User login
Stratum Corneum Absorption Kinetics of 2 Potent Topical Corticosteroid Formulations: A Pilot Study
The active ingredient of any pharmaceutical product is responsible for the agent’s efficacy and safety profile. This ingredient is extensively studied in clinical trials and evaluated by the US Food and Drug Administration before the product is commercially available. In dermatologic products, especially those for treating dermatoses, the vehicle in which the active ingredient is formulated also plays a role in drug delivery and indirectly impacts therapeutic outcomes, unlike excipients in oral medications. Topical vehicles must be stable, provide a suitable environment that will not degrade the active ingredient or affect its efficacy, and be cosmetically acceptable.1
Topical vehicles are formulated to maintain the stability of the active ingredient and allow it to readily penetrate the skin and reach its target area with minimal absorption into the bloodstream, thus avoiding systemic adverse events. A variety of vehicles can exist for a single active ingredient to accommodate different phases of disease and different anatomical sites where the disease may occur.2 For example, alcohol-based vehicles, sprays, and foams are preferred for the scalp where evaporation of the vehicle is beneficial to prevent greasiness of the hair, while ointments may be preferred due to their occlusive nature for areas with xerotic or thick skin from dermatoses.
Cosmetic acceptability of the vehicle may influence patient adherence to therapy. Housman et al3 assessed a variety of products formulated in different vehicles (ie, solutions, foams, emollients, gels, creams, ointments) for the treatment of psoriasis. Patients with psoriasis applied each test product to a quarter-sized area of normal skin on the forearm using a cotton swab and completed a preference questionnaire. By far, respondents significantly preferred solutions and foams over creams, gels, and ointments (P<.01). Side effects were rated to be the most important characteristics of topical therapy, followed by time needed for application, ease of application, and messiness.3 Presumably, if patients are frustrated with the topical product that they are using, adherence to the prescribed dosage and application instructions will diminish over time, leading to suboptimal steady-state levels of the product. If appropriate levels of the drug are not present at the target site, treatment will not be successful.
Steady-state levels of a topical drug at the site of action also are maintained via appropriate application frequency, most commonly once to 4 times daily for dermatologic products. Fluocinonide and halcinonide are class II (potent) corticosteroids indicated for the relief of inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses and usually are administered at least twice daily. In double-blind clinical studies comparing both products in the treatment of psoriasis, halcinonide resulted in more improved outcomes than fluocinonide.4-6 Sudilovsky and Clewe4 studied 140 patients with moderate to severe psoriasis. After 3 weeks of treatment, 44% showed superior results with halcinonide, 27% showed superior results with fluocinonide, 26% showed equal results with both products, and 3% showed no relief.4 Similarly, Close5 reported that 61% of patients showed superior results with halcinonide, 25% showed superior results with fluocinonide, 10% showed equal results with both products, and 4% showed no relief (N=50). Lynfield and Watsky6 reported that 56% of patients with severe psoriasis who were treated with halcinonide for 2 weeks showed improvement to normal or slight inflammation compared to 44% of patients treated with fluocinonide (N=59). All 3 studies used cream formulations of halcinonide and fluocinonide.
Recently, halcinonide cream was shown to have an immediate release into the stratum corneum that peaked within 1 hour of application and remained elevated for 6 hours before beginning to decline.7 These results support a biphasic release of halcinonide, which is in agreement with its formulation—that halcinonide exists in both a solution phase for immediate release into the skin and in a suspension phase that allows a sustained release after equilibrium is reached between the solution and suspension phases.8 Fluocinonide is not known to be formulated in a similar way. Its vehicle composition and penetration into the skin could explain the superior efficacy of halcinonide versus fluocinonide.
The current pilot study was conducted to compare the release pattern of fluocinonide cream versus halcinonide cream into the stratum corneum using an in vivo, noninvasive method. Results for halcin-onide have been previously published.7
Methods
Participants were sequestered in a controlled environment for the entire day to allow the skin to equilibrate prior to product application. The methodology for the application and quantification of halcinonide cream 0.1% into the stratum corneum of 5 participants using a tape-stripping protocol has been described elsewhere.7 Concordia Clinical Research institutional review board (Cedar Knolls, New Jersey) approved this study, which was conducted at Dermatology Consulting Services (High Point, North Carolina).
A 0.1-g dose of generic fluocinonide cream 0.05% was applied to four 2.5-cm circular sites on the forearm in 5 participants with normal skin until completely absorbed. Circular tape strips were subsequently placed on the application site at 1, 3, 6, and 9 hours posttreatment and were held for 10 seconds with a controlled pressure plunger to ensure adequate and consistent contact between the tape strip and the skin. The tape strip was removed with forceps, rolled with the skin scale inside, and placed in a glass vial. This procedure was repeated 6 times at 1 of 4 sites with a new tape strip at each time point to obtain samples from deeper skin layers. A total of 24 tape strips were collected from each participant.
All vials were frozen at -20°C and were shipped overnight to Robert Kellar, PhD, at the Center for Bioengineering Innovation at Northern Arizona University (Flagstaff, Arizona) for mass spectroscopy evaluation. Once received at the outside facility, the vials were stored at -20°C until analysis. Each sample was spiked with a known quantity of an appropriate reference standard and extracted with 1 mL acetonitrile at room temperature for 1 minute with agitation. New unused tape strips were spiked with a small amount of fluocinonide reference standard for extraction efficiency.
Extracts were evaporated to dryness under nitrogen gas, resuspended in 200 µL chromatography solvent, and quantified using liquid chromatography–mass spectrometry. To remove the skin scale from the tape strips, 10 mL of a solvent solution of 0.1 mg/mL fludrocortisone acetate in acetonitrile was dispensed into a 4 dram vial containing the tape strip. The vials were ultrasonicated and shaken for 10 to 15 minutes, and the samples were further diluted to 100-fold and were inverted several times to ensure complete dissolution of fluocinonide before liquid chromatography–mass spectrometry.
A standard curve ranging from the lower limit of quantification to the upper limit of quantification for the fluocinonide reference was used to determine the quantity of fluocinonide in each of the tape strips. Once the lower limit of quantification was reached in a given set of tape strip samples (1-, 3-, 6-, and 9-hour samples), the next 2 sequential tape strips in that set were analyzed to confirm fluocinonide was not detectable in deeper layers. Standard quality controls were analyzed to ensure run-to-run and sample-to-sample accuracy.
Each sample was analyzed in duplicate; 10 mg fluocinonide was used as a reference standard. The minimum detectable concentration of fluocinonide was 1 ng/mL.
Results
As expected, tape strip 1 from each participant contained the highest concentration of fluocinonide. This strip corresponded to the most superficial layer of skin. Concentrations decreased in deeper skin layers, as detected in strips 2 to 6.
In general, the average concentration of fluocin-onide in strip 1 for all 5 participants was highest at hour 1, with a subsequent decline at hours 3, 6, and 9; however, participant 1 showed a second peak in fluocinonide concentration at hour 6 (Figure 1). When the fluocinonide concentration in strips 1 to 6 was averaged for each participant at each time point, similar results were obtained: a general decline after hour 1, but a second prominent peak at hour 6 in participant 1 only. In participant 1, the average fluocinonide concentration for strips 1 to 6 was 393 ng/mL at hour 1 and declined to 208 ng/mL at hour 3; it increased to 451 ng/mL at hour 6 before declining again to 202 ng/mL at hour 9.
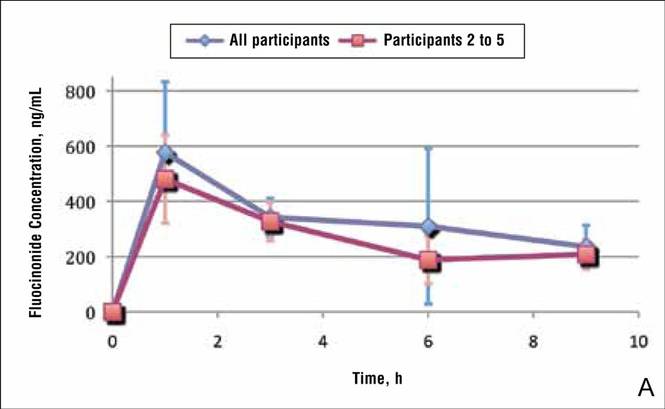
Because participant 1 was the only one to exhibit a second peak of fluocinonide concentration, it appears that measurements obtained from this participant may be outliers. When removing partici-pant 1 from the analysis of fluocinonide concentration in strip 1 at each time point, a clear decline is evident from hour 1 to hour 9 (Figure 2A, red line [partici-pants 2–5] vs blue line [participants 1–5]).
When the average concentration of fluocinonide was calculated in strips 1 to 6 from all participants, there was a general steady decline after hour 1 with a slight increase of 25 ng/mL at hour 6 (Figure 2B, blue line). This increase is due to the measurements obtained from participant 1; however, if partici-pant 1 is removed from the analysis, a constant decline is observed from hour 1 to hour 9 (Figure 2B, red line).
|
A prior study evaluated the penetration and absorption of halcinonide in the stratum corneum.7 In summary, halcinonide concentration peaked at hour 1 following application and remained elevated to hour 6, before beginning a slow decline. The average concentration of halcinonide from all participants in strips 1 to 6 reached 1350 ng/mL at hour 1, remained within 93% to 97% of this level (1253–1303 ng/mL) for the next 5 hours, and declined only 29% from the peak at hour 1 to hour 9 (958 ng/mL)(Figure 3, blue line).7 In contrast, the fluocinonide concentration in participants 2 to 5 from the current study reached 190 ng/mL at hour 1 and steadily declined 53% to 89 ng/mL by hour 9 (Figure 3, red line).
Two participants from the prior halcinonide study also were enrolled in the current fluocinonide study (referred to as participant A and B). In general, halcinonide levels in both participants remained elevated for 6 hours after application and declined 27.5% and 35.5%, respectively, by hour 9 (Figure 4). Participant A experienced a 20.5% dip in halcinonide concentration at hour 3 followed by an increase at hour 6; however, the halcinonide concentration at hour 9 was similar to hour 3.7 In contrast, fluocin-onide concentrations for these participants peaked at 1 hour and clearly declined approximately 60% over the next 8 hours.
Comment
The release of both fluocinonide and halcinonide into the skin was evaluated using dermal tape stripping on 4 sites on the forearms of healthy individuals. Cream formulations of each corticosteroid were evaluated in 5 participants, with 2 participants receiving both formulations during different study periods. In the prior study with halcinonide, the stratum corneum exhibited the highest concentration of the corticosteroid, with substantial declines beyond strip 6 (ie, strips 7–20).7 For this reason, only strips 1 to 6 were evaluated for corticosteroid penetration and absorption.
Results from strip 1 indicated immediate absorption of corticosteroid (fluocinonide and halcinonide) into the skin. Unlike the release of halcinonide, which demonstrated a clear sustained release over 6 hours before decreasing,7 fluocinonide concentrations began declining immediately after peaking at hour 1 and continued to decline up to hour 9. Only participant 1 exhibited a second peak of fluocinonide concentration at hour 6; the rest of the participants did not. This second peak is most likely an anomaly due to the small number of participants rather than a true elevation.
Given the rapid decline of fluocinonide concentration over the 9 hours compared with the more gradual decline of halcinonide concentration, there appears to be no evidence of a biphasic sustained release of fluocinonide from its vehicle. This difference in release pattern from each corticosteroid’s respective vehicle may explain in part the different clinical outcomes in comparative studies.4-6
It is known that vehicle composition affects corticosteroid diffusion from the vehicle to the skin surface and subsequent penetration into the skin.9 Either process can determine the overall effectiveness of the product. Ayres and Hooper10 evaluated the penetration of 4 topical preparations of cortisol. Product 1 delivered 16 times more cortisol to the skin than product 2, 8 times more than product 3, and 3 times more than product 4. Because all the preparations contained cortisol-free alcohol, these differences were attributed to the vehicle in which the cortisol was formulated. Products 1 and 4 both contained 10% urea, but the urea in product 1 was a powder in a cream base and the urea in product 4 was in a stabilizing emulsified base. Product 2 contained a propylene glycol/water base and product 3 was a water-miscible cream.10
Generic corticosteroid products have been observed in clinical practice and have been shown in vasoconstriction assays to be less and more potent than their brand-name equivalents.2,11 Vasoconstriction assays are the standard for assessing the potency of topical corticosteroids and predicting their clinical efficacy.2 One study reported significant differences in therapeutic effectiveness between generic formulations and their brand-name equivalents.12 Kenalog cream 0.1% (multiple manufacturers) was significantly more potent than any of the generic triamcinolone creams tested (P<.05); in fact, Kenalog cream 0.025% (multiple manufacturers) was statistically superior to all the generic triamcinolone creams 0.1%. Moreover, Artistocort A ointment 0.1% (Lederele Laboratories) and Valisone cream 0.1% (Schering Corporation) also were more potent than their generics at the same concentration in the same vehicle type.12 A second study also observed that 2 of 6 generic formulations had significantly less vasoconstriction than their respective brand-name formulations.11 A brand-name betamethasone valerate cream produced significantly greater vasoconstriction than its generic equivalent, and a brand-name betamethasone dipropionate cream produced greater vasoconstriction than one generic and equal vasoconstriction to another generic. Additionally, the vasoconstriction measured with Diprosone was greater than that measured with Diprolene, another brand-name product of betamethasone dipropionate.11 Diprosone and Diprolene differ in their vehicle content. The latter, a class I corticosteroid, contains a modified vehicle high in propylene glycol, whereas the former contains less propylene glycol and thus is classified as a class III corticosteroid. Propylene glycol allows hydrophobic molecules such as corticosteroids to dissolve more fully in the vehicle.12
Ostrenga et al1 studied the solubility of corticosteroids in different vehicles and, as expected, corticosteroids that fully solubilized in the vehicle exhibited better penetration into the skin on assessment with vasoconstriction assays. Corticosteroids in a suspension, on the other hand, showed slower penetration into the skin.1,13 A balance between the solution and suspension phase would allow a drug to rapidly penetrate the skin upon application, and when this pool of solubilized drug was depleted, additional drug could penetrate into the skin from the suspension phase. Based on the tape strip results from the current study it appears that halcinonide, which is manufactured in a biphasic formulation, follows this pattern of penetration and absorption into the stratum corneum. In contrast, fluocinonide appears to exist in a soluble state without much, if any, amount in a suspension phase because it had no sustained release during the 9 hours after application.
Common belief among dermatologists is that long-term use of corticosteroids leads to tachyphylaxis,14 which can be attributed to poor patient adherence. If patients skip doses, then the steady state of the product at the target site is not maintained. It is interesting to speculate that using agents with more sustained release beyond the time of application (such as halcinonide) may preserve steady-state levels even when patients are neglectful of the next medication application. Corticosteroids that work in 2 phases such as halcinonide may minimize tachyphylaxis experienced with prolonged use of corticosteroids.
Fluocinonide and halcinonide are both class II high-potency corticosteroids as shown on outcomes from vasoconstrictor assays, which assess the extent to which a corticosteroid causes cutaneous vasoconstriction or blanching in normal healthy individuals.15 The assay depends on the molecule diffusing from the vehicle, penetrating the skin, and causing a reaction (blanching) that is then evaluated. The assay cannot effectively evaluate the rate of continued diffusion and skin penetration beyond the appearance of blanching. In contrast, the tape-stripping method provides an inside look at the extent of penetration of the corticosteroid beyond the skin surface and the rate of its clearance from different skin layers. In the current study, the levels of fluocinonide declined after peaking at 1 hour after application, but the levels of halcinonide clearly remained elevated after peaking at the same time point. Most likely, vasoconstrictor studies would not be able to differentiate between the concentrations of the 2 products in the stratum corneum beyond the first hour after application.
Tape stripping, or dermatopharmacokinetics, has advantages over vasoconstriction assays in studying corticosteroid penetration and clearance from the stratum corneum. At one point, the US Food and Drug Administration had included tape stripping in its preliminary guidelines for generic topical bioequivalence studies until data from the same formulation generated from 2 different laboratories produced different results.16 Since that time, much work has been done with tape stripping to ensure its consistency. Weigmann et al17 demonstrated equivalent results with clobetasol using vasoconstriction and tape stripping, and Wiedersberg et al18 demonstrated the same with betamethasone. For the current study, the fluocinonide and halcinonide formulations were weighed prior to application so that the same dose was tested in all participants. A plunger was used to produce consistent pressure at all application sites to control for the amount of skin that was stripped off with the tape. Results for both corticosteroids were consistent between the participants. Variability in the data was detected; however, this observation is most likely due to the small number of participants in the studies.
Conclusion
In summary, this pilot study demonstrated that fluocinonide concentration in the stratum corneum peaks within the first hour of application before beginning a steady general decline. There was no evidence of sustained release. In contrast, halcin-onide demonstrated a sustained release for 6 hours after application. Halcinonide is formulated in a cream base in which the corticosteroid is present in a solution and suspension phase that allows for sustained delivery in skin over time. Fluocinonide does not appear to be formulated in the same way, and its concentrations in the stratum corneum begin to decline 1 hour after application.
Acknowledgement
Thank you to Robert Kellar, PhD, at the Center for Bioengineering Innovation at Northern Arizona University, Flagstaff, for conducting the liquid chromatography–mass spectrometry.
1. Ostrenga J, Haleblian J, Poulsen B, et al. Vehicle design for a new topical steroid, fluocinonide. J Invest Dermatol. 1971;56:392-399.
2. Rathi SK, D’Souza P. Rational and ethical use of topical corticosteroids based on safety and efficacy. Indian J Dermatol. 2012;57:251-259.
3. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
4. Sudilovsky A, Clewe TH. Comparative efficacy of halcin-onide and fluocinonide creams in psoriasis and eczematous dermatoses. J Clin Pharmacol. 1975;15:779-784.
5. Close JE. Double-blind comparison of topical halcinonide and fluocinonide in the treatment of psoriasis. Int J Dermatol. 1976;15:534-537.
6. Lynfield Y, Watsky M. Psoriasis: topical corticosteroid therapy. Cutis. 1976;18:133, 136-137.
7. Draelos ZD. Demonstration of the biphasic release of 0.1% halcinonide cream. J Drugs Dermatol. 2015;14:89-90.
8. Bagatell FK. Halcinonide: a new potent topical anti-inflammatory drug. Cutis. 1974;14:459-462.
9. Ostrenga J, Steinmetz C, Poulsen B. Significance of vehicle composition. I. relationship between topical vehicle composition, skin penetrability, and clinical efficacy. J Pharm Sci. 1971;60:1175-1179.
10. Ayres PJ, Hooper G. Assessment of the skin penetration properties of different carrier vehicles for topically applied cortisol. Br J Dermatol. 1978;99:307-317.
11. Olsen EA. Double-blind controlled comparison of generic and trade-name topical steroids using the vasoconstriction assay. Arch Dermatol. 1991;127:197-201.
12. Stoughton RB. Are generic formulations equivalent to trade name topical glucocorticoids? Arch Dermatol. 1987;123:1312-1314.
13. Poulsen BJ, Young E, Coquilla V, et al. Effect of topical vehicle composition on the in vitro release of fluocinolone acetonide and its acetate ester. J Pharm Sci. 1968;57:928-933.
14. Taheri A, Cantrell J, Feldman SR. Tachyphylaxis to topical glucocorticoids: what is the evidence? Dermatol Online J. 2013;19:18954.
15. Ference JD, Last AR. Choosing topical corticosteroids. Am Fam Physician. 2009;79:135-140.
16. Pershing LK, Nelson JL, Corlett JL, et al. Assessment of dermatopharmacokinetic approach in the bioequivalence determination of topical tretinoin gel products. J Am Acad Dermatol. 2003;48:740-751.
17. Weigmann H, Lademann J, v Pelchrzim R, et al. Bioavailability of clobetasol propionate-quantification of drug concentrations in the stratum corneum by dermatopharmacokinetics using tape stripping. Skin Pharmacol Appl Skin Physiol. 1999;12:46-53.
18. Wiedersberg S, Naik A, Leopold CS, et al. Pharmacodynamics and dermatopharmacokinetics of betamethasone 17-valerate: assessment of topical bioavailability. Br J Dermatol. 2009;160:676-686.
The active ingredient of any pharmaceutical product is responsible for the agent’s efficacy and safety profile. This ingredient is extensively studied in clinical trials and evaluated by the US Food and Drug Administration before the product is commercially available. In dermatologic products, especially those for treating dermatoses, the vehicle in which the active ingredient is formulated also plays a role in drug delivery and indirectly impacts therapeutic outcomes, unlike excipients in oral medications. Topical vehicles must be stable, provide a suitable environment that will not degrade the active ingredient or affect its efficacy, and be cosmetically acceptable.1
Topical vehicles are formulated to maintain the stability of the active ingredient and allow it to readily penetrate the skin and reach its target area with minimal absorption into the bloodstream, thus avoiding systemic adverse events. A variety of vehicles can exist for a single active ingredient to accommodate different phases of disease and different anatomical sites where the disease may occur.2 For example, alcohol-based vehicles, sprays, and foams are preferred for the scalp where evaporation of the vehicle is beneficial to prevent greasiness of the hair, while ointments may be preferred due to their occlusive nature for areas with xerotic or thick skin from dermatoses.
Cosmetic acceptability of the vehicle may influence patient adherence to therapy. Housman et al3 assessed a variety of products formulated in different vehicles (ie, solutions, foams, emollients, gels, creams, ointments) for the treatment of psoriasis. Patients with psoriasis applied each test product to a quarter-sized area of normal skin on the forearm using a cotton swab and completed a preference questionnaire. By far, respondents significantly preferred solutions and foams over creams, gels, and ointments (P<.01). Side effects were rated to be the most important characteristics of topical therapy, followed by time needed for application, ease of application, and messiness.3 Presumably, if patients are frustrated with the topical product that they are using, adherence to the prescribed dosage and application instructions will diminish over time, leading to suboptimal steady-state levels of the product. If appropriate levels of the drug are not present at the target site, treatment will not be successful.
Steady-state levels of a topical drug at the site of action also are maintained via appropriate application frequency, most commonly once to 4 times daily for dermatologic products. Fluocinonide and halcinonide are class II (potent) corticosteroids indicated for the relief of inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses and usually are administered at least twice daily. In double-blind clinical studies comparing both products in the treatment of psoriasis, halcinonide resulted in more improved outcomes than fluocinonide.4-6 Sudilovsky and Clewe4 studied 140 patients with moderate to severe psoriasis. After 3 weeks of treatment, 44% showed superior results with halcinonide, 27% showed superior results with fluocinonide, 26% showed equal results with both products, and 3% showed no relief.4 Similarly, Close5 reported that 61% of patients showed superior results with halcinonide, 25% showed superior results with fluocinonide, 10% showed equal results with both products, and 4% showed no relief (N=50). Lynfield and Watsky6 reported that 56% of patients with severe psoriasis who were treated with halcinonide for 2 weeks showed improvement to normal or slight inflammation compared to 44% of patients treated with fluocinonide (N=59). All 3 studies used cream formulations of halcinonide and fluocinonide.
Recently, halcinonide cream was shown to have an immediate release into the stratum corneum that peaked within 1 hour of application and remained elevated for 6 hours before beginning to decline.7 These results support a biphasic release of halcinonide, which is in agreement with its formulation—that halcinonide exists in both a solution phase for immediate release into the skin and in a suspension phase that allows a sustained release after equilibrium is reached between the solution and suspension phases.8 Fluocinonide is not known to be formulated in a similar way. Its vehicle composition and penetration into the skin could explain the superior efficacy of halcinonide versus fluocinonide.
The current pilot study was conducted to compare the release pattern of fluocinonide cream versus halcinonide cream into the stratum corneum using an in vivo, noninvasive method. Results for halcin-onide have been previously published.7
Methods
Participants were sequestered in a controlled environment for the entire day to allow the skin to equilibrate prior to product application. The methodology for the application and quantification of halcinonide cream 0.1% into the stratum corneum of 5 participants using a tape-stripping protocol has been described elsewhere.7 Concordia Clinical Research institutional review board (Cedar Knolls, New Jersey) approved this study, which was conducted at Dermatology Consulting Services (High Point, North Carolina).
A 0.1-g dose of generic fluocinonide cream 0.05% was applied to four 2.5-cm circular sites on the forearm in 5 participants with normal skin until completely absorbed. Circular tape strips were subsequently placed on the application site at 1, 3, 6, and 9 hours posttreatment and were held for 10 seconds with a controlled pressure plunger to ensure adequate and consistent contact between the tape strip and the skin. The tape strip was removed with forceps, rolled with the skin scale inside, and placed in a glass vial. This procedure was repeated 6 times at 1 of 4 sites with a new tape strip at each time point to obtain samples from deeper skin layers. A total of 24 tape strips were collected from each participant.
All vials were frozen at -20°C and were shipped overnight to Robert Kellar, PhD, at the Center for Bioengineering Innovation at Northern Arizona University (Flagstaff, Arizona) for mass spectroscopy evaluation. Once received at the outside facility, the vials were stored at -20°C until analysis. Each sample was spiked with a known quantity of an appropriate reference standard and extracted with 1 mL acetonitrile at room temperature for 1 minute with agitation. New unused tape strips were spiked with a small amount of fluocinonide reference standard for extraction efficiency.
Extracts were evaporated to dryness under nitrogen gas, resuspended in 200 µL chromatography solvent, and quantified using liquid chromatography–mass spectrometry. To remove the skin scale from the tape strips, 10 mL of a solvent solution of 0.1 mg/mL fludrocortisone acetate in acetonitrile was dispensed into a 4 dram vial containing the tape strip. The vials were ultrasonicated and shaken for 10 to 15 minutes, and the samples were further diluted to 100-fold and were inverted several times to ensure complete dissolution of fluocinonide before liquid chromatography–mass spectrometry.
A standard curve ranging from the lower limit of quantification to the upper limit of quantification for the fluocinonide reference was used to determine the quantity of fluocinonide in each of the tape strips. Once the lower limit of quantification was reached in a given set of tape strip samples (1-, 3-, 6-, and 9-hour samples), the next 2 sequential tape strips in that set were analyzed to confirm fluocinonide was not detectable in deeper layers. Standard quality controls were analyzed to ensure run-to-run and sample-to-sample accuracy.
Each sample was analyzed in duplicate; 10 mg fluocinonide was used as a reference standard. The minimum detectable concentration of fluocinonide was 1 ng/mL.
Results
As expected, tape strip 1 from each participant contained the highest concentration of fluocinonide. This strip corresponded to the most superficial layer of skin. Concentrations decreased in deeper skin layers, as detected in strips 2 to 6.
In general, the average concentration of fluocin-onide in strip 1 for all 5 participants was highest at hour 1, with a subsequent decline at hours 3, 6, and 9; however, participant 1 showed a second peak in fluocinonide concentration at hour 6 (Figure 1). When the fluocinonide concentration in strips 1 to 6 was averaged for each participant at each time point, similar results were obtained: a general decline after hour 1, but a second prominent peak at hour 6 in participant 1 only. In participant 1, the average fluocinonide concentration for strips 1 to 6 was 393 ng/mL at hour 1 and declined to 208 ng/mL at hour 3; it increased to 451 ng/mL at hour 6 before declining again to 202 ng/mL at hour 9.
Because participant 1 was the only one to exhibit a second peak of fluocinonide concentration, it appears that measurements obtained from this participant may be outliers. When removing partici-pant 1 from the analysis of fluocinonide concentration in strip 1 at each time point, a clear decline is evident from hour 1 to hour 9 (Figure 2A, red line [partici-pants 2–5] vs blue line [participants 1–5]).
When the average concentration of fluocinonide was calculated in strips 1 to 6 from all participants, there was a general steady decline after hour 1 with a slight increase of 25 ng/mL at hour 6 (Figure 2B, blue line). This increase is due to the measurements obtained from participant 1; however, if partici-pant 1 is removed from the analysis, a constant decline is observed from hour 1 to hour 9 (Figure 2B, red line).
|
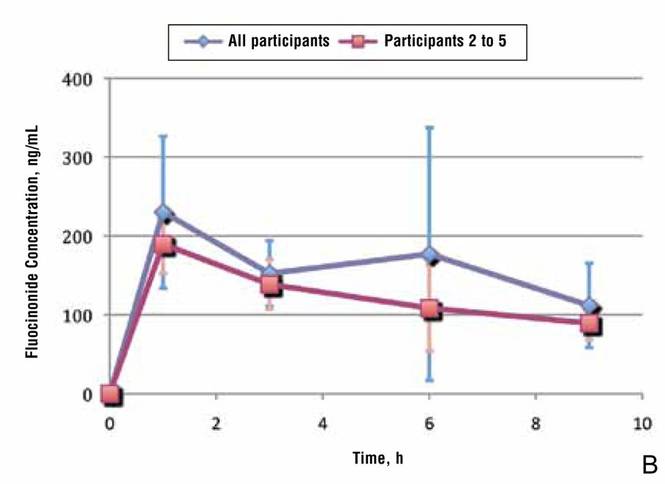
A prior study evaluated the penetration and absorption of halcinonide in the stratum corneum.7 In summary, halcinonide concentration peaked at hour 1 following application and remained elevated to hour 6, before beginning a slow decline. The average concentration of halcinonide from all participants in strips 1 to 6 reached 1350 ng/mL at hour 1, remained within 93% to 97% of this level (1253–1303 ng/mL) for the next 5 hours, and declined only 29% from the peak at hour 1 to hour 9 (958 ng/mL)(Figure 3, blue line).7 In contrast, the fluocinonide concentration in participants 2 to 5 from the current study reached 190 ng/mL at hour 1 and steadily declined 53% to 89 ng/mL by hour 9 (Figure 3, red line).
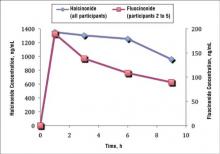
Two participants from the prior halcinonide study also were enrolled in the current fluocinonide study (referred to as participant A and B). In general, halcinonide levels in both participants remained elevated for 6 hours after application and declined 27.5% and 35.5%, respectively, by hour 9 (Figure 4). Participant A experienced a 20.5% dip in halcinonide concentration at hour 3 followed by an increase at hour 6; however, the halcinonide concentration at hour 9 was similar to hour 3.7 In contrast, fluocin-onide concentrations for these participants peaked at 1 hour and clearly declined approximately 60% over the next 8 hours.
Comment
The release of both fluocinonide and halcinonide into the skin was evaluated using dermal tape stripping on 4 sites on the forearms of healthy individuals. Cream formulations of each corticosteroid were evaluated in 5 participants, with 2 participants receiving both formulations during different study periods. In the prior study with halcinonide, the stratum corneum exhibited the highest concentration of the corticosteroid, with substantial declines beyond strip 6 (ie, strips 7–20).7 For this reason, only strips 1 to 6 were evaluated for corticosteroid penetration and absorption.
Results from strip 1 indicated immediate absorption of corticosteroid (fluocinonide and halcinonide) into the skin. Unlike the release of halcinonide, which demonstrated a clear sustained release over 6 hours before decreasing,7 fluocinonide concentrations began declining immediately after peaking at hour 1 and continued to decline up to hour 9. Only participant 1 exhibited a second peak of fluocinonide concentration at hour 6; the rest of the participants did not. This second peak is most likely an anomaly due to the small number of participants rather than a true elevation.
Given the rapid decline of fluocinonide concentration over the 9 hours compared with the more gradual decline of halcinonide concentration, there appears to be no evidence of a biphasic sustained release of fluocinonide from its vehicle. This difference in release pattern from each corticosteroid’s respective vehicle may explain in part the different clinical outcomes in comparative studies.4-6
It is known that vehicle composition affects corticosteroid diffusion from the vehicle to the skin surface and subsequent penetration into the skin.9 Either process can determine the overall effectiveness of the product. Ayres and Hooper10 evaluated the penetration of 4 topical preparations of cortisol. Product 1 delivered 16 times more cortisol to the skin than product 2, 8 times more than product 3, and 3 times more than product 4. Because all the preparations contained cortisol-free alcohol, these differences were attributed to the vehicle in which the cortisol was formulated. Products 1 and 4 both contained 10% urea, but the urea in product 1 was a powder in a cream base and the urea in product 4 was in a stabilizing emulsified base. Product 2 contained a propylene glycol/water base and product 3 was a water-miscible cream.10
Generic corticosteroid products have been observed in clinical practice and have been shown in vasoconstriction assays to be less and more potent than their brand-name equivalents.2,11 Vasoconstriction assays are the standard for assessing the potency of topical corticosteroids and predicting their clinical efficacy.2 One study reported significant differences in therapeutic effectiveness between generic formulations and their brand-name equivalents.12 Kenalog cream 0.1% (multiple manufacturers) was significantly more potent than any of the generic triamcinolone creams tested (P<.05); in fact, Kenalog cream 0.025% (multiple manufacturers) was statistically superior to all the generic triamcinolone creams 0.1%. Moreover, Artistocort A ointment 0.1% (Lederele Laboratories) and Valisone cream 0.1% (Schering Corporation) also were more potent than their generics at the same concentration in the same vehicle type.12 A second study also observed that 2 of 6 generic formulations had significantly less vasoconstriction than their respective brand-name formulations.11 A brand-name betamethasone valerate cream produced significantly greater vasoconstriction than its generic equivalent, and a brand-name betamethasone dipropionate cream produced greater vasoconstriction than one generic and equal vasoconstriction to another generic. Additionally, the vasoconstriction measured with Diprosone was greater than that measured with Diprolene, another brand-name product of betamethasone dipropionate.11 Diprosone and Diprolene differ in their vehicle content. The latter, a class I corticosteroid, contains a modified vehicle high in propylene glycol, whereas the former contains less propylene glycol and thus is classified as a class III corticosteroid. Propylene glycol allows hydrophobic molecules such as corticosteroids to dissolve more fully in the vehicle.12
Ostrenga et al1 studied the solubility of corticosteroids in different vehicles and, as expected, corticosteroids that fully solubilized in the vehicle exhibited better penetration into the skin on assessment with vasoconstriction assays. Corticosteroids in a suspension, on the other hand, showed slower penetration into the skin.1,13 A balance between the solution and suspension phase would allow a drug to rapidly penetrate the skin upon application, and when this pool of solubilized drug was depleted, additional drug could penetrate into the skin from the suspension phase. Based on the tape strip results from the current study it appears that halcinonide, which is manufactured in a biphasic formulation, follows this pattern of penetration and absorption into the stratum corneum. In contrast, fluocinonide appears to exist in a soluble state without much, if any, amount in a suspension phase because it had no sustained release during the 9 hours after application.
Common belief among dermatologists is that long-term use of corticosteroids leads to tachyphylaxis,14 which can be attributed to poor patient adherence. If patients skip doses, then the steady state of the product at the target site is not maintained. It is interesting to speculate that using agents with more sustained release beyond the time of application (such as halcinonide) may preserve steady-state levels even when patients are neglectful of the next medication application. Corticosteroids that work in 2 phases such as halcinonide may minimize tachyphylaxis experienced with prolonged use of corticosteroids.
Fluocinonide and halcinonide are both class II high-potency corticosteroids as shown on outcomes from vasoconstrictor assays, which assess the extent to which a corticosteroid causes cutaneous vasoconstriction or blanching in normal healthy individuals.15 The assay depends on the molecule diffusing from the vehicle, penetrating the skin, and causing a reaction (blanching) that is then evaluated. The assay cannot effectively evaluate the rate of continued diffusion and skin penetration beyond the appearance of blanching. In contrast, the tape-stripping method provides an inside look at the extent of penetration of the corticosteroid beyond the skin surface and the rate of its clearance from different skin layers. In the current study, the levels of fluocinonide declined after peaking at 1 hour after application, but the levels of halcinonide clearly remained elevated after peaking at the same time point. Most likely, vasoconstrictor studies would not be able to differentiate between the concentrations of the 2 products in the stratum corneum beyond the first hour after application.
Tape stripping, or dermatopharmacokinetics, has advantages over vasoconstriction assays in studying corticosteroid penetration and clearance from the stratum corneum. At one point, the US Food and Drug Administration had included tape stripping in its preliminary guidelines for generic topical bioequivalence studies until data from the same formulation generated from 2 different laboratories produced different results.16 Since that time, much work has been done with tape stripping to ensure its consistency. Weigmann et al17 demonstrated equivalent results with clobetasol using vasoconstriction and tape stripping, and Wiedersberg et al18 demonstrated the same with betamethasone. For the current study, the fluocinonide and halcinonide formulations were weighed prior to application so that the same dose was tested in all participants. A plunger was used to produce consistent pressure at all application sites to control for the amount of skin that was stripped off with the tape. Results for both corticosteroids were consistent between the participants. Variability in the data was detected; however, this observation is most likely due to the small number of participants in the studies.
Conclusion
In summary, this pilot study demonstrated that fluocinonide concentration in the stratum corneum peaks within the first hour of application before beginning a steady general decline. There was no evidence of sustained release. In contrast, halcin-onide demonstrated a sustained release for 6 hours after application. Halcinonide is formulated in a cream base in which the corticosteroid is present in a solution and suspension phase that allows for sustained delivery in skin over time. Fluocinonide does not appear to be formulated in the same way, and its concentrations in the stratum corneum begin to decline 1 hour after application.
Acknowledgement
Thank you to Robert Kellar, PhD, at the Center for Bioengineering Innovation at Northern Arizona University, Flagstaff, for conducting the liquid chromatography–mass spectrometry.
The active ingredient of any pharmaceutical product is responsible for the agent’s efficacy and safety profile. This ingredient is extensively studied in clinical trials and evaluated by the US Food and Drug Administration before the product is commercially available. In dermatologic products, especially those for treating dermatoses, the vehicle in which the active ingredient is formulated also plays a role in drug delivery and indirectly impacts therapeutic outcomes, unlike excipients in oral medications. Topical vehicles must be stable, provide a suitable environment that will not degrade the active ingredient or affect its efficacy, and be cosmetically acceptable.1
Topical vehicles are formulated to maintain the stability of the active ingredient and allow it to readily penetrate the skin and reach its target area with minimal absorption into the bloodstream, thus avoiding systemic adverse events. A variety of vehicles can exist for a single active ingredient to accommodate different phases of disease and different anatomical sites where the disease may occur.2 For example, alcohol-based vehicles, sprays, and foams are preferred for the scalp where evaporation of the vehicle is beneficial to prevent greasiness of the hair, while ointments may be preferred due to their occlusive nature for areas with xerotic or thick skin from dermatoses.
Cosmetic acceptability of the vehicle may influence patient adherence to therapy. Housman et al3 assessed a variety of products formulated in different vehicles (ie, solutions, foams, emollients, gels, creams, ointments) for the treatment of psoriasis. Patients with psoriasis applied each test product to a quarter-sized area of normal skin on the forearm using a cotton swab and completed a preference questionnaire. By far, respondents significantly preferred solutions and foams over creams, gels, and ointments (P<.01). Side effects were rated to be the most important characteristics of topical therapy, followed by time needed for application, ease of application, and messiness.3 Presumably, if patients are frustrated with the topical product that they are using, adherence to the prescribed dosage and application instructions will diminish over time, leading to suboptimal steady-state levels of the product. If appropriate levels of the drug are not present at the target site, treatment will not be successful.
Steady-state levels of a topical drug at the site of action also are maintained via appropriate application frequency, most commonly once to 4 times daily for dermatologic products. Fluocinonide and halcinonide are class II (potent) corticosteroids indicated for the relief of inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses and usually are administered at least twice daily. In double-blind clinical studies comparing both products in the treatment of psoriasis, halcinonide resulted in more improved outcomes than fluocinonide.4-6 Sudilovsky and Clewe4 studied 140 patients with moderate to severe psoriasis. After 3 weeks of treatment, 44% showed superior results with halcinonide, 27% showed superior results with fluocinonide, 26% showed equal results with both products, and 3% showed no relief.4 Similarly, Close5 reported that 61% of patients showed superior results with halcinonide, 25% showed superior results with fluocinonide, 10% showed equal results with both products, and 4% showed no relief (N=50). Lynfield and Watsky6 reported that 56% of patients with severe psoriasis who were treated with halcinonide for 2 weeks showed improvement to normal or slight inflammation compared to 44% of patients treated with fluocinonide (N=59). All 3 studies used cream formulations of halcinonide and fluocinonide.
Recently, halcinonide cream was shown to have an immediate release into the stratum corneum that peaked within 1 hour of application and remained elevated for 6 hours before beginning to decline.7 These results support a biphasic release of halcinonide, which is in agreement with its formulation—that halcinonide exists in both a solution phase for immediate release into the skin and in a suspension phase that allows a sustained release after equilibrium is reached between the solution and suspension phases.8 Fluocinonide is not known to be formulated in a similar way. Its vehicle composition and penetration into the skin could explain the superior efficacy of halcinonide versus fluocinonide.
The current pilot study was conducted to compare the release pattern of fluocinonide cream versus halcinonide cream into the stratum corneum using an in vivo, noninvasive method. Results for halcin-onide have been previously published.7
Methods
Participants were sequestered in a controlled environment for the entire day to allow the skin to equilibrate prior to product application. The methodology for the application and quantification of halcinonide cream 0.1% into the stratum corneum of 5 participants using a tape-stripping protocol has been described elsewhere.7 Concordia Clinical Research institutional review board (Cedar Knolls, New Jersey) approved this study, which was conducted at Dermatology Consulting Services (High Point, North Carolina).
A 0.1-g dose of generic fluocinonide cream 0.05% was applied to four 2.5-cm circular sites on the forearm in 5 participants with normal skin until completely absorbed. Circular tape strips were subsequently placed on the application site at 1, 3, 6, and 9 hours posttreatment and were held for 10 seconds with a controlled pressure plunger to ensure adequate and consistent contact between the tape strip and the skin. The tape strip was removed with forceps, rolled with the skin scale inside, and placed in a glass vial. This procedure was repeated 6 times at 1 of 4 sites with a new tape strip at each time point to obtain samples from deeper skin layers. A total of 24 tape strips were collected from each participant.
All vials were frozen at -20°C and were shipped overnight to Robert Kellar, PhD, at the Center for Bioengineering Innovation at Northern Arizona University (Flagstaff, Arizona) for mass spectroscopy evaluation. Once received at the outside facility, the vials were stored at -20°C until analysis. Each sample was spiked with a known quantity of an appropriate reference standard and extracted with 1 mL acetonitrile at room temperature for 1 minute with agitation. New unused tape strips were spiked with a small amount of fluocinonide reference standard for extraction efficiency.
Extracts were evaporated to dryness under nitrogen gas, resuspended in 200 µL chromatography solvent, and quantified using liquid chromatography–mass spectrometry. To remove the skin scale from the tape strips, 10 mL of a solvent solution of 0.1 mg/mL fludrocortisone acetate in acetonitrile was dispensed into a 4 dram vial containing the tape strip. The vials were ultrasonicated and shaken for 10 to 15 minutes, and the samples were further diluted to 100-fold and were inverted several times to ensure complete dissolution of fluocinonide before liquid chromatography–mass spectrometry.
A standard curve ranging from the lower limit of quantification to the upper limit of quantification for the fluocinonide reference was used to determine the quantity of fluocinonide in each of the tape strips. Once the lower limit of quantification was reached in a given set of tape strip samples (1-, 3-, 6-, and 9-hour samples), the next 2 sequential tape strips in that set were analyzed to confirm fluocinonide was not detectable in deeper layers. Standard quality controls were analyzed to ensure run-to-run and sample-to-sample accuracy.
Each sample was analyzed in duplicate; 10 mg fluocinonide was used as a reference standard. The minimum detectable concentration of fluocinonide was 1 ng/mL.
Results
As expected, tape strip 1 from each participant contained the highest concentration of fluocinonide. This strip corresponded to the most superficial layer of skin. Concentrations decreased in deeper skin layers, as detected in strips 2 to 6.
In general, the average concentration of fluocin-onide in strip 1 for all 5 participants was highest at hour 1, with a subsequent decline at hours 3, 6, and 9; however, participant 1 showed a second peak in fluocinonide concentration at hour 6 (Figure 1). When the fluocinonide concentration in strips 1 to 6 was averaged for each participant at each time point, similar results were obtained: a general decline after hour 1, but a second prominent peak at hour 6 in participant 1 only. In participant 1, the average fluocinonide concentration for strips 1 to 6 was 393 ng/mL at hour 1 and declined to 208 ng/mL at hour 3; it increased to 451 ng/mL at hour 6 before declining again to 202 ng/mL at hour 9.
Because participant 1 was the only one to exhibit a second peak of fluocinonide concentration, it appears that measurements obtained from this participant may be outliers. When removing partici-pant 1 from the analysis of fluocinonide concentration in strip 1 at each time point, a clear decline is evident from hour 1 to hour 9 (Figure 2A, red line [partici-pants 2–5] vs blue line [participants 1–5]).
When the average concentration of fluocinonide was calculated in strips 1 to 6 from all participants, there was a general steady decline after hour 1 with a slight increase of 25 ng/mL at hour 6 (Figure 2B, blue line). This increase is due to the measurements obtained from participant 1; however, if partici-pant 1 is removed from the analysis, a constant decline is observed from hour 1 to hour 9 (Figure 2B, red line).
|
A prior study evaluated the penetration and absorption of halcinonide in the stratum corneum.7 In summary, halcinonide concentration peaked at hour 1 following application and remained elevated to hour 6, before beginning a slow decline. The average concentration of halcinonide from all participants in strips 1 to 6 reached 1350 ng/mL at hour 1, remained within 93% to 97% of this level (1253–1303 ng/mL) for the next 5 hours, and declined only 29% from the peak at hour 1 to hour 9 (958 ng/mL)(Figure 3, blue line).7 In contrast, the fluocinonide concentration in participants 2 to 5 from the current study reached 190 ng/mL at hour 1 and steadily declined 53% to 89 ng/mL by hour 9 (Figure 3, red line).
Two participants from the prior halcinonide study also were enrolled in the current fluocinonide study (referred to as participant A and B). In general, halcinonide levels in both participants remained elevated for 6 hours after application and declined 27.5% and 35.5%, respectively, by hour 9 (Figure 4). Participant A experienced a 20.5% dip in halcinonide concentration at hour 3 followed by an increase at hour 6; however, the halcinonide concentration at hour 9 was similar to hour 3.7 In contrast, fluocin-onide concentrations for these participants peaked at 1 hour and clearly declined approximately 60% over the next 8 hours.
Comment
The release of both fluocinonide and halcinonide into the skin was evaluated using dermal tape stripping on 4 sites on the forearms of healthy individuals. Cream formulations of each corticosteroid were evaluated in 5 participants, with 2 participants receiving both formulations during different study periods. In the prior study with halcinonide, the stratum corneum exhibited the highest concentration of the corticosteroid, with substantial declines beyond strip 6 (ie, strips 7–20).7 For this reason, only strips 1 to 6 were evaluated for corticosteroid penetration and absorption.
Results from strip 1 indicated immediate absorption of corticosteroid (fluocinonide and halcinonide) into the skin. Unlike the release of halcinonide, which demonstrated a clear sustained release over 6 hours before decreasing,7 fluocinonide concentrations began declining immediately after peaking at hour 1 and continued to decline up to hour 9. Only participant 1 exhibited a second peak of fluocinonide concentration at hour 6; the rest of the participants did not. This second peak is most likely an anomaly due to the small number of participants rather than a true elevation.
Given the rapid decline of fluocinonide concentration over the 9 hours compared with the more gradual decline of halcinonide concentration, there appears to be no evidence of a biphasic sustained release of fluocinonide from its vehicle. This difference in release pattern from each corticosteroid’s respective vehicle may explain in part the different clinical outcomes in comparative studies.4-6
It is known that vehicle composition affects corticosteroid diffusion from the vehicle to the skin surface and subsequent penetration into the skin.9 Either process can determine the overall effectiveness of the product. Ayres and Hooper10 evaluated the penetration of 4 topical preparations of cortisol. Product 1 delivered 16 times more cortisol to the skin than product 2, 8 times more than product 3, and 3 times more than product 4. Because all the preparations contained cortisol-free alcohol, these differences were attributed to the vehicle in which the cortisol was formulated. Products 1 and 4 both contained 10% urea, but the urea in product 1 was a powder in a cream base and the urea in product 4 was in a stabilizing emulsified base. Product 2 contained a propylene glycol/water base and product 3 was a water-miscible cream.10
Generic corticosteroid products have been observed in clinical practice and have been shown in vasoconstriction assays to be less and more potent than their brand-name equivalents.2,11 Vasoconstriction assays are the standard for assessing the potency of topical corticosteroids and predicting their clinical efficacy.2 One study reported significant differences in therapeutic effectiveness between generic formulations and their brand-name equivalents.12 Kenalog cream 0.1% (multiple manufacturers) was significantly more potent than any of the generic triamcinolone creams tested (P<.05); in fact, Kenalog cream 0.025% (multiple manufacturers) was statistically superior to all the generic triamcinolone creams 0.1%. Moreover, Artistocort A ointment 0.1% (Lederele Laboratories) and Valisone cream 0.1% (Schering Corporation) also were more potent than their generics at the same concentration in the same vehicle type.12 A second study also observed that 2 of 6 generic formulations had significantly less vasoconstriction than their respective brand-name formulations.11 A brand-name betamethasone valerate cream produced significantly greater vasoconstriction than its generic equivalent, and a brand-name betamethasone dipropionate cream produced greater vasoconstriction than one generic and equal vasoconstriction to another generic. Additionally, the vasoconstriction measured with Diprosone was greater than that measured with Diprolene, another brand-name product of betamethasone dipropionate.11 Diprosone and Diprolene differ in their vehicle content. The latter, a class I corticosteroid, contains a modified vehicle high in propylene glycol, whereas the former contains less propylene glycol and thus is classified as a class III corticosteroid. Propylene glycol allows hydrophobic molecules such as corticosteroids to dissolve more fully in the vehicle.12
Ostrenga et al1 studied the solubility of corticosteroids in different vehicles and, as expected, corticosteroids that fully solubilized in the vehicle exhibited better penetration into the skin on assessment with vasoconstriction assays. Corticosteroids in a suspension, on the other hand, showed slower penetration into the skin.1,13 A balance between the solution and suspension phase would allow a drug to rapidly penetrate the skin upon application, and when this pool of solubilized drug was depleted, additional drug could penetrate into the skin from the suspension phase. Based on the tape strip results from the current study it appears that halcinonide, which is manufactured in a biphasic formulation, follows this pattern of penetration and absorption into the stratum corneum. In contrast, fluocinonide appears to exist in a soluble state without much, if any, amount in a suspension phase because it had no sustained release during the 9 hours after application.
Common belief among dermatologists is that long-term use of corticosteroids leads to tachyphylaxis,14 which can be attributed to poor patient adherence. If patients skip doses, then the steady state of the product at the target site is not maintained. It is interesting to speculate that using agents with more sustained release beyond the time of application (such as halcinonide) may preserve steady-state levels even when patients are neglectful of the next medication application. Corticosteroids that work in 2 phases such as halcinonide may minimize tachyphylaxis experienced with prolonged use of corticosteroids.
Fluocinonide and halcinonide are both class II high-potency corticosteroids as shown on outcomes from vasoconstrictor assays, which assess the extent to which a corticosteroid causes cutaneous vasoconstriction or blanching in normal healthy individuals.15 The assay depends on the molecule diffusing from the vehicle, penetrating the skin, and causing a reaction (blanching) that is then evaluated. The assay cannot effectively evaluate the rate of continued diffusion and skin penetration beyond the appearance of blanching. In contrast, the tape-stripping method provides an inside look at the extent of penetration of the corticosteroid beyond the skin surface and the rate of its clearance from different skin layers. In the current study, the levels of fluocinonide declined after peaking at 1 hour after application, but the levels of halcinonide clearly remained elevated after peaking at the same time point. Most likely, vasoconstrictor studies would not be able to differentiate between the concentrations of the 2 products in the stratum corneum beyond the first hour after application.
Tape stripping, or dermatopharmacokinetics, has advantages over vasoconstriction assays in studying corticosteroid penetration and clearance from the stratum corneum. At one point, the US Food and Drug Administration had included tape stripping in its preliminary guidelines for generic topical bioequivalence studies until data from the same formulation generated from 2 different laboratories produced different results.16 Since that time, much work has been done with tape stripping to ensure its consistency. Weigmann et al17 demonstrated equivalent results with clobetasol using vasoconstriction and tape stripping, and Wiedersberg et al18 demonstrated the same with betamethasone. For the current study, the fluocinonide and halcinonide formulations were weighed prior to application so that the same dose was tested in all participants. A plunger was used to produce consistent pressure at all application sites to control for the amount of skin that was stripped off with the tape. Results for both corticosteroids were consistent between the participants. Variability in the data was detected; however, this observation is most likely due to the small number of participants in the studies.
Conclusion
In summary, this pilot study demonstrated that fluocinonide concentration in the stratum corneum peaks within the first hour of application before beginning a steady general decline. There was no evidence of sustained release. In contrast, halcin-onide demonstrated a sustained release for 6 hours after application. Halcinonide is formulated in a cream base in which the corticosteroid is present in a solution and suspension phase that allows for sustained delivery in skin over time. Fluocinonide does not appear to be formulated in the same way, and its concentrations in the stratum corneum begin to decline 1 hour after application.
Acknowledgement
Thank you to Robert Kellar, PhD, at the Center for Bioengineering Innovation at Northern Arizona University, Flagstaff, for conducting the liquid chromatography–mass spectrometry.
1. Ostrenga J, Haleblian J, Poulsen B, et al. Vehicle design for a new topical steroid, fluocinonide. J Invest Dermatol. 1971;56:392-399.
2. Rathi SK, D’Souza P. Rational and ethical use of topical corticosteroids based on safety and efficacy. Indian J Dermatol. 2012;57:251-259.
3. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
4. Sudilovsky A, Clewe TH. Comparative efficacy of halcin-onide and fluocinonide creams in psoriasis and eczematous dermatoses. J Clin Pharmacol. 1975;15:779-784.
5. Close JE. Double-blind comparison of topical halcinonide and fluocinonide in the treatment of psoriasis. Int J Dermatol. 1976;15:534-537.
6. Lynfield Y, Watsky M. Psoriasis: topical corticosteroid therapy. Cutis. 1976;18:133, 136-137.
7. Draelos ZD. Demonstration of the biphasic release of 0.1% halcinonide cream. J Drugs Dermatol. 2015;14:89-90.
8. Bagatell FK. Halcinonide: a new potent topical anti-inflammatory drug. Cutis. 1974;14:459-462.
9. Ostrenga J, Steinmetz C, Poulsen B. Significance of vehicle composition. I. relationship between topical vehicle composition, skin penetrability, and clinical efficacy. J Pharm Sci. 1971;60:1175-1179.
10. Ayres PJ, Hooper G. Assessment of the skin penetration properties of different carrier vehicles for topically applied cortisol. Br J Dermatol. 1978;99:307-317.
11. Olsen EA. Double-blind controlled comparison of generic and trade-name topical steroids using the vasoconstriction assay. Arch Dermatol. 1991;127:197-201.
12. Stoughton RB. Are generic formulations equivalent to trade name topical glucocorticoids? Arch Dermatol. 1987;123:1312-1314.
13. Poulsen BJ, Young E, Coquilla V, et al. Effect of topical vehicle composition on the in vitro release of fluocinolone acetonide and its acetate ester. J Pharm Sci. 1968;57:928-933.
14. Taheri A, Cantrell J, Feldman SR. Tachyphylaxis to topical glucocorticoids: what is the evidence? Dermatol Online J. 2013;19:18954.
15. Ference JD, Last AR. Choosing topical corticosteroids. Am Fam Physician. 2009;79:135-140.
16. Pershing LK, Nelson JL, Corlett JL, et al. Assessment of dermatopharmacokinetic approach in the bioequivalence determination of topical tretinoin gel products. J Am Acad Dermatol. 2003;48:740-751.
17. Weigmann H, Lademann J, v Pelchrzim R, et al. Bioavailability of clobetasol propionate-quantification of drug concentrations in the stratum corneum by dermatopharmacokinetics using tape stripping. Skin Pharmacol Appl Skin Physiol. 1999;12:46-53.
18. Wiedersberg S, Naik A, Leopold CS, et al. Pharmacodynamics and dermatopharmacokinetics of betamethasone 17-valerate: assessment of topical bioavailability. Br J Dermatol. 2009;160:676-686.
1. Ostrenga J, Haleblian J, Poulsen B, et al. Vehicle design for a new topical steroid, fluocinonide. J Invest Dermatol. 1971;56:392-399.
2. Rathi SK, D’Souza P. Rational and ethical use of topical corticosteroids based on safety and efficacy. Indian J Dermatol. 2012;57:251-259.
3. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
4. Sudilovsky A, Clewe TH. Comparative efficacy of halcin-onide and fluocinonide creams in psoriasis and eczematous dermatoses. J Clin Pharmacol. 1975;15:779-784.
5. Close JE. Double-blind comparison of topical halcinonide and fluocinonide in the treatment of psoriasis. Int J Dermatol. 1976;15:534-537.
6. Lynfield Y, Watsky M. Psoriasis: topical corticosteroid therapy. Cutis. 1976;18:133, 136-137.
7. Draelos ZD. Demonstration of the biphasic release of 0.1% halcinonide cream. J Drugs Dermatol. 2015;14:89-90.
8. Bagatell FK. Halcinonide: a new potent topical anti-inflammatory drug. Cutis. 1974;14:459-462.
9. Ostrenga J, Steinmetz C, Poulsen B. Significance of vehicle composition. I. relationship between topical vehicle composition, skin penetrability, and clinical efficacy. J Pharm Sci. 1971;60:1175-1179.
10. Ayres PJ, Hooper G. Assessment of the skin penetration properties of different carrier vehicles for topically applied cortisol. Br J Dermatol. 1978;99:307-317.
11. Olsen EA. Double-blind controlled comparison of generic and trade-name topical steroids using the vasoconstriction assay. Arch Dermatol. 1991;127:197-201.
12. Stoughton RB. Are generic formulations equivalent to trade name topical glucocorticoids? Arch Dermatol. 1987;123:1312-1314.
13. Poulsen BJ, Young E, Coquilla V, et al. Effect of topical vehicle composition on the in vitro release of fluocinolone acetonide and its acetate ester. J Pharm Sci. 1968;57:928-933.
14. Taheri A, Cantrell J, Feldman SR. Tachyphylaxis to topical glucocorticoids: what is the evidence? Dermatol Online J. 2013;19:18954.
15. Ference JD, Last AR. Choosing topical corticosteroids. Am Fam Physician. 2009;79:135-140.
16. Pershing LK, Nelson JL, Corlett JL, et al. Assessment of dermatopharmacokinetic approach in the bioequivalence determination of topical tretinoin gel products. J Am Acad Dermatol. 2003;48:740-751.
17. Weigmann H, Lademann J, v Pelchrzim R, et al. Bioavailability of clobetasol propionate-quantification of drug concentrations in the stratum corneum by dermatopharmacokinetics using tape stripping. Skin Pharmacol Appl Skin Physiol. 1999;12:46-53.
18. Wiedersberg S, Naik A, Leopold CS, et al. Pharmacodynamics and dermatopharmacokinetics of betamethasone 17-valerate: assessment of topical bioavailability. Br J Dermatol. 2009;160:676-686.
Practice Points
- Fluocinonide concentration in the stratum corneum peaks within the first hour of application and then begins a steady decline.
- Halcinonide concentration also peaks within the first hour of application and remains elevated for 6 hours after application.
- Halcinonide, rather than fluocinonide, may provide clinical benefits in between doses because of its sustained release hours after application.
A Phase 3 Randomized, Double-blind, Vehicle-Controlled Trial of Azelaic Acid Foam 15% in the Treatment of Papulopustular Rosacea
Rosacea is a common dermatologic disorder that generally is characterized by erythema as well as papules and pustules on the cheeks, chin, forehead, and nose. Moreover, telangiectasia and burning or stinging sensations often occur.1,2 These clinical manifestations and other related ones frequently lead to the perception of “sensitive skin.” Rosacea patients often experience low self-esteem, anxiety, and social embarrassment.3 Reports of the gender distribution of the disease vary but often show female predominance.4 Although it also occurs in darker skin types, rosacea is more common in individuals with lighter skin.1
The etiology of rosacea is not yet fully understood, but the underlying pathology has been attributed to dysregulated immune responses. Although the flares of a typical fluctuating disease course often are caused by exogenous triggers, there is evidence that an underlying genetic component predisposes some individuals to pathologic changes associated with the condition.5 Augmented immune activity and proinflammatory signaling appear to induce the infiltration of inflammatory elements into affected areas.2 These regions show dilated vasculature and increased cutaneous blood flow secondary to inflammation. Systemic oxidative stress also may contribute to epidermal dysfunction, as the antioxidant capacity of the skin in patients with rosacea is depleted relative to that of healthy individuals. The biochemical and vascular changes characteristic of rosacea coincide with aberrant permeability of the stratum corneum.6 The resulting decreased hydration and water loss across the skin contribute to the sensitivity and irritation typical of the disease.2
Current guidelines for the optimal management of rosacea with papulopustular lesions recommend skin care, photoprotection, and topical therapy. Depending on the severity of disease and the likelihood of adherence to a topical regimen, use of oral agents may be warranted.7
Azelaic acid (AzA), an unbranched saturated dicarboxylic acid (1,7-heptanedicarboxylic acid) that occurs in plants, is one of several US Food and Drug Administration–approved topical agents for the treatment of inflammatory lesions in rosacea.8 Although the pathophysiology of rosacea is not yet fully understood, there is a growing consensus about the role of proinflammatory molecules (eg, kallikrein 5, cathelicidins) as well as reactive oxygen species (ROS).9 Azelaic acid has been demonstrated to modulate the inflammatory response in normal human keratinocytes through several pathways, including modulation of the signaling pathways of peroxisome proliferator-activated receptor g and nuclear factor kB, concurrent with the observed inhibition of proinflammatory cytokine secretion.10 Additionally, AzA can inhibit the release of ROS from neutrophils and also may reduce ROS by direct scavenging effects.11 Further, AzA shows direct inhibition of kallikrein 5 in human keratinocytes as well as a reduction of the expression of kallikrein 5 and cathelicidin in murine skin and the facial skin of patients with rosacea.12
In a series of randomized trials in patients with papulopustular rosacea (PPR), AzA has shown clinical efficacy and safety as a topical treatment.13-15 Based on these studies, a gel formulation of AzA with a 15% concentration has been approved for treating inflammatory papules and pustules of mild to moderate rosacea.16
Although AzA delivered in a gel matrix is an effective therapy, topical delivery of active pharmaceutical ingredients via foam is often preferred over traditional vehicles in patients with sensitive skin. Patient rationale for favoring foam includes improved appearance and ease of application, namely easier to spread with a reduced need to manipulate inflamed skin.17 Also, data reveal that patients may be more compliant with a treatment that meets their needs such as an optimized foam formulation.18 In addition, the lipid components of an optimized formulation are thought to contribute to an improved skin condition.19 The foam vehicle used in this study is a proprietary oil-in-water formulation that includes fatty alcohols and triglycerides. The novel delivery of AzA in a foam formulation will provide clinicians and patients with a new option for improved individualized care.
We report the primary results of a phase 3 study in patients with PPR comparing the efficacy and safety of twice-daily AzA foam 15% with vehicle foam. The phase 3 study builds on the results of a prior randomized double-blind trial (N=401) that demonstrated significant improvements relative to vehicle in therapeutic success rate (P=.017) and decreased inflammatory lesion count (ILC)(P<.001) among patients treated with AzA foam 15%.8
Methods
Study Design
This phase 3 randomized, double-blind, vehicle-controlled, parallel-group, multicenter study was conducted in patients with PPR according to Good Clinical Practice guidelines in 48 study centers in the United States. The objective was to evaluate a 12-week, twice-daily (morning and evening) course of AzA foam 15% versus vehicle.
Participants were men and women aged 18 years or older with moderate to severe PPR (as determined by investigator global assessment [IGA]) presenting with 12 to 50 papules and/or pustules and persistent erythema with or without telangiectasia. Informed consent was obtained from all participants before any study-related activities were carried out.
The study products were applied to the entire facial area each morning and evening at a dose of 0.5 g, thus administering 150 mg of AzA daily in the active arm of the trial (computerized randomization 1:1). The treatment period lasted 12 weeks, and participants were evaluated at baseline and weeks 4, 8, and 12. The follow-up period lasted 4 weeks following the end of treatment (EoT) and was concluded with one final end-of-study visit.
Efficacy Evaluations
There were 2 coprimary efficacy end points. Therapeutic success rate was evaluated using the IGA scale (clear, minimal, mild, moderate, or severe). Treatment success was defined as an IGA score of either clear or minimal (with at least a 2-step improvement) at EoT, whereas treatment failure was constituted by IGA scores of mild, moderate, or severe.
The second coprimary end point was the nominal change in ILC from baseline to EoT as determined by the total number of facial papules and pustules. Efficacy and safety parameters were evaluated at weeks 4, 8, and 12, as well as at the end of the 4-week follow-up period. Throughout the study, the investigator, participants, and all study personnel remained blinded.
Safety
Information about adverse events (AEs) was collected at each study visit, and AEs were graded according to seriousness (yes or no) and intensity (mild, moderate, or severe).
Statistical Analysis
Efficacy was confirmed by analysis of the treatment success rate at EoT with Cochran-Mantel-Haenszel test statistics, including a point estimate and 95% confidence interval (CI) for the odds ratio. Change in ILC at EoT was analyzed via an analysis of covariance model using treatment, center, and baseline lesion count as factors. (Additional methods can be found in the Appendix below.)
Results
Study Participants
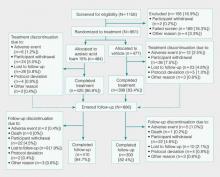
Of the 1156 patients who were screened for eligibility, 961 were randomized to treatment with AzA foam (n=484) or vehicle (n=477)(Figure 1). Sixty-four (13.2%) participants in the AzA foam group and 79 (16.6%) in the vehicle group discontinued treatment before completing the study. The most common reasons for discontinuation were participant withdrawal from the study and lost to follow-up. Six (1.2%) participants from the AzA foam group and 12 (2.5%) from the vehicle group discontinued because of AEs. All safety and efficacy data presented are based on the full analysis set, which consisted of the 961 participants randomized to treatment.
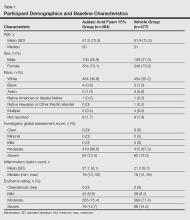
Demographic and baseline characteristics were balanced between the treatment groups (Table 1). The majority of participants were female (73.0%) and white (95.5%), reflecting the patient populations of independent studies that found a higher prevalence of rosacea in women and lighter skin types.4 There were no significant differences in baseline measures of PPR severity between the treatment groups. Participants in the AzA foam and vehicle groups had a mean ILC of 21.7 and 21.2, respectively, and 76.4% of participants had more than 14 lesions. All participants had an IGA score of moderate (86.8%) or severe (13.2%). Moderate or severe erythema was present in 91.5% of participants.
Treatment compliance, as measured by the percentage of expected doses that were actually administered, was 97.1% in the AzA foam group and 95.9% in the vehicle group.
Efficacy
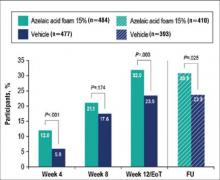
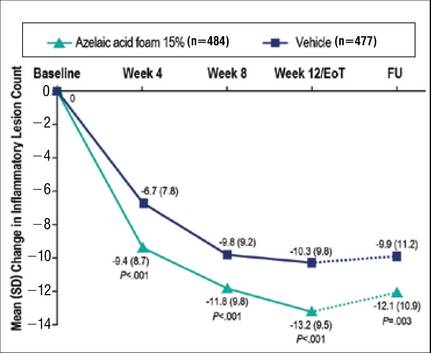
Results from both primary end points demonstrated superior efficacy of AzA foam over vehicle. The AzA foam group achieved a greater IGA success rate at EoT compared with the vehicle group (32.0% vs 23.5%; Cochran-Mantel-Haenszel test center-adjusted P<.001; odds ratio, 1.6; 95% CI, 1.2-2.2). Treatment success rate was higher in the AzA foam group than in the vehicle group at every time point past baseline (Figure 2). Similarly, the decrease in mean nominal ILC values was greater in the AzA foam group at every time point after baseline (Figure 3), and the treatment difference at EoT was statistically significant in favor of AzA foam (-2.7, F1,920=23.7, P<.001; 95% CI, -3.8 to -1.6). The divergence between treatment groups at week 4 reveals an onset of AzA effect early in the study.

|
|
Although the AzA foam group showed significantly better efficacy results than the vehicle group for the coprimary end points, participants in the vehicle group did show appreciable IGA success rates (23.5%) and changes in ILC (-10.3) at EoT (Figures 2 and 3).
Notably, the AzA foam group maintained better results than vehicle for both primary end points even at the end of the 4-week follow-up after EoT (Figures 2 and 3). Sensitivity analysis (data not shown) confirmed the findings from the full analysis set.
Safety
Adverse events were experienced by 149 (30.8%) participants in the AzA foam group and 119 (24.9%) in the vehicle group. The most common noncutaneous AEs (>1% of participants) reported during AzA foam treatment were nasopharyngitis, headache, upper respiratory tract infection, and influenza. In the vehicle group, the most common noncutaneous AEs reported were nasopharyngitis and headache. Drug-related AEs (relationship assessed by the investigator) were reported slightly more often in the AzA foam group (7.6%) than in the vehicle group (4.6%). Drug-related AEs were predominantly cutaneous and occurred at the site of application (Table 2). Drug-related cutaneous AEs were more common in the AzA foam group (7.0%) than in the vehicle group (4.4%). Although serious AEs were more common in the vehicle group, all were regarded as unrelated to the study medication. A single death occurred in the vehicle group due to an accident unrelated to the study drug.
The most frequent drug-related AEs in participants treated with AzA foam versus vehicle were application-site pain (3.5% vs 1.3%), application-site pruritus (1.4% vs 0.4%), and application-site dryness (1.0% vs 0.6%). The classical rosacea symptom of stinging is subsumed under the term application-site pain, according to MedDRA (Medical Dictionary for Regulatory Activities).
All other drug-related AEs occurred at a frequency of less than 1% in participants from both groups. Serious AEs were rare and unrelated to treatment, with 3 AEs reported in the AzA foam group and 4 in the vehicle group. Adverse events leading to study drug withdrawal occurred in less than 2% of participants and were more common in the vehicle group (2.5%) than in the AzA foam group (1.2%). Of the 3 drug-related AEs leading to withdrawal in the AzA foam group, 2 were due to cutaneous reaction and 1 was due to a burning sensation. The number of active drug-related cutaneous AEs was highest during the first 4 weeks of treatment and declined over the course of the study (eFigure).
More than 96% of AEs were resolved by the end of the study. Of the participants experiencing AEs that did not resolve during the course of the study, 16 were in the AzA foam group and 10 in the vehicle group. Six unresolved AEs were drug related, with 3 occurring in each treatment group. Unresolved drug-related cutaneous AEs in the AzA foam group were pain, pruritus, and dryness at the application site.
Comment
Overall, the results from this phase 3 trial demonstrate that the new foam formulation of AzA was efficacious and safe in a 12-week, twice-daily course of treatment for moderate to severe PPR. The AzA foam formulation was significantly superior to vehicle (P<.001) for both primary efficacy end points. Participants in the AzA foam group achieved therapeutic success at a higher rate than the vehicle group, and the change in nominal ILC at EoT was significantly greater for participants treated with AzA foam than for those treated with vehicle (P<.001). Differences between the 2 treatment groups for the coprimary end point measures arose early in the study, demonstrating that symptoms were rapidly controlled. Between weeks 8 and 12 (EoT), the rate of increase of beneficial effects in the AzA foam group remained high, while the vehicle group showed a notable slowing. There was no indication of any rebound effect in overall disease severity subsequent to EoT. After 4 weeks of follow-up, there was still a beneficial treatment effect present in favor of the AzA foam group, as indicated by the persistence of improvements in both coprimary end point measures throughout the follow-up period.
Analyses of alternative populations and secondary end points (data not shown) supported the efficacy results reported here. There was no indication of irregular study center effects, and the sensitivity analyses demonstrated robustness of the data for the observed treatment effects.
The use of vehicle foam alone appeared to be beneficial in reducing ILC and improving IGA rating, which suggests that the properties of the new foam formulation are favorable for the inflamed lesional skin of rosacea. Of note, other dermatology studies, including trials in rosacea, have reported therapeutic effects of vehicle treatment that may be attributable to the positive effects of skin care with certain formulations.20
Azelaic acid foam was well tolerated in the current study. More than 93% of AEs in either treatment group were of mild or moderate severity. The incidence of drug-related AEs was low in both groups and mainly occurred at the application site. There were no drug-related severe or serious AEs. The low incidence of reported drug-related noncutaneous AEs in the AzA foam group (dysgeusia in 1 patient and headache in 2 patients) supports the known favorable systemic tolerance profile of AzA.
Although most drug-related AEs occurred at the application site, they were generally transient, with the majority of events in the AzA foam group lasting no more than 1 hour. Most cutaneous AEs developed early in the study. In the AzA foam group, the prevalence of drug-related cutaneous AEs dropped at every time interval as the study progressed (eFigure). Very few AEs of any type persisted through the end of the study. These safety results were accompanied by a high compliance rate and a high participation rate throughout the course of the study. Taken together, the available data for this AzA foam formulation support a favorable tolerability profile. The results of this study are consistent with and expand on data from an earlier investigation of similar design.8
Conclusion
The development of an AzA foam formulation with higher lipid content was intended to expand the treatment options available to physicians and patients who are managing rosacea. Most topical dermatologic treatments are currently delivered in classical formulations such as creams or gels, but patients who use topical therapies have rated messiness and ease of application among the most important characteristics affecting quality of life.17,21 Foam formulations may offer improvements in this regard; ease of application may minimize unnecessary manipulation of inflamed skin and contribute to a high level of user satisfaction.22 However, the design of the current study was limited to evaluating only the AzA foam formulation versus a foam vehicle, and direct comparisons of clinical efficacy and tolerability to other AzA topical preparations were not performed. Nonetheless, patients have previously reported that they would be more likely to comply with a recommended course of dermatologic foam therapy than other topical formulations.18 The proposed foam formulation was designed to attend to the specific needs of the dry and sensitive skin in rosacea by combining the demonstrated efficacy properties exhibited by AzA gel 15% with the good tolerability and acceptability of a lipid-containing foam formulation. Development of this formulation was targeted to obtain a product that would be highly spreadable, dry quickly, and be easy to apply. The available data for this AzA foam formulation support the value of this option in the topical treatment of rosacea. The success in reduction of overall disease severity, lack of any rebound after EoT, and the observed tolerability and high adherence rates suggest that this novel formulation is a useful addition to current treatment options for rosacea.
Addendum
After release of the study data for unblinding and statistical evaluation, the following inconsistency regarding patient distribution was noted: 1 participant was incorrectly evaluated as part of the AzA foam analysis group when in fact this patient was randomized to vehicle and was treated throughout the study with vehicle. This participant did not experience any AE and did not show any IGA improvement at the EoT. As this single case did not have an impact on the statistical conclusions or interpretation of the results, the released study data have not been changed. This deviation was described as a database erratum in the study report.
Acknowledgement—Editorial support through inVentiv Medical Communications, New York, New York, was provided by Bayer HealthCare Pharmaceuticals Inc.
APPENDIX
Supplementary Methods
Supplementary Study Design
This study met all local legal and regulatory requirements and was conducted according to the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. Before the start of the study and implementation, the protocol and all amendments were approved by the appropriate independent ethics committee or institutional review board at each study site. Two protocol amendments were implemented before the first participant visit.
Exclusion criteria included the presence of dermatoses that could interfere with rosacea diagnosis or evaluation, facial laser surgery or topical use of any medication to treat rosacea within 6 weeks before randomization, systemic use of any medications to treat rosacea, and known unresponsiveness to AzA treatment. Further standard exclusion criteria included alcohol or drug use or parallel participation in other clinical studies, which were necessary to exclude undue influence on study evaluations and/or participant safety. The study was conducted by qualified investigators at 48 centers in the United States.
The investigational product was filled in identical containers according to the randomization list generated by a computer program using blocks. Complete blocks of study medication were distributed to the centers. Eligible participants were randomized 1:1 into either AzA foam or vehicle treatment groups by assignment of a randomization number at baseline. A blind investigational product under the same randomization number was dispensed to and returned from participants by study personnel who were not involved in the assessments. Blinding was achieved by using labels on the investigational products that did not allow identification of the true medication.
Compliance was evaluated from participant diaries as well as the number of expected doses and actually applied doses.
Additional Efficacy Evaluations
A number of secondary variables (not reported here) were assessed, including changes in other manifestations of PPR, as well as participant assessments of treatment response, tolerability, cosmetic preferences, and quality of life.
Additional Safety
Investigators reported a yes or no response as to whether there was a reasonable causal relationship between AEs and treatment. Moreover, AEs that began at the start of or during treatment were considered treatment emergent. Cutaneous AEs were further assessed regarding location and duration. An AE was deemed local if it occurred at the application site and transient if it subsided within 60 minutes of onset.
Statistical Analysis
The primary efficacy analyses presented here were based on the full analysis set of participants who were randomized and had medication dispensed. For participants with no EoT value, the last nonmissing value was used including baseline (last-observation-carried-forward methodology). Participants who discontinued treatment prematurely because of lack of efficacy were considered to be treatment failures, regardless of the reported IGA score. Statistical significance was needed for both coprimary efficacy variables at a 1-sided 2.5% significance level to show confirmed superiority of AzA foam versus vehicle.
A number of sensitivity analyses were performed, including an analysis of the coprimary end points using observed data, analysis of the per-protocol population of participants who did not prematurely discontinue treatment and had no major protocol deviations, subgroup analyses, and the use of statistical methods to investigate the effect of missing observations. Analyses of success rate and nominal change in ILC were repeated for each postbaseline visit using χ² and t tests, respectively. All summary and statistical analyses were performed according to the study protocol (unchanged after the start of the study) using SAS version 9.2.
Results from a prior study provided the basis for the sample size, which was calculated to show a significant difference in both primary efficacy end points with a power of 90%.8 To allow for dropouts, 480 participants in each treatment group were to be randomized for a total of 960 participants.
1. Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
2. Del Rosso JQ. Advances in understanding and managing rosacea: part 1: connecting the dots between pathophysiological mechanisms and common clinical features of rosacea with emphasis on vascular changes and facial erythema. J Clin Aesthet Dermatol. 2012;5:16-25.
3. Huynh TT. Burden of disease: the psychosocial impact of rosacea on a patient’s quality of life. Am Health Drug Benefits. 2013;6:348-354.
4. Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6 suppl 1):S27-S35.
5. Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 suppl 1):S15-S26.
6. Wollina U. Recent advances in the understanding and management of rosacea. F1000Prime Rep. 2014;6:50.
7. Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 5: a guide on the management of rosacea. Cutis. 2014;93:134-138.
8. Draelos ZD, Elewski B, Staedtler G, et al. Azelaic acid foam 15% in the treatment of papulopustular rosacea: a randomized, double-blind, vehicle-controlled study. Cutis. 2013;92:306-317.
9. Yamasaki K, Di Nardo A, Bardan A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975-980.
10. Mastrofrancesco A, Ottaviani M, Aspite N, et al. Azelaic acid modulates the inflammatory response in normal human keratinocytes through PPARg activation. Exp Dermatol. 2010;19:813-820.
11. Akamatsu H, Komura J, Asada Y, et al. Inhibitory effect of azelaic acid on neutrophil functions: a possible cause for its efficacy in treating pathogenetically unrelated diseases. Arch Dermatol Res. 1991;283:162-166.
12. Coda AB, Hata T, Miller J, et al. Cathelicidin, kalli-krein 5, and serine protease activity is inhibited during treatment of rosacea with azelaic acid 15% gel. J Am Acad Dermatol. 2013;69:570-577.
13. van Zuuren EJ, Kramer SF, Carter BR, et al. Effective and evidence-based management strategies for rosacea: summary of a Cochrane systematic review. Br J Dermatol. 2011;165:760-781.
14. Thiboutot D, Thieroff-Ekerdt R, Graupe K. Efficacy and safety of azelaic acid (15%) gel as a new treatment for papulopustular rosacea: results from two vehicle-controlled, randomized phase III studies. J Am Acad Dermatol. 2003;48:836-845.
15. Thiboutot DM, Fleischer AB Jr, Del Rosso JQ, et al. Azelaic acid 15% gel once daily versus twice daily in papulopustular rosacea. J Drugs Dermatol. 2008;7:541-546.
16. Finacea [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; 2015.
17. Zhao Y, Jones SA, Brown MB. Dynamic foams in topical drug delivery. J Pharm Pharmacol. 2010;62:678-684.
18. Gottlieb AB, Ford RO, Spellman MC. The efficacy and tolerability of clobetasol propionate foam 0.05% in the treatment of mild to moderate plaque-type psoriasis of nonscalp regions. J Cutan Med Surg. 2003;7:185-192.
19. Loden M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am J Clin Dermatol. 2003;4:771-788.
20. Jackson JM, Pelle M. Topical rosacea therapy: the importance of vehicles for efficacy, tolerability and compliance. J Drugs Dermatol. 2011;10:627-633.
21. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
22. Kircik LH, Bikowski JB. Vehicles matter: topical foam formulations. Practical Dermatology. January 2012(suppl):3-18.
Rosacea is a common dermatologic disorder that generally is characterized by erythema as well as papules and pustules on the cheeks, chin, forehead, and nose. Moreover, telangiectasia and burning or stinging sensations often occur.1,2 These clinical manifestations and other related ones frequently lead to the perception of “sensitive skin.” Rosacea patients often experience low self-esteem, anxiety, and social embarrassment.3 Reports of the gender distribution of the disease vary but often show female predominance.4 Although it also occurs in darker skin types, rosacea is more common in individuals with lighter skin.1
The etiology of rosacea is not yet fully understood, but the underlying pathology has been attributed to dysregulated immune responses. Although the flares of a typical fluctuating disease course often are caused by exogenous triggers, there is evidence that an underlying genetic component predisposes some individuals to pathologic changes associated with the condition.5 Augmented immune activity and proinflammatory signaling appear to induce the infiltration of inflammatory elements into affected areas.2 These regions show dilated vasculature and increased cutaneous blood flow secondary to inflammation. Systemic oxidative stress also may contribute to epidermal dysfunction, as the antioxidant capacity of the skin in patients with rosacea is depleted relative to that of healthy individuals. The biochemical and vascular changes characteristic of rosacea coincide with aberrant permeability of the stratum corneum.6 The resulting decreased hydration and water loss across the skin contribute to the sensitivity and irritation typical of the disease.2
Current guidelines for the optimal management of rosacea with papulopustular lesions recommend skin care, photoprotection, and topical therapy. Depending on the severity of disease and the likelihood of adherence to a topical regimen, use of oral agents may be warranted.7
Azelaic acid (AzA), an unbranched saturated dicarboxylic acid (1,7-heptanedicarboxylic acid) that occurs in plants, is one of several US Food and Drug Administration–approved topical agents for the treatment of inflammatory lesions in rosacea.8 Although the pathophysiology of rosacea is not yet fully understood, there is a growing consensus about the role of proinflammatory molecules (eg, kallikrein 5, cathelicidins) as well as reactive oxygen species (ROS).9 Azelaic acid has been demonstrated to modulate the inflammatory response in normal human keratinocytes through several pathways, including modulation of the signaling pathways of peroxisome proliferator-activated receptor g and nuclear factor kB, concurrent with the observed inhibition of proinflammatory cytokine secretion.10 Additionally, AzA can inhibit the release of ROS from neutrophils and also may reduce ROS by direct scavenging effects.11 Further, AzA shows direct inhibition of kallikrein 5 in human keratinocytes as well as a reduction of the expression of kallikrein 5 and cathelicidin in murine skin and the facial skin of patients with rosacea.12
In a series of randomized trials in patients with papulopustular rosacea (PPR), AzA has shown clinical efficacy and safety as a topical treatment.13-15 Based on these studies, a gel formulation of AzA with a 15% concentration has been approved for treating inflammatory papules and pustules of mild to moderate rosacea.16
Although AzA delivered in a gel matrix is an effective therapy, topical delivery of active pharmaceutical ingredients via foam is often preferred over traditional vehicles in patients with sensitive skin. Patient rationale for favoring foam includes improved appearance and ease of application, namely easier to spread with a reduced need to manipulate inflamed skin.17 Also, data reveal that patients may be more compliant with a treatment that meets their needs such as an optimized foam formulation.18 In addition, the lipid components of an optimized formulation are thought to contribute to an improved skin condition.19 The foam vehicle used in this study is a proprietary oil-in-water formulation that includes fatty alcohols and triglycerides. The novel delivery of AzA in a foam formulation will provide clinicians and patients with a new option for improved individualized care.
We report the primary results of a phase 3 study in patients with PPR comparing the efficacy and safety of twice-daily AzA foam 15% with vehicle foam. The phase 3 study builds on the results of a prior randomized double-blind trial (N=401) that demonstrated significant improvements relative to vehicle in therapeutic success rate (P=.017) and decreased inflammatory lesion count (ILC)(P<.001) among patients treated with AzA foam 15%.8
Methods
Study Design
This phase 3 randomized, double-blind, vehicle-controlled, parallel-group, multicenter study was conducted in patients with PPR according to Good Clinical Practice guidelines in 48 study centers in the United States. The objective was to evaluate a 12-week, twice-daily (morning and evening) course of AzA foam 15% versus vehicle.
Participants were men and women aged 18 years or older with moderate to severe PPR (as determined by investigator global assessment [IGA]) presenting with 12 to 50 papules and/or pustules and persistent erythema with or without telangiectasia. Informed consent was obtained from all participants before any study-related activities were carried out.
The study products were applied to the entire facial area each morning and evening at a dose of 0.5 g, thus administering 150 mg of AzA daily in the active arm of the trial (computerized randomization 1:1). The treatment period lasted 12 weeks, and participants were evaluated at baseline and weeks 4, 8, and 12. The follow-up period lasted 4 weeks following the end of treatment (EoT) and was concluded with one final end-of-study visit.
Efficacy Evaluations
There were 2 coprimary efficacy end points. Therapeutic success rate was evaluated using the IGA scale (clear, minimal, mild, moderate, or severe). Treatment success was defined as an IGA score of either clear or minimal (with at least a 2-step improvement) at EoT, whereas treatment failure was constituted by IGA scores of mild, moderate, or severe.
The second coprimary end point was the nominal change in ILC from baseline to EoT as determined by the total number of facial papules and pustules. Efficacy and safety parameters were evaluated at weeks 4, 8, and 12, as well as at the end of the 4-week follow-up period. Throughout the study, the investigator, participants, and all study personnel remained blinded.
Safety
Information about adverse events (AEs) was collected at each study visit, and AEs were graded according to seriousness (yes or no) and intensity (mild, moderate, or severe).
Statistical Analysis
Efficacy was confirmed by analysis of the treatment success rate at EoT with Cochran-Mantel-Haenszel test statistics, including a point estimate and 95% confidence interval (CI) for the odds ratio. Change in ILC at EoT was analyzed via an analysis of covariance model using treatment, center, and baseline lesion count as factors. (Additional methods can be found in the Appendix below.)
Results
Study Participants
Of the 1156 patients who were screened for eligibility, 961 were randomized to treatment with AzA foam (n=484) or vehicle (n=477)(Figure 1). Sixty-four (13.2%) participants in the AzA foam group and 79 (16.6%) in the vehicle group discontinued treatment before completing the study. The most common reasons for discontinuation were participant withdrawal from the study and lost to follow-up. Six (1.2%) participants from the AzA foam group and 12 (2.5%) from the vehicle group discontinued because of AEs. All safety and efficacy data presented are based on the full analysis set, which consisted of the 961 participants randomized to treatment.
Demographic and baseline characteristics were balanced between the treatment groups (Table 1). The majority of participants were female (73.0%) and white (95.5%), reflecting the patient populations of independent studies that found a higher prevalence of rosacea in women and lighter skin types.4 There were no significant differences in baseline measures of PPR severity between the treatment groups. Participants in the AzA foam and vehicle groups had a mean ILC of 21.7 and 21.2, respectively, and 76.4% of participants had more than 14 lesions. All participants had an IGA score of moderate (86.8%) or severe (13.2%). Moderate or severe erythema was present in 91.5% of participants.
Treatment compliance, as measured by the percentage of expected doses that were actually administered, was 97.1% in the AzA foam group and 95.9% in the vehicle group.
Efficacy
Results from both primary end points demonstrated superior efficacy of AzA foam over vehicle. The AzA foam group achieved a greater IGA success rate at EoT compared with the vehicle group (32.0% vs 23.5%; Cochran-Mantel-Haenszel test center-adjusted P<.001; odds ratio, 1.6; 95% CI, 1.2-2.2). Treatment success rate was higher in the AzA foam group than in the vehicle group at every time point past baseline (Figure 2). Similarly, the decrease in mean nominal ILC values was greater in the AzA foam group at every time point after baseline (Figure 3), and the treatment difference at EoT was statistically significant in favor of AzA foam (-2.7, F1,920=23.7, P<.001; 95% CI, -3.8 to -1.6). The divergence between treatment groups at week 4 reveals an onset of AzA effect early in the study.

|

|
Although the AzA foam group showed significantly better efficacy results than the vehicle group for the coprimary end points, participants in the vehicle group did show appreciable IGA success rates (23.5%) and changes in ILC (-10.3) at EoT (Figures 2 and 3).
Notably, the AzA foam group maintained better results than vehicle for both primary end points even at the end of the 4-week follow-up after EoT (Figures 2 and 3). Sensitivity analysis (data not shown) confirmed the findings from the full analysis set.
Safety
Adverse events were experienced by 149 (30.8%) participants in the AzA foam group and 119 (24.9%) in the vehicle group. The most common noncutaneous AEs (>1% of participants) reported during AzA foam treatment were nasopharyngitis, headache, upper respiratory tract infection, and influenza. In the vehicle group, the most common noncutaneous AEs reported were nasopharyngitis and headache. Drug-related AEs (relationship assessed by the investigator) were reported slightly more often in the AzA foam group (7.6%) than in the vehicle group (4.6%). Drug-related AEs were predominantly cutaneous and occurred at the site of application (Table 2). Drug-related cutaneous AEs were more common in the AzA foam group (7.0%) than in the vehicle group (4.4%). Although serious AEs were more common in the vehicle group, all were regarded as unrelated to the study medication. A single death occurred in the vehicle group due to an accident unrelated to the study drug.
The most frequent drug-related AEs in participants treated with AzA foam versus vehicle were application-site pain (3.5% vs 1.3%), application-site pruritus (1.4% vs 0.4%), and application-site dryness (1.0% vs 0.6%). The classical rosacea symptom of stinging is subsumed under the term application-site pain, according to MedDRA (Medical Dictionary for Regulatory Activities).
All other drug-related AEs occurred at a frequency of less than 1% in participants from both groups. Serious AEs were rare and unrelated to treatment, with 3 AEs reported in the AzA foam group and 4 in the vehicle group. Adverse events leading to study drug withdrawal occurred in less than 2% of participants and were more common in the vehicle group (2.5%) than in the AzA foam group (1.2%). Of the 3 drug-related AEs leading to withdrawal in the AzA foam group, 2 were due to cutaneous reaction and 1 was due to a burning sensation. The number of active drug-related cutaneous AEs was highest during the first 4 weeks of treatment and declined over the course of the study (eFigure).
More than 96% of AEs were resolved by the end of the study. Of the participants experiencing AEs that did not resolve during the course of the study, 16 were in the AzA foam group and 10 in the vehicle group. Six unresolved AEs were drug related, with 3 occurring in each treatment group. Unresolved drug-related cutaneous AEs in the AzA foam group were pain, pruritus, and dryness at the application site.
Comment
Overall, the results from this phase 3 trial demonstrate that the new foam formulation of AzA was efficacious and safe in a 12-week, twice-daily course of treatment for moderate to severe PPR. The AzA foam formulation was significantly superior to vehicle (P<.001) for both primary efficacy end points. Participants in the AzA foam group achieved therapeutic success at a higher rate than the vehicle group, and the change in nominal ILC at EoT was significantly greater for participants treated with AzA foam than for those treated with vehicle (P<.001). Differences between the 2 treatment groups for the coprimary end point measures arose early in the study, demonstrating that symptoms were rapidly controlled. Between weeks 8 and 12 (EoT), the rate of increase of beneficial effects in the AzA foam group remained high, while the vehicle group showed a notable slowing. There was no indication of any rebound effect in overall disease severity subsequent to EoT. After 4 weeks of follow-up, there was still a beneficial treatment effect present in favor of the AzA foam group, as indicated by the persistence of improvements in both coprimary end point measures throughout the follow-up period.
Analyses of alternative populations and secondary end points (data not shown) supported the efficacy results reported here. There was no indication of irregular study center effects, and the sensitivity analyses demonstrated robustness of the data for the observed treatment effects.
The use of vehicle foam alone appeared to be beneficial in reducing ILC and improving IGA rating, which suggests that the properties of the new foam formulation are favorable for the inflamed lesional skin of rosacea. Of note, other dermatology studies, including trials in rosacea, have reported therapeutic effects of vehicle treatment that may be attributable to the positive effects of skin care with certain formulations.20
Azelaic acid foam was well tolerated in the current study. More than 93% of AEs in either treatment group were of mild or moderate severity. The incidence of drug-related AEs was low in both groups and mainly occurred at the application site. There were no drug-related severe or serious AEs. The low incidence of reported drug-related noncutaneous AEs in the AzA foam group (dysgeusia in 1 patient and headache in 2 patients) supports the known favorable systemic tolerance profile of AzA.
Although most drug-related AEs occurred at the application site, they were generally transient, with the majority of events in the AzA foam group lasting no more than 1 hour. Most cutaneous AEs developed early in the study. In the AzA foam group, the prevalence of drug-related cutaneous AEs dropped at every time interval as the study progressed (eFigure). Very few AEs of any type persisted through the end of the study. These safety results were accompanied by a high compliance rate and a high participation rate throughout the course of the study. Taken together, the available data for this AzA foam formulation support a favorable tolerability profile. The results of this study are consistent with and expand on data from an earlier investigation of similar design.8
Conclusion
The development of an AzA foam formulation with higher lipid content was intended to expand the treatment options available to physicians and patients who are managing rosacea. Most topical dermatologic treatments are currently delivered in classical formulations such as creams or gels, but patients who use topical therapies have rated messiness and ease of application among the most important characteristics affecting quality of life.17,21 Foam formulations may offer improvements in this regard; ease of application may minimize unnecessary manipulation of inflamed skin and contribute to a high level of user satisfaction.22 However, the design of the current study was limited to evaluating only the AzA foam formulation versus a foam vehicle, and direct comparisons of clinical efficacy and tolerability to other AzA topical preparations were not performed. Nonetheless, patients have previously reported that they would be more likely to comply with a recommended course of dermatologic foam therapy than other topical formulations.18 The proposed foam formulation was designed to attend to the specific needs of the dry and sensitive skin in rosacea by combining the demonstrated efficacy properties exhibited by AzA gel 15% with the good tolerability and acceptability of a lipid-containing foam formulation. Development of this formulation was targeted to obtain a product that would be highly spreadable, dry quickly, and be easy to apply. The available data for this AzA foam formulation support the value of this option in the topical treatment of rosacea. The success in reduction of overall disease severity, lack of any rebound after EoT, and the observed tolerability and high adherence rates suggest that this novel formulation is a useful addition to current treatment options for rosacea.
Addendum
After release of the study data for unblinding and statistical evaluation, the following inconsistency regarding patient distribution was noted: 1 participant was incorrectly evaluated as part of the AzA foam analysis group when in fact this patient was randomized to vehicle and was treated throughout the study with vehicle. This participant did not experience any AE and did not show any IGA improvement at the EoT. As this single case did not have an impact on the statistical conclusions or interpretation of the results, the released study data have not been changed. This deviation was described as a database erratum in the study report.
Acknowledgement—Editorial support through inVentiv Medical Communications, New York, New York, was provided by Bayer HealthCare Pharmaceuticals Inc.
APPENDIX
Supplementary Methods
Supplementary Study Design
This study met all local legal and regulatory requirements and was conducted according to the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. Before the start of the study and implementation, the protocol and all amendments were approved by the appropriate independent ethics committee or institutional review board at each study site. Two protocol amendments were implemented before the first participant visit.
Exclusion criteria included the presence of dermatoses that could interfere with rosacea diagnosis or evaluation, facial laser surgery or topical use of any medication to treat rosacea within 6 weeks before randomization, systemic use of any medications to treat rosacea, and known unresponsiveness to AzA treatment. Further standard exclusion criteria included alcohol or drug use or parallel participation in other clinical studies, which were necessary to exclude undue influence on study evaluations and/or participant safety. The study was conducted by qualified investigators at 48 centers in the United States.
The investigational product was filled in identical containers according to the randomization list generated by a computer program using blocks. Complete blocks of study medication were distributed to the centers. Eligible participants were randomized 1:1 into either AzA foam or vehicle treatment groups by assignment of a randomization number at baseline. A blind investigational product under the same randomization number was dispensed to and returned from participants by study personnel who were not involved in the assessments. Blinding was achieved by using labels on the investigational products that did not allow identification of the true medication.
Compliance was evaluated from participant diaries as well as the number of expected doses and actually applied doses.
Additional Efficacy Evaluations
A number of secondary variables (not reported here) were assessed, including changes in other manifestations of PPR, as well as participant assessments of treatment response, tolerability, cosmetic preferences, and quality of life.
Additional Safety
Investigators reported a yes or no response as to whether there was a reasonable causal relationship between AEs and treatment. Moreover, AEs that began at the start of or during treatment were considered treatment emergent. Cutaneous AEs were further assessed regarding location and duration. An AE was deemed local if it occurred at the application site and transient if it subsided within 60 minutes of onset.
Statistical Analysis
The primary efficacy analyses presented here were based on the full analysis set of participants who were randomized and had medication dispensed. For participants with no EoT value, the last nonmissing value was used including baseline (last-observation-carried-forward methodology). Participants who discontinued treatment prematurely because of lack of efficacy were considered to be treatment failures, regardless of the reported IGA score. Statistical significance was needed for both coprimary efficacy variables at a 1-sided 2.5% significance level to show confirmed superiority of AzA foam versus vehicle.
A number of sensitivity analyses were performed, including an analysis of the coprimary end points using observed data, analysis of the per-protocol population of participants who did not prematurely discontinue treatment and had no major protocol deviations, subgroup analyses, and the use of statistical methods to investigate the effect of missing observations. Analyses of success rate and nominal change in ILC were repeated for each postbaseline visit using χ² and t tests, respectively. All summary and statistical analyses were performed according to the study protocol (unchanged after the start of the study) using SAS version 9.2.
Results from a prior study provided the basis for the sample size, which was calculated to show a significant difference in both primary efficacy end points with a power of 90%.8 To allow for dropouts, 480 participants in each treatment group were to be randomized for a total of 960 participants.
Rosacea is a common dermatologic disorder that generally is characterized by erythema as well as papules and pustules on the cheeks, chin, forehead, and nose. Moreover, telangiectasia and burning or stinging sensations often occur.1,2 These clinical manifestations and other related ones frequently lead to the perception of “sensitive skin.” Rosacea patients often experience low self-esteem, anxiety, and social embarrassment.3 Reports of the gender distribution of the disease vary but often show female predominance.4 Although it also occurs in darker skin types, rosacea is more common in individuals with lighter skin.1
The etiology of rosacea is not yet fully understood, but the underlying pathology has been attributed to dysregulated immune responses. Although the flares of a typical fluctuating disease course often are caused by exogenous triggers, there is evidence that an underlying genetic component predisposes some individuals to pathologic changes associated with the condition.5 Augmented immune activity and proinflammatory signaling appear to induce the infiltration of inflammatory elements into affected areas.2 These regions show dilated vasculature and increased cutaneous blood flow secondary to inflammation. Systemic oxidative stress also may contribute to epidermal dysfunction, as the antioxidant capacity of the skin in patients with rosacea is depleted relative to that of healthy individuals. The biochemical and vascular changes characteristic of rosacea coincide with aberrant permeability of the stratum corneum.6 The resulting decreased hydration and water loss across the skin contribute to the sensitivity and irritation typical of the disease.2
Current guidelines for the optimal management of rosacea with papulopustular lesions recommend skin care, photoprotection, and topical therapy. Depending on the severity of disease and the likelihood of adherence to a topical regimen, use of oral agents may be warranted.7
Azelaic acid (AzA), an unbranched saturated dicarboxylic acid (1,7-heptanedicarboxylic acid) that occurs in plants, is one of several US Food and Drug Administration–approved topical agents for the treatment of inflammatory lesions in rosacea.8 Although the pathophysiology of rosacea is not yet fully understood, there is a growing consensus about the role of proinflammatory molecules (eg, kallikrein 5, cathelicidins) as well as reactive oxygen species (ROS).9 Azelaic acid has been demonstrated to modulate the inflammatory response in normal human keratinocytes through several pathways, including modulation of the signaling pathways of peroxisome proliferator-activated receptor g and nuclear factor kB, concurrent with the observed inhibition of proinflammatory cytokine secretion.10 Additionally, AzA can inhibit the release of ROS from neutrophils and also may reduce ROS by direct scavenging effects.11 Further, AzA shows direct inhibition of kallikrein 5 in human keratinocytes as well as a reduction of the expression of kallikrein 5 and cathelicidin in murine skin and the facial skin of patients with rosacea.12
In a series of randomized trials in patients with papulopustular rosacea (PPR), AzA has shown clinical efficacy and safety as a topical treatment.13-15 Based on these studies, a gel formulation of AzA with a 15% concentration has been approved for treating inflammatory papules and pustules of mild to moderate rosacea.16
Although AzA delivered in a gel matrix is an effective therapy, topical delivery of active pharmaceutical ingredients via foam is often preferred over traditional vehicles in patients with sensitive skin. Patient rationale for favoring foam includes improved appearance and ease of application, namely easier to spread with a reduced need to manipulate inflamed skin.17 Also, data reveal that patients may be more compliant with a treatment that meets their needs such as an optimized foam formulation.18 In addition, the lipid components of an optimized formulation are thought to contribute to an improved skin condition.19 The foam vehicle used in this study is a proprietary oil-in-water formulation that includes fatty alcohols and triglycerides. The novel delivery of AzA in a foam formulation will provide clinicians and patients with a new option for improved individualized care.
We report the primary results of a phase 3 study in patients with PPR comparing the efficacy and safety of twice-daily AzA foam 15% with vehicle foam. The phase 3 study builds on the results of a prior randomized double-blind trial (N=401) that demonstrated significant improvements relative to vehicle in therapeutic success rate (P=.017) and decreased inflammatory lesion count (ILC)(P<.001) among patients treated with AzA foam 15%.8
Methods
Study Design
This phase 3 randomized, double-blind, vehicle-controlled, parallel-group, multicenter study was conducted in patients with PPR according to Good Clinical Practice guidelines in 48 study centers in the United States. The objective was to evaluate a 12-week, twice-daily (morning and evening) course of AzA foam 15% versus vehicle.
Participants were men and women aged 18 years or older with moderate to severe PPR (as determined by investigator global assessment [IGA]) presenting with 12 to 50 papules and/or pustules and persistent erythema with or without telangiectasia. Informed consent was obtained from all participants before any study-related activities were carried out.
The study products were applied to the entire facial area each morning and evening at a dose of 0.5 g, thus administering 150 mg of AzA daily in the active arm of the trial (computerized randomization 1:1). The treatment period lasted 12 weeks, and participants were evaluated at baseline and weeks 4, 8, and 12. The follow-up period lasted 4 weeks following the end of treatment (EoT) and was concluded with one final end-of-study visit.
Efficacy Evaluations
There were 2 coprimary efficacy end points. Therapeutic success rate was evaluated using the IGA scale (clear, minimal, mild, moderate, or severe). Treatment success was defined as an IGA score of either clear or minimal (with at least a 2-step improvement) at EoT, whereas treatment failure was constituted by IGA scores of mild, moderate, or severe.
The second coprimary end point was the nominal change in ILC from baseline to EoT as determined by the total number of facial papules and pustules. Efficacy and safety parameters were evaluated at weeks 4, 8, and 12, as well as at the end of the 4-week follow-up period. Throughout the study, the investigator, participants, and all study personnel remained blinded.
Safety
Information about adverse events (AEs) was collected at each study visit, and AEs were graded according to seriousness (yes or no) and intensity (mild, moderate, or severe).
Statistical Analysis
Efficacy was confirmed by analysis of the treatment success rate at EoT with Cochran-Mantel-Haenszel test statistics, including a point estimate and 95% confidence interval (CI) for the odds ratio. Change in ILC at EoT was analyzed via an analysis of covariance model using treatment, center, and baseline lesion count as factors. (Additional methods can be found in the Appendix below.)
Results
Study Participants
Of the 1156 patients who were screened for eligibility, 961 were randomized to treatment with AzA foam (n=484) or vehicle (n=477)(Figure 1). Sixty-four (13.2%) participants in the AzA foam group and 79 (16.6%) in the vehicle group discontinued treatment before completing the study. The most common reasons for discontinuation were participant withdrawal from the study and lost to follow-up. Six (1.2%) participants from the AzA foam group and 12 (2.5%) from the vehicle group discontinued because of AEs. All safety and efficacy data presented are based on the full analysis set, which consisted of the 961 participants randomized to treatment.
Demographic and baseline characteristics were balanced between the treatment groups (Table 1). The majority of participants were female (73.0%) and white (95.5%), reflecting the patient populations of independent studies that found a higher prevalence of rosacea in women and lighter skin types.4 There were no significant differences in baseline measures of PPR severity between the treatment groups. Participants in the AzA foam and vehicle groups had a mean ILC of 21.7 and 21.2, respectively, and 76.4% of participants had more than 14 lesions. All participants had an IGA score of moderate (86.8%) or severe (13.2%). Moderate or severe erythema was present in 91.5% of participants.
Treatment compliance, as measured by the percentage of expected doses that were actually administered, was 97.1% in the AzA foam group and 95.9% in the vehicle group.
Efficacy
Results from both primary end points demonstrated superior efficacy of AzA foam over vehicle. The AzA foam group achieved a greater IGA success rate at EoT compared with the vehicle group (32.0% vs 23.5%; Cochran-Mantel-Haenszel test center-adjusted P<.001; odds ratio, 1.6; 95% CI, 1.2-2.2). Treatment success rate was higher in the AzA foam group than in the vehicle group at every time point past baseline (Figure 2). Similarly, the decrease in mean nominal ILC values was greater in the AzA foam group at every time point after baseline (Figure 3), and the treatment difference at EoT was statistically significant in favor of AzA foam (-2.7, F1,920=23.7, P<.001; 95% CI, -3.8 to -1.6). The divergence between treatment groups at week 4 reveals an onset of AzA effect early in the study.

|

|
Although the AzA foam group showed significantly better efficacy results than the vehicle group for the coprimary end points, participants in the vehicle group did show appreciable IGA success rates (23.5%) and changes in ILC (-10.3) at EoT (Figures 2 and 3).
Notably, the AzA foam group maintained better results than vehicle for both primary end points even at the end of the 4-week follow-up after EoT (Figures 2 and 3). Sensitivity analysis (data not shown) confirmed the findings from the full analysis set.
Safety
Adverse events were experienced by 149 (30.8%) participants in the AzA foam group and 119 (24.9%) in the vehicle group. The most common noncutaneous AEs (>1% of participants) reported during AzA foam treatment were nasopharyngitis, headache, upper respiratory tract infection, and influenza. In the vehicle group, the most common noncutaneous AEs reported were nasopharyngitis and headache. Drug-related AEs (relationship assessed by the investigator) were reported slightly more often in the AzA foam group (7.6%) than in the vehicle group (4.6%). Drug-related AEs were predominantly cutaneous and occurred at the site of application (Table 2). Drug-related cutaneous AEs were more common in the AzA foam group (7.0%) than in the vehicle group (4.4%). Although serious AEs were more common in the vehicle group, all were regarded as unrelated to the study medication. A single death occurred in the vehicle group due to an accident unrelated to the study drug.
The most frequent drug-related AEs in participants treated with AzA foam versus vehicle were application-site pain (3.5% vs 1.3%), application-site pruritus (1.4% vs 0.4%), and application-site dryness (1.0% vs 0.6%). The classical rosacea symptom of stinging is subsumed under the term application-site pain, according to MedDRA (Medical Dictionary for Regulatory Activities).
All other drug-related AEs occurred at a frequency of less than 1% in participants from both groups. Serious AEs were rare and unrelated to treatment, with 3 AEs reported in the AzA foam group and 4 in the vehicle group. Adverse events leading to study drug withdrawal occurred in less than 2% of participants and were more common in the vehicle group (2.5%) than in the AzA foam group (1.2%). Of the 3 drug-related AEs leading to withdrawal in the AzA foam group, 2 were due to cutaneous reaction and 1 was due to a burning sensation. The number of active drug-related cutaneous AEs was highest during the first 4 weeks of treatment and declined over the course of the study (eFigure).
More than 96% of AEs were resolved by the end of the study. Of the participants experiencing AEs that did not resolve during the course of the study, 16 were in the AzA foam group and 10 in the vehicle group. Six unresolved AEs were drug related, with 3 occurring in each treatment group. Unresolved drug-related cutaneous AEs in the AzA foam group were pain, pruritus, and dryness at the application site.
Comment
Overall, the results from this phase 3 trial demonstrate that the new foam formulation of AzA was efficacious and safe in a 12-week, twice-daily course of treatment for moderate to severe PPR. The AzA foam formulation was significantly superior to vehicle (P<.001) for both primary efficacy end points. Participants in the AzA foam group achieved therapeutic success at a higher rate than the vehicle group, and the change in nominal ILC at EoT was significantly greater for participants treated with AzA foam than for those treated with vehicle (P<.001). Differences between the 2 treatment groups for the coprimary end point measures arose early in the study, demonstrating that symptoms were rapidly controlled. Between weeks 8 and 12 (EoT), the rate of increase of beneficial effects in the AzA foam group remained high, while the vehicle group showed a notable slowing. There was no indication of any rebound effect in overall disease severity subsequent to EoT. After 4 weeks of follow-up, there was still a beneficial treatment effect present in favor of the AzA foam group, as indicated by the persistence of improvements in both coprimary end point measures throughout the follow-up period.
Analyses of alternative populations and secondary end points (data not shown) supported the efficacy results reported here. There was no indication of irregular study center effects, and the sensitivity analyses demonstrated robustness of the data for the observed treatment effects.
The use of vehicle foam alone appeared to be beneficial in reducing ILC and improving IGA rating, which suggests that the properties of the new foam formulation are favorable for the inflamed lesional skin of rosacea. Of note, other dermatology studies, including trials in rosacea, have reported therapeutic effects of vehicle treatment that may be attributable to the positive effects of skin care with certain formulations.20
Azelaic acid foam was well tolerated in the current study. More than 93% of AEs in either treatment group were of mild or moderate severity. The incidence of drug-related AEs was low in both groups and mainly occurred at the application site. There were no drug-related severe or serious AEs. The low incidence of reported drug-related noncutaneous AEs in the AzA foam group (dysgeusia in 1 patient and headache in 2 patients) supports the known favorable systemic tolerance profile of AzA.
Although most drug-related AEs occurred at the application site, they were generally transient, with the majority of events in the AzA foam group lasting no more than 1 hour. Most cutaneous AEs developed early in the study. In the AzA foam group, the prevalence of drug-related cutaneous AEs dropped at every time interval as the study progressed (eFigure). Very few AEs of any type persisted through the end of the study. These safety results were accompanied by a high compliance rate and a high participation rate throughout the course of the study. Taken together, the available data for this AzA foam formulation support a favorable tolerability profile. The results of this study are consistent with and expand on data from an earlier investigation of similar design.8
Conclusion
The development of an AzA foam formulation with higher lipid content was intended to expand the treatment options available to physicians and patients who are managing rosacea. Most topical dermatologic treatments are currently delivered in classical formulations such as creams or gels, but patients who use topical therapies have rated messiness and ease of application among the most important characteristics affecting quality of life.17,21 Foam formulations may offer improvements in this regard; ease of application may minimize unnecessary manipulation of inflamed skin and contribute to a high level of user satisfaction.22 However, the design of the current study was limited to evaluating only the AzA foam formulation versus a foam vehicle, and direct comparisons of clinical efficacy and tolerability to other AzA topical preparations were not performed. Nonetheless, patients have previously reported that they would be more likely to comply with a recommended course of dermatologic foam therapy than other topical formulations.18 The proposed foam formulation was designed to attend to the specific needs of the dry and sensitive skin in rosacea by combining the demonstrated efficacy properties exhibited by AzA gel 15% with the good tolerability and acceptability of a lipid-containing foam formulation. Development of this formulation was targeted to obtain a product that would be highly spreadable, dry quickly, and be easy to apply. The available data for this AzA foam formulation support the value of this option in the topical treatment of rosacea. The success in reduction of overall disease severity, lack of any rebound after EoT, and the observed tolerability and high adherence rates suggest that this novel formulation is a useful addition to current treatment options for rosacea.
Addendum
After release of the study data for unblinding and statistical evaluation, the following inconsistency regarding patient distribution was noted: 1 participant was incorrectly evaluated as part of the AzA foam analysis group when in fact this patient was randomized to vehicle and was treated throughout the study with vehicle. This participant did not experience any AE and did not show any IGA improvement at the EoT. As this single case did not have an impact on the statistical conclusions or interpretation of the results, the released study data have not been changed. This deviation was described as a database erratum in the study report.
Acknowledgement—Editorial support through inVentiv Medical Communications, New York, New York, was provided by Bayer HealthCare Pharmaceuticals Inc.
APPENDIX
Supplementary Methods
Supplementary Study Design
This study met all local legal and regulatory requirements and was conducted according to the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. Before the start of the study and implementation, the protocol and all amendments were approved by the appropriate independent ethics committee or institutional review board at each study site. Two protocol amendments were implemented before the first participant visit.
Exclusion criteria included the presence of dermatoses that could interfere with rosacea diagnosis or evaluation, facial laser surgery or topical use of any medication to treat rosacea within 6 weeks before randomization, systemic use of any medications to treat rosacea, and known unresponsiveness to AzA treatment. Further standard exclusion criteria included alcohol or drug use or parallel participation in other clinical studies, which were necessary to exclude undue influence on study evaluations and/or participant safety. The study was conducted by qualified investigators at 48 centers in the United States.
The investigational product was filled in identical containers according to the randomization list generated by a computer program using blocks. Complete blocks of study medication were distributed to the centers. Eligible participants were randomized 1:1 into either AzA foam or vehicle treatment groups by assignment of a randomization number at baseline. A blind investigational product under the same randomization number was dispensed to and returned from participants by study personnel who were not involved in the assessments. Blinding was achieved by using labels on the investigational products that did not allow identification of the true medication.
Compliance was evaluated from participant diaries as well as the number of expected doses and actually applied doses.
Additional Efficacy Evaluations
A number of secondary variables (not reported here) were assessed, including changes in other manifestations of PPR, as well as participant assessments of treatment response, tolerability, cosmetic preferences, and quality of life.
Additional Safety
Investigators reported a yes or no response as to whether there was a reasonable causal relationship between AEs and treatment. Moreover, AEs that began at the start of or during treatment were considered treatment emergent. Cutaneous AEs were further assessed regarding location and duration. An AE was deemed local if it occurred at the application site and transient if it subsided within 60 minutes of onset.
Statistical Analysis
The primary efficacy analyses presented here were based on the full analysis set of participants who were randomized and had medication dispensed. For participants with no EoT value, the last nonmissing value was used including baseline (last-observation-carried-forward methodology). Participants who discontinued treatment prematurely because of lack of efficacy were considered to be treatment failures, regardless of the reported IGA score. Statistical significance was needed for both coprimary efficacy variables at a 1-sided 2.5% significance level to show confirmed superiority of AzA foam versus vehicle.
A number of sensitivity analyses were performed, including an analysis of the coprimary end points using observed data, analysis of the per-protocol population of participants who did not prematurely discontinue treatment and had no major protocol deviations, subgroup analyses, and the use of statistical methods to investigate the effect of missing observations. Analyses of success rate and nominal change in ILC were repeated for each postbaseline visit using χ² and t tests, respectively. All summary and statistical analyses were performed according to the study protocol (unchanged after the start of the study) using SAS version 9.2.
Results from a prior study provided the basis for the sample size, which was calculated to show a significant difference in both primary efficacy end points with a power of 90%.8 To allow for dropouts, 480 participants in each treatment group were to be randomized for a total of 960 participants.
1. Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
2. Del Rosso JQ. Advances in understanding and managing rosacea: part 1: connecting the dots between pathophysiological mechanisms and common clinical features of rosacea with emphasis on vascular changes and facial erythema. J Clin Aesthet Dermatol. 2012;5:16-25.
3. Huynh TT. Burden of disease: the psychosocial impact of rosacea on a patient’s quality of life. Am Health Drug Benefits. 2013;6:348-354.
4. Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6 suppl 1):S27-S35.
5. Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 suppl 1):S15-S26.
6. Wollina U. Recent advances in the understanding and management of rosacea. F1000Prime Rep. 2014;6:50.
7. Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 5: a guide on the management of rosacea. Cutis. 2014;93:134-138.
8. Draelos ZD, Elewski B, Staedtler G, et al. Azelaic acid foam 15% in the treatment of papulopustular rosacea: a randomized, double-blind, vehicle-controlled study. Cutis. 2013;92:306-317.
9. Yamasaki K, Di Nardo A, Bardan A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975-980.
10. Mastrofrancesco A, Ottaviani M, Aspite N, et al. Azelaic acid modulates the inflammatory response in normal human keratinocytes through PPARg activation. Exp Dermatol. 2010;19:813-820.
11. Akamatsu H, Komura J, Asada Y, et al. Inhibitory effect of azelaic acid on neutrophil functions: a possible cause for its efficacy in treating pathogenetically unrelated diseases. Arch Dermatol Res. 1991;283:162-166.
12. Coda AB, Hata T, Miller J, et al. Cathelicidin, kalli-krein 5, and serine protease activity is inhibited during treatment of rosacea with azelaic acid 15% gel. J Am Acad Dermatol. 2013;69:570-577.
13. van Zuuren EJ, Kramer SF, Carter BR, et al. Effective and evidence-based management strategies for rosacea: summary of a Cochrane systematic review. Br J Dermatol. 2011;165:760-781.
14. Thiboutot D, Thieroff-Ekerdt R, Graupe K. Efficacy and safety of azelaic acid (15%) gel as a new treatment for papulopustular rosacea: results from two vehicle-controlled, randomized phase III studies. J Am Acad Dermatol. 2003;48:836-845.
15. Thiboutot DM, Fleischer AB Jr, Del Rosso JQ, et al. Azelaic acid 15% gel once daily versus twice daily in papulopustular rosacea. J Drugs Dermatol. 2008;7:541-546.
16. Finacea [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; 2015.
17. Zhao Y, Jones SA, Brown MB. Dynamic foams in topical drug delivery. J Pharm Pharmacol. 2010;62:678-684.
18. Gottlieb AB, Ford RO, Spellman MC. The efficacy and tolerability of clobetasol propionate foam 0.05% in the treatment of mild to moderate plaque-type psoriasis of nonscalp regions. J Cutan Med Surg. 2003;7:185-192.
19. Loden M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am J Clin Dermatol. 2003;4:771-788.
20. Jackson JM, Pelle M. Topical rosacea therapy: the importance of vehicles for efficacy, tolerability and compliance. J Drugs Dermatol. 2011;10:627-633.
21. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
22. Kircik LH, Bikowski JB. Vehicles matter: topical foam formulations. Practical Dermatology. January 2012(suppl):3-18.
1. Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
2. Del Rosso JQ. Advances in understanding and managing rosacea: part 1: connecting the dots between pathophysiological mechanisms and common clinical features of rosacea with emphasis on vascular changes and facial erythema. J Clin Aesthet Dermatol. 2012;5:16-25.
3. Huynh TT. Burden of disease: the psychosocial impact of rosacea on a patient’s quality of life. Am Health Drug Benefits. 2013;6:348-354.
4. Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6 suppl 1):S27-S35.
5. Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 suppl 1):S15-S26.
6. Wollina U. Recent advances in the understanding and management of rosacea. F1000Prime Rep. 2014;6:50.
7. Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 5: a guide on the management of rosacea. Cutis. 2014;93:134-138.
8. Draelos ZD, Elewski B, Staedtler G, et al. Azelaic acid foam 15% in the treatment of papulopustular rosacea: a randomized, double-blind, vehicle-controlled study. Cutis. 2013;92:306-317.
9. Yamasaki K, Di Nardo A, Bardan A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975-980.
10. Mastrofrancesco A, Ottaviani M, Aspite N, et al. Azelaic acid modulates the inflammatory response in normal human keratinocytes through PPARg activation. Exp Dermatol. 2010;19:813-820.
11. Akamatsu H, Komura J, Asada Y, et al. Inhibitory effect of azelaic acid on neutrophil functions: a possible cause for its efficacy in treating pathogenetically unrelated diseases. Arch Dermatol Res. 1991;283:162-166.
12. Coda AB, Hata T, Miller J, et al. Cathelicidin, kalli-krein 5, and serine protease activity is inhibited during treatment of rosacea with azelaic acid 15% gel. J Am Acad Dermatol. 2013;69:570-577.
13. van Zuuren EJ, Kramer SF, Carter BR, et al. Effective and evidence-based management strategies for rosacea: summary of a Cochrane systematic review. Br J Dermatol. 2011;165:760-781.
14. Thiboutot D, Thieroff-Ekerdt R, Graupe K. Efficacy and safety of azelaic acid (15%) gel as a new treatment for papulopustular rosacea: results from two vehicle-controlled, randomized phase III studies. J Am Acad Dermatol. 2003;48:836-845.
15. Thiboutot DM, Fleischer AB Jr, Del Rosso JQ, et al. Azelaic acid 15% gel once daily versus twice daily in papulopustular rosacea. J Drugs Dermatol. 2008;7:541-546.
16. Finacea [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; 2015.
17. Zhao Y, Jones SA, Brown MB. Dynamic foams in topical drug delivery. J Pharm Pharmacol. 2010;62:678-684.
18. Gottlieb AB, Ford RO, Spellman MC. The efficacy and tolerability of clobetasol propionate foam 0.05% in the treatment of mild to moderate plaque-type psoriasis of nonscalp regions. J Cutan Med Surg. 2003;7:185-192.
19. Loden M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am J Clin Dermatol. 2003;4:771-788.
20. Jackson JM, Pelle M. Topical rosacea therapy: the importance of vehicles for efficacy, tolerability and compliance. J Drugs Dermatol. 2011;10:627-633.
21. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
22. Kircik LH, Bikowski JB. Vehicles matter: topical foam formulations. Practical Dermatology. January 2012(suppl):3-18.