User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Abrocitinib emerges as a potential treatment option for moderate-to-severe atopic dermatitis
Key clinical point: Preliminary evidence from a meta-analysis suggests that abrocitinib was significantly beneficial with a tolerable adverse event profile in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: At 12 weeks, patients treated with abrocitinib achieved a significantly higher Investigator’s Global Assessment response vs those treated with placebo (risk ratio [RR], 3.52; P less than .00001). Treatment-emergent adverse effects were mostly mild and manageable, with a higher risk in the abrocitinib vs placebo group (RR, 1.17; P = .002).
Study details: Findings are from a meta-analysis of 4 randomized controlled trials, which assessed clinical outcomes in 1,175 patients with moderate-to-severe AD with inadequate response to topical medications who received abrocitinib 100 or 200 mg and 334 control participants who received placebo.
Disclosures: The study did not receive any funding. No conflict of interests was reported.
Source: Meher BR et al. J Dermatol Treat. 2021 Jul 27. doi: 10.1080/09546634.2021.1961997.
Key clinical point: Preliminary evidence from a meta-analysis suggests that abrocitinib was significantly beneficial with a tolerable adverse event profile in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: At 12 weeks, patients treated with abrocitinib achieved a significantly higher Investigator’s Global Assessment response vs those treated with placebo (risk ratio [RR], 3.52; P less than .00001). Treatment-emergent adverse effects were mostly mild and manageable, with a higher risk in the abrocitinib vs placebo group (RR, 1.17; P = .002).
Study details: Findings are from a meta-analysis of 4 randomized controlled trials, which assessed clinical outcomes in 1,175 patients with moderate-to-severe AD with inadequate response to topical medications who received abrocitinib 100 or 200 mg and 334 control participants who received placebo.
Disclosures: The study did not receive any funding. No conflict of interests was reported.
Source: Meher BR et al. J Dermatol Treat. 2021 Jul 27. doi: 10.1080/09546634.2021.1961997.
Key clinical point: Preliminary evidence from a meta-analysis suggests that abrocitinib was significantly beneficial with a tolerable adverse event profile in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: At 12 weeks, patients treated with abrocitinib achieved a significantly higher Investigator’s Global Assessment response vs those treated with placebo (risk ratio [RR], 3.52; P less than .00001). Treatment-emergent adverse effects were mostly mild and manageable, with a higher risk in the abrocitinib vs placebo group (RR, 1.17; P = .002).
Study details: Findings are from a meta-analysis of 4 randomized controlled trials, which assessed clinical outcomes in 1,175 patients with moderate-to-severe AD with inadequate response to topical medications who received abrocitinib 100 or 200 mg and 334 control participants who received placebo.
Disclosures: The study did not receive any funding. No conflict of interests was reported.
Source: Meher BR et al. J Dermatol Treat. 2021 Jul 27. doi: 10.1080/09546634.2021.1961997.
Atopic dermatitis tied to increased risk for hypertension
Key clinical point: Patients with atopic dermatitis (AD), particularly those with a moderate-to-severe form of the disease, had significantly higher odds of hypertension than healthy controls.
Major finding: Overall, odds of hypertension was significantly higher in patients with AD than healthy controls (odds ratio [OR], 1.16; 95% confidence interval [CI], 1.04-1.30), particularly in those with moderate-to-severe AD (OR, 2.33; 95% CI, 1.10-4.94). Hypertension was mainly reported as an adverse event from cyclosporine A (pooled prevalence, 7.8%).
Study details: Findings are from a meta-analysis of 19 studies involving 269,861 adults with AD and 718,873 healthy controls, including 52,530 children with AD and 340,356 children as healthy controls.
Disclosures: The study did not report any source of funding. No conflict of interests was reported.
Source: Yousaf M et al. Br J Dermatol. 2021 Jul 28. doi: 10.1111/bjd.20661.
Key clinical point: Patients with atopic dermatitis (AD), particularly those with a moderate-to-severe form of the disease, had significantly higher odds of hypertension than healthy controls.
Major finding: Overall, odds of hypertension was significantly higher in patients with AD than healthy controls (odds ratio [OR], 1.16; 95% confidence interval [CI], 1.04-1.30), particularly in those with moderate-to-severe AD (OR, 2.33; 95% CI, 1.10-4.94). Hypertension was mainly reported as an adverse event from cyclosporine A (pooled prevalence, 7.8%).
Study details: Findings are from a meta-analysis of 19 studies involving 269,861 adults with AD and 718,873 healthy controls, including 52,530 children with AD and 340,356 children as healthy controls.
Disclosures: The study did not report any source of funding. No conflict of interests was reported.
Source: Yousaf M et al. Br J Dermatol. 2021 Jul 28. doi: 10.1111/bjd.20661.
Key clinical point: Patients with atopic dermatitis (AD), particularly those with a moderate-to-severe form of the disease, had significantly higher odds of hypertension than healthy controls.
Major finding: Overall, odds of hypertension was significantly higher in patients with AD than healthy controls (odds ratio [OR], 1.16; 95% confidence interval [CI], 1.04-1.30), particularly in those with moderate-to-severe AD (OR, 2.33; 95% CI, 1.10-4.94). Hypertension was mainly reported as an adverse event from cyclosporine A (pooled prevalence, 7.8%).
Study details: Findings are from a meta-analysis of 19 studies involving 269,861 adults with AD and 718,873 healthy controls, including 52,530 children with AD and 340,356 children as healthy controls.
Disclosures: The study did not report any source of funding. No conflict of interests was reported.
Source: Yousaf M et al. Br J Dermatol. 2021 Jul 28. doi: 10.1111/bjd.20661.
Comorbidities account for higher incidence of hospitalization in patients with AD
Key clinical point: Over 20 years of evidence indicates an increased incidence of hospitalizations among patients with atopic dermatitis (AD) in the United States, mainly attributed to increased burden of medical comorbidities rather than AD itself, highlighting the need for increased screening and management of comorbidities.
Major finding: The number of hospitalizations among patients with AD increased from 1.0 to 2.3 per 100,000 persons from 1998 to 2018 (adjusted P-trend less than .0001). However, the proportion of hospitalizations with AD as the principal diagnosis reduced from 11.5% in 1998 to 3.7% in 2018 (adjusted P-trend = .001). A higher proportion of patients reported Charlson Comorbidity Index score of 3 or higher in 2018 vs 1998 (27.8% vs 10.5%; adjusted P-trend less than .0001).
Study details: Findings are from a longitudinal study including 23,410 adults hospitalized with any form of AD between 1998 and 2018.
Disclosures: The study did not report any source of funding. No conflict of interests was reported.
Source: Edigin E et al. J Am Acad Dermatol. 2021 Jul 9. doi: 10.1016/j.jaad.2021.06.882.
Key clinical point: Over 20 years of evidence indicates an increased incidence of hospitalizations among patients with atopic dermatitis (AD) in the United States, mainly attributed to increased burden of medical comorbidities rather than AD itself, highlighting the need for increased screening and management of comorbidities.
Major finding: The number of hospitalizations among patients with AD increased from 1.0 to 2.3 per 100,000 persons from 1998 to 2018 (adjusted P-trend less than .0001). However, the proportion of hospitalizations with AD as the principal diagnosis reduced from 11.5% in 1998 to 3.7% in 2018 (adjusted P-trend = .001). A higher proportion of patients reported Charlson Comorbidity Index score of 3 or higher in 2018 vs 1998 (27.8% vs 10.5%; adjusted P-trend less than .0001).
Study details: Findings are from a longitudinal study including 23,410 adults hospitalized with any form of AD between 1998 and 2018.
Disclosures: The study did not report any source of funding. No conflict of interests was reported.
Source: Edigin E et al. J Am Acad Dermatol. 2021 Jul 9. doi: 10.1016/j.jaad.2021.06.882.
Key clinical point: Over 20 years of evidence indicates an increased incidence of hospitalizations among patients with atopic dermatitis (AD) in the United States, mainly attributed to increased burden of medical comorbidities rather than AD itself, highlighting the need for increased screening and management of comorbidities.
Major finding: The number of hospitalizations among patients with AD increased from 1.0 to 2.3 per 100,000 persons from 1998 to 2018 (adjusted P-trend less than .0001). However, the proportion of hospitalizations with AD as the principal diagnosis reduced from 11.5% in 1998 to 3.7% in 2018 (adjusted P-trend = .001). A higher proportion of patients reported Charlson Comorbidity Index score of 3 or higher in 2018 vs 1998 (27.8% vs 10.5%; adjusted P-trend less than .0001).
Study details: Findings are from a longitudinal study including 23,410 adults hospitalized with any form of AD between 1998 and 2018.
Disclosures: The study did not report any source of funding. No conflict of interests was reported.
Source: Edigin E et al. J Am Acad Dermatol. 2021 Jul 9. doi: 10.1016/j.jaad.2021.06.882.
Methylation on golli-MBP locus serves as an indicator of atopic dermatitis severity in children
Key clinical point: Reduced DNA methylation of golli-myelin basic protein (MBP) locus could serve as an important biomarker to determine the severity of atopic dermatitis (AD) in pediatric patients.
Major finding: Loss of DNA methylation and higher golli-MBP mRNA expression were observed in pediatric patients with AD (both P less than .0001). The score in the intensity of symptoms increased with decreasing methylation levels in cg27400313, the differentially methylated CpG cluster of MBP gene (P = .012).
Study details: The study used a discovery cohort of 24 pediatric patients with AD and 24 control participants to screen for DNA methylation. The results were further validated in an additional cohort of 224 pediatric patients with AD and 44 control participants.
Disclosures: The study was supported by grants from Ministry of Health and Welfare and Chang Gung Memorial Hospital. The authors declared no conflict of interests.
Source: Chen KD et al. J Invest Dermatol. 2021 Jul 19. doi: 10.1016/j.jid.2021.06.025.
Key clinical point: Reduced DNA methylation of golli-myelin basic protein (MBP) locus could serve as an important biomarker to determine the severity of atopic dermatitis (AD) in pediatric patients.
Major finding: Loss of DNA methylation and higher golli-MBP mRNA expression were observed in pediatric patients with AD (both P less than .0001). The score in the intensity of symptoms increased with decreasing methylation levels in cg27400313, the differentially methylated CpG cluster of MBP gene (P = .012).
Study details: The study used a discovery cohort of 24 pediatric patients with AD and 24 control participants to screen for DNA methylation. The results were further validated in an additional cohort of 224 pediatric patients with AD and 44 control participants.
Disclosures: The study was supported by grants from Ministry of Health and Welfare and Chang Gung Memorial Hospital. The authors declared no conflict of interests.
Source: Chen KD et al. J Invest Dermatol. 2021 Jul 19. doi: 10.1016/j.jid.2021.06.025.
Key clinical point: Reduced DNA methylation of golli-myelin basic protein (MBP) locus could serve as an important biomarker to determine the severity of atopic dermatitis (AD) in pediatric patients.
Major finding: Loss of DNA methylation and higher golli-MBP mRNA expression were observed in pediatric patients with AD (both P less than .0001). The score in the intensity of symptoms increased with decreasing methylation levels in cg27400313, the differentially methylated CpG cluster of MBP gene (P = .012).
Study details: The study used a discovery cohort of 24 pediatric patients with AD and 24 control participants to screen for DNA methylation. The results were further validated in an additional cohort of 224 pediatric patients with AD and 44 control participants.
Disclosures: The study was supported by grants from Ministry of Health and Welfare and Chang Gung Memorial Hospital. The authors declared no conflict of interests.
Source: Chen KD et al. J Invest Dermatol. 2021 Jul 19. doi: 10.1016/j.jid.2021.06.025.
Sleep disturbance and geriatric age in atopic dermatitis: Is there a link?
Key clinical point: Geriatric patients experienced more profound sleep disturbance (SD) despite having similar severity of atopic dermatitis (AD) as younger adult patients with AD.
Major finding: Geriatric age was not associated with severity of AD as measured by Eczema Area and Severity Index score (adjusted odds ratio [aOR], 1.47; P = .3269). However, geriatric patients with AD spent an increased number of nights with SD from eczema (aOR, 2.14; P = .0142), experienced fatigue (aOR, 1.81; P = .0313), and had trouble staying asleep (aOR, 2.26; P = .0030).
Study details: Findings are from a cross-sectional, dermatology practice-based study conducted between 2014 and 2019 and included adults diagnosed with AD.
Disclosures: This study was supported by Agency for Healthcare Research and Quality, the Dermatology Foundation, and an unrestricted research grant from Galderma. The authors declared no conflict of interests.
Source: Manjunath J and Silverberg JI. J Am Acad Dermatol. 2021 Jul 29. doi: 10.1016/j.jaad.2021.07.039.
Key clinical point: Geriatric patients experienced more profound sleep disturbance (SD) despite having similar severity of atopic dermatitis (AD) as younger adult patients with AD.
Major finding: Geriatric age was not associated with severity of AD as measured by Eczema Area and Severity Index score (adjusted odds ratio [aOR], 1.47; P = .3269). However, geriatric patients with AD spent an increased number of nights with SD from eczema (aOR, 2.14; P = .0142), experienced fatigue (aOR, 1.81; P = .0313), and had trouble staying asleep (aOR, 2.26; P = .0030).
Study details: Findings are from a cross-sectional, dermatology practice-based study conducted between 2014 and 2019 and included adults diagnosed with AD.
Disclosures: This study was supported by Agency for Healthcare Research and Quality, the Dermatology Foundation, and an unrestricted research grant from Galderma. The authors declared no conflict of interests.
Source: Manjunath J and Silverberg JI. J Am Acad Dermatol. 2021 Jul 29. doi: 10.1016/j.jaad.2021.07.039.
Key clinical point: Geriatric patients experienced more profound sleep disturbance (SD) despite having similar severity of atopic dermatitis (AD) as younger adult patients with AD.
Major finding: Geriatric age was not associated with severity of AD as measured by Eczema Area and Severity Index score (adjusted odds ratio [aOR], 1.47; P = .3269). However, geriatric patients with AD spent an increased number of nights with SD from eczema (aOR, 2.14; P = .0142), experienced fatigue (aOR, 1.81; P = .0313), and had trouble staying asleep (aOR, 2.26; P = .0030).
Study details: Findings are from a cross-sectional, dermatology practice-based study conducted between 2014 and 2019 and included adults diagnosed with AD.
Disclosures: This study was supported by Agency for Healthcare Research and Quality, the Dermatology Foundation, and an unrestricted research grant from Galderma. The authors declared no conflict of interests.
Source: Manjunath J and Silverberg JI. J Am Acad Dermatol. 2021 Jul 29. doi: 10.1016/j.jaad.2021.07.039.
Cold atmospheric plasma alleviates AD severity without any safety issues
Key clinical point: Cold atmospheric plasma (CAP) can potentially improve the clinical severity of atopic dermatitis (AD) by recovering the diversity of skin microbiome and promoting wound healing for damaged skin barriers without any safety issues.
Major finding: At the end of treatment, reduction in Staphylococcus aureus count was significantly higher for the CAP vs sham group (10.14% vs 15.29%; P = .047). In the CAP group, mean modified AD antecubital severity score reduced significantly at week 4 vs baseline (13.12 vs 33.73; P less than .001), whereas reduction in the sham group was not statistically significant (P = .114). No severe adverse events were reported.
Study details: Findings are from a prospective analysis of 22 adults with mild-to-moderate AD having symmetric lesions. For each patient, the symmetric lesions were randomly assigned to either CAP or sham treatment.
Disclosures: The study did not report any source of funding. No conflicts of interest were reported.
Source: Kim YJ et al. Sci Rep. 2021 Jul 14. doi: 10.1038/s41598-021-93941-y.
Key clinical point: Cold atmospheric plasma (CAP) can potentially improve the clinical severity of atopic dermatitis (AD) by recovering the diversity of skin microbiome and promoting wound healing for damaged skin barriers without any safety issues.
Major finding: At the end of treatment, reduction in Staphylococcus aureus count was significantly higher for the CAP vs sham group (10.14% vs 15.29%; P = .047). In the CAP group, mean modified AD antecubital severity score reduced significantly at week 4 vs baseline (13.12 vs 33.73; P less than .001), whereas reduction in the sham group was not statistically significant (P = .114). No severe adverse events were reported.
Study details: Findings are from a prospective analysis of 22 adults with mild-to-moderate AD having symmetric lesions. For each patient, the symmetric lesions were randomly assigned to either CAP or sham treatment.
Disclosures: The study did not report any source of funding. No conflicts of interest were reported.
Source: Kim YJ et al. Sci Rep. 2021 Jul 14. doi: 10.1038/s41598-021-93941-y.
Key clinical point: Cold atmospheric plasma (CAP) can potentially improve the clinical severity of atopic dermatitis (AD) by recovering the diversity of skin microbiome and promoting wound healing for damaged skin barriers without any safety issues.
Major finding: At the end of treatment, reduction in Staphylococcus aureus count was significantly higher for the CAP vs sham group (10.14% vs 15.29%; P = .047). In the CAP group, mean modified AD antecubital severity score reduced significantly at week 4 vs baseline (13.12 vs 33.73; P less than .001), whereas reduction in the sham group was not statistically significant (P = .114). No severe adverse events were reported.
Study details: Findings are from a prospective analysis of 22 adults with mild-to-moderate AD having symmetric lesions. For each patient, the symmetric lesions were randomly assigned to either CAP or sham treatment.
Disclosures: The study did not report any source of funding. No conflicts of interest were reported.
Source: Kim YJ et al. Sci Rep. 2021 Jul 14. doi: 10.1038/s41598-021-93941-y.
Difamilast ointment shows promise for pediatric atopic dermatitis in phase 3
Key clinical point: Difamilast 0.3% and 1% ointments demonstrated superiority over vehicle along with a favorable safety profile when applied twice daily for up to 4 weeks in pediatric patients with atopic dermatitis (AD).
Major finding: At week 4, the success rate in investigator global assessment score was significantly higher with difamilast 0.3% (44.6%; P = .0005) and 1% (47.1%; P less than .0001) vs vehicle (18.1%) group. Treatment-emergent adverse effects were mostly mild or moderate in severity, with adverse event profiles similar between treatment and vehicle groups.
Study details: Findings are from a double-blind phase 3 trial including 251 patients aged 2-14 years with mild-to-moderate AD who were randomly assigned to receive difamilast 0.3%, difamilast 1%, or vehicle ointment twice daily for 4 weeks.
Disclosures: This study was funded by Otsuka Pharmaceutical Co., Ltd. Dr. Saeki and Dr. Baba declared receiving consultation fees from Otsuka Pharmaceutical Co., Ltd. Dr. Ito, Dr. Yokota, and Dr. Tsubouchi declared being employees of Otsuka Pharmaceutical Co., Ltd.
Source: Saeki H et al. Br J Dermatol. 2021 Jul 21. doi: 10.1111/bjd.20655.
Key clinical point: Difamilast 0.3% and 1% ointments demonstrated superiority over vehicle along with a favorable safety profile when applied twice daily for up to 4 weeks in pediatric patients with atopic dermatitis (AD).
Major finding: At week 4, the success rate in investigator global assessment score was significantly higher with difamilast 0.3% (44.6%; P = .0005) and 1% (47.1%; P less than .0001) vs vehicle (18.1%) group. Treatment-emergent adverse effects were mostly mild or moderate in severity, with adverse event profiles similar between treatment and vehicle groups.
Study details: Findings are from a double-blind phase 3 trial including 251 patients aged 2-14 years with mild-to-moderate AD who were randomly assigned to receive difamilast 0.3%, difamilast 1%, or vehicle ointment twice daily for 4 weeks.
Disclosures: This study was funded by Otsuka Pharmaceutical Co., Ltd. Dr. Saeki and Dr. Baba declared receiving consultation fees from Otsuka Pharmaceutical Co., Ltd. Dr. Ito, Dr. Yokota, and Dr. Tsubouchi declared being employees of Otsuka Pharmaceutical Co., Ltd.
Source: Saeki H et al. Br J Dermatol. 2021 Jul 21. doi: 10.1111/bjd.20655.
Key clinical point: Difamilast 0.3% and 1% ointments demonstrated superiority over vehicle along with a favorable safety profile when applied twice daily for up to 4 weeks in pediatric patients with atopic dermatitis (AD).
Major finding: At week 4, the success rate in investigator global assessment score was significantly higher with difamilast 0.3% (44.6%; P = .0005) and 1% (47.1%; P less than .0001) vs vehicle (18.1%) group. Treatment-emergent adverse effects were mostly mild or moderate in severity, with adverse event profiles similar between treatment and vehicle groups.
Study details: Findings are from a double-blind phase 3 trial including 251 patients aged 2-14 years with mild-to-moderate AD who were randomly assigned to receive difamilast 0.3%, difamilast 1%, or vehicle ointment twice daily for 4 weeks.
Disclosures: This study was funded by Otsuka Pharmaceutical Co., Ltd. Dr. Saeki and Dr. Baba declared receiving consultation fees from Otsuka Pharmaceutical Co., Ltd. Dr. Ito, Dr. Yokota, and Dr. Tsubouchi declared being employees of Otsuka Pharmaceutical Co., Ltd.
Source: Saeki H et al. Br J Dermatol. 2021 Jul 21. doi: 10.1111/bjd.20655.
Upadacitinib shows superior efficacy over dupilumab for moderate-to-severe atopic dermatitis in phase 3b
Key clinical point: Upadacitinib demonstrated superior efficacy over dupilumab for treatment of adults with moderate-to-severe atopic dermatitis (AD) with no new safety signals identified.
Major finding: At week 16, a higher proportion of patients achieved 75% improvement in Eczema Area and Severity Index with upadacitinib vs dupilumab (71.0% vs 61.1%; P = .006). Rates of serious treatment-emergent adverse events leading to drug discontinuation in upadacitinib vs dupilumab were 2.9% vs 1.2%, with no new safety risks observed for upadacitinib.
Study details: Findings are 24-week results of Heads up, a phase 3b trial including 692 adults with moderate-to-severe AD who were randomly assigned to receive oral upadacitinib, 30 mg once daily, or subcutaneous dupilumab, 300 mg every alternate week.
Disclosures: This study was funded by AbbVie. The authors declared serving as speaker, consultant, scientific advisor, clinical study investigator, and/or receiving personal fees and grants from various sources including AbbVie. Four authors declared being employees and/or shareholders of AbbVie.
Source: Blauvelt A et al. JAMA Dermatol. 2021 Aug 4. doi: 10.1001/jamadermatol.2021.3023.
Key clinical point: Upadacitinib demonstrated superior efficacy over dupilumab for treatment of adults with moderate-to-severe atopic dermatitis (AD) with no new safety signals identified.
Major finding: At week 16, a higher proportion of patients achieved 75% improvement in Eczema Area and Severity Index with upadacitinib vs dupilumab (71.0% vs 61.1%; P = .006). Rates of serious treatment-emergent adverse events leading to drug discontinuation in upadacitinib vs dupilumab were 2.9% vs 1.2%, with no new safety risks observed for upadacitinib.
Study details: Findings are 24-week results of Heads up, a phase 3b trial including 692 adults with moderate-to-severe AD who were randomly assigned to receive oral upadacitinib, 30 mg once daily, or subcutaneous dupilumab, 300 mg every alternate week.
Disclosures: This study was funded by AbbVie. The authors declared serving as speaker, consultant, scientific advisor, clinical study investigator, and/or receiving personal fees and grants from various sources including AbbVie. Four authors declared being employees and/or shareholders of AbbVie.
Source: Blauvelt A et al. JAMA Dermatol. 2021 Aug 4. doi: 10.1001/jamadermatol.2021.3023.
Key clinical point: Upadacitinib demonstrated superior efficacy over dupilumab for treatment of adults with moderate-to-severe atopic dermatitis (AD) with no new safety signals identified.
Major finding: At week 16, a higher proportion of patients achieved 75% improvement in Eczema Area and Severity Index with upadacitinib vs dupilumab (71.0% vs 61.1%; P = .006). Rates of serious treatment-emergent adverse events leading to drug discontinuation in upadacitinib vs dupilumab were 2.9% vs 1.2%, with no new safety risks observed for upadacitinib.
Study details: Findings are 24-week results of Heads up, a phase 3b trial including 692 adults with moderate-to-severe AD who were randomly assigned to receive oral upadacitinib, 30 mg once daily, or subcutaneous dupilumab, 300 mg every alternate week.
Disclosures: This study was funded by AbbVie. The authors declared serving as speaker, consultant, scientific advisor, clinical study investigator, and/or receiving personal fees and grants from various sources including AbbVie. Four authors declared being employees and/or shareholders of AbbVie.
Source: Blauvelt A et al. JAMA Dermatol. 2021 Aug 4. doi: 10.1001/jamadermatol.2021.3023.
Cutaneous Protothecosis
To the Editor:
Protothecosis infections are caused by an achlorophyllic algae of the species Prototheca. Prototheca organisms are found mostly in soil and water.1 Human infections are rare and involve 2 species, Prototheca wickerhamii and Prototheca zopfii. The former most commonly is responsible for human infections, though P zopfii results in more serious systemic infections with a poor prognosis. There are various types of Prototheca infection presentations, with a 2007 review of 117 cases reporting that cutaneous infections are most common (66%), followed by systemic infections (19%), and olecranon bursitis (15%).2 Skin lesions most commonly occur on the extremities and face, and they present as vesiculobullous and ulcerative lesions with purulent drainage. The skin lesions also may appear as erythematous plaques or nodules, subcutaneous papules, verrucous or herpetiformis lesions, or pyogenic granuloma–like lesions.3 Protothecosis typically affects immunocompromised individuals, especially those with a history of chronic corticosteroid use, malignancy, diabetes mellitus, AIDS, and/or organ transplant.1 We present a case of cutaneous protothecosis on the dorsal distal extremity of a 94-year-old woman. History of exposure to soil while gardening was elicited from the patient, and no immunosuppressive history was present aside from the patient’s age. This case may prompt workup for malignancy or immunosuppression in this patient subset.
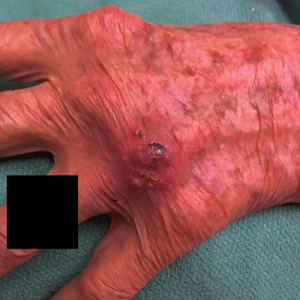
A 94-year-old woman with a medical history of cutaneous squamous cell carcinoma (SCC) presented with a growing lesion on the dorsal surface of the left fourth digit of 2 months’ duration. The patient reported the lesion was painful, and she noted preceding trauma to the area that was suspected to have occurred while gardening. Physical examination revealed an ulcerated, hypertrophic, erythematous nodule on the dorsal surface of the left fourth metacarpophalangeal joint. The differential diagnosis included SCC, inflamed cyst, verruca vulgaris, and orf virus due to the clinical presentation. A shave biopsy was performed, and the lesion subsequently was treated with electrodesiccation and curettage.
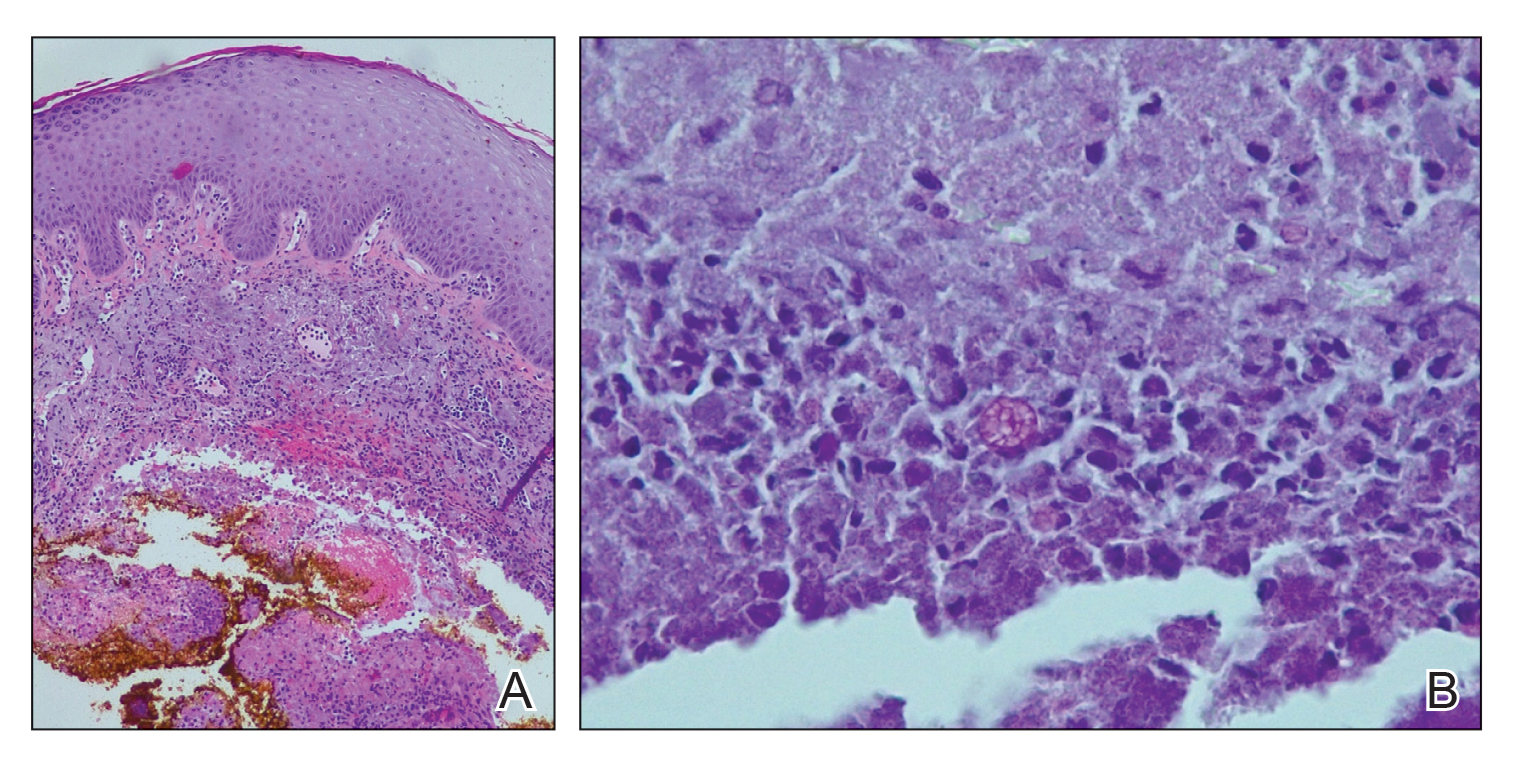
Histopathologic evaluation revealed pseudoepitheliomatous hyperplasia with a mixed inflammatory infiltrate including lymphocytes and histiocytes. A morula within the dermis was characteristic of a protothecosis infection (Figure 1). On follow-up visit 6 weeks later, the lesion had grown back to its original size and morphology (Figure 2). At this time, the lesion was again treated with shave removal, followed by electrodesiccation and curettage, and the patient was placed on oral fluconazole 200 mg daily for 1 month. When the lesion did not resolve with fluconazole, she was referred to infectious disease as well as general surgery for surgical removal and debridement of the lesion. Unfortunately, the patient was lost to follow-up.
Protothecosis is an infectious disease comprised of achlorophyllic algae found in soil and water that rarely affects humans. When it does affect humans, cutaneous infections are most common. All human cases in which organisms were identified to species level have been caused by P wickerhamii or P zopfii species.2 Inoculation is suspected to occur through trauma to affected skin, especially when in the context of contaminated water. Our patient reported history of trauma to the hand, with soil from gardening as the potential aquagenic source of the infection.

The clinical presentation of protothecosis ranges from localized cutaneous to disseminated systemic infections, with most reported cases of systemic disease occurring in immunocompromised individuals. The cutaneous lesions of protothecosis vary greatly in clinical appearance including ulcerative nodules (as in our case), papules, plaques, pustules, and vesicles with erosion or crusting.4
Cutaneous protothecosis has the potential to mimic many other skin diseases and lesions, and, given its rarity, it may not be on the radar of dermatologists. Our patient’s lesion was presumed to be a skin cancer and was treated as such because of the history of SCC and clinical presentation. Although excision of individual lesions of protothecosis can be curative, electrodesiccation and curettage does not appear to be an adequate treatment, as the lesion subsequently recurred. It also is possible that this case represents P zopfii infection, as it did not respond to treatment with oral fluconazole, though in vitro studies with fluconazole to both P zopfii and P wickerhamii had variable treatment success.2 Also, the histopathologic findings were most consistent with P wickerhamii, revealing small, round, symmetrical morula, compared to P zopfii, which typically will display oval or cylindrical, asymmetrical, random internal segmentation.5 This case may warrant determination of species, which can be accomplished by a culture on Sabouraud dextrose agar, carbohydrate and alcohol assimilation test, yeast biochemical card, serological typing by immunoblotting, immunofluorescence study using species-specific antibodies, or amplification by polymerase chain reaction for small subunit ribosomal DNA sequences.2,6-8
The natural history of isolated skin disease is an indolent progressive course; however, reports do exist noting spontaneous resolution.4,9 Treatment options for Prototheca infections can be disappointing and consist of both surgical and medical management, or a combination of the 2 approaches. Reports in the literature support the use of antifungals including ketoconazole, voriconazole, itraconazole, fluconazole, and amphotericin B, with the latter displaying the best activity against Prototheca species.2 Tetracycline has been used in combination with oral or topical amphotericin B and was found to be synergistic in vitro and in case reports at successfully treating cutaneous protothecosis infections. It is possible that our patient was not treated with fluconazole long enough for it to become therapeutic, as most reported treatment regimens are weeks to months in length. Conversely, it may have been of benefit to transition the patient to topical amphotericin B and tetracycline, as fluconazole failed in this patient. However, treatment successes and failures are limited to case reports/case series and in vitro studies, with prospective studies lacking. Due to the variability with in vitro susceptibility profiles for Prototheca species, it generally is not recommended to pursue in vitro susceptibility testing in the management of Prototheca skin infections due to the inconsistency demonstrated between in vitro activity and clinical response to therapy.2
- Silva PC, Costa e Silva SB, Lima RB, et al. Cutaneous protothecosis—case report. An Bras Dermatol. 2013;88:183-185.
- Lass-Flörl C, Mayr A. Human protothecosis. Clin Microbiol Rev. 2007;20:230-242.
- Seok JY, Lee Y, Lee H, et al. Human cutaneous protothecosis: report of a case and literature review. Korean J Pathol. 2013;47:575-578.
- Mayorga J, Barba-Gómez JF, Verduzco-Martínez AP, et al. Protothecosis. Clin Dermatol. 2012;30:432-436.
- Walsh SV, Johnson RA, Tahan SR. Protothecosis: an unusual cause of chronic subcutaneous and soft tissue infection. Am J Dermatopathol. 1998;20:379-382.
- Casal MJ, Gutierrez J. Simple new test for rapid differentiation of Prototheca wickerhamii from Prototheca zopfii. J Clin Microbiol. 1983;18:992-993.
- Arnold, P, Ahearn, DG. The systematics of the genus Prototheca with a description of a new species P. filamenta. Mycologia 1972;64:265-275.
- Roesler U, Scholz H, Hensel H. Emended phenotypic characterization of Prototheca zopfii: a proposal for three biotypes and standards for their identification. Int J Syst Evol Microbiol. 2003;53:1195-1199.
- Todd JR, King JW, Oberle A, et al. Protothecosis: report of a case with 20-year follow-up, and review of previously published cases. Med Mycol. 2012;50:673-689.
To the Editor:
Protothecosis infections are caused by an achlorophyllic algae of the species Prototheca. Prototheca organisms are found mostly in soil and water.1 Human infections are rare and involve 2 species, Prototheca wickerhamii and Prototheca zopfii. The former most commonly is responsible for human infections, though P zopfii results in more serious systemic infections with a poor prognosis. There are various types of Prototheca infection presentations, with a 2007 review of 117 cases reporting that cutaneous infections are most common (66%), followed by systemic infections (19%), and olecranon bursitis (15%).2 Skin lesions most commonly occur on the extremities and face, and they present as vesiculobullous and ulcerative lesions with purulent drainage. The skin lesions also may appear as erythematous plaques or nodules, subcutaneous papules, verrucous or herpetiformis lesions, or pyogenic granuloma–like lesions.3 Protothecosis typically affects immunocompromised individuals, especially those with a history of chronic corticosteroid use, malignancy, diabetes mellitus, AIDS, and/or organ transplant.1 We present a case of cutaneous protothecosis on the dorsal distal extremity of a 94-year-old woman. History of exposure to soil while gardening was elicited from the patient, and no immunosuppressive history was present aside from the patient’s age. This case may prompt workup for malignancy or immunosuppression in this patient subset.
A 94-year-old woman with a medical history of cutaneous squamous cell carcinoma (SCC) presented with a growing lesion on the dorsal surface of the left fourth digit of 2 months’ duration. The patient reported the lesion was painful, and she noted preceding trauma to the area that was suspected to have occurred while gardening. Physical examination revealed an ulcerated, hypertrophic, erythematous nodule on the dorsal surface of the left fourth metacarpophalangeal joint. The differential diagnosis included SCC, inflamed cyst, verruca vulgaris, and orf virus due to the clinical presentation. A shave biopsy was performed, and the lesion subsequently was treated with electrodesiccation and curettage.
Histopathologic evaluation revealed pseudoepitheliomatous hyperplasia with a mixed inflammatory infiltrate including lymphocytes and histiocytes. A morula within the dermis was characteristic of a protothecosis infection (Figure 1). On follow-up visit 6 weeks later, the lesion had grown back to its original size and morphology (Figure 2). At this time, the lesion was again treated with shave removal, followed by electrodesiccation and curettage, and the patient was placed on oral fluconazole 200 mg daily for 1 month. When the lesion did not resolve with fluconazole, she was referred to infectious disease as well as general surgery for surgical removal and debridement of the lesion. Unfortunately, the patient was lost to follow-up.

Protothecosis is an infectious disease comprised of achlorophyllic algae found in soil and water that rarely affects humans. When it does affect humans, cutaneous infections are most common. All human cases in which organisms were identified to species level have been caused by P wickerhamii or P zopfii species.2 Inoculation is suspected to occur through trauma to affected skin, especially when in the context of contaminated water. Our patient reported history of trauma to the hand, with soil from gardening as the potential aquagenic source of the infection.

The clinical presentation of protothecosis ranges from localized cutaneous to disseminated systemic infections, with most reported cases of systemic disease occurring in immunocompromised individuals. The cutaneous lesions of protothecosis vary greatly in clinical appearance including ulcerative nodules (as in our case), papules, plaques, pustules, and vesicles with erosion or crusting.4
Cutaneous protothecosis has the potential to mimic many other skin diseases and lesions, and, given its rarity, it may not be on the radar of dermatologists. Our patient’s lesion was presumed to be a skin cancer and was treated as such because of the history of SCC and clinical presentation. Although excision of individual lesions of protothecosis can be curative, electrodesiccation and curettage does not appear to be an adequate treatment, as the lesion subsequently recurred. It also is possible that this case represents P zopfii infection, as it did not respond to treatment with oral fluconazole, though in vitro studies with fluconazole to both P zopfii and P wickerhamii had variable treatment success.2 Also, the histopathologic findings were most consistent with P wickerhamii, revealing small, round, symmetrical morula, compared to P zopfii, which typically will display oval or cylindrical, asymmetrical, random internal segmentation.5 This case may warrant determination of species, which can be accomplished by a culture on Sabouraud dextrose agar, carbohydrate and alcohol assimilation test, yeast biochemical card, serological typing by immunoblotting, immunofluorescence study using species-specific antibodies, or amplification by polymerase chain reaction for small subunit ribosomal DNA sequences.2,6-8
The natural history of isolated skin disease is an indolent progressive course; however, reports do exist noting spontaneous resolution.4,9 Treatment options for Prototheca infections can be disappointing and consist of both surgical and medical management, or a combination of the 2 approaches. Reports in the literature support the use of antifungals including ketoconazole, voriconazole, itraconazole, fluconazole, and amphotericin B, with the latter displaying the best activity against Prototheca species.2 Tetracycline has been used in combination with oral or topical amphotericin B and was found to be synergistic in vitro and in case reports at successfully treating cutaneous protothecosis infections. It is possible that our patient was not treated with fluconazole long enough for it to become therapeutic, as most reported treatment regimens are weeks to months in length. Conversely, it may have been of benefit to transition the patient to topical amphotericin B and tetracycline, as fluconazole failed in this patient. However, treatment successes and failures are limited to case reports/case series and in vitro studies, with prospective studies lacking. Due to the variability with in vitro susceptibility profiles for Prototheca species, it generally is not recommended to pursue in vitro susceptibility testing in the management of Prototheca skin infections due to the inconsistency demonstrated between in vitro activity and clinical response to therapy.2
To the Editor:
Protothecosis infections are caused by an achlorophyllic algae of the species Prototheca. Prototheca organisms are found mostly in soil and water.1 Human infections are rare and involve 2 species, Prototheca wickerhamii and Prototheca zopfii. The former most commonly is responsible for human infections, though P zopfii results in more serious systemic infections with a poor prognosis. There are various types of Prototheca infection presentations, with a 2007 review of 117 cases reporting that cutaneous infections are most common (66%), followed by systemic infections (19%), and olecranon bursitis (15%).2 Skin lesions most commonly occur on the extremities and face, and they present as vesiculobullous and ulcerative lesions with purulent drainage. The skin lesions also may appear as erythematous plaques or nodules, subcutaneous papules, verrucous or herpetiformis lesions, or pyogenic granuloma–like lesions.3 Protothecosis typically affects immunocompromised individuals, especially those with a history of chronic corticosteroid use, malignancy, diabetes mellitus, AIDS, and/or organ transplant.1 We present a case of cutaneous protothecosis on the dorsal distal extremity of a 94-year-old woman. History of exposure to soil while gardening was elicited from the patient, and no immunosuppressive history was present aside from the patient’s age. This case may prompt workup for malignancy or immunosuppression in this patient subset.
A 94-year-old woman with a medical history of cutaneous squamous cell carcinoma (SCC) presented with a growing lesion on the dorsal surface of the left fourth digit of 2 months’ duration. The patient reported the lesion was painful, and she noted preceding trauma to the area that was suspected to have occurred while gardening. Physical examination revealed an ulcerated, hypertrophic, erythematous nodule on the dorsal surface of the left fourth metacarpophalangeal joint. The differential diagnosis included SCC, inflamed cyst, verruca vulgaris, and orf virus due to the clinical presentation. A shave biopsy was performed, and the lesion subsequently was treated with electrodesiccation and curettage.
Histopathologic evaluation revealed pseudoepitheliomatous hyperplasia with a mixed inflammatory infiltrate including lymphocytes and histiocytes. A morula within the dermis was characteristic of a protothecosis infection (Figure 1). On follow-up visit 6 weeks later, the lesion had grown back to its original size and morphology (Figure 2). At this time, the lesion was again treated with shave removal, followed by electrodesiccation and curettage, and the patient was placed on oral fluconazole 200 mg daily for 1 month. When the lesion did not resolve with fluconazole, she was referred to infectious disease as well as general surgery for surgical removal and debridement of the lesion. Unfortunately, the patient was lost to follow-up.

Protothecosis is an infectious disease comprised of achlorophyllic algae found in soil and water that rarely affects humans. When it does affect humans, cutaneous infections are most common. All human cases in which organisms were identified to species level have been caused by P wickerhamii or P zopfii species.2 Inoculation is suspected to occur through trauma to affected skin, especially when in the context of contaminated water. Our patient reported history of trauma to the hand, with soil from gardening as the potential aquagenic source of the infection.

The clinical presentation of protothecosis ranges from localized cutaneous to disseminated systemic infections, with most reported cases of systemic disease occurring in immunocompromised individuals. The cutaneous lesions of protothecosis vary greatly in clinical appearance including ulcerative nodules (as in our case), papules, plaques, pustules, and vesicles with erosion or crusting.4
Cutaneous protothecosis has the potential to mimic many other skin diseases and lesions, and, given its rarity, it may not be on the radar of dermatologists. Our patient’s lesion was presumed to be a skin cancer and was treated as such because of the history of SCC and clinical presentation. Although excision of individual lesions of protothecosis can be curative, electrodesiccation and curettage does not appear to be an adequate treatment, as the lesion subsequently recurred. It also is possible that this case represents P zopfii infection, as it did not respond to treatment with oral fluconazole, though in vitro studies with fluconazole to both P zopfii and P wickerhamii had variable treatment success.2 Also, the histopathologic findings were most consistent with P wickerhamii, revealing small, round, symmetrical morula, compared to P zopfii, which typically will display oval or cylindrical, asymmetrical, random internal segmentation.5 This case may warrant determination of species, which can be accomplished by a culture on Sabouraud dextrose agar, carbohydrate and alcohol assimilation test, yeast biochemical card, serological typing by immunoblotting, immunofluorescence study using species-specific antibodies, or amplification by polymerase chain reaction for small subunit ribosomal DNA sequences.2,6-8
The natural history of isolated skin disease is an indolent progressive course; however, reports do exist noting spontaneous resolution.4,9 Treatment options for Prototheca infections can be disappointing and consist of both surgical and medical management, or a combination of the 2 approaches. Reports in the literature support the use of antifungals including ketoconazole, voriconazole, itraconazole, fluconazole, and amphotericin B, with the latter displaying the best activity against Prototheca species.2 Tetracycline has been used in combination with oral or topical amphotericin B and was found to be synergistic in vitro and in case reports at successfully treating cutaneous protothecosis infections. It is possible that our patient was not treated with fluconazole long enough for it to become therapeutic, as most reported treatment regimens are weeks to months in length. Conversely, it may have been of benefit to transition the patient to topical amphotericin B and tetracycline, as fluconazole failed in this patient. However, treatment successes and failures are limited to case reports/case series and in vitro studies, with prospective studies lacking. Due to the variability with in vitro susceptibility profiles for Prototheca species, it generally is not recommended to pursue in vitro susceptibility testing in the management of Prototheca skin infections due to the inconsistency demonstrated between in vitro activity and clinical response to therapy.2
- Silva PC, Costa e Silva SB, Lima RB, et al. Cutaneous protothecosis—case report. An Bras Dermatol. 2013;88:183-185.
- Lass-Flörl C, Mayr A. Human protothecosis. Clin Microbiol Rev. 2007;20:230-242.
- Seok JY, Lee Y, Lee H, et al. Human cutaneous protothecosis: report of a case and literature review. Korean J Pathol. 2013;47:575-578.
- Mayorga J, Barba-Gómez JF, Verduzco-Martínez AP, et al. Protothecosis. Clin Dermatol. 2012;30:432-436.
- Walsh SV, Johnson RA, Tahan SR. Protothecosis: an unusual cause of chronic subcutaneous and soft tissue infection. Am J Dermatopathol. 1998;20:379-382.
- Casal MJ, Gutierrez J. Simple new test for rapid differentiation of Prototheca wickerhamii from Prototheca zopfii. J Clin Microbiol. 1983;18:992-993.
- Arnold, P, Ahearn, DG. The systematics of the genus Prototheca with a description of a new species P. filamenta. Mycologia 1972;64:265-275.
- Roesler U, Scholz H, Hensel H. Emended phenotypic characterization of Prototheca zopfii: a proposal for three biotypes and standards for their identification. Int J Syst Evol Microbiol. 2003;53:1195-1199.
- Todd JR, King JW, Oberle A, et al. Protothecosis: report of a case with 20-year follow-up, and review of previously published cases. Med Mycol. 2012;50:673-689.
- Silva PC, Costa e Silva SB, Lima RB, et al. Cutaneous protothecosis—case report. An Bras Dermatol. 2013;88:183-185.
- Lass-Flörl C, Mayr A. Human protothecosis. Clin Microbiol Rev. 2007;20:230-242.
- Seok JY, Lee Y, Lee H, et al. Human cutaneous protothecosis: report of a case and literature review. Korean J Pathol. 2013;47:575-578.
- Mayorga J, Barba-Gómez JF, Verduzco-Martínez AP, et al. Protothecosis. Clin Dermatol. 2012;30:432-436.
- Walsh SV, Johnson RA, Tahan SR. Protothecosis: an unusual cause of chronic subcutaneous and soft tissue infection. Am J Dermatopathol. 1998;20:379-382.
- Casal MJ, Gutierrez J. Simple new test for rapid differentiation of Prototheca wickerhamii from Prototheca zopfii. J Clin Microbiol. 1983;18:992-993.
- Arnold, P, Ahearn, DG. The systematics of the genus Prototheca with a description of a new species P. filamenta. Mycologia 1972;64:265-275.
- Roesler U, Scholz H, Hensel H. Emended phenotypic characterization of Prototheca zopfii: a proposal for three biotypes and standards for their identification. Int J Syst Evol Microbiol. 2003;53:1195-1199.
- Todd JR, King JW, Oberle A, et al. Protothecosis: report of a case with 20-year follow-up, and review of previously published cases. Med Mycol. 2012;50:673-689.
Practice Points
- Cutaneous protothecosis is a rare skin infection most commonly reported in immunocompromised individuals with recent exposure to contaminated soil or water. Cutaneous protothecosis has the potential to mimic many other skin diseases and lesions, including eczema; nonmelanoma skin cancer; or bacterial, viral, and fungal skin infections.
- A skin biopsy is essential for diagnosis, and histopathology is characteristic with soccer ball–appearing morula noted in a mixed inflammatory infiltrate.
Bimekizumab approved in Europe for psoriasis treatment
, according to a statement from the manufacturer.
Bimekizumab (Bimzelx), a humanized IgG1 monoclonal antibody, is the first approved treatment for moderate to severe plaque psoriasis that selectively inhibits interleukin (IL)–17A and IL-17F, the statement from UCB said.
In the United States, the Food and Drug Administration is expected to make a decision on approval of bimekizumab for treating psoriasis on Oct. 15.
Approval in the EU was based on data from three phase 3 trials including a total of 1,480 adult patients with moderate to severe psoriasis, which found that those treated with bimekizumab experienced significantly greater skin clearance, compared with placebo, ustekinumab, and adalimumab, with a favorable safety profile, according to the company.
In all three studies (BE VIVID, BE READY, and BE SURE), more than 80% of patients treated with bimekizumab showed improved skin clearance after 16 weeks, significantly more than those treated with ustekinumab, placebo, or adalimumab, based on an improvement of at least 90% in the Psoriasis Area & Severity Index (PASI 90) and an Investigator’s Global Assessment (IGA) response of clear or almost clear skin (IGA 0/1). In all three studies, these clinical responses persisted after 1 year.
The recommended dose of bimekizumab is 320 mg, given in two subcutaneous injections every 4 weeks to week 16, then every 8 weeks. However, for “some patients” weighing 120 kg or more who have not achieved complete skin clearance at 16 weeks, 320 mg every 4 weeks after that time may improve response to treatment, according to the company statement.
The most common treatment-related adverse events in the studies were upper respiratory tract infections (a majority of which were nasopharyngitis), reported by 14.5% of patients, followed by oral candidiasis, reported by 7.3%.
Results of BE READY and BE VIVID were published in The Lancet. Results of the BE SURE study were published in The New England Journal of Medicine.
Bimekizumab is contraindicated for individuals with clinically important active infections such as tuberculosis, and for individuals with any hypersensitivity to the active substance. More details on bimekizumab are available on the website of the European Medicines Agency.
, according to a statement from the manufacturer.
Bimekizumab (Bimzelx), a humanized IgG1 monoclonal antibody, is the first approved treatment for moderate to severe plaque psoriasis that selectively inhibits interleukin (IL)–17A and IL-17F, the statement from UCB said.
In the United States, the Food and Drug Administration is expected to make a decision on approval of bimekizumab for treating psoriasis on Oct. 15.
Approval in the EU was based on data from three phase 3 trials including a total of 1,480 adult patients with moderate to severe psoriasis, which found that those treated with bimekizumab experienced significantly greater skin clearance, compared with placebo, ustekinumab, and adalimumab, with a favorable safety profile, according to the company.
In all three studies (BE VIVID, BE READY, and BE SURE), more than 80% of patients treated with bimekizumab showed improved skin clearance after 16 weeks, significantly more than those treated with ustekinumab, placebo, or adalimumab, based on an improvement of at least 90% in the Psoriasis Area & Severity Index (PASI 90) and an Investigator’s Global Assessment (IGA) response of clear or almost clear skin (IGA 0/1). In all three studies, these clinical responses persisted after 1 year.
The recommended dose of bimekizumab is 320 mg, given in two subcutaneous injections every 4 weeks to week 16, then every 8 weeks. However, for “some patients” weighing 120 kg or more who have not achieved complete skin clearance at 16 weeks, 320 mg every 4 weeks after that time may improve response to treatment, according to the company statement.
The most common treatment-related adverse events in the studies were upper respiratory tract infections (a majority of which were nasopharyngitis), reported by 14.5% of patients, followed by oral candidiasis, reported by 7.3%.
Results of BE READY and BE VIVID were published in The Lancet. Results of the BE SURE study were published in The New England Journal of Medicine.
Bimekizumab is contraindicated for individuals with clinically important active infections such as tuberculosis, and for individuals with any hypersensitivity to the active substance. More details on bimekizumab are available on the website of the European Medicines Agency.
, according to a statement from the manufacturer.
Bimekizumab (Bimzelx), a humanized IgG1 monoclonal antibody, is the first approved treatment for moderate to severe plaque psoriasis that selectively inhibits interleukin (IL)–17A and IL-17F, the statement from UCB said.
In the United States, the Food and Drug Administration is expected to make a decision on approval of bimekizumab for treating psoriasis on Oct. 15.
Approval in the EU was based on data from three phase 3 trials including a total of 1,480 adult patients with moderate to severe psoriasis, which found that those treated with bimekizumab experienced significantly greater skin clearance, compared with placebo, ustekinumab, and adalimumab, with a favorable safety profile, according to the company.
In all three studies (BE VIVID, BE READY, and BE SURE), more than 80% of patients treated with bimekizumab showed improved skin clearance after 16 weeks, significantly more than those treated with ustekinumab, placebo, or adalimumab, based on an improvement of at least 90% in the Psoriasis Area & Severity Index (PASI 90) and an Investigator’s Global Assessment (IGA) response of clear or almost clear skin (IGA 0/1). In all three studies, these clinical responses persisted after 1 year.
The recommended dose of bimekizumab is 320 mg, given in two subcutaneous injections every 4 weeks to week 16, then every 8 weeks. However, for “some patients” weighing 120 kg or more who have not achieved complete skin clearance at 16 weeks, 320 mg every 4 weeks after that time may improve response to treatment, according to the company statement.
The most common treatment-related adverse events in the studies were upper respiratory tract infections (a majority of which were nasopharyngitis), reported by 14.5% of patients, followed by oral candidiasis, reported by 7.3%.
Results of BE READY and BE VIVID were published in The Lancet. Results of the BE SURE study were published in The New England Journal of Medicine.
Bimekizumab is contraindicated for individuals with clinically important active infections such as tuberculosis, and for individuals with any hypersensitivity to the active substance. More details on bimekizumab are available on the website of the European Medicines Agency.