User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Is your patient’s poor recall more than just a ‘senior moment’?
Memory and other cognitive complaints are common among the general population and become more prevalent with age.1 People who have significant emotional investment in their cognitive competence, mood disturbance, somatic symptoms, and anxiety or related disorders are likely to worry more about their cognitive functioning as they age.
Common complaints
Age-related complaints, typically beginning by age 50, often include problems retaining or retrieving names, difficulty recalling details of conversations and written materials, and hazy recollection of remote events and the time frame of recent life events. Common complaints involve difficulties with mental calculations, multi-tasking (including vulnerability to distraction), and problems keeping track of and organizing information. The most common complaint is difficulty with remembering the reason for entering a room.
More concerning are complaints involving recurrent lapses in judgment or forgetfulness with significant implications for everyday living (eg, physical safety, job performance, travel, and finances), especially when validated by friends or family members and coupled with decline in at least 1 activity of daily living, and poor insight.
Helping your forgetful patient
Office evaluation with brief cognitive screening instruments—namely, the Montreal Cognitive Assessment and the recent revision of the Mini-Mental State Examination—might help clarify the clinical presentation. Proceed with caution: Screening tests tap a limited number of neurocognitive functions and can generate a false-negative result among brighter and better educated patients and a false-positive result among the less intelligent and less educated.2 Applying age- and education-corrected norms can reduce misclassification but does not eliminate it.
Screening measures can facilitate decision-making regarding the need for more comprehensive psychometric assessment. Such evaluations sample a broader range of neurobehavioral domains, in greater depth, and provide a more nuanced picture of a patient’s neurocognition.
Findings on a battery of psychological and neuropsychological tests that might evoke concern include problems with incidental, anterograde, and recent memory that are not satisfactorily explained by: age and education or vocational training; estimated premorbid intelligence; residual neurodevelopmental disorders (attention, learning, and autistic-spectrum disorders); situational, sociocultural, and psychiatric factors; and motivational influences—notably, malingering.
Some difficulties with memory are highly associated with mild cognitive impairment or early dementia:
• anterograde memory (involving a reduced rate of verbal and nonverbal learning over repeated trials)
• poor retention
• accelerated forgetting of newly learned information
• failure to benefit from recognition and other mnemonic cues
• so-called source error confusion—a misattribution that involves difficulty differentiating target information from competing information, as reflected in confabulation errors and an elevated rate of intrusion errors.
Disclosure
Dr. Pollak reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Weiner MF, Garrett R, Bret ME. Neuropsychiatric assessment and diagnosis. In: Weiner MF, Lipton AM, eds. Clinical manual of Alzheimer disease and other dementias. Arlington, VA: American Psychiatric Publishing, Inc.; 2012: 3-46.
2. Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests: administration, norms and commentary: third edition. New York, NY: Oxford University Press; 2006.
Memory and other cognitive complaints are common among the general population and become more prevalent with age.1 People who have significant emotional investment in their cognitive competence, mood disturbance, somatic symptoms, and anxiety or related disorders are likely to worry more about their cognitive functioning as they age.
Common complaints
Age-related complaints, typically beginning by age 50, often include problems retaining or retrieving names, difficulty recalling details of conversations and written materials, and hazy recollection of remote events and the time frame of recent life events. Common complaints involve difficulties with mental calculations, multi-tasking (including vulnerability to distraction), and problems keeping track of and organizing information. The most common complaint is difficulty with remembering the reason for entering a room.
More concerning are complaints involving recurrent lapses in judgment or forgetfulness with significant implications for everyday living (eg, physical safety, job performance, travel, and finances), especially when validated by friends or family members and coupled with decline in at least 1 activity of daily living, and poor insight.
Helping your forgetful patient
Office evaluation with brief cognitive screening instruments—namely, the Montreal Cognitive Assessment and the recent revision of the Mini-Mental State Examination—might help clarify the clinical presentation. Proceed with caution: Screening tests tap a limited number of neurocognitive functions and can generate a false-negative result among brighter and better educated patients and a false-positive result among the less intelligent and less educated.2 Applying age- and education-corrected norms can reduce misclassification but does not eliminate it.
Screening measures can facilitate decision-making regarding the need for more comprehensive psychometric assessment. Such evaluations sample a broader range of neurobehavioral domains, in greater depth, and provide a more nuanced picture of a patient’s neurocognition.
Findings on a battery of psychological and neuropsychological tests that might evoke concern include problems with incidental, anterograde, and recent memory that are not satisfactorily explained by: age and education or vocational training; estimated premorbid intelligence; residual neurodevelopmental disorders (attention, learning, and autistic-spectrum disorders); situational, sociocultural, and psychiatric factors; and motivational influences—notably, malingering.
Some difficulties with memory are highly associated with mild cognitive impairment or early dementia:
• anterograde memory (involving a reduced rate of verbal and nonverbal learning over repeated trials)
• poor retention
• accelerated forgetting of newly learned information
• failure to benefit from recognition and other mnemonic cues
• so-called source error confusion—a misattribution that involves difficulty differentiating target information from competing information, as reflected in confabulation errors and an elevated rate of intrusion errors.
Disclosure
Dr. Pollak reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Memory and other cognitive complaints are common among the general population and become more prevalent with age.1 People who have significant emotional investment in their cognitive competence, mood disturbance, somatic symptoms, and anxiety or related disorders are likely to worry more about their cognitive functioning as they age.
Common complaints
Age-related complaints, typically beginning by age 50, often include problems retaining or retrieving names, difficulty recalling details of conversations and written materials, and hazy recollection of remote events and the time frame of recent life events. Common complaints involve difficulties with mental calculations, multi-tasking (including vulnerability to distraction), and problems keeping track of and organizing information. The most common complaint is difficulty with remembering the reason for entering a room.
More concerning are complaints involving recurrent lapses in judgment or forgetfulness with significant implications for everyday living (eg, physical safety, job performance, travel, and finances), especially when validated by friends or family members and coupled with decline in at least 1 activity of daily living, and poor insight.
Helping your forgetful patient
Office evaluation with brief cognitive screening instruments—namely, the Montreal Cognitive Assessment and the recent revision of the Mini-Mental State Examination—might help clarify the clinical presentation. Proceed with caution: Screening tests tap a limited number of neurocognitive functions and can generate a false-negative result among brighter and better educated patients and a false-positive result among the less intelligent and less educated.2 Applying age- and education-corrected norms can reduce misclassification but does not eliminate it.
Screening measures can facilitate decision-making regarding the need for more comprehensive psychometric assessment. Such evaluations sample a broader range of neurobehavioral domains, in greater depth, and provide a more nuanced picture of a patient’s neurocognition.
Findings on a battery of psychological and neuropsychological tests that might evoke concern include problems with incidental, anterograde, and recent memory that are not satisfactorily explained by: age and education or vocational training; estimated premorbid intelligence; residual neurodevelopmental disorders (attention, learning, and autistic-spectrum disorders); situational, sociocultural, and psychiatric factors; and motivational influences—notably, malingering.
Some difficulties with memory are highly associated with mild cognitive impairment or early dementia:
• anterograde memory (involving a reduced rate of verbal and nonverbal learning over repeated trials)
• poor retention
• accelerated forgetting of newly learned information
• failure to benefit from recognition and other mnemonic cues
• so-called source error confusion—a misattribution that involves difficulty differentiating target information from competing information, as reflected in confabulation errors and an elevated rate of intrusion errors.
Disclosure
Dr. Pollak reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Weiner MF, Garrett R, Bret ME. Neuropsychiatric assessment and diagnosis. In: Weiner MF, Lipton AM, eds. Clinical manual of Alzheimer disease and other dementias. Arlington, VA: American Psychiatric Publishing, Inc.; 2012: 3-46.
2. Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests: administration, norms and commentary: third edition. New York, NY: Oxford University Press; 2006.
1. Weiner MF, Garrett R, Bret ME. Neuropsychiatric assessment and diagnosis. In: Weiner MF, Lipton AM, eds. Clinical manual of Alzheimer disease and other dementias. Arlington, VA: American Psychiatric Publishing, Inc.; 2012: 3-46.
2. Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests: administration, norms and commentary: third edition. New York, NY: Oxford University Press; 2006.
Discharging your patients who display contingency-based suicidality: 6 steps
Discharging patients from a hospital or emergency department despite his (her) ongoing suicidal ideation is a clinical dilemma. Typically, these patients do not respond to hospital care and do not follow up after discharge. They often have a poorly treated illness and many unmet psychosocial and interpersonal needs.1 These patients may communicate their suicidality as conditional, aimed at satisfying unmet needs; secondary gain; dependency needs; or remaining in the sick role. Faced with impending discharge, such a patient might increase the intensity of his suicidal statements or engage in behaviors that subvert discharge. Some go as far as to engage in behaviors with apparent suicidal intent soon after discharge.
A complicated decision
Such patients often are at a chronically elevated risk for suicide because of mood disorders, personality pathology, substance use disorder, or a history of serious suicide attempt.2 Do not dismiss a patient’s suicidal statements; he is ill and may end his own life.
Managing these situations can put you under a variety of pressures: your own negative emotional and psychological reactions to the patient; pressure from staff to avoid admission or expedite discharge of the patient; and administrative pressure to efficiently manage resources.3 You’re faced with a difficult decision: Discharge a patient who might self-harm or commit suicide, or continue care that may be counterproductive.
We propose 6 steps that have helped us promote good clinical care while documenting the necessary information to manage risk in these complex situations.
1. Define and document the clinical situation. Summarize the clinical dilemma.
2. Assess and document current suicide risk.4 Conduct a formal suicide risk assessment; if necessary, reassess throughout care. Focus on dynamic risk factors; protective risk factors (static and dynamic); acute stressors (or lack thereof) that would increase their risk of suicide above their chronically elevated baseline; and access to lethal means—firearms, stockpiled medication, etc.
3. Document modified dynamic or protective factors. Review the dynamic risk and protective factors you have identified and how they have been modified by treatment to date. If dynamic factors have not been modified, indicate why and document the recommended plan to address these matters. You might not be able to provide relief, but you should be able to outline a plan for eventual relief.
4. Document the reasons continued care in the acute setting is not indicated. Reasons might include: the patient isn’t participating in recommended care or treatment; the patient isn’t improving, or is becoming worse, in the care environment; continued care is preventing or interfering with access to more effective care options; is counterproductive to the patient’s stated goals; or compromising the safety benefit of the structured care environment because the patient is not collaborating with his care team.
5. Document your discussion of discharge with the patient. Highlight attempts to engage the patient in adaptive problem solving. Work out a crisis or suicide safety plan and give the patient a copy and keep a copy in his (her) chart.
If the patient refuses to engage in safety planning, document it in the chart. Note the absence of any conditions that might impair the patient’s volitional capacity to not end their life—intoxication, delirium, acute psychosis, etc. Explicitly frame the patient’s responsibility for his life. Discuss and document a follow-up plan and make direct contact with providers and social supports, documenting whether contacting these providers was successful.
6. Consult with a colleague. An informal non-visit consultation with a colleague demonstrates your recognition of the complexity of the situation and your due diligence in arriving at a discharge decision. Consultation often will result in useful additional strategies for managing or engaging the patient. A colleague’s agreement helps demonstrate that “average practitioner” and “prudent practitioner” standards of care have been met with respect to clinical decision-making.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Lambert MT, Bonner J. Characteristics and six-month outcome of patients who use suicide threats to seek hospital admission. Psychiatr Serv. 1996;47(8):871-873.
2. Zaheer J, Links PS, Liu E. Assessment and emergency management of suicidality in personality disorders. Psychiatr Clin North Am. 2008;31(3):527-543, viii-ix.
3. Gutheil TG, Schetky D. A date with death: management of time-based and contingent suicidal intent. Am J Psychiatry. 1998;155(11):1502-1507.
4. Haney EM, O’Neil ME, Carson S, et al. Suicide risk factors and risk assessment tools: a systematic review. VA Evidence-based Synthesis Program Reports. Washington, DC: Department of Veterans Affairs; 2012.
Discharging patients from a hospital or emergency department despite his (her) ongoing suicidal ideation is a clinical dilemma. Typically, these patients do not respond to hospital care and do not follow up after discharge. They often have a poorly treated illness and many unmet psychosocial and interpersonal needs.1 These patients may communicate their suicidality as conditional, aimed at satisfying unmet needs; secondary gain; dependency needs; or remaining in the sick role. Faced with impending discharge, such a patient might increase the intensity of his suicidal statements or engage in behaviors that subvert discharge. Some go as far as to engage in behaviors with apparent suicidal intent soon after discharge.
A complicated decision
Such patients often are at a chronically elevated risk for suicide because of mood disorders, personality pathology, substance use disorder, or a history of serious suicide attempt.2 Do not dismiss a patient’s suicidal statements; he is ill and may end his own life.
Managing these situations can put you under a variety of pressures: your own negative emotional and psychological reactions to the patient; pressure from staff to avoid admission or expedite discharge of the patient; and administrative pressure to efficiently manage resources.3 You’re faced with a difficult decision: Discharge a patient who might self-harm or commit suicide, or continue care that may be counterproductive.
We propose 6 steps that have helped us promote good clinical care while documenting the necessary information to manage risk in these complex situations.
1. Define and document the clinical situation. Summarize the clinical dilemma.
2. Assess and document current suicide risk.4 Conduct a formal suicide risk assessment; if necessary, reassess throughout care. Focus on dynamic risk factors; protective risk factors (static and dynamic); acute stressors (or lack thereof) that would increase their risk of suicide above their chronically elevated baseline; and access to lethal means—firearms, stockpiled medication, etc.
3. Document modified dynamic or protective factors. Review the dynamic risk and protective factors you have identified and how they have been modified by treatment to date. If dynamic factors have not been modified, indicate why and document the recommended plan to address these matters. You might not be able to provide relief, but you should be able to outline a plan for eventual relief.
4. Document the reasons continued care in the acute setting is not indicated. Reasons might include: the patient isn’t participating in recommended care or treatment; the patient isn’t improving, or is becoming worse, in the care environment; continued care is preventing or interfering with access to more effective care options; is counterproductive to the patient’s stated goals; or compromising the safety benefit of the structured care environment because the patient is not collaborating with his care team.
5. Document your discussion of discharge with the patient. Highlight attempts to engage the patient in adaptive problem solving. Work out a crisis or suicide safety plan and give the patient a copy and keep a copy in his (her) chart.
If the patient refuses to engage in safety planning, document it in the chart. Note the absence of any conditions that might impair the patient’s volitional capacity to not end their life—intoxication, delirium, acute psychosis, etc. Explicitly frame the patient’s responsibility for his life. Discuss and document a follow-up plan and make direct contact with providers and social supports, documenting whether contacting these providers was successful.
6. Consult with a colleague. An informal non-visit consultation with a colleague demonstrates your recognition of the complexity of the situation and your due diligence in arriving at a discharge decision. Consultation often will result in useful additional strategies for managing or engaging the patient. A colleague’s agreement helps demonstrate that “average practitioner” and “prudent practitioner” standards of care have been met with respect to clinical decision-making.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Discharging patients from a hospital or emergency department despite his (her) ongoing suicidal ideation is a clinical dilemma. Typically, these patients do not respond to hospital care and do not follow up after discharge. They often have a poorly treated illness and many unmet psychosocial and interpersonal needs.1 These patients may communicate their suicidality as conditional, aimed at satisfying unmet needs; secondary gain; dependency needs; or remaining in the sick role. Faced with impending discharge, such a patient might increase the intensity of his suicidal statements or engage in behaviors that subvert discharge. Some go as far as to engage in behaviors with apparent suicidal intent soon after discharge.
A complicated decision
Such patients often are at a chronically elevated risk for suicide because of mood disorders, personality pathology, substance use disorder, or a history of serious suicide attempt.2 Do not dismiss a patient’s suicidal statements; he is ill and may end his own life.
Managing these situations can put you under a variety of pressures: your own negative emotional and psychological reactions to the patient; pressure from staff to avoid admission or expedite discharge of the patient; and administrative pressure to efficiently manage resources.3 You’re faced with a difficult decision: Discharge a patient who might self-harm or commit suicide, or continue care that may be counterproductive.
We propose 6 steps that have helped us promote good clinical care while documenting the necessary information to manage risk in these complex situations.
1. Define and document the clinical situation. Summarize the clinical dilemma.
2. Assess and document current suicide risk.4 Conduct a formal suicide risk assessment; if necessary, reassess throughout care. Focus on dynamic risk factors; protective risk factors (static and dynamic); acute stressors (or lack thereof) that would increase their risk of suicide above their chronically elevated baseline; and access to lethal means—firearms, stockpiled medication, etc.
3. Document modified dynamic or protective factors. Review the dynamic risk and protective factors you have identified and how they have been modified by treatment to date. If dynamic factors have not been modified, indicate why and document the recommended plan to address these matters. You might not be able to provide relief, but you should be able to outline a plan for eventual relief.
4. Document the reasons continued care in the acute setting is not indicated. Reasons might include: the patient isn’t participating in recommended care or treatment; the patient isn’t improving, or is becoming worse, in the care environment; continued care is preventing or interfering with access to more effective care options; is counterproductive to the patient’s stated goals; or compromising the safety benefit of the structured care environment because the patient is not collaborating with his care team.
5. Document your discussion of discharge with the patient. Highlight attempts to engage the patient in adaptive problem solving. Work out a crisis or suicide safety plan and give the patient a copy and keep a copy in his (her) chart.
If the patient refuses to engage in safety planning, document it in the chart. Note the absence of any conditions that might impair the patient’s volitional capacity to not end their life—intoxication, delirium, acute psychosis, etc. Explicitly frame the patient’s responsibility for his life. Discuss and document a follow-up plan and make direct contact with providers and social supports, documenting whether contacting these providers was successful.
6. Consult with a colleague. An informal non-visit consultation with a colleague demonstrates your recognition of the complexity of the situation and your due diligence in arriving at a discharge decision. Consultation often will result in useful additional strategies for managing or engaging the patient. A colleague’s agreement helps demonstrate that “average practitioner” and “prudent practitioner” standards of care have been met with respect to clinical decision-making.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Lambert MT, Bonner J. Characteristics and six-month outcome of patients who use suicide threats to seek hospital admission. Psychiatr Serv. 1996;47(8):871-873.
2. Zaheer J, Links PS, Liu E. Assessment and emergency management of suicidality in personality disorders. Psychiatr Clin North Am. 2008;31(3):527-543, viii-ix.
3. Gutheil TG, Schetky D. A date with death: management of time-based and contingent suicidal intent. Am J Psychiatry. 1998;155(11):1502-1507.
4. Haney EM, O’Neil ME, Carson S, et al. Suicide risk factors and risk assessment tools: a systematic review. VA Evidence-based Synthesis Program Reports. Washington, DC: Department of Veterans Affairs; 2012.
1. Lambert MT, Bonner J. Characteristics and six-month outcome of patients who use suicide threats to seek hospital admission. Psychiatr Serv. 1996;47(8):871-873.
2. Zaheer J, Links PS, Liu E. Assessment and emergency management of suicidality in personality disorders. Psychiatr Clin North Am. 2008;31(3):527-543, viii-ix.
3. Gutheil TG, Schetky D. A date with death: management of time-based and contingent suicidal intent. Am J Psychiatry. 1998;155(11):1502-1507.
4. Haney EM, O’Neil ME, Carson S, et al. Suicide risk factors and risk assessment tools: a systematic review. VA Evidence-based Synthesis Program Reports. Washington, DC: Department of Veterans Affairs; 2012.
Medication for alcohol use disorder: Which agents work best?
Historically, alcohol use disorder (AUD; classified as alcohol abuse or dependence in DSM-IV-TR) has been treated with psychosocial therapies, but many patients treated this way relapse into heavy drinking patterns and are unable to sustain sobriety (Box 11). Although vital for treating AUD, psychosocial methods have, to date, a modest success rate. Research has demonstrated that combining pharmacotherapy with psychosocial programs is effective for treating AUD.2
Patients and clinicians might associate AUD medications with so-called aversion therapy because, for many years, the only treatment was disulfiram, which causes unpleasant physical effects when consumed with alcohol. However, newer medications help patients maintain abstinence by targeting brain neurotransmitters relevant to addiction neurocircuitry, such as dopamine, serotonin, ϒ-aminobutyric acid (GABA), glutamate, and opioid.3 These medications may help patients with AUD achieve sobriety, avoid relapse, decrease heavy drinking days, and delay time to recurrent drinking.
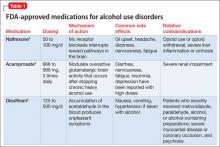
In this article, we review FDA-approved medications (Table 1)4-6 and off-label agents (Table 2)3,7-18 and provide recommendations for treating patients with AUD (Box 2).19-21
FDA-approved treatments
Naltrexone is an opiate antagonist that blocks the mu receptor and is believed to interrupt the dopamine reward pathway in the brain for alcohol. A meta-analysis of 2,861 patients in 24 combined randomized controlled trials (RCTs) demonstrated naltrexone to be an effective short-term (12 weeks) treatment for alcoholism, significantly decreasing relapses.22 The large multisite COMBINE study (N = 1,383) showed that naltrexone, 100 mg/d, and medical treatment without behavioral treatment over 16 weeks was more effective than placebo in increasing percentage of days abstinent (80.6% vs 75.1%, respectively) and reducing the percentage of patients experiencing heavy drinking days (66.2% vs 73.1%, respectively).2 Patients who have a family history of AUD or strong cravings, or both, may benefit most from naltrexone.3,7,23 Despite evidence of the effectiveness of naltrexone for AUD, not all studies have yielded positive results.24
Common side effects of naltrexone, if present, appear early in treatment and include GI upset (eg, nausea, vomiting, abdominal pain), headache, and fatigue.2,3,22,23 Hepatotoxicity has been reported with dosages of 100 to 300 mg/d, but lab values typically normalize when naltrexone is discontinued.2,3,23 Monitor markers of liver function including ϒ-glutamyltransferase, aspartate aminotransferase, alanine aminotransferase, and bilirubin before and during naltrexone treatment (we check patients 1 to 3 months after starting treatment and yearly thereafter). Obtain a negative urine drug screen for opioids before administering naltrexone, because if opioids were consumed recently naltrexone could precipitate withdrawal. Because of the unknown teratogenicity of naltrexone, women of childbearing age should undergo pregnancy testing before and periodically during naltrexone therapy.
Naltrexone also is available in a once-monthly, 380-mg injectable formulation. Studies show that, similar to its oral counterpart, injectable naltrexone effectively reduces heavy drinking days and number of drinks a day compared with placebo.25,26 Advantages of injectable naltrexone are its extended steady release of medication and its efficacy for patients who do not adhere to oral dosing.7,27 Side effects are similar to oral naltrexone, except for injection site reactions and pain.
Contraindications to naltrexone include current opioid use because its antagonistic effects on opioid receptors render opioid analgesia ineffective. Patients who have used opioids within 7 to 10 days or who may be surreptitiously using opioids should not take naltrexone because it may cause opioid withdrawal. Some patients may try to override the opioid receptor blockade of naltrexone with higher opioid doses, which could result in overdose.
Naltrexone is approved for treating opioid use disorder and may be useful for persons with comorbid opioid use disorder and AUD, if the patient has been adequately detoxified from opioids and intends to abstain from these drugs. Patients who have extensive liver damage secondary to acute hepatitis or uncompensated cirrhosis would not be good candidates for naltrexone because of a risk of hepatotoxicity.2,3,22
Because of ease of dosing, we recommend naltrexone as a first-line treatment for AUD, unless the patient requires opioids or has severe liver disease. We recommend increasing naltrexone from 50 mg to 100 mg before switching to acamprosate, based on European studies.
Acamprosate is a glutamate antagonist that is thought to modulate overactive glutamatergic brain activity that occurs after stopping chronic heavy alcohol use. In a meta-analysis of 17 studies (N = 4,087), continuous abstinence rates at 6 months were significantly higher in acamprosate-treated patients (36.1%) than in patients receiving placebo (23.4%).28 In a review of European studies, acamprosate benefited patients who have increased anxiety, physiological dependence, negative family history of AUD, and late age of onset (age >25) of alcohol dependence.7 However, in the COMBINE trial acamprosate was no more effective than placebo.2
We consider acamprosate an effective option for patients who do not respond to naltrexone or have a contraindication. Dosages of 333 mg to 666 mg, 3 times a day, are recommended, although dosages up to 3 g/d have been studied; titration is not required.2,7,28 We recommend advising patients to continue treatment even if they relapse, because these medications may mitigate relapse severity. Adherence to multiple daily doses can be problematic for some patients, but pairing medications with meals or bedtime may improve adherence.
Diarrhea is the most common side effect of acamprosate; nervousness, fatigue, insomnia, and depression have been reported with high dosages.2,3,7,28 Acamprosate is excreted through the kidney and is safe for patients with liver disorders such as acute hepatitis or cirrhosis. The drug is contraindicated in patients with acute or chronic renal failure with creatinine clearance <30 mL/min; those with less severe renal insufficiency might need a lower dosage. Obtain baseline renal function before starting acamprosate; women of childbearing age should undergo a pregnancy test.
Disulfiram inhibits alcohol metabolism, resulting in acetaldehyde accumulation, which causes unpleasant physical effects such as nausea, vomiting, and hypotension. This creates a negative rather than a positive experience with drinking. A US Veterans Administration Cooperative Study randomized 605 participants to riboflavin, disulfiram, 1 mg/d (an inactive dose), or disulfiram, 250 mg/d (standard dose). There was no difference in percentage of patients remaining abstinent or time to first drink.8 Participants receiving disulfiram, 250 mg/d, had fewer drinking days after relapse compared with the other groups.8
Adverse physical effects produced when disulfiram and alcohol interact include tremor, diaphoresis, unstable blood pressure, and severe diarrhea and vomiting. Disulfiram can cause medically serious reactions in a small percentage of patients, especially those with significant medical comorbidity or advanced age. Patients with severe hypertension, diabetes mellitus, heart disease, a history of stroke, peripheral neuropathy, epilepsy, or renal or hepatic insufficiency should not use disulfiram.3 Patients taking disulfiram should avoid casual exposures to food, aftershave, mouthwash, and hand sanitizer that might contain alcohol. Disulfiram has no significant effect on alcohol craving. Social support to help oversee dosing may enhance adherence.7
Off-label medications
Topiramate is FDA-approved to treat migraine headaches and some seizure disorders. Topiramate facilitates GABA-mediated neuronal inhibition and antagonizes certain glutamate receptor subtypes. In an RCT (N = 150), topiramate, up to 300 mg/d, was more effective than placebo at reducing heavy drinking days and number of drinks per day, increasing days abstinent, and alleviating cravings.9 In a 12-week, double-blind RCT (N = 150), topiramate increased “safe drinking”—defined as ≤1 standard drink per day for women and ≤2 per day for men—vs placebo.10 Dosages were 75 mg to 300 mg/d in twice daily divided doses. Dosing starts at 25 mg/d and increases by 25 to 50 mg a day at weekly intervals. We recommend reserving topiramate for persons who do not respond to or cannot tolerate naltrexone and acamprosate because of the slow titration needed to prevent side effects. Although not studied, it may seem that topiramate’s antiepileptic actions could prevent seizures during alcohol withdrawal, but the protracted titration would limit its utility.
Side effects of topiramate include impaired memory and concentration, paresthesia, and anorexia and are more likely to present during rapid titration or with a high dosage.7,8 Rare reports of spontaneous myopia, angle-closure glaucoma, increased intraocular pressure, ocular pain, and blurry vision have been reported, but these complications often resolve with discontinuation of topiramate.7,8
Topiramate primarily is excreted through the kidney, and its action in the renal tubules can lead to metabolic acidosis or nephrolithiasis.8 Relative contraindications include acute or chronic kidney disease, including kidney stones. Consider slower titration and a 50% reduction in dosing if creatinine clearance is <70 mL/min. Obtain renal function tests before starting topiramate and consider monitoring serum bicarbonate for metabolic acidosis (we test at 3 and 6 months, then every 6 months). Because of teratogenic effects of topiramate (eg, cleft lip and palate), rule out pregnancy in all women of childbearing age.
Baclofen is a GABAb receptor agonist that is FDA approved for treating spasticity. Because GABA transmission is down-regulated in chronic AUD, it is a commonly targeted neurotransmitter when developing medications for AUD. GABAa receptors are fast-acting inhibitory ion channels, and its agonists (eg, benzodiazepines) have a significant abuse and cross-addiction liability. GABAb receptors, however, are slow-acting through a complex cascade of intracellular signals, and therefore GABAb agonists such as baclofen have been studied for treating addiction.
In a randomized double-blind, placebo-controlled trial (N = 39), baclofen was superior to placebo in suppressing obsessive aspects of cravings and decreasing state anxiety.11 Baclofen, 10 mg 3 times daily, in another randomized double-blind, placebo-controlled trial (n = 42) reduced the number of drinks per day by 53% vs placebo; 20 mg 3 times a day resulted in a 68% reduction in drinks per day vs placebo.12 However, a placebo-controlled RCT (n = 80) reported that baclofen, 10 mg 3 times daily, was not superior to placebo for primary outcomes related to alcohol consumption, although it did significantly decrease cravings and anxiety among persons with AUD.13 Evidence suggests that baclofen might be effective for promoting abstinence, reducing the risk of relapse, and alleviating cravings and anxiety in persons with AUD, although further investigation is needed.
In studies for AUD, the side-effect profile for baclofen was relatively benign.11-13 Nausea, fatigue, sleepiness, vertigo, and abdominal pain were reported; overall, baclofen was found to be safe and to have no abuse liability.7,10,12 The addictive potential of other muscle relaxers may have dissuaded providers from using baclofen for AUD, but we consider it a reasonable alternative when FDA-approved treatments fail.
Because baclofen is primarily eliminated by the kidneys, it may be safe for people with cirrhosis or severe liver disease.12 Baseline renal labs should be performed before administering baclofen and a negative pregnancy test obtained for women of childbearing age.
Ondansetron is a serotonin receptor type 3 (5-HT3) antagonist that has shown promising results for AUD.3,7 Research suggests that 5-HT3 receptors are an action site for alcohol in the brain and are thought to play a role in its rewarding effects.7 Ondansetron may be more effective for early-onset alcoholism (EOA) than late-onset alcoholism (LOA).14,15 EOA (age ≤25) is characterized by strong family history of AUD and prominent antisocial traits. A randomized double-blind, placebo-controlled trial (n = 271) reported that ondansetron, 4 mcg/kg twice daily, was superior to placebo in reducing number of drinks per day, increasing days abstinent, and reducing cravings in patients with EOA.14 Among persons with EOA, ondansetron, 16 mcg/kg twice daily, significantly reduced the severity of symptoms of fatigue, confusion, and overall mood disturbance such as depression, anxiety, and hostility compared with placebo in an RCT (n = 321).15 The lowest available oral dosage of ondansetron is 4 mg tablets or 4 mg/5 mL solution. We have used 4 mg twice daily for patients who have failed naltrexone and acamprosate or when these agents are contraindicated.
Common side effects of ondansetron include constipation, diarrhea, elevated liver enzymes, tachycardia, headache, and fatigue. Contraindications include congenital long QT syndrome, QTc prolongation risk, or significant hepatic impairment. We suggest evaluating baseline electrocardiogram and liver function tests. Women should undergo a pregnancy test before receiving medications.
Gabapentin is an anticonvulsant that is FDA-approved for treating epilepsy and postherpetic neuralgia. Gabapentin is related structurally to GABA and may potentiate central nervous system GABA activity, inhibit glutamate activity, and reduce norepinephrine and dopamine release.16 Gabapentin is thought to balance the GABA/glutamate dysregulation found in early alcohol abstinence and reduce risk for alcohol relapse.16A randomized, double-blind, placebo-controlled trial (N = 60) demonstrated that gabapentin, 600 mg/d, significantly reduced number of drinks per day and heavy drinking days and increased days of abstinence compared with placebo over 28 days.17 Another double-blind, randomized, placebo-controlled trial of 150 people used gabapentin, 900 or 1,800 mg/d; there was a linear dose response for increased days abstinent and no heavy drinking days in favor of gabapentin.18
An RCT (N = 150) evaluated adding gabapentin, up to 1200 mg/d, to naltrexone, 50 mg/d, vs naltrexone with placebo or double placebo over 6 weeks of treatment. The combined gabapentin-naltrexone group outperformed the other 2 groups on time to heavy drinking, number of heavy drinking days, and number of drinks per day. Gabapentin’s positive effects on sleep may have mediated some of its beneficial effects.29 In an open-label pilot study, gabapentin was more effective than trazodone for insomnia during early alcohol abstinence.30 Of note, gabapentin is a safe alternative to benzodiazepines for alcohol detoxification in patients with severe hepatic disease or those at risk of interacting with alcohol (eg, outpatients at high risk to drink during detoxification).31 Gabapentin, 400 mg/d to 1,600 mg/d, generally is safe and well tolerated and has some support for improving cravings, reducing alcohol consumption, delaying relapse, and improving sleep in patients with AUD.
Side effects of gabapentin include daytime sedation, dizziness, ataxia, fatigue, and dyspepsia. Using 3 divided doses might enhance tolerability. To reduce daytime sedation, we recommend administering most of the dose at night, which also may relieve insomnia. Gabapentin is excreted through the kidney; baseline renal function tests should be performed before initiating treatment, because the dosage might need to be adjusted in people with renal insufficiency.
Bottom Line
FDA-approved (acamprosate, naltrexone, and disulfiram) and off-label (baclofen, gabapentin, ondansetron, and topiramate) agents can help patients with alcohol use disorder achieve abstinence, reduce heavy drinking days, prevent relapse, and maintain sobriety. Research supports the use of pharmacotherapy combined with psychosocial modalities, such as 12-step programs, motivational interviewing, and cognitive-behavioral therapy.
Related Resources
- National Institute on Drug Abuse. Principles of drug addiction treatment: A research-based guide (third edition). www.drugabuse.gov/publications/principles-drug-addiction-treatment-research-based-guide-third-edition/evidence-based-approaches-to-drug-addiction-treatment/pharmacotherapi-1.
- Substance Abuse and Mental Health Services Administration. Incorporating alcohol pharmacotherapies into medical practice. http://162.99.3.213/products/manuals/tips/pdf/TIP49.pdf.
- Pettinati HM, Mattson ME. Medical management treatment manual: A clinical guide for researchers and clinicians providing pharmacotherapy for alcohol dependence. http://pubs.niaaa.nih.gov/publications/MedicalManual/MMManual.pdf.
Drug Brand Names
Acamprosate • Campral Naltrexone • Vivitrol, ReVia
Baclofen • Lioresal Ondansetron • Zofran
Disulfiram • Antabuse Topiramate • Topamax
Gabapentin • Neurontin Trazodone • Desyrel, Oleptro
Metronidazole • Flagyl
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. World Health Organization. Alcohol fact sheet. http://www.who.int/mediacentre/factsheets/fs349/en/index.html. Published February 2011. Accessed April 30, 2013.
2. Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence. The COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003-2017.
3. Mann K. Pharmacotherapy of alcohol dependence a review of the clinical data. CNS Drugs. 2004;18(8):485-504.
4. ReVia [package insert]. Pomona, NY: Barr Pharmaceuticals; 2009.
5. Campral [package insert]. St. Louis, MO: Forest Pharmaceuticals; 2004.
6. Antabuse [package insert]. Pomona, NY: Barr Pharmaceuticals; 2010.
7. Johnson BA. Update on neuropharmacological treatments for alcoholism: scientific basis and clinical findings. Biochem Pharmacol. 2008;75(1):34-56.
8. Fuller RK, Branchey L, Brightwell DR, et al. Disulfiram treatment of alcoholism: a Veterans Administration cooperative study. JAMA. 1986;256(11):1449-1454.
9. Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):
1677-1685.
10. Ma JZ, Ait-Daoud N, Johnson BA. Topiramate reduces the harm of excessive drinking: implications for public health and primary care. Addiction. 2006;101:1561-1568.
11. Addolorato G, Caputo F, Capristo E, et al. Baclofen efficacy in reducing alcohol craving and intake: a preliminary double-blind randomized controlled study. Alcohol Alcohol. 2002;37(5):504-508.
12. Addolorato G, Leggio L, Ferrulli A, et al. Dose-response effect of baclofen in reducing daily alcohol intake in alcohol dependence: secondary analysis of a randomized, double-blind, placebo-controlled trial. Alcohol Alcohol. 2011;46(3):312-317.
13. Garbutt JC, Kampov-Polevoy AB, Gallop R, et al. Efficacy and safety of baclofen for alcohol dependence: a randomized, double-blind placebo-controlled trial. Alcohol Clin Exp Res. 2010;34(11):1849-1857.
14. Johnson BA, Roache JD, Javors MA, et al. Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: a randomized controlled trial. JAMA. 2000;284(8):963-971.
15. Johnson BA, Ait-Daoud N, Ma JZ, et al. Ondansetron reduces mood disturbance among biologically predisposed, alcohol-dependent individuals. Alcohol Clin Exp Res. 2003;27(11):1773-1779.
16. Myrick H, Anton R, Voronin K, et al. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res. 2007;31(2):221-227.
17. Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68:1691-1700.
18. Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial [published online November 4, 2013]. JAMA Intern Med. doi: 10.1001/jamainternmed.2013.11950.
19. The Management of Substance Use Disorders Working Group. VA/DoD clinical practice guideline for management of substance use disorders (SUD). http://www.healthquality.va.gov/sud/sud_full_601f.pdf. Published August 2009. Accessed November 22, 2013.
20. Mark TL, Kranzler HR, Song X, et al. Physicians’ opinions about medication to treat alcoholism. Addiction. 2003;98(5):617-626.
21. Thomas CP, Wallack SS, Lee S, et al. Research to practice: adoption of naltrexone in alcoholism treatment. J Subst Abuse Treat. 2003;24(1):1-11.
22. Srisurapanont M, Jarusuraisin N. Naltrexone for the treatment of alcoholism: a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2005;8:267-280.
23. Anton RF. Naltrexone for the management of alcohol dependence. N Engl J Med. 2008;359(7):715-721.
24. Gueorguieva R, Wu R, Pittman B, et al. New insights into the efficacy of naltrexone based on trajectory-based reanalysis of two negative clinical trials. Biol Psychiatry. 2007;61(11): 1290-1295.
25. Lapham S, Forman R, Alexander M, et al. The effects of extended-release naltrexone on holiday drinking in alcohol-dependent patients. J Subst Abuse. 2009;36(1):1-6.
26. Ciraulo DA, Dong Q, Silverman BL, et al. Early treatment response in alcohol dependence with extended-release naltrexone. J Clin Psychiatry. 2008;69(2):190-195.
27. Mark TL, Montejano LB, Kranzler HR, et al. Comparison of healthcare utilization among patients treated with alcoholism medications. Am J Managed Care. 2010;16(12): 879-888.
28. Mann K, Lehert P, Morgan MY. The efficacy of acamprosate in the maintenance of abstinence in alcohol-dependent individuals: results of a meta-analysis. Alcohol Clin Exp Res. 2004;28(1):51-63.
29. Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry. 2011;168(7):709-717.
30. Karam-Hage M, Brower KJ. Open pilot study of gabapentin versus trazodone to treat insomnia in alcoholic outpatients. Psychiatry Clin Neurosci. 2003;57(5):542-544.
31. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
Historically, alcohol use disorder (AUD; classified as alcohol abuse or dependence in DSM-IV-TR) has been treated with psychosocial therapies, but many patients treated this way relapse into heavy drinking patterns and are unable to sustain sobriety (Box 11). Although vital for treating AUD, psychosocial methods have, to date, a modest success rate. Research has demonstrated that combining pharmacotherapy with psychosocial programs is effective for treating AUD.2
Patients and clinicians might associate AUD medications with so-called aversion therapy because, for many years, the only treatment was disulfiram, which causes unpleasant physical effects when consumed with alcohol. However, newer medications help patients maintain abstinence by targeting brain neurotransmitters relevant to addiction neurocircuitry, such as dopamine, serotonin, ϒ-aminobutyric acid (GABA), glutamate, and opioid.3 These medications may help patients with AUD achieve sobriety, avoid relapse, decrease heavy drinking days, and delay time to recurrent drinking.
In this article, we review FDA-approved medications (Table 1)4-6 and off-label agents (Table 2)3,7-18 and provide recommendations for treating patients with AUD (Box 2).19-21
FDA-approved treatments
Naltrexone is an opiate antagonist that blocks the mu receptor and is believed to interrupt the dopamine reward pathway in the brain for alcohol. A meta-analysis of 2,861 patients in 24 combined randomized controlled trials (RCTs) demonstrated naltrexone to be an effective short-term (12 weeks) treatment for alcoholism, significantly decreasing relapses.22 The large multisite COMBINE study (N = 1,383) showed that naltrexone, 100 mg/d, and medical treatment without behavioral treatment over 16 weeks was more effective than placebo in increasing percentage of days abstinent (80.6% vs 75.1%, respectively) and reducing the percentage of patients experiencing heavy drinking days (66.2% vs 73.1%, respectively).2 Patients who have a family history of AUD or strong cravings, or both, may benefit most from naltrexone.3,7,23 Despite evidence of the effectiveness of naltrexone for AUD, not all studies have yielded positive results.24
Common side effects of naltrexone, if present, appear early in treatment and include GI upset (eg, nausea, vomiting, abdominal pain), headache, and fatigue.2,3,22,23 Hepatotoxicity has been reported with dosages of 100 to 300 mg/d, but lab values typically normalize when naltrexone is discontinued.2,3,23 Monitor markers of liver function including ϒ-glutamyltransferase, aspartate aminotransferase, alanine aminotransferase, and bilirubin before and during naltrexone treatment (we check patients 1 to 3 months after starting treatment and yearly thereafter). Obtain a negative urine drug screen for opioids before administering naltrexone, because if opioids were consumed recently naltrexone could precipitate withdrawal. Because of the unknown teratogenicity of naltrexone, women of childbearing age should undergo pregnancy testing before and periodically during naltrexone therapy.
Naltrexone also is available in a once-monthly, 380-mg injectable formulation. Studies show that, similar to its oral counterpart, injectable naltrexone effectively reduces heavy drinking days and number of drinks a day compared with placebo.25,26 Advantages of injectable naltrexone are its extended steady release of medication and its efficacy for patients who do not adhere to oral dosing.7,27 Side effects are similar to oral naltrexone, except for injection site reactions and pain.
Contraindications to naltrexone include current opioid use because its antagonistic effects on opioid receptors render opioid analgesia ineffective. Patients who have used opioids within 7 to 10 days or who may be surreptitiously using opioids should not take naltrexone because it may cause opioid withdrawal. Some patients may try to override the opioid receptor blockade of naltrexone with higher opioid doses, which could result in overdose.
Naltrexone is approved for treating opioid use disorder and may be useful for persons with comorbid opioid use disorder and AUD, if the patient has been adequately detoxified from opioids and intends to abstain from these drugs. Patients who have extensive liver damage secondary to acute hepatitis or uncompensated cirrhosis would not be good candidates for naltrexone because of a risk of hepatotoxicity.2,3,22
Because of ease of dosing, we recommend naltrexone as a first-line treatment for AUD, unless the patient requires opioids or has severe liver disease. We recommend increasing naltrexone from 50 mg to 100 mg before switching to acamprosate, based on European studies.
Acamprosate is a glutamate antagonist that is thought to modulate overactive glutamatergic brain activity that occurs after stopping chronic heavy alcohol use. In a meta-analysis of 17 studies (N = 4,087), continuous abstinence rates at 6 months were significantly higher in acamprosate-treated patients (36.1%) than in patients receiving placebo (23.4%).28 In a review of European studies, acamprosate benefited patients who have increased anxiety, physiological dependence, negative family history of AUD, and late age of onset (age >25) of alcohol dependence.7 However, in the COMBINE trial acamprosate was no more effective than placebo.2
We consider acamprosate an effective option for patients who do not respond to naltrexone or have a contraindication. Dosages of 333 mg to 666 mg, 3 times a day, are recommended, although dosages up to 3 g/d have been studied; titration is not required.2,7,28 We recommend advising patients to continue treatment even if they relapse, because these medications may mitigate relapse severity. Adherence to multiple daily doses can be problematic for some patients, but pairing medications with meals or bedtime may improve adherence.
Diarrhea is the most common side effect of acamprosate; nervousness, fatigue, insomnia, and depression have been reported with high dosages.2,3,7,28 Acamprosate is excreted through the kidney and is safe for patients with liver disorders such as acute hepatitis or cirrhosis. The drug is contraindicated in patients with acute or chronic renal failure with creatinine clearance <30 mL/min; those with less severe renal insufficiency might need a lower dosage. Obtain baseline renal function before starting acamprosate; women of childbearing age should undergo a pregnancy test.
Disulfiram inhibits alcohol metabolism, resulting in acetaldehyde accumulation, which causes unpleasant physical effects such as nausea, vomiting, and hypotension. This creates a negative rather than a positive experience with drinking. A US Veterans Administration Cooperative Study randomized 605 participants to riboflavin, disulfiram, 1 mg/d (an inactive dose), or disulfiram, 250 mg/d (standard dose). There was no difference in percentage of patients remaining abstinent or time to first drink.8 Participants receiving disulfiram, 250 mg/d, had fewer drinking days after relapse compared with the other groups.8
Adverse physical effects produced when disulfiram and alcohol interact include tremor, diaphoresis, unstable blood pressure, and severe diarrhea and vomiting. Disulfiram can cause medically serious reactions in a small percentage of patients, especially those with significant medical comorbidity or advanced age. Patients with severe hypertension, diabetes mellitus, heart disease, a history of stroke, peripheral neuropathy, epilepsy, or renal or hepatic insufficiency should not use disulfiram.3 Patients taking disulfiram should avoid casual exposures to food, aftershave, mouthwash, and hand sanitizer that might contain alcohol. Disulfiram has no significant effect on alcohol craving. Social support to help oversee dosing may enhance adherence.7
Off-label medications
Topiramate is FDA-approved to treat migraine headaches and some seizure disorders. Topiramate facilitates GABA-mediated neuronal inhibition and antagonizes certain glutamate receptor subtypes. In an RCT (N = 150), topiramate, up to 300 mg/d, was more effective than placebo at reducing heavy drinking days and number of drinks per day, increasing days abstinent, and alleviating cravings.9 In a 12-week, double-blind RCT (N = 150), topiramate increased “safe drinking”—defined as ≤1 standard drink per day for women and ≤2 per day for men—vs placebo.10 Dosages were 75 mg to 300 mg/d in twice daily divided doses. Dosing starts at 25 mg/d and increases by 25 to 50 mg a day at weekly intervals. We recommend reserving topiramate for persons who do not respond to or cannot tolerate naltrexone and acamprosate because of the slow titration needed to prevent side effects. Although not studied, it may seem that topiramate’s antiepileptic actions could prevent seizures during alcohol withdrawal, but the protracted titration would limit its utility.
Side effects of topiramate include impaired memory and concentration, paresthesia, and anorexia and are more likely to present during rapid titration or with a high dosage.7,8 Rare reports of spontaneous myopia, angle-closure glaucoma, increased intraocular pressure, ocular pain, and blurry vision have been reported, but these complications often resolve with discontinuation of topiramate.7,8
Topiramate primarily is excreted through the kidney, and its action in the renal tubules can lead to metabolic acidosis or nephrolithiasis.8 Relative contraindications include acute or chronic kidney disease, including kidney stones. Consider slower titration and a 50% reduction in dosing if creatinine clearance is <70 mL/min. Obtain renal function tests before starting topiramate and consider monitoring serum bicarbonate for metabolic acidosis (we test at 3 and 6 months, then every 6 months). Because of teratogenic effects of topiramate (eg, cleft lip and palate), rule out pregnancy in all women of childbearing age.
Baclofen is a GABAb receptor agonist that is FDA approved for treating spasticity. Because GABA transmission is down-regulated in chronic AUD, it is a commonly targeted neurotransmitter when developing medications for AUD. GABAa receptors are fast-acting inhibitory ion channels, and its agonists (eg, benzodiazepines) have a significant abuse and cross-addiction liability. GABAb receptors, however, are slow-acting through a complex cascade of intracellular signals, and therefore GABAb agonists such as baclofen have been studied for treating addiction.
In a randomized double-blind, placebo-controlled trial (N = 39), baclofen was superior to placebo in suppressing obsessive aspects of cravings and decreasing state anxiety.11 Baclofen, 10 mg 3 times daily, in another randomized double-blind, placebo-controlled trial (n = 42) reduced the number of drinks per day by 53% vs placebo; 20 mg 3 times a day resulted in a 68% reduction in drinks per day vs placebo.12 However, a placebo-controlled RCT (n = 80) reported that baclofen, 10 mg 3 times daily, was not superior to placebo for primary outcomes related to alcohol consumption, although it did significantly decrease cravings and anxiety among persons with AUD.13 Evidence suggests that baclofen might be effective for promoting abstinence, reducing the risk of relapse, and alleviating cravings and anxiety in persons with AUD, although further investigation is needed.
In studies for AUD, the side-effect profile for baclofen was relatively benign.11-13 Nausea, fatigue, sleepiness, vertigo, and abdominal pain were reported; overall, baclofen was found to be safe and to have no abuse liability.7,10,12 The addictive potential of other muscle relaxers may have dissuaded providers from using baclofen for AUD, but we consider it a reasonable alternative when FDA-approved treatments fail.
Because baclofen is primarily eliminated by the kidneys, it may be safe for people with cirrhosis or severe liver disease.12 Baseline renal labs should be performed before administering baclofen and a negative pregnancy test obtained for women of childbearing age.
Ondansetron is a serotonin receptor type 3 (5-HT3) antagonist that has shown promising results for AUD.3,7 Research suggests that 5-HT3 receptors are an action site for alcohol in the brain and are thought to play a role in its rewarding effects.7 Ondansetron may be more effective for early-onset alcoholism (EOA) than late-onset alcoholism (LOA).14,15 EOA (age ≤25) is characterized by strong family history of AUD and prominent antisocial traits. A randomized double-blind, placebo-controlled trial (n = 271) reported that ondansetron, 4 mcg/kg twice daily, was superior to placebo in reducing number of drinks per day, increasing days abstinent, and reducing cravings in patients with EOA.14 Among persons with EOA, ondansetron, 16 mcg/kg twice daily, significantly reduced the severity of symptoms of fatigue, confusion, and overall mood disturbance such as depression, anxiety, and hostility compared with placebo in an RCT (n = 321).15 The lowest available oral dosage of ondansetron is 4 mg tablets or 4 mg/5 mL solution. We have used 4 mg twice daily for patients who have failed naltrexone and acamprosate or when these agents are contraindicated.
Common side effects of ondansetron include constipation, diarrhea, elevated liver enzymes, tachycardia, headache, and fatigue. Contraindications include congenital long QT syndrome, QTc prolongation risk, or significant hepatic impairment. We suggest evaluating baseline electrocardiogram and liver function tests. Women should undergo a pregnancy test before receiving medications.
Gabapentin is an anticonvulsant that is FDA-approved for treating epilepsy and postherpetic neuralgia. Gabapentin is related structurally to GABA and may potentiate central nervous system GABA activity, inhibit glutamate activity, and reduce norepinephrine and dopamine release.16 Gabapentin is thought to balance the GABA/glutamate dysregulation found in early alcohol abstinence and reduce risk for alcohol relapse.16A randomized, double-blind, placebo-controlled trial (N = 60) demonstrated that gabapentin, 600 mg/d, significantly reduced number of drinks per day and heavy drinking days and increased days of abstinence compared with placebo over 28 days.17 Another double-blind, randomized, placebo-controlled trial of 150 people used gabapentin, 900 or 1,800 mg/d; there was a linear dose response for increased days abstinent and no heavy drinking days in favor of gabapentin.18
An RCT (N = 150) evaluated adding gabapentin, up to 1200 mg/d, to naltrexone, 50 mg/d, vs naltrexone with placebo or double placebo over 6 weeks of treatment. The combined gabapentin-naltrexone group outperformed the other 2 groups on time to heavy drinking, number of heavy drinking days, and number of drinks per day. Gabapentin’s positive effects on sleep may have mediated some of its beneficial effects.29 In an open-label pilot study, gabapentin was more effective than trazodone for insomnia during early alcohol abstinence.30 Of note, gabapentin is a safe alternative to benzodiazepines for alcohol detoxification in patients with severe hepatic disease or those at risk of interacting with alcohol (eg, outpatients at high risk to drink during detoxification).31 Gabapentin, 400 mg/d to 1,600 mg/d, generally is safe and well tolerated and has some support for improving cravings, reducing alcohol consumption, delaying relapse, and improving sleep in patients with AUD.
Side effects of gabapentin include daytime sedation, dizziness, ataxia, fatigue, and dyspepsia. Using 3 divided doses might enhance tolerability. To reduce daytime sedation, we recommend administering most of the dose at night, which also may relieve insomnia. Gabapentin is excreted through the kidney; baseline renal function tests should be performed before initiating treatment, because the dosage might need to be adjusted in people with renal insufficiency.
Bottom Line
FDA-approved (acamprosate, naltrexone, and disulfiram) and off-label (baclofen, gabapentin, ondansetron, and topiramate) agents can help patients with alcohol use disorder achieve abstinence, reduce heavy drinking days, prevent relapse, and maintain sobriety. Research supports the use of pharmacotherapy combined with psychosocial modalities, such as 12-step programs, motivational interviewing, and cognitive-behavioral therapy.
Related Resources
- National Institute on Drug Abuse. Principles of drug addiction treatment: A research-based guide (third edition). www.drugabuse.gov/publications/principles-drug-addiction-treatment-research-based-guide-third-edition/evidence-based-approaches-to-drug-addiction-treatment/pharmacotherapi-1.
- Substance Abuse and Mental Health Services Administration. Incorporating alcohol pharmacotherapies into medical practice. http://162.99.3.213/products/manuals/tips/pdf/TIP49.pdf.
- Pettinati HM, Mattson ME. Medical management treatment manual: A clinical guide for researchers and clinicians providing pharmacotherapy for alcohol dependence. http://pubs.niaaa.nih.gov/publications/MedicalManual/MMManual.pdf.
Drug Brand Names
Acamprosate • Campral Naltrexone • Vivitrol, ReVia
Baclofen • Lioresal Ondansetron • Zofran
Disulfiram • Antabuse Topiramate • Topamax
Gabapentin • Neurontin Trazodone • Desyrel, Oleptro
Metronidazole • Flagyl
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Historically, alcohol use disorder (AUD; classified as alcohol abuse or dependence in DSM-IV-TR) has been treated with psychosocial therapies, but many patients treated this way relapse into heavy drinking patterns and are unable to sustain sobriety (Box 11). Although vital for treating AUD, psychosocial methods have, to date, a modest success rate. Research has demonstrated that combining pharmacotherapy with psychosocial programs is effective for treating AUD.2
Patients and clinicians might associate AUD medications with so-called aversion therapy because, for many years, the only treatment was disulfiram, which causes unpleasant physical effects when consumed with alcohol. However, newer medications help patients maintain abstinence by targeting brain neurotransmitters relevant to addiction neurocircuitry, such as dopamine, serotonin, ϒ-aminobutyric acid (GABA), glutamate, and opioid.3 These medications may help patients with AUD achieve sobriety, avoid relapse, decrease heavy drinking days, and delay time to recurrent drinking.
In this article, we review FDA-approved medications (Table 1)4-6 and off-label agents (Table 2)3,7-18 and provide recommendations for treating patients with AUD (Box 2).19-21
FDA-approved treatments
Naltrexone is an opiate antagonist that blocks the mu receptor and is believed to interrupt the dopamine reward pathway in the brain for alcohol. A meta-analysis of 2,861 patients in 24 combined randomized controlled trials (RCTs) demonstrated naltrexone to be an effective short-term (12 weeks) treatment for alcoholism, significantly decreasing relapses.22 The large multisite COMBINE study (N = 1,383) showed that naltrexone, 100 mg/d, and medical treatment without behavioral treatment over 16 weeks was more effective than placebo in increasing percentage of days abstinent (80.6% vs 75.1%, respectively) and reducing the percentage of patients experiencing heavy drinking days (66.2% vs 73.1%, respectively).2 Patients who have a family history of AUD or strong cravings, or both, may benefit most from naltrexone.3,7,23 Despite evidence of the effectiveness of naltrexone for AUD, not all studies have yielded positive results.24
Common side effects of naltrexone, if present, appear early in treatment and include GI upset (eg, nausea, vomiting, abdominal pain), headache, and fatigue.2,3,22,23 Hepatotoxicity has been reported with dosages of 100 to 300 mg/d, but lab values typically normalize when naltrexone is discontinued.2,3,23 Monitor markers of liver function including ϒ-glutamyltransferase, aspartate aminotransferase, alanine aminotransferase, and bilirubin before and during naltrexone treatment (we check patients 1 to 3 months after starting treatment and yearly thereafter). Obtain a negative urine drug screen for opioids before administering naltrexone, because if opioids were consumed recently naltrexone could precipitate withdrawal. Because of the unknown teratogenicity of naltrexone, women of childbearing age should undergo pregnancy testing before and periodically during naltrexone therapy.
Naltrexone also is available in a once-monthly, 380-mg injectable formulation. Studies show that, similar to its oral counterpart, injectable naltrexone effectively reduces heavy drinking days and number of drinks a day compared with placebo.25,26 Advantages of injectable naltrexone are its extended steady release of medication and its efficacy for patients who do not adhere to oral dosing.7,27 Side effects are similar to oral naltrexone, except for injection site reactions and pain.
Contraindications to naltrexone include current opioid use because its antagonistic effects on opioid receptors render opioid analgesia ineffective. Patients who have used opioids within 7 to 10 days or who may be surreptitiously using opioids should not take naltrexone because it may cause opioid withdrawal. Some patients may try to override the opioid receptor blockade of naltrexone with higher opioid doses, which could result in overdose.
Naltrexone is approved for treating opioid use disorder and may be useful for persons with comorbid opioid use disorder and AUD, if the patient has been adequately detoxified from opioids and intends to abstain from these drugs. Patients who have extensive liver damage secondary to acute hepatitis or uncompensated cirrhosis would not be good candidates for naltrexone because of a risk of hepatotoxicity.2,3,22
Because of ease of dosing, we recommend naltrexone as a first-line treatment for AUD, unless the patient requires opioids or has severe liver disease. We recommend increasing naltrexone from 50 mg to 100 mg before switching to acamprosate, based on European studies.
Acamprosate is a glutamate antagonist that is thought to modulate overactive glutamatergic brain activity that occurs after stopping chronic heavy alcohol use. In a meta-analysis of 17 studies (N = 4,087), continuous abstinence rates at 6 months were significantly higher in acamprosate-treated patients (36.1%) than in patients receiving placebo (23.4%).28 In a review of European studies, acamprosate benefited patients who have increased anxiety, physiological dependence, negative family history of AUD, and late age of onset (age >25) of alcohol dependence.7 However, in the COMBINE trial acamprosate was no more effective than placebo.2
We consider acamprosate an effective option for patients who do not respond to naltrexone or have a contraindication. Dosages of 333 mg to 666 mg, 3 times a day, are recommended, although dosages up to 3 g/d have been studied; titration is not required.2,7,28 We recommend advising patients to continue treatment even if they relapse, because these medications may mitigate relapse severity. Adherence to multiple daily doses can be problematic for some patients, but pairing medications with meals or bedtime may improve adherence.
Diarrhea is the most common side effect of acamprosate; nervousness, fatigue, insomnia, and depression have been reported with high dosages.2,3,7,28 Acamprosate is excreted through the kidney and is safe for patients with liver disorders such as acute hepatitis or cirrhosis. The drug is contraindicated in patients with acute or chronic renal failure with creatinine clearance <30 mL/min; those with less severe renal insufficiency might need a lower dosage. Obtain baseline renal function before starting acamprosate; women of childbearing age should undergo a pregnancy test.
Disulfiram inhibits alcohol metabolism, resulting in acetaldehyde accumulation, which causes unpleasant physical effects such as nausea, vomiting, and hypotension. This creates a negative rather than a positive experience with drinking. A US Veterans Administration Cooperative Study randomized 605 participants to riboflavin, disulfiram, 1 mg/d (an inactive dose), or disulfiram, 250 mg/d (standard dose). There was no difference in percentage of patients remaining abstinent or time to first drink.8 Participants receiving disulfiram, 250 mg/d, had fewer drinking days after relapse compared with the other groups.8
Adverse physical effects produced when disulfiram and alcohol interact include tremor, diaphoresis, unstable blood pressure, and severe diarrhea and vomiting. Disulfiram can cause medically serious reactions in a small percentage of patients, especially those with significant medical comorbidity or advanced age. Patients with severe hypertension, diabetes mellitus, heart disease, a history of stroke, peripheral neuropathy, epilepsy, or renal or hepatic insufficiency should not use disulfiram.3 Patients taking disulfiram should avoid casual exposures to food, aftershave, mouthwash, and hand sanitizer that might contain alcohol. Disulfiram has no significant effect on alcohol craving. Social support to help oversee dosing may enhance adherence.7
Off-label medications
Topiramate is FDA-approved to treat migraine headaches and some seizure disorders. Topiramate facilitates GABA-mediated neuronal inhibition and antagonizes certain glutamate receptor subtypes. In an RCT (N = 150), topiramate, up to 300 mg/d, was more effective than placebo at reducing heavy drinking days and number of drinks per day, increasing days abstinent, and alleviating cravings.9 In a 12-week, double-blind RCT (N = 150), topiramate increased “safe drinking”—defined as ≤1 standard drink per day for women and ≤2 per day for men—vs placebo.10 Dosages were 75 mg to 300 mg/d in twice daily divided doses. Dosing starts at 25 mg/d and increases by 25 to 50 mg a day at weekly intervals. We recommend reserving topiramate for persons who do not respond to or cannot tolerate naltrexone and acamprosate because of the slow titration needed to prevent side effects. Although not studied, it may seem that topiramate’s antiepileptic actions could prevent seizures during alcohol withdrawal, but the protracted titration would limit its utility.
Side effects of topiramate include impaired memory and concentration, paresthesia, and anorexia and are more likely to present during rapid titration or with a high dosage.7,8 Rare reports of spontaneous myopia, angle-closure glaucoma, increased intraocular pressure, ocular pain, and blurry vision have been reported, but these complications often resolve with discontinuation of topiramate.7,8
Topiramate primarily is excreted through the kidney, and its action in the renal tubules can lead to metabolic acidosis or nephrolithiasis.8 Relative contraindications include acute or chronic kidney disease, including kidney stones. Consider slower titration and a 50% reduction in dosing if creatinine clearance is <70 mL/min. Obtain renal function tests before starting topiramate and consider monitoring serum bicarbonate for metabolic acidosis (we test at 3 and 6 months, then every 6 months). Because of teratogenic effects of topiramate (eg, cleft lip and palate), rule out pregnancy in all women of childbearing age.
Baclofen is a GABAb receptor agonist that is FDA approved for treating spasticity. Because GABA transmission is down-regulated in chronic AUD, it is a commonly targeted neurotransmitter when developing medications for AUD. GABAa receptors are fast-acting inhibitory ion channels, and its agonists (eg, benzodiazepines) have a significant abuse and cross-addiction liability. GABAb receptors, however, are slow-acting through a complex cascade of intracellular signals, and therefore GABAb agonists such as baclofen have been studied for treating addiction.
In a randomized double-blind, placebo-controlled trial (N = 39), baclofen was superior to placebo in suppressing obsessive aspects of cravings and decreasing state anxiety.11 Baclofen, 10 mg 3 times daily, in another randomized double-blind, placebo-controlled trial (n = 42) reduced the number of drinks per day by 53% vs placebo; 20 mg 3 times a day resulted in a 68% reduction in drinks per day vs placebo.12 However, a placebo-controlled RCT (n = 80) reported that baclofen, 10 mg 3 times daily, was not superior to placebo for primary outcomes related to alcohol consumption, although it did significantly decrease cravings and anxiety among persons with AUD.13 Evidence suggests that baclofen might be effective for promoting abstinence, reducing the risk of relapse, and alleviating cravings and anxiety in persons with AUD, although further investigation is needed.
In studies for AUD, the side-effect profile for baclofen was relatively benign.11-13 Nausea, fatigue, sleepiness, vertigo, and abdominal pain were reported; overall, baclofen was found to be safe and to have no abuse liability.7,10,12 The addictive potential of other muscle relaxers may have dissuaded providers from using baclofen for AUD, but we consider it a reasonable alternative when FDA-approved treatments fail.
Because baclofen is primarily eliminated by the kidneys, it may be safe for people with cirrhosis or severe liver disease.12 Baseline renal labs should be performed before administering baclofen and a negative pregnancy test obtained for women of childbearing age.
Ondansetron is a serotonin receptor type 3 (5-HT3) antagonist that has shown promising results for AUD.3,7 Research suggests that 5-HT3 receptors are an action site for alcohol in the brain and are thought to play a role in its rewarding effects.7 Ondansetron may be more effective for early-onset alcoholism (EOA) than late-onset alcoholism (LOA).14,15 EOA (age ≤25) is characterized by strong family history of AUD and prominent antisocial traits. A randomized double-blind, placebo-controlled trial (n = 271) reported that ondansetron, 4 mcg/kg twice daily, was superior to placebo in reducing number of drinks per day, increasing days abstinent, and reducing cravings in patients with EOA.14 Among persons with EOA, ondansetron, 16 mcg/kg twice daily, significantly reduced the severity of symptoms of fatigue, confusion, and overall mood disturbance such as depression, anxiety, and hostility compared with placebo in an RCT (n = 321).15 The lowest available oral dosage of ondansetron is 4 mg tablets or 4 mg/5 mL solution. We have used 4 mg twice daily for patients who have failed naltrexone and acamprosate or when these agents are contraindicated.
Common side effects of ondansetron include constipation, diarrhea, elevated liver enzymes, tachycardia, headache, and fatigue. Contraindications include congenital long QT syndrome, QTc prolongation risk, or significant hepatic impairment. We suggest evaluating baseline electrocardiogram and liver function tests. Women should undergo a pregnancy test before receiving medications.
Gabapentin is an anticonvulsant that is FDA-approved for treating epilepsy and postherpetic neuralgia. Gabapentin is related structurally to GABA and may potentiate central nervous system GABA activity, inhibit glutamate activity, and reduce norepinephrine and dopamine release.16 Gabapentin is thought to balance the GABA/glutamate dysregulation found in early alcohol abstinence and reduce risk for alcohol relapse.16A randomized, double-blind, placebo-controlled trial (N = 60) demonstrated that gabapentin, 600 mg/d, significantly reduced number of drinks per day and heavy drinking days and increased days of abstinence compared with placebo over 28 days.17 Another double-blind, randomized, placebo-controlled trial of 150 people used gabapentin, 900 or 1,800 mg/d; there was a linear dose response for increased days abstinent and no heavy drinking days in favor of gabapentin.18
An RCT (N = 150) evaluated adding gabapentin, up to 1200 mg/d, to naltrexone, 50 mg/d, vs naltrexone with placebo or double placebo over 6 weeks of treatment. The combined gabapentin-naltrexone group outperformed the other 2 groups on time to heavy drinking, number of heavy drinking days, and number of drinks per day. Gabapentin’s positive effects on sleep may have mediated some of its beneficial effects.29 In an open-label pilot study, gabapentin was more effective than trazodone for insomnia during early alcohol abstinence.30 Of note, gabapentin is a safe alternative to benzodiazepines for alcohol detoxification in patients with severe hepatic disease or those at risk of interacting with alcohol (eg, outpatients at high risk to drink during detoxification).31 Gabapentin, 400 mg/d to 1,600 mg/d, generally is safe and well tolerated and has some support for improving cravings, reducing alcohol consumption, delaying relapse, and improving sleep in patients with AUD.
Side effects of gabapentin include daytime sedation, dizziness, ataxia, fatigue, and dyspepsia. Using 3 divided doses might enhance tolerability. To reduce daytime sedation, we recommend administering most of the dose at night, which also may relieve insomnia. Gabapentin is excreted through the kidney; baseline renal function tests should be performed before initiating treatment, because the dosage might need to be adjusted in people with renal insufficiency.
Bottom Line
FDA-approved (acamprosate, naltrexone, and disulfiram) and off-label (baclofen, gabapentin, ondansetron, and topiramate) agents can help patients with alcohol use disorder achieve abstinence, reduce heavy drinking days, prevent relapse, and maintain sobriety. Research supports the use of pharmacotherapy combined with psychosocial modalities, such as 12-step programs, motivational interviewing, and cognitive-behavioral therapy.
Related Resources
- National Institute on Drug Abuse. Principles of drug addiction treatment: A research-based guide (third edition). www.drugabuse.gov/publications/principles-drug-addiction-treatment-research-based-guide-third-edition/evidence-based-approaches-to-drug-addiction-treatment/pharmacotherapi-1.
- Substance Abuse and Mental Health Services Administration. Incorporating alcohol pharmacotherapies into medical practice. http://162.99.3.213/products/manuals/tips/pdf/TIP49.pdf.
- Pettinati HM, Mattson ME. Medical management treatment manual: A clinical guide for researchers and clinicians providing pharmacotherapy for alcohol dependence. http://pubs.niaaa.nih.gov/publications/MedicalManual/MMManual.pdf.
Drug Brand Names
Acamprosate • Campral Naltrexone • Vivitrol, ReVia
Baclofen • Lioresal Ondansetron • Zofran
Disulfiram • Antabuse Topiramate • Topamax
Gabapentin • Neurontin Trazodone • Desyrel, Oleptro
Metronidazole • Flagyl
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. World Health Organization. Alcohol fact sheet. http://www.who.int/mediacentre/factsheets/fs349/en/index.html. Published February 2011. Accessed April 30, 2013.
2. Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence. The COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003-2017.
3. Mann K. Pharmacotherapy of alcohol dependence a review of the clinical data. CNS Drugs. 2004;18(8):485-504.
4. ReVia [package insert]. Pomona, NY: Barr Pharmaceuticals; 2009.
5. Campral [package insert]. St. Louis, MO: Forest Pharmaceuticals; 2004.
6. Antabuse [package insert]. Pomona, NY: Barr Pharmaceuticals; 2010.
7. Johnson BA. Update on neuropharmacological treatments for alcoholism: scientific basis and clinical findings. Biochem Pharmacol. 2008;75(1):34-56.
8. Fuller RK, Branchey L, Brightwell DR, et al. Disulfiram treatment of alcoholism: a Veterans Administration cooperative study. JAMA. 1986;256(11):1449-1454.
9. Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):
1677-1685.
10. Ma JZ, Ait-Daoud N, Johnson BA. Topiramate reduces the harm of excessive drinking: implications for public health and primary care. Addiction. 2006;101:1561-1568.
11. Addolorato G, Caputo F, Capristo E, et al. Baclofen efficacy in reducing alcohol craving and intake: a preliminary double-blind randomized controlled study. Alcohol Alcohol. 2002;37(5):504-508.
12. Addolorato G, Leggio L, Ferrulli A, et al. Dose-response effect of baclofen in reducing daily alcohol intake in alcohol dependence: secondary analysis of a randomized, double-blind, placebo-controlled trial. Alcohol Alcohol. 2011;46(3):312-317.
13. Garbutt JC, Kampov-Polevoy AB, Gallop R, et al. Efficacy and safety of baclofen for alcohol dependence: a randomized, double-blind placebo-controlled trial. Alcohol Clin Exp Res. 2010;34(11):1849-1857.
14. Johnson BA, Roache JD, Javors MA, et al. Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: a randomized controlled trial. JAMA. 2000;284(8):963-971.
15. Johnson BA, Ait-Daoud N, Ma JZ, et al. Ondansetron reduces mood disturbance among biologically predisposed, alcohol-dependent individuals. Alcohol Clin Exp Res. 2003;27(11):1773-1779.
16. Myrick H, Anton R, Voronin K, et al. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res. 2007;31(2):221-227.
17. Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68:1691-1700.
18. Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial [published online November 4, 2013]. JAMA Intern Med. doi: 10.1001/jamainternmed.2013.11950.
19. The Management of Substance Use Disorders Working Group. VA/DoD clinical practice guideline for management of substance use disorders (SUD). http://www.healthquality.va.gov/sud/sud_full_601f.pdf. Published August 2009. Accessed November 22, 2013.
20. Mark TL, Kranzler HR, Song X, et al. Physicians’ opinions about medication to treat alcoholism. Addiction. 2003;98(5):617-626.
21. Thomas CP, Wallack SS, Lee S, et al. Research to practice: adoption of naltrexone in alcoholism treatment. J Subst Abuse Treat. 2003;24(1):1-11.
22. Srisurapanont M, Jarusuraisin N. Naltrexone for the treatment of alcoholism: a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2005;8:267-280.
23. Anton RF. Naltrexone for the management of alcohol dependence. N Engl J Med. 2008;359(7):715-721.
24. Gueorguieva R, Wu R, Pittman B, et al. New insights into the efficacy of naltrexone based on trajectory-based reanalysis of two negative clinical trials. Biol Psychiatry. 2007;61(11): 1290-1295.
25. Lapham S, Forman R, Alexander M, et al. The effects of extended-release naltrexone on holiday drinking in alcohol-dependent patients. J Subst Abuse. 2009;36(1):1-6.
26. Ciraulo DA, Dong Q, Silverman BL, et al. Early treatment response in alcohol dependence with extended-release naltrexone. J Clin Psychiatry. 2008;69(2):190-195.
27. Mark TL, Montejano LB, Kranzler HR, et al. Comparison of healthcare utilization among patients treated with alcoholism medications. Am J Managed Care. 2010;16(12): 879-888.
28. Mann K, Lehert P, Morgan MY. The efficacy of acamprosate in the maintenance of abstinence in alcohol-dependent individuals: results of a meta-analysis. Alcohol Clin Exp Res. 2004;28(1):51-63.
29. Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry. 2011;168(7):709-717.
30. Karam-Hage M, Brower KJ. Open pilot study of gabapentin versus trazodone to treat insomnia in alcoholic outpatients. Psychiatry Clin Neurosci. 2003;57(5):542-544.
31. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
1. World Health Organization. Alcohol fact sheet. http://www.who.int/mediacentre/factsheets/fs349/en/index.html. Published February 2011. Accessed April 30, 2013.
2. Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence. The COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003-2017.
3. Mann K. Pharmacotherapy of alcohol dependence a review of the clinical data. CNS Drugs. 2004;18(8):485-504.
4. ReVia [package insert]. Pomona, NY: Barr Pharmaceuticals; 2009.
5. Campral [package insert]. St. Louis, MO: Forest Pharmaceuticals; 2004.
6. Antabuse [package insert]. Pomona, NY: Barr Pharmaceuticals; 2010.
7. Johnson BA. Update on neuropharmacological treatments for alcoholism: scientific basis and clinical findings. Biochem Pharmacol. 2008;75(1):34-56.
8. Fuller RK, Branchey L, Brightwell DR, et al. Disulfiram treatment of alcoholism: a Veterans Administration cooperative study. JAMA. 1986;256(11):1449-1454.
9. Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):
1677-1685.
10. Ma JZ, Ait-Daoud N, Johnson BA. Topiramate reduces the harm of excessive drinking: implications for public health and primary care. Addiction. 2006;101:1561-1568.
11. Addolorato G, Caputo F, Capristo E, et al. Baclofen efficacy in reducing alcohol craving and intake: a preliminary double-blind randomized controlled study. Alcohol Alcohol. 2002;37(5):504-508.
12. Addolorato G, Leggio L, Ferrulli A, et al. Dose-response effect of baclofen in reducing daily alcohol intake in alcohol dependence: secondary analysis of a randomized, double-blind, placebo-controlled trial. Alcohol Alcohol. 2011;46(3):312-317.
13. Garbutt JC, Kampov-Polevoy AB, Gallop R, et al. Efficacy and safety of baclofen for alcohol dependence: a randomized, double-blind placebo-controlled trial. Alcohol Clin Exp Res. 2010;34(11):1849-1857.
14. Johnson BA, Roache JD, Javors MA, et al. Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: a randomized controlled trial. JAMA. 2000;284(8):963-971.
15. Johnson BA, Ait-Daoud N, Ma JZ, et al. Ondansetron reduces mood disturbance among biologically predisposed, alcohol-dependent individuals. Alcohol Clin Exp Res. 2003;27(11):1773-1779.
16. Myrick H, Anton R, Voronin K, et al. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res. 2007;31(2):221-227.
17. Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68:1691-1700.
18. Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial [published online November 4, 2013]. JAMA Intern Med. doi: 10.1001/jamainternmed.2013.11950.
19. The Management of Substance Use Disorders Working Group. VA/DoD clinical practice guideline for management of substance use disorders (SUD). http://www.healthquality.va.gov/sud/sud_full_601f.pdf. Published August 2009. Accessed November 22, 2013.
20. Mark TL, Kranzler HR, Song X, et al. Physicians’ opinions about medication to treat alcoholism. Addiction. 2003;98(5):617-626.
21. Thomas CP, Wallack SS, Lee S, et al. Research to practice: adoption of naltrexone in alcoholism treatment. J Subst Abuse Treat. 2003;24(1):1-11.
22. Srisurapanont M, Jarusuraisin N. Naltrexone for the treatment of alcoholism: a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2005;8:267-280.
23. Anton RF. Naltrexone for the management of alcohol dependence. N Engl J Med. 2008;359(7):715-721.
24. Gueorguieva R, Wu R, Pittman B, et al. New insights into the efficacy of naltrexone based on trajectory-based reanalysis of two negative clinical trials. Biol Psychiatry. 2007;61(11): 1290-1295.
25. Lapham S, Forman R, Alexander M, et al. The effects of extended-release naltrexone on holiday drinking in alcohol-dependent patients. J Subst Abuse. 2009;36(1):1-6.
26. Ciraulo DA, Dong Q, Silverman BL, et al. Early treatment response in alcohol dependence with extended-release naltrexone. J Clin Psychiatry. 2008;69(2):190-195.
27. Mark TL, Montejano LB, Kranzler HR, et al. Comparison of healthcare utilization among patients treated with alcoholism medications. Am J Managed Care. 2010;16(12): 879-888.
28. Mann K, Lehert P, Morgan MY. The efficacy of acamprosate in the maintenance of abstinence in alcohol-dependent individuals: results of a meta-analysis. Alcohol Clin Exp Res. 2004;28(1):51-63.
29. Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry. 2011;168(7):709-717.
30. Karam-Hage M, Brower KJ. Open pilot study of gabapentin versus trazodone to treat insomnia in alcoholic outpatients. Psychiatry Clin Neurosci. 2003;57(5):542-544.
31. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
Performing capacity evaluations: What’s expected from your consult
One of the most common reasons medical colleagues seek consultation with a psychiatrist is to address the question of capacity. Indeed, this referral question often is posed as, “Is the patient competent?”
This referral question is incomplete and incorrectly phrased. The question should include the domain in which capacity is being questioned—for example, “Is the patient competent to refuse surgery?” Specifically identifying the area in which competency is questioned is necessary because a person might be competent in one area and incompetent in another (Box 1).
The question of competency should be modified as follows: “Does the patient have capacity to refuse surgery?” Competency is the degree of mental soundness necessary to make decisions about a specific issue or to carry out a specific act. Capacity is a person’s ability to make an informed decision. A determination of competency is a judicial finding made by the court. A physician can opine about a patient’s capacity, but cannot determine competency.
Adults are presumed to have capacity unless determined otherwise by the court. A person who lacks capacity to make an informed decision or give consent might need to be referred for a competency hearing or have a guardian appointed. Psychiatrists often are called on to provide an opinion to the court regarding a person’s capacity. Psychiatrists are particularly skilled at accessing a person’s mental status and gauging its potential for interfering with specific areas of functioning, but, in fact, any physician can make a determination of capacity.1
In this article, I:
- outline the components of a capacity evaluation
- describe the tools used in the determination of capacity
- review the typical features of patients and psychiatrists who perform capacity evaluations.
What constitutes a capacity evaluation?
The components of a capacity evaluation are comprehension, free choice, and reliability.
Comprehension refers to a patient’s factual understanding of his (her) medical condition—for example, including the risks and benefits of treatment and reasonable alternatives. The patient should show an understanding of 1) the situation as it relates to his condition, and 2) the consequences of his decisions. He also should demonstrate a rational manipulation of the information presented, applying a coherent and logical thought process to analyze possible courses of action.2
To determine if the patient has the requisite knowledge regarding his condition, the physician must be familiar with the patient’s clinical status. This might require consultation with the treating physician. Communication is a key component of capacity evaluations. Barriers to good communication can lead to the evaluating physician’s perception that the patient lacks capacity. If a patient does not understand his condition or the proposed treatments, the psychiatrist should educate him. It might be useful to arrange a meeting with the treating physician to facilitate communication.
Free choice. The patient’s decision to accept or reject a proposed treatment should be voluntary and free of coercion. In assessing a patient’s capacity, the psychiatrist should determine whether choices have been rendered impossible because of unrealistic fears or expectations about treatment, or because of impaired mental processes.
Reliability refers to a patient’s ability to provide a consistent choice over time. A patient who vacillates or is inconsistent does not have capacity to make decisions.
Features of patients referred for evaluation, and their evaluators
The most common reason for a capacity evaluation is a patient’s refusal of medical treatment. Between 3% and 25% of requests for psychiatric consultation in hospital settings involve questions about patients’ competence to make a treatment-related decision.3 Approximately 25% of adult medicine inpatients lack capacity for medical decision-making.4
Decision-making capacity is a functional evaluation. Decision-making capacity does not relate specifically to a person’s psychiatric diagnosis. In other words, the presence of a mental disorder does not render a person incapable of making decisions. However, people with Alzheimer’s disease or dementia have a high rate of impaired capacity for making treatment decisions.
Schizophrenia has been found to have the highest rate of impaired decision-making among psychiatric disorders; depression is second and bipolar disorder, third. The strongest predictor of incapacity in psychiatric patients is lack of insight.5 Positive symptoms, negative symptoms, severity of symptoms, involuntary admission, lack of insight, and treatment refusal were strong predictors of incapacity in a sample of psychiatric patients.6
The neuronal basis of decision-making is unknown. Studies have implicated functioning of the medial and lateral prefrontal cortex as an important correlate of decision-making capacity.7 As a result of these findings, a brain-based criterion could be added to the conceptual criteria of capacity. The specific neuropsychological components necessary for decision-making capacity are unknown. Some studies suggest that poor executive functioning and limited learning ability correlate with impaired decision-making capacity.8 Little is known about the relationship between emotion and capacity. Supady et al demonstrated that higher cognitive empathy and good emotion recognition were associated with increased decision-making capacity and higher rates of refusal to give informed consent.9
Physician bias has been identified in capacity evaluations. See Box 2.4,10-12
Tools used in capacity evaluations
Most capacity evaluations are conducted by clinical interview (Box 3). The reliability of physicians’ unstructured judgments of capacity has been poor.13 In a study of 5 physicians who made a determination of capacity after watching a videotape of capacity assessments, the rate of agreement among the subjects was no better than that of chance.14
There is no specific, simple, quick test to assess capacity.
Folstein Mini-Mental State Examination. The MMSE has not been found to be predictive of decision-making capacity. It has been found to correlate with clinical judgments of incapacity, and may be used to identify patients at the high and low ends of the range of capacity, especially among older persons who exhibit cognitive impairment.15 Patients who have severe dementia (MMSE score <16) have a high likelihood of being unable to consent to treatment.16
MacArthur Competence Assessment Tool-Treatment. The MacCAT-T is a structured interviewing tool used to evaluate a patient’s decision-making ability. It is the most commonly used screening tool to evaluate decision-making capacity. Advantages of the MacCAT-T include a higher inter-rater agreement and—unlike other assessment instruments—its ability to incorporate information specific to a patient’s decision-making situation.17 The MacCAT-T requires training and experience to administer.
Bottom Line
Physicians make decisions about a patient’s decision-making capacity. Courts determine competence by a formal judicial proceeding. The psychiatric consultant’s role in capacity evaluations is to determine if the patient 1) possesses the requisite knowledge about the specific referral issue and 2) demonstrates a voluntary and reliable decision.
Related Resources
- The MacArthur Treatment Competence Study. www.macarthur.virginia.edu/treatment.html.
- Resnick P, Sorrentino R: Forensic considerations (chapter. 8). In: Psychosomatic medicine. Blumenfield M, Strain JJ, eds. Baltimore, MD: Lippincott Williams & Wilkins; 2006:91-106.
- Appelbaum PS. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357(18):1834-1840.
Disclosure
Dr. Sorrentino reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Grisson T, Appelbaum PS. Assessing competence to consent to treatment—a guide for physicians and other health professionals. New York, NY: Oxford University Press; 1998.
2. Cohen LM, McCue JD, Green GM. Do clinical and formal assessments of capacity of patients in the intensive care unit to make decisions agree? Arch Intern Med. 1993;153(21): 2841-2845.
3. Farnsworth MG. Competency evaluations in a general hospital. Psychosomatics. 1990;31(1):60-66.
4. Sessums LL, Zembrzuska H, Jackson JL. Does this patient have medical decision-making capacity? JAMA. 2011; 306(4):420-427.
5. Cairns R, Maddock C, Buchanan A, et al. Prevalence and predictors of mental incapacity in psychiatric in-patients. Br J Psychiatry. 2005;187:379-385.
6. Candia PC, Barba AC. Mental capacity and consent to treatment in psychiatric patients: the state of the research. Curr Opin Psychiatry. 2011;24(5):442-446.
7. Duncan J. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23(10):475-483.
8. Mandarelli G, Parmigiani G, Tarsitani L, et al. The relationship between executive functions and capacity to consent to treatment in acute psychiatric hospitalization. J Empir Res Hum Res Ethics. 2012;7(5):63-70.
9. Supady A, Voelkel A, Witzel J, et al. How is informed consent related to emotions and empathy? An exploratory neuroethical investigation. J Med Ethics. 2011;37(5):311-317.
10. Jeste DV, Depp CA, Palmer BW. Magnitude of impairment in decisional capacity in people with schizophrenia compared to normal subjects: an overview. Schizophr Bull. 2006;32(1):121-128.
11. Feldman-Stewart D, Brundage MD. Challenges for designing and implementing decision aids. Patient Educ Couns. 2004;54(3):265-273.
12. Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44(5):681-692.
13. Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007; 357(18):1834-1840.
14. Marson DC, McIntruff B, Hawkins L, et al. Consistency of physician judgments of capacity to consent to mild Alzheimer’s disease. J Am Geriatr Soc. 1997;45(4):453-457.
15. Kim SY, Caine ED. Utility and limits of the mini mental status examination in evaluating consent capacity in Alzheimer’s disease. Psychiatr Serv. 2002;53(10):1322-1324.
16. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198.
17. Grisso T, Appelbaum PS. MacArthur competence assessment tool for treatment (MacCAT-T). Sarasota, FL: Professional Resource Press; 1998.
One of the most common reasons medical colleagues seek consultation with a psychiatrist is to address the question of capacity. Indeed, this referral question often is posed as, “Is the patient competent?”
This referral question is incomplete and incorrectly phrased. The question should include the domain in which capacity is being questioned—for example, “Is the patient competent to refuse surgery?” Specifically identifying the area in which competency is questioned is necessary because a person might be competent in one area and incompetent in another (Box 1).
The question of competency should be modified as follows: “Does the patient have capacity to refuse surgery?” Competency is the degree of mental soundness necessary to make decisions about a specific issue or to carry out a specific act. Capacity is a person’s ability to make an informed decision. A determination of competency is a judicial finding made by the court. A physician can opine about a patient’s capacity, but cannot determine competency.
Adults are presumed to have capacity unless determined otherwise by the court. A person who lacks capacity to make an informed decision or give consent might need to be referred for a competency hearing or have a guardian appointed. Psychiatrists often are called on to provide an opinion to the court regarding a person’s capacity. Psychiatrists are particularly skilled at accessing a person’s mental status and gauging its potential for interfering with specific areas of functioning, but, in fact, any physician can make a determination of capacity.1
In this article, I:
- outline the components of a capacity evaluation
- describe the tools used in the determination of capacity
- review the typical features of patients and psychiatrists who perform capacity evaluations.
What constitutes a capacity evaluation?
The components of a capacity evaluation are comprehension, free choice, and reliability.
Comprehension refers to a patient’s factual understanding of his (her) medical condition—for example, including the risks and benefits of treatment and reasonable alternatives. The patient should show an understanding of 1) the situation as it relates to his condition, and 2) the consequences of his decisions. He also should demonstrate a rational manipulation of the information presented, applying a coherent and logical thought process to analyze possible courses of action.2
To determine if the patient has the requisite knowledge regarding his condition, the physician must be familiar with the patient’s clinical status. This might require consultation with the treating physician. Communication is a key component of capacity evaluations. Barriers to good communication can lead to the evaluating physician’s perception that the patient lacks capacity. If a patient does not understand his condition or the proposed treatments, the psychiatrist should educate him. It might be useful to arrange a meeting with the treating physician to facilitate communication.
Free choice. The patient’s decision to accept or reject a proposed treatment should be voluntary and free of coercion. In assessing a patient’s capacity, the psychiatrist should determine whether choices have been rendered impossible because of unrealistic fears or expectations about treatment, or because of impaired mental processes.
Reliability refers to a patient’s ability to provide a consistent choice over time. A patient who vacillates or is inconsistent does not have capacity to make decisions.
Features of patients referred for evaluation, and their evaluators
The most common reason for a capacity evaluation is a patient’s refusal of medical treatment. Between 3% and 25% of requests for psychiatric consultation in hospital settings involve questions about patients’ competence to make a treatment-related decision.3 Approximately 25% of adult medicine inpatients lack capacity for medical decision-making.4
Decision-making capacity is a functional evaluation. Decision-making capacity does not relate specifically to a person’s psychiatric diagnosis. In other words, the presence of a mental disorder does not render a person incapable of making decisions. However, people with Alzheimer’s disease or dementia have a high rate of impaired capacity for making treatment decisions.
Schizophrenia has been found to have the highest rate of impaired decision-making among psychiatric disorders; depression is second and bipolar disorder, third. The strongest predictor of incapacity in psychiatric patients is lack of insight.5 Positive symptoms, negative symptoms, severity of symptoms, involuntary admission, lack of insight, and treatment refusal were strong predictors of incapacity in a sample of psychiatric patients.6
The neuronal basis of decision-making is unknown. Studies have implicated functioning of the medial and lateral prefrontal cortex as an important correlate of decision-making capacity.7 As a result of these findings, a brain-based criterion could be added to the conceptual criteria of capacity. The specific neuropsychological components necessary for decision-making capacity are unknown. Some studies suggest that poor executive functioning and limited learning ability correlate with impaired decision-making capacity.8 Little is known about the relationship between emotion and capacity. Supady et al demonstrated that higher cognitive empathy and good emotion recognition were associated with increased decision-making capacity and higher rates of refusal to give informed consent.9
Physician bias has been identified in capacity evaluations. See Box 2.4,10-12
Tools used in capacity evaluations
Most capacity evaluations are conducted by clinical interview (Box 3). The reliability of physicians’ unstructured judgments of capacity has been poor.13 In a study of 5 physicians who made a determination of capacity after watching a videotape of capacity assessments, the rate of agreement among the subjects was no better than that of chance.14
There is no specific, simple, quick test to assess capacity.
Folstein Mini-Mental State Examination. The MMSE has not been found to be predictive of decision-making capacity. It has been found to correlate with clinical judgments of incapacity, and may be used to identify patients at the high and low ends of the range of capacity, especially among older persons who exhibit cognitive impairment.15 Patients who have severe dementia (MMSE score <16) have a high likelihood of being unable to consent to treatment.16
MacArthur Competence Assessment Tool-Treatment. The MacCAT-T is a structured interviewing tool used to evaluate a patient’s decision-making ability. It is the most commonly used screening tool to evaluate decision-making capacity. Advantages of the MacCAT-T include a higher inter-rater agreement and—unlike other assessment instruments—its ability to incorporate information specific to a patient’s decision-making situation.17 The MacCAT-T requires training and experience to administer.
Bottom Line
Physicians make decisions about a patient’s decision-making capacity. Courts determine competence by a formal judicial proceeding. The psychiatric consultant’s role in capacity evaluations is to determine if the patient 1) possesses the requisite knowledge about the specific referral issue and 2) demonstrates a voluntary and reliable decision.
Related Resources
- The MacArthur Treatment Competence Study. www.macarthur.virginia.edu/treatment.html.
- Resnick P, Sorrentino R: Forensic considerations (chapter. 8). In: Psychosomatic medicine. Blumenfield M, Strain JJ, eds. Baltimore, MD: Lippincott Williams & Wilkins; 2006:91-106.
- Appelbaum PS. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357(18):1834-1840.
Disclosure
Dr. Sorrentino reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
One of the most common reasons medical colleagues seek consultation with a psychiatrist is to address the question of capacity. Indeed, this referral question often is posed as, “Is the patient competent?”
This referral question is incomplete and incorrectly phrased. The question should include the domain in which capacity is being questioned—for example, “Is the patient competent to refuse surgery?” Specifically identifying the area in which competency is questioned is necessary because a person might be competent in one area and incompetent in another (Box 1).
The question of competency should be modified as follows: “Does the patient have capacity to refuse surgery?” Competency is the degree of mental soundness necessary to make decisions about a specific issue or to carry out a specific act. Capacity is a person’s ability to make an informed decision. A determination of competency is a judicial finding made by the court. A physician can opine about a patient’s capacity, but cannot determine competency.
Adults are presumed to have capacity unless determined otherwise by the court. A person who lacks capacity to make an informed decision or give consent might need to be referred for a competency hearing or have a guardian appointed. Psychiatrists often are called on to provide an opinion to the court regarding a person’s capacity. Psychiatrists are particularly skilled at accessing a person’s mental status and gauging its potential for interfering with specific areas of functioning, but, in fact, any physician can make a determination of capacity.1
In this article, I:
- outline the components of a capacity evaluation
- describe the tools used in the determination of capacity
- review the typical features of patients and psychiatrists who perform capacity evaluations.
What constitutes a capacity evaluation?
The components of a capacity evaluation are comprehension, free choice, and reliability.
Comprehension refers to a patient’s factual understanding of his (her) medical condition—for example, including the risks and benefits of treatment and reasonable alternatives. The patient should show an understanding of 1) the situation as it relates to his condition, and 2) the consequences of his decisions. He also should demonstrate a rational manipulation of the information presented, applying a coherent and logical thought process to analyze possible courses of action.2
To determine if the patient has the requisite knowledge regarding his condition, the physician must be familiar with the patient’s clinical status. This might require consultation with the treating physician. Communication is a key component of capacity evaluations. Barriers to good communication can lead to the evaluating physician’s perception that the patient lacks capacity. If a patient does not understand his condition or the proposed treatments, the psychiatrist should educate him. It might be useful to arrange a meeting with the treating physician to facilitate communication.
Free choice. The patient’s decision to accept or reject a proposed treatment should be voluntary and free of coercion. In assessing a patient’s capacity, the psychiatrist should determine whether choices have been rendered impossible because of unrealistic fears or expectations about treatment, or because of impaired mental processes.
Reliability refers to a patient’s ability to provide a consistent choice over time. A patient who vacillates or is inconsistent does not have capacity to make decisions.
Features of patients referred for evaluation, and their evaluators
The most common reason for a capacity evaluation is a patient’s refusal of medical treatment. Between 3% and 25% of requests for psychiatric consultation in hospital settings involve questions about patients’ competence to make a treatment-related decision.3 Approximately 25% of adult medicine inpatients lack capacity for medical decision-making.4
Decision-making capacity is a functional evaluation. Decision-making capacity does not relate specifically to a person’s psychiatric diagnosis. In other words, the presence of a mental disorder does not render a person incapable of making decisions. However, people with Alzheimer’s disease or dementia have a high rate of impaired capacity for making treatment decisions.
Schizophrenia has been found to have the highest rate of impaired decision-making among psychiatric disorders; depression is second and bipolar disorder, third. The strongest predictor of incapacity in psychiatric patients is lack of insight.5 Positive symptoms, negative symptoms, severity of symptoms, involuntary admission, lack of insight, and treatment refusal were strong predictors of incapacity in a sample of psychiatric patients.6
The neuronal basis of decision-making is unknown. Studies have implicated functioning of the medial and lateral prefrontal cortex as an important correlate of decision-making capacity.7 As a result of these findings, a brain-based criterion could be added to the conceptual criteria of capacity. The specific neuropsychological components necessary for decision-making capacity are unknown. Some studies suggest that poor executive functioning and limited learning ability correlate with impaired decision-making capacity.8 Little is known about the relationship between emotion and capacity. Supady et al demonstrated that higher cognitive empathy and good emotion recognition were associated with increased decision-making capacity and higher rates of refusal to give informed consent.9
Physician bias has been identified in capacity evaluations. See Box 2.4,10-12
Tools used in capacity evaluations
Most capacity evaluations are conducted by clinical interview (Box 3). The reliability of physicians’ unstructured judgments of capacity has been poor.13 In a study of 5 physicians who made a determination of capacity after watching a videotape of capacity assessments, the rate of agreement among the subjects was no better than that of chance.14
There is no specific, simple, quick test to assess capacity.
Folstein Mini-Mental State Examination. The MMSE has not been found to be predictive of decision-making capacity. It has been found to correlate with clinical judgments of incapacity, and may be used to identify patients at the high and low ends of the range of capacity, especially among older persons who exhibit cognitive impairment.15 Patients who have severe dementia (MMSE score <16) have a high likelihood of being unable to consent to treatment.16
MacArthur Competence Assessment Tool-Treatment. The MacCAT-T is a structured interviewing tool used to evaluate a patient’s decision-making ability. It is the most commonly used screening tool to evaluate decision-making capacity. Advantages of the MacCAT-T include a higher inter-rater agreement and—unlike other assessment instruments—its ability to incorporate information specific to a patient’s decision-making situation.17 The MacCAT-T requires training and experience to administer.
Bottom Line
Physicians make decisions about a patient’s decision-making capacity. Courts determine competence by a formal judicial proceeding. The psychiatric consultant’s role in capacity evaluations is to determine if the patient 1) possesses the requisite knowledge about the specific referral issue and 2) demonstrates a voluntary and reliable decision.
Related Resources
- The MacArthur Treatment Competence Study. www.macarthur.virginia.edu/treatment.html.
- Resnick P, Sorrentino R: Forensic considerations (chapter. 8). In: Psychosomatic medicine. Blumenfield M, Strain JJ, eds. Baltimore, MD: Lippincott Williams & Wilkins; 2006:91-106.
- Appelbaum PS. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357(18):1834-1840.
Disclosure
Dr. Sorrentino reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Grisson T, Appelbaum PS. Assessing competence to consent to treatment—a guide for physicians and other health professionals. New York, NY: Oxford University Press; 1998.
2. Cohen LM, McCue JD, Green GM. Do clinical and formal assessments of capacity of patients in the intensive care unit to make decisions agree? Arch Intern Med. 1993;153(21): 2841-2845.
3. Farnsworth MG. Competency evaluations in a general hospital. Psychosomatics. 1990;31(1):60-66.
4. Sessums LL, Zembrzuska H, Jackson JL. Does this patient have medical decision-making capacity? JAMA. 2011; 306(4):420-427.
5. Cairns R, Maddock C, Buchanan A, et al. Prevalence and predictors of mental incapacity in psychiatric in-patients. Br J Psychiatry. 2005;187:379-385.
6. Candia PC, Barba AC. Mental capacity and consent to treatment in psychiatric patients: the state of the research. Curr Opin Psychiatry. 2011;24(5):442-446.
7. Duncan J. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23(10):475-483.
8. Mandarelli G, Parmigiani G, Tarsitani L, et al. The relationship between executive functions and capacity to consent to treatment in acute psychiatric hospitalization. J Empir Res Hum Res Ethics. 2012;7(5):63-70.
9. Supady A, Voelkel A, Witzel J, et al. How is informed consent related to emotions and empathy? An exploratory neuroethical investigation. J Med Ethics. 2011;37(5):311-317.
10. Jeste DV, Depp CA, Palmer BW. Magnitude of impairment in decisional capacity in people with schizophrenia compared to normal subjects: an overview. Schizophr Bull. 2006;32(1):121-128.
11. Feldman-Stewart D, Brundage MD. Challenges for designing and implementing decision aids. Patient Educ Couns. 2004;54(3):265-273.
12. Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44(5):681-692.
13. Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007; 357(18):1834-1840.
14. Marson DC, McIntruff B, Hawkins L, et al. Consistency of physician judgments of capacity to consent to mild Alzheimer’s disease. J Am Geriatr Soc. 1997;45(4):453-457.
15. Kim SY, Caine ED. Utility and limits of the mini mental status examination in evaluating consent capacity in Alzheimer’s disease. Psychiatr Serv. 2002;53(10):1322-1324.
16. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198.
17. Grisso T, Appelbaum PS. MacArthur competence assessment tool for treatment (MacCAT-T). Sarasota, FL: Professional Resource Press; 1998.
1. Grisson T, Appelbaum PS. Assessing competence to consent to treatment—a guide for physicians and other health professionals. New York, NY: Oxford University Press; 1998.
2. Cohen LM, McCue JD, Green GM. Do clinical and formal assessments of capacity of patients in the intensive care unit to make decisions agree? Arch Intern Med. 1993;153(21): 2841-2845.
3. Farnsworth MG. Competency evaluations in a general hospital. Psychosomatics. 1990;31(1):60-66.
4. Sessums LL, Zembrzuska H, Jackson JL. Does this patient have medical decision-making capacity? JAMA. 2011; 306(4):420-427.
5. Cairns R, Maddock C, Buchanan A, et al. Prevalence and predictors of mental incapacity in psychiatric in-patients. Br J Psychiatry. 2005;187:379-385.
6. Candia PC, Barba AC. Mental capacity and consent to treatment in psychiatric patients: the state of the research. Curr Opin Psychiatry. 2011;24(5):442-446.
7. Duncan J. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23(10):475-483.
8. Mandarelli G, Parmigiani G, Tarsitani L, et al. The relationship between executive functions and capacity to consent to treatment in acute psychiatric hospitalization. J Empir Res Hum Res Ethics. 2012;7(5):63-70.
9. Supady A, Voelkel A, Witzel J, et al. How is informed consent related to emotions and empathy? An exploratory neuroethical investigation. J Med Ethics. 2011;37(5):311-317.
10. Jeste DV, Depp CA, Palmer BW. Magnitude of impairment in decisional capacity in people with schizophrenia compared to normal subjects: an overview. Schizophr Bull. 2006;32(1):121-128.
11. Feldman-Stewart D, Brundage MD. Challenges for designing and implementing decision aids. Patient Educ Couns. 2004;54(3):265-273.
12. Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44(5):681-692.
13. Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007; 357(18):1834-1840.
14. Marson DC, McIntruff B, Hawkins L, et al. Consistency of physician judgments of capacity to consent to mild Alzheimer’s disease. J Am Geriatr Soc. 1997;45(4):453-457.
15. Kim SY, Caine ED. Utility and limits of the mini mental status examination in evaluating consent capacity in Alzheimer’s disease. Psychiatr Serv. 2002;53(10):1322-1324.
16. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198.
17. Grisso T, Appelbaum PS. MacArthur competence assessment tool for treatment (MacCAT-T). Sarasota, FL: Professional Resource Press; 1998.
What is the relevance of a 2-week response to an antipsychotic?
Mr. M, age 28, was given a diagnosis of schizophrenia 6 years ago after experiencing a psychotic break involving auditory hallucinations and paranoia. Olanzapine, 10 mg/d, relieved his symptoms, but he stopped taking the drug after gaining 40 pounds and developing diabetes mellitus. He had 2 other hospital admissions for acute psychosis and has taken at least 1 other medication, the name of which he can’t recall. Recently, Mr. M was involuntarily admitted to the psychiatric ward of his local hospital. His psychiatrist started aripiprazole, 10 mg/d, which was titrated to 30 mg/d. After 2 weeks he reports only a slight decrease in hallucinations. His mother is growing concerned about the effectiveness of this medication and wants to know if it’s time to consider another drug.
Time to onset of action of antipsychotic agents has been debated since at least 1970.1 Supporters of the delayed-onset hypothesis assert that antipsychotics take weeks or months to show significant improvement of symptoms because of the need for depolarization block for efficacy.2 Trials of 4 to 6 weeks often are recommended for patients before failure is declared,3,4 and trials of this length or longer have proved useful for first-episode patients.5-7 Recent studies suggest, however, that response is cumulative for chronically ill triglyceride, and LDL levels, and a decrease in the HDL level.2 These effects may be seen without an increase in BMI, and should be considered a direct effect of the antipsychotic.5 Although the mechanism by which dyslipidemia occurs is poorly understood, an increase in the blood glucose level is thought to be, in part, mediated by antagonism of M3 muscarinic receptors on pancreatic âpatients with most improvement occurring during weeks 1 and 2.1,8
Two meta-analyses found the greatest rate of cumulative improvement in symptoms during the first 2 weeks.1,8 These analyses included chronically ill patients with mean duration of illness of 15.5 and 10.4 years, respectively. Patients reported 21.9% and 20.5% reductions in symptoms from baseline at 2 weeks, with total responses between 30% at 4 weeks and 40% at 1 year, respectively. These meta-analyses indicate that most of the benefit from antipsychotics in this patient population occurs in the first 2 weeks, which supports the early-response hypothesis.
These observations led to questions about the predictive value of early response and minimum time to determine treatment failure. This article discusses the significance of early response and non-response to antipsychotics and their impact on treating patients with schizophrenia.
What are the predictive factors? How can they guide treatment?
Of the 8 studies in our literature review, only 2 reported early response rates >50%.9,10 (see this article at CurrentPsychiatry.com for a Box describing the literature review.) Positive predictive value (PPV) ranged from 0.51 to 0.81, meaning that 51% to 81% of early responders continued to respond. Six of the 8 studies reported PPV of 50% to 70%. 9,11-15 This appears to be true for chronic and first-episode patients, suggesting that 30% to 50% of early responders will fail to have a sustained response (Table 1,9-16Table 2,9-16 and Figure).
Compared with early response, early non-response is a more consistent predictor of final non-response. In every study of chronically ill patients, negative predictive value (NPV) was greater than PPV (Table 1).9-16 NPVs in the literature suggest that 58% to 91% of early non-responders will continue to be non-responders. This seems to be true of chronically ill patients for whom NPVs consistently were between 75% and 85%. By comparison, in first-episode patients NPVs of 58% and 66% were calculated (Table 19-16 and Figure).14,15
These observations suggest that reassessing drug therapy is indicated early in treatment for early non-responders, particularly in chronically ill patients. However, early non-response in a first-episode patient is not as strong a predictor of eventual treatment failure, supporting the idea that first-episode patients may experience a delayed response to therapy. Researchers studying onset of antipsychotic effect report that median time to response onset in first-episode patients may be ≥8 weeks.6,8 In patients who do not achieve modest early response, assess dose, adherence, substance abuse, and psychosocial stressors.3 For patients without dose, adherence, substance use, or stress issues, switching drug therapy in chronically ill early non-responders is reasonable because the probability of a late response is small.
Individual patient characteristics determine how much these data aid clinical decision-making. If a patient has a good response to an antipsychotic in the first 2 weeks, continue the drug, but observe the patient closely because response may not be sustained. In first-episode patients who fail to respond within 2 weeks of starting an antipsychotic, it is reasonable to continue the drug for several weeks because these patients may be more likely to respond later in therapy.
Clinicians treating chronically ill patients who have failed several antipsychotics and demonstrate a poor response after 2 weeks of an appropriate antipsychotic dose are justified in changing medications because later significant response is unlikely. If a patient has a poor early response but has failed several other antipsychotics with few remaining alternatives, it is reasonable to continue the maximum tolerated dose of the current therapy because the patient may be a late responder. However, early non-response predicts future non-response in many patients.
Case continued
In the case described here, Mr. M is failing his current treatment regimen with a reasonable antipsychotic dose after 2 weeks. Because Mr. M has been on 2 antipsychotics and demonstrated a good response to olanzapine, changing medications should be considered.
Related Resource
- Correll CU, Hauser M. The year in psychosis and bipolar disorder: predicting response to schizophrenia treatment. www.medscape.com/viewarticle/735211_7.
Drug Brand Names
Aripiprazole • Abilify Quetiapine • Seroquel
Haloperidol • Haldol Risperidone • Risperdal
Olanzapine • Zyprexa Ziprasidone • Geodon
Paliperidone • Invega
Disclosures
Dr. Straley owns stock in Johnson & Johnson. Dr. Webster reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Agid O, Kapur S, Arenovich T, et al. Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch Gen Psychiatry. 2003;60(12):1228-1235.
2. Grace AA, Bunney BS, Moore H, et al. Dopamine-cell depolarization block as a model for the therapeutic actions of antipsychotic drugs. Trends Neurosci. 1997;20(1):31-37.
3. Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association Steering Committee on Practice Guidelines et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
4. Meltzer HY, Bobo WV, Heckers SH, et al. Chapter 16. Schizophrenia. In: Ebert MH, Loosen PT, Nurcombe B, Leckman JF, eds. CURRENT Diagnosis & Treatment: Psychiatry. 2nd ed. New York: McGraw-Hill; 2008. http://www.accessmedicine.com/content.aspx?aID=3284037. Accessed December 5, 2013.
5. Robinson DG, Woerner MG, Alvir JM, et al. Predictors of treatment response from a first episode of schizophrenia or schizoaffective disorder. Am J Psychiatry. 1999;156(4):544-549.
6. Emsley R, Rabinowitz J, Medori R. Time course for antipsychotic treatment response in first-episode schizophrenia. Am J Psychiatry. 2006;163(4):743-745.
7. Lieberman JA, Phillips M, Gu H, et al. Atypical and conventional antipsychotic drugs in treatment-naive first-episode schizophrenia: a 52-week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacology. 2003;28(5):995-1003.
8. Leucht S, Busch R, Hamann J, et al. Early-onset hypothesis of antipsychotic drug action: a hypothesis tested, confirmed and extended. Biol Psychiatry. 2005;57(12):1543-1549.
9. Kinon BJ, Chen L, Stauffer VL, et al. Early onset of antipsychotic action in schizophrenia: evaluating the possibility of shorter acute efficacy trials. J Clin Psychopharmacol. 2010;30(3):286-289.
10. Hatta K, Otachi T, Sudo Y, et al. Difference in early prediction of antipsychotic non-response between risperidone and olanzapine in the treatment of acute-phase schizophrenia. Schizophr Res. 2011;128(1-3):127-135.
11. Glick ID, Bossie CA, Alphs L, et al. Onset and persistence of antipsychotic response in patients with schizophrenia. J Clin Psychopharmacol. 2009;29(6):542-547.
12. Kinon BJ, Chen L, Ascher-Svanum H, et al. Predicting response to atypical antipsychotics based on early response in the treatment of schizophrenia. Schizophr Res. 2008;102(1-3):230-240.
13. Jäger M, Schmauss M, Laux G, et al. Early improvement as a predictor of remission and response in schizophrenia: results from a naturalistic study. Eur Psychiatry. 2009;24(8):501-506.
14. Kinon BJ, Chen L, Ascher-Svanum H, et al. Early response to antipsychotic drug therapy as a clinical marker of subsequent response in the treatment of schizophrenia. Neuropsychopharmacology. 2010;35(2):581-590.
15. Gallego JA, Robinson DG, Sevy SM, et al. Time to treatment response in first-episode schizophrenia: should acute treatment trials last several months? J Clin Psychiatry. 2011;72(12):1691-1696.
16. Stauffer VL, Case M, Kinon BJ, et al. Early response to antipsychotic therapy as a clinical marker of subsequent response in the treatment of patients with first-episode psychosis. Psychiatry Res. 2011;187(1-2):42-48.
Mr. M, age 28, was given a diagnosis of schizophrenia 6 years ago after experiencing a psychotic break involving auditory hallucinations and paranoia. Olanzapine, 10 mg/d, relieved his symptoms, but he stopped taking the drug after gaining 40 pounds and developing diabetes mellitus. He had 2 other hospital admissions for acute psychosis and has taken at least 1 other medication, the name of which he can’t recall. Recently, Mr. M was involuntarily admitted to the psychiatric ward of his local hospital. His psychiatrist started aripiprazole, 10 mg/d, which was titrated to 30 mg/d. After 2 weeks he reports only a slight decrease in hallucinations. His mother is growing concerned about the effectiveness of this medication and wants to know if it’s time to consider another drug.
Time to onset of action of antipsychotic agents has been debated since at least 1970.1 Supporters of the delayed-onset hypothesis assert that antipsychotics take weeks or months to show significant improvement of symptoms because of the need for depolarization block for efficacy.2 Trials of 4 to 6 weeks often are recommended for patients before failure is declared,3,4 and trials of this length or longer have proved useful for first-episode patients.5-7 Recent studies suggest, however, that response is cumulative for chronically ill triglyceride, and LDL levels, and a decrease in the HDL level.2 These effects may be seen without an increase in BMI, and should be considered a direct effect of the antipsychotic.5 Although the mechanism by which dyslipidemia occurs is poorly understood, an increase in the blood glucose level is thought to be, in part, mediated by antagonism of M3 muscarinic receptors on pancreatic âpatients with most improvement occurring during weeks 1 and 2.1,8
Two meta-analyses found the greatest rate of cumulative improvement in symptoms during the first 2 weeks.1,8 These analyses included chronically ill patients with mean duration of illness of 15.5 and 10.4 years, respectively. Patients reported 21.9% and 20.5% reductions in symptoms from baseline at 2 weeks, with total responses between 30% at 4 weeks and 40% at 1 year, respectively. These meta-analyses indicate that most of the benefit from antipsychotics in this patient population occurs in the first 2 weeks, which supports the early-response hypothesis.
These observations led to questions about the predictive value of early response and minimum time to determine treatment failure. This article discusses the significance of early response and non-response to antipsychotics and their impact on treating patients with schizophrenia.
What are the predictive factors? How can they guide treatment?
Of the 8 studies in our literature review, only 2 reported early response rates >50%.9,10 (see this article at CurrentPsychiatry.com for a Box describing the literature review.) Positive predictive value (PPV) ranged from 0.51 to 0.81, meaning that 51% to 81% of early responders continued to respond. Six of the 8 studies reported PPV of 50% to 70%. 9,11-15 This appears to be true for chronic and first-episode patients, suggesting that 30% to 50% of early responders will fail to have a sustained response (Table 1,9-16Table 2,9-16 and Figure).
Compared with early response, early non-response is a more consistent predictor of final non-response. In every study of chronically ill patients, negative predictive value (NPV) was greater than PPV (Table 1).9-16 NPVs in the literature suggest that 58% to 91% of early non-responders will continue to be non-responders. This seems to be true of chronically ill patients for whom NPVs consistently were between 75% and 85%. By comparison, in first-episode patients NPVs of 58% and 66% were calculated (Table 19-16 and Figure).14,15
These observations suggest that reassessing drug therapy is indicated early in treatment for early non-responders, particularly in chronically ill patients. However, early non-response in a first-episode patient is not as strong a predictor of eventual treatment failure, supporting the idea that first-episode patients may experience a delayed response to therapy. Researchers studying onset of antipsychotic effect report that median time to response onset in first-episode patients may be ≥8 weeks.6,8 In patients who do not achieve modest early response, assess dose, adherence, substance abuse, and psychosocial stressors.3 For patients without dose, adherence, substance use, or stress issues, switching drug therapy in chronically ill early non-responders is reasonable because the probability of a late response is small.
Individual patient characteristics determine how much these data aid clinical decision-making. If a patient has a good response to an antipsychotic in the first 2 weeks, continue the drug, but observe the patient closely because response may not be sustained. In first-episode patients who fail to respond within 2 weeks of starting an antipsychotic, it is reasonable to continue the drug for several weeks because these patients may be more likely to respond later in therapy.
Clinicians treating chronically ill patients who have failed several antipsychotics and demonstrate a poor response after 2 weeks of an appropriate antipsychotic dose are justified in changing medications because later significant response is unlikely. If a patient has a poor early response but has failed several other antipsychotics with few remaining alternatives, it is reasonable to continue the maximum tolerated dose of the current therapy because the patient may be a late responder. However, early non-response predicts future non-response in many patients.
Case continued
In the case described here, Mr. M is failing his current treatment regimen with a reasonable antipsychotic dose after 2 weeks. Because Mr. M has been on 2 antipsychotics and demonstrated a good response to olanzapine, changing medications should be considered.
Related Resource
- Correll CU, Hauser M. The year in psychosis and bipolar disorder: predicting response to schizophrenia treatment. www.medscape.com/viewarticle/735211_7.
Drug Brand Names
Aripiprazole • Abilify Quetiapine • Seroquel
Haloperidol • Haldol Risperidone • Risperdal
Olanzapine • Zyprexa Ziprasidone • Geodon
Paliperidone • Invega
Disclosures
Dr. Straley owns stock in Johnson & Johnson. Dr. Webster reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Mr. M, age 28, was given a diagnosis of schizophrenia 6 years ago after experiencing a psychotic break involving auditory hallucinations and paranoia. Olanzapine, 10 mg/d, relieved his symptoms, but he stopped taking the drug after gaining 40 pounds and developing diabetes mellitus. He had 2 other hospital admissions for acute psychosis and has taken at least 1 other medication, the name of which he can’t recall. Recently, Mr. M was involuntarily admitted to the psychiatric ward of his local hospital. His psychiatrist started aripiprazole, 10 mg/d, which was titrated to 30 mg/d. After 2 weeks he reports only a slight decrease in hallucinations. His mother is growing concerned about the effectiveness of this medication and wants to know if it’s time to consider another drug.
Time to onset of action of antipsychotic agents has been debated since at least 1970.1 Supporters of the delayed-onset hypothesis assert that antipsychotics take weeks or months to show significant improvement of symptoms because of the need for depolarization block for efficacy.2 Trials of 4 to 6 weeks often are recommended for patients before failure is declared,3,4 and trials of this length or longer have proved useful for first-episode patients.5-7 Recent studies suggest, however, that response is cumulative for chronically ill triglyceride, and LDL levels, and a decrease in the HDL level.2 These effects may be seen without an increase in BMI, and should be considered a direct effect of the antipsychotic.5 Although the mechanism by which dyslipidemia occurs is poorly understood, an increase in the blood glucose level is thought to be, in part, mediated by antagonism of M3 muscarinic receptors on pancreatic âpatients with most improvement occurring during weeks 1 and 2.1,8
Two meta-analyses found the greatest rate of cumulative improvement in symptoms during the first 2 weeks.1,8 These analyses included chronically ill patients with mean duration of illness of 15.5 and 10.4 years, respectively. Patients reported 21.9% and 20.5% reductions in symptoms from baseline at 2 weeks, with total responses between 30% at 4 weeks and 40% at 1 year, respectively. These meta-analyses indicate that most of the benefit from antipsychotics in this patient population occurs in the first 2 weeks, which supports the early-response hypothesis.
These observations led to questions about the predictive value of early response and minimum time to determine treatment failure. This article discusses the significance of early response and non-response to antipsychotics and their impact on treating patients with schizophrenia.
What are the predictive factors? How can they guide treatment?
Of the 8 studies in our literature review, only 2 reported early response rates >50%.9,10 (see this article at CurrentPsychiatry.com for a Box describing the literature review.) Positive predictive value (PPV) ranged from 0.51 to 0.81, meaning that 51% to 81% of early responders continued to respond. Six of the 8 studies reported PPV of 50% to 70%. 9,11-15 This appears to be true for chronic and first-episode patients, suggesting that 30% to 50% of early responders will fail to have a sustained response (Table 1,9-16Table 2,9-16 and Figure).
Compared with early response, early non-response is a more consistent predictor of final non-response. In every study of chronically ill patients, negative predictive value (NPV) was greater than PPV (Table 1).9-16 NPVs in the literature suggest that 58% to 91% of early non-responders will continue to be non-responders. This seems to be true of chronically ill patients for whom NPVs consistently were between 75% and 85%. By comparison, in first-episode patients NPVs of 58% and 66% were calculated (Table 19-16 and Figure).14,15
These observations suggest that reassessing drug therapy is indicated early in treatment for early non-responders, particularly in chronically ill patients. However, early non-response in a first-episode patient is not as strong a predictor of eventual treatment failure, supporting the idea that first-episode patients may experience a delayed response to therapy. Researchers studying onset of antipsychotic effect report that median time to response onset in first-episode patients may be ≥8 weeks.6,8 In patients who do not achieve modest early response, assess dose, adherence, substance abuse, and psychosocial stressors.3 For patients without dose, adherence, substance use, or stress issues, switching drug therapy in chronically ill early non-responders is reasonable because the probability of a late response is small.
Individual patient characteristics determine how much these data aid clinical decision-making. If a patient has a good response to an antipsychotic in the first 2 weeks, continue the drug, but observe the patient closely because response may not be sustained. In first-episode patients who fail to respond within 2 weeks of starting an antipsychotic, it is reasonable to continue the drug for several weeks because these patients may be more likely to respond later in therapy.
Clinicians treating chronically ill patients who have failed several antipsychotics and demonstrate a poor response after 2 weeks of an appropriate antipsychotic dose are justified in changing medications because later significant response is unlikely. If a patient has a poor early response but has failed several other antipsychotics with few remaining alternatives, it is reasonable to continue the maximum tolerated dose of the current therapy because the patient may be a late responder. However, early non-response predicts future non-response in many patients.
Case continued
In the case described here, Mr. M is failing his current treatment regimen with a reasonable antipsychotic dose after 2 weeks. Because Mr. M has been on 2 antipsychotics and demonstrated a good response to olanzapine, changing medications should be considered.
Related Resource
- Correll CU, Hauser M. The year in psychosis and bipolar disorder: predicting response to schizophrenia treatment. www.medscape.com/viewarticle/735211_7.
Drug Brand Names
Aripiprazole • Abilify Quetiapine • Seroquel
Haloperidol • Haldol Risperidone • Risperdal
Olanzapine • Zyprexa Ziprasidone • Geodon
Paliperidone • Invega
Disclosures
Dr. Straley owns stock in Johnson & Johnson. Dr. Webster reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Agid O, Kapur S, Arenovich T, et al. Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch Gen Psychiatry. 2003;60(12):1228-1235.
2. Grace AA, Bunney BS, Moore H, et al. Dopamine-cell depolarization block as a model for the therapeutic actions of antipsychotic drugs. Trends Neurosci. 1997;20(1):31-37.
3. Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association Steering Committee on Practice Guidelines et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
4. Meltzer HY, Bobo WV, Heckers SH, et al. Chapter 16. Schizophrenia. In: Ebert MH, Loosen PT, Nurcombe B, Leckman JF, eds. CURRENT Diagnosis & Treatment: Psychiatry. 2nd ed. New York: McGraw-Hill; 2008. http://www.accessmedicine.com/content.aspx?aID=3284037. Accessed December 5, 2013.
5. Robinson DG, Woerner MG, Alvir JM, et al. Predictors of treatment response from a first episode of schizophrenia or schizoaffective disorder. Am J Psychiatry. 1999;156(4):544-549.
6. Emsley R, Rabinowitz J, Medori R. Time course for antipsychotic treatment response in first-episode schizophrenia. Am J Psychiatry. 2006;163(4):743-745.
7. Lieberman JA, Phillips M, Gu H, et al. Atypical and conventional antipsychotic drugs in treatment-naive first-episode schizophrenia: a 52-week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacology. 2003;28(5):995-1003.
8. Leucht S, Busch R, Hamann J, et al. Early-onset hypothesis of antipsychotic drug action: a hypothesis tested, confirmed and extended. Biol Psychiatry. 2005;57(12):1543-1549.
9. Kinon BJ, Chen L, Stauffer VL, et al. Early onset of antipsychotic action in schizophrenia: evaluating the possibility of shorter acute efficacy trials. J Clin Psychopharmacol. 2010;30(3):286-289.
10. Hatta K, Otachi T, Sudo Y, et al. Difference in early prediction of antipsychotic non-response between risperidone and olanzapine in the treatment of acute-phase schizophrenia. Schizophr Res. 2011;128(1-3):127-135.
11. Glick ID, Bossie CA, Alphs L, et al. Onset and persistence of antipsychotic response in patients with schizophrenia. J Clin Psychopharmacol. 2009;29(6):542-547.
12. Kinon BJ, Chen L, Ascher-Svanum H, et al. Predicting response to atypical antipsychotics based on early response in the treatment of schizophrenia. Schizophr Res. 2008;102(1-3):230-240.
13. Jäger M, Schmauss M, Laux G, et al. Early improvement as a predictor of remission and response in schizophrenia: results from a naturalistic study. Eur Psychiatry. 2009;24(8):501-506.
14. Kinon BJ, Chen L, Ascher-Svanum H, et al. Early response to antipsychotic drug therapy as a clinical marker of subsequent response in the treatment of schizophrenia. Neuropsychopharmacology. 2010;35(2):581-590.
15. Gallego JA, Robinson DG, Sevy SM, et al. Time to treatment response in first-episode schizophrenia: should acute treatment trials last several months? J Clin Psychiatry. 2011;72(12):1691-1696.
16. Stauffer VL, Case M, Kinon BJ, et al. Early response to antipsychotic therapy as a clinical marker of subsequent response in the treatment of patients with first-episode psychosis. Psychiatry Res. 2011;187(1-2):42-48.
1. Agid O, Kapur S, Arenovich T, et al. Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch Gen Psychiatry. 2003;60(12):1228-1235.
2. Grace AA, Bunney BS, Moore H, et al. Dopamine-cell depolarization block as a model for the therapeutic actions of antipsychotic drugs. Trends Neurosci. 1997;20(1):31-37.
3. Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association Steering Committee on Practice Guidelines et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
4. Meltzer HY, Bobo WV, Heckers SH, et al. Chapter 16. Schizophrenia. In: Ebert MH, Loosen PT, Nurcombe B, Leckman JF, eds. CURRENT Diagnosis & Treatment: Psychiatry. 2nd ed. New York: McGraw-Hill; 2008. http://www.accessmedicine.com/content.aspx?aID=3284037. Accessed December 5, 2013.
5. Robinson DG, Woerner MG, Alvir JM, et al. Predictors of treatment response from a first episode of schizophrenia or schizoaffective disorder. Am J Psychiatry. 1999;156(4):544-549.
6. Emsley R, Rabinowitz J, Medori R. Time course for antipsychotic treatment response in first-episode schizophrenia. Am J Psychiatry. 2006;163(4):743-745.
7. Lieberman JA, Phillips M, Gu H, et al. Atypical and conventional antipsychotic drugs in treatment-naive first-episode schizophrenia: a 52-week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacology. 2003;28(5):995-1003.
8. Leucht S, Busch R, Hamann J, et al. Early-onset hypothesis of antipsychotic drug action: a hypothesis tested, confirmed and extended. Biol Psychiatry. 2005;57(12):1543-1549.
9. Kinon BJ, Chen L, Stauffer VL, et al. Early onset of antipsychotic action in schizophrenia: evaluating the possibility of shorter acute efficacy trials. J Clin Psychopharmacol. 2010;30(3):286-289.
10. Hatta K, Otachi T, Sudo Y, et al. Difference in early prediction of antipsychotic non-response between risperidone and olanzapine in the treatment of acute-phase schizophrenia. Schizophr Res. 2011;128(1-3):127-135.
11. Glick ID, Bossie CA, Alphs L, et al. Onset and persistence of antipsychotic response in patients with schizophrenia. J Clin Psychopharmacol. 2009;29(6):542-547.
12. Kinon BJ, Chen L, Ascher-Svanum H, et al. Predicting response to atypical antipsychotics based on early response in the treatment of schizophrenia. Schizophr Res. 2008;102(1-3):230-240.
13. Jäger M, Schmauss M, Laux G, et al. Early improvement as a predictor of remission and response in schizophrenia: results from a naturalistic study. Eur Psychiatry. 2009;24(8):501-506.
14. Kinon BJ, Chen L, Ascher-Svanum H, et al. Early response to antipsychotic drug therapy as a clinical marker of subsequent response in the treatment of schizophrenia. Neuropsychopharmacology. 2010;35(2):581-590.
15. Gallego JA, Robinson DG, Sevy SM, et al. Time to treatment response in first-episode schizophrenia: should acute treatment trials last several months? J Clin Psychiatry. 2011;72(12):1691-1696.
16. Stauffer VL, Case M, Kinon BJ, et al. Early response to antipsychotic therapy as a clinical marker of subsequent response in the treatment of patients with first-episode psychosis. Psychiatry Res. 2011;187(1-2):42-48.
Hearing voices, time traveling, and being hit with a high-heeled shoe
CASE Grief and confusion
Mr. P, age 47, is arrested for entering the apartment of a woman he does not know and tossing her belongings out the window. When he is assessed to determine if he can participate in his legal defense, examiners find an attentive, courteous man who is baffled by his own behavior.
Mr. P says that he had been “stressed out” after the recent death of his grandmother, with whom he was close. He says he entered the apartment because voices told him to do so. He has no recent history of substance abuse or psychiatric hospitalizations, but he had a similar episode of “confusion” years before, when another close family member died.
Mr. P is found not fit to stand trial and the charges are dropped. He accepts haloperidol, 10 mg/d, and benztropine, 2 mg/d, and is transferred to a hospital for psychiatric treatment.
On interview, Mr. P is well groomed, soft-spoken, and shy, without formal thought disorder. Physical exam and routine lab tests are within normal limits. He says that 18 months before his arrest, he and his frail grandmother moved to a large city in hopes that he would find a wife. Both depended on the grandmother’s Social Security benefits while he cared for her.
In the 2 months after she died, he reports that he felt sad and alone and slept poorly, but made efforts to find a job and keep his apartment. When his efforts failed and he lost the apartment, he stayed with various friends for a few days at a time, then spent several days in the subway before ending up on the streets.
His arrest on the current charge occurred 4 days after he began walking the streets.
a) continue haloperidol to treat psychotic symptoms
b) discontinue haloperidol and observe him
c) add an antidepressant to haloperidol
HISTORY Imagining nonsense
Mr. P cannot explain why he started “trashing” the woman’s apartment, but says he entered it because he thought it was his apartment. With embarrassment and regret, he admits he has been depressed and confused, “imagining things”—“foolish things,” he admits—such as being in a different “time zone.”
Contradicting his earlier statements, Mr. P now admits that he had “a few beers” and denies that he experienced auditory hallucinations, saying he only talks to himself. He now says that within 2 days after his arrest, he was “all over it.” Mr. P denies current symptoms, including hallucinations, but, when pressed, waffles, then admits to a strange belief: that some people, including him, can move from one “time zone” to another.
Mr. P says he was treated for psychiatric problems 4 years earlier when his parents were killed in a car crash. By his recollection, his reaction to their death was similar to his reaction to his grandmother’s death: He became upset and wandered the streets for a few days, “moving between time zones” and talking to himself but not experiencing hallucinations. After he was taken to a hospital and “given an injection,” he calmed down and was released. Within a few days he recovered and returned to supporting himself and caring for his grandmother. Mr. P says the idea of travelling between “time zones” is embarrassing and nonsensical but adds that he was affected in this way because he “bickered” with his mother.
Mr. P’s grandmother raised him until he was age 15, although he frequently visited his parents, who lived nearby and worked during the day. Mr. P initially denies substance abuse, then admits to smoking marijuana every day for about a year before admission. He also admits to cocaine abuse in his 20s. He denies a history of suicide attempts.
The author’s observations
Mr. P reported only 2 episodes of “confusion” (or psychosis) and strange behavior in his life, both precipitated by the loss of a loved one, and at least 1 while under the influence of alcohol and Cannabis. He gave an inconsistent and ambiguous history of auditory hallucinations associated with episodes of confusion. He believes that time travel is possible, an idea that he acknowledged is nonsense. This alone was not enough to warrant long-term antipsychotic treatment. The most likely diagnosis seemed to be brief psychotic episode induced by Cannabis and the stressors of homelessness and his grandmother’s death.
EVALUATION Changing stories
No longer taking haloperidol, Mr. P continues to deny hallucinations and depressed mood, but keeps to himself. Nine days after admission he becomes tearful after he informs his aunt of his grandmother’s death in a telephone call, then approaches a nurse and complains of sadness and auditory hallucinations.
Mr. P confesses that he denied hallucinations on admission because he feared he would remain in the hospital for years if he revealed the truth that he had been experiencing auditory hallucinations almost continuously from age 10. He reports that the voices distracted him when he worked; seem to be male; often spoke gibberish; and alternate between deprecating and positive and supportive. Mr. P is reluctant to disclose more about what the voices actually say, although he acknowledges that they are not commenting or conversing with him, and that he has never believed the voices were his own thoughts but did believe that they came from inside his brain.
With haloperidol, the voices stopped. They resumed, however, when haloperidol was discontinued.
When we ask what happened to him at age 10, Mr. P shrugs.
a) childhood onset schizophrenia
b) substance abuse
c) posttraumatic stress disorder (PTSD)
d) none
The author’s observations
In community samples of children and adolescents, auditory hallucinations are not rare and usually do not cause distress or dysfunction. In a study of 3,870 children age 7 and 8,1 9% endorsed auditory hallucinations. Most heard 1 voice, once a week or less, at low volume. In 85% of children who experienced hallucinations, they caused minimal or no suffering; 97% reported minimal or no interference with daily functioning. Among children who experienced auditory hallucinations at age 7 or 8, 24% continued to hallucinate 5 years later.2 Persistent hallucinations were associated with more problematic behaviors at baseline and follow up.
In a group of 12-year-old twins, 4.2% reported auditory hallucinations.3 In that study, hallucinations were not related to Cannabis use; rather, they were heritable and related to risk factors such as cognitive impairment; behavioral, emotional, and educational problems at age 5; and a history of physical abuse and self-harm at age 12. The authors noted that these are risk factors and correlates of schizophrenia, but are not specific to schizophrenia.
Hallucinations and delusions have been found in 4% to 8% of children and adolescents referred for psychiatric treatment,4 far more than the prevalence of childhood-onset schizophrenia (0.01% of children).5 Psychotic symptoms in children have been associated with bipolar disorder, but also with anxiety disorders, obsessive-compulsive disorder, PTSD, pervasive developmental disorder, conduct disorder, and substance abuse.4
Childhood-onset schizophrenia is rare and would require that Mr. P have a diagnosis of schizophrenia as an adult. It is possible that Mr. P’s childhood symptoms were related to substance abuse but he was not asked for this history because it seemed unlikely in a 10-year-old boy. A PTSD diagnosis requires a traumatic event, which Mr. P did not reveal. It is possible that at age 10 he did not have a psychiatric disorder.
a) PTSD
b) dissociative disorder
c) borderline personality disorder
d) chronic schizophrenia
e) no psychiatric diagnosis
Among adults in the general population, 10% to 15% report auditory hallucinations.6 Hallucinations could be caused by substance abuse or psychiatric conditions other than schizophrenia; however, in adults—as in children—auditory hallucinations can occur in the absence of these conditions (Table 1) and rarely cause distress or dysfunction.6 In Sommer and colleagues’6 study of 103 healthy persons, none who heard voices had disorganization or negative symptoms. Those who heard voices had significantly more schizotypal symptoms and more childhood trauma, including emotional, physical, and sexual abuse, than those who did not hear voices.6
Conditions associated with hallucinations
PTSD is associated with auditory hallucinations and other psychotic symptoms.7 Most studies are of combat veterans with PTSD, in whom auditory hallucinations and delusions were associated with major depressive disorder, not a thought disorder or inappropriate affect.8 In a community sample,9 psychotic symptoms—particularly auditory hallucinations—were associated with PTSD. Subjects with PTSD and psychotic symptoms were more likely to have other psychiatric disorders, including major depressive disorder and substance use disorder, than patients with PTSD but no psychotic symptoms; however, the relationship between PTSD and psychosis remained after controlling for other psychiatric disorders.
Hallucinations can occur in persons with dissociative disorders in the absence of distinct personality states.10 Hallucinations have been seen transiently and chronically in persons with borderline personality disorder and can be associated with comorbid conditions such as substance abuse disorders, mood disorders, and PTSD.11
Mr. P lacked the reduced capacity for interpersonal relationships required for a schizotypal personality disorder diagnosis. A diagnosis of PTSD or dissociative disorder requires a history of trauma, which Mr. P did not report.
“Time travelling” with incomprehensible behavior could be interpreted as dissociation, but dissociative fugue or dissociative disorder not otherwise specified (NOS) cannot be diagnosed if symptoms might be the direct effect of a substance, such as Cannabis. Mr. P admitted to substance abuse. We can rule out borderline personality disorder because he did not display or admit to tempestuous interpersonal relationships.
A schizophrenia diagnosis requires the presence of auditory hallucinations that commented on his behavior or conversed among themselves, a second psychotic symptom for ≥1 month, or negative symptoms, which Mr. P lacked (unless belief in time travel is considered delusional).
Last, a physician might have considered malingering or a factitious disorder when Mr. P was found not able to participate in his own defense, but this seemed less likely after he revealed that he experienced auditory hallucinations since age 10.
HISTORY Bad beatings
With a few days of beginning risperidone, 4 mg/d, Mr. P reports that his hallucinations have stopped and he feels less sad. He reveals that, at age 10, when the hallucinations began, his mother hit him over the head with a high-heeled shoe, causing a scalp laceration that required a visit to the emergency room for suturing. His mother beat Mr. P for as long as he could remember. She beat him “bad” at least twice weekly, and he was taken to the hospital 7 or 8 times for injury, but she also beat him “constantly” with a belt buckle, sometimes striking his head. She instructed him to tell nobody.
The author’s observations
Auditory hallucinations in adults have been associated with childhood abuse, particularly childhood sexual abuse,12 in clinical and non-clinical samples.13 Some argue13 that child abuse itself causes hallucinations and other psychotic symptoms.
OUTCOME Depressed and sleepless
Mr. P admits that he had been smoking marijuana 2 to 3 times daily for a year. He also reports insomnia, sleeping approximately 4 hours a night and spending hours awake in bed thinking of his grandmother, with depressed mood and tearfulness. He denies suicidal ideas and hallucinations. He is treated for depressive disorder NOS first with amitriptyline, 50 mg at bedtime, for sleep, then paroxetine, 20 mg/d, for depressive symptoms, in addition to risperidone, 4 mg/d. Although Mr. P does not describe re-experiencing his childhood trauma, avoidance of stimuli associated with the trauma, or symptoms of increased arousal (except for insomnia), the treatment team did not ask, so it remains uncertain if he has PTSD (Table 2).
When Mr. P is discharged to a clinic, he smiles easily and is positive and supportive with other patients. He spruces up his appearance by wearing jewelry and works in the hospital kitchen.
Bottom Line
Chronic auditory hallucinations are associated with psychiatric illnesses other than chronic schizophrenia, particularly those resulting from trauma such as posttraumatic stress disorder. They can also occur in the absence of diagnosable psychiatric illness and rarely cause distress or functional impairment. Auditory hallucinations in adults have been associated with childhood abuse.
Related Resources
- Moskowitz A, Schafer I, Dorahy MJ. Psychosis, trauma and dissociation: emerging perspectives on severe psychopathology. West Sussex, UK: John Wiley and Sons, Ltd.; 2008.
- The International Hearing Voices Network. www.intervoiceonline.org.
Drug Brand Names
Amitriptyline • Elavil Paroxetine • Paxil
Benztropine • Cogentin Risperidone • Risperdal
Haloperidol • Haldol
Disclosure
Dr. Crowner reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Barthel-Velthuis AA, Jenner JA, van de Willige G, et al Prevalence and correlates of auditory vocal hallucinations in middle childhood. Br J Psychiatry. 2010;196(1):41-46.
2. Bartels-Velthuis AA, van de Willige G, Jenner JA, et al. Course of auditory vocal hallucinations in childhood: 5-year follow-up study. Br J Psychiatry. 2011;199(4):296-302.
3. Polanczyk G, Moffitt TE, Arsensault L, et al. Etiological and clinical features of childhood psychotic symptoms: results from a birth cohort. Arch Gen Psychiatry. 2010;67(4):328-338.
4. Biederman J, Pety C, Faracone SV, et al. Phenomenology of childhood psychosis: Findings from a large sample of psychiatrically referred youth. J Nerv Ment Dis 2004;192(9):607-614.
5. American Academy of Child and Adolescent Psychiatry. Practice parameters for the assessment and treatment of children and adolescents with schizophrenia. J Am Acad Child Adolesc Psychiatry. 2001;40(suppl 7):4SS-23S.
6. Sommer IEC, Daalman K, Rietkerk T, et al. Healthy individuals with auditory verbal hallucinations; Who are they? Psychiatric assessments of a selected sample of 103 subjects. Schizophr Bull. 2010;36(3):633-641.
7. Butler RW, Mueser KT, Sprock J, et al. Positive symptoms of psychosis in posttraumatic stress disorder. Biol Psychiatry. 1996;39:839-844.
8. David D, Kutcher GS, Jackson EI, et al Psychotic symptoms in combat-related posttraumatic stress disorder. J Clin Psychiatry. 1999;60(1):29-32.
9. Sareen J, Cox BJ, Goodwin RD, et al. Co-occurrence of posttraumatic stress disorder with positive psychotic symptoms in a nationally representative sample. J Trauma Stress. 2005;18(4):313-322.
10. Sar V, Akyuv G, Dogan O. Prevalence of dissociative disorders among women in the general population. Psychiatry Res. 2007;149:169-176.
11. Barnow S, Arens EA, Sieswerda S, et al. Borderline personality disorder and psychosis: a review. Curr Psychiatry Rep. 2010;12(3):186-195.
12. Bebbington P, Jonas S, Kuipers E, et al. Childhood sexual abuse and psychosis: data from a cross-sectional national psychiatric survey in England. Br J Psychiatry. 2011;199(1):29-37.
13. Read J, van Os J, Morrison AP, et al. Childhood trauma, psychosis and schizophrenia: a literature review with theoretical and clinical implications. Acta Psychiatr Scand. 2005;112(5):330-350.
CASE Grief and confusion
Mr. P, age 47, is arrested for entering the apartment of a woman he does not know and tossing her belongings out the window. When he is assessed to determine if he can participate in his legal defense, examiners find an attentive, courteous man who is baffled by his own behavior.
Mr. P says that he had been “stressed out” after the recent death of his grandmother, with whom he was close. He says he entered the apartment because voices told him to do so. He has no recent history of substance abuse or psychiatric hospitalizations, but he had a similar episode of “confusion” years before, when another close family member died.
Mr. P is found not fit to stand trial and the charges are dropped. He accepts haloperidol, 10 mg/d, and benztropine, 2 mg/d, and is transferred to a hospital for psychiatric treatment.
On interview, Mr. P is well groomed, soft-spoken, and shy, without formal thought disorder. Physical exam and routine lab tests are within normal limits. He says that 18 months before his arrest, he and his frail grandmother moved to a large city in hopes that he would find a wife. Both depended on the grandmother’s Social Security benefits while he cared for her.
In the 2 months after she died, he reports that he felt sad and alone and slept poorly, but made efforts to find a job and keep his apartment. When his efforts failed and he lost the apartment, he stayed with various friends for a few days at a time, then spent several days in the subway before ending up on the streets.
His arrest on the current charge occurred 4 days after he began walking the streets.
a) continue haloperidol to treat psychotic symptoms
b) discontinue haloperidol and observe him
c) add an antidepressant to haloperidol
HISTORY Imagining nonsense
Mr. P cannot explain why he started “trashing” the woman’s apartment, but says he entered it because he thought it was his apartment. With embarrassment and regret, he admits he has been depressed and confused, “imagining things”—“foolish things,” he admits—such as being in a different “time zone.”
Contradicting his earlier statements, Mr. P now admits that he had “a few beers” and denies that he experienced auditory hallucinations, saying he only talks to himself. He now says that within 2 days after his arrest, he was “all over it.” Mr. P denies current symptoms, including hallucinations, but, when pressed, waffles, then admits to a strange belief: that some people, including him, can move from one “time zone” to another.
Mr. P says he was treated for psychiatric problems 4 years earlier when his parents were killed in a car crash. By his recollection, his reaction to their death was similar to his reaction to his grandmother’s death: He became upset and wandered the streets for a few days, “moving between time zones” and talking to himself but not experiencing hallucinations. After he was taken to a hospital and “given an injection,” he calmed down and was released. Within a few days he recovered and returned to supporting himself and caring for his grandmother. Mr. P says the idea of travelling between “time zones” is embarrassing and nonsensical but adds that he was affected in this way because he “bickered” with his mother.
Mr. P’s grandmother raised him until he was age 15, although he frequently visited his parents, who lived nearby and worked during the day. Mr. P initially denies substance abuse, then admits to smoking marijuana every day for about a year before admission. He also admits to cocaine abuse in his 20s. He denies a history of suicide attempts.
The author’s observations
Mr. P reported only 2 episodes of “confusion” (or psychosis) and strange behavior in his life, both precipitated by the loss of a loved one, and at least 1 while under the influence of alcohol and Cannabis. He gave an inconsistent and ambiguous history of auditory hallucinations associated with episodes of confusion. He believes that time travel is possible, an idea that he acknowledged is nonsense. This alone was not enough to warrant long-term antipsychotic treatment. The most likely diagnosis seemed to be brief psychotic episode induced by Cannabis and the stressors of homelessness and his grandmother’s death.
EVALUATION Changing stories
No longer taking haloperidol, Mr. P continues to deny hallucinations and depressed mood, but keeps to himself. Nine days after admission he becomes tearful after he informs his aunt of his grandmother’s death in a telephone call, then approaches a nurse and complains of sadness and auditory hallucinations.
Mr. P confesses that he denied hallucinations on admission because he feared he would remain in the hospital for years if he revealed the truth that he had been experiencing auditory hallucinations almost continuously from age 10. He reports that the voices distracted him when he worked; seem to be male; often spoke gibberish; and alternate between deprecating and positive and supportive. Mr. P is reluctant to disclose more about what the voices actually say, although he acknowledges that they are not commenting or conversing with him, and that he has never believed the voices were his own thoughts but did believe that they came from inside his brain.
With haloperidol, the voices stopped. They resumed, however, when haloperidol was discontinued.
When we ask what happened to him at age 10, Mr. P shrugs.
a) childhood onset schizophrenia
b) substance abuse
c) posttraumatic stress disorder (PTSD)
d) none
The author’s observations
In community samples of children and adolescents, auditory hallucinations are not rare and usually do not cause distress or dysfunction. In a study of 3,870 children age 7 and 8,1 9% endorsed auditory hallucinations. Most heard 1 voice, once a week or less, at low volume. In 85% of children who experienced hallucinations, they caused minimal or no suffering; 97% reported minimal or no interference with daily functioning. Among children who experienced auditory hallucinations at age 7 or 8, 24% continued to hallucinate 5 years later.2 Persistent hallucinations were associated with more problematic behaviors at baseline and follow up.
In a group of 12-year-old twins, 4.2% reported auditory hallucinations.3 In that study, hallucinations were not related to Cannabis use; rather, they were heritable and related to risk factors such as cognitive impairment; behavioral, emotional, and educational problems at age 5; and a history of physical abuse and self-harm at age 12. The authors noted that these are risk factors and correlates of schizophrenia, but are not specific to schizophrenia.
Hallucinations and delusions have been found in 4% to 8% of children and adolescents referred for psychiatric treatment,4 far more than the prevalence of childhood-onset schizophrenia (0.01% of children).5 Psychotic symptoms in children have been associated with bipolar disorder, but also with anxiety disorders, obsessive-compulsive disorder, PTSD, pervasive developmental disorder, conduct disorder, and substance abuse.4
Childhood-onset schizophrenia is rare and would require that Mr. P have a diagnosis of schizophrenia as an adult. It is possible that Mr. P’s childhood symptoms were related to substance abuse but he was not asked for this history because it seemed unlikely in a 10-year-old boy. A PTSD diagnosis requires a traumatic event, which Mr. P did not reveal. It is possible that at age 10 he did not have a psychiatric disorder.
a) PTSD
b) dissociative disorder
c) borderline personality disorder
d) chronic schizophrenia
e) no psychiatric diagnosis
Among adults in the general population, 10% to 15% report auditory hallucinations.6 Hallucinations could be caused by substance abuse or psychiatric conditions other than schizophrenia; however, in adults—as in children—auditory hallucinations can occur in the absence of these conditions (Table 1) and rarely cause distress or dysfunction.6 In Sommer and colleagues’6 study of 103 healthy persons, none who heard voices had disorganization or negative symptoms. Those who heard voices had significantly more schizotypal symptoms and more childhood trauma, including emotional, physical, and sexual abuse, than those who did not hear voices.6
Conditions associated with hallucinations
PTSD is associated with auditory hallucinations and other psychotic symptoms.7 Most studies are of combat veterans with PTSD, in whom auditory hallucinations and delusions were associated with major depressive disorder, not a thought disorder or inappropriate affect.8 In a community sample,9 psychotic symptoms—particularly auditory hallucinations—were associated with PTSD. Subjects with PTSD and psychotic symptoms were more likely to have other psychiatric disorders, including major depressive disorder and substance use disorder, than patients with PTSD but no psychotic symptoms; however, the relationship between PTSD and psychosis remained after controlling for other psychiatric disorders.
Hallucinations can occur in persons with dissociative disorders in the absence of distinct personality states.10 Hallucinations have been seen transiently and chronically in persons with borderline personality disorder and can be associated with comorbid conditions such as substance abuse disorders, mood disorders, and PTSD.11
Mr. P lacked the reduced capacity for interpersonal relationships required for a schizotypal personality disorder diagnosis. A diagnosis of PTSD or dissociative disorder requires a history of trauma, which Mr. P did not report.
“Time travelling” with incomprehensible behavior could be interpreted as dissociation, but dissociative fugue or dissociative disorder not otherwise specified (NOS) cannot be diagnosed if symptoms might be the direct effect of a substance, such as Cannabis. Mr. P admitted to substance abuse. We can rule out borderline personality disorder because he did not display or admit to tempestuous interpersonal relationships.
A schizophrenia diagnosis requires the presence of auditory hallucinations that commented on his behavior or conversed among themselves, a second psychotic symptom for ≥1 month, or negative symptoms, which Mr. P lacked (unless belief in time travel is considered delusional).
Last, a physician might have considered malingering or a factitious disorder when Mr. P was found not able to participate in his own defense, but this seemed less likely after he revealed that he experienced auditory hallucinations since age 10.
HISTORY Bad beatings
With a few days of beginning risperidone, 4 mg/d, Mr. P reports that his hallucinations have stopped and he feels less sad. He reveals that, at age 10, when the hallucinations began, his mother hit him over the head with a high-heeled shoe, causing a scalp laceration that required a visit to the emergency room for suturing. His mother beat Mr. P for as long as he could remember. She beat him “bad” at least twice weekly, and he was taken to the hospital 7 or 8 times for injury, but she also beat him “constantly” with a belt buckle, sometimes striking his head. She instructed him to tell nobody.
The author’s observations
Auditory hallucinations in adults have been associated with childhood abuse, particularly childhood sexual abuse,12 in clinical and non-clinical samples.13 Some argue13 that child abuse itself causes hallucinations and other psychotic symptoms.
OUTCOME Depressed and sleepless
Mr. P admits that he had been smoking marijuana 2 to 3 times daily for a year. He also reports insomnia, sleeping approximately 4 hours a night and spending hours awake in bed thinking of his grandmother, with depressed mood and tearfulness. He denies suicidal ideas and hallucinations. He is treated for depressive disorder NOS first with amitriptyline, 50 mg at bedtime, for sleep, then paroxetine, 20 mg/d, for depressive symptoms, in addition to risperidone, 4 mg/d. Although Mr. P does not describe re-experiencing his childhood trauma, avoidance of stimuli associated with the trauma, or symptoms of increased arousal (except for insomnia), the treatment team did not ask, so it remains uncertain if he has PTSD (Table 2).
When Mr. P is discharged to a clinic, he smiles easily and is positive and supportive with other patients. He spruces up his appearance by wearing jewelry and works in the hospital kitchen.
Bottom Line
Chronic auditory hallucinations are associated with psychiatric illnesses other than chronic schizophrenia, particularly those resulting from trauma such as posttraumatic stress disorder. They can also occur in the absence of diagnosable psychiatric illness and rarely cause distress or functional impairment. Auditory hallucinations in adults have been associated with childhood abuse.
Related Resources
- Moskowitz A, Schafer I, Dorahy MJ. Psychosis, trauma and dissociation: emerging perspectives on severe psychopathology. West Sussex, UK: John Wiley and Sons, Ltd.; 2008.
- The International Hearing Voices Network. www.intervoiceonline.org.
Drug Brand Names
Amitriptyline • Elavil Paroxetine • Paxil
Benztropine • Cogentin Risperidone • Risperdal
Haloperidol • Haldol
Disclosure
Dr. Crowner reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Grief and confusion
Mr. P, age 47, is arrested for entering the apartment of a woman he does not know and tossing her belongings out the window. When he is assessed to determine if he can participate in his legal defense, examiners find an attentive, courteous man who is baffled by his own behavior.
Mr. P says that he had been “stressed out” after the recent death of his grandmother, with whom he was close. He says he entered the apartment because voices told him to do so. He has no recent history of substance abuse or psychiatric hospitalizations, but he had a similar episode of “confusion” years before, when another close family member died.
Mr. P is found not fit to stand trial and the charges are dropped. He accepts haloperidol, 10 mg/d, and benztropine, 2 mg/d, and is transferred to a hospital for psychiatric treatment.
On interview, Mr. P is well groomed, soft-spoken, and shy, without formal thought disorder. Physical exam and routine lab tests are within normal limits. He says that 18 months before his arrest, he and his frail grandmother moved to a large city in hopes that he would find a wife. Both depended on the grandmother’s Social Security benefits while he cared for her.
In the 2 months after she died, he reports that he felt sad and alone and slept poorly, but made efforts to find a job and keep his apartment. When his efforts failed and he lost the apartment, he stayed with various friends for a few days at a time, then spent several days in the subway before ending up on the streets.
His arrest on the current charge occurred 4 days after he began walking the streets.
a) continue haloperidol to treat psychotic symptoms
b) discontinue haloperidol and observe him
c) add an antidepressant to haloperidol
HISTORY Imagining nonsense
Mr. P cannot explain why he started “trashing” the woman’s apartment, but says he entered it because he thought it was his apartment. With embarrassment and regret, he admits he has been depressed and confused, “imagining things”—“foolish things,” he admits—such as being in a different “time zone.”
Contradicting his earlier statements, Mr. P now admits that he had “a few beers” and denies that he experienced auditory hallucinations, saying he only talks to himself. He now says that within 2 days after his arrest, he was “all over it.” Mr. P denies current symptoms, including hallucinations, but, when pressed, waffles, then admits to a strange belief: that some people, including him, can move from one “time zone” to another.
Mr. P says he was treated for psychiatric problems 4 years earlier when his parents were killed in a car crash. By his recollection, his reaction to their death was similar to his reaction to his grandmother’s death: He became upset and wandered the streets for a few days, “moving between time zones” and talking to himself but not experiencing hallucinations. After he was taken to a hospital and “given an injection,” he calmed down and was released. Within a few days he recovered and returned to supporting himself and caring for his grandmother. Mr. P says the idea of travelling between “time zones” is embarrassing and nonsensical but adds that he was affected in this way because he “bickered” with his mother.
Mr. P’s grandmother raised him until he was age 15, although he frequently visited his parents, who lived nearby and worked during the day. Mr. P initially denies substance abuse, then admits to smoking marijuana every day for about a year before admission. He also admits to cocaine abuse in his 20s. He denies a history of suicide attempts.
The author’s observations
Mr. P reported only 2 episodes of “confusion” (or psychosis) and strange behavior in his life, both precipitated by the loss of a loved one, and at least 1 while under the influence of alcohol and Cannabis. He gave an inconsistent and ambiguous history of auditory hallucinations associated with episodes of confusion. He believes that time travel is possible, an idea that he acknowledged is nonsense. This alone was not enough to warrant long-term antipsychotic treatment. The most likely diagnosis seemed to be brief psychotic episode induced by Cannabis and the stressors of homelessness and his grandmother’s death.
EVALUATION Changing stories
No longer taking haloperidol, Mr. P continues to deny hallucinations and depressed mood, but keeps to himself. Nine days after admission he becomes tearful after he informs his aunt of his grandmother’s death in a telephone call, then approaches a nurse and complains of sadness and auditory hallucinations.
Mr. P confesses that he denied hallucinations on admission because he feared he would remain in the hospital for years if he revealed the truth that he had been experiencing auditory hallucinations almost continuously from age 10. He reports that the voices distracted him when he worked; seem to be male; often spoke gibberish; and alternate between deprecating and positive and supportive. Mr. P is reluctant to disclose more about what the voices actually say, although he acknowledges that they are not commenting or conversing with him, and that he has never believed the voices were his own thoughts but did believe that they came from inside his brain.
With haloperidol, the voices stopped. They resumed, however, when haloperidol was discontinued.
When we ask what happened to him at age 10, Mr. P shrugs.
a) childhood onset schizophrenia
b) substance abuse
c) posttraumatic stress disorder (PTSD)
d) none
The author’s observations
In community samples of children and adolescents, auditory hallucinations are not rare and usually do not cause distress or dysfunction. In a study of 3,870 children age 7 and 8,1 9% endorsed auditory hallucinations. Most heard 1 voice, once a week or less, at low volume. In 85% of children who experienced hallucinations, they caused minimal or no suffering; 97% reported minimal or no interference with daily functioning. Among children who experienced auditory hallucinations at age 7 or 8, 24% continued to hallucinate 5 years later.2 Persistent hallucinations were associated with more problematic behaviors at baseline and follow up.
In a group of 12-year-old twins, 4.2% reported auditory hallucinations.3 In that study, hallucinations were not related to Cannabis use; rather, they were heritable and related to risk factors such as cognitive impairment; behavioral, emotional, and educational problems at age 5; and a history of physical abuse and self-harm at age 12. The authors noted that these are risk factors and correlates of schizophrenia, but are not specific to schizophrenia.
Hallucinations and delusions have been found in 4% to 8% of children and adolescents referred for psychiatric treatment,4 far more than the prevalence of childhood-onset schizophrenia (0.01% of children).5 Psychotic symptoms in children have been associated with bipolar disorder, but also with anxiety disorders, obsessive-compulsive disorder, PTSD, pervasive developmental disorder, conduct disorder, and substance abuse.4
Childhood-onset schizophrenia is rare and would require that Mr. P have a diagnosis of schizophrenia as an adult. It is possible that Mr. P’s childhood symptoms were related to substance abuse but he was not asked for this history because it seemed unlikely in a 10-year-old boy. A PTSD diagnosis requires a traumatic event, which Mr. P did not reveal. It is possible that at age 10 he did not have a psychiatric disorder.
a) PTSD
b) dissociative disorder
c) borderline personality disorder
d) chronic schizophrenia
e) no psychiatric diagnosis
Among adults in the general population, 10% to 15% report auditory hallucinations.6 Hallucinations could be caused by substance abuse or psychiatric conditions other than schizophrenia; however, in adults—as in children—auditory hallucinations can occur in the absence of these conditions (Table 1) and rarely cause distress or dysfunction.6 In Sommer and colleagues’6 study of 103 healthy persons, none who heard voices had disorganization or negative symptoms. Those who heard voices had significantly more schizotypal symptoms and more childhood trauma, including emotional, physical, and sexual abuse, than those who did not hear voices.6
Conditions associated with hallucinations
PTSD is associated with auditory hallucinations and other psychotic symptoms.7 Most studies are of combat veterans with PTSD, in whom auditory hallucinations and delusions were associated with major depressive disorder, not a thought disorder or inappropriate affect.8 In a community sample,9 psychotic symptoms—particularly auditory hallucinations—were associated with PTSD. Subjects with PTSD and psychotic symptoms were more likely to have other psychiatric disorders, including major depressive disorder and substance use disorder, than patients with PTSD but no psychotic symptoms; however, the relationship between PTSD and psychosis remained after controlling for other psychiatric disorders.
Hallucinations can occur in persons with dissociative disorders in the absence of distinct personality states.10 Hallucinations have been seen transiently and chronically in persons with borderline personality disorder and can be associated with comorbid conditions such as substance abuse disorders, mood disorders, and PTSD.11
Mr. P lacked the reduced capacity for interpersonal relationships required for a schizotypal personality disorder diagnosis. A diagnosis of PTSD or dissociative disorder requires a history of trauma, which Mr. P did not report.
“Time travelling” with incomprehensible behavior could be interpreted as dissociation, but dissociative fugue or dissociative disorder not otherwise specified (NOS) cannot be diagnosed if symptoms might be the direct effect of a substance, such as Cannabis. Mr. P admitted to substance abuse. We can rule out borderline personality disorder because he did not display or admit to tempestuous interpersonal relationships.
A schizophrenia diagnosis requires the presence of auditory hallucinations that commented on his behavior or conversed among themselves, a second psychotic symptom for ≥1 month, or negative symptoms, which Mr. P lacked (unless belief in time travel is considered delusional).
Last, a physician might have considered malingering or a factitious disorder when Mr. P was found not able to participate in his own defense, but this seemed less likely after he revealed that he experienced auditory hallucinations since age 10.
HISTORY Bad beatings
With a few days of beginning risperidone, 4 mg/d, Mr. P reports that his hallucinations have stopped and he feels less sad. He reveals that, at age 10, when the hallucinations began, his mother hit him over the head with a high-heeled shoe, causing a scalp laceration that required a visit to the emergency room for suturing. His mother beat Mr. P for as long as he could remember. She beat him “bad” at least twice weekly, and he was taken to the hospital 7 or 8 times for injury, but she also beat him “constantly” with a belt buckle, sometimes striking his head. She instructed him to tell nobody.
The author’s observations
Auditory hallucinations in adults have been associated with childhood abuse, particularly childhood sexual abuse,12 in clinical and non-clinical samples.13 Some argue13 that child abuse itself causes hallucinations and other psychotic symptoms.
OUTCOME Depressed and sleepless
Mr. P admits that he had been smoking marijuana 2 to 3 times daily for a year. He also reports insomnia, sleeping approximately 4 hours a night and spending hours awake in bed thinking of his grandmother, with depressed mood and tearfulness. He denies suicidal ideas and hallucinations. He is treated for depressive disorder NOS first with amitriptyline, 50 mg at bedtime, for sleep, then paroxetine, 20 mg/d, for depressive symptoms, in addition to risperidone, 4 mg/d. Although Mr. P does not describe re-experiencing his childhood trauma, avoidance of stimuli associated with the trauma, or symptoms of increased arousal (except for insomnia), the treatment team did not ask, so it remains uncertain if he has PTSD (Table 2).
When Mr. P is discharged to a clinic, he smiles easily and is positive and supportive with other patients. He spruces up his appearance by wearing jewelry and works in the hospital kitchen.
Bottom Line
Chronic auditory hallucinations are associated with psychiatric illnesses other than chronic schizophrenia, particularly those resulting from trauma such as posttraumatic stress disorder. They can also occur in the absence of diagnosable psychiatric illness and rarely cause distress or functional impairment. Auditory hallucinations in adults have been associated with childhood abuse.
Related Resources
- Moskowitz A, Schafer I, Dorahy MJ. Psychosis, trauma and dissociation: emerging perspectives on severe psychopathology. West Sussex, UK: John Wiley and Sons, Ltd.; 2008.
- The International Hearing Voices Network. www.intervoiceonline.org.
Drug Brand Names
Amitriptyline • Elavil Paroxetine • Paxil
Benztropine • Cogentin Risperidone • Risperdal
Haloperidol • Haldol
Disclosure
Dr. Crowner reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Barthel-Velthuis AA, Jenner JA, van de Willige G, et al Prevalence and correlates of auditory vocal hallucinations in middle childhood. Br J Psychiatry. 2010;196(1):41-46.
2. Bartels-Velthuis AA, van de Willige G, Jenner JA, et al. Course of auditory vocal hallucinations in childhood: 5-year follow-up study. Br J Psychiatry. 2011;199(4):296-302.
3. Polanczyk G, Moffitt TE, Arsensault L, et al. Etiological and clinical features of childhood psychotic symptoms: results from a birth cohort. Arch Gen Psychiatry. 2010;67(4):328-338.
4. Biederman J, Pety C, Faracone SV, et al. Phenomenology of childhood psychosis: Findings from a large sample of psychiatrically referred youth. J Nerv Ment Dis 2004;192(9):607-614.
5. American Academy of Child and Adolescent Psychiatry. Practice parameters for the assessment and treatment of children and adolescents with schizophrenia. J Am Acad Child Adolesc Psychiatry. 2001;40(suppl 7):4SS-23S.
6. Sommer IEC, Daalman K, Rietkerk T, et al. Healthy individuals with auditory verbal hallucinations; Who are they? Psychiatric assessments of a selected sample of 103 subjects. Schizophr Bull. 2010;36(3):633-641.
7. Butler RW, Mueser KT, Sprock J, et al. Positive symptoms of psychosis in posttraumatic stress disorder. Biol Psychiatry. 1996;39:839-844.
8. David D, Kutcher GS, Jackson EI, et al Psychotic symptoms in combat-related posttraumatic stress disorder. J Clin Psychiatry. 1999;60(1):29-32.
9. Sareen J, Cox BJ, Goodwin RD, et al. Co-occurrence of posttraumatic stress disorder with positive psychotic symptoms in a nationally representative sample. J Trauma Stress. 2005;18(4):313-322.
10. Sar V, Akyuv G, Dogan O. Prevalence of dissociative disorders among women in the general population. Psychiatry Res. 2007;149:169-176.
11. Barnow S, Arens EA, Sieswerda S, et al. Borderline personality disorder and psychosis: a review. Curr Psychiatry Rep. 2010;12(3):186-195.
12. Bebbington P, Jonas S, Kuipers E, et al. Childhood sexual abuse and psychosis: data from a cross-sectional national psychiatric survey in England. Br J Psychiatry. 2011;199(1):29-37.
13. Read J, van Os J, Morrison AP, et al. Childhood trauma, psychosis and schizophrenia: a literature review with theoretical and clinical implications. Acta Psychiatr Scand. 2005;112(5):330-350.
1. Barthel-Velthuis AA, Jenner JA, van de Willige G, et al Prevalence and correlates of auditory vocal hallucinations in middle childhood. Br J Psychiatry. 2010;196(1):41-46.
2. Bartels-Velthuis AA, van de Willige G, Jenner JA, et al. Course of auditory vocal hallucinations in childhood: 5-year follow-up study. Br J Psychiatry. 2011;199(4):296-302.
3. Polanczyk G, Moffitt TE, Arsensault L, et al. Etiological and clinical features of childhood psychotic symptoms: results from a birth cohort. Arch Gen Psychiatry. 2010;67(4):328-338.
4. Biederman J, Pety C, Faracone SV, et al. Phenomenology of childhood psychosis: Findings from a large sample of psychiatrically referred youth. J Nerv Ment Dis 2004;192(9):607-614.
5. American Academy of Child and Adolescent Psychiatry. Practice parameters for the assessment and treatment of children and adolescents with schizophrenia. J Am Acad Child Adolesc Psychiatry. 2001;40(suppl 7):4SS-23S.
6. Sommer IEC, Daalman K, Rietkerk T, et al. Healthy individuals with auditory verbal hallucinations; Who are they? Psychiatric assessments of a selected sample of 103 subjects. Schizophr Bull. 2010;36(3):633-641.
7. Butler RW, Mueser KT, Sprock J, et al. Positive symptoms of psychosis in posttraumatic stress disorder. Biol Psychiatry. 1996;39:839-844.
8. David D, Kutcher GS, Jackson EI, et al Psychotic symptoms in combat-related posttraumatic stress disorder. J Clin Psychiatry. 1999;60(1):29-32.
9. Sareen J, Cox BJ, Goodwin RD, et al. Co-occurrence of posttraumatic stress disorder with positive psychotic symptoms in a nationally representative sample. J Trauma Stress. 2005;18(4):313-322.
10. Sar V, Akyuv G, Dogan O. Prevalence of dissociative disorders among women in the general population. Psychiatry Res. 2007;149:169-176.
11. Barnow S, Arens EA, Sieswerda S, et al. Borderline personality disorder and psychosis: a review. Curr Psychiatry Rep. 2010;12(3):186-195.
12. Bebbington P, Jonas S, Kuipers E, et al. Childhood sexual abuse and psychosis: data from a cross-sectional national psychiatric survey in England. Br J Psychiatry. 2011;199(1):29-37.
13. Read J, van Os J, Morrison AP, et al. Childhood trauma, psychosis and schizophrenia: a literature review with theoretical and clinical implications. Acta Psychiatr Scand. 2005;112(5):330-350.
The travesty of disparity and non-parity
Staggering disparities and lack of parity mark many aspects of mental health care, at huge cost to the 25% of Americans who suffer a mental disorder. It’s time to correct this unconscionable discrepancy.
Imagine a world in which aortic stenosis is fully covered by health insurance but coverage for atrial fibrillation is carved out and assigned partial coverage, with a high copayment, a limit on physician visits and hospital days, and a lifetime cap on coverage. If it strikes you as outrageous that disorders of the same organ could be treated so differently, with irrational discrimination, think again! Such an egregious injustice has been going on for a long time against disorders of the brain.
Care provided for stroke, brain tumor, and epilepsy is given full coverage—but not so for depression, psychosis, and panic disorder. To make the matter more bizarre, depression is a known complication of certain types of stroke; some brain tumors are associated with psychosis; and panic attacks can occur with complex partial seizures—demonstrating that the pathways of so-called “mental” illnesses are as “physical” as any neurological disorder of the brain.
Welcome to the absurd world of lack of parity for psychiatric brain disorders!
Senseless, unethical discrimination against mental illness goes on unabated, despite lip service by politicians and policy-makers. The lack of parity adds insult to injury for psychiatric patients, inflicting a financial burden atop unbearable suffering and functional disability.
How long will this injustice endure?
For a civilized society, in which discrimination based on skin color, sex, sexual orientation, and religious belief is prohibited, discrimination by medical illness seems to be tolerated with impunity. This is a blind spot of unimaginable magnitude in the conscience of society and a cancerous lesion on its ethical standards.
The lack of parity in health-care coverage for psychiatric disorders is compounded by several other disparities. Consider this array of discriminatory actions and conditions directed at psychiatrists and their patients:
• The mentally ill endure a stigma that deprives them of the compassion and sympathy that people who have other medical disorders routinely receive.
• Managed-care organizations and some health-maintenance organizations are reluctant to allow the use of newer, more tolerable, and less neurotoxic psychoactive medications by psychiatrists and nurse practitioners; instead, they impose a fail-first policy with decades-old generic drugs to save money—although newer drugs are readily available for oncologists, cardiologists, and neurologists to use.
• Behavioral health is always “carved out”—that obscene word—in insurance contracts. This is overt discrimination against the mentally ill and mental health professionals that the government should not allow, in its role as a watchdog of human rights.
• Psychiatric wards usually are the most poorly renovated and oldest section of hospitals, while obstetrics, oncology, cardiology, and orthopedics units have state-of-the-art facilities. Many inner-city community mental health centers look tad better than a war zone, with dilapidated buildings, stained and broken furniture, and peeling walls. Where are the funds that were supposed to be recovered by shuttering asylums and to flow into community care for outpatient mental health care?
• It’s the height of discrimination that the relapsed mentally ill have no place to go but jail or prison. Isn’t it the worst injustice to transform medical illness into a felony? Why are there few long-term facilities for severe mental illness any more—the equivalent of refractory patients? In a civilized nation, why is homelessness among people who have a brain disorder tolerated?
• The medical records of psychiatric patients are segregated and set aside, perpetuating the unfair shame and guilt associated with mental illness. When will a history of depression and a broken spirit be no more secretive than having a broken leg?
• The lack of regard for psychiatry as a vital medical specialty reflects medieval-like ignorance and discrimination in an age when the neurobiology of psychiatric brain disorders is unfolding with dazzling scientific elegance. When will those who treat mood, thought, behavior, and cognition receive the same respect as a surgeon who transplants a liver or a cardiologist who manages heart failure?
• The disparity in access by the seriously mentally ill to primary, secondary, and tertiary care has, literally, fatal consequences. Despite the high prevalence of serious cardio-metabolic, pulmonary, infectious, and gastrointestinal disorders among the mentally ill, a large percentage do not receive the most basic primary care to manage their ailments—let alone undergo special procedures or advanced surgical interventions. This shameful neglect and health-care disparity contribute to early mortality (by 20 to 25 years) among people who suffer a serious mental disorder. This is a deadly disparity, yet it goes unaddressed.
• Last, a disparity exists in funding for research to find the causes of, and cures for, disorders of the brain’s mind. Psychiatric disorders and substance use cost society more than $300 billion annually in the United States, yet investment in research on those disorders pales compared with the support provided to the study of other medical disorders.
It’s time society closed this ugly gap
Lack of parity extends across a broad swath of issues related to mental illness, with a huge personal and material cost to the 25% of the US population that suffers a mental disorder. It’s time to close the shameful gap and end this harmful discrimination. Let’s hope that ongoing changes in health care will, finally, reverse the injustice. Given the broken promises of the past, however, let’s not count our chickens before they hatch….
Staggering disparities and lack of parity mark many aspects of mental health care, at huge cost to the 25% of Americans who suffer a mental disorder. It’s time to correct this unconscionable discrepancy.
Imagine a world in which aortic stenosis is fully covered by health insurance but coverage for atrial fibrillation is carved out and assigned partial coverage, with a high copayment, a limit on physician visits and hospital days, and a lifetime cap on coverage. If it strikes you as outrageous that disorders of the same organ could be treated so differently, with irrational discrimination, think again! Such an egregious injustice has been going on for a long time against disorders of the brain.
Care provided for stroke, brain tumor, and epilepsy is given full coverage—but not so for depression, psychosis, and panic disorder. To make the matter more bizarre, depression is a known complication of certain types of stroke; some brain tumors are associated with psychosis; and panic attacks can occur with complex partial seizures—demonstrating that the pathways of so-called “mental” illnesses are as “physical” as any neurological disorder of the brain.
Welcome to the absurd world of lack of parity for psychiatric brain disorders!
Senseless, unethical discrimination against mental illness goes on unabated, despite lip service by politicians and policy-makers. The lack of parity adds insult to injury for psychiatric patients, inflicting a financial burden atop unbearable suffering and functional disability.
How long will this injustice endure?
For a civilized society, in which discrimination based on skin color, sex, sexual orientation, and religious belief is prohibited, discrimination by medical illness seems to be tolerated with impunity. This is a blind spot of unimaginable magnitude in the conscience of society and a cancerous lesion on its ethical standards.
The lack of parity in health-care coverage for psychiatric disorders is compounded by several other disparities. Consider this array of discriminatory actions and conditions directed at psychiatrists and their patients:
• The mentally ill endure a stigma that deprives them of the compassion and sympathy that people who have other medical disorders routinely receive.
• Managed-care organizations and some health-maintenance organizations are reluctant to allow the use of newer, more tolerable, and less neurotoxic psychoactive medications by psychiatrists and nurse practitioners; instead, they impose a fail-first policy with decades-old generic drugs to save money—although newer drugs are readily available for oncologists, cardiologists, and neurologists to use.
• Behavioral health is always “carved out”—that obscene word—in insurance contracts. This is overt discrimination against the mentally ill and mental health professionals that the government should not allow, in its role as a watchdog of human rights.
• Psychiatric wards usually are the most poorly renovated and oldest section of hospitals, while obstetrics, oncology, cardiology, and orthopedics units have state-of-the-art facilities. Many inner-city community mental health centers look tad better than a war zone, with dilapidated buildings, stained and broken furniture, and peeling walls. Where are the funds that were supposed to be recovered by shuttering asylums and to flow into community care for outpatient mental health care?
• It’s the height of discrimination that the relapsed mentally ill have no place to go but jail or prison. Isn’t it the worst injustice to transform medical illness into a felony? Why are there few long-term facilities for severe mental illness any more—the equivalent of refractory patients? In a civilized nation, why is homelessness among people who have a brain disorder tolerated?
• The medical records of psychiatric patients are segregated and set aside, perpetuating the unfair shame and guilt associated with mental illness. When will a history of depression and a broken spirit be no more secretive than having a broken leg?
• The lack of regard for psychiatry as a vital medical specialty reflects medieval-like ignorance and discrimination in an age when the neurobiology of psychiatric brain disorders is unfolding with dazzling scientific elegance. When will those who treat mood, thought, behavior, and cognition receive the same respect as a surgeon who transplants a liver or a cardiologist who manages heart failure?
• The disparity in access by the seriously mentally ill to primary, secondary, and tertiary care has, literally, fatal consequences. Despite the high prevalence of serious cardio-metabolic, pulmonary, infectious, and gastrointestinal disorders among the mentally ill, a large percentage do not receive the most basic primary care to manage their ailments—let alone undergo special procedures or advanced surgical interventions. This shameful neglect and health-care disparity contribute to early mortality (by 20 to 25 years) among people who suffer a serious mental disorder. This is a deadly disparity, yet it goes unaddressed.
• Last, a disparity exists in funding for research to find the causes of, and cures for, disorders of the brain’s mind. Psychiatric disorders and substance use cost society more than $300 billion annually in the United States, yet investment in research on those disorders pales compared with the support provided to the study of other medical disorders.
It’s time society closed this ugly gap
Lack of parity extends across a broad swath of issues related to mental illness, with a huge personal and material cost to the 25% of the US population that suffers a mental disorder. It’s time to close the shameful gap and end this harmful discrimination. Let’s hope that ongoing changes in health care will, finally, reverse the injustice. Given the broken promises of the past, however, let’s not count our chickens before they hatch….
Staggering disparities and lack of parity mark many aspects of mental health care, at huge cost to the 25% of Americans who suffer a mental disorder. It’s time to correct this unconscionable discrepancy.
Imagine a world in which aortic stenosis is fully covered by health insurance but coverage for atrial fibrillation is carved out and assigned partial coverage, with a high copayment, a limit on physician visits and hospital days, and a lifetime cap on coverage. If it strikes you as outrageous that disorders of the same organ could be treated so differently, with irrational discrimination, think again! Such an egregious injustice has been going on for a long time against disorders of the brain.
Care provided for stroke, brain tumor, and epilepsy is given full coverage—but not so for depression, psychosis, and panic disorder. To make the matter more bizarre, depression is a known complication of certain types of stroke; some brain tumors are associated with psychosis; and panic attacks can occur with complex partial seizures—demonstrating that the pathways of so-called “mental” illnesses are as “physical” as any neurological disorder of the brain.
Welcome to the absurd world of lack of parity for psychiatric brain disorders!
Senseless, unethical discrimination against mental illness goes on unabated, despite lip service by politicians and policy-makers. The lack of parity adds insult to injury for psychiatric patients, inflicting a financial burden atop unbearable suffering and functional disability.
How long will this injustice endure?
For a civilized society, in which discrimination based on skin color, sex, sexual orientation, and religious belief is prohibited, discrimination by medical illness seems to be tolerated with impunity. This is a blind spot of unimaginable magnitude in the conscience of society and a cancerous lesion on its ethical standards.
The lack of parity in health-care coverage for psychiatric disorders is compounded by several other disparities. Consider this array of discriminatory actions and conditions directed at psychiatrists and their patients:
• The mentally ill endure a stigma that deprives them of the compassion and sympathy that people who have other medical disorders routinely receive.
• Managed-care organizations and some health-maintenance organizations are reluctant to allow the use of newer, more tolerable, and less neurotoxic psychoactive medications by psychiatrists and nurse practitioners; instead, they impose a fail-first policy with decades-old generic drugs to save money—although newer drugs are readily available for oncologists, cardiologists, and neurologists to use.
• Behavioral health is always “carved out”—that obscene word—in insurance contracts. This is overt discrimination against the mentally ill and mental health professionals that the government should not allow, in its role as a watchdog of human rights.
• Psychiatric wards usually are the most poorly renovated and oldest section of hospitals, while obstetrics, oncology, cardiology, and orthopedics units have state-of-the-art facilities. Many inner-city community mental health centers look tad better than a war zone, with dilapidated buildings, stained and broken furniture, and peeling walls. Where are the funds that were supposed to be recovered by shuttering asylums and to flow into community care for outpatient mental health care?
• It’s the height of discrimination that the relapsed mentally ill have no place to go but jail or prison. Isn’t it the worst injustice to transform medical illness into a felony? Why are there few long-term facilities for severe mental illness any more—the equivalent of refractory patients? In a civilized nation, why is homelessness among people who have a brain disorder tolerated?
• The medical records of psychiatric patients are segregated and set aside, perpetuating the unfair shame and guilt associated with mental illness. When will a history of depression and a broken spirit be no more secretive than having a broken leg?
• The lack of regard for psychiatry as a vital medical specialty reflects medieval-like ignorance and discrimination in an age when the neurobiology of psychiatric brain disorders is unfolding with dazzling scientific elegance. When will those who treat mood, thought, behavior, and cognition receive the same respect as a surgeon who transplants a liver or a cardiologist who manages heart failure?
• The disparity in access by the seriously mentally ill to primary, secondary, and tertiary care has, literally, fatal consequences. Despite the high prevalence of serious cardio-metabolic, pulmonary, infectious, and gastrointestinal disorders among the mentally ill, a large percentage do not receive the most basic primary care to manage their ailments—let alone undergo special procedures or advanced surgical interventions. This shameful neglect and health-care disparity contribute to early mortality (by 20 to 25 years) among people who suffer a serious mental disorder. This is a deadly disparity, yet it goes unaddressed.
• Last, a disparity exists in funding for research to find the causes of, and cures for, disorders of the brain’s mind. Psychiatric disorders and substance use cost society more than $300 billion annually in the United States, yet investment in research on those disorders pales compared with the support provided to the study of other medical disorders.
It’s time society closed this ugly gap
Lack of parity extends across a broad swath of issues related to mental illness, with a huge personal and material cost to the 25% of the US population that suffers a mental disorder. It’s time to close the shameful gap and end this harmful discrimination. Let’s hope that ongoing changes in health care will, finally, reverse the injustice. Given the broken promises of the past, however, let’s not count our chickens before they hatch….
Unifying neurology and psychiatry - a 'prescient but premature' notion?
We read with interest Dr. Henry A. Nasrallah’s call to unify psychiatry and neurology into 1 specialty (Current Psychiatry, From the Editor, August 2013, p. 8-9; http://bit.ly/16wImL3). Trends in this direction already are evident. Medical schools, including New York University School of Medicine, the University of Massachusetts Medical School, and the Medical University of South Carolina, have 6-year combined psychiatry and neurology residency programs that prepare students for board exams in either specialty or both specialties. We offer considerations that we hope will energize and inform the discussion, in turn moving us toward discovery of an optimal framework for all stakeholders.
In support of unification, Dr. Nasrallah’s editorial points to research advances in pharmacology, neuroimaging, and genetics. He writes that a “neuropharmacological revolution” is occurring, and it includes the discovery of medications that control symptoms of mood and anxiety disorders. The use of psychotropic medications certainly is escalating; >1 in every 10 Americans currently takes an antidepressant,1,2 reflecting a >400% increase over the past 2 decades.2
However, increasing use does not prove efficacy or establish causal mechanisms. Controversy persists regarding antidepressant efficacy, particularly for mild-to-moderate depression. The Sequenced Treatment Alternatives to Relieve Depression trial3 was the largest study to evaluate antidepressant effectiveness.3 Summarizing the findings, Thomas Insel, MD, Director of the National Institute of Mental Health stated, “Most important, this study demonstrates that for at least 70% of patients, appropriate treatment with an SSRI [selective serotonin reuptake inhibitor] is not enough.”4 What is clear is that the benefits of antidepressants are smaller than originally thought, and may be limited to patients with particular types of (severe) depression.
We agree that developments in neuroimaging and genetics of mental illness are exciting and some day may change the relationship between neurology and psychiatry. However, advanced neuroimaging technologies such as functional magnetic resonance imaging (fMRI) and diffusion tensor imaging (or diffusion MRI) have shown abnormalities involving brain-wide networks in psychiatric disorders5—not distinct regional abnormalities. In other words, psychiatry deals with mental phenomena that are modular in nature and not always reducible to their molecular origins, whereas in neurologic disorders, research linear cause-effect relationships and regional abnormalities are more common.
Genome-wide association studies have shown that few polymorphisms can be associated with numerous mental disorders6 (ie, multifinality), and that several genes may be associated with a particular disorder (ie, equifinality). Multifinality and equfinality are characteristics of complex disorders, with nonlinear gene × gene and gene × environment interactions. In contrast, most heritable neurologic disorders follow a Mendelian pattern of inheritance and predictable cause-effect neuropathology. Furthermore, recent advances in epigenetics—in which environmental and social factors modify gene expression and affect patterns of inheritance7—have further complicated our understanding of genetic contributions to psychiatric illness, and have revealed limitations in reductionist models.
Although neurologic and psychiatric disorders ultimately reflect cerebral pathology, the former commonly result from circumscribed lesions, and the latter are more complexly determined, resulting from disruptions in brain circuitry influenced by genetic, epigenetic, environmental, and social factors.7 As much as we might like it, research in psychiatry has not progressed to the point of fully explaining the brain-based processes underlying complex psychiatric disorders, decipher complex gene × gene and gene × environment interactions, or associate certain genes and biological pathways with specific disorders. A move to formally combine neurology and psychiatry may be prescient but premature because different sets of skills and methodologies might be required of clinicians and researchers in either specialty. More research is needed to shape and refine our disease and training models, and inform evidence-based practice.
Patrick J. Lustman, PhD
St. Louis VA Medical Center
John Cochran Hospital Division
Department of Psychiatry
Washington University School of Medicine
John M. Ray, MA
St. Louis VA Medical Center
John Cochran Hospital Division
Andrea L. Taylor, PhD
St. Louis VA Medical Center
John Cochran Hospital Division
Kenneth E. Freedland, PhD
Department of Psychiatry
Washington University School of Medicine
Dragan M. Svrakic, MD
St. Louis VA Medical Center
John Cochran Hospital Division
Department of Psychiatry
Washington University School of Medicine
St. Louis, Missouri
1. Charney DS, Nemeroff CB, Lewis L, et al. National Depressive and Manic-Depressive Association consensus statement on the use of placebo in clinical trials of mood disorders. Arch Gen Psychiatry. 2002;59(3):262-270.
2. Pratt LA, Brody DJ, Gu Q. Antidepressant use in persons aged 12 and over: United States, 2005-2008. NCHS Data Brief. 2011;(76):1-8.
3. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40.
4. Insel TR. Beyond efficacy: the STAR*D Trial. Am J Psychiatry. 2006;163(1):5-7.
5. Zhou Y, Liang M, Tian L, et al. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr Res. 2007;97(1-3):194-205.
6. Sullivan PA. A framework for interpreting genome-wide association studies of psychiatric disorders. Mol Psychiatry. 2009;14(1):10-17.
7. Svrakic DM, Zorumski CF, Svrakic NM, et al. Risk architecture of schizophrenia: the role of epigenetics. Curr Opin Psychiatry. 2013;26(2):188-195.
We read with interest Dr. Henry A. Nasrallah’s call to unify psychiatry and neurology into 1 specialty (Current Psychiatry, From the Editor, August 2013, p. 8-9; http://bit.ly/16wImL3). Trends in this direction already are evident. Medical schools, including New York University School of Medicine, the University of Massachusetts Medical School, and the Medical University of South Carolina, have 6-year combined psychiatry and neurology residency programs that prepare students for board exams in either specialty or both specialties. We offer considerations that we hope will energize and inform the discussion, in turn moving us toward discovery of an optimal framework for all stakeholders.
In support of unification, Dr. Nasrallah’s editorial points to research advances in pharmacology, neuroimaging, and genetics. He writes that a “neuropharmacological revolution” is occurring, and it includes the discovery of medications that control symptoms of mood and anxiety disorders. The use of psychotropic medications certainly is escalating; >1 in every 10 Americans currently takes an antidepressant,1,2 reflecting a >400% increase over the past 2 decades.2
However, increasing use does not prove efficacy or establish causal mechanisms. Controversy persists regarding antidepressant efficacy, particularly for mild-to-moderate depression. The Sequenced Treatment Alternatives to Relieve Depression trial3 was the largest study to evaluate antidepressant effectiveness.3 Summarizing the findings, Thomas Insel, MD, Director of the National Institute of Mental Health stated, “Most important, this study demonstrates that for at least 70% of patients, appropriate treatment with an SSRI [selective serotonin reuptake inhibitor] is not enough.”4 What is clear is that the benefits of antidepressants are smaller than originally thought, and may be limited to patients with particular types of (severe) depression.
We agree that developments in neuroimaging and genetics of mental illness are exciting and some day may change the relationship between neurology and psychiatry. However, advanced neuroimaging technologies such as functional magnetic resonance imaging (fMRI) and diffusion tensor imaging (or diffusion MRI) have shown abnormalities involving brain-wide networks in psychiatric disorders5—not distinct regional abnormalities. In other words, psychiatry deals with mental phenomena that are modular in nature and not always reducible to their molecular origins, whereas in neurologic disorders, research linear cause-effect relationships and regional abnormalities are more common.
Genome-wide association studies have shown that few polymorphisms can be associated with numerous mental disorders6 (ie, multifinality), and that several genes may be associated with a particular disorder (ie, equifinality). Multifinality and equfinality are characteristics of complex disorders, with nonlinear gene × gene and gene × environment interactions. In contrast, most heritable neurologic disorders follow a Mendelian pattern of inheritance and predictable cause-effect neuropathology. Furthermore, recent advances in epigenetics—in which environmental and social factors modify gene expression and affect patterns of inheritance7—have further complicated our understanding of genetic contributions to psychiatric illness, and have revealed limitations in reductionist models.
Although neurologic and psychiatric disorders ultimately reflect cerebral pathology, the former commonly result from circumscribed lesions, and the latter are more complexly determined, resulting from disruptions in brain circuitry influenced by genetic, epigenetic, environmental, and social factors.7 As much as we might like it, research in psychiatry has not progressed to the point of fully explaining the brain-based processes underlying complex psychiatric disorders, decipher complex gene × gene and gene × environment interactions, or associate certain genes and biological pathways with specific disorders. A move to formally combine neurology and psychiatry may be prescient but premature because different sets of skills and methodologies might be required of clinicians and researchers in either specialty. More research is needed to shape and refine our disease and training models, and inform evidence-based practice.
Patrick J. Lustman, PhD
St. Louis VA Medical Center
John Cochran Hospital Division
Department of Psychiatry
Washington University School of Medicine
John M. Ray, MA
St. Louis VA Medical Center
John Cochran Hospital Division
Andrea L. Taylor, PhD
St. Louis VA Medical Center
John Cochran Hospital Division
Kenneth E. Freedland, PhD
Department of Psychiatry
Washington University School of Medicine
Dragan M. Svrakic, MD
St. Louis VA Medical Center
John Cochran Hospital Division
Department of Psychiatry
Washington University School of Medicine
St. Louis, Missouri
We read with interest Dr. Henry A. Nasrallah’s call to unify psychiatry and neurology into 1 specialty (Current Psychiatry, From the Editor, August 2013, p. 8-9; http://bit.ly/16wImL3). Trends in this direction already are evident. Medical schools, including New York University School of Medicine, the University of Massachusetts Medical School, and the Medical University of South Carolina, have 6-year combined psychiatry and neurology residency programs that prepare students for board exams in either specialty or both specialties. We offer considerations that we hope will energize and inform the discussion, in turn moving us toward discovery of an optimal framework for all stakeholders.
In support of unification, Dr. Nasrallah’s editorial points to research advances in pharmacology, neuroimaging, and genetics. He writes that a “neuropharmacological revolution” is occurring, and it includes the discovery of medications that control symptoms of mood and anxiety disorders. The use of psychotropic medications certainly is escalating; >1 in every 10 Americans currently takes an antidepressant,1,2 reflecting a >400% increase over the past 2 decades.2
However, increasing use does not prove efficacy or establish causal mechanisms. Controversy persists regarding antidepressant efficacy, particularly for mild-to-moderate depression. The Sequenced Treatment Alternatives to Relieve Depression trial3 was the largest study to evaluate antidepressant effectiveness.3 Summarizing the findings, Thomas Insel, MD, Director of the National Institute of Mental Health stated, “Most important, this study demonstrates that for at least 70% of patients, appropriate treatment with an SSRI [selective serotonin reuptake inhibitor] is not enough.”4 What is clear is that the benefits of antidepressants are smaller than originally thought, and may be limited to patients with particular types of (severe) depression.
We agree that developments in neuroimaging and genetics of mental illness are exciting and some day may change the relationship between neurology and psychiatry. However, advanced neuroimaging technologies such as functional magnetic resonance imaging (fMRI) and diffusion tensor imaging (or diffusion MRI) have shown abnormalities involving brain-wide networks in psychiatric disorders5—not distinct regional abnormalities. In other words, psychiatry deals with mental phenomena that are modular in nature and not always reducible to their molecular origins, whereas in neurologic disorders, research linear cause-effect relationships and regional abnormalities are more common.
Genome-wide association studies have shown that few polymorphisms can be associated with numerous mental disorders6 (ie, multifinality), and that several genes may be associated with a particular disorder (ie, equifinality). Multifinality and equfinality are characteristics of complex disorders, with nonlinear gene × gene and gene × environment interactions. In contrast, most heritable neurologic disorders follow a Mendelian pattern of inheritance and predictable cause-effect neuropathology. Furthermore, recent advances in epigenetics—in which environmental and social factors modify gene expression and affect patterns of inheritance7—have further complicated our understanding of genetic contributions to psychiatric illness, and have revealed limitations in reductionist models.
Although neurologic and psychiatric disorders ultimately reflect cerebral pathology, the former commonly result from circumscribed lesions, and the latter are more complexly determined, resulting from disruptions in brain circuitry influenced by genetic, epigenetic, environmental, and social factors.7 As much as we might like it, research in psychiatry has not progressed to the point of fully explaining the brain-based processes underlying complex psychiatric disorders, decipher complex gene × gene and gene × environment interactions, or associate certain genes and biological pathways with specific disorders. A move to formally combine neurology and psychiatry may be prescient but premature because different sets of skills and methodologies might be required of clinicians and researchers in either specialty. More research is needed to shape and refine our disease and training models, and inform evidence-based practice.
Patrick J. Lustman, PhD
St. Louis VA Medical Center
John Cochran Hospital Division
Department of Psychiatry
Washington University School of Medicine
John M. Ray, MA
St. Louis VA Medical Center
John Cochran Hospital Division
Andrea L. Taylor, PhD
St. Louis VA Medical Center
John Cochran Hospital Division
Kenneth E. Freedland, PhD
Department of Psychiatry
Washington University School of Medicine
Dragan M. Svrakic, MD
St. Louis VA Medical Center
John Cochran Hospital Division
Department of Psychiatry
Washington University School of Medicine
St. Louis, Missouri
1. Charney DS, Nemeroff CB, Lewis L, et al. National Depressive and Manic-Depressive Association consensus statement on the use of placebo in clinical trials of mood disorders. Arch Gen Psychiatry. 2002;59(3):262-270.
2. Pratt LA, Brody DJ, Gu Q. Antidepressant use in persons aged 12 and over: United States, 2005-2008. NCHS Data Brief. 2011;(76):1-8.
3. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40.
4. Insel TR. Beyond efficacy: the STAR*D Trial. Am J Psychiatry. 2006;163(1):5-7.
5. Zhou Y, Liang M, Tian L, et al. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr Res. 2007;97(1-3):194-205.
6. Sullivan PA. A framework for interpreting genome-wide association studies of psychiatric disorders. Mol Psychiatry. 2009;14(1):10-17.
7. Svrakic DM, Zorumski CF, Svrakic NM, et al. Risk architecture of schizophrenia: the role of epigenetics. Curr Opin Psychiatry. 2013;26(2):188-195.
1. Charney DS, Nemeroff CB, Lewis L, et al. National Depressive and Manic-Depressive Association consensus statement on the use of placebo in clinical trials of mood disorders. Arch Gen Psychiatry. 2002;59(3):262-270.
2. Pratt LA, Brody DJ, Gu Q. Antidepressant use in persons aged 12 and over: United States, 2005-2008. NCHS Data Brief. 2011;(76):1-8.
3. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40.
4. Insel TR. Beyond efficacy: the STAR*D Trial. Am J Psychiatry. 2006;163(1):5-7.
5. Zhou Y, Liang M, Tian L, et al. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr Res. 2007;97(1-3):194-205.
6. Sullivan PA. A framework for interpreting genome-wide association studies of psychiatric disorders. Mol Psychiatry. 2009;14(1):10-17.
7. Svrakic DM, Zorumski CF, Svrakic NM, et al. Risk architecture of schizophrenia: the role of epigenetics. Curr Opin Psychiatry. 2013;26(2):188-195.
Flying high with Bromo-DragonFLY
I enjoyed reading “New ‘legal’ highs: Kratom and methoxetamine” (Current Psychiatry, Pearls, August 2013, p.54-55; http://bit.ly/1gmPVFB), which discussed legal substances of abuse with adverse effects. I appreciate Dr. Julianna Troy’s recommendation to be familiar with “legal” drugs given their prevalence and potential for adverse effects when taken alone or with psychotropics.
Another legal substance, Bromo-DragonFLY—so named for the resemblance of its chemical structure to the insect—is illegal in many European countries and is being abused in the United States, notably in New Orleans, Louisiana.1 Available as a white or off-white powder, or as paper “blotters,” the drug exerts serotonergic and noradrenergic receptor agonism.1 It is characterized by slow onset but long duration of action.
Intoxication with Bromo-Dragon-FLY beyond the usual dose of 800 to 1,300 µg can result in dystonia, tachycardia, hypertension, psychosis, tachypnea, vasoconstriction with necrosis, seizures, hepatic or renal dysfunction, and death.1-5 Treatment comprises hydration, respiratory support, and benzodiazepines, muscle relaxants, or antipsychotics.1-4
Jonathan R. Scarff, MD
VA Outpatient Clinic
Spartanburg, South Carolina
1. Pletsch G, Rodgman C. Bromo-DragonFLY-induced dystonia. Am J Psychiatry Res J. 2013; 8(2):7-8.
2. Nielsen VT, Høgberg LC, Behrens JK. Bromo-Dragonfly poisoning of 18-year-old male. Ugeskr Laeger. 2010;172(19):1461-1462.
3. Wood DM, Looker JJ, Shaikh L, et al. Delayed onset of seizures and toxicity associated with recreational use of Bromo-dragonFLY. J Med Toxicol. 2009;5(4):226-229.
4. Thorlacius K, Borna C, Personne M. Bromo-DragonFLY-life threatening drug: can cause tissue necrosis as demonstrated by the first described case. Lakartidningen. 2008;105(16):1199-1200.
5. Andreasen MF, Telving R, Birkler RI, et al. A fatal poisoning involving Bromo-Dragonfly. Forensic Sci Int. 2009;183(1-3):91-96.
I enjoyed reading “New ‘legal’ highs: Kratom and methoxetamine” (Current Psychiatry, Pearls, August 2013, p.54-55; http://bit.ly/1gmPVFB), which discussed legal substances of abuse with adverse effects. I appreciate Dr. Julianna Troy’s recommendation to be familiar with “legal” drugs given their prevalence and potential for adverse effects when taken alone or with psychotropics.
Another legal substance, Bromo-DragonFLY—so named for the resemblance of its chemical structure to the insect—is illegal in many European countries and is being abused in the United States, notably in New Orleans, Louisiana.1 Available as a white or off-white powder, or as paper “blotters,” the drug exerts serotonergic and noradrenergic receptor agonism.1 It is characterized by slow onset but long duration of action.
Intoxication with Bromo-Dragon-FLY beyond the usual dose of 800 to 1,300 µg can result in dystonia, tachycardia, hypertension, psychosis, tachypnea, vasoconstriction with necrosis, seizures, hepatic or renal dysfunction, and death.1-5 Treatment comprises hydration, respiratory support, and benzodiazepines, muscle relaxants, or antipsychotics.1-4
Jonathan R. Scarff, MD
VA Outpatient Clinic
Spartanburg, South Carolina
I enjoyed reading “New ‘legal’ highs: Kratom and methoxetamine” (Current Psychiatry, Pearls, August 2013, p.54-55; http://bit.ly/1gmPVFB), which discussed legal substances of abuse with adverse effects. I appreciate Dr. Julianna Troy’s recommendation to be familiar with “legal” drugs given their prevalence and potential for adverse effects when taken alone or with psychotropics.
Another legal substance, Bromo-DragonFLY—so named for the resemblance of its chemical structure to the insect—is illegal in many European countries and is being abused in the United States, notably in New Orleans, Louisiana.1 Available as a white or off-white powder, or as paper “blotters,” the drug exerts serotonergic and noradrenergic receptor agonism.1 It is characterized by slow onset but long duration of action.
Intoxication with Bromo-Dragon-FLY beyond the usual dose of 800 to 1,300 µg can result in dystonia, tachycardia, hypertension, psychosis, tachypnea, vasoconstriction with necrosis, seizures, hepatic or renal dysfunction, and death.1-5 Treatment comprises hydration, respiratory support, and benzodiazepines, muscle relaxants, or antipsychotics.1-4
Jonathan R. Scarff, MD
VA Outpatient Clinic
Spartanburg, South Carolina
1. Pletsch G, Rodgman C. Bromo-DragonFLY-induced dystonia. Am J Psychiatry Res J. 2013; 8(2):7-8.
2. Nielsen VT, Høgberg LC, Behrens JK. Bromo-Dragonfly poisoning of 18-year-old male. Ugeskr Laeger. 2010;172(19):1461-1462.
3. Wood DM, Looker JJ, Shaikh L, et al. Delayed onset of seizures and toxicity associated with recreational use of Bromo-dragonFLY. J Med Toxicol. 2009;5(4):226-229.
4. Thorlacius K, Borna C, Personne M. Bromo-DragonFLY-life threatening drug: can cause tissue necrosis as demonstrated by the first described case. Lakartidningen. 2008;105(16):1199-1200.
5. Andreasen MF, Telving R, Birkler RI, et al. A fatal poisoning involving Bromo-Dragonfly. Forensic Sci Int. 2009;183(1-3):91-96.
1. Pletsch G, Rodgman C. Bromo-DragonFLY-induced dystonia. Am J Psychiatry Res J. 2013; 8(2):7-8.
2. Nielsen VT, Høgberg LC, Behrens JK. Bromo-Dragonfly poisoning of 18-year-old male. Ugeskr Laeger. 2010;172(19):1461-1462.
3. Wood DM, Looker JJ, Shaikh L, et al. Delayed onset of seizures and toxicity associated with recreational use of Bromo-dragonFLY. J Med Toxicol. 2009;5(4):226-229.
4. Thorlacius K, Borna C, Personne M. Bromo-DragonFLY-life threatening drug: can cause tissue necrosis as demonstrated by the first described case. Lakartidningen. 2008;105(16):1199-1200.
5. Andreasen MF, Telving R, Birkler RI, et al. A fatal poisoning involving Bromo-Dragonfly. Forensic Sci Int. 2009;183(1-3):91-96.
Is he DISTRACTED? Considerations when diagnosing ADHD in an adult
Adult attention-deficit/hyperactivity disorder (ADHD) can be challenging to assess accurately. Adult ADHD differs significantly from childhood ADHD, in that hyperactivity often is absent or greatly diminished, comorbid disorders (depression or substance use) are common, and previously compensated attention deficits in school can manifest in the patient’s personal and professional life.1
The mnemonic DISTRACTED can help when recalling key components in assessing adult ADHD.2 Because ADHD is a developmental disorder—there are signs of onset in childhood—it is important to maintain a longitudinal view when asking about patterns of behavior or thinking.
Distractibility. Is there a pattern of getting “off track” in conversations or in school or work situations because of straying thoughts or daydreams? Is there a tendency to over-respond to extraneous stimuli (eg, cell phones, computers, television) that impedes the patient’s ability to converse, receive information, or follow directions?
Impulsivity. Does the patient have a history of saying things “off the cuff,” interrupting others, or “walking on” someone else’s words in a conversation? Is impulsivity evident in the person’s substance use or spending patterns?
School history. This domain is important in diagnosing ADHD in adults because there needs to be evidence that the disorder was present from an early age. How did the patient perform in school (ie, grades, organization, completion of homework assignments)? Was there a behavioral pattern that reflected hyperactivity (could not stay seated) or emotional dysregulation (frequent outbursts)?
Task completion. Does the patient have trouble finishing assignments at work, staying focused on a project that is considered boring, or completing a home project (eg, fixing a leaky faucet) in a timely fashion?
Rating scales. Rating scales should be used to help support the diagnosis, based on the patient’s history and life story. There are >12 scales that can be utilized in a
clinical setting3; the ADHD/Hyperactivity Disorder Self-Report Scale is a brief and easy measure of core ADHD symptoms.
Accidents. Adults with ADHD often are accident-prone because of inattention, hyperactivity, or impulsivity. Does the patient have a history of unintentionally hurting himself because he “wasn’t paying attention” (falls, burns), or was too impatient (traffic accidents or citations)?
Commitments. Does the patient fail to fulfill verbal obligations (by arriving late, forgetting to run errands)? Has this difficulty to commit created problems in relationships over time?
Time management. How difficult is it for the patient to stay organized while balancing work expectations, social obligations, and family needs? Is there a pattern of chaotic scheduling with regard to meals, work, or sleeping?
Employment. Has the patient changed jobs because the work becomes “too boring” or “uninteresting”? Is there a pattern of being terminated because of poor work quality based on time management or job performance?
Decisions. Adults with ADHD often make hasty, ill-informed choices or procrastinate so that they do not have to make a decision. Does the patient’s decision-making reveal a pattern of being too distracted to hear the information needed, or too impatient to consider all the details?
Remember: No single component of this mnemonic alone suffices to make a diagnosis of adult ADHD. However, these considerations will help clarify what lies behind your DISTRACTED patient’s search for self-understanding and appropriate medical care.
Disclosure
Dr. Christensen reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Barkley RA, Brown TE. Unrecognized attention-deficit/hyperactivity disorder in adults presenting with other psychiatric disorders. CNS Spectr. 2008;13(11):977-984.
2. Barkley R. Taking charge of adult ADHD. New York, NY: Guilford Press; 2010.
3. Attwell C. ADHD, rating scales, and your practice today. The Carlat Psychiatry Report. 2012;10(12):1,3,5-8.
Adult attention-deficit/hyperactivity disorder (ADHD) can be challenging to assess accurately. Adult ADHD differs significantly from childhood ADHD, in that hyperactivity often is absent or greatly diminished, comorbid disorders (depression or substance use) are common, and previously compensated attention deficits in school can manifest in the patient’s personal and professional life.1
The mnemonic DISTRACTED can help when recalling key components in assessing adult ADHD.2 Because ADHD is a developmental disorder—there are signs of onset in childhood—it is important to maintain a longitudinal view when asking about patterns of behavior or thinking.
Distractibility. Is there a pattern of getting “off track” in conversations or in school or work situations because of straying thoughts or daydreams? Is there a tendency to over-respond to extraneous stimuli (eg, cell phones, computers, television) that impedes the patient’s ability to converse, receive information, or follow directions?
Impulsivity. Does the patient have a history of saying things “off the cuff,” interrupting others, or “walking on” someone else’s words in a conversation? Is impulsivity evident in the person’s substance use or spending patterns?
School history. This domain is important in diagnosing ADHD in adults because there needs to be evidence that the disorder was present from an early age. How did the patient perform in school (ie, grades, organization, completion of homework assignments)? Was there a behavioral pattern that reflected hyperactivity (could not stay seated) or emotional dysregulation (frequent outbursts)?
Task completion. Does the patient have trouble finishing assignments at work, staying focused on a project that is considered boring, or completing a home project (eg, fixing a leaky faucet) in a timely fashion?
Rating scales. Rating scales should be used to help support the diagnosis, based on the patient’s history and life story. There are >12 scales that can be utilized in a
clinical setting3; the ADHD/Hyperactivity Disorder Self-Report Scale is a brief and easy measure of core ADHD symptoms.
Accidents. Adults with ADHD often are accident-prone because of inattention, hyperactivity, or impulsivity. Does the patient have a history of unintentionally hurting himself because he “wasn’t paying attention” (falls, burns), or was too impatient (traffic accidents or citations)?
Commitments. Does the patient fail to fulfill verbal obligations (by arriving late, forgetting to run errands)? Has this difficulty to commit created problems in relationships over time?
Time management. How difficult is it for the patient to stay organized while balancing work expectations, social obligations, and family needs? Is there a pattern of chaotic scheduling with regard to meals, work, or sleeping?
Employment. Has the patient changed jobs because the work becomes “too boring” or “uninteresting”? Is there a pattern of being terminated because of poor work quality based on time management or job performance?
Decisions. Adults with ADHD often make hasty, ill-informed choices or procrastinate so that they do not have to make a decision. Does the patient’s decision-making reveal a pattern of being too distracted to hear the information needed, or too impatient to consider all the details?
Remember: No single component of this mnemonic alone suffices to make a diagnosis of adult ADHD. However, these considerations will help clarify what lies behind your DISTRACTED patient’s search for self-understanding and appropriate medical care.
Disclosure
Dr. Christensen reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Adult attention-deficit/hyperactivity disorder (ADHD) can be challenging to assess accurately. Adult ADHD differs significantly from childhood ADHD, in that hyperactivity often is absent or greatly diminished, comorbid disorders (depression or substance use) are common, and previously compensated attention deficits in school can manifest in the patient’s personal and professional life.1
The mnemonic DISTRACTED can help when recalling key components in assessing adult ADHD.2 Because ADHD is a developmental disorder—there are signs of onset in childhood—it is important to maintain a longitudinal view when asking about patterns of behavior or thinking.
Distractibility. Is there a pattern of getting “off track” in conversations or in school or work situations because of straying thoughts or daydreams? Is there a tendency to over-respond to extraneous stimuli (eg, cell phones, computers, television) that impedes the patient’s ability to converse, receive information, or follow directions?
Impulsivity. Does the patient have a history of saying things “off the cuff,” interrupting others, or “walking on” someone else’s words in a conversation? Is impulsivity evident in the person’s substance use or spending patterns?
School history. This domain is important in diagnosing ADHD in adults because there needs to be evidence that the disorder was present from an early age. How did the patient perform in school (ie, grades, organization, completion of homework assignments)? Was there a behavioral pattern that reflected hyperactivity (could not stay seated) or emotional dysregulation (frequent outbursts)?
Task completion. Does the patient have trouble finishing assignments at work, staying focused on a project that is considered boring, or completing a home project (eg, fixing a leaky faucet) in a timely fashion?
Rating scales. Rating scales should be used to help support the diagnosis, based on the patient’s history and life story. There are >12 scales that can be utilized in a
clinical setting3; the ADHD/Hyperactivity Disorder Self-Report Scale is a brief and easy measure of core ADHD symptoms.
Accidents. Adults with ADHD often are accident-prone because of inattention, hyperactivity, or impulsivity. Does the patient have a history of unintentionally hurting himself because he “wasn’t paying attention” (falls, burns), or was too impatient (traffic accidents or citations)?
Commitments. Does the patient fail to fulfill verbal obligations (by arriving late, forgetting to run errands)? Has this difficulty to commit created problems in relationships over time?
Time management. How difficult is it for the patient to stay organized while balancing work expectations, social obligations, and family needs? Is there a pattern of chaotic scheduling with regard to meals, work, or sleeping?
Employment. Has the patient changed jobs because the work becomes “too boring” or “uninteresting”? Is there a pattern of being terminated because of poor work quality based on time management or job performance?
Decisions. Adults with ADHD often make hasty, ill-informed choices or procrastinate so that they do not have to make a decision. Does the patient’s decision-making reveal a pattern of being too distracted to hear the information needed, or too impatient to consider all the details?
Remember: No single component of this mnemonic alone suffices to make a diagnosis of adult ADHD. However, these considerations will help clarify what lies behind your DISTRACTED patient’s search for self-understanding and appropriate medical care.
Disclosure
Dr. Christensen reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Barkley RA, Brown TE. Unrecognized attention-deficit/hyperactivity disorder in adults presenting with other psychiatric disorders. CNS Spectr. 2008;13(11):977-984.
2. Barkley R. Taking charge of adult ADHD. New York, NY: Guilford Press; 2010.
3. Attwell C. ADHD, rating scales, and your practice today. The Carlat Psychiatry Report. 2012;10(12):1,3,5-8.
1. Barkley RA, Brown TE. Unrecognized attention-deficit/hyperactivity disorder in adults presenting with other psychiatric disorders. CNS Spectr. 2008;13(11):977-984.
2. Barkley R. Taking charge of adult ADHD. New York, NY: Guilford Press; 2010.
3. Attwell C. ADHD, rating scales, and your practice today. The Carlat Psychiatry Report. 2012;10(12):1,3,5-8.