User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Potentially dangerous mix: Antiretrovirals and drugs of abuse
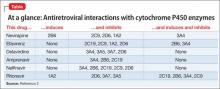
Patients with HIV often receive antiretroviral therapy, which includes non-nucleoside reverse transcriptase inhibitors and protease inhibitors. Opiate, amphetamine, cocaine, and Cannabis abuse are common among this population.1 Many of these substances and antiretroviral medications undergo hepatic metabolism by cytochrome P450 (CYP) isoenzymes, which could lead to adverse events (Table2).
MDMA
The synthetic derivative of the amphetamine 3,4-methylenedioxymethamphetamine (MDMA [“Ecstasy” or “Molly”]) is metabolized by CYP2D6; thus, coadministration of MDMA with ritonavir can result in MDMA toxicity. This can induce a dangerous, potentially fatal serotonin syndrome characterized by tachycardia, arrhythmia, hyperthermia, seizures, myocardial infarction, rhabdomyolysis, renal or liver failure, and death.3
Opiates
Opiates are metabolized by CYP2D6 and, sometimes by CYP3A4. Metabolism of opiates, such as oxycodone, is decreased when these drugs are coadministered with a CYP2D6 inhibitor—potentially leading to toxicity.
Analgesic effect may be augmented when a CYP2D6 inhibitor is started, and decreased when the agent is stopped. Inducers of CYP2D6 or CYP3A4 can lead to decreased analgesia and oxycodone withdrawal.
Efavirenz can cause methadone withdrawal. Methadone inhibition of CYP2D6 and CYP3A4 can increase the serum level of antiretroviral medications, with adverse effects and resulting poor compliance.4
Cannabis
Tetrahydrocannabinol is metabolized by CYP3A4 and CYP2D6. Cannabis and CYP3A4 inhibitor co-utilization can cause toxicity, evidenced by paranoia, hallucinations, delusions, depersonalization, tachycardia, and orthostatic hypotension. Co-exposure of antiretroviral agents in occasional Cannabis users yields only a small change metabolically; however, nonadherence has been reported more frequently in heavy users.5
Education can help
The variability of CYP genotypes makes it important to understand drug metabolism as an aid to improving outcomes among patients with HIV who abuse drugs. Because of the risk for adverse effects, discuss the dangers of substance abuse with patients for whom antiretroviral therapy has been prescribed.
Clinical education should improve compliance and prognosis in patients with HIV. Advise drug users about pharmaceutical effects and risks of co-utilization. This might help some patients limit the use of illicit substances—and will help physicians manage pharmacotherapy with greater safety.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arvguidelines/22/hiv-and-illicit-drug-users. Updated March 27, 2012. Accessed July 26, 2013.
2. Walubo A. The role of cytochrome P450 in antiretroviral drug interactions. Expert Opin Drug Metab Toxicol. 2007;3(4):583-598.
3. Papaseit E, Vázquez A, Pérez-Mañá C, et al. Surviving life-threatening MDMA (3,4-methylenedioxymethamphetamine,ecstasy) toxicity caused by ritonavir (RTV).
Intensive Care Med. 2012;38(7):1239-1240.
4. Antoniou T, Tseng AL. Interactions between recreational drugs and antiretroviral agents. Ann Pharmacother. 2002;36(10):1598-1613.
5. Bonn-Miller MO, Oser ML, Bucossi MM, et al. Cannabis use and HIV antiretroviral therapy adherence and HIV-related symptoms. J Behav Med. 2014;37(1):1-10.
3,4-methylenedioxymethamphetamine, opiates
Patients with HIV often receive antiretroviral therapy, which includes non-nucleoside reverse transcriptase inhibitors and protease inhibitors. Opiate, amphetamine, cocaine, and Cannabis abuse are common among this population.1 Many of these substances and antiretroviral medications undergo hepatic metabolism by cytochrome P450 (CYP) isoenzymes, which could lead to adverse events (Table2).
MDMA
The synthetic derivative of the amphetamine 3,4-methylenedioxymethamphetamine (MDMA [“Ecstasy” or “Molly”]) is metabolized by CYP2D6; thus, coadministration of MDMA with ritonavir can result in MDMA toxicity. This can induce a dangerous, potentially fatal serotonin syndrome characterized by tachycardia, arrhythmia, hyperthermia, seizures, myocardial infarction, rhabdomyolysis, renal or liver failure, and death.3
Opiates
Opiates are metabolized by CYP2D6 and, sometimes by CYP3A4. Metabolism of opiates, such as oxycodone, is decreased when these drugs are coadministered with a CYP2D6 inhibitor—potentially leading to toxicity.
Analgesic effect may be augmented when a CYP2D6 inhibitor is started, and decreased when the agent is stopped. Inducers of CYP2D6 or CYP3A4 can lead to decreased analgesia and oxycodone withdrawal.
Efavirenz can cause methadone withdrawal. Methadone inhibition of CYP2D6 and CYP3A4 can increase the serum level of antiretroviral medications, with adverse effects and resulting poor compliance.4
Cannabis
Tetrahydrocannabinol is metabolized by CYP3A4 and CYP2D6. Cannabis and CYP3A4 inhibitor co-utilization can cause toxicity, evidenced by paranoia, hallucinations, delusions, depersonalization, tachycardia, and orthostatic hypotension. Co-exposure of antiretroviral agents in occasional Cannabis users yields only a small change metabolically; however, nonadherence has been reported more frequently in heavy users.5
Education can help
The variability of CYP genotypes makes it important to understand drug metabolism as an aid to improving outcomes among patients with HIV who abuse drugs. Because of the risk for adverse effects, discuss the dangers of substance abuse with patients for whom antiretroviral therapy has been prescribed.
Clinical education should improve compliance and prognosis in patients with HIV. Advise drug users about pharmaceutical effects and risks of co-utilization. This might help some patients limit the use of illicit substances—and will help physicians manage pharmacotherapy with greater safety.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Patients with HIV often receive antiretroviral therapy, which includes non-nucleoside reverse transcriptase inhibitors and protease inhibitors. Opiate, amphetamine, cocaine, and Cannabis abuse are common among this population.1 Many of these substances and antiretroviral medications undergo hepatic metabolism by cytochrome P450 (CYP) isoenzymes, which could lead to adverse events (Table2).
MDMA
The synthetic derivative of the amphetamine 3,4-methylenedioxymethamphetamine (MDMA [“Ecstasy” or “Molly”]) is metabolized by CYP2D6; thus, coadministration of MDMA with ritonavir can result in MDMA toxicity. This can induce a dangerous, potentially fatal serotonin syndrome characterized by tachycardia, arrhythmia, hyperthermia, seizures, myocardial infarction, rhabdomyolysis, renal or liver failure, and death.3
Opiates
Opiates are metabolized by CYP2D6 and, sometimes by CYP3A4. Metabolism of opiates, such as oxycodone, is decreased when these drugs are coadministered with a CYP2D6 inhibitor—potentially leading to toxicity.
Analgesic effect may be augmented when a CYP2D6 inhibitor is started, and decreased when the agent is stopped. Inducers of CYP2D6 or CYP3A4 can lead to decreased analgesia and oxycodone withdrawal.
Efavirenz can cause methadone withdrawal. Methadone inhibition of CYP2D6 and CYP3A4 can increase the serum level of antiretroviral medications, with adverse effects and resulting poor compliance.4
Cannabis
Tetrahydrocannabinol is metabolized by CYP3A4 and CYP2D6. Cannabis and CYP3A4 inhibitor co-utilization can cause toxicity, evidenced by paranoia, hallucinations, delusions, depersonalization, tachycardia, and orthostatic hypotension. Co-exposure of antiretroviral agents in occasional Cannabis users yields only a small change metabolically; however, nonadherence has been reported more frequently in heavy users.5
Education can help
The variability of CYP genotypes makes it important to understand drug metabolism as an aid to improving outcomes among patients with HIV who abuse drugs. Because of the risk for adverse effects, discuss the dangers of substance abuse with patients for whom antiretroviral therapy has been prescribed.
Clinical education should improve compliance and prognosis in patients with HIV. Advise drug users about pharmaceutical effects and risks of co-utilization. This might help some patients limit the use of illicit substances—and will help physicians manage pharmacotherapy with greater safety.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arvguidelines/22/hiv-and-illicit-drug-users. Updated March 27, 2012. Accessed July 26, 2013.
2. Walubo A. The role of cytochrome P450 in antiretroviral drug interactions. Expert Opin Drug Metab Toxicol. 2007;3(4):583-598.
3. Papaseit E, Vázquez A, Pérez-Mañá C, et al. Surviving life-threatening MDMA (3,4-methylenedioxymethamphetamine,ecstasy) toxicity caused by ritonavir (RTV).
Intensive Care Med. 2012;38(7):1239-1240.
4. Antoniou T, Tseng AL. Interactions between recreational drugs and antiretroviral agents. Ann Pharmacother. 2002;36(10):1598-1613.
5. Bonn-Miller MO, Oser ML, Bucossi MM, et al. Cannabis use and HIV antiretroviral therapy adherence and HIV-related symptoms. J Behav Med. 2014;37(1):1-10.
1. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arvguidelines/22/hiv-and-illicit-drug-users. Updated March 27, 2012. Accessed July 26, 2013.
2. Walubo A. The role of cytochrome P450 in antiretroviral drug interactions. Expert Opin Drug Metab Toxicol. 2007;3(4):583-598.
3. Papaseit E, Vázquez A, Pérez-Mañá C, et al. Surviving life-threatening MDMA (3,4-methylenedioxymethamphetamine,ecstasy) toxicity caused by ritonavir (RTV).
Intensive Care Med. 2012;38(7):1239-1240.
4. Antoniou T, Tseng AL. Interactions between recreational drugs and antiretroviral agents. Ann Pharmacother. 2002;36(10):1598-1613.
5. Bonn-Miller MO, Oser ML, Bucossi MM, et al. Cannabis use and HIV antiretroviral therapy adherence and HIV-related symptoms. J Behav Med. 2014;37(1):1-10.
3,4-methylenedioxymethamphetamine, opiates
3,4-methylenedioxymethamphetamine, opiates
Acting strange after trying to ‘get numb’
CASE Numb and confused
Mr. L, age 17, is admitted to the hospital after ingesting 24 diphenhydramine 25-mg tablets in 3 hours as a possible suicide attempt. His parents witnessed him behaving strangely and brought him to the hospital. They state that their son was visibly agitated and acting inappropriately. He was seen talking to birds, trees, and the walls of the house.
Mr. L says he is upset because he broke up with his girlfriend a week earlier after she asked if they could “take a break.” He says that he took the diphenhydramine because he wanted to “get numb” to deal with the emotional stress caused by the break-up.
After the break-up, Mr. L experienced middle-to-late insomnia and was unable to get more than 3 or 4 hours of sleep a night. He reports significant fatigue, depressed mood, anhedonia, impaired concentration, and psychomotor retardation. He denies homicidal ideation or auditory and visual hallucinations.
As an aside, Mr. L reports that, for the past year, he had difficulties with gender identity, sometimes thinking that he might be better off if he had been born a girl and that he felt uncomfortable in a male body.
Which treatment option would you choose for Mr. L’s substance abuse?
a) refer him to a 12-step program
b) begin supportive measures
c) administer activated charcoal
d) prescribe a benzodiazepine to control agitation
The authors’ observations
As youths gain increasing access to medical and pharmaceutical knowledge through the Internet and other sources, it appears that adolescent drug abuse has, in part, shifted toward more easily attainable over-the-counter (OTC) medications. Diphenhydramine, a first-generation antihistamine, can be abused for its effects on the CNS, such as disturbed coordination, irritability, paresthesia, blurred vision, and depression. Effects of diphenhydramine are increased by the presence of alcohol, monoamine oxidase inhibitors, diazepam, hypnotics, sedatives, tranquilizers, and other CNS depressants. In 2011, diphenhydramine abuse was involved in 19,012 emergency room visits, of which 9,301 were for drug-related suicide attempts.1
Diphenhydramine is an inverse agonist of the histamine H1 receptor.2 It is a member of the ethanolamine subclass of antihistaminergic agents.3 By reversing the effects of histamine on capillaries, diphenhydramine can reduce the intensity of allergic symptoms. Diphenhydramine also crosses the blood–brain barrier and antagonizes H1 receptors centrally.
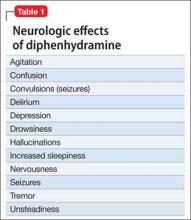
Used as a common sleep aid and allergy medication, the drug works primarily as an H1 receptor partial agonist, but also is a strong competitive antagonist at muscarinic acetylcholine receptors.4 It is abused for its sedative effects and its capacity to cause delirium and hallucinations.5 Diphenhydramine can have a stimulatory effect in children and young adults, instead of the sedating properties seen in adults.6 Such misuse is concerning because diphenhydramine overdose can lead to delirium, confusion, and hallucinations, tachycardia, seizures, mydriasis, xerostomia, urinary retention, ileus, anhidrosis, and hyperthermia. In severe cases it has been associated with cardiac arrhythmias, rhabdomyolysis, status epilepticus, and death.4,6 Neurologic symptoms of diphenhydramine overdose are listed in Table 1.
HISTORY Polysubstance abuse
Mr. L has a 2-year history of major depressive disorder and a history of Cannabis abuse with physiological dependence; Robitussin (base active ingredient, guaifenesin) and hydrocodone abuse with physiological dependence; 3,4-methylenedioxymethamphetamine (MDMA) abuse; and diphenhydramine abuse. He also has a history of gender dysphoria, although he reports that these feelings have become less severe over the past year.
Mr. L attends bi-weekly appointments with an outpatient psychiatrist and reportedly adheres to his medication regimen: fluoxetine, 40 mg/d, and risperidone, 1 mg at bedtime. He denies previous suicidal ideation, suicide attempts, homicidal ideation, or homicidal attempts. He reports no history of physical, sexual, or emotional abuse. He gets good grades in school and has no outstanding academic problems.
Mr. L began using Cannabis at age 14; his last use was 3 weeks before admission. He is guarded about his use of Robitussin, hydrocodone, and MDMA. However, Mr. L reports that he has researched diphenhydramine on the internet and believes that he can safely take up to 1,200 mg without overdosing. He reports normally taking 450 mg of diphenhydramine daily. Mr. L reports difficulty urinating after using diphenhydramine but no other physical complaints.
Mr. L lives with his father and stepmother and has a history of one psychiatric hospitalization at a different facility 2 months ago, followed by outpatient therapy. He obtained his Graduate Equivalency Diploma (GED) and plans to attend college.
At age 5, Mr. L emigrated from Turkey to the United States with his parents. His mother returned to Turkey when he was age 6 and has had no contact with her son since. Whenever Mr. L visits Turkey with his father, the patient refuses to see her, as per collaterals. He gets along well with his stepmother, who is his maternal aunt. Mr. L has been bullied at school and reportedly has few friends.
On mental status examination, Mr. L has an appropriate appearance and appears to be his stated age. He shows good eye contact and is cooperative. Muscle tone and gait are within normal limits. He has no abnormal movements. Speech, thought processes, and associations are normal. He denies auditory hallucinations, visual hallucinations, suicidal ideation (although he presented with a probable suicide attempt), or homicidal ideation. No delusions are elicited.
Mr. L shows poor judgment about his drug use and situation. He demonstrates limited insight, because he says his only goal is to get out of the hospital. He is alert, awake, and oriented to person, place, and time. He shows no memory or knowledge impairment. He appears euthymic with an inappropriate and constricted affect. On neurologic exam, he had mild tremors in his hands. The authors’ observationsTreatment for diphenhydramine overdose should begin quickly to prevent life-threatening effects and reduce the risk for mortality. The toxin can be removed from the patient’s GI tract with activated charcoal or gastric lavage if the patient presents within 1 hour of ingesting the substance. Administering IV fluids will prevent dehydration. Cardiac functioning is monitored and benzodiazepines could be administered to manage seizures.
Key elements of a toxicologic physical examination include:
• eyes: pupillary size, symmetry, and response to light (vertical or horizontal nystagmus)
• oropharynx: moist or dry mucous membranes, presence or absence of the gag reflex, distinctive odors
• abdomen: presence or absence and quality of bowel sounds
• skin: warm and dry, warm and sweaty, or cool
• neurologic: level of consciousness and mental status, presence of tremors, seizures, or other movement disorders, presence or absence and quality of deep tendon reflexes.7
If a child or adolescent patient cannot communicate how much of a drug he (she) has ingested, questions to ask parents or other informants include:
• Was the medication purchased recently, and if so was the bottle or box full before the patient took the pills?
• If the medication was not new, how many pills were in the bottle before the patient got to it?
• If the medication was prescribed, how many pills were originally prescribed, when was the medication prescribed, and how many pills were already taken prior to the patient getting to the bottle?
• How many pills were left in the bottle?
• How many pills were seen around the area where the patient was found?
• How many pills were found in the patient’s mouth?7
Recommendations
It is well known that OTC medication abuse is a growing medical problem (Table 2). Antihistamines, including diphenhydramine, are readily available to minors and adults. Because of the powerful sedating effects of antihistamines, many adolescent health practitioners give them to patients who have insomnia as a sleep aid.8 As in our case, antihistamines are used recreationally for their hallucinogenic effects, at dosages of 300 to 700 mg.9 Severe symptoms of toxicity, such as delirium and psychosis, seizures, and coma, occur at dosages ≥1,000 mg.9
With growing abuse of these medications, we aim to encourage detailed history taking about abuse of OTC drugs, especially diphenhydramine in adolescent patients.
Outcome Improvement, discharge
Mr. L is given a dual diagnosis of diphenhydramine-induced psychotic disorder with
hallucinations and diphenhydramine-induced depressive disorder, both with onset during intoxication. He also is given a provisional diagnosis of psychotic disorder not otherwise specified and major depressive disorder. Last, he is given a diagnosis of Cannabis dependence with physiological dependence, MDMA abuse, hydrocodone abuse, and Robitussin abuse.
Mr. L is maintained on fluoxetine, 40 mg/d, and risperidone, 1 mg at bedtime and 0.5 mg in the morning. He receives milieu, individual, group, recreational, and medical therapy while in the hospital. Symptoms abate and he is discharged with a plan to follow up with outpatient providers.
Bottom Line
Abuse of over-the-counter (OTC) drugs, such as diphenhydramine, among youths is a growing problem. Remember to question adolescents who appear intoxicated or to have overdosed not only about abuse of alcohol and illicit substances but also of common—and easily and legally accessible—OTC drugs.
Related Resources
• Carr BC. Efficacy, abuse, and toxicity of over-the-counter cough and cold medicines in the pediatric population. Curr Opin Pediatr. 2006;18(2):184-188.
• Thomas A, Nallur DG, Jones N, et al. Diphenhydramine abuse and detoxification: a brief review and case report. J Psychopharmacol. 2009;23(1):101-105.
Drug Brand Names
Diazepam • Valium Hydrocodone • Vicodin
Diphenhydramine • Benadryl Risperidone • Risperdal
Fluoxetine • Prozac
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. U.S. Department of Health and Human Services. Drug Abuse Warning Network, 2011: National estimates of drug-related emergency department visits. http://www.samhsa. gov/data/2k13/DAWN2k11ED/DAWN2k11ED.htm. Published May 2013. Accessed on September 29, 2014.
2. Yamashiro K, Kiryu J, Tsujikawa A, et al. Suppressive effects of histamine H1 receptor antagonist diphenhydramine on the leukocyte infiltration during endotoxin-induced uveitis. Exp Eye Res. 2001;73(1):69-80.
3. Skidgel RA, Kaplan AP, Erdos EG. Histamine, bradykinin, and their antagonists. In: Brunton L, Chabner B, Knollman B, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York, NY: McGraw Hill; 2011: 911-935.
4. Vearrier D, Curtis JA. Case files of the medical toxicology fellowship at Drexel University. Rhabdomyolysis and compartment syndrome following acute diphenhydramine overdose. J Med Toxicol. 2011;7(3):213-219.
5. Ho M, Tsai K, Liu C. Diphenhydramine overdose related delirium: a case report. Journal of Emergency and Critical Care Medicine. 2006;17(2):77-79.
6. Krenzelok EP, Anderson GM, Mirick M. Massive diphenhydramine overdose resulting in death. Ann Emerg Med. 1982;11(4):212-213.
7. Inaba AS. Toxicologic teasers: Testing your knowledge of clinical toxicology. Hawaii Med J. 1998;57(4):471-473.
8. Kaplan SL. Busner J. The use of prn and stat medication in three child psychiatric inpatient settings. Psychopharmacol Bull. 1997;33(1):161-164.
9. Radovanovic D, Meier PJ, Guirguis M, et al. Dose-dependent toxicity of diphenhydramine overdose. Hum Exp Toxicol. 2000;19(9):489-495.
CASE Numb and confused
Mr. L, age 17, is admitted to the hospital after ingesting 24 diphenhydramine 25-mg tablets in 3 hours as a possible suicide attempt. His parents witnessed him behaving strangely and brought him to the hospital. They state that their son was visibly agitated and acting inappropriately. He was seen talking to birds, trees, and the walls of the house.
Mr. L says he is upset because he broke up with his girlfriend a week earlier after she asked if they could “take a break.” He says that he took the diphenhydramine because he wanted to “get numb” to deal with the emotional stress caused by the break-up.
After the break-up, Mr. L experienced middle-to-late insomnia and was unable to get more than 3 or 4 hours of sleep a night. He reports significant fatigue, depressed mood, anhedonia, impaired concentration, and psychomotor retardation. He denies homicidal ideation or auditory and visual hallucinations.
As an aside, Mr. L reports that, for the past year, he had difficulties with gender identity, sometimes thinking that he might be better off if he had been born a girl and that he felt uncomfortable in a male body.
Which treatment option would you choose for Mr. L’s substance abuse?
a) refer him to a 12-step program
b) begin supportive measures
c) administer activated charcoal
d) prescribe a benzodiazepine to control agitation
The authors’ observations
As youths gain increasing access to medical and pharmaceutical knowledge through the Internet and other sources, it appears that adolescent drug abuse has, in part, shifted toward more easily attainable over-the-counter (OTC) medications. Diphenhydramine, a first-generation antihistamine, can be abused for its effects on the CNS, such as disturbed coordination, irritability, paresthesia, blurred vision, and depression. Effects of diphenhydramine are increased by the presence of alcohol, monoamine oxidase inhibitors, diazepam, hypnotics, sedatives, tranquilizers, and other CNS depressants. In 2011, diphenhydramine abuse was involved in 19,012 emergency room visits, of which 9,301 were for drug-related suicide attempts.1
Diphenhydramine is an inverse agonist of the histamine H1 receptor.2 It is a member of the ethanolamine subclass of antihistaminergic agents.3 By reversing the effects of histamine on capillaries, diphenhydramine can reduce the intensity of allergic symptoms. Diphenhydramine also crosses the blood–brain barrier and antagonizes H1 receptors centrally.
Used as a common sleep aid and allergy medication, the drug works primarily as an H1 receptor partial agonist, but also is a strong competitive antagonist at muscarinic acetylcholine receptors.4 It is abused for its sedative effects and its capacity to cause delirium and hallucinations.5 Diphenhydramine can have a stimulatory effect in children and young adults, instead of the sedating properties seen in adults.6 Such misuse is concerning because diphenhydramine overdose can lead to delirium, confusion, and hallucinations, tachycardia, seizures, mydriasis, xerostomia, urinary retention, ileus, anhidrosis, and hyperthermia. In severe cases it has been associated with cardiac arrhythmias, rhabdomyolysis, status epilepticus, and death.4,6 Neurologic symptoms of diphenhydramine overdose are listed in Table 1.
HISTORY Polysubstance abuse
Mr. L has a 2-year history of major depressive disorder and a history of Cannabis abuse with physiological dependence; Robitussin (base active ingredient, guaifenesin) and hydrocodone abuse with physiological dependence; 3,4-methylenedioxymethamphetamine (MDMA) abuse; and diphenhydramine abuse. He also has a history of gender dysphoria, although he reports that these feelings have become less severe over the past year.
Mr. L attends bi-weekly appointments with an outpatient psychiatrist and reportedly adheres to his medication regimen: fluoxetine, 40 mg/d, and risperidone, 1 mg at bedtime. He denies previous suicidal ideation, suicide attempts, homicidal ideation, or homicidal attempts. He reports no history of physical, sexual, or emotional abuse. He gets good grades in school and has no outstanding academic problems.
Mr. L began using Cannabis at age 14; his last use was 3 weeks before admission. He is guarded about his use of Robitussin, hydrocodone, and MDMA. However, Mr. L reports that he has researched diphenhydramine on the internet and believes that he can safely take up to 1,200 mg without overdosing. He reports normally taking 450 mg of diphenhydramine daily. Mr. L reports difficulty urinating after using diphenhydramine but no other physical complaints.
Mr. L lives with his father and stepmother and has a history of one psychiatric hospitalization at a different facility 2 months ago, followed by outpatient therapy. He obtained his Graduate Equivalency Diploma (GED) and plans to attend college.
At age 5, Mr. L emigrated from Turkey to the United States with his parents. His mother returned to Turkey when he was age 6 and has had no contact with her son since. Whenever Mr. L visits Turkey with his father, the patient refuses to see her, as per collaterals. He gets along well with his stepmother, who is his maternal aunt. Mr. L has been bullied at school and reportedly has few friends.
On mental status examination, Mr. L has an appropriate appearance and appears to be his stated age. He shows good eye contact and is cooperative. Muscle tone and gait are within normal limits. He has no abnormal movements. Speech, thought processes, and associations are normal. He denies auditory hallucinations, visual hallucinations, suicidal ideation (although he presented with a probable suicide attempt), or homicidal ideation. No delusions are elicited.
Mr. L shows poor judgment about his drug use and situation. He demonstrates limited insight, because he says his only goal is to get out of the hospital. He is alert, awake, and oriented to person, place, and time. He shows no memory or knowledge impairment. He appears euthymic with an inappropriate and constricted affect. On neurologic exam, he had mild tremors in his hands. The authors’ observationsTreatment for diphenhydramine overdose should begin quickly to prevent life-threatening effects and reduce the risk for mortality. The toxin can be removed from the patient’s GI tract with activated charcoal or gastric lavage if the patient presents within 1 hour of ingesting the substance. Administering IV fluids will prevent dehydration. Cardiac functioning is monitored and benzodiazepines could be administered to manage seizures.
Key elements of a toxicologic physical examination include:
• eyes: pupillary size, symmetry, and response to light (vertical or horizontal nystagmus)
• oropharynx: moist or dry mucous membranes, presence or absence of the gag reflex, distinctive odors
• abdomen: presence or absence and quality of bowel sounds
• skin: warm and dry, warm and sweaty, or cool
• neurologic: level of consciousness and mental status, presence of tremors, seizures, or other movement disorders, presence or absence and quality of deep tendon reflexes.7
If a child or adolescent patient cannot communicate how much of a drug he (she) has ingested, questions to ask parents or other informants include:
• Was the medication purchased recently, and if so was the bottle or box full before the patient took the pills?
• If the medication was not new, how many pills were in the bottle before the patient got to it?
• If the medication was prescribed, how many pills were originally prescribed, when was the medication prescribed, and how many pills were already taken prior to the patient getting to the bottle?
• How many pills were left in the bottle?
• How many pills were seen around the area where the patient was found?
• How many pills were found in the patient’s mouth?7
Recommendations
It is well known that OTC medication abuse is a growing medical problem (Table 2). Antihistamines, including diphenhydramine, are readily available to minors and adults. Because of the powerful sedating effects of antihistamines, many adolescent health practitioners give them to patients who have insomnia as a sleep aid.8 As in our case, antihistamines are used recreationally for their hallucinogenic effects, at dosages of 300 to 700 mg.9 Severe symptoms of toxicity, such as delirium and psychosis, seizures, and coma, occur at dosages ≥1,000 mg.9
With growing abuse of these medications, we aim to encourage detailed history taking about abuse of OTC drugs, especially diphenhydramine in adolescent patients.
Outcome Improvement, discharge
Mr. L is given a dual diagnosis of diphenhydramine-induced psychotic disorder with
hallucinations and diphenhydramine-induced depressive disorder, both with onset during intoxication. He also is given a provisional diagnosis of psychotic disorder not otherwise specified and major depressive disorder. Last, he is given a diagnosis of Cannabis dependence with physiological dependence, MDMA abuse, hydrocodone abuse, and Robitussin abuse.
Mr. L is maintained on fluoxetine, 40 mg/d, and risperidone, 1 mg at bedtime and 0.5 mg in the morning. He receives milieu, individual, group, recreational, and medical therapy while in the hospital. Symptoms abate and he is discharged with a plan to follow up with outpatient providers.
Bottom Line
Abuse of over-the-counter (OTC) drugs, such as diphenhydramine, among youths is a growing problem. Remember to question adolescents who appear intoxicated or to have overdosed not only about abuse of alcohol and illicit substances but also of common—and easily and legally accessible—OTC drugs.
Related Resources
• Carr BC. Efficacy, abuse, and toxicity of over-the-counter cough and cold medicines in the pediatric population. Curr Opin Pediatr. 2006;18(2):184-188.
• Thomas A, Nallur DG, Jones N, et al. Diphenhydramine abuse and detoxification: a brief review and case report. J Psychopharmacol. 2009;23(1):101-105.
Drug Brand Names
Diazepam • Valium Hydrocodone • Vicodin
Diphenhydramine • Benadryl Risperidone • Risperdal
Fluoxetine • Prozac
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Numb and confused
Mr. L, age 17, is admitted to the hospital after ingesting 24 diphenhydramine 25-mg tablets in 3 hours as a possible suicide attempt. His parents witnessed him behaving strangely and brought him to the hospital. They state that their son was visibly agitated and acting inappropriately. He was seen talking to birds, trees, and the walls of the house.
Mr. L says he is upset because he broke up with his girlfriend a week earlier after she asked if they could “take a break.” He says that he took the diphenhydramine because he wanted to “get numb” to deal with the emotional stress caused by the break-up.
After the break-up, Mr. L experienced middle-to-late insomnia and was unable to get more than 3 or 4 hours of sleep a night. He reports significant fatigue, depressed mood, anhedonia, impaired concentration, and psychomotor retardation. He denies homicidal ideation or auditory and visual hallucinations.
As an aside, Mr. L reports that, for the past year, he had difficulties with gender identity, sometimes thinking that he might be better off if he had been born a girl and that he felt uncomfortable in a male body.
Which treatment option would you choose for Mr. L’s substance abuse?
a) refer him to a 12-step program
b) begin supportive measures
c) administer activated charcoal
d) prescribe a benzodiazepine to control agitation
The authors’ observations
As youths gain increasing access to medical and pharmaceutical knowledge through the Internet and other sources, it appears that adolescent drug abuse has, in part, shifted toward more easily attainable over-the-counter (OTC) medications. Diphenhydramine, a first-generation antihistamine, can be abused for its effects on the CNS, such as disturbed coordination, irritability, paresthesia, blurred vision, and depression. Effects of diphenhydramine are increased by the presence of alcohol, monoamine oxidase inhibitors, diazepam, hypnotics, sedatives, tranquilizers, and other CNS depressants. In 2011, diphenhydramine abuse was involved in 19,012 emergency room visits, of which 9,301 were for drug-related suicide attempts.1
Diphenhydramine is an inverse agonist of the histamine H1 receptor.2 It is a member of the ethanolamine subclass of antihistaminergic agents.3 By reversing the effects of histamine on capillaries, diphenhydramine can reduce the intensity of allergic symptoms. Diphenhydramine also crosses the blood–brain barrier and antagonizes H1 receptors centrally.
Used as a common sleep aid and allergy medication, the drug works primarily as an H1 receptor partial agonist, but also is a strong competitive antagonist at muscarinic acetylcholine receptors.4 It is abused for its sedative effects and its capacity to cause delirium and hallucinations.5 Diphenhydramine can have a stimulatory effect in children and young adults, instead of the sedating properties seen in adults.6 Such misuse is concerning because diphenhydramine overdose can lead to delirium, confusion, and hallucinations, tachycardia, seizures, mydriasis, xerostomia, urinary retention, ileus, anhidrosis, and hyperthermia. In severe cases it has been associated with cardiac arrhythmias, rhabdomyolysis, status epilepticus, and death.4,6 Neurologic symptoms of diphenhydramine overdose are listed in Table 1.
HISTORY Polysubstance abuse
Mr. L has a 2-year history of major depressive disorder and a history of Cannabis abuse with physiological dependence; Robitussin (base active ingredient, guaifenesin) and hydrocodone abuse with physiological dependence; 3,4-methylenedioxymethamphetamine (MDMA) abuse; and diphenhydramine abuse. He also has a history of gender dysphoria, although he reports that these feelings have become less severe over the past year.
Mr. L attends bi-weekly appointments with an outpatient psychiatrist and reportedly adheres to his medication regimen: fluoxetine, 40 mg/d, and risperidone, 1 mg at bedtime. He denies previous suicidal ideation, suicide attempts, homicidal ideation, or homicidal attempts. He reports no history of physical, sexual, or emotional abuse. He gets good grades in school and has no outstanding academic problems.
Mr. L began using Cannabis at age 14; his last use was 3 weeks before admission. He is guarded about his use of Robitussin, hydrocodone, and MDMA. However, Mr. L reports that he has researched diphenhydramine on the internet and believes that he can safely take up to 1,200 mg without overdosing. He reports normally taking 450 mg of diphenhydramine daily. Mr. L reports difficulty urinating after using diphenhydramine but no other physical complaints.
Mr. L lives with his father and stepmother and has a history of one psychiatric hospitalization at a different facility 2 months ago, followed by outpatient therapy. He obtained his Graduate Equivalency Diploma (GED) and plans to attend college.
At age 5, Mr. L emigrated from Turkey to the United States with his parents. His mother returned to Turkey when he was age 6 and has had no contact with her son since. Whenever Mr. L visits Turkey with his father, the patient refuses to see her, as per collaterals. He gets along well with his stepmother, who is his maternal aunt. Mr. L has been bullied at school and reportedly has few friends.
On mental status examination, Mr. L has an appropriate appearance and appears to be his stated age. He shows good eye contact and is cooperative. Muscle tone and gait are within normal limits. He has no abnormal movements. Speech, thought processes, and associations are normal. He denies auditory hallucinations, visual hallucinations, suicidal ideation (although he presented with a probable suicide attempt), or homicidal ideation. No delusions are elicited.
Mr. L shows poor judgment about his drug use and situation. He demonstrates limited insight, because he says his only goal is to get out of the hospital. He is alert, awake, and oriented to person, place, and time. He shows no memory or knowledge impairment. He appears euthymic with an inappropriate and constricted affect. On neurologic exam, he had mild tremors in his hands. The authors’ observationsTreatment for diphenhydramine overdose should begin quickly to prevent life-threatening effects and reduce the risk for mortality. The toxin can be removed from the patient’s GI tract with activated charcoal or gastric lavage if the patient presents within 1 hour of ingesting the substance. Administering IV fluids will prevent dehydration. Cardiac functioning is monitored and benzodiazepines could be administered to manage seizures.
Key elements of a toxicologic physical examination include:
• eyes: pupillary size, symmetry, and response to light (vertical or horizontal nystagmus)
• oropharynx: moist or dry mucous membranes, presence or absence of the gag reflex, distinctive odors
• abdomen: presence or absence and quality of bowel sounds
• skin: warm and dry, warm and sweaty, or cool
• neurologic: level of consciousness and mental status, presence of tremors, seizures, or other movement disorders, presence or absence and quality of deep tendon reflexes.7
If a child or adolescent patient cannot communicate how much of a drug he (she) has ingested, questions to ask parents or other informants include:
• Was the medication purchased recently, and if so was the bottle or box full before the patient took the pills?
• If the medication was not new, how many pills were in the bottle before the patient got to it?
• If the medication was prescribed, how many pills were originally prescribed, when was the medication prescribed, and how many pills were already taken prior to the patient getting to the bottle?
• How many pills were left in the bottle?
• How many pills were seen around the area where the patient was found?
• How many pills were found in the patient’s mouth?7
Recommendations
It is well known that OTC medication abuse is a growing medical problem (Table 2). Antihistamines, including diphenhydramine, are readily available to minors and adults. Because of the powerful sedating effects of antihistamines, many adolescent health practitioners give them to patients who have insomnia as a sleep aid.8 As in our case, antihistamines are used recreationally for their hallucinogenic effects, at dosages of 300 to 700 mg.9 Severe symptoms of toxicity, such as delirium and psychosis, seizures, and coma, occur at dosages ≥1,000 mg.9
With growing abuse of these medications, we aim to encourage detailed history taking about abuse of OTC drugs, especially diphenhydramine in adolescent patients.
Outcome Improvement, discharge
Mr. L is given a dual diagnosis of diphenhydramine-induced psychotic disorder with
hallucinations and diphenhydramine-induced depressive disorder, both with onset during intoxication. He also is given a provisional diagnosis of psychotic disorder not otherwise specified and major depressive disorder. Last, he is given a diagnosis of Cannabis dependence with physiological dependence, MDMA abuse, hydrocodone abuse, and Robitussin abuse.
Mr. L is maintained on fluoxetine, 40 mg/d, and risperidone, 1 mg at bedtime and 0.5 mg in the morning. He receives milieu, individual, group, recreational, and medical therapy while in the hospital. Symptoms abate and he is discharged with a plan to follow up with outpatient providers.
Bottom Line
Abuse of over-the-counter (OTC) drugs, such as diphenhydramine, among youths is a growing problem. Remember to question adolescents who appear intoxicated or to have overdosed not only about abuse of alcohol and illicit substances but also of common—and easily and legally accessible—OTC drugs.
Related Resources
• Carr BC. Efficacy, abuse, and toxicity of over-the-counter cough and cold medicines in the pediatric population. Curr Opin Pediatr. 2006;18(2):184-188.
• Thomas A, Nallur DG, Jones N, et al. Diphenhydramine abuse and detoxification: a brief review and case report. J Psychopharmacol. 2009;23(1):101-105.
Drug Brand Names
Diazepam • Valium Hydrocodone • Vicodin
Diphenhydramine • Benadryl Risperidone • Risperdal
Fluoxetine • Prozac
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. U.S. Department of Health and Human Services. Drug Abuse Warning Network, 2011: National estimates of drug-related emergency department visits. http://www.samhsa. gov/data/2k13/DAWN2k11ED/DAWN2k11ED.htm. Published May 2013. Accessed on September 29, 2014.
2. Yamashiro K, Kiryu J, Tsujikawa A, et al. Suppressive effects of histamine H1 receptor antagonist diphenhydramine on the leukocyte infiltration during endotoxin-induced uveitis. Exp Eye Res. 2001;73(1):69-80.
3. Skidgel RA, Kaplan AP, Erdos EG. Histamine, bradykinin, and their antagonists. In: Brunton L, Chabner B, Knollman B, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York, NY: McGraw Hill; 2011: 911-935.
4. Vearrier D, Curtis JA. Case files of the medical toxicology fellowship at Drexel University. Rhabdomyolysis and compartment syndrome following acute diphenhydramine overdose. J Med Toxicol. 2011;7(3):213-219.
5. Ho M, Tsai K, Liu C. Diphenhydramine overdose related delirium: a case report. Journal of Emergency and Critical Care Medicine. 2006;17(2):77-79.
6. Krenzelok EP, Anderson GM, Mirick M. Massive diphenhydramine overdose resulting in death. Ann Emerg Med. 1982;11(4):212-213.
7. Inaba AS. Toxicologic teasers: Testing your knowledge of clinical toxicology. Hawaii Med J. 1998;57(4):471-473.
8. Kaplan SL. Busner J. The use of prn and stat medication in three child psychiatric inpatient settings. Psychopharmacol Bull. 1997;33(1):161-164.
9. Radovanovic D, Meier PJ, Guirguis M, et al. Dose-dependent toxicity of diphenhydramine overdose. Hum Exp Toxicol. 2000;19(9):489-495.
1. U.S. Department of Health and Human Services. Drug Abuse Warning Network, 2011: National estimates of drug-related emergency department visits. http://www.samhsa. gov/data/2k13/DAWN2k11ED/DAWN2k11ED.htm. Published May 2013. Accessed on September 29, 2014.
2. Yamashiro K, Kiryu J, Tsujikawa A, et al. Suppressive effects of histamine H1 receptor antagonist diphenhydramine on the leukocyte infiltration during endotoxin-induced uveitis. Exp Eye Res. 2001;73(1):69-80.
3. Skidgel RA, Kaplan AP, Erdos EG. Histamine, bradykinin, and their antagonists. In: Brunton L, Chabner B, Knollman B, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York, NY: McGraw Hill; 2011: 911-935.
4. Vearrier D, Curtis JA. Case files of the medical toxicology fellowship at Drexel University. Rhabdomyolysis and compartment syndrome following acute diphenhydramine overdose. J Med Toxicol. 2011;7(3):213-219.
5. Ho M, Tsai K, Liu C. Diphenhydramine overdose related delirium: a case report. Journal of Emergency and Critical Care Medicine. 2006;17(2):77-79.
6. Krenzelok EP, Anderson GM, Mirick M. Massive diphenhydramine overdose resulting in death. Ann Emerg Med. 1982;11(4):212-213.
7. Inaba AS. Toxicologic teasers: Testing your knowledge of clinical toxicology. Hawaii Med J. 1998;57(4):471-473.
8. Kaplan SL. Busner J. The use of prn and stat medication in three child psychiatric inpatient settings. Psychopharmacol Bull. 1997;33(1):161-164.
9. Radovanovic D, Meier PJ, Guirguis M, et al. Dose-dependent toxicity of diphenhydramine overdose. Hum Exp Toxicol. 2000;19(9):489-495.
How to assess the merits of psychological and neuropsychological test evaluations
Psychological and neuropsychological test evaluations, like all consultative diagnostic services, can vary in quality and clinical utility. Many of these examinations provide valuable insights and helpful recommendations; regrettably, some assessments are only marginally beneficial and can contribute to diagnostic confusion and uncertainty.
When weighing the pros and cons of evaluations, consider these best practices.
Gold-standard tests ought to be in-cluded in the assessment. These include (but are not limited to) the Wechsler Adult Intelligence Scale-Fourth Edition (WAIS-IV); Wechsler Memory Scale-Fourth Edition (WMS-IV); Delis-Kaplan Executive Function System (D-KEFS); Wechsler Individual Achievement Test-Third Edition (WIAT-III); and the Minnesota Multiphasic Personality Inventory-2 (MMPI-2). These tests have a strong evidence base that:
• demonstrates good reliability (ie, produce consistent and accurate scores across examiners and time intervals and are relatively free of measurement error)
• demonstrates good validity (ie, have been shown to measure aspects of psychological and neuropsychological functioning that they claim to measure).
Many gold-standard tests are normed on national samples and are stratified by age, sex, ethnicity or race, educational level, and geographic region. They also include normative data based on the performance of patients who have neuropsychiatric syndromes often seen by psychiatrists in practice.1
The test battery ought to comprise cognitive and neuropsychological measures as well as affective and behavioral measures. When feasible, these tests should be supplemented by informant-based measures of neuropsychiatric functioning to obtain a comprehensive assessment of the patient’s capacities and skills.
An estimated premorbid baseline should be established. This is done by taking a relevant history and administering tests, such as the National Adult Reading Test (NART), that can be used to compare against current test performance. This testing-in-context approach helps differentiate long-term limitations in information processing, which might be attributed to a DSM-5 intellectual disability, specific learning disorder, or other neurodevelopmental disorder, from a known or suspected recent neurobehavioral change.
Tests in the assessment should tap a broad set of neurobehavioral functions. Doing so ensures that, when a patient is referred with a change in cognition or other aspects of mental status, it will be easier to determine whether clinically significant score discrepancies exist across different ability and skill domains. Such dissociations in performance can have important implications for the differential diagnosis and everyday functioning.
Tests that are sensitive to a patient’s over-reporting of symptoms should be used as part of the evaluation in cases of suspected malingering—especially subtle simulation that might elude identification with brief screening-level measures.2 These tests can include the Test of Memory Malingering (TOMM) and the Structured Interview of Reported Symptoms, 2nd edition (SIRS-2).
Test recommendations ought to be grounded in findings; practical; and relatively easy to implement. They also should be consistent with the treatment setting and the patient’s lifestyle, values, and treatment preferences.3
Disclosure
Dr. Pollak reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Geisinger KF, Bracken BA, Carlson JF, et al, eds. APA handbook of testing and assessment in psychology. Washington, DC: American Psychological Association Press; 2013.
2. Brady MC, Scher LM, Newman W. “I just saw Big Bird. He was 100 feet tall!” Malingering in the emergency department. Current Psychiatry. 2013;12(10):33-38,40.
3. McHugh RK, Whitton SW, Peckham AD, et al. Patient p for psychological vs pharmacologic treatment of psychiatric disorders: a meta-analytic review. J Clin Psychiatry. 2013;74(6):595-602.
Psychological and neuropsychological test evaluations, like all consultative diagnostic services, can vary in quality and clinical utility. Many of these examinations provide valuable insights and helpful recommendations; regrettably, some assessments are only marginally beneficial and can contribute to diagnostic confusion and uncertainty.
When weighing the pros and cons of evaluations, consider these best practices.
Gold-standard tests ought to be in-cluded in the assessment. These include (but are not limited to) the Wechsler Adult Intelligence Scale-Fourth Edition (WAIS-IV); Wechsler Memory Scale-Fourth Edition (WMS-IV); Delis-Kaplan Executive Function System (D-KEFS); Wechsler Individual Achievement Test-Third Edition (WIAT-III); and the Minnesota Multiphasic Personality Inventory-2 (MMPI-2). These tests have a strong evidence base that:
• demonstrates good reliability (ie, produce consistent and accurate scores across examiners and time intervals and are relatively free of measurement error)
• demonstrates good validity (ie, have been shown to measure aspects of psychological and neuropsychological functioning that they claim to measure).
Many gold-standard tests are normed on national samples and are stratified by age, sex, ethnicity or race, educational level, and geographic region. They also include normative data based on the performance of patients who have neuropsychiatric syndromes often seen by psychiatrists in practice.1
The test battery ought to comprise cognitive and neuropsychological measures as well as affective and behavioral measures. When feasible, these tests should be supplemented by informant-based measures of neuropsychiatric functioning to obtain a comprehensive assessment of the patient’s capacities and skills.
An estimated premorbid baseline should be established. This is done by taking a relevant history and administering tests, such as the National Adult Reading Test (NART), that can be used to compare against current test performance. This testing-in-context approach helps differentiate long-term limitations in information processing, which might be attributed to a DSM-5 intellectual disability, specific learning disorder, or other neurodevelopmental disorder, from a known or suspected recent neurobehavioral change.
Tests in the assessment should tap a broad set of neurobehavioral functions. Doing so ensures that, when a patient is referred with a change in cognition or other aspects of mental status, it will be easier to determine whether clinically significant score discrepancies exist across different ability and skill domains. Such dissociations in performance can have important implications for the differential diagnosis and everyday functioning.
Tests that are sensitive to a patient’s over-reporting of symptoms should be used as part of the evaluation in cases of suspected malingering—especially subtle simulation that might elude identification with brief screening-level measures.2 These tests can include the Test of Memory Malingering (TOMM) and the Structured Interview of Reported Symptoms, 2nd edition (SIRS-2).
Test recommendations ought to be grounded in findings; practical; and relatively easy to implement. They also should be consistent with the treatment setting and the patient’s lifestyle, values, and treatment preferences.3
Disclosure
Dr. Pollak reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Psychological and neuropsychological test evaluations, like all consultative diagnostic services, can vary in quality and clinical utility. Many of these examinations provide valuable insights and helpful recommendations; regrettably, some assessments are only marginally beneficial and can contribute to diagnostic confusion and uncertainty.
When weighing the pros and cons of evaluations, consider these best practices.
Gold-standard tests ought to be in-cluded in the assessment. These include (but are not limited to) the Wechsler Adult Intelligence Scale-Fourth Edition (WAIS-IV); Wechsler Memory Scale-Fourth Edition (WMS-IV); Delis-Kaplan Executive Function System (D-KEFS); Wechsler Individual Achievement Test-Third Edition (WIAT-III); and the Minnesota Multiphasic Personality Inventory-2 (MMPI-2). These tests have a strong evidence base that:
• demonstrates good reliability (ie, produce consistent and accurate scores across examiners and time intervals and are relatively free of measurement error)
• demonstrates good validity (ie, have been shown to measure aspects of psychological and neuropsychological functioning that they claim to measure).
Many gold-standard tests are normed on national samples and are stratified by age, sex, ethnicity or race, educational level, and geographic region. They also include normative data based on the performance of patients who have neuropsychiatric syndromes often seen by psychiatrists in practice.1
The test battery ought to comprise cognitive and neuropsychological measures as well as affective and behavioral measures. When feasible, these tests should be supplemented by informant-based measures of neuropsychiatric functioning to obtain a comprehensive assessment of the patient’s capacities and skills.
An estimated premorbid baseline should be established. This is done by taking a relevant history and administering tests, such as the National Adult Reading Test (NART), that can be used to compare against current test performance. This testing-in-context approach helps differentiate long-term limitations in information processing, which might be attributed to a DSM-5 intellectual disability, specific learning disorder, or other neurodevelopmental disorder, from a known or suspected recent neurobehavioral change.
Tests in the assessment should tap a broad set of neurobehavioral functions. Doing so ensures that, when a patient is referred with a change in cognition or other aspects of mental status, it will be easier to determine whether clinically significant score discrepancies exist across different ability and skill domains. Such dissociations in performance can have important implications for the differential diagnosis and everyday functioning.
Tests that are sensitive to a patient’s over-reporting of symptoms should be used as part of the evaluation in cases of suspected malingering—especially subtle simulation that might elude identification with brief screening-level measures.2 These tests can include the Test of Memory Malingering (TOMM) and the Structured Interview of Reported Symptoms, 2nd edition (SIRS-2).
Test recommendations ought to be grounded in findings; practical; and relatively easy to implement. They also should be consistent with the treatment setting and the patient’s lifestyle, values, and treatment preferences.3
Disclosure
Dr. Pollak reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Geisinger KF, Bracken BA, Carlson JF, et al, eds. APA handbook of testing and assessment in psychology. Washington, DC: American Psychological Association Press; 2013.
2. Brady MC, Scher LM, Newman W. “I just saw Big Bird. He was 100 feet tall!” Malingering in the emergency department. Current Psychiatry. 2013;12(10):33-38,40.
3. McHugh RK, Whitton SW, Peckham AD, et al. Patient p for psychological vs pharmacologic treatment of psychiatric disorders: a meta-analytic review. J Clin Psychiatry. 2013;74(6):595-602.
1. Geisinger KF, Bracken BA, Carlson JF, et al, eds. APA handbook of testing and assessment in psychology. Washington, DC: American Psychological Association Press; 2013.
2. Brady MC, Scher LM, Newman W. “I just saw Big Bird. He was 100 feet tall!” Malingering in the emergency department. Current Psychiatry. 2013;12(10):33-38,40.
3. McHugh RK, Whitton SW, Peckham AD, et al. Patient p for psychological vs pharmacologic treatment of psychiatric disorders: a meta-analytic review. J Clin Psychiatry. 2013;74(6):595-602.
Treating bipolar mania in the outpatient setting: Risk vs reward
Manic episodes, by definition, are associated with significant social or occupational impairment.1 Some manic patients are violent or engage in reckless behaviors that can harm themselves or others, such as speeding, disrupting traffic, or playing with fire. When these patients present to a psychiatrist’s outpatient practice, involuntary hospitalization might be justified.
However, some manic patients, in spite of their elevated, expansive, or irritable mood state, never behave dangerously and might not meet legal criteria for involuntary hospitalization, although these criteria differ from state to state. These patients might see a psychiatrist because manic symptoms such as irritability, talkativeness, and impulsivity are bothersome to their family members but pose no serious danger (Box). In this situation, the psychiatrist can strongly encourage the patient to seek voluntary hospitalization or attend a partial hospitalization program. If the patient declines, the psychiatrist is left with 2 choices: initiate treatment in the outpatient setting or refuse to treat the patient and refer to another provider.
Treating “non-dangerous” mania in the outpatient setting is fraught with challenges:
• the possibility that the patient’s condition will progress to dangerousness
• poor adherence to treatment because of the patient’s limited insight
• the large amount of time required from the psychiatrist and care team to adequately manage the manic episode (eg, time spent with family members, frequent patient visits, and managing communications from the patient).
There are no guidelines to assist the office-based practitioner in treating mania in the outpatient setting. When considering dosing and optimal medication combinations for treating mania, clinical trials may be of limited value because most of these studies only included hospitalized manic patients.
Because of this dearth of knowledge, we provide recommendations based on our review of the literature and from our experience working with manic patients who refuse voluntary hospitalization and could not be hospitalized against their will. These recommendations are organized into 3 sections: diagnostic approach, treatment strategy, and family involvement.
Diagnostic approach
Making a diagnosis of mania might seem straightforward for clinicians who work in inpatient settings; however, mania might not present with classic florid symptoms among outpatients. Patients might have a chief concern of irritability, dysphoria, anxiety, or “insomnia,” which may lead clinicians to focus initially on non-bipolar conditions.2
During the interview, it is important to assess for any current DSM-5 symptoms of a manic episode, while being careful not to accept a patient’s denial of symptoms. Patients with mania often have poor insight and are unaware of changes from their baseline state when manic.3 Alternatively, manic patients may want you to believe that they are well and could minimize or deny all symptoms. Therefore, it is important to pay attention to mental status examination findings, such as hyperverbal speech, elated affect, psychomotor agitation, a tangential thought process, or flight of ideas.
Countertransference feelings of diagnostic confusion or frustration after long patient monologues or multiple interruptions by the patient should be incorporated into the diagnostic assessment. Family members or friends often can provide objective observations of behavioral changes necessary to secure the diagnosis.
Treatment strategy
Decision points. When treating manic outpatients, assess the need for hospitalization at each visit. Advantages of the inpatient setting include:
• the possibility of rapid medication adjustments
• continuous observation to ensure the patient’s safety
• keeping the patient temporarily removed from his community to prevent irreversible social and economic harms.
However, a challenge with hospitalization is third-party payers’ influence on a patient’s length of stay, which may lead to rapid medication changes that may not be clinically ideal.
At each outpatient visit, explore with the patient and family emerging symptoms that could justify involuntary hospitalization. Document whether you recommended inpatient hospitalization, the patient’s response to the recommendation, that you are aware and have considered the risks associated with outpatient care, and that you have discussed these risks with the patient and family.
For patients well-known to the psychiatrist, a history of dangerous mania may lead him (her) to strongly recommend hospitalization, whereas a pre-existing therapeutic alliance and no current or distant history of dangerous mania may lead the clinician to look for alternatives to inpatient care. Concomitant drug or alcohol use may increase the likelihood of mania becoming dangerous, making outpatient treatment ill-advised and riskier for everyone involved.
In exchange for agreeing to provide outpatient care for mania, it often is helpful to negotiate with the patient and family a threshold level of symptoms or behavior that will result in the patient agreeing to voluntary hospitalization (Table 1). Such an agreement can include stopping outpatient treatment if the patient does not improve significantly after 2 or 3 weeks or develops psychotic symptoms. The negotiation also can include partial hospitalization as an option, so long as the patient’s mania continues to be non-dangerous.
Obtaining pretreatment blood work can help a clinician determine whether a medication is safe to prescribe and establish causality if laboratory abnormalities arise after treatment begins. Ideally, the psychiatrist should follow consensus guidelines developed by the International Society for Bipolar Disorders4 or the American Psychiatric Association (APA)5 and order appropriate laboratory tests before prescribing anti-manic medications. Determine the pregnancy status of female patients of child-bearing age before prescribing a potentially teratogenic medication, especially because mania is associated with increased libido.6
Manic patients might be too disorganized to follow up with recommendations for laboratory testing, or could wait several days before completing blood work. Although not ideal, to avoid delaying treatment, a clinician might need to prescribe medication at the initial office visit, without pretreatment laboratory results. When the patient is more organized, complete the blood work. Keeping home pregnancy tests in the office can help rule out pregnancy before prescribing medication.
Medication. Meta-analyses have established the efficacy of mood stabilizers and antipsychotics for treating mania,7,8 and several consensus guidelines have incorporated these findings into treatment algorithms.9
For a patient already taking medications recommended by the guidelines, assess treatment adherence during the initial interview by questioning the patient and family. When the logistics of phlebotomy permit, obtaining the blood level of psychotropics can show the presence of any detectable drug concentration, which demonstrates that the patient has taken the medication recently.
If there is no evidence of nonadherence, an initial step might be to increase the dosage of the antipsychotic or mood stabilizer that the patient is already taking, ensuring that the dosage is optimized based on FDA indications and clinical trials data. The recommended rate of dosage adjustments differs among medications; however, optimal dosing should be reached quickly because a World Federation of Societies of Biological Psychiatry task force recommends that a mania treatment trial not exceed 2 weeks.10
Dosage increases can be made at weekly visits or sooner, based on treatment response and tolerability. If there is no benefit after optimizing the dosage, the next step would be to add a mood stabilizer to a second-generation antipsychotic (SGA), or vice versa to promote additive or synergistic medication effects.11 Switching one medication for the other should be avoided unless there are tolerability concerns.
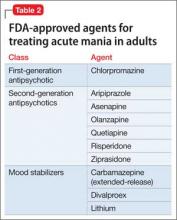
For a patient who is not taking any medications, select a treatment that balances rapid stabilization with long-term efficacy and tolerability. Table 2 lists FDA-approved treatments for mania. Lamotrigine provides prophylactic efficacy with few associated risks, but it has no anti-manic effects and would be a poor choice for most actively manic patients. Most studies indicate that antipsychotics work faster than lithium at the 1-week mark; however, this may be a function of the lithium titration schedule followed in the protocols, the severity of mania among enrolled patients, the inclusion of typically non-responsive manic patients (eg, mixed) in the analysis, and the antipsychotic’s sedative potential relative to lithium. Although the anti-manic and prophylactic potential of lithium and valproate might make them an ideal first-line option, antipsychotics could stabilize a manic patient faster, especially if agitation is present.12,13
Breaking mania quickly is important when treating patients in the outpatient setting. In these situations, a reasonable choice is to prescribe a SGA, because of their rapid onset of effect, low potential for switch to depression, and utility in treating classic, mixed, or psychotic mania.10 Oral loading of valproate (20 mg/kg) is another option. An inpatient study that used an oral-loading strategy demonstrated a similar time to response as olanzapine,14 in contrast to an inpatient15 and an outpatient study16 that employed a standard starting dosage for each patient and led to slower improvement compared with olanzapine.
SGAs should be dosed moderately and lower than if the patient were hospitalized, to avoid alienating the patient from treatment by causing intolerable side effects. In particular, patients and their families should be warned about immediate risks, such as orthostasis or extrapyramidal symptoms. Although treatment guidelines recommend combination therapy as a possible first-line option,9 in the outpatient setting, monotherapy with an optimally dosed, rapid-acting agent is preferred to promote medication adherence and avoid potentially dangerous sedation. Manic patients experience increased distractibility and verbal memory and executive function impairments that can interfere with medication adherence.17 Therefore, patients are more likely to follow a simpler regimen. If SGA or valproate monotherapy does not control mania, begin combination treatment with a mood stabilizer and SGA. If the patient experiences remission with SGA monotherapy, the risks and benefits of maintaining the SGA vs switching to a mood stabilizer can be discussed.
Provide medication “as needed” for agitation—additional SGA dosing or a benzodiazepine—and explain to family members when their use is warranted. Benzodiazepines can provide short-term benefits for manic patients: anxiety relief, sedation, and anti-manic efficacy as monotherapy18-20 and in combination with other medications.21 Studies showing monotherapy efficacy employed high dosages of benzodiazepines (lorazepam mean dosage, 14 mg/d; clonazepam mean dosage, 13 mg/d)19 and high dosages of antipsychotics as needed,18,20 and often were associated with excessive sedation and ataxia.18,19 This makes benzodiazepine monotherapy a potentially dangerous approach for outpatient treatment of mania. IM lorazepam treated manic agitation less quickly than IM olanzapine, suggesting that SGAs are preferable in the outpatient setting because rapid control of agitation is crucial.22 If prescribed, a trusted family member should dispense benzodiazepines to the patient to minimize misuse because of impulsivity, distractibility, desperation to sleep, or pleasure seeking.
SGAs have the benefit of sedation but occasionally additional sleep medications are required. Benzodiazepine receptor agonists (BzRAs), such as zolpidem, eszopiclone, and zaleplon, should be used with caution. Although these medicines are effective in treating insomnia in individuals with primary insomnia23 and major depression,24 they have not been studied in manic patients. The decreased need for sleep in mania is phenomenologically25 and perhaps biologically different than insomnia in major depression.26 Therefore, mania-associated sleep disturbance might not respond to BZRAs. BzRAs also might induce somnambulism and other parasomnias,27 especially when used in combination with psychotropics, such as valproate28; it is unclear if the manic state itself increases this risk further. Sedating antihistamines with anticholinergic blockade, such as diphenhydramine and low dosages (<100 mg/d) of quetiapine, are best used only in combination with anti-manic medications because of putative link between anticholinergic blockade and manic induction.29 Less studied but safer options include novel anticonvulsants (gabapentin, pregabalin), melatonin, and melatonin receptor agonists. Sedating antidepressants, such as mirtazapine and trazodone, should be avoided.25
Important adjunctive treatment steps include discontinuing all pro-manic agents, including antidepressants, stimulants, and steroids, and discouraging use of caffeine, energy drinks, illicit drugs, and alcohol. The patient should return for office visits at least weekly, and possibly more frequently, depending on severity. Telephone check-in calls between scheduled visits may be necessary until the mania is broken.
Psychotherapy. Other than supportive therapy and psychoeducation, other forms of psychotherapy during mania are not indicated. Psychotherapy trials in bipolar disorder do not inform anti-manic efficacy because few have enrolled acutely manic patients and most report long-term benefits rather than short-term efficacy for the index manic episode.30 Educate patients about the importance of maintaining regular social rhythms and taking medication as prescribed. Manic patients might not be aware that they are acting differently during manic episodes, therefore efforts to improve the patient’s insight are unlikely to succeed. More time should be spent emphasizing the importance of adherence to treatment and taking anti-manic medications as prescribed. This discussion can be enhanced by focusing on the medication’s potential to reduce the unpleasant symptoms of mania, including irritability, insomnia, anxiety, and racing thoughts. At the first visit, discuss setting boundaries with the patient to reduce mania-driven, intrusive phone calls. A patient might develop insight after mania has resolved and he (she) can appreciate social or economic harm that occurred while manic. This discussion might foster adherence to maintenance treatment. Advise your patient to limit activities that may increase stimulation and perpetuate the mania, such as exercise, parties, concerts, or crowded shopping malls. Also, recommend that your patient stop working temporarily, to reduce stress and prevent any manic-driven interactions that could result in job loss.
If your patient has an established relationship with a psychotherapist, discuss with the therapist the plan to initiate mania treatment in the outpatient setting and work as a collaborative team, assuming that the patient has granted permission to share information. Encourage the therapist to increase the frequency of sessions with the patient to enable greater monitoring of changes in the patient’s manic symptoms.
Family involvement
Family support is crucial when treating mania in the outpatient setting. Lacking insight and organization, manic patients require the “auxiliary” judgment of trusted family members to ensure treatment success. The family should identify a single person to act as the liaison between the family and the psychiatrist. The psychiatrist should instruct this individual to accompany the patient to each clinic visit and provide regular updates on the patient’s adherence to treatment, changes in symptoms, and any new behaviors that would justify involuntary hospitalization. The treatment plan should be clearly communicated to this individual to ensure that it is implemented correctly. Ideally, this individual would be someone who understands that bipolar disorder is a mental illness, who can tolerate the patient’s potential resentment of them for taking on this role, and who can influence the patient and the other family members to adhere to the treatment plan.
This family member also should watch the patient take medication to rule out nonadherence if the patient’s condition does not improve.
Provide extensive psychoeducation to the family (Table 3). Discuss these teaching points and their implications at length during the first visit and reinforce them at subsequent visits. Advise spouses that the acute manic period is not the time to make major decisions about their marriage or to engage in couple’s therapy. These options are better explored after the patient recovers from the manic episode.
Encourage the family to engage in mania harm-reduction techniques to the extent that the patient will allow (Table 4). In particular, they should hold onto their loved one’s credit cards and checkbook, and discourage the patient from making any major financial decisions until the mania has resolved. Additionally, patients should be relieved of childcare responsibilities during this period. If there are any child welfare safety concerns, the clinician will need to report this to authorities as required by local laws.
Advise family members or roommates to call emergency services and request a crisis intervention team, or to take the patient to an emergency room if he (she) makes verbal threats to harm themselves or others, is violent, or demonstrates behaviors that indicate that he is no longer able to care for himself. The psychiatrist should assist with completing Family and Medical Leave Act paperwork for family members who will monitor the patient at home, a work-excuse letter for the patient so he does not lose his job, and short-term disability paperwork to ensure income for the patient during the manic period.
These interventions can be challenging for the entire family system because they place family members in a paternalistic role and reduce the patient’s autonomy within the family. This is problematic when these role changes occur between spouses or between a patient-parent and his (her) children. Such changes typically need to be reversed over time and may require the help of a family or couple’s therapist. To support the psychological health of the patient’s family, refer them to the National Alliance on Mental Illness for family support groups or to individual psychotherapists.
Outpatient management can be rewarding
For “non-dangerous” manic patients who cannot be hospitalized involuntarily and refuse full or partial hospitalization, a psychiatrist must choose between beginning treatment in the clinic and referring the patient to another provider. The latter option is consistent with the APA’s ethical guidelines,31 but must be done appropriately to avoid legal liability.32 This decision may disappoint a family desperate to see their loved one recover quickly and may leave them feeling betrayed by the mental health system. On the other hand, choosing to treat mania in the outpatient setting can be rewarding when resolution of mania restores the family’s homeostasis.
To achieve this outcome, the outpatient psychiatrist must engage the patient’s family to ensure that the patient adheres to the treatment plan and monitor for potentially dangerous behavior. The psychiatrist also must use his knowledge of mood symptoms, cognitive impairments, and the psychological experience of manic patients to create a safe and effective treatment strategy that the patient and family can implement.
Because of mania’s unpredictability and destructive potential, psychiatrists who agree to treat manic patients as outpatients should be familiar with their state’s statutes and case law that pertain to the refusal to accept a new patient, patient abandonment, involuntary hospitalization, confidentiality, and mandatory reporting. They also should seek clinical or legal consultation if they feel overwhelmed or uncertain about the safest and most legally sound approach.
Bottom Line
Treating mania in the outpatient setting is risky but can be accomplished in select patients with the help of the patient’s family and a strategy that integrates evidence-based pharmacotherapeutic and psychotherapeutic strategies. Because manic patients could display dangerous behavior, be familiar with your state’s laws regarding involuntary commitment, patient abandonment, and mandatory reporting.
Related Resources
• National Alliance on Mental Illness. www.NAMI.org.
• Depression and Bipolar Support Alliance. www.DBSAlliance.org.
Drug Brand Names
Aripiprazole • Abilify Mirtazapine • Remeron
Asenapine • Saphris Olanzapine • Zyprexa
Carbamazepine • Equetro, Tegretol Pregabalin • Lyrica
Chlorpromazine • Thorazine Quetiapine • Seroquel
Clonazepam • Klonopin Risperidone • Risperdal
Diphrenhydramine • Benadryl Trazodone • Desyrel
Eszopiclone • Lunesta Valproate • Divalproex
Gabapentin • Neurontin Zaleplon • Sonata
Lamotrigine • Lamictal Ziprasidone • Geodon
Lithium • Eskalith, Lithobid Zolpidem • Ambien
Lorazepam • Ativan
Acknowledgement
The authors thank Peter Ash, MD, for carefully reviewing this manuscript and providing feedback.
Disclosures
Dr. Rakofsky receives research or grant support from Takeda. Dr. Dunlop receives research or grant support from Forest, GlaxoSmithKline, and Otsuka.
1. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Cassidy F, Murry E, Forest K, et al. Signs and symptoms of mania in pure and mixed episodes. J Affect Disord. 1998;50(2-3):187-201.
3. Yen CF, Chen CS, Ko CH, et al. Changes in insight among patients with bipolar I disorder: a 2-year prospective study. Bipolar Disord. 2007;9(3):238-242.
4. Ng F, Mammen OK, Wilting I, et al. The International Society for Bipolar Disorders (ISBD) consensus guidelines for the safety monitoring of bipolar disorder treatments. Bipolar Disord. 2009;11(6):559-595.
5. American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(suppl 4):1-50.
6. Allison JB, Wilson WP. Sexual behavior of manic patients: a preliminary report. South Med J. 1960;53:870-874.
7. Cipriani A, Barbui C, Salanti G, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet. 2011; 378(9799):1306-1315.
8. Yildiz A, Vieta E, Leucht S, et al. Efficacy of antimanic treatments: meta-analysis of randomized, controlled trials. Neuropsychopharmacology. 2011;36(2):375-389.
9. Nivoli AM, Murru A, Goikolea JM, et al. New treatment guidelines for acute bipolar mania: a critical review. J Affect Disord. 2012;140(2):125-141.
10. Grunze H, Vieta E, Goodwin GM, et al. The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: update 2009 on the treatment of acute mania. World J Biol Psychiatry. 2009;10(2):85-116.
11. Tohen M, Chengappa KN, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry. 2002;59(1):62-69.
12. Tohen M, Jacobs TG, Feldman PD. Onset of action of antipsychotics in the treatment of mania. Bipolar Disord. 2000;2(3 pt 2):261-268.
13. Goikolea JM, Colom F, Capapey J, et al. Faster onset of antimanic action with haloperidol compared to second-generation antipsychotics. A meta-analysis of randomized clinical trials in acute mania. Eur Neuropsychopharmacol. 2013;23(4):305-316.
14. Zajecka JM, Weisler R, Sachs G, et al. A comparison of the efficacy, safety, and tolerability of divalproex sodium and olanzapine in the treatment of bipolar disorder. J Clin Psychiatry. 2002;63(12):1148-1155.
15. Tohen M, Baker RW, Altshuler LL, et al. Olanzapine versus divalproex in the treatment of acute mania. Am J Psychiatry. 2002;159(6):1011-1017.
16. Tohen M, Vieta E, Goodwin GM, et al. Olanzapine versus divalproex versus placebo in the treatment of mild to moderate mania: a randomized, 12-week, double-blind study. J Clin Psychiatry. 2008;69(11):1776-1789.
17. Martínez-Arán A, Vieta E, Reinares M, et al. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am J Psychiatry. 2004; 161(2):262-270.
18. Edwards R, Stephenson U, Flewett T. Clonazepam in acute mania: a double blind trial. Aust N Z J Psychiatry. 1991;25(2):238-242.
19. Bradwejn J, Shriqui C, Koszycki D, et al. Double-blind comparison of the effects of clonazepam and lorazepam in acute mania. J Clin Psychopharmacol. 1990;10(6):403-408.
20. Clark HM, Berk M, Brook S. A randomized controlled single blind study of the efficacy of clonazepam and lithium in the treatment of acute mania. Human Psychopharmacology: Clinical and Experimental. 1997;12(4):325-328.
21. Lenox RH, Newhouse PA, Creelman WL, et al. Adjunctive treatment of manic agitation with lorazepam versus haloperidol: a double-blind study. J Clin Psychiatry. 1992;53(2):47-52.
22. Meehan K, Zhang F, David S, et al. A double-blind, randomized comparison of the efficacy and safety of intramuscular injections of olanzapine, lorazepam, or placebo in treating acutely agitated patients diagnosed with bipolar mania. J Clin Psychopharmacol. 2001;21(4):389-397.
23. Huedo-Medina TB, Kirsch I, Middlemass J, et al. Effectiveness of non-benzodiazepine hypnotics in treatment of adult insomnia: meta-analysis of data submitted to the Food and Drug Administration. BMJ. 2012;345:e8343. doi: 10.1136/bmj.e8343.
24. Fava M, Asnis GM, Shrivastava RK, et al. Improved insomnia symptoms and sleep-related next-day functioning in patients with comorbid major depressive disorder and insomnia following concomitant zolpidem extended-release 12.5 mg and escitalopram treatment: a randomized controlled trial. J Clin Psychiatry. 2011;72(7):914-928.
25. Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165(7):830-843.
26. Linkowski P, Kerkhofs M, Rielaert C, et al. Sleep during mania in manic-depressive males. Eur Arch Psychiatry Neurol Sci. 1986;235(6):339-341.
27. Poceta JS. Zolpidem ingestion, automatisms, and sleep driving: a clinical and legal case series. J Clin Sleep Med. 2011;7(6):632-638.
28. Sattar SP, Ramaswamy S, Bhatia SC, et al. Somnambulism due to probable interaction of valproic acid and zolpidem. Ann Pharmacother. 2003;37(10):1429-1433.
29. Rybakowski JK, Koszewska I, Puzynski S. Anticholinergic mechanisms: a forgotten cause of the switch process in bipolar disorder [Comment on: The neurolobiology of the switch process in bipolar disorder: a review. J Clin Psychiatry. 2010]. J Clin Psychiatry. 2010;71(12):1698-1699; author reply 1699-1700.
30. Miklowitz DJ. Adjunctive psychotherapy for bipolar disorder: state of the evidence. Am J Psychiatry. 2008;165(11):1408-1419.
31. American Psychiatric Association. The principles of medical ethics with annotations especially applicable to psychiatry. Arlington, VA: American Psychiatric Association; 2013.
32. Simon RI, Shuman DW. Clinical manual of psychiatry and law. Arlington, VA: American Psychiatric Publishing; 2007:17-36.
Manic episodes, by definition, are associated with significant social or occupational impairment.1 Some manic patients are violent or engage in reckless behaviors that can harm themselves or others, such as speeding, disrupting traffic, or playing with fire. When these patients present to a psychiatrist’s outpatient practice, involuntary hospitalization might be justified.
However, some manic patients, in spite of their elevated, expansive, or irritable mood state, never behave dangerously and might not meet legal criteria for involuntary hospitalization, although these criteria differ from state to state. These patients might see a psychiatrist because manic symptoms such as irritability, talkativeness, and impulsivity are bothersome to their family members but pose no serious danger (Box). In this situation, the psychiatrist can strongly encourage the patient to seek voluntary hospitalization or attend a partial hospitalization program. If the patient declines, the psychiatrist is left with 2 choices: initiate treatment in the outpatient setting or refuse to treat the patient and refer to another provider.
Treating “non-dangerous” mania in the outpatient setting is fraught with challenges:
• the possibility that the patient’s condition will progress to dangerousness
• poor adherence to treatment because of the patient’s limited insight
• the large amount of time required from the psychiatrist and care team to adequately manage the manic episode (eg, time spent with family members, frequent patient visits, and managing communications from the patient).
There are no guidelines to assist the office-based practitioner in treating mania in the outpatient setting. When considering dosing and optimal medication combinations for treating mania, clinical trials may be of limited value because most of these studies only included hospitalized manic patients.
Because of this dearth of knowledge, we provide recommendations based on our review of the literature and from our experience working with manic patients who refuse voluntary hospitalization and could not be hospitalized against their will. These recommendations are organized into 3 sections: diagnostic approach, treatment strategy, and family involvement.
Diagnostic approach
Making a diagnosis of mania might seem straightforward for clinicians who work in inpatient settings; however, mania might not present with classic florid symptoms among outpatients. Patients might have a chief concern of irritability, dysphoria, anxiety, or “insomnia,” which may lead clinicians to focus initially on non-bipolar conditions.2
During the interview, it is important to assess for any current DSM-5 symptoms of a manic episode, while being careful not to accept a patient’s denial of symptoms. Patients with mania often have poor insight and are unaware of changes from their baseline state when manic.3 Alternatively, manic patients may want you to believe that they are well and could minimize or deny all symptoms. Therefore, it is important to pay attention to mental status examination findings, such as hyperverbal speech, elated affect, psychomotor agitation, a tangential thought process, or flight of ideas.
Countertransference feelings of diagnostic confusion or frustration after long patient monologues or multiple interruptions by the patient should be incorporated into the diagnostic assessment. Family members or friends often can provide objective observations of behavioral changes necessary to secure the diagnosis.
Treatment strategy
Decision points. When treating manic outpatients, assess the need for hospitalization at each visit. Advantages of the inpatient setting include:
• the possibility of rapid medication adjustments
• continuous observation to ensure the patient’s safety
• keeping the patient temporarily removed from his community to prevent irreversible social and economic harms.
However, a challenge with hospitalization is third-party payers’ influence on a patient’s length of stay, which may lead to rapid medication changes that may not be clinically ideal.
At each outpatient visit, explore with the patient and family emerging symptoms that could justify involuntary hospitalization. Document whether you recommended inpatient hospitalization, the patient’s response to the recommendation, that you are aware and have considered the risks associated with outpatient care, and that you have discussed these risks with the patient and family.
For patients well-known to the psychiatrist, a history of dangerous mania may lead him (her) to strongly recommend hospitalization, whereas a pre-existing therapeutic alliance and no current or distant history of dangerous mania may lead the clinician to look for alternatives to inpatient care. Concomitant drug or alcohol use may increase the likelihood of mania becoming dangerous, making outpatient treatment ill-advised and riskier for everyone involved.
In exchange for agreeing to provide outpatient care for mania, it often is helpful to negotiate with the patient and family a threshold level of symptoms or behavior that will result in the patient agreeing to voluntary hospitalization (Table 1). Such an agreement can include stopping outpatient treatment if the patient does not improve significantly after 2 or 3 weeks or develops psychotic symptoms. The negotiation also can include partial hospitalization as an option, so long as the patient’s mania continues to be non-dangerous.
Obtaining pretreatment blood work can help a clinician determine whether a medication is safe to prescribe and establish causality if laboratory abnormalities arise after treatment begins. Ideally, the psychiatrist should follow consensus guidelines developed by the International Society for Bipolar Disorders4 or the American Psychiatric Association (APA)5 and order appropriate laboratory tests before prescribing anti-manic medications. Determine the pregnancy status of female patients of child-bearing age before prescribing a potentially teratogenic medication, especially because mania is associated with increased libido.6
Manic patients might be too disorganized to follow up with recommendations for laboratory testing, or could wait several days before completing blood work. Although not ideal, to avoid delaying treatment, a clinician might need to prescribe medication at the initial office visit, without pretreatment laboratory results. When the patient is more organized, complete the blood work. Keeping home pregnancy tests in the office can help rule out pregnancy before prescribing medication.
Medication. Meta-analyses have established the efficacy of mood stabilizers and antipsychotics for treating mania,7,8 and several consensus guidelines have incorporated these findings into treatment algorithms.9
For a patient already taking medications recommended by the guidelines, assess treatment adherence during the initial interview by questioning the patient and family. When the logistics of phlebotomy permit, obtaining the blood level of psychotropics can show the presence of any detectable drug concentration, which demonstrates that the patient has taken the medication recently.
If there is no evidence of nonadherence, an initial step might be to increase the dosage of the antipsychotic or mood stabilizer that the patient is already taking, ensuring that the dosage is optimized based on FDA indications and clinical trials data. The recommended rate of dosage adjustments differs among medications; however, optimal dosing should be reached quickly because a World Federation of Societies of Biological Psychiatry task force recommends that a mania treatment trial not exceed 2 weeks.10
Dosage increases can be made at weekly visits or sooner, based on treatment response and tolerability. If there is no benefit after optimizing the dosage, the next step would be to add a mood stabilizer to a second-generation antipsychotic (SGA), or vice versa to promote additive or synergistic medication effects.11 Switching one medication for the other should be avoided unless there are tolerability concerns.
For a patient who is not taking any medications, select a treatment that balances rapid stabilization with long-term efficacy and tolerability. Table 2 lists FDA-approved treatments for mania. Lamotrigine provides prophylactic efficacy with few associated risks, but it has no anti-manic effects and would be a poor choice for most actively manic patients. Most studies indicate that antipsychotics work faster than lithium at the 1-week mark; however, this may be a function of the lithium titration schedule followed in the protocols, the severity of mania among enrolled patients, the inclusion of typically non-responsive manic patients (eg, mixed) in the analysis, and the antipsychotic’s sedative potential relative to lithium. Although the anti-manic and prophylactic potential of lithium and valproate might make them an ideal first-line option, antipsychotics could stabilize a manic patient faster, especially if agitation is present.12,13
Breaking mania quickly is important when treating patients in the outpatient setting. In these situations, a reasonable choice is to prescribe a SGA, because of their rapid onset of effect, low potential for switch to depression, and utility in treating classic, mixed, or psychotic mania.10 Oral loading of valproate (20 mg/kg) is another option. An inpatient study that used an oral-loading strategy demonstrated a similar time to response as olanzapine,14 in contrast to an inpatient15 and an outpatient study16 that employed a standard starting dosage for each patient and led to slower improvement compared with olanzapine.
SGAs should be dosed moderately and lower than if the patient were hospitalized, to avoid alienating the patient from treatment by causing intolerable side effects. In particular, patients and their families should be warned about immediate risks, such as orthostasis or extrapyramidal symptoms. Although treatment guidelines recommend combination therapy as a possible first-line option,9 in the outpatient setting, monotherapy with an optimally dosed, rapid-acting agent is preferred to promote medication adherence and avoid potentially dangerous sedation. Manic patients experience increased distractibility and verbal memory and executive function impairments that can interfere with medication adherence.17 Therefore, patients are more likely to follow a simpler regimen. If SGA or valproate monotherapy does not control mania, begin combination treatment with a mood stabilizer and SGA. If the patient experiences remission with SGA monotherapy, the risks and benefits of maintaining the SGA vs switching to a mood stabilizer can be discussed.
Provide medication “as needed” for agitation—additional SGA dosing or a benzodiazepine—and explain to family members when their use is warranted. Benzodiazepines can provide short-term benefits for manic patients: anxiety relief, sedation, and anti-manic efficacy as monotherapy18-20 and in combination with other medications.21 Studies showing monotherapy efficacy employed high dosages of benzodiazepines (lorazepam mean dosage, 14 mg/d; clonazepam mean dosage, 13 mg/d)19 and high dosages of antipsychotics as needed,18,20 and often were associated with excessive sedation and ataxia.18,19 This makes benzodiazepine monotherapy a potentially dangerous approach for outpatient treatment of mania. IM lorazepam treated manic agitation less quickly than IM olanzapine, suggesting that SGAs are preferable in the outpatient setting because rapid control of agitation is crucial.22 If prescribed, a trusted family member should dispense benzodiazepines to the patient to minimize misuse because of impulsivity, distractibility, desperation to sleep, or pleasure seeking.
SGAs have the benefit of sedation but occasionally additional sleep medications are required. Benzodiazepine receptor agonists (BzRAs), such as zolpidem, eszopiclone, and zaleplon, should be used with caution. Although these medicines are effective in treating insomnia in individuals with primary insomnia23 and major depression,24 they have not been studied in manic patients. The decreased need for sleep in mania is phenomenologically25 and perhaps biologically different than insomnia in major depression.26 Therefore, mania-associated sleep disturbance might not respond to BZRAs. BzRAs also might induce somnambulism and other parasomnias,27 especially when used in combination with psychotropics, such as valproate28; it is unclear if the manic state itself increases this risk further. Sedating antihistamines with anticholinergic blockade, such as diphenhydramine and low dosages (<100 mg/d) of quetiapine, are best used only in combination with anti-manic medications because of putative link between anticholinergic blockade and manic induction.29 Less studied but safer options include novel anticonvulsants (gabapentin, pregabalin), melatonin, and melatonin receptor agonists. Sedating antidepressants, such as mirtazapine and trazodone, should be avoided.25
Important adjunctive treatment steps include discontinuing all pro-manic agents, including antidepressants, stimulants, and steroids, and discouraging use of caffeine, energy drinks, illicit drugs, and alcohol. The patient should return for office visits at least weekly, and possibly more frequently, depending on severity. Telephone check-in calls between scheduled visits may be necessary until the mania is broken.
Psychotherapy. Other than supportive therapy and psychoeducation, other forms of psychotherapy during mania are not indicated. Psychotherapy trials in bipolar disorder do not inform anti-manic efficacy because few have enrolled acutely manic patients and most report long-term benefits rather than short-term efficacy for the index manic episode.30 Educate patients about the importance of maintaining regular social rhythms and taking medication as prescribed. Manic patients might not be aware that they are acting differently during manic episodes, therefore efforts to improve the patient’s insight are unlikely to succeed. More time should be spent emphasizing the importance of adherence to treatment and taking anti-manic medications as prescribed. This discussion can be enhanced by focusing on the medication’s potential to reduce the unpleasant symptoms of mania, including irritability, insomnia, anxiety, and racing thoughts. At the first visit, discuss setting boundaries with the patient to reduce mania-driven, intrusive phone calls. A patient might develop insight after mania has resolved and he (she) can appreciate social or economic harm that occurred while manic. This discussion might foster adherence to maintenance treatment. Advise your patient to limit activities that may increase stimulation and perpetuate the mania, such as exercise, parties, concerts, or crowded shopping malls. Also, recommend that your patient stop working temporarily, to reduce stress and prevent any manic-driven interactions that could result in job loss.
If your patient has an established relationship with a psychotherapist, discuss with the therapist the plan to initiate mania treatment in the outpatient setting and work as a collaborative team, assuming that the patient has granted permission to share information. Encourage the therapist to increase the frequency of sessions with the patient to enable greater monitoring of changes in the patient’s manic symptoms.
Family involvement
Family support is crucial when treating mania in the outpatient setting. Lacking insight and organization, manic patients require the “auxiliary” judgment of trusted family members to ensure treatment success. The family should identify a single person to act as the liaison between the family and the psychiatrist. The psychiatrist should instruct this individual to accompany the patient to each clinic visit and provide regular updates on the patient’s adherence to treatment, changes in symptoms, and any new behaviors that would justify involuntary hospitalization. The treatment plan should be clearly communicated to this individual to ensure that it is implemented correctly. Ideally, this individual would be someone who understands that bipolar disorder is a mental illness, who can tolerate the patient’s potential resentment of them for taking on this role, and who can influence the patient and the other family members to adhere to the treatment plan.
This family member also should watch the patient take medication to rule out nonadherence if the patient’s condition does not improve.
Provide extensive psychoeducation to the family (Table 3). Discuss these teaching points and their implications at length during the first visit and reinforce them at subsequent visits. Advise spouses that the acute manic period is not the time to make major decisions about their marriage or to engage in couple’s therapy. These options are better explored after the patient recovers from the manic episode.
Encourage the family to engage in mania harm-reduction techniques to the extent that the patient will allow (Table 4). In particular, they should hold onto their loved one’s credit cards and checkbook, and discourage the patient from making any major financial decisions until the mania has resolved. Additionally, patients should be relieved of childcare responsibilities during this period. If there are any child welfare safety concerns, the clinician will need to report this to authorities as required by local laws.
Advise family members or roommates to call emergency services and request a crisis intervention team, or to take the patient to an emergency room if he (she) makes verbal threats to harm themselves or others, is violent, or demonstrates behaviors that indicate that he is no longer able to care for himself. The psychiatrist should assist with completing Family and Medical Leave Act paperwork for family members who will monitor the patient at home, a work-excuse letter for the patient so he does not lose his job, and short-term disability paperwork to ensure income for the patient during the manic period.
These interventions can be challenging for the entire family system because they place family members in a paternalistic role and reduce the patient’s autonomy within the family. This is problematic when these role changes occur between spouses or between a patient-parent and his (her) children. Such changes typically need to be reversed over time and may require the help of a family or couple’s therapist. To support the psychological health of the patient’s family, refer them to the National Alliance on Mental Illness for family support groups or to individual psychotherapists.
Outpatient management can be rewarding
For “non-dangerous” manic patients who cannot be hospitalized involuntarily and refuse full or partial hospitalization, a psychiatrist must choose between beginning treatment in the clinic and referring the patient to another provider. The latter option is consistent with the APA’s ethical guidelines,31 but must be done appropriately to avoid legal liability.32 This decision may disappoint a family desperate to see their loved one recover quickly and may leave them feeling betrayed by the mental health system. On the other hand, choosing to treat mania in the outpatient setting can be rewarding when resolution of mania restores the family’s homeostasis.
To achieve this outcome, the outpatient psychiatrist must engage the patient’s family to ensure that the patient adheres to the treatment plan and monitor for potentially dangerous behavior. The psychiatrist also must use his knowledge of mood symptoms, cognitive impairments, and the psychological experience of manic patients to create a safe and effective treatment strategy that the patient and family can implement.
Because of mania’s unpredictability and destructive potential, psychiatrists who agree to treat manic patients as outpatients should be familiar with their state’s statutes and case law that pertain to the refusal to accept a new patient, patient abandonment, involuntary hospitalization, confidentiality, and mandatory reporting. They also should seek clinical or legal consultation if they feel overwhelmed or uncertain about the safest and most legally sound approach.
Bottom Line
Treating mania in the outpatient setting is risky but can be accomplished in select patients with the help of the patient’s family and a strategy that integrates evidence-based pharmacotherapeutic and psychotherapeutic strategies. Because manic patients could display dangerous behavior, be familiar with your state’s laws regarding involuntary commitment, patient abandonment, and mandatory reporting.
Related Resources
• National Alliance on Mental Illness. www.NAMI.org.
• Depression and Bipolar Support Alliance. www.DBSAlliance.org.
Drug Brand Names
Aripiprazole • Abilify Mirtazapine • Remeron
Asenapine • Saphris Olanzapine • Zyprexa
Carbamazepine • Equetro, Tegretol Pregabalin • Lyrica
Chlorpromazine • Thorazine Quetiapine • Seroquel
Clonazepam • Klonopin Risperidone • Risperdal
Diphrenhydramine • Benadryl Trazodone • Desyrel
Eszopiclone • Lunesta Valproate • Divalproex
Gabapentin • Neurontin Zaleplon • Sonata
Lamotrigine • Lamictal Ziprasidone • Geodon
Lithium • Eskalith, Lithobid Zolpidem • Ambien
Lorazepam • Ativan
Acknowledgement
The authors thank Peter Ash, MD, for carefully reviewing this manuscript and providing feedback.
Disclosures
Dr. Rakofsky receives research or grant support from Takeda. Dr. Dunlop receives research or grant support from Forest, GlaxoSmithKline, and Otsuka.
Manic episodes, by definition, are associated with significant social or occupational impairment.1 Some manic patients are violent or engage in reckless behaviors that can harm themselves or others, such as speeding, disrupting traffic, or playing with fire. When these patients present to a psychiatrist’s outpatient practice, involuntary hospitalization might be justified.
However, some manic patients, in spite of their elevated, expansive, or irritable mood state, never behave dangerously and might not meet legal criteria for involuntary hospitalization, although these criteria differ from state to state. These patients might see a psychiatrist because manic symptoms such as irritability, talkativeness, and impulsivity are bothersome to their family members but pose no serious danger (Box). In this situation, the psychiatrist can strongly encourage the patient to seek voluntary hospitalization or attend a partial hospitalization program. If the patient declines, the psychiatrist is left with 2 choices: initiate treatment in the outpatient setting or refuse to treat the patient and refer to another provider.
Treating “non-dangerous” mania in the outpatient setting is fraught with challenges:
• the possibility that the patient’s condition will progress to dangerousness
• poor adherence to treatment because of the patient’s limited insight
• the large amount of time required from the psychiatrist and care team to adequately manage the manic episode (eg, time spent with family members, frequent patient visits, and managing communications from the patient).
There are no guidelines to assist the office-based practitioner in treating mania in the outpatient setting. When considering dosing and optimal medication combinations for treating mania, clinical trials may be of limited value because most of these studies only included hospitalized manic patients.
Because of this dearth of knowledge, we provide recommendations based on our review of the literature and from our experience working with manic patients who refuse voluntary hospitalization and could not be hospitalized against their will. These recommendations are organized into 3 sections: diagnostic approach, treatment strategy, and family involvement.
Diagnostic approach
Making a diagnosis of mania might seem straightforward for clinicians who work in inpatient settings; however, mania might not present with classic florid symptoms among outpatients. Patients might have a chief concern of irritability, dysphoria, anxiety, or “insomnia,” which may lead clinicians to focus initially on non-bipolar conditions.2
During the interview, it is important to assess for any current DSM-5 symptoms of a manic episode, while being careful not to accept a patient’s denial of symptoms. Patients with mania often have poor insight and are unaware of changes from their baseline state when manic.3 Alternatively, manic patients may want you to believe that they are well and could minimize or deny all symptoms. Therefore, it is important to pay attention to mental status examination findings, such as hyperverbal speech, elated affect, psychomotor agitation, a tangential thought process, or flight of ideas.
Countertransference feelings of diagnostic confusion or frustration after long patient monologues or multiple interruptions by the patient should be incorporated into the diagnostic assessment. Family members or friends often can provide objective observations of behavioral changes necessary to secure the diagnosis.
Treatment strategy
Decision points. When treating manic outpatients, assess the need for hospitalization at each visit. Advantages of the inpatient setting include:
• the possibility of rapid medication adjustments
• continuous observation to ensure the patient’s safety
• keeping the patient temporarily removed from his community to prevent irreversible social and economic harms.
However, a challenge with hospitalization is third-party payers’ influence on a patient’s length of stay, which may lead to rapid medication changes that may not be clinically ideal.
At each outpatient visit, explore with the patient and family emerging symptoms that could justify involuntary hospitalization. Document whether you recommended inpatient hospitalization, the patient’s response to the recommendation, that you are aware and have considered the risks associated with outpatient care, and that you have discussed these risks with the patient and family.
For patients well-known to the psychiatrist, a history of dangerous mania may lead him (her) to strongly recommend hospitalization, whereas a pre-existing therapeutic alliance and no current or distant history of dangerous mania may lead the clinician to look for alternatives to inpatient care. Concomitant drug or alcohol use may increase the likelihood of mania becoming dangerous, making outpatient treatment ill-advised and riskier for everyone involved.
In exchange for agreeing to provide outpatient care for mania, it often is helpful to negotiate with the patient and family a threshold level of symptoms or behavior that will result in the patient agreeing to voluntary hospitalization (Table 1). Such an agreement can include stopping outpatient treatment if the patient does not improve significantly after 2 or 3 weeks or develops psychotic symptoms. The negotiation also can include partial hospitalization as an option, so long as the patient’s mania continues to be non-dangerous.
Obtaining pretreatment blood work can help a clinician determine whether a medication is safe to prescribe and establish causality if laboratory abnormalities arise after treatment begins. Ideally, the psychiatrist should follow consensus guidelines developed by the International Society for Bipolar Disorders4 or the American Psychiatric Association (APA)5 and order appropriate laboratory tests before prescribing anti-manic medications. Determine the pregnancy status of female patients of child-bearing age before prescribing a potentially teratogenic medication, especially because mania is associated with increased libido.6
Manic patients might be too disorganized to follow up with recommendations for laboratory testing, or could wait several days before completing blood work. Although not ideal, to avoid delaying treatment, a clinician might need to prescribe medication at the initial office visit, without pretreatment laboratory results. When the patient is more organized, complete the blood work. Keeping home pregnancy tests in the office can help rule out pregnancy before prescribing medication.
Medication. Meta-analyses have established the efficacy of mood stabilizers and antipsychotics for treating mania,7,8 and several consensus guidelines have incorporated these findings into treatment algorithms.9
For a patient already taking medications recommended by the guidelines, assess treatment adherence during the initial interview by questioning the patient and family. When the logistics of phlebotomy permit, obtaining the blood level of psychotropics can show the presence of any detectable drug concentration, which demonstrates that the patient has taken the medication recently.
If there is no evidence of nonadherence, an initial step might be to increase the dosage of the antipsychotic or mood stabilizer that the patient is already taking, ensuring that the dosage is optimized based on FDA indications and clinical trials data. The recommended rate of dosage adjustments differs among medications; however, optimal dosing should be reached quickly because a World Federation of Societies of Biological Psychiatry task force recommends that a mania treatment trial not exceed 2 weeks.10
Dosage increases can be made at weekly visits or sooner, based on treatment response and tolerability. If there is no benefit after optimizing the dosage, the next step would be to add a mood stabilizer to a second-generation antipsychotic (SGA), or vice versa to promote additive or synergistic medication effects.11 Switching one medication for the other should be avoided unless there are tolerability concerns.
For a patient who is not taking any medications, select a treatment that balances rapid stabilization with long-term efficacy and tolerability. Table 2 lists FDA-approved treatments for mania. Lamotrigine provides prophylactic efficacy with few associated risks, but it has no anti-manic effects and would be a poor choice for most actively manic patients. Most studies indicate that antipsychotics work faster than lithium at the 1-week mark; however, this may be a function of the lithium titration schedule followed in the protocols, the severity of mania among enrolled patients, the inclusion of typically non-responsive manic patients (eg, mixed) in the analysis, and the antipsychotic’s sedative potential relative to lithium. Although the anti-manic and prophylactic potential of lithium and valproate might make them an ideal first-line option, antipsychotics could stabilize a manic patient faster, especially if agitation is present.12,13
Breaking mania quickly is important when treating patients in the outpatient setting. In these situations, a reasonable choice is to prescribe a SGA, because of their rapid onset of effect, low potential for switch to depression, and utility in treating classic, mixed, or psychotic mania.10 Oral loading of valproate (20 mg/kg) is another option. An inpatient study that used an oral-loading strategy demonstrated a similar time to response as olanzapine,14 in contrast to an inpatient15 and an outpatient study16 that employed a standard starting dosage for each patient and led to slower improvement compared with olanzapine.
SGAs should be dosed moderately and lower than if the patient were hospitalized, to avoid alienating the patient from treatment by causing intolerable side effects. In particular, patients and their families should be warned about immediate risks, such as orthostasis or extrapyramidal symptoms. Although treatment guidelines recommend combination therapy as a possible first-line option,9 in the outpatient setting, monotherapy with an optimally dosed, rapid-acting agent is preferred to promote medication adherence and avoid potentially dangerous sedation. Manic patients experience increased distractibility and verbal memory and executive function impairments that can interfere with medication adherence.17 Therefore, patients are more likely to follow a simpler regimen. If SGA or valproate monotherapy does not control mania, begin combination treatment with a mood stabilizer and SGA. If the patient experiences remission with SGA monotherapy, the risks and benefits of maintaining the SGA vs switching to a mood stabilizer can be discussed.
Provide medication “as needed” for agitation—additional SGA dosing or a benzodiazepine—and explain to family members when their use is warranted. Benzodiazepines can provide short-term benefits for manic patients: anxiety relief, sedation, and anti-manic efficacy as monotherapy18-20 and in combination with other medications.21 Studies showing monotherapy efficacy employed high dosages of benzodiazepines (lorazepam mean dosage, 14 mg/d; clonazepam mean dosage, 13 mg/d)19 and high dosages of antipsychotics as needed,18,20 and often were associated with excessive sedation and ataxia.18,19 This makes benzodiazepine monotherapy a potentially dangerous approach for outpatient treatment of mania. IM lorazepam treated manic agitation less quickly than IM olanzapine, suggesting that SGAs are preferable in the outpatient setting because rapid control of agitation is crucial.22 If prescribed, a trusted family member should dispense benzodiazepines to the patient to minimize misuse because of impulsivity, distractibility, desperation to sleep, or pleasure seeking.
SGAs have the benefit of sedation but occasionally additional sleep medications are required. Benzodiazepine receptor agonists (BzRAs), such as zolpidem, eszopiclone, and zaleplon, should be used with caution. Although these medicines are effective in treating insomnia in individuals with primary insomnia23 and major depression,24 they have not been studied in manic patients. The decreased need for sleep in mania is phenomenologically25 and perhaps biologically different than insomnia in major depression.26 Therefore, mania-associated sleep disturbance might not respond to BZRAs. BzRAs also might induce somnambulism and other parasomnias,27 especially when used in combination with psychotropics, such as valproate28; it is unclear if the manic state itself increases this risk further. Sedating antihistamines with anticholinergic blockade, such as diphenhydramine and low dosages (<100 mg/d) of quetiapine, are best used only in combination with anti-manic medications because of putative link between anticholinergic blockade and manic induction.29 Less studied but safer options include novel anticonvulsants (gabapentin, pregabalin), melatonin, and melatonin receptor agonists. Sedating antidepressants, such as mirtazapine and trazodone, should be avoided.25
Important adjunctive treatment steps include discontinuing all pro-manic agents, including antidepressants, stimulants, and steroids, and discouraging use of caffeine, energy drinks, illicit drugs, and alcohol. The patient should return for office visits at least weekly, and possibly more frequently, depending on severity. Telephone check-in calls between scheduled visits may be necessary until the mania is broken.
Psychotherapy. Other than supportive therapy and psychoeducation, other forms of psychotherapy during mania are not indicated. Psychotherapy trials in bipolar disorder do not inform anti-manic efficacy because few have enrolled acutely manic patients and most report long-term benefits rather than short-term efficacy for the index manic episode.30 Educate patients about the importance of maintaining regular social rhythms and taking medication as prescribed. Manic patients might not be aware that they are acting differently during manic episodes, therefore efforts to improve the patient’s insight are unlikely to succeed. More time should be spent emphasizing the importance of adherence to treatment and taking anti-manic medications as prescribed. This discussion can be enhanced by focusing on the medication’s potential to reduce the unpleasant symptoms of mania, including irritability, insomnia, anxiety, and racing thoughts. At the first visit, discuss setting boundaries with the patient to reduce mania-driven, intrusive phone calls. A patient might develop insight after mania has resolved and he (she) can appreciate social or economic harm that occurred while manic. This discussion might foster adherence to maintenance treatment. Advise your patient to limit activities that may increase stimulation and perpetuate the mania, such as exercise, parties, concerts, or crowded shopping malls. Also, recommend that your patient stop working temporarily, to reduce stress and prevent any manic-driven interactions that could result in job loss.
If your patient has an established relationship with a psychotherapist, discuss with the therapist the plan to initiate mania treatment in the outpatient setting and work as a collaborative team, assuming that the patient has granted permission to share information. Encourage the therapist to increase the frequency of sessions with the patient to enable greater monitoring of changes in the patient’s manic symptoms.
Family involvement
Family support is crucial when treating mania in the outpatient setting. Lacking insight and organization, manic patients require the “auxiliary” judgment of trusted family members to ensure treatment success. The family should identify a single person to act as the liaison between the family and the psychiatrist. The psychiatrist should instruct this individual to accompany the patient to each clinic visit and provide regular updates on the patient’s adherence to treatment, changes in symptoms, and any new behaviors that would justify involuntary hospitalization. The treatment plan should be clearly communicated to this individual to ensure that it is implemented correctly. Ideally, this individual would be someone who understands that bipolar disorder is a mental illness, who can tolerate the patient’s potential resentment of them for taking on this role, and who can influence the patient and the other family members to adhere to the treatment plan.
This family member also should watch the patient take medication to rule out nonadherence if the patient’s condition does not improve.
Provide extensive psychoeducation to the family (Table 3). Discuss these teaching points and their implications at length during the first visit and reinforce them at subsequent visits. Advise spouses that the acute manic period is not the time to make major decisions about their marriage or to engage in couple’s therapy. These options are better explored after the patient recovers from the manic episode.
Encourage the family to engage in mania harm-reduction techniques to the extent that the patient will allow (Table 4). In particular, they should hold onto their loved one’s credit cards and checkbook, and discourage the patient from making any major financial decisions until the mania has resolved. Additionally, patients should be relieved of childcare responsibilities during this period. If there are any child welfare safety concerns, the clinician will need to report this to authorities as required by local laws.
Advise family members or roommates to call emergency services and request a crisis intervention team, or to take the patient to an emergency room if he (she) makes verbal threats to harm themselves or others, is violent, or demonstrates behaviors that indicate that he is no longer able to care for himself. The psychiatrist should assist with completing Family and Medical Leave Act paperwork for family members who will monitor the patient at home, a work-excuse letter for the patient so he does not lose his job, and short-term disability paperwork to ensure income for the patient during the manic period.
These interventions can be challenging for the entire family system because they place family members in a paternalistic role and reduce the patient’s autonomy within the family. This is problematic when these role changes occur between spouses or between a patient-parent and his (her) children. Such changes typically need to be reversed over time and may require the help of a family or couple’s therapist. To support the psychological health of the patient’s family, refer them to the National Alliance on Mental Illness for family support groups or to individual psychotherapists.
Outpatient management can be rewarding
For “non-dangerous” manic patients who cannot be hospitalized involuntarily and refuse full or partial hospitalization, a psychiatrist must choose between beginning treatment in the clinic and referring the patient to another provider. The latter option is consistent with the APA’s ethical guidelines,31 but must be done appropriately to avoid legal liability.32 This decision may disappoint a family desperate to see their loved one recover quickly and may leave them feeling betrayed by the mental health system. On the other hand, choosing to treat mania in the outpatient setting can be rewarding when resolution of mania restores the family’s homeostasis.
To achieve this outcome, the outpatient psychiatrist must engage the patient’s family to ensure that the patient adheres to the treatment plan and monitor for potentially dangerous behavior. The psychiatrist also must use his knowledge of mood symptoms, cognitive impairments, and the psychological experience of manic patients to create a safe and effective treatment strategy that the patient and family can implement.
Because of mania’s unpredictability and destructive potential, psychiatrists who agree to treat manic patients as outpatients should be familiar with their state’s statutes and case law that pertain to the refusal to accept a new patient, patient abandonment, involuntary hospitalization, confidentiality, and mandatory reporting. They also should seek clinical or legal consultation if they feel overwhelmed or uncertain about the safest and most legally sound approach.
Bottom Line
Treating mania in the outpatient setting is risky but can be accomplished in select patients with the help of the patient’s family and a strategy that integrates evidence-based pharmacotherapeutic and psychotherapeutic strategies. Because manic patients could display dangerous behavior, be familiar with your state’s laws regarding involuntary commitment, patient abandonment, and mandatory reporting.
Related Resources
• National Alliance on Mental Illness. www.NAMI.org.
• Depression and Bipolar Support Alliance. www.DBSAlliance.org.
Drug Brand Names
Aripiprazole • Abilify Mirtazapine • Remeron
Asenapine • Saphris Olanzapine • Zyprexa
Carbamazepine • Equetro, Tegretol Pregabalin • Lyrica
Chlorpromazine • Thorazine Quetiapine • Seroquel
Clonazepam • Klonopin Risperidone • Risperdal
Diphrenhydramine • Benadryl Trazodone • Desyrel
Eszopiclone • Lunesta Valproate • Divalproex
Gabapentin • Neurontin Zaleplon • Sonata
Lamotrigine • Lamictal Ziprasidone • Geodon
Lithium • Eskalith, Lithobid Zolpidem • Ambien
Lorazepam • Ativan
Acknowledgement
The authors thank Peter Ash, MD, for carefully reviewing this manuscript and providing feedback.
Disclosures
Dr. Rakofsky receives research or grant support from Takeda. Dr. Dunlop receives research or grant support from Forest, GlaxoSmithKline, and Otsuka.
1. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Cassidy F, Murry E, Forest K, et al. Signs and symptoms of mania in pure and mixed episodes. J Affect Disord. 1998;50(2-3):187-201.
3. Yen CF, Chen CS, Ko CH, et al. Changes in insight among patients with bipolar I disorder: a 2-year prospective study. Bipolar Disord. 2007;9(3):238-242.
4. Ng F, Mammen OK, Wilting I, et al. The International Society for Bipolar Disorders (ISBD) consensus guidelines for the safety monitoring of bipolar disorder treatments. Bipolar Disord. 2009;11(6):559-595.
5. American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(suppl 4):1-50.
6. Allison JB, Wilson WP. Sexual behavior of manic patients: a preliminary report. South Med J. 1960;53:870-874.
7. Cipriani A, Barbui C, Salanti G, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet. 2011; 378(9799):1306-1315.
8. Yildiz A, Vieta E, Leucht S, et al. Efficacy of antimanic treatments: meta-analysis of randomized, controlled trials. Neuropsychopharmacology. 2011;36(2):375-389.
9. Nivoli AM, Murru A, Goikolea JM, et al. New treatment guidelines for acute bipolar mania: a critical review. J Affect Disord. 2012;140(2):125-141.
10. Grunze H, Vieta E, Goodwin GM, et al. The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: update 2009 on the treatment of acute mania. World J Biol Psychiatry. 2009;10(2):85-116.
11. Tohen M, Chengappa KN, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry. 2002;59(1):62-69.
12. Tohen M, Jacobs TG, Feldman PD. Onset of action of antipsychotics in the treatment of mania. Bipolar Disord. 2000;2(3 pt 2):261-268.
13. Goikolea JM, Colom F, Capapey J, et al. Faster onset of antimanic action with haloperidol compared to second-generation antipsychotics. A meta-analysis of randomized clinical trials in acute mania. Eur Neuropsychopharmacol. 2013;23(4):305-316.
14. Zajecka JM, Weisler R, Sachs G, et al. A comparison of the efficacy, safety, and tolerability of divalproex sodium and olanzapine in the treatment of bipolar disorder. J Clin Psychiatry. 2002;63(12):1148-1155.
15. Tohen M, Baker RW, Altshuler LL, et al. Olanzapine versus divalproex in the treatment of acute mania. Am J Psychiatry. 2002;159(6):1011-1017.
16. Tohen M, Vieta E, Goodwin GM, et al. Olanzapine versus divalproex versus placebo in the treatment of mild to moderate mania: a randomized, 12-week, double-blind study. J Clin Psychiatry. 2008;69(11):1776-1789.
17. Martínez-Arán A, Vieta E, Reinares M, et al. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am J Psychiatry. 2004; 161(2):262-270.
18. Edwards R, Stephenson U, Flewett T. Clonazepam in acute mania: a double blind trial. Aust N Z J Psychiatry. 1991;25(2):238-242.
19. Bradwejn J, Shriqui C, Koszycki D, et al. Double-blind comparison of the effects of clonazepam and lorazepam in acute mania. J Clin Psychopharmacol. 1990;10(6):403-408.
20. Clark HM, Berk M, Brook S. A randomized controlled single blind study of the efficacy of clonazepam and lithium in the treatment of acute mania. Human Psychopharmacology: Clinical and Experimental. 1997;12(4):325-328.
21. Lenox RH, Newhouse PA, Creelman WL, et al. Adjunctive treatment of manic agitation with lorazepam versus haloperidol: a double-blind study. J Clin Psychiatry. 1992;53(2):47-52.
22. Meehan K, Zhang F, David S, et al. A double-blind, randomized comparison of the efficacy and safety of intramuscular injections of olanzapine, lorazepam, or placebo in treating acutely agitated patients diagnosed with bipolar mania. J Clin Psychopharmacol. 2001;21(4):389-397.
23. Huedo-Medina TB, Kirsch I, Middlemass J, et al. Effectiveness of non-benzodiazepine hypnotics in treatment of adult insomnia: meta-analysis of data submitted to the Food and Drug Administration. BMJ. 2012;345:e8343. doi: 10.1136/bmj.e8343.
24. Fava M, Asnis GM, Shrivastava RK, et al. Improved insomnia symptoms and sleep-related next-day functioning in patients with comorbid major depressive disorder and insomnia following concomitant zolpidem extended-release 12.5 mg and escitalopram treatment: a randomized controlled trial. J Clin Psychiatry. 2011;72(7):914-928.
25. Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165(7):830-843.
26. Linkowski P, Kerkhofs M, Rielaert C, et al. Sleep during mania in manic-depressive males. Eur Arch Psychiatry Neurol Sci. 1986;235(6):339-341.
27. Poceta JS. Zolpidem ingestion, automatisms, and sleep driving: a clinical and legal case series. J Clin Sleep Med. 2011;7(6):632-638.
28. Sattar SP, Ramaswamy S, Bhatia SC, et al. Somnambulism due to probable interaction of valproic acid and zolpidem. Ann Pharmacother. 2003;37(10):1429-1433.
29. Rybakowski JK, Koszewska I, Puzynski S. Anticholinergic mechanisms: a forgotten cause of the switch process in bipolar disorder [Comment on: The neurolobiology of the switch process in bipolar disorder: a review. J Clin Psychiatry. 2010]. J Clin Psychiatry. 2010;71(12):1698-1699; author reply 1699-1700.
30. Miklowitz DJ. Adjunctive psychotherapy for bipolar disorder: state of the evidence. Am J Psychiatry. 2008;165(11):1408-1419.
31. American Psychiatric Association. The principles of medical ethics with annotations especially applicable to psychiatry. Arlington, VA: American Psychiatric Association; 2013.
32. Simon RI, Shuman DW. Clinical manual of psychiatry and law. Arlington, VA: American Psychiatric Publishing; 2007:17-36.
1. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Cassidy F, Murry E, Forest K, et al. Signs and symptoms of mania in pure and mixed episodes. J Affect Disord. 1998;50(2-3):187-201.
3. Yen CF, Chen CS, Ko CH, et al. Changes in insight among patients with bipolar I disorder: a 2-year prospective study. Bipolar Disord. 2007;9(3):238-242.
4. Ng F, Mammen OK, Wilting I, et al. The International Society for Bipolar Disorders (ISBD) consensus guidelines for the safety monitoring of bipolar disorder treatments. Bipolar Disord. 2009;11(6):559-595.
5. American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(suppl 4):1-50.
6. Allison JB, Wilson WP. Sexual behavior of manic patients: a preliminary report. South Med J. 1960;53:870-874.
7. Cipriani A, Barbui C, Salanti G, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet. 2011; 378(9799):1306-1315.
8. Yildiz A, Vieta E, Leucht S, et al. Efficacy of antimanic treatments: meta-analysis of randomized, controlled trials. Neuropsychopharmacology. 2011;36(2):375-389.
9. Nivoli AM, Murru A, Goikolea JM, et al. New treatment guidelines for acute bipolar mania: a critical review. J Affect Disord. 2012;140(2):125-141.
10. Grunze H, Vieta E, Goodwin GM, et al. The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: update 2009 on the treatment of acute mania. World J Biol Psychiatry. 2009;10(2):85-116.
11. Tohen M, Chengappa KN, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry. 2002;59(1):62-69.
12. Tohen M, Jacobs TG, Feldman PD. Onset of action of antipsychotics in the treatment of mania. Bipolar Disord. 2000;2(3 pt 2):261-268.
13. Goikolea JM, Colom F, Capapey J, et al. Faster onset of antimanic action with haloperidol compared to second-generation antipsychotics. A meta-analysis of randomized clinical trials in acute mania. Eur Neuropsychopharmacol. 2013;23(4):305-316.
14. Zajecka JM, Weisler R, Sachs G, et al. A comparison of the efficacy, safety, and tolerability of divalproex sodium and olanzapine in the treatment of bipolar disorder. J Clin Psychiatry. 2002;63(12):1148-1155.
15. Tohen M, Baker RW, Altshuler LL, et al. Olanzapine versus divalproex in the treatment of acute mania. Am J Psychiatry. 2002;159(6):1011-1017.
16. Tohen M, Vieta E, Goodwin GM, et al. Olanzapine versus divalproex versus placebo in the treatment of mild to moderate mania: a randomized, 12-week, double-blind study. J Clin Psychiatry. 2008;69(11):1776-1789.
17. Martínez-Arán A, Vieta E, Reinares M, et al. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am J Psychiatry. 2004; 161(2):262-270.
18. Edwards R, Stephenson U, Flewett T. Clonazepam in acute mania: a double blind trial. Aust N Z J Psychiatry. 1991;25(2):238-242.
19. Bradwejn J, Shriqui C, Koszycki D, et al. Double-blind comparison of the effects of clonazepam and lorazepam in acute mania. J Clin Psychopharmacol. 1990;10(6):403-408.
20. Clark HM, Berk M, Brook S. A randomized controlled single blind study of the efficacy of clonazepam and lithium in the treatment of acute mania. Human Psychopharmacology: Clinical and Experimental. 1997;12(4):325-328.
21. Lenox RH, Newhouse PA, Creelman WL, et al. Adjunctive treatment of manic agitation with lorazepam versus haloperidol: a double-blind study. J Clin Psychiatry. 1992;53(2):47-52.
22. Meehan K, Zhang F, David S, et al. A double-blind, randomized comparison of the efficacy and safety of intramuscular injections of olanzapine, lorazepam, or placebo in treating acutely agitated patients diagnosed with bipolar mania. J Clin Psychopharmacol. 2001;21(4):389-397.
23. Huedo-Medina TB, Kirsch I, Middlemass J, et al. Effectiveness of non-benzodiazepine hypnotics in treatment of adult insomnia: meta-analysis of data submitted to the Food and Drug Administration. BMJ. 2012;345:e8343. doi: 10.1136/bmj.e8343.
24. Fava M, Asnis GM, Shrivastava RK, et al. Improved insomnia symptoms and sleep-related next-day functioning in patients with comorbid major depressive disorder and insomnia following concomitant zolpidem extended-release 12.5 mg and escitalopram treatment: a randomized controlled trial. J Clin Psychiatry. 2011;72(7):914-928.
25. Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165(7):830-843.
26. Linkowski P, Kerkhofs M, Rielaert C, et al. Sleep during mania in manic-depressive males. Eur Arch Psychiatry Neurol Sci. 1986;235(6):339-341.
27. Poceta JS. Zolpidem ingestion, automatisms, and sleep driving: a clinical and legal case series. J Clin Sleep Med. 2011;7(6):632-638.
28. Sattar SP, Ramaswamy S, Bhatia SC, et al. Somnambulism due to probable interaction of valproic acid and zolpidem. Ann Pharmacother. 2003;37(10):1429-1433.
29. Rybakowski JK, Koszewska I, Puzynski S. Anticholinergic mechanisms: a forgotten cause of the switch process in bipolar disorder [Comment on: The neurolobiology of the switch process in bipolar disorder: a review. J Clin Psychiatry. 2010]. J Clin Psychiatry. 2010;71(12):1698-1699; author reply 1699-1700.
30. Miklowitz DJ. Adjunctive psychotherapy for bipolar disorder: state of the evidence. Am J Psychiatry. 2008;165(11):1408-1419.
31. American Psychiatric Association. The principles of medical ethics with annotations especially applicable to psychiatry. Arlington, VA: American Psychiatric Association; 2013.
32. Simon RI, Shuman DW. Clinical manual of psychiatry and law. Arlington, VA: American Psychiatric Publishing; 2007:17-36.
Why partner with clinical pharmacists?
While reading the “Opportunities to partner with clinical pharmacists in ambulatory care” (Current Psychiatry, Evidence-Based Reviews, July 2014, p. 23-29 [http://bit.ly/1s3yqmh], I became puzzled. Several times, I asked myself, “As a psychiatrist reasonably well-trained in psychopharmacology, why would I need or want to partner with a clinical pharmacist in this fashion?” Indeed, I was under the impression that this is what I trained to do. It called to mind a bumper sticker from the feminist movement of the 1960s that read, “A woman without a man is like a fish without a bicycle.” It then occurred to me that a psychiatrist without a clinical pharmacist would find himself or herself in that same lamentable position.
Scott D. Mendelson, MD, PhD
Roseburg, Oregon
While reading the “Opportunities to partner with clinical pharmacists in ambulatory care” (Current Psychiatry, Evidence-Based Reviews, July 2014, p. 23-29 [http://bit.ly/1s3yqmh], I became puzzled. Several times, I asked myself, “As a psychiatrist reasonably well-trained in psychopharmacology, why would I need or want to partner with a clinical pharmacist in this fashion?” Indeed, I was under the impression that this is what I trained to do. It called to mind a bumper sticker from the feminist movement of the 1960s that read, “A woman without a man is like a fish without a bicycle.” It then occurred to me that a psychiatrist without a clinical pharmacist would find himself or herself in that same lamentable position.
Scott D. Mendelson, MD, PhD
Roseburg, Oregon
While reading the “Opportunities to partner with clinical pharmacists in ambulatory care” (Current Psychiatry, Evidence-Based Reviews, July 2014, p. 23-29 [http://bit.ly/1s3yqmh], I became puzzled. Several times, I asked myself, “As a psychiatrist reasonably well-trained in psychopharmacology, why would I need or want to partner with a clinical pharmacist in this fashion?” Indeed, I was under the impression that this is what I trained to do. It called to mind a bumper sticker from the feminist movement of the 1960s that read, “A woman without a man is like a fish without a bicycle.” It then occurred to me that a psychiatrist without a clinical pharmacist would find himself or herself in that same lamentable position.
Scott D. Mendelson, MD, PhD
Roseburg, Oregon
The ‘decline’ of psychoanalysis
There are many interesting aspects of Dr. Nasrallah’s review of the changes in psychiatry in recent decades (Post-World War II psychiatry: 70 years of momentous change, Current Psychiatry, From the Editor, July 2014, p. 21-22, 49-50 [http://bit.ly/1m8HcdC]). There is no doubt that great strides have been made, particularly in the care of the more seriously ill, and that those accomplishments owe a good deal to the introduction of psychoactive agents.
However, his reference to the “decline” of psychoanalysis was unfortunate and a gratuitous insult to those of us who continue to practice psychoanalysis and who recognize how much psychoanalytic thinking has contributed to the psychotherapeutic practices of non-analyst psychiatrists. If by decline he means that patients who once were in analysis now are being treated with medication alone, he is correct. That might not always be in the best interest of patients, but it is a fact. If by decline he means that in all instances all patients benefit more from pills than they would from analysis, his viewpoint is derived from misinformation.
Since academic psychiatry and psychiatric publications became wholly owned subsidiaries of the pharmaceutical industry, this dismissive attitude about psychoanalysis has attained the status of established wisdom. Psychoanalysts understand that one size does not fit all, no single treatment is the best choice for all patients, and medications can be of great value. Why can’t psychopharmacologists show a similar respect for psychoanalysis?
Charles Goodstein, MD
Tenafly, New Jersey
Dr. Nasrallah responds
Thank you, Dr. Goodstein, for expressing your view about my editorial. However, it is unfair to describe the editorial as being dismissive and insulting toward psychoanalysts. I was simply stating undeniable historical facts about the evolution of psychiatry—one aspect was the reduced prevalence and influence of psychoanalysis over the past few decades, which was partially because of the advent of pharmacotherapy. The other reason was the emergence of other psychotherapies, such as cognitive-behavioral therapy, interpersonal psychotherapy, and dialectical behavior therapy, which are evidence-based, shorter in duration, and more cost effective.
Psychoanalysis remains an important component of contemporary psychiatry, albeit limited to a smaller subgroup of patients.
In my residency, I was heavily trained in psychodynamic therapy, and many of my supervisors were psychoanalysts. I developed my neuroscience skills in a post-residency fellowship at the National Institutes of Health. Nowadays, residency programs must provide both psychotherapeutic and psychopharmacologic training to psychiatric residents.
Your statement that medications have replaced psychotherapy is inaccurate. We train our residents to provide each outpatient with both pharmacotherapy (when indicated) side-by-side with psychotherapy—whether supportive, psychoeducational, psychodynamic, or cognitive-behavioral therapy, or a combination thereof. I continually warn residents about reducing psychiatric care to giving pills, which would be a travesty.
In addition, I regard psychotherapy as a neurobiological intervention because it modifies brain connectivity and neuroplasticity (see my December 2013 Editorial, “Repositioning psychotherapy as neurobiological intervention,” available at CurrentPsychiatry.com).
Last, I wish you would not insult academic psychiatry as being a “wholly owned subsidiary of the pharmaceutical industry.” Someone must develop new and better treatments for serious psychiatric brain disorders. The only entities dedicated to doing that, in the United States, are the pharmaceutical industry and the academic psychopharmacology experts. Together, they generate new ideas and develop innovative mechanisms of action and test them in controlled clinical trials to treat disabling mental disorders. It is not fair to impugn the integrity of academic psychiatrists when they are doing what they were trained to do. They have the integrity and objectivity to criticize the industry when necessary. (See page 50 of my editorial under the subheading “Pharmaceutical industry debacle.”)
Henry A. Nasrallah, MD
Professor and Chairman
Department of Neurology & Psychiatry
Saint Louis University School of Medicine
St. Louis, Missouri
There are many interesting aspects of Dr. Nasrallah’s review of the changes in psychiatry in recent decades (Post-World War II psychiatry: 70 years of momentous change, Current Psychiatry, From the Editor, July 2014, p. 21-22, 49-50 [http://bit.ly/1m8HcdC]). There is no doubt that great strides have been made, particularly in the care of the more seriously ill, and that those accomplishments owe a good deal to the introduction of psychoactive agents.
However, his reference to the “decline” of psychoanalysis was unfortunate and a gratuitous insult to those of us who continue to practice psychoanalysis and who recognize how much psychoanalytic thinking has contributed to the psychotherapeutic practices of non-analyst psychiatrists. If by decline he means that patients who once were in analysis now are being treated with medication alone, he is correct. That might not always be in the best interest of patients, but it is a fact. If by decline he means that in all instances all patients benefit more from pills than they would from analysis, his viewpoint is derived from misinformation.
Since academic psychiatry and psychiatric publications became wholly owned subsidiaries of the pharmaceutical industry, this dismissive attitude about psychoanalysis has attained the status of established wisdom. Psychoanalysts understand that one size does not fit all, no single treatment is the best choice for all patients, and medications can be of great value. Why can’t psychopharmacologists show a similar respect for psychoanalysis?
Charles Goodstein, MD
Tenafly, New Jersey
Dr. Nasrallah responds
Thank you, Dr. Goodstein, for expressing your view about my editorial. However, it is unfair to describe the editorial as being dismissive and insulting toward psychoanalysts. I was simply stating undeniable historical facts about the evolution of psychiatry—one aspect was the reduced prevalence and influence of psychoanalysis over the past few decades, which was partially because of the advent of pharmacotherapy. The other reason was the emergence of other psychotherapies, such as cognitive-behavioral therapy, interpersonal psychotherapy, and dialectical behavior therapy, which are evidence-based, shorter in duration, and more cost effective.
Psychoanalysis remains an important component of contemporary psychiatry, albeit limited to a smaller subgroup of patients.
In my residency, I was heavily trained in psychodynamic therapy, and many of my supervisors were psychoanalysts. I developed my neuroscience skills in a post-residency fellowship at the National Institutes of Health. Nowadays, residency programs must provide both psychotherapeutic and psychopharmacologic training to psychiatric residents.
Your statement that medications have replaced psychotherapy is inaccurate. We train our residents to provide each outpatient with both pharmacotherapy (when indicated) side-by-side with psychotherapy—whether supportive, psychoeducational, psychodynamic, or cognitive-behavioral therapy, or a combination thereof. I continually warn residents about reducing psychiatric care to giving pills, which would be a travesty.
In addition, I regard psychotherapy as a neurobiological intervention because it modifies brain connectivity and neuroplasticity (see my December 2013 Editorial, “Repositioning psychotherapy as neurobiological intervention,” available at CurrentPsychiatry.com).
Last, I wish you would not insult academic psychiatry as being a “wholly owned subsidiary of the pharmaceutical industry.” Someone must develop new and better treatments for serious psychiatric brain disorders. The only entities dedicated to doing that, in the United States, are the pharmaceutical industry and the academic psychopharmacology experts. Together, they generate new ideas and develop innovative mechanisms of action and test them in controlled clinical trials to treat disabling mental disorders. It is not fair to impugn the integrity of academic psychiatrists when they are doing what they were trained to do. They have the integrity and objectivity to criticize the industry when necessary. (See page 50 of my editorial under the subheading “Pharmaceutical industry debacle.”)
Henry A. Nasrallah, MD
Professor and Chairman
Department of Neurology & Psychiatry
Saint Louis University School of Medicine
St. Louis, Missouri
There are many interesting aspects of Dr. Nasrallah’s review of the changes in psychiatry in recent decades (Post-World War II psychiatry: 70 years of momentous change, Current Psychiatry, From the Editor, July 2014, p. 21-22, 49-50 [http://bit.ly/1m8HcdC]). There is no doubt that great strides have been made, particularly in the care of the more seriously ill, and that those accomplishments owe a good deal to the introduction of psychoactive agents.
However, his reference to the “decline” of psychoanalysis was unfortunate and a gratuitous insult to those of us who continue to practice psychoanalysis and who recognize how much psychoanalytic thinking has contributed to the psychotherapeutic practices of non-analyst psychiatrists. If by decline he means that patients who once were in analysis now are being treated with medication alone, he is correct. That might not always be in the best interest of patients, but it is a fact. If by decline he means that in all instances all patients benefit more from pills than they would from analysis, his viewpoint is derived from misinformation.
Since academic psychiatry and psychiatric publications became wholly owned subsidiaries of the pharmaceutical industry, this dismissive attitude about psychoanalysis has attained the status of established wisdom. Psychoanalysts understand that one size does not fit all, no single treatment is the best choice for all patients, and medications can be of great value. Why can’t psychopharmacologists show a similar respect for psychoanalysis?
Charles Goodstein, MD
Tenafly, New Jersey
Dr. Nasrallah responds
Thank you, Dr. Goodstein, for expressing your view about my editorial. However, it is unfair to describe the editorial as being dismissive and insulting toward psychoanalysts. I was simply stating undeniable historical facts about the evolution of psychiatry—one aspect was the reduced prevalence and influence of psychoanalysis over the past few decades, which was partially because of the advent of pharmacotherapy. The other reason was the emergence of other psychotherapies, such as cognitive-behavioral therapy, interpersonal psychotherapy, and dialectical behavior therapy, which are evidence-based, shorter in duration, and more cost effective.
Psychoanalysis remains an important component of contemporary psychiatry, albeit limited to a smaller subgroup of patients.
In my residency, I was heavily trained in psychodynamic therapy, and many of my supervisors were psychoanalysts. I developed my neuroscience skills in a post-residency fellowship at the National Institutes of Health. Nowadays, residency programs must provide both psychotherapeutic and psychopharmacologic training to psychiatric residents.
Your statement that medications have replaced psychotherapy is inaccurate. We train our residents to provide each outpatient with both pharmacotherapy (when indicated) side-by-side with psychotherapy—whether supportive, psychoeducational, psychodynamic, or cognitive-behavioral therapy, or a combination thereof. I continually warn residents about reducing psychiatric care to giving pills, which would be a travesty.
In addition, I regard psychotherapy as a neurobiological intervention because it modifies brain connectivity and neuroplasticity (see my December 2013 Editorial, “Repositioning psychotherapy as neurobiological intervention,” available at CurrentPsychiatry.com).
Last, I wish you would not insult academic psychiatry as being a “wholly owned subsidiary of the pharmaceutical industry.” Someone must develop new and better treatments for serious psychiatric brain disorders. The only entities dedicated to doing that, in the United States, are the pharmaceutical industry and the academic psychopharmacology experts. Together, they generate new ideas and develop innovative mechanisms of action and test them in controlled clinical trials to treat disabling mental disorders. It is not fair to impugn the integrity of academic psychiatrists when they are doing what they were trained to do. They have the integrity and objectivity to criticize the industry when necessary. (See page 50 of my editorial under the subheading “Pharmaceutical industry debacle.”)
Henry A. Nasrallah, MD
Professor and Chairman
Department of Neurology & Psychiatry
Saint Louis University School of Medicine
St. Louis, Missouri
Cannabis abuse and use
Cannabis abuse and THC content are on the rise
The authors of the July 2014 Residents’ Voices article (What we ought to talk about when we’re talking about decriminalizing Cannabis, Current Psychiatry, July 2014, p. 45-46 [http://bit.ly/1uAb7iK]) highlight the mental health complications of Cannabis and mention that, when Cannabis is juxtaposed with other illicit substances, it appears innocuous.
On the contrary: Data from the 2011 Drug Abuse Warning Network highlighted the rising involvement of Cannabis in emergency department (ED) visits. The report indicated that of the 1,252,500 ED visits involving illicit drugs in 2011, the most common illicit drug involved was cocaine, which accounted for 505,224 ED visits, with Cannabis a close second at 455,668 visits—not including synthetic cannabinoids, which came in fifth, with 28,531 ED visits.1
Another useful point to buttress the concerns raised by the authors is that the potency of delta-9-tetrahydrocannabinol (THC), the primary psychoactive ingredient in Cannabis, has increased gradually over the years. The University of Mississippi Potency Monitoring Project, a National Institute on Drug Abuse–funded landmark project that studied samples of Cannabis confiscated by law enforcement in the United States between 1993 and 2008, revealed that the mean THC content increased from 3.4% in 1993, to 8.8% in 2008.2 The THC content of Cannabis is responsible for most of its psychoactive effects, so that the higher the THC content, the greater the adverse effects on mental health.
A major phytocannabinoid, cannabidiol (CBD), also present in Cannabis, appears to counteract the adverse effects of THC, particularly by means of its antipsychotic property. Compared with the rising mean THC content of Cannabis from 1993 to 2008, CBD content has remained relatively the same: a mean of 0.3% in 1993 and 0.4% in 2008.3,4
Several factors have been postulated for the trend toward a high THC–low CBD profile in recent years: cultivation methods, the preference for cultivating seedless female plants (sinsemilla) that tend to have a high THC content, and global availability of seeds over the Internet. The high THC–low CBD profile has been linked to an increased risk of Cannabis dependence and increased treatment-seeking for Cannabis-related problems.3
Adegboyega Oyemade
Addiction Psychiatrist
Maryland Treatment Centers, Inc.
Attending Psychiatrist
Sinai Hospital
Baltimore, Maryland
-------------------------------------------------------------------
Research for 'Rx: Cannabis' is needed
Regarding the essay by Drs. Gershan and Gangahar on decriminalization of Cannabis, I want to comment on issues surrounding prescription Cannabis.
It is clear that Cannabis can exacerbate psychosis, among other risks, but its potential benefits remain relatively unexplored. The authors correctly point out that, among indications for Cannabis, none are FDA-approved. Yet, off-label prescribing is pervasive and accepted in psychiatry, lack of FDA approval of indications for Cannabis is not an especially compelling argument against such prescribing.*
Lack of research and funding hampers efforts to conduct trials of the therapeutic value of Cannabis, as does its Schedule I status (ie, “no currently accepted medical use and a high potential for abuse” [language of the Controlled Substances Act]). There are reports of benefit in intractable epilepsy and posttraumatic stress disorder (PTSD) that merit further investigation; however, such research is hampered, I believe, by bureaucracy.
For example, an approved study at the University of Arizona of the use of Cannabis to treat PTSD has remained in regulatory limbo for longer than 4 years because of the immense hurdles involved in performing research on this substance—despite how pressing such research is, given the large number of veterans returning from active duty with this diagnosis and the paucity of treatment options.
Perhaps, there also is something “missing” in the debate about research into Cannabis.
Wesley Ryan, MD
PGY-5 Addiction Psychiatry Fellow
University of Washington
Seattle, Washington
*Editor's Note: An earlier version of this article stated, "Yet, off-labeling prescribing of Cannabis is pervasive, and I've found, accepted in psychiatry," which does not reflect the author's opinion or intended meaning. The sentence has been corrected to read, "Yet, because off-label prescribing is pervasive and accepted in psychiatry, lack of FDA approval of indications for Cannabis is not an especially compelling argument against such prescribing."
1. U.S. Department of Health and Human Services. Drug Abuse Warning Network, 2011: National estimates of drug-related emergency department visits. http://www.samhsa.gov/data/2k13/ DAWN2k11ED/DAWN2k11ED.htm. Published May 2013. Accessed on July 26, 2014.
2. Mehmedic Z, Chandra S, Slade D, et al. Potency trends of Δ9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. J Forensic Sci. 2010;55(5):1209-1217.
3. Swift W, Wong A, Li KM, et al. Analysis of cannabis seizures in NSW, Australia: cannabis potency and cannabinoid profile. PLos One. 2013;8(7):e70052. doi: 10.1371/journal.pone.0070052.
4. Morrison P. It’s the lack of balance in cannabis that does the harm. http://www.beckleyfoundation. org/2010/10/its-the-lack-of-balance-in-cannabis-that-does-the-harm. Published October 21, 2010. Accessed July 26, 2014.
Cannabis abuse and THC content are on the rise
The authors of the July 2014 Residents’ Voices article (What we ought to talk about when we’re talking about decriminalizing Cannabis, Current Psychiatry, July 2014, p. 45-46 [http://bit.ly/1uAb7iK]) highlight the mental health complications of Cannabis and mention that, when Cannabis is juxtaposed with other illicit substances, it appears innocuous.
On the contrary: Data from the 2011 Drug Abuse Warning Network highlighted the rising involvement of Cannabis in emergency department (ED) visits. The report indicated that of the 1,252,500 ED visits involving illicit drugs in 2011, the most common illicit drug involved was cocaine, which accounted for 505,224 ED visits, with Cannabis a close second at 455,668 visits—not including synthetic cannabinoids, which came in fifth, with 28,531 ED visits.1
Another useful point to buttress the concerns raised by the authors is that the potency of delta-9-tetrahydrocannabinol (THC), the primary psychoactive ingredient in Cannabis, has increased gradually over the years. The University of Mississippi Potency Monitoring Project, a National Institute on Drug Abuse–funded landmark project that studied samples of Cannabis confiscated by law enforcement in the United States between 1993 and 2008, revealed that the mean THC content increased from 3.4% in 1993, to 8.8% in 2008.2 The THC content of Cannabis is responsible for most of its psychoactive effects, so that the higher the THC content, the greater the adverse effects on mental health.
A major phytocannabinoid, cannabidiol (CBD), also present in Cannabis, appears to counteract the adverse effects of THC, particularly by means of its antipsychotic property. Compared with the rising mean THC content of Cannabis from 1993 to 2008, CBD content has remained relatively the same: a mean of 0.3% in 1993 and 0.4% in 2008.3,4
Several factors have been postulated for the trend toward a high THC–low CBD profile in recent years: cultivation methods, the preference for cultivating seedless female plants (sinsemilla) that tend to have a high THC content, and global availability of seeds over the Internet. The high THC–low CBD profile has been linked to an increased risk of Cannabis dependence and increased treatment-seeking for Cannabis-related problems.3
Adegboyega Oyemade
Addiction Psychiatrist
Maryland Treatment Centers, Inc.
Attending Psychiatrist
Sinai Hospital
Baltimore, Maryland
-------------------------------------------------------------------
Research for 'Rx: Cannabis' is needed
Regarding the essay by Drs. Gershan and Gangahar on decriminalization of Cannabis, I want to comment on issues surrounding prescription Cannabis.
It is clear that Cannabis can exacerbate psychosis, among other risks, but its potential benefits remain relatively unexplored. The authors correctly point out that, among indications for Cannabis, none are FDA-approved. Yet, off-label prescribing is pervasive and accepted in psychiatry, lack of FDA approval of indications for Cannabis is not an especially compelling argument against such prescribing.*
Lack of research and funding hampers efforts to conduct trials of the therapeutic value of Cannabis, as does its Schedule I status (ie, “no currently accepted medical use and a high potential for abuse” [language of the Controlled Substances Act]). There are reports of benefit in intractable epilepsy and posttraumatic stress disorder (PTSD) that merit further investigation; however, such research is hampered, I believe, by bureaucracy.
For example, an approved study at the University of Arizona of the use of Cannabis to treat PTSD has remained in regulatory limbo for longer than 4 years because of the immense hurdles involved in performing research on this substance—despite how pressing such research is, given the large number of veterans returning from active duty with this diagnosis and the paucity of treatment options.
Perhaps, there also is something “missing” in the debate about research into Cannabis.
Wesley Ryan, MD
PGY-5 Addiction Psychiatry Fellow
University of Washington
Seattle, Washington
*Editor's Note: An earlier version of this article stated, "Yet, off-labeling prescribing of Cannabis is pervasive, and I've found, accepted in psychiatry," which does not reflect the author's opinion or intended meaning. The sentence has been corrected to read, "Yet, because off-label prescribing is pervasive and accepted in psychiatry, lack of FDA approval of indications for Cannabis is not an especially compelling argument against such prescribing."
Cannabis abuse and THC content are on the rise
The authors of the July 2014 Residents’ Voices article (What we ought to talk about when we’re talking about decriminalizing Cannabis, Current Psychiatry, July 2014, p. 45-46 [http://bit.ly/1uAb7iK]) highlight the mental health complications of Cannabis and mention that, when Cannabis is juxtaposed with other illicit substances, it appears innocuous.
On the contrary: Data from the 2011 Drug Abuse Warning Network highlighted the rising involvement of Cannabis in emergency department (ED) visits. The report indicated that of the 1,252,500 ED visits involving illicit drugs in 2011, the most common illicit drug involved was cocaine, which accounted for 505,224 ED visits, with Cannabis a close second at 455,668 visits—not including synthetic cannabinoids, which came in fifth, with 28,531 ED visits.1
Another useful point to buttress the concerns raised by the authors is that the potency of delta-9-tetrahydrocannabinol (THC), the primary psychoactive ingredient in Cannabis, has increased gradually over the years. The University of Mississippi Potency Monitoring Project, a National Institute on Drug Abuse–funded landmark project that studied samples of Cannabis confiscated by law enforcement in the United States between 1993 and 2008, revealed that the mean THC content increased from 3.4% in 1993, to 8.8% in 2008.2 The THC content of Cannabis is responsible for most of its psychoactive effects, so that the higher the THC content, the greater the adverse effects on mental health.
A major phytocannabinoid, cannabidiol (CBD), also present in Cannabis, appears to counteract the adverse effects of THC, particularly by means of its antipsychotic property. Compared with the rising mean THC content of Cannabis from 1993 to 2008, CBD content has remained relatively the same: a mean of 0.3% in 1993 and 0.4% in 2008.3,4
Several factors have been postulated for the trend toward a high THC–low CBD profile in recent years: cultivation methods, the preference for cultivating seedless female plants (sinsemilla) that tend to have a high THC content, and global availability of seeds over the Internet. The high THC–low CBD profile has been linked to an increased risk of Cannabis dependence and increased treatment-seeking for Cannabis-related problems.3
Adegboyega Oyemade
Addiction Psychiatrist
Maryland Treatment Centers, Inc.
Attending Psychiatrist
Sinai Hospital
Baltimore, Maryland
-------------------------------------------------------------------
Research for 'Rx: Cannabis' is needed
Regarding the essay by Drs. Gershan and Gangahar on decriminalization of Cannabis, I want to comment on issues surrounding prescription Cannabis.
It is clear that Cannabis can exacerbate psychosis, among other risks, but its potential benefits remain relatively unexplored. The authors correctly point out that, among indications for Cannabis, none are FDA-approved. Yet, off-label prescribing is pervasive and accepted in psychiatry, lack of FDA approval of indications for Cannabis is not an especially compelling argument against such prescribing.*
Lack of research and funding hampers efforts to conduct trials of the therapeutic value of Cannabis, as does its Schedule I status (ie, “no currently accepted medical use and a high potential for abuse” [language of the Controlled Substances Act]). There are reports of benefit in intractable epilepsy and posttraumatic stress disorder (PTSD) that merit further investigation; however, such research is hampered, I believe, by bureaucracy.
For example, an approved study at the University of Arizona of the use of Cannabis to treat PTSD has remained in regulatory limbo for longer than 4 years because of the immense hurdles involved in performing research on this substance—despite how pressing such research is, given the large number of veterans returning from active duty with this diagnosis and the paucity of treatment options.
Perhaps, there also is something “missing” in the debate about research into Cannabis.
Wesley Ryan, MD
PGY-5 Addiction Psychiatry Fellow
University of Washington
Seattle, Washington
*Editor's Note: An earlier version of this article stated, "Yet, off-labeling prescribing of Cannabis is pervasive, and I've found, accepted in psychiatry," which does not reflect the author's opinion or intended meaning. The sentence has been corrected to read, "Yet, because off-label prescribing is pervasive and accepted in psychiatry, lack of FDA approval of indications for Cannabis is not an especially compelling argument against such prescribing."
1. U.S. Department of Health and Human Services. Drug Abuse Warning Network, 2011: National estimates of drug-related emergency department visits. http://www.samhsa.gov/data/2k13/ DAWN2k11ED/DAWN2k11ED.htm. Published May 2013. Accessed on July 26, 2014.
2. Mehmedic Z, Chandra S, Slade D, et al. Potency trends of Δ9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. J Forensic Sci. 2010;55(5):1209-1217.
3. Swift W, Wong A, Li KM, et al. Analysis of cannabis seizures in NSW, Australia: cannabis potency and cannabinoid profile. PLos One. 2013;8(7):e70052. doi: 10.1371/journal.pone.0070052.
4. Morrison P. It’s the lack of balance in cannabis that does the harm. http://www.beckleyfoundation. org/2010/10/its-the-lack-of-balance-in-cannabis-that-does-the-harm. Published October 21, 2010. Accessed July 26, 2014.
1. U.S. Department of Health and Human Services. Drug Abuse Warning Network, 2011: National estimates of drug-related emergency department visits. http://www.samhsa.gov/data/2k13/ DAWN2k11ED/DAWN2k11ED.htm. Published May 2013. Accessed on July 26, 2014.
2. Mehmedic Z, Chandra S, Slade D, et al. Potency trends of Δ9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. J Forensic Sci. 2010;55(5):1209-1217.
3. Swift W, Wong A, Li KM, et al. Analysis of cannabis seizures in NSW, Australia: cannabis potency and cannabinoid profile. PLos One. 2013;8(7):e70052. doi: 10.1371/journal.pone.0070052.
4. Morrison P. It’s the lack of balance in cannabis that does the harm. http://www.beckleyfoundation. org/2010/10/its-the-lack-of-balance-in-cannabis-that-does-the-harm. Published October 21, 2010. Accessed July 26, 2014.
Suicide assessment and management self-test: How do you score?
As explained in the first part of this article in the October 2014 issue of Current Psychiatry, assessing and managing suicide risk are complex, difficult tasks without clear-cut, easy solutions. The case-based, multiple-choice self-test, with accompanying commentary, presented here is designed to enhance one’s ability to provide care for patients at risk for suicide. Part 2 of this article poses the remaining 7 of 15 questions, which are based on clinical experience and the referenced work of others.
Question 9
Mr. N, age 62, will be discharged from the psychiatric unit tomorrow. He was admitted after an overdose suicide attempt. Mr. N was depressed after the loss of his business and was “treating” his depression and anxiety with alcohol. He is successfully withdrawn from alcohol and responds to medication and supportive psychotherapy. During a family meeting with staff, Mr. N’s wife states that he keeps a gun by his bedside. Mr. N has improved and is eager to go home.
Before discharging Mr. N, the psychiatrist or staff should:
a) instruct Mr. N to remove the gun from his bedside
b) instruct his wife to remove the gun from the home
c) instruct the wife to look for >1 gun
d) instruct the wife, before Mr. N’s discharge, to call the staff once guns and
ammunition are safely removed according to the pre-arranged safety plan
e) instruct the wife to lock up the gun in a place that is not known to the patient
The best response option is D
Guns in the home are associated with a significant increase in suicide. All patients at risk for suicide must be asked if guns are available at home or easily accessible elsewhere, or if they intend to purchase a gun. Gun safety management requires a collaborative team approach including the clinician, patient, and person designated responsible for removing guns from the home.1 The responsible person should be required to call the clinician to confirm that the guns have been removed and secured according to the plan. The principles of gun safety management apply to outpatients, inpatients, and emergency patients, although implementation varies according to the clinical setting.
Asking the patient to remove guns from the home is too risky. Guns must be safely secured before the patient is discharged. Asking a spouse, other family member, or partner is necessary. The person asked must be willing to remove guns and ammunition according to a pre-arranged plan requiring a callback upon completion. A callback is essential because a family member in denial may do nothing to remove the guns or lock or “hide” them in the home where they will be found by a determined suicidal patient. Guns may be available outside the home, such as in the car, at the work place, or for purchase.
The essence of gun safety management is verification. Trust but verify or, better yet, verify, then trust.
Question 10
A recently admitted 56-year-old inpatient was discovered wrapping a towel around her neck. She denied suicidal intent; however, the treatment team viewed the incident as a suicide rehearsal. She was placed on one-to-one close observation.
Inpatient suicides frequently occur:
a) shortly after admission
b) during staff shift changes
c) at meal times
d) shortly after discharge
e) all of the above
The best response option is E
Inpatient suicides also occur at increased frequency when psychiatric residents finish their rotations and in understaffed psychiatric units.2 Undue delay in the evaluation of a newly admitted acute, high-risk patient might allow the patient to commit suicide.
Most patient suicides occur shortly after hospital discharge (a few hours, days, or weeks later). Appleby et al3 found that the highest number of suicides occurred during the first week after discharge. Meehan et al4 found that suicide occurred most frequently during the first 2 weeks post-discharge; the highest number of suicides occurred on the first day after discharge.
Question 11
Ms. G, a 43-year-old, single woman in acute suicide crisis, is admitted to the psychiatric unit of a general hospital. She is diagnosed with bipolar I disorder, most recent episode depressed, and borderline personality disorder. She has had multiple psychiatric hospitalizations, all precipitated by a suicide crisis. The average length of stay on the psychiatric unit is 6.3 days. After 7 days of intensive treatment, Ms. G is stabilized and suicide risk is reduced. The treatment team prepares for her discharge.
Ms. G’s suicide risk at discharge is most likely at:
a) indeterminate risk
b) low risk
c) moderate risk
d) chronic high risk
e) acute high risk
The best response option is D
The length of stay in many acute care psychiatric facilities is <7 days. The goal of hospitalization is to stabilize the patient and discharge to appropriate community mental health resources. Discharge planning begins at the time of admission.
Reducing Ms. G’s suicide risk to low or moderate is unlikely because of her diagnoses, frequent hospitalizations, and acute high risk for suicide on admission. After acute, high-risk suicidal patients are treated, many revert to chronic high risk for suicide.
Patients at chronic high risk for suicide often are treated as outpatients, except when an acute suicidal crisis requires hospitalization.5 At discharge from the hospital, the goal is to return the patient to outpatient treatment.
A discharge note identifies the acute suicide risk factors that have abated and the chronic (long-term) suicide risk factors that remain. The discharge note also addresses a patient’s chronic vulnerability to suicide. For example, a patient can become acutely suicidal again, depending on a number of factors, including the nature and cause of the psychiatric illness, adequacy of future treatment, adherence to treatment recommendations, and unforeseeable life vicissitudes.
Question 12
A 20-year-old college student is hospitalized after an overdose suicide attempt. Failing grades, panic attacks, and depression precipitated the suicide attempt. After 8 days of hospitalization, she is much improved and ready for discharge. She is assessed to be at low to moderate suicide risk. The treating psychiatrist and social worker convene a family meeting with both parents and an older brother. The family’s role after discharge is discussed.
All of the following options are helpful family roles except:
a) provide constant 24-hour family supervision
b) provide emotional support
c) observe and report symptoms and behaviors of concern
d) encourage adherence with treatment
e) provide helpful feedback about the patient’s thoughts and behavior
The best response option is A
The family’s role is important, but it is not a substitute for constant safety management provided by trained mental health professionals on an inpatient psychiatric unit.5 Early discharge of an inpatient by relying on family supervision can be precarious. Most inpatients are discharged at some level of suicide risk, given the short length of hospital stay. If an outpatient at risk of suicide requires constant 24-hour family supervision, then psychiatric hospitalization is indicated.
Patients who are intent on killing themselves can find ingenious ways to attempt or commit suicide. Asking family members to keep a constant watch often fails. Most family members will not follow the patient into the bathroom or be able to stay up all night to observe the patient. Moreover, family members find reasons to make exceptions to constant surveillance because of denial, fatigue, or the need to attend to other pressing matters.
Question 13
During the initial evaluation of a patient, it is the psychiatrist’s practice to routinely inquire about current and past suicide ideation. An affirmative answer prompts a systematic suicide risk assessment. In the absence of current risk, if exploration of the patient’s history reveals chronic suicide risk factors, the psychiatrist conducts a systematic suicide risk assessment.
The chronic risk factor that has the highest association with suicide is:
a) family history of mental illness or suicide
b) childhood abuse
c) history of a suicide attempt
d) impulsivity or aggression
e) prior psychiatric hospitalization
The best response option is C
A comprehensive suicide risk assessment may not be required at the initial outpatient evaluation in the absence of acute suicide risk factors. However, chronic suicide risk factors may be present.
The Standard Mortality Ratio (SMR) for prior suicide attempts by any method was 38.61.6 Suicide risk was highest in the 2 years after the first attempt. The SMR is a measure of the relative risk of suicide compared with the expected rate in the general population (SMR of 1).
Some chronic suicide risk factors are static: for example, a family history of psychiatric illness or earlier suicide attempt. Other chronic risk factors, usually a trait characteristic, can become acute: for example, impulsivity or aggression, or deliberate self-harm. The presence of chronic suicide risk factors should prompt a systematic suicide risk assessment. Evaluation of chronic suicide risk factors is an essential component of comprehensive assessment.5
Question 14
A psychiatrist is treating Dr. R, a 43-year-old physician, for anxiety and depression. The psychiatrist sees Dr. R twice a week for psychotherapy and medication management. A recent lawsuit filed against Dr. R has severely exacerbated her symptoms. She can sleep for only a few hours. Suicide ideation has emerged, frightening Dr. R and her family. The psychiatrist performs a systematic suicide risk assessment and determines that Dr. R is at acute high risk for suicide.
The psychiatrist recommends immediate hospitalization, but Dr. R adamantly refuses. The psychiatrist decides not to involuntarily hospitalize her because she does not meet the substantive criteria of the state involuntary commitment statute (eg, overt suicidal behaviors). The psychiatrist chooses to continue outpatient treatment.
Clinical interventions to reduce Dr. R’s suicide risk include:
a) see her more often
b) adjust medications
c) obtain a consult
d) refer her to an intensive outpatient program
e) all of the above
The best response option is E
To hospitalize or not to hospitalize— that is the conundrum that psychiatrists often face with high-risk suicidal patients. The decision is more complicated when the need for hospitalization is clear but the patient refuses. The decisions that the psychiatrist makes at this point are crucial for treatment and risk management.5
If the patient disagrees with the psychiatrist’s recommendation to hospitalize, refusal should be addressed as a treatment issue. When the need for hospitalization is acute, a prolonged inquiry is not possible. In addition, the therapeutic alliance may become strained. This clinical situation tries a clinician’s professional mettle.
Consultation and referral are options to consider if time and the patient’s condition allows. A psychiatric clinician should never worry alone; sleepless nights benefit neither the psychiatrist nor the patient.
As Dr. R’s case shows, a psychiatrist might decide not to hospitalize a patient who is assessed to be at moderate or high risk of suicide. Protective factors may allow continuing outpatient treatment. A good therapeutic alliance may be present if the psychiatrist has worked with the patient for some time. Family support also may be available.
The clinician must determine if the patient’s suicide risk can be managed by more frequent visits and treatment adjustments. Also, supportive family members can help by providing observational data. Protective factors can be overwhelmed by a severe mental illness. In contrast, a patient assessed as being at moderate risk of suicide might need to be hospitalized when protective factors are few or absent.
The psychiatrist may determine that a patient at high risk of suicide who refuses hospitalization does not meet criteria for involuntary hospitalization. For example, criteria might require that the patient must have made a suicide attempt within a specified period of time. States have provisions in their commitment statutes granting immunity from liability if the clinician uses reasonable clinical judgment and acts in good faith when involuntarily hospitalizing a patient.7
Question 15
Mr. U, a 39-year-old, married engineer, is ready to be discharged from the inpatient unit. He was admitted 7 days earlier for acute alcohol intoxication and suicidal threats. He has undergone successful detoxification. Mr. U has had 2 similar episodes within the past year.
The treatment team conducts a risk-benefit analysis for both discharge and continued hospitalization. A consultation also is obtained.
The discharge decision will be most influenced by:
a) presence of family support
b) compliance with follow-up care
c) availability of dual diagnosis programs
d) systematic suicide risk assessment
e) consultation
The best response option is D
All of the options in Question 15 concerning discharge planning of patients at risk for suicide are important. However, conducting a systematic suicide risk assessment to inform discharge planning is the most critical. Mr. U had 2 previous psychiatric admissions for alcohol abuse and suicidal ideation. He is a chronic suicide risk who becomes high risk when intoxicated.
Discharge planning begins at admission and is refined during the patient’s stay. Before a patient is discharged, a final post-discharge treatment and aftercare plan is necessary. After discharge, suicide risk increases as the intensity of treatment decreases.8
The patient’s willingness to cooperate with discharge and aftercare planning is critical in establishing contact with follow-up treaters. The treatment team should structure the follow-up plan to encourage compliance. For example, psychotic patients at risk of suicide who have a history of stopping medications after discharge can be given a long-acting IM antipsychotic that will last until they reach aftercare. Patients with comorbid drug and alcohol abuse disorders are referred to agencies equipped to manage dual-diagnosis patients.
Psychiatrists’ ability to ensure follow-up treatment is limited, a fact that must be acknowledged by the psychiatric and legal communities. Beyond patient stabilization, a clinician’s options to bring about positive changes can be limited or nonexistent. Also, the patient’s failure to adhere to post-discharge plans and treatment often leads to rehospitalization, hopelessness, and greater suicide risk.
Psychiatric patients at moderate or moderate-to-high risk for suicide increasingly are treated in outpatient settings. It is the responsibility of the clinician and the treatment team to competently hand off the patient to appropriate outpatient aftercare. With the patient’s permission, the psychiatrist or social worker should call the follow-up agency or therapist before discharge to provide information about the patient’s diagnosis, treatment, and hospital course.
Last, follow-up appointments should be made as close to the time of discharge as possible. Suicide often occurs on the first day after discharge.3
Bottom Line
Fully commit time and effort to the ongoing assessment, treatment, and management of patients at suicide risk. Suicide risk assessment is a process, not an event. Conduct a suicide risk assessment at important clinical junctures (eg, initial evaluation, discharge, changing observation levels). Contemporaneously, document suicide risk assessments. This self-assessment helps clinicians gauge their strengths and identify skills that need further development.
Disclosure
Dr. Simon reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Simon is the co-editor of The American Psychiatry Publishing textbook of Suicide Assessment and Management, 2nd edition, from which this article is adapted, by permission of the publisher, American Psychiatry Publishing, Inc. ©2012.
1. Simon RI. Gun safety management with patients at risk for suicide. Suicide Life Threat Behav. 2007;37(5):518-526.
2. Qin P, Nordenoft M. Suicide risk in relation to psychiatric hospitalization: evidence based on longitudinal registers. Arch Gen Psychiatry. 2005;62(4):427-432.
3. Appleby L, Shaw J, Amos T, et al. Suicide within 12 months of contact with mental health services: national clinical survey. BMJ. 1999;318(7193):1235-1239.
4. Meehan J, Kapur N, Hunt IM, et al. Suicide in mental health in-patients within 3 months of discharge. National clinical survey. Br J Psychiatry. 2006;188:129-134.
5. Simon RI. Preventing patient suicide: clinical assessment and management. Arlington, VA: American Psychiatric Publishing, Inc.; 2011.
6. Harris CE, Barraclough B. Suicide as an outcome for mental disorders. A meta-analysis. Br J Psychiatry. 1997;170:205-228.
7. Simon RI, Shuman DW. Clinical manual of psychiatry and law. Arlington, VA: American Psychiatric Publishing, Inc.; 2007.
8. Appleby L, Dennehy JA, Thomas CS, et al. Aftercare and clinical characteristics of people with mental illness who commit suicide: a case-control study. Lancet. 1999;353(9162):1397-1400.
As explained in the first part of this article in the October 2014 issue of Current Psychiatry, assessing and managing suicide risk are complex, difficult tasks without clear-cut, easy solutions. The case-based, multiple-choice self-test, with accompanying commentary, presented here is designed to enhance one’s ability to provide care for patients at risk for suicide. Part 2 of this article poses the remaining 7 of 15 questions, which are based on clinical experience and the referenced work of others.
Question 9
Mr. N, age 62, will be discharged from the psychiatric unit tomorrow. He was admitted after an overdose suicide attempt. Mr. N was depressed after the loss of his business and was “treating” his depression and anxiety with alcohol. He is successfully withdrawn from alcohol and responds to medication and supportive psychotherapy. During a family meeting with staff, Mr. N’s wife states that he keeps a gun by his bedside. Mr. N has improved and is eager to go home.
Before discharging Mr. N, the psychiatrist or staff should:
a) instruct Mr. N to remove the gun from his bedside
b) instruct his wife to remove the gun from the home
c) instruct the wife to look for >1 gun
d) instruct the wife, before Mr. N’s discharge, to call the staff once guns and
ammunition are safely removed according to the pre-arranged safety plan
e) instruct the wife to lock up the gun in a place that is not known to the patient
The best response option is D
Guns in the home are associated with a significant increase in suicide. All patients at risk for suicide must be asked if guns are available at home or easily accessible elsewhere, or if they intend to purchase a gun. Gun safety management requires a collaborative team approach including the clinician, patient, and person designated responsible for removing guns from the home.1 The responsible person should be required to call the clinician to confirm that the guns have been removed and secured according to the plan. The principles of gun safety management apply to outpatients, inpatients, and emergency patients, although implementation varies according to the clinical setting.
Asking the patient to remove guns from the home is too risky. Guns must be safely secured before the patient is discharged. Asking a spouse, other family member, or partner is necessary. The person asked must be willing to remove guns and ammunition according to a pre-arranged plan requiring a callback upon completion. A callback is essential because a family member in denial may do nothing to remove the guns or lock or “hide” them in the home where they will be found by a determined suicidal patient. Guns may be available outside the home, such as in the car, at the work place, or for purchase.
The essence of gun safety management is verification. Trust but verify or, better yet, verify, then trust.
Question 10
A recently admitted 56-year-old inpatient was discovered wrapping a towel around her neck. She denied suicidal intent; however, the treatment team viewed the incident as a suicide rehearsal. She was placed on one-to-one close observation.
Inpatient suicides frequently occur:
a) shortly after admission
b) during staff shift changes
c) at meal times
d) shortly after discharge
e) all of the above
The best response option is E
Inpatient suicides also occur at increased frequency when psychiatric residents finish their rotations and in understaffed psychiatric units.2 Undue delay in the evaluation of a newly admitted acute, high-risk patient might allow the patient to commit suicide.
Most patient suicides occur shortly after hospital discharge (a few hours, days, or weeks later). Appleby et al3 found that the highest number of suicides occurred during the first week after discharge. Meehan et al4 found that suicide occurred most frequently during the first 2 weeks post-discharge; the highest number of suicides occurred on the first day after discharge.
Question 11
Ms. G, a 43-year-old, single woman in acute suicide crisis, is admitted to the psychiatric unit of a general hospital. She is diagnosed with bipolar I disorder, most recent episode depressed, and borderline personality disorder. She has had multiple psychiatric hospitalizations, all precipitated by a suicide crisis. The average length of stay on the psychiatric unit is 6.3 days. After 7 days of intensive treatment, Ms. G is stabilized and suicide risk is reduced. The treatment team prepares for her discharge.
Ms. G’s suicide risk at discharge is most likely at:
a) indeterminate risk
b) low risk
c) moderate risk
d) chronic high risk
e) acute high risk
The best response option is D
The length of stay in many acute care psychiatric facilities is <7 days. The goal of hospitalization is to stabilize the patient and discharge to appropriate community mental health resources. Discharge planning begins at the time of admission.
Reducing Ms. G’s suicide risk to low or moderate is unlikely because of her diagnoses, frequent hospitalizations, and acute high risk for suicide on admission. After acute, high-risk suicidal patients are treated, many revert to chronic high risk for suicide.
Patients at chronic high risk for suicide often are treated as outpatients, except when an acute suicidal crisis requires hospitalization.5 At discharge from the hospital, the goal is to return the patient to outpatient treatment.
A discharge note identifies the acute suicide risk factors that have abated and the chronic (long-term) suicide risk factors that remain. The discharge note also addresses a patient’s chronic vulnerability to suicide. For example, a patient can become acutely suicidal again, depending on a number of factors, including the nature and cause of the psychiatric illness, adequacy of future treatment, adherence to treatment recommendations, and unforeseeable life vicissitudes.
Question 12
A 20-year-old college student is hospitalized after an overdose suicide attempt. Failing grades, panic attacks, and depression precipitated the suicide attempt. After 8 days of hospitalization, she is much improved and ready for discharge. She is assessed to be at low to moderate suicide risk. The treating psychiatrist and social worker convene a family meeting with both parents and an older brother. The family’s role after discharge is discussed.
All of the following options are helpful family roles except:
a) provide constant 24-hour family supervision
b) provide emotional support
c) observe and report symptoms and behaviors of concern
d) encourage adherence with treatment
e) provide helpful feedback about the patient’s thoughts and behavior
The best response option is A
The family’s role is important, but it is not a substitute for constant safety management provided by trained mental health professionals on an inpatient psychiatric unit.5 Early discharge of an inpatient by relying on family supervision can be precarious. Most inpatients are discharged at some level of suicide risk, given the short length of hospital stay. If an outpatient at risk of suicide requires constant 24-hour family supervision, then psychiatric hospitalization is indicated.
Patients who are intent on killing themselves can find ingenious ways to attempt or commit suicide. Asking family members to keep a constant watch often fails. Most family members will not follow the patient into the bathroom or be able to stay up all night to observe the patient. Moreover, family members find reasons to make exceptions to constant surveillance because of denial, fatigue, or the need to attend to other pressing matters.
Question 13
During the initial evaluation of a patient, it is the psychiatrist’s practice to routinely inquire about current and past suicide ideation. An affirmative answer prompts a systematic suicide risk assessment. In the absence of current risk, if exploration of the patient’s history reveals chronic suicide risk factors, the psychiatrist conducts a systematic suicide risk assessment.
The chronic risk factor that has the highest association with suicide is:
a) family history of mental illness or suicide
b) childhood abuse
c) history of a suicide attempt
d) impulsivity or aggression
e) prior psychiatric hospitalization
The best response option is C
A comprehensive suicide risk assessment may not be required at the initial outpatient evaluation in the absence of acute suicide risk factors. However, chronic suicide risk factors may be present.
The Standard Mortality Ratio (SMR) for prior suicide attempts by any method was 38.61.6 Suicide risk was highest in the 2 years after the first attempt. The SMR is a measure of the relative risk of suicide compared with the expected rate in the general population (SMR of 1).
Some chronic suicide risk factors are static: for example, a family history of psychiatric illness or earlier suicide attempt. Other chronic risk factors, usually a trait characteristic, can become acute: for example, impulsivity or aggression, or deliberate self-harm. The presence of chronic suicide risk factors should prompt a systematic suicide risk assessment. Evaluation of chronic suicide risk factors is an essential component of comprehensive assessment.5
Question 14
A psychiatrist is treating Dr. R, a 43-year-old physician, for anxiety and depression. The psychiatrist sees Dr. R twice a week for psychotherapy and medication management. A recent lawsuit filed against Dr. R has severely exacerbated her symptoms. She can sleep for only a few hours. Suicide ideation has emerged, frightening Dr. R and her family. The psychiatrist performs a systematic suicide risk assessment and determines that Dr. R is at acute high risk for suicide.
The psychiatrist recommends immediate hospitalization, but Dr. R adamantly refuses. The psychiatrist decides not to involuntarily hospitalize her because she does not meet the substantive criteria of the state involuntary commitment statute (eg, overt suicidal behaviors). The psychiatrist chooses to continue outpatient treatment.
Clinical interventions to reduce Dr. R’s suicide risk include:
a) see her more often
b) adjust medications
c) obtain a consult
d) refer her to an intensive outpatient program
e) all of the above
The best response option is E
To hospitalize or not to hospitalize— that is the conundrum that psychiatrists often face with high-risk suicidal patients. The decision is more complicated when the need for hospitalization is clear but the patient refuses. The decisions that the psychiatrist makes at this point are crucial for treatment and risk management.5
If the patient disagrees with the psychiatrist’s recommendation to hospitalize, refusal should be addressed as a treatment issue. When the need for hospitalization is acute, a prolonged inquiry is not possible. In addition, the therapeutic alliance may become strained. This clinical situation tries a clinician’s professional mettle.
Consultation and referral are options to consider if time and the patient’s condition allows. A psychiatric clinician should never worry alone; sleepless nights benefit neither the psychiatrist nor the patient.
As Dr. R’s case shows, a psychiatrist might decide not to hospitalize a patient who is assessed to be at moderate or high risk of suicide. Protective factors may allow continuing outpatient treatment. A good therapeutic alliance may be present if the psychiatrist has worked with the patient for some time. Family support also may be available.
The clinician must determine if the patient’s suicide risk can be managed by more frequent visits and treatment adjustments. Also, supportive family members can help by providing observational data. Protective factors can be overwhelmed by a severe mental illness. In contrast, a patient assessed as being at moderate risk of suicide might need to be hospitalized when protective factors are few or absent.
The psychiatrist may determine that a patient at high risk of suicide who refuses hospitalization does not meet criteria for involuntary hospitalization. For example, criteria might require that the patient must have made a suicide attempt within a specified period of time. States have provisions in their commitment statutes granting immunity from liability if the clinician uses reasonable clinical judgment and acts in good faith when involuntarily hospitalizing a patient.7
Question 15
Mr. U, a 39-year-old, married engineer, is ready to be discharged from the inpatient unit. He was admitted 7 days earlier for acute alcohol intoxication and suicidal threats. He has undergone successful detoxification. Mr. U has had 2 similar episodes within the past year.
The treatment team conducts a risk-benefit analysis for both discharge and continued hospitalization. A consultation also is obtained.
The discharge decision will be most influenced by:
a) presence of family support
b) compliance with follow-up care
c) availability of dual diagnosis programs
d) systematic suicide risk assessment
e) consultation
The best response option is D
All of the options in Question 15 concerning discharge planning of patients at risk for suicide are important. However, conducting a systematic suicide risk assessment to inform discharge planning is the most critical. Mr. U had 2 previous psychiatric admissions for alcohol abuse and suicidal ideation. He is a chronic suicide risk who becomes high risk when intoxicated.
Discharge planning begins at admission and is refined during the patient’s stay. Before a patient is discharged, a final post-discharge treatment and aftercare plan is necessary. After discharge, suicide risk increases as the intensity of treatment decreases.8
The patient’s willingness to cooperate with discharge and aftercare planning is critical in establishing contact with follow-up treaters. The treatment team should structure the follow-up plan to encourage compliance. For example, psychotic patients at risk of suicide who have a history of stopping medications after discharge can be given a long-acting IM antipsychotic that will last until they reach aftercare. Patients with comorbid drug and alcohol abuse disorders are referred to agencies equipped to manage dual-diagnosis patients.
Psychiatrists’ ability to ensure follow-up treatment is limited, a fact that must be acknowledged by the psychiatric and legal communities. Beyond patient stabilization, a clinician’s options to bring about positive changes can be limited or nonexistent. Also, the patient’s failure to adhere to post-discharge plans and treatment often leads to rehospitalization, hopelessness, and greater suicide risk.
Psychiatric patients at moderate or moderate-to-high risk for suicide increasingly are treated in outpatient settings. It is the responsibility of the clinician and the treatment team to competently hand off the patient to appropriate outpatient aftercare. With the patient’s permission, the psychiatrist or social worker should call the follow-up agency or therapist before discharge to provide information about the patient’s diagnosis, treatment, and hospital course.
Last, follow-up appointments should be made as close to the time of discharge as possible. Suicide often occurs on the first day after discharge.3
Bottom Line
Fully commit time and effort to the ongoing assessment, treatment, and management of patients at suicide risk. Suicide risk assessment is a process, not an event. Conduct a suicide risk assessment at important clinical junctures (eg, initial evaluation, discharge, changing observation levels). Contemporaneously, document suicide risk assessments. This self-assessment helps clinicians gauge their strengths and identify skills that need further development.
Disclosure
Dr. Simon reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Simon is the co-editor of The American Psychiatry Publishing textbook of Suicide Assessment and Management, 2nd edition, from which this article is adapted, by permission of the publisher, American Psychiatry Publishing, Inc. ©2012.
As explained in the first part of this article in the October 2014 issue of Current Psychiatry, assessing and managing suicide risk are complex, difficult tasks without clear-cut, easy solutions. The case-based, multiple-choice self-test, with accompanying commentary, presented here is designed to enhance one’s ability to provide care for patients at risk for suicide. Part 2 of this article poses the remaining 7 of 15 questions, which are based on clinical experience and the referenced work of others.
Question 9
Mr. N, age 62, will be discharged from the psychiatric unit tomorrow. He was admitted after an overdose suicide attempt. Mr. N was depressed after the loss of his business and was “treating” his depression and anxiety with alcohol. He is successfully withdrawn from alcohol and responds to medication and supportive psychotherapy. During a family meeting with staff, Mr. N’s wife states that he keeps a gun by his bedside. Mr. N has improved and is eager to go home.
Before discharging Mr. N, the psychiatrist or staff should:
a) instruct Mr. N to remove the gun from his bedside
b) instruct his wife to remove the gun from the home
c) instruct the wife to look for >1 gun
d) instruct the wife, before Mr. N’s discharge, to call the staff once guns and
ammunition are safely removed according to the pre-arranged safety plan
e) instruct the wife to lock up the gun in a place that is not known to the patient
The best response option is D
Guns in the home are associated with a significant increase in suicide. All patients at risk for suicide must be asked if guns are available at home or easily accessible elsewhere, or if they intend to purchase a gun. Gun safety management requires a collaborative team approach including the clinician, patient, and person designated responsible for removing guns from the home.1 The responsible person should be required to call the clinician to confirm that the guns have been removed and secured according to the plan. The principles of gun safety management apply to outpatients, inpatients, and emergency patients, although implementation varies according to the clinical setting.
Asking the patient to remove guns from the home is too risky. Guns must be safely secured before the patient is discharged. Asking a spouse, other family member, or partner is necessary. The person asked must be willing to remove guns and ammunition according to a pre-arranged plan requiring a callback upon completion. A callback is essential because a family member in denial may do nothing to remove the guns or lock or “hide” them in the home where they will be found by a determined suicidal patient. Guns may be available outside the home, such as in the car, at the work place, or for purchase.
The essence of gun safety management is verification. Trust but verify or, better yet, verify, then trust.
Question 10
A recently admitted 56-year-old inpatient was discovered wrapping a towel around her neck. She denied suicidal intent; however, the treatment team viewed the incident as a suicide rehearsal. She was placed on one-to-one close observation.
Inpatient suicides frequently occur:
a) shortly after admission
b) during staff shift changes
c) at meal times
d) shortly after discharge
e) all of the above
The best response option is E
Inpatient suicides also occur at increased frequency when psychiatric residents finish their rotations and in understaffed psychiatric units.2 Undue delay in the evaluation of a newly admitted acute, high-risk patient might allow the patient to commit suicide.
Most patient suicides occur shortly after hospital discharge (a few hours, days, or weeks later). Appleby et al3 found that the highest number of suicides occurred during the first week after discharge. Meehan et al4 found that suicide occurred most frequently during the first 2 weeks post-discharge; the highest number of suicides occurred on the first day after discharge.
Question 11
Ms. G, a 43-year-old, single woman in acute suicide crisis, is admitted to the psychiatric unit of a general hospital. She is diagnosed with bipolar I disorder, most recent episode depressed, and borderline personality disorder. She has had multiple psychiatric hospitalizations, all precipitated by a suicide crisis. The average length of stay on the psychiatric unit is 6.3 days. After 7 days of intensive treatment, Ms. G is stabilized and suicide risk is reduced. The treatment team prepares for her discharge.
Ms. G’s suicide risk at discharge is most likely at:
a) indeterminate risk
b) low risk
c) moderate risk
d) chronic high risk
e) acute high risk
The best response option is D
The length of stay in many acute care psychiatric facilities is <7 days. The goal of hospitalization is to stabilize the patient and discharge to appropriate community mental health resources. Discharge planning begins at the time of admission.
Reducing Ms. G’s suicide risk to low or moderate is unlikely because of her diagnoses, frequent hospitalizations, and acute high risk for suicide on admission. After acute, high-risk suicidal patients are treated, many revert to chronic high risk for suicide.
Patients at chronic high risk for suicide often are treated as outpatients, except when an acute suicidal crisis requires hospitalization.5 At discharge from the hospital, the goal is to return the patient to outpatient treatment.
A discharge note identifies the acute suicide risk factors that have abated and the chronic (long-term) suicide risk factors that remain. The discharge note also addresses a patient’s chronic vulnerability to suicide. For example, a patient can become acutely suicidal again, depending on a number of factors, including the nature and cause of the psychiatric illness, adequacy of future treatment, adherence to treatment recommendations, and unforeseeable life vicissitudes.
Question 12
A 20-year-old college student is hospitalized after an overdose suicide attempt. Failing grades, panic attacks, and depression precipitated the suicide attempt. After 8 days of hospitalization, she is much improved and ready for discharge. She is assessed to be at low to moderate suicide risk. The treating psychiatrist and social worker convene a family meeting with both parents and an older brother. The family’s role after discharge is discussed.
All of the following options are helpful family roles except:
a) provide constant 24-hour family supervision
b) provide emotional support
c) observe and report symptoms and behaviors of concern
d) encourage adherence with treatment
e) provide helpful feedback about the patient’s thoughts and behavior
The best response option is A
The family’s role is important, but it is not a substitute for constant safety management provided by trained mental health professionals on an inpatient psychiatric unit.5 Early discharge of an inpatient by relying on family supervision can be precarious. Most inpatients are discharged at some level of suicide risk, given the short length of hospital stay. If an outpatient at risk of suicide requires constant 24-hour family supervision, then psychiatric hospitalization is indicated.
Patients who are intent on killing themselves can find ingenious ways to attempt or commit suicide. Asking family members to keep a constant watch often fails. Most family members will not follow the patient into the bathroom or be able to stay up all night to observe the patient. Moreover, family members find reasons to make exceptions to constant surveillance because of denial, fatigue, or the need to attend to other pressing matters.
Question 13
During the initial evaluation of a patient, it is the psychiatrist’s practice to routinely inquire about current and past suicide ideation. An affirmative answer prompts a systematic suicide risk assessment. In the absence of current risk, if exploration of the patient’s history reveals chronic suicide risk factors, the psychiatrist conducts a systematic suicide risk assessment.
The chronic risk factor that has the highest association with suicide is:
a) family history of mental illness or suicide
b) childhood abuse
c) history of a suicide attempt
d) impulsivity or aggression
e) prior psychiatric hospitalization
The best response option is C
A comprehensive suicide risk assessment may not be required at the initial outpatient evaluation in the absence of acute suicide risk factors. However, chronic suicide risk factors may be present.
The Standard Mortality Ratio (SMR) for prior suicide attempts by any method was 38.61.6 Suicide risk was highest in the 2 years after the first attempt. The SMR is a measure of the relative risk of suicide compared with the expected rate in the general population (SMR of 1).
Some chronic suicide risk factors are static: for example, a family history of psychiatric illness or earlier suicide attempt. Other chronic risk factors, usually a trait characteristic, can become acute: for example, impulsivity or aggression, or deliberate self-harm. The presence of chronic suicide risk factors should prompt a systematic suicide risk assessment. Evaluation of chronic suicide risk factors is an essential component of comprehensive assessment.5
Question 14
A psychiatrist is treating Dr. R, a 43-year-old physician, for anxiety and depression. The psychiatrist sees Dr. R twice a week for psychotherapy and medication management. A recent lawsuit filed against Dr. R has severely exacerbated her symptoms. She can sleep for only a few hours. Suicide ideation has emerged, frightening Dr. R and her family. The psychiatrist performs a systematic suicide risk assessment and determines that Dr. R is at acute high risk for suicide.
The psychiatrist recommends immediate hospitalization, but Dr. R adamantly refuses. The psychiatrist decides not to involuntarily hospitalize her because she does not meet the substantive criteria of the state involuntary commitment statute (eg, overt suicidal behaviors). The psychiatrist chooses to continue outpatient treatment.
Clinical interventions to reduce Dr. R’s suicide risk include:
a) see her more often
b) adjust medications
c) obtain a consult
d) refer her to an intensive outpatient program
e) all of the above
The best response option is E
To hospitalize or not to hospitalize— that is the conundrum that psychiatrists often face with high-risk suicidal patients. The decision is more complicated when the need for hospitalization is clear but the patient refuses. The decisions that the psychiatrist makes at this point are crucial for treatment and risk management.5
If the patient disagrees with the psychiatrist’s recommendation to hospitalize, refusal should be addressed as a treatment issue. When the need for hospitalization is acute, a prolonged inquiry is not possible. In addition, the therapeutic alliance may become strained. This clinical situation tries a clinician’s professional mettle.
Consultation and referral are options to consider if time and the patient’s condition allows. A psychiatric clinician should never worry alone; sleepless nights benefit neither the psychiatrist nor the patient.
As Dr. R’s case shows, a psychiatrist might decide not to hospitalize a patient who is assessed to be at moderate or high risk of suicide. Protective factors may allow continuing outpatient treatment. A good therapeutic alliance may be present if the psychiatrist has worked with the patient for some time. Family support also may be available.
The clinician must determine if the patient’s suicide risk can be managed by more frequent visits and treatment adjustments. Also, supportive family members can help by providing observational data. Protective factors can be overwhelmed by a severe mental illness. In contrast, a patient assessed as being at moderate risk of suicide might need to be hospitalized when protective factors are few or absent.
The psychiatrist may determine that a patient at high risk of suicide who refuses hospitalization does not meet criteria for involuntary hospitalization. For example, criteria might require that the patient must have made a suicide attempt within a specified period of time. States have provisions in their commitment statutes granting immunity from liability if the clinician uses reasonable clinical judgment and acts in good faith when involuntarily hospitalizing a patient.7
Question 15
Mr. U, a 39-year-old, married engineer, is ready to be discharged from the inpatient unit. He was admitted 7 days earlier for acute alcohol intoxication and suicidal threats. He has undergone successful detoxification. Mr. U has had 2 similar episodes within the past year.
The treatment team conducts a risk-benefit analysis for both discharge and continued hospitalization. A consultation also is obtained.
The discharge decision will be most influenced by:
a) presence of family support
b) compliance with follow-up care
c) availability of dual diagnosis programs
d) systematic suicide risk assessment
e) consultation
The best response option is D
All of the options in Question 15 concerning discharge planning of patients at risk for suicide are important. However, conducting a systematic suicide risk assessment to inform discharge planning is the most critical. Mr. U had 2 previous psychiatric admissions for alcohol abuse and suicidal ideation. He is a chronic suicide risk who becomes high risk when intoxicated.
Discharge planning begins at admission and is refined during the patient’s stay. Before a patient is discharged, a final post-discharge treatment and aftercare plan is necessary. After discharge, suicide risk increases as the intensity of treatment decreases.8
The patient’s willingness to cooperate with discharge and aftercare planning is critical in establishing contact with follow-up treaters. The treatment team should structure the follow-up plan to encourage compliance. For example, psychotic patients at risk of suicide who have a history of stopping medications after discharge can be given a long-acting IM antipsychotic that will last until they reach aftercare. Patients with comorbid drug and alcohol abuse disorders are referred to agencies equipped to manage dual-diagnosis patients.
Psychiatrists’ ability to ensure follow-up treatment is limited, a fact that must be acknowledged by the psychiatric and legal communities. Beyond patient stabilization, a clinician’s options to bring about positive changes can be limited or nonexistent. Also, the patient’s failure to adhere to post-discharge plans and treatment often leads to rehospitalization, hopelessness, and greater suicide risk.
Psychiatric patients at moderate or moderate-to-high risk for suicide increasingly are treated in outpatient settings. It is the responsibility of the clinician and the treatment team to competently hand off the patient to appropriate outpatient aftercare. With the patient’s permission, the psychiatrist or social worker should call the follow-up agency or therapist before discharge to provide information about the patient’s diagnosis, treatment, and hospital course.
Last, follow-up appointments should be made as close to the time of discharge as possible. Suicide often occurs on the first day after discharge.3
Bottom Line
Fully commit time and effort to the ongoing assessment, treatment, and management of patients at suicide risk. Suicide risk assessment is a process, not an event. Conduct a suicide risk assessment at important clinical junctures (eg, initial evaluation, discharge, changing observation levels). Contemporaneously, document suicide risk assessments. This self-assessment helps clinicians gauge their strengths and identify skills that need further development.
Disclosure
Dr. Simon reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Simon is the co-editor of The American Psychiatry Publishing textbook of Suicide Assessment and Management, 2nd edition, from which this article is adapted, by permission of the publisher, American Psychiatry Publishing, Inc. ©2012.
1. Simon RI. Gun safety management with patients at risk for suicide. Suicide Life Threat Behav. 2007;37(5):518-526.
2. Qin P, Nordenoft M. Suicide risk in relation to psychiatric hospitalization: evidence based on longitudinal registers. Arch Gen Psychiatry. 2005;62(4):427-432.
3. Appleby L, Shaw J, Amos T, et al. Suicide within 12 months of contact with mental health services: national clinical survey. BMJ. 1999;318(7193):1235-1239.
4. Meehan J, Kapur N, Hunt IM, et al. Suicide in mental health in-patients within 3 months of discharge. National clinical survey. Br J Psychiatry. 2006;188:129-134.
5. Simon RI. Preventing patient suicide: clinical assessment and management. Arlington, VA: American Psychiatric Publishing, Inc.; 2011.
6. Harris CE, Barraclough B. Suicide as an outcome for mental disorders. A meta-analysis. Br J Psychiatry. 1997;170:205-228.
7. Simon RI, Shuman DW. Clinical manual of psychiatry and law. Arlington, VA: American Psychiatric Publishing, Inc.; 2007.
8. Appleby L, Dennehy JA, Thomas CS, et al. Aftercare and clinical characteristics of people with mental illness who commit suicide: a case-control study. Lancet. 1999;353(9162):1397-1400.
1. Simon RI. Gun safety management with patients at risk for suicide. Suicide Life Threat Behav. 2007;37(5):518-526.
2. Qin P, Nordenoft M. Suicide risk in relation to psychiatric hospitalization: evidence based on longitudinal registers. Arch Gen Psychiatry. 2005;62(4):427-432.
3. Appleby L, Shaw J, Amos T, et al. Suicide within 12 months of contact with mental health services: national clinical survey. BMJ. 1999;318(7193):1235-1239.
4. Meehan J, Kapur N, Hunt IM, et al. Suicide in mental health in-patients within 3 months of discharge. National clinical survey. Br J Psychiatry. 2006;188:129-134.
5. Simon RI. Preventing patient suicide: clinical assessment and management. Arlington, VA: American Psychiatric Publishing, Inc.; 2011.
6. Harris CE, Barraclough B. Suicide as an outcome for mental disorders. A meta-analysis. Br J Psychiatry. 1997;170:205-228.
7. Simon RI, Shuman DW. Clinical manual of psychiatry and law. Arlington, VA: American Psychiatric Publishing, Inc.; 2007.
8. Appleby L, Dennehy JA, Thomas CS, et al. Aftercare and clinical characteristics of people with mental illness who commit suicide: a case-control study. Lancet. 1999;353(9162):1397-1400.
The sins and peccadillos of psychiatric practice
But as professionals, and by virtue of our rigorous training, we continually reflect on our thoughts and feelings when we care for our patients, and we examine the effect of our behavior and communication patterns on them. Such self-reflection is especially important when it comes to countertransference while treating a person who has been made vulnerable by emotional turmoil and who develops strong transference feelings toward the treating psychiatrist.
Personal integrity is paramount in psychiatric practice; it’s an indispensable ingredient when dealing with the intimate thoughts, feelings, and impulses of people who are seeking psychiatric help. In addition, the wisdom to recognize one’s limitations as a provider of care is an important attributes of seasoned practitioners. Patients might perceive us as demigods, but we know better than to be carried away with hubris and pretend that we are.
Exercising sound judgment isn’t easy
This is especially true when dealing with the varying degrees of ambiguity that shroud complex psychiatric conditions. The overriding principle for good clinical judgment in any medical practice is the patient’s welfare, and that must dominate the moral, ethical, medical, and scientific decision-making of all psychiatrists. Those of us in charge of training medical students and residents emphasize this principle every day at the bedside and in the clinic. Upholding those principles, side by side with the knowledge and skills of psychiatric practice, are the hallmarks of good medical training.
But missteps occur. Peccadillos, infractions, and even transgressions happen—accidentally by competent, ethical psychiatrists; deliberately, sometimes, by a few unethical scoundrels. The sins of psychiatric practice come in a range of gravity and consequences. Here are some I’ve observed among colleagues over the years:
Becoming sexually intimate with one’s patient. Violating the sacred boundary of the doctor-patient relationship is unforgivable; it’s a sin that scars the patient and can destroy the psychiatrist’s reputation and career. We must respect and uphold that boundary—not only during active treatment but even after care is terminated.
Divulging clinical details to other without the patient’s consent. Breach of trust is not only an ethical misstep; it is a violation of the Health Insurance Portability and Accountability Act of 1996 (HIPAA), with substantial penalties attached.
Treating the mind while ignoring the body and brain. We are physicians first, psychiatrists second. We must fully assess patients who present with psychiatric signs and symptoms to rule out a general medical condition that could be generating behavioral symptoms. Such co-occurring medical conditions might involve various organ systems, such as endocrinopathies, or might emanate from brain lesions, whether traumatic, degenerative, demyelinating, infectious, or neoplastic. Without careful medical evaluation, the wrong diagnosis might be made and inappropriate, even harmful, treatment provided while necessary care is delayed.
Treatment plans can sometimes overlook potential harmful effects of some medications on physical health— whether metabolic, cardiovascular, neurologic, gastrointestinal, hormonal, hematologic, or dermatologic. An optimal treatment plan embarks on healing the mind without ignoring or harming the body.
Failing to obtain additional information from sources who know the patient or who can provide old records. Such information is vital in psychiatry, because the patient’s account often is incomplete, even distorted, because of cognitive deficits or psychopathologic factors. Additional information can be corroborative or contradictory, and is sometimes critical—significantly influencing the diagnosis or the treatment plan, or both.
Allowing personal beliefs to influence care. This transgression includes inserting one’s views about religion, politics, sexual orientation, ethnic origin, and socioeconomic class into medical care. The same unimpeachable, high caliber of care must be provided to every patient, and must not differ in any way from the care that we would recommend to our own family members.
Reducing psychiatry to prescribing a pill. However unacceptable and deficient this reductionist degradation of psychiatric management is, it is sometimes imposed by organizations in which the caseload is huge and the number of providers insufficient. We must resist the temptation to compromise, and must strive to address not only the biological aspects of illness but psychological and social dimensions as well. Patients will not have an optimal if we don’t.
Practicing with an outdated knowledge base—one acquired during residency years, often years or even decades, earlier. There is no medical or ethical justification for using 1985, or even 2005, standards of psychiatric care in 2015.
Psychiatry is rapidly evolving, with many ongoing changes and advances. Updating one’s practice pattern through lifelong learning is an absolute must for psychiatrists (and for all health care professionals). Utilizing the latest, evidence-based data to guide diagnosis and treatment is an indispensable component of good psychiatric care.
Neglecting to consider treatment options. Consider just a few scenarios: The recurrently relapsing patient with psychosis who is not switched to a long-acting injectable formulation; the persistently psychotic patient who does not receive a trial of clozapine; the treatment-resistant depression patient who is not referred for electroconvulsive therapy or transcranial magnetic stimulation; and the patient receiving an atypical antipsychotic who is monitored inconsistently for metabolic dysregulation.
Treating patients but not vigorously advocating for them—thus allowing a broken, convoluted mental health system to delay or prevent access to care; incarcerate relapsed patients instead of hospitalizing them; permit insurance companies to discriminate against coverage of mental illness; and tie the hands of psychiatrists who want to select medication they judge best for their patients.
None of us is 'without sin'
We all aspire to help our patients in the best way we can, and to avoid errors. However, even a seasoned psychiatrist can stumble unwittingly, and that is understandable and forgivable. It is willful, recurring neglect of the patient’s welfare that can be deleterious and that, in my opinion, qualifies as a cardinal sin. Fortunately, such neglect is a low-frequency event in psychiatric practice, but even a single occurrence is one too many.
But as professionals, and by virtue of our rigorous training, we continually reflect on our thoughts and feelings when we care for our patients, and we examine the effect of our behavior and communication patterns on them. Such self-reflection is especially important when it comes to countertransference while treating a person who has been made vulnerable by emotional turmoil and who develops strong transference feelings toward the treating psychiatrist.
Personal integrity is paramount in psychiatric practice; it’s an indispensable ingredient when dealing with the intimate thoughts, feelings, and impulses of people who are seeking psychiatric help. In addition, the wisdom to recognize one’s limitations as a provider of care is an important attributes of seasoned practitioners. Patients might perceive us as demigods, but we know better than to be carried away with hubris and pretend that we are.
Exercising sound judgment isn’t easy
This is especially true when dealing with the varying degrees of ambiguity that shroud complex psychiatric conditions. The overriding principle for good clinical judgment in any medical practice is the patient’s welfare, and that must dominate the moral, ethical, medical, and scientific decision-making of all psychiatrists. Those of us in charge of training medical students and residents emphasize this principle every day at the bedside and in the clinic. Upholding those principles, side by side with the knowledge and skills of psychiatric practice, are the hallmarks of good medical training.
But missteps occur. Peccadillos, infractions, and even transgressions happen—accidentally by competent, ethical psychiatrists; deliberately, sometimes, by a few unethical scoundrels. The sins of psychiatric practice come in a range of gravity and consequences. Here are some I’ve observed among colleagues over the years:
Becoming sexually intimate with one’s patient. Violating the sacred boundary of the doctor-patient relationship is unforgivable; it’s a sin that scars the patient and can destroy the psychiatrist’s reputation and career. We must respect and uphold that boundary—not only during active treatment but even after care is terminated.
Divulging clinical details to other without the patient’s consent. Breach of trust is not only an ethical misstep; it is a violation of the Health Insurance Portability and Accountability Act of 1996 (HIPAA), with substantial penalties attached.
Treating the mind while ignoring the body and brain. We are physicians first, psychiatrists second. We must fully assess patients who present with psychiatric signs and symptoms to rule out a general medical condition that could be generating behavioral symptoms. Such co-occurring medical conditions might involve various organ systems, such as endocrinopathies, or might emanate from brain lesions, whether traumatic, degenerative, demyelinating, infectious, or neoplastic. Without careful medical evaluation, the wrong diagnosis might be made and inappropriate, even harmful, treatment provided while necessary care is delayed.
Treatment plans can sometimes overlook potential harmful effects of some medications on physical health— whether metabolic, cardiovascular, neurologic, gastrointestinal, hormonal, hematologic, or dermatologic. An optimal treatment plan embarks on healing the mind without ignoring or harming the body.
Failing to obtain additional information from sources who know the patient or who can provide old records. Such information is vital in psychiatry, because the patient’s account often is incomplete, even distorted, because of cognitive deficits or psychopathologic factors. Additional information can be corroborative or contradictory, and is sometimes critical—significantly influencing the diagnosis or the treatment plan, or both.
Allowing personal beliefs to influence care. This transgression includes inserting one’s views about religion, politics, sexual orientation, ethnic origin, and socioeconomic class into medical care. The same unimpeachable, high caliber of care must be provided to every patient, and must not differ in any way from the care that we would recommend to our own family members.
Reducing psychiatry to prescribing a pill. However unacceptable and deficient this reductionist degradation of psychiatric management is, it is sometimes imposed by organizations in which the caseload is huge and the number of providers insufficient. We must resist the temptation to compromise, and must strive to address not only the biological aspects of illness but psychological and social dimensions as well. Patients will not have an optimal if we don’t.
Practicing with an outdated knowledge base—one acquired during residency years, often years or even decades, earlier. There is no medical or ethical justification for using 1985, or even 2005, standards of psychiatric care in 2015.
Psychiatry is rapidly evolving, with many ongoing changes and advances. Updating one’s practice pattern through lifelong learning is an absolute must for psychiatrists (and for all health care professionals). Utilizing the latest, evidence-based data to guide diagnosis and treatment is an indispensable component of good psychiatric care.
Neglecting to consider treatment options. Consider just a few scenarios: The recurrently relapsing patient with psychosis who is not switched to a long-acting injectable formulation; the persistently psychotic patient who does not receive a trial of clozapine; the treatment-resistant depression patient who is not referred for electroconvulsive therapy or transcranial magnetic stimulation; and the patient receiving an atypical antipsychotic who is monitored inconsistently for metabolic dysregulation.
Treating patients but not vigorously advocating for them—thus allowing a broken, convoluted mental health system to delay or prevent access to care; incarcerate relapsed patients instead of hospitalizing them; permit insurance companies to discriminate against coverage of mental illness; and tie the hands of psychiatrists who want to select medication they judge best for their patients.
None of us is 'without sin'
We all aspire to help our patients in the best way we can, and to avoid errors. However, even a seasoned psychiatrist can stumble unwittingly, and that is understandable and forgivable. It is willful, recurring neglect of the patient’s welfare that can be deleterious and that, in my opinion, qualifies as a cardinal sin. Fortunately, such neglect is a low-frequency event in psychiatric practice, but even a single occurrence is one too many.
But as professionals, and by virtue of our rigorous training, we continually reflect on our thoughts and feelings when we care for our patients, and we examine the effect of our behavior and communication patterns on them. Such self-reflection is especially important when it comes to countertransference while treating a person who has been made vulnerable by emotional turmoil and who develops strong transference feelings toward the treating psychiatrist.
Personal integrity is paramount in psychiatric practice; it’s an indispensable ingredient when dealing with the intimate thoughts, feelings, and impulses of people who are seeking psychiatric help. In addition, the wisdom to recognize one’s limitations as a provider of care is an important attributes of seasoned practitioners. Patients might perceive us as demigods, but we know better than to be carried away with hubris and pretend that we are.
Exercising sound judgment isn’t easy
This is especially true when dealing with the varying degrees of ambiguity that shroud complex psychiatric conditions. The overriding principle for good clinical judgment in any medical practice is the patient’s welfare, and that must dominate the moral, ethical, medical, and scientific decision-making of all psychiatrists. Those of us in charge of training medical students and residents emphasize this principle every day at the bedside and in the clinic. Upholding those principles, side by side with the knowledge and skills of psychiatric practice, are the hallmarks of good medical training.
But missteps occur. Peccadillos, infractions, and even transgressions happen—accidentally by competent, ethical psychiatrists; deliberately, sometimes, by a few unethical scoundrels. The sins of psychiatric practice come in a range of gravity and consequences. Here are some I’ve observed among colleagues over the years:
Becoming sexually intimate with one’s patient. Violating the sacred boundary of the doctor-patient relationship is unforgivable; it’s a sin that scars the patient and can destroy the psychiatrist’s reputation and career. We must respect and uphold that boundary—not only during active treatment but even after care is terminated.
Divulging clinical details to other without the patient’s consent. Breach of trust is not only an ethical misstep; it is a violation of the Health Insurance Portability and Accountability Act of 1996 (HIPAA), with substantial penalties attached.
Treating the mind while ignoring the body and brain. We are physicians first, psychiatrists second. We must fully assess patients who present with psychiatric signs and symptoms to rule out a general medical condition that could be generating behavioral symptoms. Such co-occurring medical conditions might involve various organ systems, such as endocrinopathies, or might emanate from brain lesions, whether traumatic, degenerative, demyelinating, infectious, or neoplastic. Without careful medical evaluation, the wrong diagnosis might be made and inappropriate, even harmful, treatment provided while necessary care is delayed.
Treatment plans can sometimes overlook potential harmful effects of some medications on physical health— whether metabolic, cardiovascular, neurologic, gastrointestinal, hormonal, hematologic, or dermatologic. An optimal treatment plan embarks on healing the mind without ignoring or harming the body.
Failing to obtain additional information from sources who know the patient or who can provide old records. Such information is vital in psychiatry, because the patient’s account often is incomplete, even distorted, because of cognitive deficits or psychopathologic factors. Additional information can be corroborative or contradictory, and is sometimes critical—significantly influencing the diagnosis or the treatment plan, or both.
Allowing personal beliefs to influence care. This transgression includes inserting one’s views about religion, politics, sexual orientation, ethnic origin, and socioeconomic class into medical care. The same unimpeachable, high caliber of care must be provided to every patient, and must not differ in any way from the care that we would recommend to our own family members.
Reducing psychiatry to prescribing a pill. However unacceptable and deficient this reductionist degradation of psychiatric management is, it is sometimes imposed by organizations in which the caseload is huge and the number of providers insufficient. We must resist the temptation to compromise, and must strive to address not only the biological aspects of illness but psychological and social dimensions as well. Patients will not have an optimal if we don’t.
Practicing with an outdated knowledge base—one acquired during residency years, often years or even decades, earlier. There is no medical or ethical justification for using 1985, or even 2005, standards of psychiatric care in 2015.
Psychiatry is rapidly evolving, with many ongoing changes and advances. Updating one’s practice pattern through lifelong learning is an absolute must for psychiatrists (and for all health care professionals). Utilizing the latest, evidence-based data to guide diagnosis and treatment is an indispensable component of good psychiatric care.
Neglecting to consider treatment options. Consider just a few scenarios: The recurrently relapsing patient with psychosis who is not switched to a long-acting injectable formulation; the persistently psychotic patient who does not receive a trial of clozapine; the treatment-resistant depression patient who is not referred for electroconvulsive therapy or transcranial magnetic stimulation; and the patient receiving an atypical antipsychotic who is monitored inconsistently for metabolic dysregulation.
Treating patients but not vigorously advocating for them—thus allowing a broken, convoluted mental health system to delay or prevent access to care; incarcerate relapsed patients instead of hospitalizing them; permit insurance companies to discriminate against coverage of mental illness; and tie the hands of psychiatrists who want to select medication they judge best for their patients.
None of us is 'without sin'
We all aspire to help our patients in the best way we can, and to avoid errors. However, even a seasoned psychiatrist can stumble unwittingly, and that is understandable and forgivable. It is willful, recurring neglect of the patient’s welfare that can be deleterious and that, in my opinion, qualifies as a cardinal sin. Fortunately, such neglect is a low-frequency event in psychiatric practice, but even a single occurrence is one too many.
Living with schizophrenia