User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Stem cell transplants could be ‘transformational’ in type 1 diabetes
NEW ORLEANS – Two patients with type 1 diabetes have now experienced improved blood glucose control with Vertex Pharmaceutical’s investigational allogeneic stem cell–derived islets (VX-880), with the first person now completely insulin independent at 9 months post transplant.
Prior to the procedure, both patients had hypoglycemic unawareness and had experienced multiple episodes of severe hypoglycemia, conditions considered severe enough to justify the risk of immune suppression (which is required for such stem cell–derived islet transplants as they are “foreign” to the recipient).
The first patient, a 64-year-old man with type 1 diabetes for more than 40 years, now has a hemoglobin A1c in the normal range without taking any insulin more than 9 months after the procedure. The second, a 35-year-old woman with type 1 diabetes for 10.7 years, experienced a 30% reduction in insulin use and significant increased time spent in target glucose range, by 5 months post transplant. Both patients were given just half the targeted VX-880 dose.
Data for those two patients – the first in Vertex’s phase 1/2 multicenter, single-arm, open-label clinical trial of VX-880 – were reported at the annual scientific sessions of the American Diabetes Association, by James F. Markmann, MD, PhD.
He has been transplanting pancreatic islet cells from deceased donors into humans via infusion into the hepatic portal vein for over 20 years.
Transplantation of pancreatic islet cells obtained from cadavers have been shown to eliminate severe hypoglycemia and improve glycemic control in patients with type 1 diabetes, but they’re limited in quantity and are of variable quality. Islets that are manufactured via differentiation from human pluripotent stem cells represent an alternative, explained Dr. Markmann, chief of the division of transplant surgery at Massachusetts General Hospital, Boston.
“This is a new area. ... We hope this will be the same or potentially better. With stem cell–derived islets the quality, consistency, and reliability might produce a better result than with cadaveric islets,” he commented during a press briefing here.
A third patient has recently received the full targeted VX-880 dose but was not part of the current report. The planned enrollment is 17 patients. The trial is currently on clinical hold per the Food and Drug Administration concerning the criteria around dose escalation, but Vertex is working with the FDA to sort that out. Meanwhile, enrollment remains open in Canada, Dr. Markmann said.
In answer to a question about how patient 1 is doing now, Dr. Markmann replied, “He’s doing great. He’s probably the most appreciative patient I’ve ever met. His life was being destroyed by diabetes. He couldn’t work. He crashed his motorcycle from [low blood sugar]. He really was tremendously appreciative that he could participate.”
When Dr. Markmann explained the potential uncertainties and risks to the patient prior to the procedure, the patient replied: “I want to participate. If I die from this and I help somebody else I’d be happy, but I can’t go on living the way I’m living.”
“These people really suffer and this, I think, brings hope to them,” Dr. Markmann said.
‘Beautiful data’ seen in two patients, with ‘transformational’ potential
Asked to comment, Marlon Pragnell, PhD, vice president for Research & Science at the ADA, told this news organization: “It’s beautiful data. People who have type 1 diabetes lack [pancreatic] beta cells ... it was impossible to get sufficient beta cells from cadaveric transplants. It’s just nowhere near enough. If this is safe and effective, if they continue to show safety and efficacy like patient 1, this will be transformational.”
Dr. Markmann presented data for the most recent study visit for each of the two patients, 270 days for patient 1 and 150 days for patient 2. Prior to the transplants, patient 1 had experienced five severe hypoglycemic events and patient 2 had experienced three.
Both had undetectable C-peptide levels at baseline. In response to a mixed-meal tolerance test, patient 1 showed a “robust” C-peptide response by day 90, which increased by day 180. Those levels had dropped but were still detectable by day 270, “possibly due to improved insulin sensitivity,” Dr. Markmann said.
Similarly, Patient 2 also had increased C-peptide that increased to detectable range by day 90 with improved glucose disposal.
Hemoglobin A1c dropped in patient 1 from 8.6% at baseline to 6.9% at day 180, to a “remarkable” 5.2% at day 270. For patient 2, the drop was from 7.5% to a nadir of 6.4% by day 57, then reversing back to 7.1% at day 150.
Both patients also had significant reductions in insulin dose. For patient 1, the dose reduction was more than 90% – from 34 units at baseline to 2.6 units by day 90. By day 210 he was able to stop insulin and by day 270 he met formal criteria for insulin independence.
Patient 2 also had a significant reduction in insulin dose, from 25.9 units to 18.7 by day 57 and remained stable thereafter, at 18.2 units by day 150.
Asked why Patient 2’s results weren’t quite as impressive as patient 1’s, Dr. Markmann replied “I think what’s important is that both patients did great. And since this was a half-dose, we might have expected that the outcome was going to be more like patient 2 rather than patient 1. So, I think we’re just going to have to [study this in] more patients to understand where it falls.”
Although patient 1 experienced a cluster of six severe hypoglycemic events early in the posttransplant period, he had no further events after day 35. Patient 2 had no severe hypoglycemic events.
Other safety events were generally consistent with that seen with the immunosuppressive regimen in the perioperative period. Patient 1 had a “mild and self-limited” rise in liver function test and also experienced two severe adverse events: A rash from the immune suppression that resolved spontaneously, and dehydration requiring hospitalization on day 186. Patient 2’s adverse events were all mild to moderate, most commonly headache and hypomagnesemia and not related to VX-880.
Immunosuppression: Work is ongoing
The immunosuppression regimen used comprises a depletion of lymphocytes at induction, followed by a maintenance regimen of two standard agents used in kidney transplant patients and found to be well tolerated, Dr. Markmann said.
Still, the risk of immunosuppression generally outweighs the potential benefit for most people with type 1 diabetes who are managing reasonably well with insulin treatment.
“This is part one of a two-part problem. One is to have a reliable, consistent, effective cell therapy. The second is to develop an approach that doesn’t require immunosuppression. ... But if we had a way of transplanting the cells without the need for immunosuppression, then it could be really widely available. That’s an opportunity for the future since these cells can be made in unlimited quantities,” Dr. Markmann commented during the press briefing.
Asked for his thoughts about the immunosuppression aspect, Dr. Pragnell told this news organization: “Immune suppression is a concern, but I feel that this is just the start of so much research in this area. They’re going to take this step by step. This is just the start. My understanding is they have additional strategies around immune suppression, and in the future they might not even need immunosuppression. But even at this stage right now, it’s amazing.”
He added: “The ‘artificial pancreas’ is a huge step forward, but it’s just a bridge to a cure, whereas if they’re able to show safety and efficacy, this is potentially a cure. ... I’m very excited about it.”
Dr. Markmann serves on advisory boards for iTolerance, eGenesis, and QihanBio. He is a consultant to Vertex Pharmaceuticals. Dr. Pragnell is an ADA employee and has no further disclosures.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – Two patients with type 1 diabetes have now experienced improved blood glucose control with Vertex Pharmaceutical’s investigational allogeneic stem cell–derived islets (VX-880), with the first person now completely insulin independent at 9 months post transplant.
Prior to the procedure, both patients had hypoglycemic unawareness and had experienced multiple episodes of severe hypoglycemia, conditions considered severe enough to justify the risk of immune suppression (which is required for such stem cell–derived islet transplants as they are “foreign” to the recipient).
The first patient, a 64-year-old man with type 1 diabetes for more than 40 years, now has a hemoglobin A1c in the normal range without taking any insulin more than 9 months after the procedure. The second, a 35-year-old woman with type 1 diabetes for 10.7 years, experienced a 30% reduction in insulin use and significant increased time spent in target glucose range, by 5 months post transplant. Both patients were given just half the targeted VX-880 dose.
Data for those two patients – the first in Vertex’s phase 1/2 multicenter, single-arm, open-label clinical trial of VX-880 – were reported at the annual scientific sessions of the American Diabetes Association, by James F. Markmann, MD, PhD.
He has been transplanting pancreatic islet cells from deceased donors into humans via infusion into the hepatic portal vein for over 20 years.
Transplantation of pancreatic islet cells obtained from cadavers have been shown to eliminate severe hypoglycemia and improve glycemic control in patients with type 1 diabetes, but they’re limited in quantity and are of variable quality. Islets that are manufactured via differentiation from human pluripotent stem cells represent an alternative, explained Dr. Markmann, chief of the division of transplant surgery at Massachusetts General Hospital, Boston.
“This is a new area. ... We hope this will be the same or potentially better. With stem cell–derived islets the quality, consistency, and reliability might produce a better result than with cadaveric islets,” he commented during a press briefing here.
A third patient has recently received the full targeted VX-880 dose but was not part of the current report. The planned enrollment is 17 patients. The trial is currently on clinical hold per the Food and Drug Administration concerning the criteria around dose escalation, but Vertex is working with the FDA to sort that out. Meanwhile, enrollment remains open in Canada, Dr. Markmann said.
In answer to a question about how patient 1 is doing now, Dr. Markmann replied, “He’s doing great. He’s probably the most appreciative patient I’ve ever met. His life was being destroyed by diabetes. He couldn’t work. He crashed his motorcycle from [low blood sugar]. He really was tremendously appreciative that he could participate.”
When Dr. Markmann explained the potential uncertainties and risks to the patient prior to the procedure, the patient replied: “I want to participate. If I die from this and I help somebody else I’d be happy, but I can’t go on living the way I’m living.”
“These people really suffer and this, I think, brings hope to them,” Dr. Markmann said.
‘Beautiful data’ seen in two patients, with ‘transformational’ potential
Asked to comment, Marlon Pragnell, PhD, vice president for Research & Science at the ADA, told this news organization: “It’s beautiful data. People who have type 1 diabetes lack [pancreatic] beta cells ... it was impossible to get sufficient beta cells from cadaveric transplants. It’s just nowhere near enough. If this is safe and effective, if they continue to show safety and efficacy like patient 1, this will be transformational.”
Dr. Markmann presented data for the most recent study visit for each of the two patients, 270 days for patient 1 and 150 days for patient 2. Prior to the transplants, patient 1 had experienced five severe hypoglycemic events and patient 2 had experienced three.
Both had undetectable C-peptide levels at baseline. In response to a mixed-meal tolerance test, patient 1 showed a “robust” C-peptide response by day 90, which increased by day 180. Those levels had dropped but were still detectable by day 270, “possibly due to improved insulin sensitivity,” Dr. Markmann said.
Similarly, Patient 2 also had increased C-peptide that increased to detectable range by day 90 with improved glucose disposal.
Hemoglobin A1c dropped in patient 1 from 8.6% at baseline to 6.9% at day 180, to a “remarkable” 5.2% at day 270. For patient 2, the drop was from 7.5% to a nadir of 6.4% by day 57, then reversing back to 7.1% at day 150.
Both patients also had significant reductions in insulin dose. For patient 1, the dose reduction was more than 90% – from 34 units at baseline to 2.6 units by day 90. By day 210 he was able to stop insulin and by day 270 he met formal criteria for insulin independence.
Patient 2 also had a significant reduction in insulin dose, from 25.9 units to 18.7 by day 57 and remained stable thereafter, at 18.2 units by day 150.
Asked why Patient 2’s results weren’t quite as impressive as patient 1’s, Dr. Markmann replied “I think what’s important is that both patients did great. And since this was a half-dose, we might have expected that the outcome was going to be more like patient 2 rather than patient 1. So, I think we’re just going to have to [study this in] more patients to understand where it falls.”
Although patient 1 experienced a cluster of six severe hypoglycemic events early in the posttransplant period, he had no further events after day 35. Patient 2 had no severe hypoglycemic events.
Other safety events were generally consistent with that seen with the immunosuppressive regimen in the perioperative period. Patient 1 had a “mild and self-limited” rise in liver function test and also experienced two severe adverse events: A rash from the immune suppression that resolved spontaneously, and dehydration requiring hospitalization on day 186. Patient 2’s adverse events were all mild to moderate, most commonly headache and hypomagnesemia and not related to VX-880.
Immunosuppression: Work is ongoing
The immunosuppression regimen used comprises a depletion of lymphocytes at induction, followed by a maintenance regimen of two standard agents used in kidney transplant patients and found to be well tolerated, Dr. Markmann said.
Still, the risk of immunosuppression generally outweighs the potential benefit for most people with type 1 diabetes who are managing reasonably well with insulin treatment.
“This is part one of a two-part problem. One is to have a reliable, consistent, effective cell therapy. The second is to develop an approach that doesn’t require immunosuppression. ... But if we had a way of transplanting the cells without the need for immunosuppression, then it could be really widely available. That’s an opportunity for the future since these cells can be made in unlimited quantities,” Dr. Markmann commented during the press briefing.
Asked for his thoughts about the immunosuppression aspect, Dr. Pragnell told this news organization: “Immune suppression is a concern, but I feel that this is just the start of so much research in this area. They’re going to take this step by step. This is just the start. My understanding is they have additional strategies around immune suppression, and in the future they might not even need immunosuppression. But even at this stage right now, it’s amazing.”
He added: “The ‘artificial pancreas’ is a huge step forward, but it’s just a bridge to a cure, whereas if they’re able to show safety and efficacy, this is potentially a cure. ... I’m very excited about it.”
Dr. Markmann serves on advisory boards for iTolerance, eGenesis, and QihanBio. He is a consultant to Vertex Pharmaceuticals. Dr. Pragnell is an ADA employee and has no further disclosures.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – Two patients with type 1 diabetes have now experienced improved blood glucose control with Vertex Pharmaceutical’s investigational allogeneic stem cell–derived islets (VX-880), with the first person now completely insulin independent at 9 months post transplant.
Prior to the procedure, both patients had hypoglycemic unawareness and had experienced multiple episodes of severe hypoglycemia, conditions considered severe enough to justify the risk of immune suppression (which is required for such stem cell–derived islet transplants as they are “foreign” to the recipient).
The first patient, a 64-year-old man with type 1 diabetes for more than 40 years, now has a hemoglobin A1c in the normal range without taking any insulin more than 9 months after the procedure. The second, a 35-year-old woman with type 1 diabetes for 10.7 years, experienced a 30% reduction in insulin use and significant increased time spent in target glucose range, by 5 months post transplant. Both patients were given just half the targeted VX-880 dose.
Data for those two patients – the first in Vertex’s phase 1/2 multicenter, single-arm, open-label clinical trial of VX-880 – were reported at the annual scientific sessions of the American Diabetes Association, by James F. Markmann, MD, PhD.
He has been transplanting pancreatic islet cells from deceased donors into humans via infusion into the hepatic portal vein for over 20 years.
Transplantation of pancreatic islet cells obtained from cadavers have been shown to eliminate severe hypoglycemia and improve glycemic control in patients with type 1 diabetes, but they’re limited in quantity and are of variable quality. Islets that are manufactured via differentiation from human pluripotent stem cells represent an alternative, explained Dr. Markmann, chief of the division of transplant surgery at Massachusetts General Hospital, Boston.
“This is a new area. ... We hope this will be the same or potentially better. With stem cell–derived islets the quality, consistency, and reliability might produce a better result than with cadaveric islets,” he commented during a press briefing here.
A third patient has recently received the full targeted VX-880 dose but was not part of the current report. The planned enrollment is 17 patients. The trial is currently on clinical hold per the Food and Drug Administration concerning the criteria around dose escalation, but Vertex is working with the FDA to sort that out. Meanwhile, enrollment remains open in Canada, Dr. Markmann said.
In answer to a question about how patient 1 is doing now, Dr. Markmann replied, “He’s doing great. He’s probably the most appreciative patient I’ve ever met. His life was being destroyed by diabetes. He couldn’t work. He crashed his motorcycle from [low blood sugar]. He really was tremendously appreciative that he could participate.”
When Dr. Markmann explained the potential uncertainties and risks to the patient prior to the procedure, the patient replied: “I want to participate. If I die from this and I help somebody else I’d be happy, but I can’t go on living the way I’m living.”
“These people really suffer and this, I think, brings hope to them,” Dr. Markmann said.
‘Beautiful data’ seen in two patients, with ‘transformational’ potential
Asked to comment, Marlon Pragnell, PhD, vice president for Research & Science at the ADA, told this news organization: “It’s beautiful data. People who have type 1 diabetes lack [pancreatic] beta cells ... it was impossible to get sufficient beta cells from cadaveric transplants. It’s just nowhere near enough. If this is safe and effective, if they continue to show safety and efficacy like patient 1, this will be transformational.”
Dr. Markmann presented data for the most recent study visit for each of the two patients, 270 days for patient 1 and 150 days for patient 2. Prior to the transplants, patient 1 had experienced five severe hypoglycemic events and patient 2 had experienced three.
Both had undetectable C-peptide levels at baseline. In response to a mixed-meal tolerance test, patient 1 showed a “robust” C-peptide response by day 90, which increased by day 180. Those levels had dropped but were still detectable by day 270, “possibly due to improved insulin sensitivity,” Dr. Markmann said.
Similarly, Patient 2 also had increased C-peptide that increased to detectable range by day 90 with improved glucose disposal.
Hemoglobin A1c dropped in patient 1 from 8.6% at baseline to 6.9% at day 180, to a “remarkable” 5.2% at day 270. For patient 2, the drop was from 7.5% to a nadir of 6.4% by day 57, then reversing back to 7.1% at day 150.
Both patients also had significant reductions in insulin dose. For patient 1, the dose reduction was more than 90% – from 34 units at baseline to 2.6 units by day 90. By day 210 he was able to stop insulin and by day 270 he met formal criteria for insulin independence.
Patient 2 also had a significant reduction in insulin dose, from 25.9 units to 18.7 by day 57 and remained stable thereafter, at 18.2 units by day 150.
Asked why Patient 2’s results weren’t quite as impressive as patient 1’s, Dr. Markmann replied “I think what’s important is that both patients did great. And since this was a half-dose, we might have expected that the outcome was going to be more like patient 2 rather than patient 1. So, I think we’re just going to have to [study this in] more patients to understand where it falls.”
Although patient 1 experienced a cluster of six severe hypoglycemic events early in the posttransplant period, he had no further events after day 35. Patient 2 had no severe hypoglycemic events.
Other safety events were generally consistent with that seen with the immunosuppressive regimen in the perioperative period. Patient 1 had a “mild and self-limited” rise in liver function test and also experienced two severe adverse events: A rash from the immune suppression that resolved spontaneously, and dehydration requiring hospitalization on day 186. Patient 2’s adverse events were all mild to moderate, most commonly headache and hypomagnesemia and not related to VX-880.
Immunosuppression: Work is ongoing
The immunosuppression regimen used comprises a depletion of lymphocytes at induction, followed by a maintenance regimen of two standard agents used in kidney transplant patients and found to be well tolerated, Dr. Markmann said.
Still, the risk of immunosuppression generally outweighs the potential benefit for most people with type 1 diabetes who are managing reasonably well with insulin treatment.
“This is part one of a two-part problem. One is to have a reliable, consistent, effective cell therapy. The second is to develop an approach that doesn’t require immunosuppression. ... But if we had a way of transplanting the cells without the need for immunosuppression, then it could be really widely available. That’s an opportunity for the future since these cells can be made in unlimited quantities,” Dr. Markmann commented during the press briefing.
Asked for his thoughts about the immunosuppression aspect, Dr. Pragnell told this news organization: “Immune suppression is a concern, but I feel that this is just the start of so much research in this area. They’re going to take this step by step. This is just the start. My understanding is they have additional strategies around immune suppression, and in the future they might not even need immunosuppression. But even at this stage right now, it’s amazing.”
He added: “The ‘artificial pancreas’ is a huge step forward, but it’s just a bridge to a cure, whereas if they’re able to show safety and efficacy, this is potentially a cure. ... I’m very excited about it.”
Dr. Markmann serves on advisory boards for iTolerance, eGenesis, and QihanBio. He is a consultant to Vertex Pharmaceuticals. Dr. Pragnell is an ADA employee and has no further disclosures.
A version of this article first appeared on Medscape.com.
AT ADA 2022
Substance use the main cause of physician license actions
Despite a sharp uptick in 2011, substance use–specific license actions taken against physicians dropped in frequency between 2004 and 2020.
More than three fourths (76.3%) of license actions taken against physicians were related to substance use, according to a recent study published in JAMA. Psychological impairment was the reason associated with more than 1 in 10 (11.5%) actions taken against physicians’ licenses, while physical impairment was the reason behind approximately 12% of such actions, per the study.
Researchers analyzed 5032 actions taken against the licenses of U.S. physicians. The actions were reported to the National Practitioner Data Bank and were related to substance use, psychological impairment, and physical impairment. The National Practitioner Data Bank is a web-based repository of reports with information on medical malpractice payments and certain adverse actions related to healthcare practitioners, providers, and suppliers. It is provided by the Department of Health & Human Services.
“While there has been increased attention [on] the mental health of physicians, we wanted to understand the extent to which changes in attitudes and practices were reflected in actions taken by hospitals or licensing boards, which are reported in the National Practitioner Data Bank,” Lisa Rotenstein, MD, a primary care physician at Boston’s Brigham and Women’s Hospital and lead author of the study, told this news organization.
Dr. Rotenstein, who is an assistant professor at Harvard Medical School, Boston, studies issues of mental health among physicians and trainees. Dr. Rotenstein was the lead author of a 2016 study that found that more than a quarter (27.2%) of medical students have depressive symptoms. She was also lead author of a 2018 study published in JAMA on the prevalence of burnout among attending physicians.
Actions against physicians trending downward
2011 marked the peak in actions taken against physicians’ licenses for substance use, per the study, but actions related to substance use have otherwise maintained a steady decline over the past 17 years. Researchers found that physicians with license actions as a result of substance use or psychological impairment were more likely to receive indefinite penalties, while also having emergency action taken against their license to practice.
In addition, physicians who had actions taken against their licenses because of substance use or psychological impairment were more likely to accrue a greater number of actions over the course of their careers, according to the study.
About 47% of physicians reported experiencing burnout per Medscape’s Physician Burnout and Depression Report 2022: Stress, Anxiety, and Anger report. Burnout among emergency physicians spiked from 43% in 2020 to 60% in 2021, according to the report.
More than one quarter (26%) of physicians reported drinking alcohol to cope with burnout in 2020, according to Medscape’s 2021 Physician Burnout and Suicide Report. Per the 2021 report, 48% of physicians chose exercise to deal with burnout, while 35% indulged in eating junk food.
Peter Grinspoon, MD, a Boston-based primary care physician, wrote in The Los Angeles Times in 2016 that the rate of substance abuse among physicians starts at 10% and can go as high as 15%; by comparison, rates of substance use among the general population are 8%-10%. “What appears to account for the difference is physician distress, and in the case of drug abuse, plentiful access,” he added.
Dr. Grinspoon wrote a 2016 book called “Free Refills: A Doctor Confronts His Addiction,” which chronicles his experience in recovery and relapse as a physician who was dependent on opioid painkillers.
The findings from the recent study in JAMA “suggest we have made some progress in addressing issues related to substance use in ways that don’t result in license actions or even in meeting physicians’ need for support related to substance use,” said Dr. Rotenstein.
Still, she insists that there’s “substantial opportunity to improve mental health and support offerings for physicians and to reduce stigma related to seeking and receiving mental health support, ideally averting the need for license actions.”
According to Dr. Rotenstein, the cases listed in the National Practitioner Data Bank represent the most severe cases; these reports have risen to a high level of attention or concern and are the result of adverse action reports submitted by healthcare institutions and state licensing boards.
“There are many, many more physicians whose cases are not represented here but who struggle with depression, anxiety, substance use, and more,” said Dr. Rotenstein.
A version of this article first appeared on Medscape.com.
Despite a sharp uptick in 2011, substance use–specific license actions taken against physicians dropped in frequency between 2004 and 2020.
More than three fourths (76.3%) of license actions taken against physicians were related to substance use, according to a recent study published in JAMA. Psychological impairment was the reason associated with more than 1 in 10 (11.5%) actions taken against physicians’ licenses, while physical impairment was the reason behind approximately 12% of such actions, per the study.
Researchers analyzed 5032 actions taken against the licenses of U.S. physicians. The actions were reported to the National Practitioner Data Bank and were related to substance use, psychological impairment, and physical impairment. The National Practitioner Data Bank is a web-based repository of reports with information on medical malpractice payments and certain adverse actions related to healthcare practitioners, providers, and suppliers. It is provided by the Department of Health & Human Services.
“While there has been increased attention [on] the mental health of physicians, we wanted to understand the extent to which changes in attitudes and practices were reflected in actions taken by hospitals or licensing boards, which are reported in the National Practitioner Data Bank,” Lisa Rotenstein, MD, a primary care physician at Boston’s Brigham and Women’s Hospital and lead author of the study, told this news organization.
Dr. Rotenstein, who is an assistant professor at Harvard Medical School, Boston, studies issues of mental health among physicians and trainees. Dr. Rotenstein was the lead author of a 2016 study that found that more than a quarter (27.2%) of medical students have depressive symptoms. She was also lead author of a 2018 study published in JAMA on the prevalence of burnout among attending physicians.
Actions against physicians trending downward
2011 marked the peak in actions taken against physicians’ licenses for substance use, per the study, but actions related to substance use have otherwise maintained a steady decline over the past 17 years. Researchers found that physicians with license actions as a result of substance use or psychological impairment were more likely to receive indefinite penalties, while also having emergency action taken against their license to practice.
In addition, physicians who had actions taken against their licenses because of substance use or psychological impairment were more likely to accrue a greater number of actions over the course of their careers, according to the study.
About 47% of physicians reported experiencing burnout per Medscape’s Physician Burnout and Depression Report 2022: Stress, Anxiety, and Anger report. Burnout among emergency physicians spiked from 43% in 2020 to 60% in 2021, according to the report.
More than one quarter (26%) of physicians reported drinking alcohol to cope with burnout in 2020, according to Medscape’s 2021 Physician Burnout and Suicide Report. Per the 2021 report, 48% of physicians chose exercise to deal with burnout, while 35% indulged in eating junk food.
Peter Grinspoon, MD, a Boston-based primary care physician, wrote in The Los Angeles Times in 2016 that the rate of substance abuse among physicians starts at 10% and can go as high as 15%; by comparison, rates of substance use among the general population are 8%-10%. “What appears to account for the difference is physician distress, and in the case of drug abuse, plentiful access,” he added.
Dr. Grinspoon wrote a 2016 book called “Free Refills: A Doctor Confronts His Addiction,” which chronicles his experience in recovery and relapse as a physician who was dependent on opioid painkillers.
The findings from the recent study in JAMA “suggest we have made some progress in addressing issues related to substance use in ways that don’t result in license actions or even in meeting physicians’ need for support related to substance use,” said Dr. Rotenstein.
Still, she insists that there’s “substantial opportunity to improve mental health and support offerings for physicians and to reduce stigma related to seeking and receiving mental health support, ideally averting the need for license actions.”
According to Dr. Rotenstein, the cases listed in the National Practitioner Data Bank represent the most severe cases; these reports have risen to a high level of attention or concern and are the result of adverse action reports submitted by healthcare institutions and state licensing boards.
“There are many, many more physicians whose cases are not represented here but who struggle with depression, anxiety, substance use, and more,” said Dr. Rotenstein.
A version of this article first appeared on Medscape.com.
Despite a sharp uptick in 2011, substance use–specific license actions taken against physicians dropped in frequency between 2004 and 2020.
More than three fourths (76.3%) of license actions taken against physicians were related to substance use, according to a recent study published in JAMA. Psychological impairment was the reason associated with more than 1 in 10 (11.5%) actions taken against physicians’ licenses, while physical impairment was the reason behind approximately 12% of such actions, per the study.
Researchers analyzed 5032 actions taken against the licenses of U.S. physicians. The actions were reported to the National Practitioner Data Bank and were related to substance use, psychological impairment, and physical impairment. The National Practitioner Data Bank is a web-based repository of reports with information on medical malpractice payments and certain adverse actions related to healthcare practitioners, providers, and suppliers. It is provided by the Department of Health & Human Services.
“While there has been increased attention [on] the mental health of physicians, we wanted to understand the extent to which changes in attitudes and practices were reflected in actions taken by hospitals or licensing boards, which are reported in the National Practitioner Data Bank,” Lisa Rotenstein, MD, a primary care physician at Boston’s Brigham and Women’s Hospital and lead author of the study, told this news organization.
Dr. Rotenstein, who is an assistant professor at Harvard Medical School, Boston, studies issues of mental health among physicians and trainees. Dr. Rotenstein was the lead author of a 2016 study that found that more than a quarter (27.2%) of medical students have depressive symptoms. She was also lead author of a 2018 study published in JAMA on the prevalence of burnout among attending physicians.
Actions against physicians trending downward
2011 marked the peak in actions taken against physicians’ licenses for substance use, per the study, but actions related to substance use have otherwise maintained a steady decline over the past 17 years. Researchers found that physicians with license actions as a result of substance use or psychological impairment were more likely to receive indefinite penalties, while also having emergency action taken against their license to practice.
In addition, physicians who had actions taken against their licenses because of substance use or psychological impairment were more likely to accrue a greater number of actions over the course of their careers, according to the study.
About 47% of physicians reported experiencing burnout per Medscape’s Physician Burnout and Depression Report 2022: Stress, Anxiety, and Anger report. Burnout among emergency physicians spiked from 43% in 2020 to 60% in 2021, according to the report.
More than one quarter (26%) of physicians reported drinking alcohol to cope with burnout in 2020, according to Medscape’s 2021 Physician Burnout and Suicide Report. Per the 2021 report, 48% of physicians chose exercise to deal with burnout, while 35% indulged in eating junk food.
Peter Grinspoon, MD, a Boston-based primary care physician, wrote in The Los Angeles Times in 2016 that the rate of substance abuse among physicians starts at 10% and can go as high as 15%; by comparison, rates of substance use among the general population are 8%-10%. “What appears to account for the difference is physician distress, and in the case of drug abuse, plentiful access,” he added.
Dr. Grinspoon wrote a 2016 book called “Free Refills: A Doctor Confronts His Addiction,” which chronicles his experience in recovery and relapse as a physician who was dependent on opioid painkillers.
The findings from the recent study in JAMA “suggest we have made some progress in addressing issues related to substance use in ways that don’t result in license actions or even in meeting physicians’ need for support related to substance use,” said Dr. Rotenstein.
Still, she insists that there’s “substantial opportunity to improve mental health and support offerings for physicians and to reduce stigma related to seeking and receiving mental health support, ideally averting the need for license actions.”
According to Dr. Rotenstein, the cases listed in the National Practitioner Data Bank represent the most severe cases; these reports have risen to a high level of attention or concern and are the result of adverse action reports submitted by healthcare institutions and state licensing boards.
“There are many, many more physicians whose cases are not represented here but who struggle with depression, anxiety, substance use, and more,” said Dr. Rotenstein.
A version of this article first appeared on Medscape.com.
FROM JAMA
FDA panel strongly backs protein-based Novavax COVID-19 vaccine
than the cutting-edge technology used in mRNA-based shots.
The Vaccines and Related Biological Products Advisory Committee of the Food and Drug Administration voted almost unanimously June 7 in favor of Novavax’s two-dose COVID-19 vaccine for those 18 or older – despite some concerns over rare events of myocarditis and pericarditis.
The tally was 21 “yes” votes, without any “no” votes, but one abstention from a panelist who then offered a largely positive take on this vaccine.
Panelist Bruce Gellin, MD, explained at the end of the meeting that he would have cast a conditional vote in favor of the Novavax vaccine, called NVX-CoV2373, had that been an option. Dr. Gellin, chief of global public health strategy for the Rockefeller Foundation and a vaccine expert, said he didn’t want his abstention to be considered as signaling opposition to the Novavax shot.
Instead, he said, he expects FDA officials will gather more data and evidence about the Novavax vaccine, especially in relation to certain manufacturing issues, before making its decision on the company’s application.
Earlier in the day, a top FDA vaccine reviewer, Doran Fink, MD, PhD, noted that there were important manufacturing differences between the Novavax vaccine supply used in different projects, complicating efforts to assess the company’s application for emergency use authorization (EUA).
But Dr. Fink noted that the FDA staff already had made a convincing case in its briefing document, with enough evidence for an initial conditional clearance to be found in available data.
The FDA is not bound to follow the suggestions of its advisory committees but it often does.
Using the ‘bully pulpit’
At the beginning of the meeting, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said he was seizing the “bully pulpit” in addressing the need to persuade more people in the United States to take shots against COVID-19.
About 67% of people in the United States aged 18 and older are fully vaccinated, but only about 50% of those in this group have had a first booster, according to the Centers for Disease Control and Prevention.
The two-dose mRNA vaccines from Pfizer and Moderna have been the subject of intense misinformation campaigns on social media, despite efforts by the FDA and other public health officials to convey the message about their strong benefit-risk profile. The FDA in May limited the authorized use of Johnson & Johnson’s single-dose COVID-19 shot, which is based on a different technology, because of concerns about rare and potentially life-threatening blood clots.
Novavax has been described as a more traditional vaccine – a protein subunit shot similar to one people have long received for protection against influenza, pertussis (whooping cough), diphtheria, and tetanus.
“Having a protein-based alternative may be more comfortable for some in terms of their acceptance of vaccines,” Dr. Marks said. “We do have a problem with vaccine uptake that is very serious in the United States. And anything we can do to get people more comfortable to be able to accept these potentially life-saving medical products is something that we feel we are compelled to do.”
Dr. Marks offered these remarks in answer to an FDA panelist’s question about the need to consider an EUA for yet another vaccine.
EUAs are special clearances the FDA can grant in connection with public health emergencies such as the pandemic. The FDA used EUAs for the initial December 2020 clearances of the Pfizer-BioNTech and Moderna vaccines. It has since granted normal approvals for both of these mRNA-based vaccines, based on larger bodies of evidence gathered and submitted by their developers.
During the meeting, the FDA panelists in general appeared comfortable with the idea of granting another EUA for a vaccine. There was agreement that the shot appeared to work in key tests, although these were done before the rise of the Omicron variant.
In a key test, known as study 301, the Novavax vaccine was judged to be 90.4% effective. In the study, 17 of the 17,272 people who got the Novavax vaccine developed COVID-19, compared with 79 of the 8,385 in the placebo group.
Panelists expressed disappointment with the lack of information about how the shot would work now.
“We’re looking at the efficacy against strains that don’t exist any longer,” said panelist Eric J. Rubin, MD, PhD, a Harvard professor and editor of the New England Journal of Medicine.
Still, Dr. Rubin added that he agreed with the argument the FDA’s Dr. Marks had made earlier for an EUA for the Novavax vaccine.
“If there really is a population of patients who are willing to take this and not willing to take the existing vaccines, I think it’s pretty compelling,” Dr. Rubin said.
Other FDA panelists were skeptical of this argument. Jay Portnoy, MD, who was listed on the FDA roster as the panel’s consumer representative, said he has close friends who are vaccine skeptics.
“Their hesitancy is more ideological than technological,” said Dr. Portnoy of Children’s Mercy Hospital, Kansas City, Mo. “So I really doubt that this vaccine is going to crack that nut, but perhaps some individuals would get this when they wouldn’t get the other ones.”
Myocarditis, pericarditis
The Novavax vaccine is already authorized in other countries, including Canada. Novavax in February announced that it had begun shipping its first doses of the vaccine to European Union member states. The vaccine can be moved through existing vaccine supply and cold chain channels instead of requiring complex new delivery procedures.
That could prove an advantage in time, said FDA panelist Michael Nelson, MD, PhD, of the University of Virginia, Charlottesville.
“Who knows even with supply chain challenges down the road, it will be nice to have options going forward,” Dr. Nelson said.
As with other COVID-19 vaccines, clinicians and researchers are still working to understand the potential risk for inflammation of heart muscle and nearby tissue with vaccination. Most patients with myocarditis or pericarditis who sought medical care for these conditions responded well to medicine and rest and felt better quickly, the CDC says on its website. They usually return to their normal daily activities after their symptoms improve.
At the June 7 meeting, Dr. Nelson said there may be cases of myocarditis that go undetected.
“Our signals are those who get admitted to the emergency room and the hospital,” he said. “I’m quite convinced that there are others who are experiencing cardiac events of lesser severity that are worthy of being studied, both from mechanistic and outcomes standpoints. So we have a lot of work to do.”
In looking at results for an initial pool of 40,000 people who received the Novavax vaccine, there were five reported cases of myocarditis or pericarditis developing within 20 days of people getting the shot, the FDA staff said in its presentation on safety.
In a briefing document released ahead of the advisory committee meeting, the FDA staff flagged this number of cases in a relatively small database as a concern, noting it “could be higher than reported during postauthorization use of mRNA COVID-19 vaccines (for which no cases were identified in preauthorization evaluation).”
Novavax officials took a somewhat unusual step of responding in public. The Gaithersburg, Md.–based company on June 3 issued a statement saying researchers had come to “expect to see natural background events of myocarditis in any sufficiently large database, and that young males are at higher risk.”
The data from the company’s placebo-controlled studies show that, overall, in its clinical development program, the rate of myocarditis was balanced between the vaccine and placebo arms (0.007% and 0.005%), Novavax said.
At the June 7 meeting, FDA panelists including Dr. Nelson, and Paul A. Offit, MD, of Children’s Hospital of Philadelphia, urged continued study to try to determine whether and how the vaccines could trigger myocarditis. Investments made now in pursuing these questions related to COVID-19 shots may pay off later, Dr. Offit said.
“We can use that knowledge to make safer vaccines for a disease that is going to be with us for decades, if not longer,” he said.
A version of this article first appeared on Medscape.com.
than the cutting-edge technology used in mRNA-based shots.
The Vaccines and Related Biological Products Advisory Committee of the Food and Drug Administration voted almost unanimously June 7 in favor of Novavax’s two-dose COVID-19 vaccine for those 18 or older – despite some concerns over rare events of myocarditis and pericarditis.
The tally was 21 “yes” votes, without any “no” votes, but one abstention from a panelist who then offered a largely positive take on this vaccine.
Panelist Bruce Gellin, MD, explained at the end of the meeting that he would have cast a conditional vote in favor of the Novavax vaccine, called NVX-CoV2373, had that been an option. Dr. Gellin, chief of global public health strategy for the Rockefeller Foundation and a vaccine expert, said he didn’t want his abstention to be considered as signaling opposition to the Novavax shot.
Instead, he said, he expects FDA officials will gather more data and evidence about the Novavax vaccine, especially in relation to certain manufacturing issues, before making its decision on the company’s application.
Earlier in the day, a top FDA vaccine reviewer, Doran Fink, MD, PhD, noted that there were important manufacturing differences between the Novavax vaccine supply used in different projects, complicating efforts to assess the company’s application for emergency use authorization (EUA).
But Dr. Fink noted that the FDA staff already had made a convincing case in its briefing document, with enough evidence for an initial conditional clearance to be found in available data.
The FDA is not bound to follow the suggestions of its advisory committees but it often does.
Using the ‘bully pulpit’
At the beginning of the meeting, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said he was seizing the “bully pulpit” in addressing the need to persuade more people in the United States to take shots against COVID-19.
About 67% of people in the United States aged 18 and older are fully vaccinated, but only about 50% of those in this group have had a first booster, according to the Centers for Disease Control and Prevention.
The two-dose mRNA vaccines from Pfizer and Moderna have been the subject of intense misinformation campaigns on social media, despite efforts by the FDA and other public health officials to convey the message about their strong benefit-risk profile. The FDA in May limited the authorized use of Johnson & Johnson’s single-dose COVID-19 shot, which is based on a different technology, because of concerns about rare and potentially life-threatening blood clots.
Novavax has been described as a more traditional vaccine – a protein subunit shot similar to one people have long received for protection against influenza, pertussis (whooping cough), diphtheria, and tetanus.
“Having a protein-based alternative may be more comfortable for some in terms of their acceptance of vaccines,” Dr. Marks said. “We do have a problem with vaccine uptake that is very serious in the United States. And anything we can do to get people more comfortable to be able to accept these potentially life-saving medical products is something that we feel we are compelled to do.”
Dr. Marks offered these remarks in answer to an FDA panelist’s question about the need to consider an EUA for yet another vaccine.
EUAs are special clearances the FDA can grant in connection with public health emergencies such as the pandemic. The FDA used EUAs for the initial December 2020 clearances of the Pfizer-BioNTech and Moderna vaccines. It has since granted normal approvals for both of these mRNA-based vaccines, based on larger bodies of evidence gathered and submitted by their developers.
During the meeting, the FDA panelists in general appeared comfortable with the idea of granting another EUA for a vaccine. There was agreement that the shot appeared to work in key tests, although these were done before the rise of the Omicron variant.
In a key test, known as study 301, the Novavax vaccine was judged to be 90.4% effective. In the study, 17 of the 17,272 people who got the Novavax vaccine developed COVID-19, compared with 79 of the 8,385 in the placebo group.
Panelists expressed disappointment with the lack of information about how the shot would work now.
“We’re looking at the efficacy against strains that don’t exist any longer,” said panelist Eric J. Rubin, MD, PhD, a Harvard professor and editor of the New England Journal of Medicine.
Still, Dr. Rubin added that he agreed with the argument the FDA’s Dr. Marks had made earlier for an EUA for the Novavax vaccine.
“If there really is a population of patients who are willing to take this and not willing to take the existing vaccines, I think it’s pretty compelling,” Dr. Rubin said.
Other FDA panelists were skeptical of this argument. Jay Portnoy, MD, who was listed on the FDA roster as the panel’s consumer representative, said he has close friends who are vaccine skeptics.
“Their hesitancy is more ideological than technological,” said Dr. Portnoy of Children’s Mercy Hospital, Kansas City, Mo. “So I really doubt that this vaccine is going to crack that nut, but perhaps some individuals would get this when they wouldn’t get the other ones.”
Myocarditis, pericarditis
The Novavax vaccine is already authorized in other countries, including Canada. Novavax in February announced that it had begun shipping its first doses of the vaccine to European Union member states. The vaccine can be moved through existing vaccine supply and cold chain channels instead of requiring complex new delivery procedures.
That could prove an advantage in time, said FDA panelist Michael Nelson, MD, PhD, of the University of Virginia, Charlottesville.
“Who knows even with supply chain challenges down the road, it will be nice to have options going forward,” Dr. Nelson said.
As with other COVID-19 vaccines, clinicians and researchers are still working to understand the potential risk for inflammation of heart muscle and nearby tissue with vaccination. Most patients with myocarditis or pericarditis who sought medical care for these conditions responded well to medicine and rest and felt better quickly, the CDC says on its website. They usually return to their normal daily activities after their symptoms improve.
At the June 7 meeting, Dr. Nelson said there may be cases of myocarditis that go undetected.
“Our signals are those who get admitted to the emergency room and the hospital,” he said. “I’m quite convinced that there are others who are experiencing cardiac events of lesser severity that are worthy of being studied, both from mechanistic and outcomes standpoints. So we have a lot of work to do.”
In looking at results for an initial pool of 40,000 people who received the Novavax vaccine, there were five reported cases of myocarditis or pericarditis developing within 20 days of people getting the shot, the FDA staff said in its presentation on safety.
In a briefing document released ahead of the advisory committee meeting, the FDA staff flagged this number of cases in a relatively small database as a concern, noting it “could be higher than reported during postauthorization use of mRNA COVID-19 vaccines (for which no cases were identified in preauthorization evaluation).”
Novavax officials took a somewhat unusual step of responding in public. The Gaithersburg, Md.–based company on June 3 issued a statement saying researchers had come to “expect to see natural background events of myocarditis in any sufficiently large database, and that young males are at higher risk.”
The data from the company’s placebo-controlled studies show that, overall, in its clinical development program, the rate of myocarditis was balanced between the vaccine and placebo arms (0.007% and 0.005%), Novavax said.
At the June 7 meeting, FDA panelists including Dr. Nelson, and Paul A. Offit, MD, of Children’s Hospital of Philadelphia, urged continued study to try to determine whether and how the vaccines could trigger myocarditis. Investments made now in pursuing these questions related to COVID-19 shots may pay off later, Dr. Offit said.
“We can use that knowledge to make safer vaccines for a disease that is going to be with us for decades, if not longer,” he said.
A version of this article first appeared on Medscape.com.
than the cutting-edge technology used in mRNA-based shots.
The Vaccines and Related Biological Products Advisory Committee of the Food and Drug Administration voted almost unanimously June 7 in favor of Novavax’s two-dose COVID-19 vaccine for those 18 or older – despite some concerns over rare events of myocarditis and pericarditis.
The tally was 21 “yes” votes, without any “no” votes, but one abstention from a panelist who then offered a largely positive take on this vaccine.
Panelist Bruce Gellin, MD, explained at the end of the meeting that he would have cast a conditional vote in favor of the Novavax vaccine, called NVX-CoV2373, had that been an option. Dr. Gellin, chief of global public health strategy for the Rockefeller Foundation and a vaccine expert, said he didn’t want his abstention to be considered as signaling opposition to the Novavax shot.
Instead, he said, he expects FDA officials will gather more data and evidence about the Novavax vaccine, especially in relation to certain manufacturing issues, before making its decision on the company’s application.
Earlier in the day, a top FDA vaccine reviewer, Doran Fink, MD, PhD, noted that there were important manufacturing differences between the Novavax vaccine supply used in different projects, complicating efforts to assess the company’s application for emergency use authorization (EUA).
But Dr. Fink noted that the FDA staff already had made a convincing case in its briefing document, with enough evidence for an initial conditional clearance to be found in available data.
The FDA is not bound to follow the suggestions of its advisory committees but it often does.
Using the ‘bully pulpit’
At the beginning of the meeting, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said he was seizing the “bully pulpit” in addressing the need to persuade more people in the United States to take shots against COVID-19.
About 67% of people in the United States aged 18 and older are fully vaccinated, but only about 50% of those in this group have had a first booster, according to the Centers for Disease Control and Prevention.
The two-dose mRNA vaccines from Pfizer and Moderna have been the subject of intense misinformation campaigns on social media, despite efforts by the FDA and other public health officials to convey the message about their strong benefit-risk profile. The FDA in May limited the authorized use of Johnson & Johnson’s single-dose COVID-19 shot, which is based on a different technology, because of concerns about rare and potentially life-threatening blood clots.
Novavax has been described as a more traditional vaccine – a protein subunit shot similar to one people have long received for protection against influenza, pertussis (whooping cough), diphtheria, and tetanus.
“Having a protein-based alternative may be more comfortable for some in terms of their acceptance of vaccines,” Dr. Marks said. “We do have a problem with vaccine uptake that is very serious in the United States. And anything we can do to get people more comfortable to be able to accept these potentially life-saving medical products is something that we feel we are compelled to do.”
Dr. Marks offered these remarks in answer to an FDA panelist’s question about the need to consider an EUA for yet another vaccine.
EUAs are special clearances the FDA can grant in connection with public health emergencies such as the pandemic. The FDA used EUAs for the initial December 2020 clearances of the Pfizer-BioNTech and Moderna vaccines. It has since granted normal approvals for both of these mRNA-based vaccines, based on larger bodies of evidence gathered and submitted by their developers.
During the meeting, the FDA panelists in general appeared comfortable with the idea of granting another EUA for a vaccine. There was agreement that the shot appeared to work in key tests, although these were done before the rise of the Omicron variant.
In a key test, known as study 301, the Novavax vaccine was judged to be 90.4% effective. In the study, 17 of the 17,272 people who got the Novavax vaccine developed COVID-19, compared with 79 of the 8,385 in the placebo group.
Panelists expressed disappointment with the lack of information about how the shot would work now.
“We’re looking at the efficacy against strains that don’t exist any longer,” said panelist Eric J. Rubin, MD, PhD, a Harvard professor and editor of the New England Journal of Medicine.
Still, Dr. Rubin added that he agreed with the argument the FDA’s Dr. Marks had made earlier for an EUA for the Novavax vaccine.
“If there really is a population of patients who are willing to take this and not willing to take the existing vaccines, I think it’s pretty compelling,” Dr. Rubin said.
Other FDA panelists were skeptical of this argument. Jay Portnoy, MD, who was listed on the FDA roster as the panel’s consumer representative, said he has close friends who are vaccine skeptics.
“Their hesitancy is more ideological than technological,” said Dr. Portnoy of Children’s Mercy Hospital, Kansas City, Mo. “So I really doubt that this vaccine is going to crack that nut, but perhaps some individuals would get this when they wouldn’t get the other ones.”
Myocarditis, pericarditis
The Novavax vaccine is already authorized in other countries, including Canada. Novavax in February announced that it had begun shipping its first doses of the vaccine to European Union member states. The vaccine can be moved through existing vaccine supply and cold chain channels instead of requiring complex new delivery procedures.
That could prove an advantage in time, said FDA panelist Michael Nelson, MD, PhD, of the University of Virginia, Charlottesville.
“Who knows even with supply chain challenges down the road, it will be nice to have options going forward,” Dr. Nelson said.
As with other COVID-19 vaccines, clinicians and researchers are still working to understand the potential risk for inflammation of heart muscle and nearby tissue with vaccination. Most patients with myocarditis or pericarditis who sought medical care for these conditions responded well to medicine and rest and felt better quickly, the CDC says on its website. They usually return to their normal daily activities after their symptoms improve.
At the June 7 meeting, Dr. Nelson said there may be cases of myocarditis that go undetected.
“Our signals are those who get admitted to the emergency room and the hospital,” he said. “I’m quite convinced that there are others who are experiencing cardiac events of lesser severity that are worthy of being studied, both from mechanistic and outcomes standpoints. So we have a lot of work to do.”
In looking at results for an initial pool of 40,000 people who received the Novavax vaccine, there were five reported cases of myocarditis or pericarditis developing within 20 days of people getting the shot, the FDA staff said in its presentation on safety.
In a briefing document released ahead of the advisory committee meeting, the FDA staff flagged this number of cases in a relatively small database as a concern, noting it “could be higher than reported during postauthorization use of mRNA COVID-19 vaccines (for which no cases were identified in preauthorization evaluation).”
Novavax officials took a somewhat unusual step of responding in public. The Gaithersburg, Md.–based company on June 3 issued a statement saying researchers had come to “expect to see natural background events of myocarditis in any sufficiently large database, and that young males are at higher risk.”
The data from the company’s placebo-controlled studies show that, overall, in its clinical development program, the rate of myocarditis was balanced between the vaccine and placebo arms (0.007% and 0.005%), Novavax said.
At the June 7 meeting, FDA panelists including Dr. Nelson, and Paul A. Offit, MD, of Children’s Hospital of Philadelphia, urged continued study to try to determine whether and how the vaccines could trigger myocarditis. Investments made now in pursuing these questions related to COVID-19 shots may pay off later, Dr. Offit said.
“We can use that knowledge to make safer vaccines for a disease that is going to be with us for decades, if not longer,” he said.
A version of this article first appeared on Medscape.com.
‘Sit less, move more’ to reduce stroke risk
in a population-based study of middle aged and older adults.
The study also found relatively short periods of moderate to vigorous exercise were associated with reduced stroke risk.
“Our results suggest there are a number of ways to reduce stroke risk simply by moving about,” said lead author Steven P. Hooker, PhD, San Diego State University. “This could be with short periods of moderate to vigorous activity each day, longer periods of light activity, or just sedentary for shorter periods of time. All these things can make a difference.”
Dr. Hooker explained that, while it has been found previously that moderate to vigorous exercise reduces stroke risk, this study gives more information on light-intensity activities and sedentary behavior and the risk of stroke.
“Our results suggest that you don’t have to be a chronic exerciser to reduce stroke risk. Replacing sedentary time with light-intensity activity will be beneficial. Just go for a short walk, get up from your desk and move around the house at regular intervals. That can help to reduce stroke risk,” Dr. Hooker said.
“Our message is basically to sit less and move more,” he added.
The study was published online in JAMA Network Open.
The study involved 7,607 U.S. individuals without a history of stroke, with oversampling from the southeastern “Stroke Belt,” who were participating in the REGARDS cohort study.
The participants wore an accelerometer to measure physical activity and sedentary behavior for 7 consecutive days. The mean age of the individuals was 63 years; 54% were female, 32% were Black.
Over a mean follow-up of 7.4 years, 286 incident stroke cases occurred.
Results showed that increased levels of physical activity were associated with reduced risk of stroke.
For moderate to vigorous activity, compared with participants in the lowest tertile, those in the highest tertile of total daily time in moderate to vigorous activity had a 43% lower risk of stroke.
In the current study, the amount of moderate to vigorous activity associated with a significant reduction in stroke risk was approximately 25 minutes per day (3 hours per week).
Dr. Hooker noted that moderate to vigorous activity included things such as brisk walking, jogging, bike riding, swimming, or playing tennis or soccer. “Doing such activities for just 25 minutes per day reduced risk of stroke by 43%. This is very doable. Just commuting to work by bicycle would cover you here,” he said.
In terms of light-intensity activity, individuals who did 4-5 hours of light activities each day had a 26% reduced risk for first stroke, compared with those doing less than 3 hours of such light activities.
Dr. Hooker explained that examples of light activity included household chores, such as vacuuming, washing dishes, or going for a gentle stroll. “These activities do not require heaving breathing, increased heart rate or breaking into a sweat. They are activities of daily living and relatively easy to engage in.”
But he pointed out that the 4-5 hours of light activity every day linked to a reduction in stroke risk may be more difficult to achieve than the 25 minutes of moderate to vigorous activity, saying: “You have to have some intentionality here.”
Long bouts of sedentary time are harmful
The study also showed that sedentary time was associated with a higher risk for stroke.
The authors noted that time spent in sedentary behavior is of interest because most adults spend most of their awake time being physically inactive.
They report that participants in the highest tertile of sedentary time (more than 13 hours/day) exhibited a 44% increase in risk of stroke, compared with those in the lowest tertile (less than 11 hours/day), and the association remained significant when adjusted for several covariates, including moderate to vigorous activity.
“Even when controlling for the amount of other physical activity, sedentary behavior is still highly associated with risk of stroke. So even if you are active, long bouts of sedentary behavior are harmful,” Dr. Hooker commented.
They also found that longer bouts of sedentary time (more than 17 minutes at a time) were associated with a 54% higher risk of stroke than shorter bouts (less than 8 minutes).
“This suggests that breaking up periods of sedentary behavior into shorter bouts would be beneficial,” Dr. Hooker said.
“If you are going to spend the evening on the couch watching television, try to stand up and walk around every few minutes. Same for if you are sitting at a computer all day – try having a standing workstation, or at least take regular breaks to walk around,” he added.
This research was supported by grants from the National Institute of Neurological Disorders and Stroke and the National Institute on Aging. Additional funding was provided by an unrestricted grant from the Coca-Cola Company. The authors report no disclosures.
A version of this article first appeared on Medscape.com.
in a population-based study of middle aged and older adults.
The study also found relatively short periods of moderate to vigorous exercise were associated with reduced stroke risk.
“Our results suggest there are a number of ways to reduce stroke risk simply by moving about,” said lead author Steven P. Hooker, PhD, San Diego State University. “This could be with short periods of moderate to vigorous activity each day, longer periods of light activity, or just sedentary for shorter periods of time. All these things can make a difference.”
Dr. Hooker explained that, while it has been found previously that moderate to vigorous exercise reduces stroke risk, this study gives more information on light-intensity activities and sedentary behavior and the risk of stroke.
“Our results suggest that you don’t have to be a chronic exerciser to reduce stroke risk. Replacing sedentary time with light-intensity activity will be beneficial. Just go for a short walk, get up from your desk and move around the house at regular intervals. That can help to reduce stroke risk,” Dr. Hooker said.
“Our message is basically to sit less and move more,” he added.
The study was published online in JAMA Network Open.
The study involved 7,607 U.S. individuals without a history of stroke, with oversampling from the southeastern “Stroke Belt,” who were participating in the REGARDS cohort study.
The participants wore an accelerometer to measure physical activity and sedentary behavior for 7 consecutive days. The mean age of the individuals was 63 years; 54% were female, 32% were Black.
Over a mean follow-up of 7.4 years, 286 incident stroke cases occurred.
Results showed that increased levels of physical activity were associated with reduced risk of stroke.
For moderate to vigorous activity, compared with participants in the lowest tertile, those in the highest tertile of total daily time in moderate to vigorous activity had a 43% lower risk of stroke.
In the current study, the amount of moderate to vigorous activity associated with a significant reduction in stroke risk was approximately 25 minutes per day (3 hours per week).
Dr. Hooker noted that moderate to vigorous activity included things such as brisk walking, jogging, bike riding, swimming, or playing tennis or soccer. “Doing such activities for just 25 minutes per day reduced risk of stroke by 43%. This is very doable. Just commuting to work by bicycle would cover you here,” he said.
In terms of light-intensity activity, individuals who did 4-5 hours of light activities each day had a 26% reduced risk for first stroke, compared with those doing less than 3 hours of such light activities.
Dr. Hooker explained that examples of light activity included household chores, such as vacuuming, washing dishes, or going for a gentle stroll. “These activities do not require heaving breathing, increased heart rate or breaking into a sweat. They are activities of daily living and relatively easy to engage in.”
But he pointed out that the 4-5 hours of light activity every day linked to a reduction in stroke risk may be more difficult to achieve than the 25 minutes of moderate to vigorous activity, saying: “You have to have some intentionality here.”
Long bouts of sedentary time are harmful
The study also showed that sedentary time was associated with a higher risk for stroke.
The authors noted that time spent in sedentary behavior is of interest because most adults spend most of their awake time being physically inactive.
They report that participants in the highest tertile of sedentary time (more than 13 hours/day) exhibited a 44% increase in risk of stroke, compared with those in the lowest tertile (less than 11 hours/day), and the association remained significant when adjusted for several covariates, including moderate to vigorous activity.
“Even when controlling for the amount of other physical activity, sedentary behavior is still highly associated with risk of stroke. So even if you are active, long bouts of sedentary behavior are harmful,” Dr. Hooker commented.
They also found that longer bouts of sedentary time (more than 17 minutes at a time) were associated with a 54% higher risk of stroke than shorter bouts (less than 8 minutes).
“This suggests that breaking up periods of sedentary behavior into shorter bouts would be beneficial,” Dr. Hooker said.
“If you are going to spend the evening on the couch watching television, try to stand up and walk around every few minutes. Same for if you are sitting at a computer all day – try having a standing workstation, or at least take regular breaks to walk around,” he added.
This research was supported by grants from the National Institute of Neurological Disorders and Stroke and the National Institute on Aging. Additional funding was provided by an unrestricted grant from the Coca-Cola Company. The authors report no disclosures.
A version of this article first appeared on Medscape.com.
in a population-based study of middle aged and older adults.
The study also found relatively short periods of moderate to vigorous exercise were associated with reduced stroke risk.
“Our results suggest there are a number of ways to reduce stroke risk simply by moving about,” said lead author Steven P. Hooker, PhD, San Diego State University. “This could be with short periods of moderate to vigorous activity each day, longer periods of light activity, or just sedentary for shorter periods of time. All these things can make a difference.”
Dr. Hooker explained that, while it has been found previously that moderate to vigorous exercise reduces stroke risk, this study gives more information on light-intensity activities and sedentary behavior and the risk of stroke.
“Our results suggest that you don’t have to be a chronic exerciser to reduce stroke risk. Replacing sedentary time with light-intensity activity will be beneficial. Just go for a short walk, get up from your desk and move around the house at regular intervals. That can help to reduce stroke risk,” Dr. Hooker said.
“Our message is basically to sit less and move more,” he added.
The study was published online in JAMA Network Open.
The study involved 7,607 U.S. individuals without a history of stroke, with oversampling from the southeastern “Stroke Belt,” who were participating in the REGARDS cohort study.
The participants wore an accelerometer to measure physical activity and sedentary behavior for 7 consecutive days. The mean age of the individuals was 63 years; 54% were female, 32% were Black.
Over a mean follow-up of 7.4 years, 286 incident stroke cases occurred.
Results showed that increased levels of physical activity were associated with reduced risk of stroke.
For moderate to vigorous activity, compared with participants in the lowest tertile, those in the highest tertile of total daily time in moderate to vigorous activity had a 43% lower risk of stroke.
In the current study, the amount of moderate to vigorous activity associated with a significant reduction in stroke risk was approximately 25 minutes per day (3 hours per week).
Dr. Hooker noted that moderate to vigorous activity included things such as brisk walking, jogging, bike riding, swimming, or playing tennis or soccer. “Doing such activities for just 25 minutes per day reduced risk of stroke by 43%. This is very doable. Just commuting to work by bicycle would cover you here,” he said.
In terms of light-intensity activity, individuals who did 4-5 hours of light activities each day had a 26% reduced risk for first stroke, compared with those doing less than 3 hours of such light activities.
Dr. Hooker explained that examples of light activity included household chores, such as vacuuming, washing dishes, or going for a gentle stroll. “These activities do not require heaving breathing, increased heart rate or breaking into a sweat. They are activities of daily living and relatively easy to engage in.”
But he pointed out that the 4-5 hours of light activity every day linked to a reduction in stroke risk may be more difficult to achieve than the 25 minutes of moderate to vigorous activity, saying: “You have to have some intentionality here.”
Long bouts of sedentary time are harmful
The study also showed that sedentary time was associated with a higher risk for stroke.
The authors noted that time spent in sedentary behavior is of interest because most adults spend most of their awake time being physically inactive.
They report that participants in the highest tertile of sedentary time (more than 13 hours/day) exhibited a 44% increase in risk of stroke, compared with those in the lowest tertile (less than 11 hours/day), and the association remained significant when adjusted for several covariates, including moderate to vigorous activity.
“Even when controlling for the amount of other physical activity, sedentary behavior is still highly associated with risk of stroke. So even if you are active, long bouts of sedentary behavior are harmful,” Dr. Hooker commented.
They also found that longer bouts of sedentary time (more than 17 minutes at a time) were associated with a 54% higher risk of stroke than shorter bouts (less than 8 minutes).
“This suggests that breaking up periods of sedentary behavior into shorter bouts would be beneficial,” Dr. Hooker said.
“If you are going to spend the evening on the couch watching television, try to stand up and walk around every few minutes. Same for if you are sitting at a computer all day – try having a standing workstation, or at least take regular breaks to walk around,” he added.
This research was supported by grants from the National Institute of Neurological Disorders and Stroke and the National Institute on Aging. Additional funding was provided by an unrestricted grant from the Coca-Cola Company. The authors report no disclosures.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Antipsychotic tied to dose-related weight gain, higher cholesterol
new research suggests.
Investigators analyzed 1-year data for more than 400 patients who were taking risperidone and/or its metabolite paliperidone (Invega). Results showed increments of 1 mg of risperidone-equivalent doses were associated with an increase of 0.25% of weight within a year of follow-up.
“Although our findings report a positive and statistically significant dose-dependence of weight gain and cholesterol, both total and LDL [cholesterol], the size of the predicted changes of metabolic effects is clinically nonrelevant,” lead author Marianna Piras, PharmD, Centre for Psychiatric Neuroscience, Lausanne (Switzerland) University Hospital, said in an interview.
“Therefore, dose lowering would not have a beneficial effect on attenuating weight gain or cholesterol increases and could lead to psychiatric decompensation,” said Ms. Piras, who is also a PhD candidate in the unit of pharmacogenetics and clinical psychopharmacology at the University of Lausanne.
However, she added that because dose increments could increase risk for significant weight gain in the first month of treatment – the dose can be increased typically in a range of 1-10 grams – and strong dose increments could contribute to metabolic worsening over time, “risperidone minimum effective doses should be preferred.”
The findings were published online in the Journal of Clinical Psychiatry.
‘Serious public health issue’
Compared with the general population, patients with mental illness present with a greater prevalence of metabolic disorders. In addition, several psychotropic medications, including antipsychotics, can induce metabolic alterations such as weight gain, the investigators noted.
Antipsychotic-induced metabolic adverse effects “constitute a serious public health issue” because they are risk factors for cardiovascular diseases such as obesity and/or dyslipidemia, “which have been associated with a 10-year reduced life expectancy in the psychiatric population,” Ms. Piras said.
“The dose-dependence of metabolic adverse effects is a debated subject that needs to be assessed for each psychotropic drug known to induce weight gain,” she added.
Several previous studies have examined whether there is a dose-related effect of antipsychotics on metabolic parameters, “with some results suggesting that [weight gain] seems to develop even when low off-label doses are prescribed,” Ms. Piras noted.
She and her colleagues had already studied dose-related metabolic effects of quetiapine (Seroquel) and olanzapine (Zyprexa).
Risperidone is an antipsychotic with a “medium to high metabolic risk profile,” the researchers note, and few studies have examined the impact of risperidone on metabolic parameters other than weight gain.
For the current analysis, they analyzed data from a longitudinal study that included 438 patients (mean age, 40.7 years; 50.7% men) who started treatment with risperidone and/or paliperidone between 2007 and 2018.
The participants had diagnoses of schizophrenia, schizoaffective disorder, bipolar disorder, depression, “other,” or “unknown.”
Clinical follow-up periods were up to a year, but were no shorter than 3 weeks. The investigators also assessed the data at different time intervals at 1, 3, 6, and 12 months “to appreciate the evolution of the metabolic parameters.”
In addition, they collected demographic and clinical information, such as comorbidities, and measured patients’ weight, height, waist circumference, blood pressure, plasma glucose, and lipids at baseline and at 1, 3, and 12 months and then annually. Weight, waist circumference, and BP were also assessed at 2 and 6 months.
Doses of paliperidone were converted into risperidone-equivalent doses.
Significant weight gain over time
The mean duration of follow-up for the participants, of whom 374 were being treated with risperidone and 64 with paliperidone, was 153 days. Close to half (48.2%) were taking other psychotropic medications known to be associated with some degree of metabolic risk.
Patients were divided into two cohorts based on their daily dose intake (DDI): less than 3 mg/day (n = 201) and at least 3 mg/day (n = 237).
In the overall cohort, a “significant effect of time on weight change was found for each time point,” the investigators reported.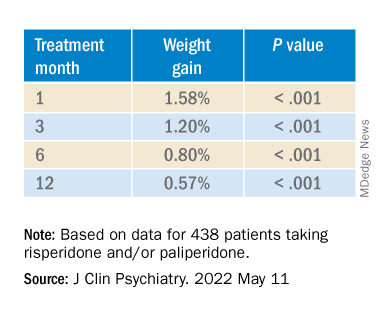
When the researchers looked at the changes according to DDI, they found that each 1-mg dose increase was associated with incremental weight gain at each time point.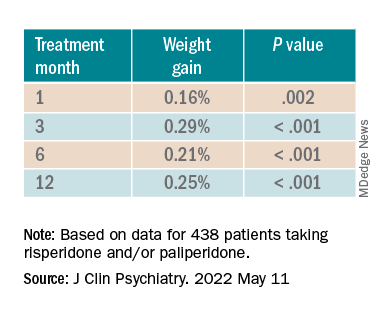
Patients who had 5% or greater weight gain in the first month continued to gain weight more than patients who did not reach that threshold, leading the researchers to call that early threshold a “strong predictor of important weight gain in the long term.” There was a weight gain of 6.68% at 3 months, of 7.36% at 6 months, and of 7.7% at 12 months.
After the patients were stratified by age, there were differences in the effect of DDI on various age groups at different time points.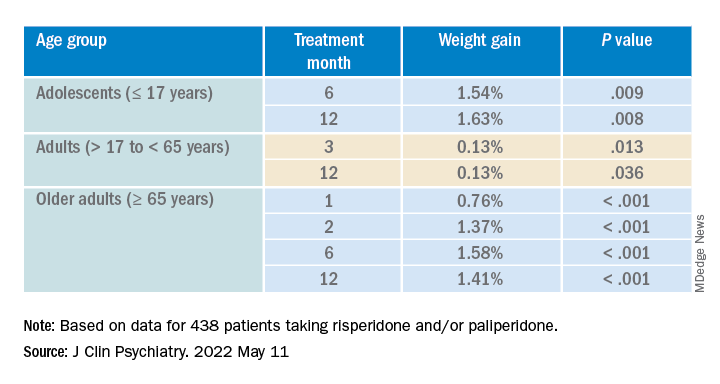
Dose was shown to have a significant effect on weight gain for women at all four time points (P ≥ .001), but for men only at 3 months (P = .003).
For each additional 1-mg dose, there was a 0.05 mmol/L (1.93 mg/dL) increase in total cholesterol (P = .018) after 1 year and a 0.04 mmol/L (1.54 mg/dL) increase in LDL cholesterol (P = .011).
There were no significant effects of time or DDI on triglycerides, HDL cholesterol, glucose levels, and systolic BP, and there was a negative effect of DDI on diastolic BP (P = .001).
The findings “provide evidence for a small dose effect of risperidone” on weight gain and total and LDL cholesterol levels, the investigators note.
Ms. Piras added that because each antipsychotic differs in its metabolic risk profile, “further analyses on other antipsychotics are ongoing in our laboratory, so far confirming our findings.”
Small increases, big changes
Commenting on the study, Erika Nurmi, MD, PhD, associate professor in the department of psychiatry and biobehavioral sciences at the Semel Institute for Neuroscience, University of California, Los Angeles, said the study is “unique in the field.”
It “leverages real-world data from a large patient registry to ask a long-unanswered question: Are weight and metabolic adverse effects proportional to dose? Big data approaches like these are very powerful, given the large number of participants that can be included,” said Dr. Nurmi, who was not involved with the research.
However, she cautioned, the “biggest drawback [is that] these data are by nature much more complex and prone to confounding effects.”
In this case, a “critical confounder” for the study was that the majority of individuals taking higher risperidone doses were also taking other drugs known to cause weight gain, whereas the majority of those on lower risperidone doses were not. “This difference may explain the dose relationship observed,” she said.
Because real-world, big data are “valuable but also messy, conclusions drawn from them must be interpreted with caution,” Dr. Nurmi said.
She added that it is generally wise to use the lowest effective dose possible.
“Clinicians should appreciate that even small doses of antipsychotics can cause big changes in weight. Risks and benefits of medications must be carefully considered in clinical practice,” Dr. Nurmi said.
The research was funded in part by the Swiss National Research Foundation. Piras reports no relevant financial relationships. The other investigators’ disclosures are listed in the original article. Dr. Nurmi reported no relevant financial relationships, but she is an unpaid member of the Tourette Association of America’s medical advisory board and of the Myriad Genetics scientific advisory board.
A version of this article first appeared on Medscape.com.
new research suggests.
Investigators analyzed 1-year data for more than 400 patients who were taking risperidone and/or its metabolite paliperidone (Invega). Results showed increments of 1 mg of risperidone-equivalent doses were associated with an increase of 0.25% of weight within a year of follow-up.
“Although our findings report a positive and statistically significant dose-dependence of weight gain and cholesterol, both total and LDL [cholesterol], the size of the predicted changes of metabolic effects is clinically nonrelevant,” lead author Marianna Piras, PharmD, Centre for Psychiatric Neuroscience, Lausanne (Switzerland) University Hospital, said in an interview.
“Therefore, dose lowering would not have a beneficial effect on attenuating weight gain or cholesterol increases and could lead to psychiatric decompensation,” said Ms. Piras, who is also a PhD candidate in the unit of pharmacogenetics and clinical psychopharmacology at the University of Lausanne.
However, she added that because dose increments could increase risk for significant weight gain in the first month of treatment – the dose can be increased typically in a range of 1-10 grams – and strong dose increments could contribute to metabolic worsening over time, “risperidone minimum effective doses should be preferred.”
The findings were published online in the Journal of Clinical Psychiatry.
‘Serious public health issue’
Compared with the general population, patients with mental illness present with a greater prevalence of metabolic disorders. In addition, several psychotropic medications, including antipsychotics, can induce metabolic alterations such as weight gain, the investigators noted.
Antipsychotic-induced metabolic adverse effects “constitute a serious public health issue” because they are risk factors for cardiovascular diseases such as obesity and/or dyslipidemia, “which have been associated with a 10-year reduced life expectancy in the psychiatric population,” Ms. Piras said.
“The dose-dependence of metabolic adverse effects is a debated subject that needs to be assessed for each psychotropic drug known to induce weight gain,” she added.
Several previous studies have examined whether there is a dose-related effect of antipsychotics on metabolic parameters, “with some results suggesting that [weight gain] seems to develop even when low off-label doses are prescribed,” Ms. Piras noted.
She and her colleagues had already studied dose-related metabolic effects of quetiapine (Seroquel) and olanzapine (Zyprexa).
Risperidone is an antipsychotic with a “medium to high metabolic risk profile,” the researchers note, and few studies have examined the impact of risperidone on metabolic parameters other than weight gain.
For the current analysis, they analyzed data from a longitudinal study that included 438 patients (mean age, 40.7 years; 50.7% men) who started treatment with risperidone and/or paliperidone between 2007 and 2018.
The participants had diagnoses of schizophrenia, schizoaffective disorder, bipolar disorder, depression, “other,” or “unknown.”
Clinical follow-up periods were up to a year, but were no shorter than 3 weeks. The investigators also assessed the data at different time intervals at 1, 3, 6, and 12 months “to appreciate the evolution of the metabolic parameters.”
In addition, they collected demographic and clinical information, such as comorbidities, and measured patients’ weight, height, waist circumference, blood pressure, plasma glucose, and lipids at baseline and at 1, 3, and 12 months and then annually. Weight, waist circumference, and BP were also assessed at 2 and 6 months.
Doses of paliperidone were converted into risperidone-equivalent doses.
Significant weight gain over time
The mean duration of follow-up for the participants, of whom 374 were being treated with risperidone and 64 with paliperidone, was 153 days. Close to half (48.2%) were taking other psychotropic medications known to be associated with some degree of metabolic risk.
Patients were divided into two cohorts based on their daily dose intake (DDI): less than 3 mg/day (n = 201) and at least 3 mg/day (n = 237).
In the overall cohort, a “significant effect of time on weight change was found for each time point,” the investigators reported.
When the researchers looked at the changes according to DDI, they found that each 1-mg dose increase was associated with incremental weight gain at each time point.
Patients who had 5% or greater weight gain in the first month continued to gain weight more than patients who did not reach that threshold, leading the researchers to call that early threshold a “strong predictor of important weight gain in the long term.” There was a weight gain of 6.68% at 3 months, of 7.36% at 6 months, and of 7.7% at 12 months.
After the patients were stratified by age, there were differences in the effect of DDI on various age groups at different time points.
Dose was shown to have a significant effect on weight gain for women at all four time points (P ≥ .001), but for men only at 3 months (P = .003).
For each additional 1-mg dose, there was a 0.05 mmol/L (1.93 mg/dL) increase in total cholesterol (P = .018) after 1 year and a 0.04 mmol/L (1.54 mg/dL) increase in LDL cholesterol (P = .011).
There were no significant effects of time or DDI on triglycerides, HDL cholesterol, glucose levels, and systolic BP, and there was a negative effect of DDI on diastolic BP (P = .001).
The findings “provide evidence for a small dose effect of risperidone” on weight gain and total and LDL cholesterol levels, the investigators note.
Ms. Piras added that because each antipsychotic differs in its metabolic risk profile, “further analyses on other antipsychotics are ongoing in our laboratory, so far confirming our findings.”
Small increases, big changes
Commenting on the study, Erika Nurmi, MD, PhD, associate professor in the department of psychiatry and biobehavioral sciences at the Semel Institute for Neuroscience, University of California, Los Angeles, said the study is “unique in the field.”
It “leverages real-world data from a large patient registry to ask a long-unanswered question: Are weight and metabolic adverse effects proportional to dose? Big data approaches like these are very powerful, given the large number of participants that can be included,” said Dr. Nurmi, who was not involved with the research.
However, she cautioned, the “biggest drawback [is that] these data are by nature much more complex and prone to confounding effects.”
In this case, a “critical confounder” for the study was that the majority of individuals taking higher risperidone doses were also taking other drugs known to cause weight gain, whereas the majority of those on lower risperidone doses were not. “This difference may explain the dose relationship observed,” she said.
Because real-world, big data are “valuable but also messy, conclusions drawn from them must be interpreted with caution,” Dr. Nurmi said.
She added that it is generally wise to use the lowest effective dose possible.
“Clinicians should appreciate that even small doses of antipsychotics can cause big changes in weight. Risks and benefits of medications must be carefully considered in clinical practice,” Dr. Nurmi said.
The research was funded in part by the Swiss National Research Foundation. Piras reports no relevant financial relationships. The other investigators’ disclosures are listed in the original article. Dr. Nurmi reported no relevant financial relationships, but she is an unpaid member of the Tourette Association of America’s medical advisory board and of the Myriad Genetics scientific advisory board.
A version of this article first appeared on Medscape.com.
new research suggests.
Investigators analyzed 1-year data for more than 400 patients who were taking risperidone and/or its metabolite paliperidone (Invega). Results showed increments of 1 mg of risperidone-equivalent doses were associated with an increase of 0.25% of weight within a year of follow-up.
“Although our findings report a positive and statistically significant dose-dependence of weight gain and cholesterol, both total and LDL [cholesterol], the size of the predicted changes of metabolic effects is clinically nonrelevant,” lead author Marianna Piras, PharmD, Centre for Psychiatric Neuroscience, Lausanne (Switzerland) University Hospital, said in an interview.
“Therefore, dose lowering would not have a beneficial effect on attenuating weight gain or cholesterol increases and could lead to psychiatric decompensation,” said Ms. Piras, who is also a PhD candidate in the unit of pharmacogenetics and clinical psychopharmacology at the University of Lausanne.
However, she added that because dose increments could increase risk for significant weight gain in the first month of treatment – the dose can be increased typically in a range of 1-10 grams – and strong dose increments could contribute to metabolic worsening over time, “risperidone minimum effective doses should be preferred.”
The findings were published online in the Journal of Clinical Psychiatry.
‘Serious public health issue’
Compared with the general population, patients with mental illness present with a greater prevalence of metabolic disorders. In addition, several psychotropic medications, including antipsychotics, can induce metabolic alterations such as weight gain, the investigators noted.
Antipsychotic-induced metabolic adverse effects “constitute a serious public health issue” because they are risk factors for cardiovascular diseases such as obesity and/or dyslipidemia, “which have been associated with a 10-year reduced life expectancy in the psychiatric population,” Ms. Piras said.
“The dose-dependence of metabolic adverse effects is a debated subject that needs to be assessed for each psychotropic drug known to induce weight gain,” she added.
Several previous studies have examined whether there is a dose-related effect of antipsychotics on metabolic parameters, “with some results suggesting that [weight gain] seems to develop even when low off-label doses are prescribed,” Ms. Piras noted.
She and her colleagues had already studied dose-related metabolic effects of quetiapine (Seroquel) and olanzapine (Zyprexa).
Risperidone is an antipsychotic with a “medium to high metabolic risk profile,” the researchers note, and few studies have examined the impact of risperidone on metabolic parameters other than weight gain.
For the current analysis, they analyzed data from a longitudinal study that included 438 patients (mean age, 40.7 years; 50.7% men) who started treatment with risperidone and/or paliperidone between 2007 and 2018.
The participants had diagnoses of schizophrenia, schizoaffective disorder, bipolar disorder, depression, “other,” or “unknown.”
Clinical follow-up periods were up to a year, but were no shorter than 3 weeks. The investigators also assessed the data at different time intervals at 1, 3, 6, and 12 months “to appreciate the evolution of the metabolic parameters.”
In addition, they collected demographic and clinical information, such as comorbidities, and measured patients’ weight, height, waist circumference, blood pressure, plasma glucose, and lipids at baseline and at 1, 3, and 12 months and then annually. Weight, waist circumference, and BP were also assessed at 2 and 6 months.
Doses of paliperidone were converted into risperidone-equivalent doses.
Significant weight gain over time
The mean duration of follow-up for the participants, of whom 374 were being treated with risperidone and 64 with paliperidone, was 153 days. Close to half (48.2%) were taking other psychotropic medications known to be associated with some degree of metabolic risk.
Patients were divided into two cohorts based on their daily dose intake (DDI): less than 3 mg/day (n = 201) and at least 3 mg/day (n = 237).
In the overall cohort, a “significant effect of time on weight change was found for each time point,” the investigators reported.
When the researchers looked at the changes according to DDI, they found that each 1-mg dose increase was associated with incremental weight gain at each time point.
Patients who had 5% or greater weight gain in the first month continued to gain weight more than patients who did not reach that threshold, leading the researchers to call that early threshold a “strong predictor of important weight gain in the long term.” There was a weight gain of 6.68% at 3 months, of 7.36% at 6 months, and of 7.7% at 12 months.
After the patients were stratified by age, there were differences in the effect of DDI on various age groups at different time points.
Dose was shown to have a significant effect on weight gain for women at all four time points (P ≥ .001), but for men only at 3 months (P = .003).
For each additional 1-mg dose, there was a 0.05 mmol/L (1.93 mg/dL) increase in total cholesterol (P = .018) after 1 year and a 0.04 mmol/L (1.54 mg/dL) increase in LDL cholesterol (P = .011).
There were no significant effects of time or DDI on triglycerides, HDL cholesterol, glucose levels, and systolic BP, and there was a negative effect of DDI on diastolic BP (P = .001).
The findings “provide evidence for a small dose effect of risperidone” on weight gain and total and LDL cholesterol levels, the investigators note.
Ms. Piras added that because each antipsychotic differs in its metabolic risk profile, “further analyses on other antipsychotics are ongoing in our laboratory, so far confirming our findings.”
Small increases, big changes
Commenting on the study, Erika Nurmi, MD, PhD, associate professor in the department of psychiatry and biobehavioral sciences at the Semel Institute for Neuroscience, University of California, Los Angeles, said the study is “unique in the field.”
It “leverages real-world data from a large patient registry to ask a long-unanswered question: Are weight and metabolic adverse effects proportional to dose? Big data approaches like these are very powerful, given the large number of participants that can be included,” said Dr. Nurmi, who was not involved with the research.
However, she cautioned, the “biggest drawback [is that] these data are by nature much more complex and prone to confounding effects.”
In this case, a “critical confounder” for the study was that the majority of individuals taking higher risperidone doses were also taking other drugs known to cause weight gain, whereas the majority of those on lower risperidone doses were not. “This difference may explain the dose relationship observed,” she said.
Because real-world, big data are “valuable but also messy, conclusions drawn from them must be interpreted with caution,” Dr. Nurmi said.
She added that it is generally wise to use the lowest effective dose possible.
“Clinicians should appreciate that even small doses of antipsychotics can cause big changes in weight. Risks and benefits of medications must be carefully considered in clinical practice,” Dr. Nurmi said.
The research was funded in part by the Swiss National Research Foundation. Piras reports no relevant financial relationships. The other investigators’ disclosures are listed in the original article. Dr. Nurmi reported no relevant financial relationships, but she is an unpaid member of the Tourette Association of America’s medical advisory board and of the Myriad Genetics scientific advisory board.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
‘Remission is possible’ for patients with type 2 diabetes
A novel approach that involves sensors, artificial intelligence, and real-time individualized lifestyle guidance from an app and live coaches led to a high rate of remission of type 2 diabetes in a new study.
Specifically, among 199 patients with type 2 diabetes in India who received the app-delivered lifestyle guidance developed by Twin Health, Mountain View, Calif., mean hemoglobin A1c dropped from 9.0% to 5.7% at 6 months.
This is “huge,” Paramesh Shamanna, MD, told a press briefing at the annual scientific sessions of the American Diabetes Association. The research was presented as three posters by the group at the meeting.
Patients were a mean age of 43 and had diabetes for a mean of 3.7 years and up to 8 years.
An “unprecedented” 84% of patients had remission of diabetes at 6 months, Dr. Shamanna, medical director at Twin Health, noted.
Diabetes remission was defined according to the 2021 joint consensus statement from the ADA and other organizations as an A1c less than 6.5% without the use of diabetes medications for at least 3 months.
Importantly, patients’ time in range (percentage of time spent in target blood glucose range) increased from 53% to 81%, Dr. Shamanna pointed out. On average, patients’ waist circumference decreased by 10 cm (3.9 inches) and their weight dropped from 79 kg (approximately 174 lb) to 68 kg (150 lb).
These results are driven by “the continuous individualized and precise guidance regarding nutrition, activity, and sleep,” Dr. Shamanna said in an interview.
Remission is not reversal or cure ...
“Remission” from type 2 diabetes is not “reversal” or a “cure,” Robert A. Gabbay, MD, PhD, chief scientific and medical officer of the ADA, stressed to the press. Just like cancer, diabetes can return after remission
Therefore, it is important to follow the lifestyle guidance. Patients may still be at risk for diabetes complications after diabetes remission, so it’s also important to continue to be screened for eye disease, nerve damage, and lipid levels.
However, “remission can be made to last,” Dr. Shamanna said, by continuing to follow the lifestyle advice and getting back on track after a relapse.
“We’re in a different time right now,” Lisa Shah, MD, chief medical officer, Twin Health, noted. “This is very different from management of blood glucose to a certain number.”
This study shows that “remission [from type 2 diabetes] is possible. How you achieve it can be precise for you.”
The program is designed to consider the health and happiness of the patient, added Shashank R. Joshi, MD, chief scientist, Twin Health. “We want remission to be complication free. These findings give patients hope.”
“It’s exciting now that we can really start thinking about remission as an option for people with [type 2] diabetes, and that just provides such incredible hope for all of those living with [type 2] diabetes,” Dr. Gabbay said in an interview.
How the intervention works
The Twin Precision Treatment (TPT) intervention integrates multiple data – glucose values from a continuous glucose monitor (CGM); heart rate, activity, and sleep time from a fitness tracker; blood pressure values from a blood pressure cuff; food intake from the patient’s food log; and weight and body fat data from a smart scale – and provides the patient with precise, individualized nutrition and health guidance.
The four most critical sensors are the CGM, the fitness tracker, the smart scale, and the blood pressure cuff, Dr. Shah explained. The system gathers thousands of signals combined with patient self-reported data including mood or anxiety.
The CGM is used to build the initial nutrition guidance during the first 30 days. Once a patient is in remission, he or she can just keep the fitness tracker and smart scale.
The coaches who are part of this program include dietitians who are trained to provide compassionate patient education and help patients avoid diabetes relapse, and they are overseen by a licensed provider.
The program does not restrict calories. “It is not a diet,” Dr. Shah stressed.
The algorithm makes mini adjustments to the food a person is already eating to improve nutrition, Dr. Joshi explained. “This is personalized medicine at its best.” Patients eat food that they like and are guided to make small changes to get glucose under control and avoid glucose spikes.
The program is designed to safely deescalate diabetes medications as A1c decreases, Dr. Shamanna added.
U.S. clinical trial, health insurance coverage
The 1-year results of the current trial are expected in August, and the trial will continue for 2-=5 years, Dr. Shamanna said.
The company has started a clinical trial in the United States, with 5-year results expected in 2027.
“Currently, in the United States, we are partnering with self-insured employers and select health plans that offer [Twin Precision Treatment ] as an available benefit for their members,” Dr. Shah said. It “is suitable for most members living with type 2 diabetes, with rare exclusion situations.”
The study was funded by Twin Health. Dr. Shamanna, Dr. Shah, and Dr. Joshi are employees of Twin Health.
A version of this article first appeared on Medscape.com.
A novel approach that involves sensors, artificial intelligence, and real-time individualized lifestyle guidance from an app and live coaches led to a high rate of remission of type 2 diabetes in a new study.
Specifically, among 199 patients with type 2 diabetes in India who received the app-delivered lifestyle guidance developed by Twin Health, Mountain View, Calif., mean hemoglobin A1c dropped from 9.0% to 5.7% at 6 months.
This is “huge,” Paramesh Shamanna, MD, told a press briefing at the annual scientific sessions of the American Diabetes Association. The research was presented as three posters by the group at the meeting.
Patients were a mean age of 43 and had diabetes for a mean of 3.7 years and up to 8 years.
An “unprecedented” 84% of patients had remission of diabetes at 6 months, Dr. Shamanna, medical director at Twin Health, noted.
Diabetes remission was defined according to the 2021 joint consensus statement from the ADA and other organizations as an A1c less than 6.5% without the use of diabetes medications for at least 3 months.
Importantly, patients’ time in range (percentage of time spent in target blood glucose range) increased from 53% to 81%, Dr. Shamanna pointed out. On average, patients’ waist circumference decreased by 10 cm (3.9 inches) and their weight dropped from 79 kg (approximately 174 lb) to 68 kg (150 lb).
These results are driven by “the continuous individualized and precise guidance regarding nutrition, activity, and sleep,” Dr. Shamanna said in an interview.
Remission is not reversal or cure ...
“Remission” from type 2 diabetes is not “reversal” or a “cure,” Robert A. Gabbay, MD, PhD, chief scientific and medical officer of the ADA, stressed to the press. Just like cancer, diabetes can return after remission
Therefore, it is important to follow the lifestyle guidance. Patients may still be at risk for diabetes complications after diabetes remission, so it’s also important to continue to be screened for eye disease, nerve damage, and lipid levels.
However, “remission can be made to last,” Dr. Shamanna said, by continuing to follow the lifestyle advice and getting back on track after a relapse.
“We’re in a different time right now,” Lisa Shah, MD, chief medical officer, Twin Health, noted. “This is very different from management of blood glucose to a certain number.”
This study shows that “remission [from type 2 diabetes] is possible. How you achieve it can be precise for you.”
The program is designed to consider the health and happiness of the patient, added Shashank R. Joshi, MD, chief scientist, Twin Health. “We want remission to be complication free. These findings give patients hope.”
“It’s exciting now that we can really start thinking about remission as an option for people with [type 2] diabetes, and that just provides such incredible hope for all of those living with [type 2] diabetes,” Dr. Gabbay said in an interview.
How the intervention works
The Twin Precision Treatment (TPT) intervention integrates multiple data – glucose values from a continuous glucose monitor (CGM); heart rate, activity, and sleep time from a fitness tracker; blood pressure values from a blood pressure cuff; food intake from the patient’s food log; and weight and body fat data from a smart scale – and provides the patient with precise, individualized nutrition and health guidance.
The four most critical sensors are the CGM, the fitness tracker, the smart scale, and the blood pressure cuff, Dr. Shah explained. The system gathers thousands of signals combined with patient self-reported data including mood or anxiety.
The CGM is used to build the initial nutrition guidance during the first 30 days. Once a patient is in remission, he or she can just keep the fitness tracker and smart scale.
The coaches who are part of this program include dietitians who are trained to provide compassionate patient education and help patients avoid diabetes relapse, and they are overseen by a licensed provider.
The program does not restrict calories. “It is not a diet,” Dr. Shah stressed.
The algorithm makes mini adjustments to the food a person is already eating to improve nutrition, Dr. Joshi explained. “This is personalized medicine at its best.” Patients eat food that they like and are guided to make small changes to get glucose under control and avoid glucose spikes.
The program is designed to safely deescalate diabetes medications as A1c decreases, Dr. Shamanna added.
U.S. clinical trial, health insurance coverage
The 1-year results of the current trial are expected in August, and the trial will continue for 2-=5 years, Dr. Shamanna said.
The company has started a clinical trial in the United States, with 5-year results expected in 2027.
“Currently, in the United States, we are partnering with self-insured employers and select health plans that offer [Twin Precision Treatment ] as an available benefit for their members,” Dr. Shah said. It “is suitable for most members living with type 2 diabetes, with rare exclusion situations.”
The study was funded by Twin Health. Dr. Shamanna, Dr. Shah, and Dr. Joshi are employees of Twin Health.
A version of this article first appeared on Medscape.com.
A novel approach that involves sensors, artificial intelligence, and real-time individualized lifestyle guidance from an app and live coaches led to a high rate of remission of type 2 diabetes in a new study.
Specifically, among 199 patients with type 2 diabetes in India who received the app-delivered lifestyle guidance developed by Twin Health, Mountain View, Calif., mean hemoglobin A1c dropped from 9.0% to 5.7% at 6 months.
This is “huge,” Paramesh Shamanna, MD, told a press briefing at the annual scientific sessions of the American Diabetes Association. The research was presented as three posters by the group at the meeting.
Patients were a mean age of 43 and had diabetes for a mean of 3.7 years and up to 8 years.
An “unprecedented” 84% of patients had remission of diabetes at 6 months, Dr. Shamanna, medical director at Twin Health, noted.
Diabetes remission was defined according to the 2021 joint consensus statement from the ADA and other organizations as an A1c less than 6.5% without the use of diabetes medications for at least 3 months.
Importantly, patients’ time in range (percentage of time spent in target blood glucose range) increased from 53% to 81%, Dr. Shamanna pointed out. On average, patients’ waist circumference decreased by 10 cm (3.9 inches) and their weight dropped from 79 kg (approximately 174 lb) to 68 kg (150 lb).
These results are driven by “the continuous individualized and precise guidance regarding nutrition, activity, and sleep,” Dr. Shamanna said in an interview.
Remission is not reversal or cure ...
“Remission” from type 2 diabetes is not “reversal” or a “cure,” Robert A. Gabbay, MD, PhD, chief scientific and medical officer of the ADA, stressed to the press. Just like cancer, diabetes can return after remission
Therefore, it is important to follow the lifestyle guidance. Patients may still be at risk for diabetes complications after diabetes remission, so it’s also important to continue to be screened for eye disease, nerve damage, and lipid levels.
However, “remission can be made to last,” Dr. Shamanna said, by continuing to follow the lifestyle advice and getting back on track after a relapse.
“We’re in a different time right now,” Lisa Shah, MD, chief medical officer, Twin Health, noted. “This is very different from management of blood glucose to a certain number.”
This study shows that “remission [from type 2 diabetes] is possible. How you achieve it can be precise for you.”
The program is designed to consider the health and happiness of the patient, added Shashank R. Joshi, MD, chief scientist, Twin Health. “We want remission to be complication free. These findings give patients hope.”
“It’s exciting now that we can really start thinking about remission as an option for people with [type 2] diabetes, and that just provides such incredible hope for all of those living with [type 2] diabetes,” Dr. Gabbay said in an interview.
How the intervention works
The Twin Precision Treatment (TPT) intervention integrates multiple data – glucose values from a continuous glucose monitor (CGM); heart rate, activity, and sleep time from a fitness tracker; blood pressure values from a blood pressure cuff; food intake from the patient’s food log; and weight and body fat data from a smart scale – and provides the patient with precise, individualized nutrition and health guidance.
The four most critical sensors are the CGM, the fitness tracker, the smart scale, and the blood pressure cuff, Dr. Shah explained. The system gathers thousands of signals combined with patient self-reported data including mood or anxiety.
The CGM is used to build the initial nutrition guidance during the first 30 days. Once a patient is in remission, he or she can just keep the fitness tracker and smart scale.
The coaches who are part of this program include dietitians who are trained to provide compassionate patient education and help patients avoid diabetes relapse, and they are overseen by a licensed provider.
The program does not restrict calories. “It is not a diet,” Dr. Shah stressed.
The algorithm makes mini adjustments to the food a person is already eating to improve nutrition, Dr. Joshi explained. “This is personalized medicine at its best.” Patients eat food that they like and are guided to make small changes to get glucose under control and avoid glucose spikes.
The program is designed to safely deescalate diabetes medications as A1c decreases, Dr. Shamanna added.
U.S. clinical trial, health insurance coverage
The 1-year results of the current trial are expected in August, and the trial will continue for 2-=5 years, Dr. Shamanna said.
The company has started a clinical trial in the United States, with 5-year results expected in 2027.
“Currently, in the United States, we are partnering with self-insured employers and select health plans that offer [Twin Precision Treatment ] as an available benefit for their members,” Dr. Shah said. It “is suitable for most members living with type 2 diabetes, with rare exclusion situations.”
The study was funded by Twin Health. Dr. Shamanna, Dr. Shah, and Dr. Joshi are employees of Twin Health.
A version of this article first appeared on Medscape.com.
FROM ADA 2022
Antidiabetes drug costs keep patients away
NEW ORLEANS – , according to findings from two separate studies.
One study looked at the insurance records of more than 70,000 U.S. patients with type 2 diabetes and established cardiovascular disease who were already on metformin. The findings showed that, after adjustment for confounders, the quartile of patients with the highest out-of-pocket cost for an agent from the sodium-glucose cotransporter 2 (SGLT2)–inhibitor class filled a prescription for one of these drugs a significant 21% less often than did patients from the quartile with the lowest personal expense, after adjustment for a variety of potential confounding factors, reported Jing Luo, MD, at the annual scientific sessions of the American Diabetes Association.
A similar analysis run by Dr. Luo and his associates looking at glucagonlike peptide-1 (GLP-1) receptor agonists showed that the quartile of patients who had to pay the most for one of those drugs had an adjusted 12% lower rate of filling a prescription, compared with those with the lowest out-of-pocket expense, a difference that fell just short of significance.
“If we consistently see that high drug costs affect use of highly effective medications in patients with type 2 diabetes and risk factors, it’s quite problematic because it’s not just a matter of money, but it also makes a difference in the patient’s quality of care,” Dr. Luo said in an interview.
Prevention drug lists can help
Consistency turned up in a second report at the same ADA session that retrospectively reviewed data collected during 2004-2017 by a single large U.S. health insurer to identify 3,315 matched pairs of children and adults with diabetes who all had high-deductible health plans for their medical insurance, along with an associated health savings account.
One set of patients in each matched pair began to receive, at some point during follow-up, coverage with a prevention drug list (PDL; also called a formulary) that provided them with a variety of specified agents at no charge. They included oral antidiabetes agents, insulin, antihypertensives, and lipid-lowering drugs. The other half of the matched pairs of patients received no PDL coverage and had copays for their antidiabetes medications.
The findings showed that the rates of out-of-pocket costs for antidiabetes drugs, antidiabetic medications used, and acute diabetes complications all tracked extremely closely between the matched pairs before half of them started to receive their PDL coverage. However, after PDL coverage kicked in, out of pocket costs dropped by 32% for the people with PDL coverage, compared with those who did not receive this coverage. Oral antidiabetes medication use rose modestly, but acute diabetes complications “declined substantially,” with a 14% relative reduction overall in those with PDL coverage, compared with those without, reported J. Franklin Wharam, MBBCh, a professor and health policy researcher at Duke University in Durham, N.C. In the roughly half of the study cohort who fell into a low-income category based on where they lived, the rate of excess acute diabetes complications was 23% higher for those without a PDL, compared with those who had that coverage.
PDL coverage linked with “large reductions in acute, preventable diabetes complications,” concluded Dr. Wharam. “Policy makers and employers should incentivize PDL uptake among low-income patients with diabetes.”
Newer, more effective drugs cost a lot
“The more comorbidities that patients have, the greater is the strength of the evidence for using newer antidiabetes drugs that are more expensive,” but that would mean spending much more on this part of patient care, noted Dr. Luo, an internal medicine physician and researcher at the University of Pittsburgh. “It will cost a lot of money, and I’m not sure what the solution is. It’s a huge conundrum.”
About 30 million Americans have type 2 diabetes. If every one of them went on an SGLT2 inhibitor, or went on an SGLT2 inhibitor plus a GLP-1 receptor agonist, “it would bankrupt the U.S. health care system, so we can’t do that,” commented Sylvio E. Inzucchi, MD, in an interview. “The only thing holding this back is cost. We target these drugs to the patients most apt to benefit from them. If they were generic they would be used much more widely,” noted Dr. Inzucchi, professor and clinical chief of endocrinology at Yale University in New Haven, Conn.
The study run by Dr. Luo and his associates retrospectively reviewed data from 72,743 U.S. adults included in the Optum Clinformatics database during December 2017–December 2019. All included patients had type 2 diabetes, received metformin monotherapy, and had established atherosclerotic cardiovascular disease. They averaged 72 years of age, 56% were men, and 88% were on a Medicare Advantage plan, while the remainder had commercial insurance. Their average hemoglobin A1c level was 6.8%.
People in the quartile with the lowest copays spent an average of about $20/month for either an SGLT2 inhibitor or a GLP-1 receptor agonist. Those in the quartile with the highest copays spent roughly $100/month for agents from each of these two classes. The analysis followed patients for a median of 914 days.
In addition to finding disparate rates of drug use between these two quartiles, the analysis also showed that higher copays linked with longer times to initially fill prescriptions for these drugs. But while those with higher copays took longer to start both classes than did those with the smallest copays, even those with the lowest out-of-pocket costs averaged about a year to initiate treatment.
Dr. Luo attributed this delay to other factors besides costs to patients, such as clinicians prescribing other classes of second-line oral antidiabetes agents, clinical inertia, and lack of awareness by clinicians of the special benefits of SGLT2 inhibitors and GLP-1 receptor antagonists for patients with type 2 diabetes and cardiovascular disease.
“A lot of clinical and social factors drive medication use,” not just out-of-pocket cost, he explained.
Dr. Luo is a consultant to Alosa Health. Dr. Wharam had no disclosures. Dr. Inzucchi is an adviser to Abbott Diagnostics, Esperion Therapeutics, and vTv Therapeutics, a consultant to Merck and Pfizer, and has other relationships with AstraZeneca, Boehringer Ingelheim, Lexicon, and Novo Nordisk.
NEW ORLEANS – , according to findings from two separate studies.
One study looked at the insurance records of more than 70,000 U.S. patients with type 2 diabetes and established cardiovascular disease who were already on metformin. The findings showed that, after adjustment for confounders, the quartile of patients with the highest out-of-pocket cost for an agent from the sodium-glucose cotransporter 2 (SGLT2)–inhibitor class filled a prescription for one of these drugs a significant 21% less often than did patients from the quartile with the lowest personal expense, after adjustment for a variety of potential confounding factors, reported Jing Luo, MD, at the annual scientific sessions of the American Diabetes Association.
A similar analysis run by Dr. Luo and his associates looking at glucagonlike peptide-1 (GLP-1) receptor agonists showed that the quartile of patients who had to pay the most for one of those drugs had an adjusted 12% lower rate of filling a prescription, compared with those with the lowest out-of-pocket expense, a difference that fell just short of significance.
“If we consistently see that high drug costs affect use of highly effective medications in patients with type 2 diabetes and risk factors, it’s quite problematic because it’s not just a matter of money, but it also makes a difference in the patient’s quality of care,” Dr. Luo said in an interview.
Prevention drug lists can help
Consistency turned up in a second report at the same ADA session that retrospectively reviewed data collected during 2004-2017 by a single large U.S. health insurer to identify 3,315 matched pairs of children and adults with diabetes who all had high-deductible health plans for their medical insurance, along with an associated health savings account.
One set of patients in each matched pair began to receive, at some point during follow-up, coverage with a prevention drug list (PDL; also called a formulary) that provided them with a variety of specified agents at no charge. They included oral antidiabetes agents, insulin, antihypertensives, and lipid-lowering drugs. The other half of the matched pairs of patients received no PDL coverage and had copays for their antidiabetes medications.
The findings showed that the rates of out-of-pocket costs for antidiabetes drugs, antidiabetic medications used, and acute diabetes complications all tracked extremely closely between the matched pairs before half of them started to receive their PDL coverage. However, after PDL coverage kicked in, out of pocket costs dropped by 32% for the people with PDL coverage, compared with those who did not receive this coverage. Oral antidiabetes medication use rose modestly, but acute diabetes complications “declined substantially,” with a 14% relative reduction overall in those with PDL coverage, compared with those without, reported J. Franklin Wharam, MBBCh, a professor and health policy researcher at Duke University in Durham, N.C. In the roughly half of the study cohort who fell into a low-income category based on where they lived, the rate of excess acute diabetes complications was 23% higher for those without a PDL, compared with those who had that coverage.
PDL coverage linked with “large reductions in acute, preventable diabetes complications,” concluded Dr. Wharam. “Policy makers and employers should incentivize PDL uptake among low-income patients with diabetes.”
Newer, more effective drugs cost a lot
“The more comorbidities that patients have, the greater is the strength of the evidence for using newer antidiabetes drugs that are more expensive,” but that would mean spending much more on this part of patient care, noted Dr. Luo, an internal medicine physician and researcher at the University of Pittsburgh. “It will cost a lot of money, and I’m not sure what the solution is. It’s a huge conundrum.”
About 30 million Americans have type 2 diabetes. If every one of them went on an SGLT2 inhibitor, or went on an SGLT2 inhibitor plus a GLP-1 receptor agonist, “it would bankrupt the U.S. health care system, so we can’t do that,” commented Sylvio E. Inzucchi, MD, in an interview. “The only thing holding this back is cost. We target these drugs to the patients most apt to benefit from them. If they were generic they would be used much more widely,” noted Dr. Inzucchi, professor and clinical chief of endocrinology at Yale University in New Haven, Conn.
The study run by Dr. Luo and his associates retrospectively reviewed data from 72,743 U.S. adults included in the Optum Clinformatics database during December 2017–December 2019. All included patients had type 2 diabetes, received metformin monotherapy, and had established atherosclerotic cardiovascular disease. They averaged 72 years of age, 56% were men, and 88% were on a Medicare Advantage plan, while the remainder had commercial insurance. Their average hemoglobin A1c level was 6.8%.
People in the quartile with the lowest copays spent an average of about $20/month for either an SGLT2 inhibitor or a GLP-1 receptor agonist. Those in the quartile with the highest copays spent roughly $100/month for agents from each of these two classes. The analysis followed patients for a median of 914 days.
In addition to finding disparate rates of drug use between these two quartiles, the analysis also showed that higher copays linked with longer times to initially fill prescriptions for these drugs. But while those with higher copays took longer to start both classes than did those with the smallest copays, even those with the lowest out-of-pocket costs averaged about a year to initiate treatment.
Dr. Luo attributed this delay to other factors besides costs to patients, such as clinicians prescribing other classes of second-line oral antidiabetes agents, clinical inertia, and lack of awareness by clinicians of the special benefits of SGLT2 inhibitors and GLP-1 receptor antagonists for patients with type 2 diabetes and cardiovascular disease.
“A lot of clinical and social factors drive medication use,” not just out-of-pocket cost, he explained.
Dr. Luo is a consultant to Alosa Health. Dr. Wharam had no disclosures. Dr. Inzucchi is an adviser to Abbott Diagnostics, Esperion Therapeutics, and vTv Therapeutics, a consultant to Merck and Pfizer, and has other relationships with AstraZeneca, Boehringer Ingelheim, Lexicon, and Novo Nordisk.
NEW ORLEANS – , according to findings from two separate studies.
One study looked at the insurance records of more than 70,000 U.S. patients with type 2 diabetes and established cardiovascular disease who were already on metformin. The findings showed that, after adjustment for confounders, the quartile of patients with the highest out-of-pocket cost for an agent from the sodium-glucose cotransporter 2 (SGLT2)–inhibitor class filled a prescription for one of these drugs a significant 21% less often than did patients from the quartile with the lowest personal expense, after adjustment for a variety of potential confounding factors, reported Jing Luo, MD, at the annual scientific sessions of the American Diabetes Association.
A similar analysis run by Dr. Luo and his associates looking at glucagonlike peptide-1 (GLP-1) receptor agonists showed that the quartile of patients who had to pay the most for one of those drugs had an adjusted 12% lower rate of filling a prescription, compared with those with the lowest out-of-pocket expense, a difference that fell just short of significance.
“If we consistently see that high drug costs affect use of highly effective medications in patients with type 2 diabetes and risk factors, it’s quite problematic because it’s not just a matter of money, but it also makes a difference in the patient’s quality of care,” Dr. Luo said in an interview.
Prevention drug lists can help
Consistency turned up in a second report at the same ADA session that retrospectively reviewed data collected during 2004-2017 by a single large U.S. health insurer to identify 3,315 matched pairs of children and adults with diabetes who all had high-deductible health plans for their medical insurance, along with an associated health savings account.
One set of patients in each matched pair began to receive, at some point during follow-up, coverage with a prevention drug list (PDL; also called a formulary) that provided them with a variety of specified agents at no charge. They included oral antidiabetes agents, insulin, antihypertensives, and lipid-lowering drugs. The other half of the matched pairs of patients received no PDL coverage and had copays for their antidiabetes medications.
The findings showed that the rates of out-of-pocket costs for antidiabetes drugs, antidiabetic medications used, and acute diabetes complications all tracked extremely closely between the matched pairs before half of them started to receive their PDL coverage. However, after PDL coverage kicked in, out of pocket costs dropped by 32% for the people with PDL coverage, compared with those who did not receive this coverage. Oral antidiabetes medication use rose modestly, but acute diabetes complications “declined substantially,” with a 14% relative reduction overall in those with PDL coverage, compared with those without, reported J. Franklin Wharam, MBBCh, a professor and health policy researcher at Duke University in Durham, N.C. In the roughly half of the study cohort who fell into a low-income category based on where they lived, the rate of excess acute diabetes complications was 23% higher for those without a PDL, compared with those who had that coverage.
PDL coverage linked with “large reductions in acute, preventable diabetes complications,” concluded Dr. Wharam. “Policy makers and employers should incentivize PDL uptake among low-income patients with diabetes.”
Newer, more effective drugs cost a lot
“The more comorbidities that patients have, the greater is the strength of the evidence for using newer antidiabetes drugs that are more expensive,” but that would mean spending much more on this part of patient care, noted Dr. Luo, an internal medicine physician and researcher at the University of Pittsburgh. “It will cost a lot of money, and I’m not sure what the solution is. It’s a huge conundrum.”
About 30 million Americans have type 2 diabetes. If every one of them went on an SGLT2 inhibitor, or went on an SGLT2 inhibitor plus a GLP-1 receptor agonist, “it would bankrupt the U.S. health care system, so we can’t do that,” commented Sylvio E. Inzucchi, MD, in an interview. “The only thing holding this back is cost. We target these drugs to the patients most apt to benefit from them. If they were generic they would be used much more widely,” noted Dr. Inzucchi, professor and clinical chief of endocrinology at Yale University in New Haven, Conn.
The study run by Dr. Luo and his associates retrospectively reviewed data from 72,743 U.S. adults included in the Optum Clinformatics database during December 2017–December 2019. All included patients had type 2 diabetes, received metformin monotherapy, and had established atherosclerotic cardiovascular disease. They averaged 72 years of age, 56% were men, and 88% were on a Medicare Advantage plan, while the remainder had commercial insurance. Their average hemoglobin A1c level was 6.8%.
People in the quartile with the lowest copays spent an average of about $20/month for either an SGLT2 inhibitor or a GLP-1 receptor agonist. Those in the quartile with the highest copays spent roughly $100/month for agents from each of these two classes. The analysis followed patients for a median of 914 days.
In addition to finding disparate rates of drug use between these two quartiles, the analysis also showed that higher copays linked with longer times to initially fill prescriptions for these drugs. But while those with higher copays took longer to start both classes than did those with the smallest copays, even those with the lowest out-of-pocket costs averaged about a year to initiate treatment.
Dr. Luo attributed this delay to other factors besides costs to patients, such as clinicians prescribing other classes of second-line oral antidiabetes agents, clinical inertia, and lack of awareness by clinicians of the special benefits of SGLT2 inhibitors and GLP-1 receptor antagonists for patients with type 2 diabetes and cardiovascular disease.
“A lot of clinical and social factors drive medication use,” not just out-of-pocket cost, he explained.
Dr. Luo is a consultant to Alosa Health. Dr. Wharam had no disclosures. Dr. Inzucchi is an adviser to Abbott Diagnostics, Esperion Therapeutics, and vTv Therapeutics, a consultant to Merck and Pfizer, and has other relationships with AstraZeneca, Boehringer Ingelheim, Lexicon, and Novo Nordisk.
AT ADA 2022
Obesity in adolescence raises risk for adult type 1 diabetes
NEW ORLEANS – Obesity in adolescence is linked to an increased risk for type 1 diabetes onset in adulthood, new research suggests.
These new data, from Israeli military recruits followed for over a decade, suggest that obesity may be playing a causal role in type 1 as well as type 2 diabetes.
The incidence of type 1 diabetes has been increasing by about 2%-3% annually over recent decades, but the reasons aren’t clear. The study is the first to examine the role of obesity in adolescence and type 1 diabetes in young adulthood, and also the first to examine the question of using antibody status as part of the criteria for a type 1 diagnosis.
The findings were reported at the annual scientific sessions of the American Diabetes Association by Gilad Twig, MD, PhD, professor of medicine at Sheba Medical Center, Tel HaShomer, Israel. “For people who might have a high risk for developing type 1 diabetes, these results emphasize the importance of maintaining a normal weight,” he said in an interview. He noted that, although this recommendation applies to everyone, “here it’s becoming more precise for the population – more individualized in the sense that this might specifically help you.”
Naveed Sattar, PhD, professor and honorary consultant in cardiovascular and medical sciences at the University of Glasgow, said in an interview that carrying too much weight “will make the pancreas have to work harder to make insulin to keep the sugar normal. So, if you’re stressing the system and the pancreas is already likely to fail, it will fail faster.”
Clinically, Dr. Sattar said, “Lifestyle does matter to the risk of developing type 1 diabetes. The weighting may be different [from type 2]. The major factor in type 1 is still the genetics, but if you have a family history of type 1 and your genetic potential is greater, you will minimize your risk by staying leaner.”
Study highlights that type 1 is not always ‘juvenile’
In addition to countering the long-held belief that type 1 diabetes is primarily a condition of thin individuals and unrelated to obesity, the data also reinforce the emerging recognition that type 1 diabetes isn’t always “juvenile” and in fact often arises in adulthood.
“About half of all cases of type 1 diabetes develop after age 18. By reputation, people think it’s a disease of children. But it’s begun to grow so that now 50% of cases occur after late adolescence,” noted Dr. Twig.
Dr. Sattar pointed to a UK Biobank study showing that nearly half of all cases of type 1 diabetes arise after age 30 years. “You absolutely can get type 1 in adulthood. It’s not rare.”
Direct correlation seen in otherwise healthy young people
The retrospective nationwide cohort study included 1,426,362 17-year-olds (834,050 male and 592,312 female) who underwent medical evaluation prior to military conscription starting in January 1996 and followed them through 2016. At baseline, none had a history of dysglycemia.
The data were linked with information about adult-onset type 1 diabetes in the Israeli National Diabetes Registry. In all, 777 incident type 1 diabetes cases were recorded over the study period, with a rate of 4.9 cases per 100,000 person-years.
Over a median follow-up of 11.2 years, there was a graded incidence of type 1 diabetes across BMI groups from underweight to obesity, from 3.6 to 8.4 cases per 100,000 person-years.
After adjustment for sex, birth year, age at study entry, education, and cognitive performance with BMI 5th-49th percentiles as the reference, the hazard ratios were 1.05 for the 50th-74th BMI percentiles, 1.41 for 75th-84th, 1.54 for those who were overweight, and 2.05 for those with obesity.
Every 5-unit increment in BMI corresponded to a 35% greater incidence of type 1 diabetes (adjusted hazard ratio 1.35) and every one increment was associated with a 35% greater risk (1.25), both values significant.
Sensitivity analyses resulted in similar findings for those with no other chronic health conditions at baseline. The results also didn’t change in a separate analysis of 574,720 subjects in whom autoantibody data were available to confirm the type 1 diabetes diagnosis.
Hypotheses for mechanisms
The mechanism for the association isn’t clear, but in a simultaneously published article in Diabetologia, Dr. Twig and colleagues outline several hypotheses. One relates to the growing evidence of a link between various autoimmune conditions, which point to the possibility of elevated adipokines and cytokines in obesity diminishing self-tolerance by promoting proinflammatory processes.
The authors cite data from the TrialNet Pathway to Prevention study of relatives of people with type 1 diabetes in which participants who were overweight and obese had an increased risk of islet autoantibody expression. However, not all data have supported this finding.
“Obesity is related to several other autoimmune conditions, so it’s not a complete surprise it might be related to another,” Dr. Twig noted.
Other possibilities include vitamin D deficiency, a high-fat diet, and alterations in gut microbiota.
And then there’s the “accelerator hypothesis,” suggesting that both type 1 and type 2 diabetes result from insulin resistance and genetic background that affect the rate of beta cell loss and the disease phenotype. Dr. Sattar said that the accelerator hypotheses “makes complete sense to me. Because the population is so obese, we’re seeing it more now whereas we might not have seen it 40 years ago when the BMI differentials were far less in society.”
Dr. Twig has no disclosures. Dr. Sattar has consulted for or received lecture fees from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Hanmi Pharmaceutical, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, and Sanofi, and received grant support from AstraZeneca, Boehringer Ingelheim, Novartis, and Roche Diagnostics through his institution.
NEW ORLEANS – Obesity in adolescence is linked to an increased risk for type 1 diabetes onset in adulthood, new research suggests.
These new data, from Israeli military recruits followed for over a decade, suggest that obesity may be playing a causal role in type 1 as well as type 2 diabetes.
The incidence of type 1 diabetes has been increasing by about 2%-3% annually over recent decades, but the reasons aren’t clear. The study is the first to examine the role of obesity in adolescence and type 1 diabetes in young adulthood, and also the first to examine the question of using antibody status as part of the criteria for a type 1 diagnosis.
The findings were reported at the annual scientific sessions of the American Diabetes Association by Gilad Twig, MD, PhD, professor of medicine at Sheba Medical Center, Tel HaShomer, Israel. “For people who might have a high risk for developing type 1 diabetes, these results emphasize the importance of maintaining a normal weight,” he said in an interview. He noted that, although this recommendation applies to everyone, “here it’s becoming more precise for the population – more individualized in the sense that this might specifically help you.”
Naveed Sattar, PhD, professor and honorary consultant in cardiovascular and medical sciences at the University of Glasgow, said in an interview that carrying too much weight “will make the pancreas have to work harder to make insulin to keep the sugar normal. So, if you’re stressing the system and the pancreas is already likely to fail, it will fail faster.”
Clinically, Dr. Sattar said, “Lifestyle does matter to the risk of developing type 1 diabetes. The weighting may be different [from type 2]. The major factor in type 1 is still the genetics, but if you have a family history of type 1 and your genetic potential is greater, you will minimize your risk by staying leaner.”
Study highlights that type 1 is not always ‘juvenile’
In addition to countering the long-held belief that type 1 diabetes is primarily a condition of thin individuals and unrelated to obesity, the data also reinforce the emerging recognition that type 1 diabetes isn’t always “juvenile” and in fact often arises in adulthood.
“About half of all cases of type 1 diabetes develop after age 18. By reputation, people think it’s a disease of children. But it’s begun to grow so that now 50% of cases occur after late adolescence,” noted Dr. Twig.
Dr. Sattar pointed to a UK Biobank study showing that nearly half of all cases of type 1 diabetes arise after age 30 years. “You absolutely can get type 1 in adulthood. It’s not rare.”
Direct correlation seen in otherwise healthy young people
The retrospective nationwide cohort study included 1,426,362 17-year-olds (834,050 male and 592,312 female) who underwent medical evaluation prior to military conscription starting in January 1996 and followed them through 2016. At baseline, none had a history of dysglycemia.
The data were linked with information about adult-onset type 1 diabetes in the Israeli National Diabetes Registry. In all, 777 incident type 1 diabetes cases were recorded over the study period, with a rate of 4.9 cases per 100,000 person-years.
Over a median follow-up of 11.2 years, there was a graded incidence of type 1 diabetes across BMI groups from underweight to obesity, from 3.6 to 8.4 cases per 100,000 person-years.
After adjustment for sex, birth year, age at study entry, education, and cognitive performance with BMI 5th-49th percentiles as the reference, the hazard ratios were 1.05 for the 50th-74th BMI percentiles, 1.41 for 75th-84th, 1.54 for those who were overweight, and 2.05 for those with obesity.
Every 5-unit increment in BMI corresponded to a 35% greater incidence of type 1 diabetes (adjusted hazard ratio 1.35) and every one increment was associated with a 35% greater risk (1.25), both values significant.
Sensitivity analyses resulted in similar findings for those with no other chronic health conditions at baseline. The results also didn’t change in a separate analysis of 574,720 subjects in whom autoantibody data were available to confirm the type 1 diabetes diagnosis.
Hypotheses for mechanisms
The mechanism for the association isn’t clear, but in a simultaneously published article in Diabetologia, Dr. Twig and colleagues outline several hypotheses. One relates to the growing evidence of a link between various autoimmune conditions, which point to the possibility of elevated adipokines and cytokines in obesity diminishing self-tolerance by promoting proinflammatory processes.
The authors cite data from the TrialNet Pathway to Prevention study of relatives of people with type 1 diabetes in which participants who were overweight and obese had an increased risk of islet autoantibody expression. However, not all data have supported this finding.
“Obesity is related to several other autoimmune conditions, so it’s not a complete surprise it might be related to another,” Dr. Twig noted.
Other possibilities include vitamin D deficiency, a high-fat diet, and alterations in gut microbiota.
And then there’s the “accelerator hypothesis,” suggesting that both type 1 and type 2 diabetes result from insulin resistance and genetic background that affect the rate of beta cell loss and the disease phenotype. Dr. Sattar said that the accelerator hypotheses “makes complete sense to me. Because the population is so obese, we’re seeing it more now whereas we might not have seen it 40 years ago when the BMI differentials were far less in society.”
Dr. Twig has no disclosures. Dr. Sattar has consulted for or received lecture fees from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Hanmi Pharmaceutical, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, and Sanofi, and received grant support from AstraZeneca, Boehringer Ingelheim, Novartis, and Roche Diagnostics through his institution.
NEW ORLEANS – Obesity in adolescence is linked to an increased risk for type 1 diabetes onset in adulthood, new research suggests.
These new data, from Israeli military recruits followed for over a decade, suggest that obesity may be playing a causal role in type 1 as well as type 2 diabetes.
The incidence of type 1 diabetes has been increasing by about 2%-3% annually over recent decades, but the reasons aren’t clear. The study is the first to examine the role of obesity in adolescence and type 1 diabetes in young adulthood, and also the first to examine the question of using antibody status as part of the criteria for a type 1 diagnosis.
The findings were reported at the annual scientific sessions of the American Diabetes Association by Gilad Twig, MD, PhD, professor of medicine at Sheba Medical Center, Tel HaShomer, Israel. “For people who might have a high risk for developing type 1 diabetes, these results emphasize the importance of maintaining a normal weight,” he said in an interview. He noted that, although this recommendation applies to everyone, “here it’s becoming more precise for the population – more individualized in the sense that this might specifically help you.”
Naveed Sattar, PhD, professor and honorary consultant in cardiovascular and medical sciences at the University of Glasgow, said in an interview that carrying too much weight “will make the pancreas have to work harder to make insulin to keep the sugar normal. So, if you’re stressing the system and the pancreas is already likely to fail, it will fail faster.”
Clinically, Dr. Sattar said, “Lifestyle does matter to the risk of developing type 1 diabetes. The weighting may be different [from type 2]. The major factor in type 1 is still the genetics, but if you have a family history of type 1 and your genetic potential is greater, you will minimize your risk by staying leaner.”
Study highlights that type 1 is not always ‘juvenile’
In addition to countering the long-held belief that type 1 diabetes is primarily a condition of thin individuals and unrelated to obesity, the data also reinforce the emerging recognition that type 1 diabetes isn’t always “juvenile” and in fact often arises in adulthood.
“About half of all cases of type 1 diabetes develop after age 18. By reputation, people think it’s a disease of children. But it’s begun to grow so that now 50% of cases occur after late adolescence,” noted Dr. Twig.
Dr. Sattar pointed to a UK Biobank study showing that nearly half of all cases of type 1 diabetes arise after age 30 years. “You absolutely can get type 1 in adulthood. It’s not rare.”
Direct correlation seen in otherwise healthy young people
The retrospective nationwide cohort study included 1,426,362 17-year-olds (834,050 male and 592,312 female) who underwent medical evaluation prior to military conscription starting in January 1996 and followed them through 2016. At baseline, none had a history of dysglycemia.
The data were linked with information about adult-onset type 1 diabetes in the Israeli National Diabetes Registry. In all, 777 incident type 1 diabetes cases were recorded over the study period, with a rate of 4.9 cases per 100,000 person-years.
Over a median follow-up of 11.2 years, there was a graded incidence of type 1 diabetes across BMI groups from underweight to obesity, from 3.6 to 8.4 cases per 100,000 person-years.
After adjustment for sex, birth year, age at study entry, education, and cognitive performance with BMI 5th-49th percentiles as the reference, the hazard ratios were 1.05 for the 50th-74th BMI percentiles, 1.41 for 75th-84th, 1.54 for those who were overweight, and 2.05 for those with obesity.
Every 5-unit increment in BMI corresponded to a 35% greater incidence of type 1 diabetes (adjusted hazard ratio 1.35) and every one increment was associated with a 35% greater risk (1.25), both values significant.
Sensitivity analyses resulted in similar findings for those with no other chronic health conditions at baseline. The results also didn’t change in a separate analysis of 574,720 subjects in whom autoantibody data were available to confirm the type 1 diabetes diagnosis.
Hypotheses for mechanisms
The mechanism for the association isn’t clear, but in a simultaneously published article in Diabetologia, Dr. Twig and colleagues outline several hypotheses. One relates to the growing evidence of a link between various autoimmune conditions, which point to the possibility of elevated adipokines and cytokines in obesity diminishing self-tolerance by promoting proinflammatory processes.
The authors cite data from the TrialNet Pathway to Prevention study of relatives of people with type 1 diabetes in which participants who were overweight and obese had an increased risk of islet autoantibody expression. However, not all data have supported this finding.
“Obesity is related to several other autoimmune conditions, so it’s not a complete surprise it might be related to another,” Dr. Twig noted.
Other possibilities include vitamin D deficiency, a high-fat diet, and alterations in gut microbiota.
And then there’s the “accelerator hypothesis,” suggesting that both type 1 and type 2 diabetes result from insulin resistance and genetic background that affect the rate of beta cell loss and the disease phenotype. Dr. Sattar said that the accelerator hypotheses “makes complete sense to me. Because the population is so obese, we’re seeing it more now whereas we might not have seen it 40 years ago when the BMI differentials were far less in society.”
Dr. Twig has no disclosures. Dr. Sattar has consulted for or received lecture fees from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Hanmi Pharmaceutical, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, and Sanofi, and received grant support from AstraZeneca, Boehringer Ingelheim, Novartis, and Roche Diagnostics through his institution.
AT ADA 2022
Weekly dulaglutide promising in youth with type 2 diabetes
Another glucagonlike peptide-1 (GLP1) agonist, dulaglutide (Trulicity, Lilly), is poised to be a new option for glycemic control in youth aged 10-18 years with type 2 diabetes, given as a weekly injection, based on the AWARD-PEDS clinical trial.
The U.S. Food and Drug Administration has already approved daily injection liraglutide (Victoza, Novo Nordisk) in 2019 and weekly exenatide (Bydureon/Bydureon BCise, AstraZeneca) in 2021 for glycemic control in young patients with type 2 diabetes, both of which are also GLP-1 agonists.
AWARD-PEDS showed that youth with type 2 diabetes and obesity treated with or without metformin or basal insulin who received weekly injections of 0.75 mg or 1.5 mg of dulaglutide had lower hemoglobin A1c at 26 weeks than patients who received placebo.
Eli Lilly is now submitting these trial results to the FDA for this indication.
Dulaglutide was cleared for use in adults with type 2 diabetes in the United States in 2014 and was additionally approved for reducing the risk of major adverse cardiovascular events (MACE) in adults with type 2 diabetes at high risk of such events in 2020.
The most common adverse symptoms were gastrointestinal, and the safety profile was consistent with that in adults. However, the drug had no effect on body mass index.
The study was simultaneously published in the New England Journal of Medicine and presented as a late-breaking poster at the annual scientific sessions of the American Diabetes Association in New Orleans.
Might dulaglutide target pathophysiologic impairments in youth?
Dulaglutide would “offer a new treatment that targets the pathophysiologic impairments of type 2 diabetes in youth,” Silva A. Arslanian, MD, lead investigator, told this news organization.
Exenatide is also given as a weekly injection but is associated with a smaller decrease in A1c and does not improve fasting glucose concentrations, plus it requires more steps compared with the dulaglutide single-use pen, said Dr. Arslanian, who is scientific director at the Center for Pediatric Research in Obesity & Metabolism, UPMC Children’s Hospital of Pittsburgh.
“Liraglutide is a daily injection, and I believe most patients, particularly adolescents, would prefer a weekly injection,” she added.
Invited to comment, Elvira Isganaitis, MD, MPH, said “the significance of this paper lies in the fact that options for treating type 2 diabetes in children are currently much more limited than in adults – which is a major problem given recent studies that show that type 2 diabetes in youth is much more aggressive and more likely to cause complications early in the disease course.”
Dr. Isganaitis was not involved with the trial but is an investigator for the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study.
“With supply chain shortages and health insurance coverage issues that are common in the U.S., it would be helpful to have more than one FDA-approved option for a weekly GLP-1 receptor agonist in children [and] access to other classes of medications,” added Dr. Isganaitis, a pediatric endocrinologist at the Joslin Diabetes Center, Boston.
Phase 3 trials of sodium-glucose cotransporter 2 (SGLT2) inhibitors and dipeptidyl peptidase-4 (DPP-4) inhibitors in youth with type 2 diabetes are also ongoing, Dr. Arslanian noted, “but as always, recruitment is slow with adolescents.”
“I am not optimistic that DPP4 inhibitors will have a place in the treatment of youth with type 2 diabetes,” she said. A recent study showed the addition of sitagliptin to metformin in youth with type 2 diabetes did not provide durable improvement in glycemic control.
Potentially promising therapy
In their published article, Dr. Arslanian and colleagues write that “considering the progressive increase in [A1c] over time that was observed in the TODAY trial, with 34% of youths having [an A1c] of at least 10% after up to 15 years of follow-up, we believe that the effects of dulaglutide therapy appear to be potentially promising.”
The TODAY trial showed that more than 50% of youth with type 2 diabetes taking metformin failed to maintain glycemic control within a median of 11.5 months, Dr. Arslanian elaborated, and over time their A1c escalated while their beta-cell function deteriorated rapidly, and complications progressed quickly.
“Therefore,” she noted, “considering that dulaglutide and the GLP-1 receptor agonist class of drugs improve A1c, improve beta-cell function, suppress glucagon concentrations, and improve insulin sensitivity, dulaglutide would provide a promising new treatment option for youth with type 2 diabetes.”
Phase 3 superiority trial
The AWARD-PEDS trial included 154 youth with type 2 diabetes and a BMI greater than the 85th percentile for their age and sex at 46 centers in nine countries. Researchers randomized participants 1:1:1 to the two doses of dulaglutide or placebo for 26 weeks, followed by a 26-week open-label study (during which the placebo group received 0.75 mg dulaglutide) and a 4-week safety extension.
Participants were a mean age of 14.5 years and had a mean BMI of 34 kg/m2.
In each of the dulaglutide groups, roughly 66% of patients were female and 58% were White, 18% were Black, and about 57% were Hispanic. They had a mean weight of 91 kg (200 lb) and a mean A1c of about 8%; 62% were taking metformin only, 27% were taking metformin plus basal insulin, 3% were taking basal insulin only, and 10% were on diet and exercise only.
At 26 weeks, mean A1c increased by 0.6% in the placebo group but decreased by 0.6% in the 0.75-mg dulaglutide group and by 0.9% in the 1.5-mg dulaglutide group (P < .001 for both comparisons versus placebo).
Also at 26 weeks, more participants in the pooled dulaglutide groups than in the placebo group had an A1c <7.0% (51% vs. 14%; P < .001).
Fasting glucose concentration increased in the placebo group (+17.1 mg/dL ) and decreased in the pooled dulaglutide groups (–18.9 mg/dL; P < .001).
There were no group differences in BMI or adiposity-related parameters even at 52 weeks.
“I believe adolescents may be somewhat resistant to the weight-reducing effects of GLP-1 agonists in diabetes trials (liraglutide and exenatide youth type 2 diabetes trials showed the same thing) and they may need higher doses,” Dr. Arslanian speculated.
“Only future studies will be able to address this issue,” she concluded.
The study was funded by Eli Lilly. Dr. Arslanian has disclosed being a consultant for Eli Lilly, Novo Nordisk, and Rhythm Pharmaceuticals; participating in data safety monitoring for AstraZeneca and Eli Lilly trials; and receiving institutional research funding from Eli Lilly and Novo Nordisk. Dr. Isganaitis has disclosed receiving research funding (paid to her institution) from Dexcom and AstraZeneca.
A version of this article first appeared on Medscape.com.
Another glucagonlike peptide-1 (GLP1) agonist, dulaglutide (Trulicity, Lilly), is poised to be a new option for glycemic control in youth aged 10-18 years with type 2 diabetes, given as a weekly injection, based on the AWARD-PEDS clinical trial.
The U.S. Food and Drug Administration has already approved daily injection liraglutide (Victoza, Novo Nordisk) in 2019 and weekly exenatide (Bydureon/Bydureon BCise, AstraZeneca) in 2021 for glycemic control in young patients with type 2 diabetes, both of which are also GLP-1 agonists.
AWARD-PEDS showed that youth with type 2 diabetes and obesity treated with or without metformin or basal insulin who received weekly injections of 0.75 mg or 1.5 mg of dulaglutide had lower hemoglobin A1c at 26 weeks than patients who received placebo.
Eli Lilly is now submitting these trial results to the FDA for this indication.
Dulaglutide was cleared for use in adults with type 2 diabetes in the United States in 2014 and was additionally approved for reducing the risk of major adverse cardiovascular events (MACE) in adults with type 2 diabetes at high risk of such events in 2020.
The most common adverse symptoms were gastrointestinal, and the safety profile was consistent with that in adults. However, the drug had no effect on body mass index.
The study was simultaneously published in the New England Journal of Medicine and presented as a late-breaking poster at the annual scientific sessions of the American Diabetes Association in New Orleans.
Might dulaglutide target pathophysiologic impairments in youth?
Dulaglutide would “offer a new treatment that targets the pathophysiologic impairments of type 2 diabetes in youth,” Silva A. Arslanian, MD, lead investigator, told this news organization.
Exenatide is also given as a weekly injection but is associated with a smaller decrease in A1c and does not improve fasting glucose concentrations, plus it requires more steps compared with the dulaglutide single-use pen, said Dr. Arslanian, who is scientific director at the Center for Pediatric Research in Obesity & Metabolism, UPMC Children’s Hospital of Pittsburgh.
“Liraglutide is a daily injection, and I believe most patients, particularly adolescents, would prefer a weekly injection,” she added.
Invited to comment, Elvira Isganaitis, MD, MPH, said “the significance of this paper lies in the fact that options for treating type 2 diabetes in children are currently much more limited than in adults – which is a major problem given recent studies that show that type 2 diabetes in youth is much more aggressive and more likely to cause complications early in the disease course.”
Dr. Isganaitis was not involved with the trial but is an investigator for the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study.
“With supply chain shortages and health insurance coverage issues that are common in the U.S., it would be helpful to have more than one FDA-approved option for a weekly GLP-1 receptor agonist in children [and] access to other classes of medications,” added Dr. Isganaitis, a pediatric endocrinologist at the Joslin Diabetes Center, Boston.
Phase 3 trials of sodium-glucose cotransporter 2 (SGLT2) inhibitors and dipeptidyl peptidase-4 (DPP-4) inhibitors in youth with type 2 diabetes are also ongoing, Dr. Arslanian noted, “but as always, recruitment is slow with adolescents.”
“I am not optimistic that DPP4 inhibitors will have a place in the treatment of youth with type 2 diabetes,” she said. A recent study showed the addition of sitagliptin to metformin in youth with type 2 diabetes did not provide durable improvement in glycemic control.
Potentially promising therapy
In their published article, Dr. Arslanian and colleagues write that “considering the progressive increase in [A1c] over time that was observed in the TODAY trial, with 34% of youths having [an A1c] of at least 10% after up to 15 years of follow-up, we believe that the effects of dulaglutide therapy appear to be potentially promising.”
The TODAY trial showed that more than 50% of youth with type 2 diabetes taking metformin failed to maintain glycemic control within a median of 11.5 months, Dr. Arslanian elaborated, and over time their A1c escalated while their beta-cell function deteriorated rapidly, and complications progressed quickly.
“Therefore,” she noted, “considering that dulaglutide and the GLP-1 receptor agonist class of drugs improve A1c, improve beta-cell function, suppress glucagon concentrations, and improve insulin sensitivity, dulaglutide would provide a promising new treatment option for youth with type 2 diabetes.”
Phase 3 superiority trial
The AWARD-PEDS trial included 154 youth with type 2 diabetes and a BMI greater than the 85th percentile for their age and sex at 46 centers in nine countries. Researchers randomized participants 1:1:1 to the two doses of dulaglutide or placebo for 26 weeks, followed by a 26-week open-label study (during which the placebo group received 0.75 mg dulaglutide) and a 4-week safety extension.
Participants were a mean age of 14.5 years and had a mean BMI of 34 kg/m2.
In each of the dulaglutide groups, roughly 66% of patients were female and 58% were White, 18% were Black, and about 57% were Hispanic. They had a mean weight of 91 kg (200 lb) and a mean A1c of about 8%; 62% were taking metformin only, 27% were taking metformin plus basal insulin, 3% were taking basal insulin only, and 10% were on diet and exercise only.
At 26 weeks, mean A1c increased by 0.6% in the placebo group but decreased by 0.6% in the 0.75-mg dulaglutide group and by 0.9% in the 1.5-mg dulaglutide group (P < .001 for both comparisons versus placebo).
Also at 26 weeks, more participants in the pooled dulaglutide groups than in the placebo group had an A1c <7.0% (51% vs. 14%; P < .001).
Fasting glucose concentration increased in the placebo group (+17.1 mg/dL ) and decreased in the pooled dulaglutide groups (–18.9 mg/dL; P < .001).
There were no group differences in BMI or adiposity-related parameters even at 52 weeks.
“I believe adolescents may be somewhat resistant to the weight-reducing effects of GLP-1 agonists in diabetes trials (liraglutide and exenatide youth type 2 diabetes trials showed the same thing) and they may need higher doses,” Dr. Arslanian speculated.
“Only future studies will be able to address this issue,” she concluded.
The study was funded by Eli Lilly. Dr. Arslanian has disclosed being a consultant for Eli Lilly, Novo Nordisk, and Rhythm Pharmaceuticals; participating in data safety monitoring for AstraZeneca and Eli Lilly trials; and receiving institutional research funding from Eli Lilly and Novo Nordisk. Dr. Isganaitis has disclosed receiving research funding (paid to her institution) from Dexcom and AstraZeneca.
A version of this article first appeared on Medscape.com.
Another glucagonlike peptide-1 (GLP1) agonist, dulaglutide (Trulicity, Lilly), is poised to be a new option for glycemic control in youth aged 10-18 years with type 2 diabetes, given as a weekly injection, based on the AWARD-PEDS clinical trial.
The U.S. Food and Drug Administration has already approved daily injection liraglutide (Victoza, Novo Nordisk) in 2019 and weekly exenatide (Bydureon/Bydureon BCise, AstraZeneca) in 2021 for glycemic control in young patients with type 2 diabetes, both of which are also GLP-1 agonists.
AWARD-PEDS showed that youth with type 2 diabetes and obesity treated with or without metformin or basal insulin who received weekly injections of 0.75 mg or 1.5 mg of dulaglutide had lower hemoglobin A1c at 26 weeks than patients who received placebo.
Eli Lilly is now submitting these trial results to the FDA for this indication.
Dulaglutide was cleared for use in adults with type 2 diabetes in the United States in 2014 and was additionally approved for reducing the risk of major adverse cardiovascular events (MACE) in adults with type 2 diabetes at high risk of such events in 2020.
The most common adverse symptoms were gastrointestinal, and the safety profile was consistent with that in adults. However, the drug had no effect on body mass index.
The study was simultaneously published in the New England Journal of Medicine and presented as a late-breaking poster at the annual scientific sessions of the American Diabetes Association in New Orleans.
Might dulaglutide target pathophysiologic impairments in youth?
Dulaglutide would “offer a new treatment that targets the pathophysiologic impairments of type 2 diabetes in youth,” Silva A. Arslanian, MD, lead investigator, told this news organization.
Exenatide is also given as a weekly injection but is associated with a smaller decrease in A1c and does not improve fasting glucose concentrations, plus it requires more steps compared with the dulaglutide single-use pen, said Dr. Arslanian, who is scientific director at the Center for Pediatric Research in Obesity & Metabolism, UPMC Children’s Hospital of Pittsburgh.
“Liraglutide is a daily injection, and I believe most patients, particularly adolescents, would prefer a weekly injection,” she added.
Invited to comment, Elvira Isganaitis, MD, MPH, said “the significance of this paper lies in the fact that options for treating type 2 diabetes in children are currently much more limited than in adults – which is a major problem given recent studies that show that type 2 diabetes in youth is much more aggressive and more likely to cause complications early in the disease course.”
Dr. Isganaitis was not involved with the trial but is an investigator for the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study.
“With supply chain shortages and health insurance coverage issues that are common in the U.S., it would be helpful to have more than one FDA-approved option for a weekly GLP-1 receptor agonist in children [and] access to other classes of medications,” added Dr. Isganaitis, a pediatric endocrinologist at the Joslin Diabetes Center, Boston.
Phase 3 trials of sodium-glucose cotransporter 2 (SGLT2) inhibitors and dipeptidyl peptidase-4 (DPP-4) inhibitors in youth with type 2 diabetes are also ongoing, Dr. Arslanian noted, “but as always, recruitment is slow with adolescents.”
“I am not optimistic that DPP4 inhibitors will have a place in the treatment of youth with type 2 diabetes,” she said. A recent study showed the addition of sitagliptin to metformin in youth with type 2 diabetes did not provide durable improvement in glycemic control.
Potentially promising therapy
In their published article, Dr. Arslanian and colleagues write that “considering the progressive increase in [A1c] over time that was observed in the TODAY trial, with 34% of youths having [an A1c] of at least 10% after up to 15 years of follow-up, we believe that the effects of dulaglutide therapy appear to be potentially promising.”
The TODAY trial showed that more than 50% of youth with type 2 diabetes taking metformin failed to maintain glycemic control within a median of 11.5 months, Dr. Arslanian elaborated, and over time their A1c escalated while their beta-cell function deteriorated rapidly, and complications progressed quickly.
“Therefore,” she noted, “considering that dulaglutide and the GLP-1 receptor agonist class of drugs improve A1c, improve beta-cell function, suppress glucagon concentrations, and improve insulin sensitivity, dulaglutide would provide a promising new treatment option for youth with type 2 diabetes.”
Phase 3 superiority trial
The AWARD-PEDS trial included 154 youth with type 2 diabetes and a BMI greater than the 85th percentile for their age and sex at 46 centers in nine countries. Researchers randomized participants 1:1:1 to the two doses of dulaglutide or placebo for 26 weeks, followed by a 26-week open-label study (during which the placebo group received 0.75 mg dulaglutide) and a 4-week safety extension.
Participants were a mean age of 14.5 years and had a mean BMI of 34 kg/m2.
In each of the dulaglutide groups, roughly 66% of patients were female and 58% were White, 18% were Black, and about 57% were Hispanic. They had a mean weight of 91 kg (200 lb) and a mean A1c of about 8%; 62% were taking metformin only, 27% were taking metformin plus basal insulin, 3% were taking basal insulin only, and 10% were on diet and exercise only.
At 26 weeks, mean A1c increased by 0.6% in the placebo group but decreased by 0.6% in the 0.75-mg dulaglutide group and by 0.9% in the 1.5-mg dulaglutide group (P < .001 for both comparisons versus placebo).
Also at 26 weeks, more participants in the pooled dulaglutide groups than in the placebo group had an A1c <7.0% (51% vs. 14%; P < .001).
Fasting glucose concentration increased in the placebo group (+17.1 mg/dL ) and decreased in the pooled dulaglutide groups (–18.9 mg/dL; P < .001).
There were no group differences in BMI or adiposity-related parameters even at 52 weeks.
“I believe adolescents may be somewhat resistant to the weight-reducing effects of GLP-1 agonists in diabetes trials (liraglutide and exenatide youth type 2 diabetes trials showed the same thing) and they may need higher doses,” Dr. Arslanian speculated.
“Only future studies will be able to address this issue,” she concluded.
The study was funded by Eli Lilly. Dr. Arslanian has disclosed being a consultant for Eli Lilly, Novo Nordisk, and Rhythm Pharmaceuticals; participating in data safety monitoring for AstraZeneca and Eli Lilly trials; and receiving institutional research funding from Eli Lilly and Novo Nordisk. Dr. Isganaitis has disclosed receiving research funding (paid to her institution) from Dexcom and AstraZeneca.
A version of this article first appeared on Medscape.com.
FROM ADA 2022
Tirzepatide powers ‘unprecedented’ weight loss in SURMOUNT-1
NEW ORLEANS – Treatment of people with obesity but no diabetes with the dual–incretin agonist tirzepatide safely produced “unprecedented” levels of weight loss in the vast majority of patients in SURMOUNT-1, a placebo-controlled trial with more than 2,500 people with obesity or overweight plus at least one weight-related complication.
Although the pivotal trial did not directly compare weekly subcutaneous injection with the twincretin tirzepatide (at 5 mg, 10 mg, or 15 mg) with either bariatric surgery or what has been the reigning champ of weight-loss agents, a 2.4-mg/week injection of semaglutide (Wegovy), the new findings are impressive because they eclipsed semaglutide’s past performance in at least three important ways, said Ania M. Jastreboff, MD, PhD, SURMOUNT-1’s lead investigator, at the annual scientific sessions of the American Diabetes Association.
First, the highest-tested dosage of tirzepatide, 15 mg/week, for 72 weeks, produced a 5% or greater loss in baseline weight in 91%-96% of patients, an effect “not previously seen” in any prior phase 3 trial of a weight-loss agent, noted Dr. Jastreboff, an endocrinologist and director of Weight Management & Obesity Prevention at Yale University in New Haven, Conn.
Second, the average level of weight loss among the 630 people who received 15 mg/week was 22.5% in the on-treatment analysis, and 20.9% in the intention-to-treat analysis, again a magnitude of effect never before seen with any other medical intervention.
And in an exploratory analysis, 40% of people who received the highest-tested tirzepatide dose of 15 mg/week had at least a 25% loss in baseline weight in the on-treatment analysis, another example of unprecedented weight-loss achievement, said Dr. Jastreboff.
Looking at the data another way, the average baseline weight of those in the trial was 104 kg (230 lb) at the start, and the average weight loss was between 35 and 52 lbs by 72 weeks on treatment, Dr. Jastreboff said in a press conference.
She noted, however, that not everyone will respond to tirzepatide, “but if you do respond to this medicine, you will feel full earlier, you won’t want to go back for seconds, and you may eat smaller amounts more often.”
Such weight-loss agents will need to be taken chronically, in the same way that medications are for hypertension or dyslipidemia, Dr. Jastreboff stressed. “If you stop the antiobesity medication then the body fat mass set point will go back up so this necessitates long-term treatment.”
A new era: Weight loss ‘in the range of bariatric surgery’
Tirzepatide, developed by Lilly, has recently been approved in the United States for the treatment of type 2 diabetes, under the brand name Mounjaro.
SURMOUNT-1 was designed to examine the effect of the agent in overweight/obesity, and the company will be filing for the additional indication of weight loss in the future. Top-line results of SURMOUNT-1 generated much excitement when Lilly reported them back in April, including a story in The New York Times.
Semaglutide, a Novo Nordisk drug, is approved in the United States for type 2 diabetes (as Ozempic at doses of either 1 mg or 2 mg per week) and also for weight loss, as Wegovy, at the higher dose of 2.4 mg per week. When Wegovy was given the green light by the Food and Drug Administration a year ago, it too was hailed as a “game changer” for obesity.
The weight-loss results seen in SURMOUNT-1 “put tirzepatide squarely in the range of weight loss achieved with bariatric surgery,” concluded Louis J. Aronne, MD, a coinvestigator on the trial, professor at Weill-Cornell Medicine in New York, and director of the Center for Weight Management and Metabolic Clinical Research of Weill-Cornell.
The results are “amazing,” and propel the weight-loss field into “a new era of obesity treatment,” commented Lee M. Kaplan, MD, who was not involved in the study and served as designated discussant for the trial.
Despite the lack of direct comparison, the findings indicate that “tirzepatide causes more weight loss than semaglutide,” and it provides “an opportunity to meet or exceed” the weight-loss effects of bariatric surgery, added Dr. Kaplan, director of the Obesity, Metabolism and Nutrition Institute at Massachusetts General Hospital in Boston.
Simultaneously with Dr. Jastreboff’s report at the meeting, the results were published online in The New England Journal of Medicine.
An accompanying editorial agrees with Dr. Kaplan: “It is remarkable that the magnitude of weight loss with tirzepatide was similar to that with gastric bypass, which raises the potential for alternative medical approaches to the treatment of obesity.”
“The tides are shifting, and there are now more options for people with obesity to lose weight,” write Clifford J. Rosen, MD, of Tufts University, Boston, and Julie R. Ingelfinger, MD, of Harvard University and Massachusetts General Hospital, Boston.
Dual incretin agonism ‘enhances activity,’ says expert
Tirzepatide is the first agent on the U.S. market from a novel class of dual-incretin agonists, with a molecular structure engineered to activate both the glucagonlike protein-1 (GLP-1) receptor and the glucose-dependent insulinotropic polypeptide (GIP), the two predominant incretins in the human gut. This combined activity has led to the twincretin nickname for tirzepatide.
Semaglutide is a single-incretin agonist, with its activity focused exclusively on the GLP-1 receptor.
Dr. Aronne tied the apparently superior efficacy of tirzepatide relative to semaglutide directly to the added incretin activity of tirzepatide. “The dual approach enhances efficacy,” he proposed during his presentation at the meeting.
The impressive efficacy and reassuring safety profile reported from SURMOUNT-1 opens the door to a new approach to treating obesity, which in the past has often taken a back seat to treatments for dyslipidemia, hypertension, and diabetes.
“Now that we can treat obesity safely and effectively, it makes sense to treat obesity first,” Dr. Aronne recommended.
Dr. Jastreboff agreed: “Perhaps we can prevent diabetes by treating obesity head-on,” she remarked.
Weight-loss agents gain U.S. traction
There have been concerns about patient access to these newer weight-loss drugs in the United States, given that the retail cost of semaglutide for obesity exceeds $1,000/month, but Dr. Aronne reported data that painted a more optimistic picture.
His numbers showed that during the first months that semaglutide was on the U.S. market as a weight-loss agent, the number of U.S. prescriptions written for branded antiobesity medications roughly doubled, a spike that seemed mostly driven by the introduction and growing use of semaglutide.
With tirzepatide, every prespecified cardiometabolic parameter assessed in the trial showed clinically meaningful improvements, reported Dr. Jastreboff, including an average 17% reduction in waist circumference in patients on either of the highest two dosages, a 34% average drop in total fat mass, an average 0.5–percentage point cut in baseline hemoglobin A1c at the highest two dosages, substantial cuts in fasting plasma glucose and fasting insulin levels, an average 28% drop in triglyceride levels, and an average systolic blood pressure reduction of about 8 mm Hg that occurred within 24 weeks on treatment.
“I think that insurers will sign up” for tirzepatide coverage based on benefits like this, Dr. Aronne predicted.
SURMOUNT-1 randomized 2,539 patients with obesity or with overweight plus at least one weight-related complication at any of 119 sites in nine countries. They had a body mass index of 30 kg/m2 or more, or 27 kg/m2 or more and at least one weight-related complication, excluding diabetes. They were randomized in a 1:1:1:1 ratio to receive once-weekly, subcutaneous tirzepatide (5 mg, 10 mg, or 15 mg) or placebo for 72 weeks, including a 20-week dose-escalation period.
The study’s two primary endpoints were the average percentage change in body weight from entry to 72 weeks, and the percentage of participants reaching at least a 5% reduction in their baseline body weight by 72 weeks.
The most common adverse events with tirzepatide were gastrointestinal, and most were mild to moderate in severity, occurring primarily during dose escalation. Adverse events caused treatment discontinuation in 4.3%, 7.1%, 6.2%, and 2.6% of participants receiving 5-mg, 10-mg, and 15-mg tirzepatide doses and placebo, respectively
The trial ran from December 2019 to April 2022, so during the peak of the COVID-19 pandemic, which Dr. Jastreboff described as an “amazing feat.”
Jamy Ard, MD, who chaired the SURMOUNT-1 session quipped, after hearing the results, “Wow; that’s exciting. If you’re not excited by the results, you’d better check your pulse.”
Dr. Ard is a professor at Wake Forest University, Winston-Salem, N.C., and codirector of the Wake Forest Baptist Health Weight Management Center in Winston-Salem.
SURMOUNT-1 was sponsored by Eli Lilly, the company that markets tirzepatide (Mounjaro). Dr. Jastreboff has been an advisor or consultant to Eli Lilly, as well as to Boehringer Ingelheim, Intellihealth, Novo Nordisk, Pfizer, Rhythm Pharmaceuticals, Scholar Rock, and Weight Watchers, and she has received research funding from Eli Lilly and Novo Nordisk. Dr. Aronne has been a consultant or advisor to, speaker on behalf of, or received research funding from Eli Lilly as well as from Altimmune, Amgen, Allurion, Intellihealth, Janssen, Novo Nordisk, Pfizer, and United Health group; he has an ownership interest in ERX, Gelesis, and Intellihealth; and he serves on the board of ERX, Jamieson Wellness, and Intellihealth. Dr. Kaplan has been a consultant to Eli Lilly, as well as to Amgen, Boehringer Ingelheim, Gelesis, Gilead, Novo Nordisk, Optum Health, Pfizer, Rhythm Pharmaceuticals, the Obesity and Nutrition Institute, and Xeno Biosciences. Dr. Ard has been a consultant to Eli Lilly, as well as to Nestle Health Sciences and Novo Nordisk, and he has received research funding from Boehringer Ingelheim, Epitomee, Medical, and United Health Group.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – Treatment of people with obesity but no diabetes with the dual–incretin agonist tirzepatide safely produced “unprecedented” levels of weight loss in the vast majority of patients in SURMOUNT-1, a placebo-controlled trial with more than 2,500 people with obesity or overweight plus at least one weight-related complication.
Although the pivotal trial did not directly compare weekly subcutaneous injection with the twincretin tirzepatide (at 5 mg, 10 mg, or 15 mg) with either bariatric surgery or what has been the reigning champ of weight-loss agents, a 2.4-mg/week injection of semaglutide (Wegovy), the new findings are impressive because they eclipsed semaglutide’s past performance in at least three important ways, said Ania M. Jastreboff, MD, PhD, SURMOUNT-1’s lead investigator, at the annual scientific sessions of the American Diabetes Association.
First, the highest-tested dosage of tirzepatide, 15 mg/week, for 72 weeks, produced a 5% or greater loss in baseline weight in 91%-96% of patients, an effect “not previously seen” in any prior phase 3 trial of a weight-loss agent, noted Dr. Jastreboff, an endocrinologist and director of Weight Management & Obesity Prevention at Yale University in New Haven, Conn.
Second, the average level of weight loss among the 630 people who received 15 mg/week was 22.5% in the on-treatment analysis, and 20.9% in the intention-to-treat analysis, again a magnitude of effect never before seen with any other medical intervention.
And in an exploratory analysis, 40% of people who received the highest-tested tirzepatide dose of 15 mg/week had at least a 25% loss in baseline weight in the on-treatment analysis, another example of unprecedented weight-loss achievement, said Dr. Jastreboff.
Looking at the data another way, the average baseline weight of those in the trial was 104 kg (230 lb) at the start, and the average weight loss was between 35 and 52 lbs by 72 weeks on treatment, Dr. Jastreboff said in a press conference.
She noted, however, that not everyone will respond to tirzepatide, “but if you do respond to this medicine, you will feel full earlier, you won’t want to go back for seconds, and you may eat smaller amounts more often.”
Such weight-loss agents will need to be taken chronically, in the same way that medications are for hypertension or dyslipidemia, Dr. Jastreboff stressed. “If you stop the antiobesity medication then the body fat mass set point will go back up so this necessitates long-term treatment.”
A new era: Weight loss ‘in the range of bariatric surgery’
Tirzepatide, developed by Lilly, has recently been approved in the United States for the treatment of type 2 diabetes, under the brand name Mounjaro.
SURMOUNT-1 was designed to examine the effect of the agent in overweight/obesity, and the company will be filing for the additional indication of weight loss in the future. Top-line results of SURMOUNT-1 generated much excitement when Lilly reported them back in April, including a story in The New York Times.
Semaglutide, a Novo Nordisk drug, is approved in the United States for type 2 diabetes (as Ozempic at doses of either 1 mg or 2 mg per week) and also for weight loss, as Wegovy, at the higher dose of 2.4 mg per week. When Wegovy was given the green light by the Food and Drug Administration a year ago, it too was hailed as a “game changer” for obesity.
The weight-loss results seen in SURMOUNT-1 “put tirzepatide squarely in the range of weight loss achieved with bariatric surgery,” concluded Louis J. Aronne, MD, a coinvestigator on the trial, professor at Weill-Cornell Medicine in New York, and director of the Center for Weight Management and Metabolic Clinical Research of Weill-Cornell.
The results are “amazing,” and propel the weight-loss field into “a new era of obesity treatment,” commented Lee M. Kaplan, MD, who was not involved in the study and served as designated discussant for the trial.
Despite the lack of direct comparison, the findings indicate that “tirzepatide causes more weight loss than semaglutide,” and it provides “an opportunity to meet or exceed” the weight-loss effects of bariatric surgery, added Dr. Kaplan, director of the Obesity, Metabolism and Nutrition Institute at Massachusetts General Hospital in Boston.
Simultaneously with Dr. Jastreboff’s report at the meeting, the results were published online in The New England Journal of Medicine.
An accompanying editorial agrees with Dr. Kaplan: “It is remarkable that the magnitude of weight loss with tirzepatide was similar to that with gastric bypass, which raises the potential for alternative medical approaches to the treatment of obesity.”
“The tides are shifting, and there are now more options for people with obesity to lose weight,” write Clifford J. Rosen, MD, of Tufts University, Boston, and Julie R. Ingelfinger, MD, of Harvard University and Massachusetts General Hospital, Boston.
Dual incretin agonism ‘enhances activity,’ says expert
Tirzepatide is the first agent on the U.S. market from a novel class of dual-incretin agonists, with a molecular structure engineered to activate both the glucagonlike protein-1 (GLP-1) receptor and the glucose-dependent insulinotropic polypeptide (GIP), the two predominant incretins in the human gut. This combined activity has led to the twincretin nickname for tirzepatide.
Semaglutide is a single-incretin agonist, with its activity focused exclusively on the GLP-1 receptor.
Dr. Aronne tied the apparently superior efficacy of tirzepatide relative to semaglutide directly to the added incretin activity of tirzepatide. “The dual approach enhances efficacy,” he proposed during his presentation at the meeting.
The impressive efficacy and reassuring safety profile reported from SURMOUNT-1 opens the door to a new approach to treating obesity, which in the past has often taken a back seat to treatments for dyslipidemia, hypertension, and diabetes.
“Now that we can treat obesity safely and effectively, it makes sense to treat obesity first,” Dr. Aronne recommended.
Dr. Jastreboff agreed: “Perhaps we can prevent diabetes by treating obesity head-on,” she remarked.
Weight-loss agents gain U.S. traction
There have been concerns about patient access to these newer weight-loss drugs in the United States, given that the retail cost of semaglutide for obesity exceeds $1,000/month, but Dr. Aronne reported data that painted a more optimistic picture.
His numbers showed that during the first months that semaglutide was on the U.S. market as a weight-loss agent, the number of U.S. prescriptions written for branded antiobesity medications roughly doubled, a spike that seemed mostly driven by the introduction and growing use of semaglutide.
With tirzepatide, every prespecified cardiometabolic parameter assessed in the trial showed clinically meaningful improvements, reported Dr. Jastreboff, including an average 17% reduction in waist circumference in patients on either of the highest two dosages, a 34% average drop in total fat mass, an average 0.5–percentage point cut in baseline hemoglobin A1c at the highest two dosages, substantial cuts in fasting plasma glucose and fasting insulin levels, an average 28% drop in triglyceride levels, and an average systolic blood pressure reduction of about 8 mm Hg that occurred within 24 weeks on treatment.
“I think that insurers will sign up” for tirzepatide coverage based on benefits like this, Dr. Aronne predicted.
SURMOUNT-1 randomized 2,539 patients with obesity or with overweight plus at least one weight-related complication at any of 119 sites in nine countries. They had a body mass index of 30 kg/m2 or more, or 27 kg/m2 or more and at least one weight-related complication, excluding diabetes. They were randomized in a 1:1:1:1 ratio to receive once-weekly, subcutaneous tirzepatide (5 mg, 10 mg, or 15 mg) or placebo for 72 weeks, including a 20-week dose-escalation period.
The study’s two primary endpoints were the average percentage change in body weight from entry to 72 weeks, and the percentage of participants reaching at least a 5% reduction in their baseline body weight by 72 weeks.
The most common adverse events with tirzepatide were gastrointestinal, and most were mild to moderate in severity, occurring primarily during dose escalation. Adverse events caused treatment discontinuation in 4.3%, 7.1%, 6.2%, and 2.6% of participants receiving 5-mg, 10-mg, and 15-mg tirzepatide doses and placebo, respectively
The trial ran from December 2019 to April 2022, so during the peak of the COVID-19 pandemic, which Dr. Jastreboff described as an “amazing feat.”
Jamy Ard, MD, who chaired the SURMOUNT-1 session quipped, after hearing the results, “Wow; that’s exciting. If you’re not excited by the results, you’d better check your pulse.”
Dr. Ard is a professor at Wake Forest University, Winston-Salem, N.C., and codirector of the Wake Forest Baptist Health Weight Management Center in Winston-Salem.
SURMOUNT-1 was sponsored by Eli Lilly, the company that markets tirzepatide (Mounjaro). Dr. Jastreboff has been an advisor or consultant to Eli Lilly, as well as to Boehringer Ingelheim, Intellihealth, Novo Nordisk, Pfizer, Rhythm Pharmaceuticals, Scholar Rock, and Weight Watchers, and she has received research funding from Eli Lilly and Novo Nordisk. Dr. Aronne has been a consultant or advisor to, speaker on behalf of, or received research funding from Eli Lilly as well as from Altimmune, Amgen, Allurion, Intellihealth, Janssen, Novo Nordisk, Pfizer, and United Health group; he has an ownership interest in ERX, Gelesis, and Intellihealth; and he serves on the board of ERX, Jamieson Wellness, and Intellihealth. Dr. Kaplan has been a consultant to Eli Lilly, as well as to Amgen, Boehringer Ingelheim, Gelesis, Gilead, Novo Nordisk, Optum Health, Pfizer, Rhythm Pharmaceuticals, the Obesity and Nutrition Institute, and Xeno Biosciences. Dr. Ard has been a consultant to Eli Lilly, as well as to Nestle Health Sciences and Novo Nordisk, and he has received research funding from Boehringer Ingelheim, Epitomee, Medical, and United Health Group.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – Treatment of people with obesity but no diabetes with the dual–incretin agonist tirzepatide safely produced “unprecedented” levels of weight loss in the vast majority of patients in SURMOUNT-1, a placebo-controlled trial with more than 2,500 people with obesity or overweight plus at least one weight-related complication.
Although the pivotal trial did not directly compare weekly subcutaneous injection with the twincretin tirzepatide (at 5 mg, 10 mg, or 15 mg) with either bariatric surgery or what has been the reigning champ of weight-loss agents, a 2.4-mg/week injection of semaglutide (Wegovy), the new findings are impressive because they eclipsed semaglutide’s past performance in at least three important ways, said Ania M. Jastreboff, MD, PhD, SURMOUNT-1’s lead investigator, at the annual scientific sessions of the American Diabetes Association.
First, the highest-tested dosage of tirzepatide, 15 mg/week, for 72 weeks, produced a 5% or greater loss in baseline weight in 91%-96% of patients, an effect “not previously seen” in any prior phase 3 trial of a weight-loss agent, noted Dr. Jastreboff, an endocrinologist and director of Weight Management & Obesity Prevention at Yale University in New Haven, Conn.
Second, the average level of weight loss among the 630 people who received 15 mg/week was 22.5% in the on-treatment analysis, and 20.9% in the intention-to-treat analysis, again a magnitude of effect never before seen with any other medical intervention.
And in an exploratory analysis, 40% of people who received the highest-tested tirzepatide dose of 15 mg/week had at least a 25% loss in baseline weight in the on-treatment analysis, another example of unprecedented weight-loss achievement, said Dr. Jastreboff.
Looking at the data another way, the average baseline weight of those in the trial was 104 kg (230 lb) at the start, and the average weight loss was between 35 and 52 lbs by 72 weeks on treatment, Dr. Jastreboff said in a press conference.
She noted, however, that not everyone will respond to tirzepatide, “but if you do respond to this medicine, you will feel full earlier, you won’t want to go back for seconds, and you may eat smaller amounts more often.”
Such weight-loss agents will need to be taken chronically, in the same way that medications are for hypertension or dyslipidemia, Dr. Jastreboff stressed. “If you stop the antiobesity medication then the body fat mass set point will go back up so this necessitates long-term treatment.”
A new era: Weight loss ‘in the range of bariatric surgery’
Tirzepatide, developed by Lilly, has recently been approved in the United States for the treatment of type 2 diabetes, under the brand name Mounjaro.
SURMOUNT-1 was designed to examine the effect of the agent in overweight/obesity, and the company will be filing for the additional indication of weight loss in the future. Top-line results of SURMOUNT-1 generated much excitement when Lilly reported them back in April, including a story in The New York Times.
Semaglutide, a Novo Nordisk drug, is approved in the United States for type 2 diabetes (as Ozempic at doses of either 1 mg or 2 mg per week) and also for weight loss, as Wegovy, at the higher dose of 2.4 mg per week. When Wegovy was given the green light by the Food and Drug Administration a year ago, it too was hailed as a “game changer” for obesity.
The weight-loss results seen in SURMOUNT-1 “put tirzepatide squarely in the range of weight loss achieved with bariatric surgery,” concluded Louis J. Aronne, MD, a coinvestigator on the trial, professor at Weill-Cornell Medicine in New York, and director of the Center for Weight Management and Metabolic Clinical Research of Weill-Cornell.
The results are “amazing,” and propel the weight-loss field into “a new era of obesity treatment,” commented Lee M. Kaplan, MD, who was not involved in the study and served as designated discussant for the trial.
Despite the lack of direct comparison, the findings indicate that “tirzepatide causes more weight loss than semaglutide,” and it provides “an opportunity to meet or exceed” the weight-loss effects of bariatric surgery, added Dr. Kaplan, director of the Obesity, Metabolism and Nutrition Institute at Massachusetts General Hospital in Boston.
Simultaneously with Dr. Jastreboff’s report at the meeting, the results were published online in The New England Journal of Medicine.
An accompanying editorial agrees with Dr. Kaplan: “It is remarkable that the magnitude of weight loss with tirzepatide was similar to that with gastric bypass, which raises the potential for alternative medical approaches to the treatment of obesity.”
“The tides are shifting, and there are now more options for people with obesity to lose weight,” write Clifford J. Rosen, MD, of Tufts University, Boston, and Julie R. Ingelfinger, MD, of Harvard University and Massachusetts General Hospital, Boston.
Dual incretin agonism ‘enhances activity,’ says expert
Tirzepatide is the first agent on the U.S. market from a novel class of dual-incretin agonists, with a molecular structure engineered to activate both the glucagonlike protein-1 (GLP-1) receptor and the glucose-dependent insulinotropic polypeptide (GIP), the two predominant incretins in the human gut. This combined activity has led to the twincretin nickname for tirzepatide.
Semaglutide is a single-incretin agonist, with its activity focused exclusively on the GLP-1 receptor.
Dr. Aronne tied the apparently superior efficacy of tirzepatide relative to semaglutide directly to the added incretin activity of tirzepatide. “The dual approach enhances efficacy,” he proposed during his presentation at the meeting.
The impressive efficacy and reassuring safety profile reported from SURMOUNT-1 opens the door to a new approach to treating obesity, which in the past has often taken a back seat to treatments for dyslipidemia, hypertension, and diabetes.
“Now that we can treat obesity safely and effectively, it makes sense to treat obesity first,” Dr. Aronne recommended.
Dr. Jastreboff agreed: “Perhaps we can prevent diabetes by treating obesity head-on,” she remarked.
Weight-loss agents gain U.S. traction
There have been concerns about patient access to these newer weight-loss drugs in the United States, given that the retail cost of semaglutide for obesity exceeds $1,000/month, but Dr. Aronne reported data that painted a more optimistic picture.
His numbers showed that during the first months that semaglutide was on the U.S. market as a weight-loss agent, the number of U.S. prescriptions written for branded antiobesity medications roughly doubled, a spike that seemed mostly driven by the introduction and growing use of semaglutide.
With tirzepatide, every prespecified cardiometabolic parameter assessed in the trial showed clinically meaningful improvements, reported Dr. Jastreboff, including an average 17% reduction in waist circumference in patients on either of the highest two dosages, a 34% average drop in total fat mass, an average 0.5–percentage point cut in baseline hemoglobin A1c at the highest two dosages, substantial cuts in fasting plasma glucose and fasting insulin levels, an average 28% drop in triglyceride levels, and an average systolic blood pressure reduction of about 8 mm Hg that occurred within 24 weeks on treatment.
“I think that insurers will sign up” for tirzepatide coverage based on benefits like this, Dr. Aronne predicted.
SURMOUNT-1 randomized 2,539 patients with obesity or with overweight plus at least one weight-related complication at any of 119 sites in nine countries. They had a body mass index of 30 kg/m2 or more, or 27 kg/m2 or more and at least one weight-related complication, excluding diabetes. They were randomized in a 1:1:1:1 ratio to receive once-weekly, subcutaneous tirzepatide (5 mg, 10 mg, or 15 mg) or placebo for 72 weeks, including a 20-week dose-escalation period.
The study’s two primary endpoints were the average percentage change in body weight from entry to 72 weeks, and the percentage of participants reaching at least a 5% reduction in their baseline body weight by 72 weeks.
The most common adverse events with tirzepatide were gastrointestinal, and most were mild to moderate in severity, occurring primarily during dose escalation. Adverse events caused treatment discontinuation in 4.3%, 7.1%, 6.2%, and 2.6% of participants receiving 5-mg, 10-mg, and 15-mg tirzepatide doses and placebo, respectively
The trial ran from December 2019 to April 2022, so during the peak of the COVID-19 pandemic, which Dr. Jastreboff described as an “amazing feat.”
Jamy Ard, MD, who chaired the SURMOUNT-1 session quipped, after hearing the results, “Wow; that’s exciting. If you’re not excited by the results, you’d better check your pulse.”
Dr. Ard is a professor at Wake Forest University, Winston-Salem, N.C., and codirector of the Wake Forest Baptist Health Weight Management Center in Winston-Salem.
SURMOUNT-1 was sponsored by Eli Lilly, the company that markets tirzepatide (Mounjaro). Dr. Jastreboff has been an advisor or consultant to Eli Lilly, as well as to Boehringer Ingelheim, Intellihealth, Novo Nordisk, Pfizer, Rhythm Pharmaceuticals, Scholar Rock, and Weight Watchers, and she has received research funding from Eli Lilly and Novo Nordisk. Dr. Aronne has been a consultant or advisor to, speaker on behalf of, or received research funding from Eli Lilly as well as from Altimmune, Amgen, Allurion, Intellihealth, Janssen, Novo Nordisk, Pfizer, and United Health group; he has an ownership interest in ERX, Gelesis, and Intellihealth; and he serves on the board of ERX, Jamieson Wellness, and Intellihealth. Dr. Kaplan has been a consultant to Eli Lilly, as well as to Amgen, Boehringer Ingelheim, Gelesis, Gilead, Novo Nordisk, Optum Health, Pfizer, Rhythm Pharmaceuticals, the Obesity and Nutrition Institute, and Xeno Biosciences. Dr. Ard has been a consultant to Eli Lilly, as well as to Nestle Health Sciences and Novo Nordisk, and he has received research funding from Boehringer Ingelheim, Epitomee, Medical, and United Health Group.
A version of this article first appeared on Medscape.com.
AT ADA 2022















