User login
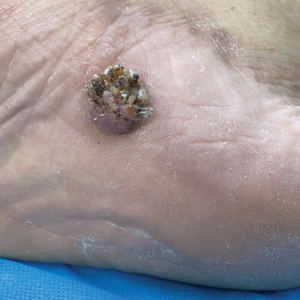
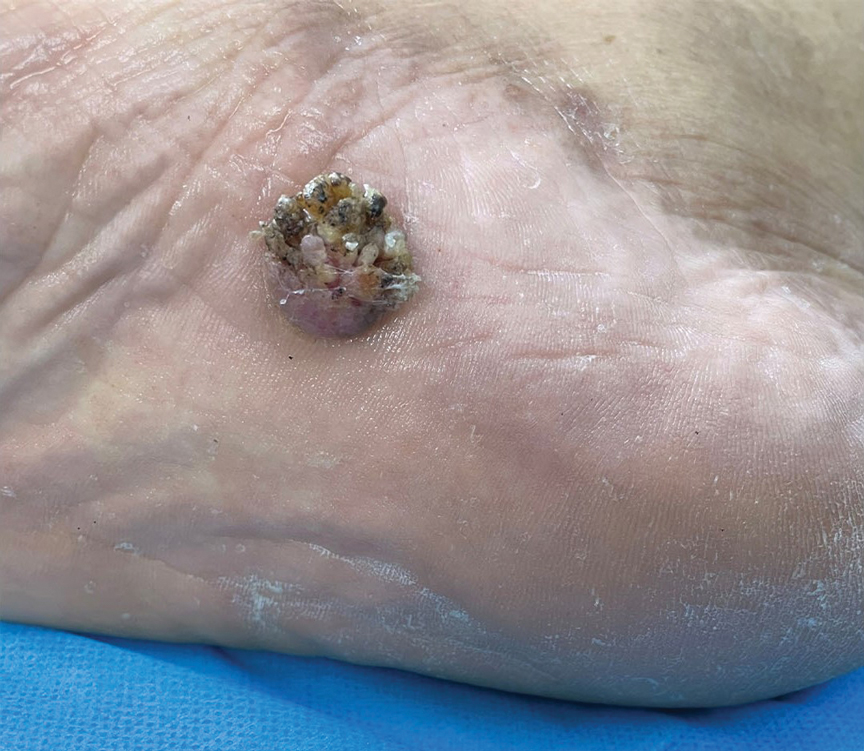
Verrucous Plaque on the Foot
The Diagnosis: Eccrine Poroma
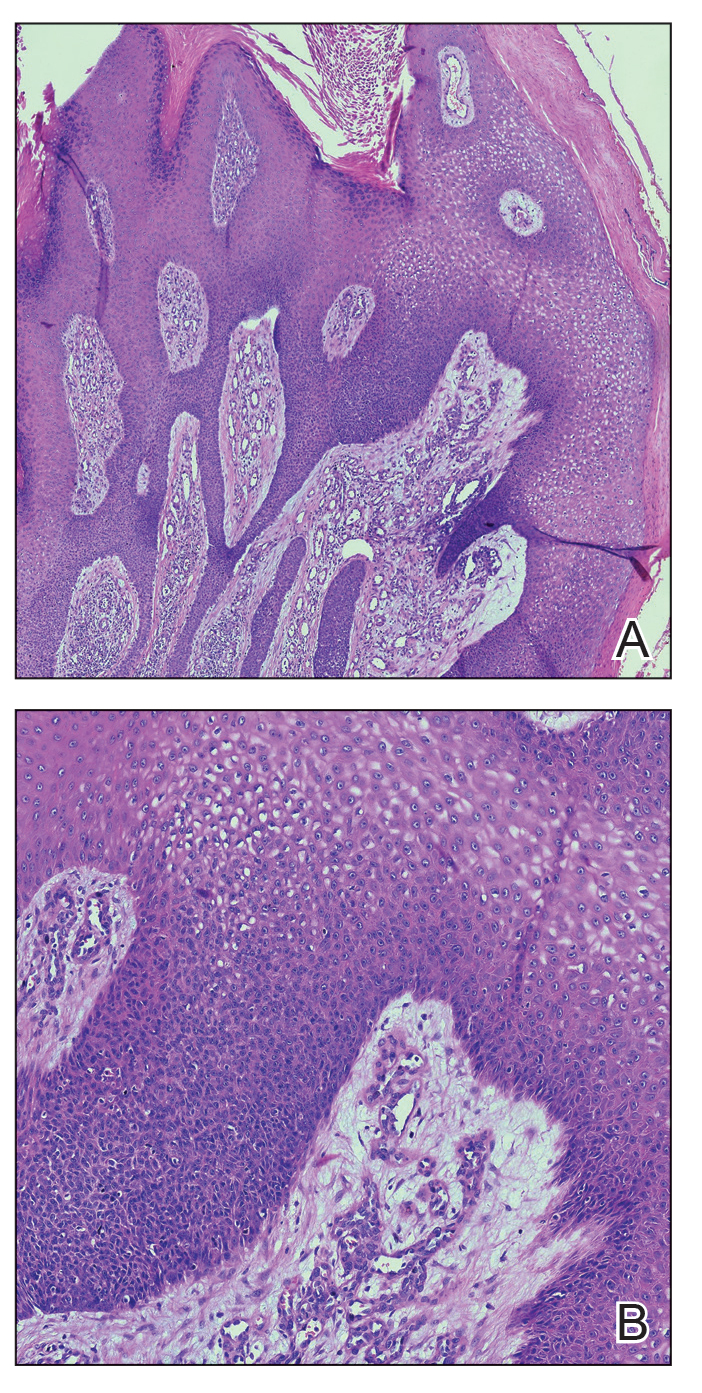
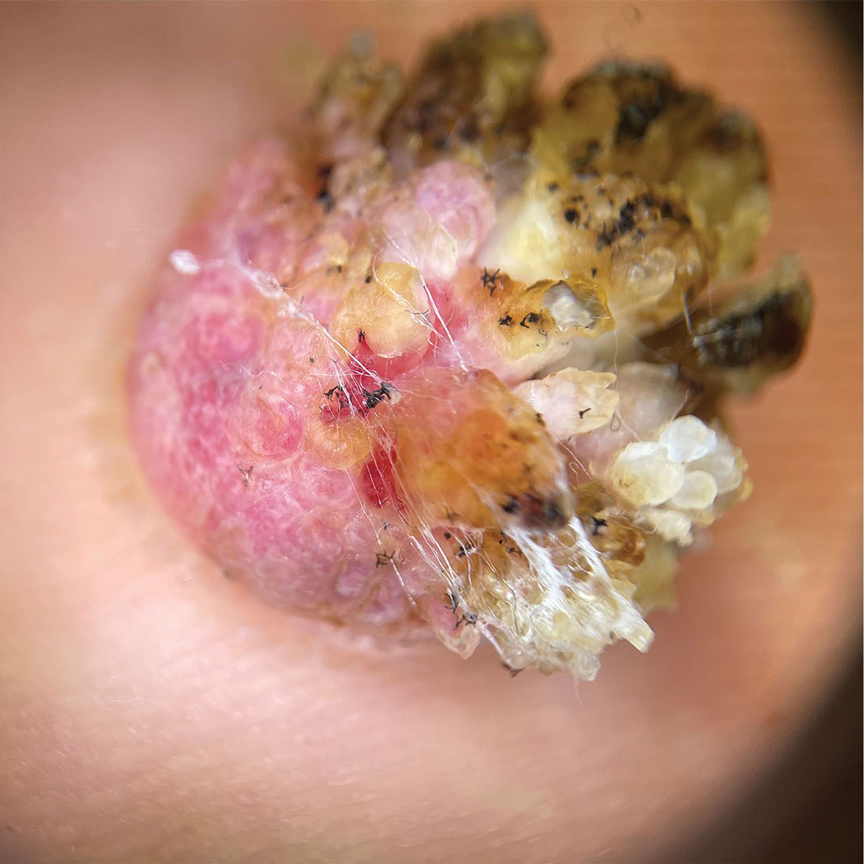
Histopathology demonstrated epidermal thickening, epidermal protrusions, a well-defined mass of tumor cells that extended from the epidermis down to the dermis, and luminal structures. Poroid cells and ovoid nuclei with basophilic cytoplasm also were evident (Figure 1). Dermoscopy showed papillomatous growth, milky-red areas, and dotted vessels (Figure 2). Reflectance confocal microscopy (RCM) at the spinous layer showed hyporefractile, dark, roundish lumina surrounded by keratinocytes (Figure 3). Based on the histologic, dermoscopic, and RCM findings, our patient was diagnosed with eccrine poroma.
Goldman et al1 first described poroma in 1956. Poromas, which include eccrine poroma, are a group of benign cutaneous neoplasms arising from the terminal eccrine or apocrine sweat gland ducts.2 Histologically, poroid cells appear as cuboidal keratinocytes with monomorphous ovoid nuclei and discrete nucleoli.3 They usually appear as nodules or plaques with colors varying from flesh colored to red, brown, or bluish, and they clinically mimic several benign and malignant skin tumors. The differential diagnosis may include keratoacanthoma, plantar wart, verrucous carcinoma, basal cell carcinoma, and squamous cell carcinoma. Poromas can be of eccrine or apocrine origin.4 They also belong to a broad group of neoplasms, including nodular hidradenomas, clear cell hidradenomas, hidroacanthoma simplex, dermal duct tumors, and hidradenomas.5 Four subtypes—poroma, poroid hidradenoma, hidroacanthoma simplex, and dermal duct tumor—have been documented.6 Because poromas have nonspecific and variable clinical presentations, they often are misdiagnosed as other skin neoplasms, and differentiation may be difficult. For example, some cases of poroma present with follicular, sebaceous, and/or apocrine differentiation, leading to difficulty in diagnosis.
Characteristic features of eccrine poroma seen on dermoscopy and RCM have the potential to aid in the diagnosis compared to histopathology. Marchetti et al7 proposed 4 patterns of characteristic dermoscopic findings. Pattern 1 refers to the classic description with bleeding spots, a structureless yellow appearance, milkyred globules, and branched vessels. Patterns 2 and 3 simulate basal cell carcinoma, dermal nevus, or vascular tumors. Pattern 4 refers to tumors that are large in size and resemble keratinizing neoplasms.7 Brugués et al8 described poromas with the following RCM findings: an atypical honeycomb shape that was well separated from the normal epithelium, hyporefractile nests with atypical cells, lack of palisading, and dark holes. One study described RCM parameters as cords without palisading, dark holes, prominent vascularization, and abundant stroma—findings that were positively associated with poroma in a univariate analysis. These findings assist in distinguishing poromas from other conditions in the differential diagnosis.9
There is a substantial overlap in clinical appearance with malignant conditions, including basal cell carcinoma, squamous cell carcinoma, cutaneous metastases, and Paget disease; therefore, the use of dermoscopy and RCM may be helpful in the diagnosis and recognition of specific features, as well as the corresponding patterns of poroma. Poromas commonly display vascularized features due to the variability of dermoscopic patterns of eccrine poroma, and further studies are required to establish the specificity of vascularized features.
Acral lesions are more likely to show the classic clinical features of erythema and exophytic growth. A case of a collision tumor with the verrucous changes of poroma, seborrheic keratosis, and viral wart has been described.10 The verrucous changes may lead to misdiagnosis as plantar warts or other neoplasms. Clinicians also should consider conditions that are induced by friction or trauma. In our patient, dermoscopy and RCM aided in the diagnosis of eccrine poroma due to the interference of prominent overlying verrucous changes.
Treatment of poroma is optional. Deeper lesions can be treated with surgical excision, and superficial lesions may be treated with electrosurgical destruction. Our patient was treated with surgical excision followed by repair of the surgical defect with a double V-Y flap.
- Goldman P, Pinkus H, Rogin JR. Eccrine poroma; tumors exhibiting features of the epidermal sweat duct unit. AMA Arch Derm. 1956; 74:511-521.
- Miller AC, Adjei S, Temiz LA, et al. Dermal duct tumor: a diagnostic dilemma [published online January 28, 2022]. Dermatopathology (Basel). 2022;9:36-47. doi:10.3390/dermatopathology9010007
- Ahmed Jan N, Masood S. Poroma. StatPearls [Internet]. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK560909/
- Casper DJ, Glass LF, Shenefelt PD. An unusually large eccrine poroma: a case report and review of the literature. Cutis. 2011; 88:227-229.
- Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms. Int J Dermatol. 2014;53:1053-1061.
- Betti R, Bombonato C, Cerri A, et al. Unusual sites for poromas are not very unusual: a survey of 101 cases. Clin Exp Dermatol. 2014; 39:119-122.
- Marchetti MA, Marino ML, Virmani P, et al. Dermoscopic features and patterns of poromas: a multicenter observational case-control study conducted by the International Dermoscopy Society (IDS). J Eur Acad Dermatol Venereol. 2018;32:1263-1271.
- Brugués A, Gamboa M, Alós L, et al. The challenging diagnosis of eccrine poromas. J Am Acad Dermatol. 2016;74:E113-E115.
- Di Tullio F, Mandel VD, Ignazio S, et al. The role of reflectance confocal microscopy in the diagnosis of eccrine poroma: a retrospective casecontrol study. Exp Dermatol. 2022;31:1779-1790.
- Bloom BS, Kamino H, Hale CS, et al. Collision tumor of eccrine poroma, seborrheic keratosis, and a viral wart. Dermatol Online J. 2014;20:13030/qt8tm0r9b9.
The Diagnosis: Eccrine Poroma
Histopathology demonstrated epidermal thickening, epidermal protrusions, a well-defined mass of tumor cells that extended from the epidermis down to the dermis, and luminal structures. Poroid cells and ovoid nuclei with basophilic cytoplasm also were evident (Figure 1). Dermoscopy showed papillomatous growth, milky-red areas, and dotted vessels (Figure 2). Reflectance confocal microscopy (RCM) at the spinous layer showed hyporefractile, dark, roundish lumina surrounded by keratinocytes (Figure 3). Based on the histologic, dermoscopic, and RCM findings, our patient was diagnosed with eccrine poroma.

Goldman et al1 first described poroma in 1956. Poromas, which include eccrine poroma, are a group of benign cutaneous neoplasms arising from the terminal eccrine or apocrine sweat gland ducts.2 Histologically, poroid cells appear as cuboidal keratinocytes with monomorphous ovoid nuclei and discrete nucleoli.3 They usually appear as nodules or plaques with colors varying from flesh colored to red, brown, or bluish, and they clinically mimic several benign and malignant skin tumors. The differential diagnosis may include keratoacanthoma, plantar wart, verrucous carcinoma, basal cell carcinoma, and squamous cell carcinoma. Poromas can be of eccrine or apocrine origin.4 They also belong to a broad group of neoplasms, including nodular hidradenomas, clear cell hidradenomas, hidroacanthoma simplex, dermal duct tumors, and hidradenomas.5 Four subtypes—poroma, poroid hidradenoma, hidroacanthoma simplex, and dermal duct tumor—have been documented.6 Because poromas have nonspecific and variable clinical presentations, they often are misdiagnosed as other skin neoplasms, and differentiation may be difficult. For example, some cases of poroma present with follicular, sebaceous, and/or apocrine differentiation, leading to difficulty in diagnosis.

Characteristic features of eccrine poroma seen on dermoscopy and RCM have the potential to aid in the diagnosis compared to histopathology. Marchetti et al7 proposed 4 patterns of characteristic dermoscopic findings. Pattern 1 refers to the classic description with bleeding spots, a structureless yellow appearance, milkyred globules, and branched vessels. Patterns 2 and 3 simulate basal cell carcinoma, dermal nevus, or vascular tumors. Pattern 4 refers to tumors that are large in size and resemble keratinizing neoplasms.7 Brugués et al8 described poromas with the following RCM findings: an atypical honeycomb shape that was well separated from the normal epithelium, hyporefractile nests with atypical cells, lack of palisading, and dark holes. One study described RCM parameters as cords without palisading, dark holes, prominent vascularization, and abundant stroma—findings that were positively associated with poroma in a univariate analysis. These findings assist in distinguishing poromas from other conditions in the differential diagnosis.9

There is a substantial overlap in clinical appearance with malignant conditions, including basal cell carcinoma, squamous cell carcinoma, cutaneous metastases, and Paget disease; therefore, the use of dermoscopy and RCM may be helpful in the diagnosis and recognition of specific features, as well as the corresponding patterns of poroma. Poromas commonly display vascularized features due to the variability of dermoscopic patterns of eccrine poroma, and further studies are required to establish the specificity of vascularized features.
Acral lesions are more likely to show the classic clinical features of erythema and exophytic growth. A case of a collision tumor with the verrucous changes of poroma, seborrheic keratosis, and viral wart has been described.10 The verrucous changes may lead to misdiagnosis as plantar warts or other neoplasms. Clinicians also should consider conditions that are induced by friction or trauma. In our patient, dermoscopy and RCM aided in the diagnosis of eccrine poroma due to the interference of prominent overlying verrucous changes.
Treatment of poroma is optional. Deeper lesions can be treated with surgical excision, and superficial lesions may be treated with electrosurgical destruction. Our patient was treated with surgical excision followed by repair of the surgical defect with a double V-Y flap.
The Diagnosis: Eccrine Poroma
Histopathology demonstrated epidermal thickening, epidermal protrusions, a well-defined mass of tumor cells that extended from the epidermis down to the dermis, and luminal structures. Poroid cells and ovoid nuclei with basophilic cytoplasm also were evident (Figure 1). Dermoscopy showed papillomatous growth, milky-red areas, and dotted vessels (Figure 2). Reflectance confocal microscopy (RCM) at the spinous layer showed hyporefractile, dark, roundish lumina surrounded by keratinocytes (Figure 3). Based on the histologic, dermoscopic, and RCM findings, our patient was diagnosed with eccrine poroma.

Goldman et al1 first described poroma in 1956. Poromas, which include eccrine poroma, are a group of benign cutaneous neoplasms arising from the terminal eccrine or apocrine sweat gland ducts.2 Histologically, poroid cells appear as cuboidal keratinocytes with monomorphous ovoid nuclei and discrete nucleoli.3 They usually appear as nodules or plaques with colors varying from flesh colored to red, brown, or bluish, and they clinically mimic several benign and malignant skin tumors. The differential diagnosis may include keratoacanthoma, plantar wart, verrucous carcinoma, basal cell carcinoma, and squamous cell carcinoma. Poromas can be of eccrine or apocrine origin.4 They also belong to a broad group of neoplasms, including nodular hidradenomas, clear cell hidradenomas, hidroacanthoma simplex, dermal duct tumors, and hidradenomas.5 Four subtypes—poroma, poroid hidradenoma, hidroacanthoma simplex, and dermal duct tumor—have been documented.6 Because poromas have nonspecific and variable clinical presentations, they often are misdiagnosed as other skin neoplasms, and differentiation may be difficult. For example, some cases of poroma present with follicular, sebaceous, and/or apocrine differentiation, leading to difficulty in diagnosis.

Characteristic features of eccrine poroma seen on dermoscopy and RCM have the potential to aid in the diagnosis compared to histopathology. Marchetti et al7 proposed 4 patterns of characteristic dermoscopic findings. Pattern 1 refers to the classic description with bleeding spots, a structureless yellow appearance, milkyred globules, and branched vessels. Patterns 2 and 3 simulate basal cell carcinoma, dermal nevus, or vascular tumors. Pattern 4 refers to tumors that are large in size and resemble keratinizing neoplasms.7 Brugués et al8 described poromas with the following RCM findings: an atypical honeycomb shape that was well separated from the normal epithelium, hyporefractile nests with atypical cells, lack of palisading, and dark holes. One study described RCM parameters as cords without palisading, dark holes, prominent vascularization, and abundant stroma—findings that were positively associated with poroma in a univariate analysis. These findings assist in distinguishing poromas from other conditions in the differential diagnosis.9

There is a substantial overlap in clinical appearance with malignant conditions, including basal cell carcinoma, squamous cell carcinoma, cutaneous metastases, and Paget disease; therefore, the use of dermoscopy and RCM may be helpful in the diagnosis and recognition of specific features, as well as the corresponding patterns of poroma. Poromas commonly display vascularized features due to the variability of dermoscopic patterns of eccrine poroma, and further studies are required to establish the specificity of vascularized features.
Acral lesions are more likely to show the classic clinical features of erythema and exophytic growth. A case of a collision tumor with the verrucous changes of poroma, seborrheic keratosis, and viral wart has been described.10 The verrucous changes may lead to misdiagnosis as plantar warts or other neoplasms. Clinicians also should consider conditions that are induced by friction or trauma. In our patient, dermoscopy and RCM aided in the diagnosis of eccrine poroma due to the interference of prominent overlying verrucous changes.
Treatment of poroma is optional. Deeper lesions can be treated with surgical excision, and superficial lesions may be treated with electrosurgical destruction. Our patient was treated with surgical excision followed by repair of the surgical defect with a double V-Y flap.
- Goldman P, Pinkus H, Rogin JR. Eccrine poroma; tumors exhibiting features of the epidermal sweat duct unit. AMA Arch Derm. 1956; 74:511-521.
- Miller AC, Adjei S, Temiz LA, et al. Dermal duct tumor: a diagnostic dilemma [published online January 28, 2022]. Dermatopathology (Basel). 2022;9:36-47. doi:10.3390/dermatopathology9010007
- Ahmed Jan N, Masood S. Poroma. StatPearls [Internet]. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK560909/
- Casper DJ, Glass LF, Shenefelt PD. An unusually large eccrine poroma: a case report and review of the literature. Cutis. 2011; 88:227-229.
- Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms. Int J Dermatol. 2014;53:1053-1061.
- Betti R, Bombonato C, Cerri A, et al. Unusual sites for poromas are not very unusual: a survey of 101 cases. Clin Exp Dermatol. 2014; 39:119-122.
- Marchetti MA, Marino ML, Virmani P, et al. Dermoscopic features and patterns of poromas: a multicenter observational case-control study conducted by the International Dermoscopy Society (IDS). J Eur Acad Dermatol Venereol. 2018;32:1263-1271.
- Brugués A, Gamboa M, Alós L, et al. The challenging diagnosis of eccrine poromas. J Am Acad Dermatol. 2016;74:E113-E115.
- Di Tullio F, Mandel VD, Ignazio S, et al. The role of reflectance confocal microscopy in the diagnosis of eccrine poroma: a retrospective casecontrol study. Exp Dermatol. 2022;31:1779-1790.
- Bloom BS, Kamino H, Hale CS, et al. Collision tumor of eccrine poroma, seborrheic keratosis, and a viral wart. Dermatol Online J. 2014;20:13030/qt8tm0r9b9.
- Goldman P, Pinkus H, Rogin JR. Eccrine poroma; tumors exhibiting features of the epidermal sweat duct unit. AMA Arch Derm. 1956; 74:511-521.
- Miller AC, Adjei S, Temiz LA, et al. Dermal duct tumor: a diagnostic dilemma [published online January 28, 2022]. Dermatopathology (Basel). 2022;9:36-47. doi:10.3390/dermatopathology9010007
- Ahmed Jan N, Masood S. Poroma. StatPearls [Internet]. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK560909/
- Casper DJ, Glass LF, Shenefelt PD. An unusually large eccrine poroma: a case report and review of the literature. Cutis. 2011; 88:227-229.
- Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms. Int J Dermatol. 2014;53:1053-1061.
- Betti R, Bombonato C, Cerri A, et al. Unusual sites for poromas are not very unusual: a survey of 101 cases. Clin Exp Dermatol. 2014; 39:119-122.
- Marchetti MA, Marino ML, Virmani P, et al. Dermoscopic features and patterns of poromas: a multicenter observational case-control study conducted by the International Dermoscopy Society (IDS). J Eur Acad Dermatol Venereol. 2018;32:1263-1271.
- Brugués A, Gamboa M, Alós L, et al. The challenging diagnosis of eccrine poromas. J Am Acad Dermatol. 2016;74:E113-E115.
- Di Tullio F, Mandel VD, Ignazio S, et al. The role of reflectance confocal microscopy in the diagnosis of eccrine poroma: a retrospective casecontrol study. Exp Dermatol. 2022;31:1779-1790.
- Bloom BS, Kamino H, Hale CS, et al. Collision tumor of eccrine poroma, seborrheic keratosis, and a viral wart. Dermatol Online J. 2014;20:13030/qt8tm0r9b9.
A 62-year-old man presented with an enlarging plaque on the foot of 3 years’ duration. He experienced minor pain while walking but reported no other symptoms. His family history was negative for similar anomalies, and his medical history was negative for the presence of malignant tumors. Physical examination revealed a 2-mm erythematous plaque on the plantar aspect of the right foot with prominent overlying verrucous changes and no ulceration or regional lymphadenopathy. Dermoscopy and reflectance confocal microscopy of the lesion were performed along with a histopathologic examination after complete surgical excision.
A Rare Case of Leptomeningeal Carcinomatosis From Gastroesophageal Adenocarcinoma Masquerading as Polyneuropathy
INTRODUCTION
Leptomeningeal metastasis (LM) is an extremely rare complication of gastroesophageal (GE) cancer. Diagnosis is challenging due to frequently nonspecific clinical presentations, limited sensitivity of diagnostic testing, and potential overlap with neurologic immune-related adverse events (irAE). We describe a case of metastatic gastroesophageal cancer on immunotherapy presenting with LM masquerading as polyneuropathy.
CASE REPORT
A 74-year-old male with HER2+ GE junction cancer with peritoneal metastases diagnosed 6 months ago, on maintenance trastuzumab/pembrolizumab and with no previous history of cranial or spinal disease, presented with worsening ataxia, headache, and diplopia for one month with multiple negative outpatient MRIs. Examination showed left abducens nerve palsy, dysmetria and absent deep tendon reflexes in upper and lower extremities. CT head was unremarkable, and MRI showed non-specific mild enhancement of the right optic nerve, symmetrical lumbosacral nerve roots and cauda equina concerning for paraneoplastic versus immunotherapy-related polyneuropathy. He was started on empiric high-dose corticosteroids. PET-CT was negative for FDG-avid lesions. Cerebrospinal fluid (CSF) analysis revealed moderate pleocytosis with many large atypical cells, elevated protein (118 mg/dL) and LDH (28 IU/L). Immunohistochemistry was positive for CDX2, CA 19-9, CK7, and pankeratin, consistent with metastatic adenocarcinoma, negative for HER2 in contrast to the original tumor. He subsequently developed hydrocephalus requiring a ventriculoperitoneal shunt. He received ten fractions of whole brain irradiation before electing to pursue hospice care.
DISCUSSION
LM is an extremely rare complication of GE cancer with an incidence of <0.2% and carries a poor prognosis. Differentiation between LM and irAE in patients on immunotherapy can be challenging. Diagnosis relies mostly on CSF cytology, and lumbar puncture should not be delayed in patients with new neurologic symptoms. Treatment options are intrathecal chemotherapy, radiation and steroids. A recent phase II trial has shown promise for intrathecal trastuzumab in patients with HER2+ cancers, but options for HER2 negative disease remain mostly palliative.
CONCLUSIONS
Our case highlights the need for suspecting this rare metastatic site, as early diagnosis and genetic characterization allow for exploring more treatment options including targeted therapies which may improve overall survival and quality of life.
INTRODUCTION
Leptomeningeal metastasis (LM) is an extremely rare complication of gastroesophageal (GE) cancer. Diagnosis is challenging due to frequently nonspecific clinical presentations, limited sensitivity of diagnostic testing, and potential overlap with neurologic immune-related adverse events (irAE). We describe a case of metastatic gastroesophageal cancer on immunotherapy presenting with LM masquerading as polyneuropathy.
CASE REPORT
A 74-year-old male with HER2+ GE junction cancer with peritoneal metastases diagnosed 6 months ago, on maintenance trastuzumab/pembrolizumab and with no previous history of cranial or spinal disease, presented with worsening ataxia, headache, and diplopia for one month with multiple negative outpatient MRIs. Examination showed left abducens nerve palsy, dysmetria and absent deep tendon reflexes in upper and lower extremities. CT head was unremarkable, and MRI showed non-specific mild enhancement of the right optic nerve, symmetrical lumbosacral nerve roots and cauda equina concerning for paraneoplastic versus immunotherapy-related polyneuropathy. He was started on empiric high-dose corticosteroids. PET-CT was negative for FDG-avid lesions. Cerebrospinal fluid (CSF) analysis revealed moderate pleocytosis with many large atypical cells, elevated protein (118 mg/dL) and LDH (28 IU/L). Immunohistochemistry was positive for CDX2, CA 19-9, CK7, and pankeratin, consistent with metastatic adenocarcinoma, negative for HER2 in contrast to the original tumor. He subsequently developed hydrocephalus requiring a ventriculoperitoneal shunt. He received ten fractions of whole brain irradiation before electing to pursue hospice care.
DISCUSSION
LM is an extremely rare complication of GE cancer with an incidence of <0.2% and carries a poor prognosis. Differentiation between LM and irAE in patients on immunotherapy can be challenging. Diagnosis relies mostly on CSF cytology, and lumbar puncture should not be delayed in patients with new neurologic symptoms. Treatment options are intrathecal chemotherapy, radiation and steroids. A recent phase II trial has shown promise for intrathecal trastuzumab in patients with HER2+ cancers, but options for HER2 negative disease remain mostly palliative.
CONCLUSIONS
Our case highlights the need for suspecting this rare metastatic site, as early diagnosis and genetic characterization allow for exploring more treatment options including targeted therapies which may improve overall survival and quality of life.
INTRODUCTION
Leptomeningeal metastasis (LM) is an extremely rare complication of gastroesophageal (GE) cancer. Diagnosis is challenging due to frequently nonspecific clinical presentations, limited sensitivity of diagnostic testing, and potential overlap with neurologic immune-related adverse events (irAE). We describe a case of metastatic gastroesophageal cancer on immunotherapy presenting with LM masquerading as polyneuropathy.
CASE REPORT
A 74-year-old male with HER2+ GE junction cancer with peritoneal metastases diagnosed 6 months ago, on maintenance trastuzumab/pembrolizumab and with no previous history of cranial or spinal disease, presented with worsening ataxia, headache, and diplopia for one month with multiple negative outpatient MRIs. Examination showed left abducens nerve palsy, dysmetria and absent deep tendon reflexes in upper and lower extremities. CT head was unremarkable, and MRI showed non-specific mild enhancement of the right optic nerve, symmetrical lumbosacral nerve roots and cauda equina concerning for paraneoplastic versus immunotherapy-related polyneuropathy. He was started on empiric high-dose corticosteroids. PET-CT was negative for FDG-avid lesions. Cerebrospinal fluid (CSF) analysis revealed moderate pleocytosis with many large atypical cells, elevated protein (118 mg/dL) and LDH (28 IU/L). Immunohistochemistry was positive for CDX2, CA 19-9, CK7, and pankeratin, consistent with metastatic adenocarcinoma, negative for HER2 in contrast to the original tumor. He subsequently developed hydrocephalus requiring a ventriculoperitoneal shunt. He received ten fractions of whole brain irradiation before electing to pursue hospice care.
DISCUSSION
LM is an extremely rare complication of GE cancer with an incidence of <0.2% and carries a poor prognosis. Differentiation between LM and irAE in patients on immunotherapy can be challenging. Diagnosis relies mostly on CSF cytology, and lumbar puncture should not be delayed in patients with new neurologic symptoms. Treatment options are intrathecal chemotherapy, radiation and steroids. A recent phase II trial has shown promise for intrathecal trastuzumab in patients with HER2+ cancers, but options for HER2 negative disease remain mostly palliative.
CONCLUSIONS
Our case highlights the need for suspecting this rare metastatic site, as early diagnosis and genetic characterization allow for exploring more treatment options including targeted therapies which may improve overall survival and quality of life.