User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Augmenting antidepressants with triiodothyronine: An underutilized strategy
Discuss this article at www.facebook.com/CurrentPsychiatry
Partial responsiveness to antidepressant monotherapy is a struggle for many depressed patients. The literature supports the effectiveness of augmenting tricyclic antidepressants (TCAs) with triiodothyronine (T3) in unipolar depression. One meta-analysis suggests that T3 may accelerate antidepressant response in patients with treatment-resistant depression.1 Likewise, T3 augmentation can improve depressive symptoms in patients without subclinical hypothyroidism whose depression did not fully respond to selective serotonin reuptake inhibitors (SSRIs).2 In the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, patients received lithium or T3 augmentation after they failed 2 antidepressant trials.3 Patients receiving lithium or T3 augmentation showed similar remission rates, but the latter was associated with lower discontinuation rates because of its more favorable side effect profile.
Despite its reported effectiveness, T3 may be underutilized or misunderstood by prescribers.4 Familiarity and use of liothyronine (the exogenous levorotatory form of T3) augmentation may improve response rates for depressed patients taking antidepressants.
Thyroid workup
Untreated thyroid conditions may reduce a depressed patient’s response to antidepressants.1 Check thyroid-stimulating hormone (TSH) levels as part of a medical workup for depressed mood before initiating T3. If TSH is elevated, a free thyroxine (T4) level should be ordered to detect clinical hypothyroidism. T4 as a sole initial screening test will not reveal subclinical hypothyroidism. Likewise, unbound T3 levels may be normal in patients with hypothyroidism, which could demonstrate endogenous adaptations in deiodination. Consult with an endocrinologist if you suspect Hashimoto’s thyroiditis or if a patient has other abnormal laboratory values.
Patient selection
Preliminary data suggest that women respond better to T3 augmentation than men,1 possibly because of women’s greater susceptibility to clinical and subclinical hypothyroidism. Patients also should demonstrate partial response to a TCA or SSRI at an adequate dose and duration. Reconsider augmentation if you are concerned that a patient might divert or abuse T3, such as patients with eating disorders.
Safety considerations
In general, most patients tolerate T3. However, risks of T3 supplementation include hyperthyroidism. Severely depressed TSH levels (<0.1 mIU/mL) may predispose patients to atrial arrhythmias or osteoporosis, which compromise the benefits of thyroid augmentation. Low serum TSH increases the risk of atrial fibrillation, and higher free T4 levels are associated with a graded risk of atrial fibrillation.5 Thyroid hormone may cause bone resorption. Although 1 study did not demonstrate accelerated bone loss with exogenous levothyroxine,6 caution is warranted in vulnerable populations, such as postmenopausal women.
T3 is a FDA pregnancy category A medication, making it a viable augmentation agent for pregnant women when lithium use may not be possible. However, we do not recommend T3 use in pregnant women without obstetric consultation.
Drug interactions
Cholestyramine decreases T3’s clinical effect, as can antacids and iron and calcium supplements.7 Similarly, carbamazepine will decrease T3 effectiveness by inducing hepatic metabolism. T3 may enhance warfarin’s anticoagulant effect and increase insulin requirements among diabetic individuals.
Augmentation dosing
Liothyronine should be started at 25 mcg/d taken on an empty stomach in the morning at least 30 minutes before eating. Signs of hyperthyroidism, such as sweating, anxiety, loose stools, heat intolerance, irritability, and tachycardia, suggest that the patient may not tolerate further increases. In geriatric patients or those with elevated cardiovascular risk, consider starting T3 at 12.5 mcg/d.
After 1 to 2 weeks, increase to 37.5 or 50 mcg/d. Evidence is limited for doses >50 mcg/d, the maximum dose used in the STAR*D study. Be aware that depressed patients may be tempted to increase their T3 dose, especially if they experience increased energy or weight loss. Doses >75 mcg/d are associated with an increased mortality rate.8
Monitor TSH levels after the first month of augmentation or when adding medications that change absorption or metabolism of thyroid hormones. If the T3 dose is stable, annual TSH measurements are adequate. Using standard hypothyroidism guidelines, maintain serum TSH between 0.4 and 2.0 mU/L.9 T3 levels do not need monitoring.
Response monitoring
As highlighted by STAR*D, the goal of depression treatment is remission, and symptom severity should be tracked. The self-reported 9-item Patient Health Questionnaire can complement clinical impressions as a quick and easy outcome measure when administered every 2 weeks. In STAR*D, the mean response time and time to remission for patients receiving T3 augmentation was 6 weeks and 6.6 weeks, respectively. However, 28% of patients did not achieve remission until week 14,3 which highlights the need for an adequate trial of T3.
Disclosures
Dr. Casher is a speaker for AstraZeneca, Pfizer Inc, and Sunovion Pharmaceuticals.
Drs. Gih and Bostwick report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Altshuler LL, Bauer M, Frye MA, et al. Does thyroid supplementation accelerate tricyclic antidepressant response? A review and meta-analysis of the literature. Am J Psychiatry. 2001;158:1617-1622.
2. Abraham G, Milev R, Stuart Lawson J. T3 augmentation of SSRI resistant depression. J Affect Disord. 2006;91:211-215.
3. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163:1519-1530.
4. Thase ME. Therapeutic alternatives for difficult-to-treat depression: a narrative review of the state of the evidence. CNS Spectr. 2004;9:808-816, 818-821.
5. Heeringa J, Hoogendoorn EH, van der Deure WM, et al. High-normal thyroid function and risk of atrial fibrillation: the Rotterdam study. Arch Intern Med. 2008;168:2219-2224.
6. Bauer M, Fairbanks L, Berghöfer A, et al. Bone mineral density during maintenance treatment with supraphysiological doses of levothyroxine in affective disorders: a longitudinal study. J Affect Disord. 2004;83:183-190.
7. Micromedex Healthcare Series [database online] Greenwood Village CO: Thomson Healthcare; 2010.
8. Yamamoto T, Fukuyama J, Fujiyoshi A. Factors associated with mortality of myxedema coma: report of eight cases and literature survey. Thyroid. 1999;9:1167-1174.
9. Devdhar M, Ousman YH, Burman KD. Hypothyroidism. Endocrinol Metab Clin North Am. 2007;36:595-615 v.
Discuss this article at www.facebook.com/CurrentPsychiatry
Partial responsiveness to antidepressant monotherapy is a struggle for many depressed patients. The literature supports the effectiveness of augmenting tricyclic antidepressants (TCAs) with triiodothyronine (T3) in unipolar depression. One meta-analysis suggests that T3 may accelerate antidepressant response in patients with treatment-resistant depression.1 Likewise, T3 augmentation can improve depressive symptoms in patients without subclinical hypothyroidism whose depression did not fully respond to selective serotonin reuptake inhibitors (SSRIs).2 In the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, patients received lithium or T3 augmentation after they failed 2 antidepressant trials.3 Patients receiving lithium or T3 augmentation showed similar remission rates, but the latter was associated with lower discontinuation rates because of its more favorable side effect profile.
Despite its reported effectiveness, T3 may be underutilized or misunderstood by prescribers.4 Familiarity and use of liothyronine (the exogenous levorotatory form of T3) augmentation may improve response rates for depressed patients taking antidepressants.
Thyroid workup
Untreated thyroid conditions may reduce a depressed patient’s response to antidepressants.1 Check thyroid-stimulating hormone (TSH) levels as part of a medical workup for depressed mood before initiating T3. If TSH is elevated, a free thyroxine (T4) level should be ordered to detect clinical hypothyroidism. T4 as a sole initial screening test will not reveal subclinical hypothyroidism. Likewise, unbound T3 levels may be normal in patients with hypothyroidism, which could demonstrate endogenous adaptations in deiodination. Consult with an endocrinologist if you suspect Hashimoto’s thyroiditis or if a patient has other abnormal laboratory values.
Patient selection
Preliminary data suggest that women respond better to T3 augmentation than men,1 possibly because of women’s greater susceptibility to clinical and subclinical hypothyroidism. Patients also should demonstrate partial response to a TCA or SSRI at an adequate dose and duration. Reconsider augmentation if you are concerned that a patient might divert or abuse T3, such as patients with eating disorders.
Safety considerations
In general, most patients tolerate T3. However, risks of T3 supplementation include hyperthyroidism. Severely depressed TSH levels (<0.1 mIU/mL) may predispose patients to atrial arrhythmias or osteoporosis, which compromise the benefits of thyroid augmentation. Low serum TSH increases the risk of atrial fibrillation, and higher free T4 levels are associated with a graded risk of atrial fibrillation.5 Thyroid hormone may cause bone resorption. Although 1 study did not demonstrate accelerated bone loss with exogenous levothyroxine,6 caution is warranted in vulnerable populations, such as postmenopausal women.
T3 is a FDA pregnancy category A medication, making it a viable augmentation agent for pregnant women when lithium use may not be possible. However, we do not recommend T3 use in pregnant women without obstetric consultation.
Drug interactions
Cholestyramine decreases T3’s clinical effect, as can antacids and iron and calcium supplements.7 Similarly, carbamazepine will decrease T3 effectiveness by inducing hepatic metabolism. T3 may enhance warfarin’s anticoagulant effect and increase insulin requirements among diabetic individuals.
Augmentation dosing
Liothyronine should be started at 25 mcg/d taken on an empty stomach in the morning at least 30 minutes before eating. Signs of hyperthyroidism, such as sweating, anxiety, loose stools, heat intolerance, irritability, and tachycardia, suggest that the patient may not tolerate further increases. In geriatric patients or those with elevated cardiovascular risk, consider starting T3 at 12.5 mcg/d.
After 1 to 2 weeks, increase to 37.5 or 50 mcg/d. Evidence is limited for doses >50 mcg/d, the maximum dose used in the STAR*D study. Be aware that depressed patients may be tempted to increase their T3 dose, especially if they experience increased energy or weight loss. Doses >75 mcg/d are associated with an increased mortality rate.8
Monitor TSH levels after the first month of augmentation or when adding medications that change absorption or metabolism of thyroid hormones. If the T3 dose is stable, annual TSH measurements are adequate. Using standard hypothyroidism guidelines, maintain serum TSH between 0.4 and 2.0 mU/L.9 T3 levels do not need monitoring.
Response monitoring
As highlighted by STAR*D, the goal of depression treatment is remission, and symptom severity should be tracked. The self-reported 9-item Patient Health Questionnaire can complement clinical impressions as a quick and easy outcome measure when administered every 2 weeks. In STAR*D, the mean response time and time to remission for patients receiving T3 augmentation was 6 weeks and 6.6 weeks, respectively. However, 28% of patients did not achieve remission until week 14,3 which highlights the need for an adequate trial of T3.
Disclosures
Dr. Casher is a speaker for AstraZeneca, Pfizer Inc, and Sunovion Pharmaceuticals.
Drs. Gih and Bostwick report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
Partial responsiveness to antidepressant monotherapy is a struggle for many depressed patients. The literature supports the effectiveness of augmenting tricyclic antidepressants (TCAs) with triiodothyronine (T3) in unipolar depression. One meta-analysis suggests that T3 may accelerate antidepressant response in patients with treatment-resistant depression.1 Likewise, T3 augmentation can improve depressive symptoms in patients without subclinical hypothyroidism whose depression did not fully respond to selective serotonin reuptake inhibitors (SSRIs).2 In the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, patients received lithium or T3 augmentation after they failed 2 antidepressant trials.3 Patients receiving lithium or T3 augmentation showed similar remission rates, but the latter was associated with lower discontinuation rates because of its more favorable side effect profile.
Despite its reported effectiveness, T3 may be underutilized or misunderstood by prescribers.4 Familiarity and use of liothyronine (the exogenous levorotatory form of T3) augmentation may improve response rates for depressed patients taking antidepressants.
Thyroid workup
Untreated thyroid conditions may reduce a depressed patient’s response to antidepressants.1 Check thyroid-stimulating hormone (TSH) levels as part of a medical workup for depressed mood before initiating T3. If TSH is elevated, a free thyroxine (T4) level should be ordered to detect clinical hypothyroidism. T4 as a sole initial screening test will not reveal subclinical hypothyroidism. Likewise, unbound T3 levels may be normal in patients with hypothyroidism, which could demonstrate endogenous adaptations in deiodination. Consult with an endocrinologist if you suspect Hashimoto’s thyroiditis or if a patient has other abnormal laboratory values.
Patient selection
Preliminary data suggest that women respond better to T3 augmentation than men,1 possibly because of women’s greater susceptibility to clinical and subclinical hypothyroidism. Patients also should demonstrate partial response to a TCA or SSRI at an adequate dose and duration. Reconsider augmentation if you are concerned that a patient might divert or abuse T3, such as patients with eating disorders.
Safety considerations
In general, most patients tolerate T3. However, risks of T3 supplementation include hyperthyroidism. Severely depressed TSH levels (<0.1 mIU/mL) may predispose patients to atrial arrhythmias or osteoporosis, which compromise the benefits of thyroid augmentation. Low serum TSH increases the risk of atrial fibrillation, and higher free T4 levels are associated with a graded risk of atrial fibrillation.5 Thyroid hormone may cause bone resorption. Although 1 study did not demonstrate accelerated bone loss with exogenous levothyroxine,6 caution is warranted in vulnerable populations, such as postmenopausal women.
T3 is a FDA pregnancy category A medication, making it a viable augmentation agent for pregnant women when lithium use may not be possible. However, we do not recommend T3 use in pregnant women without obstetric consultation.
Drug interactions
Cholestyramine decreases T3’s clinical effect, as can antacids and iron and calcium supplements.7 Similarly, carbamazepine will decrease T3 effectiveness by inducing hepatic metabolism. T3 may enhance warfarin’s anticoagulant effect and increase insulin requirements among diabetic individuals.
Augmentation dosing
Liothyronine should be started at 25 mcg/d taken on an empty stomach in the morning at least 30 minutes before eating. Signs of hyperthyroidism, such as sweating, anxiety, loose stools, heat intolerance, irritability, and tachycardia, suggest that the patient may not tolerate further increases. In geriatric patients or those with elevated cardiovascular risk, consider starting T3 at 12.5 mcg/d.
After 1 to 2 weeks, increase to 37.5 or 50 mcg/d. Evidence is limited for doses >50 mcg/d, the maximum dose used in the STAR*D study. Be aware that depressed patients may be tempted to increase their T3 dose, especially if they experience increased energy or weight loss. Doses >75 mcg/d are associated with an increased mortality rate.8
Monitor TSH levels after the first month of augmentation or when adding medications that change absorption or metabolism of thyroid hormones. If the T3 dose is stable, annual TSH measurements are adequate. Using standard hypothyroidism guidelines, maintain serum TSH between 0.4 and 2.0 mU/L.9 T3 levels do not need monitoring.
Response monitoring
As highlighted by STAR*D, the goal of depression treatment is remission, and symptom severity should be tracked. The self-reported 9-item Patient Health Questionnaire can complement clinical impressions as a quick and easy outcome measure when administered every 2 weeks. In STAR*D, the mean response time and time to remission for patients receiving T3 augmentation was 6 weeks and 6.6 weeks, respectively. However, 28% of patients did not achieve remission until week 14,3 which highlights the need for an adequate trial of T3.
Disclosures
Dr. Casher is a speaker for AstraZeneca, Pfizer Inc, and Sunovion Pharmaceuticals.
Drs. Gih and Bostwick report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Altshuler LL, Bauer M, Frye MA, et al. Does thyroid supplementation accelerate tricyclic antidepressant response? A review and meta-analysis of the literature. Am J Psychiatry. 2001;158:1617-1622.
2. Abraham G, Milev R, Stuart Lawson J. T3 augmentation of SSRI resistant depression. J Affect Disord. 2006;91:211-215.
3. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163:1519-1530.
4. Thase ME. Therapeutic alternatives for difficult-to-treat depression: a narrative review of the state of the evidence. CNS Spectr. 2004;9:808-816, 818-821.
5. Heeringa J, Hoogendoorn EH, van der Deure WM, et al. High-normal thyroid function and risk of atrial fibrillation: the Rotterdam study. Arch Intern Med. 2008;168:2219-2224.
6. Bauer M, Fairbanks L, Berghöfer A, et al. Bone mineral density during maintenance treatment with supraphysiological doses of levothyroxine in affective disorders: a longitudinal study. J Affect Disord. 2004;83:183-190.
7. Micromedex Healthcare Series [database online] Greenwood Village CO: Thomson Healthcare; 2010.
8. Yamamoto T, Fukuyama J, Fujiyoshi A. Factors associated with mortality of myxedema coma: report of eight cases and literature survey. Thyroid. 1999;9:1167-1174.
9. Devdhar M, Ousman YH, Burman KD. Hypothyroidism. Endocrinol Metab Clin North Am. 2007;36:595-615 v.
1. Altshuler LL, Bauer M, Frye MA, et al. Does thyroid supplementation accelerate tricyclic antidepressant response? A review and meta-analysis of the literature. Am J Psychiatry. 2001;158:1617-1622.
2. Abraham G, Milev R, Stuart Lawson J. T3 augmentation of SSRI resistant depression. J Affect Disord. 2006;91:211-215.
3. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163:1519-1530.
4. Thase ME. Therapeutic alternatives for difficult-to-treat depression: a narrative review of the state of the evidence. CNS Spectr. 2004;9:808-816, 818-821.
5. Heeringa J, Hoogendoorn EH, van der Deure WM, et al. High-normal thyroid function and risk of atrial fibrillation: the Rotterdam study. Arch Intern Med. 2008;168:2219-2224.
6. Bauer M, Fairbanks L, Berghöfer A, et al. Bone mineral density during maintenance treatment with supraphysiological doses of levothyroxine in affective disorders: a longitudinal study. J Affect Disord. 2004;83:183-190.
7. Micromedex Healthcare Series [database online] Greenwood Village CO: Thomson Healthcare; 2010.
8. Yamamoto T, Fukuyama J, Fujiyoshi A. Factors associated with mortality of myxedema coma: report of eight cases and literature survey. Thyroid. 1999;9:1167-1174.
9. Devdhar M, Ousman YH, Burman KD. Hypothyroidism. Endocrinol Metab Clin North Am. 2007;36:595-615 v.
Medically unexplained physical symptoms: Evidence-based interventions
Discuss this article at www.facebook.com/CurrentPsychiatry
Mrs. B, age 45, is referred by her primary care physician (PCP) for treatment of depressive symptoms that have worsened over the last 6 months. Her depressed mood is associated with worsening of multiple chronic, physical symptoms that began 4 years ago with several musculoskeletal complaints. Three years ago she developed recurrent abdominal discomfort and bloating, followed by recurrent chest pain. These symptoms have resulted in multiple trips to the hospital, several invasive procedures, and extensive medical consultation. After repeated workups, her symptoms are medically unexplained. These symptoms interfere with her ability to engage in and enjoy life.
Even after thorough investigation, up to one-third of patients’ physical symptoms remain unexplained.1-3 Most patients with unexplained symptoms improve; however, a small proportion do not. Such patients often are referred for psychiatric consultation.
Workup may reveal psychiatric etiology of a patient’s medically unexplained physical symptoms (MUPS). Clinicians need to take a unique approach to caring for patients whose symptoms remain unexplained after workup because diagnostic features may emerge over time and a collaborative, unbiased, integrated approach eventually may reveal a treatable diagnosis. This type of approach also is important when no medical or psychiatric diagnosis can be reached.
This article reviews the prevalence, comorbidity, and treatment challenges of patients whose physical symptoms are medically unexplained, and recommends evidence-based treatment strategies.
Inconsistent terminology
The terms MUPS, medically unexplained symptoms, somatoform disorder, somatization, and the functional syndromes (eg, irritable bowel syndrome [IBS], fibromyalgia, interstitial cystitis, chronic fatigue, etc.) often are used interchangeably. This inconsistent nomenclature creates classification difficulties because several of these terms assume a different etiology for the patient’s physical symptoms (ie, medical vs psychiatric).
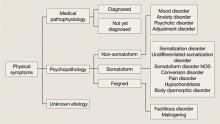
Physical symptoms typically are explained by:
- medical pathophysiology
- psychopathology, or
- unknown etiology (Figure).
MUPS typically are defined as physical or somatic symptoms without a known etiology after appropriate testing, workup, and referrals. Workup may be limited or extensive, may evolve over time (eg, diagnosis may be made 2 years after primary symptom onset), and often involves collaboration among several specialists.
The above definition does not specify severity, medical/psychiatric comorbidity, or number or duration of symptoms. A proposed classification, medically unexplained symptoms spectrum disorder, attempts to categorize patients with MUPS based on severity and duration of symptoms, as well as medical and/or psychiatric comorbidities (Table).4 MUPS may account for up to two-thirds of physical symptoms in specialty clinics; to read about the prevalence of MUPS, see the Box below.
Figure: Typical etiology of physical symptoms
NOS: Not otherwise specifiedTable
Medically unexplained symptoms spectrum disorder
| Severity | Mild, moderate, severe |
| Duration | Acute (days to weeks), subacute (<6 months), chronic (>6 months) |
| Comorbidity | Psychiatric, medical, none |
| Source: Reference 4 | |
CASE CONTINUED: Abuse and assault
Mrs. B has been married for 10 years and has 2 children. She denies using tobacco, alcohol, or illicit drugs. Both of her parents were in good physical health. As a child Mrs. B was physically and verbally abused by her father, and her parents divorced when she was 15. She was sexually assaulted while in college.
Mrs. B has had 1 previous depressive episode, which occurred shortly after the sexual assault. During that time she was hospitalized twice for attempting suicide by overdosing on prescription medications. She was stabilized on fluoxetine, 40 mg/d, which was tapered and discontinued after 2 years.
Factors linked to MUPS
Young women (age 16 to 25) are more likely to receive an MUPS diagnosis than men or older individuals. Employment, socioeconomic background, and educational level are not consistently associated with MUPS.5 Patients with MUPS have higher rates of physical and sexual abuse.6
Several studies have shown an association with childhood parental ill health and development of MUPS, but the exact nature of “ill health” was not clearly defined. Parental death was not associated with MUPS, which suggests that the association to parental ill health is related to non-threatening physical disease.6
Approximately 60% of patients with MUPS have a comorbid non-somatoform DSM-IV-TR diagnosis.7-9 Symptoms and rates of depressive, anxiety, and panic disorders are higher in patients with MUPS than either healthy controls or patients with similar diseases of known organic pathology.7,8
The estimated prevalence of somatoform disorder in patients with MUPS is approximately 4%, which is higher than in the general population (.2% to 2%).7,8 On measures of mental and physical function, patients with MUPS who have somatoform disorders have been found to be more distressed than normal controls and patients with MUPS without somatoform disorders.8
Although few studies have directly examined the relationship between personality disorders and MUPS, there is evidence of an association between certain personality traits (eg, neuroticism, alexithymia, negative affect) and MUPS.10,11
CASE CONTINUED: Rejected advice
Mrs. B has been worked up multiple times for acute coronary syndrome; been unsuccessfully treated for gastroesophageal reflux disease, lactose intolerance, and IBS; had a negative rheumatologic workup; and tried several medication regimens with no improvement in symptoms. Three years ago Mrs. B’s gastroenterologist implied her abdominal symptoms were caused by her history of sexual assault and suggested she seek psychiatric consultation. Offended, Mrs. B sought a second opinion and no longer sees her first gastroenterologist.
Barriers to treatment
Despite having high levels of psychosocial distress, health care utilization, and medical disability, patients with MUPS often are suboptimally treated. Factors that might contribute to this include:
- inadequate identification
- bias in diagnosis and treatment
- poor follow-up on referrals
- an absence of treatment guidelines.7,12,13
Many clinicians are unaware of the high prevalence of MUPS, which often leads to repeated referral to specialty clinics, even when patients already have received an MUPS diagnosis.12,14 Additionally, clinicians often are unaware of how individual biases influence their diagnostic thought process. A “difficult patient” may receive a MUPS diagnosis more readily than a “pleasant patient,” which could contribute to an incomplete workup. An epidemiologic study revealed that the strongest predictor of misdiagnosing MUPS is doctor dissatisfaction with the clinical encounter.15 Younger, unmarried, anxious patients receiving disability benefits are more likely to be incorrectly labeled as having MUPS, only to later receive a non-MUPS diagnosis.15
Bias in treatment and intervention also exists. Qualitative analysis of consultations suggests that physicians’ decisions to offer patients somatic treatments (eg, investigation, add/change medications, referral to specialists) are responses to patients’ extended and complex accounts of their symptoms.17 The likelihood of intervention was unrelated to patients’ request for treatment, and intervention became less likely when patients described psychosocial problems.16
Patients with MUPS and comorbid psychiatric disorders often are referred for psychosocial treatment, but 1 study found that as few as 10% of such patients follow up on a referral.17 In that study, 81% of MUPS patients were willing to receive psychosocial treatment in a primary care setting by their physician. Although there are many reasons patients with MUPS resist referral to mental health professionals, be aware that many of these individuals do not attribute their symptoms to psychosocial problems or experience their symptoms psychologically. To these patients, psychiatric referral may seem inappropriate or be perceived as belittling and minimizing their symptoms.
CASE CONTINUED: Frustration and guilt
Mrs. B’s depressive symptoms began 18 months ago with fatigue, poor sleep, and withdrawal from her children and husband. She struggles with hopelessness that her physical symptoms will not resolve and guilt because of the financial strain her medical care has placed on the family. She is extremely frustrated that her doctors are unable to find a medical diagnosis for her symptoms and fears that without a diagnosis she will be perceived as “crazy.” She is not certain if there is a medical explanation for her symptoms but vehemently believes they are not associated with her mood or psychosocial stress.
Treatment strategies
A collaborative, unbiased, integrated approach to treatment can address some of the challenges that arise when patients with MUPS confront the limitations of modern medicine. Integrated care involves ongoing communication among medical and psychiatric specialists, as well as collaboration with social workers, physical therapists, nutritionists, or pain management specialists when indicated.
Although the primary care provider often coordinates a MUPS patient’s medical treatment, a consulting psychiatrist plays an important educational, diagnostic, and therapeutic role. The therapeutic role is especially important because patients with MUPS frequently view their general practitioner as having a limited role in managing psychosocial problems.18
Because physical illness and psychosocial stress frequently coexist and compound each other, diagnostic efforts should focus on medical and psychiatric illness. Review the patient’s medical workup of the unexplained symptoms and, when indicated, request further testing. Evaluate the risks and benefits of additional testing and discuss them with the patient; additional testing carries a risk of iatrogenic harm, higher false-positive rates, and increased costs. Avoiding iatrogenic harm and unnecessary, overly aggressive testing is essential.
Identifying primary or comorbid psychiatric disease and psychosocial issues also is integral to managing patients with MUPS. This may be difficult because some patients might be hesitant to discuss psychosocial issues, whereas others may be unaware of psychiatric symptomatology or the connection between mental and physical illness. When possible, it may be useful to clarify symptomatology as:
- primarily somatic (expression of psychological illness through physical means)
- primarily psychiatric (psychiatric illness presenting with physical symptoms) or
- bordering between somatic and psychiatric.
CASE CONTINUED: Collaboration and improvement
You diagnose Mrs. B with major depressive disorder and prescribe fluoxetine, titrating her up to 40 mg/d. Mrs. B also begins weekly psychodynamic psychotherapy. In collaboration with her PCP, you decide to refer Mrs. B to physical therapy and direct psychotherapy toward coping strategies, with the hope of improving functionality. Although she continues to have musculoskeletal symptoms after completing physical therapy, Mrs. B notices moderate improvement and feels less distressed by these symptoms.
After 1 year of fluoxetine treatment, Mrs. B’s depressive symptoms improve. In psychotherapy, her fixation on physical symptoms and desire to establish a diagnosis gradually lessen. As her emotional trauma from childhood abuse unravels, psychotherapy shifts toward improving affect regulation. During this time Mrs. B experiences an increase in unexplained chest pain and shortness of breath, which later abate.
Continued follow-up with a gastroenterologist leads to a diagnosis of celiac disease. With treatment, her GI symptoms resolve.
What do patients want?
Begin MUPS treatment by developing a supportive, empathic relationship with the patient. Carefully listen to the patient’s description of his or her symptoms. Elucidating patients’ experience often is challenging because their narratives frequently are complex, nonlinear, and limited by time.18 Patients’ models for understanding their symptoms also may be complex.18 They may be reluctant to share their explanations, fearing they will be unable to communicate the complexity of their beliefs or their symptoms will be oversimplified.18
Focus on understanding what the patient seeks from the physician—emotional support vs diagnosis vs treatment. In a prospective naturalistic study, the content of MUPS patients’ narratives was correlated with what they sought from their physician.17 Patients who sought emotional support frequently discussed psychosocial problems, issues, and management. Patients who wanted an explanation for their symptoms often mentioned physical symptoms, explanations, and diseases. Those who were looking for additional testing or intervention often directly addressed this with the physician.17
Although many patients desire a diagnosis and somatic treatment, this is not always their primary agenda. Many MUPS patients seek emotional support or confirmation of their explanatory model.17,18 Patients’ desires for emotional support, medical explanation, diagnosis, or somatic intervention often are neither clearly nor explicitly stated. Despite this, patients hope their physician understands the extent of their problems and value those who help them make sense of their narratives.18 Misunderstanding patients’ agendas can result in a mismatch of treatment expectations and fracture the patient-physician relationship. Developing mutual expectations is crucial to building rapport, improving collaborative care, and avoiding unnecessary, potentially harmful interventions.
Psychotherapic interventions
Psychopharmacologic treatment is indicated for MUPS patients who have comorbid psychiatric conditions.
Research of psychotherapy in MUPS has been plagued by methodologic problems and inconsistent results.3 Group therapy, short-term dynamic therapy, hypnotherapy, and cognitive-behavioral therapy (CBT) have been studied. In a trial of 140 MUPS patients who received 1 session of CBT, 71% experienced improvement in physical symptoms, 47% in functional status, and 38% in measures of psychological distress.19 A review of 34 randomized controlled trials involving 3,922 patients with somatoform disorders who received CBT found that some patients with MUPS responded after 5 to 6 sessions.3
Cognitive techniques focus on identifying and restructuring automatic, dysfunctional thoughts that may compound, perpetuate, or worsen somatic symptoms. Behavioral techniques include relaxation and efforts to increase motivation. A CBT treatment plan may involve establishing goals, addressing patients’ understanding of their symptoms, obtaining a commitment for treatment, and negotiating the details of the treatment plan.8,12
Supportive techniques also are valuable in treating MUPS patients. Educate patients and treating physicians that there is a neurophysiologic basis for the patient’s physical symptoms and that symptoms may wax and wane. Reinforcement of functional improvement through concrete, practical solutions can help patients develop healthy, adaptive coping skills. Encouraging patients to move beyond somatic complaints to discuss social and personal difficulties can lead to more effective management of these problems.
Clearly communicate your initial impressions, diagnoses, and treatment plan to other members of the treatment team. A consultation letter from the psychiatrist to the PCP has been shown to decrease costs and slightly improve the patient’s functional status, symptoms, and quality of life.20 When possible, educate the PCP and specialists about the dynamics, challenges, biases, and frustrations physicians commonly face when caring for MUPS patients.
Related Resources
- Burton C. Beyond somatization: a review of the understanding and treatment of medically unexplained physical symptoms. Brit J Gen Pract. 2003;53:233-239.
- Creed F. The outcome of medically unexplained symptoms—will DSM-V improve on DSM-IV somatoform disorders? J Psychosom Res. 2009;66:379-381.
- Katon W, Sullivan M, Walker E. Medical symptoms without identified pathology: relationship to psychiatric disorders, childhood and adult trauma, and personality traits. Ann Intern Med. 2001;134(9 Pt 2):917-925.
- Sharpe M, Mayou R, Walker J. Bodily symptoms: new approaches to classification. J Psychosom Res. 2006;60: 353-356.
Drug Brand Names
- Fluoxetine • Prozac
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Medically unexplained physical symptoms (MUPS) has been found to make up 10% to 30% of the physical symptoms in primary care clinics and 37% to 66% in specialty clinics.a-c The latter statistic is based on a cross-sectional survey of 899 consecutive new patients from 7 outpatient clinics in London, United Kingdom. Sixty-five percent responded and 52% of respondents had at least 1 medically unexplained symptom, diagnosed 3 months after initial clinic presentation.c
Patients with MUPS carry significant clinical importance. They are more likely to have a relatively poor quality of life and higher rates of disability.d,e They tend to be higher utilizers of health care.c,f High utilization of services and potentially unnecessary lab testing and consultation result in increased costs and high rates of iatrogenic complications.d-f
References
a. McCarron RM, Xiong GL, Bourgeois JA. Lippincott’s primary care psychiatry. Philadelphia, PA: Lippincott Williams and Wilkins; 2009.
b. Richardson RD, Engel CC. Evaluation and management of medically unexplained physical symptoms. Neurologist. 2004;10:18-30.
c. Nimnuan C, Hotopf M, Wessely S. Medically unexplained symptoms. An epidemiological study in seven specialties. J Psychosom Res. 2001;51:361-367.
d. Reid S, Wessely S, Crayford T, et al. Medically unexplained symptoms in frequent attenders of secondary health care: retrospective cohort study. BMJ. 2001;322:1-4.
e. Smith BJ, McGorn KJ, Weller D, et al. The identification in primary care of patients who have been repeatedly referred to hospital for medically unexplained symptoms: a pilot study. J Psychosom Res. 2009;67:207-211.
f. Smith RC, Lein C, Collins C, et al. Treating patients with medically unexplained symptoms in primary care. J Gen Intern Med. 2003;18:478-489.
1. Jackson JL, Kroenke K. Prevalence impact, and prognosis of multisomatoform disorder in primary care: a 5-year follow-up study. Psychosom Med. 2008;70:430-434.
2. Jackson JL, George S, Hinchey S. Medically unexplained physical symptoms. J Gen Intern Med. 2009;24:540-542.
3. Kroenke K. Efficacy of treatment for somatoform disorders: a review of randomized controlled trials. Psychosom Med. 2007;69:881-888.
4. Smith RC, Dwamena FC. Classification and diagnosis of patients with medically unexplained symptoms. J Gen Intern Med. 2007;22:685-691.
5. Nimnuan C, Hotopf M, Wessely S. Medically unexplained symptoms. An epidemiological study in seven specialties. J Psychosom Res. 2001;51:361-367.
6. Hotopf M, Mayou R, Wadsworth M, et al. Childhood risk factors for adults with medically unexplained symptoms: results from a national birth cohort study. Am J Psychiatry. 1999;156(11):1796-1800.
7. Smith RC, Gardiner JC, Lyles JS, et al. Exploration of DSM-IV criteria in primary care patients with medically unexplained symptoms. Psychosom Med. 2005;67:123-129.
8. Smith RC, Lyles JS, Gardiner JC, et al. Primary care clinicians treat patients with medically unexplained symptoms: a randomized controlled trial. J Gen Intern Med. 2006;21:671-677.
9. Henningsen P, Zimmermann T, Sattel H. Medically unexplained physical symptoms anxiety, and depression. A meta-analytic review. Psychosom Med. 2003;65:528-533.
10. Costa PT, McCrae RR. Neuroticism somatic complaints, and disease: is the bark worse than the bite? J Pers. 1987;55(2):299-315.
11. Gucht VD, Fischler B, Heiser W. Personality and affect as determinants of medically unexplained symptoms in primary care. A follow-up study. J Psychosom Res. 2004;56:279-285.
12. Smith RC, Lein C, Collins C, et al. Treating patients with medically unexplained symptoms in primary care. J Gen Intern Med. 2003;18:478-489.
13. McFarlane AC, Ellis N, Fasphm F, et al. The conundrum of medically unexplained symptoms: questions to consider. Psychosomatics. 2008;49(5):369-377.
14. Reid S, Wessely S, Crayford T, et al. Medically unexplained symptoms in frequent attenders of secondary health care: retrospective cohort study. BMJ. 2001;322:1-4.
15. Nimnuan C, Hotopf M, Wessely S. Medically unexplained symptoms: how often and why are they missed? QJM. 2000;93:21-28.
16. Salmon P, Humphris GM, Ring A, et al. Primary care consultations about medically unexplained symptoms: patient presentations and doctor responses that influence the probability of somatic intervention. Psychosom Med. 2007;69:571-577.
17. Salmon P, Ring A, Humphris GM, et al. Primary care consultations about medically unexplained symptoms: how do patients indicate what they want? J Gen Intern Med. 2009;24(4):450-456.
18. Peters S, Rogers A, Salmon P, et al. What do patients choose to tell their doctors? Qualitative analysis of potential barriers to reattributing medically unexplained symptoms. J Gen Intern Med. 2008;24(4):443-449.
19. Martin A, Rauh E, Fichter M, et al. A one-session treatment for patients suffering from medically unexplained symptoms in primary care: a randomized clinical trial. Psychosomatics. 2007;48:294-303.
20. Smith BJ, McGorn KJ, Weller D, et al. The identification in primary care of patients who have been repeatedly referred to hospital for medically unexplained symptoms: a pilot study. J Psychosom Res. 2009;67:207-211.
Discuss this article at www.facebook.com/CurrentPsychiatry
Mrs. B, age 45, is referred by her primary care physician (PCP) for treatment of depressive symptoms that have worsened over the last 6 months. Her depressed mood is associated with worsening of multiple chronic, physical symptoms that began 4 years ago with several musculoskeletal complaints. Three years ago she developed recurrent abdominal discomfort and bloating, followed by recurrent chest pain. These symptoms have resulted in multiple trips to the hospital, several invasive procedures, and extensive medical consultation. After repeated workups, her symptoms are medically unexplained. These symptoms interfere with her ability to engage in and enjoy life.
Even after thorough investigation, up to one-third of patients’ physical symptoms remain unexplained.1-3 Most patients with unexplained symptoms improve; however, a small proportion do not. Such patients often are referred for psychiatric consultation.
Workup may reveal psychiatric etiology of a patient’s medically unexplained physical symptoms (MUPS). Clinicians need to take a unique approach to caring for patients whose symptoms remain unexplained after workup because diagnostic features may emerge over time and a collaborative, unbiased, integrated approach eventually may reveal a treatable diagnosis. This type of approach also is important when no medical or psychiatric diagnosis can be reached.
This article reviews the prevalence, comorbidity, and treatment challenges of patients whose physical symptoms are medically unexplained, and recommends evidence-based treatment strategies.
Inconsistent terminology
The terms MUPS, medically unexplained symptoms, somatoform disorder, somatization, and the functional syndromes (eg, irritable bowel syndrome [IBS], fibromyalgia, interstitial cystitis, chronic fatigue, etc.) often are used interchangeably. This inconsistent nomenclature creates classification difficulties because several of these terms assume a different etiology for the patient’s physical symptoms (ie, medical vs psychiatric).
Physical symptoms typically are explained by:
- medical pathophysiology
- psychopathology, or
- unknown etiology (Figure).
MUPS typically are defined as physical or somatic symptoms without a known etiology after appropriate testing, workup, and referrals. Workup may be limited or extensive, may evolve over time (eg, diagnosis may be made 2 years after primary symptom onset), and often involves collaboration among several specialists.
The above definition does not specify severity, medical/psychiatric comorbidity, or number or duration of symptoms. A proposed classification, medically unexplained symptoms spectrum disorder, attempts to categorize patients with MUPS based on severity and duration of symptoms, as well as medical and/or psychiatric comorbidities (Table).4 MUPS may account for up to two-thirds of physical symptoms in specialty clinics; to read about the prevalence of MUPS, see the Box below.
Figure: Typical etiology of physical symptoms
NOS: Not otherwise specifiedTable
Medically unexplained symptoms spectrum disorder
| Severity | Mild, moderate, severe |
| Duration | Acute (days to weeks), subacute (<6 months), chronic (>6 months) |
| Comorbidity | Psychiatric, medical, none |
| Source: Reference 4 | |
CASE CONTINUED: Abuse and assault
Mrs. B has been married for 10 years and has 2 children. She denies using tobacco, alcohol, or illicit drugs. Both of her parents were in good physical health. As a child Mrs. B was physically and verbally abused by her father, and her parents divorced when she was 15. She was sexually assaulted while in college.
Mrs. B has had 1 previous depressive episode, which occurred shortly after the sexual assault. During that time she was hospitalized twice for attempting suicide by overdosing on prescription medications. She was stabilized on fluoxetine, 40 mg/d, which was tapered and discontinued after 2 years.
Factors linked to MUPS
Young women (age 16 to 25) are more likely to receive an MUPS diagnosis than men or older individuals. Employment, socioeconomic background, and educational level are not consistently associated with MUPS.5 Patients with MUPS have higher rates of physical and sexual abuse.6
Several studies have shown an association with childhood parental ill health and development of MUPS, but the exact nature of “ill health” was not clearly defined. Parental death was not associated with MUPS, which suggests that the association to parental ill health is related to non-threatening physical disease.6
Approximately 60% of patients with MUPS have a comorbid non-somatoform DSM-IV-TR diagnosis.7-9 Symptoms and rates of depressive, anxiety, and panic disorders are higher in patients with MUPS than either healthy controls or patients with similar diseases of known organic pathology.7,8
The estimated prevalence of somatoform disorder in patients with MUPS is approximately 4%, which is higher than in the general population (.2% to 2%).7,8 On measures of mental and physical function, patients with MUPS who have somatoform disorders have been found to be more distressed than normal controls and patients with MUPS without somatoform disorders.8
Although few studies have directly examined the relationship between personality disorders and MUPS, there is evidence of an association between certain personality traits (eg, neuroticism, alexithymia, negative affect) and MUPS.10,11
CASE CONTINUED: Rejected advice
Mrs. B has been worked up multiple times for acute coronary syndrome; been unsuccessfully treated for gastroesophageal reflux disease, lactose intolerance, and IBS; had a negative rheumatologic workup; and tried several medication regimens with no improvement in symptoms. Three years ago Mrs. B’s gastroenterologist implied her abdominal symptoms were caused by her history of sexual assault and suggested she seek psychiatric consultation. Offended, Mrs. B sought a second opinion and no longer sees her first gastroenterologist.
Barriers to treatment
Despite having high levels of psychosocial distress, health care utilization, and medical disability, patients with MUPS often are suboptimally treated. Factors that might contribute to this include:
- inadequate identification
- bias in diagnosis and treatment
- poor follow-up on referrals
- an absence of treatment guidelines.7,12,13
Many clinicians are unaware of the high prevalence of MUPS, which often leads to repeated referral to specialty clinics, even when patients already have received an MUPS diagnosis.12,14 Additionally, clinicians often are unaware of how individual biases influence their diagnostic thought process. A “difficult patient” may receive a MUPS diagnosis more readily than a “pleasant patient,” which could contribute to an incomplete workup. An epidemiologic study revealed that the strongest predictor of misdiagnosing MUPS is doctor dissatisfaction with the clinical encounter.15 Younger, unmarried, anxious patients receiving disability benefits are more likely to be incorrectly labeled as having MUPS, only to later receive a non-MUPS diagnosis.15
Bias in treatment and intervention also exists. Qualitative analysis of consultations suggests that physicians’ decisions to offer patients somatic treatments (eg, investigation, add/change medications, referral to specialists) are responses to patients’ extended and complex accounts of their symptoms.17 The likelihood of intervention was unrelated to patients’ request for treatment, and intervention became less likely when patients described psychosocial problems.16
Patients with MUPS and comorbid psychiatric disorders often are referred for psychosocial treatment, but 1 study found that as few as 10% of such patients follow up on a referral.17 In that study, 81% of MUPS patients were willing to receive psychosocial treatment in a primary care setting by their physician. Although there are many reasons patients with MUPS resist referral to mental health professionals, be aware that many of these individuals do not attribute their symptoms to psychosocial problems or experience their symptoms psychologically. To these patients, psychiatric referral may seem inappropriate or be perceived as belittling and minimizing their symptoms.
CASE CONTINUED: Frustration and guilt
Mrs. B’s depressive symptoms began 18 months ago with fatigue, poor sleep, and withdrawal from her children and husband. She struggles with hopelessness that her physical symptoms will not resolve and guilt because of the financial strain her medical care has placed on the family. She is extremely frustrated that her doctors are unable to find a medical diagnosis for her symptoms and fears that without a diagnosis she will be perceived as “crazy.” She is not certain if there is a medical explanation for her symptoms but vehemently believes they are not associated with her mood or psychosocial stress.
Treatment strategies
A collaborative, unbiased, integrated approach to treatment can address some of the challenges that arise when patients with MUPS confront the limitations of modern medicine. Integrated care involves ongoing communication among medical and psychiatric specialists, as well as collaboration with social workers, physical therapists, nutritionists, or pain management specialists when indicated.
Although the primary care provider often coordinates a MUPS patient’s medical treatment, a consulting psychiatrist plays an important educational, diagnostic, and therapeutic role. The therapeutic role is especially important because patients with MUPS frequently view their general practitioner as having a limited role in managing psychosocial problems.18
Because physical illness and psychosocial stress frequently coexist and compound each other, diagnostic efforts should focus on medical and psychiatric illness. Review the patient’s medical workup of the unexplained symptoms and, when indicated, request further testing. Evaluate the risks and benefits of additional testing and discuss them with the patient; additional testing carries a risk of iatrogenic harm, higher false-positive rates, and increased costs. Avoiding iatrogenic harm and unnecessary, overly aggressive testing is essential.
Identifying primary or comorbid psychiatric disease and psychosocial issues also is integral to managing patients with MUPS. This may be difficult because some patients might be hesitant to discuss psychosocial issues, whereas others may be unaware of psychiatric symptomatology or the connection between mental and physical illness. When possible, it may be useful to clarify symptomatology as:
- primarily somatic (expression of psychological illness through physical means)
- primarily psychiatric (psychiatric illness presenting with physical symptoms) or
- bordering between somatic and psychiatric.
CASE CONTINUED: Collaboration and improvement
You diagnose Mrs. B with major depressive disorder and prescribe fluoxetine, titrating her up to 40 mg/d. Mrs. B also begins weekly psychodynamic psychotherapy. In collaboration with her PCP, you decide to refer Mrs. B to physical therapy and direct psychotherapy toward coping strategies, with the hope of improving functionality. Although she continues to have musculoskeletal symptoms after completing physical therapy, Mrs. B notices moderate improvement and feels less distressed by these symptoms.
After 1 year of fluoxetine treatment, Mrs. B’s depressive symptoms improve. In psychotherapy, her fixation on physical symptoms and desire to establish a diagnosis gradually lessen. As her emotional trauma from childhood abuse unravels, psychotherapy shifts toward improving affect regulation. During this time Mrs. B experiences an increase in unexplained chest pain and shortness of breath, which later abate.
Continued follow-up with a gastroenterologist leads to a diagnosis of celiac disease. With treatment, her GI symptoms resolve.
What do patients want?
Begin MUPS treatment by developing a supportive, empathic relationship with the patient. Carefully listen to the patient’s description of his or her symptoms. Elucidating patients’ experience often is challenging because their narratives frequently are complex, nonlinear, and limited by time.18 Patients’ models for understanding their symptoms also may be complex.18 They may be reluctant to share their explanations, fearing they will be unable to communicate the complexity of their beliefs or their symptoms will be oversimplified.18
Focus on understanding what the patient seeks from the physician—emotional support vs diagnosis vs treatment. In a prospective naturalistic study, the content of MUPS patients’ narratives was correlated with what they sought from their physician.17 Patients who sought emotional support frequently discussed psychosocial problems, issues, and management. Patients who wanted an explanation for their symptoms often mentioned physical symptoms, explanations, and diseases. Those who were looking for additional testing or intervention often directly addressed this with the physician.17
Although many patients desire a diagnosis and somatic treatment, this is not always their primary agenda. Many MUPS patients seek emotional support or confirmation of their explanatory model.17,18 Patients’ desires for emotional support, medical explanation, diagnosis, or somatic intervention often are neither clearly nor explicitly stated. Despite this, patients hope their physician understands the extent of their problems and value those who help them make sense of their narratives.18 Misunderstanding patients’ agendas can result in a mismatch of treatment expectations and fracture the patient-physician relationship. Developing mutual expectations is crucial to building rapport, improving collaborative care, and avoiding unnecessary, potentially harmful interventions.
Psychotherapic interventions
Psychopharmacologic treatment is indicated for MUPS patients who have comorbid psychiatric conditions.
Research of psychotherapy in MUPS has been plagued by methodologic problems and inconsistent results.3 Group therapy, short-term dynamic therapy, hypnotherapy, and cognitive-behavioral therapy (CBT) have been studied. In a trial of 140 MUPS patients who received 1 session of CBT, 71% experienced improvement in physical symptoms, 47% in functional status, and 38% in measures of psychological distress.19 A review of 34 randomized controlled trials involving 3,922 patients with somatoform disorders who received CBT found that some patients with MUPS responded after 5 to 6 sessions.3
Cognitive techniques focus on identifying and restructuring automatic, dysfunctional thoughts that may compound, perpetuate, or worsen somatic symptoms. Behavioral techniques include relaxation and efforts to increase motivation. A CBT treatment plan may involve establishing goals, addressing patients’ understanding of their symptoms, obtaining a commitment for treatment, and negotiating the details of the treatment plan.8,12
Supportive techniques also are valuable in treating MUPS patients. Educate patients and treating physicians that there is a neurophysiologic basis for the patient’s physical symptoms and that symptoms may wax and wane. Reinforcement of functional improvement through concrete, practical solutions can help patients develop healthy, adaptive coping skills. Encouraging patients to move beyond somatic complaints to discuss social and personal difficulties can lead to more effective management of these problems.
Clearly communicate your initial impressions, diagnoses, and treatment plan to other members of the treatment team. A consultation letter from the psychiatrist to the PCP has been shown to decrease costs and slightly improve the patient’s functional status, symptoms, and quality of life.20 When possible, educate the PCP and specialists about the dynamics, challenges, biases, and frustrations physicians commonly face when caring for MUPS patients.
Related Resources
- Burton C. Beyond somatization: a review of the understanding and treatment of medically unexplained physical symptoms. Brit J Gen Pract. 2003;53:233-239.
- Creed F. The outcome of medically unexplained symptoms—will DSM-V improve on DSM-IV somatoform disorders? J Psychosom Res. 2009;66:379-381.
- Katon W, Sullivan M, Walker E. Medical symptoms without identified pathology: relationship to psychiatric disorders, childhood and adult trauma, and personality traits. Ann Intern Med. 2001;134(9 Pt 2):917-925.
- Sharpe M, Mayou R, Walker J. Bodily symptoms: new approaches to classification. J Psychosom Res. 2006;60: 353-356.
Drug Brand Names
- Fluoxetine • Prozac
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Medically unexplained physical symptoms (MUPS) has been found to make up 10% to 30% of the physical symptoms in primary care clinics and 37% to 66% in specialty clinics.a-c The latter statistic is based on a cross-sectional survey of 899 consecutive new patients from 7 outpatient clinics in London, United Kingdom. Sixty-five percent responded and 52% of respondents had at least 1 medically unexplained symptom, diagnosed 3 months after initial clinic presentation.c
Patients with MUPS carry significant clinical importance. They are more likely to have a relatively poor quality of life and higher rates of disability.d,e They tend to be higher utilizers of health care.c,f High utilization of services and potentially unnecessary lab testing and consultation result in increased costs and high rates of iatrogenic complications.d-f
References
a. McCarron RM, Xiong GL, Bourgeois JA. Lippincott’s primary care psychiatry. Philadelphia, PA: Lippincott Williams and Wilkins; 2009.
b. Richardson RD, Engel CC. Evaluation and management of medically unexplained physical symptoms. Neurologist. 2004;10:18-30.
c. Nimnuan C, Hotopf M, Wessely S. Medically unexplained symptoms. An epidemiological study in seven specialties. J Psychosom Res. 2001;51:361-367.
d. Reid S, Wessely S, Crayford T, et al. Medically unexplained symptoms in frequent attenders of secondary health care: retrospective cohort study. BMJ. 2001;322:1-4.
e. Smith BJ, McGorn KJ, Weller D, et al. The identification in primary care of patients who have been repeatedly referred to hospital for medically unexplained symptoms: a pilot study. J Psychosom Res. 2009;67:207-211.
f. Smith RC, Lein C, Collins C, et al. Treating patients with medically unexplained symptoms in primary care. J Gen Intern Med. 2003;18:478-489.
Discuss this article at www.facebook.com/CurrentPsychiatry
Mrs. B, age 45, is referred by her primary care physician (PCP) for treatment of depressive symptoms that have worsened over the last 6 months. Her depressed mood is associated with worsening of multiple chronic, physical symptoms that began 4 years ago with several musculoskeletal complaints. Three years ago she developed recurrent abdominal discomfort and bloating, followed by recurrent chest pain. These symptoms have resulted in multiple trips to the hospital, several invasive procedures, and extensive medical consultation. After repeated workups, her symptoms are medically unexplained. These symptoms interfere with her ability to engage in and enjoy life.
Even after thorough investigation, up to one-third of patients’ physical symptoms remain unexplained.1-3 Most patients with unexplained symptoms improve; however, a small proportion do not. Such patients often are referred for psychiatric consultation.
Workup may reveal psychiatric etiology of a patient’s medically unexplained physical symptoms (MUPS). Clinicians need to take a unique approach to caring for patients whose symptoms remain unexplained after workup because diagnostic features may emerge over time and a collaborative, unbiased, integrated approach eventually may reveal a treatable diagnosis. This type of approach also is important when no medical or psychiatric diagnosis can be reached.
This article reviews the prevalence, comorbidity, and treatment challenges of patients whose physical symptoms are medically unexplained, and recommends evidence-based treatment strategies.
Inconsistent terminology
The terms MUPS, medically unexplained symptoms, somatoform disorder, somatization, and the functional syndromes (eg, irritable bowel syndrome [IBS], fibromyalgia, interstitial cystitis, chronic fatigue, etc.) often are used interchangeably. This inconsistent nomenclature creates classification difficulties because several of these terms assume a different etiology for the patient’s physical symptoms (ie, medical vs psychiatric).
Physical symptoms typically are explained by:
- medical pathophysiology
- psychopathology, or
- unknown etiology (Figure).
MUPS typically are defined as physical or somatic symptoms without a known etiology after appropriate testing, workup, and referrals. Workup may be limited or extensive, may evolve over time (eg, diagnosis may be made 2 years after primary symptom onset), and often involves collaboration among several specialists.
The above definition does not specify severity, medical/psychiatric comorbidity, or number or duration of symptoms. A proposed classification, medically unexplained symptoms spectrum disorder, attempts to categorize patients with MUPS based on severity and duration of symptoms, as well as medical and/or psychiatric comorbidities (Table).4 MUPS may account for up to two-thirds of physical symptoms in specialty clinics; to read about the prevalence of MUPS, see the Box below.
Figure: Typical etiology of physical symptoms
NOS: Not otherwise specifiedTable
Medically unexplained symptoms spectrum disorder
| Severity | Mild, moderate, severe |
| Duration | Acute (days to weeks), subacute (<6 months), chronic (>6 months) |
| Comorbidity | Psychiatric, medical, none |
| Source: Reference 4 | |
CASE CONTINUED: Abuse and assault
Mrs. B has been married for 10 years and has 2 children. She denies using tobacco, alcohol, or illicit drugs. Both of her parents were in good physical health. As a child Mrs. B was physically and verbally abused by her father, and her parents divorced when she was 15. She was sexually assaulted while in college.
Mrs. B has had 1 previous depressive episode, which occurred shortly after the sexual assault. During that time she was hospitalized twice for attempting suicide by overdosing on prescription medications. She was stabilized on fluoxetine, 40 mg/d, which was tapered and discontinued after 2 years.
Factors linked to MUPS
Young women (age 16 to 25) are more likely to receive an MUPS diagnosis than men or older individuals. Employment, socioeconomic background, and educational level are not consistently associated with MUPS.5 Patients with MUPS have higher rates of physical and sexual abuse.6
Several studies have shown an association with childhood parental ill health and development of MUPS, but the exact nature of “ill health” was not clearly defined. Parental death was not associated with MUPS, which suggests that the association to parental ill health is related to non-threatening physical disease.6
Approximately 60% of patients with MUPS have a comorbid non-somatoform DSM-IV-TR diagnosis.7-9 Symptoms and rates of depressive, anxiety, and panic disorders are higher in patients with MUPS than either healthy controls or patients with similar diseases of known organic pathology.7,8
The estimated prevalence of somatoform disorder in patients with MUPS is approximately 4%, which is higher than in the general population (.2% to 2%).7,8 On measures of mental and physical function, patients with MUPS who have somatoform disorders have been found to be more distressed than normal controls and patients with MUPS without somatoform disorders.8
Although few studies have directly examined the relationship between personality disorders and MUPS, there is evidence of an association between certain personality traits (eg, neuroticism, alexithymia, negative affect) and MUPS.10,11
CASE CONTINUED: Rejected advice
Mrs. B has been worked up multiple times for acute coronary syndrome; been unsuccessfully treated for gastroesophageal reflux disease, lactose intolerance, and IBS; had a negative rheumatologic workup; and tried several medication regimens with no improvement in symptoms. Three years ago Mrs. B’s gastroenterologist implied her abdominal symptoms were caused by her history of sexual assault and suggested she seek psychiatric consultation. Offended, Mrs. B sought a second opinion and no longer sees her first gastroenterologist.
Barriers to treatment
Despite having high levels of psychosocial distress, health care utilization, and medical disability, patients with MUPS often are suboptimally treated. Factors that might contribute to this include:
- inadequate identification
- bias in diagnosis and treatment
- poor follow-up on referrals
- an absence of treatment guidelines.7,12,13
Many clinicians are unaware of the high prevalence of MUPS, which often leads to repeated referral to specialty clinics, even when patients already have received an MUPS diagnosis.12,14 Additionally, clinicians often are unaware of how individual biases influence their diagnostic thought process. A “difficult patient” may receive a MUPS diagnosis more readily than a “pleasant patient,” which could contribute to an incomplete workup. An epidemiologic study revealed that the strongest predictor of misdiagnosing MUPS is doctor dissatisfaction with the clinical encounter.15 Younger, unmarried, anxious patients receiving disability benefits are more likely to be incorrectly labeled as having MUPS, only to later receive a non-MUPS diagnosis.15
Bias in treatment and intervention also exists. Qualitative analysis of consultations suggests that physicians’ decisions to offer patients somatic treatments (eg, investigation, add/change medications, referral to specialists) are responses to patients’ extended and complex accounts of their symptoms.17 The likelihood of intervention was unrelated to patients’ request for treatment, and intervention became less likely when patients described psychosocial problems.16
Patients with MUPS and comorbid psychiatric disorders often are referred for psychosocial treatment, but 1 study found that as few as 10% of such patients follow up on a referral.17 In that study, 81% of MUPS patients were willing to receive psychosocial treatment in a primary care setting by their physician. Although there are many reasons patients with MUPS resist referral to mental health professionals, be aware that many of these individuals do not attribute their symptoms to psychosocial problems or experience their symptoms psychologically. To these patients, psychiatric referral may seem inappropriate or be perceived as belittling and minimizing their symptoms.
CASE CONTINUED: Frustration and guilt
Mrs. B’s depressive symptoms began 18 months ago with fatigue, poor sleep, and withdrawal from her children and husband. She struggles with hopelessness that her physical symptoms will not resolve and guilt because of the financial strain her medical care has placed on the family. She is extremely frustrated that her doctors are unable to find a medical diagnosis for her symptoms and fears that without a diagnosis she will be perceived as “crazy.” She is not certain if there is a medical explanation for her symptoms but vehemently believes they are not associated with her mood or psychosocial stress.
Treatment strategies
A collaborative, unbiased, integrated approach to treatment can address some of the challenges that arise when patients with MUPS confront the limitations of modern medicine. Integrated care involves ongoing communication among medical and psychiatric specialists, as well as collaboration with social workers, physical therapists, nutritionists, or pain management specialists when indicated.
Although the primary care provider often coordinates a MUPS patient’s medical treatment, a consulting psychiatrist plays an important educational, diagnostic, and therapeutic role. The therapeutic role is especially important because patients with MUPS frequently view their general practitioner as having a limited role in managing psychosocial problems.18
Because physical illness and psychosocial stress frequently coexist and compound each other, diagnostic efforts should focus on medical and psychiatric illness. Review the patient’s medical workup of the unexplained symptoms and, when indicated, request further testing. Evaluate the risks and benefits of additional testing and discuss them with the patient; additional testing carries a risk of iatrogenic harm, higher false-positive rates, and increased costs. Avoiding iatrogenic harm and unnecessary, overly aggressive testing is essential.
Identifying primary or comorbid psychiatric disease and psychosocial issues also is integral to managing patients with MUPS. This may be difficult because some patients might be hesitant to discuss psychosocial issues, whereas others may be unaware of psychiatric symptomatology or the connection between mental and physical illness. When possible, it may be useful to clarify symptomatology as:
- primarily somatic (expression of psychological illness through physical means)
- primarily psychiatric (psychiatric illness presenting with physical symptoms) or
- bordering between somatic and psychiatric.
CASE CONTINUED: Collaboration and improvement
You diagnose Mrs. B with major depressive disorder and prescribe fluoxetine, titrating her up to 40 mg/d. Mrs. B also begins weekly psychodynamic psychotherapy. In collaboration with her PCP, you decide to refer Mrs. B to physical therapy and direct psychotherapy toward coping strategies, with the hope of improving functionality. Although she continues to have musculoskeletal symptoms after completing physical therapy, Mrs. B notices moderate improvement and feels less distressed by these symptoms.
After 1 year of fluoxetine treatment, Mrs. B’s depressive symptoms improve. In psychotherapy, her fixation on physical symptoms and desire to establish a diagnosis gradually lessen. As her emotional trauma from childhood abuse unravels, psychotherapy shifts toward improving affect regulation. During this time Mrs. B experiences an increase in unexplained chest pain and shortness of breath, which later abate.
Continued follow-up with a gastroenterologist leads to a diagnosis of celiac disease. With treatment, her GI symptoms resolve.
What do patients want?
Begin MUPS treatment by developing a supportive, empathic relationship with the patient. Carefully listen to the patient’s description of his or her symptoms. Elucidating patients’ experience often is challenging because their narratives frequently are complex, nonlinear, and limited by time.18 Patients’ models for understanding their symptoms also may be complex.18 They may be reluctant to share their explanations, fearing they will be unable to communicate the complexity of their beliefs or their symptoms will be oversimplified.18
Focus on understanding what the patient seeks from the physician—emotional support vs diagnosis vs treatment. In a prospective naturalistic study, the content of MUPS patients’ narratives was correlated with what they sought from their physician.17 Patients who sought emotional support frequently discussed psychosocial problems, issues, and management. Patients who wanted an explanation for their symptoms often mentioned physical symptoms, explanations, and diseases. Those who were looking for additional testing or intervention often directly addressed this with the physician.17
Although many patients desire a diagnosis and somatic treatment, this is not always their primary agenda. Many MUPS patients seek emotional support or confirmation of their explanatory model.17,18 Patients’ desires for emotional support, medical explanation, diagnosis, or somatic intervention often are neither clearly nor explicitly stated. Despite this, patients hope their physician understands the extent of their problems and value those who help them make sense of their narratives.18 Misunderstanding patients’ agendas can result in a mismatch of treatment expectations and fracture the patient-physician relationship. Developing mutual expectations is crucial to building rapport, improving collaborative care, and avoiding unnecessary, potentially harmful interventions.
Psychotherapic interventions
Psychopharmacologic treatment is indicated for MUPS patients who have comorbid psychiatric conditions.
Research of psychotherapy in MUPS has been plagued by methodologic problems and inconsistent results.3 Group therapy, short-term dynamic therapy, hypnotherapy, and cognitive-behavioral therapy (CBT) have been studied. In a trial of 140 MUPS patients who received 1 session of CBT, 71% experienced improvement in physical symptoms, 47% in functional status, and 38% in measures of psychological distress.19 A review of 34 randomized controlled trials involving 3,922 patients with somatoform disorders who received CBT found that some patients with MUPS responded after 5 to 6 sessions.3
Cognitive techniques focus on identifying and restructuring automatic, dysfunctional thoughts that may compound, perpetuate, or worsen somatic symptoms. Behavioral techniques include relaxation and efforts to increase motivation. A CBT treatment plan may involve establishing goals, addressing patients’ understanding of their symptoms, obtaining a commitment for treatment, and negotiating the details of the treatment plan.8,12
Supportive techniques also are valuable in treating MUPS patients. Educate patients and treating physicians that there is a neurophysiologic basis for the patient’s physical symptoms and that symptoms may wax and wane. Reinforcement of functional improvement through concrete, practical solutions can help patients develop healthy, adaptive coping skills. Encouraging patients to move beyond somatic complaints to discuss social and personal difficulties can lead to more effective management of these problems.
Clearly communicate your initial impressions, diagnoses, and treatment plan to other members of the treatment team. A consultation letter from the psychiatrist to the PCP has been shown to decrease costs and slightly improve the patient’s functional status, symptoms, and quality of life.20 When possible, educate the PCP and specialists about the dynamics, challenges, biases, and frustrations physicians commonly face when caring for MUPS patients.
Related Resources
- Burton C. Beyond somatization: a review of the understanding and treatment of medically unexplained physical symptoms. Brit J Gen Pract. 2003;53:233-239.
- Creed F. The outcome of medically unexplained symptoms—will DSM-V improve on DSM-IV somatoform disorders? J Psychosom Res. 2009;66:379-381.
- Katon W, Sullivan M, Walker E. Medical symptoms without identified pathology: relationship to psychiatric disorders, childhood and adult trauma, and personality traits. Ann Intern Med. 2001;134(9 Pt 2):917-925.
- Sharpe M, Mayou R, Walker J. Bodily symptoms: new approaches to classification. J Psychosom Res. 2006;60: 353-356.
Drug Brand Names
- Fluoxetine • Prozac
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Medically unexplained physical symptoms (MUPS) has been found to make up 10% to 30% of the physical symptoms in primary care clinics and 37% to 66% in specialty clinics.a-c The latter statistic is based on a cross-sectional survey of 899 consecutive new patients from 7 outpatient clinics in London, United Kingdom. Sixty-five percent responded and 52% of respondents had at least 1 medically unexplained symptom, diagnosed 3 months after initial clinic presentation.c
Patients with MUPS carry significant clinical importance. They are more likely to have a relatively poor quality of life and higher rates of disability.d,e They tend to be higher utilizers of health care.c,f High utilization of services and potentially unnecessary lab testing and consultation result in increased costs and high rates of iatrogenic complications.d-f
References
a. McCarron RM, Xiong GL, Bourgeois JA. Lippincott’s primary care psychiatry. Philadelphia, PA: Lippincott Williams and Wilkins; 2009.
b. Richardson RD, Engel CC. Evaluation and management of medically unexplained physical symptoms. Neurologist. 2004;10:18-30.
c. Nimnuan C, Hotopf M, Wessely S. Medically unexplained symptoms. An epidemiological study in seven specialties. J Psychosom Res. 2001;51:361-367.
d. Reid S, Wessely S, Crayford T, et al. Medically unexplained symptoms in frequent attenders of secondary health care: retrospective cohort study. BMJ. 2001;322:1-4.
e. Smith BJ, McGorn KJ, Weller D, et al. The identification in primary care of patients who have been repeatedly referred to hospital for medically unexplained symptoms: a pilot study. J Psychosom Res. 2009;67:207-211.
f. Smith RC, Lein C, Collins C, et al. Treating patients with medically unexplained symptoms in primary care. J Gen Intern Med. 2003;18:478-489.
1. Jackson JL, Kroenke K. Prevalence impact, and prognosis of multisomatoform disorder in primary care: a 5-year follow-up study. Psychosom Med. 2008;70:430-434.
2. Jackson JL, George S, Hinchey S. Medically unexplained physical symptoms. J Gen Intern Med. 2009;24:540-542.
3. Kroenke K. Efficacy of treatment for somatoform disorders: a review of randomized controlled trials. Psychosom Med. 2007;69:881-888.
4. Smith RC, Dwamena FC. Classification and diagnosis of patients with medically unexplained symptoms. J Gen Intern Med. 2007;22:685-691.
5. Nimnuan C, Hotopf M, Wessely S. Medically unexplained symptoms. An epidemiological study in seven specialties. J Psychosom Res. 2001;51:361-367.
6. Hotopf M, Mayou R, Wadsworth M, et al. Childhood risk factors for adults with medically unexplained symptoms: results from a national birth cohort study. Am J Psychiatry. 1999;156(11):1796-1800.
7. Smith RC, Gardiner JC, Lyles JS, et al. Exploration of DSM-IV criteria in primary care patients with medically unexplained symptoms. Psychosom Med. 2005;67:123-129.
8. Smith RC, Lyles JS, Gardiner JC, et al. Primary care clinicians treat patients with medically unexplained symptoms: a randomized controlled trial. J Gen Intern Med. 2006;21:671-677.
9. Henningsen P, Zimmermann T, Sattel H. Medically unexplained physical symptoms anxiety, and depression. A meta-analytic review. Psychosom Med. 2003;65:528-533.
10. Costa PT, McCrae RR. Neuroticism somatic complaints, and disease: is the bark worse than the bite? J Pers. 1987;55(2):299-315.
11. Gucht VD, Fischler B, Heiser W. Personality and affect as determinants of medically unexplained symptoms in primary care. A follow-up study. J Psychosom Res. 2004;56:279-285.
12. Smith RC, Lein C, Collins C, et al. Treating patients with medically unexplained symptoms in primary care. J Gen Intern Med. 2003;18:478-489.
13. McFarlane AC, Ellis N, Fasphm F, et al. The conundrum of medically unexplained symptoms: questions to consider. Psychosomatics. 2008;49(5):369-377.
14. Reid S, Wessely S, Crayford T, et al. Medically unexplained symptoms in frequent attenders of secondary health care: retrospective cohort study. BMJ. 2001;322:1-4.
15. Nimnuan C, Hotopf M, Wessely S. Medically unexplained symptoms: how often and why are they missed? QJM. 2000;93:21-28.
16. Salmon P, Humphris GM, Ring A, et al. Primary care consultations about medically unexplained symptoms: patient presentations and doctor responses that influence the probability of somatic intervention. Psychosom Med. 2007;69:571-577.
17. Salmon P, Ring A, Humphris GM, et al. Primary care consultations about medically unexplained symptoms: how do patients indicate what they want? J Gen Intern Med. 2009;24(4):450-456.
18. Peters S, Rogers A, Salmon P, et al. What do patients choose to tell their doctors? Qualitative analysis of potential barriers to reattributing medically unexplained symptoms. J Gen Intern Med. 2008;24(4):443-449.
19. Martin A, Rauh E, Fichter M, et al. A one-session treatment for patients suffering from medically unexplained symptoms in primary care: a randomized clinical trial. Psychosomatics. 2007;48:294-303.
20. Smith BJ, McGorn KJ, Weller D, et al. The identification in primary care of patients who have been repeatedly referred to hospital for medically unexplained symptoms: a pilot study. J Psychosom Res. 2009;67:207-211.
1. Jackson JL, Kroenke K. Prevalence impact, and prognosis of multisomatoform disorder in primary care: a 5-year follow-up study. Psychosom Med. 2008;70:430-434.
2. Jackson JL, George S, Hinchey S. Medically unexplained physical symptoms. J Gen Intern Med. 2009;24:540-542.
3. Kroenke K. Efficacy of treatment for somatoform disorders: a review of randomized controlled trials. Psychosom Med. 2007;69:881-888.
4. Smith RC, Dwamena FC. Classification and diagnosis of patients with medically unexplained symptoms. J Gen Intern Med. 2007;22:685-691.
5. Nimnuan C, Hotopf M, Wessely S. Medically unexplained symptoms. An epidemiological study in seven specialties. J Psychosom Res. 2001;51:361-367.
6. Hotopf M, Mayou R, Wadsworth M, et al. Childhood risk factors for adults with medically unexplained symptoms: results from a national birth cohort study. Am J Psychiatry. 1999;156(11):1796-1800.
7. Smith RC, Gardiner JC, Lyles JS, et al. Exploration of DSM-IV criteria in primary care patients with medically unexplained symptoms. Psychosom Med. 2005;67:123-129.
8. Smith RC, Lyles JS, Gardiner JC, et al. Primary care clinicians treat patients with medically unexplained symptoms: a randomized controlled trial. J Gen Intern Med. 2006;21:671-677.
9. Henningsen P, Zimmermann T, Sattel H. Medically unexplained physical symptoms anxiety, and depression. A meta-analytic review. Psychosom Med. 2003;65:528-533.
10. Costa PT, McCrae RR. Neuroticism somatic complaints, and disease: is the bark worse than the bite? J Pers. 1987;55(2):299-315.
11. Gucht VD, Fischler B, Heiser W. Personality and affect as determinants of medically unexplained symptoms in primary care. A follow-up study. J Psychosom Res. 2004;56:279-285.
12. Smith RC, Lein C, Collins C, et al. Treating patients with medically unexplained symptoms in primary care. J Gen Intern Med. 2003;18:478-489.
13. McFarlane AC, Ellis N, Fasphm F, et al. The conundrum of medically unexplained symptoms: questions to consider. Psychosomatics. 2008;49(5):369-377.
14. Reid S, Wessely S, Crayford T, et al. Medically unexplained symptoms in frequent attenders of secondary health care: retrospective cohort study. BMJ. 2001;322:1-4.
15. Nimnuan C, Hotopf M, Wessely S. Medically unexplained symptoms: how often and why are they missed? QJM. 2000;93:21-28.
16. Salmon P, Humphris GM, Ring A, et al. Primary care consultations about medically unexplained symptoms: patient presentations and doctor responses that influence the probability of somatic intervention. Psychosom Med. 2007;69:571-577.
17. Salmon P, Ring A, Humphris GM, et al. Primary care consultations about medically unexplained symptoms: how do patients indicate what they want? J Gen Intern Med. 2009;24(4):450-456.
18. Peters S, Rogers A, Salmon P, et al. What do patients choose to tell their doctors? Qualitative analysis of potential barriers to reattributing medically unexplained symptoms. J Gen Intern Med. 2008;24(4):443-449.
19. Martin A, Rauh E, Fichter M, et al. A one-session treatment for patients suffering from medically unexplained symptoms in primary care: a randomized clinical trial. Psychosomatics. 2007;48:294-303.
20. Smith BJ, McGorn KJ, Weller D, et al. The identification in primary care of patients who have been repeatedly referred to hospital for medically unexplained symptoms: a pilot study. J Psychosom Res. 2009;67:207-211.
Harnessing epigenetics for psychiatry
It sounds like science fiction, but it may soon become a reality. The young science of epigenetics is revolutionizing the traditional principles of genetics and weaving them into countless environmental factors, from biologic to behavioral, that can modify and direct gene expression toward health or disease. When nature and nurture collide, epigenetic events occur. The most exciting implications of gene-environment interactions are the endless possibilities to exploit and manipulate epigenetics to prevent or ameliorate psychiatric disorders.
Lamarckism, the discredited “inheritance of acquired characteristics,” was a primitive version of epigenetics proposed by Jean-Baptiste Lamarck (1744-1829), to build on Charles Darwin’s theory of evolution and explain adaptation to the environment. Lamarck’s ideas preceded the discovery of “heredity” introduced by the Austrian monk Gregor Johann Mendel in 1865. The terms “gene” and “genetics” were coined by William Bateson in 1902 and the location of genes within the chromosomes was discovered in 1910 by Thomas Morgan. The term “epigenetics” was coined in 1940 by Conrad Hal Waddington, and this science has accelerated dramatically over the past decade when it was discovered that:
- Certain biologic substances such as histones and methyl groups can attach to the “promoter” region (ie, the switch) of a gene and either silence the gene or turn it on to increase its expression. This explains how >200 distinctly different tissues develop in a fetus although every single cell of those tissues contains the same 23 chromosomes, with a total of approximately 20,000 genes.
- Behaviors or activities by adults may, unbeknownst to them, lead to disease in their yet-to-be-born offspring by switching certain genes on or off, without any change in the structure or sequence of DNA. For example, one study found that men who began to smoke before puberty (when sperm production begins) had offspring with significantly higher body weight than men who did not smoke before puberty.1 Animal studies show that organisms subjected to caloric restriction will live 30% longer than those with normal caloric intake, and their offspring have 20% longer longevity without being subjected to caloric restriction!2 That’s Lamarckism, but a much more sophisticated version than the original.
How does this link to psychiatry? We already have a large body of evidence that the environment can have a substantial deleterious or salutary impact on the development of psychiatric brain disorders. One set of evidence comes from studying that most important environment, the womb. Prenatal, neonatal (obstetric), and postnatal adverse events can disrupt brain development and lead to serious psychopathology, such as schizophrenia. Another type of evidence is seen in childhood abuse (physical, sexual, or verbal), which can lead to sensitization of the HPA axis and result in serious mood and anxiety disorders in adulthood. On the positive side, an enriched environment during infancy can stimulate neuroplasticity, connectivity, and enhanced brain growth.
Imagine what we will be able to do once research elucidates more than the epigenetic changes caused by adverse intrauterine events for a fetus or unhealthy pre-conception behaviors in future parents. Imagine, too, if psychiatric scientists discover parental behaviors that may improve offspring temperament, bolster resilience under stress, blunt impulsivity, suppress antisocial behavior, reduce the susceptibility to addiction(s), enhance cognitive ability, inhibit emergence of psychiatric symptoms, increase motivation, and, importantly, extinguish inherited suicidal or homicidal urges.
Of all medical disciplines, I believe psychiatry stands to benefit the most from advances in what may be designated as the field of “therapeutic epigenetics.” Two reasons justify this assertion:
- A very high proportion (50%) of our 20,000 genes are expressed only in the brain, creating huge opportunities for “interventional epigenetics” to prevent, modify, modulate, or ameliorate psychiatric diseases by silencing abnormal genes (once we identify them) or turbocharging the expression of adaptive genes (once they are identified).
- The brain is the most plastic organ in the body, changing on an ongoing basis by interacting with the environment (ie, the human experiences of everyday life). The unique ongoing neuroplasticity of the brain is driven by gene expression stimulated by environmental stimuli, which can be targeted for therapeutic purposes. In fact, it is likely that psychotherapy and pharmacotherapy exert their efficacy and certain adverse effects via selective gene expression.
Yes, it does sound like science fiction that some day we will be able to capitalize on gene-environment interactions to heal the body, the brain, and the mind, thus circumventing the use of medications. Psychiatric destiny is not in the DNA but in how targeted environmental influences can bring out the best in DNA, that magically malleable matter of life.
1. Pembrey ME, Bygren LO, Kaati G, et al. ALSPAC Study Team. Sex-specific, male-line transgenerational responses in humans. Eur J Hum Genet. 2006;14(2):159-166.
2. Kaneko G, Yoshinaga T, Yanagawa Y, et al. Calorie restriction-induced maternal longevity is transmitted to their daughters in a rotifer. Functional Ecology. 2011;25(1):209-216.
It sounds like science fiction, but it may soon become a reality. The young science of epigenetics is revolutionizing the traditional principles of genetics and weaving them into countless environmental factors, from biologic to behavioral, that can modify and direct gene expression toward health or disease. When nature and nurture collide, epigenetic events occur. The most exciting implications of gene-environment interactions are the endless possibilities to exploit and manipulate epigenetics to prevent or ameliorate psychiatric disorders.
Lamarckism, the discredited “inheritance of acquired characteristics,” was a primitive version of epigenetics proposed by Jean-Baptiste Lamarck (1744-1829), to build on Charles Darwin’s theory of evolution and explain adaptation to the environment. Lamarck’s ideas preceded the discovery of “heredity” introduced by the Austrian monk Gregor Johann Mendel in 1865. The terms “gene” and “genetics” were coined by William Bateson in 1902 and the location of genes within the chromosomes was discovered in 1910 by Thomas Morgan. The term “epigenetics” was coined in 1940 by Conrad Hal Waddington, and this science has accelerated dramatically over the past decade when it was discovered that:
- Certain biologic substances such as histones and methyl groups can attach to the “promoter” region (ie, the switch) of a gene and either silence the gene or turn it on to increase its expression. This explains how >200 distinctly different tissues develop in a fetus although every single cell of those tissues contains the same 23 chromosomes, with a total of approximately 20,000 genes.
- Behaviors or activities by adults may, unbeknownst to them, lead to disease in their yet-to-be-born offspring by switching certain genes on or off, without any change in the structure or sequence of DNA. For example, one study found that men who began to smoke before puberty (when sperm production begins) had offspring with significantly higher body weight than men who did not smoke before puberty.1 Animal studies show that organisms subjected to caloric restriction will live 30% longer than those with normal caloric intake, and their offspring have 20% longer longevity without being subjected to caloric restriction!2 That’s Lamarckism, but a much more sophisticated version than the original.
How does this link to psychiatry? We already have a large body of evidence that the environment can have a substantial deleterious or salutary impact on the development of psychiatric brain disorders. One set of evidence comes from studying that most important environment, the womb. Prenatal, neonatal (obstetric), and postnatal adverse events can disrupt brain development and lead to serious psychopathology, such as schizophrenia. Another type of evidence is seen in childhood abuse (physical, sexual, or verbal), which can lead to sensitization of the HPA axis and result in serious mood and anxiety disorders in adulthood. On the positive side, an enriched environment during infancy can stimulate neuroplasticity, connectivity, and enhanced brain growth.
Imagine what we will be able to do once research elucidates more than the epigenetic changes caused by adverse intrauterine events for a fetus or unhealthy pre-conception behaviors in future parents. Imagine, too, if psychiatric scientists discover parental behaviors that may improve offspring temperament, bolster resilience under stress, blunt impulsivity, suppress antisocial behavior, reduce the susceptibility to addiction(s), enhance cognitive ability, inhibit emergence of psychiatric symptoms, increase motivation, and, importantly, extinguish inherited suicidal or homicidal urges.
Of all medical disciplines, I believe psychiatry stands to benefit the most from advances in what may be designated as the field of “therapeutic epigenetics.” Two reasons justify this assertion:
- A very high proportion (50%) of our 20,000 genes are expressed only in the brain, creating huge opportunities for “interventional epigenetics” to prevent, modify, modulate, or ameliorate psychiatric diseases by silencing abnormal genes (once we identify them) or turbocharging the expression of adaptive genes (once they are identified).
- The brain is the most plastic organ in the body, changing on an ongoing basis by interacting with the environment (ie, the human experiences of everyday life). The unique ongoing neuroplasticity of the brain is driven by gene expression stimulated by environmental stimuli, which can be targeted for therapeutic purposes. In fact, it is likely that psychotherapy and pharmacotherapy exert their efficacy and certain adverse effects via selective gene expression.
Yes, it does sound like science fiction that some day we will be able to capitalize on gene-environment interactions to heal the body, the brain, and the mind, thus circumventing the use of medications. Psychiatric destiny is not in the DNA but in how targeted environmental influences can bring out the best in DNA, that magically malleable matter of life.
It sounds like science fiction, but it may soon become a reality. The young science of epigenetics is revolutionizing the traditional principles of genetics and weaving them into countless environmental factors, from biologic to behavioral, that can modify and direct gene expression toward health or disease. When nature and nurture collide, epigenetic events occur. The most exciting implications of gene-environment interactions are the endless possibilities to exploit and manipulate epigenetics to prevent or ameliorate psychiatric disorders.
Lamarckism, the discredited “inheritance of acquired characteristics,” was a primitive version of epigenetics proposed by Jean-Baptiste Lamarck (1744-1829), to build on Charles Darwin’s theory of evolution and explain adaptation to the environment. Lamarck’s ideas preceded the discovery of “heredity” introduced by the Austrian monk Gregor Johann Mendel in 1865. The terms “gene” and “genetics” were coined by William Bateson in 1902 and the location of genes within the chromosomes was discovered in 1910 by Thomas Morgan. The term “epigenetics” was coined in 1940 by Conrad Hal Waddington, and this science has accelerated dramatically over the past decade when it was discovered that:
- Certain biologic substances such as histones and methyl groups can attach to the “promoter” region (ie, the switch) of a gene and either silence the gene or turn it on to increase its expression. This explains how >200 distinctly different tissues develop in a fetus although every single cell of those tissues contains the same 23 chromosomes, with a total of approximately 20,000 genes.
- Behaviors or activities by adults may, unbeknownst to them, lead to disease in their yet-to-be-born offspring by switching certain genes on or off, without any change in the structure or sequence of DNA. For example, one study found that men who began to smoke before puberty (when sperm production begins) had offspring with significantly higher body weight than men who did not smoke before puberty.1 Animal studies show that organisms subjected to caloric restriction will live 30% longer than those with normal caloric intake, and their offspring have 20% longer longevity without being subjected to caloric restriction!2 That’s Lamarckism, but a much more sophisticated version than the original.
How does this link to psychiatry? We already have a large body of evidence that the environment can have a substantial deleterious or salutary impact on the development of psychiatric brain disorders. One set of evidence comes from studying that most important environment, the womb. Prenatal, neonatal (obstetric), and postnatal adverse events can disrupt brain development and lead to serious psychopathology, such as schizophrenia. Another type of evidence is seen in childhood abuse (physical, sexual, or verbal), which can lead to sensitization of the HPA axis and result in serious mood and anxiety disorders in adulthood. On the positive side, an enriched environment during infancy can stimulate neuroplasticity, connectivity, and enhanced brain growth.
Imagine what we will be able to do once research elucidates more than the epigenetic changes caused by adverse intrauterine events for a fetus or unhealthy pre-conception behaviors in future parents. Imagine, too, if psychiatric scientists discover parental behaviors that may improve offspring temperament, bolster resilience under stress, blunt impulsivity, suppress antisocial behavior, reduce the susceptibility to addiction(s), enhance cognitive ability, inhibit emergence of psychiatric symptoms, increase motivation, and, importantly, extinguish inherited suicidal or homicidal urges.
Of all medical disciplines, I believe psychiatry stands to benefit the most from advances in what may be designated as the field of “therapeutic epigenetics.” Two reasons justify this assertion:
- A very high proportion (50%) of our 20,000 genes are expressed only in the brain, creating huge opportunities for “interventional epigenetics” to prevent, modify, modulate, or ameliorate psychiatric diseases by silencing abnormal genes (once we identify them) or turbocharging the expression of adaptive genes (once they are identified).
- The brain is the most plastic organ in the body, changing on an ongoing basis by interacting with the environment (ie, the human experiences of everyday life). The unique ongoing neuroplasticity of the brain is driven by gene expression stimulated by environmental stimuli, which can be targeted for therapeutic purposes. In fact, it is likely that psychotherapy and pharmacotherapy exert their efficacy and certain adverse effects via selective gene expression.
Yes, it does sound like science fiction that some day we will be able to capitalize on gene-environment interactions to heal the body, the brain, and the mind, thus circumventing the use of medications. Psychiatric destiny is not in the DNA but in how targeted environmental influences can bring out the best in DNA, that magically malleable matter of life.
1. Pembrey ME, Bygren LO, Kaati G, et al. ALSPAC Study Team. Sex-specific, male-line transgenerational responses in humans. Eur J Hum Genet. 2006;14(2):159-166.
2. Kaneko G, Yoshinaga T, Yanagawa Y, et al. Calorie restriction-induced maternal longevity is transmitted to their daughters in a rotifer. Functional Ecology. 2011;25(1):209-216.
1. Pembrey ME, Bygren LO, Kaati G, et al. ALSPAC Study Team. Sex-specific, male-line transgenerational responses in humans. Eur J Hum Genet. 2006;14(2):159-166.
2. Kaneko G, Yoshinaga T, Yanagawa Y, et al. Calorie restriction-induced maternal longevity is transmitted to their daughters in a rotifer. Functional Ecology. 2011;25(1):209-216.
Promoting treatment adherence in patients with bipolar disorder
Discuss this article at www.facebook.com/CurrentPsychiatry
Treatment nonadherence among patients with chronic illness is high, and bipolar disorder (BD) is no exception. Approximately 21% to 50% of patients with BD do not adhere to their recommended treatment regimen,1 which adds to the burden of illness and worsens prognosis.
Although treatment nonadherence is a concern with any psychiatric disorder, we focus on BD because of the high prevalence of the disorder, the lifelong nature of the illness, and its resulting disability. BD is challenging to treat even with motivated patients, and psychiatrists cannot count on individuals to follow their prescribed regimen just because they were told to do so. Choosing the best treatment for each patient is complicated, and as physicians, we need to learn how to connect with our patients, increase our insight into their concerns, and work collaboratively to find a treatment they can follow.
This article describes methods of assessing adherence, factors that affect adherence, and pharmacologic and psychosocial interventions to enhance adherence and improve outcomes.
What is adherence?
As the doctor-patient relationship and medical treatment evolved to become more patient-centered, so have the terms used to describe individuals’ treatment-related behavior. Compliance, a physician-centered term that mandates following instructions to achieve treatment goals, evolved to adherence, the extent to which a person fulfills their part of an agreed-upon treatment plan, followed by concordance, which describes a decision-making alliance between patient and provider that strongly considers patients’ input.
Adherence is considered adequate when it occurs at the minimum level necessary for the patient to respond to treatment and avoid relapse.2 Research on adherence in BD can be difficult to interpret because results may be influenced by:
- selection bias (patients who are adherent and insightful are more likely to consent to research)
- complications caused by polypharmacy and comorbidity
- investigators’ ability to choose the proper measure to delineate medication adherence attitudes and behaviors
- patients’ compliance with the adherence-enhancing interventions.2
Assessment methods. Several tools can be used to measure adherence to mental illness treatment. Attitudinal scales capture a person’s subjective feelings (such as being on a medication, insight, perceived strength of the therapeutic alliance, and level of stigma faced) and can reflect attitude change that may result from adherence-enhancing interventions. Adherence behavior scales may be convenient to administer in the office but tend to overestimate patients’ adherence (Table 1).3-7
Pill counts are inexpensive but patients can manipulate unused medication. Prescription refill counts are easy to obtain but do not confirm that the patient took the medication. Electronic medication monitors capture the time of specific doses and can calculate the adherence rate, but they are expensive and do not ensure that the medication was ingested. Measuring the drug in urine or blood is an objective measure of adherence and can serve as clinical guide to pharmacotherapy, but offers limited correlation with the amount of medication taken and is expensive. A combination of measures to estimate adherence may be best.2
Table 1
Tools for measuring adherence to medications
| Components/characteristics | Advantages | Disadvantages |
|---|---|---|
| Rating of Medication Influences3 | ||
| 19 items. Subscales: Reasons for adherence (prevention, influence of others, medication affinity), reasons for nonadherence (denial, dysphoria, logistical problems, label rejection, family influence, negative therapeutic alliance) | Valid, reliable. Correlates with other scales (DAI) | Developed on a population including only patients with schizophrenia treated with antipsychotics. Requires a trained rater |
| Drug Attitude Inventory4 | ||
| 30 items. Reflects patients’ attitudes about medication | Self-rated. High internal consistency. Accurately discriminates between adherent and nonadherent patients | Developed on a population including only patients with schizophrenia |
| Lithium Attitudes Questionnaire5 | ||
| 19 items. Areas of assessment: opposition to continue lithium, therapeutic effectiveness of lithium not accepted, difficulty with pill-taking routine, denial of illness severity, subcultural attitudes opposed to drug treatment, dissatisfaction with factual knowledge of lithium | Self-rated. Developed on patients with BD attending a lithium clinic. Good test/retest reliability for most items | The questionnaire is fairly long; shorter versions were adapted from original version |
| Medication Adherence Rating Scale6 | ||
| 10 items that assess medication adherence behavior, attitudes toward taking medication, negative side effects, attitudes toward psychotropic medication, measures adherence in past week | Self-rated. Validated on patients with various diagnoses, including BD. Correlates well with DAI, MAQ, and mood stabilizer drug levels (lithium and carbamazepine) | Validation methods may be limited by the other measures (for example, medication levels can be influenced by metabolism) |
| Brief Adherence Rating Scale7 | ||
| 3 items. Number of pills prescribed daily, days with no medication taken, and days with medication taken less than prescribed. Nonadherence defined as <70% of doses taken. Measures adherence in past month | Clinician–rated. Short. Good correlation with electronic medication monitoring. High internal reliability. Good test/retest reliability. Greater adherence on BARS correlates with lower psychotic symptom scores. Sensitive and specific in identifying nonadherence | Validation study only on patients with schizophrenia and schizoaffective disorder taking antipsychotics |
| BARS: Brief Adherence Rating Scale; BD: bipolar disorder; DAI: Drug Attitude Inventory; MAQ: Medication Adherence Questionnaire | ||
BD adherence studies
Treatment adherence in BD is challenged by the chronic remission-relapse pattern of the disorder. Manic episodes carry the highest risk of nonadherence.2 Scott and Pope8 evaluated self-reported adherence to mood stabilizers (lithium, carbamazepine, or valproate) among 98 patients with major depressive disorder and 78 with BD. They found that 32% of patients were partially adherent (defined as having missed >30% of doses in the past month) and >60% of these patients had sub-therapeutic plasma levels of mood stabilizers.
In a study of 106 BD outpatients treated with lithium who completed scales regarding their attitudes toward and knowledge of lithium and the Medication Adherence Rating Scale (MARS), 86% of patients had a therapeutic serum lithium level (.6 to 1.2 mEq/L), and knowledge of lithium was correlated with adherence.9 Jónsdóttir et al10 looked at medication adherence among 280 patients with schizophrenia and BD by comparing patient self-reports to provider reports and measuring serum drug concentrations; adherence was defined as having a serum concentration within the reference level for the specific medication. BD patients had an adherence rate of 66%, and self-reported adherence as measured by MARS and provider reports correlated with serum concentrations.
In a study of 71 adolescents with BD followed for 1 year after their first hospitalization for a manic or mixed episode, DelBello et al11 defined nonadherence as taking medication <25% of the time and partial adherence as taking medication 25% to 75% of the time. They found that 42% of patients were partially adherent and 23% were nonadherent.
Strakowski12 followed 46 adults from Taiwan and 96 from the United States for 1 year after their first manic or mixed episode and found that 79% of the Taiwanese patients and 50% of U.S. patients were adherent. Using the medication possession ratio (MPR)—which is calculated based the number of days between expected and actual prescription refills—to determine adherence, Sajatovic13 found that 54% of 44,637 veterans being treated for BD with lithium or anticonvulsants were fully adherent (MPR >.80), 25% were partially adherent (MPR >.50 to .80), and 21% were nonadherent (MPR ≤.50). In a survey of 131 randomly selected psychiatrists and 429 of their adult BD patients, Baldessarini14 found that 34% of patients reported missing ≥1 medication dose in past 10 days, but psychiatrists recognized only 18% of patients as nonadherent.
What affects adherence?
Although all BD patients share the same diagnosis, the factors that ultimately result in their medication adherence are as variable as the individuals themselves. Patients’ age, sex, culture, symptom severity, worldview, socioeconomic status, opinion of mental illness, and self-image influence their individual decisions on adhering to a prescribed medication regimen.1,15
Perception of medication efficacy. Not surprisingly, if a medication does not seem to decrease debilitating symptoms, a patient is unlikely to continue taking it. Patients with BD feel more affected by depressive symptoms than by manic symptoms, and have indicated that they are more likely to adhere to and view as successful treatments that reduce depressive symptoms.16,17
Tolerability. In an Internet-based survey, 469 patients with BD indicated that medication-related weight gain and cognitive impairment were the most important factors that affected adherence.16 Individuals’ concerns about possible side effects may contribute more to nonadherence than actually experiencing side effects.17 Concerns about long-term metabolic side effects from atypical antipsychotics also may limit adherence.17
Neurocognitive impairment. Whether caused by BD, aging, or a combination of these factors, deficits in memory, attention, and executive functioning can lead to unintentional nonadherence. In a study that assessed medication management ability among middle-aged and older adults, patients with BD were found to make 2.8 times more errors than healthy controls.18
Therapeutic alliance and psychoeducation. Patients’ expectations for pharmacotherapy vary from specific symptom relief to hopes for a complete cure, and their fears may be influenced by media and advertisements.17 Nonetheless a positive therapeutic alliance with the treating provider improves illness outcomes.19
A clinician’s ability to help patients build insight is invaluable for their current and future treatment. In a survey of 435 veterans with BD, nonadherence was greater among patients with limited insight about the role of medication in their illness.20 A study of 65 BD patients that evaluated insight into medication adherence at initial interview and 1 year later found that difficulty with adherence at the initial interview predicted future nonadherence and was correlated with lack of insight.21 Rosa et al9 found that BD patients in denial of their illness and those who had little psychoeducation were more frequently nonadherent with lithium treatment.
Other factors that may contribute to medication nonadherence in BD patients include comorbid substance abuse or personality disorders, both of which are associated with more frequent relapse.15 Marriage has a beneficial affect on adherence.15 A good support system may contribute to treatment adherence; in a study of 107 children and adolescents with BD, nonadherent patients were more likely to experience family dysfunction and have a parental history of psychiatric hospitalization.22
Adherence and BD course
Treatment adherence decreases the suicide rate among BD patients. Angst et al23 evaluated the rate of suicide among 406 patients with BD and unipolar depression who were followed for 40 years. They found that 11% committed suicide; untreated patients had significantly higher standardized mortality rates than of those who were treated with lithium, antipsychotics, or antidepressants. Other studies confirm this finding.15
Repeated relapse may predict poorer cognitive performance. Lopez-Jaramillo et al24 showed that patients with BD who had more manic episodes performed poorer on cognitive tests assessing attention, memory, and executive functioning compared with patients with less episodes and with normal subjects.
Medication adherence in BD is a priority because of potential neurodegeneration in BD and the neuroprotective effects of mood stabilizers and some atypical antipsychotics (Box).
As emerging studies document morphologic brain changes associated with bipolar disorder (BD), researchers have been relating these changes to the duration and progression of illness. A longer duration of illness is associated with a smaller total gray matter volume on brain MRI of BD patients compared with unipolar patients and normal controls.a Brain MRI analysis of grey and white matter in elderly patients with longstanding BD who underwent neuropsychological testing to rule out dementia showed a decreased concentration of grey matter in the anterior limbic areas as well as reduced fiber tract coherence in the corpus callosum when compared with normal controls.b
Additionally, microstructural brain changes have been associated with acute mood states, in particular bipolar depression.c Lithium, valproate, olanzapine, and clozapine are neuroprotective in cultures of human-derived neuroblastoma cells, by enhancing the cells’ proliferation and survival.d
Source:
a. Frey BN, Zunta-Soares GB, Caetano SC, et al. Illness duration and total brain gray matter in bipolar disorder: evidence for neurodegeneration? European Neuropsychopharm. 2008;18:717-722.
b. Haller S, Xekardaki A, Delaloye C, et al. Combined analysis of grey matter voxel-based morphometry and white matter tract-based spatial statistics in late-life bipolar disorder. J Psychiatry Neurosci. 2011;36(1):100140.
c. Zanetti MV, Jackowski MP, Versace A, et al. State-dependent microstructural white matter changes in bipolar I depression. Eur Arch Psychiatry Clin Neurosci. 2009;259(6):316-328.
d. Aubry J, Schwald M, Ballmann E, et al. Early effects of mood stabilizers on the Akt/GSK-3ß signaling pathway and on cell survival and proliferation. Psychopharmacology. 2009;205:419-429.
Increasing adherence
Pharmacologic strategies. Adherence in BD often is difficult when patients require a complex medication regimen to control their illness. Patients and clinicians may prefer to use once-daily dosing drug formulations, which can provide consistent serum levels and fewer adverse effects. Divalproex extended-release (ER) allows once-daily dosing and improved tolerability by reducing fluctuations in valproic acid serum concentrations compared with the delayed-release formulation. In a retrospective chart review,25 most patients (62%) who switched to divalproex ER from divalproex delayed-release preferred the ER formulation; 52% showed clinical improvement, 81% did not experience side effects, and 8% demonstrated higher adherence after switching.25 Similarly, an extended-release formulation of carbamazepine is approved for treating acute mania.
Many atypical antipsychotics are FDA-approved for acute mania, acute bipolar depression, and/or maintenance (Table 2). Long-acting injectable formulations (LAIs) may be used as maintenance treatment if nonadherence is an issue. LAI risperidone, which is FDA-approved for maintenance treatment of bipolar I disorder (BDI), was found to be safe and effective in stable BD patients who were switched from an oral antipsychotic.26 Asenapine is provided in a rapidly absorbed, sublingual form and is FDA-approved for treating acute mania or mixed episodes associated with BDI.27 Overall, however, only slightly more than one-half of BD patients are adherent to atypical antipsychotics.15
Although antidepressant use in BD is controversial, Sajatovic17 found 44% of depressed BDI patients were treated with antidepressants. Novel extended-release antidepressant formulations—including controlled-release fluvoxamine, paroxetine, extended-release bupropion and venlafaxine, once-weekly fluoxetine, rapidly dissolving mirtazapine, and transdermal selegiline—can optimize drug delivery, minimize side effects, and delay onset of action.1
Psychosocial strategies used in BD include psychoeducation, cognitive-behavioral therapy (CBT), family-focused interventions, and interpersonal and social rhythm therapy (IPSRT) (Table 3).28-30 Psychoeducation alone or combined with other interventions can decrease the risk of relapse and hospitalization and improve adherence.28 In a 2-year study of 50 euthymic BD patients treated with lithium who participated in a brief hospital-based psychoeducation program, Even et al31 found patients’ knowledge about lithium but not their attitudes changed significantly after the program. The changes persisted 2 years after the intervention, with a trend toward a decreased hospitalization rate.
Miklowitz32 reported on 293 BD patients randomized to receive collaborative care (3 psychoeducational sessions delivered over 6 weeks) or 1 of 3 types of intensive psychotherapy: CBT, IPSRT, or family-focused therapy. Attrition was similar for both groups. Compared with those receiving collaborative care, significantly more patients receiving intensive psychotherapy recovered after 1 year, and did so in shorter time.
In a 3-year, multi-site Veterans Administration (VA) study, 306 BD patients received psychoeducation and support from nurse care coordinators who were responsible for access, continuity of care, and information flow to psychiatrists or usual care according to VA guidelines.33 Compared with the usual care group, patients who received psychoeducation and support from nurse care coordinators had shorter duration of manic episodes and improved function and quality of life. A meta-analysis30 of 12 randomized controlled trials of CBT in BD showed a medium effect size of CBT on adherence at 6 months post-treatment.
Table 2
FDA-approved medications for adult bipolar disorder
| Bipolar disorder indication | Medications |
|---|---|
| Acute treatment of mania/mixed episodes | Aripiprazole,a,b asenapine,a carbamazepine extended release,a divalproex sodium,a lithium,a quetiapine,a risperidone,a-c ziprasidonea,b |
| Depressive episodes | Olanzapine/fluoxetine,a quetiapinea |
| Maintenance treatment | Aripiprazole (as monotherapy and as adjunct to lithium or divalproex sodium),a,b asenapine,d lamotrigine,a lithium,a olanzapine,a-c quetiapine (as adjunct to lithium or divalproex sodium),a risperidone,e ziprasidone (as adjunct to lithium or divalproex sodium)a |
| apill form bintramuscular for acute agitation cdisintegrating tablet dsublingual tablet elong-acting injectable | |
Table 3
Psychosocial interventions for bipolar disorder
| Intervention | Description | Results in bipolar disorder | Optimal stage of illness for intervention |
|---|---|---|---|
| Individual and family psycho-education28,29 | Strategies to educate the patient about the illness, medications, side effects, and relapse prevention | Decreases relapse, (particularly manic episodes) and hospitalizations. Increases adherence | Manic episodes |
| Cognitive-behavioral therapy28-30 | Focuses on understanding patient’s perceptions of illness and treatment. Equates resistance with exploring, rather than challenging resistance to take medication. Identifies and modifies negative automatic thoughts about medication. Motivation techniques useful in comorbid substance use | Decreases clinical symptoms. Increases adherence, quality of life, and social functioning | Depressive episodes |
| IPSRT28,29 | Uses motivational interviewing and CBT techniques to stabilize daily routines and resolve interpersonal problems | Prevents relapse | Depressive episodes |
| Family-focused therapy28,29 | A combination of psychoeducation, communication, and problem-solving skills training | Reduces mood symptoms, number of depressive relapses, and time depressed. Increases adherence | Depressive episodes |
| IPSRT: interpersonal and social rhythm therapy | |||
Related Resource
- Deegan PE. The importance of personal medicine: a qualitative study of resilience in people with psychiatric disabilities. Scand J Public Health Suppl. 2005;66:29-35.
Drug Brand Names
- Aripiprazole • Abilify
- Asenapine • Saphris
- Bupropion • Wellbutrin
- Carbamazepine • Carbatrol, Tegretol
- Carbamazepine extended- release • Equetro
- Clozapine • Clozaril
- Divalproex • Depakote, Depakote ER
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid
- Mirtazapine • Remeron
- Olanzapine • Zyprexa
- Olanzapine/fluoxetine • Symbyax
- Paroxetine • Paxil
- Quetiapine • Seroquel, Seroquel XR
- Risperidone • Risperdal
- Risperidone long-acting injectable • Risperdal Consta
- Selegiline • Eldepryl, Emsam
- Valproate • Depacon
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosures
Dr. Foster receives research/grant support from the American Psychiatric Foundation, the National Institute of Mental Health, and Sunovion Pharmaceuticals.
Dr. Sheehan and Ms. Johns report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Buckley PF, Foster AE, Patel NC, et al. Adherence to mental health treatment. New York, NY: Oxford University Press; 2009;1-10:53-69.
2. Velligan D, Sajatovic M, Valenstein M, et al. Methodological challenges in psychiatric treatment adherence research. Clin Schizophr Relat Psychoses. 2010;4(1):74-91.
3. Weiden P, Rapkin B, Mott T, et al. Rating of Medication Influences (ROMI) scale in schizophrenia. Schizophr Bull. 1994;20:297-310.
4. Hogan TP, Awad AG, Eastwood R. A self-report scale predictive of drug compliance in schizophrenics: reliability and discriminative validity. Psychol Med. 1983;13(1):177-183.
5. Harvey NS. The development and descriptive use of the Lithium Attitudes Questionnaire. J Affect Disord. 1991;22(4):211-219.
6. Thompson K, Kulkarni J, Sergejew AA. Reliability and validity of a new Medication Adherence Rating Scale (MARS). Schizophr Res. 2000;42:241-247.
7. Byerly MJ, Nazonezny PA, Rush AJ. The Brief Adherence Rating Scale (BARS) validated against electronic monitoring in assessing the antipsychotic medication adherence of outpatients with schizophrenia and schizoaffective disorder. Schizophr Res. 2008;100(1-3):60-69.
8. Scott J, Pope M. Non-adherence with mood stabilizers: prevalence and predictors. J Clin Psychiatry. 2002;63:384-390.
9. Rosa AR, Marco M, Fachel JM, et al. Correlation between drug treatment adherence and lithium treatment attitudes and knowledge in bipolar patients. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:217-224.
10. Jónsdóttir H, Opjordsmoen S, Birkenaes A, et al. Medication adherence in outpatients with severe mental disorders, relation between self-reports and serum level. J Clin Psychopharmacol. 2010;30:169-175.
11. DelBello M, Hanserman D, Adler CM, et al. Twelve-month outcome of adolescents with bipolar disorder following first hospitalization for a manic or mixed episode. Am J Psychiatry. 2007;164:582-590.
12. Strakowski SM, Tsai SY, DelBello MP, et al. Outcome following a first manic episode: cross national US and Taiwan comparison. Bipolar Disord. 2007;9:820-827.
13. Sajatovic M, Valenstein M, Blow F, et al. Treatment adherence with lithium and anticonvulsant medications among patients with bipolar disorder. Psychiatr Serv. 2007;58:855-863.
14. Baldessarini RJ, Perry R, Pike J. Factors associated with treatment nonadherence among US bipolar patients. Hum Psychopharmacol. 2008;23:95-105.
15. Berk L, Hallam KT, Colom F, et al. Enhancing medication adherence in patients with bipolar disorder. Hum Psychopharmacol. 2010;25(1):1-16.
16. Johnson FR, Ozdemir S, Manjunath R, et al. Factors that affect adherence to bipolar disorder treatments: a stated-preference approach. Med Care. 2007;45(6):545-552.
17. Sajatovic M, Jenkins JH, Cassidy KA, et al. Medication treatment perceptions, concerns and expectations among depressed individuals with type I bipolar disorder. J Affect Disord. 2009;115(3):360-366.
18. Depp CA, Cain AE, Palmer BW, et al. Assessment of medication management ability in middle-aged and older adults with bipolar disorder. J Clin Psychopharmacol. 2008;28(2):225-229.
19. Gaudiano BA, Miller IW. Patients’ expectancies the alliance in pharmacotherapy, and treatment outcomes in bipolar disorder. J Consult Clin Psychol. 2006;74(4):671-676.
20. Copeland LA, Zeber JE, Salloum IM, et al. Treatment adherence and illness insight in veterans with bipolar disorder. J Nerv Ment Dis. 2008;196(1):16-21.
21. Yen CF, Chen CS, Ko CH, et al. Relationships between insight and medication adherence in outpatients with schizophrenia and bipolar disorder: prospective study. Psychiatry Clin Neurosci. 2005;59(4):403-409.
22. Drotar D, Greenley RN, Demeter CA, et al. Adherence to pharmacological treatment for juvenile bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):831-839.
23. Angst J, Angst F, Gerber-Werder R, et al. Suicide in 406 mood-disorder patients with and without long–term medication: a 40 to 44 years’ follow-up. Arch Suicide Res. 2005;9:279-300.
24. Lopez-Jaramillo C, Lopera-Vasquez J, Aurora G, et al. Effects of recurrence on the cognitive performance of patients with bipolar I disorder: implications for relapse prevention and treatment adherence. Bipolar Disord. 2010;12:557-567.
25. Minirth FB, Neal V. Assessment of patient preference and side effects in patients switched from divalproex sodium delayed release to divalproex sodium extended release. J Clin Psychopharmacol. 2005;25:99-101.
26. Han C, Lee MS, Pae CU, et al. Usefulness of long-acting injectable risperidone during 12-month maintenance therapy of bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1219-1223.
27. McIntyre RS, Cohen M, Zhao J, et al. Asenapine for long term treatment of bipolar disorder: a double blind 40-week extension study. J Affect Disord. 2010;126:358-365.
28. Velligan DI, Weiden PJ, Sajatovic M, et al. Strategies for addressing adherence problems in patients with serious and persistent mental illness: recommendations from expert consensus guidelines. J Psychiatr Pract. 2010;16(5):306-324.
29. Miklowitz DJ. Adjunctive psychotherapy for bipolar disorder: state of the evidence. Am J Psychiatry. 2008;165(11):1408-1419.
30. Szentagotai A, David D. The efficacy of cognitive-behavioral therapy in bipolar disorder: a quantitative meta-analysis. J Clin Psychiatry. 2010;71(1):66-72.
31. Even C, Thuile J, Stern K, et al. Psychoeducation for patients with bipolar disorder receiving lithium: short and long term impact on locus of control and knowledge about lithium. J Affect Disord. 2010;123:299-302.
32. Miklowitz DJ, Otto MW, Frank E, et al. Psychosocial treatments for bipolar depression: A 1-year randomized trial from the Systematic Treatment Enhancement Program. Arch Gen Psychiatry. 2007;64:419-426.
33. Bauer MS, McBride L, Williford WO, et al. Collaborative care for bipolar disorder, part II. Impact on clinical outcome, function and costs. Psychiatr Serv. 2006;57:937-945.
Discuss this article at www.facebook.com/CurrentPsychiatry
Treatment nonadherence among patients with chronic illness is high, and bipolar disorder (BD) is no exception. Approximately 21% to 50% of patients with BD do not adhere to their recommended treatment regimen,1 which adds to the burden of illness and worsens prognosis.
Although treatment nonadherence is a concern with any psychiatric disorder, we focus on BD because of the high prevalence of the disorder, the lifelong nature of the illness, and its resulting disability. BD is challenging to treat even with motivated patients, and psychiatrists cannot count on individuals to follow their prescribed regimen just because they were told to do so. Choosing the best treatment for each patient is complicated, and as physicians, we need to learn how to connect with our patients, increase our insight into their concerns, and work collaboratively to find a treatment they can follow.
This article describes methods of assessing adherence, factors that affect adherence, and pharmacologic and psychosocial interventions to enhance adherence and improve outcomes.
What is adherence?
As the doctor-patient relationship and medical treatment evolved to become more patient-centered, so have the terms used to describe individuals’ treatment-related behavior. Compliance, a physician-centered term that mandates following instructions to achieve treatment goals, evolved to adherence, the extent to which a person fulfills their part of an agreed-upon treatment plan, followed by concordance, which describes a decision-making alliance between patient and provider that strongly considers patients’ input.
Adherence is considered adequate when it occurs at the minimum level necessary for the patient to respond to treatment and avoid relapse.2 Research on adherence in BD can be difficult to interpret because results may be influenced by:
- selection bias (patients who are adherent and insightful are more likely to consent to research)
- complications caused by polypharmacy and comorbidity
- investigators’ ability to choose the proper measure to delineate medication adherence attitudes and behaviors
- patients’ compliance with the adherence-enhancing interventions.2
Assessment methods. Several tools can be used to measure adherence to mental illness treatment. Attitudinal scales capture a person’s subjective feelings (such as being on a medication, insight, perceived strength of the therapeutic alliance, and level of stigma faced) and can reflect attitude change that may result from adherence-enhancing interventions. Adherence behavior scales may be convenient to administer in the office but tend to overestimate patients’ adherence (Table 1).3-7
Pill counts are inexpensive but patients can manipulate unused medication. Prescription refill counts are easy to obtain but do not confirm that the patient took the medication. Electronic medication monitors capture the time of specific doses and can calculate the adherence rate, but they are expensive and do not ensure that the medication was ingested. Measuring the drug in urine or blood is an objective measure of adherence and can serve as clinical guide to pharmacotherapy, but offers limited correlation with the amount of medication taken and is expensive. A combination of measures to estimate adherence may be best.2
Table 1
Tools for measuring adherence to medications
| Components/characteristics | Advantages | Disadvantages |
|---|---|---|
| Rating of Medication Influences3 | ||
| 19 items. Subscales: Reasons for adherence (prevention, influence of others, medication affinity), reasons for nonadherence (denial, dysphoria, logistical problems, label rejection, family influence, negative therapeutic alliance) | Valid, reliable. Correlates with other scales (DAI) | Developed on a population including only patients with schizophrenia treated with antipsychotics. Requires a trained rater |
| Drug Attitude Inventory4 | ||
| 30 items. Reflects patients’ attitudes about medication | Self-rated. High internal consistency. Accurately discriminates between adherent and nonadherent patients | Developed on a population including only patients with schizophrenia |
| Lithium Attitudes Questionnaire5 | ||
| 19 items. Areas of assessment: opposition to continue lithium, therapeutic effectiveness of lithium not accepted, difficulty with pill-taking routine, denial of illness severity, subcultural attitudes opposed to drug treatment, dissatisfaction with factual knowledge of lithium | Self-rated. Developed on patients with BD attending a lithium clinic. Good test/retest reliability for most items | The questionnaire is fairly long; shorter versions were adapted from original version |
| Medication Adherence Rating Scale6 | ||
| 10 items that assess medication adherence behavior, attitudes toward taking medication, negative side effects, attitudes toward psychotropic medication, measures adherence in past week | Self-rated. Validated on patients with various diagnoses, including BD. Correlates well with DAI, MAQ, and mood stabilizer drug levels (lithium and carbamazepine) | Validation methods may be limited by the other measures (for example, medication levels can be influenced by metabolism) |
| Brief Adherence Rating Scale7 | ||
| 3 items. Number of pills prescribed daily, days with no medication taken, and days with medication taken less than prescribed. Nonadherence defined as <70% of doses taken. Measures adherence in past month | Clinician–rated. Short. Good correlation with electronic medication monitoring. High internal reliability. Good test/retest reliability. Greater adherence on BARS correlates with lower psychotic symptom scores. Sensitive and specific in identifying nonadherence | Validation study only on patients with schizophrenia and schizoaffective disorder taking antipsychotics |
| BARS: Brief Adherence Rating Scale; BD: bipolar disorder; DAI: Drug Attitude Inventory; MAQ: Medication Adherence Questionnaire | ||
BD adherence studies
Treatment adherence in BD is challenged by the chronic remission-relapse pattern of the disorder. Manic episodes carry the highest risk of nonadherence.2 Scott and Pope8 evaluated self-reported adherence to mood stabilizers (lithium, carbamazepine, or valproate) among 98 patients with major depressive disorder and 78 with BD. They found that 32% of patients were partially adherent (defined as having missed >30% of doses in the past month) and >60% of these patients had sub-therapeutic plasma levels of mood stabilizers.
In a study of 106 BD outpatients treated with lithium who completed scales regarding their attitudes toward and knowledge of lithium and the Medication Adherence Rating Scale (MARS), 86% of patients had a therapeutic serum lithium level (.6 to 1.2 mEq/L), and knowledge of lithium was correlated with adherence.9 Jónsdóttir et al10 looked at medication adherence among 280 patients with schizophrenia and BD by comparing patient self-reports to provider reports and measuring serum drug concentrations; adherence was defined as having a serum concentration within the reference level for the specific medication. BD patients had an adherence rate of 66%, and self-reported adherence as measured by MARS and provider reports correlated with serum concentrations.
In a study of 71 adolescents with BD followed for 1 year after their first hospitalization for a manic or mixed episode, DelBello et al11 defined nonadherence as taking medication <25% of the time and partial adherence as taking medication 25% to 75% of the time. They found that 42% of patients were partially adherent and 23% were nonadherent.
Strakowski12 followed 46 adults from Taiwan and 96 from the United States for 1 year after their first manic or mixed episode and found that 79% of the Taiwanese patients and 50% of U.S. patients were adherent. Using the medication possession ratio (MPR)—which is calculated based the number of days between expected and actual prescription refills—to determine adherence, Sajatovic13 found that 54% of 44,637 veterans being treated for BD with lithium or anticonvulsants were fully adherent (MPR >.80), 25% were partially adherent (MPR >.50 to .80), and 21% were nonadherent (MPR ≤.50). In a survey of 131 randomly selected psychiatrists and 429 of their adult BD patients, Baldessarini14 found that 34% of patients reported missing ≥1 medication dose in past 10 days, but psychiatrists recognized only 18% of patients as nonadherent.
What affects adherence?
Although all BD patients share the same diagnosis, the factors that ultimately result in their medication adherence are as variable as the individuals themselves. Patients’ age, sex, culture, symptom severity, worldview, socioeconomic status, opinion of mental illness, and self-image influence their individual decisions on adhering to a prescribed medication regimen.1,15
Perception of medication efficacy. Not surprisingly, if a medication does not seem to decrease debilitating symptoms, a patient is unlikely to continue taking it. Patients with BD feel more affected by depressive symptoms than by manic symptoms, and have indicated that they are more likely to adhere to and view as successful treatments that reduce depressive symptoms.16,17
Tolerability. In an Internet-based survey, 469 patients with BD indicated that medication-related weight gain and cognitive impairment were the most important factors that affected adherence.16 Individuals’ concerns about possible side effects may contribute more to nonadherence than actually experiencing side effects.17 Concerns about long-term metabolic side effects from atypical antipsychotics also may limit adherence.17
Neurocognitive impairment. Whether caused by BD, aging, or a combination of these factors, deficits in memory, attention, and executive functioning can lead to unintentional nonadherence. In a study that assessed medication management ability among middle-aged and older adults, patients with BD were found to make 2.8 times more errors than healthy controls.18
Therapeutic alliance and psychoeducation. Patients’ expectations for pharmacotherapy vary from specific symptom relief to hopes for a complete cure, and their fears may be influenced by media and advertisements.17 Nonetheless a positive therapeutic alliance with the treating provider improves illness outcomes.19
A clinician’s ability to help patients build insight is invaluable for their current and future treatment. In a survey of 435 veterans with BD, nonadherence was greater among patients with limited insight about the role of medication in their illness.20 A study of 65 BD patients that evaluated insight into medication adherence at initial interview and 1 year later found that difficulty with adherence at the initial interview predicted future nonadherence and was correlated with lack of insight.21 Rosa et al9 found that BD patients in denial of their illness and those who had little psychoeducation were more frequently nonadherent with lithium treatment.
Other factors that may contribute to medication nonadherence in BD patients include comorbid substance abuse or personality disorders, both of which are associated with more frequent relapse.15 Marriage has a beneficial affect on adherence.15 A good support system may contribute to treatment adherence; in a study of 107 children and adolescents with BD, nonadherent patients were more likely to experience family dysfunction and have a parental history of psychiatric hospitalization.22
Adherence and BD course
Treatment adherence decreases the suicide rate among BD patients. Angst et al23 evaluated the rate of suicide among 406 patients with BD and unipolar depression who were followed for 40 years. They found that 11% committed suicide; untreated patients had significantly higher standardized mortality rates than of those who were treated with lithium, antipsychotics, or antidepressants. Other studies confirm this finding.15
Repeated relapse may predict poorer cognitive performance. Lopez-Jaramillo et al24 showed that patients with BD who had more manic episodes performed poorer on cognitive tests assessing attention, memory, and executive functioning compared with patients with less episodes and with normal subjects.
Medication adherence in BD is a priority because of potential neurodegeneration in BD and the neuroprotective effects of mood stabilizers and some atypical antipsychotics (Box).
As emerging studies document morphologic brain changes associated with bipolar disorder (BD), researchers have been relating these changes to the duration and progression of illness. A longer duration of illness is associated with a smaller total gray matter volume on brain MRI of BD patients compared with unipolar patients and normal controls.a Brain MRI analysis of grey and white matter in elderly patients with longstanding BD who underwent neuropsychological testing to rule out dementia showed a decreased concentration of grey matter in the anterior limbic areas as well as reduced fiber tract coherence in the corpus callosum when compared with normal controls.b
Additionally, microstructural brain changes have been associated with acute mood states, in particular bipolar depression.c Lithium, valproate, olanzapine, and clozapine are neuroprotective in cultures of human-derived neuroblastoma cells, by enhancing the cells’ proliferation and survival.d
Source:
a. Frey BN, Zunta-Soares GB, Caetano SC, et al. Illness duration and total brain gray matter in bipolar disorder: evidence for neurodegeneration? European Neuropsychopharm. 2008;18:717-722.
b. Haller S, Xekardaki A, Delaloye C, et al. Combined analysis of grey matter voxel-based morphometry and white matter tract-based spatial statistics in late-life bipolar disorder. J Psychiatry Neurosci. 2011;36(1):100140.
c. Zanetti MV, Jackowski MP, Versace A, et al. State-dependent microstructural white matter changes in bipolar I depression. Eur Arch Psychiatry Clin Neurosci. 2009;259(6):316-328.
d. Aubry J, Schwald M, Ballmann E, et al. Early effects of mood stabilizers on the Akt/GSK-3ß signaling pathway and on cell survival and proliferation. Psychopharmacology. 2009;205:419-429.
Increasing adherence
Pharmacologic strategies. Adherence in BD often is difficult when patients require a complex medication regimen to control their illness. Patients and clinicians may prefer to use once-daily dosing drug formulations, which can provide consistent serum levels and fewer adverse effects. Divalproex extended-release (ER) allows once-daily dosing and improved tolerability by reducing fluctuations in valproic acid serum concentrations compared with the delayed-release formulation. In a retrospective chart review,25 most patients (62%) who switched to divalproex ER from divalproex delayed-release preferred the ER formulation; 52% showed clinical improvement, 81% did not experience side effects, and 8% demonstrated higher adherence after switching.25 Similarly, an extended-release formulation of carbamazepine is approved for treating acute mania.
Many atypical antipsychotics are FDA-approved for acute mania, acute bipolar depression, and/or maintenance (Table 2). Long-acting injectable formulations (LAIs) may be used as maintenance treatment if nonadherence is an issue. LAI risperidone, which is FDA-approved for maintenance treatment of bipolar I disorder (BDI), was found to be safe and effective in stable BD patients who were switched from an oral antipsychotic.26 Asenapine is provided in a rapidly absorbed, sublingual form and is FDA-approved for treating acute mania or mixed episodes associated with BDI.27 Overall, however, only slightly more than one-half of BD patients are adherent to atypical antipsychotics.15
Although antidepressant use in BD is controversial, Sajatovic17 found 44% of depressed BDI patients were treated with antidepressants. Novel extended-release antidepressant formulations—including controlled-release fluvoxamine, paroxetine, extended-release bupropion and venlafaxine, once-weekly fluoxetine, rapidly dissolving mirtazapine, and transdermal selegiline—can optimize drug delivery, minimize side effects, and delay onset of action.1
Psychosocial strategies used in BD include psychoeducation, cognitive-behavioral therapy (CBT), family-focused interventions, and interpersonal and social rhythm therapy (IPSRT) (Table 3).28-30 Psychoeducation alone or combined with other interventions can decrease the risk of relapse and hospitalization and improve adherence.28 In a 2-year study of 50 euthymic BD patients treated with lithium who participated in a brief hospital-based psychoeducation program, Even et al31 found patients’ knowledge about lithium but not their attitudes changed significantly after the program. The changes persisted 2 years after the intervention, with a trend toward a decreased hospitalization rate.
Miklowitz32 reported on 293 BD patients randomized to receive collaborative care (3 psychoeducational sessions delivered over 6 weeks) or 1 of 3 types of intensive psychotherapy: CBT, IPSRT, or family-focused therapy. Attrition was similar for both groups. Compared with those receiving collaborative care, significantly more patients receiving intensive psychotherapy recovered after 1 year, and did so in shorter time.
In a 3-year, multi-site Veterans Administration (VA) study, 306 BD patients received psychoeducation and support from nurse care coordinators who were responsible for access, continuity of care, and information flow to psychiatrists or usual care according to VA guidelines.33 Compared with the usual care group, patients who received psychoeducation and support from nurse care coordinators had shorter duration of manic episodes and improved function and quality of life. A meta-analysis30 of 12 randomized controlled trials of CBT in BD showed a medium effect size of CBT on adherence at 6 months post-treatment.
Table 2
FDA-approved medications for adult bipolar disorder
| Bipolar disorder indication | Medications |
|---|---|
| Acute treatment of mania/mixed episodes | Aripiprazole,a,b asenapine,a carbamazepine extended release,a divalproex sodium,a lithium,a quetiapine,a risperidone,a-c ziprasidonea,b |
| Depressive episodes | Olanzapine/fluoxetine,a quetiapinea |
| Maintenance treatment | Aripiprazole (as monotherapy and as adjunct to lithium or divalproex sodium),a,b asenapine,d lamotrigine,a lithium,a olanzapine,a-c quetiapine (as adjunct to lithium or divalproex sodium),a risperidone,e ziprasidone (as adjunct to lithium or divalproex sodium)a |
| apill form bintramuscular for acute agitation cdisintegrating tablet dsublingual tablet elong-acting injectable | |
Table 3
Psychosocial interventions for bipolar disorder
| Intervention | Description | Results in bipolar disorder | Optimal stage of illness for intervention |
|---|---|---|---|
| Individual and family psycho-education28,29 | Strategies to educate the patient about the illness, medications, side effects, and relapse prevention | Decreases relapse, (particularly manic episodes) and hospitalizations. Increases adherence | Manic episodes |
| Cognitive-behavioral therapy28-30 | Focuses on understanding patient’s perceptions of illness and treatment. Equates resistance with exploring, rather than challenging resistance to take medication. Identifies and modifies negative automatic thoughts about medication. Motivation techniques useful in comorbid substance use | Decreases clinical symptoms. Increases adherence, quality of life, and social functioning | Depressive episodes |
| IPSRT28,29 | Uses motivational interviewing and CBT techniques to stabilize daily routines and resolve interpersonal problems | Prevents relapse | Depressive episodes |
| Family-focused therapy28,29 | A combination of psychoeducation, communication, and problem-solving skills training | Reduces mood symptoms, number of depressive relapses, and time depressed. Increases adherence | Depressive episodes |
| IPSRT: interpersonal and social rhythm therapy | |||
Related Resource
- Deegan PE. The importance of personal medicine: a qualitative study of resilience in people with psychiatric disabilities. Scand J Public Health Suppl. 2005;66:29-35.
Drug Brand Names
- Aripiprazole • Abilify
- Asenapine • Saphris
- Bupropion • Wellbutrin
- Carbamazepine • Carbatrol, Tegretol
- Carbamazepine extended- release • Equetro
- Clozapine • Clozaril
- Divalproex • Depakote, Depakote ER
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid
- Mirtazapine • Remeron
- Olanzapine • Zyprexa
- Olanzapine/fluoxetine • Symbyax
- Paroxetine • Paxil
- Quetiapine • Seroquel, Seroquel XR
- Risperidone • Risperdal
- Risperidone long-acting injectable • Risperdal Consta
- Selegiline • Eldepryl, Emsam
- Valproate • Depacon
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosures
Dr. Foster receives research/grant support from the American Psychiatric Foundation, the National Institute of Mental Health, and Sunovion Pharmaceuticals.
Dr. Sheehan and Ms. Johns report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
Treatment nonadherence among patients with chronic illness is high, and bipolar disorder (BD) is no exception. Approximately 21% to 50% of patients with BD do not adhere to their recommended treatment regimen,1 which adds to the burden of illness and worsens prognosis.
Although treatment nonadherence is a concern with any psychiatric disorder, we focus on BD because of the high prevalence of the disorder, the lifelong nature of the illness, and its resulting disability. BD is challenging to treat even with motivated patients, and psychiatrists cannot count on individuals to follow their prescribed regimen just because they were told to do so. Choosing the best treatment for each patient is complicated, and as physicians, we need to learn how to connect with our patients, increase our insight into their concerns, and work collaboratively to find a treatment they can follow.
This article describes methods of assessing adherence, factors that affect adherence, and pharmacologic and psychosocial interventions to enhance adherence and improve outcomes.
What is adherence?
As the doctor-patient relationship and medical treatment evolved to become more patient-centered, so have the terms used to describe individuals’ treatment-related behavior. Compliance, a physician-centered term that mandates following instructions to achieve treatment goals, evolved to adherence, the extent to which a person fulfills their part of an agreed-upon treatment plan, followed by concordance, which describes a decision-making alliance between patient and provider that strongly considers patients’ input.
Adherence is considered adequate when it occurs at the minimum level necessary for the patient to respond to treatment and avoid relapse.2 Research on adherence in BD can be difficult to interpret because results may be influenced by:
- selection bias (patients who are adherent and insightful are more likely to consent to research)
- complications caused by polypharmacy and comorbidity
- investigators’ ability to choose the proper measure to delineate medication adherence attitudes and behaviors
- patients’ compliance with the adherence-enhancing interventions.2
Assessment methods. Several tools can be used to measure adherence to mental illness treatment. Attitudinal scales capture a person’s subjective feelings (such as being on a medication, insight, perceived strength of the therapeutic alliance, and level of stigma faced) and can reflect attitude change that may result from adherence-enhancing interventions. Adherence behavior scales may be convenient to administer in the office but tend to overestimate patients’ adherence (Table 1).3-7
Pill counts are inexpensive but patients can manipulate unused medication. Prescription refill counts are easy to obtain but do not confirm that the patient took the medication. Electronic medication monitors capture the time of specific doses and can calculate the adherence rate, but they are expensive and do not ensure that the medication was ingested. Measuring the drug in urine or blood is an objective measure of adherence and can serve as clinical guide to pharmacotherapy, but offers limited correlation with the amount of medication taken and is expensive. A combination of measures to estimate adherence may be best.2
Table 1
Tools for measuring adherence to medications
| Components/characteristics | Advantages | Disadvantages |
|---|---|---|
| Rating of Medication Influences3 | ||
| 19 items. Subscales: Reasons for adherence (prevention, influence of others, medication affinity), reasons for nonadherence (denial, dysphoria, logistical problems, label rejection, family influence, negative therapeutic alliance) | Valid, reliable. Correlates with other scales (DAI) | Developed on a population including only patients with schizophrenia treated with antipsychotics. Requires a trained rater |
| Drug Attitude Inventory4 | ||
| 30 items. Reflects patients’ attitudes about medication | Self-rated. High internal consistency. Accurately discriminates between adherent and nonadherent patients | Developed on a population including only patients with schizophrenia |
| Lithium Attitudes Questionnaire5 | ||
| 19 items. Areas of assessment: opposition to continue lithium, therapeutic effectiveness of lithium not accepted, difficulty with pill-taking routine, denial of illness severity, subcultural attitudes opposed to drug treatment, dissatisfaction with factual knowledge of lithium | Self-rated. Developed on patients with BD attending a lithium clinic. Good test/retest reliability for most items | The questionnaire is fairly long; shorter versions were adapted from original version |
| Medication Adherence Rating Scale6 | ||
| 10 items that assess medication adherence behavior, attitudes toward taking medication, negative side effects, attitudes toward psychotropic medication, measures adherence in past week | Self-rated. Validated on patients with various diagnoses, including BD. Correlates well with DAI, MAQ, and mood stabilizer drug levels (lithium and carbamazepine) | Validation methods may be limited by the other measures (for example, medication levels can be influenced by metabolism) |
| Brief Adherence Rating Scale7 | ||
| 3 items. Number of pills prescribed daily, days with no medication taken, and days with medication taken less than prescribed. Nonadherence defined as <70% of doses taken. Measures adherence in past month | Clinician–rated. Short. Good correlation with electronic medication monitoring. High internal reliability. Good test/retest reliability. Greater adherence on BARS correlates with lower psychotic symptom scores. Sensitive and specific in identifying nonadherence | Validation study only on patients with schizophrenia and schizoaffective disorder taking antipsychotics |
| BARS: Brief Adherence Rating Scale; BD: bipolar disorder; DAI: Drug Attitude Inventory; MAQ: Medication Adherence Questionnaire | ||
BD adherence studies
Treatment adherence in BD is challenged by the chronic remission-relapse pattern of the disorder. Manic episodes carry the highest risk of nonadherence.2 Scott and Pope8 evaluated self-reported adherence to mood stabilizers (lithium, carbamazepine, or valproate) among 98 patients with major depressive disorder and 78 with BD. They found that 32% of patients were partially adherent (defined as having missed >30% of doses in the past month) and >60% of these patients had sub-therapeutic plasma levels of mood stabilizers.
In a study of 106 BD outpatients treated with lithium who completed scales regarding their attitudes toward and knowledge of lithium and the Medication Adherence Rating Scale (MARS), 86% of patients had a therapeutic serum lithium level (.6 to 1.2 mEq/L), and knowledge of lithium was correlated with adherence.9 Jónsdóttir et al10 looked at medication adherence among 280 patients with schizophrenia and BD by comparing patient self-reports to provider reports and measuring serum drug concentrations; adherence was defined as having a serum concentration within the reference level for the specific medication. BD patients had an adherence rate of 66%, and self-reported adherence as measured by MARS and provider reports correlated with serum concentrations.
In a study of 71 adolescents with BD followed for 1 year after their first hospitalization for a manic or mixed episode, DelBello et al11 defined nonadherence as taking medication <25% of the time and partial adherence as taking medication 25% to 75% of the time. They found that 42% of patients were partially adherent and 23% were nonadherent.
Strakowski12 followed 46 adults from Taiwan and 96 from the United States for 1 year after their first manic or mixed episode and found that 79% of the Taiwanese patients and 50% of U.S. patients were adherent. Using the medication possession ratio (MPR)—which is calculated based the number of days between expected and actual prescription refills—to determine adherence, Sajatovic13 found that 54% of 44,637 veterans being treated for BD with lithium or anticonvulsants were fully adherent (MPR >.80), 25% were partially adherent (MPR >.50 to .80), and 21% were nonadherent (MPR ≤.50). In a survey of 131 randomly selected psychiatrists and 429 of their adult BD patients, Baldessarini14 found that 34% of patients reported missing ≥1 medication dose in past 10 days, but psychiatrists recognized only 18% of patients as nonadherent.
What affects adherence?
Although all BD patients share the same diagnosis, the factors that ultimately result in their medication adherence are as variable as the individuals themselves. Patients’ age, sex, culture, symptom severity, worldview, socioeconomic status, opinion of mental illness, and self-image influence their individual decisions on adhering to a prescribed medication regimen.1,15
Perception of medication efficacy. Not surprisingly, if a medication does not seem to decrease debilitating symptoms, a patient is unlikely to continue taking it. Patients with BD feel more affected by depressive symptoms than by manic symptoms, and have indicated that they are more likely to adhere to and view as successful treatments that reduce depressive symptoms.16,17
Tolerability. In an Internet-based survey, 469 patients with BD indicated that medication-related weight gain and cognitive impairment were the most important factors that affected adherence.16 Individuals’ concerns about possible side effects may contribute more to nonadherence than actually experiencing side effects.17 Concerns about long-term metabolic side effects from atypical antipsychotics also may limit adherence.17
Neurocognitive impairment. Whether caused by BD, aging, or a combination of these factors, deficits in memory, attention, and executive functioning can lead to unintentional nonadherence. In a study that assessed medication management ability among middle-aged and older adults, patients with BD were found to make 2.8 times more errors than healthy controls.18
Therapeutic alliance and psychoeducation. Patients’ expectations for pharmacotherapy vary from specific symptom relief to hopes for a complete cure, and their fears may be influenced by media and advertisements.17 Nonetheless a positive therapeutic alliance with the treating provider improves illness outcomes.19
A clinician’s ability to help patients build insight is invaluable for their current and future treatment. In a survey of 435 veterans with BD, nonadherence was greater among patients with limited insight about the role of medication in their illness.20 A study of 65 BD patients that evaluated insight into medication adherence at initial interview and 1 year later found that difficulty with adherence at the initial interview predicted future nonadherence and was correlated with lack of insight.21 Rosa et al9 found that BD patients in denial of their illness and those who had little psychoeducation were more frequently nonadherent with lithium treatment.
Other factors that may contribute to medication nonadherence in BD patients include comorbid substance abuse or personality disorders, both of which are associated with more frequent relapse.15 Marriage has a beneficial affect on adherence.15 A good support system may contribute to treatment adherence; in a study of 107 children and adolescents with BD, nonadherent patients were more likely to experience family dysfunction and have a parental history of psychiatric hospitalization.22
Adherence and BD course
Treatment adherence decreases the suicide rate among BD patients. Angst et al23 evaluated the rate of suicide among 406 patients with BD and unipolar depression who were followed for 40 years. They found that 11% committed suicide; untreated patients had significantly higher standardized mortality rates than of those who were treated with lithium, antipsychotics, or antidepressants. Other studies confirm this finding.15
Repeated relapse may predict poorer cognitive performance. Lopez-Jaramillo et al24 showed that patients with BD who had more manic episodes performed poorer on cognitive tests assessing attention, memory, and executive functioning compared with patients with less episodes and with normal subjects.
Medication adherence in BD is a priority because of potential neurodegeneration in BD and the neuroprotective effects of mood stabilizers and some atypical antipsychotics (Box).
As emerging studies document morphologic brain changes associated with bipolar disorder (BD), researchers have been relating these changes to the duration and progression of illness. A longer duration of illness is associated with a smaller total gray matter volume on brain MRI of BD patients compared with unipolar patients and normal controls.a Brain MRI analysis of grey and white matter in elderly patients with longstanding BD who underwent neuropsychological testing to rule out dementia showed a decreased concentration of grey matter in the anterior limbic areas as well as reduced fiber tract coherence in the corpus callosum when compared with normal controls.b
Additionally, microstructural brain changes have been associated with acute mood states, in particular bipolar depression.c Lithium, valproate, olanzapine, and clozapine are neuroprotective in cultures of human-derived neuroblastoma cells, by enhancing the cells’ proliferation and survival.d
Source:
a. Frey BN, Zunta-Soares GB, Caetano SC, et al. Illness duration and total brain gray matter in bipolar disorder: evidence for neurodegeneration? European Neuropsychopharm. 2008;18:717-722.
b. Haller S, Xekardaki A, Delaloye C, et al. Combined analysis of grey matter voxel-based morphometry and white matter tract-based spatial statistics in late-life bipolar disorder. J Psychiatry Neurosci. 2011;36(1):100140.
c. Zanetti MV, Jackowski MP, Versace A, et al. State-dependent microstructural white matter changes in bipolar I depression. Eur Arch Psychiatry Clin Neurosci. 2009;259(6):316-328.
d. Aubry J, Schwald M, Ballmann E, et al. Early effects of mood stabilizers on the Akt/GSK-3ß signaling pathway and on cell survival and proliferation. Psychopharmacology. 2009;205:419-429.
Increasing adherence
Pharmacologic strategies. Adherence in BD often is difficult when patients require a complex medication regimen to control their illness. Patients and clinicians may prefer to use once-daily dosing drug formulations, which can provide consistent serum levels and fewer adverse effects. Divalproex extended-release (ER) allows once-daily dosing and improved tolerability by reducing fluctuations in valproic acid serum concentrations compared with the delayed-release formulation. In a retrospective chart review,25 most patients (62%) who switched to divalproex ER from divalproex delayed-release preferred the ER formulation; 52% showed clinical improvement, 81% did not experience side effects, and 8% demonstrated higher adherence after switching.25 Similarly, an extended-release formulation of carbamazepine is approved for treating acute mania.
Many atypical antipsychotics are FDA-approved for acute mania, acute bipolar depression, and/or maintenance (Table 2). Long-acting injectable formulations (LAIs) may be used as maintenance treatment if nonadherence is an issue. LAI risperidone, which is FDA-approved for maintenance treatment of bipolar I disorder (BDI), was found to be safe and effective in stable BD patients who were switched from an oral antipsychotic.26 Asenapine is provided in a rapidly absorbed, sublingual form and is FDA-approved for treating acute mania or mixed episodes associated with BDI.27 Overall, however, only slightly more than one-half of BD patients are adherent to atypical antipsychotics.15
Although antidepressant use in BD is controversial, Sajatovic17 found 44% of depressed BDI patients were treated with antidepressants. Novel extended-release antidepressant formulations—including controlled-release fluvoxamine, paroxetine, extended-release bupropion and venlafaxine, once-weekly fluoxetine, rapidly dissolving mirtazapine, and transdermal selegiline—can optimize drug delivery, minimize side effects, and delay onset of action.1
Psychosocial strategies used in BD include psychoeducation, cognitive-behavioral therapy (CBT), family-focused interventions, and interpersonal and social rhythm therapy (IPSRT) (Table 3).28-30 Psychoeducation alone or combined with other interventions can decrease the risk of relapse and hospitalization and improve adherence.28 In a 2-year study of 50 euthymic BD patients treated with lithium who participated in a brief hospital-based psychoeducation program, Even et al31 found patients’ knowledge about lithium but not their attitudes changed significantly after the program. The changes persisted 2 years after the intervention, with a trend toward a decreased hospitalization rate.
Miklowitz32 reported on 293 BD patients randomized to receive collaborative care (3 psychoeducational sessions delivered over 6 weeks) or 1 of 3 types of intensive psychotherapy: CBT, IPSRT, or family-focused therapy. Attrition was similar for both groups. Compared with those receiving collaborative care, significantly more patients receiving intensive psychotherapy recovered after 1 year, and did so in shorter time.
In a 3-year, multi-site Veterans Administration (VA) study, 306 BD patients received psychoeducation and support from nurse care coordinators who were responsible for access, continuity of care, and information flow to psychiatrists or usual care according to VA guidelines.33 Compared with the usual care group, patients who received psychoeducation and support from nurse care coordinators had shorter duration of manic episodes and improved function and quality of life. A meta-analysis30 of 12 randomized controlled trials of CBT in BD showed a medium effect size of CBT on adherence at 6 months post-treatment.
Table 2
FDA-approved medications for adult bipolar disorder
| Bipolar disorder indication | Medications |
|---|---|
| Acute treatment of mania/mixed episodes | Aripiprazole,a,b asenapine,a carbamazepine extended release,a divalproex sodium,a lithium,a quetiapine,a risperidone,a-c ziprasidonea,b |
| Depressive episodes | Olanzapine/fluoxetine,a quetiapinea |
| Maintenance treatment | Aripiprazole (as monotherapy and as adjunct to lithium or divalproex sodium),a,b asenapine,d lamotrigine,a lithium,a olanzapine,a-c quetiapine (as adjunct to lithium or divalproex sodium),a risperidone,e ziprasidone (as adjunct to lithium or divalproex sodium)a |
| apill form bintramuscular for acute agitation cdisintegrating tablet dsublingual tablet elong-acting injectable | |
Table 3
Psychosocial interventions for bipolar disorder
| Intervention | Description | Results in bipolar disorder | Optimal stage of illness for intervention |
|---|---|---|---|
| Individual and family psycho-education28,29 | Strategies to educate the patient about the illness, medications, side effects, and relapse prevention | Decreases relapse, (particularly manic episodes) and hospitalizations. Increases adherence | Manic episodes |
| Cognitive-behavioral therapy28-30 | Focuses on understanding patient’s perceptions of illness and treatment. Equates resistance with exploring, rather than challenging resistance to take medication. Identifies and modifies negative automatic thoughts about medication. Motivation techniques useful in comorbid substance use | Decreases clinical symptoms. Increases adherence, quality of life, and social functioning | Depressive episodes |
| IPSRT28,29 | Uses motivational interviewing and CBT techniques to stabilize daily routines and resolve interpersonal problems | Prevents relapse | Depressive episodes |
| Family-focused therapy28,29 | A combination of psychoeducation, communication, and problem-solving skills training | Reduces mood symptoms, number of depressive relapses, and time depressed. Increases adherence | Depressive episodes |
| IPSRT: interpersonal and social rhythm therapy | |||
Related Resource
- Deegan PE. The importance of personal medicine: a qualitative study of resilience in people with psychiatric disabilities. Scand J Public Health Suppl. 2005;66:29-35.
Drug Brand Names
- Aripiprazole • Abilify
- Asenapine • Saphris
- Bupropion • Wellbutrin
- Carbamazepine • Carbatrol, Tegretol
- Carbamazepine extended- release • Equetro
- Clozapine • Clozaril
- Divalproex • Depakote, Depakote ER
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid
- Mirtazapine • Remeron
- Olanzapine • Zyprexa
- Olanzapine/fluoxetine • Symbyax
- Paroxetine • Paxil
- Quetiapine • Seroquel, Seroquel XR
- Risperidone • Risperdal
- Risperidone long-acting injectable • Risperdal Consta
- Selegiline • Eldepryl, Emsam
- Valproate • Depacon
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosures
Dr. Foster receives research/grant support from the American Psychiatric Foundation, the National Institute of Mental Health, and Sunovion Pharmaceuticals.
Dr. Sheehan and Ms. Johns report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Buckley PF, Foster AE, Patel NC, et al. Adherence to mental health treatment. New York, NY: Oxford University Press; 2009;1-10:53-69.
2. Velligan D, Sajatovic M, Valenstein M, et al. Methodological challenges in psychiatric treatment adherence research. Clin Schizophr Relat Psychoses. 2010;4(1):74-91.
3. Weiden P, Rapkin B, Mott T, et al. Rating of Medication Influences (ROMI) scale in schizophrenia. Schizophr Bull. 1994;20:297-310.
4. Hogan TP, Awad AG, Eastwood R. A self-report scale predictive of drug compliance in schizophrenics: reliability and discriminative validity. Psychol Med. 1983;13(1):177-183.
5. Harvey NS. The development and descriptive use of the Lithium Attitudes Questionnaire. J Affect Disord. 1991;22(4):211-219.
6. Thompson K, Kulkarni J, Sergejew AA. Reliability and validity of a new Medication Adherence Rating Scale (MARS). Schizophr Res. 2000;42:241-247.
7. Byerly MJ, Nazonezny PA, Rush AJ. The Brief Adherence Rating Scale (BARS) validated against electronic monitoring in assessing the antipsychotic medication adherence of outpatients with schizophrenia and schizoaffective disorder. Schizophr Res. 2008;100(1-3):60-69.
8. Scott J, Pope M. Non-adherence with mood stabilizers: prevalence and predictors. J Clin Psychiatry. 2002;63:384-390.
9. Rosa AR, Marco M, Fachel JM, et al. Correlation between drug treatment adherence and lithium treatment attitudes and knowledge in bipolar patients. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:217-224.
10. Jónsdóttir H, Opjordsmoen S, Birkenaes A, et al. Medication adherence in outpatients with severe mental disorders, relation between self-reports and serum level. J Clin Psychopharmacol. 2010;30:169-175.
11. DelBello M, Hanserman D, Adler CM, et al. Twelve-month outcome of adolescents with bipolar disorder following first hospitalization for a manic or mixed episode. Am J Psychiatry. 2007;164:582-590.
12. Strakowski SM, Tsai SY, DelBello MP, et al. Outcome following a first manic episode: cross national US and Taiwan comparison. Bipolar Disord. 2007;9:820-827.
13. Sajatovic M, Valenstein M, Blow F, et al. Treatment adherence with lithium and anticonvulsant medications among patients with bipolar disorder. Psychiatr Serv. 2007;58:855-863.
14. Baldessarini RJ, Perry R, Pike J. Factors associated with treatment nonadherence among US bipolar patients. Hum Psychopharmacol. 2008;23:95-105.
15. Berk L, Hallam KT, Colom F, et al. Enhancing medication adherence in patients with bipolar disorder. Hum Psychopharmacol. 2010;25(1):1-16.
16. Johnson FR, Ozdemir S, Manjunath R, et al. Factors that affect adherence to bipolar disorder treatments: a stated-preference approach. Med Care. 2007;45(6):545-552.
17. Sajatovic M, Jenkins JH, Cassidy KA, et al. Medication treatment perceptions, concerns and expectations among depressed individuals with type I bipolar disorder. J Affect Disord. 2009;115(3):360-366.
18. Depp CA, Cain AE, Palmer BW, et al. Assessment of medication management ability in middle-aged and older adults with bipolar disorder. J Clin Psychopharmacol. 2008;28(2):225-229.
19. Gaudiano BA, Miller IW. Patients’ expectancies the alliance in pharmacotherapy, and treatment outcomes in bipolar disorder. J Consult Clin Psychol. 2006;74(4):671-676.
20. Copeland LA, Zeber JE, Salloum IM, et al. Treatment adherence and illness insight in veterans with bipolar disorder. J Nerv Ment Dis. 2008;196(1):16-21.
21. Yen CF, Chen CS, Ko CH, et al. Relationships between insight and medication adherence in outpatients with schizophrenia and bipolar disorder: prospective study. Psychiatry Clin Neurosci. 2005;59(4):403-409.
22. Drotar D, Greenley RN, Demeter CA, et al. Adherence to pharmacological treatment for juvenile bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):831-839.
23. Angst J, Angst F, Gerber-Werder R, et al. Suicide in 406 mood-disorder patients with and without long–term medication: a 40 to 44 years’ follow-up. Arch Suicide Res. 2005;9:279-300.
24. Lopez-Jaramillo C, Lopera-Vasquez J, Aurora G, et al. Effects of recurrence on the cognitive performance of patients with bipolar I disorder: implications for relapse prevention and treatment adherence. Bipolar Disord. 2010;12:557-567.
25. Minirth FB, Neal V. Assessment of patient preference and side effects in patients switched from divalproex sodium delayed release to divalproex sodium extended release. J Clin Psychopharmacol. 2005;25:99-101.
26. Han C, Lee MS, Pae CU, et al. Usefulness of long-acting injectable risperidone during 12-month maintenance therapy of bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1219-1223.
27. McIntyre RS, Cohen M, Zhao J, et al. Asenapine for long term treatment of bipolar disorder: a double blind 40-week extension study. J Affect Disord. 2010;126:358-365.
28. Velligan DI, Weiden PJ, Sajatovic M, et al. Strategies for addressing adherence problems in patients with serious and persistent mental illness: recommendations from expert consensus guidelines. J Psychiatr Pract. 2010;16(5):306-324.
29. Miklowitz DJ. Adjunctive psychotherapy for bipolar disorder: state of the evidence. Am J Psychiatry. 2008;165(11):1408-1419.
30. Szentagotai A, David D. The efficacy of cognitive-behavioral therapy in bipolar disorder: a quantitative meta-analysis. J Clin Psychiatry. 2010;71(1):66-72.
31. Even C, Thuile J, Stern K, et al. Psychoeducation for patients with bipolar disorder receiving lithium: short and long term impact on locus of control and knowledge about lithium. J Affect Disord. 2010;123:299-302.
32. Miklowitz DJ, Otto MW, Frank E, et al. Psychosocial treatments for bipolar depression: A 1-year randomized trial from the Systematic Treatment Enhancement Program. Arch Gen Psychiatry. 2007;64:419-426.
33. Bauer MS, McBride L, Williford WO, et al. Collaborative care for bipolar disorder, part II. Impact on clinical outcome, function and costs. Psychiatr Serv. 2006;57:937-945.
1. Buckley PF, Foster AE, Patel NC, et al. Adherence to mental health treatment. New York, NY: Oxford University Press; 2009;1-10:53-69.
2. Velligan D, Sajatovic M, Valenstein M, et al. Methodological challenges in psychiatric treatment adherence research. Clin Schizophr Relat Psychoses. 2010;4(1):74-91.
3. Weiden P, Rapkin B, Mott T, et al. Rating of Medication Influences (ROMI) scale in schizophrenia. Schizophr Bull. 1994;20:297-310.
4. Hogan TP, Awad AG, Eastwood R. A self-report scale predictive of drug compliance in schizophrenics: reliability and discriminative validity. Psychol Med. 1983;13(1):177-183.
5. Harvey NS. The development and descriptive use of the Lithium Attitudes Questionnaire. J Affect Disord. 1991;22(4):211-219.
6. Thompson K, Kulkarni J, Sergejew AA. Reliability and validity of a new Medication Adherence Rating Scale (MARS). Schizophr Res. 2000;42:241-247.
7. Byerly MJ, Nazonezny PA, Rush AJ. The Brief Adherence Rating Scale (BARS) validated against electronic monitoring in assessing the antipsychotic medication adherence of outpatients with schizophrenia and schizoaffective disorder. Schizophr Res. 2008;100(1-3):60-69.
8. Scott J, Pope M. Non-adherence with mood stabilizers: prevalence and predictors. J Clin Psychiatry. 2002;63:384-390.
9. Rosa AR, Marco M, Fachel JM, et al. Correlation between drug treatment adherence and lithium treatment attitudes and knowledge in bipolar patients. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:217-224.
10. Jónsdóttir H, Opjordsmoen S, Birkenaes A, et al. Medication adherence in outpatients with severe mental disorders, relation between self-reports and serum level. J Clin Psychopharmacol. 2010;30:169-175.
11. DelBello M, Hanserman D, Adler CM, et al. Twelve-month outcome of adolescents with bipolar disorder following first hospitalization for a manic or mixed episode. Am J Psychiatry. 2007;164:582-590.
12. Strakowski SM, Tsai SY, DelBello MP, et al. Outcome following a first manic episode: cross national US and Taiwan comparison. Bipolar Disord. 2007;9:820-827.
13. Sajatovic M, Valenstein M, Blow F, et al. Treatment adherence with lithium and anticonvulsant medications among patients with bipolar disorder. Psychiatr Serv. 2007;58:855-863.
14. Baldessarini RJ, Perry R, Pike J. Factors associated with treatment nonadherence among US bipolar patients. Hum Psychopharmacol. 2008;23:95-105.
15. Berk L, Hallam KT, Colom F, et al. Enhancing medication adherence in patients with bipolar disorder. Hum Psychopharmacol. 2010;25(1):1-16.
16. Johnson FR, Ozdemir S, Manjunath R, et al. Factors that affect adherence to bipolar disorder treatments: a stated-preference approach. Med Care. 2007;45(6):545-552.
17. Sajatovic M, Jenkins JH, Cassidy KA, et al. Medication treatment perceptions, concerns and expectations among depressed individuals with type I bipolar disorder. J Affect Disord. 2009;115(3):360-366.
18. Depp CA, Cain AE, Palmer BW, et al. Assessment of medication management ability in middle-aged and older adults with bipolar disorder. J Clin Psychopharmacol. 2008;28(2):225-229.
19. Gaudiano BA, Miller IW. Patients’ expectancies the alliance in pharmacotherapy, and treatment outcomes in bipolar disorder. J Consult Clin Psychol. 2006;74(4):671-676.
20. Copeland LA, Zeber JE, Salloum IM, et al. Treatment adherence and illness insight in veterans with bipolar disorder. J Nerv Ment Dis. 2008;196(1):16-21.
21. Yen CF, Chen CS, Ko CH, et al. Relationships between insight and medication adherence in outpatients with schizophrenia and bipolar disorder: prospective study. Psychiatry Clin Neurosci. 2005;59(4):403-409.
22. Drotar D, Greenley RN, Demeter CA, et al. Adherence to pharmacological treatment for juvenile bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):831-839.
23. Angst J, Angst F, Gerber-Werder R, et al. Suicide in 406 mood-disorder patients with and without long–term medication: a 40 to 44 years’ follow-up. Arch Suicide Res. 2005;9:279-300.
24. Lopez-Jaramillo C, Lopera-Vasquez J, Aurora G, et al. Effects of recurrence on the cognitive performance of patients with bipolar I disorder: implications for relapse prevention and treatment adherence. Bipolar Disord. 2010;12:557-567.
25. Minirth FB, Neal V. Assessment of patient preference and side effects in patients switched from divalproex sodium delayed release to divalproex sodium extended release. J Clin Psychopharmacol. 2005;25:99-101.
26. Han C, Lee MS, Pae CU, et al. Usefulness of long-acting injectable risperidone during 12-month maintenance therapy of bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1219-1223.
27. McIntyre RS, Cohen M, Zhao J, et al. Asenapine for long term treatment of bipolar disorder: a double blind 40-week extension study. J Affect Disord. 2010;126:358-365.
28. Velligan DI, Weiden PJ, Sajatovic M, et al. Strategies for addressing adherence problems in patients with serious and persistent mental illness: recommendations from expert consensus guidelines. J Psychiatr Pract. 2010;16(5):306-324.
29. Miklowitz DJ. Adjunctive psychotherapy for bipolar disorder: state of the evidence. Am J Psychiatry. 2008;165(11):1408-1419.
30. Szentagotai A, David D. The efficacy of cognitive-behavioral therapy in bipolar disorder: a quantitative meta-analysis. J Clin Psychiatry. 2010;71(1):66-72.
31. Even C, Thuile J, Stern K, et al. Psychoeducation for patients with bipolar disorder receiving lithium: short and long term impact on locus of control and knowledge about lithium. J Affect Disord. 2010;123:299-302.
32. Miklowitz DJ, Otto MW, Frank E, et al. Psychosocial treatments for bipolar depression: A 1-year randomized trial from the Systematic Treatment Enhancement Program. Arch Gen Psychiatry. 2007;64:419-426.
33. Bauer MS, McBride L, Williford WO, et al. Collaborative care for bipolar disorder, part II. Impact on clinical outcome, function and costs. Psychiatr Serv. 2006;57:937-945.
How to prevent adverse drug events
Medication errors due to system-, provider-, or patient-related factors contribute significantly to increased costs, adverse drug events (ADEs), and morbidity and mortality.1 One study found >60% of ADEs that led to hospitalization could have been prevented by strategies such as adequate monitoring or appropriate prescribing.2 Psychiatrists have an opportunity to reduce rates of ADEs; however, the possibility of disease symptoms overlapping with these adverse events is 1 of many obstacles prescribing clinicians face.1 Prescribers also must contend with adverse effects of polypharmacy, which are common among psychiatric patients. Patient-related factors of concern include:
- seeing multiple prescribers
- medication nonadherence
- failure to communicate use of herbal or over-the-counter products
- lack of insight
- comorbid medical and psychiatric diagnoses, such as dementia.1
This article highlights potential ADEs and major medication safety concerns that may contribute to morbidity and mortality among patients taking psychotropics. Although many factors are beyond the prescribing clinician’s control—such as medication dispensing and administration errors—psychiatrists can substantially reduce ADEs. We will cover potential adverse events associated with key medications or medication classes, drug interactions with potentially devastating consequences, and strategies to minimize risks of ADEs, including enhanced awareness and monitoring (Table 1).
Table 1
How to avoid ADEs with psychotropics
| Establish a collaborative practice among physicians, pharmacists, nurses, and social workers to enhance patient care and reduce the risk of medication errors and negative outcomes |
| Educate patients to increase their understanding of psychiatric diseases and medications and increase compliance with therapy. This may lead the patient to self-monitor drug efficacy and adverse effects |
| Be aware of psychotropic medications’ ‘black-box’ warnings that guide their safe use |
| Pay particular attention to drugs with a narrow therapeutic index, such as lithium and tricyclic antidepressants, which have small safety margins and are lethal in overdose |
| Avoid using 1 drug to treat the side effects of another. Minimizing polypharmacy can reduce medication errors, DDIs, and ADEs |
| Remain vigilant for DDIs, which can be serious and life-threatening. Examples include sudden cardiac death from additive QTc prolongation effects and NMS. Early detection of NMS and discontinuing the offending agent(s) can help prevent patient morbidity and mortality |
| Stay up-to-date on literature and drug warnings to employ best practices and avoid potentially serious adverse and/or lethal outcomes |
| Encourage patients to disclose any prescription drugs, over-the-counter medications, and herbal therapies they are taking |
| Develop strategies to prevent ADEs, such as personal formularies, suicide assessments, prescribing limited quantities, ‘eyes on’ medication administration, therapeutic drug monitoring, utilizing databases and resources for drug information, and patient education |
| ADEs: adverse drug events; DDIs: drug-drug interactions; NMS: neuroleptic malignant syndrome |
Prescription drug overdose
Each year, unintentional drug overdoses account for >20,000 deaths in the United States.3 Prescription medications, particularly opioid analgesics, have contributed to the doubling of overdose mortality rates in recent years. A recent study reported that nearly 50% of unintentional drug overdose deaths were associated with psychotropics and one-third of these deaths were associated with benzodiazepines, many of which were not prescribed to the individual.4
The risk of mortality from intentional drug overdose also must be assessed. Tricyclic antidepressants (TCAs) are a particularly lethal class of medications in suicide attempts and may result in arrhythmias, coma, seizures, respiratory failure, and death.5 Venlafaxine and mirtazapine are associated with greater risk of death and toxicity in overdose, respectively, than selective serotonin reuptake inhibitors (SSRIs).6 Lithium toxicity in overdose may lead to bradycardia, seizure, coma, hyperventilation, serotonin syndrome, respiratory failure, or death.5 The risk of death with lithium or benzodiazepine monotherapy is low when these agents are taken as prescribed. However, prescribers must exercise caution when these agents are used in combination. Interactions involving drugs with a narrow therapeutic index—such as lithium and TCAs—are more likely to be clinically significant because small increases in drug concentration can lead to serious adverse effects or death. See Related Resources for a review article on appropriate use and monitoring of lithium.
Drug-drug interactions
Many Americans take multiple prescription and nonprescription drugs, and psychiatric patients are more likely than other individuals to have more complex medication regimens.7 This can result in polypharmacy and drug-drug interactions (DDIs), which can lead to undesired medication effects and serious, potentially fatal ADEs.
Pharmacokinetic interactions typically affect drug concentrations and occur when 1 drug interferes with the absorption, distribution, metabolism, or excretion of another drug. Many common pharmacokinetic interactions involve the liver cytochrome P450 (CYP) system, which is responsible for metabolizing many medications.8 DDIs can occur when CYP enzymes are modified by inhibitors or inducers, which can decrease or increase drug clearance, respectively. Table 2 5,7,9 provides examples of common CYP450 substrates, inhibitors, and inducers. Polymorphisms in the pharmacogenetics of CYP450 also can affect overall drug clearance and the impact of DDIs.8
Pharmacodynamic interactions are caused by additive or competing effects of multiple drugs. The most serious of these involve medications that increase a patient’s risk of serotonin syndrome or neuroleptic malignant syndrome (NMS); both are medical emergencies that require immediate hospitalization.
Although any medication with serotonergic activity can induce serotonin syndrome, combinations of serotonergic drugs in particular are associated with increased risk.10 Serotonin syndrome is characterized by hyperthermia, altered muscle tone, altered mental status, and autonomic instability; rhabdomyolysis and disseminated intravascular coagulation are potential lethal complications.10 A high index of suspicion can help clinicians rapidly detect serotonin syndrome, discontinue offending agents, and initiate supportive treatments.
NMS is a life-threatening complication of antipsychotics characterized by fever, delirium, muscle rigidity, autonomic instability, and abnormal laboratory findings that include elevated white blood count and increased creatinine kinase from muscle injury. In early stages, NMS may be mistaken for extrapyramidal symptoms. Although NMS can occur with any antipsychotic as monotherapy, additive antidopaminergic effects increase the risk. Patients with a compromised CNS as a result of mental retardation, traumatic brain injury, or metabolic abnormalities also are at increased risk of developing NMS.11
Other pharmacodynamic interactions involve medications that may have additive effects on prolonging QTc intervals. For example, TCAs are pro-arrhythmic and have quinidine-like effects, which can cause cardiac conduction abnormalities and prolonged PR and QTc intervals.12 Employ routine ECG monitoring when prescribing multiple medications known to cause QTc prolongation, such as TCAs (Table 3).13,14 The Arizona Center for Education and Research on Therapeutics (www.azcert.org) provides a searchable list of QT-prolonging drugs (see Related Resources).
Medications also can interact with food, disease states, and herbal supplements. Alcohol interacts with many CNS-active medications, including many psychotropics. Patients taking benzodiazepines may experience oversedation and respiratory depression from alcohol’s additive sedating effects.5 Advise patients to limit their alcohol intake while taking CNS-depressing psychotropics such as benzodiazepines, antipsychotics, and some antidepressants. Monoamine oxidase inhibitors (MAOIs) and tyramine-containing food—such as cheese, beer, preserved meat, and soy sauce—can lead to a dangerous hypertensive crisis that requires immediate medical intervention to prevent life-threatening complications.5 Hypertensive crisis may be more significant in patients who have pre-existing hypertension. Finally, herbal supplements also can interact with medications. Patients who take St. John’s wort for depressive symptoms might not realize that it can reduce the efficacy of other drugs or increase their risk of serotonin syndrome.9
Table 2
Cytochrome P450 substrates, inhibitors, and inducers
| 3A4 | 2D6 | 2C9 | 2C19 | 1A2 | |
|---|---|---|---|---|---|
| Substrates | Carbamazepine Citalopram Fluoxetine Haloperidol Mirtazapine Oxcarbazepine Quetiapine Sertraline Ziprasidone | Aripiprazole Citalopram Duloxetine Fluoxetine Haloperidol Mirtazapine Paroxetine Risperidone Sertraline Venlafaxine TCAs | Amitriptyline Carbamazepine Sertraline Valproic acid | Citalopram Clomipramine Sertraline Valproic acid | Carbamazepine Clozapine Olanzapine |
| Inhibitors | Amiodarone Aprepitant Azole antifungals Carbamazepine Cimetidine Diltiazem Erythromycin Fluoxetine (norfloxetine) Grapefruit juice Imatinib Paroxetine Ritonavir Sertraline Verapamil | Amiodarone Bupropion Cimetidine Duloxetine Fluoxetine Methadone Paroxetine Ritonavir Sertraline | Amiodarone Fluconazole Isoniazid Sertraline Trimethoprim-sulfamethoxazole Valproic acid | Cimetidine Fluoxetine Ketoconazole Omeprazole Sertraline Valproic acid | Amiodarone Cimetidine Fluoroquinolones |
| Inducers | Carbamazepine Phenobarbital Phenytoin Rifampin St. John’s wort | Rifampin | Phenobarbital Rifampin | Carbamazepine Rifampin | Nafcillin Phenobarbital Rifampin Smoking |
| TCAs: tricyclic antidepressants Source: References 5,7,9 | |||||
Table 3
Psychotropics associated with QT prolongation
| Class | Agents |
|---|---|
| Antidepressants | Mirtazapine, SNRIs (desvenlafaxine, venlafaxine), SSRIs (citalopram, fluoxetine, paroxetine, sertraline), TCAs (amitriptyline, clomipramine, desipramine, doxepin, imipramine, protriptyline, trimipramine), trazodone |
| Typical antipsychotics | Chlorpromazine, fluphenazine, haloperidol, perphenazine, thioridazine, trifluoperazine |
| Atypical antipsychotics | Aripiprazole, asenapine, clozapine, iloperidone, paliperidone, quetiapine, risperidone, ziprasidone |
| Mood stabilizers | Lithium |
| Miscellaneous agents | Amantadine, atomoxetine, chloral hydrate, diphenhydramine, galantamine |
| Stimulants | Amphetamine/dextroamphetamine products, methylphenidate/dexmethylphenidate |
| SNRIs: serotonin-norepinephrine reuptake inhibitors; SSRIs: selective serotonin reuptake inhibitors; TCAs: tricyclic antidepressants Source: Adapted from references 13,14 | |
“Black-box” warnings issued by the FDA are included in the package insert to highlight a medication’s risks of dangerous and potentially lethal adverse effects. Table 4 highlights current black-box warnings for various psychotropics.5,14-16
Antidepressants and suicide. All medications with antidepressant indications carry a black-box warning for risk of suicidal ideation and behavior in children, adolescents, and young adults during the early months of medication therapy. This includes not only SSRIs and serotonin-norepinephrine reuptake inhibitors, but also anticonvulsants and atypical antipsychotics indicated for treating mood disorders. Monitor young patients carefully and advise family members to alert clinicians of any signs of suicidality or unusual behavior.
Lamotrigine and aseptic meningitis. Aseptic meningitis—inflammation of the meninges that is not caused by bacteria—is a rare but serious adverse effect of lamotrigine. Symptoms include headache, fever, stiff neck, nausea and vomiting, delirium, rash, and sensitivity to light.5 Forty cases of aseptic meningitis in children and adults were reported over 15 years, representing <.01% of all lamotrigine prescriptions.5 Most of these patients required hospitalization, but symptoms resolved after lamotrigine was discontinued. Prompt identification and management of aseptic meningitis are necessary to prevent permanent brain damage and death. Other complications of aseptic meningitis include long-term neurologic sequelae such as cognitive impairment, seizure disorders, and behavioral disturbances.
Table 4
Which psychotropics carry ‘black-box’ warnings?
| Warning | Class or medication affected | Comments |
|---|---|---|
| Suicidality | Antidepressants Antipsychotics indicated for mood disorders Anticonvulsants | See ‘Black-box warnings’ |
| Serious, life-threatening rashes such as Stevens-Johnson syndrome or toxic epidermal necrolysis | Lamotrigine Carbamazepine | Lamotrigine’s risk of severe dermatologic reactions necessitates slow titration during drug initiation Carbamazepine warning includes a recommendation for genetic screening in Asian patients because Stevens-Johnson syndrome is associated with the HLA-B*1502 allele found primarily in the Asian population |
| Increased mortality in elderly patients with dementia-related psychosis | Antipsychotics | A study of >10,000 geriatric patients with dementia showed mortality rates of 22.6% to 29.1% among those who took antipsychotics compared with 14.6% for patients taking other psychiatric medications. When antipsychotics are used in older adults, well-documented informed consent from the patient or substitute decision-maker is required |
| Other effects | Clozapine | Agranulocytosis occurs in 1% to 2% of clozapine patients, necessitating WBC/ANC monitoring Clozapine-induced myocarditis, generally accompanied by peripheral eosinophilia, usually occurs within the first 2 months of treatment, and can result in significant mortality from resultant cardiomyopathy. Early warning signs of fever, fatigue, and tachycardia are easily mistaken for the more benign effects of clozapine titration Seizures are more likely with higher doses. Cautious use is advised with patients who have an underlying seizure disorder Other cardiovascular and respiratory effects: Hypotension has been associated with rapid initial titration. Cardiac and respiratory arrest and circulatory collapse have occurred rarely. Respiratory complications are more likely when clozapine is used in combination with benzodiazepines |
| ANC: absolute neutrophil count; WBC: white blood cell Source: References 5,14-16 | ||
Other complications
Hematologic effects. All classes of psychotropics carry a risk (1 to 2 cases per year per 100,00 patients) of serious hematologic complications, including neutropenia, agranulocytosis, eosinophilia, thrombocytopenia, purpura, and anemia.17 Agranulocytosis has been associated most commonly with clozapine, carbamazepine, and typical antipsychotics.17 SSRIs, which are widely prescribed, are associated with increased risk of bruising and bleeding. Patients with bleeding or platelet disorders are at an increased risk for these complications.17
Seizures. Several classes of psychotropics are associated with an increased risk of seizures. Among antipsychotics, clozapine and chlorpromazine are the most seizurogenic.18 Among antidepressants, bupropion and clomipramine are most likely to lower seizure thresholds.18 Psychotropics’ seizure-inducing effects are dose-related. Vulnerability to seizures while taking psychotropics is related to having a history of epilepsy or brain injury.18 Seizures also can occur when benzodiazepines or anticonvulsants are withdrawn too quickly.
Heat stroke. Although a rare occurrence, psychotropics with anticholinergic side effects can contribute to heat stroke. Older patients are particularly vulnerable to the risk of body temperature dysregulation.19
Ketoacidosis and hyperosmolar coma. Medication-related deaths have occurred as a result of ketoacidosis and hyperosmolar coma associated with atypical antipsychotics. These hyperglycemia-related fatalities are most likely with clozapine and olanzapine.20
Hip fractures and falls. Geriatric patients are vulnerable to falls and resultant hip fractures related to use of TCAs, SSRIs, benzodiazepines, and antipsychotics. This is not a trivial matter; hip fractures increase the mortality rate by 12% to 20% in the year after the injury.21 The risk of falls is related to sedation, orthostatic hypotension, arrhythmias, and confusion associated with psychotropics.21,22
Akathisia and suicide. Unrecognized or undertreated akathisia is most commonly associated with antipsychotics, but also can occur with SSRIs. Although akathisia is commonly thought of as a motor syndrome of restlessness, patients may find the less-recognized psychic symptoms of increased inner turmoil and hallucinations just as distressing. This complex of symptoms is associated with an increased risk of suicide.23 If discontinuing the offending agent is not feasible, akathisia can be treated with beta blockers, benzodiazepines, or anticholinergics.24
Hepatotoxicity. Hepatotoxicity from psychotropics occurs in only a small percentage of patients, and can range from transient elevations in liver enzymes to fulminant liver failure. Adverse hepatic effects may be a manifestation of a hypersensitivity reaction accompanied by rash and eosinophilia.25 MAOIs and TCAs can cause cholestatic liver injury, whereas nefazodone has been associated with fulminant liver failure. Other psychotropics—including SSRIs, antipsychotics, benzodiazepines, and older antiepileptics—can cause negative hepatic effects but rarely are associated with acute liver failure.25,26 Although few medications can cause complete liver failure on their own, hepatotoxicity from medications may precipitate severe, potentially fatal outcomes in patients with underlying liver diseases such as hepatitis and cirrhosis. Additive hepatotoxicity from multiple medications also can be problematic. Although psychotropic-induced hepatotoxicity is rare, assess psychotropic doses in patients with liver dysfunction, because drug clearance may be altered, which increases the risk for other serious adverse events.25
Suicide assessment is key
Ongoing monitoring for current or developing suicidal ideation is an important strategy to prevent medication-related mortality in patients vulnerable to self-harm. Initial assessments and follow-up appointments should include a detailed inquiry about suicidal ideations, plans, and behaviors. Patients taking medications that carry black-box warnings for suicide risk should be seen frequently during the first few months of treatment. Patients receiving medications that are lethal in overdose (eg, lithium and TCAs) should be carefully screened for suicide risk. Prescribe medications in limited quantities or arrange for a family member to monitor the patient if necessary. Patients with a history of suicide attempts and current suicide plans may require close observation and initiating medications while hospitalized.
Other prevention strategies
Prescribing psychotropics in a manner that promotes mental well being while minimizing negative outcomes can be challenging. By developing a personal formulary of drugs commonly encountered and prescribed in their practice, psychiatrists can increase their awareness of serious safety concerns, potential DDIs, and appropriate use based on available literature.7,27
Medication histories and drug reconciliation—comparing a patient’s medication orders to all of the medications the patient has been taking—can help clinicians avoid making inappropriate dose adjustments, duplicating therapy, or prescribing medications patients previously have failed or did not tolerate. Establishing a collaborative practice environment with physicians, pharmacists, nurses, and social workers can minimize medication errors and risk of adverse outcomes by increasing communication regarding the patient’s treatment.7
Computerized drug databases and other electronic resources and consultation with pharmacists can help prescribers identify, avoid, and manage clinically significant DDIs.27 Medications could interact with other drugs as long as their effects persist in the body, which could be days to months after the drug is discontinued. Future research may lead to tools to identify patient pharmacogenetic profiles.
Recognizing psychotropic DDIs and adverse effects remains a challenge because of the complexity of the affected organ, the brain. Clinicians should be vigilant to changes in a patient’s presentation because they may be a manifestation of a medication side effect.7 Appropriate therapeutic drug monitoring should occur on a routine, scheduled basis. Closer monitoring may be necessary with dose changes, potential DDIs, signs and symptoms of toxicity/efficacy failure, and renal or hepatic function changes.
Lastly, patients’ education and involvement in their health care may increase their awareness, responsibility, and medication adherence. For challenging patients, family involvement and “eyes on” medication administration can increase adherence and prevent psychotropic misuse.
- Arizona Center for Education and Research on Therapeutics. Drugs that prolong the QT interval and/or induce Torsades de Pointes ventricular arrhythmia. www.azcert.org/medical-pros/drug-lists/drug-lists.cfm.
- Cates ME, Sims PJ. Therapeutic drug management of lithium. Am J Pharm Educ. 2005;69(5):88.
- Wren P, Frizzell LA, Keltner NL, et al. Three potentially fatal adverse effects of psychotropic medications. Perspect Psychiatr Care. 2003;39(2):75-81.
- Bishop JR, Bishop DL. How to prevent serotonin syndrome from drug-drug interactions. Current Psychiatry. 2011;10(3):81-83.
Drug Brand Names
- Amantadine • Symmetrel
- Amiodarone • Cordarone, Pacerone
- Amitriptyline • Elavil
- Amphetamine/dextroamphetamine • Adderall, others
- Aprepitant • Emend
- Aripiprazole • Abilify
- Asenapine • Saphris
- Atomoxetine • Strattera
- Bupropion • Wellbutrin, Zyban
- Carbamazepine • Tegretol, others
- Chloral hydrate • Somnote
- Chlorpromazine • Thorazine
- Cimetidine • Tagamet
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clozapine • Clozaril
- Desipramine • Norpramin
- Desvenlafaxine • Pristiq
- Diltiazem • Cardia, others
- Diphenhydramine • Benadryl, others
- Doxepin • Sinequan, Silenor
- Duloxetine • Cymbalta
- Erythromycin • Ery-Tab, others
- Fluconazole • Diflucan
- Fluoxetine • Prozac
- Fluphenazine • Prolixin
- Galantamine • Razadyne
- Haloperidol • Haldol
- Iloperidone • Fanapt
- Imatinib • Gleevec
- Imipramine • Tofranil
- Isoniazid • Nydrazid, others
- Ketoconazole • Nizoral, others
- Lamotrigine • Lamictal
- Lithium • Eskalith, others
- Methadone • Dolophine, Methadose
- Methylphenidate/ dexmethylphenidate • Ritalin, others
- Mirtazapine • Remeron
- Nafcillin • Nafcil, others
- Nefazodone • Serzone
- Olanzapine • Zyprexa
- Omeprazole • Prilosec
- Oxcarbazepine • Trileptal
- Paliperidone • Invega
- Paroxetine • Paxil
- Perphenazine • Trilafon
- Phenobarbital • Luminal, others
- Phenytoin • Dilantin
- Protriptyline • Vivactil
- Quetiapine • Seroquel
- Rifampin • Rifadin, others
- Risperidone • Risperdal
- Ritonavir • Norvir
- Sertraline • Zoloft
- Thioridazine • Mellaril
- Trazodone • Desyrel, Oleptro
- Trifluoperazine • Stelazine
- Trimethoprim/Sulfamethoxazole • Bactrim, Septra
- Trimipramine • Surmontil
- Valproic acid • Depakote, others
- Venlafaxine • Effexor
- Verapamil • Calan, others
- Ziprasidone • Geodon
Disclosure
Drs. Yu and Bostwick report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Casher is a speaker for AstraZeneca, Pfizer Inc, and Sunovion Pharmaceuticals.
1. Procyshyn RM, Barr AM, Brickell T, et al. Medication errors in psychiatry: a comprehensive review. CNS Drugs. 2004;24(7):595-609.
2. McDonnell PJ, Jacobs MR. Hospital admissions resulting from preventable adverse drug reactions. Ann Pharmacother. 2002;36(9):1331-1336.
3. Centers for Disease Control and Prevention. Prescription drug overdose: state health agencies respond. 2008. Available at: http://www.cdc.gov/HomeandRecreationalSafety/pubs/RXReport_web-a.pdf. Accessed February 15 2011.
4. Toblin RL, Paulozzi LJ, Logan JE, et al. Mental illness and psychotropic drug use among prescription drug overdose deaths: a medical examiner chart review. J Clin Psychiatry. 2010;71(4):491-496.
5. Micromedex Healthcare Series (electronic version). 2011. Available at: http://www.micromedex.com. Accessed February 15 2011.
6. Hawton K, Bergen H, Simkin S, et al. Toxicity of antidepressants: rates of suicide relative to prescribing and non-fatal overdose. Br J Psychiatry. 2010;196(5):354-358.
7. Preskorn SH, Flockhart D. 2010 guide to psychiatric drug interactions. Primary Psychiatry. 2009;16(12):45-74.
8. Lin JH, Lu AY. Inhibition and induction of cytochrome P450 and the clinical implications. Clin Pharmacokinet. 1998;35(5):361-390.
9. Kutscher EC, Alexander B. A review of drug interactions with psychiatric medicines for the pharmacy practitioner. J Pharm Pract. 2007;20(4):327-333.
10. Wren P, Frizzell LA, Keltner NL, et al. Three potentially fatal adverse effects of psychotropic medications. Perspect Psychiatr Care. 2003;39(2):75-81.
11. Dave M, Miceli K, Modha P. Psychiatric medicine. The psychiatrist’s guide to the treatment of common medical illnesses. Philadelphia PA: Lippincott Williams & Wilkins; 2007.
12. Mir S, Taylor D. The adverse effects of antidepressants. Curr Opin Psychiatry. 1997;10(2):88-94.
13. Drug-induced long QT interval. Pharmacist’s Letter/Prescriber’s Letter. 2010;26(4):260421.-
14. Drug-induced prolongation of the QT interval and torsades de pointes. 2011. Available at: http://online.factsandcomparisons.com. Accessed February 15 2011.
15. Kales HC, Valenstein M, Kim HM, et al. Mortality risk in patients with dementia treated with antipsychotics versus other psychiatric medications. Am J Psychiatry. 2007;164(10):1568-1576; quiz 1623.
16. Merrill DB, Ahmari SE, Bradford JM, et al. Myocarditis during clozapine treatment. Am J Psychiatry. 2006;163(2):204-208.
17. Oyesanmi O, Kunkel EJ, Monti DA, et al. Hematologic side effects of psychotropics. Psychosomatics. 1999;40(5):414-421.
18. Pisani F, Oteri G, Costa C, et al. Effects of psychotropic drugs on seizure threshold. Drug Saf. 2002;25(2):91-110.
19. Martin-Latry K, Goumy M, Latry P, et al. Psychotropic drugs use and risk of heat-related hospitalization. Eur Psychiatry. 2007;22:335-338.
20. Melkersson K, Dahl ML. Adverse metabolic effects associated with atypical antipsychotics: literature review and clinical implications. Drugs. 2004;64(7):701-723.
21. Liu B, Anderson G, Mittmann N, et al. Use of selective serotonin-reuptake inhibitors or tricyclic antidepressants and risk of hip fractures in elderly people. Lancet. 1998;351(9112):1303-1307.
22. Cumming RG, Le Couteur DG. Benzodiazepines and risk of hip fractures in older people: a review of the evidence. CNS Drugs. 2003;17(11):825-837.
23. Hirose S. The causes of underdiagnosing akathisia. Schizophr Bull. 2003;29(3):547-558.
24. Kane JM, Fleischhacker WW, Hansen L, et al. Akathisia: an updated review focusing on second-generation antipsychotics. J Clin Psychiatry. 2009;70(5):627-643.
25. Selim K, Kaplowitz N. Hepatotoxicity of psychotropic drugs. Hepatology. 1999;29(5):1347-1351.
26. Chitturi S, George J. Hepatotoxicity of commonly used drugs: nonsteroidal anti-inflammatory drugs antihypertensives, antidiabetic agents, anticonvulsants, lipid-lowering agents, psychotropic drugs. Semin Liver Dis. 2002;22(2):169-183.
27. Sandson NB, Armstrong SC, Cozza KL. An overview of psychotropic drug-drug interactions. Psychosomatics. 2005;46(5):464-494.
Medication errors due to system-, provider-, or patient-related factors contribute significantly to increased costs, adverse drug events (ADEs), and morbidity and mortality.1 One study found >60% of ADEs that led to hospitalization could have been prevented by strategies such as adequate monitoring or appropriate prescribing.2 Psychiatrists have an opportunity to reduce rates of ADEs; however, the possibility of disease symptoms overlapping with these adverse events is 1 of many obstacles prescribing clinicians face.1 Prescribers also must contend with adverse effects of polypharmacy, which are common among psychiatric patients. Patient-related factors of concern include:
- seeing multiple prescribers
- medication nonadherence
- failure to communicate use of herbal or over-the-counter products
- lack of insight
- comorbid medical and psychiatric diagnoses, such as dementia.1
This article highlights potential ADEs and major medication safety concerns that may contribute to morbidity and mortality among patients taking psychotropics. Although many factors are beyond the prescribing clinician’s control—such as medication dispensing and administration errors—psychiatrists can substantially reduce ADEs. We will cover potential adverse events associated with key medications or medication classes, drug interactions with potentially devastating consequences, and strategies to minimize risks of ADEs, including enhanced awareness and monitoring (Table 1).
Table 1
How to avoid ADEs with psychotropics
| Establish a collaborative practice among physicians, pharmacists, nurses, and social workers to enhance patient care and reduce the risk of medication errors and negative outcomes |
| Educate patients to increase their understanding of psychiatric diseases and medications and increase compliance with therapy. This may lead the patient to self-monitor drug efficacy and adverse effects |
| Be aware of psychotropic medications’ ‘black-box’ warnings that guide their safe use |
| Pay particular attention to drugs with a narrow therapeutic index, such as lithium and tricyclic antidepressants, which have small safety margins and are lethal in overdose |
| Avoid using 1 drug to treat the side effects of another. Minimizing polypharmacy can reduce medication errors, DDIs, and ADEs |
| Remain vigilant for DDIs, which can be serious and life-threatening. Examples include sudden cardiac death from additive QTc prolongation effects and NMS. Early detection of NMS and discontinuing the offending agent(s) can help prevent patient morbidity and mortality |
| Stay up-to-date on literature and drug warnings to employ best practices and avoid potentially serious adverse and/or lethal outcomes |
| Encourage patients to disclose any prescription drugs, over-the-counter medications, and herbal therapies they are taking |
| Develop strategies to prevent ADEs, such as personal formularies, suicide assessments, prescribing limited quantities, ‘eyes on’ medication administration, therapeutic drug monitoring, utilizing databases and resources for drug information, and patient education |
| ADEs: adverse drug events; DDIs: drug-drug interactions; NMS: neuroleptic malignant syndrome |
Prescription drug overdose
Each year, unintentional drug overdoses account for >20,000 deaths in the United States.3 Prescription medications, particularly opioid analgesics, have contributed to the doubling of overdose mortality rates in recent years. A recent study reported that nearly 50% of unintentional drug overdose deaths were associated with psychotropics and one-third of these deaths were associated with benzodiazepines, many of which were not prescribed to the individual.4
The risk of mortality from intentional drug overdose also must be assessed. Tricyclic antidepressants (TCAs) are a particularly lethal class of medications in suicide attempts and may result in arrhythmias, coma, seizures, respiratory failure, and death.5 Venlafaxine and mirtazapine are associated with greater risk of death and toxicity in overdose, respectively, than selective serotonin reuptake inhibitors (SSRIs).6 Lithium toxicity in overdose may lead to bradycardia, seizure, coma, hyperventilation, serotonin syndrome, respiratory failure, or death.5 The risk of death with lithium or benzodiazepine monotherapy is low when these agents are taken as prescribed. However, prescribers must exercise caution when these agents are used in combination. Interactions involving drugs with a narrow therapeutic index—such as lithium and TCAs—are more likely to be clinically significant because small increases in drug concentration can lead to serious adverse effects or death. See Related Resources for a review article on appropriate use and monitoring of lithium.
Drug-drug interactions
Many Americans take multiple prescription and nonprescription drugs, and psychiatric patients are more likely than other individuals to have more complex medication regimens.7 This can result in polypharmacy and drug-drug interactions (DDIs), which can lead to undesired medication effects and serious, potentially fatal ADEs.
Pharmacokinetic interactions typically affect drug concentrations and occur when 1 drug interferes with the absorption, distribution, metabolism, or excretion of another drug. Many common pharmacokinetic interactions involve the liver cytochrome P450 (CYP) system, which is responsible for metabolizing many medications.8 DDIs can occur when CYP enzymes are modified by inhibitors or inducers, which can decrease or increase drug clearance, respectively. Table 2 5,7,9 provides examples of common CYP450 substrates, inhibitors, and inducers. Polymorphisms in the pharmacogenetics of CYP450 also can affect overall drug clearance and the impact of DDIs.8
Pharmacodynamic interactions are caused by additive or competing effects of multiple drugs. The most serious of these involve medications that increase a patient’s risk of serotonin syndrome or neuroleptic malignant syndrome (NMS); both are medical emergencies that require immediate hospitalization.
Although any medication with serotonergic activity can induce serotonin syndrome, combinations of serotonergic drugs in particular are associated with increased risk.10 Serotonin syndrome is characterized by hyperthermia, altered muscle tone, altered mental status, and autonomic instability; rhabdomyolysis and disseminated intravascular coagulation are potential lethal complications.10 A high index of suspicion can help clinicians rapidly detect serotonin syndrome, discontinue offending agents, and initiate supportive treatments.
NMS is a life-threatening complication of antipsychotics characterized by fever, delirium, muscle rigidity, autonomic instability, and abnormal laboratory findings that include elevated white blood count and increased creatinine kinase from muscle injury. In early stages, NMS may be mistaken for extrapyramidal symptoms. Although NMS can occur with any antipsychotic as monotherapy, additive antidopaminergic effects increase the risk. Patients with a compromised CNS as a result of mental retardation, traumatic brain injury, or metabolic abnormalities also are at increased risk of developing NMS.11
Other pharmacodynamic interactions involve medications that may have additive effects on prolonging QTc intervals. For example, TCAs are pro-arrhythmic and have quinidine-like effects, which can cause cardiac conduction abnormalities and prolonged PR and QTc intervals.12 Employ routine ECG monitoring when prescribing multiple medications known to cause QTc prolongation, such as TCAs (Table 3).13,14 The Arizona Center for Education and Research on Therapeutics (www.azcert.org) provides a searchable list of QT-prolonging drugs (see Related Resources).
Medications also can interact with food, disease states, and herbal supplements. Alcohol interacts with many CNS-active medications, including many psychotropics. Patients taking benzodiazepines may experience oversedation and respiratory depression from alcohol’s additive sedating effects.5 Advise patients to limit their alcohol intake while taking CNS-depressing psychotropics such as benzodiazepines, antipsychotics, and some antidepressants. Monoamine oxidase inhibitors (MAOIs) and tyramine-containing food—such as cheese, beer, preserved meat, and soy sauce—can lead to a dangerous hypertensive crisis that requires immediate medical intervention to prevent life-threatening complications.5 Hypertensive crisis may be more significant in patients who have pre-existing hypertension. Finally, herbal supplements also can interact with medications. Patients who take St. John’s wort for depressive symptoms might not realize that it can reduce the efficacy of other drugs or increase their risk of serotonin syndrome.9
Table 2
Cytochrome P450 substrates, inhibitors, and inducers
| 3A4 | 2D6 | 2C9 | 2C19 | 1A2 | |
|---|---|---|---|---|---|
| Substrates | Carbamazepine Citalopram Fluoxetine Haloperidol Mirtazapine Oxcarbazepine Quetiapine Sertraline Ziprasidone | Aripiprazole Citalopram Duloxetine Fluoxetine Haloperidol Mirtazapine Paroxetine Risperidone Sertraline Venlafaxine TCAs | Amitriptyline Carbamazepine Sertraline Valproic acid | Citalopram Clomipramine Sertraline Valproic acid | Carbamazepine Clozapine Olanzapine |
| Inhibitors | Amiodarone Aprepitant Azole antifungals Carbamazepine Cimetidine Diltiazem Erythromycin Fluoxetine (norfloxetine) Grapefruit juice Imatinib Paroxetine Ritonavir Sertraline Verapamil | Amiodarone Bupropion Cimetidine Duloxetine Fluoxetine Methadone Paroxetine Ritonavir Sertraline | Amiodarone Fluconazole Isoniazid Sertraline Trimethoprim-sulfamethoxazole Valproic acid | Cimetidine Fluoxetine Ketoconazole Omeprazole Sertraline Valproic acid | Amiodarone Cimetidine Fluoroquinolones |
| Inducers | Carbamazepine Phenobarbital Phenytoin Rifampin St. John’s wort | Rifampin | Phenobarbital Rifampin | Carbamazepine Rifampin | Nafcillin Phenobarbital Rifampin Smoking |
| TCAs: tricyclic antidepressants Source: References 5,7,9 | |||||
Table 3
Psychotropics associated with QT prolongation
| Class | Agents |
|---|---|
| Antidepressants | Mirtazapine, SNRIs (desvenlafaxine, venlafaxine), SSRIs (citalopram, fluoxetine, paroxetine, sertraline), TCAs (amitriptyline, clomipramine, desipramine, doxepin, imipramine, protriptyline, trimipramine), trazodone |
| Typical antipsychotics | Chlorpromazine, fluphenazine, haloperidol, perphenazine, thioridazine, trifluoperazine |
| Atypical antipsychotics | Aripiprazole, asenapine, clozapine, iloperidone, paliperidone, quetiapine, risperidone, ziprasidone |
| Mood stabilizers | Lithium |
| Miscellaneous agents | Amantadine, atomoxetine, chloral hydrate, diphenhydramine, galantamine |
| Stimulants | Amphetamine/dextroamphetamine products, methylphenidate/dexmethylphenidate |
| SNRIs: serotonin-norepinephrine reuptake inhibitors; SSRIs: selective serotonin reuptake inhibitors; TCAs: tricyclic antidepressants Source: Adapted from references 13,14 | |
“Black-box” warnings issued by the FDA are included in the package insert to highlight a medication’s risks of dangerous and potentially lethal adverse effects. Table 4 highlights current black-box warnings for various psychotropics.5,14-16
Antidepressants and suicide. All medications with antidepressant indications carry a black-box warning for risk of suicidal ideation and behavior in children, adolescents, and young adults during the early months of medication therapy. This includes not only SSRIs and serotonin-norepinephrine reuptake inhibitors, but also anticonvulsants and atypical antipsychotics indicated for treating mood disorders. Monitor young patients carefully and advise family members to alert clinicians of any signs of suicidality or unusual behavior.
Lamotrigine and aseptic meningitis. Aseptic meningitis—inflammation of the meninges that is not caused by bacteria—is a rare but serious adverse effect of lamotrigine. Symptoms include headache, fever, stiff neck, nausea and vomiting, delirium, rash, and sensitivity to light.5 Forty cases of aseptic meningitis in children and adults were reported over 15 years, representing <.01% of all lamotrigine prescriptions.5 Most of these patients required hospitalization, but symptoms resolved after lamotrigine was discontinued. Prompt identification and management of aseptic meningitis are necessary to prevent permanent brain damage and death. Other complications of aseptic meningitis include long-term neurologic sequelae such as cognitive impairment, seizure disorders, and behavioral disturbances.
Table 4
Which psychotropics carry ‘black-box’ warnings?
| Warning | Class or medication affected | Comments |
|---|---|---|
| Suicidality | Antidepressants Antipsychotics indicated for mood disorders Anticonvulsants | See ‘Black-box warnings’ |
| Serious, life-threatening rashes such as Stevens-Johnson syndrome or toxic epidermal necrolysis | Lamotrigine Carbamazepine | Lamotrigine’s risk of severe dermatologic reactions necessitates slow titration during drug initiation Carbamazepine warning includes a recommendation for genetic screening in Asian patients because Stevens-Johnson syndrome is associated with the HLA-B*1502 allele found primarily in the Asian population |
| Increased mortality in elderly patients with dementia-related psychosis | Antipsychotics | A study of >10,000 geriatric patients with dementia showed mortality rates of 22.6% to 29.1% among those who took antipsychotics compared with 14.6% for patients taking other psychiatric medications. When antipsychotics are used in older adults, well-documented informed consent from the patient or substitute decision-maker is required |
| Other effects | Clozapine | Agranulocytosis occurs in 1% to 2% of clozapine patients, necessitating WBC/ANC monitoring Clozapine-induced myocarditis, generally accompanied by peripheral eosinophilia, usually occurs within the first 2 months of treatment, and can result in significant mortality from resultant cardiomyopathy. Early warning signs of fever, fatigue, and tachycardia are easily mistaken for the more benign effects of clozapine titration Seizures are more likely with higher doses. Cautious use is advised with patients who have an underlying seizure disorder Other cardiovascular and respiratory effects: Hypotension has been associated with rapid initial titration. Cardiac and respiratory arrest and circulatory collapse have occurred rarely. Respiratory complications are more likely when clozapine is used in combination with benzodiazepines |
| ANC: absolute neutrophil count; WBC: white blood cell Source: References 5,14-16 | ||
Other complications
Hematologic effects. All classes of psychotropics carry a risk (1 to 2 cases per year per 100,00 patients) of serious hematologic complications, including neutropenia, agranulocytosis, eosinophilia, thrombocytopenia, purpura, and anemia.17 Agranulocytosis has been associated most commonly with clozapine, carbamazepine, and typical antipsychotics.17 SSRIs, which are widely prescribed, are associated with increased risk of bruising and bleeding. Patients with bleeding or platelet disorders are at an increased risk for these complications.17
Seizures. Several classes of psychotropics are associated with an increased risk of seizures. Among antipsychotics, clozapine and chlorpromazine are the most seizurogenic.18 Among antidepressants, bupropion and clomipramine are most likely to lower seizure thresholds.18 Psychotropics’ seizure-inducing effects are dose-related. Vulnerability to seizures while taking psychotropics is related to having a history of epilepsy or brain injury.18 Seizures also can occur when benzodiazepines or anticonvulsants are withdrawn too quickly.
Heat stroke. Although a rare occurrence, psychotropics with anticholinergic side effects can contribute to heat stroke. Older patients are particularly vulnerable to the risk of body temperature dysregulation.19
Ketoacidosis and hyperosmolar coma. Medication-related deaths have occurred as a result of ketoacidosis and hyperosmolar coma associated with atypical antipsychotics. These hyperglycemia-related fatalities are most likely with clozapine and olanzapine.20
Hip fractures and falls. Geriatric patients are vulnerable to falls and resultant hip fractures related to use of TCAs, SSRIs, benzodiazepines, and antipsychotics. This is not a trivial matter; hip fractures increase the mortality rate by 12% to 20% in the year after the injury.21 The risk of falls is related to sedation, orthostatic hypotension, arrhythmias, and confusion associated with psychotropics.21,22
Akathisia and suicide. Unrecognized or undertreated akathisia is most commonly associated with antipsychotics, but also can occur with SSRIs. Although akathisia is commonly thought of as a motor syndrome of restlessness, patients may find the less-recognized psychic symptoms of increased inner turmoil and hallucinations just as distressing. This complex of symptoms is associated with an increased risk of suicide.23 If discontinuing the offending agent is not feasible, akathisia can be treated with beta blockers, benzodiazepines, or anticholinergics.24
Hepatotoxicity. Hepatotoxicity from psychotropics occurs in only a small percentage of patients, and can range from transient elevations in liver enzymes to fulminant liver failure. Adverse hepatic effects may be a manifestation of a hypersensitivity reaction accompanied by rash and eosinophilia.25 MAOIs and TCAs can cause cholestatic liver injury, whereas nefazodone has been associated with fulminant liver failure. Other psychotropics—including SSRIs, antipsychotics, benzodiazepines, and older antiepileptics—can cause negative hepatic effects but rarely are associated with acute liver failure.25,26 Although few medications can cause complete liver failure on their own, hepatotoxicity from medications may precipitate severe, potentially fatal outcomes in patients with underlying liver diseases such as hepatitis and cirrhosis. Additive hepatotoxicity from multiple medications also can be problematic. Although psychotropic-induced hepatotoxicity is rare, assess psychotropic doses in patients with liver dysfunction, because drug clearance may be altered, which increases the risk for other serious adverse events.25
Suicide assessment is key
Ongoing monitoring for current or developing suicidal ideation is an important strategy to prevent medication-related mortality in patients vulnerable to self-harm. Initial assessments and follow-up appointments should include a detailed inquiry about suicidal ideations, plans, and behaviors. Patients taking medications that carry black-box warnings for suicide risk should be seen frequently during the first few months of treatment. Patients receiving medications that are lethal in overdose (eg, lithium and TCAs) should be carefully screened for suicide risk. Prescribe medications in limited quantities or arrange for a family member to monitor the patient if necessary. Patients with a history of suicide attempts and current suicide plans may require close observation and initiating medications while hospitalized.
Other prevention strategies
Prescribing psychotropics in a manner that promotes mental well being while minimizing negative outcomes can be challenging. By developing a personal formulary of drugs commonly encountered and prescribed in their practice, psychiatrists can increase their awareness of serious safety concerns, potential DDIs, and appropriate use based on available literature.7,27
Medication histories and drug reconciliation—comparing a patient’s medication orders to all of the medications the patient has been taking—can help clinicians avoid making inappropriate dose adjustments, duplicating therapy, or prescribing medications patients previously have failed or did not tolerate. Establishing a collaborative practice environment with physicians, pharmacists, nurses, and social workers can minimize medication errors and risk of adverse outcomes by increasing communication regarding the patient’s treatment.7
Computerized drug databases and other electronic resources and consultation with pharmacists can help prescribers identify, avoid, and manage clinically significant DDIs.27 Medications could interact with other drugs as long as their effects persist in the body, which could be days to months after the drug is discontinued. Future research may lead to tools to identify patient pharmacogenetic profiles.
Recognizing psychotropic DDIs and adverse effects remains a challenge because of the complexity of the affected organ, the brain. Clinicians should be vigilant to changes in a patient’s presentation because they may be a manifestation of a medication side effect.7 Appropriate therapeutic drug monitoring should occur on a routine, scheduled basis. Closer monitoring may be necessary with dose changes, potential DDIs, signs and symptoms of toxicity/efficacy failure, and renal or hepatic function changes.
Lastly, patients’ education and involvement in their health care may increase their awareness, responsibility, and medication adherence. For challenging patients, family involvement and “eyes on” medication administration can increase adherence and prevent psychotropic misuse.
- Arizona Center for Education and Research on Therapeutics. Drugs that prolong the QT interval and/or induce Torsades de Pointes ventricular arrhythmia. www.azcert.org/medical-pros/drug-lists/drug-lists.cfm.
- Cates ME, Sims PJ. Therapeutic drug management of lithium. Am J Pharm Educ. 2005;69(5):88.
- Wren P, Frizzell LA, Keltner NL, et al. Three potentially fatal adverse effects of psychotropic medications. Perspect Psychiatr Care. 2003;39(2):75-81.
- Bishop JR, Bishop DL. How to prevent serotonin syndrome from drug-drug interactions. Current Psychiatry. 2011;10(3):81-83.
Drug Brand Names
- Amantadine • Symmetrel
- Amiodarone • Cordarone, Pacerone
- Amitriptyline • Elavil
- Amphetamine/dextroamphetamine • Adderall, others
- Aprepitant • Emend
- Aripiprazole • Abilify
- Asenapine • Saphris
- Atomoxetine • Strattera
- Bupropion • Wellbutrin, Zyban
- Carbamazepine • Tegretol, others
- Chloral hydrate • Somnote
- Chlorpromazine • Thorazine
- Cimetidine • Tagamet
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clozapine • Clozaril
- Desipramine • Norpramin
- Desvenlafaxine • Pristiq
- Diltiazem • Cardia, others
- Diphenhydramine • Benadryl, others
- Doxepin • Sinequan, Silenor
- Duloxetine • Cymbalta
- Erythromycin • Ery-Tab, others
- Fluconazole • Diflucan
- Fluoxetine • Prozac
- Fluphenazine • Prolixin
- Galantamine • Razadyne
- Haloperidol • Haldol
- Iloperidone • Fanapt
- Imatinib • Gleevec
- Imipramine • Tofranil
- Isoniazid • Nydrazid, others
- Ketoconazole • Nizoral, others
- Lamotrigine • Lamictal
- Lithium • Eskalith, others
- Methadone • Dolophine, Methadose
- Methylphenidate/ dexmethylphenidate • Ritalin, others
- Mirtazapine • Remeron
- Nafcillin • Nafcil, others
- Nefazodone • Serzone
- Olanzapine • Zyprexa
- Omeprazole • Prilosec
- Oxcarbazepine • Trileptal
- Paliperidone • Invega
- Paroxetine • Paxil
- Perphenazine • Trilafon
- Phenobarbital • Luminal, others
- Phenytoin • Dilantin
- Protriptyline • Vivactil
- Quetiapine • Seroquel
- Rifampin • Rifadin, others
- Risperidone • Risperdal
- Ritonavir • Norvir
- Sertraline • Zoloft
- Thioridazine • Mellaril
- Trazodone • Desyrel, Oleptro
- Trifluoperazine • Stelazine
- Trimethoprim/Sulfamethoxazole • Bactrim, Septra
- Trimipramine • Surmontil
- Valproic acid • Depakote, others
- Venlafaxine • Effexor
- Verapamil • Calan, others
- Ziprasidone • Geodon
Disclosure
Drs. Yu and Bostwick report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Casher is a speaker for AstraZeneca, Pfizer Inc, and Sunovion Pharmaceuticals.
Medication errors due to system-, provider-, or patient-related factors contribute significantly to increased costs, adverse drug events (ADEs), and morbidity and mortality.1 One study found >60% of ADEs that led to hospitalization could have been prevented by strategies such as adequate monitoring or appropriate prescribing.2 Psychiatrists have an opportunity to reduce rates of ADEs; however, the possibility of disease symptoms overlapping with these adverse events is 1 of many obstacles prescribing clinicians face.1 Prescribers also must contend with adverse effects of polypharmacy, which are common among psychiatric patients. Patient-related factors of concern include:
- seeing multiple prescribers
- medication nonadherence
- failure to communicate use of herbal or over-the-counter products
- lack of insight
- comorbid medical and psychiatric diagnoses, such as dementia.1
This article highlights potential ADEs and major medication safety concerns that may contribute to morbidity and mortality among patients taking psychotropics. Although many factors are beyond the prescribing clinician’s control—such as medication dispensing and administration errors—psychiatrists can substantially reduce ADEs. We will cover potential adverse events associated with key medications or medication classes, drug interactions with potentially devastating consequences, and strategies to minimize risks of ADEs, including enhanced awareness and monitoring (Table 1).
Table 1
How to avoid ADEs with psychotropics
| Establish a collaborative practice among physicians, pharmacists, nurses, and social workers to enhance patient care and reduce the risk of medication errors and negative outcomes |
| Educate patients to increase their understanding of psychiatric diseases and medications and increase compliance with therapy. This may lead the patient to self-monitor drug efficacy and adverse effects |
| Be aware of psychotropic medications’ ‘black-box’ warnings that guide their safe use |
| Pay particular attention to drugs with a narrow therapeutic index, such as lithium and tricyclic antidepressants, which have small safety margins and are lethal in overdose |
| Avoid using 1 drug to treat the side effects of another. Minimizing polypharmacy can reduce medication errors, DDIs, and ADEs |
| Remain vigilant for DDIs, which can be serious and life-threatening. Examples include sudden cardiac death from additive QTc prolongation effects and NMS. Early detection of NMS and discontinuing the offending agent(s) can help prevent patient morbidity and mortality |
| Stay up-to-date on literature and drug warnings to employ best practices and avoid potentially serious adverse and/or lethal outcomes |
| Encourage patients to disclose any prescription drugs, over-the-counter medications, and herbal therapies they are taking |
| Develop strategies to prevent ADEs, such as personal formularies, suicide assessments, prescribing limited quantities, ‘eyes on’ medication administration, therapeutic drug monitoring, utilizing databases and resources for drug information, and patient education |
| ADEs: adverse drug events; DDIs: drug-drug interactions; NMS: neuroleptic malignant syndrome |
Prescription drug overdose
Each year, unintentional drug overdoses account for >20,000 deaths in the United States.3 Prescription medications, particularly opioid analgesics, have contributed to the doubling of overdose mortality rates in recent years. A recent study reported that nearly 50% of unintentional drug overdose deaths were associated with psychotropics and one-third of these deaths were associated with benzodiazepines, many of which were not prescribed to the individual.4
The risk of mortality from intentional drug overdose also must be assessed. Tricyclic antidepressants (TCAs) are a particularly lethal class of medications in suicide attempts and may result in arrhythmias, coma, seizures, respiratory failure, and death.5 Venlafaxine and mirtazapine are associated with greater risk of death and toxicity in overdose, respectively, than selective serotonin reuptake inhibitors (SSRIs).6 Lithium toxicity in overdose may lead to bradycardia, seizure, coma, hyperventilation, serotonin syndrome, respiratory failure, or death.5 The risk of death with lithium or benzodiazepine monotherapy is low when these agents are taken as prescribed. However, prescribers must exercise caution when these agents are used in combination. Interactions involving drugs with a narrow therapeutic index—such as lithium and TCAs—are more likely to be clinically significant because small increases in drug concentration can lead to serious adverse effects or death. See Related Resources for a review article on appropriate use and monitoring of lithium.
Drug-drug interactions
Many Americans take multiple prescription and nonprescription drugs, and psychiatric patients are more likely than other individuals to have more complex medication regimens.7 This can result in polypharmacy and drug-drug interactions (DDIs), which can lead to undesired medication effects and serious, potentially fatal ADEs.
Pharmacokinetic interactions typically affect drug concentrations and occur when 1 drug interferes with the absorption, distribution, metabolism, or excretion of another drug. Many common pharmacokinetic interactions involve the liver cytochrome P450 (CYP) system, which is responsible for metabolizing many medications.8 DDIs can occur when CYP enzymes are modified by inhibitors or inducers, which can decrease or increase drug clearance, respectively. Table 2 5,7,9 provides examples of common CYP450 substrates, inhibitors, and inducers. Polymorphisms in the pharmacogenetics of CYP450 also can affect overall drug clearance and the impact of DDIs.8
Pharmacodynamic interactions are caused by additive or competing effects of multiple drugs. The most serious of these involve medications that increase a patient’s risk of serotonin syndrome or neuroleptic malignant syndrome (NMS); both are medical emergencies that require immediate hospitalization.
Although any medication with serotonergic activity can induce serotonin syndrome, combinations of serotonergic drugs in particular are associated with increased risk.10 Serotonin syndrome is characterized by hyperthermia, altered muscle tone, altered mental status, and autonomic instability; rhabdomyolysis and disseminated intravascular coagulation are potential lethal complications.10 A high index of suspicion can help clinicians rapidly detect serotonin syndrome, discontinue offending agents, and initiate supportive treatments.
NMS is a life-threatening complication of antipsychotics characterized by fever, delirium, muscle rigidity, autonomic instability, and abnormal laboratory findings that include elevated white blood count and increased creatinine kinase from muscle injury. In early stages, NMS may be mistaken for extrapyramidal symptoms. Although NMS can occur with any antipsychotic as monotherapy, additive antidopaminergic effects increase the risk. Patients with a compromised CNS as a result of mental retardation, traumatic brain injury, or metabolic abnormalities also are at increased risk of developing NMS.11
Other pharmacodynamic interactions involve medications that may have additive effects on prolonging QTc intervals. For example, TCAs are pro-arrhythmic and have quinidine-like effects, which can cause cardiac conduction abnormalities and prolonged PR and QTc intervals.12 Employ routine ECG monitoring when prescribing multiple medications known to cause QTc prolongation, such as TCAs (Table 3).13,14 The Arizona Center for Education and Research on Therapeutics (www.azcert.org) provides a searchable list of QT-prolonging drugs (see Related Resources).
Medications also can interact with food, disease states, and herbal supplements. Alcohol interacts with many CNS-active medications, including many psychotropics. Patients taking benzodiazepines may experience oversedation and respiratory depression from alcohol’s additive sedating effects.5 Advise patients to limit their alcohol intake while taking CNS-depressing psychotropics such as benzodiazepines, antipsychotics, and some antidepressants. Monoamine oxidase inhibitors (MAOIs) and tyramine-containing food—such as cheese, beer, preserved meat, and soy sauce—can lead to a dangerous hypertensive crisis that requires immediate medical intervention to prevent life-threatening complications.5 Hypertensive crisis may be more significant in patients who have pre-existing hypertension. Finally, herbal supplements also can interact with medications. Patients who take St. John’s wort for depressive symptoms might not realize that it can reduce the efficacy of other drugs or increase their risk of serotonin syndrome.9
Table 2
Cytochrome P450 substrates, inhibitors, and inducers
| 3A4 | 2D6 | 2C9 | 2C19 | 1A2 | |
|---|---|---|---|---|---|
| Substrates | Carbamazepine Citalopram Fluoxetine Haloperidol Mirtazapine Oxcarbazepine Quetiapine Sertraline Ziprasidone | Aripiprazole Citalopram Duloxetine Fluoxetine Haloperidol Mirtazapine Paroxetine Risperidone Sertraline Venlafaxine TCAs | Amitriptyline Carbamazepine Sertraline Valproic acid | Citalopram Clomipramine Sertraline Valproic acid | Carbamazepine Clozapine Olanzapine |
| Inhibitors | Amiodarone Aprepitant Azole antifungals Carbamazepine Cimetidine Diltiazem Erythromycin Fluoxetine (norfloxetine) Grapefruit juice Imatinib Paroxetine Ritonavir Sertraline Verapamil | Amiodarone Bupropion Cimetidine Duloxetine Fluoxetine Methadone Paroxetine Ritonavir Sertraline | Amiodarone Fluconazole Isoniazid Sertraline Trimethoprim-sulfamethoxazole Valproic acid | Cimetidine Fluoxetine Ketoconazole Omeprazole Sertraline Valproic acid | Amiodarone Cimetidine Fluoroquinolones |
| Inducers | Carbamazepine Phenobarbital Phenytoin Rifampin St. John’s wort | Rifampin | Phenobarbital Rifampin | Carbamazepine Rifampin | Nafcillin Phenobarbital Rifampin Smoking |
| TCAs: tricyclic antidepressants Source: References 5,7,9 | |||||
Table 3
Psychotropics associated with QT prolongation
| Class | Agents |
|---|---|
| Antidepressants | Mirtazapine, SNRIs (desvenlafaxine, venlafaxine), SSRIs (citalopram, fluoxetine, paroxetine, sertraline), TCAs (amitriptyline, clomipramine, desipramine, doxepin, imipramine, protriptyline, trimipramine), trazodone |
| Typical antipsychotics | Chlorpromazine, fluphenazine, haloperidol, perphenazine, thioridazine, trifluoperazine |
| Atypical antipsychotics | Aripiprazole, asenapine, clozapine, iloperidone, paliperidone, quetiapine, risperidone, ziprasidone |
| Mood stabilizers | Lithium |
| Miscellaneous agents | Amantadine, atomoxetine, chloral hydrate, diphenhydramine, galantamine |
| Stimulants | Amphetamine/dextroamphetamine products, methylphenidate/dexmethylphenidate |
| SNRIs: serotonin-norepinephrine reuptake inhibitors; SSRIs: selective serotonin reuptake inhibitors; TCAs: tricyclic antidepressants Source: Adapted from references 13,14 | |
“Black-box” warnings issued by the FDA are included in the package insert to highlight a medication’s risks of dangerous and potentially lethal adverse effects. Table 4 highlights current black-box warnings for various psychotropics.5,14-16
Antidepressants and suicide. All medications with antidepressant indications carry a black-box warning for risk of suicidal ideation and behavior in children, adolescents, and young adults during the early months of medication therapy. This includes not only SSRIs and serotonin-norepinephrine reuptake inhibitors, but also anticonvulsants and atypical antipsychotics indicated for treating mood disorders. Monitor young patients carefully and advise family members to alert clinicians of any signs of suicidality or unusual behavior.
Lamotrigine and aseptic meningitis. Aseptic meningitis—inflammation of the meninges that is not caused by bacteria—is a rare but serious adverse effect of lamotrigine. Symptoms include headache, fever, stiff neck, nausea and vomiting, delirium, rash, and sensitivity to light.5 Forty cases of aseptic meningitis in children and adults were reported over 15 years, representing <.01% of all lamotrigine prescriptions.5 Most of these patients required hospitalization, but symptoms resolved after lamotrigine was discontinued. Prompt identification and management of aseptic meningitis are necessary to prevent permanent brain damage and death. Other complications of aseptic meningitis include long-term neurologic sequelae such as cognitive impairment, seizure disorders, and behavioral disturbances.
Table 4
Which psychotropics carry ‘black-box’ warnings?
| Warning | Class or medication affected | Comments |
|---|---|---|
| Suicidality | Antidepressants Antipsychotics indicated for mood disorders Anticonvulsants | See ‘Black-box warnings’ |
| Serious, life-threatening rashes such as Stevens-Johnson syndrome or toxic epidermal necrolysis | Lamotrigine Carbamazepine | Lamotrigine’s risk of severe dermatologic reactions necessitates slow titration during drug initiation Carbamazepine warning includes a recommendation for genetic screening in Asian patients because Stevens-Johnson syndrome is associated with the HLA-B*1502 allele found primarily in the Asian population |
| Increased mortality in elderly patients with dementia-related psychosis | Antipsychotics | A study of >10,000 geriatric patients with dementia showed mortality rates of 22.6% to 29.1% among those who took antipsychotics compared with 14.6% for patients taking other psychiatric medications. When antipsychotics are used in older adults, well-documented informed consent from the patient or substitute decision-maker is required |
| Other effects | Clozapine | Agranulocytosis occurs in 1% to 2% of clozapine patients, necessitating WBC/ANC monitoring Clozapine-induced myocarditis, generally accompanied by peripheral eosinophilia, usually occurs within the first 2 months of treatment, and can result in significant mortality from resultant cardiomyopathy. Early warning signs of fever, fatigue, and tachycardia are easily mistaken for the more benign effects of clozapine titration Seizures are more likely with higher doses. Cautious use is advised with patients who have an underlying seizure disorder Other cardiovascular and respiratory effects: Hypotension has been associated with rapid initial titration. Cardiac and respiratory arrest and circulatory collapse have occurred rarely. Respiratory complications are more likely when clozapine is used in combination with benzodiazepines |
| ANC: absolute neutrophil count; WBC: white blood cell Source: References 5,14-16 | ||
Other complications
Hematologic effects. All classes of psychotropics carry a risk (1 to 2 cases per year per 100,00 patients) of serious hematologic complications, including neutropenia, agranulocytosis, eosinophilia, thrombocytopenia, purpura, and anemia.17 Agranulocytosis has been associated most commonly with clozapine, carbamazepine, and typical antipsychotics.17 SSRIs, which are widely prescribed, are associated with increased risk of bruising and bleeding. Patients with bleeding or platelet disorders are at an increased risk for these complications.17
Seizures. Several classes of psychotropics are associated with an increased risk of seizures. Among antipsychotics, clozapine and chlorpromazine are the most seizurogenic.18 Among antidepressants, bupropion and clomipramine are most likely to lower seizure thresholds.18 Psychotropics’ seizure-inducing effects are dose-related. Vulnerability to seizures while taking psychotropics is related to having a history of epilepsy or brain injury.18 Seizures also can occur when benzodiazepines or anticonvulsants are withdrawn too quickly.
Heat stroke. Although a rare occurrence, psychotropics with anticholinergic side effects can contribute to heat stroke. Older patients are particularly vulnerable to the risk of body temperature dysregulation.19
Ketoacidosis and hyperosmolar coma. Medication-related deaths have occurred as a result of ketoacidosis and hyperosmolar coma associated with atypical antipsychotics. These hyperglycemia-related fatalities are most likely with clozapine and olanzapine.20
Hip fractures and falls. Geriatric patients are vulnerable to falls and resultant hip fractures related to use of TCAs, SSRIs, benzodiazepines, and antipsychotics. This is not a trivial matter; hip fractures increase the mortality rate by 12% to 20% in the year after the injury.21 The risk of falls is related to sedation, orthostatic hypotension, arrhythmias, and confusion associated with psychotropics.21,22
Akathisia and suicide. Unrecognized or undertreated akathisia is most commonly associated with antipsychotics, but also can occur with SSRIs. Although akathisia is commonly thought of as a motor syndrome of restlessness, patients may find the less-recognized psychic symptoms of increased inner turmoil and hallucinations just as distressing. This complex of symptoms is associated with an increased risk of suicide.23 If discontinuing the offending agent is not feasible, akathisia can be treated with beta blockers, benzodiazepines, or anticholinergics.24
Hepatotoxicity. Hepatotoxicity from psychotropics occurs in only a small percentage of patients, and can range from transient elevations in liver enzymes to fulminant liver failure. Adverse hepatic effects may be a manifestation of a hypersensitivity reaction accompanied by rash and eosinophilia.25 MAOIs and TCAs can cause cholestatic liver injury, whereas nefazodone has been associated with fulminant liver failure. Other psychotropics—including SSRIs, antipsychotics, benzodiazepines, and older antiepileptics—can cause negative hepatic effects but rarely are associated with acute liver failure.25,26 Although few medications can cause complete liver failure on their own, hepatotoxicity from medications may precipitate severe, potentially fatal outcomes in patients with underlying liver diseases such as hepatitis and cirrhosis. Additive hepatotoxicity from multiple medications also can be problematic. Although psychotropic-induced hepatotoxicity is rare, assess psychotropic doses in patients with liver dysfunction, because drug clearance may be altered, which increases the risk for other serious adverse events.25
Suicide assessment is key
Ongoing monitoring for current or developing suicidal ideation is an important strategy to prevent medication-related mortality in patients vulnerable to self-harm. Initial assessments and follow-up appointments should include a detailed inquiry about suicidal ideations, plans, and behaviors. Patients taking medications that carry black-box warnings for suicide risk should be seen frequently during the first few months of treatment. Patients receiving medications that are lethal in overdose (eg, lithium and TCAs) should be carefully screened for suicide risk. Prescribe medications in limited quantities or arrange for a family member to monitor the patient if necessary. Patients with a history of suicide attempts and current suicide plans may require close observation and initiating medications while hospitalized.
Other prevention strategies
Prescribing psychotropics in a manner that promotes mental well being while minimizing negative outcomes can be challenging. By developing a personal formulary of drugs commonly encountered and prescribed in their practice, psychiatrists can increase their awareness of serious safety concerns, potential DDIs, and appropriate use based on available literature.7,27
Medication histories and drug reconciliation—comparing a patient’s medication orders to all of the medications the patient has been taking—can help clinicians avoid making inappropriate dose adjustments, duplicating therapy, or prescribing medications patients previously have failed or did not tolerate. Establishing a collaborative practice environment with physicians, pharmacists, nurses, and social workers can minimize medication errors and risk of adverse outcomes by increasing communication regarding the patient’s treatment.7
Computerized drug databases and other electronic resources and consultation with pharmacists can help prescribers identify, avoid, and manage clinically significant DDIs.27 Medications could interact with other drugs as long as their effects persist in the body, which could be days to months after the drug is discontinued. Future research may lead to tools to identify patient pharmacogenetic profiles.
Recognizing psychotropic DDIs and adverse effects remains a challenge because of the complexity of the affected organ, the brain. Clinicians should be vigilant to changes in a patient’s presentation because they may be a manifestation of a medication side effect.7 Appropriate therapeutic drug monitoring should occur on a routine, scheduled basis. Closer monitoring may be necessary with dose changes, potential DDIs, signs and symptoms of toxicity/efficacy failure, and renal or hepatic function changes.
Lastly, patients’ education and involvement in their health care may increase their awareness, responsibility, and medication adherence. For challenging patients, family involvement and “eyes on” medication administration can increase adherence and prevent psychotropic misuse.
- Arizona Center for Education and Research on Therapeutics. Drugs that prolong the QT interval and/or induce Torsades de Pointes ventricular arrhythmia. www.azcert.org/medical-pros/drug-lists/drug-lists.cfm.
- Cates ME, Sims PJ. Therapeutic drug management of lithium. Am J Pharm Educ. 2005;69(5):88.
- Wren P, Frizzell LA, Keltner NL, et al. Three potentially fatal adverse effects of psychotropic medications. Perspect Psychiatr Care. 2003;39(2):75-81.
- Bishop JR, Bishop DL. How to prevent serotonin syndrome from drug-drug interactions. Current Psychiatry. 2011;10(3):81-83.
Drug Brand Names
- Amantadine • Symmetrel
- Amiodarone • Cordarone, Pacerone
- Amitriptyline • Elavil
- Amphetamine/dextroamphetamine • Adderall, others
- Aprepitant • Emend
- Aripiprazole • Abilify
- Asenapine • Saphris
- Atomoxetine • Strattera
- Bupropion • Wellbutrin, Zyban
- Carbamazepine • Tegretol, others
- Chloral hydrate • Somnote
- Chlorpromazine • Thorazine
- Cimetidine • Tagamet
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clozapine • Clozaril
- Desipramine • Norpramin
- Desvenlafaxine • Pristiq
- Diltiazem • Cardia, others
- Diphenhydramine • Benadryl, others
- Doxepin • Sinequan, Silenor
- Duloxetine • Cymbalta
- Erythromycin • Ery-Tab, others
- Fluconazole • Diflucan
- Fluoxetine • Prozac
- Fluphenazine • Prolixin
- Galantamine • Razadyne
- Haloperidol • Haldol
- Iloperidone • Fanapt
- Imatinib • Gleevec
- Imipramine • Tofranil
- Isoniazid • Nydrazid, others
- Ketoconazole • Nizoral, others
- Lamotrigine • Lamictal
- Lithium • Eskalith, others
- Methadone • Dolophine, Methadose
- Methylphenidate/ dexmethylphenidate • Ritalin, others
- Mirtazapine • Remeron
- Nafcillin • Nafcil, others
- Nefazodone • Serzone
- Olanzapine • Zyprexa
- Omeprazole • Prilosec
- Oxcarbazepine • Trileptal
- Paliperidone • Invega
- Paroxetine • Paxil
- Perphenazine • Trilafon
- Phenobarbital • Luminal, others
- Phenytoin • Dilantin
- Protriptyline • Vivactil
- Quetiapine • Seroquel
- Rifampin • Rifadin, others
- Risperidone • Risperdal
- Ritonavir • Norvir
- Sertraline • Zoloft
- Thioridazine • Mellaril
- Trazodone • Desyrel, Oleptro
- Trifluoperazine • Stelazine
- Trimethoprim/Sulfamethoxazole • Bactrim, Septra
- Trimipramine • Surmontil
- Valproic acid • Depakote, others
- Venlafaxine • Effexor
- Verapamil • Calan, others
- Ziprasidone • Geodon
Disclosure
Drs. Yu and Bostwick report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Casher is a speaker for AstraZeneca, Pfizer Inc, and Sunovion Pharmaceuticals.
1. Procyshyn RM, Barr AM, Brickell T, et al. Medication errors in psychiatry: a comprehensive review. CNS Drugs. 2004;24(7):595-609.
2. McDonnell PJ, Jacobs MR. Hospital admissions resulting from preventable adverse drug reactions. Ann Pharmacother. 2002;36(9):1331-1336.
3. Centers for Disease Control and Prevention. Prescription drug overdose: state health agencies respond. 2008. Available at: http://www.cdc.gov/HomeandRecreationalSafety/pubs/RXReport_web-a.pdf. Accessed February 15 2011.
4. Toblin RL, Paulozzi LJ, Logan JE, et al. Mental illness and psychotropic drug use among prescription drug overdose deaths: a medical examiner chart review. J Clin Psychiatry. 2010;71(4):491-496.
5. Micromedex Healthcare Series (electronic version). 2011. Available at: http://www.micromedex.com. Accessed February 15 2011.
6. Hawton K, Bergen H, Simkin S, et al. Toxicity of antidepressants: rates of suicide relative to prescribing and non-fatal overdose. Br J Psychiatry. 2010;196(5):354-358.
7. Preskorn SH, Flockhart D. 2010 guide to psychiatric drug interactions. Primary Psychiatry. 2009;16(12):45-74.
8. Lin JH, Lu AY. Inhibition and induction of cytochrome P450 and the clinical implications. Clin Pharmacokinet. 1998;35(5):361-390.
9. Kutscher EC, Alexander B. A review of drug interactions with psychiatric medicines for the pharmacy practitioner. J Pharm Pract. 2007;20(4):327-333.
10. Wren P, Frizzell LA, Keltner NL, et al. Three potentially fatal adverse effects of psychotropic medications. Perspect Psychiatr Care. 2003;39(2):75-81.
11. Dave M, Miceli K, Modha P. Psychiatric medicine. The psychiatrist’s guide to the treatment of common medical illnesses. Philadelphia PA: Lippincott Williams & Wilkins; 2007.
12. Mir S, Taylor D. The adverse effects of antidepressants. Curr Opin Psychiatry. 1997;10(2):88-94.
13. Drug-induced long QT interval. Pharmacist’s Letter/Prescriber’s Letter. 2010;26(4):260421.-
14. Drug-induced prolongation of the QT interval and torsades de pointes. 2011. Available at: http://online.factsandcomparisons.com. Accessed February 15 2011.
15. Kales HC, Valenstein M, Kim HM, et al. Mortality risk in patients with dementia treated with antipsychotics versus other psychiatric medications. Am J Psychiatry. 2007;164(10):1568-1576; quiz 1623.
16. Merrill DB, Ahmari SE, Bradford JM, et al. Myocarditis during clozapine treatment. Am J Psychiatry. 2006;163(2):204-208.
17. Oyesanmi O, Kunkel EJ, Monti DA, et al. Hematologic side effects of psychotropics. Psychosomatics. 1999;40(5):414-421.
18. Pisani F, Oteri G, Costa C, et al. Effects of psychotropic drugs on seizure threshold. Drug Saf. 2002;25(2):91-110.
19. Martin-Latry K, Goumy M, Latry P, et al. Psychotropic drugs use and risk of heat-related hospitalization. Eur Psychiatry. 2007;22:335-338.
20. Melkersson K, Dahl ML. Adverse metabolic effects associated with atypical antipsychotics: literature review and clinical implications. Drugs. 2004;64(7):701-723.
21. Liu B, Anderson G, Mittmann N, et al. Use of selective serotonin-reuptake inhibitors or tricyclic antidepressants and risk of hip fractures in elderly people. Lancet. 1998;351(9112):1303-1307.
22. Cumming RG, Le Couteur DG. Benzodiazepines and risk of hip fractures in older people: a review of the evidence. CNS Drugs. 2003;17(11):825-837.
23. Hirose S. The causes of underdiagnosing akathisia. Schizophr Bull. 2003;29(3):547-558.
24. Kane JM, Fleischhacker WW, Hansen L, et al. Akathisia: an updated review focusing on second-generation antipsychotics. J Clin Psychiatry. 2009;70(5):627-643.
25. Selim K, Kaplowitz N. Hepatotoxicity of psychotropic drugs. Hepatology. 1999;29(5):1347-1351.
26. Chitturi S, George J. Hepatotoxicity of commonly used drugs: nonsteroidal anti-inflammatory drugs antihypertensives, antidiabetic agents, anticonvulsants, lipid-lowering agents, psychotropic drugs. Semin Liver Dis. 2002;22(2):169-183.
27. Sandson NB, Armstrong SC, Cozza KL. An overview of psychotropic drug-drug interactions. Psychosomatics. 2005;46(5):464-494.
1. Procyshyn RM, Barr AM, Brickell T, et al. Medication errors in psychiatry: a comprehensive review. CNS Drugs. 2004;24(7):595-609.
2. McDonnell PJ, Jacobs MR. Hospital admissions resulting from preventable adverse drug reactions. Ann Pharmacother. 2002;36(9):1331-1336.
3. Centers for Disease Control and Prevention. Prescription drug overdose: state health agencies respond. 2008. Available at: http://www.cdc.gov/HomeandRecreationalSafety/pubs/RXReport_web-a.pdf. Accessed February 15 2011.
4. Toblin RL, Paulozzi LJ, Logan JE, et al. Mental illness and psychotropic drug use among prescription drug overdose deaths: a medical examiner chart review. J Clin Psychiatry. 2010;71(4):491-496.
5. Micromedex Healthcare Series (electronic version). 2011. Available at: http://www.micromedex.com. Accessed February 15 2011.
6. Hawton K, Bergen H, Simkin S, et al. Toxicity of antidepressants: rates of suicide relative to prescribing and non-fatal overdose. Br J Psychiatry. 2010;196(5):354-358.
7. Preskorn SH, Flockhart D. 2010 guide to psychiatric drug interactions. Primary Psychiatry. 2009;16(12):45-74.
8. Lin JH, Lu AY. Inhibition and induction of cytochrome P450 and the clinical implications. Clin Pharmacokinet. 1998;35(5):361-390.
9. Kutscher EC, Alexander B. A review of drug interactions with psychiatric medicines for the pharmacy practitioner. J Pharm Pract. 2007;20(4):327-333.
10. Wren P, Frizzell LA, Keltner NL, et al. Three potentially fatal adverse effects of psychotropic medications. Perspect Psychiatr Care. 2003;39(2):75-81.
11. Dave M, Miceli K, Modha P. Psychiatric medicine. The psychiatrist’s guide to the treatment of common medical illnesses. Philadelphia PA: Lippincott Williams & Wilkins; 2007.
12. Mir S, Taylor D. The adverse effects of antidepressants. Curr Opin Psychiatry. 1997;10(2):88-94.
13. Drug-induced long QT interval. Pharmacist’s Letter/Prescriber’s Letter. 2010;26(4):260421.-
14. Drug-induced prolongation of the QT interval and torsades de pointes. 2011. Available at: http://online.factsandcomparisons.com. Accessed February 15 2011.
15. Kales HC, Valenstein M, Kim HM, et al. Mortality risk in patients with dementia treated with antipsychotics versus other psychiatric medications. Am J Psychiatry. 2007;164(10):1568-1576; quiz 1623.
16. Merrill DB, Ahmari SE, Bradford JM, et al. Myocarditis during clozapine treatment. Am J Psychiatry. 2006;163(2):204-208.
17. Oyesanmi O, Kunkel EJ, Monti DA, et al. Hematologic side effects of psychotropics. Psychosomatics. 1999;40(5):414-421.
18. Pisani F, Oteri G, Costa C, et al. Effects of psychotropic drugs on seizure threshold. Drug Saf. 2002;25(2):91-110.
19. Martin-Latry K, Goumy M, Latry P, et al. Psychotropic drugs use and risk of heat-related hospitalization. Eur Psychiatry. 2007;22:335-338.
20. Melkersson K, Dahl ML. Adverse metabolic effects associated with atypical antipsychotics: literature review and clinical implications. Drugs. 2004;64(7):701-723.
21. Liu B, Anderson G, Mittmann N, et al. Use of selective serotonin-reuptake inhibitors or tricyclic antidepressants and risk of hip fractures in elderly people. Lancet. 1998;351(9112):1303-1307.
22. Cumming RG, Le Couteur DG. Benzodiazepines and risk of hip fractures in older people: a review of the evidence. CNS Drugs. 2003;17(11):825-837.
23. Hirose S. The causes of underdiagnosing akathisia. Schizophr Bull. 2003;29(3):547-558.
24. Kane JM, Fleischhacker WW, Hansen L, et al. Akathisia: an updated review focusing on second-generation antipsychotics. J Clin Psychiatry. 2009;70(5):627-643.
25. Selim K, Kaplowitz N. Hepatotoxicity of psychotropic drugs. Hepatology. 1999;29(5):1347-1351.
26. Chitturi S, George J. Hepatotoxicity of commonly used drugs: nonsteroidal anti-inflammatory drugs antihypertensives, antidiabetic agents, anticonvulsants, lipid-lowering agents, psychotropic drugs. Semin Liver Dis. 2002;22(2):169-183.
27. Sandson NB, Armstrong SC, Cozza KL. An overview of psychotropic drug-drug interactions. Psychosomatics. 2005;46(5):464-494.
How to best help patients with residual schizophrenia symptoms
Pregnant and moving involuntarily
CASE: Abnormal movements
Pregnant and unsure of her due date, Ms. A, age 35, presents to the emergency room complaining of hourly uterine contractions for the last 3 days and new onset vaginal bleeding. Ms. A is admitted to the obstetrics (OB) service for preterm labor at 34 and 3/7 weeks as dated by a triage ultrasound.
During initial examination by the OB service, Ms. A’s blood pressure is 155/112 mm Hg with a pulse of 126. Her cervix is dilated to 4 centimeters. Her physical exam is notable for rapid, repetitive, involuntary movements in her upper extremities and to a lesser degree in lower extremities. Ms. A is started on IV fluids and hydralazine, 10 mg/d, for elevated blood pressure. Later that day, she delivers a preterm female weighing 2,360 grams in a spontaneous vaginal delivery without any complications.
After delivery, the OB service requests a psychiatric consultation to evaluate Ms. A’s “blunted affect,” history of heavy alcohol use, and abnormal movements. During examination, Ms. A is alert and oriented to her surroundings. She states that this was her eleventh pregnancy; however, she is unable to recall details of most previous pregnancies. She also cannot remember any significant medical, surgical, or mental health history. Ms. A appears distracted, has difficulty participating in the interview, and gives contradictory histories to different team members. She is well groomed but shows repetitive circular movements of her hands, feet, and jaw that are nearly continuous. In addition, Ms. A has intermittent lip biting and smacking. Her speech is delayed, with increased latency of her responses to basic questions.
Her mood is neutral, her affect is blunted, and she denies any current suicidal or homicidal ideations, delusions, and auditory or visual hallucinations. Although her chart indicates a history of alcohol abuse, she denies this history and current drug or alcohol use. Her Mini-Mental State Exam score is a 22/30, missing points in her ability to copy shapes and write a sentence, complicated by her chorea-like upper body movements. She also demonstrates marked inattentiveness and is unwilling to cooperate with spelling “world.” On physical exam, her gait is wide-based but steady.
The authors’ observations
Determining the cause of Ms. A’s abnormal movements, delayed speech, and neutral mood initially proves difficult because she is minimally cooperative with the interview and we find discrepancies between information she provides and her medical records from previous OB admissions. It is unclear whether these inconsistencies are because of her faltering memory—which she admits has worsened in the last year—or unwillingness to provide a complete medical history.
We consider possible substance intoxication given her documented history of substance use. However, an extended drug screen is negative and her laboratory values do not suggest heavy alcohol use.
HISTORY: Depression and confusion
The next day, Ms. A is more cooperative with the interview. She says that she began feeling depressed 8 years ago, around the time her brother was killed in a violent crime. She denies previous psychiatric hospitalizations, but says she attempted suicide 4 years ago by stabbing herself in the throat with a fork. After that attempt, she was referred to an outpatient psychiatrist whom she continues to see intermittently. She says that her abnormal movements started 2 years before she first saw her outpatient psychiatrist.
She says she has been prescribed several medications, but remembers only taking quetiapine for depressive symptoms and insomnia. After a discussion with her psychiatrist about the possible effects of quetiapine on the fetus, she discontinued the drug approximately 8 weeks into her pregnancy. Quetiapine decreased her movement symptoms slightly, and she feels her movements have become uncontrollable since discontinuing it.
She reports increased feelings of sadness, worthlessness, guilt, decreased energy, irritability, and difficulty sleeping during her pregnancy. She denies current or past psychotic symptoms or mania. Ms. A says she has noticed problems with her memory as well as increased confusion over recent months. She often gets lost and cannot remember where she lives after leaving her home.
Based on hospital records, we learn that an MRI of the brain without contrast was completed 1 year ago to “evaluate choreiform movements.” The scan showed mild atrophy and abnormal signal within the caudate and putamen, as well as volume loss. We consult with the neurology service to evaluate Ms. A’s abnormal movements and her previous abnormal brain imaging. The neurologic exam notes that Ms. A has orofacial dyskinesias and near-continuous choreiform movements in her arms and hands. Her gait remains wide-based and she is unable to tandem walk. Because Ms. A shows no new neurologic symptoms, the neurology service does not feel that additional neuroimaging is indicated.
The authors’ observations
In consultation with neurology, the leading differential diagnoses include tardive dyskinesia, chorea gravidarum, and Huntington’s disease. See the Table1,2 for the differential diagnosis of chorea.
Ms. A reports taking quetiapine for 3 years, which suggests possible tardive dyskinesia. Although second-generation antipsychotics have a lower incidence of movement disorders than first-generation antipsychotics, the risk still exists. Withdrawal dyskinesias can occur after suddenly stopping or tapering antipsychotics and appear as extrapyramidal symptoms, including choreoathetosis similar to what Ms. A experienced.3,4 This type of dyskinesia is thought to be secondary to chronic dopamine antagonism leading to increased postsynaptic receptors and dopamine hypersensitivity.5 Because Ms. A discontinued quetiapine early in her pregnancy, withdrawal dyskinesias are less likely.
Because Ms. A presented with a movement disorder while pregnant, the neurology service considers chorea gravidarum, the term given to chorea occurring during pregnancy. This syndrome is thought to be caused by the effects of pregnancy on the basal ganglia.6 Historically, chorea gravidarum was associated with rheumatic fever (RF); however, with the decline in prevalence of RF, most choreiform movements that appear during pregnancy typically are caused by other diseases, such as systemic lupus erythematosus or Huntington’s disease. Approximately one-half of chorea gravidarum cases are idiopathic, with RF and antiphospholipid syndrome accounting for the remainder.7 Huntington’s disease during pregnancy is rare because it tends to present in women beyond childbearing age.
Based on Ms. A’s symptoms and previous MRI findings, we ask her if she has a known family history of Huntington’s disease. She denies this, but says she has not seen her father since she was very young and is uncertain of his medical history.
Table
Differential diagnosis for chorea
| Genetic | Huntington’s disease, benign hereditary chorea, neuroacanthocytosis, dentatorubral-pallidoluysian atrophy, Wilson’s disease, spinocerebellar ataxia, Friedreich’s ataxia |
| Rheumatic disorders | Sydenham’s chorea, chorea gravidarum |
| Drug-induced/toxicity | Neuroleptic drugs, steroids, anticonvulsants, antiparkinson agents, stimulants (amphetamines, cocaine), lithium, dopamine agonists |
| Systemic disorders | Systemic lupus erythematosus, thyrotoxicosis, polycythemia vera, hyperglycemia, AIDS, paraneoplastic syndrome |
| Vascular/trauma | Cerebral hemorrhage, vasculitis, stroke, antiphospholipid antibody syndrome |
| AIDS: acquired immune deficiency syndrome Source: References 1,2 | |
TREATMENT: Restart medication
Ms. A’s laboratory results show a slightly low hemoglobin of 10.5 g/dL and hematocrit of 32.8%. Her mean corpuscular volume is slightly decreased at 77 fL. Her urinalysis is negative, and blood glucose and thyroid-stimulating hormone are within normal limits. Rapid plasma regain, anti-nuclear antibody, and human immunodeficiency virus (HIV) are negative. Based on hospital records, we learn that during the previous admission a year ago a serum ceruloplasmin and serum copper were drawn and were normal.
We contact Ms. A’s outpatient psychiatrist for collateral information. The psychiatrist says he first evaluated Ms. A 3 years ago after a friend brought her in because of strange behavior, including talking to herself, making odd facial gestures, and laughing inappropriately. Although Ms. A denies past psychiatric hospitalizations, her psychiatrist states that she was hospitalized for 1 week after the suicide attempt 4 years ago and prescribed lorazepam and sertraline during that admission. He speculates that the suicide attempt may have been related to 5 of her children being taken from her by the Department of Family and Child Services after police raided her home to search for drugs. Custody was awarded to their respective fathers, causing Ms. A to “snap,” according to her friend.
Since then, neither Ms. A nor her psychiatrist have reported any further psychotic symptoms. Her psychiatrist confirms that Ms. A’s abnormal movements were present before her first appointment with him. He says that he referred Ms. A to a local hospital for a neurology work-up, but she did not schedule an appointment.
When we follow up with Ms. A 2 days after delivery, she continues to deny depressive symptoms, although her affect remains blunted. She says she is looking forward to going home with the baby, whom she plans to bottle feed. Her choreiform movements appear unchanged. She also continues to experience lip smacking. Although Ms. A recognizes that she has some movements, she minimizes them and says they do not bother her. She continues to demonstrate latency in her verbal responses to questions. Based on the collateral history and positive response with quetiapine, we recommend that Ms. A be restarted on quetiapine, 200 mg/d.
The authors’ observations
Ms. A’s choreiform movements started before her psychotic symptoms and subsequent usage of neuroleptic medication, which makes tardive dyskinesia less likely. Laboratory studies rule out systemic lupus erythematosus, HIV, and Wilson’s disease as the cause of her abnormal movements.
Ms. A’s history is highly suggestive of Huntington’s disease. She exhibits classic motor signs, including involuntary choreiform movements in her extremities. She also has psychiatric symptoms that are commonly associated with Huntington’s disease, including depression—which preceded her motor symptoms—cognitive decline, apathy, and psychotic symptoms. In addition, her MRI findings of volume changes in the caudate nucleus and the putamen and inability to rule out a family history make Huntington’s disease more likely (Box).1,8-11
Huntington’s disease is an autosomal dominant disorder characterized by progressive motor, cognitive, and psychiatric disturbances and is the most common genetic cause of chorea. The underlying genetic mutation is a CAG repeat expansion in the Huntington’s disease gene. A Huntington’s disease diagnosis generally is considered in the presence of the characteristic choreiform movements and slowly progressive cognitive decline.8 Physical symptoms can present at any age, although they usually begin between age 35 and 44. In early stages of the disease, patients may experience subtle changes in personality, cognition, and physical skills. Although most Huntington’s disease patients eventually exhibit similar physical symptoms, the onset, progression, and extent of cognitive and psychiatric symptoms vary among individuals. However, psychiatric symptoms frequently are present during the early stages of the disease, often before motor symptoms begin and can include personality changes, irritability, agitation, apathy, and depression. In addition, up to 23% of patients with Huntington’s disease develop psychotic symptoms.1,9 There is no cure for Huntington’s disease, and mean disease duration is 17 to 20 years. The most common cause of death among Huntington’s disease patients is pneumonia, followed by suicide.1
A Huntington’s disease diagnosis is based on clinical symptoms and signs in an individual who has a parent with proven Huntington’s disease and is confirmed by DNA tests.1 Typical neuroanatomic findings include initial neuronal loss in the striatum followed by a diffuse involvement of cortical and subcortical areas.10 Volume changes in the caudate nucleus and the putamen may be a reliable measure of Huntington’s disease and potentially serve as a biomarker.11
Psychiatric symptoms
Psychiatric symptoms frequently are evident in the early stages of Huntington’s disease, often before onset of motor symptoms.1 Depression is the most common sign, and can be difficult to diagnose because weight loss, apathy, and inactivity also occur in Huntington’s disease. Feelings of low self-esteem, guilt, and anxiety can help distinguish depression from symptoms of Huntington’s disease. Cognitive decline also may present before the first motor symptoms occur. Cognitive changes typically are related to executive functions and affected individuals may develop impairments in organization and planning. Psychotic symptoms may be present, but are more common in later stages of the disease.1
Ms. A reported that quetiapine seemed to lessen her choreiform movements, and dopamine receptor blocking agents (ie, antipsychotics) often are considered for managing chorea and psychosis in Huntington’s disease. However, there are few double-blind, placebo-controlled studies evaluating the efficacy of these agents.12 Small, uncontrolled, nonrandomized trials found quetiapine has some efficacy for both motor and psychiatric symptoms in Huntington’s disease.12-15
OUTCOME: Lost to follow-up
Ms. A is discharged from the hospital 3 days after she delivers her daughter and is given an appointment in 6 weeks at an affiliated movement disorders clinic. Before discharge, she is tested for the Huntington’s disease gene mutation with a plan to receive her results during her follow-up visit. During the informed consent process for the genetic testing, Ms. A states that she was tested previously and was quite sure that the test was positive for Huntington’s disease, although she could not recall where or when this testing was completed.
Ms. A also is scheduled to follow up with her obstetrician for a 6-week postpartum check-up and tubal ligation. We encourage Ms. A to make an appointment with her psychiatrist soon after discharge. We also make a referral to the Department of Family and Children Services to provide adequate support and resources to her and her children because of her physical and psychiatric issues.
Ms. A does not show up for her follow-up appointment at the movement disorders clinic. The genetic test is not completed during this admission because of a clerical error, and the serum sample subsequently expires.
The authors’ observations
Although Huntington’s disease is the most likely cause of Ms. A’s presentation, we were unable to confirm the diagnosis with genetic testing. If Ms. A returns to the neurology service and the genetic test is negative for Huntington’s disease, other causes of chorea must be investigated.
Related Resources
- De Marchi N, Mennella R. Huntington’s disease and its association with psychopathology. Harv Rev Psychiatry. 2000; 7(5):278-289.
- Revilla FJ, Grutzendler J, Larsh TR. Huntington disease. Medscape. http://emedicine.medscape.com/article/1150165-overview.
Drug Brand Names
- Hydralazine • Apresoline
- Lithium • Eskalith, Lithobid, others
- Lorazepam • Ativan
- Quetiapine • Seroquel
- Sertraline • Zoloft
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Roos RA. Huntington’s disease: a clinical review. Orphanet J Rare Dis. 2010;5(1):40.-
2. Wild EJ, Tabrizi SJ. The differential diagnosis of chorea. Pract Neurol. 2007;7:360-373.
3. Urbano M, Spiegel D, Rai A. Atypical antipsychotic withdrawal dyskinesia in 4 patients with mood disorders. J Clin Psychopharmacol. 2007;27(6):705-707.
4. Kafantaris V, Hirsch J, Saito E, et al. Treatment of withdrawal dyskinesia. J Am Acad Child Adolesc Psychiatry. 2005;44(11):1102-1103.
5. Creese I, Burt DR, Snyder SH. Dopamine receptor binding enhancement accompanies lesion-induced behavioral supersensitivity. Science. 1977;197(4303):596-598.
6. Kranick SM, Mowry EM, Colcher A, et al. Movement disorders and pregnancy: a review of the literature. Mov Disord. 2010;25(6):665-671.
7. Ramachandran TS. Chorea gravidarum. Medscape. Available at: http://emedicine.medscape.com/article/1149725-overview. Accessed May 4 2011.
8. Panegyres PK, Goh JG. The neurology and natural history of patients with indeterminate CAG repeat length mutations of the Huntington disease gene. J Neurol Sci. 2011;301(1-2):14-20.
9. Shiwach R. Psychopathology in Huntington’s disease patients. Acta Psychiatr Scand. 1994;90:241-246.
10. De Marchi N, Mennella R. Huntington’s disease and its association with psychopathology. Harv Rev Psychiatry. 2000;7:278-289.
11. van den Bogaard SJ, Dumas EM, Acharya TP, et al. and the TRACK-HD Investigator Group. Early atrophy of pallidum and accumbens nucleus in Huntington’s disease. J Neurol. 2011;258(3):412-420.
12. Frank S, Jankovic J. Advances in the pharmacological management of Huntington’s disease. Drugs. 2010;70(5):561-571.
13. Alpay M, Koroshetz WJ. Quetiapine in the treatment of behavioral disturbances in patients with Huntington’s disease. Psychosomatics. 2006;47(1):70-72.
14. Seitz DP, Millson RC. Quetiapine in the management of psychosis secondary to Huntington’s disease: a case report. Can J Psychiatry. 2004;49(6):413.-
15. Bonelli RM, Niederwieser G. Quetiapine in Huntington’s disease: a first case report. J Neurol. 2002;249(8):1114-1115.
CASE: Abnormal movements
Pregnant and unsure of her due date, Ms. A, age 35, presents to the emergency room complaining of hourly uterine contractions for the last 3 days and new onset vaginal bleeding. Ms. A is admitted to the obstetrics (OB) service for preterm labor at 34 and 3/7 weeks as dated by a triage ultrasound.
During initial examination by the OB service, Ms. A’s blood pressure is 155/112 mm Hg with a pulse of 126. Her cervix is dilated to 4 centimeters. Her physical exam is notable for rapid, repetitive, involuntary movements in her upper extremities and to a lesser degree in lower extremities. Ms. A is started on IV fluids and hydralazine, 10 mg/d, for elevated blood pressure. Later that day, she delivers a preterm female weighing 2,360 grams in a spontaneous vaginal delivery without any complications.
After delivery, the OB service requests a psychiatric consultation to evaluate Ms. A’s “blunted affect,” history of heavy alcohol use, and abnormal movements. During examination, Ms. A is alert and oriented to her surroundings. She states that this was her eleventh pregnancy; however, she is unable to recall details of most previous pregnancies. She also cannot remember any significant medical, surgical, or mental health history. Ms. A appears distracted, has difficulty participating in the interview, and gives contradictory histories to different team members. She is well groomed but shows repetitive circular movements of her hands, feet, and jaw that are nearly continuous. In addition, Ms. A has intermittent lip biting and smacking. Her speech is delayed, with increased latency of her responses to basic questions.
Her mood is neutral, her affect is blunted, and she denies any current suicidal or homicidal ideations, delusions, and auditory or visual hallucinations. Although her chart indicates a history of alcohol abuse, she denies this history and current drug or alcohol use. Her Mini-Mental State Exam score is a 22/30, missing points in her ability to copy shapes and write a sentence, complicated by her chorea-like upper body movements. She also demonstrates marked inattentiveness and is unwilling to cooperate with spelling “world.” On physical exam, her gait is wide-based but steady.
The authors’ observations
Determining the cause of Ms. A’s abnormal movements, delayed speech, and neutral mood initially proves difficult because she is minimally cooperative with the interview and we find discrepancies between information she provides and her medical records from previous OB admissions. It is unclear whether these inconsistencies are because of her faltering memory—which she admits has worsened in the last year—or unwillingness to provide a complete medical history.
We consider possible substance intoxication given her documented history of substance use. However, an extended drug screen is negative and her laboratory values do not suggest heavy alcohol use.
HISTORY: Depression and confusion
The next day, Ms. A is more cooperative with the interview. She says that she began feeling depressed 8 years ago, around the time her brother was killed in a violent crime. She denies previous psychiatric hospitalizations, but says she attempted suicide 4 years ago by stabbing herself in the throat with a fork. After that attempt, she was referred to an outpatient psychiatrist whom she continues to see intermittently. She says that her abnormal movements started 2 years before she first saw her outpatient psychiatrist.
She says she has been prescribed several medications, but remembers only taking quetiapine for depressive symptoms and insomnia. After a discussion with her psychiatrist about the possible effects of quetiapine on the fetus, she discontinued the drug approximately 8 weeks into her pregnancy. Quetiapine decreased her movement symptoms slightly, and she feels her movements have become uncontrollable since discontinuing it.
She reports increased feelings of sadness, worthlessness, guilt, decreased energy, irritability, and difficulty sleeping during her pregnancy. She denies current or past psychotic symptoms or mania. Ms. A says she has noticed problems with her memory as well as increased confusion over recent months. She often gets lost and cannot remember where she lives after leaving her home.
Based on hospital records, we learn that an MRI of the brain without contrast was completed 1 year ago to “evaluate choreiform movements.” The scan showed mild atrophy and abnormal signal within the caudate and putamen, as well as volume loss. We consult with the neurology service to evaluate Ms. A’s abnormal movements and her previous abnormal brain imaging. The neurologic exam notes that Ms. A has orofacial dyskinesias and near-continuous choreiform movements in her arms and hands. Her gait remains wide-based and she is unable to tandem walk. Because Ms. A shows no new neurologic symptoms, the neurology service does not feel that additional neuroimaging is indicated.
The authors’ observations
In consultation with neurology, the leading differential diagnoses include tardive dyskinesia, chorea gravidarum, and Huntington’s disease. See the Table1,2 for the differential diagnosis of chorea.
Ms. A reports taking quetiapine for 3 years, which suggests possible tardive dyskinesia. Although second-generation antipsychotics have a lower incidence of movement disorders than first-generation antipsychotics, the risk still exists. Withdrawal dyskinesias can occur after suddenly stopping or tapering antipsychotics and appear as extrapyramidal symptoms, including choreoathetosis similar to what Ms. A experienced.3,4 This type of dyskinesia is thought to be secondary to chronic dopamine antagonism leading to increased postsynaptic receptors and dopamine hypersensitivity.5 Because Ms. A discontinued quetiapine early in her pregnancy, withdrawal dyskinesias are less likely.
Because Ms. A presented with a movement disorder while pregnant, the neurology service considers chorea gravidarum, the term given to chorea occurring during pregnancy. This syndrome is thought to be caused by the effects of pregnancy on the basal ganglia.6 Historically, chorea gravidarum was associated with rheumatic fever (RF); however, with the decline in prevalence of RF, most choreiform movements that appear during pregnancy typically are caused by other diseases, such as systemic lupus erythematosus or Huntington’s disease. Approximately one-half of chorea gravidarum cases are idiopathic, with RF and antiphospholipid syndrome accounting for the remainder.7 Huntington’s disease during pregnancy is rare because it tends to present in women beyond childbearing age.
Based on Ms. A’s symptoms and previous MRI findings, we ask her if she has a known family history of Huntington’s disease. She denies this, but says she has not seen her father since she was very young and is uncertain of his medical history.
Table
Differential diagnosis for chorea
| Genetic | Huntington’s disease, benign hereditary chorea, neuroacanthocytosis, dentatorubral-pallidoluysian atrophy, Wilson’s disease, spinocerebellar ataxia, Friedreich’s ataxia |
| Rheumatic disorders | Sydenham’s chorea, chorea gravidarum |
| Drug-induced/toxicity | Neuroleptic drugs, steroids, anticonvulsants, antiparkinson agents, stimulants (amphetamines, cocaine), lithium, dopamine agonists |
| Systemic disorders | Systemic lupus erythematosus, thyrotoxicosis, polycythemia vera, hyperglycemia, AIDS, paraneoplastic syndrome |
| Vascular/trauma | Cerebral hemorrhage, vasculitis, stroke, antiphospholipid antibody syndrome |
| AIDS: acquired immune deficiency syndrome Source: References 1,2 | |
TREATMENT: Restart medication
Ms. A’s laboratory results show a slightly low hemoglobin of 10.5 g/dL and hematocrit of 32.8%. Her mean corpuscular volume is slightly decreased at 77 fL. Her urinalysis is negative, and blood glucose and thyroid-stimulating hormone are within normal limits. Rapid plasma regain, anti-nuclear antibody, and human immunodeficiency virus (HIV) are negative. Based on hospital records, we learn that during the previous admission a year ago a serum ceruloplasmin and serum copper were drawn and were normal.
We contact Ms. A’s outpatient psychiatrist for collateral information. The psychiatrist says he first evaluated Ms. A 3 years ago after a friend brought her in because of strange behavior, including talking to herself, making odd facial gestures, and laughing inappropriately. Although Ms. A denies past psychiatric hospitalizations, her psychiatrist states that she was hospitalized for 1 week after the suicide attempt 4 years ago and prescribed lorazepam and sertraline during that admission. He speculates that the suicide attempt may have been related to 5 of her children being taken from her by the Department of Family and Child Services after police raided her home to search for drugs. Custody was awarded to their respective fathers, causing Ms. A to “snap,” according to her friend.
Since then, neither Ms. A nor her psychiatrist have reported any further psychotic symptoms. Her psychiatrist confirms that Ms. A’s abnormal movements were present before her first appointment with him. He says that he referred Ms. A to a local hospital for a neurology work-up, but she did not schedule an appointment.
When we follow up with Ms. A 2 days after delivery, she continues to deny depressive symptoms, although her affect remains blunted. She says she is looking forward to going home with the baby, whom she plans to bottle feed. Her choreiform movements appear unchanged. She also continues to experience lip smacking. Although Ms. A recognizes that she has some movements, she minimizes them and says they do not bother her. She continues to demonstrate latency in her verbal responses to questions. Based on the collateral history and positive response with quetiapine, we recommend that Ms. A be restarted on quetiapine, 200 mg/d.
The authors’ observations
Ms. A’s choreiform movements started before her psychotic symptoms and subsequent usage of neuroleptic medication, which makes tardive dyskinesia less likely. Laboratory studies rule out systemic lupus erythematosus, HIV, and Wilson’s disease as the cause of her abnormal movements.
Ms. A’s history is highly suggestive of Huntington’s disease. She exhibits classic motor signs, including involuntary choreiform movements in her extremities. She also has psychiatric symptoms that are commonly associated with Huntington’s disease, including depression—which preceded her motor symptoms—cognitive decline, apathy, and psychotic symptoms. In addition, her MRI findings of volume changes in the caudate nucleus and the putamen and inability to rule out a family history make Huntington’s disease more likely (Box).1,8-11
Huntington’s disease is an autosomal dominant disorder characterized by progressive motor, cognitive, and psychiatric disturbances and is the most common genetic cause of chorea. The underlying genetic mutation is a CAG repeat expansion in the Huntington’s disease gene. A Huntington’s disease diagnosis generally is considered in the presence of the characteristic choreiform movements and slowly progressive cognitive decline.8 Physical symptoms can present at any age, although they usually begin between age 35 and 44. In early stages of the disease, patients may experience subtle changes in personality, cognition, and physical skills. Although most Huntington’s disease patients eventually exhibit similar physical symptoms, the onset, progression, and extent of cognitive and psychiatric symptoms vary among individuals. However, psychiatric symptoms frequently are present during the early stages of the disease, often before motor symptoms begin and can include personality changes, irritability, agitation, apathy, and depression. In addition, up to 23% of patients with Huntington’s disease develop psychotic symptoms.1,9 There is no cure for Huntington’s disease, and mean disease duration is 17 to 20 years. The most common cause of death among Huntington’s disease patients is pneumonia, followed by suicide.1
A Huntington’s disease diagnosis is based on clinical symptoms and signs in an individual who has a parent with proven Huntington’s disease and is confirmed by DNA tests.1 Typical neuroanatomic findings include initial neuronal loss in the striatum followed by a diffuse involvement of cortical and subcortical areas.10 Volume changes in the caudate nucleus and the putamen may be a reliable measure of Huntington’s disease and potentially serve as a biomarker.11
Psychiatric symptoms
Psychiatric symptoms frequently are evident in the early stages of Huntington’s disease, often before onset of motor symptoms.1 Depression is the most common sign, and can be difficult to diagnose because weight loss, apathy, and inactivity also occur in Huntington’s disease. Feelings of low self-esteem, guilt, and anxiety can help distinguish depression from symptoms of Huntington’s disease. Cognitive decline also may present before the first motor symptoms occur. Cognitive changes typically are related to executive functions and affected individuals may develop impairments in organization and planning. Psychotic symptoms may be present, but are more common in later stages of the disease.1
Ms. A reported that quetiapine seemed to lessen her choreiform movements, and dopamine receptor blocking agents (ie, antipsychotics) often are considered for managing chorea and psychosis in Huntington’s disease. However, there are few double-blind, placebo-controlled studies evaluating the efficacy of these agents.12 Small, uncontrolled, nonrandomized trials found quetiapine has some efficacy for both motor and psychiatric symptoms in Huntington’s disease.12-15
OUTCOME: Lost to follow-up
Ms. A is discharged from the hospital 3 days after she delivers her daughter and is given an appointment in 6 weeks at an affiliated movement disorders clinic. Before discharge, she is tested for the Huntington’s disease gene mutation with a plan to receive her results during her follow-up visit. During the informed consent process for the genetic testing, Ms. A states that she was tested previously and was quite sure that the test was positive for Huntington’s disease, although she could not recall where or when this testing was completed.
Ms. A also is scheduled to follow up with her obstetrician for a 6-week postpartum check-up and tubal ligation. We encourage Ms. A to make an appointment with her psychiatrist soon after discharge. We also make a referral to the Department of Family and Children Services to provide adequate support and resources to her and her children because of her physical and psychiatric issues.
Ms. A does not show up for her follow-up appointment at the movement disorders clinic. The genetic test is not completed during this admission because of a clerical error, and the serum sample subsequently expires.
The authors’ observations
Although Huntington’s disease is the most likely cause of Ms. A’s presentation, we were unable to confirm the diagnosis with genetic testing. If Ms. A returns to the neurology service and the genetic test is negative for Huntington’s disease, other causes of chorea must be investigated.
Related Resources
- De Marchi N, Mennella R. Huntington’s disease and its association with psychopathology. Harv Rev Psychiatry. 2000; 7(5):278-289.
- Revilla FJ, Grutzendler J, Larsh TR. Huntington disease. Medscape. http://emedicine.medscape.com/article/1150165-overview.
Drug Brand Names
- Hydralazine • Apresoline
- Lithium • Eskalith, Lithobid, others
- Lorazepam • Ativan
- Quetiapine • Seroquel
- Sertraline • Zoloft
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Abnormal movements
Pregnant and unsure of her due date, Ms. A, age 35, presents to the emergency room complaining of hourly uterine contractions for the last 3 days and new onset vaginal bleeding. Ms. A is admitted to the obstetrics (OB) service for preterm labor at 34 and 3/7 weeks as dated by a triage ultrasound.
During initial examination by the OB service, Ms. A’s blood pressure is 155/112 mm Hg with a pulse of 126. Her cervix is dilated to 4 centimeters. Her physical exam is notable for rapid, repetitive, involuntary movements in her upper extremities and to a lesser degree in lower extremities. Ms. A is started on IV fluids and hydralazine, 10 mg/d, for elevated blood pressure. Later that day, she delivers a preterm female weighing 2,360 grams in a spontaneous vaginal delivery without any complications.
After delivery, the OB service requests a psychiatric consultation to evaluate Ms. A’s “blunted affect,” history of heavy alcohol use, and abnormal movements. During examination, Ms. A is alert and oriented to her surroundings. She states that this was her eleventh pregnancy; however, she is unable to recall details of most previous pregnancies. She also cannot remember any significant medical, surgical, or mental health history. Ms. A appears distracted, has difficulty participating in the interview, and gives contradictory histories to different team members. She is well groomed but shows repetitive circular movements of her hands, feet, and jaw that are nearly continuous. In addition, Ms. A has intermittent lip biting and smacking. Her speech is delayed, with increased latency of her responses to basic questions.
Her mood is neutral, her affect is blunted, and she denies any current suicidal or homicidal ideations, delusions, and auditory or visual hallucinations. Although her chart indicates a history of alcohol abuse, she denies this history and current drug or alcohol use. Her Mini-Mental State Exam score is a 22/30, missing points in her ability to copy shapes and write a sentence, complicated by her chorea-like upper body movements. She also demonstrates marked inattentiveness and is unwilling to cooperate with spelling “world.” On physical exam, her gait is wide-based but steady.
The authors’ observations
Determining the cause of Ms. A’s abnormal movements, delayed speech, and neutral mood initially proves difficult because she is minimally cooperative with the interview and we find discrepancies between information she provides and her medical records from previous OB admissions. It is unclear whether these inconsistencies are because of her faltering memory—which she admits has worsened in the last year—or unwillingness to provide a complete medical history.
We consider possible substance intoxication given her documented history of substance use. However, an extended drug screen is negative and her laboratory values do not suggest heavy alcohol use.
HISTORY: Depression and confusion
The next day, Ms. A is more cooperative with the interview. She says that she began feeling depressed 8 years ago, around the time her brother was killed in a violent crime. She denies previous psychiatric hospitalizations, but says she attempted suicide 4 years ago by stabbing herself in the throat with a fork. After that attempt, she was referred to an outpatient psychiatrist whom she continues to see intermittently. She says that her abnormal movements started 2 years before she first saw her outpatient psychiatrist.
She says she has been prescribed several medications, but remembers only taking quetiapine for depressive symptoms and insomnia. After a discussion with her psychiatrist about the possible effects of quetiapine on the fetus, she discontinued the drug approximately 8 weeks into her pregnancy. Quetiapine decreased her movement symptoms slightly, and she feels her movements have become uncontrollable since discontinuing it.
She reports increased feelings of sadness, worthlessness, guilt, decreased energy, irritability, and difficulty sleeping during her pregnancy. She denies current or past psychotic symptoms or mania. Ms. A says she has noticed problems with her memory as well as increased confusion over recent months. She often gets lost and cannot remember where she lives after leaving her home.
Based on hospital records, we learn that an MRI of the brain without contrast was completed 1 year ago to “evaluate choreiform movements.” The scan showed mild atrophy and abnormal signal within the caudate and putamen, as well as volume loss. We consult with the neurology service to evaluate Ms. A’s abnormal movements and her previous abnormal brain imaging. The neurologic exam notes that Ms. A has orofacial dyskinesias and near-continuous choreiform movements in her arms and hands. Her gait remains wide-based and she is unable to tandem walk. Because Ms. A shows no new neurologic symptoms, the neurology service does not feel that additional neuroimaging is indicated.
The authors’ observations
In consultation with neurology, the leading differential diagnoses include tardive dyskinesia, chorea gravidarum, and Huntington’s disease. See the Table1,2 for the differential diagnosis of chorea.
Ms. A reports taking quetiapine for 3 years, which suggests possible tardive dyskinesia. Although second-generation antipsychotics have a lower incidence of movement disorders than first-generation antipsychotics, the risk still exists. Withdrawal dyskinesias can occur after suddenly stopping or tapering antipsychotics and appear as extrapyramidal symptoms, including choreoathetosis similar to what Ms. A experienced.3,4 This type of dyskinesia is thought to be secondary to chronic dopamine antagonism leading to increased postsynaptic receptors and dopamine hypersensitivity.5 Because Ms. A discontinued quetiapine early in her pregnancy, withdrawal dyskinesias are less likely.
Because Ms. A presented with a movement disorder while pregnant, the neurology service considers chorea gravidarum, the term given to chorea occurring during pregnancy. This syndrome is thought to be caused by the effects of pregnancy on the basal ganglia.6 Historically, chorea gravidarum was associated with rheumatic fever (RF); however, with the decline in prevalence of RF, most choreiform movements that appear during pregnancy typically are caused by other diseases, such as systemic lupus erythematosus or Huntington’s disease. Approximately one-half of chorea gravidarum cases are idiopathic, with RF and antiphospholipid syndrome accounting for the remainder.7 Huntington’s disease during pregnancy is rare because it tends to present in women beyond childbearing age.
Based on Ms. A’s symptoms and previous MRI findings, we ask her if she has a known family history of Huntington’s disease. She denies this, but says she has not seen her father since she was very young and is uncertain of his medical history.
Table
Differential diagnosis for chorea
| Genetic | Huntington’s disease, benign hereditary chorea, neuroacanthocytosis, dentatorubral-pallidoluysian atrophy, Wilson’s disease, spinocerebellar ataxia, Friedreich’s ataxia |
| Rheumatic disorders | Sydenham’s chorea, chorea gravidarum |
| Drug-induced/toxicity | Neuroleptic drugs, steroids, anticonvulsants, antiparkinson agents, stimulants (amphetamines, cocaine), lithium, dopamine agonists |
| Systemic disorders | Systemic lupus erythematosus, thyrotoxicosis, polycythemia vera, hyperglycemia, AIDS, paraneoplastic syndrome |
| Vascular/trauma | Cerebral hemorrhage, vasculitis, stroke, antiphospholipid antibody syndrome |
| AIDS: acquired immune deficiency syndrome Source: References 1,2 | |
TREATMENT: Restart medication
Ms. A’s laboratory results show a slightly low hemoglobin of 10.5 g/dL and hematocrit of 32.8%. Her mean corpuscular volume is slightly decreased at 77 fL. Her urinalysis is negative, and blood glucose and thyroid-stimulating hormone are within normal limits. Rapid plasma regain, anti-nuclear antibody, and human immunodeficiency virus (HIV) are negative. Based on hospital records, we learn that during the previous admission a year ago a serum ceruloplasmin and serum copper were drawn and were normal.
We contact Ms. A’s outpatient psychiatrist for collateral information. The psychiatrist says he first evaluated Ms. A 3 years ago after a friend brought her in because of strange behavior, including talking to herself, making odd facial gestures, and laughing inappropriately. Although Ms. A denies past psychiatric hospitalizations, her psychiatrist states that she was hospitalized for 1 week after the suicide attempt 4 years ago and prescribed lorazepam and sertraline during that admission. He speculates that the suicide attempt may have been related to 5 of her children being taken from her by the Department of Family and Child Services after police raided her home to search for drugs. Custody was awarded to their respective fathers, causing Ms. A to “snap,” according to her friend.
Since then, neither Ms. A nor her psychiatrist have reported any further psychotic symptoms. Her psychiatrist confirms that Ms. A’s abnormal movements were present before her first appointment with him. He says that he referred Ms. A to a local hospital for a neurology work-up, but she did not schedule an appointment.
When we follow up with Ms. A 2 days after delivery, she continues to deny depressive symptoms, although her affect remains blunted. She says she is looking forward to going home with the baby, whom she plans to bottle feed. Her choreiform movements appear unchanged. She also continues to experience lip smacking. Although Ms. A recognizes that she has some movements, she minimizes them and says they do not bother her. She continues to demonstrate latency in her verbal responses to questions. Based on the collateral history and positive response with quetiapine, we recommend that Ms. A be restarted on quetiapine, 200 mg/d.
The authors’ observations
Ms. A’s choreiform movements started before her psychotic symptoms and subsequent usage of neuroleptic medication, which makes tardive dyskinesia less likely. Laboratory studies rule out systemic lupus erythematosus, HIV, and Wilson’s disease as the cause of her abnormal movements.
Ms. A’s history is highly suggestive of Huntington’s disease. She exhibits classic motor signs, including involuntary choreiform movements in her extremities. She also has psychiatric symptoms that are commonly associated with Huntington’s disease, including depression—which preceded her motor symptoms—cognitive decline, apathy, and psychotic symptoms. In addition, her MRI findings of volume changes in the caudate nucleus and the putamen and inability to rule out a family history make Huntington’s disease more likely (Box).1,8-11
Huntington’s disease is an autosomal dominant disorder characterized by progressive motor, cognitive, and psychiatric disturbances and is the most common genetic cause of chorea. The underlying genetic mutation is a CAG repeat expansion in the Huntington’s disease gene. A Huntington’s disease diagnosis generally is considered in the presence of the characteristic choreiform movements and slowly progressive cognitive decline.8 Physical symptoms can present at any age, although they usually begin between age 35 and 44. In early stages of the disease, patients may experience subtle changes in personality, cognition, and physical skills. Although most Huntington’s disease patients eventually exhibit similar physical symptoms, the onset, progression, and extent of cognitive and psychiatric symptoms vary among individuals. However, psychiatric symptoms frequently are present during the early stages of the disease, often before motor symptoms begin and can include personality changes, irritability, agitation, apathy, and depression. In addition, up to 23% of patients with Huntington’s disease develop psychotic symptoms.1,9 There is no cure for Huntington’s disease, and mean disease duration is 17 to 20 years. The most common cause of death among Huntington’s disease patients is pneumonia, followed by suicide.1
A Huntington’s disease diagnosis is based on clinical symptoms and signs in an individual who has a parent with proven Huntington’s disease and is confirmed by DNA tests.1 Typical neuroanatomic findings include initial neuronal loss in the striatum followed by a diffuse involvement of cortical and subcortical areas.10 Volume changes in the caudate nucleus and the putamen may be a reliable measure of Huntington’s disease and potentially serve as a biomarker.11
Psychiatric symptoms
Psychiatric symptoms frequently are evident in the early stages of Huntington’s disease, often before onset of motor symptoms.1 Depression is the most common sign, and can be difficult to diagnose because weight loss, apathy, and inactivity also occur in Huntington’s disease. Feelings of low self-esteem, guilt, and anxiety can help distinguish depression from symptoms of Huntington’s disease. Cognitive decline also may present before the first motor symptoms occur. Cognitive changes typically are related to executive functions and affected individuals may develop impairments in organization and planning. Psychotic symptoms may be present, but are more common in later stages of the disease.1
Ms. A reported that quetiapine seemed to lessen her choreiform movements, and dopamine receptor blocking agents (ie, antipsychotics) often are considered for managing chorea and psychosis in Huntington’s disease. However, there are few double-blind, placebo-controlled studies evaluating the efficacy of these agents.12 Small, uncontrolled, nonrandomized trials found quetiapine has some efficacy for both motor and psychiatric symptoms in Huntington’s disease.12-15
OUTCOME: Lost to follow-up
Ms. A is discharged from the hospital 3 days after she delivers her daughter and is given an appointment in 6 weeks at an affiliated movement disorders clinic. Before discharge, she is tested for the Huntington’s disease gene mutation with a plan to receive her results during her follow-up visit. During the informed consent process for the genetic testing, Ms. A states that she was tested previously and was quite sure that the test was positive for Huntington’s disease, although she could not recall where or when this testing was completed.
Ms. A also is scheduled to follow up with her obstetrician for a 6-week postpartum check-up and tubal ligation. We encourage Ms. A to make an appointment with her psychiatrist soon after discharge. We also make a referral to the Department of Family and Children Services to provide adequate support and resources to her and her children because of her physical and psychiatric issues.
Ms. A does not show up for her follow-up appointment at the movement disorders clinic. The genetic test is not completed during this admission because of a clerical error, and the serum sample subsequently expires.
The authors’ observations
Although Huntington’s disease is the most likely cause of Ms. A’s presentation, we were unable to confirm the diagnosis with genetic testing. If Ms. A returns to the neurology service and the genetic test is negative for Huntington’s disease, other causes of chorea must be investigated.
Related Resources
- De Marchi N, Mennella R. Huntington’s disease and its association with psychopathology. Harv Rev Psychiatry. 2000; 7(5):278-289.
- Revilla FJ, Grutzendler J, Larsh TR. Huntington disease. Medscape. http://emedicine.medscape.com/article/1150165-overview.
Drug Brand Names
- Hydralazine • Apresoline
- Lithium • Eskalith, Lithobid, others
- Lorazepam • Ativan
- Quetiapine • Seroquel
- Sertraline • Zoloft
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Roos RA. Huntington’s disease: a clinical review. Orphanet J Rare Dis. 2010;5(1):40.-
2. Wild EJ, Tabrizi SJ. The differential diagnosis of chorea. Pract Neurol. 2007;7:360-373.
3. Urbano M, Spiegel D, Rai A. Atypical antipsychotic withdrawal dyskinesia in 4 patients with mood disorders. J Clin Psychopharmacol. 2007;27(6):705-707.
4. Kafantaris V, Hirsch J, Saito E, et al. Treatment of withdrawal dyskinesia. J Am Acad Child Adolesc Psychiatry. 2005;44(11):1102-1103.
5. Creese I, Burt DR, Snyder SH. Dopamine receptor binding enhancement accompanies lesion-induced behavioral supersensitivity. Science. 1977;197(4303):596-598.
6. Kranick SM, Mowry EM, Colcher A, et al. Movement disorders and pregnancy: a review of the literature. Mov Disord. 2010;25(6):665-671.
7. Ramachandran TS. Chorea gravidarum. Medscape. Available at: http://emedicine.medscape.com/article/1149725-overview. Accessed May 4 2011.
8. Panegyres PK, Goh JG. The neurology and natural history of patients with indeterminate CAG repeat length mutations of the Huntington disease gene. J Neurol Sci. 2011;301(1-2):14-20.
9. Shiwach R. Psychopathology in Huntington’s disease patients. Acta Psychiatr Scand. 1994;90:241-246.
10. De Marchi N, Mennella R. Huntington’s disease and its association with psychopathology. Harv Rev Psychiatry. 2000;7:278-289.
11. van den Bogaard SJ, Dumas EM, Acharya TP, et al. and the TRACK-HD Investigator Group. Early atrophy of pallidum and accumbens nucleus in Huntington’s disease. J Neurol. 2011;258(3):412-420.
12. Frank S, Jankovic J. Advances in the pharmacological management of Huntington’s disease. Drugs. 2010;70(5):561-571.
13. Alpay M, Koroshetz WJ. Quetiapine in the treatment of behavioral disturbances in patients with Huntington’s disease. Psychosomatics. 2006;47(1):70-72.
14. Seitz DP, Millson RC. Quetiapine in the management of psychosis secondary to Huntington’s disease: a case report. Can J Psychiatry. 2004;49(6):413.-
15. Bonelli RM, Niederwieser G. Quetiapine in Huntington’s disease: a first case report. J Neurol. 2002;249(8):1114-1115.
1. Roos RA. Huntington’s disease: a clinical review. Orphanet J Rare Dis. 2010;5(1):40.-
2. Wild EJ, Tabrizi SJ. The differential diagnosis of chorea. Pract Neurol. 2007;7:360-373.
3. Urbano M, Spiegel D, Rai A. Atypical antipsychotic withdrawal dyskinesia in 4 patients with mood disorders. J Clin Psychopharmacol. 2007;27(6):705-707.
4. Kafantaris V, Hirsch J, Saito E, et al. Treatment of withdrawal dyskinesia. J Am Acad Child Adolesc Psychiatry. 2005;44(11):1102-1103.
5. Creese I, Burt DR, Snyder SH. Dopamine receptor binding enhancement accompanies lesion-induced behavioral supersensitivity. Science. 1977;197(4303):596-598.
6. Kranick SM, Mowry EM, Colcher A, et al. Movement disorders and pregnancy: a review of the literature. Mov Disord. 2010;25(6):665-671.
7. Ramachandran TS. Chorea gravidarum. Medscape. Available at: http://emedicine.medscape.com/article/1149725-overview. Accessed May 4 2011.
8. Panegyres PK, Goh JG. The neurology and natural history of patients with indeterminate CAG repeat length mutations of the Huntington disease gene. J Neurol Sci. 2011;301(1-2):14-20.
9. Shiwach R. Psychopathology in Huntington’s disease patients. Acta Psychiatr Scand. 1994;90:241-246.
10. De Marchi N, Mennella R. Huntington’s disease and its association with psychopathology. Harv Rev Psychiatry. 2000;7:278-289.
11. van den Bogaard SJ, Dumas EM, Acharya TP, et al. and the TRACK-HD Investigator Group. Early atrophy of pallidum and accumbens nucleus in Huntington’s disease. J Neurol. 2011;258(3):412-420.
12. Frank S, Jankovic J. Advances in the pharmacological management of Huntington’s disease. Drugs. 2010;70(5):561-571.
13. Alpay M, Koroshetz WJ. Quetiapine in the treatment of behavioral disturbances in patients with Huntington’s disease. Psychosomatics. 2006;47(1):70-72.
14. Seitz DP, Millson RC. Quetiapine in the management of psychosis secondary to Huntington’s disease: a case report. Can J Psychiatry. 2004;49(6):413.-
15. Bonelli RM, Niederwieser G. Quetiapine in Huntington’s disease: a first case report. J Neurol. 2002;249(8):1114-1115.
Ketamine and glutamate
I enjoyed the article “Glutamate: New hope for schizophrenia treatment” (Current Psychiatry, April 2011, p. 68-74) by Drs. Kantrowitz and Javitt. However, perhaps due to my own ignorance of the subject, I remain puzzled about their suggestion that the glutamatergic model of schizophrenia is supported by evidence showing that agonists at presynaptic mGluR2/3 receptors reverse the psychotomimetic effects of the N-methyl-D-aspartate (NMDA) receptor antagonist ketamine. Indeed, one would think that action of glutamatergic agonists at presynaptic autoreceptors would reduce glutamatergic activity and therefore mimic, rather than block, the effects of the antagonist ketamine.
Scott Mendelson, MD, PhD
Psychiatrist
Roseburg VA Hospital
Roseburg, OR
The authors respond
Thank you for your interest. We agree that this is a paradoxical effect of glutamate that is based largely upon the work of Bita Moghaddam, PhD, John Krystal, MD, and others. The basic finding is that blocking postsynaptic NMDA receptors leads to a rebound increase in presynaptic glutamate release that is pathological. Dr. Moghaddam showed that treatment with mGluR2/3 agonists reversed dopaminergic abnormalities and cognitive deficits induced by NMDAR antagonists in rodents.1 Dr. Krystal showed that the presynaptic glutamate release antagonist lamotrigine blocked psychotomimetic effects of ketamine in normal human volunteers.2 These findings have led to the hypothesis that blocking presynaptic glutamate may restore balance between glutamate and GABA systems, particularly in frontal brain regions. This theory also was supported by 1 successful clinical trial of a mGluR2/3 agonist,3 although replication studies are ongoing. When interpreting these findings, it is important to keep in mind that glutamate acts at several receptor types in addition to NMDA, and it is the balance between these receptors, as well as the balance between excitatory glutamatergic vs inhibitory GABAergic neurotransmission, that may be critical in psychosis.
Joshua T. Kantrowitz, MD
Assistant Professor
Department of Psychiatry
Columbia College of Physicians and Surgeons
New York, NY
Schizophrenia Research Center
Nathan Kline Institute for Psychiatric Research
Orangeburg, NY
Daniel C. Javitt, MD, PhD
Director, Schizophrenia Research
Nathan Kline Institute for Psychiatric Research
Orangeburg, NY
Professor of Psychiatry and Neuroscience
New York University School of Medicine
New York, NY
1. Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281(5381):1349-1352.
2. Krystal JH, Abi-Saab W, Perry E, et al. Preliminary evidence of attenuation of the disruptive effects of the NMDA glutamate receptor antagonist, ketamine, on working memory by pretreatment with the group II metabotropic glutamate receptor agonist, LY354740, in healthy human subjects. Psychopharmacology (Berl). 2005;179(1):303-309.
3. Patil ST, Zhang L, Martenyi F, et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med. 2007;13(9):1102-1107.
I enjoyed the article “Glutamate: New hope for schizophrenia treatment” (Current Psychiatry, April 2011, p. 68-74) by Drs. Kantrowitz and Javitt. However, perhaps due to my own ignorance of the subject, I remain puzzled about their suggestion that the glutamatergic model of schizophrenia is supported by evidence showing that agonists at presynaptic mGluR2/3 receptors reverse the psychotomimetic effects of the N-methyl-D-aspartate (NMDA) receptor antagonist ketamine. Indeed, one would think that action of glutamatergic agonists at presynaptic autoreceptors would reduce glutamatergic activity and therefore mimic, rather than block, the effects of the antagonist ketamine.
Scott Mendelson, MD, PhD
Psychiatrist
Roseburg VA Hospital
Roseburg, OR
The authors respond
Thank you for your interest. We agree that this is a paradoxical effect of glutamate that is based largely upon the work of Bita Moghaddam, PhD, John Krystal, MD, and others. The basic finding is that blocking postsynaptic NMDA receptors leads to a rebound increase in presynaptic glutamate release that is pathological. Dr. Moghaddam showed that treatment with mGluR2/3 agonists reversed dopaminergic abnormalities and cognitive deficits induced by NMDAR antagonists in rodents.1 Dr. Krystal showed that the presynaptic glutamate release antagonist lamotrigine blocked psychotomimetic effects of ketamine in normal human volunteers.2 These findings have led to the hypothesis that blocking presynaptic glutamate may restore balance between glutamate and GABA systems, particularly in frontal brain regions. This theory also was supported by 1 successful clinical trial of a mGluR2/3 agonist,3 although replication studies are ongoing. When interpreting these findings, it is important to keep in mind that glutamate acts at several receptor types in addition to NMDA, and it is the balance between these receptors, as well as the balance between excitatory glutamatergic vs inhibitory GABAergic neurotransmission, that may be critical in psychosis.
Joshua T. Kantrowitz, MD
Assistant Professor
Department of Psychiatry
Columbia College of Physicians and Surgeons
New York, NY
Schizophrenia Research Center
Nathan Kline Institute for Psychiatric Research
Orangeburg, NY
Daniel C. Javitt, MD, PhD
Director, Schizophrenia Research
Nathan Kline Institute for Psychiatric Research
Orangeburg, NY
Professor of Psychiatry and Neuroscience
New York University School of Medicine
New York, NY
I enjoyed the article “Glutamate: New hope for schizophrenia treatment” (Current Psychiatry, April 2011, p. 68-74) by Drs. Kantrowitz and Javitt. However, perhaps due to my own ignorance of the subject, I remain puzzled about their suggestion that the glutamatergic model of schizophrenia is supported by evidence showing that agonists at presynaptic mGluR2/3 receptors reverse the psychotomimetic effects of the N-methyl-D-aspartate (NMDA) receptor antagonist ketamine. Indeed, one would think that action of glutamatergic agonists at presynaptic autoreceptors would reduce glutamatergic activity and therefore mimic, rather than block, the effects of the antagonist ketamine.
Scott Mendelson, MD, PhD
Psychiatrist
Roseburg VA Hospital
Roseburg, OR
The authors respond
Thank you for your interest. We agree that this is a paradoxical effect of glutamate that is based largely upon the work of Bita Moghaddam, PhD, John Krystal, MD, and others. The basic finding is that blocking postsynaptic NMDA receptors leads to a rebound increase in presynaptic glutamate release that is pathological. Dr. Moghaddam showed that treatment with mGluR2/3 agonists reversed dopaminergic abnormalities and cognitive deficits induced by NMDAR antagonists in rodents.1 Dr. Krystal showed that the presynaptic glutamate release antagonist lamotrigine blocked psychotomimetic effects of ketamine in normal human volunteers.2 These findings have led to the hypothesis that blocking presynaptic glutamate may restore balance between glutamate and GABA systems, particularly in frontal brain regions. This theory also was supported by 1 successful clinical trial of a mGluR2/3 agonist,3 although replication studies are ongoing. When interpreting these findings, it is important to keep in mind that glutamate acts at several receptor types in addition to NMDA, and it is the balance between these receptors, as well as the balance between excitatory glutamatergic vs inhibitory GABAergic neurotransmission, that may be critical in psychosis.
Joshua T. Kantrowitz, MD
Assistant Professor
Department of Psychiatry
Columbia College of Physicians and Surgeons
New York, NY
Schizophrenia Research Center
Nathan Kline Institute for Psychiatric Research
Orangeburg, NY
Daniel C. Javitt, MD, PhD
Director, Schizophrenia Research
Nathan Kline Institute for Psychiatric Research
Orangeburg, NY
Professor of Psychiatry and Neuroscience
New York University School of Medicine
New York, NY
1. Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281(5381):1349-1352.
2. Krystal JH, Abi-Saab W, Perry E, et al. Preliminary evidence of attenuation of the disruptive effects of the NMDA glutamate receptor antagonist, ketamine, on working memory by pretreatment with the group II metabotropic glutamate receptor agonist, LY354740, in healthy human subjects. Psychopharmacology (Berl). 2005;179(1):303-309.
3. Patil ST, Zhang L, Martenyi F, et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med. 2007;13(9):1102-1107.
1. Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281(5381):1349-1352.
2. Krystal JH, Abi-Saab W, Perry E, et al. Preliminary evidence of attenuation of the disruptive effects of the NMDA glutamate receptor antagonist, ketamine, on working memory by pretreatment with the group II metabotropic glutamate receptor agonist, LY354740, in healthy human subjects. Psychopharmacology (Berl). 2005;179(1):303-309.
3. Patil ST, Zhang L, Martenyi F, et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med. 2007;13(9):1102-1107.
Hazardous polypharmacy
I am writing to compliment Dr. Henry A. Nasrallah for having the guts to write “Polypharmacy subtypes” (From the Editor, Current Psychiatry, April 2011, p. 10-12). I try hard to have patients on no more than 4 medications, 1 from each class if indicated. In California, what Dr. Nasrallah described as ridiculous and hazardous is all too rampant. I have inherited patients taking as many as 7 psychotropics and the initial evaluation of these patients usually begins with families stating that they are angry about their loved ones being “doped up to the point of being zombies.” When I am finally able to get a good history of symptoms, I typically find that patients do not meet DSM-IV-TR criteria for some diagnoses. I then wean them off the medications, see what symptoms emerge, and then “reinvent the wheel” with their medication regimens. I was verbally reprimanded by the medical director at 1 job because he thought a patient was “doing well” on 7 medications despite the fact he was oversedated. I also have inherited patients on medications that interacted with other medications and were causing medical problems.
Problems with polypharmacy in California are, as I said, rampant.
Terry Roh, MD
Child and Adult Psychiatrist
Lutheran Social Services of Southern California
Big Bear Lake, CA
I am writing to compliment Dr. Henry A. Nasrallah for having the guts to write “Polypharmacy subtypes” (From the Editor, Current Psychiatry, April 2011, p. 10-12). I try hard to have patients on no more than 4 medications, 1 from each class if indicated. In California, what Dr. Nasrallah described as ridiculous and hazardous is all too rampant. I have inherited patients taking as many as 7 psychotropics and the initial evaluation of these patients usually begins with families stating that they are angry about their loved ones being “doped up to the point of being zombies.” When I am finally able to get a good history of symptoms, I typically find that patients do not meet DSM-IV-TR criteria for some diagnoses. I then wean them off the medications, see what symptoms emerge, and then “reinvent the wheel” with their medication regimens. I was verbally reprimanded by the medical director at 1 job because he thought a patient was “doing well” on 7 medications despite the fact he was oversedated. I also have inherited patients on medications that interacted with other medications and were causing medical problems.
Problems with polypharmacy in California are, as I said, rampant.
Terry Roh, MD
Child and Adult Psychiatrist
Lutheran Social Services of Southern California
Big Bear Lake, CA
I am writing to compliment Dr. Henry A. Nasrallah for having the guts to write “Polypharmacy subtypes” (From the Editor, Current Psychiatry, April 2011, p. 10-12). I try hard to have patients on no more than 4 medications, 1 from each class if indicated. In California, what Dr. Nasrallah described as ridiculous and hazardous is all too rampant. I have inherited patients taking as many as 7 psychotropics and the initial evaluation of these patients usually begins with families stating that they are angry about their loved ones being “doped up to the point of being zombies.” When I am finally able to get a good history of symptoms, I typically find that patients do not meet DSM-IV-TR criteria for some diagnoses. I then wean them off the medications, see what symptoms emerge, and then “reinvent the wheel” with their medication regimens. I was verbally reprimanded by the medical director at 1 job because he thought a patient was “doing well” on 7 medications despite the fact he was oversedated. I also have inherited patients on medications that interacted with other medications and were causing medical problems.
Problems with polypharmacy in California are, as I said, rampant.
Terry Roh, MD
Child and Adult Psychiatrist
Lutheran Social Services of Southern California
Big Bear Lake, CA
Comments & Controversies
Hazardous polypharmacy
I am writing to compliment Dr. Henry A. Nasrallah for having the guts to write “Polypharmacy subtypes” (From the Editor, Current Psychiatry, April 2011, p. 10-12). I try hard to have patients on no more than 4 medications, 1 from each class if indicated. In California, what Dr. Nasrallah described as ridiculous and hazardous is all too rampant. I have inherited patients taking as many as 7 psychotropics and the initial evaluation of these patients usually begins with families stating that they are angry about their loved ones being “doped up to the point of being zombies.” When I am finally able to get a good history of symptoms, I typically find that patients do not meet DSM-IV-TR criteria for some diagnoses. I then wean them off the medications, see what symptoms emerge, and then “reinvent the wheel” with their medication regimens. I was verbally reprimanded by the medical director at 1 job because he thought a patient was “doing well” on 7 medications despite the fact he was oversedated. I also have inherited patients on medications that interacted with other medications and were causing medical problems.
Problems with polypharmacy in California are, as I said, rampant.
Terry Roh, MD
Child and Adult Psychiatrist
Lutheran Social Services of Southern California
Big Bear Lake, CA
Ketamine and glutamate
I enjoyed the article “Glutamate: New hope for schizophrenia treatment” (Current Psychiatry, April 2011, p. 68-74) by Drs. Kantrowitz and Javitt. However, perhaps due to my own ignorance of the subject, I remain puzzled about their suggestion that the glutamatergic model of schizophrenia is supported by evidence showing that agonists at presynaptic mGluR2/3 receptors reverse the psychotomimetic effects of the N-methyl-D-aspartate (NMDA) receptor antagonist ketamine. Indeed, one would think that action of glutamatergic agonists at presynaptic autoreceptors would reduce glutamatergic activity and therefore mimic, rather than block, the effects of the antagonist ketamine.
Scott Mendelson, MD, PhD
Psychiatrist
Roseburg VA Hospital
Roseburg, OR
The authors respond
Thank you for your interest. We agree that this is a paradoxical effect of glutamate that is based largely upon the work of Bita Moghaddam, PhD, John Krystal, MD, and others. The basic finding is that blocking postsynaptic NMDA receptors leads to a rebound increase in presynaptic glutamate release that is pathological. Dr. Moghaddam showed that treatment with mGluR2/3 agonists reversed dopaminergic abnormalities and cognitive deficits induced by NMDAR antagonists in rodents.1 Dr. Krystal showed that the presynaptic glutamate release antagonist lamotrigine blocked psychotomimetic effects of ketamine in normal human volunteers.2 These findings have led to the hypothesis that blocking presynaptic glutamate may restore balance between glutamate and GABA systems, particularly in frontal brain regions. This theory also was supported by 1 successful clinical trial of a mGluR2/3 agonist,3 although replication studies are ongoing. When interpreting these findings, it is important to keep in mind that glutamate acts at several receptor types in addition to NMDA, and it is the balance between these receptors, as well as the balance between excitatory glutamatergic vs inhibitory GABAergic neurotransmission, that may be critical in psychosis.
Joshua T. Kantrowitz, MD
Assistant Professor
Department of Psychiatry
Columbia College of Physicians and Surgeons
New York, NY
Schizophrenia Research Center
Nathan Kline Institute for Psychiatric Research
Orangeburg, NY
Daniel C. Javitt, MD, PhD
Director, Schizophrenia Research
Nathan Kline Institute for Psychiatric Research
Orangeburg, NY
Professor of Psychiatry and Neuroscience
New York University School of Medicine
New York, NY
1. Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281(5381):1349-1352.
2. Krystal JH, Abi-Saab W, Perry E, et al. Preliminary evidence of attenuation of the disruptive effects of the NMDA glutamate receptor antagonist, ketamine, on working memory by pretreatment with the group II metabotropic glutamate receptor agonist, LY354740, in healthy human subjects. Psychopharmacology (Berl). 2005;179(1):303-309.
3. Patil ST, Zhang L, Martenyi F, et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med. 2007;13(9):1102-1107.
Hazardous polypharmacy
I am writing to compliment Dr. Henry A. Nasrallah for having the guts to write “Polypharmacy subtypes” (From the Editor, Current Psychiatry, April 2011, p. 10-12). I try hard to have patients on no more than 4 medications, 1 from each class if indicated. In California, what Dr. Nasrallah described as ridiculous and hazardous is all too rampant. I have inherited patients taking as many as 7 psychotropics and the initial evaluation of these patients usually begins with families stating that they are angry about their loved ones being “doped up to the point of being zombies.” When I am finally able to get a good history of symptoms, I typically find that patients do not meet DSM-IV-TR criteria for some diagnoses. I then wean them off the medications, see what symptoms emerge, and then “reinvent the wheel” with their medication regimens. I was verbally reprimanded by the medical director at 1 job because he thought a patient was “doing well” on 7 medications despite the fact he was oversedated. I also have inherited patients on medications that interacted with other medications and were causing medical problems.
Problems with polypharmacy in California are, as I said, rampant.
Terry Roh, MD
Child and Adult Psychiatrist
Lutheran Social Services of Southern California
Big Bear Lake, CA
Ketamine and glutamate
I enjoyed the article “Glutamate: New hope for schizophrenia treatment” (Current Psychiatry, April 2011, p. 68-74) by Drs. Kantrowitz and Javitt. However, perhaps due to my own ignorance of the subject, I remain puzzled about their suggestion that the glutamatergic model of schizophrenia is supported by evidence showing that agonists at presynaptic mGluR2/3 receptors reverse the psychotomimetic effects of the N-methyl-D-aspartate (NMDA) receptor antagonist ketamine. Indeed, one would think that action of glutamatergic agonists at presynaptic autoreceptors would reduce glutamatergic activity and therefore mimic, rather than block, the effects of the antagonist ketamine.
Scott Mendelson, MD, PhD
Psychiatrist
Roseburg VA Hospital
Roseburg, OR
The authors respond
Thank you for your interest. We agree that this is a paradoxical effect of glutamate that is based largely upon the work of Bita Moghaddam, PhD, John Krystal, MD, and others. The basic finding is that blocking postsynaptic NMDA receptors leads to a rebound increase in presynaptic glutamate release that is pathological. Dr. Moghaddam showed that treatment with mGluR2/3 agonists reversed dopaminergic abnormalities and cognitive deficits induced by NMDAR antagonists in rodents.1 Dr. Krystal showed that the presynaptic glutamate release antagonist lamotrigine blocked psychotomimetic effects of ketamine in normal human volunteers.2 These findings have led to the hypothesis that blocking presynaptic glutamate may restore balance between glutamate and GABA systems, particularly in frontal brain regions. This theory also was supported by 1 successful clinical trial of a mGluR2/3 agonist,3 although replication studies are ongoing. When interpreting these findings, it is important to keep in mind that glutamate acts at several receptor types in addition to NMDA, and it is the balance between these receptors, as well as the balance between excitatory glutamatergic vs inhibitory GABAergic neurotransmission, that may be critical in psychosis.
Joshua T. Kantrowitz, MD
Assistant Professor
Department of Psychiatry
Columbia College of Physicians and Surgeons
New York, NY
Schizophrenia Research Center
Nathan Kline Institute for Psychiatric Research
Orangeburg, NY
Daniel C. Javitt, MD, PhD
Director, Schizophrenia Research
Nathan Kline Institute for Psychiatric Research
Orangeburg, NY
Professor of Psychiatry and Neuroscience
New York University School of Medicine
New York, NY
Hazardous polypharmacy
I am writing to compliment Dr. Henry A. Nasrallah for having the guts to write “Polypharmacy subtypes” (From the Editor, Current Psychiatry, April 2011, p. 10-12). I try hard to have patients on no more than 4 medications, 1 from each class if indicated. In California, what Dr. Nasrallah described as ridiculous and hazardous is all too rampant. I have inherited patients taking as many as 7 psychotropics and the initial evaluation of these patients usually begins with families stating that they are angry about their loved ones being “doped up to the point of being zombies.” When I am finally able to get a good history of symptoms, I typically find that patients do not meet DSM-IV-TR criteria for some diagnoses. I then wean them off the medications, see what symptoms emerge, and then “reinvent the wheel” with their medication regimens. I was verbally reprimanded by the medical director at 1 job because he thought a patient was “doing well” on 7 medications despite the fact he was oversedated. I also have inherited patients on medications that interacted with other medications and were causing medical problems.
Problems with polypharmacy in California are, as I said, rampant.
Terry Roh, MD
Child and Adult Psychiatrist
Lutheran Social Services of Southern California
Big Bear Lake, CA
Ketamine and glutamate
I enjoyed the article “Glutamate: New hope for schizophrenia treatment” (Current Psychiatry, April 2011, p. 68-74) by Drs. Kantrowitz and Javitt. However, perhaps due to my own ignorance of the subject, I remain puzzled about their suggestion that the glutamatergic model of schizophrenia is supported by evidence showing that agonists at presynaptic mGluR2/3 receptors reverse the psychotomimetic effects of the N-methyl-D-aspartate (NMDA) receptor antagonist ketamine. Indeed, one would think that action of glutamatergic agonists at presynaptic autoreceptors would reduce glutamatergic activity and therefore mimic, rather than block, the effects of the antagonist ketamine.
Scott Mendelson, MD, PhD
Psychiatrist
Roseburg VA Hospital
Roseburg, OR
The authors respond
Thank you for your interest. We agree that this is a paradoxical effect of glutamate that is based largely upon the work of Bita Moghaddam, PhD, John Krystal, MD, and others. The basic finding is that blocking postsynaptic NMDA receptors leads to a rebound increase in presynaptic glutamate release that is pathological. Dr. Moghaddam showed that treatment with mGluR2/3 agonists reversed dopaminergic abnormalities and cognitive deficits induced by NMDAR antagonists in rodents.1 Dr. Krystal showed that the presynaptic glutamate release antagonist lamotrigine blocked psychotomimetic effects of ketamine in normal human volunteers.2 These findings have led to the hypothesis that blocking presynaptic glutamate may restore balance between glutamate and GABA systems, particularly in frontal brain regions. This theory also was supported by 1 successful clinical trial of a mGluR2/3 agonist,3 although replication studies are ongoing. When interpreting these findings, it is important to keep in mind that glutamate acts at several receptor types in addition to NMDA, and it is the balance between these receptors, as well as the balance between excitatory glutamatergic vs inhibitory GABAergic neurotransmission, that may be critical in psychosis.
Joshua T. Kantrowitz, MD
Assistant Professor
Department of Psychiatry
Columbia College of Physicians and Surgeons
New York, NY
Schizophrenia Research Center
Nathan Kline Institute for Psychiatric Research
Orangeburg, NY
Daniel C. Javitt, MD, PhD
Director, Schizophrenia Research
Nathan Kline Institute for Psychiatric Research
Orangeburg, NY
Professor of Psychiatry and Neuroscience
New York University School of Medicine
New York, NY
1. Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281(5381):1349-1352.
2. Krystal JH, Abi-Saab W, Perry E, et al. Preliminary evidence of attenuation of the disruptive effects of the NMDA glutamate receptor antagonist, ketamine, on working memory by pretreatment with the group II metabotropic glutamate receptor agonist, LY354740, in healthy human subjects. Psychopharmacology (Berl). 2005;179(1):303-309.
3. Patil ST, Zhang L, Martenyi F, et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med. 2007;13(9):1102-1107.
1. Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281(5381):1349-1352.
2. Krystal JH, Abi-Saab W, Perry E, et al. Preliminary evidence of attenuation of the disruptive effects of the NMDA glutamate receptor antagonist, ketamine, on working memory by pretreatment with the group II metabotropic glutamate receptor agonist, LY354740, in healthy human subjects. Psychopharmacology (Berl). 2005;179(1):303-309.
3. Patil ST, Zhang L, Martenyi F, et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med. 2007;13(9):1102-1107.