User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
‘DIY’ artificial pancreas systems found to be safe, effective: CREATE trial
NEW ORLEANS – Open-source automated insulin delivery systems appear to be both effective and safe in adults and children, new research finds.
Automated insulin delivery (AID) system, also known as closed-loop systems or an artificial pancreas, link an insulin pump and a continuous glucose monitor (CGM) with an algorithm that automatically adjusts insulin delivery to optimize glycemic control.
Prior to the availability of commercial AID systems, Dana Lewis, a patient with type 1 diabetes, and her partner codeveloped an algorithm that could link older versions of an insulin pump and CGM.
In 2015, they made the code and all related materials open-source, so that anyone who wanted to create their own AID system could do so. Today thousands of people worldwide with type 1 diabetes are using the systems, which are sometimes called “do-it-yourself (DIY)” AID systems although the approach has been community based.
AID systems are not approved by any regulatory body, and despite several nonrandomized studies demonstrating their effectiveness and safety, there is still concern among some health professionals about their safety. In 2019, the U.S. Food and Drug Administration warned against the use of any nonapproved devices or algorithms. (Now, though, at least one open-source AID system algorithm is under FDA review.)
Aimed at addressing those concerns, CREATE (Community Derived Automated Insulin Delivery) is the first randomized controlled clinical trial to compare an open-source AID system to insulin pump therapy and CGM (without any communication between the two) in patients with type 1 diabetes, most of whom were naive to AID systems.
Doctors uncomfortable with open source; study provides reassurance
The findings were presented at the American Diabetes Association scientific sessions by Martin I. de Bock, PhD, a pediatric endocrinologist and senior lecturer at the University of Otago, Christchurch, New Zealand.
The study compared the most commonly used open-source AID system (using the OpenAPS algorithm from a version of AndroidAPS implemented in a smartphone with the DANA-i insulin pump and Dexcom G6 CGM) to any insulin pump plus CGM as a comparator group.
The open-source AID system led to a significant reduction in hemoglobin A1c with no major safety issues.
“The acceptance [among clinicians] of open-source systems is diverse and complicated, [with varying] personal comfort levels of seeing someone using an AID system that has no regulatory approval,” Dr. de Bock told this news organization.
“This is one of the reasons that it was so important to conduct the CREATE trial for the many thousands of open-source AID users. Given that the trial demonstrated safety and efficacy using the most robust scientific methodology available – a long-term randomized controlled trial – it may go some way to provide assurance for providers when they are seeing people using an open-source automated system,” he said.
Asked for comment, session moderator Diana Isaacs, PharmD, CDCES, an endocrine clinical pharmacist at the Cleveland Clinic, told this news organization: “There has been concern that these systems aren’t safe, so showing the safety is important. I think people deserve choice. As long as they’re safe, patients should be able to use what they want to use, and we should support them.”
Dr. Isaacs pointed out that an advantage of open-source systems over current commercial AIDs for patients is the ability to customize glucose targets, but in CREATE, those targets were established in the protocol by the investigators.
“I think it’s nice having the data, although in the trial they had specific requirements. They had a target range and active insulin time that they were recommending. So it’s a little different than true DIY where you don’t really have those guidelines you have to follow. It is exciting, it’s very interesting, but I wouldn’t say it’s a true mirror of the real world.”
Open-source systems improved time-in-range, no safety issues
For the CREATE study, 100 participants were enrolled, including 50 children aged 7-15 years and 50 adults aged 16-70 years. All participants had been using insulin pumps for at least 6 months. Most of the children and about two-thirds of the adults were also using CGMs, but just 6% of the children and 18% of the adults had prior experience with AID systems.
Baseline A1c in children was 7.5% and in adults was 7.7%.
After a 4-week run-in, all patients were randomized to the open-source AID or insulin pump plus CGM for 6 months.
The final group analyzed consisted of 42 patients in the open-source AID group and 53 patients in the comparator group.
The primary outcome, the adjusted mean difference in percent time-in-range (glucose of 70-180 mg/dL) during the final 2 weeks of the 6-month trial, showed a significant difference of 14% (P < .001) with open-source AID compared with pump plus CGM only.
Time-in-range in the open-source AID group rose from 61.2% to 71.2%, while it actually dropped slightly in the comparator group, from 57.7% to 54.5%.
The proportion of patients achieving time-in-range greater than 70% with open-source AID was 60% versus just 15% with pump plus CGM.
Glycemic improvements with open-source AID were significant for adults and children and were greater for those with higher baseline A1c levels. The effect was immediate and sustained throughout the study period, “which is super-pleasing, because there was a worry that the technical burden of open source might be [leading to] dropout, but we didn’t see that. It was sustained right through to the end of the trial,” Dr. de Bock commented.
Hypoglycemic rates didn’t differ between groups, and there were no episodes of severe hypoglycemia or diabetic ketoacidosis.
No more waiting: What is the future of open-source AID?
When the open-source APS was first developed, users coined the motto: “We are not waiting.” But now that the “wait” is over and several commercial AIDs have been approved by regulatory bodies, with others still in the pipeline, will people still use open-source systems?
There are no current data on people moving from DIY to commercial systems. However, Dr. de Bock said, “For most who undertook an open-source option, the precision of the settings that they can use and enjoy would mean that most would likely stick to their open source.”
Dr. Isaacs agrees: “I actually don’t think it’s going to go away in the near future, because the FDA has very specific criteria for where these [formally approved] devices can be in terms of their target ranges and requirements versus with open source you can really customize. So I still think there’s going to be a subset of people who want that customization, who want the lower targets.”
Dana Lewis, the originator of the DIY system and a CREATE coauthor, told this news organization: “I don’t believe there has been a fall-off, and in fact, I think open-source AID has continued to have ongoing uptake as awareness increases about options and as more pumps and CGMs become interoperable with various open-source AID choices.”
“I think uptake increasing is also influenced by the fact that in places like Europe, Asia, and Australia there are in-warranty on-the-market pumps that are compatible and interoperable with open-source AID. I think awareness of AID overall increases uptake of commercial and open source alike,” she said.
“Clinicians, as emphasized in recent position statements, must maintain support of the person with diabetes, irrespective of the mode of treatment they are on. ... Health care providers should be encouraged to learn from the experiences of the people who have stuck with open-source AID or switched, so that they can inform themselves of the relative strengths and benefits of each system,” Dr. de Bock advised.
Ms. Lewis noted: “We are seeing increasing awareness and comfort in endocrinologists from the community perspective, and we do hope that this study helps increase conversation and awareness of the safety and efficacy of open-source AID systems as an option for people with diabetes.”
In fact, the team published an article specifically about clinicians’ experience in CREATE. “The learning curve is similar across AID technology,” she observed.
Findings of a 6-month continuation phase of CREATE, in which all participants used the open-source AID, are scheduled to be presented in September at the European Association for the Study of Diabetes annual meeting.
The study was funded by the Health Research Council of New Zealand, with hardware support from SOOIL Developments, South Korea; Dexcom; and Vodafone New Zealand. Dr. de Bock has reported receiving honoraria and/or research funding from Novo Nordisk, Sanofi, Pfizer, Medtronic, Lilly, Ypsomed, and Dexcom. Dr. Isaacs has reported serving as a consultant for LifeScan, Lilly, and Insulet, and as a speaker for Dexcom, Medtronic, Abbott, and Novo Nordisk. Ms. Lewis has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – Open-source automated insulin delivery systems appear to be both effective and safe in adults and children, new research finds.
Automated insulin delivery (AID) system, also known as closed-loop systems or an artificial pancreas, link an insulin pump and a continuous glucose monitor (CGM) with an algorithm that automatically adjusts insulin delivery to optimize glycemic control.
Prior to the availability of commercial AID systems, Dana Lewis, a patient with type 1 diabetes, and her partner codeveloped an algorithm that could link older versions of an insulin pump and CGM.
In 2015, they made the code and all related materials open-source, so that anyone who wanted to create their own AID system could do so. Today thousands of people worldwide with type 1 diabetes are using the systems, which are sometimes called “do-it-yourself (DIY)” AID systems although the approach has been community based.
AID systems are not approved by any regulatory body, and despite several nonrandomized studies demonstrating their effectiveness and safety, there is still concern among some health professionals about their safety. In 2019, the U.S. Food and Drug Administration warned against the use of any nonapproved devices or algorithms. (Now, though, at least one open-source AID system algorithm is under FDA review.)
Aimed at addressing those concerns, CREATE (Community Derived Automated Insulin Delivery) is the first randomized controlled clinical trial to compare an open-source AID system to insulin pump therapy and CGM (without any communication between the two) in patients with type 1 diabetes, most of whom were naive to AID systems.
Doctors uncomfortable with open source; study provides reassurance
The findings were presented at the American Diabetes Association scientific sessions by Martin I. de Bock, PhD, a pediatric endocrinologist and senior lecturer at the University of Otago, Christchurch, New Zealand.
The study compared the most commonly used open-source AID system (using the OpenAPS algorithm from a version of AndroidAPS implemented in a smartphone with the DANA-i insulin pump and Dexcom G6 CGM) to any insulin pump plus CGM as a comparator group.
The open-source AID system led to a significant reduction in hemoglobin A1c with no major safety issues.
“The acceptance [among clinicians] of open-source systems is diverse and complicated, [with varying] personal comfort levels of seeing someone using an AID system that has no regulatory approval,” Dr. de Bock told this news organization.
“This is one of the reasons that it was so important to conduct the CREATE trial for the many thousands of open-source AID users. Given that the trial demonstrated safety and efficacy using the most robust scientific methodology available – a long-term randomized controlled trial – it may go some way to provide assurance for providers when they are seeing people using an open-source automated system,” he said.
Asked for comment, session moderator Diana Isaacs, PharmD, CDCES, an endocrine clinical pharmacist at the Cleveland Clinic, told this news organization: “There has been concern that these systems aren’t safe, so showing the safety is important. I think people deserve choice. As long as they’re safe, patients should be able to use what they want to use, and we should support them.”
Dr. Isaacs pointed out that an advantage of open-source systems over current commercial AIDs for patients is the ability to customize glucose targets, but in CREATE, those targets were established in the protocol by the investigators.
“I think it’s nice having the data, although in the trial they had specific requirements. They had a target range and active insulin time that they were recommending. So it’s a little different than true DIY where you don’t really have those guidelines you have to follow. It is exciting, it’s very interesting, but I wouldn’t say it’s a true mirror of the real world.”
Open-source systems improved time-in-range, no safety issues
For the CREATE study, 100 participants were enrolled, including 50 children aged 7-15 years and 50 adults aged 16-70 years. All participants had been using insulin pumps for at least 6 months. Most of the children and about two-thirds of the adults were also using CGMs, but just 6% of the children and 18% of the adults had prior experience with AID systems.
Baseline A1c in children was 7.5% and in adults was 7.7%.
After a 4-week run-in, all patients were randomized to the open-source AID or insulin pump plus CGM for 6 months.
The final group analyzed consisted of 42 patients in the open-source AID group and 53 patients in the comparator group.
The primary outcome, the adjusted mean difference in percent time-in-range (glucose of 70-180 mg/dL) during the final 2 weeks of the 6-month trial, showed a significant difference of 14% (P < .001) with open-source AID compared with pump plus CGM only.
Time-in-range in the open-source AID group rose from 61.2% to 71.2%, while it actually dropped slightly in the comparator group, from 57.7% to 54.5%.
The proportion of patients achieving time-in-range greater than 70% with open-source AID was 60% versus just 15% with pump plus CGM.
Glycemic improvements with open-source AID were significant for adults and children and were greater for those with higher baseline A1c levels. The effect was immediate and sustained throughout the study period, “which is super-pleasing, because there was a worry that the technical burden of open source might be [leading to] dropout, but we didn’t see that. It was sustained right through to the end of the trial,” Dr. de Bock commented.
Hypoglycemic rates didn’t differ between groups, and there were no episodes of severe hypoglycemia or diabetic ketoacidosis.
No more waiting: What is the future of open-source AID?
When the open-source APS was first developed, users coined the motto: “We are not waiting.” But now that the “wait” is over and several commercial AIDs have been approved by regulatory bodies, with others still in the pipeline, will people still use open-source systems?
There are no current data on people moving from DIY to commercial systems. However, Dr. de Bock said, “For most who undertook an open-source option, the precision of the settings that they can use and enjoy would mean that most would likely stick to their open source.”
Dr. Isaacs agrees: “I actually don’t think it’s going to go away in the near future, because the FDA has very specific criteria for where these [formally approved] devices can be in terms of their target ranges and requirements versus with open source you can really customize. So I still think there’s going to be a subset of people who want that customization, who want the lower targets.”
Dana Lewis, the originator of the DIY system and a CREATE coauthor, told this news organization: “I don’t believe there has been a fall-off, and in fact, I think open-source AID has continued to have ongoing uptake as awareness increases about options and as more pumps and CGMs become interoperable with various open-source AID choices.”
“I think uptake increasing is also influenced by the fact that in places like Europe, Asia, and Australia there are in-warranty on-the-market pumps that are compatible and interoperable with open-source AID. I think awareness of AID overall increases uptake of commercial and open source alike,” she said.
“Clinicians, as emphasized in recent position statements, must maintain support of the person with diabetes, irrespective of the mode of treatment they are on. ... Health care providers should be encouraged to learn from the experiences of the people who have stuck with open-source AID or switched, so that they can inform themselves of the relative strengths and benefits of each system,” Dr. de Bock advised.
Ms. Lewis noted: “We are seeing increasing awareness and comfort in endocrinologists from the community perspective, and we do hope that this study helps increase conversation and awareness of the safety and efficacy of open-source AID systems as an option for people with diabetes.”
In fact, the team published an article specifically about clinicians’ experience in CREATE. “The learning curve is similar across AID technology,” she observed.
Findings of a 6-month continuation phase of CREATE, in which all participants used the open-source AID, are scheduled to be presented in September at the European Association for the Study of Diabetes annual meeting.
The study was funded by the Health Research Council of New Zealand, with hardware support from SOOIL Developments, South Korea; Dexcom; and Vodafone New Zealand. Dr. de Bock has reported receiving honoraria and/or research funding from Novo Nordisk, Sanofi, Pfizer, Medtronic, Lilly, Ypsomed, and Dexcom. Dr. Isaacs has reported serving as a consultant for LifeScan, Lilly, and Insulet, and as a speaker for Dexcom, Medtronic, Abbott, and Novo Nordisk. Ms. Lewis has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – Open-source automated insulin delivery systems appear to be both effective and safe in adults and children, new research finds.
Automated insulin delivery (AID) system, also known as closed-loop systems or an artificial pancreas, link an insulin pump and a continuous glucose monitor (CGM) with an algorithm that automatically adjusts insulin delivery to optimize glycemic control.
Prior to the availability of commercial AID systems, Dana Lewis, a patient with type 1 diabetes, and her partner codeveloped an algorithm that could link older versions of an insulin pump and CGM.
In 2015, they made the code and all related materials open-source, so that anyone who wanted to create their own AID system could do so. Today thousands of people worldwide with type 1 diabetes are using the systems, which are sometimes called “do-it-yourself (DIY)” AID systems although the approach has been community based.
AID systems are not approved by any regulatory body, and despite several nonrandomized studies demonstrating their effectiveness and safety, there is still concern among some health professionals about their safety. In 2019, the U.S. Food and Drug Administration warned against the use of any nonapproved devices or algorithms. (Now, though, at least one open-source AID system algorithm is under FDA review.)
Aimed at addressing those concerns, CREATE (Community Derived Automated Insulin Delivery) is the first randomized controlled clinical trial to compare an open-source AID system to insulin pump therapy and CGM (without any communication between the two) in patients with type 1 diabetes, most of whom were naive to AID systems.
Doctors uncomfortable with open source; study provides reassurance
The findings were presented at the American Diabetes Association scientific sessions by Martin I. de Bock, PhD, a pediatric endocrinologist and senior lecturer at the University of Otago, Christchurch, New Zealand.
The study compared the most commonly used open-source AID system (using the OpenAPS algorithm from a version of AndroidAPS implemented in a smartphone with the DANA-i insulin pump and Dexcom G6 CGM) to any insulin pump plus CGM as a comparator group.
The open-source AID system led to a significant reduction in hemoglobin A1c with no major safety issues.
“The acceptance [among clinicians] of open-source systems is diverse and complicated, [with varying] personal comfort levels of seeing someone using an AID system that has no regulatory approval,” Dr. de Bock told this news organization.
“This is one of the reasons that it was so important to conduct the CREATE trial for the many thousands of open-source AID users. Given that the trial demonstrated safety and efficacy using the most robust scientific methodology available – a long-term randomized controlled trial – it may go some way to provide assurance for providers when they are seeing people using an open-source automated system,” he said.
Asked for comment, session moderator Diana Isaacs, PharmD, CDCES, an endocrine clinical pharmacist at the Cleveland Clinic, told this news organization: “There has been concern that these systems aren’t safe, so showing the safety is important. I think people deserve choice. As long as they’re safe, patients should be able to use what they want to use, and we should support them.”
Dr. Isaacs pointed out that an advantage of open-source systems over current commercial AIDs for patients is the ability to customize glucose targets, but in CREATE, those targets were established in the protocol by the investigators.
“I think it’s nice having the data, although in the trial they had specific requirements. They had a target range and active insulin time that they were recommending. So it’s a little different than true DIY where you don’t really have those guidelines you have to follow. It is exciting, it’s very interesting, but I wouldn’t say it’s a true mirror of the real world.”
Open-source systems improved time-in-range, no safety issues
For the CREATE study, 100 participants were enrolled, including 50 children aged 7-15 years and 50 adults aged 16-70 years. All participants had been using insulin pumps for at least 6 months. Most of the children and about two-thirds of the adults were also using CGMs, but just 6% of the children and 18% of the adults had prior experience with AID systems.
Baseline A1c in children was 7.5% and in adults was 7.7%.
After a 4-week run-in, all patients were randomized to the open-source AID or insulin pump plus CGM for 6 months.
The final group analyzed consisted of 42 patients in the open-source AID group and 53 patients in the comparator group.
The primary outcome, the adjusted mean difference in percent time-in-range (glucose of 70-180 mg/dL) during the final 2 weeks of the 6-month trial, showed a significant difference of 14% (P < .001) with open-source AID compared with pump plus CGM only.
Time-in-range in the open-source AID group rose from 61.2% to 71.2%, while it actually dropped slightly in the comparator group, from 57.7% to 54.5%.
The proportion of patients achieving time-in-range greater than 70% with open-source AID was 60% versus just 15% with pump plus CGM.
Glycemic improvements with open-source AID were significant for adults and children and were greater for those with higher baseline A1c levels. The effect was immediate and sustained throughout the study period, “which is super-pleasing, because there was a worry that the technical burden of open source might be [leading to] dropout, but we didn’t see that. It was sustained right through to the end of the trial,” Dr. de Bock commented.
Hypoglycemic rates didn’t differ between groups, and there were no episodes of severe hypoglycemia or diabetic ketoacidosis.
No more waiting: What is the future of open-source AID?
When the open-source APS was first developed, users coined the motto: “We are not waiting.” But now that the “wait” is over and several commercial AIDs have been approved by regulatory bodies, with others still in the pipeline, will people still use open-source systems?
There are no current data on people moving from DIY to commercial systems. However, Dr. de Bock said, “For most who undertook an open-source option, the precision of the settings that they can use and enjoy would mean that most would likely stick to their open source.”
Dr. Isaacs agrees: “I actually don’t think it’s going to go away in the near future, because the FDA has very specific criteria for where these [formally approved] devices can be in terms of their target ranges and requirements versus with open source you can really customize. So I still think there’s going to be a subset of people who want that customization, who want the lower targets.”
Dana Lewis, the originator of the DIY system and a CREATE coauthor, told this news organization: “I don’t believe there has been a fall-off, and in fact, I think open-source AID has continued to have ongoing uptake as awareness increases about options and as more pumps and CGMs become interoperable with various open-source AID choices.”
“I think uptake increasing is also influenced by the fact that in places like Europe, Asia, and Australia there are in-warranty on-the-market pumps that are compatible and interoperable with open-source AID. I think awareness of AID overall increases uptake of commercial and open source alike,” she said.
“Clinicians, as emphasized in recent position statements, must maintain support of the person with diabetes, irrespective of the mode of treatment they are on. ... Health care providers should be encouraged to learn from the experiences of the people who have stuck with open-source AID or switched, so that they can inform themselves of the relative strengths and benefits of each system,” Dr. de Bock advised.
Ms. Lewis noted: “We are seeing increasing awareness and comfort in endocrinologists from the community perspective, and we do hope that this study helps increase conversation and awareness of the safety and efficacy of open-source AID systems as an option for people with diabetes.”
In fact, the team published an article specifically about clinicians’ experience in CREATE. “The learning curve is similar across AID technology,” she observed.
Findings of a 6-month continuation phase of CREATE, in which all participants used the open-source AID, are scheduled to be presented in September at the European Association for the Study of Diabetes annual meeting.
The study was funded by the Health Research Council of New Zealand, with hardware support from SOOIL Developments, South Korea; Dexcom; and Vodafone New Zealand. Dr. de Bock has reported receiving honoraria and/or research funding from Novo Nordisk, Sanofi, Pfizer, Medtronic, Lilly, Ypsomed, and Dexcom. Dr. Isaacs has reported serving as a consultant for LifeScan, Lilly, and Insulet, and as a speaker for Dexcom, Medtronic, Abbott, and Novo Nordisk. Ms. Lewis has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ADA 2022
Surgery during a pandemic? COVID vaccination status matters – or not
An online survey captured mixed information about people’s willingness to undergo surgery during a viral pandemic in relation to the vaccine status of the patient and staff. The findings showcase opportunities for public education and “skillful messaging,” researchers report.
In survey scenarios that asked people to imagine their vaccination status, people were more willing to undergo surgery if it was lifesaving, rather than elective, especially if vaccinated. The prospect of no hospital stay tipped the scales further toward surgery. The vaccination status of hospital staff played only a minor role in decision making, according to the study, which was published in Vaccine.
But as a post hoc analysis revealed, it was participants who were not vaccinated against COVID-19 in real life who were more willing to undergo surgery, compared with those who had one or two shots.
In either case, too many people were unwilling to undergo lifesaving surgery, even though the risk of hospital-acquired COVID-19 is low. “Making this choice for an actual health problem would result in an unacceptably high rate of potential morbidity attributable to pandemic-related fears, the authors wrote.
In an unusual approach, the researchers used Amazon’s Mechanical Turk to electronically recruit 2,006 adults. The participants answered a 26-item survey about a hypothetical surgery in an unnamed pandemic with different combinations of vaccine status for patient and staff.
Coauthor and anesthesiologist Keith J. Ruskin, MD, of the University of Chicago, told this news organization that they “wanted to make this timeless” and independent of COVID “so that when the next thing came about, the paper would still be relevant.”
The researchers were surprised by the findings at the extreme ends of attitudes toward surgery. Some were still willing to have elective surgery with (hypothetically) unvaccinated patients and staff.
“And people at the other end, even though they are vaccinated, the hospital staff is vaccinated, and the surgery is lifesaving, they absolutely won’t have surgery,” Dr. Ruskin said.
He viewed these two groups as opportunities for education. “You can present information in the most positive light to get them to do the right thing with what’s best for themselves,” he said.
As an example, Dr. Ruskin pointed to an ad in Illinois. “It’s not only people saying I’m getting vaccinated for myself and my family, but there are people who said I got vaccinated and I still got COVID, but it could have been much worse. Please, if you’re on the fence, just get vaccinated,” he said.
Coauthor Anna Clebone Ruskin, MD, an anesthesiologist at the University of Chicago, said, “Humans are programmed to see things in extremes. With surgery, people tend to think of surgery as a monolith – surgery is all good, or surgery is all bad, where there is a huge in between. So we saw those extremes. ... Seeing that dichotomy with people on either end was pretty surprising.
“Getting surgery is not always good. Getting surgery is not always bad. It’s a risk-versus-benefit analysis and educating the public to consider the risks and benefits of medical decisions, in general, would be enormously beneficial,” she said.
A post hoc analysis found that “participants who were not actually vaccinated against COVID-19 were generally more willing to undergo surgery compared to those who had one vaccination or two vaccinations,” the authors wrote.
In a second post hoc finding, participants who reported high wariness of vaccines were generally more likely to be willing to undergo surgery. Notably, 15% of participants “were unwilling to undergo lifesaving surgery during a pandemic even when they and the health care staff were vaccinated,” the authors wrote.
Dr. Keith J. Ruskin hypothesized about this result, saying, “What we think is that potentially actually getting vaccinated against COVID-19 may indicate that you have a lower risk tolerance. So you may be less likely to do anything you perceive to be risky if you’re vaccinated against COVID-19.”
The authors stated that “the risk of hospital-acquired COVID-19 even prior to vaccination is vanishingly small.” The risk of nosocomial COVID varies among different studies. An EPIC-based study between April 2020 and October 2021 found the risk to be 1.8%; EPIC describes the fears of a patient catching COVID at a hospital as “likely unfounded.”
In the United Kingdom, the risk was as high as 24% earlier in the pandemic and then declined to approximately 5% a year ago. Omicron also brought more infections. Rates varied significantly among hospitals – and, notably, the risk of death from a nosocomial COVID infection was 21% in April-September 2020.
Emily Landon, MD, an epidemiologist and executive medical director for infection prevention and control at the University of Chicago Medicine, told this news organization that the study’s data were collected during Delta, a “time when we thought that this was a pandemic of the unvaccinated. But there was serious politicization of the vaccine.”
Dr. Landon said one of the study’s strengths was the large number of participants. A limitation was, “You’re going to have less participants who are generally poor and indigent, and fewer old participants, probably because they’re less likely to respond to an online survey.
“But the most interesting results are that people who were wary of vaccines or who hadn’t been vaccinated, were much more willing to undergo surgical procedures in the time of a pandemic, regardless of status, which reflects the fact that not being vaccinated correlates with not worrying much about COVID. Vaccinated individuals had a lot more wariness about undergoing surgical procedures during a pandemic.”
It appeared “individuals who were vaccinated in real life [were] worried about staff vaccination,” Dr. Landon noted. She concluded, “I think it supports the need for mandatory vaccinations in health care workers.”
The study has implications for hospital vaccination policies and practices. In Cumberland, Md., when COVID was high and vaccines first became available, the Maryland Hospital Association said that all health care staff should be vaccinated. The local hospital, UPMC–Western Maryland Hospital, refused.
Two months later, the local news reporter, Teresa McMinn, wrote, “While Maryland’s largest hospital systems have ‘led by example by mandating vaccines for all of their hospital staff,’ other facilities – including UPMC Western Maryland and Garrett Regional Medical Center – have taken no such action even though it’s been 8 months since vaccines were made available to health care workers.”
The hospital would not tell patients whether staff were vaccinated, either. An ongoing concern for members of the community is the lack of communication with UPMC, which erodes trust in the health system – the only hospital available in this rural community.
This vaccine study supports that the vaccination status of the staff may influence some patients’ decision on whether to have surgery.
The Ruskins and Dr. Landon have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An online survey captured mixed information about people’s willingness to undergo surgery during a viral pandemic in relation to the vaccine status of the patient and staff. The findings showcase opportunities for public education and “skillful messaging,” researchers report.
In survey scenarios that asked people to imagine their vaccination status, people were more willing to undergo surgery if it was lifesaving, rather than elective, especially if vaccinated. The prospect of no hospital stay tipped the scales further toward surgery. The vaccination status of hospital staff played only a minor role in decision making, according to the study, which was published in Vaccine.
But as a post hoc analysis revealed, it was participants who were not vaccinated against COVID-19 in real life who were more willing to undergo surgery, compared with those who had one or two shots.
In either case, too many people were unwilling to undergo lifesaving surgery, even though the risk of hospital-acquired COVID-19 is low. “Making this choice for an actual health problem would result in an unacceptably high rate of potential morbidity attributable to pandemic-related fears, the authors wrote.
In an unusual approach, the researchers used Amazon’s Mechanical Turk to electronically recruit 2,006 adults. The participants answered a 26-item survey about a hypothetical surgery in an unnamed pandemic with different combinations of vaccine status for patient and staff.
Coauthor and anesthesiologist Keith J. Ruskin, MD, of the University of Chicago, told this news organization that they “wanted to make this timeless” and independent of COVID “so that when the next thing came about, the paper would still be relevant.”
The researchers were surprised by the findings at the extreme ends of attitudes toward surgery. Some were still willing to have elective surgery with (hypothetically) unvaccinated patients and staff.
“And people at the other end, even though they are vaccinated, the hospital staff is vaccinated, and the surgery is lifesaving, they absolutely won’t have surgery,” Dr. Ruskin said.
He viewed these two groups as opportunities for education. “You can present information in the most positive light to get them to do the right thing with what’s best for themselves,” he said.
As an example, Dr. Ruskin pointed to an ad in Illinois. “It’s not only people saying I’m getting vaccinated for myself and my family, but there are people who said I got vaccinated and I still got COVID, but it could have been much worse. Please, if you’re on the fence, just get vaccinated,” he said.
Coauthor Anna Clebone Ruskin, MD, an anesthesiologist at the University of Chicago, said, “Humans are programmed to see things in extremes. With surgery, people tend to think of surgery as a monolith – surgery is all good, or surgery is all bad, where there is a huge in between. So we saw those extremes. ... Seeing that dichotomy with people on either end was pretty surprising.
“Getting surgery is not always good. Getting surgery is not always bad. It’s a risk-versus-benefit analysis and educating the public to consider the risks and benefits of medical decisions, in general, would be enormously beneficial,” she said.
A post hoc analysis found that “participants who were not actually vaccinated against COVID-19 were generally more willing to undergo surgery compared to those who had one vaccination or two vaccinations,” the authors wrote.
In a second post hoc finding, participants who reported high wariness of vaccines were generally more likely to be willing to undergo surgery. Notably, 15% of participants “were unwilling to undergo lifesaving surgery during a pandemic even when they and the health care staff were vaccinated,” the authors wrote.
Dr. Keith J. Ruskin hypothesized about this result, saying, “What we think is that potentially actually getting vaccinated against COVID-19 may indicate that you have a lower risk tolerance. So you may be less likely to do anything you perceive to be risky if you’re vaccinated against COVID-19.”
The authors stated that “the risk of hospital-acquired COVID-19 even prior to vaccination is vanishingly small.” The risk of nosocomial COVID varies among different studies. An EPIC-based study between April 2020 and October 2021 found the risk to be 1.8%; EPIC describes the fears of a patient catching COVID at a hospital as “likely unfounded.”
In the United Kingdom, the risk was as high as 24% earlier in the pandemic and then declined to approximately 5% a year ago. Omicron also brought more infections. Rates varied significantly among hospitals – and, notably, the risk of death from a nosocomial COVID infection was 21% in April-September 2020.
Emily Landon, MD, an epidemiologist and executive medical director for infection prevention and control at the University of Chicago Medicine, told this news organization that the study’s data were collected during Delta, a “time when we thought that this was a pandemic of the unvaccinated. But there was serious politicization of the vaccine.”
Dr. Landon said one of the study’s strengths was the large number of participants. A limitation was, “You’re going to have less participants who are generally poor and indigent, and fewer old participants, probably because they’re less likely to respond to an online survey.
“But the most interesting results are that people who were wary of vaccines or who hadn’t been vaccinated, were much more willing to undergo surgical procedures in the time of a pandemic, regardless of status, which reflects the fact that not being vaccinated correlates with not worrying much about COVID. Vaccinated individuals had a lot more wariness about undergoing surgical procedures during a pandemic.”
It appeared “individuals who were vaccinated in real life [were] worried about staff vaccination,” Dr. Landon noted. She concluded, “I think it supports the need for mandatory vaccinations in health care workers.”
The study has implications for hospital vaccination policies and practices. In Cumberland, Md., when COVID was high and vaccines first became available, the Maryland Hospital Association said that all health care staff should be vaccinated. The local hospital, UPMC–Western Maryland Hospital, refused.
Two months later, the local news reporter, Teresa McMinn, wrote, “While Maryland’s largest hospital systems have ‘led by example by mandating vaccines for all of their hospital staff,’ other facilities – including UPMC Western Maryland and Garrett Regional Medical Center – have taken no such action even though it’s been 8 months since vaccines were made available to health care workers.”
The hospital would not tell patients whether staff were vaccinated, either. An ongoing concern for members of the community is the lack of communication with UPMC, which erodes trust in the health system – the only hospital available in this rural community.
This vaccine study supports that the vaccination status of the staff may influence some patients’ decision on whether to have surgery.
The Ruskins and Dr. Landon have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An online survey captured mixed information about people’s willingness to undergo surgery during a viral pandemic in relation to the vaccine status of the patient and staff. The findings showcase opportunities for public education and “skillful messaging,” researchers report.
In survey scenarios that asked people to imagine their vaccination status, people were more willing to undergo surgery if it was lifesaving, rather than elective, especially if vaccinated. The prospect of no hospital stay tipped the scales further toward surgery. The vaccination status of hospital staff played only a minor role in decision making, according to the study, which was published in Vaccine.
But as a post hoc analysis revealed, it was participants who were not vaccinated against COVID-19 in real life who were more willing to undergo surgery, compared with those who had one or two shots.
In either case, too many people were unwilling to undergo lifesaving surgery, even though the risk of hospital-acquired COVID-19 is low. “Making this choice for an actual health problem would result in an unacceptably high rate of potential morbidity attributable to pandemic-related fears, the authors wrote.
In an unusual approach, the researchers used Amazon’s Mechanical Turk to electronically recruit 2,006 adults. The participants answered a 26-item survey about a hypothetical surgery in an unnamed pandemic with different combinations of vaccine status for patient and staff.
Coauthor and anesthesiologist Keith J. Ruskin, MD, of the University of Chicago, told this news organization that they “wanted to make this timeless” and independent of COVID “so that when the next thing came about, the paper would still be relevant.”
The researchers were surprised by the findings at the extreme ends of attitudes toward surgery. Some were still willing to have elective surgery with (hypothetically) unvaccinated patients and staff.
“And people at the other end, even though they are vaccinated, the hospital staff is vaccinated, and the surgery is lifesaving, they absolutely won’t have surgery,” Dr. Ruskin said.
He viewed these two groups as opportunities for education. “You can present information in the most positive light to get them to do the right thing with what’s best for themselves,” he said.
As an example, Dr. Ruskin pointed to an ad in Illinois. “It’s not only people saying I’m getting vaccinated for myself and my family, but there are people who said I got vaccinated and I still got COVID, but it could have been much worse. Please, if you’re on the fence, just get vaccinated,” he said.
Coauthor Anna Clebone Ruskin, MD, an anesthesiologist at the University of Chicago, said, “Humans are programmed to see things in extremes. With surgery, people tend to think of surgery as a monolith – surgery is all good, or surgery is all bad, where there is a huge in between. So we saw those extremes. ... Seeing that dichotomy with people on either end was pretty surprising.
“Getting surgery is not always good. Getting surgery is not always bad. It’s a risk-versus-benefit analysis and educating the public to consider the risks and benefits of medical decisions, in general, would be enormously beneficial,” she said.
A post hoc analysis found that “participants who were not actually vaccinated against COVID-19 were generally more willing to undergo surgery compared to those who had one vaccination or two vaccinations,” the authors wrote.
In a second post hoc finding, participants who reported high wariness of vaccines were generally more likely to be willing to undergo surgery. Notably, 15% of participants “were unwilling to undergo lifesaving surgery during a pandemic even when they and the health care staff were vaccinated,” the authors wrote.
Dr. Keith J. Ruskin hypothesized about this result, saying, “What we think is that potentially actually getting vaccinated against COVID-19 may indicate that you have a lower risk tolerance. So you may be less likely to do anything you perceive to be risky if you’re vaccinated against COVID-19.”
The authors stated that “the risk of hospital-acquired COVID-19 even prior to vaccination is vanishingly small.” The risk of nosocomial COVID varies among different studies. An EPIC-based study between April 2020 and October 2021 found the risk to be 1.8%; EPIC describes the fears of a patient catching COVID at a hospital as “likely unfounded.”
In the United Kingdom, the risk was as high as 24% earlier in the pandemic and then declined to approximately 5% a year ago. Omicron also brought more infections. Rates varied significantly among hospitals – and, notably, the risk of death from a nosocomial COVID infection was 21% in April-September 2020.
Emily Landon, MD, an epidemiologist and executive medical director for infection prevention and control at the University of Chicago Medicine, told this news organization that the study’s data were collected during Delta, a “time when we thought that this was a pandemic of the unvaccinated. But there was serious politicization of the vaccine.”
Dr. Landon said one of the study’s strengths was the large number of participants. A limitation was, “You’re going to have less participants who are generally poor and indigent, and fewer old participants, probably because they’re less likely to respond to an online survey.
“But the most interesting results are that people who were wary of vaccines or who hadn’t been vaccinated, were much more willing to undergo surgical procedures in the time of a pandemic, regardless of status, which reflects the fact that not being vaccinated correlates with not worrying much about COVID. Vaccinated individuals had a lot more wariness about undergoing surgical procedures during a pandemic.”
It appeared “individuals who were vaccinated in real life [were] worried about staff vaccination,” Dr. Landon noted. She concluded, “I think it supports the need for mandatory vaccinations in health care workers.”
The study has implications for hospital vaccination policies and practices. In Cumberland, Md., when COVID was high and vaccines first became available, the Maryland Hospital Association said that all health care staff should be vaccinated. The local hospital, UPMC–Western Maryland Hospital, refused.
Two months later, the local news reporter, Teresa McMinn, wrote, “While Maryland’s largest hospital systems have ‘led by example by mandating vaccines for all of their hospital staff,’ other facilities – including UPMC Western Maryland and Garrett Regional Medical Center – have taken no such action even though it’s been 8 months since vaccines were made available to health care workers.”
The hospital would not tell patients whether staff were vaccinated, either. An ongoing concern for members of the community is the lack of communication with UPMC, which erodes trust in the health system – the only hospital available in this rural community.
This vaccine study supports that the vaccination status of the staff may influence some patients’ decision on whether to have surgery.
The Ruskins and Dr. Landon have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
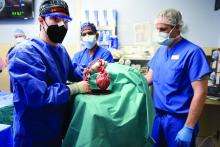
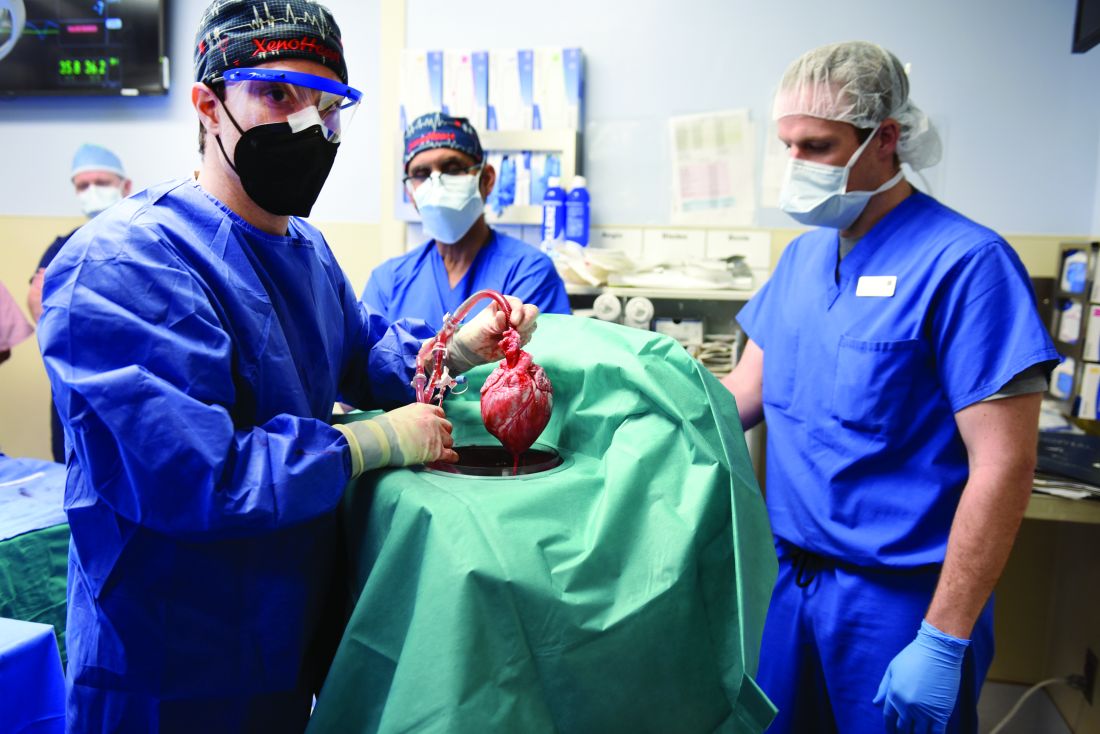
Cause of death in pig heart recipient: New clues
The underlying cause of David Bennett’s death on March 8, two months after he received the heart of a genetically altered pig, remains unknown and is only slightly less mysterious for what can likely be ruled out, suggests a progress report on the case from the director of the cardiac xenotransplantation program where the pioneering surgery took place.
Mr. Bennett died in “diastolic heart failure,” reported Muhammad M. Mohiuddin, MBBS, University of Maryland School of Medicine, Baltimore, “but the mechanism is still under investigation.”
Although the immediate cause could have been single or multiple, evidence so far does not point to immune rejection nor does it support a role for a recently proposed suspect, infection by porcine cytomegalovirus (PCMV), Dr. Mohiuddin observed in front of a standing-room-only audience June 6 at the American Transplant Congress (ATC) in Boston. The congress is a joint meeting of the American Society of Transplant Surgeons (ASTS) and the American Society of Transplantation (AST).
Rocky clinical course
Early characterizations of the patient’s death focused more on his diminished, end-stage clinical condition at the time of the surgery than on immune rejection or other direct effects of the xenograft or on the first-of-its-kind procedure itself.
The 57-year-old Mr. Bennett had presented to the University of Maryland team with nonischemic cardiomyopathy, on multiple inotropes, and requiring an intra-aortic balloon pump, Dr. Mohiuddin said in his ATC presentation. The patient had suffered multiple arrests and resuscitations, and by the time of surgery had been hospitalized for almost 2 months, including 40 days on veno-arterial extracorporeal membrane oxygenation (ECMO).
The transplant procedure itself went as planned until removal of the aortic cross clamp, which triggered a type-A aortic dissection. “We put a graft in the ascending aorta and a stent in the descending aorta. Even after 2 days, we found the dissection extending to the renal artery, so we had to go back and also put a stent in the renal artery,” Dr. Mohiuddin said.
Mr. Bennett also underwent two exploratory laparotomies in the first 10 days after transplantation, after CT imaging revealed signs of possible bowel inflammation and ischemia.
Further, he had to fight back a series of infections that led to major changes to his experimental drug regimen, which included immunosuppressants methylprednisolone and mycophenolate mofetil (MMF), the investigational anti-CD40 antibody KPL-404 (Kiniksa Pharmaceuticals), and the anti-inflammatories etanercept (Enbrel) and tocilizumab (Actemra).
One episode of sepsis, in particular, forced temporary withdrawal of MMF and a reduction in methylprednisolone dosage. It’s unknown whether the 30-day MMF suspension played a role in Mr. Bennett’s ultimate clinical deterioration and death, but it’s “highly possible,” Dr. Mohiuddin said in an interview.
Realistically, Mr. Bennett’s death was likely “multifactorial,” Dr. Mohiuddin said. He was in such poor clinical condition going into the procedure, and afterward confronted so many clinical challenges, that “it’s very difficult to say that one thing caused it.”
That hasn’t lessened speculation that the patient’s heart failed secondary to immunologic rejection or PCMV infection, either in Mr. Bennett or the donor pig.
A role for PCMV?
Weeks after Mr. Bennett’s death, as previously reported, his surgeon announced at a public forum that PCMV had been identified in the transplanted heart and in tissues of the donor pig. Mr. Bennett’s circulation showed traces of the viral DNA but not of the virus itself.
The presence of PCMV in transplanted porcine hearts is a well-recognized potential hazard in animal models but is considered avoidable with proper screening. In Mr. Bennett’s case, preoperative screening of the pig donor missed signs of the virus.
Still, PCMV could potentially have contributed to Mr. Bennett’s death, acknowledged Bartley P. Griffith, MD, University of Maryland School of Medicine, who had announced the PCMV finding in an AST-sponsored April 20 webcast.
Preclinical evidence does suggest that PCMV can harm a xenograft organ, observed David H. Sachs, MD, Columbia University Medical Center, New York, from the audience during the comment period after Dr. Mohiuddin’s presentation.
“Each species has a CMV, and they’re quite species-specific,” observed the renowned surgeon and xenotransplantation immunologist. “We showed almost 10 years ago that if PCMV was in a pig kidney, it led to a much shortened survival of the pig kidney in a baboon. There was never any evidence, however, that the CMV infected the baboon or any baboon cells.”
Dr. Sachs asked Dr. Mohiuddin for confirmation that Mr. Bennett displayed no more than DNAemia, circulating cell-free PCMV DNA presumably shed from the porcine heart, but no sign of the virus itself outside of the heart’s porcine cells.
Cell-free DNA had shown up in Mr. Bennett’s circulation about 20 days after the surgery, with concentrations rising until at least day 50. Post-hoc polymerase chain reaction (PCR) testing disclosed PCMV only in the pig’s spleen and porcine cells of the transplanted heart, Dr. Mohiuddin noted.
“We have not found any evidence that the patient was infected by PCMV,” nor was there evidence of any disease related to PCMV, Dr. Mohiuddin replied.
Nor of ongoing rejection
Mr. Bennett’s new heart passed a critical test in the first post-implantation hours by avoiding acute rejection, a potentially disastrous outcome that three of the pig’s 10 gene edits had been designed to prevent.
Although chronic immune rejection was always a concern despite Mr. Bennett’s novel immunosuppressant regimen, myocardial biopsy on postoperative days 34, 50, and 56 and necropsy showed “no signs of typical xenograft rejection,” Dr. Mohiuddin said at the ATC presentation. But “there’s a chance of atypical rejection which we were not accustomed to.”
By day 50, his diastolic function showed echocardiographic signs of deterioration, and “we started seeing interstitial edema with some extravasation of red blood cells, which we thought would resolve over a period of time,” he said. Eventually, however, “we saw that turn into fibroblasts and scar tissue.”
Mr. Bennett once again went on veno-arterial ECMO but died 10 days later. Once they had seen histologic evidence of fibrosis, Dr. Mohiuddin told this news organization, the team believed the myocardial injury was irreversible. “That was the reason we gave up on recovery.”
Mr. Bennett’s xenotransplantation journey has taught the field a lot, he said. “By no means was this a failure; we consider this a huge success. You can do all the experiments in animal models, but you won’t find out the true mechanism of rejection unless you do these kinds of human experiments.”
Looking ahead to clinical trials
Research involving humans is always subject to vagaries of human nature, including degree of adherence to prescribed therapy and – in xenotransplantation – precautions in place to mitigate any risks to public health. Such risks theoretically include transfer of porcine viruses or other pathogens to the patient and subsequent release into the general population.
Looking ahead to the possibility of clinical trials after this successful xenotransplantation experience, transplant nephrologist and epidemiologist Peter P. Reese, MD, PhD, University of Pennsylvania, Philadelphia, raised the potentially controversial issue in discussion following Dr. Mohiuddin’s presentation.
It’s known that Mr. Bennett had been repeatedly turned down for a conventional allograft transplant primarily because of his history of treatment noncompliance. Should such a record, Dr. Reese asked, be a relative contraindication to enrollment in any future xenotransplantation trials? Or does the field need a standardized gauge of a patient’s readiness, once discharged, to adhere not only to all medications – including those that fight infection – but also with rules established for public safety, such as routine contact reporting?
“It makes me wonder about choosing a noncompliant patient for these trials,” Dr. Reese said. “If we discharge a patient from the hospital who is at risk for a zoonotic infection that could spread if they basically refuse to cooperate with us or with public health authorities, it really could have negative consequences for the reputation of the field.”
Dr. Mohiuddin agreed such concerns are valid. Mr. Bennett “and all his immediate contacts” signed consent forms acknowledging their willingness to be followed should he be discharged. Mr. Bennett himself “signed a consent to inform us if he has any other intimate contact with someone,” he said in an interview.
“But those are only on paper.” Had Mr. Bennett survived to be discharged, Dr. Mohuiddin said, “no one knows how he would have behaved.”
Dr. Mohiuddin said the research staff had prepared to monitor Mr. Bennett at his home if that’s what it took. “We were ready to follow him as long as we could. There was a surveillance plan in place.”
A version of this article first appeared on Medscape.com.
The underlying cause of David Bennett’s death on March 8, two months after he received the heart of a genetically altered pig, remains unknown and is only slightly less mysterious for what can likely be ruled out, suggests a progress report on the case from the director of the cardiac xenotransplantation program where the pioneering surgery took place.
Mr. Bennett died in “diastolic heart failure,” reported Muhammad M. Mohiuddin, MBBS, University of Maryland School of Medicine, Baltimore, “but the mechanism is still under investigation.”
Although the immediate cause could have been single or multiple, evidence so far does not point to immune rejection nor does it support a role for a recently proposed suspect, infection by porcine cytomegalovirus (PCMV), Dr. Mohiuddin observed in front of a standing-room-only audience June 6 at the American Transplant Congress (ATC) in Boston. The congress is a joint meeting of the American Society of Transplant Surgeons (ASTS) and the American Society of Transplantation (AST).
Rocky clinical course
Early characterizations of the patient’s death focused more on his diminished, end-stage clinical condition at the time of the surgery than on immune rejection or other direct effects of the xenograft or on the first-of-its-kind procedure itself.
The 57-year-old Mr. Bennett had presented to the University of Maryland team with nonischemic cardiomyopathy, on multiple inotropes, and requiring an intra-aortic balloon pump, Dr. Mohiuddin said in his ATC presentation. The patient had suffered multiple arrests and resuscitations, and by the time of surgery had been hospitalized for almost 2 months, including 40 days on veno-arterial extracorporeal membrane oxygenation (ECMO).
The transplant procedure itself went as planned until removal of the aortic cross clamp, which triggered a type-A aortic dissection. “We put a graft in the ascending aorta and a stent in the descending aorta. Even after 2 days, we found the dissection extending to the renal artery, so we had to go back and also put a stent in the renal artery,” Dr. Mohiuddin said.
Mr. Bennett also underwent two exploratory laparotomies in the first 10 days after transplantation, after CT imaging revealed signs of possible bowel inflammation and ischemia.
Further, he had to fight back a series of infections that led to major changes to his experimental drug regimen, which included immunosuppressants methylprednisolone and mycophenolate mofetil (MMF), the investigational anti-CD40 antibody KPL-404 (Kiniksa Pharmaceuticals), and the anti-inflammatories etanercept (Enbrel) and tocilizumab (Actemra).
One episode of sepsis, in particular, forced temporary withdrawal of MMF and a reduction in methylprednisolone dosage. It’s unknown whether the 30-day MMF suspension played a role in Mr. Bennett’s ultimate clinical deterioration and death, but it’s “highly possible,” Dr. Mohiuddin said in an interview.
Realistically, Mr. Bennett’s death was likely “multifactorial,” Dr. Mohiuddin said. He was in such poor clinical condition going into the procedure, and afterward confronted so many clinical challenges, that “it’s very difficult to say that one thing caused it.”
That hasn’t lessened speculation that the patient’s heart failed secondary to immunologic rejection or PCMV infection, either in Mr. Bennett or the donor pig.
A role for PCMV?
Weeks after Mr. Bennett’s death, as previously reported, his surgeon announced at a public forum that PCMV had been identified in the transplanted heart and in tissues of the donor pig. Mr. Bennett’s circulation showed traces of the viral DNA but not of the virus itself.
The presence of PCMV in transplanted porcine hearts is a well-recognized potential hazard in animal models but is considered avoidable with proper screening. In Mr. Bennett’s case, preoperative screening of the pig donor missed signs of the virus.
Still, PCMV could potentially have contributed to Mr. Bennett’s death, acknowledged Bartley P. Griffith, MD, University of Maryland School of Medicine, who had announced the PCMV finding in an AST-sponsored April 20 webcast.
Preclinical evidence does suggest that PCMV can harm a xenograft organ, observed David H. Sachs, MD, Columbia University Medical Center, New York, from the audience during the comment period after Dr. Mohiuddin’s presentation.
“Each species has a CMV, and they’re quite species-specific,” observed the renowned surgeon and xenotransplantation immunologist. “We showed almost 10 years ago that if PCMV was in a pig kidney, it led to a much shortened survival of the pig kidney in a baboon. There was never any evidence, however, that the CMV infected the baboon or any baboon cells.”
Dr. Sachs asked Dr. Mohiuddin for confirmation that Mr. Bennett displayed no more than DNAemia, circulating cell-free PCMV DNA presumably shed from the porcine heart, but no sign of the virus itself outside of the heart’s porcine cells.
Cell-free DNA had shown up in Mr. Bennett’s circulation about 20 days after the surgery, with concentrations rising until at least day 50. Post-hoc polymerase chain reaction (PCR) testing disclosed PCMV only in the pig’s spleen and porcine cells of the transplanted heart, Dr. Mohiuddin noted.
“We have not found any evidence that the patient was infected by PCMV,” nor was there evidence of any disease related to PCMV, Dr. Mohiuddin replied.
Nor of ongoing rejection
Mr. Bennett’s new heart passed a critical test in the first post-implantation hours by avoiding acute rejection, a potentially disastrous outcome that three of the pig’s 10 gene edits had been designed to prevent.
Although chronic immune rejection was always a concern despite Mr. Bennett’s novel immunosuppressant regimen, myocardial biopsy on postoperative days 34, 50, and 56 and necropsy showed “no signs of typical xenograft rejection,” Dr. Mohiuddin said at the ATC presentation. But “there’s a chance of atypical rejection which we were not accustomed to.”
By day 50, his diastolic function showed echocardiographic signs of deterioration, and “we started seeing interstitial edema with some extravasation of red blood cells, which we thought would resolve over a period of time,” he said. Eventually, however, “we saw that turn into fibroblasts and scar tissue.”
Mr. Bennett once again went on veno-arterial ECMO but died 10 days later. Once they had seen histologic evidence of fibrosis, Dr. Mohiuddin told this news organization, the team believed the myocardial injury was irreversible. “That was the reason we gave up on recovery.”
Mr. Bennett’s xenotransplantation journey has taught the field a lot, he said. “By no means was this a failure; we consider this a huge success. You can do all the experiments in animal models, but you won’t find out the true mechanism of rejection unless you do these kinds of human experiments.”
Looking ahead to clinical trials
Research involving humans is always subject to vagaries of human nature, including degree of adherence to prescribed therapy and – in xenotransplantation – precautions in place to mitigate any risks to public health. Such risks theoretically include transfer of porcine viruses or other pathogens to the patient and subsequent release into the general population.
Looking ahead to the possibility of clinical trials after this successful xenotransplantation experience, transplant nephrologist and epidemiologist Peter P. Reese, MD, PhD, University of Pennsylvania, Philadelphia, raised the potentially controversial issue in discussion following Dr. Mohiuddin’s presentation.
It’s known that Mr. Bennett had been repeatedly turned down for a conventional allograft transplant primarily because of his history of treatment noncompliance. Should such a record, Dr. Reese asked, be a relative contraindication to enrollment in any future xenotransplantation trials? Or does the field need a standardized gauge of a patient’s readiness, once discharged, to adhere not only to all medications – including those that fight infection – but also with rules established for public safety, such as routine contact reporting?
“It makes me wonder about choosing a noncompliant patient for these trials,” Dr. Reese said. “If we discharge a patient from the hospital who is at risk for a zoonotic infection that could spread if they basically refuse to cooperate with us or with public health authorities, it really could have negative consequences for the reputation of the field.”
Dr. Mohiuddin agreed such concerns are valid. Mr. Bennett “and all his immediate contacts” signed consent forms acknowledging their willingness to be followed should he be discharged. Mr. Bennett himself “signed a consent to inform us if he has any other intimate contact with someone,” he said in an interview.
“But those are only on paper.” Had Mr. Bennett survived to be discharged, Dr. Mohuiddin said, “no one knows how he would have behaved.”
Dr. Mohiuddin said the research staff had prepared to monitor Mr. Bennett at his home if that’s what it took. “We were ready to follow him as long as we could. There was a surveillance plan in place.”
A version of this article first appeared on Medscape.com.
The underlying cause of David Bennett’s death on March 8, two months after he received the heart of a genetically altered pig, remains unknown and is only slightly less mysterious for what can likely be ruled out, suggests a progress report on the case from the director of the cardiac xenotransplantation program where the pioneering surgery took place.
Mr. Bennett died in “diastolic heart failure,” reported Muhammad M. Mohiuddin, MBBS, University of Maryland School of Medicine, Baltimore, “but the mechanism is still under investigation.”
Although the immediate cause could have been single or multiple, evidence so far does not point to immune rejection nor does it support a role for a recently proposed suspect, infection by porcine cytomegalovirus (PCMV), Dr. Mohiuddin observed in front of a standing-room-only audience June 6 at the American Transplant Congress (ATC) in Boston. The congress is a joint meeting of the American Society of Transplant Surgeons (ASTS) and the American Society of Transplantation (AST).
Rocky clinical course
Early characterizations of the patient’s death focused more on his diminished, end-stage clinical condition at the time of the surgery than on immune rejection or other direct effects of the xenograft or on the first-of-its-kind procedure itself.
The 57-year-old Mr. Bennett had presented to the University of Maryland team with nonischemic cardiomyopathy, on multiple inotropes, and requiring an intra-aortic balloon pump, Dr. Mohiuddin said in his ATC presentation. The patient had suffered multiple arrests and resuscitations, and by the time of surgery had been hospitalized for almost 2 months, including 40 days on veno-arterial extracorporeal membrane oxygenation (ECMO).
The transplant procedure itself went as planned until removal of the aortic cross clamp, which triggered a type-A aortic dissection. “We put a graft in the ascending aorta and a stent in the descending aorta. Even after 2 days, we found the dissection extending to the renal artery, so we had to go back and also put a stent in the renal artery,” Dr. Mohiuddin said.
Mr. Bennett also underwent two exploratory laparotomies in the first 10 days after transplantation, after CT imaging revealed signs of possible bowel inflammation and ischemia.
Further, he had to fight back a series of infections that led to major changes to his experimental drug regimen, which included immunosuppressants methylprednisolone and mycophenolate mofetil (MMF), the investigational anti-CD40 antibody KPL-404 (Kiniksa Pharmaceuticals), and the anti-inflammatories etanercept (Enbrel) and tocilizumab (Actemra).
One episode of sepsis, in particular, forced temporary withdrawal of MMF and a reduction in methylprednisolone dosage. It’s unknown whether the 30-day MMF suspension played a role in Mr. Bennett’s ultimate clinical deterioration and death, but it’s “highly possible,” Dr. Mohiuddin said in an interview.
Realistically, Mr. Bennett’s death was likely “multifactorial,” Dr. Mohiuddin said. He was in such poor clinical condition going into the procedure, and afterward confronted so many clinical challenges, that “it’s very difficult to say that one thing caused it.”
That hasn’t lessened speculation that the patient’s heart failed secondary to immunologic rejection or PCMV infection, either in Mr. Bennett or the donor pig.
A role for PCMV?
Weeks after Mr. Bennett’s death, as previously reported, his surgeon announced at a public forum that PCMV had been identified in the transplanted heart and in tissues of the donor pig. Mr. Bennett’s circulation showed traces of the viral DNA but not of the virus itself.
The presence of PCMV in transplanted porcine hearts is a well-recognized potential hazard in animal models but is considered avoidable with proper screening. In Mr. Bennett’s case, preoperative screening of the pig donor missed signs of the virus.
Still, PCMV could potentially have contributed to Mr. Bennett’s death, acknowledged Bartley P. Griffith, MD, University of Maryland School of Medicine, who had announced the PCMV finding in an AST-sponsored April 20 webcast.
Preclinical evidence does suggest that PCMV can harm a xenograft organ, observed David H. Sachs, MD, Columbia University Medical Center, New York, from the audience during the comment period after Dr. Mohiuddin’s presentation.
“Each species has a CMV, and they’re quite species-specific,” observed the renowned surgeon and xenotransplantation immunologist. “We showed almost 10 years ago that if PCMV was in a pig kidney, it led to a much shortened survival of the pig kidney in a baboon. There was never any evidence, however, that the CMV infected the baboon or any baboon cells.”
Dr. Sachs asked Dr. Mohiuddin for confirmation that Mr. Bennett displayed no more than DNAemia, circulating cell-free PCMV DNA presumably shed from the porcine heart, but no sign of the virus itself outside of the heart’s porcine cells.
Cell-free DNA had shown up in Mr. Bennett’s circulation about 20 days after the surgery, with concentrations rising until at least day 50. Post-hoc polymerase chain reaction (PCR) testing disclosed PCMV only in the pig’s spleen and porcine cells of the transplanted heart, Dr. Mohiuddin noted.
“We have not found any evidence that the patient was infected by PCMV,” nor was there evidence of any disease related to PCMV, Dr. Mohiuddin replied.
Nor of ongoing rejection
Mr. Bennett’s new heart passed a critical test in the first post-implantation hours by avoiding acute rejection, a potentially disastrous outcome that three of the pig’s 10 gene edits had been designed to prevent.
Although chronic immune rejection was always a concern despite Mr. Bennett’s novel immunosuppressant regimen, myocardial biopsy on postoperative days 34, 50, and 56 and necropsy showed “no signs of typical xenograft rejection,” Dr. Mohiuddin said at the ATC presentation. But “there’s a chance of atypical rejection which we were not accustomed to.”
By day 50, his diastolic function showed echocardiographic signs of deterioration, and “we started seeing interstitial edema with some extravasation of red blood cells, which we thought would resolve over a period of time,” he said. Eventually, however, “we saw that turn into fibroblasts and scar tissue.”
Mr. Bennett once again went on veno-arterial ECMO but died 10 days later. Once they had seen histologic evidence of fibrosis, Dr. Mohiuddin told this news organization, the team believed the myocardial injury was irreversible. “That was the reason we gave up on recovery.”
Mr. Bennett’s xenotransplantation journey has taught the field a lot, he said. “By no means was this a failure; we consider this a huge success. You can do all the experiments in animal models, but you won’t find out the true mechanism of rejection unless you do these kinds of human experiments.”
Looking ahead to clinical trials
Research involving humans is always subject to vagaries of human nature, including degree of adherence to prescribed therapy and – in xenotransplantation – precautions in place to mitigate any risks to public health. Such risks theoretically include transfer of porcine viruses or other pathogens to the patient and subsequent release into the general population.
Looking ahead to the possibility of clinical trials after this successful xenotransplantation experience, transplant nephrologist and epidemiologist Peter P. Reese, MD, PhD, University of Pennsylvania, Philadelphia, raised the potentially controversial issue in discussion following Dr. Mohiuddin’s presentation.
It’s known that Mr. Bennett had been repeatedly turned down for a conventional allograft transplant primarily because of his history of treatment noncompliance. Should such a record, Dr. Reese asked, be a relative contraindication to enrollment in any future xenotransplantation trials? Or does the field need a standardized gauge of a patient’s readiness, once discharged, to adhere not only to all medications – including those that fight infection – but also with rules established for public safety, such as routine contact reporting?
“It makes me wonder about choosing a noncompliant patient for these trials,” Dr. Reese said. “If we discharge a patient from the hospital who is at risk for a zoonotic infection that could spread if they basically refuse to cooperate with us or with public health authorities, it really could have negative consequences for the reputation of the field.”
Dr. Mohiuddin agreed such concerns are valid. Mr. Bennett “and all his immediate contacts” signed consent forms acknowledging their willingness to be followed should he be discharged. Mr. Bennett himself “signed a consent to inform us if he has any other intimate contact with someone,” he said in an interview.
“But those are only on paper.” Had Mr. Bennett survived to be discharged, Dr. Mohuiddin said, “no one knows how he would have behaved.”
Dr. Mohiuddin said the research staff had prepared to monitor Mr. Bennett at his home if that’s what it took. “We were ready to follow him as long as we could. There was a surveillance plan in place.”
A version of this article first appeared on Medscape.com.
Prediabetes is linked independently to myocardial infarction
Prediabetes is not only a predictor of diabetes and the cardiovascular complications that ensue, but it is also a risk factor by itself for myocardial infarction, according to data drawn from almost 1.8 million patients hospitalized for MI.
“Our study serves as a wakeup call for clinicians and patients to shift the focus to preventing prediabetes, and not just diabetes, said Geethika Thota, MD, at the annual meeting of the Endocrine Society.
There are plenty of data suggesting that prediabetes places patients on a trajectory toward cardiovascular disease. In a meta-analysis of 129 studies published 2 years ago, prediabetes was not only associated with a statistically significant 16% increase in coronary heart disease, but also a 13% increased risk of all-cause mortality relative to those with normoglycemia.
Data drawn from 1.8 million patients
In this study, 1,794,149 weighted patient hospitalizations for MI were drawn from the National Inpatient Sample database. Excluding patients who eventually developed diabetes, roughly 1% of these patients had a history of prediabetes in the past, according to a search of ICD-10 codes.
Before adjustment for other risk factors, prediabetes was linked to a greater than 40% increased odds of MI (odds ratio, 1.41; P < .01). After adjustment for a large array of known MI risk factors – including prior history of MI, dyslipidemia, hypertension, nicotine dependence, and obesity – prediabetes remained an independent risk factor, corresponding with a 25% increased risk of MI (OR, 1.25; P < .01).
A history of prediabetes was also an independent risk factor for percutaneous intervention and coronary artery bypass grafting, with increased risk of 45% and 95%, respectively.
As a retrospective study looking at prediabetes as a risk factor in those who already had a MI, it is possible that not all patients with prediabetes were properly coded, but Dr. Thota said that was unlikely to have been an issue of sufficient magnitude to have affected the major conclusions.
Relevance seen for community care
Although the study was drawn from hospitalized patients, its relevance is for the community setting, where screening and intervention for prediabetes has the potential to alter the risk, according to Dr. Thota.
Most clinicians are likely aware of the value of screening for prediabetes, which was defined in this study as a hemoglobin A1c of 5.7%-6.4%, but Dr. Thota suggested that many might not fully grasp the full scope of goals. Early detection and prevention will prevent diabetes and, by extension, cardiovascular disease, but her data suggest that control of prediabetes with lower cardiovascular risk by a more direct route.
“Despite mounting evidence, many clinicians are unaware that prediabetes is also a major risk factor for atherosclerotic cardiovascular disease,” said Dr. Thota, an internal medicine resident at Saint Peter’s University Hospital, New Brunswick, N.J.
Like diabetes, the prevalence of prediabetes is growing rapidly, according to data from the Centers for Disease Control that Dr. Thota cited. In 2020, the Centers for Disease Control and Prevention estimated that 38% of the adult population have prediabetes. By 2030, one model predicts a further 25% growth.
Screening for hyperglycemia is part of routine patient evaluations at Dr. Thota’s center. In an interview, she said that once a diagnosis of prediabetes is entered in the electronic medical record, the history is carried forward so that changes in status are continually monitored.
Worsening prediabetes should be addressed
“Prediabetes is not treated with medication, at least initially,” Dr. Thota explained. Rather, patients are educated about important lifestyle changes, such as diet and physical activity, that can reverse the diagnosis. However, patients who remain on a path of worsening hyperglycemia are candidates for more intensive lifestyle intervention and might be considered selectively for metformin.
“Early recognition of prediabetes through screening is important,” Dr. Thota emphasized. The benefit for preventing patients from progressing to diabetes is well recognized, but these data provide the basis for incentivizing lifestyle changes in patients with prediabetes by telling them that it can reduce their risk for MI.
These data have an important message, but they are not surprising, according to Deepak L. Bhatt, MD, executive director, interventional cardiovascular programs, Brigham and Women’s Hospital Heart & Vascular Center, Boston.
“In fact, in daily practice we see a substantial percentage of patients with MI who have prediabetes that had not been previously recognized or formally diagnosed,” Dr. Bhatt said in an interview.
“Identifying these patients – preferably prior to coming in with cardiovascular complications – is important both to reduce cardiovascular risk but also to try and prevent progression at diabetes,” he added.
Dr. Bhatt went on to say that this large analysis, confirming that prediabetes is independently associated with MI, should prompt clinicians to screen patients rigorously for this condition.
“At a minimum, such patients would be candidates for intensive lifestyle modification aimed at weight loss and treatment of frequent coexistent conditions, such as hypertension and dyslipidemia,” Dr. Bhatt said.
Dr. Thota reports no potential conflicts of interest. Dr. Bhatt has financial relationships with more than 30 pharmaceutical companies, many of which make products relevant to the management of diabetes and cardiovascular disease.
Prediabetes is not only a predictor of diabetes and the cardiovascular complications that ensue, but it is also a risk factor by itself for myocardial infarction, according to data drawn from almost 1.8 million patients hospitalized for MI.
“Our study serves as a wakeup call for clinicians and patients to shift the focus to preventing prediabetes, and not just diabetes, said Geethika Thota, MD, at the annual meeting of the Endocrine Society.
There are plenty of data suggesting that prediabetes places patients on a trajectory toward cardiovascular disease. In a meta-analysis of 129 studies published 2 years ago, prediabetes was not only associated with a statistically significant 16% increase in coronary heart disease, but also a 13% increased risk of all-cause mortality relative to those with normoglycemia.
Data drawn from 1.8 million patients
In this study, 1,794,149 weighted patient hospitalizations for MI were drawn from the National Inpatient Sample database. Excluding patients who eventually developed diabetes, roughly 1% of these patients had a history of prediabetes in the past, according to a search of ICD-10 codes.
Before adjustment for other risk factors, prediabetes was linked to a greater than 40% increased odds of MI (odds ratio, 1.41; P < .01). After adjustment for a large array of known MI risk factors – including prior history of MI, dyslipidemia, hypertension, nicotine dependence, and obesity – prediabetes remained an independent risk factor, corresponding with a 25% increased risk of MI (OR, 1.25; P < .01).
A history of prediabetes was also an independent risk factor for percutaneous intervention and coronary artery bypass grafting, with increased risk of 45% and 95%, respectively.
As a retrospective study looking at prediabetes as a risk factor in those who already had a MI, it is possible that not all patients with prediabetes were properly coded, but Dr. Thota said that was unlikely to have been an issue of sufficient magnitude to have affected the major conclusions.
Relevance seen for community care
Although the study was drawn from hospitalized patients, its relevance is for the community setting, where screening and intervention for prediabetes has the potential to alter the risk, according to Dr. Thota.
Most clinicians are likely aware of the value of screening for prediabetes, which was defined in this study as a hemoglobin A1c of 5.7%-6.4%, but Dr. Thota suggested that many might not fully grasp the full scope of goals. Early detection and prevention will prevent diabetes and, by extension, cardiovascular disease, but her data suggest that control of prediabetes with lower cardiovascular risk by a more direct route.
“Despite mounting evidence, many clinicians are unaware that prediabetes is also a major risk factor for atherosclerotic cardiovascular disease,” said Dr. Thota, an internal medicine resident at Saint Peter’s University Hospital, New Brunswick, N.J.
Like diabetes, the prevalence of prediabetes is growing rapidly, according to data from the Centers for Disease Control that Dr. Thota cited. In 2020, the Centers for Disease Control and Prevention estimated that 38% of the adult population have prediabetes. By 2030, one model predicts a further 25% growth.
Screening for hyperglycemia is part of routine patient evaluations at Dr. Thota’s center. In an interview, she said that once a diagnosis of prediabetes is entered in the electronic medical record, the history is carried forward so that changes in status are continually monitored.
Worsening prediabetes should be addressed
“Prediabetes is not treated with medication, at least initially,” Dr. Thota explained. Rather, patients are educated about important lifestyle changes, such as diet and physical activity, that can reverse the diagnosis. However, patients who remain on a path of worsening hyperglycemia are candidates for more intensive lifestyle intervention and might be considered selectively for metformin.
“Early recognition of prediabetes through screening is important,” Dr. Thota emphasized. The benefit for preventing patients from progressing to diabetes is well recognized, but these data provide the basis for incentivizing lifestyle changes in patients with prediabetes by telling them that it can reduce their risk for MI.
These data have an important message, but they are not surprising, according to Deepak L. Bhatt, MD, executive director, interventional cardiovascular programs, Brigham and Women’s Hospital Heart & Vascular Center, Boston.
“In fact, in daily practice we see a substantial percentage of patients with MI who have prediabetes that had not been previously recognized or formally diagnosed,” Dr. Bhatt said in an interview.
“Identifying these patients – preferably prior to coming in with cardiovascular complications – is important both to reduce cardiovascular risk but also to try and prevent progression at diabetes,” he added.
Dr. Bhatt went on to say that this large analysis, confirming that prediabetes is independently associated with MI, should prompt clinicians to screen patients rigorously for this condition.
“At a minimum, such patients would be candidates for intensive lifestyle modification aimed at weight loss and treatment of frequent coexistent conditions, such as hypertension and dyslipidemia,” Dr. Bhatt said.
Dr. Thota reports no potential conflicts of interest. Dr. Bhatt has financial relationships with more than 30 pharmaceutical companies, many of which make products relevant to the management of diabetes and cardiovascular disease.
Prediabetes is not only a predictor of diabetes and the cardiovascular complications that ensue, but it is also a risk factor by itself for myocardial infarction, according to data drawn from almost 1.8 million patients hospitalized for MI.
“Our study serves as a wakeup call for clinicians and patients to shift the focus to preventing prediabetes, and not just diabetes, said Geethika Thota, MD, at the annual meeting of the Endocrine Society.
There are plenty of data suggesting that prediabetes places patients on a trajectory toward cardiovascular disease. In a meta-analysis of 129 studies published 2 years ago, prediabetes was not only associated with a statistically significant 16% increase in coronary heart disease, but also a 13% increased risk of all-cause mortality relative to those with normoglycemia.
Data drawn from 1.8 million patients
In this study, 1,794,149 weighted patient hospitalizations for MI were drawn from the National Inpatient Sample database. Excluding patients who eventually developed diabetes, roughly 1% of these patients had a history of prediabetes in the past, according to a search of ICD-10 codes.
Before adjustment for other risk factors, prediabetes was linked to a greater than 40% increased odds of MI (odds ratio, 1.41; P < .01). After adjustment for a large array of known MI risk factors – including prior history of MI, dyslipidemia, hypertension, nicotine dependence, and obesity – prediabetes remained an independent risk factor, corresponding with a 25% increased risk of MI (OR, 1.25; P < .01).
A history of prediabetes was also an independent risk factor for percutaneous intervention and coronary artery bypass grafting, with increased risk of 45% and 95%, respectively.
As a retrospective study looking at prediabetes as a risk factor in those who already had a MI, it is possible that not all patients with prediabetes were properly coded, but Dr. Thota said that was unlikely to have been an issue of sufficient magnitude to have affected the major conclusions.
Relevance seen for community care
Although the study was drawn from hospitalized patients, its relevance is for the community setting, where screening and intervention for prediabetes has the potential to alter the risk, according to Dr. Thota.
Most clinicians are likely aware of the value of screening for prediabetes, which was defined in this study as a hemoglobin A1c of 5.7%-6.4%, but Dr. Thota suggested that many might not fully grasp the full scope of goals. Early detection and prevention will prevent diabetes and, by extension, cardiovascular disease, but her data suggest that control of prediabetes with lower cardiovascular risk by a more direct route.
“Despite mounting evidence, many clinicians are unaware that prediabetes is also a major risk factor for atherosclerotic cardiovascular disease,” said Dr. Thota, an internal medicine resident at Saint Peter’s University Hospital, New Brunswick, N.J.
Like diabetes, the prevalence of prediabetes is growing rapidly, according to data from the Centers for Disease Control that Dr. Thota cited. In 2020, the Centers for Disease Control and Prevention estimated that 38% of the adult population have prediabetes. By 2030, one model predicts a further 25% growth.
Screening for hyperglycemia is part of routine patient evaluations at Dr. Thota’s center. In an interview, she said that once a diagnosis of prediabetes is entered in the electronic medical record, the history is carried forward so that changes in status are continually monitored.
Worsening prediabetes should be addressed
“Prediabetes is not treated with medication, at least initially,” Dr. Thota explained. Rather, patients are educated about important lifestyle changes, such as diet and physical activity, that can reverse the diagnosis. However, patients who remain on a path of worsening hyperglycemia are candidates for more intensive lifestyle intervention and might be considered selectively for metformin.
“Early recognition of prediabetes through screening is important,” Dr. Thota emphasized. The benefit for preventing patients from progressing to diabetes is well recognized, but these data provide the basis for incentivizing lifestyle changes in patients with prediabetes by telling them that it can reduce their risk for MI.
These data have an important message, but they are not surprising, according to Deepak L. Bhatt, MD, executive director, interventional cardiovascular programs, Brigham and Women’s Hospital Heart & Vascular Center, Boston.
“In fact, in daily practice we see a substantial percentage of patients with MI who have prediabetes that had not been previously recognized or formally diagnosed,” Dr. Bhatt said in an interview.
“Identifying these patients – preferably prior to coming in with cardiovascular complications – is important both to reduce cardiovascular risk but also to try and prevent progression at diabetes,” he added.
Dr. Bhatt went on to say that this large analysis, confirming that prediabetes is independently associated with MI, should prompt clinicians to screen patients rigorously for this condition.
“At a minimum, such patients would be candidates for intensive lifestyle modification aimed at weight loss and treatment of frequent coexistent conditions, such as hypertension and dyslipidemia,” Dr. Bhatt said.
Dr. Thota reports no potential conflicts of interest. Dr. Bhatt has financial relationships with more than 30 pharmaceutical companies, many of which make products relevant to the management of diabetes and cardiovascular disease.
FROM ENDO 2022
Avexitide promising for hypoglycemia after weight-loss surgery
Avexitide (Eiger Biopharmaceuticals), a first-in-class glucagonlike peptide (GLP)–1 receptor blocker, significantly reduced hypoglycemia in patients with refractory postbariatric hypoglycemia, new research finds.
Postbariatric hypoglycemia is a complication of bariatric surgery that is estimated to occur in about 29%-34% of people who undergo Roux-en-Y gastric bypass and in 11%-23% of those who undergo vertical sleeve gastrectomy. It typically manifests about 1-3 hours after meals and can lead to severe neuroglycopenic symptoms including blurred vision, confusion, drowsiness, and incoordination.
In addition, more than one-third (37%) with the condition have hypoglycemic unawareness. This can lead to seizures in about 59%, loss of consciousness and hospitalization in 50%, motor vehicle accidents, and even death. More than 90% with the condition consider themselves disabled, and 41% report being unable to work.
There are no currently approved medical treatments for postbariatric hypoglycemia. The standard of care is medical nutrition therapy involving a low-carbohydrate diet with carb restriction and small, frequent mixed meals. If this doesn’t work, off-label stepped pharmacotherapy has been tried, including acarbose (Precose), octreotide (Sandostatin), and diazoxide (Proglycem).
But “these are limited by efficacy and tolerability,” said Marilyn Tan, MD, who presented the findings from the phase 2 trial of avexitide at the annual meeting of the Endocrine Society.
In very severe cases, gastrostomy tubes or bypass reversal are options but those lead to weight regain and incomplete efficacy. “Safe, effective, and targeted therapies are needed urgently for postbariatric hypoglycemia,” said Dr. Tan, of the department of endocrinology at Stanford (Calif.) University.
The pathophysiology isn’t fully understood, but there appears to be an exaggerated GLP-1 response that leads to abnormal insulin secretion and symptomatic hyperinsulinemic hypoglycemia. Avexitide (formerly exendin 9-39), blocks the GLP-1 receptor and mitigates the excessive GLP-1 response, she explained.
Asked to comment, session moderator Michelle Van Name, MD, told this news organization, “This is a problem and it’s important for us to understand more about it and to identify different treatment options so these patients can continue to live their full, healthy lives post bariatric surgery.”
And, avexitide also holds potential for treating congenital hyperinsulinism, “which is a very challenging disease to treat in babies,” noted Dr. Van Name, a pediatric endocrinologist at Yale University, New Haven, Conn.
Drug reduced all levels of hypoglycemia, across surgery types
The study enrolled 14 women and 2 men with severe refractory postbariatric surgery hypoglycemia despite medical nutrition therapy. A majority (9) had undergone Roux-en-Y gastric bypass, 4 had vertical sleeve gastrectomy, 2 gastrectomy, and 1 had Nissen fundoplication. Seven patients (43.7%) had experienced loss of consciousness from hyperinsulinemic hypoglycemia. None had diabetes.
They were randomly assigned to either subcutaneous 45 mg of avexitide twice daily or 90 mg once daily for 14 days each, with a 2-day washout period followed by a switch to the other dose.
Both doses resulted in significant reductions in hypoglycemia as measured by self–blood glucose monitoring. The once-daily dose reduced level 1 hypoglycemia (glucose < 70 mg/dL) by 67.5% and it reduced level 2 (< 54 mg/dL) by 53.3% (P = .0043).
Even greater reductions were seen in severe hypoglycemia (that is, altered mental status/requiring assistance) – by 67.5% for the twice-daily dose (P = .0003) and by 66.1% with the once-daily dose (P = .0003).
“This is consistent with what we’ve seen in prior avexitide trials,” Dr. Tan noted.
More hypoglycemic events were captured using blinded continuous glucose monitoring (CGM), since it picked up episodes of which the patient was unaware. There were significant reductions in percentage time spent in level 1 and level 2 hypoglycemia, as well as in absolute number of hypoglycemic events over 14 days.
Here, the effect was greater with the once-daily 90 mg dose, with reductions of up to 65% in time spent and number of events, but results for the twice-daily dose were also significant, Dr. Tan said.
The drug was effective across all surgical subtypes. Patients who underwent vertical sleeve gastrectomy/gastrectomy had greater rates of hypoglycemia at baseline and “robust responses to avexitide subcutaneous injections. This supports the critical role of GLP-1,” Dr. Tan said.
There were no severe adverse events. The most commonly reported adverse events were diarrhea, headache, bloating, and injection-site reaction/bruising. All were mild and self-limited and resolved without treatment. No participants withdrew from the study.
Eiger Biopharmaceuticals is currently working with the U.S. Food and Drug Administration and the European Medicines Agency to design a phase 3 trial.
The study is an investor-initiated trial with funding from Eiger Biopharmaceuticals. Dr. Tan receives research support from Eiger Biopharmaceuticals as a site investigator. Dr. Van Name is an investigator for a trial sponsored by Provention Bio.
A version of this article first appeared on Medscape.com.
Avexitide (Eiger Biopharmaceuticals), a first-in-class glucagonlike peptide (GLP)–1 receptor blocker, significantly reduced hypoglycemia in patients with refractory postbariatric hypoglycemia, new research finds.
Postbariatric hypoglycemia is a complication of bariatric surgery that is estimated to occur in about 29%-34% of people who undergo Roux-en-Y gastric bypass and in 11%-23% of those who undergo vertical sleeve gastrectomy. It typically manifests about 1-3 hours after meals and can lead to severe neuroglycopenic symptoms including blurred vision, confusion, drowsiness, and incoordination.
In addition, more than one-third (37%) with the condition have hypoglycemic unawareness. This can lead to seizures in about 59%, loss of consciousness and hospitalization in 50%, motor vehicle accidents, and even death. More than 90% with the condition consider themselves disabled, and 41% report being unable to work.
There are no currently approved medical treatments for postbariatric hypoglycemia. The standard of care is medical nutrition therapy involving a low-carbohydrate diet with carb restriction and small, frequent mixed meals. If this doesn’t work, off-label stepped pharmacotherapy has been tried, including acarbose (Precose), octreotide (Sandostatin), and diazoxide (Proglycem).
But “these are limited by efficacy and tolerability,” said Marilyn Tan, MD, who presented the findings from the phase 2 trial of avexitide at the annual meeting of the Endocrine Society.
In very severe cases, gastrostomy tubes or bypass reversal are options but those lead to weight regain and incomplete efficacy. “Safe, effective, and targeted therapies are needed urgently for postbariatric hypoglycemia,” said Dr. Tan, of the department of endocrinology at Stanford (Calif.) University.
The pathophysiology isn’t fully understood, but there appears to be an exaggerated GLP-1 response that leads to abnormal insulin secretion and symptomatic hyperinsulinemic hypoglycemia. Avexitide (formerly exendin 9-39), blocks the GLP-1 receptor and mitigates the excessive GLP-1 response, she explained.
Asked to comment, session moderator Michelle Van Name, MD, told this news organization, “This is a problem and it’s important for us to understand more about it and to identify different treatment options so these patients can continue to live their full, healthy lives post bariatric surgery.”
And, avexitide also holds potential for treating congenital hyperinsulinism, “which is a very challenging disease to treat in babies,” noted Dr. Van Name, a pediatric endocrinologist at Yale University, New Haven, Conn.
Drug reduced all levels of hypoglycemia, across surgery types
The study enrolled 14 women and 2 men with severe refractory postbariatric surgery hypoglycemia despite medical nutrition therapy. A majority (9) had undergone Roux-en-Y gastric bypass, 4 had vertical sleeve gastrectomy, 2 gastrectomy, and 1 had Nissen fundoplication. Seven patients (43.7%) had experienced loss of consciousness from hyperinsulinemic hypoglycemia. None had diabetes.
They were randomly assigned to either subcutaneous 45 mg of avexitide twice daily or 90 mg once daily for 14 days each, with a 2-day washout period followed by a switch to the other dose.
Both doses resulted in significant reductions in hypoglycemia as measured by self–blood glucose monitoring. The once-daily dose reduced level 1 hypoglycemia (glucose < 70 mg/dL) by 67.5% and it reduced level 2 (< 54 mg/dL) by 53.3% (P = .0043).
Even greater reductions were seen in severe hypoglycemia (that is, altered mental status/requiring assistance) – by 67.5% for the twice-daily dose (P = .0003) and by 66.1% with the once-daily dose (P = .0003).
“This is consistent with what we’ve seen in prior avexitide trials,” Dr. Tan noted.
More hypoglycemic events were captured using blinded continuous glucose monitoring (CGM), since it picked up episodes of which the patient was unaware. There were significant reductions in percentage time spent in level 1 and level 2 hypoglycemia, as well as in absolute number of hypoglycemic events over 14 days.
Here, the effect was greater with the once-daily 90 mg dose, with reductions of up to 65% in time spent and number of events, but results for the twice-daily dose were also significant, Dr. Tan said.
The drug was effective across all surgical subtypes. Patients who underwent vertical sleeve gastrectomy/gastrectomy had greater rates of hypoglycemia at baseline and “robust responses to avexitide subcutaneous injections. This supports the critical role of GLP-1,” Dr. Tan said.
There were no severe adverse events. The most commonly reported adverse events were diarrhea, headache, bloating, and injection-site reaction/bruising. All were mild and self-limited and resolved without treatment. No participants withdrew from the study.
Eiger Biopharmaceuticals is currently working with the U.S. Food and Drug Administration and the European Medicines Agency to design a phase 3 trial.
The study is an investor-initiated trial with funding from Eiger Biopharmaceuticals. Dr. Tan receives research support from Eiger Biopharmaceuticals as a site investigator. Dr. Van Name is an investigator for a trial sponsored by Provention Bio.
A version of this article first appeared on Medscape.com.
Avexitide (Eiger Biopharmaceuticals), a first-in-class glucagonlike peptide (GLP)–1 receptor blocker, significantly reduced hypoglycemia in patients with refractory postbariatric hypoglycemia, new research finds.
Postbariatric hypoglycemia is a complication of bariatric surgery that is estimated to occur in about 29%-34% of people who undergo Roux-en-Y gastric bypass and in 11%-23% of those who undergo vertical sleeve gastrectomy. It typically manifests about 1-3 hours after meals and can lead to severe neuroglycopenic symptoms including blurred vision, confusion, drowsiness, and incoordination.
In addition, more than one-third (37%) with the condition have hypoglycemic unawareness. This can lead to seizures in about 59%, loss of consciousness and hospitalization in 50%, motor vehicle accidents, and even death. More than 90% with the condition consider themselves disabled, and 41% report being unable to work.
There are no currently approved medical treatments for postbariatric hypoglycemia. The standard of care is medical nutrition therapy involving a low-carbohydrate diet with carb restriction and small, frequent mixed meals. If this doesn’t work, off-label stepped pharmacotherapy has been tried, including acarbose (Precose), octreotide (Sandostatin), and diazoxide (Proglycem).
But “these are limited by efficacy and tolerability,” said Marilyn Tan, MD, who presented the findings from the phase 2 trial of avexitide at the annual meeting of the Endocrine Society.
In very severe cases, gastrostomy tubes or bypass reversal are options but those lead to weight regain and incomplete efficacy. “Safe, effective, and targeted therapies are needed urgently for postbariatric hypoglycemia,” said Dr. Tan, of the department of endocrinology at Stanford (Calif.) University.
The pathophysiology isn’t fully understood, but there appears to be an exaggerated GLP-1 response that leads to abnormal insulin secretion and symptomatic hyperinsulinemic hypoglycemia. Avexitide (formerly exendin 9-39), blocks the GLP-1 receptor and mitigates the excessive GLP-1 response, she explained.
Asked to comment, session moderator Michelle Van Name, MD, told this news organization, “This is a problem and it’s important for us to understand more about it and to identify different treatment options so these patients can continue to live their full, healthy lives post bariatric surgery.”
And, avexitide also holds potential for treating congenital hyperinsulinism, “which is a very challenging disease to treat in babies,” noted Dr. Van Name, a pediatric endocrinologist at Yale University, New Haven, Conn.
Drug reduced all levels of hypoglycemia, across surgery types
The study enrolled 14 women and 2 men with severe refractory postbariatric surgery hypoglycemia despite medical nutrition therapy. A majority (9) had undergone Roux-en-Y gastric bypass, 4 had vertical sleeve gastrectomy, 2 gastrectomy, and 1 had Nissen fundoplication. Seven patients (43.7%) had experienced loss of consciousness from hyperinsulinemic hypoglycemia. None had diabetes.
They were randomly assigned to either subcutaneous 45 mg of avexitide twice daily or 90 mg once daily for 14 days each, with a 2-day washout period followed by a switch to the other dose.
Both doses resulted in significant reductions in hypoglycemia as measured by self–blood glucose monitoring. The once-daily dose reduced level 1 hypoglycemia (glucose < 70 mg/dL) by 67.5% and it reduced level 2 (< 54 mg/dL) by 53.3% (P = .0043).
Even greater reductions were seen in severe hypoglycemia (that is, altered mental status/requiring assistance) – by 67.5% for the twice-daily dose (P = .0003) and by 66.1% with the once-daily dose (P = .0003).
“This is consistent with what we’ve seen in prior avexitide trials,” Dr. Tan noted.
More hypoglycemic events were captured using blinded continuous glucose monitoring (CGM), since it picked up episodes of which the patient was unaware. There were significant reductions in percentage time spent in level 1 and level 2 hypoglycemia, as well as in absolute number of hypoglycemic events over 14 days.
Here, the effect was greater with the once-daily 90 mg dose, with reductions of up to 65% in time spent and number of events, but results for the twice-daily dose were also significant, Dr. Tan said.
The drug was effective across all surgical subtypes. Patients who underwent vertical sleeve gastrectomy/gastrectomy had greater rates of hypoglycemia at baseline and “robust responses to avexitide subcutaneous injections. This supports the critical role of GLP-1,” Dr. Tan said.
There were no severe adverse events. The most commonly reported adverse events were diarrhea, headache, bloating, and injection-site reaction/bruising. All were mild and self-limited and resolved without treatment. No participants withdrew from the study.
Eiger Biopharmaceuticals is currently working with the U.S. Food and Drug Administration and the European Medicines Agency to design a phase 3 trial.
The study is an investor-initiated trial with funding from Eiger Biopharmaceuticals. Dr. Tan receives research support from Eiger Biopharmaceuticals as a site investigator. Dr. Van Name is an investigator for a trial sponsored by Provention Bio.
A version of this article first appeared on Medscape.com.
FROM ENDO 2022
Just 20 minutes of vigorous activity daily benefits teens
Vigorous physical activity for 20 minutes a day was enough to maximize cardiorespiratory benefits in adolescents, based on data from more than 300 individuals.
Current recommendations for physical activity in children and adolescents from the World Health Organization call for moderate to vigorous physical activity (MVPA) for an average of 60 minutes a day for physical and mental health; however, guidance on how much physical activity teens need to maximize cardiorespiratory fitness (CRF) has not been determined, Samuel Joseph Burden, BMedSci, of John Radcliffe Hospital Oxford (England), and colleagues wrote.
“Although data in young people are limited, adult studies have shown that regular, brief vigorous physical activity is highly effective at improving health markers, including CRF, which is also an important marker of health in youth,” the researchers wrote.
In a study published in Pediatrics, the researchers examined the associations between physical activity intensity and maximal CRF. The study population included 339 adolescents aged 13-14 years who were evaluated during the 2018-2019 and 2019-2020 school years. Participants wore wrist accelerometers to measure the intensity of physical activity and participated in 20-meter shuttle runs to demonstrate CRF. The researchers used partial multivariable linear regression to assess variables at different intensities including moderate physical activity (MPA), light physical activity (LPA), and sedentary time, as well as vigorous physical activity (VPA).
The wrist monitors measured the intensities of physical activity based on the bandpass-filtered followed by Euclidean norm metric (BFEN), a validated metric. “Previously validated thresholds for BFEN were used to determine the average duration of daily physical activity at each intensity: 0.1 g for LPA, 0.314 g for MPA, and 0.998 g for VPA,” the researchers wrote. Physical activity below the threshold for LPA was categorized as sedentary time.
Participants wore the accelerometers for 1 week; value recording included at least 3 weekdays and 1 weekend day, and each valid day required more than 6 hours of awake time.
Overall, VPA for up to 20 minutes was significantly associated with improved CRF. However, the benefits on CRF plateaued after that time, and longer duration of VPA was not associated with significantly greater improvements in CRF. Neither MPA nor LPA were associated with any improvements in CRF.
Participants who engaged in an average of 14 minutes (range, 12-17 minutes) of VPA per day met the median CRF.
The researchers also conducted independent t tests to assess differences in VPA at different CRF thresholds.
Those in the highest quartile of VPA had CRF z scores 1.03 higher, compared with those in the lower quartiles.
Given that current PA guidelines involve a combination of moderate and vigorous PA that could be met by MPA with no VPA, the current findings have public health implications for improving CRF in adolescents, the researchers wrote.
Even with MPA as an option, most adolescents fail to meet the recommendations of at least 60 minutes of MVPA, they said. “One possible reason is that this duration is quite long, requiring a daily time commitment that some may find difficult to maintain. A shorter target of 20 minutes might be easier to schedule daily and a focus on VPA would simplify messages about the intensity of activity that is likely to improve CRF.”
The study findings were limited by several factors including the use of data from only two schools in the United Kingdom, which may limit generalizability, and future research would ideally include a more direct assessment of VO2 max, the researchers wrote. However, the results were strengthened by the large and diverse study population, including teens with a wide range of body mass index as well as CRF.
Future research is needed to test whether interventions based on a target of 20 minutes of VPA creates significant improvements in adolescent cardiometabolic health, the researchers concluded.
Any activity has value for sedentary teens
The current study suggests that counseling teens about physical activity may be less challenging for clinicians if optimal cardiorespiratory benefits can be reached with shorter bouts of activity, Michele LaBotz, MD, of Intermed Sports Medicine, South Portland, Maine, and Sarah Hoffman, DO, of Tufts University, Boston, wrote in an accompanying editorial.
The results have two key implications for pediatricians, the authors said. First, “optimal CRF can be achieved with much shorter periods of activity than previously recommended.” Second, “current ‘moderate to vigorous’ PA recommendations may not be sufficient to improve CRF in adolescents, if achieved through moderate activity only.”
However, the editorialists emphasized that, although shorter periods of higher-intensity exercise reduce the time burden for teens and families, specific education is needed to explain the extra effort involved in exercising vigorously enough for cardiorespiratory benefits.
Patients can be counseled that activity is vigorous when they start to sweat, their face gets red, and they feel short of breath and unable to talk during activity,” they explained. These sensations may be new and uncomfortable for children and teens who have been quite sedentary or used to low-intensity activity. Dr. LaBotz and Dr. Hoffman advised pediatricians to counsel patients to build intensity gradually, with “exercise snacks,” that involve several minutes of activity that become more challenging over time.
“Exercise snacks can include anything that elevates the heart rate for a minute or more, such as running up and down the stairs a few times; chasing the dog around the backyard; or just putting on some music and dancing hard,” the editorialists wrote.
“Some exercise is better than none, and extrapolating from adult data, the biggest benefit likely occurs when we can help our most sedentary and least fit patients become a bit more active, even if it falls short of currently recommended levels,” they concluded.
The study was supported by grants to various researchers from the British Heart Foundation, the Elizabeth Casson Trust, the U.K. National Institute of Health Research, the Professor Nigel Groome Studentship scheme (Oxford Brookes University), and the U.K. Department of Health. The researchers had no financial conflicts to disclose. The editorialists had no financial conflicts to disclose.
Vigorous physical activity for 20 minutes a day was enough to maximize cardiorespiratory benefits in adolescents, based on data from more than 300 individuals.
Current recommendations for physical activity in children and adolescents from the World Health Organization call for moderate to vigorous physical activity (MVPA) for an average of 60 minutes a day for physical and mental health; however, guidance on how much physical activity teens need to maximize cardiorespiratory fitness (CRF) has not been determined, Samuel Joseph Burden, BMedSci, of John Radcliffe Hospital Oxford (England), and colleagues wrote.
“Although data in young people are limited, adult studies have shown that regular, brief vigorous physical activity is highly effective at improving health markers, including CRF, which is also an important marker of health in youth,” the researchers wrote.
In a study published in Pediatrics, the researchers examined the associations between physical activity intensity and maximal CRF. The study population included 339 adolescents aged 13-14 years who were evaluated during the 2018-2019 and 2019-2020 school years. Participants wore wrist accelerometers to measure the intensity of physical activity and participated in 20-meter shuttle runs to demonstrate CRF. The researchers used partial multivariable linear regression to assess variables at different intensities including moderate physical activity (MPA), light physical activity (LPA), and sedentary time, as well as vigorous physical activity (VPA).
The wrist monitors measured the intensities of physical activity based on the bandpass-filtered followed by Euclidean norm metric (BFEN), a validated metric. “Previously validated thresholds for BFEN were used to determine the average duration of daily physical activity at each intensity: 0.1 g for LPA, 0.314 g for MPA, and 0.998 g for VPA,” the researchers wrote. Physical activity below the threshold for LPA was categorized as sedentary time.
Participants wore the accelerometers for 1 week; value recording included at least 3 weekdays and 1 weekend day, and each valid day required more than 6 hours of awake time.
Overall, VPA for up to 20 minutes was significantly associated with improved CRF. However, the benefits on CRF plateaued after that time, and longer duration of VPA was not associated with significantly greater improvements in CRF. Neither MPA nor LPA were associated with any improvements in CRF.
Participants who engaged in an average of 14 minutes (range, 12-17 minutes) of VPA per day met the median CRF.
The researchers also conducted independent t tests to assess differences in VPA at different CRF thresholds.
Those in the highest quartile of VPA had CRF z scores 1.03 higher, compared with those in the lower quartiles.
Given that current PA guidelines involve a combination of moderate and vigorous PA that could be met by MPA with no VPA, the current findings have public health implications for improving CRF in adolescents, the researchers wrote.
Even with MPA as an option, most adolescents fail to meet the recommendations of at least 60 minutes of MVPA, they said. “One possible reason is that this duration is quite long, requiring a daily time commitment that some may find difficult to maintain. A shorter target of 20 minutes might be easier to schedule daily and a focus on VPA would simplify messages about the intensity of activity that is likely to improve CRF.”
The study findings were limited by several factors including the use of data from only two schools in the United Kingdom, which may limit generalizability, and future research would ideally include a more direct assessment of VO2 max, the researchers wrote. However, the results were strengthened by the large and diverse study population, including teens with a wide range of body mass index as well as CRF.
Future research is needed to test whether interventions based on a target of 20 minutes of VPA creates significant improvements in adolescent cardiometabolic health, the researchers concluded.
Any activity has value for sedentary teens
The current study suggests that counseling teens about physical activity may be less challenging for clinicians if optimal cardiorespiratory benefits can be reached with shorter bouts of activity, Michele LaBotz, MD, of Intermed Sports Medicine, South Portland, Maine, and Sarah Hoffman, DO, of Tufts University, Boston, wrote in an accompanying editorial.
The results have two key implications for pediatricians, the authors said. First, “optimal CRF can be achieved with much shorter periods of activity than previously recommended.” Second, “current ‘moderate to vigorous’ PA recommendations may not be sufficient to improve CRF in adolescents, if achieved through moderate activity only.”
However, the editorialists emphasized that, although shorter periods of higher-intensity exercise reduce the time burden for teens and families, specific education is needed to explain the extra effort involved in exercising vigorously enough for cardiorespiratory benefits.
Patients can be counseled that activity is vigorous when they start to sweat, their face gets red, and they feel short of breath and unable to talk during activity,” they explained. These sensations may be new and uncomfortable for children and teens who have been quite sedentary or used to low-intensity activity. Dr. LaBotz and Dr. Hoffman advised pediatricians to counsel patients to build intensity gradually, with “exercise snacks,” that involve several minutes of activity that become more challenging over time.
“Exercise snacks can include anything that elevates the heart rate for a minute or more, such as running up and down the stairs a few times; chasing the dog around the backyard; or just putting on some music and dancing hard,” the editorialists wrote.
“Some exercise is better than none, and extrapolating from adult data, the biggest benefit likely occurs when we can help our most sedentary and least fit patients become a bit more active, even if it falls short of currently recommended levels,” they concluded.
The study was supported by grants to various researchers from the British Heart Foundation, the Elizabeth Casson Trust, the U.K. National Institute of Health Research, the Professor Nigel Groome Studentship scheme (Oxford Brookes University), and the U.K. Department of Health. The researchers had no financial conflicts to disclose. The editorialists had no financial conflicts to disclose.
Vigorous physical activity for 20 minutes a day was enough to maximize cardiorespiratory benefits in adolescents, based on data from more than 300 individuals.
Current recommendations for physical activity in children and adolescents from the World Health Organization call for moderate to vigorous physical activity (MVPA) for an average of 60 minutes a day for physical and mental health; however, guidance on how much physical activity teens need to maximize cardiorespiratory fitness (CRF) has not been determined, Samuel Joseph Burden, BMedSci, of John Radcliffe Hospital Oxford (England), and colleagues wrote.
“Although data in young people are limited, adult studies have shown that regular, brief vigorous physical activity is highly effective at improving health markers, including CRF, which is also an important marker of health in youth,” the researchers wrote.
In a study published in Pediatrics, the researchers examined the associations between physical activity intensity and maximal CRF. The study population included 339 adolescents aged 13-14 years who were evaluated during the 2018-2019 and 2019-2020 school years. Participants wore wrist accelerometers to measure the intensity of physical activity and participated in 20-meter shuttle runs to demonstrate CRF. The researchers used partial multivariable linear regression to assess variables at different intensities including moderate physical activity (MPA), light physical activity (LPA), and sedentary time, as well as vigorous physical activity (VPA).
The wrist monitors measured the intensities of physical activity based on the bandpass-filtered followed by Euclidean norm metric (BFEN), a validated metric. “Previously validated thresholds for BFEN were used to determine the average duration of daily physical activity at each intensity: 0.1 g for LPA, 0.314 g for MPA, and 0.998 g for VPA,” the researchers wrote. Physical activity below the threshold for LPA was categorized as sedentary time.
Participants wore the accelerometers for 1 week; value recording included at least 3 weekdays and 1 weekend day, and each valid day required more than 6 hours of awake time.
Overall, VPA for up to 20 minutes was significantly associated with improved CRF. However, the benefits on CRF plateaued after that time, and longer duration of VPA was not associated with significantly greater improvements in CRF. Neither MPA nor LPA were associated with any improvements in CRF.
Participants who engaged in an average of 14 minutes (range, 12-17 minutes) of VPA per day met the median CRF.
The researchers also conducted independent t tests to assess differences in VPA at different CRF thresholds.
Those in the highest quartile of VPA had CRF z scores 1.03 higher, compared with those in the lower quartiles.
Given that current PA guidelines involve a combination of moderate and vigorous PA that could be met by MPA with no VPA, the current findings have public health implications for improving CRF in adolescents, the researchers wrote.
Even with MPA as an option, most adolescents fail to meet the recommendations of at least 60 minutes of MVPA, they said. “One possible reason is that this duration is quite long, requiring a daily time commitment that some may find difficult to maintain. A shorter target of 20 minutes might be easier to schedule daily and a focus on VPA would simplify messages about the intensity of activity that is likely to improve CRF.”
The study findings were limited by several factors including the use of data from only two schools in the United Kingdom, which may limit generalizability, and future research would ideally include a more direct assessment of VO2 max, the researchers wrote. However, the results were strengthened by the large and diverse study population, including teens with a wide range of body mass index as well as CRF.
Future research is needed to test whether interventions based on a target of 20 minutes of VPA creates significant improvements in adolescent cardiometabolic health, the researchers concluded.
Any activity has value for sedentary teens
The current study suggests that counseling teens about physical activity may be less challenging for clinicians if optimal cardiorespiratory benefits can be reached with shorter bouts of activity, Michele LaBotz, MD, of Intermed Sports Medicine, South Portland, Maine, and Sarah Hoffman, DO, of Tufts University, Boston, wrote in an accompanying editorial.
The results have two key implications for pediatricians, the authors said. First, “optimal CRF can be achieved with much shorter periods of activity than previously recommended.” Second, “current ‘moderate to vigorous’ PA recommendations may not be sufficient to improve CRF in adolescents, if achieved through moderate activity only.”
However, the editorialists emphasized that, although shorter periods of higher-intensity exercise reduce the time burden for teens and families, specific education is needed to explain the extra effort involved in exercising vigorously enough for cardiorespiratory benefits.
Patients can be counseled that activity is vigorous when they start to sweat, their face gets red, and they feel short of breath and unable to talk during activity,” they explained. These sensations may be new and uncomfortable for children and teens who have been quite sedentary or used to low-intensity activity. Dr. LaBotz and Dr. Hoffman advised pediatricians to counsel patients to build intensity gradually, with “exercise snacks,” that involve several minutes of activity that become more challenging over time.
“Exercise snacks can include anything that elevates the heart rate for a minute or more, such as running up and down the stairs a few times; chasing the dog around the backyard; or just putting on some music and dancing hard,” the editorialists wrote.
“Some exercise is better than none, and extrapolating from adult data, the biggest benefit likely occurs when we can help our most sedentary and least fit patients become a bit more active, even if it falls short of currently recommended levels,” they concluded.
The study was supported by grants to various researchers from the British Heart Foundation, the Elizabeth Casson Trust, the U.K. National Institute of Health Research, the Professor Nigel Groome Studentship scheme (Oxford Brookes University), and the U.K. Department of Health. The researchers had no financial conflicts to disclose. The editorialists had no financial conflicts to disclose.
FROM PEDIATRICS
SGLT2 inhibitors cut AFib risk in real-word analysis
NEW ORLEANS – The case continues to grow for prioritizing a sodium-glucose transporter 2 (SGLT2) inhibitor in patients with type 2 diabetes, as real-world evidence of benefit and safety accumulates on top of the data from randomized trials that first established this class as a management pillar.
Another important effect of these agents gaining increasing currency, on top of their well-established benefits in patients with type 2 diabetes for preventing acute heart failure exacerbations and slowing progression of diabetic kidney disease, is that they cut the incidence of new-onset atrial fibrillation (AFib). That effect was confirmed in an analysis of data from about 300,000 U.S. patients included in recent Medicare records, Elisabetta Patorno, MD, reported at the annual scientific sessions of the American Diabetes Association.
But despite documentation like this, real-world evidence also continues to show limited uptake of SGLT2 inhibitors in U.S. patients with type 2 diabetes. Records from more than 1.3 million patients with type 2 diabetes managed in the Veterans Affairs Healthcare System during 2019 or 2022 documented that just 10% of these patients received an agent from this class, even though all were eligible to receive it, according to findings in a separate report at the meeting.
The AFib analysis analyzed two sets of propensity score–matched Medicare patients during 2013-2018 aged 65 years or older with type 2 diabetes and no history of AFib. One analysis focused on 80,475 matched patients who started on treatment with either an SGLT2 inhibitor or a glucagonlike peptide–1 (GLP-1) receptor agonist, and a second on 74,868 matched patients who began either an SGTL2 inhibitor or a dipeptidyl peptidase–4 (DPP4) inhibitor. In both analyses, matching involved more than 130 variables. In both pair sets, patients at baseline averaged about 72 years old, nearly two-thirds were women, about 8%-9% had heart failure, 77%-80% were on metformin, and 20%-25% were using insulin.
The study’s primary endpoint was the incidence of hospitalization for AFib, which occurred a significant 18% less often in the patients who started on an SGLT2, compared with those who started a DPP4 inhibitor during median follow-up of 6.7 months, and a significant 10% less often, compared with those starting a GLP-1 receptor agonist during a median follow-up of 6.0 months, Elisabetta Patorno, MD, DrPH, reported at the meeting. This worked out to 3.7 fewer hospitalizations for AFib per 1,000 patient-years of follow-up among the people who received an SGLT2 inhibitor, compared with a DPP4 inhibitor, and a decrease of 1.8 hospitalizations/1,000 patient-years when compared against patients in a GLP-1 receptor agonist.
Two secondary outcomes showed significantly fewer episodes of newly diagnosed AFib, and significantly fewer patients initiating AFib treatment among those who received an SGLT2 inhibitor relative to the comparator groups. In addition, these associations were consistent across subgroup analyses that divided patients by their age, sex, history of heart failure, and history of atherosclerotic cardiovascular disease.
AFib effects add to benefits
The findings “suggest that initiation of an SGLT2 inhibitor may be beneficial in older adults with type 2 diabetes who are at risk for AFib,” said Dr. Patorno, a researcher in the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital, Boston. “These new findings on AFib may be helpful when weighing the potential risks and benefits of various glucose-lowering drugs in older patients with type 2 diabetes.”
This new evidence follows several prior reports from other research groups of data supporting an AFib benefit from SGLT2 inhibitors. The earlier reports include a post hoc analysis of more than 17,000 patients enrolled in the DECLARE-TIMI 58 cardiovascular outcome trial of dapagliflozin (Farxiga), which showed a 19% relative decrease in the rate of incident AFib or atrial flutter events during a median 4.2 year follow-up.
Other prior reports that found a reduced incidence of AFib events linked with SGLT2 inhibitor treatment include a 2020 meta-analysis based on data from more than 38,000 patients with type 2 diabetes enrolled in any of 16 randomized, controlled trials, which found a 24% relative risk reduction. And an as-yet unpublished report from researchers at the University of Rochester (N.Y.) and their associates presented in November 2021 at the annual scientific sessions of the American Heart Association that documented a significant 24% relative risk reduction in incident AFib events linked to SGLT2 inhibitor treatment in a prospective study of 13,890 patients at several hospitals in Israel or the United States.
Evidence ‘convincing’ in totality
The accumulated evidence for a reduced incidence of AFib when patients were on treatment with an SGLT2 inhibitor are “convincing because it’s real world data that complements what we know from clinical trials,” commented Silvio E. Inzucchi, MD, professor of medicine at Yale University and director of the Yale Medicine Diabetes Center in New Haven, Conn., who was not involved with the study.
“If these drugs reduce heart failure, they may also reduce AFib. Heart failure patients easily slip into AFib,” he noted in an interview, but added that “I don’t think this explains all cases” of the reduced AFib incidence.
Dr. Patorno offered a few other possible mechanisms for the observed effect. The class may work by reducing blood pressure, weight, inflammation, and oxidative stress, mitochondrial dysfunction, atrial remodeling, and AFib susceptibility. These agents are also known to cause natriuresis and diuresis, which could reduce atrial dilation, a mechanism that again relates the AFib effect to the better documented reduction in acute heart failure exacerbations.
“With the diuretic effect, we’d expect less overload at the atrium and less dilation, and the same mechanism would reduce heart failure,” she said in an interview.
“If you reduce preload and afterload you may reduce stress on the ventricle and reduce atrial stretch, and that might have a significant effect on atrial arrhythmia,” agreed Dr. Inzucchi.
EMPRISE produces more real-world evidence
A pair of additional reports at the meeting that Dr. Patorno coauthored provided real-world evidence supporting the dramatic heart failure benefit of the SGLT2 inhibitor empagliflozin (Jardiance) in U.S. patients with type 2 diabetes, compared with alternative drug classes. The EMPRISE study used data from the Medicare, Optum Clinformatics, and MarketScan databases during the period from August 2014, when empagliflozin became available, to September 2019. The study used more than 140 variables to match patients treated with either empagliflozin or a comparator agent.
The results showed that, in an analysis of more than 130,000 matched pairs, treatment with empagliflozin was linked to a significant 30% reduction in the incidence of hospitalization for heart failure, compared with patients treated with a GLP-1 receptor agonist. Analysis of more than 116,000 matched pairs of patients showed that treatment with empagliflozin linked with a significant 29%-50% reduced rate of hospitalization for heart failure, compared with matched patients treated with a DPP4 inhibitor.
These findings “add to the pool of information” on the efficacy of agents from the SGLT2 inhibitor class, Dr. Patorno said in an interview. “We wanted to look at the full range of patients with type 2 diabetes who we see in practice,” rather than the more selected group of patients enrolled in randomized trials.
SGLT2 inhibitor use lags even when cost isn’t an issue
Despite all the accumulated evidence for efficacy and safety of the class, usage remains low, Julio A. Lamprea-Montealegre, MD, PhD, a cardiologist at the University of California, San Francisco, reported in a separate talk at the meeting. The study he presented examined records for 1,319,500 adults with type 2 diabetes managed in the VA Healthcare System during 2019 and 2020. Despite being in a system that “removes the influence of cost,” just 10% of these patients received treatment with an SGLT2 inhibitor, and 7% received treatment with a GLP-1 receptor agonist.
Notably, his analysis further showed that treatment with an SGLT2 inhibitor was especially depressed among patients with an estimated glomerular filtration rate (eGFR) of 30-44 mL/min per 1.73m2. In this subgroup, usage of a drug from this class was at two-thirds of the rate, compared with patients with an eGFR of at least 90 mL/min per 1.73m2. His findings also documented lower rates of use in patients with higher risk for atherosclerotic cardiovascular disease. Dr. Lamprea-Montealegre called this a “treatment paradox,” in which patients likely to get the most benefit from an SGLT2 inhibitor were also less likely to actually receive it.
While his findings from the VA System suggest that drug cost is not the only factor driving underuse, the high price set for the SGLT2 inhibitor drugs that all currently remain on U.S. patents is widely considered an important factor.
“There is a big problem of affordability,” said Dr. Patorno.
“SGLT2 inhibitors should probably be first-line therapy” for many patients with type 2 diabetes, said Dr. Inzucchi. “The only thing holding it back is cost,” a situation that he hopes will dramatically shift once agents from this class become generic and have substantially lower price tags.
The EMPRISE study received funding from Boehringer Ingelheim, the company that markets empagliflozin (Jardiance). Dr. Patorno had no relevant commercial disclosures. Dr. Inzucchi is an adviser to Abbott Diagnostics, Esperion Therapeutics, and vTv Therapeutics, a consultant to Merck and Pfizer, and has other relationships with AstraZeneca, Boehringer Ingelheim, Lexicon, and Novo Nordisk. Dr. Lamprea-Montealegre had received research funding from Bayer.
NEW ORLEANS – The case continues to grow for prioritizing a sodium-glucose transporter 2 (SGLT2) inhibitor in patients with type 2 diabetes, as real-world evidence of benefit and safety accumulates on top of the data from randomized trials that first established this class as a management pillar.
Another important effect of these agents gaining increasing currency, on top of their well-established benefits in patients with type 2 diabetes for preventing acute heart failure exacerbations and slowing progression of diabetic kidney disease, is that they cut the incidence of new-onset atrial fibrillation (AFib). That effect was confirmed in an analysis of data from about 300,000 U.S. patients included in recent Medicare records, Elisabetta Patorno, MD, reported at the annual scientific sessions of the American Diabetes Association.
But despite documentation like this, real-world evidence also continues to show limited uptake of SGLT2 inhibitors in U.S. patients with type 2 diabetes. Records from more than 1.3 million patients with type 2 diabetes managed in the Veterans Affairs Healthcare System during 2019 or 2022 documented that just 10% of these patients received an agent from this class, even though all were eligible to receive it, according to findings in a separate report at the meeting.
The AFib analysis analyzed two sets of propensity score–matched Medicare patients during 2013-2018 aged 65 years or older with type 2 diabetes and no history of AFib. One analysis focused on 80,475 matched patients who started on treatment with either an SGLT2 inhibitor or a glucagonlike peptide–1 (GLP-1) receptor agonist, and a second on 74,868 matched patients who began either an SGTL2 inhibitor or a dipeptidyl peptidase–4 (DPP4) inhibitor. In both analyses, matching involved more than 130 variables. In both pair sets, patients at baseline averaged about 72 years old, nearly two-thirds were women, about 8%-9% had heart failure, 77%-80% were on metformin, and 20%-25% were using insulin.
The study’s primary endpoint was the incidence of hospitalization for AFib, which occurred a significant 18% less often in the patients who started on an SGLT2, compared with those who started a DPP4 inhibitor during median follow-up of 6.7 months, and a significant 10% less often, compared with those starting a GLP-1 receptor agonist during a median follow-up of 6.0 months, Elisabetta Patorno, MD, DrPH, reported at the meeting. This worked out to 3.7 fewer hospitalizations for AFib per 1,000 patient-years of follow-up among the people who received an SGLT2 inhibitor, compared with a DPP4 inhibitor, and a decrease of 1.8 hospitalizations/1,000 patient-years when compared against patients in a GLP-1 receptor agonist.
Two secondary outcomes showed significantly fewer episodes of newly diagnosed AFib, and significantly fewer patients initiating AFib treatment among those who received an SGLT2 inhibitor relative to the comparator groups. In addition, these associations were consistent across subgroup analyses that divided patients by their age, sex, history of heart failure, and history of atherosclerotic cardiovascular disease.
AFib effects add to benefits
The findings “suggest that initiation of an SGLT2 inhibitor may be beneficial in older adults with type 2 diabetes who are at risk for AFib,” said Dr. Patorno, a researcher in the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital, Boston. “These new findings on AFib may be helpful when weighing the potential risks and benefits of various glucose-lowering drugs in older patients with type 2 diabetes.”
This new evidence follows several prior reports from other research groups of data supporting an AFib benefit from SGLT2 inhibitors. The earlier reports include a post hoc analysis of more than 17,000 patients enrolled in the DECLARE-TIMI 58 cardiovascular outcome trial of dapagliflozin (Farxiga), which showed a 19% relative decrease in the rate of incident AFib or atrial flutter events during a median 4.2 year follow-up.
Other prior reports that found a reduced incidence of AFib events linked with SGLT2 inhibitor treatment include a 2020 meta-analysis based on data from more than 38,000 patients with type 2 diabetes enrolled in any of 16 randomized, controlled trials, which found a 24% relative risk reduction. And an as-yet unpublished report from researchers at the University of Rochester (N.Y.) and their associates presented in November 2021 at the annual scientific sessions of the American Heart Association that documented a significant 24% relative risk reduction in incident AFib events linked to SGLT2 inhibitor treatment in a prospective study of 13,890 patients at several hospitals in Israel or the United States.
Evidence ‘convincing’ in totality
The accumulated evidence for a reduced incidence of AFib when patients were on treatment with an SGLT2 inhibitor are “convincing because it’s real world data that complements what we know from clinical trials,” commented Silvio E. Inzucchi, MD, professor of medicine at Yale University and director of the Yale Medicine Diabetes Center in New Haven, Conn., who was not involved with the study.
“If these drugs reduce heart failure, they may also reduce AFib. Heart failure patients easily slip into AFib,” he noted in an interview, but added that “I don’t think this explains all cases” of the reduced AFib incidence.
Dr. Patorno offered a few other possible mechanisms for the observed effect. The class may work by reducing blood pressure, weight, inflammation, and oxidative stress, mitochondrial dysfunction, atrial remodeling, and AFib susceptibility. These agents are also known to cause natriuresis and diuresis, which could reduce atrial dilation, a mechanism that again relates the AFib effect to the better documented reduction in acute heart failure exacerbations.
“With the diuretic effect, we’d expect less overload at the atrium and less dilation, and the same mechanism would reduce heart failure,” she said in an interview.
“If you reduce preload and afterload you may reduce stress on the ventricle and reduce atrial stretch, and that might have a significant effect on atrial arrhythmia,” agreed Dr. Inzucchi.
EMPRISE produces more real-world evidence
A pair of additional reports at the meeting that Dr. Patorno coauthored provided real-world evidence supporting the dramatic heart failure benefit of the SGLT2 inhibitor empagliflozin (Jardiance) in U.S. patients with type 2 diabetes, compared with alternative drug classes. The EMPRISE study used data from the Medicare, Optum Clinformatics, and MarketScan databases during the period from August 2014, when empagliflozin became available, to September 2019. The study used more than 140 variables to match patients treated with either empagliflozin or a comparator agent.
The results showed that, in an analysis of more than 130,000 matched pairs, treatment with empagliflozin was linked to a significant 30% reduction in the incidence of hospitalization for heart failure, compared with patients treated with a GLP-1 receptor agonist. Analysis of more than 116,000 matched pairs of patients showed that treatment with empagliflozin linked with a significant 29%-50% reduced rate of hospitalization for heart failure, compared with matched patients treated with a DPP4 inhibitor.
These findings “add to the pool of information” on the efficacy of agents from the SGLT2 inhibitor class, Dr. Patorno said in an interview. “We wanted to look at the full range of patients with type 2 diabetes who we see in practice,” rather than the more selected group of patients enrolled in randomized trials.
SGLT2 inhibitor use lags even when cost isn’t an issue
Despite all the accumulated evidence for efficacy and safety of the class, usage remains low, Julio A. Lamprea-Montealegre, MD, PhD, a cardiologist at the University of California, San Francisco, reported in a separate talk at the meeting. The study he presented examined records for 1,319,500 adults with type 2 diabetes managed in the VA Healthcare System during 2019 and 2020. Despite being in a system that “removes the influence of cost,” just 10% of these patients received treatment with an SGLT2 inhibitor, and 7% received treatment with a GLP-1 receptor agonist.
Notably, his analysis further showed that treatment with an SGLT2 inhibitor was especially depressed among patients with an estimated glomerular filtration rate (eGFR) of 30-44 mL/min per 1.73m2. In this subgroup, usage of a drug from this class was at two-thirds of the rate, compared with patients with an eGFR of at least 90 mL/min per 1.73m2. His findings also documented lower rates of use in patients with higher risk for atherosclerotic cardiovascular disease. Dr. Lamprea-Montealegre called this a “treatment paradox,” in which patients likely to get the most benefit from an SGLT2 inhibitor were also less likely to actually receive it.
While his findings from the VA System suggest that drug cost is not the only factor driving underuse, the high price set for the SGLT2 inhibitor drugs that all currently remain on U.S. patents is widely considered an important factor.
“There is a big problem of affordability,” said Dr. Patorno.
“SGLT2 inhibitors should probably be first-line therapy” for many patients with type 2 diabetes, said Dr. Inzucchi. “The only thing holding it back is cost,” a situation that he hopes will dramatically shift once agents from this class become generic and have substantially lower price tags.
The EMPRISE study received funding from Boehringer Ingelheim, the company that markets empagliflozin (Jardiance). Dr. Patorno had no relevant commercial disclosures. Dr. Inzucchi is an adviser to Abbott Diagnostics, Esperion Therapeutics, and vTv Therapeutics, a consultant to Merck and Pfizer, and has other relationships with AstraZeneca, Boehringer Ingelheim, Lexicon, and Novo Nordisk. Dr. Lamprea-Montealegre had received research funding from Bayer.
NEW ORLEANS – The case continues to grow for prioritizing a sodium-glucose transporter 2 (SGLT2) inhibitor in patients with type 2 diabetes, as real-world evidence of benefit and safety accumulates on top of the data from randomized trials that first established this class as a management pillar.
Another important effect of these agents gaining increasing currency, on top of their well-established benefits in patients with type 2 diabetes for preventing acute heart failure exacerbations and slowing progression of diabetic kidney disease, is that they cut the incidence of new-onset atrial fibrillation (AFib). That effect was confirmed in an analysis of data from about 300,000 U.S. patients included in recent Medicare records, Elisabetta Patorno, MD, reported at the annual scientific sessions of the American Diabetes Association.
But despite documentation like this, real-world evidence also continues to show limited uptake of SGLT2 inhibitors in U.S. patients with type 2 diabetes. Records from more than 1.3 million patients with type 2 diabetes managed in the Veterans Affairs Healthcare System during 2019 or 2022 documented that just 10% of these patients received an agent from this class, even though all were eligible to receive it, according to findings in a separate report at the meeting.
The AFib analysis analyzed two sets of propensity score–matched Medicare patients during 2013-2018 aged 65 years or older with type 2 diabetes and no history of AFib. One analysis focused on 80,475 matched patients who started on treatment with either an SGLT2 inhibitor or a glucagonlike peptide–1 (GLP-1) receptor agonist, and a second on 74,868 matched patients who began either an SGTL2 inhibitor or a dipeptidyl peptidase–4 (DPP4) inhibitor. In both analyses, matching involved more than 130 variables. In both pair sets, patients at baseline averaged about 72 years old, nearly two-thirds were women, about 8%-9% had heart failure, 77%-80% were on metformin, and 20%-25% were using insulin.
The study’s primary endpoint was the incidence of hospitalization for AFib, which occurred a significant 18% less often in the patients who started on an SGLT2, compared with those who started a DPP4 inhibitor during median follow-up of 6.7 months, and a significant 10% less often, compared with those starting a GLP-1 receptor agonist during a median follow-up of 6.0 months, Elisabetta Patorno, MD, DrPH, reported at the meeting. This worked out to 3.7 fewer hospitalizations for AFib per 1,000 patient-years of follow-up among the people who received an SGLT2 inhibitor, compared with a DPP4 inhibitor, and a decrease of 1.8 hospitalizations/1,000 patient-years when compared against patients in a GLP-1 receptor agonist.
Two secondary outcomes showed significantly fewer episodes of newly diagnosed AFib, and significantly fewer patients initiating AFib treatment among those who received an SGLT2 inhibitor relative to the comparator groups. In addition, these associations were consistent across subgroup analyses that divided patients by their age, sex, history of heart failure, and history of atherosclerotic cardiovascular disease.
AFib effects add to benefits
The findings “suggest that initiation of an SGLT2 inhibitor may be beneficial in older adults with type 2 diabetes who are at risk for AFib,” said Dr. Patorno, a researcher in the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital, Boston. “These new findings on AFib may be helpful when weighing the potential risks and benefits of various glucose-lowering drugs in older patients with type 2 diabetes.”
This new evidence follows several prior reports from other research groups of data supporting an AFib benefit from SGLT2 inhibitors. The earlier reports include a post hoc analysis of more than 17,000 patients enrolled in the DECLARE-TIMI 58 cardiovascular outcome trial of dapagliflozin (Farxiga), which showed a 19% relative decrease in the rate of incident AFib or atrial flutter events during a median 4.2 year follow-up.
Other prior reports that found a reduced incidence of AFib events linked with SGLT2 inhibitor treatment include a 2020 meta-analysis based on data from more than 38,000 patients with type 2 diabetes enrolled in any of 16 randomized, controlled trials, which found a 24% relative risk reduction. And an as-yet unpublished report from researchers at the University of Rochester (N.Y.) and their associates presented in November 2021 at the annual scientific sessions of the American Heart Association that documented a significant 24% relative risk reduction in incident AFib events linked to SGLT2 inhibitor treatment in a prospective study of 13,890 patients at several hospitals in Israel or the United States.
Evidence ‘convincing’ in totality
The accumulated evidence for a reduced incidence of AFib when patients were on treatment with an SGLT2 inhibitor are “convincing because it’s real world data that complements what we know from clinical trials,” commented Silvio E. Inzucchi, MD, professor of medicine at Yale University and director of the Yale Medicine Diabetes Center in New Haven, Conn., who was not involved with the study.
“If these drugs reduce heart failure, they may also reduce AFib. Heart failure patients easily slip into AFib,” he noted in an interview, but added that “I don’t think this explains all cases” of the reduced AFib incidence.
Dr. Patorno offered a few other possible mechanisms for the observed effect. The class may work by reducing blood pressure, weight, inflammation, and oxidative stress, mitochondrial dysfunction, atrial remodeling, and AFib susceptibility. These agents are also known to cause natriuresis and diuresis, which could reduce atrial dilation, a mechanism that again relates the AFib effect to the better documented reduction in acute heart failure exacerbations.
“With the diuretic effect, we’d expect less overload at the atrium and less dilation, and the same mechanism would reduce heart failure,” she said in an interview.
“If you reduce preload and afterload you may reduce stress on the ventricle and reduce atrial stretch, and that might have a significant effect on atrial arrhythmia,” agreed Dr. Inzucchi.
EMPRISE produces more real-world evidence
A pair of additional reports at the meeting that Dr. Patorno coauthored provided real-world evidence supporting the dramatic heart failure benefit of the SGLT2 inhibitor empagliflozin (Jardiance) in U.S. patients with type 2 diabetes, compared with alternative drug classes. The EMPRISE study used data from the Medicare, Optum Clinformatics, and MarketScan databases during the period from August 2014, when empagliflozin became available, to September 2019. The study used more than 140 variables to match patients treated with either empagliflozin or a comparator agent.
The results showed that, in an analysis of more than 130,000 matched pairs, treatment with empagliflozin was linked to a significant 30% reduction in the incidence of hospitalization for heart failure, compared with patients treated with a GLP-1 receptor agonist. Analysis of more than 116,000 matched pairs of patients showed that treatment with empagliflozin linked with a significant 29%-50% reduced rate of hospitalization for heart failure, compared with matched patients treated with a DPP4 inhibitor.
These findings “add to the pool of information” on the efficacy of agents from the SGLT2 inhibitor class, Dr. Patorno said in an interview. “We wanted to look at the full range of patients with type 2 diabetes who we see in practice,” rather than the more selected group of patients enrolled in randomized trials.
SGLT2 inhibitor use lags even when cost isn’t an issue
Despite all the accumulated evidence for efficacy and safety of the class, usage remains low, Julio A. Lamprea-Montealegre, MD, PhD, a cardiologist at the University of California, San Francisco, reported in a separate talk at the meeting. The study he presented examined records for 1,319,500 adults with type 2 diabetes managed in the VA Healthcare System during 2019 and 2020. Despite being in a system that “removes the influence of cost,” just 10% of these patients received treatment with an SGLT2 inhibitor, and 7% received treatment with a GLP-1 receptor agonist.
Notably, his analysis further showed that treatment with an SGLT2 inhibitor was especially depressed among patients with an estimated glomerular filtration rate (eGFR) of 30-44 mL/min per 1.73m2. In this subgroup, usage of a drug from this class was at two-thirds of the rate, compared with patients with an eGFR of at least 90 mL/min per 1.73m2. His findings also documented lower rates of use in patients with higher risk for atherosclerotic cardiovascular disease. Dr. Lamprea-Montealegre called this a “treatment paradox,” in which patients likely to get the most benefit from an SGLT2 inhibitor were also less likely to actually receive it.
While his findings from the VA System suggest that drug cost is not the only factor driving underuse, the high price set for the SGLT2 inhibitor drugs that all currently remain on U.S. patents is widely considered an important factor.
“There is a big problem of affordability,” said Dr. Patorno.
“SGLT2 inhibitors should probably be first-line therapy” for many patients with type 2 diabetes, said Dr. Inzucchi. “The only thing holding it back is cost,” a situation that he hopes will dramatically shift once agents from this class become generic and have substantially lower price tags.
The EMPRISE study received funding from Boehringer Ingelheim, the company that markets empagliflozin (Jardiance). Dr. Patorno had no relevant commercial disclosures. Dr. Inzucchi is an adviser to Abbott Diagnostics, Esperion Therapeutics, and vTv Therapeutics, a consultant to Merck and Pfizer, and has other relationships with AstraZeneca, Boehringer Ingelheim, Lexicon, and Novo Nordisk. Dr. Lamprea-Montealegre had received research funding from Bayer.
AT ADA 2022
Emergency angiography for cardiac arrest without ST elevation?
Patients successfully resuscitated after an out-of-hospital cardiac arrest who did not have ST-segment elevation on their electrocardiogram did not benefit from emergency coronary angiography in a new randomized clinical trial.
In the EMERGE trial, a strategy of emergency coronary angiography was not found to be better than a strategy of delayed coronary angiography with respect to the 180-day survival rate with no or minimal neurologic sequelae.
The authors note that, although the study was underpowered, the results are consistent with previously published studies and do not support routine emergency coronary angiography in survivors of out-of-hospital cardiac arrest without ST elevation.
But senior author, Christian Spaulding, MD, PhD, European Hospital Georges Pompidou, Paris, believes some such patients may still benefit from emergency angiography.
“Most patients who have been resuscitated after out of hospital cardiac arrest will have neurological damage, which will be the primary cause of death,” Dr. Spaulding told this news organization. “It will not make any difference to these patients if they have a coronary lesion treated. So, going forward, I think we need to look for patients who are likely not to have a high degree of neurological damage and who could still benefit from early angiography.”
The EMERGE study was published online in JAMA Cardiology.
In patients who have suffered an out-of-hospital cardiac arrest with no obvious noncardiac cause such as trauma, it is believed that the cardiac arrest is caused by coronary occlusions, and emergency angiography may be able to improve survival in these patients, Dr. Spaulding explained.
In about one-third of such patients, the ECG before hospitalization shows ST elevation, and in this group, there is a high probability (around 70%-80%) that there is going to be a coronary occlusion, so these patients are usually taken directly to emergency angiography.
But, in the other two-thirds of patients, there is no ST elevation on the ECG, and in these patients the chances of finding a coronary occlusion are lower (around 25%-35%).
The EMERGE trial was conducted in this latter group without ST elevation.
For the study, which was conducted in 22 French centers, 279 such patients (mean age, 64 years) were randomized to either emergency or delayed (48-96 hours) coronary angiography. The mean time delay between randomization and coronary angiography was 0.6 hours in the emergency group and 55.1 hours in the delayed group.
The primary outcome was the 180-day survival rate with minimal neurological damage, defined as Cerebral Performance Category of 2 or less. This occurred in 34.1% of the emergency angiography group and 30.7% of the delayed angiography group (hazard ratio, 0.87; 95% confidence interval, 0.65-1.15; P = .32).
There was also no difference in the overall survival rate at 180 days (36.2% vs. 33.3%; HR, 0.86; P = .31) and in secondary outcomes between the two groups.
Dr. Spaulding noted that three other randomized trials in a similar patient population have all shown similar results, with no difference in survival found between patients who have emergency coronary angiography as soon as they are admitted to hospital and those in whom angiography was not performed until a couple of days later.
However, several registry studies in a total of more than 6,000 patients have suggested a benefit of immediate angiography in these patients. “So, there is some disconnect here,” he said.
Dr. Spaulding believes the reason for this disconnect may be that the registry studies may have included patients with less neurological damage so more likely to survive and to benefit from having coronary lesions treated promptly.
“Paramedics sometimes make a judgment on which patients may have minimal neurological damage, and this may affect the choice of hospital a patient is taken to, and then the emergency department doctor may again assess whether a patient should go for immediate angiography or not. So, patients in these registry studies who received emergency angiography were likely already preselected to some extent,” he suggested.
In contrast, the randomized trials have accepted all patients, so there were probably more with neurological damage. “In our trial, almost 70% of patients were in asystole. These are the ones who [are] the most likely to have neurological damage,” he pointed out.
“Because there was such a striking difference in the registry studies, I think there is a group of patients [who] will benefit from immediate emergency coronary angiography, but we have to work out how to select these patients,” he commented.
Dr. Spaulding noted that a recent registry study published in JACC: Cardiovascular Interventions used a score known as MIRACLE2, (which takes into account various factors including age of patient and type of rhythm on ECG) and the degree of cardiogenic shock on arrival at hospital as measured by the SCAI shock score to define a potential cohort of patients at low risk for neurologic injury who benefit most from immediate coronary angiography.
“In my practice at present, I would advise the emergency team that a young patient who had had resuscitation started quickly, had been defibrillated early, and got to hospital quickly should go for an immediate coronary angiogram. It can’t do any harm, and there may be a benefit in such patients,” Dr. Spaulding added.The EMERGE study was supported in part by Assistance Publique–Hôpitaux de Paris and the French Ministry of Health, through the national Programme Hospitalier de Recherche Clinique. Dr. Spaulding reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients successfully resuscitated after an out-of-hospital cardiac arrest who did not have ST-segment elevation on their electrocardiogram did not benefit from emergency coronary angiography in a new randomized clinical trial.
In the EMERGE trial, a strategy of emergency coronary angiography was not found to be better than a strategy of delayed coronary angiography with respect to the 180-day survival rate with no or minimal neurologic sequelae.
The authors note that, although the study was underpowered, the results are consistent with previously published studies and do not support routine emergency coronary angiography in survivors of out-of-hospital cardiac arrest without ST elevation.
But senior author, Christian Spaulding, MD, PhD, European Hospital Georges Pompidou, Paris, believes some such patients may still benefit from emergency angiography.
“Most patients who have been resuscitated after out of hospital cardiac arrest will have neurological damage, which will be the primary cause of death,” Dr. Spaulding told this news organization. “It will not make any difference to these patients if they have a coronary lesion treated. So, going forward, I think we need to look for patients who are likely not to have a high degree of neurological damage and who could still benefit from early angiography.”
The EMERGE study was published online in JAMA Cardiology.
In patients who have suffered an out-of-hospital cardiac arrest with no obvious noncardiac cause such as trauma, it is believed that the cardiac arrest is caused by coronary occlusions, and emergency angiography may be able to improve survival in these patients, Dr. Spaulding explained.
In about one-third of such patients, the ECG before hospitalization shows ST elevation, and in this group, there is a high probability (around 70%-80%) that there is going to be a coronary occlusion, so these patients are usually taken directly to emergency angiography.
But, in the other two-thirds of patients, there is no ST elevation on the ECG, and in these patients the chances of finding a coronary occlusion are lower (around 25%-35%).
The EMERGE trial was conducted in this latter group without ST elevation.
For the study, which was conducted in 22 French centers, 279 such patients (mean age, 64 years) were randomized to either emergency or delayed (48-96 hours) coronary angiography. The mean time delay between randomization and coronary angiography was 0.6 hours in the emergency group and 55.1 hours in the delayed group.
The primary outcome was the 180-day survival rate with minimal neurological damage, defined as Cerebral Performance Category of 2 or less. This occurred in 34.1% of the emergency angiography group and 30.7% of the delayed angiography group (hazard ratio, 0.87; 95% confidence interval, 0.65-1.15; P = .32).
There was also no difference in the overall survival rate at 180 days (36.2% vs. 33.3%; HR, 0.86; P = .31) and in secondary outcomes between the two groups.
Dr. Spaulding noted that three other randomized trials in a similar patient population have all shown similar results, with no difference in survival found between patients who have emergency coronary angiography as soon as they are admitted to hospital and those in whom angiography was not performed until a couple of days later.
However, several registry studies in a total of more than 6,000 patients have suggested a benefit of immediate angiography in these patients. “So, there is some disconnect here,” he said.
Dr. Spaulding believes the reason for this disconnect may be that the registry studies may have included patients with less neurological damage so more likely to survive and to benefit from having coronary lesions treated promptly.
“Paramedics sometimes make a judgment on which patients may have minimal neurological damage, and this may affect the choice of hospital a patient is taken to, and then the emergency department doctor may again assess whether a patient should go for immediate angiography or not. So, patients in these registry studies who received emergency angiography were likely already preselected to some extent,” he suggested.
In contrast, the randomized trials have accepted all patients, so there were probably more with neurological damage. “In our trial, almost 70% of patients were in asystole. These are the ones who [are] the most likely to have neurological damage,” he pointed out.
“Because there was such a striking difference in the registry studies, I think there is a group of patients [who] will benefit from immediate emergency coronary angiography, but we have to work out how to select these patients,” he commented.
Dr. Spaulding noted that a recent registry study published in JACC: Cardiovascular Interventions used a score known as MIRACLE2, (which takes into account various factors including age of patient and type of rhythm on ECG) and the degree of cardiogenic shock on arrival at hospital as measured by the SCAI shock score to define a potential cohort of patients at low risk for neurologic injury who benefit most from immediate coronary angiography.
“In my practice at present, I would advise the emergency team that a young patient who had had resuscitation started quickly, had been defibrillated early, and got to hospital quickly should go for an immediate coronary angiogram. It can’t do any harm, and there may be a benefit in such patients,” Dr. Spaulding added.The EMERGE study was supported in part by Assistance Publique–Hôpitaux de Paris and the French Ministry of Health, through the national Programme Hospitalier de Recherche Clinique. Dr. Spaulding reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients successfully resuscitated after an out-of-hospital cardiac arrest who did not have ST-segment elevation on their electrocardiogram did not benefit from emergency coronary angiography in a new randomized clinical trial.
In the EMERGE trial, a strategy of emergency coronary angiography was not found to be better than a strategy of delayed coronary angiography with respect to the 180-day survival rate with no or minimal neurologic sequelae.
The authors note that, although the study was underpowered, the results are consistent with previously published studies and do not support routine emergency coronary angiography in survivors of out-of-hospital cardiac arrest without ST elevation.
But senior author, Christian Spaulding, MD, PhD, European Hospital Georges Pompidou, Paris, believes some such patients may still benefit from emergency angiography.
“Most patients who have been resuscitated after out of hospital cardiac arrest will have neurological damage, which will be the primary cause of death,” Dr. Spaulding told this news organization. “It will not make any difference to these patients if they have a coronary lesion treated. So, going forward, I think we need to look for patients who are likely not to have a high degree of neurological damage and who could still benefit from early angiography.”
The EMERGE study was published online in JAMA Cardiology.
In patients who have suffered an out-of-hospital cardiac arrest with no obvious noncardiac cause such as trauma, it is believed that the cardiac arrest is caused by coronary occlusions, and emergency angiography may be able to improve survival in these patients, Dr. Spaulding explained.
In about one-third of such patients, the ECG before hospitalization shows ST elevation, and in this group, there is a high probability (around 70%-80%) that there is going to be a coronary occlusion, so these patients are usually taken directly to emergency angiography.
But, in the other two-thirds of patients, there is no ST elevation on the ECG, and in these patients the chances of finding a coronary occlusion are lower (around 25%-35%).
The EMERGE trial was conducted in this latter group without ST elevation.
For the study, which was conducted in 22 French centers, 279 such patients (mean age, 64 years) were randomized to either emergency or delayed (48-96 hours) coronary angiography. The mean time delay between randomization and coronary angiography was 0.6 hours in the emergency group and 55.1 hours in the delayed group.
The primary outcome was the 180-day survival rate with minimal neurological damage, defined as Cerebral Performance Category of 2 or less. This occurred in 34.1% of the emergency angiography group and 30.7% of the delayed angiography group (hazard ratio, 0.87; 95% confidence interval, 0.65-1.15; P = .32).
There was also no difference in the overall survival rate at 180 days (36.2% vs. 33.3%; HR, 0.86; P = .31) and in secondary outcomes between the two groups.
Dr. Spaulding noted that three other randomized trials in a similar patient population have all shown similar results, with no difference in survival found between patients who have emergency coronary angiography as soon as they are admitted to hospital and those in whom angiography was not performed until a couple of days later.
However, several registry studies in a total of more than 6,000 patients have suggested a benefit of immediate angiography in these patients. “So, there is some disconnect here,” he said.
Dr. Spaulding believes the reason for this disconnect may be that the registry studies may have included patients with less neurological damage so more likely to survive and to benefit from having coronary lesions treated promptly.
“Paramedics sometimes make a judgment on which patients may have minimal neurological damage, and this may affect the choice of hospital a patient is taken to, and then the emergency department doctor may again assess whether a patient should go for immediate angiography or not. So, patients in these registry studies who received emergency angiography were likely already preselected to some extent,” he suggested.
In contrast, the randomized trials have accepted all patients, so there were probably more with neurological damage. “In our trial, almost 70% of patients were in asystole. These are the ones who [are] the most likely to have neurological damage,” he pointed out.
“Because there was such a striking difference in the registry studies, I think there is a group of patients [who] will benefit from immediate emergency coronary angiography, but we have to work out how to select these patients,” he commented.
Dr. Spaulding noted that a recent registry study published in JACC: Cardiovascular Interventions used a score known as MIRACLE2, (which takes into account various factors including age of patient and type of rhythm on ECG) and the degree of cardiogenic shock on arrival at hospital as measured by the SCAI shock score to define a potential cohort of patients at low risk for neurologic injury who benefit most from immediate coronary angiography.
“In my practice at present, I would advise the emergency team that a young patient who had had resuscitation started quickly, had been defibrillated early, and got to hospital quickly should go for an immediate coronary angiogram. It can’t do any harm, and there may be a benefit in such patients,” Dr. Spaulding added.The EMERGE study was supported in part by Assistance Publique–Hôpitaux de Paris and the French Ministry of Health, through the national Programme Hospitalier de Recherche Clinique. Dr. Spaulding reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New guideline for in-hospital care of diabetes says use CGMs
Goal-directed glycemic management – which may include new technologies for glucose monitoring – for non–critically ill hospitalized patients who have diabetes or newly recognized hyperglycemia can improve outcomes, according to a new practice guideline from the Endocrine Society.
Even though roughly 35% of hospitalized patients have diabetes or newly discovered hyperglycemia, there is “wide variability in glycemic management in clinical practice,” writing panel chair Mary Korytkowski, MD, from the University of Pittsburgh, said at the annual meeting of the Endocrine Society. “These patients get admitted to every patient service in the hospital, meaning that every clinical service will encounter this group of patients, and their glycemic management can have a major effect on their outcomes. Both short term and long term.”
This guideline provides strategies “to achieve previously recommended glycemic goals while also reducing the risk for hypoglycemia, and this includes inpatient use of insulin pump therapy or continuous glucose monitoring [CGM] devices, among others,” she said.
It also includes “recommendations for preoperative glycemic goals as well as when the use of correctional insulin – well known as sliding scale insulin – may be appropriate” and when it is not.
The document, which replaces a 2012 guideline, was published online in the Journal of Clinical Endocrinology & Metabolism.
A multidisciplinary panel developed the document over the last 3 years to answer 10 clinical practice questions related to management of non–critically ill hospitalized patients with diabetes or newly discovered hyperglycemia.
Use of CGM devices in hospital
The first recommendation is: “In adults with insulin-treated diabetes hospitalized for noncritical illness who are at high risk of hypoglycemia, we suggest the use of real-time [CGM] with confirmatory bedside point-of-care blood glucose monitoring for adjustments in insulin dosing rather than point-of-care blood glucose rather than testing alone in hospital settings where resources and training are available.” (Conditional recommendation. Low certainty of evidence).
“We were actually very careful in terms of looking at the data” for use of CGMs, Dr. Korytkowski said in an interview.
Although CGMs are approved by the Food and Drug Administration in the outpatient setting, and that’s becoming the standard of care there, they are not yet approved for in-hospital use.
However, the FDA granted an emergency allowance for use of CGMs in hospitals during the COVID-19 pandemic.
That was “when everyone was scrambling for what to do,” Dr. Korytkowski noted. “There was a shortage of personal protective equipment and a real interest in trying to limit the amount of exposure of healthcare personnel in some of these really critically ill patients for whom intravenous insulin therapy was used to control their glucose level.”
On March 1, the FDA granted Breakthrough Devices Designation for Dexcom CGM use in the hospital setting.
The new guideline suggests CGM be used to detect trends in glycemic management, with insulin dosing decisions made with point-of-care glucose measure (the standard of care).
To implement CGM for glycemic management in hospitals, Dr. Korytkowski said, would require “extensive staff and nursing education to have people with expertise available to provide support to nursing personnel who are both placing these devices, changing these devices, looking at trends, and then knowing when to remove them for certain procedures such as MRI or radiologic procedures.”
“We know that not all hospitals may be readily available to use these devices,” she said. “It is an area of active research. But the use of these devices during the pandemic, in both critical care and non–critical care setting has really provided us with a lot of information that was used to formulate this suggestion in the guideline.”
The document addresses the following areas: CGM, continuous subcutaneous insulin infusion pump therapy, inpatient diabetes education, prespecified preoperative glycemic targets, use of neutral protamine Hagedorn insulin for glucocorticoid or enteral nutrition-associated hyperglycemia, noninsulin therapies, preoperative carbohydrate-containing oral fluids, carbohydrate counting for prandial (mealtime) insulin dosing, and correctional and scheduled (basal or basal bolus) insulin therapies.
Nine key recommendations
Dr. Korytkowski identified nine key recommendations:
- CGM systems can help guide glycemic management with reduced risk for hypoglycemia.
- Patients experiencing glucocorticoid- or enteral nutrition–associated hyperglycemia require scheduled insulin therapy to address anticipated glucose excursions.
- Selected patients using insulin pump therapy prior to a hospital admission can continue to use these devices in the hospital if they have the mental and physical capacity to do so with knowledgeable hospital personnel.
- Diabetes self-management education provided to hospitalized patients can promote improved glycemic control following discharge with reductions in the risk for hospital readmission. “We know that is recommended for patients in the outpatient setting but often they do not get this,” she said. “We were able to observe that this can also impact long-term outcomes “
- Patients with diabetes scheduled for elective surgery may have improved postoperative outcomes when preoperative hemoglobin A1c is 8% or less and preoperative blood glucose is less than 180 mg/dL. “This recommendation answers the question: ‘Where should glycemic goals be for people who are undergoing surgery?’ ”
- Providing preoperative carbohydrate-containing beverages to patients with known diabetes is not recommended.
- Patients with newly recognized hyperglycemia or well-managed diabetes on noninsulin therapy may be treated with correctional insulin alone as initial therapy at hospital admission.
- Some noninsulin diabetes therapies can be used in combination with correction insulin for patients with type 2 diabetes who have mild hyperglycemia.
- Correctional insulin – “otherwise known as sliding-scale insulin” – can be used as initial therapy for patients with newly recognized hyperglycemia or type 2 diabetes treated with noninsulin therapy prior to hospital admission.
- Scheduled insulin therapy is preferred for patients experiencing persistent blood glucose values greater than 180 mg/dL and is recommended for patients using insulin therapy prior to admission.
The guideline writers’ hopes
“We hope that this guideline will resolve debates” about appropriate preoperative glycemic management and when sliding-scale insulin can be used and should not be used, said Dr. Korytkowski.
The authors also hope that “it will stimulate research funding for this very important aspect of diabetes care, and that hospitals will recognize the importance of having access to knowledgeable diabetes care and education specialists who can provide staff education regarding inpatient glycemic management, provide oversight for patients using insulin pump therapy or CGM devices, and empower hospital nurses to provide diabetes [self-management] education prior to patient discharge.”
Claire Pegg, the patient representative on the panel, hopes “that this guideline serves as the beginning of a conversation that will allow inpatient caregivers to provide individualized care to patients – some of whom may be self-sufficient with their glycemic management and others who need additional assistance.”
Development of the guideline was funded by the Endocrine Society. Dr. Korytkowski has reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Goal-directed glycemic management – which may include new technologies for glucose monitoring – for non–critically ill hospitalized patients who have diabetes or newly recognized hyperglycemia can improve outcomes, according to a new practice guideline from the Endocrine Society.
Even though roughly 35% of hospitalized patients have diabetes or newly discovered hyperglycemia, there is “wide variability in glycemic management in clinical practice,” writing panel chair Mary Korytkowski, MD, from the University of Pittsburgh, said at the annual meeting of the Endocrine Society. “These patients get admitted to every patient service in the hospital, meaning that every clinical service will encounter this group of patients, and their glycemic management can have a major effect on their outcomes. Both short term and long term.”
This guideline provides strategies “to achieve previously recommended glycemic goals while also reducing the risk for hypoglycemia, and this includes inpatient use of insulin pump therapy or continuous glucose monitoring [CGM] devices, among others,” she said.
It also includes “recommendations for preoperative glycemic goals as well as when the use of correctional insulin – well known as sliding scale insulin – may be appropriate” and when it is not.
The document, which replaces a 2012 guideline, was published online in the Journal of Clinical Endocrinology & Metabolism.
A multidisciplinary panel developed the document over the last 3 years to answer 10 clinical practice questions related to management of non–critically ill hospitalized patients with diabetes or newly discovered hyperglycemia.
Use of CGM devices in hospital
The first recommendation is: “In adults with insulin-treated diabetes hospitalized for noncritical illness who are at high risk of hypoglycemia, we suggest the use of real-time [CGM] with confirmatory bedside point-of-care blood glucose monitoring for adjustments in insulin dosing rather than point-of-care blood glucose rather than testing alone in hospital settings where resources and training are available.” (Conditional recommendation. Low certainty of evidence).
“We were actually very careful in terms of looking at the data” for use of CGMs, Dr. Korytkowski said in an interview.
Although CGMs are approved by the Food and Drug Administration in the outpatient setting, and that’s becoming the standard of care there, they are not yet approved for in-hospital use.
However, the FDA granted an emergency allowance for use of CGMs in hospitals during the COVID-19 pandemic.
That was “when everyone was scrambling for what to do,” Dr. Korytkowski noted. “There was a shortage of personal protective equipment and a real interest in trying to limit the amount of exposure of healthcare personnel in some of these really critically ill patients for whom intravenous insulin therapy was used to control their glucose level.”
On March 1, the FDA granted Breakthrough Devices Designation for Dexcom CGM use in the hospital setting.
The new guideline suggests CGM be used to detect trends in glycemic management, with insulin dosing decisions made with point-of-care glucose measure (the standard of care).
To implement CGM for glycemic management in hospitals, Dr. Korytkowski said, would require “extensive staff and nursing education to have people with expertise available to provide support to nursing personnel who are both placing these devices, changing these devices, looking at trends, and then knowing when to remove them for certain procedures such as MRI or radiologic procedures.”
“We know that not all hospitals may be readily available to use these devices,” she said. “It is an area of active research. But the use of these devices during the pandemic, in both critical care and non–critical care setting has really provided us with a lot of information that was used to formulate this suggestion in the guideline.”
The document addresses the following areas: CGM, continuous subcutaneous insulin infusion pump therapy, inpatient diabetes education, prespecified preoperative glycemic targets, use of neutral protamine Hagedorn insulin for glucocorticoid or enteral nutrition-associated hyperglycemia, noninsulin therapies, preoperative carbohydrate-containing oral fluids, carbohydrate counting for prandial (mealtime) insulin dosing, and correctional and scheduled (basal or basal bolus) insulin therapies.
Nine key recommendations
Dr. Korytkowski identified nine key recommendations:
- CGM systems can help guide glycemic management with reduced risk for hypoglycemia.
- Patients experiencing glucocorticoid- or enteral nutrition–associated hyperglycemia require scheduled insulin therapy to address anticipated glucose excursions.
- Selected patients using insulin pump therapy prior to a hospital admission can continue to use these devices in the hospital if they have the mental and physical capacity to do so with knowledgeable hospital personnel.
- Diabetes self-management education provided to hospitalized patients can promote improved glycemic control following discharge with reductions in the risk for hospital readmission. “We know that is recommended for patients in the outpatient setting but often they do not get this,” she said. “We were able to observe that this can also impact long-term outcomes “
- Patients with diabetes scheduled for elective surgery may have improved postoperative outcomes when preoperative hemoglobin A1c is 8% or less and preoperative blood glucose is less than 180 mg/dL. “This recommendation answers the question: ‘Where should glycemic goals be for people who are undergoing surgery?’ ”
- Providing preoperative carbohydrate-containing beverages to patients with known diabetes is not recommended.
- Patients with newly recognized hyperglycemia or well-managed diabetes on noninsulin therapy may be treated with correctional insulin alone as initial therapy at hospital admission.
- Some noninsulin diabetes therapies can be used in combination with correction insulin for patients with type 2 diabetes who have mild hyperglycemia.
- Correctional insulin – “otherwise known as sliding-scale insulin” – can be used as initial therapy for patients with newly recognized hyperglycemia or type 2 diabetes treated with noninsulin therapy prior to hospital admission.
- Scheduled insulin therapy is preferred for patients experiencing persistent blood glucose values greater than 180 mg/dL and is recommended for patients using insulin therapy prior to admission.
The guideline writers’ hopes
“We hope that this guideline will resolve debates” about appropriate preoperative glycemic management and when sliding-scale insulin can be used and should not be used, said Dr. Korytkowski.
The authors also hope that “it will stimulate research funding for this very important aspect of diabetes care, and that hospitals will recognize the importance of having access to knowledgeable diabetes care and education specialists who can provide staff education regarding inpatient glycemic management, provide oversight for patients using insulin pump therapy or CGM devices, and empower hospital nurses to provide diabetes [self-management] education prior to patient discharge.”
Claire Pegg, the patient representative on the panel, hopes “that this guideline serves as the beginning of a conversation that will allow inpatient caregivers to provide individualized care to patients – some of whom may be self-sufficient with their glycemic management and others who need additional assistance.”
Development of the guideline was funded by the Endocrine Society. Dr. Korytkowski has reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Goal-directed glycemic management – which may include new technologies for glucose monitoring – for non–critically ill hospitalized patients who have diabetes or newly recognized hyperglycemia can improve outcomes, according to a new practice guideline from the Endocrine Society.
Even though roughly 35% of hospitalized patients have diabetes or newly discovered hyperglycemia, there is “wide variability in glycemic management in clinical practice,” writing panel chair Mary Korytkowski, MD, from the University of Pittsburgh, said at the annual meeting of the Endocrine Society. “These patients get admitted to every patient service in the hospital, meaning that every clinical service will encounter this group of patients, and their glycemic management can have a major effect on their outcomes. Both short term and long term.”
This guideline provides strategies “to achieve previously recommended glycemic goals while also reducing the risk for hypoglycemia, and this includes inpatient use of insulin pump therapy or continuous glucose monitoring [CGM] devices, among others,” she said.
It also includes “recommendations for preoperative glycemic goals as well as when the use of correctional insulin – well known as sliding scale insulin – may be appropriate” and when it is not.
The document, which replaces a 2012 guideline, was published online in the Journal of Clinical Endocrinology & Metabolism.
A multidisciplinary panel developed the document over the last 3 years to answer 10 clinical practice questions related to management of non–critically ill hospitalized patients with diabetes or newly discovered hyperglycemia.
Use of CGM devices in hospital
The first recommendation is: “In adults with insulin-treated diabetes hospitalized for noncritical illness who are at high risk of hypoglycemia, we suggest the use of real-time [CGM] with confirmatory bedside point-of-care blood glucose monitoring for adjustments in insulin dosing rather than point-of-care blood glucose rather than testing alone in hospital settings where resources and training are available.” (Conditional recommendation. Low certainty of evidence).
“We were actually very careful in terms of looking at the data” for use of CGMs, Dr. Korytkowski said in an interview.
Although CGMs are approved by the Food and Drug Administration in the outpatient setting, and that’s becoming the standard of care there, they are not yet approved for in-hospital use.
However, the FDA granted an emergency allowance for use of CGMs in hospitals during the COVID-19 pandemic.
That was “when everyone was scrambling for what to do,” Dr. Korytkowski noted. “There was a shortage of personal protective equipment and a real interest in trying to limit the amount of exposure of healthcare personnel in some of these really critically ill patients for whom intravenous insulin therapy was used to control their glucose level.”
On March 1, the FDA granted Breakthrough Devices Designation for Dexcom CGM use in the hospital setting.
The new guideline suggests CGM be used to detect trends in glycemic management, with insulin dosing decisions made with point-of-care glucose measure (the standard of care).
To implement CGM for glycemic management in hospitals, Dr. Korytkowski said, would require “extensive staff and nursing education to have people with expertise available to provide support to nursing personnel who are both placing these devices, changing these devices, looking at trends, and then knowing when to remove them for certain procedures such as MRI or radiologic procedures.”
“We know that not all hospitals may be readily available to use these devices,” she said. “It is an area of active research. But the use of these devices during the pandemic, in both critical care and non–critical care setting has really provided us with a lot of information that was used to formulate this suggestion in the guideline.”
The document addresses the following areas: CGM, continuous subcutaneous insulin infusion pump therapy, inpatient diabetes education, prespecified preoperative glycemic targets, use of neutral protamine Hagedorn insulin for glucocorticoid or enteral nutrition-associated hyperglycemia, noninsulin therapies, preoperative carbohydrate-containing oral fluids, carbohydrate counting for prandial (mealtime) insulin dosing, and correctional and scheduled (basal or basal bolus) insulin therapies.
Nine key recommendations
Dr. Korytkowski identified nine key recommendations:
- CGM systems can help guide glycemic management with reduced risk for hypoglycemia.
- Patients experiencing glucocorticoid- or enteral nutrition–associated hyperglycemia require scheduled insulin therapy to address anticipated glucose excursions.
- Selected patients using insulin pump therapy prior to a hospital admission can continue to use these devices in the hospital if they have the mental and physical capacity to do so with knowledgeable hospital personnel.
- Diabetes self-management education provided to hospitalized patients can promote improved glycemic control following discharge with reductions in the risk for hospital readmission. “We know that is recommended for patients in the outpatient setting but often they do not get this,” she said. “We were able to observe that this can also impact long-term outcomes “
- Patients with diabetes scheduled for elective surgery may have improved postoperative outcomes when preoperative hemoglobin A1c is 8% or less and preoperative blood glucose is less than 180 mg/dL. “This recommendation answers the question: ‘Where should glycemic goals be for people who are undergoing surgery?’ ”
- Providing preoperative carbohydrate-containing beverages to patients with known diabetes is not recommended.
- Patients with newly recognized hyperglycemia or well-managed diabetes on noninsulin therapy may be treated with correctional insulin alone as initial therapy at hospital admission.
- Some noninsulin diabetes therapies can be used in combination with correction insulin for patients with type 2 diabetes who have mild hyperglycemia.
- Correctional insulin – “otherwise known as sliding-scale insulin” – can be used as initial therapy for patients with newly recognized hyperglycemia or type 2 diabetes treated with noninsulin therapy prior to hospital admission.
- Scheduled insulin therapy is preferred for patients experiencing persistent blood glucose values greater than 180 mg/dL and is recommended for patients using insulin therapy prior to admission.
The guideline writers’ hopes
“We hope that this guideline will resolve debates” about appropriate preoperative glycemic management and when sliding-scale insulin can be used and should not be used, said Dr. Korytkowski.
The authors also hope that “it will stimulate research funding for this very important aspect of diabetes care, and that hospitals will recognize the importance of having access to knowledgeable diabetes care and education specialists who can provide staff education regarding inpatient glycemic management, provide oversight for patients using insulin pump therapy or CGM devices, and empower hospital nurses to provide diabetes [self-management] education prior to patient discharge.”
Claire Pegg, the patient representative on the panel, hopes “that this guideline serves as the beginning of a conversation that will allow inpatient caregivers to provide individualized care to patients – some of whom may be self-sufficient with their glycemic management and others who need additional assistance.”
Development of the guideline was funded by the Endocrine Society. Dr. Korytkowski has reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FROM ENDO 2022
Self-injury and suicide ‘all too common’ in type 1 diabetes
Depression, self-harm, and suicide among people with type 1 and type 2 diabetes are “underappreciated” among health care practitioners, according to Katharine Barnard-Kelly, PhD, who founded the Reducing Suicide Rates Among Individuals With Diabetes (RESCUE) advocacy group in 2021.
“We have the most advanced technology to achieve glycemic control, but the mental burden remains underappreciated,” she said at a symposium with other speakers from RESCUE during the annual scientific sessions of the American Diabetes Association.
Notably, suicide and self-harm are “all too common” among young adults with type 1 diabetes who are receiving insulin, said Dr. Barnard-Kelly, a psychologist and visiting professor at Southern Health NHS Foundation Trust, Southampton, United Kingdom. And insulin under- or overdosing is the most common method of self-harm.
However, “with a multipronged approach to awareness, education, and identification, we have the opportunity to intervene on the link between suicide and diabetes,” she said, noting that the aim is to “raise awareness and arm [doctors and others] with messages that can ultimately save a young person’s life if adopted in clinical practice and through mental health screenings.”
The rationale behind the RESCUE initiative is also described in a brief report published in Diabetes Technology & Therapeutics.
Six key messages
RESCUE now has “approximately 30 members across academia, clinical practice, industry, advocacy, government, regulatory bodies [including the U.S. Food and Drug Administration], and people with diabetes from several countries,” Dr. Barnard-Kelly told this news organization.
She identified six key messages from the symposium:
- “Suicide prevalence is considerably higher among people with diabetes than the general population.
- Talking about suicide does not increase an individual’s risk of suicide.
- Current screening tools for depression and suicide are not sufficiently sensitive to be effective among people with diabetes.
- Identification of suicidal acts among people with diabetes is extremely difficult.
- For every suicide, the World Health Organization reports there are 20 suicide attempts.
- Health care providers often underestimate the prevalence of suicidality among their patient population and feel ill-equipped to initiate conversations with their patients about suicide.”
Dr. Barnard-Kelly also presented some sobering statistics that highlight the need for increased awareness.
A study reported that, of 160 cases of insulin overdose, 90% were suicides.
Adolescents and young adults with type 2 diabetes are 61% more likely to report suicidal thoughts than those without diabetes.
The risk of depression is two- to three-times higher in people with diabetes. According to another study, 7% of deaths in individuals with type 1 diabetes are estimated to be from suicide.
Survey about screening for depression, suicide risk in diabetes
During the symposium, Daniel R. Chernavvsky, MD, reported results from a small online survey of health care professionals who treat patients with type 1 or type 2 diabetes, which identified their concerns about screening for depression and assessing suicide risk in patients with diabetes.
Respondents were mainly from the United States (103) but were also from the United Kingdom (18), Slovenia, and the Netherlands (5), said Dr. Chernavvsky, who is senior director of medical affairs at Dexcom, Charlottesville, Va.
They included 59 doctors, 21 nurses,17 diabetes educators, 15 psychologists, seven dieticians, four social workers, and six “other” health care professionals, with a mean age of 46 (range, 25-72 years old) who had been working on average 14 years (range, 0.5-45 years).
Close to three-quarters (72%) reported that at least one of their patients had attempted suicide. The most common self-harm behaviors in their patients were insulin omission or a too large insulin bolus, and less often, binge eating.
Almost all respondents (95%) believed that routine visits to the diabetes clinic were appropriate times to discuss depression, self-injury, and suicidal ideation – at every visit (42% of respondents) or some visits (52%).
Only 30% were comfortable asking patients about self-harm or suicide.
Psychologists and social workers were very comfortable, but others were less comfortable or not comfortable at all.
Many respondents expressed concerns such as, “What do I do?” “Would I make the problem worse?” “Would I give the patient the idea?” Some reported they had “limited resources” or it “feels invasive.”
They identified a need for “a better understanding of what [they could] do to support and care for patients,” and “more knowledge about how to deal with [patients’] answers” to screening questionnaires.
Screening for psychological morbidities in diabetes
Guidelines from the ADA and the International Society for Pediatric and Adolescent Diabetes recommend routine screening of patients with diabetes for psychological morbidities, including depression, said Shideh Majidi, MD.
Depression is associated with higher A1c, noted Dr. Majidi, who is associate director, childhood and adolescent diabetes program at Children’s National Hospital, Washington, D.C.
She identified the following topics that need to be addressed when considering implementing a program for depression screening and suicide risk assessment in a diabetes clinic:
- Conducting screening: Which screening questionnaire will you use? Who will do it? Where? How often?
- Scoring screening questionnaires: Who will do it?
- Depression screening discussion: Who will do it? How will the person be notified of the score?
- Suicide risk assessment: Who will conduct it? What is the process to get someone to the emergency department?
- Resources/referral: Who will initiate and follow-up?
Next steps
The RESCUE advocacy group is preparing educational and support materials for health care professionals who treat patients with diabetes, as well as other materials for patients themselves.
A version of this article first appeared on Medscape.com.
Depression, self-harm, and suicide among people with type 1 and type 2 diabetes are “underappreciated” among health care practitioners, according to Katharine Barnard-Kelly, PhD, who founded the Reducing Suicide Rates Among Individuals With Diabetes (RESCUE) advocacy group in 2021.
“We have the most advanced technology to achieve glycemic control, but the mental burden remains underappreciated,” she said at a symposium with other speakers from RESCUE during the annual scientific sessions of the American Diabetes Association.
Notably, suicide and self-harm are “all too common” among young adults with type 1 diabetes who are receiving insulin, said Dr. Barnard-Kelly, a psychologist and visiting professor at Southern Health NHS Foundation Trust, Southampton, United Kingdom. And insulin under- or overdosing is the most common method of self-harm.
However, “with a multipronged approach to awareness, education, and identification, we have the opportunity to intervene on the link between suicide and diabetes,” she said, noting that the aim is to “raise awareness and arm [doctors and others] with messages that can ultimately save a young person’s life if adopted in clinical practice and through mental health screenings.”
The rationale behind the RESCUE initiative is also described in a brief report published in Diabetes Technology & Therapeutics.
Six key messages
RESCUE now has “approximately 30 members across academia, clinical practice, industry, advocacy, government, regulatory bodies [including the U.S. Food and Drug Administration], and people with diabetes from several countries,” Dr. Barnard-Kelly told this news organization.
She identified six key messages from the symposium:
- “Suicide prevalence is considerably higher among people with diabetes than the general population.
- Talking about suicide does not increase an individual’s risk of suicide.
- Current screening tools for depression and suicide are not sufficiently sensitive to be effective among people with diabetes.
- Identification of suicidal acts among people with diabetes is extremely difficult.
- For every suicide, the World Health Organization reports there are 20 suicide attempts.
- Health care providers often underestimate the prevalence of suicidality among their patient population and feel ill-equipped to initiate conversations with their patients about suicide.”
Dr. Barnard-Kelly also presented some sobering statistics that highlight the need for increased awareness.
A study reported that, of 160 cases of insulin overdose, 90% were suicides.
Adolescents and young adults with type 2 diabetes are 61% more likely to report suicidal thoughts than those without diabetes.
The risk of depression is two- to three-times higher in people with diabetes. According to another study, 7% of deaths in individuals with type 1 diabetes are estimated to be from suicide.
Survey about screening for depression, suicide risk in diabetes
During the symposium, Daniel R. Chernavvsky, MD, reported results from a small online survey of health care professionals who treat patients with type 1 or type 2 diabetes, which identified their concerns about screening for depression and assessing suicide risk in patients with diabetes.
Respondents were mainly from the United States (103) but were also from the United Kingdom (18), Slovenia, and the Netherlands (5), said Dr. Chernavvsky, who is senior director of medical affairs at Dexcom, Charlottesville, Va.
They included 59 doctors, 21 nurses,17 diabetes educators, 15 psychologists, seven dieticians, four social workers, and six “other” health care professionals, with a mean age of 46 (range, 25-72 years old) who had been working on average 14 years (range, 0.5-45 years).
Close to three-quarters (72%) reported that at least one of their patients had attempted suicide. The most common self-harm behaviors in their patients were insulin omission or a too large insulin bolus, and less often, binge eating.
Almost all respondents (95%) believed that routine visits to the diabetes clinic were appropriate times to discuss depression, self-injury, and suicidal ideation – at every visit (42% of respondents) or some visits (52%).
Only 30% were comfortable asking patients about self-harm or suicide.
Psychologists and social workers were very comfortable, but others were less comfortable or not comfortable at all.
Many respondents expressed concerns such as, “What do I do?” “Would I make the problem worse?” “Would I give the patient the idea?” Some reported they had “limited resources” or it “feels invasive.”
They identified a need for “a better understanding of what [they could] do to support and care for patients,” and “more knowledge about how to deal with [patients’] answers” to screening questionnaires.
Screening for psychological morbidities in diabetes
Guidelines from the ADA and the International Society for Pediatric and Adolescent Diabetes recommend routine screening of patients with diabetes for psychological morbidities, including depression, said Shideh Majidi, MD.
Depression is associated with higher A1c, noted Dr. Majidi, who is associate director, childhood and adolescent diabetes program at Children’s National Hospital, Washington, D.C.
She identified the following topics that need to be addressed when considering implementing a program for depression screening and suicide risk assessment in a diabetes clinic:
- Conducting screening: Which screening questionnaire will you use? Who will do it? Where? How often?
- Scoring screening questionnaires: Who will do it?
- Depression screening discussion: Who will do it? How will the person be notified of the score?
- Suicide risk assessment: Who will conduct it? What is the process to get someone to the emergency department?
- Resources/referral: Who will initiate and follow-up?
Next steps
The RESCUE advocacy group is preparing educational and support materials for health care professionals who treat patients with diabetes, as well as other materials for patients themselves.
A version of this article first appeared on Medscape.com.
Depression, self-harm, and suicide among people with type 1 and type 2 diabetes are “underappreciated” among health care practitioners, according to Katharine Barnard-Kelly, PhD, who founded the Reducing Suicide Rates Among Individuals With Diabetes (RESCUE) advocacy group in 2021.
“We have the most advanced technology to achieve glycemic control, but the mental burden remains underappreciated,” she said at a symposium with other speakers from RESCUE during the annual scientific sessions of the American Diabetes Association.
Notably, suicide and self-harm are “all too common” among young adults with type 1 diabetes who are receiving insulin, said Dr. Barnard-Kelly, a psychologist and visiting professor at Southern Health NHS Foundation Trust, Southampton, United Kingdom. And insulin under- or overdosing is the most common method of self-harm.
However, “with a multipronged approach to awareness, education, and identification, we have the opportunity to intervene on the link between suicide and diabetes,” she said, noting that the aim is to “raise awareness and arm [doctors and others] with messages that can ultimately save a young person’s life if adopted in clinical practice and through mental health screenings.”
The rationale behind the RESCUE initiative is also described in a brief report published in Diabetes Technology & Therapeutics.
Six key messages
RESCUE now has “approximately 30 members across academia, clinical practice, industry, advocacy, government, regulatory bodies [including the U.S. Food and Drug Administration], and people with diabetes from several countries,” Dr. Barnard-Kelly told this news organization.
She identified six key messages from the symposium:
- “Suicide prevalence is considerably higher among people with diabetes than the general population.
- Talking about suicide does not increase an individual’s risk of suicide.
- Current screening tools for depression and suicide are not sufficiently sensitive to be effective among people with diabetes.
- Identification of suicidal acts among people with diabetes is extremely difficult.
- For every suicide, the World Health Organization reports there are 20 suicide attempts.
- Health care providers often underestimate the prevalence of suicidality among their patient population and feel ill-equipped to initiate conversations with their patients about suicide.”
Dr. Barnard-Kelly also presented some sobering statistics that highlight the need for increased awareness.
A study reported that, of 160 cases of insulin overdose, 90% were suicides.
Adolescents and young adults with type 2 diabetes are 61% more likely to report suicidal thoughts than those without diabetes.
The risk of depression is two- to three-times higher in people with diabetes. According to another study, 7% of deaths in individuals with type 1 diabetes are estimated to be from suicide.
Survey about screening for depression, suicide risk in diabetes
During the symposium, Daniel R. Chernavvsky, MD, reported results from a small online survey of health care professionals who treat patients with type 1 or type 2 diabetes, which identified their concerns about screening for depression and assessing suicide risk in patients with diabetes.
Respondents were mainly from the United States (103) but were also from the United Kingdom (18), Slovenia, and the Netherlands (5), said Dr. Chernavvsky, who is senior director of medical affairs at Dexcom, Charlottesville, Va.
They included 59 doctors, 21 nurses,17 diabetes educators, 15 psychologists, seven dieticians, four social workers, and six “other” health care professionals, with a mean age of 46 (range, 25-72 years old) who had been working on average 14 years (range, 0.5-45 years).
Close to three-quarters (72%) reported that at least one of their patients had attempted suicide. The most common self-harm behaviors in their patients were insulin omission or a too large insulin bolus, and less often, binge eating.
Almost all respondents (95%) believed that routine visits to the diabetes clinic were appropriate times to discuss depression, self-injury, and suicidal ideation – at every visit (42% of respondents) or some visits (52%).
Only 30% were comfortable asking patients about self-harm or suicide.
Psychologists and social workers were very comfortable, but others were less comfortable or not comfortable at all.
Many respondents expressed concerns such as, “What do I do?” “Would I make the problem worse?” “Would I give the patient the idea?” Some reported they had “limited resources” or it “feels invasive.”
They identified a need for “a better understanding of what [they could] do to support and care for patients,” and “more knowledge about how to deal with [patients’] answers” to screening questionnaires.
Screening for psychological morbidities in diabetes
Guidelines from the ADA and the International Society for Pediatric and Adolescent Diabetes recommend routine screening of patients with diabetes for psychological morbidities, including depression, said Shideh Majidi, MD.
Depression is associated with higher A1c, noted Dr. Majidi, who is associate director, childhood and adolescent diabetes program at Children’s National Hospital, Washington, D.C.
She identified the following topics that need to be addressed when considering implementing a program for depression screening and suicide risk assessment in a diabetes clinic:
- Conducting screening: Which screening questionnaire will you use? Who will do it? Where? How often?
- Scoring screening questionnaires: Who will do it?
- Depression screening discussion: Who will do it? How will the person be notified of the score?
- Suicide risk assessment: Who will conduct it? What is the process to get someone to the emergency department?
- Resources/referral: Who will initiate and follow-up?
Next steps
The RESCUE advocacy group is preparing educational and support materials for health care professionals who treat patients with diabetes, as well as other materials for patients themselves.
A version of this article first appeared on Medscape.com.
FROM ADA 2022