User login
Screening for Meaning: Do Skin Cancer Screening Events Accomplish Anything?
Screening for Meaning: Do Skin Cancer Screening Events Accomplish Anything?
When Skin Cancer Awareness Month rolls around every May, my social media feed is inundated with posts extolling the benefits of total body skin examinations and the life-saving potential of skin cancer screenings; however, time and again the US Preventive Services Task Force (USPSTF)—the leading authority on evidence-based public health recommendations in the United States—has found the evidence supporting skin cancer screenings to be insufficient. The USPSTF has cited a lack of high-quality studies and inadequate data to recommend screening for the general population, excluding those at elevated risk due to personal, family, or occupational history.1 A 2019 Cochrane review went further, concluding that current evidence refutes the utility of population-based screening for melanoma.2
Despite these findings, skin cancer screenings and total body skin examinations remain popular among patients both with and without a personal or family history of cutaneous malignancy. Indeed, the anecdotal experience of dermatologists worldwide suggests an intangible benefit to screening that persists, even if robust data to support it remain elusive.
Putting aside studies that suggest these screenings help identify melanomas at earlier stages and with reduced Breslow thicknesses,3 there is a crucial benefit from face-to-face interaction between medical professionals and the public during skin cancer screening events or health fairs. This interaction has become especially important in an era when misinformation thrives online and so-called skin care “experts” with no formal training can amass tens of thousands—or even millions—of followers on social media.
So, what are the intangible benefits of the face-to-face interactions that occur naturally during skin cancer screenings? The most obvious is education. While the USPSTF may not recommend routine screening for skin cancer in the general population, it does endorse education for children, adolescents, and adults on the importance of minimizing exposure to UV radiation, particularly those with lighter skin tones.4 Publicly advertised skin cancer screenings at health fairs or other community events may offer an opportunity to raise awareness about sun safety and protection, including the value of peak UV avoidance, sun-protective clothing, and proper sunscreen use; these settings also serve as platforms for health care providers to counter misinformation, including concerns about sunscreen safety both for the patient and the environment, overhyped risks for vitamin D deficiency from sun avoidance, and myths about low skin cancer risk in patients with skin of color.
While the benefits of skin self-examination (SSE) remain uncertain, especially in low-risk populations, screening events provide an opportunity to educate patients on who is most likely to benefit from SSE and in whom the practice may cause more harm than good.5 For higher-risk individuals such as melanoma survivors or those with a strong family history, screening fairs can serve as meaningful touchpoints that reinforce the importance of sun protection and regular examinations with a health care provider. For those eager to perform SSEs, these events offer the chance to teach best practices—how to conduct SSEs effectively, what features to look for (eg, the ABCDE method or the ugly duckling sign), and when to seek professional care.
Finally (and importantly), skin cancer screening events provide peace of mind for patients. Reassurance from a professional about a benign skin lesion can alleviate anxiety that might otherwise lead to emergency or urgent care visits. While cellulitis and other skin infections are the most common dermatologic conditions seen in emergency settings, benign neoplasms and similar nonurgent conditions still contribute a substantial burden to urgent care systems in the United States.6 Outside emergency care, systems-level data support what many of us observe in practice: two of the most common reasons for referral to dermatology are benign neoplasms and epidermoid cysts, accounting for millions of visits annually.7 In fact, recent claims data suggest that the most common diagnosis made in US dermatology clinics in 2023 was (you guessed it!) seborrheic keratosis.8
What if instead of requiring a patient to wait weeks for a primary care appointment and months for a dermatology referral—all while worrying about a rapidly growing pigmented lesion and incurring costs in copays, travel, lost wages, and time away from work—we offered a fast, trustworthy, and free evaluation that meets the patient where they live, work, or socialize? An evaluation that not only eases their fears but also provides meaningful education about skin cancer prevention and screening guidelines? While precautions must of course be taken to ensure that the quality and completeness of such an examination equals that of an in-clinic evaluation, if services of this quality can be provided, public screening events may offer a simple, accessible, and valuable solution that delivers peace of mind and helps reduce unnecessary strain on emergency, primary, and specialty care networks.
- US Preventive Services Task Force; Mangione CM, Barry MJ, Nicholson WK, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2023;329:1290-1295. doi:10.1001/jama.2023.4342
- Johansson M, Brodersen J, Gøtzsche PC. Screening for reducing morbidity and mortality in malignant melanoma. Cochrane Database Syst Rev. 2019;6:CD012352. doi:10.1002/14651858.CD012352.pub2
- Matsumoto M, Wack S, Weinstock MA, et al. Five-year outcomes of a melanoma screening initiative in a large health care system. JAMA Dermatol. 2022;158:504-512. doi:10.1001/jamadermatol.2022.0253
- Grossman DC, Curry SJ, Owens DK, et al. Behavioral counseling to prevent skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1134-1142.
- Ersser SJ, Effah A, Dyson J, et al. Effectiveness of interventions to support the early detection of skin cancer through skin self‐examination: a systematic review and meta‐analysis. Br J Dermatol. 2019;180:1339-1347. doi:10.1111/bjd.17529
- Nadkarni A, Domeisen N, Hill D, et al. The most common dermatology diagnoses in the emergency department. J Am Acad Dermatol. 2016;75:1261-1266. doi:10.1016/j.jaad.2016.07.054
- Grada A, Muddasani S, Fleischer AB Jr. Trends in office visits for the five most common skin diseases in the United States. J Clin Aesthet Dermatol. 2022;15:E82-E86.
- Definitive Healthcare. What are the most common diagnoses by dermatologists? Published January 31, 2024. Accessed May 5, 2025. https://www.definitivehc.com/resources/healthcare-insights/top-dermatologist-diagnoses
When Skin Cancer Awareness Month rolls around every May, my social media feed is inundated with posts extolling the benefits of total body skin examinations and the life-saving potential of skin cancer screenings; however, time and again the US Preventive Services Task Force (USPSTF)—the leading authority on evidence-based public health recommendations in the United States—has found the evidence supporting skin cancer screenings to be insufficient. The USPSTF has cited a lack of high-quality studies and inadequate data to recommend screening for the general population, excluding those at elevated risk due to personal, family, or occupational history.1 A 2019 Cochrane review went further, concluding that current evidence refutes the utility of population-based screening for melanoma.2
Despite these findings, skin cancer screenings and total body skin examinations remain popular among patients both with and without a personal or family history of cutaneous malignancy. Indeed, the anecdotal experience of dermatologists worldwide suggests an intangible benefit to screening that persists, even if robust data to support it remain elusive.
Putting aside studies that suggest these screenings help identify melanomas at earlier stages and with reduced Breslow thicknesses,3 there is a crucial benefit from face-to-face interaction between medical professionals and the public during skin cancer screening events or health fairs. This interaction has become especially important in an era when misinformation thrives online and so-called skin care “experts” with no formal training can amass tens of thousands—or even millions—of followers on social media.
So, what are the intangible benefits of the face-to-face interactions that occur naturally during skin cancer screenings? The most obvious is education. While the USPSTF may not recommend routine screening for skin cancer in the general population, it does endorse education for children, adolescents, and adults on the importance of minimizing exposure to UV radiation, particularly those with lighter skin tones.4 Publicly advertised skin cancer screenings at health fairs or other community events may offer an opportunity to raise awareness about sun safety and protection, including the value of peak UV avoidance, sun-protective clothing, and proper sunscreen use; these settings also serve as platforms for health care providers to counter misinformation, including concerns about sunscreen safety both for the patient and the environment, overhyped risks for vitamin D deficiency from sun avoidance, and myths about low skin cancer risk in patients with skin of color.
While the benefits of skin self-examination (SSE) remain uncertain, especially in low-risk populations, screening events provide an opportunity to educate patients on who is most likely to benefit from SSE and in whom the practice may cause more harm than good.5 For higher-risk individuals such as melanoma survivors or those with a strong family history, screening fairs can serve as meaningful touchpoints that reinforce the importance of sun protection and regular examinations with a health care provider. For those eager to perform SSEs, these events offer the chance to teach best practices—how to conduct SSEs effectively, what features to look for (eg, the ABCDE method or the ugly duckling sign), and when to seek professional care.
Finally (and importantly), skin cancer screening events provide peace of mind for patients. Reassurance from a professional about a benign skin lesion can alleviate anxiety that might otherwise lead to emergency or urgent care visits. While cellulitis and other skin infections are the most common dermatologic conditions seen in emergency settings, benign neoplasms and similar nonurgent conditions still contribute a substantial burden to urgent care systems in the United States.6 Outside emergency care, systems-level data support what many of us observe in practice: two of the most common reasons for referral to dermatology are benign neoplasms and epidermoid cysts, accounting for millions of visits annually.7 In fact, recent claims data suggest that the most common diagnosis made in US dermatology clinics in 2023 was (you guessed it!) seborrheic keratosis.8
What if instead of requiring a patient to wait weeks for a primary care appointment and months for a dermatology referral—all while worrying about a rapidly growing pigmented lesion and incurring costs in copays, travel, lost wages, and time away from work—we offered a fast, trustworthy, and free evaluation that meets the patient where they live, work, or socialize? An evaluation that not only eases their fears but also provides meaningful education about skin cancer prevention and screening guidelines? While precautions must of course be taken to ensure that the quality and completeness of such an examination equals that of an in-clinic evaluation, if services of this quality can be provided, public screening events may offer a simple, accessible, and valuable solution that delivers peace of mind and helps reduce unnecessary strain on emergency, primary, and specialty care networks.
When Skin Cancer Awareness Month rolls around every May, my social media feed is inundated with posts extolling the benefits of total body skin examinations and the life-saving potential of skin cancer screenings; however, time and again the US Preventive Services Task Force (USPSTF)—the leading authority on evidence-based public health recommendations in the United States—has found the evidence supporting skin cancer screenings to be insufficient. The USPSTF has cited a lack of high-quality studies and inadequate data to recommend screening for the general population, excluding those at elevated risk due to personal, family, or occupational history.1 A 2019 Cochrane review went further, concluding that current evidence refutes the utility of population-based screening for melanoma.2
Despite these findings, skin cancer screenings and total body skin examinations remain popular among patients both with and without a personal or family history of cutaneous malignancy. Indeed, the anecdotal experience of dermatologists worldwide suggests an intangible benefit to screening that persists, even if robust data to support it remain elusive.
Putting aside studies that suggest these screenings help identify melanomas at earlier stages and with reduced Breslow thicknesses,3 there is a crucial benefit from face-to-face interaction between medical professionals and the public during skin cancer screening events or health fairs. This interaction has become especially important in an era when misinformation thrives online and so-called skin care “experts” with no formal training can amass tens of thousands—or even millions—of followers on social media.
So, what are the intangible benefits of the face-to-face interactions that occur naturally during skin cancer screenings? The most obvious is education. While the USPSTF may not recommend routine screening for skin cancer in the general population, it does endorse education for children, adolescents, and adults on the importance of minimizing exposure to UV radiation, particularly those with lighter skin tones.4 Publicly advertised skin cancer screenings at health fairs or other community events may offer an opportunity to raise awareness about sun safety and protection, including the value of peak UV avoidance, sun-protective clothing, and proper sunscreen use; these settings also serve as platforms for health care providers to counter misinformation, including concerns about sunscreen safety both for the patient and the environment, overhyped risks for vitamin D deficiency from sun avoidance, and myths about low skin cancer risk in patients with skin of color.
While the benefits of skin self-examination (SSE) remain uncertain, especially in low-risk populations, screening events provide an opportunity to educate patients on who is most likely to benefit from SSE and in whom the practice may cause more harm than good.5 For higher-risk individuals such as melanoma survivors or those with a strong family history, screening fairs can serve as meaningful touchpoints that reinforce the importance of sun protection and regular examinations with a health care provider. For those eager to perform SSEs, these events offer the chance to teach best practices—how to conduct SSEs effectively, what features to look for (eg, the ABCDE method or the ugly duckling sign), and when to seek professional care.
Finally (and importantly), skin cancer screening events provide peace of mind for patients. Reassurance from a professional about a benign skin lesion can alleviate anxiety that might otherwise lead to emergency or urgent care visits. While cellulitis and other skin infections are the most common dermatologic conditions seen in emergency settings, benign neoplasms and similar nonurgent conditions still contribute a substantial burden to urgent care systems in the United States.6 Outside emergency care, systems-level data support what many of us observe in practice: two of the most common reasons for referral to dermatology are benign neoplasms and epidermoid cysts, accounting for millions of visits annually.7 In fact, recent claims data suggest that the most common diagnosis made in US dermatology clinics in 2023 was (you guessed it!) seborrheic keratosis.8
What if instead of requiring a patient to wait weeks for a primary care appointment and months for a dermatology referral—all while worrying about a rapidly growing pigmented lesion and incurring costs in copays, travel, lost wages, and time away from work—we offered a fast, trustworthy, and free evaluation that meets the patient where they live, work, or socialize? An evaluation that not only eases their fears but also provides meaningful education about skin cancer prevention and screening guidelines? While precautions must of course be taken to ensure that the quality and completeness of such an examination equals that of an in-clinic evaluation, if services of this quality can be provided, public screening events may offer a simple, accessible, and valuable solution that delivers peace of mind and helps reduce unnecessary strain on emergency, primary, and specialty care networks.
- US Preventive Services Task Force; Mangione CM, Barry MJ, Nicholson WK, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2023;329:1290-1295. doi:10.1001/jama.2023.4342
- Johansson M, Brodersen J, Gøtzsche PC. Screening for reducing morbidity and mortality in malignant melanoma. Cochrane Database Syst Rev. 2019;6:CD012352. doi:10.1002/14651858.CD012352.pub2
- Matsumoto M, Wack S, Weinstock MA, et al. Five-year outcomes of a melanoma screening initiative in a large health care system. JAMA Dermatol. 2022;158:504-512. doi:10.1001/jamadermatol.2022.0253
- Grossman DC, Curry SJ, Owens DK, et al. Behavioral counseling to prevent skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1134-1142.
- Ersser SJ, Effah A, Dyson J, et al. Effectiveness of interventions to support the early detection of skin cancer through skin self‐examination: a systematic review and meta‐analysis. Br J Dermatol. 2019;180:1339-1347. doi:10.1111/bjd.17529
- Nadkarni A, Domeisen N, Hill D, et al. The most common dermatology diagnoses in the emergency department. J Am Acad Dermatol. 2016;75:1261-1266. doi:10.1016/j.jaad.2016.07.054
- Grada A, Muddasani S, Fleischer AB Jr. Trends in office visits for the five most common skin diseases in the United States. J Clin Aesthet Dermatol. 2022;15:E82-E86.
- Definitive Healthcare. What are the most common diagnoses by dermatologists? Published January 31, 2024. Accessed May 5, 2025. https://www.definitivehc.com/resources/healthcare-insights/top-dermatologist-diagnoses
- US Preventive Services Task Force; Mangione CM, Barry MJ, Nicholson WK, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2023;329:1290-1295. doi:10.1001/jama.2023.4342
- Johansson M, Brodersen J, Gøtzsche PC. Screening for reducing morbidity and mortality in malignant melanoma. Cochrane Database Syst Rev. 2019;6:CD012352. doi:10.1002/14651858.CD012352.pub2
- Matsumoto M, Wack S, Weinstock MA, et al. Five-year outcomes of a melanoma screening initiative in a large health care system. JAMA Dermatol. 2022;158:504-512. doi:10.1001/jamadermatol.2022.0253
- Grossman DC, Curry SJ, Owens DK, et al. Behavioral counseling to prevent skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1134-1142.
- Ersser SJ, Effah A, Dyson J, et al. Effectiveness of interventions to support the early detection of skin cancer through skin self‐examination: a systematic review and meta‐analysis. Br J Dermatol. 2019;180:1339-1347. doi:10.1111/bjd.17529
- Nadkarni A, Domeisen N, Hill D, et al. The most common dermatology diagnoses in the emergency department. J Am Acad Dermatol. 2016;75:1261-1266. doi:10.1016/j.jaad.2016.07.054
- Grada A, Muddasani S, Fleischer AB Jr. Trends in office visits for the five most common skin diseases in the United States. J Clin Aesthet Dermatol. 2022;15:E82-E86.
- Definitive Healthcare. What are the most common diagnoses by dermatologists? Published January 31, 2024. Accessed May 5, 2025. https://www.definitivehc.com/resources/healthcare-insights/top-dermatologist-diagnoses
Screening for Meaning: Do Skin Cancer Screening Events Accomplish Anything?
Screening for Meaning: Do Skin Cancer Screening Events Accomplish Anything?
Operational Risk Management in Dermatologic Procedures
Operational Risk Management in Dermatologic Procedures
Operational risk management (ORM) refers to the systematic identification and assessment of daily operational risks within an organization designed to mitigate negative financial, reputational, and safety outcomes while maximizing efficiency and achievement of objectives.1 Operational risk management is indispensable to modern military operations, optimizing mission readiness while minimizing complications and personnel morbidity. Application of ORM in medicine holds considerable promise due to the emphasis on precise and efficient decision-making in high-stakes environments, where the margin for error is minimal. In this article, we propose integrating ORM principles into dermatologic surgery to enhance patient-centered care through improved counseling, risk assessment, and procedural outcomes.
Principles and Processes of ORM
The ORM framework is built on 4 fundamental principles: accept risk when benefits outweigh the cost, accept no unnecessary risk, anticipate and manage risk by planning, and make risk decisions at the right level.2 These principles form the foundation of the ORM’s systematic 5-step approach to identify hazards, assess hazards, make risk decisions, implement controls, and supervise. Key to the ORM process is the use of risk assessment codes and the risk assessment matrix to quantify and prioritize risks. Risk assessment codes are numerical values assigned to hazards based on their assessed severity and probability. The risk assessment matrix is a tool that plots the severity of a hazard against its probability. By locating a hazard on the matrix, users can visualize its risk level in terms of severity and probability. Building and using the risk assessment matrix begins with determining severity by assessing the potential impact of a hazard and categorizing it into levels (catastrophic, critical, moderate, or negligible). Next, probability is determined by evaluating the likelihood of occurrence (frequent, likely, occasional, seldom, or unlikely). Finally, the severity and probability are combined to assign a risk assessment code, which indicates the risk level and helps visualize criticality. Systematically applying these principles and processes enables users to make informed decisions that balance mission objectives with safety.
Proposed Framework for ORM in Dermatology Surgery
Current risk mitigation in dermatologic surgery includes strict medication oversight, sterilization protocols, and photography to prevent wrong-site surgeries. Preoperative risk assessment through conducting a thorough patient history is vital, considering factors such as pregnancy, allergies, bleeding history, cardiac devices, and keloid propensity, all of which impact surgical outcomes.3-5 After gathering the patient’s history, dermatologists determine appropriateness for surgery and its inherent risks, typically via an informed consent process outlining the diagnosis and procedure purpose as well as a list of risks, benefits, and alternatives, including forgoing treatment.
Importantly, the standard process for dermatologic risk evaluation often lacks a comprehensive systematic approach seen in other higher-risk surgical fields. For example, general surgeons frequently utilize risk assessment calculators such as the one developed by the American College of Surgeons’ National Surgical Quality Improvement Program to estimate surgical complications.6 While specific guidelines exist for evaluating factors such as hypertension or anticoagulant use, no single tool synthesizes all patient risk factors for a unified assessment. Therefore, we propose integrating ORM as a structured decision-making process that offers a more consistent means for dermatologists to evaluate, synthesize, categorize, and present risks to patients. Our proposed process includes translating military mishap severity into a framework that helps patients better understand decisions about their health care when using ORM (eTable 1). The proposed process also provides dermatologists with a systematic, proactive, and iterative approach to assessing risks that allows them to consistently qualify medical decisions (eTable 2).
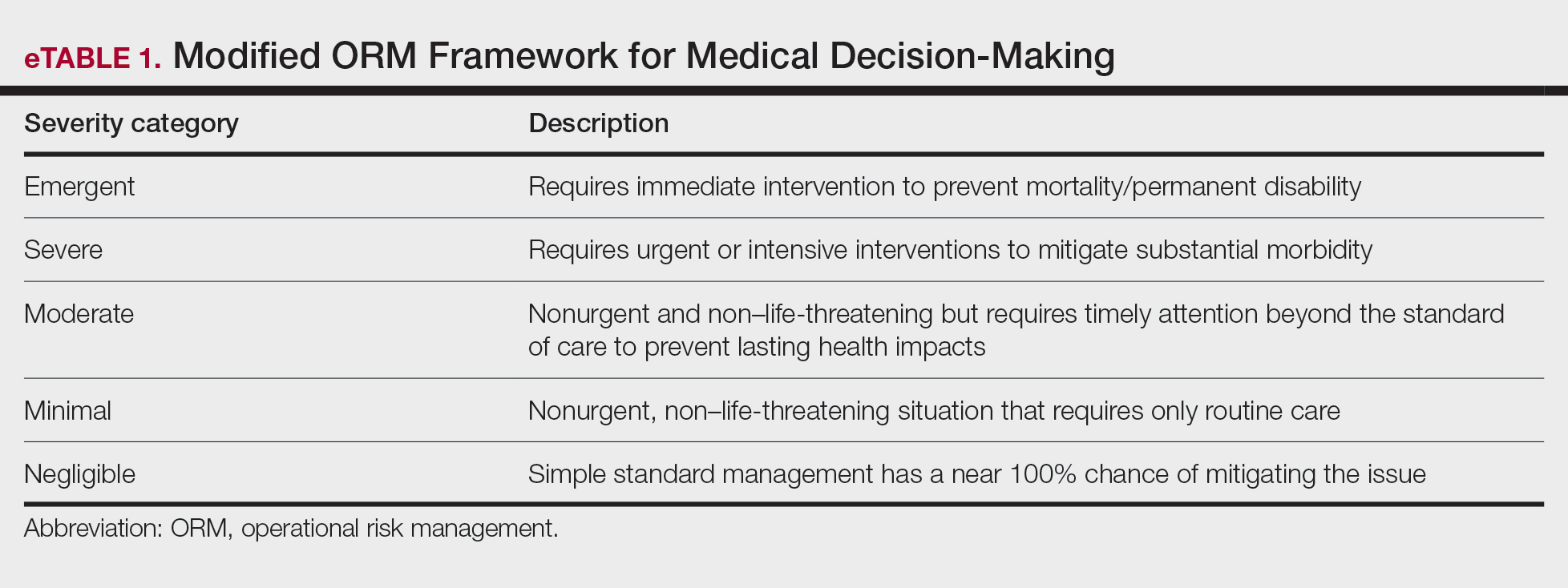
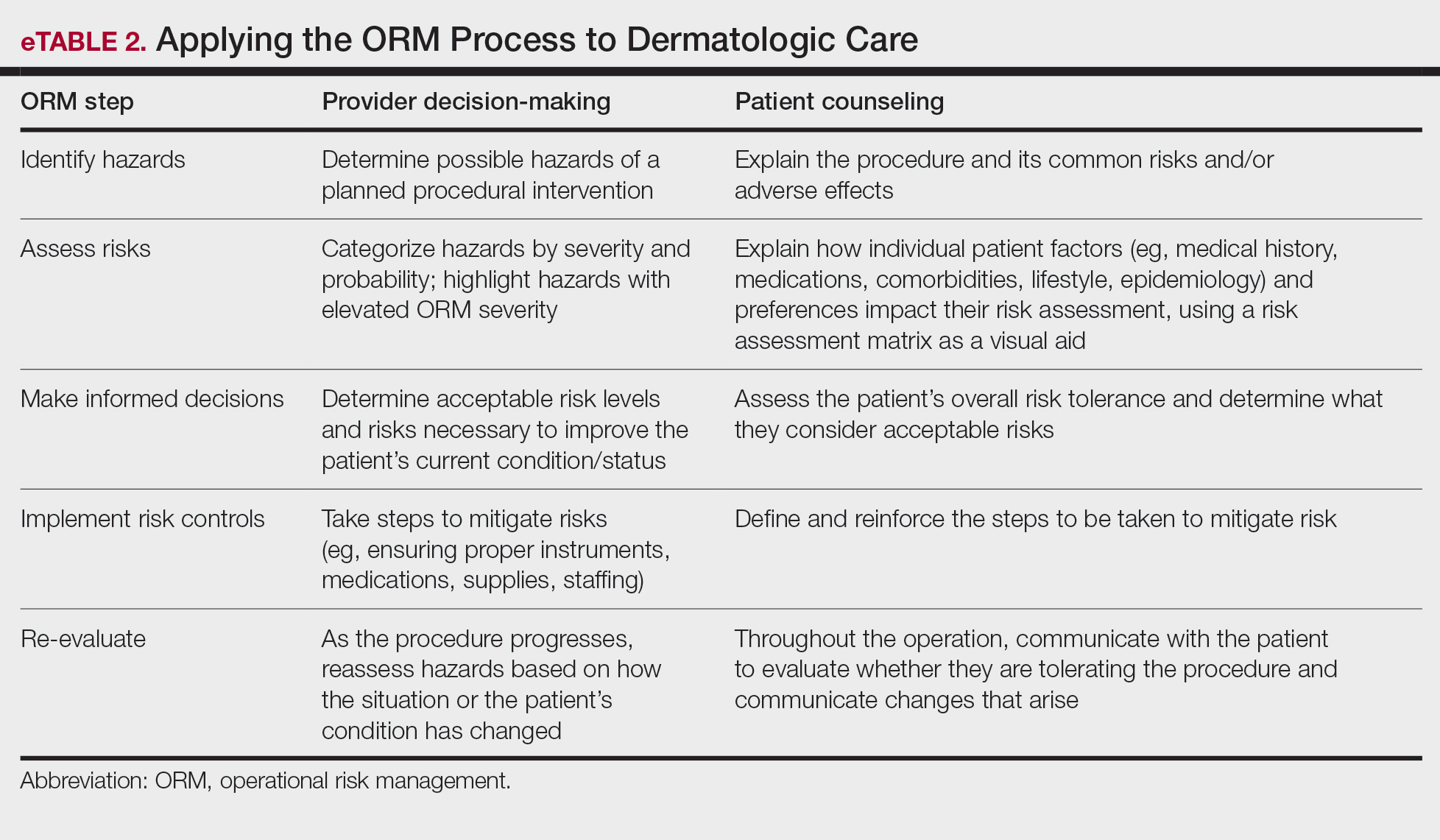
Patients often struggle to understand surgical risk severity, including overestimating the risks of routine minor procedures or underestimating the risks of more intensive procedures.7,8 Incorporating ORM into patient communication mirrors the provider’s process but uses patient-friendly terminology—it is discussion based and integrates patient preferences and tolerances (eTable 2). These steps often occur informally in dermatologic counseling; however, an organized structured approach, especially using a visual aid such as a risk assessment matrix, enhances patient comprehension, recall, and satisfaction.9
Practical Scenarios
Integrating ORM into dermatologic surgery is a proactive iterative process for both provider decision-making and patient communication. Leveraging a risk assessment matrix as a visual aid allows for clear identification, evaluation, and mitigation of hazards, fostering collaborative choices with regard to the treatment approach. Here we provide 2 case scenarios highlighting how ORM and the risk assessment matrix can be used in the management of a complex patient with a lesion in a high-risk location as well as to address patient anxiety and comorbidities. It is important to note that the way the matrices are completed in the examples provided may differ compared to other providers. The purpose of ORM is not to dictate risk categories but to serve as a tool for providers to take their own experiences and knowledge of the patient to guide their decision-making and counseling processes.
Case Scenario 1—An elderly man with a history of diabetes, cardiovascular accident, coronary artery bypass grafting, and multiple squamous cell carcinoma excisions presents for evaluation of a 1-cm squamous cell carcinoma in situ on the left leg. His current medications include an anticoagulant and antihypertensives.
In this scenario, the provider would apply ORM by identifying and assessing hazards, making risk decisions, implementing controls, and supervising care.
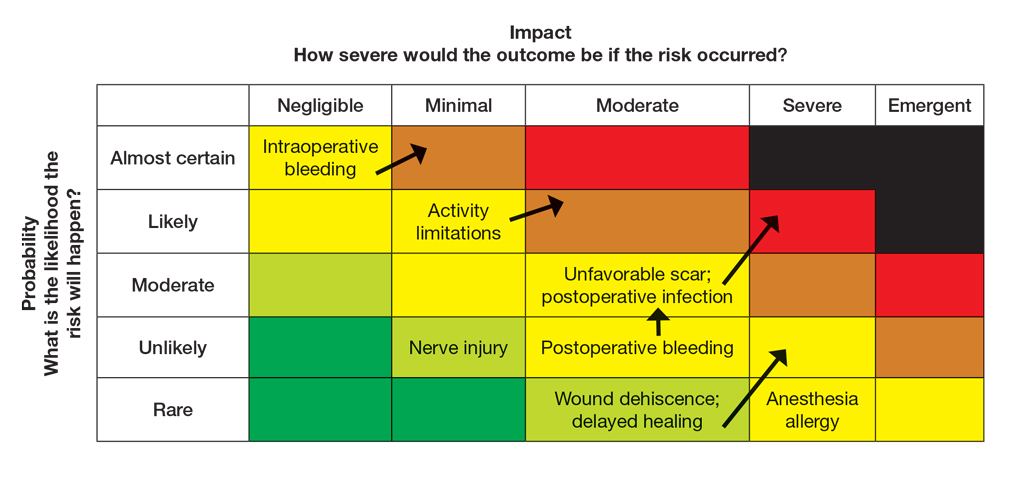
General hazards for excision on the leg include bleeding, infection, scarring, pain, delayed healing, activity limitations, and possible further procedures. Before the visit, the provider should prepare baseline risk matrices for 2 potential treatment options: wide local excision and electrodessication and curettage. For example, surgical bleeding may be assessed as negligible severity and almost certain probability for a general excision.
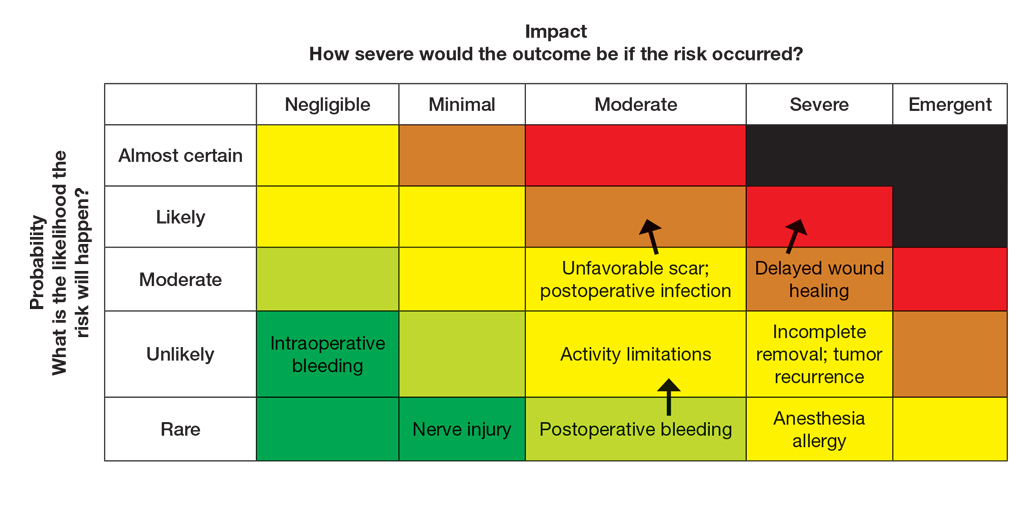
Next, the provider would incorporate the patient’s unique history in the risk matrices (eFigures 1 and 2). The patient’s use of an anticoagulant indicates a bleeding risk; therefore, the provider may shift the severity to minimal clinical concern, understanding the need for enhanced perioperative management. The history of diabetes also has a considerable impact on wound healing, so the provider might elevate the probability of delayed wound healing from rare to unlikely and the severity from moderate to severe. The prior cardiovascular accident also raises concerns about mobility and activity limitations during recovery, which could be escalated from minimal to moderate clinical concern if postoperative limitations on ambulation increase the risk for new clots. Based on this internal assessment, the provider identifies which risks are elevated and require further attention and discussion with the patient, helping tailor the counseling approach and potential treatment plan. The provider should begin to consider initial control measures such as coordinating anticoagulant management, ensuring diabetes is well controlled, and planning for postoperative ambulation support.
Once the provider has conducted the internal assessment, the ORM matrices become powerful tools for shared decision-making with the patient. The provider can walk the patient through the procedures and their common risks and then explain how their individual situation modifies the risks. The visual and explicit upgrade on the matrices allows the patient to clearly see how unique factors influence their personal risk profile, moving beyond a generic list of complications. The provider then should engage the patient in a discussion about their risk tolerance, which is crucial for mutual agreement on whether to proceed with treatment and, if so, which procedure is most appropriate given the patient’s comfort level with their individualized risk profile. Then the provider should reinforce the proactive steps planned to mitigate the identified risks to provide assurance and reinforce the collaborative approach to safety.
Finally, throughout the preoperative and postoperative phases, the provider should continuously monitor the patient’s condition and the effectiveness of the control measures, adjusting the plan as needed.
In this scenario, both the provider and the patient participated in the risk assessment, with the provider completing the assessment before the visit and presenting it to the patient or performing the assessment in real time with the patient present to explain the reasoning behind assignment of risk based on each procedure and the patient’s unique risk factors.
Case Scenario 2—A 38-year-old woman with a history of hypertension and procedural anxiety presents for evaluation of a biopsy-proven basal cell carcinoma on the nasal ala. The patient is taking diltiazem for hypertension and is compliant with her medication. Her blood pressure at the current visit is 148/96 mm Hg, which she attributes to white coat syndrome. Mohs micrographic surgery generally is the gold standard treatment for this case.
The provider’s ORM process, conducted either before or in real time during the visit, would begin with identification and assessment of the hazards. For Mohs surgery on the nasal ala, common hazards would include scarring, pain, infection, bleeding, and potential cosmetic distortion. Unique to this patient are the procedural anxiety and hypertension.
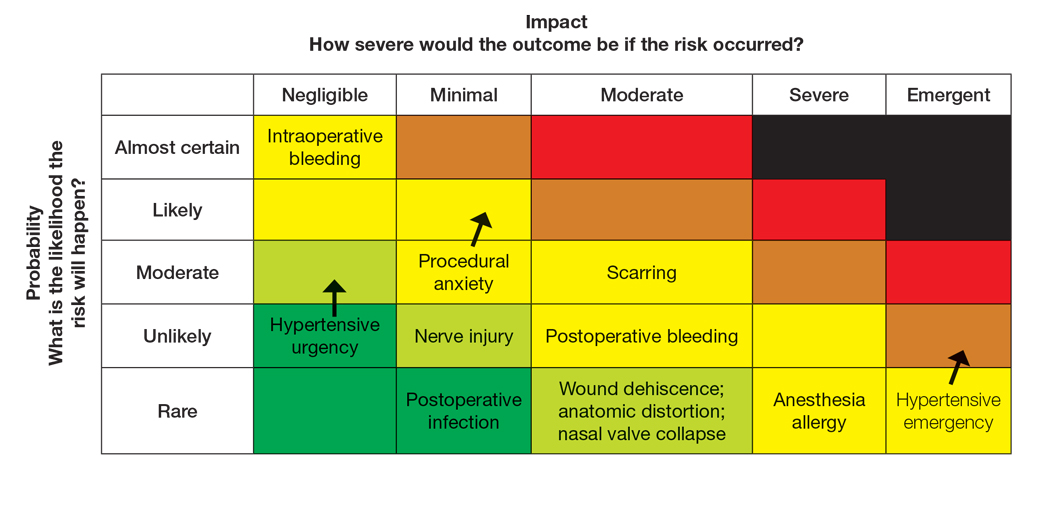
To populate the risk assessment matrix (eFigure 3), the provider would first map the baseline risks of Mohs surgery, which include considerable scarring as a moderate clinical concern but a seldom probability. Because the patient’s procedural anxiety directly increases the probability of intraoperative distress or elevated blood pressure during the procedure, the provider might assess patient distress/anxiety as a moderate clinical concern with a likely probability. While the patient’s blood pressure is controlled, the white coat syndrome raises the probability of hypertensive urgency/emergency during surgery; this might be elevated from unlikely to occasional or likely probability, and severity might increase from minimal to moderate due to its potential impact on procedural safety. The provider should consider strategies to address these elevated risks during the consultation. Then, as part of preprocedure planning, the provider should consider discussing anxiolytics, emphasizing medication compliance, and ensuring a calm environment for the patient’s surgery.
For this patient, the risk assessment matrix becomes a powerful tool to address fears and proactively manage her unique risk factors. To start the counseling process, the provider should explain the procedure, its benefits, and potential adverse effects. Then, the patient’s individualized risks can be visualized using the matrix, which also is an opportunity for reassurance, as it can alleviate patient fears by contextualizing rare but impactful outcomes.9
Now the provider can assess the patient’s risk tolerance. This discussion ensures that the patient’s comfort level and preferences are central to the treatment decision, even for a gold-standard procedure such as Mohs surgery. By listening and responding to the patient’s input, the provider can build trust and discuss strategies that can help control for some risk factors.
Finally, the provider would re-evaluate throughout the procedure by continuously monitoring the patient’s anxiety and vital signs. The provider should also be ready to adjust pain management or employ anxiety-reduction techniques.
Final Thoughts
Reviewing the risk assessment matrix can be an effective way to nonjudgmentally discuss a patient’s unique risk factors and provide a complete understanding of the planned treatment or procedure. It conveys to the patient that, as the provider, you are taking their health seriously when considering treatment options and can be a means to build patient rapport and trust. This approach mirrors risk communication strategies long employed in military operational planning, where transparency and structured risk evaluation are essential to maintaining mission readiness and unit cohesion.
- The OR Society. The history of OR. The OR Society. Published 2023.
- Naval Postgraduate School. ORM: operational risk management. Accessed September 12, 2025. https://nps.edu/web/safety/orm
- Smith C, Srivastava D, Nijhawan RI. Optimizing patient safety in dermatologic surgery. Dermatol Clin. 2019;37:319-328.
- Minkis K, Whittington A, Alam M. Dermatologic surgery emergencies: complications caused by systemic reactions, high-energy systems, and trauma. J Am Acad Dermatol. 2016;75:265-284.
- Pomerantz RG, Lee DA, Siegel DM. Risk assessment in surgical patients: balancing iatrogenic risks and benefits. Clin Dermatol. 2011;29:669-677.
- Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surgeons. 2013;217:833-842.
- Lloyd AJ. The extent of patients’ understanding of the risk of treatments. BMJ Qual Saf. 2001;10:i14-i18.
- Falagas ME, Korbila IP, Giannopoulou KP, et al. Informed consent: how much and what do patients understand? Am J Surg. 2009;198:420-435.
- Cohen SM, Baimas-George M, Ponce C, et al. Is a picture worth a thousand words? a scoping review of the impact of visual aids on patients undergoing surgery. J Surg Educ. 2024;81:1276-1292.
Operational risk management (ORM) refers to the systematic identification and assessment of daily operational risks within an organization designed to mitigate negative financial, reputational, and safety outcomes while maximizing efficiency and achievement of objectives.1 Operational risk management is indispensable to modern military operations, optimizing mission readiness while minimizing complications and personnel morbidity. Application of ORM in medicine holds considerable promise due to the emphasis on precise and efficient decision-making in high-stakes environments, where the margin for error is minimal. In this article, we propose integrating ORM principles into dermatologic surgery to enhance patient-centered care through improved counseling, risk assessment, and procedural outcomes.
Principles and Processes of ORM
The ORM framework is built on 4 fundamental principles: accept risk when benefits outweigh the cost, accept no unnecessary risk, anticipate and manage risk by planning, and make risk decisions at the right level.2 These principles form the foundation of the ORM’s systematic 5-step approach to identify hazards, assess hazards, make risk decisions, implement controls, and supervise. Key to the ORM process is the use of risk assessment codes and the risk assessment matrix to quantify and prioritize risks. Risk assessment codes are numerical values assigned to hazards based on their assessed severity and probability. The risk assessment matrix is a tool that plots the severity of a hazard against its probability. By locating a hazard on the matrix, users can visualize its risk level in terms of severity and probability. Building and using the risk assessment matrix begins with determining severity by assessing the potential impact of a hazard and categorizing it into levels (catastrophic, critical, moderate, or negligible). Next, probability is determined by evaluating the likelihood of occurrence (frequent, likely, occasional, seldom, or unlikely). Finally, the severity and probability are combined to assign a risk assessment code, which indicates the risk level and helps visualize criticality. Systematically applying these principles and processes enables users to make informed decisions that balance mission objectives with safety.
Proposed Framework for ORM in Dermatology Surgery
Current risk mitigation in dermatologic surgery includes strict medication oversight, sterilization protocols, and photography to prevent wrong-site surgeries. Preoperative risk assessment through conducting a thorough patient history is vital, considering factors such as pregnancy, allergies, bleeding history, cardiac devices, and keloid propensity, all of which impact surgical outcomes.3-5 After gathering the patient’s history, dermatologists determine appropriateness for surgery and its inherent risks, typically via an informed consent process outlining the diagnosis and procedure purpose as well as a list of risks, benefits, and alternatives, including forgoing treatment.
Importantly, the standard process for dermatologic risk evaluation often lacks a comprehensive systematic approach seen in other higher-risk surgical fields. For example, general surgeons frequently utilize risk assessment calculators such as the one developed by the American College of Surgeons’ National Surgical Quality Improvement Program to estimate surgical complications.6 While specific guidelines exist for evaluating factors such as hypertension or anticoagulant use, no single tool synthesizes all patient risk factors for a unified assessment. Therefore, we propose integrating ORM as a structured decision-making process that offers a more consistent means for dermatologists to evaluate, synthesize, categorize, and present risks to patients. Our proposed process includes translating military mishap severity into a framework that helps patients better understand decisions about their health care when using ORM (eTable 1). The proposed process also provides dermatologists with a systematic, proactive, and iterative approach to assessing risks that allows them to consistently qualify medical decisions (eTable 2).


Patients often struggle to understand surgical risk severity, including overestimating the risks of routine minor procedures or underestimating the risks of more intensive procedures.7,8 Incorporating ORM into patient communication mirrors the provider’s process but uses patient-friendly terminology—it is discussion based and integrates patient preferences and tolerances (eTable 2). These steps often occur informally in dermatologic counseling; however, an organized structured approach, especially using a visual aid such as a risk assessment matrix, enhances patient comprehension, recall, and satisfaction.9
Practical Scenarios
Integrating ORM into dermatologic surgery is a proactive iterative process for both provider decision-making and patient communication. Leveraging a risk assessment matrix as a visual aid allows for clear identification, evaluation, and mitigation of hazards, fostering collaborative choices with regard to the treatment approach. Here we provide 2 case scenarios highlighting how ORM and the risk assessment matrix can be used in the management of a complex patient with a lesion in a high-risk location as well as to address patient anxiety and comorbidities. It is important to note that the way the matrices are completed in the examples provided may differ compared to other providers. The purpose of ORM is not to dictate risk categories but to serve as a tool for providers to take their own experiences and knowledge of the patient to guide their decision-making and counseling processes.
Case Scenario 1—An elderly man with a history of diabetes, cardiovascular accident, coronary artery bypass grafting, and multiple squamous cell carcinoma excisions presents for evaluation of a 1-cm squamous cell carcinoma in situ on the left leg. His current medications include an anticoagulant and antihypertensives.
In this scenario, the provider would apply ORM by identifying and assessing hazards, making risk decisions, implementing controls, and supervising care.
General hazards for excision on the leg include bleeding, infection, scarring, pain, delayed healing, activity limitations, and possible further procedures. Before the visit, the provider should prepare baseline risk matrices for 2 potential treatment options: wide local excision and electrodessication and curettage. For example, surgical bleeding may be assessed as negligible severity and almost certain probability for a general excision.
Next, the provider would incorporate the patient’s unique history in the risk matrices (eFigures 1 and 2). The patient’s use of an anticoagulant indicates a bleeding risk; therefore, the provider may shift the severity to minimal clinical concern, understanding the need for enhanced perioperative management. The history of diabetes also has a considerable impact on wound healing, so the provider might elevate the probability of delayed wound healing from rare to unlikely and the severity from moderate to severe. The prior cardiovascular accident also raises concerns about mobility and activity limitations during recovery, which could be escalated from minimal to moderate clinical concern if postoperative limitations on ambulation increase the risk for new clots. Based on this internal assessment, the provider identifies which risks are elevated and require further attention and discussion with the patient, helping tailor the counseling approach and potential treatment plan. The provider should begin to consider initial control measures such as coordinating anticoagulant management, ensuring diabetes is well controlled, and planning for postoperative ambulation support.


Once the provider has conducted the internal assessment, the ORM matrices become powerful tools for shared decision-making with the patient. The provider can walk the patient through the procedures and their common risks and then explain how their individual situation modifies the risks. The visual and explicit upgrade on the matrices allows the patient to clearly see how unique factors influence their personal risk profile, moving beyond a generic list of complications. The provider then should engage the patient in a discussion about their risk tolerance, which is crucial for mutual agreement on whether to proceed with treatment and, if so, which procedure is most appropriate given the patient’s comfort level with their individualized risk profile. Then the provider should reinforce the proactive steps planned to mitigate the identified risks to provide assurance and reinforce the collaborative approach to safety.
Finally, throughout the preoperative and postoperative phases, the provider should continuously monitor the patient’s condition and the effectiveness of the control measures, adjusting the plan as needed.
In this scenario, both the provider and the patient participated in the risk assessment, with the provider completing the assessment before the visit and presenting it to the patient or performing the assessment in real time with the patient present to explain the reasoning behind assignment of risk based on each procedure and the patient’s unique risk factors.
Case Scenario 2—A 38-year-old woman with a history of hypertension and procedural anxiety presents for evaluation of a biopsy-proven basal cell carcinoma on the nasal ala. The patient is taking diltiazem for hypertension and is compliant with her medication. Her blood pressure at the current visit is 148/96 mm Hg, which she attributes to white coat syndrome. Mohs micrographic surgery generally is the gold standard treatment for this case.
The provider’s ORM process, conducted either before or in real time during the visit, would begin with identification and assessment of the hazards. For Mohs surgery on the nasal ala, common hazards would include scarring, pain, infection, bleeding, and potential cosmetic distortion. Unique to this patient are the procedural anxiety and hypertension.
To populate the risk assessment matrix (eFigure 3), the provider would first map the baseline risks of Mohs surgery, which include considerable scarring as a moderate clinical concern but a seldom probability. Because the patient’s procedural anxiety directly increases the probability of intraoperative distress or elevated blood pressure during the procedure, the provider might assess patient distress/anxiety as a moderate clinical concern with a likely probability. While the patient’s blood pressure is controlled, the white coat syndrome raises the probability of hypertensive urgency/emergency during surgery; this might be elevated from unlikely to occasional or likely probability, and severity might increase from minimal to moderate due to its potential impact on procedural safety. The provider should consider strategies to address these elevated risks during the consultation. Then, as part of preprocedure planning, the provider should consider discussing anxiolytics, emphasizing medication compliance, and ensuring a calm environment for the patient’s surgery.

For this patient, the risk assessment matrix becomes a powerful tool to address fears and proactively manage her unique risk factors. To start the counseling process, the provider should explain the procedure, its benefits, and potential adverse effects. Then, the patient’s individualized risks can be visualized using the matrix, which also is an opportunity for reassurance, as it can alleviate patient fears by contextualizing rare but impactful outcomes.9
Now the provider can assess the patient’s risk tolerance. This discussion ensures that the patient’s comfort level and preferences are central to the treatment decision, even for a gold-standard procedure such as Mohs surgery. By listening and responding to the patient’s input, the provider can build trust and discuss strategies that can help control for some risk factors.
Finally, the provider would re-evaluate throughout the procedure by continuously monitoring the patient’s anxiety and vital signs. The provider should also be ready to adjust pain management or employ anxiety-reduction techniques.
Final Thoughts
Reviewing the risk assessment matrix can be an effective way to nonjudgmentally discuss a patient’s unique risk factors and provide a complete understanding of the planned treatment or procedure. It conveys to the patient that, as the provider, you are taking their health seriously when considering treatment options and can be a means to build patient rapport and trust. This approach mirrors risk communication strategies long employed in military operational planning, where transparency and structured risk evaluation are essential to maintaining mission readiness and unit cohesion.
Operational risk management (ORM) refers to the systematic identification and assessment of daily operational risks within an organization designed to mitigate negative financial, reputational, and safety outcomes while maximizing efficiency and achievement of objectives.1 Operational risk management is indispensable to modern military operations, optimizing mission readiness while minimizing complications and personnel morbidity. Application of ORM in medicine holds considerable promise due to the emphasis on precise and efficient decision-making in high-stakes environments, where the margin for error is minimal. In this article, we propose integrating ORM principles into dermatologic surgery to enhance patient-centered care through improved counseling, risk assessment, and procedural outcomes.
Principles and Processes of ORM
The ORM framework is built on 4 fundamental principles: accept risk when benefits outweigh the cost, accept no unnecessary risk, anticipate and manage risk by planning, and make risk decisions at the right level.2 These principles form the foundation of the ORM’s systematic 5-step approach to identify hazards, assess hazards, make risk decisions, implement controls, and supervise. Key to the ORM process is the use of risk assessment codes and the risk assessment matrix to quantify and prioritize risks. Risk assessment codes are numerical values assigned to hazards based on their assessed severity and probability. The risk assessment matrix is a tool that plots the severity of a hazard against its probability. By locating a hazard on the matrix, users can visualize its risk level in terms of severity and probability. Building and using the risk assessment matrix begins with determining severity by assessing the potential impact of a hazard and categorizing it into levels (catastrophic, critical, moderate, or negligible). Next, probability is determined by evaluating the likelihood of occurrence (frequent, likely, occasional, seldom, or unlikely). Finally, the severity and probability are combined to assign a risk assessment code, which indicates the risk level and helps visualize criticality. Systematically applying these principles and processes enables users to make informed decisions that balance mission objectives with safety.
Proposed Framework for ORM in Dermatology Surgery
Current risk mitigation in dermatologic surgery includes strict medication oversight, sterilization protocols, and photography to prevent wrong-site surgeries. Preoperative risk assessment through conducting a thorough patient history is vital, considering factors such as pregnancy, allergies, bleeding history, cardiac devices, and keloid propensity, all of which impact surgical outcomes.3-5 After gathering the patient’s history, dermatologists determine appropriateness for surgery and its inherent risks, typically via an informed consent process outlining the diagnosis and procedure purpose as well as a list of risks, benefits, and alternatives, including forgoing treatment.
Importantly, the standard process for dermatologic risk evaluation often lacks a comprehensive systematic approach seen in other higher-risk surgical fields. For example, general surgeons frequently utilize risk assessment calculators such as the one developed by the American College of Surgeons’ National Surgical Quality Improvement Program to estimate surgical complications.6 While specific guidelines exist for evaluating factors such as hypertension or anticoagulant use, no single tool synthesizes all patient risk factors for a unified assessment. Therefore, we propose integrating ORM as a structured decision-making process that offers a more consistent means for dermatologists to evaluate, synthesize, categorize, and present risks to patients. Our proposed process includes translating military mishap severity into a framework that helps patients better understand decisions about their health care when using ORM (eTable 1). The proposed process also provides dermatologists with a systematic, proactive, and iterative approach to assessing risks that allows them to consistently qualify medical decisions (eTable 2).


Patients often struggle to understand surgical risk severity, including overestimating the risks of routine minor procedures or underestimating the risks of more intensive procedures.7,8 Incorporating ORM into patient communication mirrors the provider’s process but uses patient-friendly terminology—it is discussion based and integrates patient preferences and tolerances (eTable 2). These steps often occur informally in dermatologic counseling; however, an organized structured approach, especially using a visual aid such as a risk assessment matrix, enhances patient comprehension, recall, and satisfaction.9
Practical Scenarios
Integrating ORM into dermatologic surgery is a proactive iterative process for both provider decision-making and patient communication. Leveraging a risk assessment matrix as a visual aid allows for clear identification, evaluation, and mitigation of hazards, fostering collaborative choices with regard to the treatment approach. Here we provide 2 case scenarios highlighting how ORM and the risk assessment matrix can be used in the management of a complex patient with a lesion in a high-risk location as well as to address patient anxiety and comorbidities. It is important to note that the way the matrices are completed in the examples provided may differ compared to other providers. The purpose of ORM is not to dictate risk categories but to serve as a tool for providers to take their own experiences and knowledge of the patient to guide their decision-making and counseling processes.
Case Scenario 1—An elderly man with a history of diabetes, cardiovascular accident, coronary artery bypass grafting, and multiple squamous cell carcinoma excisions presents for evaluation of a 1-cm squamous cell carcinoma in situ on the left leg. His current medications include an anticoagulant and antihypertensives.
In this scenario, the provider would apply ORM by identifying and assessing hazards, making risk decisions, implementing controls, and supervising care.
General hazards for excision on the leg include bleeding, infection, scarring, pain, delayed healing, activity limitations, and possible further procedures. Before the visit, the provider should prepare baseline risk matrices for 2 potential treatment options: wide local excision and electrodessication and curettage. For example, surgical bleeding may be assessed as negligible severity and almost certain probability for a general excision.
Next, the provider would incorporate the patient’s unique history in the risk matrices (eFigures 1 and 2). The patient’s use of an anticoagulant indicates a bleeding risk; therefore, the provider may shift the severity to minimal clinical concern, understanding the need for enhanced perioperative management. The history of diabetes also has a considerable impact on wound healing, so the provider might elevate the probability of delayed wound healing from rare to unlikely and the severity from moderate to severe. The prior cardiovascular accident also raises concerns about mobility and activity limitations during recovery, which could be escalated from minimal to moderate clinical concern if postoperative limitations on ambulation increase the risk for new clots. Based on this internal assessment, the provider identifies which risks are elevated and require further attention and discussion with the patient, helping tailor the counseling approach and potential treatment plan. The provider should begin to consider initial control measures such as coordinating anticoagulant management, ensuring diabetes is well controlled, and planning for postoperative ambulation support.


Once the provider has conducted the internal assessment, the ORM matrices become powerful tools for shared decision-making with the patient. The provider can walk the patient through the procedures and their common risks and then explain how their individual situation modifies the risks. The visual and explicit upgrade on the matrices allows the patient to clearly see how unique factors influence their personal risk profile, moving beyond a generic list of complications. The provider then should engage the patient in a discussion about their risk tolerance, which is crucial for mutual agreement on whether to proceed with treatment and, if so, which procedure is most appropriate given the patient’s comfort level with their individualized risk profile. Then the provider should reinforce the proactive steps planned to mitigate the identified risks to provide assurance and reinforce the collaborative approach to safety.
Finally, throughout the preoperative and postoperative phases, the provider should continuously monitor the patient’s condition and the effectiveness of the control measures, adjusting the plan as needed.
In this scenario, both the provider and the patient participated in the risk assessment, with the provider completing the assessment before the visit and presenting it to the patient or performing the assessment in real time with the patient present to explain the reasoning behind assignment of risk based on each procedure and the patient’s unique risk factors.
Case Scenario 2—A 38-year-old woman with a history of hypertension and procedural anxiety presents for evaluation of a biopsy-proven basal cell carcinoma on the nasal ala. The patient is taking diltiazem for hypertension and is compliant with her medication. Her blood pressure at the current visit is 148/96 mm Hg, which she attributes to white coat syndrome. Mohs micrographic surgery generally is the gold standard treatment for this case.
The provider’s ORM process, conducted either before or in real time during the visit, would begin with identification and assessment of the hazards. For Mohs surgery on the nasal ala, common hazards would include scarring, pain, infection, bleeding, and potential cosmetic distortion. Unique to this patient are the procedural anxiety and hypertension.
To populate the risk assessment matrix (eFigure 3), the provider would first map the baseline risks of Mohs surgery, which include considerable scarring as a moderate clinical concern but a seldom probability. Because the patient’s procedural anxiety directly increases the probability of intraoperative distress or elevated blood pressure during the procedure, the provider might assess patient distress/anxiety as a moderate clinical concern with a likely probability. While the patient’s blood pressure is controlled, the white coat syndrome raises the probability of hypertensive urgency/emergency during surgery; this might be elevated from unlikely to occasional or likely probability, and severity might increase from minimal to moderate due to its potential impact on procedural safety. The provider should consider strategies to address these elevated risks during the consultation. Then, as part of preprocedure planning, the provider should consider discussing anxiolytics, emphasizing medication compliance, and ensuring a calm environment for the patient’s surgery.

For this patient, the risk assessment matrix becomes a powerful tool to address fears and proactively manage her unique risk factors. To start the counseling process, the provider should explain the procedure, its benefits, and potential adverse effects. Then, the patient’s individualized risks can be visualized using the matrix, which also is an opportunity for reassurance, as it can alleviate patient fears by contextualizing rare but impactful outcomes.9
Now the provider can assess the patient’s risk tolerance. This discussion ensures that the patient’s comfort level and preferences are central to the treatment decision, even for a gold-standard procedure such as Mohs surgery. By listening and responding to the patient’s input, the provider can build trust and discuss strategies that can help control for some risk factors.
Finally, the provider would re-evaluate throughout the procedure by continuously monitoring the patient’s anxiety and vital signs. The provider should also be ready to adjust pain management or employ anxiety-reduction techniques.
Final Thoughts
Reviewing the risk assessment matrix can be an effective way to nonjudgmentally discuss a patient’s unique risk factors and provide a complete understanding of the planned treatment or procedure. It conveys to the patient that, as the provider, you are taking their health seriously when considering treatment options and can be a means to build patient rapport and trust. This approach mirrors risk communication strategies long employed in military operational planning, where transparency and structured risk evaluation are essential to maintaining mission readiness and unit cohesion.
- The OR Society. The history of OR. The OR Society. Published 2023.
- Naval Postgraduate School. ORM: operational risk management. Accessed September 12, 2025. https://nps.edu/web/safety/orm
- Smith C, Srivastava D, Nijhawan RI. Optimizing patient safety in dermatologic surgery. Dermatol Clin. 2019;37:319-328.
- Minkis K, Whittington A, Alam M. Dermatologic surgery emergencies: complications caused by systemic reactions, high-energy systems, and trauma. J Am Acad Dermatol. 2016;75:265-284.
- Pomerantz RG, Lee DA, Siegel DM. Risk assessment in surgical patients: balancing iatrogenic risks and benefits. Clin Dermatol. 2011;29:669-677.
- Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surgeons. 2013;217:833-842.
- Lloyd AJ. The extent of patients’ understanding of the risk of treatments. BMJ Qual Saf. 2001;10:i14-i18.
- Falagas ME, Korbila IP, Giannopoulou KP, et al. Informed consent: how much and what do patients understand? Am J Surg. 2009;198:420-435.
- Cohen SM, Baimas-George M, Ponce C, et al. Is a picture worth a thousand words? a scoping review of the impact of visual aids on patients undergoing surgery. J Surg Educ. 2024;81:1276-1292.
- The OR Society. The history of OR. The OR Society. Published 2023.
- Naval Postgraduate School. ORM: operational risk management. Accessed September 12, 2025. https://nps.edu/web/safety/orm
- Smith C, Srivastava D, Nijhawan RI. Optimizing patient safety in dermatologic surgery. Dermatol Clin. 2019;37:319-328.
- Minkis K, Whittington A, Alam M. Dermatologic surgery emergencies: complications caused by systemic reactions, high-energy systems, and trauma. J Am Acad Dermatol. 2016;75:265-284.
- Pomerantz RG, Lee DA, Siegel DM. Risk assessment in surgical patients: balancing iatrogenic risks and benefits. Clin Dermatol. 2011;29:669-677.
- Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surgeons. 2013;217:833-842.
- Lloyd AJ. The extent of patients’ understanding of the risk of treatments. BMJ Qual Saf. 2001;10:i14-i18.
- Falagas ME, Korbila IP, Giannopoulou KP, et al. Informed consent: how much and what do patients understand? Am J Surg. 2009;198:420-435.
- Cohen SM, Baimas-George M, Ponce C, et al. Is a picture worth a thousand words? a scoping review of the impact of visual aids on patients undergoing surgery. J Surg Educ. 2024;81:1276-1292.
Operational Risk Management in Dermatologic Procedures
Operational Risk Management in Dermatologic Procedures
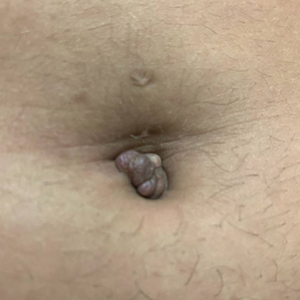
Tender Nodule on the Umbilicus
Tender Nodule on the Umbilicus
THE DIAGNOSIS: Villar Nodule
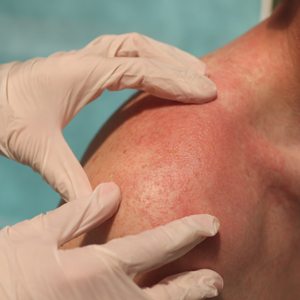
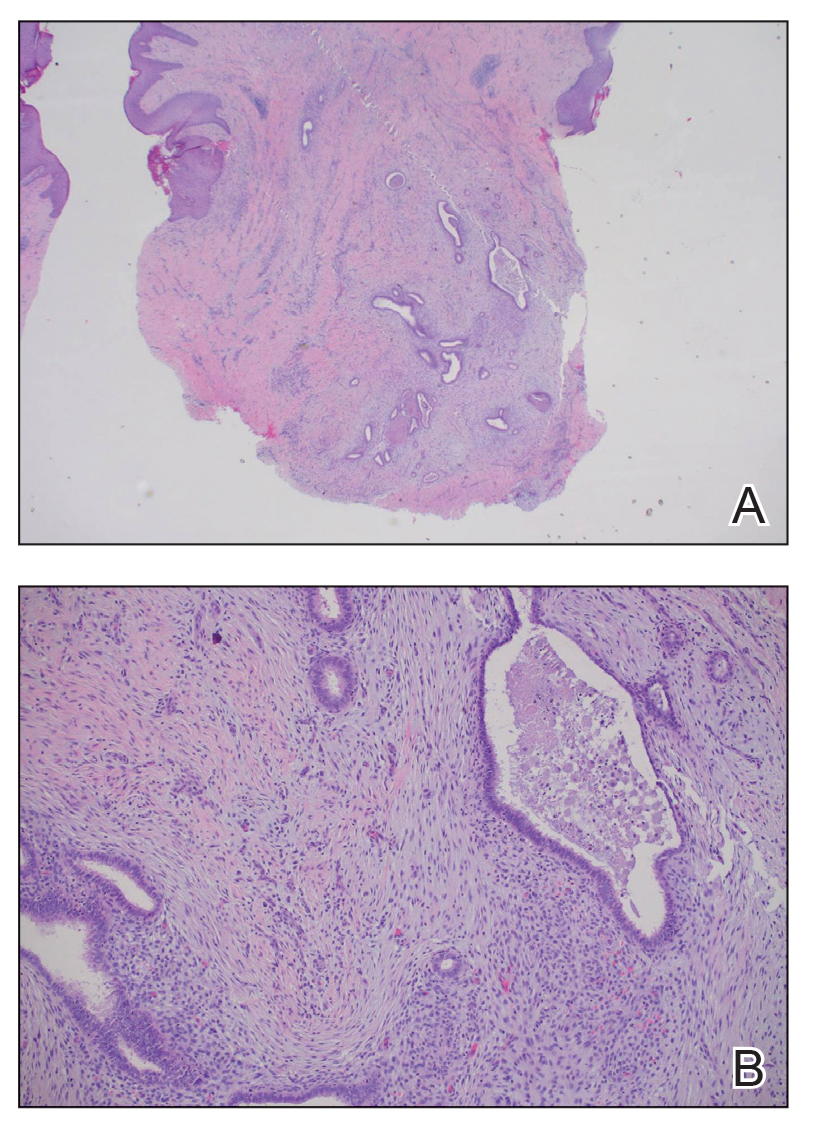
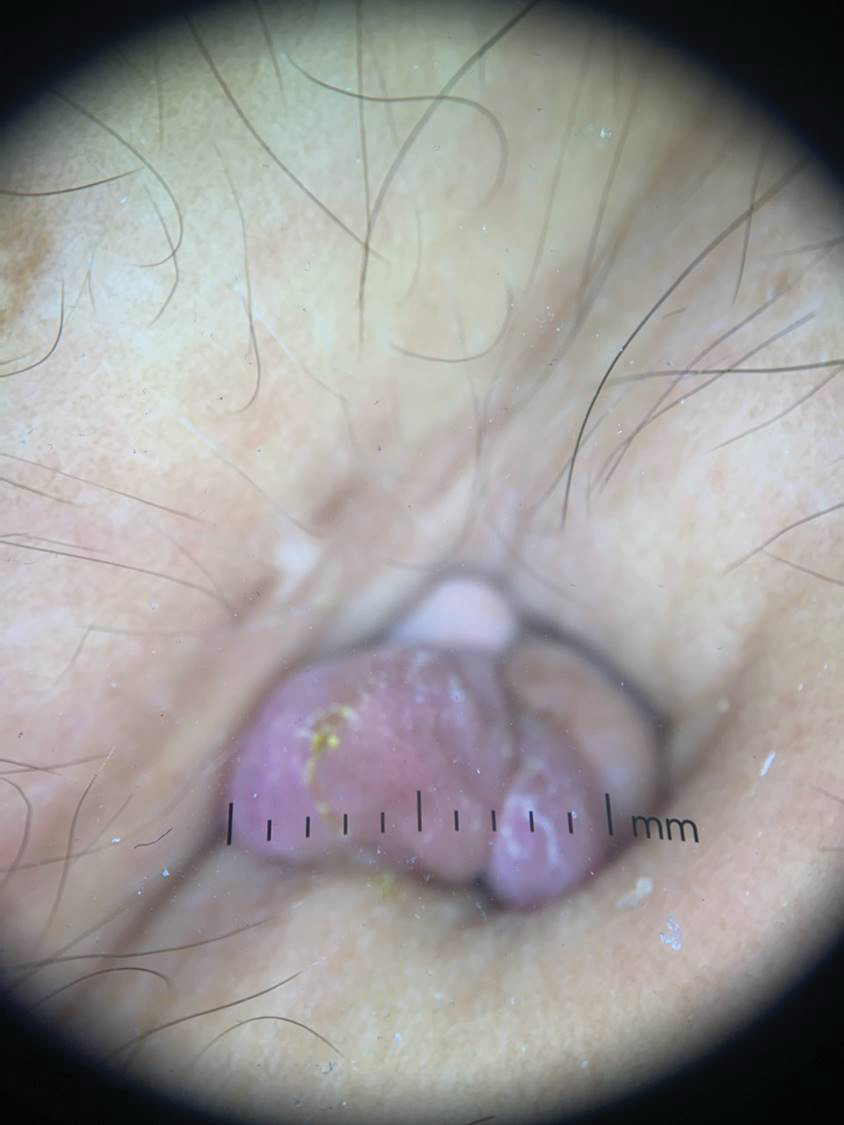
The biopsy revealed features consistent with cutaneous endometriosis in the setting of a painful, tender, multilobulated nodule with a cyclical bleeding pattern (Figure 1). The bleeding pattern of the nodule during menses and lack of surgical history supported the diagnosis of primary cutaneous endometriosis in our patient. She was diagnosed with endometriosis by gynecology, and her primary care physician started her on an oral contraceptive based on this diagnosis. She also was referred to gynecology and plastic surgery for a joint surgical consultation to remove the nodule. She initially decided to do a trial of the oral contraceptive but subsequently underwent umbilical endometrioma excision with neo-umbilicus creation with no evidence of recurrence.
Primary cutaneous endometriosis should be considered in young females who present with tender umbilical nodules. Endometriosis refers to the presence of an endometriumlike epithelium outside the endometrium and myometrium.1 The condition affects 10% to 15% of reproductive-aged (ie, 18-49 years) women in the United States and typically involves tissues within the pelvis, such as the ovaries, pouch of Douglas, or pelvic ligaments.2 Cutaneous endometriosis is the growth of endometrial tissue in the skin and is rare, accounting for less than 5.5% of cases of extrapelvic endometriosis worldwide, affecting primarily the umbilicus, abdominal wall, and vulva.3,4
The 2 main types of cutaneous endometriosis are primary (spontaneous) and secondary. Primary lesions develop in patients without prior surgical history, and secondary lesions occur within previous surgical incision sites, often scars from cesarean delivery.5 Less than 30% of cases of cutaneous endometriosis are primary disease.6 Primary cutaneous endometriosis of the umbilicus, known as Villar nodule, was first described in 1886.3,7 Up to 40% of patients with extrapelvic endometriosis worldwide presented with Villar nodules in a systematic literature review.6 The prevalence of these nodules is unknown, but the incidence is less than 1% of cases of extragenital endometriosis.4
There are 2 leading theories of primary cutaneous endometriosis pathogenesis. The first is the transportation theory, in which endometrial cells are transported outside the uterus via the lymphatic system.8 The second is the metaplasia theory, which proposed that endometrial cells develop in the coelomic mesothelium in the presence of high estrogen levels.8,9
Secondary cutaneous endometriosis, also known as scar endometriosis, is suspected to be caused by an iatrogenic implantation of endometrial cells at the scar of a prior surgical site.9 Although our patient had an existing umbilicus scar from a piercing, it was improbable for that to have been the nidus, as the keloid scar was superficial and did not have contact with the abdominal cavity for iatrogenic implantation. Clinical diagnosis for secondary cutaneous endometriosis often is made based on a triad of features: a nonmalignant abdominal mass, recurring pain and bleeding of the lesion with menses, and prior history of abdominal surgery.9,10 On clinical examination, these features typically manifest as a palpable subcutaneous mass that is black, blue, brown, or red. Often, the lesions enlarge and bleed during the menstrual cycle, causing pain, tenderness, or pruritus.3 Dermoscopic features of secondary cutaneous endometriosis are erythematous umbilical nodules with a homogeneous vascular pattern that appears red with a brownish hue (Figure 2).9,11 Dermoscopic features may vary with the hormone cycle; for example, the follicular phase (correlating with day 7 of menses) demonstrates polypoid projections, erythematous violaceous color, dark-brown spots, and active bleeding of the lesion.12 Clinical and dermoscopic examination are useful tools in this diagnosis.
Imaging such as ultrasonography, computed tomography, or magnetic resonance imaging may be useful in identifying abdominal endometriomas.8,13,14 Pelvic involvement of endometriosis was found in approximately 15% of patients in a case series,4 with concurrent primary umbilical endometriosis. Imaging studies may assist evaluation for fistula formation, presence of malignancies, and the extent of endometriosis within the abdominal cavity.
Histopathology is key to confirming cutaneous endometriosis and shows multiple bland-appearing glands of varying sizes with loose, concentric, edematous, or fibromyxoid stroma (Figure 1).3 Red blood cells sometimes are found with hemosiderin within the stroma. Immunohistochemical staining with estrogen receptors may aid in identifying the endometriumlike epithelial cells.13
Standard treatment involves surgical excision with 1-cm margins and umbilical preservation, which results in a recurrence rate of less than 10%.4,10 Medical therapy, such as aromatase inhibitors, progestogens, antiprogestogens, combined oral contraceptives, or gonadotropin-releasing hormone agonists or antagonists may help manage pain or reduce the size of the nodule.4,15 Simple observation also is a potential course for patients who decline treatment options.
Differential diagnoses include lobular capillary hemangioma, also known as pyogenic granuloma; Sister Mary Joseph nodule; umbilical hernia; and dermatofibrosarcoma protuberans. Lobular capillary hemangiomas commonly are acquired benign vascular proliferations of the skin that are friable and tend to ulcerate.16 These lesions typically grow rapidly and often are located on the face, lips, mucosae, and fingers. Histopathologic examination may show an exophytic lesion with lobules of proliferating capillaries within an edematous matrix, superficial ulceration, and an epithelial collarette.17 Treatment includes surgical excision, cauterization, laser treatments, sclerotherapy, injectable medications, and topical medications, but recurrence is possible with any of these interventions.18
Cutaneous metastasis of an internal solid organ cancer, commonly known as a Sister Mary Joseph nodule, typically manifests as an erythematous, irregularly shaped nodule that may protrude from the umbilicus.14 Gastrointestinal symptoms such as change in bowel habits or obstructive symptoms in the setting of a progressive malignancy are common.14 Clinical features include a firm fixed lesion, oozing, and ulceration.19 On dermoscopy, polymorphous vascular patterns, milky red structureless areas, and white lines typically are present.11 Although dermoscopic features may differentiate this entity from cutaneous endometriosis, tissue sampling and histologic examination are crucial diagnostic tools to identify malignant vs benign lesions.
An umbilical hernia is a protrusion of omentum, bowel, or other intra-abdominal organs in an abdominal wall defect. Clinical presentation includes a soft protrusion that may be reduced on palpation if nonstrangulated.20 Treatment includes watchful waiting or surgical repair. The reducibility and presence of an abdominal wall defect may point to this diagnosis. Imaging also may aid in the diagnosis if the history and physical examination are unclear.
Dermatofibrosarcoma protuberans is a slow-developing, low- to intermediate-grade, soft-tissue sarcoma that occurs in less than 0.1% of all cancers in the United States.21 Lesions often manifest as small, firm, slow-growing, painless, flesh-colored dermal plaques; subcutaneous thickening; or atrophic nonprotuberant lesions typically involving the trunk.21 Histopathologically, they are composed of uniform spindle-cell proliferation growing in a storiform pattern and subcutaneous fat trapping that has strong and diffuse CD34 immunoreactivity.21,22 Pathologic examination typically distinguishes this diagnosis from cutaneous endometriosis. Treatment includes tumor resection that may or may not involve radiotherapy and targeted therapy, as recurrence and metastases are possible.
Primary cutaneous endometriosis is a rare but important diagnosis for dermatologists to consider when evaluating umbilical nodules. Clinical features may include bleeding masses during menses in females of reproductive age. Dermoscopic examination aids in workup, and histopathologic testing can confirm the diagnosis and rule out malignancies. Surgical excision is the treatment of choice with a low rate of recurrence.
- International Working Group of AAGL, ESGE, ESHRE and WES; Tomassetti C, Johnson NP, et al. An international terminology for endometriosis, 2021. Hum Reprod Open. 2021;2021:hoab029. doi:10.1093/hropen/hoab029
- Batista M, Alves F, Cardoso J, et al. Cutaneous endometriosis: a differential diagnosis of umbilical nodule. Acta Med Port. 2020; 33:282-284. doi:10.20344/amp.10966
- Brown ME, Osswald S, Biediger T. Cutaneous endometriosis of the umbilicus (Villar’s nodule). Int J Womens Dermatol. 2020;6:214-215. doi:10.1016/j.ijwd.2020.01.001
- Bindra V, Sampurna S, Kade S, et al. Primary umbilical endometriosis - case series and review of clinical presentation, diagnosis and management. Int J Surg Case Rep. 2022;94:107134. doi:10.1016/j.ijscr.2022.107134
- Loh SH, Lew BL, Sim WY. Primary cutaneous endometriosis of umbilicus. Ann Dermatol. 2017;29:621-625. doi:10.5021/ad.2017.29.5.621
- Victory R, Diamond MP, Johns DA. Villar’s nodule: a case report and systematic literature review of endometriosis externa of the umbilicus. J Minim Invasive Gynecol. 2007;14:23-32. doi:10.1016/j.jmig.2006.07.01
- Van den Nouland D, Kaur M. Primary umbilical endometriosis: a case report. Facts Views Vis Obgyn. 2017;9:115-119.
- Machairiotis N, Stylianaki A, Dryllis G, et al. Extrapelvic endometriosis: a rare entity or an under diagnosed condition? Diagn Pathol. 2013;8:194. doi:10.1186/1746-1596-8-194
- Huang QF, Jiang B, Yang X, et al. Primary versus secondary cutaneous endometriosis: literature review and case study. Heliyon. 2023;9:E20094. doi:10.1016/j.heliyon.2023.e20094
- Gonzalez RH, Singh MS, Hamza SA. Cutaneous endometriosis: a case report and review of the literature. Am J Case Rep. 2021;22:E932493. doi:10.12659/AJCR.932493
- Buljan M, Arzberger E, Šitum M, et al. The use of dermoscopy in differentiating Sister Mary Joseph nodule and cutaneous endometriosis. Australas J Dermatol. 2019;60:E233-E235. doi:10.1111/ajd.12980
- Costa IM, Gomes CM, Morais OO, et al. Cutaneous endometriosis: dermoscopic findings related to phases of the female hormonal cycle. Int J Dermatol. 2014;53:E130-E132. doi:10.1111 /j.1365-4632.2012.05854.x
- Mohaghegh F, Hatami P, Rajabi P, et al. Coexistence of cutaneous endometriosis and ovarian endometrioma: a case report. J Med Case Rep. 2022;16:256. doi:10.1186/s13256-022-03483-8
- Raffi L, Suresh R, McCalmont TH, et al. Cutaneous endometriosis. Int J Womens Dermatol. 2019;5:384-386. doi:10.1016 /j.ijwd.2019.06.025
- Saunders PTK, Horne AW. Endometriosis: etiology, pathobiology, and therapeutic prospects. Cell. 2021;184:2807-2824. doi:10.1016 /j.cell.2021.04.041
- Habif TP. Clinical Dermatology a Color Guide to Diagnosis and Therapy. St. Louis, Mo. Elsevier; 2016.
- Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8:267-276. doi:10.1111/j.15251470.1991.tb00931.x
- Kaleeny JD, Janis JE. Pyogenic granuloma diagnosis and management: a practical review. Plast Reconstr Surg Glob Open. 2024;12:E6160. doi:10.1097/GOX.0000000000006160
- Ha DL, Yang MY, Shin JO, et al. Benign umbilical tumors resembling Sister Mary Joseph nodule. Clin Med Insights Oncol. 2021;15:1179554921995022. doi:10.1177/1179554921995022
- Lawrence PF, Smeds M, Jessica Beth O’connell. Essentials of General Surgery and Surgical Specialties. Wolters Kluwer Health; 2019.
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752. doi:10.3390/jcm9061752
- Allen A, Ahn C, Sangüeza OP. Dermatofibrosarcoma protuberans. Dermatol Clin. 2019;37:483-488. doi:10.1016/j.det.2019.05.006
THE DIAGNOSIS: Villar Nodule
The biopsy revealed features consistent with cutaneous endometriosis in the setting of a painful, tender, multilobulated nodule with a cyclical bleeding pattern (Figure 1). The bleeding pattern of the nodule during menses and lack of surgical history supported the diagnosis of primary cutaneous endometriosis in our patient. She was diagnosed with endometriosis by gynecology, and her primary care physician started her on an oral contraceptive based on this diagnosis. She also was referred to gynecology and plastic surgery for a joint surgical consultation to remove the nodule. She initially decided to do a trial of the oral contraceptive but subsequently underwent umbilical endometrioma excision with neo-umbilicus creation with no evidence of recurrence.

Primary cutaneous endometriosis should be considered in young females who present with tender umbilical nodules. Endometriosis refers to the presence of an endometriumlike epithelium outside the endometrium and myometrium.1 The condition affects 10% to 15% of reproductive-aged (ie, 18-49 years) women in the United States and typically involves tissues within the pelvis, such as the ovaries, pouch of Douglas, or pelvic ligaments.2 Cutaneous endometriosis is the growth of endometrial tissue in the skin and is rare, accounting for less than 5.5% of cases of extrapelvic endometriosis worldwide, affecting primarily the umbilicus, abdominal wall, and vulva.3,4
The 2 main types of cutaneous endometriosis are primary (spontaneous) and secondary. Primary lesions develop in patients without prior surgical history, and secondary lesions occur within previous surgical incision sites, often scars from cesarean delivery.5 Less than 30% of cases of cutaneous endometriosis are primary disease.6 Primary cutaneous endometriosis of the umbilicus, known as Villar nodule, was first described in 1886.3,7 Up to 40% of patients with extrapelvic endometriosis worldwide presented with Villar nodules in a systematic literature review.6 The prevalence of these nodules is unknown, but the incidence is less than 1% of cases of extragenital endometriosis.4
There are 2 leading theories of primary cutaneous endometriosis pathogenesis. The first is the transportation theory, in which endometrial cells are transported outside the uterus via the lymphatic system.8 The second is the metaplasia theory, which proposed that endometrial cells develop in the coelomic mesothelium in the presence of high estrogen levels.8,9
Secondary cutaneous endometriosis, also known as scar endometriosis, is suspected to be caused by an iatrogenic implantation of endometrial cells at the scar of a prior surgical site.9 Although our patient had an existing umbilicus scar from a piercing, it was improbable for that to have been the nidus, as the keloid scar was superficial and did not have contact with the abdominal cavity for iatrogenic implantation. Clinical diagnosis for secondary cutaneous endometriosis often is made based on a triad of features: a nonmalignant abdominal mass, recurring pain and bleeding of the lesion with menses, and prior history of abdominal surgery.9,10 On clinical examination, these features typically manifest as a palpable subcutaneous mass that is black, blue, brown, or red. Often, the lesions enlarge and bleed during the menstrual cycle, causing pain, tenderness, or pruritus.3 Dermoscopic features of secondary cutaneous endometriosis are erythematous umbilical nodules with a homogeneous vascular pattern that appears red with a brownish hue (Figure 2).9,11 Dermoscopic features may vary with the hormone cycle; for example, the follicular phase (correlating with day 7 of menses) demonstrates polypoid projections, erythematous violaceous color, dark-brown spots, and active bleeding of the lesion.12 Clinical and dermoscopic examination are useful tools in this diagnosis.

Imaging such as ultrasonography, computed tomography, or magnetic resonance imaging may be useful in identifying abdominal endometriomas.8,13,14 Pelvic involvement of endometriosis was found in approximately 15% of patients in a case series,4 with concurrent primary umbilical endometriosis. Imaging studies may assist evaluation for fistula formation, presence of malignancies, and the extent of endometriosis within the abdominal cavity.
Histopathology is key to confirming cutaneous endometriosis and shows multiple bland-appearing glands of varying sizes with loose, concentric, edematous, or fibromyxoid stroma (Figure 1).3 Red blood cells sometimes are found with hemosiderin within the stroma. Immunohistochemical staining with estrogen receptors may aid in identifying the endometriumlike epithelial cells.13
Standard treatment involves surgical excision with 1-cm margins and umbilical preservation, which results in a recurrence rate of less than 10%.4,10 Medical therapy, such as aromatase inhibitors, progestogens, antiprogestogens, combined oral contraceptives, or gonadotropin-releasing hormone agonists or antagonists may help manage pain or reduce the size of the nodule.4,15 Simple observation also is a potential course for patients who decline treatment options.
Differential diagnoses include lobular capillary hemangioma, also known as pyogenic granuloma; Sister Mary Joseph nodule; umbilical hernia; and dermatofibrosarcoma protuberans. Lobular capillary hemangiomas commonly are acquired benign vascular proliferations of the skin that are friable and tend to ulcerate.16 These lesions typically grow rapidly and often are located on the face, lips, mucosae, and fingers. Histopathologic examination may show an exophytic lesion with lobules of proliferating capillaries within an edematous matrix, superficial ulceration, and an epithelial collarette.17 Treatment includes surgical excision, cauterization, laser treatments, sclerotherapy, injectable medications, and topical medications, but recurrence is possible with any of these interventions.18
Cutaneous metastasis of an internal solid organ cancer, commonly known as a Sister Mary Joseph nodule, typically manifests as an erythematous, irregularly shaped nodule that may protrude from the umbilicus.14 Gastrointestinal symptoms such as change in bowel habits or obstructive symptoms in the setting of a progressive malignancy are common.14 Clinical features include a firm fixed lesion, oozing, and ulceration.19 On dermoscopy, polymorphous vascular patterns, milky red structureless areas, and white lines typically are present.11 Although dermoscopic features may differentiate this entity from cutaneous endometriosis, tissue sampling and histologic examination are crucial diagnostic tools to identify malignant vs benign lesions.
An umbilical hernia is a protrusion of omentum, bowel, or other intra-abdominal organs in an abdominal wall defect. Clinical presentation includes a soft protrusion that may be reduced on palpation if nonstrangulated.20 Treatment includes watchful waiting or surgical repair. The reducibility and presence of an abdominal wall defect may point to this diagnosis. Imaging also may aid in the diagnosis if the history and physical examination are unclear.
Dermatofibrosarcoma protuberans is a slow-developing, low- to intermediate-grade, soft-tissue sarcoma that occurs in less than 0.1% of all cancers in the United States.21 Lesions often manifest as small, firm, slow-growing, painless, flesh-colored dermal plaques; subcutaneous thickening; or atrophic nonprotuberant lesions typically involving the trunk.21 Histopathologically, they are composed of uniform spindle-cell proliferation growing in a storiform pattern and subcutaneous fat trapping that has strong and diffuse CD34 immunoreactivity.21,22 Pathologic examination typically distinguishes this diagnosis from cutaneous endometriosis. Treatment includes tumor resection that may or may not involve radiotherapy and targeted therapy, as recurrence and metastases are possible.
Primary cutaneous endometriosis is a rare but important diagnosis for dermatologists to consider when evaluating umbilical nodules. Clinical features may include bleeding masses during menses in females of reproductive age. Dermoscopic examination aids in workup, and histopathologic testing can confirm the diagnosis and rule out malignancies. Surgical excision is the treatment of choice with a low rate of recurrence.
THE DIAGNOSIS: Villar Nodule
The biopsy revealed features consistent with cutaneous endometriosis in the setting of a painful, tender, multilobulated nodule with a cyclical bleeding pattern (Figure 1). The bleeding pattern of the nodule during menses and lack of surgical history supported the diagnosis of primary cutaneous endometriosis in our patient. She was diagnosed with endometriosis by gynecology, and her primary care physician started her on an oral contraceptive based on this diagnosis. She also was referred to gynecology and plastic surgery for a joint surgical consultation to remove the nodule. She initially decided to do a trial of the oral contraceptive but subsequently underwent umbilical endometrioma excision with neo-umbilicus creation with no evidence of recurrence.

Primary cutaneous endometriosis should be considered in young females who present with tender umbilical nodules. Endometriosis refers to the presence of an endometriumlike epithelium outside the endometrium and myometrium.1 The condition affects 10% to 15% of reproductive-aged (ie, 18-49 years) women in the United States and typically involves tissues within the pelvis, such as the ovaries, pouch of Douglas, or pelvic ligaments.2 Cutaneous endometriosis is the growth of endometrial tissue in the skin and is rare, accounting for less than 5.5% of cases of extrapelvic endometriosis worldwide, affecting primarily the umbilicus, abdominal wall, and vulva.3,4
The 2 main types of cutaneous endometriosis are primary (spontaneous) and secondary. Primary lesions develop in patients without prior surgical history, and secondary lesions occur within previous surgical incision sites, often scars from cesarean delivery.5 Less than 30% of cases of cutaneous endometriosis are primary disease.6 Primary cutaneous endometriosis of the umbilicus, known as Villar nodule, was first described in 1886.3,7 Up to 40% of patients with extrapelvic endometriosis worldwide presented with Villar nodules in a systematic literature review.6 The prevalence of these nodules is unknown, but the incidence is less than 1% of cases of extragenital endometriosis.4
There are 2 leading theories of primary cutaneous endometriosis pathogenesis. The first is the transportation theory, in which endometrial cells are transported outside the uterus via the lymphatic system.8 The second is the metaplasia theory, which proposed that endometrial cells develop in the coelomic mesothelium in the presence of high estrogen levels.8,9
Secondary cutaneous endometriosis, also known as scar endometriosis, is suspected to be caused by an iatrogenic implantation of endometrial cells at the scar of a prior surgical site.9 Although our patient had an existing umbilicus scar from a piercing, it was improbable for that to have been the nidus, as the keloid scar was superficial and did not have contact with the abdominal cavity for iatrogenic implantation. Clinical diagnosis for secondary cutaneous endometriosis often is made based on a triad of features: a nonmalignant abdominal mass, recurring pain and bleeding of the lesion with menses, and prior history of abdominal surgery.9,10 On clinical examination, these features typically manifest as a palpable subcutaneous mass that is black, blue, brown, or red. Often, the lesions enlarge and bleed during the menstrual cycle, causing pain, tenderness, or pruritus.3 Dermoscopic features of secondary cutaneous endometriosis are erythematous umbilical nodules with a homogeneous vascular pattern that appears red with a brownish hue (Figure 2).9,11 Dermoscopic features may vary with the hormone cycle; for example, the follicular phase (correlating with day 7 of menses) demonstrates polypoid projections, erythematous violaceous color, dark-brown spots, and active bleeding of the lesion.12 Clinical and dermoscopic examination are useful tools in this diagnosis.

Imaging such as ultrasonography, computed tomography, or magnetic resonance imaging may be useful in identifying abdominal endometriomas.8,13,14 Pelvic involvement of endometriosis was found in approximately 15% of patients in a case series,4 with concurrent primary umbilical endometriosis. Imaging studies may assist evaluation for fistula formation, presence of malignancies, and the extent of endometriosis within the abdominal cavity.
Histopathology is key to confirming cutaneous endometriosis and shows multiple bland-appearing glands of varying sizes with loose, concentric, edematous, or fibromyxoid stroma (Figure 1).3 Red blood cells sometimes are found with hemosiderin within the stroma. Immunohistochemical staining with estrogen receptors may aid in identifying the endometriumlike epithelial cells.13
Standard treatment involves surgical excision with 1-cm margins and umbilical preservation, which results in a recurrence rate of less than 10%.4,10 Medical therapy, such as aromatase inhibitors, progestogens, antiprogestogens, combined oral contraceptives, or gonadotropin-releasing hormone agonists or antagonists may help manage pain or reduce the size of the nodule.4,15 Simple observation also is a potential course for patients who decline treatment options.
Differential diagnoses include lobular capillary hemangioma, also known as pyogenic granuloma; Sister Mary Joseph nodule; umbilical hernia; and dermatofibrosarcoma protuberans. Lobular capillary hemangiomas commonly are acquired benign vascular proliferations of the skin that are friable and tend to ulcerate.16 These lesions typically grow rapidly and often are located on the face, lips, mucosae, and fingers. Histopathologic examination may show an exophytic lesion with lobules of proliferating capillaries within an edematous matrix, superficial ulceration, and an epithelial collarette.17 Treatment includes surgical excision, cauterization, laser treatments, sclerotherapy, injectable medications, and topical medications, but recurrence is possible with any of these interventions.18
Cutaneous metastasis of an internal solid organ cancer, commonly known as a Sister Mary Joseph nodule, typically manifests as an erythematous, irregularly shaped nodule that may protrude from the umbilicus.14 Gastrointestinal symptoms such as change in bowel habits or obstructive symptoms in the setting of a progressive malignancy are common.14 Clinical features include a firm fixed lesion, oozing, and ulceration.19 On dermoscopy, polymorphous vascular patterns, milky red structureless areas, and white lines typically are present.11 Although dermoscopic features may differentiate this entity from cutaneous endometriosis, tissue sampling and histologic examination are crucial diagnostic tools to identify malignant vs benign lesions.
An umbilical hernia is a protrusion of omentum, bowel, or other intra-abdominal organs in an abdominal wall defect. Clinical presentation includes a soft protrusion that may be reduced on palpation if nonstrangulated.20 Treatment includes watchful waiting or surgical repair. The reducibility and presence of an abdominal wall defect may point to this diagnosis. Imaging also may aid in the diagnosis if the history and physical examination are unclear.
Dermatofibrosarcoma protuberans is a slow-developing, low- to intermediate-grade, soft-tissue sarcoma that occurs in less than 0.1% of all cancers in the United States.21 Lesions often manifest as small, firm, slow-growing, painless, flesh-colored dermal plaques; subcutaneous thickening; or atrophic nonprotuberant lesions typically involving the trunk.21 Histopathologically, they are composed of uniform spindle-cell proliferation growing in a storiform pattern and subcutaneous fat trapping that has strong and diffuse CD34 immunoreactivity.21,22 Pathologic examination typically distinguishes this diagnosis from cutaneous endometriosis. Treatment includes tumor resection that may or may not involve radiotherapy and targeted therapy, as recurrence and metastases are possible.
Primary cutaneous endometriosis is a rare but important diagnosis for dermatologists to consider when evaluating umbilical nodules. Clinical features may include bleeding masses during menses in females of reproductive age. Dermoscopic examination aids in workup, and histopathologic testing can confirm the diagnosis and rule out malignancies. Surgical excision is the treatment of choice with a low rate of recurrence.
- International Working Group of AAGL, ESGE, ESHRE and WES; Tomassetti C, Johnson NP, et al. An international terminology for endometriosis, 2021. Hum Reprod Open. 2021;2021:hoab029. doi:10.1093/hropen/hoab029
- Batista M, Alves F, Cardoso J, et al. Cutaneous endometriosis: a differential diagnosis of umbilical nodule. Acta Med Port. 2020; 33:282-284. doi:10.20344/amp.10966
- Brown ME, Osswald S, Biediger T. Cutaneous endometriosis of the umbilicus (Villar’s nodule). Int J Womens Dermatol. 2020;6:214-215. doi:10.1016/j.ijwd.2020.01.001
- Bindra V, Sampurna S, Kade S, et al. Primary umbilical endometriosis - case series and review of clinical presentation, diagnosis and management. Int J Surg Case Rep. 2022;94:107134. doi:10.1016/j.ijscr.2022.107134
- Loh SH, Lew BL, Sim WY. Primary cutaneous endometriosis of umbilicus. Ann Dermatol. 2017;29:621-625. doi:10.5021/ad.2017.29.5.621
- Victory R, Diamond MP, Johns DA. Villar’s nodule: a case report and systematic literature review of endometriosis externa of the umbilicus. J Minim Invasive Gynecol. 2007;14:23-32. doi:10.1016/j.jmig.2006.07.01
- Van den Nouland D, Kaur M. Primary umbilical endometriosis: a case report. Facts Views Vis Obgyn. 2017;9:115-119.
- Machairiotis N, Stylianaki A, Dryllis G, et al. Extrapelvic endometriosis: a rare entity or an under diagnosed condition? Diagn Pathol. 2013;8:194. doi:10.1186/1746-1596-8-194
- Huang QF, Jiang B, Yang X, et al. Primary versus secondary cutaneous endometriosis: literature review and case study. Heliyon. 2023;9:E20094. doi:10.1016/j.heliyon.2023.e20094
- Gonzalez RH, Singh MS, Hamza SA. Cutaneous endometriosis: a case report and review of the literature. Am J Case Rep. 2021;22:E932493. doi:10.12659/AJCR.932493
- Buljan M, Arzberger E, Šitum M, et al. The use of dermoscopy in differentiating Sister Mary Joseph nodule and cutaneous endometriosis. Australas J Dermatol. 2019;60:E233-E235. doi:10.1111/ajd.12980
- Costa IM, Gomes CM, Morais OO, et al. Cutaneous endometriosis: dermoscopic findings related to phases of the female hormonal cycle. Int J Dermatol. 2014;53:E130-E132. doi:10.1111 /j.1365-4632.2012.05854.x
- Mohaghegh F, Hatami P, Rajabi P, et al. Coexistence of cutaneous endometriosis and ovarian endometrioma: a case report. J Med Case Rep. 2022;16:256. doi:10.1186/s13256-022-03483-8
- Raffi L, Suresh R, McCalmont TH, et al. Cutaneous endometriosis. Int J Womens Dermatol. 2019;5:384-386. doi:10.1016 /j.ijwd.2019.06.025
- Saunders PTK, Horne AW. Endometriosis: etiology, pathobiology, and therapeutic prospects. Cell. 2021;184:2807-2824. doi:10.1016 /j.cell.2021.04.041
- Habif TP. Clinical Dermatology a Color Guide to Diagnosis and Therapy. St. Louis, Mo. Elsevier; 2016.
- Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8:267-276. doi:10.1111/j.15251470.1991.tb00931.x
- Kaleeny JD, Janis JE. Pyogenic granuloma diagnosis and management: a practical review. Plast Reconstr Surg Glob Open. 2024;12:E6160. doi:10.1097/GOX.0000000000006160
- Ha DL, Yang MY, Shin JO, et al. Benign umbilical tumors resembling Sister Mary Joseph nodule. Clin Med Insights Oncol. 2021;15:1179554921995022. doi:10.1177/1179554921995022
- Lawrence PF, Smeds M, Jessica Beth O’connell. Essentials of General Surgery and Surgical Specialties. Wolters Kluwer Health; 2019.
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752. doi:10.3390/jcm9061752
- Allen A, Ahn C, Sangüeza OP. Dermatofibrosarcoma protuberans. Dermatol Clin. 2019;37:483-488. doi:10.1016/j.det.2019.05.006
- International Working Group of AAGL, ESGE, ESHRE and WES; Tomassetti C, Johnson NP, et al. An international terminology for endometriosis, 2021. Hum Reprod Open. 2021;2021:hoab029. doi:10.1093/hropen/hoab029
- Batista M, Alves F, Cardoso J, et al. Cutaneous endometriosis: a differential diagnosis of umbilical nodule. Acta Med Port. 2020; 33:282-284. doi:10.20344/amp.10966
- Brown ME, Osswald S, Biediger T. Cutaneous endometriosis of the umbilicus (Villar’s nodule). Int J Womens Dermatol. 2020;6:214-215. doi:10.1016/j.ijwd.2020.01.001
- Bindra V, Sampurna S, Kade S, et al. Primary umbilical endometriosis - case series and review of clinical presentation, diagnosis and management. Int J Surg Case Rep. 2022;94:107134. doi:10.1016/j.ijscr.2022.107134
- Loh SH, Lew BL, Sim WY. Primary cutaneous endometriosis of umbilicus. Ann Dermatol. 2017;29:621-625. doi:10.5021/ad.2017.29.5.621
- Victory R, Diamond MP, Johns DA. Villar’s nodule: a case report and systematic literature review of endometriosis externa of the umbilicus. J Minim Invasive Gynecol. 2007;14:23-32. doi:10.1016/j.jmig.2006.07.01
- Van den Nouland D, Kaur M. Primary umbilical endometriosis: a case report. Facts Views Vis Obgyn. 2017;9:115-119.
- Machairiotis N, Stylianaki A, Dryllis G, et al. Extrapelvic endometriosis: a rare entity or an under diagnosed condition? Diagn Pathol. 2013;8:194. doi:10.1186/1746-1596-8-194
- Huang QF, Jiang B, Yang X, et al. Primary versus secondary cutaneous endometriosis: literature review and case study. Heliyon. 2023;9:E20094. doi:10.1016/j.heliyon.2023.e20094
- Gonzalez RH, Singh MS, Hamza SA. Cutaneous endometriosis: a case report and review of the literature. Am J Case Rep. 2021;22:E932493. doi:10.12659/AJCR.932493
- Buljan M, Arzberger E, Šitum M, et al. The use of dermoscopy in differentiating Sister Mary Joseph nodule and cutaneous endometriosis. Australas J Dermatol. 2019;60:E233-E235. doi:10.1111/ajd.12980
- Costa IM, Gomes CM, Morais OO, et al. Cutaneous endometriosis: dermoscopic findings related to phases of the female hormonal cycle. Int J Dermatol. 2014;53:E130-E132. doi:10.1111 /j.1365-4632.2012.05854.x
- Mohaghegh F, Hatami P, Rajabi P, et al. Coexistence of cutaneous endometriosis and ovarian endometrioma: a case report. J Med Case Rep. 2022;16:256. doi:10.1186/s13256-022-03483-8
- Raffi L, Suresh R, McCalmont TH, et al. Cutaneous endometriosis. Int J Womens Dermatol. 2019;5:384-386. doi:10.1016 /j.ijwd.2019.06.025
- Saunders PTK, Horne AW. Endometriosis: etiology, pathobiology, and therapeutic prospects. Cell. 2021;184:2807-2824. doi:10.1016 /j.cell.2021.04.041
- Habif TP. Clinical Dermatology a Color Guide to Diagnosis and Therapy. St. Louis, Mo. Elsevier; 2016.
- Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8:267-276. doi:10.1111/j.15251470.1991.tb00931.x
- Kaleeny JD, Janis JE. Pyogenic granuloma diagnosis and management: a practical review. Plast Reconstr Surg Glob Open. 2024;12:E6160. doi:10.1097/GOX.0000000000006160
- Ha DL, Yang MY, Shin JO, et al. Benign umbilical tumors resembling Sister Mary Joseph nodule. Clin Med Insights Oncol. 2021;15:1179554921995022. doi:10.1177/1179554921995022
- Lawrence PF, Smeds M, Jessica Beth O’connell. Essentials of General Surgery and Surgical Specialties. Wolters Kluwer Health; 2019.
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752. doi:10.3390/jcm9061752
- Allen A, Ahn C, Sangüeza OP. Dermatofibrosarcoma protuberans. Dermatol Clin. 2019;37:483-488. doi:10.1016/j.det.2019.05.006
Tender Nodule on the Umbilicus
Tender Nodule on the Umbilicus
A 25-year-old woman was referred to the dermatology clinic by her primary care provider for evaluation of a tender nodule on the inferior umbilicus of 2 years' duration at the site of a preexisting keloid scar. The patient reported that the lesion caused occasional pain and tenderness. A few weeks prior to the current presentation, a dark-red bloody discharge developed at the superior aspect of the lesion that subsequently crusted over. The patient denied any use of oral contraceptives or history of abdominal surgery.
The original keloid scar had been treated successfully by an outside physician with intralesional steroid injections, and the patient was interested in a similar procedure for the current nodule. She also had a history of a hyperpigmented hypertrophic scar on the superior periumbilical area from a previous piercing that had resolved several years prior to presentation.
Physical examination of the lesion revealed a 1.2-cm, soft, tender, violaceous nodule with scant yellow crust along the superior surface of the umbilicus. There was no palpable abdominal wall defect, and the nodule was not reducible into the abdominal cavity. An interval history revealed bleeding of the lesion during the patient's menstrual cycle with persistent pain and tenderness. A punch biopsy was performed.
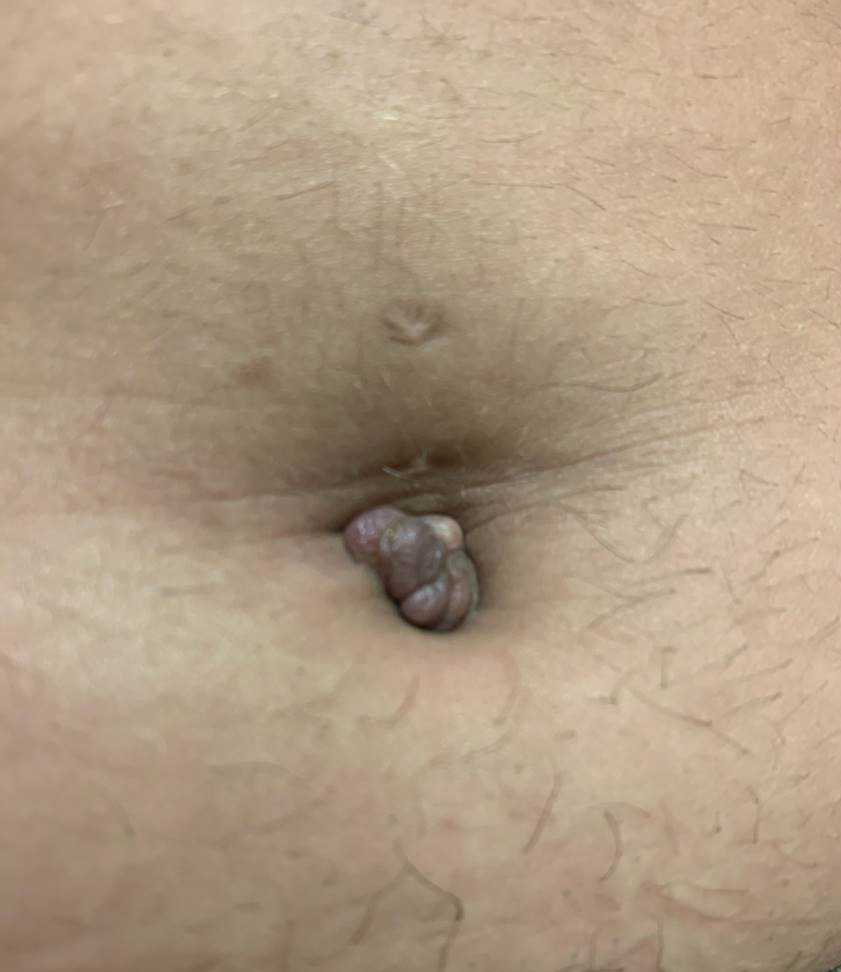
The Burden of Skin Cancer in the Military Health System, 2017-2022
This retrospective observational study investigates skin cancer prevalence and care patterns within the Military Health System (MHS) from 2017 to 2022. Utilizing the MHS Management Analysis and Reporting Tool (most commonly called M2), we analyzed more than 5 million patient encounters and documented skin cancer prevalence in the MHS beneficiary population utilizing available demographic data. Notable findings included an increased prevalence of skin cancer in the military population compared with the civilian population, a substantial decline in direct care (DC) visits at military treatment facilities compared with civilian purchased care (PC) visits, and a decreased total number of visits during COVID-19 restrictions.
The Military Health System (MHS) is a worldwide health care delivery system that serves 9.6 million beneficiaries, including military service members, retirees, and their families.1 Its mission is 2-fold: provide a medically ready force, and provide a medical benefit in keeping with the service and sacrifice of active-duty personnel, military retirees, and their families. For fiscal year (FY) 2022, active-duty service members and their families comprised 16.7% and 19.9% of beneficiaries, respectively, while retired service members and their families comprised 27% and 32% of beneficiaries, respectively.
The MHS operates under the authority of the Department of Defense (DoD) and is supported by an annual budget of approximately $50 billion.1 Health care provision within the MHS is managed by TRICARE regional networks.2 Within these networks, MHS beneficiaries may receive health care in 2 categories: direct care (DC) and purchased care (PC). Direct care is rendered in military treatment facilities by military or civilian providers contracted by the DoD, and PC is administered by civilian providers at civilian health care facilities within the TRICARE network, which is comprised of individual providers, clinics, and hospitals that have agreed to accept TRICARE beneficiaries.1 Purchased care is fee-for-service and paid for by the MHS. Of note, the MHS differs from the Veterans Affairs health care system in that the MHS through DC and PC sees only active-duty service members, active-duty dependents, retirees, and retirees’ dependents (primarily spouses), whereas Veterans Affairs sees only veterans (not necessarily retirees) discharged from military service with compensable medical conditions or disabilities.
Skin cancer presents a notable concern for the US Military, as the risk for skin cancer is thought to be higher than in the general population.3,4 This elevated risk is attributed to numerous factors inherent to active-duty service, including time spent in tropical environments, increased exposure to UV radiation, time spent at high altitudes, and decreased rates of sun-protective behaviors.3 Although numerous studies have explored the mechanisms that contribute to service members’ increased skin cancer risk, there are few (if any) that discuss the burden of skin cancer on the MHS and where its beneficiaries receive their skin cancer care. This study evaluated the burden of skin cancer within the MHS, as demonstrated by the period prevalence of skin cancer among its beneficiaries and the number and distribution of patient visits for skin cancer across both DC and PC from 2017 to 2022.
Methods
Data Collection—This retrospective observational study was designed to describe trends in outpatient visits with a skin cancer diagnosis and annual prevalence of skin cancer types in the MHS. Data are from all MHS beneficiaries who were eligible or enrolled in the analysis year. Our data source was the MHS Management Analysis and Reporting Tool (most commonly called M2), a query tool that contains the current and most recent 5 full FYs of Defense Health Agency corporate health care data including aggregated FY and calendar-year counts of MHS beneficiaries from 2017 to 2022 using encounter and claims data tables from both DC and PC. Data in M2 are coded using a pseudo-person identification number, and queries performed for this study were limited to de-identified visit and patient counts.
Skin cancer diagnoses were defined by relevant International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes recorded from outpatient visits in DC and PC. The M2 database was queried to find aggregate counts of visits and unique MHS beneficiaries with one or more diagnoses of a skin cancer type of interest (defined by relevant ICD-10-CM code) over the study period stratified by year and by patient demographic characteristics. Skin cancer types by ICD-10-CM code group included basal cell carcinoma (BCC), squamous cell carcinoma (SCC), malignant melanoma (MM), and other (including Merkel cell carcinoma and sebaceous carcinoma). Demographic strata included age, sex, military status (active duty, dependents of active duty, retired, or all others), sponsor military rank, and sponsor branch (army, air force, marine corps, or navy). Visit counts included diagnoses from any ICD position (for encounters that contained multiple ICD codes) to describe the total volume of care that addressed a diagnosed skin cancer. Counts of unique patients in prevalence analyses included relevant diagnoses in the primary ICD position only to increase the specificity of prevalence estimates.
Data Analysis—Descriptive analyses included the total number of outpatient visits with a skin cancer diagnosis in DC and PC over the study period, with percentages of total visits by year and by demographic strata. Separate analyses estimated annual prevalences of skin cancer types in the MHS by study year and within 2022 by demographic strata. Numerators in prevalence analyses were defined as the number of unique individuals with one or more relevant ICD codes in the analysis year. Denominators were defined as the total number of MHS beneficiaries in the analysis year and resulting period prevalences reported. Observed prevalences were qualitatively described, and trends were compared with prevalences in nonmilitary populations reported in the literature.
Ethics—This study was conducted as part of a study using secondary analyses of de-identified data from the M2 database. The study was reviewed and approved by the Walter Reed National Military Medical Center institutional review board.
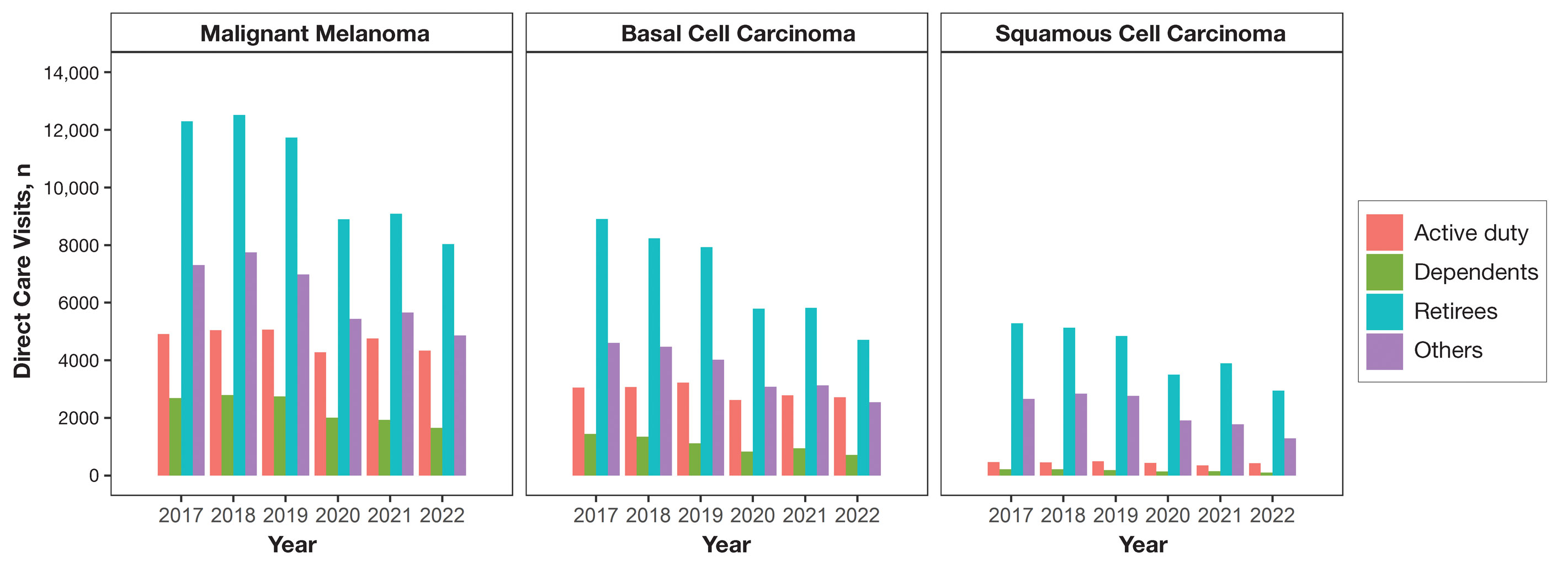
Results
Encounter data were analyzed from a total of 5,374,348 visits between DC and PC over the study period for each cancer type of interest. Figures 1 and 2 show temporal trends in DC visits compared with PC visits in each beneficiary category. The percentage of total DC visits subsequently declined each year throughout the study period, with percentage decreases from 2017 to 2022 of 1.45% or 8200 fewer visits for MM, 3.41% or 7280 fewer visits for BCC, and 2.26% or 3673 fewer visits for SCC.
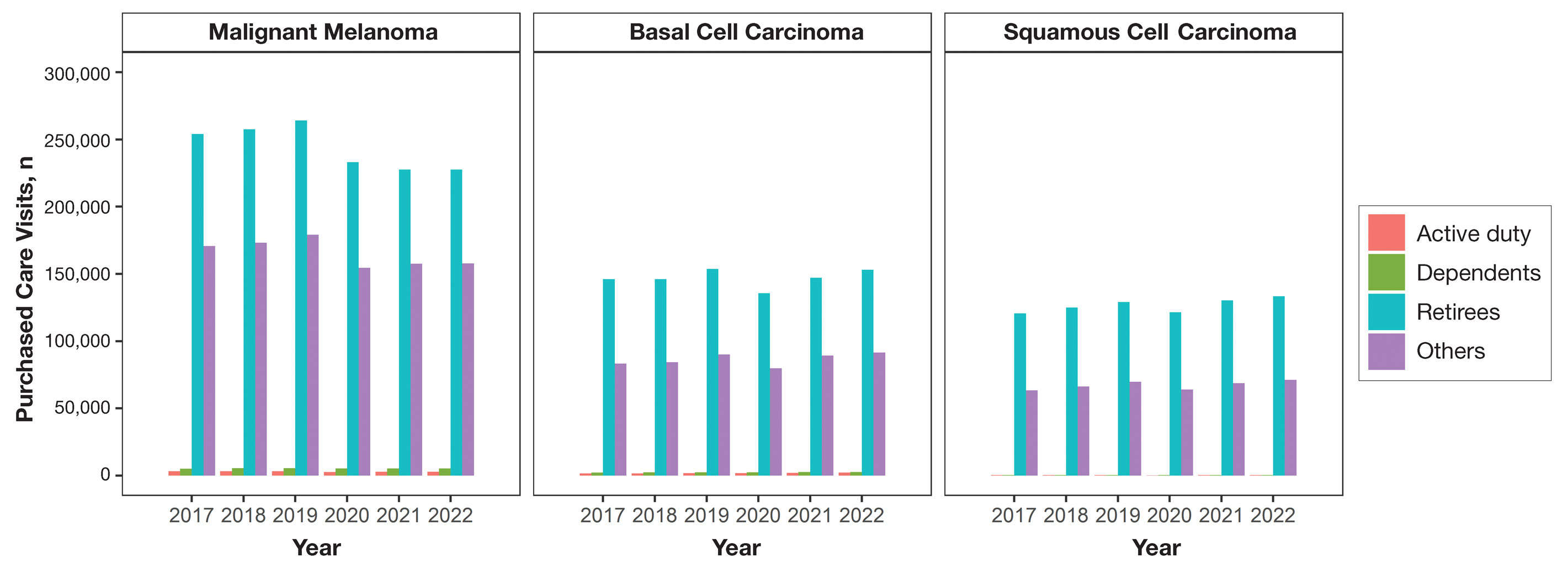
When stratified by beneficiary category, this trend remained consistent among dependents and retirees, with the most notable annual percentage decrease from 2019 to 2020. A higher proportion of younger adults and active-duty beneficiaries was seen in DC relative to PC, in which most visits were among retirees and others (primarily dependents of retirees, survivors, and Guard/Reserve on active duty, as well as inactive Guard/Reserve). No linear trends over time were apparent for active duty in DC and for dependents and retirees in PC. eTable 1 summarizes the demographic characteristics of MHS beneficiaries being seen in DC and PC over the study period for each cancer type of interest.
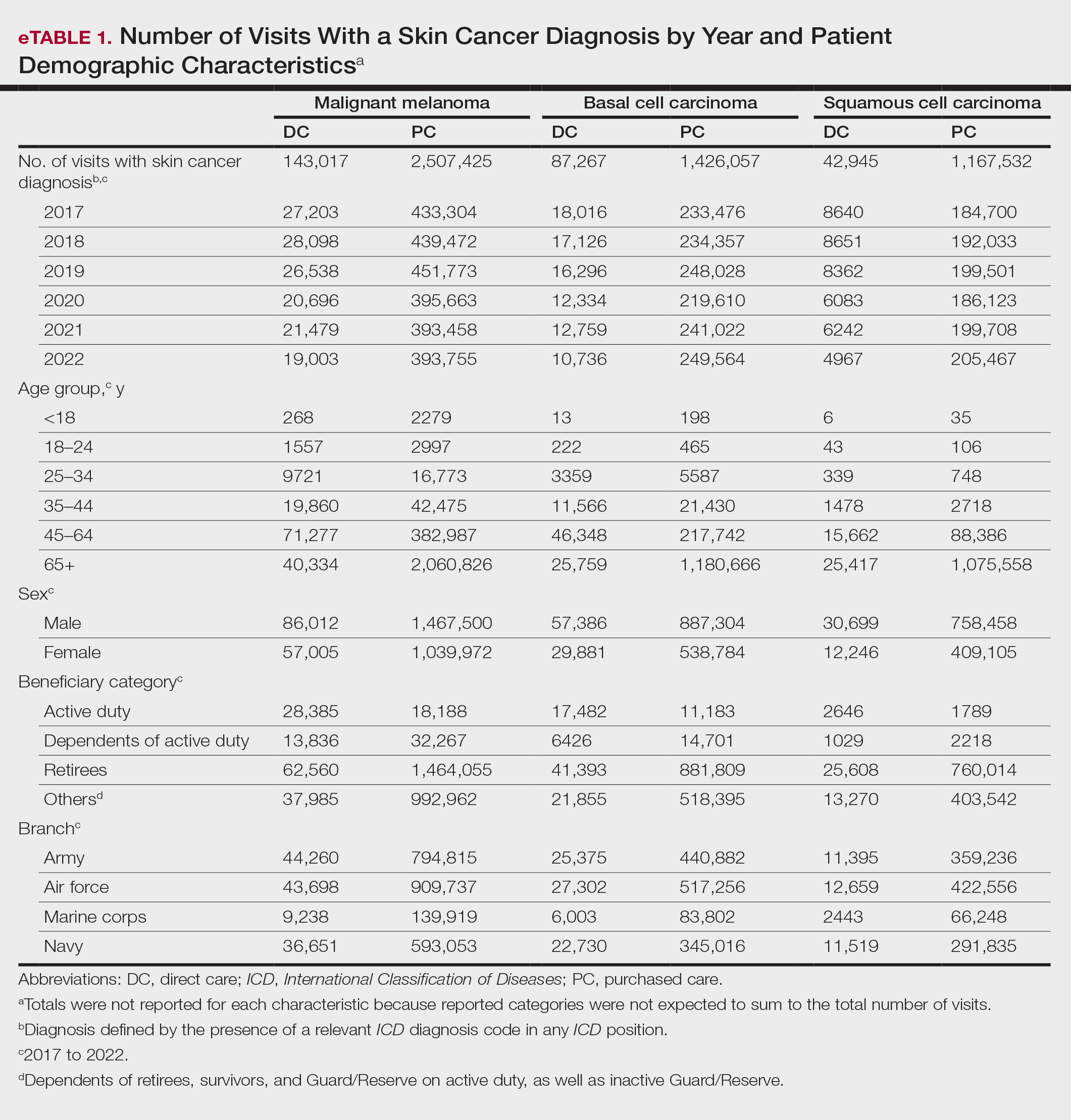
The Table shows the period prevalence of skin cancer diagnoses within the MHS beneficiary population from 2017 to 2022. These data were further analyzed by MM, BCC, and SCC (eTable 2) and demographics of interest for the year 2022. By beneficiary category, the period prevalence of MM was 0.08% in active duty, 0.06% in dependents, 0.48% in others, and 1.10% in retirees; the period prevalence of BCC was 0.12% in active duty, 0.07% in dependents, 0.91% in others, and 2.50% in retirees; and the period prevalence of SCC was 0.02% in active duty, 0.01% in dependents, 0.63% in others, and 1.87% in retirees. By sponsor branch, the period prevalence of MM was 0.35% in the army, 0.62% in the air force, 0.35% in the marine corps, and 0.65% in the navy; the period prevalence of BCC was 0.74% in the army, 1.30% in the air force, 0.74% in the marine corps, and 1.36% in the navy; and the period prevalence of SCC was 0.52% in the army, 0.92% in the air force, 0.51% in the marine corps, and 0.97% in the navy.
Comment
This study aimed to provide insight into the burden of skin cancer within the MHS beneficiary population and to identify temporal trends in where these beneficiaries receive their care. We examined patient encounter data from more than 9.6 million MHS beneficiaries.
The utilization of ICD codes from patient encounters to estimate the prevalence of nonmelanoma skin cancer (NMSC) has demonstrated a high positive predictive value. In one study, NMSC cases were confirmed in 96.5% of ICD code–identified patients.5 We presented an extensive collection of epidemiologic data on BCC and SCC, which posed unique challenges for tracking, as they are not reported to or monitored by cancer registries such as the Surveillance, Epidemiology, and End Results (SEER) Program.6
MHS Compared to the US Population—A study using the Global Burden of Disease 2019 database revealed an increasing trend in the incidence and prevalence of NMSC and melanoma since 1990. The same study found the period prevalence in 2019 of MM, SCC, and BCC in the general US population to be 0.13%, 0.31%, and 0.05%, respectively.7 In contrast, among MHS beneficiaries, we observed a higher prevalence in the same year, with figures of 0.66% for MM, 0.72% for SCC, and 1.02% for BCC. According to the SEER database, the period prevalence of MM within the general US population in 2020 was 0.4%.8 That same year, we identified a higher period prevalence of MM—0.54%—within the MHS beneficiary population. Specifically, within the MHS retiree population, the prevalence in 2022 was double that of the general MHS population, with a rate of 1.10%, underscoring the importance of skin cancer screening in older, at-risk adult populations. Prior studies similarly found increased rates of skin cancer within the military beneficiary population. Further studies are needed to compare age-adjusted rates in the MHS vs US population.9-11
COVID-19 Trends—Our data showed an overall decreasing prevalence of skin cancer in the MHS from 2019 to 2021. We suspect that the apparent decrease in skin cancer prevalence may be attributed to underdiagnosis from COVID-19 pandemic restrictions. During that time, many dermatology clinics at military treatment facilities underwent temporary closures, and some dermatologists were sent on nondermatologic utilization tours. Likewise, a US multi-institutional study described declining rates of new melanomas from 2020 to 2021, with an increased proportion of patient presentations with advanced melanoma, suggesting an underdiagnosis of melanoma cases during pandemic restrictions. That study also noted an increased rate of patient-identified melanomas and a decreased rate of provider-identified melanomas during that time.12 Contributing factors may include excess hospital demand, increased patient complexity and acute care needs, and long outpatient clinic backlogs during this time.13Financial Burden—Over our 5-year study period, there were 5,374,348 patient encounters addressing skin cancer, both in DC and PC (Figures 1 and 2; eTable 1). In 2016 to 2018, the average annual cost of treating skin cancer in the US civilian, noninstitutionalized population was $1243 for NMSC (BCC and SCC) and $2430 for melanoma.6 Using this metric, the estimated total cost of care rendered in the MHS in 2018 for NMSC and melanoma was $202,510,803 and $156,516,300, respectively.
Trends in DC vs PC—In the years examined, we found a notable decrease in the number of beneficiaries receiving treatment for MM, BCC, and SCC in DC. Simultaneously, there has been an increase in the number of beneficiaries receiving PC for BCC and SCC, though this trend was not apparent for MM.
Our data provided interesting insights into the percentage of PC compared with DC offered within the MHS. Importantly, our findings suggested that the majority of skin cancer in active-duty service members is managed with DC within the military treatment facility setting (61% DC management over the period analyzed). This finding was true across all years of data analyzed, suggesting that the COVID-19 pandemic did not result in a quantifiable shift in care of skin cancer within the active-duty component to outside providers. One of the critical roles of dermatologists in the MHS is to diagnose and treat skin cancer, and our study suggested that the current global manning and staffing for MHS dermatologists may not be sufficient to meet the burden of skin cancers encountered within our active-duty troops, as only 61% are managed with DC. In particular, service members in more austere and/or overseas locations may not have ready access to a dermatologist.
The burden of skin cancer shifts dramatically when analyzing care of all other populations included in these data, including dependents of active-duty service members, retirees, and the category of “other” (ie, principally dependents of retirees). Within these populations, the rate of DC falls to 30%, with 70% of active-duty dependent care being deferred to network. The findings are even more noticeable for retirees and others within these 2 cohorts in all types of skin cancer analyzed, where DC only accounted for 5.2% of those skin cancers encountered and managed across TRICARE-eligible beneficiaries. For MM, BCC, and SCC, percentages of DC were 5.4%, 5.8%, and 3.5%, respectively. Although it is interesting to note the lower percentage of SCC managed via DC, our data did not allow for extrapolation as to why more SCC cases may be deferred to network. The shift to PC may align with DoD initiatives to increase the private sector’s involvement in military medicine and transition to civilianizing the MHS.14 In the end, the findings are remarkable, with approximately 95% of skin cancer care and management provided overall via PC.
These findings differ from previously published data regarding DC and PC from other specialty areas. Results from an analysis of DC vs PC for plastic surgery for the entire MHS from 2016 to 2019 found 83.2% of cases were deferred to network.15 A similar publication in the orthopedics literature examined TRICARE claims for patients who underwent total hip or knee arthroplasties between 2006 and 2019 and found 84.6% of cases were referred for PC. Notably, the authors utilized generalized linear models for cost analysis and found that DC was more expensive than PC, though this likely was a result of higher rates of hospital readmission within DC cases.16 Lastly, an article on the DC vs PC disposition of MHS patients with breast cancer from 2003 to 2008 found 46% of cases managed with DC vs 26.% with PC and 27.8% receiving a combination. In this case, the authors found a reduced cost associated with DC vs PC.17
Little additional literature exists regarding the costs of DC vs PC. An article published in 2016 designed to assess costs of DC vs PC showed that almost all military treatment facilities have higher costs than their private sector counterparts, with a few exceptions.18 This does not assess the costs of specific procedures, however, and only the overall cost to maintain a treatment facility. Importantly, this study was based on data from FY 2014 and has not been updated to reflect complex changes within the MHS system and the private health care system. Indeed, a US Government Accountability Office FY 2023 study highlighted staffing and efficiency issues within this transition to civilian medicine; subsequently, the 2024 President’s Budget suspended all planned clinical medical military end strength divestitures, underscoring the potential ineffectiveness of a civilianized MHS at meeting the health care needs of its beneficiaries.19,20 Future research on a national scale will be necessary to see if there is a reversal of this trend to PC and if doing so has any impact on access to DC for active-duty troops or active-duty dependents.
In addition to PC vs DC trends, we also can get a sense of the impact of the COVID pandemic restrictions on access to DC vs PC by assessing the change in rates seen in the data from the pre-COVID years (2017-2019) to the “post-COVID” years (2020-2022) included. Overall, rates of DC decreased uniformly from their already low percentages. In our study, rates of DC decreased from 5.8% in 2019 to 4.8% in 2022 for MM, from 6.6% to 4.3% for BCC, and from 4.2% to 2.9% for SCC. Although these changes seem small at first, they represent a 30.6% overall decrease in DC for BCC and an overall decrease of 55.4% in DC for SCC. Although our data do not allow us to extrapolate the real cost of this reduction across a nationwide health care system and more than 5 million care encounters, the financial and personal (ie, lost man-hours) costs of this decrease in DC likely are substantial.
In addition to costs, qualitative aspects that contribute to the burden of skin cancer include treatment-related morbidity, such as scarring, pain, and time spent away from family, work, and hobbies, as well as overall patient satisfaction with the quality of care they receive.21 Future work is critical to assess the real cost of this immense burden of PC for the treatment and management of skin cancers within the DoD beneficiary population.
Limitations—This study is limited by its observational nature. Given the mechanism of our data collection, we may have underestimated disease prevalence, as not all patients are seen for their diagnosis annually. Furthermore, reported demographic strata (eg, age, sex) were limited to those available and valid in the M2 reporting system. Finally, our study only collected data from those service members or former service members seen within the MHS and does not reflect any care rendered to those who are no longer active duty but did not officially retire from the military (ie, nonretired service members receiving care in the Veterans Affairs system for skin cancer).
Conclusion
We describe the annual burden of care for skin cancer in the MHS beneficiary population. Noteworthy findings observed were an overall decrease in beneficiaries being treated for skin cancer through DC; a decreasing annual prevalence of skin cancer diagnosis between 2019 and 2021, which may represent underdiagnosis or decreased follow-up in the setting of the COVID-19 pandemic; and a higher rate of skin cancer in the military beneficiary population compared to the civilian population.
- US Department of Defense. Military health. Accessed October 5, 2023. https://www.defense.gov/
- Wooten NR, Brittingham JA, Pitner RO, et al. Purchased behavioral health care received by Military Health System beneficiaries in civilian medical facilities, 2000-2014. Mil Med. 2018;183:E278-E290. doi:10.1093/milmed/usx101
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192. doi:10.1016/j.jaad.2017.11.062
- American Academy of Dermatology. Skin cancer. Updated April 22, 2022. Accessed April 17, 2024. https://www.aad.org/media/stats-skin-cancer
- Eide MJ, Krajenta R, Johnson D, et al. Identification of patients with nonmelanoma skin cancer using health maintenance organization claims data. Am J Epidemiol. 2010;171:123-128. doi:10.1093/aje/kwp352
- Kao SYZ, Ekwueme DU, Holman DM, et al. Economic burden of skin cancer treatment in the USA: an analysis of the Medical Expenditure Panel Survey Data, 2012-2018. Cancer Causes Control. 2023;34:205-212. doi:10.1007/s10552-022-01644-0
- Aggarwal P, Knabel P, Fleischer AB. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J Am Acad Dermatol. 2021;85:388-395. doi:10.1016/j.jaad.2021.03.109
- SEER*Explorer. SEER Incidence Data, November 2023 Submission (1975-2021). National Cancer Institute; 2024. Accessed April 17, 2024. https://seer.cancer.gov/statistics-network/explorer/application.html?site=53&data_type=1&graph_type=1&compareBy=sex&chk_sex_1=1&chk_sex_3=3&chk_sex_2=2&rate_type=2&race=1&age_range=1&advopt_precision=1&advopt_show_ci=on&hdn_view=1&advopt_show_apc=on&advopt_display=1
- Brown J, Kopf AW, Rigel DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663. doi:10.1111/j.1365-4362.1984.tb01228.x
- Page WF, Whiteman D, Murphy M. A comparison of melanoma mortality among WWII veterans of the Pacific and European theaters. Ann Epidemiol. 2000;10:192-195. doi:10.1016/s1047-2797(99)00050-2
- Ramani ML, Bennett RG. High prevalence of skin cancer in World War II servicemen stationed in the Pacific theater. J Am Acad Dermatol. 1993;28:733-737. doi:10.1016/0190-9622(93)70102-Y
- Trepanowski N, Chang MS, Zhou G, et al. Delays in melanoma presentation during the COVID-19 pandemic: a nationwide multi-institutional cohort study. J Am Acad Dermatol. 2022;87:1217-1219. doi:10.1016/j.jaad.2022.06.031
- Gibbs A. COVID-19 shutdowns caused delays in melanoma diagnoses, study finds. OHSU News. August 4, 2022. Accessed April 17, 2024. https://news.ohsu.edu/2022/08/04/covid-19-shutdowns-caused-delays-in-melanoma-diagnoses-study-finds
- Kime P. Pentagon budget calls for ‘civilianizing’ military hospitals. Military Times. Published February 10, 2020. Accessed April 17, 2024. https://www.militarytimes.com/news/your-military/2020/02/10/pentagon-budget-calls-for-civilianizing-military-hospitals/
- O’Reilly EB, Norris E, Ortiz-Pomales YT, et al. A comparison of direct care at military medical treatment facilities with purchased care in plastic surgery operative volume. Plast Reconstr Surg Glob Open. 2022;10(10 suppl):124-125. doi:10.1097/01.GOX.0000898976.03344.62
- Haag A, Hosein S, Lyon S, et al. Outcomes for arthroplasties in military health: a retrospective analysis of direct versus purchased care. Mil Med. 2023;188(suppl 6):45-51. doi:10.1093/milmed/usac441
- Eaglehouse YL, Georg MW, Richard P, et al. Cost-efficiency of breast cancer care in the US Military Health System: an economic evaluation in direct and purchased care. Mil Med. 2019;184:e494-e501. doi:10.1093/milmed/usz025
- Lurie PM. Comparing the cost of military treatment facilities with private sector care. Institute for Defense Analyses; February 2016. Accessed April 17, 2024. https://www.ida.org/research-and-publications/publications/all/c/co/comparing-the-costs-of-military-treatment-facilities-with-private-sector-care
- Defense Health Program. Fiscal Year (FY) 2024 President’s Budget: Operation and Maintenance Procurement Research, Development, Test and Evaluation. Department of Defense; March 2023. Accessed April 17, 2024. https://comptroller.defense.gov/Portals/45/Documents/defbudget/fy2024/budget_justification/pdfs/09_Defense_Health_Program/00-DHP_Vols_I_II_and_III_PB24.pdf
- US Government Accountability Office. Defense Health Care. DOD should reevaluate market structure for military medical treatment facility management. Published August 21, 2023. Accessed April 17, 2024. https://www.gao.gov/products/gao-23-105441
- Rosenberg A, Cho S. We can do better at protecting our service members from skin cancer. Mil Med. 2022;187:311-313. doi:10.1093/milmed/usac198
This retrospective observational study investigates skin cancer prevalence and care patterns within the Military Health System (MHS) from 2017 to 2022. Utilizing the MHS Management Analysis and Reporting Tool (most commonly called M2), we analyzed more than 5 million patient encounters and documented skin cancer prevalence in the MHS beneficiary population utilizing available demographic data. Notable findings included an increased prevalence of skin cancer in the military population compared with the civilian population, a substantial decline in direct care (DC) visits at military treatment facilities compared with civilian purchased care (PC) visits, and a decreased total number of visits during COVID-19 restrictions.
The Military Health System (MHS) is a worldwide health care delivery system that serves 9.6 million beneficiaries, including military service members, retirees, and their families.1 Its mission is 2-fold: provide a medically ready force, and provide a medical benefit in keeping with the service and sacrifice of active-duty personnel, military retirees, and their families. For fiscal year (FY) 2022, active-duty service members and their families comprised 16.7% and 19.9% of beneficiaries, respectively, while retired service members and their families comprised 27% and 32% of beneficiaries, respectively.
The MHS operates under the authority of the Department of Defense (DoD) and is supported by an annual budget of approximately $50 billion.1 Health care provision within the MHS is managed by TRICARE regional networks.2 Within these networks, MHS beneficiaries may receive health care in 2 categories: direct care (DC) and purchased care (PC). Direct care is rendered in military treatment facilities by military or civilian providers contracted by the DoD, and PC is administered by civilian providers at civilian health care facilities within the TRICARE network, which is comprised of individual providers, clinics, and hospitals that have agreed to accept TRICARE beneficiaries.1 Purchased care is fee-for-service and paid for by the MHS. Of note, the MHS differs from the Veterans Affairs health care system in that the MHS through DC and PC sees only active-duty service members, active-duty dependents, retirees, and retirees’ dependents (primarily spouses), whereas Veterans Affairs sees only veterans (not necessarily retirees) discharged from military service with compensable medical conditions or disabilities.
Skin cancer presents a notable concern for the US Military, as the risk for skin cancer is thought to be higher than in the general population.3,4 This elevated risk is attributed to numerous factors inherent to active-duty service, including time spent in tropical environments, increased exposure to UV radiation, time spent at high altitudes, and decreased rates of sun-protective behaviors.3 Although numerous studies have explored the mechanisms that contribute to service members’ increased skin cancer risk, there are few (if any) that discuss the burden of skin cancer on the MHS and where its beneficiaries receive their skin cancer care. This study evaluated the burden of skin cancer within the MHS, as demonstrated by the period prevalence of skin cancer among its beneficiaries and the number and distribution of patient visits for skin cancer across both DC and PC from 2017 to 2022.
Methods
Data Collection—This retrospective observational study was designed to describe trends in outpatient visits with a skin cancer diagnosis and annual prevalence of skin cancer types in the MHS. Data are from all MHS beneficiaries who were eligible or enrolled in the analysis year. Our data source was the MHS Management Analysis and Reporting Tool (most commonly called M2), a query tool that contains the current and most recent 5 full FYs of Defense Health Agency corporate health care data including aggregated FY and calendar-year counts of MHS beneficiaries from 2017 to 2022 using encounter and claims data tables from both DC and PC. Data in M2 are coded using a pseudo-person identification number, and queries performed for this study were limited to de-identified visit and patient counts.
Skin cancer diagnoses were defined by relevant International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes recorded from outpatient visits in DC and PC. The M2 database was queried to find aggregate counts of visits and unique MHS beneficiaries with one or more diagnoses of a skin cancer type of interest (defined by relevant ICD-10-CM code) over the study period stratified by year and by patient demographic characteristics. Skin cancer types by ICD-10-CM code group included basal cell carcinoma (BCC), squamous cell carcinoma (SCC), malignant melanoma (MM), and other (including Merkel cell carcinoma and sebaceous carcinoma). Demographic strata included age, sex, military status (active duty, dependents of active duty, retired, or all others), sponsor military rank, and sponsor branch (army, air force, marine corps, or navy). Visit counts included diagnoses from any ICD position (for encounters that contained multiple ICD codes) to describe the total volume of care that addressed a diagnosed skin cancer. Counts of unique patients in prevalence analyses included relevant diagnoses in the primary ICD position only to increase the specificity of prevalence estimates.
Data Analysis—Descriptive analyses included the total number of outpatient visits with a skin cancer diagnosis in DC and PC over the study period, with percentages of total visits by year and by demographic strata. Separate analyses estimated annual prevalences of skin cancer types in the MHS by study year and within 2022 by demographic strata. Numerators in prevalence analyses were defined as the number of unique individuals with one or more relevant ICD codes in the analysis year. Denominators were defined as the total number of MHS beneficiaries in the analysis year and resulting period prevalences reported. Observed prevalences were qualitatively described, and trends were compared with prevalences in nonmilitary populations reported in the literature.
Ethics—This study was conducted as part of a study using secondary analyses of de-identified data from the M2 database. The study was reviewed and approved by the Walter Reed National Military Medical Center institutional review board.

Results
Encounter data were analyzed from a total of 5,374,348 visits between DC and PC over the study period for each cancer type of interest. Figures 1 and 2 show temporal trends in DC visits compared with PC visits in each beneficiary category. The percentage of total DC visits subsequently declined each year throughout the study period, with percentage decreases from 2017 to 2022 of 1.45% or 8200 fewer visits for MM, 3.41% or 7280 fewer visits for BCC, and 2.26% or 3673 fewer visits for SCC.

When stratified by beneficiary category, this trend remained consistent among dependents and retirees, with the most notable annual percentage decrease from 2019 to 2020. A higher proportion of younger adults and active-duty beneficiaries was seen in DC relative to PC, in which most visits were among retirees and others (primarily dependents of retirees, survivors, and Guard/Reserve on active duty, as well as inactive Guard/Reserve). No linear trends over time were apparent for active duty in DC and for dependents and retirees in PC. eTable 1 summarizes the demographic characteristics of MHS beneficiaries being seen in DC and PC over the study period for each cancer type of interest.

The Table shows the period prevalence of skin cancer diagnoses within the MHS beneficiary population from 2017 to 2022. These data were further analyzed by MM, BCC, and SCC (eTable 2) and demographics of interest for the year 2022. By beneficiary category, the period prevalence of MM was 0.08% in active duty, 0.06% in dependents, 0.48% in others, and 1.10% in retirees; the period prevalence of BCC was 0.12% in active duty, 0.07% in dependents, 0.91% in others, and 2.50% in retirees; and the period prevalence of SCC was 0.02% in active duty, 0.01% in dependents, 0.63% in others, and 1.87% in retirees. By sponsor branch, the period prevalence of MM was 0.35% in the army, 0.62% in the air force, 0.35% in the marine corps, and 0.65% in the navy; the period prevalence of BCC was 0.74% in the army, 1.30% in the air force, 0.74% in the marine corps, and 1.36% in the navy; and the period prevalence of SCC was 0.52% in the army, 0.92% in the air force, 0.51% in the marine corps, and 0.97% in the navy.


Comment
This study aimed to provide insight into the burden of skin cancer within the MHS beneficiary population and to identify temporal trends in where these beneficiaries receive their care. We examined patient encounter data from more than 9.6 million MHS beneficiaries.
The utilization of ICD codes from patient encounters to estimate the prevalence of nonmelanoma skin cancer (NMSC) has demonstrated a high positive predictive value. In one study, NMSC cases were confirmed in 96.5% of ICD code–identified patients.5 We presented an extensive collection of epidemiologic data on BCC and SCC, which posed unique challenges for tracking, as they are not reported to or monitored by cancer registries such as the Surveillance, Epidemiology, and End Results (SEER) Program.6
MHS Compared to the US Population—A study using the Global Burden of Disease 2019 database revealed an increasing trend in the incidence and prevalence of NMSC and melanoma since 1990. The same study found the period prevalence in 2019 of MM, SCC, and BCC in the general US population to be 0.13%, 0.31%, and 0.05%, respectively.7 In contrast, among MHS beneficiaries, we observed a higher prevalence in the same year, with figures of 0.66% for MM, 0.72% for SCC, and 1.02% for BCC. According to the SEER database, the period prevalence of MM within the general US population in 2020 was 0.4%.8 That same year, we identified a higher period prevalence of MM—0.54%—within the MHS beneficiary population. Specifically, within the MHS retiree population, the prevalence in 2022 was double that of the general MHS population, with a rate of 1.10%, underscoring the importance of skin cancer screening in older, at-risk adult populations. Prior studies similarly found increased rates of skin cancer within the military beneficiary population. Further studies are needed to compare age-adjusted rates in the MHS vs US population.9-11
COVID-19 Trends—Our data showed an overall decreasing prevalence of skin cancer in the MHS from 2019 to 2021. We suspect that the apparent decrease in skin cancer prevalence may be attributed to underdiagnosis from COVID-19 pandemic restrictions. During that time, many dermatology clinics at military treatment facilities underwent temporary closures, and some dermatologists were sent on nondermatologic utilization tours. Likewise, a US multi-institutional study described declining rates of new melanomas from 2020 to 2021, with an increased proportion of patient presentations with advanced melanoma, suggesting an underdiagnosis of melanoma cases during pandemic restrictions. That study also noted an increased rate of patient-identified melanomas and a decreased rate of provider-identified melanomas during that time.12 Contributing factors may include excess hospital demand, increased patient complexity and acute care needs, and long outpatient clinic backlogs during this time.13Financial Burden—Over our 5-year study period, there were 5,374,348 patient encounters addressing skin cancer, both in DC and PC (Figures 1 and 2; eTable 1). In 2016 to 2018, the average annual cost of treating skin cancer in the US civilian, noninstitutionalized population was $1243 for NMSC (BCC and SCC) and $2430 for melanoma.6 Using this metric, the estimated total cost of care rendered in the MHS in 2018 for NMSC and melanoma was $202,510,803 and $156,516,300, respectively.
Trends in DC vs PC—In the years examined, we found a notable decrease in the number of beneficiaries receiving treatment for MM, BCC, and SCC in DC. Simultaneously, there has been an increase in the number of beneficiaries receiving PC for BCC and SCC, though this trend was not apparent for MM.
Our data provided interesting insights into the percentage of PC compared with DC offered within the MHS. Importantly, our findings suggested that the majority of skin cancer in active-duty service members is managed with DC within the military treatment facility setting (61% DC management over the period analyzed). This finding was true across all years of data analyzed, suggesting that the COVID-19 pandemic did not result in a quantifiable shift in care of skin cancer within the active-duty component to outside providers. One of the critical roles of dermatologists in the MHS is to diagnose and treat skin cancer, and our study suggested that the current global manning and staffing for MHS dermatologists may not be sufficient to meet the burden of skin cancers encountered within our active-duty troops, as only 61% are managed with DC. In particular, service members in more austere and/or overseas locations may not have ready access to a dermatologist.
The burden of skin cancer shifts dramatically when analyzing care of all other populations included in these data, including dependents of active-duty service members, retirees, and the category of “other” (ie, principally dependents of retirees). Within these populations, the rate of DC falls to 30%, with 70% of active-duty dependent care being deferred to network. The findings are even more noticeable for retirees and others within these 2 cohorts in all types of skin cancer analyzed, where DC only accounted for 5.2% of those skin cancers encountered and managed across TRICARE-eligible beneficiaries. For MM, BCC, and SCC, percentages of DC were 5.4%, 5.8%, and 3.5%, respectively. Although it is interesting to note the lower percentage of SCC managed via DC, our data did not allow for extrapolation as to why more SCC cases may be deferred to network. The shift to PC may align with DoD initiatives to increase the private sector’s involvement in military medicine and transition to civilianizing the MHS.14 In the end, the findings are remarkable, with approximately 95% of skin cancer care and management provided overall via PC.
These findings differ from previously published data regarding DC and PC from other specialty areas. Results from an analysis of DC vs PC for plastic surgery for the entire MHS from 2016 to 2019 found 83.2% of cases were deferred to network.15 A similar publication in the orthopedics literature examined TRICARE claims for patients who underwent total hip or knee arthroplasties between 2006 and 2019 and found 84.6% of cases were referred for PC. Notably, the authors utilized generalized linear models for cost analysis and found that DC was more expensive than PC, though this likely was a result of higher rates of hospital readmission within DC cases.16 Lastly, an article on the DC vs PC disposition of MHS patients with breast cancer from 2003 to 2008 found 46% of cases managed with DC vs 26.% with PC and 27.8% receiving a combination. In this case, the authors found a reduced cost associated with DC vs PC.17
Little additional literature exists regarding the costs of DC vs PC. An article published in 2016 designed to assess costs of DC vs PC showed that almost all military treatment facilities have higher costs than their private sector counterparts, with a few exceptions.18 This does not assess the costs of specific procedures, however, and only the overall cost to maintain a treatment facility. Importantly, this study was based on data from FY 2014 and has not been updated to reflect complex changes within the MHS system and the private health care system. Indeed, a US Government Accountability Office FY 2023 study highlighted staffing and efficiency issues within this transition to civilian medicine; subsequently, the 2024 President’s Budget suspended all planned clinical medical military end strength divestitures, underscoring the potential ineffectiveness of a civilianized MHS at meeting the health care needs of its beneficiaries.19,20 Future research on a national scale will be necessary to see if there is a reversal of this trend to PC and if doing so has any impact on access to DC for active-duty troops or active-duty dependents.
In addition to PC vs DC trends, we also can get a sense of the impact of the COVID pandemic restrictions on access to DC vs PC by assessing the change in rates seen in the data from the pre-COVID years (2017-2019) to the “post-COVID” years (2020-2022) included. Overall, rates of DC decreased uniformly from their already low percentages. In our study, rates of DC decreased from 5.8% in 2019 to 4.8% in 2022 for MM, from 6.6% to 4.3% for BCC, and from 4.2% to 2.9% for SCC. Although these changes seem small at first, they represent a 30.6% overall decrease in DC for BCC and an overall decrease of 55.4% in DC for SCC. Although our data do not allow us to extrapolate the real cost of this reduction across a nationwide health care system and more than 5 million care encounters, the financial and personal (ie, lost man-hours) costs of this decrease in DC likely are substantial.
In addition to costs, qualitative aspects that contribute to the burden of skin cancer include treatment-related morbidity, such as scarring, pain, and time spent away from family, work, and hobbies, as well as overall patient satisfaction with the quality of care they receive.21 Future work is critical to assess the real cost of this immense burden of PC for the treatment and management of skin cancers within the DoD beneficiary population.
Limitations—This study is limited by its observational nature. Given the mechanism of our data collection, we may have underestimated disease prevalence, as not all patients are seen for their diagnosis annually. Furthermore, reported demographic strata (eg, age, sex) were limited to those available and valid in the M2 reporting system. Finally, our study only collected data from those service members or former service members seen within the MHS and does not reflect any care rendered to those who are no longer active duty but did not officially retire from the military (ie, nonretired service members receiving care in the Veterans Affairs system for skin cancer).
Conclusion
We describe the annual burden of care for skin cancer in the MHS beneficiary population. Noteworthy findings observed were an overall decrease in beneficiaries being treated for skin cancer through DC; a decreasing annual prevalence of skin cancer diagnosis between 2019 and 2021, which may represent underdiagnosis or decreased follow-up in the setting of the COVID-19 pandemic; and a higher rate of skin cancer in the military beneficiary population compared to the civilian population.
This retrospective observational study investigates skin cancer prevalence and care patterns within the Military Health System (MHS) from 2017 to 2022. Utilizing the MHS Management Analysis and Reporting Tool (most commonly called M2), we analyzed more than 5 million patient encounters and documented skin cancer prevalence in the MHS beneficiary population utilizing available demographic data. Notable findings included an increased prevalence of skin cancer in the military population compared with the civilian population, a substantial decline in direct care (DC) visits at military treatment facilities compared with civilian purchased care (PC) visits, and a decreased total number of visits during COVID-19 restrictions.
The Military Health System (MHS) is a worldwide health care delivery system that serves 9.6 million beneficiaries, including military service members, retirees, and their families.1 Its mission is 2-fold: provide a medically ready force, and provide a medical benefit in keeping with the service and sacrifice of active-duty personnel, military retirees, and their families. For fiscal year (FY) 2022, active-duty service members and their families comprised 16.7% and 19.9% of beneficiaries, respectively, while retired service members and their families comprised 27% and 32% of beneficiaries, respectively.
The MHS operates under the authority of the Department of Defense (DoD) and is supported by an annual budget of approximately $50 billion.1 Health care provision within the MHS is managed by TRICARE regional networks.2 Within these networks, MHS beneficiaries may receive health care in 2 categories: direct care (DC) and purchased care (PC). Direct care is rendered in military treatment facilities by military or civilian providers contracted by the DoD, and PC is administered by civilian providers at civilian health care facilities within the TRICARE network, which is comprised of individual providers, clinics, and hospitals that have agreed to accept TRICARE beneficiaries.1 Purchased care is fee-for-service and paid for by the MHS. Of note, the MHS differs from the Veterans Affairs health care system in that the MHS through DC and PC sees only active-duty service members, active-duty dependents, retirees, and retirees’ dependents (primarily spouses), whereas Veterans Affairs sees only veterans (not necessarily retirees) discharged from military service with compensable medical conditions or disabilities.
Skin cancer presents a notable concern for the US Military, as the risk for skin cancer is thought to be higher than in the general population.3,4 This elevated risk is attributed to numerous factors inherent to active-duty service, including time spent in tropical environments, increased exposure to UV radiation, time spent at high altitudes, and decreased rates of sun-protective behaviors.3 Although numerous studies have explored the mechanisms that contribute to service members’ increased skin cancer risk, there are few (if any) that discuss the burden of skin cancer on the MHS and where its beneficiaries receive their skin cancer care. This study evaluated the burden of skin cancer within the MHS, as demonstrated by the period prevalence of skin cancer among its beneficiaries and the number and distribution of patient visits for skin cancer across both DC and PC from 2017 to 2022.
Methods
Data Collection—This retrospective observational study was designed to describe trends in outpatient visits with a skin cancer diagnosis and annual prevalence of skin cancer types in the MHS. Data are from all MHS beneficiaries who were eligible or enrolled in the analysis year. Our data source was the MHS Management Analysis and Reporting Tool (most commonly called M2), a query tool that contains the current and most recent 5 full FYs of Defense Health Agency corporate health care data including aggregated FY and calendar-year counts of MHS beneficiaries from 2017 to 2022 using encounter and claims data tables from both DC and PC. Data in M2 are coded using a pseudo-person identification number, and queries performed for this study were limited to de-identified visit and patient counts.
Skin cancer diagnoses were defined by relevant International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes recorded from outpatient visits in DC and PC. The M2 database was queried to find aggregate counts of visits and unique MHS beneficiaries with one or more diagnoses of a skin cancer type of interest (defined by relevant ICD-10-CM code) over the study period stratified by year and by patient demographic characteristics. Skin cancer types by ICD-10-CM code group included basal cell carcinoma (BCC), squamous cell carcinoma (SCC), malignant melanoma (MM), and other (including Merkel cell carcinoma and sebaceous carcinoma). Demographic strata included age, sex, military status (active duty, dependents of active duty, retired, or all others), sponsor military rank, and sponsor branch (army, air force, marine corps, or navy). Visit counts included diagnoses from any ICD position (for encounters that contained multiple ICD codes) to describe the total volume of care that addressed a diagnosed skin cancer. Counts of unique patients in prevalence analyses included relevant diagnoses in the primary ICD position only to increase the specificity of prevalence estimates.
Data Analysis—Descriptive analyses included the total number of outpatient visits with a skin cancer diagnosis in DC and PC over the study period, with percentages of total visits by year and by demographic strata. Separate analyses estimated annual prevalences of skin cancer types in the MHS by study year and within 2022 by demographic strata. Numerators in prevalence analyses were defined as the number of unique individuals with one or more relevant ICD codes in the analysis year. Denominators were defined as the total number of MHS beneficiaries in the analysis year and resulting period prevalences reported. Observed prevalences were qualitatively described, and trends were compared with prevalences in nonmilitary populations reported in the literature.
Ethics—This study was conducted as part of a study using secondary analyses of de-identified data from the M2 database. The study was reviewed and approved by the Walter Reed National Military Medical Center institutional review board.

Results
Encounter data were analyzed from a total of 5,374,348 visits between DC and PC over the study period for each cancer type of interest. Figures 1 and 2 show temporal trends in DC visits compared with PC visits in each beneficiary category. The percentage of total DC visits subsequently declined each year throughout the study period, with percentage decreases from 2017 to 2022 of 1.45% or 8200 fewer visits for MM, 3.41% or 7280 fewer visits for BCC, and 2.26% or 3673 fewer visits for SCC.

When stratified by beneficiary category, this trend remained consistent among dependents and retirees, with the most notable annual percentage decrease from 2019 to 2020. A higher proportion of younger adults and active-duty beneficiaries was seen in DC relative to PC, in which most visits were among retirees and others (primarily dependents of retirees, survivors, and Guard/Reserve on active duty, as well as inactive Guard/Reserve). No linear trends over time were apparent for active duty in DC and for dependents and retirees in PC. eTable 1 summarizes the demographic characteristics of MHS beneficiaries being seen in DC and PC over the study period for each cancer type of interest.

The Table shows the period prevalence of skin cancer diagnoses within the MHS beneficiary population from 2017 to 2022. These data were further analyzed by MM, BCC, and SCC (eTable 2) and demographics of interest for the year 2022. By beneficiary category, the period prevalence of MM was 0.08% in active duty, 0.06% in dependents, 0.48% in others, and 1.10% in retirees; the period prevalence of BCC was 0.12% in active duty, 0.07% in dependents, 0.91% in others, and 2.50% in retirees; and the period prevalence of SCC was 0.02% in active duty, 0.01% in dependents, 0.63% in others, and 1.87% in retirees. By sponsor branch, the period prevalence of MM was 0.35% in the army, 0.62% in the air force, 0.35% in the marine corps, and 0.65% in the navy; the period prevalence of BCC was 0.74% in the army, 1.30% in the air force, 0.74% in the marine corps, and 1.36% in the navy; and the period prevalence of SCC was 0.52% in the army, 0.92% in the air force, 0.51% in the marine corps, and 0.97% in the navy.


Comment
This study aimed to provide insight into the burden of skin cancer within the MHS beneficiary population and to identify temporal trends in where these beneficiaries receive their care. We examined patient encounter data from more than 9.6 million MHS beneficiaries.
The utilization of ICD codes from patient encounters to estimate the prevalence of nonmelanoma skin cancer (NMSC) has demonstrated a high positive predictive value. In one study, NMSC cases were confirmed in 96.5% of ICD code–identified patients.5 We presented an extensive collection of epidemiologic data on BCC and SCC, which posed unique challenges for tracking, as they are not reported to or monitored by cancer registries such as the Surveillance, Epidemiology, and End Results (SEER) Program.6
MHS Compared to the US Population—A study using the Global Burden of Disease 2019 database revealed an increasing trend in the incidence and prevalence of NMSC and melanoma since 1990. The same study found the period prevalence in 2019 of MM, SCC, and BCC in the general US population to be 0.13%, 0.31%, and 0.05%, respectively.7 In contrast, among MHS beneficiaries, we observed a higher prevalence in the same year, with figures of 0.66% for MM, 0.72% for SCC, and 1.02% for BCC. According to the SEER database, the period prevalence of MM within the general US population in 2020 was 0.4%.8 That same year, we identified a higher period prevalence of MM—0.54%—within the MHS beneficiary population. Specifically, within the MHS retiree population, the prevalence in 2022 was double that of the general MHS population, with a rate of 1.10%, underscoring the importance of skin cancer screening in older, at-risk adult populations. Prior studies similarly found increased rates of skin cancer within the military beneficiary population. Further studies are needed to compare age-adjusted rates in the MHS vs US population.9-11
COVID-19 Trends—Our data showed an overall decreasing prevalence of skin cancer in the MHS from 2019 to 2021. We suspect that the apparent decrease in skin cancer prevalence may be attributed to underdiagnosis from COVID-19 pandemic restrictions. During that time, many dermatology clinics at military treatment facilities underwent temporary closures, and some dermatologists were sent on nondermatologic utilization tours. Likewise, a US multi-institutional study described declining rates of new melanomas from 2020 to 2021, with an increased proportion of patient presentations with advanced melanoma, suggesting an underdiagnosis of melanoma cases during pandemic restrictions. That study also noted an increased rate of patient-identified melanomas and a decreased rate of provider-identified melanomas during that time.12 Contributing factors may include excess hospital demand, increased patient complexity and acute care needs, and long outpatient clinic backlogs during this time.13Financial Burden—Over our 5-year study period, there were 5,374,348 patient encounters addressing skin cancer, both in DC and PC (Figures 1 and 2; eTable 1). In 2016 to 2018, the average annual cost of treating skin cancer in the US civilian, noninstitutionalized population was $1243 for NMSC (BCC and SCC) and $2430 for melanoma.6 Using this metric, the estimated total cost of care rendered in the MHS in 2018 for NMSC and melanoma was $202,510,803 and $156,516,300, respectively.
Trends in DC vs PC—In the years examined, we found a notable decrease in the number of beneficiaries receiving treatment for MM, BCC, and SCC in DC. Simultaneously, there has been an increase in the number of beneficiaries receiving PC for BCC and SCC, though this trend was not apparent for MM.
Our data provided interesting insights into the percentage of PC compared with DC offered within the MHS. Importantly, our findings suggested that the majority of skin cancer in active-duty service members is managed with DC within the military treatment facility setting (61% DC management over the period analyzed). This finding was true across all years of data analyzed, suggesting that the COVID-19 pandemic did not result in a quantifiable shift in care of skin cancer within the active-duty component to outside providers. One of the critical roles of dermatologists in the MHS is to diagnose and treat skin cancer, and our study suggested that the current global manning and staffing for MHS dermatologists may not be sufficient to meet the burden of skin cancers encountered within our active-duty troops, as only 61% are managed with DC. In particular, service members in more austere and/or overseas locations may not have ready access to a dermatologist.
The burden of skin cancer shifts dramatically when analyzing care of all other populations included in these data, including dependents of active-duty service members, retirees, and the category of “other” (ie, principally dependents of retirees). Within these populations, the rate of DC falls to 30%, with 70% of active-duty dependent care being deferred to network. The findings are even more noticeable for retirees and others within these 2 cohorts in all types of skin cancer analyzed, where DC only accounted for 5.2% of those skin cancers encountered and managed across TRICARE-eligible beneficiaries. For MM, BCC, and SCC, percentages of DC were 5.4%, 5.8%, and 3.5%, respectively. Although it is interesting to note the lower percentage of SCC managed via DC, our data did not allow for extrapolation as to why more SCC cases may be deferred to network. The shift to PC may align with DoD initiatives to increase the private sector’s involvement in military medicine and transition to civilianizing the MHS.14 In the end, the findings are remarkable, with approximately 95% of skin cancer care and management provided overall via PC.
These findings differ from previously published data regarding DC and PC from other specialty areas. Results from an analysis of DC vs PC for plastic surgery for the entire MHS from 2016 to 2019 found 83.2% of cases were deferred to network.15 A similar publication in the orthopedics literature examined TRICARE claims for patients who underwent total hip or knee arthroplasties between 2006 and 2019 and found 84.6% of cases were referred for PC. Notably, the authors utilized generalized linear models for cost analysis and found that DC was more expensive than PC, though this likely was a result of higher rates of hospital readmission within DC cases.16 Lastly, an article on the DC vs PC disposition of MHS patients with breast cancer from 2003 to 2008 found 46% of cases managed with DC vs 26.% with PC and 27.8% receiving a combination. In this case, the authors found a reduced cost associated with DC vs PC.17
Little additional literature exists regarding the costs of DC vs PC. An article published in 2016 designed to assess costs of DC vs PC showed that almost all military treatment facilities have higher costs than their private sector counterparts, with a few exceptions.18 This does not assess the costs of specific procedures, however, and only the overall cost to maintain a treatment facility. Importantly, this study was based on data from FY 2014 and has not been updated to reflect complex changes within the MHS system and the private health care system. Indeed, a US Government Accountability Office FY 2023 study highlighted staffing and efficiency issues within this transition to civilian medicine; subsequently, the 2024 President’s Budget suspended all planned clinical medical military end strength divestitures, underscoring the potential ineffectiveness of a civilianized MHS at meeting the health care needs of its beneficiaries.19,20 Future research on a national scale will be necessary to see if there is a reversal of this trend to PC and if doing so has any impact on access to DC for active-duty troops or active-duty dependents.
In addition to PC vs DC trends, we also can get a sense of the impact of the COVID pandemic restrictions on access to DC vs PC by assessing the change in rates seen in the data from the pre-COVID years (2017-2019) to the “post-COVID” years (2020-2022) included. Overall, rates of DC decreased uniformly from their already low percentages. In our study, rates of DC decreased from 5.8% in 2019 to 4.8% in 2022 for MM, from 6.6% to 4.3% for BCC, and from 4.2% to 2.9% for SCC. Although these changes seem small at first, they represent a 30.6% overall decrease in DC for BCC and an overall decrease of 55.4% in DC for SCC. Although our data do not allow us to extrapolate the real cost of this reduction across a nationwide health care system and more than 5 million care encounters, the financial and personal (ie, lost man-hours) costs of this decrease in DC likely are substantial.
In addition to costs, qualitative aspects that contribute to the burden of skin cancer include treatment-related morbidity, such as scarring, pain, and time spent away from family, work, and hobbies, as well as overall patient satisfaction with the quality of care they receive.21 Future work is critical to assess the real cost of this immense burden of PC for the treatment and management of skin cancers within the DoD beneficiary population.
Limitations—This study is limited by its observational nature. Given the mechanism of our data collection, we may have underestimated disease prevalence, as not all patients are seen for their diagnosis annually. Furthermore, reported demographic strata (eg, age, sex) were limited to those available and valid in the M2 reporting system. Finally, our study only collected data from those service members or former service members seen within the MHS and does not reflect any care rendered to those who are no longer active duty but did not officially retire from the military (ie, nonretired service members receiving care in the Veterans Affairs system for skin cancer).
Conclusion
We describe the annual burden of care for skin cancer in the MHS beneficiary population. Noteworthy findings observed were an overall decrease in beneficiaries being treated for skin cancer through DC; a decreasing annual prevalence of skin cancer diagnosis between 2019 and 2021, which may represent underdiagnosis or decreased follow-up in the setting of the COVID-19 pandemic; and a higher rate of skin cancer in the military beneficiary population compared to the civilian population.
- US Department of Defense. Military health. Accessed October 5, 2023. https://www.defense.gov/
- Wooten NR, Brittingham JA, Pitner RO, et al. Purchased behavioral health care received by Military Health System beneficiaries in civilian medical facilities, 2000-2014. Mil Med. 2018;183:E278-E290. doi:10.1093/milmed/usx101
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192. doi:10.1016/j.jaad.2017.11.062
- American Academy of Dermatology. Skin cancer. Updated April 22, 2022. Accessed April 17, 2024. https://www.aad.org/media/stats-skin-cancer
- Eide MJ, Krajenta R, Johnson D, et al. Identification of patients with nonmelanoma skin cancer using health maintenance organization claims data. Am J Epidemiol. 2010;171:123-128. doi:10.1093/aje/kwp352
- Kao SYZ, Ekwueme DU, Holman DM, et al. Economic burden of skin cancer treatment in the USA: an analysis of the Medical Expenditure Panel Survey Data, 2012-2018. Cancer Causes Control. 2023;34:205-212. doi:10.1007/s10552-022-01644-0
- Aggarwal P, Knabel P, Fleischer AB. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J Am Acad Dermatol. 2021;85:388-395. doi:10.1016/j.jaad.2021.03.109
- SEER*Explorer. SEER Incidence Data, November 2023 Submission (1975-2021). National Cancer Institute; 2024. Accessed April 17, 2024. https://seer.cancer.gov/statistics-network/explorer/application.html?site=53&data_type=1&graph_type=1&compareBy=sex&chk_sex_1=1&chk_sex_3=3&chk_sex_2=2&rate_type=2&race=1&age_range=1&advopt_precision=1&advopt_show_ci=on&hdn_view=1&advopt_show_apc=on&advopt_display=1
- Brown J, Kopf AW, Rigel DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663. doi:10.1111/j.1365-4362.1984.tb01228.x
- Page WF, Whiteman D, Murphy M. A comparison of melanoma mortality among WWII veterans of the Pacific and European theaters. Ann Epidemiol. 2000;10:192-195. doi:10.1016/s1047-2797(99)00050-2
- Ramani ML, Bennett RG. High prevalence of skin cancer in World War II servicemen stationed in the Pacific theater. J Am Acad Dermatol. 1993;28:733-737. doi:10.1016/0190-9622(93)70102-Y
- Trepanowski N, Chang MS, Zhou G, et al. Delays in melanoma presentation during the COVID-19 pandemic: a nationwide multi-institutional cohort study. J Am Acad Dermatol. 2022;87:1217-1219. doi:10.1016/j.jaad.2022.06.031
- Gibbs A. COVID-19 shutdowns caused delays in melanoma diagnoses, study finds. OHSU News. August 4, 2022. Accessed April 17, 2024. https://news.ohsu.edu/2022/08/04/covid-19-shutdowns-caused-delays-in-melanoma-diagnoses-study-finds
- Kime P. Pentagon budget calls for ‘civilianizing’ military hospitals. Military Times. Published February 10, 2020. Accessed April 17, 2024. https://www.militarytimes.com/news/your-military/2020/02/10/pentagon-budget-calls-for-civilianizing-military-hospitals/
- O’Reilly EB, Norris E, Ortiz-Pomales YT, et al. A comparison of direct care at military medical treatment facilities with purchased care in plastic surgery operative volume. Plast Reconstr Surg Glob Open. 2022;10(10 suppl):124-125. doi:10.1097/01.GOX.0000898976.03344.62
- Haag A, Hosein S, Lyon S, et al. Outcomes for arthroplasties in military health: a retrospective analysis of direct versus purchased care. Mil Med. 2023;188(suppl 6):45-51. doi:10.1093/milmed/usac441
- Eaglehouse YL, Georg MW, Richard P, et al. Cost-efficiency of breast cancer care in the US Military Health System: an economic evaluation in direct and purchased care. Mil Med. 2019;184:e494-e501. doi:10.1093/milmed/usz025
- Lurie PM. Comparing the cost of military treatment facilities with private sector care. Institute for Defense Analyses; February 2016. Accessed April 17, 2024. https://www.ida.org/research-and-publications/publications/all/c/co/comparing-the-costs-of-military-treatment-facilities-with-private-sector-care
- Defense Health Program. Fiscal Year (FY) 2024 President’s Budget: Operation and Maintenance Procurement Research, Development, Test and Evaluation. Department of Defense; March 2023. Accessed April 17, 2024. https://comptroller.defense.gov/Portals/45/Documents/defbudget/fy2024/budget_justification/pdfs/09_Defense_Health_Program/00-DHP_Vols_I_II_and_III_PB24.pdf
- US Government Accountability Office. Defense Health Care. DOD should reevaluate market structure for military medical treatment facility management. Published August 21, 2023. Accessed April 17, 2024. https://www.gao.gov/products/gao-23-105441
- Rosenberg A, Cho S. We can do better at protecting our service members from skin cancer. Mil Med. 2022;187:311-313. doi:10.1093/milmed/usac198
- US Department of Defense. Military health. Accessed October 5, 2023. https://www.defense.gov/
- Wooten NR, Brittingham JA, Pitner RO, et al. Purchased behavioral health care received by Military Health System beneficiaries in civilian medical facilities, 2000-2014. Mil Med. 2018;183:E278-E290. doi:10.1093/milmed/usx101
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192. doi:10.1016/j.jaad.2017.11.062
- American Academy of Dermatology. Skin cancer. Updated April 22, 2022. Accessed April 17, 2024. https://www.aad.org/media/stats-skin-cancer
- Eide MJ, Krajenta R, Johnson D, et al. Identification of patients with nonmelanoma skin cancer using health maintenance organization claims data. Am J Epidemiol. 2010;171:123-128. doi:10.1093/aje/kwp352
- Kao SYZ, Ekwueme DU, Holman DM, et al. Economic burden of skin cancer treatment in the USA: an analysis of the Medical Expenditure Panel Survey Data, 2012-2018. Cancer Causes Control. 2023;34:205-212. doi:10.1007/s10552-022-01644-0
- Aggarwal P, Knabel P, Fleischer AB. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J Am Acad Dermatol. 2021;85:388-395. doi:10.1016/j.jaad.2021.03.109
- SEER*Explorer. SEER Incidence Data, November 2023 Submission (1975-2021). National Cancer Institute; 2024. Accessed April 17, 2024. https://seer.cancer.gov/statistics-network/explorer/application.html?site=53&data_type=1&graph_type=1&compareBy=sex&chk_sex_1=1&chk_sex_3=3&chk_sex_2=2&rate_type=2&race=1&age_range=1&advopt_precision=1&advopt_show_ci=on&hdn_view=1&advopt_show_apc=on&advopt_display=1
- Brown J, Kopf AW, Rigel DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663. doi:10.1111/j.1365-4362.1984.tb01228.x
- Page WF, Whiteman D, Murphy M. A comparison of melanoma mortality among WWII veterans of the Pacific and European theaters. Ann Epidemiol. 2000;10:192-195. doi:10.1016/s1047-2797(99)00050-2
- Ramani ML, Bennett RG. High prevalence of skin cancer in World War II servicemen stationed in the Pacific theater. J Am Acad Dermatol. 1993;28:733-737. doi:10.1016/0190-9622(93)70102-Y
- Trepanowski N, Chang MS, Zhou G, et al. Delays in melanoma presentation during the COVID-19 pandemic: a nationwide multi-institutional cohort study. J Am Acad Dermatol. 2022;87:1217-1219. doi:10.1016/j.jaad.2022.06.031
- Gibbs A. COVID-19 shutdowns caused delays in melanoma diagnoses, study finds. OHSU News. August 4, 2022. Accessed April 17, 2024. https://news.ohsu.edu/2022/08/04/covid-19-shutdowns-caused-delays-in-melanoma-diagnoses-study-finds
- Kime P. Pentagon budget calls for ‘civilianizing’ military hospitals. Military Times. Published February 10, 2020. Accessed April 17, 2024. https://www.militarytimes.com/news/your-military/2020/02/10/pentagon-budget-calls-for-civilianizing-military-hospitals/
- O’Reilly EB, Norris E, Ortiz-Pomales YT, et al. A comparison of direct care at military medical treatment facilities with purchased care in plastic surgery operative volume. Plast Reconstr Surg Glob Open. 2022;10(10 suppl):124-125. doi:10.1097/01.GOX.0000898976.03344.62
- Haag A, Hosein S, Lyon S, et al. Outcomes for arthroplasties in military health: a retrospective analysis of direct versus purchased care. Mil Med. 2023;188(suppl 6):45-51. doi:10.1093/milmed/usac441
- Eaglehouse YL, Georg MW, Richard P, et al. Cost-efficiency of breast cancer care in the US Military Health System: an economic evaluation in direct and purchased care. Mil Med. 2019;184:e494-e501. doi:10.1093/milmed/usz025
- Lurie PM. Comparing the cost of military treatment facilities with private sector care. Institute for Defense Analyses; February 2016. Accessed April 17, 2024. https://www.ida.org/research-and-publications/publications/all/c/co/comparing-the-costs-of-military-treatment-facilities-with-private-sector-care
- Defense Health Program. Fiscal Year (FY) 2024 President’s Budget: Operation and Maintenance Procurement Research, Development, Test and Evaluation. Department of Defense; March 2023. Accessed April 17, 2024. https://comptroller.defense.gov/Portals/45/Documents/defbudget/fy2024/budget_justification/pdfs/09_Defense_Health_Program/00-DHP_Vols_I_II_and_III_PB24.pdf
- US Government Accountability Office. Defense Health Care. DOD should reevaluate market structure for military medical treatment facility management. Published August 21, 2023. Accessed April 17, 2024. https://www.gao.gov/products/gao-23-105441
- Rosenberg A, Cho S. We can do better at protecting our service members from skin cancer. Mil Med. 2022;187:311-313. doi:10.1093/milmed/usac198
PRACTICE POINTS
- Study data showed an overall decreasing prevalence of skin cancer in the Military Health System (MHS) from 2019 to 2021, possibly attributable to underdiagnosis resulting from the COVID-19 pandemic. Providers should be mindful of this trend when screening patients who have experienced interruptions in care.
- An overall increased prevalence of skin cancer was noted in the military beneficiary population compared with publicly available civilian data—and thus this diagnosis should be given special consideration within this population.