User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Do stimulants for ADHD increase the risk of substance use disorders?
Discuss this article at www.facebook.com/CurrentPsychiatry
Does prescribing stimulants to patients with attention-deficit/hyperactivity disorder (ADHD) increase their risk of future substance abuse? Because ADHD is a common pediatric condition with symptoms that often persist into adulthood, and stimulants are an efficacious first-line therapy, this possible association is a concern for psychiatrists whether they treat children or adults.
Some researchers have expressed concerns that stimulant exposure could predispose patients to future substance abuse.1 Proponents of the biologic model of “kindling” hypothesize early exposure to stimulants could increase the risk of later substance use disorders (SUDs) by modifying or “priming” the brain, which then becomes more receptive to illicit drug exposure. Although there is some evidence that stimulant use does increase SUD risk, other evidence suggests stimulant use does not increase susceptibility to SUDs2,3 and some studies have suggested stimulant use in ADHD patients may protect against SUDs.4,5
This article reviews shared characteristics of ADHD and SUDs and the latest research on the association between the clinical use of stimulants and later development of SUDs. We also offer clinical recommendations for assessing and treating ADHD and comorbid SUD.
ADHD/SUD overlap
Compared with those without the disorder, patients with ADHD have a 6.2 times higher risk of developing an SUD.6 Individuals with ADHD experience an earlier age of onset and a longer duration of SUDs.7 Several retrospective and prospective studies reveal ADHD is a risk factor for SUDs.8 A longitudinal study that tracked teenage males with or without ADHD into young adulthood found SUDs were 4 times more common among those with ADHD.9 Up to 45% of adults with ADHD have a history of alcohol abuse or dependence, and up to 30% have a history of illegal drug abuse or dependence.10
Conversely, an estimated 35% to 71% of alcohol abusers and 15% to 25% of substance-dependent patients have ADHD.11 Adults with ADHD and comorbid SUD report earlier onset12 and greater severity13 of substance abuse than adults without ADHD. Patients with ADHD experience earlier onset and higher rates of tobacco smoking by mid-adolescence.14
Developmental psychopathology. Longitudinal studies have suggested certain psychopathologic characteristics of ADHD can predispose an individual to SUDs independent of stimulant exposure. For example, inattention, impulsivity, and hyperactivity predispose an individual to develop an SUD and also are core symptoms of ADHD.15 Another study found impulsivity, impersistence, and difficulty sitting still at age 3 predicted alcohol abuse at age 21.16 A different longitudinal study found novelty-seeking behavior (restlessness, running/jumping and not keeping still, being squirmy and fidgety) between age 6 to 10 predicted adolescent drug abuse and cigarette smoking.17 Poor response inhibition is a key characteristic of ADHD and has been linked to adolescent drinking.18
ADHD may be an independent risk factor for SUD because a common neurobiologic psychopathology may predispose an individual to develop both conditions. The dopamine system has been implicated in SUD, and dysfunction in the dopaminergic circuits—mostly in basal and frontal cortex with consequent defects in executive function and reward system—also has been found in ADHD.19 Cognitive dysfunction associated with ADHD may decrease a patient’s ability to estimate the negative consequences of substance abuse and to delay immediate gratification from drug or alcohol use.
ADHD patients are more vulnerable to SUDs if they have a comorbid condition, such as oppositional defiant disorder,13,20 bipolar disorder,20,21 or conduct disorder (CD).20,22 Patients with ADHD and comorbid CD are estimated to be 8.8 times more likely to have an SUD before age 18 compared with those with ADHD alone.23 Comorbid ADHD and CD may increase patients’ predisposition to develop dependence on highly addictive drugs, such as cocaine or methamphetamine.24 Impaired executive function, behavioral dyscontrol, impulsivity, and peer rejection are common in both ADHD and CD and may increase the risk of developing SUDs in individuals who have both conditions.25 Other risk factors for SUDs in patients with ADHD are listed in Table 1.26
Table 1
Risk factors for SUDs in patients with ADHD
| Presence of comorbid conditions (ie, oppositional defiant disorder, conduct disorder, bipolar disorder, eating disorder) |
| White or Hispanic race |
| Partially treated or residual ADHD symptoms |
| Attending a competitive college program |
| College youth who had late onset of stimulant treatment |
| Member of a college sorority/fraternity |
| ADHD: attention-deficit/hyperactivity disorder; SUDs: substance use disorders Source: Reference 26 |
Stimulants’ affect on SUD risk
Increased risk. Limited studies suggest exposure to stimulants is a risk factor for developing SUDs. In a longitudinal study, Lambert et al27 followed 218 patients with ADHD and 182 without ADHD into adulthood and found a linear trend between duration of stimulant treatment and prevalence of cocaine dependence. ADHD patients exposed to stimulants for >1 year had the highest prevalence of cocaine abuse (27%), compared with untreated subjects (15%), or those treated with stimulants for <1 year (18%). However, the study did not control for comorbid contributing factors, such as CD.
No change. In a 10-year naturalistic study, Biederman et al28 followed 109 children with ADHD age 7 to 12 into adulthood. These children had a developmental reading disorder but no other psychiatric comorbidities. When comparing patients who were treated with methylphenidate (n = 43) with those who did not receive stimulants (n = 66), Bierderman et al found no significant difference between the 2 groups in the prevalence of SUD for any of the 7 drug categories studied.
Decreased risk. Two meta-analyses found children with ADHD who were treated with stimulants and followed until adolescence were 5.8 times less likely to develop SUDs compared with those who did not receive stimulants.28,29 This protective effect diminished when patients were followed into adulthood, but individuals treated with stimulants were 1.4 times less likely to develop SUDs than those not treated with stimulants.30 In a prospective case-control, 5-year follow-up study of 114 patients with ADHD treated with stimulants, Wilens et al31 found significant protective effects of stimulant treatment on the development of any SUD. They found no effects from time of onset or duration of stimulant therapy on subsequent risk of SUDs or cigarette smoking.
One possible explanation for stimulants’ apparently reduced protective effect among adults is for patients with ADHD, stimulant use might delay but not prevent SUDs. It also is likely that by adulthood, loss of parental supervision leads to poor medication adherence and increased susceptibility to SUDs.30
Other studies have found exposure to stimulants may protect against SUDs. Katusic et al23 reviewed medical records for documented SUDs in 295 adults with ADHD treated with stimulants and 84 who did not receive stimulants. They found 20% of patients who received stimulants had a documented SUD compared with 27% of those not treated with stimulants. Barkely et al32 followed 98 stimulant-treated and 21 untreated ADHD patients with a mean age of 15 and 21, respectively. They found stimulant treatment did not increase the risk for substance use or abuse in either group.
ADHD and stimulant abuse
The prevalence of stimulant misuse is as high as 9% in patients in grade school and high school and up to 35% in college-age individuals.33 ADHD patients who misuse stimulants (eg, escalating dose without authorization) or skip stimulant doses to use illicit drugs or alcohol are more likely to sell their medication.34 Immediate-release stimulant formulations are more liable to be abused than extended-release drugs because they achieve earlier peak drug concentrations and dopamine blockade, indicating rapid drug absorption and central drug activity. Close monitoring and use of extended-release formulations are useful deterrents against stimulant abuse.
Clinical recommendations
Detecting and treating SUDs in patients with ADHD can be challenging. Ideally, the best time to assess for ADHD symptoms is after a prolonged abstinence from any influencing substance. However, in most clinical situations this is not practical. A better approach is a longitudinal assessment for ADHD symptoms. Detecting evidence of early childhood onset of ADHD symptoms before the patient began using substances can be helpful in conducting a proper differential diagnosis. Assessing for symptoms of SUDs in early adolescence, along with serial assessment of ADHD symptoms, also can be helpful. Symptoms secondary to ADHD are likely to show a consistent pattern, whereas symptoms secondary to an SUD may be sporadic.
When assessing SUD risk, consider the patient’s clinical condition, history of comorbidities that suggest SUDs, and overall functional status. Collateral information about the patient’s behavior and substance abuse from family members is important. A history of CD, bipolar disorder, or antisocial personality disorder should raise concerns about potential future stimulant abuse or diversion. Close monitoring of patients suspected of having an SUD is essential to detect stimulant abuse or diversion, which often manifests as weight loss, requests for higher doses, requests to switch from long-acting or extended-release formulations to immediate-release formulations, and repeated and suspicious “lost prescriptions.” Close observation for other subtle signs—such as changes in personality or mood and unexplained accidents or injuries—also may be needed.35
Challenges of treating ADHD and co-occurring SUD include poor medication adherence, need for a higher therapeutic stimulant dose, and difficulty in assessing the therapeutic benefit of pharmacotherapy in the presence of an SUD.36 Treating ADHD comorbid with SUD requires a collaborative approach that involves a psychiatrist, family members, and a behavioral care provider in addition to frequent monitoring.34
In the absence of treatment guidelines for treating ADHD with comorbid SUDs, some clinicians prefer to stabilize the SUD before initiating stimulants. Others prefer to use nonstimulants (such as atomoxetine, guanfacine, bupropion, venlafaxine, tricyclic antidepressants, or modafinil) as a first-line treatment. However, nonstimulants have not demonstrated efficacy comparable to that of stimulants for ADHD.35
Table 2 offers clinical recommendations to minimize the risk of SUDs when treating ADHD patients with stimulants. Long-acting stimulant formulations are preferred over short-acting medications because they are less likely to be abused. Psychosocial interventions for treating ADHD and co-occurring SUD disorder include cognitive-behavioral therapy with emphasis on structured skills training and cognitive remediation.
Table 2
Minimizing SUD risk when treating ADHD patients with stimulants
| Assess symptom burden and psychosocial impairment |
| Establish a treatment contract and boundaries at the onset of treatment, including your right to terminate treatment if you suspect stimulant misuse |
| Assess for comorbidities that may increase your patient’s SUD risk (see Table 1) |
| Emphasize strict adherence to treatment recommendations |
| Involve the patient’s family as much as possible |
| Obtain collateral information on the patient’s history of ADHD-related symptoms from parents, siblings, significant others, etc. |
| Distinguish between patients with substance use vs an SUD or a history of an SUD |
| Obtain urine toxicology screening as appropriate |
| Carefully document dispensed stimulants– strength of medication, number of capsules, pills, patches, etc. Note date of dispensation and refill dates |
| Select delayed- or extended-release stimulant formulations |
| Consider prescribing nonstimulants if appropriate |
| Use rating scales such as Conners Adult ADHD Rating Scale to monitor ADHD symptom severity and response to treatment |
| Schedule frequent, face-to-face clinical monitoring visits |
| ADHD: attention-deficit/hyperactivity disorder; SUD: substance use disorder |
Related Resource
- Faraone SV, Wilens T. Does stimulant treatment lead to substance use disorders? J Clin Psychiatry. 2003;64(suppl 11):9-13.
- Upadhyaya HP, Rose K, Wang W, et al. Attention deficit hyperactivity disorder medication and substance use patterns among adolescents and young adults. J Child Adolesc Psychopharmacol. 2005;15:799-809.
- Mariani JJ, Levin FR. Treatment strategies for co-occurring ADHD and substance use disorders. Am J Addict. 2007;16(suppl 1):45-56.
Drug Brand Names
- Atomoxetine • Strattera
- Bupropion • Wellbutrin, Zyban
- Guanfacine • Tenex, Intuniv
- Methylphenidate • Ritalin
- Modafinil • Provigil
- Venlafaxine • Effexor
Disclosures
Dr. Shailesh Jain and Dr. Islam report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Rakesh Jain has received research support from, is a consultant to, and/or is a speaker for Addrenex Pharmaceuticals, AstraZeneca, Eli Lilly and Company, Forest Pharmaceuticals, Merck, Pamlab, Pfizer Inc., Shionogi Inc., Shire, and Sunovion Pharmaceuticals.
1. Lambert NM, McLeod M, Schenk S. Subjective responses to initial experience with cocaine: an exploration of the incentive-sensitization theory of drug abuse. Addiction. 2006;101(5):713-725.
2. Mannuzza S, Klein RG, Moulton JL. Does stimulant treatment place children at risk for adult substance abuse? A controlled prospective follow-up study. J Child Adolesc Psychopharmacol. 2003;13(3):273-282.
3. Katusic SK, Barbaresi WJ, Colligan RC, et al. Psychostimulant treatment and risk for substance abuse among young adults with a history of attention-deficit/hyperactivity disorder: a population-based, birth cohort study. J Child Adolesc Psychopharmacol. 2005;15(5):764-776.
4. Wilens TE, Faraone SV, Biederman J, et al. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111(1):179-185.
5. Biederman J, Wilens T, Mick E, et al. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics. 1999;104(2):e20.-
6. Katusic SK, Barbaresi WJ, Colligan RC, et al. Psychostimulant treatment and risk for substance abuse among young adults with a history of attention-deficit/hyperactivity disorder: a population-based, birth cohort study. J Child Adolesc Psychopharmacol. 2005;15(5):764-776.
7. Wilens TE. Impact of ADHD and its treatment on substance abuse in adults. J Clin Psychiatry. 2004;65(suppl 3):38-45.
8. Barkley RA, Fischer M, Smallish L, et al. Young adult follow-up of hyperactive children: antisocial activities and drug use. J Child Psychol Psychiatry. 2004;45(2):195-221.
9. Mannuzza S, Klein RG, Bessler A, et al. Adult outcome of hyperactive boys. Educational achievement, occupational rank, and psychiatric status. Arch Gen Psychiatry. 1993;50(7):565-576.
10. Wilens TE. Attention-deficit/hyperactivity disorder and the substance use disorders: the nature of the relationship subtypes at risk, and treatment issues. Psychiatr Clin North Am. 2004;27(2):283-301.
11. Wilens TE. AOD use and attention deficit/hyperactivity disorder. Alcohol Health Res World. 1998;22(2):127-130.
12. Wilens TE, Biederman J, Abrantes AM, et al. Clinical characteristics of psychiatrically referred adolescent outpatients with substance use disorder. J Am Acad Child Adolesc Psychiatry. 1997;36(7):941-947.
13. Schubiner H, Tzelepis A, Milberger S, et al. Prevalence of attention-deficit/hyperactivity disorder and conduct disorder among substance abusers. J Clin Psychiatry. 2000;61(4):244-251.
14. Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. J Learn Disabil. 1998;31(6):533-544.
15. Zucker RA. Alcohol use and the alcohol use disorders: a developmental biopsychosocial systems formulation covering the life course. In: Cicchetti D Cohen D, eds. Developmental psychopathology. 2nd ed. Hoboken, NJ: John Wiley & Sons Inc; 2006;620-656.
16. Caspi A, Moffitt TE, Newman DL, et al. Behavioral observations at age 3 years predict adult psychiatric disorders. Longitudinal evidence from a birth cohort. Arch Gen Psychiatry. 1996;53(11):1033-1039.
17. Màsse LC, Tremblay RE. Behavior of boys in kindergarten and the onset of substance use during adolescence. Arch Gen Psychiatry. 1997;54(1):62-68.
18. Nigg JT, Wong MM, Martel MM, et al. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(4):468-475.
19. Seidman LJ, Valera EM, Makris N. Structural brain imaging of attention-deficit hyperactivity disorder. Biol Psychiatry. 2005;57(11):1263-1272.
20. Biederman J, Wilens T, Mick E, et al. Is ADHD a risk factor for psychoactive substance use disorders? Findings from a four-year prospective follow-up study. J Am Acad Child Adolesc Psychiatry. 1997;36(1):21-29.
21. Wilens TE, Biederman J, Millstein RB, et al. Risk for substance use disorders in youths with child- and adolescent-onset bipolar disorder. J Am Acad Child Adolesc Psychiatry. 1999;38(6):680-685.
22. Schubiner H, Saules KK, Arfken CL, et al. Double-blind placebo-controlled trial of methylphenidate in the treatment of adult ADHD patients with comorbid cocaine dependence. Exp Clin Psychopharmacol. 2002;10(3):286-294.
23. Katusic SK, Barbaresi WJ, Colligan RC, et al. Psychostimulant treatment and risk for substance abuse among young adults with a history of attention-deficit/hyperactivity disorder: a population-based, birth cohort study. J Child Adolesc Psychopharmacol. 2005;15(5):764-776.
24. Flory K, Milich R, Lynam DR, et al. Relation between childhood disruptive behavior disorders and substance use and dependence symptoms in young adulthood: individuals with symptoms of attention-deficit/hyperactivity disorder and conduct disorder are uniquely at risk. Psychol Addict Behav. 2003;17(2):151-158.
25. Wilens TE. Attention-deficit/hyperactivity disorder and the substance use disorders: the nature of the relationship subtypes at risk, and treatment issues. Psychiatr Clin North Am. 2004;27(2):283-301.
26. Wilson JJ. ADHD and substance use disorders: developmental aspects and the impact of stimulant treatment. Am J Addict. 2007;16(suppl 1):5-11.
27. Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. J Learn Disabil. 1998;31(6):533-544.
28. Biederman J, Monuteaux MC, Spencer T, et al. Stimulant therapy and risk for subsequent substance use disorders in male adults with ADHD: a naturalistic controlled 10-year follow-up study. Am J Psychiatry. 2008;165(5):597-603.
29. Wilens TE, Faraone SV, Biederman J, et al. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111(1):179-185.
30. Faraone SV, Wilens TE. Effect of stimulant medications for attention-deficit/hyperactivity disorder on later substance use and the potential for stimulant misuse abuse, and diversion. J Clin Psychiatry. 2007;68(suppl 11):15-22.
31. Wilens TE, Adamson J, Monuteaux MC, et al. Effect of prior stimulant treatment for attention-deficit/hyperactivity disorder on subsequent risk for cigarette smoking and alcohol and drug use disorders in adolescents. Arch Pediatr Adolesc Med. 2008;162(10):916-921.
32. Barkley RA, Fischer M, Smallish L, et al. Does the treatment of attention-deficit/hyperactivity disorder with stimulants contribute to drug use/abuse? A 13-year prospective study. Pediatrics. 2003;111(1):97-109.
33. Wilens TE, Adler LA, Adams J, et al. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47(1):21-31.
34. Upadhyaya HP, Rose K, Wang W, et al. Attention-deficit/hyperactivity disorder, medication treatment, and substance use patterns among adolescents and young adults. J Child Adolesc Psychopharmacol. 2005;15(5):799-809.
35. Kollins SH. A qualitative review of issues arising in the use of psycho-stimulant medications in patients with ADHD and co-morbid substance use disorders. Curr Med Res Opin. 2008;24(5):1345-1357.
36. Faraone SV, Biederman J, Wilens TE, et al. A naturalistic study of the effects of pharmacotherapy on substance use disorders among ADHD adults. Psychol Med. 2007;37(12):1743-1752.
Discuss this article at www.facebook.com/CurrentPsychiatry
Does prescribing stimulants to patients with attention-deficit/hyperactivity disorder (ADHD) increase their risk of future substance abuse? Because ADHD is a common pediatric condition with symptoms that often persist into adulthood, and stimulants are an efficacious first-line therapy, this possible association is a concern for psychiatrists whether they treat children or adults.
Some researchers have expressed concerns that stimulant exposure could predispose patients to future substance abuse.1 Proponents of the biologic model of “kindling” hypothesize early exposure to stimulants could increase the risk of later substance use disorders (SUDs) by modifying or “priming” the brain, which then becomes more receptive to illicit drug exposure. Although there is some evidence that stimulant use does increase SUD risk, other evidence suggests stimulant use does not increase susceptibility to SUDs2,3 and some studies have suggested stimulant use in ADHD patients may protect against SUDs.4,5
This article reviews shared characteristics of ADHD and SUDs and the latest research on the association between the clinical use of stimulants and later development of SUDs. We also offer clinical recommendations for assessing and treating ADHD and comorbid SUD.
ADHD/SUD overlap
Compared with those without the disorder, patients with ADHD have a 6.2 times higher risk of developing an SUD.6 Individuals with ADHD experience an earlier age of onset and a longer duration of SUDs.7 Several retrospective and prospective studies reveal ADHD is a risk factor for SUDs.8 A longitudinal study that tracked teenage males with or without ADHD into young adulthood found SUDs were 4 times more common among those with ADHD.9 Up to 45% of adults with ADHD have a history of alcohol abuse or dependence, and up to 30% have a history of illegal drug abuse or dependence.10
Conversely, an estimated 35% to 71% of alcohol abusers and 15% to 25% of substance-dependent patients have ADHD.11 Adults with ADHD and comorbid SUD report earlier onset12 and greater severity13 of substance abuse than adults without ADHD. Patients with ADHD experience earlier onset and higher rates of tobacco smoking by mid-adolescence.14
Developmental psychopathology. Longitudinal studies have suggested certain psychopathologic characteristics of ADHD can predispose an individual to SUDs independent of stimulant exposure. For example, inattention, impulsivity, and hyperactivity predispose an individual to develop an SUD and also are core symptoms of ADHD.15 Another study found impulsivity, impersistence, and difficulty sitting still at age 3 predicted alcohol abuse at age 21.16 A different longitudinal study found novelty-seeking behavior (restlessness, running/jumping and not keeping still, being squirmy and fidgety) between age 6 to 10 predicted adolescent drug abuse and cigarette smoking.17 Poor response inhibition is a key characteristic of ADHD and has been linked to adolescent drinking.18
ADHD may be an independent risk factor for SUD because a common neurobiologic psychopathology may predispose an individual to develop both conditions. The dopamine system has been implicated in SUD, and dysfunction in the dopaminergic circuits—mostly in basal and frontal cortex with consequent defects in executive function and reward system—also has been found in ADHD.19 Cognitive dysfunction associated with ADHD may decrease a patient’s ability to estimate the negative consequences of substance abuse and to delay immediate gratification from drug or alcohol use.
ADHD patients are more vulnerable to SUDs if they have a comorbid condition, such as oppositional defiant disorder,13,20 bipolar disorder,20,21 or conduct disorder (CD).20,22 Patients with ADHD and comorbid CD are estimated to be 8.8 times more likely to have an SUD before age 18 compared with those with ADHD alone.23 Comorbid ADHD and CD may increase patients’ predisposition to develop dependence on highly addictive drugs, such as cocaine or methamphetamine.24 Impaired executive function, behavioral dyscontrol, impulsivity, and peer rejection are common in both ADHD and CD and may increase the risk of developing SUDs in individuals who have both conditions.25 Other risk factors for SUDs in patients with ADHD are listed in Table 1.26
Table 1
Risk factors for SUDs in patients with ADHD
| Presence of comorbid conditions (ie, oppositional defiant disorder, conduct disorder, bipolar disorder, eating disorder) |
| White or Hispanic race |
| Partially treated or residual ADHD symptoms |
| Attending a competitive college program |
| College youth who had late onset of stimulant treatment |
| Member of a college sorority/fraternity |
| ADHD: attention-deficit/hyperactivity disorder; SUDs: substance use disorders Source: Reference 26 |
Stimulants’ affect on SUD risk
Increased risk. Limited studies suggest exposure to stimulants is a risk factor for developing SUDs. In a longitudinal study, Lambert et al27 followed 218 patients with ADHD and 182 without ADHD into adulthood and found a linear trend between duration of stimulant treatment and prevalence of cocaine dependence. ADHD patients exposed to stimulants for >1 year had the highest prevalence of cocaine abuse (27%), compared with untreated subjects (15%), or those treated with stimulants for <1 year (18%). However, the study did not control for comorbid contributing factors, such as CD.
No change. In a 10-year naturalistic study, Biederman et al28 followed 109 children with ADHD age 7 to 12 into adulthood. These children had a developmental reading disorder but no other psychiatric comorbidities. When comparing patients who were treated with methylphenidate (n = 43) with those who did not receive stimulants (n = 66), Bierderman et al found no significant difference between the 2 groups in the prevalence of SUD for any of the 7 drug categories studied.
Decreased risk. Two meta-analyses found children with ADHD who were treated with stimulants and followed until adolescence were 5.8 times less likely to develop SUDs compared with those who did not receive stimulants.28,29 This protective effect diminished when patients were followed into adulthood, but individuals treated with stimulants were 1.4 times less likely to develop SUDs than those not treated with stimulants.30 In a prospective case-control, 5-year follow-up study of 114 patients with ADHD treated with stimulants, Wilens et al31 found significant protective effects of stimulant treatment on the development of any SUD. They found no effects from time of onset or duration of stimulant therapy on subsequent risk of SUDs or cigarette smoking.
One possible explanation for stimulants’ apparently reduced protective effect among adults is for patients with ADHD, stimulant use might delay but not prevent SUDs. It also is likely that by adulthood, loss of parental supervision leads to poor medication adherence and increased susceptibility to SUDs.30
Other studies have found exposure to stimulants may protect against SUDs. Katusic et al23 reviewed medical records for documented SUDs in 295 adults with ADHD treated with stimulants and 84 who did not receive stimulants. They found 20% of patients who received stimulants had a documented SUD compared with 27% of those not treated with stimulants. Barkely et al32 followed 98 stimulant-treated and 21 untreated ADHD patients with a mean age of 15 and 21, respectively. They found stimulant treatment did not increase the risk for substance use or abuse in either group.
ADHD and stimulant abuse
The prevalence of stimulant misuse is as high as 9% in patients in grade school and high school and up to 35% in college-age individuals.33 ADHD patients who misuse stimulants (eg, escalating dose without authorization) or skip stimulant doses to use illicit drugs or alcohol are more likely to sell their medication.34 Immediate-release stimulant formulations are more liable to be abused than extended-release drugs because they achieve earlier peak drug concentrations and dopamine blockade, indicating rapid drug absorption and central drug activity. Close monitoring and use of extended-release formulations are useful deterrents against stimulant abuse.
Clinical recommendations
Detecting and treating SUDs in patients with ADHD can be challenging. Ideally, the best time to assess for ADHD symptoms is after a prolonged abstinence from any influencing substance. However, in most clinical situations this is not practical. A better approach is a longitudinal assessment for ADHD symptoms. Detecting evidence of early childhood onset of ADHD symptoms before the patient began using substances can be helpful in conducting a proper differential diagnosis. Assessing for symptoms of SUDs in early adolescence, along with serial assessment of ADHD symptoms, also can be helpful. Symptoms secondary to ADHD are likely to show a consistent pattern, whereas symptoms secondary to an SUD may be sporadic.
When assessing SUD risk, consider the patient’s clinical condition, history of comorbidities that suggest SUDs, and overall functional status. Collateral information about the patient’s behavior and substance abuse from family members is important. A history of CD, bipolar disorder, or antisocial personality disorder should raise concerns about potential future stimulant abuse or diversion. Close monitoring of patients suspected of having an SUD is essential to detect stimulant abuse or diversion, which often manifests as weight loss, requests for higher doses, requests to switch from long-acting or extended-release formulations to immediate-release formulations, and repeated and suspicious “lost prescriptions.” Close observation for other subtle signs—such as changes in personality or mood and unexplained accidents or injuries—also may be needed.35
Challenges of treating ADHD and co-occurring SUD include poor medication adherence, need for a higher therapeutic stimulant dose, and difficulty in assessing the therapeutic benefit of pharmacotherapy in the presence of an SUD.36 Treating ADHD comorbid with SUD requires a collaborative approach that involves a psychiatrist, family members, and a behavioral care provider in addition to frequent monitoring.34
In the absence of treatment guidelines for treating ADHD with comorbid SUDs, some clinicians prefer to stabilize the SUD before initiating stimulants. Others prefer to use nonstimulants (such as atomoxetine, guanfacine, bupropion, venlafaxine, tricyclic antidepressants, or modafinil) as a first-line treatment. However, nonstimulants have not demonstrated efficacy comparable to that of stimulants for ADHD.35
Table 2 offers clinical recommendations to minimize the risk of SUDs when treating ADHD patients with stimulants. Long-acting stimulant formulations are preferred over short-acting medications because they are less likely to be abused. Psychosocial interventions for treating ADHD and co-occurring SUD disorder include cognitive-behavioral therapy with emphasis on structured skills training and cognitive remediation.
Table 2
Minimizing SUD risk when treating ADHD patients with stimulants
| Assess symptom burden and psychosocial impairment |
| Establish a treatment contract and boundaries at the onset of treatment, including your right to terminate treatment if you suspect stimulant misuse |
| Assess for comorbidities that may increase your patient’s SUD risk (see Table 1) |
| Emphasize strict adherence to treatment recommendations |
| Involve the patient’s family as much as possible |
| Obtain collateral information on the patient’s history of ADHD-related symptoms from parents, siblings, significant others, etc. |
| Distinguish between patients with substance use vs an SUD or a history of an SUD |
| Obtain urine toxicology screening as appropriate |
| Carefully document dispensed stimulants– strength of medication, number of capsules, pills, patches, etc. Note date of dispensation and refill dates |
| Select delayed- or extended-release stimulant formulations |
| Consider prescribing nonstimulants if appropriate |
| Use rating scales such as Conners Adult ADHD Rating Scale to monitor ADHD symptom severity and response to treatment |
| Schedule frequent, face-to-face clinical monitoring visits |
| ADHD: attention-deficit/hyperactivity disorder; SUD: substance use disorder |
Related Resource
- Faraone SV, Wilens T. Does stimulant treatment lead to substance use disorders? J Clin Psychiatry. 2003;64(suppl 11):9-13.
- Upadhyaya HP, Rose K, Wang W, et al. Attention deficit hyperactivity disorder medication and substance use patterns among adolescents and young adults. J Child Adolesc Psychopharmacol. 2005;15:799-809.
- Mariani JJ, Levin FR. Treatment strategies for co-occurring ADHD and substance use disorders. Am J Addict. 2007;16(suppl 1):45-56.
Drug Brand Names
- Atomoxetine • Strattera
- Bupropion • Wellbutrin, Zyban
- Guanfacine • Tenex, Intuniv
- Methylphenidate • Ritalin
- Modafinil • Provigil
- Venlafaxine • Effexor
Disclosures
Dr. Shailesh Jain and Dr. Islam report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Rakesh Jain has received research support from, is a consultant to, and/or is a speaker for Addrenex Pharmaceuticals, AstraZeneca, Eli Lilly and Company, Forest Pharmaceuticals, Merck, Pamlab, Pfizer Inc., Shionogi Inc., Shire, and Sunovion Pharmaceuticals.
Discuss this article at www.facebook.com/CurrentPsychiatry
Does prescribing stimulants to patients with attention-deficit/hyperactivity disorder (ADHD) increase their risk of future substance abuse? Because ADHD is a common pediatric condition with symptoms that often persist into adulthood, and stimulants are an efficacious first-line therapy, this possible association is a concern for psychiatrists whether they treat children or adults.
Some researchers have expressed concerns that stimulant exposure could predispose patients to future substance abuse.1 Proponents of the biologic model of “kindling” hypothesize early exposure to stimulants could increase the risk of later substance use disorders (SUDs) by modifying or “priming” the brain, which then becomes more receptive to illicit drug exposure. Although there is some evidence that stimulant use does increase SUD risk, other evidence suggests stimulant use does not increase susceptibility to SUDs2,3 and some studies have suggested stimulant use in ADHD patients may protect against SUDs.4,5
This article reviews shared characteristics of ADHD and SUDs and the latest research on the association between the clinical use of stimulants and later development of SUDs. We also offer clinical recommendations for assessing and treating ADHD and comorbid SUD.
ADHD/SUD overlap
Compared with those without the disorder, patients with ADHD have a 6.2 times higher risk of developing an SUD.6 Individuals with ADHD experience an earlier age of onset and a longer duration of SUDs.7 Several retrospective and prospective studies reveal ADHD is a risk factor for SUDs.8 A longitudinal study that tracked teenage males with or without ADHD into young adulthood found SUDs were 4 times more common among those with ADHD.9 Up to 45% of adults with ADHD have a history of alcohol abuse or dependence, and up to 30% have a history of illegal drug abuse or dependence.10
Conversely, an estimated 35% to 71% of alcohol abusers and 15% to 25% of substance-dependent patients have ADHD.11 Adults with ADHD and comorbid SUD report earlier onset12 and greater severity13 of substance abuse than adults without ADHD. Patients with ADHD experience earlier onset and higher rates of tobacco smoking by mid-adolescence.14
Developmental psychopathology. Longitudinal studies have suggested certain psychopathologic characteristics of ADHD can predispose an individual to SUDs independent of stimulant exposure. For example, inattention, impulsivity, and hyperactivity predispose an individual to develop an SUD and also are core symptoms of ADHD.15 Another study found impulsivity, impersistence, and difficulty sitting still at age 3 predicted alcohol abuse at age 21.16 A different longitudinal study found novelty-seeking behavior (restlessness, running/jumping and not keeping still, being squirmy and fidgety) between age 6 to 10 predicted adolescent drug abuse and cigarette smoking.17 Poor response inhibition is a key characteristic of ADHD and has been linked to adolescent drinking.18
ADHD may be an independent risk factor for SUD because a common neurobiologic psychopathology may predispose an individual to develop both conditions. The dopamine system has been implicated in SUD, and dysfunction in the dopaminergic circuits—mostly in basal and frontal cortex with consequent defects in executive function and reward system—also has been found in ADHD.19 Cognitive dysfunction associated with ADHD may decrease a patient’s ability to estimate the negative consequences of substance abuse and to delay immediate gratification from drug or alcohol use.
ADHD patients are more vulnerable to SUDs if they have a comorbid condition, such as oppositional defiant disorder,13,20 bipolar disorder,20,21 or conduct disorder (CD).20,22 Patients with ADHD and comorbid CD are estimated to be 8.8 times more likely to have an SUD before age 18 compared with those with ADHD alone.23 Comorbid ADHD and CD may increase patients’ predisposition to develop dependence on highly addictive drugs, such as cocaine or methamphetamine.24 Impaired executive function, behavioral dyscontrol, impulsivity, and peer rejection are common in both ADHD and CD and may increase the risk of developing SUDs in individuals who have both conditions.25 Other risk factors for SUDs in patients with ADHD are listed in Table 1.26
Table 1
Risk factors for SUDs in patients with ADHD
| Presence of comorbid conditions (ie, oppositional defiant disorder, conduct disorder, bipolar disorder, eating disorder) |
| White or Hispanic race |
| Partially treated or residual ADHD symptoms |
| Attending a competitive college program |
| College youth who had late onset of stimulant treatment |
| Member of a college sorority/fraternity |
| ADHD: attention-deficit/hyperactivity disorder; SUDs: substance use disorders Source: Reference 26 |
Stimulants’ affect on SUD risk
Increased risk. Limited studies suggest exposure to stimulants is a risk factor for developing SUDs. In a longitudinal study, Lambert et al27 followed 218 patients with ADHD and 182 without ADHD into adulthood and found a linear trend between duration of stimulant treatment and prevalence of cocaine dependence. ADHD patients exposed to stimulants for >1 year had the highest prevalence of cocaine abuse (27%), compared with untreated subjects (15%), or those treated with stimulants for <1 year (18%). However, the study did not control for comorbid contributing factors, such as CD.
No change. In a 10-year naturalistic study, Biederman et al28 followed 109 children with ADHD age 7 to 12 into adulthood. These children had a developmental reading disorder but no other psychiatric comorbidities. When comparing patients who were treated with methylphenidate (n = 43) with those who did not receive stimulants (n = 66), Bierderman et al found no significant difference between the 2 groups in the prevalence of SUD for any of the 7 drug categories studied.
Decreased risk. Two meta-analyses found children with ADHD who were treated with stimulants and followed until adolescence were 5.8 times less likely to develop SUDs compared with those who did not receive stimulants.28,29 This protective effect diminished when patients were followed into adulthood, but individuals treated with stimulants were 1.4 times less likely to develop SUDs than those not treated with stimulants.30 In a prospective case-control, 5-year follow-up study of 114 patients with ADHD treated with stimulants, Wilens et al31 found significant protective effects of stimulant treatment on the development of any SUD. They found no effects from time of onset or duration of stimulant therapy on subsequent risk of SUDs or cigarette smoking.
One possible explanation for stimulants’ apparently reduced protective effect among adults is for patients with ADHD, stimulant use might delay but not prevent SUDs. It also is likely that by adulthood, loss of parental supervision leads to poor medication adherence and increased susceptibility to SUDs.30
Other studies have found exposure to stimulants may protect against SUDs. Katusic et al23 reviewed medical records for documented SUDs in 295 adults with ADHD treated with stimulants and 84 who did not receive stimulants. They found 20% of patients who received stimulants had a documented SUD compared with 27% of those not treated with stimulants. Barkely et al32 followed 98 stimulant-treated and 21 untreated ADHD patients with a mean age of 15 and 21, respectively. They found stimulant treatment did not increase the risk for substance use or abuse in either group.
ADHD and stimulant abuse
The prevalence of stimulant misuse is as high as 9% in patients in grade school and high school and up to 35% in college-age individuals.33 ADHD patients who misuse stimulants (eg, escalating dose without authorization) or skip stimulant doses to use illicit drugs or alcohol are more likely to sell their medication.34 Immediate-release stimulant formulations are more liable to be abused than extended-release drugs because they achieve earlier peak drug concentrations and dopamine blockade, indicating rapid drug absorption and central drug activity. Close monitoring and use of extended-release formulations are useful deterrents against stimulant abuse.
Clinical recommendations
Detecting and treating SUDs in patients with ADHD can be challenging. Ideally, the best time to assess for ADHD symptoms is after a prolonged abstinence from any influencing substance. However, in most clinical situations this is not practical. A better approach is a longitudinal assessment for ADHD symptoms. Detecting evidence of early childhood onset of ADHD symptoms before the patient began using substances can be helpful in conducting a proper differential diagnosis. Assessing for symptoms of SUDs in early adolescence, along with serial assessment of ADHD symptoms, also can be helpful. Symptoms secondary to ADHD are likely to show a consistent pattern, whereas symptoms secondary to an SUD may be sporadic.
When assessing SUD risk, consider the patient’s clinical condition, history of comorbidities that suggest SUDs, and overall functional status. Collateral information about the patient’s behavior and substance abuse from family members is important. A history of CD, bipolar disorder, or antisocial personality disorder should raise concerns about potential future stimulant abuse or diversion. Close monitoring of patients suspected of having an SUD is essential to detect stimulant abuse or diversion, which often manifests as weight loss, requests for higher doses, requests to switch from long-acting or extended-release formulations to immediate-release formulations, and repeated and suspicious “lost prescriptions.” Close observation for other subtle signs—such as changes in personality or mood and unexplained accidents or injuries—also may be needed.35
Challenges of treating ADHD and co-occurring SUD include poor medication adherence, need for a higher therapeutic stimulant dose, and difficulty in assessing the therapeutic benefit of pharmacotherapy in the presence of an SUD.36 Treating ADHD comorbid with SUD requires a collaborative approach that involves a psychiatrist, family members, and a behavioral care provider in addition to frequent monitoring.34
In the absence of treatment guidelines for treating ADHD with comorbid SUDs, some clinicians prefer to stabilize the SUD before initiating stimulants. Others prefer to use nonstimulants (such as atomoxetine, guanfacine, bupropion, venlafaxine, tricyclic antidepressants, or modafinil) as a first-line treatment. However, nonstimulants have not demonstrated efficacy comparable to that of stimulants for ADHD.35
Table 2 offers clinical recommendations to minimize the risk of SUDs when treating ADHD patients with stimulants. Long-acting stimulant formulations are preferred over short-acting medications because they are less likely to be abused. Psychosocial interventions for treating ADHD and co-occurring SUD disorder include cognitive-behavioral therapy with emphasis on structured skills training and cognitive remediation.
Table 2
Minimizing SUD risk when treating ADHD patients with stimulants
| Assess symptom burden and psychosocial impairment |
| Establish a treatment contract and boundaries at the onset of treatment, including your right to terminate treatment if you suspect stimulant misuse |
| Assess for comorbidities that may increase your patient’s SUD risk (see Table 1) |
| Emphasize strict adherence to treatment recommendations |
| Involve the patient’s family as much as possible |
| Obtain collateral information on the patient’s history of ADHD-related symptoms from parents, siblings, significant others, etc. |
| Distinguish between patients with substance use vs an SUD or a history of an SUD |
| Obtain urine toxicology screening as appropriate |
| Carefully document dispensed stimulants– strength of medication, number of capsules, pills, patches, etc. Note date of dispensation and refill dates |
| Select delayed- or extended-release stimulant formulations |
| Consider prescribing nonstimulants if appropriate |
| Use rating scales such as Conners Adult ADHD Rating Scale to monitor ADHD symptom severity and response to treatment |
| Schedule frequent, face-to-face clinical monitoring visits |
| ADHD: attention-deficit/hyperactivity disorder; SUD: substance use disorder |
Related Resource
- Faraone SV, Wilens T. Does stimulant treatment lead to substance use disorders? J Clin Psychiatry. 2003;64(suppl 11):9-13.
- Upadhyaya HP, Rose K, Wang W, et al. Attention deficit hyperactivity disorder medication and substance use patterns among adolescents and young adults. J Child Adolesc Psychopharmacol. 2005;15:799-809.
- Mariani JJ, Levin FR. Treatment strategies for co-occurring ADHD and substance use disorders. Am J Addict. 2007;16(suppl 1):45-56.
Drug Brand Names
- Atomoxetine • Strattera
- Bupropion • Wellbutrin, Zyban
- Guanfacine • Tenex, Intuniv
- Methylphenidate • Ritalin
- Modafinil • Provigil
- Venlafaxine • Effexor
Disclosures
Dr. Shailesh Jain and Dr. Islam report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Rakesh Jain has received research support from, is a consultant to, and/or is a speaker for Addrenex Pharmaceuticals, AstraZeneca, Eli Lilly and Company, Forest Pharmaceuticals, Merck, Pamlab, Pfizer Inc., Shionogi Inc., Shire, and Sunovion Pharmaceuticals.
1. Lambert NM, McLeod M, Schenk S. Subjective responses to initial experience with cocaine: an exploration of the incentive-sensitization theory of drug abuse. Addiction. 2006;101(5):713-725.
2. Mannuzza S, Klein RG, Moulton JL. Does stimulant treatment place children at risk for adult substance abuse? A controlled prospective follow-up study. J Child Adolesc Psychopharmacol. 2003;13(3):273-282.
3. Katusic SK, Barbaresi WJ, Colligan RC, et al. Psychostimulant treatment and risk for substance abuse among young adults with a history of attention-deficit/hyperactivity disorder: a population-based, birth cohort study. J Child Adolesc Psychopharmacol. 2005;15(5):764-776.
4. Wilens TE, Faraone SV, Biederman J, et al. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111(1):179-185.
5. Biederman J, Wilens T, Mick E, et al. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics. 1999;104(2):e20.-
6. Katusic SK, Barbaresi WJ, Colligan RC, et al. Psychostimulant treatment and risk for substance abuse among young adults with a history of attention-deficit/hyperactivity disorder: a population-based, birth cohort study. J Child Adolesc Psychopharmacol. 2005;15(5):764-776.
7. Wilens TE. Impact of ADHD and its treatment on substance abuse in adults. J Clin Psychiatry. 2004;65(suppl 3):38-45.
8. Barkley RA, Fischer M, Smallish L, et al. Young adult follow-up of hyperactive children: antisocial activities and drug use. J Child Psychol Psychiatry. 2004;45(2):195-221.
9. Mannuzza S, Klein RG, Bessler A, et al. Adult outcome of hyperactive boys. Educational achievement, occupational rank, and psychiatric status. Arch Gen Psychiatry. 1993;50(7):565-576.
10. Wilens TE. Attention-deficit/hyperactivity disorder and the substance use disorders: the nature of the relationship subtypes at risk, and treatment issues. Psychiatr Clin North Am. 2004;27(2):283-301.
11. Wilens TE. AOD use and attention deficit/hyperactivity disorder. Alcohol Health Res World. 1998;22(2):127-130.
12. Wilens TE, Biederman J, Abrantes AM, et al. Clinical characteristics of psychiatrically referred adolescent outpatients with substance use disorder. J Am Acad Child Adolesc Psychiatry. 1997;36(7):941-947.
13. Schubiner H, Tzelepis A, Milberger S, et al. Prevalence of attention-deficit/hyperactivity disorder and conduct disorder among substance abusers. J Clin Psychiatry. 2000;61(4):244-251.
14. Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. J Learn Disabil. 1998;31(6):533-544.
15. Zucker RA. Alcohol use and the alcohol use disorders: a developmental biopsychosocial systems formulation covering the life course. In: Cicchetti D Cohen D, eds. Developmental psychopathology. 2nd ed. Hoboken, NJ: John Wiley & Sons Inc; 2006;620-656.
16. Caspi A, Moffitt TE, Newman DL, et al. Behavioral observations at age 3 years predict adult psychiatric disorders. Longitudinal evidence from a birth cohort. Arch Gen Psychiatry. 1996;53(11):1033-1039.
17. Màsse LC, Tremblay RE. Behavior of boys in kindergarten and the onset of substance use during adolescence. Arch Gen Psychiatry. 1997;54(1):62-68.
18. Nigg JT, Wong MM, Martel MM, et al. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(4):468-475.
19. Seidman LJ, Valera EM, Makris N. Structural brain imaging of attention-deficit hyperactivity disorder. Biol Psychiatry. 2005;57(11):1263-1272.
20. Biederman J, Wilens T, Mick E, et al. Is ADHD a risk factor for psychoactive substance use disorders? Findings from a four-year prospective follow-up study. J Am Acad Child Adolesc Psychiatry. 1997;36(1):21-29.
21. Wilens TE, Biederman J, Millstein RB, et al. Risk for substance use disorders in youths with child- and adolescent-onset bipolar disorder. J Am Acad Child Adolesc Psychiatry. 1999;38(6):680-685.
22. Schubiner H, Saules KK, Arfken CL, et al. Double-blind placebo-controlled trial of methylphenidate in the treatment of adult ADHD patients with comorbid cocaine dependence. Exp Clin Psychopharmacol. 2002;10(3):286-294.
23. Katusic SK, Barbaresi WJ, Colligan RC, et al. Psychostimulant treatment and risk for substance abuse among young adults with a history of attention-deficit/hyperactivity disorder: a population-based, birth cohort study. J Child Adolesc Psychopharmacol. 2005;15(5):764-776.
24. Flory K, Milich R, Lynam DR, et al. Relation between childhood disruptive behavior disorders and substance use and dependence symptoms in young adulthood: individuals with symptoms of attention-deficit/hyperactivity disorder and conduct disorder are uniquely at risk. Psychol Addict Behav. 2003;17(2):151-158.
25. Wilens TE. Attention-deficit/hyperactivity disorder and the substance use disorders: the nature of the relationship subtypes at risk, and treatment issues. Psychiatr Clin North Am. 2004;27(2):283-301.
26. Wilson JJ. ADHD and substance use disorders: developmental aspects and the impact of stimulant treatment. Am J Addict. 2007;16(suppl 1):5-11.
27. Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. J Learn Disabil. 1998;31(6):533-544.
28. Biederman J, Monuteaux MC, Spencer T, et al. Stimulant therapy and risk for subsequent substance use disorders in male adults with ADHD: a naturalistic controlled 10-year follow-up study. Am J Psychiatry. 2008;165(5):597-603.
29. Wilens TE, Faraone SV, Biederman J, et al. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111(1):179-185.
30. Faraone SV, Wilens TE. Effect of stimulant medications for attention-deficit/hyperactivity disorder on later substance use and the potential for stimulant misuse abuse, and diversion. J Clin Psychiatry. 2007;68(suppl 11):15-22.
31. Wilens TE, Adamson J, Monuteaux MC, et al. Effect of prior stimulant treatment for attention-deficit/hyperactivity disorder on subsequent risk for cigarette smoking and alcohol and drug use disorders in adolescents. Arch Pediatr Adolesc Med. 2008;162(10):916-921.
32. Barkley RA, Fischer M, Smallish L, et al. Does the treatment of attention-deficit/hyperactivity disorder with stimulants contribute to drug use/abuse? A 13-year prospective study. Pediatrics. 2003;111(1):97-109.
33. Wilens TE, Adler LA, Adams J, et al. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47(1):21-31.
34. Upadhyaya HP, Rose K, Wang W, et al. Attention-deficit/hyperactivity disorder, medication treatment, and substance use patterns among adolescents and young adults. J Child Adolesc Psychopharmacol. 2005;15(5):799-809.
35. Kollins SH. A qualitative review of issues arising in the use of psycho-stimulant medications in patients with ADHD and co-morbid substance use disorders. Curr Med Res Opin. 2008;24(5):1345-1357.
36. Faraone SV, Biederman J, Wilens TE, et al. A naturalistic study of the effects of pharmacotherapy on substance use disorders among ADHD adults. Psychol Med. 2007;37(12):1743-1752.
1. Lambert NM, McLeod M, Schenk S. Subjective responses to initial experience with cocaine: an exploration of the incentive-sensitization theory of drug abuse. Addiction. 2006;101(5):713-725.
2. Mannuzza S, Klein RG, Moulton JL. Does stimulant treatment place children at risk for adult substance abuse? A controlled prospective follow-up study. J Child Adolesc Psychopharmacol. 2003;13(3):273-282.
3. Katusic SK, Barbaresi WJ, Colligan RC, et al. Psychostimulant treatment and risk for substance abuse among young adults with a history of attention-deficit/hyperactivity disorder: a population-based, birth cohort study. J Child Adolesc Psychopharmacol. 2005;15(5):764-776.
4. Wilens TE, Faraone SV, Biederman J, et al. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111(1):179-185.
5. Biederman J, Wilens T, Mick E, et al. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics. 1999;104(2):e20.-
6. Katusic SK, Barbaresi WJ, Colligan RC, et al. Psychostimulant treatment and risk for substance abuse among young adults with a history of attention-deficit/hyperactivity disorder: a population-based, birth cohort study. J Child Adolesc Psychopharmacol. 2005;15(5):764-776.
7. Wilens TE. Impact of ADHD and its treatment on substance abuse in adults. J Clin Psychiatry. 2004;65(suppl 3):38-45.
8. Barkley RA, Fischer M, Smallish L, et al. Young adult follow-up of hyperactive children: antisocial activities and drug use. J Child Psychol Psychiatry. 2004;45(2):195-221.
9. Mannuzza S, Klein RG, Bessler A, et al. Adult outcome of hyperactive boys. Educational achievement, occupational rank, and psychiatric status. Arch Gen Psychiatry. 1993;50(7):565-576.
10. Wilens TE. Attention-deficit/hyperactivity disorder and the substance use disorders: the nature of the relationship subtypes at risk, and treatment issues. Psychiatr Clin North Am. 2004;27(2):283-301.
11. Wilens TE. AOD use and attention deficit/hyperactivity disorder. Alcohol Health Res World. 1998;22(2):127-130.
12. Wilens TE, Biederman J, Abrantes AM, et al. Clinical characteristics of psychiatrically referred adolescent outpatients with substance use disorder. J Am Acad Child Adolesc Psychiatry. 1997;36(7):941-947.
13. Schubiner H, Tzelepis A, Milberger S, et al. Prevalence of attention-deficit/hyperactivity disorder and conduct disorder among substance abusers. J Clin Psychiatry. 2000;61(4):244-251.
14. Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. J Learn Disabil. 1998;31(6):533-544.
15. Zucker RA. Alcohol use and the alcohol use disorders: a developmental biopsychosocial systems formulation covering the life course. In: Cicchetti D Cohen D, eds. Developmental psychopathology. 2nd ed. Hoboken, NJ: John Wiley & Sons Inc; 2006;620-656.
16. Caspi A, Moffitt TE, Newman DL, et al. Behavioral observations at age 3 years predict adult psychiatric disorders. Longitudinal evidence from a birth cohort. Arch Gen Psychiatry. 1996;53(11):1033-1039.
17. Màsse LC, Tremblay RE. Behavior of boys in kindergarten and the onset of substance use during adolescence. Arch Gen Psychiatry. 1997;54(1):62-68.
18. Nigg JT, Wong MM, Martel MM, et al. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(4):468-475.
19. Seidman LJ, Valera EM, Makris N. Structural brain imaging of attention-deficit hyperactivity disorder. Biol Psychiatry. 2005;57(11):1263-1272.
20. Biederman J, Wilens T, Mick E, et al. Is ADHD a risk factor for psychoactive substance use disorders? Findings from a four-year prospective follow-up study. J Am Acad Child Adolesc Psychiatry. 1997;36(1):21-29.
21. Wilens TE, Biederman J, Millstein RB, et al. Risk for substance use disorders in youths with child- and adolescent-onset bipolar disorder. J Am Acad Child Adolesc Psychiatry. 1999;38(6):680-685.
22. Schubiner H, Saules KK, Arfken CL, et al. Double-blind placebo-controlled trial of methylphenidate in the treatment of adult ADHD patients with comorbid cocaine dependence. Exp Clin Psychopharmacol. 2002;10(3):286-294.
23. Katusic SK, Barbaresi WJ, Colligan RC, et al. Psychostimulant treatment and risk for substance abuse among young adults with a history of attention-deficit/hyperactivity disorder: a population-based, birth cohort study. J Child Adolesc Psychopharmacol. 2005;15(5):764-776.
24. Flory K, Milich R, Lynam DR, et al. Relation between childhood disruptive behavior disorders and substance use and dependence symptoms in young adulthood: individuals with symptoms of attention-deficit/hyperactivity disorder and conduct disorder are uniquely at risk. Psychol Addict Behav. 2003;17(2):151-158.
25. Wilens TE. Attention-deficit/hyperactivity disorder and the substance use disorders: the nature of the relationship subtypes at risk, and treatment issues. Psychiatr Clin North Am. 2004;27(2):283-301.
26. Wilson JJ. ADHD and substance use disorders: developmental aspects and the impact of stimulant treatment. Am J Addict. 2007;16(suppl 1):5-11.
27. Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. J Learn Disabil. 1998;31(6):533-544.
28. Biederman J, Monuteaux MC, Spencer T, et al. Stimulant therapy and risk for subsequent substance use disorders in male adults with ADHD: a naturalistic controlled 10-year follow-up study. Am J Psychiatry. 2008;165(5):597-603.
29. Wilens TE, Faraone SV, Biederman J, et al. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111(1):179-185.
30. Faraone SV, Wilens TE. Effect of stimulant medications for attention-deficit/hyperactivity disorder on later substance use and the potential for stimulant misuse abuse, and diversion. J Clin Psychiatry. 2007;68(suppl 11):15-22.
31. Wilens TE, Adamson J, Monuteaux MC, et al. Effect of prior stimulant treatment for attention-deficit/hyperactivity disorder on subsequent risk for cigarette smoking and alcohol and drug use disorders in adolescents. Arch Pediatr Adolesc Med. 2008;162(10):916-921.
32. Barkley RA, Fischer M, Smallish L, et al. Does the treatment of attention-deficit/hyperactivity disorder with stimulants contribute to drug use/abuse? A 13-year prospective study. Pediatrics. 2003;111(1):97-109.
33. Wilens TE, Adler LA, Adams J, et al. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47(1):21-31.
34. Upadhyaya HP, Rose K, Wang W, et al. Attention-deficit/hyperactivity disorder, medication treatment, and substance use patterns among adolescents and young adults. J Child Adolesc Psychopharmacol. 2005;15(5):799-809.
35. Kollins SH. A qualitative review of issues arising in the use of psycho-stimulant medications in patients with ADHD and co-morbid substance use disorders. Curr Med Res Opin. 2008;24(5):1345-1357.
36. Faraone SV, Biederman J, Wilens TE, et al. A naturalistic study of the effects of pharmacotherapy on substance use disorders among ADHD adults. Psychol Med. 2007;37(12):1743-1752.
Strategies to help patients break the chains of tobacco addiction
Discuss this article at www.facebook.com/CurrentPsychiatry
You are treating Mr. P, age 34, for schizoaffective disorder. He smokes 1 pack of cigarettes per day and has smoked for approximately 17 years. He has tried to stop but never has been able to quit for more than a few weeks. He reveals whenever he tries to quit, he starts feeling extremely lethargic and “depressed” and resumes smoking to prevent these symptoms from worsening. However, Mr. P expresses some interest in trying to quit again and asks whether any medications could prevent him from becoming depressed while he tries to quit.
Cigarette smoking is overrepresented and undertreated among individuals with psychiatric illness, in part because of the largely unfounded belief held by some patients and clinicians that smoking cessation might worsen psychiatric symptoms. In this article, we argue this challenge can be overcome and psychiatrists and other mental health professionals can and should help their patients reap the innumerable benefits of quitting smoking. We discuss:
- the short- and long-term effects of smoking cessation
- evidence-based treatment guidelines for working with motivated and unmotivated smokers
- unique issues that may arise when treating smokers who have psychiatric disorders.
Quitting: Profound benefits
Quitting smoking has substantial benefits beginning within minutes after taking the last puff. Some of the benefits that occur within the first few days of quitting include:
- decreased blood pressure and pulse rate
- improved circulation
- improved ability to smell and taste
- easier breathing.
Longer-term smoking abstinence drastically reduces risk of heart attack, stroke, cancer, respiratory disease, and a host of other illnesses that affect—and kill—individuals with psychiatric disorders several decades earlier than their counterparts in the general population.1 There also are financial benefits to quitting; using the 2009 national average of $5.33 per pack, a 1-pack-per-day smoker who quits would save >$150 per month, which accounts for only the direct cost of cigarettes.2
Although the beneficial effects of quitting smoking are profound and far-reaching, in the short-term they are counterbalanced by nicotine withdrawal symptoms—including restlessness, irritability, depressed mood, concentration problems, and increased appetite/weight gain—that are formidable distractions from the positive aspects of quitting. Additionally, nicotine withdrawal symptoms tend to be more severe in smokers who have a psychiatric disorder.3 Fortunately, there are effective, evidence-based methods of reducing withdrawal symptoms and helping smokers cope with these and other challenges of quitting.
Combined treatment is best
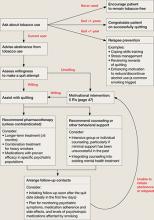
Current treatment guidelines4 suggest all smokers should be offered pharmacotherapy and counseling to aid quitting because this combined approach has the highest success rate (Algorithm). Table 1 4 provides information about dosing, efficacy, and side effect profile of each of the 7 FDA-approved medications for smoking cessation. Using any of the approved medications at least doubles the odds of successful quitting compared with placebo.4 These pharmacotherapies can reduce or prevent nicotine withdrawal symptoms and—at least in the case of bupropion and varenicline—decrease reinforcement from smoking, thereby lowering the likelihood a lapse (ie, smoking ≥1 cigarettes without returning to regular smoking) will develop into a full-blown relapse (ie, return to regular smoking).
Algorithm: Tobacco cessation treatment for psychiatric patients
Source: Adapted from reference 4 Medication selection depends on many factors, including:
- the patient’s psychiatric illness
- her/his prior response to smoking cessation pharmacotherapies
- concomitant psychiatric medications
- patient preference.5
Placebo-controlled trials of smoking cessation aids in psychiatrically ill patients are limited, but several studies of smokers with a history of major depression indicate treatment with bupropion SR or nortriptyline is effective.6 Similarly, although relapse rates generally are higher in patients with schizophrenia compared with non-mentally ill smokers, nicotine replacement therapy and bupropion SR are more effective than placebo in patients with this disorder.7,8 When we prescribe these treatments, we tend to extend the duration of treatment beyond those described in Table 1 ,4 and to use combined treatments (eg, a transdermal patch with a shorter-acting gum or lozenge preparation) to better target the marked withdrawal symptoms more severely nicotine-dependent patients frequently experience.
Table 1
First-line pharmacotherapies for smoking cessation
| Medication | Standard dosage | Efficacy (OR, % abstinent at 6 mos. [with 95% CI]) | Contraindications (C) and precautions (P) | Common side effects |
|---|---|---|---|---|
| Non-nicotine medications | ||||
| Bupropion | Days 1-3: 150 mg/d Days 4-8: 150 mg bid Continue for 7-12 weeks at 150 mg bid | 2.0 (1.8-2.2), 24% (22%-26%) | C: Eating disorders, seizure history, taking bupropion, MAOI in past 2 weeks P: Pregnancy, cardiovascular disease, warning for emergent psychiatric symptoms | Insomnia, dry mouth |
| Varenicline | Days 1-3: 0.5 mg/d Days 4-7: 0.5 mg bid Day 8+: 1 mg bid Continue 11 weeks at 1 mg bid; up to 6 months for maintenance | 3.1 (2.5-3.8), 33% (29%-38%) | P: Warning for emergent psychiatric symptoms | Nausea, sleep problems, abnormal dreams |
| Nicotine replacement therapies | ||||
| Nicotine gum | 1 piece every 1-2 hours for 6-12 weeks <20 cigarettes/d: 2 mg gum ≥20 cigarettes/d: 4 mg gum | 1.5 (1.2-1.7), 19% (17%-22%) | P: Pregnancy, recent myocardial infarction, serious arrhythmia, unstable angina | Mouth soreness, hiccups, dyspepsia |
| Nicotine inhaler | 6-16 cartridges/d, up to 6 months | 2.1 (1.5-2.9), 25% (19%-32%) | Same as above | Mouth/throat irritation, coughing, rhinitis |
| Nicotine lozenge | 9-20 lozenges/d, up to 12 weeks Smoke ≤30 minutes after waking: 4 mg lozenge Smoke >30 minutes after waking: 2 mg lozenge | 2.0 (1.6-2.5)a | Same as above | Nausea, hiccups, heartburn |
| Nicotine nasal spray | 1-2 doses/hour, 8-40 doses/d for 3-6 months | 2.3 (1.7-3.0), 27% (22%-33%) | C: Severe reactive airway disease P: Same as above | Nasal irritation, higher risk of dependency |
| Nicotine patch | 1 patch/d, step-down dosing over 8 weeks Weeks 1-4: 21 mg patch Weeks 5-6: 14 mg patch Weeks 7-8: 7 mg patch | 1.9 (1.7-2.2) 23% (21%-26%) | P: Same as above | Skin reactions, sleep problems, abnormal dreams |
| aStead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2008;1:CD000146. bid: twice a day; CI: confidence interval; MAOI: monoamine oxidase inhibitor; OR: odds ratio Source: Adapted from reference 4 | ||||
Counseling. All smokers should be provided with brief interventions consistent with the 5 A’s—Ask, Advise, Assess, Assist, and Arrange (Table 2).4 For smokers who are not motivated to quit, the recommended approach follows the principles of the 5 R’s—Relevance, Risks, Rewards, Roadblocks, and Repetition (Table 3).4 Smokers who are motivated to quit and willing to participate in more intensive treatment may be offered face-to-face individual or group counseling (depending upon availability) or referred to a telephone quit line (see Related Resources). Intensive treatments such as these typically provide social support and assistance overcoming barriers to cessation and developing skills to initiate and maintain abstinence (eg, coping with a lapse or handling cravings, identifying and avoiding high-risk situations for smoking). As a general rule, greater intensity of counseling is associated with a greater likelihood of quitting.4
Table 2
The 5 A’s of tobacco treatment
| Intervention | Example | |
|---|---|---|
| Ask | Systematically inquire about tobacco use | “Do you currently use, or have you ever used, tobacco products?” |
| Advise | Counsel all tobacco users to quit in a clear, strong, and personalized manner | “I think it is very important for you quit smoking to keep your breathing problems from getting any worse” |
| Assess | Determine the tobacco user’s willingness to make a quit attempt | “What do you think? Are you ready to quit?” |
| Assist | Offer or refer to treatment/support (if ready to quit; if not ready, see Table 3 for recommended interventions) | “I’m here to help you with this. Let me start by letting you know about the many options available to help you quit” |
| Arrange | Plan for follow-up contacts (at least 1, preferably within 1 week of the quit date) | “I would like to give you a call within the next week to see how you did with your quit date. Would that be OK with you?” |
| Source: Adapted from reference 4 | ||
Table 3
The 5 R’s: Principles of interventions for smokers not ready to quit
| Principle | Example | |
|---|---|---|
| Relevance | Why is quitting smoking personally relevant? | “You’ve told me your kids sometimes make comments to you about quitting smoking. How does that affect you?” |
| Risks | What are the negative consequences of smoking? | “What don’t you like about smoking? What problems have you had from smoking?” |
| Rewards | What are the benefits of quitting smoking? | “Can you think of anything that would be good about quitting? Tell me about that” |
| Roadblocks | What are the barriers to quitting? | “What worries do you have about trying to quit? What happened the last time you tried to quit smoking?” |
| Repetition | Message repeated at every visit | “I know we have talked about quitting smoking before, but things may have changed since then. I also think that this is such an important issue we should keep it on the table for discussion. What do you think?” |
| Source: Adapted from reference 4 | ||
Q&A about treatment
How effective are smoking cessation interventions for individuals with psychiatric disorders? Several studies have demonstrated, on any given quit attempt, smokers with psychiatric or substance use disorders can be as successful as smokers without these disorders.9-11 In fact, quit rates as high as approximately 70% for end-of-treatment11 and 30% for 6-month follow-up10 have been reported. Of course, effectiveness varies by type and intensity of treatment as well as by individual characteristics of the smoker. Smokers with psychiatric disorders may fare better with more intensive interventions than briefer ones,12,13 and factors such as high levels of nicotine dependence and exposure to smoking environments—both of which are characteristic of smokers with serious mental illness—can negatively impact treatment outcomes.4
Should the nature of the psychiatric disorder(s) guide decisions about the optimal pharmacotherapy or counseling approach? There have been numerous attempts to investigate the effectiveness of targeted interventions for particular subgroups of smokers with psychiatric disorders, including:
- studies of the efficacy of the antidepressants bupropion14 and nortriptyline15 as well as cognitive-behavioral therapy-based mood management counseling16 for depressed smokers
- integrative treatment approaches for smokers with posttraumatic stress disorder (PTSD)17
- group counseling designed specifically for smokers with schizophrenia.18,19
Although more research is needed and there have been some promising early results (eg, McFall et al17), current literature does not provide consistent evidence supporting treatment matching solely on the basis of the psychiatric disorder. Rather, patient preference, safety considerations (eg, use of medications in children/adolescents, pregnant women), medication side effect profiles, prior experience with the treatment approach, and cost/availability of treatment should guide development of the treatment plan. When results from placebo-controlled trials are available for subgroups of patients (eg, those with a history of major depression), consider this information when selecting a pharmacologic smoking cessation aid.
What is the risk of psychiatric symptoms worsening as a result of quitting smoking? Little research on this topic is available because more often than not, smokers with psychiatric disorders are excluded from tobacco treatment studies. However, research examining psychiatric status changes among recent quitters with schizophrenia,20,21 depression,22,23 PTSD,17 and substance use disorders24 suggests smoking cessation does not worsen symptoms of these disorders, and may be associated with symptom improvement.17 Nonetheless, driven largely by anecdotal evidence, the misconception that smoking cessation worsens psychiatric symptoms remains a substantial barrier to treatment.
Mr. P’s case is an example of how not probing about the nature of psychiatric complaints can be problematic. Mr. P reported what on first glance appeared to be a worsening of psychiatric symptoms starting when he stopped smoking and resolved when he resumed smoking. However, without gathering additional information about these events, we cannot conclude stopping smoking caused his psychiatric symptoms to worsen. Other potential explanations include nicotine withdrawal symptoms, side effects of smoking cessation medications, an increase in levels of psychotropic medications for which metabolism is affected by tobacco smoke, or the natural course of his mood disorder. The timing of the onset and offset of symptoms seems to argue against Mr. P’s symptoms reflecting the natural course of his mood disorder, but the other 3 explanations remain plausible.
It is important to distinguish whether Mr. P’s worsening symptoms are consistent with a depressive episode or whether they are a manifestation of the transient dysphoria that accompanies nicotine withdrawal. Assessing the severity and persistence of the mood disturbance as well as the timing of onset could help make this determination. Nicotine withdrawal symptoms typically emerge within 24 hours of quitting or significantly reducing smoking and tend to peak within approximately 1 week. Thus, depressive symptoms that develop after weeks or months of abstinence would be less consistent with nicotine withdrawal. Additionally, the lethargy Mr. P reported may be a symptom of depression, or it may stem from a cessation-induced increase in antipsychotic serum levels. Because tobacco smoke increases the metabolism of several antipsychotics and antidepressants—including olanzapine, clozapine, haloperidol, and fluoxetine25—stopping smoking may increase medication levels and side effects. To rule out medication side effects as a cause of post-cessation mood changes, the psychiatrist should ask Mr. P about which smoking cessation pharmacotherapies (if any) he was using and which psychotropic medications he was taking. Unfortunately, such a detailed history is not always taken, and patient-generated theories of smoking cessation causing worsening psychiatric symptoms often are taken at face value.
When should smokers with psychiatric disorders be encouraged to quit? Are there times when smoking cessation should be discouraged? Tobacco treatment guidelines4 recommend advising users to quit at every clinical encounter, but there has been some debate about the timing of tobacco treatment for smokers with psychiatric disorders. There is minimal research to guide such treatment decisions. However, even if quit attempts are more successful during times of symptomatic stability—and there is no conclusive evidence to indicate they are—waiting for perfect mental health before initiating smoking cessation treatment is unnecessary and ill-advised. In some situations, such as when a patient has experienced an acute increase in psychiatric symptoms or when psychotropics are being titrated, a short-term postponement of quitting may be reasonable. However, discouraging smokers from trying to quit when they express readiness to try should be done sparingly, because it is uncertain how long that window of opportunity will be open, and the consequences of missed opportunities can be fatal.
- National Tobacco Quitline. 1-800-QUIT-NOW. www.smokefree.gov.
- University of California, San Francisco, Schools of Pharmacy and Medicine. Rx for Change (free online training program for clinicians). http://rxforchange.ucsf.edu.
- National Association of State Mental Health Program Directors. Tobacco-free living in psychiatric settings: a best-practices toolkit promoting wellness and recovery. www.nasmhpd.org/general_files/publications/NASMHPD.toolkitfinalupdated90707.pdf.
Drug Brand Names
- Bupropion • Wellbutrin, Zyban
- Clozapine • Clozaril
- Fluoxetine • Prozac
- Haloperidol • Haldol
- Nortriptyline • Aventyl, Pamelor
- Olanzapine • Zyprexa
- Varenicline • Chantix
Disclosures
Dr. Heffner was supported by National Institute on Drug Abuse grant#026517. She is a consultant to Pfizer Inc.
Dr. Anthenelli is supported by National Institute on Alcohol Abuse and Alcoholism grant#AA19720 and by the Department of Veterans Affairs. He is a consultant to GlaxoSmithKline and Pfizer Inc.
The Tri-State Tobacco and Alcohol Research Center receives research support from Eli Lilly and Company, Nabi Biopharmaceuticals, Pfizer Inc., and sanofi-aventis.
1. Colton CW, Manderscheid RW. Congruencies in increased mortality rates years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.-
2. Centers for Disease Control and Prevention. Trends in state and federal cigarette tax and retail price—1970-2009. 2010. Available at: http://www.cdc.gov/tobacco/data_statistics/tables/economics/trends. Accessed June 28 2011.
3. Xian H, Scherrer JF, Eisen SA, et al. Nicotine dependence subtypes: association with smoking history, diagnostic criteria and psychiatric disorders in 5440 regular smokers from the Vietnam Era Twin Registry. Addict Behav. 2007;32(1):137-147.
4. Fiore MC, Jaén CR, Baker TB, et al. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U. S. Public Health Service report. Am J Prev Med. 2008;35(2):158-176.
5. Anthenelli RM. How–and why–to help psychiatric patients stop smoking. Current Psychiatry. 2005;4(1):77-87.
6. Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2007;1:CD000031.-
7. Williams JM, Foulds J. Successful tobacco dependence treatment in schizophrenia. Am J Psychiatry. 2007;164(2):222-227.
8. George TP, Vessicchio JC, Termine A, et al. A placebo controlled trial of bupropion for smoking cessation in schizophrenia. Biol Psychiatry. 2002;52(1):53-61.
9. Hughes JR, Kalman D. Do smokers with alcohol problems have more difficulty quitting? Drug Alcohol Depend. 2006;82(2):91-102.
10. McClure JB, Swan GE, Catz SL, et al. Smoking outcome by psychiatric history after behavioral and varenicline treatment. J Subst Abuse Treat. 2010;38(4):394-402.
11. Stapleton JA, Watson L, Spirling LI, et al. Varenicline in the routine treatment of tobacco dependence: a pre-post comparison with nicotine replacement therapy and an evaluation in those with mental illness. Addiction. 2008;103(1):146-154.
12. Hall SM, Muñoz RF, Reus VI. Cognitive-behavioral intervention increases abstinence rates for depressive-history smokers. J Consult Clin Psychol. 1994;62(1):141-146.
13. Hall SM, Muñoz RF, Reus VI, et al. Mood management and nicotine gum in smoking treatment: a therapeutic contact and placebo-controlled study. J Consult Clin Psychol. 1996;64(5):1003-1009.
14. Evins AE, Culhane MA, Alpert JE, et al. A controlled trial of bupropion added to nicotine patch and behavioral therapy for smoking cessation in adults with unipolar depressive disorders. J Clin Psychopharmacol. 2008;28(6):660-666.
15. Hall SM, Reus VI, Muñoz RF, et al. Nortriptyline and cognitive-behavioral therapy in the treatment of cigarette smoking. Arch Gen Psychiatry. 1998;55(8):683-690.
16. Brown RA, Kahler CW, Niaura R, et al. Cognitive-behavioral treatment for depression in smoking cessation. J Consult Clin Psychol. 2001;69(3):471-480.
17. McFall M, Saxon AJ, Malte CA, et al. Integrating tobacco cessation into mental health care for posttraumatic stress disorder: a randomized controlled trial. JAMA. 2010;304(22):2485-2493.
18. George TP, Ziedonis DM, Feingold A, et al. Nicotine transdermal patch and atypical antipsychotic medications for smoking cessation in schizophrenia. Am J Psychiatry. 2000;157(11):1835-1842.
19. Williams JM, Steinberg ML, Zimmermann MH, et al. Comparison of two intensities of tobacco dependence counseling in schizophrenia and schizoaffective disorder. J Subst Abuse Treat. 2010;38(4):384-393.
20. Evins AE, Cather C, Culhane MA, et al. A 12-week double-blind, placebo-controlled study of bupropion sr added to high-dose dual nicotine replacement therapy for smoking cessation or reduction in schizophrenia. J Clin Psychopharmacol. 2007;27(4):380-386.
21. Weinberger AH, Hitsman B, Papandonatos GD, et al. Predictors of abstinence and changes in psychiatric symptoms in a pooled sample of smokers with schizophrenia receiving combination pharmacotherapy and behavioral therapy for smoking cessation. J Clin Psychopharmacol. 2009;29(6):601-603.
22. Prochaska JJ, Hall SM, Tsoh JY, et al. Treating tobacco dependence in clinically depressed smokers: effect of smoking cessation on mental health functioning. Am J Public Health. 2008;98(3):446-448.
23. Tsoh JY, Humfleet GL, Muñoz RF, et al. Development of major depression after treatment for smoking cessation. Am J Psychiatry. 2000;157(3):368-374.
24. Prochaska JJ, Delucchi K, Hall SM. A meta-analysis of smoking cessation interventions with individuals in substance abuse treatment or recovery. J Consult Clin Psychol. 2004;72(6):1144-1156.
25. Zevin S, Benowitz NL. Drug interactions with tobacco smoking. An update. Clin Pharmacokinet. 1999;36(6):425-438.
Discuss this article at www.facebook.com/CurrentPsychiatry
You are treating Mr. P, age 34, for schizoaffective disorder. He smokes 1 pack of cigarettes per day and has smoked for approximately 17 years. He has tried to stop but never has been able to quit for more than a few weeks. He reveals whenever he tries to quit, he starts feeling extremely lethargic and “depressed” and resumes smoking to prevent these symptoms from worsening. However, Mr. P expresses some interest in trying to quit again and asks whether any medications could prevent him from becoming depressed while he tries to quit.
Cigarette smoking is overrepresented and undertreated among individuals with psychiatric illness, in part because of the largely unfounded belief held by some patients and clinicians that smoking cessation might worsen psychiatric symptoms. In this article, we argue this challenge can be overcome and psychiatrists and other mental health professionals can and should help their patients reap the innumerable benefits of quitting smoking. We discuss:
- the short- and long-term effects of smoking cessation
- evidence-based treatment guidelines for working with motivated and unmotivated smokers
- unique issues that may arise when treating smokers who have psychiatric disorders.
Quitting: Profound benefits
Quitting smoking has substantial benefits beginning within minutes after taking the last puff. Some of the benefits that occur within the first few days of quitting include:
- decreased blood pressure and pulse rate
- improved circulation
- improved ability to smell and taste
- easier breathing.
Longer-term smoking abstinence drastically reduces risk of heart attack, stroke, cancer, respiratory disease, and a host of other illnesses that affect—and kill—individuals with psychiatric disorders several decades earlier than their counterparts in the general population.1 There also are financial benefits to quitting; using the 2009 national average of $5.33 per pack, a 1-pack-per-day smoker who quits would save >$150 per month, which accounts for only the direct cost of cigarettes.2
Although the beneficial effects of quitting smoking are profound and far-reaching, in the short-term they are counterbalanced by nicotine withdrawal symptoms—including restlessness, irritability, depressed mood, concentration problems, and increased appetite/weight gain—that are formidable distractions from the positive aspects of quitting. Additionally, nicotine withdrawal symptoms tend to be more severe in smokers who have a psychiatric disorder.3 Fortunately, there are effective, evidence-based methods of reducing withdrawal symptoms and helping smokers cope with these and other challenges of quitting.
Combined treatment is best
Current treatment guidelines4 suggest all smokers should be offered pharmacotherapy and counseling to aid quitting because this combined approach has the highest success rate (Algorithm). Table 1 4 provides information about dosing, efficacy, and side effect profile of each of the 7 FDA-approved medications for smoking cessation. Using any of the approved medications at least doubles the odds of successful quitting compared with placebo.4 These pharmacotherapies can reduce or prevent nicotine withdrawal symptoms and—at least in the case of bupropion and varenicline—decrease reinforcement from smoking, thereby lowering the likelihood a lapse (ie, smoking ≥1 cigarettes without returning to regular smoking) will develop into a full-blown relapse (ie, return to regular smoking).
Algorithm: Tobacco cessation treatment for psychiatric patients
Source: Adapted from reference 4 Medication selection depends on many factors, including:
- the patient’s psychiatric illness
- her/his prior response to smoking cessation pharmacotherapies
- concomitant psychiatric medications
- patient preference.5
Placebo-controlled trials of smoking cessation aids in psychiatrically ill patients are limited, but several studies of smokers with a history of major depression indicate treatment with bupropion SR or nortriptyline is effective.6 Similarly, although relapse rates generally are higher in patients with schizophrenia compared with non-mentally ill smokers, nicotine replacement therapy and bupropion SR are more effective than placebo in patients with this disorder.7,8 When we prescribe these treatments, we tend to extend the duration of treatment beyond those described in Table 1 ,4 and to use combined treatments (eg, a transdermal patch with a shorter-acting gum or lozenge preparation) to better target the marked withdrawal symptoms more severely nicotine-dependent patients frequently experience.
Table 1
First-line pharmacotherapies for smoking cessation
| Medication | Standard dosage | Efficacy (OR, % abstinent at 6 mos. [with 95% CI]) | Contraindications (C) and precautions (P) | Common side effects |
|---|---|---|---|---|
| Non-nicotine medications | ||||
| Bupropion | Days 1-3: 150 mg/d Days 4-8: 150 mg bid Continue for 7-12 weeks at 150 mg bid | 2.0 (1.8-2.2), 24% (22%-26%) | C: Eating disorders, seizure history, taking bupropion, MAOI in past 2 weeks P: Pregnancy, cardiovascular disease, warning for emergent psychiatric symptoms | Insomnia, dry mouth |
| Varenicline | Days 1-3: 0.5 mg/d Days 4-7: 0.5 mg bid Day 8+: 1 mg bid Continue 11 weeks at 1 mg bid; up to 6 months for maintenance | 3.1 (2.5-3.8), 33% (29%-38%) | P: Warning for emergent psychiatric symptoms | Nausea, sleep problems, abnormal dreams |
| Nicotine replacement therapies | ||||
| Nicotine gum | 1 piece every 1-2 hours for 6-12 weeks <20 cigarettes/d: 2 mg gum ≥20 cigarettes/d: 4 mg gum | 1.5 (1.2-1.7), 19% (17%-22%) | P: Pregnancy, recent myocardial infarction, serious arrhythmia, unstable angina | Mouth soreness, hiccups, dyspepsia |
| Nicotine inhaler | 6-16 cartridges/d, up to 6 months | 2.1 (1.5-2.9), 25% (19%-32%) | Same as above | Mouth/throat irritation, coughing, rhinitis |
| Nicotine lozenge | 9-20 lozenges/d, up to 12 weeks Smoke ≤30 minutes after waking: 4 mg lozenge Smoke >30 minutes after waking: 2 mg lozenge | 2.0 (1.6-2.5)a | Same as above | Nausea, hiccups, heartburn |
| Nicotine nasal spray | 1-2 doses/hour, 8-40 doses/d for 3-6 months | 2.3 (1.7-3.0), 27% (22%-33%) | C: Severe reactive airway disease P: Same as above | Nasal irritation, higher risk of dependency |
| Nicotine patch | 1 patch/d, step-down dosing over 8 weeks Weeks 1-4: 21 mg patch Weeks 5-6: 14 mg patch Weeks 7-8: 7 mg patch | 1.9 (1.7-2.2) 23% (21%-26%) | P: Same as above | Skin reactions, sleep problems, abnormal dreams |
| aStead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2008;1:CD000146. bid: twice a day; CI: confidence interval; MAOI: monoamine oxidase inhibitor; OR: odds ratio Source: Adapted from reference 4 | ||||
Counseling. All smokers should be provided with brief interventions consistent with the 5 A’s—Ask, Advise, Assess, Assist, and Arrange (Table 2).4 For smokers who are not motivated to quit, the recommended approach follows the principles of the 5 R’s—Relevance, Risks, Rewards, Roadblocks, and Repetition (Table 3).4 Smokers who are motivated to quit and willing to participate in more intensive treatment may be offered face-to-face individual or group counseling (depending upon availability) or referred to a telephone quit line (see Related Resources). Intensive treatments such as these typically provide social support and assistance overcoming barriers to cessation and developing skills to initiate and maintain abstinence (eg, coping with a lapse or handling cravings, identifying and avoiding high-risk situations for smoking). As a general rule, greater intensity of counseling is associated with a greater likelihood of quitting.4
Table 2
The 5 A’s of tobacco treatment
| Intervention | Example | |
|---|---|---|
| Ask | Systematically inquire about tobacco use | “Do you currently use, or have you ever used, tobacco products?” |
| Advise | Counsel all tobacco users to quit in a clear, strong, and personalized manner | “I think it is very important for you quit smoking to keep your breathing problems from getting any worse” |
| Assess | Determine the tobacco user’s willingness to make a quit attempt | “What do you think? Are you ready to quit?” |
| Assist | Offer or refer to treatment/support (if ready to quit; if not ready, see Table 3 for recommended interventions) | “I’m here to help you with this. Let me start by letting you know about the many options available to help you quit” |
| Arrange | Plan for follow-up contacts (at least 1, preferably within 1 week of the quit date) | “I would like to give you a call within the next week to see how you did with your quit date. Would that be OK with you?” |
| Source: Adapted from reference 4 | ||
Table 3
The 5 R’s: Principles of interventions for smokers not ready to quit
| Principle | Example | |
|---|---|---|
| Relevance | Why is quitting smoking personally relevant? | “You’ve told me your kids sometimes make comments to you about quitting smoking. How does that affect you?” |
| Risks | What are the negative consequences of smoking? | “What don’t you like about smoking? What problems have you had from smoking?” |
| Rewards | What are the benefits of quitting smoking? | “Can you think of anything that would be good about quitting? Tell me about that” |
| Roadblocks | What are the barriers to quitting? | “What worries do you have about trying to quit? What happened the last time you tried to quit smoking?” |
| Repetition | Message repeated at every visit | “I know we have talked about quitting smoking before, but things may have changed since then. I also think that this is such an important issue we should keep it on the table for discussion. What do you think?” |
| Source: Adapted from reference 4 | ||
Q&A about treatment
How effective are smoking cessation interventions for individuals with psychiatric disorders? Several studies have demonstrated, on any given quit attempt, smokers with psychiatric or substance use disorders can be as successful as smokers without these disorders.9-11 In fact, quit rates as high as approximately 70% for end-of-treatment11 and 30% for 6-month follow-up10 have been reported. Of course, effectiveness varies by type and intensity of treatment as well as by individual characteristics of the smoker. Smokers with psychiatric disorders may fare better with more intensive interventions than briefer ones,12,13 and factors such as high levels of nicotine dependence and exposure to smoking environments—both of which are characteristic of smokers with serious mental illness—can negatively impact treatment outcomes.4
Should the nature of the psychiatric disorder(s) guide decisions about the optimal pharmacotherapy or counseling approach? There have been numerous attempts to investigate the effectiveness of targeted interventions for particular subgroups of smokers with psychiatric disorders, including:
- studies of the efficacy of the antidepressants bupropion14 and nortriptyline15 as well as cognitive-behavioral therapy-based mood management counseling16 for depressed smokers
- integrative treatment approaches for smokers with posttraumatic stress disorder (PTSD)17
- group counseling designed specifically for smokers with schizophrenia.18,19
Although more research is needed and there have been some promising early results (eg, McFall et al17), current literature does not provide consistent evidence supporting treatment matching solely on the basis of the psychiatric disorder. Rather, patient preference, safety considerations (eg, use of medications in children/adolescents, pregnant women), medication side effect profiles, prior experience with the treatment approach, and cost/availability of treatment should guide development of the treatment plan. When results from placebo-controlled trials are available for subgroups of patients (eg, those with a history of major depression), consider this information when selecting a pharmacologic smoking cessation aid.
What is the risk of psychiatric symptoms worsening as a result of quitting smoking? Little research on this topic is available because more often than not, smokers with psychiatric disorders are excluded from tobacco treatment studies. However, research examining psychiatric status changes among recent quitters with schizophrenia,20,21 depression,22,23 PTSD,17 and substance use disorders24 suggests smoking cessation does not worsen symptoms of these disorders, and may be associated with symptom improvement.17 Nonetheless, driven largely by anecdotal evidence, the misconception that smoking cessation worsens psychiatric symptoms remains a substantial barrier to treatment.
Mr. P’s case is an example of how not probing about the nature of psychiatric complaints can be problematic. Mr. P reported what on first glance appeared to be a worsening of psychiatric symptoms starting when he stopped smoking and resolved when he resumed smoking. However, without gathering additional information about these events, we cannot conclude stopping smoking caused his psychiatric symptoms to worsen. Other potential explanations include nicotine withdrawal symptoms, side effects of smoking cessation medications, an increase in levels of psychotropic medications for which metabolism is affected by tobacco smoke, or the natural course of his mood disorder. The timing of the onset and offset of symptoms seems to argue against Mr. P’s symptoms reflecting the natural course of his mood disorder, but the other 3 explanations remain plausible.
It is important to distinguish whether Mr. P’s worsening symptoms are consistent with a depressive episode or whether they are a manifestation of the transient dysphoria that accompanies nicotine withdrawal. Assessing the severity and persistence of the mood disturbance as well as the timing of onset could help make this determination. Nicotine withdrawal symptoms typically emerge within 24 hours of quitting or significantly reducing smoking and tend to peak within approximately 1 week. Thus, depressive symptoms that develop after weeks or months of abstinence would be less consistent with nicotine withdrawal. Additionally, the lethargy Mr. P reported may be a symptom of depression, or it may stem from a cessation-induced increase in antipsychotic serum levels. Because tobacco smoke increases the metabolism of several antipsychotics and antidepressants—including olanzapine, clozapine, haloperidol, and fluoxetine25—stopping smoking may increase medication levels and side effects. To rule out medication side effects as a cause of post-cessation mood changes, the psychiatrist should ask Mr. P about which smoking cessation pharmacotherapies (if any) he was using and which psychotropic medications he was taking. Unfortunately, such a detailed history is not always taken, and patient-generated theories of smoking cessation causing worsening psychiatric symptoms often are taken at face value.
When should smokers with psychiatric disorders be encouraged to quit? Are there times when smoking cessation should be discouraged? Tobacco treatment guidelines4 recommend advising users to quit at every clinical encounter, but there has been some debate about the timing of tobacco treatment for smokers with psychiatric disorders. There is minimal research to guide such treatment decisions. However, even if quit attempts are more successful during times of symptomatic stability—and there is no conclusive evidence to indicate they are—waiting for perfect mental health before initiating smoking cessation treatment is unnecessary and ill-advised. In some situations, such as when a patient has experienced an acute increase in psychiatric symptoms or when psychotropics are being titrated, a short-term postponement of quitting may be reasonable. However, discouraging smokers from trying to quit when they express readiness to try should be done sparingly, because it is uncertain how long that window of opportunity will be open, and the consequences of missed opportunities can be fatal.
- National Tobacco Quitline. 1-800-QUIT-NOW. www.smokefree.gov.
- University of California, San Francisco, Schools of Pharmacy and Medicine. Rx for Change (free online training program for clinicians). http://rxforchange.ucsf.edu.
- National Association of State Mental Health Program Directors. Tobacco-free living in psychiatric settings: a best-practices toolkit promoting wellness and recovery. www.nasmhpd.org/general_files/publications/NASMHPD.toolkitfinalupdated90707.pdf.
Drug Brand Names
- Bupropion • Wellbutrin, Zyban
- Clozapine • Clozaril
- Fluoxetine • Prozac
- Haloperidol • Haldol
- Nortriptyline • Aventyl, Pamelor
- Olanzapine • Zyprexa
- Varenicline • Chantix
Disclosures
Dr. Heffner was supported by National Institute on Drug Abuse grant#026517. She is a consultant to Pfizer Inc.
Dr. Anthenelli is supported by National Institute on Alcohol Abuse and Alcoholism grant#AA19720 and by the Department of Veterans Affairs. He is a consultant to GlaxoSmithKline and Pfizer Inc.
The Tri-State Tobacco and Alcohol Research Center receives research support from Eli Lilly and Company, Nabi Biopharmaceuticals, Pfizer Inc., and sanofi-aventis.
Discuss this article at www.facebook.com/CurrentPsychiatry
You are treating Mr. P, age 34, for schizoaffective disorder. He smokes 1 pack of cigarettes per day and has smoked for approximately 17 years. He has tried to stop but never has been able to quit for more than a few weeks. He reveals whenever he tries to quit, he starts feeling extremely lethargic and “depressed” and resumes smoking to prevent these symptoms from worsening. However, Mr. P expresses some interest in trying to quit again and asks whether any medications could prevent him from becoming depressed while he tries to quit.
Cigarette smoking is overrepresented and undertreated among individuals with psychiatric illness, in part because of the largely unfounded belief held by some patients and clinicians that smoking cessation might worsen psychiatric symptoms. In this article, we argue this challenge can be overcome and psychiatrists and other mental health professionals can and should help their patients reap the innumerable benefits of quitting smoking. We discuss:
- the short- and long-term effects of smoking cessation
- evidence-based treatment guidelines for working with motivated and unmotivated smokers
- unique issues that may arise when treating smokers who have psychiatric disorders.
Quitting: Profound benefits
Quitting smoking has substantial benefits beginning within minutes after taking the last puff. Some of the benefits that occur within the first few days of quitting include:
- decreased blood pressure and pulse rate
- improved circulation
- improved ability to smell and taste
- easier breathing.
Longer-term smoking abstinence drastically reduces risk of heart attack, stroke, cancer, respiratory disease, and a host of other illnesses that affect—and kill—individuals with psychiatric disorders several decades earlier than their counterparts in the general population.1 There also are financial benefits to quitting; using the 2009 national average of $5.33 per pack, a 1-pack-per-day smoker who quits would save >$150 per month, which accounts for only the direct cost of cigarettes.2
Although the beneficial effects of quitting smoking are profound and far-reaching, in the short-term they are counterbalanced by nicotine withdrawal symptoms—including restlessness, irritability, depressed mood, concentration problems, and increased appetite/weight gain—that are formidable distractions from the positive aspects of quitting. Additionally, nicotine withdrawal symptoms tend to be more severe in smokers who have a psychiatric disorder.3 Fortunately, there are effective, evidence-based methods of reducing withdrawal symptoms and helping smokers cope with these and other challenges of quitting.
Combined treatment is best
Current treatment guidelines4 suggest all smokers should be offered pharmacotherapy and counseling to aid quitting because this combined approach has the highest success rate (Algorithm). Table 1 4 provides information about dosing, efficacy, and side effect profile of each of the 7 FDA-approved medications for smoking cessation. Using any of the approved medications at least doubles the odds of successful quitting compared with placebo.4 These pharmacotherapies can reduce or prevent nicotine withdrawal symptoms and—at least in the case of bupropion and varenicline—decrease reinforcement from smoking, thereby lowering the likelihood a lapse (ie, smoking ≥1 cigarettes without returning to regular smoking) will develop into a full-blown relapse (ie, return to regular smoking).
Algorithm: Tobacco cessation treatment for psychiatric patients
Source: Adapted from reference 4 Medication selection depends on many factors, including:
- the patient’s psychiatric illness
- her/his prior response to smoking cessation pharmacotherapies
- concomitant psychiatric medications
- patient preference.5
Placebo-controlled trials of smoking cessation aids in psychiatrically ill patients are limited, but several studies of smokers with a history of major depression indicate treatment with bupropion SR or nortriptyline is effective.6 Similarly, although relapse rates generally are higher in patients with schizophrenia compared with non-mentally ill smokers, nicotine replacement therapy and bupropion SR are more effective than placebo in patients with this disorder.7,8 When we prescribe these treatments, we tend to extend the duration of treatment beyond those described in Table 1 ,4 and to use combined treatments (eg, a transdermal patch with a shorter-acting gum or lozenge preparation) to better target the marked withdrawal symptoms more severely nicotine-dependent patients frequently experience.
Table 1
First-line pharmacotherapies for smoking cessation
| Medication | Standard dosage | Efficacy (OR, % abstinent at 6 mos. [with 95% CI]) | Contraindications (C) and precautions (P) | Common side effects |
|---|---|---|---|---|
| Non-nicotine medications | ||||
| Bupropion | Days 1-3: 150 mg/d Days 4-8: 150 mg bid Continue for 7-12 weeks at 150 mg bid | 2.0 (1.8-2.2), 24% (22%-26%) | C: Eating disorders, seizure history, taking bupropion, MAOI in past 2 weeks P: Pregnancy, cardiovascular disease, warning for emergent psychiatric symptoms | Insomnia, dry mouth |
| Varenicline | Days 1-3: 0.5 mg/d Days 4-7: 0.5 mg bid Day 8+: 1 mg bid Continue 11 weeks at 1 mg bid; up to 6 months for maintenance | 3.1 (2.5-3.8), 33% (29%-38%) | P: Warning for emergent psychiatric symptoms | Nausea, sleep problems, abnormal dreams |
| Nicotine replacement therapies | ||||
| Nicotine gum | 1 piece every 1-2 hours for 6-12 weeks <20 cigarettes/d: 2 mg gum ≥20 cigarettes/d: 4 mg gum | 1.5 (1.2-1.7), 19% (17%-22%) | P: Pregnancy, recent myocardial infarction, serious arrhythmia, unstable angina | Mouth soreness, hiccups, dyspepsia |
| Nicotine inhaler | 6-16 cartridges/d, up to 6 months | 2.1 (1.5-2.9), 25% (19%-32%) | Same as above | Mouth/throat irritation, coughing, rhinitis |
| Nicotine lozenge | 9-20 lozenges/d, up to 12 weeks Smoke ≤30 minutes after waking: 4 mg lozenge Smoke >30 minutes after waking: 2 mg lozenge | 2.0 (1.6-2.5)a | Same as above | Nausea, hiccups, heartburn |
| Nicotine nasal spray | 1-2 doses/hour, 8-40 doses/d for 3-6 months | 2.3 (1.7-3.0), 27% (22%-33%) | C: Severe reactive airway disease P: Same as above | Nasal irritation, higher risk of dependency |
| Nicotine patch | 1 patch/d, step-down dosing over 8 weeks Weeks 1-4: 21 mg patch Weeks 5-6: 14 mg patch Weeks 7-8: 7 mg patch | 1.9 (1.7-2.2) 23% (21%-26%) | P: Same as above | Skin reactions, sleep problems, abnormal dreams |
| aStead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2008;1:CD000146. bid: twice a day; CI: confidence interval; MAOI: monoamine oxidase inhibitor; OR: odds ratio Source: Adapted from reference 4 | ||||
Counseling. All smokers should be provided with brief interventions consistent with the 5 A’s—Ask, Advise, Assess, Assist, and Arrange (Table 2).4 For smokers who are not motivated to quit, the recommended approach follows the principles of the 5 R’s—Relevance, Risks, Rewards, Roadblocks, and Repetition (Table 3).4 Smokers who are motivated to quit and willing to participate in more intensive treatment may be offered face-to-face individual or group counseling (depending upon availability) or referred to a telephone quit line (see Related Resources). Intensive treatments such as these typically provide social support and assistance overcoming barriers to cessation and developing skills to initiate and maintain abstinence (eg, coping with a lapse or handling cravings, identifying and avoiding high-risk situations for smoking). As a general rule, greater intensity of counseling is associated with a greater likelihood of quitting.4
Table 2
The 5 A’s of tobacco treatment
| Intervention | Example | |
|---|---|---|
| Ask | Systematically inquire about tobacco use | “Do you currently use, or have you ever used, tobacco products?” |
| Advise | Counsel all tobacco users to quit in a clear, strong, and personalized manner | “I think it is very important for you quit smoking to keep your breathing problems from getting any worse” |
| Assess | Determine the tobacco user’s willingness to make a quit attempt | “What do you think? Are you ready to quit?” |
| Assist | Offer or refer to treatment/support (if ready to quit; if not ready, see Table 3 for recommended interventions) | “I’m here to help you with this. Let me start by letting you know about the many options available to help you quit” |
| Arrange | Plan for follow-up contacts (at least 1, preferably within 1 week of the quit date) | “I would like to give you a call within the next week to see how you did with your quit date. Would that be OK with you?” |
| Source: Adapted from reference 4 | ||
Table 3
The 5 R’s: Principles of interventions for smokers not ready to quit
| Principle | Example | |
|---|---|---|
| Relevance | Why is quitting smoking personally relevant? | “You’ve told me your kids sometimes make comments to you about quitting smoking. How does that affect you?” |
| Risks | What are the negative consequences of smoking? | “What don’t you like about smoking? What problems have you had from smoking?” |
| Rewards | What are the benefits of quitting smoking? | “Can you think of anything that would be good about quitting? Tell me about that” |
| Roadblocks | What are the barriers to quitting? | “What worries do you have about trying to quit? What happened the last time you tried to quit smoking?” |
| Repetition | Message repeated at every visit | “I know we have talked about quitting smoking before, but things may have changed since then. I also think that this is such an important issue we should keep it on the table for discussion. What do you think?” |
| Source: Adapted from reference 4 | ||
Q&A about treatment
How effective are smoking cessation interventions for individuals with psychiatric disorders? Several studies have demonstrated, on any given quit attempt, smokers with psychiatric or substance use disorders can be as successful as smokers without these disorders.9-11 In fact, quit rates as high as approximately 70% for end-of-treatment11 and 30% for 6-month follow-up10 have been reported. Of course, effectiveness varies by type and intensity of treatment as well as by individual characteristics of the smoker. Smokers with psychiatric disorders may fare better with more intensive interventions than briefer ones,12,13 and factors such as high levels of nicotine dependence and exposure to smoking environments—both of which are characteristic of smokers with serious mental illness—can negatively impact treatment outcomes.4
Should the nature of the psychiatric disorder(s) guide decisions about the optimal pharmacotherapy or counseling approach? There have been numerous attempts to investigate the effectiveness of targeted interventions for particular subgroups of smokers with psychiatric disorders, including:
- studies of the efficacy of the antidepressants bupropion14 and nortriptyline15 as well as cognitive-behavioral therapy-based mood management counseling16 for depressed smokers
- integrative treatment approaches for smokers with posttraumatic stress disorder (PTSD)17
- group counseling designed specifically for smokers with schizophrenia.18,19
Although more research is needed and there have been some promising early results (eg, McFall et al17), current literature does not provide consistent evidence supporting treatment matching solely on the basis of the psychiatric disorder. Rather, patient preference, safety considerations (eg, use of medications in children/adolescents, pregnant women), medication side effect profiles, prior experience with the treatment approach, and cost/availability of treatment should guide development of the treatment plan. When results from placebo-controlled trials are available for subgroups of patients (eg, those with a history of major depression), consider this information when selecting a pharmacologic smoking cessation aid.
What is the risk of psychiatric symptoms worsening as a result of quitting smoking? Little research on this topic is available because more often than not, smokers with psychiatric disorders are excluded from tobacco treatment studies. However, research examining psychiatric status changes among recent quitters with schizophrenia,20,21 depression,22,23 PTSD,17 and substance use disorders24 suggests smoking cessation does not worsen symptoms of these disorders, and may be associated with symptom improvement.17 Nonetheless, driven largely by anecdotal evidence, the misconception that smoking cessation worsens psychiatric symptoms remains a substantial barrier to treatment.
Mr. P’s case is an example of how not probing about the nature of psychiatric complaints can be problematic. Mr. P reported what on first glance appeared to be a worsening of psychiatric symptoms starting when he stopped smoking and resolved when he resumed smoking. However, without gathering additional information about these events, we cannot conclude stopping smoking caused his psychiatric symptoms to worsen. Other potential explanations include nicotine withdrawal symptoms, side effects of smoking cessation medications, an increase in levels of psychotropic medications for which metabolism is affected by tobacco smoke, or the natural course of his mood disorder. The timing of the onset and offset of symptoms seems to argue against Mr. P’s symptoms reflecting the natural course of his mood disorder, but the other 3 explanations remain plausible.
It is important to distinguish whether Mr. P’s worsening symptoms are consistent with a depressive episode or whether they are a manifestation of the transient dysphoria that accompanies nicotine withdrawal. Assessing the severity and persistence of the mood disturbance as well as the timing of onset could help make this determination. Nicotine withdrawal symptoms typically emerge within 24 hours of quitting or significantly reducing smoking and tend to peak within approximately 1 week. Thus, depressive symptoms that develop after weeks or months of abstinence would be less consistent with nicotine withdrawal. Additionally, the lethargy Mr. P reported may be a symptom of depression, or it may stem from a cessation-induced increase in antipsychotic serum levels. Because tobacco smoke increases the metabolism of several antipsychotics and antidepressants—including olanzapine, clozapine, haloperidol, and fluoxetine25—stopping smoking may increase medication levels and side effects. To rule out medication side effects as a cause of post-cessation mood changes, the psychiatrist should ask Mr. P about which smoking cessation pharmacotherapies (if any) he was using and which psychotropic medications he was taking. Unfortunately, such a detailed history is not always taken, and patient-generated theories of smoking cessation causing worsening psychiatric symptoms often are taken at face value.
When should smokers with psychiatric disorders be encouraged to quit? Are there times when smoking cessation should be discouraged? Tobacco treatment guidelines4 recommend advising users to quit at every clinical encounter, but there has been some debate about the timing of tobacco treatment for smokers with psychiatric disorders. There is minimal research to guide such treatment decisions. However, even if quit attempts are more successful during times of symptomatic stability—and there is no conclusive evidence to indicate they are—waiting for perfect mental health before initiating smoking cessation treatment is unnecessary and ill-advised. In some situations, such as when a patient has experienced an acute increase in psychiatric symptoms or when psychotropics are being titrated, a short-term postponement of quitting may be reasonable. However, discouraging smokers from trying to quit when they express readiness to try should be done sparingly, because it is uncertain how long that window of opportunity will be open, and the consequences of missed opportunities can be fatal.
- National Tobacco Quitline. 1-800-QUIT-NOW. www.smokefree.gov.
- University of California, San Francisco, Schools of Pharmacy and Medicine. Rx for Change (free online training program for clinicians). http://rxforchange.ucsf.edu.
- National Association of State Mental Health Program Directors. Tobacco-free living in psychiatric settings: a best-practices toolkit promoting wellness and recovery. www.nasmhpd.org/general_files/publications/NASMHPD.toolkitfinalupdated90707.pdf.
Drug Brand Names
- Bupropion • Wellbutrin, Zyban
- Clozapine • Clozaril
- Fluoxetine • Prozac
- Haloperidol • Haldol
- Nortriptyline • Aventyl, Pamelor
- Olanzapine • Zyprexa
- Varenicline • Chantix
Disclosures
Dr. Heffner was supported by National Institute on Drug Abuse grant#026517. She is a consultant to Pfizer Inc.
Dr. Anthenelli is supported by National Institute on Alcohol Abuse and Alcoholism grant#AA19720 and by the Department of Veterans Affairs. He is a consultant to GlaxoSmithKline and Pfizer Inc.
The Tri-State Tobacco and Alcohol Research Center receives research support from Eli Lilly and Company, Nabi Biopharmaceuticals, Pfizer Inc., and sanofi-aventis.
1. Colton CW, Manderscheid RW. Congruencies in increased mortality rates years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.-
2. Centers for Disease Control and Prevention. Trends in state and federal cigarette tax and retail price—1970-2009. 2010. Available at: http://www.cdc.gov/tobacco/data_statistics/tables/economics/trends. Accessed June 28 2011.
3. Xian H, Scherrer JF, Eisen SA, et al. Nicotine dependence subtypes: association with smoking history, diagnostic criteria and psychiatric disorders in 5440 regular smokers from the Vietnam Era Twin Registry. Addict Behav. 2007;32(1):137-147.
4. Fiore MC, Jaén CR, Baker TB, et al. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U. S. Public Health Service report. Am J Prev Med. 2008;35(2):158-176.
5. Anthenelli RM. How–and why–to help psychiatric patients stop smoking. Current Psychiatry. 2005;4(1):77-87.
6. Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2007;1:CD000031.-
7. Williams JM, Foulds J. Successful tobacco dependence treatment in schizophrenia. Am J Psychiatry. 2007;164(2):222-227.
8. George TP, Vessicchio JC, Termine A, et al. A placebo controlled trial of bupropion for smoking cessation in schizophrenia. Biol Psychiatry. 2002;52(1):53-61.
9. Hughes JR, Kalman D. Do smokers with alcohol problems have more difficulty quitting? Drug Alcohol Depend. 2006;82(2):91-102.
10. McClure JB, Swan GE, Catz SL, et al. Smoking outcome by psychiatric history after behavioral and varenicline treatment. J Subst Abuse Treat. 2010;38(4):394-402.
11. Stapleton JA, Watson L, Spirling LI, et al. Varenicline in the routine treatment of tobacco dependence: a pre-post comparison with nicotine replacement therapy and an evaluation in those with mental illness. Addiction. 2008;103(1):146-154.
12. Hall SM, Muñoz RF, Reus VI. Cognitive-behavioral intervention increases abstinence rates for depressive-history smokers. J Consult Clin Psychol. 1994;62(1):141-146.
13. Hall SM, Muñoz RF, Reus VI, et al. Mood management and nicotine gum in smoking treatment: a therapeutic contact and placebo-controlled study. J Consult Clin Psychol. 1996;64(5):1003-1009.
14. Evins AE, Culhane MA, Alpert JE, et al. A controlled trial of bupropion added to nicotine patch and behavioral therapy for smoking cessation in adults with unipolar depressive disorders. J Clin Psychopharmacol. 2008;28(6):660-666.
15. Hall SM, Reus VI, Muñoz RF, et al. Nortriptyline and cognitive-behavioral therapy in the treatment of cigarette smoking. Arch Gen Psychiatry. 1998;55(8):683-690.
16. Brown RA, Kahler CW, Niaura R, et al. Cognitive-behavioral treatment for depression in smoking cessation. J Consult Clin Psychol. 2001;69(3):471-480.
17. McFall M, Saxon AJ, Malte CA, et al. Integrating tobacco cessation into mental health care for posttraumatic stress disorder: a randomized controlled trial. JAMA. 2010;304(22):2485-2493.
18. George TP, Ziedonis DM, Feingold A, et al. Nicotine transdermal patch and atypical antipsychotic medications for smoking cessation in schizophrenia. Am J Psychiatry. 2000;157(11):1835-1842.
19. Williams JM, Steinberg ML, Zimmermann MH, et al. Comparison of two intensities of tobacco dependence counseling in schizophrenia and schizoaffective disorder. J Subst Abuse Treat. 2010;38(4):384-393.
20. Evins AE, Cather C, Culhane MA, et al. A 12-week double-blind, placebo-controlled study of bupropion sr added to high-dose dual nicotine replacement therapy for smoking cessation or reduction in schizophrenia. J Clin Psychopharmacol. 2007;27(4):380-386.
21. Weinberger AH, Hitsman B, Papandonatos GD, et al. Predictors of abstinence and changes in psychiatric symptoms in a pooled sample of smokers with schizophrenia receiving combination pharmacotherapy and behavioral therapy for smoking cessation. J Clin Psychopharmacol. 2009;29(6):601-603.
22. Prochaska JJ, Hall SM, Tsoh JY, et al. Treating tobacco dependence in clinically depressed smokers: effect of smoking cessation on mental health functioning. Am J Public Health. 2008;98(3):446-448.
23. Tsoh JY, Humfleet GL, Muñoz RF, et al. Development of major depression after treatment for smoking cessation. Am J Psychiatry. 2000;157(3):368-374.
24. Prochaska JJ, Delucchi K, Hall SM. A meta-analysis of smoking cessation interventions with individuals in substance abuse treatment or recovery. J Consult Clin Psychol. 2004;72(6):1144-1156.
25. Zevin S, Benowitz NL. Drug interactions with tobacco smoking. An update. Clin Pharmacokinet. 1999;36(6):425-438.
1. Colton CW, Manderscheid RW. Congruencies in increased mortality rates years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.-
2. Centers for Disease Control and Prevention. Trends in state and federal cigarette tax and retail price—1970-2009. 2010. Available at: http://www.cdc.gov/tobacco/data_statistics/tables/economics/trends. Accessed June 28 2011.
3. Xian H, Scherrer JF, Eisen SA, et al. Nicotine dependence subtypes: association with smoking history, diagnostic criteria and psychiatric disorders in 5440 regular smokers from the Vietnam Era Twin Registry. Addict Behav. 2007;32(1):137-147.
4. Fiore MC, Jaén CR, Baker TB, et al. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U. S. Public Health Service report. Am J Prev Med. 2008;35(2):158-176.
5. Anthenelli RM. How–and why–to help psychiatric patients stop smoking. Current Psychiatry. 2005;4(1):77-87.
6. Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2007;1:CD000031.-
7. Williams JM, Foulds J. Successful tobacco dependence treatment in schizophrenia. Am J Psychiatry. 2007;164(2):222-227.
8. George TP, Vessicchio JC, Termine A, et al. A placebo controlled trial of bupropion for smoking cessation in schizophrenia. Biol Psychiatry. 2002;52(1):53-61.
9. Hughes JR, Kalman D. Do smokers with alcohol problems have more difficulty quitting? Drug Alcohol Depend. 2006;82(2):91-102.
10. McClure JB, Swan GE, Catz SL, et al. Smoking outcome by psychiatric history after behavioral and varenicline treatment. J Subst Abuse Treat. 2010;38(4):394-402.
11. Stapleton JA, Watson L, Spirling LI, et al. Varenicline in the routine treatment of tobacco dependence: a pre-post comparison with nicotine replacement therapy and an evaluation in those with mental illness. Addiction. 2008;103(1):146-154.
12. Hall SM, Muñoz RF, Reus VI. Cognitive-behavioral intervention increases abstinence rates for depressive-history smokers. J Consult Clin Psychol. 1994;62(1):141-146.
13. Hall SM, Muñoz RF, Reus VI, et al. Mood management and nicotine gum in smoking treatment: a therapeutic contact and placebo-controlled study. J Consult Clin Psychol. 1996;64(5):1003-1009.
14. Evins AE, Culhane MA, Alpert JE, et al. A controlled trial of bupropion added to nicotine patch and behavioral therapy for smoking cessation in adults with unipolar depressive disorders. J Clin Psychopharmacol. 2008;28(6):660-666.
15. Hall SM, Reus VI, Muñoz RF, et al. Nortriptyline and cognitive-behavioral therapy in the treatment of cigarette smoking. Arch Gen Psychiatry. 1998;55(8):683-690.
16. Brown RA, Kahler CW, Niaura R, et al. Cognitive-behavioral treatment for depression in smoking cessation. J Consult Clin Psychol. 2001;69(3):471-480.
17. McFall M, Saxon AJ, Malte CA, et al. Integrating tobacco cessation into mental health care for posttraumatic stress disorder: a randomized controlled trial. JAMA. 2010;304(22):2485-2493.
18. George TP, Ziedonis DM, Feingold A, et al. Nicotine transdermal patch and atypical antipsychotic medications for smoking cessation in schizophrenia. Am J Psychiatry. 2000;157(11):1835-1842.
19. Williams JM, Steinberg ML, Zimmermann MH, et al. Comparison of two intensities of tobacco dependence counseling in schizophrenia and schizoaffective disorder. J Subst Abuse Treat. 2010;38(4):384-393.
20. Evins AE, Cather C, Culhane MA, et al. A 12-week double-blind, placebo-controlled study of bupropion sr added to high-dose dual nicotine replacement therapy for smoking cessation or reduction in schizophrenia. J Clin Psychopharmacol. 2007;27(4):380-386.
21. Weinberger AH, Hitsman B, Papandonatos GD, et al. Predictors of abstinence and changes in psychiatric symptoms in a pooled sample of smokers with schizophrenia receiving combination pharmacotherapy and behavioral therapy for smoking cessation. J Clin Psychopharmacol. 2009;29(6):601-603.
22. Prochaska JJ, Hall SM, Tsoh JY, et al. Treating tobacco dependence in clinically depressed smokers: effect of smoking cessation on mental health functioning. Am J Public Health. 2008;98(3):446-448.
23. Tsoh JY, Humfleet GL, Muñoz RF, et al. Development of major depression after treatment for smoking cessation. Am J Psychiatry. 2000;157(3):368-374.
24. Prochaska JJ, Delucchi K, Hall SM. A meta-analysis of smoking cessation interventions with individuals in substance abuse treatment or recovery. J Consult Clin Psychol. 2004;72(6):1144-1156.
25. Zevin S, Benowitz NL. Drug interactions with tobacco smoking. An update. Clin Pharmacokinet. 1999;36(6):425-438.
How to assess for possible drug-drug interactions
A dangerous GI complication
Discuss this article at www.facebook.com/CurrentPsychiatry
CASE: Gl surgery
Ms. X, age 61, presents to the emergency department (ED) complaining of nausea, vomiting, and abdominal pain and distension. CT scan of her abdomen reveals segmental ischemia in her colon with abscess formation, which leads to immediate surgery, including ileocecostomy with primary anastomosis. After surgery, Ms. X suffers from gastrointestinal (GI) dysmotility. The gastroenterology team recommends daily enemas along with a soft diet after she is discharged.
Ms. X has chronic paranoid schizophrenia, which has been treated successfully for 18 years with clozapine, 500 mg/d. During acute psychotic episodes, she experienced paranoid delusions and command auditory hallucinations telling her to kill herself. She had previous trials of several antipsychotics, including quetiapine, thiothixene, thioridazine, trifluoperazine, chlorpromazine, and haloperidol, all of which were ineffective and poorly tolerated because of serious side effects.
Within 1 month of discharge, Ms. X returns to the ED with nausea, vomiting, and abdominal distension. Abdominal CT scan suggests partial small bowel obstruction and significantly dilated loops of small bowel with decompressed rectum and sigmoid colon. Considering her recent GI surgery and absence of abdominal pain, she is managed with conservative measures, including nasogastric tube decompression and total parenteral nutrition. CT enterography demonstrates no areas of stricture formation with interval decompression.
The psychiatric service is consulted to evaluate the possibility of clozapine-induced paralytic ileus. During initial assessment, Ms. X denies any psychotic symptoms, including paranoid ideations, delusions, and auditory or visual hallucinations, and firmly believes that clozapine helps keep her stable. She also denies mood symptoms that could indicate mania or depression. She shows no signs or symptoms that suggest anticholinergic delirium.
The authors’ observations
Clozapine has proven efficacy in managing treatment-resistant schizophrenia,1-3 but the drug has been associated with life-threatening side effects, including agranulocytosis/neutropenia, myocarditis/cardiomyopathy, arrhythmia, seizures, diabetic ketoacidosis, fulminant hepatic failure, pulmonary embolism, and GI complications.4
Clozapine-induced GI side effects include anorexia, nausea, vomiting, heartburn, abdominal discomfort, diarrhea, and constipation. Clozapine-induced gastrointestinal hypomotility (CIGH) can lead to fecalith formation, which may result in intestinal obstruction/pseudo-obstruction, intestinal distension, necrosis, perforation, sepsis, aspiration from inhalation of feculent vomitus, or dysphagia.5 Constipation has been reported in 14% to 60% of patients who take clozapine,6 although other psychiatric medications also can cause constipation (Table 1). Severe constipation can lead to potentially fatal GI complications such as intestinal obstruction, necrosis, perforation, and sepsis, which is associated with significant morbidity due to bowel resection and a 27.5% mortality rate.5
The underlying mechanism of clozapine-induced constipation has been well established. The gut is innervated mainly by cholinergic and serotonergic receptors (5-HT3) and these receptors are responsible for peristalsis. Clozapine has a potent anticholinergic effect and acts as a strong antagonist of serotonin receptors (5-HT2, 5-HT3, 5-HT6, 5-HT7), which can lead to gut hypomotility.7 Risk factors associated with CIGH include:
- high dose of clozapine (mean dosage >428 mg/d)
- high serum clozapine levels (>500 ng/mL)
- coadministration of anticholinergic medications
- concomitant use of cytochrome P450 (CYP) enzyme inhibitors (medications inhibiting CYP1A2 enzyme)
- comorbid medical illnesses
- fever
- history of surgical bowel resection, GI pathology, and constipation.5
Table 1
Psychotropics associated with constipation
| Class | Medications |
|---|---|
| Atypical antipsychotics | Clozapine, risperidone |
| Typical antipsychotics | Chlorpromazine, haloperidol, pimozide, thioridazine, thiothixene, trifluoperazine |
| Anticholinergics | Benztropine, trihexyphenidyl |
| Antidepressants | Amitriptyline, clomipramine, doxepin, imipramine, nortriptyline, trimipramine |
HISTORY: Medical comorbidities
Ms. X’s medical history is significant for chronic constipation, hypertension, obstructive pulmonary disease, and hyperthyroidism. Her medications include trazodone, 25 mg/d; fluoxetine, 40 mg/d, for negative symptoms and insomnia; docusate sodium, 200 mg/d; polyethylene glycol, 17 g/d; and bisacodyl suppository, 10 mg as needed for constipation. On admission, her laboratory test results—including complete blood count, liver function tests, kidney function tests, thyroid function profile, and serum calcium levels—all were within normal range.
The authors’ observations
Because the prevalence and severity of clozapine-induced constipation seem to be dose-dependent,8 minimizing the dosage is a logical management strategy.9 The life-threatening nature of clozapine-induced GI complications may require rapid dose reduction, which could compromise a patient’s stability. There is a little evidence regarding systematic management of clozapine-induced GI complications (Table 2).
Table 2
Clinical pearls for treating clozapine-induced constipation
| Serum clozapine levels >500 to 700 ng/mL have been associated with increased incidence of severe GI complications |
| Serum clozapine levels can guide reduction of clozapine dosage because of its linear kinetics (ie, halving the clozapine dose will halve the serum clozapine level) |
| Clozapine dosages should be reduced by no more than 25 mg/d to a maximum of 100 mg/week |
TREATMENT: Clozapine reduction
We obtain a serum clozapine level, which is elevated at 553 ng/mL. We recommend gradual reducing Ms. X’s clozapine dosage by 50 mg every 3 to 4 days to reach a target dose of 300 to 350 mg/d, to attain serum clozapine levels 350 to 400 ng/mL. Because of trazodone’s potential anticholinergic action, which could be worsening Ms. X’s constipation, we stop the drug and begin zolpidem, 5 to 10 mg/d, to manage her insomnia. During these medication changes, we closely monitor Ms. X for reemerging psychotic symptoms.
The authors’ observations
In addition to risk factors such as chronic constipation and recent GI surgery, Ms. X’s supra-therapeutic serum clozapine level (553 ng/mL) significantly increased her risk of clozapine-induced paralytic ileus. Antidepressants such as selective serotonin reuptake inhibitors (SSRIs) are known to increase tissue concentrations of clozapine and its major metabolite, norclozapine, by primarily inhibiting CYP1A2 and perhaps CYP2D6.10 As a potent inhibitor of CYPA12, fluvoxamine can inhibit clozapine metabolism, resulting in higher plasma concentrations.11 In Ms. X’s case, fluoxetine could have increased serum clozapine levels because of its ability to inhibit clozapine metabolism via CYP2D6-mediated mechanisms.12
Although clozapine serum levels are not routinely measured, such testing may be indicated in patients who do not respond to or are unable to tolerate clozapine. Clozapine levels should be obtained 12 hours after the bedtime dose (trough levels), several days after clozapine initiation. Serum clozapine levels <350 ng/mL are associated with lack of clinical response.13 Higher serum levels (500 to 700 ng/mL) have been associated with greater incidences of serious GI complications. Serum clozapine levels also help guide clozapine dosage reduction because of its linear kinetics—halving the dose will halve the serum clozapine level.14
OUTCOME: GI symptoms improve
Ms. X shows improved GI motility within few days of the first decrease in her clozapine dosage. Nausea, vomiting, and abdominal distension gradually resolve over 2 weeks with concomitant reduction in clozapine dosage to 300 mg/d (50 mg in the morning and 250 mg at bedtime) without reemergence of psychotic symptoms. She is able to tolerate a soft diet, and conservative GI measures are no longer required. She is discharged home with outpatient surgical and psychiatric follow-up.
The authors’ observations
Successful reversal of severe clozapine-induced constipation—occurring at serum clozapine level of 490 ng/mL—has been reported in a 45-year-old man with treatment-resistant schizophrenia. This was accomplished by cautious reduction of clozapine dosage (400 mg/d to 250 mg/d) over 1 week.15 Slower clozapine titration—reducing the dose by no more than 25 mg/d to a maximum of 100 mg/week—has been recommended.16 It also has been suggested to replace part of the clozapine dose with a less antimuscarinic antipsychotic, such as quetiapine or haloperidol, thereby using the second antipsychotic as a clozapine-sparing agent.9 For example, the clozapine dose could be reduced by 25% by substituting 2 mg of quetiapine for every 1 mg of clozapine.
Prevention
Psychiatrists who prescribe clozapine should take a careful history of risk factors that might predispose patients to clozapine-induced GI side effects. Caution patients to whom you prescribe clozapine about possible development of constipation and the risk of serious GI complications. Enlist family members and caseworkers to keep a close eye on GI side effects in patients receiving clozapine. Advise patients to prevent constipation by eating a high fiber diet, drinking adequate fluids, and getting regular exercise. Patients should be treated aggressively with laxatives to relieve constipation and educated about the warning signs of intestinal obstruction, such as worsening constipation, abdominal pain, vomiting, and inability to pass flatus.17
Rapidly fatal bowel ischemia caused by clozapine has been reported.18 Therefore, urgently refer patients for medical evaluation if you have any concerns about worsening constipation or observe signs of intestinal obstruction. Vigilant consideration of clozapine as a likely culprit in severe GI complications in inpatient settings can prevent morbidity and mortality.
In our case, cautious reduction of clozapine dosage, guided by serum clozapine levels, had obviated the need for surgery and prevented reemergence of psychotic symptoms.
Related Resources
- Drew L, Herdson P. Clozapine and constipation: a serious issue. Aust N Z J Psychiatry. 1997;31(1):149-150.
- Winstead NS, Winstead DK. 5-step plan to treat constipation in psychiatric patients. Current Psychiatry. 2008;7(5):29-39.
Drug Brand Names
- Amitriptyline • Elavil
- Benztropine • Cogentin
- Bisacodyl suppository • Dulcolax, others
- Chlorpromazine • Thorazine
- Clomipramine • Anafranil
- Clozapine • Clozaril
- Docusate sodium • Colace, others
- Doxepin • Adapin, Sinequan
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Haloperidol • Haldol
- Imipramine • Tofranil
- Nortriptyline • Aventyl, Pamelor
- Pimozide • Orap
- Polyethylene glycol • MiraLax
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Thioridazine • Mellaril
- Thiothixene • Navane
- Trazodone • Desyrel, Oleptro
- Trifluoperazine • Stelazine
- Trihexyphenidyl • Artane, Trihexane
- Trimipramine • Surmontil
- Zolpidem • Ambien
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. American Psychiatric Association. Treatment of patients with schizophrenia, second edition. Recommendations for patients with schizophrenia. Arlington, VA: American Psychiatric Publishing, Inc; 2004.
2. Stahl S. Essential psychopharmacology. Cambridge, United Kingdom: Cambridge University Press; 1999.
3. Hardman J, Limbird L, Molinoff P. Goodman & Gilman’s pharmacological basis of therapeutics. New York, NY: McGraw-Hill; 1996.
4. Flanagan RJ, Ball RY. Gastrointestinal hypomotility: an under-recognized life-threatening adverse effect of clozapine. Forensic Sci Int. 2011;206(1-3):e31-36.
5. Palmer SE, McLean RM, Ellis PM, et al. Life-threatening clozapine-induced gastrointestinal hypomotility: an analysis of 102 cases. J Clin Psychiatry. 2008;69(5):759-768.
6. Claghorn J, Honigfeld G, Abuzzahab F, Sr, et al. The risks and benefits of clozapine versus chlorpromazine. J Clin Psychopharmacol. 1987;7:337-384.
7. Perrott J. Serious gastrointestinal adverse effects of clozapine. Psychopharmacology Newsletter. 2009;1-5.
8. Pare J, Riffand P, Baurdeix I. The clozapine in France. Information Psychiatric. 1993;4:389-397.
9. Levin TT, Barrett J, Mendelowitz A. Death from clozapine-induced constipation: case report and literature review. Psychosomatics. 2002;43:71-73.
10. Centorrino F, Baldessarini RJ, Frankenburg FR, et al. Serum levels of clozapine and norclozapine in patients treated with selective serotonin reuptake inhibitors. Am J Psychiatry. 1996;153(6):820-822.
11. Sproule BA, Naranjo CA, Brenmer KE, et al. Selective serotonin reuptake inhibitors and CNS drug interactions. A critical review of the evidence. Clin Pharmacokinet. 1997;33(6):454-471.
12. Urichuk L, Prior TI, Dursun S, et al. Metabolism of atypical antipsychotics: involvement of cytochrome p450 enzymes and relevance for drug-drug interactions. Curr Drug Metab. 2008;9(5):410-418.
13. Perry P, Miller DD, Arndt SV, et al. Clozapine and norclozapine plasma concentrations and clinical responses of treatment-refractory schizophrenic patients. Am J Psychiatry. 1991;148:231-235.
14. Freudenreich O. Clozapine drug levels guide dosing. Current Psychiatry. 2009;8(3):78.-
15. Pelizza L, De Luca P, La Pesa M, et al. Clozapine-induced intestinal occlusion: a serious side effect. Acta Biomed. 2007;78:144-148.
16. Hayes G, Gibler B. Clozapine-induced constipation. Am J Psychiatry. 1995;152:298.-
17. American College of Gastroenterology Chronic Constipation Task Force. An evidence-based approach to the management of chronic constipation in North America. Am J Gastroenterol. 2005;100(suppl 1):S1-4.
18. Townsend G, Curtis D. Case report: rapidly fatal bowel ischaemia on clozapine treatment. BMC Psychiatry. 2006;6:43.-
Discuss this article at www.facebook.com/CurrentPsychiatry
CASE: Gl surgery
Ms. X, age 61, presents to the emergency department (ED) complaining of nausea, vomiting, and abdominal pain and distension. CT scan of her abdomen reveals segmental ischemia in her colon with abscess formation, which leads to immediate surgery, including ileocecostomy with primary anastomosis. After surgery, Ms. X suffers from gastrointestinal (GI) dysmotility. The gastroenterology team recommends daily enemas along with a soft diet after she is discharged.
Ms. X has chronic paranoid schizophrenia, which has been treated successfully for 18 years with clozapine, 500 mg/d. During acute psychotic episodes, she experienced paranoid delusions and command auditory hallucinations telling her to kill herself. She had previous trials of several antipsychotics, including quetiapine, thiothixene, thioridazine, trifluoperazine, chlorpromazine, and haloperidol, all of which were ineffective and poorly tolerated because of serious side effects.
Within 1 month of discharge, Ms. X returns to the ED with nausea, vomiting, and abdominal distension. Abdominal CT scan suggests partial small bowel obstruction and significantly dilated loops of small bowel with decompressed rectum and sigmoid colon. Considering her recent GI surgery and absence of abdominal pain, she is managed with conservative measures, including nasogastric tube decompression and total parenteral nutrition. CT enterography demonstrates no areas of stricture formation with interval decompression.
The psychiatric service is consulted to evaluate the possibility of clozapine-induced paralytic ileus. During initial assessment, Ms. X denies any psychotic symptoms, including paranoid ideations, delusions, and auditory or visual hallucinations, and firmly believes that clozapine helps keep her stable. She also denies mood symptoms that could indicate mania or depression. She shows no signs or symptoms that suggest anticholinergic delirium.
The authors’ observations
Clozapine has proven efficacy in managing treatment-resistant schizophrenia,1-3 but the drug has been associated with life-threatening side effects, including agranulocytosis/neutropenia, myocarditis/cardiomyopathy, arrhythmia, seizures, diabetic ketoacidosis, fulminant hepatic failure, pulmonary embolism, and GI complications.4
Clozapine-induced GI side effects include anorexia, nausea, vomiting, heartburn, abdominal discomfort, diarrhea, and constipation. Clozapine-induced gastrointestinal hypomotility (CIGH) can lead to fecalith formation, which may result in intestinal obstruction/pseudo-obstruction, intestinal distension, necrosis, perforation, sepsis, aspiration from inhalation of feculent vomitus, or dysphagia.5 Constipation has been reported in 14% to 60% of patients who take clozapine,6 although other psychiatric medications also can cause constipation (Table 1). Severe constipation can lead to potentially fatal GI complications such as intestinal obstruction, necrosis, perforation, and sepsis, which is associated with significant morbidity due to bowel resection and a 27.5% mortality rate.5
The underlying mechanism of clozapine-induced constipation has been well established. The gut is innervated mainly by cholinergic and serotonergic receptors (5-HT3) and these receptors are responsible for peristalsis. Clozapine has a potent anticholinergic effect and acts as a strong antagonist of serotonin receptors (5-HT2, 5-HT3, 5-HT6, 5-HT7), which can lead to gut hypomotility.7 Risk factors associated with CIGH include:
- high dose of clozapine (mean dosage >428 mg/d)
- high serum clozapine levels (>500 ng/mL)
- coadministration of anticholinergic medications
- concomitant use of cytochrome P450 (CYP) enzyme inhibitors (medications inhibiting CYP1A2 enzyme)
- comorbid medical illnesses
- fever
- history of surgical bowel resection, GI pathology, and constipation.5
Table 1
Psychotropics associated with constipation
| Class | Medications |
|---|---|
| Atypical antipsychotics | Clozapine, risperidone |
| Typical antipsychotics | Chlorpromazine, haloperidol, pimozide, thioridazine, thiothixene, trifluoperazine |
| Anticholinergics | Benztropine, trihexyphenidyl |
| Antidepressants | Amitriptyline, clomipramine, doxepin, imipramine, nortriptyline, trimipramine |
HISTORY: Medical comorbidities
Ms. X’s medical history is significant for chronic constipation, hypertension, obstructive pulmonary disease, and hyperthyroidism. Her medications include trazodone, 25 mg/d; fluoxetine, 40 mg/d, for negative symptoms and insomnia; docusate sodium, 200 mg/d; polyethylene glycol, 17 g/d; and bisacodyl suppository, 10 mg as needed for constipation. On admission, her laboratory test results—including complete blood count, liver function tests, kidney function tests, thyroid function profile, and serum calcium levels—all were within normal range.
The authors’ observations
Because the prevalence and severity of clozapine-induced constipation seem to be dose-dependent,8 minimizing the dosage is a logical management strategy.9 The life-threatening nature of clozapine-induced GI complications may require rapid dose reduction, which could compromise a patient’s stability. There is a little evidence regarding systematic management of clozapine-induced GI complications (Table 2).
Table 2
Clinical pearls for treating clozapine-induced constipation
| Serum clozapine levels >500 to 700 ng/mL have been associated with increased incidence of severe GI complications |
| Serum clozapine levels can guide reduction of clozapine dosage because of its linear kinetics (ie, halving the clozapine dose will halve the serum clozapine level) |
| Clozapine dosages should be reduced by no more than 25 mg/d to a maximum of 100 mg/week |
TREATMENT: Clozapine reduction
We obtain a serum clozapine level, which is elevated at 553 ng/mL. We recommend gradual reducing Ms. X’s clozapine dosage by 50 mg every 3 to 4 days to reach a target dose of 300 to 350 mg/d, to attain serum clozapine levels 350 to 400 ng/mL. Because of trazodone’s potential anticholinergic action, which could be worsening Ms. X’s constipation, we stop the drug and begin zolpidem, 5 to 10 mg/d, to manage her insomnia. During these medication changes, we closely monitor Ms. X for reemerging psychotic symptoms.
The authors’ observations
In addition to risk factors such as chronic constipation and recent GI surgery, Ms. X’s supra-therapeutic serum clozapine level (553 ng/mL) significantly increased her risk of clozapine-induced paralytic ileus. Antidepressants such as selective serotonin reuptake inhibitors (SSRIs) are known to increase tissue concentrations of clozapine and its major metabolite, norclozapine, by primarily inhibiting CYP1A2 and perhaps CYP2D6.10 As a potent inhibitor of CYPA12, fluvoxamine can inhibit clozapine metabolism, resulting in higher plasma concentrations.11 In Ms. X’s case, fluoxetine could have increased serum clozapine levels because of its ability to inhibit clozapine metabolism via CYP2D6-mediated mechanisms.12
Although clozapine serum levels are not routinely measured, such testing may be indicated in patients who do not respond to or are unable to tolerate clozapine. Clozapine levels should be obtained 12 hours after the bedtime dose (trough levels), several days after clozapine initiation. Serum clozapine levels <350 ng/mL are associated with lack of clinical response.13 Higher serum levels (500 to 700 ng/mL) have been associated with greater incidences of serious GI complications. Serum clozapine levels also help guide clozapine dosage reduction because of its linear kinetics—halving the dose will halve the serum clozapine level.14
OUTCOME: GI symptoms improve
Ms. X shows improved GI motility within few days of the first decrease in her clozapine dosage. Nausea, vomiting, and abdominal distension gradually resolve over 2 weeks with concomitant reduction in clozapine dosage to 300 mg/d (50 mg in the morning and 250 mg at bedtime) without reemergence of psychotic symptoms. She is able to tolerate a soft diet, and conservative GI measures are no longer required. She is discharged home with outpatient surgical and psychiatric follow-up.
The authors’ observations
Successful reversal of severe clozapine-induced constipation—occurring at serum clozapine level of 490 ng/mL—has been reported in a 45-year-old man with treatment-resistant schizophrenia. This was accomplished by cautious reduction of clozapine dosage (400 mg/d to 250 mg/d) over 1 week.15 Slower clozapine titration—reducing the dose by no more than 25 mg/d to a maximum of 100 mg/week—has been recommended.16 It also has been suggested to replace part of the clozapine dose with a less antimuscarinic antipsychotic, such as quetiapine or haloperidol, thereby using the second antipsychotic as a clozapine-sparing agent.9 For example, the clozapine dose could be reduced by 25% by substituting 2 mg of quetiapine for every 1 mg of clozapine.
Prevention
Psychiatrists who prescribe clozapine should take a careful history of risk factors that might predispose patients to clozapine-induced GI side effects. Caution patients to whom you prescribe clozapine about possible development of constipation and the risk of serious GI complications. Enlist family members and caseworkers to keep a close eye on GI side effects in patients receiving clozapine. Advise patients to prevent constipation by eating a high fiber diet, drinking adequate fluids, and getting regular exercise. Patients should be treated aggressively with laxatives to relieve constipation and educated about the warning signs of intestinal obstruction, such as worsening constipation, abdominal pain, vomiting, and inability to pass flatus.17
Rapidly fatal bowel ischemia caused by clozapine has been reported.18 Therefore, urgently refer patients for medical evaluation if you have any concerns about worsening constipation or observe signs of intestinal obstruction. Vigilant consideration of clozapine as a likely culprit in severe GI complications in inpatient settings can prevent morbidity and mortality.
In our case, cautious reduction of clozapine dosage, guided by serum clozapine levels, had obviated the need for surgery and prevented reemergence of psychotic symptoms.
Related Resources
- Drew L, Herdson P. Clozapine and constipation: a serious issue. Aust N Z J Psychiatry. 1997;31(1):149-150.
- Winstead NS, Winstead DK. 5-step plan to treat constipation in psychiatric patients. Current Psychiatry. 2008;7(5):29-39.
Drug Brand Names
- Amitriptyline • Elavil
- Benztropine • Cogentin
- Bisacodyl suppository • Dulcolax, others
- Chlorpromazine • Thorazine
- Clomipramine • Anafranil
- Clozapine • Clozaril
- Docusate sodium • Colace, others
- Doxepin • Adapin, Sinequan
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Haloperidol • Haldol
- Imipramine • Tofranil
- Nortriptyline • Aventyl, Pamelor
- Pimozide • Orap
- Polyethylene glycol • MiraLax
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Thioridazine • Mellaril
- Thiothixene • Navane
- Trazodone • Desyrel, Oleptro
- Trifluoperazine • Stelazine
- Trihexyphenidyl • Artane, Trihexane
- Trimipramine • Surmontil
- Zolpidem • Ambien
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
CASE: Gl surgery
Ms. X, age 61, presents to the emergency department (ED) complaining of nausea, vomiting, and abdominal pain and distension. CT scan of her abdomen reveals segmental ischemia in her colon with abscess formation, which leads to immediate surgery, including ileocecostomy with primary anastomosis. After surgery, Ms. X suffers from gastrointestinal (GI) dysmotility. The gastroenterology team recommends daily enemas along with a soft diet after she is discharged.
Ms. X has chronic paranoid schizophrenia, which has been treated successfully for 18 years with clozapine, 500 mg/d. During acute psychotic episodes, she experienced paranoid delusions and command auditory hallucinations telling her to kill herself. She had previous trials of several antipsychotics, including quetiapine, thiothixene, thioridazine, trifluoperazine, chlorpromazine, and haloperidol, all of which were ineffective and poorly tolerated because of serious side effects.
Within 1 month of discharge, Ms. X returns to the ED with nausea, vomiting, and abdominal distension. Abdominal CT scan suggests partial small bowel obstruction and significantly dilated loops of small bowel with decompressed rectum and sigmoid colon. Considering her recent GI surgery and absence of abdominal pain, she is managed with conservative measures, including nasogastric tube decompression and total parenteral nutrition. CT enterography demonstrates no areas of stricture formation with interval decompression.
The psychiatric service is consulted to evaluate the possibility of clozapine-induced paralytic ileus. During initial assessment, Ms. X denies any psychotic symptoms, including paranoid ideations, delusions, and auditory or visual hallucinations, and firmly believes that clozapine helps keep her stable. She also denies mood symptoms that could indicate mania or depression. She shows no signs or symptoms that suggest anticholinergic delirium.
The authors’ observations
Clozapine has proven efficacy in managing treatment-resistant schizophrenia,1-3 but the drug has been associated with life-threatening side effects, including agranulocytosis/neutropenia, myocarditis/cardiomyopathy, arrhythmia, seizures, diabetic ketoacidosis, fulminant hepatic failure, pulmonary embolism, and GI complications.4
Clozapine-induced GI side effects include anorexia, nausea, vomiting, heartburn, abdominal discomfort, diarrhea, and constipation. Clozapine-induced gastrointestinal hypomotility (CIGH) can lead to fecalith formation, which may result in intestinal obstruction/pseudo-obstruction, intestinal distension, necrosis, perforation, sepsis, aspiration from inhalation of feculent vomitus, or dysphagia.5 Constipation has been reported in 14% to 60% of patients who take clozapine,6 although other psychiatric medications also can cause constipation (Table 1). Severe constipation can lead to potentially fatal GI complications such as intestinal obstruction, necrosis, perforation, and sepsis, which is associated with significant morbidity due to bowel resection and a 27.5% mortality rate.5
The underlying mechanism of clozapine-induced constipation has been well established. The gut is innervated mainly by cholinergic and serotonergic receptors (5-HT3) and these receptors are responsible for peristalsis. Clozapine has a potent anticholinergic effect and acts as a strong antagonist of serotonin receptors (5-HT2, 5-HT3, 5-HT6, 5-HT7), which can lead to gut hypomotility.7 Risk factors associated with CIGH include:
- high dose of clozapine (mean dosage >428 mg/d)
- high serum clozapine levels (>500 ng/mL)
- coadministration of anticholinergic medications
- concomitant use of cytochrome P450 (CYP) enzyme inhibitors (medications inhibiting CYP1A2 enzyme)
- comorbid medical illnesses
- fever
- history of surgical bowel resection, GI pathology, and constipation.5
Table 1
Psychotropics associated with constipation
| Class | Medications |
|---|---|
| Atypical antipsychotics | Clozapine, risperidone |
| Typical antipsychotics | Chlorpromazine, haloperidol, pimozide, thioridazine, thiothixene, trifluoperazine |
| Anticholinergics | Benztropine, trihexyphenidyl |
| Antidepressants | Amitriptyline, clomipramine, doxepin, imipramine, nortriptyline, trimipramine |
HISTORY: Medical comorbidities
Ms. X’s medical history is significant for chronic constipation, hypertension, obstructive pulmonary disease, and hyperthyroidism. Her medications include trazodone, 25 mg/d; fluoxetine, 40 mg/d, for negative symptoms and insomnia; docusate sodium, 200 mg/d; polyethylene glycol, 17 g/d; and bisacodyl suppository, 10 mg as needed for constipation. On admission, her laboratory test results—including complete blood count, liver function tests, kidney function tests, thyroid function profile, and serum calcium levels—all were within normal range.
The authors’ observations
Because the prevalence and severity of clozapine-induced constipation seem to be dose-dependent,8 minimizing the dosage is a logical management strategy.9 The life-threatening nature of clozapine-induced GI complications may require rapid dose reduction, which could compromise a patient’s stability. There is a little evidence regarding systematic management of clozapine-induced GI complications (Table 2).
Table 2
Clinical pearls for treating clozapine-induced constipation
| Serum clozapine levels >500 to 700 ng/mL have been associated with increased incidence of severe GI complications |
| Serum clozapine levels can guide reduction of clozapine dosage because of its linear kinetics (ie, halving the clozapine dose will halve the serum clozapine level) |
| Clozapine dosages should be reduced by no more than 25 mg/d to a maximum of 100 mg/week |
TREATMENT: Clozapine reduction
We obtain a serum clozapine level, which is elevated at 553 ng/mL. We recommend gradual reducing Ms. X’s clozapine dosage by 50 mg every 3 to 4 days to reach a target dose of 300 to 350 mg/d, to attain serum clozapine levels 350 to 400 ng/mL. Because of trazodone’s potential anticholinergic action, which could be worsening Ms. X’s constipation, we stop the drug and begin zolpidem, 5 to 10 mg/d, to manage her insomnia. During these medication changes, we closely monitor Ms. X for reemerging psychotic symptoms.
The authors’ observations
In addition to risk factors such as chronic constipation and recent GI surgery, Ms. X’s supra-therapeutic serum clozapine level (553 ng/mL) significantly increased her risk of clozapine-induced paralytic ileus. Antidepressants such as selective serotonin reuptake inhibitors (SSRIs) are known to increase tissue concentrations of clozapine and its major metabolite, norclozapine, by primarily inhibiting CYP1A2 and perhaps CYP2D6.10 As a potent inhibitor of CYPA12, fluvoxamine can inhibit clozapine metabolism, resulting in higher plasma concentrations.11 In Ms. X’s case, fluoxetine could have increased serum clozapine levels because of its ability to inhibit clozapine metabolism via CYP2D6-mediated mechanisms.12
Although clozapine serum levels are not routinely measured, such testing may be indicated in patients who do not respond to or are unable to tolerate clozapine. Clozapine levels should be obtained 12 hours after the bedtime dose (trough levels), several days after clozapine initiation. Serum clozapine levels <350 ng/mL are associated with lack of clinical response.13 Higher serum levels (500 to 700 ng/mL) have been associated with greater incidences of serious GI complications. Serum clozapine levels also help guide clozapine dosage reduction because of its linear kinetics—halving the dose will halve the serum clozapine level.14
OUTCOME: GI symptoms improve
Ms. X shows improved GI motility within few days of the first decrease in her clozapine dosage. Nausea, vomiting, and abdominal distension gradually resolve over 2 weeks with concomitant reduction in clozapine dosage to 300 mg/d (50 mg in the morning and 250 mg at bedtime) without reemergence of psychotic symptoms. She is able to tolerate a soft diet, and conservative GI measures are no longer required. She is discharged home with outpatient surgical and psychiatric follow-up.
The authors’ observations
Successful reversal of severe clozapine-induced constipation—occurring at serum clozapine level of 490 ng/mL—has been reported in a 45-year-old man with treatment-resistant schizophrenia. This was accomplished by cautious reduction of clozapine dosage (400 mg/d to 250 mg/d) over 1 week.15 Slower clozapine titration—reducing the dose by no more than 25 mg/d to a maximum of 100 mg/week—has been recommended.16 It also has been suggested to replace part of the clozapine dose with a less antimuscarinic antipsychotic, such as quetiapine or haloperidol, thereby using the second antipsychotic as a clozapine-sparing agent.9 For example, the clozapine dose could be reduced by 25% by substituting 2 mg of quetiapine for every 1 mg of clozapine.
Prevention
Psychiatrists who prescribe clozapine should take a careful history of risk factors that might predispose patients to clozapine-induced GI side effects. Caution patients to whom you prescribe clozapine about possible development of constipation and the risk of serious GI complications. Enlist family members and caseworkers to keep a close eye on GI side effects in patients receiving clozapine. Advise patients to prevent constipation by eating a high fiber diet, drinking adequate fluids, and getting regular exercise. Patients should be treated aggressively with laxatives to relieve constipation and educated about the warning signs of intestinal obstruction, such as worsening constipation, abdominal pain, vomiting, and inability to pass flatus.17
Rapidly fatal bowel ischemia caused by clozapine has been reported.18 Therefore, urgently refer patients for medical evaluation if you have any concerns about worsening constipation or observe signs of intestinal obstruction. Vigilant consideration of clozapine as a likely culprit in severe GI complications in inpatient settings can prevent morbidity and mortality.
In our case, cautious reduction of clozapine dosage, guided by serum clozapine levels, had obviated the need for surgery and prevented reemergence of psychotic symptoms.
Related Resources
- Drew L, Herdson P. Clozapine and constipation: a serious issue. Aust N Z J Psychiatry. 1997;31(1):149-150.
- Winstead NS, Winstead DK. 5-step plan to treat constipation in psychiatric patients. Current Psychiatry. 2008;7(5):29-39.
Drug Brand Names
- Amitriptyline • Elavil
- Benztropine • Cogentin
- Bisacodyl suppository • Dulcolax, others
- Chlorpromazine • Thorazine
- Clomipramine • Anafranil
- Clozapine • Clozaril
- Docusate sodium • Colace, others
- Doxepin • Adapin, Sinequan
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Haloperidol • Haldol
- Imipramine • Tofranil
- Nortriptyline • Aventyl, Pamelor
- Pimozide • Orap
- Polyethylene glycol • MiraLax
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Thioridazine • Mellaril
- Thiothixene • Navane
- Trazodone • Desyrel, Oleptro
- Trifluoperazine • Stelazine
- Trihexyphenidyl • Artane, Trihexane
- Trimipramine • Surmontil
- Zolpidem • Ambien
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. American Psychiatric Association. Treatment of patients with schizophrenia, second edition. Recommendations for patients with schizophrenia. Arlington, VA: American Psychiatric Publishing, Inc; 2004.
2. Stahl S. Essential psychopharmacology. Cambridge, United Kingdom: Cambridge University Press; 1999.
3. Hardman J, Limbird L, Molinoff P. Goodman & Gilman’s pharmacological basis of therapeutics. New York, NY: McGraw-Hill; 1996.
4. Flanagan RJ, Ball RY. Gastrointestinal hypomotility: an under-recognized life-threatening adverse effect of clozapine. Forensic Sci Int. 2011;206(1-3):e31-36.
5. Palmer SE, McLean RM, Ellis PM, et al. Life-threatening clozapine-induced gastrointestinal hypomotility: an analysis of 102 cases. J Clin Psychiatry. 2008;69(5):759-768.
6. Claghorn J, Honigfeld G, Abuzzahab F, Sr, et al. The risks and benefits of clozapine versus chlorpromazine. J Clin Psychopharmacol. 1987;7:337-384.
7. Perrott J. Serious gastrointestinal adverse effects of clozapine. Psychopharmacology Newsletter. 2009;1-5.
8. Pare J, Riffand P, Baurdeix I. The clozapine in France. Information Psychiatric. 1993;4:389-397.
9. Levin TT, Barrett J, Mendelowitz A. Death from clozapine-induced constipation: case report and literature review. Psychosomatics. 2002;43:71-73.
10. Centorrino F, Baldessarini RJ, Frankenburg FR, et al. Serum levels of clozapine and norclozapine in patients treated with selective serotonin reuptake inhibitors. Am J Psychiatry. 1996;153(6):820-822.
11. Sproule BA, Naranjo CA, Brenmer KE, et al. Selective serotonin reuptake inhibitors and CNS drug interactions. A critical review of the evidence. Clin Pharmacokinet. 1997;33(6):454-471.
12. Urichuk L, Prior TI, Dursun S, et al. Metabolism of atypical antipsychotics: involvement of cytochrome p450 enzymes and relevance for drug-drug interactions. Curr Drug Metab. 2008;9(5):410-418.
13. Perry P, Miller DD, Arndt SV, et al. Clozapine and norclozapine plasma concentrations and clinical responses of treatment-refractory schizophrenic patients. Am J Psychiatry. 1991;148:231-235.
14. Freudenreich O. Clozapine drug levels guide dosing. Current Psychiatry. 2009;8(3):78.-
15. Pelizza L, De Luca P, La Pesa M, et al. Clozapine-induced intestinal occlusion: a serious side effect. Acta Biomed. 2007;78:144-148.
16. Hayes G, Gibler B. Clozapine-induced constipation. Am J Psychiatry. 1995;152:298.-
17. American College of Gastroenterology Chronic Constipation Task Force. An evidence-based approach to the management of chronic constipation in North America. Am J Gastroenterol. 2005;100(suppl 1):S1-4.
18. Townsend G, Curtis D. Case report: rapidly fatal bowel ischaemia on clozapine treatment. BMC Psychiatry. 2006;6:43.-
1. American Psychiatric Association. Treatment of patients with schizophrenia, second edition. Recommendations for patients with schizophrenia. Arlington, VA: American Psychiatric Publishing, Inc; 2004.
2. Stahl S. Essential psychopharmacology. Cambridge, United Kingdom: Cambridge University Press; 1999.
3. Hardman J, Limbird L, Molinoff P. Goodman & Gilman’s pharmacological basis of therapeutics. New York, NY: McGraw-Hill; 1996.
4. Flanagan RJ, Ball RY. Gastrointestinal hypomotility: an under-recognized life-threatening adverse effect of clozapine. Forensic Sci Int. 2011;206(1-3):e31-36.
5. Palmer SE, McLean RM, Ellis PM, et al. Life-threatening clozapine-induced gastrointestinal hypomotility: an analysis of 102 cases. J Clin Psychiatry. 2008;69(5):759-768.
6. Claghorn J, Honigfeld G, Abuzzahab F, Sr, et al. The risks and benefits of clozapine versus chlorpromazine. J Clin Psychopharmacol. 1987;7:337-384.
7. Perrott J. Serious gastrointestinal adverse effects of clozapine. Psychopharmacology Newsletter. 2009;1-5.
8. Pare J, Riffand P, Baurdeix I. The clozapine in France. Information Psychiatric. 1993;4:389-397.
9. Levin TT, Barrett J, Mendelowitz A. Death from clozapine-induced constipation: case report and literature review. Psychosomatics. 2002;43:71-73.
10. Centorrino F, Baldessarini RJ, Frankenburg FR, et al. Serum levels of clozapine and norclozapine in patients treated with selective serotonin reuptake inhibitors. Am J Psychiatry. 1996;153(6):820-822.
11. Sproule BA, Naranjo CA, Brenmer KE, et al. Selective serotonin reuptake inhibitors and CNS drug interactions. A critical review of the evidence. Clin Pharmacokinet. 1997;33(6):454-471.
12. Urichuk L, Prior TI, Dursun S, et al. Metabolism of atypical antipsychotics: involvement of cytochrome p450 enzymes and relevance for drug-drug interactions. Curr Drug Metab. 2008;9(5):410-418.
13. Perry P, Miller DD, Arndt SV, et al. Clozapine and norclozapine plasma concentrations and clinical responses of treatment-refractory schizophrenic patients. Am J Psychiatry. 1991;148:231-235.
14. Freudenreich O. Clozapine drug levels guide dosing. Current Psychiatry. 2009;8(3):78.-
15. Pelizza L, De Luca P, La Pesa M, et al. Clozapine-induced intestinal occlusion: a serious side effect. Acta Biomed. 2007;78:144-148.
16. Hayes G, Gibler B. Clozapine-induced constipation. Am J Psychiatry. 1995;152:298.-
17. American College of Gastroenterology Chronic Constipation Task Force. An evidence-based approach to the management of chronic constipation in North America. Am J Gastroenterol. 2005;100(suppl 1):S1-4.
18. Townsend G, Curtis D. Case report: rapidly fatal bowel ischaemia on clozapine treatment. BMC Psychiatry. 2006;6:43.-
Treating delirium
Discuss this article at www.facebook.com/CurrentPsychiatry
Regarding “Atypical antipsychotics for delirium: A reasonable alternative to haloperidol?” (Current Psychiatry, January 2011, p. 37-46): Delirium usually is an acute encephalopathy with cerebral dysfunction caused by varying pathologies, most of which are extracranial. Examples include mental confusion induced by hypoxia or hypoglycemia. Primary treatment of delirium must be aimed at the specific cause.
Antipsychotic drugs are only a symptomatic intervention for delirium. They can and do provide behavioral control; however, these medications may worsen cases of alcohol or sedative withdrawal, ictal-related problems, neuroleptic malignant syndrome, etc. An antipsychotic may complicate other conditions, thus creating additional clinical difficulties for some patients. Recommending antipsychotics as a treatment focuses on symptomatic aspects; however, the critical mandate is to diagnose and specifically manage the etiology. Once the cause is corrected, the delirium usually resolves.
While treating the primary pathology, if behavioral issues still urgently require immediate control, a benzodiazepine is safer than an antipsychotic. Both medications provide symptomatic control, but a benzodiazepine is less likely to add new clinical problems. The only major precaution with a benzodiazepine is to avoid overprescribing. It is simply safer to rely on benzodiazepines for short-term behavioral management.
Steven Lippmann, MD
Professor of Psychiatry
University of Louisville School of Medicine
Louisville, KY
Discuss this article at www.facebook.com/CurrentPsychiatry
Regarding “Atypical antipsychotics for delirium: A reasonable alternative to haloperidol?” (Current Psychiatry, January 2011, p. 37-46): Delirium usually is an acute encephalopathy with cerebral dysfunction caused by varying pathologies, most of which are extracranial. Examples include mental confusion induced by hypoxia or hypoglycemia. Primary treatment of delirium must be aimed at the specific cause.
Antipsychotic drugs are only a symptomatic intervention for delirium. They can and do provide behavioral control; however, these medications may worsen cases of alcohol or sedative withdrawal, ictal-related problems, neuroleptic malignant syndrome, etc. An antipsychotic may complicate other conditions, thus creating additional clinical difficulties for some patients. Recommending antipsychotics as a treatment focuses on symptomatic aspects; however, the critical mandate is to diagnose and specifically manage the etiology. Once the cause is corrected, the delirium usually resolves.
While treating the primary pathology, if behavioral issues still urgently require immediate control, a benzodiazepine is safer than an antipsychotic. Both medications provide symptomatic control, but a benzodiazepine is less likely to add new clinical problems. The only major precaution with a benzodiazepine is to avoid overprescribing. It is simply safer to rely on benzodiazepines for short-term behavioral management.
Steven Lippmann, MD
Professor of Psychiatry
University of Louisville School of Medicine
Louisville, KY
Discuss this article at www.facebook.com/CurrentPsychiatry
Regarding “Atypical antipsychotics for delirium: A reasonable alternative to haloperidol?” (Current Psychiatry, January 2011, p. 37-46): Delirium usually is an acute encephalopathy with cerebral dysfunction caused by varying pathologies, most of which are extracranial. Examples include mental confusion induced by hypoxia or hypoglycemia. Primary treatment of delirium must be aimed at the specific cause.
Antipsychotic drugs are only a symptomatic intervention for delirium. They can and do provide behavioral control; however, these medications may worsen cases of alcohol or sedative withdrawal, ictal-related problems, neuroleptic malignant syndrome, etc. An antipsychotic may complicate other conditions, thus creating additional clinical difficulties for some patients. Recommending antipsychotics as a treatment focuses on symptomatic aspects; however, the critical mandate is to diagnose and specifically manage the etiology. Once the cause is corrected, the delirium usually resolves.
While treating the primary pathology, if behavioral issues still urgently require immediate control, a benzodiazepine is safer than an antipsychotic. Both medications provide symptomatic control, but a benzodiazepine is less likely to add new clinical problems. The only major precaution with a benzodiazepine is to avoid overprescribing. It is simply safer to rely on benzodiazepines for short-term behavioral management.
Steven Lippmann, MD
Professor of Psychiatry
University of Louisville School of Medicine
Louisville, KY
Glutamatergic dysfunction
Discuss this article at www.facebook.com/CurrentPsychiatry
I read with interest the article by Drs. Kantrowitz and Javitt (“Glutamate: New hope for schizophrenia treatment,” Current Psychiatry, April 2011, p. 68-74) describing the glutamatergic model of schizophrenia. There is extensive evidence that in addition to neurocognitive deficits, schizophrenia is characterized by various neuromuscular abnormalities, including skeletal muscle fiber changes, alterations of alpha-motor neuron excitability, increased motor unit fiber densities, increased branching of terminal motor nerves, and elevated levels of muscular enzymes.1,2 These neuromuscular abnormalities also are found in healthy first-degree relatives of patients with schizophrenia.3 Although the precise cause of these neuromuscular abnormalities has not been elucidated, one possible explanation is that they may be the result of neuronal injury mediated by excitatory amino acids.1 For example, Stevens4 suggested that abnormal sprouting and reinnervation of neurons in schizophrenia might be caused by such injury.
Amyotrophic lateral sclerosis (ALS) is a progressive degenerative syndrome involving upper and lower alpha-motor neuron systems. A substantial body of evidence supports the hypothesis that glutamate-mediated excito-toxicity is responsible for the death of motor neurons in ALS.5 Evidence suggests that having schizophrenia may be associated with an increased risk of developing ALS, and this risk might be explained by the toxic effects of excitatory amino acids on neuronal function.1,6 Recently, Stommel et al7 hypothesized that treating schizophrenia could protect against development of ALS, which is of interest because antipsychotics may have direct and indirect effects on modulating glutamate receptor systems.8
Robert H. Howland, MD
Associate Professor of Psychiatry
University of Pittsburgh School of Medicine
Western Psychiatric Institute and Clinic
Pittsburgh, PA
1. Howland RH. Excitatory amino acids schizophrenia, and amyotrophic lateral sclerosis. Integrative Psychiatry. 1993;9(2):72-76.
2. Flyckt L, Borg J, Borg K, et al. Muscle biopsy, macro EMG, and clinical characteristics in patients with schizophrenia. Biol Psychiatry. 2000;47:991-999.
3. Flyckt L, Wiesel FA, Borg J, et al. Neuromuscular and psychomotor abnormalities in patients with schizophrenia and their first-degree relatives. J Psychiatr Res. 2000;34:355-364.
4. Stevens JR. Abnormal reinnervation as a basis for schizophrenia: a hypothesis. Arch Gen Psychiatry. 1992;49:238-243.
5. Neale JH, Olszewski RT, Gehl LM, et al. The neurotransmitter N-acetylaspartyl-glutamate in models of pain, ALS, diabetic neuropathy, CNS injury and schizophrenia. Trends Pharmacol Sci. 2005;26:477-484.
6. Howland RH. Schizophrenia and amyotrophic lateral sclerosis. Compr Psychiatry. 1990;31(4):327-336.
7. Stommel EW, Graber D, Montanye J, et al. Does treating schizophrenia reduce the chances of developing amyotrophic lateral sclerosis? Med Hypoth. 2007;69:1021-1028.
8. Homayoun H, Moghaddam B. Orbitofrontal cortex neurons as a common target for classic and glutamatergic antipsychotic drugs. PNAS. 2008;105(46):18041-18046.
Discuss this article at www.facebook.com/CurrentPsychiatry
I read with interest the article by Drs. Kantrowitz and Javitt (“Glutamate: New hope for schizophrenia treatment,” Current Psychiatry, April 2011, p. 68-74) describing the glutamatergic model of schizophrenia. There is extensive evidence that in addition to neurocognitive deficits, schizophrenia is characterized by various neuromuscular abnormalities, including skeletal muscle fiber changes, alterations of alpha-motor neuron excitability, increased motor unit fiber densities, increased branching of terminal motor nerves, and elevated levels of muscular enzymes.1,2 These neuromuscular abnormalities also are found in healthy first-degree relatives of patients with schizophrenia.3 Although the precise cause of these neuromuscular abnormalities has not been elucidated, one possible explanation is that they may be the result of neuronal injury mediated by excitatory amino acids.1 For example, Stevens4 suggested that abnormal sprouting and reinnervation of neurons in schizophrenia might be caused by such injury.
Amyotrophic lateral sclerosis (ALS) is a progressive degenerative syndrome involving upper and lower alpha-motor neuron systems. A substantial body of evidence supports the hypothesis that glutamate-mediated excito-toxicity is responsible for the death of motor neurons in ALS.5 Evidence suggests that having schizophrenia may be associated with an increased risk of developing ALS, and this risk might be explained by the toxic effects of excitatory amino acids on neuronal function.1,6 Recently, Stommel et al7 hypothesized that treating schizophrenia could protect against development of ALS, which is of interest because antipsychotics may have direct and indirect effects on modulating glutamate receptor systems.8
Robert H. Howland, MD
Associate Professor of Psychiatry
University of Pittsburgh School of Medicine
Western Psychiatric Institute and Clinic
Pittsburgh, PA
Discuss this article at www.facebook.com/CurrentPsychiatry
I read with interest the article by Drs. Kantrowitz and Javitt (“Glutamate: New hope for schizophrenia treatment,” Current Psychiatry, April 2011, p. 68-74) describing the glutamatergic model of schizophrenia. There is extensive evidence that in addition to neurocognitive deficits, schizophrenia is characterized by various neuromuscular abnormalities, including skeletal muscle fiber changes, alterations of alpha-motor neuron excitability, increased motor unit fiber densities, increased branching of terminal motor nerves, and elevated levels of muscular enzymes.1,2 These neuromuscular abnormalities also are found in healthy first-degree relatives of patients with schizophrenia.3 Although the precise cause of these neuromuscular abnormalities has not been elucidated, one possible explanation is that they may be the result of neuronal injury mediated by excitatory amino acids.1 For example, Stevens4 suggested that abnormal sprouting and reinnervation of neurons in schizophrenia might be caused by such injury.
Amyotrophic lateral sclerosis (ALS) is a progressive degenerative syndrome involving upper and lower alpha-motor neuron systems. A substantial body of evidence supports the hypothesis that glutamate-mediated excito-toxicity is responsible for the death of motor neurons in ALS.5 Evidence suggests that having schizophrenia may be associated with an increased risk of developing ALS, and this risk might be explained by the toxic effects of excitatory amino acids on neuronal function.1,6 Recently, Stommel et al7 hypothesized that treating schizophrenia could protect against development of ALS, which is of interest because antipsychotics may have direct and indirect effects on modulating glutamate receptor systems.8
Robert H. Howland, MD
Associate Professor of Psychiatry
University of Pittsburgh School of Medicine
Western Psychiatric Institute and Clinic
Pittsburgh, PA
1. Howland RH. Excitatory amino acids schizophrenia, and amyotrophic lateral sclerosis. Integrative Psychiatry. 1993;9(2):72-76.
2. Flyckt L, Borg J, Borg K, et al. Muscle biopsy, macro EMG, and clinical characteristics in patients with schizophrenia. Biol Psychiatry. 2000;47:991-999.
3. Flyckt L, Wiesel FA, Borg J, et al. Neuromuscular and psychomotor abnormalities in patients with schizophrenia and their first-degree relatives. J Psychiatr Res. 2000;34:355-364.
4. Stevens JR. Abnormal reinnervation as a basis for schizophrenia: a hypothesis. Arch Gen Psychiatry. 1992;49:238-243.
5. Neale JH, Olszewski RT, Gehl LM, et al. The neurotransmitter N-acetylaspartyl-glutamate in models of pain, ALS, diabetic neuropathy, CNS injury and schizophrenia. Trends Pharmacol Sci. 2005;26:477-484.
6. Howland RH. Schizophrenia and amyotrophic lateral sclerosis. Compr Psychiatry. 1990;31(4):327-336.
7. Stommel EW, Graber D, Montanye J, et al. Does treating schizophrenia reduce the chances of developing amyotrophic lateral sclerosis? Med Hypoth. 2007;69:1021-1028.
8. Homayoun H, Moghaddam B. Orbitofrontal cortex neurons as a common target for classic and glutamatergic antipsychotic drugs. PNAS. 2008;105(46):18041-18046.
1. Howland RH. Excitatory amino acids schizophrenia, and amyotrophic lateral sclerosis. Integrative Psychiatry. 1993;9(2):72-76.
2. Flyckt L, Borg J, Borg K, et al. Muscle biopsy, macro EMG, and clinical characteristics in patients with schizophrenia. Biol Psychiatry. 2000;47:991-999.
3. Flyckt L, Wiesel FA, Borg J, et al. Neuromuscular and psychomotor abnormalities in patients with schizophrenia and their first-degree relatives. J Psychiatr Res. 2000;34:355-364.
4. Stevens JR. Abnormal reinnervation as a basis for schizophrenia: a hypothesis. Arch Gen Psychiatry. 1992;49:238-243.
5. Neale JH, Olszewski RT, Gehl LM, et al. The neurotransmitter N-acetylaspartyl-glutamate in models of pain, ALS, diabetic neuropathy, CNS injury and schizophrenia. Trends Pharmacol Sci. 2005;26:477-484.
6. Howland RH. Schizophrenia and amyotrophic lateral sclerosis. Compr Psychiatry. 1990;31(4):327-336.
7. Stommel EW, Graber D, Montanye J, et al. Does treating schizophrenia reduce the chances of developing amyotrophic lateral sclerosis? Med Hypoth. 2007;69:1021-1028.
8. Homayoun H, Moghaddam B. Orbitofrontal cortex neurons as a common target for classic and glutamatergic antipsychotic drugs. PNAS. 2008;105(46):18041-18046.
Efficient pharmacotherapy
Discuss this article at www.facebook.com/CurrentPsychiatry
I agree with Dr. Nasrallah’s assertions that a single psychotropic can treat different disorders (“Parsimonious pharmacotherapy,” From the Editor, Current Psychiatry, May 2011, p. 12-16). During my consultation-liaison fellowship at MD Anderson Cancer Center, different forms of delirium were a daily occurrence in our hospitalized patients. A low dose of psychotropic—usually quetiapine—proved effective in controlling delirium; in acute and severe cases, low-dose IV haloperidol helped control agitation and psychosis.
Low-dose quetiapine also was beneficial in treating comorbidities such as increased anxiety, depression, pain, and insomnia, with minimal side effects. Because most of our patients suffered from several medical conditions and powerful, multiple-medication chemotherapies, in most cases psychotropic polypharmacy was contraindicated and avoided at all costs.
Corey Roman, MD
Psychiatrist, private practice
Miami, FL
Discuss this article at www.facebook.com/CurrentPsychiatry
I agree with Dr. Nasrallah’s assertions that a single psychotropic can treat different disorders (“Parsimonious pharmacotherapy,” From the Editor, Current Psychiatry, May 2011, p. 12-16). During my consultation-liaison fellowship at MD Anderson Cancer Center, different forms of delirium were a daily occurrence in our hospitalized patients. A low dose of psychotropic—usually quetiapine—proved effective in controlling delirium; in acute and severe cases, low-dose IV haloperidol helped control agitation and psychosis.
Low-dose quetiapine also was beneficial in treating comorbidities such as increased anxiety, depression, pain, and insomnia, with minimal side effects. Because most of our patients suffered from several medical conditions and powerful, multiple-medication chemotherapies, in most cases psychotropic polypharmacy was contraindicated and avoided at all costs.
Corey Roman, MD
Psychiatrist, private practice
Miami, FL
Discuss this article at www.facebook.com/CurrentPsychiatry
I agree with Dr. Nasrallah’s assertions that a single psychotropic can treat different disorders (“Parsimonious pharmacotherapy,” From the Editor, Current Psychiatry, May 2011, p. 12-16). During my consultation-liaison fellowship at MD Anderson Cancer Center, different forms of delirium were a daily occurrence in our hospitalized patients. A low dose of psychotropic—usually quetiapine—proved effective in controlling delirium; in acute and severe cases, low-dose IV haloperidol helped control agitation and psychosis.
Low-dose quetiapine also was beneficial in treating comorbidities such as increased anxiety, depression, pain, and insomnia, with minimal side effects. Because most of our patients suffered from several medical conditions and powerful, multiple-medication chemotherapies, in most cases psychotropic polypharmacy was contraindicated and avoided at all costs.
Corey Roman, MD
Psychiatrist, private practice
Miami, FL
Differentiating NLD
“Does your patient have a psychiatric illness or nonverbal learning disorder?” (Current Psychiatry, May 2011, p. 17-35) reviews the differential diagnosis of “nonverbal learning disorder.”
Nonverbal learning disorder (NLD) has yet to be established as a distinct, valid neurodevelopmental syndrome. Many of the proffered diagnostic criteria, clinical presentations, and laboratory findings (especially results from psychological/neuropsychological and psychoeducational testing) overlap considerably with other neurodevelopmental syndromes characterized by social inadequacy and peculiarity, including Asperger’s disorder.
Therefore, rather than representing a discrete diagnostic category with overlapping features as suggested by this article, this putative syndrome probably is best conceptualized as falling within the milder end of a neurodevelopmental disorder spectrum characterized by deficits in social competence, judgment, and perception, accompanied by impaired daily functioning referable to these difficulties. The DSM-5 workgroup appears to recognize this problem of “splitting” vs “lumping.” Reports indicate that Asperger’s disorder may no longer be considered a “standalone” clinical syndrome and likely will be reconceptualized as a mild form of autism.
Many individuals have cognitive/neuropsychological and academic/learning difficulties compatible with nonverbal learning problems and do not exhibit the difficulties with social interactions the article cites. One way to advocate for NLD as a distinct clinical syndrome is to limit the diagnosis to a certain constellation of cognitive/neuropsychological and academic impairments that lead to educational, interpersonal, and/or vocational difficulties. Patients who also display clinically significant neurodevelopmentally based deficits in social competence/skills would fall outside this category and would be placed within the milder end of the autistic spectrum, or perhaps included within a broader “neuro-social disorder” spectrum. As the authors aptly point out, psychometric testing is important for diagnosis and psychoeducational planning for patients with the information processing difficulties and life struggles well described in this article.
Jerrold Pollak, PhD
Coordinator, Program in Medical and
Forensic Neuropsychology
Seacoast Mental Health Center
Portsmouth, NH
The authors respond
We thank Dr. Pollak for his comments. Although considerable overlap may exist among “neurodevelopmental syndromes characterized by social inadequacy and peculiarity,” we sought to draw attention to the specific neurocognitive differences in NLD as compared with other disorders that, despite potentially sharing features with NLD, are distinct disorders.
We agree with Dr. Pollak’s suggestion that NLD someday might be classified within the DSM along a “neurodevelopmental disorder spectrum characterized by deficits in social competence, judgment, and perception accompanied by impaired everyday functioning referable to these difficulties.” This process will require careful characterization of individuals with NLD—as well as other individuals with difficulties in visual-spatial integration, attention, nonverbal memory, and expression and integration of emotion—to establish convergent and discriminant validity for NLD. Further, we believe that recognition of NLD as a distinct disorder will facilitate research and development of specific treatment interventions as compared with other conditions, despite potential syndromic or symptomatic overlap with NLD.
Sergio V. Delgado, MD
Professor of Psychiatry, Pediatrics,
and Psychoanalysis
Elizabeth Wassenaar, MD
Resident in Psychiatry, Child and Adolescent
Psychiatry, and Pediatrics
Jeffrey R. Strawn, MD
Assistant Professor of Psychiatry
and Pediatrics
Cincinnati Children’s Hospital Medical Center
University of Cincinnati College of Medicine
Cincinnati, OH
“Does your patient have a psychiatric illness or nonverbal learning disorder?” (Current Psychiatry, May 2011, p. 17-35) reviews the differential diagnosis of “nonverbal learning disorder.”
Nonverbal learning disorder (NLD) has yet to be established as a distinct, valid neurodevelopmental syndrome. Many of the proffered diagnostic criteria, clinical presentations, and laboratory findings (especially results from psychological/neuropsychological and psychoeducational testing) overlap considerably with other neurodevelopmental syndromes characterized by social inadequacy and peculiarity, including Asperger’s disorder.
Therefore, rather than representing a discrete diagnostic category with overlapping features as suggested by this article, this putative syndrome probably is best conceptualized as falling within the milder end of a neurodevelopmental disorder spectrum characterized by deficits in social competence, judgment, and perception, accompanied by impaired daily functioning referable to these difficulties. The DSM-5 workgroup appears to recognize this problem of “splitting” vs “lumping.” Reports indicate that Asperger’s disorder may no longer be considered a “standalone” clinical syndrome and likely will be reconceptualized as a mild form of autism.
Many individuals have cognitive/neuropsychological and academic/learning difficulties compatible with nonverbal learning problems and do not exhibit the difficulties with social interactions the article cites. One way to advocate for NLD as a distinct clinical syndrome is to limit the diagnosis to a certain constellation of cognitive/neuropsychological and academic impairments that lead to educational, interpersonal, and/or vocational difficulties. Patients who also display clinically significant neurodevelopmentally based deficits in social competence/skills would fall outside this category and would be placed within the milder end of the autistic spectrum, or perhaps included within a broader “neuro-social disorder” spectrum. As the authors aptly point out, psychometric testing is important for diagnosis and psychoeducational planning for patients with the information processing difficulties and life struggles well described in this article.
Jerrold Pollak, PhD
Coordinator, Program in Medical and
Forensic Neuropsychology
Seacoast Mental Health Center
Portsmouth, NH
The authors respond
We thank Dr. Pollak for his comments. Although considerable overlap may exist among “neurodevelopmental syndromes characterized by social inadequacy and peculiarity,” we sought to draw attention to the specific neurocognitive differences in NLD as compared with other disorders that, despite potentially sharing features with NLD, are distinct disorders.
We agree with Dr. Pollak’s suggestion that NLD someday might be classified within the DSM along a “neurodevelopmental disorder spectrum characterized by deficits in social competence, judgment, and perception accompanied by impaired everyday functioning referable to these difficulties.” This process will require careful characterization of individuals with NLD—as well as other individuals with difficulties in visual-spatial integration, attention, nonverbal memory, and expression and integration of emotion—to establish convergent and discriminant validity for NLD. Further, we believe that recognition of NLD as a distinct disorder will facilitate research and development of specific treatment interventions as compared with other conditions, despite potential syndromic or symptomatic overlap with NLD.
Sergio V. Delgado, MD
Professor of Psychiatry, Pediatrics,
and Psychoanalysis
Elizabeth Wassenaar, MD
Resident in Psychiatry, Child and Adolescent
Psychiatry, and Pediatrics
Jeffrey R. Strawn, MD
Assistant Professor of Psychiatry
and Pediatrics
Cincinnati Children’s Hospital Medical Center
University of Cincinnati College of Medicine
Cincinnati, OH
“Does your patient have a psychiatric illness or nonverbal learning disorder?” (Current Psychiatry, May 2011, p. 17-35) reviews the differential diagnosis of “nonverbal learning disorder.”
Nonverbal learning disorder (NLD) has yet to be established as a distinct, valid neurodevelopmental syndrome. Many of the proffered diagnostic criteria, clinical presentations, and laboratory findings (especially results from psychological/neuropsychological and psychoeducational testing) overlap considerably with other neurodevelopmental syndromes characterized by social inadequacy and peculiarity, including Asperger’s disorder.
Therefore, rather than representing a discrete diagnostic category with overlapping features as suggested by this article, this putative syndrome probably is best conceptualized as falling within the milder end of a neurodevelopmental disorder spectrum characterized by deficits in social competence, judgment, and perception, accompanied by impaired daily functioning referable to these difficulties. The DSM-5 workgroup appears to recognize this problem of “splitting” vs “lumping.” Reports indicate that Asperger’s disorder may no longer be considered a “standalone” clinical syndrome and likely will be reconceptualized as a mild form of autism.
Many individuals have cognitive/neuropsychological and academic/learning difficulties compatible with nonverbal learning problems and do not exhibit the difficulties with social interactions the article cites. One way to advocate for NLD as a distinct clinical syndrome is to limit the diagnosis to a certain constellation of cognitive/neuropsychological and academic impairments that lead to educational, interpersonal, and/or vocational difficulties. Patients who also display clinically significant neurodevelopmentally based deficits in social competence/skills would fall outside this category and would be placed within the milder end of the autistic spectrum, or perhaps included within a broader “neuro-social disorder” spectrum. As the authors aptly point out, psychometric testing is important for diagnosis and psychoeducational planning for patients with the information processing difficulties and life struggles well described in this article.
Jerrold Pollak, PhD
Coordinator, Program in Medical and
Forensic Neuropsychology
Seacoast Mental Health Center
Portsmouth, NH
The authors respond
We thank Dr. Pollak for his comments. Although considerable overlap may exist among “neurodevelopmental syndromes characterized by social inadequacy and peculiarity,” we sought to draw attention to the specific neurocognitive differences in NLD as compared with other disorders that, despite potentially sharing features with NLD, are distinct disorders.
We agree with Dr. Pollak’s suggestion that NLD someday might be classified within the DSM along a “neurodevelopmental disorder spectrum characterized by deficits in social competence, judgment, and perception accompanied by impaired everyday functioning referable to these difficulties.” This process will require careful characterization of individuals with NLD—as well as other individuals with difficulties in visual-spatial integration, attention, nonverbal memory, and expression and integration of emotion—to establish convergent and discriminant validity for NLD. Further, we believe that recognition of NLD as a distinct disorder will facilitate research and development of specific treatment interventions as compared with other conditions, despite potential syndromic or symptomatic overlap with NLD.
Sergio V. Delgado, MD
Professor of Psychiatry, Pediatrics,
and Psychoanalysis
Elizabeth Wassenaar, MD
Resident in Psychiatry, Child and Adolescent
Psychiatry, and Pediatrics
Jeffrey R. Strawn, MD
Assistant Professor of Psychiatry
and Pediatrics
Cincinnati Children’s Hospital Medical Center
University of Cincinnati College of Medicine
Cincinnati, OH
Comments & Controversies
Discuss this article at www.facebook.com/CurrentPsychiatry
Differentiating NLD
“Does your patient have a psychiatric illness or nonverbal learning disorder?” (Current Psychiatry, May 2011, p. 17-35) reviews the differential diagnosis of “nonverbal learning disorder.”
Nonverbal learning disorder (NLD) has yet to be established as a distinct, valid neurodevelopmental syndrome. Many of the proffered diagnostic criteria, clinical presentations, and laboratory findings (especially results from psychological/neuropsychological and psychoeducational testing) overlap considerably with other neurodevelopmental syndromes characterized by social inadequacy and peculiarity, including Asperger’s disorder.
Therefore, rather than representing a discrete diagnostic category with overlapping features as suggested by this article, this putative syndrome probably is best conceptualized as falling within the milder end of a neurodevelopmental disorder spectrum characterized by deficits in social competence, judgment, and perception, accompanied by impaired daily functioning referable to these difficulties. The DSM-5 workgroup appears to recognize this problem of “splitting” vs “lumping.” Reports indicate that Asperger’s disorder may no longer be considered a “standalone” clinical syndrome and likely will be reconceptualized as a mild form of autism.
Many individuals have cognitive/neuropsychological and academic/learning difficulties compatible with nonverbal learning problems and do not exhibit the difficulties with social interactions the article cites. One way to advocate for NLD as a distinct clinical syndrome is to limit the diagnosis to a certain constellation of cognitive/neuropsychological and academic impairments that lead to educational, interpersonal, and/or vocational difficulties. Patients who also display clinically significant neurodevelopmentally based deficits in social competence/skills would fall outside this category and would be placed within the milder end of the autistic spectrum, or perhaps included within a broader “neuro-social disorder” spectrum. As the authors aptly point out, psychometric testing is important for diagnosis and psychoeducational planning for patients with the information processing difficulties and life struggles well described in this article.
Jerrold Pollak, PhD
Coordinator, Program in Medical and
Forensic Neuropsychology
Seacoast Mental Health Center
Portsmouth, NH
The authors respond
We thank Dr. Pollak for his comments. Although considerable overlap may exist among “neurodevelopmental syndromes characterized by social inadequacy and peculiarity,” we sought to draw attention to the specific neurocognitive differences in NLD as compared with other disorders that, despite potentially sharing features with NLD, are distinct disorders.
We agree with Dr. Pollak’s suggestion that NLD someday might be classified within the DSM along a “neurodevelopmental disorder spectrum characterized by deficits in social competence, judgment, and perception accompanied by impaired everyday functioning referable to these difficulties.” This process will require careful characterization of individuals with NLD—as well as other individuals with difficulties in visual-spatial integration, attention, nonverbal memory, and expression and integration of emotion—to establish convergent and discriminant validity for NLD. Further, we believe that recognition of NLD as a distinct disorder will facilitate research and development of specific treatment interventions as compared with other conditions, despite potential syndromic or symptomatic overlap with NLD.
Sergio V. Delgado, MD
Professor of Psychiatry, Pediatrics,
and Psychoanalysis
Elizabeth Wassenaar, MD
Resident in Psychiatry, Child and Adolescent
Psychiatry, and Pediatrics
Jeffrey R. Strawn, MD
Assistant Professor of Psychiatry
and Pediatrics
Cincinnati Children’s Hospital Medical Center
University of Cincinnati College of Medicine
Cincinnati, OH
Efficient pharmacotherapy
I agree with Dr. Nasrallah’s assertions that a single psychotropic can treat different disorders (“Parsimonious pharmacotherapy,” From the Editor, Current Psychiatry, May 2011, p. 12-16). During my consultation-liaison fellowship at MD Anderson Cancer Center, different forms of delirium were a daily occurrence in our hospitalized patients. A low dose of psychotropic—usually quetiapine—proved effective in controlling delirium; in acute and severe cases, low-dose IV haloperidol helped control agitation and psychosis.
Low-dose quetiapine also was beneficial in treating comorbidities such as increased anxiety, depression, pain, and insomnia, with minimal side effects. Because most of our patients suffered from several medical conditions and powerful, multiple-medication chemotherapies, in most cases psychotropic polypharmacy was contraindicated and avoided at all costs.
Corey Roman, MD
Psychiatrist, private practice
Miami, FL
Glutamatergic dysfunction
I read with interest the article by Drs. Kantrowitz and Javitt (“Glutamate: New hope for schizophrenia treatment,” Current Psychiatry, April 2011, p. 68-74) describing the glutamatergic model of schizophrenia. There is extensive evidence that in addition to neurocognitive deficits, schizophrenia is characterized by various neuromuscular abnormalities, including skeletal muscle fiber changes, alterations of alpha-motor neuron excitability, increased motor unit fiber densities, increased branching of terminal motor nerves, and elevated levels of muscular enzymes.1,2 These neuromuscular abnormalities also are found in healthy first-degree relatives of patients with schizophrenia.3 Although the precise cause of these neuromuscular abnormalities has not been elucidated, one possible explanation is that they may be the result of neuronal injury mediated by excitatory amino acids.1 For example, Stevens4 suggested that abnormal sprouting and reinnervation of neurons in schizophrenia might be caused by such injury.
Amyotrophic lateral sclerosis (ALS) is a progressive degenerative syndrome involving upper and lower alpha-motor neuron systems. A substantial body of evidence supports the hypothesis that glutamate-mediated excito-toxicity is responsible for the death of motor neurons in ALS.5 Evidence suggests that having schizophrenia may be associated with an increased risk of developing ALS, and this risk might be explained by the toxic effects of excitatory amino acids on neuronal function.1,6 Recently, Stommel et al7 hypothesized that treating schizophrenia could protect against development of ALS, which is of interest because antipsychotics may have direct and indirect effects on modulating glutamate receptor systems.8
Robert H. Howland, MD
Associate Professor of Psychiatry
University of Pittsburgh School of Medicine
Western Psychiatric Institute and Clinic
Pittsburgh, PA
Treating delirium
Regarding “Atypical antipsychotics for delirium: A reasonable alternative to haloperidol?” (Current Psychiatry, January 2011, p. 37-46): Delirium usually is an acute encephalopathy with cerebral dysfunction caused by varying pathologies, most of which are extracranial. Examples include mental confusion induced by hypoxia or hypoglycemia. Primary treatment of delirium must be aimed at the specific cause.
Antipsychotic drugs are only a symptomatic intervention for delirium. They can and do provide behavioral control; however, these medications may worsen cases of alcohol or sedative withdrawal, ictal-related problems, neuroleptic malignant syndrome, etc. An antipsychotic may complicate other conditions, thus creating additional clinical difficulties for some patients. Recommending antipsychotics as a treatment focuses on symptomatic aspects; however, the critical mandate is to diagnose and specifically manage the etiology. Once the cause is corrected, the delirium usually resolves.
While treating the primary pathology, if behavioral issues still urgently require immediate control, a benzodiazepine is safer than an antipsychotic. Both medications provide symptomatic control, but a benzodiazepine is less likely to add new clinical problems. The only major precaution with a benzodiazepine is to avoid overprescribing. It is simply safer to rely on benzodiazepines for short-term behavioral management.
Steven Lippmann, MD
Professor of Psychiatry
University of Louisville School of Medicine
Louisville, KY
Discuss this article at www.facebook.com/CurrentPsychiatry
Differentiating NLD
“Does your patient have a psychiatric illness or nonverbal learning disorder?” (Current Psychiatry, May 2011, p. 17-35) reviews the differential diagnosis of “nonverbal learning disorder.”
Nonverbal learning disorder (NLD) has yet to be established as a distinct, valid neurodevelopmental syndrome. Many of the proffered diagnostic criteria, clinical presentations, and laboratory findings (especially results from psychological/neuropsychological and psychoeducational testing) overlap considerably with other neurodevelopmental syndromes characterized by social inadequacy and peculiarity, including Asperger’s disorder.
Therefore, rather than representing a discrete diagnostic category with overlapping features as suggested by this article, this putative syndrome probably is best conceptualized as falling within the milder end of a neurodevelopmental disorder spectrum characterized by deficits in social competence, judgment, and perception, accompanied by impaired daily functioning referable to these difficulties. The DSM-5 workgroup appears to recognize this problem of “splitting” vs “lumping.” Reports indicate that Asperger’s disorder may no longer be considered a “standalone” clinical syndrome and likely will be reconceptualized as a mild form of autism.
Many individuals have cognitive/neuropsychological and academic/learning difficulties compatible with nonverbal learning problems and do not exhibit the difficulties with social interactions the article cites. One way to advocate for NLD as a distinct clinical syndrome is to limit the diagnosis to a certain constellation of cognitive/neuropsychological and academic impairments that lead to educational, interpersonal, and/or vocational difficulties. Patients who also display clinically significant neurodevelopmentally based deficits in social competence/skills would fall outside this category and would be placed within the milder end of the autistic spectrum, or perhaps included within a broader “neuro-social disorder” spectrum. As the authors aptly point out, psychometric testing is important for diagnosis and psychoeducational planning for patients with the information processing difficulties and life struggles well described in this article.
Jerrold Pollak, PhD
Coordinator, Program in Medical and
Forensic Neuropsychology
Seacoast Mental Health Center
Portsmouth, NH
The authors respond
We thank Dr. Pollak for his comments. Although considerable overlap may exist among “neurodevelopmental syndromes characterized by social inadequacy and peculiarity,” we sought to draw attention to the specific neurocognitive differences in NLD as compared with other disorders that, despite potentially sharing features with NLD, are distinct disorders.
We agree with Dr. Pollak’s suggestion that NLD someday might be classified within the DSM along a “neurodevelopmental disorder spectrum characterized by deficits in social competence, judgment, and perception accompanied by impaired everyday functioning referable to these difficulties.” This process will require careful characterization of individuals with NLD—as well as other individuals with difficulties in visual-spatial integration, attention, nonverbal memory, and expression and integration of emotion—to establish convergent and discriminant validity for NLD. Further, we believe that recognition of NLD as a distinct disorder will facilitate research and development of specific treatment interventions as compared with other conditions, despite potential syndromic or symptomatic overlap with NLD.
Sergio V. Delgado, MD
Professor of Psychiatry, Pediatrics,
and Psychoanalysis
Elizabeth Wassenaar, MD
Resident in Psychiatry, Child and Adolescent
Psychiatry, and Pediatrics
Jeffrey R. Strawn, MD
Assistant Professor of Psychiatry
and Pediatrics
Cincinnati Children’s Hospital Medical Center
University of Cincinnati College of Medicine
Cincinnati, OH
Efficient pharmacotherapy
I agree with Dr. Nasrallah’s assertions that a single psychotropic can treat different disorders (“Parsimonious pharmacotherapy,” From the Editor, Current Psychiatry, May 2011, p. 12-16). During my consultation-liaison fellowship at MD Anderson Cancer Center, different forms of delirium were a daily occurrence in our hospitalized patients. A low dose of psychotropic—usually quetiapine—proved effective in controlling delirium; in acute and severe cases, low-dose IV haloperidol helped control agitation and psychosis.
Low-dose quetiapine also was beneficial in treating comorbidities such as increased anxiety, depression, pain, and insomnia, with minimal side effects. Because most of our patients suffered from several medical conditions and powerful, multiple-medication chemotherapies, in most cases psychotropic polypharmacy was contraindicated and avoided at all costs.
Corey Roman, MD
Psychiatrist, private practice
Miami, FL
Glutamatergic dysfunction
I read with interest the article by Drs. Kantrowitz and Javitt (“Glutamate: New hope for schizophrenia treatment,” Current Psychiatry, April 2011, p. 68-74) describing the glutamatergic model of schizophrenia. There is extensive evidence that in addition to neurocognitive deficits, schizophrenia is characterized by various neuromuscular abnormalities, including skeletal muscle fiber changes, alterations of alpha-motor neuron excitability, increased motor unit fiber densities, increased branching of terminal motor nerves, and elevated levels of muscular enzymes.1,2 These neuromuscular abnormalities also are found in healthy first-degree relatives of patients with schizophrenia.3 Although the precise cause of these neuromuscular abnormalities has not been elucidated, one possible explanation is that they may be the result of neuronal injury mediated by excitatory amino acids.1 For example, Stevens4 suggested that abnormal sprouting and reinnervation of neurons in schizophrenia might be caused by such injury.
Amyotrophic lateral sclerosis (ALS) is a progressive degenerative syndrome involving upper and lower alpha-motor neuron systems. A substantial body of evidence supports the hypothesis that glutamate-mediated excito-toxicity is responsible for the death of motor neurons in ALS.5 Evidence suggests that having schizophrenia may be associated with an increased risk of developing ALS, and this risk might be explained by the toxic effects of excitatory amino acids on neuronal function.1,6 Recently, Stommel et al7 hypothesized that treating schizophrenia could protect against development of ALS, which is of interest because antipsychotics may have direct and indirect effects on modulating glutamate receptor systems.8
Robert H. Howland, MD
Associate Professor of Psychiatry
University of Pittsburgh School of Medicine
Western Psychiatric Institute and Clinic
Pittsburgh, PA
Treating delirium
Regarding “Atypical antipsychotics for delirium: A reasonable alternative to haloperidol?” (Current Psychiatry, January 2011, p. 37-46): Delirium usually is an acute encephalopathy with cerebral dysfunction caused by varying pathologies, most of which are extracranial. Examples include mental confusion induced by hypoxia or hypoglycemia. Primary treatment of delirium must be aimed at the specific cause.
Antipsychotic drugs are only a symptomatic intervention for delirium. They can and do provide behavioral control; however, these medications may worsen cases of alcohol or sedative withdrawal, ictal-related problems, neuroleptic malignant syndrome, etc. An antipsychotic may complicate other conditions, thus creating additional clinical difficulties for some patients. Recommending antipsychotics as a treatment focuses on symptomatic aspects; however, the critical mandate is to diagnose and specifically manage the etiology. Once the cause is corrected, the delirium usually resolves.
While treating the primary pathology, if behavioral issues still urgently require immediate control, a benzodiazepine is safer than an antipsychotic. Both medications provide symptomatic control, but a benzodiazepine is less likely to add new clinical problems. The only major precaution with a benzodiazepine is to avoid overprescribing. It is simply safer to rely on benzodiazepines for short-term behavioral management.
Steven Lippmann, MD
Professor of Psychiatry
University of Louisville School of Medicine
Louisville, KY
Discuss this article at www.facebook.com/CurrentPsychiatry
Differentiating NLD
“Does your patient have a psychiatric illness or nonverbal learning disorder?” (Current Psychiatry, May 2011, p. 17-35) reviews the differential diagnosis of “nonverbal learning disorder.”
Nonverbal learning disorder (NLD) has yet to be established as a distinct, valid neurodevelopmental syndrome. Many of the proffered diagnostic criteria, clinical presentations, and laboratory findings (especially results from psychological/neuropsychological and psychoeducational testing) overlap considerably with other neurodevelopmental syndromes characterized by social inadequacy and peculiarity, including Asperger’s disorder.
Therefore, rather than representing a discrete diagnostic category with overlapping features as suggested by this article, this putative syndrome probably is best conceptualized as falling within the milder end of a neurodevelopmental disorder spectrum characterized by deficits in social competence, judgment, and perception, accompanied by impaired daily functioning referable to these difficulties. The DSM-5 workgroup appears to recognize this problem of “splitting” vs “lumping.” Reports indicate that Asperger’s disorder may no longer be considered a “standalone” clinical syndrome and likely will be reconceptualized as a mild form of autism.
Many individuals have cognitive/neuropsychological and academic/learning difficulties compatible with nonverbal learning problems and do not exhibit the difficulties with social interactions the article cites. One way to advocate for NLD as a distinct clinical syndrome is to limit the diagnosis to a certain constellation of cognitive/neuropsychological and academic impairments that lead to educational, interpersonal, and/or vocational difficulties. Patients who also display clinically significant neurodevelopmentally based deficits in social competence/skills would fall outside this category and would be placed within the milder end of the autistic spectrum, or perhaps included within a broader “neuro-social disorder” spectrum. As the authors aptly point out, psychometric testing is important for diagnosis and psychoeducational planning for patients with the information processing difficulties and life struggles well described in this article.
Jerrold Pollak, PhD
Coordinator, Program in Medical and
Forensic Neuropsychology
Seacoast Mental Health Center
Portsmouth, NH
The authors respond
We thank Dr. Pollak for his comments. Although considerable overlap may exist among “neurodevelopmental syndromes characterized by social inadequacy and peculiarity,” we sought to draw attention to the specific neurocognitive differences in NLD as compared with other disorders that, despite potentially sharing features with NLD, are distinct disorders.
We agree with Dr. Pollak’s suggestion that NLD someday might be classified within the DSM along a “neurodevelopmental disorder spectrum characterized by deficits in social competence, judgment, and perception accompanied by impaired everyday functioning referable to these difficulties.” This process will require careful characterization of individuals with NLD—as well as other individuals with difficulties in visual-spatial integration, attention, nonverbal memory, and expression and integration of emotion—to establish convergent and discriminant validity for NLD. Further, we believe that recognition of NLD as a distinct disorder will facilitate research and development of specific treatment interventions as compared with other conditions, despite potential syndromic or symptomatic overlap with NLD.
Sergio V. Delgado, MD
Professor of Psychiatry, Pediatrics,
and Psychoanalysis
Elizabeth Wassenaar, MD
Resident in Psychiatry, Child and Adolescent
Psychiatry, and Pediatrics
Jeffrey R. Strawn, MD
Assistant Professor of Psychiatry
and Pediatrics
Cincinnati Children’s Hospital Medical Center
University of Cincinnati College of Medicine
Cincinnati, OH
Efficient pharmacotherapy
I agree with Dr. Nasrallah’s assertions that a single psychotropic can treat different disorders (“Parsimonious pharmacotherapy,” From the Editor, Current Psychiatry, May 2011, p. 12-16). During my consultation-liaison fellowship at MD Anderson Cancer Center, different forms of delirium were a daily occurrence in our hospitalized patients. A low dose of psychotropic—usually quetiapine—proved effective in controlling delirium; in acute and severe cases, low-dose IV haloperidol helped control agitation and psychosis.
Low-dose quetiapine also was beneficial in treating comorbidities such as increased anxiety, depression, pain, and insomnia, with minimal side effects. Because most of our patients suffered from several medical conditions and powerful, multiple-medication chemotherapies, in most cases psychotropic polypharmacy was contraindicated and avoided at all costs.
Corey Roman, MD
Psychiatrist, private practice
Miami, FL
Glutamatergic dysfunction
I read with interest the article by Drs. Kantrowitz and Javitt (“Glutamate: New hope for schizophrenia treatment,” Current Psychiatry, April 2011, p. 68-74) describing the glutamatergic model of schizophrenia. There is extensive evidence that in addition to neurocognitive deficits, schizophrenia is characterized by various neuromuscular abnormalities, including skeletal muscle fiber changes, alterations of alpha-motor neuron excitability, increased motor unit fiber densities, increased branching of terminal motor nerves, and elevated levels of muscular enzymes.1,2 These neuromuscular abnormalities also are found in healthy first-degree relatives of patients with schizophrenia.3 Although the precise cause of these neuromuscular abnormalities has not been elucidated, one possible explanation is that they may be the result of neuronal injury mediated by excitatory amino acids.1 For example, Stevens4 suggested that abnormal sprouting and reinnervation of neurons in schizophrenia might be caused by such injury.
Amyotrophic lateral sclerosis (ALS) is a progressive degenerative syndrome involving upper and lower alpha-motor neuron systems. A substantial body of evidence supports the hypothesis that glutamate-mediated excito-toxicity is responsible for the death of motor neurons in ALS.5 Evidence suggests that having schizophrenia may be associated with an increased risk of developing ALS, and this risk might be explained by the toxic effects of excitatory amino acids on neuronal function.1,6 Recently, Stommel et al7 hypothesized that treating schizophrenia could protect against development of ALS, which is of interest because antipsychotics may have direct and indirect effects on modulating glutamate receptor systems.8
Robert H. Howland, MD
Associate Professor of Psychiatry
University of Pittsburgh School of Medicine
Western Psychiatric Institute and Clinic
Pittsburgh, PA
Treating delirium
Regarding “Atypical antipsychotics for delirium: A reasonable alternative to haloperidol?” (Current Psychiatry, January 2011, p. 37-46): Delirium usually is an acute encephalopathy with cerebral dysfunction caused by varying pathologies, most of which are extracranial. Examples include mental confusion induced by hypoxia or hypoglycemia. Primary treatment of delirium must be aimed at the specific cause.
Antipsychotic drugs are only a symptomatic intervention for delirium. They can and do provide behavioral control; however, these medications may worsen cases of alcohol or sedative withdrawal, ictal-related problems, neuroleptic malignant syndrome, etc. An antipsychotic may complicate other conditions, thus creating additional clinical difficulties for some patients. Recommending antipsychotics as a treatment focuses on symptomatic aspects; however, the critical mandate is to diagnose and specifically manage the etiology. Once the cause is corrected, the delirium usually resolves.
While treating the primary pathology, if behavioral issues still urgently require immediate control, a benzodiazepine is safer than an antipsychotic. Both medications provide symptomatic control, but a benzodiazepine is less likely to add new clinical problems. The only major precaution with a benzodiazepine is to avoid overprescribing. It is simply safer to rely on benzodiazepines for short-term behavioral management.
Steven Lippmann, MD
Professor of Psychiatry
University of Louisville School of Medicine
Louisville, KY
The ABCDEs of identifying eating disorders
Although eating disorders can be life-threatening,1 many patients remain undiagnosed until late in the disease course. Early identification and treatment may reduce the risk of chronic health consequences and mortality.
Based on the DSM-IV-TR categorical approach, many clinicians think of anorexia nervosa and bulimia nervosa as the primary eating disorders. However, eating disorder not otherwise specified tends to be the most common diagnosis.2 Several authors have suggested that combining categorical and dimensional approaches may be useful in diagnosing these patients.2
It is easy to suspect an eating disorder in patients of very low weight, but patients who are of normal weight or obese also may have an eating disorder. In addition to measuring body mass index, inquire about patients’ lowest and highest adult, nonpregnant weights and what they consider to be their “ideal” weight. The mnemonic ABCDE can help you remember key components of assessing patients who might have an eating disorder.
Associated health problems. Anorexia patients commonly present with emaciation, skin and hair dryness, cold intolerance, bradycardia, and orthostatic hypotension. Look for calluses on dorsum of the hands, parotid enlargement, mouth ulcers, dental caries, and edema, which may be found in bulimia patients.
Body image. Determine whether your patients’ self esteem is correlated with body weight and shape, how often they weigh themselves, and if they are satisfied with the way their body is proportioned. Ask if they fear weight gain or are driven to be thin.
Compensatory behaviors may include self-induced vomiting or excessive use of diet pills, diuretics, or laxatives. Other examples are restricting food, chewing and spitting out food, and over-exercising (especially lengthy cardiovascular workouts).
Diet. Inquire whether your patients have ever been on a diet, reduced the amount of food they consume, or used medications indicated for obesity. Patients may avoid entire categories of foods (lipids, carbohydrates), which may lead to malnutrition and vitamin deficiencies.
Eating behaviors. Patients may eat very small meals or excessive portions of certain foods. They might skip meals or eat only alone or at night. Finishing meals very slowly or very quickly, mixing food on their plate, eating with tiny bites, or drinking a lot of water with and between meals also may be clues to disordered eating. None of these behaviors by itself indicate an eating disorder if not accompanied by other symptoms.
Disclosure
The authors report no financial relationship with any manufacturer whose products are mentioned in this article or with manufacturers of competing products.
Although eating disorders can be life-threatening,1 many patients remain undiagnosed until late in the disease course. Early identification and treatment may reduce the risk of chronic health consequences and mortality.
Based on the DSM-IV-TR categorical approach, many clinicians think of anorexia nervosa and bulimia nervosa as the primary eating disorders. However, eating disorder not otherwise specified tends to be the most common diagnosis.2 Several authors have suggested that combining categorical and dimensional approaches may be useful in diagnosing these patients.2
It is easy to suspect an eating disorder in patients of very low weight, but patients who are of normal weight or obese also may have an eating disorder. In addition to measuring body mass index, inquire about patients’ lowest and highest adult, nonpregnant weights and what they consider to be their “ideal” weight. The mnemonic ABCDE can help you remember key components of assessing patients who might have an eating disorder.
Associated health problems. Anorexia patients commonly present with emaciation, skin and hair dryness, cold intolerance, bradycardia, and orthostatic hypotension. Look for calluses on dorsum of the hands, parotid enlargement, mouth ulcers, dental caries, and edema, which may be found in bulimia patients.
Body image. Determine whether your patients’ self esteem is correlated with body weight and shape, how often they weigh themselves, and if they are satisfied with the way their body is proportioned. Ask if they fear weight gain or are driven to be thin.
Compensatory behaviors may include self-induced vomiting or excessive use of diet pills, diuretics, or laxatives. Other examples are restricting food, chewing and spitting out food, and over-exercising (especially lengthy cardiovascular workouts).
Diet. Inquire whether your patients have ever been on a diet, reduced the amount of food they consume, or used medications indicated for obesity. Patients may avoid entire categories of foods (lipids, carbohydrates), which may lead to malnutrition and vitamin deficiencies.
Eating behaviors. Patients may eat very small meals or excessive portions of certain foods. They might skip meals or eat only alone or at night. Finishing meals very slowly or very quickly, mixing food on their plate, eating with tiny bites, or drinking a lot of water with and between meals also may be clues to disordered eating. None of these behaviors by itself indicate an eating disorder if not accompanied by other symptoms.
Disclosure
The authors report no financial relationship with any manufacturer whose products are mentioned in this article or with manufacturers of competing products.
Although eating disorders can be life-threatening,1 many patients remain undiagnosed until late in the disease course. Early identification and treatment may reduce the risk of chronic health consequences and mortality.
Based on the DSM-IV-TR categorical approach, many clinicians think of anorexia nervosa and bulimia nervosa as the primary eating disorders. However, eating disorder not otherwise specified tends to be the most common diagnosis.2 Several authors have suggested that combining categorical and dimensional approaches may be useful in diagnosing these patients.2
It is easy to suspect an eating disorder in patients of very low weight, but patients who are of normal weight or obese also may have an eating disorder. In addition to measuring body mass index, inquire about patients’ lowest and highest adult, nonpregnant weights and what they consider to be their “ideal” weight. The mnemonic ABCDE can help you remember key components of assessing patients who might have an eating disorder.
Associated health problems. Anorexia patients commonly present with emaciation, skin and hair dryness, cold intolerance, bradycardia, and orthostatic hypotension. Look for calluses on dorsum of the hands, parotid enlargement, mouth ulcers, dental caries, and edema, which may be found in bulimia patients.
Body image. Determine whether your patients’ self esteem is correlated with body weight and shape, how often they weigh themselves, and if they are satisfied with the way their body is proportioned. Ask if they fear weight gain or are driven to be thin.
Compensatory behaviors may include self-induced vomiting or excessive use of diet pills, diuretics, or laxatives. Other examples are restricting food, chewing and spitting out food, and over-exercising (especially lengthy cardiovascular workouts).
Diet. Inquire whether your patients have ever been on a diet, reduced the amount of food they consume, or used medications indicated for obesity. Patients may avoid entire categories of foods (lipids, carbohydrates), which may lead to malnutrition and vitamin deficiencies.
Eating behaviors. Patients may eat very small meals or excessive portions of certain foods. They might skip meals or eat only alone or at night. Finishing meals very slowly or very quickly, mixing food on their plate, eating with tiny bites, or drinking a lot of water with and between meals also may be clues to disordered eating. None of these behaviors by itself indicate an eating disorder if not accompanied by other symptoms.
Disclosure
The authors report no financial relationship with any manufacturer whose products are mentioned in this article or with manufacturers of competing products.