User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Psoriatic arthritis health care costs continue to rise over time
Annual health care costs for patients with psoriatic arthritis rose over recent 5-year periods across all categories of resource use to a significantly greater extent than among patients with psoriasis only or those without any psoriatic disease diagnoses, according to commercial insurance claims data.
Using an IBM MarketScan Commercial Database, researchers examined claims data for 208,434 patients with psoriasis, 47,274 with PsA, and 255,708 controls who had neither psoriasis nor PsA. Controls were matched for age and sex. Those with RA, ankylosing spondylitis, Crohn’s disease, or ulcerative colitis were excluded.
The investigators examined data for 2009-2020, following patients for 5 years within that period. They looked at hospitalizations, outpatient and pharmacy services, lab services, and office visits, Steven Peterson, director of market access for rheumatology at Janssen Pharmaceuticals, said in his presentation of the data at the Pan American League of Associations for Rheumatology 2021 annual meeting, held recently as a virtual event.
The research was also published online May 2, 2021, in Clinical Rheumatology.
Big differences between the groups were seen in the first year, when the average health care costs for the PsA group were $28,322, about half of which was outpatient drug costs. That compared with $12,039 for the psoriasis group and $6,672 for the control group.
The differences tended to widen over time. By the fifth year, average costs for the PsA group were $34,290, nearly 60% of which were drug costs. That compared with $12,877 for the psoriasis group and $8,569 for the control group. In each year examined, outpatient drug costs accounted for less than half of the expenses for the psoriasis group and about a quarter for the control group.
Researchers found that the PsA group needed 28.7 prescriptions per person per year, compared with 17.0 and 12.7 in the psoriasis and control groups, respectively, Mr. Peterson said. He also noted that patients with PsA and psoriasis tended to have higher rates of hypertension, depression, and anxiety.
“The cost and resource utilization disparity between these patient groups demonstrates the high remaining unmet medical need for patients with psoriasis and psoriatic arthritis,” Mr. Peterson said during the virtual proceedings.
Do findings reflect treatment advances?
Elaine Husni, MD, MPH, director of the Arthritis and Musculoskeletal Center at the Cleveland Clinic, where she studies health outcomes in PsA, said the findings are helpful in pointing to a trend across a large sample. But she added it’s important to remember that the increasing costs could reflect recent advances in PsA treatment, which include costly biologic drugs.
“There’s a ton more treatments for psoriasis and psoriatic arthritis than there were even just 5 years ago,” she said in an interview. She was not involved in the research.
Dr. Husni would like to see a more detailed look at the costs, from the categories of expenses to the patients who are incurring the highest costs.
“Is it just a couple of percent of really sick patients that are driving the psoriatic arthritis group?” she wondered.
She also pointed out that PsA is going to be more expensive by its very nature. PsA tends to develop 3-10 years after psoriasis, adding to the costs for someone who already has psoriasis and at a time when they are older and likely have higher health care costs because of comorbidities that develop with age.
Dr. Husni said she does think about treatment costs, in that a less expensive first-line drug might be more appropriate than going straight to a more expensive biologic, especially because they also tend to be safer. She said it’s not just a simple question of curbing costs.
“Is there a way that we can personalize medicine?” she asked. “Is there a way that we can be more accurate about which people may need the more expensive drugs, and which patients may need the less expensive drugs? Are we getting better at monitoring so we can avoid high-cost events?”
Mr. Peterson is an employee of Janssen Pharmaceuticals. Dr. Husni reported serving as a consultant to AbbVie, Amgen, Bristol-Myers Squibb, UCB, Novartis, Lilly, and Pfizer.
* Update, 9/28/21: The headline and parts of this story were updated to better reflect the study on which it reports.
A version of this article first appeared on Medscape.com.
Annual health care costs for patients with psoriatic arthritis rose over recent 5-year periods across all categories of resource use to a significantly greater extent than among patients with psoriasis only or those without any psoriatic disease diagnoses, according to commercial insurance claims data.
Using an IBM MarketScan Commercial Database, researchers examined claims data for 208,434 patients with psoriasis, 47,274 with PsA, and 255,708 controls who had neither psoriasis nor PsA. Controls were matched for age and sex. Those with RA, ankylosing spondylitis, Crohn’s disease, or ulcerative colitis were excluded.
The investigators examined data for 2009-2020, following patients for 5 years within that period. They looked at hospitalizations, outpatient and pharmacy services, lab services, and office visits, Steven Peterson, director of market access for rheumatology at Janssen Pharmaceuticals, said in his presentation of the data at the Pan American League of Associations for Rheumatology 2021 annual meeting, held recently as a virtual event.
The research was also published online May 2, 2021, in Clinical Rheumatology.
Big differences between the groups were seen in the first year, when the average health care costs for the PsA group were $28,322, about half of which was outpatient drug costs. That compared with $12,039 for the psoriasis group and $6,672 for the control group.
The differences tended to widen over time. By the fifth year, average costs for the PsA group were $34,290, nearly 60% of which were drug costs. That compared with $12,877 for the psoriasis group and $8,569 for the control group. In each year examined, outpatient drug costs accounted for less than half of the expenses for the psoriasis group and about a quarter for the control group.
Researchers found that the PsA group needed 28.7 prescriptions per person per year, compared with 17.0 and 12.7 in the psoriasis and control groups, respectively, Mr. Peterson said. He also noted that patients with PsA and psoriasis tended to have higher rates of hypertension, depression, and anxiety.
“The cost and resource utilization disparity between these patient groups demonstrates the high remaining unmet medical need for patients with psoriasis and psoriatic arthritis,” Mr. Peterson said during the virtual proceedings.
Do findings reflect treatment advances?
Elaine Husni, MD, MPH, director of the Arthritis and Musculoskeletal Center at the Cleveland Clinic, where she studies health outcomes in PsA, said the findings are helpful in pointing to a trend across a large sample. But she added it’s important to remember that the increasing costs could reflect recent advances in PsA treatment, which include costly biologic drugs.
“There’s a ton more treatments for psoriasis and psoriatic arthritis than there were even just 5 years ago,” she said in an interview. She was not involved in the research.
Dr. Husni would like to see a more detailed look at the costs, from the categories of expenses to the patients who are incurring the highest costs.
“Is it just a couple of percent of really sick patients that are driving the psoriatic arthritis group?” she wondered.
She also pointed out that PsA is going to be more expensive by its very nature. PsA tends to develop 3-10 years after psoriasis, adding to the costs for someone who already has psoriasis and at a time when they are older and likely have higher health care costs because of comorbidities that develop with age.
Dr. Husni said she does think about treatment costs, in that a less expensive first-line drug might be more appropriate than going straight to a more expensive biologic, especially because they also tend to be safer. She said it’s not just a simple question of curbing costs.
“Is there a way that we can personalize medicine?” she asked. “Is there a way that we can be more accurate about which people may need the more expensive drugs, and which patients may need the less expensive drugs? Are we getting better at monitoring so we can avoid high-cost events?”
Mr. Peterson is an employee of Janssen Pharmaceuticals. Dr. Husni reported serving as a consultant to AbbVie, Amgen, Bristol-Myers Squibb, UCB, Novartis, Lilly, and Pfizer.
* Update, 9/28/21: The headline and parts of this story were updated to better reflect the study on which it reports.
A version of this article first appeared on Medscape.com.
Annual health care costs for patients with psoriatic arthritis rose over recent 5-year periods across all categories of resource use to a significantly greater extent than among patients with psoriasis only or those without any psoriatic disease diagnoses, according to commercial insurance claims data.
Using an IBM MarketScan Commercial Database, researchers examined claims data for 208,434 patients with psoriasis, 47,274 with PsA, and 255,708 controls who had neither psoriasis nor PsA. Controls were matched for age and sex. Those with RA, ankylosing spondylitis, Crohn’s disease, or ulcerative colitis were excluded.
The investigators examined data for 2009-2020, following patients for 5 years within that period. They looked at hospitalizations, outpatient and pharmacy services, lab services, and office visits, Steven Peterson, director of market access for rheumatology at Janssen Pharmaceuticals, said in his presentation of the data at the Pan American League of Associations for Rheumatology 2021 annual meeting, held recently as a virtual event.
The research was also published online May 2, 2021, in Clinical Rheumatology.
Big differences between the groups were seen in the first year, when the average health care costs for the PsA group were $28,322, about half of which was outpatient drug costs. That compared with $12,039 for the psoriasis group and $6,672 for the control group.
The differences tended to widen over time. By the fifth year, average costs for the PsA group were $34,290, nearly 60% of which were drug costs. That compared with $12,877 for the psoriasis group and $8,569 for the control group. In each year examined, outpatient drug costs accounted for less than half of the expenses for the psoriasis group and about a quarter for the control group.
Researchers found that the PsA group needed 28.7 prescriptions per person per year, compared with 17.0 and 12.7 in the psoriasis and control groups, respectively, Mr. Peterson said. He also noted that patients with PsA and psoriasis tended to have higher rates of hypertension, depression, and anxiety.
“The cost and resource utilization disparity between these patient groups demonstrates the high remaining unmet medical need for patients with psoriasis and psoriatic arthritis,” Mr. Peterson said during the virtual proceedings.
Do findings reflect treatment advances?
Elaine Husni, MD, MPH, director of the Arthritis and Musculoskeletal Center at the Cleveland Clinic, where she studies health outcomes in PsA, said the findings are helpful in pointing to a trend across a large sample. But she added it’s important to remember that the increasing costs could reflect recent advances in PsA treatment, which include costly biologic drugs.
“There’s a ton more treatments for psoriasis and psoriatic arthritis than there were even just 5 years ago,” she said in an interview. She was not involved in the research.
Dr. Husni would like to see a more detailed look at the costs, from the categories of expenses to the patients who are incurring the highest costs.
“Is it just a couple of percent of really sick patients that are driving the psoriatic arthritis group?” she wondered.
She also pointed out that PsA is going to be more expensive by its very nature. PsA tends to develop 3-10 years after psoriasis, adding to the costs for someone who already has psoriasis and at a time when they are older and likely have higher health care costs because of comorbidities that develop with age.
Dr. Husni said she does think about treatment costs, in that a less expensive first-line drug might be more appropriate than going straight to a more expensive biologic, especially because they also tend to be safer. She said it’s not just a simple question of curbing costs.
“Is there a way that we can personalize medicine?” she asked. “Is there a way that we can be more accurate about which people may need the more expensive drugs, and which patients may need the less expensive drugs? Are we getting better at monitoring so we can avoid high-cost events?”
Mr. Peterson is an employee of Janssen Pharmaceuticals. Dr. Husni reported serving as a consultant to AbbVie, Amgen, Bristol-Myers Squibb, UCB, Novartis, Lilly, and Pfizer.
* Update, 9/28/21: The headline and parts of this story were updated to better reflect the study on which it reports.
A version of this article first appeared on Medscape.com.
Review eyes nail unit toxicities secondary to targeted cancer therapy
while damage to other nail unit anatomic areas can be wide-ranging.
Those are key findings from an evidence-based literature review published on July 21, 2021, in the Journal of the American Academy of Dermatology, as a letter to the editor. “Dermatologic toxicities are often the earliest-presenting and highest-incidence adverse events due to targeted anticancer therapies and immunotherapies,” corresponding author Anisha B. Patel, MD, of the department of dermatology at the University of Texas MD Anderson Cancer Center, Houston, and colleagues wrote. “Nail unit toxicities due to immunotherapy are caused by nonspecific immune activation. Targeted therapies, particularly mitogen-activated protein kinase pathway inhibitors, lead to epidermal thinning of the nail folds and periungual tissue, increasing susceptibility to trauma and penetration by nail plate fragments. Although cutaneous toxicities have been well described, further characterization of nail unit toxicities is needed.”
The researchers searched the PubMed database using the terms nail, nail toxicity, nail dystrophy, paronychia, onycholysis, pyogenic granuloma, onychopathy, targeted therapy, and immunotherapy, and reviewed relevant articles for clinical presentation, diagnosis, incidence, outcomes, and references. They also proposed treatment algorithms for this patient population based on the existing literature and the authors’ collective clinical experience.
Dr. Patel and colleagues found that paronychia and periungual pyogenic granulomas were the most common nail unit toxicities caused by targeted therapy. “Damage to other nail unit anatomic areas includes drug induced or exacerbated lichen planus and psoriasis as well as pigmentary and neoplastic changes,” they wrote. “Onycholysis, onychoschizia, paronychia, psoriasis, lichen planus, and dermatomyositis have been reported with immune checkpoint inhibitors,” with the time of onset during the first week of treatment to several months after treatment has started.
According to National Cancer Institute criteria, nail adverse events associated with medical treatment include nail changes, discoloration, ridging, paronychia, and infection. The severity of nail loss, paronychia, and infection can be graded up to 3 (defined as “severe or medically significant but not life threatening”), while the remainder of nail toxicities may be categorized only as grade 1 (defined as “mild,” with “intervention not indicated”). “High-grade toxicities have been reported, especially with pan-fibroblast growth factor receptor inhibitors,” the authors wrote, referring to a previous study.
The review includes treatment algorithms for paronychia, periungual pyogenic granuloma, nail lichen planus, and psoriasis. “Long-acting and nonselective immunosuppressants are reserved for dose-limiting toxicities, given their unknown effects on already-immunosuppressed patients with cancer and on cancer therapy,” the authors wrote. “A discussion with the oncology department is essential before starting an immunomodulator or immunosuppressant.”
To manage onycholysis, Dr. Patel and colleagues recommended trimming the onycholytic nail plate to its attachment point. “Partial avulsion is used to treat a refractory abscess or painful hemorrhage,” they wrote. “A Pseudomonas superinfection is treated twice daily with a topical antibiotic solution. Brittle nail syndrome is managed with emollients or the application of polyureaurethane, a 16% nail solution, or a hydrosoluble nail lacquer,” they wrote, adding that biotin supplementation is not recommended.
Jonathan Leventhal, MD, who was asked to comment on the study, said that nail toxicity from targeted cancer therapy is one of the most common reasons for consultation in his role as director of the Yale University oncodermatology program at Smilow Cancer Hospital, New Haven, Conn. “When severe, these reactions frequently impact patients’ quality of life,” he said.
“This study is helpful for all dermatologists caring for cancer patients,” with strengths that include “succinctly summarizing the most prevalent conditions and providing a clear and practical algorithm for approaching these nail toxicities,” he said. In addition to targeted agents and immunotherapy, “we commonly see nail toxicities from cytotoxic chemotherapy, which was not reviewed in this paper. Multidisciplinary evaluation and dermatologic involvement is certainly beneficial to make accurate diagnoses and promptly manage these conditions, helping patients stay on their oncologic therapies.”
The researchers reported no financial disclosures. Dr. Leventhal disclosed that he is a member of the advisory board for Regeneron, Sanofi, Bristol-Myers Squibb, and La Roche–Posay. He has also received research funding from Azitra and OnQuality.
while damage to other nail unit anatomic areas can be wide-ranging.
Those are key findings from an evidence-based literature review published on July 21, 2021, in the Journal of the American Academy of Dermatology, as a letter to the editor. “Dermatologic toxicities are often the earliest-presenting and highest-incidence adverse events due to targeted anticancer therapies and immunotherapies,” corresponding author Anisha B. Patel, MD, of the department of dermatology at the University of Texas MD Anderson Cancer Center, Houston, and colleagues wrote. “Nail unit toxicities due to immunotherapy are caused by nonspecific immune activation. Targeted therapies, particularly mitogen-activated protein kinase pathway inhibitors, lead to epidermal thinning of the nail folds and periungual tissue, increasing susceptibility to trauma and penetration by nail plate fragments. Although cutaneous toxicities have been well described, further characterization of nail unit toxicities is needed.”
The researchers searched the PubMed database using the terms nail, nail toxicity, nail dystrophy, paronychia, onycholysis, pyogenic granuloma, onychopathy, targeted therapy, and immunotherapy, and reviewed relevant articles for clinical presentation, diagnosis, incidence, outcomes, and references. They also proposed treatment algorithms for this patient population based on the existing literature and the authors’ collective clinical experience.
Dr. Patel and colleagues found that paronychia and periungual pyogenic granulomas were the most common nail unit toxicities caused by targeted therapy. “Damage to other nail unit anatomic areas includes drug induced or exacerbated lichen planus and psoriasis as well as pigmentary and neoplastic changes,” they wrote. “Onycholysis, onychoschizia, paronychia, psoriasis, lichen planus, and dermatomyositis have been reported with immune checkpoint inhibitors,” with the time of onset during the first week of treatment to several months after treatment has started.
According to National Cancer Institute criteria, nail adverse events associated with medical treatment include nail changes, discoloration, ridging, paronychia, and infection. The severity of nail loss, paronychia, and infection can be graded up to 3 (defined as “severe or medically significant but not life threatening”), while the remainder of nail toxicities may be categorized only as grade 1 (defined as “mild,” with “intervention not indicated”). “High-grade toxicities have been reported, especially with pan-fibroblast growth factor receptor inhibitors,” the authors wrote, referring to a previous study.
The review includes treatment algorithms for paronychia, periungual pyogenic granuloma, nail lichen planus, and psoriasis. “Long-acting and nonselective immunosuppressants are reserved for dose-limiting toxicities, given their unknown effects on already-immunosuppressed patients with cancer and on cancer therapy,” the authors wrote. “A discussion with the oncology department is essential before starting an immunomodulator or immunosuppressant.”
To manage onycholysis, Dr. Patel and colleagues recommended trimming the onycholytic nail plate to its attachment point. “Partial avulsion is used to treat a refractory abscess or painful hemorrhage,” they wrote. “A Pseudomonas superinfection is treated twice daily with a topical antibiotic solution. Brittle nail syndrome is managed with emollients or the application of polyureaurethane, a 16% nail solution, or a hydrosoluble nail lacquer,” they wrote, adding that biotin supplementation is not recommended.
Jonathan Leventhal, MD, who was asked to comment on the study, said that nail toxicity from targeted cancer therapy is one of the most common reasons for consultation in his role as director of the Yale University oncodermatology program at Smilow Cancer Hospital, New Haven, Conn. “When severe, these reactions frequently impact patients’ quality of life,” he said.
“This study is helpful for all dermatologists caring for cancer patients,” with strengths that include “succinctly summarizing the most prevalent conditions and providing a clear and practical algorithm for approaching these nail toxicities,” he said. In addition to targeted agents and immunotherapy, “we commonly see nail toxicities from cytotoxic chemotherapy, which was not reviewed in this paper. Multidisciplinary evaluation and dermatologic involvement is certainly beneficial to make accurate diagnoses and promptly manage these conditions, helping patients stay on their oncologic therapies.”
The researchers reported no financial disclosures. Dr. Leventhal disclosed that he is a member of the advisory board for Regeneron, Sanofi, Bristol-Myers Squibb, and La Roche–Posay. He has also received research funding from Azitra and OnQuality.
while damage to other nail unit anatomic areas can be wide-ranging.
Those are key findings from an evidence-based literature review published on July 21, 2021, in the Journal of the American Academy of Dermatology, as a letter to the editor. “Dermatologic toxicities are often the earliest-presenting and highest-incidence adverse events due to targeted anticancer therapies and immunotherapies,” corresponding author Anisha B. Patel, MD, of the department of dermatology at the University of Texas MD Anderson Cancer Center, Houston, and colleagues wrote. “Nail unit toxicities due to immunotherapy are caused by nonspecific immune activation. Targeted therapies, particularly mitogen-activated protein kinase pathway inhibitors, lead to epidermal thinning of the nail folds and periungual tissue, increasing susceptibility to trauma and penetration by nail plate fragments. Although cutaneous toxicities have been well described, further characterization of nail unit toxicities is needed.”
The researchers searched the PubMed database using the terms nail, nail toxicity, nail dystrophy, paronychia, onycholysis, pyogenic granuloma, onychopathy, targeted therapy, and immunotherapy, and reviewed relevant articles for clinical presentation, diagnosis, incidence, outcomes, and references. They also proposed treatment algorithms for this patient population based on the existing literature and the authors’ collective clinical experience.
Dr. Patel and colleagues found that paronychia and periungual pyogenic granulomas were the most common nail unit toxicities caused by targeted therapy. “Damage to other nail unit anatomic areas includes drug induced or exacerbated lichen planus and psoriasis as well as pigmentary and neoplastic changes,” they wrote. “Onycholysis, onychoschizia, paronychia, psoriasis, lichen planus, and dermatomyositis have been reported with immune checkpoint inhibitors,” with the time of onset during the first week of treatment to several months after treatment has started.
According to National Cancer Institute criteria, nail adverse events associated with medical treatment include nail changes, discoloration, ridging, paronychia, and infection. The severity of nail loss, paronychia, and infection can be graded up to 3 (defined as “severe or medically significant but not life threatening”), while the remainder of nail toxicities may be categorized only as grade 1 (defined as “mild,” with “intervention not indicated”). “High-grade toxicities have been reported, especially with pan-fibroblast growth factor receptor inhibitors,” the authors wrote, referring to a previous study.
The review includes treatment algorithms for paronychia, periungual pyogenic granuloma, nail lichen planus, and psoriasis. “Long-acting and nonselective immunosuppressants are reserved for dose-limiting toxicities, given their unknown effects on already-immunosuppressed patients with cancer and on cancer therapy,” the authors wrote. “A discussion with the oncology department is essential before starting an immunomodulator or immunosuppressant.”
To manage onycholysis, Dr. Patel and colleagues recommended trimming the onycholytic nail plate to its attachment point. “Partial avulsion is used to treat a refractory abscess or painful hemorrhage,” they wrote. “A Pseudomonas superinfection is treated twice daily with a topical antibiotic solution. Brittle nail syndrome is managed with emollients or the application of polyureaurethane, a 16% nail solution, or a hydrosoluble nail lacquer,” they wrote, adding that biotin supplementation is not recommended.
Jonathan Leventhal, MD, who was asked to comment on the study, said that nail toxicity from targeted cancer therapy is one of the most common reasons for consultation in his role as director of the Yale University oncodermatology program at Smilow Cancer Hospital, New Haven, Conn. “When severe, these reactions frequently impact patients’ quality of life,” he said.
“This study is helpful for all dermatologists caring for cancer patients,” with strengths that include “succinctly summarizing the most prevalent conditions and providing a clear and practical algorithm for approaching these nail toxicities,” he said. In addition to targeted agents and immunotherapy, “we commonly see nail toxicities from cytotoxic chemotherapy, which was not reviewed in this paper. Multidisciplinary evaluation and dermatologic involvement is certainly beneficial to make accurate diagnoses and promptly manage these conditions, helping patients stay on their oncologic therapies.”
The researchers reported no financial disclosures. Dr. Leventhal disclosed that he is a member of the advisory board for Regeneron, Sanofi, Bristol-Myers Squibb, and La Roche–Posay. He has also received research funding from Azitra and OnQuality.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Sudden-Onset Blistering Rash
The Diagnosis: Generalized Bullous Fixed Drug Eruption
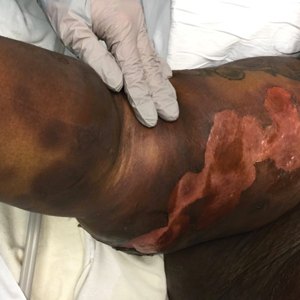
A punch biopsy from the left thigh revealed a vacuolar interface dermatitis with full-thickness necrosis of the epidermis and a patchy lichenoid inflammatory cell infiltrate in the superficial dermis consistent with a generalized bullous fixed drug eruption (GBFDE). The patient received supportive care and methylprednisolone with improvement of symptoms.
Generalized bullous fixed drug eruption is a rare, potentially life-threatening form of a fixed drug eruption (FDE), a cutaneous drug reaction that occurs in response to a causative medication. It typically presents with welldemarcated, dusky, erythematous patches or plaques that recur in the same sites with repeat exposure.1 The pathogenesis of FDE has been hypothesized to involve epidermal CD8+ T cells, which are activated by drug exposure and release cytotoxic molecules including Fas, Fas ligand, perforin, and granzyme B, resulting in lysis of the surrounding keratinocytes.1-3 Common eliciting drugs include nonsteroidal anti-inflammatory drugs, antibacterial agents (particularly trimethoprim-sulfamethoxazole), barbiturates, acetaminophen, and antimalarials.1 In addition to the findings seen in FDE, GBFDE is characterized by widespread bullous skin lesions.1-4 Typical histologic patterns seen in GBFDE are dispersed epidermal apoptotic keratinocytes, prominent dermal eosinophilic and lymphocytic infiltrates, and dermal melanophages.3 Discontinuing the causative agent and diligent prevention of re-exposure are the most important steps in management, as additional exposures can increase the number of lesions and overall severity. Symptoms typically resolve 7 to 14 days after drug discontinuation, often with postinflammatory hyperpigmentation.3
Generalized bullous fixed drug eruption presents a diagnostic challenge, as it sometimes involves the oral mucosa and can exhibit the Nikolsky sign. Thus, it often is confused with Stevens-Johnson syndrome (SJS) or toxic epidermal necrolysis (TEN).1,4 Stevens-Johnson syndrome and TEN are severe cutaneous drug eruptions that also can present with diffuse bullous skin lesions. Stevens-Johnson syndrome and TEN are thought to be a spectrum of the same disease that initially presents with dusky red macules that can coalesce, develop central blistering, and lead to skin detachment.5 Stevens-Johnson syndrome is defined as skin detachment of less than 10% body surface area (BSA); TEN is defined as skin detachment of more than 30% BSA. Stevens-Johnson syndrome/TEN overlap syndrome includes skin detachment of 10% to 30% BSA.5
Causative medications overlap substantially with GBFDE and include anticonvulsants, sulfa-containing drugs, antibiotics, nonsteroidal anti-inflammatory drugs, and uric acid–lowering agents. The histology of SJS/TEN also is quite similar to GBFDE, and these entities may be indistinguishable without clinical information.5 Lee et al1 found that absence of grouped necrotic keratinocytes (fire flag sign), deep inflammatory infiltrates, notable pigment incontinence, and higher eosinophil counts appear to be more common in GBFDE than SJS/TEN. Constitutional symptoms and mucosal involvement also were more frequent in SJS/TEN.
The timing of clinical presentation and medical history can be useful in differentiating between SJS/TEN and GBFDE. In SJS/TEN, drug exposure typically occurs 1 to 3 weeks before onset of symptoms vs 30 minutes to 24 hours in GBFDE.3 Additionally, a history of similar eruption in the same location is pathognomonic for GBFDE. Although GBFDE has been thought to have a better prognosis than SJS/TEN, more recent data suggest mortality rates may be similar.3 A case-control study found a mortality rate of 22% (13/58) in patients with GBFDE compared to 28% (n=170) in SJS/TEN patients.4
Erythema multiforme (EM) is an uncommon immunemediated disorder that typically presents as targetoid lesions with central epidermal necrosis in an acral distribution. Erythema multiforme can arise from a variety of factors, but up to 90% of cases are due to infection, most commonly herpes simplex virus; medications account for less than 10% of cases.6 Previously, EM has been thought to be on the same disease spectrum as SJS and TEN. It is now clear that EM is a separate entity with similar mucosal erosions but different cutaneous findings,6 mainly typical target lesions that differ from the atypical targets seen in SJS.
Staphylococcal scalded skin syndrome is a blistering skin disorder associated with local Staphylococcus aureus infection. It most commonly is seen in children and rarely occurs in adults who are not on dialysis. Some Staphylococcus strains produce exfoliative toxins A and B, which are serine proteases that target and cleave desmoglein 1, a mediator of keratinocyte adhesion. Staphylococcal scalded skin syndrome initially presents with erythema accentuated in the skin folds that becomes generalized. The disruption of keratinocyte adhesion leads to bullae formation in areas of erythema and diffuse sheetlike desquamation. Pathology reveals subcorneal rather than subepidermal blistering, which is seen in GBFDE and SJS/TEN. Treatment involves antistaphylococcal antibiotics and supportive care. With proper treatment, most cases resolve within 2 to 3 weeks.7
Mycoplasma pneumoniae–induced rash and mucositis presents with prominent mucositis and can have cutaneous findings of sparse vesiculobullous or targetoid eruption.8 Mycoplasma pneumoniae typically infects the lungs and is a leading cause of community-acquired pneumonia. However, a subset of patients can have extrapulmonary disease presenting as mucocutaneous eruptions, which is preceded by an approximately weeklong prodrome of fever, cough, and malaise.7 Mycoplasma pneumoniae–induced rash and mucositis also affect children and young patients and is more common in males.8
- Lee CH, Chen YC, Cho YT, et al. Fixed-drug eruption: a retrospective study in a single referral center in northern Taiwan. Dermatologica Sinica. 2012;30:11-15. doi:10.1016/j.dsi.2012.02.002
- Cho Y-T, Lin J-W, Chen Y-C, et al. Generalized bullous fixed drug eruption is distinct from Stevens-Johnson syndrome/toxic epidermal necrolysis by immunohistopathological features. J Am Acad Dermatol. 2014;70:539-548. doi:10.1016/j.jaad.2013.11.015
- Mitre V, Applebaum DS, Albahrani Y, et al. Generalized bullous fixed drug eruption imitating toxic epidermal necrolysis: a case report and literature review. Dermatol Online J. 2017;23: 13030/qt25v009gs.
- Lipowicz S, Sekula P, Ingen-Housz-Oro S, et al. Prognosis of generalized bullous fixed drug eruption: comparison with StevensJohnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 2013;168:726-732. doi:10.1111/bjd.12133
- Cho Y-T, Chu C-Y. Treatments for severe cutaneous adverse reactions [published online December 27, 2017]. J Immunol Res. doi:10.1155/2017/1503709
- Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902. doi:10.1111/j.1365-4632.2011.05348.x
- Leung AKC, Barankin B, Leong KF. Staphylococcal-scalded skin syndrome: evaluation, diagnosis, and management. World J Pediatr. 2018;14:116-120.
- Canavan TN, Mathes EF, Frieden I, et al. Mycoplasma pneumoniae–induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol. 2015;72:239-245. doi:10.1016/j .jaad.2014.06.026
The Diagnosis: Generalized Bullous Fixed Drug Eruption
A punch biopsy from the left thigh revealed a vacuolar interface dermatitis with full-thickness necrosis of the epidermis and a patchy lichenoid inflammatory cell infiltrate in the superficial dermis consistent with a generalized bullous fixed drug eruption (GBFDE). The patient received supportive care and methylprednisolone with improvement of symptoms.
Generalized bullous fixed drug eruption is a rare, potentially life-threatening form of a fixed drug eruption (FDE), a cutaneous drug reaction that occurs in response to a causative medication. It typically presents with welldemarcated, dusky, erythematous patches or plaques that recur in the same sites with repeat exposure.1 The pathogenesis of FDE has been hypothesized to involve epidermal CD8+ T cells, which are activated by drug exposure and release cytotoxic molecules including Fas, Fas ligand, perforin, and granzyme B, resulting in lysis of the surrounding keratinocytes.1-3 Common eliciting drugs include nonsteroidal anti-inflammatory drugs, antibacterial agents (particularly trimethoprim-sulfamethoxazole), barbiturates, acetaminophen, and antimalarials.1 In addition to the findings seen in FDE, GBFDE is characterized by widespread bullous skin lesions.1-4 Typical histologic patterns seen in GBFDE are dispersed epidermal apoptotic keratinocytes, prominent dermal eosinophilic and lymphocytic infiltrates, and dermal melanophages.3 Discontinuing the causative agent and diligent prevention of re-exposure are the most important steps in management, as additional exposures can increase the number of lesions and overall severity. Symptoms typically resolve 7 to 14 days after drug discontinuation, often with postinflammatory hyperpigmentation.3
Generalized bullous fixed drug eruption presents a diagnostic challenge, as it sometimes involves the oral mucosa and can exhibit the Nikolsky sign. Thus, it often is confused with Stevens-Johnson syndrome (SJS) or toxic epidermal necrolysis (TEN).1,4 Stevens-Johnson syndrome and TEN are severe cutaneous drug eruptions that also can present with diffuse bullous skin lesions. Stevens-Johnson syndrome and TEN are thought to be a spectrum of the same disease that initially presents with dusky red macules that can coalesce, develop central blistering, and lead to skin detachment.5 Stevens-Johnson syndrome is defined as skin detachment of less than 10% body surface area (BSA); TEN is defined as skin detachment of more than 30% BSA. Stevens-Johnson syndrome/TEN overlap syndrome includes skin detachment of 10% to 30% BSA.5
Causative medications overlap substantially with GBFDE and include anticonvulsants, sulfa-containing drugs, antibiotics, nonsteroidal anti-inflammatory drugs, and uric acid–lowering agents. The histology of SJS/TEN also is quite similar to GBFDE, and these entities may be indistinguishable without clinical information.5 Lee et al1 found that absence of grouped necrotic keratinocytes (fire flag sign), deep inflammatory infiltrates, notable pigment incontinence, and higher eosinophil counts appear to be more common in GBFDE than SJS/TEN. Constitutional symptoms and mucosal involvement also were more frequent in SJS/TEN.
The timing of clinical presentation and medical history can be useful in differentiating between SJS/TEN and GBFDE. In SJS/TEN, drug exposure typically occurs 1 to 3 weeks before onset of symptoms vs 30 minutes to 24 hours in GBFDE.3 Additionally, a history of similar eruption in the same location is pathognomonic for GBFDE. Although GBFDE has been thought to have a better prognosis than SJS/TEN, more recent data suggest mortality rates may be similar.3 A case-control study found a mortality rate of 22% (13/58) in patients with GBFDE compared to 28% (n=170) in SJS/TEN patients.4
Erythema multiforme (EM) is an uncommon immunemediated disorder that typically presents as targetoid lesions with central epidermal necrosis in an acral distribution. Erythema multiforme can arise from a variety of factors, but up to 90% of cases are due to infection, most commonly herpes simplex virus; medications account for less than 10% of cases.6 Previously, EM has been thought to be on the same disease spectrum as SJS and TEN. It is now clear that EM is a separate entity with similar mucosal erosions but different cutaneous findings,6 mainly typical target lesions that differ from the atypical targets seen in SJS.
Staphylococcal scalded skin syndrome is a blistering skin disorder associated with local Staphylococcus aureus infection. It most commonly is seen in children and rarely occurs in adults who are not on dialysis. Some Staphylococcus strains produce exfoliative toxins A and B, which are serine proteases that target and cleave desmoglein 1, a mediator of keratinocyte adhesion. Staphylococcal scalded skin syndrome initially presents with erythema accentuated in the skin folds that becomes generalized. The disruption of keratinocyte adhesion leads to bullae formation in areas of erythema and diffuse sheetlike desquamation. Pathology reveals subcorneal rather than subepidermal blistering, which is seen in GBFDE and SJS/TEN. Treatment involves antistaphylococcal antibiotics and supportive care. With proper treatment, most cases resolve within 2 to 3 weeks.7
Mycoplasma pneumoniae–induced rash and mucositis presents with prominent mucositis and can have cutaneous findings of sparse vesiculobullous or targetoid eruption.8 Mycoplasma pneumoniae typically infects the lungs and is a leading cause of community-acquired pneumonia. However, a subset of patients can have extrapulmonary disease presenting as mucocutaneous eruptions, which is preceded by an approximately weeklong prodrome of fever, cough, and malaise.7 Mycoplasma pneumoniae–induced rash and mucositis also affect children and young patients and is more common in males.8
The Diagnosis: Generalized Bullous Fixed Drug Eruption
A punch biopsy from the left thigh revealed a vacuolar interface dermatitis with full-thickness necrosis of the epidermis and a patchy lichenoid inflammatory cell infiltrate in the superficial dermis consistent with a generalized bullous fixed drug eruption (GBFDE). The patient received supportive care and methylprednisolone with improvement of symptoms.
Generalized bullous fixed drug eruption is a rare, potentially life-threatening form of a fixed drug eruption (FDE), a cutaneous drug reaction that occurs in response to a causative medication. It typically presents with welldemarcated, dusky, erythematous patches or plaques that recur in the same sites with repeat exposure.1 The pathogenesis of FDE has been hypothesized to involve epidermal CD8+ T cells, which are activated by drug exposure and release cytotoxic molecules including Fas, Fas ligand, perforin, and granzyme B, resulting in lysis of the surrounding keratinocytes.1-3 Common eliciting drugs include nonsteroidal anti-inflammatory drugs, antibacterial agents (particularly trimethoprim-sulfamethoxazole), barbiturates, acetaminophen, and antimalarials.1 In addition to the findings seen in FDE, GBFDE is characterized by widespread bullous skin lesions.1-4 Typical histologic patterns seen in GBFDE are dispersed epidermal apoptotic keratinocytes, prominent dermal eosinophilic and lymphocytic infiltrates, and dermal melanophages.3 Discontinuing the causative agent and diligent prevention of re-exposure are the most important steps in management, as additional exposures can increase the number of lesions and overall severity. Symptoms typically resolve 7 to 14 days after drug discontinuation, often with postinflammatory hyperpigmentation.3
Generalized bullous fixed drug eruption presents a diagnostic challenge, as it sometimes involves the oral mucosa and can exhibit the Nikolsky sign. Thus, it often is confused with Stevens-Johnson syndrome (SJS) or toxic epidermal necrolysis (TEN).1,4 Stevens-Johnson syndrome and TEN are severe cutaneous drug eruptions that also can present with diffuse bullous skin lesions. Stevens-Johnson syndrome and TEN are thought to be a spectrum of the same disease that initially presents with dusky red macules that can coalesce, develop central blistering, and lead to skin detachment.5 Stevens-Johnson syndrome is defined as skin detachment of less than 10% body surface area (BSA); TEN is defined as skin detachment of more than 30% BSA. Stevens-Johnson syndrome/TEN overlap syndrome includes skin detachment of 10% to 30% BSA.5
Causative medications overlap substantially with GBFDE and include anticonvulsants, sulfa-containing drugs, antibiotics, nonsteroidal anti-inflammatory drugs, and uric acid–lowering agents. The histology of SJS/TEN also is quite similar to GBFDE, and these entities may be indistinguishable without clinical information.5 Lee et al1 found that absence of grouped necrotic keratinocytes (fire flag sign), deep inflammatory infiltrates, notable pigment incontinence, and higher eosinophil counts appear to be more common in GBFDE than SJS/TEN. Constitutional symptoms and mucosal involvement also were more frequent in SJS/TEN.
The timing of clinical presentation and medical history can be useful in differentiating between SJS/TEN and GBFDE. In SJS/TEN, drug exposure typically occurs 1 to 3 weeks before onset of symptoms vs 30 minutes to 24 hours in GBFDE.3 Additionally, a history of similar eruption in the same location is pathognomonic for GBFDE. Although GBFDE has been thought to have a better prognosis than SJS/TEN, more recent data suggest mortality rates may be similar.3 A case-control study found a mortality rate of 22% (13/58) in patients with GBFDE compared to 28% (n=170) in SJS/TEN patients.4
Erythema multiforme (EM) is an uncommon immunemediated disorder that typically presents as targetoid lesions with central epidermal necrosis in an acral distribution. Erythema multiforme can arise from a variety of factors, but up to 90% of cases are due to infection, most commonly herpes simplex virus; medications account for less than 10% of cases.6 Previously, EM has been thought to be on the same disease spectrum as SJS and TEN. It is now clear that EM is a separate entity with similar mucosal erosions but different cutaneous findings,6 mainly typical target lesions that differ from the atypical targets seen in SJS.
Staphylococcal scalded skin syndrome is a blistering skin disorder associated with local Staphylococcus aureus infection. It most commonly is seen in children and rarely occurs in adults who are not on dialysis. Some Staphylococcus strains produce exfoliative toxins A and B, which are serine proteases that target and cleave desmoglein 1, a mediator of keratinocyte adhesion. Staphylococcal scalded skin syndrome initially presents with erythema accentuated in the skin folds that becomes generalized. The disruption of keratinocyte adhesion leads to bullae formation in areas of erythema and diffuse sheetlike desquamation. Pathology reveals subcorneal rather than subepidermal blistering, which is seen in GBFDE and SJS/TEN. Treatment involves antistaphylococcal antibiotics and supportive care. With proper treatment, most cases resolve within 2 to 3 weeks.7
Mycoplasma pneumoniae–induced rash and mucositis presents with prominent mucositis and can have cutaneous findings of sparse vesiculobullous or targetoid eruption.8 Mycoplasma pneumoniae typically infects the lungs and is a leading cause of community-acquired pneumonia. However, a subset of patients can have extrapulmonary disease presenting as mucocutaneous eruptions, which is preceded by an approximately weeklong prodrome of fever, cough, and malaise.7 Mycoplasma pneumoniae–induced rash and mucositis also affect children and young patients and is more common in males.8
- Lee CH, Chen YC, Cho YT, et al. Fixed-drug eruption: a retrospective study in a single referral center in northern Taiwan. Dermatologica Sinica. 2012;30:11-15. doi:10.1016/j.dsi.2012.02.002
- Cho Y-T, Lin J-W, Chen Y-C, et al. Generalized bullous fixed drug eruption is distinct from Stevens-Johnson syndrome/toxic epidermal necrolysis by immunohistopathological features. J Am Acad Dermatol. 2014;70:539-548. doi:10.1016/j.jaad.2013.11.015
- Mitre V, Applebaum DS, Albahrani Y, et al. Generalized bullous fixed drug eruption imitating toxic epidermal necrolysis: a case report and literature review. Dermatol Online J. 2017;23: 13030/qt25v009gs.
- Lipowicz S, Sekula P, Ingen-Housz-Oro S, et al. Prognosis of generalized bullous fixed drug eruption: comparison with StevensJohnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 2013;168:726-732. doi:10.1111/bjd.12133
- Cho Y-T, Chu C-Y. Treatments for severe cutaneous adverse reactions [published online December 27, 2017]. J Immunol Res. doi:10.1155/2017/1503709
- Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902. doi:10.1111/j.1365-4632.2011.05348.x
- Leung AKC, Barankin B, Leong KF. Staphylococcal-scalded skin syndrome: evaluation, diagnosis, and management. World J Pediatr. 2018;14:116-120.
- Canavan TN, Mathes EF, Frieden I, et al. Mycoplasma pneumoniae–induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol. 2015;72:239-245. doi:10.1016/j .jaad.2014.06.026
- Lee CH, Chen YC, Cho YT, et al. Fixed-drug eruption: a retrospective study in a single referral center in northern Taiwan. Dermatologica Sinica. 2012;30:11-15. doi:10.1016/j.dsi.2012.02.002
- Cho Y-T, Lin J-W, Chen Y-C, et al. Generalized bullous fixed drug eruption is distinct from Stevens-Johnson syndrome/toxic epidermal necrolysis by immunohistopathological features. J Am Acad Dermatol. 2014;70:539-548. doi:10.1016/j.jaad.2013.11.015
- Mitre V, Applebaum DS, Albahrani Y, et al. Generalized bullous fixed drug eruption imitating toxic epidermal necrolysis: a case report and literature review. Dermatol Online J. 2017;23: 13030/qt25v009gs.
- Lipowicz S, Sekula P, Ingen-Housz-Oro S, et al. Prognosis of generalized bullous fixed drug eruption: comparison with StevensJohnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 2013;168:726-732. doi:10.1111/bjd.12133
- Cho Y-T, Chu C-Y. Treatments for severe cutaneous adverse reactions [published online December 27, 2017]. J Immunol Res. doi:10.1155/2017/1503709
- Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902. doi:10.1111/j.1365-4632.2011.05348.x
- Leung AKC, Barankin B, Leong KF. Staphylococcal-scalded skin syndrome: evaluation, diagnosis, and management. World J Pediatr. 2018;14:116-120.
- Canavan TN, Mathes EF, Frieden I, et al. Mycoplasma pneumoniae–induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol. 2015;72:239-245. doi:10.1016/j .jaad.2014.06.026
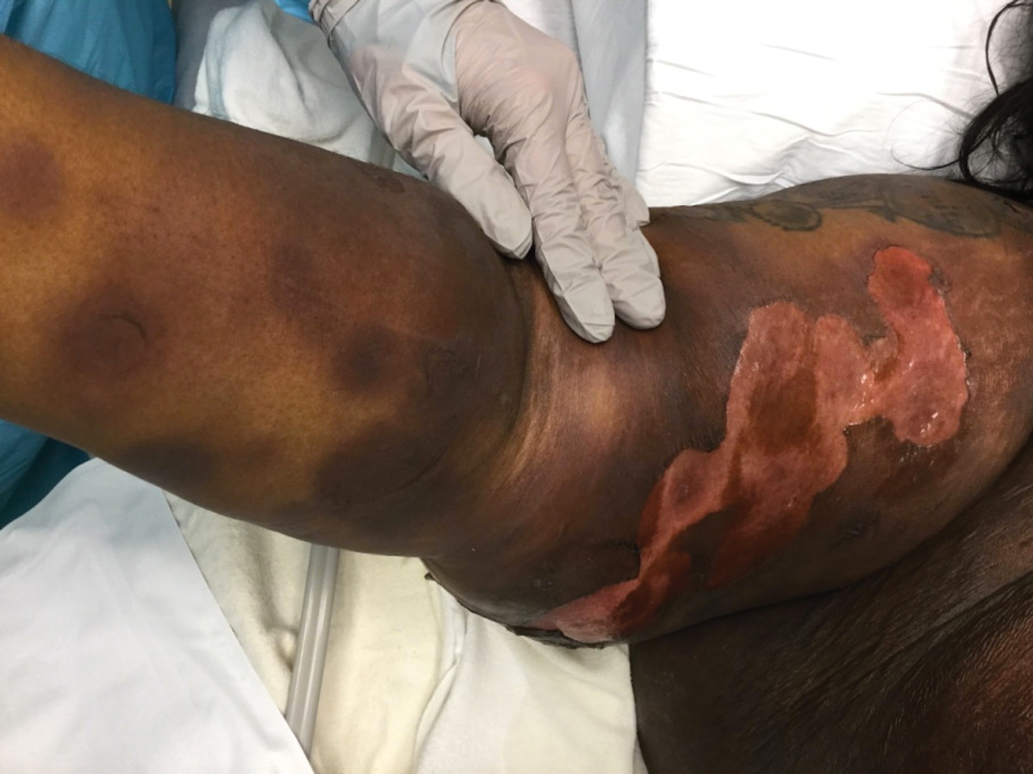
A 45-year-old woman presented with a diffuse rash 2 days after receiving ondansetron. She developed blisters on the arms, legs, trunk, and face 2 hours after exposure. There was no oral or vaginal involvement. She reported a history of leg blisters after prior exposure to ondansetron that were not as severe or numerous as the current episode. Physical examination revealed innumerable coalescing, ovoid and circular, dusky patches, some with central flaccid bullae, along with large areas of denuded skin on the trunk, arms, legs, and face. There were erosions on the lower eyelids without conjunctival or other mucosal involvement.
Health care workers eager for COVID booster shots
As COVID vaccine boosters move closer to reality, most physicians and nurses are ready and willing to get another shot in the arm, according to a new Medscape survey.
Altogether, 93% of physicians and 87% of nurses/advanced practice nurses (APNs) said they wanted to get a booster, although the timing of when they wanted the shots differed somewhat between the two groups surveyed Aug. 4-15.
Among the 732 physicians polled, 50% wanted to get their shot immediately, compared with 38% of the 1,193 nurses/APNs who responded, while 44% of physicians and 50% of nurses/APNs said that they would wait until the vaccine booster was authorized and recommended.
At this point in time, almost all of the health care workers surveyed – 98% of physicians and 94% of nurses/APNs – have been fully vaccinated against COVID-19. A small proportion of each group, however, received the Johnson & Johnson vaccine (1% of physicians and 3% of nurses) and are not included in the current plan for booster shots.
The Medscape survey sample did include one group that is already eligible for a third dose: About 20% of physicians and 26% of nurses/ANPs said they have a condition or take a medication that compromises their immune system.
Respondents’ experiences with patient requests for boosters suggest a somewhat lower level of interest. About two-thirds of the health care workers (69% of physicians and 63% of nurses) said that patients frequently or sometimes asked about COVID boosters, compared with 13% (physicians) and 19% (nurses) who said their patients had never asked.
Interest lower among general population
In a separate survey conducted by WebMD, 82% of those who have been at least partially vaccinated said they want to get a COVID vaccine booster (14% immediately and 68% after authorization and recommendation). Of the remaining vaccinees, 7% said they do not want to get a booster and 11% were unsure.
The full sample of 592 respondents surveyed Aug. 5-10, however, included 19% who do not plan to get vaccinated and 6% who are planning to be vaccinated but have not yet done so.
The proportion of immunocompromised individuals in the two survey groups was similar, with about 25% of those in the WebMD survey reporting they have a condition or take a medication that compromises their immune system. Those respondents were more than twice as likely to want to get a booster immediately, compared to those with an uncompromised immune system (24% vs. 11%).
The distribution of vaccines received by brand was also comparable between the two groups surveyed. Of health care workers and readers, over half of each group received the Pfizer/BioNTech vaccine (59% vs. 54%), followed by Moderna (38% vs. 40%) and Johnson & Johnson (3% vs. 5%).
A version of this article first appeared on Medscape.com.
As COVID vaccine boosters move closer to reality, most physicians and nurses are ready and willing to get another shot in the arm, according to a new Medscape survey.
Altogether, 93% of physicians and 87% of nurses/advanced practice nurses (APNs) said they wanted to get a booster, although the timing of when they wanted the shots differed somewhat between the two groups surveyed Aug. 4-15.
Among the 732 physicians polled, 50% wanted to get their shot immediately, compared with 38% of the 1,193 nurses/APNs who responded, while 44% of physicians and 50% of nurses/APNs said that they would wait until the vaccine booster was authorized and recommended.
At this point in time, almost all of the health care workers surveyed – 98% of physicians and 94% of nurses/APNs – have been fully vaccinated against COVID-19. A small proportion of each group, however, received the Johnson & Johnson vaccine (1% of physicians and 3% of nurses) and are not included in the current plan for booster shots.
The Medscape survey sample did include one group that is already eligible for a third dose: About 20% of physicians and 26% of nurses/ANPs said they have a condition or take a medication that compromises their immune system.
Respondents’ experiences with patient requests for boosters suggest a somewhat lower level of interest. About two-thirds of the health care workers (69% of physicians and 63% of nurses) said that patients frequently or sometimes asked about COVID boosters, compared with 13% (physicians) and 19% (nurses) who said their patients had never asked.
Interest lower among general population
In a separate survey conducted by WebMD, 82% of those who have been at least partially vaccinated said they want to get a COVID vaccine booster (14% immediately and 68% after authorization and recommendation). Of the remaining vaccinees, 7% said they do not want to get a booster and 11% were unsure.
The full sample of 592 respondents surveyed Aug. 5-10, however, included 19% who do not plan to get vaccinated and 6% who are planning to be vaccinated but have not yet done so.
The proportion of immunocompromised individuals in the two survey groups was similar, with about 25% of those in the WebMD survey reporting they have a condition or take a medication that compromises their immune system. Those respondents were more than twice as likely to want to get a booster immediately, compared to those with an uncompromised immune system (24% vs. 11%).
The distribution of vaccines received by brand was also comparable between the two groups surveyed. Of health care workers and readers, over half of each group received the Pfizer/BioNTech vaccine (59% vs. 54%), followed by Moderna (38% vs. 40%) and Johnson & Johnson (3% vs. 5%).
A version of this article first appeared on Medscape.com.
As COVID vaccine boosters move closer to reality, most physicians and nurses are ready and willing to get another shot in the arm, according to a new Medscape survey.
Altogether, 93% of physicians and 87% of nurses/advanced practice nurses (APNs) said they wanted to get a booster, although the timing of when they wanted the shots differed somewhat between the two groups surveyed Aug. 4-15.
Among the 732 physicians polled, 50% wanted to get their shot immediately, compared with 38% of the 1,193 nurses/APNs who responded, while 44% of physicians and 50% of nurses/APNs said that they would wait until the vaccine booster was authorized and recommended.
At this point in time, almost all of the health care workers surveyed – 98% of physicians and 94% of nurses/APNs – have been fully vaccinated against COVID-19. A small proportion of each group, however, received the Johnson & Johnson vaccine (1% of physicians and 3% of nurses) and are not included in the current plan for booster shots.
The Medscape survey sample did include one group that is already eligible for a third dose: About 20% of physicians and 26% of nurses/ANPs said they have a condition or take a medication that compromises their immune system.
Respondents’ experiences with patient requests for boosters suggest a somewhat lower level of interest. About two-thirds of the health care workers (69% of physicians and 63% of nurses) said that patients frequently or sometimes asked about COVID boosters, compared with 13% (physicians) and 19% (nurses) who said their patients had never asked.
Interest lower among general population
In a separate survey conducted by WebMD, 82% of those who have been at least partially vaccinated said they want to get a COVID vaccine booster (14% immediately and 68% after authorization and recommendation). Of the remaining vaccinees, 7% said they do not want to get a booster and 11% were unsure.
The full sample of 592 respondents surveyed Aug. 5-10, however, included 19% who do not plan to get vaccinated and 6% who are planning to be vaccinated but have not yet done so.
The proportion of immunocompromised individuals in the two survey groups was similar, with about 25% of those in the WebMD survey reporting they have a condition or take a medication that compromises their immune system. Those respondents were more than twice as likely to want to get a booster immediately, compared to those with an uncompromised immune system (24% vs. 11%).
The distribution of vaccines received by brand was also comparable between the two groups surveyed. Of health care workers and readers, over half of each group received the Pfizer/BioNTech vaccine (59% vs. 54%), followed by Moderna (38% vs. 40%) and Johnson & Johnson (3% vs. 5%).
A version of this article first appeared on Medscape.com.
Plastic barriers may not stop COVID-19 spread, experts say
Plastic barriers that separate people in stores, restaurants, and classrooms may not be as effective at stopping the spread of COVID-19 as originally thought, according to The New York Times.
Scientists who study air flow, ventilation, and aerosol droplets say the barriers may not help, and in fact, could make the situation worse by blocking normal air flow, the newspaper reported.
Typically, as people interact and breathe in a room, currents and ventilation systems recirculate the air and disperse the exhaled particles. With plastic barriers, however, particles could get trapped in “dead zones” and build up.
“If you have a forest of barriers in a classroom, it’s going to interfere with proper ventilation of that room,” Linsey Marr, professor of civil and environmental engineering at Virginia Tech, told the newspaper.
“Everybody’s aerosols are going to be trapped and stuck there and building up, and they will end up spreading beyond your own desk,” she said.
Several variables factor into the efficacy of plastic barriers, The New York Times reported. Shields may stop big respiratory droplets from coughs and sneezes, for instance, but they may not do much to prevent small aerosol particles from viruses such as COVID-19 from spreading.
“We have shown this effect of blocking larger particles, but also that the smaller aerosols travel over the screen and become mixed in the room air within about 5 minutes,” Catherine Noakes, professor of environment engineering at the University of Leeds, told the newspaper.
“This means if people are interacting for more than a few minutes, they would likely be exposed to the virus regardless of the screen,” she said.
The effectiveness of plastic barriers likely also depends on the location and setup, the newspaper reported. A bus driver with a large barrier, for instance, may be able to avoid inhaling the particles that passengers are exhaling. A bank cashier or store clerk behind a large barrier may also be partly protected.
Even still, scientists say more research is needed. For instance, taller barriers are more likely to be effective. However, a large number of barriers in one room could likely block air flow.
Researchers have recommended that schools and offices focus on ventilation, masks, and vaccines to slow the spread of the coronavirus.
“Air flow in rooms is pretty complicated,” Richard Corsi, dean of engineering at the University of California at Davis, told the newspaper.
“Every room is different in terms of the arrangement of furniture, the height of the walls and ceilings, the vents, where the bookshelves are,” he said. “All of these things have a huge impact on the actual flow and air distribution in a room.”
A version of this article first appeared on WebMD.com.
Plastic barriers that separate people in stores, restaurants, and classrooms may not be as effective at stopping the spread of COVID-19 as originally thought, according to The New York Times.
Scientists who study air flow, ventilation, and aerosol droplets say the barriers may not help, and in fact, could make the situation worse by blocking normal air flow, the newspaper reported.
Typically, as people interact and breathe in a room, currents and ventilation systems recirculate the air and disperse the exhaled particles. With plastic barriers, however, particles could get trapped in “dead zones” and build up.
“If you have a forest of barriers in a classroom, it’s going to interfere with proper ventilation of that room,” Linsey Marr, professor of civil and environmental engineering at Virginia Tech, told the newspaper.
“Everybody’s aerosols are going to be trapped and stuck there and building up, and they will end up spreading beyond your own desk,” she said.
Several variables factor into the efficacy of plastic barriers, The New York Times reported. Shields may stop big respiratory droplets from coughs and sneezes, for instance, but they may not do much to prevent small aerosol particles from viruses such as COVID-19 from spreading.
“We have shown this effect of blocking larger particles, but also that the smaller aerosols travel over the screen and become mixed in the room air within about 5 minutes,” Catherine Noakes, professor of environment engineering at the University of Leeds, told the newspaper.
“This means if people are interacting for more than a few minutes, they would likely be exposed to the virus regardless of the screen,” she said.
The effectiveness of plastic barriers likely also depends on the location and setup, the newspaper reported. A bus driver with a large barrier, for instance, may be able to avoid inhaling the particles that passengers are exhaling. A bank cashier or store clerk behind a large barrier may also be partly protected.
Even still, scientists say more research is needed. For instance, taller barriers are more likely to be effective. However, a large number of barriers in one room could likely block air flow.
Researchers have recommended that schools and offices focus on ventilation, masks, and vaccines to slow the spread of the coronavirus.
“Air flow in rooms is pretty complicated,” Richard Corsi, dean of engineering at the University of California at Davis, told the newspaper.
“Every room is different in terms of the arrangement of furniture, the height of the walls and ceilings, the vents, where the bookshelves are,” he said. “All of these things have a huge impact on the actual flow and air distribution in a room.”
A version of this article first appeared on WebMD.com.
Plastic barriers that separate people in stores, restaurants, and classrooms may not be as effective at stopping the spread of COVID-19 as originally thought, according to The New York Times.
Scientists who study air flow, ventilation, and aerosol droplets say the barriers may not help, and in fact, could make the situation worse by blocking normal air flow, the newspaper reported.
Typically, as people interact and breathe in a room, currents and ventilation systems recirculate the air and disperse the exhaled particles. With plastic barriers, however, particles could get trapped in “dead zones” and build up.
“If you have a forest of barriers in a classroom, it’s going to interfere with proper ventilation of that room,” Linsey Marr, professor of civil and environmental engineering at Virginia Tech, told the newspaper.
“Everybody’s aerosols are going to be trapped and stuck there and building up, and they will end up spreading beyond your own desk,” she said.
Several variables factor into the efficacy of plastic barriers, The New York Times reported. Shields may stop big respiratory droplets from coughs and sneezes, for instance, but they may not do much to prevent small aerosol particles from viruses such as COVID-19 from spreading.
“We have shown this effect of blocking larger particles, but also that the smaller aerosols travel over the screen and become mixed in the room air within about 5 minutes,” Catherine Noakes, professor of environment engineering at the University of Leeds, told the newspaper.
“This means if people are interacting for more than a few minutes, they would likely be exposed to the virus regardless of the screen,” she said.
The effectiveness of plastic barriers likely also depends on the location and setup, the newspaper reported. A bus driver with a large barrier, for instance, may be able to avoid inhaling the particles that passengers are exhaling. A bank cashier or store clerk behind a large barrier may also be partly protected.
Even still, scientists say more research is needed. For instance, taller barriers are more likely to be effective. However, a large number of barriers in one room could likely block air flow.
Researchers have recommended that schools and offices focus on ventilation, masks, and vaccines to slow the spread of the coronavirus.
“Air flow in rooms is pretty complicated,” Richard Corsi, dean of engineering at the University of California at Davis, told the newspaper.
“Every room is different in terms of the arrangement of furniture, the height of the walls and ceilings, the vents, where the bookshelves are,” he said. “All of these things have a huge impact on the actual flow and air distribution in a room.”
A version of this article first appeared on WebMD.com.
FDA fully approves Pfizer COVID-19 vaccine
The Food and Drug Administration has granted a biological license application, more commonly known as “full approval,” to the Pfizer-BioNTech COVID-19 vaccine.
It is the first COVID-19 vaccine to be fully licensed in the United States. It will be marketed under the trade name Comirnaty.
The approval applies to individuals ages 16 years and older. The vaccine is still available for emergency use for those ages 12-15.
The FDA’s stamp of approval is somewhat anticlimactic, following months of real-world use and millions of doses doled out to the general population. It comes after months of scrutiny by the agency of the clinical trial data.
Still, the approval puts the vaccines on firmer legal footing and is expected to spur a raft of new vaccination requirements by employers, schools, and universities.
“The FDA approval is the gold standard,” President Joe Biden said from the White House. “Those who have been waiting for full approval should go and get your shot now.”
“It could save your life or the lives of those you love,” he said.
Biden also called on businesses to mandate COVID vaccines for their employees.
Indeed, soon after the approval was announced, Defense Secretary Lloyd Austin said the vaccines would be required for all 1.4 million active duty service members.
Public health advocates have seen full approval as an important tool to increase U.S. vaccination rates and had criticized the FDA for taking so long to grant the license.
In a news briefing on the approval, Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, said the agency had not dragged its feet.
Marks noted that his team had reviewed tens of thousands of pages of clinical trial data -- down to the level of individual patients. They also inspected clinical trial sites and manufacturing facilities, and reviewed information gathered after the vaccines were authorized for use.
“It’s been 97 days since Pfizer completed the role of its [application for approval] and the clock started, which means that we completed this in about 40% of the normal clock time for a submission of this magnitude,” he said. “People worked day and night.”
The agency resisted pressure to speed up its process, saying a thorough review was necessary to ensure public confidence.
“While millions of people have already safely received COVID-19 vaccines, we recognize that for some, the FDA approval of a vaccine may now instill additional confidence to get vaccinated. Today’s milestone puts us one step closer to altering the course of this pandemic in the U.S.,” acting FDA Commissioner Janet Woodcock said in a FDA news release.
Experts agreed the move would increase public confidence.
“I don't expect a big line outside of vaccination sites this afternoon or tomorrow morning, but it will persuade some,” said William Schaffner, MD, a professor of infectious diseases at Vanderbilt University in Nashville.
A recent Kaiser Family Foundation poll found that 3 in 10 unvaccinated adults said they would be more likely to get vaccinated if the vaccines were given full approval.
More importantly, Schaffner said, the FDA’s approval would lay the groundwork for vaccine mandates. “I think those kinds of mandates are going to be necessary to get us up over 80% vaccinated.”
In granting the approval, the agency reviewed a record amount of data from more than 40,000 people who took part in clinical trials. About 12,000 recipients have been followed for at least 6 months, the agency said.
The FDA also reviewed safety data collected since it issued its emergency use authorization for the shots in December.
Based on the results from the clinical trials, the vaccine was 91% effective at preventing COVID-19 disease. But that estimate came from data collected before the Delta variant became widespread.
The most commonly reported side effects in the clinical trials were pain, redness and swelling at the injection site, fatigue, headache, muscle or joint pain, chills, and fever.
The FDA said the vaccine is effective in preventing COVID-19 and potentially serious outcomes, including hospitalization and death.
Based on safety data reviewed since the two-dose vaccine was approved, the FDA said the data demonstrates a higher risk for heart inflammation -- clinically known as myocarditis or pericarditis -- especially within 7 days after the second dose of the shots. The risk is highest for men under age 40, compared to women and older men.
The prescription information includes warnings about these risks. The FDA said the drugmakers must continue to study the risks and long-term effects on people who have myocarditis after vaccination.
A version of this article first appeared on Medscape.com.
This article was updated on 8/24/21.
The Food and Drug Administration has granted a biological license application, more commonly known as “full approval,” to the Pfizer-BioNTech COVID-19 vaccine.
It is the first COVID-19 vaccine to be fully licensed in the United States. It will be marketed under the trade name Comirnaty.
The approval applies to individuals ages 16 years and older. The vaccine is still available for emergency use for those ages 12-15.
The FDA’s stamp of approval is somewhat anticlimactic, following months of real-world use and millions of doses doled out to the general population. It comes after months of scrutiny by the agency of the clinical trial data.
Still, the approval puts the vaccines on firmer legal footing and is expected to spur a raft of new vaccination requirements by employers, schools, and universities.
“The FDA approval is the gold standard,” President Joe Biden said from the White House. “Those who have been waiting for full approval should go and get your shot now.”
“It could save your life or the lives of those you love,” he said.
Biden also called on businesses to mandate COVID vaccines for their employees.
Indeed, soon after the approval was announced, Defense Secretary Lloyd Austin said the vaccines would be required for all 1.4 million active duty service members.
Public health advocates have seen full approval as an important tool to increase U.S. vaccination rates and had criticized the FDA for taking so long to grant the license.
In a news briefing on the approval, Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, said the agency had not dragged its feet.
Marks noted that his team had reviewed tens of thousands of pages of clinical trial data -- down to the level of individual patients. They also inspected clinical trial sites and manufacturing facilities, and reviewed information gathered after the vaccines were authorized for use.
“It’s been 97 days since Pfizer completed the role of its [application for approval] and the clock started, which means that we completed this in about 40% of the normal clock time for a submission of this magnitude,” he said. “People worked day and night.”
The agency resisted pressure to speed up its process, saying a thorough review was necessary to ensure public confidence.
“While millions of people have already safely received COVID-19 vaccines, we recognize that for some, the FDA approval of a vaccine may now instill additional confidence to get vaccinated. Today’s milestone puts us one step closer to altering the course of this pandemic in the U.S.,” acting FDA Commissioner Janet Woodcock said in a FDA news release.
Experts agreed the move would increase public confidence.
“I don't expect a big line outside of vaccination sites this afternoon or tomorrow morning, but it will persuade some,” said William Schaffner, MD, a professor of infectious diseases at Vanderbilt University in Nashville.
A recent Kaiser Family Foundation poll found that 3 in 10 unvaccinated adults said they would be more likely to get vaccinated if the vaccines were given full approval.
More importantly, Schaffner said, the FDA’s approval would lay the groundwork for vaccine mandates. “I think those kinds of mandates are going to be necessary to get us up over 80% vaccinated.”
In granting the approval, the agency reviewed a record amount of data from more than 40,000 people who took part in clinical trials. About 12,000 recipients have been followed for at least 6 months, the agency said.
The FDA also reviewed safety data collected since it issued its emergency use authorization for the shots in December.
Based on the results from the clinical trials, the vaccine was 91% effective at preventing COVID-19 disease. But that estimate came from data collected before the Delta variant became widespread.
The most commonly reported side effects in the clinical trials were pain, redness and swelling at the injection site, fatigue, headache, muscle or joint pain, chills, and fever.
The FDA said the vaccine is effective in preventing COVID-19 and potentially serious outcomes, including hospitalization and death.
Based on safety data reviewed since the two-dose vaccine was approved, the FDA said the data demonstrates a higher risk for heart inflammation -- clinically known as myocarditis or pericarditis -- especially within 7 days after the second dose of the shots. The risk is highest for men under age 40, compared to women and older men.
The prescription information includes warnings about these risks. The FDA said the drugmakers must continue to study the risks and long-term effects on people who have myocarditis after vaccination.
A version of this article first appeared on Medscape.com.
This article was updated on 8/24/21.
The Food and Drug Administration has granted a biological license application, more commonly known as “full approval,” to the Pfizer-BioNTech COVID-19 vaccine.
It is the first COVID-19 vaccine to be fully licensed in the United States. It will be marketed under the trade name Comirnaty.
The approval applies to individuals ages 16 years and older. The vaccine is still available for emergency use for those ages 12-15.
The FDA’s stamp of approval is somewhat anticlimactic, following months of real-world use and millions of doses doled out to the general population. It comes after months of scrutiny by the agency of the clinical trial data.
Still, the approval puts the vaccines on firmer legal footing and is expected to spur a raft of new vaccination requirements by employers, schools, and universities.
“The FDA approval is the gold standard,” President Joe Biden said from the White House. “Those who have been waiting for full approval should go and get your shot now.”
“It could save your life or the lives of those you love,” he said.
Biden also called on businesses to mandate COVID vaccines for their employees.
Indeed, soon after the approval was announced, Defense Secretary Lloyd Austin said the vaccines would be required for all 1.4 million active duty service members.
Public health advocates have seen full approval as an important tool to increase U.S. vaccination rates and had criticized the FDA for taking so long to grant the license.
In a news briefing on the approval, Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, said the agency had not dragged its feet.
Marks noted that his team had reviewed tens of thousands of pages of clinical trial data -- down to the level of individual patients. They also inspected clinical trial sites and manufacturing facilities, and reviewed information gathered after the vaccines were authorized for use.
“It’s been 97 days since Pfizer completed the role of its [application for approval] and the clock started, which means that we completed this in about 40% of the normal clock time for a submission of this magnitude,” he said. “People worked day and night.”
The agency resisted pressure to speed up its process, saying a thorough review was necessary to ensure public confidence.
“While millions of people have already safely received COVID-19 vaccines, we recognize that for some, the FDA approval of a vaccine may now instill additional confidence to get vaccinated. Today’s milestone puts us one step closer to altering the course of this pandemic in the U.S.,” acting FDA Commissioner Janet Woodcock said in a FDA news release.
Experts agreed the move would increase public confidence.
“I don't expect a big line outside of vaccination sites this afternoon or tomorrow morning, but it will persuade some,” said William Schaffner, MD, a professor of infectious diseases at Vanderbilt University in Nashville.
A recent Kaiser Family Foundation poll found that 3 in 10 unvaccinated adults said they would be more likely to get vaccinated if the vaccines were given full approval.
More importantly, Schaffner said, the FDA’s approval would lay the groundwork for vaccine mandates. “I think those kinds of mandates are going to be necessary to get us up over 80% vaccinated.”
In granting the approval, the agency reviewed a record amount of data from more than 40,000 people who took part in clinical trials. About 12,000 recipients have been followed for at least 6 months, the agency said.
The FDA also reviewed safety data collected since it issued its emergency use authorization for the shots in December.
Based on the results from the clinical trials, the vaccine was 91% effective at preventing COVID-19 disease. But that estimate came from data collected before the Delta variant became widespread.
The most commonly reported side effects in the clinical trials were pain, redness and swelling at the injection site, fatigue, headache, muscle or joint pain, chills, and fever.
The FDA said the vaccine is effective in preventing COVID-19 and potentially serious outcomes, including hospitalization and death.
Based on safety data reviewed since the two-dose vaccine was approved, the FDA said the data demonstrates a higher risk for heart inflammation -- clinically known as myocarditis or pericarditis -- especially within 7 days after the second dose of the shots. The risk is highest for men under age 40, compared to women and older men.
The prescription information includes warnings about these risks. The FDA said the drugmakers must continue to study the risks and long-term effects on people who have myocarditis after vaccination.
A version of this article first appeared on Medscape.com.
This article was updated on 8/24/21.
Shouldn’t docs who spread false COVID-19 info lose their licenses?
A tall, distinguished-looking physician in shirtsleeves and suspenders walked to the microphone at the Mt. Vernon, Ind., school board meeting on a Friday evening in early August. He launched into an impassioned, 7-minute attack on the public health establishment’s medical guidelines for COVID-19.
“The Center for Disease Control and the Indiana State [Department] of Health are giving you very bad scientific guidance,” said Daniel Stock, MD, a primary care physician with a concierge practice in Noblesville, Ind., He described himself as a “functional family medicine physician,” though he is not board certified in family medicine.
Dr. Stock told the school board members that COVID-19 vaccines are counterproductive because they make coronavirus infections worse. He claimed his treatment of “over 15” COVID-19 patients with vitamin D, ivermectin, and zinc has kept them out of the hospital, and that those treatments reduce mortality risk from the disease by 75%. (A study released in mid-August found that ivermectin is ineffective in treating COVID-19).
In response to Dr. Stock’s remarks, the state health department quickly issued a statement reaffirming that COVID-19 vaccines “are highly effective at preventing hospitalizations and deaths.” But by then, the YouTube video of Dr. Stock’s comments had garnered nearly 600,000 views as of Aug. 12 and had been shared over 10,000 times on Facebook. Opponents of COVID-19 vaccines and masking policies across the country have been citing his comments.
Across the country, state medical licensing boards and state and national medical associations are struggling with how to respond to scientifically baseless public statements about COVID-19 by some physicians such as Dr. Stock. They fear such statements are increasing public confusion and are heightening political conflict. Physicians accused of spreading false information include public officials such as Scott Atlas, MD, who served as President Donald Trump’s COVID-19 advisor, and Kentucky Sen. Rand Paul, an ophthalmologist, whose YouTube account was temporarily suspended in August after he posted a video disputing the effectiveness of masking in stopping the spread of COVID-19.
“That’s the problem – those types of viral videos of someone somewhere who thinks they know something the rest of us don’t,” lamented Jennifer Bryan, MD, board chair of the Mississippi State Medical Association. “I don’t know any good reason why a physician should be advising against vaccination. It’s appropriate for medical boards to look into those situations.”
The Federation of State Medical Boards agrees. In July, it warned that physicians who willfully spread false information about COVID-19 risk suspension or revocation of their medical license. The federation cited a “dramatic increase in the dissemination of COVID-19 vaccine misinformation and disinformation by physicians.” That’s particularly dangerous, it said, because physicians enjoy a high degree of public credibility.
Medical boards will particularly examine cases in which there is a pattern of misinformation or disinformation showing that a physician poses a continuing threat to public health, said Hank Chaudhry, DO, the federation’s CEO. In some cases, he said, boards have contacted physicians and have persuaded them to voluntarily refrain from making false public statements, without taking disciplinary action.
“Words matter,” he said. “Physicians have a really big platform, whether they realize it or not. Misinformation or disinformation in the context of COVID can not only cause harm but also death. We felt it was appropriate to remind physicians to be careful.”
Although medical leaders stress that most physicians are promoting solid science on COVID-19, the London-based Center for Countering Digital Hate, in a May report titled “The Disinformation Dozen,” named four U.S. physicians among 12 people who it said produce 65% of the misleading claims and lies about COVID-19 vaccines that abound on Facebook, Instagram, and Twitter. The leading spreader of false claims, the group said, is Joseph Mercola, MD, an Illinois-licensed osteopath living in Cape Coral, Fla. He did not respond to requests for comment.
But so far, state licensing boards and federal and state medical associations generally have been reluctant to discipline or publicly call out physicians who have spread misinformation about the causes, treatments, vaccines, and prevention strategies for COVID-19. Some of these physicians, such as Dr. Mercola, have a long history predating the COVID-19 pandemic of disseminating scientifically baseless information, often in connection with their marketing of products and services.
For instance, the Medical Licensing Board of Indiana and the state attorney general’s office, which brings medical disciplinary actions, declined to comment on Dr. Stock’s public statements at the August school board meeting. When asked about Dr. Stock, the Indiana State Medical Association, without mentioning his name, said: “We urge Hoosier physicians to share the proven facts [about public health measures recommended by the CDC and the Indiana Department of Health] with their patients and their communities.” Dr. Stock did not respond to a request for comment.
Experts say state medical boards are ill equipped and are often unwilling to address the challenge of disciplining physicians who disseminate dangerously false medical information. That enforcement gap is particularly troubling in the middle of a deadly pandemic such as this one.
“Unless you can show a harm to an individual patient, it’s pretty tough to get the boards to do much,” said Art Caplan, PhD, a professor of bioethics at New York University. “I wish they would, but they just don’t.”
That’s partly because state laws require the boards to engage in lengthy, confidential investigations and adversarial legal processes before imposing disciplinary actions. The laws generally require patients or members of the public to file a complaint before an investigation can start. Some states, however, do allow their medical boards to take rapid emergency action if a physician poses an immediate threat to patients or the public.
Another hurdle is that medical boards that seek to sanction physicians for making dangerously misleading public statements could face lawsuits alleging that such actions violate the physicians’ constitutional free speech rights or their professional autonomy.
“We have free speech, and you can get away with a lot of stuff,” said Stephen Barrett, MD, who for many years has critically documented examples of medical fraud on his website, Quackwatch. “Some doctors would sue if they were challenged by medical boards, and I’m not sure the boards would win that court fight. People have written books with advice that killed people, and I’m not aware of a single case where the author was disciplined.”
In addition, it’s not clear that U.S. physicians who are not government officials have any legal obligation – as opposed to a moral obligation – to the government or the public to promote public health, said Jonathan Moreno, PhD, a professor of medical ethics at University of Pennsylvania, Philadelphia. “Is transmitting misinformation about COVID-19 public health malpractice?” he asked. “Do we as a society see physicians having a special role as guides in an emergency? I’d like to think we do, but we don’t have a strong tradition like that in the U.S.”
But California State Sen. Richard Pan, MD, a pediatrician who represents the Sacramento area, doesn’t buy the arguments about why medical boards can’t discipline physicians for spreading misinformation. He successfully sponsored a 2019 bill that strengthens the medical board’s ability to discipline physicians who dole out medically unjustified vaccine exemptions to children.
“A medical license is a privilege. It’s an imprimatur from the state that the person is someone who upholds professional standards,” Dr. Pan said. “If someone is intentionally spreading disinformation for personal gain and that’s putting the public at risk, the medical board has a duty to act.”
There have been only a few publicly announced disciplinary actions related to COVID-19 misinformation so far.
Last December, the Oregon Medical Board, on an emergency basis, suspended the license of Steven LaTulippe, MD, of Dallas, Ore. He had publicly announced that he and his staff were not wearing masks in his clinic. In addition, he compared COVID-19 to the common cold and denied the governor’s legal authority to adopt public health protection measures. A recorded message on his office phone said he’s challenging the licensure action in court.
Last January, the Medical Board of California made Thomas Cowan, MD, of San Francisco surrender his license after Dr. Cowan posted a YouTube video, which went viral last year, that claimed that 5G Internet networks cause COVID-19. He did not respond to a request for comment.
In May, the College of Physicians and Surgeons of British Columbia reprimanded Stephen Malthouse, MD, and forbade him from speaking on issues related to COVID-19. He had written a widely circulated open letter to the province’s chief health office claiming that the pandemic was “over” and that measures to control the spread of COVID-19 were worse than the virus. He has challenged the disciplinary action in court, alleging it violates his right to free speech.
Attacking the problem from a different angle, the U.S. Federal Trade Commission has issued enforcement actions in cases in which physicians and other health care professionals engaged in deceptive business practices related to COVID-19. That approach may be applicable to a number of physicians accused of spreading COVID-19 misinformation, who allegedly have done so at least partly to sell unproven products and services to prevent or treat the disease.
In June, the FTC settled a case against Stephen Meis, MD, of Porterville, Calif. The settlement required that he stop making unsupported claims that his company’s dietary supplements effectively treat COVID-19 symptoms and that he pay $103,420 in refunds to defrauded customers.
State medical boards in the United States generally are not allowed to disclose investigations or disciplinary processes until they finalize a disciplinary action, so other investigations that have not been publicly disclosed may be pending.
A spokesperson for the Medical Board of California said the board is aware of questionable statements about COVID-19 made by several physicians and “will be looking into it.” That comment was in response to a question about statements made at a news conference last year by two Bakersfield emergency physicians, Artin Massihi, MD, and Dan Erickson, DO. They claimed that their COVID-19 testing data showed that the virus is not that dangerous. Dr. Erickson is an osteopath and is regulated by the Osteopathic Medical Board of California.
The two physicians’ news conference prompted an unusual joint statement from the American College of Emergency Medicine and the American Academy of Emergency Medicine in April 2020 declaring that they “emphatically condemn” Dr. Massihi’s and Dr. Erickson’s “reckless and untested musings.” The groups added that it appeared that the physicians issued the comments “to advance their personal financial interests without regard for the public’s health.”
Neither Dr. Massihi nor Dr. Erickson responded to a request for comment.
As for the physician dubbed by the Center for Countering Digital Hate as the world’s most influential spreader of COVID-19 misinformation on social media: No recent public complaints have been filed, and no disciplinary action has been taken against Dr. Mercola, according to a spokesman for the Illinois Department of Financial and Professional Regulation.
According to court records, Dr. Mercola faced disciplinary complaints from the Illinois board in the early 2000s for allegedly providing false and potentially harmful medical advice on his website. There is no record of any final disciplinary action taken against him.
In widely disseminated online posts, Dr. Mercola has called the COVID-19 pandemic a “scam” and said “forced vaccination” is part of a plan to re-set the global economic system. He called COVID-19 vaccines “a medical fraud,” claiming they “alter your genetic coding.” In February, the U.S. Food and Drug Administration ordered Dr. Mercola to stop saying on his website that various vitamins and dietary supplements he sells through his website are effective in preventing or treating COVID-19.
The New York Times reported in July that Dr. Mercola’s English-language Facebook page has more than 1.7 million followers, that his Spanish-language page has one million, and that he has 300,000 followers on Twitter and 400,000 on YouTube.
In August, Dr. Mercola announced that he was deleting the large archive of articles he’s written on his website but would continue to post articles every day that would be available on the site for only 48 hours. He explained his decision by saying he’s facing “blatant censorship” as part of a “McCarthyism-like attack” from “the sitting President of the United States.” He encouraged people to read his book, “The Truth about COVID-19.”
The lack of action against Dr. Mercola for his lengthy list of scientifically unfounded statements and marketing claims about COVID-19 and other medical conditions infuriates Quackwatch’s Dr. Barrett. He’s amazed that the Illinois board did not discipline Dr. Mercola despite a number of enforcement actions against him by the FTC and the FDA.
“If a doctor were to say to a patient, ‘Don’t wear a mask and don’t get vaccinated,’ the doctor would be held responsible for a bad outcome,” he said. “But if you say it to millions and as a direct result a dozen people die, shouldn’t the doctor also be held responsible for that misinformation? I think he should lose his license.”
Another of the four physicians cited in the “Disinformation Dozen” report is Sherri Tenpenny, DO, an osteopath licensed in Ohio, who has published posts on social media advocating against masking, testing, and vaccines to prevent COVID-19 infections. A spokesperson for the State Medical Board of Ohio said Dr. Tenpenny’s license expires on Oct. 1, 2021, and that any investigation would be confidential. She added that grounds for disciplinary action include “making a false, fraudulent, deceptive, or misleading statement in relation to the practice of medicine and surgery.” Dr. Tenpenny could not be reached for comment.
A third physician named in the report is Christiane Northrup, MD, an ob.gyn. formerly licensed in Maine, who has published posts advocating unproven cures for COVID-19 and claiming that vaccines increase chronic illness. Dennis Smith, executive director of the Maine Board of Licensure in Medicine, said the board received complaints about Dr. Northrup’s posts but can’t act because she withdrew her Maine license in 2015. He added that the Maine board can issue sanctions against physicians who engage in fraud, deceit, or misrepresentation or who post scientifically unfounded statements online.
The fourth physician identified in the “Disinformation Dozen” report is Rashid Buttar, DO, an osteopath practicing in Mooresville, N.C., who has claimed in social media posts that COVID-19 vaccines cause infertility and that COVID-19 tests contain living microorganisms. A spokeswoman for the North Carolina Medical Board said she could not confirm or deny the existence of any investigation of Dr. Buttar, who signed a consent order with the medical board in 2010 following charges of exorbitant fees, worthless tests and treatment, and false diagnoses. Undisclosed conditions were placed on his medical license in 2013. The spokesperson added that the board would investigate any information alleging that a physician spread false information about COVID-19.
Another physician who has caused widespread consternation over scientifically unfounded statements about COVID-19 is Simone Gold, MD, formerly an emergency department physician in Los Angeles. She founded a group called America’s Frontline Doctors, which filed a federal lawsuit in Alabama this spring to block the FDA from issuing an emergency use authorization allowing teenagers to receive COVID-19 vaccinations. She called the vaccines “an experimental biological agent whose harms are well-documented.”
Last summer, Dr. Gold and other physicians in her group held a news conference on the steps of the U.S. Supreme Court Building promoting hydroxychloroquine as a COVID-19 treatment. They declared that masks don’t work and that the virus isn’t deadly, and made other false claims. The news conference was livestreamed by conservative media outlets, was promoted on Twitter by then-President Trump and his family, and was viewed online more than 14 million times.
One of the participating physicians, Stella Immanuel, MD, of Houston, claimed in a video that went viral that she had successfully used hydroxychloroquine for more than 400 patients to cure the disease. In response, the Texas Medical Board, without naming Dr. Immanuel, warned that if it received a complaint about any physician who made a false claim about having a cure for COVID-19, it would investigate and potentially take disciplinary action.
Although no publicly known disciplinary action has been taken against Dr. Gold, she told The Washington Post last January that after participating in that July 2020 news conference, she was fired from her emergency department job at two hospitals and that she hasn’t worked as a physician since. Dr. Gold did not respond to a request for comment.
The outcome in her situation is consistent with the view of NYU’s Dr. Caplan that methods other than medical board discipline – such as action by employers, social media pressure, and reprimands from professional societies –will have to be used to hold physicians accountable for spreading COVID-19 misinformation.
“I’m disappointed to have to say it, but I don’t think medical boards are going to be effective,” he said. “We don’t know how to manage misinformation despite being in a plague. We just don’t.
A version of this article first appeared on Medscape.com.
A tall, distinguished-looking physician in shirtsleeves and suspenders walked to the microphone at the Mt. Vernon, Ind., school board meeting on a Friday evening in early August. He launched into an impassioned, 7-minute attack on the public health establishment’s medical guidelines for COVID-19.
“The Center for Disease Control and the Indiana State [Department] of Health are giving you very bad scientific guidance,” said Daniel Stock, MD, a primary care physician with a concierge practice in Noblesville, Ind., He described himself as a “functional family medicine physician,” though he is not board certified in family medicine.
Dr. Stock told the school board members that COVID-19 vaccines are counterproductive because they make coronavirus infections worse. He claimed his treatment of “over 15” COVID-19 patients with vitamin D, ivermectin, and zinc has kept them out of the hospital, and that those treatments reduce mortality risk from the disease by 75%. (A study released in mid-August found that ivermectin is ineffective in treating COVID-19).
In response to Dr. Stock’s remarks, the state health department quickly issued a statement reaffirming that COVID-19 vaccines “are highly effective at preventing hospitalizations and deaths.” But by then, the YouTube video of Dr. Stock’s comments had garnered nearly 600,000 views as of Aug. 12 and had been shared over 10,000 times on Facebook. Opponents of COVID-19 vaccines and masking policies across the country have been citing his comments.
Across the country, state medical licensing boards and state and national medical associations are struggling with how to respond to scientifically baseless public statements about COVID-19 by some physicians such as Dr. Stock. They fear such statements are increasing public confusion and are heightening political conflict. Physicians accused of spreading false information include public officials such as Scott Atlas, MD, who served as President Donald Trump’s COVID-19 advisor, and Kentucky Sen. Rand Paul, an ophthalmologist, whose YouTube account was temporarily suspended in August after he posted a video disputing the effectiveness of masking in stopping the spread of COVID-19.
“That’s the problem – those types of viral videos of someone somewhere who thinks they know something the rest of us don’t,” lamented Jennifer Bryan, MD, board chair of the Mississippi State Medical Association. “I don’t know any good reason why a physician should be advising against vaccination. It’s appropriate for medical boards to look into those situations.”
The Federation of State Medical Boards agrees. In July, it warned that physicians who willfully spread false information about COVID-19 risk suspension or revocation of their medical license. The federation cited a “dramatic increase in the dissemination of COVID-19 vaccine misinformation and disinformation by physicians.” That’s particularly dangerous, it said, because physicians enjoy a high degree of public credibility.
Medical boards will particularly examine cases in which there is a pattern of misinformation or disinformation showing that a physician poses a continuing threat to public health, said Hank Chaudhry, DO, the federation’s CEO. In some cases, he said, boards have contacted physicians and have persuaded them to voluntarily refrain from making false public statements, without taking disciplinary action.
“Words matter,” he said. “Physicians have a really big platform, whether they realize it or not. Misinformation or disinformation in the context of COVID can not only cause harm but also death. We felt it was appropriate to remind physicians to be careful.”
Although medical leaders stress that most physicians are promoting solid science on COVID-19, the London-based Center for Countering Digital Hate, in a May report titled “The Disinformation Dozen,” named four U.S. physicians among 12 people who it said produce 65% of the misleading claims and lies about COVID-19 vaccines that abound on Facebook, Instagram, and Twitter. The leading spreader of false claims, the group said, is Joseph Mercola, MD, an Illinois-licensed osteopath living in Cape Coral, Fla. He did not respond to requests for comment.
But so far, state licensing boards and federal and state medical associations generally have been reluctant to discipline or publicly call out physicians who have spread misinformation about the causes, treatments, vaccines, and prevention strategies for COVID-19. Some of these physicians, such as Dr. Mercola, have a long history predating the COVID-19 pandemic of disseminating scientifically baseless information, often in connection with their marketing of products and services.
For instance, the Medical Licensing Board of Indiana and the state attorney general’s office, which brings medical disciplinary actions, declined to comment on Dr. Stock’s public statements at the August school board meeting. When asked about Dr. Stock, the Indiana State Medical Association, without mentioning his name, said: “We urge Hoosier physicians to share the proven facts [about public health measures recommended by the CDC and the Indiana Department of Health] with their patients and their communities.” Dr. Stock did not respond to a request for comment.
Experts say state medical boards are ill equipped and are often unwilling to address the challenge of disciplining physicians who disseminate dangerously false medical information. That enforcement gap is particularly troubling in the middle of a deadly pandemic such as this one.
“Unless you can show a harm to an individual patient, it’s pretty tough to get the boards to do much,” said Art Caplan, PhD, a professor of bioethics at New York University. “I wish they would, but they just don’t.”
That’s partly because state laws require the boards to engage in lengthy, confidential investigations and adversarial legal processes before imposing disciplinary actions. The laws generally require patients or members of the public to file a complaint before an investigation can start. Some states, however, do allow their medical boards to take rapid emergency action if a physician poses an immediate threat to patients or the public.
Another hurdle is that medical boards that seek to sanction physicians for making dangerously misleading public statements could face lawsuits alleging that such actions violate the physicians’ constitutional free speech rights or their professional autonomy.
“We have free speech, and you can get away with a lot of stuff,” said Stephen Barrett, MD, who for many years has critically documented examples of medical fraud on his website, Quackwatch. “Some doctors would sue if they were challenged by medical boards, and I’m not sure the boards would win that court fight. People have written books with advice that killed people, and I’m not aware of a single case where the author was disciplined.”
In addition, it’s not clear that U.S. physicians who are not government officials have any legal obligation – as opposed to a moral obligation – to the government or the public to promote public health, said Jonathan Moreno, PhD, a professor of medical ethics at University of Pennsylvania, Philadelphia. “Is transmitting misinformation about COVID-19 public health malpractice?” he asked. “Do we as a society see physicians having a special role as guides in an emergency? I’d like to think we do, but we don’t have a strong tradition like that in the U.S.”
But California State Sen. Richard Pan, MD, a pediatrician who represents the Sacramento area, doesn’t buy the arguments about why medical boards can’t discipline physicians for spreading misinformation. He successfully sponsored a 2019 bill that strengthens the medical board’s ability to discipline physicians who dole out medically unjustified vaccine exemptions to children.
“A medical license is a privilege. It’s an imprimatur from the state that the person is someone who upholds professional standards,” Dr. Pan said. “If someone is intentionally spreading disinformation for personal gain and that’s putting the public at risk, the medical board has a duty to act.”
There have been only a few publicly announced disciplinary actions related to COVID-19 misinformation so far.
Last December, the Oregon Medical Board, on an emergency basis, suspended the license of Steven LaTulippe, MD, of Dallas, Ore. He had publicly announced that he and his staff were not wearing masks in his clinic. In addition, he compared COVID-19 to the common cold and denied the governor’s legal authority to adopt public health protection measures. A recorded message on his office phone said he’s challenging the licensure action in court.
Last January, the Medical Board of California made Thomas Cowan, MD, of San Francisco surrender his license after Dr. Cowan posted a YouTube video, which went viral last year, that claimed that 5G Internet networks cause COVID-19. He did not respond to a request for comment.
In May, the College of Physicians and Surgeons of British Columbia reprimanded Stephen Malthouse, MD, and forbade him from speaking on issues related to COVID-19. He had written a widely circulated open letter to the province’s chief health office claiming that the pandemic was “over” and that measures to control the spread of COVID-19 were worse than the virus. He has challenged the disciplinary action in court, alleging it violates his right to free speech.
Attacking the problem from a different angle, the U.S. Federal Trade Commission has issued enforcement actions in cases in which physicians and other health care professionals engaged in deceptive business practices related to COVID-19. That approach may be applicable to a number of physicians accused of spreading COVID-19 misinformation, who allegedly have done so at least partly to sell unproven products and services to prevent or treat the disease.
In June, the FTC settled a case against Stephen Meis, MD, of Porterville, Calif. The settlement required that he stop making unsupported claims that his company’s dietary supplements effectively treat COVID-19 symptoms and that he pay $103,420 in refunds to defrauded customers.
State medical boards in the United States generally are not allowed to disclose investigations or disciplinary processes until they finalize a disciplinary action, so other investigations that have not been publicly disclosed may be pending.
A spokesperson for the Medical Board of California said the board is aware of questionable statements about COVID-19 made by several physicians and “will be looking into it.” That comment was in response to a question about statements made at a news conference last year by two Bakersfield emergency physicians, Artin Massihi, MD, and Dan Erickson, DO. They claimed that their COVID-19 testing data showed that the virus is not that dangerous. Dr. Erickson is an osteopath and is regulated by the Osteopathic Medical Board of California.
The two physicians’ news conference prompted an unusual joint statement from the American College of Emergency Medicine and the American Academy of Emergency Medicine in April 2020 declaring that they “emphatically condemn” Dr. Massihi’s and Dr. Erickson’s “reckless and untested musings.” The groups added that it appeared that the physicians issued the comments “to advance their personal financial interests without regard for the public’s health.”
Neither Dr. Massihi nor Dr. Erickson responded to a request for comment.
As for the physician dubbed by the Center for Countering Digital Hate as the world’s most influential spreader of COVID-19 misinformation on social media: No recent public complaints have been filed, and no disciplinary action has been taken against Dr. Mercola, according to a spokesman for the Illinois Department of Financial and Professional Regulation.
According to court records, Dr. Mercola faced disciplinary complaints from the Illinois board in the early 2000s for allegedly providing false and potentially harmful medical advice on his website. There is no record of any final disciplinary action taken against him.
In widely disseminated online posts, Dr. Mercola has called the COVID-19 pandemic a “scam” and said “forced vaccination” is part of a plan to re-set the global economic system. He called COVID-19 vaccines “a medical fraud,” claiming they “alter your genetic coding.” In February, the U.S. Food and Drug Administration ordered Dr. Mercola to stop saying on his website that various vitamins and dietary supplements he sells through his website are effective in preventing or treating COVID-19.
The New York Times reported in July that Dr. Mercola’s English-language Facebook page has more than 1.7 million followers, that his Spanish-language page has one million, and that he has 300,000 followers on Twitter and 400,000 on YouTube.
In August, Dr. Mercola announced that he was deleting the large archive of articles he’s written on his website but would continue to post articles every day that would be available on the site for only 48 hours. He explained his decision by saying he’s facing “blatant censorship” as part of a “McCarthyism-like attack” from “the sitting President of the United States.” He encouraged people to read his book, “The Truth about COVID-19.”
The lack of action against Dr. Mercola for his lengthy list of scientifically unfounded statements and marketing claims about COVID-19 and other medical conditions infuriates Quackwatch’s Dr. Barrett. He’s amazed that the Illinois board did not discipline Dr. Mercola despite a number of enforcement actions against him by the FTC and the FDA.
“If a doctor were to say to a patient, ‘Don’t wear a mask and don’t get vaccinated,’ the doctor would be held responsible for a bad outcome,” he said. “But if you say it to millions and as a direct result a dozen people die, shouldn’t the doctor also be held responsible for that misinformation? I think he should lose his license.”
Another of the four physicians cited in the “Disinformation Dozen” report is Sherri Tenpenny, DO, an osteopath licensed in Ohio, who has published posts on social media advocating against masking, testing, and vaccines to prevent COVID-19 infections. A spokesperson for the State Medical Board of Ohio said Dr. Tenpenny’s license expires on Oct. 1, 2021, and that any investigation would be confidential. She added that grounds for disciplinary action include “making a false, fraudulent, deceptive, or misleading statement in relation to the practice of medicine and surgery.” Dr. Tenpenny could not be reached for comment.
A third physician named in the report is Christiane Northrup, MD, an ob.gyn. formerly licensed in Maine, who has published posts advocating unproven cures for COVID-19 and claiming that vaccines increase chronic illness. Dennis Smith, executive director of the Maine Board of Licensure in Medicine, said the board received complaints about Dr. Northrup’s posts but can’t act because she withdrew her Maine license in 2015. He added that the Maine board can issue sanctions against physicians who engage in fraud, deceit, or misrepresentation or who post scientifically unfounded statements online.
The fourth physician identified in the “Disinformation Dozen” report is Rashid Buttar, DO, an osteopath practicing in Mooresville, N.C., who has claimed in social media posts that COVID-19 vaccines cause infertility and that COVID-19 tests contain living microorganisms. A spokeswoman for the North Carolina Medical Board said she could not confirm or deny the existence of any investigation of Dr. Buttar, who signed a consent order with the medical board in 2010 following charges of exorbitant fees, worthless tests and treatment, and false diagnoses. Undisclosed conditions were placed on his medical license in 2013. The spokesperson added that the board would investigate any information alleging that a physician spread false information about COVID-19.
Another physician who has caused widespread consternation over scientifically unfounded statements about COVID-19 is Simone Gold, MD, formerly an emergency department physician in Los Angeles. She founded a group called America’s Frontline Doctors, which filed a federal lawsuit in Alabama this spring to block the FDA from issuing an emergency use authorization allowing teenagers to receive COVID-19 vaccinations. She called the vaccines “an experimental biological agent whose harms are well-documented.”
Last summer, Dr. Gold and other physicians in her group held a news conference on the steps of the U.S. Supreme Court Building promoting hydroxychloroquine as a COVID-19 treatment. They declared that masks don’t work and that the virus isn’t deadly, and made other false claims. The news conference was livestreamed by conservative media outlets, was promoted on Twitter by then-President Trump and his family, and was viewed online more than 14 million times.
One of the participating physicians, Stella Immanuel, MD, of Houston, claimed in a video that went viral that she had successfully used hydroxychloroquine for more than 400 patients to cure the disease. In response, the Texas Medical Board, without naming Dr. Immanuel, warned that if it received a complaint about any physician who made a false claim about having a cure for COVID-19, it would investigate and potentially take disciplinary action.
Although no publicly known disciplinary action has been taken against Dr. Gold, she told The Washington Post last January that after participating in that July 2020 news conference, she was fired from her emergency department job at two hospitals and that she hasn’t worked as a physician since. Dr. Gold did not respond to a request for comment.
The outcome in her situation is consistent with the view of NYU’s Dr. Caplan that methods other than medical board discipline – such as action by employers, social media pressure, and reprimands from professional societies –will have to be used to hold physicians accountable for spreading COVID-19 misinformation.
“I’m disappointed to have to say it, but I don’t think medical boards are going to be effective,” he said. “We don’t know how to manage misinformation despite being in a plague. We just don’t.
A version of this article first appeared on Medscape.com.
A tall, distinguished-looking physician in shirtsleeves and suspenders walked to the microphone at the Mt. Vernon, Ind., school board meeting on a Friday evening in early August. He launched into an impassioned, 7-minute attack on the public health establishment’s medical guidelines for COVID-19.
“The Center for Disease Control and the Indiana State [Department] of Health are giving you very bad scientific guidance,” said Daniel Stock, MD, a primary care physician with a concierge practice in Noblesville, Ind., He described himself as a “functional family medicine physician,” though he is not board certified in family medicine.
Dr. Stock told the school board members that COVID-19 vaccines are counterproductive because they make coronavirus infections worse. He claimed his treatment of “over 15” COVID-19 patients with vitamin D, ivermectin, and zinc has kept them out of the hospital, and that those treatments reduce mortality risk from the disease by 75%. (A study released in mid-August found that ivermectin is ineffective in treating COVID-19).
In response to Dr. Stock’s remarks, the state health department quickly issued a statement reaffirming that COVID-19 vaccines “are highly effective at preventing hospitalizations and deaths.” But by then, the YouTube video of Dr. Stock’s comments had garnered nearly 600,000 views as of Aug. 12 and had been shared over 10,000 times on Facebook. Opponents of COVID-19 vaccines and masking policies across the country have been citing his comments.
Across the country, state medical licensing boards and state and national medical associations are struggling with how to respond to scientifically baseless public statements about COVID-19 by some physicians such as Dr. Stock. They fear such statements are increasing public confusion and are heightening political conflict. Physicians accused of spreading false information include public officials such as Scott Atlas, MD, who served as President Donald Trump’s COVID-19 advisor, and Kentucky Sen. Rand Paul, an ophthalmologist, whose YouTube account was temporarily suspended in August after he posted a video disputing the effectiveness of masking in stopping the spread of COVID-19.
“That’s the problem – those types of viral videos of someone somewhere who thinks they know something the rest of us don’t,” lamented Jennifer Bryan, MD, board chair of the Mississippi State Medical Association. “I don’t know any good reason why a physician should be advising against vaccination. It’s appropriate for medical boards to look into those situations.”
The Federation of State Medical Boards agrees. In July, it warned that physicians who willfully spread false information about COVID-19 risk suspension or revocation of their medical license. The federation cited a “dramatic increase in the dissemination of COVID-19 vaccine misinformation and disinformation by physicians.” That’s particularly dangerous, it said, because physicians enjoy a high degree of public credibility.
Medical boards will particularly examine cases in which there is a pattern of misinformation or disinformation showing that a physician poses a continuing threat to public health, said Hank Chaudhry, DO, the federation’s CEO. In some cases, he said, boards have contacted physicians and have persuaded them to voluntarily refrain from making false public statements, without taking disciplinary action.
“Words matter,” he said. “Physicians have a really big platform, whether they realize it or not. Misinformation or disinformation in the context of COVID can not only cause harm but also death. We felt it was appropriate to remind physicians to be careful.”
Although medical leaders stress that most physicians are promoting solid science on COVID-19, the London-based Center for Countering Digital Hate, in a May report titled “The Disinformation Dozen,” named four U.S. physicians among 12 people who it said produce 65% of the misleading claims and lies about COVID-19 vaccines that abound on Facebook, Instagram, and Twitter. The leading spreader of false claims, the group said, is Joseph Mercola, MD, an Illinois-licensed osteopath living in Cape Coral, Fla. He did not respond to requests for comment.
But so far, state licensing boards and federal and state medical associations generally have been reluctant to discipline or publicly call out physicians who have spread misinformation about the causes, treatments, vaccines, and prevention strategies for COVID-19. Some of these physicians, such as Dr. Mercola, have a long history predating the COVID-19 pandemic of disseminating scientifically baseless information, often in connection with their marketing of products and services.
For instance, the Medical Licensing Board of Indiana and the state attorney general’s office, which brings medical disciplinary actions, declined to comment on Dr. Stock’s public statements at the August school board meeting. When asked about Dr. Stock, the Indiana State Medical Association, without mentioning his name, said: “We urge Hoosier physicians to share the proven facts [about public health measures recommended by the CDC and the Indiana Department of Health] with their patients and their communities.” Dr. Stock did not respond to a request for comment.
Experts say state medical boards are ill equipped and are often unwilling to address the challenge of disciplining physicians who disseminate dangerously false medical information. That enforcement gap is particularly troubling in the middle of a deadly pandemic such as this one.
“Unless you can show a harm to an individual patient, it’s pretty tough to get the boards to do much,” said Art Caplan, PhD, a professor of bioethics at New York University. “I wish they would, but they just don’t.”
That’s partly because state laws require the boards to engage in lengthy, confidential investigations and adversarial legal processes before imposing disciplinary actions. The laws generally require patients or members of the public to file a complaint before an investigation can start. Some states, however, do allow their medical boards to take rapid emergency action if a physician poses an immediate threat to patients or the public.
Another hurdle is that medical boards that seek to sanction physicians for making dangerously misleading public statements could face lawsuits alleging that such actions violate the physicians’ constitutional free speech rights or their professional autonomy.
“We have free speech, and you can get away with a lot of stuff,” said Stephen Barrett, MD, who for many years has critically documented examples of medical fraud on his website, Quackwatch. “Some doctors would sue if they were challenged by medical boards, and I’m not sure the boards would win that court fight. People have written books with advice that killed people, and I’m not aware of a single case where the author was disciplined.”
In addition, it’s not clear that U.S. physicians who are not government officials have any legal obligation – as opposed to a moral obligation – to the government or the public to promote public health, said Jonathan Moreno, PhD, a professor of medical ethics at University of Pennsylvania, Philadelphia. “Is transmitting misinformation about COVID-19 public health malpractice?” he asked. “Do we as a society see physicians having a special role as guides in an emergency? I’d like to think we do, but we don’t have a strong tradition like that in the U.S.”
But California State Sen. Richard Pan, MD, a pediatrician who represents the Sacramento area, doesn’t buy the arguments about why medical boards can’t discipline physicians for spreading misinformation. He successfully sponsored a 2019 bill that strengthens the medical board’s ability to discipline physicians who dole out medically unjustified vaccine exemptions to children.
“A medical license is a privilege. It’s an imprimatur from the state that the person is someone who upholds professional standards,” Dr. Pan said. “If someone is intentionally spreading disinformation for personal gain and that’s putting the public at risk, the medical board has a duty to act.”
There have been only a few publicly announced disciplinary actions related to COVID-19 misinformation so far.
Last December, the Oregon Medical Board, on an emergency basis, suspended the license of Steven LaTulippe, MD, of Dallas, Ore. He had publicly announced that he and his staff were not wearing masks in his clinic. In addition, he compared COVID-19 to the common cold and denied the governor’s legal authority to adopt public health protection measures. A recorded message on his office phone said he’s challenging the licensure action in court.
Last January, the Medical Board of California made Thomas Cowan, MD, of San Francisco surrender his license after Dr. Cowan posted a YouTube video, which went viral last year, that claimed that 5G Internet networks cause COVID-19. He did not respond to a request for comment.
In May, the College of Physicians and Surgeons of British Columbia reprimanded Stephen Malthouse, MD, and forbade him from speaking on issues related to COVID-19. He had written a widely circulated open letter to the province’s chief health office claiming that the pandemic was “over” and that measures to control the spread of COVID-19 were worse than the virus. He has challenged the disciplinary action in court, alleging it violates his right to free speech.
Attacking the problem from a different angle, the U.S. Federal Trade Commission has issued enforcement actions in cases in which physicians and other health care professionals engaged in deceptive business practices related to COVID-19. That approach may be applicable to a number of physicians accused of spreading COVID-19 misinformation, who allegedly have done so at least partly to sell unproven products and services to prevent or treat the disease.
In June, the FTC settled a case against Stephen Meis, MD, of Porterville, Calif. The settlement required that he stop making unsupported claims that his company’s dietary supplements effectively treat COVID-19 symptoms and that he pay $103,420 in refunds to defrauded customers.
State medical boards in the United States generally are not allowed to disclose investigations or disciplinary processes until they finalize a disciplinary action, so other investigations that have not been publicly disclosed may be pending.
A spokesperson for the Medical Board of California said the board is aware of questionable statements about COVID-19 made by several physicians and “will be looking into it.” That comment was in response to a question about statements made at a news conference last year by two Bakersfield emergency physicians, Artin Massihi, MD, and Dan Erickson, DO. They claimed that their COVID-19 testing data showed that the virus is not that dangerous. Dr. Erickson is an osteopath and is regulated by the Osteopathic Medical Board of California.
The two physicians’ news conference prompted an unusual joint statement from the American College of Emergency Medicine and the American Academy of Emergency Medicine in April 2020 declaring that they “emphatically condemn” Dr. Massihi’s and Dr. Erickson’s “reckless and untested musings.” The groups added that it appeared that the physicians issued the comments “to advance their personal financial interests without regard for the public’s health.”
Neither Dr. Massihi nor Dr. Erickson responded to a request for comment.
As for the physician dubbed by the Center for Countering Digital Hate as the world’s most influential spreader of COVID-19 misinformation on social media: No recent public complaints have been filed, and no disciplinary action has been taken against Dr. Mercola, according to a spokesman for the Illinois Department of Financial and Professional Regulation.
According to court records, Dr. Mercola faced disciplinary complaints from the Illinois board in the early 2000s for allegedly providing false and potentially harmful medical advice on his website. There is no record of any final disciplinary action taken against him.
In widely disseminated online posts, Dr. Mercola has called the COVID-19 pandemic a “scam” and said “forced vaccination” is part of a plan to re-set the global economic system. He called COVID-19 vaccines “a medical fraud,” claiming they “alter your genetic coding.” In February, the U.S. Food and Drug Administration ordered Dr. Mercola to stop saying on his website that various vitamins and dietary supplements he sells through his website are effective in preventing or treating COVID-19.
The New York Times reported in July that Dr. Mercola’s English-language Facebook page has more than 1.7 million followers, that his Spanish-language page has one million, and that he has 300,000 followers on Twitter and 400,000 on YouTube.
In August, Dr. Mercola announced that he was deleting the large archive of articles he’s written on his website but would continue to post articles every day that would be available on the site for only 48 hours. He explained his decision by saying he’s facing “blatant censorship” as part of a “McCarthyism-like attack” from “the sitting President of the United States.” He encouraged people to read his book, “The Truth about COVID-19.”
The lack of action against Dr. Mercola for his lengthy list of scientifically unfounded statements and marketing claims about COVID-19 and other medical conditions infuriates Quackwatch’s Dr. Barrett. He’s amazed that the Illinois board did not discipline Dr. Mercola despite a number of enforcement actions against him by the FTC and the FDA.
“If a doctor were to say to a patient, ‘Don’t wear a mask and don’t get vaccinated,’ the doctor would be held responsible for a bad outcome,” he said. “But if you say it to millions and as a direct result a dozen people die, shouldn’t the doctor also be held responsible for that misinformation? I think he should lose his license.”
Another of the four physicians cited in the “Disinformation Dozen” report is Sherri Tenpenny, DO, an osteopath licensed in Ohio, who has published posts on social media advocating against masking, testing, and vaccines to prevent COVID-19 infections. A spokesperson for the State Medical Board of Ohio said Dr. Tenpenny’s license expires on Oct. 1, 2021, and that any investigation would be confidential. She added that grounds for disciplinary action include “making a false, fraudulent, deceptive, or misleading statement in relation to the practice of medicine and surgery.” Dr. Tenpenny could not be reached for comment.
A third physician named in the report is Christiane Northrup, MD, an ob.gyn. formerly licensed in Maine, who has published posts advocating unproven cures for COVID-19 and claiming that vaccines increase chronic illness. Dennis Smith, executive director of the Maine Board of Licensure in Medicine, said the board received complaints about Dr. Northrup’s posts but can’t act because she withdrew her Maine license in 2015. He added that the Maine board can issue sanctions against physicians who engage in fraud, deceit, or misrepresentation or who post scientifically unfounded statements online.
The fourth physician identified in the “Disinformation Dozen” report is Rashid Buttar, DO, an osteopath practicing in Mooresville, N.C., who has claimed in social media posts that COVID-19 vaccines cause infertility and that COVID-19 tests contain living microorganisms. A spokeswoman for the North Carolina Medical Board said she could not confirm or deny the existence of any investigation of Dr. Buttar, who signed a consent order with the medical board in 2010 following charges of exorbitant fees, worthless tests and treatment, and false diagnoses. Undisclosed conditions were placed on his medical license in 2013. The spokesperson added that the board would investigate any information alleging that a physician spread false information about COVID-19.
Another physician who has caused widespread consternation over scientifically unfounded statements about COVID-19 is Simone Gold, MD, formerly an emergency department physician in Los Angeles. She founded a group called America’s Frontline Doctors, which filed a federal lawsuit in Alabama this spring to block the FDA from issuing an emergency use authorization allowing teenagers to receive COVID-19 vaccinations. She called the vaccines “an experimental biological agent whose harms are well-documented.”
Last summer, Dr. Gold and other physicians in her group held a news conference on the steps of the U.S. Supreme Court Building promoting hydroxychloroquine as a COVID-19 treatment. They declared that masks don’t work and that the virus isn’t deadly, and made other false claims. The news conference was livestreamed by conservative media outlets, was promoted on Twitter by then-President Trump and his family, and was viewed online more than 14 million times.
One of the participating physicians, Stella Immanuel, MD, of Houston, claimed in a video that went viral that she had successfully used hydroxychloroquine for more than 400 patients to cure the disease. In response, the Texas Medical Board, without naming Dr. Immanuel, warned that if it received a complaint about any physician who made a false claim about having a cure for COVID-19, it would investigate and potentially take disciplinary action.
Although no publicly known disciplinary action has been taken against Dr. Gold, she told The Washington Post last January that after participating in that July 2020 news conference, she was fired from her emergency department job at two hospitals and that she hasn’t worked as a physician since. Dr. Gold did not respond to a request for comment.
The outcome in her situation is consistent with the view of NYU’s Dr. Caplan that methods other than medical board discipline – such as action by employers, social media pressure, and reprimands from professional societies –will have to be used to hold physicians accountable for spreading COVID-19 misinformation.
“I’m disappointed to have to say it, but I don’t think medical boards are going to be effective,” he said. “We don’t know how to manage misinformation despite being in a plague. We just don’t.
A version of this article first appeared on Medscape.com.
Delta whiplash: How the new surge is affecting mental health
Thanks to the rollout of the COVID-19 vaccines, more than just flowers were blooming this past spring. People came out of lockdown like bears emerging from hibernation, making plans to reunite with friends and loved ones they hadn’t seen in months. But with the tremendous surge in cases brought by the Delta variant, this summer has been anything but sunny and carefree. Case counts have once more reached prevaccination levels. In a repeat of last summer, people are canceling travel plans, and the lead-up to the new school year has become fraught and stressful.
“This whiplash is causing people to feel a variety of emotions: disappointment, uncertainty, anxiety, possibly anger and frustration,” says Vaile Wright, Ph.D., senior director of health care innovation at the American Psychological Association. “When it seemed like there was a light at the end of the tunnel, and we have the tools to overcome [the virus], and we’re not really using them, it can be hard for people to understand.”
The importance of hope
For decades, researchers have been digging into the crucial role hopefulness plays in mental health. The vaccine rollout, earlier than anticipated, provided a much needed burst of hope after months of bad news.
“It was a feeling of almost euphoria in June: ‘We’re going to see everybody!’” says Rachel Goldenberg, a rabbi in Jackson Heights, NY. “We have a theme for our High Holidays, and this year’s is very hopeful: Sow in tears, reap in joy. It felt like the sowing in tears part was behind us, and we were looking forward to reaping in joy. Slowly but surely, with Delta, everything has turned upside down.”
For Roxanne Hawn, a writer in Golden, Colo., vaccination offered a glimpse of something like normal life.
“I wore cute clothes. I stopped and got takeout for lunch. I bought myself flowers. I even had a little uplifting soundtrack for that time of hope and relief,” she says. “With the Delta variant, it feels like that window of normalcy closed quickly.”
Having that little bit of hope dashed can wear down even the sturdiest spirits, says Marissa King, PhD, author of “Social Chemistry: Decoding the Elements of Human Connection”.
“There was a moment when we were able to reconnect, to experience joy and the hope of being able to revitalize relationships,” she says. “The loss of that hope and the fear of being isolated again is causing so much distress.”
A new kind of loneliness
When the pandemic started, mom of three Julie Schwietert Collazo formed a WhatsApp group with several friends who were taking lockdown seriously. They got each other through months of isolation and celebrated the idea of reopening. Then Ms. Collazo’s oldest got COVID, just 5 weeks before her 12th birthday, and their family went back into quarantine. Her moms’ group is no longer on the same page about precautions.
“Last year we were doing it together, and it made it feel a bit easier,” she says. “As things started to normalize, everybody started thinking and moving in different directions. It feels like we’re not working through the same issues collectively like before.”
Dr. King says the feeling Ms. Collazo describes is quite common these days.
“A profound sense of loneliness comes from feeling like you’re the only one,” she says. “There’s such disagreement about the best path forward, it can feel lonely just because you think differently.”
An epidemic of anxiety
As the Delta variant drives case numbers back up again, worries increase as well.
“Is this ever going to end?” asks Ms. Collazo. “Is this our new reality, constantly having to order our lives around COVID?”
This uneasiness affects our well-being.
The National Center for Health Statistics and the Census Bureau have monitored the nation’s mental health via the ongoing Household Pulse Survey during the pandemic. It asks participants about their symptoms of either anxiety or depression. Throughout, more people have reported feeling anxious than depressed.
Anxiety peaked around Thanksgiving and Christmas, with nearly 38% of people reporting symptoms. The first vaccines began to roll out around that time, and anxiety levels steadily went down through the spring and early summer, dipping below 25% in late June. But those numbers have begun to creep back up – the most recent data, which goes through Aug. 2, found 27% of Americans reporting symptoms of anxiety.
“Nervous is the new normal,” says Vivian Pender, MD, president of the American Psychiatric Association. “Uncertainty makes people feel anxious.”
Empathy vs. anger
The way politics play into basic measures like mask-wearing and vaccination adds its own layer of stress. Physical altercations have resulted: In Los Angeles, a participant was stabbed at an antivaccination protest. At an Austin, Tex., elementary school, angry parents physically and verbally assaulted teachers who wore masks. Things have gotten so heated, the Department of Homeland Security issued a National Terrorism Advisory System bulletin last week. It warns that extremists could use new COVID-driven public health restrictions as an excuse to commit domestic terrorism.
Anger goes in the opposite direction, too, with people who’ve been following recommended procedures becoming increasingly fed up with those who flout them. Those intense emotions may not lead to violence, but they do make it harder for us to feel secure.
“It’s a public health crisis, and it’s turned into something different. When we get into us/them situations, we start to lose empathy. Empathy is important to identify solutions and work together as a community,” says Dr. Wright. “That’s what sparks the anger: the sense of ‘You aren’t doing what you’re supposed to be doing.’”
How to cope
Loneliness, anxiety, and anger may be swirling all around you right now. But that doesn’t make you powerless to boost your mental health. These suggestions may help:
- Trust your gut. If your community is reopening faster than feels comfortable to you, do whatever makes your family feel safe. “Ask yourself how you’re feeling, and use your feelings to guide your decisions,” says Dr. Pender. “Get more information, then follow the science.”
- Stop judging yourself. If you’re feeling lonely or mourning the losses COVID has brought, don’t fight it, says Wright. “Let it be an emotion that comes and goes, and try to find ways to feel connected to other people.”
- Practice self-care. It may sound simplistic, but eating healthy foods, exercising, and getting a good night’s sleep can all contribute to a more positive
- Try to ease anxiety. Meditation, calming self-talk, and soothing music can all lift your spirits. Or try diaphragmatic breathing: Breathe in for 5 seconds, hold for 2, and breathe out for 5. Even squeezing a stress ball can give you a tangible sense of
- Take action. Both Rabbi Goldenberg and Ms. Collazo, who runs a nonprofit that works to reunite immigrant families, say helping their community helps them feel better. “To sing and lead Shabbat services, even on Zoom, to see the faces of my people, it’s very healing,” says Rabbi Goldenberg. One small thing you can do: If you have family or friends who are hesitant about vaccination, Dr. Wright suggests having gentle conversations to convince them. “You can be way more influential than a celebrity,” she says.
- Remember you’re not alone. Whether you’re physically isolated from others or just feel like nobody else is following the same protocols as you, there are ways to feel connected. “Reach out to people you’ve been close with in the past, but you may have lost touch,” says Dr. King. “It gives you an opportunity to rekindle joy. Particularly in this moment, when a lot of people are so afraid, it’s easier to reach out to those you already know than try to meet new people.” Dr. King’s research has found it takes as few as two close connections to make people feel supported.
- Stay in the present. Instead of stressing over what’s already happened or worrying about what might still come, just think about today. “We’ve learned a lot about the coronavirus, and we’re still learning more,” says Dr. Wright. “We don’t know what the future looks like, but it won’t be like this forever.”
A version of this article first appeared on WebMD.com.
Thanks to the rollout of the COVID-19 vaccines, more than just flowers were blooming this past spring. People came out of lockdown like bears emerging from hibernation, making plans to reunite with friends and loved ones they hadn’t seen in months. But with the tremendous surge in cases brought by the Delta variant, this summer has been anything but sunny and carefree. Case counts have once more reached prevaccination levels. In a repeat of last summer, people are canceling travel plans, and the lead-up to the new school year has become fraught and stressful.
“This whiplash is causing people to feel a variety of emotions: disappointment, uncertainty, anxiety, possibly anger and frustration,” says Vaile Wright, Ph.D., senior director of health care innovation at the American Psychological Association. “When it seemed like there was a light at the end of the tunnel, and we have the tools to overcome [the virus], and we’re not really using them, it can be hard for people to understand.”
The importance of hope
For decades, researchers have been digging into the crucial role hopefulness plays in mental health. The vaccine rollout, earlier than anticipated, provided a much needed burst of hope after months of bad news.
“It was a feeling of almost euphoria in June: ‘We’re going to see everybody!’” says Rachel Goldenberg, a rabbi in Jackson Heights, NY. “We have a theme for our High Holidays, and this year’s is very hopeful: Sow in tears, reap in joy. It felt like the sowing in tears part was behind us, and we were looking forward to reaping in joy. Slowly but surely, with Delta, everything has turned upside down.”
For Roxanne Hawn, a writer in Golden, Colo., vaccination offered a glimpse of something like normal life.
“I wore cute clothes. I stopped and got takeout for lunch. I bought myself flowers. I even had a little uplifting soundtrack for that time of hope and relief,” she says. “With the Delta variant, it feels like that window of normalcy closed quickly.”
Having that little bit of hope dashed can wear down even the sturdiest spirits, says Marissa King, PhD, author of “Social Chemistry: Decoding the Elements of Human Connection”.
“There was a moment when we were able to reconnect, to experience joy and the hope of being able to revitalize relationships,” she says. “The loss of that hope and the fear of being isolated again is causing so much distress.”
A new kind of loneliness
When the pandemic started, mom of three Julie Schwietert Collazo formed a WhatsApp group with several friends who were taking lockdown seriously. They got each other through months of isolation and celebrated the idea of reopening. Then Ms. Collazo’s oldest got COVID, just 5 weeks before her 12th birthday, and their family went back into quarantine. Her moms’ group is no longer on the same page about precautions.
“Last year we were doing it together, and it made it feel a bit easier,” she says. “As things started to normalize, everybody started thinking and moving in different directions. It feels like we’re not working through the same issues collectively like before.”
Dr. King says the feeling Ms. Collazo describes is quite common these days.
“A profound sense of loneliness comes from feeling like you’re the only one,” she says. “There’s such disagreement about the best path forward, it can feel lonely just because you think differently.”
An epidemic of anxiety
As the Delta variant drives case numbers back up again, worries increase as well.
“Is this ever going to end?” asks Ms. Collazo. “Is this our new reality, constantly having to order our lives around COVID?”
This uneasiness affects our well-being.
The National Center for Health Statistics and the Census Bureau have monitored the nation’s mental health via the ongoing Household Pulse Survey during the pandemic. It asks participants about their symptoms of either anxiety or depression. Throughout, more people have reported feeling anxious than depressed.
Anxiety peaked around Thanksgiving and Christmas, with nearly 38% of people reporting symptoms. The first vaccines began to roll out around that time, and anxiety levels steadily went down through the spring and early summer, dipping below 25% in late June. But those numbers have begun to creep back up – the most recent data, which goes through Aug. 2, found 27% of Americans reporting symptoms of anxiety.
“Nervous is the new normal,” says Vivian Pender, MD, president of the American Psychiatric Association. “Uncertainty makes people feel anxious.”
Empathy vs. anger
The way politics play into basic measures like mask-wearing and vaccination adds its own layer of stress. Physical altercations have resulted: In Los Angeles, a participant was stabbed at an antivaccination protest. At an Austin, Tex., elementary school, angry parents physically and verbally assaulted teachers who wore masks. Things have gotten so heated, the Department of Homeland Security issued a National Terrorism Advisory System bulletin last week. It warns that extremists could use new COVID-driven public health restrictions as an excuse to commit domestic terrorism.
Anger goes in the opposite direction, too, with people who’ve been following recommended procedures becoming increasingly fed up with those who flout them. Those intense emotions may not lead to violence, but they do make it harder for us to feel secure.
“It’s a public health crisis, and it’s turned into something different. When we get into us/them situations, we start to lose empathy. Empathy is important to identify solutions and work together as a community,” says Dr. Wright. “That’s what sparks the anger: the sense of ‘You aren’t doing what you’re supposed to be doing.’”
How to cope
Loneliness, anxiety, and anger may be swirling all around you right now. But that doesn’t make you powerless to boost your mental health. These suggestions may help:
- Trust your gut. If your community is reopening faster than feels comfortable to you, do whatever makes your family feel safe. “Ask yourself how you’re feeling, and use your feelings to guide your decisions,” says Dr. Pender. “Get more information, then follow the science.”
- Stop judging yourself. If you’re feeling lonely or mourning the losses COVID has brought, don’t fight it, says Wright. “Let it be an emotion that comes and goes, and try to find ways to feel connected to other people.”
- Practice self-care. It may sound simplistic, but eating healthy foods, exercising, and getting a good night’s sleep can all contribute to a more positive
- Try to ease anxiety. Meditation, calming self-talk, and soothing music can all lift your spirits. Or try diaphragmatic breathing: Breathe in for 5 seconds, hold for 2, and breathe out for 5. Even squeezing a stress ball can give you a tangible sense of
- Take action. Both Rabbi Goldenberg and Ms. Collazo, who runs a nonprofit that works to reunite immigrant families, say helping their community helps them feel better. “To sing and lead Shabbat services, even on Zoom, to see the faces of my people, it’s very healing,” says Rabbi Goldenberg. One small thing you can do: If you have family or friends who are hesitant about vaccination, Dr. Wright suggests having gentle conversations to convince them. “You can be way more influential than a celebrity,” she says.
- Remember you’re not alone. Whether you’re physically isolated from others or just feel like nobody else is following the same protocols as you, there are ways to feel connected. “Reach out to people you’ve been close with in the past, but you may have lost touch,” says Dr. King. “It gives you an opportunity to rekindle joy. Particularly in this moment, when a lot of people are so afraid, it’s easier to reach out to those you already know than try to meet new people.” Dr. King’s research has found it takes as few as two close connections to make people feel supported.
- Stay in the present. Instead of stressing over what’s already happened or worrying about what might still come, just think about today. “We’ve learned a lot about the coronavirus, and we’re still learning more,” says Dr. Wright. “We don’t know what the future looks like, but it won’t be like this forever.”
A version of this article first appeared on WebMD.com.
Thanks to the rollout of the COVID-19 vaccines, more than just flowers were blooming this past spring. People came out of lockdown like bears emerging from hibernation, making plans to reunite with friends and loved ones they hadn’t seen in months. But with the tremendous surge in cases brought by the Delta variant, this summer has been anything but sunny and carefree. Case counts have once more reached prevaccination levels. In a repeat of last summer, people are canceling travel plans, and the lead-up to the new school year has become fraught and stressful.
“This whiplash is causing people to feel a variety of emotions: disappointment, uncertainty, anxiety, possibly anger and frustration,” says Vaile Wright, Ph.D., senior director of health care innovation at the American Psychological Association. “When it seemed like there was a light at the end of the tunnel, and we have the tools to overcome [the virus], and we’re not really using them, it can be hard for people to understand.”
The importance of hope
For decades, researchers have been digging into the crucial role hopefulness plays in mental health. The vaccine rollout, earlier than anticipated, provided a much needed burst of hope after months of bad news.
“It was a feeling of almost euphoria in June: ‘We’re going to see everybody!’” says Rachel Goldenberg, a rabbi in Jackson Heights, NY. “We have a theme for our High Holidays, and this year’s is very hopeful: Sow in tears, reap in joy. It felt like the sowing in tears part was behind us, and we were looking forward to reaping in joy. Slowly but surely, with Delta, everything has turned upside down.”
For Roxanne Hawn, a writer in Golden, Colo., vaccination offered a glimpse of something like normal life.
“I wore cute clothes. I stopped and got takeout for lunch. I bought myself flowers. I even had a little uplifting soundtrack for that time of hope and relief,” she says. “With the Delta variant, it feels like that window of normalcy closed quickly.”
Having that little bit of hope dashed can wear down even the sturdiest spirits, says Marissa King, PhD, author of “Social Chemistry: Decoding the Elements of Human Connection”.
“There was a moment when we were able to reconnect, to experience joy and the hope of being able to revitalize relationships,” she says. “The loss of that hope and the fear of being isolated again is causing so much distress.”
A new kind of loneliness
When the pandemic started, mom of three Julie Schwietert Collazo formed a WhatsApp group with several friends who were taking lockdown seriously. They got each other through months of isolation and celebrated the idea of reopening. Then Ms. Collazo’s oldest got COVID, just 5 weeks before her 12th birthday, and their family went back into quarantine. Her moms’ group is no longer on the same page about precautions.
“Last year we were doing it together, and it made it feel a bit easier,” she says. “As things started to normalize, everybody started thinking and moving in different directions. It feels like we’re not working through the same issues collectively like before.”
Dr. King says the feeling Ms. Collazo describes is quite common these days.
“A profound sense of loneliness comes from feeling like you’re the only one,” she says. “There’s such disagreement about the best path forward, it can feel lonely just because you think differently.”
An epidemic of anxiety
As the Delta variant drives case numbers back up again, worries increase as well.
“Is this ever going to end?” asks Ms. Collazo. “Is this our new reality, constantly having to order our lives around COVID?”
This uneasiness affects our well-being.
The National Center for Health Statistics and the Census Bureau have monitored the nation’s mental health via the ongoing Household Pulse Survey during the pandemic. It asks participants about their symptoms of either anxiety or depression. Throughout, more people have reported feeling anxious than depressed.
Anxiety peaked around Thanksgiving and Christmas, with nearly 38% of people reporting symptoms. The first vaccines began to roll out around that time, and anxiety levels steadily went down through the spring and early summer, dipping below 25% in late June. But those numbers have begun to creep back up – the most recent data, which goes through Aug. 2, found 27% of Americans reporting symptoms of anxiety.
“Nervous is the new normal,” says Vivian Pender, MD, president of the American Psychiatric Association. “Uncertainty makes people feel anxious.”
Empathy vs. anger
The way politics play into basic measures like mask-wearing and vaccination adds its own layer of stress. Physical altercations have resulted: In Los Angeles, a participant was stabbed at an antivaccination protest. At an Austin, Tex., elementary school, angry parents physically and verbally assaulted teachers who wore masks. Things have gotten so heated, the Department of Homeland Security issued a National Terrorism Advisory System bulletin last week. It warns that extremists could use new COVID-driven public health restrictions as an excuse to commit domestic terrorism.
Anger goes in the opposite direction, too, with people who’ve been following recommended procedures becoming increasingly fed up with those who flout them. Those intense emotions may not lead to violence, but they do make it harder for us to feel secure.
“It’s a public health crisis, and it’s turned into something different. When we get into us/them situations, we start to lose empathy. Empathy is important to identify solutions and work together as a community,” says Dr. Wright. “That’s what sparks the anger: the sense of ‘You aren’t doing what you’re supposed to be doing.’”
How to cope
Loneliness, anxiety, and anger may be swirling all around you right now. But that doesn’t make you powerless to boost your mental health. These suggestions may help:
- Trust your gut. If your community is reopening faster than feels comfortable to you, do whatever makes your family feel safe. “Ask yourself how you’re feeling, and use your feelings to guide your decisions,” says Dr. Pender. “Get more information, then follow the science.”
- Stop judging yourself. If you’re feeling lonely or mourning the losses COVID has brought, don’t fight it, says Wright. “Let it be an emotion that comes and goes, and try to find ways to feel connected to other people.”
- Practice self-care. It may sound simplistic, but eating healthy foods, exercising, and getting a good night’s sleep can all contribute to a more positive
- Try to ease anxiety. Meditation, calming self-talk, and soothing music can all lift your spirits. Or try diaphragmatic breathing: Breathe in for 5 seconds, hold for 2, and breathe out for 5. Even squeezing a stress ball can give you a tangible sense of
- Take action. Both Rabbi Goldenberg and Ms. Collazo, who runs a nonprofit that works to reunite immigrant families, say helping their community helps them feel better. “To sing and lead Shabbat services, even on Zoom, to see the faces of my people, it’s very healing,” says Rabbi Goldenberg. One small thing you can do: If you have family or friends who are hesitant about vaccination, Dr. Wright suggests having gentle conversations to convince them. “You can be way more influential than a celebrity,” she says.
- Remember you’re not alone. Whether you’re physically isolated from others or just feel like nobody else is following the same protocols as you, there are ways to feel connected. “Reach out to people you’ve been close with in the past, but you may have lost touch,” says Dr. King. “It gives you an opportunity to rekindle joy. Particularly in this moment, when a lot of people are so afraid, it’s easier to reach out to those you already know than try to meet new people.” Dr. King’s research has found it takes as few as two close connections to make people feel supported.
- Stay in the present. Instead of stressing over what’s already happened or worrying about what might still come, just think about today. “We’ve learned a lot about the coronavirus, and we’re still learning more,” says Dr. Wright. “We don’t know what the future looks like, but it won’t be like this forever.”
A version of this article first appeared on WebMD.com.
How to pick the best face masks for children, according to the experts
One essential back-to-school item for children this fall is a face mask – the Centers for Disease Control and Prevention and the American Academy of Pediatrics both recommend them – but finding one that’s actually protective for a child is not a straightforward task, as many parents can attest.
There’s little in the way of official guidance or research to inform evidence-based recommendations on what type of face masks works best for children.
Search for children’s face masks on Amazon and you’ll run into a smorgasbord of options: masks with three, four, or five layers, different designs, and different materials. There’s one company selling a mask it calls an m95 model, a term the company devised.
It’s almost impossible to verify many of the claims being made by the manufacturers, or to know if they will fit your child’s face until you order some, which can get expensive.
But it’s worth looking for a good mask. A large study of more than 1 million people being conducted online by Facebook and Carnegie Mellon University found that students who wore face masks in school had a reduced risk for testing positive for the virus and getting sick with COVID-19 symptoms. The study was published in June in the journal Science.
Delta more contagious
The Delta variant of the new coronavirus is much more contagious than previous versions of the virus. Studies have shown that infected people carry 1,000 times more virus in their noses and throats than with the viruses that circulated last winter and spring. They shed more viral particles into the air when they talk or yell or sing, making this COVID-19–causing virus much more transmissible than in the past.
What that means, says Kimberly Prather, PhD, an aerosol scientist and distinguished professor at the Scripps Institution of Oceanography in La Jolla, California, is that if it once took about 15 minutes of proximity to an infected person to catch the infection, that window of risk is now much shorter.
“If you believe the 15-minute magical number, now if you take 1,000 times the viral load, basically in 1 second you could inhale that same amount of virus. So it’s gone from 15 minutes to 1 second,” Dr. Prather said in an online seminar on school safety she helped to organize.
A better mask
What that means is that we need to upgrade our face masks, switching away from ill-fitting fabric masks, which can offer varying degrees of protection depending on the number of layers and type of fabric that’s used, to more highly protective surgical masks or better yet, N95 respirators, which provide the highest level of filtration.
That’s harder to do for children, who have much smaller faces.
Any masks that gapes around the edges isn’t going to work well, no matter how well it filters.
“N95s are not made to fit kids. They do not come in kid sizes, so I do not recommend N95s for kids,” said Linsey Marr, an environmental engineer at Virginia Tech, who tests face masks in her lab.
Ms. Marr says parents need to consider the attributes masks in this order of priority:
Comfort: “If your kid won’t wear it, it’s not helping at all,” she said.
Fit: “Leaks around the sides are like having a hole in your mask and aerosols carrying the virus can get right through,” Ms. Marr said.
Filtration: How well the mask blocks small particles.
One option to improve fit is to layer a fabric mask over a surgical mask. The fabric mask helps to hold the edges of the surgical mask more tightly to a person’s face. The surgical mask creates better filtration.
Ms. Marr said KN94 or KN95 masks, which are being manufactured in China and Korea, are good choices. They offer nearly the same degree of filtration as an N95, and they fit closely to the face, to minimize leaks.
Check for counterfeits
The KN94 and KN95 masks for children are widely available, but Ms. Marr said parents do need to watch out for counterfeits, which don’t perform as well.
The National Institute for Occupational Safety and Health gives examples of counterfeit products here.
There’s also a type of cloth mask that has a built-in, edge-to-edge filter layer that is made for children.
“Some of these filter out more than 99% of particles and those can be very effective, if they fit well,” Ms. Marr said.
She has compiled and publicly posted a list of her recommendations for masks for children.
There’s also a new standard for face masks. It’s called ASTM F3502-21, and it’s published by an international organization that sets voluntary standards for thousands of products. To claim that a mask meets this standard, a manufacturer has to have its mask tested and demonstrate that it provides a certain level of filtration and breathability.
A version of this article first appeared on Medscape.com.
One essential back-to-school item for children this fall is a face mask – the Centers for Disease Control and Prevention and the American Academy of Pediatrics both recommend them – but finding one that’s actually protective for a child is not a straightforward task, as many parents can attest.
There’s little in the way of official guidance or research to inform evidence-based recommendations on what type of face masks works best for children.
Search for children’s face masks on Amazon and you’ll run into a smorgasbord of options: masks with three, four, or five layers, different designs, and different materials. There’s one company selling a mask it calls an m95 model, a term the company devised.
It’s almost impossible to verify many of the claims being made by the manufacturers, or to know if they will fit your child’s face until you order some, which can get expensive.
But it’s worth looking for a good mask. A large study of more than 1 million people being conducted online by Facebook and Carnegie Mellon University found that students who wore face masks in school had a reduced risk for testing positive for the virus and getting sick with COVID-19 symptoms. The study was published in June in the journal Science.
Delta more contagious
The Delta variant of the new coronavirus is much more contagious than previous versions of the virus. Studies have shown that infected people carry 1,000 times more virus in their noses and throats than with the viruses that circulated last winter and spring. They shed more viral particles into the air when they talk or yell or sing, making this COVID-19–causing virus much more transmissible than in the past.
What that means, says Kimberly Prather, PhD, an aerosol scientist and distinguished professor at the Scripps Institution of Oceanography in La Jolla, California, is that if it once took about 15 minutes of proximity to an infected person to catch the infection, that window of risk is now much shorter.
“If you believe the 15-minute magical number, now if you take 1,000 times the viral load, basically in 1 second you could inhale that same amount of virus. So it’s gone from 15 minutes to 1 second,” Dr. Prather said in an online seminar on school safety she helped to organize.
A better mask
What that means is that we need to upgrade our face masks, switching away from ill-fitting fabric masks, which can offer varying degrees of protection depending on the number of layers and type of fabric that’s used, to more highly protective surgical masks or better yet, N95 respirators, which provide the highest level of filtration.
That’s harder to do for children, who have much smaller faces.
Any masks that gapes around the edges isn’t going to work well, no matter how well it filters.
“N95s are not made to fit kids. They do not come in kid sizes, so I do not recommend N95s for kids,” said Linsey Marr, an environmental engineer at Virginia Tech, who tests face masks in her lab.
Ms. Marr says parents need to consider the attributes masks in this order of priority:
Comfort: “If your kid won’t wear it, it’s not helping at all,” she said.
Fit: “Leaks around the sides are like having a hole in your mask and aerosols carrying the virus can get right through,” Ms. Marr said.
Filtration: How well the mask blocks small particles.
One option to improve fit is to layer a fabric mask over a surgical mask. The fabric mask helps to hold the edges of the surgical mask more tightly to a person’s face. The surgical mask creates better filtration.
Ms. Marr said KN94 or KN95 masks, which are being manufactured in China and Korea, are good choices. They offer nearly the same degree of filtration as an N95, and they fit closely to the face, to minimize leaks.
Check for counterfeits
The KN94 and KN95 masks for children are widely available, but Ms. Marr said parents do need to watch out for counterfeits, which don’t perform as well.
The National Institute for Occupational Safety and Health gives examples of counterfeit products here.
There’s also a type of cloth mask that has a built-in, edge-to-edge filter layer that is made for children.
“Some of these filter out more than 99% of particles and those can be very effective, if they fit well,” Ms. Marr said.
She has compiled and publicly posted a list of her recommendations for masks for children.
There’s also a new standard for face masks. It’s called ASTM F3502-21, and it’s published by an international organization that sets voluntary standards for thousands of products. To claim that a mask meets this standard, a manufacturer has to have its mask tested and demonstrate that it provides a certain level of filtration and breathability.
A version of this article first appeared on Medscape.com.
One essential back-to-school item for children this fall is a face mask – the Centers for Disease Control and Prevention and the American Academy of Pediatrics both recommend them – but finding one that’s actually protective for a child is not a straightforward task, as many parents can attest.
There’s little in the way of official guidance or research to inform evidence-based recommendations on what type of face masks works best for children.
Search for children’s face masks on Amazon and you’ll run into a smorgasbord of options: masks with three, four, or five layers, different designs, and different materials. There’s one company selling a mask it calls an m95 model, a term the company devised.
It’s almost impossible to verify many of the claims being made by the manufacturers, or to know if they will fit your child’s face until you order some, which can get expensive.
But it’s worth looking for a good mask. A large study of more than 1 million people being conducted online by Facebook and Carnegie Mellon University found that students who wore face masks in school had a reduced risk for testing positive for the virus and getting sick with COVID-19 symptoms. The study was published in June in the journal Science.
Delta more contagious
The Delta variant of the new coronavirus is much more contagious than previous versions of the virus. Studies have shown that infected people carry 1,000 times more virus in their noses and throats than with the viruses that circulated last winter and spring. They shed more viral particles into the air when they talk or yell or sing, making this COVID-19–causing virus much more transmissible than in the past.
What that means, says Kimberly Prather, PhD, an aerosol scientist and distinguished professor at the Scripps Institution of Oceanography in La Jolla, California, is that if it once took about 15 minutes of proximity to an infected person to catch the infection, that window of risk is now much shorter.
“If you believe the 15-minute magical number, now if you take 1,000 times the viral load, basically in 1 second you could inhale that same amount of virus. So it’s gone from 15 minutes to 1 second,” Dr. Prather said in an online seminar on school safety she helped to organize.
A better mask
What that means is that we need to upgrade our face masks, switching away from ill-fitting fabric masks, which can offer varying degrees of protection depending on the number of layers and type of fabric that’s used, to more highly protective surgical masks or better yet, N95 respirators, which provide the highest level of filtration.
That’s harder to do for children, who have much smaller faces.
Any masks that gapes around the edges isn’t going to work well, no matter how well it filters.
“N95s are not made to fit kids. They do not come in kid sizes, so I do not recommend N95s for kids,” said Linsey Marr, an environmental engineer at Virginia Tech, who tests face masks in her lab.
Ms. Marr says parents need to consider the attributes masks in this order of priority:
Comfort: “If your kid won’t wear it, it’s not helping at all,” she said.
Fit: “Leaks around the sides are like having a hole in your mask and aerosols carrying the virus can get right through,” Ms. Marr said.
Filtration: How well the mask blocks small particles.
One option to improve fit is to layer a fabric mask over a surgical mask. The fabric mask helps to hold the edges of the surgical mask more tightly to a person’s face. The surgical mask creates better filtration.
Ms. Marr said KN94 or KN95 masks, which are being manufactured in China and Korea, are good choices. They offer nearly the same degree of filtration as an N95, and they fit closely to the face, to minimize leaks.
Check for counterfeits
The KN94 and KN95 masks for children are widely available, but Ms. Marr said parents do need to watch out for counterfeits, which don’t perform as well.
The National Institute for Occupational Safety and Health gives examples of counterfeit products here.
There’s also a type of cloth mask that has a built-in, edge-to-edge filter layer that is made for children.
“Some of these filter out more than 99% of particles and those can be very effective, if they fit well,” Ms. Marr said.
She has compiled and publicly posted a list of her recommendations for masks for children.
There’s also a new standard for face masks. It’s called ASTM F3502-21, and it’s published by an international organization that sets voluntary standards for thousands of products. To claim that a mask meets this standard, a manufacturer has to have its mask tested and demonstrate that it provides a certain level of filtration and breathability.
A version of this article first appeared on Medscape.com.
Inflation will be the end of medicine
Inflation is the senility of democracies.
–Sylvia Townsend Warner
Electronic medical records? No, all of these are minor annoyances in the face of the practice killer, inflation.
Physicians live in a closed box where medical reimbursements are fixed, directly or by contract proxy to the government (Medicare) pay rate. Inflation is projected to be between 5% and 10% this year. We cannot increase our rates to increase the salaries of our employees, cover our increased medical disposable costs, and pay more for our state licensures and DEA registrations. No, we must try to find savings in our budget, which we have been squeezing for years.
Currently, medicine is facing a 9.75% cut in Medicare reimbursements, which will reset most private insurance rates, based on a percentage of Medicare. The temporary 3.75% conversion factor (CF) increase for all services is expiring. Also expiring is the 2% sequester from the Budget Control Act of 2011 (BCA), signed into law in August 2011. This was originally scheduled to sunset in 2021, but is going to continue to 2030.
A 4% statutory pay-as-you-go (PAYGO) sequester resulting from passage of the American Rescue Plan Act is being imposed. Statutory PAYGO is a policy written into law (it can be changed only through new legislation) that requires deficit neutrality overall in the laws (other than annual appropriations) enacted by Congress and imposes automatic spending reductions at the end of the year if such laws increase the deficit when they are added together.
There is a statutory freeze on Medicare Physician Fee Schedule (PFS) updates until 2026, at which time an annual increase of 0.25%, which is lower than inflation, will be enacted. This adds up to a 9.75% cut in Medicare pay until at least 2026. Recall that almost all of your private insurance contracts are tied to Medicare (some more, some less) and that this cut to the physician is doubled if your overhead is fixed at the typical 50% for most practices. This means an almost 20% cut in take-home pay for most physicians.
Now, when considering the most recent inflation number, which projects 5%-10% inflation for this year and at least 2% annually in the future, which compounds yearly (the Fed target), you are looking at catastrophic numbers.
The conversion factor – the pool of money doled out to physicians – has failed to keep pace with inflation – even at 2%-3% a year – and reimbursement is only 50% of what it was when created in 1998, despite small increases by Congress along the way. A recent Wall Street Journal guest editorial claimed that Medicare payments benefited from cost of living adjustments, same as Social Security. I do not agree, hence the 50% pay gap since 1998.
In addition, the costs of running a practice have increased by 37% between 2001 and 2020, 1.7% per year, according to the Medicare Economic Index.
Some of this may include general inflation, but certainly new OSHA rules, electronic medical records, Medicare quality improvement measures, and assorted other costs do not. So based on my own conservative estimate, on top of the 50% decline in the payment pool, physicians’ noninflationary operating costs increased by at least another 10% over the last 20 odd years. This is a 60% decline in reimbursements!
Medicare payments have been under pressure from the Centers for Medicare & Medicaid Services (CMS) anti-inflationary payment policies for more than 20 years. While physician services represent a very modest portion of the overall growth in health care costs, they are an easy target for cuts when policymakers seek to limit spending. Although we avoided direct cuts to reimbursements caused by the Medicare sustainable growth rate formula (SGR) – which was enacted in 1997 and repealed in 2015 – Medicare provider payments have remained constrained by a budget-neutral financing system.
There used to be ways out of the box. Physicians could go to work for hospitals or have their practices acquired by them, resulting in much better hospital-based reimbursement. This has been eliminated by site-neutral payments, which while instituted by President Trump, are unopposed by President Biden. You could also join larger groups with some loss of autonomy, which could presumably negotiate better rates with private insurers as another way out, but these rates are almost always based on a percentage of Medicare as noted above.
There may be a bit of good news, with price transparency being instituted, which again is unopposed by the Biden administration. At least private practice physicians may be able to show their services are a bargain compared to hospitals.
One could also take the low road, and sell out to private equity, but I suspect these deals will become much less attractive since some of these entities are going broke and all will feel the bite of lower reimbursements.
Physicians and patients should rise up and demand better reimbursements for physicians, or there will be no physicians to see. This is not greed, a bigger house, or a newer car, this is becoming a matter of practice survival. And seniors are not greedy, they have paid hundreds of thousands of dollars into Medicare in taxes for health insurance in retirement.
Physicians and retirees should contact their federal legislators and let them know a 9.75% cut is untenable and ask for Medicare rates to be fixed to the cost of living, just as Social Security is. Before we fund trillions of dollars in new government programs, perhaps we should look to the solvency of the existing ones we have.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
Inflation is the senility of democracies.
–Sylvia Townsend Warner
Electronic medical records? No, all of these are minor annoyances in the face of the practice killer, inflation.
Physicians live in a closed box where medical reimbursements are fixed, directly or by contract proxy to the government (Medicare) pay rate. Inflation is projected to be between 5% and 10% this year. We cannot increase our rates to increase the salaries of our employees, cover our increased medical disposable costs, and pay more for our state licensures and DEA registrations. No, we must try to find savings in our budget, which we have been squeezing for years.
Currently, medicine is facing a 9.75% cut in Medicare reimbursements, which will reset most private insurance rates, based on a percentage of Medicare. The temporary 3.75% conversion factor (CF) increase for all services is expiring. Also expiring is the 2% sequester from the Budget Control Act of 2011 (BCA), signed into law in August 2011. This was originally scheduled to sunset in 2021, but is going to continue to 2030.
A 4% statutory pay-as-you-go (PAYGO) sequester resulting from passage of the American Rescue Plan Act is being imposed. Statutory PAYGO is a policy written into law (it can be changed only through new legislation) that requires deficit neutrality overall in the laws (other than annual appropriations) enacted by Congress and imposes automatic spending reductions at the end of the year if such laws increase the deficit when they are added together.
There is a statutory freeze on Medicare Physician Fee Schedule (PFS) updates until 2026, at which time an annual increase of 0.25%, which is lower than inflation, will be enacted. This adds up to a 9.75% cut in Medicare pay until at least 2026. Recall that almost all of your private insurance contracts are tied to Medicare (some more, some less) and that this cut to the physician is doubled if your overhead is fixed at the typical 50% for most practices. This means an almost 20% cut in take-home pay for most physicians.
Now, when considering the most recent inflation number, which projects 5%-10% inflation for this year and at least 2% annually in the future, which compounds yearly (the Fed target), you are looking at catastrophic numbers.
The conversion factor – the pool of money doled out to physicians – has failed to keep pace with inflation – even at 2%-3% a year – and reimbursement is only 50% of what it was when created in 1998, despite small increases by Congress along the way. A recent Wall Street Journal guest editorial claimed that Medicare payments benefited from cost of living adjustments, same as Social Security. I do not agree, hence the 50% pay gap since 1998.
In addition, the costs of running a practice have increased by 37% between 2001 and 2020, 1.7% per year, according to the Medicare Economic Index.
Some of this may include general inflation, but certainly new OSHA rules, electronic medical records, Medicare quality improvement measures, and assorted other costs do not. So based on my own conservative estimate, on top of the 50% decline in the payment pool, physicians’ noninflationary operating costs increased by at least another 10% over the last 20 odd years. This is a 60% decline in reimbursements!
Medicare payments have been under pressure from the Centers for Medicare & Medicaid Services (CMS) anti-inflationary payment policies for more than 20 years. While physician services represent a very modest portion of the overall growth in health care costs, they are an easy target for cuts when policymakers seek to limit spending. Although we avoided direct cuts to reimbursements caused by the Medicare sustainable growth rate formula (SGR) – which was enacted in 1997 and repealed in 2015 – Medicare provider payments have remained constrained by a budget-neutral financing system.
There used to be ways out of the box. Physicians could go to work for hospitals or have their practices acquired by them, resulting in much better hospital-based reimbursement. This has been eliminated by site-neutral payments, which while instituted by President Trump, are unopposed by President Biden. You could also join larger groups with some loss of autonomy, which could presumably negotiate better rates with private insurers as another way out, but these rates are almost always based on a percentage of Medicare as noted above.
There may be a bit of good news, with price transparency being instituted, which again is unopposed by the Biden administration. At least private practice physicians may be able to show their services are a bargain compared to hospitals.
One could also take the low road, and sell out to private equity, but I suspect these deals will become much less attractive since some of these entities are going broke and all will feel the bite of lower reimbursements.
Physicians and patients should rise up and demand better reimbursements for physicians, or there will be no physicians to see. This is not greed, a bigger house, or a newer car, this is becoming a matter of practice survival. And seniors are not greedy, they have paid hundreds of thousands of dollars into Medicare in taxes for health insurance in retirement.
Physicians and retirees should contact their federal legislators and let them know a 9.75% cut is untenable and ask for Medicare rates to be fixed to the cost of living, just as Social Security is. Before we fund trillions of dollars in new government programs, perhaps we should look to the solvency of the existing ones we have.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
Inflation is the senility of democracies.
–Sylvia Townsend Warner
Electronic medical records? No, all of these are minor annoyances in the face of the practice killer, inflation.
Physicians live in a closed box where medical reimbursements are fixed, directly or by contract proxy to the government (Medicare) pay rate. Inflation is projected to be between 5% and 10% this year. We cannot increase our rates to increase the salaries of our employees, cover our increased medical disposable costs, and pay more for our state licensures and DEA registrations. No, we must try to find savings in our budget, which we have been squeezing for years.
Currently, medicine is facing a 9.75% cut in Medicare reimbursements, which will reset most private insurance rates, based on a percentage of Medicare. The temporary 3.75% conversion factor (CF) increase for all services is expiring. Also expiring is the 2% sequester from the Budget Control Act of 2011 (BCA), signed into law in August 2011. This was originally scheduled to sunset in 2021, but is going to continue to 2030.
A 4% statutory pay-as-you-go (PAYGO) sequester resulting from passage of the American Rescue Plan Act is being imposed. Statutory PAYGO is a policy written into law (it can be changed only through new legislation) that requires deficit neutrality overall in the laws (other than annual appropriations) enacted by Congress and imposes automatic spending reductions at the end of the year if such laws increase the deficit when they are added together.
There is a statutory freeze on Medicare Physician Fee Schedule (PFS) updates until 2026, at which time an annual increase of 0.25%, which is lower than inflation, will be enacted. This adds up to a 9.75% cut in Medicare pay until at least 2026. Recall that almost all of your private insurance contracts are tied to Medicare (some more, some less) and that this cut to the physician is doubled if your overhead is fixed at the typical 50% for most practices. This means an almost 20% cut in take-home pay for most physicians.
Now, when considering the most recent inflation number, which projects 5%-10% inflation for this year and at least 2% annually in the future, which compounds yearly (the Fed target), you are looking at catastrophic numbers.
The conversion factor – the pool of money doled out to physicians – has failed to keep pace with inflation – even at 2%-3% a year – and reimbursement is only 50% of what it was when created in 1998, despite small increases by Congress along the way. A recent Wall Street Journal guest editorial claimed that Medicare payments benefited from cost of living adjustments, same as Social Security. I do not agree, hence the 50% pay gap since 1998.
In addition, the costs of running a practice have increased by 37% between 2001 and 2020, 1.7% per year, according to the Medicare Economic Index.
Some of this may include general inflation, but certainly new OSHA rules, electronic medical records, Medicare quality improvement measures, and assorted other costs do not. So based on my own conservative estimate, on top of the 50% decline in the payment pool, physicians’ noninflationary operating costs increased by at least another 10% over the last 20 odd years. This is a 60% decline in reimbursements!
Medicare payments have been under pressure from the Centers for Medicare & Medicaid Services (CMS) anti-inflationary payment policies for more than 20 years. While physician services represent a very modest portion of the overall growth in health care costs, they are an easy target for cuts when policymakers seek to limit spending. Although we avoided direct cuts to reimbursements caused by the Medicare sustainable growth rate formula (SGR) – which was enacted in 1997 and repealed in 2015 – Medicare provider payments have remained constrained by a budget-neutral financing system.
There used to be ways out of the box. Physicians could go to work for hospitals or have their practices acquired by them, resulting in much better hospital-based reimbursement. This has been eliminated by site-neutral payments, which while instituted by President Trump, are unopposed by President Biden. You could also join larger groups with some loss of autonomy, which could presumably negotiate better rates with private insurers as another way out, but these rates are almost always based on a percentage of Medicare as noted above.
There may be a bit of good news, with price transparency being instituted, which again is unopposed by the Biden administration. At least private practice physicians may be able to show their services are a bargain compared to hospitals.
One could also take the low road, and sell out to private equity, but I suspect these deals will become much less attractive since some of these entities are going broke and all will feel the bite of lower reimbursements.
Physicians and patients should rise up and demand better reimbursements for physicians, or there will be no physicians to see. This is not greed, a bigger house, or a newer car, this is becoming a matter of practice survival. And seniors are not greedy, they have paid hundreds of thousands of dollars into Medicare in taxes for health insurance in retirement.
Physicians and retirees should contact their federal legislators and let them know a 9.75% cut is untenable and ask for Medicare rates to be fixed to the cost of living, just as Social Security is. Before we fund trillions of dollars in new government programs, perhaps we should look to the solvency of the existing ones we have.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].