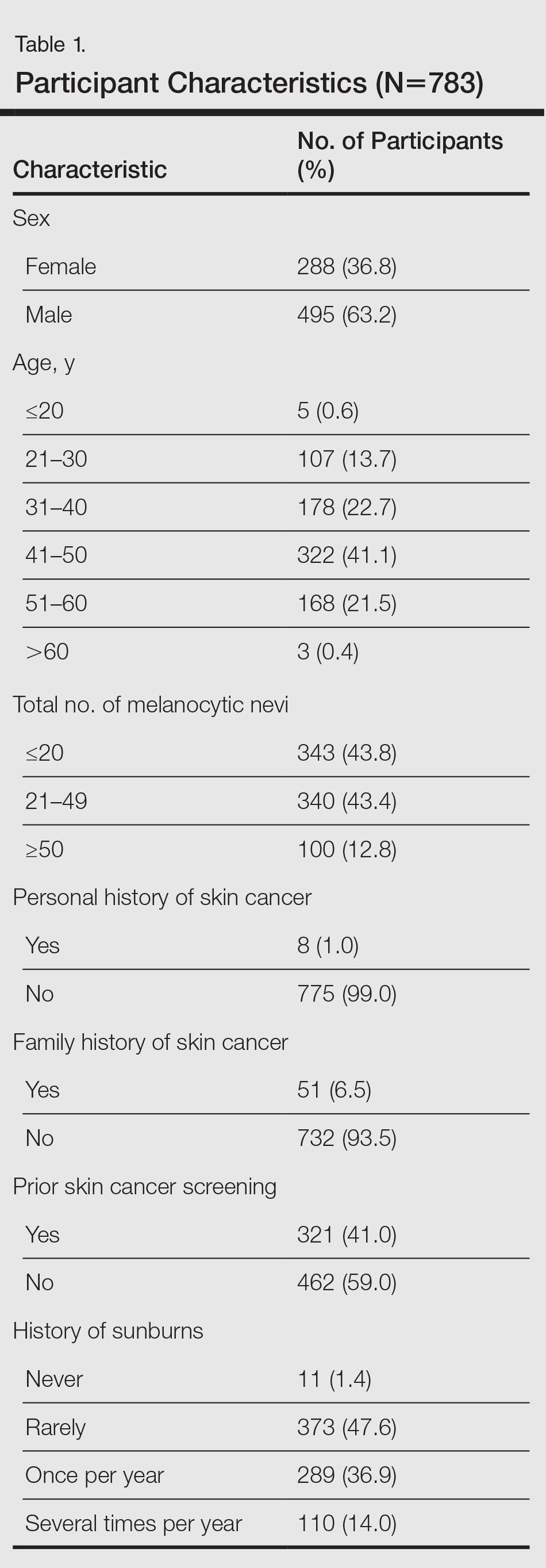
The results of the clinical examinations showed that 43.8% of participants had 20 or fewer melanocytic nevi, 43.4% had 21 to 49 melanocytic nevi, and 12.8% had 50 or more melanocytic nevi. Significantly more women than men had 20 or fewer melanocytic nevi (P<.05).
Approximately 92% of participants assessed themselves as having Fitzpatrick skin types II (35.2%) or III (56.7%), while only approximately 3.6% and 4.5% assessed themselves as having skin types I and IV, respectively. The results of the Fitzpatrick skin type assessments made by dermatologists were similar: 96.9% of participants were assessed as having Fitzpatrick skin types II (43.0%) and III (53.8%); approximately 1.9% and 1.3% were assessed as having Fitzpatrick skin types I and IV, respectively. Results showed that 80.2% of all participants assessed their skin type in the same way as the dermatologist; 13.5% assessed their skin type as darker and 6.3% (49/783) assessed it as lighter. A quantitative analysis of Fitzpatrick skin type and sex showed that significantly more male participants than female participants assessed their Fitzpatrick skin type darker than their actual skin type (P<.01).
Overall, 47.6% of participants reported having had sunburn rarely in the past, while 36.9% and 14.0% had experienced sunburn once per year and several times per year, respectively. Approximately 1.4% of participants reported never having a sunburn. More of the male participants made use of comprehensive sun protection using all methods listed (34.5%; P<.05) or a combination of sunscreen and sun-protective clothing (14.9%; P<.01) than the female participants who relied more frequently on sunscreen alone (29.5%; P<.01) or a combination of sunscreen and staying in the shade (29.5%; P<.01)
In general it was clear that sunscreen, either alone or in combination with other sun-protection methods, was used most frequently (88.0%); 58.0% protected themselves by staying in the shade, while 48.0% used suitable sun-protective clothing. Only 3.6% of participants did not protect themselves using any of the suggested methods.
A total of 661 categorized skin lesions were found in 377 participants. Of these lesions, 491 were Nc and 121 were Ne. Twenty-four of the skin lesions were suspected precancerous lesions, 13 were suspected BCC, 2 were suspected MM, and 10 were suspected other skin tumor (Table 2). Overall, male participants who were diagnosed with at least 1 skin lesion (average age, 44.0 years) were significantly older than the women (average age, 39.3 years)(P<.01). Similar findings were observed in participants with at least 1 Nc (men, 43.3 years; women, 38.7 years; P<.01) and at least 1 Ne (men, 44.2 years; women, 38.0 years; P<.05). With regard to the individual risk for developing skin cancer, 32.6% of participants were considered to be at low risk, 64.9% were at moderate risk, and 2.6% were at high risk.

Approximately 61.5% of 377 participants who were diagnosed with at least 1 categorized skin lesion were advised to have a specific skin lesion checked by a dermatologist or to have a full examination for skin cancer once every 12 months. Furthermore, 22.5% were advised to follow-up biannually and 11.7% were advised to follow-up once every 2 years. Of the remaining participants who were advised to have follow-ups, 0.3% were advised to have a skin examination once every 3 months after having had MM, and 4.0% were advised to have follow-up once every 18 months. Overall, follow-up was recommended within 1 year in 84.4% of cases and within 1 to 2 years in 15.6% (Table 3).

Subsequent histological analysis of the excised tissue resulted in a diagnosis of only 21 clinically significant skin conditions. One case of Bowen disease and 1 case of BCC was confirmed. Histological analysis identified the remaining 19 excised skin lesions, which included the 2 suspected MMs, as dysplastic nevi.
Comment
The aim of this cross-sectional study was to examine the importance and effectiveness of employee skin cancer screening programs. In comparison with the total workforce, significantly more women took part than men. Female participants were significantly younger than male participants, which mirrors the findings of prior studies showing that screening programs reach women more frequently than men and that women who participate in screenings are also younger on average in comparison to men.7-13 Men and older individuals usually are underrepresented.7,13 The average age of participants in our study was 42.3 years, which is lower than in the SCREEN (Skin Cancer Research to Provide Evidence for Effectiveness of Screening in Northern Germany) study (average age, 49.7 years).13 The average age in our study also is likely to be lower than patients who undergo skin cancer screenings offered by statutory health insurance providers in Germany, which has a minimum age restriction of 35 years; however, it is comparable to the average age of participants in other employee screening programs and therefore represents the average age of individuals employed in Germany.15,16
The employee skin cancer screening program in this study generated a high level of interest, indicated by the fact that all available appointments had been booked just 36 hours after the screening was announced. Furthermore, there was a waiting list of approximately 300 employees who were not able to undergo a skin examination. For logistical reasons, the number of participants was limited to 10% of the workforce. The high level of interest is an indication of increased awareness of the importance of recognizing skin tumors early and the associated need for information as well as the need to undergo screening for skin cancer as a precaution. This observation also can be made with regard to the skin cancer screening introduced by statutory health insurance providers in Germany. Studies published by Augustin et al20 and Kornek et al21,22 confirm that skin cancer screenings have gained wide acceptance in Germany because they were introduced by statutory health insurance providers in 2008. The number of skin cancer screenings carried out by dermatologists in Germany also is increasing.20-22 Although approximately 19% of those eligible to participate took part in the SCREEN pilot project,13 approximately 31% of individuals who were eligible to participate took part in skin cancer screenings offered by statutory health insurance providers in Germany in 2012, and the percentage is rising.23 Two important factors affecting the high level of interest in the employee screening program used in our study were undoubtedly the advantages of the examination taking place during working hours and being held on the occupational health services’ premises in the workplace, which helped participants avoid the cost of travel and wait times associated with visiting a medical practice.
Of 783 participants included in this study, 377 displayed at least 1 categorized skin lesion; the majority were suspicious melanocytic nevi. This high incidence rate suggested that regular skin cancer screenings are useful, as it has been shown that there is a correlation between higher numbers of melanocytic nevi and increased risk for developing melanoma.24
In a study by Winkler et al,25 a skin cancer screening of 1658 bank and insurance employees found that 33.8% of those examined displayed at least 1 atypical melanocytic nevus and 27.2% displayed more than 50 melanocytic nevi (compared to 12.8% with ≥50 melanocytic nevi in the current study). The risk for developing skin cancer was classified as intermediate or high in 54.5% (compared to 67.5% at moderate or high risk in the current study).25 Therefore, the rate of suspicious skin lesions was lower in the population of the study by Winkler et al25 in comparison to the population of the current study. As the overall number of melanocytic nevi and the individual risk for skin cancer, however, was underestimated by the majority of the bank and insurance employees,25 employee skin cancer screening programs can be used as a potentially effective tool to make employees aware of the issue and sensitizing them to it. Employee screening in addition to a final diagnosis can contribute to ensuring suitable treatment is started. For example, in the large-scale employee screening published by Schaefer et al15 and Augustin et al,16 48,665 and 90,880 employees, respectively, were screened for inflammatory and noninflammatory skin diseases, and 19% and 27% of participants, respectively, were diagnosed with skin lesions that required treatment.
Participants in the current study were given no further treatment or advice. Recommendations were made that participants monitor suspicious skin lesions or have them removed. With regard to future screening, 84.4% of participants with at least 1 categorized skin lesion were advised to have a regular follow-up within 1 year, while 15.6% were advised to follow-up within 1 to 2 years. Therefore, a period of 2 years before the next checkup, the period between screenings offered by statutory health insurance providers in Germany,12 was considered too long for the majority of participants, according to the dermatologists involved with our study.
Conclusion
The high rate of suspicious skin lesions diagnosed demonstrated the effectiveness of skin cancer screenings organized in the workplace, which should be recommended for all employees, not only those who are at high risk for developing skin cancer due to the nature of their work, such as those who work outdoors. It should be noted that the study group examined in the current study was a homogeneous group of employees of a technical company only and is therefore relatively selective. Nevertheless, despite the comparatively selective and young participant group, these examinations provide evidence of the importance of skin cancer screening programs for a wider population.
Acknowledgments
The authors thank Heidi Seybold, MD; Petra Wörl, MD; Sybille Thoma-Uszynski, MD; and Jens Bussmann, MD (all from Erlangen, Germany), for their support and active assistance in the practical implementation of this study.
- Boniol M, Autier P, Boyle P, et al. Cutaneous melanoma attributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:e4757.
- Gilchrest BA, Eller MS, Geller AC, et al. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 1999;340:1341-1348.
- Rigel DS. Cutaneous ultraviolet exposure and its relationship to the development of skin cancer. J Am Acad Dermatol. 2008;58:129-132.
- El Ghissassi F, Baan R, Straif K, et al; WHO International Agency for Research on Cancer Monograph Working Group. A review of human carcinogens—part D: radiation. Lancet Oncol. 2009;10:751-752.
- MacLennan R, Green AC, McLeod GR, et al. Increasing incidence of cutaneous melanoma in Queensland, Australia. J Natl Cancer Inst. 1992;84:1427-1432.
- Heinzerling LM, Dummer R, Panizzon RG, et al. Prevention campaign against skin cancer. Dermatology. 2002;205:229-233.
- Stratigos A, Nikolaou V, Kedicoglou S, et al. Melanoma/skin cancer screening in a Mediterranean country: results of the Euromelanoma Screening Day Campaign in Greece. J Eur Acad Dermatol Venereol. 2007;21:56-62.
- Garbe C, Hauschild A, Volkenandt M, et al. Evidence and interdisciplinary consense-based German guidelines: diagnosis and surveillance of melanoma. Melanoma Res. 2007;17:393-399.
- Choudhury K, Volkmer B, Greinert R, et al. Effectiveness of skin cancer screening programmes. Br J Dermatol. 2012;167:94-98.
- Eisemann N, Waldmann A, Geller AC, et al. Non-melanoma skin cancer incidence and impact of skin cancer screening on incidence. J Invest Dermatol. 2014;134:43-50.
- Katalinic A, Waldmann A, Weinstock MA, et al. Does skin cancer screening save lives? an observational study comparing trends in melanoma mortality in regions with and without screening. Cancer. 2012;118:5395-5402.
- Bekanntmachung (1430 A) eines Beschlusses des Gemeinsamen Bundeausschusses über eine Änderung der Krebsfrüherkennungs-Richtlinien: Hautkrebs-Screening [press release]. Berlin, Germany: Bundesministerium für Gesundheit (Federal Ministry of Health, Germany); vom 15. November 2007.
- Breitbart EW, Waldmann A, Nolte S, et al. Systematic skin cancer screening in Northern Germany. J Am Acad Dermatol. 2012;66:201-211.
- Waldmann A, Nolte S, Weinstock MA, et al. Skin cancer screening participation and impact on melanoma incidence in Germany—an observational study on incidence trends in regions with and without population-based screening. Br J Cancer. 2012;106:970-974.
- Schaefer I, Rustenbach SJ, Zimmer L, et al. Prevalence of skin diseases in a cohort of 48,665 employees in Germany. Dermatology. 2008;217:169-172.
- Augustin M, Herberger K, Hintzen S, et al. Prevalence of skin lesions and need for treatment in a cohort of 90880 workers. Br J Dermatol. 2011;165:865-873.
- LeBlanc WG, Vidal L, Kirsner RS, et al. Reported skin cancer screening of US adult workers. J Am Acad Dermatol. 2008;59:55-63.
- Harbauer A, Binder M, Pehamberger H, et al. Validity of an unsupervised self-administered questionnaire for self-assessment of melanoma risk. Melanoma Res. 2003;13:537-542.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Augustin M, Stadler R, Reusch M, et al. Skin cancer screening in Germany—perception by the public. J Dtsch Dermatol Ges. 2012;10:42-49.
- Kornek T, Augustin M. Skin cancer prevention. J Dtsch Dermatol Ges. 2013;11:283-296.
- Kornek T, Schäfer I, Reusch M, et al. Routine skin cancer screening in Germany: four years of experience from the dermatologists’ perspective. Dermatology. 2012;225:289-293.
- Barmer GEK Arztreport 2014 [press release]. Berlin, Germany: Barmer GEK; February 4, 2014.
- Bauer J, Garbe C. Acquired melanocytic nevi as riskfactor for melanoma development. a comprehensive review of epidemiological data. Pigment Cell Res. 2003;16:297-306.
- Winkler A, Plugfelder A, Weide B, et al. Screening for skin cancer in bank and insurance employees: risk profile and correlation of self and physician’s assessment. Int J Dermatol. 2015;54:419-423.
- Boniol M, Autier P, Boyle P, et al. Cutaneous melanoma attributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:e4757.
- Gilchrest BA, Eller MS, Geller AC, et al. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 1999;340:1341-1348.
- Rigel DS. Cutaneous ultraviolet exposure and its relationship to the development of skin cancer. J Am Acad Dermatol. 2008;58:129-132.
- El Ghissassi F, Baan R, Straif K, et al; WHO International Agency for Research on Cancer Monograph Working Group. A review of human carcinogens—part D: radiation. Lancet Oncol. 2009;10:751-752.
- MacLennan R, Green AC, McLeod GR, et al. Increasing incidence of cutaneous melanoma in Queensland, Australia. J Natl Cancer Inst. 1992;84:1427-1432.
- Heinzerling LM, Dummer R, Panizzon RG, et al. Prevention campaign against skin cancer. Dermatology. 2002;205:229-233.
- Stratigos A, Nikolaou V, Kedicoglou S, et al. Melanoma/skin cancer screening in a Mediterranean country: results of the Euromelanoma Screening Day Campaign in Greece. J Eur Acad Dermatol Venereol. 2007;21:56-62.
- Garbe C, Hauschild A, Volkenandt M, et al. Evidence and interdisciplinary consense-based German guidelines: diagnosis and surveillance of melanoma. Melanoma Res. 2007;17:393-399.
- Choudhury K, Volkmer B, Greinert R, et al. Effectiveness of skin cancer screening programmes. Br J Dermatol. 2012;167:94-98.
- Eisemann N, Waldmann A, Geller AC, et al. Non-melanoma skin cancer incidence and impact of skin cancer screening on incidence. J Invest Dermatol. 2014;134:43-50.
- Katalinic A, Waldmann A, Weinstock MA, et al. Does skin cancer screening save lives? an observational study comparing trends in melanoma mortality in regions with and without screening. Cancer. 2012;118:5395-5402.
- Bekanntmachung (1430 A) eines Beschlusses des Gemeinsamen Bundeausschusses über eine Änderung der Krebsfrüherkennungs-Richtlinien: Hautkrebs-Screening [press release]. Berlin, Germany: Bundesministerium für Gesundheit (Federal Ministry of Health, Germany); vom 15. November 2007.
- Breitbart EW, Waldmann A, Nolte S, et al. Systematic skin cancer screening in Northern Germany. J Am Acad Dermatol. 2012;66:201-211.
- Waldmann A, Nolte S, Weinstock MA, et al. Skin cancer screening participation and impact on melanoma incidence in Germany—an observational study on incidence trends in regions with and without population-based screening. Br J Cancer. 2012;106:970-974.
- Schaefer I, Rustenbach SJ, Zimmer L, et al. Prevalence of skin diseases in a cohort of 48,665 employees in Germany. Dermatology. 2008;217:169-172.
- Augustin M, Herberger K, Hintzen S, et al. Prevalence of skin lesions and need for treatment in a cohort of 90880 workers. Br J Dermatol. 2011;165:865-873.
- LeBlanc WG, Vidal L, Kirsner RS, et al. Reported skin cancer screening of US adult workers. J Am Acad Dermatol. 2008;59:55-63.
- Harbauer A, Binder M, Pehamberger H, et al. Validity of an unsupervised self-administered questionnaire for self-assessment of melanoma risk. Melanoma Res. 2003;13:537-542.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Augustin M, Stadler R, Reusch M, et al. Skin cancer screening in Germany—perception by the public. J Dtsch Dermatol Ges. 2012;10:42-49.
- Kornek T, Augustin M. Skin cancer prevention. J Dtsch Dermatol Ges. 2013;11:283-296.
- Kornek T, Schäfer I, Reusch M, et al. Routine skin cancer screening in Germany: four years of experience from the dermatologists’ perspective. Dermatology. 2012;225:289-293.
- Barmer GEK Arztreport 2014 [press release]. Berlin, Germany: Barmer GEK; February 4, 2014.
- Bauer J, Garbe C. Acquired melanocytic nevi as riskfactor for melanoma development. a comprehensive review of epidemiological data. Pigment Cell Res. 2003;16:297-306.
- Winkler A, Plugfelder A, Weide B, et al. Screening for skin cancer in bank and insurance employees: risk profile and correlation of self and physician’s assessment. Int J Dermatol. 2015;54:419-423.
Practice Points
- Employee skin cancer screening programs are an important method of examining high numbers of individuals quickly and efficiently and should be used as an important tool for secondary skin cancer prevention.
- The high rate of suspicious skin lesions diagnosed in this study demonstrates the effectiveness of skin cancer screenings organized in the workplace and provides evidence of the importance of skin cancer screening programs for a wider population.
- Employee skin cancer screening programs should be recommended for all employees, not only those who are at high risk for developing skin cancer due to the nature of their work, such as those who work outdoors.
Shiitake Mushroom Dermatitis
To the Editor:
The shiitake mushroom (Lentinula edodes) is a popular Asian food and represents the second most consumed mushroom in the world. It is known for having a range of strong health benefits including antihypertensive, anti-inflammatory, and immunomodulatory effects. Especially in Asia, this mushroom has been used in patients with cancers of the gastrointestinal tract and also may be helpful in the treatment of human immunodeficiency virus.1,2 The source of these effects is lentinan, a polysaccharide in the mushroom. However, lentinan also can cause a toxic reaction of the skin when the mushrooms are eaten raw or undercooked. These reactions are mainly reported in Asia, but more cases have been published in the last decade in Europe and the United States, evidence that the incidence of this adverse effect has increased in the Western world.
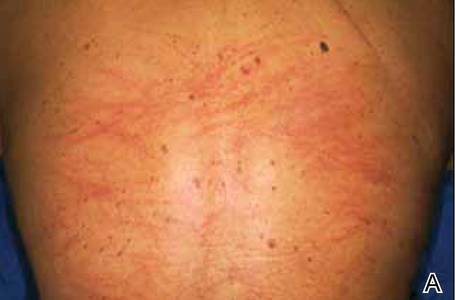
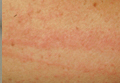
A 65-year-old woman with no notable medical history presented to our outpatient practice with sudden onset of a pruritic, erythematous, papular eruption on the neck. The eruption began that morning. The diagnosis of eczematous dermatitis was made and hydrocortisone cream 2.5% was started. Three days later, she returned with spread of the rash to the trunk, arms, and legs despite the topical treatment. She denied fevers, chills, or constitutional symptoms. The patient also denied recent travel or bug bites. However, she reported that she recently had started using raw shiitake mushrooms in her salad; the first time was 3 days before the symptoms appeared. Physical examination revealed erythematous skin with long flagellate streaks composed of petechiae, papules, and vesicles involving the trunk, arms, and legs (Figure). Oral and nasal mucosae were uninvolved. Dermatographism was negative. The diagnosis of flagellate dermatitis from shiitake mushrooms was made given the patient’s history and the unique clinical findings of the skin. Blood work and a biopsy were not performed. Instead, the patient was advised to avoid shiitake mushrooms and use clobetasol propionate cream 0.05% twice daily for 2 weeks on the affected areas. The symptoms resolved within 10 days.
|
The first known case of toxicoderma to shiitake mushrooms was reported in Japan by Nakamura3 in 1977. Since this seminal report, numerous cases have followed. This disorder is mainly seen in Asia.
Patients usually present with linear groups of pruritic, papular, petechial, and vesicular lesions in a flagellate pattern, most commonly localized on the trunk, arms, and legs. Oral and nasal mucosae usually are not involved, and fever and malaise may be associated. All symptoms typically occur 1 to 2 days after ingestion of the mushrooms. The patient’s history and typical clinical findings lead to a diagnosis; however, blood tests may show inflammation with leukocytosis and elevated C-reactive protein levels. Biopsy of the skin shows lymphocytic dermal infiltrates with spongiosis and necrotic cells within the epidermis.4
Differential diagnoses include flagellate dermatitis associated with bleomycin, a glycopeptide antibiotic produced by the bacterium Streptomyces verticillus. Because it causes breaks in the DNA, bleomycin is commonly used as a chemotherapeutic agent in treating Hodgkin lymphoma and other malignancies. It presents with linear postinflammatory hyperpigmentation of the skin. However, unlike shiitake dermatitis, there is a lack of papules. Another differential diagnosis includes herpes zoster virus, which should be ruled out clinically.
All symptoms in shiitake dermatitis usually resolve within 1 to 8 weeks of avoidance of the culprit food. Topical steroids and antihistamines can be given.
The underlying pathology is a toxic reaction to the polysaccharide lentinan in the mushrooms, which is known as a thermolabile agent.5 Therefore, it may only cause a toxic reaction when the mushrooms are consumed raw or undercooked. Prick testing is usually negative in these patients, which suggests a toxic and not an immunologic reaction of the human body.6 Other forms of reaction to shiitake mushrooms include contact dermatitis after skin contact and allergic alveolitis after occupational exposure to mushroom spores, mainly in individuals cultivating shiitake mushrooms (mushroom worker’s lung). In these forms of the disease, prick testing may be positive.7,8
Flagellate dermatitis caused by shiitake mushrooms is still an uncommon dermatologic phenomenon in the Western world. Future studies and cases should be reported to increase the awareness of this disorder. Although the patients present with typical clinical findings, the diagnosis can be missed if history is not carefully considered.
1. Ng ML, Yap AT. Inhibition of human colon carcinoma development by lentinan from shiitake mushrooms (Lentinus edodes). J Altern Complement Med. 2002;8:581-589.
2. Gordon M, Bihari B, Goosby E, et al. A placebo-controlled trial of the immune modulator, lentinan, in HIV-positive patients: a phase I/II trial. J Med. 1998;29:305-330.
3. Nakamura T. Toxicoderma caused by shiitake. Jpn J Clin Dermatol. 1977;31:65-68.
4. Hanada K, Hashimoto I. Flagellate mushroom (shiitake) dermatitis and photosensitivity. Dermatology. 1998;197:255-257.
5. Lippert U, Martin V, Schwertfeger C, et al. Shiitake dermatitis. Br J Dermatol. 2003;148:178-179.
6. Nakamura T. Shiitake (Lentinus edodes) dermatitis. Contact Dermatitis. 1992;27:6570.
7. Ueda A, Obama K, Aoyama K, et al. Allergic contact dermatitis in shiitake (Lentinus edodes (Berk) Sing) growers. Contact Dermatitis. 1992;26:228-233.
8. Ampere A, Delhaes L, Soots J, et al. Hypersensitivity pneumonitis induced by shiitake mushroom spores. Med Mycol. 2012;50:654-657.
To the Editor:
The shiitake mushroom (Lentinula edodes) is a popular Asian food and represents the second most consumed mushroom in the world. It is known for having a range of strong health benefits including antihypertensive, anti-inflammatory, and immunomodulatory effects. Especially in Asia, this mushroom has been used in patients with cancers of the gastrointestinal tract and also may be helpful in the treatment of human immunodeficiency virus.1,2 The source of these effects is lentinan, a polysaccharide in the mushroom. However, lentinan also can cause a toxic reaction of the skin when the mushrooms are eaten raw or undercooked. These reactions are mainly reported in Asia, but more cases have been published in the last decade in Europe and the United States, evidence that the incidence of this adverse effect has increased in the Western world.
A 65-year-old woman with no notable medical history presented to our outpatient practice with sudden onset of a pruritic, erythematous, papular eruption on the neck. The eruption began that morning. The diagnosis of eczematous dermatitis was made and hydrocortisone cream 2.5% was started. Three days later, she returned with spread of the rash to the trunk, arms, and legs despite the topical treatment. She denied fevers, chills, or constitutional symptoms. The patient also denied recent travel or bug bites. However, she reported that she recently had started using raw shiitake mushrooms in her salad; the first time was 3 days before the symptoms appeared. Physical examination revealed erythematous skin with long flagellate streaks composed of petechiae, papules, and vesicles involving the trunk, arms, and legs (Figure). Oral and nasal mucosae were uninvolved. Dermatographism was negative. The diagnosis of flagellate dermatitis from shiitake mushrooms was made given the patient’s history and the unique clinical findings of the skin. Blood work and a biopsy were not performed. Instead, the patient was advised to avoid shiitake mushrooms and use clobetasol propionate cream 0.05% twice daily for 2 weeks on the affected areas. The symptoms resolved within 10 days.
|
The first known case of toxicoderma to shiitake mushrooms was reported in Japan by Nakamura3 in 1977. Since this seminal report, numerous cases have followed. This disorder is mainly seen in Asia.
Patients usually present with linear groups of pruritic, papular, petechial, and vesicular lesions in a flagellate pattern, most commonly localized on the trunk, arms, and legs. Oral and nasal mucosae usually are not involved, and fever and malaise may be associated. All symptoms typically occur 1 to 2 days after ingestion of the mushrooms. The patient’s history and typical clinical findings lead to a diagnosis; however, blood tests may show inflammation with leukocytosis and elevated C-reactive protein levels. Biopsy of the skin shows lymphocytic dermal infiltrates with spongiosis and necrotic cells within the epidermis.4
Differential diagnoses include flagellate dermatitis associated with bleomycin, a glycopeptide antibiotic produced by the bacterium Streptomyces verticillus. Because it causes breaks in the DNA, bleomycin is commonly used as a chemotherapeutic agent in treating Hodgkin lymphoma and other malignancies. It presents with linear postinflammatory hyperpigmentation of the skin. However, unlike shiitake dermatitis, there is a lack of papules. Another differential diagnosis includes herpes zoster virus, which should be ruled out clinically.
All symptoms in shiitake dermatitis usually resolve within 1 to 8 weeks of avoidance of the culprit food. Topical steroids and antihistamines can be given.
The underlying pathology is a toxic reaction to the polysaccharide lentinan in the mushrooms, which is known as a thermolabile agent.5 Therefore, it may only cause a toxic reaction when the mushrooms are consumed raw or undercooked. Prick testing is usually negative in these patients, which suggests a toxic and not an immunologic reaction of the human body.6 Other forms of reaction to shiitake mushrooms include contact dermatitis after skin contact and allergic alveolitis after occupational exposure to mushroom spores, mainly in individuals cultivating shiitake mushrooms (mushroom worker’s lung). In these forms of the disease, prick testing may be positive.7,8
Flagellate dermatitis caused by shiitake mushrooms is still an uncommon dermatologic phenomenon in the Western world. Future studies and cases should be reported to increase the awareness of this disorder. Although the patients present with typical clinical findings, the diagnosis can be missed if history is not carefully considered.
To the Editor:
The shiitake mushroom (Lentinula edodes) is a popular Asian food and represents the second most consumed mushroom in the world. It is known for having a range of strong health benefits including antihypertensive, anti-inflammatory, and immunomodulatory effects. Especially in Asia, this mushroom has been used in patients with cancers of the gastrointestinal tract and also may be helpful in the treatment of human immunodeficiency virus.1,2 The source of these effects is lentinan, a polysaccharide in the mushroom. However, lentinan also can cause a toxic reaction of the skin when the mushrooms are eaten raw or undercooked. These reactions are mainly reported in Asia, but more cases have been published in the last decade in Europe and the United States, evidence that the incidence of this adverse effect has increased in the Western world.
A 65-year-old woman with no notable medical history presented to our outpatient practice with sudden onset of a pruritic, erythematous, papular eruption on the neck. The eruption began that morning. The diagnosis of eczematous dermatitis was made and hydrocortisone cream 2.5% was started. Three days later, she returned with spread of the rash to the trunk, arms, and legs despite the topical treatment. She denied fevers, chills, or constitutional symptoms. The patient also denied recent travel or bug bites. However, she reported that she recently had started using raw shiitake mushrooms in her salad; the first time was 3 days before the symptoms appeared. Physical examination revealed erythematous skin with long flagellate streaks composed of petechiae, papules, and vesicles involving the trunk, arms, and legs (Figure). Oral and nasal mucosae were uninvolved. Dermatographism was negative. The diagnosis of flagellate dermatitis from shiitake mushrooms was made given the patient’s history and the unique clinical findings of the skin. Blood work and a biopsy were not performed. Instead, the patient was advised to avoid shiitake mushrooms and use clobetasol propionate cream 0.05% twice daily for 2 weeks on the affected areas. The symptoms resolved within 10 days.
|
The first known case of toxicoderma to shiitake mushrooms was reported in Japan by Nakamura3 in 1977. Since this seminal report, numerous cases have followed. This disorder is mainly seen in Asia.
Patients usually present with linear groups of pruritic, papular, petechial, and vesicular lesions in a flagellate pattern, most commonly localized on the trunk, arms, and legs. Oral and nasal mucosae usually are not involved, and fever and malaise may be associated. All symptoms typically occur 1 to 2 days after ingestion of the mushrooms. The patient’s history and typical clinical findings lead to a diagnosis; however, blood tests may show inflammation with leukocytosis and elevated C-reactive protein levels. Biopsy of the skin shows lymphocytic dermal infiltrates with spongiosis and necrotic cells within the epidermis.4
Differential diagnoses include flagellate dermatitis associated with bleomycin, a glycopeptide antibiotic produced by the bacterium Streptomyces verticillus. Because it causes breaks in the DNA, bleomycin is commonly used as a chemotherapeutic agent in treating Hodgkin lymphoma and other malignancies. It presents with linear postinflammatory hyperpigmentation of the skin. However, unlike shiitake dermatitis, there is a lack of papules. Another differential diagnosis includes herpes zoster virus, which should be ruled out clinically.
All symptoms in shiitake dermatitis usually resolve within 1 to 8 weeks of avoidance of the culprit food. Topical steroids and antihistamines can be given.
The underlying pathology is a toxic reaction to the polysaccharide lentinan in the mushrooms, which is known as a thermolabile agent.5 Therefore, it may only cause a toxic reaction when the mushrooms are consumed raw or undercooked. Prick testing is usually negative in these patients, which suggests a toxic and not an immunologic reaction of the human body.6 Other forms of reaction to shiitake mushrooms include contact dermatitis after skin contact and allergic alveolitis after occupational exposure to mushroom spores, mainly in individuals cultivating shiitake mushrooms (mushroom worker’s lung). In these forms of the disease, prick testing may be positive.7,8
Flagellate dermatitis caused by shiitake mushrooms is still an uncommon dermatologic phenomenon in the Western world. Future studies and cases should be reported to increase the awareness of this disorder. Although the patients present with typical clinical findings, the diagnosis can be missed if history is not carefully considered.
1. Ng ML, Yap AT. Inhibition of human colon carcinoma development by lentinan from shiitake mushrooms (Lentinus edodes). J Altern Complement Med. 2002;8:581-589.
2. Gordon M, Bihari B, Goosby E, et al. A placebo-controlled trial of the immune modulator, lentinan, in HIV-positive patients: a phase I/II trial. J Med. 1998;29:305-330.
3. Nakamura T. Toxicoderma caused by shiitake. Jpn J Clin Dermatol. 1977;31:65-68.
4. Hanada K, Hashimoto I. Flagellate mushroom (shiitake) dermatitis and photosensitivity. Dermatology. 1998;197:255-257.
5. Lippert U, Martin V, Schwertfeger C, et al. Shiitake dermatitis. Br J Dermatol. 2003;148:178-179.
6. Nakamura T. Shiitake (Lentinus edodes) dermatitis. Contact Dermatitis. 1992;27:6570.
7. Ueda A, Obama K, Aoyama K, et al. Allergic contact dermatitis in shiitake (Lentinus edodes (Berk) Sing) growers. Contact Dermatitis. 1992;26:228-233.
8. Ampere A, Delhaes L, Soots J, et al. Hypersensitivity pneumonitis induced by shiitake mushroom spores. Med Mycol. 2012;50:654-657.
1. Ng ML, Yap AT. Inhibition of human colon carcinoma development by lentinan from shiitake mushrooms (Lentinus edodes). J Altern Complement Med. 2002;8:581-589.
2. Gordon M, Bihari B, Goosby E, et al. A placebo-controlled trial of the immune modulator, lentinan, in HIV-positive patients: a phase I/II trial. J Med. 1998;29:305-330.
3. Nakamura T. Toxicoderma caused by shiitake. Jpn J Clin Dermatol. 1977;31:65-68.
4. Hanada K, Hashimoto I. Flagellate mushroom (shiitake) dermatitis and photosensitivity. Dermatology. 1998;197:255-257.
5. Lippert U, Martin V, Schwertfeger C, et al. Shiitake dermatitis. Br J Dermatol. 2003;148:178-179.
6. Nakamura T. Shiitake (Lentinus edodes) dermatitis. Contact Dermatitis. 1992;27:6570.
7. Ueda A, Obama K, Aoyama K, et al. Allergic contact dermatitis in shiitake (Lentinus edodes (Berk) Sing) growers. Contact Dermatitis. 1992;26:228-233.
8. Ampere A, Delhaes L, Soots J, et al. Hypersensitivity pneumonitis induced by shiitake mushroom spores. Med Mycol. 2012;50:654-657.