User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
More mask wearing could save 130,000 US lives by end of February
A cumulative 511,000 lives could be lost from COVID-19 in the United States by the end of February 2021, a new prediction study reveals.
However, if universal mask wearing is adopted — defined as 95% of Americans complying with the protective measure — along with social distancing mandates as warranted, nearly 130,000 of those lives could be saved.
And if even 85% of Americans comply, an additional 95,800 lives would be spared before March of next year, researchers at the University of Washington Institute for Health Metrics and Evaluation (IHME) report.
The study was published online October 23 in Nature Medicine.
“The study is sound and makes the case for mandatory mask policies,” said Arthur L. Caplan, PhD, a professor of bioethics at NYU Langone Health in New York City, who frequently provides commentary for Medscape.
Without mandatory mask requirements, he added, “we will see a pandemic slaughter and an overwhelmed healthcare system and workforce.”
The IHME team evaluated COVID-19 data for cases and related deaths between February 1 and September 21. Based on this data, they predicted the likely future of SARS-CoV-2 infections on a state level from September 22, 2020, to February 2021.
An Optimistic Projection
Lead author Robert C. Reiner Jr and colleagues looked at five scenarios. For example, they calculated likely deaths associated with COVID-19 if adoption of mask and social distancing recommendations were nearly universal. They note that Singapore achieved a 95% compliance rate with masks and used this as their “best-case scenario” model.
An estimated 129,574 (range, 85,284–170,867) additional lives could be saved if 95% of Americans wore masks in public, their research reveals. This optimistic scenario includes a “plausible reference” in which any US state reaching 8 COVID-19 deaths per 1 million residents would enact 6 weeks of social distancing mandates (SDMs).
Achieving this level of mask compliance in the United States “could be sufficient to ameliorate the worst effects of epidemic resurgences in many states,” the researchers note.
In contrast, the proportion of Americans wearing masks in public as of September 22 was 49%, according to IHME data.
Universal mask use unlikely
“I’m not a modeling expert, but it is an interesting, and as far as I can judge, well-conducted study which looks, state by state, at what might happen in various scenarios around masking policies going forward — and in particular the effect that mandated masking might have,” Trish Greenhalgh, MD, told Medscape Medical News.
“However, the scenario is a thought experiment. Near-universal mask use is not going to happen in the USA, nor indeed in any individual state, right now, given how emotive the issue has become,” added Greenhalgh, professor in the Nuffield Department of Primary Care Health Sciences at Oxford University, UK. She was not affiliated with the study.
“Hence, whilst I am broadly supportive of the science,” she said, “I’m not confident that this paper will be able to change policy.”
Other ‘What if?’ scenarios
The authors also predicted the mortality implications associated with lower adherence to masks, the presence or absence of SDMs, and what could happen if mandates continue to ease at their current rate.
For example, they considered a scenario with less-than-universal mask use in public, 85%, along with SDMs being reinstated based on the mortality rate threshold. In this instance, they found an additional 95,814 (range, 60,731–133,077) lives could be spared by February 28.
Another calculation looked at outcomes if 95% of Americans wore masks going forward without states instituting SDMs at any point. In this case, the researchers predict that 490,437 Americans would die from COVID-19 by February 2021.
A fourth analysis revealed what would happen without greater mask use if the mortality threshold triggered 6 weeks of SDMs as warranted. Under this ‘plausible reference’ calculation, a total 511,373 Americans would die from COVID-19 by the end of February.
A fifth scenario predicted potential mortality if states continue easing SDMs at the current pace. “This is an alternative scenario to the more probable situation where states are expected to respond to an impending health crisis by reinstating some SDMs,” the authors note. The predicted number of American deaths appears more dire in this calculation. The investigators predict cumulative total deaths could reach 1,053,206 (range, 759,693–1,452,397) by the end of February 2021.
The death toll would likely vary among states in this scenario. California, Florida, and Pennsylvania would like account for approximately one third of all deaths.
All the modeling scenarios considered other factors including pneumonia seasonality, mobility, testing rates, and mask use per capita.
“I have seen the IHME study and I agree with the broad conclusions,” Richard Stutt, PhD, of the Epidemiology and Modelling Group at the University of Cambridge, UK, told Medscape Medical News.
“Case numbers are climbing in the US, and without further intervention, there will be a significant number of deaths over the coming months,” he said.
Masks are low cost and widely available, Stutt said. “I am hopeful that even if masks are not widely adopted, we will not see as many deaths as predicted here, as these outbreaks can be significantly reduced by increased social distancing or lockdowns.”
“However this comes at a far higher economic cost than the use of masks, and still requires action,” added Stutt, who authored a study in June that modeled facemasks in combination with “lock-down” measures for managing the COVID-19 pandemic.
Modeling study results depend on the assumptions researchers make, and the IHME team rightly tested a number of different assumptions, Greenhalgh said.
“The key conclusion,” she added, “is here: ‘The implementation of SDMs as soon as individual states reach a threshold of 8 daily deaths per million could dramatically ameliorate the effects of the disease; achieving near-universal mask use could delay, or in many states, possibly prevent, this threshold from being reached and has the potential to save the most lives while minimizing damage to the economy.’ “
“This is a useful piece of information and I think is borne out by their data,” added Greenhalgh, lead author of an April study on face masks for the public during the pandemic.
You can visit the IHME website for the most current mortality projections.
Caplan, Greenhalgh, and Stutt have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
A cumulative 511,000 lives could be lost from COVID-19 in the United States by the end of February 2021, a new prediction study reveals.
However, if universal mask wearing is adopted — defined as 95% of Americans complying with the protective measure — along with social distancing mandates as warranted, nearly 130,000 of those lives could be saved.
And if even 85% of Americans comply, an additional 95,800 lives would be spared before March of next year, researchers at the University of Washington Institute for Health Metrics and Evaluation (IHME) report.
The study was published online October 23 in Nature Medicine.
“The study is sound and makes the case for mandatory mask policies,” said Arthur L. Caplan, PhD, a professor of bioethics at NYU Langone Health in New York City, who frequently provides commentary for Medscape.
Without mandatory mask requirements, he added, “we will see a pandemic slaughter and an overwhelmed healthcare system and workforce.”
The IHME team evaluated COVID-19 data for cases and related deaths between February 1 and September 21. Based on this data, they predicted the likely future of SARS-CoV-2 infections on a state level from September 22, 2020, to February 2021.
An Optimistic Projection
Lead author Robert C. Reiner Jr and colleagues looked at five scenarios. For example, they calculated likely deaths associated with COVID-19 if adoption of mask and social distancing recommendations were nearly universal. They note that Singapore achieved a 95% compliance rate with masks and used this as their “best-case scenario” model.
An estimated 129,574 (range, 85,284–170,867) additional lives could be saved if 95% of Americans wore masks in public, their research reveals. This optimistic scenario includes a “plausible reference” in which any US state reaching 8 COVID-19 deaths per 1 million residents would enact 6 weeks of social distancing mandates (SDMs).
Achieving this level of mask compliance in the United States “could be sufficient to ameliorate the worst effects of epidemic resurgences in many states,” the researchers note.
In contrast, the proportion of Americans wearing masks in public as of September 22 was 49%, according to IHME data.
Universal mask use unlikely
“I’m not a modeling expert, but it is an interesting, and as far as I can judge, well-conducted study which looks, state by state, at what might happen in various scenarios around masking policies going forward — and in particular the effect that mandated masking might have,” Trish Greenhalgh, MD, told Medscape Medical News.
“However, the scenario is a thought experiment. Near-universal mask use is not going to happen in the USA, nor indeed in any individual state, right now, given how emotive the issue has become,” added Greenhalgh, professor in the Nuffield Department of Primary Care Health Sciences at Oxford University, UK. She was not affiliated with the study.
“Hence, whilst I am broadly supportive of the science,” she said, “I’m not confident that this paper will be able to change policy.”
Other ‘What if?’ scenarios
The authors also predicted the mortality implications associated with lower adherence to masks, the presence or absence of SDMs, and what could happen if mandates continue to ease at their current rate.
For example, they considered a scenario with less-than-universal mask use in public, 85%, along with SDMs being reinstated based on the mortality rate threshold. In this instance, they found an additional 95,814 (range, 60,731–133,077) lives could be spared by February 28.
Another calculation looked at outcomes if 95% of Americans wore masks going forward without states instituting SDMs at any point. In this case, the researchers predict that 490,437 Americans would die from COVID-19 by February 2021.
A fourth analysis revealed what would happen without greater mask use if the mortality threshold triggered 6 weeks of SDMs as warranted. Under this ‘plausible reference’ calculation, a total 511,373 Americans would die from COVID-19 by the end of February.
A fifth scenario predicted potential mortality if states continue easing SDMs at the current pace. “This is an alternative scenario to the more probable situation where states are expected to respond to an impending health crisis by reinstating some SDMs,” the authors note. The predicted number of American deaths appears more dire in this calculation. The investigators predict cumulative total deaths could reach 1,053,206 (range, 759,693–1,452,397) by the end of February 2021.
The death toll would likely vary among states in this scenario. California, Florida, and Pennsylvania would like account for approximately one third of all deaths.
All the modeling scenarios considered other factors including pneumonia seasonality, mobility, testing rates, and mask use per capita.
“I have seen the IHME study and I agree with the broad conclusions,” Richard Stutt, PhD, of the Epidemiology and Modelling Group at the University of Cambridge, UK, told Medscape Medical News.
“Case numbers are climbing in the US, and without further intervention, there will be a significant number of deaths over the coming months,” he said.
Masks are low cost and widely available, Stutt said. “I am hopeful that even if masks are not widely adopted, we will not see as many deaths as predicted here, as these outbreaks can be significantly reduced by increased social distancing or lockdowns.”
“However this comes at a far higher economic cost than the use of masks, and still requires action,” added Stutt, who authored a study in June that modeled facemasks in combination with “lock-down” measures for managing the COVID-19 pandemic.
Modeling study results depend on the assumptions researchers make, and the IHME team rightly tested a number of different assumptions, Greenhalgh said.
“The key conclusion,” she added, “is here: ‘The implementation of SDMs as soon as individual states reach a threshold of 8 daily deaths per million could dramatically ameliorate the effects of the disease; achieving near-universal mask use could delay, or in many states, possibly prevent, this threshold from being reached and has the potential to save the most lives while minimizing damage to the economy.’ “
“This is a useful piece of information and I think is borne out by their data,” added Greenhalgh, lead author of an April study on face masks for the public during the pandemic.
You can visit the IHME website for the most current mortality projections.
Caplan, Greenhalgh, and Stutt have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
A cumulative 511,000 lives could be lost from COVID-19 in the United States by the end of February 2021, a new prediction study reveals.
However, if universal mask wearing is adopted — defined as 95% of Americans complying with the protective measure — along with social distancing mandates as warranted, nearly 130,000 of those lives could be saved.
And if even 85% of Americans comply, an additional 95,800 lives would be spared before March of next year, researchers at the University of Washington Institute for Health Metrics and Evaluation (IHME) report.
The study was published online October 23 in Nature Medicine.
“The study is sound and makes the case for mandatory mask policies,” said Arthur L. Caplan, PhD, a professor of bioethics at NYU Langone Health in New York City, who frequently provides commentary for Medscape.
Without mandatory mask requirements, he added, “we will see a pandemic slaughter and an overwhelmed healthcare system and workforce.”
The IHME team evaluated COVID-19 data for cases and related deaths between February 1 and September 21. Based on this data, they predicted the likely future of SARS-CoV-2 infections on a state level from September 22, 2020, to February 2021.
An Optimistic Projection
Lead author Robert C. Reiner Jr and colleagues looked at five scenarios. For example, they calculated likely deaths associated with COVID-19 if adoption of mask and social distancing recommendations were nearly universal. They note that Singapore achieved a 95% compliance rate with masks and used this as their “best-case scenario” model.
An estimated 129,574 (range, 85,284–170,867) additional lives could be saved if 95% of Americans wore masks in public, their research reveals. This optimistic scenario includes a “plausible reference” in which any US state reaching 8 COVID-19 deaths per 1 million residents would enact 6 weeks of social distancing mandates (SDMs).
Achieving this level of mask compliance in the United States “could be sufficient to ameliorate the worst effects of epidemic resurgences in many states,” the researchers note.
In contrast, the proportion of Americans wearing masks in public as of September 22 was 49%, according to IHME data.
Universal mask use unlikely
“I’m not a modeling expert, but it is an interesting, and as far as I can judge, well-conducted study which looks, state by state, at what might happen in various scenarios around masking policies going forward — and in particular the effect that mandated masking might have,” Trish Greenhalgh, MD, told Medscape Medical News.
“However, the scenario is a thought experiment. Near-universal mask use is not going to happen in the USA, nor indeed in any individual state, right now, given how emotive the issue has become,” added Greenhalgh, professor in the Nuffield Department of Primary Care Health Sciences at Oxford University, UK. She was not affiliated with the study.
“Hence, whilst I am broadly supportive of the science,” she said, “I’m not confident that this paper will be able to change policy.”
Other ‘What if?’ scenarios
The authors also predicted the mortality implications associated with lower adherence to masks, the presence or absence of SDMs, and what could happen if mandates continue to ease at their current rate.
For example, they considered a scenario with less-than-universal mask use in public, 85%, along with SDMs being reinstated based on the mortality rate threshold. In this instance, they found an additional 95,814 (range, 60,731–133,077) lives could be spared by February 28.
Another calculation looked at outcomes if 95% of Americans wore masks going forward without states instituting SDMs at any point. In this case, the researchers predict that 490,437 Americans would die from COVID-19 by February 2021.
A fourth analysis revealed what would happen without greater mask use if the mortality threshold triggered 6 weeks of SDMs as warranted. Under this ‘plausible reference’ calculation, a total 511,373 Americans would die from COVID-19 by the end of February.
A fifth scenario predicted potential mortality if states continue easing SDMs at the current pace. “This is an alternative scenario to the more probable situation where states are expected to respond to an impending health crisis by reinstating some SDMs,” the authors note. The predicted number of American deaths appears more dire in this calculation. The investigators predict cumulative total deaths could reach 1,053,206 (range, 759,693–1,452,397) by the end of February 2021.
The death toll would likely vary among states in this scenario. California, Florida, and Pennsylvania would like account for approximately one third of all deaths.
All the modeling scenarios considered other factors including pneumonia seasonality, mobility, testing rates, and mask use per capita.
“I have seen the IHME study and I agree with the broad conclusions,” Richard Stutt, PhD, of the Epidemiology and Modelling Group at the University of Cambridge, UK, told Medscape Medical News.
“Case numbers are climbing in the US, and without further intervention, there will be a significant number of deaths over the coming months,” he said.
Masks are low cost and widely available, Stutt said. “I am hopeful that even if masks are not widely adopted, we will not see as many deaths as predicted here, as these outbreaks can be significantly reduced by increased social distancing or lockdowns.”
“However this comes at a far higher economic cost than the use of masks, and still requires action,” added Stutt, who authored a study in June that modeled facemasks in combination with “lock-down” measures for managing the COVID-19 pandemic.
Modeling study results depend on the assumptions researchers make, and the IHME team rightly tested a number of different assumptions, Greenhalgh said.
“The key conclusion,” she added, “is here: ‘The implementation of SDMs as soon as individual states reach a threshold of 8 daily deaths per million could dramatically ameliorate the effects of the disease; achieving near-universal mask use could delay, or in many states, possibly prevent, this threshold from being reached and has the potential to save the most lives while minimizing damage to the economy.’ “
“This is a useful piece of information and I think is borne out by their data,” added Greenhalgh, lead author of an April study on face masks for the public during the pandemic.
You can visit the IHME website for the most current mortality projections.
Caplan, Greenhalgh, and Stutt have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Chinese American families suffer discrimination related to COVID-19
according to results from a survey study.
In the United States, where public officials continue to refer to SARS-CoV-2 as the “China virus” and have often sought to draw attention to its origins in Wuhan, China, “the associations between discrimination triggered by the racialization of this acute public health crisis and mental health are unknown,” Charissa S.L. Cheah, PhD, of the University of Maryland, Baltimore County, and colleagues wrote.
For their research published Oct. 29 in Pediatrics, Dr. Cheah and colleagues recruited a cohort of 543 Chinese American parents of school-age children, and 230 of their children aged 10-18 years, to complete online surveys between mid-March and late May 2020. Parents in the cohort were largely foreign born, with all identifying as ethnically Chinese, while their children were mostly U.S. born.
Evidence of discrimination against Chinese Americans
Half of parents and their children (51% of parents and 50% of youth) reported experiencing at least one in-person incident of direct discrimination (assessed using questions derived from a validated scale of racial aggression) related to the pandemic. Dr. Cheah and colleagues also reported a high incidence of direct discrimination online (32% of parents and 46% of youth). Additionally, the researchers measured reports of vicarious or indirect discrimination – such as hearing jokes or disparaging remarks about one’s ethnic group – which they used a different adapted scale to capture. More than three-quarters of the cohort reported such experiences.
The experiences of discrimination likely bore on the mental health of both parents and youth. Using a series of instruments designed to measure overall psychological well-being as well as symptoms of depression, anxiety, and certain emotional and behavioral outcomes, Dr. Cheah and colleagues reported significant negative associations between direct online or in-person discrimination and psychological health. For parents and children alike, anxiety and depressive symptoms were positively associated with all varieties of discrimination experiences measured in the study.
About a fifth of the youth in the study were deemed, based on the symptom scales used in the study, to have an elevated risk of clinically significant mental health problems, higher than the 10%-15% that would be expected for these age groups in the United States.
“This study revealed that a high percentage of Chinese American parents and their children personally experienced or witnessed anti-Chinese or anti–Asian American racial discrimination both online and in person due to the COVID-19 pandemic,” the investigators wrote. “Most respondents reported directly experiencing or witnessing racial discrimination against other Chinese or Asian American individuals due to COVID-19 at least once.”
Dr. Cheah and colleagues noted that their cross-sectional study did not lend itself to causal interpretations and was vulnerable to certain types of reporting bias. Nonetheless, they argued, as the pandemic continues, “pediatricians should be sensitive to the potential mental health needs of Chinese American youth and their parents related to various forms of racism, in addition to other stressors, as the foundations of perceptions of racial-ethnic discrimination and their consequences may be set during this period.”
COVID-19 didn’t only bring infection
In an accompanying editorial, Tina L. Cheng, MD, of Johns Hopkins University, Baltimore, and her daughter Alison M. Conca-Cheng, a medical student at Brown University, Providence, R.I., remarked that the study’s findings were consistent with recent research that found “4 in 10 Americans reported that it has become more common since COVID-19 for people to express racist views about Asian Americans,” and also described an increase in complaints of discriminatory experiences by Asian Americans.
In this context, a link to poor mental health “should be no surprise,” Dr. Cheng and Ms. Conca-Cheng argued, and urged pediatricians to consult the American Academy of Pediatrics’ 2019 policy statement on racism and on child and adolescent health. “It calls for us to optimize clinical practice, improve workforce development and professional education, strengthen research, and deploy systems through community engagement, advocacy, and public policy.”
David Rettew, MD, a child and adolescent psychiatrist and associate professor of psychiatry and pediatrics at the University of Vermont, Burlington, called the study’s main points “clear and disturbing.”
“While it is difficult to find much in the way here of a silver lining, these alarming reports have helped people working in health care and mental health to understand racism as another form of trauma and abuse which, like other types, can have real negative effects on health,” Dr. Rettew said in an interview. “The more we as mental health professions ask about racism and offer resources for people who have experienced it, just as we would people who have endured other types of trauma, the more we can help people heal. That said, it would be better just to stop this from happening in the first place.”
Dr. Cheah and colleagues’ study was supported by a National Science Foundation grant. The investigators disclosed no conflicts of interest. Dr. Cheng and Ms. Conca-Cheng disclosed no financial conflicts of interest related to their editorial. Dr. Rettew said he had no relevant financial disclosures.
SOURCE: Cheah CSL et al. Pediatrics. 2020;146(5):e2020021816.
according to results from a survey study.
In the United States, where public officials continue to refer to SARS-CoV-2 as the “China virus” and have often sought to draw attention to its origins in Wuhan, China, “the associations between discrimination triggered by the racialization of this acute public health crisis and mental health are unknown,” Charissa S.L. Cheah, PhD, of the University of Maryland, Baltimore County, and colleagues wrote.
For their research published Oct. 29 in Pediatrics, Dr. Cheah and colleagues recruited a cohort of 543 Chinese American parents of school-age children, and 230 of their children aged 10-18 years, to complete online surveys between mid-March and late May 2020. Parents in the cohort were largely foreign born, with all identifying as ethnically Chinese, while their children were mostly U.S. born.
Evidence of discrimination against Chinese Americans
Half of parents and their children (51% of parents and 50% of youth) reported experiencing at least one in-person incident of direct discrimination (assessed using questions derived from a validated scale of racial aggression) related to the pandemic. Dr. Cheah and colleagues also reported a high incidence of direct discrimination online (32% of parents and 46% of youth). Additionally, the researchers measured reports of vicarious or indirect discrimination – such as hearing jokes or disparaging remarks about one’s ethnic group – which they used a different adapted scale to capture. More than three-quarters of the cohort reported such experiences.
The experiences of discrimination likely bore on the mental health of both parents and youth. Using a series of instruments designed to measure overall psychological well-being as well as symptoms of depression, anxiety, and certain emotional and behavioral outcomes, Dr. Cheah and colleagues reported significant negative associations between direct online or in-person discrimination and psychological health. For parents and children alike, anxiety and depressive symptoms were positively associated with all varieties of discrimination experiences measured in the study.
About a fifth of the youth in the study were deemed, based on the symptom scales used in the study, to have an elevated risk of clinically significant mental health problems, higher than the 10%-15% that would be expected for these age groups in the United States.
“This study revealed that a high percentage of Chinese American parents and their children personally experienced or witnessed anti-Chinese or anti–Asian American racial discrimination both online and in person due to the COVID-19 pandemic,” the investigators wrote. “Most respondents reported directly experiencing or witnessing racial discrimination against other Chinese or Asian American individuals due to COVID-19 at least once.”
Dr. Cheah and colleagues noted that their cross-sectional study did not lend itself to causal interpretations and was vulnerable to certain types of reporting bias. Nonetheless, they argued, as the pandemic continues, “pediatricians should be sensitive to the potential mental health needs of Chinese American youth and their parents related to various forms of racism, in addition to other stressors, as the foundations of perceptions of racial-ethnic discrimination and their consequences may be set during this period.”
COVID-19 didn’t only bring infection
In an accompanying editorial, Tina L. Cheng, MD, of Johns Hopkins University, Baltimore, and her daughter Alison M. Conca-Cheng, a medical student at Brown University, Providence, R.I., remarked that the study’s findings were consistent with recent research that found “4 in 10 Americans reported that it has become more common since COVID-19 for people to express racist views about Asian Americans,” and also described an increase in complaints of discriminatory experiences by Asian Americans.
In this context, a link to poor mental health “should be no surprise,” Dr. Cheng and Ms. Conca-Cheng argued, and urged pediatricians to consult the American Academy of Pediatrics’ 2019 policy statement on racism and on child and adolescent health. “It calls for us to optimize clinical practice, improve workforce development and professional education, strengthen research, and deploy systems through community engagement, advocacy, and public policy.”
David Rettew, MD, a child and adolescent psychiatrist and associate professor of psychiatry and pediatrics at the University of Vermont, Burlington, called the study’s main points “clear and disturbing.”
“While it is difficult to find much in the way here of a silver lining, these alarming reports have helped people working in health care and mental health to understand racism as another form of trauma and abuse which, like other types, can have real negative effects on health,” Dr. Rettew said in an interview. “The more we as mental health professions ask about racism and offer resources for people who have experienced it, just as we would people who have endured other types of trauma, the more we can help people heal. That said, it would be better just to stop this from happening in the first place.”
Dr. Cheah and colleagues’ study was supported by a National Science Foundation grant. The investigators disclosed no conflicts of interest. Dr. Cheng and Ms. Conca-Cheng disclosed no financial conflicts of interest related to their editorial. Dr. Rettew said he had no relevant financial disclosures.
SOURCE: Cheah CSL et al. Pediatrics. 2020;146(5):e2020021816.
according to results from a survey study.
In the United States, where public officials continue to refer to SARS-CoV-2 as the “China virus” and have often sought to draw attention to its origins in Wuhan, China, “the associations between discrimination triggered by the racialization of this acute public health crisis and mental health are unknown,” Charissa S.L. Cheah, PhD, of the University of Maryland, Baltimore County, and colleagues wrote.
For their research published Oct. 29 in Pediatrics, Dr. Cheah and colleagues recruited a cohort of 543 Chinese American parents of school-age children, and 230 of their children aged 10-18 years, to complete online surveys between mid-March and late May 2020. Parents in the cohort were largely foreign born, with all identifying as ethnically Chinese, while their children were mostly U.S. born.
Evidence of discrimination against Chinese Americans
Half of parents and their children (51% of parents and 50% of youth) reported experiencing at least one in-person incident of direct discrimination (assessed using questions derived from a validated scale of racial aggression) related to the pandemic. Dr. Cheah and colleagues also reported a high incidence of direct discrimination online (32% of parents and 46% of youth). Additionally, the researchers measured reports of vicarious or indirect discrimination – such as hearing jokes or disparaging remarks about one’s ethnic group – which they used a different adapted scale to capture. More than three-quarters of the cohort reported such experiences.
The experiences of discrimination likely bore on the mental health of both parents and youth. Using a series of instruments designed to measure overall psychological well-being as well as symptoms of depression, anxiety, and certain emotional and behavioral outcomes, Dr. Cheah and colleagues reported significant negative associations between direct online or in-person discrimination and psychological health. For parents and children alike, anxiety and depressive symptoms were positively associated with all varieties of discrimination experiences measured in the study.
About a fifth of the youth in the study were deemed, based on the symptom scales used in the study, to have an elevated risk of clinically significant mental health problems, higher than the 10%-15% that would be expected for these age groups in the United States.
“This study revealed that a high percentage of Chinese American parents and their children personally experienced or witnessed anti-Chinese or anti–Asian American racial discrimination both online and in person due to the COVID-19 pandemic,” the investigators wrote. “Most respondents reported directly experiencing or witnessing racial discrimination against other Chinese or Asian American individuals due to COVID-19 at least once.”
Dr. Cheah and colleagues noted that their cross-sectional study did not lend itself to causal interpretations and was vulnerable to certain types of reporting bias. Nonetheless, they argued, as the pandemic continues, “pediatricians should be sensitive to the potential mental health needs of Chinese American youth and their parents related to various forms of racism, in addition to other stressors, as the foundations of perceptions of racial-ethnic discrimination and their consequences may be set during this period.”
COVID-19 didn’t only bring infection
In an accompanying editorial, Tina L. Cheng, MD, of Johns Hopkins University, Baltimore, and her daughter Alison M. Conca-Cheng, a medical student at Brown University, Providence, R.I., remarked that the study’s findings were consistent with recent research that found “4 in 10 Americans reported that it has become more common since COVID-19 for people to express racist views about Asian Americans,” and also described an increase in complaints of discriminatory experiences by Asian Americans.
In this context, a link to poor mental health “should be no surprise,” Dr. Cheng and Ms. Conca-Cheng argued, and urged pediatricians to consult the American Academy of Pediatrics’ 2019 policy statement on racism and on child and adolescent health. “It calls for us to optimize clinical practice, improve workforce development and professional education, strengthen research, and deploy systems through community engagement, advocacy, and public policy.”
David Rettew, MD, a child and adolescent psychiatrist and associate professor of psychiatry and pediatrics at the University of Vermont, Burlington, called the study’s main points “clear and disturbing.”
“While it is difficult to find much in the way here of a silver lining, these alarming reports have helped people working in health care and mental health to understand racism as another form of trauma and abuse which, like other types, can have real negative effects on health,” Dr. Rettew said in an interview. “The more we as mental health professions ask about racism and offer resources for people who have experienced it, just as we would people who have endured other types of trauma, the more we can help people heal. That said, it would be better just to stop this from happening in the first place.”
Dr. Cheah and colleagues’ study was supported by a National Science Foundation grant. The investigators disclosed no conflicts of interest. Dr. Cheng and Ms. Conca-Cheng disclosed no financial conflicts of interest related to their editorial. Dr. Rettew said he had no relevant financial disclosures.
SOURCE: Cheah CSL et al. Pediatrics. 2020;146(5):e2020021816.
FROM PEDIATRICS
Novel drug slows progression of diabetic kidney disease
For patients with diabetic kidney disease, finerenone, an agent from a new class of selective, nonsteroidal mineralocorticoid receptor antagonists, led to significant reductions in combined adverse renal outcomes and in combined adverse cardiovascular outcomes in the pivotal FIDELIO-DKD trial.
And the safety results showed a good level of tolerability. The rate of hyperkalemia was higher with finerenone than with placebo, but the rate of drug discontinuations for elevated potassium was lower than that seen with spironolactone, a steroidal mineralocorticoid receptor antagonist (MRA).
“An ideal drug would cause no hyperkalemia, but the absolute risk we saw is a fraction of what we see with spironolactone in this vulnerable patient population,” said Rajiv Agarwal, MD, from Indiana in Indianapolis, during a press briefing.
After a median follow-up of 2.6 years, finerenone was associated with a 3.4% absolute reduction in the rate of combined adverse renal events, the study’s primary end point, which comprised kidney failure, renal death, and a drop in estimated glomerular filtration rate (eGFR) of at least 40% from baseline. This produced a significant relative risk reduction of 18%, with a number needed to treat of 32 to prevent one of these events, Dr. Agarwal reported at Kidney Week 2020. Findings from the FIDELIO-DKD trial were published simultaneously in the New England Journal of Medicine.
Finerenone was also associated with an absolute 2.4% reduction in the rate of combined adverse cardiovascular events, the study’s “key secondary end point,” which included cardiovascular death, nonfatal MI, nonfatal stroke, and hospitalization for heart failure. This translated into a significant relative risk reduction of 14% and a number needed to treat of 42 to prevent one of these events.
FIDELIO-DKD assessed 5,734 patients with type 2 diabetes and chronic kidney disease from more than 1,000 sites in 48 countries, including the United States, from 2015 to 2018. In the study cohort, average age was slightly more than 65 years, average baseline systolic blood pressure was 138 mm Hg, average duration of diabetes was nearly 17 years, average baseline glycated hemoglobin (A1c) was 7.7%, and fewer than 5% of patients were Black, 25% were Asian, and about 63% were White.
A suggestion of less severe hyperkalemia
Finerenone was well tolerated by the participants, and the findings suggest that it caused less clinically meaningful hyperkalemia than spironolactone, the most established and widely used MRA.
Like all MRA drugs, finerenone led to an increase in serum potassium in all patient subgroups – in this case 0.2 mmol/L – unlike placebo, said Dr. Agarwal.
The overall incidence of hyperkalemia was 16% in the 2,827 evaluable patients in the finerenone group and 8% in the 2,831 evaluable patients in the placebo group. Fewer than 10% of patients in the trial received a potassium-binding agent.
The rate of hyperkalemia leading to treatment discontinuation was higher in the finerenone group than in the placebo group (2.3% vs. 0.9%).
That 2.3% rate is 10 times lower than the 23.0% rate of hyperkalemia-related treatment discontinuation in patients who received spironolactone and no potassium-binding agent, said Dr. Agarwal, citing a previous study he was involved with.
He hypothesized that finerenone might cause less clinically meaningful hyperkalemia because it creates no active metabolites that linger in the body, whereas spironolactone produces active metabolites with a half life of about 1 week.
“The risk for hyperkalemia is clearly increased with finerenone compared with placebo, and in the absence of head-to-head studies, it’s hard to know how it compares with spironolactone or eplerenone [Inspra],” the other agents in the MRA class, said Mikhail N. Kosiborod, MD, from the University of Missouri–Kansas City.
“The rates of hyperkalemia observed in FIDELIO-DKD were overall comparable to what we would expect from eplerenone. But the rate of serious hyperkalemia was quite low with finerenone, which is reassuring,” Dr. Kosiborod said in an interview.
And the adverse-effect profile showed that finerenone “is as safe as you could expect from an MRA,” said Janani Rangaswami, MD, from the Einstein Medical Center in Philadelphia.
The rate of hyperkalemia should be interpreted in the context of the high risk the enrolled patients faced, given that they all had moderate to severe diabetic kidney disease with albuminuria and, in some cases, eGFR rates as low as 25 mL/min per 1.73m2, she explained. In addition, all patients were on maximally tolerated treatment with either an angiotensin-converting–enzyme inhibitor or an angiotensin receptor blocker to inhibit the renin angiotensin system (RAS).
“Considering this background, it’s a very acceptable adverse-event profile,” Dr. Rangaswami said in an interview.
Renal drugs that could work together
More than 99% of patients in FIDELIO-DKD were on an RAS inhibitor, but fewer than 5% were on a sodium glucose cotransporter 2 (SGLT2) inhibitor at baseline, and fewer than 10% started on this drug class during the course of the study.
Despite that, both Dr. Kosiborod and Dr. Rangaswami are enthusiastic about the prospect of using the three drugs in combination to maximize renal and cardiovascular benefits in FIDELIO-DKD–type patients. Recent results from the CREDENCE study of canagliflozin (Invokana) and from the DAPA-CKD study of dapagluflozin (Farxiga) have established SGLT2 inhibitors – at least those two – as key agents for patients with chronic kidney disease.
Dual treatment with an RAS inhibitor and an SGLT2 inhibitor is “clearly established” for patients with diabetic kidney disease, said Dr. Agarwal.
“After CREDENCE, DAPA-CKD, and now FIDELIO-DKD, we need to seriously consider triple therapy as the future of treatment for diabetic kidney disease to prevent both cardiovascular and kidney complications,” said Dr. Kosiborod. The approach will mimic the multidrug therapy that’s now standard for patients with heart failure with reduced ejection fraction (HFrEF). But he cautioned that this triple combination needs further testing.
“Triple therapy will be the standard of care” for patients with diabetic kidney disease, Dr. Rangaswami agreed, but she cautioned that she would not currently expand the target population for finerenone to patients without type 2 diabetes or to patients without the level of albuminuria required for entry into FIDELIO-DKD: at least 30 mg/g of creatinine per day. And patients with HFrEF were excluded from FIDELIO-DKD, so that limitation on finerenone use should remain for the time being, she added.
Dr. Rangaswami said she is optimistic about the potential efficacy of finerenone added to an SGLT2 inhibitor because of the likelihood that the two drug classes work in different but complementary ways. SGLT2 inhibitors seem to exert their renal protective effects largely through hemodynamic effects, whereas it is likely that finerenone exerts its effects largely as an anti-inflammatory and antifibrotic agent, she speculated. The FIDELIO-DKD results appear to rule out any major effect of finerenone on blood pressure lowering because average systolic pressure fell by only about 2 mm Hg in the treatment group.
“The benefits of finerenone for cardiorenal outcomes are substantial and clinically meaningful,” Dr. Kosiborod said. “We cannot assume that other MRAs, such as spironolactone, provide similar benefits,” he cautioned, but the results are “very good news for patients with type 2 diabetes and chronic kidney disease. We now have another effective intervention with a different mechanism of action.”
FIDELIO-DKD was sponsored by Bayer, the company developing finerenone (BAY 94-8862). Dr. Agarwal has been a consultant to and has received honoraria from Bayer and from several other companies. Dr. Kosiborod has been a consultant to Bayer and to AstraZeneca, Boehringer Ingelheim, Jansse, Merck, and Vifor and has received research funding from AstraZeneca and Boehringer Ingelheim. Dr. Rangaswami has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
For patients with diabetic kidney disease, finerenone, an agent from a new class of selective, nonsteroidal mineralocorticoid receptor antagonists, led to significant reductions in combined adverse renal outcomes and in combined adverse cardiovascular outcomes in the pivotal FIDELIO-DKD trial.
And the safety results showed a good level of tolerability. The rate of hyperkalemia was higher with finerenone than with placebo, but the rate of drug discontinuations for elevated potassium was lower than that seen with spironolactone, a steroidal mineralocorticoid receptor antagonist (MRA).
“An ideal drug would cause no hyperkalemia, but the absolute risk we saw is a fraction of what we see with spironolactone in this vulnerable patient population,” said Rajiv Agarwal, MD, from Indiana in Indianapolis, during a press briefing.
After a median follow-up of 2.6 years, finerenone was associated with a 3.4% absolute reduction in the rate of combined adverse renal events, the study’s primary end point, which comprised kidney failure, renal death, and a drop in estimated glomerular filtration rate (eGFR) of at least 40% from baseline. This produced a significant relative risk reduction of 18%, with a number needed to treat of 32 to prevent one of these events, Dr. Agarwal reported at Kidney Week 2020. Findings from the FIDELIO-DKD trial were published simultaneously in the New England Journal of Medicine.
Finerenone was also associated with an absolute 2.4% reduction in the rate of combined adverse cardiovascular events, the study’s “key secondary end point,” which included cardiovascular death, nonfatal MI, nonfatal stroke, and hospitalization for heart failure. This translated into a significant relative risk reduction of 14% and a number needed to treat of 42 to prevent one of these events.
FIDELIO-DKD assessed 5,734 patients with type 2 diabetes and chronic kidney disease from more than 1,000 sites in 48 countries, including the United States, from 2015 to 2018. In the study cohort, average age was slightly more than 65 years, average baseline systolic blood pressure was 138 mm Hg, average duration of diabetes was nearly 17 years, average baseline glycated hemoglobin (A1c) was 7.7%, and fewer than 5% of patients were Black, 25% were Asian, and about 63% were White.
A suggestion of less severe hyperkalemia
Finerenone was well tolerated by the participants, and the findings suggest that it caused less clinically meaningful hyperkalemia than spironolactone, the most established and widely used MRA.
Like all MRA drugs, finerenone led to an increase in serum potassium in all patient subgroups – in this case 0.2 mmol/L – unlike placebo, said Dr. Agarwal.
The overall incidence of hyperkalemia was 16% in the 2,827 evaluable patients in the finerenone group and 8% in the 2,831 evaluable patients in the placebo group. Fewer than 10% of patients in the trial received a potassium-binding agent.
The rate of hyperkalemia leading to treatment discontinuation was higher in the finerenone group than in the placebo group (2.3% vs. 0.9%).
That 2.3% rate is 10 times lower than the 23.0% rate of hyperkalemia-related treatment discontinuation in patients who received spironolactone and no potassium-binding agent, said Dr. Agarwal, citing a previous study he was involved with.
He hypothesized that finerenone might cause less clinically meaningful hyperkalemia because it creates no active metabolites that linger in the body, whereas spironolactone produces active metabolites with a half life of about 1 week.
“The risk for hyperkalemia is clearly increased with finerenone compared with placebo, and in the absence of head-to-head studies, it’s hard to know how it compares with spironolactone or eplerenone [Inspra],” the other agents in the MRA class, said Mikhail N. Kosiborod, MD, from the University of Missouri–Kansas City.
“The rates of hyperkalemia observed in FIDELIO-DKD were overall comparable to what we would expect from eplerenone. But the rate of serious hyperkalemia was quite low with finerenone, which is reassuring,” Dr. Kosiborod said in an interview.
And the adverse-effect profile showed that finerenone “is as safe as you could expect from an MRA,” said Janani Rangaswami, MD, from the Einstein Medical Center in Philadelphia.
The rate of hyperkalemia should be interpreted in the context of the high risk the enrolled patients faced, given that they all had moderate to severe diabetic kidney disease with albuminuria and, in some cases, eGFR rates as low as 25 mL/min per 1.73m2, she explained. In addition, all patients were on maximally tolerated treatment with either an angiotensin-converting–enzyme inhibitor or an angiotensin receptor blocker to inhibit the renin angiotensin system (RAS).
“Considering this background, it’s a very acceptable adverse-event profile,” Dr. Rangaswami said in an interview.
Renal drugs that could work together
More than 99% of patients in FIDELIO-DKD were on an RAS inhibitor, but fewer than 5% were on a sodium glucose cotransporter 2 (SGLT2) inhibitor at baseline, and fewer than 10% started on this drug class during the course of the study.
Despite that, both Dr. Kosiborod and Dr. Rangaswami are enthusiastic about the prospect of using the three drugs in combination to maximize renal and cardiovascular benefits in FIDELIO-DKD–type patients. Recent results from the CREDENCE study of canagliflozin (Invokana) and from the DAPA-CKD study of dapagluflozin (Farxiga) have established SGLT2 inhibitors – at least those two – as key agents for patients with chronic kidney disease.
Dual treatment with an RAS inhibitor and an SGLT2 inhibitor is “clearly established” for patients with diabetic kidney disease, said Dr. Agarwal.
“After CREDENCE, DAPA-CKD, and now FIDELIO-DKD, we need to seriously consider triple therapy as the future of treatment for diabetic kidney disease to prevent both cardiovascular and kidney complications,” said Dr. Kosiborod. The approach will mimic the multidrug therapy that’s now standard for patients with heart failure with reduced ejection fraction (HFrEF). But he cautioned that this triple combination needs further testing.
“Triple therapy will be the standard of care” for patients with diabetic kidney disease, Dr. Rangaswami agreed, but she cautioned that she would not currently expand the target population for finerenone to patients without type 2 diabetes or to patients without the level of albuminuria required for entry into FIDELIO-DKD: at least 30 mg/g of creatinine per day. And patients with HFrEF were excluded from FIDELIO-DKD, so that limitation on finerenone use should remain for the time being, she added.
Dr. Rangaswami said she is optimistic about the potential efficacy of finerenone added to an SGLT2 inhibitor because of the likelihood that the two drug classes work in different but complementary ways. SGLT2 inhibitors seem to exert their renal protective effects largely through hemodynamic effects, whereas it is likely that finerenone exerts its effects largely as an anti-inflammatory and antifibrotic agent, she speculated. The FIDELIO-DKD results appear to rule out any major effect of finerenone on blood pressure lowering because average systolic pressure fell by only about 2 mm Hg in the treatment group.
“The benefits of finerenone for cardiorenal outcomes are substantial and clinically meaningful,” Dr. Kosiborod said. “We cannot assume that other MRAs, such as spironolactone, provide similar benefits,” he cautioned, but the results are “very good news for patients with type 2 diabetes and chronic kidney disease. We now have another effective intervention with a different mechanism of action.”
FIDELIO-DKD was sponsored by Bayer, the company developing finerenone (BAY 94-8862). Dr. Agarwal has been a consultant to and has received honoraria from Bayer and from several other companies. Dr. Kosiborod has been a consultant to Bayer and to AstraZeneca, Boehringer Ingelheim, Jansse, Merck, and Vifor and has received research funding from AstraZeneca and Boehringer Ingelheim. Dr. Rangaswami has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
For patients with diabetic kidney disease, finerenone, an agent from a new class of selective, nonsteroidal mineralocorticoid receptor antagonists, led to significant reductions in combined adverse renal outcomes and in combined adverse cardiovascular outcomes in the pivotal FIDELIO-DKD trial.
And the safety results showed a good level of tolerability. The rate of hyperkalemia was higher with finerenone than with placebo, but the rate of drug discontinuations for elevated potassium was lower than that seen with spironolactone, a steroidal mineralocorticoid receptor antagonist (MRA).
“An ideal drug would cause no hyperkalemia, but the absolute risk we saw is a fraction of what we see with spironolactone in this vulnerable patient population,” said Rajiv Agarwal, MD, from Indiana in Indianapolis, during a press briefing.
After a median follow-up of 2.6 years, finerenone was associated with a 3.4% absolute reduction in the rate of combined adverse renal events, the study’s primary end point, which comprised kidney failure, renal death, and a drop in estimated glomerular filtration rate (eGFR) of at least 40% from baseline. This produced a significant relative risk reduction of 18%, with a number needed to treat of 32 to prevent one of these events, Dr. Agarwal reported at Kidney Week 2020. Findings from the FIDELIO-DKD trial were published simultaneously in the New England Journal of Medicine.
Finerenone was also associated with an absolute 2.4% reduction in the rate of combined adverse cardiovascular events, the study’s “key secondary end point,” which included cardiovascular death, nonfatal MI, nonfatal stroke, and hospitalization for heart failure. This translated into a significant relative risk reduction of 14% and a number needed to treat of 42 to prevent one of these events.
FIDELIO-DKD assessed 5,734 patients with type 2 diabetes and chronic kidney disease from more than 1,000 sites in 48 countries, including the United States, from 2015 to 2018. In the study cohort, average age was slightly more than 65 years, average baseline systolic blood pressure was 138 mm Hg, average duration of diabetes was nearly 17 years, average baseline glycated hemoglobin (A1c) was 7.7%, and fewer than 5% of patients were Black, 25% were Asian, and about 63% were White.
A suggestion of less severe hyperkalemia
Finerenone was well tolerated by the participants, and the findings suggest that it caused less clinically meaningful hyperkalemia than spironolactone, the most established and widely used MRA.
Like all MRA drugs, finerenone led to an increase in serum potassium in all patient subgroups – in this case 0.2 mmol/L – unlike placebo, said Dr. Agarwal.
The overall incidence of hyperkalemia was 16% in the 2,827 evaluable patients in the finerenone group and 8% in the 2,831 evaluable patients in the placebo group. Fewer than 10% of patients in the trial received a potassium-binding agent.
The rate of hyperkalemia leading to treatment discontinuation was higher in the finerenone group than in the placebo group (2.3% vs. 0.9%).
That 2.3% rate is 10 times lower than the 23.0% rate of hyperkalemia-related treatment discontinuation in patients who received spironolactone and no potassium-binding agent, said Dr. Agarwal, citing a previous study he was involved with.
He hypothesized that finerenone might cause less clinically meaningful hyperkalemia because it creates no active metabolites that linger in the body, whereas spironolactone produces active metabolites with a half life of about 1 week.
“The risk for hyperkalemia is clearly increased with finerenone compared with placebo, and in the absence of head-to-head studies, it’s hard to know how it compares with spironolactone or eplerenone [Inspra],” the other agents in the MRA class, said Mikhail N. Kosiborod, MD, from the University of Missouri–Kansas City.
“The rates of hyperkalemia observed in FIDELIO-DKD were overall comparable to what we would expect from eplerenone. But the rate of serious hyperkalemia was quite low with finerenone, which is reassuring,” Dr. Kosiborod said in an interview.
And the adverse-effect profile showed that finerenone “is as safe as you could expect from an MRA,” said Janani Rangaswami, MD, from the Einstein Medical Center in Philadelphia.
The rate of hyperkalemia should be interpreted in the context of the high risk the enrolled patients faced, given that they all had moderate to severe diabetic kidney disease with albuminuria and, in some cases, eGFR rates as low as 25 mL/min per 1.73m2, she explained. In addition, all patients were on maximally tolerated treatment with either an angiotensin-converting–enzyme inhibitor or an angiotensin receptor blocker to inhibit the renin angiotensin system (RAS).
“Considering this background, it’s a very acceptable adverse-event profile,” Dr. Rangaswami said in an interview.
Renal drugs that could work together
More than 99% of patients in FIDELIO-DKD were on an RAS inhibitor, but fewer than 5% were on a sodium glucose cotransporter 2 (SGLT2) inhibitor at baseline, and fewer than 10% started on this drug class during the course of the study.
Despite that, both Dr. Kosiborod and Dr. Rangaswami are enthusiastic about the prospect of using the three drugs in combination to maximize renal and cardiovascular benefits in FIDELIO-DKD–type patients. Recent results from the CREDENCE study of canagliflozin (Invokana) and from the DAPA-CKD study of dapagluflozin (Farxiga) have established SGLT2 inhibitors – at least those two – as key agents for patients with chronic kidney disease.
Dual treatment with an RAS inhibitor and an SGLT2 inhibitor is “clearly established” for patients with diabetic kidney disease, said Dr. Agarwal.
“After CREDENCE, DAPA-CKD, and now FIDELIO-DKD, we need to seriously consider triple therapy as the future of treatment for diabetic kidney disease to prevent both cardiovascular and kidney complications,” said Dr. Kosiborod. The approach will mimic the multidrug therapy that’s now standard for patients with heart failure with reduced ejection fraction (HFrEF). But he cautioned that this triple combination needs further testing.
“Triple therapy will be the standard of care” for patients with diabetic kidney disease, Dr. Rangaswami agreed, but she cautioned that she would not currently expand the target population for finerenone to patients without type 2 diabetes or to patients without the level of albuminuria required for entry into FIDELIO-DKD: at least 30 mg/g of creatinine per day. And patients with HFrEF were excluded from FIDELIO-DKD, so that limitation on finerenone use should remain for the time being, she added.
Dr. Rangaswami said she is optimistic about the potential efficacy of finerenone added to an SGLT2 inhibitor because of the likelihood that the two drug classes work in different but complementary ways. SGLT2 inhibitors seem to exert their renal protective effects largely through hemodynamic effects, whereas it is likely that finerenone exerts its effects largely as an anti-inflammatory and antifibrotic agent, she speculated. The FIDELIO-DKD results appear to rule out any major effect of finerenone on blood pressure lowering because average systolic pressure fell by only about 2 mm Hg in the treatment group.
“The benefits of finerenone for cardiorenal outcomes are substantial and clinically meaningful,” Dr. Kosiborod said. “We cannot assume that other MRAs, such as spironolactone, provide similar benefits,” he cautioned, but the results are “very good news for patients with type 2 diabetes and chronic kidney disease. We now have another effective intervention with a different mechanism of action.”
FIDELIO-DKD was sponsored by Bayer, the company developing finerenone (BAY 94-8862). Dr. Agarwal has been a consultant to and has received honoraria from Bayer and from several other companies. Dr. Kosiborod has been a consultant to Bayer and to AstraZeneca, Boehringer Ingelheim, Jansse, Merck, and Vifor and has received research funding from AstraZeneca and Boehringer Ingelheim. Dr. Rangaswami has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM KIDNEY WEEK
Tocilizumab stumbles as COVID-19 treatment, narrow role possible
Tocilizumab (Actemra/RoActemra) was not found to have any clear role as a treatment for COVID-19 in four new studies.
Three randomized controlled trials showed that the drug either had no benefit or only a modest one, contradicting a large retrospective study that had hinted at a more robust effect.
“This is not a blockbuster,” said David Cennimo, MD, an infectious disease expert at Rutgers New Jersey Medical School, Newark, New Jersey. “This is not something that’s going to revolutionize our treatment of COVID-19.”
But some researchers still regard these studies as showing evidence that the drug benefits certain patients with severe inflammation.
The immune response to SARS-CoV-2 includes elevated levels of the cytokine interleukin-6 (IL-6). In some patients, this response becomes a nonspecific inflammation, a “cytokine storm,” involving edema and inflammatory cell infiltration in the lungs. These cases are among the most severe.
Dexamethasone has proved effective in controlling this inflammation in some patients. Researchers have theorized that a more targeted suppression of IL-6 could be even more effective or work in cases that don’t respond to dexamethasone.
A recombinant monoclonal antibody, tocilizumab blocks IL-6 receptors. It is approved by the US Food and Drug Administration for use in patients with rheumatologic disorders and cytokine release syndrome induced by chimeric antigen receptor T-cell therapy.
Current National Institutes of Health (NIH) guidelines recommend against the use of tocilizumab as a treatment for COVID-19, despite earlier observational studies that suggested the drug might help patients with moderate to severe disease. Controlled trials were lacking until now.
The most hopeful results in this batch came from the CORIMUNO-19 platform of open-label, randomized controlled trials of immune modulatory treatments for moderate or severe COVID-19 in France.
Published in JAMA Internal Medicine , the trial recruited patients from nine French hospitals. Patients were eligible if they required at least 3 L/min of oxygen without ventilation or admission to the intensive care unit.
The investigators randomly assigned 64 patients to receive tocilizumab 8 mg/kg body weight intravenously plus usual care and 67 patients to usual care alone. Usual care included antibiotic agents, antiviral agents, corticosteroids, vasopressor support, and anticoagulants.
After 4 days, the investigators scored patients on the World Health Organization 10-point Clinical Progression Scale. Twelve of the patients who received tocilizumab scored higher than 5 vs 19 of the patients in the usual care group, with higher scores indicating clinical deterioration.
After 14 days, 24% of the patients taking tocilizumab required either noninvasive ventilation or mechanical ventilation or had died, vs 36% in the usual care group (median posterior hazard ratio [HR], 0.58; 90% credible interval, 0.33 – 1.00).
“We reduced the risk of dying or requiring mechanical ventilation, so for me, the study was positive,” said Olivier Hermine, MD, PhD, a professor of hematology at Paris Descartes University in Paris, France.
However, there was no difference in mortality at 28 days. Hermine hopes to have longer-term outcomes soon, he told Medscape Medical News.
A second randomized controlled trial, also published in JAMA Internal Medicine , provided less hope. In this RCT-TCZ-COVID-19 Study Group trial, conducted at 24 Italian centers, patients were enrolled if their partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2/FiO2) ratios were between 200 and 300 mm Hg and if their inflammatory phenotypes were defined by fever and elevated C-reactive protein level.
The investigators randomly assigned 60 patients to receive tocilizumab 8 mg/kg up to a maximum of 800 mg within 8 hours of randomization, followed by a second dose after 12 hours. They assigned 66 patients to a control group that received supportive care until clinical worsening, at which point patients could receive tocilizumab as a rescue therapy.
Of the patients who received tocilizumab, 28.3% showed clinical worsening within 14 days, compared to 27.0% in the control group (rate ratio, 1.05; 95% CI, 0.59 – 1.86). There was no significant difference between the groups in terms of the proportion admitted to intensive care. The researchers stopped the trial prematurely because tocilizumab did not seem to be making a difference.
The BACC Bay Tocilizumab Trial was conducted at seven Boston hospitals. The results, which were published in The New England Journal of Medicine, were also discouraging.
In that trial, enrolled patients met two sets of parameters. First, the patients had at least one of the following signs: C-reactive protein level higher than 50 mg/L, ferritin level higher than 500 ng/mL, D-dimer level higher than 1000 ng/mL, or a lactate dehydrogenase level higher than 250 U/L. Second, the patients had to have at least two of the following signs: body temperature >38° C, pulmonary infiltrates, or the need for supplemental oxygen to maintain an oxygen saturation greater than 92%.
The investigators randomly assigned 161 patients to receive intravenous tocilizumab 8 mg/kg up to 800 mg and 81 to receive a placebo.
They didn’t find a statistically significant difference between the groups. The hazard ratio for intubation or death in the tocilizumab group as compared with the placebo group was 0.83 (95% CI, 0.38 – 1.81; P = .64). The hazard ratio for disease worsening was 1.11 (95% CI, 0.59 – 2.10; P = .73). At 14 days, the conditions of 18.0% of the patients who received tocilizumab and 14.9% of the patients who received the placebo worsened.
In contrast to these randomized trials, STOP-COVID, a retrospective analysis of 3924 patients, also published in JAMA Internal Medicine, found that the risk for death was lower for patients treated with tocilizumab compared with those not treated with tocilizumab (HR, 0.71; 95% CI, 0.56 – 0.92) over a median follow-up period of 27 days.
Also on the bright side, none of the new studies showed significant adverse reactions to tocilizumab.
More randomized clinical trials are underway. In press releases announcing topline data, Roche reported mostly negative results in its phase 3 COVACTA trial but noted a 44% reduction in the risk for progression to death or ventilation in its phase 3 IMPACTA trial. Roche did not comment on the ethnicity of its COVACTA patients; it said IMPACTA enrolled a majority of Hispanic patients and included large representations of Native American and Black patients.
Results don’t support routine use
Commenting on the new studies, editorialists in both JAMA Internal Medicine and The New England Journal of Medicine concluded that the tocilizumab results were not strong enough to support routine use.
“My take-home point from looking at all of these together is that, even if it does help, it’s most likely in a small subset of the population and/or a small effect,” Cennimo told Medscape Medical News.
But the NIH recommendation against tocilizumab goes too far, argued Cristina Mussini, MD, a professor of infectious diseases at the University of Modena and Reggio Emilia in Italy, who is a coauthor of a cohort study of tocilizumab and served on the CORIMUNO-19 Data Safety and Monitoring Board.
“I really think it’s too early to recommend against it because at least two clinical trials showed protection against mechanical ventilation and death,” she said.
She prescribes tocilizumab for patients who have not been helped by dexamethasone. “It’s just a rescue drug,” she told Medscape Medical News. “It’s not something you use for everybody, but it’s the only weapon we have now when the patient is really going to the intensive care unit.”
The BACC Bay Tocilizumab Trial was funded by Genentech/Roche. Genentech/Roche provided the drug for the CORIMUNO and RCT-TCZ-COVID-19 trials. The STOP-COVID study was supported by grants from the NIH and by the Frankel Cardiovascular Center COVID-19: Impact Research Ignitor. Cennimo, Hermine, and Mussini have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Tocilizumab (Actemra/RoActemra) was not found to have any clear role as a treatment for COVID-19 in four new studies.
Three randomized controlled trials showed that the drug either had no benefit or only a modest one, contradicting a large retrospective study that had hinted at a more robust effect.
“This is not a blockbuster,” said David Cennimo, MD, an infectious disease expert at Rutgers New Jersey Medical School, Newark, New Jersey. “This is not something that’s going to revolutionize our treatment of COVID-19.”
But some researchers still regard these studies as showing evidence that the drug benefits certain patients with severe inflammation.
The immune response to SARS-CoV-2 includes elevated levels of the cytokine interleukin-6 (IL-6). In some patients, this response becomes a nonspecific inflammation, a “cytokine storm,” involving edema and inflammatory cell infiltration in the lungs. These cases are among the most severe.
Dexamethasone has proved effective in controlling this inflammation in some patients. Researchers have theorized that a more targeted suppression of IL-6 could be even more effective or work in cases that don’t respond to dexamethasone.
A recombinant monoclonal antibody, tocilizumab blocks IL-6 receptors. It is approved by the US Food and Drug Administration for use in patients with rheumatologic disorders and cytokine release syndrome induced by chimeric antigen receptor T-cell therapy.
Current National Institutes of Health (NIH) guidelines recommend against the use of tocilizumab as a treatment for COVID-19, despite earlier observational studies that suggested the drug might help patients with moderate to severe disease. Controlled trials were lacking until now.
The most hopeful results in this batch came from the CORIMUNO-19 platform of open-label, randomized controlled trials of immune modulatory treatments for moderate or severe COVID-19 in France.
Published in JAMA Internal Medicine , the trial recruited patients from nine French hospitals. Patients were eligible if they required at least 3 L/min of oxygen without ventilation or admission to the intensive care unit.
The investigators randomly assigned 64 patients to receive tocilizumab 8 mg/kg body weight intravenously plus usual care and 67 patients to usual care alone. Usual care included antibiotic agents, antiviral agents, corticosteroids, vasopressor support, and anticoagulants.
After 4 days, the investigators scored patients on the World Health Organization 10-point Clinical Progression Scale. Twelve of the patients who received tocilizumab scored higher than 5 vs 19 of the patients in the usual care group, with higher scores indicating clinical deterioration.
After 14 days, 24% of the patients taking tocilizumab required either noninvasive ventilation or mechanical ventilation or had died, vs 36% in the usual care group (median posterior hazard ratio [HR], 0.58; 90% credible interval, 0.33 – 1.00).
“We reduced the risk of dying or requiring mechanical ventilation, so for me, the study was positive,” said Olivier Hermine, MD, PhD, a professor of hematology at Paris Descartes University in Paris, France.
However, there was no difference in mortality at 28 days. Hermine hopes to have longer-term outcomes soon, he told Medscape Medical News.
A second randomized controlled trial, also published in JAMA Internal Medicine , provided less hope. In this RCT-TCZ-COVID-19 Study Group trial, conducted at 24 Italian centers, patients were enrolled if their partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2/FiO2) ratios were between 200 and 300 mm Hg and if their inflammatory phenotypes were defined by fever and elevated C-reactive protein level.
The investigators randomly assigned 60 patients to receive tocilizumab 8 mg/kg up to a maximum of 800 mg within 8 hours of randomization, followed by a second dose after 12 hours. They assigned 66 patients to a control group that received supportive care until clinical worsening, at which point patients could receive tocilizumab as a rescue therapy.
Of the patients who received tocilizumab, 28.3% showed clinical worsening within 14 days, compared to 27.0% in the control group (rate ratio, 1.05; 95% CI, 0.59 – 1.86). There was no significant difference between the groups in terms of the proportion admitted to intensive care. The researchers stopped the trial prematurely because tocilizumab did not seem to be making a difference.
The BACC Bay Tocilizumab Trial was conducted at seven Boston hospitals. The results, which were published in The New England Journal of Medicine, were also discouraging.
In that trial, enrolled patients met two sets of parameters. First, the patients had at least one of the following signs: C-reactive protein level higher than 50 mg/L, ferritin level higher than 500 ng/mL, D-dimer level higher than 1000 ng/mL, or a lactate dehydrogenase level higher than 250 U/L. Second, the patients had to have at least two of the following signs: body temperature >38° C, pulmonary infiltrates, or the need for supplemental oxygen to maintain an oxygen saturation greater than 92%.
The investigators randomly assigned 161 patients to receive intravenous tocilizumab 8 mg/kg up to 800 mg and 81 to receive a placebo.
They didn’t find a statistically significant difference between the groups. The hazard ratio for intubation or death in the tocilizumab group as compared with the placebo group was 0.83 (95% CI, 0.38 – 1.81; P = .64). The hazard ratio for disease worsening was 1.11 (95% CI, 0.59 – 2.10; P = .73). At 14 days, the conditions of 18.0% of the patients who received tocilizumab and 14.9% of the patients who received the placebo worsened.
In contrast to these randomized trials, STOP-COVID, a retrospective analysis of 3924 patients, also published in JAMA Internal Medicine, found that the risk for death was lower for patients treated with tocilizumab compared with those not treated with tocilizumab (HR, 0.71; 95% CI, 0.56 – 0.92) over a median follow-up period of 27 days.
Also on the bright side, none of the new studies showed significant adverse reactions to tocilizumab.
More randomized clinical trials are underway. In press releases announcing topline data, Roche reported mostly negative results in its phase 3 COVACTA trial but noted a 44% reduction in the risk for progression to death or ventilation in its phase 3 IMPACTA trial. Roche did not comment on the ethnicity of its COVACTA patients; it said IMPACTA enrolled a majority of Hispanic patients and included large representations of Native American and Black patients.
Results don’t support routine use
Commenting on the new studies, editorialists in both JAMA Internal Medicine and The New England Journal of Medicine concluded that the tocilizumab results were not strong enough to support routine use.
“My take-home point from looking at all of these together is that, even if it does help, it’s most likely in a small subset of the population and/or a small effect,” Cennimo told Medscape Medical News.
But the NIH recommendation against tocilizumab goes too far, argued Cristina Mussini, MD, a professor of infectious diseases at the University of Modena and Reggio Emilia in Italy, who is a coauthor of a cohort study of tocilizumab and served on the CORIMUNO-19 Data Safety and Monitoring Board.
“I really think it’s too early to recommend against it because at least two clinical trials showed protection against mechanical ventilation and death,” she said.
She prescribes tocilizumab for patients who have not been helped by dexamethasone. “It’s just a rescue drug,” she told Medscape Medical News. “It’s not something you use for everybody, but it’s the only weapon we have now when the patient is really going to the intensive care unit.”
The BACC Bay Tocilizumab Trial was funded by Genentech/Roche. Genentech/Roche provided the drug for the CORIMUNO and RCT-TCZ-COVID-19 trials. The STOP-COVID study was supported by grants from the NIH and by the Frankel Cardiovascular Center COVID-19: Impact Research Ignitor. Cennimo, Hermine, and Mussini have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Tocilizumab (Actemra/RoActemra) was not found to have any clear role as a treatment for COVID-19 in four new studies.
Three randomized controlled trials showed that the drug either had no benefit or only a modest one, contradicting a large retrospective study that had hinted at a more robust effect.
“This is not a blockbuster,” said David Cennimo, MD, an infectious disease expert at Rutgers New Jersey Medical School, Newark, New Jersey. “This is not something that’s going to revolutionize our treatment of COVID-19.”
But some researchers still regard these studies as showing evidence that the drug benefits certain patients with severe inflammation.
The immune response to SARS-CoV-2 includes elevated levels of the cytokine interleukin-6 (IL-6). In some patients, this response becomes a nonspecific inflammation, a “cytokine storm,” involving edema and inflammatory cell infiltration in the lungs. These cases are among the most severe.
Dexamethasone has proved effective in controlling this inflammation in some patients. Researchers have theorized that a more targeted suppression of IL-6 could be even more effective or work in cases that don’t respond to dexamethasone.
A recombinant monoclonal antibody, tocilizumab blocks IL-6 receptors. It is approved by the US Food and Drug Administration for use in patients with rheumatologic disorders and cytokine release syndrome induced by chimeric antigen receptor T-cell therapy.
Current National Institutes of Health (NIH) guidelines recommend against the use of tocilizumab as a treatment for COVID-19, despite earlier observational studies that suggested the drug might help patients with moderate to severe disease. Controlled trials were lacking until now.
The most hopeful results in this batch came from the CORIMUNO-19 platform of open-label, randomized controlled trials of immune modulatory treatments for moderate or severe COVID-19 in France.
Published in JAMA Internal Medicine , the trial recruited patients from nine French hospitals. Patients were eligible if they required at least 3 L/min of oxygen without ventilation or admission to the intensive care unit.
The investigators randomly assigned 64 patients to receive tocilizumab 8 mg/kg body weight intravenously plus usual care and 67 patients to usual care alone. Usual care included antibiotic agents, antiviral agents, corticosteroids, vasopressor support, and anticoagulants.
After 4 days, the investigators scored patients on the World Health Organization 10-point Clinical Progression Scale. Twelve of the patients who received tocilizumab scored higher than 5 vs 19 of the patients in the usual care group, with higher scores indicating clinical deterioration.
After 14 days, 24% of the patients taking tocilizumab required either noninvasive ventilation or mechanical ventilation or had died, vs 36% in the usual care group (median posterior hazard ratio [HR], 0.58; 90% credible interval, 0.33 – 1.00).
“We reduced the risk of dying or requiring mechanical ventilation, so for me, the study was positive,” said Olivier Hermine, MD, PhD, a professor of hematology at Paris Descartes University in Paris, France.
However, there was no difference in mortality at 28 days. Hermine hopes to have longer-term outcomes soon, he told Medscape Medical News.
A second randomized controlled trial, also published in JAMA Internal Medicine , provided less hope. In this RCT-TCZ-COVID-19 Study Group trial, conducted at 24 Italian centers, patients were enrolled if their partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2/FiO2) ratios were between 200 and 300 mm Hg and if their inflammatory phenotypes were defined by fever and elevated C-reactive protein level.
The investigators randomly assigned 60 patients to receive tocilizumab 8 mg/kg up to a maximum of 800 mg within 8 hours of randomization, followed by a second dose after 12 hours. They assigned 66 patients to a control group that received supportive care until clinical worsening, at which point patients could receive tocilizumab as a rescue therapy.
Of the patients who received tocilizumab, 28.3% showed clinical worsening within 14 days, compared to 27.0% in the control group (rate ratio, 1.05; 95% CI, 0.59 – 1.86). There was no significant difference between the groups in terms of the proportion admitted to intensive care. The researchers stopped the trial prematurely because tocilizumab did not seem to be making a difference.
The BACC Bay Tocilizumab Trial was conducted at seven Boston hospitals. The results, which were published in The New England Journal of Medicine, were also discouraging.
In that trial, enrolled patients met two sets of parameters. First, the patients had at least one of the following signs: C-reactive protein level higher than 50 mg/L, ferritin level higher than 500 ng/mL, D-dimer level higher than 1000 ng/mL, or a lactate dehydrogenase level higher than 250 U/L. Second, the patients had to have at least two of the following signs: body temperature >38° C, pulmonary infiltrates, or the need for supplemental oxygen to maintain an oxygen saturation greater than 92%.
The investigators randomly assigned 161 patients to receive intravenous tocilizumab 8 mg/kg up to 800 mg and 81 to receive a placebo.
They didn’t find a statistically significant difference between the groups. The hazard ratio for intubation or death in the tocilizumab group as compared with the placebo group was 0.83 (95% CI, 0.38 – 1.81; P = .64). The hazard ratio for disease worsening was 1.11 (95% CI, 0.59 – 2.10; P = .73). At 14 days, the conditions of 18.0% of the patients who received tocilizumab and 14.9% of the patients who received the placebo worsened.
In contrast to these randomized trials, STOP-COVID, a retrospective analysis of 3924 patients, also published in JAMA Internal Medicine, found that the risk for death was lower for patients treated with tocilizumab compared with those not treated with tocilizumab (HR, 0.71; 95% CI, 0.56 – 0.92) over a median follow-up period of 27 days.
Also on the bright side, none of the new studies showed significant adverse reactions to tocilizumab.
More randomized clinical trials are underway. In press releases announcing topline data, Roche reported mostly negative results in its phase 3 COVACTA trial but noted a 44% reduction in the risk for progression to death or ventilation in its phase 3 IMPACTA trial. Roche did not comment on the ethnicity of its COVACTA patients; it said IMPACTA enrolled a majority of Hispanic patients and included large representations of Native American and Black patients.
Results don’t support routine use
Commenting on the new studies, editorialists in both JAMA Internal Medicine and The New England Journal of Medicine concluded that the tocilizumab results were not strong enough to support routine use.
“My take-home point from looking at all of these together is that, even if it does help, it’s most likely in a small subset of the population and/or a small effect,” Cennimo told Medscape Medical News.
But the NIH recommendation against tocilizumab goes too far, argued Cristina Mussini, MD, a professor of infectious diseases at the University of Modena and Reggio Emilia in Italy, who is a coauthor of a cohort study of tocilizumab and served on the CORIMUNO-19 Data Safety and Monitoring Board.
“I really think it’s too early to recommend against it because at least two clinical trials showed protection against mechanical ventilation and death,” she said.
She prescribes tocilizumab for patients who have not been helped by dexamethasone. “It’s just a rescue drug,” she told Medscape Medical News. “It’s not something you use for everybody, but it’s the only weapon we have now when the patient is really going to the intensive care unit.”
The BACC Bay Tocilizumab Trial was funded by Genentech/Roche. Genentech/Roche provided the drug for the CORIMUNO and RCT-TCZ-COVID-19 trials. The STOP-COVID study was supported by grants from the NIH and by the Frankel Cardiovascular Center COVID-19: Impact Research Ignitor. Cennimo, Hermine, and Mussini have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
ACC expert consensus on post-TAVR arrhythmias
The American College of Cardiology (ACC) has released a new Expert Consensus Decision Pathway (ECDP) on the management of conduction disturbances after transcatheter aortic valve replacement (TAVR).
The document provides guidance to clinicians in identifying and managing this common complication of TAVR, covering the pre-TAVR, periprocedural and post-TAVR periods.
“Conduction disturbances after TAVR are common and there is currently heterogeneity in how they’re managed, ranging from a casual observational approach to invasive electrophysiological studies and preemptive pacemaker implantation,” said writing committee chair Scott Lilly, MD, PhD, from the Ohio State Wexner Medical Center in Columbus.
“We felt this kind of collaborative effort to review what little research there is on this topic and come to [an] expert consensus was long overdue,” he added.
The document was published online Oct. 21 in the Journal of the American College of Cardiology.
Dr. Lilly stressed in an interview that this effort is an ECDP and not a guideline “because there is not data out there to solidly stand on and say, ‘This is the way we should do things.’ “
His hope is that this document will generate more discussion on this topic and spur some (probably National Institutes of Health–sponsored) clinical trials to better guide practice.
Not uncommon and not decreasing
Complete heart block requiring permanent pacemaker (PPM) implantation is seen in about 15% of patients within 30 days after TAVR. While this is a clear indication for PPM, there is no consensus on the management of less severe conduction disturbances such as new bundle branch or transient complete atrioventricular (AV) heart block.
Unlike the rates of bleeding, vascular injury, and stroke, which have decreased over time, the rates of in-hospital PPM implantation after TAVR have not changed significantly since commercialization in 2012. This is a concern because TAVR is increasingly used in younger, lower-risk patients.
“The pacemaker rate really hasn’t improved at a clip we would like to see if it was going to be a durable technology,” Dr. Lilly said.
Consensus regarding a reasonable strategy to manage cardiac conduction disturbances after TAVR has been elusive. This is a result of several things: a dearth of adequately powered, randomized controlled trials; the often transient nature of the conduction disturbances; evolving technologies; and the interplay of cardiology subspecialties involved.
The 2013 European Society of Cardiology guidelines address pacing post-TAVR, but do not provide in-depth discussion on the topic. This is the first effort sponsored by a cardiovascular society in the United States to review the existing data and experience and propose evidence-based expert guidance.
Pre-TAVR assessment
Pre-TAVR assessment should consider the patient’s risk for postprocedure conduction disturbances, the authors said. Since bradyarrythmias and aortic stenosis may present similarly (fatigue, lightheadedness, and syncope being hallmarks of both), a careful history is needed to determine if bradyarrhythmia is present.
An electrocardiogram (ECG) or ambulatory rhythm monitoring may identify baseline conduction abnormalities and help predict the need for post-TAVR PPM.
“In this section, we underscored some of the literature that has raised awareness about the presence of preexisting arrhythmias in TAVR patients and suggest that monitoring in selected patients before the procedure is reasonable, particularly those presenting with syncope or lightheadedness,” said Dr. Lilly.
Intraprocedural management
On the day of the procedure, patients determined to have elevated risk for complete AV heart block require careful perioperative ECG and hemodynamic monitoring. Regardless of preexisting risk, said the authors that all patients should be monitored on a telemetry unit during the procedure with ability to do emergency pacing if necessary.
“In the periprocedural section, we address the role of electrophysiological studies for identifying patients at high-risk of subsequent heart block,” said Dr. Lilly. “That’s a practice that’s occurring at a number of centers, but the data out there is insufficient to establish it as a pacemaker indication. Routine EP testing for patients deemed at risk for conduction disturbances after TAVR is not guideline-based and more research is needed.”
The document also outlines the effects of medications and anesthesia on postprocedure conduction abnormalities.
Post-TAVR management
The authors define post-TAVR management as continuing through 30-days after discharge.
The ECDP carefully outlines which patients can be discharged without monitoring and those for whom outpatient monitoring can be considered.
“If I’m going to pick one thing from this section, it’s the monitoring piece. A lot of patients that have a conduction disturbance right after TAVR – but you’re not sure if it’s going to progress and require a pacemaker – might stay in the hospital for an extended time waiting to see if the heart holds up,” reported Dr. Lilly.
“But a number of centers are now discharging people at 1 or 2 days, which begs the question: What do you do with these folks? Our group has published data showing that 30-day monitoring in select patients is a safe approach,” said Dr. Lilly.
There are shortcomings, however, in existing data, and recommendations will likely change as more data are collected, he explained.
As well, there remains uncertainty in how conduction block should be managed after TAVR, and clinical judgment is “foundational” in this, wrote the authors.
“This document is meant to help programs deal with these situations right now, acknowledging full and well, that really good randomized clinical data is not available,” said Dr. Lilly.
Dr. Lilly has disclosed no relevant financial relationships. The work of the writing committee was supported exclusively by the American College of Cardiology without commercial support.
A version of this article originally appeared on Medscape.com.
The American College of Cardiology (ACC) has released a new Expert Consensus Decision Pathway (ECDP) on the management of conduction disturbances after transcatheter aortic valve replacement (TAVR).
The document provides guidance to clinicians in identifying and managing this common complication of TAVR, covering the pre-TAVR, periprocedural and post-TAVR periods.
“Conduction disturbances after TAVR are common and there is currently heterogeneity in how they’re managed, ranging from a casual observational approach to invasive electrophysiological studies and preemptive pacemaker implantation,” said writing committee chair Scott Lilly, MD, PhD, from the Ohio State Wexner Medical Center in Columbus.
“We felt this kind of collaborative effort to review what little research there is on this topic and come to [an] expert consensus was long overdue,” he added.
The document was published online Oct. 21 in the Journal of the American College of Cardiology.
Dr. Lilly stressed in an interview that this effort is an ECDP and not a guideline “because there is not data out there to solidly stand on and say, ‘This is the way we should do things.’ “
His hope is that this document will generate more discussion on this topic and spur some (probably National Institutes of Health–sponsored) clinical trials to better guide practice.
Not uncommon and not decreasing
Complete heart block requiring permanent pacemaker (PPM) implantation is seen in about 15% of patients within 30 days after TAVR. While this is a clear indication for PPM, there is no consensus on the management of less severe conduction disturbances such as new bundle branch or transient complete atrioventricular (AV) heart block.
Unlike the rates of bleeding, vascular injury, and stroke, which have decreased over time, the rates of in-hospital PPM implantation after TAVR have not changed significantly since commercialization in 2012. This is a concern because TAVR is increasingly used in younger, lower-risk patients.
“The pacemaker rate really hasn’t improved at a clip we would like to see if it was going to be a durable technology,” Dr. Lilly said.
Consensus regarding a reasonable strategy to manage cardiac conduction disturbances after TAVR has been elusive. This is a result of several things: a dearth of adequately powered, randomized controlled trials; the often transient nature of the conduction disturbances; evolving technologies; and the interplay of cardiology subspecialties involved.
The 2013 European Society of Cardiology guidelines address pacing post-TAVR, but do not provide in-depth discussion on the topic. This is the first effort sponsored by a cardiovascular society in the United States to review the existing data and experience and propose evidence-based expert guidance.
Pre-TAVR assessment
Pre-TAVR assessment should consider the patient’s risk for postprocedure conduction disturbances, the authors said. Since bradyarrythmias and aortic stenosis may present similarly (fatigue, lightheadedness, and syncope being hallmarks of both), a careful history is needed to determine if bradyarrhythmia is present.
An electrocardiogram (ECG) or ambulatory rhythm monitoring may identify baseline conduction abnormalities and help predict the need for post-TAVR PPM.
“In this section, we underscored some of the literature that has raised awareness about the presence of preexisting arrhythmias in TAVR patients and suggest that monitoring in selected patients before the procedure is reasonable, particularly those presenting with syncope or lightheadedness,” said Dr. Lilly.
Intraprocedural management
On the day of the procedure, patients determined to have elevated risk for complete AV heart block require careful perioperative ECG and hemodynamic monitoring. Regardless of preexisting risk, said the authors that all patients should be monitored on a telemetry unit during the procedure with ability to do emergency pacing if necessary.
“In the periprocedural section, we address the role of electrophysiological studies for identifying patients at high-risk of subsequent heart block,” said Dr. Lilly. “That’s a practice that’s occurring at a number of centers, but the data out there is insufficient to establish it as a pacemaker indication. Routine EP testing for patients deemed at risk for conduction disturbances after TAVR is not guideline-based and more research is needed.”
The document also outlines the effects of medications and anesthesia on postprocedure conduction abnormalities.
Post-TAVR management
The authors define post-TAVR management as continuing through 30-days after discharge.
The ECDP carefully outlines which patients can be discharged without monitoring and those for whom outpatient monitoring can be considered.
“If I’m going to pick one thing from this section, it’s the monitoring piece. A lot of patients that have a conduction disturbance right after TAVR – but you’re not sure if it’s going to progress and require a pacemaker – might stay in the hospital for an extended time waiting to see if the heart holds up,” reported Dr. Lilly.
“But a number of centers are now discharging people at 1 or 2 days, which begs the question: What do you do with these folks? Our group has published data showing that 30-day monitoring in select patients is a safe approach,” said Dr. Lilly.
There are shortcomings, however, in existing data, and recommendations will likely change as more data are collected, he explained.
As well, there remains uncertainty in how conduction block should be managed after TAVR, and clinical judgment is “foundational” in this, wrote the authors.
“This document is meant to help programs deal with these situations right now, acknowledging full and well, that really good randomized clinical data is not available,” said Dr. Lilly.
Dr. Lilly has disclosed no relevant financial relationships. The work of the writing committee was supported exclusively by the American College of Cardiology without commercial support.
A version of this article originally appeared on Medscape.com.
The American College of Cardiology (ACC) has released a new Expert Consensus Decision Pathway (ECDP) on the management of conduction disturbances after transcatheter aortic valve replacement (TAVR).
The document provides guidance to clinicians in identifying and managing this common complication of TAVR, covering the pre-TAVR, periprocedural and post-TAVR periods.
“Conduction disturbances after TAVR are common and there is currently heterogeneity in how they’re managed, ranging from a casual observational approach to invasive electrophysiological studies and preemptive pacemaker implantation,” said writing committee chair Scott Lilly, MD, PhD, from the Ohio State Wexner Medical Center in Columbus.
“We felt this kind of collaborative effort to review what little research there is on this topic and come to [an] expert consensus was long overdue,” he added.
The document was published online Oct. 21 in the Journal of the American College of Cardiology.
Dr. Lilly stressed in an interview that this effort is an ECDP and not a guideline “because there is not data out there to solidly stand on and say, ‘This is the way we should do things.’ “
His hope is that this document will generate more discussion on this topic and spur some (probably National Institutes of Health–sponsored) clinical trials to better guide practice.
Not uncommon and not decreasing
Complete heart block requiring permanent pacemaker (PPM) implantation is seen in about 15% of patients within 30 days after TAVR. While this is a clear indication for PPM, there is no consensus on the management of less severe conduction disturbances such as new bundle branch or transient complete atrioventricular (AV) heart block.
Unlike the rates of bleeding, vascular injury, and stroke, which have decreased over time, the rates of in-hospital PPM implantation after TAVR have not changed significantly since commercialization in 2012. This is a concern because TAVR is increasingly used in younger, lower-risk patients.
“The pacemaker rate really hasn’t improved at a clip we would like to see if it was going to be a durable technology,” Dr. Lilly said.
Consensus regarding a reasonable strategy to manage cardiac conduction disturbances after TAVR has been elusive. This is a result of several things: a dearth of adequately powered, randomized controlled trials; the often transient nature of the conduction disturbances; evolving technologies; and the interplay of cardiology subspecialties involved.
The 2013 European Society of Cardiology guidelines address pacing post-TAVR, but do not provide in-depth discussion on the topic. This is the first effort sponsored by a cardiovascular society in the United States to review the existing data and experience and propose evidence-based expert guidance.
Pre-TAVR assessment
Pre-TAVR assessment should consider the patient’s risk for postprocedure conduction disturbances, the authors said. Since bradyarrythmias and aortic stenosis may present similarly (fatigue, lightheadedness, and syncope being hallmarks of both), a careful history is needed to determine if bradyarrhythmia is present.
An electrocardiogram (ECG) or ambulatory rhythm monitoring may identify baseline conduction abnormalities and help predict the need for post-TAVR PPM.
“In this section, we underscored some of the literature that has raised awareness about the presence of preexisting arrhythmias in TAVR patients and suggest that monitoring in selected patients before the procedure is reasonable, particularly those presenting with syncope or lightheadedness,” said Dr. Lilly.
Intraprocedural management
On the day of the procedure, patients determined to have elevated risk for complete AV heart block require careful perioperative ECG and hemodynamic monitoring. Regardless of preexisting risk, said the authors that all patients should be monitored on a telemetry unit during the procedure with ability to do emergency pacing if necessary.
“In the periprocedural section, we address the role of electrophysiological studies for identifying patients at high-risk of subsequent heart block,” said Dr. Lilly. “That’s a practice that’s occurring at a number of centers, but the data out there is insufficient to establish it as a pacemaker indication. Routine EP testing for patients deemed at risk for conduction disturbances after TAVR is not guideline-based and more research is needed.”
The document also outlines the effects of medications and anesthesia on postprocedure conduction abnormalities.
Post-TAVR management
The authors define post-TAVR management as continuing through 30-days after discharge.
The ECDP carefully outlines which patients can be discharged without monitoring and those for whom outpatient monitoring can be considered.
“If I’m going to pick one thing from this section, it’s the monitoring piece. A lot of patients that have a conduction disturbance right after TAVR – but you’re not sure if it’s going to progress and require a pacemaker – might stay in the hospital for an extended time waiting to see if the heart holds up,” reported Dr. Lilly.
“But a number of centers are now discharging people at 1 or 2 days, which begs the question: What do you do with these folks? Our group has published data showing that 30-day monitoring in select patients is a safe approach,” said Dr. Lilly.
There are shortcomings, however, in existing data, and recommendations will likely change as more data are collected, he explained.
As well, there remains uncertainty in how conduction block should be managed after TAVR, and clinical judgment is “foundational” in this, wrote the authors.
“This document is meant to help programs deal with these situations right now, acknowledging full and well, that really good randomized clinical data is not available,” said Dr. Lilly.
Dr. Lilly has disclosed no relevant financial relationships. The work of the writing committee was supported exclusively by the American College of Cardiology without commercial support.
A version of this article originally appeared on Medscape.com.
Lilly stops antibody trial in hospitalized COVID-19 patients, other trials continue
Eli Lilly announced it will halt its ACTIV-3 trial evaluating the antibody bamlanivimab in combination with remdesivir for people hospitalized with COVID-19, after new evidence regarding efficacy emerged.
The new data from the National Institutes of Health suggest that the experimental neutralizing antibody therapy does not offer significant clinical benefit for people with more advanced COVID-19 illness, according to a company statement.
Eli Lilly also announced it plans to continue its other trials evaluating the antibody, including those assessing a potential role in treating people in the earlier stages of COVID-19.
“While there was insufficient evidence that bamlanivimab improved clinical outcomes when added to other treatments in hospitalized patients with COVID-19, we remain confident based on data from Lilly’s BLAZE-1 study that bamlanivimab monotherapy may prevent progression of disease for those earlier in the course of COVID-19,” the statement reads.
The ACTIV-3 trial was paused on October 13 after a data and safety monitoring board cited safety concerns.
The most recent data update that triggered an end to the trial did not reveal any significant differences in safety, though.
This article first appeared on Medscape.com.
Eli Lilly announced it will halt its ACTIV-3 trial evaluating the antibody bamlanivimab in combination with remdesivir for people hospitalized with COVID-19, after new evidence regarding efficacy emerged.
The new data from the National Institutes of Health suggest that the experimental neutralizing antibody therapy does not offer significant clinical benefit for people with more advanced COVID-19 illness, according to a company statement.
Eli Lilly also announced it plans to continue its other trials evaluating the antibody, including those assessing a potential role in treating people in the earlier stages of COVID-19.
“While there was insufficient evidence that bamlanivimab improved clinical outcomes when added to other treatments in hospitalized patients with COVID-19, we remain confident based on data from Lilly’s BLAZE-1 study that bamlanivimab monotherapy may prevent progression of disease for those earlier in the course of COVID-19,” the statement reads.
The ACTIV-3 trial was paused on October 13 after a data and safety monitoring board cited safety concerns.
The most recent data update that triggered an end to the trial did not reveal any significant differences in safety, though.
This article first appeared on Medscape.com.
Eli Lilly announced it will halt its ACTIV-3 trial evaluating the antibody bamlanivimab in combination with remdesivir for people hospitalized with COVID-19, after new evidence regarding efficacy emerged.
The new data from the National Institutes of Health suggest that the experimental neutralizing antibody therapy does not offer significant clinical benefit for people with more advanced COVID-19 illness, according to a company statement.
Eli Lilly also announced it plans to continue its other trials evaluating the antibody, including those assessing a potential role in treating people in the earlier stages of COVID-19.
“While there was insufficient evidence that bamlanivimab improved clinical outcomes when added to other treatments in hospitalized patients with COVID-19, we remain confident based on data from Lilly’s BLAZE-1 study that bamlanivimab monotherapy may prevent progression of disease for those earlier in the course of COVID-19,” the statement reads.
The ACTIV-3 trial was paused on October 13 after a data and safety monitoring board cited safety concerns.
The most recent data update that triggered an end to the trial did not reveal any significant differences in safety, though.
This article first appeared on Medscape.com.
AACE issues ‘cookbook’ algorithm to manage dyslipidemia
A new algorithm on lipid management and prevention of cardiovascular disease from the American Association of Clinical Endocrinologists* (AACE) and the American College of Endocrinology (ACE) is “a nice cookbook” that many clinicians, especially those who are not lipid experts, will find useful, according to writing committee chair Yehuda Handelsman, MD.
The algorithm, published Oct. 10 in Endocrine Practice as 10 slides, or as part of a more detailed consensus statement, is a companion to the 2017 AACE/ACE guidelines for lipid management and includes more recent information about new therapies.
“What we’re trying to do here is to say, ‘focus on LDL-C, triglycerides, high-risk patients, and lifestyle. Understand all the medications available to you to reduce LDL-C and reduce triglycerides,’ ” Dr. Handelsman, of the Metabolic Institute of America, Tarzana, Calif., explained in an interview.
“We touch on lipoprotein(a), which we still don’t have medication for, but it identifies people at high risk, and we need that.”
Clinicians also need to know “that we’ve got some newer drugs in the market that can manage people who have statin intolerance,” Dr. Handelsman added.
“We introduced new therapies like icosapent ethyl” (Vascepa, Amarin) for hypertriglyceridemia, “when to use it, and how to use it. Even though it was not part of the 2017 guideline, we gave recommendations based on current data in the algorithm.”
Although there is no good evidence that lowering triglycerides reduces heart disease, he continued, many experts believe that the target triglyceride level should be less than 150 mg/dL, and the algorithm explains how to treat to this goal.
“Last, and most importantly, I cannot fail to underscore the fact that lifestyle is very important,” he emphasized.
Robert H. Eckel, MD, of the University of Colorado at Denver, Aurora, and president of medicine and science at the American Diabetes Association, who was not involved with this algorithm, said in an interview that the algorithm is important since it offers “the clinician or health care practitioner an approach, a kind of a cookbook or application of the guidelines, for how to manage lipid disorders in patients at risk ... It’s geared for the nonexperts too,” he said.
Dyslipidemia treatment summarized in 10 slides
The AACE/ACE algorithm comprises 10 slides, one each for dyslipidemic states, secondary causes of lipid disorders, screening for and assessing lipid disorders and atherosclerotic CVD (ASCVD) risk, ASCVD risk categories and treatment goals, lifestyle recommendations, treating LDL-C to goal, managing statin intolerance and safety, management of hypertriglyceridemia and the role of icosapent ethyl, assessment and management of elevated lipoprotein(a), and profiles of medications for dyslipidemia.
The algorithm defines five ASCVD risk categories and recommends increasingly lower LDL-C, non–HDL-C, and apo B target levels with increasing risk, but the same triglyceride target for all.
First, “treatment of lipid disorders begins with lifestyle therapy to improve nutrition, physical activity, weight, and other factors that affect lipids,” the consensus statement authors stress.
Next, “LDL-C has been, and remains, the main focus of efforts to improve lipid profiles in individuals at risk for ASCVD” (see table).
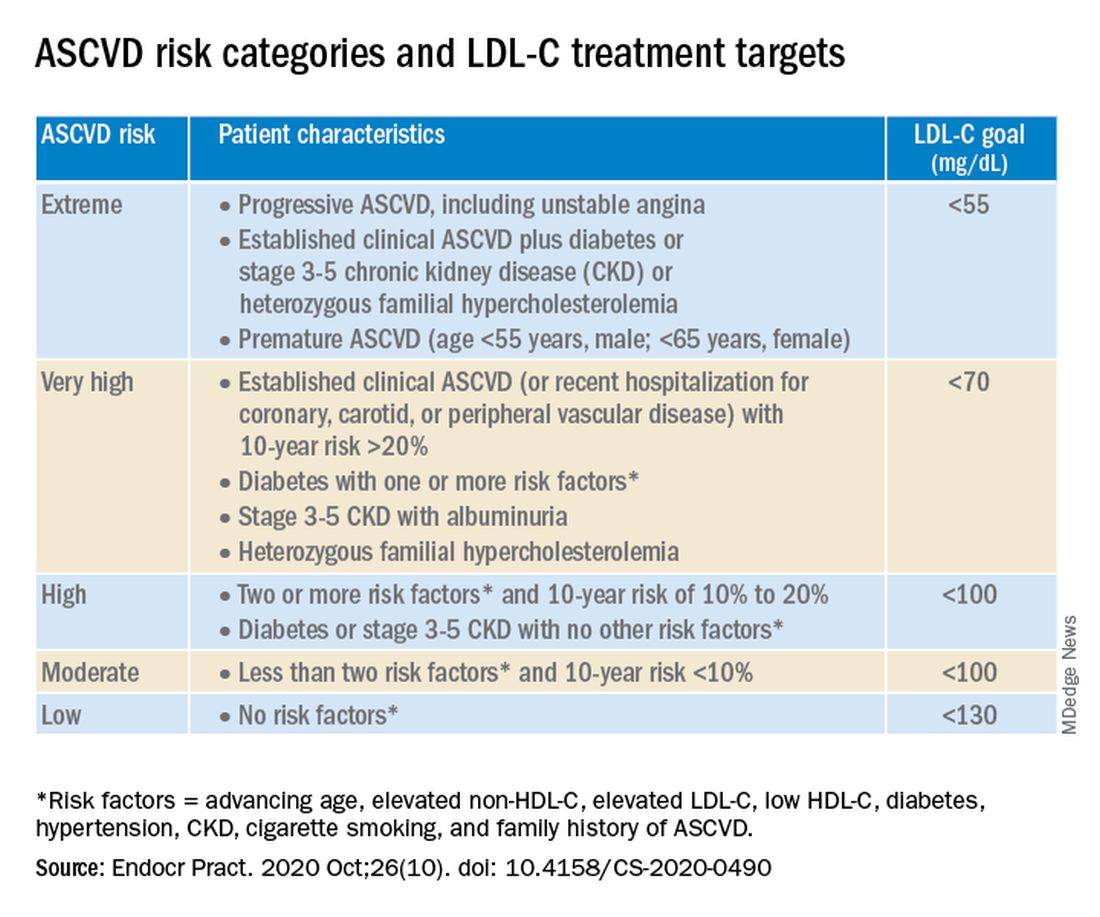
“We stratify [LDL-C] not as a one-treatment-target-for-all,” but rather as extreme, very high, high, moderate, and low ASCVD risk, Dr. Handelsman explained, with different treatment pathways (specified in another slide) to reach different risk-dependent goals.
“Unlike the ACC [American College of Cardiology] guideline, which shows if you want to further reduce LDL after statin give ezetimibe first, we say ‘no’,” he noted. “If somebody has an extreme risk, and you don’t think ezetimibe will get to a goal below 55 mg/dL, you should go first with a PCSK9 [proprotein convertase subtilisin/kexin type 9] inhibitor, and only then add ezetimibe or [colesevelam] or other drugs,” he said.
The consensus statement authors expand on this scenario. “Treatment for patients at extreme risk should begin with lifestyle therapy plus a high-intensity statin (atorvastatin 40 to 80 mg or rosuvastatin 20 to 40 mg, or the highest tolerated statin dose) to achieve an LDL-C goal of less than 55 mg/dL.”
“If LDL-C remains above goal after 3 months,” a PCSK9 inhibitor (evolocumab [Repatha, Amgen] or alirocumab [Praluent, Sanofi/Regeneron]), the cholesterol absorption inhibitor ezetimibe, or the bile acid sequestrant colesevelam (Welchol, Daiichi Sankyo) or the adenosine triphosphate-citrate lyase (ACL) inhibitor bempedoic acid (Nexletol, Esperion) “should be added, depending on required LDL-C lowering, and a third agent should be added if the combination fails to achieve the goal.”
However, “because the cost of ezetimibe is low, it may be preferred over PCSK9 inhibitors as second-line therapy to achieve an LDL-C below 70 mg/dL for patients who require no more than 15%-20% further reduction to reach goals.”
For patients at moderate or high risk, lipid management should begin with a moderate-intensity statin and be increased to a high-intensity statin before adding a second lipid-lowering medication to reach an LDL-C below 100 mg/dL.
According to the consensus statement, the desirable goal for triglycerides is less than 150 mg/dL.
In all patients with triglyceride levels of at least 500 mg/dL, statin therapy should be combined with a fibrate, prescription-grade omega-3 fatty acid, and/or niacin to reduce triglycerides.
In any patient with established ASCVD or diabetes with at least 2 ASCVD risk factors and triglycerides of 135-499 mg/dL, icosapent ethyl should be added to a statin to prevent ASCVD events.
Statement aligns with major guidelines
In general, the 2017 AACE/ACE guidelines and algorithm are “pretty similar” to other guidelines such as the 2018 ACC/American Heart Association (AHA) guidelines for cholesterol management, the 2019 ACC/AHA guidelines for primary prevention of CVD, and the 2019 European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) guidelines for the management of dyslipidemia, according to Dr. Eckel.
They have “all have now taken into consideration the evidence behind PCSK9 inhibitors,” he noted. “That’s important because those drugs have proven to be effective.”
Two differences, he pointed out, are that the 2019 ESC/EAS guidelines suggest that lipoprotein(a) measurement be considered at least once in every adult’s lifetime, and they recommend apo B analysis in people with high triglycerides but normal LDL (or no higher than 100 mg/dL), to identify additional risk.
*AACE changes its name, broadens focus
Shortly after its algorithm was published, AACE announced that it has a new organization name and brand, the American Association of Clinical Endocrinology, which “more clearly defines AACE as a community of individuals who work together to elevate the practice of clinical endocrinology,” according to an Oct. 20 statement.
The change is meant to acknowledge AACE’s “more modern, inclusive approach to endocrinology that supports multidisciplinary care teams – with endocrinologists leading the way.”
Along with the name change is a new global website. The statement notes that “health care professionals and community members can access all of the valuable clinical content such as guidelines, disease state networks and important education by visiting the pro portal in the top right corner of the site, or by going directly to pro.aace.com.”
Dr. Handelsman discloses that he receives research grant support from Amgen, Applied Therapeutics, AstraZeneca, BMS, Gan & Lee, Novo Nordisk, and Sanofi, and he is a consultant and/or speaker for Amarin, BI-Lilly, and Sanofi.
Dr. Eckel has received consultant/advisory board fees from Kowa, Novo Nordisk, and Provention Bio.
A new algorithm on lipid management and prevention of cardiovascular disease from the American Association of Clinical Endocrinologists* (AACE) and the American College of Endocrinology (ACE) is “a nice cookbook” that many clinicians, especially those who are not lipid experts, will find useful, according to writing committee chair Yehuda Handelsman, MD.
The algorithm, published Oct. 10 in Endocrine Practice as 10 slides, or as part of a more detailed consensus statement, is a companion to the 2017 AACE/ACE guidelines for lipid management and includes more recent information about new therapies.
“What we’re trying to do here is to say, ‘focus on LDL-C, triglycerides, high-risk patients, and lifestyle. Understand all the medications available to you to reduce LDL-C and reduce triglycerides,’ ” Dr. Handelsman, of the Metabolic Institute of America, Tarzana, Calif., explained in an interview.
“We touch on lipoprotein(a), which we still don’t have medication for, but it identifies people at high risk, and we need that.”
Clinicians also need to know “that we’ve got some newer drugs in the market that can manage people who have statin intolerance,” Dr. Handelsman added.
“We introduced new therapies like icosapent ethyl” (Vascepa, Amarin) for hypertriglyceridemia, “when to use it, and how to use it. Even though it was not part of the 2017 guideline, we gave recommendations based on current data in the algorithm.”
Although there is no good evidence that lowering triglycerides reduces heart disease, he continued, many experts believe that the target triglyceride level should be less than 150 mg/dL, and the algorithm explains how to treat to this goal.
“Last, and most importantly, I cannot fail to underscore the fact that lifestyle is very important,” he emphasized.
Robert H. Eckel, MD, of the University of Colorado at Denver, Aurora, and president of medicine and science at the American Diabetes Association, who was not involved with this algorithm, said in an interview that the algorithm is important since it offers “the clinician or health care practitioner an approach, a kind of a cookbook or application of the guidelines, for how to manage lipid disorders in patients at risk ... It’s geared for the nonexperts too,” he said.
Dyslipidemia treatment summarized in 10 slides
The AACE/ACE algorithm comprises 10 slides, one each for dyslipidemic states, secondary causes of lipid disorders, screening for and assessing lipid disorders and atherosclerotic CVD (ASCVD) risk, ASCVD risk categories and treatment goals, lifestyle recommendations, treating LDL-C to goal, managing statin intolerance and safety, management of hypertriglyceridemia and the role of icosapent ethyl, assessment and management of elevated lipoprotein(a), and profiles of medications for dyslipidemia.
The algorithm defines five ASCVD risk categories and recommends increasingly lower LDL-C, non–HDL-C, and apo B target levels with increasing risk, but the same triglyceride target for all.
First, “treatment of lipid disorders begins with lifestyle therapy to improve nutrition, physical activity, weight, and other factors that affect lipids,” the consensus statement authors stress.
Next, “LDL-C has been, and remains, the main focus of efforts to improve lipid profiles in individuals at risk for ASCVD” (see table).

“We stratify [LDL-C] not as a one-treatment-target-for-all,” but rather as extreme, very high, high, moderate, and low ASCVD risk, Dr. Handelsman explained, with different treatment pathways (specified in another slide) to reach different risk-dependent goals.
“Unlike the ACC [American College of Cardiology] guideline, which shows if you want to further reduce LDL after statin give ezetimibe first, we say ‘no’,” he noted. “If somebody has an extreme risk, and you don’t think ezetimibe will get to a goal below 55 mg/dL, you should go first with a PCSK9 [proprotein convertase subtilisin/kexin type 9] inhibitor, and only then add ezetimibe or [colesevelam] or other drugs,” he said.
The consensus statement authors expand on this scenario. “Treatment for patients at extreme risk should begin with lifestyle therapy plus a high-intensity statin (atorvastatin 40 to 80 mg or rosuvastatin 20 to 40 mg, or the highest tolerated statin dose) to achieve an LDL-C goal of less than 55 mg/dL.”
“If LDL-C remains above goal after 3 months,” a PCSK9 inhibitor (evolocumab [Repatha, Amgen] or alirocumab [Praluent, Sanofi/Regeneron]), the cholesterol absorption inhibitor ezetimibe, or the bile acid sequestrant colesevelam (Welchol, Daiichi Sankyo) or the adenosine triphosphate-citrate lyase (ACL) inhibitor bempedoic acid (Nexletol, Esperion) “should be added, depending on required LDL-C lowering, and a third agent should be added if the combination fails to achieve the goal.”
However, “because the cost of ezetimibe is low, it may be preferred over PCSK9 inhibitors as second-line therapy to achieve an LDL-C below 70 mg/dL for patients who require no more than 15%-20% further reduction to reach goals.”
For patients at moderate or high risk, lipid management should begin with a moderate-intensity statin and be increased to a high-intensity statin before adding a second lipid-lowering medication to reach an LDL-C below 100 mg/dL.
According to the consensus statement, the desirable goal for triglycerides is less than 150 mg/dL.
In all patients with triglyceride levels of at least 500 mg/dL, statin therapy should be combined with a fibrate, prescription-grade omega-3 fatty acid, and/or niacin to reduce triglycerides.
In any patient with established ASCVD or diabetes with at least 2 ASCVD risk factors and triglycerides of 135-499 mg/dL, icosapent ethyl should be added to a statin to prevent ASCVD events.
Statement aligns with major guidelines
In general, the 2017 AACE/ACE guidelines and algorithm are “pretty similar” to other guidelines such as the 2018 ACC/American Heart Association (AHA) guidelines for cholesterol management, the 2019 ACC/AHA guidelines for primary prevention of CVD, and the 2019 European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) guidelines for the management of dyslipidemia, according to Dr. Eckel.
They have “all have now taken into consideration the evidence behind PCSK9 inhibitors,” he noted. “That’s important because those drugs have proven to be effective.”
Two differences, he pointed out, are that the 2019 ESC/EAS guidelines suggest that lipoprotein(a) measurement be considered at least once in every adult’s lifetime, and they recommend apo B analysis in people with high triglycerides but normal LDL (or no higher than 100 mg/dL), to identify additional risk.
*AACE changes its name, broadens focus
Shortly after its algorithm was published, AACE announced that it has a new organization name and brand, the American Association of Clinical Endocrinology, which “more clearly defines AACE as a community of individuals who work together to elevate the practice of clinical endocrinology,” according to an Oct. 20 statement.
The change is meant to acknowledge AACE’s “more modern, inclusive approach to endocrinology that supports multidisciplinary care teams – with endocrinologists leading the way.”
Along with the name change is a new global website. The statement notes that “health care professionals and community members can access all of the valuable clinical content such as guidelines, disease state networks and important education by visiting the pro portal in the top right corner of the site, or by going directly to pro.aace.com.”
Dr. Handelsman discloses that he receives research grant support from Amgen, Applied Therapeutics, AstraZeneca, BMS, Gan & Lee, Novo Nordisk, and Sanofi, and he is a consultant and/or speaker for Amarin, BI-Lilly, and Sanofi.
Dr. Eckel has received consultant/advisory board fees from Kowa, Novo Nordisk, and Provention Bio.
A new algorithm on lipid management and prevention of cardiovascular disease from the American Association of Clinical Endocrinologists* (AACE) and the American College of Endocrinology (ACE) is “a nice cookbook” that many clinicians, especially those who are not lipid experts, will find useful, according to writing committee chair Yehuda Handelsman, MD.
The algorithm, published Oct. 10 in Endocrine Practice as 10 slides, or as part of a more detailed consensus statement, is a companion to the 2017 AACE/ACE guidelines for lipid management and includes more recent information about new therapies.
“What we’re trying to do here is to say, ‘focus on LDL-C, triglycerides, high-risk patients, and lifestyle. Understand all the medications available to you to reduce LDL-C and reduce triglycerides,’ ” Dr. Handelsman, of the Metabolic Institute of America, Tarzana, Calif., explained in an interview.
“We touch on lipoprotein(a), which we still don’t have medication for, but it identifies people at high risk, and we need that.”
Clinicians also need to know “that we’ve got some newer drugs in the market that can manage people who have statin intolerance,” Dr. Handelsman added.
“We introduced new therapies like icosapent ethyl” (Vascepa, Amarin) for hypertriglyceridemia, “when to use it, and how to use it. Even though it was not part of the 2017 guideline, we gave recommendations based on current data in the algorithm.”
Although there is no good evidence that lowering triglycerides reduces heart disease, he continued, many experts believe that the target triglyceride level should be less than 150 mg/dL, and the algorithm explains how to treat to this goal.
“Last, and most importantly, I cannot fail to underscore the fact that lifestyle is very important,” he emphasized.
Robert H. Eckel, MD, of the University of Colorado at Denver, Aurora, and president of medicine and science at the American Diabetes Association, who was not involved with this algorithm, said in an interview that the algorithm is important since it offers “the clinician or health care practitioner an approach, a kind of a cookbook or application of the guidelines, for how to manage lipid disorders in patients at risk ... It’s geared for the nonexperts too,” he said.
Dyslipidemia treatment summarized in 10 slides
The AACE/ACE algorithm comprises 10 slides, one each for dyslipidemic states, secondary causes of lipid disorders, screening for and assessing lipid disorders and atherosclerotic CVD (ASCVD) risk, ASCVD risk categories and treatment goals, lifestyle recommendations, treating LDL-C to goal, managing statin intolerance and safety, management of hypertriglyceridemia and the role of icosapent ethyl, assessment and management of elevated lipoprotein(a), and profiles of medications for dyslipidemia.
The algorithm defines five ASCVD risk categories and recommends increasingly lower LDL-C, non–HDL-C, and apo B target levels with increasing risk, but the same triglyceride target for all.
First, “treatment of lipid disorders begins with lifestyle therapy to improve nutrition, physical activity, weight, and other factors that affect lipids,” the consensus statement authors stress.
Next, “LDL-C has been, and remains, the main focus of efforts to improve lipid profiles in individuals at risk for ASCVD” (see table).

“We stratify [LDL-C] not as a one-treatment-target-for-all,” but rather as extreme, very high, high, moderate, and low ASCVD risk, Dr. Handelsman explained, with different treatment pathways (specified in another slide) to reach different risk-dependent goals.
“Unlike the ACC [American College of Cardiology] guideline, which shows if you want to further reduce LDL after statin give ezetimibe first, we say ‘no’,” he noted. “If somebody has an extreme risk, and you don’t think ezetimibe will get to a goal below 55 mg/dL, you should go first with a PCSK9 [proprotein convertase subtilisin/kexin type 9] inhibitor, and only then add ezetimibe or [colesevelam] or other drugs,” he said.
The consensus statement authors expand on this scenario. “Treatment for patients at extreme risk should begin with lifestyle therapy plus a high-intensity statin (atorvastatin 40 to 80 mg or rosuvastatin 20 to 40 mg, or the highest tolerated statin dose) to achieve an LDL-C goal of less than 55 mg/dL.”
“If LDL-C remains above goal after 3 months,” a PCSK9 inhibitor (evolocumab [Repatha, Amgen] or alirocumab [Praluent, Sanofi/Regeneron]), the cholesterol absorption inhibitor ezetimibe, or the bile acid sequestrant colesevelam (Welchol, Daiichi Sankyo) or the adenosine triphosphate-citrate lyase (ACL) inhibitor bempedoic acid (Nexletol, Esperion) “should be added, depending on required LDL-C lowering, and a third agent should be added if the combination fails to achieve the goal.”
However, “because the cost of ezetimibe is low, it may be preferred over PCSK9 inhibitors as second-line therapy to achieve an LDL-C below 70 mg/dL for patients who require no more than 15%-20% further reduction to reach goals.”
For patients at moderate or high risk, lipid management should begin with a moderate-intensity statin and be increased to a high-intensity statin before adding a second lipid-lowering medication to reach an LDL-C below 100 mg/dL.
According to the consensus statement, the desirable goal for triglycerides is less than 150 mg/dL.
In all patients with triglyceride levels of at least 500 mg/dL, statin therapy should be combined with a fibrate, prescription-grade omega-3 fatty acid, and/or niacin to reduce triglycerides.
In any patient with established ASCVD or diabetes with at least 2 ASCVD risk factors and triglycerides of 135-499 mg/dL, icosapent ethyl should be added to a statin to prevent ASCVD events.
Statement aligns with major guidelines
In general, the 2017 AACE/ACE guidelines and algorithm are “pretty similar” to other guidelines such as the 2018 ACC/American Heart Association (AHA) guidelines for cholesterol management, the 2019 ACC/AHA guidelines for primary prevention of CVD, and the 2019 European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) guidelines for the management of dyslipidemia, according to Dr. Eckel.
They have “all have now taken into consideration the evidence behind PCSK9 inhibitors,” he noted. “That’s important because those drugs have proven to be effective.”
Two differences, he pointed out, are that the 2019 ESC/EAS guidelines suggest that lipoprotein(a) measurement be considered at least once in every adult’s lifetime, and they recommend apo B analysis in people with high triglycerides but normal LDL (or no higher than 100 mg/dL), to identify additional risk.
*AACE changes its name, broadens focus
Shortly after its algorithm was published, AACE announced that it has a new organization name and brand, the American Association of Clinical Endocrinology, which “more clearly defines AACE as a community of individuals who work together to elevate the practice of clinical endocrinology,” according to an Oct. 20 statement.
The change is meant to acknowledge AACE’s “more modern, inclusive approach to endocrinology that supports multidisciplinary care teams – with endocrinologists leading the way.”
Along with the name change is a new global website. The statement notes that “health care professionals and community members can access all of the valuable clinical content such as guidelines, disease state networks and important education by visiting the pro portal in the top right corner of the site, or by going directly to pro.aace.com.”
Dr. Handelsman discloses that he receives research grant support from Amgen, Applied Therapeutics, AstraZeneca, BMS, Gan & Lee, Novo Nordisk, and Sanofi, and he is a consultant and/or speaker for Amarin, BI-Lilly, and Sanofi.
Dr. Eckel has received consultant/advisory board fees from Kowa, Novo Nordisk, and Provention Bio.
Higher serum omega-3 tied to better outcome after STEMI
Regular consumption of foods rich in omega-3 fatty acids was associated with improved prognosis after ST-segment myocardial infarction (STEMI) in a new observational study.
The prospective study, which involved 944 patients with STEMI who underwent primary percutaneous coronary intervention (PCI), showed that plasma levels of fatty acids at the time of the STEMI were inversely associated with both incident major adverse cardiovascular events (MACE) and cardiovascular readmissions (adjusted hazard ratio, 0.76 and 0.74 for 1-SD increase; for both, P < .05).
No association was seen for the endpoint of all-cause mortality.
“What we showed is that your consumption of fish and other sources of omega-3 fatty acids before the heart attack impacts your prognosis after the heart attack. It’s a novel approach because it’s not primary prevention or secondary prevention,” said Aleix Sala-Vila, PharmD, PhD, from the Institut Hospital del Mar d’Investigacions Mèdiques (IMIM) in Barcelona, Spain.
Sala-Vila, co–senior author Antoni Bayés-Genís, MD, PhD, Heart Universitari Germans Trias I Pujol, Barcelona, and first author Iolanda Lázaro, PhD, also from IMIM, reported their findings online Oct. 26 in the Journal of the American College of Cardiology.
It has been established that dietary omega-3 eicosapentaenoic acid (EPA) has cardioprotective properties, but observational studies and randomized trials of EPA intake have yielded disparate findings.
This study avoided the usual traps of nutritional epidemiology research – self-reported food diaries and intake questionnaires. For this study, the researchers measured tissue levels of EPA and alpha-linolenic acid (ALA) by measuring serum phosphatidylcholine (PC) levels, which reflect dietary intake during the previous 3 or 4 weeks.
This technique, said Sala-Vila, not only provides a more reliable measure of fatty acid intake over time but also avoids measurement errors related to fatty acid content variation.
For example, “The EPA content of a piece of fish eaten in January could be very different from one eaten in June,” explained Sala-Vila.
That said, he acknowledged that this technique, which uses gas chromatography, does not at present have a clear clinical application. “It’s quite difficult just to convert levels of serum-PC EPA into consumption of fatty fish. We feel that the best advice at this point is that given by the American Heart Association to eat two servings of fatty fish a week.”
EPA and ALA: Partners in prevention?
In addition to the findings regarding EPA, the researchers also found that serum-PC ALA was inversely related to all-cause mortality after STEMI (HR, 0.65 for 1-SD increase; P < .05).
A trend was seen for an association between ALA and lower risk for incident MACE (P = .093).
ALA is readily available from inexpensive plant sources (eg, chia seeds, flax seeds, walnuts, soy beans) and has been associated with lower all-cause mortality in high-risk individuals.
This omega-3 fatty acid is often given short shrift in the fatty acid world because of the seven-step enzymatic process needed to convert it into more beneficial forms.
“We know that the conversion of ALA to EPA or DHA [docohexaenoic acid] is marginal, but we decided to include it in the study because we feel that this fatty acid is becoming more important because there are some issues with fish consumption – people are concerned about pollutants and sustainability, and some just don’t like it,” explained Sala-Vila.
“We were shocked to see that the marine-derived and vegetable-derived fatty acids don’t appear to compete, but rather they act synergistically,” said Sala-Villa. The researchers suggested that marine and vegetable omega-3 fatty acids may act as “partners in prevention.”
“We are not metabolically adapted to converting ALA to EPA, but despite this, there is a large body of evidence showing that one way to increase the status of EPA and DHA in our membranes is by eating these sources of fatty acids,” said Sala-Vila.
For almost 20 years, Sala-Vila has been studying how the consumption of foods rich in omega-3 affects disease. Two of his current projects involve studying levels of ALA in red blood cell membranes as a risk factor for ischemic stroke and omega-3 status in individuals with cognitive impairment who are at high risk for Alzheimer’s disease.
Applicable to all patients with atherosclerosis
In comments to theheart.org | Medscape Cardiology, Deepak Bhatt, MD, called the study “terrific,” adding that the effort is “as good as it gets” for observational nutrition research.
“I think one has to view these findings in the larger universe of what is really a revolution in omega-3 fatty acid research,” said Bhatt.
This universe, he said, includes a wealth of observational research showing the benefits of omega-3s, two outcome trials – JELIS and REDUCE-IT – that showed the benefits of EPA supplementation, and two imaging studies – EVAPORATE and CHERRY – that showed favorable effects of EPA on the vasculature.
REDUCE-IT, for which Bhatt served as principal investigator, showed that treatment with icosapent ethyl (Vascepa), a high-dose purified form of EPA, led to a 25% relative risk reduction in MACE in an at-risk Western population.
The results, said Bhatt, who co-wrote an editorial that accompanies the current Sala-Vila article, “likely apply to all patients with atherosclerosis or who are at high risk for it” and supports the practice of counseling patients to increase their intake of food rich in omega-3 fatty acids.
The field may be due for a shake-up, he noted. At next month’s American Heart Association meeting, the results of another trial of another prescription-grade EPA/DHA supplement will be presented, and they are expected to be negative.
AstraZeneca announced in January 2020 the early closure of the STRENGTH trial of Epanova after an interim analysis showed a low likelihood of their product demonstrating benefit in the enrolled population.
Epanova is a fish-oil derived mixture of free fatty acids, primarily EPA and DHA. It is approved in the United States and is indicated as an adjunct to diet to reduce triglyceride levels in adults with severe (≥500 mg/dL) hypertriglyceridemia. This indication is not affected by the data from the STRENGTH trial, according to a company press release.
Sala-Vila has received grants and support from the California Walnut Commission, including a grant to support part of this study. Bayés-Genís and Bhatt have relationships with a number of companies.
This article first appeared on Medscape.com.
Regular consumption of foods rich in omega-3 fatty acids was associated with improved prognosis after ST-segment myocardial infarction (STEMI) in a new observational study.
The prospective study, which involved 944 patients with STEMI who underwent primary percutaneous coronary intervention (PCI), showed that plasma levels of fatty acids at the time of the STEMI were inversely associated with both incident major adverse cardiovascular events (MACE) and cardiovascular readmissions (adjusted hazard ratio, 0.76 and 0.74 for 1-SD increase; for both, P < .05).
No association was seen for the endpoint of all-cause mortality.
“What we showed is that your consumption of fish and other sources of omega-3 fatty acids before the heart attack impacts your prognosis after the heart attack. It’s a novel approach because it’s not primary prevention or secondary prevention,” said Aleix Sala-Vila, PharmD, PhD, from the Institut Hospital del Mar d’Investigacions Mèdiques (IMIM) in Barcelona, Spain.
Sala-Vila, co–senior author Antoni Bayés-Genís, MD, PhD, Heart Universitari Germans Trias I Pujol, Barcelona, and first author Iolanda Lázaro, PhD, also from IMIM, reported their findings online Oct. 26 in the Journal of the American College of Cardiology.
It has been established that dietary omega-3 eicosapentaenoic acid (EPA) has cardioprotective properties, but observational studies and randomized trials of EPA intake have yielded disparate findings.
This study avoided the usual traps of nutritional epidemiology research – self-reported food diaries and intake questionnaires. For this study, the researchers measured tissue levels of EPA and alpha-linolenic acid (ALA) by measuring serum phosphatidylcholine (PC) levels, which reflect dietary intake during the previous 3 or 4 weeks.
This technique, said Sala-Vila, not only provides a more reliable measure of fatty acid intake over time but also avoids measurement errors related to fatty acid content variation.
For example, “The EPA content of a piece of fish eaten in January could be very different from one eaten in June,” explained Sala-Vila.
That said, he acknowledged that this technique, which uses gas chromatography, does not at present have a clear clinical application. “It’s quite difficult just to convert levels of serum-PC EPA into consumption of fatty fish. We feel that the best advice at this point is that given by the American Heart Association to eat two servings of fatty fish a week.”
EPA and ALA: Partners in prevention?
In addition to the findings regarding EPA, the researchers also found that serum-PC ALA was inversely related to all-cause mortality after STEMI (HR, 0.65 for 1-SD increase; P < .05).
A trend was seen for an association between ALA and lower risk for incident MACE (P = .093).
ALA is readily available from inexpensive plant sources (eg, chia seeds, flax seeds, walnuts, soy beans) and has been associated with lower all-cause mortality in high-risk individuals.
This omega-3 fatty acid is often given short shrift in the fatty acid world because of the seven-step enzymatic process needed to convert it into more beneficial forms.
“We know that the conversion of ALA to EPA or DHA [docohexaenoic acid] is marginal, but we decided to include it in the study because we feel that this fatty acid is becoming more important because there are some issues with fish consumption – people are concerned about pollutants and sustainability, and some just don’t like it,” explained Sala-Vila.
“We were shocked to see that the marine-derived and vegetable-derived fatty acids don’t appear to compete, but rather they act synergistically,” said Sala-Villa. The researchers suggested that marine and vegetable omega-3 fatty acids may act as “partners in prevention.”
“We are not metabolically adapted to converting ALA to EPA, but despite this, there is a large body of evidence showing that one way to increase the status of EPA and DHA in our membranes is by eating these sources of fatty acids,” said Sala-Vila.
For almost 20 years, Sala-Vila has been studying how the consumption of foods rich in omega-3 affects disease. Two of his current projects involve studying levels of ALA in red blood cell membranes as a risk factor for ischemic stroke and omega-3 status in individuals with cognitive impairment who are at high risk for Alzheimer’s disease.
Applicable to all patients with atherosclerosis
In comments to theheart.org | Medscape Cardiology, Deepak Bhatt, MD, called the study “terrific,” adding that the effort is “as good as it gets” for observational nutrition research.
“I think one has to view these findings in the larger universe of what is really a revolution in omega-3 fatty acid research,” said Bhatt.
This universe, he said, includes a wealth of observational research showing the benefits of omega-3s, two outcome trials – JELIS and REDUCE-IT – that showed the benefits of EPA supplementation, and two imaging studies – EVAPORATE and CHERRY – that showed favorable effects of EPA on the vasculature.
REDUCE-IT, for which Bhatt served as principal investigator, showed that treatment with icosapent ethyl (Vascepa), a high-dose purified form of EPA, led to a 25% relative risk reduction in MACE in an at-risk Western population.
The results, said Bhatt, who co-wrote an editorial that accompanies the current Sala-Vila article, “likely apply to all patients with atherosclerosis or who are at high risk for it” and supports the practice of counseling patients to increase their intake of food rich in omega-3 fatty acids.
The field may be due for a shake-up, he noted. At next month’s American Heart Association meeting, the results of another trial of another prescription-grade EPA/DHA supplement will be presented, and they are expected to be negative.
AstraZeneca announced in January 2020 the early closure of the STRENGTH trial of Epanova after an interim analysis showed a low likelihood of their product demonstrating benefit in the enrolled population.
Epanova is a fish-oil derived mixture of free fatty acids, primarily EPA and DHA. It is approved in the United States and is indicated as an adjunct to diet to reduce triglyceride levels in adults with severe (≥500 mg/dL) hypertriglyceridemia. This indication is not affected by the data from the STRENGTH trial, according to a company press release.
Sala-Vila has received grants and support from the California Walnut Commission, including a grant to support part of this study. Bayés-Genís and Bhatt have relationships with a number of companies.
This article first appeared on Medscape.com.
Regular consumption of foods rich in omega-3 fatty acids was associated with improved prognosis after ST-segment myocardial infarction (STEMI) in a new observational study.
The prospective study, which involved 944 patients with STEMI who underwent primary percutaneous coronary intervention (PCI), showed that plasma levels of fatty acids at the time of the STEMI were inversely associated with both incident major adverse cardiovascular events (MACE) and cardiovascular readmissions (adjusted hazard ratio, 0.76 and 0.74 for 1-SD increase; for both, P < .05).
No association was seen for the endpoint of all-cause mortality.
“What we showed is that your consumption of fish and other sources of omega-3 fatty acids before the heart attack impacts your prognosis after the heart attack. It’s a novel approach because it’s not primary prevention or secondary prevention,” said Aleix Sala-Vila, PharmD, PhD, from the Institut Hospital del Mar d’Investigacions Mèdiques (IMIM) in Barcelona, Spain.
Sala-Vila, co–senior author Antoni Bayés-Genís, MD, PhD, Heart Universitari Germans Trias I Pujol, Barcelona, and first author Iolanda Lázaro, PhD, also from IMIM, reported their findings online Oct. 26 in the Journal of the American College of Cardiology.
It has been established that dietary omega-3 eicosapentaenoic acid (EPA) has cardioprotective properties, but observational studies and randomized trials of EPA intake have yielded disparate findings.
This study avoided the usual traps of nutritional epidemiology research – self-reported food diaries and intake questionnaires. For this study, the researchers measured tissue levels of EPA and alpha-linolenic acid (ALA) by measuring serum phosphatidylcholine (PC) levels, which reflect dietary intake during the previous 3 or 4 weeks.
This technique, said Sala-Vila, not only provides a more reliable measure of fatty acid intake over time but also avoids measurement errors related to fatty acid content variation.
For example, “The EPA content of a piece of fish eaten in January could be very different from one eaten in June,” explained Sala-Vila.
That said, he acknowledged that this technique, which uses gas chromatography, does not at present have a clear clinical application. “It’s quite difficult just to convert levels of serum-PC EPA into consumption of fatty fish. We feel that the best advice at this point is that given by the American Heart Association to eat two servings of fatty fish a week.”
EPA and ALA: Partners in prevention?
In addition to the findings regarding EPA, the researchers also found that serum-PC ALA was inversely related to all-cause mortality after STEMI (HR, 0.65 for 1-SD increase; P < .05).
A trend was seen for an association between ALA and lower risk for incident MACE (P = .093).
ALA is readily available from inexpensive plant sources (eg, chia seeds, flax seeds, walnuts, soy beans) and has been associated with lower all-cause mortality in high-risk individuals.
This omega-3 fatty acid is often given short shrift in the fatty acid world because of the seven-step enzymatic process needed to convert it into more beneficial forms.
“We know that the conversion of ALA to EPA or DHA [docohexaenoic acid] is marginal, but we decided to include it in the study because we feel that this fatty acid is becoming more important because there are some issues with fish consumption – people are concerned about pollutants and sustainability, and some just don’t like it,” explained Sala-Vila.
“We were shocked to see that the marine-derived and vegetable-derived fatty acids don’t appear to compete, but rather they act synergistically,” said Sala-Villa. The researchers suggested that marine and vegetable omega-3 fatty acids may act as “partners in prevention.”
“We are not metabolically adapted to converting ALA to EPA, but despite this, there is a large body of evidence showing that one way to increase the status of EPA and DHA in our membranes is by eating these sources of fatty acids,” said Sala-Vila.
For almost 20 years, Sala-Vila has been studying how the consumption of foods rich in omega-3 affects disease. Two of his current projects involve studying levels of ALA in red blood cell membranes as a risk factor for ischemic stroke and omega-3 status in individuals with cognitive impairment who are at high risk for Alzheimer’s disease.
Applicable to all patients with atherosclerosis
In comments to theheart.org | Medscape Cardiology, Deepak Bhatt, MD, called the study “terrific,” adding that the effort is “as good as it gets” for observational nutrition research.
“I think one has to view these findings in the larger universe of what is really a revolution in omega-3 fatty acid research,” said Bhatt.
This universe, he said, includes a wealth of observational research showing the benefits of omega-3s, two outcome trials – JELIS and REDUCE-IT – that showed the benefits of EPA supplementation, and two imaging studies – EVAPORATE and CHERRY – that showed favorable effects of EPA on the vasculature.
REDUCE-IT, for which Bhatt served as principal investigator, showed that treatment with icosapent ethyl (Vascepa), a high-dose purified form of EPA, led to a 25% relative risk reduction in MACE in an at-risk Western population.
The results, said Bhatt, who co-wrote an editorial that accompanies the current Sala-Vila article, “likely apply to all patients with atherosclerosis or who are at high risk for it” and supports the practice of counseling patients to increase their intake of food rich in omega-3 fatty acids.
The field may be due for a shake-up, he noted. At next month’s American Heart Association meeting, the results of another trial of another prescription-grade EPA/DHA supplement will be presented, and they are expected to be negative.
AstraZeneca announced in January 2020 the early closure of the STRENGTH trial of Epanova after an interim analysis showed a low likelihood of their product demonstrating benefit in the enrolled population.
Epanova is a fish-oil derived mixture of free fatty acids, primarily EPA and DHA. It is approved in the United States and is indicated as an adjunct to diet to reduce triglyceride levels in adults with severe (≥500 mg/dL) hypertriglyceridemia. This indication is not affected by the data from the STRENGTH trial, according to a company press release.
Sala-Vila has received grants and support from the California Walnut Commission, including a grant to support part of this study. Bayés-Genís and Bhatt have relationships with a number of companies.
This article first appeared on Medscape.com.
Vertebral fractures in COVID-19 linked to mortality
Vertebral fractures appear to be common in people with severe COVID-19, and also raise the mortality risk, findings from a retrospective cohort suggest.
Among 114 patients with COVID-19 who underwent lateral chest x-rays at the San Raffaele Hospital ED in Milan, more than a third were found to have thoracic vertebral fractures. And, those individuals were more than twice as likely to die as were those without vertebral fractures.
“Morphometric vertebral fractures are one of the most common comorbidities among adults hospitalized with COVID-19, and the presence of such fractures may predict the severity of disease outcomes,” lead investigator Andrea Giustina, MD, said in an interview.
This is the first study to examine vertebral fracture prevalence in any coronavirus disease, but such fractures have been linked to an increased risk of pneumonia and impaired respiratory function, including restrictive pulmonary dysfunction. One possible mechanism may be that they cause anatomical changes, such as kyphosis, which negatively impact respiratory function by decreasing vital capacity, forced expiratory volume in 1 second, and inspiratory time, explained Dr. Giustina, professor of endocrinology, San Raffaele Vita Salute University, Milan, and president of the European Society of Endocrinology. The results were published in the Journal of Clinical Endocrinology and Metabolism.
Clinically, the findings suggest that all patients with COVID-19 who are undergoing chest x-rays should have morphometric vertebral x-ray evaluation, said Dr. Giustina.
“One interesting aspect of the study is that without morphometry, approximatively two thirds of vertebral fractures [would have been] missed. Therefore, they are largely underestimated in clinical practice,” he noted.
Thoracic vertebral fractures assessed via lateral chest x-rays
The 114 study subjects included were those whose lateral chest x-rays allowed for a high-quality assessment and in which all the thoracic tract of T4-T12 were viewable and assessable. None had been using glucocorticoids and only 3% had a prior diagnosis of osteoporosis.
The majority (75%) were male, and median age was 57 years. Most (79%) were hospitalized after evaluation in the ED. Of those, 12% (13) were admitted to the ICU and 15% (16) died.
Thoracic vertebral fractures were detected on the lateral chest x-rays in 36% (41) of the patients. In contrast, in studies of women aged 50 years and older from the general European population, morphometric vertebral fracture prevalence ranged from 18% to 26%, the investigators noted.
Of the total 65 vertebral fractures detected, 60% were classified as mild (height ratio decrease <25%), 33.3% as moderate (25%-40% decrease) and 7.7% as severe (>40%). Patients with more than one vertebral fracture were classified by their most severe one.
Those with versus without vertebral fractures didn’t differ by sex, body mass index, or clinical or biological parameters evaluated in the ED. But, compared with those without vertebral fractures, those with them were significantly older (68 vs. 54 years) and were more likely to have arterial hypertension (56% vs. 30%) and coronary artery disease (22% vs. 7%).
In multivariate analysis, age was the only statistically significant predictor of vertebral fractures (odds ratio, 1.04; P < .001).
Mortality doubled, though not significantly
Those with vertebral fractures were more likely to be hospitalized, although not significantly (88% vs. 74%). There was no significant difference in ICU admission (11% vs. 12.5%).
However, those with vertebral fractures required noninvasive mechanical ventilation significantly more often (48.8% vs. 27.4%; P = .02), and were more than twice as likely to die (22% vs. 10%; P = .07). While the difference in overall mortality wasn’t quite statistically significant, those with severe vertebral fractures were significantly more likely to die, compared with those with mild or moderate fractures (60%, 7%, 24%, respectively, for severe, moderate, and mild; P = .04), despite no significant differences in clinical or laboratory parameters.
“Our data from the field reinforce the need of implementing previously published recommendations concerning the importance of bone fragility care during the COVID pandemic with at least those patients already treated with antiosteoporotic drugs maintaining their adherence to treatments including vitamin D, which have also been suggested very recently to have no relevant predisposing effect on COVID-19,” Dr. Giustina and colleagues wrote.
Moreover, they added, “continuity of care should also include bone density monitoring despite very restricted access to clinical facilities, during the COVID-19 pandemic. Finally, all patients with fractures should start antiresorptive treatment right away, even during hospital stay.”
The authors reported having no disclosures.
SOURCE: Giustina A et al. J Clin Endocrinol Metab. 2020 Oct 21. doi: 10.1210/clinem/dgaa738.
Vertebral fractures appear to be common in people with severe COVID-19, and also raise the mortality risk, findings from a retrospective cohort suggest.
Among 114 patients with COVID-19 who underwent lateral chest x-rays at the San Raffaele Hospital ED in Milan, more than a third were found to have thoracic vertebral fractures. And, those individuals were more than twice as likely to die as were those without vertebral fractures.
“Morphometric vertebral fractures are one of the most common comorbidities among adults hospitalized with COVID-19, and the presence of such fractures may predict the severity of disease outcomes,” lead investigator Andrea Giustina, MD, said in an interview.
This is the first study to examine vertebral fracture prevalence in any coronavirus disease, but such fractures have been linked to an increased risk of pneumonia and impaired respiratory function, including restrictive pulmonary dysfunction. One possible mechanism may be that they cause anatomical changes, such as kyphosis, which negatively impact respiratory function by decreasing vital capacity, forced expiratory volume in 1 second, and inspiratory time, explained Dr. Giustina, professor of endocrinology, San Raffaele Vita Salute University, Milan, and president of the European Society of Endocrinology. The results were published in the Journal of Clinical Endocrinology and Metabolism.
Clinically, the findings suggest that all patients with COVID-19 who are undergoing chest x-rays should have morphometric vertebral x-ray evaluation, said Dr. Giustina.
“One interesting aspect of the study is that without morphometry, approximatively two thirds of vertebral fractures [would have been] missed. Therefore, they are largely underestimated in clinical practice,” he noted.
Thoracic vertebral fractures assessed via lateral chest x-rays
The 114 study subjects included were those whose lateral chest x-rays allowed for a high-quality assessment and in which all the thoracic tract of T4-T12 were viewable and assessable. None had been using glucocorticoids and only 3% had a prior diagnosis of osteoporosis.
The majority (75%) were male, and median age was 57 years. Most (79%) were hospitalized after evaluation in the ED. Of those, 12% (13) were admitted to the ICU and 15% (16) died.
Thoracic vertebral fractures were detected on the lateral chest x-rays in 36% (41) of the patients. In contrast, in studies of women aged 50 years and older from the general European population, morphometric vertebral fracture prevalence ranged from 18% to 26%, the investigators noted.
Of the total 65 vertebral fractures detected, 60% were classified as mild (height ratio decrease <25%), 33.3% as moderate (25%-40% decrease) and 7.7% as severe (>40%). Patients with more than one vertebral fracture were classified by their most severe one.
Those with versus without vertebral fractures didn’t differ by sex, body mass index, or clinical or biological parameters evaluated in the ED. But, compared with those without vertebral fractures, those with them were significantly older (68 vs. 54 years) and were more likely to have arterial hypertension (56% vs. 30%) and coronary artery disease (22% vs. 7%).
In multivariate analysis, age was the only statistically significant predictor of vertebral fractures (odds ratio, 1.04; P < .001).
Mortality doubled, though not significantly
Those with vertebral fractures were more likely to be hospitalized, although not significantly (88% vs. 74%). There was no significant difference in ICU admission (11% vs. 12.5%).
However, those with vertebral fractures required noninvasive mechanical ventilation significantly more often (48.8% vs. 27.4%; P = .02), and were more than twice as likely to die (22% vs. 10%; P = .07). While the difference in overall mortality wasn’t quite statistically significant, those with severe vertebral fractures were significantly more likely to die, compared with those with mild or moderate fractures (60%, 7%, 24%, respectively, for severe, moderate, and mild; P = .04), despite no significant differences in clinical or laboratory parameters.
“Our data from the field reinforce the need of implementing previously published recommendations concerning the importance of bone fragility care during the COVID pandemic with at least those patients already treated with antiosteoporotic drugs maintaining their adherence to treatments including vitamin D, which have also been suggested very recently to have no relevant predisposing effect on COVID-19,” Dr. Giustina and colleagues wrote.
Moreover, they added, “continuity of care should also include bone density monitoring despite very restricted access to clinical facilities, during the COVID-19 pandemic. Finally, all patients with fractures should start antiresorptive treatment right away, even during hospital stay.”
The authors reported having no disclosures.
SOURCE: Giustina A et al. J Clin Endocrinol Metab. 2020 Oct 21. doi: 10.1210/clinem/dgaa738.
Vertebral fractures appear to be common in people with severe COVID-19, and also raise the mortality risk, findings from a retrospective cohort suggest.
Among 114 patients with COVID-19 who underwent lateral chest x-rays at the San Raffaele Hospital ED in Milan, more than a third were found to have thoracic vertebral fractures. And, those individuals were more than twice as likely to die as were those without vertebral fractures.
“Morphometric vertebral fractures are one of the most common comorbidities among adults hospitalized with COVID-19, and the presence of such fractures may predict the severity of disease outcomes,” lead investigator Andrea Giustina, MD, said in an interview.
This is the first study to examine vertebral fracture prevalence in any coronavirus disease, but such fractures have been linked to an increased risk of pneumonia and impaired respiratory function, including restrictive pulmonary dysfunction. One possible mechanism may be that they cause anatomical changes, such as kyphosis, which negatively impact respiratory function by decreasing vital capacity, forced expiratory volume in 1 second, and inspiratory time, explained Dr. Giustina, professor of endocrinology, San Raffaele Vita Salute University, Milan, and president of the European Society of Endocrinology. The results were published in the Journal of Clinical Endocrinology and Metabolism.
Clinically, the findings suggest that all patients with COVID-19 who are undergoing chest x-rays should have morphometric vertebral x-ray evaluation, said Dr. Giustina.
“One interesting aspect of the study is that without morphometry, approximatively two thirds of vertebral fractures [would have been] missed. Therefore, they are largely underestimated in clinical practice,” he noted.
Thoracic vertebral fractures assessed via lateral chest x-rays
The 114 study subjects included were those whose lateral chest x-rays allowed for a high-quality assessment and in which all the thoracic tract of T4-T12 were viewable and assessable. None had been using glucocorticoids and only 3% had a prior diagnosis of osteoporosis.
The majority (75%) were male, and median age was 57 years. Most (79%) were hospitalized after evaluation in the ED. Of those, 12% (13) were admitted to the ICU and 15% (16) died.
Thoracic vertebral fractures were detected on the lateral chest x-rays in 36% (41) of the patients. In contrast, in studies of women aged 50 years and older from the general European population, morphometric vertebral fracture prevalence ranged from 18% to 26%, the investigators noted.
Of the total 65 vertebral fractures detected, 60% were classified as mild (height ratio decrease <25%), 33.3% as moderate (25%-40% decrease) and 7.7% as severe (>40%). Patients with more than one vertebral fracture were classified by their most severe one.
Those with versus without vertebral fractures didn’t differ by sex, body mass index, or clinical or biological parameters evaluated in the ED. But, compared with those without vertebral fractures, those with them were significantly older (68 vs. 54 years) and were more likely to have arterial hypertension (56% vs. 30%) and coronary artery disease (22% vs. 7%).
In multivariate analysis, age was the only statistically significant predictor of vertebral fractures (odds ratio, 1.04; P < .001).
Mortality doubled, though not significantly
Those with vertebral fractures were more likely to be hospitalized, although not significantly (88% vs. 74%). There was no significant difference in ICU admission (11% vs. 12.5%).
However, those with vertebral fractures required noninvasive mechanical ventilation significantly more often (48.8% vs. 27.4%; P = .02), and were more than twice as likely to die (22% vs. 10%; P = .07). While the difference in overall mortality wasn’t quite statistically significant, those with severe vertebral fractures were significantly more likely to die, compared with those with mild or moderate fractures (60%, 7%, 24%, respectively, for severe, moderate, and mild; P = .04), despite no significant differences in clinical or laboratory parameters.
“Our data from the field reinforce the need of implementing previously published recommendations concerning the importance of bone fragility care during the COVID pandemic with at least those patients already treated with antiosteoporotic drugs maintaining their adherence to treatments including vitamin D, which have also been suggested very recently to have no relevant predisposing effect on COVID-19,” Dr. Giustina and colleagues wrote.
Moreover, they added, “continuity of care should also include bone density monitoring despite very restricted access to clinical facilities, during the COVID-19 pandemic. Finally, all patients with fractures should start antiresorptive treatment right away, even during hospital stay.”
The authors reported having no disclosures.
SOURCE: Giustina A et al. J Clin Endocrinol Metab. 2020 Oct 21. doi: 10.1210/clinem/dgaa738.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY AND METABOLISM
COVID-19: Immunity from antibodies may decline rapidly
Antibody response to the SARS-CoV-2 virus wanes over time, latest research has suggested.
An ongoing study led by Imperial College London (ICL) found that the proportion of people testing positive for COVID-19 antibodies dropped by 26.5% over a 3-month period between June and September.
The findings from a non–peer reviewed preprint suggested that infection with SARS-CoV-2 confers only limited protection against reinfection.
Professor Paul Elliott, director of the REACT-2 programme at ICL, said: “Testing positive for antibodies does not mean you are immune to COVID-19.
“It remains unclear what level of immunity antibodies provide, or for how long this immunity lasts.”
Experts said that, while the findings suggested that immunity might fade over time, the severity of illness from further infections could be reduced.
Antibody prevalence declined in all adults
Results from cross-sectional studies over the 3-month period involved 365,104 adults who self-administered a lateral flow immunoassay test.
There were 17,576 positive tests over the three rounds.
Antibody prevalence, adjusted for test characteristics and weighted to the adult population of England, declined from 6.0% to 4.4%, a reduction of 26.5% over the 3 months.
The decline was seen in all age groups. However, the lowest prevalence of a positive test, and the largest fall, was seen in those aged 75 years and older.
No change was seen in positive antibody tests in health care workers over the 3 months.
The results suggested that people who did not show symptoms of COVID-19 were more likely to lose detectable antibodies sooner than those who did show symptoms.
Prof Helen Ward, one of the lead authors of the report said that, while it was clear that the proportion of people with antibodies was falling over time, “We don’t yet know whether this will leave these people at risk of reinfection with the virus that causes COVID-19, but it is essential that everyone continues to follow guidance to reduce the risk to themselves and others.”
Results ‘weaken argument for herd immunity’
Commenting on the results to the Science Media Centre, Rowland Kao, professor of veterinary epidemiology and data science at the University of Edinburgh, warned that, if the results were correct, “any strategy that relies on ‘herd immunity’ lacks credibility.”
However, he added that, “while the decline is substantial, nevertheless substantial proportions of the population do retain some immune response, over 4 months after the peak of the epidemic”.
Eleanor Riley, professor of immunology and infectious disease, also from the University of Edinburgh, said it was too early to assume that immunity to SARS-CoV-2 did not last because “the study does not look at antibody concentrations, antibody function, or other aspects of immunity such as T-cell immunity and does not look at the trajectory of antibody levels in the same individuals over time”.
However, she said the findings did not mean that a vaccine would be ineffective because vaccines contained adjuvants that could induce durable immune responses, particularly with multiple immunizations.
“What is not clear is how quickly antibody levels would rise again if a person encounters the SARS-CoV-2 virus a second time. It is possible they will still rapidly respond, and either have a milder illness, or remain protected through immune memory,” commented Dr. Alexander Edwards, associate professor in biomedical technology at the University of Reading.
Health Minister Lord Bethell said: “Regardless of the result of an antibody test, everyone must continue to comply with government guidelines including social distancing, self-isolating, and getting a test if you have symptoms, and always remember: hands, face, space.”
This article first appeared on Medscape.com.
Antibody response to the SARS-CoV-2 virus wanes over time, latest research has suggested.
An ongoing study led by Imperial College London (ICL) found that the proportion of people testing positive for COVID-19 antibodies dropped by 26.5% over a 3-month period between June and September.
The findings from a non–peer reviewed preprint suggested that infection with SARS-CoV-2 confers only limited protection against reinfection.
Professor Paul Elliott, director of the REACT-2 programme at ICL, said: “Testing positive for antibodies does not mean you are immune to COVID-19.
“It remains unclear what level of immunity antibodies provide, or for how long this immunity lasts.”
Experts said that, while the findings suggested that immunity might fade over time, the severity of illness from further infections could be reduced.
Antibody prevalence declined in all adults
Results from cross-sectional studies over the 3-month period involved 365,104 adults who self-administered a lateral flow immunoassay test.
There were 17,576 positive tests over the three rounds.
Antibody prevalence, adjusted for test characteristics and weighted to the adult population of England, declined from 6.0% to 4.4%, a reduction of 26.5% over the 3 months.
The decline was seen in all age groups. However, the lowest prevalence of a positive test, and the largest fall, was seen in those aged 75 years and older.
No change was seen in positive antibody tests in health care workers over the 3 months.
The results suggested that people who did not show symptoms of COVID-19 were more likely to lose detectable antibodies sooner than those who did show symptoms.
Prof Helen Ward, one of the lead authors of the report said that, while it was clear that the proportion of people with antibodies was falling over time, “We don’t yet know whether this will leave these people at risk of reinfection with the virus that causes COVID-19, but it is essential that everyone continues to follow guidance to reduce the risk to themselves and others.”
Results ‘weaken argument for herd immunity’
Commenting on the results to the Science Media Centre, Rowland Kao, professor of veterinary epidemiology and data science at the University of Edinburgh, warned that, if the results were correct, “any strategy that relies on ‘herd immunity’ lacks credibility.”
However, he added that, “while the decline is substantial, nevertheless substantial proportions of the population do retain some immune response, over 4 months after the peak of the epidemic”.
Eleanor Riley, professor of immunology and infectious disease, also from the University of Edinburgh, said it was too early to assume that immunity to SARS-CoV-2 did not last because “the study does not look at antibody concentrations, antibody function, or other aspects of immunity such as T-cell immunity and does not look at the trajectory of antibody levels in the same individuals over time”.
However, she said the findings did not mean that a vaccine would be ineffective because vaccines contained adjuvants that could induce durable immune responses, particularly with multiple immunizations.
“What is not clear is how quickly antibody levels would rise again if a person encounters the SARS-CoV-2 virus a second time. It is possible they will still rapidly respond, and either have a milder illness, or remain protected through immune memory,” commented Dr. Alexander Edwards, associate professor in biomedical technology at the University of Reading.
Health Minister Lord Bethell said: “Regardless of the result of an antibody test, everyone must continue to comply with government guidelines including social distancing, self-isolating, and getting a test if you have symptoms, and always remember: hands, face, space.”
This article first appeared on Medscape.com.
Antibody response to the SARS-CoV-2 virus wanes over time, latest research has suggested.
An ongoing study led by Imperial College London (ICL) found that the proportion of people testing positive for COVID-19 antibodies dropped by 26.5% over a 3-month period between June and September.
The findings from a non–peer reviewed preprint suggested that infection with SARS-CoV-2 confers only limited protection against reinfection.
Professor Paul Elliott, director of the REACT-2 programme at ICL, said: “Testing positive for antibodies does not mean you are immune to COVID-19.
“It remains unclear what level of immunity antibodies provide, or for how long this immunity lasts.”
Experts said that, while the findings suggested that immunity might fade over time, the severity of illness from further infections could be reduced.
Antibody prevalence declined in all adults
Results from cross-sectional studies over the 3-month period involved 365,104 adults who self-administered a lateral flow immunoassay test.
There were 17,576 positive tests over the three rounds.
Antibody prevalence, adjusted for test characteristics and weighted to the adult population of England, declined from 6.0% to 4.4%, a reduction of 26.5% over the 3 months.
The decline was seen in all age groups. However, the lowest prevalence of a positive test, and the largest fall, was seen in those aged 75 years and older.
No change was seen in positive antibody tests in health care workers over the 3 months.
The results suggested that people who did not show symptoms of COVID-19 were more likely to lose detectable antibodies sooner than those who did show symptoms.
Prof Helen Ward, one of the lead authors of the report said that, while it was clear that the proportion of people with antibodies was falling over time, “We don’t yet know whether this will leave these people at risk of reinfection with the virus that causes COVID-19, but it is essential that everyone continues to follow guidance to reduce the risk to themselves and others.”
Results ‘weaken argument for herd immunity’
Commenting on the results to the Science Media Centre, Rowland Kao, professor of veterinary epidemiology and data science at the University of Edinburgh, warned that, if the results were correct, “any strategy that relies on ‘herd immunity’ lacks credibility.”
However, he added that, “while the decline is substantial, nevertheless substantial proportions of the population do retain some immune response, over 4 months after the peak of the epidemic”.
Eleanor Riley, professor of immunology and infectious disease, also from the University of Edinburgh, said it was too early to assume that immunity to SARS-CoV-2 did not last because “the study does not look at antibody concentrations, antibody function, or other aspects of immunity such as T-cell immunity and does not look at the trajectory of antibody levels in the same individuals over time”.
However, she said the findings did not mean that a vaccine would be ineffective because vaccines contained adjuvants that could induce durable immune responses, particularly with multiple immunizations.
“What is not clear is how quickly antibody levels would rise again if a person encounters the SARS-CoV-2 virus a second time. It is possible they will still rapidly respond, and either have a milder illness, or remain protected through immune memory,” commented Dr. Alexander Edwards, associate professor in biomedical technology at the University of Reading.
Health Minister Lord Bethell said: “Regardless of the result of an antibody test, everyone must continue to comply with government guidelines including social distancing, self-isolating, and getting a test if you have symptoms, and always remember: hands, face, space.”
This article first appeared on Medscape.com.




