User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Can anti-inflammatory medications improve symptoms and reduce mortality in schizophrenia?
Consider 3 observations:
- Evidence is mounting that cytokine abnormalities are present in schizophrenia (Box1-8).
- Reduced arterial compliance (change in volume divided by change in pressure [ΔV/ΔP] in an artery during the cardiac cycle) is an early marker of cardiovascular disease (CVD) and a robust predictor of mortality, and is associated with cytokine abnormalities.
- People with schizophrenia experience increased mortality from CVD.
Taken together, the 3 statements hint at a hypothesis: a common inflammatory process involving cytokine imbalance is associated with symptoms of schizophrenia, reduced arterial compliance, and CVD.
Anti-inflammatory therapeutics that target specific cytokines might both decrease psychiatric symptoms and reduce cardiac mortality in people with schizophrenia. In this article, we (1) highlight the potential role of anti-inflammatory medications in reducing both psychiatric symptoms and cardiac mortality in people with schizophrenia and (2) review the pathophysiological basis of this inflammatory commonality and the evidence for its presence in schizophrenia.
The ‘membrane hypothesis’ of schizophrenia
In this hypothesis, a disturbance in the synthesis and structure of membrane phospholipids results in a subsequent disturbance in the function of neuronal membrane proteins, which might be associated with symptoms and mortality in schizophrenia.9-12 The synaptic vesicle protein synaptophysin, a marker for synaptic density, was found to be decreased in postmortem tissue from the gyrus cinguli in 11 patients with schizophrenia, compared with 13 controls.10 Intracellular phospholipases A2 (inPLA2) act as key enzymes in cell membrane repair and remodeling and in neuroplasticity, neurodevelopment, apoptosis, synaptic pruning, neurodegenerative processes, and neuroinflammation.
In a study, people with first-episode schizophrenia (n = 24) who were drug-naïve or off antipsychotic medication were compared with 25 healthy controls using voxel-based morphometry analysis of T1 high-resolution MRI. inPLA2 activity was increased in the patient group compared with controls; the analysis revealed abnormalities of the frontal and medial temporal cortices, hippocampus, and left-middle and superior temporal gyri in first-episode patients.11 In another study, inPLA2 activity was increased in 35 people with first-episode schizophrenia, compared with 22 controls, and was associated with symptom severity and outcome after 12 weeks of antipsychotic treatment.12
Early CVD mortality in schizophrenia
People with schizophrenia have an elevated rate of CVD compared with the general population; in part, this elevation is linked to magnified risk factors for CVD, including obesity, metabolic syndrome, cigarette smoking, and diabetes13-17; furthermore, most antipsychotics can cause or worsen metabolic syndrome.17
CVD is one of the most common causes of death among people with schizophrenia.17,18 Their life expectancy is reported to be 51 to 61 years—20 to 25 years less than what is seen in the general population.19-21
Arterial compliance in schizophrenia
Reduced arterial compliance has been found to be a robust predictor of atherosclerosis, stroke, and myocardial infarction22-29:
- In 376 subjects who had routine diagnostic coronary angiography associated with coronary stenosis, arterial compliance was reduced significantly—even after controlling for age, sex, smoking, diabetes, hypertension, hyperlipidemia, and obesity.24
In a cross-sectional study, 63 male U.S. veterans age 18 to 70 who had a psychiatric diagnosis (16 taking quetiapine, 19 taking risperidone, and 28 treated in the past but off antipsychotics for 2 months) had significantly reduced compliance in thigh- and calf-level arteries than male controls (n = 111), adjusting for body mass index and Framingham Risk Score (FRS). Of the 63 patients, 23 had a diagnosis of schizophrenia or schizoaffective disorder.30 (The FRS is an estimate of a person’s 10-year cardiovascular risk, calculated using age, sex, total cholesterol, high-density lipoprotein, smoker or not, systolic blood pressure, and whether taking an antihypertensive or not. Compliance was measured using computerized plethysmography). Although not statistically significant, secondary analyses from this data set (n = 77, including men for whom factors for metabolic syndrome were available) showed that calf-level compliance (1.82 vs 2.06 mL) and thigh-level compliance (3.6 vs 4.26 mL; P = .06) were reduced in subjects with schizophrenia, compared with those who had another psychiatric diagnosis.31
- In another study, arterial compliance was significantly reduced in 10 subjects with schizophrenia, compared with 10 healthy controls.32
- Last, reduced total arterial compliance has been shown to be a robust predictor of mortality in older people, compared with reduced local or regional arterial compliance.33
Cytokine abnormalities in arterial compliance
The mechanism by which reduced arterial compliance is associated with cardiovascular pathology is not entirely clear. Arterial compliance is a predictor of cardiovascular disorders independent of hypertension.34 Two studies show that vascular inflammation is associated with reduced arterial compliance.35,36 Reduced arterial compliance is associated with increased angiotensin II activity; increased nicotinamide adenine dinucleotide phosphate oxidase activity; reduced nitric oxide activity; and increased reactive oxygen species.37-39 Angiotensin-II signaling activates transforming growth factor-β, tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-17, IL-6, and C-reactive protein (CRP)—all of which are associated with reduced arterial compliance.39-46 In addition, high-sensitivity CRP is significantly associated with reduced arterial compliance.47-49
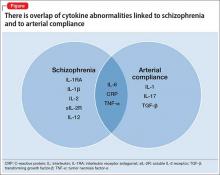
The overlap of cytokine abnormalities linked to schizophrenia and to arterial compliance is depicted in the Figure.
Anti-inflammatory medications and arterial compliance
Evidence suggests that anti-inflammatory medications increase arterial compliance:
- In 10 patients who had coronary artery disease or diabetes, or both, simvastatin (40 mg/d) was administered for 4 months. Arterial compliance improved in all 10 after 2 months of treatment and increased by 34% after 4 months.27
- Evidence also suggests that the use of omega-3 fatty acids was associated with increased arterial compliance in people with dyslipidemia.50
- Last, in people with rheumatoid arthritis, infliximab, a monoclonal antibody against TNF-Symbol Stdα, reduced aortic inflammation; this effect correlated with an increase in aortic compliance.51
Anti-inflammatory medications in schizophrenia
Two studies have yielded notable findings:
- A meta-analysis of 5 randomized controlled trials (RCTs) involving 264 subjects, comprising 4 studies of celecoxib and 1 of acetylsalicylic acid, had an effect size of 0.43 on total symptom severity. Investigators argued that acetylsalicylic acid might have the additional benefit of decreasing the risk of cardiac death in schizophrenia.52
- A review of 26 RCTs examined the efficacy of anti-inflammatory medications on symptom severity in schizophrenia. Acetylsalicylic acid, N-acetylcysteine, and estrogens had an effect size of 0.3, 0.45, and 0.51, respectively.53
Significance of these findings
A revelation that cytokine abnormalities are associated with schizophrenia symptoms and co-occurring somatic illness might offer an important new avenue of therapeutic discovery. On average, people with schizophrenia die 20 to 25 years earlier than the general population; CVD is the major cause of their death. Measuring arterial compliance, a novel noninvasive technology in psychiatry, as well as metabolic parameters, could serve as an early biomarker for assessing risk of CVD.
Implications for psychiatric practice. If inflammation plays a role in CVD in schizophrenia—either independently of factors such as metabolic syndrome, obesity, and smoking, or on the causal pathway linking these factors to reduced arterial compliance and to CVD—treatment with anti-inflammatory medications might reduce the alarming disparity of mortality that accompanies schizophrenia. In short, anti-inflammatory medications may offer a double benefit in this setting. Furthermore, success in this approach could spur clarification of the role of abnormal cytokines in other psychiatric disorders.
At this time, for your patients, consider that anti-inflammatory medications routinely used in medical practice, such as nonsteroidal anti-inflammatory drugs, omega-3 fatty acids, and statins, might alleviate psychiatric symptoms and might reduce cardiovascular mortality in schizophrenia.
Future directions
Perhaps only a limited number of cytokines are common to schizophrenia and reduced arterial compliance. Targeting those specific cytokines might, however, provide the dual benefit in schizophrenia of:
- alleviating symptoms
- reducing the rate of CVD-related mortality.
Studies are warranted to determine the value of (1) anti-inflammatory medications, such as N-acetylcysteine and infliximab and (2) anti-inflammatory combination therapy for this dual purpose. In fact, recruitment of subjects is underway for a study, Anti-Inflammatory Combination Therapy for the Treatment of Schizophrenia, at the University of Maryland (ClinicalTrials.gov Identifier: NCT01514682).
1. Potvin S, Stip E, Sepehry AA, et al. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63(8):801-808.
2. Miller BJ, Buckley P, Seabolt W, et al. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663-671.
3. Frydecka D, Misiak B, Pawlak-Adamska E, et al. Interleukin-6: the missing element of the neurocognitive deterioration in schizophrenia? The focus on genetic underpinnings, cognitive impairment and clinical manifestation. Eur Arch Psychiatry Clin Neurosci. 2015;265(6):449-459.
4. Dickerson F, Stallings C, Origoni A, et al. Additive effects of elevated C-reactive protein and exposure to herpes simplex virus type 1 on cognitive impairment in individuals with schizophrenia. Schizophr Res. 2012;134(1):83-88.
5. Dickerson F, Stallings C, Origoni A, et al. C-reactive protein is associated with the severity of cognitive impairment but not of psychiatric symptoms in individuals with schizophrenia. Schizophr Res. 2007;93(1-3):261-265.
6. Asevedo E, Rizzo LB, Gadelha A, et al. Peripheral interleukin-2 level is associated with negative symptoms and cognitive performance in schizophrenia. Physiol Behav. 2014;129:194-198.
7. Miller BJ, Culpepper N, Rapaport MH. C-reactive protein levels in schizophrenia: a review and meta-analysis. Clin Schizophr Relat Psychoses. 2014;7(4):223-230.
8. Micoulaud-Franchi JA, Faugere M, Boyer L, et al. Elevated C-reactive protein is associated with sensory gating deficit in schizophrenia. Schizophr Res. 2015;165(1):94-96.
9. Horrobin DF. The membrane phospholipid hypothesis as a biochemical basis for the neurodevelopmental concept of schizophrenia. Schizophr Res. 1998;30(3):193-208.
10. Landén M, Davidsson P, Gottfries CG, et al. Reduction of the synaptophysin level but normal levels of glycerophospholipids in the gyrus cinguli in schizophrenia. Schizophr Res. 2002;55(1-2):83-98.
11. Smesny S, Milleit B, Nenadic I, et al. Phospholipase A2 activity is associated with structural brain changes in schizophrenia. Neuroimage. 2010;52(4):1314-1327.
12. Smesny S, Kunstmann C, Kunstmann S, et al. Phospholipase A2 activity in first episode schizophrenia: associations with symptom severity and outcome at week 12. World J Biol Psychiatry. 2011;12(8):598-607.
13. Fontaine KR, Heo M, Harrigan EP, et al. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res. 2001;101(3):277-288.
14. Homel P, Casey D, Allison DB. Changes in body mass index for individuals with and without schizophrenia, 1987-1996. Schizophr Res. 2002;55(3):277-284.
15. Hedley AA, Ogden CL, Johnson CL, et al. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. JAMA. 2004;291(23):2847-2850.
16. Dickerson FB, Brown CH, Kreyenbuhl JA, et al. Obesity among individuals with serious mental illness. Acta Psychiatr Scand. 2006;113(4):306-313.
17. Newcomer JW. Metabolic syndrome and mental illness. Am J Manag Care. 2007;13(suppl 7):S170-S177.
18. Healy D, Le Noury J, Harris M, et al. Mortality in schizophrenia and related psychoses: data from two cohorts, 1875-1924 and 1994-2010. BMJ Open. 2012;2(5). doi: 10.1136/bmjopen-2012-001810.
19. Newman SC, Bland RC. Mortality in a cohort of patients with schizophrenia: a record linkage study. Can J Psychiatry. 1991;36(4):239-245.
20. Harris EC, Barraclough B. Excess mortality of mental disorder. Br J Psychiatry. 1998;173:11-53.
21. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
22. Farrar DJ, Bond MG, Riley WA, et al. Anatomic correlates of aortic pulse wave velocity and carotid artery elasticity during atherosclerosis progression and regression in monkeys. Circulation. 1991;83(5):1754-1763.
23. Wada T, Kodaira K, Fujishiro K, et al. Correlation of ultrasound-measured common carotid artery stiffness with pathological findings. Arterioscler Thromb. 1994;14(3):479-482.
24. Herrington DM, Kesler K, Reiber JH, et al. Arterial compliance adds to conventional risk factors for prediction of angiographic coronary artery disease. Am Heart J. 2013;146(4):662-667.
25. Willens HJ, Davis W, Herrington DM, et al. Relationship of peripheral arterial compliance and standard cardiovascular risk factors. Vasc Endovascular Surg. 2003;37(3):197-206.
26. Herrington DM, Brown WV, Mosca L, et al. Relationship between arterial stiffness and subclinical aortic atherosclerosis. Circulation. 2004;110(4):432-437.
27. Saliashvili G, Davis WW, Harris MT, et al. Simvastatin improved arterial compliance in high-risk patients. Vasc Endovascular Surg. 2004;38(6):519-523.
28. Le NA, Brown WV, Davis WW, et al. Comparison of the relation of triglyceride-rich lipoproteins and muscular artery compliance in healthy women versus healthy men. Am J Cardiol. 2005;95(9):1049-1054.
29. Willens HJ, Chirinos JA, Brown WV, et al. Usefulness of arterial compliance in the thigh in predicting exercise capacity in individuals without coronary heart disease. Am J Cardiol. 2005;96(2):306-310.
30. Koola MM, Brown WV, Qualls C, et al. Reduced arterial compliance in patients with psychiatric diagnoses. Schizophr Res. 2012;137(1-3):251-253.
31. Koola MM, Sorkin JD, Fargotstein M, et al. Predictors of calf arterial compliance in male veterans with psychiatric diagnoses. The Primary Care Companion for CNS Disorders. In press.
32. Phillips AA, Warburton DE, Flynn SW, et al. Assessment of arterial stiffness among schizophrenia-spectrum disorders using aortic pulse wave velocity and arterial compliance: a pilot study. Psychiatry Res. 2014;215(1):14-19.
33. Papaioannou TG, Protogerou AD, Stergiopulos N, et al. Total arterial compliance estimated by a novel method and all-cause mortality in the elderly: the PROTEGER study. Age (Dordr). 2014;36(3):9661.
34. Park S, Lakatta EG. Role of inflammation in the pathogenesis of arterial stiffness. Yonsei Med J. 2012;53(2):258-261.
35. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107(2):346-354.
36. Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25(5):932-943.
37. van der Loo B, Labugger R, Skepper JN, et al. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192(12):1731-1744.
38. Csiszar A, Ungvari Z, Edwards JG, et al. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90(11):1159-1166.
39. Wang MC, Tsai WC, Chen JY, et al. Arterial stiffness correlated with cardiac remodelling in patients with chronic kidney disease. Nephrology (Carlton). 2007;12(6):591-597.
40. Belmin J, Bernard C, Corman B, et al. Increased production of tumor necrosis factor and interleukin-6 by arterial wall of aged rats. Am J Physiol. 1995;268(6 pt 2):H2288-2293.
41. Gerli R, Monti D, Bistoni O, et al. Chemokines, sTNF-Rs and sCD30 serum levels in healthy aged people and centenarians. Mech Ageing Dev. 2000;121(1-3):37-46.
42. Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102(18):2165-2168.
43. Torzewski M, Rist C, Mortensen RF, et al. C-reactive protein in the arterial intima: role of C-reactive protein receptor-dependent monocyte recruitment in atherogenesis. Arterioscler Thromb Vasc Biol. 2000;20(9):2094-2099.
44. Venugopal SK, Devaraj S, Yuhanna I, et al. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation. 2002;106(12):1439-1441.
45. Csiszar A, Ungvari Z, Koller A, et al. Aging-induced proinflammatory shift in cytokine expression profile in coronary arteries. FASEB J. 2003;17(9):1183-1185.
46. Spinetti G, Wang M, Monticone R, et al. Rat aortic MCP-1 and its receptor CCR2 increase with age and alter vascular smooth muscle cell function. Arterioscler Thromb Vasc Biol. 2004;24(8):1397-1402.
47. Mattace-Raso FU, van der Cammen TJ, van der Meer IM, et al. C-reactive protein and arterial stiffness in older adults: the Rotterdam Study. Atherosclerosis. 2004;176(1):111-116.
48. Mahmud A, Feely J. Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension. 2005;46(5):1118-1122.
49. Nagano M, Nakamura M, Sato K, et al. Association between serum C-reactive protein levels and pulse wave velocity: a population-based cross-sectional study in a general population. Atherosclerosis. 2005;180(1):189-195.
50. Nestel P, Shige H, Pomeroy S, et al. The n-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid increase systemic arterial compliance in humans. Am J Clin Nutr. 2002;76(2):326-330.
51. Mäki-Petäjä KM, Elkhawad M, Cheriyan J, et al. Anti-tumor necrosis factor-α therapy reduces aortic inflammation and stiffness in patients with rheumatoid arthritis. Circulation. 2012;126(21):2473-2480.
52. Sommer IE, de Witte L, Begemann M, et al. Nonsteroidal anti-inflammatory drugs in schizophrenia: ready for practice or a good start? A meta-analysis. J Clin Psychiatry. 2012;73(4):414-419.
53. Sommer IE, van Westrhenen R, Begemann MJ, et al. Efficacy of anti-inflammatory agents to improve symptoms in patients with schizophrenia: an update. Schizophr Bull. 2014;40(1):181-191.
Consider 3 observations:
- Evidence is mounting that cytokine abnormalities are present in schizophrenia (Box1-8).
- Reduced arterial compliance (change in volume divided by change in pressure [ΔV/ΔP] in an artery during the cardiac cycle) is an early marker of cardiovascular disease (CVD) and a robust predictor of mortality, and is associated with cytokine abnormalities.
- People with schizophrenia experience increased mortality from CVD.
Taken together, the 3 statements hint at a hypothesis: a common inflammatory process involving cytokine imbalance is associated with symptoms of schizophrenia, reduced arterial compliance, and CVD.
Anti-inflammatory therapeutics that target specific cytokines might both decrease psychiatric symptoms and reduce cardiac mortality in people with schizophrenia. In this article, we (1) highlight the potential role of anti-inflammatory medications in reducing both psychiatric symptoms and cardiac mortality in people with schizophrenia and (2) review the pathophysiological basis of this inflammatory commonality and the evidence for its presence in schizophrenia.
The ‘membrane hypothesis’ of schizophrenia
In this hypothesis, a disturbance in the synthesis and structure of membrane phospholipids results in a subsequent disturbance in the function of neuronal membrane proteins, which might be associated with symptoms and mortality in schizophrenia.9-12 The synaptic vesicle protein synaptophysin, a marker for synaptic density, was found to be decreased in postmortem tissue from the gyrus cinguli in 11 patients with schizophrenia, compared with 13 controls.10 Intracellular phospholipases A2 (inPLA2) act as key enzymes in cell membrane repair and remodeling and in neuroplasticity, neurodevelopment, apoptosis, synaptic pruning, neurodegenerative processes, and neuroinflammation.
In a study, people with first-episode schizophrenia (n = 24) who were drug-naïve or off antipsychotic medication were compared with 25 healthy controls using voxel-based morphometry analysis of T1 high-resolution MRI. inPLA2 activity was increased in the patient group compared with controls; the analysis revealed abnormalities of the frontal and medial temporal cortices, hippocampus, and left-middle and superior temporal gyri in first-episode patients.11 In another study, inPLA2 activity was increased in 35 people with first-episode schizophrenia, compared with 22 controls, and was associated with symptom severity and outcome after 12 weeks of antipsychotic treatment.12
Early CVD mortality in schizophrenia
People with schizophrenia have an elevated rate of CVD compared with the general population; in part, this elevation is linked to magnified risk factors for CVD, including obesity, metabolic syndrome, cigarette smoking, and diabetes13-17; furthermore, most antipsychotics can cause or worsen metabolic syndrome.17
CVD is one of the most common causes of death among people with schizophrenia.17,18 Their life expectancy is reported to be 51 to 61 years—20 to 25 years less than what is seen in the general population.19-21
Arterial compliance in schizophrenia
Reduced arterial compliance has been found to be a robust predictor of atherosclerosis, stroke, and myocardial infarction22-29:
- In 376 subjects who had routine diagnostic coronary angiography associated with coronary stenosis, arterial compliance was reduced significantly—even after controlling for age, sex, smoking, diabetes, hypertension, hyperlipidemia, and obesity.24
In a cross-sectional study, 63 male U.S. veterans age 18 to 70 who had a psychiatric diagnosis (16 taking quetiapine, 19 taking risperidone, and 28 treated in the past but off antipsychotics for 2 months) had significantly reduced compliance in thigh- and calf-level arteries than male controls (n = 111), adjusting for body mass index and Framingham Risk Score (FRS). Of the 63 patients, 23 had a diagnosis of schizophrenia or schizoaffective disorder.30 (The FRS is an estimate of a person’s 10-year cardiovascular risk, calculated using age, sex, total cholesterol, high-density lipoprotein, smoker or not, systolic blood pressure, and whether taking an antihypertensive or not. Compliance was measured using computerized plethysmography). Although not statistically significant, secondary analyses from this data set (n = 77, including men for whom factors for metabolic syndrome were available) showed that calf-level compliance (1.82 vs 2.06 mL) and thigh-level compliance (3.6 vs 4.26 mL; P = .06) were reduced in subjects with schizophrenia, compared with those who had another psychiatric diagnosis.31
- In another study, arterial compliance was significantly reduced in 10 subjects with schizophrenia, compared with 10 healthy controls.32
- Last, reduced total arterial compliance has been shown to be a robust predictor of mortality in older people, compared with reduced local or regional arterial compliance.33
Cytokine abnormalities in arterial compliance
The mechanism by which reduced arterial compliance is associated with cardiovascular pathology is not entirely clear. Arterial compliance is a predictor of cardiovascular disorders independent of hypertension.34 Two studies show that vascular inflammation is associated with reduced arterial compliance.35,36 Reduced arterial compliance is associated with increased angiotensin II activity; increased nicotinamide adenine dinucleotide phosphate oxidase activity; reduced nitric oxide activity; and increased reactive oxygen species.37-39 Angiotensin-II signaling activates transforming growth factor-β, tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-17, IL-6, and C-reactive protein (CRP)—all of which are associated with reduced arterial compliance.39-46 In addition, high-sensitivity CRP is significantly associated with reduced arterial compliance.47-49
The overlap of cytokine abnormalities linked to schizophrenia and to arterial compliance is depicted in the Figure.
Anti-inflammatory medications and arterial compliance
Evidence suggests that anti-inflammatory medications increase arterial compliance:
- In 10 patients who had coronary artery disease or diabetes, or both, simvastatin (40 mg/d) was administered for 4 months. Arterial compliance improved in all 10 after 2 months of treatment and increased by 34% after 4 months.27
- Evidence also suggests that the use of omega-3 fatty acids was associated with increased arterial compliance in people with dyslipidemia.50
- Last, in people with rheumatoid arthritis, infliximab, a monoclonal antibody against TNF-Symbol Stdα, reduced aortic inflammation; this effect correlated with an increase in aortic compliance.51
Anti-inflammatory medications in schizophrenia
Two studies have yielded notable findings:
- A meta-analysis of 5 randomized controlled trials (RCTs) involving 264 subjects, comprising 4 studies of celecoxib and 1 of acetylsalicylic acid, had an effect size of 0.43 on total symptom severity. Investigators argued that acetylsalicylic acid might have the additional benefit of decreasing the risk of cardiac death in schizophrenia.52
- A review of 26 RCTs examined the efficacy of anti-inflammatory medications on symptom severity in schizophrenia. Acetylsalicylic acid, N-acetylcysteine, and estrogens had an effect size of 0.3, 0.45, and 0.51, respectively.53
Significance of these findings
A revelation that cytokine abnormalities are associated with schizophrenia symptoms and co-occurring somatic illness might offer an important new avenue of therapeutic discovery. On average, people with schizophrenia die 20 to 25 years earlier than the general population; CVD is the major cause of their death. Measuring arterial compliance, a novel noninvasive technology in psychiatry, as well as metabolic parameters, could serve as an early biomarker for assessing risk of CVD.
Implications for psychiatric practice. If inflammation plays a role in CVD in schizophrenia—either independently of factors such as metabolic syndrome, obesity, and smoking, or on the causal pathway linking these factors to reduced arterial compliance and to CVD—treatment with anti-inflammatory medications might reduce the alarming disparity of mortality that accompanies schizophrenia. In short, anti-inflammatory medications may offer a double benefit in this setting. Furthermore, success in this approach could spur clarification of the role of abnormal cytokines in other psychiatric disorders.
At this time, for your patients, consider that anti-inflammatory medications routinely used in medical practice, such as nonsteroidal anti-inflammatory drugs, omega-3 fatty acids, and statins, might alleviate psychiatric symptoms and might reduce cardiovascular mortality in schizophrenia.
Future directions
Perhaps only a limited number of cytokines are common to schizophrenia and reduced arterial compliance. Targeting those specific cytokines might, however, provide the dual benefit in schizophrenia of:
- alleviating symptoms
- reducing the rate of CVD-related mortality.
Studies are warranted to determine the value of (1) anti-inflammatory medications, such as N-acetylcysteine and infliximab and (2) anti-inflammatory combination therapy for this dual purpose. In fact, recruitment of subjects is underway for a study, Anti-Inflammatory Combination Therapy for the Treatment of Schizophrenia, at the University of Maryland (ClinicalTrials.gov Identifier: NCT01514682).
Consider 3 observations:
- Evidence is mounting that cytokine abnormalities are present in schizophrenia (Box1-8).
- Reduced arterial compliance (change in volume divided by change in pressure [ΔV/ΔP] in an artery during the cardiac cycle) is an early marker of cardiovascular disease (CVD) and a robust predictor of mortality, and is associated with cytokine abnormalities.
- People with schizophrenia experience increased mortality from CVD.
Taken together, the 3 statements hint at a hypothesis: a common inflammatory process involving cytokine imbalance is associated with symptoms of schizophrenia, reduced arterial compliance, and CVD.
Anti-inflammatory therapeutics that target specific cytokines might both decrease psychiatric symptoms and reduce cardiac mortality in people with schizophrenia. In this article, we (1) highlight the potential role of anti-inflammatory medications in reducing both psychiatric symptoms and cardiac mortality in people with schizophrenia and (2) review the pathophysiological basis of this inflammatory commonality and the evidence for its presence in schizophrenia.
The ‘membrane hypothesis’ of schizophrenia
In this hypothesis, a disturbance in the synthesis and structure of membrane phospholipids results in a subsequent disturbance in the function of neuronal membrane proteins, which might be associated with symptoms and mortality in schizophrenia.9-12 The synaptic vesicle protein synaptophysin, a marker for synaptic density, was found to be decreased in postmortem tissue from the gyrus cinguli in 11 patients with schizophrenia, compared with 13 controls.10 Intracellular phospholipases A2 (inPLA2) act as key enzymes in cell membrane repair and remodeling and in neuroplasticity, neurodevelopment, apoptosis, synaptic pruning, neurodegenerative processes, and neuroinflammation.
In a study, people with first-episode schizophrenia (n = 24) who were drug-naïve or off antipsychotic medication were compared with 25 healthy controls using voxel-based morphometry analysis of T1 high-resolution MRI. inPLA2 activity was increased in the patient group compared with controls; the analysis revealed abnormalities of the frontal and medial temporal cortices, hippocampus, and left-middle and superior temporal gyri in first-episode patients.11 In another study, inPLA2 activity was increased in 35 people with first-episode schizophrenia, compared with 22 controls, and was associated with symptom severity and outcome after 12 weeks of antipsychotic treatment.12
Early CVD mortality in schizophrenia
People with schizophrenia have an elevated rate of CVD compared with the general population; in part, this elevation is linked to magnified risk factors for CVD, including obesity, metabolic syndrome, cigarette smoking, and diabetes13-17; furthermore, most antipsychotics can cause or worsen metabolic syndrome.17
CVD is one of the most common causes of death among people with schizophrenia.17,18 Their life expectancy is reported to be 51 to 61 years—20 to 25 years less than what is seen in the general population.19-21
Arterial compliance in schizophrenia
Reduced arterial compliance has been found to be a robust predictor of atherosclerosis, stroke, and myocardial infarction22-29:
- In 376 subjects who had routine diagnostic coronary angiography associated with coronary stenosis, arterial compliance was reduced significantly—even after controlling for age, sex, smoking, diabetes, hypertension, hyperlipidemia, and obesity.24
In a cross-sectional study, 63 male U.S. veterans age 18 to 70 who had a psychiatric diagnosis (16 taking quetiapine, 19 taking risperidone, and 28 treated in the past but off antipsychotics for 2 months) had significantly reduced compliance in thigh- and calf-level arteries than male controls (n = 111), adjusting for body mass index and Framingham Risk Score (FRS). Of the 63 patients, 23 had a diagnosis of schizophrenia or schizoaffective disorder.30 (The FRS is an estimate of a person’s 10-year cardiovascular risk, calculated using age, sex, total cholesterol, high-density lipoprotein, smoker or not, systolic blood pressure, and whether taking an antihypertensive or not. Compliance was measured using computerized plethysmography). Although not statistically significant, secondary analyses from this data set (n = 77, including men for whom factors for metabolic syndrome were available) showed that calf-level compliance (1.82 vs 2.06 mL) and thigh-level compliance (3.6 vs 4.26 mL; P = .06) were reduced in subjects with schizophrenia, compared with those who had another psychiatric diagnosis.31
- In another study, arterial compliance was significantly reduced in 10 subjects with schizophrenia, compared with 10 healthy controls.32
- Last, reduced total arterial compliance has been shown to be a robust predictor of mortality in older people, compared with reduced local or regional arterial compliance.33
Cytokine abnormalities in arterial compliance
The mechanism by which reduced arterial compliance is associated with cardiovascular pathology is not entirely clear. Arterial compliance is a predictor of cardiovascular disorders independent of hypertension.34 Two studies show that vascular inflammation is associated with reduced arterial compliance.35,36 Reduced arterial compliance is associated with increased angiotensin II activity; increased nicotinamide adenine dinucleotide phosphate oxidase activity; reduced nitric oxide activity; and increased reactive oxygen species.37-39 Angiotensin-II signaling activates transforming growth factor-β, tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-17, IL-6, and C-reactive protein (CRP)—all of which are associated with reduced arterial compliance.39-46 In addition, high-sensitivity CRP is significantly associated with reduced arterial compliance.47-49
The overlap of cytokine abnormalities linked to schizophrenia and to arterial compliance is depicted in the Figure.
Anti-inflammatory medications and arterial compliance
Evidence suggests that anti-inflammatory medications increase arterial compliance:
- In 10 patients who had coronary artery disease or diabetes, or both, simvastatin (40 mg/d) was administered for 4 months. Arterial compliance improved in all 10 after 2 months of treatment and increased by 34% after 4 months.27
- Evidence also suggests that the use of omega-3 fatty acids was associated with increased arterial compliance in people with dyslipidemia.50
- Last, in people with rheumatoid arthritis, infliximab, a monoclonal antibody against TNF-Symbol Stdα, reduced aortic inflammation; this effect correlated with an increase in aortic compliance.51
Anti-inflammatory medications in schizophrenia
Two studies have yielded notable findings:
- A meta-analysis of 5 randomized controlled trials (RCTs) involving 264 subjects, comprising 4 studies of celecoxib and 1 of acetylsalicylic acid, had an effect size of 0.43 on total symptom severity. Investigators argued that acetylsalicylic acid might have the additional benefit of decreasing the risk of cardiac death in schizophrenia.52
- A review of 26 RCTs examined the efficacy of anti-inflammatory medications on symptom severity in schizophrenia. Acetylsalicylic acid, N-acetylcysteine, and estrogens had an effect size of 0.3, 0.45, and 0.51, respectively.53
Significance of these findings
A revelation that cytokine abnormalities are associated with schizophrenia symptoms and co-occurring somatic illness might offer an important new avenue of therapeutic discovery. On average, people with schizophrenia die 20 to 25 years earlier than the general population; CVD is the major cause of their death. Measuring arterial compliance, a novel noninvasive technology in psychiatry, as well as metabolic parameters, could serve as an early biomarker for assessing risk of CVD.
Implications for psychiatric practice. If inflammation plays a role in CVD in schizophrenia—either independently of factors such as metabolic syndrome, obesity, and smoking, or on the causal pathway linking these factors to reduced arterial compliance and to CVD—treatment with anti-inflammatory medications might reduce the alarming disparity of mortality that accompanies schizophrenia. In short, anti-inflammatory medications may offer a double benefit in this setting. Furthermore, success in this approach could spur clarification of the role of abnormal cytokines in other psychiatric disorders.
At this time, for your patients, consider that anti-inflammatory medications routinely used in medical practice, such as nonsteroidal anti-inflammatory drugs, omega-3 fatty acids, and statins, might alleviate psychiatric symptoms and might reduce cardiovascular mortality in schizophrenia.
Future directions
Perhaps only a limited number of cytokines are common to schizophrenia and reduced arterial compliance. Targeting those specific cytokines might, however, provide the dual benefit in schizophrenia of:
- alleviating symptoms
- reducing the rate of CVD-related mortality.
Studies are warranted to determine the value of (1) anti-inflammatory medications, such as N-acetylcysteine and infliximab and (2) anti-inflammatory combination therapy for this dual purpose. In fact, recruitment of subjects is underway for a study, Anti-Inflammatory Combination Therapy for the Treatment of Schizophrenia, at the University of Maryland (ClinicalTrials.gov Identifier: NCT01514682).
1. Potvin S, Stip E, Sepehry AA, et al. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63(8):801-808.
2. Miller BJ, Buckley P, Seabolt W, et al. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663-671.
3. Frydecka D, Misiak B, Pawlak-Adamska E, et al. Interleukin-6: the missing element of the neurocognitive deterioration in schizophrenia? The focus on genetic underpinnings, cognitive impairment and clinical manifestation. Eur Arch Psychiatry Clin Neurosci. 2015;265(6):449-459.
4. Dickerson F, Stallings C, Origoni A, et al. Additive effects of elevated C-reactive protein and exposure to herpes simplex virus type 1 on cognitive impairment in individuals with schizophrenia. Schizophr Res. 2012;134(1):83-88.
5. Dickerson F, Stallings C, Origoni A, et al. C-reactive protein is associated with the severity of cognitive impairment but not of psychiatric symptoms in individuals with schizophrenia. Schizophr Res. 2007;93(1-3):261-265.
6. Asevedo E, Rizzo LB, Gadelha A, et al. Peripheral interleukin-2 level is associated with negative symptoms and cognitive performance in schizophrenia. Physiol Behav. 2014;129:194-198.
7. Miller BJ, Culpepper N, Rapaport MH. C-reactive protein levels in schizophrenia: a review and meta-analysis. Clin Schizophr Relat Psychoses. 2014;7(4):223-230.
8. Micoulaud-Franchi JA, Faugere M, Boyer L, et al. Elevated C-reactive protein is associated with sensory gating deficit in schizophrenia. Schizophr Res. 2015;165(1):94-96.
9. Horrobin DF. The membrane phospholipid hypothesis as a biochemical basis for the neurodevelopmental concept of schizophrenia. Schizophr Res. 1998;30(3):193-208.
10. Landén M, Davidsson P, Gottfries CG, et al. Reduction of the synaptophysin level but normal levels of glycerophospholipids in the gyrus cinguli in schizophrenia. Schizophr Res. 2002;55(1-2):83-98.
11. Smesny S, Milleit B, Nenadic I, et al. Phospholipase A2 activity is associated with structural brain changes in schizophrenia. Neuroimage. 2010;52(4):1314-1327.
12. Smesny S, Kunstmann C, Kunstmann S, et al. Phospholipase A2 activity in first episode schizophrenia: associations with symptom severity and outcome at week 12. World J Biol Psychiatry. 2011;12(8):598-607.
13. Fontaine KR, Heo M, Harrigan EP, et al. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res. 2001;101(3):277-288.
14. Homel P, Casey D, Allison DB. Changes in body mass index for individuals with and without schizophrenia, 1987-1996. Schizophr Res. 2002;55(3):277-284.
15. Hedley AA, Ogden CL, Johnson CL, et al. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. JAMA. 2004;291(23):2847-2850.
16. Dickerson FB, Brown CH, Kreyenbuhl JA, et al. Obesity among individuals with serious mental illness. Acta Psychiatr Scand. 2006;113(4):306-313.
17. Newcomer JW. Metabolic syndrome and mental illness. Am J Manag Care. 2007;13(suppl 7):S170-S177.
18. Healy D, Le Noury J, Harris M, et al. Mortality in schizophrenia and related psychoses: data from two cohorts, 1875-1924 and 1994-2010. BMJ Open. 2012;2(5). doi: 10.1136/bmjopen-2012-001810.
19. Newman SC, Bland RC. Mortality in a cohort of patients with schizophrenia: a record linkage study. Can J Psychiatry. 1991;36(4):239-245.
20. Harris EC, Barraclough B. Excess mortality of mental disorder. Br J Psychiatry. 1998;173:11-53.
21. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
22. Farrar DJ, Bond MG, Riley WA, et al. Anatomic correlates of aortic pulse wave velocity and carotid artery elasticity during atherosclerosis progression and regression in monkeys. Circulation. 1991;83(5):1754-1763.
23. Wada T, Kodaira K, Fujishiro K, et al. Correlation of ultrasound-measured common carotid artery stiffness with pathological findings. Arterioscler Thromb. 1994;14(3):479-482.
24. Herrington DM, Kesler K, Reiber JH, et al. Arterial compliance adds to conventional risk factors for prediction of angiographic coronary artery disease. Am Heart J. 2013;146(4):662-667.
25. Willens HJ, Davis W, Herrington DM, et al. Relationship of peripheral arterial compliance and standard cardiovascular risk factors. Vasc Endovascular Surg. 2003;37(3):197-206.
26. Herrington DM, Brown WV, Mosca L, et al. Relationship between arterial stiffness and subclinical aortic atherosclerosis. Circulation. 2004;110(4):432-437.
27. Saliashvili G, Davis WW, Harris MT, et al. Simvastatin improved arterial compliance in high-risk patients. Vasc Endovascular Surg. 2004;38(6):519-523.
28. Le NA, Brown WV, Davis WW, et al. Comparison of the relation of triglyceride-rich lipoproteins and muscular artery compliance in healthy women versus healthy men. Am J Cardiol. 2005;95(9):1049-1054.
29. Willens HJ, Chirinos JA, Brown WV, et al. Usefulness of arterial compliance in the thigh in predicting exercise capacity in individuals without coronary heart disease. Am J Cardiol. 2005;96(2):306-310.
30. Koola MM, Brown WV, Qualls C, et al. Reduced arterial compliance in patients with psychiatric diagnoses. Schizophr Res. 2012;137(1-3):251-253.
31. Koola MM, Sorkin JD, Fargotstein M, et al. Predictors of calf arterial compliance in male veterans with psychiatric diagnoses. The Primary Care Companion for CNS Disorders. In press.
32. Phillips AA, Warburton DE, Flynn SW, et al. Assessment of arterial stiffness among schizophrenia-spectrum disorders using aortic pulse wave velocity and arterial compliance: a pilot study. Psychiatry Res. 2014;215(1):14-19.
33. Papaioannou TG, Protogerou AD, Stergiopulos N, et al. Total arterial compliance estimated by a novel method and all-cause mortality in the elderly: the PROTEGER study. Age (Dordr). 2014;36(3):9661.
34. Park S, Lakatta EG. Role of inflammation in the pathogenesis of arterial stiffness. Yonsei Med J. 2012;53(2):258-261.
35. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107(2):346-354.
36. Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25(5):932-943.
37. van der Loo B, Labugger R, Skepper JN, et al. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192(12):1731-1744.
38. Csiszar A, Ungvari Z, Edwards JG, et al. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90(11):1159-1166.
39. Wang MC, Tsai WC, Chen JY, et al. Arterial stiffness correlated with cardiac remodelling in patients with chronic kidney disease. Nephrology (Carlton). 2007;12(6):591-597.
40. Belmin J, Bernard C, Corman B, et al. Increased production of tumor necrosis factor and interleukin-6 by arterial wall of aged rats. Am J Physiol. 1995;268(6 pt 2):H2288-2293.
41. Gerli R, Monti D, Bistoni O, et al. Chemokines, sTNF-Rs and sCD30 serum levels in healthy aged people and centenarians. Mech Ageing Dev. 2000;121(1-3):37-46.
42. Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102(18):2165-2168.
43. Torzewski M, Rist C, Mortensen RF, et al. C-reactive protein in the arterial intima: role of C-reactive protein receptor-dependent monocyte recruitment in atherogenesis. Arterioscler Thromb Vasc Biol. 2000;20(9):2094-2099.
44. Venugopal SK, Devaraj S, Yuhanna I, et al. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation. 2002;106(12):1439-1441.
45. Csiszar A, Ungvari Z, Koller A, et al. Aging-induced proinflammatory shift in cytokine expression profile in coronary arteries. FASEB J. 2003;17(9):1183-1185.
46. Spinetti G, Wang M, Monticone R, et al. Rat aortic MCP-1 and its receptor CCR2 increase with age and alter vascular smooth muscle cell function. Arterioscler Thromb Vasc Biol. 2004;24(8):1397-1402.
47. Mattace-Raso FU, van der Cammen TJ, van der Meer IM, et al. C-reactive protein and arterial stiffness in older adults: the Rotterdam Study. Atherosclerosis. 2004;176(1):111-116.
48. Mahmud A, Feely J. Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension. 2005;46(5):1118-1122.
49. Nagano M, Nakamura M, Sato K, et al. Association between serum C-reactive protein levels and pulse wave velocity: a population-based cross-sectional study in a general population. Atherosclerosis. 2005;180(1):189-195.
50. Nestel P, Shige H, Pomeroy S, et al. The n-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid increase systemic arterial compliance in humans. Am J Clin Nutr. 2002;76(2):326-330.
51. Mäki-Petäjä KM, Elkhawad M, Cheriyan J, et al. Anti-tumor necrosis factor-α therapy reduces aortic inflammation and stiffness in patients with rheumatoid arthritis. Circulation. 2012;126(21):2473-2480.
52. Sommer IE, de Witte L, Begemann M, et al. Nonsteroidal anti-inflammatory drugs in schizophrenia: ready for practice or a good start? A meta-analysis. J Clin Psychiatry. 2012;73(4):414-419.
53. Sommer IE, van Westrhenen R, Begemann MJ, et al. Efficacy of anti-inflammatory agents to improve symptoms in patients with schizophrenia: an update. Schizophr Bull. 2014;40(1):181-191.
1. Potvin S, Stip E, Sepehry AA, et al. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63(8):801-808.
2. Miller BJ, Buckley P, Seabolt W, et al. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663-671.
3. Frydecka D, Misiak B, Pawlak-Adamska E, et al. Interleukin-6: the missing element of the neurocognitive deterioration in schizophrenia? The focus on genetic underpinnings, cognitive impairment and clinical manifestation. Eur Arch Psychiatry Clin Neurosci. 2015;265(6):449-459.
4. Dickerson F, Stallings C, Origoni A, et al. Additive effects of elevated C-reactive protein and exposure to herpes simplex virus type 1 on cognitive impairment in individuals with schizophrenia. Schizophr Res. 2012;134(1):83-88.
5. Dickerson F, Stallings C, Origoni A, et al. C-reactive protein is associated with the severity of cognitive impairment but not of psychiatric symptoms in individuals with schizophrenia. Schizophr Res. 2007;93(1-3):261-265.
6. Asevedo E, Rizzo LB, Gadelha A, et al. Peripheral interleukin-2 level is associated with negative symptoms and cognitive performance in schizophrenia. Physiol Behav. 2014;129:194-198.
7. Miller BJ, Culpepper N, Rapaport MH. C-reactive protein levels in schizophrenia: a review and meta-analysis. Clin Schizophr Relat Psychoses. 2014;7(4):223-230.
8. Micoulaud-Franchi JA, Faugere M, Boyer L, et al. Elevated C-reactive protein is associated with sensory gating deficit in schizophrenia. Schizophr Res. 2015;165(1):94-96.
9. Horrobin DF. The membrane phospholipid hypothesis as a biochemical basis for the neurodevelopmental concept of schizophrenia. Schizophr Res. 1998;30(3):193-208.
10. Landén M, Davidsson P, Gottfries CG, et al. Reduction of the synaptophysin level but normal levels of glycerophospholipids in the gyrus cinguli in schizophrenia. Schizophr Res. 2002;55(1-2):83-98.
11. Smesny S, Milleit B, Nenadic I, et al. Phospholipase A2 activity is associated with structural brain changes in schizophrenia. Neuroimage. 2010;52(4):1314-1327.
12. Smesny S, Kunstmann C, Kunstmann S, et al. Phospholipase A2 activity in first episode schizophrenia: associations with symptom severity and outcome at week 12. World J Biol Psychiatry. 2011;12(8):598-607.
13. Fontaine KR, Heo M, Harrigan EP, et al. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res. 2001;101(3):277-288.
14. Homel P, Casey D, Allison DB. Changes in body mass index for individuals with and without schizophrenia, 1987-1996. Schizophr Res. 2002;55(3):277-284.
15. Hedley AA, Ogden CL, Johnson CL, et al. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. JAMA. 2004;291(23):2847-2850.
16. Dickerson FB, Brown CH, Kreyenbuhl JA, et al. Obesity among individuals with serious mental illness. Acta Psychiatr Scand. 2006;113(4):306-313.
17. Newcomer JW. Metabolic syndrome and mental illness. Am J Manag Care. 2007;13(suppl 7):S170-S177.
18. Healy D, Le Noury J, Harris M, et al. Mortality in schizophrenia and related psychoses: data from two cohorts, 1875-1924 and 1994-2010. BMJ Open. 2012;2(5). doi: 10.1136/bmjopen-2012-001810.
19. Newman SC, Bland RC. Mortality in a cohort of patients with schizophrenia: a record linkage study. Can J Psychiatry. 1991;36(4):239-245.
20. Harris EC, Barraclough B. Excess mortality of mental disorder. Br J Psychiatry. 1998;173:11-53.
21. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
22. Farrar DJ, Bond MG, Riley WA, et al. Anatomic correlates of aortic pulse wave velocity and carotid artery elasticity during atherosclerosis progression and regression in monkeys. Circulation. 1991;83(5):1754-1763.
23. Wada T, Kodaira K, Fujishiro K, et al. Correlation of ultrasound-measured common carotid artery stiffness with pathological findings. Arterioscler Thromb. 1994;14(3):479-482.
24. Herrington DM, Kesler K, Reiber JH, et al. Arterial compliance adds to conventional risk factors for prediction of angiographic coronary artery disease. Am Heart J. 2013;146(4):662-667.
25. Willens HJ, Davis W, Herrington DM, et al. Relationship of peripheral arterial compliance and standard cardiovascular risk factors. Vasc Endovascular Surg. 2003;37(3):197-206.
26. Herrington DM, Brown WV, Mosca L, et al. Relationship between arterial stiffness and subclinical aortic atherosclerosis. Circulation. 2004;110(4):432-437.
27. Saliashvili G, Davis WW, Harris MT, et al. Simvastatin improved arterial compliance in high-risk patients. Vasc Endovascular Surg. 2004;38(6):519-523.
28. Le NA, Brown WV, Davis WW, et al. Comparison of the relation of triglyceride-rich lipoproteins and muscular artery compliance in healthy women versus healthy men. Am J Cardiol. 2005;95(9):1049-1054.
29. Willens HJ, Chirinos JA, Brown WV, et al. Usefulness of arterial compliance in the thigh in predicting exercise capacity in individuals without coronary heart disease. Am J Cardiol. 2005;96(2):306-310.
30. Koola MM, Brown WV, Qualls C, et al. Reduced arterial compliance in patients with psychiatric diagnoses. Schizophr Res. 2012;137(1-3):251-253.
31. Koola MM, Sorkin JD, Fargotstein M, et al. Predictors of calf arterial compliance in male veterans with psychiatric diagnoses. The Primary Care Companion for CNS Disorders. In press.
32. Phillips AA, Warburton DE, Flynn SW, et al. Assessment of arterial stiffness among schizophrenia-spectrum disorders using aortic pulse wave velocity and arterial compliance: a pilot study. Psychiatry Res. 2014;215(1):14-19.
33. Papaioannou TG, Protogerou AD, Stergiopulos N, et al. Total arterial compliance estimated by a novel method and all-cause mortality in the elderly: the PROTEGER study. Age (Dordr). 2014;36(3):9661.
34. Park S, Lakatta EG. Role of inflammation in the pathogenesis of arterial stiffness. Yonsei Med J. 2012;53(2):258-261.
35. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107(2):346-354.
36. Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25(5):932-943.
37. van der Loo B, Labugger R, Skepper JN, et al. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192(12):1731-1744.
38. Csiszar A, Ungvari Z, Edwards JG, et al. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90(11):1159-1166.
39. Wang MC, Tsai WC, Chen JY, et al. Arterial stiffness correlated with cardiac remodelling in patients with chronic kidney disease. Nephrology (Carlton). 2007;12(6):591-597.
40. Belmin J, Bernard C, Corman B, et al. Increased production of tumor necrosis factor and interleukin-6 by arterial wall of aged rats. Am J Physiol. 1995;268(6 pt 2):H2288-2293.
41. Gerli R, Monti D, Bistoni O, et al. Chemokines, sTNF-Rs and sCD30 serum levels in healthy aged people and centenarians. Mech Ageing Dev. 2000;121(1-3):37-46.
42. Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102(18):2165-2168.
43. Torzewski M, Rist C, Mortensen RF, et al. C-reactive protein in the arterial intima: role of C-reactive protein receptor-dependent monocyte recruitment in atherogenesis. Arterioscler Thromb Vasc Biol. 2000;20(9):2094-2099.
44. Venugopal SK, Devaraj S, Yuhanna I, et al. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation. 2002;106(12):1439-1441.
45. Csiszar A, Ungvari Z, Koller A, et al. Aging-induced proinflammatory shift in cytokine expression profile in coronary arteries. FASEB J. 2003;17(9):1183-1185.
46. Spinetti G, Wang M, Monticone R, et al. Rat aortic MCP-1 and its receptor CCR2 increase with age and alter vascular smooth muscle cell function. Arterioscler Thromb Vasc Biol. 2004;24(8):1397-1402.
47. Mattace-Raso FU, van der Cammen TJ, van der Meer IM, et al. C-reactive protein and arterial stiffness in older adults: the Rotterdam Study. Atherosclerosis. 2004;176(1):111-116.
48. Mahmud A, Feely J. Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension. 2005;46(5):1118-1122.
49. Nagano M, Nakamura M, Sato K, et al. Association between serum C-reactive protein levels and pulse wave velocity: a population-based cross-sectional study in a general population. Atherosclerosis. 2005;180(1):189-195.
50. Nestel P, Shige H, Pomeroy S, et al. The n-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid increase systemic arterial compliance in humans. Am J Clin Nutr. 2002;76(2):326-330.
51. Mäki-Petäjä KM, Elkhawad M, Cheriyan J, et al. Anti-tumor necrosis factor-α therapy reduces aortic inflammation and stiffness in patients with rheumatoid arthritis. Circulation. 2012;126(21):2473-2480.
52. Sommer IE, de Witte L, Begemann M, et al. Nonsteroidal anti-inflammatory drugs in schizophrenia: ready for practice or a good start? A meta-analysis. J Clin Psychiatry. 2012;73(4):414-419.
53. Sommer IE, van Westrhenen R, Begemann MJ, et al. Efficacy of anti-inflammatory agents to improve symptoms in patients with schizophrenia: an update. Schizophr Bull. 2014;40(1):181-191.
Is it a 'senior moment' or early dementia? Addressing memory concerns in older patients
Many older patients are concerned about their memory. The “worried well” may come into your office with a list of things they can’t recall, yet they remember each “deficit” quite well. Anticipatory anxiety about one’s own decline is common, and is most often concerned with changes in memory.1,2
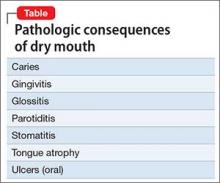
Patients with dementia or early cognitive decline often are oblivious to their cognitive changes, however. Of particular concern is progressive dementia, especially Alzheimer’s disease (AD). Although jokes about “senior moments” are common, concern about AD incurs deep-seated worry. It is essential for clinicians to differentiate normal cognitive changes of aging—particularly those in memory—from early signs of neurodegenerative disease (Table 13).
In this article, we review typical memory changes in persons age >65, and differentiate these from mild cognitive impairment (MCI), an increasingly recognized prodrome of AD. Clinicians armed with knowledge of MCI are able to reassure the worried well, or recommend neuropsychological testing as indicated.
Is memory change inevitable with aging?
Memory loss is a common problem in aging, with variable severity. Research is establishing norms in cognitive functioning through the ninth decade of life.4 Controversy about sampling, measures, and methods abound,5 and drives prolific research on the subject, which is beyond the scope of this article. It has been demonstrated that there are a few “optimally aging” persons who avoid memory decline altogether.5,6 Most researchers and clinicians agree, however, that memory change is pervasive with advancing age.
Memory change follows a gradient with recent memories lost to a greater degree than remote memories (Ribot’s Law).7 Forgetfulness is characteristic of normal aging, and frequently manifests with misplaced objects and short-term lapses. However, this is not pathological—as long as the item or memory is recalled within 24 to 48 hours.
Compared with younger adults, healthy older adults are less efficient at encoding new information. Subsequently, they have more difficulty retrieving data, particularly after a delay. The time needed to learn and use new information increases, which is referred to as processing inefficiency. This influences changes in test performance across all cognitive domains, with decreases in measures of mental processing speed, working memory, and problem-solving.
Many patients who complain about “forgetfulness” are experiencing this normal change. It is not uncommon for a patient to offer a list of things she has forgotten recently, along with the dates and circumstances in which she forgot them. Because she sometimes forgets things, but remembers them later, there likely is nothing to worry about. If reminders—such as her list—help, this too is a good sign, because it shows her resourcefulness in using accommodations. If the patient is managing her normal activities, reassurance is warranted.
Mild cognitive impairment
Since at least 1958,8 clinical observations and research have recognized a prodrome that differentiates cognitive changes predictive of dementia from those that represent typical aging. Several studies and methods have converged toward consensus that MCI is a valid construct for that purpose, with ecological validity and sound predictive value. Clinical value is evident when a patient does not meet criteria for MCI; in this case, the clinician can reassure the worried well with conviction.
Revealing the diagnosis of MCI to patients requires sensitivity and assurance that you will reevaluate the condition annually. Although there is no evidence-based remedy for MCI or means to slow its progression to dementia, data are rapidly accruing regarding the value of lifestyle changes and other nonpharmacologic interventions.9
Recognizing MCI most simply requires 2 criteria:
The patient’s expressed concern about decline in cognitive functioning from a previous level of performance. Alternately, a caretaker’s report is valuable because the patient might lack insight. You are not looking for an inability to perform activities of daily living, which is indicative of frank dementia; rather, you want to determine whether the person’s independence in functional abilities is preserved, although less efficient. Patients might repeatedly report occurrences of new problems, although modest, in some cases. Although problems with memory often are the most frequently reported symptoms, changes can be observed in any cognitive domain. Uncharacteristic inability to understand instructions, frustration with new tasks, and inflexibility are common.
Quantified clinical assessment that the patient’s cognitive decline exceeds norms of his age cohort. Clinicians are already familiar with many of these tests (5-minute recall, clock face drawing, etc.). For MCI, we recommend the Montreal Cognitive Assessment (MoCA), which is specifically designed for MCI.10 It takes only 10 minutes to administer. Multiple versions of the MoCA, and instructions for its administration are available for provider use at www.mocatest.org.
When these criteria are met—a decline in previous functioning and an objective clinical confirmation—referral for neuropsychological testing is recommended. Subtypes of MCI—amnestic and non-amnestic—have been employed to specify the subtype (amnesic) that is most consistent with prodromal AD. However, this dichotomous scheme does not adequately explain or capture the heterogeneity of MCI.11,12
Medical considerations
Just as all domains of cognition are correlated to some degree, the overall health status of a person influences evaluation of memory. Variables, such as fatigue, test anxiety, mood, motivation, visual and auditory acuity, education, language fluency, attention, and pain, affect test performance. In addition, clinician rapport and the manner in which tests are administered must be considered.
Depression can mimic MCI. A depressed patient often has poor expectations of himself and slowed thinking, and might exaggerate symptoms. He might give up on tests or refuse to complete them. His presentation initially could suggest cognitive decline, but depression is revealed when the clinician pays attention to vegetative signs (insomnia, poor appetite) or suicidal ideation. There is growing evidence that subjective complaints of memory loss are more frequently associated with depression than with objective measures of cognitive impairment.13,14
Other treatable conditions can present with cognitive change (the so-called reversible dementias). A deficiency of vitamin B12, thiamine, or folate often is seen because quality of nutrition generally decreases with age. Hyponatremia and dehydration can present with confusion and memory impairment. Other treatable conditions include:
- cerebral vasculitis, which could improve with immune suppressants
- endocrine diseases, which might respond to hormonal or surgical treatment
- normal pressure hydrocephalus, which can be relieved by surgical placement of a shunt.
Take a complete history. What exactly is the nature of the patient or caregiver’s complaint? You need to attempt to engage the patient in conversation, observing his behavior during the evaluation. Is there notable delay in response, difficulty in attention and focus, or in understanding questions?
The content of speech is an indicator of the patient’s information processing. Ask the patient to recite as many animals from the jungle as possible. Most people can come up with at least 15. The person with MCI will likely name fewer animals, but may respond well to cueing, and perform better in recognition (eg, pictures or drawings) vs retrieval. When asked to describe a typical day, the patient may offer a vague, nonchalant response eg, “I keep busy watching the news.” This kind of response may be evidence of confabulation; with further questioning, he is unable to identify current issues of interest.
Substance abuse. It is essential that clinicians recognize that elders are not exempt from alcohol and other drug abuse that affects cognition. Skilled history taking, including attention to non-verbal responses, is indicated. A defensive tone, rolling of eyes, or silent yet affirmative nodding are means by which caregivers offer essential “clues” to the provider.
A quick screening tool for the office is valuable; many clinicians are most familiar with the Mini-Mental State Examination or the Saint Louis University Mental Status Examination, which are known to be sensitive in detecting memory problems and other cognitive defects. As we noted, the MoCA is now recommended for differentiating more subtle changes of MCI.10,15 It is important to remember that common conditions such as an urinary tract infection or trauma after anesthesia for routine procedures such as colonoscopy can cause cognitive impairment. Again, eliciting history from a family member is valuable because the patient may have forgotten vital data.
A good physical exam is important when evaluating for dementia. Look for any neurologic anomaly. Check for disinhibition of primitive reflexes, eg, abnormal grasp or snout response or Babinski sign. Compare the symmetry and strength of deep tendon reflexes. Look for neurologic soft signs. Any pathological reflex response can be an important clue about neurodegeneration or space-occupying lesions. We recall seeing a 62-year-old man whose spouse brought him for evaluation for new-onset reckless driving and marked inattention to personal hygiene that developed over the previous 3 months. On examination, he appeared disheveled and had a dull affect, although disinhibited and careless. His mentation and gait were slowed. He denied distress of any kind. Frontal release signs were noted on exam. An MRI revealed a space-occupying lesion of the frontal lobe measuring 3 cm wide with a thickness of 2 cm, which pathology confirmed as a benign tumor.
Always check for arrhythmia and hypertension. These are significant risk factors for ischemic brain disease, multiple-infarct stroke, or other forms of vascular dementia. A shuffling gait suggests Parkinson’s disease, or even Lewy body dementia, or medication-related conditions, for example, from antipsychotics.
Take a medication history. Many common treatments for anxiety and insomnia can cause symptoms that mimic dementia. Digitalis toxicity results in poor recall and confusion. Combinations of common medicines (antacids, antihistamines, and others) compete for metabolic pathways and lead to altered mental status. Referencing the Beers List16 is valuable; anticholinergics, benzodiazepines, and narcotic analgesics are of special concern. The latter could still be useful for comfort care at the end of life.
It is common for seniors to take a variety of untested and unproven supplements in the hope of preventing or lessening memory problems. In addition to incurring significant costs, the indiscriminate use of supplements poses risks of toxicity, including unintended interactions with prescribed medications. Many older adults do not disclose their use of these supplements to providers because they do not consider them “medicine.”
Labs. The next level of evaluation calls for a basic laboratory workup. Check complete blood count, liver enzymes, thyroid function tests, vitamin D, B12 and folate levels; perform urinalysis and a complete metabolic panel. Look at a general hormone panel; abnormal values could reveal a pituitary adenoma. (In the past 33 years, the first author has found 42 pituitary tumors in the workup of mental status change.)
We use imaging, such as a CT or MRI of the brain, in almost all cases of suspected dementia. Cerebral atrophy, space-occupying lesions, and shifting of the ventricles often correspond with cognitive decline.
Treatment
Effective treatment of dementia remains elusive. Other than for the “reversible dementias,” pharmacotherapy has shown less progress than had been expected. Donepezil, galantamine, rivastigmine, and memantine could slow disease progression in some cases. There have been many studies for dementia preventives and treatments. Extensive reviews and meta-analyses, including those of randomized controlled trials17-19 abound for a variety of herbs, supplements, and antioxidants; none have shown compelling results. Table 2 lists Institute of Medicine recommendations supported by evidence that could reduce effects of cognitive aging.20
Recommendations from collaboration between the National Institute on Aging and the Alzheimer’s Association21 state that research should focus on biomarkers, such as neural substrates or genotypes. Indicators of oxidative stress (cytokines) and inflammation (isoprostanes) show promise as measures of brain changes that correspond with increased risk of AD or other dementias.
Summing up
Older adults are a heterogeneous group. Intellectual capacity does not diminish with advancing age. Many elders now exceed expectations for productivity, athletic ability, scientific achievement, and the creative arts. Others live longer with diminished quality of life, their health compromised by progressive neurodegenerative disease.
Age-associated memory change often is exaggerated and feared by older adults and, regrettably, is associated with inevitable functional impairment and is seen as heralding the loss of autonomy. The worried well are anxious, although the stigma associated with cognitive decline may preclude confiding their concerns.
Providers need the tools and acumen to treat patients along an increasingly long continuum of time, including conveyance of evidence-based encouragement toward optimal health and vitality.
1. Serby MJ, Yhap C, Landron EY. A study of herbal remedies for memory complaints. J Neuropsychiatry Clin Neurosci. 2010;22(3):345-347.
2. Jaremka LM, Derry HM, Bornstein R, et al. Omega-3 supplementation and loneliness-related memory problems: secondary analyses of a randomized controlled trial. Psychosom Med. 2014;76(8):650-658.
3. Depp CA, Harmell A, Vania IV. Successful cognitive aging. In: Pardon MC, Bondi MW, eds. Behavioral neurobiology of aging. New York, NY: Springer-Verlag; 2012:35-50.
4. Invik RJ, Malec JF, Smith GE, et al. Mayo’s older Americans normative studies: WAIS-R, WMS-R, and AVLT norms for ages 56 to 97. Clin Neuropsychol. 1992;6(suppl 1):1-104.
5. Powell DH, Whitla DK. Profiles in cognitive aging. Boston, MA: Harvard University Press; 1994.
6. Negash S, Smith GE, Pankratz SE, et al. Successful aging: definitions and prediction of longevity and conversion to mild cognitive impairment. Am J Geriatr Psychiatry. 2011;19(6):581-588.
7. Ribot T. Diseases of memory: an essay in the positive psychology. London, United Kingdom: Kegan Paul Trench; 1882.
8. Kral VA. Neuropsychiatric observations in old peoples home: studies of memory dysfunction in senescence. J Gerontol. 1958;13(2):169-176.
9. Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308(19):2020-2029.
10. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive assessment. J Am Geriatr Soc. 2005;53(4):695-699.
11. Clark LR, Delano-Wood L, Lisbon DJ, et al. Are empirically-derived subtypes of mild cognitive impairment consistent with conventional subtypes? J Intl Neuropsychol Soc. 2013;19(6):1-11.
12. Ganguli M, Snitz BE, Saxton JA, et al. Outcomes of mild cognitive impairment by definition: a population study. Arch Neurol. 2011;68(6):761-767.
13. Bartley M, Bokde AL, Ewers M, et al. Subjective memory complaints in community dwelling older people: the influence of brain and psychopathology. Intl J Geriatr Psychiatry. 2012;27(8):836-843.
14. Chung JC, Man DW. Self-appraised, informant-reported, and objective memory and cognitive function in mild cognitive impairment. Dement Geriatr Cogn Disord. 2009;27(2):187-193.
15. Tsoi KK, Chan JY, Hirai HW, et al. Cognitive tests to detect dementia: a systematic review and meta-analysis. JAMA Intern Med. 2015;175(9):1450-1458.
16. American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
17. May BH, Yang AW, Zhang AL, et al. Chinese herbal medicine for mild cognitive impairment and age associated memory impairment: a review of randomised controlled trials. Biogerontology. 2009;10(2):109-123.
18. Loef M, Walach H. The omega-6/omega-3 ratio and dementia or cognitive decline: a systematic review on human studies and biological evidence. J Nutr Gerontol Geriatr. 2013;32(1):1-23.
19. Solfrizzi VP, Panza F. Plant-based nutraceutical interventions against cognitive impairment and dementia: meta-analytic evidence of efficacy of a standardized Gingko biloba extract. J Alzheimers Dis. 2015;43(2):605-611.
20. Institute of Medicine. Cognitive aging: progress in understanding and opportunities for action. Washington, DC: National Academies Press; 2015.
21. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270-279.
Many older patients are concerned about their memory. The “worried well” may come into your office with a list of things they can’t recall, yet they remember each “deficit” quite well. Anticipatory anxiety about one’s own decline is common, and is most often concerned with changes in memory.1,2
Patients with dementia or early cognitive decline often are oblivious to their cognitive changes, however. Of particular concern is progressive dementia, especially Alzheimer’s disease (AD). Although jokes about “senior moments” are common, concern about AD incurs deep-seated worry. It is essential for clinicians to differentiate normal cognitive changes of aging—particularly those in memory—from early signs of neurodegenerative disease (Table 13).
In this article, we review typical memory changes in persons age >65, and differentiate these from mild cognitive impairment (MCI), an increasingly recognized prodrome of AD. Clinicians armed with knowledge of MCI are able to reassure the worried well, or recommend neuropsychological testing as indicated.
Is memory change inevitable with aging?
Memory loss is a common problem in aging, with variable severity. Research is establishing norms in cognitive functioning through the ninth decade of life.4 Controversy about sampling, measures, and methods abound,5 and drives prolific research on the subject, which is beyond the scope of this article. It has been demonstrated that there are a few “optimally aging” persons who avoid memory decline altogether.5,6 Most researchers and clinicians agree, however, that memory change is pervasive with advancing age.
Memory change follows a gradient with recent memories lost to a greater degree than remote memories (Ribot’s Law).7 Forgetfulness is characteristic of normal aging, and frequently manifests with misplaced objects and short-term lapses. However, this is not pathological—as long as the item or memory is recalled within 24 to 48 hours.
Compared with younger adults, healthy older adults are less efficient at encoding new information. Subsequently, they have more difficulty retrieving data, particularly after a delay. The time needed to learn and use new information increases, which is referred to as processing inefficiency. This influences changes in test performance across all cognitive domains, with decreases in measures of mental processing speed, working memory, and problem-solving.
Many patients who complain about “forgetfulness” are experiencing this normal change. It is not uncommon for a patient to offer a list of things she has forgotten recently, along with the dates and circumstances in which she forgot them. Because she sometimes forgets things, but remembers them later, there likely is nothing to worry about. If reminders—such as her list—help, this too is a good sign, because it shows her resourcefulness in using accommodations. If the patient is managing her normal activities, reassurance is warranted.
Mild cognitive impairment
Since at least 1958,8 clinical observations and research have recognized a prodrome that differentiates cognitive changes predictive of dementia from those that represent typical aging. Several studies and methods have converged toward consensus that MCI is a valid construct for that purpose, with ecological validity and sound predictive value. Clinical value is evident when a patient does not meet criteria for MCI; in this case, the clinician can reassure the worried well with conviction.
Revealing the diagnosis of MCI to patients requires sensitivity and assurance that you will reevaluate the condition annually. Although there is no evidence-based remedy for MCI or means to slow its progression to dementia, data are rapidly accruing regarding the value of lifestyle changes and other nonpharmacologic interventions.9
Recognizing MCI most simply requires 2 criteria:
The patient’s expressed concern about decline in cognitive functioning from a previous level of performance. Alternately, a caretaker’s report is valuable because the patient might lack insight. You are not looking for an inability to perform activities of daily living, which is indicative of frank dementia; rather, you want to determine whether the person’s independence in functional abilities is preserved, although less efficient. Patients might repeatedly report occurrences of new problems, although modest, in some cases. Although problems with memory often are the most frequently reported symptoms, changes can be observed in any cognitive domain. Uncharacteristic inability to understand instructions, frustration with new tasks, and inflexibility are common.
Quantified clinical assessment that the patient’s cognitive decline exceeds norms of his age cohort. Clinicians are already familiar with many of these tests (5-minute recall, clock face drawing, etc.). For MCI, we recommend the Montreal Cognitive Assessment (MoCA), which is specifically designed for MCI.10 It takes only 10 minutes to administer. Multiple versions of the MoCA, and instructions for its administration are available for provider use at www.mocatest.org.
When these criteria are met—a decline in previous functioning and an objective clinical confirmation—referral for neuropsychological testing is recommended. Subtypes of MCI—amnestic and non-amnestic—have been employed to specify the subtype (amnesic) that is most consistent with prodromal AD. However, this dichotomous scheme does not adequately explain or capture the heterogeneity of MCI.11,12
Medical considerations
Just as all domains of cognition are correlated to some degree, the overall health status of a person influences evaluation of memory. Variables, such as fatigue, test anxiety, mood, motivation, visual and auditory acuity, education, language fluency, attention, and pain, affect test performance. In addition, clinician rapport and the manner in which tests are administered must be considered.
Depression can mimic MCI. A depressed patient often has poor expectations of himself and slowed thinking, and might exaggerate symptoms. He might give up on tests or refuse to complete them. His presentation initially could suggest cognitive decline, but depression is revealed when the clinician pays attention to vegetative signs (insomnia, poor appetite) or suicidal ideation. There is growing evidence that subjective complaints of memory loss are more frequently associated with depression than with objective measures of cognitive impairment.13,14
Other treatable conditions can present with cognitive change (the so-called reversible dementias). A deficiency of vitamin B12, thiamine, or folate often is seen because quality of nutrition generally decreases with age. Hyponatremia and dehydration can present with confusion and memory impairment. Other treatable conditions include:
- cerebral vasculitis, which could improve with immune suppressants
- endocrine diseases, which might respond to hormonal or surgical treatment
- normal pressure hydrocephalus, which can be relieved by surgical placement of a shunt.
Take a complete history. What exactly is the nature of the patient or caregiver’s complaint? You need to attempt to engage the patient in conversation, observing his behavior during the evaluation. Is there notable delay in response, difficulty in attention and focus, or in understanding questions?
The content of speech is an indicator of the patient’s information processing. Ask the patient to recite as many animals from the jungle as possible. Most people can come up with at least 15. The person with MCI will likely name fewer animals, but may respond well to cueing, and perform better in recognition (eg, pictures or drawings) vs retrieval. When asked to describe a typical day, the patient may offer a vague, nonchalant response eg, “I keep busy watching the news.” This kind of response may be evidence of confabulation; with further questioning, he is unable to identify current issues of interest.
Substance abuse. It is essential that clinicians recognize that elders are not exempt from alcohol and other drug abuse that affects cognition. Skilled history taking, including attention to non-verbal responses, is indicated. A defensive tone, rolling of eyes, or silent yet affirmative nodding are means by which caregivers offer essential “clues” to the provider.
A quick screening tool for the office is valuable; many clinicians are most familiar with the Mini-Mental State Examination or the Saint Louis University Mental Status Examination, which are known to be sensitive in detecting memory problems and other cognitive defects. As we noted, the MoCA is now recommended for differentiating more subtle changes of MCI.10,15 It is important to remember that common conditions such as an urinary tract infection or trauma after anesthesia for routine procedures such as colonoscopy can cause cognitive impairment. Again, eliciting history from a family member is valuable because the patient may have forgotten vital data.
A good physical exam is important when evaluating for dementia. Look for any neurologic anomaly. Check for disinhibition of primitive reflexes, eg, abnormal grasp or snout response or Babinski sign. Compare the symmetry and strength of deep tendon reflexes. Look for neurologic soft signs. Any pathological reflex response can be an important clue about neurodegeneration or space-occupying lesions. We recall seeing a 62-year-old man whose spouse brought him for evaluation for new-onset reckless driving and marked inattention to personal hygiene that developed over the previous 3 months. On examination, he appeared disheveled and had a dull affect, although disinhibited and careless. His mentation and gait were slowed. He denied distress of any kind. Frontal release signs were noted on exam. An MRI revealed a space-occupying lesion of the frontal lobe measuring 3 cm wide with a thickness of 2 cm, which pathology confirmed as a benign tumor.
Always check for arrhythmia and hypertension. These are significant risk factors for ischemic brain disease, multiple-infarct stroke, or other forms of vascular dementia. A shuffling gait suggests Parkinson’s disease, or even Lewy body dementia, or medication-related conditions, for example, from antipsychotics.
Take a medication history. Many common treatments for anxiety and insomnia can cause symptoms that mimic dementia. Digitalis toxicity results in poor recall and confusion. Combinations of common medicines (antacids, antihistamines, and others) compete for metabolic pathways and lead to altered mental status. Referencing the Beers List16 is valuable; anticholinergics, benzodiazepines, and narcotic analgesics are of special concern. The latter could still be useful for comfort care at the end of life.
It is common for seniors to take a variety of untested and unproven supplements in the hope of preventing or lessening memory problems. In addition to incurring significant costs, the indiscriminate use of supplements poses risks of toxicity, including unintended interactions with prescribed medications. Many older adults do not disclose their use of these supplements to providers because they do not consider them “medicine.”
Labs. The next level of evaluation calls for a basic laboratory workup. Check complete blood count, liver enzymes, thyroid function tests, vitamin D, B12 and folate levels; perform urinalysis and a complete metabolic panel. Look at a general hormone panel; abnormal values could reveal a pituitary adenoma. (In the past 33 years, the first author has found 42 pituitary tumors in the workup of mental status change.)
We use imaging, such as a CT or MRI of the brain, in almost all cases of suspected dementia. Cerebral atrophy, space-occupying lesions, and shifting of the ventricles often correspond with cognitive decline.
Treatment
Effective treatment of dementia remains elusive. Other than for the “reversible dementias,” pharmacotherapy has shown less progress than had been expected. Donepezil, galantamine, rivastigmine, and memantine could slow disease progression in some cases. There have been many studies for dementia preventives and treatments. Extensive reviews and meta-analyses, including those of randomized controlled trials17-19 abound for a variety of herbs, supplements, and antioxidants; none have shown compelling results. Table 2 lists Institute of Medicine recommendations supported by evidence that could reduce effects of cognitive aging.20
Recommendations from collaboration between the National Institute on Aging and the Alzheimer’s Association21 state that research should focus on biomarkers, such as neural substrates or genotypes. Indicators of oxidative stress (cytokines) and inflammation (isoprostanes) show promise as measures of brain changes that correspond with increased risk of AD or other dementias.
Summing up
Older adults are a heterogeneous group. Intellectual capacity does not diminish with advancing age. Many elders now exceed expectations for productivity, athletic ability, scientific achievement, and the creative arts. Others live longer with diminished quality of life, their health compromised by progressive neurodegenerative disease.
Age-associated memory change often is exaggerated and feared by older adults and, regrettably, is associated with inevitable functional impairment and is seen as heralding the loss of autonomy. The worried well are anxious, although the stigma associated with cognitive decline may preclude confiding their concerns.
Providers need the tools and acumen to treat patients along an increasingly long continuum of time, including conveyance of evidence-based encouragement toward optimal health and vitality.
Many older patients are concerned about their memory. The “worried well” may come into your office with a list of things they can’t recall, yet they remember each “deficit” quite well. Anticipatory anxiety about one’s own decline is common, and is most often concerned with changes in memory.1,2
Patients with dementia or early cognitive decline often are oblivious to their cognitive changes, however. Of particular concern is progressive dementia, especially Alzheimer’s disease (AD). Although jokes about “senior moments” are common, concern about AD incurs deep-seated worry. It is essential for clinicians to differentiate normal cognitive changes of aging—particularly those in memory—from early signs of neurodegenerative disease (Table 13).
In this article, we review typical memory changes in persons age >65, and differentiate these from mild cognitive impairment (MCI), an increasingly recognized prodrome of AD. Clinicians armed with knowledge of MCI are able to reassure the worried well, or recommend neuropsychological testing as indicated.
Is memory change inevitable with aging?
Memory loss is a common problem in aging, with variable severity. Research is establishing norms in cognitive functioning through the ninth decade of life.4 Controversy about sampling, measures, and methods abound,5 and drives prolific research on the subject, which is beyond the scope of this article. It has been demonstrated that there are a few “optimally aging” persons who avoid memory decline altogether.5,6 Most researchers and clinicians agree, however, that memory change is pervasive with advancing age.
Memory change follows a gradient with recent memories lost to a greater degree than remote memories (Ribot’s Law).7 Forgetfulness is characteristic of normal aging, and frequently manifests with misplaced objects and short-term lapses. However, this is not pathological—as long as the item or memory is recalled within 24 to 48 hours.
Compared with younger adults, healthy older adults are less efficient at encoding new information. Subsequently, they have more difficulty retrieving data, particularly after a delay. The time needed to learn and use new information increases, which is referred to as processing inefficiency. This influences changes in test performance across all cognitive domains, with decreases in measures of mental processing speed, working memory, and problem-solving.
Many patients who complain about “forgetfulness” are experiencing this normal change. It is not uncommon for a patient to offer a list of things she has forgotten recently, along with the dates and circumstances in which she forgot them. Because she sometimes forgets things, but remembers them later, there likely is nothing to worry about. If reminders—such as her list—help, this too is a good sign, because it shows her resourcefulness in using accommodations. If the patient is managing her normal activities, reassurance is warranted.
Mild cognitive impairment
Since at least 1958,8 clinical observations and research have recognized a prodrome that differentiates cognitive changes predictive of dementia from those that represent typical aging. Several studies and methods have converged toward consensus that MCI is a valid construct for that purpose, with ecological validity and sound predictive value. Clinical value is evident when a patient does not meet criteria for MCI; in this case, the clinician can reassure the worried well with conviction.
Revealing the diagnosis of MCI to patients requires sensitivity and assurance that you will reevaluate the condition annually. Although there is no evidence-based remedy for MCI or means to slow its progression to dementia, data are rapidly accruing regarding the value of lifestyle changes and other nonpharmacologic interventions.9
Recognizing MCI most simply requires 2 criteria:
The patient’s expressed concern about decline in cognitive functioning from a previous level of performance. Alternately, a caretaker’s report is valuable because the patient might lack insight. You are not looking for an inability to perform activities of daily living, which is indicative of frank dementia; rather, you want to determine whether the person’s independence in functional abilities is preserved, although less efficient. Patients might repeatedly report occurrences of new problems, although modest, in some cases. Although problems with memory often are the most frequently reported symptoms, changes can be observed in any cognitive domain. Uncharacteristic inability to understand instructions, frustration with new tasks, and inflexibility are common.
Quantified clinical assessment that the patient’s cognitive decline exceeds norms of his age cohort. Clinicians are already familiar with many of these tests (5-minute recall, clock face drawing, etc.). For MCI, we recommend the Montreal Cognitive Assessment (MoCA), which is specifically designed for MCI.10 It takes only 10 minutes to administer. Multiple versions of the MoCA, and instructions for its administration are available for provider use at www.mocatest.org.
When these criteria are met—a decline in previous functioning and an objective clinical confirmation—referral for neuropsychological testing is recommended. Subtypes of MCI—amnestic and non-amnestic—have been employed to specify the subtype (amnesic) that is most consistent with prodromal AD. However, this dichotomous scheme does not adequately explain or capture the heterogeneity of MCI.11,12
Medical considerations
Just as all domains of cognition are correlated to some degree, the overall health status of a person influences evaluation of memory. Variables, such as fatigue, test anxiety, mood, motivation, visual and auditory acuity, education, language fluency, attention, and pain, affect test performance. In addition, clinician rapport and the manner in which tests are administered must be considered.
Depression can mimic MCI. A depressed patient often has poor expectations of himself and slowed thinking, and might exaggerate symptoms. He might give up on tests or refuse to complete them. His presentation initially could suggest cognitive decline, but depression is revealed when the clinician pays attention to vegetative signs (insomnia, poor appetite) or suicidal ideation. There is growing evidence that subjective complaints of memory loss are more frequently associated with depression than with objective measures of cognitive impairment.13,14
Other treatable conditions can present with cognitive change (the so-called reversible dementias). A deficiency of vitamin B12, thiamine, or folate often is seen because quality of nutrition generally decreases with age. Hyponatremia and dehydration can present with confusion and memory impairment. Other treatable conditions include:
- cerebral vasculitis, which could improve with immune suppressants
- endocrine diseases, which might respond to hormonal or surgical treatment
- normal pressure hydrocephalus, which can be relieved by surgical placement of a shunt.
Take a complete history. What exactly is the nature of the patient or caregiver’s complaint? You need to attempt to engage the patient in conversation, observing his behavior during the evaluation. Is there notable delay in response, difficulty in attention and focus, or in understanding questions?
The content of speech is an indicator of the patient’s information processing. Ask the patient to recite as many animals from the jungle as possible. Most people can come up with at least 15. The person with MCI will likely name fewer animals, but may respond well to cueing, and perform better in recognition (eg, pictures or drawings) vs retrieval. When asked to describe a typical day, the patient may offer a vague, nonchalant response eg, “I keep busy watching the news.” This kind of response may be evidence of confabulation; with further questioning, he is unable to identify current issues of interest.
Substance abuse. It is essential that clinicians recognize that elders are not exempt from alcohol and other drug abuse that affects cognition. Skilled history taking, including attention to non-verbal responses, is indicated. A defensive tone, rolling of eyes, or silent yet affirmative nodding are means by which caregivers offer essential “clues” to the provider.
A quick screening tool for the office is valuable; many clinicians are most familiar with the Mini-Mental State Examination or the Saint Louis University Mental Status Examination, which are known to be sensitive in detecting memory problems and other cognitive defects. As we noted, the MoCA is now recommended for differentiating more subtle changes of MCI.10,15 It is important to remember that common conditions such as an urinary tract infection or trauma after anesthesia for routine procedures such as colonoscopy can cause cognitive impairment. Again, eliciting history from a family member is valuable because the patient may have forgotten vital data.
A good physical exam is important when evaluating for dementia. Look for any neurologic anomaly. Check for disinhibition of primitive reflexes, eg, abnormal grasp or snout response or Babinski sign. Compare the symmetry and strength of deep tendon reflexes. Look for neurologic soft signs. Any pathological reflex response can be an important clue about neurodegeneration or space-occupying lesions. We recall seeing a 62-year-old man whose spouse brought him for evaluation for new-onset reckless driving and marked inattention to personal hygiene that developed over the previous 3 months. On examination, he appeared disheveled and had a dull affect, although disinhibited and careless. His mentation and gait were slowed. He denied distress of any kind. Frontal release signs were noted on exam. An MRI revealed a space-occupying lesion of the frontal lobe measuring 3 cm wide with a thickness of 2 cm, which pathology confirmed as a benign tumor.
Always check for arrhythmia and hypertension. These are significant risk factors for ischemic brain disease, multiple-infarct stroke, or other forms of vascular dementia. A shuffling gait suggests Parkinson’s disease, or even Lewy body dementia, or medication-related conditions, for example, from antipsychotics.
Take a medication history. Many common treatments for anxiety and insomnia can cause symptoms that mimic dementia. Digitalis toxicity results in poor recall and confusion. Combinations of common medicines (antacids, antihistamines, and others) compete for metabolic pathways and lead to altered mental status. Referencing the Beers List16 is valuable; anticholinergics, benzodiazepines, and narcotic analgesics are of special concern. The latter could still be useful for comfort care at the end of life.
It is common for seniors to take a variety of untested and unproven supplements in the hope of preventing or lessening memory problems. In addition to incurring significant costs, the indiscriminate use of supplements poses risks of toxicity, including unintended interactions with prescribed medications. Many older adults do not disclose their use of these supplements to providers because they do not consider them “medicine.”
Labs. The next level of evaluation calls for a basic laboratory workup. Check complete blood count, liver enzymes, thyroid function tests, vitamin D, B12 and folate levels; perform urinalysis and a complete metabolic panel. Look at a general hormone panel; abnormal values could reveal a pituitary adenoma. (In the past 33 years, the first author has found 42 pituitary tumors in the workup of mental status change.)
We use imaging, such as a CT or MRI of the brain, in almost all cases of suspected dementia. Cerebral atrophy, space-occupying lesions, and shifting of the ventricles often correspond with cognitive decline.
Treatment
Effective treatment of dementia remains elusive. Other than for the “reversible dementias,” pharmacotherapy has shown less progress than had been expected. Donepezil, galantamine, rivastigmine, and memantine could slow disease progression in some cases. There have been many studies for dementia preventives and treatments. Extensive reviews and meta-analyses, including those of randomized controlled trials17-19 abound for a variety of herbs, supplements, and antioxidants; none have shown compelling results. Table 2 lists Institute of Medicine recommendations supported by evidence that could reduce effects of cognitive aging.20
Recommendations from collaboration between the National Institute on Aging and the Alzheimer’s Association21 state that research should focus on biomarkers, such as neural substrates or genotypes. Indicators of oxidative stress (cytokines) and inflammation (isoprostanes) show promise as measures of brain changes that correspond with increased risk of AD or other dementias.
Summing up
Older adults are a heterogeneous group. Intellectual capacity does not diminish with advancing age. Many elders now exceed expectations for productivity, athletic ability, scientific achievement, and the creative arts. Others live longer with diminished quality of life, their health compromised by progressive neurodegenerative disease.
Age-associated memory change often is exaggerated and feared by older adults and, regrettably, is associated with inevitable functional impairment and is seen as heralding the loss of autonomy. The worried well are anxious, although the stigma associated with cognitive decline may preclude confiding their concerns.
Providers need the tools and acumen to treat patients along an increasingly long continuum of time, including conveyance of evidence-based encouragement toward optimal health and vitality.
1. Serby MJ, Yhap C, Landron EY. A study of herbal remedies for memory complaints. J Neuropsychiatry Clin Neurosci. 2010;22(3):345-347.
2. Jaremka LM, Derry HM, Bornstein R, et al. Omega-3 supplementation and loneliness-related memory problems: secondary analyses of a randomized controlled trial. Psychosom Med. 2014;76(8):650-658.
3. Depp CA, Harmell A, Vania IV. Successful cognitive aging. In: Pardon MC, Bondi MW, eds. Behavioral neurobiology of aging. New York, NY: Springer-Verlag; 2012:35-50.
4. Invik RJ, Malec JF, Smith GE, et al. Mayo’s older Americans normative studies: WAIS-R, WMS-R, and AVLT norms for ages 56 to 97. Clin Neuropsychol. 1992;6(suppl 1):1-104.
5. Powell DH, Whitla DK. Profiles in cognitive aging. Boston, MA: Harvard University Press; 1994.
6. Negash S, Smith GE, Pankratz SE, et al. Successful aging: definitions and prediction of longevity and conversion to mild cognitive impairment. Am J Geriatr Psychiatry. 2011;19(6):581-588.
7. Ribot T. Diseases of memory: an essay in the positive psychology. London, United Kingdom: Kegan Paul Trench; 1882.
8. Kral VA. Neuropsychiatric observations in old peoples home: studies of memory dysfunction in senescence. J Gerontol. 1958;13(2):169-176.
9. Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308(19):2020-2029.
10. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive assessment. J Am Geriatr Soc. 2005;53(4):695-699.
11. Clark LR, Delano-Wood L, Lisbon DJ, et al. Are empirically-derived subtypes of mild cognitive impairment consistent with conventional subtypes? J Intl Neuropsychol Soc. 2013;19(6):1-11.
12. Ganguli M, Snitz BE, Saxton JA, et al. Outcomes of mild cognitive impairment by definition: a population study. Arch Neurol. 2011;68(6):761-767.
13. Bartley M, Bokde AL, Ewers M, et al. Subjective memory complaints in community dwelling older people: the influence of brain and psychopathology. Intl J Geriatr Psychiatry. 2012;27(8):836-843.
14. Chung JC, Man DW. Self-appraised, informant-reported, and objective memory and cognitive function in mild cognitive impairment. Dement Geriatr Cogn Disord. 2009;27(2):187-193.
15. Tsoi KK, Chan JY, Hirai HW, et al. Cognitive tests to detect dementia: a systematic review and meta-analysis. JAMA Intern Med. 2015;175(9):1450-1458.
16. American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
17. May BH, Yang AW, Zhang AL, et al. Chinese herbal medicine for mild cognitive impairment and age associated memory impairment: a review of randomised controlled trials. Biogerontology. 2009;10(2):109-123.
18. Loef M, Walach H. The omega-6/omega-3 ratio and dementia or cognitive decline: a systematic review on human studies and biological evidence. J Nutr Gerontol Geriatr. 2013;32(1):1-23.
19. Solfrizzi VP, Panza F. Plant-based nutraceutical interventions against cognitive impairment and dementia: meta-analytic evidence of efficacy of a standardized Gingko biloba extract. J Alzheimers Dis. 2015;43(2):605-611.
20. Institute of Medicine. Cognitive aging: progress in understanding and opportunities for action. Washington, DC: National Academies Press; 2015.
21. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270-279.
1. Serby MJ, Yhap C, Landron EY. A study of herbal remedies for memory complaints. J Neuropsychiatry Clin Neurosci. 2010;22(3):345-347.
2. Jaremka LM, Derry HM, Bornstein R, et al. Omega-3 supplementation and loneliness-related memory problems: secondary analyses of a randomized controlled trial. Psychosom Med. 2014;76(8):650-658.
3. Depp CA, Harmell A, Vania IV. Successful cognitive aging. In: Pardon MC, Bondi MW, eds. Behavioral neurobiology of aging. New York, NY: Springer-Verlag; 2012:35-50.
4. Invik RJ, Malec JF, Smith GE, et al. Mayo’s older Americans normative studies: WAIS-R, WMS-R, and AVLT norms for ages 56 to 97. Clin Neuropsychol. 1992;6(suppl 1):1-104.
5. Powell DH, Whitla DK. Profiles in cognitive aging. Boston, MA: Harvard University Press; 1994.
6. Negash S, Smith GE, Pankratz SE, et al. Successful aging: definitions and prediction of longevity and conversion to mild cognitive impairment. Am J Geriatr Psychiatry. 2011;19(6):581-588.
7. Ribot T. Diseases of memory: an essay in the positive psychology. London, United Kingdom: Kegan Paul Trench; 1882.
8. Kral VA. Neuropsychiatric observations in old peoples home: studies of memory dysfunction in senescence. J Gerontol. 1958;13(2):169-176.
9. Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308(19):2020-2029.
10. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive assessment. J Am Geriatr Soc. 2005;53(4):695-699.
11. Clark LR, Delano-Wood L, Lisbon DJ, et al. Are empirically-derived subtypes of mild cognitive impairment consistent with conventional subtypes? J Intl Neuropsychol Soc. 2013;19(6):1-11.
12. Ganguli M, Snitz BE, Saxton JA, et al. Outcomes of mild cognitive impairment by definition: a population study. Arch Neurol. 2011;68(6):761-767.
13. Bartley M, Bokde AL, Ewers M, et al. Subjective memory complaints in community dwelling older people: the influence of brain and psychopathology. Intl J Geriatr Psychiatry. 2012;27(8):836-843.
14. Chung JC, Man DW. Self-appraised, informant-reported, and objective memory and cognitive function in mild cognitive impairment. Dement Geriatr Cogn Disord. 2009;27(2):187-193.
15. Tsoi KK, Chan JY, Hirai HW, et al. Cognitive tests to detect dementia: a systematic review and meta-analysis. JAMA Intern Med. 2015;175(9):1450-1458.
16. American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
17. May BH, Yang AW, Zhang AL, et al. Chinese herbal medicine for mild cognitive impairment and age associated memory impairment: a review of randomised controlled trials. Biogerontology. 2009;10(2):109-123.
18. Loef M, Walach H. The omega-6/omega-3 ratio and dementia or cognitive decline: a systematic review on human studies and biological evidence. J Nutr Gerontol Geriatr. 2013;32(1):1-23.
19. Solfrizzi VP, Panza F. Plant-based nutraceutical interventions against cognitive impairment and dementia: meta-analytic evidence of efficacy of a standardized Gingko biloba extract. J Alzheimers Dis. 2015;43(2):605-611.
20. Institute of Medicine. Cognitive aging: progress in understanding and opportunities for action. Washington, DC: National Academies Press; 2015.
21. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270-279.
Don’t assume that psychiatric patients lack capacity to make decisions about care
Some practitioners of medicine—including psychiatrists—might equate “psychosis” with incapacity, but that isn’t necessarily true. Even patients who, by most measures, are deemed psychotic might be capable of making wise and thoughtful decisions about their life. The case I describe in this article demonstrates that fact.
While rotating on a busy consultation service, I was asked to evaluate the capacity of a woman who had a diagnosis of schizophrenia and was being seen for worsening auditory hallucinations and progressive weight loss. She had a complicated medical course that eventually led to multiple requests to the consult team for a capacity evaluation.
The question of capacity in this patient, and in the psychiatric population generally, motivated me to review the literature, because the assumption by many on the medical teams involved in this patient’s care was that psychiatric patients do not have the capacity to participate in their own care. My goal here is to clarify the misconceptions in regard to this situation.
CASE REPORT
Schizophrenia, weight loss, back pain
Ms. V, age 67, a resident of a group home for the past 6 years, was brought to the emergency department (ED) because of weight loss and auditory hallucinations that had developed during the past few months. She had a history of paranoid schizophrenia that included several psychiatric hospitalizations but no known medical history.
The patient appeared cachectic and dehydrated. When approached, she was pleasant and reported hearing voices of the “devil.”
“They are not scary,” she confided. “They talk to me about art and literature.”
Over the past 6 months, Ms. V had lost 60 lb; she was now bedridden because of back pain. Collateral information obtained from staff members at the group home indicated that she had refused to get out of bed, and only intermittently took her medications or ate meals during the past few months. In general, however, she had been relatively stable over the course of her psychiatric illness, was adherent to psychiatric treatment, and had had no psychiatric hospitalizations in the past 3 decades.
Ominous development. Although the ED staff was convinced that Ms. V needed psychiatric admission, we (the consult team) first requested a detailed medical workup, including imaging studies. A CT scan showed multiple metastatic foci throughout her spine. She was admitted to the medical service.
Respiratory distress developed; her condition deteriorated. Numerous capacity consults were requested because she refused a medical workup or to sign do-not-resuscitate and do-not-intubate orders. Each time an evaluation was performed, Ms. V was deemed by various clinicians on the consult service to have decision-making capacity.
The patient grew unhappy with the staff’s insistence that she undergo more tests regardless of her stated wishes. The palliative care service determined that further workup would not benefit her medically: Ms. V’s condition would be grave and her prognosis poor regardless of what treatment she received.
The medical team continued to believe that, because that this patient had a mental illness and was actively hallucinating, she did not have the capacity to refuse any proposed treatments and tests.
What is capacity?
Capacity is an assessment of a person’s ability to make rational decisions. This includes the ability to understand, appreciate, and manipulate information in reaching such decisions. Determining whether a patient has the capacity to accept or refuse treatment is a medical decision that any physician can make; however, consultation−liaison psychiatrists are the experts who often are involved in this activity, particularly in patients who have a psychiatric comorbidity.
Capacity is evaluated by assessing 4 standards; that is, whether a patient can:
- communicate choice about proposed treatment
- understand her (his) medical situation
- appreciate the situation and its consequences
- manipulate information to reach a rational decision.1-3
- manipulate information to reach a rational decision.
CASE REPORT continued
Although Ms. V’s health was deteriorating and her auditory hallucinations were becoming worse, she appeared insightful about her medical problems, understood her prognosis, and wanted comfort care. She understood that having multiple metastases meant a poor prognosis, and that a biopsy might yield a medical diagnosis. She stated, “If it were caught earlier and I was better able to tolerate treatment, it would make sense to know for sure, but now it doesn’t make sense. I just want to have no pain in my end.”
Misconceptions
In a study by Ganzini et al,4,5 395 consultation−liaison psychiatrists, geriatricians, and geriatric psychologists responded to a survey in which they rated types of misunderstandings by clinicians who refer patients for assessment of decision-making capacity. Seventy percent reported that it is common that, when a patient has a mental illness such as schizophrenia, practitioners think that the patient lacks capacity to make medical decisions. However, results of a meta-analysis by Jeste et al,6 in which the magnitude of impairment of decisional capacity in patients with schizophrenia was assessed in comparison to that of normal subjects, suggest that the presence of schizophrenia does not necessarily mean the patient has impaired capacity.
Voluntary participation in research. Many patients with schizophrenia volunteer to participate in clinical trials even when they are acutely psychotic and admitted to a psychiatric hospital. Given the importance placed on participants’ voluntary informed consent as a prerequisite for ethical conduct of research, the cognitive and emotional impairments associated with schizophrenia raise questions about patients’ capacity to consent.
As is true in other areas of functional capacity, the ability of patients with schizophrenia to make competent decisions relates more to their overall cognitive functioning than to the presence or absence of specific symptoms of the disorder.7 Documentation of longitudinal consent-related abilities among research participants with schizophrenia in the long-term Clinical Antipsychotic Trials of Intervention Effectiveness study indicated that most participants had stable or improved consent-related abilities. Although almost 25% of participants experienced substantial worsening, only 4% fell below the study’s capacity threshold for enrollment.8
What I learned from Ms. V
A diagnosis of schizophrenia does not automatically render a person unable to make decisions about medical care. Even patients who have severe mental illness might have significant intact areas of reality testing. Ethically, it is important to at least consider that the chronically mentally ill can understand treatment options and express consistent choices.
Healthcare providers might tend to exclude psychiatric patients from end-of-life decisions because they (1) are worried about the emotional fragility of such patients and (2) assume that patients lack capacity to participate in making such important decisions. The case presented here is an example of a patient with a severe psychiatric diagnosis being able to participate in her care despite her mental state.
1. Appelbaum PS, Grisso T. Assessing patients’ capacities to consent to treatment. N Engl J Med. 1988;319(25):1635-1638.
2. Leo RJ. Competency and the capacity to make treatment decisions: a primer for primary care physicians. Prim Care Companion J Clin Psychiatry. 1999;1(5):131-141.
3. White MM, Lofwall MR. Challenges of the capacity evaluation for the consultation-liaison psychiatrist. J Psychiatr Pract. 2015;21(2):160-170.
4. Ganzini L, Volicer L, Nelson W, et al. Pitfalls in assessment of decision-making capacity. Psychosomatics. 2003;44(3):237-243.
5. Ganzini L, Volicer L, Nelson WA, et al. Ten myths about decision-making capacity. J Am Med Dir Assoc. 2005;6(3):S100-S104.
6. Jeste DV, Depp CA, Palmer BW. Magnitude of impairment in decisional capacity in people with schizophrenia compared to normal subjects: an overview. Schizophr Bull. 2005;32(1):121-128.
7. Appelbaum PS. Decisional capacity of patients with schizophrenia to consent to research: taking stock. Schizophr Bull. 2005;32(1):22-25.
8. Stroup TS, Appelbaum PS, Gu H, et al. Longitudinal consent-related abilities among research participants with schizophrenia: results from the CATIE study. Schizophr Res. 2011;130(1-3):47-52.
Some practitioners of medicine—including psychiatrists—might equate “psychosis” with incapacity, but that isn’t necessarily true. Even patients who, by most measures, are deemed psychotic might be capable of making wise and thoughtful decisions about their life. The case I describe in this article demonstrates that fact.
While rotating on a busy consultation service, I was asked to evaluate the capacity of a woman who had a diagnosis of schizophrenia and was being seen for worsening auditory hallucinations and progressive weight loss. She had a complicated medical course that eventually led to multiple requests to the consult team for a capacity evaluation.
The question of capacity in this patient, and in the psychiatric population generally, motivated me to review the literature, because the assumption by many on the medical teams involved in this patient’s care was that psychiatric patients do not have the capacity to participate in their own care. My goal here is to clarify the misconceptions in regard to this situation.
CASE REPORT
Schizophrenia, weight loss, back pain
Ms. V, age 67, a resident of a group home for the past 6 years, was brought to the emergency department (ED) because of weight loss and auditory hallucinations that had developed during the past few months. She had a history of paranoid schizophrenia that included several psychiatric hospitalizations but no known medical history.
The patient appeared cachectic and dehydrated. When approached, she was pleasant and reported hearing voices of the “devil.”
“They are not scary,” she confided. “They talk to me about art and literature.”
Over the past 6 months, Ms. V had lost 60 lb; she was now bedridden because of back pain. Collateral information obtained from staff members at the group home indicated that she had refused to get out of bed, and only intermittently took her medications or ate meals during the past few months. In general, however, she had been relatively stable over the course of her psychiatric illness, was adherent to psychiatric treatment, and had had no psychiatric hospitalizations in the past 3 decades.
Ominous development. Although the ED staff was convinced that Ms. V needed psychiatric admission, we (the consult team) first requested a detailed medical workup, including imaging studies. A CT scan showed multiple metastatic foci throughout her spine. She was admitted to the medical service.
Respiratory distress developed; her condition deteriorated. Numerous capacity consults were requested because she refused a medical workup or to sign do-not-resuscitate and do-not-intubate orders. Each time an evaluation was performed, Ms. V was deemed by various clinicians on the consult service to have decision-making capacity.
The patient grew unhappy with the staff’s insistence that she undergo more tests regardless of her stated wishes. The palliative care service determined that further workup would not benefit her medically: Ms. V’s condition would be grave and her prognosis poor regardless of what treatment she received.
The medical team continued to believe that, because that this patient had a mental illness and was actively hallucinating, she did not have the capacity to refuse any proposed treatments and tests.
What is capacity?
Capacity is an assessment of a person’s ability to make rational decisions. This includes the ability to understand, appreciate, and manipulate information in reaching such decisions. Determining whether a patient has the capacity to accept or refuse treatment is a medical decision that any physician can make; however, consultation−liaison psychiatrists are the experts who often are involved in this activity, particularly in patients who have a psychiatric comorbidity.
Capacity is evaluated by assessing 4 standards; that is, whether a patient can:
- communicate choice about proposed treatment
- understand her (his) medical situation
- appreciate the situation and its consequences
- manipulate information to reach a rational decision.1-3
- manipulate information to reach a rational decision.
CASE REPORT continued
Although Ms. V’s health was deteriorating and her auditory hallucinations were becoming worse, she appeared insightful about her medical problems, understood her prognosis, and wanted comfort care. She understood that having multiple metastases meant a poor prognosis, and that a biopsy might yield a medical diagnosis. She stated, “If it were caught earlier and I was better able to tolerate treatment, it would make sense to know for sure, but now it doesn’t make sense. I just want to have no pain in my end.”
Misconceptions
In a study by Ganzini et al,4,5 395 consultation−liaison psychiatrists, geriatricians, and geriatric psychologists responded to a survey in which they rated types of misunderstandings by clinicians who refer patients for assessment of decision-making capacity. Seventy percent reported that it is common that, when a patient has a mental illness such as schizophrenia, practitioners think that the patient lacks capacity to make medical decisions. However, results of a meta-analysis by Jeste et al,6 in which the magnitude of impairment of decisional capacity in patients with schizophrenia was assessed in comparison to that of normal subjects, suggest that the presence of schizophrenia does not necessarily mean the patient has impaired capacity.
Voluntary participation in research. Many patients with schizophrenia volunteer to participate in clinical trials even when they are acutely psychotic and admitted to a psychiatric hospital. Given the importance placed on participants’ voluntary informed consent as a prerequisite for ethical conduct of research, the cognitive and emotional impairments associated with schizophrenia raise questions about patients’ capacity to consent.
As is true in other areas of functional capacity, the ability of patients with schizophrenia to make competent decisions relates more to their overall cognitive functioning than to the presence or absence of specific symptoms of the disorder.7 Documentation of longitudinal consent-related abilities among research participants with schizophrenia in the long-term Clinical Antipsychotic Trials of Intervention Effectiveness study indicated that most participants had stable or improved consent-related abilities. Although almost 25% of participants experienced substantial worsening, only 4% fell below the study’s capacity threshold for enrollment.8
What I learned from Ms. V
A diagnosis of schizophrenia does not automatically render a person unable to make decisions about medical care. Even patients who have severe mental illness might have significant intact areas of reality testing. Ethically, it is important to at least consider that the chronically mentally ill can understand treatment options and express consistent choices.
Healthcare providers might tend to exclude psychiatric patients from end-of-life decisions because they (1) are worried about the emotional fragility of such patients and (2) assume that patients lack capacity to participate in making such important decisions. The case presented here is an example of a patient with a severe psychiatric diagnosis being able to participate in her care despite her mental state.
Some practitioners of medicine—including psychiatrists—might equate “psychosis” with incapacity, but that isn’t necessarily true. Even patients who, by most measures, are deemed psychotic might be capable of making wise and thoughtful decisions about their life. The case I describe in this article demonstrates that fact.
While rotating on a busy consultation service, I was asked to evaluate the capacity of a woman who had a diagnosis of schizophrenia and was being seen for worsening auditory hallucinations and progressive weight loss. She had a complicated medical course that eventually led to multiple requests to the consult team for a capacity evaluation.
The question of capacity in this patient, and in the psychiatric population generally, motivated me to review the literature, because the assumption by many on the medical teams involved in this patient’s care was that psychiatric patients do not have the capacity to participate in their own care. My goal here is to clarify the misconceptions in regard to this situation.
CASE REPORT
Schizophrenia, weight loss, back pain
Ms. V, age 67, a resident of a group home for the past 6 years, was brought to the emergency department (ED) because of weight loss and auditory hallucinations that had developed during the past few months. She had a history of paranoid schizophrenia that included several psychiatric hospitalizations but no known medical history.
The patient appeared cachectic and dehydrated. When approached, she was pleasant and reported hearing voices of the “devil.”
“They are not scary,” she confided. “They talk to me about art and literature.”
Over the past 6 months, Ms. V had lost 60 lb; she was now bedridden because of back pain. Collateral information obtained from staff members at the group home indicated that she had refused to get out of bed, and only intermittently took her medications or ate meals during the past few months. In general, however, she had been relatively stable over the course of her psychiatric illness, was adherent to psychiatric treatment, and had had no psychiatric hospitalizations in the past 3 decades.
Ominous development. Although the ED staff was convinced that Ms. V needed psychiatric admission, we (the consult team) first requested a detailed medical workup, including imaging studies. A CT scan showed multiple metastatic foci throughout her spine. She was admitted to the medical service.
Respiratory distress developed; her condition deteriorated. Numerous capacity consults were requested because she refused a medical workup or to sign do-not-resuscitate and do-not-intubate orders. Each time an evaluation was performed, Ms. V was deemed by various clinicians on the consult service to have decision-making capacity.
The patient grew unhappy with the staff’s insistence that she undergo more tests regardless of her stated wishes. The palliative care service determined that further workup would not benefit her medically: Ms. V’s condition would be grave and her prognosis poor regardless of what treatment she received.
The medical team continued to believe that, because that this patient had a mental illness and was actively hallucinating, she did not have the capacity to refuse any proposed treatments and tests.
What is capacity?
Capacity is an assessment of a person’s ability to make rational decisions. This includes the ability to understand, appreciate, and manipulate information in reaching such decisions. Determining whether a patient has the capacity to accept or refuse treatment is a medical decision that any physician can make; however, consultation−liaison psychiatrists are the experts who often are involved in this activity, particularly in patients who have a psychiatric comorbidity.
Capacity is evaluated by assessing 4 standards; that is, whether a patient can:
- communicate choice about proposed treatment
- understand her (his) medical situation
- appreciate the situation and its consequences
- manipulate information to reach a rational decision.1-3
- manipulate information to reach a rational decision.
CASE REPORT continued
Although Ms. V’s health was deteriorating and her auditory hallucinations were becoming worse, she appeared insightful about her medical problems, understood her prognosis, and wanted comfort care. She understood that having multiple metastases meant a poor prognosis, and that a biopsy might yield a medical diagnosis. She stated, “If it were caught earlier and I was better able to tolerate treatment, it would make sense to know for sure, but now it doesn’t make sense. I just want to have no pain in my end.”
Misconceptions
In a study by Ganzini et al,4,5 395 consultation−liaison psychiatrists, geriatricians, and geriatric psychologists responded to a survey in which they rated types of misunderstandings by clinicians who refer patients for assessment of decision-making capacity. Seventy percent reported that it is common that, when a patient has a mental illness such as schizophrenia, practitioners think that the patient lacks capacity to make medical decisions. However, results of a meta-analysis by Jeste et al,6 in which the magnitude of impairment of decisional capacity in patients with schizophrenia was assessed in comparison to that of normal subjects, suggest that the presence of schizophrenia does not necessarily mean the patient has impaired capacity.
Voluntary participation in research. Many patients with schizophrenia volunteer to participate in clinical trials even when they are acutely psychotic and admitted to a psychiatric hospital. Given the importance placed on participants’ voluntary informed consent as a prerequisite for ethical conduct of research, the cognitive and emotional impairments associated with schizophrenia raise questions about patients’ capacity to consent.
As is true in other areas of functional capacity, the ability of patients with schizophrenia to make competent decisions relates more to their overall cognitive functioning than to the presence or absence of specific symptoms of the disorder.7 Documentation of longitudinal consent-related abilities among research participants with schizophrenia in the long-term Clinical Antipsychotic Trials of Intervention Effectiveness study indicated that most participants had stable or improved consent-related abilities. Although almost 25% of participants experienced substantial worsening, only 4% fell below the study’s capacity threshold for enrollment.8
What I learned from Ms. V
A diagnosis of schizophrenia does not automatically render a person unable to make decisions about medical care. Even patients who have severe mental illness might have significant intact areas of reality testing. Ethically, it is important to at least consider that the chronically mentally ill can understand treatment options and express consistent choices.
Healthcare providers might tend to exclude psychiatric patients from end-of-life decisions because they (1) are worried about the emotional fragility of such patients and (2) assume that patients lack capacity to participate in making such important decisions. The case presented here is an example of a patient with a severe psychiatric diagnosis being able to participate in her care despite her mental state.
1. Appelbaum PS, Grisso T. Assessing patients’ capacities to consent to treatment. N Engl J Med. 1988;319(25):1635-1638.
2. Leo RJ. Competency and the capacity to make treatment decisions: a primer for primary care physicians. Prim Care Companion J Clin Psychiatry. 1999;1(5):131-141.
3. White MM, Lofwall MR. Challenges of the capacity evaluation for the consultation-liaison psychiatrist. J Psychiatr Pract. 2015;21(2):160-170.
4. Ganzini L, Volicer L, Nelson W, et al. Pitfalls in assessment of decision-making capacity. Psychosomatics. 2003;44(3):237-243.
5. Ganzini L, Volicer L, Nelson WA, et al. Ten myths about decision-making capacity. J Am Med Dir Assoc. 2005;6(3):S100-S104.
6. Jeste DV, Depp CA, Palmer BW. Magnitude of impairment in decisional capacity in people with schizophrenia compared to normal subjects: an overview. Schizophr Bull. 2005;32(1):121-128.
7. Appelbaum PS. Decisional capacity of patients with schizophrenia to consent to research: taking stock. Schizophr Bull. 2005;32(1):22-25.
8. Stroup TS, Appelbaum PS, Gu H, et al. Longitudinal consent-related abilities among research participants with schizophrenia: results from the CATIE study. Schizophr Res. 2011;130(1-3):47-52.
1. Appelbaum PS, Grisso T. Assessing patients’ capacities to consent to treatment. N Engl J Med. 1988;319(25):1635-1638.
2. Leo RJ. Competency and the capacity to make treatment decisions: a primer for primary care physicians. Prim Care Companion J Clin Psychiatry. 1999;1(5):131-141.
3. White MM, Lofwall MR. Challenges of the capacity evaluation for the consultation-liaison psychiatrist. J Psychiatr Pract. 2015;21(2):160-170.
4. Ganzini L, Volicer L, Nelson W, et al. Pitfalls in assessment of decision-making capacity. Psychosomatics. 2003;44(3):237-243.
5. Ganzini L, Volicer L, Nelson WA, et al. Ten myths about decision-making capacity. J Am Med Dir Assoc. 2005;6(3):S100-S104.
6. Jeste DV, Depp CA, Palmer BW. Magnitude of impairment in decisional capacity in people with schizophrenia compared to normal subjects: an overview. Schizophr Bull. 2005;32(1):121-128.
7. Appelbaum PS. Decisional capacity of patients with schizophrenia to consent to research: taking stock. Schizophr Bull. 2005;32(1):22-25.
8. Stroup TS, Appelbaum PS, Gu H, et al. Longitudinal consent-related abilities among research participants with schizophrenia: results from the CATIE study. Schizophr Res. 2011;130(1-3):47-52.
The ‘worried well’ and the ‘walking wounded’: How will we know them?; ‘Struggling with inner demons’
The ‘worried well’ and the ‘walking wounded’: How will we know them?
One of Dr. Henry A. Nasrallah’s resolutions (16 New Year’s resolutions for psychiatrists in 2016, From the Editor, January 2016, p. 23,24) stated that a significant percentage of one’s practice should be dedicated to the sickest patients, followed by the statement, “There are enough non-physician mental health professionals to handle the walking wounded and worried well.”
Who are the “walking wounded” and the “worried well”? These are commonly used terms, but who falls into these categories? I think it is important to get a sense of who is in these groups, because my takeaway from this editorial is that it is acceptable to let the walking wounded and worried well be treated by lesser-trained clinicians. Do these terms refer to a diagnostic group? Level of functioning? Severity of symptoms? Or severity plus chronicity? Level of suffering? Ability to “fake” looking less severe?
I wonder, am I a walking wounded or worried well? Are some of my friends, or my family members? When I see a patient, I ask myself if he (she) might be in that category.
Susan Fredriksen, MD
Private Practice
Hayesville, North Carolina
Dr. Nasrallah responds
I use those terms to refer to persons who have psychiatric symptoms but are not disabled socially or vocationally. They deserve a full psychiatric evaluation when they initially seek help, but generally do well with various types of psychotherapy, including cognitive-behavioral therapy, interpersonal therapy, psychodynamic therapy, or dialectic behavior therapy. There are many well-trained psychologists and licensed therapists who can administer those therapies as well as, or better than, some psychiatrists.
I recommended that psychiatrists dedicate a significant percentage (more than 50%) of their practice to more severely ill patients (those with psychosis, bipolar disorder, major depressive disorder, panic disorders, obsessive-compulsive disorder, posttraumatic stress disorder, etc.) because we are the only mental health professionals who can competently integrate biopsychosocial treatments for these patients and administer pharmacotherapeutic agents in addition to non-drug approaches. The supply of psychiatrists is short, and the number of seriously ill patients who need the medical expertise we can provide is large.
Henry A. Nasrallah, MD
Professor and Chair
Department of Psychiatry
Saint Louis University School of Medicine
St. Louis, Missouri
‘Struggling with inner demons’
I would hope that Dr. Nasrallah would understand that the use of the metaphor, “struggling with inner demons,” does not suggest “stupid” (Stop blaming ‘demons’ for bizarre delusions or behavior!, From the Editor, February 2016, p. 19,20,22). A celebrity, or any other person, might be struggling with intense, conflicting emotions that create chaos and distress. I would shudder if I read in The New York Times, “Well known actor’s divorce and drug use clearly leading to hypertrophied amygdala.” The term inner demons does not necessarily imply medieval superstition, but rather a well-established use of creative language.
Ron Samarian, MD
Chief, Department of Psychiatry
William Beaumont Hospital
Royal Oak, Michigan
Chair, Oakland University
William Beaumont Medical School
Rochester, Michigan
Dr. Nasrallah responds
Dr. Samarian missed the reason for my umbrage with the “inner demons” metaphor. As a psychiatrist, educator, and researcher, I am exquisitely sensitive to the poor understanding of mental illness and the rampant stigma associated with psychiatric disorders despite the incredible neurobiologic advances. Thus, I regard the metaphor that employs words like “demons” when describing intense struggles with emotional upheavals and stress as having an unfortunate connotation to the obsolete beliefs that abnormal behavior, thoughts, or mood are due to the devil and his nefarious demons.
I would welcome a metaphor that describes a depressed person as having a shrunken hippocampus, which would regrow with antidepressant or electroconvulsive therapy, because that’s the biologic truth and has no misleading connotations; the same with Dr. Samarian’s example of a hypertrophied amygdala in a person with chronic stress.
The ‘worried well’ and the ‘walking wounded’: How will we know them?
One of Dr. Henry A. Nasrallah’s resolutions (16 New Year’s resolutions for psychiatrists in 2016, From the Editor, January 2016, p. 23,24) stated that a significant percentage of one’s practice should be dedicated to the sickest patients, followed by the statement, “There are enough non-physician mental health professionals to handle the walking wounded and worried well.”
Who are the “walking wounded” and the “worried well”? These are commonly used terms, but who falls into these categories? I think it is important to get a sense of who is in these groups, because my takeaway from this editorial is that it is acceptable to let the walking wounded and worried well be treated by lesser-trained clinicians. Do these terms refer to a diagnostic group? Level of functioning? Severity of symptoms? Or severity plus chronicity? Level of suffering? Ability to “fake” looking less severe?
I wonder, am I a walking wounded or worried well? Are some of my friends, or my family members? When I see a patient, I ask myself if he (she) might be in that category.
Susan Fredriksen, MD
Private Practice
Hayesville, North Carolina
Dr. Nasrallah responds
I use those terms to refer to persons who have psychiatric symptoms but are not disabled socially or vocationally. They deserve a full psychiatric evaluation when they initially seek help, but generally do well with various types of psychotherapy, including cognitive-behavioral therapy, interpersonal therapy, psychodynamic therapy, or dialectic behavior therapy. There are many well-trained psychologists and licensed therapists who can administer those therapies as well as, or better than, some psychiatrists.
I recommended that psychiatrists dedicate a significant percentage (more than 50%) of their practice to more severely ill patients (those with psychosis, bipolar disorder, major depressive disorder, panic disorders, obsessive-compulsive disorder, posttraumatic stress disorder, etc.) because we are the only mental health professionals who can competently integrate biopsychosocial treatments for these patients and administer pharmacotherapeutic agents in addition to non-drug approaches. The supply of psychiatrists is short, and the number of seriously ill patients who need the medical expertise we can provide is large.
Henry A. Nasrallah, MD
Professor and Chair
Department of Psychiatry
Saint Louis University School of Medicine
St. Louis, Missouri
‘Struggling with inner demons’
I would hope that Dr. Nasrallah would understand that the use of the metaphor, “struggling with inner demons,” does not suggest “stupid” (Stop blaming ‘demons’ for bizarre delusions or behavior!, From the Editor, February 2016, p. 19,20,22). A celebrity, or any other person, might be struggling with intense, conflicting emotions that create chaos and distress. I would shudder if I read in The New York Times, “Well known actor’s divorce and drug use clearly leading to hypertrophied amygdala.” The term inner demons does not necessarily imply medieval superstition, but rather a well-established use of creative language.
Ron Samarian, MD
Chief, Department of Psychiatry
William Beaumont Hospital
Royal Oak, Michigan
Chair, Oakland University
William Beaumont Medical School
Rochester, Michigan
Dr. Nasrallah responds
Dr. Samarian missed the reason for my umbrage with the “inner demons” metaphor. As a psychiatrist, educator, and researcher, I am exquisitely sensitive to the poor understanding of mental illness and the rampant stigma associated with psychiatric disorders despite the incredible neurobiologic advances. Thus, I regard the metaphor that employs words like “demons” when describing intense struggles with emotional upheavals and stress as having an unfortunate connotation to the obsolete beliefs that abnormal behavior, thoughts, or mood are due to the devil and his nefarious demons.
I would welcome a metaphor that describes a depressed person as having a shrunken hippocampus, which would regrow with antidepressant or electroconvulsive therapy, because that’s the biologic truth and has no misleading connotations; the same with Dr. Samarian’s example of a hypertrophied amygdala in a person with chronic stress.
The ‘worried well’ and the ‘walking wounded’: How will we know them?
One of Dr. Henry A. Nasrallah’s resolutions (16 New Year’s resolutions for psychiatrists in 2016, From the Editor, January 2016, p. 23,24) stated that a significant percentage of one’s practice should be dedicated to the sickest patients, followed by the statement, “There are enough non-physician mental health professionals to handle the walking wounded and worried well.”
Who are the “walking wounded” and the “worried well”? These are commonly used terms, but who falls into these categories? I think it is important to get a sense of who is in these groups, because my takeaway from this editorial is that it is acceptable to let the walking wounded and worried well be treated by lesser-trained clinicians. Do these terms refer to a diagnostic group? Level of functioning? Severity of symptoms? Or severity plus chronicity? Level of suffering? Ability to “fake” looking less severe?
I wonder, am I a walking wounded or worried well? Are some of my friends, or my family members? When I see a patient, I ask myself if he (she) might be in that category.
Susan Fredriksen, MD
Private Practice
Hayesville, North Carolina
Dr. Nasrallah responds
I use those terms to refer to persons who have psychiatric symptoms but are not disabled socially or vocationally. They deserve a full psychiatric evaluation when they initially seek help, but generally do well with various types of psychotherapy, including cognitive-behavioral therapy, interpersonal therapy, psychodynamic therapy, or dialectic behavior therapy. There are many well-trained psychologists and licensed therapists who can administer those therapies as well as, or better than, some psychiatrists.
I recommended that psychiatrists dedicate a significant percentage (more than 50%) of their practice to more severely ill patients (those with psychosis, bipolar disorder, major depressive disorder, panic disorders, obsessive-compulsive disorder, posttraumatic stress disorder, etc.) because we are the only mental health professionals who can competently integrate biopsychosocial treatments for these patients and administer pharmacotherapeutic agents in addition to non-drug approaches. The supply of psychiatrists is short, and the number of seriously ill patients who need the medical expertise we can provide is large.
Henry A. Nasrallah, MD
Professor and Chair
Department of Psychiatry
Saint Louis University School of Medicine
St. Louis, Missouri
‘Struggling with inner demons’
I would hope that Dr. Nasrallah would understand that the use of the metaphor, “struggling with inner demons,” does not suggest “stupid” (Stop blaming ‘demons’ for bizarre delusions or behavior!, From the Editor, February 2016, p. 19,20,22). A celebrity, or any other person, might be struggling with intense, conflicting emotions that create chaos and distress. I would shudder if I read in The New York Times, “Well known actor’s divorce and drug use clearly leading to hypertrophied amygdala.” The term inner demons does not necessarily imply medieval superstition, but rather a well-established use of creative language.
Ron Samarian, MD
Chief, Department of Psychiatry
William Beaumont Hospital
Royal Oak, Michigan
Chair, Oakland University
William Beaumont Medical School
Rochester, Michigan
Dr. Nasrallah responds
Dr. Samarian missed the reason for my umbrage with the “inner demons” metaphor. As a psychiatrist, educator, and researcher, I am exquisitely sensitive to the poor understanding of mental illness and the rampant stigma associated with psychiatric disorders despite the incredible neurobiologic advances. Thus, I regard the metaphor that employs words like “demons” when describing intense struggles with emotional upheavals and stress as having an unfortunate connotation to the obsolete beliefs that abnormal behavior, thoughts, or mood are due to the devil and his nefarious demons.
I would welcome a metaphor that describes a depressed person as having a shrunken hippocampus, which would regrow with antidepressant or electroconvulsive therapy, because that’s the biologic truth and has no misleading connotations; the same with Dr. Samarian’s example of a hypertrophied amygdala in a person with chronic stress.
Treat insomnia in depressed, even suicidal, people
Antipsychotic polypharmacy back to monotherapy
1. Godleski LS, Kerler R, Barber JW, et al. Multiple versus single antipsychotic drug treatment in chronic psychosis. J Nerv Ment Dis. 1989;177(11):686-689.
2. Suzuki T, Uchida H, Tanaka KF, et al. Revising polypharmacy to a single antipsychotic regimen for patients with chronic schizophrenia. Int J Neuropsychopharmacol. 2004;7(2):133-142.
3. Essock SM, Schooler NR, Stroup TS, et al; Schizophrenia Trials Network. Effectiveness of switching from antipsychotic polypharmacy to monotherapy. Am J Psychiatry. 2011;168(7):702-708.
4. Hori H, Yoshimura R, Katsuki A, et al. Switching to antipsychotic monotherapy can improve attention and processing speed, and social activity in chronic schizophrenia patients. J Psychiatr Res. 2013;47(12):1843-1848.
5. Constantine RJ. Andel R. McPherson M, et al. The risks and benefits of switching patients with schizophrenia or schizoaffective disorder from two to one antipsychotic medication: a randomized controlled trial. Schizophr Res. 2015;166(1-3):194-200.
1. Godleski LS, Kerler R, Barber JW, et al. Multiple versus single antipsychotic drug treatment in chronic psychosis. J Nerv Ment Dis. 1989;177(11):686-689.
2. Suzuki T, Uchida H, Tanaka KF, et al. Revising polypharmacy to a single antipsychotic regimen for patients with chronic schizophrenia. Int J Neuropsychopharmacol. 2004;7(2):133-142.
3. Essock SM, Schooler NR, Stroup TS, et al; Schizophrenia Trials Network. Effectiveness of switching from antipsychotic polypharmacy to monotherapy. Am J Psychiatry. 2011;168(7):702-708.
4. Hori H, Yoshimura R, Katsuki A, et al. Switching to antipsychotic monotherapy can improve attention and processing speed, and social activity in chronic schizophrenia patients. J Psychiatr Res. 2013;47(12):1843-1848.
5. Constantine RJ. Andel R. McPherson M, et al. The risks and benefits of switching patients with schizophrenia or schizoaffective disorder from two to one antipsychotic medication: a randomized controlled trial. Schizophr Res. 2015;166(1-3):194-200.
1. Godleski LS, Kerler R, Barber JW, et al. Multiple versus single antipsychotic drug treatment in chronic psychosis. J Nerv Ment Dis. 1989;177(11):686-689.
2. Suzuki T, Uchida H, Tanaka KF, et al. Revising polypharmacy to a single antipsychotic regimen for patients with chronic schizophrenia. Int J Neuropsychopharmacol. 2004;7(2):133-142.
3. Essock SM, Schooler NR, Stroup TS, et al; Schizophrenia Trials Network. Effectiveness of switching from antipsychotic polypharmacy to monotherapy. Am J Psychiatry. 2011;168(7):702-708.
4. Hori H, Yoshimura R, Katsuki A, et al. Switching to antipsychotic monotherapy can improve attention and processing speed, and social activity in chronic schizophrenia patients. J Psychiatr Res. 2013;47(12):1843-1848.
5. Constantine RJ. Andel R. McPherson M, et al. The risks and benefits of switching patients with schizophrenia or schizoaffective disorder from two to one antipsychotic medication: a randomized controlled trial. Schizophr Res. 2015;166(1-3):194-200.
Manic after taking a vacation
CASE From soft-spoken to manic
Mr. K, age 36, an Asian male with no psychiatric history, arrives at the outpatient psychiatry clinic accompanied by his wife, after being referred from the emergency room the night before. He reports racing thoughts, euphoric mood, increased speech, hypergraphia, elevated self-esteem, decreased need for sleep, distractibility, and increased goal-directed activity. Notably, Mr. K states that he likes how he is feeling.
Mr. K’s wife says that his condition is a clear change from his baseline demeanor: soft-spoken and mild-mannered.
Mr. K reports that his symptoms started approximately 10 days earlier, after he returned from a cruise with his wife. During the cruise, he used a scopolamine patch to prevent motion sickness. Mr. K and his wife say that they believe that the scopolamine patch caused his symptoms.
Can scopolamine cause mania?
a) No
b) Yes; this is well-documented in the literature
c) It is theoretically possible because of scopolamine’s antidepressant and central anticholinergic effects
TREATMENT Lithium, close follow up
Mr. K has no history of psychiatric illness or substance use and no recent use of psychoactive substances—other than scopolamine—that could trigger a manic episode. His family history is significant for a younger brother who had a single manic episode at age 12 and a suicide attempt as a young adult.
Mr. K works full-time on rotating shifts—including some overnight shifts—as a manufacturing supervisor at a biotechnology company. He has been unable to work since returning from the cruise because of his psychiatric symptoms.
Mr. K is started on sustained-release (SR) lithium, 900 mg/d. In addition, the psychiatrist advises Mr. K to continue taking clonazepam, 0.5 to 1 mg as needed, which the emergency medicine physician prescribed, for insomnia. Mr. K is referred to a psychiatric intensive outpatient program (IOP), 3 days a week for 2 weeks, and is advised to stay home from work until symptoms stabilize.
Mr. K follows up closely with the psychiatrist in the clinic, every 1 to 2 weeks for the first month, as well as by several telephone and e-mail contacts. Lithium SR is titrated to 1,200 mg/d, to a therapeutic serum level of 1.1 mEq/L. Clonazepam is switched to quetiapine, 25 to 50 mg as needed, to address ongoing insomnia and to reduce the risk of dependency on clonazepam.
Mr. K’s mania gradually abates. He finishes the IOP and returns to work 3 weeks after his initial presentation. At an office visit, Mr. K’s wife gives the psychiatrist 2 scientific articles documenting the antidepressant effect of scopolamine.1,2 Mr. K and his wife both continue to believe that Mr. K’s manic episode was triggered by the scopolamine patch he used while on the cruise. They think this is important because Mr. K believes he would not have developed mania otherwise, and he does not want to take a mood stabilizer for the rest of his life.
The author’s observations
There are several proposed mechanisms for scopolamine’s antidepressant effect (Table 1).3-9 Scopolamine blocks central muscarinic cholinergic receptors, which reduces production of glutamate receptors and leads to reduced glutamate transmission and neurotoxicity.3,4 Scopolamine—similar to ketamine—could enhance synaptogenesis and synaptic signaling.5,6 Also, by blocking muscarinic autoreceptors, scopolamine results in an acute upregulation of acetylcholine release, which, in turn, influences the nicotinic, dopamine, serotonin, and neuropeptide Y systems. This action could contribute to anti-inflammatory effects, all of which can benefit mood.7-9 These antidepressant mechanisms also could explain why, theoretically, scopolamine could precipitate mania in a person predisposed to mental illness.
Proposed by Janowsky et al10 in 1972, the cholinergic−adrenergic balance hypothesis of affective disorders suggests that depression represents an excess of central cholinergic tone over adrenergic tone, and that mania represents the opposite imbalance. Several lines of evidence in the literature support this theory. For example, depressed patients have been found to have hypersensitive central cholinergic receptors.11,12 Also, central cholinesterase inhibition has been shown to affect pituitary hormone and epinephrine levels via central muscarinic receptors.13 In addition, scopolamine has been shown to attenuate these effects via the central anti-muscarinic mechanism.14
Rapid antidepressant therapy. Scopolamine is being studied as a rapid antidepressant treatment, although it usually is administered via IV infusion, rather than patch form, in trials.15-17 IV ketamine is another therapy being investigated for rapid treatment of depression, which might have downstream mechanisms of action related to scopolamine.5,18 Electroconvulsive therapy is a well-known for its quick antidepressant effect, which could involve synaptogenesis or effects on the neuroendocrine system.19 Sleep deprivation also can produce a rapid antidepressant effect20 (Table 21,2,5,6,15,16,18-20).
OUTCOME Prone to motion sickness
Approximately 3.5 months after his initial presentation, Mr. K continues to do well with treatment. He is euthymic and functioning well at work. He and his wife are preparing for the birth of their first child.
Mr. K is prone to motion sickness, and asks if he can take over-the-counter dimenhydrinate tablets for long car rides. He reports that dimenhydrinate has worked well for him in the past without triggering manic episodes, and he did not anticipate needing to take it very often.
What would you tell Mr. K about dimenhydrinate for motion sickness during car rides?
a) Mr. K should not take dimenhydrinate to prevent motion sickness because he experienced a manic episode triggered by a scopolamine patch
b) Mr. K can use dimenhydrinate as much as he wants to prevent motion sickness because it poses no risk of mania
c) Mr. K can use dimenhydrinate with caution and sparingly on a trial basis, as long as he is taking his mood stabilizer
FOLLOW UP Cautious use
The psychiatrist advised Mr. K to take dimenhydrinate cautiously when needed for long car rides. The psychiatrist feels this is safe because Mr. K is taking a mood stabilizer (lithium). Also, although dimenhydrinate has anticholinergic properties, occasional use is thought to pose less risk of triggering mania than the constant anticholinergic exposure over several days with a scopolamine patch. (The scopolamine patch contains 1.5 mg of the drug delivered over 3 days [ie, 0.5 mg/d]. In trials of IV scopolamine for depression, the dosage was 0.4 mcg/kg/d administered over 3 consecutive days.15-17 For an adult weighing 70 kg, this would be equivalent to 0.24 mg/d. Therefore, using a scopolamine patch over 3 days would appear to deliver a robust antidepressant-level dosage, even taking into account possible lower bioavailability with transdermal administration compared with IV infusion.)
The psychiatrist concludes that sporadic use of dimenhydrinate tablets for motion sickness during occasional long car rides poses less of a risk for Mr. K of triggering mania than repeat use of a scopolamine patch.
The author’s observations
Mr. K’s case is notable for several reasons:
- Novelty. This might be the first report of scopolamine-induced mania in the literature. In clinical trials by Furey and Drevets,15 Drevets and Furey,16 and Ellis et al,17 no study participants who received scopolamine infusion developed mania or hypomania. Although it is possible that Mr. K’s manic episode could have occurred spontaneously and was coincidental to his scopolamine use, there are valid reasons why scopolamine could trigger mania in a vulnerable person.
- Biochemical insight. The case underscores the role of the muscarinic cholinergic system in regulating mood.10
- Rational medical care. Sensible clinical decision-making was needed when Mr. K asked about using dimenhydrinate for motion sickness during car rides. Although there might not be definitively correct answers for questions that arose during Mr. K’s care (in the absence of research literature), theoretical understanding of the antidepressant effects of anticholinergic medications helped inform the psychiatrist’s responses to Mr. K and his wife.
1. Drevets WC, Zarate CA Jr, Furey ML. Antidepressant effects of the muscarinic cholinergic receptor antagonist scopolamine: a review. Biol Psychiatry. 2013;73(12):1156-1163.
2. Jaffe RJ, Novakovic V, Peselow ED. Scopolamine as an antidepressant: a systematic review. Clin Neuropharmacol. 2013;36(1):24-26.
3. Rami A, Ausmeir F, Winckler J, et al. Differential effects of scopolamine on neuronal survival in ischemia and glutamate neurotoxicity: relationships to the excessive vulnerability of the dorsoseptal hippocampus. J Chem Neuroanat. 1997;13(3):201-208.
4. Benveniste M, Wilhelm J, Dingledine RJ, et al. Subunit-dependent modulation of kainate receptors by muscarinic acetylcholine receptors. Brain Res. 2010;1352:61-69.
5. Duman RS, Li N, Liu RJ, et al. Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology. 2012;62(1):35-41.
6. Voleti B, Navarria A, Liu R, et al. Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biol Psychiatry. 2013;74(10):742-749.
7. Overstreet DH, Friedman E, Mathé AA, et al. The Flinders Sensitive Line rat: a selectively bred putative animal model of depression. Neurosci Biobehav Rev. 2005;29(4-5):739-759.
8. Tizabi Y, Getachew B, Rezvani AH, et al. Antidepressant-like effects of nicotine and reduced nicotinic receptor binding in the Fawn-Hooded rat, an animal model of co-morbid depression and alcoholism. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(3):398-402.
9. Wang DW, Zhou RB, Yao YM. Role of cholinergic anti-inflammatory pathway in regulating host response and its interventional strategy for inflammatory diseases. Chin J Traumatol. 2009;12(6):355-364.
10. Janowsky DS, el-Yousef MK, Davis JM, et al. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2(7778):632-635.
11. Risch SC, Kalin NH, Janowsky DS. Cholinergic challenges in affective illness: behavioral and neuroendocrine correlates. J Clin Psychopharmacol. 1981;1(4):186-192.
12. Risch SC, Janowsky DS, Gillin JC. Muscarinic supersensitivity of anterior pituitary ACTH and β-endorphin release in major depressive illness. Peptides. 1983;4(5):789-792.
13. Risch SC, Janowsky DS, Mott MA, et al. Central and peripheral cholinesterase inhibition: effects on anterior pituitary and sympathomimetic function. Psychoneuroendocrinology. 1986;11(2):221-230.
14. Janowsky DS, Risch SC, Kennedy B, et al. Central muscarinic effects of physostigmine on mood, cardiovascular function, pituitary and adrenal neuroendocrine release. Psychopharmacology (Berl). 1986;89(2):150-154.
15. Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2006;63(10):1121-1129.
16. Drevets WC, Furey ML. Replication of scopolamine’s antidepressant efficacy in major depressive disorder: a randomized, placebo-controlled clinical trial. Biol Psychiatry. 2010;67(5):432-438.
17. Ellis JS, Zarate CA Jr, Luckenbaugh DA, et al. Antidepressant treatment history as a predictor of response to scopolamine: clinical implications. J Affect Disord. 2014;162:39-42.
18. Newport DJ, Carpenter LL, McDonald WM, et al. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172(10):950-966.
19. Bolwig TG. How does electroconvulsive therapy work? Theories on its mechanism. Can J Psychiatry. 2011;56(1):13-18.
20. Wu JC, Kelsoe JR, Schachat C, et al. Rapid and sustained antidepressant response with sleep deprivation and chronotherapy in bipolar disorder. Biol Psychiatry. 2009;66(3):298-301.
CASE From soft-spoken to manic
Mr. K, age 36, an Asian male with no psychiatric history, arrives at the outpatient psychiatry clinic accompanied by his wife, after being referred from the emergency room the night before. He reports racing thoughts, euphoric mood, increased speech, hypergraphia, elevated self-esteem, decreased need for sleep, distractibility, and increased goal-directed activity. Notably, Mr. K states that he likes how he is feeling.
Mr. K’s wife says that his condition is a clear change from his baseline demeanor: soft-spoken and mild-mannered.
Mr. K reports that his symptoms started approximately 10 days earlier, after he returned from a cruise with his wife. During the cruise, he used a scopolamine patch to prevent motion sickness. Mr. K and his wife say that they believe that the scopolamine patch caused his symptoms.
Can scopolamine cause mania?
a) No
b) Yes; this is well-documented in the literature
c) It is theoretically possible because of scopolamine’s antidepressant and central anticholinergic effects
TREATMENT Lithium, close follow up
Mr. K has no history of psychiatric illness or substance use and no recent use of psychoactive substances—other than scopolamine—that could trigger a manic episode. His family history is significant for a younger brother who had a single manic episode at age 12 and a suicide attempt as a young adult.
Mr. K works full-time on rotating shifts—including some overnight shifts—as a manufacturing supervisor at a biotechnology company. He has been unable to work since returning from the cruise because of his psychiatric symptoms.
Mr. K is started on sustained-release (SR) lithium, 900 mg/d. In addition, the psychiatrist advises Mr. K to continue taking clonazepam, 0.5 to 1 mg as needed, which the emergency medicine physician prescribed, for insomnia. Mr. K is referred to a psychiatric intensive outpatient program (IOP), 3 days a week for 2 weeks, and is advised to stay home from work until symptoms stabilize.
Mr. K follows up closely with the psychiatrist in the clinic, every 1 to 2 weeks for the first month, as well as by several telephone and e-mail contacts. Lithium SR is titrated to 1,200 mg/d, to a therapeutic serum level of 1.1 mEq/L. Clonazepam is switched to quetiapine, 25 to 50 mg as needed, to address ongoing insomnia and to reduce the risk of dependency on clonazepam.
Mr. K’s mania gradually abates. He finishes the IOP and returns to work 3 weeks after his initial presentation. At an office visit, Mr. K’s wife gives the psychiatrist 2 scientific articles documenting the antidepressant effect of scopolamine.1,2 Mr. K and his wife both continue to believe that Mr. K’s manic episode was triggered by the scopolamine patch he used while on the cruise. They think this is important because Mr. K believes he would not have developed mania otherwise, and he does not want to take a mood stabilizer for the rest of his life.
The author’s observations
There are several proposed mechanisms for scopolamine’s antidepressant effect (Table 1).3-9 Scopolamine blocks central muscarinic cholinergic receptors, which reduces production of glutamate receptors and leads to reduced glutamate transmission and neurotoxicity.3,4 Scopolamine—similar to ketamine—could enhance synaptogenesis and synaptic signaling.5,6 Also, by blocking muscarinic autoreceptors, scopolamine results in an acute upregulation of acetylcholine release, which, in turn, influences the nicotinic, dopamine, serotonin, and neuropeptide Y systems. This action could contribute to anti-inflammatory effects, all of which can benefit mood.7-9 These antidepressant mechanisms also could explain why, theoretically, scopolamine could precipitate mania in a person predisposed to mental illness.
Proposed by Janowsky et al10 in 1972, the cholinergic−adrenergic balance hypothesis of affective disorders suggests that depression represents an excess of central cholinergic tone over adrenergic tone, and that mania represents the opposite imbalance. Several lines of evidence in the literature support this theory. For example, depressed patients have been found to have hypersensitive central cholinergic receptors.11,12 Also, central cholinesterase inhibition has been shown to affect pituitary hormone and epinephrine levels via central muscarinic receptors.13 In addition, scopolamine has been shown to attenuate these effects via the central anti-muscarinic mechanism.14
Rapid antidepressant therapy. Scopolamine is being studied as a rapid antidepressant treatment, although it usually is administered via IV infusion, rather than patch form, in trials.15-17 IV ketamine is another therapy being investigated for rapid treatment of depression, which might have downstream mechanisms of action related to scopolamine.5,18 Electroconvulsive therapy is a well-known for its quick antidepressant effect, which could involve synaptogenesis or effects on the neuroendocrine system.19 Sleep deprivation also can produce a rapid antidepressant effect20 (Table 21,2,5,6,15,16,18-20).
OUTCOME Prone to motion sickness
Approximately 3.5 months after his initial presentation, Mr. K continues to do well with treatment. He is euthymic and functioning well at work. He and his wife are preparing for the birth of their first child.
Mr. K is prone to motion sickness, and asks if he can take over-the-counter dimenhydrinate tablets for long car rides. He reports that dimenhydrinate has worked well for him in the past without triggering manic episodes, and he did not anticipate needing to take it very often.
What would you tell Mr. K about dimenhydrinate for motion sickness during car rides?
a) Mr. K should not take dimenhydrinate to prevent motion sickness because he experienced a manic episode triggered by a scopolamine patch
b) Mr. K can use dimenhydrinate as much as he wants to prevent motion sickness because it poses no risk of mania
c) Mr. K can use dimenhydrinate with caution and sparingly on a trial basis, as long as he is taking his mood stabilizer
FOLLOW UP Cautious use
The psychiatrist advised Mr. K to take dimenhydrinate cautiously when needed for long car rides. The psychiatrist feels this is safe because Mr. K is taking a mood stabilizer (lithium). Also, although dimenhydrinate has anticholinergic properties, occasional use is thought to pose less risk of triggering mania than the constant anticholinergic exposure over several days with a scopolamine patch. (The scopolamine patch contains 1.5 mg of the drug delivered over 3 days [ie, 0.5 mg/d]. In trials of IV scopolamine for depression, the dosage was 0.4 mcg/kg/d administered over 3 consecutive days.15-17 For an adult weighing 70 kg, this would be equivalent to 0.24 mg/d. Therefore, using a scopolamine patch over 3 days would appear to deliver a robust antidepressant-level dosage, even taking into account possible lower bioavailability with transdermal administration compared with IV infusion.)
The psychiatrist concludes that sporadic use of dimenhydrinate tablets for motion sickness during occasional long car rides poses less of a risk for Mr. K of triggering mania than repeat use of a scopolamine patch.
The author’s observations
Mr. K’s case is notable for several reasons:
- Novelty. This might be the first report of scopolamine-induced mania in the literature. In clinical trials by Furey and Drevets,15 Drevets and Furey,16 and Ellis et al,17 no study participants who received scopolamine infusion developed mania or hypomania. Although it is possible that Mr. K’s manic episode could have occurred spontaneously and was coincidental to his scopolamine use, there are valid reasons why scopolamine could trigger mania in a vulnerable person.
- Biochemical insight. The case underscores the role of the muscarinic cholinergic system in regulating mood.10
- Rational medical care. Sensible clinical decision-making was needed when Mr. K asked about using dimenhydrinate for motion sickness during car rides. Although there might not be definitively correct answers for questions that arose during Mr. K’s care (in the absence of research literature), theoretical understanding of the antidepressant effects of anticholinergic medications helped inform the psychiatrist’s responses to Mr. K and his wife.
CASE From soft-spoken to manic
Mr. K, age 36, an Asian male with no psychiatric history, arrives at the outpatient psychiatry clinic accompanied by his wife, after being referred from the emergency room the night before. He reports racing thoughts, euphoric mood, increased speech, hypergraphia, elevated self-esteem, decreased need for sleep, distractibility, and increased goal-directed activity. Notably, Mr. K states that he likes how he is feeling.
Mr. K’s wife says that his condition is a clear change from his baseline demeanor: soft-spoken and mild-mannered.
Mr. K reports that his symptoms started approximately 10 days earlier, after he returned from a cruise with his wife. During the cruise, he used a scopolamine patch to prevent motion sickness. Mr. K and his wife say that they believe that the scopolamine patch caused his symptoms.
Can scopolamine cause mania?
a) No
b) Yes; this is well-documented in the literature
c) It is theoretically possible because of scopolamine’s antidepressant and central anticholinergic effects
TREATMENT Lithium, close follow up
Mr. K has no history of psychiatric illness or substance use and no recent use of psychoactive substances—other than scopolamine—that could trigger a manic episode. His family history is significant for a younger brother who had a single manic episode at age 12 and a suicide attempt as a young adult.
Mr. K works full-time on rotating shifts—including some overnight shifts—as a manufacturing supervisor at a biotechnology company. He has been unable to work since returning from the cruise because of his psychiatric symptoms.
Mr. K is started on sustained-release (SR) lithium, 900 mg/d. In addition, the psychiatrist advises Mr. K to continue taking clonazepam, 0.5 to 1 mg as needed, which the emergency medicine physician prescribed, for insomnia. Mr. K is referred to a psychiatric intensive outpatient program (IOP), 3 days a week for 2 weeks, and is advised to stay home from work until symptoms stabilize.
Mr. K follows up closely with the psychiatrist in the clinic, every 1 to 2 weeks for the first month, as well as by several telephone and e-mail contacts. Lithium SR is titrated to 1,200 mg/d, to a therapeutic serum level of 1.1 mEq/L. Clonazepam is switched to quetiapine, 25 to 50 mg as needed, to address ongoing insomnia and to reduce the risk of dependency on clonazepam.
Mr. K’s mania gradually abates. He finishes the IOP and returns to work 3 weeks after his initial presentation. At an office visit, Mr. K’s wife gives the psychiatrist 2 scientific articles documenting the antidepressant effect of scopolamine.1,2 Mr. K and his wife both continue to believe that Mr. K’s manic episode was triggered by the scopolamine patch he used while on the cruise. They think this is important because Mr. K believes he would not have developed mania otherwise, and he does not want to take a mood stabilizer for the rest of his life.
The author’s observations
There are several proposed mechanisms for scopolamine’s antidepressant effect (Table 1).3-9 Scopolamine blocks central muscarinic cholinergic receptors, which reduces production of glutamate receptors and leads to reduced glutamate transmission and neurotoxicity.3,4 Scopolamine—similar to ketamine—could enhance synaptogenesis and synaptic signaling.5,6 Also, by blocking muscarinic autoreceptors, scopolamine results in an acute upregulation of acetylcholine release, which, in turn, influences the nicotinic, dopamine, serotonin, and neuropeptide Y systems. This action could contribute to anti-inflammatory effects, all of which can benefit mood.7-9 These antidepressant mechanisms also could explain why, theoretically, scopolamine could precipitate mania in a person predisposed to mental illness.
Proposed by Janowsky et al10 in 1972, the cholinergic−adrenergic balance hypothesis of affective disorders suggests that depression represents an excess of central cholinergic tone over adrenergic tone, and that mania represents the opposite imbalance. Several lines of evidence in the literature support this theory. For example, depressed patients have been found to have hypersensitive central cholinergic receptors.11,12 Also, central cholinesterase inhibition has been shown to affect pituitary hormone and epinephrine levels via central muscarinic receptors.13 In addition, scopolamine has been shown to attenuate these effects via the central anti-muscarinic mechanism.14
Rapid antidepressant therapy. Scopolamine is being studied as a rapid antidepressant treatment, although it usually is administered via IV infusion, rather than patch form, in trials.15-17 IV ketamine is another therapy being investigated for rapid treatment of depression, which might have downstream mechanisms of action related to scopolamine.5,18 Electroconvulsive therapy is a well-known for its quick antidepressant effect, which could involve synaptogenesis or effects on the neuroendocrine system.19 Sleep deprivation also can produce a rapid antidepressant effect20 (Table 21,2,5,6,15,16,18-20).
OUTCOME Prone to motion sickness
Approximately 3.5 months after his initial presentation, Mr. K continues to do well with treatment. He is euthymic and functioning well at work. He and his wife are preparing for the birth of their first child.
Mr. K is prone to motion sickness, and asks if he can take over-the-counter dimenhydrinate tablets for long car rides. He reports that dimenhydrinate has worked well for him in the past without triggering manic episodes, and he did not anticipate needing to take it very often.
What would you tell Mr. K about dimenhydrinate for motion sickness during car rides?
a) Mr. K should not take dimenhydrinate to prevent motion sickness because he experienced a manic episode triggered by a scopolamine patch
b) Mr. K can use dimenhydrinate as much as he wants to prevent motion sickness because it poses no risk of mania
c) Mr. K can use dimenhydrinate with caution and sparingly on a trial basis, as long as he is taking his mood stabilizer
FOLLOW UP Cautious use
The psychiatrist advised Mr. K to take dimenhydrinate cautiously when needed for long car rides. The psychiatrist feels this is safe because Mr. K is taking a mood stabilizer (lithium). Also, although dimenhydrinate has anticholinergic properties, occasional use is thought to pose less risk of triggering mania than the constant anticholinergic exposure over several days with a scopolamine patch. (The scopolamine patch contains 1.5 mg of the drug delivered over 3 days [ie, 0.5 mg/d]. In trials of IV scopolamine for depression, the dosage was 0.4 mcg/kg/d administered over 3 consecutive days.15-17 For an adult weighing 70 kg, this would be equivalent to 0.24 mg/d. Therefore, using a scopolamine patch over 3 days would appear to deliver a robust antidepressant-level dosage, even taking into account possible lower bioavailability with transdermal administration compared with IV infusion.)
The psychiatrist concludes that sporadic use of dimenhydrinate tablets for motion sickness during occasional long car rides poses less of a risk for Mr. K of triggering mania than repeat use of a scopolamine patch.
The author’s observations
Mr. K’s case is notable for several reasons:
- Novelty. This might be the first report of scopolamine-induced mania in the literature. In clinical trials by Furey and Drevets,15 Drevets and Furey,16 and Ellis et al,17 no study participants who received scopolamine infusion developed mania or hypomania. Although it is possible that Mr. K’s manic episode could have occurred spontaneously and was coincidental to his scopolamine use, there are valid reasons why scopolamine could trigger mania in a vulnerable person.
- Biochemical insight. The case underscores the role of the muscarinic cholinergic system in regulating mood.10
- Rational medical care. Sensible clinical decision-making was needed when Mr. K asked about using dimenhydrinate for motion sickness during car rides. Although there might not be definitively correct answers for questions that arose during Mr. K’s care (in the absence of research literature), theoretical understanding of the antidepressant effects of anticholinergic medications helped inform the psychiatrist’s responses to Mr. K and his wife.
1. Drevets WC, Zarate CA Jr, Furey ML. Antidepressant effects of the muscarinic cholinergic receptor antagonist scopolamine: a review. Biol Psychiatry. 2013;73(12):1156-1163.
2. Jaffe RJ, Novakovic V, Peselow ED. Scopolamine as an antidepressant: a systematic review. Clin Neuropharmacol. 2013;36(1):24-26.
3. Rami A, Ausmeir F, Winckler J, et al. Differential effects of scopolamine on neuronal survival in ischemia and glutamate neurotoxicity: relationships to the excessive vulnerability of the dorsoseptal hippocampus. J Chem Neuroanat. 1997;13(3):201-208.
4. Benveniste M, Wilhelm J, Dingledine RJ, et al. Subunit-dependent modulation of kainate receptors by muscarinic acetylcholine receptors. Brain Res. 2010;1352:61-69.
5. Duman RS, Li N, Liu RJ, et al. Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology. 2012;62(1):35-41.
6. Voleti B, Navarria A, Liu R, et al. Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biol Psychiatry. 2013;74(10):742-749.
7. Overstreet DH, Friedman E, Mathé AA, et al. The Flinders Sensitive Line rat: a selectively bred putative animal model of depression. Neurosci Biobehav Rev. 2005;29(4-5):739-759.
8. Tizabi Y, Getachew B, Rezvani AH, et al. Antidepressant-like effects of nicotine and reduced nicotinic receptor binding in the Fawn-Hooded rat, an animal model of co-morbid depression and alcoholism. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(3):398-402.
9. Wang DW, Zhou RB, Yao YM. Role of cholinergic anti-inflammatory pathway in regulating host response and its interventional strategy for inflammatory diseases. Chin J Traumatol. 2009;12(6):355-364.
10. Janowsky DS, el-Yousef MK, Davis JM, et al. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2(7778):632-635.
11. Risch SC, Kalin NH, Janowsky DS. Cholinergic challenges in affective illness: behavioral and neuroendocrine correlates. J Clin Psychopharmacol. 1981;1(4):186-192.
12. Risch SC, Janowsky DS, Gillin JC. Muscarinic supersensitivity of anterior pituitary ACTH and β-endorphin release in major depressive illness. Peptides. 1983;4(5):789-792.
13. Risch SC, Janowsky DS, Mott MA, et al. Central and peripheral cholinesterase inhibition: effects on anterior pituitary and sympathomimetic function. Psychoneuroendocrinology. 1986;11(2):221-230.
14. Janowsky DS, Risch SC, Kennedy B, et al. Central muscarinic effects of physostigmine on mood, cardiovascular function, pituitary and adrenal neuroendocrine release. Psychopharmacology (Berl). 1986;89(2):150-154.
15. Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2006;63(10):1121-1129.
16. Drevets WC, Furey ML. Replication of scopolamine’s antidepressant efficacy in major depressive disorder: a randomized, placebo-controlled clinical trial. Biol Psychiatry. 2010;67(5):432-438.
17. Ellis JS, Zarate CA Jr, Luckenbaugh DA, et al. Antidepressant treatment history as a predictor of response to scopolamine: clinical implications. J Affect Disord. 2014;162:39-42.
18. Newport DJ, Carpenter LL, McDonald WM, et al. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172(10):950-966.
19. Bolwig TG. How does electroconvulsive therapy work? Theories on its mechanism. Can J Psychiatry. 2011;56(1):13-18.
20. Wu JC, Kelsoe JR, Schachat C, et al. Rapid and sustained antidepressant response with sleep deprivation and chronotherapy in bipolar disorder. Biol Psychiatry. 2009;66(3):298-301.
1. Drevets WC, Zarate CA Jr, Furey ML. Antidepressant effects of the muscarinic cholinergic receptor antagonist scopolamine: a review. Biol Psychiatry. 2013;73(12):1156-1163.
2. Jaffe RJ, Novakovic V, Peselow ED. Scopolamine as an antidepressant: a systematic review. Clin Neuropharmacol. 2013;36(1):24-26.
3. Rami A, Ausmeir F, Winckler J, et al. Differential effects of scopolamine on neuronal survival in ischemia and glutamate neurotoxicity: relationships to the excessive vulnerability of the dorsoseptal hippocampus. J Chem Neuroanat. 1997;13(3):201-208.
4. Benveniste M, Wilhelm J, Dingledine RJ, et al. Subunit-dependent modulation of kainate receptors by muscarinic acetylcholine receptors. Brain Res. 2010;1352:61-69.
5. Duman RS, Li N, Liu RJ, et al. Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology. 2012;62(1):35-41.
6. Voleti B, Navarria A, Liu R, et al. Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biol Psychiatry. 2013;74(10):742-749.
7. Overstreet DH, Friedman E, Mathé AA, et al. The Flinders Sensitive Line rat: a selectively bred putative animal model of depression. Neurosci Biobehav Rev. 2005;29(4-5):739-759.
8. Tizabi Y, Getachew B, Rezvani AH, et al. Antidepressant-like effects of nicotine and reduced nicotinic receptor binding in the Fawn-Hooded rat, an animal model of co-morbid depression and alcoholism. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(3):398-402.
9. Wang DW, Zhou RB, Yao YM. Role of cholinergic anti-inflammatory pathway in regulating host response and its interventional strategy for inflammatory diseases. Chin J Traumatol. 2009;12(6):355-364.
10. Janowsky DS, el-Yousef MK, Davis JM, et al. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2(7778):632-635.
11. Risch SC, Kalin NH, Janowsky DS. Cholinergic challenges in affective illness: behavioral and neuroendocrine correlates. J Clin Psychopharmacol. 1981;1(4):186-192.
12. Risch SC, Janowsky DS, Gillin JC. Muscarinic supersensitivity of anterior pituitary ACTH and β-endorphin release in major depressive illness. Peptides. 1983;4(5):789-792.
13. Risch SC, Janowsky DS, Mott MA, et al. Central and peripheral cholinesterase inhibition: effects on anterior pituitary and sympathomimetic function. Psychoneuroendocrinology. 1986;11(2):221-230.
14. Janowsky DS, Risch SC, Kennedy B, et al. Central muscarinic effects of physostigmine on mood, cardiovascular function, pituitary and adrenal neuroendocrine release. Psychopharmacology (Berl). 1986;89(2):150-154.
15. Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2006;63(10):1121-1129.
16. Drevets WC, Furey ML. Replication of scopolamine’s antidepressant efficacy in major depressive disorder: a randomized, placebo-controlled clinical trial. Biol Psychiatry. 2010;67(5):432-438.
17. Ellis JS, Zarate CA Jr, Luckenbaugh DA, et al. Antidepressant treatment history as a predictor of response to scopolamine: clinical implications. J Affect Disord. 2014;162:39-42.
18. Newport DJ, Carpenter LL, McDonald WM, et al. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172(10):950-966.
19. Bolwig TG. How does electroconvulsive therapy work? Theories on its mechanism. Can J Psychiatry. 2011;56(1):13-18.
20. Wu JC, Kelsoe JR, Schachat C, et al. Rapid and sustained antidepressant response with sleep deprivation and chronotherapy in bipolar disorder. Biol Psychiatry. 2009;66(3):298-301.
Be an activist to prevent edentulism among the mentally ill
Poor dental hygiene is a serious and prevalent problem among people with mental illness or cognitive impairment: Dental caries and periodontal disease are 3.4 times more common among the mentally ill than among the general population.1 Little has been published on the causes and prevention of these diseases among the mentally ill, however. Interprofessional education provides the opportunity to reinforce the connection between oral health and systemic health.
Untreated dental disease can result in edentulism (partial or complete tooth loss). Often, this condition leads to embarrassment, poor self-image, and social isolation—all of which can exacerbate the psychotic state and its symptoms. Working with your patient to improve oral health can, in turn, lead to better mental and physical health.
CASE REPORT
Edentulism in a man with schizophrenia
A 34-year-old man, given a diagnosis of schizophrenia at age 17, is admitted to the inpatient psychiatry unit for bizarre behavior. The next day, 4 maxillary and incisor teeth fall out suddenly while he is brushing his teeth. The patient is brought to emergency dental services.
Factors contributing to his tooth loss include:
- schizophrenia
- neglected oral hygiene
- adverse effects of antipsychotic medication
- lack of advice on the importance of oral hygiene
- failure to recognize signs of a dental problem.
What else can lead to edentulism?
Breakdown of the periodontal attachment2 also can be caused by disinterest in oral hygiene practices; craving of, and preference for, carbohydrates because of reduced central serotonin activity3,4; and xerostomia.
Xerostomia, or dry mouth, caused by psychotropic agents and an altered immune response, facilitates growth of pathogenic bacteria and can lead to several dental diseases (Table). These conditions are exacerbated by consumption of chewing gum, sweets, and sugary drinks in response to constantly feeling thirsty from xerostomia. Advise patients to take frequent sips of fluid or let ice cubes melt in their mouth.
Bruxism. Patients taking a selective serotonin reuptake inhibitor or an atypical antipsychotic can develop a movement disorder (eg, extrapyramidal symptoms or tardive dyskinesia) that includes clenching, grinding of the teeth (bruxism), or both, which can worsen their periodontal condition.
Lack of skills, physical dexterity, and motivation to maintain good oral hygiene are common among people with mental illness. Most patients visit a dentist only when they experience a serious oral problem or an emergency (ie, trauma). Many dentists treat psychiatric patients by extracting the tooth that is causing the pain, instead of pursuing complex tooth preservation or restoration techniques because of (1) the extent of the disease, (2) lack of knowledge related to psychiatric illnesses, and (3) frequent and timely follow-ups.5
Providing education about oral health to patients, implementing preventive steps, and educating other medical specialities about the link between oral health and systemic health can help to reduce the burden of dental problems among mentally ill patients.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products
1. Persson K, Axtelius B, Söderfeldt B, et al. Oral health-related quality of life and dental status in an outpatient psychiatric population: a multivariate approach. Int J Ment Health Nurs. 2010;19(1):62-70.
2. Lalloo R, Kisely S, Amarasinghe H, et al. Oral health of patients on psychotropic medications: a study of outpatients in Queensland. Australas Psychiatry. 2013;21(4):338-342.
3. O’Neil A, Berk M, Venugopal K, et al. The association between poor dental health and depression: findings from a large-scale, population-based study (the NHANES study). Gen Hosp Psychiatry. 2014;36(3):266-270.
4. Kisely S, Quek LH, Paris J, et al. Advanced dental disease in people with severe mental illness: systematic review and meta-analysis. Br J Psychiatry. 2011;199(3):187-193.
5. Arnaiz A, Zumárraga M, Díez-Altuna I, et al. Oral health and the symptoms of schizophrenia. Psychiatry Res. 2011;188(1):24-28.
Poor dental hygiene is a serious and prevalent problem among people with mental illness or cognitive impairment: Dental caries and periodontal disease are 3.4 times more common among the mentally ill than among the general population.1 Little has been published on the causes and prevention of these diseases among the mentally ill, however. Interprofessional education provides the opportunity to reinforce the connection between oral health and systemic health.
Untreated dental disease can result in edentulism (partial or complete tooth loss). Often, this condition leads to embarrassment, poor self-image, and social isolation—all of which can exacerbate the psychotic state and its symptoms. Working with your patient to improve oral health can, in turn, lead to better mental and physical health.
CASE REPORT
Edentulism in a man with schizophrenia
A 34-year-old man, given a diagnosis of schizophrenia at age 17, is admitted to the inpatient psychiatry unit for bizarre behavior. The next day, 4 maxillary and incisor teeth fall out suddenly while he is brushing his teeth. The patient is brought to emergency dental services.
Factors contributing to his tooth loss include:
- schizophrenia
- neglected oral hygiene
- adverse effects of antipsychotic medication
- lack of advice on the importance of oral hygiene
- failure to recognize signs of a dental problem.
What else can lead to edentulism?
Breakdown of the periodontal attachment2 also can be caused by disinterest in oral hygiene practices; craving of, and preference for, carbohydrates because of reduced central serotonin activity3,4; and xerostomia.
Xerostomia, or dry mouth, caused by psychotropic agents and an altered immune response, facilitates growth of pathogenic bacteria and can lead to several dental diseases (Table). These conditions are exacerbated by consumption of chewing gum, sweets, and sugary drinks in response to constantly feeling thirsty from xerostomia. Advise patients to take frequent sips of fluid or let ice cubes melt in their mouth.
Bruxism. Patients taking a selective serotonin reuptake inhibitor or an atypical antipsychotic can develop a movement disorder (eg, extrapyramidal symptoms or tardive dyskinesia) that includes clenching, grinding of the teeth (bruxism), or both, which can worsen their periodontal condition.
Lack of skills, physical dexterity, and motivation to maintain good oral hygiene are common among people with mental illness. Most patients visit a dentist only when they experience a serious oral problem or an emergency (ie, trauma). Many dentists treat psychiatric patients by extracting the tooth that is causing the pain, instead of pursuing complex tooth preservation or restoration techniques because of (1) the extent of the disease, (2) lack of knowledge related to psychiatric illnesses, and (3) frequent and timely follow-ups.5
Providing education about oral health to patients, implementing preventive steps, and educating other medical specialities about the link between oral health and systemic health can help to reduce the burden of dental problems among mentally ill patients.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products
Poor dental hygiene is a serious and prevalent problem among people with mental illness or cognitive impairment: Dental caries and periodontal disease are 3.4 times more common among the mentally ill than among the general population.1 Little has been published on the causes and prevention of these diseases among the mentally ill, however. Interprofessional education provides the opportunity to reinforce the connection between oral health and systemic health.
Untreated dental disease can result in edentulism (partial or complete tooth loss). Often, this condition leads to embarrassment, poor self-image, and social isolation—all of which can exacerbate the psychotic state and its symptoms. Working with your patient to improve oral health can, in turn, lead to better mental and physical health.
CASE REPORT
Edentulism in a man with schizophrenia
A 34-year-old man, given a diagnosis of schizophrenia at age 17, is admitted to the inpatient psychiatry unit for bizarre behavior. The next day, 4 maxillary and incisor teeth fall out suddenly while he is brushing his teeth. The patient is brought to emergency dental services.
Factors contributing to his tooth loss include:
- schizophrenia
- neglected oral hygiene
- adverse effects of antipsychotic medication
- lack of advice on the importance of oral hygiene
- failure to recognize signs of a dental problem.
What else can lead to edentulism?
Breakdown of the periodontal attachment2 also can be caused by disinterest in oral hygiene practices; craving of, and preference for, carbohydrates because of reduced central serotonin activity3,4; and xerostomia.
Xerostomia, or dry mouth, caused by psychotropic agents and an altered immune response, facilitates growth of pathogenic bacteria and can lead to several dental diseases (Table). These conditions are exacerbated by consumption of chewing gum, sweets, and sugary drinks in response to constantly feeling thirsty from xerostomia. Advise patients to take frequent sips of fluid or let ice cubes melt in their mouth.
Bruxism. Patients taking a selective serotonin reuptake inhibitor or an atypical antipsychotic can develop a movement disorder (eg, extrapyramidal symptoms or tardive dyskinesia) that includes clenching, grinding of the teeth (bruxism), or both, which can worsen their periodontal condition.
Lack of skills, physical dexterity, and motivation to maintain good oral hygiene are common among people with mental illness. Most patients visit a dentist only when they experience a serious oral problem or an emergency (ie, trauma). Many dentists treat psychiatric patients by extracting the tooth that is causing the pain, instead of pursuing complex tooth preservation or restoration techniques because of (1) the extent of the disease, (2) lack of knowledge related to psychiatric illnesses, and (3) frequent and timely follow-ups.5
Providing education about oral health to patients, implementing preventive steps, and educating other medical specialities about the link between oral health and systemic health can help to reduce the burden of dental problems among mentally ill patients.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products
1. Persson K, Axtelius B, Söderfeldt B, et al. Oral health-related quality of life and dental status in an outpatient psychiatric population: a multivariate approach. Int J Ment Health Nurs. 2010;19(1):62-70.
2. Lalloo R, Kisely S, Amarasinghe H, et al. Oral health of patients on psychotropic medications: a study of outpatients in Queensland. Australas Psychiatry. 2013;21(4):338-342.
3. O’Neil A, Berk M, Venugopal K, et al. The association between poor dental health and depression: findings from a large-scale, population-based study (the NHANES study). Gen Hosp Psychiatry. 2014;36(3):266-270.
4. Kisely S, Quek LH, Paris J, et al. Advanced dental disease in people with severe mental illness: systematic review and meta-analysis. Br J Psychiatry. 2011;199(3):187-193.
5. Arnaiz A, Zumárraga M, Díez-Altuna I, et al. Oral health and the symptoms of schizophrenia. Psychiatry Res. 2011;188(1):24-28.
1. Persson K, Axtelius B, Söderfeldt B, et al. Oral health-related quality of life and dental status in an outpatient psychiatric population: a multivariate approach. Int J Ment Health Nurs. 2010;19(1):62-70.
2. Lalloo R, Kisely S, Amarasinghe H, et al. Oral health of patients on psychotropic medications: a study of outpatients in Queensland. Australas Psychiatry. 2013;21(4):338-342.
3. O’Neil A, Berk M, Venugopal K, et al. The association between poor dental health and depression: findings from a large-scale, population-based study (the NHANES study). Gen Hosp Psychiatry. 2014;36(3):266-270.
4. Kisely S, Quek LH, Paris J, et al. Advanced dental disease in people with severe mental illness: systematic review and meta-analysis. Br J Psychiatry. 2011;199(3):187-193.
5. Arnaiz A, Zumárraga M, Díez-Altuna I, et al. Oral health and the symptoms of schizophrenia. Psychiatry Res. 2011;188(1):24-28.
Technology offers tools for ensuring adherence to medical therapy
Nonadherence to medical therapy is a widespread and complex problem that is a significant variable in the treatment of psychiatric illness and in patients’ prognosis. More than 50% of people who have a chronic illness struggle to comply with their medication regimen—for many reasons.1
Many variables predict poor adherence, so it cannot be expected that a single solution will solve the problem entirely.2 Novel adherence technologies are available, as we discuss in this article, and more are in development.
What is nonadherence to medical therapy?
Nonadherence can be defined primarily as not taking prescribed medication in the recommended dosage or frequency, or not taking prescribed medication at all.3 Nonadherence can result in an increased risk of relapse, hospitalization, poor therapeutic response, and delayed remission and recovery.
Secondarily, non-attendance or irregular attendance at appointments with providers is a form of nonadherence that can have a negative impact on treatment outcomes.4
Why is medical adherence important in psychiatry?
Medication nonadherence has major consequences for psychiatric patients5 and for the greater health care system; it is estimated that, in the United States, the cost of nonadherence is as high as $300 billion a year.6 In psychiatry, the rate of nonadherence to medical therapy has been reported to be 11% to 80% of patients with schizophrenia; 12% to 64% with bipolar disorders; and 30% to 60% with depression.7-9 These surprising statistics make it imperative to design treatment strategies that include an effective patient-centric medication adherence plan, based on diagnosis, patient need, education, and support.
Why are patients nonadherent?
Many variables lead to patient nonadherence (Figure 1). The most common reason is that patients simply forget to take their medication.10 Among psychiatric patients, other reasons are:
- lack of insight
- negative emotional reaction to taking medication11
- feeling better and no longer believing that the medication is needed12,13
- distress associated with side effects14,15
- high cost of medication15
- patient’s perception that medication won’t be effective16,17
- concern about substance abuse18
- fear of dependency19
- complicated dosing regimen20
- general lack of motivation.21
Emotional barriers to medication nonadherence are an underestimated area that can benefit greatly from the expertise and understanding of psychiatrists. These barriers include a sense of losing control, self-stigmatization, denial, poor insight, and beliefs about illness and medications.
Additional patient variables that contribute to nonadherence include:
- suboptimal health literacy
- stigma and shame about the need for psychiatric treatment
- lack of patient involvement in treatment decision-making.
Who is responsible for adherence?
Adherence to medical therapy is not the patient’s responsibility, exclusively. Rather, it is a collection of complex components that generally includes physicians and the health care system. Because barriers to medication adherence are complex and varied, solutions to improve adherence must be multifaceted.
Providers. Patients’ care often is managed by multiple physicians, which can lead to communication lapses about complicated drug regimens and potential adverse effects. To assist patients in adhering to their medication regimen, physicians should recognize, and acknowledge to the patient, that many psychiatric patients have difficulty taking their medications and provide advice and information in how to address this problem.
Families. Likewise, it is important to educate patients and their family about the need for medication—helping the patient see that it is his (her) choice and, indeed, his direct responsibility to take his medication and improve his health. The risk–benefit balance of treatment should be explained to the patient and his family, as well as the nature of the psychiatric diagnosis and how effective patient–physician collaboration can help him function and adhere to his medication regimen in a consistent, reliable manner.
The larger system. Health care systems can contribute to medication adherence by reducing time constraints on visits to providers, to allow time to discuss all aspects of medication adherence. Limited visits in the clinic means physicians are not able to (1) spend adequate time discussing the medication regimen to ensure full patient comprehension and (2) conduct an assessment of medication-taking behaviors. Team-based approaches could improve efficiency, patient understanding, adherence, and early detection of adherence issues.22,23
Strategies such as additional clinic visits and reminder calls to discuss adherence carry a cost, but their long-term advantage is that, if patients understand how to better adhere to their medication regimens, their actions will have a positive impact on their health care costs and outcomes and on the wider health economy—as a result of reduced hospital admissions and reduced need to care for patients whose condition deteriorates because of nonadherence. It is imperative that we build strong relationships with other providers to show that we are committed to building supportive, effective adherence support programs that focus on the individual patient’s needs.
What is the available technology?
There is no standard way to measure nonadherence. The most common, and simplest, measure—asking the patient—is unreliable and severely overestimates adherence.
Direct measures of adherence include observing the patient taking his medications and testing for the concentration of those medications in blood or urine. Indirect adherence assessment methods, such as pill counts, a medication diary, self-report, clinician ratings, pharmacy chart review, and electronic devices that monitor the opening of a lid or tablet strip, have all been used; yet reviews of those methods have shown less than favorable results.6
Pre-packaged pill packs have helped some patients with a simple method for medication management.
Electronic monitoring, using a medication vial cap device (Figure 2) that electronically records the date and time of bottle opening, has become common in general medicine and among patients with schizophrenia.6,13,24-26 Diaz et al24 reported that electronic monitoring detected a greater nonadherence rate (57%) than what prescribers reported (7%) or patients self-reported (5%)—demonstrating that prescribers and patients grossly overestimate adherence. In another study that looked at electronic monitoring, researchers reported that adherence was much higher in depressed youth (87%)27 than what had been seen in adults (67%) in a similar study.13
The downside to pill packs and electronic monitoring? There is no guarantee the patient has actually taken the medication despite the data reported by the system.
Event marker-signaling devices. Novel technologies have been developed to measure adherence:
Proteus Digital Health feedback system (www.proteus.com) requires that patients ingest a tablet containing a tiny, dietary mineral-based “ingestible event marker.” Upon contact with gastric fluid electrolytes, the event marker emits a unique signal that is transmitted through bodily tissue to a small receiver in a patch worn on the torso. The receiver then transmits a signal to a cellular phone, indicating the time and date when the medication was ingested (Figure 3).
A 4-week pilot study28 found that the ingestible event marker is feasible and acceptable to patients: 27 of 28 participants (96%) completed the study, with a mean adherence rate of 74%. Although the system identifies ingestible sensors with high accuracy and is easily tolerated by patients, the pilot study was brief; a longer duration of adherence while wearing the patch needs to be studied.
Breath analysis, facial recognition. Even directly observing ingestion of a medication can be problematic: Some patients don’t swallow the medication and spit it out later. One way around that subterfuge is to consider using other advanced medication adherence solutions that are breath-based or use facial recognition technology and confirm ingestion.
Xhale SMART (www.xhale.com/smart) is a handheld device that generates a reminder to the patient to take his medication; afterward, he (she) must blow into the device so that ingestion of the medication is detected (Figure 4). The medication has breath-detectable adherence markers already incorporated. The adherence marker then is released into the stomach and small intestine, where the adherence marker metabolite is transported through the bloodstream into the lungs and exhaled. The patient must breathe into a breath analysis device, which measures medication ingestion compared with a baseline breath print.
Several articles in the literature have reported the accuracy of this device in detecting the ingested metabolite in every participant, without adverse effects.29,30 Clinical data on the use of the breath-based detector is not available to the public at this time.
AiCure (www.aicure.com) is a facial recognition-based technology platform that can work through any smartphone. The device is powered by artificial intelligence software and motion-sensing technology that can detect, in real time, whether the patient is taking the medication as prescribed. Patients who take an incorrect dose, or who do not use the software, are automatically flagged for immediate follow-up. This technology enables real-time intervention by a provider with the nonadherent patient.
An important note: These innovative technological advances are tools that can help clinicians manage an important aspect of treatment, but they do not show the entire picture: The physician−patient relationship and the therapeutic alliance are key to optimal treatment adherence.
Engage and empower the patient
Novel adherence technologies are, as we’ve described, available, and more are being developed. Incorporating these technologies into clinical care requires continued input and support from clinicians and patients. Digital and mobile health applications are multi-beneficial: They can empower patients to self-manage medication regimens and appointments while they also receive social and psychological information and support as needed. Understanding one’s own illness can, ultimately, improve outcomes and significantly reduce health care costs.
Patient empowerment is key. The physician is an important influencer in this regard.
The role of the physician must not be undervalued in maintaining adherence to therapy; she (he) plays a vital role in continued patient engagement and behavioral training. Integrating physician-led oversight, patient education, and commitment, and novel digital mobile adherence technologies will help deliver better outcomes.
The push to engage. A “one size fits all” approach to maintaining adherence won’t be effective. We need to better understand the individual patient’s underlying cause(s) for nonadherence, then to tailor a solution to influence and change that behavior. One way to do this is by interacting and engaging more directly (and in a digital manner) with patients to monitor adherence.
A recent example of the move toward direct patient engagement is the agreement entered by Otsuka Pharmaceuticals and Proteus Digital Health to develop novel digital health products. The FDA has accepted for review the combination product of Otsuka’s brand of aripiprazole and Proteus’s ingestible sensor. If the product is approved by the FDA, physicians will be able to prescribe aripiprazole with the ingestible sensor embedded in the tablet and then measure medication adherence and other patient physiologic metrics (eg, activity, rest) through the wearable sensor patch and medical software application designed specifically for patient and physician use.
This technology could have huge potential in mental health care, where patients struggle with both adhering to their medication regimen and communicating with the health care team. Physicians could measure adherence when treating adults with schizophrenia, bipolar disorders, and major depressive disorder; flag those who are not adhering as having higher risk of disease progression and poorer outcome; and allow decisions to be made more quickly based on treatment need.
Developing and enhancing these collaborative and patient-centric approaches will increase self-monitoring and patient responsibility, and encourage behavior change.
‘All-in’ strategy. By continuing to use the latest technologies and connecting them to the range of stakeholders—physicians, nurses, pharmacists, payers—we will develop an all-inclusive adherence intervention strategy. All patients will be integrated, and all of them, and their family, will be provided with positive psychoeducational care and motivational counseling (Figure 5). In addition, such a support-based patient experience must be aligned with the work of clinical care providers. Compliance therapy and behavioral training, together with active patient engagement, can help improve insight, acceptance of treatment, and, over the long term, adherence.31,32
1. World Health Organization. Adherence to long-term therapies: evidence for action. Geneva, Switzerland: World Health Organization; 2003.
2. Bosworth HB, Granger BB, Mendys P, et al. Medication adherence: a call for action. Am Heart J. 2011;162(3):412-424.
3. Crowe M, Wilson L, Inder M. Patients’ reports of the factors influencing medication adherence in bipolar disorder – an integrative review of the literature. Int J Nurs Stud. 2011;48(7):894-903.
4. Mert D, Turgut NH, Kelleci M, et al. Perspectives on reasons of medication nonadherence in psychiatric patients. Patient Prefer Adherence. 2015;9:87-93.
5. Chapman SC, Horne R. Medication nonadherence and psychiatry. Curr Opin Psychiatry. 2013;26(5):446-452.
6. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487-497.
7. Thompson L, McCabe R. The effect of clinician-patient alliance and communication on treatment adherence in mental health care: a systematic review. BMC Psychiatry. 2012;12:87.
8. Yilmaz S, Buzlu S. Antipsikotik kullanan hastalarda ilaç yan etkileri ve ilaç uyumu. Florence Nightingale Hem˘girelik Dergisi. 2012;20(2):93-103.
9. Kelleci M, Ata EE. Psikiyatri Klini˘ginde yatan hastaların ilaç uyumları ve sosyal destekle iliskisi. [Drug compliance of patients hospitalized in the psychiatry clinic and the relationship with social support]. Psikiyatri Hemsireli˘gi Dergisi. 2011;2(suppl 3):105-110.
10. Bulloch AG, Patten SB. Non-adherence with psychotropic medications in the general population. Soc Psychiatry Psychiatr Epidemiol. 2010;45(1):47-56.
11. Rosenbaum L. Beyond belief—how people feel about taking medications for heart disease. N Engl J Med. 2015;372(2):183-187.
12. Cramer J, Rosenheck R, Kirk G, et al. Medication compliance feedback and monitoring in a clinical trial: predictions and outcomes. Value Health. 2003;6(5):566-573.
13. Nakonezny PA, Byerly MJ, Rush AJ. Electronic monitoring of antipsychotic medication adherence in outpatients with schizophrenia or schizoaffective disorder: an empirical evaluation of its reliability and predictive validity. Psychiatry Res. 2008;157(1-3):259-263.
14. Fortney JC, Pyne JM, Edlund MJ, et al. Reasons for antidepressant nonadherence among veterans treated in primary care clinics. J Clin Psychiatry. 2011;72(6):827-834.
15. Kennedy J, Tuleu I, Mackay K. Unfilled prescriptions of medicare beneficiaries: prevalence, reasons, and types of medicines prescribed. J Manag Care Pharm. 2008;14(6):553-560.
16. Hoencamp E, Stevens A, Haffmans J. Patients’ attitudes toward antidepressants. Psychiatr Serv. 2002;53(9):1180-1181.
17. Keller MB, Hirschfeld RM, Demyttenaere K, et al. Optimizing outcomes in depression: focus on antidepressant compliance. Int Clin Psychopharmacol. 2002;17(6):265-271.
18. Akerblad AC, Bengtsson F, Holgersson M, et al. Identification of primary care patients at risk of nonadherence to antidepressant treatment. Patient Prefer Adherence. 2008;2:376-386.
19. Brown C, Battista DR, Bruehlman R, et al. Beliefs about antidepressant medications in primary care patients: relationship to self-reported adherence. Med Care. 2005;43(12):1203-1207.
20. Demyttenaere K, Adelin A, Patrick M, et al. Six-month compliance with antidepressant medication in the treatment of major depressive disorder. Int Clin Psychopharmacol. 2008;23(1):36-42.
21. Massand PS. Tolerability and adherence issues in antidepressant therapy. Clin Ther. 2003;25(8):2289-2304.
22. Medicare Prescription Drug, Improvement, and Modernization Act of 2003. Pub L No. 108-173, 117 Stat 2066.
23. Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011;86(4):304-314.
24. Diaz E, Neuse E, Sullivan MC, et al. Adherence to conventional and atypical antipsychotics after hospital discharge. J Clin Psychiatry. 2004;65(3):354-360.
25. Byerly M, Fisher R, Whatley K, et al. A comparison of electronic monitoring vs. clinician rating of antipsychotic adherence in outpatients with schizophrenia. Psychiatry Res. 2005;133(2-3):129-133.
26. Byerly MJ, Nakonezny PA, Lescouflair E. Antipsychotic medication adherence in schizophrenia. Psychiatr Clin North Am. 2007;30(3):437-452.
27. Nakonezny PA, Hughes CW, Mayes TL, et al. A comparison of various methods of measuring antidepressant medication adherence among children and adolescents with major depressive disorder in a 12-week open trial of fluoxetine. J Child Adolesc Psychopharmacol. 2010;20(5):431-439.
28. Kane JM, Perlis RH, DiCarlo LA, et al. First experience with a wireless system incorporating physiologic assessments and direct confirmation of digital tablet ingestions in ambulatory patients with schizophrenia or bipolar disorder. J Clin Psychiatry. 2013;74(6):e533-e540. doi: 10.4088/JCP.12m08222.
29. Morey TE, Booth MM, Prather RA, et al. Measurement of ethanol in gaseous breath using a miniature gas chromatograph. J Anal Toxicol. 2011;35(3):134-142.
30. Morey TE, Booth M, Wasdo S, et al. Oral adherence monitoring using a breath test to supplement highly active antiretroviral therapy. AIDS Behav. 2013;17(1):298-306.
31. Torem MS. Participatory pharmacotherapy: 10 strategies for enhancing adherence. Current Psychiatry. 2013;12(7):21-25.
32. Zygmunt A, Olfson M, Boyer CA, et al. Interventions to improve medication adherence in schizophrenia. Am J Psychiatry. 2002;159(10):1653-1664.
Nonadherence to medical therapy is a widespread and complex problem that is a significant variable in the treatment of psychiatric illness and in patients’ prognosis. More than 50% of people who have a chronic illness struggle to comply with their medication regimen—for many reasons.1
Many variables predict poor adherence, so it cannot be expected that a single solution will solve the problem entirely.2 Novel adherence technologies are available, as we discuss in this article, and more are in development.
What is nonadherence to medical therapy?
Nonadherence can be defined primarily as not taking prescribed medication in the recommended dosage or frequency, or not taking prescribed medication at all.3 Nonadherence can result in an increased risk of relapse, hospitalization, poor therapeutic response, and delayed remission and recovery.
Secondarily, non-attendance or irregular attendance at appointments with providers is a form of nonadherence that can have a negative impact on treatment outcomes.4
Why is medical adherence important in psychiatry?
Medication nonadherence has major consequences for psychiatric patients5 and for the greater health care system; it is estimated that, in the United States, the cost of nonadherence is as high as $300 billion a year.6 In psychiatry, the rate of nonadherence to medical therapy has been reported to be 11% to 80% of patients with schizophrenia; 12% to 64% with bipolar disorders; and 30% to 60% with depression.7-9 These surprising statistics make it imperative to design treatment strategies that include an effective patient-centric medication adherence plan, based on diagnosis, patient need, education, and support.
Why are patients nonadherent?
Many variables lead to patient nonadherence (Figure 1). The most common reason is that patients simply forget to take their medication.10 Among psychiatric patients, other reasons are:
- lack of insight
- negative emotional reaction to taking medication11
- feeling better and no longer believing that the medication is needed12,13
- distress associated with side effects14,15
- high cost of medication15
- patient’s perception that medication won’t be effective16,17
- concern about substance abuse18
- fear of dependency19
- complicated dosing regimen20
- general lack of motivation.21
Emotional barriers to medication nonadherence are an underestimated area that can benefit greatly from the expertise and understanding of psychiatrists. These barriers include a sense of losing control, self-stigmatization, denial, poor insight, and beliefs about illness and medications.
Additional patient variables that contribute to nonadherence include:
- suboptimal health literacy
- stigma and shame about the need for psychiatric treatment
- lack of patient involvement in treatment decision-making.
Who is responsible for adherence?
Adherence to medical therapy is not the patient’s responsibility, exclusively. Rather, it is a collection of complex components that generally includes physicians and the health care system. Because barriers to medication adherence are complex and varied, solutions to improve adherence must be multifaceted.
Providers. Patients’ care often is managed by multiple physicians, which can lead to communication lapses about complicated drug regimens and potential adverse effects. To assist patients in adhering to their medication regimen, physicians should recognize, and acknowledge to the patient, that many psychiatric patients have difficulty taking their medications and provide advice and information in how to address this problem.
Families. Likewise, it is important to educate patients and their family about the need for medication—helping the patient see that it is his (her) choice and, indeed, his direct responsibility to take his medication and improve his health. The risk–benefit balance of treatment should be explained to the patient and his family, as well as the nature of the psychiatric diagnosis and how effective patient–physician collaboration can help him function and adhere to his medication regimen in a consistent, reliable manner.
The larger system. Health care systems can contribute to medication adherence by reducing time constraints on visits to providers, to allow time to discuss all aspects of medication adherence. Limited visits in the clinic means physicians are not able to (1) spend adequate time discussing the medication regimen to ensure full patient comprehension and (2) conduct an assessment of medication-taking behaviors. Team-based approaches could improve efficiency, patient understanding, adherence, and early detection of adherence issues.22,23
Strategies such as additional clinic visits and reminder calls to discuss adherence carry a cost, but their long-term advantage is that, if patients understand how to better adhere to their medication regimens, their actions will have a positive impact on their health care costs and outcomes and on the wider health economy—as a result of reduced hospital admissions and reduced need to care for patients whose condition deteriorates because of nonadherence. It is imperative that we build strong relationships with other providers to show that we are committed to building supportive, effective adherence support programs that focus on the individual patient’s needs.
What is the available technology?
There is no standard way to measure nonadherence. The most common, and simplest, measure—asking the patient—is unreliable and severely overestimates adherence.
Direct measures of adherence include observing the patient taking his medications and testing for the concentration of those medications in blood or urine. Indirect adherence assessment methods, such as pill counts, a medication diary, self-report, clinician ratings, pharmacy chart review, and electronic devices that monitor the opening of a lid or tablet strip, have all been used; yet reviews of those methods have shown less than favorable results.6
Pre-packaged pill packs have helped some patients with a simple method for medication management.
Electronic monitoring, using a medication vial cap device (Figure 2) that electronically records the date and time of bottle opening, has become common in general medicine and among patients with schizophrenia.6,13,24-26 Diaz et al24 reported that electronic monitoring detected a greater nonadherence rate (57%) than what prescribers reported (7%) or patients self-reported (5%)—demonstrating that prescribers and patients grossly overestimate adherence. In another study that looked at electronic monitoring, researchers reported that adherence was much higher in depressed youth (87%)27 than what had been seen in adults (67%) in a similar study.13
The downside to pill packs and electronic monitoring? There is no guarantee the patient has actually taken the medication despite the data reported by the system.
Event marker-signaling devices. Novel technologies have been developed to measure adherence:
Proteus Digital Health feedback system (www.proteus.com) requires that patients ingest a tablet containing a tiny, dietary mineral-based “ingestible event marker.” Upon contact with gastric fluid electrolytes, the event marker emits a unique signal that is transmitted through bodily tissue to a small receiver in a patch worn on the torso. The receiver then transmits a signal to a cellular phone, indicating the time and date when the medication was ingested (Figure 3).
A 4-week pilot study28 found that the ingestible event marker is feasible and acceptable to patients: 27 of 28 participants (96%) completed the study, with a mean adherence rate of 74%. Although the system identifies ingestible sensors with high accuracy and is easily tolerated by patients, the pilot study was brief; a longer duration of adherence while wearing the patch needs to be studied.
Breath analysis, facial recognition. Even directly observing ingestion of a medication can be problematic: Some patients don’t swallow the medication and spit it out later. One way around that subterfuge is to consider using other advanced medication adherence solutions that are breath-based or use facial recognition technology and confirm ingestion.
Xhale SMART (www.xhale.com/smart) is a handheld device that generates a reminder to the patient to take his medication; afterward, he (she) must blow into the device so that ingestion of the medication is detected (Figure 4). The medication has breath-detectable adherence markers already incorporated. The adherence marker then is released into the stomach and small intestine, where the adherence marker metabolite is transported through the bloodstream into the lungs and exhaled. The patient must breathe into a breath analysis device, which measures medication ingestion compared with a baseline breath print.
Several articles in the literature have reported the accuracy of this device in detecting the ingested metabolite in every participant, without adverse effects.29,30 Clinical data on the use of the breath-based detector is not available to the public at this time.
AiCure (www.aicure.com) is a facial recognition-based technology platform that can work through any smartphone. The device is powered by artificial intelligence software and motion-sensing technology that can detect, in real time, whether the patient is taking the medication as prescribed. Patients who take an incorrect dose, or who do not use the software, are automatically flagged for immediate follow-up. This technology enables real-time intervention by a provider with the nonadherent patient.
An important note: These innovative technological advances are tools that can help clinicians manage an important aspect of treatment, but they do not show the entire picture: The physician−patient relationship and the therapeutic alliance are key to optimal treatment adherence.
Engage and empower the patient
Novel adherence technologies are, as we’ve described, available, and more are being developed. Incorporating these technologies into clinical care requires continued input and support from clinicians and patients. Digital and mobile health applications are multi-beneficial: They can empower patients to self-manage medication regimens and appointments while they also receive social and psychological information and support as needed. Understanding one’s own illness can, ultimately, improve outcomes and significantly reduce health care costs.
Patient empowerment is key. The physician is an important influencer in this regard.
The role of the physician must not be undervalued in maintaining adherence to therapy; she (he) plays a vital role in continued patient engagement and behavioral training. Integrating physician-led oversight, patient education, and commitment, and novel digital mobile adherence technologies will help deliver better outcomes.
The push to engage. A “one size fits all” approach to maintaining adherence won’t be effective. We need to better understand the individual patient’s underlying cause(s) for nonadherence, then to tailor a solution to influence and change that behavior. One way to do this is by interacting and engaging more directly (and in a digital manner) with patients to monitor adherence.
A recent example of the move toward direct patient engagement is the agreement entered by Otsuka Pharmaceuticals and Proteus Digital Health to develop novel digital health products. The FDA has accepted for review the combination product of Otsuka’s brand of aripiprazole and Proteus’s ingestible sensor. If the product is approved by the FDA, physicians will be able to prescribe aripiprazole with the ingestible sensor embedded in the tablet and then measure medication adherence and other patient physiologic metrics (eg, activity, rest) through the wearable sensor patch and medical software application designed specifically for patient and physician use.
This technology could have huge potential in mental health care, where patients struggle with both adhering to their medication regimen and communicating with the health care team. Physicians could measure adherence when treating adults with schizophrenia, bipolar disorders, and major depressive disorder; flag those who are not adhering as having higher risk of disease progression and poorer outcome; and allow decisions to be made more quickly based on treatment need.
Developing and enhancing these collaborative and patient-centric approaches will increase self-monitoring and patient responsibility, and encourage behavior change.
‘All-in’ strategy. By continuing to use the latest technologies and connecting them to the range of stakeholders—physicians, nurses, pharmacists, payers—we will develop an all-inclusive adherence intervention strategy. All patients will be integrated, and all of them, and their family, will be provided with positive psychoeducational care and motivational counseling (Figure 5). In addition, such a support-based patient experience must be aligned with the work of clinical care providers. Compliance therapy and behavioral training, together with active patient engagement, can help improve insight, acceptance of treatment, and, over the long term, adherence.31,32
Nonadherence to medical therapy is a widespread and complex problem that is a significant variable in the treatment of psychiatric illness and in patients’ prognosis. More than 50% of people who have a chronic illness struggle to comply with their medication regimen—for many reasons.1
Many variables predict poor adherence, so it cannot be expected that a single solution will solve the problem entirely.2 Novel adherence technologies are available, as we discuss in this article, and more are in development.
What is nonadherence to medical therapy?
Nonadherence can be defined primarily as not taking prescribed medication in the recommended dosage or frequency, or not taking prescribed medication at all.3 Nonadherence can result in an increased risk of relapse, hospitalization, poor therapeutic response, and delayed remission and recovery.
Secondarily, non-attendance or irregular attendance at appointments with providers is a form of nonadherence that can have a negative impact on treatment outcomes.4
Why is medical adherence important in psychiatry?
Medication nonadherence has major consequences for psychiatric patients5 and for the greater health care system; it is estimated that, in the United States, the cost of nonadherence is as high as $300 billion a year.6 In psychiatry, the rate of nonadherence to medical therapy has been reported to be 11% to 80% of patients with schizophrenia; 12% to 64% with bipolar disorders; and 30% to 60% with depression.7-9 These surprising statistics make it imperative to design treatment strategies that include an effective patient-centric medication adherence plan, based on diagnosis, patient need, education, and support.
Why are patients nonadherent?
Many variables lead to patient nonadherence (Figure 1). The most common reason is that patients simply forget to take their medication.10 Among psychiatric patients, other reasons are:
- lack of insight
- negative emotional reaction to taking medication11
- feeling better and no longer believing that the medication is needed12,13
- distress associated with side effects14,15
- high cost of medication15
- patient’s perception that medication won’t be effective16,17
- concern about substance abuse18
- fear of dependency19
- complicated dosing regimen20
- general lack of motivation.21
Emotional barriers to medication nonadherence are an underestimated area that can benefit greatly from the expertise and understanding of psychiatrists. These barriers include a sense of losing control, self-stigmatization, denial, poor insight, and beliefs about illness and medications.
Additional patient variables that contribute to nonadherence include:
- suboptimal health literacy
- stigma and shame about the need for psychiatric treatment
- lack of patient involvement in treatment decision-making.
Who is responsible for adherence?
Adherence to medical therapy is not the patient’s responsibility, exclusively. Rather, it is a collection of complex components that generally includes physicians and the health care system. Because barriers to medication adherence are complex and varied, solutions to improve adherence must be multifaceted.
Providers. Patients’ care often is managed by multiple physicians, which can lead to communication lapses about complicated drug regimens and potential adverse effects. To assist patients in adhering to their medication regimen, physicians should recognize, and acknowledge to the patient, that many psychiatric patients have difficulty taking their medications and provide advice and information in how to address this problem.
Families. Likewise, it is important to educate patients and their family about the need for medication—helping the patient see that it is his (her) choice and, indeed, his direct responsibility to take his medication and improve his health. The risk–benefit balance of treatment should be explained to the patient and his family, as well as the nature of the psychiatric diagnosis and how effective patient–physician collaboration can help him function and adhere to his medication regimen in a consistent, reliable manner.
The larger system. Health care systems can contribute to medication adherence by reducing time constraints on visits to providers, to allow time to discuss all aspects of medication adherence. Limited visits in the clinic means physicians are not able to (1) spend adequate time discussing the medication regimen to ensure full patient comprehension and (2) conduct an assessment of medication-taking behaviors. Team-based approaches could improve efficiency, patient understanding, adherence, and early detection of adherence issues.22,23
Strategies such as additional clinic visits and reminder calls to discuss adherence carry a cost, but their long-term advantage is that, if patients understand how to better adhere to their medication regimens, their actions will have a positive impact on their health care costs and outcomes and on the wider health economy—as a result of reduced hospital admissions and reduced need to care for patients whose condition deteriorates because of nonadherence. It is imperative that we build strong relationships with other providers to show that we are committed to building supportive, effective adherence support programs that focus on the individual patient’s needs.
What is the available technology?
There is no standard way to measure nonadherence. The most common, and simplest, measure—asking the patient—is unreliable and severely overestimates adherence.
Direct measures of adherence include observing the patient taking his medications and testing for the concentration of those medications in blood or urine. Indirect adherence assessment methods, such as pill counts, a medication diary, self-report, clinician ratings, pharmacy chart review, and electronic devices that monitor the opening of a lid or tablet strip, have all been used; yet reviews of those methods have shown less than favorable results.6
Pre-packaged pill packs have helped some patients with a simple method for medication management.
Electronic monitoring, using a medication vial cap device (Figure 2) that electronically records the date and time of bottle opening, has become common in general medicine and among patients with schizophrenia.6,13,24-26 Diaz et al24 reported that electronic monitoring detected a greater nonadherence rate (57%) than what prescribers reported (7%) or patients self-reported (5%)—demonstrating that prescribers and patients grossly overestimate adherence. In another study that looked at electronic monitoring, researchers reported that adherence was much higher in depressed youth (87%)27 than what had been seen in adults (67%) in a similar study.13
The downside to pill packs and electronic monitoring? There is no guarantee the patient has actually taken the medication despite the data reported by the system.
Event marker-signaling devices. Novel technologies have been developed to measure adherence:
Proteus Digital Health feedback system (www.proteus.com) requires that patients ingest a tablet containing a tiny, dietary mineral-based “ingestible event marker.” Upon contact with gastric fluid electrolytes, the event marker emits a unique signal that is transmitted through bodily tissue to a small receiver in a patch worn on the torso. The receiver then transmits a signal to a cellular phone, indicating the time and date when the medication was ingested (Figure 3).
A 4-week pilot study28 found that the ingestible event marker is feasible and acceptable to patients: 27 of 28 participants (96%) completed the study, with a mean adherence rate of 74%. Although the system identifies ingestible sensors with high accuracy and is easily tolerated by patients, the pilot study was brief; a longer duration of adherence while wearing the patch needs to be studied.
Breath analysis, facial recognition. Even directly observing ingestion of a medication can be problematic: Some patients don’t swallow the medication and spit it out later. One way around that subterfuge is to consider using other advanced medication adherence solutions that are breath-based or use facial recognition technology and confirm ingestion.
Xhale SMART (www.xhale.com/smart) is a handheld device that generates a reminder to the patient to take his medication; afterward, he (she) must blow into the device so that ingestion of the medication is detected (Figure 4). The medication has breath-detectable adherence markers already incorporated. The adherence marker then is released into the stomach and small intestine, where the adherence marker metabolite is transported through the bloodstream into the lungs and exhaled. The patient must breathe into a breath analysis device, which measures medication ingestion compared with a baseline breath print.
Several articles in the literature have reported the accuracy of this device in detecting the ingested metabolite in every participant, without adverse effects.29,30 Clinical data on the use of the breath-based detector is not available to the public at this time.
AiCure (www.aicure.com) is a facial recognition-based technology platform that can work through any smartphone. The device is powered by artificial intelligence software and motion-sensing technology that can detect, in real time, whether the patient is taking the medication as prescribed. Patients who take an incorrect dose, or who do not use the software, are automatically flagged for immediate follow-up. This technology enables real-time intervention by a provider with the nonadherent patient.
An important note: These innovative technological advances are tools that can help clinicians manage an important aspect of treatment, but they do not show the entire picture: The physician−patient relationship and the therapeutic alliance are key to optimal treatment adherence.
Engage and empower the patient
Novel adherence technologies are, as we’ve described, available, and more are being developed. Incorporating these technologies into clinical care requires continued input and support from clinicians and patients. Digital and mobile health applications are multi-beneficial: They can empower patients to self-manage medication regimens and appointments while they also receive social and psychological information and support as needed. Understanding one’s own illness can, ultimately, improve outcomes and significantly reduce health care costs.
Patient empowerment is key. The physician is an important influencer in this regard.
The role of the physician must not be undervalued in maintaining adherence to therapy; she (he) plays a vital role in continued patient engagement and behavioral training. Integrating physician-led oversight, patient education, and commitment, and novel digital mobile adherence technologies will help deliver better outcomes.
The push to engage. A “one size fits all” approach to maintaining adherence won’t be effective. We need to better understand the individual patient’s underlying cause(s) for nonadherence, then to tailor a solution to influence and change that behavior. One way to do this is by interacting and engaging more directly (and in a digital manner) with patients to monitor adherence.
A recent example of the move toward direct patient engagement is the agreement entered by Otsuka Pharmaceuticals and Proteus Digital Health to develop novel digital health products. The FDA has accepted for review the combination product of Otsuka’s brand of aripiprazole and Proteus’s ingestible sensor. If the product is approved by the FDA, physicians will be able to prescribe aripiprazole with the ingestible sensor embedded in the tablet and then measure medication adherence and other patient physiologic metrics (eg, activity, rest) through the wearable sensor patch and medical software application designed specifically for patient and physician use.
This technology could have huge potential in mental health care, where patients struggle with both adhering to their medication regimen and communicating with the health care team. Physicians could measure adherence when treating adults with schizophrenia, bipolar disorders, and major depressive disorder; flag those who are not adhering as having higher risk of disease progression and poorer outcome; and allow decisions to be made more quickly based on treatment need.
Developing and enhancing these collaborative and patient-centric approaches will increase self-monitoring and patient responsibility, and encourage behavior change.
‘All-in’ strategy. By continuing to use the latest technologies and connecting them to the range of stakeholders—physicians, nurses, pharmacists, payers—we will develop an all-inclusive adherence intervention strategy. All patients will be integrated, and all of them, and their family, will be provided with positive psychoeducational care and motivational counseling (Figure 5). In addition, such a support-based patient experience must be aligned with the work of clinical care providers. Compliance therapy and behavioral training, together with active patient engagement, can help improve insight, acceptance of treatment, and, over the long term, adherence.31,32
1. World Health Organization. Adherence to long-term therapies: evidence for action. Geneva, Switzerland: World Health Organization; 2003.
2. Bosworth HB, Granger BB, Mendys P, et al. Medication adherence: a call for action. Am Heart J. 2011;162(3):412-424.
3. Crowe M, Wilson L, Inder M. Patients’ reports of the factors influencing medication adherence in bipolar disorder – an integrative review of the literature. Int J Nurs Stud. 2011;48(7):894-903.
4. Mert D, Turgut NH, Kelleci M, et al. Perspectives on reasons of medication nonadherence in psychiatric patients. Patient Prefer Adherence. 2015;9:87-93.
5. Chapman SC, Horne R. Medication nonadherence and psychiatry. Curr Opin Psychiatry. 2013;26(5):446-452.
6. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487-497.
7. Thompson L, McCabe R. The effect of clinician-patient alliance and communication on treatment adherence in mental health care: a systematic review. BMC Psychiatry. 2012;12:87.
8. Yilmaz S, Buzlu S. Antipsikotik kullanan hastalarda ilaç yan etkileri ve ilaç uyumu. Florence Nightingale Hem˘girelik Dergisi. 2012;20(2):93-103.
9. Kelleci M, Ata EE. Psikiyatri Klini˘ginde yatan hastaların ilaç uyumları ve sosyal destekle iliskisi. [Drug compliance of patients hospitalized in the psychiatry clinic and the relationship with social support]. Psikiyatri Hemsireli˘gi Dergisi. 2011;2(suppl 3):105-110.
10. Bulloch AG, Patten SB. Non-adherence with psychotropic medications in the general population. Soc Psychiatry Psychiatr Epidemiol. 2010;45(1):47-56.
11. Rosenbaum L. Beyond belief—how people feel about taking medications for heart disease. N Engl J Med. 2015;372(2):183-187.
12. Cramer J, Rosenheck R, Kirk G, et al. Medication compliance feedback and monitoring in a clinical trial: predictions and outcomes. Value Health. 2003;6(5):566-573.
13. Nakonezny PA, Byerly MJ, Rush AJ. Electronic monitoring of antipsychotic medication adherence in outpatients with schizophrenia or schizoaffective disorder: an empirical evaluation of its reliability and predictive validity. Psychiatry Res. 2008;157(1-3):259-263.
14. Fortney JC, Pyne JM, Edlund MJ, et al. Reasons for antidepressant nonadherence among veterans treated in primary care clinics. J Clin Psychiatry. 2011;72(6):827-834.
15. Kennedy J, Tuleu I, Mackay K. Unfilled prescriptions of medicare beneficiaries: prevalence, reasons, and types of medicines prescribed. J Manag Care Pharm. 2008;14(6):553-560.
16. Hoencamp E, Stevens A, Haffmans J. Patients’ attitudes toward antidepressants. Psychiatr Serv. 2002;53(9):1180-1181.
17. Keller MB, Hirschfeld RM, Demyttenaere K, et al. Optimizing outcomes in depression: focus on antidepressant compliance. Int Clin Psychopharmacol. 2002;17(6):265-271.
18. Akerblad AC, Bengtsson F, Holgersson M, et al. Identification of primary care patients at risk of nonadherence to antidepressant treatment. Patient Prefer Adherence. 2008;2:376-386.
19. Brown C, Battista DR, Bruehlman R, et al. Beliefs about antidepressant medications in primary care patients: relationship to self-reported adherence. Med Care. 2005;43(12):1203-1207.
20. Demyttenaere K, Adelin A, Patrick M, et al. Six-month compliance with antidepressant medication in the treatment of major depressive disorder. Int Clin Psychopharmacol. 2008;23(1):36-42.
21. Massand PS. Tolerability and adherence issues in antidepressant therapy. Clin Ther. 2003;25(8):2289-2304.
22. Medicare Prescription Drug, Improvement, and Modernization Act of 2003. Pub L No. 108-173, 117 Stat 2066.
23. Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011;86(4):304-314.
24. Diaz E, Neuse E, Sullivan MC, et al. Adherence to conventional and atypical antipsychotics after hospital discharge. J Clin Psychiatry. 2004;65(3):354-360.
25. Byerly M, Fisher R, Whatley K, et al. A comparison of electronic monitoring vs. clinician rating of antipsychotic adherence in outpatients with schizophrenia. Psychiatry Res. 2005;133(2-3):129-133.
26. Byerly MJ, Nakonezny PA, Lescouflair E. Antipsychotic medication adherence in schizophrenia. Psychiatr Clin North Am. 2007;30(3):437-452.
27. Nakonezny PA, Hughes CW, Mayes TL, et al. A comparison of various methods of measuring antidepressant medication adherence among children and adolescents with major depressive disorder in a 12-week open trial of fluoxetine. J Child Adolesc Psychopharmacol. 2010;20(5):431-439.
28. Kane JM, Perlis RH, DiCarlo LA, et al. First experience with a wireless system incorporating physiologic assessments and direct confirmation of digital tablet ingestions in ambulatory patients with schizophrenia or bipolar disorder. J Clin Psychiatry. 2013;74(6):e533-e540. doi: 10.4088/JCP.12m08222.
29. Morey TE, Booth MM, Prather RA, et al. Measurement of ethanol in gaseous breath using a miniature gas chromatograph. J Anal Toxicol. 2011;35(3):134-142.
30. Morey TE, Booth M, Wasdo S, et al. Oral adherence monitoring using a breath test to supplement highly active antiretroviral therapy. AIDS Behav. 2013;17(1):298-306.
31. Torem MS. Participatory pharmacotherapy: 10 strategies for enhancing adherence. Current Psychiatry. 2013;12(7):21-25.
32. Zygmunt A, Olfson M, Boyer CA, et al. Interventions to improve medication adherence in schizophrenia. Am J Psychiatry. 2002;159(10):1653-1664.
1. World Health Organization. Adherence to long-term therapies: evidence for action. Geneva, Switzerland: World Health Organization; 2003.
2. Bosworth HB, Granger BB, Mendys P, et al. Medication adherence: a call for action. Am Heart J. 2011;162(3):412-424.
3. Crowe M, Wilson L, Inder M. Patients’ reports of the factors influencing medication adherence in bipolar disorder – an integrative review of the literature. Int J Nurs Stud. 2011;48(7):894-903.
4. Mert D, Turgut NH, Kelleci M, et al. Perspectives on reasons of medication nonadherence in psychiatric patients. Patient Prefer Adherence. 2015;9:87-93.
5. Chapman SC, Horne R. Medication nonadherence and psychiatry. Curr Opin Psychiatry. 2013;26(5):446-452.
6. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487-497.
7. Thompson L, McCabe R. The effect of clinician-patient alliance and communication on treatment adherence in mental health care: a systematic review. BMC Psychiatry. 2012;12:87.
8. Yilmaz S, Buzlu S. Antipsikotik kullanan hastalarda ilaç yan etkileri ve ilaç uyumu. Florence Nightingale Hem˘girelik Dergisi. 2012;20(2):93-103.
9. Kelleci M, Ata EE. Psikiyatri Klini˘ginde yatan hastaların ilaç uyumları ve sosyal destekle iliskisi. [Drug compliance of patients hospitalized in the psychiatry clinic and the relationship with social support]. Psikiyatri Hemsireli˘gi Dergisi. 2011;2(suppl 3):105-110.
10. Bulloch AG, Patten SB. Non-adherence with psychotropic medications in the general population. Soc Psychiatry Psychiatr Epidemiol. 2010;45(1):47-56.
11. Rosenbaum L. Beyond belief—how people feel about taking medications for heart disease. N Engl J Med. 2015;372(2):183-187.
12. Cramer J, Rosenheck R, Kirk G, et al. Medication compliance feedback and monitoring in a clinical trial: predictions and outcomes. Value Health. 2003;6(5):566-573.
13. Nakonezny PA, Byerly MJ, Rush AJ. Electronic monitoring of antipsychotic medication adherence in outpatients with schizophrenia or schizoaffective disorder: an empirical evaluation of its reliability and predictive validity. Psychiatry Res. 2008;157(1-3):259-263.
14. Fortney JC, Pyne JM, Edlund MJ, et al. Reasons for antidepressant nonadherence among veterans treated in primary care clinics. J Clin Psychiatry. 2011;72(6):827-834.
15. Kennedy J, Tuleu I, Mackay K. Unfilled prescriptions of medicare beneficiaries: prevalence, reasons, and types of medicines prescribed. J Manag Care Pharm. 2008;14(6):553-560.
16. Hoencamp E, Stevens A, Haffmans J. Patients’ attitudes toward antidepressants. Psychiatr Serv. 2002;53(9):1180-1181.
17. Keller MB, Hirschfeld RM, Demyttenaere K, et al. Optimizing outcomes in depression: focus on antidepressant compliance. Int Clin Psychopharmacol. 2002;17(6):265-271.
18. Akerblad AC, Bengtsson F, Holgersson M, et al. Identification of primary care patients at risk of nonadherence to antidepressant treatment. Patient Prefer Adherence. 2008;2:376-386.
19. Brown C, Battista DR, Bruehlman R, et al. Beliefs about antidepressant medications in primary care patients: relationship to self-reported adherence. Med Care. 2005;43(12):1203-1207.
20. Demyttenaere K, Adelin A, Patrick M, et al. Six-month compliance with antidepressant medication in the treatment of major depressive disorder. Int Clin Psychopharmacol. 2008;23(1):36-42.
21. Massand PS. Tolerability and adherence issues in antidepressant therapy. Clin Ther. 2003;25(8):2289-2304.
22. Medicare Prescription Drug, Improvement, and Modernization Act of 2003. Pub L No. 108-173, 117 Stat 2066.
23. Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011;86(4):304-314.
24. Diaz E, Neuse E, Sullivan MC, et al. Adherence to conventional and atypical antipsychotics after hospital discharge. J Clin Psychiatry. 2004;65(3):354-360.
25. Byerly M, Fisher R, Whatley K, et al. A comparison of electronic monitoring vs. clinician rating of antipsychotic adherence in outpatients with schizophrenia. Psychiatry Res. 2005;133(2-3):129-133.
26. Byerly MJ, Nakonezny PA, Lescouflair E. Antipsychotic medication adherence in schizophrenia. Psychiatr Clin North Am. 2007;30(3):437-452.
27. Nakonezny PA, Hughes CW, Mayes TL, et al. A comparison of various methods of measuring antidepressant medication adherence among children and adolescents with major depressive disorder in a 12-week open trial of fluoxetine. J Child Adolesc Psychopharmacol. 2010;20(5):431-439.
28. Kane JM, Perlis RH, DiCarlo LA, et al. First experience with a wireless system incorporating physiologic assessments and direct confirmation of digital tablet ingestions in ambulatory patients with schizophrenia or bipolar disorder. J Clin Psychiatry. 2013;74(6):e533-e540. doi: 10.4088/JCP.12m08222.
29. Morey TE, Booth MM, Prather RA, et al. Measurement of ethanol in gaseous breath using a miniature gas chromatograph. J Anal Toxicol. 2011;35(3):134-142.
30. Morey TE, Booth M, Wasdo S, et al. Oral adherence monitoring using a breath test to supplement highly active antiretroviral therapy. AIDS Behav. 2013;17(1):298-306.
31. Torem MS. Participatory pharmacotherapy: 10 strategies for enhancing adherence. Current Psychiatry. 2013;12(7):21-25.
32. Zygmunt A, Olfson M, Boyer CA, et al. Interventions to improve medication adherence in schizophrenia. Am J Psychiatry. 2002;159(10):1653-1664.
When and why to initiate antipsychotic polypharmacy, and with which agents
Mr. C, age 31, who has a 7-year history of schizophrenia and is currently on perphenazine, 24 mg twice a day, presents for psychiatric admission after experiencing paranoid delusions. Notable symptoms include delusions of reference and persecution, along with affective flattening and intermittent suicidal ideation. Perphenazine is tapered, and he is started on quetiapine, titrated to 600 mg/d.
Past antipsychotic trials include aripiprazole, olanzapine, paliperidone, haloperidol,
and ziprasidone. Because of his refractory symptoms and tolerability issues with other antipsychotics, Mr. C is switched to clozapine, 400 mg/d. His symptoms improve, but he experiences dose-limiting sialorrhea. Risperidone, 1 mg/d, is added to clozapine, which helps his psychosis and improves his functional status. Additionally, Mr. C develops enough insight to recognize his delusions and use skills learned in psychotherapy to cope with them.
Antipsychotic polypharmacy (APP), the concurrent use of ≥2 antipsychotics, is a topic of debate among mental health care providers. Studies indicate the prevalence of APP can reach upwards of 40%, with 1 systematic review citing more recent median APP prevalence in North America as 17%, an increase from a median of 12.7% in the 1980s.1 Other studies cite more recent figures as around 20%.2,3
The literature lists several reasons for use of long-term APP, including:
- incomplete cross-titration
- accidental continuation of APP that was intended to be temporary
- monotherapy failure
- mitigation or enhancement of effects of other antipsychotics (Table 1).1,4
Other factors include direct-to-consumer advertising, external pressures to decrease hospital stays, and low doctor-to-patient ratios.5 Although it can take as long as 16 weeks to see clinically significant improvement with an antipsychotic, prescribers might expect results after 4 weeks of treatment.6 Therefore, treatments could be labeled ineffective because trials did not last long enough, leading to premature use of polypharmacy. Combinations of a first- and second-generation antipsychotic (SGA) or 2 SGAs are most common.2,7,8
Treatment guidelines (Table 2)9-17 suggest APP could be considered after several failures of monotherapy, including clozapine monotherapy, although some guidelines do not address the issue or recommend against APP because of lack of efficacy and safety data. Additionally, APP poses safety concerns (Table 3).18-22 Recommendations for APP with combinations that do not include clozapine generally are not provided, because high-level evidence to support this strategy is lacking. Data on safety and efficacy of APP are mixed, with much of the literature dominated by case reports and uncontrolled studies.19
What to initiate
Clozapine. Higher-level evidence is available for clozapine APP. The combination of clozapine and risperidone is one of the most thoroughly studied and, therefore, is a reasonable first choice. Randomized controlled trials (RCTs) examining clozapine plus risperidone23-29 have yielded mixed results and have not provided conclusive information regarding benefit for positive vs negative symptoms.24-28
One RCT reported a significant change in Brief Psychiatric Rating Scale (BPRS) total and positive symptom scores.27 Other RCTs have shown a non-significant trend toward greater change in total, positive, and negative symptom scores with the clozapine-risperidone combination compared with clozapine monotherapy.25,28 In terms of cognition, this combination provided no additional benefit.23 Response, defined as ≥20% reduction in total BPRS or Positive and Negative Syndrome Scale (PANSS) scores, for clozapine plus risperidone range from 13% to 83%, compared with 8% to 29% for clozapine plus placebo.24,25,27,29
Data from 1 study27 suggest a number needed to treat of 4 to achieve at least a 20% improvement in BPRS scores with clozapine plus risperidone vs clozapine monotherapy. Across these studies, the average risperidone dosage was 4 mg/d, although using the lowest effective dosage is encouraged. A small number of RCTs and articles examining other APP combinations (Table 4)30-33 have yielded mixed results.
Overall, APP appears to be well-tolerated, although it is associated with an increased risk of adverse effects, including sedation, extrapyramidal symptoms, hyperprolactinemia, sexual dysfunction, cognitive impairment, anticholinergic effects, hyperlipidemia, and diabetes.23,24,34-36 Surprisingly, 1 literature review36 found no association between APP and increased risk of orthostasis. Increased occurrence of sedation, hyperprolactinemia, and an elevated fasting blood glucose level have been found for clozapine plus risperidone compared with clozapine monotherapy.24-26,28
Aripiprazole. Adjunctive aripiprazole, a dopamine partial agonist, could reduce elevated prolactin levels caused by other antipsychotics.32 In a study37 of 56 patients taking haloperidol who had hyperprolactinemia, prolactin levels normalized in 88.5% of patients taking adjunctive aripiprazole, 30 mg/d, compared with 3.6% of those with added placebo. Furthermore, results from 2 RCTs38,39 of patients taking clozapine or olanzapine suggest adjunctive aripiprazole could improve weight and metabolic profile. Therefore, adding aripiprazole to existing antipsychotic regimens is reasonable for patients with drug-induced symptomatic hyperprolactinemia or metabolic effects and who cannot be easily switched to another antipsychotic.
When to initiate
Most treatment guidelines9-17 recommend clozapine only after monotherapy with at least 2 other antipsychotics fails. It is reasonable to add an antipsychotic to clozapine in patients who have shown a partial response to clozapine after a minimum of 3 months. Non-clozapine APP should be considered when:
- a patient derives no benefit from clozapine
- refuses clozapine
- clozapine is contraindicated
- APP is initiated to mitigate side effects from another antipsychotic.
Antipsychotics could take up to 16 weeks to achieve full efficacy,6 therefore, an adequate trial period within the target dosage range is advised for all antipsychotics (Table 5).13,40
Why initiate
Based on available data, partial response to maximum recommended dosages of antipsychotic monotherapy, including clozapine, or inability to tolerate higher dosages, provides a reason for initiating APP. Non-clozapine APP generally should be considered only in patients who refuse, cannot tolerate, or do not respond to clozapine. Consider using validated rating scales to track treatment outcomes (ideally, a ≥20% symptomatic reduction on the BPRS or PANSS), although there is no formal guidance regarding their use or benefit in APP.
Summing up
APP is a fairly common prescribing practice, even though safety and efficacy data are mixed. The issue of APP has become prevalent enough that regulatory bodies are involved in its monitoring and documentation.41
Clozapine APP, especially with risperidone, has the most substantial evidence to support it. Although APP generally is well tolerated, the overall dearth of conclusive safety and efficacy data indicates that this practice should be reserved for patients who have not responded adequately to monotherapy, including clozapine. Adjunctive aripiprazole could be considered for addressing symptomatic hyperprolactinemia or other metabolic effects caused by other antipsychotics.
An adequate trial as long as 16 weeks is advised before assessing the efficacy of any antipsychotic regimen. If APP provides inadequate response, or if there is no clear indication for APP, consider switching the patient back to monotherapy.42-44
1. Gallego JA, Bonetti J, Zhang J, et al. Prevalence and correlates of antipsychotic polypharmacy: a systematic review and meta-regression of global and regional trends from the 1970s to 2009. Schizophr Res. 2012;138(1):18-28.
2. Gören JL, Meterko M, Williams S, et al. Antipsychotic prescribing pathways, polypharmacy, and clozapine use in treatment of schizophrenia. Psychiatr Serv. 2013;64(6):527-533.
3. Sun F, Stock EM, Copeland LA, et al. Polypharmacy with antipsychotic drugs in patients with schizophrenia: trends in multiple health care systems. Am J Health Syst Pharm. 2014;71(9):728-738.
4. Tapp A, Wood AE, Secrest L, et al. Combination antipsychotic therapy in clinical practice. Psychiatr Serv. 2003;54(1):55-59.
5. Ananth J, Parameswaran S, Gunatilake S. Antipsychotic polypharmacy. Curr Pharm Des. 2004;10(18):2231-2238.
6. Stahl SM. Antipsychotic polypharmacy: evidence based or eminence based? Acta Psychiatr Scand. 2002;106(5):321-322.
7. Botts S, Hines H, Littrell R. Antipsychotic polypharmacy in the ambulatory care setting, 1993-2000. Psychiatr Serv. 2003;54(8):1086.
8. Santone G, Bellantuono C, Rucci P, et al. Patient characteristics and process factors associated with antipsychotic polypharmacy in a nationwide sample of psychiatric inpatients in Italy. Pharmacoepidemiol Drug Saf. 2011;20(5):441-449.
9. American Psychiatric Association. Practice guideline for the treatment of patients with schizophrenia, second edition. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/schizophrenia.pdf. Updated September 2009. Accessed September 20, 2014.
10. Barnes TRE; Schizophrenia Consensus Group of the British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. http://www.bap.org.uk/pdfs/Schizophrenia_Consensus_Guideline_Document.pdf. Updated 2011. Accessed September 20, 2014.
11. National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: prevention and management. http://www.nice.org.uk/guidance/cg178. Published February 2014. Accessed September 20, 2014.
12. Texas Medication Algorithm Project. Schizophrenia treatment algorithms. http://www.jpshealthnet.org/sites/default/files/tmapalgorithmforschizophrenia.pdf. Updated April 2008. Accessed September 20, 2014.
13. Hasan A, Falkai P, Wobrock T, et al; World Federation of Societies of Biological Psychiatry (WFSBP). World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry. 2012;13(5):318-378.
14. Canadian Psychiatric Association. Clinical practice guidelines: treatment of schizophrenia. https://ww1.cpa-apc.org/Publications/Clinical_Guidelines/schizophrenia/november2005/index.asp. Updated November 2005. Accessed February 26, 2016.
15. Royal Australian and New Zealand College of Psychiatrists. Clinical practice guidelines for the treatment of schizophrenia and related disorders. http://www.ranzcp.org/Files/ranzcp-attachments/Resources/Publications/CPG/Clinician/CPG_Clinician_Full_Schizophrenia-pdf.aspx. Updated May 2005. Accessed February 26, 2016.
16. Scottish Intercollegiate Guidelines Network. Management of schizophrenia: a national clinical guideline. http://www.sign.ac.uk/guidelines/fulltext/131/index.html. Updated March 2013. Accessed September 20, 2014.
17. Buchanan RW, Kreyenbuhl J, Kelly DL, et al; Schizophrenia Patient Outcomes Research Team (PORT). The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71-93.
18. Correll CU, Gallego JA. Antipsychotic polypharmacy: a comprehensive evaluation of relevant correlates of a long-standing clinical practice. Psychiatr Clin North Am. 2012;35(3):661-681.
19. Tranulis C, Skalli L, Lalonde P, et al. Benefits and risks of antipsychotic polypharmacy: an evidence-based review of the literature. Drug Saf. 2008;31(1):7-20.
20. Barnes TR, Paton C. Antipsychotic polypharmacy in schizophrenia: benefits and risks. CNS Drugs. 2011;25(5):383-399.
21. Lochmann van Bennekom MW, Gijsman HJ, Zitman FG. Antipsychotic polypharmacy in psychotic disorders: a critical review of neurobiology, efficacy, tolerability and cost effectiveness. J Psychopharmacol. 2013;27(4):327-336.
22. Weinmann S, Read J, Aderhold V. Influence of antipsychotics on mortality in schizophrenia: systematic review. Schizophr Res. 2009;113(1):1-11.
23. Akdede BB, Anil Ya˘gcio˘glu AE, Alptekin K, et al. A double-blind study of combination of clozapine with risperidone in patients with schizophrenia: effects on cognition. J Clin Psychiatry. 2006;67(12):1912-1919.
24. Anil Ya˘gcio˘glu AE, Kivircik Akdede BB, Turgut TI, et al. A double-blind controlled study of adjunctive treatment with risperidone in schizophrenic patients partially responsive to clozapine: efficacy and safety. J Clin Psychiatry. 2005;66(1):63-72.
25. Freudenreich O, Henderson DC, Walsh JP, et al. Risperidone augmentation for schizophrenia partially responsive to clozapine: a double-blind, placebo-controlled trial. Schizophr Res. 2007;92(1-3):90-94.
26. Honer WG, Thornton AE, Chen EY, et al; Clozapine and Risperidone Enhancement (CARE) Study Group. Clozapine alone versus clozapine and risperidone with refractory schizophrenia. N Engl J Med. 2006;354(5):472-482.
27. Josiassen RC, Joseph A, Kohegyi E, et al. Clozapine augmented with risperidone in the treatment of schizophrenia: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2005;162(1):130-136.
28. Weiner E, Conley RR, Ball MP, et al. Adjunctive risperidone for partially responsive people with schizophrenia treated with clozapine. Neuropsychopharmacology. 2010;35(11):2274-2283.
29. Zink M, Kuwilsky A, Krumm B, et al. Efficacy and tolerability of ziprasidone versus risperidone as augmentation in patients partially responsive to clozapine: a randomized controlled clinical trial. J Psychopharmacol. 2009;23(3):305-314.
30. Canadian Agency for Drugs and Technology in Health. Current utilization of antipsychotic agents for schizophrenia: combination and high-dose therapies. https://www.cadth.ca/sites/default/files/pdf/H0503_AAP-Current-Utilization-Report_e.pdf. Published August 2012. Accessed February 26, 2016.
31. Chang JS, Ahn YM, Park HJ, et al. Aripiprazole augmentation in clozapine treated patients with refractory schizophrenia: an 8-week, randomized, double blind, placebo-controlled trial. J Clin Psychiatry. 2008;69(5):720-731.
32. Kane JM, Correll CU, Goff DC, et al. A multicenter, randomized, double-blind, placebo-controlled, 16-week study of adjunctive aripiprazole for schizophrenia or schizoaffective disorder inadequately treated with quetiapine or risperidone monotherapy. J Clin Psychiatry. 2009;70(10):1348-1357.
33. Velligan DI, Carroll C, Lage MJ, et al. Outcomes of medicaid beneficiaries with schizophrenia receiving clozapine only or antipsychotic combinations. Psychiatr Serv. 2015;66(2):127-133.
34. Citrome L, Jaffe A, Levine J, et al. Relationship between antipsychotic medication treatment and new cases of diabetes among psychiatric inpatients. Psychiatr Serv. 2004;55(9):1006-1013.
35. Correll CU, Frederickson AM, Kane JM, et al. Does antipsychotic polypharmacy increase the risk for metabolic syndrome? Schizophr Res. 2007;89(1-3):91-100.
36. Gallego JA, Nielsen J, De Hert M, et al. Safety and tolerability of antipsychotic polypharmacy. Expert Opin Drug Saf. 2012;11(4):527-542.
37. Shim JC, Shin JG, Kelly DL, et al. Adjunctive treatment with a dopamine partial agonist, aripiprazole, for antipsychotic-induced hyperprolactinemia: a placebo-controlled trial. Am J Psychiatry. 2007;164(9):1404-1410.
38. Fan X, Borba CP, Copeland P, et al. Metabolic effects of adjunctive aripiprazole in clozapine-treated patients with schizophrenia. Acta Psychiatr Scand. 2013;127(3):217-226.
39. Henderson DC, Fan X, Copeland PM, et al. Aripiprazole added to overweight and obese olanzapine-treated schizophrenia patients. J Clin Psychopharmacol. 2009;26(2):165-169.
40. Drug Information Handbook, 22th ed. Hudson, OH: Lexi-Comp, Inc.; 2013:1143-1147.
41. The Joint Commission. Specifications Manual for Joint Commission National Quality Measures (v2013A1). https://manual.jointcommission.org/releases/TJC2013A/. Accessed on May 13, 2015.
42. Essock SM, Schooler NR, Stroup TS, et al; Schizophrenia Trials Network. Effectiveness of switching from antipsychotic polypharmacy to monotherapy. Am J Psychiatry. 2011;168(7):702-708.
43. Godleski LS, Kerler R, Barber JW, et al. Multiple versus single antipsychotic drug treatment in chronic psychosis. J Nerv Ment Dis. 1989;177(11):686-689.
44. Suzuki T, Uchida H, Tanaka KF, et al. Revising polypharmacy to a single antipsychotic regimen for patients with chronic schizophrenia. Int J Neuropsychopharmacol. 2004;7(2):133-142.
Mr. C, age 31, who has a 7-year history of schizophrenia and is currently on perphenazine, 24 mg twice a day, presents for psychiatric admission after experiencing paranoid delusions. Notable symptoms include delusions of reference and persecution, along with affective flattening and intermittent suicidal ideation. Perphenazine is tapered, and he is started on quetiapine, titrated to 600 mg/d.
Past antipsychotic trials include aripiprazole, olanzapine, paliperidone, haloperidol,
and ziprasidone. Because of his refractory symptoms and tolerability issues with other antipsychotics, Mr. C is switched to clozapine, 400 mg/d. His symptoms improve, but he experiences dose-limiting sialorrhea. Risperidone, 1 mg/d, is added to clozapine, which helps his psychosis and improves his functional status. Additionally, Mr. C develops enough insight to recognize his delusions and use skills learned in psychotherapy to cope with them.
Antipsychotic polypharmacy (APP), the concurrent use of ≥2 antipsychotics, is a topic of debate among mental health care providers. Studies indicate the prevalence of APP can reach upwards of 40%, with 1 systematic review citing more recent median APP prevalence in North America as 17%, an increase from a median of 12.7% in the 1980s.1 Other studies cite more recent figures as around 20%.2,3
The literature lists several reasons for use of long-term APP, including:
- incomplete cross-titration
- accidental continuation of APP that was intended to be temporary
- monotherapy failure
- mitigation or enhancement of effects of other antipsychotics (Table 1).1,4
Other factors include direct-to-consumer advertising, external pressures to decrease hospital stays, and low doctor-to-patient ratios.5 Although it can take as long as 16 weeks to see clinically significant improvement with an antipsychotic, prescribers might expect results after 4 weeks of treatment.6 Therefore, treatments could be labeled ineffective because trials did not last long enough, leading to premature use of polypharmacy. Combinations of a first- and second-generation antipsychotic (SGA) or 2 SGAs are most common.2,7,8
Treatment guidelines (Table 2)9-17 suggest APP could be considered after several failures of monotherapy, including clozapine monotherapy, although some guidelines do not address the issue or recommend against APP because of lack of efficacy and safety data. Additionally, APP poses safety concerns (Table 3).18-22 Recommendations for APP with combinations that do not include clozapine generally are not provided, because high-level evidence to support this strategy is lacking. Data on safety and efficacy of APP are mixed, with much of the literature dominated by case reports and uncontrolled studies.19
What to initiate
Clozapine. Higher-level evidence is available for clozapine APP. The combination of clozapine and risperidone is one of the most thoroughly studied and, therefore, is a reasonable first choice. Randomized controlled trials (RCTs) examining clozapine plus risperidone23-29 have yielded mixed results and have not provided conclusive information regarding benefit for positive vs negative symptoms.24-28
One RCT reported a significant change in Brief Psychiatric Rating Scale (BPRS) total and positive symptom scores.27 Other RCTs have shown a non-significant trend toward greater change in total, positive, and negative symptom scores with the clozapine-risperidone combination compared with clozapine monotherapy.25,28 In terms of cognition, this combination provided no additional benefit.23 Response, defined as ≥20% reduction in total BPRS or Positive and Negative Syndrome Scale (PANSS) scores, for clozapine plus risperidone range from 13% to 83%, compared with 8% to 29% for clozapine plus placebo.24,25,27,29
Data from 1 study27 suggest a number needed to treat of 4 to achieve at least a 20% improvement in BPRS scores with clozapine plus risperidone vs clozapine monotherapy. Across these studies, the average risperidone dosage was 4 mg/d, although using the lowest effective dosage is encouraged. A small number of RCTs and articles examining other APP combinations (Table 4)30-33 have yielded mixed results.
Overall, APP appears to be well-tolerated, although it is associated with an increased risk of adverse effects, including sedation, extrapyramidal symptoms, hyperprolactinemia, sexual dysfunction, cognitive impairment, anticholinergic effects, hyperlipidemia, and diabetes.23,24,34-36 Surprisingly, 1 literature review36 found no association between APP and increased risk of orthostasis. Increased occurrence of sedation, hyperprolactinemia, and an elevated fasting blood glucose level have been found for clozapine plus risperidone compared with clozapine monotherapy.24-26,28
Aripiprazole. Adjunctive aripiprazole, a dopamine partial agonist, could reduce elevated prolactin levels caused by other antipsychotics.32 In a study37 of 56 patients taking haloperidol who had hyperprolactinemia, prolactin levels normalized in 88.5% of patients taking adjunctive aripiprazole, 30 mg/d, compared with 3.6% of those with added placebo. Furthermore, results from 2 RCTs38,39 of patients taking clozapine or olanzapine suggest adjunctive aripiprazole could improve weight and metabolic profile. Therefore, adding aripiprazole to existing antipsychotic regimens is reasonable for patients with drug-induced symptomatic hyperprolactinemia or metabolic effects and who cannot be easily switched to another antipsychotic.
When to initiate
Most treatment guidelines9-17 recommend clozapine only after monotherapy with at least 2 other antipsychotics fails. It is reasonable to add an antipsychotic to clozapine in patients who have shown a partial response to clozapine after a minimum of 3 months. Non-clozapine APP should be considered when:
- a patient derives no benefit from clozapine
- refuses clozapine
- clozapine is contraindicated
- APP is initiated to mitigate side effects from another antipsychotic.
Antipsychotics could take up to 16 weeks to achieve full efficacy,6 therefore, an adequate trial period within the target dosage range is advised for all antipsychotics (Table 5).13,40
Why initiate
Based on available data, partial response to maximum recommended dosages of antipsychotic monotherapy, including clozapine, or inability to tolerate higher dosages, provides a reason for initiating APP. Non-clozapine APP generally should be considered only in patients who refuse, cannot tolerate, or do not respond to clozapine. Consider using validated rating scales to track treatment outcomes (ideally, a ≥20% symptomatic reduction on the BPRS or PANSS), although there is no formal guidance regarding their use or benefit in APP.
Summing up
APP is a fairly common prescribing practice, even though safety and efficacy data are mixed. The issue of APP has become prevalent enough that regulatory bodies are involved in its monitoring and documentation.41
Clozapine APP, especially with risperidone, has the most substantial evidence to support it. Although APP generally is well tolerated, the overall dearth of conclusive safety and efficacy data indicates that this practice should be reserved for patients who have not responded adequately to monotherapy, including clozapine. Adjunctive aripiprazole could be considered for addressing symptomatic hyperprolactinemia or other metabolic effects caused by other antipsychotics.
An adequate trial as long as 16 weeks is advised before assessing the efficacy of any antipsychotic regimen. If APP provides inadequate response, or if there is no clear indication for APP, consider switching the patient back to monotherapy.42-44
Mr. C, age 31, who has a 7-year history of schizophrenia and is currently on perphenazine, 24 mg twice a day, presents for psychiatric admission after experiencing paranoid delusions. Notable symptoms include delusions of reference and persecution, along with affective flattening and intermittent suicidal ideation. Perphenazine is tapered, and he is started on quetiapine, titrated to 600 mg/d.
Past antipsychotic trials include aripiprazole, olanzapine, paliperidone, haloperidol,
and ziprasidone. Because of his refractory symptoms and tolerability issues with other antipsychotics, Mr. C is switched to clozapine, 400 mg/d. His symptoms improve, but he experiences dose-limiting sialorrhea. Risperidone, 1 mg/d, is added to clozapine, which helps his psychosis and improves his functional status. Additionally, Mr. C develops enough insight to recognize his delusions and use skills learned in psychotherapy to cope with them.
Antipsychotic polypharmacy (APP), the concurrent use of ≥2 antipsychotics, is a topic of debate among mental health care providers. Studies indicate the prevalence of APP can reach upwards of 40%, with 1 systematic review citing more recent median APP prevalence in North America as 17%, an increase from a median of 12.7% in the 1980s.1 Other studies cite more recent figures as around 20%.2,3
The literature lists several reasons for use of long-term APP, including:
- incomplete cross-titration
- accidental continuation of APP that was intended to be temporary
- monotherapy failure
- mitigation or enhancement of effects of other antipsychotics (Table 1).1,4
Other factors include direct-to-consumer advertising, external pressures to decrease hospital stays, and low doctor-to-patient ratios.5 Although it can take as long as 16 weeks to see clinically significant improvement with an antipsychotic, prescribers might expect results after 4 weeks of treatment.6 Therefore, treatments could be labeled ineffective because trials did not last long enough, leading to premature use of polypharmacy. Combinations of a first- and second-generation antipsychotic (SGA) or 2 SGAs are most common.2,7,8
Treatment guidelines (Table 2)9-17 suggest APP could be considered after several failures of monotherapy, including clozapine monotherapy, although some guidelines do not address the issue or recommend against APP because of lack of efficacy and safety data. Additionally, APP poses safety concerns (Table 3).18-22 Recommendations for APP with combinations that do not include clozapine generally are not provided, because high-level evidence to support this strategy is lacking. Data on safety and efficacy of APP are mixed, with much of the literature dominated by case reports and uncontrolled studies.19
What to initiate
Clozapine. Higher-level evidence is available for clozapine APP. The combination of clozapine and risperidone is one of the most thoroughly studied and, therefore, is a reasonable first choice. Randomized controlled trials (RCTs) examining clozapine plus risperidone23-29 have yielded mixed results and have not provided conclusive information regarding benefit for positive vs negative symptoms.24-28
One RCT reported a significant change in Brief Psychiatric Rating Scale (BPRS) total and positive symptom scores.27 Other RCTs have shown a non-significant trend toward greater change in total, positive, and negative symptom scores with the clozapine-risperidone combination compared with clozapine monotherapy.25,28 In terms of cognition, this combination provided no additional benefit.23 Response, defined as ≥20% reduction in total BPRS or Positive and Negative Syndrome Scale (PANSS) scores, for clozapine plus risperidone range from 13% to 83%, compared with 8% to 29% for clozapine plus placebo.24,25,27,29
Data from 1 study27 suggest a number needed to treat of 4 to achieve at least a 20% improvement in BPRS scores with clozapine plus risperidone vs clozapine monotherapy. Across these studies, the average risperidone dosage was 4 mg/d, although using the lowest effective dosage is encouraged. A small number of RCTs and articles examining other APP combinations (Table 4)30-33 have yielded mixed results.
Overall, APP appears to be well-tolerated, although it is associated with an increased risk of adverse effects, including sedation, extrapyramidal symptoms, hyperprolactinemia, sexual dysfunction, cognitive impairment, anticholinergic effects, hyperlipidemia, and diabetes.23,24,34-36 Surprisingly, 1 literature review36 found no association between APP and increased risk of orthostasis. Increased occurrence of sedation, hyperprolactinemia, and an elevated fasting blood glucose level have been found for clozapine plus risperidone compared with clozapine monotherapy.24-26,28
Aripiprazole. Adjunctive aripiprazole, a dopamine partial agonist, could reduce elevated prolactin levels caused by other antipsychotics.32 In a study37 of 56 patients taking haloperidol who had hyperprolactinemia, prolactin levels normalized in 88.5% of patients taking adjunctive aripiprazole, 30 mg/d, compared with 3.6% of those with added placebo. Furthermore, results from 2 RCTs38,39 of patients taking clozapine or olanzapine suggest adjunctive aripiprazole could improve weight and metabolic profile. Therefore, adding aripiprazole to existing antipsychotic regimens is reasonable for patients with drug-induced symptomatic hyperprolactinemia or metabolic effects and who cannot be easily switched to another antipsychotic.
When to initiate
Most treatment guidelines9-17 recommend clozapine only after monotherapy with at least 2 other antipsychotics fails. It is reasonable to add an antipsychotic to clozapine in patients who have shown a partial response to clozapine after a minimum of 3 months. Non-clozapine APP should be considered when:
- a patient derives no benefit from clozapine
- refuses clozapine
- clozapine is contraindicated
- APP is initiated to mitigate side effects from another antipsychotic.
Antipsychotics could take up to 16 weeks to achieve full efficacy,6 therefore, an adequate trial period within the target dosage range is advised for all antipsychotics (Table 5).13,40
Why initiate
Based on available data, partial response to maximum recommended dosages of antipsychotic monotherapy, including clozapine, or inability to tolerate higher dosages, provides a reason for initiating APP. Non-clozapine APP generally should be considered only in patients who refuse, cannot tolerate, or do not respond to clozapine. Consider using validated rating scales to track treatment outcomes (ideally, a ≥20% symptomatic reduction on the BPRS or PANSS), although there is no formal guidance regarding their use or benefit in APP.
Summing up
APP is a fairly common prescribing practice, even though safety and efficacy data are mixed. The issue of APP has become prevalent enough that regulatory bodies are involved in its monitoring and documentation.41
Clozapine APP, especially with risperidone, has the most substantial evidence to support it. Although APP generally is well tolerated, the overall dearth of conclusive safety and efficacy data indicates that this practice should be reserved for patients who have not responded adequately to monotherapy, including clozapine. Adjunctive aripiprazole could be considered for addressing symptomatic hyperprolactinemia or other metabolic effects caused by other antipsychotics.
An adequate trial as long as 16 weeks is advised before assessing the efficacy of any antipsychotic regimen. If APP provides inadequate response, or if there is no clear indication for APP, consider switching the patient back to monotherapy.42-44
1. Gallego JA, Bonetti J, Zhang J, et al. Prevalence and correlates of antipsychotic polypharmacy: a systematic review and meta-regression of global and regional trends from the 1970s to 2009. Schizophr Res. 2012;138(1):18-28.
2. Gören JL, Meterko M, Williams S, et al. Antipsychotic prescribing pathways, polypharmacy, and clozapine use in treatment of schizophrenia. Psychiatr Serv. 2013;64(6):527-533.
3. Sun F, Stock EM, Copeland LA, et al. Polypharmacy with antipsychotic drugs in patients with schizophrenia: trends in multiple health care systems. Am J Health Syst Pharm. 2014;71(9):728-738.
4. Tapp A, Wood AE, Secrest L, et al. Combination antipsychotic therapy in clinical practice. Psychiatr Serv. 2003;54(1):55-59.
5. Ananth J, Parameswaran S, Gunatilake S. Antipsychotic polypharmacy. Curr Pharm Des. 2004;10(18):2231-2238.
6. Stahl SM. Antipsychotic polypharmacy: evidence based or eminence based? Acta Psychiatr Scand. 2002;106(5):321-322.
7. Botts S, Hines H, Littrell R. Antipsychotic polypharmacy in the ambulatory care setting, 1993-2000. Psychiatr Serv. 2003;54(8):1086.
8. Santone G, Bellantuono C, Rucci P, et al. Patient characteristics and process factors associated with antipsychotic polypharmacy in a nationwide sample of psychiatric inpatients in Italy. Pharmacoepidemiol Drug Saf. 2011;20(5):441-449.
9. American Psychiatric Association. Practice guideline for the treatment of patients with schizophrenia, second edition. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/schizophrenia.pdf. Updated September 2009. Accessed September 20, 2014.
10. Barnes TRE; Schizophrenia Consensus Group of the British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. http://www.bap.org.uk/pdfs/Schizophrenia_Consensus_Guideline_Document.pdf. Updated 2011. Accessed September 20, 2014.
11. National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: prevention and management. http://www.nice.org.uk/guidance/cg178. Published February 2014. Accessed September 20, 2014.
12. Texas Medication Algorithm Project. Schizophrenia treatment algorithms. http://www.jpshealthnet.org/sites/default/files/tmapalgorithmforschizophrenia.pdf. Updated April 2008. Accessed September 20, 2014.
13. Hasan A, Falkai P, Wobrock T, et al; World Federation of Societies of Biological Psychiatry (WFSBP). World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry. 2012;13(5):318-378.
14. Canadian Psychiatric Association. Clinical practice guidelines: treatment of schizophrenia. https://ww1.cpa-apc.org/Publications/Clinical_Guidelines/schizophrenia/november2005/index.asp. Updated November 2005. Accessed February 26, 2016.
15. Royal Australian and New Zealand College of Psychiatrists. Clinical practice guidelines for the treatment of schizophrenia and related disorders. http://www.ranzcp.org/Files/ranzcp-attachments/Resources/Publications/CPG/Clinician/CPG_Clinician_Full_Schizophrenia-pdf.aspx. Updated May 2005. Accessed February 26, 2016.
16. Scottish Intercollegiate Guidelines Network. Management of schizophrenia: a national clinical guideline. http://www.sign.ac.uk/guidelines/fulltext/131/index.html. Updated March 2013. Accessed September 20, 2014.
17. Buchanan RW, Kreyenbuhl J, Kelly DL, et al; Schizophrenia Patient Outcomes Research Team (PORT). The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71-93.
18. Correll CU, Gallego JA. Antipsychotic polypharmacy: a comprehensive evaluation of relevant correlates of a long-standing clinical practice. Psychiatr Clin North Am. 2012;35(3):661-681.
19. Tranulis C, Skalli L, Lalonde P, et al. Benefits and risks of antipsychotic polypharmacy: an evidence-based review of the literature. Drug Saf. 2008;31(1):7-20.
20. Barnes TR, Paton C. Antipsychotic polypharmacy in schizophrenia: benefits and risks. CNS Drugs. 2011;25(5):383-399.
21. Lochmann van Bennekom MW, Gijsman HJ, Zitman FG. Antipsychotic polypharmacy in psychotic disorders: a critical review of neurobiology, efficacy, tolerability and cost effectiveness. J Psychopharmacol. 2013;27(4):327-336.
22. Weinmann S, Read J, Aderhold V. Influence of antipsychotics on mortality in schizophrenia: systematic review. Schizophr Res. 2009;113(1):1-11.
23. Akdede BB, Anil Ya˘gcio˘glu AE, Alptekin K, et al. A double-blind study of combination of clozapine with risperidone in patients with schizophrenia: effects on cognition. J Clin Psychiatry. 2006;67(12):1912-1919.
24. Anil Ya˘gcio˘glu AE, Kivircik Akdede BB, Turgut TI, et al. A double-blind controlled study of adjunctive treatment with risperidone in schizophrenic patients partially responsive to clozapine: efficacy and safety. J Clin Psychiatry. 2005;66(1):63-72.
25. Freudenreich O, Henderson DC, Walsh JP, et al. Risperidone augmentation for schizophrenia partially responsive to clozapine: a double-blind, placebo-controlled trial. Schizophr Res. 2007;92(1-3):90-94.
26. Honer WG, Thornton AE, Chen EY, et al; Clozapine and Risperidone Enhancement (CARE) Study Group. Clozapine alone versus clozapine and risperidone with refractory schizophrenia. N Engl J Med. 2006;354(5):472-482.
27. Josiassen RC, Joseph A, Kohegyi E, et al. Clozapine augmented with risperidone in the treatment of schizophrenia: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2005;162(1):130-136.
28. Weiner E, Conley RR, Ball MP, et al. Adjunctive risperidone for partially responsive people with schizophrenia treated with clozapine. Neuropsychopharmacology. 2010;35(11):2274-2283.
29. Zink M, Kuwilsky A, Krumm B, et al. Efficacy and tolerability of ziprasidone versus risperidone as augmentation in patients partially responsive to clozapine: a randomized controlled clinical trial. J Psychopharmacol. 2009;23(3):305-314.
30. Canadian Agency for Drugs and Technology in Health. Current utilization of antipsychotic agents for schizophrenia: combination and high-dose therapies. https://www.cadth.ca/sites/default/files/pdf/H0503_AAP-Current-Utilization-Report_e.pdf. Published August 2012. Accessed February 26, 2016.
31. Chang JS, Ahn YM, Park HJ, et al. Aripiprazole augmentation in clozapine treated patients with refractory schizophrenia: an 8-week, randomized, double blind, placebo-controlled trial. J Clin Psychiatry. 2008;69(5):720-731.
32. Kane JM, Correll CU, Goff DC, et al. A multicenter, randomized, double-blind, placebo-controlled, 16-week study of adjunctive aripiprazole for schizophrenia or schizoaffective disorder inadequately treated with quetiapine or risperidone monotherapy. J Clin Psychiatry. 2009;70(10):1348-1357.
33. Velligan DI, Carroll C, Lage MJ, et al. Outcomes of medicaid beneficiaries with schizophrenia receiving clozapine only or antipsychotic combinations. Psychiatr Serv. 2015;66(2):127-133.
34. Citrome L, Jaffe A, Levine J, et al. Relationship between antipsychotic medication treatment and new cases of diabetes among psychiatric inpatients. Psychiatr Serv. 2004;55(9):1006-1013.
35. Correll CU, Frederickson AM, Kane JM, et al. Does antipsychotic polypharmacy increase the risk for metabolic syndrome? Schizophr Res. 2007;89(1-3):91-100.
36. Gallego JA, Nielsen J, De Hert M, et al. Safety and tolerability of antipsychotic polypharmacy. Expert Opin Drug Saf. 2012;11(4):527-542.
37. Shim JC, Shin JG, Kelly DL, et al. Adjunctive treatment with a dopamine partial agonist, aripiprazole, for antipsychotic-induced hyperprolactinemia: a placebo-controlled trial. Am J Psychiatry. 2007;164(9):1404-1410.
38. Fan X, Borba CP, Copeland P, et al. Metabolic effects of adjunctive aripiprazole in clozapine-treated patients with schizophrenia. Acta Psychiatr Scand. 2013;127(3):217-226.
39. Henderson DC, Fan X, Copeland PM, et al. Aripiprazole added to overweight and obese olanzapine-treated schizophrenia patients. J Clin Psychopharmacol. 2009;26(2):165-169.
40. Drug Information Handbook, 22th ed. Hudson, OH: Lexi-Comp, Inc.; 2013:1143-1147.
41. The Joint Commission. Specifications Manual for Joint Commission National Quality Measures (v2013A1). https://manual.jointcommission.org/releases/TJC2013A/. Accessed on May 13, 2015.
42. Essock SM, Schooler NR, Stroup TS, et al; Schizophrenia Trials Network. Effectiveness of switching from antipsychotic polypharmacy to monotherapy. Am J Psychiatry. 2011;168(7):702-708.
43. Godleski LS, Kerler R, Barber JW, et al. Multiple versus single antipsychotic drug treatment in chronic psychosis. J Nerv Ment Dis. 1989;177(11):686-689.
44. Suzuki T, Uchida H, Tanaka KF, et al. Revising polypharmacy to a single antipsychotic regimen for patients with chronic schizophrenia. Int J Neuropsychopharmacol. 2004;7(2):133-142.
1. Gallego JA, Bonetti J, Zhang J, et al. Prevalence and correlates of antipsychotic polypharmacy: a systematic review and meta-regression of global and regional trends from the 1970s to 2009. Schizophr Res. 2012;138(1):18-28.
2. Gören JL, Meterko M, Williams S, et al. Antipsychotic prescribing pathways, polypharmacy, and clozapine use in treatment of schizophrenia. Psychiatr Serv. 2013;64(6):527-533.
3. Sun F, Stock EM, Copeland LA, et al. Polypharmacy with antipsychotic drugs in patients with schizophrenia: trends in multiple health care systems. Am J Health Syst Pharm. 2014;71(9):728-738.
4. Tapp A, Wood AE, Secrest L, et al. Combination antipsychotic therapy in clinical practice. Psychiatr Serv. 2003;54(1):55-59.
5. Ananth J, Parameswaran S, Gunatilake S. Antipsychotic polypharmacy. Curr Pharm Des. 2004;10(18):2231-2238.
6. Stahl SM. Antipsychotic polypharmacy: evidence based or eminence based? Acta Psychiatr Scand. 2002;106(5):321-322.
7. Botts S, Hines H, Littrell R. Antipsychotic polypharmacy in the ambulatory care setting, 1993-2000. Psychiatr Serv. 2003;54(8):1086.
8. Santone G, Bellantuono C, Rucci P, et al. Patient characteristics and process factors associated with antipsychotic polypharmacy in a nationwide sample of psychiatric inpatients in Italy. Pharmacoepidemiol Drug Saf. 2011;20(5):441-449.
9. American Psychiatric Association. Practice guideline for the treatment of patients with schizophrenia, second edition. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/schizophrenia.pdf. Updated September 2009. Accessed September 20, 2014.
10. Barnes TRE; Schizophrenia Consensus Group of the British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. http://www.bap.org.uk/pdfs/Schizophrenia_Consensus_Guideline_Document.pdf. Updated 2011. Accessed September 20, 2014.
11. National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: prevention and management. http://www.nice.org.uk/guidance/cg178. Published February 2014. Accessed September 20, 2014.
12. Texas Medication Algorithm Project. Schizophrenia treatment algorithms. http://www.jpshealthnet.org/sites/default/files/tmapalgorithmforschizophrenia.pdf. Updated April 2008. Accessed September 20, 2014.
13. Hasan A, Falkai P, Wobrock T, et al; World Federation of Societies of Biological Psychiatry (WFSBP). World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry. 2012;13(5):318-378.
14. Canadian Psychiatric Association. Clinical practice guidelines: treatment of schizophrenia. https://ww1.cpa-apc.org/Publications/Clinical_Guidelines/schizophrenia/november2005/index.asp. Updated November 2005. Accessed February 26, 2016.
15. Royal Australian and New Zealand College of Psychiatrists. Clinical practice guidelines for the treatment of schizophrenia and related disorders. http://www.ranzcp.org/Files/ranzcp-attachments/Resources/Publications/CPG/Clinician/CPG_Clinician_Full_Schizophrenia-pdf.aspx. Updated May 2005. Accessed February 26, 2016.
16. Scottish Intercollegiate Guidelines Network. Management of schizophrenia: a national clinical guideline. http://www.sign.ac.uk/guidelines/fulltext/131/index.html. Updated March 2013. Accessed September 20, 2014.
17. Buchanan RW, Kreyenbuhl J, Kelly DL, et al; Schizophrenia Patient Outcomes Research Team (PORT). The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71-93.
18. Correll CU, Gallego JA. Antipsychotic polypharmacy: a comprehensive evaluation of relevant correlates of a long-standing clinical practice. Psychiatr Clin North Am. 2012;35(3):661-681.
19. Tranulis C, Skalli L, Lalonde P, et al. Benefits and risks of antipsychotic polypharmacy: an evidence-based review of the literature. Drug Saf. 2008;31(1):7-20.
20. Barnes TR, Paton C. Antipsychotic polypharmacy in schizophrenia: benefits and risks. CNS Drugs. 2011;25(5):383-399.
21. Lochmann van Bennekom MW, Gijsman HJ, Zitman FG. Antipsychotic polypharmacy in psychotic disorders: a critical review of neurobiology, efficacy, tolerability and cost effectiveness. J Psychopharmacol. 2013;27(4):327-336.
22. Weinmann S, Read J, Aderhold V. Influence of antipsychotics on mortality in schizophrenia: systematic review. Schizophr Res. 2009;113(1):1-11.
23. Akdede BB, Anil Ya˘gcio˘glu AE, Alptekin K, et al. A double-blind study of combination of clozapine with risperidone in patients with schizophrenia: effects on cognition. J Clin Psychiatry. 2006;67(12):1912-1919.
24. Anil Ya˘gcio˘glu AE, Kivircik Akdede BB, Turgut TI, et al. A double-blind controlled study of adjunctive treatment with risperidone in schizophrenic patients partially responsive to clozapine: efficacy and safety. J Clin Psychiatry. 2005;66(1):63-72.
25. Freudenreich O, Henderson DC, Walsh JP, et al. Risperidone augmentation for schizophrenia partially responsive to clozapine: a double-blind, placebo-controlled trial. Schizophr Res. 2007;92(1-3):90-94.
26. Honer WG, Thornton AE, Chen EY, et al; Clozapine and Risperidone Enhancement (CARE) Study Group. Clozapine alone versus clozapine and risperidone with refractory schizophrenia. N Engl J Med. 2006;354(5):472-482.
27. Josiassen RC, Joseph A, Kohegyi E, et al. Clozapine augmented with risperidone in the treatment of schizophrenia: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2005;162(1):130-136.
28. Weiner E, Conley RR, Ball MP, et al. Adjunctive risperidone for partially responsive people with schizophrenia treated with clozapine. Neuropsychopharmacology. 2010;35(11):2274-2283.
29. Zink M, Kuwilsky A, Krumm B, et al. Efficacy and tolerability of ziprasidone versus risperidone as augmentation in patients partially responsive to clozapine: a randomized controlled clinical trial. J Psychopharmacol. 2009;23(3):305-314.
30. Canadian Agency for Drugs and Technology in Health. Current utilization of antipsychotic agents for schizophrenia: combination and high-dose therapies. https://www.cadth.ca/sites/default/files/pdf/H0503_AAP-Current-Utilization-Report_e.pdf. Published August 2012. Accessed February 26, 2016.
31. Chang JS, Ahn YM, Park HJ, et al. Aripiprazole augmentation in clozapine treated patients with refractory schizophrenia: an 8-week, randomized, double blind, placebo-controlled trial. J Clin Psychiatry. 2008;69(5):720-731.
32. Kane JM, Correll CU, Goff DC, et al. A multicenter, randomized, double-blind, placebo-controlled, 16-week study of adjunctive aripiprazole for schizophrenia or schizoaffective disorder inadequately treated with quetiapine or risperidone monotherapy. J Clin Psychiatry. 2009;70(10):1348-1357.
33. Velligan DI, Carroll C, Lage MJ, et al. Outcomes of medicaid beneficiaries with schizophrenia receiving clozapine only or antipsychotic combinations. Psychiatr Serv. 2015;66(2):127-133.
34. Citrome L, Jaffe A, Levine J, et al. Relationship between antipsychotic medication treatment and new cases of diabetes among psychiatric inpatients. Psychiatr Serv. 2004;55(9):1006-1013.
35. Correll CU, Frederickson AM, Kane JM, et al. Does antipsychotic polypharmacy increase the risk for metabolic syndrome? Schizophr Res. 2007;89(1-3):91-100.
36. Gallego JA, Nielsen J, De Hert M, et al. Safety and tolerability of antipsychotic polypharmacy. Expert Opin Drug Saf. 2012;11(4):527-542.
37. Shim JC, Shin JG, Kelly DL, et al. Adjunctive treatment with a dopamine partial agonist, aripiprazole, for antipsychotic-induced hyperprolactinemia: a placebo-controlled trial. Am J Psychiatry. 2007;164(9):1404-1410.
38. Fan X, Borba CP, Copeland P, et al. Metabolic effects of adjunctive aripiprazole in clozapine-treated patients with schizophrenia. Acta Psychiatr Scand. 2013;127(3):217-226.
39. Henderson DC, Fan X, Copeland PM, et al. Aripiprazole added to overweight and obese olanzapine-treated schizophrenia patients. J Clin Psychopharmacol. 2009;26(2):165-169.
40. Drug Information Handbook, 22th ed. Hudson, OH: Lexi-Comp, Inc.; 2013:1143-1147.
41. The Joint Commission. Specifications Manual for Joint Commission National Quality Measures (v2013A1). https://manual.jointcommission.org/releases/TJC2013A/. Accessed on May 13, 2015.
42. Essock SM, Schooler NR, Stroup TS, et al; Schizophrenia Trials Network. Effectiveness of switching from antipsychotic polypharmacy to monotherapy. Am J Psychiatry. 2011;168(7):702-708.
43. Godleski LS, Kerler R, Barber JW, et al. Multiple versus single antipsychotic drug treatment in chronic psychosis. J Nerv Ment Dis. 1989;177(11):686-689.
44. Suzuki T, Uchida H, Tanaka KF, et al. Revising polypharmacy to a single antipsychotic regimen for patients with chronic schizophrenia. Int J Neuropsychopharmacol. 2004;7(2):133-142.