User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Off-label prescribing: 7 steps for safer, more effective treatment
Have you noticed two curious patterns in off-label prescribing? Psychiatrists avoid agents approved for treating insomnia but prescribe anticonvulsants for a variety of unapproved uses.
Most of us prescribe medications for therapeutic uses not found in FDA-approved labeling. Among 200 psychiatrists surveyed, 65% said they had prescribed off label in the previous month, and only 4% had ever received a patient complaint about the practice.Malpractice Verdicts).
Box
Private insurance. Psychotropic costs are rising 20% a year, contributing to the nation’s annual 13% overall prescription drug cost increase.15 To control rising costs, some medical insurance plans consider off-label use as “unapproved and experimental” and deny coverage. Pharmacy benefit and self-insured employer plans may act similarly, although some states require insurers to cover off-label use of all approved edications.
Government programs. Medicaid does not exclude coverage of off-label prescriptions. How the new Medicare prescription drug plan (Part D) handles off-label prescribing remains unclear.
Why psychiatrists prescribe off-label
| Therapeutic reasons |
| Patient has a disorder for which no drug is labeled |
| Patient falls outside of labeled age or demographic group, such as children, older patients, and pregnant women |
| Patient fails to respond to labeled products |
| Off-label product may potentiate response to a labeled agent or minimize its adverse effects |
| Preferences |
| Manufacturers and respected peers promote use of off-label products as first- or second-line agents18 |
| Practitioner wishes to foster innovative treatments |
| Patients or families request an off-label drug instead of labeled alternatives |
| Practitioner avoids using a particular labeled drug or drug class |
Most state medical practice laws spell out the information required in the patient chart to demonstrate informed consent, defined variously as:
- what a reasonable provider would tell a patient
- what a reasonable patient would expect to hear from the provider
- what a patient would need to hear before deciding on a treatment course.
What the law says
Off-label prescribing is legal, common, necessary, and recognized in some states by statute and by U.S. Supreme Court review.
Court decisions. In a class action suit before the top court (Buckman Company vs. Plaintiff’s Legal Commission, 2001), 5,000 plaintiffs claimed damages from orthopedic screws and plates that were FDA-approved for use in long bones but not for use in the spine. A unanimous court held that such off-label use is an accepted and necessary offshoot of FDA regulatory function and does not interfere with the practice of medicine.
The courts also have determined that off-label use does not mean “experimental” and itself is not a risk. Off-label use may be consistent with the standard of care and does not categorically indicate negligence (though a practitioner who prescribes negligently—such as prescribing a drug to which a patient is known to be allergic—may be found liable).
Drug manufacturers’ risk. The courts recognize that patients receive prescription drugs from doctors, not directly from the manufacturers. The law thus provides some immunity to manufacturers if your patient is injured by a drug you prescribe off-label. The learned-intermediary rule says anufacturers must warn you adequately of a drug’s foreseeable risks, and you then assume the responsibility to warn the patient.
The courts recognize exceptions, though, and have required manufacturers to warn patients directly about vaccines given in mass immunizations, drugs withdrawn from the market, drugs advertised directly to consumers, and other risks.
- BMJ Publishing Group. Clinical evidence. Summary of what is known—and not known—about more than 200 medical disorders and 2,000 treatments. www.clinicalevidence.com.
- Cochrane Library of evidence-based clinical reviews. www.cochrane.org.
- Agency for Health Care Research and Quality. Draft comparative effectiveness review of off-label use of atypical antipsychotic drugs. http://effectivehealthcare.ahrq.gov/synthesize/reports/draft.cfm.
- Amitriptyline • Elavil, others
- Carbamazepine • Carbetrol; Epitol; Equetro; Tegretol
- Gabapentin • Gabarone; Neurontin
- Lamotrigine • Lamictal
- Trazodone • Desyrel; Trialodine
- Valproate • Depakote; Depakene
Dr. Kramer reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. McCall receives research support from GlaxoSmithKline, Sanofi-Aventis, Sepracor, Takeda, and Wyeth, is an advisor to King Pharmaceuticals and Sepracor, and is a speaker for GlaxoSmtihKline, Sepracor, and Wyeth.
1. Lowe-Ponsford FL, Baldwin DS. Off-label prescribing by psychiatrists. Psychiatr Bull 2000;24(11):415-17.
2. Zito JM, Safer DJ, dosReis S, et al. Trends in the prescribing of psychotropic medications to preschoolers. JAMA 2000;283(8):1025-30.
3. Kelly DL, Love RC, Mackowick M, et al. Atypical antipsychotic use in a state hospital inpatient adolescent population. J Child Adolesc Psychopharmacol 2004;14(1):75-85.
4. Rosenheck R, Leslie D, Sernyak M. From clinical trials to real-world practice: use of atypical antipsychotic medication nationally in the Department of Veterans Affairs. Med Care 2001;39(3):302-8.
5. Fountoulakis KN, Nimatoudis I, Iacovides A, Kaprinis G. Off-label indications for atypical antipsychotics: A systematic review. Ann Gen Hosp Psychiatry 2004;3(1):4.-
6. Pomerantz JM, Finkelstein SN, Berndt ER, et al. Prescriber intent, off-label usage, and early discontinuation of antidepressants: a retrospective physician survey and data analysis. J Clin Psychiatry 2004;65(3):395-404.
1. Beck JM, Azari ED. FDA, off-label use, and informed consent. Food Drug Law J 1998;53:71-104.
8. Hepper F, Fellow-Smith E. Off-label prescribing in a community child and adolescent mental health service: Implications for information giving and informed consent. Clin Manag 2005;13(1):29-33.
9. O’Reilly JD, Dalal A. Off-label or out of bounds? Prescriber and marketer liability for unapproved uses of FDA-approved drugs. Ann Health Law 2003;12:295-324.
10. Ware JC, Pittard JT. Increased deep sleep after trazodone use: a double-blind placebo-controlled study in healthy young adults. J Clin Psychiatry 1990;51:18-22.
11. McCall WV. Use of off-label medications in the treatment of chronic insomnia. J Clin Sleep Med 2005;1(4):e494-5.
12. Chen H, Deshpande AD, Jiang R, Martin BC. An epidemiological investigation of off-label anticonvulsant drug use in the Georgia Medicaid population. Pharmacoepidemiol Drug Saf 2005;14(9):629-38.
13. Mack A. Examination of the evidence for off-label use of gabapentin. J Manag Care Pharm 2003;9(6):559-68.
14. Citrome L, Levine J, Allingham B. Utilization of valproate: extent of inpatient use in the New York State Office of Mental Health. Psychiatr Q 1998;69(4):283-300.
15. De Leon O. Antiepileptic drugs for the acute and maintenance treatment of bipolar disorder. Harv Rev Psychiatry 2001;9(5):209-22.
16. Le Bon O, Murphy JR, Staner L, et al. Double-blind, placebocontrolled study of the efficacy of trazodone in alcohol postwithdrawal syndrome: polysomnographic and clinical evaluations. J Clin Psychopharmacol 2003;23(4):377-83.
17. Zuvekas SH. Prescription drugs and the changing patterns of treatment for mental disorders, 1996-2001. Health Aff (Millwood) 2005;24(1):195-205.
18. Glick ID, Murray SR, Vasudevan P, et al. Treatment with atypical antipsychotics: new indications and new populations. J Psychiatr Res 2001;35(3):187-91.
Have you noticed two curious patterns in off-label prescribing? Psychiatrists avoid agents approved for treating insomnia but prescribe anticonvulsants for a variety of unapproved uses.
Most of us prescribe medications for therapeutic uses not found in FDA-approved labeling. Among 200 psychiatrists surveyed, 65% said they had prescribed off label in the previous month, and only 4% had ever received a patient complaint about the practice.Malpractice Verdicts).
Box
Private insurance. Psychotropic costs are rising 20% a year, contributing to the nation’s annual 13% overall prescription drug cost increase.15 To control rising costs, some medical insurance plans consider off-label use as “unapproved and experimental” and deny coverage. Pharmacy benefit and self-insured employer plans may act similarly, although some states require insurers to cover off-label use of all approved edications.
Government programs. Medicaid does not exclude coverage of off-label prescriptions. How the new Medicare prescription drug plan (Part D) handles off-label prescribing remains unclear.
Why psychiatrists prescribe off-label
| Therapeutic reasons |
| Patient has a disorder for which no drug is labeled |
| Patient falls outside of labeled age or demographic group, such as children, older patients, and pregnant women |
| Patient fails to respond to labeled products |
| Off-label product may potentiate response to a labeled agent or minimize its adverse effects |
| Preferences |
| Manufacturers and respected peers promote use of off-label products as first- or second-line agents18 |
| Practitioner wishes to foster innovative treatments |
| Patients or families request an off-label drug instead of labeled alternatives |
| Practitioner avoids using a particular labeled drug or drug class |
Most state medical practice laws spell out the information required in the patient chart to demonstrate informed consent, defined variously as:
- what a reasonable provider would tell a patient
- what a reasonable patient would expect to hear from the provider
- what a patient would need to hear before deciding on a treatment course.
What the law says
Off-label prescribing is legal, common, necessary, and recognized in some states by statute and by U.S. Supreme Court review.
Court decisions. In a class action suit before the top court (Buckman Company vs. Plaintiff’s Legal Commission, 2001), 5,000 plaintiffs claimed damages from orthopedic screws and plates that were FDA-approved for use in long bones but not for use in the spine. A unanimous court held that such off-label use is an accepted and necessary offshoot of FDA regulatory function and does not interfere with the practice of medicine.
The courts also have determined that off-label use does not mean “experimental” and itself is not a risk. Off-label use may be consistent with the standard of care and does not categorically indicate negligence (though a practitioner who prescribes negligently—such as prescribing a drug to which a patient is known to be allergic—may be found liable).
Drug manufacturers’ risk. The courts recognize that patients receive prescription drugs from doctors, not directly from the manufacturers. The law thus provides some immunity to manufacturers if your patient is injured by a drug you prescribe off-label. The learned-intermediary rule says anufacturers must warn you adequately of a drug’s foreseeable risks, and you then assume the responsibility to warn the patient.
The courts recognize exceptions, though, and have required manufacturers to warn patients directly about vaccines given in mass immunizations, drugs withdrawn from the market, drugs advertised directly to consumers, and other risks.
- BMJ Publishing Group. Clinical evidence. Summary of what is known—and not known—about more than 200 medical disorders and 2,000 treatments. www.clinicalevidence.com.
- Cochrane Library of evidence-based clinical reviews. www.cochrane.org.
- Agency for Health Care Research and Quality. Draft comparative effectiveness review of off-label use of atypical antipsychotic drugs. http://effectivehealthcare.ahrq.gov/synthesize/reports/draft.cfm.
- Amitriptyline • Elavil, others
- Carbamazepine • Carbetrol; Epitol; Equetro; Tegretol
- Gabapentin • Gabarone; Neurontin
- Lamotrigine • Lamictal
- Trazodone • Desyrel; Trialodine
- Valproate • Depakote; Depakene
Dr. Kramer reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. McCall receives research support from GlaxoSmithKline, Sanofi-Aventis, Sepracor, Takeda, and Wyeth, is an advisor to King Pharmaceuticals and Sepracor, and is a speaker for GlaxoSmtihKline, Sepracor, and Wyeth.
Have you noticed two curious patterns in off-label prescribing? Psychiatrists avoid agents approved for treating insomnia but prescribe anticonvulsants for a variety of unapproved uses.
Most of us prescribe medications for therapeutic uses not found in FDA-approved labeling. Among 200 psychiatrists surveyed, 65% said they had prescribed off label in the previous month, and only 4% had ever received a patient complaint about the practice.Malpractice Verdicts).
Box
Private insurance. Psychotropic costs are rising 20% a year, contributing to the nation’s annual 13% overall prescription drug cost increase.15 To control rising costs, some medical insurance plans consider off-label use as “unapproved and experimental” and deny coverage. Pharmacy benefit and self-insured employer plans may act similarly, although some states require insurers to cover off-label use of all approved edications.
Government programs. Medicaid does not exclude coverage of off-label prescriptions. How the new Medicare prescription drug plan (Part D) handles off-label prescribing remains unclear.
Why psychiatrists prescribe off-label
| Therapeutic reasons |
| Patient has a disorder for which no drug is labeled |
| Patient falls outside of labeled age or demographic group, such as children, older patients, and pregnant women |
| Patient fails to respond to labeled products |
| Off-label product may potentiate response to a labeled agent or minimize its adverse effects |
| Preferences |
| Manufacturers and respected peers promote use of off-label products as first- or second-line agents18 |
| Practitioner wishes to foster innovative treatments |
| Patients or families request an off-label drug instead of labeled alternatives |
| Practitioner avoids using a particular labeled drug or drug class |
Most state medical practice laws spell out the information required in the patient chart to demonstrate informed consent, defined variously as:
- what a reasonable provider would tell a patient
- what a reasonable patient would expect to hear from the provider
- what a patient would need to hear before deciding on a treatment course.
What the law says
Off-label prescribing is legal, common, necessary, and recognized in some states by statute and by U.S. Supreme Court review.
Court decisions. In a class action suit before the top court (Buckman Company vs. Plaintiff’s Legal Commission, 2001), 5,000 plaintiffs claimed damages from orthopedic screws and plates that were FDA-approved for use in long bones but not for use in the spine. A unanimous court held that such off-label use is an accepted and necessary offshoot of FDA regulatory function and does not interfere with the practice of medicine.
The courts also have determined that off-label use does not mean “experimental” and itself is not a risk. Off-label use may be consistent with the standard of care and does not categorically indicate negligence (though a practitioner who prescribes negligently—such as prescribing a drug to which a patient is known to be allergic—may be found liable).
Drug manufacturers’ risk. The courts recognize that patients receive prescription drugs from doctors, not directly from the manufacturers. The law thus provides some immunity to manufacturers if your patient is injured by a drug you prescribe off-label. The learned-intermediary rule says anufacturers must warn you adequately of a drug’s foreseeable risks, and you then assume the responsibility to warn the patient.
The courts recognize exceptions, though, and have required manufacturers to warn patients directly about vaccines given in mass immunizations, drugs withdrawn from the market, drugs advertised directly to consumers, and other risks.
- BMJ Publishing Group. Clinical evidence. Summary of what is known—and not known—about more than 200 medical disorders and 2,000 treatments. www.clinicalevidence.com.
- Cochrane Library of evidence-based clinical reviews. www.cochrane.org.
- Agency for Health Care Research and Quality. Draft comparative effectiveness review of off-label use of atypical antipsychotic drugs. http://effectivehealthcare.ahrq.gov/synthesize/reports/draft.cfm.
- Amitriptyline • Elavil, others
- Carbamazepine • Carbetrol; Epitol; Equetro; Tegretol
- Gabapentin • Gabarone; Neurontin
- Lamotrigine • Lamictal
- Trazodone • Desyrel; Trialodine
- Valproate • Depakote; Depakene
Dr. Kramer reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. McCall receives research support from GlaxoSmithKline, Sanofi-Aventis, Sepracor, Takeda, and Wyeth, is an advisor to King Pharmaceuticals and Sepracor, and is a speaker for GlaxoSmtihKline, Sepracor, and Wyeth.
1. Lowe-Ponsford FL, Baldwin DS. Off-label prescribing by psychiatrists. Psychiatr Bull 2000;24(11):415-17.
2. Zito JM, Safer DJ, dosReis S, et al. Trends in the prescribing of psychotropic medications to preschoolers. JAMA 2000;283(8):1025-30.
3. Kelly DL, Love RC, Mackowick M, et al. Atypical antipsychotic use in a state hospital inpatient adolescent population. J Child Adolesc Psychopharmacol 2004;14(1):75-85.
4. Rosenheck R, Leslie D, Sernyak M. From clinical trials to real-world practice: use of atypical antipsychotic medication nationally in the Department of Veterans Affairs. Med Care 2001;39(3):302-8.
5. Fountoulakis KN, Nimatoudis I, Iacovides A, Kaprinis G. Off-label indications for atypical antipsychotics: A systematic review. Ann Gen Hosp Psychiatry 2004;3(1):4.-
6. Pomerantz JM, Finkelstein SN, Berndt ER, et al. Prescriber intent, off-label usage, and early discontinuation of antidepressants: a retrospective physician survey and data analysis. J Clin Psychiatry 2004;65(3):395-404.
1. Beck JM, Azari ED. FDA, off-label use, and informed consent. Food Drug Law J 1998;53:71-104.
8. Hepper F, Fellow-Smith E. Off-label prescribing in a community child and adolescent mental health service: Implications for information giving and informed consent. Clin Manag 2005;13(1):29-33.
9. O’Reilly JD, Dalal A. Off-label or out of bounds? Prescriber and marketer liability for unapproved uses of FDA-approved drugs. Ann Health Law 2003;12:295-324.
10. Ware JC, Pittard JT. Increased deep sleep after trazodone use: a double-blind placebo-controlled study in healthy young adults. J Clin Psychiatry 1990;51:18-22.
11. McCall WV. Use of off-label medications in the treatment of chronic insomnia. J Clin Sleep Med 2005;1(4):e494-5.
12. Chen H, Deshpande AD, Jiang R, Martin BC. An epidemiological investigation of off-label anticonvulsant drug use in the Georgia Medicaid population. Pharmacoepidemiol Drug Saf 2005;14(9):629-38.
13. Mack A. Examination of the evidence for off-label use of gabapentin. J Manag Care Pharm 2003;9(6):559-68.
14. Citrome L, Levine J, Allingham B. Utilization of valproate: extent of inpatient use in the New York State Office of Mental Health. Psychiatr Q 1998;69(4):283-300.
15. De Leon O. Antiepileptic drugs for the acute and maintenance treatment of bipolar disorder. Harv Rev Psychiatry 2001;9(5):209-22.
16. Le Bon O, Murphy JR, Staner L, et al. Double-blind, placebocontrolled study of the efficacy of trazodone in alcohol postwithdrawal syndrome: polysomnographic and clinical evaluations. J Clin Psychopharmacol 2003;23(4):377-83.
17. Zuvekas SH. Prescription drugs and the changing patterns of treatment for mental disorders, 1996-2001. Health Aff (Millwood) 2005;24(1):195-205.
18. Glick ID, Murray SR, Vasudevan P, et al. Treatment with atypical antipsychotics: new indications and new populations. J Psychiatr Res 2001;35(3):187-91.
1. Lowe-Ponsford FL, Baldwin DS. Off-label prescribing by psychiatrists. Psychiatr Bull 2000;24(11):415-17.
2. Zito JM, Safer DJ, dosReis S, et al. Trends in the prescribing of psychotropic medications to preschoolers. JAMA 2000;283(8):1025-30.
3. Kelly DL, Love RC, Mackowick M, et al. Atypical antipsychotic use in a state hospital inpatient adolescent population. J Child Adolesc Psychopharmacol 2004;14(1):75-85.
4. Rosenheck R, Leslie D, Sernyak M. From clinical trials to real-world practice: use of atypical antipsychotic medication nationally in the Department of Veterans Affairs. Med Care 2001;39(3):302-8.
5. Fountoulakis KN, Nimatoudis I, Iacovides A, Kaprinis G. Off-label indications for atypical antipsychotics: A systematic review. Ann Gen Hosp Psychiatry 2004;3(1):4.-
6. Pomerantz JM, Finkelstein SN, Berndt ER, et al. Prescriber intent, off-label usage, and early discontinuation of antidepressants: a retrospective physician survey and data analysis. J Clin Psychiatry 2004;65(3):395-404.
1. Beck JM, Azari ED. FDA, off-label use, and informed consent. Food Drug Law J 1998;53:71-104.
8. Hepper F, Fellow-Smith E. Off-label prescribing in a community child and adolescent mental health service: Implications for information giving and informed consent. Clin Manag 2005;13(1):29-33.
9. O’Reilly JD, Dalal A. Off-label or out of bounds? Prescriber and marketer liability for unapproved uses of FDA-approved drugs. Ann Health Law 2003;12:295-324.
10. Ware JC, Pittard JT. Increased deep sleep after trazodone use: a double-blind placebo-controlled study in healthy young adults. J Clin Psychiatry 1990;51:18-22.
11. McCall WV. Use of off-label medications in the treatment of chronic insomnia. J Clin Sleep Med 2005;1(4):e494-5.
12. Chen H, Deshpande AD, Jiang R, Martin BC. An epidemiological investigation of off-label anticonvulsant drug use in the Georgia Medicaid population. Pharmacoepidemiol Drug Saf 2005;14(9):629-38.
13. Mack A. Examination of the evidence for off-label use of gabapentin. J Manag Care Pharm 2003;9(6):559-68.
14. Citrome L, Levine J, Allingham B. Utilization of valproate: extent of inpatient use in the New York State Office of Mental Health. Psychiatr Q 1998;69(4):283-300.
15. De Leon O. Antiepileptic drugs for the acute and maintenance treatment of bipolar disorder. Harv Rev Psychiatry 2001;9(5):209-22.
16. Le Bon O, Murphy JR, Staner L, et al. Double-blind, placebocontrolled study of the efficacy of trazodone in alcohol postwithdrawal syndrome: polysomnographic and clinical evaluations. J Clin Psychopharmacol 2003;23(4):377-83.
17. Zuvekas SH. Prescription drugs and the changing patterns of treatment for mental disorders, 1996-2001. Health Aff (Millwood) 2005;24(1):195-205.
18. Glick ID, Murray SR, Vasudevan P, et al. Treatment with atypical antipsychotics: new indications and new populations. J Psychiatr Res 2001;35(3):187-91.
Intramuscular naltrexone
A long-acting, intramuscular (IM) naltrexone formulation—which at press time awaited FDA approval (Table)—could improve adherence to alcohol dependency pharmacotherapy.
Oral naltrexone can reduce alcohol consumption1 and relapse rates,1,2 but patients often stop taking it3 and increase their risk of relapse.2 Once-daily dosing, inconsistent motivation toward treatment, and cognitive impairment secondary to chronic alcohol dependence often thwart oral naltrexone therapy.
By contrast, IM naltrexone surmounts most compliance issues because you or a clinical assistant administer the drug. Short-term side effects—such as nausea for 2 days—are less likely to affect adherence because the medication keeps working weeks after side effects abate. This gives you time before the next dose to reassure the patient and gives the patient the benefits of continued treatment.
Table
IM naltrexone: Fast facts
| Drug brand name: Vivitrol |
| Class: Opioid antagonist |
| Prospective indication: Alcohol dependence |
| FDA action: Issued approvable letter Dec. 28, 2005 |
| Manufacturer: Alkermes |
| Dosing forms: 380 mg suspension via IM injection |
| Recommended dosage: 380 mg once monthly |
| Estimated date of availability: Spring 2006 |
How naltrexone works
Alcohol stimulates release of endogenous opioids, which in turn stimulate release of dopamine, which mediates reinforcement.4 Opioid receptor stimulation not associated with dopamine also reinforces alcohol use.5 Persons vulnerable to alcohol dependence generally have lower basal levels of opioid secretion and are stimulated at higher levels.6 Opioids also increase dopamine by inhibiting GABA neurons, which suppress dopamine release when uninhibited.
As an opioid antagonist, naltrexone prevents opioids from binding with μ-opioid receptors and modulates dopamine production. This may make drinking less “rewarding” and may reduce craving triggered by conditioned cues associated with alcohol use.
IM naltrexone is packaged in biodegradable microspheres that slowly release naltrexone for 1 month after injection. The microspheres are made of a polyactide-co-glycolide polymer used in other extended-release drugs and in absorbable sutures.
Pharmacokinetics
IM naltrexone plasma levels peak 2 to 3 days after injection, then decline gradually over 30 days. Oral naltrexone dosed at 50 mg/d for 30 days—a cumulative dose of 1,500 mg/month—produces daily peak plasma levels of approximately 10 ng/mL and troughs approaching zero. A once-monthly IM naltrexone injection results in a lower net dose but more-sustained naltrexone levels.
Efficacy
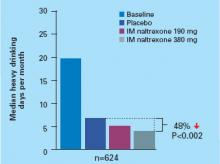
IM naltrexone significantly reduced heavy drinking among alcohol-dependent patients in a phase 3 randomized, placebo-controlled, multicenter trial.7 Actively drinking adults who met DSM-IV criteria for alcohol dependence (N=624) received IM naltrexone, 190 or 380 mg, or placebo every 4 weeks for 6 months. Oral naltrexone lead-in doses were not given. All patients also received 12 sessions of standardized supportive psychosocial therapy during the study.
The primary efficacy measure was event rate of heavy drinking, defined as number of heavy drinking days (≥5 drinks/day for men, ≥4 drinks/day for women) divided by number of days in the study. An event rate ratio (treatment-group to placebo-group event rate) was then estimated over time, taking into account patients who discontinued the study.
After 6 months, event rate of heavy drinking fell 25% among patients receiving 380 mg of IM naltrexone and supportive therapy, compared with patients receiving placebo and supportive therapy (P=0.02). That rate decreased 17% among patients who received 190 mg of IM naltrexone compared with placebo, but the difference between the two treatment groups was not statistically significant (P=0.07).
The median number of heavy drinking days per month decreased substantially across 6 months among all study groups. The decrease was more substantial among patients taking IM naltrexone, 380 mg, than among the placebo group (Figure).
Roughly 8% of patients abstained from drinking for 7 days before entering the study. Among patients who received 380 mg of IM naltrexone:
- those who were abstinent before the study had an 80% greater reduction in event rate of heavy drinking compared with placebo
- nonabstinent patients showed a 21% greater reduction in event rate of heavy drinking compared with placebo.
These findings suggest that IM naltrexone is more effective in persons abstaining from drinking but can also help actively drinking patients.
IM naltrexone also reduced heavy drinking among patients who entered a 1-year open-label extension study after completing the 6-month study.8 Drinking reductions were greater among patients who received 380 mg of naltrexone during both the 6-month and 1-year trials than among those who received placebo for 6 months and were switched to naltrexone, 380 mg, in the 1-year extension.
Figure Median heavy drinking days after 6 months of IM naltrexone or placebo
Source: Reference 7
Tolerability
IM naltrexone was well-tolerated in the phase 3 trial.7 Most-common adverse effects included
- nausea (reported by 33% of patients receiving 380 mg [n=205] and 25% of those receiving 190 mg [n=210])
- headache (22%, 16%)
- fatigue (20%, 16%).
At 380 mg, decreased appetite (13%), dizziness (13%), and injection site pain (12%) also differed significantly from placebo. Nausea was rated as mild or moderate in 95% of cases, usually occurred only after the first injection, and lasted 1 to 2 days on average.
Nine percent of patients taking naltrexone, 190 mg, or placebo also reported injection site pain. Approximately 1% of all patients dropped out because of injection site reactions.
Patients generally adhered to treatment, with 64% receiving 6 injections and 74% receiving at least 4. By comparison, a meta-analysis3 of oral naltrexone clinical trials showed an average 50% retention rate across studies, most of which lasted only 3 months. Study withdrawals because of adverse events were more prevalent among patients receiving IM naltrexone, 380 mg (14.1%), than among the placebo group (6.7%), but the number of serious adverse events differed little.7
Liver enzymes (AST and ALT) did not change significantly during the study. Gamma-glutamyltransferase decreased in all patients, consistent with reduced drinking.
Interactions between IM naltrexone and other medications are probably similar to those observed with oral naltrexone.
Contraindications
Although product labeling was not available when this article was written, IM naltrexone, like its oral form, will likely be contraindicated for patients who:
- are taking opioid analgesics
- are in acute opioid withdrawal
- test positive on urine screen for opioids
- have acute hepatitis or liver failure
- are taking maintenance methadone or buprenorphine or are opioid-dependent.
Patients should be opioid-free for 7 to 10 days before starting IM naltrexone to avoid acute withdrawal symptoms.
Before starting IM naltrexone in patients with a history of opioid abuse, give naloxone, 0.8 mg, to test for withdrawal. Do not start naltrexone if the patient shows signs of opioid withdrawal within 20 minutes of receiving naloxone.
Clinical implications
Long-acting IM naltrexone will make it easier to ensure treatment adherence, compared with oral naltrexone. Giving the drug during the office visit will change your practice patterns, but this increase in hands-on care could strengthen the therapeutic alliance. Compared with interpreting patient self-reports, you can also more accurately document adherence to IM naltrexone therapy.
All alcohol-dependence medications work best when combined with psychosocial treatment, and monthly medication visits alone will not provide patients the cognitive and skill-building work they need to recover. Patients early in recovery need to be seen much more often by you and/or another provider of recovery-oriented psychosocial treatment.
Which patients will be more receptive to in-office treatment is unclear. Patients who have relapsed because of nonadherence to oral medications may be more willing to try IM therapy after you explain its benefits. Similarly, IM naltrexone may be more beneficial to patients who:
- cannot adhere to oral medication because of cognitive problems or impulsivity
- face severe consequences—such as legal problems, loss of parental custody, or loss of employment—if treatment fails.
The optimal duration of IM naltrexone therapy is not known, but the injectable has shown efficacy after 6 months7 and 1 year.8 Some patients have taken it for more than 3 years.8 Before stopping IM naltrexone, consider whether the patient:
- has achieved sobriety
- has developed skills and external support to maintain sobriety
- has reduced craving intensity or time spent preoccupied with alcohol
- shows improved global psychosocial function as reflected in improved relationships, work performance, and general health.
Patients with family histories of alcohol dependence and who reduce days of heavy drinking but do not achieve sobriety on IM naltrexone are probably at higher risk of relapse to heavy drinking after stopping the medication.
Related resources
- Injectable naltrexone Web site. http://alkermes2005.ifactory.com/products/naltrexone.html.
Drug brand names
- IM naltrexone • Vivitrol
- Oral naltrexone • Depade
- Naloxone • Narcan
Disclosure
Dr. Rosenthal is a consultant for Forest Laboratories and Alkermes.
1. Anton RF, Moak DH, Waid LR, et al. Naltrexone and cognitive behavioral therapy for the treatment of outpatient alcoholics: results of a placebo-controlled trial. Am J Psychiatry 1999;156:1758-64.
2. Pettinati HM, Volpicelli JR, Pierce JD, Jr, O’Brien CP. Improving naltrexone response: An intervention for medical practitioners toenhance medication compliance in alcohol dependent patients. J Addict Dis 2000;19:71-83.
3. Bouza C, Magro A, Munoz A, Amate J. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: a systematic review. Addiction 2004;99:811-28.
4. Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther 1993;267:250-8.
5. Pettit HO, Ettenberg A, Bloom FE, Koob GF. Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology (Berl) 1984;84:167-73.
6. Gianoulakis C. Characterization of the effects of acute ethanol administration on the release of beta-endorphin peptides by the rat hypothalamus. Eur J Pharmacol 1990;180:21-9.
7. Garbutt JC, Kranzler HR, O’Malley SS, et al. Vivitrex Study Group. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA 2005;293:1617-25.
8. Gastfriend DR, Dong Q, Loewy J, et al. Durability of effect of long-acting injectable naltrexone. Presented at: Annual Meeting of the American Psychiatric Association; 2005; Atlanta, GA.
A long-acting, intramuscular (IM) naltrexone formulation—which at press time awaited FDA approval (Table)—could improve adherence to alcohol dependency pharmacotherapy.
Oral naltrexone can reduce alcohol consumption1 and relapse rates,1,2 but patients often stop taking it3 and increase their risk of relapse.2 Once-daily dosing, inconsistent motivation toward treatment, and cognitive impairment secondary to chronic alcohol dependence often thwart oral naltrexone therapy.
By contrast, IM naltrexone surmounts most compliance issues because you or a clinical assistant administer the drug. Short-term side effects—such as nausea for 2 days—are less likely to affect adherence because the medication keeps working weeks after side effects abate. This gives you time before the next dose to reassure the patient and gives the patient the benefits of continued treatment.
Table
IM naltrexone: Fast facts
| Drug brand name: Vivitrol |
| Class: Opioid antagonist |
| Prospective indication: Alcohol dependence |
| FDA action: Issued approvable letter Dec. 28, 2005 |
| Manufacturer: Alkermes |
| Dosing forms: 380 mg suspension via IM injection |
| Recommended dosage: 380 mg once monthly |
| Estimated date of availability: Spring 2006 |
How naltrexone works
Alcohol stimulates release of endogenous opioids, which in turn stimulate release of dopamine, which mediates reinforcement.4 Opioid receptor stimulation not associated with dopamine also reinforces alcohol use.5 Persons vulnerable to alcohol dependence generally have lower basal levels of opioid secretion and are stimulated at higher levels.6 Opioids also increase dopamine by inhibiting GABA neurons, which suppress dopamine release when uninhibited.
As an opioid antagonist, naltrexone prevents opioids from binding with μ-opioid receptors and modulates dopamine production. This may make drinking less “rewarding” and may reduce craving triggered by conditioned cues associated with alcohol use.
IM naltrexone is packaged in biodegradable microspheres that slowly release naltrexone for 1 month after injection. The microspheres are made of a polyactide-co-glycolide polymer used in other extended-release drugs and in absorbable sutures.
Pharmacokinetics
IM naltrexone plasma levels peak 2 to 3 days after injection, then decline gradually over 30 days. Oral naltrexone dosed at 50 mg/d for 30 days—a cumulative dose of 1,500 mg/month—produces daily peak plasma levels of approximately 10 ng/mL and troughs approaching zero. A once-monthly IM naltrexone injection results in a lower net dose but more-sustained naltrexone levels.
Efficacy
IM naltrexone significantly reduced heavy drinking among alcohol-dependent patients in a phase 3 randomized, placebo-controlled, multicenter trial.7 Actively drinking adults who met DSM-IV criteria for alcohol dependence (N=624) received IM naltrexone, 190 or 380 mg, or placebo every 4 weeks for 6 months. Oral naltrexone lead-in doses were not given. All patients also received 12 sessions of standardized supportive psychosocial therapy during the study.
The primary efficacy measure was event rate of heavy drinking, defined as number of heavy drinking days (≥5 drinks/day for men, ≥4 drinks/day for women) divided by number of days in the study. An event rate ratio (treatment-group to placebo-group event rate) was then estimated over time, taking into account patients who discontinued the study.
After 6 months, event rate of heavy drinking fell 25% among patients receiving 380 mg of IM naltrexone and supportive therapy, compared with patients receiving placebo and supportive therapy (P=0.02). That rate decreased 17% among patients who received 190 mg of IM naltrexone compared with placebo, but the difference between the two treatment groups was not statistically significant (P=0.07).
The median number of heavy drinking days per month decreased substantially across 6 months among all study groups. The decrease was more substantial among patients taking IM naltrexone, 380 mg, than among the placebo group (Figure).
Roughly 8% of patients abstained from drinking for 7 days before entering the study. Among patients who received 380 mg of IM naltrexone:
- those who were abstinent before the study had an 80% greater reduction in event rate of heavy drinking compared with placebo
- nonabstinent patients showed a 21% greater reduction in event rate of heavy drinking compared with placebo.
These findings suggest that IM naltrexone is more effective in persons abstaining from drinking but can also help actively drinking patients.
IM naltrexone also reduced heavy drinking among patients who entered a 1-year open-label extension study after completing the 6-month study.8 Drinking reductions were greater among patients who received 380 mg of naltrexone during both the 6-month and 1-year trials than among those who received placebo for 6 months and were switched to naltrexone, 380 mg, in the 1-year extension.
Figure Median heavy drinking days after 6 months of IM naltrexone or placebo
Source: Reference 7
Tolerability
IM naltrexone was well-tolerated in the phase 3 trial.7 Most-common adverse effects included
- nausea (reported by 33% of patients receiving 380 mg [n=205] and 25% of those receiving 190 mg [n=210])
- headache (22%, 16%)
- fatigue (20%, 16%).
At 380 mg, decreased appetite (13%), dizziness (13%), and injection site pain (12%) also differed significantly from placebo. Nausea was rated as mild or moderate in 95% of cases, usually occurred only after the first injection, and lasted 1 to 2 days on average.
Nine percent of patients taking naltrexone, 190 mg, or placebo also reported injection site pain. Approximately 1% of all patients dropped out because of injection site reactions.
Patients generally adhered to treatment, with 64% receiving 6 injections and 74% receiving at least 4. By comparison, a meta-analysis3 of oral naltrexone clinical trials showed an average 50% retention rate across studies, most of which lasted only 3 months. Study withdrawals because of adverse events were more prevalent among patients receiving IM naltrexone, 380 mg (14.1%), than among the placebo group (6.7%), but the number of serious adverse events differed little.7
Liver enzymes (AST and ALT) did not change significantly during the study. Gamma-glutamyltransferase decreased in all patients, consistent with reduced drinking.
Interactions between IM naltrexone and other medications are probably similar to those observed with oral naltrexone.
Contraindications
Although product labeling was not available when this article was written, IM naltrexone, like its oral form, will likely be contraindicated for patients who:
- are taking opioid analgesics
- are in acute opioid withdrawal
- test positive on urine screen for opioids
- have acute hepatitis or liver failure
- are taking maintenance methadone or buprenorphine or are opioid-dependent.
Patients should be opioid-free for 7 to 10 days before starting IM naltrexone to avoid acute withdrawal symptoms.
Before starting IM naltrexone in patients with a history of opioid abuse, give naloxone, 0.8 mg, to test for withdrawal. Do not start naltrexone if the patient shows signs of opioid withdrawal within 20 minutes of receiving naloxone.
Clinical implications
Long-acting IM naltrexone will make it easier to ensure treatment adherence, compared with oral naltrexone. Giving the drug during the office visit will change your practice patterns, but this increase in hands-on care could strengthen the therapeutic alliance. Compared with interpreting patient self-reports, you can also more accurately document adherence to IM naltrexone therapy.
All alcohol-dependence medications work best when combined with psychosocial treatment, and monthly medication visits alone will not provide patients the cognitive and skill-building work they need to recover. Patients early in recovery need to be seen much more often by you and/or another provider of recovery-oriented psychosocial treatment.
Which patients will be more receptive to in-office treatment is unclear. Patients who have relapsed because of nonadherence to oral medications may be more willing to try IM therapy after you explain its benefits. Similarly, IM naltrexone may be more beneficial to patients who:
- cannot adhere to oral medication because of cognitive problems or impulsivity
- face severe consequences—such as legal problems, loss of parental custody, or loss of employment—if treatment fails.
The optimal duration of IM naltrexone therapy is not known, but the injectable has shown efficacy after 6 months7 and 1 year.8 Some patients have taken it for more than 3 years.8 Before stopping IM naltrexone, consider whether the patient:
- has achieved sobriety
- has developed skills and external support to maintain sobriety
- has reduced craving intensity or time spent preoccupied with alcohol
- shows improved global psychosocial function as reflected in improved relationships, work performance, and general health.
Patients with family histories of alcohol dependence and who reduce days of heavy drinking but do not achieve sobriety on IM naltrexone are probably at higher risk of relapse to heavy drinking after stopping the medication.
Related resources
- Injectable naltrexone Web site. http://alkermes2005.ifactory.com/products/naltrexone.html.
Drug brand names
- IM naltrexone • Vivitrol
- Oral naltrexone • Depade
- Naloxone • Narcan
Disclosure
Dr. Rosenthal is a consultant for Forest Laboratories and Alkermes.
A long-acting, intramuscular (IM) naltrexone formulation—which at press time awaited FDA approval (Table)—could improve adherence to alcohol dependency pharmacotherapy.
Oral naltrexone can reduce alcohol consumption1 and relapse rates,1,2 but patients often stop taking it3 and increase their risk of relapse.2 Once-daily dosing, inconsistent motivation toward treatment, and cognitive impairment secondary to chronic alcohol dependence often thwart oral naltrexone therapy.
By contrast, IM naltrexone surmounts most compliance issues because you or a clinical assistant administer the drug. Short-term side effects—such as nausea for 2 days—are less likely to affect adherence because the medication keeps working weeks after side effects abate. This gives you time before the next dose to reassure the patient and gives the patient the benefits of continued treatment.
Table
IM naltrexone: Fast facts
| Drug brand name: Vivitrol |
| Class: Opioid antagonist |
| Prospective indication: Alcohol dependence |
| FDA action: Issued approvable letter Dec. 28, 2005 |
| Manufacturer: Alkermes |
| Dosing forms: 380 mg suspension via IM injection |
| Recommended dosage: 380 mg once monthly |
| Estimated date of availability: Spring 2006 |
How naltrexone works
Alcohol stimulates release of endogenous opioids, which in turn stimulate release of dopamine, which mediates reinforcement.4 Opioid receptor stimulation not associated with dopamine also reinforces alcohol use.5 Persons vulnerable to alcohol dependence generally have lower basal levels of opioid secretion and are stimulated at higher levels.6 Opioids also increase dopamine by inhibiting GABA neurons, which suppress dopamine release when uninhibited.
As an opioid antagonist, naltrexone prevents opioids from binding with μ-opioid receptors and modulates dopamine production. This may make drinking less “rewarding” and may reduce craving triggered by conditioned cues associated with alcohol use.
IM naltrexone is packaged in biodegradable microspheres that slowly release naltrexone for 1 month after injection. The microspheres are made of a polyactide-co-glycolide polymer used in other extended-release drugs and in absorbable sutures.
Pharmacokinetics
IM naltrexone plasma levels peak 2 to 3 days after injection, then decline gradually over 30 days. Oral naltrexone dosed at 50 mg/d for 30 days—a cumulative dose of 1,500 mg/month—produces daily peak plasma levels of approximately 10 ng/mL and troughs approaching zero. A once-monthly IM naltrexone injection results in a lower net dose but more-sustained naltrexone levels.
Efficacy
IM naltrexone significantly reduced heavy drinking among alcohol-dependent patients in a phase 3 randomized, placebo-controlled, multicenter trial.7 Actively drinking adults who met DSM-IV criteria for alcohol dependence (N=624) received IM naltrexone, 190 or 380 mg, or placebo every 4 weeks for 6 months. Oral naltrexone lead-in doses were not given. All patients also received 12 sessions of standardized supportive psychosocial therapy during the study.
The primary efficacy measure was event rate of heavy drinking, defined as number of heavy drinking days (≥5 drinks/day for men, ≥4 drinks/day for women) divided by number of days in the study. An event rate ratio (treatment-group to placebo-group event rate) was then estimated over time, taking into account patients who discontinued the study.
After 6 months, event rate of heavy drinking fell 25% among patients receiving 380 mg of IM naltrexone and supportive therapy, compared with patients receiving placebo and supportive therapy (P=0.02). That rate decreased 17% among patients who received 190 mg of IM naltrexone compared with placebo, but the difference between the two treatment groups was not statistically significant (P=0.07).
The median number of heavy drinking days per month decreased substantially across 6 months among all study groups. The decrease was more substantial among patients taking IM naltrexone, 380 mg, than among the placebo group (Figure).
Roughly 8% of patients abstained from drinking for 7 days before entering the study. Among patients who received 380 mg of IM naltrexone:
- those who were abstinent before the study had an 80% greater reduction in event rate of heavy drinking compared with placebo
- nonabstinent patients showed a 21% greater reduction in event rate of heavy drinking compared with placebo.
These findings suggest that IM naltrexone is more effective in persons abstaining from drinking but can also help actively drinking patients.
IM naltrexone also reduced heavy drinking among patients who entered a 1-year open-label extension study after completing the 6-month study.8 Drinking reductions were greater among patients who received 380 mg of naltrexone during both the 6-month and 1-year trials than among those who received placebo for 6 months and were switched to naltrexone, 380 mg, in the 1-year extension.
Figure Median heavy drinking days after 6 months of IM naltrexone or placebo
Source: Reference 7
Tolerability
IM naltrexone was well-tolerated in the phase 3 trial.7 Most-common adverse effects included
- nausea (reported by 33% of patients receiving 380 mg [n=205] and 25% of those receiving 190 mg [n=210])
- headache (22%, 16%)
- fatigue (20%, 16%).
At 380 mg, decreased appetite (13%), dizziness (13%), and injection site pain (12%) also differed significantly from placebo. Nausea was rated as mild or moderate in 95% of cases, usually occurred only after the first injection, and lasted 1 to 2 days on average.
Nine percent of patients taking naltrexone, 190 mg, or placebo also reported injection site pain. Approximately 1% of all patients dropped out because of injection site reactions.
Patients generally adhered to treatment, with 64% receiving 6 injections and 74% receiving at least 4. By comparison, a meta-analysis3 of oral naltrexone clinical trials showed an average 50% retention rate across studies, most of which lasted only 3 months. Study withdrawals because of adverse events were more prevalent among patients receiving IM naltrexone, 380 mg (14.1%), than among the placebo group (6.7%), but the number of serious adverse events differed little.7
Liver enzymes (AST and ALT) did not change significantly during the study. Gamma-glutamyltransferase decreased in all patients, consistent with reduced drinking.
Interactions between IM naltrexone and other medications are probably similar to those observed with oral naltrexone.
Contraindications
Although product labeling was not available when this article was written, IM naltrexone, like its oral form, will likely be contraindicated for patients who:
- are taking opioid analgesics
- are in acute opioid withdrawal
- test positive on urine screen for opioids
- have acute hepatitis or liver failure
- are taking maintenance methadone or buprenorphine or are opioid-dependent.
Patients should be opioid-free for 7 to 10 days before starting IM naltrexone to avoid acute withdrawal symptoms.
Before starting IM naltrexone in patients with a history of opioid abuse, give naloxone, 0.8 mg, to test for withdrawal. Do not start naltrexone if the patient shows signs of opioid withdrawal within 20 minutes of receiving naloxone.
Clinical implications
Long-acting IM naltrexone will make it easier to ensure treatment adherence, compared with oral naltrexone. Giving the drug during the office visit will change your practice patterns, but this increase in hands-on care could strengthen the therapeutic alliance. Compared with interpreting patient self-reports, you can also more accurately document adherence to IM naltrexone therapy.
All alcohol-dependence medications work best when combined with psychosocial treatment, and monthly medication visits alone will not provide patients the cognitive and skill-building work they need to recover. Patients early in recovery need to be seen much more often by you and/or another provider of recovery-oriented psychosocial treatment.
Which patients will be more receptive to in-office treatment is unclear. Patients who have relapsed because of nonadherence to oral medications may be more willing to try IM therapy after you explain its benefits. Similarly, IM naltrexone may be more beneficial to patients who:
- cannot adhere to oral medication because of cognitive problems or impulsivity
- face severe consequences—such as legal problems, loss of parental custody, or loss of employment—if treatment fails.
The optimal duration of IM naltrexone therapy is not known, but the injectable has shown efficacy after 6 months7 and 1 year.8 Some patients have taken it for more than 3 years.8 Before stopping IM naltrexone, consider whether the patient:
- has achieved sobriety
- has developed skills and external support to maintain sobriety
- has reduced craving intensity or time spent preoccupied with alcohol
- shows improved global psychosocial function as reflected in improved relationships, work performance, and general health.
Patients with family histories of alcohol dependence and who reduce days of heavy drinking but do not achieve sobriety on IM naltrexone are probably at higher risk of relapse to heavy drinking after stopping the medication.
Related resources
- Injectable naltrexone Web site. http://alkermes2005.ifactory.com/products/naltrexone.html.
Drug brand names
- IM naltrexone • Vivitrol
- Oral naltrexone • Depade
- Naloxone • Narcan
Disclosure
Dr. Rosenthal is a consultant for Forest Laboratories and Alkermes.
1. Anton RF, Moak DH, Waid LR, et al. Naltrexone and cognitive behavioral therapy for the treatment of outpatient alcoholics: results of a placebo-controlled trial. Am J Psychiatry 1999;156:1758-64.
2. Pettinati HM, Volpicelli JR, Pierce JD, Jr, O’Brien CP. Improving naltrexone response: An intervention for medical practitioners toenhance medication compliance in alcohol dependent patients. J Addict Dis 2000;19:71-83.
3. Bouza C, Magro A, Munoz A, Amate J. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: a systematic review. Addiction 2004;99:811-28.
4. Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther 1993;267:250-8.
5. Pettit HO, Ettenberg A, Bloom FE, Koob GF. Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology (Berl) 1984;84:167-73.
6. Gianoulakis C. Characterization of the effects of acute ethanol administration on the release of beta-endorphin peptides by the rat hypothalamus. Eur J Pharmacol 1990;180:21-9.
7. Garbutt JC, Kranzler HR, O’Malley SS, et al. Vivitrex Study Group. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA 2005;293:1617-25.
8. Gastfriend DR, Dong Q, Loewy J, et al. Durability of effect of long-acting injectable naltrexone. Presented at: Annual Meeting of the American Psychiatric Association; 2005; Atlanta, GA.
1. Anton RF, Moak DH, Waid LR, et al. Naltrexone and cognitive behavioral therapy for the treatment of outpatient alcoholics: results of a placebo-controlled trial. Am J Psychiatry 1999;156:1758-64.
2. Pettinati HM, Volpicelli JR, Pierce JD, Jr, O’Brien CP. Improving naltrexone response: An intervention for medical practitioners toenhance medication compliance in alcohol dependent patients. J Addict Dis 2000;19:71-83.
3. Bouza C, Magro A, Munoz A, Amate J. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: a systematic review. Addiction 2004;99:811-28.
4. Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther 1993;267:250-8.
5. Pettit HO, Ettenberg A, Bloom FE, Koob GF. Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology (Berl) 1984;84:167-73.
6. Gianoulakis C. Characterization of the effects of acute ethanol administration on the release of beta-endorphin peptides by the rat hypothalamus. Eur J Pharmacol 1990;180:21-9.
7. Garbutt JC, Kranzler HR, O’Malley SS, et al. Vivitrex Study Group. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA 2005;293:1617-25.
8. Gastfriend DR, Dong Q, Loewy J, et al. Durability of effect of long-acting injectable naltrexone. Presented at: Annual Meeting of the American Psychiatric Association; 2005; Atlanta, GA.
Oral board jitters? Try these rehearsal tips
Many candidates become anxious before and during the American Board of Psychiatry and Neurology (ABPN) part II oral exam, especially if they failed before. As many as 50% of candidates fail the part II oral exam on the first try, and those who fail a second time risk failing several times.1
Studying more and worrying less can improve your chance of passing, whether it’s your first time taking the test or after an initial failure. You may also perform better if you begin the certification process promptly after residency training.2 By fitting rehearsal opportunities into a busy schedule, you—or residents you supervise—can prepare for the exam’s oral portion and reduce test-taking anxiety.
Form a study group. Take turns performing oral board-type interviews with other candidates. Give and receive informal feedback while you practice.
Conduct many mock board interviews. Mock interviews with volunteer patients who have given written or verbal consent are the most-thorough form of exam preparation, especially when supervised by psychiatrists who have been examiners. Try to do as many as your schedule permits.
Practice on your patients. When you see a new patient, keep the board process in mind and try to do a 30-minute interview. Imagine you are in front of the examiners.
Dictate evaluations as if presenting to examiners. Tape-recording dictations allows you to review your presentation later and critique yourself.
Practice for the exam’s video portion. Because this part is commonly overlooked, many candidates pass the live interview but fail the video portion.
For the video portion, you are asked to watch a short video of a patient interview and present a case based on information from the video. You can purchase sample videos from commercial test preparation organizations, which often advertise in American Psychiatric Association newsletters.
Videotape yourself interviewing and presenting the case. Reviewing the videos can help you identify and correct body language problems so that you convey warmth, empathy, and confidence. You can videotape an interview after obtaining written consent from the patient. If no patients agree, videotape yourself giving the case presentation only.
Practice in inpatient, outpatient, and office settings. This gives you a chance to practice interviewing patients with a variety of psychiatric conditions.
These tips can help you make the 30-minute interview and presentation second nature, reduce exam anxiety, and increase your chance of passing.
1. Moran M. Project helps candidate succeed on ABPN exam. Psychiatr News 2005;40(17):22.-
2. Juul D, Scully JH, Jr, Scheiber SC. Achieving board certification in psychiatry: a cohort study. Am J Psychiatry 2003;160(3):563-5.
Dr. Khawaja is staff psychiatrist, VA Medical Center, Minneapolis, and has recently been appointed assistant professor, department of psychiatry, University of Minnesota.
Many candidates become anxious before and during the American Board of Psychiatry and Neurology (ABPN) part II oral exam, especially if they failed before. As many as 50% of candidates fail the part II oral exam on the first try, and those who fail a second time risk failing several times.1
Studying more and worrying less can improve your chance of passing, whether it’s your first time taking the test or after an initial failure. You may also perform better if you begin the certification process promptly after residency training.2 By fitting rehearsal opportunities into a busy schedule, you—or residents you supervise—can prepare for the exam’s oral portion and reduce test-taking anxiety.
Form a study group. Take turns performing oral board-type interviews with other candidates. Give and receive informal feedback while you practice.
Conduct many mock board interviews. Mock interviews with volunteer patients who have given written or verbal consent are the most-thorough form of exam preparation, especially when supervised by psychiatrists who have been examiners. Try to do as many as your schedule permits.
Practice on your patients. When you see a new patient, keep the board process in mind and try to do a 30-minute interview. Imagine you are in front of the examiners.
Dictate evaluations as if presenting to examiners. Tape-recording dictations allows you to review your presentation later and critique yourself.
Practice for the exam’s video portion. Because this part is commonly overlooked, many candidates pass the live interview but fail the video portion.
For the video portion, you are asked to watch a short video of a patient interview and present a case based on information from the video. You can purchase sample videos from commercial test preparation organizations, which often advertise in American Psychiatric Association newsletters.
Videotape yourself interviewing and presenting the case. Reviewing the videos can help you identify and correct body language problems so that you convey warmth, empathy, and confidence. You can videotape an interview after obtaining written consent from the patient. If no patients agree, videotape yourself giving the case presentation only.
Practice in inpatient, outpatient, and office settings. This gives you a chance to practice interviewing patients with a variety of psychiatric conditions.
These tips can help you make the 30-minute interview and presentation second nature, reduce exam anxiety, and increase your chance of passing.
Many candidates become anxious before and during the American Board of Psychiatry and Neurology (ABPN) part II oral exam, especially if they failed before. As many as 50% of candidates fail the part II oral exam on the first try, and those who fail a second time risk failing several times.1
Studying more and worrying less can improve your chance of passing, whether it’s your first time taking the test or after an initial failure. You may also perform better if you begin the certification process promptly after residency training.2 By fitting rehearsal opportunities into a busy schedule, you—or residents you supervise—can prepare for the exam’s oral portion and reduce test-taking anxiety.
Form a study group. Take turns performing oral board-type interviews with other candidates. Give and receive informal feedback while you practice.
Conduct many mock board interviews. Mock interviews with volunteer patients who have given written or verbal consent are the most-thorough form of exam preparation, especially when supervised by psychiatrists who have been examiners. Try to do as many as your schedule permits.
Practice on your patients. When you see a new patient, keep the board process in mind and try to do a 30-minute interview. Imagine you are in front of the examiners.
Dictate evaluations as if presenting to examiners. Tape-recording dictations allows you to review your presentation later and critique yourself.
Practice for the exam’s video portion. Because this part is commonly overlooked, many candidates pass the live interview but fail the video portion.
For the video portion, you are asked to watch a short video of a patient interview and present a case based on information from the video. You can purchase sample videos from commercial test preparation organizations, which often advertise in American Psychiatric Association newsletters.
Videotape yourself interviewing and presenting the case. Reviewing the videos can help you identify and correct body language problems so that you convey warmth, empathy, and confidence. You can videotape an interview after obtaining written consent from the patient. If no patients agree, videotape yourself giving the case presentation only.
Practice in inpatient, outpatient, and office settings. This gives you a chance to practice interviewing patients with a variety of psychiatric conditions.
These tips can help you make the 30-minute interview and presentation second nature, reduce exam anxiety, and increase your chance of passing.
1. Moran M. Project helps candidate succeed on ABPN exam. Psychiatr News 2005;40(17):22.-
2. Juul D, Scully JH, Jr, Scheiber SC. Achieving board certification in psychiatry: a cohort study. Am J Psychiatry 2003;160(3):563-5.
Dr. Khawaja is staff psychiatrist, VA Medical Center, Minneapolis, and has recently been appointed assistant professor, department of psychiatry, University of Minnesota.
1. Moran M. Project helps candidate succeed on ABPN exam. Psychiatr News 2005;40(17):22.-
2. Juul D, Scully JH, Jr, Scheiber SC. Achieving board certification in psychiatry: a cohort study. Am J Psychiatry 2003;160(3):563-5.
Dr. Khawaja is staff psychiatrist, VA Medical Center, Minneapolis, and has recently been appointed assistant professor, department of psychiatry, University of Minnesota.
4 ECT electrode options: Which is best for your patient?
Patients with severe mood disorders tend to respond more favorably to electroconvulsive therapy (ECT) when electrode placement has been individually selected for them. Yet most ECT practitioners use one electrode placement—bitemporal—for all patients (Figure 1).1
While standard bitemporal ECT is generally most effective, it also produces the greatest degree of disorientation, which can prolong a hospital stay or increase the need for supportive care.
Bifrontal ECT (Figure 2, part A) has been shown as efficacious as bitemporal placement while producing less disorientation.2 I have found left anterior right temporal (LART) placement (Figure 2, part B), to be equally effective with fewer side effects.
Figure 1 Bitemporal electrode placement
This generally effective and most widely used electrode placement causes the greatest post-ECT disorientation.
Figure 2a Bifrontal placement
Figure 2b Left anterior right temporal (LART) placement
Figure 2c Right unilateral placement
Side Effects of Lart
Side effects such as disorientation and loss of self-care should be less severe and prevalent with LART than with other bilateral placements because:
- the left electrode is far anterior to the temporal lobe, rather than close to it as in bitemporal placement.
- LART avoids symmetrical effect, which can block compensation by the opposite hemisphere.
Hypothetically, some patients might not respond to bifrontal or LART placement but respond to bitemporal ECT, although no such instances have been reported in the literature. By contrast, response to bitemporal ECT after failure of right unilateral ECT (Figure 2, part C) is well known; indeed, studies of unilateral ECT typically include provisions for changing to bitemporal ECT. Moreover, early relapse (within 2 months) appears more frequently after unilateral ECT.
Patient Selection and Dosing
Low-dose right unilateral ECT should suffice in men younger than age 40 because they usually develop vigorous seizures without substantial disorientation afterward. Low dose in unilateral ECT is millicoulomb (mC) charge less than 4 times the patient’s age in years.
In patients who do not develop vigorous seizures—a common problem in men age >65—unilateral ECT is more likely to produce disappointing results than other ECT configurations. Moreover, severe confusion from unilateral ECT is not rare in patients older than 65. Unilateral ECT should not be a routine choice for this age group.
Stimulus dosing in effect alters electrode placement. High dose spreads the stimulus as if the electrodes were much larger. As a result, all forms of placement at high stimulus doses more closely resemble bitemporal ECT, increasing side effect risk and negating differences among the forms of electrode placement. In fact side effects from unilateral ECT at high stimulus doses approximate those of bitemporal ECT.3 Right unilateral ECT in this context has no apparent advantages.
When intervention with ECT is urgently needed—such as for patients with severe catatonia, inanition, or active suicidality—efficacy is paramount and bitemporal ECT is the usual choice. Typical starting dose in mC is 2.5 times age.
In nonemergency circumstances, my experience with LART placement has resulted in strong enthusiasm for ECT by nursing staff and patients who recognize improvement without noticeable side effects.
Clinicians who use ECT should obtain first-hand experience with all four methods of electrode placement. In addition, use of brief-pulse rather than sine-wave stimuli is as important to minimizing side effects as electrode placement.
Disclosure
Dr. Swartz has equity interests in Somatics, LLC, a manufacturer of ECT instruments.
1. Prudic J, Olfson M, Sackeim HA. Electro-convulsive therapy practices in the community. Psychol Med 2001;31:929-34.
2. Swartz CM, Nelson AI. Rational electroconvulsive therapy electrode placement. Psychiatry 2005 2005;2(7):37-43.Available at: http://psychiatrymmc.com/displayArticle.cfm?articleID=article14. Accessed February 6, 2006.
3. McCall WV, Dunn A, Rosenquist PB, Hughes D. Markedly suprathreshold right unilateral ECT versus minimally suprathreshold bilateral ECT: antidepressant and memory effects. J ECT 2002;3:126-9.
Dr. Swartz is professor and chief, division of psychiatric research, Southern Illinois University School of Medicine, Springfield, IL.
Patients with severe mood disorders tend to respond more favorably to electroconvulsive therapy (ECT) when electrode placement has been individually selected for them. Yet most ECT practitioners use one electrode placement—bitemporal—for all patients (Figure 1).1
While standard bitemporal ECT is generally most effective, it also produces the greatest degree of disorientation, which can prolong a hospital stay or increase the need for supportive care.
Bifrontal ECT (Figure 2, part A) has been shown as efficacious as bitemporal placement while producing less disorientation.2 I have found left anterior right temporal (LART) placement (Figure 2, part B), to be equally effective with fewer side effects.
Figure 1 Bitemporal electrode placement
This generally effective and most widely used electrode placement causes the greatest post-ECT disorientation.
Figure 2a Bifrontal placement
Figure 2b Left anterior right temporal (LART) placement
Figure 2c Right unilateral placement
Side Effects of Lart
Side effects such as disorientation and loss of self-care should be less severe and prevalent with LART than with other bilateral placements because:
- the left electrode is far anterior to the temporal lobe, rather than close to it as in bitemporal placement.
- LART avoids symmetrical effect, which can block compensation by the opposite hemisphere.
Hypothetically, some patients might not respond to bifrontal or LART placement but respond to bitemporal ECT, although no such instances have been reported in the literature. By contrast, response to bitemporal ECT after failure of right unilateral ECT (Figure 2, part C) is well known; indeed, studies of unilateral ECT typically include provisions for changing to bitemporal ECT. Moreover, early relapse (within 2 months) appears more frequently after unilateral ECT.
Patient Selection and Dosing
Low-dose right unilateral ECT should suffice in men younger than age 40 because they usually develop vigorous seizures without substantial disorientation afterward. Low dose in unilateral ECT is millicoulomb (mC) charge less than 4 times the patient’s age in years.
In patients who do not develop vigorous seizures—a common problem in men age >65—unilateral ECT is more likely to produce disappointing results than other ECT configurations. Moreover, severe confusion from unilateral ECT is not rare in patients older than 65. Unilateral ECT should not be a routine choice for this age group.
Stimulus dosing in effect alters electrode placement. High dose spreads the stimulus as if the electrodes were much larger. As a result, all forms of placement at high stimulus doses more closely resemble bitemporal ECT, increasing side effect risk and negating differences among the forms of electrode placement. In fact side effects from unilateral ECT at high stimulus doses approximate those of bitemporal ECT.3 Right unilateral ECT in this context has no apparent advantages.
When intervention with ECT is urgently needed—such as for patients with severe catatonia, inanition, or active suicidality—efficacy is paramount and bitemporal ECT is the usual choice. Typical starting dose in mC is 2.5 times age.
In nonemergency circumstances, my experience with LART placement has resulted in strong enthusiasm for ECT by nursing staff and patients who recognize improvement without noticeable side effects.
Clinicians who use ECT should obtain first-hand experience with all four methods of electrode placement. In addition, use of brief-pulse rather than sine-wave stimuli is as important to minimizing side effects as electrode placement.
Disclosure
Dr. Swartz has equity interests in Somatics, LLC, a manufacturer of ECT instruments.
Patients with severe mood disorders tend to respond more favorably to electroconvulsive therapy (ECT) when electrode placement has been individually selected for them. Yet most ECT practitioners use one electrode placement—bitemporal—for all patients (Figure 1).1
While standard bitemporal ECT is generally most effective, it also produces the greatest degree of disorientation, which can prolong a hospital stay or increase the need for supportive care.
Bifrontal ECT (Figure 2, part A) has been shown as efficacious as bitemporal placement while producing less disorientation.2 I have found left anterior right temporal (LART) placement (Figure 2, part B), to be equally effective with fewer side effects.
Figure 1 Bitemporal electrode placement
This generally effective and most widely used electrode placement causes the greatest post-ECT disorientation.
Figure 2a Bifrontal placement
Figure 2b Left anterior right temporal (LART) placement
Figure 2c Right unilateral placement
Side Effects of Lart
Side effects such as disorientation and loss of self-care should be less severe and prevalent with LART than with other bilateral placements because:
- the left electrode is far anterior to the temporal lobe, rather than close to it as in bitemporal placement.
- LART avoids symmetrical effect, which can block compensation by the opposite hemisphere.
Hypothetically, some patients might not respond to bifrontal or LART placement but respond to bitemporal ECT, although no such instances have been reported in the literature. By contrast, response to bitemporal ECT after failure of right unilateral ECT (Figure 2, part C) is well known; indeed, studies of unilateral ECT typically include provisions for changing to bitemporal ECT. Moreover, early relapse (within 2 months) appears more frequently after unilateral ECT.
Patient Selection and Dosing
Low-dose right unilateral ECT should suffice in men younger than age 40 because they usually develop vigorous seizures without substantial disorientation afterward. Low dose in unilateral ECT is millicoulomb (mC) charge less than 4 times the patient’s age in years.
In patients who do not develop vigorous seizures—a common problem in men age >65—unilateral ECT is more likely to produce disappointing results than other ECT configurations. Moreover, severe confusion from unilateral ECT is not rare in patients older than 65. Unilateral ECT should not be a routine choice for this age group.
Stimulus dosing in effect alters electrode placement. High dose spreads the stimulus as if the electrodes were much larger. As a result, all forms of placement at high stimulus doses more closely resemble bitemporal ECT, increasing side effect risk and negating differences among the forms of electrode placement. In fact side effects from unilateral ECT at high stimulus doses approximate those of bitemporal ECT.3 Right unilateral ECT in this context has no apparent advantages.
When intervention with ECT is urgently needed—such as for patients with severe catatonia, inanition, or active suicidality—efficacy is paramount and bitemporal ECT is the usual choice. Typical starting dose in mC is 2.5 times age.
In nonemergency circumstances, my experience with LART placement has resulted in strong enthusiasm for ECT by nursing staff and patients who recognize improvement without noticeable side effects.
Clinicians who use ECT should obtain first-hand experience with all four methods of electrode placement. In addition, use of brief-pulse rather than sine-wave stimuli is as important to minimizing side effects as electrode placement.
Disclosure
Dr. Swartz has equity interests in Somatics, LLC, a manufacturer of ECT instruments.
1. Prudic J, Olfson M, Sackeim HA. Electro-convulsive therapy practices in the community. Psychol Med 2001;31:929-34.
2. Swartz CM, Nelson AI. Rational electroconvulsive therapy electrode placement. Psychiatry 2005 2005;2(7):37-43.Available at: http://psychiatrymmc.com/displayArticle.cfm?articleID=article14. Accessed February 6, 2006.
3. McCall WV, Dunn A, Rosenquist PB, Hughes D. Markedly suprathreshold right unilateral ECT versus minimally suprathreshold bilateral ECT: antidepressant and memory effects. J ECT 2002;3:126-9.
Dr. Swartz is professor and chief, division of psychiatric research, Southern Illinois University School of Medicine, Springfield, IL.
1. Prudic J, Olfson M, Sackeim HA. Electro-convulsive therapy practices in the community. Psychol Med 2001;31:929-34.
2. Swartz CM, Nelson AI. Rational electroconvulsive therapy electrode placement. Psychiatry 2005 2005;2(7):37-43.Available at: http://psychiatrymmc.com/displayArticle.cfm?articleID=article14. Accessed February 6, 2006.
3. McCall WV, Dunn A, Rosenquist PB, Hughes D. Markedly suprathreshold right unilateral ECT versus minimally suprathreshold bilateral ECT: antidepressant and memory effects. J ECT 2002;3:126-9.
Dr. Swartz is professor and chief, division of psychiatric research, Southern Illinois University School of Medicine, Springfield, IL.
Glutamatergic TB drug ‘cools off’ anxiety disorders
Think of anxiety disorders as an overactive brain alarm that psychotropics and exposure therapy quiet via separate mechanisms. Psychotropics “cool off” the alarm by curtailing excitability of the amygdala, brainstem nuclei, and hypothalamus.1 Psychological treatments, particularly exposure therapy, seek to teach the brain not to fear the dreaded object.2
One would assume that combining medication and exposure therapy for anxiety would be beneficial, but results have been disappointing.3 Anxiolytics do not enhance—and many impede—learning that occurs during psychotherapy. When the medications are tapered, patients who receive psychotherapy plus placebo typically experience more-enduring benefit than those receiving psychotherapy plus active medication.4-6
An unlikely candidate—a glutamatergic tuberculosis (TB) drug—may offer a solution. The drug and others in its class may potentiate psychotherapy’s effects by enhancing learning rather than relief.7
Glutamate/learning link
Glutamate neurons have 3 types of glutamatergic receptors, with the NMDA and AMPA types perceived as most important because of their possible role in memory development. Creating new memories may involve strengthening signals between glutamate neurons. The exact cellular mechanism is unknown, but it may involve greater release of neurotransmitters or formation of new synapses.
Stronger signaling—and hence learning—may depend on opening the NMDA receptor to enhance postsynaptic potential.8 Opening both NMDA and AMPA receptors generates a stronger signal and allows calcium influx, compared with opening the AMPA receptor alone (Figure). This combination can activate genes that control protein synthesis and result in structural changes necessary for developing long-term memories.
D-cycloserine—a partial agonist at the NMDA receptor—is usually used as an antibiotic to treat TB. The drug also has been shown to enhance the learning process that underlies fear extinction in rats. A group at Emory University studied the effect of adding the medication to exposure therapy in humans with acrophobia.7
FigureNMDA receptor agonists may enhance learning in psychotherapy
Normally, only glutamate neurons’ AMPA receptors activate in response to glutamate release (A). Both AMPA and NMDA receptors open in response to NMDA receptor agonists like D-cycloserine (B). Consequent stronger signaling may improve memory.
Going ‘up’
The researchers developed a virtual reality exposure system in which participants felt as if they were standing in an elevator, watching the floors recede as they rose 19 stories. Exposure therapy—seven weekly 35- to 45-minute sessions—has been shown to reduce the fear patients with acrophobia experience in virtual elevators.
Of 27 subjects, 10 received placebo and 17 received D-cycloserine, 50 mg or 500 mg. Subjects took their pills 2 to 4 hours before an exposure session. All participants experienced 2 virtual exposure sessions 1 to 2 weeks apart, which is considered suboptimal treatment for acrophobia.
Three months after the study, the D-cycloserine groups showed a markedly reduced fear of heights on the virtual elevator, while the placebo group showed no change from baseline. Fear levels were measured by subjective report of discomfort at each “floor.” D-cycloserine subjects also reported significantly greater reductions in measures of acrophobia in their daily lives.
Interestingly, both the D-cycloserine and placebo groups were equally frightened during virtual reality exposure. Only later did the D-cycloserine groups report less fear when exposed to heights, indicating that D-cycloserine enhanced learning that occurred during exposure therapy.
It’s exciting to think that medications could enhance and accelerate healing by activating the appropriate receptors during psychotherapy and give patients enduring benefits without the need for continued treatment.
If shown to be effective in larger studies, glutamatergic medications plus psychotherapy could provide more effective therapy for anxiety disorder. This approach is reported to be under investigation for treating anorexia nervosa, social phobia, panic disorder, and obsessive-compulsive disorder.9
1. Lydiard RB. Break the ‘fear circuit’ in resistant panic disorder. Current Psychiatry 2003;2(11):12-22.
2. Tynes LL, Tynes SF. Panic attacks: help sufferers recover with cognitive-behavioral therapy. Current Psychiatry 2005;4(11):51-60.
3. Otto MW, Smits JAJ, Reese HE. Combined psychotherapy and pharmacotherapy for mood and anxiety disorders in adults: review and analysis. Clinical Psychology: Science & Practice 2005;12(1):72-86.
4. Barlow DH, Gorman JM, Shear MK, Woods SW. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: A randomized controlled trial. JAMA 2000;283(19):2529-36.
5. Haug TT, Blomhoff S, Hellstrom K, et al. Exposure therapy and sertraline in social phobia: I-year follow-up of a randomised controlled trial. Br J Psychiatry 2003;182:312-8.
6. Marks IM, Swinson RP, Basoglu M, et al. Alprazolam and exposure alone and combined in panic disorder with agoraphobia. A controlled study in London and Toronto. Br J Psychiatry 1993;162:776-87.
7. Ressler KJ, Rothbaum BO, Tannenbaum L, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry 2004;61(11):1136-44.
8. Purves D, Augustine GJ, Fitzpatrick D, et al. Plasticity of mature synapses and circuits. In: Neuroscience (3rd ed). Sunderland, MA: Sinauer; 2004:575-610.
9. O’Connor A. A pill that helps ease grip of irrational fears. New York Times March 22, 2005.
Think of anxiety disorders as an overactive brain alarm that psychotropics and exposure therapy quiet via separate mechanisms. Psychotropics “cool off” the alarm by curtailing excitability of the amygdala, brainstem nuclei, and hypothalamus.1 Psychological treatments, particularly exposure therapy, seek to teach the brain not to fear the dreaded object.2
One would assume that combining medication and exposure therapy for anxiety would be beneficial, but results have been disappointing.3 Anxiolytics do not enhance—and many impede—learning that occurs during psychotherapy. When the medications are tapered, patients who receive psychotherapy plus placebo typically experience more-enduring benefit than those receiving psychotherapy plus active medication.4-6
An unlikely candidate—a glutamatergic tuberculosis (TB) drug—may offer a solution. The drug and others in its class may potentiate psychotherapy’s effects by enhancing learning rather than relief.7
Glutamate/learning link
Glutamate neurons have 3 types of glutamatergic receptors, with the NMDA and AMPA types perceived as most important because of their possible role in memory development. Creating new memories may involve strengthening signals between glutamate neurons. The exact cellular mechanism is unknown, but it may involve greater release of neurotransmitters or formation of new synapses.
Stronger signaling—and hence learning—may depend on opening the NMDA receptor to enhance postsynaptic potential.8 Opening both NMDA and AMPA receptors generates a stronger signal and allows calcium influx, compared with opening the AMPA receptor alone (Figure). This combination can activate genes that control protein synthesis and result in structural changes necessary for developing long-term memories.
D-cycloserine—a partial agonist at the NMDA receptor—is usually used as an antibiotic to treat TB. The drug also has been shown to enhance the learning process that underlies fear extinction in rats. A group at Emory University studied the effect of adding the medication to exposure therapy in humans with acrophobia.7
FigureNMDA receptor agonists may enhance learning in psychotherapy
Normally, only glutamate neurons’ AMPA receptors activate in response to glutamate release (A). Both AMPA and NMDA receptors open in response to NMDA receptor agonists like D-cycloserine (B). Consequent stronger signaling may improve memory.
Going ‘up’
The researchers developed a virtual reality exposure system in which participants felt as if they were standing in an elevator, watching the floors recede as they rose 19 stories. Exposure therapy—seven weekly 35- to 45-minute sessions—has been shown to reduce the fear patients with acrophobia experience in virtual elevators.
Of 27 subjects, 10 received placebo and 17 received D-cycloserine, 50 mg or 500 mg. Subjects took their pills 2 to 4 hours before an exposure session. All participants experienced 2 virtual exposure sessions 1 to 2 weeks apart, which is considered suboptimal treatment for acrophobia.
Three months after the study, the D-cycloserine groups showed a markedly reduced fear of heights on the virtual elevator, while the placebo group showed no change from baseline. Fear levels were measured by subjective report of discomfort at each “floor.” D-cycloserine subjects also reported significantly greater reductions in measures of acrophobia in their daily lives.
Interestingly, both the D-cycloserine and placebo groups were equally frightened during virtual reality exposure. Only later did the D-cycloserine groups report less fear when exposed to heights, indicating that D-cycloserine enhanced learning that occurred during exposure therapy.
It’s exciting to think that medications could enhance and accelerate healing by activating the appropriate receptors during psychotherapy and give patients enduring benefits without the need for continued treatment.
If shown to be effective in larger studies, glutamatergic medications plus psychotherapy could provide more effective therapy for anxiety disorder. This approach is reported to be under investigation for treating anorexia nervosa, social phobia, panic disorder, and obsessive-compulsive disorder.9
Think of anxiety disorders as an overactive brain alarm that psychotropics and exposure therapy quiet via separate mechanisms. Psychotropics “cool off” the alarm by curtailing excitability of the amygdala, brainstem nuclei, and hypothalamus.1 Psychological treatments, particularly exposure therapy, seek to teach the brain not to fear the dreaded object.2
One would assume that combining medication and exposure therapy for anxiety would be beneficial, but results have been disappointing.3 Anxiolytics do not enhance—and many impede—learning that occurs during psychotherapy. When the medications are tapered, patients who receive psychotherapy plus placebo typically experience more-enduring benefit than those receiving psychotherapy plus active medication.4-6
An unlikely candidate—a glutamatergic tuberculosis (TB) drug—may offer a solution. The drug and others in its class may potentiate psychotherapy’s effects by enhancing learning rather than relief.7
Glutamate/learning link
Glutamate neurons have 3 types of glutamatergic receptors, with the NMDA and AMPA types perceived as most important because of their possible role in memory development. Creating new memories may involve strengthening signals between glutamate neurons. The exact cellular mechanism is unknown, but it may involve greater release of neurotransmitters or formation of new synapses.
Stronger signaling—and hence learning—may depend on opening the NMDA receptor to enhance postsynaptic potential.8 Opening both NMDA and AMPA receptors generates a stronger signal and allows calcium influx, compared with opening the AMPA receptor alone (Figure). This combination can activate genes that control protein synthesis and result in structural changes necessary for developing long-term memories.
D-cycloserine—a partial agonist at the NMDA receptor—is usually used as an antibiotic to treat TB. The drug also has been shown to enhance the learning process that underlies fear extinction in rats. A group at Emory University studied the effect of adding the medication to exposure therapy in humans with acrophobia.7
FigureNMDA receptor agonists may enhance learning in psychotherapy
Normally, only glutamate neurons’ AMPA receptors activate in response to glutamate release (A). Both AMPA and NMDA receptors open in response to NMDA receptor agonists like D-cycloserine (B). Consequent stronger signaling may improve memory.
Going ‘up’
The researchers developed a virtual reality exposure system in which participants felt as if they were standing in an elevator, watching the floors recede as they rose 19 stories. Exposure therapy—seven weekly 35- to 45-minute sessions—has been shown to reduce the fear patients with acrophobia experience in virtual elevators.
Of 27 subjects, 10 received placebo and 17 received D-cycloserine, 50 mg or 500 mg. Subjects took their pills 2 to 4 hours before an exposure session. All participants experienced 2 virtual exposure sessions 1 to 2 weeks apart, which is considered suboptimal treatment for acrophobia.
Three months after the study, the D-cycloserine groups showed a markedly reduced fear of heights on the virtual elevator, while the placebo group showed no change from baseline. Fear levels were measured by subjective report of discomfort at each “floor.” D-cycloserine subjects also reported significantly greater reductions in measures of acrophobia in their daily lives.
Interestingly, both the D-cycloserine and placebo groups were equally frightened during virtual reality exposure. Only later did the D-cycloserine groups report less fear when exposed to heights, indicating that D-cycloserine enhanced learning that occurred during exposure therapy.
It’s exciting to think that medications could enhance and accelerate healing by activating the appropriate receptors during psychotherapy and give patients enduring benefits without the need for continued treatment.
If shown to be effective in larger studies, glutamatergic medications plus psychotherapy could provide more effective therapy for anxiety disorder. This approach is reported to be under investigation for treating anorexia nervosa, social phobia, panic disorder, and obsessive-compulsive disorder.9
1. Lydiard RB. Break the ‘fear circuit’ in resistant panic disorder. Current Psychiatry 2003;2(11):12-22.
2. Tynes LL, Tynes SF. Panic attacks: help sufferers recover with cognitive-behavioral therapy. Current Psychiatry 2005;4(11):51-60.
3. Otto MW, Smits JAJ, Reese HE. Combined psychotherapy and pharmacotherapy for mood and anxiety disorders in adults: review and analysis. Clinical Psychology: Science & Practice 2005;12(1):72-86.
4. Barlow DH, Gorman JM, Shear MK, Woods SW. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: A randomized controlled trial. JAMA 2000;283(19):2529-36.
5. Haug TT, Blomhoff S, Hellstrom K, et al. Exposure therapy and sertraline in social phobia: I-year follow-up of a randomised controlled trial. Br J Psychiatry 2003;182:312-8.
6. Marks IM, Swinson RP, Basoglu M, et al. Alprazolam and exposure alone and combined in panic disorder with agoraphobia. A controlled study in London and Toronto. Br J Psychiatry 1993;162:776-87.
7. Ressler KJ, Rothbaum BO, Tannenbaum L, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry 2004;61(11):1136-44.
8. Purves D, Augustine GJ, Fitzpatrick D, et al. Plasticity of mature synapses and circuits. In: Neuroscience (3rd ed). Sunderland, MA: Sinauer; 2004:575-610.
9. O’Connor A. A pill that helps ease grip of irrational fears. New York Times March 22, 2005.
1. Lydiard RB. Break the ‘fear circuit’ in resistant panic disorder. Current Psychiatry 2003;2(11):12-22.
2. Tynes LL, Tynes SF. Panic attacks: help sufferers recover with cognitive-behavioral therapy. Current Psychiatry 2005;4(11):51-60.
3. Otto MW, Smits JAJ, Reese HE. Combined psychotherapy and pharmacotherapy for mood and anxiety disorders in adults: review and analysis. Clinical Psychology: Science & Practice 2005;12(1):72-86.
4. Barlow DH, Gorman JM, Shear MK, Woods SW. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: A randomized controlled trial. JAMA 2000;283(19):2529-36.
5. Haug TT, Blomhoff S, Hellstrom K, et al. Exposure therapy and sertraline in social phobia: I-year follow-up of a randomised controlled trial. Br J Psychiatry 2003;182:312-8.
6. Marks IM, Swinson RP, Basoglu M, et al. Alprazolam and exposure alone and combined in panic disorder with agoraphobia. A controlled study in London and Toronto. Br J Psychiatry 1993;162:776-87.
7. Ressler KJ, Rothbaum BO, Tannenbaum L, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry 2004;61(11):1136-44.
8. Purves D, Augustine GJ, Fitzpatrick D, et al. Plasticity of mature synapses and circuits. In: Neuroscience (3rd ed). Sunderland, MA: Sinauer; 2004:575-610.
9. O’Connor A. A pill that helps ease grip of irrational fears. New York Times March 22, 2005.
2 therapies lift mood in chronic fatigue syndrome
Chronic fatigue syndrome (CFS) continues to puzzle and provoke. Years of research have failed to find a biomedical cause or to answer fundamental questions, such as “What is it?” and “Does it exist?”
Up to two-thirds of CFS patients have psychiatric disorders,1 but psychiatrists are not the first physicians CFS patients usually see. Primary care physicians may ask you to help confirm the diagnosis, manage patients’ anxiety and depression, differentiate CFS from somatoform disorders, or provide psychotherapy for patients and their families.
Knowing what transpires in the referring physician’s office is key to helping a patient function despite CFS. This article describes:
- CFS clinical features and possible causes
- psychiatric comorbidities and exclusions
- cognitive behavioral therapy (CBT) and graded exercise, the only two therapies shown to improve CFS patients’ daily function.
Case: Anxious, depressed, and tired
Mr. A, age 43, is referred to you with anxiety and depressed mood associated with CFS, diagnosed 2 years ago. Fatigue predated Mr. A’s depression and anxiety, which his primary care physician considers consequences of CFS.
Mr. A is married, has three children, and owns a successful accounting practice. CFS was diagnosed from the classic presentation: abrupt onset of fatigue despite good health and a promising career. Now, overwhelming fatigue reduces his productivity. He makes up for frequent rest breaks by working in snatches of time, even at 4 AM. He is spending little time with his wife and children.
Defining CFS
The Centers for Disease Control and Prevention (CDC) defined CFS in 1988 while investigating infectious causes of fatigue (Table 1).2,3 Epstein-Barr virus was thought to be the cause of CFS.
Most patients who present to their primary care physicians with complaints of fatigue do not meet CDC criteria for CFS, however. These “non-CFS” patients are diagnosed as having “idiopathic chronic fatigue,” a term that is not particularly helpful because CFS remains an idiopathic disorder.
Clinical findings. As with Mr. A, many CFS patients’ fatigue begins suddenly, often with flu-like symptoms. Patients lose tolerance for exercise and alcohol and become unable to work or socialize at pre-illness levels.
Medical symptoms can overlap those of other conditions, such as fibromyalgia, chemical sensitivities, and irritable bowel disorder. These associations make the condition difficult to assess and contribute to some physicians’ difficulty in accepting CFS as a biomedical condition. Mistrust and a poor patient-physician relationship can result when the physician doubts the symptoms’ “medical” nature and the patient resents the implication that the suffering is “all in your head.”
What causes CFS? No consistent factor has been identified that explains the pathophysiology of CFS symptoms. Many possibilities have been examined, but the evidence is confusing and contradictory.
A few preliminary studies suggest possible familial (shared environmental) and genetic components, but data are sparse and no more than suggestive.4 Findings of CNS studies are inconsistent, and the search for a change in immune function or an infectious agent has been fruitless despite some patients’ infection-like symptoms. The early hypothesis that Epstein-Barr virus was responsible has been disproved.
Imaging studies, psychological testing, and neuroendocrine investigations have identified abnormalities in some patients with CFS. The most-promising findings point to abnormalities in the hypothalamic-pituitary-adrenal axis and in serotonergic neurotransmission,5 suggesting an abnormal stress response in some patients.
Table 1
CDC diagnostic criteria for chronic fatigue syndrome
| At least 6 months of fatigue sufficient to “substantially reduce” patient’s level of activity |
4 or more of 8 concurrent symptoms:
|
| No obvious medical or psychiatric causes, such as eating disorders, psychosis, bipolar disorder, melancholia, or substance abuse* |
| CDC: Centers for Disease Control and Prevention |
| * Many nonpsychotic psychiatric disorders (such as atypical depression) do not exclude a CFS diagnosis |
| Source: References 1 and 2 |
Case continued: Test results are normal
Mr. A has undergone extensive medical assessment (complete blood cell count; renal, hepatic, and thyroid function tests; calcium, phosphate, and glucose determinations; and urinalysis), which yielded normal results. Brain MRI findings were also normal; specifically, no evidence of multiple sclerosis.
Even so, he has had nonspecific symptoms of impaired concentration, sore throat, tender cervical nodes, muscle pain, and nonrefreshing sleep. Physical exertion can leave him drained for at least 1 or 2 days.
Psychiatric Disorders
As many as 66% of CFS patients may have one or more psychiatric comorbidities; the most common are generalized anxiety disorder, panic disorder, depression, and somatoform disorder.1 Because CFS symptoms are regarded as being not fully explained by a known medical disorder, patients are often diagnosed as having an undifferentiated somatoform disorder. Either disorder could be diagnosed in some cases, but this differentiation sheds no new light on the condition.
CFS and depression. Could CFS and depression be one and the same? Proponents of that position point out the similarity of symptoms, loss of function, and—in at least some cases—favorable response to antidepressants. Opponents cite other factors such as:
- presence of sore throat, lymphadenopathy, and post-exercise fatigue
- differences in sleep patterns
- frequent absence of psychiatric illness before fatigue onset
- evidence of hypocortisolism (also seen in patients with melancholic depression) in some CFS patients.
Primary Care Workup
Complaints of long-lasting, debilitating fatigue should alert the primary care physician to CFS. Like somatization disorder, CFS requires a physical workup, though as few as 2% of CFS patients are found to have an undiagnosed medical illness that explains the symptoms.9 The evaluation’s goal is not so much to find out what’s causing the fatigue as to reassure the patient that all avenues are considered before the diagnosis is made. When this is accomplished well, the patient is likely to accept psychiatric referral or treatment, if needed.
Two-part initial evaluation. If the initial physical exam and laboratory work find no biomedical cause for the patient’s chronic fatigue symptoms, we recommend a two-part primary care evaluation. This includes a focused discussion with the patient about CFS (Table 2).10 Goals of the first session are to:
- establish a relationship that will survive difficult times
- teach the patient to think of complex medical problems as having psychological and social consequences, if not causes.
Primary care physicians usually request a psychiatric consultation to confirm or rule out psychiatric conditions that exclude a CFS diagnosis (melancholic depression, bipolar disorder, schizophrenia, anorexia nervosa or bulimia, and recent substance abuse). They also may refer in cases of other common disorders with poorly explained symptoms such as fibromyalgia and chemical sensitivity disorder.
Table 2
Two-part initial primary care evaluation of chronic fatigue
First session
|
Case continued: High anxiety
Mr. A describes how fatigue is affecting his work and home life. He is especially worried that he will not be attentive enough to catch accounting errors by his employees.
Interestingly, his anxiety remits but fatigue continues when he goes on vacation. He has no history of melancholic depression, bipolar disorder, psychosis, or substance abuse.
Psychiatric Assessment
The referring physician should provide a full account of the medical workup. This:
- assures you that possible medical causes of fatigue have been excluded
- provides information on psychiatric history and previous treatments
- delineates information on initial treatment efforts.
Realize how defensive a patient may feel about being given a vague and disputed diagnosis such as CFS. Because the diagnosis depends somewhat on examining his or her volitional contribution to the symptoms,6 your listening skills are key to building the patient-physician relationship. Taking the patient’s suffering seriously is essential and may provide great relief.
When you confirm a CFS diagnosis, the next step is to identify any frequently occurring psychiatric comorbidities, such as nonmelancholic depression, anxiety, and somatoform disorders.
Psychiatric Treatment
CBT and exercise. Only CBT and graded exercise therapy yielded “promising results” in a systematic review of all CFS treatments studied in 44 controlled treatment trials.11 By comparison, evidence is inconclusive or insufficient to support the use of:
- immunoglobulins or hydrocortisone
- most psychotropics—including all classes of antidepressants.
- Patients learn about their illness, develop a realistic assessment of their limitations, and come to understand that physical activity will not harm them.
- Patients begin graded exercises designed to slowly extend their exercise tolerance and widen their range of daily activities.
Trained psychologists usually do this work, and your role is to be aware of the key part this approach plays in managing CFS patients and to set up appropriate referrals.
Case continued: Relief and acceptance
Mr. A continues to see you and a therapist for treatment of mild depression and severe anxiety. His behavioral therapy focuses on helping him cope with how his illness limits his relationship to work and family. His therapist also explores with him the personal meanings of his new situation, his feelings about issues such as dependence, and limitations imposed on his life goals.
You start a trial of fluoxetine (up to 40 mg/d for several months) with minimal benefit. You then try nortriptyline, 25 mg nightly, and clonazepam, 0.5 mg bid. Although these drugs can be sedating, Mr. A reports feeling no more fatigued than he was before taking them. He improves slightly after 7 months but not enough that he wants to continue the medication.
Medications. Neither psychiatric nor other medication classes have shown efficacy in treating CFS core symptoms.15 One recent study16 found citalopram helped reduce chronic fatigue, but the study was small and uncontrolled. Although the subjects had chronic fatigue, not all met the formal definition of CFS for study inclusion.
Medication does play an important role in treating comorbid anxiety and depression. Usual psychopharmacologic strategies are appropriate. As in Mr. A’s case, most psychiatrists use SSRIs as first-line medications, but side effects are probably the most useful guide to medication choice.
Case continued: Additional treatment
Because medication has had little effect on Mr. A’s anxiety and depressed mood, you suggest adding a graded exercise program to his treatment plan. He improves steadily over time and says he is pleased. Although progress is slow, he finds it reassuring to be accomplishing realistic goals. He realizes that you and the therapist do not have the answer to his illness, but he trusts you and is comforted that you accept his condition and are willing to listen and help.
Related resources
- Afari N, Buchwald D. Chronic fatigue syndrome: a review. Am J Psychiatry 2003;160:221-36.
- Reid S, Chalder T, Cleare A, et al. Chronic fatigue syndrome. Clin Evid 2004;12:1578-93.
- Centers for Disease Control and Prevention. Chronic fatigue syndrome. http://www.cdc.gov/ncidod/diseases/cfs/.
- International Association for Chronic Fatigue Syndrome. http://www.aacfs.org.
- Citalopram • Celexa, others
- Clonazepam • Lorazepam, others
- Fluoxetine • Prozac
- Nortriptyline • Aventyl, others
The authors report no financial relationship with any company whos products are mentioned in this article or with manufacturers of competing products.
1. Kroenke K, Wood DR, Mangelsdorff AD, et al. Chronic fatigue in primary care: prevalence, patient characteristics, and outcome. JAMA 1988;260:929-34.
2. Holmes GP, Kaplan JE, Gantz NM, et al. Chronic fatigue syndrome: a working case definition. Ann Intern Med 1988;108:387-9.
3. Goshorn RK. Chronic fatigue syndrome: a review for clinicians. Semin Neurol 1998;18:237-42.
4. Afari N, Buchwald D. Chronic fatigue syndrome: a review. Am J Psychiatry 2003;160:221-36.
5. Parker AJR, Wessely S, Cleare AJ. The neuroendocrinology of chronic fatigue syndrome and fibromyalgia. Psychol Med 2001;31:1331-45.
6. Clarke JN, James S. The radicalized self: the impact on the self of the contested nature of the diagnosis of chronic fatigue syndrome. Soc Sci Med 2003;57:1387-95.
7. Demitrack MA. The psychobiology of chronic fatigue: the central nervous system as a final common pathway. In: Demitrack MA, Abbey SE (eds). Chronic fatigue syndrome: an integrative approach to evaluation and treatment. New York: Guilford Press; 1996:72-109.
8. Cassem EH. Depression and anxiety secondary to medical illness. Psychiatr Clin North Am 1990;13:597-612.
9. Lane TJ, Matthews DA, Manu P. The low yield of physical examinations and laboratory investigations of patients with chronic fatigue. Am J Med Sci 1990;299:313-18.
10. Ruffin MT, Margo GM, Margo KL. Puzzling physical conditions (monograph, edition No. 209). Home study self-assessment program. Presented at: American Academy of Family Physicians; October 1996; Kansas City, MO.
11. Whiting P, Bagnall A, Sowden A, et al. Interventions for the treatment and management of chronic fatigue syndrome: a systematic review. JAMA 2001;286:1360-8.
12. Deale A, Husain K, Chalder T, Wessely S. Long-term outcome of cognitive behavior therapy versus relaxation therapy for chronic fatigue syndrome: a 5-year follow-up study. Am J Psychiatry 2001;158:2038-42.
13. Sharpe M. Cognitive behavior therapy for chronic fatigue syndrome: efficacy and implications. Am J Med 1998;105:104S-9S.
14. Stulemeijer M, de Jong LW, Fiselier TJ, et al. Cognitive behavior therapy for adolescents with chronic fatigue syndrome: randomized control trial. BMJ 2005;330:1418.-
15. Straus SE. Pharmacotherapy of chronic fatigue syndrome: another gallant attempt. JAMA 2004;292:1234-5.
16. Hartz AJ, Bentler SE, Brake KA, Kelly MW. The effectiveness of citalopram for idiopathic chronic fatigue. J Clin Psychiatry 2003;64:927-35.
Chronic fatigue syndrome (CFS) continues to puzzle and provoke. Years of research have failed to find a biomedical cause or to answer fundamental questions, such as “What is it?” and “Does it exist?”
Up to two-thirds of CFS patients have psychiatric disorders,1 but psychiatrists are not the first physicians CFS patients usually see. Primary care physicians may ask you to help confirm the diagnosis, manage patients’ anxiety and depression, differentiate CFS from somatoform disorders, or provide psychotherapy for patients and their families.
Knowing what transpires in the referring physician’s office is key to helping a patient function despite CFS. This article describes:
- CFS clinical features and possible causes
- psychiatric comorbidities and exclusions
- cognitive behavioral therapy (CBT) and graded exercise, the only two therapies shown to improve CFS patients’ daily function.
Case: Anxious, depressed, and tired
Mr. A, age 43, is referred to you with anxiety and depressed mood associated with CFS, diagnosed 2 years ago. Fatigue predated Mr. A’s depression and anxiety, which his primary care physician considers consequences of CFS.
Mr. A is married, has three children, and owns a successful accounting practice. CFS was diagnosed from the classic presentation: abrupt onset of fatigue despite good health and a promising career. Now, overwhelming fatigue reduces his productivity. He makes up for frequent rest breaks by working in snatches of time, even at 4 AM. He is spending little time with his wife and children.
Defining CFS
The Centers for Disease Control and Prevention (CDC) defined CFS in 1988 while investigating infectious causes of fatigue (Table 1).2,3 Epstein-Barr virus was thought to be the cause of CFS.
Most patients who present to their primary care physicians with complaints of fatigue do not meet CDC criteria for CFS, however. These “non-CFS” patients are diagnosed as having “idiopathic chronic fatigue,” a term that is not particularly helpful because CFS remains an idiopathic disorder.
Clinical findings. As with Mr. A, many CFS patients’ fatigue begins suddenly, often with flu-like symptoms. Patients lose tolerance for exercise and alcohol and become unable to work or socialize at pre-illness levels.
Medical symptoms can overlap those of other conditions, such as fibromyalgia, chemical sensitivities, and irritable bowel disorder. These associations make the condition difficult to assess and contribute to some physicians’ difficulty in accepting CFS as a biomedical condition. Mistrust and a poor patient-physician relationship can result when the physician doubts the symptoms’ “medical” nature and the patient resents the implication that the suffering is “all in your head.”
What causes CFS? No consistent factor has been identified that explains the pathophysiology of CFS symptoms. Many possibilities have been examined, but the evidence is confusing and contradictory.
A few preliminary studies suggest possible familial (shared environmental) and genetic components, but data are sparse and no more than suggestive.4 Findings of CNS studies are inconsistent, and the search for a change in immune function or an infectious agent has been fruitless despite some patients’ infection-like symptoms. The early hypothesis that Epstein-Barr virus was responsible has been disproved.
Imaging studies, psychological testing, and neuroendocrine investigations have identified abnormalities in some patients with CFS. The most-promising findings point to abnormalities in the hypothalamic-pituitary-adrenal axis and in serotonergic neurotransmission,5 suggesting an abnormal stress response in some patients.
Table 1
CDC diagnostic criteria for chronic fatigue syndrome
| At least 6 months of fatigue sufficient to “substantially reduce” patient’s level of activity |
4 or more of 8 concurrent symptoms:
|
| No obvious medical or psychiatric causes, such as eating disorders, psychosis, bipolar disorder, melancholia, or substance abuse* |
| CDC: Centers for Disease Control and Prevention |
| * Many nonpsychotic psychiatric disorders (such as atypical depression) do not exclude a CFS diagnosis |
| Source: References 1 and 2 |
Case continued: Test results are normal
Mr. A has undergone extensive medical assessment (complete blood cell count; renal, hepatic, and thyroid function tests; calcium, phosphate, and glucose determinations; and urinalysis), which yielded normal results. Brain MRI findings were also normal; specifically, no evidence of multiple sclerosis.
Even so, he has had nonspecific symptoms of impaired concentration, sore throat, tender cervical nodes, muscle pain, and nonrefreshing sleep. Physical exertion can leave him drained for at least 1 or 2 days.
Psychiatric Disorders
As many as 66% of CFS patients may have one or more psychiatric comorbidities; the most common are generalized anxiety disorder, panic disorder, depression, and somatoform disorder.1 Because CFS symptoms are regarded as being not fully explained by a known medical disorder, patients are often diagnosed as having an undifferentiated somatoform disorder. Either disorder could be diagnosed in some cases, but this differentiation sheds no new light on the condition.
CFS and depression. Could CFS and depression be one and the same? Proponents of that position point out the similarity of symptoms, loss of function, and—in at least some cases—favorable response to antidepressants. Opponents cite other factors such as:
- presence of sore throat, lymphadenopathy, and post-exercise fatigue
- differences in sleep patterns
- frequent absence of psychiatric illness before fatigue onset
- evidence of hypocortisolism (also seen in patients with melancholic depression) in some CFS patients.
Primary Care Workup
Complaints of long-lasting, debilitating fatigue should alert the primary care physician to CFS. Like somatization disorder, CFS requires a physical workup, though as few as 2% of CFS patients are found to have an undiagnosed medical illness that explains the symptoms.9 The evaluation’s goal is not so much to find out what’s causing the fatigue as to reassure the patient that all avenues are considered before the diagnosis is made. When this is accomplished well, the patient is likely to accept psychiatric referral or treatment, if needed.
Two-part initial evaluation. If the initial physical exam and laboratory work find no biomedical cause for the patient’s chronic fatigue symptoms, we recommend a two-part primary care evaluation. This includes a focused discussion with the patient about CFS (Table 2).10 Goals of the first session are to:
- establish a relationship that will survive difficult times
- teach the patient to think of complex medical problems as having psychological and social consequences, if not causes.
Primary care physicians usually request a psychiatric consultation to confirm or rule out psychiatric conditions that exclude a CFS diagnosis (melancholic depression, bipolar disorder, schizophrenia, anorexia nervosa or bulimia, and recent substance abuse). They also may refer in cases of other common disorders with poorly explained symptoms such as fibromyalgia and chemical sensitivity disorder.
Table 2
Two-part initial primary care evaluation of chronic fatigue
First session
|
Case continued: High anxiety
Mr. A describes how fatigue is affecting his work and home life. He is especially worried that he will not be attentive enough to catch accounting errors by his employees.
Interestingly, his anxiety remits but fatigue continues when he goes on vacation. He has no history of melancholic depression, bipolar disorder, psychosis, or substance abuse.
Psychiatric Assessment
The referring physician should provide a full account of the medical workup. This:
- assures you that possible medical causes of fatigue have been excluded
- provides information on psychiatric history and previous treatments
- delineates information on initial treatment efforts.
Realize how defensive a patient may feel about being given a vague and disputed diagnosis such as CFS. Because the diagnosis depends somewhat on examining his or her volitional contribution to the symptoms,6 your listening skills are key to building the patient-physician relationship. Taking the patient’s suffering seriously is essential and may provide great relief.
When you confirm a CFS diagnosis, the next step is to identify any frequently occurring psychiatric comorbidities, such as nonmelancholic depression, anxiety, and somatoform disorders.
Psychiatric Treatment
CBT and exercise. Only CBT and graded exercise therapy yielded “promising results” in a systematic review of all CFS treatments studied in 44 controlled treatment trials.11 By comparison, evidence is inconclusive or insufficient to support the use of:
- immunoglobulins or hydrocortisone
- most psychotropics—including all classes of antidepressants.
- Patients learn about their illness, develop a realistic assessment of their limitations, and come to understand that physical activity will not harm them.
- Patients begin graded exercises designed to slowly extend their exercise tolerance and widen their range of daily activities.
Trained psychologists usually do this work, and your role is to be aware of the key part this approach plays in managing CFS patients and to set up appropriate referrals.
Case continued: Relief and acceptance
Mr. A continues to see you and a therapist for treatment of mild depression and severe anxiety. His behavioral therapy focuses on helping him cope with how his illness limits his relationship to work and family. His therapist also explores with him the personal meanings of his new situation, his feelings about issues such as dependence, and limitations imposed on his life goals.
You start a trial of fluoxetine (up to 40 mg/d for several months) with minimal benefit. You then try nortriptyline, 25 mg nightly, and clonazepam, 0.5 mg bid. Although these drugs can be sedating, Mr. A reports feeling no more fatigued than he was before taking them. He improves slightly after 7 months but not enough that he wants to continue the medication.
Medications. Neither psychiatric nor other medication classes have shown efficacy in treating CFS core symptoms.15 One recent study16 found citalopram helped reduce chronic fatigue, but the study was small and uncontrolled. Although the subjects had chronic fatigue, not all met the formal definition of CFS for study inclusion.
Medication does play an important role in treating comorbid anxiety and depression. Usual psychopharmacologic strategies are appropriate. As in Mr. A’s case, most psychiatrists use SSRIs as first-line medications, but side effects are probably the most useful guide to medication choice.
Case continued: Additional treatment
Because medication has had little effect on Mr. A’s anxiety and depressed mood, you suggest adding a graded exercise program to his treatment plan. He improves steadily over time and says he is pleased. Although progress is slow, he finds it reassuring to be accomplishing realistic goals. He realizes that you and the therapist do not have the answer to his illness, but he trusts you and is comforted that you accept his condition and are willing to listen and help.
Related resources
- Afari N, Buchwald D. Chronic fatigue syndrome: a review. Am J Psychiatry 2003;160:221-36.
- Reid S, Chalder T, Cleare A, et al. Chronic fatigue syndrome. Clin Evid 2004;12:1578-93.
- Centers for Disease Control and Prevention. Chronic fatigue syndrome. http://www.cdc.gov/ncidod/diseases/cfs/.
- International Association for Chronic Fatigue Syndrome. http://www.aacfs.org.
- Citalopram • Celexa, others
- Clonazepam • Lorazepam, others
- Fluoxetine • Prozac
- Nortriptyline • Aventyl, others
The authors report no financial relationship with any company whos products are mentioned in this article or with manufacturers of competing products.
Chronic fatigue syndrome (CFS) continues to puzzle and provoke. Years of research have failed to find a biomedical cause or to answer fundamental questions, such as “What is it?” and “Does it exist?”
Up to two-thirds of CFS patients have psychiatric disorders,1 but psychiatrists are not the first physicians CFS patients usually see. Primary care physicians may ask you to help confirm the diagnosis, manage patients’ anxiety and depression, differentiate CFS from somatoform disorders, or provide psychotherapy for patients and their families.
Knowing what transpires in the referring physician’s office is key to helping a patient function despite CFS. This article describes:
- CFS clinical features and possible causes
- psychiatric comorbidities and exclusions
- cognitive behavioral therapy (CBT) and graded exercise, the only two therapies shown to improve CFS patients’ daily function.
Case: Anxious, depressed, and tired
Mr. A, age 43, is referred to you with anxiety and depressed mood associated with CFS, diagnosed 2 years ago. Fatigue predated Mr. A’s depression and anxiety, which his primary care physician considers consequences of CFS.
Mr. A is married, has three children, and owns a successful accounting practice. CFS was diagnosed from the classic presentation: abrupt onset of fatigue despite good health and a promising career. Now, overwhelming fatigue reduces his productivity. He makes up for frequent rest breaks by working in snatches of time, even at 4 AM. He is spending little time with his wife and children.
Defining CFS
The Centers for Disease Control and Prevention (CDC) defined CFS in 1988 while investigating infectious causes of fatigue (Table 1).2,3 Epstein-Barr virus was thought to be the cause of CFS.
Most patients who present to their primary care physicians with complaints of fatigue do not meet CDC criteria for CFS, however. These “non-CFS” patients are diagnosed as having “idiopathic chronic fatigue,” a term that is not particularly helpful because CFS remains an idiopathic disorder.
Clinical findings. As with Mr. A, many CFS patients’ fatigue begins suddenly, often with flu-like symptoms. Patients lose tolerance for exercise and alcohol and become unable to work or socialize at pre-illness levels.
Medical symptoms can overlap those of other conditions, such as fibromyalgia, chemical sensitivities, and irritable bowel disorder. These associations make the condition difficult to assess and contribute to some physicians’ difficulty in accepting CFS as a biomedical condition. Mistrust and a poor patient-physician relationship can result when the physician doubts the symptoms’ “medical” nature and the patient resents the implication that the suffering is “all in your head.”
What causes CFS? No consistent factor has been identified that explains the pathophysiology of CFS symptoms. Many possibilities have been examined, but the evidence is confusing and contradictory.
A few preliminary studies suggest possible familial (shared environmental) and genetic components, but data are sparse and no more than suggestive.4 Findings of CNS studies are inconsistent, and the search for a change in immune function or an infectious agent has been fruitless despite some patients’ infection-like symptoms. The early hypothesis that Epstein-Barr virus was responsible has been disproved.
Imaging studies, psychological testing, and neuroendocrine investigations have identified abnormalities in some patients with CFS. The most-promising findings point to abnormalities in the hypothalamic-pituitary-adrenal axis and in serotonergic neurotransmission,5 suggesting an abnormal stress response in some patients.
Table 1
CDC diagnostic criteria for chronic fatigue syndrome
| At least 6 months of fatigue sufficient to “substantially reduce” patient’s level of activity |
4 or more of 8 concurrent symptoms:
|
| No obvious medical or psychiatric causes, such as eating disorders, psychosis, bipolar disorder, melancholia, or substance abuse* |
| CDC: Centers for Disease Control and Prevention |
| * Many nonpsychotic psychiatric disorders (such as atypical depression) do not exclude a CFS diagnosis |
| Source: References 1 and 2 |
Case continued: Test results are normal
Mr. A has undergone extensive medical assessment (complete blood cell count; renal, hepatic, and thyroid function tests; calcium, phosphate, and glucose determinations; and urinalysis), which yielded normal results. Brain MRI findings were also normal; specifically, no evidence of multiple sclerosis.
Even so, he has had nonspecific symptoms of impaired concentration, sore throat, tender cervical nodes, muscle pain, and nonrefreshing sleep. Physical exertion can leave him drained for at least 1 or 2 days.
Psychiatric Disorders
As many as 66% of CFS patients may have one or more psychiatric comorbidities; the most common are generalized anxiety disorder, panic disorder, depression, and somatoform disorder.1 Because CFS symptoms are regarded as being not fully explained by a known medical disorder, patients are often diagnosed as having an undifferentiated somatoform disorder. Either disorder could be diagnosed in some cases, but this differentiation sheds no new light on the condition.
CFS and depression. Could CFS and depression be one and the same? Proponents of that position point out the similarity of symptoms, loss of function, and—in at least some cases—favorable response to antidepressants. Opponents cite other factors such as:
- presence of sore throat, lymphadenopathy, and post-exercise fatigue
- differences in sleep patterns
- frequent absence of psychiatric illness before fatigue onset
- evidence of hypocortisolism (also seen in patients with melancholic depression) in some CFS patients.
Primary Care Workup
Complaints of long-lasting, debilitating fatigue should alert the primary care physician to CFS. Like somatization disorder, CFS requires a physical workup, though as few as 2% of CFS patients are found to have an undiagnosed medical illness that explains the symptoms.9 The evaluation’s goal is not so much to find out what’s causing the fatigue as to reassure the patient that all avenues are considered before the diagnosis is made. When this is accomplished well, the patient is likely to accept psychiatric referral or treatment, if needed.
Two-part initial evaluation. If the initial physical exam and laboratory work find no biomedical cause for the patient’s chronic fatigue symptoms, we recommend a two-part primary care evaluation. This includes a focused discussion with the patient about CFS (Table 2).10 Goals of the first session are to:
- establish a relationship that will survive difficult times
- teach the patient to think of complex medical problems as having psychological and social consequences, if not causes.
Primary care physicians usually request a psychiatric consultation to confirm or rule out psychiatric conditions that exclude a CFS diagnosis (melancholic depression, bipolar disorder, schizophrenia, anorexia nervosa or bulimia, and recent substance abuse). They also may refer in cases of other common disorders with poorly explained symptoms such as fibromyalgia and chemical sensitivity disorder.
Table 2
Two-part initial primary care evaluation of chronic fatigue
First session
|
Case continued: High anxiety
Mr. A describes how fatigue is affecting his work and home life. He is especially worried that he will not be attentive enough to catch accounting errors by his employees.
Interestingly, his anxiety remits but fatigue continues when he goes on vacation. He has no history of melancholic depression, bipolar disorder, psychosis, or substance abuse.
Psychiatric Assessment
The referring physician should provide a full account of the medical workup. This:
- assures you that possible medical causes of fatigue have been excluded
- provides information on psychiatric history and previous treatments
- delineates information on initial treatment efforts.
Realize how defensive a patient may feel about being given a vague and disputed diagnosis such as CFS. Because the diagnosis depends somewhat on examining his or her volitional contribution to the symptoms,6 your listening skills are key to building the patient-physician relationship. Taking the patient’s suffering seriously is essential and may provide great relief.
When you confirm a CFS diagnosis, the next step is to identify any frequently occurring psychiatric comorbidities, such as nonmelancholic depression, anxiety, and somatoform disorders.
Psychiatric Treatment
CBT and exercise. Only CBT and graded exercise therapy yielded “promising results” in a systematic review of all CFS treatments studied in 44 controlled treatment trials.11 By comparison, evidence is inconclusive or insufficient to support the use of:
- immunoglobulins or hydrocortisone
- most psychotropics—including all classes of antidepressants.
- Patients learn about their illness, develop a realistic assessment of their limitations, and come to understand that physical activity will not harm them.
- Patients begin graded exercises designed to slowly extend their exercise tolerance and widen their range of daily activities.
Trained psychologists usually do this work, and your role is to be aware of the key part this approach plays in managing CFS patients and to set up appropriate referrals.
Case continued: Relief and acceptance
Mr. A continues to see you and a therapist for treatment of mild depression and severe anxiety. His behavioral therapy focuses on helping him cope with how his illness limits his relationship to work and family. His therapist also explores with him the personal meanings of his new situation, his feelings about issues such as dependence, and limitations imposed on his life goals.
You start a trial of fluoxetine (up to 40 mg/d for several months) with minimal benefit. You then try nortriptyline, 25 mg nightly, and clonazepam, 0.5 mg bid. Although these drugs can be sedating, Mr. A reports feeling no more fatigued than he was before taking them. He improves slightly after 7 months but not enough that he wants to continue the medication.
Medications. Neither psychiatric nor other medication classes have shown efficacy in treating CFS core symptoms.15 One recent study16 found citalopram helped reduce chronic fatigue, but the study was small and uncontrolled. Although the subjects had chronic fatigue, not all met the formal definition of CFS for study inclusion.
Medication does play an important role in treating comorbid anxiety and depression. Usual psychopharmacologic strategies are appropriate. As in Mr. A’s case, most psychiatrists use SSRIs as first-line medications, but side effects are probably the most useful guide to medication choice.
Case continued: Additional treatment
Because medication has had little effect on Mr. A’s anxiety and depressed mood, you suggest adding a graded exercise program to his treatment plan. He improves steadily over time and says he is pleased. Although progress is slow, he finds it reassuring to be accomplishing realistic goals. He realizes that you and the therapist do not have the answer to his illness, but he trusts you and is comforted that you accept his condition and are willing to listen and help.
Related resources
- Afari N, Buchwald D. Chronic fatigue syndrome: a review. Am J Psychiatry 2003;160:221-36.
- Reid S, Chalder T, Cleare A, et al. Chronic fatigue syndrome. Clin Evid 2004;12:1578-93.
- Centers for Disease Control and Prevention. Chronic fatigue syndrome. http://www.cdc.gov/ncidod/diseases/cfs/.
- International Association for Chronic Fatigue Syndrome. http://www.aacfs.org.
- Citalopram • Celexa, others
- Clonazepam • Lorazepam, others
- Fluoxetine • Prozac
- Nortriptyline • Aventyl, others
The authors report no financial relationship with any company whos products are mentioned in this article or with manufacturers of competing products.
1. Kroenke K, Wood DR, Mangelsdorff AD, et al. Chronic fatigue in primary care: prevalence, patient characteristics, and outcome. JAMA 1988;260:929-34.
2. Holmes GP, Kaplan JE, Gantz NM, et al. Chronic fatigue syndrome: a working case definition. Ann Intern Med 1988;108:387-9.
3. Goshorn RK. Chronic fatigue syndrome: a review for clinicians. Semin Neurol 1998;18:237-42.
4. Afari N, Buchwald D. Chronic fatigue syndrome: a review. Am J Psychiatry 2003;160:221-36.
5. Parker AJR, Wessely S, Cleare AJ. The neuroendocrinology of chronic fatigue syndrome and fibromyalgia. Psychol Med 2001;31:1331-45.
6. Clarke JN, James S. The radicalized self: the impact on the self of the contested nature of the diagnosis of chronic fatigue syndrome. Soc Sci Med 2003;57:1387-95.
7. Demitrack MA. The psychobiology of chronic fatigue: the central nervous system as a final common pathway. In: Demitrack MA, Abbey SE (eds). Chronic fatigue syndrome: an integrative approach to evaluation and treatment. New York: Guilford Press; 1996:72-109.
8. Cassem EH. Depression and anxiety secondary to medical illness. Psychiatr Clin North Am 1990;13:597-612.
9. Lane TJ, Matthews DA, Manu P. The low yield of physical examinations and laboratory investigations of patients with chronic fatigue. Am J Med Sci 1990;299:313-18.
10. Ruffin MT, Margo GM, Margo KL. Puzzling physical conditions (monograph, edition No. 209). Home study self-assessment program. Presented at: American Academy of Family Physicians; October 1996; Kansas City, MO.
11. Whiting P, Bagnall A, Sowden A, et al. Interventions for the treatment and management of chronic fatigue syndrome: a systematic review. JAMA 2001;286:1360-8.
12. Deale A, Husain K, Chalder T, Wessely S. Long-term outcome of cognitive behavior therapy versus relaxation therapy for chronic fatigue syndrome: a 5-year follow-up study. Am J Psychiatry 2001;158:2038-42.
13. Sharpe M. Cognitive behavior therapy for chronic fatigue syndrome: efficacy and implications. Am J Med 1998;105:104S-9S.
14. Stulemeijer M, de Jong LW, Fiselier TJ, et al. Cognitive behavior therapy for adolescents with chronic fatigue syndrome: randomized control trial. BMJ 2005;330:1418.-
15. Straus SE. Pharmacotherapy of chronic fatigue syndrome: another gallant attempt. JAMA 2004;292:1234-5.
16. Hartz AJ, Bentler SE, Brake KA, Kelly MW. The effectiveness of citalopram for idiopathic chronic fatigue. J Clin Psychiatry 2003;64:927-35.
1. Kroenke K, Wood DR, Mangelsdorff AD, et al. Chronic fatigue in primary care: prevalence, patient characteristics, and outcome. JAMA 1988;260:929-34.
2. Holmes GP, Kaplan JE, Gantz NM, et al. Chronic fatigue syndrome: a working case definition. Ann Intern Med 1988;108:387-9.
3. Goshorn RK. Chronic fatigue syndrome: a review for clinicians. Semin Neurol 1998;18:237-42.
4. Afari N, Buchwald D. Chronic fatigue syndrome: a review. Am J Psychiatry 2003;160:221-36.
5. Parker AJR, Wessely S, Cleare AJ. The neuroendocrinology of chronic fatigue syndrome and fibromyalgia. Psychol Med 2001;31:1331-45.
6. Clarke JN, James S. The radicalized self: the impact on the self of the contested nature of the diagnosis of chronic fatigue syndrome. Soc Sci Med 2003;57:1387-95.
7. Demitrack MA. The psychobiology of chronic fatigue: the central nervous system as a final common pathway. In: Demitrack MA, Abbey SE (eds). Chronic fatigue syndrome: an integrative approach to evaluation and treatment. New York: Guilford Press; 1996:72-109.
8. Cassem EH. Depression and anxiety secondary to medical illness. Psychiatr Clin North Am 1990;13:597-612.
9. Lane TJ, Matthews DA, Manu P. The low yield of physical examinations and laboratory investigations of patients with chronic fatigue. Am J Med Sci 1990;299:313-18.
10. Ruffin MT, Margo GM, Margo KL. Puzzling physical conditions (monograph, edition No. 209). Home study self-assessment program. Presented at: American Academy of Family Physicians; October 1996; Kansas City, MO.
11. Whiting P, Bagnall A, Sowden A, et al. Interventions for the treatment and management of chronic fatigue syndrome: a systematic review. JAMA 2001;286:1360-8.
12. Deale A, Husain K, Chalder T, Wessely S. Long-term outcome of cognitive behavior therapy versus relaxation therapy for chronic fatigue syndrome: a 5-year follow-up study. Am J Psychiatry 2001;158:2038-42.
13. Sharpe M. Cognitive behavior therapy for chronic fatigue syndrome: efficacy and implications. Am J Med 1998;105:104S-9S.
14. Stulemeijer M, de Jong LW, Fiselier TJ, et al. Cognitive behavior therapy for adolescents with chronic fatigue syndrome: randomized control trial. BMJ 2005;330:1418.-
15. Straus SE. Pharmacotherapy of chronic fatigue syndrome: another gallant attempt. JAMA 2004;292:1234-5.
16. Hartz AJ, Bentler SE, Brake KA, Kelly MW. The effectiveness of citalopram for idiopathic chronic fatigue. J Clin Psychiatry 2003;64:927-35.
Music memories quell addictions
A friend and colleague recently gave me the book Live at the Fillmore East: A Photographic Memoir (Thunder’s Mouth Press, 1999), in which photographer Amalie R. Rothschild chronicles the celebrated rock music venue on New York’s Lower East Side.
The book has been a powerful therapeutic tool for three of my patients:
1) Mr. S, age 56, has a long history of alcoholism and depression and had been fighting with his wife over his impulsive purchase of a guitar that belonged to Pete Townshend of The Who. For Mr. S, the guitar brings back memories of the Fillmore, where he met Townshend backstage. He considers that period the high point of his life, which has since been a series of personal and professional disappointments.
When I showed Mr. S the book, he wept inconsolably for his lost youth. I encouraged him to tell his wife about what he experienced. The couple talked honestly for the first time in 20 years, and he made a commitment to her to stop drinking. He remains sober.
2) Mr. Z, a 22-year-old paralegal, battled marijuana dependence and an anxiety disorder. His parents divorced when he was age 4. His mother and stepfather have been pushing him to go to law school, while his father, a former music producer who is bedridden with amyotrophic lateral sclerosis, feels he should do whatever makes him happy. Mr. Z grew up listening to 1960’s music with his father.
Mr. Z noticed the Fillmore East book during a recent session. He convulsed with tears as he looked through it and spoke lovingly of his father for the first time. He has since stopped smoking marijuana and has reconnected with his dying father. He is now going to law school and wants to specialize in entertainment law.
3) Mr. M, age 33, has been treated for alcoholism and attention deficit disorder. Unable to stop drinking as an outpatient, he checked into the Crossroads Rehabilitation Centre in Antigua, West Indies, for 1 month. When he returned he “felt like an adult for the first time.” He regularly attends Alcoholics Anonymous meetings and feels “spirituality” replacing the anger he once felt toward himself and the world.
Upon seeing the Fillmore East book, Mr. M cried over the photos of Eric Clapton. He is inspired by Clapton’s recovery from addiction and how Clapton, after his preschool son’s death, founded Crossroads, which provides free substance abuse treatment for Antiguans.
Like Clapton, Mr. M wants to help other alcoholics recover. He is applying to school to become a substance abuse counselor.
Jeffrey S. Rosecan, MD
New York, NY
A friend and colleague recently gave me the book Live at the Fillmore East: A Photographic Memoir (Thunder’s Mouth Press, 1999), in which photographer Amalie R. Rothschild chronicles the celebrated rock music venue on New York’s Lower East Side.
The book has been a powerful therapeutic tool for three of my patients:
1) Mr. S, age 56, has a long history of alcoholism and depression and had been fighting with his wife over his impulsive purchase of a guitar that belonged to Pete Townshend of The Who. For Mr. S, the guitar brings back memories of the Fillmore, where he met Townshend backstage. He considers that period the high point of his life, which has since been a series of personal and professional disappointments.
When I showed Mr. S the book, he wept inconsolably for his lost youth. I encouraged him to tell his wife about what he experienced. The couple talked honestly for the first time in 20 years, and he made a commitment to her to stop drinking. He remains sober.
2) Mr. Z, a 22-year-old paralegal, battled marijuana dependence and an anxiety disorder. His parents divorced when he was age 4. His mother and stepfather have been pushing him to go to law school, while his father, a former music producer who is bedridden with amyotrophic lateral sclerosis, feels he should do whatever makes him happy. Mr. Z grew up listening to 1960’s music with his father.
Mr. Z noticed the Fillmore East book during a recent session. He convulsed with tears as he looked through it and spoke lovingly of his father for the first time. He has since stopped smoking marijuana and has reconnected with his dying father. He is now going to law school and wants to specialize in entertainment law.
3) Mr. M, age 33, has been treated for alcoholism and attention deficit disorder. Unable to stop drinking as an outpatient, he checked into the Crossroads Rehabilitation Centre in Antigua, West Indies, for 1 month. When he returned he “felt like an adult for the first time.” He regularly attends Alcoholics Anonymous meetings and feels “spirituality” replacing the anger he once felt toward himself and the world.
Upon seeing the Fillmore East book, Mr. M cried over the photos of Eric Clapton. He is inspired by Clapton’s recovery from addiction and how Clapton, after his preschool son’s death, founded Crossroads, which provides free substance abuse treatment for Antiguans.
Like Clapton, Mr. M wants to help other alcoholics recover. He is applying to school to become a substance abuse counselor.
Jeffrey S. Rosecan, MD
New York, NY
A friend and colleague recently gave me the book Live at the Fillmore East: A Photographic Memoir (Thunder’s Mouth Press, 1999), in which photographer Amalie R. Rothschild chronicles the celebrated rock music venue on New York’s Lower East Side.
The book has been a powerful therapeutic tool for three of my patients:
1) Mr. S, age 56, has a long history of alcoholism and depression and had been fighting with his wife over his impulsive purchase of a guitar that belonged to Pete Townshend of The Who. For Mr. S, the guitar brings back memories of the Fillmore, where he met Townshend backstage. He considers that period the high point of his life, which has since been a series of personal and professional disappointments.
When I showed Mr. S the book, he wept inconsolably for his lost youth. I encouraged him to tell his wife about what he experienced. The couple talked honestly for the first time in 20 years, and he made a commitment to her to stop drinking. He remains sober.
2) Mr. Z, a 22-year-old paralegal, battled marijuana dependence and an anxiety disorder. His parents divorced when he was age 4. His mother and stepfather have been pushing him to go to law school, while his father, a former music producer who is bedridden with amyotrophic lateral sclerosis, feels he should do whatever makes him happy. Mr. Z grew up listening to 1960’s music with his father.
Mr. Z noticed the Fillmore East book during a recent session. He convulsed with tears as he looked through it and spoke lovingly of his father for the first time. He has since stopped smoking marijuana and has reconnected with his dying father. He is now going to law school and wants to specialize in entertainment law.
3) Mr. M, age 33, has been treated for alcoholism and attention deficit disorder. Unable to stop drinking as an outpatient, he checked into the Crossroads Rehabilitation Centre in Antigua, West Indies, for 1 month. When he returned he “felt like an adult for the first time.” He regularly attends Alcoholics Anonymous meetings and feels “spirituality” replacing the anger he once felt toward himself and the world.
Upon seeing the Fillmore East book, Mr. M cried over the photos of Eric Clapton. He is inspired by Clapton’s recovery from addiction and how Clapton, after his preschool son’s death, founded Crossroads, which provides free substance abuse treatment for Antiguans.
Like Clapton, Mr. M wants to help other alcoholics recover. He is applying to school to become a substance abuse counselor.
Jeffrey S. Rosecan, MD
New York, NY
Med checks: 15 minutes can make a difference
“How to avoid burning out and keep your spark” by Drs. Phil Bohnert and Anne O’Connell (Current Psychiatry, January 2006) offers welcome insights in this time of high stress for clinical psychiatrists. The authors’ analysis and suggestions seem worthwhile.
When discussing “External causes for burnout,” however, the authors took a cheap, unnecessarily harsh shot at the “15-minute” medication check as “probably the most demoralizing hazard.” That would only be true if the psychiatrist does not see the value of such brief interactions or cannot do them properly.
We can indeed help patients within 15 minutes and make empathetic connections by asking about their lives and what they value. Ignoring this skill slights the many psychiatrists who—because they want to serve the underserved but lack adequate funding—do quite well within that time frame.
Further, some patients, depending on their pathology and personality, prefer shorter visits. A truncated visit may help these patients stay in treatment.
Finally, the 15-minute visit is not all managed care’s doing: Brief med checks were common in community psychiatry long before the ascendance of managed care.
As the old saying goes for child rearing, it’s not (only) the quantity of time, it’s the quality of time together.
H. Steven Moffic, MD
Milwaukee, WI
The authors respond
We thank Dr. Moffic for his thoughtful comments. We do not wish to negate the value of the 15-minute medication check or its utility in increasing public access to psychiatric care.
It must be noted that psychiatrists have reported anecdotally and in the literature1,2 that such a brief visit can frustrate the desire to handle both medications and psychotherapy. For those who envisioned a psychotherapy-based practice, it may well remain a burnout hazard.
We welcome further discussion of the pros and cons of the 15-minute med check.
Phil Bohnert, MD
Anne O’Connell, MD, PhD
University of Hawaii, Honolulu
1. Regestein Q. Psychiatrists’ views of managed care and the future of psychiatry. Gen Hosp Psychiatry 2000;22:97-106.
2. Kalman TP, Goldstein MA. Satisfaction of Manhattan psychiatrists with private practice: assessing the impact of managed care. Medscape Psychiatry & Mental Health eJournal 1998;3(1).
“How to avoid burning out and keep your spark” by Drs. Phil Bohnert and Anne O’Connell (Current Psychiatry, January 2006) offers welcome insights in this time of high stress for clinical psychiatrists. The authors’ analysis and suggestions seem worthwhile.
When discussing “External causes for burnout,” however, the authors took a cheap, unnecessarily harsh shot at the “15-minute” medication check as “probably the most demoralizing hazard.” That would only be true if the psychiatrist does not see the value of such brief interactions or cannot do them properly.
We can indeed help patients within 15 minutes and make empathetic connections by asking about their lives and what they value. Ignoring this skill slights the many psychiatrists who—because they want to serve the underserved but lack adequate funding—do quite well within that time frame.
Further, some patients, depending on their pathology and personality, prefer shorter visits. A truncated visit may help these patients stay in treatment.
Finally, the 15-minute visit is not all managed care’s doing: Brief med checks were common in community psychiatry long before the ascendance of managed care.
As the old saying goes for child rearing, it’s not (only) the quantity of time, it’s the quality of time together.
H. Steven Moffic, MD
Milwaukee, WI
The authors respond
We thank Dr. Moffic for his thoughtful comments. We do not wish to negate the value of the 15-minute medication check or its utility in increasing public access to psychiatric care.
It must be noted that psychiatrists have reported anecdotally and in the literature1,2 that such a brief visit can frustrate the desire to handle both medications and psychotherapy. For those who envisioned a psychotherapy-based practice, it may well remain a burnout hazard.
We welcome further discussion of the pros and cons of the 15-minute med check.
Phil Bohnert, MD
Anne O’Connell, MD, PhD
University of Hawaii, Honolulu
“How to avoid burning out and keep your spark” by Drs. Phil Bohnert and Anne O’Connell (Current Psychiatry, January 2006) offers welcome insights in this time of high stress for clinical psychiatrists. The authors’ analysis and suggestions seem worthwhile.
When discussing “External causes for burnout,” however, the authors took a cheap, unnecessarily harsh shot at the “15-minute” medication check as “probably the most demoralizing hazard.” That would only be true if the psychiatrist does not see the value of such brief interactions or cannot do them properly.
We can indeed help patients within 15 minutes and make empathetic connections by asking about their lives and what they value. Ignoring this skill slights the many psychiatrists who—because they want to serve the underserved but lack adequate funding—do quite well within that time frame.
Further, some patients, depending on their pathology and personality, prefer shorter visits. A truncated visit may help these patients stay in treatment.
Finally, the 15-minute visit is not all managed care’s doing: Brief med checks were common in community psychiatry long before the ascendance of managed care.
As the old saying goes for child rearing, it’s not (only) the quantity of time, it’s the quality of time together.
H. Steven Moffic, MD
Milwaukee, WI
The authors respond
We thank Dr. Moffic for his thoughtful comments. We do not wish to negate the value of the 15-minute medication check or its utility in increasing public access to psychiatric care.
It must be noted that psychiatrists have reported anecdotally and in the literature1,2 that such a brief visit can frustrate the desire to handle both medications and psychotherapy. For those who envisioned a psychotherapy-based practice, it may well remain a burnout hazard.
We welcome further discussion of the pros and cons of the 15-minute med check.
Phil Bohnert, MD
Anne O’Connell, MD, PhD
University of Hawaii, Honolulu
1. Regestein Q. Psychiatrists’ views of managed care and the future of psychiatry. Gen Hosp Psychiatry 2000;22:97-106.
2. Kalman TP, Goldstein MA. Satisfaction of Manhattan psychiatrists with private practice: assessing the impact of managed care. Medscape Psychiatry & Mental Health eJournal 1998;3(1).
1. Regestein Q. Psychiatrists’ views of managed care and the future of psychiatry. Gen Hosp Psychiatry 2000;22:97-106.
2. Kalman TP, Goldstein MA. Satisfaction of Manhattan psychiatrists with private practice: assessing the impact of managed care. Medscape Psychiatry & Mental Health eJournal 1998;3(1).
No such thing as schizophrenia?
This issue of Current Psychiatry poses the question: “Does schizophrenia exist?” Isn’t it shocking that we are still asking this in 2006? After all, our specialty treats a sizable proportion of patients diagnosed with schizophrenia.
I have been trying to think of analogous questions other Quadrant HealthCom Inc. specialty journals might consider:
- Would Contemporary Surgery ask “Does appendicitis exist?”
- Would The Journal of Family Practice ask “Does the common cold exist?”
- Would OBG Management ask “Does pregnancy exist?” (Well, maybe our question about schizophrenia is not quite that extreme.)
C. Raymond Lake, MD, PhD, professor of psychiatry at the University of Kansas School of Medicine, and Nathaniel Hurwitz, MD, assistant professor of psychiatry at Yale University School of Medicine, make a strong case that psychiatrists frequently fail to recognize severe bipolar disorder by assuming that psychosis means schizophrenia. These authors then examine recent evidence that schizophrenia may not exist as an independent disorder.
Because Drs. Lake and Hurwitz acknowledge that their view is a minority opinion, we asked Henry A. Nasrallah, MD, editor of Schizophrenia Research, for his perspective. He argues persuasively for a fundamental distinction between schizophrenia and bipolar disorder.
Discussions such as this reinforce my belief that psychiatry continues to evolve as a medical specialty. What do you think? To voice your opinion, click here. We will publish selected letters in Current Psychiatry.
This issue of Current Psychiatry poses the question: “Does schizophrenia exist?” Isn’t it shocking that we are still asking this in 2006? After all, our specialty treats a sizable proportion of patients diagnosed with schizophrenia.
I have been trying to think of analogous questions other Quadrant HealthCom Inc. specialty journals might consider:
- Would Contemporary Surgery ask “Does appendicitis exist?”
- Would The Journal of Family Practice ask “Does the common cold exist?”
- Would OBG Management ask “Does pregnancy exist?” (Well, maybe our question about schizophrenia is not quite that extreme.)
C. Raymond Lake, MD, PhD, professor of psychiatry at the University of Kansas School of Medicine, and Nathaniel Hurwitz, MD, assistant professor of psychiatry at Yale University School of Medicine, make a strong case that psychiatrists frequently fail to recognize severe bipolar disorder by assuming that psychosis means schizophrenia. These authors then examine recent evidence that schizophrenia may not exist as an independent disorder.
Because Drs. Lake and Hurwitz acknowledge that their view is a minority opinion, we asked Henry A. Nasrallah, MD, editor of Schizophrenia Research, for his perspective. He argues persuasively for a fundamental distinction between schizophrenia and bipolar disorder.
Discussions such as this reinforce my belief that psychiatry continues to evolve as a medical specialty. What do you think? To voice your opinion, click here. We will publish selected letters in Current Psychiatry.
This issue of Current Psychiatry poses the question: “Does schizophrenia exist?” Isn’t it shocking that we are still asking this in 2006? After all, our specialty treats a sizable proportion of patients diagnosed with schizophrenia.
I have been trying to think of analogous questions other Quadrant HealthCom Inc. specialty journals might consider:
- Would Contemporary Surgery ask “Does appendicitis exist?”
- Would The Journal of Family Practice ask “Does the common cold exist?”
- Would OBG Management ask “Does pregnancy exist?” (Well, maybe our question about schizophrenia is not quite that extreme.)
C. Raymond Lake, MD, PhD, professor of psychiatry at the University of Kansas School of Medicine, and Nathaniel Hurwitz, MD, assistant professor of psychiatry at Yale University School of Medicine, make a strong case that psychiatrists frequently fail to recognize severe bipolar disorder by assuming that psychosis means schizophrenia. These authors then examine recent evidence that schizophrenia may not exist as an independent disorder.
Because Drs. Lake and Hurwitz acknowledge that their view is a minority opinion, we asked Henry A. Nasrallah, MD, editor of Schizophrenia Research, for his perspective. He argues persuasively for a fundamental distinction between schizophrenia and bipolar disorder.
Discussions such as this reinforce my belief that psychiatry continues to evolve as a medical specialty. What do you think? To voice your opinion, click here. We will publish selected letters in Current Psychiatry.
Schizophrenia is psychotic bipolar disorder? What a polarizing idea!
A hundred years ago, KraepelinDrs. Lake and Hurwitz for highlighting the diagnostic and treatment errors in a bipolar patient with severe psychotic features who was misdiagnosed as having schizophrenia. Errors such as this were common with DSM I and II but declined with the more reliable diagnostic schemas of DSM III and IV. I am puzzled, however, by their leap to the radical conclusion that schizophrenia does not exist and that all patients diagnosed with schizophrenia have psychotic bipolar disorder. This is not as egregious as Szasz’ absurd proclamation 4 decades ago that schizophrenia is a “myth,” but it is a significant scientific “transgression,” given the evidence that distinguishes schizophrenia from bipolar disorder.
Symptoms. Beyond a doubt, these two brain diseases have overlapping clinical features, pharmacotherapies, and even outcomes in a subgroup of patients. However, these diseases have major differences, as outlined in the accompanying table.
Brain anomalies. Neuroimaging studies indicate that schizophrenia is associated with more-severe and pervasive morphologic brain anomalies (dysplasia and hypoplasia) than bipolar disorder, although some bipolar patients have reduced cerebral and frontal volumes and marked cognitive deficits.3 Progressive neuro tissue loss has been observed early in schizophrenia but not in bipolar disorder.
Recent genetic studies indicate that several genes are found exclusively in schizophrenia or in bipolar disorder cohorts,4 but some are shared by both disorders and may be related to delusional symptoms.5 Familial transmission appears to differ: transgenerational studies find an abundance of mood disorders in family members of bipolar probands but relatively sparse occurrence of psychosis in families of probands with schizophrenia.
Table
Symptom differences between schizophrenia and bipolar disorder
| Symptom | Schizophrenia | Bipolar disorder |
|---|---|---|
| Psychosis | Auditory hallucinations and bizarre delusions are more common | Grandiosity is more common |
| Paranoia | Occurs in both, but more systematic in schizophrenia | |
| Core psychopathology | Far more negative symptoms and cognition dysfunction | Far more mood lability and affective cyclicity |
| Thought disorder | Far more disorganized and derailed thoughts | More likely to have racing thoughts and flight of ideas |
| Between-episode interpersonal skills | Withdrawn, alogic, seclusive | Much more interactive and verbal |
Treatment. There is no doubt that monotherapy with antipsychotics (old and new) has similar efficacy6 in schizophrenia and bipolar mania (and even in bipolar depression, with some atypicals7). However, there is minimal, if any, evidence that monotherapy mood stabilizers (lithium or anti-convulsants) have any tangible efficacy in schizophrenia. Electroconvulsive therapy is remarkably efficious in all phases of bipolar disorder but of dubious, if any, lasting benefit in schizophrenia.
Course. Both the premorbid history and post-treatment functional outcome tend to be more favorable in patients with bipolar disorder than schizophrenia. Most patients with schizophrenia experience significant clinical, social, and vocational deterioration, compared with a relative minority of bipolar patients.
In summary, schizophrenia and bipolar disorder are clearly distinct in their pure forms, although many patients have varying mixtures of both. Schizoaffective disorder is one of the most extensively investigated. Although many scholars have studied schizoaffective disorder, the evidence defies lumping it with either end of the continuum.
I agree with Drs. Lake and Hurwitz that most cases of schizoaffective disorder, especially the “schizomanic” type, are probably bipolar disorder with severe psychotic features. To assert, however, that schizophrenia does not exist at all and should be reclassified as bipolar disorder with psychotic features would contradict a massive body of clinical and biological evidence. It would cause Kraepelin to squirm in his grave.
1. Kraepelin E, Lange J. Psychiatrie. In: Klinische Psychiatrie, vol 3 (8th ed). Leipzig, Germany: Barth; 1923.
2. Nasrallah HA. The continuum of psychoses between schizophrenia and bipolar disorder. Neurol Psychiatry Brain Res 1994;2:206-9.
3. Coffman JA, Bornstein RA, Olson SC, et al. Cognitive impairment and cerebral structure by MRI in bipolar disorder. Biol Psychiatry 1990;27(11):1188-96.
4. Weinberger DR. Genetic mechanisms of psychosis: in vivo and postmortem genomics. Clin Ther 2005;27(suppl):8-15.
5. Schulze TG, Ohlfaun S, Czerski PM, et al. Genotype-phenotype studies in bipolar disorder showing association between the DAOA/G30 locus and persecutory delusions: a first step toward a molecular genetic classification of psychiatric phenotypes. Arch Gen Psychiatry 2005;162:2101-8.
6. Tandon R, Fleischhacker WW. Comparative efficacy of antipsy chotics in the treatment of schizophrenia: a critical assessment. Schizophr Res 2005;79:145-55.
7. Calabrese JR, Keck P, Jr, McFadden W, et al:. A randomized double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression. Am J Psychiatry 2005;162:1351-60.
A hundred years ago, KraepelinDrs. Lake and Hurwitz for highlighting the diagnostic and treatment errors in a bipolar patient with severe psychotic features who was misdiagnosed as having schizophrenia. Errors such as this were common with DSM I and II but declined with the more reliable diagnostic schemas of DSM III and IV. I am puzzled, however, by their leap to the radical conclusion that schizophrenia does not exist and that all patients diagnosed with schizophrenia have psychotic bipolar disorder. This is not as egregious as Szasz’ absurd proclamation 4 decades ago that schizophrenia is a “myth,” but it is a significant scientific “transgression,” given the evidence that distinguishes schizophrenia from bipolar disorder.
Symptoms. Beyond a doubt, these two brain diseases have overlapping clinical features, pharmacotherapies, and even outcomes in a subgroup of patients. However, these diseases have major differences, as outlined in the accompanying table.
Brain anomalies. Neuroimaging studies indicate that schizophrenia is associated with more-severe and pervasive morphologic brain anomalies (dysplasia and hypoplasia) than bipolar disorder, although some bipolar patients have reduced cerebral and frontal volumes and marked cognitive deficits.3 Progressive neuro tissue loss has been observed early in schizophrenia but not in bipolar disorder.
Recent genetic studies indicate that several genes are found exclusively in schizophrenia or in bipolar disorder cohorts,4 but some are shared by both disorders and may be related to delusional symptoms.5 Familial transmission appears to differ: transgenerational studies find an abundance of mood disorders in family members of bipolar probands but relatively sparse occurrence of psychosis in families of probands with schizophrenia.
Table
Symptom differences between schizophrenia and bipolar disorder
| Symptom | Schizophrenia | Bipolar disorder |
|---|---|---|
| Psychosis | Auditory hallucinations and bizarre delusions are more common | Grandiosity is more common |
| Paranoia | Occurs in both, but more systematic in schizophrenia | |
| Core psychopathology | Far more negative symptoms and cognition dysfunction | Far more mood lability and affective cyclicity |
| Thought disorder | Far more disorganized and derailed thoughts | More likely to have racing thoughts and flight of ideas |
| Between-episode interpersonal skills | Withdrawn, alogic, seclusive | Much more interactive and verbal |
Treatment. There is no doubt that monotherapy with antipsychotics (old and new) has similar efficacy6 in schizophrenia and bipolar mania (and even in bipolar depression, with some atypicals7). However, there is minimal, if any, evidence that monotherapy mood stabilizers (lithium or anti-convulsants) have any tangible efficacy in schizophrenia. Electroconvulsive therapy is remarkably efficious in all phases of bipolar disorder but of dubious, if any, lasting benefit in schizophrenia.
Course. Both the premorbid history and post-treatment functional outcome tend to be more favorable in patients with bipolar disorder than schizophrenia. Most patients with schizophrenia experience significant clinical, social, and vocational deterioration, compared with a relative minority of bipolar patients.
In summary, schizophrenia and bipolar disorder are clearly distinct in their pure forms, although many patients have varying mixtures of both. Schizoaffective disorder is one of the most extensively investigated. Although many scholars have studied schizoaffective disorder, the evidence defies lumping it with either end of the continuum.
I agree with Drs. Lake and Hurwitz that most cases of schizoaffective disorder, especially the “schizomanic” type, are probably bipolar disorder with severe psychotic features. To assert, however, that schizophrenia does not exist at all and should be reclassified as bipolar disorder with psychotic features would contradict a massive body of clinical and biological evidence. It would cause Kraepelin to squirm in his grave.
A hundred years ago, KraepelinDrs. Lake and Hurwitz for highlighting the diagnostic and treatment errors in a bipolar patient with severe psychotic features who was misdiagnosed as having schizophrenia. Errors such as this were common with DSM I and II but declined with the more reliable diagnostic schemas of DSM III and IV. I am puzzled, however, by their leap to the radical conclusion that schizophrenia does not exist and that all patients diagnosed with schizophrenia have psychotic bipolar disorder. This is not as egregious as Szasz’ absurd proclamation 4 decades ago that schizophrenia is a “myth,” but it is a significant scientific “transgression,” given the evidence that distinguishes schizophrenia from bipolar disorder.
Symptoms. Beyond a doubt, these two brain diseases have overlapping clinical features, pharmacotherapies, and even outcomes in a subgroup of patients. However, these diseases have major differences, as outlined in the accompanying table.
Brain anomalies. Neuroimaging studies indicate that schizophrenia is associated with more-severe and pervasive morphologic brain anomalies (dysplasia and hypoplasia) than bipolar disorder, although some bipolar patients have reduced cerebral and frontal volumes and marked cognitive deficits.3 Progressive neuro tissue loss has been observed early in schizophrenia but not in bipolar disorder.
Recent genetic studies indicate that several genes are found exclusively in schizophrenia or in bipolar disorder cohorts,4 but some are shared by both disorders and may be related to delusional symptoms.5 Familial transmission appears to differ: transgenerational studies find an abundance of mood disorders in family members of bipolar probands but relatively sparse occurrence of psychosis in families of probands with schizophrenia.
Table
Symptom differences between schizophrenia and bipolar disorder
| Symptom | Schizophrenia | Bipolar disorder |
|---|---|---|
| Psychosis | Auditory hallucinations and bizarre delusions are more common | Grandiosity is more common |
| Paranoia | Occurs in both, but more systematic in schizophrenia | |
| Core psychopathology | Far more negative symptoms and cognition dysfunction | Far more mood lability and affective cyclicity |
| Thought disorder | Far more disorganized and derailed thoughts | More likely to have racing thoughts and flight of ideas |
| Between-episode interpersonal skills | Withdrawn, alogic, seclusive | Much more interactive and verbal |
Treatment. There is no doubt that monotherapy with antipsychotics (old and new) has similar efficacy6 in schizophrenia and bipolar mania (and even in bipolar depression, with some atypicals7). However, there is minimal, if any, evidence that monotherapy mood stabilizers (lithium or anti-convulsants) have any tangible efficacy in schizophrenia. Electroconvulsive therapy is remarkably efficious in all phases of bipolar disorder but of dubious, if any, lasting benefit in schizophrenia.
Course. Both the premorbid history and post-treatment functional outcome tend to be more favorable in patients with bipolar disorder than schizophrenia. Most patients with schizophrenia experience significant clinical, social, and vocational deterioration, compared with a relative minority of bipolar patients.
In summary, schizophrenia and bipolar disorder are clearly distinct in their pure forms, although many patients have varying mixtures of both. Schizoaffective disorder is one of the most extensively investigated. Although many scholars have studied schizoaffective disorder, the evidence defies lumping it with either end of the continuum.
I agree with Drs. Lake and Hurwitz that most cases of schizoaffective disorder, especially the “schizomanic” type, are probably bipolar disorder with severe psychotic features. To assert, however, that schizophrenia does not exist at all and should be reclassified as bipolar disorder with psychotic features would contradict a massive body of clinical and biological evidence. It would cause Kraepelin to squirm in his grave.
1. Kraepelin E, Lange J. Psychiatrie. In: Klinische Psychiatrie, vol 3 (8th ed). Leipzig, Germany: Barth; 1923.
2. Nasrallah HA. The continuum of psychoses between schizophrenia and bipolar disorder. Neurol Psychiatry Brain Res 1994;2:206-9.
3. Coffman JA, Bornstein RA, Olson SC, et al. Cognitive impairment and cerebral structure by MRI in bipolar disorder. Biol Psychiatry 1990;27(11):1188-96.
4. Weinberger DR. Genetic mechanisms of psychosis: in vivo and postmortem genomics. Clin Ther 2005;27(suppl):8-15.
5. Schulze TG, Ohlfaun S, Czerski PM, et al. Genotype-phenotype studies in bipolar disorder showing association between the DAOA/G30 locus and persecutory delusions: a first step toward a molecular genetic classification of psychiatric phenotypes. Arch Gen Psychiatry 2005;162:2101-8.
6. Tandon R, Fleischhacker WW. Comparative efficacy of antipsy chotics in the treatment of schizophrenia: a critical assessment. Schizophr Res 2005;79:145-55.
7. Calabrese JR, Keck P, Jr, McFadden W, et al:. A randomized double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression. Am J Psychiatry 2005;162:1351-60.
1. Kraepelin E, Lange J. Psychiatrie. In: Klinische Psychiatrie, vol 3 (8th ed). Leipzig, Germany: Barth; 1923.
2. Nasrallah HA. The continuum of psychoses between schizophrenia and bipolar disorder. Neurol Psychiatry Brain Res 1994;2:206-9.
3. Coffman JA, Bornstein RA, Olson SC, et al. Cognitive impairment and cerebral structure by MRI in bipolar disorder. Biol Psychiatry 1990;27(11):1188-96.
4. Weinberger DR. Genetic mechanisms of psychosis: in vivo and postmortem genomics. Clin Ther 2005;27(suppl):8-15.
5. Schulze TG, Ohlfaun S, Czerski PM, et al. Genotype-phenotype studies in bipolar disorder showing association between the DAOA/G30 locus and persecutory delusions: a first step toward a molecular genetic classification of psychiatric phenotypes. Arch Gen Psychiatry 2005;162:2101-8.
6. Tandon R, Fleischhacker WW. Comparative efficacy of antipsy chotics in the treatment of schizophrenia: a critical assessment. Schizophr Res 2005;79:145-55.
7. Calabrese JR, Keck P, Jr, McFadden W, et al:. A randomized double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression. Am J Psychiatry 2005;162:1351-60.