User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Opiates and psychotropics: Pharmacokinetics for practitioners
• When choosing pharmacologic therapy, make sure that all medications your patient takes are documented, consider drug-drug interactions, and instruct the patient to notify you of any new medications.
• In addition to toxicity, loss of efficacy of some opiate drugs may occur as a result of metabolic inhibition or induction by psychotropic medications.
• Collaborate with the physician who is prescribing the opioid if psychotropic choices are limited. The patient’s pain may be treated adequately with another analgesic that does not interact with the psychotropic that has been chosen.
As prescribed by his internist, Mr. G, age 44, takes 10 mg of methadone every 4 hours for chronic back pain secondary to a work-related injury 3 years ago. He experiences minimal sedation. Mr. G presents for psychiatric evaluation with complaints of increasing irritability, poor focus, low energy, and lack of interest in usual activities. The psychiatrist diagnoses him with depressive disorder not otherwise specified, and prescribes fluoxetine, 20 mg/d. Three weeks later, Mr. G’s wife contacts the psychiatrist reporting that her husband seems “overmedicated” and describes excess drowsiness and slowed thought processing.
After discussion with Mr. G’s internist and pharmacist, the psychiatrist decides that this oversedation may represent a drug-drug interaction between methadone and fluoxetine resulting in higher-than-expected methadone serum levels. Mr. G is instructed to stop fluoxetine with no taper, and his methadone dose is lowered with good results. Over the next 2 weeks Mr. G is titrated back to his original methadone dose and is re-evaluated by the psychiatrist to discuss medication options to address his depression.
Psychiatrists commonly encounter patients who receive opiate medications for chronic pain. Being aware of potential drug-drug interactions between opiate medications and psychotropics can help avoid adverse effects and combinations that may affect the efficacy of either drug. Pharmacokinetic interactions may affect your choice of psychiatric medication and should be taken into account when addressing adverse effects in any patient who takes opiates and psychotropics.
Metabolic pathways
The primary metabolic pathways for opiate metabolism are the cytochrome P450 (CYP) 2D6 and 3A4 isoenzymes. Depending on the agent used, prescribers may need to consider interactions for both pathways (Table 11,2 and Table 21). For example, oxycodone is metabolized via 2D6 and 3A4 isoenzymes and is a potent analgesic with oxymorphone and noroxycodone as its active metabolites. These metabolites, however, make a negligible contribution to oxycodone’s analgesic effect.3,4 Metabolism by the 3A4 isoenzyme is the principal oxidative pathway and the 2D6 site accounts for approximately 10% of oxycodone metabolism. A randomized, placebo-controlled, crossover study showed that 2D6 inhibition by paroxetine had no significant effect on oxycodone levels; however, a combination of paroxetine and itraconazole, a potent 3A4 inhibitor, resulted in substantial increases in oxycodone plasma levels.5 Remain vigilant for possible opiate toxicity when administering oxycodone with 3A4 inhibitors.
Methadone and meperidine also involve dual pathways. Methadone is metabolized primarily by 3A4 and 2B6, with 2D6 playing a smaller role.6 CYP2D6 seems to play an important part in metabolizing the R-enantiomer of methadone, which is largely responsible for the drug’s opiate effects, such as analgesia and respiratory depression.7,8 Induction of the 3A4 isoenzyme may result in methadone withdrawal, and inhibition may cause methadone toxicity.9 Inducers of 3A4, such as carbamazepine, and inhibitors, such as fluoxetine and fluvoxamine, should be avoided or used very cautiously in patients taking methadone. The 2B6 and 2D6 isoenzymes also may increase or decrease methadone levels and should be treated similarly. In Mr. G’s case, fluoxetine inhibited all 3 isoenzymes that are primarily responsible for methadone metabolism. A better antidepressant choice for Mr. G may have been venlafaxine, which is known to only mildly inhibit 2D6, or mirtazapine, which does not seem to inhibit the major CYP isoforms to an appreciable degree.10
Although the full scope of meperidine metabolism has not been identified,9 an in vitro test demonstrated that 2B6 and 3A4 play important roles in metabolizing meperidine to normeperidine, its major metabolite.11 Normeperidine does not provide analgesia and is associated with neurotoxicity, including anxiety, tremor, muscle twitching, and seizure.12 Agents that induce 3A4—such as carbamazepine or St. John’s wort—may contribute to neurotoxicity.9 Inhibition of these isoenzymes may increase meperidine levels and lead to anticholinergic toxicity or respiratory and central nervous system depression.13,14
Opiates metabolized by the 2D6 isoenzyme include codeine, hydrocodone, and tramadol. The analgesic effect of codeine seems dependent on 2D6 metabolism. Via this pathway, codeine is converted into morphine, which has a 300-times stronger affinity for the μ opioid receptor compared with codeine. 2D6 poor metabolizers have shown codeine intolerance and toxicity.3 Psychotropics known to strongly inhibit 2D6 isoenzyme processes—such as paroxetine, fluoxetine, and bupropion—should be avoided in patients taking codeine to prevent adverse effects and potential loss of efficacy. Better antidepressant choices include citalopram or venlafaxine, which inhibit 2D6 to a lesser degree.
Hydrocodone may be a viable option for patients taking 2D6 inhibitors. Hydrocodone is metabolized by 2D6 into hydromorphone, which is 7 to 33 times more potent than hydrocodone.15 Unlike codeine, 2D6 inhibition may have little effect on hydrocodone’s analgesic properties. Animal studies have shown that inhibition of the CYP analog to 2D6 does not affect analgesic response. In humans, 2D6 inhibition does not seem to affect hydrocodone’s abuse liability.16 Two case reports describe known 2D6 poor metabolizers who showed at least a partial response to hydrocodone.15,16
Tramadol’s analgesic properties may be related to serotonin and norepinephrine reuptake inhibition. It is less potent than codeine but is metabolized via the 2D6 isoenzyme into 0-desmethyltramadol, which is up to 200 times stronger than its parent compound.17 Clinicians should be aware that tramadol’s efficacy may be decreased when coadministered with 2D6 inhibitors. In a randomized, placebo-controlled trial, paroxetine, a potent 2D6 inhibitor, was shown to lessen the analgesic effect of tramadol.18
The 3A4 site is the primary pathway for fentanyl metabolism. Agents that inhibit 3A4 could increase fentanyl plasma concentration, leading to respiratory depression.19 Examples of 3A4 inhibitors include fluoxetine and fluvoxamine.
Psychotropics may inhibit or induce P450 isoenzymes to varying degrees. For example, paroxetine and citalopram are known to inhibit 2D6 but paroxetine is a stronger inhibitor; therefore, a significant drug-drug interaction is more likely with paroxetine and a 2D6 substrate than the same substrate administered with citalopram.
Table 1
Cytochrome P450 isoenzymes inhibited and induced by psychotropics
| Isoenzyme | Potency | Psychotropic(s) |
|---|---|---|
| 2B6 inducer | Moderate | Carbamazepine |
| 2B6 inhibitors | Mild to moderate | Fluoxetine, fluvoxamine |
| Moderate | Sertraline | |
| Potent | Paroxetine | |
| 2D6 inhibitors | Mild | Venlafaxine |
| Mild to moderate | Citalopram, escitalopram, fluvoxamine, risperidone | |
| Moderate | Duloxetine | |
| Moderate to potent | Bupropion | |
| Potent | Fluoxetine, haloperidol, paroxetine | |
| Dose-dependent | Sertraline | |
| 3A4 inducer | Potent | Carbamazepine |
| 3A4 inhibitors | Mild | Sertraline |
| Mild to moderate | Fluoxetine, fluvoxamine | |
| Source: References 1,2 | ||
Table 2
Cytochrome P450 isoenzymes inhibiting and inducing opiate metabolism
| Isoenzyme | Opiates |
|---|---|
| 2B6 inducer | Methadone |
| 2B6 inhibitors | Meperidine, methadone |
| 2D6 inhibitors | Codeine (may involve loss of efficacy as well as toxicity), methadone, tramadol (may involve loss of efficacy) |
| 3A4 inducer | Meperidine, methadone |
| 3A4 inhibitors | Fentanyl, oxycodone, meperidine, methadone |
| Source: Reference 1 | |
Other considerations
In addition to pharmacokinetic interactions, it is important to consider synergistic effects of some opiates and psychotropics. Examples include:
- additive effect on respiratory depression by benzodiazepines and opiates
- increased risk of serotonin syndrome and seizure when using tramadol with selective serotonin reuptake inhibitors or tricyclic antidepressants
- additive prolongation of the QTc interval by methadone when used with psychotropics known to prolong the QTc, such as ziprasidone.9,17,20
Careful attention to these interactions and collaboration among providers can ensure the best outcome for our patients. In Mr. G’s case, collaboration with his internist would be in order, particularly if antidepressant choices are limited. In consultation with the psychiatrist, the internist might choose another opiate to treat Mr. G’s pain that would not interact with fluoxetine. If Mr. G and his physician have struggled to manage his pain and if he is stable on the current regimen, selecting a different antidepressant may be warranted.
Related Resources
- Indiana University School of Medicine drug interactions: cytochrome P450 drug interaction table. http://medicine.iupui.edu/clinpharm/ddis/table.asp.
- Ferrando SJ, Levenson JL, Owen JA, eds. Clinical manual of psychopharmacology in the medically ill. Arlington, VA: American Psychiatric Publishing, Inc; 2010.
Drug Brand Names
- Bupropion • Wellbutrin, Zyban
- Carbamazepine • Tegretol
- Citalopram • Celexa
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fentanyl • Duragesic, Actiq
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Haloperidol • Haldol
- Hydrocodone • Lortab, Vicodin, others
- Itraconazole • Sporanox
- Meperidine • Demerol
- Methadone • Dolophine, Methadose
- Mirtazapine • Remeron
- Morphine • Avinza, Duramorph, others
- Oxycodone • OxyContin, Roxicodone
- Paroxetine • Paxil
- Risperidone • Risperdal
- Sertraline • Zoloft
- Tramadol • Ultram
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Sandson NB, Armstrong SC, Cozza KL. An overview of psychotropic drug-drug interactions. Psychosomatics. 2005;46:464-494.
2. Faucette SR, Wang H, Hamilton GA. Regulation of CYP2B6 in primary human hepatocytes by prototypical inducers. Drug Metab Dispos. 2004;32(3):348-358.
3. Smith HS. Opioid metabolism. Mayo Clin Proc. 2009;84:613-624.
4. Armstrong SC, Cozza KL. Pharmacokinetic drug interactions of morphine codeine, and their derivatives: theory and clinical reality, part II. Psychosomatics. 2003;44:515-520.
5. Grönlund J, Saari TI, Hagelberg NM, et al. Exposure to oral oxycodone is increased by concomitant inhibition of CYP2D6 and 3A4 pathways, but not by inhibition of CYP2D6 alone. Br J Clin Pharmacol. 2010;70:78-87.
6. Leavitt SB. Methadone-drug* interactions. (*medications illicit drugs, and other substances). 3rd ed. Mundelein, IL: Addiction Treatment Forum; 2005.
7. Pérez de los Cobos J, Siñol N, Trujols J, et al. Association of CYP2D6 ultrarapid metabolizer genotype with deficient patient satisfaction regarding methadone maintenance treatment. Drug Alcohol Depend. 2007;89:190-194.
8. Kristensen K, Christensen CB, Christrup LL. The mu1 mu2, delta, kappa opioid receptor binding profiles of methadone stereoisomers and morphine. Life Sci. 1995;56:PL45-50.
9. Armstrong SC, Wynn GH, Sandson NB. Pharmacokinetic drug interactions of synthetic opiate analgesics. Psychosomatics. 2009;50:169-176.
10. Spina E, Santoro V, D’Arrigo C. Clinically relevant pharmacokinetic drug interactions with second-generation antidepressants: an update. Clin Ther. 2008;30:1206-1227.
11. Ramírez J, Innocenti F, Schuetz EG, et al. CYP2B6, CYP3A4, and CYP2C19 are responsible for the in vitro N-demethylation of meperidine in human liver microsomes. Drug Metab Dispos. 2004;32:930-936.
12. Kaiko RF, Foley KM, Grabinski PY, et al. Central nervous system excitatory effects of meperidine in cancer patients. Ann Neurol. 1983;13:180-185.
13. Chalverus C. Clinically important meperidine toxicities. Journal of Pharmaceutical Care in Pain and Symptom Control. 2001;9:37-55.
14. Beckwith MC, Fox ER, Chandramouli J. Removing meperidine from the health-system formulary—frequently asked questions. J Pain Palliat Care Pharmacother. 2002;16:45-59.
15. Foster A, Mobley E, Wang Z. Complicated pain management in a CYP450 2D6 poor metabolizer. Pain Pract. 2007;7:352-356.
16. Susce MT, Murray-Carmichael E, de Leon J. Response to hydrocodone codeine and oxycodone in a CYP2D6 poor metabolizer. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1356-1358.
17. Sansone RA, Sansone LA. Tramadol: seizures serotonin syndrome, and coadministered antidepressants. Psychiatry (Edgmont). 2009;6:17-21.
18. Laugesen S, Enggaard TP, Pedersen RS, et al. Paroxetine, a cytochrome P450 2D6 inhibitor, diminishes the stereoselective O-demethylation and reduces the hypoalgesic effect of tramadol. Clin Pharmacol Ther. 2005;77:312-323.
19. Duragesic [package insert]. Raritan NJ: Ortho-McNeil-Janssen Pharmaceuticals, Inc; 2009.
20. Caplehorn JR, Drummer OH. Fatal methadone toxicity: signs and circumstances and the role of benzodiazepines. Aust N Z J Public Health. 2002;26:358-362;discussion 362–363.
• When choosing pharmacologic therapy, make sure that all medications your patient takes are documented, consider drug-drug interactions, and instruct the patient to notify you of any new medications.
• In addition to toxicity, loss of efficacy of some opiate drugs may occur as a result of metabolic inhibition or induction by psychotropic medications.
• Collaborate with the physician who is prescribing the opioid if psychotropic choices are limited. The patient’s pain may be treated adequately with another analgesic that does not interact with the psychotropic that has been chosen.
As prescribed by his internist, Mr. G, age 44, takes 10 mg of methadone every 4 hours for chronic back pain secondary to a work-related injury 3 years ago. He experiences minimal sedation. Mr. G presents for psychiatric evaluation with complaints of increasing irritability, poor focus, low energy, and lack of interest in usual activities. The psychiatrist diagnoses him with depressive disorder not otherwise specified, and prescribes fluoxetine, 20 mg/d. Three weeks later, Mr. G’s wife contacts the psychiatrist reporting that her husband seems “overmedicated” and describes excess drowsiness and slowed thought processing.
After discussion with Mr. G’s internist and pharmacist, the psychiatrist decides that this oversedation may represent a drug-drug interaction between methadone and fluoxetine resulting in higher-than-expected methadone serum levels. Mr. G is instructed to stop fluoxetine with no taper, and his methadone dose is lowered with good results. Over the next 2 weeks Mr. G is titrated back to his original methadone dose and is re-evaluated by the psychiatrist to discuss medication options to address his depression.
Psychiatrists commonly encounter patients who receive opiate medications for chronic pain. Being aware of potential drug-drug interactions between opiate medications and psychotropics can help avoid adverse effects and combinations that may affect the efficacy of either drug. Pharmacokinetic interactions may affect your choice of psychiatric medication and should be taken into account when addressing adverse effects in any patient who takes opiates and psychotropics.
Metabolic pathways
The primary metabolic pathways for opiate metabolism are the cytochrome P450 (CYP) 2D6 and 3A4 isoenzymes. Depending on the agent used, prescribers may need to consider interactions for both pathways (Table 11,2 and Table 21). For example, oxycodone is metabolized via 2D6 and 3A4 isoenzymes and is a potent analgesic with oxymorphone and noroxycodone as its active metabolites. These metabolites, however, make a negligible contribution to oxycodone’s analgesic effect.3,4 Metabolism by the 3A4 isoenzyme is the principal oxidative pathway and the 2D6 site accounts for approximately 10% of oxycodone metabolism. A randomized, placebo-controlled, crossover study showed that 2D6 inhibition by paroxetine had no significant effect on oxycodone levels; however, a combination of paroxetine and itraconazole, a potent 3A4 inhibitor, resulted in substantial increases in oxycodone plasma levels.5 Remain vigilant for possible opiate toxicity when administering oxycodone with 3A4 inhibitors.
Methadone and meperidine also involve dual pathways. Methadone is metabolized primarily by 3A4 and 2B6, with 2D6 playing a smaller role.6 CYP2D6 seems to play an important part in metabolizing the R-enantiomer of methadone, which is largely responsible for the drug’s opiate effects, such as analgesia and respiratory depression.7,8 Induction of the 3A4 isoenzyme may result in methadone withdrawal, and inhibition may cause methadone toxicity.9 Inducers of 3A4, such as carbamazepine, and inhibitors, such as fluoxetine and fluvoxamine, should be avoided or used very cautiously in patients taking methadone. The 2B6 and 2D6 isoenzymes also may increase or decrease methadone levels and should be treated similarly. In Mr. G’s case, fluoxetine inhibited all 3 isoenzymes that are primarily responsible for methadone metabolism. A better antidepressant choice for Mr. G may have been venlafaxine, which is known to only mildly inhibit 2D6, or mirtazapine, which does not seem to inhibit the major CYP isoforms to an appreciable degree.10
Although the full scope of meperidine metabolism has not been identified,9 an in vitro test demonstrated that 2B6 and 3A4 play important roles in metabolizing meperidine to normeperidine, its major metabolite.11 Normeperidine does not provide analgesia and is associated with neurotoxicity, including anxiety, tremor, muscle twitching, and seizure.12 Agents that induce 3A4—such as carbamazepine or St. John’s wort—may contribute to neurotoxicity.9 Inhibition of these isoenzymes may increase meperidine levels and lead to anticholinergic toxicity or respiratory and central nervous system depression.13,14
Opiates metabolized by the 2D6 isoenzyme include codeine, hydrocodone, and tramadol. The analgesic effect of codeine seems dependent on 2D6 metabolism. Via this pathway, codeine is converted into morphine, which has a 300-times stronger affinity for the μ opioid receptor compared with codeine. 2D6 poor metabolizers have shown codeine intolerance and toxicity.3 Psychotropics known to strongly inhibit 2D6 isoenzyme processes—such as paroxetine, fluoxetine, and bupropion—should be avoided in patients taking codeine to prevent adverse effects and potential loss of efficacy. Better antidepressant choices include citalopram or venlafaxine, which inhibit 2D6 to a lesser degree.
Hydrocodone may be a viable option for patients taking 2D6 inhibitors. Hydrocodone is metabolized by 2D6 into hydromorphone, which is 7 to 33 times more potent than hydrocodone.15 Unlike codeine, 2D6 inhibition may have little effect on hydrocodone’s analgesic properties. Animal studies have shown that inhibition of the CYP analog to 2D6 does not affect analgesic response. In humans, 2D6 inhibition does not seem to affect hydrocodone’s abuse liability.16 Two case reports describe known 2D6 poor metabolizers who showed at least a partial response to hydrocodone.15,16
Tramadol’s analgesic properties may be related to serotonin and norepinephrine reuptake inhibition. It is less potent than codeine but is metabolized via the 2D6 isoenzyme into 0-desmethyltramadol, which is up to 200 times stronger than its parent compound.17 Clinicians should be aware that tramadol’s efficacy may be decreased when coadministered with 2D6 inhibitors. In a randomized, placebo-controlled trial, paroxetine, a potent 2D6 inhibitor, was shown to lessen the analgesic effect of tramadol.18
The 3A4 site is the primary pathway for fentanyl metabolism. Agents that inhibit 3A4 could increase fentanyl plasma concentration, leading to respiratory depression.19 Examples of 3A4 inhibitors include fluoxetine and fluvoxamine.
Psychotropics may inhibit or induce P450 isoenzymes to varying degrees. For example, paroxetine and citalopram are known to inhibit 2D6 but paroxetine is a stronger inhibitor; therefore, a significant drug-drug interaction is more likely with paroxetine and a 2D6 substrate than the same substrate administered with citalopram.
Table 1
Cytochrome P450 isoenzymes inhibited and induced by psychotropics
| Isoenzyme | Potency | Psychotropic(s) |
|---|---|---|
| 2B6 inducer | Moderate | Carbamazepine |
| 2B6 inhibitors | Mild to moderate | Fluoxetine, fluvoxamine |
| Moderate | Sertraline | |
| Potent | Paroxetine | |
| 2D6 inhibitors | Mild | Venlafaxine |
| Mild to moderate | Citalopram, escitalopram, fluvoxamine, risperidone | |
| Moderate | Duloxetine | |
| Moderate to potent | Bupropion | |
| Potent | Fluoxetine, haloperidol, paroxetine | |
| Dose-dependent | Sertraline | |
| 3A4 inducer | Potent | Carbamazepine |
| 3A4 inhibitors | Mild | Sertraline |
| Mild to moderate | Fluoxetine, fluvoxamine | |
| Source: References 1,2 | ||
Table 2
Cytochrome P450 isoenzymes inhibiting and inducing opiate metabolism
| Isoenzyme | Opiates |
|---|---|
| 2B6 inducer | Methadone |
| 2B6 inhibitors | Meperidine, methadone |
| 2D6 inhibitors | Codeine (may involve loss of efficacy as well as toxicity), methadone, tramadol (may involve loss of efficacy) |
| 3A4 inducer | Meperidine, methadone |
| 3A4 inhibitors | Fentanyl, oxycodone, meperidine, methadone |
| Source: Reference 1 | |
Other considerations
In addition to pharmacokinetic interactions, it is important to consider synergistic effects of some opiates and psychotropics. Examples include:
- additive effect on respiratory depression by benzodiazepines and opiates
- increased risk of serotonin syndrome and seizure when using tramadol with selective serotonin reuptake inhibitors or tricyclic antidepressants
- additive prolongation of the QTc interval by methadone when used with psychotropics known to prolong the QTc, such as ziprasidone.9,17,20
Careful attention to these interactions and collaboration among providers can ensure the best outcome for our patients. In Mr. G’s case, collaboration with his internist would be in order, particularly if antidepressant choices are limited. In consultation with the psychiatrist, the internist might choose another opiate to treat Mr. G’s pain that would not interact with fluoxetine. If Mr. G and his physician have struggled to manage his pain and if he is stable on the current regimen, selecting a different antidepressant may be warranted.
Related Resources
- Indiana University School of Medicine drug interactions: cytochrome P450 drug interaction table. http://medicine.iupui.edu/clinpharm/ddis/table.asp.
- Ferrando SJ, Levenson JL, Owen JA, eds. Clinical manual of psychopharmacology in the medically ill. Arlington, VA: American Psychiatric Publishing, Inc; 2010.
Drug Brand Names
- Bupropion • Wellbutrin, Zyban
- Carbamazepine • Tegretol
- Citalopram • Celexa
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fentanyl • Duragesic, Actiq
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Haloperidol • Haldol
- Hydrocodone • Lortab, Vicodin, others
- Itraconazole • Sporanox
- Meperidine • Demerol
- Methadone • Dolophine, Methadose
- Mirtazapine • Remeron
- Morphine • Avinza, Duramorph, others
- Oxycodone • OxyContin, Roxicodone
- Paroxetine • Paxil
- Risperidone • Risperdal
- Sertraline • Zoloft
- Tramadol • Ultram
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
• When choosing pharmacologic therapy, make sure that all medications your patient takes are documented, consider drug-drug interactions, and instruct the patient to notify you of any new medications.
• In addition to toxicity, loss of efficacy of some opiate drugs may occur as a result of metabolic inhibition or induction by psychotropic medications.
• Collaborate with the physician who is prescribing the opioid if psychotropic choices are limited. The patient’s pain may be treated adequately with another analgesic that does not interact with the psychotropic that has been chosen.
As prescribed by his internist, Mr. G, age 44, takes 10 mg of methadone every 4 hours for chronic back pain secondary to a work-related injury 3 years ago. He experiences minimal sedation. Mr. G presents for psychiatric evaluation with complaints of increasing irritability, poor focus, low energy, and lack of interest in usual activities. The psychiatrist diagnoses him with depressive disorder not otherwise specified, and prescribes fluoxetine, 20 mg/d. Three weeks later, Mr. G’s wife contacts the psychiatrist reporting that her husband seems “overmedicated” and describes excess drowsiness and slowed thought processing.
After discussion with Mr. G’s internist and pharmacist, the psychiatrist decides that this oversedation may represent a drug-drug interaction between methadone and fluoxetine resulting in higher-than-expected methadone serum levels. Mr. G is instructed to stop fluoxetine with no taper, and his methadone dose is lowered with good results. Over the next 2 weeks Mr. G is titrated back to his original methadone dose and is re-evaluated by the psychiatrist to discuss medication options to address his depression.
Psychiatrists commonly encounter patients who receive opiate medications for chronic pain. Being aware of potential drug-drug interactions between opiate medications and psychotropics can help avoid adverse effects and combinations that may affect the efficacy of either drug. Pharmacokinetic interactions may affect your choice of psychiatric medication and should be taken into account when addressing adverse effects in any patient who takes opiates and psychotropics.
Metabolic pathways
The primary metabolic pathways for opiate metabolism are the cytochrome P450 (CYP) 2D6 and 3A4 isoenzymes. Depending on the agent used, prescribers may need to consider interactions for both pathways (Table 11,2 and Table 21). For example, oxycodone is metabolized via 2D6 and 3A4 isoenzymes and is a potent analgesic with oxymorphone and noroxycodone as its active metabolites. These metabolites, however, make a negligible contribution to oxycodone’s analgesic effect.3,4 Metabolism by the 3A4 isoenzyme is the principal oxidative pathway and the 2D6 site accounts for approximately 10% of oxycodone metabolism. A randomized, placebo-controlled, crossover study showed that 2D6 inhibition by paroxetine had no significant effect on oxycodone levels; however, a combination of paroxetine and itraconazole, a potent 3A4 inhibitor, resulted in substantial increases in oxycodone plasma levels.5 Remain vigilant for possible opiate toxicity when administering oxycodone with 3A4 inhibitors.
Methadone and meperidine also involve dual pathways. Methadone is metabolized primarily by 3A4 and 2B6, with 2D6 playing a smaller role.6 CYP2D6 seems to play an important part in metabolizing the R-enantiomer of methadone, which is largely responsible for the drug’s opiate effects, such as analgesia and respiratory depression.7,8 Induction of the 3A4 isoenzyme may result in methadone withdrawal, and inhibition may cause methadone toxicity.9 Inducers of 3A4, such as carbamazepine, and inhibitors, such as fluoxetine and fluvoxamine, should be avoided or used very cautiously in patients taking methadone. The 2B6 and 2D6 isoenzymes also may increase or decrease methadone levels and should be treated similarly. In Mr. G’s case, fluoxetine inhibited all 3 isoenzymes that are primarily responsible for methadone metabolism. A better antidepressant choice for Mr. G may have been venlafaxine, which is known to only mildly inhibit 2D6, or mirtazapine, which does not seem to inhibit the major CYP isoforms to an appreciable degree.10
Although the full scope of meperidine metabolism has not been identified,9 an in vitro test demonstrated that 2B6 and 3A4 play important roles in metabolizing meperidine to normeperidine, its major metabolite.11 Normeperidine does not provide analgesia and is associated with neurotoxicity, including anxiety, tremor, muscle twitching, and seizure.12 Agents that induce 3A4—such as carbamazepine or St. John’s wort—may contribute to neurotoxicity.9 Inhibition of these isoenzymes may increase meperidine levels and lead to anticholinergic toxicity or respiratory and central nervous system depression.13,14
Opiates metabolized by the 2D6 isoenzyme include codeine, hydrocodone, and tramadol. The analgesic effect of codeine seems dependent on 2D6 metabolism. Via this pathway, codeine is converted into morphine, which has a 300-times stronger affinity for the μ opioid receptor compared with codeine. 2D6 poor metabolizers have shown codeine intolerance and toxicity.3 Psychotropics known to strongly inhibit 2D6 isoenzyme processes—such as paroxetine, fluoxetine, and bupropion—should be avoided in patients taking codeine to prevent adverse effects and potential loss of efficacy. Better antidepressant choices include citalopram or venlafaxine, which inhibit 2D6 to a lesser degree.
Hydrocodone may be a viable option for patients taking 2D6 inhibitors. Hydrocodone is metabolized by 2D6 into hydromorphone, which is 7 to 33 times more potent than hydrocodone.15 Unlike codeine, 2D6 inhibition may have little effect on hydrocodone’s analgesic properties. Animal studies have shown that inhibition of the CYP analog to 2D6 does not affect analgesic response. In humans, 2D6 inhibition does not seem to affect hydrocodone’s abuse liability.16 Two case reports describe known 2D6 poor metabolizers who showed at least a partial response to hydrocodone.15,16
Tramadol’s analgesic properties may be related to serotonin and norepinephrine reuptake inhibition. It is less potent than codeine but is metabolized via the 2D6 isoenzyme into 0-desmethyltramadol, which is up to 200 times stronger than its parent compound.17 Clinicians should be aware that tramadol’s efficacy may be decreased when coadministered with 2D6 inhibitors. In a randomized, placebo-controlled trial, paroxetine, a potent 2D6 inhibitor, was shown to lessen the analgesic effect of tramadol.18
The 3A4 site is the primary pathway for fentanyl metabolism. Agents that inhibit 3A4 could increase fentanyl plasma concentration, leading to respiratory depression.19 Examples of 3A4 inhibitors include fluoxetine and fluvoxamine.
Psychotropics may inhibit or induce P450 isoenzymes to varying degrees. For example, paroxetine and citalopram are known to inhibit 2D6 but paroxetine is a stronger inhibitor; therefore, a significant drug-drug interaction is more likely with paroxetine and a 2D6 substrate than the same substrate administered with citalopram.
Table 1
Cytochrome P450 isoenzymes inhibited and induced by psychotropics
| Isoenzyme | Potency | Psychotropic(s) |
|---|---|---|
| 2B6 inducer | Moderate | Carbamazepine |
| 2B6 inhibitors | Mild to moderate | Fluoxetine, fluvoxamine |
| Moderate | Sertraline | |
| Potent | Paroxetine | |
| 2D6 inhibitors | Mild | Venlafaxine |
| Mild to moderate | Citalopram, escitalopram, fluvoxamine, risperidone | |
| Moderate | Duloxetine | |
| Moderate to potent | Bupropion | |
| Potent | Fluoxetine, haloperidol, paroxetine | |
| Dose-dependent | Sertraline | |
| 3A4 inducer | Potent | Carbamazepine |
| 3A4 inhibitors | Mild | Sertraline |
| Mild to moderate | Fluoxetine, fluvoxamine | |
| Source: References 1,2 | ||
Table 2
Cytochrome P450 isoenzymes inhibiting and inducing opiate metabolism
| Isoenzyme | Opiates |
|---|---|
| 2B6 inducer | Methadone |
| 2B6 inhibitors | Meperidine, methadone |
| 2D6 inhibitors | Codeine (may involve loss of efficacy as well as toxicity), methadone, tramadol (may involve loss of efficacy) |
| 3A4 inducer | Meperidine, methadone |
| 3A4 inhibitors | Fentanyl, oxycodone, meperidine, methadone |
| Source: Reference 1 | |
Other considerations
In addition to pharmacokinetic interactions, it is important to consider synergistic effects of some opiates and psychotropics. Examples include:
- additive effect on respiratory depression by benzodiazepines and opiates
- increased risk of serotonin syndrome and seizure when using tramadol with selective serotonin reuptake inhibitors or tricyclic antidepressants
- additive prolongation of the QTc interval by methadone when used with psychotropics known to prolong the QTc, such as ziprasidone.9,17,20
Careful attention to these interactions and collaboration among providers can ensure the best outcome for our patients. In Mr. G’s case, collaboration with his internist would be in order, particularly if antidepressant choices are limited. In consultation with the psychiatrist, the internist might choose another opiate to treat Mr. G’s pain that would not interact with fluoxetine. If Mr. G and his physician have struggled to manage his pain and if he is stable on the current regimen, selecting a different antidepressant may be warranted.
Related Resources
- Indiana University School of Medicine drug interactions: cytochrome P450 drug interaction table. http://medicine.iupui.edu/clinpharm/ddis/table.asp.
- Ferrando SJ, Levenson JL, Owen JA, eds. Clinical manual of psychopharmacology in the medically ill. Arlington, VA: American Psychiatric Publishing, Inc; 2010.
Drug Brand Names
- Bupropion • Wellbutrin, Zyban
- Carbamazepine • Tegretol
- Citalopram • Celexa
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fentanyl • Duragesic, Actiq
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Haloperidol • Haldol
- Hydrocodone • Lortab, Vicodin, others
- Itraconazole • Sporanox
- Meperidine • Demerol
- Methadone • Dolophine, Methadose
- Mirtazapine • Remeron
- Morphine • Avinza, Duramorph, others
- Oxycodone • OxyContin, Roxicodone
- Paroxetine • Paxil
- Risperidone • Risperdal
- Sertraline • Zoloft
- Tramadol • Ultram
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Sandson NB, Armstrong SC, Cozza KL. An overview of psychotropic drug-drug interactions. Psychosomatics. 2005;46:464-494.
2. Faucette SR, Wang H, Hamilton GA. Regulation of CYP2B6 in primary human hepatocytes by prototypical inducers. Drug Metab Dispos. 2004;32(3):348-358.
3. Smith HS. Opioid metabolism. Mayo Clin Proc. 2009;84:613-624.
4. Armstrong SC, Cozza KL. Pharmacokinetic drug interactions of morphine codeine, and their derivatives: theory and clinical reality, part II. Psychosomatics. 2003;44:515-520.
5. Grönlund J, Saari TI, Hagelberg NM, et al. Exposure to oral oxycodone is increased by concomitant inhibition of CYP2D6 and 3A4 pathways, but not by inhibition of CYP2D6 alone. Br J Clin Pharmacol. 2010;70:78-87.
6. Leavitt SB. Methadone-drug* interactions. (*medications illicit drugs, and other substances). 3rd ed. Mundelein, IL: Addiction Treatment Forum; 2005.
7. Pérez de los Cobos J, Siñol N, Trujols J, et al. Association of CYP2D6 ultrarapid metabolizer genotype with deficient patient satisfaction regarding methadone maintenance treatment. Drug Alcohol Depend. 2007;89:190-194.
8. Kristensen K, Christensen CB, Christrup LL. The mu1 mu2, delta, kappa opioid receptor binding profiles of methadone stereoisomers and morphine. Life Sci. 1995;56:PL45-50.
9. Armstrong SC, Wynn GH, Sandson NB. Pharmacokinetic drug interactions of synthetic opiate analgesics. Psychosomatics. 2009;50:169-176.
10. Spina E, Santoro V, D’Arrigo C. Clinically relevant pharmacokinetic drug interactions with second-generation antidepressants: an update. Clin Ther. 2008;30:1206-1227.
11. Ramírez J, Innocenti F, Schuetz EG, et al. CYP2B6, CYP3A4, and CYP2C19 are responsible for the in vitro N-demethylation of meperidine in human liver microsomes. Drug Metab Dispos. 2004;32:930-936.
12. Kaiko RF, Foley KM, Grabinski PY, et al. Central nervous system excitatory effects of meperidine in cancer patients. Ann Neurol. 1983;13:180-185.
13. Chalverus C. Clinically important meperidine toxicities. Journal of Pharmaceutical Care in Pain and Symptom Control. 2001;9:37-55.
14. Beckwith MC, Fox ER, Chandramouli J. Removing meperidine from the health-system formulary—frequently asked questions. J Pain Palliat Care Pharmacother. 2002;16:45-59.
15. Foster A, Mobley E, Wang Z. Complicated pain management in a CYP450 2D6 poor metabolizer. Pain Pract. 2007;7:352-356.
16. Susce MT, Murray-Carmichael E, de Leon J. Response to hydrocodone codeine and oxycodone in a CYP2D6 poor metabolizer. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1356-1358.
17. Sansone RA, Sansone LA. Tramadol: seizures serotonin syndrome, and coadministered antidepressants. Psychiatry (Edgmont). 2009;6:17-21.
18. Laugesen S, Enggaard TP, Pedersen RS, et al. Paroxetine, a cytochrome P450 2D6 inhibitor, diminishes the stereoselective O-demethylation and reduces the hypoalgesic effect of tramadol. Clin Pharmacol Ther. 2005;77:312-323.
19. Duragesic [package insert]. Raritan NJ: Ortho-McNeil-Janssen Pharmaceuticals, Inc; 2009.
20. Caplehorn JR, Drummer OH. Fatal methadone toxicity: signs and circumstances and the role of benzodiazepines. Aust N Z J Public Health. 2002;26:358-362;discussion 362–363.
1. Sandson NB, Armstrong SC, Cozza KL. An overview of psychotropic drug-drug interactions. Psychosomatics. 2005;46:464-494.
2. Faucette SR, Wang H, Hamilton GA. Regulation of CYP2B6 in primary human hepatocytes by prototypical inducers. Drug Metab Dispos. 2004;32(3):348-358.
3. Smith HS. Opioid metabolism. Mayo Clin Proc. 2009;84:613-624.
4. Armstrong SC, Cozza KL. Pharmacokinetic drug interactions of morphine codeine, and their derivatives: theory and clinical reality, part II. Psychosomatics. 2003;44:515-520.
5. Grönlund J, Saari TI, Hagelberg NM, et al. Exposure to oral oxycodone is increased by concomitant inhibition of CYP2D6 and 3A4 pathways, but not by inhibition of CYP2D6 alone. Br J Clin Pharmacol. 2010;70:78-87.
6. Leavitt SB. Methadone-drug* interactions. (*medications illicit drugs, and other substances). 3rd ed. Mundelein, IL: Addiction Treatment Forum; 2005.
7. Pérez de los Cobos J, Siñol N, Trujols J, et al. Association of CYP2D6 ultrarapid metabolizer genotype with deficient patient satisfaction regarding methadone maintenance treatment. Drug Alcohol Depend. 2007;89:190-194.
8. Kristensen K, Christensen CB, Christrup LL. The mu1 mu2, delta, kappa opioid receptor binding profiles of methadone stereoisomers and morphine. Life Sci. 1995;56:PL45-50.
9. Armstrong SC, Wynn GH, Sandson NB. Pharmacokinetic drug interactions of synthetic opiate analgesics. Psychosomatics. 2009;50:169-176.
10. Spina E, Santoro V, D’Arrigo C. Clinically relevant pharmacokinetic drug interactions with second-generation antidepressants: an update. Clin Ther. 2008;30:1206-1227.
11. Ramírez J, Innocenti F, Schuetz EG, et al. CYP2B6, CYP3A4, and CYP2C19 are responsible for the in vitro N-demethylation of meperidine in human liver microsomes. Drug Metab Dispos. 2004;32:930-936.
12. Kaiko RF, Foley KM, Grabinski PY, et al. Central nervous system excitatory effects of meperidine in cancer patients. Ann Neurol. 1983;13:180-185.
13. Chalverus C. Clinically important meperidine toxicities. Journal of Pharmaceutical Care in Pain and Symptom Control. 2001;9:37-55.
14. Beckwith MC, Fox ER, Chandramouli J. Removing meperidine from the health-system formulary—frequently asked questions. J Pain Palliat Care Pharmacother. 2002;16:45-59.
15. Foster A, Mobley E, Wang Z. Complicated pain management in a CYP450 2D6 poor metabolizer. Pain Pract. 2007;7:352-356.
16. Susce MT, Murray-Carmichael E, de Leon J. Response to hydrocodone codeine and oxycodone in a CYP2D6 poor metabolizer. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1356-1358.
17. Sansone RA, Sansone LA. Tramadol: seizures serotonin syndrome, and coadministered antidepressants. Psychiatry (Edgmont). 2009;6:17-21.
18. Laugesen S, Enggaard TP, Pedersen RS, et al. Paroxetine, a cytochrome P450 2D6 inhibitor, diminishes the stereoselective O-demethylation and reduces the hypoalgesic effect of tramadol. Clin Pharmacol Ther. 2005;77:312-323.
19. Duragesic [package insert]. Raritan NJ: Ortho-McNeil-Janssen Pharmaceuticals, Inc; 2009.
20. Caplehorn JR, Drummer OH. Fatal methadone toxicity: signs and circumstances and the role of benzodiazepines. Aust N Z J Public Health. 2002;26:358-362;discussion 362–363.
Evaluating medication outcomes: 3 key questions
Post hoc ergo propter hoc—”after this, therefore because of this”—suggests that 2 distinct events linked temporally are related causally. Clinicians often apply this dictum when monitoring effects of psychotropics. Because of stigma associated with psychiatric medications, and the readiness with which many practitioners blame them for unexpected results, it is important to develop a rational approach to evaluating outcomes—particularly adverse ones—after administering psychotropic agents.
Consider a geriatric patient admitted to the hospital for a urinary tract infection. He becomes verbally aggressive and is given IV haloperidol. Five minutes later he strikes a nurse and receives lorazepam. Twenty minutes later, he is lying calmly in his bed. The nursing staff and primary team conclude that the patient’s agitation worsened because of the antipsychotic and responded to the benzodiazepine; the physician documents in the patient’s chart that he had an adverse reaction to haloperidol.
In light of what we know about psychotropic medications’ mechanism of action, a more plausible explanation is that whatever caused the patient to become agitated (delirium) resulted in physical aggression. Haloperidol simply did not have enough time to exert its effect before the patient hit the nurse. It also would be wrong to automatically conclude that the last intervention (a benzodiazepine) produced the beneficial outcome. Was it the lorazepam, the haloperidol finally “kicking in,” or a combination of both? Perhaps it was none of the above but rather a worsening infection or irregular waxing and waning of delirium that was the culprit.
To avoid incorrectly attributing negative outcomes to medications, we suggest asking yourself 3 questions:
1. Is the negative outcome a potential consequence of the underlying condition?
Consider the possibility that the medication did not cause the adverse event but merely failed to adequately treat the underlying problem. A teenager who attempts suicide 2 weeks after starting an antidepressant may be exhibiting symptoms related to depression rather than behavior caused by the medication.
2. Are other medical conditions or medications responsible for the negative outcome?
Weigh the relative likelihood that these factors are contributing to your patient’s presentation. In a surgical patient who is overly somnolent after receiving an anxiolytic, consider the possibility that a narcotic or worsening hypoxia are contributing to her somnolence.
3. Is the negative outcome likely to have occurred spontaneously?
Consider the possibility of coincidence. Lithium might not be causing declining renal function in an older patient. A dosing adjustment based on the patient’s current renal function may be a better harm-reduction strategy than discontinuing a potentially useful medication.
Careful evaluation of these potential confounding factors will greatly reduce the likelihood of falsely identifying psychotropic medications as responsible for negative outcomes. After this, but not always because of this.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Post hoc ergo propter hoc—”after this, therefore because of this”—suggests that 2 distinct events linked temporally are related causally. Clinicians often apply this dictum when monitoring effects of psychotropics. Because of stigma associated with psychiatric medications, and the readiness with which many practitioners blame them for unexpected results, it is important to develop a rational approach to evaluating outcomes—particularly adverse ones—after administering psychotropic agents.
Consider a geriatric patient admitted to the hospital for a urinary tract infection. He becomes verbally aggressive and is given IV haloperidol. Five minutes later he strikes a nurse and receives lorazepam. Twenty minutes later, he is lying calmly in his bed. The nursing staff and primary team conclude that the patient’s agitation worsened because of the antipsychotic and responded to the benzodiazepine; the physician documents in the patient’s chart that he had an adverse reaction to haloperidol.
In light of what we know about psychotropic medications’ mechanism of action, a more plausible explanation is that whatever caused the patient to become agitated (delirium) resulted in physical aggression. Haloperidol simply did not have enough time to exert its effect before the patient hit the nurse. It also would be wrong to automatically conclude that the last intervention (a benzodiazepine) produced the beneficial outcome. Was it the lorazepam, the haloperidol finally “kicking in,” or a combination of both? Perhaps it was none of the above but rather a worsening infection or irregular waxing and waning of delirium that was the culprit.
To avoid incorrectly attributing negative outcomes to medications, we suggest asking yourself 3 questions:
1. Is the negative outcome a potential consequence of the underlying condition?
Consider the possibility that the medication did not cause the adverse event but merely failed to adequately treat the underlying problem. A teenager who attempts suicide 2 weeks after starting an antidepressant may be exhibiting symptoms related to depression rather than behavior caused by the medication.
2. Are other medical conditions or medications responsible for the negative outcome?
Weigh the relative likelihood that these factors are contributing to your patient’s presentation. In a surgical patient who is overly somnolent after receiving an anxiolytic, consider the possibility that a narcotic or worsening hypoxia are contributing to her somnolence.
3. Is the negative outcome likely to have occurred spontaneously?
Consider the possibility of coincidence. Lithium might not be causing declining renal function in an older patient. A dosing adjustment based on the patient’s current renal function may be a better harm-reduction strategy than discontinuing a potentially useful medication.
Careful evaluation of these potential confounding factors will greatly reduce the likelihood of falsely identifying psychotropic medications as responsible for negative outcomes. After this, but not always because of this.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Post hoc ergo propter hoc—”after this, therefore because of this”—suggests that 2 distinct events linked temporally are related causally. Clinicians often apply this dictum when monitoring effects of psychotropics. Because of stigma associated with psychiatric medications, and the readiness with which many practitioners blame them for unexpected results, it is important to develop a rational approach to evaluating outcomes—particularly adverse ones—after administering psychotropic agents.
Consider a geriatric patient admitted to the hospital for a urinary tract infection. He becomes verbally aggressive and is given IV haloperidol. Five minutes later he strikes a nurse and receives lorazepam. Twenty minutes later, he is lying calmly in his bed. The nursing staff and primary team conclude that the patient’s agitation worsened because of the antipsychotic and responded to the benzodiazepine; the physician documents in the patient’s chart that he had an adverse reaction to haloperidol.
In light of what we know about psychotropic medications’ mechanism of action, a more plausible explanation is that whatever caused the patient to become agitated (delirium) resulted in physical aggression. Haloperidol simply did not have enough time to exert its effect before the patient hit the nurse. It also would be wrong to automatically conclude that the last intervention (a benzodiazepine) produced the beneficial outcome. Was it the lorazepam, the haloperidol finally “kicking in,” or a combination of both? Perhaps it was none of the above but rather a worsening infection or irregular waxing and waning of delirium that was the culprit.
To avoid incorrectly attributing negative outcomes to medications, we suggest asking yourself 3 questions:
1. Is the negative outcome a potential consequence of the underlying condition?
Consider the possibility that the medication did not cause the adverse event but merely failed to adequately treat the underlying problem. A teenager who attempts suicide 2 weeks after starting an antidepressant may be exhibiting symptoms related to depression rather than behavior caused by the medication.
2. Are other medical conditions or medications responsible for the negative outcome?
Weigh the relative likelihood that these factors are contributing to your patient’s presentation. In a surgical patient who is overly somnolent after receiving an anxiolytic, consider the possibility that a narcotic or worsening hypoxia are contributing to her somnolence.
3. Is the negative outcome likely to have occurred spontaneously?
Consider the possibility of coincidence. Lithium might not be causing declining renal function in an older patient. A dosing adjustment based on the patient’s current renal function may be a better harm-reduction strategy than discontinuing a potentially useful medication.
Careful evaluation of these potential confounding factors will greatly reduce the likelihood of falsely identifying psychotropic medications as responsible for negative outcomes. After this, but not always because of this.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Is your depressed postpartum patient bipolar?
Many individuals think of postpartum depression as an episode of major depressive disorder within 4 weeks of delivery; however, postpartum depressive symptoms also can occur in the context of bipolar I disorder (BD I) or bipolar II disorder (BD II). Despite the high prevalence of postpartum hypomania (9% to 20%), clinicians often fail to screen for symptoms of mania or hypomania.1 Determining if your patient’s postpartum depressive episode is caused by BD is essential when formulating an appropriate treatment plan that protects the mother and child.
Postpartum depression literature offers little guidance on the recognition and management of postpartum bipolar disorder.2 For example, there is scant evidence on pharmacologic or psychotherapeutic treatment of postpartum bipolar depression, and no studies have evaluated the use of screening instruments such as the Edinburgh Postnatal Depression Scale to detect bipolar disorder.
Misdiagnosis of postpartum depression is more likely in cases of subtle bipolarity—BD II and BD not otherwise specified—than in BD I because:3
- physicians often fail to ask postpartum patients about hypomanic symptoms such as feelings of elation, being overly talkative, racing thoughts, and decreased need for sleep
- DSM-IV-TR does not recognize hypomania with a postpartum onset specifier
- hypomania symptoms overlap normal feelings of elation and sleep disturbance following childbirth.
Clues to postpartum bipolar disorder include:
- hypomania: persistently elevated, expansive, or irritable mood
- depression onset immediately after delivery
- atypical features such as hypersomnia, leaden paralysis, and increased appetite
- racing thoughts
- concomitant psychotic symptoms
- history of BD in a first-degree relative
- antidepressants “misadventures” (rapid response; loss of response; induction of mania, hypomania, or depressive mixed episodes; and poor response).
Treatment strategies
Avoid antidepressants. Bipolar depression does not respond to antidepressants as well as unipolar depression. Moreover, antidepressants can induce mania, hypomania, or mixed states, and can increase mood cycle frequency.
Administer mood stabilizers such as lithium or lamotrigine, or atypical antipsychotics such as quetiapine.
In breast-feeding women the benefits of treatment should be balanced carefully against the risk of infant exposure to medications. Lamotrigine should be used cautiously because of concerns of skin rash and higher-than-expected drug levels in the infant. In light of recent data showing no significant adverse clinical or behavioral effects in infants, breast-feeding while taking lithium should be considered in carefully selected women. The preliminary evidence supporting the use of quetiapine during breast-feeding appears promising; however, data on the safety of atypical antipsychotics in lactating women are limited.
Promote sleep. Sleep disruption can be a symptom of as well as a trigger for postpartum bipolar depression. In women with BD, the benefits of breast-feeding should be balanced carefully against the potential for the deleterious effects of sleep deprivation in triggering mood episodes. Women should consider using a breast pump allowing others to assist with feeding or supplementing breast milk with formula to help get uninterrupted sleep.3
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Heron J, Craddock N, Jones I. Postnatal euphoria: are ‘the highs’ an indicator of bipolarity? Bipolar Disord. 2005;7:103-110.
2. Sharma V, Khan M, Corpse C, et al. Missed bipolarity and psychiatric comorbidity in women with postpartum depression. Bipolar Disord. 2008;10:742-747.
3. Sharma V, Burt VK, Ritchie, HL. Bipolar II postpartum depression: detection, diagnosis, and treatment. Am J Psychiatry. 2009;166:1217-1221.
Many individuals think of postpartum depression as an episode of major depressive disorder within 4 weeks of delivery; however, postpartum depressive symptoms also can occur in the context of bipolar I disorder (BD I) or bipolar II disorder (BD II). Despite the high prevalence of postpartum hypomania (9% to 20%), clinicians often fail to screen for symptoms of mania or hypomania.1 Determining if your patient’s postpartum depressive episode is caused by BD is essential when formulating an appropriate treatment plan that protects the mother and child.
Postpartum depression literature offers little guidance on the recognition and management of postpartum bipolar disorder.2 For example, there is scant evidence on pharmacologic or psychotherapeutic treatment of postpartum bipolar depression, and no studies have evaluated the use of screening instruments such as the Edinburgh Postnatal Depression Scale to detect bipolar disorder.
Misdiagnosis of postpartum depression is more likely in cases of subtle bipolarity—BD II and BD not otherwise specified—than in BD I because:3
- physicians often fail to ask postpartum patients about hypomanic symptoms such as feelings of elation, being overly talkative, racing thoughts, and decreased need for sleep
- DSM-IV-TR does not recognize hypomania with a postpartum onset specifier
- hypomania symptoms overlap normal feelings of elation and sleep disturbance following childbirth.
Clues to postpartum bipolar disorder include:
- hypomania: persistently elevated, expansive, or irritable mood
- depression onset immediately after delivery
- atypical features such as hypersomnia, leaden paralysis, and increased appetite
- racing thoughts
- concomitant psychotic symptoms
- history of BD in a first-degree relative
- antidepressants “misadventures” (rapid response; loss of response; induction of mania, hypomania, or depressive mixed episodes; and poor response).
Treatment strategies
Avoid antidepressants. Bipolar depression does not respond to antidepressants as well as unipolar depression. Moreover, antidepressants can induce mania, hypomania, or mixed states, and can increase mood cycle frequency.
Administer mood stabilizers such as lithium or lamotrigine, or atypical antipsychotics such as quetiapine.
In breast-feeding women the benefits of treatment should be balanced carefully against the risk of infant exposure to medications. Lamotrigine should be used cautiously because of concerns of skin rash and higher-than-expected drug levels in the infant. In light of recent data showing no significant adverse clinical or behavioral effects in infants, breast-feeding while taking lithium should be considered in carefully selected women. The preliminary evidence supporting the use of quetiapine during breast-feeding appears promising; however, data on the safety of atypical antipsychotics in lactating women are limited.
Promote sleep. Sleep disruption can be a symptom of as well as a trigger for postpartum bipolar depression. In women with BD, the benefits of breast-feeding should be balanced carefully against the potential for the deleterious effects of sleep deprivation in triggering mood episodes. Women should consider using a breast pump allowing others to assist with feeding or supplementing breast milk with formula to help get uninterrupted sleep.3
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Many individuals think of postpartum depression as an episode of major depressive disorder within 4 weeks of delivery; however, postpartum depressive symptoms also can occur in the context of bipolar I disorder (BD I) or bipolar II disorder (BD II). Despite the high prevalence of postpartum hypomania (9% to 20%), clinicians often fail to screen for symptoms of mania or hypomania.1 Determining if your patient’s postpartum depressive episode is caused by BD is essential when formulating an appropriate treatment plan that protects the mother and child.
Postpartum depression literature offers little guidance on the recognition and management of postpartum bipolar disorder.2 For example, there is scant evidence on pharmacologic or psychotherapeutic treatment of postpartum bipolar depression, and no studies have evaluated the use of screening instruments such as the Edinburgh Postnatal Depression Scale to detect bipolar disorder.
Misdiagnosis of postpartum depression is more likely in cases of subtle bipolarity—BD II and BD not otherwise specified—than in BD I because:3
- physicians often fail to ask postpartum patients about hypomanic symptoms such as feelings of elation, being overly talkative, racing thoughts, and decreased need for sleep
- DSM-IV-TR does not recognize hypomania with a postpartum onset specifier
- hypomania symptoms overlap normal feelings of elation and sleep disturbance following childbirth.
Clues to postpartum bipolar disorder include:
- hypomania: persistently elevated, expansive, or irritable mood
- depression onset immediately after delivery
- atypical features such as hypersomnia, leaden paralysis, and increased appetite
- racing thoughts
- concomitant psychotic symptoms
- history of BD in a first-degree relative
- antidepressants “misadventures” (rapid response; loss of response; induction of mania, hypomania, or depressive mixed episodes; and poor response).
Treatment strategies
Avoid antidepressants. Bipolar depression does not respond to antidepressants as well as unipolar depression. Moreover, antidepressants can induce mania, hypomania, or mixed states, and can increase mood cycle frequency.
Administer mood stabilizers such as lithium or lamotrigine, or atypical antipsychotics such as quetiapine.
In breast-feeding women the benefits of treatment should be balanced carefully against the risk of infant exposure to medications. Lamotrigine should be used cautiously because of concerns of skin rash and higher-than-expected drug levels in the infant. In light of recent data showing no significant adverse clinical or behavioral effects in infants, breast-feeding while taking lithium should be considered in carefully selected women. The preliminary evidence supporting the use of quetiapine during breast-feeding appears promising; however, data on the safety of atypical antipsychotics in lactating women are limited.
Promote sleep. Sleep disruption can be a symptom of as well as a trigger for postpartum bipolar depression. In women with BD, the benefits of breast-feeding should be balanced carefully against the potential for the deleterious effects of sleep deprivation in triggering mood episodes. Women should consider using a breast pump allowing others to assist with feeding or supplementing breast milk with formula to help get uninterrupted sleep.3
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Heron J, Craddock N, Jones I. Postnatal euphoria: are ‘the highs’ an indicator of bipolarity? Bipolar Disord. 2005;7:103-110.
2. Sharma V, Khan M, Corpse C, et al. Missed bipolarity and psychiatric comorbidity in women with postpartum depression. Bipolar Disord. 2008;10:742-747.
3. Sharma V, Burt VK, Ritchie, HL. Bipolar II postpartum depression: detection, diagnosis, and treatment. Am J Psychiatry. 2009;166:1217-1221.
1. Heron J, Craddock N, Jones I. Postnatal euphoria: are ‘the highs’ an indicator of bipolarity? Bipolar Disord. 2005;7:103-110.
2. Sharma V, Khan M, Corpse C, et al. Missed bipolarity and psychiatric comorbidity in women with postpartum depression. Bipolar Disord. 2008;10:742-747.
3. Sharma V, Burt VK, Ritchie, HL. Bipolar II postpartum depression: detection, diagnosis, and treatment. Am J Psychiatry. 2009;166:1217-1221.
The ABCs of estimating adherence to antipsychotics
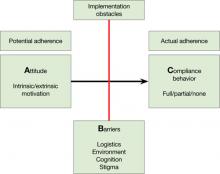
Adequate adherence to antipsychotics is critical for most patients with a chronic psychotic disorder. Therefore, appraisal of medication adherence—also called compliance in the older literature—is crucial at all visits.
Medication adherence is both an attitude and a behavior,1 and you need to assess both. Without some motivation to take medications (ie, a positive drug attitude), adherence is unlikely. On the other hand, motivation alone does not guarantee compliance behavior, and you need to assess barriers to adherence even in motivated patients.
To arrive at a clinical estimate of adherence to antipsychotics, ask yourself:
A What is my patient’s Attitude toward antipsychotics?
Expect a positive drug attitude if the medication is perceived as warranted, potentially effective, and tolerable. Inquire about prior experience with medications and psychiatrists, including unpleasant somatic experiences, periods of coercion, specific benefits, and fears. Remember that the perceived harm from—vs the perceived need for—medications is judged from the patient’s point of view, not the clinician’s. For example, some patients are motivated to take an antipsychotic because it helps them sleep. Make note if motivation is intrinsic or extrinsic. You might start by asking, “Why do you think taking this medication is a good idea?”
Figure: Attitude, Barriers, and Compliance behavior
B Are there Barriers for a motivated patient to implement optimal adherence?
Ask, “What gets in the way of taking your medication?” Identifying barriers for the motivated patient forces you to look at the individual’s real-life situation. Can the patient afford the co-pay? Is the family against the patient taking an antipsychotic? Does the patient lack a routine that would help him or her remember to take pills? Is the patient too ashamed of the stigma of taking psychiatric medications? Does the patient have cognitive impairment that leads to forgetting to take pills?
C What is my best quantitative estimate of Compliance behavior?
Ask your patient, “In the last 7 days, how many pills have you missed?” Inquire also about names of pills and dosages, number of pills prescribed, and how they are taken to get a sense of your patient’s routines and cognitive competence. This is the time to get collateral information, such as when the last prescription was filled. After collecting this information, you should be able to estimate a patient’s level of adherence (eg, almost 100%, partial 50% to 75%, none). Be aware that both clinicians and patients overestimate actual adherence.
Disclosures
Drs. Kontos and Querques report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Freudenreich receives grant/research support from Pfizer Inc. He is a consultant to Transcept Pharmaceuticals, Inc. and Beacon Health Strategies, LLC and a speaker for Reed Medical Education.
1. Velligan DI, Weiden PJ, Sajatovic M, et al. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70(suppl 4):1-46.
Adequate adherence to antipsychotics is critical for most patients with a chronic psychotic disorder. Therefore, appraisal of medication adherence—also called compliance in the older literature—is crucial at all visits.
Medication adherence is both an attitude and a behavior,1 and you need to assess both. Without some motivation to take medications (ie, a positive drug attitude), adherence is unlikely. On the other hand, motivation alone does not guarantee compliance behavior, and you need to assess barriers to adherence even in motivated patients.
To arrive at a clinical estimate of adherence to antipsychotics, ask yourself:
A What is my patient’s Attitude toward antipsychotics?
Expect a positive drug attitude if the medication is perceived as warranted, potentially effective, and tolerable. Inquire about prior experience with medications and psychiatrists, including unpleasant somatic experiences, periods of coercion, specific benefits, and fears. Remember that the perceived harm from—vs the perceived need for—medications is judged from the patient’s point of view, not the clinician’s. For example, some patients are motivated to take an antipsychotic because it helps them sleep. Make note if motivation is intrinsic or extrinsic. You might start by asking, “Why do you think taking this medication is a good idea?”
Figure: Attitude, Barriers, and Compliance behavior
B Are there Barriers for a motivated patient to implement optimal adherence?
Ask, “What gets in the way of taking your medication?” Identifying barriers for the motivated patient forces you to look at the individual’s real-life situation. Can the patient afford the co-pay? Is the family against the patient taking an antipsychotic? Does the patient lack a routine that would help him or her remember to take pills? Is the patient too ashamed of the stigma of taking psychiatric medications? Does the patient have cognitive impairment that leads to forgetting to take pills?
C What is my best quantitative estimate of Compliance behavior?
Ask your patient, “In the last 7 days, how many pills have you missed?” Inquire also about names of pills and dosages, number of pills prescribed, and how they are taken to get a sense of your patient’s routines and cognitive competence. This is the time to get collateral information, such as when the last prescription was filled. After collecting this information, you should be able to estimate a patient’s level of adherence (eg, almost 100%, partial 50% to 75%, none). Be aware that both clinicians and patients overestimate actual adherence.
Disclosures
Drs. Kontos and Querques report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Freudenreich receives grant/research support from Pfizer Inc. He is a consultant to Transcept Pharmaceuticals, Inc. and Beacon Health Strategies, LLC and a speaker for Reed Medical Education.
Adequate adherence to antipsychotics is critical for most patients with a chronic psychotic disorder. Therefore, appraisal of medication adherence—also called compliance in the older literature—is crucial at all visits.
Medication adherence is both an attitude and a behavior,1 and you need to assess both. Without some motivation to take medications (ie, a positive drug attitude), adherence is unlikely. On the other hand, motivation alone does not guarantee compliance behavior, and you need to assess barriers to adherence even in motivated patients.
To arrive at a clinical estimate of adherence to antipsychotics, ask yourself:
A What is my patient’s Attitude toward antipsychotics?
Expect a positive drug attitude if the medication is perceived as warranted, potentially effective, and tolerable. Inquire about prior experience with medications and psychiatrists, including unpleasant somatic experiences, periods of coercion, specific benefits, and fears. Remember that the perceived harm from—vs the perceived need for—medications is judged from the patient’s point of view, not the clinician’s. For example, some patients are motivated to take an antipsychotic because it helps them sleep. Make note if motivation is intrinsic or extrinsic. You might start by asking, “Why do you think taking this medication is a good idea?”
Figure: Attitude, Barriers, and Compliance behavior
B Are there Barriers for a motivated patient to implement optimal adherence?
Ask, “What gets in the way of taking your medication?” Identifying barriers for the motivated patient forces you to look at the individual’s real-life situation. Can the patient afford the co-pay? Is the family against the patient taking an antipsychotic? Does the patient lack a routine that would help him or her remember to take pills? Is the patient too ashamed of the stigma of taking psychiatric medications? Does the patient have cognitive impairment that leads to forgetting to take pills?
C What is my best quantitative estimate of Compliance behavior?
Ask your patient, “In the last 7 days, how many pills have you missed?” Inquire also about names of pills and dosages, number of pills prescribed, and how they are taken to get a sense of your patient’s routines and cognitive competence. This is the time to get collateral information, such as when the last prescription was filled. After collecting this information, you should be able to estimate a patient’s level of adherence (eg, almost 100%, partial 50% to 75%, none). Be aware that both clinicians and patients overestimate actual adherence.
Disclosures
Drs. Kontos and Querques report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Freudenreich receives grant/research support from Pfizer Inc. He is a consultant to Transcept Pharmaceuticals, Inc. and Beacon Health Strategies, LLC and a speaker for Reed Medical Education.
1. Velligan DI, Weiden PJ, Sajatovic M, et al. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70(suppl 4):1-46.
1. Velligan DI, Weiden PJ, Sajatovic M, et al. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70(suppl 4):1-46.
Should you prescribe medications for family and friends?
Dear Dr. Mossman:
On a recent golf outing, my buddy Mike told me about his trouble staying “focused” while studying for his grad school exams. He asked me to write him a prescription for methylphenidate, which he had taken in high school and college. I want to help Mike, but I’m worried about my liability if something goes wrong. What should I do?—Submitted by “Dr. C”
Doctors learn early in their careers that family, friends, or coworkers often seek informal medical advice and ask for prescriptions. Also, doctors commonly diagnose and medicate themselves rather than seek care from other professionals.1,2
In this article, we use the phrase “casual prescribing” to describe activities related to prescribing drugs for individuals such as Mike, a friend who has sought medication outside Dr. C’s customary practice setting. Despite having good intentions, you’re probably increasing your malpractice liability whenever you casually prescribe medication. Even more serious, if you casually prescribe controlled substances (eg, stimulants), you risk investigation and potential sanction by your state medical licensing agency.
- Submit your malpractice-related questions to Dr. Mossman at [email protected].
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
To decide whether, how, and when you may prescribe drugs for yourself, family members, colleagues, or friends, you need to:
- anticipate being asked to casually prescribe
- understand the emotions and forces that drive casual prescribing
- know your state medical board’s rules and regulations
- be prepared with an appropriate response.
After we explore these points, we’ll consider what Dr. C might do.
A common request
People often seek medical advice outside doctors’ offices. Playing a sport together, sitting on an airplane, or sharing other social activities strips away the veneer of formality, lets people relax, makes doctors seem more approachable, and allows medical concerns to come forth more easily.3
Access to medical care is a problem for lay people and doctors alike. In many locales, simply getting an appointment with a primary care physician or psychiatrist is difficult.4,5 Navigating health insurance rules and referral lists is frustrating. When people find a provider, they may feel guilty about taking a slot from someone else. Job expectations or a tough economy can make employees reluctant to take time off work6,7 or concerned that they’ll miss productivity goals because of illness.1
Doctors often self-prescribe to avoid facing the stigma of being ill. Although doctors should know better, many of us don’t want to experience the vulnerability that comes with being sick and needing health care. Some doctors fear colleagues’ scrutiny if their serious mental illness (eg, depression) becomes known, or they would rather treat themselves than seek professional help.1 The most formidable obstacle physicians face is time—or lack of it. Many doctors work >60 hours per week, and their dedication and altruism causes them to neglect their own health until illness interferes with their professional lives.8
Emotional factors
Doctors pride themselves on knowing how to help people, and when loved ones or colleagues ask for our help, it’s gratifying and flattering.3 Such feelings may help explain why the largest numbers of prescriptions written for non-patients are for family members and friends, followed by prescriptions written by residents for fellow house officers.9
The circumstances surrounding casual prescribing usually make it difficult to maintain objectivity, avoid substandard care, uphold ethical principles, and handle discomfort. Your professional objectivity and clinical judgment likely are compromised when a close friend, an immediate family member, or you yourself are the patient.10 Treating loved ones and close friends can make it awkward to ask about sensitive matters (eg, “How much alcohol do you drink?”) or to perform intimate parts of a physical examination. Physicians who want to “go the extra mile” for family members or friends may try to treat problems that are beyond their expertise or training—a setup for failing to meet your legal and medical obligations to conform to the prevailing standard of care.11
State medical board rules
The American Medical Association, British Medical Association, and Canadian Medical Association all discourage physicians from prescribing for themselves or family members.2Table 110,12-16 gives examples of states’ comments and guidelines relevant to casual prescribing. Overwhelmingly, state medical boards tell you that casual prescribing is ill-advised. However, in emergencies or in isolated settings where no other qualified physician is readily available, you should provide needed treatment for yourself, family, friends, or colleagues until another physician can assume care. Physicians should not be the primary or regular care providers for their immediate family members, but giving routine care for short-term, minor problems may be acceptable.14 Although state medical boards use differing language, all agree that casual prescribing requires assessment and documentation similar to what you do for patients seen in your regular practice setting. Table 2 summarizes appropriate casual prescribing practices, but you should also know the boards’ rules in the locales where you work.
Restrictions and rules for prescribing controlled substances are stricter, despite many doctors’ sometime-lax attitudes. State medical boards tell doctors not to prescribe controlled substances for friends, family, or themselves except in emergencies. Yet studies have found that house officers often write prescriptions for psychoactive drugs (including narcotics) for family members, friends, and colleagues9 and that residents are willing to prescribe codeine for a hypothetical colleague with pain from a fractured finger.17
Table 1
Selected state medical board rules and comments on casual prescribing
| State | Rules |
|---|---|
| California12 | ‘[E]valuating, diagnosing, treating, or prescribing to family members, co-workers, or friends…is discouraged’ and requires ‘the same practice/protocol for any patient in which medications are prescribed,’ including a ‘good faith exam’ and documentation that justifies the prescription |
| Montana13 | Although prescribing for one’s family or oneself is not prohibited, doing so ‘arguably…does not meet the general accepted standards of practice, and is therefore unprofessional conduct [that] may subject the physician to license discipline’ |
| New Hampshire14 | ‘Physicians generally should not treat themselves or members of their immediate families.…Except in emergencies, it is not appropriate for physicians to write prescriptions for controlled substances for themselves or immediate family members’ |
| Ohio15 | ‘[I]t is almost always a bad idea to treat a family member’s chronic condition, serious illness, or psychiatric/emotional problems’ |
| South Carolina10 | Treating immediate family members may produce less than optimal care. ‘[P]rescribing controlled substances for family members is outside the scope of good medical practice except for a bona fide emergency situation’ |
| Virginia16 | Prescriptions ‘must be based on a bona fide practitioner-patient relationship. Practitioners should obtain a medical or drug history, provide information about risks, benefits, and side effects, perform an exam, and initiate follow-up care. Practitioners should not prescribe controlled substances for themselves or family members except in emergencies, isolated settings, or for a single episode of an acute illness’ |
Table 2
Cautions and recommendations for casual prescribing
| Avoid doing it in non-emergencies |
| Obtain a medical and drug history |
| Perform an appropriate, good-faith exam |
| Create a medical record that documents the need for a prescription |
| Prescribe controlled substances only in emergencies or isolated settings |
| Inform your patient about risks, benefits, and side effects |
| Initiate needed additional interventions and follow-up care |
| Maintain confidentiality and respect HIPAA rules |
| Ask yourself, ‘Can I avoid this—is there another option?’ If the answer is ‘yes,’ don’t do it |
| HIPAA: Health Insurance Portability and Accountability Act |
Liability risk
Most residents are unaware of federal or state regulations addressing the appropriateness of prescription writing for non-patients.18 A survey of U.S. internal medicine and family practice residents at a teaching hospital found that less than a quarter believed that ethical guidelines on prescription writing existed.17 Such deficits can make malpractice liability more likely if something “goes wrong” with your casually prescribed treatment. Friends and relatives do sue doctors whom they have consulted informally,18 and casual prescribing can be hard to defend in court because it usually looks suspicious and is not well documented.
Revisiting Mike’s case
Understandably, Dr. C wants to help Mike and may even think he has a condition (eg, adult attention-deficit/hyperactivity disorder) for which a stimulant would be appropriate. But respect for Mike’s humanity—the paramount value in medical practice19—suggests that his treatment should occur after and because of a careful medical assessment rather than a golf game. Moreover, prescribing a controlled substance in a non-emergency likely would violate standards of practice promulgated by Dr. C’s medical board. Dr. C should tell Mike that his problem deserves thoughtful evaluation and suggest that Mike see his primary physician. Dr. C also could recommend psychiatrists whom Mike might consult.
Related Resource
- Aboff B, Collier V, Farber N. Residents’ prescription writing for nonpatients. JAMA. 2002;288:381-385.
Drug Brand Names
- Codeine • Tylenol with Codeine, others
- Methylphenidate • Ritalin
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Be prepared to be asked for advice and prescriptions in casual settings. When this happens, it’s fine to provide general medical information, but it’s best not to give specific advice or engage in “casual prescribing.” You can maintain social connections, be caring, and avoid boundary violations by responding with tact, referral information, and good judgment.19,20
1. Balon R. Psychiatrist attitudes toward self-treatment of their own depression. Psychother Psychosom. 2007;76:306-310.
2. Walter JK, Lang CW, Ross LF. When physicians forego the doctor-patient relationship should they elect to self-prescribe or curbside? An empirical and ethical analysis. J Med Ethics. 2010;36:19-23.
3. Reynolds H. Medical ear in the early morning tennis group—when to advise and what to say. Pharos Alpha Omega Alpha Honor Med Soc. 2010;73:14-15 discussion 16.
4. Sataline S, Wang SS. Medical schools can’t keep up: as ranks of insured expand nation faces shortage of 150,000 doctors in 15 years. Available at: http://online.wsj.com/article/SB10001424052702304506904575180331528424238.html. Accessed March 21, 2011.
5. Steinberg S. Of medical specialties demand for psychiatrists growing fastest. USA Today. July 1, 2010:6D.
6. Leonhardt D. A labor market punishing to mothers. New York Times. August 4 2010:B1.
7. Madden K. Reluctant to go on vacation? Available at: http://www.cnn.com/2010/LIVING/08/04/cb.reluctant.to.take.vacation/index.html. Accessed March 20 2011.
8. Meier DE, Back AL, Morrison RS. The inner life of physicians and care of the seriously ill. JAMA. 2001;286:3007-3014.
9. Clark A, Kau J. Patterns of psychoactive drug prescriptions by house officers for nonpatients. J Med Educ. 1988;63:44-50.
10. State Medical Board of South Carolina. Prescribing for family members. Available at: http://www.llr.state.sc.us/pol/medical/index.asp?file=Policies/MEPRESCRIBEFAM.HTM. Accessed March 20 2011.
11. Dietz LH, Jacobs A, Leming TL, et al. Physicians, surgeons, and other healers, §§130, 216-218. In: American jurisprudence. vol 61. 2nd ed. New York, NY: Thomson Reuters; 2010.
12. Medical Board of California. General office practices/protocols-frequently asked questions. Available at: http://www.medbd.ca.gov/consumer/complaint_info_questions_practice.html#13. Accessed March 20 2011.
13. Montana Board of Medical Examiners. Statement of physician prescribing for self or members of the physician’s immediate family. Available at: http://bsd.dli.mt.gov/license/bsd_boards/med_board/pdf/prescribing_self.pdf. Accessed March 20 2011.
14. New Hampshire Medical Board. Guidelines for self-prescribing and prescribing for family members. Available at: http://www.nh.gov/medicine/aboutus/self_presc.htm. Accessed March 21 2011.
15. State Medical Board of Ohio. Frequently asked questions. Available at: http://www.med.ohio.gov/professional_guidelines.htm. Accessed March 20 2011.
16. Virginia Board of Medicine. Can I prescribe for my family and myself? Available at: http://www.dhp.virginia.gov/Medicine/medicine_faq.htm#Prescribe. Accessed March 20 2011.
17. Aboff B, Collier V, Farber N. Residents’ prescription writing for nonpatients. JAMA. 2002;288:381-385.
18. Johnson LJ. Malpractice consult. Should you give informal medical advice? Med Econ. 2007;84:36.-
19. Nisselle P. Danger zone: when boundaries are crossed in the doctor-patient relationship. Aust Family Physician. 2000;29:541-544.
20. Eastwood GL. When relatives and friends ask physicians for medical advice: ethical legal, and practical considerations. J Gen Intern Med. 2009;24:1333-1335.
Dear Dr. Mossman:
On a recent golf outing, my buddy Mike told me about his trouble staying “focused” while studying for his grad school exams. He asked me to write him a prescription for methylphenidate, which he had taken in high school and college. I want to help Mike, but I’m worried about my liability if something goes wrong. What should I do?—Submitted by “Dr. C”
Doctors learn early in their careers that family, friends, or coworkers often seek informal medical advice and ask for prescriptions. Also, doctors commonly diagnose and medicate themselves rather than seek care from other professionals.1,2
In this article, we use the phrase “casual prescribing” to describe activities related to prescribing drugs for individuals such as Mike, a friend who has sought medication outside Dr. C’s customary practice setting. Despite having good intentions, you’re probably increasing your malpractice liability whenever you casually prescribe medication. Even more serious, if you casually prescribe controlled substances (eg, stimulants), you risk investigation and potential sanction by your state medical licensing agency.
- Submit your malpractice-related questions to Dr. Mossman at [email protected].
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
To decide whether, how, and when you may prescribe drugs for yourself, family members, colleagues, or friends, you need to:
- anticipate being asked to casually prescribe
- understand the emotions and forces that drive casual prescribing
- know your state medical board’s rules and regulations
- be prepared with an appropriate response.
After we explore these points, we’ll consider what Dr. C might do.
A common request
People often seek medical advice outside doctors’ offices. Playing a sport together, sitting on an airplane, or sharing other social activities strips away the veneer of formality, lets people relax, makes doctors seem more approachable, and allows medical concerns to come forth more easily.3
Access to medical care is a problem for lay people and doctors alike. In many locales, simply getting an appointment with a primary care physician or psychiatrist is difficult.4,5 Navigating health insurance rules and referral lists is frustrating. When people find a provider, they may feel guilty about taking a slot from someone else. Job expectations or a tough economy can make employees reluctant to take time off work6,7 or concerned that they’ll miss productivity goals because of illness.1
Doctors often self-prescribe to avoid facing the stigma of being ill. Although doctors should know better, many of us don’t want to experience the vulnerability that comes with being sick and needing health care. Some doctors fear colleagues’ scrutiny if their serious mental illness (eg, depression) becomes known, or they would rather treat themselves than seek professional help.1 The most formidable obstacle physicians face is time—or lack of it. Many doctors work >60 hours per week, and their dedication and altruism causes them to neglect their own health until illness interferes with their professional lives.8
Emotional factors
Doctors pride themselves on knowing how to help people, and when loved ones or colleagues ask for our help, it’s gratifying and flattering.3 Such feelings may help explain why the largest numbers of prescriptions written for non-patients are for family members and friends, followed by prescriptions written by residents for fellow house officers.9
The circumstances surrounding casual prescribing usually make it difficult to maintain objectivity, avoid substandard care, uphold ethical principles, and handle discomfort. Your professional objectivity and clinical judgment likely are compromised when a close friend, an immediate family member, or you yourself are the patient.10 Treating loved ones and close friends can make it awkward to ask about sensitive matters (eg, “How much alcohol do you drink?”) or to perform intimate parts of a physical examination. Physicians who want to “go the extra mile” for family members or friends may try to treat problems that are beyond their expertise or training—a setup for failing to meet your legal and medical obligations to conform to the prevailing standard of care.11
State medical board rules
The American Medical Association, British Medical Association, and Canadian Medical Association all discourage physicians from prescribing for themselves or family members.2Table 110,12-16 gives examples of states’ comments and guidelines relevant to casual prescribing. Overwhelmingly, state medical boards tell you that casual prescribing is ill-advised. However, in emergencies or in isolated settings where no other qualified physician is readily available, you should provide needed treatment for yourself, family, friends, or colleagues until another physician can assume care. Physicians should not be the primary or regular care providers for their immediate family members, but giving routine care for short-term, minor problems may be acceptable.14 Although state medical boards use differing language, all agree that casual prescribing requires assessment and documentation similar to what you do for patients seen in your regular practice setting. Table 2 summarizes appropriate casual prescribing practices, but you should also know the boards’ rules in the locales where you work.
Restrictions and rules for prescribing controlled substances are stricter, despite many doctors’ sometime-lax attitudes. State medical boards tell doctors not to prescribe controlled substances for friends, family, or themselves except in emergencies. Yet studies have found that house officers often write prescriptions for psychoactive drugs (including narcotics) for family members, friends, and colleagues9 and that residents are willing to prescribe codeine for a hypothetical colleague with pain from a fractured finger.17
Table 1
Selected state medical board rules and comments on casual prescribing
| State | Rules |
|---|---|
| California12 | ‘[E]valuating, diagnosing, treating, or prescribing to family members, co-workers, or friends…is discouraged’ and requires ‘the same practice/protocol for any patient in which medications are prescribed,’ including a ‘good faith exam’ and documentation that justifies the prescription |
| Montana13 | Although prescribing for one’s family or oneself is not prohibited, doing so ‘arguably…does not meet the general accepted standards of practice, and is therefore unprofessional conduct [that] may subject the physician to license discipline’ |
| New Hampshire14 | ‘Physicians generally should not treat themselves or members of their immediate families.…Except in emergencies, it is not appropriate for physicians to write prescriptions for controlled substances for themselves or immediate family members’ |
| Ohio15 | ‘[I]t is almost always a bad idea to treat a family member’s chronic condition, serious illness, or psychiatric/emotional problems’ |
| South Carolina10 | Treating immediate family members may produce less than optimal care. ‘[P]rescribing controlled substances for family members is outside the scope of good medical practice except for a bona fide emergency situation’ |
| Virginia16 | Prescriptions ‘must be based on a bona fide practitioner-patient relationship. Practitioners should obtain a medical or drug history, provide information about risks, benefits, and side effects, perform an exam, and initiate follow-up care. Practitioners should not prescribe controlled substances for themselves or family members except in emergencies, isolated settings, or for a single episode of an acute illness’ |
Table 2
Cautions and recommendations for casual prescribing
| Avoid doing it in non-emergencies |
| Obtain a medical and drug history |
| Perform an appropriate, good-faith exam |
| Create a medical record that documents the need for a prescription |
| Prescribe controlled substances only in emergencies or isolated settings |
| Inform your patient about risks, benefits, and side effects |
| Initiate needed additional interventions and follow-up care |
| Maintain confidentiality and respect HIPAA rules |
| Ask yourself, ‘Can I avoid this—is there another option?’ If the answer is ‘yes,’ don’t do it |
| HIPAA: Health Insurance Portability and Accountability Act |
Liability risk
Most residents are unaware of federal or state regulations addressing the appropriateness of prescription writing for non-patients.18 A survey of U.S. internal medicine and family practice residents at a teaching hospital found that less than a quarter believed that ethical guidelines on prescription writing existed.17 Such deficits can make malpractice liability more likely if something “goes wrong” with your casually prescribed treatment. Friends and relatives do sue doctors whom they have consulted informally,18 and casual prescribing can be hard to defend in court because it usually looks suspicious and is not well documented.
Revisiting Mike’s case
Understandably, Dr. C wants to help Mike and may even think he has a condition (eg, adult attention-deficit/hyperactivity disorder) for which a stimulant would be appropriate. But respect for Mike’s humanity—the paramount value in medical practice19—suggests that his treatment should occur after and because of a careful medical assessment rather than a golf game. Moreover, prescribing a controlled substance in a non-emergency likely would violate standards of practice promulgated by Dr. C’s medical board. Dr. C should tell Mike that his problem deserves thoughtful evaluation and suggest that Mike see his primary physician. Dr. C also could recommend psychiatrists whom Mike might consult.
Related Resource
- Aboff B, Collier V, Farber N. Residents’ prescription writing for nonpatients. JAMA. 2002;288:381-385.
Drug Brand Names
- Codeine • Tylenol with Codeine, others
- Methylphenidate • Ritalin
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Be prepared to be asked for advice and prescriptions in casual settings. When this happens, it’s fine to provide general medical information, but it’s best not to give specific advice or engage in “casual prescribing.” You can maintain social connections, be caring, and avoid boundary violations by responding with tact, referral information, and good judgment.19,20
Dear Dr. Mossman:
On a recent golf outing, my buddy Mike told me about his trouble staying “focused” while studying for his grad school exams. He asked me to write him a prescription for methylphenidate, which he had taken in high school and college. I want to help Mike, but I’m worried about my liability if something goes wrong. What should I do?—Submitted by “Dr. C”
Doctors learn early in their careers that family, friends, or coworkers often seek informal medical advice and ask for prescriptions. Also, doctors commonly diagnose and medicate themselves rather than seek care from other professionals.1,2
In this article, we use the phrase “casual prescribing” to describe activities related to prescribing drugs for individuals such as Mike, a friend who has sought medication outside Dr. C’s customary practice setting. Despite having good intentions, you’re probably increasing your malpractice liability whenever you casually prescribe medication. Even more serious, if you casually prescribe controlled substances (eg, stimulants), you risk investigation and potential sanction by your state medical licensing agency.
- Submit your malpractice-related questions to Dr. Mossman at [email protected].
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
To decide whether, how, and when you may prescribe drugs for yourself, family members, colleagues, or friends, you need to:
- anticipate being asked to casually prescribe
- understand the emotions and forces that drive casual prescribing
- know your state medical board’s rules and regulations
- be prepared with an appropriate response.
After we explore these points, we’ll consider what Dr. C might do.
A common request
People often seek medical advice outside doctors’ offices. Playing a sport together, sitting on an airplane, or sharing other social activities strips away the veneer of formality, lets people relax, makes doctors seem more approachable, and allows medical concerns to come forth more easily.3
Access to medical care is a problem for lay people and doctors alike. In many locales, simply getting an appointment with a primary care physician or psychiatrist is difficult.4,5 Navigating health insurance rules and referral lists is frustrating. When people find a provider, they may feel guilty about taking a slot from someone else. Job expectations or a tough economy can make employees reluctant to take time off work6,7 or concerned that they’ll miss productivity goals because of illness.1
Doctors often self-prescribe to avoid facing the stigma of being ill. Although doctors should know better, many of us don’t want to experience the vulnerability that comes with being sick and needing health care. Some doctors fear colleagues’ scrutiny if their serious mental illness (eg, depression) becomes known, or they would rather treat themselves than seek professional help.1 The most formidable obstacle physicians face is time—or lack of it. Many doctors work >60 hours per week, and their dedication and altruism causes them to neglect their own health until illness interferes with their professional lives.8
Emotional factors
Doctors pride themselves on knowing how to help people, and when loved ones or colleagues ask for our help, it’s gratifying and flattering.3 Such feelings may help explain why the largest numbers of prescriptions written for non-patients are for family members and friends, followed by prescriptions written by residents for fellow house officers.9
The circumstances surrounding casual prescribing usually make it difficult to maintain objectivity, avoid substandard care, uphold ethical principles, and handle discomfort. Your professional objectivity and clinical judgment likely are compromised when a close friend, an immediate family member, or you yourself are the patient.10 Treating loved ones and close friends can make it awkward to ask about sensitive matters (eg, “How much alcohol do you drink?”) or to perform intimate parts of a physical examination. Physicians who want to “go the extra mile” for family members or friends may try to treat problems that are beyond their expertise or training—a setup for failing to meet your legal and medical obligations to conform to the prevailing standard of care.11
State medical board rules
The American Medical Association, British Medical Association, and Canadian Medical Association all discourage physicians from prescribing for themselves or family members.2Table 110,12-16 gives examples of states’ comments and guidelines relevant to casual prescribing. Overwhelmingly, state medical boards tell you that casual prescribing is ill-advised. However, in emergencies or in isolated settings where no other qualified physician is readily available, you should provide needed treatment for yourself, family, friends, or colleagues until another physician can assume care. Physicians should not be the primary or regular care providers for their immediate family members, but giving routine care for short-term, minor problems may be acceptable.14 Although state medical boards use differing language, all agree that casual prescribing requires assessment and documentation similar to what you do for patients seen in your regular practice setting. Table 2 summarizes appropriate casual prescribing practices, but you should also know the boards’ rules in the locales where you work.
Restrictions and rules for prescribing controlled substances are stricter, despite many doctors’ sometime-lax attitudes. State medical boards tell doctors not to prescribe controlled substances for friends, family, or themselves except in emergencies. Yet studies have found that house officers often write prescriptions for psychoactive drugs (including narcotics) for family members, friends, and colleagues9 and that residents are willing to prescribe codeine for a hypothetical colleague with pain from a fractured finger.17
Table 1
Selected state medical board rules and comments on casual prescribing
| State | Rules |
|---|---|
| California12 | ‘[E]valuating, diagnosing, treating, or prescribing to family members, co-workers, or friends…is discouraged’ and requires ‘the same practice/protocol for any patient in which medications are prescribed,’ including a ‘good faith exam’ and documentation that justifies the prescription |
| Montana13 | Although prescribing for one’s family or oneself is not prohibited, doing so ‘arguably…does not meet the general accepted standards of practice, and is therefore unprofessional conduct [that] may subject the physician to license discipline’ |
| New Hampshire14 | ‘Physicians generally should not treat themselves or members of their immediate families.…Except in emergencies, it is not appropriate for physicians to write prescriptions for controlled substances for themselves or immediate family members’ |
| Ohio15 | ‘[I]t is almost always a bad idea to treat a family member’s chronic condition, serious illness, or psychiatric/emotional problems’ |
| South Carolina10 | Treating immediate family members may produce less than optimal care. ‘[P]rescribing controlled substances for family members is outside the scope of good medical practice except for a bona fide emergency situation’ |
| Virginia16 | Prescriptions ‘must be based on a bona fide practitioner-patient relationship. Practitioners should obtain a medical or drug history, provide information about risks, benefits, and side effects, perform an exam, and initiate follow-up care. Practitioners should not prescribe controlled substances for themselves or family members except in emergencies, isolated settings, or for a single episode of an acute illness’ |
Table 2
Cautions and recommendations for casual prescribing
| Avoid doing it in non-emergencies |
| Obtain a medical and drug history |
| Perform an appropriate, good-faith exam |
| Create a medical record that documents the need for a prescription |
| Prescribe controlled substances only in emergencies or isolated settings |
| Inform your patient about risks, benefits, and side effects |
| Initiate needed additional interventions and follow-up care |
| Maintain confidentiality and respect HIPAA rules |
| Ask yourself, ‘Can I avoid this—is there another option?’ If the answer is ‘yes,’ don’t do it |
| HIPAA: Health Insurance Portability and Accountability Act |
Liability risk
Most residents are unaware of federal or state regulations addressing the appropriateness of prescription writing for non-patients.18 A survey of U.S. internal medicine and family practice residents at a teaching hospital found that less than a quarter believed that ethical guidelines on prescription writing existed.17 Such deficits can make malpractice liability more likely if something “goes wrong” with your casually prescribed treatment. Friends and relatives do sue doctors whom they have consulted informally,18 and casual prescribing can be hard to defend in court because it usually looks suspicious and is not well documented.
Revisiting Mike’s case
Understandably, Dr. C wants to help Mike and may even think he has a condition (eg, adult attention-deficit/hyperactivity disorder) for which a stimulant would be appropriate. But respect for Mike’s humanity—the paramount value in medical practice19—suggests that his treatment should occur after and because of a careful medical assessment rather than a golf game. Moreover, prescribing a controlled substance in a non-emergency likely would violate standards of practice promulgated by Dr. C’s medical board. Dr. C should tell Mike that his problem deserves thoughtful evaluation and suggest that Mike see his primary physician. Dr. C also could recommend psychiatrists whom Mike might consult.
Related Resource
- Aboff B, Collier V, Farber N. Residents’ prescription writing for nonpatients. JAMA. 2002;288:381-385.
Drug Brand Names
- Codeine • Tylenol with Codeine, others
- Methylphenidate • Ritalin
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Be prepared to be asked for advice and prescriptions in casual settings. When this happens, it’s fine to provide general medical information, but it’s best not to give specific advice or engage in “casual prescribing.” You can maintain social connections, be caring, and avoid boundary violations by responding with tact, referral information, and good judgment.19,20
1. Balon R. Psychiatrist attitudes toward self-treatment of their own depression. Psychother Psychosom. 2007;76:306-310.
2. Walter JK, Lang CW, Ross LF. When physicians forego the doctor-patient relationship should they elect to self-prescribe or curbside? An empirical and ethical analysis. J Med Ethics. 2010;36:19-23.
3. Reynolds H. Medical ear in the early morning tennis group—when to advise and what to say. Pharos Alpha Omega Alpha Honor Med Soc. 2010;73:14-15 discussion 16.
4. Sataline S, Wang SS. Medical schools can’t keep up: as ranks of insured expand nation faces shortage of 150,000 doctors in 15 years. Available at: http://online.wsj.com/article/SB10001424052702304506904575180331528424238.html. Accessed March 21, 2011.
5. Steinberg S. Of medical specialties demand for psychiatrists growing fastest. USA Today. July 1, 2010:6D.
6. Leonhardt D. A labor market punishing to mothers. New York Times. August 4 2010:B1.
7. Madden K. Reluctant to go on vacation? Available at: http://www.cnn.com/2010/LIVING/08/04/cb.reluctant.to.take.vacation/index.html. Accessed March 20 2011.
8. Meier DE, Back AL, Morrison RS. The inner life of physicians and care of the seriously ill. JAMA. 2001;286:3007-3014.
9. Clark A, Kau J. Patterns of psychoactive drug prescriptions by house officers for nonpatients. J Med Educ. 1988;63:44-50.
10. State Medical Board of South Carolina. Prescribing for family members. Available at: http://www.llr.state.sc.us/pol/medical/index.asp?file=Policies/MEPRESCRIBEFAM.HTM. Accessed March 20 2011.
11. Dietz LH, Jacobs A, Leming TL, et al. Physicians, surgeons, and other healers, §§130, 216-218. In: American jurisprudence. vol 61. 2nd ed. New York, NY: Thomson Reuters; 2010.
12. Medical Board of California. General office practices/protocols-frequently asked questions. Available at: http://www.medbd.ca.gov/consumer/complaint_info_questions_practice.html#13. Accessed March 20 2011.
13. Montana Board of Medical Examiners. Statement of physician prescribing for self or members of the physician’s immediate family. Available at: http://bsd.dli.mt.gov/license/bsd_boards/med_board/pdf/prescribing_self.pdf. Accessed March 20 2011.
14. New Hampshire Medical Board. Guidelines for self-prescribing and prescribing for family members. Available at: http://www.nh.gov/medicine/aboutus/self_presc.htm. Accessed March 21 2011.
15. State Medical Board of Ohio. Frequently asked questions. Available at: http://www.med.ohio.gov/professional_guidelines.htm. Accessed March 20 2011.
16. Virginia Board of Medicine. Can I prescribe for my family and myself? Available at: http://www.dhp.virginia.gov/Medicine/medicine_faq.htm#Prescribe. Accessed March 20 2011.
17. Aboff B, Collier V, Farber N. Residents’ prescription writing for nonpatients. JAMA. 2002;288:381-385.
18. Johnson LJ. Malpractice consult. Should you give informal medical advice? Med Econ. 2007;84:36.-
19. Nisselle P. Danger zone: when boundaries are crossed in the doctor-patient relationship. Aust Family Physician. 2000;29:541-544.
20. Eastwood GL. When relatives and friends ask physicians for medical advice: ethical legal, and practical considerations. J Gen Intern Med. 2009;24:1333-1335.
1. Balon R. Psychiatrist attitudes toward self-treatment of their own depression. Psychother Psychosom. 2007;76:306-310.
2. Walter JK, Lang CW, Ross LF. When physicians forego the doctor-patient relationship should they elect to self-prescribe or curbside? An empirical and ethical analysis. J Med Ethics. 2010;36:19-23.
3. Reynolds H. Medical ear in the early morning tennis group—when to advise and what to say. Pharos Alpha Omega Alpha Honor Med Soc. 2010;73:14-15 discussion 16.
4. Sataline S, Wang SS. Medical schools can’t keep up: as ranks of insured expand nation faces shortage of 150,000 doctors in 15 years. Available at: http://online.wsj.com/article/SB10001424052702304506904575180331528424238.html. Accessed March 21, 2011.
5. Steinberg S. Of medical specialties demand for psychiatrists growing fastest. USA Today. July 1, 2010:6D.
6. Leonhardt D. A labor market punishing to mothers. New York Times. August 4 2010:B1.
7. Madden K. Reluctant to go on vacation? Available at: http://www.cnn.com/2010/LIVING/08/04/cb.reluctant.to.take.vacation/index.html. Accessed March 20 2011.
8. Meier DE, Back AL, Morrison RS. The inner life of physicians and care of the seriously ill. JAMA. 2001;286:3007-3014.
9. Clark A, Kau J. Patterns of psychoactive drug prescriptions by house officers for nonpatients. J Med Educ. 1988;63:44-50.
10. State Medical Board of South Carolina. Prescribing for family members. Available at: http://www.llr.state.sc.us/pol/medical/index.asp?file=Policies/MEPRESCRIBEFAM.HTM. Accessed March 20 2011.
11. Dietz LH, Jacobs A, Leming TL, et al. Physicians, surgeons, and other healers, §§130, 216-218. In: American jurisprudence. vol 61. 2nd ed. New York, NY: Thomson Reuters; 2010.
12. Medical Board of California. General office practices/protocols-frequently asked questions. Available at: http://www.medbd.ca.gov/consumer/complaint_info_questions_practice.html#13. Accessed March 20 2011.
13. Montana Board of Medical Examiners. Statement of physician prescribing for self or members of the physician’s immediate family. Available at: http://bsd.dli.mt.gov/license/bsd_boards/med_board/pdf/prescribing_self.pdf. Accessed March 20 2011.
14. New Hampshire Medical Board. Guidelines for self-prescribing and prescribing for family members. Available at: http://www.nh.gov/medicine/aboutus/self_presc.htm. Accessed March 21 2011.
15. State Medical Board of Ohio. Frequently asked questions. Available at: http://www.med.ohio.gov/professional_guidelines.htm. Accessed March 20 2011.
16. Virginia Board of Medicine. Can I prescribe for my family and myself? Available at: http://www.dhp.virginia.gov/Medicine/medicine_faq.htm#Prescribe. Accessed March 20 2011.
17. Aboff B, Collier V, Farber N. Residents’ prescription writing for nonpatients. JAMA. 2002;288:381-385.
18. Johnson LJ. Malpractice consult. Should you give informal medical advice? Med Econ. 2007;84:36.-
19. Nisselle P. Danger zone: when boundaries are crossed in the doctor-patient relationship. Aust Family Physician. 2000;29:541-544.
20. Eastwood GL. When relatives and friends ask physicians for medical advice: ethical legal, and practical considerations. J Gen Intern Med. 2009;24:1333-1335.
A skeptical view of ‘progress’ in psychiatry
Everybody loves progress. People feel uplifted by the notion of progress, by the dynamic feeling it evokes of “moving forward,” of achieving new milestones and reaching new heights. Progress implies improvement in the human condition and an upgrade in quality of life. In medical disciplines such as psychiatry, it connotes less suffering, better treatments, more hope, improved social and vocational functioning, and full restoration of wellness.
But let’s be realistic. Overall evolution of psychiatry is not as “progressive” as we like to believe. Yes, there are thrilling breakthroughs in basic neuroscience research and understanding brain structure and function at the cellular and molecular levels. However, in many other areas of psychiatric practice, I feel we have moved backward since I began my career 3 decades ago. Egress, not progress, appears to be the state of psychiatry. In a tango-like fashion, psychiatry seems to take 1 step forward on 1 level (science and discovery) and 2 steps back on another level (practice realities). As an optimistic person, it pains me to admit that we have moved backward in several aspects of psychiatry:
- The discovery of chlorpromazine, the first antipsychotic, was a miraculous event for our field, but was it “progress” for our patients? Their symptoms improved partially but they developed serious side effects and remained functionally disabled throughout their lives. Patients were “freed” from locked hospital wards, then hurled into a poorly prepared and under-resourced community mental health care system, resulting in revolving door relapses, extensive drug abuse, rampant stigma, abject poverty, physical neglect, early death, homelessness, and for many psychiatric patients, incarceration in jails and prisons, an environment more restrictive than the reviled asylums. Our patients who were medically ill individuals cared for by doctors, nurses, and other health professionals are now lowly felons. It seems that those unfortunate enough to suffer from a psychotic brain disorder are destined to be further punished for it, a great injustice in the name of “progress.”
- Insurance hassles for serious mental illness did not exist in the asylum era. If an individual developed a psychotic disorder, he or she was admitted to the nearest state hospital without hesitation and provided medical and psychosocial care, even if the stay lasted months or years. Now, the same patient cannot afford psychiatric hospitalization even if he or she has “health insurance” (a euphemism for “restricted health coverage”). Equality of psychiatric disorders with other medical and surgical disorders remains a farce, and the lack of parity for mental illness has deprived millions of patients from adequate care. How many victims of mental illness have suffered or died in the name of “progress” in the health insurance industry?
- Who is the “genius” who stipulated that a psychotic, bipolar, suicidal, or homicidal patient could be effectively treated after 3 to 4 days of hospitalization? How did patients become widgets on an assembly line? Medical students and residents on inpatient wards no longer have the rewarding experience or witnessing full improvement in their patients. Is it progress when a patient with schizophrenia or severe depression is discharged after barely 30% to 40% improvement in symptoms? No wonder relapse, suicide, and homicide rates are very high in the 3 weeks after discharge. Long-term hospitals, the last refuge for severely disabled patients who cannot care for themselves, now are rare. Is that progress?
- Why are psychiatrists shackled by more legal constraints than physicians in other medical specialties? Why should lawyers and judges tell us how to practice medicine and who, when, and how to treat? Legal progress sounds like an oxymoron to me.
- Why is the public mental health system so broken in every state? Why is it so ineffective, chaotic, underfunded, hard to navigate, and demedicalized? Why have psychiatrists—the traditional leaders in mental health—been marginalized to sign prescriptions instead of being executives and policy-setters for mental illness? Respiratory and physical therapists have important roles but the pulmonologist or the orthopedist runs the clinic. Why is it not so in public psychiatry? This is not progress, but a travesty.
- Why is psychiatry now referred to as “behavioral health”? Who decided to fix the name of psychiatric care when the original term is much more comprehensive, factual, and inclusive and uses medical terminology (iatros = “healer” or “medicine”). It is not progress to reduce to “behavior” psychiatric illnesses that involve a broad spectrum of pathologies, including thought disorders, mood disorders, perceptual disorders, cognitive disorders, pain, addictions, and many general medical conditions that manifest with psychiatric signs and symptoms. Redefining psychiatric care with inaccurate terminology certainly is not progress.
- Why are pharmaceutical companies, the only source of drug development, abandoning CNS research? Is it because cardiovascular, oncologic, and GI drugs are more profitable and less “challenging” to develop? Is it progress to turn away from the most critical medical frontier, the human brain, and its diseases? At a time when 80% of psychiatric disorders have no approved medication, it is inexcusable to shirk from discovering drugs that trigger hope for recovery for patients with untreatable mental illness.
Ogden Nash once wrote: “Progress might have been all right once, but it has gone on too long.” I will add to that for psychiatry, progress isn’t what it used to be.
Everybody loves progress. People feel uplifted by the notion of progress, by the dynamic feeling it evokes of “moving forward,” of achieving new milestones and reaching new heights. Progress implies improvement in the human condition and an upgrade in quality of life. In medical disciplines such as psychiatry, it connotes less suffering, better treatments, more hope, improved social and vocational functioning, and full restoration of wellness.
But let’s be realistic. Overall evolution of psychiatry is not as “progressive” as we like to believe. Yes, there are thrilling breakthroughs in basic neuroscience research and understanding brain structure and function at the cellular and molecular levels. However, in many other areas of psychiatric practice, I feel we have moved backward since I began my career 3 decades ago. Egress, not progress, appears to be the state of psychiatry. In a tango-like fashion, psychiatry seems to take 1 step forward on 1 level (science and discovery) and 2 steps back on another level (practice realities). As an optimistic person, it pains me to admit that we have moved backward in several aspects of psychiatry:
- The discovery of chlorpromazine, the first antipsychotic, was a miraculous event for our field, but was it “progress” for our patients? Their symptoms improved partially but they developed serious side effects and remained functionally disabled throughout their lives. Patients were “freed” from locked hospital wards, then hurled into a poorly prepared and under-resourced community mental health care system, resulting in revolving door relapses, extensive drug abuse, rampant stigma, abject poverty, physical neglect, early death, homelessness, and for many psychiatric patients, incarceration in jails and prisons, an environment more restrictive than the reviled asylums. Our patients who were medically ill individuals cared for by doctors, nurses, and other health professionals are now lowly felons. It seems that those unfortunate enough to suffer from a psychotic brain disorder are destined to be further punished for it, a great injustice in the name of “progress.”
- Insurance hassles for serious mental illness did not exist in the asylum era. If an individual developed a psychotic disorder, he or she was admitted to the nearest state hospital without hesitation and provided medical and psychosocial care, even if the stay lasted months or years. Now, the same patient cannot afford psychiatric hospitalization even if he or she has “health insurance” (a euphemism for “restricted health coverage”). Equality of psychiatric disorders with other medical and surgical disorders remains a farce, and the lack of parity for mental illness has deprived millions of patients from adequate care. How many victims of mental illness have suffered or died in the name of “progress” in the health insurance industry?
- Who is the “genius” who stipulated that a psychotic, bipolar, suicidal, or homicidal patient could be effectively treated after 3 to 4 days of hospitalization? How did patients become widgets on an assembly line? Medical students and residents on inpatient wards no longer have the rewarding experience or witnessing full improvement in their patients. Is it progress when a patient with schizophrenia or severe depression is discharged after barely 30% to 40% improvement in symptoms? No wonder relapse, suicide, and homicide rates are very high in the 3 weeks after discharge. Long-term hospitals, the last refuge for severely disabled patients who cannot care for themselves, now are rare. Is that progress?
- Why are psychiatrists shackled by more legal constraints than physicians in other medical specialties? Why should lawyers and judges tell us how to practice medicine and who, when, and how to treat? Legal progress sounds like an oxymoron to me.
- Why is the public mental health system so broken in every state? Why is it so ineffective, chaotic, underfunded, hard to navigate, and demedicalized? Why have psychiatrists—the traditional leaders in mental health—been marginalized to sign prescriptions instead of being executives and policy-setters for mental illness? Respiratory and physical therapists have important roles but the pulmonologist or the orthopedist runs the clinic. Why is it not so in public psychiatry? This is not progress, but a travesty.
- Why is psychiatry now referred to as “behavioral health”? Who decided to fix the name of psychiatric care when the original term is much more comprehensive, factual, and inclusive and uses medical terminology (iatros = “healer” or “medicine”). It is not progress to reduce to “behavior” psychiatric illnesses that involve a broad spectrum of pathologies, including thought disorders, mood disorders, perceptual disorders, cognitive disorders, pain, addictions, and many general medical conditions that manifest with psychiatric signs and symptoms. Redefining psychiatric care with inaccurate terminology certainly is not progress.
- Why are pharmaceutical companies, the only source of drug development, abandoning CNS research? Is it because cardiovascular, oncologic, and GI drugs are more profitable and less “challenging” to develop? Is it progress to turn away from the most critical medical frontier, the human brain, and its diseases? At a time when 80% of psychiatric disorders have no approved medication, it is inexcusable to shirk from discovering drugs that trigger hope for recovery for patients with untreatable mental illness.
Ogden Nash once wrote: “Progress might have been all right once, but it has gone on too long.” I will add to that for psychiatry, progress isn’t what it used to be.
Everybody loves progress. People feel uplifted by the notion of progress, by the dynamic feeling it evokes of “moving forward,” of achieving new milestones and reaching new heights. Progress implies improvement in the human condition and an upgrade in quality of life. In medical disciplines such as psychiatry, it connotes less suffering, better treatments, more hope, improved social and vocational functioning, and full restoration of wellness.
But let’s be realistic. Overall evolution of psychiatry is not as “progressive” as we like to believe. Yes, there are thrilling breakthroughs in basic neuroscience research and understanding brain structure and function at the cellular and molecular levels. However, in many other areas of psychiatric practice, I feel we have moved backward since I began my career 3 decades ago. Egress, not progress, appears to be the state of psychiatry. In a tango-like fashion, psychiatry seems to take 1 step forward on 1 level (science and discovery) and 2 steps back on another level (practice realities). As an optimistic person, it pains me to admit that we have moved backward in several aspects of psychiatry:
- The discovery of chlorpromazine, the first antipsychotic, was a miraculous event for our field, but was it “progress” for our patients? Their symptoms improved partially but they developed serious side effects and remained functionally disabled throughout their lives. Patients were “freed” from locked hospital wards, then hurled into a poorly prepared and under-resourced community mental health care system, resulting in revolving door relapses, extensive drug abuse, rampant stigma, abject poverty, physical neglect, early death, homelessness, and for many psychiatric patients, incarceration in jails and prisons, an environment more restrictive than the reviled asylums. Our patients who were medically ill individuals cared for by doctors, nurses, and other health professionals are now lowly felons. It seems that those unfortunate enough to suffer from a psychotic brain disorder are destined to be further punished for it, a great injustice in the name of “progress.”
- Insurance hassles for serious mental illness did not exist in the asylum era. If an individual developed a psychotic disorder, he or she was admitted to the nearest state hospital without hesitation and provided medical and psychosocial care, even if the stay lasted months or years. Now, the same patient cannot afford psychiatric hospitalization even if he or she has “health insurance” (a euphemism for “restricted health coverage”). Equality of psychiatric disorders with other medical and surgical disorders remains a farce, and the lack of parity for mental illness has deprived millions of patients from adequate care. How many victims of mental illness have suffered or died in the name of “progress” in the health insurance industry?
- Who is the “genius” who stipulated that a psychotic, bipolar, suicidal, or homicidal patient could be effectively treated after 3 to 4 days of hospitalization? How did patients become widgets on an assembly line? Medical students and residents on inpatient wards no longer have the rewarding experience or witnessing full improvement in their patients. Is it progress when a patient with schizophrenia or severe depression is discharged after barely 30% to 40% improvement in symptoms? No wonder relapse, suicide, and homicide rates are very high in the 3 weeks after discharge. Long-term hospitals, the last refuge for severely disabled patients who cannot care for themselves, now are rare. Is that progress?
- Why are psychiatrists shackled by more legal constraints than physicians in other medical specialties? Why should lawyers and judges tell us how to practice medicine and who, when, and how to treat? Legal progress sounds like an oxymoron to me.
- Why is the public mental health system so broken in every state? Why is it so ineffective, chaotic, underfunded, hard to navigate, and demedicalized? Why have psychiatrists—the traditional leaders in mental health—been marginalized to sign prescriptions instead of being executives and policy-setters for mental illness? Respiratory and physical therapists have important roles but the pulmonologist or the orthopedist runs the clinic. Why is it not so in public psychiatry? This is not progress, but a travesty.
- Why is psychiatry now referred to as “behavioral health”? Who decided to fix the name of psychiatric care when the original term is much more comprehensive, factual, and inclusive and uses medical terminology (iatros = “healer” or “medicine”). It is not progress to reduce to “behavior” psychiatric illnesses that involve a broad spectrum of pathologies, including thought disorders, mood disorders, perceptual disorders, cognitive disorders, pain, addictions, and many general medical conditions that manifest with psychiatric signs and symptoms. Redefining psychiatric care with inaccurate terminology certainly is not progress.
- Why are pharmaceutical companies, the only source of drug development, abandoning CNS research? Is it because cardiovascular, oncologic, and GI drugs are more profitable and less “challenging” to develop? Is it progress to turn away from the most critical medical frontier, the human brain, and its diseases? At a time when 80% of psychiatric disorders have no approved medication, it is inexcusable to shirk from discovering drugs that trigger hope for recovery for patients with untreatable mental illness.
Ogden Nash once wrote: “Progress might have been all right once, but it has gone on too long.” I will add to that for psychiatry, progress isn’t what it used to be.
Dissociative identity disorder: No excuse for criminal activity
Formerly called multiple personality disorder, dissociative identity disorder (DID) is a controversial diagnosis that challenges forensic psychiatrists, other mental health clinicians, legal professionals, the media, and the public. DID cases often present in the criminal justice system rather than in the mental health system, and the illness perplexes experts in both professions.
Individuals may commit criminal acts while in a dissociated state. A study that tracked 21 reported DID cases found that 47% of men and 35% of women reported engaging in criminal activity, including 19% of men and 7% of women who committed homicide.1 Defendants occasionally use DID as a basis for pleading not guilty by reason of insanity (NGRI). Controversy over the DID diagnosis has contributed to debates about the disorder’s role in criminal responsibility.
The DID diagnosis
An American Psychiatric Association Work Group has proposed new diagnostic criteria for DID for DSM-5, which is scheduled to be published in May 2013.2 Presently, DID is listed in DSM-IV-TR as an axis I disorder.3 Criteria for DID include the presence of ≥2 distinctive identities or personality states that recurrently take control of an individual’s behavior (Table 1).3 This is accompanied by an inability to recall important personal information to an extent that cannot be explained by ordinary forgetfulness. Patients with DID typically have a primary identity that is passive, dependent, guilty, and depressed, and alternate identities with characteristics that differ from the primary identity, commonly in reported age and gender, vocabulary, general knowledge, or predominant affect.3
Dissociative pathology may result from trauma, comorbid mental illness, or other medical issues, including complex partial seizures. Developmental theorists have proposed that severe sexual, physical, or psychological trauma in childhood predisposes an individual to develop DID.4 Theoretically, harm by a trusted caregiver forces a child to split off awareness and memory of the trauma to survive in the relationship. Later these memories and feelings are experienced as a separate personality. Because this process happens repeatedly, the patient develops multiple personalities; each has different memories and performs different functions, which may be helpful or destructive. Later, dissociation becomes a coping mechanism when individuals face stressful situations.5
Personality traits that may predispose patients to develop a dissociative disorder include mental absorption, suggestibility, ability to be easily hypnotized, and tendency to fantasize.6 Patients with dissociation also may meet criteria for posttraumatic stress disorder, borderline personality disorder, somatoform disorder, eating disorder, or substance use disorders.7
Table 1
DSM-IV-TR criteria for dissociative identity disorder
| The presence of ≥2 distinct identities or personality states, each with its own relatively enduring pattern of perceiving, relating to, and thinking about the environment and self |
| At least 2 of these identities or personality states must recurrently take control of the person’s behavior |
| An inability to recall important personal information that is too extensive to be explained by ordinary forgetfulness |
| The disturbance is not due to the direct physiological effects of substance or a general medical condition. In children, the symptoms are not attributable to imaginary playmates or other fantasy play |
| Source: Reference 3 |
DID and NGRI
An insanity defense is raised in <1% of felony cases, and is successful in only a fraction of those.8 A criminal defendant who claims NGRI asserts that he committed the offense and asks the court to find him not culpable because of his mental state when the offense occurred.
The legal approach used by the defense in cases of NGRI due to DID will be determined by the jurisdiction in which the case is tried. The “Alter-in-control” approach considers the key issue as which “alter” (personality) was in control at the time of the offense and whether he or she met the insanity standard, the “Each-alter” approach considers whether each personality met the insanity standard, and the “Host-alter” approach considers whether the dominant or primary personality met the insanity standard.9
Legal and mental health professionals are divided on whether DID warrants an acquittal for insanity. The first time DID was recognized as a mental disorder that could excuse criminal responsibility occurred in State v Milligan (1978).10 The court declared serial rapist Billy Milligan insane due to lack of one integrated personality and therefore not culpable of the crimes he committed. Public outrage was extraordinary. Since this case, most DID defenses have not been successful (Table 2).10-16
In a 1980 court case,11 a defendant charged with murder pleaded NGRI due to having multiple personalities. The court found that having alter personalities was not necessarily a mental disease that would preclude responsibility for the murder.
In State v Grimsley (1982),12 the defense used NGRI due to multiple personalities in a drunk driving case. The court ruled that it is immaterial what state of consciousness or personality the defendant was in as long as the personality controlling the behavior was conscious and aware of his or her actions.
In Kirkland v State (1983),13 attorneys for a woman who committed a bank robbery asserted an insanity defense based on a psychogenic fugue, which is sudden, unexpected travel away from home accompanied by inability to recall one’s past and confusion about identity or assumption of a new identity.3 The court found that the law adjudges criminal liability according to the person’s state of mind at the time of the act and will not inquire whether an individual possesses other personalities, fugues, or even moods in which he would not have performed the crime.
In State v Jones (1988),14 the court found the defendant guilty of murdering a woman he met at a bar despite expert testimony that his multiple personalities “paralyzed” him from knowing right from wrong.
More recently, courts have rejected the admissibility of DID evidence, including expert testimony, because the scientific evidence failed to meet reliability standards, and therefore is not ultimately useful to the judge or jury. In State v Greene (1998),15 the defendant claimed that 1 of his 24 alters was responsible for killing his therapist. The Supreme Court of Washington affirmed that evidence of Mr. Greene’s DID, including expert testimony, was not reliable and not admissible.
Similarly, in State v Lockhart (2000),16 Mr. Lockhart contested his conviction of first degree sexual assault on the basis that he was not permitted to present evidence of DID to support his insanity defense. The West Virginia Court held that the diagnosis of DID was speculative and therefore did not meet reliability standards for evidence.
Table 2
Using dissociative identity disorder* as a basis for not guilty by reason of insanity
| Case | Year | Charge | Defense | Court ruling |
|---|---|---|---|---|
| State v Milligan10 | 1978 | Rape | NGRI-MPD | Lack of an integrated personality meant the defendant was not culpable |
| State v Darnall11 | 1980 | Murder | NGRI-MPD | Multiple personalities do not preclude criminal responsibility |
| State v Grimsley12 | 1982 | Drunk driving | NGRI-MPD; primary personality had no control over the ‘alter’ | State of consciousness or personality of defendant is immaterial |
| Kirkland v State13 | 1983 | Bank robbery | NGRI-psychogenic fugue | Law does not inquire about other personalities, fugue states, or moods in cases of criminal liability |
| State v Jones14 | 1988 | Murder | NGRI-MPD | Alter personalities will not be an excuse for inability to distinguish right from wrong |
| State v Greene15 | 1998 | Murder | NGRI-DID; primary personality was ‘unconscious’ | Evidence of DID, including expert testimony, was not admissible because it did not meet reliability standards |
| State v Lockhart16 | 2000 | Sexual assault | NGRI-DID | DID was not allowed into evidence by the West Virginia Court due to lack of scientific evidence |
| *Dissociative identity disorder formerly was referred to as multiple personality disorder DID: dissociative identity disorder; MPD: multiple personality disorder; NGRI: not guilty by reason of insanity. | ||||
Evaluating DID
Because the courts may ask psychiatrists to provide expert opinion on DID to assist with legal rulings, clinicians must remain vigilant to the possibility of DID as well as to defendants who may malinger multiple personalities to evade punishment. In such situations, factors to consider include the mental status examination, data and history collection, collateral information, criminal background, mental health history, history of abuse, and objective assessment tools.
Extensive field testing has shown that the Structured Clinical Interview for Dissociative Disorders (SCID-D) has good reliability and excellent validity.17 The SCID-D allows a trained interviewer to assess the severity of 5 dissociative symptoms: amnesia, depersonalization, derealization, identity confusion, and identity alteration.17 Other tools that may help assess a patient with suspected DID are listed in Table 3.
Patients who commit criminal acts while in a dissociated state may assert a defense of NGRI due to DID, but rarely has this defense been successful. Although a patient may have distinct personalities that control his or her behavior, this condition does not preclude criminal responsibility.
The role of hypnosis in evaluating DID is controversial. The introduction of pseudo-memories and potential for iatrogenic DID may complicate the clinical presentation and subsequent diagnosis.18
Table 3
Tools for diagnosing dissociative identity disorder
| Structured Clinical Interview for Dissociative Disorders |
| Dissociative Disorder Interview Schedule |
| Dissociative Experiences Scale |
| Childhood Trauma Questionnaire |
Related Resource
- West S, Noffsinger S. Is this patient not guilty by reason of insanity? Current Psychiatry. 2006;5(8):54-62.
Disclosure
Dr. Farrell reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Putnam. Diagnosis and treatment of multiple personality disorder. New York, NY: The Guilford Press; 1989.
2. American Psychiatric Association. DSM-5 Development. 300. 14. Dissociative identity disorder. Proposed revision. Available at: http://www.dsm5.org/ProposedRevisions/Pages/proposedrevision.aspx?rid=57. Accessed April 22 2011.
3. Diagnostic and statistical manual of mental disorders. 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
4. Sadock BJ, Sadock VA. eds. Dissociative disorders. In: Sadock BJ, Sadock VA, eds. Kaplan & Sadock’s comprehensive textbook of psychiatry. 7th ed. New York, NY: Lippincott Williams & Wilkins; 2000:1544–1576.
5. Lyons-Ruth K, Dutra L, Schuder MR, et al. From infant attachment disorganization to adult dissociation: relational adaptations or traumatic experiences? Psychiatr Clin North Am. 2006;29(1):63-86, viii.
6. Brahams D. Automatism and post-traumatic stress disorder. Lancet. 1990;335(8701):1333.-
7. Simeon D, Guralnik O, Knutelska M, et al. Personality factors associated with dissociation: temperament, defenses, and cognitive schemata. Am J Psychiatry. 2002;159(3):489-491.
8. Perlin M. The jurisprudence of the insanity defense. Durham NC: Carolina Academic Press; 1994.
9. Steinberg M, Bancroft J, Buchanan J. Multiple personality disorder in criminal law. Bull Am Acad Psychiatry Law. 1993;21(3):345-356.
10. State v Milligan, No 77-CR-11-2908 (Franklin County, Ohio, December 4 1978).
11. State v Darnall, 47 Or App 161, 614 P2d 120 (1980).
12. State v Grimsley, 3 Ohio App 3d 165 444 NE2d 1071 (1982).
13. Kirkland v State, 166 Ga App 478, 304 SE2d 561 (1983).
14. State v Jones, 743 P2d 176 (Wash Ct App 1987) aff’d, 759 P2d 1183, 1185 (Wash 1998).
15. State v Greene, 92 Wn App 80, 960 P2d 980 (1998).
16. State v Lockhart, 208 W Va 622 (2000).
17. Steinberg M, Rounsaville B, Cicchetti D. Detection of dissociative disorders in psychiatric patients by a screening instrument and a structured diagnostic interview. Am J Psychiatry. 1991;148(8):1050-1054.
18. Putnam FW. Dissociative phenomena. In: Tasman A ed. Annual review of psychiatry. Washington, DC: American Psychiatric Press; 1991:145–160.
Formerly called multiple personality disorder, dissociative identity disorder (DID) is a controversial diagnosis that challenges forensic psychiatrists, other mental health clinicians, legal professionals, the media, and the public. DID cases often present in the criminal justice system rather than in the mental health system, and the illness perplexes experts in both professions.
Individuals may commit criminal acts while in a dissociated state. A study that tracked 21 reported DID cases found that 47% of men and 35% of women reported engaging in criminal activity, including 19% of men and 7% of women who committed homicide.1 Defendants occasionally use DID as a basis for pleading not guilty by reason of insanity (NGRI). Controversy over the DID diagnosis has contributed to debates about the disorder’s role in criminal responsibility.
The DID diagnosis
An American Psychiatric Association Work Group has proposed new diagnostic criteria for DID for DSM-5, which is scheduled to be published in May 2013.2 Presently, DID is listed in DSM-IV-TR as an axis I disorder.3 Criteria for DID include the presence of ≥2 distinctive identities or personality states that recurrently take control of an individual’s behavior (Table 1).3 This is accompanied by an inability to recall important personal information to an extent that cannot be explained by ordinary forgetfulness. Patients with DID typically have a primary identity that is passive, dependent, guilty, and depressed, and alternate identities with characteristics that differ from the primary identity, commonly in reported age and gender, vocabulary, general knowledge, or predominant affect.3
Dissociative pathology may result from trauma, comorbid mental illness, or other medical issues, including complex partial seizures. Developmental theorists have proposed that severe sexual, physical, or psychological trauma in childhood predisposes an individual to develop DID.4 Theoretically, harm by a trusted caregiver forces a child to split off awareness and memory of the trauma to survive in the relationship. Later these memories and feelings are experienced as a separate personality. Because this process happens repeatedly, the patient develops multiple personalities; each has different memories and performs different functions, which may be helpful or destructive. Later, dissociation becomes a coping mechanism when individuals face stressful situations.5
Personality traits that may predispose patients to develop a dissociative disorder include mental absorption, suggestibility, ability to be easily hypnotized, and tendency to fantasize.6 Patients with dissociation also may meet criteria for posttraumatic stress disorder, borderline personality disorder, somatoform disorder, eating disorder, or substance use disorders.7
Table 1
DSM-IV-TR criteria for dissociative identity disorder
| The presence of ≥2 distinct identities or personality states, each with its own relatively enduring pattern of perceiving, relating to, and thinking about the environment and self |
| At least 2 of these identities or personality states must recurrently take control of the person’s behavior |
| An inability to recall important personal information that is too extensive to be explained by ordinary forgetfulness |
| The disturbance is not due to the direct physiological effects of substance or a general medical condition. In children, the symptoms are not attributable to imaginary playmates or other fantasy play |
| Source: Reference 3 |
DID and NGRI
An insanity defense is raised in <1% of felony cases, and is successful in only a fraction of those.8 A criminal defendant who claims NGRI asserts that he committed the offense and asks the court to find him not culpable because of his mental state when the offense occurred.
The legal approach used by the defense in cases of NGRI due to DID will be determined by the jurisdiction in which the case is tried. The “Alter-in-control” approach considers the key issue as which “alter” (personality) was in control at the time of the offense and whether he or she met the insanity standard, the “Each-alter” approach considers whether each personality met the insanity standard, and the “Host-alter” approach considers whether the dominant or primary personality met the insanity standard.9
Legal and mental health professionals are divided on whether DID warrants an acquittal for insanity. The first time DID was recognized as a mental disorder that could excuse criminal responsibility occurred in State v Milligan (1978).10 The court declared serial rapist Billy Milligan insane due to lack of one integrated personality and therefore not culpable of the crimes he committed. Public outrage was extraordinary. Since this case, most DID defenses have not been successful (Table 2).10-16
In a 1980 court case,11 a defendant charged with murder pleaded NGRI due to having multiple personalities. The court found that having alter personalities was not necessarily a mental disease that would preclude responsibility for the murder.
In State v Grimsley (1982),12 the defense used NGRI due to multiple personalities in a drunk driving case. The court ruled that it is immaterial what state of consciousness or personality the defendant was in as long as the personality controlling the behavior was conscious and aware of his or her actions.
In Kirkland v State (1983),13 attorneys for a woman who committed a bank robbery asserted an insanity defense based on a psychogenic fugue, which is sudden, unexpected travel away from home accompanied by inability to recall one’s past and confusion about identity or assumption of a new identity.3 The court found that the law adjudges criminal liability according to the person’s state of mind at the time of the act and will not inquire whether an individual possesses other personalities, fugues, or even moods in which he would not have performed the crime.
In State v Jones (1988),14 the court found the defendant guilty of murdering a woman he met at a bar despite expert testimony that his multiple personalities “paralyzed” him from knowing right from wrong.
More recently, courts have rejected the admissibility of DID evidence, including expert testimony, because the scientific evidence failed to meet reliability standards, and therefore is not ultimately useful to the judge or jury. In State v Greene (1998),15 the defendant claimed that 1 of his 24 alters was responsible for killing his therapist. The Supreme Court of Washington affirmed that evidence of Mr. Greene’s DID, including expert testimony, was not reliable and not admissible.
Similarly, in State v Lockhart (2000),16 Mr. Lockhart contested his conviction of first degree sexual assault on the basis that he was not permitted to present evidence of DID to support his insanity defense. The West Virginia Court held that the diagnosis of DID was speculative and therefore did not meet reliability standards for evidence.
Table 2
Using dissociative identity disorder* as a basis for not guilty by reason of insanity
| Case | Year | Charge | Defense | Court ruling |
|---|---|---|---|---|
| State v Milligan10 | 1978 | Rape | NGRI-MPD | Lack of an integrated personality meant the defendant was not culpable |
| State v Darnall11 | 1980 | Murder | NGRI-MPD | Multiple personalities do not preclude criminal responsibility |
| State v Grimsley12 | 1982 | Drunk driving | NGRI-MPD; primary personality had no control over the ‘alter’ | State of consciousness or personality of defendant is immaterial |
| Kirkland v State13 | 1983 | Bank robbery | NGRI-psychogenic fugue | Law does not inquire about other personalities, fugue states, or moods in cases of criminal liability |
| State v Jones14 | 1988 | Murder | NGRI-MPD | Alter personalities will not be an excuse for inability to distinguish right from wrong |
| State v Greene15 | 1998 | Murder | NGRI-DID; primary personality was ‘unconscious’ | Evidence of DID, including expert testimony, was not admissible because it did not meet reliability standards |
| State v Lockhart16 | 2000 | Sexual assault | NGRI-DID | DID was not allowed into evidence by the West Virginia Court due to lack of scientific evidence |
| *Dissociative identity disorder formerly was referred to as multiple personality disorder DID: dissociative identity disorder; MPD: multiple personality disorder; NGRI: not guilty by reason of insanity. | ||||
Evaluating DID
Because the courts may ask psychiatrists to provide expert opinion on DID to assist with legal rulings, clinicians must remain vigilant to the possibility of DID as well as to defendants who may malinger multiple personalities to evade punishment. In such situations, factors to consider include the mental status examination, data and history collection, collateral information, criminal background, mental health history, history of abuse, and objective assessment tools.
Extensive field testing has shown that the Structured Clinical Interview for Dissociative Disorders (SCID-D) has good reliability and excellent validity.17 The SCID-D allows a trained interviewer to assess the severity of 5 dissociative symptoms: amnesia, depersonalization, derealization, identity confusion, and identity alteration.17 Other tools that may help assess a patient with suspected DID are listed in Table 3.
Patients who commit criminal acts while in a dissociated state may assert a defense of NGRI due to DID, but rarely has this defense been successful. Although a patient may have distinct personalities that control his or her behavior, this condition does not preclude criminal responsibility.
The role of hypnosis in evaluating DID is controversial. The introduction of pseudo-memories and potential for iatrogenic DID may complicate the clinical presentation and subsequent diagnosis.18
Table 3
Tools for diagnosing dissociative identity disorder
| Structured Clinical Interview for Dissociative Disorders |
| Dissociative Disorder Interview Schedule |
| Dissociative Experiences Scale |
| Childhood Trauma Questionnaire |
Related Resource
- West S, Noffsinger S. Is this patient not guilty by reason of insanity? Current Psychiatry. 2006;5(8):54-62.
Disclosure
Dr. Farrell reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Formerly called multiple personality disorder, dissociative identity disorder (DID) is a controversial diagnosis that challenges forensic psychiatrists, other mental health clinicians, legal professionals, the media, and the public. DID cases often present in the criminal justice system rather than in the mental health system, and the illness perplexes experts in both professions.
Individuals may commit criminal acts while in a dissociated state. A study that tracked 21 reported DID cases found that 47% of men and 35% of women reported engaging in criminal activity, including 19% of men and 7% of women who committed homicide.1 Defendants occasionally use DID as a basis for pleading not guilty by reason of insanity (NGRI). Controversy over the DID diagnosis has contributed to debates about the disorder’s role in criminal responsibility.
The DID diagnosis
An American Psychiatric Association Work Group has proposed new diagnostic criteria for DID for DSM-5, which is scheduled to be published in May 2013.2 Presently, DID is listed in DSM-IV-TR as an axis I disorder.3 Criteria for DID include the presence of ≥2 distinctive identities or personality states that recurrently take control of an individual’s behavior (Table 1).3 This is accompanied by an inability to recall important personal information to an extent that cannot be explained by ordinary forgetfulness. Patients with DID typically have a primary identity that is passive, dependent, guilty, and depressed, and alternate identities with characteristics that differ from the primary identity, commonly in reported age and gender, vocabulary, general knowledge, or predominant affect.3
Dissociative pathology may result from trauma, comorbid mental illness, or other medical issues, including complex partial seizures. Developmental theorists have proposed that severe sexual, physical, or psychological trauma in childhood predisposes an individual to develop DID.4 Theoretically, harm by a trusted caregiver forces a child to split off awareness and memory of the trauma to survive in the relationship. Later these memories and feelings are experienced as a separate personality. Because this process happens repeatedly, the patient develops multiple personalities; each has different memories and performs different functions, which may be helpful or destructive. Later, dissociation becomes a coping mechanism when individuals face stressful situations.5
Personality traits that may predispose patients to develop a dissociative disorder include mental absorption, suggestibility, ability to be easily hypnotized, and tendency to fantasize.6 Patients with dissociation also may meet criteria for posttraumatic stress disorder, borderline personality disorder, somatoform disorder, eating disorder, or substance use disorders.7
Table 1
DSM-IV-TR criteria for dissociative identity disorder
| The presence of ≥2 distinct identities or personality states, each with its own relatively enduring pattern of perceiving, relating to, and thinking about the environment and self |
| At least 2 of these identities or personality states must recurrently take control of the person’s behavior |
| An inability to recall important personal information that is too extensive to be explained by ordinary forgetfulness |
| The disturbance is not due to the direct physiological effects of substance or a general medical condition. In children, the symptoms are not attributable to imaginary playmates or other fantasy play |
| Source: Reference 3 |
DID and NGRI
An insanity defense is raised in <1% of felony cases, and is successful in only a fraction of those.8 A criminal defendant who claims NGRI asserts that he committed the offense and asks the court to find him not culpable because of his mental state when the offense occurred.
The legal approach used by the defense in cases of NGRI due to DID will be determined by the jurisdiction in which the case is tried. The “Alter-in-control” approach considers the key issue as which “alter” (personality) was in control at the time of the offense and whether he or she met the insanity standard, the “Each-alter” approach considers whether each personality met the insanity standard, and the “Host-alter” approach considers whether the dominant or primary personality met the insanity standard.9
Legal and mental health professionals are divided on whether DID warrants an acquittal for insanity. The first time DID was recognized as a mental disorder that could excuse criminal responsibility occurred in State v Milligan (1978).10 The court declared serial rapist Billy Milligan insane due to lack of one integrated personality and therefore not culpable of the crimes he committed. Public outrage was extraordinary. Since this case, most DID defenses have not been successful (Table 2).10-16
In a 1980 court case,11 a defendant charged with murder pleaded NGRI due to having multiple personalities. The court found that having alter personalities was not necessarily a mental disease that would preclude responsibility for the murder.
In State v Grimsley (1982),12 the defense used NGRI due to multiple personalities in a drunk driving case. The court ruled that it is immaterial what state of consciousness or personality the defendant was in as long as the personality controlling the behavior was conscious and aware of his or her actions.
In Kirkland v State (1983),13 attorneys for a woman who committed a bank robbery asserted an insanity defense based on a psychogenic fugue, which is sudden, unexpected travel away from home accompanied by inability to recall one’s past and confusion about identity or assumption of a new identity.3 The court found that the law adjudges criminal liability according to the person’s state of mind at the time of the act and will not inquire whether an individual possesses other personalities, fugues, or even moods in which he would not have performed the crime.
In State v Jones (1988),14 the court found the defendant guilty of murdering a woman he met at a bar despite expert testimony that his multiple personalities “paralyzed” him from knowing right from wrong.
More recently, courts have rejected the admissibility of DID evidence, including expert testimony, because the scientific evidence failed to meet reliability standards, and therefore is not ultimately useful to the judge or jury. In State v Greene (1998),15 the defendant claimed that 1 of his 24 alters was responsible for killing his therapist. The Supreme Court of Washington affirmed that evidence of Mr. Greene’s DID, including expert testimony, was not reliable and not admissible.
Similarly, in State v Lockhart (2000),16 Mr. Lockhart contested his conviction of first degree sexual assault on the basis that he was not permitted to present evidence of DID to support his insanity defense. The West Virginia Court held that the diagnosis of DID was speculative and therefore did not meet reliability standards for evidence.
Table 2
Using dissociative identity disorder* as a basis for not guilty by reason of insanity
| Case | Year | Charge | Defense | Court ruling |
|---|---|---|---|---|
| State v Milligan10 | 1978 | Rape | NGRI-MPD | Lack of an integrated personality meant the defendant was not culpable |
| State v Darnall11 | 1980 | Murder | NGRI-MPD | Multiple personalities do not preclude criminal responsibility |
| State v Grimsley12 | 1982 | Drunk driving | NGRI-MPD; primary personality had no control over the ‘alter’ | State of consciousness or personality of defendant is immaterial |
| Kirkland v State13 | 1983 | Bank robbery | NGRI-psychogenic fugue | Law does not inquire about other personalities, fugue states, or moods in cases of criminal liability |
| State v Jones14 | 1988 | Murder | NGRI-MPD | Alter personalities will not be an excuse for inability to distinguish right from wrong |
| State v Greene15 | 1998 | Murder | NGRI-DID; primary personality was ‘unconscious’ | Evidence of DID, including expert testimony, was not admissible because it did not meet reliability standards |
| State v Lockhart16 | 2000 | Sexual assault | NGRI-DID | DID was not allowed into evidence by the West Virginia Court due to lack of scientific evidence |
| *Dissociative identity disorder formerly was referred to as multiple personality disorder DID: dissociative identity disorder; MPD: multiple personality disorder; NGRI: not guilty by reason of insanity. | ||||
Evaluating DID
Because the courts may ask psychiatrists to provide expert opinion on DID to assist with legal rulings, clinicians must remain vigilant to the possibility of DID as well as to defendants who may malinger multiple personalities to evade punishment. In such situations, factors to consider include the mental status examination, data and history collection, collateral information, criminal background, mental health history, history of abuse, and objective assessment tools.
Extensive field testing has shown that the Structured Clinical Interview for Dissociative Disorders (SCID-D) has good reliability and excellent validity.17 The SCID-D allows a trained interviewer to assess the severity of 5 dissociative symptoms: amnesia, depersonalization, derealization, identity confusion, and identity alteration.17 Other tools that may help assess a patient with suspected DID are listed in Table 3.
Patients who commit criminal acts while in a dissociated state may assert a defense of NGRI due to DID, but rarely has this defense been successful. Although a patient may have distinct personalities that control his or her behavior, this condition does not preclude criminal responsibility.
The role of hypnosis in evaluating DID is controversial. The introduction of pseudo-memories and potential for iatrogenic DID may complicate the clinical presentation and subsequent diagnosis.18
Table 3
Tools for diagnosing dissociative identity disorder
| Structured Clinical Interview for Dissociative Disorders |
| Dissociative Disorder Interview Schedule |
| Dissociative Experiences Scale |
| Childhood Trauma Questionnaire |
Related Resource
- West S, Noffsinger S. Is this patient not guilty by reason of insanity? Current Psychiatry. 2006;5(8):54-62.
Disclosure
Dr. Farrell reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Putnam. Diagnosis and treatment of multiple personality disorder. New York, NY: The Guilford Press; 1989.
2. American Psychiatric Association. DSM-5 Development. 300. 14. Dissociative identity disorder. Proposed revision. Available at: http://www.dsm5.org/ProposedRevisions/Pages/proposedrevision.aspx?rid=57. Accessed April 22 2011.
3. Diagnostic and statistical manual of mental disorders. 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
4. Sadock BJ, Sadock VA. eds. Dissociative disorders. In: Sadock BJ, Sadock VA, eds. Kaplan & Sadock’s comprehensive textbook of psychiatry. 7th ed. New York, NY: Lippincott Williams & Wilkins; 2000:1544–1576.
5. Lyons-Ruth K, Dutra L, Schuder MR, et al. From infant attachment disorganization to adult dissociation: relational adaptations or traumatic experiences? Psychiatr Clin North Am. 2006;29(1):63-86, viii.
6. Brahams D. Automatism and post-traumatic stress disorder. Lancet. 1990;335(8701):1333.-
7. Simeon D, Guralnik O, Knutelska M, et al. Personality factors associated with dissociation: temperament, defenses, and cognitive schemata. Am J Psychiatry. 2002;159(3):489-491.
8. Perlin M. The jurisprudence of the insanity defense. Durham NC: Carolina Academic Press; 1994.
9. Steinberg M, Bancroft J, Buchanan J. Multiple personality disorder in criminal law. Bull Am Acad Psychiatry Law. 1993;21(3):345-356.
10. State v Milligan, No 77-CR-11-2908 (Franklin County, Ohio, December 4 1978).
11. State v Darnall, 47 Or App 161, 614 P2d 120 (1980).
12. State v Grimsley, 3 Ohio App 3d 165 444 NE2d 1071 (1982).
13. Kirkland v State, 166 Ga App 478, 304 SE2d 561 (1983).
14. State v Jones, 743 P2d 176 (Wash Ct App 1987) aff’d, 759 P2d 1183, 1185 (Wash 1998).
15. State v Greene, 92 Wn App 80, 960 P2d 980 (1998).
16. State v Lockhart, 208 W Va 622 (2000).
17. Steinberg M, Rounsaville B, Cicchetti D. Detection of dissociative disorders in psychiatric patients by a screening instrument and a structured diagnostic interview. Am J Psychiatry. 1991;148(8):1050-1054.
18. Putnam FW. Dissociative phenomena. In: Tasman A ed. Annual review of psychiatry. Washington, DC: American Psychiatric Press; 1991:145–160.
1. Putnam. Diagnosis and treatment of multiple personality disorder. New York, NY: The Guilford Press; 1989.
2. American Psychiatric Association. DSM-5 Development. 300. 14. Dissociative identity disorder. Proposed revision. Available at: http://www.dsm5.org/ProposedRevisions/Pages/proposedrevision.aspx?rid=57. Accessed April 22 2011.
3. Diagnostic and statistical manual of mental disorders. 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
4. Sadock BJ, Sadock VA. eds. Dissociative disorders. In: Sadock BJ, Sadock VA, eds. Kaplan & Sadock’s comprehensive textbook of psychiatry. 7th ed. New York, NY: Lippincott Williams & Wilkins; 2000:1544–1576.
5. Lyons-Ruth K, Dutra L, Schuder MR, et al. From infant attachment disorganization to adult dissociation: relational adaptations or traumatic experiences? Psychiatr Clin North Am. 2006;29(1):63-86, viii.
6. Brahams D. Automatism and post-traumatic stress disorder. Lancet. 1990;335(8701):1333.-
7. Simeon D, Guralnik O, Knutelska M, et al. Personality factors associated with dissociation: temperament, defenses, and cognitive schemata. Am J Psychiatry. 2002;159(3):489-491.
8. Perlin M. The jurisprudence of the insanity defense. Durham NC: Carolina Academic Press; 1994.
9. Steinberg M, Bancroft J, Buchanan J. Multiple personality disorder in criminal law. Bull Am Acad Psychiatry Law. 1993;21(3):345-356.
10. State v Milligan, No 77-CR-11-2908 (Franklin County, Ohio, December 4 1978).
11. State v Darnall, 47 Or App 161, 614 P2d 120 (1980).
12. State v Grimsley, 3 Ohio App 3d 165 444 NE2d 1071 (1982).
13. Kirkland v State, 166 Ga App 478, 304 SE2d 561 (1983).
14. State v Jones, 743 P2d 176 (Wash Ct App 1987) aff’d, 759 P2d 1183, 1185 (Wash 1998).
15. State v Greene, 92 Wn App 80, 960 P2d 980 (1998).
16. State v Lockhart, 208 W Va 622 (2000).
17. Steinberg M, Rounsaville B, Cicchetti D. Detection of dissociative disorders in psychiatric patients by a screening instrument and a structured diagnostic interview. Am J Psychiatry. 1991;148(8):1050-1054.
18. Putnam FW. Dissociative phenomena. In: Tasman A ed. Annual review of psychiatry. Washington, DC: American Psychiatric Press; 1991:145–160.
Identifying and treating depression across the life span
Discuss this article at www.facebook.com/CurrentPsychiatry
Most clinical trials of major depressive disorder (MDD) have focused on diagnosis and treatment of adults, but many younger and older patients also suffer from this condition. The prevalence of MDD is estimated to be 2% in children and 6% in adolescents.1 Up to 25% of adults age >60 experience MDD, dysthymic disorder, or “minor” depression.2
Although diagnosis and treatment of depression is similar regardless of a patient’s age, younger and older patients may not exhibit typical depressive symptoms (Table 1).1,2 For example, older adults may be more likely to report a lack of emotions than depressed mood. Vigilance for these types of distinct clinical manifestations can improve early recognition and treatment. In addition, evidence suggests there are differences in MDD treatment for younger and older patients.
This article reviews common challenges in recognizing and treating MDD in children, adolescents, and older adults.
Table 1
Major depressive disorder: Age-related differences
| Children/adolescents | Adults | Older adults | |
|---|---|---|---|
| Prevalence | 2% in children; 6% in adolescents | 20% | 25% |
| Male-to-female ratio | 1:1 in children; 1:2 in adolescents | 1:2 | 1:2 |
| DSM-IV-TR criteria | Similar | Similar | Similar |
| Clinical features | Irritability, temper tantrums, somatic complaints, hypersomina, weight gain, auditory hallucinations, psychomotor agitation, separation anxiety, social phobia, panic disorder, drug abuse, poor self-esteem | Typical DSM-IV-TR features. Psychomotor retardation, middle and terminal insomnia | Irritability, motor agitation, restlessness, somatic complaints, diarrhea and constipation, decreased libido, cognitive impairment, delusions, anxiety, panic, worsening of medical comorbidities |
| Source: References 1,2 | |||
Varying clinical features
Children/adolescents. The clinical presentation of MDD in children and adolescents is similar to that of adults. Children usually display anxiety, irritability, temper tantrums, and somatic complaints before verbally expressing depressive feelings. Psychotic depression in children manifests more often as auditory hallucinations than delusions.1
Younger vs middle-age adults. Researchers who evaluated baseline clinical and sociodemographic information of 1,498 patients enrolled in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study found that the presentation of depressive symptoms in young adult patients (age 18 to 35) differed from those of middle-age (age 36 to 50) patients.3 Younger patients were more likely to be irritable, complain of weight gain and hypersomnia, and have a negative view of life and the future. They also were more likely to report previous suicide attempts and endorse symptoms consistent with generalized anxiety disorder, social phobia, panic disorder, and drug abuse. Middle-age patients had more depressive episodes, deceased libido, and middle insomnia, and more frequently reported gastrointestinal symptoms such as diarrhea or constipation.3
Older adults. In our experience, typical MDD mood symptoms often are absent in older patients. Frequently, we see somatic complaints, motor restlessness, or psychomotor retardation; these symptoms may be attributable to a concurrent medical illness. This in turn may worsen the physical illness, leading to social isolation and considerable medical morbidity.4
Pain plays an important role in depression, particularly in older adults. Chronic pain affects up to 65% of older adults who live in the community and up to 80% of those who are institutionalized.5 The most common causes of pain in these patients are osteoarthritis, osteoporosis, fibromyalgia, degenerative disk disease, lumbar spinal stenosis, and scoliosis. In addition, neuropathic pain, such as post-herpetic neuralgia and peripheral neuropathy, and injuries resulting from falls often cause long-lasting pain.6
The presence of pain tends to negatively affect recognizing and treating depression. Regardless of their age, when a patient presents with pain or depression, investigate and consider treating both conditions.7
Memory decline is likely to be depressed older adults’ chief complaint, and when objectively tested these patients often show cognitive impairment.8 Whether depressive symptoms in this age group are a reaction to early cognitive deficits or are an early symptom of neurodegeneration remains controversial.9 Some case-control studies have found a link between a history of depression and Alzheimer’s disease (AD).10,11 In general, older patients whose first episode of depression occurs in late life have a higher relative risk of developing some form of dementia; research suggests that 50% of late-life MDD patients will develop dementia within 5 years.12
Researchers have considered the possibility that mild cognitive impairment (MCI) and dementia are a continuum of depression. In 1 study, 29 patients with MCI and 31 with MCI and MDD were assessed annually for an average of 4.3 years.13 Thirty-six patients with MCI (60%) progressed to AD. Presence of depression at the time of MCI diagnosis did not predict conversion to AD but persistence of depression for 2 to 3 years and the presence of melancholic features were associated with higher risk for AD.
Alexopoulos et al14 proposed the “vascular hypothesis theory” that cerebrovascular disease can predispose patients, particularly older adults, to depressive symptoms (Table 2).14 Whether vascular depression is a subtype of MDD remains controversial.
Table 2
Features of vascular depression
| Onset after age 50 |
| Family history of mood disorders is less common |
| Apathy |
| Marked loss of interest in activities |
| Lack of insight |
| Executive dysfunction (problems with planning, organizing, sequencing, abstracting), impaired memory or speed of processing of information |
| History of hypertension, diabetes, or cardiovascular disease |
| May have a neurologic event such as stroke or transient ischemic attack |
| White or gray matter hyperintensities |
| Source: Reference 14 |
Course and prognosis
MDD has been characterized as a self-limited disease, with an average duration of 6 to 9 months. However, newer prospective studies suggest that a substantial number of patients recover more slowly or do not ever fully recover.15 Several factors, such as genetic/biologic vulnerability and psychosocial factors, influence the courses, prognosis, and risk of relapse/recurrence of MDD in all age groups.
Children and adolescents. The typical duration of a major depressive episode for clinically referred children and adolescents is 8 to 13 months.1,16 Approximately 90% of these patients’ major depressive episodes remit by 2 years, but up to 10% persist.1,16 Within 5 years of MDD onset, up to 70% of children and adolescents will experience a recurrence,17 a rate comparable to adults.
Anxiety disorders, panic disorders, phobias, substance abuse, conduct and oppositional disorders, and attention-deficit/hyperactivity disorder occur 2 to 6 times more frequently in children and adolescents with MDD.18,19 Children with MDD who have significant psychiatric and psychosocial comorbidity experience poorer outcomes.18
Older adults. Despite optimal treatment conditions, ≥50% of older patients fail to respond adequately to first-line antidepressant pharmacotherapy.20 Treatment-resistant MDD in older patients increases:
- nonadherence to treatment for comorbid medical disorders
- disability and cognitive impairment
- burden on caregivers
- risk for early mortality, including suicide.20
Differences in treatment
Although MDD often is recurrent, episodic, and in some patients chronic, in general earlier treatment and quicker response lead to better outcomes. A large, naturalistic German study of 795 inpatients with major depression found that early improvement (20% reduction in Hamilton Depression Rating Scale-21 score within the first 2 weeks) with antidepressant therapy may predict later response and remission.21
Regardless of a patient’s age, MDD treatment should begin with education. All patients should be involved in their treatment. Encourage patients to become familiar with their triggers and stressors, improve their coping skills, and adopt a healthy lifestyle, which includes a nutritious diet, frequent exercise, and adequate sleep. As maintenance treatment we recommend that patients participate in frequent socialization and activities (Table 3). Refer patients to self-help books, online help guides, and handouts from sources such as National Institute of Mental Health.22,23 Encourage patients to have patience and perseverance, and guide them through each step of recovery.
In addition to lifestyle modification, other treatment options for depression include pharmacotherapy, interpersonal psychotherapy, cognitive-behavioral therapy (CBT), and electroconvulsive therapy (ECT). All these modalities are effective for acute and maintenance treatment and should be considered when determining the best approach for each patient.
The effectiveness of antidepressants in general is comparable among and within classes.2 Base your initial selection on the patient’s previous response to antidepressants and the medication’s side effects profile and cost.
The benefits of exercise for all patients cannot be underestimated.24 Prescribe 20 to 30 minutes of daily exercise as part of recommended lifestyle changes. Writing “daily exercise” on a prescription pad can effectively remind patients that exercise needs to be taken as seriously as medication compliance.
Children and adolescents. For mild depression, supportive therapy seems to be as effective as CBT and medications.25 A randomized controlled trial of 439 depressed adolescents found that CBT plus fluoxetine conferred quicker benefit, but in the long run may not be any more efficacious than pharmacotherapy alone.25 Researchers also found that CBT plus fluoxetine was no more effective than pharmacotherapy alone for adolescents with moderate to severe depression.25
Older adults. Compared with younger patients, geriatric patients typically require lower antidepressant dosages to achieve a specific blood level, but the blood levels at which antidepressants are most effective appear to be similar.2 Older patients also may be more likely to relapse and less likely to achieve full response to antidepressants than younger patients.2 In older adults, amitriptyline, imipramine, and doxepin are not preferred because these agents may cause orthostatic hypotension and urinary retention.2 A depressed older adult who experiences weight loss might benefit from an antidepressant that improves appetite, such as mirtazapine.26 Some research suggests that maintenance antidepressant therapy in older patients experiencing a first-time episode of MDD should continue for up to 2 years.27
A meta-analysis of 12 studies of CBT in depressed adults age ≥60 with chronic pain found that CBT was effective at improving self-reported pain but had no significant effect on depressive symptoms, physical function, or medication use.6 ECT often is prescribed for depressed older adults because its safety and efficacy for these patients has been well documented.28 Other neuromodulation therapies include vagus nerve, repetitive transcranial magnetic stimulation, and deep brain stimulation, but none of these treatments have been extensively evaluated in older patients.
Table 3
Avoiding reoccurrence of depression—a ‘prescription’ for patients
| Take medication as prescribed until your doctor instructs you to stop |
| Eat 3 nutritious meals every day |
| Sleep 6 to 8 hours each night |
| Walk/exercise for 20 minutes every day |
| Relax and do breathing exercises as taught 3 times a day |
| Talk with a friend or family member each day |
| Develop a hobby |
| Remain active |
| See your doctor once a month |
Related Resource
- American Psychiatric Association. Treatment of patients with major depressive disorder. 3rd ed. www.psychiatryonline.com/pracGuide/pracGuideChapToc_7.aspx.
Drug Brand Names
- Amitriptyline • Elavil
- Doxepin • Aponal, Silenor
- Fluoxetine • Prozac
- Imipramine • Tofranil
- Mirtazapine • Remeron
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Birmaher B, Brent D. AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(11):1503-1526.
2. American Psychiatric Association. Treatment of patients with major depressive disorder. 3rd ed. Available at: http://www.psychiatryonline.com/pracGuide/pracGuideChapToc_7.aspx. Accessed April 4 2011.
3. Husain MM, Rush AJ, Sakheim HA. Age-related characteristics of major depression: a preliminary STAR*D report. Am J Geriatr Psychiatry. 2005;13(10):852-862.
4. Gonda X, Molnár E, Torzsa P, et al. Characteristics of depression in the elderly. Psychiatr Hung. 2009;24(3):166-174.
5. Gibson, SJ. Pain and aging: a comparison of the pain experience over the adult life span. In: Dostrovsky JO, Carr DB, Koltzenburg M, eds. Proceedings of the 10th World Congress on Pain. Progress in pain research and management. Seattle, WA: IASP Press; 2003:767–790.
6. Lunde LH, Nordhus IH, Pallesen H. The effectiveness of cognitive and behavioural treatment of chronic pain in the elderly: a quantitative review. J Clin Psychol Med Settings. 2009;16:254-262.
7. Bair MJ, Robinson RL, Katon W, et al. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433-2445.
8. Reid LM, Machullich AM. Subjective memory complaints and cognitive impairment in older people. Dement Geriatr Cogn Disord. 2006;22(5-6):471-485.
9. Tsuno N, Homma A. What is the association between depression and Alzheimer’s disease? Expert Rev Neurother. 2009;9(11):]1667-1676.
10. Tsolaki M, Fountalakis K, Chantzi E, et al. Risk factors for clinically diagnosed Alzheimer’s disease: a case-control study of a Greek population. Int Psychogeriatr. 1997;9(3):327-341.
11. Speck CE, Kukull WA, Brenner DE, et al. History of depression as a risk factor for Alzheimer’s disease. Epidemiology. 1995;6(4):366-369.
12. Alexopoulos GS. Depression in elderly. Lancet. 2005;365:1961-1970.
13. Houde M, Bergman H, Whitehead V, et al. Predictive depression pattern in mild cognitive impairment. Int J Geriatr Psychiatry. 2008;23:1028-1033.
14. Alexopoulos GS, Meyers BS, Young RC, et al. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997;54(10):915-922.
15. Angst J, Gamma A, Rossler W, et al. Long-term depression versus episodic major depression: results from the prospective Zurich study of a community sample. J Affect Disord. 2009;115(1-2):112-121.
16. Birmaher B, Arbelaez C, Brent D. Course and outcome of child and adolescent major depressive disorder. Child Adolesc Psychiatr Clin N Am. 2002;11(3):619-637 x.
17. Rao U, Ryan ND, Birmaher B, et al. Unipolar depression in adolescents: clinical outcome in adulthood. J Am Acad Child Adolesc Psychiatry. 1995;34:566-578.
18. Angold A, Costello EJ, Erkanli A. Comorbidity. J Child Psych Psychiatry. 1999;40(1):57-87.
19. Costello EJ, Pine DS, Hammen C, et al. Development and natural history of mood disorders. Biol Psychiatry. 2002;52(6):529-542.
20. Lenze EJ, Sheffrin M, Driscoll HC, et al. Incomplete response in late-life depression: getting to remission. Dialogues Clin Neurosci. 2008;10(4):419-430.
21. Henkel V, Seemuller F, Obermeier M, et al. Does early improvement triggered by antidepressant predict response/remission? Analysis of data from a naturalistic study on a large sample of inpatients with major depression. J Affect Disord. 2009;115(3):439-449.
22. National Institute of Mental Health. Depression. NIH publication No. 083561. 2008. Available at: http://www.nimh.nih.gov/health/publications/depression/complete-index.shtml. Accessed April 4 2011.
23. Helpguide.org. Depression. Available at: http://www.helpguide.org/topics/depression.htm. Accessed April 4, 2011.
24. Sidhu K, Vandana P, Balon R. Exercise prescription. A practical and effective therapy for depression. Current Psychiatry. 2009;8(6):39-51.
25. Curry J, Rohde P, Simons A, et al. Predictors and moderators of acute outcome in the Treatment for Adolescents with Depression Study (TADS). J Am Acad Child Adolesc Psychiatry. 2006;45(12):1427-1439.
26. Rajii TK, Mulsant BH, Lotrich FE, et al. Use of antidepressant in late-life depression. Drugs Aging. 2008;25(10):841-853.
27. Rush AJ, Trivedi MH, Weiniewski SR, et al. Acute and longer term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917.
28. Van der Wurff FB, Stek ML, Hoogendijk WJ. The efficacy and safety of ECT in depressed older adults: a literature review. Int J Geriatr Psychiatry. 2003;18(10):894-904.
Discuss this article at www.facebook.com/CurrentPsychiatry
Most clinical trials of major depressive disorder (MDD) have focused on diagnosis and treatment of adults, but many younger and older patients also suffer from this condition. The prevalence of MDD is estimated to be 2% in children and 6% in adolescents.1 Up to 25% of adults age >60 experience MDD, dysthymic disorder, or “minor” depression.2
Although diagnosis and treatment of depression is similar regardless of a patient’s age, younger and older patients may not exhibit typical depressive symptoms (Table 1).1,2 For example, older adults may be more likely to report a lack of emotions than depressed mood. Vigilance for these types of distinct clinical manifestations can improve early recognition and treatment. In addition, evidence suggests there are differences in MDD treatment for younger and older patients.
This article reviews common challenges in recognizing and treating MDD in children, adolescents, and older adults.
Table 1
Major depressive disorder: Age-related differences
| Children/adolescents | Adults | Older adults | |
|---|---|---|---|
| Prevalence | 2% in children; 6% in adolescents | 20% | 25% |
| Male-to-female ratio | 1:1 in children; 1:2 in adolescents | 1:2 | 1:2 |
| DSM-IV-TR criteria | Similar | Similar | Similar |
| Clinical features | Irritability, temper tantrums, somatic complaints, hypersomina, weight gain, auditory hallucinations, psychomotor agitation, separation anxiety, social phobia, panic disorder, drug abuse, poor self-esteem | Typical DSM-IV-TR features. Psychomotor retardation, middle and terminal insomnia | Irritability, motor agitation, restlessness, somatic complaints, diarrhea and constipation, decreased libido, cognitive impairment, delusions, anxiety, panic, worsening of medical comorbidities |
| Source: References 1,2 | |||
Varying clinical features
Children/adolescents. The clinical presentation of MDD in children and adolescents is similar to that of adults. Children usually display anxiety, irritability, temper tantrums, and somatic complaints before verbally expressing depressive feelings. Psychotic depression in children manifests more often as auditory hallucinations than delusions.1
Younger vs middle-age adults. Researchers who evaluated baseline clinical and sociodemographic information of 1,498 patients enrolled in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study found that the presentation of depressive symptoms in young adult patients (age 18 to 35) differed from those of middle-age (age 36 to 50) patients.3 Younger patients were more likely to be irritable, complain of weight gain and hypersomnia, and have a negative view of life and the future. They also were more likely to report previous suicide attempts and endorse symptoms consistent with generalized anxiety disorder, social phobia, panic disorder, and drug abuse. Middle-age patients had more depressive episodes, deceased libido, and middle insomnia, and more frequently reported gastrointestinal symptoms such as diarrhea or constipation.3
Older adults. In our experience, typical MDD mood symptoms often are absent in older patients. Frequently, we see somatic complaints, motor restlessness, or psychomotor retardation; these symptoms may be attributable to a concurrent medical illness. This in turn may worsen the physical illness, leading to social isolation and considerable medical morbidity.4
Pain plays an important role in depression, particularly in older adults. Chronic pain affects up to 65% of older adults who live in the community and up to 80% of those who are institutionalized.5 The most common causes of pain in these patients are osteoarthritis, osteoporosis, fibromyalgia, degenerative disk disease, lumbar spinal stenosis, and scoliosis. In addition, neuropathic pain, such as post-herpetic neuralgia and peripheral neuropathy, and injuries resulting from falls often cause long-lasting pain.6
The presence of pain tends to negatively affect recognizing and treating depression. Regardless of their age, when a patient presents with pain or depression, investigate and consider treating both conditions.7
Memory decline is likely to be depressed older adults’ chief complaint, and when objectively tested these patients often show cognitive impairment.8 Whether depressive symptoms in this age group are a reaction to early cognitive deficits or are an early symptom of neurodegeneration remains controversial.9 Some case-control studies have found a link between a history of depression and Alzheimer’s disease (AD).10,11 In general, older patients whose first episode of depression occurs in late life have a higher relative risk of developing some form of dementia; research suggests that 50% of late-life MDD patients will develop dementia within 5 years.12
Researchers have considered the possibility that mild cognitive impairment (MCI) and dementia are a continuum of depression. In 1 study, 29 patients with MCI and 31 with MCI and MDD were assessed annually for an average of 4.3 years.13 Thirty-six patients with MCI (60%) progressed to AD. Presence of depression at the time of MCI diagnosis did not predict conversion to AD but persistence of depression for 2 to 3 years and the presence of melancholic features were associated with higher risk for AD.
Alexopoulos et al14 proposed the “vascular hypothesis theory” that cerebrovascular disease can predispose patients, particularly older adults, to depressive symptoms (Table 2).14 Whether vascular depression is a subtype of MDD remains controversial.
Table 2
Features of vascular depression
| Onset after age 50 |
| Family history of mood disorders is less common |
| Apathy |
| Marked loss of interest in activities |
| Lack of insight |
| Executive dysfunction (problems with planning, organizing, sequencing, abstracting), impaired memory or speed of processing of information |
| History of hypertension, diabetes, or cardiovascular disease |
| May have a neurologic event such as stroke or transient ischemic attack |
| White or gray matter hyperintensities |
| Source: Reference 14 |
Course and prognosis
MDD has been characterized as a self-limited disease, with an average duration of 6 to 9 months. However, newer prospective studies suggest that a substantial number of patients recover more slowly or do not ever fully recover.15 Several factors, such as genetic/biologic vulnerability and psychosocial factors, influence the courses, prognosis, and risk of relapse/recurrence of MDD in all age groups.
Children and adolescents. The typical duration of a major depressive episode for clinically referred children and adolescents is 8 to 13 months.1,16 Approximately 90% of these patients’ major depressive episodes remit by 2 years, but up to 10% persist.1,16 Within 5 years of MDD onset, up to 70% of children and adolescents will experience a recurrence,17 a rate comparable to adults.
Anxiety disorders, panic disorders, phobias, substance abuse, conduct and oppositional disorders, and attention-deficit/hyperactivity disorder occur 2 to 6 times more frequently in children and adolescents with MDD.18,19 Children with MDD who have significant psychiatric and psychosocial comorbidity experience poorer outcomes.18
Older adults. Despite optimal treatment conditions, ≥50% of older patients fail to respond adequately to first-line antidepressant pharmacotherapy.20 Treatment-resistant MDD in older patients increases:
- nonadherence to treatment for comorbid medical disorders
- disability and cognitive impairment
- burden on caregivers
- risk for early mortality, including suicide.20
Differences in treatment
Although MDD often is recurrent, episodic, and in some patients chronic, in general earlier treatment and quicker response lead to better outcomes. A large, naturalistic German study of 795 inpatients with major depression found that early improvement (20% reduction in Hamilton Depression Rating Scale-21 score within the first 2 weeks) with antidepressant therapy may predict later response and remission.21
Regardless of a patient’s age, MDD treatment should begin with education. All patients should be involved in their treatment. Encourage patients to become familiar with their triggers and stressors, improve their coping skills, and adopt a healthy lifestyle, which includes a nutritious diet, frequent exercise, and adequate sleep. As maintenance treatment we recommend that patients participate in frequent socialization and activities (Table 3). Refer patients to self-help books, online help guides, and handouts from sources such as National Institute of Mental Health.22,23 Encourage patients to have patience and perseverance, and guide them through each step of recovery.
In addition to lifestyle modification, other treatment options for depression include pharmacotherapy, interpersonal psychotherapy, cognitive-behavioral therapy (CBT), and electroconvulsive therapy (ECT). All these modalities are effective for acute and maintenance treatment and should be considered when determining the best approach for each patient.
The effectiveness of antidepressants in general is comparable among and within classes.2 Base your initial selection on the patient’s previous response to antidepressants and the medication’s side effects profile and cost.
The benefits of exercise for all patients cannot be underestimated.24 Prescribe 20 to 30 minutes of daily exercise as part of recommended lifestyle changes. Writing “daily exercise” on a prescription pad can effectively remind patients that exercise needs to be taken as seriously as medication compliance.
Children and adolescents. For mild depression, supportive therapy seems to be as effective as CBT and medications.25 A randomized controlled trial of 439 depressed adolescents found that CBT plus fluoxetine conferred quicker benefit, but in the long run may not be any more efficacious than pharmacotherapy alone.25 Researchers also found that CBT plus fluoxetine was no more effective than pharmacotherapy alone for adolescents with moderate to severe depression.25
Older adults. Compared with younger patients, geriatric patients typically require lower antidepressant dosages to achieve a specific blood level, but the blood levels at which antidepressants are most effective appear to be similar.2 Older patients also may be more likely to relapse and less likely to achieve full response to antidepressants than younger patients.2 In older adults, amitriptyline, imipramine, and doxepin are not preferred because these agents may cause orthostatic hypotension and urinary retention.2 A depressed older adult who experiences weight loss might benefit from an antidepressant that improves appetite, such as mirtazapine.26 Some research suggests that maintenance antidepressant therapy in older patients experiencing a first-time episode of MDD should continue for up to 2 years.27
A meta-analysis of 12 studies of CBT in depressed adults age ≥60 with chronic pain found that CBT was effective at improving self-reported pain but had no significant effect on depressive symptoms, physical function, or medication use.6 ECT often is prescribed for depressed older adults because its safety and efficacy for these patients has been well documented.28 Other neuromodulation therapies include vagus nerve, repetitive transcranial magnetic stimulation, and deep brain stimulation, but none of these treatments have been extensively evaluated in older patients.
Table 3
Avoiding reoccurrence of depression—a ‘prescription’ for patients
| Take medication as prescribed until your doctor instructs you to stop |
| Eat 3 nutritious meals every day |
| Sleep 6 to 8 hours each night |
| Walk/exercise for 20 minutes every day |
| Relax and do breathing exercises as taught 3 times a day |
| Talk with a friend or family member each day |
| Develop a hobby |
| Remain active |
| See your doctor once a month |
Related Resource
- American Psychiatric Association. Treatment of patients with major depressive disorder. 3rd ed. www.psychiatryonline.com/pracGuide/pracGuideChapToc_7.aspx.
Drug Brand Names
- Amitriptyline • Elavil
- Doxepin • Aponal, Silenor
- Fluoxetine • Prozac
- Imipramine • Tofranil
- Mirtazapine • Remeron
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
Most clinical trials of major depressive disorder (MDD) have focused on diagnosis and treatment of adults, but many younger and older patients also suffer from this condition. The prevalence of MDD is estimated to be 2% in children and 6% in adolescents.1 Up to 25% of adults age >60 experience MDD, dysthymic disorder, or “minor” depression.2
Although diagnosis and treatment of depression is similar regardless of a patient’s age, younger and older patients may not exhibit typical depressive symptoms (Table 1).1,2 For example, older adults may be more likely to report a lack of emotions than depressed mood. Vigilance for these types of distinct clinical manifestations can improve early recognition and treatment. In addition, evidence suggests there are differences in MDD treatment for younger and older patients.
This article reviews common challenges in recognizing and treating MDD in children, adolescents, and older adults.
Table 1
Major depressive disorder: Age-related differences
| Children/adolescents | Adults | Older adults | |
|---|---|---|---|
| Prevalence | 2% in children; 6% in adolescents | 20% | 25% |
| Male-to-female ratio | 1:1 in children; 1:2 in adolescents | 1:2 | 1:2 |
| DSM-IV-TR criteria | Similar | Similar | Similar |
| Clinical features | Irritability, temper tantrums, somatic complaints, hypersomina, weight gain, auditory hallucinations, psychomotor agitation, separation anxiety, social phobia, panic disorder, drug abuse, poor self-esteem | Typical DSM-IV-TR features. Psychomotor retardation, middle and terminal insomnia | Irritability, motor agitation, restlessness, somatic complaints, diarrhea and constipation, decreased libido, cognitive impairment, delusions, anxiety, panic, worsening of medical comorbidities |
| Source: References 1,2 | |||
Varying clinical features
Children/adolescents. The clinical presentation of MDD in children and adolescents is similar to that of adults. Children usually display anxiety, irritability, temper tantrums, and somatic complaints before verbally expressing depressive feelings. Psychotic depression in children manifests more often as auditory hallucinations than delusions.1
Younger vs middle-age adults. Researchers who evaluated baseline clinical and sociodemographic information of 1,498 patients enrolled in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study found that the presentation of depressive symptoms in young adult patients (age 18 to 35) differed from those of middle-age (age 36 to 50) patients.3 Younger patients were more likely to be irritable, complain of weight gain and hypersomnia, and have a negative view of life and the future. They also were more likely to report previous suicide attempts and endorse symptoms consistent with generalized anxiety disorder, social phobia, panic disorder, and drug abuse. Middle-age patients had more depressive episodes, deceased libido, and middle insomnia, and more frequently reported gastrointestinal symptoms such as diarrhea or constipation.3
Older adults. In our experience, typical MDD mood symptoms often are absent in older patients. Frequently, we see somatic complaints, motor restlessness, or psychomotor retardation; these symptoms may be attributable to a concurrent medical illness. This in turn may worsen the physical illness, leading to social isolation and considerable medical morbidity.4
Pain plays an important role in depression, particularly in older adults. Chronic pain affects up to 65% of older adults who live in the community and up to 80% of those who are institutionalized.5 The most common causes of pain in these patients are osteoarthritis, osteoporosis, fibromyalgia, degenerative disk disease, lumbar spinal stenosis, and scoliosis. In addition, neuropathic pain, such as post-herpetic neuralgia and peripheral neuropathy, and injuries resulting from falls often cause long-lasting pain.6
The presence of pain tends to negatively affect recognizing and treating depression. Regardless of their age, when a patient presents with pain or depression, investigate and consider treating both conditions.7
Memory decline is likely to be depressed older adults’ chief complaint, and when objectively tested these patients often show cognitive impairment.8 Whether depressive symptoms in this age group are a reaction to early cognitive deficits or are an early symptom of neurodegeneration remains controversial.9 Some case-control studies have found a link between a history of depression and Alzheimer’s disease (AD).10,11 In general, older patients whose first episode of depression occurs in late life have a higher relative risk of developing some form of dementia; research suggests that 50% of late-life MDD patients will develop dementia within 5 years.12
Researchers have considered the possibility that mild cognitive impairment (MCI) and dementia are a continuum of depression. In 1 study, 29 patients with MCI and 31 with MCI and MDD were assessed annually for an average of 4.3 years.13 Thirty-six patients with MCI (60%) progressed to AD. Presence of depression at the time of MCI diagnosis did not predict conversion to AD but persistence of depression for 2 to 3 years and the presence of melancholic features were associated with higher risk for AD.
Alexopoulos et al14 proposed the “vascular hypothesis theory” that cerebrovascular disease can predispose patients, particularly older adults, to depressive symptoms (Table 2).14 Whether vascular depression is a subtype of MDD remains controversial.
Table 2
Features of vascular depression
| Onset after age 50 |
| Family history of mood disorders is less common |
| Apathy |
| Marked loss of interest in activities |
| Lack of insight |
| Executive dysfunction (problems with planning, organizing, sequencing, abstracting), impaired memory or speed of processing of information |
| History of hypertension, diabetes, or cardiovascular disease |
| May have a neurologic event such as stroke or transient ischemic attack |
| White or gray matter hyperintensities |
| Source: Reference 14 |
Course and prognosis
MDD has been characterized as a self-limited disease, with an average duration of 6 to 9 months. However, newer prospective studies suggest that a substantial number of patients recover more slowly or do not ever fully recover.15 Several factors, such as genetic/biologic vulnerability and psychosocial factors, influence the courses, prognosis, and risk of relapse/recurrence of MDD in all age groups.
Children and adolescents. The typical duration of a major depressive episode for clinically referred children and adolescents is 8 to 13 months.1,16 Approximately 90% of these patients’ major depressive episodes remit by 2 years, but up to 10% persist.1,16 Within 5 years of MDD onset, up to 70% of children and adolescents will experience a recurrence,17 a rate comparable to adults.
Anxiety disorders, panic disorders, phobias, substance abuse, conduct and oppositional disorders, and attention-deficit/hyperactivity disorder occur 2 to 6 times more frequently in children and adolescents with MDD.18,19 Children with MDD who have significant psychiatric and psychosocial comorbidity experience poorer outcomes.18
Older adults. Despite optimal treatment conditions, ≥50% of older patients fail to respond adequately to first-line antidepressant pharmacotherapy.20 Treatment-resistant MDD in older patients increases:
- nonadherence to treatment for comorbid medical disorders
- disability and cognitive impairment
- burden on caregivers
- risk for early mortality, including suicide.20
Differences in treatment
Although MDD often is recurrent, episodic, and in some patients chronic, in general earlier treatment and quicker response lead to better outcomes. A large, naturalistic German study of 795 inpatients with major depression found that early improvement (20% reduction in Hamilton Depression Rating Scale-21 score within the first 2 weeks) with antidepressant therapy may predict later response and remission.21
Regardless of a patient’s age, MDD treatment should begin with education. All patients should be involved in their treatment. Encourage patients to become familiar with their triggers and stressors, improve their coping skills, and adopt a healthy lifestyle, which includes a nutritious diet, frequent exercise, and adequate sleep. As maintenance treatment we recommend that patients participate in frequent socialization and activities (Table 3). Refer patients to self-help books, online help guides, and handouts from sources such as National Institute of Mental Health.22,23 Encourage patients to have patience and perseverance, and guide them through each step of recovery.
In addition to lifestyle modification, other treatment options for depression include pharmacotherapy, interpersonal psychotherapy, cognitive-behavioral therapy (CBT), and electroconvulsive therapy (ECT). All these modalities are effective for acute and maintenance treatment and should be considered when determining the best approach for each patient.
The effectiveness of antidepressants in general is comparable among and within classes.2 Base your initial selection on the patient’s previous response to antidepressants and the medication’s side effects profile and cost.
The benefits of exercise for all patients cannot be underestimated.24 Prescribe 20 to 30 minutes of daily exercise as part of recommended lifestyle changes. Writing “daily exercise” on a prescription pad can effectively remind patients that exercise needs to be taken as seriously as medication compliance.
Children and adolescents. For mild depression, supportive therapy seems to be as effective as CBT and medications.25 A randomized controlled trial of 439 depressed adolescents found that CBT plus fluoxetine conferred quicker benefit, but in the long run may not be any more efficacious than pharmacotherapy alone.25 Researchers also found that CBT plus fluoxetine was no more effective than pharmacotherapy alone for adolescents with moderate to severe depression.25
Older adults. Compared with younger patients, geriatric patients typically require lower antidepressant dosages to achieve a specific blood level, but the blood levels at which antidepressants are most effective appear to be similar.2 Older patients also may be more likely to relapse and less likely to achieve full response to antidepressants than younger patients.2 In older adults, amitriptyline, imipramine, and doxepin are not preferred because these agents may cause orthostatic hypotension and urinary retention.2 A depressed older adult who experiences weight loss might benefit from an antidepressant that improves appetite, such as mirtazapine.26 Some research suggests that maintenance antidepressant therapy in older patients experiencing a first-time episode of MDD should continue for up to 2 years.27
A meta-analysis of 12 studies of CBT in depressed adults age ≥60 with chronic pain found that CBT was effective at improving self-reported pain but had no significant effect on depressive symptoms, physical function, or medication use.6 ECT often is prescribed for depressed older adults because its safety and efficacy for these patients has been well documented.28 Other neuromodulation therapies include vagus nerve, repetitive transcranial magnetic stimulation, and deep brain stimulation, but none of these treatments have been extensively evaluated in older patients.
Table 3
Avoiding reoccurrence of depression—a ‘prescription’ for patients
| Take medication as prescribed until your doctor instructs you to stop |
| Eat 3 nutritious meals every day |
| Sleep 6 to 8 hours each night |
| Walk/exercise for 20 minutes every day |
| Relax and do breathing exercises as taught 3 times a day |
| Talk with a friend or family member each day |
| Develop a hobby |
| Remain active |
| See your doctor once a month |
Related Resource
- American Psychiatric Association. Treatment of patients with major depressive disorder. 3rd ed. www.psychiatryonline.com/pracGuide/pracGuideChapToc_7.aspx.
Drug Brand Names
- Amitriptyline • Elavil
- Doxepin • Aponal, Silenor
- Fluoxetine • Prozac
- Imipramine • Tofranil
- Mirtazapine • Remeron
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Birmaher B, Brent D. AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(11):1503-1526.
2. American Psychiatric Association. Treatment of patients with major depressive disorder. 3rd ed. Available at: http://www.psychiatryonline.com/pracGuide/pracGuideChapToc_7.aspx. Accessed April 4 2011.
3. Husain MM, Rush AJ, Sakheim HA. Age-related characteristics of major depression: a preliminary STAR*D report. Am J Geriatr Psychiatry. 2005;13(10):852-862.
4. Gonda X, Molnár E, Torzsa P, et al. Characteristics of depression in the elderly. Psychiatr Hung. 2009;24(3):166-174.
5. Gibson, SJ. Pain and aging: a comparison of the pain experience over the adult life span. In: Dostrovsky JO, Carr DB, Koltzenburg M, eds. Proceedings of the 10th World Congress on Pain. Progress in pain research and management. Seattle, WA: IASP Press; 2003:767–790.
6. Lunde LH, Nordhus IH, Pallesen H. The effectiveness of cognitive and behavioural treatment of chronic pain in the elderly: a quantitative review. J Clin Psychol Med Settings. 2009;16:254-262.
7. Bair MJ, Robinson RL, Katon W, et al. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433-2445.
8. Reid LM, Machullich AM. Subjective memory complaints and cognitive impairment in older people. Dement Geriatr Cogn Disord. 2006;22(5-6):471-485.
9. Tsuno N, Homma A. What is the association between depression and Alzheimer’s disease? Expert Rev Neurother. 2009;9(11):]1667-1676.
10. Tsolaki M, Fountalakis K, Chantzi E, et al. Risk factors for clinically diagnosed Alzheimer’s disease: a case-control study of a Greek population. Int Psychogeriatr. 1997;9(3):327-341.
11. Speck CE, Kukull WA, Brenner DE, et al. History of depression as a risk factor for Alzheimer’s disease. Epidemiology. 1995;6(4):366-369.
12. Alexopoulos GS. Depression in elderly. Lancet. 2005;365:1961-1970.
13. Houde M, Bergman H, Whitehead V, et al. Predictive depression pattern in mild cognitive impairment. Int J Geriatr Psychiatry. 2008;23:1028-1033.
14. Alexopoulos GS, Meyers BS, Young RC, et al. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997;54(10):915-922.
15. Angst J, Gamma A, Rossler W, et al. Long-term depression versus episodic major depression: results from the prospective Zurich study of a community sample. J Affect Disord. 2009;115(1-2):112-121.
16. Birmaher B, Arbelaez C, Brent D. Course and outcome of child and adolescent major depressive disorder. Child Adolesc Psychiatr Clin N Am. 2002;11(3):619-637 x.
17. Rao U, Ryan ND, Birmaher B, et al. Unipolar depression in adolescents: clinical outcome in adulthood. J Am Acad Child Adolesc Psychiatry. 1995;34:566-578.
18. Angold A, Costello EJ, Erkanli A. Comorbidity. J Child Psych Psychiatry. 1999;40(1):57-87.
19. Costello EJ, Pine DS, Hammen C, et al. Development and natural history of mood disorders. Biol Psychiatry. 2002;52(6):529-542.
20. Lenze EJ, Sheffrin M, Driscoll HC, et al. Incomplete response in late-life depression: getting to remission. Dialogues Clin Neurosci. 2008;10(4):419-430.
21. Henkel V, Seemuller F, Obermeier M, et al. Does early improvement triggered by antidepressant predict response/remission? Analysis of data from a naturalistic study on a large sample of inpatients with major depression. J Affect Disord. 2009;115(3):439-449.
22. National Institute of Mental Health. Depression. NIH publication No. 083561. 2008. Available at: http://www.nimh.nih.gov/health/publications/depression/complete-index.shtml. Accessed April 4 2011.
23. Helpguide.org. Depression. Available at: http://www.helpguide.org/topics/depression.htm. Accessed April 4, 2011.
24. Sidhu K, Vandana P, Balon R. Exercise prescription. A practical and effective therapy for depression. Current Psychiatry. 2009;8(6):39-51.
25. Curry J, Rohde P, Simons A, et al. Predictors and moderators of acute outcome in the Treatment for Adolescents with Depression Study (TADS). J Am Acad Child Adolesc Psychiatry. 2006;45(12):1427-1439.
26. Rajii TK, Mulsant BH, Lotrich FE, et al. Use of antidepressant in late-life depression. Drugs Aging. 2008;25(10):841-853.
27. Rush AJ, Trivedi MH, Weiniewski SR, et al. Acute and longer term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917.
28. Van der Wurff FB, Stek ML, Hoogendijk WJ. The efficacy and safety of ECT in depressed older adults: a literature review. Int J Geriatr Psychiatry. 2003;18(10):894-904.
1. Birmaher B, Brent D. AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(11):1503-1526.
2. American Psychiatric Association. Treatment of patients with major depressive disorder. 3rd ed. Available at: http://www.psychiatryonline.com/pracGuide/pracGuideChapToc_7.aspx. Accessed April 4 2011.
3. Husain MM, Rush AJ, Sakheim HA. Age-related characteristics of major depression: a preliminary STAR*D report. Am J Geriatr Psychiatry. 2005;13(10):852-862.
4. Gonda X, Molnár E, Torzsa P, et al. Characteristics of depression in the elderly. Psychiatr Hung. 2009;24(3):166-174.
5. Gibson, SJ. Pain and aging: a comparison of the pain experience over the adult life span. In: Dostrovsky JO, Carr DB, Koltzenburg M, eds. Proceedings of the 10th World Congress on Pain. Progress in pain research and management. Seattle, WA: IASP Press; 2003:767–790.
6. Lunde LH, Nordhus IH, Pallesen H. The effectiveness of cognitive and behavioural treatment of chronic pain in the elderly: a quantitative review. J Clin Psychol Med Settings. 2009;16:254-262.
7. Bair MJ, Robinson RL, Katon W, et al. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433-2445.
8. Reid LM, Machullich AM. Subjective memory complaints and cognitive impairment in older people. Dement Geriatr Cogn Disord. 2006;22(5-6):471-485.
9. Tsuno N, Homma A. What is the association between depression and Alzheimer’s disease? Expert Rev Neurother. 2009;9(11):]1667-1676.
10. Tsolaki M, Fountalakis K, Chantzi E, et al. Risk factors for clinically diagnosed Alzheimer’s disease: a case-control study of a Greek population. Int Psychogeriatr. 1997;9(3):327-341.
11. Speck CE, Kukull WA, Brenner DE, et al. History of depression as a risk factor for Alzheimer’s disease. Epidemiology. 1995;6(4):366-369.
12. Alexopoulos GS. Depression in elderly. Lancet. 2005;365:1961-1970.
13. Houde M, Bergman H, Whitehead V, et al. Predictive depression pattern in mild cognitive impairment. Int J Geriatr Psychiatry. 2008;23:1028-1033.
14. Alexopoulos GS, Meyers BS, Young RC, et al. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997;54(10):915-922.
15. Angst J, Gamma A, Rossler W, et al. Long-term depression versus episodic major depression: results from the prospective Zurich study of a community sample. J Affect Disord. 2009;115(1-2):112-121.
16. Birmaher B, Arbelaez C, Brent D. Course and outcome of child and adolescent major depressive disorder. Child Adolesc Psychiatr Clin N Am. 2002;11(3):619-637 x.
17. Rao U, Ryan ND, Birmaher B, et al. Unipolar depression in adolescents: clinical outcome in adulthood. J Am Acad Child Adolesc Psychiatry. 1995;34:566-578.
18. Angold A, Costello EJ, Erkanli A. Comorbidity. J Child Psych Psychiatry. 1999;40(1):57-87.
19. Costello EJ, Pine DS, Hammen C, et al. Development and natural history of mood disorders. Biol Psychiatry. 2002;52(6):529-542.
20. Lenze EJ, Sheffrin M, Driscoll HC, et al. Incomplete response in late-life depression: getting to remission. Dialogues Clin Neurosci. 2008;10(4):419-430.
21. Henkel V, Seemuller F, Obermeier M, et al. Does early improvement triggered by antidepressant predict response/remission? Analysis of data from a naturalistic study on a large sample of inpatients with major depression. J Affect Disord. 2009;115(3):439-449.
22. National Institute of Mental Health. Depression. NIH publication No. 083561. 2008. Available at: http://www.nimh.nih.gov/health/publications/depression/complete-index.shtml. Accessed April 4 2011.
23. Helpguide.org. Depression. Available at: http://www.helpguide.org/topics/depression.htm. Accessed April 4, 2011.
24. Sidhu K, Vandana P, Balon R. Exercise prescription. A practical and effective therapy for depression. Current Psychiatry. 2009;8(6):39-51.
25. Curry J, Rohde P, Simons A, et al. Predictors and moderators of acute outcome in the Treatment for Adolescents with Depression Study (TADS). J Am Acad Child Adolesc Psychiatry. 2006;45(12):1427-1439.
26. Rajii TK, Mulsant BH, Lotrich FE, et al. Use of antidepressant in late-life depression. Drugs Aging. 2008;25(10):841-853.
27. Rush AJ, Trivedi MH, Weiniewski SR, et al. Acute and longer term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917.
28. Van der Wurff FB, Stek ML, Hoogendijk WJ. The efficacy and safety of ECT in depressed older adults: a literature review. Int J Geriatr Psychiatry. 2003;18(10):894-904.
Treatment-resistant schizophrenia: What can we do about it?
Discuss this article at www.facebook.com/CurrentPsychiatry
Patients with treatment-resistant schizophrenia can be broadly defined to include any persons with residual symptoms that cause distress or impairment despite several treatment attempts. Unfortunately, this definition may include most of our patients with schizophrenia.
Clinical trial data on treatment-resistant schizophrenia can be contradictory, leaving “N of 1” empirical treatment trials for individual patients as the current state of the art. This article presents data from clinical trials for pharmacologic and nonpharmacologic options and offers recommendations to try to help our treatment-resistant patients.
Defining treatment resistance
Research reports regarding treatment-resistant or treatment-refractory schizophrenia have relied on operational criteria such as that found in the pivotal study for clozapine1:
- at least 3 periods of treatment in the preceding 5 years with neuroleptic agents from at least 2 different chemical classes at dosages equivalent to ≥1000 mg/d of chlorpromazine for 6 weeks, each without significant symptomatic relief, and
- no period of good functioning within the preceding 5 years.1 In that study, patients also underwent a prospective treatment trial with what we now know are high doses of haloperidol (up to 60 mg/d or higher) and benztropine mesylate (6 mg/d) for a period of 6 weeks to confirm lack of drug responsiveness. Other studies have more relaxed criteria, such as:
- persistent positive symptoms—hallucinations, delusions, or marked thought disorder—after at least 6 contiguous weeks of past or present treatment, with ≥1 typical antipsychotics at doses of ≥600 mg/d in chlorpromazine equivalents
- a poor level of functioning over the past 2 years, as defined by the lack of competitive employment or enrollment in an academic or vocational program and not having age-expected interpersonal relations with someone outside the biologic family with whom ongoing regular contacts were maintained.2
In this study, no prospective period of treatment to confirm lack of drug responsiveness was required.
The most clinically relevant definition of treatment resistance depends on the patient’s individual circumstances. For some patients, targeting positive symptoms is a high priority; for others it may be negative and cognitive symptoms; for others, it may be excitement. Moreover, families may complain of symptoms or behavior that are of little or no concern to your patient.
Although we desire treatment response and remission for our patients, definitions for remission and functional recovery are in flux. Proposed criteria define symptomatic remission as 6-month maintenance of simultaneous ratings of mild or less on delusions, hallucinations, disorganized speech, grossly disorganized or catatonic behavior, and negative symptoms.3,4 Emsley et al4 note that reported remission rates vary widely across studies (17% to 88%) and that patients in remission do better than their non-remitted counterparts in several other outcome domains. Also, patients move in and out of remission over time. Predictors of remission include:
- early treatment response
- baseline symptom severity
- subjective well-being.4
Recovery is a more complex construct than remission and includes social outcomes. Although recovery lacks a standard definition, it is the implied goal of treatment. Anything short of recovery can be viewed as inadequate. If we set the bar at this height, many or most of the patients we treat for schizophrenia could be considered treatment-resistant.
Confounding factors
Before concluding that a patient is treatment-resistant, address medication adherence and possible substance use. Partial or nonadherence with antipsychotic treatment is common—approximately one-half of patients are nonadherent5—and associated with relapse and re-hospitalization.6 In addition, an estimated one-half of all individuals with schizophrenia also use substances.7
Be aware of the optimal dose for any particular antipsychotic and factors that can interfere with achieving adequate plasma levels. This means acknowledging that dosing ranges established during registration studies may not reflect the needs of day-to-day clinical practice.8 Pharmacokinetic interactions with other medications, such as carbamazepine or rifampin, can induce liver enzymes and result in subtherapeutic antipsychotic levels. Cigarette smoking also may have this effect. Lowered clozapine or olanzapine plasma levels have been observed in patients who resume smoking after being discharged from a non-smoking inpatient environment. Some antipsychotics, such as ziprasidone and lurasidone, must be taken with food in order to have sufficient bioavailability.9
What does a patient want?
Patients with schizophrenia often have limited insight into their psychotic symptoms.10 Savvy clinicians will attempt to leverage a patient’s insight into ancillary symptoms—such as impaired sleep, anxiety, and dysphoria—to encourage a therapeutic alliance and therefore adherence. If patients feel their concerns are not addressed, they may consider treatment inadequate even though the intensity of their hallucinations and delusions may have decreased.
Which antipsychotic is best?
Meta-analyses of randomized controlled trials (RCTs) of antipsychotic treatment for schizophrenia found that, although individual response will vary, clozapine generally has better efficacy that other antipsychotics.11-13 Olanzapine, risperidone, and amisulpride (which is not available in the United States) appear to be more efficacious than first-generation antipsychotics. Other second-generation antipsychotics do not consistently show greater efficacy than first-generation antipsychotics, although their tolerability profiles vary greatly.11-13
Antipsychotic monotherapy. More than 25 RCTs have focused on antipsychotic monotherapy for treatment-resistant patients; for a bibliography of these studies, click here. For the most part, clozapine has consistently demonstrated superiority over comparators. Because not all patients with schizophrenia can tolerate clozapine or are willing to have their blood monitored as required, other second-generation antipsychotics have been suggested as possible substitutes. Olanzapine has established superior efficacy to first-generation antipsychotics11-13 and perhaps comparable efficacy to clozapine in some studies.2,14-17 Risperidone appeared to be comparable to clozapine in some studies,18,19 whereas clozapine’s superiority was evident in others.14,20,21 Although an RCT found comparable efficacy for ziprasidone vs clozapine,22 patients enrolled in this study may not have been treatment-resistant regarding efficacy but instead could not tolerate prior treatments. Enrolling patients on the basis of poor efficacy and/or poor tolerability to their prior antipsychotic regimen also has complicated the interpretation of studies comparing olanzapine with clozapine16 and risperidone with clozapine.18
Antipsychotic combinations. Combinations of antipsychotics are used commonly when treating chronic schizophrenia.23 Of the approximately 20 RCTs of antipsychotic combination therapy, most tested clozapine combined with other second-generation antipsychotics, such as risperidone. For a bibliography of these studies, click here. Only 5 studies support a combination approach (Table 1).
Table 1
Antipsychotic combinations: Few studies support efficacy
| Study | Design | Patients | Results |
|---|---|---|---|
| Shiloh et al, 1997a | 10-week, double-blind, placebo-controlled | 28 patients nonresponsive to typical antipsychotics and partially responsive to clozapine received add-on sulpiride,* 600 mg/d, or placebo | The sulpiride group showed improvements in positive and negative symptoms |
| Josiassen et al, 2005b | 12-week, randomized, double-blind, placebo-controlled | 40 schizophrenia patients unresponsive or partially responsive to clozapine randomized to clozapine + placebo or clozapine + risperidone, 6 mg/d | Mean BPRS total and positive symptom subscale scores reduced in both groups but reductions were greater in the clozapine/risperidone group; reduction in SANS also was observed in the clozapine/risperidone group |
| Genç et al, 2007c | 8-week, randomized, single-blind | 56 treatment-resistant schizophrenia patients randomly assigned to clozapine + amisulpride* or clozapine + quetiapine | Both groups improved at week 8 as measured by BPRS, SANS, SAPS, and CGI; however, patients receiving amisulpride showed greater improvement |
| Muscatello et al, 2011d | 24-week, randomized, double-blind, placebo-controlled | 31 treatment-resistant schizophrenia patients receiving clozapine randomized to receive adjunctive aripiprazole or placebo | Aripiprazole showed beneficial effect on positive and general psychopathologic symptomatology, but no significant effects on executive cognitive function |
| Takahashi et al, 1999e | 8-week, randomized, single-blind, crossover | 10 neuroleptic-treated patients received add-on risperidone and mosapramine* | Both additions resulted in significant, yet modest, improvement; no significant difference in PANSS between risperidone and mosapramine |
| *Not available in the United States BPRS: Brief Psychiatric Rating Scale; CGI: Clinical Global Impression; PANSS: Positive and Negative Syndrome Scale; SANS: Scale for the Assessment of Negative Symptoms; SAPS: Scale for the Assessment of Positive Symptoms | |||
| Source: References a. Shiloh R, Zemishlany Z, Aizenberg D, et al. Sulpiride augmentation in people with schizophrenia partially responsive to clozapine. A double-blind, placebo-controlled study. Br J Psychiatry. 1997;171:569-573. b. Josiassen RC, Joseph A, Kohegyi E, et al. Clozapine augmented with risperidone in the treatment of schizophrenia: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2005;162(1):130-136. c. Genç Y, Taner E, Candansayar S. Comparison of clozapine-amisulpride and clozapine-quetiapine combinations for patients with schizophrenia who are partially responsive to clozapine: a single-blind randomized study. Adv Ther. 2007;24(1):1-13. d. Muscatello MR, Bruno A, Pandolfo G, et al. Effect of aripiprazole augmentation of clozapine in schizophrenia: a double-blind, placebo-controlled study. Schizophr Res. 2011;127(1-3):93-99. e. Takahashi N, Terao T, Oga T, et al. Comparison of risperidone and mosapramine addition to neuroleptic treatment in chronic schizophrenia. Neuropsychobiology. 1999;39(2):81-85. | |||
What about augmentation?
Adjunctive non-antipsychotics also are commonly used when treating patients with chronic schizophrenia. For example, lithium and anticonvulsants are used in approximately one-half of all inpatients with schizophrenia in facilities operated by the State of New York Office of Mental Health.24,25 The evidence base for these agents as adjuncts to antipsychotics generally is weak.26 Specifically, early reports of benefit with adjunctive lithium have been negated by later studies. Similarly, large trials of adjunctive valproate and lamotrigine have failed to replicate early and promising efficacy signals from smaller trials, although the larger studies did not specifically target treatment-resistant schizophrenia.
Among mood stabilizers, lamotrigine may be the most promising for treatment-resistant schizophrenia. In a meta-analysis of clinical trials examining schizophrenia patients receiving clozapine (N=161) who were randomized to receive adjunctive lamotrigine or adjunctive placebo, lamotrigine was superior to placebo in total score for psychosis symptoms and scores for positive and negative symptoms.27
More than 125 published RCTs have studied a wide variety of adjunctive agents other than lithium or anticonvulsants for treating persistent schizophrenia symptoms (Table 2).
Only some of the approximately 40 RCTs regarding adjunctive antidepressants in patients with chronic schizophrenia focused on patients with ongoing depressive symptoms. For a bibliography of these studies, click here. In a meta-analysis measuring improvement of negative symptoms from 23 trials (N=819),28 the effect size was moderate in favor of antidepressants. Subgroup analysis revealed significant responses for fluoxetine, trazodone, and ritanserin.
More than 50 RCTs have focused on augmenting medications for cognitive dysfunction in chronic schizophrenia. Unfortunately, agents used to treat Alzheimer’s disease have shown disappointing results when tested in patients with schizophrenia, as have agents prescribed for attention-deficit/hyperactivity disorder (methylphenidate, guanfacine, atomoxetine) or agents used to promote alertness (modafinil and armodafinil).
Medications that act on glutamate receptors may offer another potential solution, although not in combination with clozapine.29
Other agents that require further study where ≥2 positive studies have been reported (with ≤2 negative studies) include celecoxib, neurosteroids and hormones, purinergic agents, serotonin 5-HT1A receptor agonists, and serotonin 5-HT3 receptor antagonists.
Table 2
Agents studied as adjuncts to antipsychotics
| Acetylsalicylic acid and nonsteroidal anti-inflammatory agents |
| Anticonvulsants and lithium |
| Antidepressants |
| Antiglucocorticoids |
| Agents used to treat attention-deficit/hyperactivity disorder |
| Beta blockers |
| Cholinesterase inhibitors and other agents used to treat Alzheimer’s disease |
| Experimental agents that act on glutamate receptors |
| GABAA receptor drugs |
| Neurosteroids and hormones |
| Omega-3 fatty acids |
| Opioid system agents |
| Peptides |
| Purinergic agents |
| Serotonin 5-HT1A receptor agonists |
| Serotonin 5-HT3 receptor antagonists |
| Wakefulness promoting agents |
Therapeutic neuromodulation
More than 10 RCTs of repetitive transcranial magnetic stimulation (rTMS) in patients with refractory symptoms of schizophrenia have been published; the results were mixed. For a bibliography of these studies, click here. In a meta-analysis of 9 trials (n=213),30 prefrontal rTMS for treating negative symptoms demonstrated a small-to-medium effect size. In another meta-analysis31 of all prospective studies of rTMS for negative symptoms and for auditory hallucinations and overall positive symptoms in refractory schizophrenia, the effect sizes showed moderate effects.
Fewer controlled trials are available for electroconvulsive therapy,32,33 but its use with clozapine appears encouraging.34
Psychological and behavioral intervention. Cognitive-behavioral therapy, although labor-intensive, can be helpful even in patients considered treatment-resistant (Table 3). These interventions generally are provided together with pharmacotherapy.
Complementary and alternative therapies. Patients and their families may ask about complementary and alternative therapies, particularly when conventional approaches have not been successful. A meta-analysis of 6 studies (n=828)35 that reviewed adjunctive use of ginkgo in patients with chronic schizophrenia found statistically significant moderate improvement in total and negative symptoms. Negative reports also are available, including a 5-month study of adjunctive megavitamins that did not demonstrate any benefits.36 In a review of 13 RCTs of acupuncture for schizophrenia, Lee et al found the overall methodological quality was too low to draw firm conclusions.37
Table 3
Cognitive-behavioral therapy for schizophrenia
| Study | Design | Patients | Results |
|---|---|---|---|
| Pinto et al, 1999a | 6-month, randomized controlled | 37 treatment-resistant schizophrenia patients were randomized to CBT plus social skills training or supportive therapy | Both groups showed statistically significant improvement on the BPRS, SAPS, and SANS; however, patients in the CBT group had lower BPRS and SAPS scores. No difference on SANS scores |
| Barretto et al, 2009b | 21-week, controlled (nonrandom-ized) | Patients refractory to clozapine were placed in a CBT or befriending control group | The CBT group showed significant improvement in PANSS total score and general psychopathology subscale score, as well as an improvement of QLS; improvement persisted at 6-month follow-up |
| BPRS: Brief Psychiatric Rating Scale; CBT: cognitive-behavioral therapy; PANSS: Positive and Negative Syndrome Scale; QLS: Quality of Life Scale; SANS: Scale for the Assessment of Negative Symptoms; SAPS: Scale for the Assessment of Positive Symptoms | |||
| Source: References a. Pinto A, La Pia S, Mennella R, et al. Cognitive-behavioral therapy and clozapine for clients with treatment-refractory schizophrenia. Psychiatr Serv. 1999;50(7):901-904. b. Barretto EM, Kayo M, Avrichir BS, et al. A preliminary controlled trial of cognitive behavioral therapy in clozapine-resistant schizophrenia. J Nerv Ment Dis. 2009;197(11):865-868. | |||
Clinical recommendations
Before declaring a patient with schizophrenia as treatment-resistant, ensure that an adequate trial of medication did take place. This includes consideration of adequate dosing and pharmacokinetic issues. Awareness of potential substance use and/or partial adherence or nonadherence also is critical because these factors can impact treatment response.
When prescribing for a treatment-resistant schizophrenia patient, identify specific target symptoms to better inform medication selection—especially for symptoms that the patient feels are important. For example, consider an antidepressant for patients who have negative or depressive symptoms. Also take into account other patient-centered concerns, such as tolerability issues that may have interfered with adherence and response in the past.
Clozapine remains the medication of choice for treatment-resistant schizophrenia. Despite dozens of RCTs of potential adjunctive agents for treatment-resistant schizophrenia, no single approach has consistently shown efficacy in reducing symptoms, improving cognition, or increasing a patient’s level of function. Individual response can vary, and our search for the “outlier” who does respond to an adjunctive agent can explain our use of these strategies in clinical practice.
Related Resources
- Cochrane Database of Systematic Reviews. www.cochrane.org/reviews. This database contains reviews of additional therapeutic options for patients with treatment-resistant schizophrenia. As of February 23, 2011, 157 reviews were available.
- Citrome L. Treatment-refractory schizophrenia: What it is and what’s been done about it. Neuropsychiatry. 2011. Epub ahead of print.
- Citrome L. Clozapine for schizophrenia. Life-threatening or life-saving treatment? Current Psychiatry. 2009;8(12):56-63.
Drug Brand Names
- Aripiprazole • Abilify
- Armodafinil • Nuvigil
- Atomoxetine • Strattera
- Benztropine mesylate • Cogentin
- Carbamazepine • Tegretol
- Celecoxib • Celebrex
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Fluoxetine • Prozac
- Guanfacine • Tenex
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid, others
- Lurasidone • Latuda
- Methylphenidate • Ritalin, Methylin, others
- Modafinil • Provigil
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Rifampin • Rifadin
- Risperidone • Risperdal
- Trazodone • Desyrel, Oleptro
- Valproate (Divalproex) • Depakote, Depakote ER
- Ziprasidone • Geodon
Disclosure
No writing assistance or external financial support was used for this article. Dr. Citrome is a consultant for, has received honoraria from, or has conducted clinical research supported by Abbott Laboratories, AstraZeneca Pharmaceuticals, Avanir Pharmaceuticals, Azur Pharma Inc., Barr Laboratories, Bristol-Myers Squibb, Eli Lilly and Company, Forest Research Institute, GlaxoSmithKline, Janssen Pharmaceuticals, Jazz Pharmaceuticals, Merck, Novartis, Pfizer Inc., Sunovion, Valeant Pharmaceuticals, and Vanda Pharmaceuticals.
1. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
2. Volavka J, Czobor P, Sheitman B, et al. Clozapine, olanzapine, risperidone, and haloperidol in the treatment of patients with chronic schizophrenia and schizoaffective disorder. Am J Psychiatry. 2002;159(2):255-262.
3. Andreasen NC, Carpenter WT Jr, Kane JM, et al. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry. 2005;162(3):441-449.
4. Emsley R, Chiliza B, Asmal L, et al. The concepts of remission and recovery in schizophrenia. Curr Opin Psychiatry. 2011;24(2):114-121.
5. Lacro JP, Dunn LB, Dolder CR, et al. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002;63(10):892-909.
6. Robinson DG, Woerner MG, Delman HM, et al. Pharmacological treatments for first-episode schizophrenia. Schizophr Bull. 2005;31(3):705-722.
7. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264(19):2511-2518.
8. Citrome L, Volavka J. Optimal dosing of atypical antipsychotics in adults: a review of the current evidence. Harv Rev Psychiatry. 2002;10(5):280-291.
9. Citrome L. Iloperidone asenapine and lurasidone. A brief overview of three new second-generation antipsychotics. Postgrad Med. 2011;123(2):153-162.
10. Lincoln TM, Lüllmann E, Rief W. Correlates and long-term consequences of poor insight in patients with schizophrenia. A systematic review. Schizophr Bull. 2007;33(6):1324-1342.
11. Leucht S, Corves C, Arbter D, et al. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373(9657):31-41.
12. Leucht S, Komossa K, Rummel-Kluge C, et al. A meta-analysis of head-to-head comparisons of second-generation antipsychotics in the treatment of schizophrenia. Am J Psychiatry. 2009;166(2):152-163.
13. Leucht S, Arbter D, Engel RR, et al. How effective are second-generation antipsychotic drugs? A meta-analysis of placebo-controlled trials. Mol Psychiatry. 2009;14(4):429-447.
14. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600-610.
15. Tollefson GD, Birkett MA, Kiesler GM, et al. Double-blind comparison of olanzapine versus clozapine in schizophrenic patients clinically eligible for treatment with clozapine. Biol Psychiatry. 2001;49(1):52-63.
16. Bitter I, Dossenbach MR, Brook S, et al. Olanzapine versus clozapine in treatment-resistant or treatment-intolerant schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(1):173-180.
17. Meltzer HY, Bobo WV, Roy A, et al. A randomized, double-blind comparison of clozapine and high-dose olanzapine in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2008;69(2):274-285.
18. Bondolfi G, Dufour H, Patris M, et al. Risperidone versus clozapine in treatment-resistant chronic schizophrenia: a randomized double-blind study. Am J Psychiatry. 1998;155(4):499-504.
19. Wahlbeck K, Cheine M, Tuisku K, et al. Risperidone versus clozapine in treatment-resistant schizophrenia: a randomized pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24(6):911-922.
20. Breier AF, Malhotra AK, Su TP, et al. Clozapine and risperidone in chronic schizophrenia: effects on symptoms, parkinsonian side effects, and neuroendocrine response. Am J Psychiatry. 1999;156(2):294-298.
21. Azorin JM, Spiegel R, Remington G, et al. A double-blind comparative study of clozapine and risperidone in the management of severe chronic schizophrenia. Am J Psychiatry. 2001;158(8):1305-1313.
22. Sacchetti E, Galluzzo A, Valsecchi P, et al. Ziprasidone vs clozapine in schizophrenia patients refractory to multiple antipsychotic treatments: the MOZART study. Schizophr Res. 2009;113(1):112-121.
23. Jaffe AB, Levine J. Antipsychotic medication coprescribing in a large state hospital system. Pharmacoepidemiol Drug Saf. 2003;12(1):41-48.
24. Citrome L, Levine J, Allingham B. Changes in use of valproate and other mood stabilizers for patients with schizophrenia from 1994 to 1998. Psychiatr Serv. 2000;51(5):634-638.
25. Citrome L, Jaffe A, Levine J, et al. Use of mood stabilizers among patients with schizophrenia, 1994-2001. Psychiatr Serv. 2002;53(10):1212.-
26. Citrome L. Adjunctive lithium and anticonvulsants for the treatment of schizophrenia: what is the evidence? Expert Rev Neurother. 2009;9(1):55-71.
27. Tiihonen J, Wahlbeck K, Kiviniemi V. The efficacy of lamotrigine in clozapine-resistant schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2009;109(1-3):10-14.
28. Singh SP, Singh V, Kar N, et al. Efficacy of antidepressants in treating the negative symptoms of chronic schizophrenia: meta-analysis. Br J Psychiatry. 2010;197(3):174-179.
29. Kantrowitz JT, Javitt DC. Thinking glutamatergically: changing concepts of schizophrenia based upon changing neurochemical models. Clin Schizophr Relat Psychoses. 2010;4(3):189-200.
30. Dlabac-de Lange JJ, Knegtering R, Aleman A, et al. Repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: review and meta-analysis. J Clin Psychiatry. 2010;71(4):411-418.
31. Freitas C, Fregni F, Pascual-Leone A. Meta-analysis of the effects of repetitive transcranial magnetic stimulation (rTMS) on negative and positive symptoms in schizophrenia. Schizophr Res. 2009;108(1-3):11-24.
32. Chanpattana W, Chakrabhand ML, Sackeim HA, et al. Continuation ECT in treatment-resistant schizophrenia: a controlled study. J ECT. 1999;15(3):178-192.
33. Goswami U, Kumar U, Singh B. Efficacy of electroconvulsive therapy in treatment resistant schizophrenia: a double-blind study. Indian J Psychiatry. 2003;45(1):26-29.
34. Braga RJ, Petrides G. The combined use of electroconvulsive therapy and antipsychotics in patients with schizophrenia. J ECT. 2005;21(2):75-83.
35. Singh V, Singh SP, Chan K. Review and meta-analysis of usage of ginkgo as an adjunct therapy in chronic schizophrenia. Int J Neuropsychopharmacol. 2010;13(2):257-271.
36. Vaughan K, McConaghy N. Megavitamin and dietary treatment in schizophrenia: a randomised controlled trial. Aust N Z J Psychiatry. 1999;33(1):84-88.
37. Lee MS, Shin BC, Ronan P, et al. Acupuncture for schizophrenia: a systematic review and meta-analysis. Int J Clin Pract. 2009;63(11):1622-1633.
Discuss this article at www.facebook.com/CurrentPsychiatry
Patients with treatment-resistant schizophrenia can be broadly defined to include any persons with residual symptoms that cause distress or impairment despite several treatment attempts. Unfortunately, this definition may include most of our patients with schizophrenia.
Clinical trial data on treatment-resistant schizophrenia can be contradictory, leaving “N of 1” empirical treatment trials for individual patients as the current state of the art. This article presents data from clinical trials for pharmacologic and nonpharmacologic options and offers recommendations to try to help our treatment-resistant patients.
Defining treatment resistance
Research reports regarding treatment-resistant or treatment-refractory schizophrenia have relied on operational criteria such as that found in the pivotal study for clozapine1:
- at least 3 periods of treatment in the preceding 5 years with neuroleptic agents from at least 2 different chemical classes at dosages equivalent to ≥1000 mg/d of chlorpromazine for 6 weeks, each without significant symptomatic relief, and
- no period of good functioning within the preceding 5 years.1 In that study, patients also underwent a prospective treatment trial with what we now know are high doses of haloperidol (up to 60 mg/d or higher) and benztropine mesylate (6 mg/d) for a period of 6 weeks to confirm lack of drug responsiveness. Other studies have more relaxed criteria, such as:
- persistent positive symptoms—hallucinations, delusions, or marked thought disorder—after at least 6 contiguous weeks of past or present treatment, with ≥1 typical antipsychotics at doses of ≥600 mg/d in chlorpromazine equivalents
- a poor level of functioning over the past 2 years, as defined by the lack of competitive employment or enrollment in an academic or vocational program and not having age-expected interpersonal relations with someone outside the biologic family with whom ongoing regular contacts were maintained.2
In this study, no prospective period of treatment to confirm lack of drug responsiveness was required.
The most clinically relevant definition of treatment resistance depends on the patient’s individual circumstances. For some patients, targeting positive symptoms is a high priority; for others it may be negative and cognitive symptoms; for others, it may be excitement. Moreover, families may complain of symptoms or behavior that are of little or no concern to your patient.
Although we desire treatment response and remission for our patients, definitions for remission and functional recovery are in flux. Proposed criteria define symptomatic remission as 6-month maintenance of simultaneous ratings of mild or less on delusions, hallucinations, disorganized speech, grossly disorganized or catatonic behavior, and negative symptoms.3,4 Emsley et al4 note that reported remission rates vary widely across studies (17% to 88%) and that patients in remission do better than their non-remitted counterparts in several other outcome domains. Also, patients move in and out of remission over time. Predictors of remission include:
- early treatment response
- baseline symptom severity
- subjective well-being.4
Recovery is a more complex construct than remission and includes social outcomes. Although recovery lacks a standard definition, it is the implied goal of treatment. Anything short of recovery can be viewed as inadequate. If we set the bar at this height, many or most of the patients we treat for schizophrenia could be considered treatment-resistant.
Confounding factors
Before concluding that a patient is treatment-resistant, address medication adherence and possible substance use. Partial or nonadherence with antipsychotic treatment is common—approximately one-half of patients are nonadherent5—and associated with relapse and re-hospitalization.6 In addition, an estimated one-half of all individuals with schizophrenia also use substances.7
Be aware of the optimal dose for any particular antipsychotic and factors that can interfere with achieving adequate plasma levels. This means acknowledging that dosing ranges established during registration studies may not reflect the needs of day-to-day clinical practice.8 Pharmacokinetic interactions with other medications, such as carbamazepine or rifampin, can induce liver enzymes and result in subtherapeutic antipsychotic levels. Cigarette smoking also may have this effect. Lowered clozapine or olanzapine plasma levels have been observed in patients who resume smoking after being discharged from a non-smoking inpatient environment. Some antipsychotics, such as ziprasidone and lurasidone, must be taken with food in order to have sufficient bioavailability.9
What does a patient want?
Patients with schizophrenia often have limited insight into their psychotic symptoms.10 Savvy clinicians will attempt to leverage a patient’s insight into ancillary symptoms—such as impaired sleep, anxiety, and dysphoria—to encourage a therapeutic alliance and therefore adherence. If patients feel their concerns are not addressed, they may consider treatment inadequate even though the intensity of their hallucinations and delusions may have decreased.
Which antipsychotic is best?
Meta-analyses of randomized controlled trials (RCTs) of antipsychotic treatment for schizophrenia found that, although individual response will vary, clozapine generally has better efficacy that other antipsychotics.11-13 Olanzapine, risperidone, and amisulpride (which is not available in the United States) appear to be more efficacious than first-generation antipsychotics. Other second-generation antipsychotics do not consistently show greater efficacy than first-generation antipsychotics, although their tolerability profiles vary greatly.11-13
Antipsychotic monotherapy. More than 25 RCTs have focused on antipsychotic monotherapy for treatment-resistant patients; for a bibliography of these studies, click here. For the most part, clozapine has consistently demonstrated superiority over comparators. Because not all patients with schizophrenia can tolerate clozapine or are willing to have their blood monitored as required, other second-generation antipsychotics have been suggested as possible substitutes. Olanzapine has established superior efficacy to first-generation antipsychotics11-13 and perhaps comparable efficacy to clozapine in some studies.2,14-17 Risperidone appeared to be comparable to clozapine in some studies,18,19 whereas clozapine’s superiority was evident in others.14,20,21 Although an RCT found comparable efficacy for ziprasidone vs clozapine,22 patients enrolled in this study may not have been treatment-resistant regarding efficacy but instead could not tolerate prior treatments. Enrolling patients on the basis of poor efficacy and/or poor tolerability to their prior antipsychotic regimen also has complicated the interpretation of studies comparing olanzapine with clozapine16 and risperidone with clozapine.18
Antipsychotic combinations. Combinations of antipsychotics are used commonly when treating chronic schizophrenia.23 Of the approximately 20 RCTs of antipsychotic combination therapy, most tested clozapine combined with other second-generation antipsychotics, such as risperidone. For a bibliography of these studies, click here. Only 5 studies support a combination approach (Table 1).
Table 1
Antipsychotic combinations: Few studies support efficacy
| Study | Design | Patients | Results |
|---|---|---|---|
| Shiloh et al, 1997a | 10-week, double-blind, placebo-controlled | 28 patients nonresponsive to typical antipsychotics and partially responsive to clozapine received add-on sulpiride,* 600 mg/d, or placebo | The sulpiride group showed improvements in positive and negative symptoms |
| Josiassen et al, 2005b | 12-week, randomized, double-blind, placebo-controlled | 40 schizophrenia patients unresponsive or partially responsive to clozapine randomized to clozapine + placebo or clozapine + risperidone, 6 mg/d | Mean BPRS total and positive symptom subscale scores reduced in both groups but reductions were greater in the clozapine/risperidone group; reduction in SANS also was observed in the clozapine/risperidone group |
| Genç et al, 2007c | 8-week, randomized, single-blind | 56 treatment-resistant schizophrenia patients randomly assigned to clozapine + amisulpride* or clozapine + quetiapine | Both groups improved at week 8 as measured by BPRS, SANS, SAPS, and CGI; however, patients receiving amisulpride showed greater improvement |
| Muscatello et al, 2011d | 24-week, randomized, double-blind, placebo-controlled | 31 treatment-resistant schizophrenia patients receiving clozapine randomized to receive adjunctive aripiprazole or placebo | Aripiprazole showed beneficial effect on positive and general psychopathologic symptomatology, but no significant effects on executive cognitive function |
| Takahashi et al, 1999e | 8-week, randomized, single-blind, crossover | 10 neuroleptic-treated patients received add-on risperidone and mosapramine* | Both additions resulted in significant, yet modest, improvement; no significant difference in PANSS between risperidone and mosapramine |
| *Not available in the United States BPRS: Brief Psychiatric Rating Scale; CGI: Clinical Global Impression; PANSS: Positive and Negative Syndrome Scale; SANS: Scale for the Assessment of Negative Symptoms; SAPS: Scale for the Assessment of Positive Symptoms | |||
| Source: References a. Shiloh R, Zemishlany Z, Aizenberg D, et al. Sulpiride augmentation in people with schizophrenia partially responsive to clozapine. A double-blind, placebo-controlled study. Br J Psychiatry. 1997;171:569-573. b. Josiassen RC, Joseph A, Kohegyi E, et al. Clozapine augmented with risperidone in the treatment of schizophrenia: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2005;162(1):130-136. c. Genç Y, Taner E, Candansayar S. Comparison of clozapine-amisulpride and clozapine-quetiapine combinations for patients with schizophrenia who are partially responsive to clozapine: a single-blind randomized study. Adv Ther. 2007;24(1):1-13. d. Muscatello MR, Bruno A, Pandolfo G, et al. Effect of aripiprazole augmentation of clozapine in schizophrenia: a double-blind, placebo-controlled study. Schizophr Res. 2011;127(1-3):93-99. e. Takahashi N, Terao T, Oga T, et al. Comparison of risperidone and mosapramine addition to neuroleptic treatment in chronic schizophrenia. Neuropsychobiology. 1999;39(2):81-85. | |||
What about augmentation?
Adjunctive non-antipsychotics also are commonly used when treating patients with chronic schizophrenia. For example, lithium and anticonvulsants are used in approximately one-half of all inpatients with schizophrenia in facilities operated by the State of New York Office of Mental Health.24,25 The evidence base for these agents as adjuncts to antipsychotics generally is weak.26 Specifically, early reports of benefit with adjunctive lithium have been negated by later studies. Similarly, large trials of adjunctive valproate and lamotrigine have failed to replicate early and promising efficacy signals from smaller trials, although the larger studies did not specifically target treatment-resistant schizophrenia.
Among mood stabilizers, lamotrigine may be the most promising for treatment-resistant schizophrenia. In a meta-analysis of clinical trials examining schizophrenia patients receiving clozapine (N=161) who were randomized to receive adjunctive lamotrigine or adjunctive placebo, lamotrigine was superior to placebo in total score for psychosis symptoms and scores for positive and negative symptoms.27
More than 125 published RCTs have studied a wide variety of adjunctive agents other than lithium or anticonvulsants for treating persistent schizophrenia symptoms (Table 2).
Only some of the approximately 40 RCTs regarding adjunctive antidepressants in patients with chronic schizophrenia focused on patients with ongoing depressive symptoms. For a bibliography of these studies, click here. In a meta-analysis measuring improvement of negative symptoms from 23 trials (N=819),28 the effect size was moderate in favor of antidepressants. Subgroup analysis revealed significant responses for fluoxetine, trazodone, and ritanserin.
More than 50 RCTs have focused on augmenting medications for cognitive dysfunction in chronic schizophrenia. Unfortunately, agents used to treat Alzheimer’s disease have shown disappointing results when tested in patients with schizophrenia, as have agents prescribed for attention-deficit/hyperactivity disorder (methylphenidate, guanfacine, atomoxetine) or agents used to promote alertness (modafinil and armodafinil).
Medications that act on glutamate receptors may offer another potential solution, although not in combination with clozapine.29
Other agents that require further study where ≥2 positive studies have been reported (with ≤2 negative studies) include celecoxib, neurosteroids and hormones, purinergic agents, serotonin 5-HT1A receptor agonists, and serotonin 5-HT3 receptor antagonists.
Table 2
Agents studied as adjuncts to antipsychotics
| Acetylsalicylic acid and nonsteroidal anti-inflammatory agents |
| Anticonvulsants and lithium |
| Antidepressants |
| Antiglucocorticoids |
| Agents used to treat attention-deficit/hyperactivity disorder |
| Beta blockers |
| Cholinesterase inhibitors and other agents used to treat Alzheimer’s disease |
| Experimental agents that act on glutamate receptors |
| GABAA receptor drugs |
| Neurosteroids and hormones |
| Omega-3 fatty acids |
| Opioid system agents |
| Peptides |
| Purinergic agents |
| Serotonin 5-HT1A receptor agonists |
| Serotonin 5-HT3 receptor antagonists |
| Wakefulness promoting agents |
Therapeutic neuromodulation
More than 10 RCTs of repetitive transcranial magnetic stimulation (rTMS) in patients with refractory symptoms of schizophrenia have been published; the results were mixed. For a bibliography of these studies, click here. In a meta-analysis of 9 trials (n=213),30 prefrontal rTMS for treating negative symptoms demonstrated a small-to-medium effect size. In another meta-analysis31 of all prospective studies of rTMS for negative symptoms and for auditory hallucinations and overall positive symptoms in refractory schizophrenia, the effect sizes showed moderate effects.
Fewer controlled trials are available for electroconvulsive therapy,32,33 but its use with clozapine appears encouraging.34
Psychological and behavioral intervention. Cognitive-behavioral therapy, although labor-intensive, can be helpful even in patients considered treatment-resistant (Table 3). These interventions generally are provided together with pharmacotherapy.
Complementary and alternative therapies. Patients and their families may ask about complementary and alternative therapies, particularly when conventional approaches have not been successful. A meta-analysis of 6 studies (n=828)35 that reviewed adjunctive use of ginkgo in patients with chronic schizophrenia found statistically significant moderate improvement in total and negative symptoms. Negative reports also are available, including a 5-month study of adjunctive megavitamins that did not demonstrate any benefits.36 In a review of 13 RCTs of acupuncture for schizophrenia, Lee et al found the overall methodological quality was too low to draw firm conclusions.37
Table 3
Cognitive-behavioral therapy for schizophrenia
| Study | Design | Patients | Results |
|---|---|---|---|
| Pinto et al, 1999a | 6-month, randomized controlled | 37 treatment-resistant schizophrenia patients were randomized to CBT plus social skills training or supportive therapy | Both groups showed statistically significant improvement on the BPRS, SAPS, and SANS; however, patients in the CBT group had lower BPRS and SAPS scores. No difference on SANS scores |
| Barretto et al, 2009b | 21-week, controlled (nonrandom-ized) | Patients refractory to clozapine were placed in a CBT or befriending control group | The CBT group showed significant improvement in PANSS total score and general psychopathology subscale score, as well as an improvement of QLS; improvement persisted at 6-month follow-up |
| BPRS: Brief Psychiatric Rating Scale; CBT: cognitive-behavioral therapy; PANSS: Positive and Negative Syndrome Scale; QLS: Quality of Life Scale; SANS: Scale for the Assessment of Negative Symptoms; SAPS: Scale for the Assessment of Positive Symptoms | |||
| Source: References a. Pinto A, La Pia S, Mennella R, et al. Cognitive-behavioral therapy and clozapine for clients with treatment-refractory schizophrenia. Psychiatr Serv. 1999;50(7):901-904. b. Barretto EM, Kayo M, Avrichir BS, et al. A preliminary controlled trial of cognitive behavioral therapy in clozapine-resistant schizophrenia. J Nerv Ment Dis. 2009;197(11):865-868. | |||
Clinical recommendations
Before declaring a patient with schizophrenia as treatment-resistant, ensure that an adequate trial of medication did take place. This includes consideration of adequate dosing and pharmacokinetic issues. Awareness of potential substance use and/or partial adherence or nonadherence also is critical because these factors can impact treatment response.
When prescribing for a treatment-resistant schizophrenia patient, identify specific target symptoms to better inform medication selection—especially for symptoms that the patient feels are important. For example, consider an antidepressant for patients who have negative or depressive symptoms. Also take into account other patient-centered concerns, such as tolerability issues that may have interfered with adherence and response in the past.
Clozapine remains the medication of choice for treatment-resistant schizophrenia. Despite dozens of RCTs of potential adjunctive agents for treatment-resistant schizophrenia, no single approach has consistently shown efficacy in reducing symptoms, improving cognition, or increasing a patient’s level of function. Individual response can vary, and our search for the “outlier” who does respond to an adjunctive agent can explain our use of these strategies in clinical practice.
Related Resources
- Cochrane Database of Systematic Reviews. www.cochrane.org/reviews. This database contains reviews of additional therapeutic options for patients with treatment-resistant schizophrenia. As of February 23, 2011, 157 reviews were available.
- Citrome L. Treatment-refractory schizophrenia: What it is and what’s been done about it. Neuropsychiatry. 2011. Epub ahead of print.
- Citrome L. Clozapine for schizophrenia. Life-threatening or life-saving treatment? Current Psychiatry. 2009;8(12):56-63.
Drug Brand Names
- Aripiprazole • Abilify
- Armodafinil • Nuvigil
- Atomoxetine • Strattera
- Benztropine mesylate • Cogentin
- Carbamazepine • Tegretol
- Celecoxib • Celebrex
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Fluoxetine • Prozac
- Guanfacine • Tenex
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid, others
- Lurasidone • Latuda
- Methylphenidate • Ritalin, Methylin, others
- Modafinil • Provigil
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Rifampin • Rifadin
- Risperidone • Risperdal
- Trazodone • Desyrel, Oleptro
- Valproate (Divalproex) • Depakote, Depakote ER
- Ziprasidone • Geodon
Disclosure
No writing assistance or external financial support was used for this article. Dr. Citrome is a consultant for, has received honoraria from, or has conducted clinical research supported by Abbott Laboratories, AstraZeneca Pharmaceuticals, Avanir Pharmaceuticals, Azur Pharma Inc., Barr Laboratories, Bristol-Myers Squibb, Eli Lilly and Company, Forest Research Institute, GlaxoSmithKline, Janssen Pharmaceuticals, Jazz Pharmaceuticals, Merck, Novartis, Pfizer Inc., Sunovion, Valeant Pharmaceuticals, and Vanda Pharmaceuticals.
Discuss this article at www.facebook.com/CurrentPsychiatry
Patients with treatment-resistant schizophrenia can be broadly defined to include any persons with residual symptoms that cause distress or impairment despite several treatment attempts. Unfortunately, this definition may include most of our patients with schizophrenia.
Clinical trial data on treatment-resistant schizophrenia can be contradictory, leaving “N of 1” empirical treatment trials for individual patients as the current state of the art. This article presents data from clinical trials for pharmacologic and nonpharmacologic options and offers recommendations to try to help our treatment-resistant patients.
Defining treatment resistance
Research reports regarding treatment-resistant or treatment-refractory schizophrenia have relied on operational criteria such as that found in the pivotal study for clozapine1:
- at least 3 periods of treatment in the preceding 5 years with neuroleptic agents from at least 2 different chemical classes at dosages equivalent to ≥1000 mg/d of chlorpromazine for 6 weeks, each without significant symptomatic relief, and
- no period of good functioning within the preceding 5 years.1 In that study, patients also underwent a prospective treatment trial with what we now know are high doses of haloperidol (up to 60 mg/d or higher) and benztropine mesylate (6 mg/d) for a period of 6 weeks to confirm lack of drug responsiveness. Other studies have more relaxed criteria, such as:
- persistent positive symptoms—hallucinations, delusions, or marked thought disorder—after at least 6 contiguous weeks of past or present treatment, with ≥1 typical antipsychotics at doses of ≥600 mg/d in chlorpromazine equivalents
- a poor level of functioning over the past 2 years, as defined by the lack of competitive employment or enrollment in an academic or vocational program and not having age-expected interpersonal relations with someone outside the biologic family with whom ongoing regular contacts were maintained.2
In this study, no prospective period of treatment to confirm lack of drug responsiveness was required.
The most clinically relevant definition of treatment resistance depends on the patient’s individual circumstances. For some patients, targeting positive symptoms is a high priority; for others it may be negative and cognitive symptoms; for others, it may be excitement. Moreover, families may complain of symptoms or behavior that are of little or no concern to your patient.
Although we desire treatment response and remission for our patients, definitions for remission and functional recovery are in flux. Proposed criteria define symptomatic remission as 6-month maintenance of simultaneous ratings of mild or less on delusions, hallucinations, disorganized speech, grossly disorganized or catatonic behavior, and negative symptoms.3,4 Emsley et al4 note that reported remission rates vary widely across studies (17% to 88%) and that patients in remission do better than their non-remitted counterparts in several other outcome domains. Also, patients move in and out of remission over time. Predictors of remission include:
- early treatment response
- baseline symptom severity
- subjective well-being.4
Recovery is a more complex construct than remission and includes social outcomes. Although recovery lacks a standard definition, it is the implied goal of treatment. Anything short of recovery can be viewed as inadequate. If we set the bar at this height, many or most of the patients we treat for schizophrenia could be considered treatment-resistant.
Confounding factors
Before concluding that a patient is treatment-resistant, address medication adherence and possible substance use. Partial or nonadherence with antipsychotic treatment is common—approximately one-half of patients are nonadherent5—and associated with relapse and re-hospitalization.6 In addition, an estimated one-half of all individuals with schizophrenia also use substances.7
Be aware of the optimal dose for any particular antipsychotic and factors that can interfere with achieving adequate plasma levels. This means acknowledging that dosing ranges established during registration studies may not reflect the needs of day-to-day clinical practice.8 Pharmacokinetic interactions with other medications, such as carbamazepine or rifampin, can induce liver enzymes and result in subtherapeutic antipsychotic levels. Cigarette smoking also may have this effect. Lowered clozapine or olanzapine plasma levels have been observed in patients who resume smoking after being discharged from a non-smoking inpatient environment. Some antipsychotics, such as ziprasidone and lurasidone, must be taken with food in order to have sufficient bioavailability.9
What does a patient want?
Patients with schizophrenia often have limited insight into their psychotic symptoms.10 Savvy clinicians will attempt to leverage a patient’s insight into ancillary symptoms—such as impaired sleep, anxiety, and dysphoria—to encourage a therapeutic alliance and therefore adherence. If patients feel their concerns are not addressed, they may consider treatment inadequate even though the intensity of their hallucinations and delusions may have decreased.
Which antipsychotic is best?
Meta-analyses of randomized controlled trials (RCTs) of antipsychotic treatment for schizophrenia found that, although individual response will vary, clozapine generally has better efficacy that other antipsychotics.11-13 Olanzapine, risperidone, and amisulpride (which is not available in the United States) appear to be more efficacious than first-generation antipsychotics. Other second-generation antipsychotics do not consistently show greater efficacy than first-generation antipsychotics, although their tolerability profiles vary greatly.11-13
Antipsychotic monotherapy. More than 25 RCTs have focused on antipsychotic monotherapy for treatment-resistant patients; for a bibliography of these studies, click here. For the most part, clozapine has consistently demonstrated superiority over comparators. Because not all patients with schizophrenia can tolerate clozapine or are willing to have their blood monitored as required, other second-generation antipsychotics have been suggested as possible substitutes. Olanzapine has established superior efficacy to first-generation antipsychotics11-13 and perhaps comparable efficacy to clozapine in some studies.2,14-17 Risperidone appeared to be comparable to clozapine in some studies,18,19 whereas clozapine’s superiority was evident in others.14,20,21 Although an RCT found comparable efficacy for ziprasidone vs clozapine,22 patients enrolled in this study may not have been treatment-resistant regarding efficacy but instead could not tolerate prior treatments. Enrolling patients on the basis of poor efficacy and/or poor tolerability to their prior antipsychotic regimen also has complicated the interpretation of studies comparing olanzapine with clozapine16 and risperidone with clozapine.18
Antipsychotic combinations. Combinations of antipsychotics are used commonly when treating chronic schizophrenia.23 Of the approximately 20 RCTs of antipsychotic combination therapy, most tested clozapine combined with other second-generation antipsychotics, such as risperidone. For a bibliography of these studies, click here. Only 5 studies support a combination approach (Table 1).
Table 1
Antipsychotic combinations: Few studies support efficacy
| Study | Design | Patients | Results |
|---|---|---|---|
| Shiloh et al, 1997a | 10-week, double-blind, placebo-controlled | 28 patients nonresponsive to typical antipsychotics and partially responsive to clozapine received add-on sulpiride,* 600 mg/d, or placebo | The sulpiride group showed improvements in positive and negative symptoms |
| Josiassen et al, 2005b | 12-week, randomized, double-blind, placebo-controlled | 40 schizophrenia patients unresponsive or partially responsive to clozapine randomized to clozapine + placebo or clozapine + risperidone, 6 mg/d | Mean BPRS total and positive symptom subscale scores reduced in both groups but reductions were greater in the clozapine/risperidone group; reduction in SANS also was observed in the clozapine/risperidone group |
| Genç et al, 2007c | 8-week, randomized, single-blind | 56 treatment-resistant schizophrenia patients randomly assigned to clozapine + amisulpride* or clozapine + quetiapine | Both groups improved at week 8 as measured by BPRS, SANS, SAPS, and CGI; however, patients receiving amisulpride showed greater improvement |
| Muscatello et al, 2011d | 24-week, randomized, double-blind, placebo-controlled | 31 treatment-resistant schizophrenia patients receiving clozapine randomized to receive adjunctive aripiprazole or placebo | Aripiprazole showed beneficial effect on positive and general psychopathologic symptomatology, but no significant effects on executive cognitive function |
| Takahashi et al, 1999e | 8-week, randomized, single-blind, crossover | 10 neuroleptic-treated patients received add-on risperidone and mosapramine* | Both additions resulted in significant, yet modest, improvement; no significant difference in PANSS between risperidone and mosapramine |
| *Not available in the United States BPRS: Brief Psychiatric Rating Scale; CGI: Clinical Global Impression; PANSS: Positive and Negative Syndrome Scale; SANS: Scale for the Assessment of Negative Symptoms; SAPS: Scale for the Assessment of Positive Symptoms | |||
| Source: References a. Shiloh R, Zemishlany Z, Aizenberg D, et al. Sulpiride augmentation in people with schizophrenia partially responsive to clozapine. A double-blind, placebo-controlled study. Br J Psychiatry. 1997;171:569-573. b. Josiassen RC, Joseph A, Kohegyi E, et al. Clozapine augmented with risperidone in the treatment of schizophrenia: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2005;162(1):130-136. c. Genç Y, Taner E, Candansayar S. Comparison of clozapine-amisulpride and clozapine-quetiapine combinations for patients with schizophrenia who are partially responsive to clozapine: a single-blind randomized study. Adv Ther. 2007;24(1):1-13. d. Muscatello MR, Bruno A, Pandolfo G, et al. Effect of aripiprazole augmentation of clozapine in schizophrenia: a double-blind, placebo-controlled study. Schizophr Res. 2011;127(1-3):93-99. e. Takahashi N, Terao T, Oga T, et al. Comparison of risperidone and mosapramine addition to neuroleptic treatment in chronic schizophrenia. Neuropsychobiology. 1999;39(2):81-85. | |||
What about augmentation?
Adjunctive non-antipsychotics also are commonly used when treating patients with chronic schizophrenia. For example, lithium and anticonvulsants are used in approximately one-half of all inpatients with schizophrenia in facilities operated by the State of New York Office of Mental Health.24,25 The evidence base for these agents as adjuncts to antipsychotics generally is weak.26 Specifically, early reports of benefit with adjunctive lithium have been negated by later studies. Similarly, large trials of adjunctive valproate and lamotrigine have failed to replicate early and promising efficacy signals from smaller trials, although the larger studies did not specifically target treatment-resistant schizophrenia.
Among mood stabilizers, lamotrigine may be the most promising for treatment-resistant schizophrenia. In a meta-analysis of clinical trials examining schizophrenia patients receiving clozapine (N=161) who were randomized to receive adjunctive lamotrigine or adjunctive placebo, lamotrigine was superior to placebo in total score for psychosis symptoms and scores for positive and negative symptoms.27
More than 125 published RCTs have studied a wide variety of adjunctive agents other than lithium or anticonvulsants for treating persistent schizophrenia symptoms (Table 2).
Only some of the approximately 40 RCTs regarding adjunctive antidepressants in patients with chronic schizophrenia focused on patients with ongoing depressive symptoms. For a bibliography of these studies, click here. In a meta-analysis measuring improvement of negative symptoms from 23 trials (N=819),28 the effect size was moderate in favor of antidepressants. Subgroup analysis revealed significant responses for fluoxetine, trazodone, and ritanserin.
More than 50 RCTs have focused on augmenting medications for cognitive dysfunction in chronic schizophrenia. Unfortunately, agents used to treat Alzheimer’s disease have shown disappointing results when tested in patients with schizophrenia, as have agents prescribed for attention-deficit/hyperactivity disorder (methylphenidate, guanfacine, atomoxetine) or agents used to promote alertness (modafinil and armodafinil).
Medications that act on glutamate receptors may offer another potential solution, although not in combination with clozapine.29
Other agents that require further study where ≥2 positive studies have been reported (with ≤2 negative studies) include celecoxib, neurosteroids and hormones, purinergic agents, serotonin 5-HT1A receptor agonists, and serotonin 5-HT3 receptor antagonists.
Table 2
Agents studied as adjuncts to antipsychotics
| Acetylsalicylic acid and nonsteroidal anti-inflammatory agents |
| Anticonvulsants and lithium |
| Antidepressants |
| Antiglucocorticoids |
| Agents used to treat attention-deficit/hyperactivity disorder |
| Beta blockers |
| Cholinesterase inhibitors and other agents used to treat Alzheimer’s disease |
| Experimental agents that act on glutamate receptors |
| GABAA receptor drugs |
| Neurosteroids and hormones |
| Omega-3 fatty acids |
| Opioid system agents |
| Peptides |
| Purinergic agents |
| Serotonin 5-HT1A receptor agonists |
| Serotonin 5-HT3 receptor antagonists |
| Wakefulness promoting agents |
Therapeutic neuromodulation
More than 10 RCTs of repetitive transcranial magnetic stimulation (rTMS) in patients with refractory symptoms of schizophrenia have been published; the results were mixed. For a bibliography of these studies, click here. In a meta-analysis of 9 trials (n=213),30 prefrontal rTMS for treating negative symptoms demonstrated a small-to-medium effect size. In another meta-analysis31 of all prospective studies of rTMS for negative symptoms and for auditory hallucinations and overall positive symptoms in refractory schizophrenia, the effect sizes showed moderate effects.
Fewer controlled trials are available for electroconvulsive therapy,32,33 but its use with clozapine appears encouraging.34
Psychological and behavioral intervention. Cognitive-behavioral therapy, although labor-intensive, can be helpful even in patients considered treatment-resistant (Table 3). These interventions generally are provided together with pharmacotherapy.
Complementary and alternative therapies. Patients and their families may ask about complementary and alternative therapies, particularly when conventional approaches have not been successful. A meta-analysis of 6 studies (n=828)35 that reviewed adjunctive use of ginkgo in patients with chronic schizophrenia found statistically significant moderate improvement in total and negative symptoms. Negative reports also are available, including a 5-month study of adjunctive megavitamins that did not demonstrate any benefits.36 In a review of 13 RCTs of acupuncture for schizophrenia, Lee et al found the overall methodological quality was too low to draw firm conclusions.37
Table 3
Cognitive-behavioral therapy for schizophrenia
| Study | Design | Patients | Results |
|---|---|---|---|
| Pinto et al, 1999a | 6-month, randomized controlled | 37 treatment-resistant schizophrenia patients were randomized to CBT plus social skills training or supportive therapy | Both groups showed statistically significant improvement on the BPRS, SAPS, and SANS; however, patients in the CBT group had lower BPRS and SAPS scores. No difference on SANS scores |
| Barretto et al, 2009b | 21-week, controlled (nonrandom-ized) | Patients refractory to clozapine were placed in a CBT or befriending control group | The CBT group showed significant improvement in PANSS total score and general psychopathology subscale score, as well as an improvement of QLS; improvement persisted at 6-month follow-up |
| BPRS: Brief Psychiatric Rating Scale; CBT: cognitive-behavioral therapy; PANSS: Positive and Negative Syndrome Scale; QLS: Quality of Life Scale; SANS: Scale for the Assessment of Negative Symptoms; SAPS: Scale for the Assessment of Positive Symptoms | |||
| Source: References a. Pinto A, La Pia S, Mennella R, et al. Cognitive-behavioral therapy and clozapine for clients with treatment-refractory schizophrenia. Psychiatr Serv. 1999;50(7):901-904. b. Barretto EM, Kayo M, Avrichir BS, et al. A preliminary controlled trial of cognitive behavioral therapy in clozapine-resistant schizophrenia. J Nerv Ment Dis. 2009;197(11):865-868. | |||
Clinical recommendations
Before declaring a patient with schizophrenia as treatment-resistant, ensure that an adequate trial of medication did take place. This includes consideration of adequate dosing and pharmacokinetic issues. Awareness of potential substance use and/or partial adherence or nonadherence also is critical because these factors can impact treatment response.
When prescribing for a treatment-resistant schizophrenia patient, identify specific target symptoms to better inform medication selection—especially for symptoms that the patient feels are important. For example, consider an antidepressant for patients who have negative or depressive symptoms. Also take into account other patient-centered concerns, such as tolerability issues that may have interfered with adherence and response in the past.
Clozapine remains the medication of choice for treatment-resistant schizophrenia. Despite dozens of RCTs of potential adjunctive agents for treatment-resistant schizophrenia, no single approach has consistently shown efficacy in reducing symptoms, improving cognition, or increasing a patient’s level of function. Individual response can vary, and our search for the “outlier” who does respond to an adjunctive agent can explain our use of these strategies in clinical practice.
Related Resources
- Cochrane Database of Systematic Reviews. www.cochrane.org/reviews. This database contains reviews of additional therapeutic options for patients with treatment-resistant schizophrenia. As of February 23, 2011, 157 reviews were available.
- Citrome L. Treatment-refractory schizophrenia: What it is and what’s been done about it. Neuropsychiatry. 2011. Epub ahead of print.
- Citrome L. Clozapine for schizophrenia. Life-threatening or life-saving treatment? Current Psychiatry. 2009;8(12):56-63.
Drug Brand Names
- Aripiprazole • Abilify
- Armodafinil • Nuvigil
- Atomoxetine • Strattera
- Benztropine mesylate • Cogentin
- Carbamazepine • Tegretol
- Celecoxib • Celebrex
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Fluoxetine • Prozac
- Guanfacine • Tenex
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid, others
- Lurasidone • Latuda
- Methylphenidate • Ritalin, Methylin, others
- Modafinil • Provigil
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Rifampin • Rifadin
- Risperidone • Risperdal
- Trazodone • Desyrel, Oleptro
- Valproate (Divalproex) • Depakote, Depakote ER
- Ziprasidone • Geodon
Disclosure
No writing assistance or external financial support was used for this article. Dr. Citrome is a consultant for, has received honoraria from, or has conducted clinical research supported by Abbott Laboratories, AstraZeneca Pharmaceuticals, Avanir Pharmaceuticals, Azur Pharma Inc., Barr Laboratories, Bristol-Myers Squibb, Eli Lilly and Company, Forest Research Institute, GlaxoSmithKline, Janssen Pharmaceuticals, Jazz Pharmaceuticals, Merck, Novartis, Pfizer Inc., Sunovion, Valeant Pharmaceuticals, and Vanda Pharmaceuticals.
1. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
2. Volavka J, Czobor P, Sheitman B, et al. Clozapine, olanzapine, risperidone, and haloperidol in the treatment of patients with chronic schizophrenia and schizoaffective disorder. Am J Psychiatry. 2002;159(2):255-262.
3. Andreasen NC, Carpenter WT Jr, Kane JM, et al. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry. 2005;162(3):441-449.
4. Emsley R, Chiliza B, Asmal L, et al. The concepts of remission and recovery in schizophrenia. Curr Opin Psychiatry. 2011;24(2):114-121.
5. Lacro JP, Dunn LB, Dolder CR, et al. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002;63(10):892-909.
6. Robinson DG, Woerner MG, Delman HM, et al. Pharmacological treatments for first-episode schizophrenia. Schizophr Bull. 2005;31(3):705-722.
7. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264(19):2511-2518.
8. Citrome L, Volavka J. Optimal dosing of atypical antipsychotics in adults: a review of the current evidence. Harv Rev Psychiatry. 2002;10(5):280-291.
9. Citrome L. Iloperidone asenapine and lurasidone. A brief overview of three new second-generation antipsychotics. Postgrad Med. 2011;123(2):153-162.
10. Lincoln TM, Lüllmann E, Rief W. Correlates and long-term consequences of poor insight in patients with schizophrenia. A systematic review. Schizophr Bull. 2007;33(6):1324-1342.
11. Leucht S, Corves C, Arbter D, et al. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373(9657):31-41.
12. Leucht S, Komossa K, Rummel-Kluge C, et al. A meta-analysis of head-to-head comparisons of second-generation antipsychotics in the treatment of schizophrenia. Am J Psychiatry. 2009;166(2):152-163.
13. Leucht S, Arbter D, Engel RR, et al. How effective are second-generation antipsychotic drugs? A meta-analysis of placebo-controlled trials. Mol Psychiatry. 2009;14(4):429-447.
14. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600-610.
15. Tollefson GD, Birkett MA, Kiesler GM, et al. Double-blind comparison of olanzapine versus clozapine in schizophrenic patients clinically eligible for treatment with clozapine. Biol Psychiatry. 2001;49(1):52-63.
16. Bitter I, Dossenbach MR, Brook S, et al. Olanzapine versus clozapine in treatment-resistant or treatment-intolerant schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(1):173-180.
17. Meltzer HY, Bobo WV, Roy A, et al. A randomized, double-blind comparison of clozapine and high-dose olanzapine in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2008;69(2):274-285.
18. Bondolfi G, Dufour H, Patris M, et al. Risperidone versus clozapine in treatment-resistant chronic schizophrenia: a randomized double-blind study. Am J Psychiatry. 1998;155(4):499-504.
19. Wahlbeck K, Cheine M, Tuisku K, et al. Risperidone versus clozapine in treatment-resistant schizophrenia: a randomized pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24(6):911-922.
20. Breier AF, Malhotra AK, Su TP, et al. Clozapine and risperidone in chronic schizophrenia: effects on symptoms, parkinsonian side effects, and neuroendocrine response. Am J Psychiatry. 1999;156(2):294-298.
21. Azorin JM, Spiegel R, Remington G, et al. A double-blind comparative study of clozapine and risperidone in the management of severe chronic schizophrenia. Am J Psychiatry. 2001;158(8):1305-1313.
22. Sacchetti E, Galluzzo A, Valsecchi P, et al. Ziprasidone vs clozapine in schizophrenia patients refractory to multiple antipsychotic treatments: the MOZART study. Schizophr Res. 2009;113(1):112-121.
23. Jaffe AB, Levine J. Antipsychotic medication coprescribing in a large state hospital system. Pharmacoepidemiol Drug Saf. 2003;12(1):41-48.
24. Citrome L, Levine J, Allingham B. Changes in use of valproate and other mood stabilizers for patients with schizophrenia from 1994 to 1998. Psychiatr Serv. 2000;51(5):634-638.
25. Citrome L, Jaffe A, Levine J, et al. Use of mood stabilizers among patients with schizophrenia, 1994-2001. Psychiatr Serv. 2002;53(10):1212.-
26. Citrome L. Adjunctive lithium and anticonvulsants for the treatment of schizophrenia: what is the evidence? Expert Rev Neurother. 2009;9(1):55-71.
27. Tiihonen J, Wahlbeck K, Kiviniemi V. The efficacy of lamotrigine in clozapine-resistant schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2009;109(1-3):10-14.
28. Singh SP, Singh V, Kar N, et al. Efficacy of antidepressants in treating the negative symptoms of chronic schizophrenia: meta-analysis. Br J Psychiatry. 2010;197(3):174-179.
29. Kantrowitz JT, Javitt DC. Thinking glutamatergically: changing concepts of schizophrenia based upon changing neurochemical models. Clin Schizophr Relat Psychoses. 2010;4(3):189-200.
30. Dlabac-de Lange JJ, Knegtering R, Aleman A, et al. Repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: review and meta-analysis. J Clin Psychiatry. 2010;71(4):411-418.
31. Freitas C, Fregni F, Pascual-Leone A. Meta-analysis of the effects of repetitive transcranial magnetic stimulation (rTMS) on negative and positive symptoms in schizophrenia. Schizophr Res. 2009;108(1-3):11-24.
32. Chanpattana W, Chakrabhand ML, Sackeim HA, et al. Continuation ECT in treatment-resistant schizophrenia: a controlled study. J ECT. 1999;15(3):178-192.
33. Goswami U, Kumar U, Singh B. Efficacy of electroconvulsive therapy in treatment resistant schizophrenia: a double-blind study. Indian J Psychiatry. 2003;45(1):26-29.
34. Braga RJ, Petrides G. The combined use of electroconvulsive therapy and antipsychotics in patients with schizophrenia. J ECT. 2005;21(2):75-83.
35. Singh V, Singh SP, Chan K. Review and meta-analysis of usage of ginkgo as an adjunct therapy in chronic schizophrenia. Int J Neuropsychopharmacol. 2010;13(2):257-271.
36. Vaughan K, McConaghy N. Megavitamin and dietary treatment in schizophrenia: a randomised controlled trial. Aust N Z J Psychiatry. 1999;33(1):84-88.
37. Lee MS, Shin BC, Ronan P, et al. Acupuncture for schizophrenia: a systematic review and meta-analysis. Int J Clin Pract. 2009;63(11):1622-1633.
1. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
2. Volavka J, Czobor P, Sheitman B, et al. Clozapine, olanzapine, risperidone, and haloperidol in the treatment of patients with chronic schizophrenia and schizoaffective disorder. Am J Psychiatry. 2002;159(2):255-262.
3. Andreasen NC, Carpenter WT Jr, Kane JM, et al. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry. 2005;162(3):441-449.
4. Emsley R, Chiliza B, Asmal L, et al. The concepts of remission and recovery in schizophrenia. Curr Opin Psychiatry. 2011;24(2):114-121.
5. Lacro JP, Dunn LB, Dolder CR, et al. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002;63(10):892-909.
6. Robinson DG, Woerner MG, Delman HM, et al. Pharmacological treatments for first-episode schizophrenia. Schizophr Bull. 2005;31(3):705-722.
7. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264(19):2511-2518.
8. Citrome L, Volavka J. Optimal dosing of atypical antipsychotics in adults: a review of the current evidence. Harv Rev Psychiatry. 2002;10(5):280-291.
9. Citrome L. Iloperidone asenapine and lurasidone. A brief overview of three new second-generation antipsychotics. Postgrad Med. 2011;123(2):153-162.
10. Lincoln TM, Lüllmann E, Rief W. Correlates and long-term consequences of poor insight in patients with schizophrenia. A systematic review. Schizophr Bull. 2007;33(6):1324-1342.
11. Leucht S, Corves C, Arbter D, et al. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373(9657):31-41.
12. Leucht S, Komossa K, Rummel-Kluge C, et al. A meta-analysis of head-to-head comparisons of second-generation antipsychotics in the treatment of schizophrenia. Am J Psychiatry. 2009;166(2):152-163.
13. Leucht S, Arbter D, Engel RR, et al. How effective are second-generation antipsychotic drugs? A meta-analysis of placebo-controlled trials. Mol Psychiatry. 2009;14(4):429-447.
14. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600-610.
15. Tollefson GD, Birkett MA, Kiesler GM, et al. Double-blind comparison of olanzapine versus clozapine in schizophrenic patients clinically eligible for treatment with clozapine. Biol Psychiatry. 2001;49(1):52-63.
16. Bitter I, Dossenbach MR, Brook S, et al. Olanzapine versus clozapine in treatment-resistant or treatment-intolerant schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(1):173-180.
17. Meltzer HY, Bobo WV, Roy A, et al. A randomized, double-blind comparison of clozapine and high-dose olanzapine in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2008;69(2):274-285.
18. Bondolfi G, Dufour H, Patris M, et al. Risperidone versus clozapine in treatment-resistant chronic schizophrenia: a randomized double-blind study. Am J Psychiatry. 1998;155(4):499-504.
19. Wahlbeck K, Cheine M, Tuisku K, et al. Risperidone versus clozapine in treatment-resistant schizophrenia: a randomized pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24(6):911-922.
20. Breier AF, Malhotra AK, Su TP, et al. Clozapine and risperidone in chronic schizophrenia: effects on symptoms, parkinsonian side effects, and neuroendocrine response. Am J Psychiatry. 1999;156(2):294-298.
21. Azorin JM, Spiegel R, Remington G, et al. A double-blind comparative study of clozapine and risperidone in the management of severe chronic schizophrenia. Am J Psychiatry. 2001;158(8):1305-1313.
22. Sacchetti E, Galluzzo A, Valsecchi P, et al. Ziprasidone vs clozapine in schizophrenia patients refractory to multiple antipsychotic treatments: the MOZART study. Schizophr Res. 2009;113(1):112-121.
23. Jaffe AB, Levine J. Antipsychotic medication coprescribing in a large state hospital system. Pharmacoepidemiol Drug Saf. 2003;12(1):41-48.
24. Citrome L, Levine J, Allingham B. Changes in use of valproate and other mood stabilizers for patients with schizophrenia from 1994 to 1998. Psychiatr Serv. 2000;51(5):634-638.
25. Citrome L, Jaffe A, Levine J, et al. Use of mood stabilizers among patients with schizophrenia, 1994-2001. Psychiatr Serv. 2002;53(10):1212.-
26. Citrome L. Adjunctive lithium and anticonvulsants for the treatment of schizophrenia: what is the evidence? Expert Rev Neurother. 2009;9(1):55-71.
27. Tiihonen J, Wahlbeck K, Kiviniemi V. The efficacy of lamotrigine in clozapine-resistant schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2009;109(1-3):10-14.
28. Singh SP, Singh V, Kar N, et al. Efficacy of antidepressants in treating the negative symptoms of chronic schizophrenia: meta-analysis. Br J Psychiatry. 2010;197(3):174-179.
29. Kantrowitz JT, Javitt DC. Thinking glutamatergically: changing concepts of schizophrenia based upon changing neurochemical models. Clin Schizophr Relat Psychoses. 2010;4(3):189-200.
30. Dlabac-de Lange JJ, Knegtering R, Aleman A, et al. Repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: review and meta-analysis. J Clin Psychiatry. 2010;71(4):411-418.
31. Freitas C, Fregni F, Pascual-Leone A. Meta-analysis of the effects of repetitive transcranial magnetic stimulation (rTMS) on negative and positive symptoms in schizophrenia. Schizophr Res. 2009;108(1-3):11-24.
32. Chanpattana W, Chakrabhand ML, Sackeim HA, et al. Continuation ECT in treatment-resistant schizophrenia: a controlled study. J ECT. 1999;15(3):178-192.
33. Goswami U, Kumar U, Singh B. Efficacy of electroconvulsive therapy in treatment resistant schizophrenia: a double-blind study. Indian J Psychiatry. 2003;45(1):26-29.
34. Braga RJ, Petrides G. The combined use of electroconvulsive therapy and antipsychotics in patients with schizophrenia. J ECT. 2005;21(2):75-83.
35. Singh V, Singh SP, Chan K. Review and meta-analysis of usage of ginkgo as an adjunct therapy in chronic schizophrenia. Int J Neuropsychopharmacol. 2010;13(2):257-271.
36. Vaughan K, McConaghy N. Megavitamin and dietary treatment in schizophrenia: a randomised controlled trial. Aust N Z J Psychiatry. 1999;33(1):84-88.
37. Lee MS, Shin BC, Ronan P, et al. Acupuncture for schizophrenia: a systematic review and meta-analysis. Int J Clin Pract. 2009;63(11):1622-1633.