User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Evaluation of daily persistent headache
In the drafty call room, a miracle unfolds
I’ve found that, as a resident in psychiatry, it’s rare to experience a moment of truly unbridled achievement while on call. Manning the revolving door of acute psychiatric admissions can be frustrating, not to mention unfulfilling. Maybe that’s why accomplishing a small miracle, you might say, while on call recently felt so satisfying.
Broken window = workplace woes
When working a 12-hour shift, especially overnight, it’s important to have an environment that is conducive to work. As fatigue and stress build, physical comfort means a lot.
Our problem finding physical comfort in the psychiatry resident call room at Saint Louis University was that a fixture on one of the windows had been broken for several years. You could push the window open, but you could not close it. If you called the janitor, he would come and close the window, but there was no guarantee when he’d show up. You might end up typing your notes all evening in the path of a chilly stream of air.
The residents had made a formal request to have the window repaired in a more permanent manner, but this resulted in it being bolted shut. That was a solution, but an imperfect one: Now we had no way to cool the call room in the winter, and it was beginning to smell of body odor.
The psychiatry resident call room is one of the nicer ones I’ve seen, but the building it occupies is a few decades old, and no replacement parts were available for the fixtures. We were stuck with a closed window—so I thought.
That miraculous morning
I was supervising an intern one Saturday, and she had not been paged yet to see patients. The call room was a mess; I telephoned housekeeping to have the beds changed, and maintenance to unclog the sink. When the maintenance man (I’ll call him “Tom”) arrived and fixed the sink, I praised him and asked him to take a look at the window.
“It’s my dream,” I said to no one in particular, “to have a window we can open and shut.”
I didn’t get angry or exert pressure. Tom explained to me that there were no replacement parts.
“Hmm… I see…,” I said.
To my delight, Tom seemed excited to be given a problem to solve. He left to pilfer parts from other windows on the floor.
No luck. The parts were all gone. Tom apologized and suggested we purchase a suction cup, with a cord attached, to pull the window closed.
“Good idea!” I said, thanking him as he went on his way.
But 2 hours later, our maintenance hero, Tom reappeared in the doorway.
“I’ve been thinking about your window all morning,” he announced.
Tom approached the window, unbolted it, and screwed one end of a chain into the frame, creating a makeshift handle. He demonstrated how to pull the window shut.
Voilà! A window we could open and close. The intern’s jaw dropped in amazement. I turned to dance a little jig.
Satisfaction
It’s important to be able to control the temperature in the call room; even more important to have a comfortable, healthy work environment. But knowing I can influence my surroundings to get what I need at work? That’s more important than anything else at all.
Disclosure
Dr. Jennings reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
I’ve found that, as a resident in psychiatry, it’s rare to experience a moment of truly unbridled achievement while on call. Manning the revolving door of acute psychiatric admissions can be frustrating, not to mention unfulfilling. Maybe that’s why accomplishing a small miracle, you might say, while on call recently felt so satisfying.
Broken window = workplace woes
When working a 12-hour shift, especially overnight, it’s important to have an environment that is conducive to work. As fatigue and stress build, physical comfort means a lot.
Our problem finding physical comfort in the psychiatry resident call room at Saint Louis University was that a fixture on one of the windows had been broken for several years. You could push the window open, but you could not close it. If you called the janitor, he would come and close the window, but there was no guarantee when he’d show up. You might end up typing your notes all evening in the path of a chilly stream of air.
The residents had made a formal request to have the window repaired in a more permanent manner, but this resulted in it being bolted shut. That was a solution, but an imperfect one: Now we had no way to cool the call room in the winter, and it was beginning to smell of body odor.
The psychiatry resident call room is one of the nicer ones I’ve seen, but the building it occupies is a few decades old, and no replacement parts were available for the fixtures. We were stuck with a closed window—so I thought.
That miraculous morning
I was supervising an intern one Saturday, and she had not been paged yet to see patients. The call room was a mess; I telephoned housekeeping to have the beds changed, and maintenance to unclog the sink. When the maintenance man (I’ll call him “Tom”) arrived and fixed the sink, I praised him and asked him to take a look at the window.
“It’s my dream,” I said to no one in particular, “to have a window we can open and shut.”
I didn’t get angry or exert pressure. Tom explained to me that there were no replacement parts.
“Hmm… I see…,” I said.
To my delight, Tom seemed excited to be given a problem to solve. He left to pilfer parts from other windows on the floor.
No luck. The parts were all gone. Tom apologized and suggested we purchase a suction cup, with a cord attached, to pull the window closed.
“Good idea!” I said, thanking him as he went on his way.
But 2 hours later, our maintenance hero, Tom reappeared in the doorway.
“I’ve been thinking about your window all morning,” he announced.
Tom approached the window, unbolted it, and screwed one end of a chain into the frame, creating a makeshift handle. He demonstrated how to pull the window shut.
Voilà! A window we could open and close. The intern’s jaw dropped in amazement. I turned to dance a little jig.
Satisfaction
It’s important to be able to control the temperature in the call room; even more important to have a comfortable, healthy work environment. But knowing I can influence my surroundings to get what I need at work? That’s more important than anything else at all.
Disclosure
Dr. Jennings reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
I’ve found that, as a resident in psychiatry, it’s rare to experience a moment of truly unbridled achievement while on call. Manning the revolving door of acute psychiatric admissions can be frustrating, not to mention unfulfilling. Maybe that’s why accomplishing a small miracle, you might say, while on call recently felt so satisfying.
Broken window = workplace woes
When working a 12-hour shift, especially overnight, it’s important to have an environment that is conducive to work. As fatigue and stress build, physical comfort means a lot.
Our problem finding physical comfort in the psychiatry resident call room at Saint Louis University was that a fixture on one of the windows had been broken for several years. You could push the window open, but you could not close it. If you called the janitor, he would come and close the window, but there was no guarantee when he’d show up. You might end up typing your notes all evening in the path of a chilly stream of air.
The residents had made a formal request to have the window repaired in a more permanent manner, but this resulted in it being bolted shut. That was a solution, but an imperfect one: Now we had no way to cool the call room in the winter, and it was beginning to smell of body odor.
The psychiatry resident call room is one of the nicer ones I’ve seen, but the building it occupies is a few decades old, and no replacement parts were available for the fixtures. We were stuck with a closed window—so I thought.
That miraculous morning
I was supervising an intern one Saturday, and she had not been paged yet to see patients. The call room was a mess; I telephoned housekeeping to have the beds changed, and maintenance to unclog the sink. When the maintenance man (I’ll call him “Tom”) arrived and fixed the sink, I praised him and asked him to take a look at the window.
“It’s my dream,” I said to no one in particular, “to have a window we can open and shut.”
I didn’t get angry or exert pressure. Tom explained to me that there were no replacement parts.
“Hmm… I see…,” I said.
To my delight, Tom seemed excited to be given a problem to solve. He left to pilfer parts from other windows on the floor.
No luck. The parts were all gone. Tom apologized and suggested we purchase a suction cup, with a cord attached, to pull the window closed.
“Good idea!” I said, thanking him as he went on his way.
But 2 hours later, our maintenance hero, Tom reappeared in the doorway.
“I’ve been thinking about your window all morning,” he announced.
Tom approached the window, unbolted it, and screwed one end of a chain into the frame, creating a makeshift handle. He demonstrated how to pull the window shut.
Voilà! A window we could open and close. The intern’s jaw dropped in amazement. I turned to dance a little jig.
Satisfaction
It’s important to be able to control the temperature in the call room; even more important to have a comfortable, healthy work environment. But knowing I can influence my surroundings to get what I need at work? That’s more important than anything else at all.
Disclosure
Dr. Jennings reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Be prepared to adjust dosing of psychotropics after bariatric surgery
Approximately 113,000 bariatric surgeries were performed in the United States in 2010; as many as 80% of persons seeking weight loss surgery have a history of a psychiatric disorder.1,2
Bariatric surgery can be “restrictive” (limiting food intake) or “malabsorptive” (limiting food absorption). Both types of procedures can cause significant changes in pharmacokinetics. Bariatric surgery patients who take a psychotropic are at risk of toxicity or relapse of their psychiatric illness because of inappropriate formulations— immediate-release vs sustained-release—or incomplete absorption of medications. You need to anticipate potential pharmacokinetic alterations after bariatric surgery and make appropriate changes to the patient’s medication regimen.
Pharmacokinetic concerns
Roux-en-Y surgery is a malabsorptive procedure that causes food to bypass the stomach, duodenum, and a variable length of jejunum. Secondary to bypass, iron deficiency anemia is a common nutritional complication.
Other changes that affect the pharmacokinetics of psychotropics after bariatric surgery include:
• an increase in percentage of lean body mass as weight loss occurs
• a decrease in glomerular filtration rate as kidney size decreases with postsurgical weight reduction
• reversal of obesity-associated fatty liver and cirrhotic changes.
With time, intestinal adaptation occurs to compensate for the reduced length of the intestinal tract; this adaptation produces mucosal hypertrophy and increases absorptive capacity.3
Medications to taper or avoid
The absorption and bioavailability of a medication depend on its dissolvability; the pH of the medium; surface area for absorption; and GI blood flow.4 Medications that have a long absorptive phase—namely, sustained-release, extended-release, long-acting, and enteric-coated formulations—show compromised dissolvability and absorption and reduced efficacy after bariatric surgery.
Avoid slow-release formulations, including ion-exchange resins with a semipermeable membrane and those with slowly dissolving characteristics; substitute an immediate-release formulation.
Medications that require acidic pH are incompletely absorbed because gastric exposure is reduced.
Lipophilic medications depend on bile availability; impaired enterohepatic circulation because of reduced intestinal absorptive surface causes loss of bile and, therefore, impaired absorption of lipophilic medications.
Medications that are poorly intrinsically absorbed and undergo enterohepatic circulation are likely to be underabsorbed after a malabsorptive bariatric procedure.
Lamotrigine, olanzapine, and quetiapine may show decreased efficacy because of possible reduced absorption.
The lithium level, which is influenced by volume of distribution, can become toxic postoperatively; consider measuring the serum lithium level.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Livingston EH. The incidence of bariatric surgery has plateaued in the U.S. Am J Surg. 2010;200(3):378-385.
2. Jones WR, Morgan JF. Obesity surgery. Psychiatric needs must be considered. BMJ. 2010;341:c5298. doi: 10.1136/bmj.c5298.
3. Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11(1):41-50.
4. Lizer MH, Papageorgeon H, Glembot TM. Nutritional and pharmacologic challenges in the bariatric surgery patient. Obes Surg. 2010;20(12):1654-1659.
Approximately 113,000 bariatric surgeries were performed in the United States in 2010; as many as 80% of persons seeking weight loss surgery have a history of a psychiatric disorder.1,2
Bariatric surgery can be “restrictive” (limiting food intake) or “malabsorptive” (limiting food absorption). Both types of procedures can cause significant changes in pharmacokinetics. Bariatric surgery patients who take a psychotropic are at risk of toxicity or relapse of their psychiatric illness because of inappropriate formulations— immediate-release vs sustained-release—or incomplete absorption of medications. You need to anticipate potential pharmacokinetic alterations after bariatric surgery and make appropriate changes to the patient’s medication regimen.
Pharmacokinetic concerns
Roux-en-Y surgery is a malabsorptive procedure that causes food to bypass the stomach, duodenum, and a variable length of jejunum. Secondary to bypass, iron deficiency anemia is a common nutritional complication.
Other changes that affect the pharmacokinetics of psychotropics after bariatric surgery include:
• an increase in percentage of lean body mass as weight loss occurs
• a decrease in glomerular filtration rate as kidney size decreases with postsurgical weight reduction
• reversal of obesity-associated fatty liver and cirrhotic changes.
With time, intestinal adaptation occurs to compensate for the reduced length of the intestinal tract; this adaptation produces mucosal hypertrophy and increases absorptive capacity.3
Medications to taper or avoid
The absorption and bioavailability of a medication depend on its dissolvability; the pH of the medium; surface area for absorption; and GI blood flow.4 Medications that have a long absorptive phase—namely, sustained-release, extended-release, long-acting, and enteric-coated formulations—show compromised dissolvability and absorption and reduced efficacy after bariatric surgery.
Avoid slow-release formulations, including ion-exchange resins with a semipermeable membrane and those with slowly dissolving characteristics; substitute an immediate-release formulation.
Medications that require acidic pH are incompletely absorbed because gastric exposure is reduced.
Lipophilic medications depend on bile availability; impaired enterohepatic circulation because of reduced intestinal absorptive surface causes loss of bile and, therefore, impaired absorption of lipophilic medications.
Medications that are poorly intrinsically absorbed and undergo enterohepatic circulation are likely to be underabsorbed after a malabsorptive bariatric procedure.
Lamotrigine, olanzapine, and quetiapine may show decreased efficacy because of possible reduced absorption.
The lithium level, which is influenced by volume of distribution, can become toxic postoperatively; consider measuring the serum lithium level.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Approximately 113,000 bariatric surgeries were performed in the United States in 2010; as many as 80% of persons seeking weight loss surgery have a history of a psychiatric disorder.1,2
Bariatric surgery can be “restrictive” (limiting food intake) or “malabsorptive” (limiting food absorption). Both types of procedures can cause significant changes in pharmacokinetics. Bariatric surgery patients who take a psychotropic are at risk of toxicity or relapse of their psychiatric illness because of inappropriate formulations— immediate-release vs sustained-release—or incomplete absorption of medications. You need to anticipate potential pharmacokinetic alterations after bariatric surgery and make appropriate changes to the patient’s medication regimen.
Pharmacokinetic concerns
Roux-en-Y surgery is a malabsorptive procedure that causes food to bypass the stomach, duodenum, and a variable length of jejunum. Secondary to bypass, iron deficiency anemia is a common nutritional complication.
Other changes that affect the pharmacokinetics of psychotropics after bariatric surgery include:
• an increase in percentage of lean body mass as weight loss occurs
• a decrease in glomerular filtration rate as kidney size decreases with postsurgical weight reduction
• reversal of obesity-associated fatty liver and cirrhotic changes.
With time, intestinal adaptation occurs to compensate for the reduced length of the intestinal tract; this adaptation produces mucosal hypertrophy and increases absorptive capacity.3
Medications to taper or avoid
The absorption and bioavailability of a medication depend on its dissolvability; the pH of the medium; surface area for absorption; and GI blood flow.4 Medications that have a long absorptive phase—namely, sustained-release, extended-release, long-acting, and enteric-coated formulations—show compromised dissolvability and absorption and reduced efficacy after bariatric surgery.
Avoid slow-release formulations, including ion-exchange resins with a semipermeable membrane and those with slowly dissolving characteristics; substitute an immediate-release formulation.
Medications that require acidic pH are incompletely absorbed because gastric exposure is reduced.
Lipophilic medications depend on bile availability; impaired enterohepatic circulation because of reduced intestinal absorptive surface causes loss of bile and, therefore, impaired absorption of lipophilic medications.
Medications that are poorly intrinsically absorbed and undergo enterohepatic circulation are likely to be underabsorbed after a malabsorptive bariatric procedure.
Lamotrigine, olanzapine, and quetiapine may show decreased efficacy because of possible reduced absorption.
The lithium level, which is influenced by volume of distribution, can become toxic postoperatively; consider measuring the serum lithium level.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Livingston EH. The incidence of bariatric surgery has plateaued in the U.S. Am J Surg. 2010;200(3):378-385.
2. Jones WR, Morgan JF. Obesity surgery. Psychiatric needs must be considered. BMJ. 2010;341:c5298. doi: 10.1136/bmj.c5298.
3. Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11(1):41-50.
4. Lizer MH, Papageorgeon H, Glembot TM. Nutritional and pharmacologic challenges in the bariatric surgery patient. Obes Surg. 2010;20(12):1654-1659.
1. Livingston EH. The incidence of bariatric surgery has plateaued in the U.S. Am J Surg. 2010;200(3):378-385.
2. Jones WR, Morgan JF. Obesity surgery. Psychiatric needs must be considered. BMJ. 2010;341:c5298. doi: 10.1136/bmj.c5298.
3. Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11(1):41-50.
4. Lizer MH, Papageorgeon H, Glembot TM. Nutritional and pharmacologic challenges in the bariatric surgery patient. Obes Surg. 2010;20(12):1654-1659.
Depressed, suicidal, and brittle in her bones
CASE Broken down
Ms. E, age 20, is a college student who has had major depressive disorder for several years and a genetic bone disease (osteogenesis imperfecta, mixed type III and IV). She presents with depression, anxiety, and suicidal ideation. She reports recent worsening of her depressive symptoms, including anhedonia, excessive sleep, difficulty concentrating, and feeling overwhelmed, hopeless, and worthless. She also describes frequent thoughts of suicide with the plan of putting herself in oncoming traffic, although she has no history of suicide attempts.
Previously, her primary care physician prescribed lorazepam, 0.5 mg, as needed for anxiety, and sertraline, 100 mg/d, for depression and anxiety. She experienced only partial improvement in symptoms, however.
In addition to depressive symptoms, Ms. E describes manic symptoms lasting for as long as 3 to 5 days, including decreased need for sleep, increased energy, pressured speech, racing thoughts, distractibility, spending excessive money on cosmetics, and risking her safety—given her skeletal disorder— by participating in high-impact stage-combat classes. She denies auditory and visual hallucinations, homicidal ideation, and delusions.
The medical history is significant for osteogenesis imperfecta, which has caused 62 fractures and required 16 surgeries. Ms. E is a theater major who, despite her short stature and wheelchair use, reports enjoying her acting career and says she does not feel demoralized by her medical condition. She describes overcoming her physical disabilities with pride and confidence. However, her recent worsening mood symptoms have left her unable to concentrate and feeling overwhelmed with school.
Ms. E is voluntarily admitted to an inpatient psychiatric unit with a diagnosis of bipolar I disorder with rapid cycling, most recent episode mixed. Because of her bone fragility, the treatment team considers what would be an appropriate course of drug treatment to control bipolar symptoms while minimizing risk of bone loss.
Which medications are associated with decreased bone mineral density?
a) citalopram
b) haloperidol
c) carbamazepine
d) paliperidone
e) all of the above
The authors’ observations
Osteogenesis imperfecta is a genetic condition caused by mutations in genes implicated in collagen production. As a result, bones are brittle and prone to fracture. Different classes of psychotropics have been shown to increase risk of bone fractures through a variety of mechanisms. Clinicians often must choose appropriate pharmacotherapy for patients at high risk of fracture, including postmenopausal women, older patients, malnourished persons, and those with hormonal deficiencies leading to osteoporosis.
To assist our clinical decision-making, we reviewed the literature to establish appropriate management of a patient with increased bone fragility and new-onset bipolar disorder. We considered all classes of medications used to treat bipolar disorder, including antipsychotics, antidepressants, lithium, and anticonvulsants.
Antipsychotics
In population-based studies, prolactin-elevating antipsychotics have been associated with decreased bone mineral density and increased risk of fracture.1 Additional studies on geriatric and non-geriatric populations have supported these findings.2,3
The mechanism through which fracture risk is increased likely is related to antipsychotics’ effect on serum prolactin and cortisol levels. Antipsychotics act as antagonists on D2 receptors in the hypothalamic tubero-infundibular pathway, therefore preventing inhibition of prolactin. Long-term elevation in serum prolactin can cause loss of bone mineral density through secondary hypogonadism and direct effects on target tissues. Additional modifying factors include smoking and estrogen use.
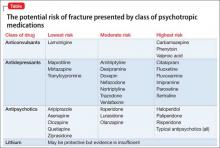
The degree to which antipsychotics increase fracture risk might be related to the degree of serum prolactin elevation.4 Antipsychotics previously have been grouped by the degree of prolactin elevation, categorizing them as high, medium, and low or no potential to elevate serum prolactin.4 Based on this classification, typical antipsychotics, risperidone, and paliperidone have the highest potential to elevate prolactin. Accordingly, antipsychotics with the lowest fracture risk are those that have the lowest risk of serum prolactin elevation: ziprasidone, asenapine, quetiapine, and clozapine. Aripiprazole may lower prolactin in some patients. This is supported by studies noting reduced bone mineral density5,6 and increased risk of fracture1 with high-potential vs low- or no-potential antipsychotics. Because of these findings, it is crucial to consider the potential risk of prolactin elevation when treating patients at increased risk of fracture. Providers should consider low/no potential antipsychotic medications before considering those with medium or high potential (Table).
Antidepressants
In a meta-analysis, antidepressants were shown to increase fracture risk by 70% to 90%.2 However, the relative risk varies by antidepressant class. Several studies have shown that selective serotonin reuptake inhibitors (SSRIs) are associated with a higher risk of fracture compared with tricyclic antidepressants (TCAs).7 In addition, antidepressants with a high affinity for the serotonin transporter, including citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and imipramine, have been associated with greater risk of osteoporotic fracture compared with those with low affinity.8
The mechanisms by which antidepressants increase fracture risk are complex, although the strongest evidence implicates a direct effect on bone metabolism via the 5-HTT receptor. This receptor, found on osteoblasts and osteoclasts, plays an important role in bone metabolism; it is through this receptor that SSRIs might inhibit osteoblasts and promote osteoclast activity, thereby disrupting bone microarchitecture. Additional studies are needed to further describe the mechanism of the association among antidepressants, bone mineral density, and fracture risk.
Fracture risk is associated with duration of use rather than dosage. Population-based studies show a higher fracture risk for new users of TCAs compared with continuous users, and the risk of fracture with SSRIs seems to increase slightly over time.9 No association has been identified between fracture risk and antidepressant dosage. According to the literature, drugs with low affinity for the serotonin transporter, such as maprotiline and mirtazapine, likely are the safest antidepressants for patients at increased risk of fracture. Options also include other TCAs and any antidepressant with low affinity for the serotonin receptor.7,8
Lithium
Studies on lithium and bone mineral density have shown mixed results. Older studies found that lithium had a negative or no effect on bone mineral density or the parathyroid hormone level.10 More recent investigations, however, suggest that the drug has a protective effect on bone mineral density, although this has not been replicated in all studies.
In a mouse model, lithium has been shown to enhance bone formation and improve bone mass, at least in part by activation of the Wnt signaling pathway through an inhibitory effect on glycogen synthase kinase-3β.11 In humans, lithium-treated adults had lower serum alkaline phosphate, osteocalcin, and C-telopeptide levels compared with controls, suggesting a state of decreased bone remodeling and increased turnover.12 There is a paucity of clinical data on the effect of lithium on fracture risk. Additional studies are necessary to elucidate lithium’s mechanism on bone mineral density and determine the magnitude of the clinical effect.
Anticonvulsants
The association among anticonvulsants, decreased bone mineral density, and increased risk of fracture is well-established in the literature.13 However, causality is difficult to determine, because many studies were of patients with a seizure disorder, who often have additional risk factors for fracture, including seizure-related trauma, drowsiness, and slowed reflexes.
Mechanisms through which anticonvulsants increase fracture risk include increased bone resorption, secondary hypoparathyroidism, and pseudohypoparathyroidism. Markers of bone resorption were elevated in patients receiving an antiepileptic.14 This effect might be enhanced by co-administration of cytochrome P450 (CYP450) enzyme-inducing anticonvulsants and CYP450 enzyme-inhibiting medications, such as valproate. Long-term treatment with valproate may produce reduction of bone mass and increased risk of fractures; however, other studies disagree with this finding.15
In addition to CYP450-inducing effects, phenytoin, carbamezapine, and phenobarbital can increase catabolism of vitamin D, which is associated with osteomalacia.14 This results in decreased intestinal absorption of calcium, hypocalcemia, and secondary hyperparathyroidism, which also increases fracture risk. Anticonvulsants also might increase resistance to pseudohypoparathyroidism and inhibit calcitonin secretion.
Lamotrigine has not been shown to interfere with bone accrual16 and may be a safer mood stabilizer for patients at high risk of fracture. For patients at increased risk of fracture, it is important to select an anticonvulsant wisely to minimize fracture risk.
How would you treat Ms. E during her hospitalization for bipolar disorder?
a) carbamazepine
b) lithium
c) risperidone
d) mirtazapine
TREATMENT Minimizing polypharmacy
Because many pharmacotherapeutic options for managing bipolar disorder can increase the risk of fracture, clinicians must be aware of the relative risk of each class of medication and each individual drug. We initiated lithium, 300 mg, 3 times a day, to stabilize Ms. E’s mood. Although clinical data are inconclusive regarding lithium’s effect on fracture risk, we felt that the benefit of acute mood stabilization outweighed the risk of decreased bone mineral index.
We selected aripiprazole, 10 mg/d, as an adjunctive treatment because of its minimal effect on serum prolactin levels.4 We considered prescribing an antidepressant but decided against it because we were concerned about manic switching.
Polypharmacy is another important consideration for Ms. E. Several studies have identified polypharmacy, particularly with antipsychotics, as an independent risk factor for fracture.3 Therefore, we sought to minimize the number of medications Ms. E receives. Although lithium monotherapy is an option, we thought that her mood symptoms were severe enough that the risk of inadequately treating her bipolar symptoms outweighed the additional risk of fracture from dual therapy with lithium and aripiprazole. Untreated or inadequately treated depression is associated with a higher fracture risk. Therefore, we avoided prescribing >2 medications to mitigate any excessive risk of fracture from polypharmacy.
Bottom Line
Different classes of medications—antipsychotics, anticonvulsants, antidepressants, and lithium—used for treating bipolar disorder have been shown to increase risk of bone fracture through a variety of mechanisms. Anticonvulsants and prolactin-elevating antipsychotics are associated with increased fracture risk; evidence on lithium is mixed. Fracture risk with antidepressants is associated with duration of use, rather than dosage.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Howard L, Kirkwood G, Leese M. Risk of hip fracture in patients with a history of schizophrenia. Br J Psychiatry. 2007;190:129-134.
2. Takkouche B, Montes-Martínez A, Gill SS, et al. Psychotropic medications and the risk of fracture: a meta-analysis. Drug Saf. 2007;30(2):171-184.
3. Sørensen HJ, Jensen SO, Nielsen J. Schizophrenia, antipsychotics and risk of hip fracture: a population-based analysis. Eur Neuropsychopharmacol. 2013;23(8):872-878.
4. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
5. Bilici M, Cakirbay H, Guler M, et al. Classical and atypical neuroleptics, and bone mineral density, in patients with schizophrenia. Int J Neurosci. 2002;112(7):817-828.
6. Becker D, Liver O, Mester R, et al. Risperidone, but not olanzapine, decreases bone mineral density in female premenopausal schizophrenia patients. J Clin Psychiatry. 2003;64(7):761-766.
7. Bolton JM, Metge C, Lix L, et al. Fracture risk from psychotropic medications: a population-based analysis. J Clin Psychopharmacol. 2008;28(4):384-391.
8. Verdel BM, Souverein PC, Egberts TC, et al. Use of antidepressant drugs and risk of osteoporotic and non-osteoporotic fractures. Bone. 2010;47(3):604-609.
9. Diem SJ, Ruppert K, Cauley JA. Rates of bone loss among women initiating antidepressant medication use in midlife. J Clin Endocrinol Metab. 2013;(11):4355-4363.
10. Plenge P, Rafaelsen OJ. Lithium effects on calcium, magnesium and phosphate in man: effects on balance, bone mineral content, faecal and urinary excretion. Acta Psychiatr Scand. 1982;66(5):361-373.
11. Clément-Lacroix P, Ai M, Morvan F, et al. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci U S A. 2005;102(48):17406-17411.
12. Zamani A, Omrani GR, Nasab MM. Lithium’s effect on bone mineral density. Bone. 2009;44(2):331-334.
13. Swanton J, Simister R, Altmann D, et al. Bone mineral density in institutionalised patients with refractory epilepsy. Seizure. 2007;16(6):538-541.
14. Pack AM, Morrell MJ. Epilepsy and bone health in adults. Epilepsy Behav. 2004;5(suppl 2):S24-S29.
15. Pack AM. Bone disease in epilepsy. Curr Neurol Neurosci Rep. 2004;4(4):329-334.
16. Sheth RD, Hermann BP. Bone mineral density with lamotrigine monotherapy for epilepsy. Pediatr Neurol. 2007;37(4):250-254.
CASE Broken down
Ms. E, age 20, is a college student who has had major depressive disorder for several years and a genetic bone disease (osteogenesis imperfecta, mixed type III and IV). She presents with depression, anxiety, and suicidal ideation. She reports recent worsening of her depressive symptoms, including anhedonia, excessive sleep, difficulty concentrating, and feeling overwhelmed, hopeless, and worthless. She also describes frequent thoughts of suicide with the plan of putting herself in oncoming traffic, although she has no history of suicide attempts.
Previously, her primary care physician prescribed lorazepam, 0.5 mg, as needed for anxiety, and sertraline, 100 mg/d, for depression and anxiety. She experienced only partial improvement in symptoms, however.
In addition to depressive symptoms, Ms. E describes manic symptoms lasting for as long as 3 to 5 days, including decreased need for sleep, increased energy, pressured speech, racing thoughts, distractibility, spending excessive money on cosmetics, and risking her safety—given her skeletal disorder— by participating in high-impact stage-combat classes. She denies auditory and visual hallucinations, homicidal ideation, and delusions.
The medical history is significant for osteogenesis imperfecta, which has caused 62 fractures and required 16 surgeries. Ms. E is a theater major who, despite her short stature and wheelchair use, reports enjoying her acting career and says she does not feel demoralized by her medical condition. She describes overcoming her physical disabilities with pride and confidence. However, her recent worsening mood symptoms have left her unable to concentrate and feeling overwhelmed with school.
Ms. E is voluntarily admitted to an inpatient psychiatric unit with a diagnosis of bipolar I disorder with rapid cycling, most recent episode mixed. Because of her bone fragility, the treatment team considers what would be an appropriate course of drug treatment to control bipolar symptoms while minimizing risk of bone loss.
Which medications are associated with decreased bone mineral density?
a) citalopram
b) haloperidol
c) carbamazepine
d) paliperidone
e) all of the above
The authors’ observations
Osteogenesis imperfecta is a genetic condition caused by mutations in genes implicated in collagen production. As a result, bones are brittle and prone to fracture. Different classes of psychotropics have been shown to increase risk of bone fractures through a variety of mechanisms. Clinicians often must choose appropriate pharmacotherapy for patients at high risk of fracture, including postmenopausal women, older patients, malnourished persons, and those with hormonal deficiencies leading to osteoporosis.
To assist our clinical decision-making, we reviewed the literature to establish appropriate management of a patient with increased bone fragility and new-onset bipolar disorder. We considered all classes of medications used to treat bipolar disorder, including antipsychotics, antidepressants, lithium, and anticonvulsants.
Antipsychotics
In population-based studies, prolactin-elevating antipsychotics have been associated with decreased bone mineral density and increased risk of fracture.1 Additional studies on geriatric and non-geriatric populations have supported these findings.2,3
The mechanism through which fracture risk is increased likely is related to antipsychotics’ effect on serum prolactin and cortisol levels. Antipsychotics act as antagonists on D2 receptors in the hypothalamic tubero-infundibular pathway, therefore preventing inhibition of prolactin. Long-term elevation in serum prolactin can cause loss of bone mineral density through secondary hypogonadism and direct effects on target tissues. Additional modifying factors include smoking and estrogen use.
The degree to which antipsychotics increase fracture risk might be related to the degree of serum prolactin elevation.4 Antipsychotics previously have been grouped by the degree of prolactin elevation, categorizing them as high, medium, and low or no potential to elevate serum prolactin.4 Based on this classification, typical antipsychotics, risperidone, and paliperidone have the highest potential to elevate prolactin. Accordingly, antipsychotics with the lowest fracture risk are those that have the lowest risk of serum prolactin elevation: ziprasidone, asenapine, quetiapine, and clozapine. Aripiprazole may lower prolactin in some patients. This is supported by studies noting reduced bone mineral density5,6 and increased risk of fracture1 with high-potential vs low- or no-potential antipsychotics. Because of these findings, it is crucial to consider the potential risk of prolactin elevation when treating patients at increased risk of fracture. Providers should consider low/no potential antipsychotic medications before considering those with medium or high potential (Table).
Antidepressants
In a meta-analysis, antidepressants were shown to increase fracture risk by 70% to 90%.2 However, the relative risk varies by antidepressant class. Several studies have shown that selective serotonin reuptake inhibitors (SSRIs) are associated with a higher risk of fracture compared with tricyclic antidepressants (TCAs).7 In addition, antidepressants with a high affinity for the serotonin transporter, including citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and imipramine, have been associated with greater risk of osteoporotic fracture compared with those with low affinity.8
The mechanisms by which antidepressants increase fracture risk are complex, although the strongest evidence implicates a direct effect on bone metabolism via the 5-HTT receptor. This receptor, found on osteoblasts and osteoclasts, plays an important role in bone metabolism; it is through this receptor that SSRIs might inhibit osteoblasts and promote osteoclast activity, thereby disrupting bone microarchitecture. Additional studies are needed to further describe the mechanism of the association among antidepressants, bone mineral density, and fracture risk.
Fracture risk is associated with duration of use rather than dosage. Population-based studies show a higher fracture risk for new users of TCAs compared with continuous users, and the risk of fracture with SSRIs seems to increase slightly over time.9 No association has been identified between fracture risk and antidepressant dosage. According to the literature, drugs with low affinity for the serotonin transporter, such as maprotiline and mirtazapine, likely are the safest antidepressants for patients at increased risk of fracture. Options also include other TCAs and any antidepressant with low affinity for the serotonin receptor.7,8
Lithium
Studies on lithium and bone mineral density have shown mixed results. Older studies found that lithium had a negative or no effect on bone mineral density or the parathyroid hormone level.10 More recent investigations, however, suggest that the drug has a protective effect on bone mineral density, although this has not been replicated in all studies.
In a mouse model, lithium has been shown to enhance bone formation and improve bone mass, at least in part by activation of the Wnt signaling pathway through an inhibitory effect on glycogen synthase kinase-3β.11 In humans, lithium-treated adults had lower serum alkaline phosphate, osteocalcin, and C-telopeptide levels compared with controls, suggesting a state of decreased bone remodeling and increased turnover.12 There is a paucity of clinical data on the effect of lithium on fracture risk. Additional studies are necessary to elucidate lithium’s mechanism on bone mineral density and determine the magnitude of the clinical effect.
Anticonvulsants
The association among anticonvulsants, decreased bone mineral density, and increased risk of fracture is well-established in the literature.13 However, causality is difficult to determine, because many studies were of patients with a seizure disorder, who often have additional risk factors for fracture, including seizure-related trauma, drowsiness, and slowed reflexes.
Mechanisms through which anticonvulsants increase fracture risk include increased bone resorption, secondary hypoparathyroidism, and pseudohypoparathyroidism. Markers of bone resorption were elevated in patients receiving an antiepileptic.14 This effect might be enhanced by co-administration of cytochrome P450 (CYP450) enzyme-inducing anticonvulsants and CYP450 enzyme-inhibiting medications, such as valproate. Long-term treatment with valproate may produce reduction of bone mass and increased risk of fractures; however, other studies disagree with this finding.15
In addition to CYP450-inducing effects, phenytoin, carbamezapine, and phenobarbital can increase catabolism of vitamin D, which is associated with osteomalacia.14 This results in decreased intestinal absorption of calcium, hypocalcemia, and secondary hyperparathyroidism, which also increases fracture risk. Anticonvulsants also might increase resistance to pseudohypoparathyroidism and inhibit calcitonin secretion.
Lamotrigine has not been shown to interfere with bone accrual16 and may be a safer mood stabilizer for patients at high risk of fracture. For patients at increased risk of fracture, it is important to select an anticonvulsant wisely to minimize fracture risk.
How would you treat Ms. E during her hospitalization for bipolar disorder?
a) carbamazepine
b) lithium
c) risperidone
d) mirtazapine
TREATMENT Minimizing polypharmacy
Because many pharmacotherapeutic options for managing bipolar disorder can increase the risk of fracture, clinicians must be aware of the relative risk of each class of medication and each individual drug. We initiated lithium, 300 mg, 3 times a day, to stabilize Ms. E’s mood. Although clinical data are inconclusive regarding lithium’s effect on fracture risk, we felt that the benefit of acute mood stabilization outweighed the risk of decreased bone mineral index.
We selected aripiprazole, 10 mg/d, as an adjunctive treatment because of its minimal effect on serum prolactin levels.4 We considered prescribing an antidepressant but decided against it because we were concerned about manic switching.
Polypharmacy is another important consideration for Ms. E. Several studies have identified polypharmacy, particularly with antipsychotics, as an independent risk factor for fracture.3 Therefore, we sought to minimize the number of medications Ms. E receives. Although lithium monotherapy is an option, we thought that her mood symptoms were severe enough that the risk of inadequately treating her bipolar symptoms outweighed the additional risk of fracture from dual therapy with lithium and aripiprazole. Untreated or inadequately treated depression is associated with a higher fracture risk. Therefore, we avoided prescribing >2 medications to mitigate any excessive risk of fracture from polypharmacy.
Bottom Line
Different classes of medications—antipsychotics, anticonvulsants, antidepressants, and lithium—used for treating bipolar disorder have been shown to increase risk of bone fracture through a variety of mechanisms. Anticonvulsants and prolactin-elevating antipsychotics are associated with increased fracture risk; evidence on lithium is mixed. Fracture risk with antidepressants is associated with duration of use, rather than dosage.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Broken down
Ms. E, age 20, is a college student who has had major depressive disorder for several years and a genetic bone disease (osteogenesis imperfecta, mixed type III and IV). She presents with depression, anxiety, and suicidal ideation. She reports recent worsening of her depressive symptoms, including anhedonia, excessive sleep, difficulty concentrating, and feeling overwhelmed, hopeless, and worthless. She also describes frequent thoughts of suicide with the plan of putting herself in oncoming traffic, although she has no history of suicide attempts.
Previously, her primary care physician prescribed lorazepam, 0.5 mg, as needed for anxiety, and sertraline, 100 mg/d, for depression and anxiety. She experienced only partial improvement in symptoms, however.
In addition to depressive symptoms, Ms. E describes manic symptoms lasting for as long as 3 to 5 days, including decreased need for sleep, increased energy, pressured speech, racing thoughts, distractibility, spending excessive money on cosmetics, and risking her safety—given her skeletal disorder— by participating in high-impact stage-combat classes. She denies auditory and visual hallucinations, homicidal ideation, and delusions.
The medical history is significant for osteogenesis imperfecta, which has caused 62 fractures and required 16 surgeries. Ms. E is a theater major who, despite her short stature and wheelchair use, reports enjoying her acting career and says she does not feel demoralized by her medical condition. She describes overcoming her physical disabilities with pride and confidence. However, her recent worsening mood symptoms have left her unable to concentrate and feeling overwhelmed with school.
Ms. E is voluntarily admitted to an inpatient psychiatric unit with a diagnosis of bipolar I disorder with rapid cycling, most recent episode mixed. Because of her bone fragility, the treatment team considers what would be an appropriate course of drug treatment to control bipolar symptoms while minimizing risk of bone loss.
Which medications are associated with decreased bone mineral density?
a) citalopram
b) haloperidol
c) carbamazepine
d) paliperidone
e) all of the above
The authors’ observations
Osteogenesis imperfecta is a genetic condition caused by mutations in genes implicated in collagen production. As a result, bones are brittle and prone to fracture. Different classes of psychotropics have been shown to increase risk of bone fractures through a variety of mechanisms. Clinicians often must choose appropriate pharmacotherapy for patients at high risk of fracture, including postmenopausal women, older patients, malnourished persons, and those with hormonal deficiencies leading to osteoporosis.
To assist our clinical decision-making, we reviewed the literature to establish appropriate management of a patient with increased bone fragility and new-onset bipolar disorder. We considered all classes of medications used to treat bipolar disorder, including antipsychotics, antidepressants, lithium, and anticonvulsants.
Antipsychotics
In population-based studies, prolactin-elevating antipsychotics have been associated with decreased bone mineral density and increased risk of fracture.1 Additional studies on geriatric and non-geriatric populations have supported these findings.2,3
The mechanism through which fracture risk is increased likely is related to antipsychotics’ effect on serum prolactin and cortisol levels. Antipsychotics act as antagonists on D2 receptors in the hypothalamic tubero-infundibular pathway, therefore preventing inhibition of prolactin. Long-term elevation in serum prolactin can cause loss of bone mineral density through secondary hypogonadism and direct effects on target tissues. Additional modifying factors include smoking and estrogen use.
The degree to which antipsychotics increase fracture risk might be related to the degree of serum prolactin elevation.4 Antipsychotics previously have been grouped by the degree of prolactin elevation, categorizing them as high, medium, and low or no potential to elevate serum prolactin.4 Based on this classification, typical antipsychotics, risperidone, and paliperidone have the highest potential to elevate prolactin. Accordingly, antipsychotics with the lowest fracture risk are those that have the lowest risk of serum prolactin elevation: ziprasidone, asenapine, quetiapine, and clozapine. Aripiprazole may lower prolactin in some patients. This is supported by studies noting reduced bone mineral density5,6 and increased risk of fracture1 with high-potential vs low- or no-potential antipsychotics. Because of these findings, it is crucial to consider the potential risk of prolactin elevation when treating patients at increased risk of fracture. Providers should consider low/no potential antipsychotic medications before considering those with medium or high potential (Table).
Antidepressants
In a meta-analysis, antidepressants were shown to increase fracture risk by 70% to 90%.2 However, the relative risk varies by antidepressant class. Several studies have shown that selective serotonin reuptake inhibitors (SSRIs) are associated with a higher risk of fracture compared with tricyclic antidepressants (TCAs).7 In addition, antidepressants with a high affinity for the serotonin transporter, including citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and imipramine, have been associated with greater risk of osteoporotic fracture compared with those with low affinity.8
The mechanisms by which antidepressants increase fracture risk are complex, although the strongest evidence implicates a direct effect on bone metabolism via the 5-HTT receptor. This receptor, found on osteoblasts and osteoclasts, plays an important role in bone metabolism; it is through this receptor that SSRIs might inhibit osteoblasts and promote osteoclast activity, thereby disrupting bone microarchitecture. Additional studies are needed to further describe the mechanism of the association among antidepressants, bone mineral density, and fracture risk.
Fracture risk is associated with duration of use rather than dosage. Population-based studies show a higher fracture risk for new users of TCAs compared with continuous users, and the risk of fracture with SSRIs seems to increase slightly over time.9 No association has been identified between fracture risk and antidepressant dosage. According to the literature, drugs with low affinity for the serotonin transporter, such as maprotiline and mirtazapine, likely are the safest antidepressants for patients at increased risk of fracture. Options also include other TCAs and any antidepressant with low affinity for the serotonin receptor.7,8
Lithium
Studies on lithium and bone mineral density have shown mixed results. Older studies found that lithium had a negative or no effect on bone mineral density or the parathyroid hormone level.10 More recent investigations, however, suggest that the drug has a protective effect on bone mineral density, although this has not been replicated in all studies.
In a mouse model, lithium has been shown to enhance bone formation and improve bone mass, at least in part by activation of the Wnt signaling pathway through an inhibitory effect on glycogen synthase kinase-3β.11 In humans, lithium-treated adults had lower serum alkaline phosphate, osteocalcin, and C-telopeptide levels compared with controls, suggesting a state of decreased bone remodeling and increased turnover.12 There is a paucity of clinical data on the effect of lithium on fracture risk. Additional studies are necessary to elucidate lithium’s mechanism on bone mineral density and determine the magnitude of the clinical effect.
Anticonvulsants
The association among anticonvulsants, decreased bone mineral density, and increased risk of fracture is well-established in the literature.13 However, causality is difficult to determine, because many studies were of patients with a seizure disorder, who often have additional risk factors for fracture, including seizure-related trauma, drowsiness, and slowed reflexes.
Mechanisms through which anticonvulsants increase fracture risk include increased bone resorption, secondary hypoparathyroidism, and pseudohypoparathyroidism. Markers of bone resorption were elevated in patients receiving an antiepileptic.14 This effect might be enhanced by co-administration of cytochrome P450 (CYP450) enzyme-inducing anticonvulsants and CYP450 enzyme-inhibiting medications, such as valproate. Long-term treatment with valproate may produce reduction of bone mass and increased risk of fractures; however, other studies disagree with this finding.15
In addition to CYP450-inducing effects, phenytoin, carbamezapine, and phenobarbital can increase catabolism of vitamin D, which is associated with osteomalacia.14 This results in decreased intestinal absorption of calcium, hypocalcemia, and secondary hyperparathyroidism, which also increases fracture risk. Anticonvulsants also might increase resistance to pseudohypoparathyroidism and inhibit calcitonin secretion.
Lamotrigine has not been shown to interfere with bone accrual16 and may be a safer mood stabilizer for patients at high risk of fracture. For patients at increased risk of fracture, it is important to select an anticonvulsant wisely to minimize fracture risk.
How would you treat Ms. E during her hospitalization for bipolar disorder?
a) carbamazepine
b) lithium
c) risperidone
d) mirtazapine
TREATMENT Minimizing polypharmacy
Because many pharmacotherapeutic options for managing bipolar disorder can increase the risk of fracture, clinicians must be aware of the relative risk of each class of medication and each individual drug. We initiated lithium, 300 mg, 3 times a day, to stabilize Ms. E’s mood. Although clinical data are inconclusive regarding lithium’s effect on fracture risk, we felt that the benefit of acute mood stabilization outweighed the risk of decreased bone mineral index.
We selected aripiprazole, 10 mg/d, as an adjunctive treatment because of its minimal effect on serum prolactin levels.4 We considered prescribing an antidepressant but decided against it because we were concerned about manic switching.
Polypharmacy is another important consideration for Ms. E. Several studies have identified polypharmacy, particularly with antipsychotics, as an independent risk factor for fracture.3 Therefore, we sought to minimize the number of medications Ms. E receives. Although lithium monotherapy is an option, we thought that her mood symptoms were severe enough that the risk of inadequately treating her bipolar symptoms outweighed the additional risk of fracture from dual therapy with lithium and aripiprazole. Untreated or inadequately treated depression is associated with a higher fracture risk. Therefore, we avoided prescribing >2 medications to mitigate any excessive risk of fracture from polypharmacy.
Bottom Line
Different classes of medications—antipsychotics, anticonvulsants, antidepressants, and lithium—used for treating bipolar disorder have been shown to increase risk of bone fracture through a variety of mechanisms. Anticonvulsants and prolactin-elevating antipsychotics are associated with increased fracture risk; evidence on lithium is mixed. Fracture risk with antidepressants is associated with duration of use, rather than dosage.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Howard L, Kirkwood G, Leese M. Risk of hip fracture in patients with a history of schizophrenia. Br J Psychiatry. 2007;190:129-134.
2. Takkouche B, Montes-Martínez A, Gill SS, et al. Psychotropic medications and the risk of fracture: a meta-analysis. Drug Saf. 2007;30(2):171-184.
3. Sørensen HJ, Jensen SO, Nielsen J. Schizophrenia, antipsychotics and risk of hip fracture: a population-based analysis. Eur Neuropsychopharmacol. 2013;23(8):872-878.
4. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
5. Bilici M, Cakirbay H, Guler M, et al. Classical and atypical neuroleptics, and bone mineral density, in patients with schizophrenia. Int J Neurosci. 2002;112(7):817-828.
6. Becker D, Liver O, Mester R, et al. Risperidone, but not olanzapine, decreases bone mineral density in female premenopausal schizophrenia patients. J Clin Psychiatry. 2003;64(7):761-766.
7. Bolton JM, Metge C, Lix L, et al. Fracture risk from psychotropic medications: a population-based analysis. J Clin Psychopharmacol. 2008;28(4):384-391.
8. Verdel BM, Souverein PC, Egberts TC, et al. Use of antidepressant drugs and risk of osteoporotic and non-osteoporotic fractures. Bone. 2010;47(3):604-609.
9. Diem SJ, Ruppert K, Cauley JA. Rates of bone loss among women initiating antidepressant medication use in midlife. J Clin Endocrinol Metab. 2013;(11):4355-4363.
10. Plenge P, Rafaelsen OJ. Lithium effects on calcium, magnesium and phosphate in man: effects on balance, bone mineral content, faecal and urinary excretion. Acta Psychiatr Scand. 1982;66(5):361-373.
11. Clément-Lacroix P, Ai M, Morvan F, et al. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci U S A. 2005;102(48):17406-17411.
12. Zamani A, Omrani GR, Nasab MM. Lithium’s effect on bone mineral density. Bone. 2009;44(2):331-334.
13. Swanton J, Simister R, Altmann D, et al. Bone mineral density in institutionalised patients with refractory epilepsy. Seizure. 2007;16(6):538-541.
14. Pack AM, Morrell MJ. Epilepsy and bone health in adults. Epilepsy Behav. 2004;5(suppl 2):S24-S29.
15. Pack AM. Bone disease in epilepsy. Curr Neurol Neurosci Rep. 2004;4(4):329-334.
16. Sheth RD, Hermann BP. Bone mineral density with lamotrigine monotherapy for epilepsy. Pediatr Neurol. 2007;37(4):250-254.
1. Howard L, Kirkwood G, Leese M. Risk of hip fracture in patients with a history of schizophrenia. Br J Psychiatry. 2007;190:129-134.
2. Takkouche B, Montes-Martínez A, Gill SS, et al. Psychotropic medications and the risk of fracture: a meta-analysis. Drug Saf. 2007;30(2):171-184.
3. Sørensen HJ, Jensen SO, Nielsen J. Schizophrenia, antipsychotics and risk of hip fracture: a population-based analysis. Eur Neuropsychopharmacol. 2013;23(8):872-878.
4. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
5. Bilici M, Cakirbay H, Guler M, et al. Classical and atypical neuroleptics, and bone mineral density, in patients with schizophrenia. Int J Neurosci. 2002;112(7):817-828.
6. Becker D, Liver O, Mester R, et al. Risperidone, but not olanzapine, decreases bone mineral density in female premenopausal schizophrenia patients. J Clin Psychiatry. 2003;64(7):761-766.
7. Bolton JM, Metge C, Lix L, et al. Fracture risk from psychotropic medications: a population-based analysis. J Clin Psychopharmacol. 2008;28(4):384-391.
8. Verdel BM, Souverein PC, Egberts TC, et al. Use of antidepressant drugs and risk of osteoporotic and non-osteoporotic fractures. Bone. 2010;47(3):604-609.
9. Diem SJ, Ruppert K, Cauley JA. Rates of bone loss among women initiating antidepressant medication use in midlife. J Clin Endocrinol Metab. 2013;(11):4355-4363.
10. Plenge P, Rafaelsen OJ. Lithium effects on calcium, magnesium and phosphate in man: effects on balance, bone mineral content, faecal and urinary excretion. Acta Psychiatr Scand. 1982;66(5):361-373.
11. Clément-Lacroix P, Ai M, Morvan F, et al. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci U S A. 2005;102(48):17406-17411.
12. Zamani A, Omrani GR, Nasab MM. Lithium’s effect on bone mineral density. Bone. 2009;44(2):331-334.
13. Swanton J, Simister R, Altmann D, et al. Bone mineral density in institutionalised patients with refractory epilepsy. Seizure. 2007;16(6):538-541.
14. Pack AM, Morrell MJ. Epilepsy and bone health in adults. Epilepsy Behav. 2004;5(suppl 2):S24-S29.
15. Pack AM. Bone disease in epilepsy. Curr Neurol Neurosci Rep. 2004;4(4):329-334.
16. Sheth RD, Hermann BP. Bone mineral density with lamotrigine monotherapy for epilepsy. Pediatr Neurol. 2007;37(4):250-254.
Will finding the depression−inflammation link lead to tailored treatments for MDD?
There is an association between inflammation and depression: Patients with a major depressive disorder (MDD) have elevated levels of pro-inflammatory cytokines interleukin (IL)-1, IL-6, tumor necrosis factor-alpha (TNF-α), and C-reactive protein (CRP). Abnormal cell-mediated immunity and lymphocyte proliferation also have been reported in patients with MDD1-2 (Box).1,3-7
What remains unclear is whether inflammation is causative in affective illness,1-4 and how the association might be exploited for the benefit of a subset of MDD patients.
Underpinnings of pathophysiology
Immune system activation leads to production of cytokines, which 1) influences the synthesis, reuptake, and release of neurotransmitters and 2) stimulates the manifestations of depression.1,2 Interferon-γ and TNF-α are involved in neuronal degeneration and inhibition of neurogenesis in the brain, especially the hippocampus— thereby explaining observed cognitive deficits in depression.
Production of cytokines in serum and cerebrospinal fluid can be triggered by psychosocial stress, administration of interferon-α or IL-2, and acute stimulation of the immune system after vaccination; this production of cytokines is associated with development of MDD.1-3 Inflammatory disorders raise a person’s vulnerability to MDD; affective illness is the most common psychiatric condition seen in association with multiple sclerosis, for example.2
Principal receptor targets
Glucocorticoid receptors. Synchrony between the hypothalamic-pituitary-adrenal axis and adrenal function occurs during stressful circumstances.2 Down-regulation, or reduced activity, of glucocorticoid receptors in depression leads to glucocorticoid resistance, resulting in hyperactivity of this axis. TNF-α is associated with glucocorticoid resistance by its action in opposing the influx of the cortisol-glucocorticoid receptor complex into the nucleus and inhibiting its linkage with DNA. Cytokines increase levels of corticotropin-releasing hormone and adrenocorticotrophic hormone, leading to a higher-than-normal cortisol concentration in depressed patients.8
N-methyl-d-aspartate (NMDA) receptors are involved in the monoamine and glutamatergic pathways that are associated with depression.2 NMDA-receptor activation raises the intracellular calcium concentration, causing neuronal cell death. Inflammatory mediators, including TNF-α, induce activation of the kyneurin pathway. Thus, instead of serotonin production, tryptophan is diverted to the synthesis of the NMDA-receptor agonists kynurenine and quinolinic acid, which leads to apoptosis.
The glutamatergic pathway involves binding of IL-1β and IL-1R complexes to hippocampal NMDA receptors.2 Persistent activation of these receptors results in calcium toxicity and neuronal death. Reuptake inhibition of neurotransmitters is explained by the action of IL-1β on reuptake of glutamate, which enhances its availability to stimulate NMDA-receptor activation.
Any prospects for therapeutics?
As described, an association exists between inflammation and depression. Psychosocial stresses initiate inflammatory responses that might result in affective illness. In treating depression and preventing its relapse, the question is whether psychotherapy provides clinical efficacy through stress reduction, thereby leading to potential anti-inflammatory action.1
Inflammation has a detrimental influence in a subset of MDD cases.9 Identification of those patients through genetic research is ongoing, with the goal of establishing specific anti-inflammatory or antidepressant therapies.
Anti-inflammatory drugs such as aspirin, celecoxib, and etanercept do induce antidepressant effects and augment the antidepressant response to other therapies.1,3 In the future, anti-inflammatory treatments might become an option for select MDD patients.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Zunszain PA, Hepgul N, Pariante CM. Inflammation and depression. Curr Top Behav Neurosci. 2013;14:135-151.
2. Krishnadas R, Cavanagh J. Depression: an inflammatory illness? J Neurol Neurosurg Psychiatry. 2012;83(5):495-502.
3. Lotrich FE, El-Gabalawy H, Guenther LC, et al. The role of inflammation in the pathophysiology of depression: different treatments and their effects. J Rheumatol Suppl. 2011;88:48-54.
4. Gimeno D, Marmot MG, Singh-Manoux A. Inflammatory markers and cognitive function in middle-aged adults: the Whitehall II study. Psychoneuroendocrinology. 2008; 33(10):1322-1334.
5. Copeland WE, Shanahan L, Worthman C, et al. Cumulative depression episodes predict later C-reactive protein levels: a prospective analysis. Biol Psychiatry. 2012;71(1):15-21.
6. Chida Y, Sudo N, Sonoda J, et al. Early-life psychological stress exacerbates adult mouse asthma via the hypothalamus-pituitary-adrenal axis. Am J Respir Crit Care Med. 2007;175(4):316-322.
7. Carpenter LL, Gawuga CE, Tyrka AR, et al. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology. 2010;35(13):2617-2623.
8. Messay B, Lim A, Marsland AL. Current understanding of the bi-directional relationship of major depression with inflammation. Biol Mood Anxiety Disord. 2012;2(1):4.
9. Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7(6):323-331.
There is an association between inflammation and depression: Patients with a major depressive disorder (MDD) have elevated levels of pro-inflammatory cytokines interleukin (IL)-1, IL-6, tumor necrosis factor-alpha (TNF-α), and C-reactive protein (CRP). Abnormal cell-mediated immunity and lymphocyte proliferation also have been reported in patients with MDD1-2 (Box).1,3-7
What remains unclear is whether inflammation is causative in affective illness,1-4 and how the association might be exploited for the benefit of a subset of MDD patients.
Underpinnings of pathophysiology
Immune system activation leads to production of cytokines, which 1) influences the synthesis, reuptake, and release of neurotransmitters and 2) stimulates the manifestations of depression.1,2 Interferon-γ and TNF-α are involved in neuronal degeneration and inhibition of neurogenesis in the brain, especially the hippocampus— thereby explaining observed cognitive deficits in depression.
Production of cytokines in serum and cerebrospinal fluid can be triggered by psychosocial stress, administration of interferon-α or IL-2, and acute stimulation of the immune system after vaccination; this production of cytokines is associated with development of MDD.1-3 Inflammatory disorders raise a person’s vulnerability to MDD; affective illness is the most common psychiatric condition seen in association with multiple sclerosis, for example.2
Principal receptor targets
Glucocorticoid receptors. Synchrony between the hypothalamic-pituitary-adrenal axis and adrenal function occurs during stressful circumstances.2 Down-regulation, or reduced activity, of glucocorticoid receptors in depression leads to glucocorticoid resistance, resulting in hyperactivity of this axis. TNF-α is associated with glucocorticoid resistance by its action in opposing the influx of the cortisol-glucocorticoid receptor complex into the nucleus and inhibiting its linkage with DNA. Cytokines increase levels of corticotropin-releasing hormone and adrenocorticotrophic hormone, leading to a higher-than-normal cortisol concentration in depressed patients.8
N-methyl-d-aspartate (NMDA) receptors are involved in the monoamine and glutamatergic pathways that are associated with depression.2 NMDA-receptor activation raises the intracellular calcium concentration, causing neuronal cell death. Inflammatory mediators, including TNF-α, induce activation of the kyneurin pathway. Thus, instead of serotonin production, tryptophan is diverted to the synthesis of the NMDA-receptor agonists kynurenine and quinolinic acid, which leads to apoptosis.
The glutamatergic pathway involves binding of IL-1β and IL-1R complexes to hippocampal NMDA receptors.2 Persistent activation of these receptors results in calcium toxicity and neuronal death. Reuptake inhibition of neurotransmitters is explained by the action of IL-1β on reuptake of glutamate, which enhances its availability to stimulate NMDA-receptor activation.
Any prospects for therapeutics?
As described, an association exists between inflammation and depression. Psychosocial stresses initiate inflammatory responses that might result in affective illness. In treating depression and preventing its relapse, the question is whether psychotherapy provides clinical efficacy through stress reduction, thereby leading to potential anti-inflammatory action.1
Inflammation has a detrimental influence in a subset of MDD cases.9 Identification of those patients through genetic research is ongoing, with the goal of establishing specific anti-inflammatory or antidepressant therapies.
Anti-inflammatory drugs such as aspirin, celecoxib, and etanercept do induce antidepressant effects and augment the antidepressant response to other therapies.1,3 In the future, anti-inflammatory treatments might become an option for select MDD patients.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
There is an association between inflammation and depression: Patients with a major depressive disorder (MDD) have elevated levels of pro-inflammatory cytokines interleukin (IL)-1, IL-6, tumor necrosis factor-alpha (TNF-α), and C-reactive protein (CRP). Abnormal cell-mediated immunity and lymphocyte proliferation also have been reported in patients with MDD1-2 (Box).1,3-7
What remains unclear is whether inflammation is causative in affective illness,1-4 and how the association might be exploited for the benefit of a subset of MDD patients.
Underpinnings of pathophysiology
Immune system activation leads to production of cytokines, which 1) influences the synthesis, reuptake, and release of neurotransmitters and 2) stimulates the manifestations of depression.1,2 Interferon-γ and TNF-α are involved in neuronal degeneration and inhibition of neurogenesis in the brain, especially the hippocampus— thereby explaining observed cognitive deficits in depression.
Production of cytokines in serum and cerebrospinal fluid can be triggered by psychosocial stress, administration of interferon-α or IL-2, and acute stimulation of the immune system after vaccination; this production of cytokines is associated with development of MDD.1-3 Inflammatory disorders raise a person’s vulnerability to MDD; affective illness is the most common psychiatric condition seen in association with multiple sclerosis, for example.2
Principal receptor targets
Glucocorticoid receptors. Synchrony between the hypothalamic-pituitary-adrenal axis and adrenal function occurs during stressful circumstances.2 Down-regulation, or reduced activity, of glucocorticoid receptors in depression leads to glucocorticoid resistance, resulting in hyperactivity of this axis. TNF-α is associated with glucocorticoid resistance by its action in opposing the influx of the cortisol-glucocorticoid receptor complex into the nucleus and inhibiting its linkage with DNA. Cytokines increase levels of corticotropin-releasing hormone and adrenocorticotrophic hormone, leading to a higher-than-normal cortisol concentration in depressed patients.8
N-methyl-d-aspartate (NMDA) receptors are involved in the monoamine and glutamatergic pathways that are associated with depression.2 NMDA-receptor activation raises the intracellular calcium concentration, causing neuronal cell death. Inflammatory mediators, including TNF-α, induce activation of the kyneurin pathway. Thus, instead of serotonin production, tryptophan is diverted to the synthesis of the NMDA-receptor agonists kynurenine and quinolinic acid, which leads to apoptosis.
The glutamatergic pathway involves binding of IL-1β and IL-1R complexes to hippocampal NMDA receptors.2 Persistent activation of these receptors results in calcium toxicity and neuronal death. Reuptake inhibition of neurotransmitters is explained by the action of IL-1β on reuptake of glutamate, which enhances its availability to stimulate NMDA-receptor activation.
Any prospects for therapeutics?
As described, an association exists between inflammation and depression. Psychosocial stresses initiate inflammatory responses that might result in affective illness. In treating depression and preventing its relapse, the question is whether psychotherapy provides clinical efficacy through stress reduction, thereby leading to potential anti-inflammatory action.1
Inflammation has a detrimental influence in a subset of MDD cases.9 Identification of those patients through genetic research is ongoing, with the goal of establishing specific anti-inflammatory or antidepressant therapies.
Anti-inflammatory drugs such as aspirin, celecoxib, and etanercept do induce antidepressant effects and augment the antidepressant response to other therapies.1,3 In the future, anti-inflammatory treatments might become an option for select MDD patients.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Zunszain PA, Hepgul N, Pariante CM. Inflammation and depression. Curr Top Behav Neurosci. 2013;14:135-151.
2. Krishnadas R, Cavanagh J. Depression: an inflammatory illness? J Neurol Neurosurg Psychiatry. 2012;83(5):495-502.
3. Lotrich FE, El-Gabalawy H, Guenther LC, et al. The role of inflammation in the pathophysiology of depression: different treatments and their effects. J Rheumatol Suppl. 2011;88:48-54.
4. Gimeno D, Marmot MG, Singh-Manoux A. Inflammatory markers and cognitive function in middle-aged adults: the Whitehall II study. Psychoneuroendocrinology. 2008; 33(10):1322-1334.
5. Copeland WE, Shanahan L, Worthman C, et al. Cumulative depression episodes predict later C-reactive protein levels: a prospective analysis. Biol Psychiatry. 2012;71(1):15-21.
6. Chida Y, Sudo N, Sonoda J, et al. Early-life psychological stress exacerbates adult mouse asthma via the hypothalamus-pituitary-adrenal axis. Am J Respir Crit Care Med. 2007;175(4):316-322.
7. Carpenter LL, Gawuga CE, Tyrka AR, et al. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology. 2010;35(13):2617-2623.
8. Messay B, Lim A, Marsland AL. Current understanding of the bi-directional relationship of major depression with inflammation. Biol Mood Anxiety Disord. 2012;2(1):4.
9. Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7(6):323-331.
1. Zunszain PA, Hepgul N, Pariante CM. Inflammation and depression. Curr Top Behav Neurosci. 2013;14:135-151.
2. Krishnadas R, Cavanagh J. Depression: an inflammatory illness? J Neurol Neurosurg Psychiatry. 2012;83(5):495-502.
3. Lotrich FE, El-Gabalawy H, Guenther LC, et al. The role of inflammation in the pathophysiology of depression: different treatments and their effects. J Rheumatol Suppl. 2011;88:48-54.
4. Gimeno D, Marmot MG, Singh-Manoux A. Inflammatory markers and cognitive function in middle-aged adults: the Whitehall II study. Psychoneuroendocrinology. 2008; 33(10):1322-1334.
5. Copeland WE, Shanahan L, Worthman C, et al. Cumulative depression episodes predict later C-reactive protein levels: a prospective analysis. Biol Psychiatry. 2012;71(1):15-21.
6. Chida Y, Sudo N, Sonoda J, et al. Early-life psychological stress exacerbates adult mouse asthma via the hypothalamus-pituitary-adrenal axis. Am J Respir Crit Care Med. 2007;175(4):316-322.
7. Carpenter LL, Gawuga CE, Tyrka AR, et al. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology. 2010;35(13):2617-2623.
8. Messay B, Lim A, Marsland AL. Current understanding of the bi-directional relationship of major depression with inflammation. Biol Mood Anxiety Disord. 2012;2(1):4.
9. Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7(6):323-331.
Sedative-hypnotics for sleepless geriatric patients
Mr. R, 75, is having difficulty sleeping. When he goes to bed, he lies there for what seems like forever, unable to fall asleep. He feels “so tired” and ends up taking naps during the day, but he cannot break this cycle. He has tried using over-the-counter products with little relief.
Mr. R’s primary care physician prescribes zaleplon, 10 mg/d, and asks him to call the clinic in 2 weeks to discuss his progress. He takes zaleplon as directed for several nights and begins to feel “sluggish” during the day, both mentally and physically, despite reporting an increase in the overall amount of sleep at night.
Sedative-hypnotic drugs are among the most commonly used medications in the United States. Use of these drugs, as well as anxiolytics, has increased from 2.8% between 1988 and 1994 to 4.7% between 2007 and 2010, according to the Department of Health and Human Services.1 In 2011, drugs categorized as sedative-hypnotics or antipsychotics were involved in 6.1% of all human exposures identified in the American Association of Poison Control Centers’ National Poison Data System.2 Therefore, an understanding of clinical and pharmacological variables related to safe and effective use is important for clinicians prescribing and monitoring therapy with these agents.
Neuropsychiatric disorders are prevalent among geriatric patients and are associated with age-related physiologic changes in the CNS.3 Such changes involve:
• neuroanatomy (brain atrophy, decreased neuronal density, increased plaque formation)
• neurotransmitters (reduced cholinergic transmission, decreased synthesis of dopamine and catecholamines), and
• neurophysiology (reduced cerebral blood flow).
These physiologic processes manifest as alterations in mental status, reflexes, sensation, gait, balance, and sleep. Examples of sleep changes among geriatric patients include decreased sleep efficiency, more frequent awakenings, and more variable sleep duration.3,4 Sleep disorders also may be related to mental disorders and other medical conditions.5 For example, the prevalence of sleep-related respiratory disorders, such as obstructive sleep apnea and central sleep apnea, increases with age.6
Sleep disorders are common among geriatric patients. In a large epidemiologic study of sleep complaints in patients age ≥65, more than one-half of patients had at least 1 sleep complaint (ie, difficulty falling asleep, trouble waking up, early awakening, need for naps, and feeling ill-rested).7 As many as 34% of patients reported symptoms of insomnia. In an analysis of National Ambulatory Medical Survey Data over 6 years, 24.8% to 27.9% of sleep-related medical office visits were attributed to patients age ≥65.8
Pharmacology in aging
Prescribing sedative-hypnotic drugs is not routinely recommended for older patients with a sleep disorder. Geriatric patients, compared with younger patients, are at higher risk of iatrogenic complications because of polypharmacy, comorbidities, relative renal and hepatic insufficiency, and other physiologic changes leading to alterations in drug exposure and metabolism (Table 1).9-12
Aging is associated with changes in body composition, including an increase in total body fat and decrease in lean body mass and total body water. These changes, as well as a prolonged GI transit time, decrease in active gut transporters, decreased blood perfusion, and decrease in plasma proteins such as albumin (because of reduced liver function or malnutrition), may lead to alteration in drug absorption patterns and may increase the volume of distribution for lipophilic drugs. Additionally, the elimination half-life of some drugs may increase with age because of larger volumes of distribution and reduction in hepatic or renal clearance.
The clinical significance of these changes is not well established. Although the process of drug absorption can change with age, the amount of drug absorbed might not be significantly affected. An increase in the volume of distribution and reduction in drug metabolism and clearance might lead to increasing amounts of circulating drug and duration of drug exposure, putting geriatric patients at an increased risk for adverse effects and drug toxicity.9
Among these mechanisms, Dolder et al11 hypothesized that drug metabolism catalyzed by cytochrome P450 (CYP) enzymes and renal excretion may be of greatest concern. Although in vitro studies suggest that concentration of CYP enzymes does not decline with age, in vivo studies have demonstrated reduced CYP activity in geriatric patients.11,12 Theoretically, a reduction in CYP activity would increase the bioavailability of drugs, especially those that are subject to extensive first-pass (ie, hepatic) metabolism, and may lead to a reduction in systemic clearance.
Independent of metabolic changes, geriatric patients are at risk of reduced renal clearance because of age-related changes in glomerular filtration rate. Pharmacodynamic changes might be observed in older patients and could be a concern even in the setting of unaltered pharmacokinetic factors.9 These changes usually require administering smaller drug dosages.
Sedative-hypnotic medications
Sedative-hypnotic agents include several barbiturates, benzodiazepines (BZDs), non-BZD benzodiazepine-receptor agonists (BzRAs), a melatonin-receptor agonist (ie, ramelteon), and an orexin-receptor antagonist (ie, suvorexant).13,14Table 214-29 summarizes selected sedative-hypnotic drugs. Additional drug classes used to treat insomnia include:
• sedating antidepressants (trazodone, amitriptyline, doxepin, mirtazapine)
• antiepileptic drugs (gabapentin, tiagabine)
• atypical antipsychotics (quetiapine, olanzapine).
FDA-approved agents for treating insomnia include amobarbital, butabarbital, pentobarbital, phenobarbital, secobarbital, chloral hydrate, diphenhydramine, doxylamine, doxepin, estazolam, flurazepam, lorazepam, quazepam, temazepam, triazolam, eszopiclone, zaleplon, zolpidem, ramelteon, and suvorexant. Not all of these drugs are recommended for use in geriatric patients. Barbiturates, for example, should be avoided.30
Pharmacokinetic characteristics vary among drugs and drug classes. Choice of pharmacotherapy should account for patient and drug characteristics and the specific sleep complaint. Sleep disorders may be variously characterized as difficulty with sleep initiation, duration, consolidation, or quality.13 Therefore, onset and duration of effect are important drug-related considerations. Sedative-hypnotic drugs with a short time-to-onset may be ideal for patients with sleep-onset insomnia.
The drugs’ duration of effect (eg, presence of active metabolites, long elimination half-life) also must be reviewed. A long elimination half-life may lead to increased drug exposure and unwanted side effects such as residual daytime drowsiness. Despite this, sedative-hypnotic drugs with a longer duration of effect (eg, intermediate- or long-acting drugs) may be best for patients with insomnia defined by difficulty maintaining sleep.
Benzodiazepines vary in their time to onset of effect, rate of elimination, and metabolism.15-21 BZDs that are FDA- approved for use as sedative-hypnotics are listed in Table 2.14-29 These BZDs have different onsets of effect as evidenced by time to achieve maximum plasma concentration (Tmax), ranging from 0.5 hours (flurazepam) to 2 hours (estazolam, quazepam, triazolam). The elimination half-life varies widely among these medications, from 1.5 hours (triazolam) to >100 hours (flurazepam). Flurazepam’s long half-life is attributable to its active major metabolite. Although most BZDs are metabolized hepatically, temazepam is subject to minimal hepatic metabolism.
Benzodiazepine-receptor agonists. There is substantial variation in the pharmacokinetic characteristics of BzRAs.15,16,22-28 There also are differences among the zolpidem dosage forms; sublingual formulations have the shortest onset of effect. Eszopiclone and zaleplon have low protein binding compared with zolpidem. Elimination half-lives vary among drugs with the shortest attributed to zaleplon (1 hour) and longest to eszopiclone (6 hours). All BzRAs are subject to extensive hepatic metabolism.
Ramelteon. Singular in its class, ramelteon is a treatment option for insomnia.29 This drug has a short onset of effect, moderate protein binding, and extensive hepatic metabolism. Ramelteon is primarily excreted in the urine as its metabolites, and the drug half-life is relatively short.
Suvorexant is the latest addition to the sedative-hypnotic armamentarium, approved by the FDA in August 2014 for difficulty with sleep onset and/or sleep maintenance.14 As an orexin-receptor antagonist, suvorexant represents a novel pharmacologic class. Suvorexant exhibits moderately rapid absorption with time to peak concentration ranging from 30 minutes to 6 hours in fasting conditions; absorption is delayed when taken with or soon after a meal. The drug is highly protein bound and extensively metabolized, primarily through CYP3A. The manufacturer recommends dose reduction (5 mg at bedtime) in patients taking moderate CYP3A inhibitors and avoiding suvorexant in patients taking strong CYP3A inhibitors. Suvorexant is primarily excreted through feces and the mean half-life is relatively long.
Considering these characteristics and age-related physiologic changes, the practitioner should be concerned about drugs that undergo extensive hepatic metabolism. Age-related reductions in CYP activity may lead to an increase in drug bioavailability and a decrease in the systemic clearance,11 which might be associated with an increase in elimination half-life and duration of action. Dosage adjustments are recommended for several BZDs (lower initial and maximum dosages for most agents) and BzRAs.17-28 No dosage adjustments for ramelteon or suvorexant in geriatric patients have been specified14,29; the manufacturers for both products assert that no differences in safety and efficacy have been observed between older and younger adult patients.
Alternative and complementary medications
Several non-prescription products, including over-the-counter drugs (eg, diphenhy-dramine, doxylamine) and herbal therapies (eg, melatonin, valerian), are used for their sedative-hypnotic properties. There is a lack of evidence supporting using diphenhydra-mine in patients with chronic insomnia, and tolerance to its hypnotic effect has been reported with repeated use.31 Concerns about anticholinergic toxicity and CNS depression limit its use in geriatric patients. Among herbal therapies, melatonin may have the strongest evidence for its ability to alleviate sleep disorders in geriatric patients32; however, meta-analyses have demonstrated small effects of melatonin on sleep latency and minimal differences in wake time after sleep onset and total sleep time.13
Clinical practice guidelines
Non-pharmacotherapeutic interventions, such as behavioral (eg, sleep hygiene measures) and psychological therapy, are recommended for initial management of sleep disorders in geriatric patients.13,33 In conjunction, the American Medical Directors Association (AMDA) recommends address ing underlying causes and exacerbating factors (eg, medical condition or medication).33 The AMDA recommends avoiding long-term pharmacotherapy and advises caution with BZD-hypnotic drugs, tricyclic antidepressants, and antihistamines. The American Academy of Sleep Medicine (AASM) recommends an initial treatment period of 2 to 4 weeks, followed by re-evaluation of continued need for treatment.13 The AASM recommends short- or intermediate-acting BzRAs or ramelteon for initial pharmacologic management of primary insomnias and insomnias comorbid with other conditions. The AASM also recommends specific dosages of BzRAs and BZDs for geriatric patients, which coincide with manufacturer-recommended dosages (Table 2).14-29
Barbiturates, chloral hydrate, and non-barbiturate, non-BZD drugs such as meprobamate are not recommended because of potential significant adverse effects and tolerance/dependence, and low therapeutic index. The AASM advises caution when using prescription drugs off-label for insomnia (eg, antidepressants, antiepileptics, antipsychotics) and recommends avoiding them, if possible, because of limited evidence supporting their use.13
Safety concerns
Two commonly used references contain recommendations for sedative-hypnotic medication use in geriatric patients.30,34 According to Gallagher et al’s34 Screening Tool of Older Person’s Prescriptions (STOPP), long-term (>1 month) use of long-acting BZDs (eg, flurazepam, diazepam) and prolonged use (>1 week) of first-generation antihistamines (eg, diphenhydramine, doxylamine) should be avoided in patients age ≥65 because of the risk of sedation, confusion, and anticholinergic side effects. STOPP recognizes that any use of BZDs, neuroleptics, or first-generation antihistamines may contribute to postural imbalance; therefore these agents are not recommended in older patients at risk for falls.
In the 2012 American Geriatrics Society (AGS) Beers Criteria, the AGS recommends avoiding barbiturates in older adults because of the high rate of physical dependence, tolerance to sleep effects, and overdose risk at low dosages.30 The AGS also recommends avoiding BZDs, stating that older adults have increased sensitivity to these agents and are at an increased risk of cognitive impairment, delirium, falls, fractures, and motor vehicle accidents when taking these drugs. Non-BZD BzRAs also should not be prescribed to patients with a history of falls or fractures, unless safer alternatives are not available.
The FDA has issued several advisory reports regarding sedative-hypnotic drugs. In 2007, all manufacturers of sedative-hypnotic drugs were required to modify their product labeling to include stronger language about potential risks.35 Among these changes, warnings for anaphylaxis and complex sleep-related behaviors were added. Also, the FDA requested that manufacturers of sedative-hypnotic drugs develop and provide patient medication guides, advising consumers on the potential risks and precautions associated with these drugs. More recently, the FDA announced changes to dosing recommendations for zolpidem-containing products because of the risk of impaired mental alertness36; manufacturers were required to lower the recommended dosages for each product.
Manufacturers of FDA-approved sedative-hypnotic drugs urge caution when prescribing these medications for geriatric patients, citing the potential for increased sensitivity, manifesting as marked excitement, depression, or confusion (eg, barbiturates), and greater risk for dosage-related adverse effects (eg, oversedation, dizziness, confusion, impaired psychomotor performance, ataxia).17-29
Use in clinical practice
Several variables should be considered when evaluating appropriateness of pharmacotherapy, including characteristics of the drug and the patient. Geriatric patients may be prone to comorbidities resulting from age-related physiologic changes. These diseases may be confounding (ie, contributing to sleep disorders); examples include medical illnesses, such as hyperthyroidism and arthritis, and psychiatric illnesses, such as depression and anxiety.37 Other conditions, such as renal and hepatic dysfunction, may lead to alteration in drug exposure. These conditions should be assessed through routine renal function tests (eg, serum creatinine and glomerular filtration rate) and liver function tests (eg, serum albumin and liver transaminases).
Multiple comorbidities suggest a higher likelihood of polypharmacy, leading to other drug-related issues (eg, drug-drug interactions). Although these issues may guide therapy by restricting medication options, their potential contribution to the underlying sleep complaints should be considered.37 Several drugs commonly used by geriatric patients may affect wakefulness (eg, analgesics, antidepressants, and antihypertensives [sedating], and thyroid hormones, corticosteroids, and CNS stimulants [alerting]).
CASE CONTINUED
In Mr. R’s case, zaleplon was initiated at 10 mg/d. Because of his age and the nature of his sleep disorder, the choice of sedative-hypnotic was suitable; however, the prescribed dosage was inappropriate. The sluggishness Mr. R experienced likely was a manifestation of increased exposure to the drug. According to manufacturer and AASM recommendations, a more appropriate dosage is 5 mg/d.13,23 Mr. R’s medical history and current medications, and his hepatic and renal function, should be assessed. If Mr. R continues to have issues with sleep initiation, zaleplon, 5 mg at bedtime, should be considered.
Related Resources
• Institute for Safe Medication Practices. www.ismp.org.
• MedWatch: The FDA Safety Information and Adverse Event Reporting Program. www.fda.gov/Safety/MedWatch/default.htm.
Drug Brand Names
Amitriptyline • Elavil Mirtazapine • Remeron
Amobarbital • Amytal Olanzapine • Zyprexa
Butabarbital • Butisol Pentobarbital • Nembutal
Chloral hydrate • Somnote Phenobarbital • Luminal
Diazepam • Valium Quazepam • Doral
Diphenhydramine • Benadryl, others Quetiapine • Seroquel
Doxepin • Silenor Ramelteon • Rozerem
Doxylamine • Unisom, others Secobarbital • Seconal
Estazolam • ProSom Suvorexant • Belsomra
Eszopiclone • Lunesta Temazepam • Restoril
Flurazepam • Dalmane Tiagabine • Gabitril
Gabapentin • Neurontin, Trazodone • Desyrel
Gralise, Horizant Zaleplon • Sonata
Lorazepam • Ativan Zolpidem • Ambien, Edluar,
Meprobamate • Equanil Intermezzo, Zolpimist
Acknowledgement
Vicki L. Ellingrod, PharmD, FCCP, is the series editor of Savvy Psychopharmacology.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. National Center for Health Statistics. Health, United States, 2012, with special feature on emergency care. http://www. cdc.gov/nchs/data/hus/hus12.pdf. Published May 2013. Accessed August 22, 2014.
2. Bronstein AC, Spyker DA, Cantilena LR Jr, et al. 2011 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 29th annual report. Clin Toxicol (Phila). 2012;50(10): 911-1164.
3. Inouye SK. Neuropsychiatric aspects of aging. In: Goldman L, Schafer AI, eds. Goldman’s cecil medicine. 24th ed. Philadelphia, PA: Elsevier Saunders; 2011:114-116.
4. Diagnostic and statistical manual of mental disorders, 4th ed. Washington, DC: American Psychiatric Association; 1994.
5. American Psychiatric Association. Highlights of changes from DSM-IV-TR to DSM-5. http://www.psychiatry. org/File%20Library/Practice/DSM/DSM-5/Changes-from-DSM-IV-TR--to-DSM-5.pdf. 2013. Accessed August 22, 2014.
6. Edwards BA, O’Driscoll DM, Ali A, et al. Aging and sleep: physiology and pathophysiology. Semin Respir Crit Care Med. 2010;31(5):618-633.
7. Foley DJ, Monjan AA, Brown SL, et al. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18(6):425-432.
8. Morlock RJ, Tan M, Mitchell DY. Patient characteristics and patterns of drug use for sleep complaints in the United States: analysis of National Ambulatory Medical Survey Data, 1997-2002. Clin Ther. 2006;28(7):1044-1053.
9. Diasio RB. Principles of drug therapy. In: Goldman L, Schafer AI, eds. Goldman’s Cecil medicine. 24th ed. Philadelphia, PA: Elsevier Saunders; 2011:124-132.
10. Hilmer SN, Ford GA. General principles of pharmacology. In: Halter JB, Ouslander JG, Tinetti ME, et al, eds. Hazzard’s geriatric medicine and gerontology. 6th ed. New York, NY: McGraw-Hill; 2009:103-122.
11. Dolder C, Nelson M, McKinsey J. Use of non-benzodiazepine hypnotics in the elderly: are all agents the same? CNS Drugs. 2007;21(5):389-405.
12. Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009;41(2):67-76.
13. Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487-504.
14. Belsomra [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.; 2014.
15. Micromedex Healthcare Series. Greenwood Village, CO: Thomson Healthcare. http://micromedex.com. Accessed August 22, 2014.
16. Lexicomp. St. Louis, MO: Wolters Kluwer Health. http:// www.lexi.com. Accessed August 22, 2014.
17. Estazolam [package insert]. Corona, CA: Watson Pharma, Inc; 2008.
18. Flurazepam [package insert]. Eatontown, NJ: West-Ward Pharmaceutical Corp; 2010.
19. Doral [package insert]. Las Vegas, NV: Nuro Pharma, Inc; 2013.
20. Restoril [package insert]. Hazelwood, MO: Mallinckrodt Inc; 2010.
21. Halcion [package insert]. New York, NY: Pharmacia & Upjohn Co; 2013.
22. Lunesta [package insert]. Marlborough, MA: Sunovion Pharmaceuticals Inc; 2012.
23. Sonata [package insert]. New York, NY: Pfizer Inc; 2013.
24. Ambien [package insert]. Bridgewater, NJ: Sanofi-Aventis; 2013.
25. Ambien CR [package insert]. Bridgewater, NJ: Sanofi- Aventis; 2013.
26. Edluar [package insert]. Somerset, NJ: Meda Pharmaceuticals Inc; 2009.
27. Intermezzo [package insert]. Point Richmond, CA: Transcept Pharmaceuticals, Inc; 2011.
28. Zolpimist [package insert]. Richmond, VA: ECR Pharmaceuticals; 2013.
29. Rozerem [package insert]. Deerfield, IL: Takeda Pharmaceuticals America, Inc; 2010.
30. The American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
31. Kirkwood CK, Melton ST. Insomnia, drowsiness, and fatigue. In: Krinsky DL, Berardi RR, Ferreri SP, et al, eds. Handbook of nonprescription drugs: an interactive approach to self-care. 17th ed. Washington, DC: American Pharmacists Association; 2012:867-884.
32. Insomnia. In: Natural Standard. Somerville, MA: Natural Standard. https://naturalmedicines.therapeuticresearch. com/databases/medical-conditions/i/insomnia.aspx. Accessed August 22, 2014.
33. American Medical Directors Association. Clinical practice guideline: sleep disorders. Columbia, MD: American Medical Directors Association; 2006.
34. Gallagher P, Ryan C, Byrne S, et al. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72-83.
35. Food and Drug Administration. FDA requests label change for all sleep disorder drug products. http://www.fda. gov/newsevents/newsroom/pressannouncements/2007/ ucm108868.htm. Published March 14, 2007. Accessed August 22, 2014.
36. Food and Drug Administration. FDA drug safety communication: risk of next-morning impairment after use of insomnia drugs; FDA requires lower recommended doses for certain drugs containing zolpidem (Ambien, Ambien CR, Edluar, and Zolpimist). http://www.fda.gov/drugs/ drugsafety/ucm334033.htm. Published January 10, 2013. Accessed August 22, 2014.
37. Cohen-Zion M, Ancoli-Israel S. Sleep disorders. In: Hazzard’s geriatric medicine and gerontology. 6th ed. New York, NY: McGraw-Hill; 2009:671-682.
Mr. R, 75, is having difficulty sleeping. When he goes to bed, he lies there for what seems like forever, unable to fall asleep. He feels “so tired” and ends up taking naps during the day, but he cannot break this cycle. He has tried using over-the-counter products with little relief.
Mr. R’s primary care physician prescribes zaleplon, 10 mg/d, and asks him to call the clinic in 2 weeks to discuss his progress. He takes zaleplon as directed for several nights and begins to feel “sluggish” during the day, both mentally and physically, despite reporting an increase in the overall amount of sleep at night.
Sedative-hypnotic drugs are among the most commonly used medications in the United States. Use of these drugs, as well as anxiolytics, has increased from 2.8% between 1988 and 1994 to 4.7% between 2007 and 2010, according to the Department of Health and Human Services.1 In 2011, drugs categorized as sedative-hypnotics or antipsychotics were involved in 6.1% of all human exposures identified in the American Association of Poison Control Centers’ National Poison Data System.2 Therefore, an understanding of clinical and pharmacological variables related to safe and effective use is important for clinicians prescribing and monitoring therapy with these agents.
Neuropsychiatric disorders are prevalent among geriatric patients and are associated with age-related physiologic changes in the CNS.3 Such changes involve:
• neuroanatomy (brain atrophy, decreased neuronal density, increased plaque formation)
• neurotransmitters (reduced cholinergic transmission, decreased synthesis of dopamine and catecholamines), and
• neurophysiology (reduced cerebral blood flow).
These physiologic processes manifest as alterations in mental status, reflexes, sensation, gait, balance, and sleep. Examples of sleep changes among geriatric patients include decreased sleep efficiency, more frequent awakenings, and more variable sleep duration.3,4 Sleep disorders also may be related to mental disorders and other medical conditions.5 For example, the prevalence of sleep-related respiratory disorders, such as obstructive sleep apnea and central sleep apnea, increases with age.6
Sleep disorders are common among geriatric patients. In a large epidemiologic study of sleep complaints in patients age ≥65, more than one-half of patients had at least 1 sleep complaint (ie, difficulty falling asleep, trouble waking up, early awakening, need for naps, and feeling ill-rested).7 As many as 34% of patients reported symptoms of insomnia. In an analysis of National Ambulatory Medical Survey Data over 6 years, 24.8% to 27.9% of sleep-related medical office visits were attributed to patients age ≥65.8
Pharmacology in aging
Prescribing sedative-hypnotic drugs is not routinely recommended for older patients with a sleep disorder. Geriatric patients, compared with younger patients, are at higher risk of iatrogenic complications because of polypharmacy, comorbidities, relative renal and hepatic insufficiency, and other physiologic changes leading to alterations in drug exposure and metabolism (Table 1).9-12
Aging is associated with changes in body composition, including an increase in total body fat and decrease in lean body mass and total body water. These changes, as well as a prolonged GI transit time, decrease in active gut transporters, decreased blood perfusion, and decrease in plasma proteins such as albumin (because of reduced liver function or malnutrition), may lead to alteration in drug absorption patterns and may increase the volume of distribution for lipophilic drugs. Additionally, the elimination half-life of some drugs may increase with age because of larger volumes of distribution and reduction in hepatic or renal clearance.
The clinical significance of these changes is not well established. Although the process of drug absorption can change with age, the amount of drug absorbed might not be significantly affected. An increase in the volume of distribution and reduction in drug metabolism and clearance might lead to increasing amounts of circulating drug and duration of drug exposure, putting geriatric patients at an increased risk for adverse effects and drug toxicity.9
Among these mechanisms, Dolder et al11 hypothesized that drug metabolism catalyzed by cytochrome P450 (CYP) enzymes and renal excretion may be of greatest concern. Although in vitro studies suggest that concentration of CYP enzymes does not decline with age, in vivo studies have demonstrated reduced CYP activity in geriatric patients.11,12 Theoretically, a reduction in CYP activity would increase the bioavailability of drugs, especially those that are subject to extensive first-pass (ie, hepatic) metabolism, and may lead to a reduction in systemic clearance.
Independent of metabolic changes, geriatric patients are at risk of reduced renal clearance because of age-related changes in glomerular filtration rate. Pharmacodynamic changes might be observed in older patients and could be a concern even in the setting of unaltered pharmacokinetic factors.9 These changes usually require administering smaller drug dosages.
Sedative-hypnotic medications
Sedative-hypnotic agents include several barbiturates, benzodiazepines (BZDs), non-BZD benzodiazepine-receptor agonists (BzRAs), a melatonin-receptor agonist (ie, ramelteon), and an orexin-receptor antagonist (ie, suvorexant).13,14Table 214-29 summarizes selected sedative-hypnotic drugs. Additional drug classes used to treat insomnia include:
• sedating antidepressants (trazodone, amitriptyline, doxepin, mirtazapine)
• antiepileptic drugs (gabapentin, tiagabine)
• atypical antipsychotics (quetiapine, olanzapine).
FDA-approved agents for treating insomnia include amobarbital, butabarbital, pentobarbital, phenobarbital, secobarbital, chloral hydrate, diphenhydramine, doxylamine, doxepin, estazolam, flurazepam, lorazepam, quazepam, temazepam, triazolam, eszopiclone, zaleplon, zolpidem, ramelteon, and suvorexant. Not all of these drugs are recommended for use in geriatric patients. Barbiturates, for example, should be avoided.30
Pharmacokinetic characteristics vary among drugs and drug classes. Choice of pharmacotherapy should account for patient and drug characteristics and the specific sleep complaint. Sleep disorders may be variously characterized as difficulty with sleep initiation, duration, consolidation, or quality.13 Therefore, onset and duration of effect are important drug-related considerations. Sedative-hypnotic drugs with a short time-to-onset may be ideal for patients with sleep-onset insomnia.
The drugs’ duration of effect (eg, presence of active metabolites, long elimination half-life) also must be reviewed. A long elimination half-life may lead to increased drug exposure and unwanted side effects such as residual daytime drowsiness. Despite this, sedative-hypnotic drugs with a longer duration of effect (eg, intermediate- or long-acting drugs) may be best for patients with insomnia defined by difficulty maintaining sleep.
Benzodiazepines vary in their time to onset of effect, rate of elimination, and metabolism.15-21 BZDs that are FDA- approved for use as sedative-hypnotics are listed in Table 2.14-29 These BZDs have different onsets of effect as evidenced by time to achieve maximum plasma concentration (Tmax), ranging from 0.5 hours (flurazepam) to 2 hours (estazolam, quazepam, triazolam). The elimination half-life varies widely among these medications, from 1.5 hours (triazolam) to >100 hours (flurazepam). Flurazepam’s long half-life is attributable to its active major metabolite. Although most BZDs are metabolized hepatically, temazepam is subject to minimal hepatic metabolism.
Benzodiazepine-receptor agonists. There is substantial variation in the pharmacokinetic characteristics of BzRAs.15,16,22-28 There also are differences among the zolpidem dosage forms; sublingual formulations have the shortest onset of effect. Eszopiclone and zaleplon have low protein binding compared with zolpidem. Elimination half-lives vary among drugs with the shortest attributed to zaleplon (1 hour) and longest to eszopiclone (6 hours). All BzRAs are subject to extensive hepatic metabolism.
Ramelteon. Singular in its class, ramelteon is a treatment option for insomnia.29 This drug has a short onset of effect, moderate protein binding, and extensive hepatic metabolism. Ramelteon is primarily excreted in the urine as its metabolites, and the drug half-life is relatively short.
Suvorexant is the latest addition to the sedative-hypnotic armamentarium, approved by the FDA in August 2014 for difficulty with sleep onset and/or sleep maintenance.14 As an orexin-receptor antagonist, suvorexant represents a novel pharmacologic class. Suvorexant exhibits moderately rapid absorption with time to peak concentration ranging from 30 minutes to 6 hours in fasting conditions; absorption is delayed when taken with or soon after a meal. The drug is highly protein bound and extensively metabolized, primarily through CYP3A. The manufacturer recommends dose reduction (5 mg at bedtime) in patients taking moderate CYP3A inhibitors and avoiding suvorexant in patients taking strong CYP3A inhibitors. Suvorexant is primarily excreted through feces and the mean half-life is relatively long.
Considering these characteristics and age-related physiologic changes, the practitioner should be concerned about drugs that undergo extensive hepatic metabolism. Age-related reductions in CYP activity may lead to an increase in drug bioavailability and a decrease in the systemic clearance,11 which might be associated with an increase in elimination half-life and duration of action. Dosage adjustments are recommended for several BZDs (lower initial and maximum dosages for most agents) and BzRAs.17-28 No dosage adjustments for ramelteon or suvorexant in geriatric patients have been specified14,29; the manufacturers for both products assert that no differences in safety and efficacy have been observed between older and younger adult patients.
Alternative and complementary medications
Several non-prescription products, including over-the-counter drugs (eg, diphenhy-dramine, doxylamine) and herbal therapies (eg, melatonin, valerian), are used for their sedative-hypnotic properties. There is a lack of evidence supporting using diphenhydra-mine in patients with chronic insomnia, and tolerance to its hypnotic effect has been reported with repeated use.31 Concerns about anticholinergic toxicity and CNS depression limit its use in geriatric patients. Among herbal therapies, melatonin may have the strongest evidence for its ability to alleviate sleep disorders in geriatric patients32; however, meta-analyses have demonstrated small effects of melatonin on sleep latency and minimal differences in wake time after sleep onset and total sleep time.13
Clinical practice guidelines
Non-pharmacotherapeutic interventions, such as behavioral (eg, sleep hygiene measures) and psychological therapy, are recommended for initial management of sleep disorders in geriatric patients.13,33 In conjunction, the American Medical Directors Association (AMDA) recommends address ing underlying causes and exacerbating factors (eg, medical condition or medication).33 The AMDA recommends avoiding long-term pharmacotherapy and advises caution with BZD-hypnotic drugs, tricyclic antidepressants, and antihistamines. The American Academy of Sleep Medicine (AASM) recommends an initial treatment period of 2 to 4 weeks, followed by re-evaluation of continued need for treatment.13 The AASM recommends short- or intermediate-acting BzRAs or ramelteon for initial pharmacologic management of primary insomnias and insomnias comorbid with other conditions. The AASM also recommends specific dosages of BzRAs and BZDs for geriatric patients, which coincide with manufacturer-recommended dosages (Table 2).14-29
Barbiturates, chloral hydrate, and non-barbiturate, non-BZD drugs such as meprobamate are not recommended because of potential significant adverse effects and tolerance/dependence, and low therapeutic index. The AASM advises caution when using prescription drugs off-label for insomnia (eg, antidepressants, antiepileptics, antipsychotics) and recommends avoiding them, if possible, because of limited evidence supporting their use.13
Safety concerns
Two commonly used references contain recommendations for sedative-hypnotic medication use in geriatric patients.30,34 According to Gallagher et al’s34 Screening Tool of Older Person’s Prescriptions (STOPP), long-term (>1 month) use of long-acting BZDs (eg, flurazepam, diazepam) and prolonged use (>1 week) of first-generation antihistamines (eg, diphenhydramine, doxylamine) should be avoided in patients age ≥65 because of the risk of sedation, confusion, and anticholinergic side effects. STOPP recognizes that any use of BZDs, neuroleptics, or first-generation antihistamines may contribute to postural imbalance; therefore these agents are not recommended in older patients at risk for falls.
In the 2012 American Geriatrics Society (AGS) Beers Criteria, the AGS recommends avoiding barbiturates in older adults because of the high rate of physical dependence, tolerance to sleep effects, and overdose risk at low dosages.30 The AGS also recommends avoiding BZDs, stating that older adults have increased sensitivity to these agents and are at an increased risk of cognitive impairment, delirium, falls, fractures, and motor vehicle accidents when taking these drugs. Non-BZD BzRAs also should not be prescribed to patients with a history of falls or fractures, unless safer alternatives are not available.
The FDA has issued several advisory reports regarding sedative-hypnotic drugs. In 2007, all manufacturers of sedative-hypnotic drugs were required to modify their product labeling to include stronger language about potential risks.35 Among these changes, warnings for anaphylaxis and complex sleep-related behaviors were added. Also, the FDA requested that manufacturers of sedative-hypnotic drugs develop and provide patient medication guides, advising consumers on the potential risks and precautions associated with these drugs. More recently, the FDA announced changes to dosing recommendations for zolpidem-containing products because of the risk of impaired mental alertness36; manufacturers were required to lower the recommended dosages for each product.
Manufacturers of FDA-approved sedative-hypnotic drugs urge caution when prescribing these medications for geriatric patients, citing the potential for increased sensitivity, manifesting as marked excitement, depression, or confusion (eg, barbiturates), and greater risk for dosage-related adverse effects (eg, oversedation, dizziness, confusion, impaired psychomotor performance, ataxia).17-29
Use in clinical practice
Several variables should be considered when evaluating appropriateness of pharmacotherapy, including characteristics of the drug and the patient. Geriatric patients may be prone to comorbidities resulting from age-related physiologic changes. These diseases may be confounding (ie, contributing to sleep disorders); examples include medical illnesses, such as hyperthyroidism and arthritis, and psychiatric illnesses, such as depression and anxiety.37 Other conditions, such as renal and hepatic dysfunction, may lead to alteration in drug exposure. These conditions should be assessed through routine renal function tests (eg, serum creatinine and glomerular filtration rate) and liver function tests (eg, serum albumin and liver transaminases).
Multiple comorbidities suggest a higher likelihood of polypharmacy, leading to other drug-related issues (eg, drug-drug interactions). Although these issues may guide therapy by restricting medication options, their potential contribution to the underlying sleep complaints should be considered.37 Several drugs commonly used by geriatric patients may affect wakefulness (eg, analgesics, antidepressants, and antihypertensives [sedating], and thyroid hormones, corticosteroids, and CNS stimulants [alerting]).
CASE CONTINUED
In Mr. R’s case, zaleplon was initiated at 10 mg/d. Because of his age and the nature of his sleep disorder, the choice of sedative-hypnotic was suitable; however, the prescribed dosage was inappropriate. The sluggishness Mr. R experienced likely was a manifestation of increased exposure to the drug. According to manufacturer and AASM recommendations, a more appropriate dosage is 5 mg/d.13,23 Mr. R’s medical history and current medications, and his hepatic and renal function, should be assessed. If Mr. R continues to have issues with sleep initiation, zaleplon, 5 mg at bedtime, should be considered.
Related Resources
• Institute for Safe Medication Practices. www.ismp.org.
• MedWatch: The FDA Safety Information and Adverse Event Reporting Program. www.fda.gov/Safety/MedWatch/default.htm.
Drug Brand Names
Amitriptyline • Elavil Mirtazapine • Remeron
Amobarbital • Amytal Olanzapine • Zyprexa
Butabarbital • Butisol Pentobarbital • Nembutal
Chloral hydrate • Somnote Phenobarbital • Luminal
Diazepam • Valium Quazepam • Doral
Diphenhydramine • Benadryl, others Quetiapine • Seroquel
Doxepin • Silenor Ramelteon • Rozerem
Doxylamine • Unisom, others Secobarbital • Seconal
Estazolam • ProSom Suvorexant • Belsomra
Eszopiclone • Lunesta Temazepam • Restoril
Flurazepam • Dalmane Tiagabine • Gabitril
Gabapentin • Neurontin, Trazodone • Desyrel
Gralise, Horizant Zaleplon • Sonata
Lorazepam • Ativan Zolpidem • Ambien, Edluar,
Meprobamate • Equanil Intermezzo, Zolpimist
Acknowledgement
Vicki L. Ellingrod, PharmD, FCCP, is the series editor of Savvy Psychopharmacology.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Mr. R, 75, is having difficulty sleeping. When he goes to bed, he lies there for what seems like forever, unable to fall asleep. He feels “so tired” and ends up taking naps during the day, but he cannot break this cycle. He has tried using over-the-counter products with little relief.
Mr. R’s primary care physician prescribes zaleplon, 10 mg/d, and asks him to call the clinic in 2 weeks to discuss his progress. He takes zaleplon as directed for several nights and begins to feel “sluggish” during the day, both mentally and physically, despite reporting an increase in the overall amount of sleep at night.
Sedative-hypnotic drugs are among the most commonly used medications in the United States. Use of these drugs, as well as anxiolytics, has increased from 2.8% between 1988 and 1994 to 4.7% between 2007 and 2010, according to the Department of Health and Human Services.1 In 2011, drugs categorized as sedative-hypnotics or antipsychotics were involved in 6.1% of all human exposures identified in the American Association of Poison Control Centers’ National Poison Data System.2 Therefore, an understanding of clinical and pharmacological variables related to safe and effective use is important for clinicians prescribing and monitoring therapy with these agents.
Neuropsychiatric disorders are prevalent among geriatric patients and are associated with age-related physiologic changes in the CNS.3 Such changes involve:
• neuroanatomy (brain atrophy, decreased neuronal density, increased plaque formation)
• neurotransmitters (reduced cholinergic transmission, decreased synthesis of dopamine and catecholamines), and
• neurophysiology (reduced cerebral blood flow).
These physiologic processes manifest as alterations in mental status, reflexes, sensation, gait, balance, and sleep. Examples of sleep changes among geriatric patients include decreased sleep efficiency, more frequent awakenings, and more variable sleep duration.3,4 Sleep disorders also may be related to mental disorders and other medical conditions.5 For example, the prevalence of sleep-related respiratory disorders, such as obstructive sleep apnea and central sleep apnea, increases with age.6
Sleep disorders are common among geriatric patients. In a large epidemiologic study of sleep complaints in patients age ≥65, more than one-half of patients had at least 1 sleep complaint (ie, difficulty falling asleep, trouble waking up, early awakening, need for naps, and feeling ill-rested).7 As many as 34% of patients reported symptoms of insomnia. In an analysis of National Ambulatory Medical Survey Data over 6 years, 24.8% to 27.9% of sleep-related medical office visits were attributed to patients age ≥65.8
Pharmacology in aging
Prescribing sedative-hypnotic drugs is not routinely recommended for older patients with a sleep disorder. Geriatric patients, compared with younger patients, are at higher risk of iatrogenic complications because of polypharmacy, comorbidities, relative renal and hepatic insufficiency, and other physiologic changes leading to alterations in drug exposure and metabolism (Table 1).9-12
Aging is associated with changes in body composition, including an increase in total body fat and decrease in lean body mass and total body water. These changes, as well as a prolonged GI transit time, decrease in active gut transporters, decreased blood perfusion, and decrease in plasma proteins such as albumin (because of reduced liver function or malnutrition), may lead to alteration in drug absorption patterns and may increase the volume of distribution for lipophilic drugs. Additionally, the elimination half-life of some drugs may increase with age because of larger volumes of distribution and reduction in hepatic or renal clearance.
The clinical significance of these changes is not well established. Although the process of drug absorption can change with age, the amount of drug absorbed might not be significantly affected. An increase in the volume of distribution and reduction in drug metabolism and clearance might lead to increasing amounts of circulating drug and duration of drug exposure, putting geriatric patients at an increased risk for adverse effects and drug toxicity.9
Among these mechanisms, Dolder et al11 hypothesized that drug metabolism catalyzed by cytochrome P450 (CYP) enzymes and renal excretion may be of greatest concern. Although in vitro studies suggest that concentration of CYP enzymes does not decline with age, in vivo studies have demonstrated reduced CYP activity in geriatric patients.11,12 Theoretically, a reduction in CYP activity would increase the bioavailability of drugs, especially those that are subject to extensive first-pass (ie, hepatic) metabolism, and may lead to a reduction in systemic clearance.
Independent of metabolic changes, geriatric patients are at risk of reduced renal clearance because of age-related changes in glomerular filtration rate. Pharmacodynamic changes might be observed in older patients and could be a concern even in the setting of unaltered pharmacokinetic factors.9 These changes usually require administering smaller drug dosages.
Sedative-hypnotic medications
Sedative-hypnotic agents include several barbiturates, benzodiazepines (BZDs), non-BZD benzodiazepine-receptor agonists (BzRAs), a melatonin-receptor agonist (ie, ramelteon), and an orexin-receptor antagonist (ie, suvorexant).13,14Table 214-29 summarizes selected sedative-hypnotic drugs. Additional drug classes used to treat insomnia include:
• sedating antidepressants (trazodone, amitriptyline, doxepin, mirtazapine)
• antiepileptic drugs (gabapentin, tiagabine)
• atypical antipsychotics (quetiapine, olanzapine).
FDA-approved agents for treating insomnia include amobarbital, butabarbital, pentobarbital, phenobarbital, secobarbital, chloral hydrate, diphenhydramine, doxylamine, doxepin, estazolam, flurazepam, lorazepam, quazepam, temazepam, triazolam, eszopiclone, zaleplon, zolpidem, ramelteon, and suvorexant. Not all of these drugs are recommended for use in geriatric patients. Barbiturates, for example, should be avoided.30
Pharmacokinetic characteristics vary among drugs and drug classes. Choice of pharmacotherapy should account for patient and drug characteristics and the specific sleep complaint. Sleep disorders may be variously characterized as difficulty with sleep initiation, duration, consolidation, or quality.13 Therefore, onset and duration of effect are important drug-related considerations. Sedative-hypnotic drugs with a short time-to-onset may be ideal for patients with sleep-onset insomnia.
The drugs’ duration of effect (eg, presence of active metabolites, long elimination half-life) also must be reviewed. A long elimination half-life may lead to increased drug exposure and unwanted side effects such as residual daytime drowsiness. Despite this, sedative-hypnotic drugs with a longer duration of effect (eg, intermediate- or long-acting drugs) may be best for patients with insomnia defined by difficulty maintaining sleep.
Benzodiazepines vary in their time to onset of effect, rate of elimination, and metabolism.15-21 BZDs that are FDA- approved for use as sedative-hypnotics are listed in Table 2.14-29 These BZDs have different onsets of effect as evidenced by time to achieve maximum plasma concentration (Tmax), ranging from 0.5 hours (flurazepam) to 2 hours (estazolam, quazepam, triazolam). The elimination half-life varies widely among these medications, from 1.5 hours (triazolam) to >100 hours (flurazepam). Flurazepam’s long half-life is attributable to its active major metabolite. Although most BZDs are metabolized hepatically, temazepam is subject to minimal hepatic metabolism.
Benzodiazepine-receptor agonists. There is substantial variation in the pharmacokinetic characteristics of BzRAs.15,16,22-28 There also are differences among the zolpidem dosage forms; sublingual formulations have the shortest onset of effect. Eszopiclone and zaleplon have low protein binding compared with zolpidem. Elimination half-lives vary among drugs with the shortest attributed to zaleplon (1 hour) and longest to eszopiclone (6 hours). All BzRAs are subject to extensive hepatic metabolism.
Ramelteon. Singular in its class, ramelteon is a treatment option for insomnia.29 This drug has a short onset of effect, moderate protein binding, and extensive hepatic metabolism. Ramelteon is primarily excreted in the urine as its metabolites, and the drug half-life is relatively short.
Suvorexant is the latest addition to the sedative-hypnotic armamentarium, approved by the FDA in August 2014 for difficulty with sleep onset and/or sleep maintenance.14 As an orexin-receptor antagonist, suvorexant represents a novel pharmacologic class. Suvorexant exhibits moderately rapid absorption with time to peak concentration ranging from 30 minutes to 6 hours in fasting conditions; absorption is delayed when taken with or soon after a meal. The drug is highly protein bound and extensively metabolized, primarily through CYP3A. The manufacturer recommends dose reduction (5 mg at bedtime) in patients taking moderate CYP3A inhibitors and avoiding suvorexant in patients taking strong CYP3A inhibitors. Suvorexant is primarily excreted through feces and the mean half-life is relatively long.
Considering these characteristics and age-related physiologic changes, the practitioner should be concerned about drugs that undergo extensive hepatic metabolism. Age-related reductions in CYP activity may lead to an increase in drug bioavailability and a decrease in the systemic clearance,11 which might be associated with an increase in elimination half-life and duration of action. Dosage adjustments are recommended for several BZDs (lower initial and maximum dosages for most agents) and BzRAs.17-28 No dosage adjustments for ramelteon or suvorexant in geriatric patients have been specified14,29; the manufacturers for both products assert that no differences in safety and efficacy have been observed between older and younger adult patients.
Alternative and complementary medications
Several non-prescription products, including over-the-counter drugs (eg, diphenhy-dramine, doxylamine) and herbal therapies (eg, melatonin, valerian), are used for their sedative-hypnotic properties. There is a lack of evidence supporting using diphenhydra-mine in patients with chronic insomnia, and tolerance to its hypnotic effect has been reported with repeated use.31 Concerns about anticholinergic toxicity and CNS depression limit its use in geriatric patients. Among herbal therapies, melatonin may have the strongest evidence for its ability to alleviate sleep disorders in geriatric patients32; however, meta-analyses have demonstrated small effects of melatonin on sleep latency and minimal differences in wake time after sleep onset and total sleep time.13
Clinical practice guidelines
Non-pharmacotherapeutic interventions, such as behavioral (eg, sleep hygiene measures) and psychological therapy, are recommended for initial management of sleep disorders in geriatric patients.13,33 In conjunction, the American Medical Directors Association (AMDA) recommends address ing underlying causes and exacerbating factors (eg, medical condition or medication).33 The AMDA recommends avoiding long-term pharmacotherapy and advises caution with BZD-hypnotic drugs, tricyclic antidepressants, and antihistamines. The American Academy of Sleep Medicine (AASM) recommends an initial treatment period of 2 to 4 weeks, followed by re-evaluation of continued need for treatment.13 The AASM recommends short- or intermediate-acting BzRAs or ramelteon for initial pharmacologic management of primary insomnias and insomnias comorbid with other conditions. The AASM also recommends specific dosages of BzRAs and BZDs for geriatric patients, which coincide with manufacturer-recommended dosages (Table 2).14-29
Barbiturates, chloral hydrate, and non-barbiturate, non-BZD drugs such as meprobamate are not recommended because of potential significant adverse effects and tolerance/dependence, and low therapeutic index. The AASM advises caution when using prescription drugs off-label for insomnia (eg, antidepressants, antiepileptics, antipsychotics) and recommends avoiding them, if possible, because of limited evidence supporting their use.13
Safety concerns
Two commonly used references contain recommendations for sedative-hypnotic medication use in geriatric patients.30,34 According to Gallagher et al’s34 Screening Tool of Older Person’s Prescriptions (STOPP), long-term (>1 month) use of long-acting BZDs (eg, flurazepam, diazepam) and prolonged use (>1 week) of first-generation antihistamines (eg, diphenhydramine, doxylamine) should be avoided in patients age ≥65 because of the risk of sedation, confusion, and anticholinergic side effects. STOPP recognizes that any use of BZDs, neuroleptics, or first-generation antihistamines may contribute to postural imbalance; therefore these agents are not recommended in older patients at risk for falls.
In the 2012 American Geriatrics Society (AGS) Beers Criteria, the AGS recommends avoiding barbiturates in older adults because of the high rate of physical dependence, tolerance to sleep effects, and overdose risk at low dosages.30 The AGS also recommends avoiding BZDs, stating that older adults have increased sensitivity to these agents and are at an increased risk of cognitive impairment, delirium, falls, fractures, and motor vehicle accidents when taking these drugs. Non-BZD BzRAs also should not be prescribed to patients with a history of falls or fractures, unless safer alternatives are not available.
The FDA has issued several advisory reports regarding sedative-hypnotic drugs. In 2007, all manufacturers of sedative-hypnotic drugs were required to modify their product labeling to include stronger language about potential risks.35 Among these changes, warnings for anaphylaxis and complex sleep-related behaviors were added. Also, the FDA requested that manufacturers of sedative-hypnotic drugs develop and provide patient medication guides, advising consumers on the potential risks and precautions associated with these drugs. More recently, the FDA announced changes to dosing recommendations for zolpidem-containing products because of the risk of impaired mental alertness36; manufacturers were required to lower the recommended dosages for each product.
Manufacturers of FDA-approved sedative-hypnotic drugs urge caution when prescribing these medications for geriatric patients, citing the potential for increased sensitivity, manifesting as marked excitement, depression, or confusion (eg, barbiturates), and greater risk for dosage-related adverse effects (eg, oversedation, dizziness, confusion, impaired psychomotor performance, ataxia).17-29
Use in clinical practice
Several variables should be considered when evaluating appropriateness of pharmacotherapy, including characteristics of the drug and the patient. Geriatric patients may be prone to comorbidities resulting from age-related physiologic changes. These diseases may be confounding (ie, contributing to sleep disorders); examples include medical illnesses, such as hyperthyroidism and arthritis, and psychiatric illnesses, such as depression and anxiety.37 Other conditions, such as renal and hepatic dysfunction, may lead to alteration in drug exposure. These conditions should be assessed through routine renal function tests (eg, serum creatinine and glomerular filtration rate) and liver function tests (eg, serum albumin and liver transaminases).
Multiple comorbidities suggest a higher likelihood of polypharmacy, leading to other drug-related issues (eg, drug-drug interactions). Although these issues may guide therapy by restricting medication options, their potential contribution to the underlying sleep complaints should be considered.37 Several drugs commonly used by geriatric patients may affect wakefulness (eg, analgesics, antidepressants, and antihypertensives [sedating], and thyroid hormones, corticosteroids, and CNS stimulants [alerting]).
CASE CONTINUED
In Mr. R’s case, zaleplon was initiated at 10 mg/d. Because of his age and the nature of his sleep disorder, the choice of sedative-hypnotic was suitable; however, the prescribed dosage was inappropriate. The sluggishness Mr. R experienced likely was a manifestation of increased exposure to the drug. According to manufacturer and AASM recommendations, a more appropriate dosage is 5 mg/d.13,23 Mr. R’s medical history and current medications, and his hepatic and renal function, should be assessed. If Mr. R continues to have issues with sleep initiation, zaleplon, 5 mg at bedtime, should be considered.
Related Resources
• Institute for Safe Medication Practices. www.ismp.org.
• MedWatch: The FDA Safety Information and Adverse Event Reporting Program. www.fda.gov/Safety/MedWatch/default.htm.
Drug Brand Names
Amitriptyline • Elavil Mirtazapine • Remeron
Amobarbital • Amytal Olanzapine • Zyprexa
Butabarbital • Butisol Pentobarbital • Nembutal
Chloral hydrate • Somnote Phenobarbital • Luminal
Diazepam • Valium Quazepam • Doral
Diphenhydramine • Benadryl, others Quetiapine • Seroquel
Doxepin • Silenor Ramelteon • Rozerem
Doxylamine • Unisom, others Secobarbital • Seconal
Estazolam • ProSom Suvorexant • Belsomra
Eszopiclone • Lunesta Temazepam • Restoril
Flurazepam • Dalmane Tiagabine • Gabitril
Gabapentin • Neurontin, Trazodone • Desyrel
Gralise, Horizant Zaleplon • Sonata
Lorazepam • Ativan Zolpidem • Ambien, Edluar,
Meprobamate • Equanil Intermezzo, Zolpimist
Acknowledgement
Vicki L. Ellingrod, PharmD, FCCP, is the series editor of Savvy Psychopharmacology.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. National Center for Health Statistics. Health, United States, 2012, with special feature on emergency care. http://www. cdc.gov/nchs/data/hus/hus12.pdf. Published May 2013. Accessed August 22, 2014.
2. Bronstein AC, Spyker DA, Cantilena LR Jr, et al. 2011 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 29th annual report. Clin Toxicol (Phila). 2012;50(10): 911-1164.
3. Inouye SK. Neuropsychiatric aspects of aging. In: Goldman L, Schafer AI, eds. Goldman’s cecil medicine. 24th ed. Philadelphia, PA: Elsevier Saunders; 2011:114-116.
4. Diagnostic and statistical manual of mental disorders, 4th ed. Washington, DC: American Psychiatric Association; 1994.
5. American Psychiatric Association. Highlights of changes from DSM-IV-TR to DSM-5. http://www.psychiatry. org/File%20Library/Practice/DSM/DSM-5/Changes-from-DSM-IV-TR--to-DSM-5.pdf. 2013. Accessed August 22, 2014.
6. Edwards BA, O’Driscoll DM, Ali A, et al. Aging and sleep: physiology and pathophysiology. Semin Respir Crit Care Med. 2010;31(5):618-633.
7. Foley DJ, Monjan AA, Brown SL, et al. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18(6):425-432.
8. Morlock RJ, Tan M, Mitchell DY. Patient characteristics and patterns of drug use for sleep complaints in the United States: analysis of National Ambulatory Medical Survey Data, 1997-2002. Clin Ther. 2006;28(7):1044-1053.
9. Diasio RB. Principles of drug therapy. In: Goldman L, Schafer AI, eds. Goldman’s Cecil medicine. 24th ed. Philadelphia, PA: Elsevier Saunders; 2011:124-132.
10. Hilmer SN, Ford GA. General principles of pharmacology. In: Halter JB, Ouslander JG, Tinetti ME, et al, eds. Hazzard’s geriatric medicine and gerontology. 6th ed. New York, NY: McGraw-Hill; 2009:103-122.
11. Dolder C, Nelson M, McKinsey J. Use of non-benzodiazepine hypnotics in the elderly: are all agents the same? CNS Drugs. 2007;21(5):389-405.
12. Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009;41(2):67-76.
13. Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487-504.
14. Belsomra [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.; 2014.
15. Micromedex Healthcare Series. Greenwood Village, CO: Thomson Healthcare. http://micromedex.com. Accessed August 22, 2014.
16. Lexicomp. St. Louis, MO: Wolters Kluwer Health. http:// www.lexi.com. Accessed August 22, 2014.
17. Estazolam [package insert]. Corona, CA: Watson Pharma, Inc; 2008.
18. Flurazepam [package insert]. Eatontown, NJ: West-Ward Pharmaceutical Corp; 2010.
19. Doral [package insert]. Las Vegas, NV: Nuro Pharma, Inc; 2013.
20. Restoril [package insert]. Hazelwood, MO: Mallinckrodt Inc; 2010.
21. Halcion [package insert]. New York, NY: Pharmacia & Upjohn Co; 2013.
22. Lunesta [package insert]. Marlborough, MA: Sunovion Pharmaceuticals Inc; 2012.
23. Sonata [package insert]. New York, NY: Pfizer Inc; 2013.
24. Ambien [package insert]. Bridgewater, NJ: Sanofi-Aventis; 2013.
25. Ambien CR [package insert]. Bridgewater, NJ: Sanofi- Aventis; 2013.
26. Edluar [package insert]. Somerset, NJ: Meda Pharmaceuticals Inc; 2009.
27. Intermezzo [package insert]. Point Richmond, CA: Transcept Pharmaceuticals, Inc; 2011.
28. Zolpimist [package insert]. Richmond, VA: ECR Pharmaceuticals; 2013.
29. Rozerem [package insert]. Deerfield, IL: Takeda Pharmaceuticals America, Inc; 2010.
30. The American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
31. Kirkwood CK, Melton ST. Insomnia, drowsiness, and fatigue. In: Krinsky DL, Berardi RR, Ferreri SP, et al, eds. Handbook of nonprescription drugs: an interactive approach to self-care. 17th ed. Washington, DC: American Pharmacists Association; 2012:867-884.
32. Insomnia. In: Natural Standard. Somerville, MA: Natural Standard. https://naturalmedicines.therapeuticresearch. com/databases/medical-conditions/i/insomnia.aspx. Accessed August 22, 2014.
33. American Medical Directors Association. Clinical practice guideline: sleep disorders. Columbia, MD: American Medical Directors Association; 2006.
34. Gallagher P, Ryan C, Byrne S, et al. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72-83.
35. Food and Drug Administration. FDA requests label change for all sleep disorder drug products. http://www.fda. gov/newsevents/newsroom/pressannouncements/2007/ ucm108868.htm. Published March 14, 2007. Accessed August 22, 2014.
36. Food and Drug Administration. FDA drug safety communication: risk of next-morning impairment after use of insomnia drugs; FDA requires lower recommended doses for certain drugs containing zolpidem (Ambien, Ambien CR, Edluar, and Zolpimist). http://www.fda.gov/drugs/ drugsafety/ucm334033.htm. Published January 10, 2013. Accessed August 22, 2014.
37. Cohen-Zion M, Ancoli-Israel S. Sleep disorders. In: Hazzard’s geriatric medicine and gerontology. 6th ed. New York, NY: McGraw-Hill; 2009:671-682.
1. National Center for Health Statistics. Health, United States, 2012, with special feature on emergency care. http://www. cdc.gov/nchs/data/hus/hus12.pdf. Published May 2013. Accessed August 22, 2014.
2. Bronstein AC, Spyker DA, Cantilena LR Jr, et al. 2011 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 29th annual report. Clin Toxicol (Phila). 2012;50(10): 911-1164.
3. Inouye SK. Neuropsychiatric aspects of aging. In: Goldman L, Schafer AI, eds. Goldman’s cecil medicine. 24th ed. Philadelphia, PA: Elsevier Saunders; 2011:114-116.
4. Diagnostic and statistical manual of mental disorders, 4th ed. Washington, DC: American Psychiatric Association; 1994.
5. American Psychiatric Association. Highlights of changes from DSM-IV-TR to DSM-5. http://www.psychiatry. org/File%20Library/Practice/DSM/DSM-5/Changes-from-DSM-IV-TR--to-DSM-5.pdf. 2013. Accessed August 22, 2014.
6. Edwards BA, O’Driscoll DM, Ali A, et al. Aging and sleep: physiology and pathophysiology. Semin Respir Crit Care Med. 2010;31(5):618-633.
7. Foley DJ, Monjan AA, Brown SL, et al. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18(6):425-432.
8. Morlock RJ, Tan M, Mitchell DY. Patient characteristics and patterns of drug use for sleep complaints in the United States: analysis of National Ambulatory Medical Survey Data, 1997-2002. Clin Ther. 2006;28(7):1044-1053.
9. Diasio RB. Principles of drug therapy. In: Goldman L, Schafer AI, eds. Goldman’s Cecil medicine. 24th ed. Philadelphia, PA: Elsevier Saunders; 2011:124-132.
10. Hilmer SN, Ford GA. General principles of pharmacology. In: Halter JB, Ouslander JG, Tinetti ME, et al, eds. Hazzard’s geriatric medicine and gerontology. 6th ed. New York, NY: McGraw-Hill; 2009:103-122.
11. Dolder C, Nelson M, McKinsey J. Use of non-benzodiazepine hypnotics in the elderly: are all agents the same? CNS Drugs. 2007;21(5):389-405.
12. Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009;41(2):67-76.
13. Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487-504.
14. Belsomra [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.; 2014.
15. Micromedex Healthcare Series. Greenwood Village, CO: Thomson Healthcare. http://micromedex.com. Accessed August 22, 2014.
16. Lexicomp. St. Louis, MO: Wolters Kluwer Health. http:// www.lexi.com. Accessed August 22, 2014.
17. Estazolam [package insert]. Corona, CA: Watson Pharma, Inc; 2008.
18. Flurazepam [package insert]. Eatontown, NJ: West-Ward Pharmaceutical Corp; 2010.
19. Doral [package insert]. Las Vegas, NV: Nuro Pharma, Inc; 2013.
20. Restoril [package insert]. Hazelwood, MO: Mallinckrodt Inc; 2010.
21. Halcion [package insert]. New York, NY: Pharmacia & Upjohn Co; 2013.
22. Lunesta [package insert]. Marlborough, MA: Sunovion Pharmaceuticals Inc; 2012.
23. Sonata [package insert]. New York, NY: Pfizer Inc; 2013.
24. Ambien [package insert]. Bridgewater, NJ: Sanofi-Aventis; 2013.
25. Ambien CR [package insert]. Bridgewater, NJ: Sanofi- Aventis; 2013.
26. Edluar [package insert]. Somerset, NJ: Meda Pharmaceuticals Inc; 2009.
27. Intermezzo [package insert]. Point Richmond, CA: Transcept Pharmaceuticals, Inc; 2011.
28. Zolpimist [package insert]. Richmond, VA: ECR Pharmaceuticals; 2013.
29. Rozerem [package insert]. Deerfield, IL: Takeda Pharmaceuticals America, Inc; 2010.
30. The American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
31. Kirkwood CK, Melton ST. Insomnia, drowsiness, and fatigue. In: Krinsky DL, Berardi RR, Ferreri SP, et al, eds. Handbook of nonprescription drugs: an interactive approach to self-care. 17th ed. Washington, DC: American Pharmacists Association; 2012:867-884.
32. Insomnia. In: Natural Standard. Somerville, MA: Natural Standard. https://naturalmedicines.therapeuticresearch. com/databases/medical-conditions/i/insomnia.aspx. Accessed August 22, 2014.
33. American Medical Directors Association. Clinical practice guideline: sleep disorders. Columbia, MD: American Medical Directors Association; 2006.
34. Gallagher P, Ryan C, Byrne S, et al. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72-83.
35. Food and Drug Administration. FDA requests label change for all sleep disorder drug products. http://www.fda. gov/newsevents/newsroom/pressannouncements/2007/ ucm108868.htm. Published March 14, 2007. Accessed August 22, 2014.
36. Food and Drug Administration. FDA drug safety communication: risk of next-morning impairment after use of insomnia drugs; FDA requires lower recommended doses for certain drugs containing zolpidem (Ambien, Ambien CR, Edluar, and Zolpimist). http://www.fda.gov/drugs/ drugsafety/ucm334033.htm. Published January 10, 2013. Accessed August 22, 2014.
37. Cohen-Zion M, Ancoli-Israel S. Sleep disorders. In: Hazzard’s geriatric medicine and gerontology. 6th ed. New York, NY: McGraw-Hill; 2009:671-682.
How to document SUICIDE risk
Despite the high prevalence of suicide and its impact on society,1 psychiatric practitioners achieve only modest success at predicting and preventing suicide. With this in mind, evaluating suicide risk when designing a safe treatment plan for a patient admitted with acute suicidal ideation or after a suicide attempt can be daunting. The mnemonic SUICIDE can remind you of the key elements to include in these patients’ charts.
Suicide assessment. Evaluate the patient for suicide risk factors and protective factors.2 Key risk factors include previous suicide attempts, family history of suicide, access to lethal means, history of a psychiatric disorder, history of alcohol and substance abuse, recent loss of a loved one, and severe hopelessness. Protective factors can be family and community support, problem-solving skills, and religious beliefs that discourage suicide.
Unpredictable and unpreventable. You are responsible for performing a comprehensive risk assessment, responding appropriately to those risks, and instituting a safe discharge plan. Despite your best effort, you might not be able to avert a suicide, and you must provide the patient and family members with this information and document it.
Interventions. Proceed with biological, psychological, and social treatment options. Review the patient’s medications and, when appropriate, consider suicide protective drugs, such as clozapine or lithium.3 Advocate for substance abuse rehabilitation. Encourage psychotherapy and work with your patient to improve social stressors.
Clear and comprehensive documentation. Detailed, accurate, and thorough daily notes are key. Go beyond reporting “no SI/ HI” (suicidal ideation/homicidal ideation). Report what the patient said and how she (he) said it. If the patient denies current suicidal ideation, ask when her (his) last suicidal thought occurred. How did the patient respond to that thought? Why? Avoid using a suicide contract—it is not a substitute for a thorough suicide assessment; it isn’t a legal document; it does not protect against legal liability; and it is ineffective.4
Intent. Evaluate the lethality of the suicidal attempt or plan. Ask about the means of suicide that were used or considered, whether the patient made provisions to ensure or avoid discovery, and how long she (he) has been planning the attempt.
Discuss the treatment plan with a collateral informant (family, friend, community support, outpatient providers). Facilitate safe discharge by including social support in planning. Request that all methods of self-harm, including guns and old prescriptions, be removed from the patient’s home. Help family members and friends identify signs of suicidal ideation.
Educate, engage, and empathize with the patent. Use the Collaborative Assessment and Management of Suicidality (CAMS), which is a structured, evidence-based method of risk assessment and treatment planning. CAMS provides a framework to involve the patient in the assessment of her suicidality and to design a suicide-specific treatment plan.5
A mnemonic can’t prevent all suicides
But using SUICIDE can ensure that the key elements of the evaluation, treatment, and discharge of a patient admitted for acute suicidal ideation or after a suicide attempt are addressed to the best of your ability during an inpatient hospitalization. In doing so, you’ll better identify modifiable risk and protective factors that will inform this plan.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. National Vital Statistics Reports, vol 61 no 4. Hyattsville, MD: National Center for Health Statistics. http://www.cdc.gov/nchs/data/nvsr/nvsr61/ nvsr61_04.pdf. Published May 8, 2013. Accessed August 18, 2014.
2. Fowler JC. Suicide risk assessment in clinical practice: pragmatic guidelines for imperfect assessments. Psychotherapy (Chic). 2012;49(1):81-90.
3. Wasserman D, Rihmer Z, Rujescu D, et al; European Psychiatric Association. The European Psychiatric Association (EPA) guidance on suicide treatment and prevention. Eur Psychiatry. 2012;27(2):129-141.
4. Garvey KA, Penn JV, Campbell AL, et al. Contracting for safety with patients: clinical practice and forensic implications. J Am Acad Psychiatry Law. 2009;37(3): 363-370.
5. Jobes DA. The Collaborative Assessment and Management of Suicidality (CAMS): an evolving evidence‐based clinical approach to suicidal risk. Suicide Life Threat Behav. 2012;42(6):640-653.
Despite the high prevalence of suicide and its impact on society,1 psychiatric practitioners achieve only modest success at predicting and preventing suicide. With this in mind, evaluating suicide risk when designing a safe treatment plan for a patient admitted with acute suicidal ideation or after a suicide attempt can be daunting. The mnemonic SUICIDE can remind you of the key elements to include in these patients’ charts.
Suicide assessment. Evaluate the patient for suicide risk factors and protective factors.2 Key risk factors include previous suicide attempts, family history of suicide, access to lethal means, history of a psychiatric disorder, history of alcohol and substance abuse, recent loss of a loved one, and severe hopelessness. Protective factors can be family and community support, problem-solving skills, and religious beliefs that discourage suicide.
Unpredictable and unpreventable. You are responsible for performing a comprehensive risk assessment, responding appropriately to those risks, and instituting a safe discharge plan. Despite your best effort, you might not be able to avert a suicide, and you must provide the patient and family members with this information and document it.
Interventions. Proceed with biological, psychological, and social treatment options. Review the patient’s medications and, when appropriate, consider suicide protective drugs, such as clozapine or lithium.3 Advocate for substance abuse rehabilitation. Encourage psychotherapy and work with your patient to improve social stressors.
Clear and comprehensive documentation. Detailed, accurate, and thorough daily notes are key. Go beyond reporting “no SI/ HI” (suicidal ideation/homicidal ideation). Report what the patient said and how she (he) said it. If the patient denies current suicidal ideation, ask when her (his) last suicidal thought occurred. How did the patient respond to that thought? Why? Avoid using a suicide contract—it is not a substitute for a thorough suicide assessment; it isn’t a legal document; it does not protect against legal liability; and it is ineffective.4
Intent. Evaluate the lethality of the suicidal attempt or plan. Ask about the means of suicide that were used or considered, whether the patient made provisions to ensure or avoid discovery, and how long she (he) has been planning the attempt.
Discuss the treatment plan with a collateral informant (family, friend, community support, outpatient providers). Facilitate safe discharge by including social support in planning. Request that all methods of self-harm, including guns and old prescriptions, be removed from the patient’s home. Help family members and friends identify signs of suicidal ideation.
Educate, engage, and empathize with the patent. Use the Collaborative Assessment and Management of Suicidality (CAMS), which is a structured, evidence-based method of risk assessment and treatment planning. CAMS provides a framework to involve the patient in the assessment of her suicidality and to design a suicide-specific treatment plan.5
A mnemonic can’t prevent all suicides
But using SUICIDE can ensure that the key elements of the evaluation, treatment, and discharge of a patient admitted for acute suicidal ideation or after a suicide attempt are addressed to the best of your ability during an inpatient hospitalization. In doing so, you’ll better identify modifiable risk and protective factors that will inform this plan.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Despite the high prevalence of suicide and its impact on society,1 psychiatric practitioners achieve only modest success at predicting and preventing suicide. With this in mind, evaluating suicide risk when designing a safe treatment plan for a patient admitted with acute suicidal ideation or after a suicide attempt can be daunting. The mnemonic SUICIDE can remind you of the key elements to include in these patients’ charts.
Suicide assessment. Evaluate the patient for suicide risk factors and protective factors.2 Key risk factors include previous suicide attempts, family history of suicide, access to lethal means, history of a psychiatric disorder, history of alcohol and substance abuse, recent loss of a loved one, and severe hopelessness. Protective factors can be family and community support, problem-solving skills, and religious beliefs that discourage suicide.
Unpredictable and unpreventable. You are responsible for performing a comprehensive risk assessment, responding appropriately to those risks, and instituting a safe discharge plan. Despite your best effort, you might not be able to avert a suicide, and you must provide the patient and family members with this information and document it.
Interventions. Proceed with biological, psychological, and social treatment options. Review the patient’s medications and, when appropriate, consider suicide protective drugs, such as clozapine or lithium.3 Advocate for substance abuse rehabilitation. Encourage psychotherapy and work with your patient to improve social stressors.
Clear and comprehensive documentation. Detailed, accurate, and thorough daily notes are key. Go beyond reporting “no SI/ HI” (suicidal ideation/homicidal ideation). Report what the patient said and how she (he) said it. If the patient denies current suicidal ideation, ask when her (his) last suicidal thought occurred. How did the patient respond to that thought? Why? Avoid using a suicide contract—it is not a substitute for a thorough suicide assessment; it isn’t a legal document; it does not protect against legal liability; and it is ineffective.4
Intent. Evaluate the lethality of the suicidal attempt or plan. Ask about the means of suicide that were used or considered, whether the patient made provisions to ensure or avoid discovery, and how long she (he) has been planning the attempt.
Discuss the treatment plan with a collateral informant (family, friend, community support, outpatient providers). Facilitate safe discharge by including social support in planning. Request that all methods of self-harm, including guns and old prescriptions, be removed from the patient’s home. Help family members and friends identify signs of suicidal ideation.
Educate, engage, and empathize with the patent. Use the Collaborative Assessment and Management of Suicidality (CAMS), which is a structured, evidence-based method of risk assessment and treatment planning. CAMS provides a framework to involve the patient in the assessment of her suicidality and to design a suicide-specific treatment plan.5
A mnemonic can’t prevent all suicides
But using SUICIDE can ensure that the key elements of the evaluation, treatment, and discharge of a patient admitted for acute suicidal ideation or after a suicide attempt are addressed to the best of your ability during an inpatient hospitalization. In doing so, you’ll better identify modifiable risk and protective factors that will inform this plan.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. National Vital Statistics Reports, vol 61 no 4. Hyattsville, MD: National Center for Health Statistics. http://www.cdc.gov/nchs/data/nvsr/nvsr61/ nvsr61_04.pdf. Published May 8, 2013. Accessed August 18, 2014.
2. Fowler JC. Suicide risk assessment in clinical practice: pragmatic guidelines for imperfect assessments. Psychotherapy (Chic). 2012;49(1):81-90.
3. Wasserman D, Rihmer Z, Rujescu D, et al; European Psychiatric Association. The European Psychiatric Association (EPA) guidance on suicide treatment and prevention. Eur Psychiatry. 2012;27(2):129-141.
4. Garvey KA, Penn JV, Campbell AL, et al. Contracting for safety with patients: clinical practice and forensic implications. J Am Acad Psychiatry Law. 2009;37(3): 363-370.
5. Jobes DA. The Collaborative Assessment and Management of Suicidality (CAMS): an evolving evidence‐based clinical approach to suicidal risk. Suicide Life Threat Behav. 2012;42(6):640-653.
1. Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. National Vital Statistics Reports, vol 61 no 4. Hyattsville, MD: National Center for Health Statistics. http://www.cdc.gov/nchs/data/nvsr/nvsr61/ nvsr61_04.pdf. Published May 8, 2013. Accessed August 18, 2014.
2. Fowler JC. Suicide risk assessment in clinical practice: pragmatic guidelines for imperfect assessments. Psychotherapy (Chic). 2012;49(1):81-90.
3. Wasserman D, Rihmer Z, Rujescu D, et al; European Psychiatric Association. The European Psychiatric Association (EPA) guidance on suicide treatment and prevention. Eur Psychiatry. 2012;27(2):129-141.
4. Garvey KA, Penn JV, Campbell AL, et al. Contracting for safety with patients: clinical practice and forensic implications. J Am Acad Psychiatry Law. 2009;37(3): 363-370.
5. Jobes DA. The Collaborative Assessment and Management of Suicidality (CAMS): an evolving evidence‐based clinical approach to suicidal risk. Suicide Life Threat Behav. 2012;42(6):640-653.
How do you score on this self-assessment of suicide risk management?: First of 2 parts
The assessment and management of suicide risk are complex and difficult tasks that raise clinical issues without clear-cut, easy answers. This case-based, multiple-choice self-assessment with accompanying commentaries is a teaching instrument that I designed to enhance a clinician’s ability to provide care for patients at risk for suicide. Part 1 of this article poses 8 of the 15 questions; the balance of questions will appear in Part 2, in the November 2014 issue of Current Psychiatry.
The questions and commentaries in this self-assessment originate in the referenced work of others and my clinical experience. Therefore, I use the preferred “best response” option—not the customary and more restrictive “correct answer” format.
How do you score?
Question 1
Mr. J, age 34, is a professional basketball player complaining of weight loss, early morning waking, and a dysphoric mood lasting for 1 month. His performance on the basketball court has declined and his wife is seeking a separation. He describes “fleeting” suicidal thoughts. He has no history of suicide attempts or depression. The patient does not abuse alcohol or drugs.
The initial assessment approach is to:
a) obtain a suicide prevention contract
b) assess suicide risk and protective factors
c) determine the cause of Mr. J’s depression
d) have Mr. J complete a suicide risk self-assessment form
e) contact his wife for additional history
The best response option is B
Suicide prevention contracts do not prevent suicide.1 Contacting the patient’s wife may be an option at a later stage of evaluation or treatment, if Mr. J grants permission. Determining the cause of his depression likely will require ongoing work up. Assessing suicide risk factors without also looking at protective factors is a common error. A comprehensive suicide risk assessment evaluation requires evaluating both risk and protective factors.2,3 Suicide risk assessment forms often omit questions about protective factors.4 Do not rely on self-assessment suicide risk forms because they are dependent on the patient’s truthfulness. Patients who are determined to commit suicide might regard the psychiatrist and other mental health professionals as the enemy.5
Question 2
Ms. P, a 56-year-old, single schoolteacher, is admitted to a psychiatric unit for severe depression and suicidal ideation without a plan. She is devoutly religious, stating, “I won’t kill myself, because I don’t want to go to hell.” Ms. P attends religious services regularly. She has a history of chronic recurrent depression with suicidal ideation and no history of suicide attempts. You suspect a diagnosis of bipolar II disorder.
In assessing religious affiliation as a protective factor against suicide, you should consider:
a) the nature of the patient’s religious conviction
b) the religion’s stated position on suicide
c) severity of the patient’s illness
d) presence of delusional religious beliefs
e) all of the above
The best response option is E
Dervic et al6 evaluated 371 depressed inpatients according to their religious or non-religious affiliation. Patients with no religious affiliation made significantly more suicide attempts, had more first-degree relatives who committed suicide, were younger, were less likely to be married or have children, and had fewer contacts with family members.
In general, religious affiliation is a protective factor against suicide but may not be a protective factor in an individual patient. Religious affiliation, similar to other presummed general protective factors, requires further scrutiny. Avoid making assumptions. For example, a depressed, devoutly religious patient may curse God for abandonment. A patient with bipolar disorder may believe that God would forgive her for committing suicide. A presumed protective factor may not be protective or might even be a risk factor, such as psychotic patients with religious delusions.
Abrahamic religions—ie, Judaism, Christianity, and Islam—prohibit suicide. Severe mental illness, however, can overcome the strongest religious prohibitions against suicide, including the fear of eternal damnation. For many psychiatric patients, religious affiliations and beliefs are protective factors against suicide, but only relatively. No protective factor against suicide, however strong, provides absolute protection against suicide. Moreover, other risk and protective factors also must be assessed comprehensively.
Question 3
Mr. W, age 18, is admitted to an inpatient psychiatric unit with severe agitation, thought disorder, disorganization, and auditory hallucinations. He is threatening to jump from a nearby building. He has no history of substance abuse.
The psychiatrist conducts a comprehensive suicide risk assessment that includes the patient’s psychiatric diagnosis as a risk factor.
Which psychiatric disorder has the highest associated suicide mortality rate?
a) schizophrenia
b) eating disorders
c) bipolar disorder
d) major depressive disorder
e) borderline personality disorder
The best response option is B
Harris and Barraclough (Table)7 calculated the standardized mortality ratio (SMR) for suicide among psychiatric disorders. SMR is calculated by dividing observed mortality by suicide by the expected mortality by suicide in the general population. Every psychiatric disorder in their study, except for mental retardation, was associated with a varying degree of suicide risk. Eating disorders had the highest SMR. The patient’s psychiatric diagnosis is a risk factor that informs the clinician’s suicide risk assessment.
Question 4
Mr. Z, a 64-year-old, recently divorced lawyer, is admitted to the psychiatric unit from the emergency room. His colleagues brought Mr. Z to the emergency room because of his suicide threats.
On the unit, Mr. Z denies suicidal ideation, plan, or intent. Agitation and suspiciousness are prominent. He refuses to authorize staff to contact his colleagues, his ex-wife, and other family members. Mr. Z demands immediate discharge and forbids contact with his outpatient psychotherapist. He is placed on 72-hour hold as a conditional voluntary admission.
The clinician should:
a) contact Mr. Z’s family, as an emergency exception to confidentiality
b) e-mail his family members with questions
c) contact the patient’s psychotherapist as permitted by the Health Insurance Portability and Accountability Act of 1996 (HIPAA)
d) try to develop a therapeutic alliance with Mr. Z
e) none of the above
The best response option is C
HIPAA permits psychiatrists and other health care providers who are treating the same patient to communicate with each other about medical treatment without obtaining permission from the patient.8 However, mental health professionals cannot share psychotherapy notes without a patient’s consent, except when legally required, such as reporting abuse or duty to warn. This is the most expeditious and productive way of obtaining essential clinical information. E-mail merely changes the mode of unauthorized communication with significant others.
Mr. Z is agitated and suspicious, and developing a therapeutic alliance would require time. It is necessary to gather information about his psychiatric condition as soon as possible. An emergency exception to maintaining confidentiality is another option.9 The definition of emergency varies among jurisdictions. Consulting with a knowledgeable attorney may be necessary, but it usually takes time. Ethically, it is permissible to breach confidentiality to protect the suicidal patient.10
Question 5
Mr. G, a 42-year-old engineer, is re-hospitalized after a failed hanging attempt. Initially, he is profoundly depressed but improves suddenly and requests discharge. The psychiatrist and clinical staff are perplexed. Is the sudden improvement real or feigned?
The treatment team should consider all of the following options except:
a) obtain records of earlier hospitalizations
b) check collateral sources of information
c) assess Mr. G’s compliance with treatment
d) obtain psychological testing to evaluate Mr. G’s honesty
e) determine whether behavioral signs of depression are present
The best response option is D
Short length of hospital stay makes it difficult to assess sudden patient improvement.11 Real improvement in a high-risk suicidal patient is a process, even when it occurs quickly. Feigned improvement is an event. Obtaining patient information from collateral sources is crucial. Sudden improvement might be caused by the patient’s resolve to complete suicide. Identifying behavioral risk factors associated with psychiatric disorders informs the clinician’s systematic suicide risk assessment of a guarded or dissimulative patient. Psychological testing will take critical time and is not a substitute for careful clinical assessment.
Question 6
In mid-winter, Ms. M, a 42-year-old homeless woman, is seen in the emergency room of a general hospital. She complains of depression and auditory hallucinations commanding her to commit suicide. Ms. M has 5 earlier admissions to the psychiatry unit for similar complaints.
The psychiatrist conducts a comprehensive suicide risk assessment. Acute and chronic risk factors for suicide are identified. Protective factors also are assessed. The psychiatrist weighs and synthesizes risk and protective factors into an overall assessment of Ms. M’s suicide risk.
The main purpose of suicide risk assessment is to:
a) predict the likelihood of suicide
b) determine imminence of suicide
c) inform patient treatment and safety management
d) identify malingered suicidal ideation
e) provide a legal defense against a malpractice claim
The best response option is C
Suicide cannot be predicted.12 The term imminent suicide is a veiled attempt to predict when a patient will attempt suicide.13 The process of a comprehensive or systematic suicide risk assessment encompasses identification, analysis, and synthesis of risk and protective factors that inform the treatment and safety management of the patient.3 The overall suicide assessment is a clinical judgment call that determines risk along a continuum of low to high. In Ms. M’s case, comprehensive suicide risk assessment will assist the clinician in determining the patient’s overall suicide risk and make an appropriate disposition. Without a systematic suicide risk assessment methodology, the clinician is at the mercy of the pejoratively labeled “frequent flyer” who is looking for sustenance and lodging. The frustrated clinician is left with little choice but to admit the patient.
Although not the main purpose, systematic suicide risk assessment can help provide a sound legal defense if a suicide malpractice claim is filed against the clinician alleging negligent assessment.14
Question 7
A psychiatrist is treating Mr. S, a 36-year-old computer analyst, with once-a-week psychotherapy and medication management for panic and depressive symptoms that emerged abruptly after the break-up of a romantic relationship. Mr. S is using alcohol to sleep. He reports occasional suicidal ideation but no plan. He finds the idea of suicide to be morally repugnant. A therapeutic alliance develops.
The psychiatrist is concerned about Mr. S’s suicide risk and the need for hospitalization. The psychiatrist performs a systematic suicide risk assessment that includes identification of individual and evidence-based protective factors. For example, Mr. S continued to pursue his interests and to participate in civil causes. The overall suicide risk is determined by the assessment of individual and evidence-based protective factors.
All of the following options are evidence-based protective factors except:
a) therapeutic alliance
b) survival and coping beliefs
c) responsibility to family
d) fear of suicide
e) moral objections to suicide
The best response option is A
Clinical consensus holds that the therapeutic alliance is an important protective factor against suicide. However, no evidence-based research supports or refutes this widely held belief among clinicians.
Linehan et al15 developed the Reasons for Living Inventory, a self-report instrument that identifies 6 subscales:
• survival and coping beliefs
• responsibility to family
• child-related concerns
• fear of suicide
• fear of social disapproval
• moral objections to suicide.
Survival and coping beliefs, responsibility to family, and child-related concerns were useful in differentiating between suicidal and non-suicidal individuals. Malone et al16 administered the Reasons for Living Inventory to 84 inpatients with major depression; 45 had attempted suicide. Depressed patients who had not attempted suicide demonstrated more sense of responsibility toward family, more fear of social disapproval, more moral objections to suicide, greater survival and coping skills, and greater fear of suicide than patients who attempted suicide. The authors recommended adding the Reasons for Living Inventory to the assessment of patients at risk for suicide.
Question 8
A 38-year-old mother of a newborn child is admitted to the psychiatric unit after expressing suicidal thoughts to her husband. She has been hospitalized previously after a hypomanic episode and severe depression; she has no history of suicide attempts. A psychiatrist diagnoses bipolar II disorder (recurrent major episodes with hypomanic episodes). The patient’s maternal aunt has bipolar disorder. Her paternal grandfather committed suicide.
The psychiatrist conducts a systematic suicide risk assessment and determines the patient is at high risk of suicide. He considers a suicide-risk reduction drug.
Which one of the following drugs has been shown to reduce suicide and suicide attempts in bipolar II patients?
a) clozapine
b) clonazepam
c) lorazepam
d) lithium
e) quetiapine
The best response option is D
Prospective, randomized and controlled trials consistently have found lower rates of completed suicides and suicide attempts during lithium maintenance treatments for patients with bipolar disorder and other major affective disorders.17
Bottom Line
Suicide risk assessment and management are challenging for even experienced clinicians. Suicide risk assessment guides appropriate treatment and management for patients at risk for suicide. This self-assessment helps mental health professionals identify potential gaps in their knowledge and reinforce best practices.
Related Resources
• Simon RI. Passive suicidal ideation: Still a high-risk clinical scenario. Current Psychiatry. 2014;13(3):13-15.
• Simon RI. Suicide rehearsals: A high-risk psychiatric emergency. Current Psychiatry. 2012;11(7):28-32.
• Bongar B, Sullivan GR. The suicidal patient: Clinical and legal standards of care. Washington, DC: American Psychological Association; 2013.
Drug Brand Names
Clonazepam • Klonopin Lorazepam • Ativan
Clozapine • Clozaril Quetiapine • Seroquel
Lithium • Eskalith, Lithobid
Disclosure
Dr. Simon reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Adapted with permission from: Simon RI. Preventing patient suicide: clinical assessment and management, Arlington VA: American Psychiatric Publishing; 2011.
Editor’s note: Part 2 of this self-assessment on suicide assessment and management in the November 2014 issue of Current Psychiatry poses 7 additional questions.
1. Stanford EJ, Goetz RR, Bloom JD. The No Harm Contract in the emergency assessment of suicide risk. J Clin Psychiatry. 1994;55(8):344-348.
2. Simon RI, Hales RE, eds. Textbook of suicide assessment and management. 2nd ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2012.
3. Practice guidelines for the assessment and treatment of patients with suicidal behaviors [Erratum in Am J Psychiatry. 2004;161(4):776]. Am J Psychiatry. 2003;160(suppl 11):1-60.
4. Simon RI. Suicide risk assessment forms: form over substance? J Am Acad Psychiatry Law. 2009;37(3): 290-293.
5. Resnick PJ. Recognizing that the suicidal patient views you as an ‘adversary.’ Current Psychiatry. 2002;1(1):8.
6. Dervic K, Oquendo MA, Grunebaum MF, et al. Religious affiliation and suicide attempt. Am J Psychiatry. 2004; 161(12):2303-2308.
7. Harris CE, Barraclough B. Suicide as an outcome for mental disorders. A meta-analysis. Br J Psychiatry. 1997;170:205-228.
8. Health insurance portability and accountability act of 1996. Pub L No. 104-191.
9. Simon RI, Shuman DW. Clinical manual of psychiatry and law. Arlington, VA: American Psychiatric Publishing, Inc; 2007.
10. American Psychiatric Association. Principles of medical ethics with annotations especially applicable to psychiatry. Section 4, annotation 8. Washington, DC: American Psychiatric Publishing, Inc; 2001.
11. Simon RI, Gutheil TG. Sudden improvement in high-risk suicidal patients: should it be trusted? Psych Serv. 2009; 60(3):387-389.
12. Pokorny AD. Prediction of suicide in psychiatric patients. Report of a prospective study. Arch Gen Psychiatry. 1983; 4(3):249-257.
13. Simon RI. Imminent suicide: the illusion of short-term prediction. Suicide Life Threat Behav. 2006;36(3): 296-301.
14. Simon RI, Shuman DW. Therapeutic risk management of clinical-legal dilemmas: should it be a core competency? J Am Acad Psychiatry Law. 2009;37(2):155-161.
15. Linehan MM, Goodstein JL, Nielsen SL, et al. Reasons for staying alive when you are thinking of killing yourself: the reasons for living inventory. J Consult Clin Psychol. 1983;51(2):276-286.
16. Malone KM, Oquendo MA, Hass GL, et al. Protective factors against suicidal acts in major depression: reasons for living. Am J Psychiatry. 2000;157(7):1084-1088.
17. Baldessarini RJ, Pompili M, Tondo L. Bipolar disorder. In: Simon RI, Hales RE, eds. Textbook of suicide assessment and management. Arlington, VA: American Psychiatric Publishing, Inc; 2006:159-176.
The assessment and management of suicide risk are complex and difficult tasks that raise clinical issues without clear-cut, easy answers. This case-based, multiple-choice self-assessment with accompanying commentaries is a teaching instrument that I designed to enhance a clinician’s ability to provide care for patients at risk for suicide. Part 1 of this article poses 8 of the 15 questions; the balance of questions will appear in Part 2, in the November 2014 issue of Current Psychiatry.
The questions and commentaries in this self-assessment originate in the referenced work of others and my clinical experience. Therefore, I use the preferred “best response” option—not the customary and more restrictive “correct answer” format.
How do you score?
Question 1
Mr. J, age 34, is a professional basketball player complaining of weight loss, early morning waking, and a dysphoric mood lasting for 1 month. His performance on the basketball court has declined and his wife is seeking a separation. He describes “fleeting” suicidal thoughts. He has no history of suicide attempts or depression. The patient does not abuse alcohol or drugs.
The initial assessment approach is to:
a) obtain a suicide prevention contract
b) assess suicide risk and protective factors
c) determine the cause of Mr. J’s depression
d) have Mr. J complete a suicide risk self-assessment form
e) contact his wife for additional history
The best response option is B
Suicide prevention contracts do not prevent suicide.1 Contacting the patient’s wife may be an option at a later stage of evaluation or treatment, if Mr. J grants permission. Determining the cause of his depression likely will require ongoing work up. Assessing suicide risk factors without also looking at protective factors is a common error. A comprehensive suicide risk assessment evaluation requires evaluating both risk and protective factors.2,3 Suicide risk assessment forms often omit questions about protective factors.4 Do not rely on self-assessment suicide risk forms because they are dependent on the patient’s truthfulness. Patients who are determined to commit suicide might regard the psychiatrist and other mental health professionals as the enemy.5
Question 2
Ms. P, a 56-year-old, single schoolteacher, is admitted to a psychiatric unit for severe depression and suicidal ideation without a plan. She is devoutly religious, stating, “I won’t kill myself, because I don’t want to go to hell.” Ms. P attends religious services regularly. She has a history of chronic recurrent depression with suicidal ideation and no history of suicide attempts. You suspect a diagnosis of bipolar II disorder.
In assessing religious affiliation as a protective factor against suicide, you should consider:
a) the nature of the patient’s religious conviction
b) the religion’s stated position on suicide
c) severity of the patient’s illness
d) presence of delusional religious beliefs
e) all of the above
The best response option is E
Dervic et al6 evaluated 371 depressed inpatients according to their religious or non-religious affiliation. Patients with no religious affiliation made significantly more suicide attempts, had more first-degree relatives who committed suicide, were younger, were less likely to be married or have children, and had fewer contacts with family members.
In general, religious affiliation is a protective factor against suicide but may not be a protective factor in an individual patient. Religious affiliation, similar to other presummed general protective factors, requires further scrutiny. Avoid making assumptions. For example, a depressed, devoutly religious patient may curse God for abandonment. A patient with bipolar disorder may believe that God would forgive her for committing suicide. A presumed protective factor may not be protective or might even be a risk factor, such as psychotic patients with religious delusions.
Abrahamic religions—ie, Judaism, Christianity, and Islam—prohibit suicide. Severe mental illness, however, can overcome the strongest religious prohibitions against suicide, including the fear of eternal damnation. For many psychiatric patients, religious affiliations and beliefs are protective factors against suicide, but only relatively. No protective factor against suicide, however strong, provides absolute protection against suicide. Moreover, other risk and protective factors also must be assessed comprehensively.
Question 3
Mr. W, age 18, is admitted to an inpatient psychiatric unit with severe agitation, thought disorder, disorganization, and auditory hallucinations. He is threatening to jump from a nearby building. He has no history of substance abuse.
The psychiatrist conducts a comprehensive suicide risk assessment that includes the patient’s psychiatric diagnosis as a risk factor.
Which psychiatric disorder has the highest associated suicide mortality rate?
a) schizophrenia
b) eating disorders
c) bipolar disorder
d) major depressive disorder
e) borderline personality disorder
The best response option is B
Harris and Barraclough (Table)7 calculated the standardized mortality ratio (SMR) for suicide among psychiatric disorders. SMR is calculated by dividing observed mortality by suicide by the expected mortality by suicide in the general population. Every psychiatric disorder in their study, except for mental retardation, was associated with a varying degree of suicide risk. Eating disorders had the highest SMR. The patient’s psychiatric diagnosis is a risk factor that informs the clinician’s suicide risk assessment.
Question 4
Mr. Z, a 64-year-old, recently divorced lawyer, is admitted to the psychiatric unit from the emergency room. His colleagues brought Mr. Z to the emergency room because of his suicide threats.
On the unit, Mr. Z denies suicidal ideation, plan, or intent. Agitation and suspiciousness are prominent. He refuses to authorize staff to contact his colleagues, his ex-wife, and other family members. Mr. Z demands immediate discharge and forbids contact with his outpatient psychotherapist. He is placed on 72-hour hold as a conditional voluntary admission.
The clinician should:
a) contact Mr. Z’s family, as an emergency exception to confidentiality
b) e-mail his family members with questions
c) contact the patient’s psychotherapist as permitted by the Health Insurance Portability and Accountability Act of 1996 (HIPAA)
d) try to develop a therapeutic alliance with Mr. Z
e) none of the above
The best response option is C
HIPAA permits psychiatrists and other health care providers who are treating the same patient to communicate with each other about medical treatment without obtaining permission from the patient.8 However, mental health professionals cannot share psychotherapy notes without a patient’s consent, except when legally required, such as reporting abuse or duty to warn. This is the most expeditious and productive way of obtaining essential clinical information. E-mail merely changes the mode of unauthorized communication with significant others.
Mr. Z is agitated and suspicious, and developing a therapeutic alliance would require time. It is necessary to gather information about his psychiatric condition as soon as possible. An emergency exception to maintaining confidentiality is another option.9 The definition of emergency varies among jurisdictions. Consulting with a knowledgeable attorney may be necessary, but it usually takes time. Ethically, it is permissible to breach confidentiality to protect the suicidal patient.10
Question 5
Mr. G, a 42-year-old engineer, is re-hospitalized after a failed hanging attempt. Initially, he is profoundly depressed but improves suddenly and requests discharge. The psychiatrist and clinical staff are perplexed. Is the sudden improvement real or feigned?
The treatment team should consider all of the following options except:
a) obtain records of earlier hospitalizations
b) check collateral sources of information
c) assess Mr. G’s compliance with treatment
d) obtain psychological testing to evaluate Mr. G’s honesty
e) determine whether behavioral signs of depression are present
The best response option is D
Short length of hospital stay makes it difficult to assess sudden patient improvement.11 Real improvement in a high-risk suicidal patient is a process, even when it occurs quickly. Feigned improvement is an event. Obtaining patient information from collateral sources is crucial. Sudden improvement might be caused by the patient’s resolve to complete suicide. Identifying behavioral risk factors associated with psychiatric disorders informs the clinician’s systematic suicide risk assessment of a guarded or dissimulative patient. Psychological testing will take critical time and is not a substitute for careful clinical assessment.
Question 6
In mid-winter, Ms. M, a 42-year-old homeless woman, is seen in the emergency room of a general hospital. She complains of depression and auditory hallucinations commanding her to commit suicide. Ms. M has 5 earlier admissions to the psychiatry unit for similar complaints.
The psychiatrist conducts a comprehensive suicide risk assessment. Acute and chronic risk factors for suicide are identified. Protective factors also are assessed. The psychiatrist weighs and synthesizes risk and protective factors into an overall assessment of Ms. M’s suicide risk.
The main purpose of suicide risk assessment is to:
a) predict the likelihood of suicide
b) determine imminence of suicide
c) inform patient treatment and safety management
d) identify malingered suicidal ideation
e) provide a legal defense against a malpractice claim
The best response option is C
Suicide cannot be predicted.12 The term imminent suicide is a veiled attempt to predict when a patient will attempt suicide.13 The process of a comprehensive or systematic suicide risk assessment encompasses identification, analysis, and synthesis of risk and protective factors that inform the treatment and safety management of the patient.3 The overall suicide assessment is a clinical judgment call that determines risk along a continuum of low to high. In Ms. M’s case, comprehensive suicide risk assessment will assist the clinician in determining the patient’s overall suicide risk and make an appropriate disposition. Without a systematic suicide risk assessment methodology, the clinician is at the mercy of the pejoratively labeled “frequent flyer” who is looking for sustenance and lodging. The frustrated clinician is left with little choice but to admit the patient.
Although not the main purpose, systematic suicide risk assessment can help provide a sound legal defense if a suicide malpractice claim is filed against the clinician alleging negligent assessment.14
Question 7
A psychiatrist is treating Mr. S, a 36-year-old computer analyst, with once-a-week psychotherapy and medication management for panic and depressive symptoms that emerged abruptly after the break-up of a romantic relationship. Mr. S is using alcohol to sleep. He reports occasional suicidal ideation but no plan. He finds the idea of suicide to be morally repugnant. A therapeutic alliance develops.
The psychiatrist is concerned about Mr. S’s suicide risk and the need for hospitalization. The psychiatrist performs a systematic suicide risk assessment that includes identification of individual and evidence-based protective factors. For example, Mr. S continued to pursue his interests and to participate in civil causes. The overall suicide risk is determined by the assessment of individual and evidence-based protective factors.
All of the following options are evidence-based protective factors except:
a) therapeutic alliance
b) survival and coping beliefs
c) responsibility to family
d) fear of suicide
e) moral objections to suicide
The best response option is A
Clinical consensus holds that the therapeutic alliance is an important protective factor against suicide. However, no evidence-based research supports or refutes this widely held belief among clinicians.
Linehan et al15 developed the Reasons for Living Inventory, a self-report instrument that identifies 6 subscales:
• survival and coping beliefs
• responsibility to family
• child-related concerns
• fear of suicide
• fear of social disapproval
• moral objections to suicide.
Survival and coping beliefs, responsibility to family, and child-related concerns were useful in differentiating between suicidal and non-suicidal individuals. Malone et al16 administered the Reasons for Living Inventory to 84 inpatients with major depression; 45 had attempted suicide. Depressed patients who had not attempted suicide demonstrated more sense of responsibility toward family, more fear of social disapproval, more moral objections to suicide, greater survival and coping skills, and greater fear of suicide than patients who attempted suicide. The authors recommended adding the Reasons for Living Inventory to the assessment of patients at risk for suicide.
Question 8
A 38-year-old mother of a newborn child is admitted to the psychiatric unit after expressing suicidal thoughts to her husband. She has been hospitalized previously after a hypomanic episode and severe depression; she has no history of suicide attempts. A psychiatrist diagnoses bipolar II disorder (recurrent major episodes with hypomanic episodes). The patient’s maternal aunt has bipolar disorder. Her paternal grandfather committed suicide.
The psychiatrist conducts a systematic suicide risk assessment and determines the patient is at high risk of suicide. He considers a suicide-risk reduction drug.
Which one of the following drugs has been shown to reduce suicide and suicide attempts in bipolar II patients?
a) clozapine
b) clonazepam
c) lorazepam
d) lithium
e) quetiapine
The best response option is D
Prospective, randomized and controlled trials consistently have found lower rates of completed suicides and suicide attempts during lithium maintenance treatments for patients with bipolar disorder and other major affective disorders.17
Bottom Line
Suicide risk assessment and management are challenging for even experienced clinicians. Suicide risk assessment guides appropriate treatment and management for patients at risk for suicide. This self-assessment helps mental health professionals identify potential gaps in their knowledge and reinforce best practices.
Related Resources
• Simon RI. Passive suicidal ideation: Still a high-risk clinical scenario. Current Psychiatry. 2014;13(3):13-15.
• Simon RI. Suicide rehearsals: A high-risk psychiatric emergency. Current Psychiatry. 2012;11(7):28-32.
• Bongar B, Sullivan GR. The suicidal patient: Clinical and legal standards of care. Washington, DC: American Psychological Association; 2013.
Drug Brand Names
Clonazepam • Klonopin Lorazepam • Ativan
Clozapine • Clozaril Quetiapine • Seroquel
Lithium • Eskalith, Lithobid
Disclosure
Dr. Simon reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Adapted with permission from: Simon RI. Preventing patient suicide: clinical assessment and management, Arlington VA: American Psychiatric Publishing; 2011.
Editor’s note: Part 2 of this self-assessment on suicide assessment and management in the November 2014 issue of Current Psychiatry poses 7 additional questions.
The assessment and management of suicide risk are complex and difficult tasks that raise clinical issues without clear-cut, easy answers. This case-based, multiple-choice self-assessment with accompanying commentaries is a teaching instrument that I designed to enhance a clinician’s ability to provide care for patients at risk for suicide. Part 1 of this article poses 8 of the 15 questions; the balance of questions will appear in Part 2, in the November 2014 issue of Current Psychiatry.
The questions and commentaries in this self-assessment originate in the referenced work of others and my clinical experience. Therefore, I use the preferred “best response” option—not the customary and more restrictive “correct answer” format.
How do you score?
Question 1
Mr. J, age 34, is a professional basketball player complaining of weight loss, early morning waking, and a dysphoric mood lasting for 1 month. His performance on the basketball court has declined and his wife is seeking a separation. He describes “fleeting” suicidal thoughts. He has no history of suicide attempts or depression. The patient does not abuse alcohol or drugs.
The initial assessment approach is to:
a) obtain a suicide prevention contract
b) assess suicide risk and protective factors
c) determine the cause of Mr. J’s depression
d) have Mr. J complete a suicide risk self-assessment form
e) contact his wife for additional history
The best response option is B
Suicide prevention contracts do not prevent suicide.1 Contacting the patient’s wife may be an option at a later stage of evaluation or treatment, if Mr. J grants permission. Determining the cause of his depression likely will require ongoing work up. Assessing suicide risk factors without also looking at protective factors is a common error. A comprehensive suicide risk assessment evaluation requires evaluating both risk and protective factors.2,3 Suicide risk assessment forms often omit questions about protective factors.4 Do not rely on self-assessment suicide risk forms because they are dependent on the patient’s truthfulness. Patients who are determined to commit suicide might regard the psychiatrist and other mental health professionals as the enemy.5
Question 2
Ms. P, a 56-year-old, single schoolteacher, is admitted to a psychiatric unit for severe depression and suicidal ideation without a plan. She is devoutly religious, stating, “I won’t kill myself, because I don’t want to go to hell.” Ms. P attends religious services regularly. She has a history of chronic recurrent depression with suicidal ideation and no history of suicide attempts. You suspect a diagnosis of bipolar II disorder.
In assessing religious affiliation as a protective factor against suicide, you should consider:
a) the nature of the patient’s religious conviction
b) the religion’s stated position on suicide
c) severity of the patient’s illness
d) presence of delusional religious beliefs
e) all of the above
The best response option is E
Dervic et al6 evaluated 371 depressed inpatients according to their religious or non-religious affiliation. Patients with no religious affiliation made significantly more suicide attempts, had more first-degree relatives who committed suicide, were younger, were less likely to be married or have children, and had fewer contacts with family members.
In general, religious affiliation is a protective factor against suicide but may not be a protective factor in an individual patient. Religious affiliation, similar to other presummed general protective factors, requires further scrutiny. Avoid making assumptions. For example, a depressed, devoutly religious patient may curse God for abandonment. A patient with bipolar disorder may believe that God would forgive her for committing suicide. A presumed protective factor may not be protective or might even be a risk factor, such as psychotic patients with religious delusions.
Abrahamic religions—ie, Judaism, Christianity, and Islam—prohibit suicide. Severe mental illness, however, can overcome the strongest religious prohibitions against suicide, including the fear of eternal damnation. For many psychiatric patients, religious affiliations and beliefs are protective factors against suicide, but only relatively. No protective factor against suicide, however strong, provides absolute protection against suicide. Moreover, other risk and protective factors also must be assessed comprehensively.
Question 3
Mr. W, age 18, is admitted to an inpatient psychiatric unit with severe agitation, thought disorder, disorganization, and auditory hallucinations. He is threatening to jump from a nearby building. He has no history of substance abuse.
The psychiatrist conducts a comprehensive suicide risk assessment that includes the patient’s psychiatric diagnosis as a risk factor.
Which psychiatric disorder has the highest associated suicide mortality rate?
a) schizophrenia
b) eating disorders
c) bipolar disorder
d) major depressive disorder
e) borderline personality disorder
The best response option is B
Harris and Barraclough (Table)7 calculated the standardized mortality ratio (SMR) for suicide among psychiatric disorders. SMR is calculated by dividing observed mortality by suicide by the expected mortality by suicide in the general population. Every psychiatric disorder in their study, except for mental retardation, was associated with a varying degree of suicide risk. Eating disorders had the highest SMR. The patient’s psychiatric diagnosis is a risk factor that informs the clinician’s suicide risk assessment.
Question 4
Mr. Z, a 64-year-old, recently divorced lawyer, is admitted to the psychiatric unit from the emergency room. His colleagues brought Mr. Z to the emergency room because of his suicide threats.
On the unit, Mr. Z denies suicidal ideation, plan, or intent. Agitation and suspiciousness are prominent. He refuses to authorize staff to contact his colleagues, his ex-wife, and other family members. Mr. Z demands immediate discharge and forbids contact with his outpatient psychotherapist. He is placed on 72-hour hold as a conditional voluntary admission.
The clinician should:
a) contact Mr. Z’s family, as an emergency exception to confidentiality
b) e-mail his family members with questions
c) contact the patient’s psychotherapist as permitted by the Health Insurance Portability and Accountability Act of 1996 (HIPAA)
d) try to develop a therapeutic alliance with Mr. Z
e) none of the above
The best response option is C
HIPAA permits psychiatrists and other health care providers who are treating the same patient to communicate with each other about medical treatment without obtaining permission from the patient.8 However, mental health professionals cannot share psychotherapy notes without a patient’s consent, except when legally required, such as reporting abuse or duty to warn. This is the most expeditious and productive way of obtaining essential clinical information. E-mail merely changes the mode of unauthorized communication with significant others.
Mr. Z is agitated and suspicious, and developing a therapeutic alliance would require time. It is necessary to gather information about his psychiatric condition as soon as possible. An emergency exception to maintaining confidentiality is another option.9 The definition of emergency varies among jurisdictions. Consulting with a knowledgeable attorney may be necessary, but it usually takes time. Ethically, it is permissible to breach confidentiality to protect the suicidal patient.10
Question 5
Mr. G, a 42-year-old engineer, is re-hospitalized after a failed hanging attempt. Initially, he is profoundly depressed but improves suddenly and requests discharge. The psychiatrist and clinical staff are perplexed. Is the sudden improvement real or feigned?
The treatment team should consider all of the following options except:
a) obtain records of earlier hospitalizations
b) check collateral sources of information
c) assess Mr. G’s compliance with treatment
d) obtain psychological testing to evaluate Mr. G’s honesty
e) determine whether behavioral signs of depression are present
The best response option is D
Short length of hospital stay makes it difficult to assess sudden patient improvement.11 Real improvement in a high-risk suicidal patient is a process, even when it occurs quickly. Feigned improvement is an event. Obtaining patient information from collateral sources is crucial. Sudden improvement might be caused by the patient’s resolve to complete suicide. Identifying behavioral risk factors associated with psychiatric disorders informs the clinician’s systematic suicide risk assessment of a guarded or dissimulative patient. Psychological testing will take critical time and is not a substitute for careful clinical assessment.
Question 6
In mid-winter, Ms. M, a 42-year-old homeless woman, is seen in the emergency room of a general hospital. She complains of depression and auditory hallucinations commanding her to commit suicide. Ms. M has 5 earlier admissions to the psychiatry unit for similar complaints.
The psychiatrist conducts a comprehensive suicide risk assessment. Acute and chronic risk factors for suicide are identified. Protective factors also are assessed. The psychiatrist weighs and synthesizes risk and protective factors into an overall assessment of Ms. M’s suicide risk.
The main purpose of suicide risk assessment is to:
a) predict the likelihood of suicide
b) determine imminence of suicide
c) inform patient treatment and safety management
d) identify malingered suicidal ideation
e) provide a legal defense against a malpractice claim
The best response option is C
Suicide cannot be predicted.12 The term imminent suicide is a veiled attempt to predict when a patient will attempt suicide.13 The process of a comprehensive or systematic suicide risk assessment encompasses identification, analysis, and synthesis of risk and protective factors that inform the treatment and safety management of the patient.3 The overall suicide assessment is a clinical judgment call that determines risk along a continuum of low to high. In Ms. M’s case, comprehensive suicide risk assessment will assist the clinician in determining the patient’s overall suicide risk and make an appropriate disposition. Without a systematic suicide risk assessment methodology, the clinician is at the mercy of the pejoratively labeled “frequent flyer” who is looking for sustenance and lodging. The frustrated clinician is left with little choice but to admit the patient.
Although not the main purpose, systematic suicide risk assessment can help provide a sound legal defense if a suicide malpractice claim is filed against the clinician alleging negligent assessment.14
Question 7
A psychiatrist is treating Mr. S, a 36-year-old computer analyst, with once-a-week psychotherapy and medication management for panic and depressive symptoms that emerged abruptly after the break-up of a romantic relationship. Mr. S is using alcohol to sleep. He reports occasional suicidal ideation but no plan. He finds the idea of suicide to be morally repugnant. A therapeutic alliance develops.
The psychiatrist is concerned about Mr. S’s suicide risk and the need for hospitalization. The psychiatrist performs a systematic suicide risk assessment that includes identification of individual and evidence-based protective factors. For example, Mr. S continued to pursue his interests and to participate in civil causes. The overall suicide risk is determined by the assessment of individual and evidence-based protective factors.
All of the following options are evidence-based protective factors except:
a) therapeutic alliance
b) survival and coping beliefs
c) responsibility to family
d) fear of suicide
e) moral objections to suicide
The best response option is A
Clinical consensus holds that the therapeutic alliance is an important protective factor against suicide. However, no evidence-based research supports or refutes this widely held belief among clinicians.
Linehan et al15 developed the Reasons for Living Inventory, a self-report instrument that identifies 6 subscales:
• survival and coping beliefs
• responsibility to family
• child-related concerns
• fear of suicide
• fear of social disapproval
• moral objections to suicide.
Survival and coping beliefs, responsibility to family, and child-related concerns were useful in differentiating between suicidal and non-suicidal individuals. Malone et al16 administered the Reasons for Living Inventory to 84 inpatients with major depression; 45 had attempted suicide. Depressed patients who had not attempted suicide demonstrated more sense of responsibility toward family, more fear of social disapproval, more moral objections to suicide, greater survival and coping skills, and greater fear of suicide than patients who attempted suicide. The authors recommended adding the Reasons for Living Inventory to the assessment of patients at risk for suicide.
Question 8
A 38-year-old mother of a newborn child is admitted to the psychiatric unit after expressing suicidal thoughts to her husband. She has been hospitalized previously after a hypomanic episode and severe depression; she has no history of suicide attempts. A psychiatrist diagnoses bipolar II disorder (recurrent major episodes with hypomanic episodes). The patient’s maternal aunt has bipolar disorder. Her paternal grandfather committed suicide.
The psychiatrist conducts a systematic suicide risk assessment and determines the patient is at high risk of suicide. He considers a suicide-risk reduction drug.
Which one of the following drugs has been shown to reduce suicide and suicide attempts in bipolar II patients?
a) clozapine
b) clonazepam
c) lorazepam
d) lithium
e) quetiapine
The best response option is D
Prospective, randomized and controlled trials consistently have found lower rates of completed suicides and suicide attempts during lithium maintenance treatments for patients with bipolar disorder and other major affective disorders.17
Bottom Line
Suicide risk assessment and management are challenging for even experienced clinicians. Suicide risk assessment guides appropriate treatment and management for patients at risk for suicide. This self-assessment helps mental health professionals identify potential gaps in their knowledge and reinforce best practices.
Related Resources
• Simon RI. Passive suicidal ideation: Still a high-risk clinical scenario. Current Psychiatry. 2014;13(3):13-15.
• Simon RI. Suicide rehearsals: A high-risk psychiatric emergency. Current Psychiatry. 2012;11(7):28-32.
• Bongar B, Sullivan GR. The suicidal patient: Clinical and legal standards of care. Washington, DC: American Psychological Association; 2013.
Drug Brand Names
Clonazepam • Klonopin Lorazepam • Ativan
Clozapine • Clozaril Quetiapine • Seroquel
Lithium • Eskalith, Lithobid
Disclosure
Dr. Simon reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Adapted with permission from: Simon RI. Preventing patient suicide: clinical assessment and management, Arlington VA: American Psychiatric Publishing; 2011.
Editor’s note: Part 2 of this self-assessment on suicide assessment and management in the November 2014 issue of Current Psychiatry poses 7 additional questions.
1. Stanford EJ, Goetz RR, Bloom JD. The No Harm Contract in the emergency assessment of suicide risk. J Clin Psychiatry. 1994;55(8):344-348.
2. Simon RI, Hales RE, eds. Textbook of suicide assessment and management. 2nd ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2012.
3. Practice guidelines for the assessment and treatment of patients with suicidal behaviors [Erratum in Am J Psychiatry. 2004;161(4):776]. Am J Psychiatry. 2003;160(suppl 11):1-60.
4. Simon RI. Suicide risk assessment forms: form over substance? J Am Acad Psychiatry Law. 2009;37(3): 290-293.
5. Resnick PJ. Recognizing that the suicidal patient views you as an ‘adversary.’ Current Psychiatry. 2002;1(1):8.
6. Dervic K, Oquendo MA, Grunebaum MF, et al. Religious affiliation and suicide attempt. Am J Psychiatry. 2004; 161(12):2303-2308.
7. Harris CE, Barraclough B. Suicide as an outcome for mental disorders. A meta-analysis. Br J Psychiatry. 1997;170:205-228.
8. Health insurance portability and accountability act of 1996. Pub L No. 104-191.
9. Simon RI, Shuman DW. Clinical manual of psychiatry and law. Arlington, VA: American Psychiatric Publishing, Inc; 2007.
10. American Psychiatric Association. Principles of medical ethics with annotations especially applicable to psychiatry. Section 4, annotation 8. Washington, DC: American Psychiatric Publishing, Inc; 2001.
11. Simon RI, Gutheil TG. Sudden improvement in high-risk suicidal patients: should it be trusted? Psych Serv. 2009; 60(3):387-389.
12. Pokorny AD. Prediction of suicide in psychiatric patients. Report of a prospective study. Arch Gen Psychiatry. 1983; 4(3):249-257.
13. Simon RI. Imminent suicide: the illusion of short-term prediction. Suicide Life Threat Behav. 2006;36(3): 296-301.
14. Simon RI, Shuman DW. Therapeutic risk management of clinical-legal dilemmas: should it be a core competency? J Am Acad Psychiatry Law. 2009;37(2):155-161.
15. Linehan MM, Goodstein JL, Nielsen SL, et al. Reasons for staying alive when you are thinking of killing yourself: the reasons for living inventory. J Consult Clin Psychol. 1983;51(2):276-286.
16. Malone KM, Oquendo MA, Hass GL, et al. Protective factors against suicidal acts in major depression: reasons for living. Am J Psychiatry. 2000;157(7):1084-1088.
17. Baldessarini RJ, Pompili M, Tondo L. Bipolar disorder. In: Simon RI, Hales RE, eds. Textbook of suicide assessment and management. Arlington, VA: American Psychiatric Publishing, Inc; 2006:159-176.
1. Stanford EJ, Goetz RR, Bloom JD. The No Harm Contract in the emergency assessment of suicide risk. J Clin Psychiatry. 1994;55(8):344-348.
2. Simon RI, Hales RE, eds. Textbook of suicide assessment and management. 2nd ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2012.
3. Practice guidelines for the assessment and treatment of patients with suicidal behaviors [Erratum in Am J Psychiatry. 2004;161(4):776]. Am J Psychiatry. 2003;160(suppl 11):1-60.
4. Simon RI. Suicide risk assessment forms: form over substance? J Am Acad Psychiatry Law. 2009;37(3): 290-293.
5. Resnick PJ. Recognizing that the suicidal patient views you as an ‘adversary.’ Current Psychiatry. 2002;1(1):8.
6. Dervic K, Oquendo MA, Grunebaum MF, et al. Religious affiliation and suicide attempt. Am J Psychiatry. 2004; 161(12):2303-2308.
7. Harris CE, Barraclough B. Suicide as an outcome for mental disorders. A meta-analysis. Br J Psychiatry. 1997;170:205-228.
8. Health insurance portability and accountability act of 1996. Pub L No. 104-191.
9. Simon RI, Shuman DW. Clinical manual of psychiatry and law. Arlington, VA: American Psychiatric Publishing, Inc; 2007.
10. American Psychiatric Association. Principles of medical ethics with annotations especially applicable to psychiatry. Section 4, annotation 8. Washington, DC: American Psychiatric Publishing, Inc; 2001.
11. Simon RI, Gutheil TG. Sudden improvement in high-risk suicidal patients: should it be trusted? Psych Serv. 2009; 60(3):387-389.
12. Pokorny AD. Prediction of suicide in psychiatric patients. Report of a prospective study. Arch Gen Psychiatry. 1983; 4(3):249-257.
13. Simon RI. Imminent suicide: the illusion of short-term prediction. Suicide Life Threat Behav. 2006;36(3): 296-301.
14. Simon RI, Shuman DW. Therapeutic risk management of clinical-legal dilemmas: should it be a core competency? J Am Acad Psychiatry Law. 2009;37(2):155-161.
15. Linehan MM, Goodstein JL, Nielsen SL, et al. Reasons for staying alive when you are thinking of killing yourself: the reasons for living inventory. J Consult Clin Psychol. 1983;51(2):276-286.
16. Malone KM, Oquendo MA, Hass GL, et al. Protective factors against suicidal acts in major depression: reasons for living. Am J Psychiatry. 2000;157(7):1084-1088.
17. Baldessarini RJ, Pompili M, Tondo L. Bipolar disorder. In: Simon RI, Hales RE, eds. Textbook of suicide assessment and management. Arlington, VA: American Psychiatric Publishing, Inc; 2006:159-176.
Clozapine is a vastly underutilized, unique agent with multiple applications
Since clozapine was launched in 1989, miraculous improvements and “awakenings” have been reported in many patients afflicted with severe schizophrenia and considered hopelessly refractory to antipsychotic pharmacotherapy. Not only do severely disabled patients regain their sanity and return to normal functioning, but the joy that their family and treating psychiatrist experience is priceless.
That’s why I am perplexed by how infrequently clozapine is used in the United States (in about 5% of patients)—even though approximately 25% of patients who have schizophrenia are either treatment-resistant or have refractory hallucinations or delusions.
Consider Bethany’s case. She was one of my young patients, who, after taking clozapine, recovered fully and resumed a productive life, after years of homelessness during which she was controlled by auditory hallucinations.
Bethany’s story began well…
Bethany grew up in a loving home, smart and talented, an “A” student in high school and talented violinist. She received a scholarship to a prestigious private university at 16 and left her parent’s home in Ohio to major in molecular biology. Her goal was to attend medical school. She excelled during her first 3 years of college, and even published 2 papers in top-tier science journals.
In her senior year, after returning from a trip to Africa, Bethany began to change. She neglected her studies and focused on raising money for HIV clinics in Africa. She began getting F’s instead of A’s, lost her scholarship and her residence hall room, and had to drop out of college. Soon, she began hearing voices commanding her every action.
..but took a really bad turn
Bethany became homeless for the next 4.5 years. She ate discarded food from garbage cans, had no change of clothes, and slept on a concrete slab behind a downtown church in a major city in California. Her parents lost track of her, although her mother, a retired nurse, frantically and relentlessly tried to find out what happened to her only daughter during that time.
Eventually, Bethany was arrested when she was found screaming back at the voices, at midnight in a residential area of the city. She was hospitalized on a psychiatric ward and given antipsychotics, but with only modest improvement.
Her parents were contacted; immediately, they flew to California to see her. The treating psychiatrist told them that their daughter had schizophrenia, and that they should lower their expectations because she would be totally disabled for the rest of her life. They brought Bethany back to Ohio where, after a tumultuous year of failed trials of several antipsychotics to suppress the auditory hallucinations, we gave her clozapine.
Gradually, Bethany improved, but she still could not read a book or magazine (which I urged her to do) without the voices intensifying and preventing her from reading.
Bethany recovers
After 6 to 8 months on clozapine, however, Bethany’s auditory hallucinations faded away. With my encouragement, she enrolled at the University of Cincinnati and took 1 course at a time. She began to get A’s again—in advanced courses, such as genetics, physics, and molecular biology. She completed her degree requirements and graduated with honors, with a Bachelor of Science degree in molecular biology. She also served as a marshal in the commencement ceremony procession.
Over the next year, with strong encouragement, Bethany wrote a book about her remarkable recovery from refractory psychosis.1 In addition, her mother wrote a deeply emotional book that described the gut-wrenching ordeal that she and her husband went through during the years that Bethany disappeared.2 I urge you to read these inspiring books (Figure) about the remarkable recovery from refractory psychosis and the heavy family burden of schizophrenia.
Back to clozapine
Although the package insert for clozapine contains 5 black-box warnings (for agranulocytosis, seizures, myocarditis, respiratory effects, and increased mortality in geriatric patients with psychosis associated with dementia), the drug is a useful last-resort medication for several approved indications and off-label uses. In addition to the official, evidence-based indication for treatment-resistant and refractory schizophrenia,3 clozapine is FDA-approved for suicidality in schizophrenia.4 Clinically reported, but unapproved, uses are listed in the Table.5-13
A little-known advantage of clozapine is its salutary effect on mortality. In a Finnish study of 66,881 persons who had schizophrenia,14 those taking clozapine had, overall, lower mortality during the treatment period than those taking any of the 6 most commonly used antipsychotic drugs.
No doubt, clozapine is associated with serious side effects15—but so is chemotherapy for cancer, and oncologists do not hesitate to use it to save their patients from physical death. Severe schizophrenia is like a cancer of the mind, and clozapine is its chemotherapy.
Fortunately for Bethany, she had almost no physical adverse effects from clozapine except for intense sedation, which was mitigated with modafinil.
We should use clozapine more than we do
Clozapine has the potential to have a healing effect for many patients whose schizophrenia is resistant to treatment. Most such patients, however, never receive a trial of the drug. Furthermore, few practitioners use clozapine for schizophrenia patients with suicidal tendencies, despite the high rate of suicide completion in schizophrenia.16
Clozapine remains, regrettably, an underutilized agent in psychiatry. Until other breakthrough drugs are discovered, its use ought to be double or triple what it is now because there are many people like Bethany who are not being given a chance to recover from their illness.
1. Yeiser B. Mind estranged. My journey from schizophrenia and homelessness to recovery. North Charleston, SC: CreateSpace Independent Publishing Platform; 2014.
2. Yeiser KS. Flight from reason: a mother’s story of schizophrenia, recovery and hope. North Charleston, SC: CreateSpace Independent Publishing Platform; 2014.
3. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
4. Meltzer HY, Alphs L, Green AI, et al. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT) [Erratum in: Arch Gen Psychiatry. 2003;60(7):735.] Arch Gen Psychiatry. 2003;60(1):82-91.
5. Frogley C, Taylor D, Dickens G, et al. A systematic review of the evidence of clozapine’s anti-aggressive effects. Int J Neuropsychopharmacol. 2012;15(9):1351-1371.
6. Margetié B, Aukst-Margetié B, Zarkovié-Palijan T. Successful treatment of polydipsia, water intoxication, and delusional jealousy in an alcohol dependent patient with clozapine. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(7):1347-1349.
7. Cupina D, Boulton M. Secondary delusional parasitosis treated successfully with a combination of clozapine and citalopram. Psychosomatics. 2012;53(3):301-302.
8. Connolly BD, Lang AE. Pharmacolgoic treatment of Parkinson disease: a review. JAMA. 2014;311(16):1670-1683.
9. Hazari N, Kate N, Grover S, et al. Clozapine and tardive movement disorders: a review. Asian J Psychiatry. 2013;6(6):439-451.
10. Zarzar T, McEvoy J. Clozapine for self-injurious behavior in individuals with borderline personality disorder. Ther Adv Psychopharmacol. 2013;3(5):272-274.
11. Vohra AK. Treatment of severe borderline personality disorder with clozapine. Indian J Psychiatry. 2010;52(3):267-269.
12. Ifteni P, Correll CU, Nielse J, et al. Rapid clozapine titration in treatment-refractory bipolar disorder. J Affect Disord. 2014;166:168-172.
13. Rogoz Z. Combined treatment with atypical antipsychotics and antidepressants in treatment-resistant depression: preclinical and clinical efficacy. Pharmacol Rep. 2013;65(6)1535-1544.
14. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
15. Raja M, Raja S. Clozapine safety, 40 years later [published online April 28, 2014]. Curr Drug Saf. doi: 10.2174/1574886309666140428115040.
16. Siris SG. Suicide and schizophrenia. J Psychopharmacol. 2001;15(2):127-135.
Since clozapine was launched in 1989, miraculous improvements and “awakenings” have been reported in many patients afflicted with severe schizophrenia and considered hopelessly refractory to antipsychotic pharmacotherapy. Not only do severely disabled patients regain their sanity and return to normal functioning, but the joy that their family and treating psychiatrist experience is priceless.
That’s why I am perplexed by how infrequently clozapine is used in the United States (in about 5% of patients)—even though approximately 25% of patients who have schizophrenia are either treatment-resistant or have refractory hallucinations or delusions.
Consider Bethany’s case. She was one of my young patients, who, after taking clozapine, recovered fully and resumed a productive life, after years of homelessness during which she was controlled by auditory hallucinations.
Bethany’s story began well…
Bethany grew up in a loving home, smart and talented, an “A” student in high school and talented violinist. She received a scholarship to a prestigious private university at 16 and left her parent’s home in Ohio to major in molecular biology. Her goal was to attend medical school. She excelled during her first 3 years of college, and even published 2 papers in top-tier science journals.
In her senior year, after returning from a trip to Africa, Bethany began to change. She neglected her studies and focused on raising money for HIV clinics in Africa. She began getting F’s instead of A’s, lost her scholarship and her residence hall room, and had to drop out of college. Soon, she began hearing voices commanding her every action.
..but took a really bad turn
Bethany became homeless for the next 4.5 years. She ate discarded food from garbage cans, had no change of clothes, and slept on a concrete slab behind a downtown church in a major city in California. Her parents lost track of her, although her mother, a retired nurse, frantically and relentlessly tried to find out what happened to her only daughter during that time.
Eventually, Bethany was arrested when she was found screaming back at the voices, at midnight in a residential area of the city. She was hospitalized on a psychiatric ward and given antipsychotics, but with only modest improvement.
Her parents were contacted; immediately, they flew to California to see her. The treating psychiatrist told them that their daughter had schizophrenia, and that they should lower their expectations because she would be totally disabled for the rest of her life. They brought Bethany back to Ohio where, after a tumultuous year of failed trials of several antipsychotics to suppress the auditory hallucinations, we gave her clozapine.
Gradually, Bethany improved, but she still could not read a book or magazine (which I urged her to do) without the voices intensifying and preventing her from reading.
Bethany recovers
After 6 to 8 months on clozapine, however, Bethany’s auditory hallucinations faded away. With my encouragement, she enrolled at the University of Cincinnati and took 1 course at a time. She began to get A’s again—in advanced courses, such as genetics, physics, and molecular biology. She completed her degree requirements and graduated with honors, with a Bachelor of Science degree in molecular biology. She also served as a marshal in the commencement ceremony procession.
Over the next year, with strong encouragement, Bethany wrote a book about her remarkable recovery from refractory psychosis.1 In addition, her mother wrote a deeply emotional book that described the gut-wrenching ordeal that she and her husband went through during the years that Bethany disappeared.2 I urge you to read these inspiring books (Figure) about the remarkable recovery from refractory psychosis and the heavy family burden of schizophrenia.
Back to clozapine
Although the package insert for clozapine contains 5 black-box warnings (for agranulocytosis, seizures, myocarditis, respiratory effects, and increased mortality in geriatric patients with psychosis associated with dementia), the drug is a useful last-resort medication for several approved indications and off-label uses. In addition to the official, evidence-based indication for treatment-resistant and refractory schizophrenia,3 clozapine is FDA-approved for suicidality in schizophrenia.4 Clinically reported, but unapproved, uses are listed in the Table.5-13
A little-known advantage of clozapine is its salutary effect on mortality. In a Finnish study of 66,881 persons who had schizophrenia,14 those taking clozapine had, overall, lower mortality during the treatment period than those taking any of the 6 most commonly used antipsychotic drugs.
No doubt, clozapine is associated with serious side effects15—but so is chemotherapy for cancer, and oncologists do not hesitate to use it to save their patients from physical death. Severe schizophrenia is like a cancer of the mind, and clozapine is its chemotherapy.
Fortunately for Bethany, she had almost no physical adverse effects from clozapine except for intense sedation, which was mitigated with modafinil.
We should use clozapine more than we do
Clozapine has the potential to have a healing effect for many patients whose schizophrenia is resistant to treatment. Most such patients, however, never receive a trial of the drug. Furthermore, few practitioners use clozapine for schizophrenia patients with suicidal tendencies, despite the high rate of suicide completion in schizophrenia.16
Clozapine remains, regrettably, an underutilized agent in psychiatry. Until other breakthrough drugs are discovered, its use ought to be double or triple what it is now because there are many people like Bethany who are not being given a chance to recover from their illness.
Since clozapine was launched in 1989, miraculous improvements and “awakenings” have been reported in many patients afflicted with severe schizophrenia and considered hopelessly refractory to antipsychotic pharmacotherapy. Not only do severely disabled patients regain their sanity and return to normal functioning, but the joy that their family and treating psychiatrist experience is priceless.
That’s why I am perplexed by how infrequently clozapine is used in the United States (in about 5% of patients)—even though approximately 25% of patients who have schizophrenia are either treatment-resistant or have refractory hallucinations or delusions.
Consider Bethany’s case. She was one of my young patients, who, after taking clozapine, recovered fully and resumed a productive life, after years of homelessness during which she was controlled by auditory hallucinations.
Bethany’s story began well…
Bethany grew up in a loving home, smart and talented, an “A” student in high school and talented violinist. She received a scholarship to a prestigious private university at 16 and left her parent’s home in Ohio to major in molecular biology. Her goal was to attend medical school. She excelled during her first 3 years of college, and even published 2 papers in top-tier science journals.
In her senior year, after returning from a trip to Africa, Bethany began to change. She neglected her studies and focused on raising money for HIV clinics in Africa. She began getting F’s instead of A’s, lost her scholarship and her residence hall room, and had to drop out of college. Soon, she began hearing voices commanding her every action.
..but took a really bad turn
Bethany became homeless for the next 4.5 years. She ate discarded food from garbage cans, had no change of clothes, and slept on a concrete slab behind a downtown church in a major city in California. Her parents lost track of her, although her mother, a retired nurse, frantically and relentlessly tried to find out what happened to her only daughter during that time.
Eventually, Bethany was arrested when she was found screaming back at the voices, at midnight in a residential area of the city. She was hospitalized on a psychiatric ward and given antipsychotics, but with only modest improvement.
Her parents were contacted; immediately, they flew to California to see her. The treating psychiatrist told them that their daughter had schizophrenia, and that they should lower their expectations because she would be totally disabled for the rest of her life. They brought Bethany back to Ohio where, after a tumultuous year of failed trials of several antipsychotics to suppress the auditory hallucinations, we gave her clozapine.
Gradually, Bethany improved, but she still could not read a book or magazine (which I urged her to do) without the voices intensifying and preventing her from reading.
Bethany recovers
After 6 to 8 months on clozapine, however, Bethany’s auditory hallucinations faded away. With my encouragement, she enrolled at the University of Cincinnati and took 1 course at a time. She began to get A’s again—in advanced courses, such as genetics, physics, and molecular biology. She completed her degree requirements and graduated with honors, with a Bachelor of Science degree in molecular biology. She also served as a marshal in the commencement ceremony procession.
Over the next year, with strong encouragement, Bethany wrote a book about her remarkable recovery from refractory psychosis.1 In addition, her mother wrote a deeply emotional book that described the gut-wrenching ordeal that she and her husband went through during the years that Bethany disappeared.2 I urge you to read these inspiring books (Figure) about the remarkable recovery from refractory psychosis and the heavy family burden of schizophrenia.
Back to clozapine
Although the package insert for clozapine contains 5 black-box warnings (for agranulocytosis, seizures, myocarditis, respiratory effects, and increased mortality in geriatric patients with psychosis associated with dementia), the drug is a useful last-resort medication for several approved indications and off-label uses. In addition to the official, evidence-based indication for treatment-resistant and refractory schizophrenia,3 clozapine is FDA-approved for suicidality in schizophrenia.4 Clinically reported, but unapproved, uses are listed in the Table.5-13
A little-known advantage of clozapine is its salutary effect on mortality. In a Finnish study of 66,881 persons who had schizophrenia,14 those taking clozapine had, overall, lower mortality during the treatment period than those taking any of the 6 most commonly used antipsychotic drugs.
No doubt, clozapine is associated with serious side effects15—but so is chemotherapy for cancer, and oncologists do not hesitate to use it to save their patients from physical death. Severe schizophrenia is like a cancer of the mind, and clozapine is its chemotherapy.
Fortunately for Bethany, she had almost no physical adverse effects from clozapine except for intense sedation, which was mitigated with modafinil.
We should use clozapine more than we do
Clozapine has the potential to have a healing effect for many patients whose schizophrenia is resistant to treatment. Most such patients, however, never receive a trial of the drug. Furthermore, few practitioners use clozapine for schizophrenia patients with suicidal tendencies, despite the high rate of suicide completion in schizophrenia.16
Clozapine remains, regrettably, an underutilized agent in psychiatry. Until other breakthrough drugs are discovered, its use ought to be double or triple what it is now because there are many people like Bethany who are not being given a chance to recover from their illness.
1. Yeiser B. Mind estranged. My journey from schizophrenia and homelessness to recovery. North Charleston, SC: CreateSpace Independent Publishing Platform; 2014.
2. Yeiser KS. Flight from reason: a mother’s story of schizophrenia, recovery and hope. North Charleston, SC: CreateSpace Independent Publishing Platform; 2014.
3. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
4. Meltzer HY, Alphs L, Green AI, et al. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT) [Erratum in: Arch Gen Psychiatry. 2003;60(7):735.] Arch Gen Psychiatry. 2003;60(1):82-91.
5. Frogley C, Taylor D, Dickens G, et al. A systematic review of the evidence of clozapine’s anti-aggressive effects. Int J Neuropsychopharmacol. 2012;15(9):1351-1371.
6. Margetié B, Aukst-Margetié B, Zarkovié-Palijan T. Successful treatment of polydipsia, water intoxication, and delusional jealousy in an alcohol dependent patient with clozapine. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(7):1347-1349.
7. Cupina D, Boulton M. Secondary delusional parasitosis treated successfully with a combination of clozapine and citalopram. Psychosomatics. 2012;53(3):301-302.
8. Connolly BD, Lang AE. Pharmacolgoic treatment of Parkinson disease: a review. JAMA. 2014;311(16):1670-1683.
9. Hazari N, Kate N, Grover S, et al. Clozapine and tardive movement disorders: a review. Asian J Psychiatry. 2013;6(6):439-451.
10. Zarzar T, McEvoy J. Clozapine for self-injurious behavior in individuals with borderline personality disorder. Ther Adv Psychopharmacol. 2013;3(5):272-274.
11. Vohra AK. Treatment of severe borderline personality disorder with clozapine. Indian J Psychiatry. 2010;52(3):267-269.
12. Ifteni P, Correll CU, Nielse J, et al. Rapid clozapine titration in treatment-refractory bipolar disorder. J Affect Disord. 2014;166:168-172.
13. Rogoz Z. Combined treatment with atypical antipsychotics and antidepressants in treatment-resistant depression: preclinical and clinical efficacy. Pharmacol Rep. 2013;65(6)1535-1544.
14. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
15. Raja M, Raja S. Clozapine safety, 40 years later [published online April 28, 2014]. Curr Drug Saf. doi: 10.2174/1574886309666140428115040.
16. Siris SG. Suicide and schizophrenia. J Psychopharmacol. 2001;15(2):127-135.
1. Yeiser B. Mind estranged. My journey from schizophrenia and homelessness to recovery. North Charleston, SC: CreateSpace Independent Publishing Platform; 2014.
2. Yeiser KS. Flight from reason: a mother’s story of schizophrenia, recovery and hope. North Charleston, SC: CreateSpace Independent Publishing Platform; 2014.
3. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
4. Meltzer HY, Alphs L, Green AI, et al. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT) [Erratum in: Arch Gen Psychiatry. 2003;60(7):735.] Arch Gen Psychiatry. 2003;60(1):82-91.
5. Frogley C, Taylor D, Dickens G, et al. A systematic review of the evidence of clozapine’s anti-aggressive effects. Int J Neuropsychopharmacol. 2012;15(9):1351-1371.
6. Margetié B, Aukst-Margetié B, Zarkovié-Palijan T. Successful treatment of polydipsia, water intoxication, and delusional jealousy in an alcohol dependent patient with clozapine. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(7):1347-1349.
7. Cupina D, Boulton M. Secondary delusional parasitosis treated successfully with a combination of clozapine and citalopram. Psychosomatics. 2012;53(3):301-302.
8. Connolly BD, Lang AE. Pharmacolgoic treatment of Parkinson disease: a review. JAMA. 2014;311(16):1670-1683.
9. Hazari N, Kate N, Grover S, et al. Clozapine and tardive movement disorders: a review. Asian J Psychiatry. 2013;6(6):439-451.
10. Zarzar T, McEvoy J. Clozapine for self-injurious behavior in individuals with borderline personality disorder. Ther Adv Psychopharmacol. 2013;3(5):272-274.
11. Vohra AK. Treatment of severe borderline personality disorder with clozapine. Indian J Psychiatry. 2010;52(3):267-269.
12. Ifteni P, Correll CU, Nielse J, et al. Rapid clozapine titration in treatment-refractory bipolar disorder. J Affect Disord. 2014;166:168-172.
13. Rogoz Z. Combined treatment with atypical antipsychotics and antidepressants in treatment-resistant depression: preclinical and clinical efficacy. Pharmacol Rep. 2013;65(6)1535-1544.
14. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
15. Raja M, Raja S. Clozapine safety, 40 years later [published online April 28, 2014]. Curr Drug Saf. doi: 10.2174/1574886309666140428115040.
16. Siris SG. Suicide and schizophrenia. J Psychopharmacol. 2001;15(2):127-135.
Monitoring calcium with lithium treatment
I appreciate Dr. McInnis’s article and his recommendation to monitor the comprehensive metabolic profile, including the calcium level, before and during lithium treatment. There is an association among lithium treatment, hypercalcemia, and hyperparathyroidism.1,2 This can occur by lithium reducing parathyroid hormone suppression or stimulating parathyroid glands.3
Surprisingly, many guidelines do not include a recommendation to monitor the calcium level; however, the International Society for Bipolar Disorders and other experts do recommend obtaining a calcium level before initiating lithium therapy and at least annually thereafter.1,4 If hypercalcemia is present, assessing lithium and the parathyroid hormone level is recommended.3
Clinicians can continue lithium and monitor calcium if treatment is beneficial, hypercalcemia is mild, and the patient is asymptomatic.2 For a symptomatic patient or one who has significant hypercalcemia, clinicians should consider discontinuing lithium and monitoring for a normalizing calcium level.2 For patients with significant hypercalcemia who need lithium therapy, consultation with an endocrinologist is advised.3
Jonathan R. Scarff, MD
VA Outpatient Clinic
Spartanburg, South Carolina
Dr. McInnis responds
Generally, calcium is included in the comprehensive biochemistry panel (Table 1). Typically, magnesium or phosphorus is overlooked, and therefore was specifically included in the table of recommendations. There is a complex relationship between lithium and calcium; Dr. Scarff’s points highlight this. It is noteworthy that lithium normalizes the calcium amplitude during action potentials in neurons derived from induced pluripotent stem cells from persons with BD1; this suggests that there might be a direct mode of action in BD involving lithium and calcium. This finding further emphasizes the importance of monitoring calcium, and the wise clinician will verify that it is included in the comprehensive biochemistry panel.
1. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
2. Lehmann SW, Lee J. Lithium-associated hyper-calcemia and hyperparathyroidism in the elderly: what do we know? J Affect Disord. 2013;146(2): 151-157.
3. Broome JT, Solorzano CC. Lithium use and primary hyperparathyroidism. Endocr Pract. 2011; 17(suppl 1):31-35.
4. Ng F, Mammen OK, Wilting I, et al. The International Society for Bipolar Disorders (ISBD) consensus guidelines for the safety monitoring of bipolar disorder treatments. Bipolar Disord. 2009; 11(6):559-595.
Reference
1. Chen HM, DeLong CJ, Bame M, et al. Transcripts involved in calcium signaling and telencephalic neuronal fate are altered in induced pluripotent stem cells from bipolar disorder patients. Transl Psychiatry. 2014;4:e375. doi:10.1038/tp.2014.12.
I appreciate Dr. McInnis’s article and his recommendation to monitor the comprehensive metabolic profile, including the calcium level, before and during lithium treatment. There is an association among lithium treatment, hypercalcemia, and hyperparathyroidism.1,2 This can occur by lithium reducing parathyroid hormone suppression or stimulating parathyroid glands.3
Surprisingly, many guidelines do not include a recommendation to monitor the calcium level; however, the International Society for Bipolar Disorders and other experts do recommend obtaining a calcium level before initiating lithium therapy and at least annually thereafter.1,4 If hypercalcemia is present, assessing lithium and the parathyroid hormone level is recommended.3
Clinicians can continue lithium and monitor calcium if treatment is beneficial, hypercalcemia is mild, and the patient is asymptomatic.2 For a symptomatic patient or one who has significant hypercalcemia, clinicians should consider discontinuing lithium and monitoring for a normalizing calcium level.2 For patients with significant hypercalcemia who need lithium therapy, consultation with an endocrinologist is advised.3
Jonathan R. Scarff, MD
VA Outpatient Clinic
Spartanburg, South Carolina
Dr. McInnis responds
Generally, calcium is included in the comprehensive biochemistry panel (Table 1). Typically, magnesium or phosphorus is overlooked, and therefore was specifically included in the table of recommendations. There is a complex relationship between lithium and calcium; Dr. Scarff’s points highlight this. It is noteworthy that lithium normalizes the calcium amplitude during action potentials in neurons derived from induced pluripotent stem cells from persons with BD1; this suggests that there might be a direct mode of action in BD involving lithium and calcium. This finding further emphasizes the importance of monitoring calcium, and the wise clinician will verify that it is included in the comprehensive biochemistry panel.
I appreciate Dr. McInnis’s article and his recommendation to monitor the comprehensive metabolic profile, including the calcium level, before and during lithium treatment. There is an association among lithium treatment, hypercalcemia, and hyperparathyroidism.1,2 This can occur by lithium reducing parathyroid hormone suppression or stimulating parathyroid glands.3
Surprisingly, many guidelines do not include a recommendation to monitor the calcium level; however, the International Society for Bipolar Disorders and other experts do recommend obtaining a calcium level before initiating lithium therapy and at least annually thereafter.1,4 If hypercalcemia is present, assessing lithium and the parathyroid hormone level is recommended.3
Clinicians can continue lithium and monitor calcium if treatment is beneficial, hypercalcemia is mild, and the patient is asymptomatic.2 For a symptomatic patient or one who has significant hypercalcemia, clinicians should consider discontinuing lithium and monitoring for a normalizing calcium level.2 For patients with significant hypercalcemia who need lithium therapy, consultation with an endocrinologist is advised.3
Jonathan R. Scarff, MD
VA Outpatient Clinic
Spartanburg, South Carolina
Dr. McInnis responds
Generally, calcium is included in the comprehensive biochemistry panel (Table 1). Typically, magnesium or phosphorus is overlooked, and therefore was specifically included in the table of recommendations. There is a complex relationship between lithium and calcium; Dr. Scarff’s points highlight this. It is noteworthy that lithium normalizes the calcium amplitude during action potentials in neurons derived from induced pluripotent stem cells from persons with BD1; this suggests that there might be a direct mode of action in BD involving lithium and calcium. This finding further emphasizes the importance of monitoring calcium, and the wise clinician will verify that it is included in the comprehensive biochemistry panel.
1. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
2. Lehmann SW, Lee J. Lithium-associated hyper-calcemia and hyperparathyroidism in the elderly: what do we know? J Affect Disord. 2013;146(2): 151-157.
3. Broome JT, Solorzano CC. Lithium use and primary hyperparathyroidism. Endocr Pract. 2011; 17(suppl 1):31-35.
4. Ng F, Mammen OK, Wilting I, et al. The International Society for Bipolar Disorders (ISBD) consensus guidelines for the safety monitoring of bipolar disorder treatments. Bipolar Disord. 2009; 11(6):559-595.
Reference
1. Chen HM, DeLong CJ, Bame M, et al. Transcripts involved in calcium signaling and telencephalic neuronal fate are altered in induced pluripotent stem cells from bipolar disorder patients. Transl Psychiatry. 2014;4:e375. doi:10.1038/tp.2014.12.
1. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
2. Lehmann SW, Lee J. Lithium-associated hyper-calcemia and hyperparathyroidism in the elderly: what do we know? J Affect Disord. 2013;146(2): 151-157.
3. Broome JT, Solorzano CC. Lithium use and primary hyperparathyroidism. Endocr Pract. 2011; 17(suppl 1):31-35.
4. Ng F, Mammen OK, Wilting I, et al. The International Society for Bipolar Disorders (ISBD) consensus guidelines for the safety monitoring of bipolar disorder treatments. Bipolar Disord. 2009; 11(6):559-595.
Reference
1. Chen HM, DeLong CJ, Bame M, et al. Transcripts involved in calcium signaling and telencephalic neuronal fate are altered in induced pluripotent stem cells from bipolar disorder patients. Transl Psychiatry. 2014;4:e375. doi:10.1038/tp.2014.12.